falseFY00017159250001715925ipa:CapitalManagementMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:VehiclesMember2023-04-300001715925ipa:FindersWarrantsMember2023-05-012024-04-300001715925ipa:AccumulatedDepreciationAmountMember2024-04-300001715925ipa:AccumulatedAmortizationMemberipa:IntellectualPropertiesMember2023-05-012024-04-300001715925ipa:OptionTwentyOneMember2024-05-012025-04-300001715925ipa:ProprietaryProcessesMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CertificationsMember2024-05-012025-04-300001715925ifrs-full:LeaseLiabilitiesMember2022-05-012023-04-300001715925ipa:AccumulatedAmortizationMemberipa:ProprietaryProcessesMember2024-04-300001715925ipa:UProteinIpaEuropeAndBioStrandMember2024-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2024-04-300001715925ipa:BiostrandMember2025-04-300001715925ipa:NetBookValueMemberifrs-full:VehiclesMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:AutomobileMember2023-04-300001715925ipa:NetBookValueMemberipa:IntellectualPropertiesMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:FixturesAndFittingsMember2024-04-300001715925ipa:AccumulatedAmortizationMemberipa:InternallyGeneratedDevelopmentCostsMember2023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:LabEquipmentMember2024-05-012025-04-300001715925ifrs-full:AccumulatedOtherComprehensiveIncomeMember2025-04-300001715925ipa:LabEquipmentMemberipa:AccumulatedDepreciationAmountMember2024-05-012025-04-300001715925ipa:RangeTwoMemberipa:FindersWarrantsMember2025-04-300001715925ipa:BiostrandB.v.Member2024-04-300001715925ipa:OptionElevenMember2025-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:BuildingsMember2024-04-300001715925ipa:OptionNineMember2024-05-012025-04-300001715925ipa:NetBookValueMember2025-04-300001715925ipa:BiostrandB.v.Member2025-04-300001715925country:NL2023-04-300001715925ipa:AccumulatedAmortizationMember2024-04-300001715925ipa:ConvertibleDebenturesMember2023-04-300001715925ipa:ProprietaryProcessesMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2023-05-012024-04-300001715925ipa:OptionFourteenMember2024-05-012025-04-300001715925ipa:BiostrandB.v.Memberipa:TrancheOneMemberipa:EscrowAgreementMember2022-04-300001715925country:BE2022-05-012023-04-300001715925ifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:ComputerHardwareMember2023-05-012024-04-300001715925ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-05-012024-04-300001715925ifrs-full:AdditionalPaidinCapitalMember2025-04-300001715925srt:NorthAmericaMember2022-05-012023-04-300001715925ifrs-full:TopOfRangeMemberipa:LabAndOfficeFacilitiesMember2024-05-012025-04-300001715925ipa:ProprietaryProcessesMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925country:BE2023-05-012024-04-300001715925country:CA2022-05-012023-04-300001715925ifrs-full:LeaseLiabilitiesMember2022-04-300001715925country:US2023-05-012024-04-300001715925currency:EUR2024-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:LabEquipmentMember2025-04-300001715925ipa:OssMember2024-04-300001715925ipa:WorkInProgressLeaseholdImrpvementsMemberifrs-full:GrossCarryingAmountMember2024-04-3000017159252025-04-300001715925ipa:ProprietaryProcessesMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ipa:IdeaFamilyB.vMember2022-05-012023-04-300001715925ifrs-full:IssuedCapitalMemberifrs-full:OrdinarySharesMember2024-05-012025-04-300001715925ipa:AllOtherCountriesMember2023-05-012024-04-300001715925country:NL2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:BuildingsMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:BuildingsMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:BuildingsMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesUSALtdIPAUSAMember2024-05-012025-04-300001715925ipa:LabEquipmentMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:UtrechtMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:WorkInProgressLeaseholdImrpvementsMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:LabEquipmentMember2024-05-012025-04-300001715925ipa:RangeTwoMemberipa:FindersWarrantsMember2024-05-012025-04-300001715925ifrs-full:RestrictedShareUnitsMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:ComputerHardwareMember2024-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:BuildingsMember2023-04-300001715925ipa:BiostrandB.v.Member2024-05-012025-04-300001715925ipa:UProteinIpaEuropeAndBioStrandMember2023-04-300001715925ifrs-full:GoodsOrServicesTransferredOverTimeMember2024-05-012025-04-300001715925ifrs-full:RetainedEarningsMember2023-04-300001715925ipa:ImmunoPreciseAntibodiesCanadaLtdMember2023-05-012024-04-300001715925ifrs-full:LiquidityRiskMemberifrs-full:LaterThanFiveYearsMember2025-04-300001715925ipa:OneJanuaryTwentyTwentyFourMemberipa:EmployeesMember2024-05-012025-04-300001715925ipa:BiostrandB.v.Member2022-05-012023-04-300001715925ipa:CustomerListMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:BidCommonSharesMemberifrs-full:BottomOfRangeMember2025-07-132025-07-130001715925ifrs-full:GrossCarryingAmountMemberipa:LabEquipmentMember2023-04-300001715925ipa:OptionTwentyMember2025-04-300001715925srt:EuropeMember2023-05-012024-04-300001715925ipa:OptionSevenMember2025-04-300001715925country:US2024-05-012025-04-300001715925ipa:AccumulatedDepreciationAmountMember2024-05-012025-04-300001715925ifrs-full:VehiclesMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ipa:TalemTherapeuticsLLCTalemMember2024-05-012025-04-300001715925ifrs-full:LaterThanThreeYearsAndNotLaterThanFourYearsMember2025-04-300001715925ifrs-full:BuildingsMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:NetBookValueMemberipa:WorkInProgressLeaseholdImrpvementsMember2025-04-300001715925ipa:NineteenFebruaryTwentyTwentyThreeMemberipa:OfficersAndEmployeesMember2025-04-300001715925ipa:FinancialAssetsCashMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:InternallyGeneratedDevelopmentCostsMember2023-05-012024-04-300001715925ipa:CustomerListMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:ConvertibleDebenturesMember2024-04-300001715925ipa:FindersWarrantsMemberipa:RangeOneMember2025-04-300001715925ipa:NetBookValueMemberipa:AutomobileMember2025-04-300001715925ifrs-full:BuildingsMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:OssMember2024-04-300001715925ipa:BiostrandMember2024-04-300001715925ipa:IntellectualPropertiesMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:LeaseholdImprovementsMember2025-04-300001715925ipa:VictoriaOneMember2023-05-012024-04-300001715925ipa:ProjectRevenueMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMember2024-04-300001715925ipa:OptionThirteenMember2025-04-300001715925ipa:AtTheMarketEquityOfferingFacilityMember2024-05-012025-04-300001715925ipa:WorkInProgressLeaseholdImrpvementsMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:ConvertibleDebenturesMemberipa:YaIiPnLtdMember2024-07-160001715925srt:NorthAmericaMember2023-04-300001715925ipa:LabEquipmentMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:AutomobileMember2024-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:VehiclesMember2024-05-012025-04-300001715925ifrs-full:RestrictedShareUnitsMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2025-04-300001715925ifrs-full:RetainedEarningsMember2023-05-012024-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2023-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2024-04-300001715925ipa:NetBookValueMemberifrs-full:BuildingsMember2024-04-300001715925ipa:ImmunoPreciseAntibodiesEuropeBVIPAEuropeFormerlyModiQuestResearchBVMember2022-05-012023-04-300001715925ipa:LabEquipmentMember2024-05-012025-04-300001715925ipa:OptionTwelveMember2024-05-012025-04-300001715925ipa:TalemTherapeuticsLLCTalemMember2023-05-012024-04-300001715925ifrs-full:TopOfRangeMemberipa:IntellectualPropertiesMember2024-05-012025-04-300001715925ipa:OptionEightMember2025-04-300001715925ipa:OptionTwelveMember2025-04-300001715925ifrs-full:LiquidityRiskMemberifrs-full:LaterThanTwoYearsAndNotLaterThanFiveYearsMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:AutomobileMember2023-04-300001715925ipa:OptionElevenMember2024-05-012025-04-300001715925ipa:LabEquipmentMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2024-05-012025-04-300001715925ifrs-full:BuildingsMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:IssuedCapitalMemberifrs-full:OrdinarySharesMember2023-04-300001715925ifrs-full:LeaseLiabilitiesMember2023-04-300001715925ipa:AccumulatedAmortizationMember2023-05-012024-04-300001715925ipa:BioStrandOneMember2024-05-012025-04-300001715925ipa:VictoriaMember2023-05-012024-04-300001715925country:AU2024-05-012025-04-300001715925ifrs-full:AccumulatedOtherComprehensiveIncomeMember2024-05-012025-04-300001715925ifrs-full:NotLaterThanOneYearMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesUSALtdIPAUSAMember2023-05-012024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:LabEquipmentMember2023-05-012024-04-300001715925ipa:LabAndOfficeFacilitiesMember2025-04-300001715925ipa:NetBookValueMemberipa:LabEquipmentMember2025-04-300001715925ifrs-full:LiquidityRiskMemberifrs-full:NotLaterThanOneYearMember2025-04-300001715925ipa:AmountsReceivableMember2024-05-012025-04-300001715925country:BE2025-04-300001715925ifrs-full:TopOfRangeMember2024-05-012025-04-300001715925ipa:NetBookValueMemberipa:InternallyGeneratedDevelopmentCostsMember2024-04-300001715925ipa:OptionNineMember2025-04-300001715925ifrs-full:GoodsOrServicesTransferredAtPointInTimeMember2024-05-012025-04-300001715925ipa:OptionFifteenMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:LeaseholdImprovementsMember2024-04-300001715925ipa:NetBookValueMemberifrs-full:FixturesAndFittingsMember2024-04-300001715925ipa:NineteenFebruaryTwentyTwentyThreeMembersrt:DirectorMember2025-04-300001715925ipa:UProteinIpaEuropeAndBioStrandMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:WorkInProgressLeaseholdImrpvementsMember2024-05-012025-04-300001715925ipa:BiostrandMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:LabEquipmentMember2023-05-012024-04-300001715925ipa:IntellectualPropertiesMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:ConvertibleDebenturesMemberipa:YaIiPnLtdMemberipa:TrancheOneMember2024-07-160001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:AutomobileMember2024-05-012025-04-300001715925ifrs-full:ComputerEquipmentMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:ComputerHardwareMember2024-05-012025-04-300001715925ipa:ProductSalesMember2022-05-012023-04-300001715925ifrs-full:LaterThanTwoYearsAndNotLaterThanThreeYearsMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:IntellectualPropertiesMember2023-04-300001715925ipa:ImmunoPreciseAntibodiesMALLCMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:ProprietaryProcessesMember2023-04-300001715925ipa:BiostrandB.v.Member2021-05-012022-04-300001715925ifrs-full:VehiclesMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:OptionFiveMember2025-04-300001715925ipa:NineteenFebruaryTwentyTwentyThreeMemberipa:OfficersAndEmployeesMember2024-05-012025-04-300001715925country:CA2023-05-012024-04-300001715925ifrs-full:EquityInvestmentsMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:InternallyGeneratedDevelopmentCostsMember2024-04-300001715925ipa:DeferredAcquisitionPaymentsMember2024-05-012025-04-300001715925ifrs-full:FinancialEffectOfCorrectionsOfAccountingErrorsMember2024-04-300001715925srt:NorthAmericaMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LeaseholdImprovementsMember2023-04-300001715925ipa:ProjectRevenueMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:LabEquipmentMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2024-04-300001715925ipa:CustomerListMember2024-05-012025-04-300001715925ipa:WorkInProgressLeaseholdImrpvementsMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:CertificationsMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:FixturesAndFittingsMember2023-05-012024-04-300001715925ipa:UntrechtMember2023-04-300001715925ipa:TrancheTwoMemberipa:BiostrandB.v.Memberipa:EscrowAgreementMember2022-04-3000017159252022-04-300001715925country:US2022-05-012023-04-300001715925ipa:AccumulatedDepreciationAmountMemberipa:LabEquipmentMember2025-04-300001715925ipa:NetBookValueMemberipa:ComputerHardwareMember2025-04-300001715925ipa:AugustThreeTwoThousandTwentyFourMemberipa:OfficersAndEmployeesMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CustomerListMember2024-04-300001715925ipa:ProprietaryProcessesMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:BuildingsMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:ComputerHardwareMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesUSALtdIPAUSAMember2022-05-012023-04-300001715925ipa:AccumulatedDepreciationAmountMember2025-04-300001715925ipa:EmployeesMemberipa:ThirteenNovemberTwentyTwentyThreeMember2024-05-012025-04-300001715925ifrs-full:IssuedCapitalMember2023-05-012024-04-300001715925ifrs-full:BottomOfRangeMember2024-05-012025-04-300001715925ipa:BiostrandMember2025-04-3000017159252022-05-012023-04-300001715925ipa:CertificationsMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:IntellectualPropertiesMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:OptionSixteenMember2025-04-300001715925ipa:NetBookValueMemberifrs-full:LeaseholdImprovementsMember2024-04-300001715925ipa:LabEquipmentMemberifrs-full:WeightedAverageMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMember2023-04-300001715925ifrs-full:ComputerSoftwareMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMember2024-05-012025-04-300001715925ipa:OptionTenToTwentyThreeMember2025-04-300001715925ipa:OptionSixteenMember2024-05-012025-04-300001715925ipa:ProductSalesMember2023-05-012024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-04-300001715925ipa:ImmunoPreciseAntibodiesEuropeBVIPAEuropeFormerlyModiQuestResearchBVMember2024-05-012025-04-300001715925ifrs-full:FinancialEffectOfCorrectionsOfAccountingErrorsMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:AdditionalPaidinCapitalMember2024-05-012025-04-300001715925ipa:OptionTwentyOneMember2025-04-300001715925ipa:NineteenFebruaryTwentyTwentyThreeMembersrt:DirectorMember2024-05-012025-04-300001715925ipa:CryoStorageMember2023-05-012024-04-300001715925ifrs-full:BuildingsMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2023-05-012024-04-300001715925ipa:EmployeesMemberipa:TwentyThreeMayTwentyTwentyThreeMember2024-05-012025-04-300001715925ipa:UntrechtMember2025-04-300001715925ipa:NineteenJanuaryTwentyTwentyFourMembersrt:DirectorMember2025-04-300001715925ipa:EmployeesMemberipa:ElevenJuneTwentyTwentyThreeMember2025-04-300001715925ipa:NetBookValueMemberifrs-full:BuildingsMember2025-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:VehiclesMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2025-04-300001715925ifrs-full:TopOfRangeMember2024-08-192024-08-190001715925ifrs-full:GrossCarryingAmountMemberipa:AutomobileMember2023-05-012024-04-300001715925ifrs-full:CurrencyRiskMember2025-04-300001715925ipa:NetBookValueMemberipa:ComputerHardwareMember2024-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:BuildingsMember2024-05-012025-04-300001715925ipa:EmployeesMemberipa:NineteenFebruaryTwentyTwentyFourMember2025-04-300001715925ipa:OptionFourMember2025-04-300001715925ipa:AtTheMarketEquityOfferingFacilityMember2025-04-300001715925ipa:NetBookValueMemberipa:LabEquipmentMember2025-04-300001715925ifrs-full:RetainedEarningsMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2024-05-012025-04-300001715925ipa:OptionNineteenMember2024-05-012025-04-300001715925dei:BusinessContactMember2024-05-012025-04-300001715925ipa:AccountsPayablesAndAccruedLiabilitiesMember2024-05-012025-04-300001715925ipa:LabEquipmentMember2024-05-012025-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2025-04-300001715925ipa:OptionSevenMember2024-05-012025-04-300001715925ipa:ConvertibleDebenturesMember2025-04-300001715925ipa:OptionEighteenMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesEuropeBVIPAEuropeFormerlyModiQuestResearchBVMember2023-05-012024-04-300001715925ifrs-full:IssuedCapitalMember2023-04-300001715925ipa:CertificationsMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:OssMember2023-05-012024-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:BuildingsMember2023-05-012024-04-300001715925ifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:NetBookValueMemberipa:ProprietaryProcessesMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:FixturesAndFittingsMember2023-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:VehiclesMember2024-04-300001715925ipa:DeferredAcquisitionPaymentsMember2022-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:WorkInProgressLeaseholdImrpvementsMember2023-04-300001715925ipa:NetBookValueMemberipa:CertificationsMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:LeaseholdImprovementsMember2023-04-300001715925ipa:TwentyThreeJanuaryTwentyTwentyThreeMemberipa:EmployeesMember2025-04-300001715925ipa:FifteenMayTwentyTwentyTwoMemberipa:EmployeesMember2024-05-012025-04-300001715925ipa:InternallyGeneratedDevelopmentCostsMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925country:NL2023-05-012024-04-300001715925srt:EuropeMember2022-05-012023-04-300001715925ifrs-full:GoodsOrServicesTransferredOverTimeMember2023-05-012024-04-300001715925ipa:FindersWarrantsMember2022-05-012023-04-300001715925ipa:OptionSevenAndEightMember2025-04-300001715925ipa:ProductSalesMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:FixturesAndFittingsMember2025-04-300001715925ipa:AllOtherCountriesMember2024-05-012025-04-300001715925ipa:OptionThreeMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2023-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:VehiclesMember2024-05-012025-04-300001715925ifrs-full:IssuedCapitalMember2024-05-012025-04-300001715925ipa:ConvertibleDebenturesMember2022-05-012023-04-300001715925ipa:BiostrandB.v.Member2024-05-012025-04-300001715925ipa:ImmunoPreciseAntibodiesNDLtdMember2024-05-012025-04-300001715925ifrs-full:RetainedEarningsMember2024-05-012025-04-300001715925srt:NorthAmericaMember2025-04-300001715925ifrs-full:VehiclesMember2025-04-300001715925ifrs-full:IssuedCapitalMember2024-04-300001715925ipa:FindersWarrantsMemberipa:RangeOneMember2024-05-012025-04-300001715925ifrs-full:AdditionalPaidinCapitalMember2023-05-012024-04-300001715925ifrs-full:IssuedCapitalMemberifrs-full:OrdinarySharesMember2022-05-012023-04-300001715925ipa:NetBookValueMemberifrs-full:FixturesAndFittingsMember2025-04-300001715925ipa:AllOtherCountriesMember2022-05-012023-04-300001715925ipa:ProprietaryProcessesMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:AtTheMarketEquityOfferingFacilityMember2024-04-300001715925ipa:CryoStorageMember2022-05-012023-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:VehiclesMember2023-04-300001715925ipa:AccumulatedAmortizationMemberipa:InternallyGeneratedDevelopmentCostsMember2025-04-300001715925ipa:BiokeyB.vMember2023-05-012024-04-300001715925ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-04-300001715925ipa:AccumulatedDepreciationAmountMember2023-05-012024-04-300001715925ipa:InternallyGeneratedDevelopmentCostsMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ipa:TwentyThreeJanuaryTwentyTwentyThreeMemberipa:EmployeesMember2024-05-012025-04-300001715925country:AU2023-05-012024-04-300001715925ifrs-full:LaterThanFiveYearsMember2025-04-300001715925ipa:EmployeesMemberipa:NineteenFebruaryTwentyTwentyFourMember2024-05-012025-04-300001715925ipa:CertificationsMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:NetBookValueMemberipa:WorkInProgressLeaseholdImrpvementsMember2024-04-3000017159252022-05-012022-07-310001715925ifrs-full:BuildingsMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ifrs-full:LeaseLiabilitiesMember2025-04-300001715925ipa:ImmunoPreciseNetherlandsBVMember2023-05-012024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:BuildingsMember2024-04-300001715925ipa:ConvertibleDebenturesMember2024-05-012025-04-300001715925ipa:ImmunoPreciseAntibodiesNDLtdMember2023-05-012024-04-300001715925ipa:ImmunoPreciseAntibodiesMALLCMember2022-05-012023-04-300001715925ipa:TwentyFebruaryTwentyTwentyFourMemberipa:EmployeesMember2025-04-300001715925ipa:OptionThirteenMember2024-05-012025-04-300001715925ipa:NetBookValueMemberipa:ProprietaryProcessesMember2025-04-300001715925ipa:BiostrandB.v.Memberipa:ThreeYearsMember2022-04-132022-04-130001715925ipa:OssMember2025-04-300001715925ipa:NetBookValueMemberipa:InternallyGeneratedDevelopmentCostsMember2025-04-300001715925ipa:ConvertibleDebenturesMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:IntellectualPropertiesMember2024-04-300001715925ipa:NetBookValueMemberipa:CertificationsMember2024-04-300001715925ipa:OptionSixMember2024-05-012025-04-300001715925ipa:LabAndOfficeFacilitiesMemberifrs-full:WeightedAverageMember2024-05-012025-04-300001715925ipa:NetBookValueMemberipa:IntellectualPropertiesMember2025-04-300001715925ipa:AccumulatedAmortizationMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:ProprietaryProcessesMember2025-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2023-05-012024-04-300001715925ipa:InternallyGeneratedDevelopmentCostsMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:LeaseholdImprovementsMember2023-05-012024-04-300001715925ipa:OptionNineteenMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LeaseholdImprovementsMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LeaseholdImprovementsMember2024-04-300001715925ipa:EightAugustTwentyTwentyThreeMemberipa:EmployeesMember2025-04-300001715925ifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2025-04-300001715925ipa:FindersWarrantsMember2023-04-300001715925ipa:WorkInProgressLeaseholdImrpvementsMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LeaseholdImprovementsMember2025-04-300001715925ifrs-full:PreviouslyStatedMember2023-05-012024-04-300001715925ipa:BiostrandMember2024-05-012025-04-3000017159252024-05-012025-04-300001715925ifrs-full:IssuedCapitalMemberifrs-full:OrdinarySharesMember2025-04-300001715925ipa:NetBookValueMember2024-04-300001715925ipa:OneJanuaryTwentyTwentyFourMemberipa:EmployeesMember2025-04-300001715925currency:USD2024-04-300001715925ifrs-full:AdditionalPaidinCapitalMember2024-04-300001715925ipa:TalemTherapeuticsLLCTalemMember2022-05-012023-04-300001715925ipa:NetBookValueMemberifrs-full:BuildingsMember2025-04-300001715925ipa:IntellectualPropertiesMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:LabEquipmentMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ifrs-full:PotentialOrdinaryShareTransactionsMemberifrs-full:TopOfRangeMemberipa:AtTheMarketEquityOfferingFacilityMember2024-02-232024-02-230001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:VehiclesMember2023-05-012024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:FixturesAndFittingsMember2024-05-012025-04-300001715925ifrs-full:TopOfRangeMemberifrs-full:VehiclesMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:LabEquipmentMember2025-04-300001715925ifrs-full:BottomOfRangeMemberifrs-full:VehiclesMember2024-05-012025-04-300001715925ipa:UProteinIpaEuropeAndBioStrandMember2025-04-300001715925ipa:OptionEighteenMember2024-05-012025-04-300001715925ipa:CertificationsMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:LeaseLiabilitiesMember2024-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:ComputerHardwareMember2023-04-300001715925ipa:OptionSeventeenMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:VehiclesMember2025-04-300001715925ipa:UProteinIpaEuropeAndBioStrandMember2023-05-012024-04-300001715925ipa:AtTheMarketEquityOfferingFacilityMember2023-05-012024-04-3000017159252024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:ComputerHardwareMember2023-05-012024-04-300001715925ipa:AccumulatedAmortizationMemberipa:CertificationsMember2024-04-300001715925ipa:BiostrandB.v.Member2023-05-012024-04-300001715925ipa:NetBookValueMemberipa:AutomobileMember2024-04-300001715925country:NL2022-05-012023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:AutomobileMember2023-05-012024-04-300001715925ipa:AccumulatedAmortizationMemberipa:CertificationsMember2025-04-300001715925ipa:FirstMarchTwentyTwentyThreeMemberipa:EmployeesMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:LabEquipmentMember2024-04-300001715925ipa:EightAugustTwentyTwentyThreeMemberipa:EmployeesMember2024-05-012025-04-300001715925ifrs-full:BottomOfRangeMemberipa:LabAndOfficeFacilitiesMember2024-05-012025-04-300001715925ipa:VictoriaMember2025-04-300001715925ipa:VictoriaMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMember2025-04-300001715925ifrs-full:BuildingsMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ipa:BioclueB.vMember2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:WorkInProgressLeaseholdImrpvementsMember2025-04-300001715925ipa:FindersWarrantsMember2024-04-300001715925ifrs-full:LeaseLiabilitiesMember2024-05-012025-04-300001715925ipa:BioStrandTwoMember2025-04-300001715925ipa:BiostrandB.v.Memberipa:NinetyDaysMember2022-04-132022-04-130001715925ipa:OssMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:InternallyGeneratedDevelopmentCostsMember2024-05-012025-04-300001715925ipa:EmployeesMemberipa:EightMayTwentyTwentyThreeMember2025-04-300001715925country:BE2024-04-300001715925ipa:BiostrandB.v.Member2023-04-300001715925ipa:IntellectualPropertiesMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:AutomobileMember2024-04-300001715925ipa:BiostrandB.v.Member2022-04-130001715925ipa:NetBookValueMemberipa:CustomerListMember2025-04-300001715925ifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:FindersWarrantsMember2024-05-012025-04-3000017159252022-05-310001715925ipa:InternallyGeneratedDevelopmentCostsMemberifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:OptionSixMember2025-04-300001715925ipa:DeferredAcquisitionPaymentsMember2023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:AutomobileMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CertificationsMember2023-05-012024-04-300001715925ipa:BioclueB.vMember2023-05-012024-04-300001715925ipa:CustomerListMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:LeaseLiabilitiesMember2023-05-012024-04-300001715925ipa:ImmunoPreciseNetherlandsBVMember2024-05-012025-04-300001715925ipa:UntrechtMember2024-04-300001715925ifrs-full:GrossCarryingAmountMember2024-05-012025-04-300001715925ipa:OptionThreeMember2024-05-012025-04-300001715925ipa:NetBookValueMemberipa:CustomerListMember2024-04-300001715925ipa:AccumulatedAmortizationMemberipa:CustomerListMember2025-04-300001715925ipa:OptionTwentyMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2023-05-012024-04-300001715925ipa:EmployeesMemberipa:EightMayTwentyTwentyThreeMember2024-05-012025-04-300001715925ifrs-full:VehiclesMemberifrs-full:WeightedAverageMember2024-05-012025-04-300001715925ipa:FifteenMayTwentyTwentyTwoMemberipa:EmployeesMember2025-04-300001715925ipa:OptionTenMember2025-04-300001715925ifrs-full:GrossCarryingAmountMember2025-04-300001715925country:CA2024-05-012025-04-300001715925ipa:TwentyFebruaryTwentyTwentyFourMemberipa:EmployeesMember2024-05-012025-04-300001715925country:BE2024-05-012025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMember2023-05-012024-04-300001715925ifrs-full:LiquidityRiskMember2025-04-300001715925ifrs-full:GrossCarryingAmountMember2023-04-300001715925ifrs-full:BottomOfRangeMemberipa:IntellectualPropertiesMember2024-05-012025-04-300001715925ipa:FirstMarchTwentyTwentyThreeMemberipa:EmployeesMember2024-05-012025-04-300001715925ifrs-full:VehiclesMember2024-04-300001715925ifrs-full:AdditionalPaidinCapitalMember2023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:ComputerHardwareMember2024-05-012025-04-300001715925ipa:InternallyGeneratedDevelopmentCostsMemberifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:CryoStorageMember2024-05-012025-04-300001715925ifrs-full:RetainedEarningsMember2024-04-300001715925ipa:BiostrandMember2022-05-012023-04-300001715925ipa:ProjectRevenueMember2022-05-012023-04-300001715925ipa:OptionEightMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:ComputerHardwareMember2023-04-300001715925ipa:BiostrandB.v.Memberifrs-full:OrdinarySharesMember2022-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMember2024-04-300001715925ipa:OneFebruaryTwentyTwentyFourMemberipa:EmployeesMember2025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:BuildingsMember2025-04-300001715925ifrs-full:GoodsOrServicesTransferredAtPointInTimeMember2023-05-012024-04-300001715925ipa:EmployeesMemberipa:TwentyThreeMayTwentyTwentyThreeMember2025-04-300001715925ipa:TrancheThreeMemberipa:BiostrandB.v.Memberipa:EscrowAgreementMember2022-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:LeaseholdImprovementsMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2023-04-300001715925country:AU2022-05-012023-04-300001715925ipa:DeferredAcquisitionPaymentsMember2022-05-012023-04-300001715925ipa:OptionFourMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:ComputerHardwareMember2024-04-300001715925ipa:AccumulatedDepreciationAmountMember2023-04-300001715925ipa:NineteenJanuaryTwentyTwentyFourMembersrt:DirectorMember2024-05-012025-04-300001715925ipa:BioStrandTwoMember2024-05-012025-04-300001715925ipa:InternallyGeneratedDevelopmentCostsMember2024-05-012025-04-300001715925country:NL2024-05-012025-04-300001715925ipa:OptionFifteenMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:LeaseholdImprovementsMember2023-05-012024-04-300001715925ipa:CustomerListMemberifrs-full:GrossCarryingAmountMember2024-04-300001715925ifrs-full:PreviouslyStatedMember2024-04-300001715925ipa:BiokeyB.vMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:ComputerHardwareMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CustomerListMember2023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:LabEquipmentMember2023-04-300001715925ipa:FourJanuaryTwentyTwentyThreeMemberipa:EmployeesMember2025-04-300001715925ipa:BiostrandB.v.Memberifrs-full:OrdinarySharesMember2022-04-130001715925ipa:AccumulatedAmortizationMemberipa:IntellectualPropertiesMember2024-05-012025-04-300001715925ipa:NetBookValueMemberifrs-full:ComputerSoftwareMember2025-04-300001715925ipa:VictoriaMember2024-04-300001715925ipa:IntellectualPropertiesMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CustomerListMember2023-05-012024-04-300001715925ipa:EmployeesMemberipa:ElevenJuneTwentyTwentyThreeMember2024-05-012025-04-300001715925ipa:SecondAprilTwentyTwentyThreeMemberipa:EmployeesMember2024-05-012025-04-300001715925ipa:OptionTwoMember2025-04-300001715925ifrs-full:GrossCarryingAmountMember2023-04-300001715925ipa:NetBookValueMemberifrs-full:LeaseholdImprovementsMember2025-04-300001715925ipa:BiostrandMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesCanadaLtdMember2022-05-012023-04-300001715925ifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:ComputerSoftwareMember2023-04-300001715925ipa:ImmunoPreciseAntibodiesNDLtdMember2022-05-012023-04-300001715925country:BE2023-04-3000017159252023-04-300001715925ipa:BiostrandB.v.Member2023-05-012024-04-300001715925ipa:BiokeyB.vMember2022-05-012023-04-300001715925ipa:EmployeesMemberipa:ThirteenNovemberTwentyTwentyThreeMember2025-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2022-05-012023-04-300001715925ifrs-full:VehiclesMember2024-05-012025-04-300001715925srt:NorthAmericaMemberipa:CorporateSegmentsMember2024-04-300001715925ipa:IdeaFamilyB.vMember2024-05-012025-04-300001715925ipa:AugustThreeTwoThousandTwentyFourMemberipa:OfficersAndEmployeesMember2025-04-300001715925ipa:CustomerListMemberifrs-full:GrossCarryingAmountMember2023-05-012024-04-300001715925ipa:AccumulatedDepreciationAmountMemberifrs-full:BuildingsMember2024-05-012025-04-300001715925srt:EuropeMember2024-05-012025-04-300001715925ipa:OptionOneMember2025-04-300001715925ipa:UtrechtMember2024-04-300001715925ipa:DeferredAcquisitionPaymentsMember2023-05-012024-04-300001715925ipa:NetBookValueMemberipa:LabEquipmentMember2024-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:AutomobileMember2024-05-012025-04-300001715925ipa:EmployeesMemberipa:FifteenMarchTwentyTwentyThreeMember2024-05-012025-04-300001715925ipa:OptionTwoMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:BuildingsMember2023-05-012024-04-300001715925ifrs-full:IssuedCapitalMember2025-04-300001715925ipa:OptionFourteenMember2025-04-300001715925srt:NorthAmericaMember2024-04-300001715925ipa:ConvertibleDebenturesMemberipa:YaIiPnLtdMemberipa:TrancheTwoMember2024-07-160001715925ipa:DeferredAcquisitionPaymentsMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CustomerListMember2024-05-012025-04-300001715925ifrs-full:RestrictedShareUnitsMember2024-05-012025-04-300001715925ifrs-full:FixturesAndFittingsMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:IntellectualPropertiesMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:ProprietaryProcessesMember2023-05-012024-04-300001715925ipa:OptionTenMember2024-05-012025-04-300001715925ipa:OptionFiveMember2024-05-012025-04-300001715925ipa:EmployeesMemberipa:FifteenMarchTwentyTwentyThreeMember2025-04-300001715925ipa:OneFebruaryTwentyTwentyFourMemberipa:EmployeesMember2024-05-012025-04-300001715925ipa:SecondAprilTwentyTwentyThreeMemberipa:EmployeesMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:BuildingsMember2023-04-300001715925ifrs-full:GrossCarryingAmountMemberifrs-full:BuildingsMember2023-04-300001715925ipa:NetBookValueMemberifrs-full:ComputerSoftwareMember2024-04-300001715925ifrs-full:GoodsOrServicesTransferredAtPointInTimeMember2022-05-012023-04-300001715925ipa:ImmunoPreciseAntibodiesMALLCMember2023-05-012024-04-300001715925ipa:ConvertibleDebenturesMember2022-04-300001715925ifrs-full:LiquidityRiskMemberifrs-full:LaterThanOneYearAndNotLaterThanTwoYearsMember2025-04-300001715925ipa:OptionSeventeenMember2025-04-300001715925ipa:FindersWarrantsMember2025-04-300001715925ipa:AccumulatedAmortizationMemberipa:ProprietaryProcessesMember2024-05-012025-04-300001715925ipa:AccumulatedAmortizationMemberipa:CertificationsMember2023-04-300001715925ipa:AccumulatedAmortizationMember2025-04-300001715925ipa:BioclueB.vMember2022-05-012023-04-300001715925ifrs-full:GoodsOrServicesTransferredOverTimeMember2022-05-012023-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberipa:WorkInProgressLeaseholdImrpvementsMember2024-04-300001715925ipa:VictoriaOneMember2024-04-300001715925ipa:OptionOneMember2024-05-012025-04-300001715925ipa:FourJanuaryTwentyTwentyThreeMemberipa:EmployeesMember2024-05-012025-04-300001715925ifrs-full:AccumulatedOtherComprehensiveIncomeMember2024-04-300001715925country:NL2025-04-300001715925ipa:BioStrandOneMember2025-04-300001715925ipa:ImmunoPreciseAntibodiesCanadaLtdMember2024-05-012025-04-300001715925ifrs-full:LaterThanFourYearsAndNotLaterThanFiveYearsMember2025-04-300001715925ipa:IdeaFamilyB.vMember2023-05-012024-04-300001715925ipa:WorkInProgressLeaseholdImrpvementsMemberifrs-full:GrossCarryingAmountMember2025-04-300001715925ifrs-full:AccumulatedDepreciationAndAmortisationMemberifrs-full:BuildingsMember2024-05-012025-04-300001715925srt:NorthAmericaMember2023-05-012024-04-3000017159252023-05-012024-04-300001715925ifrs-full:LeaseholdImprovementsMember2024-05-012025-04-300001715925ifrs-full:BuildingsMember2024-05-012025-04-300001715925ifrs-full:GrossCarryingAmountMemberipa:AutomobileMember2025-04-300001715925ipa:ImmunoPreciseNetherlandsBVMember2022-05-012023-04-300001715925ipa:CertificationsMemberifrs-full:GrossCarryingAmountMember2025-04-30iso4217:EURxbrli:purexbrli:sharesiso4217:CADiso4217:USDutr:Y
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
|
|
☐ |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended April 30, 2025 |
OR
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from ____________________ to ____________________ |
OR
|
|
☐ |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
Commission file number: 001-39530
ImmunoPrecise Antibodies Ltd.
(Exact name of Registrant as specified in its charter)
British Columbia
(Jurisdiction of incorporation or organization)
Industrious 823 Congress Ave Suite 300 Austin, Texas 78701, United States
(Address of principal executive offices)
Joseph Scheffler, 250-483-0308, jscheffler@ipatherapeutics.com
Industrious 823 Congress Ave Suite 300 Austin, Texas 78701, United States
(Name, Telephone, E-Mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol |
Name of each exchange on which registered |
Common Shares, no par value |
IPA |
The Nasdaq Stock Market, LLC |
Securities registered or to be registered pursuant to Section 12(g) of the Act: N/A
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: 46,154,118 Common Shares
Indicate by check mark if the Company is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
If this report is an annual or transition report, indicate by check mark if the Company is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. Yes ☐ No ☒
Indicate by check mark whether the Company (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or such shorter period that the Company was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Company has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Company was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark whether the Company is a large accelerated filer, an accelerated filer, a non-accelerated filer or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
Large accelerated filer ☐ |
Accelerated filer ☐ |
Non-accelerated filer ☒ |
Emerging growth company ☒ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the Company has used to prepare the financial statements included in this filing:
|
|
|
U.S. GAAP ☐ |
International Financial Reporting Standards as issued By the International Accounting Standards Board ☒ |
Other ☐ |
If “Other” has been checked in response to previous question, indicate by check mark which financial statement item the Company has elected to follow. Item 17☐ Item 18☐
If this is an annual report, indicate by check mark whether the Company is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
INTRODUCTION
In this Annual Report on Form 20-F (the “Annual Report”), “IPA,” “Company,” “we,” “us” and “our” refer to ImmunoPrecise Antibodies Ltd. and its consolidated subsidiaries.
Information contained in this Annual Report is given as of April 30, 2025, the fiscal year end of Company, unless otherwise specifically stated.
Market and industry data used throughout this Annual Report was obtained from various publicly available sources. Although the Company believes that these independent sources are generally reliable, the accuracy and completeness of such information are not guaranteed and have not been verified due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and the limitations and uncertainty inherent in any statistical survey of market size, conditions and prospects.
Statements made in this Annual Report concerning the contents of any contract, agreement or other document are summaries of such contracts, agreements or documents and are not complete descriptions of all of their terms. If we file any of these documents as an exhibit to this Annual Report, you may read the document itself for a complete description of its terms.
The Company reports under International Financial Reporting Standards as issued by the International Accounting Standards Board. None of the consolidated financial statements contained in this Annual Report were prepared in accordance with generally accepted accounting principles in the United States. The Company's financial statements are presented in Canadian dollars. In this Annual Report, unless otherwise indicated, all dollar amounts and references to "$" or "CAD$" are to Canadian dollars and references to "U.S.$" are to United States dollars, but most of the figures included in this Annual Report, including the Company's financial statements, are in Canadian dollars.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains “forward-looking statements” and “forward-looking information” within the meaning of United States and Canadian securities laws (collectively, “forward-looking statements”) about the Company which reflect management's expectations regarding the Company's future growth, results of operations, operational and financial performance and business prospects and opportunities. In addition, the Company may make or approve certain statements in future filings with Canadian and United States regulatory authorities, in news releases, or in oral or written presentations by representatives of the Company that are not statements of historical fact and may also constitute forward-looking statements. All statements, other than statements of historical fact, made by the Company that address activities, events or developments that the Company expects or anticipates will or may occur in the future are forward-looking statements, including, but not limited to statements preceded by, followed by, or that include words such as "may", "would", "could", "will", "likely", "expect", "anticipate", "believe", "intends", "plan", "forecast", "budget", "schedule", "project", "estimate", "outlook", or the negative of those words or other similar or comparable words.
Forward-looking statements involve significant risks, assumptions, uncertainties, and other factors that may cause actual future performance, achievements, or other realities to differ materiality from those expressed or implied in any forward-looking statements and, accordingly, should not be read as guarantees of future performance, achievements, or realities. Although the forward-looking statements contained in this Annual Report and the documents incorporated by reference herein and therein reflect management's current beliefs based upon information currently available to management and based upon what management believes to be reasonable assumptions, the Company cannot be certain that actual results will be consistent with these forward-looking statements. A number of risks and factors could cause actual results, performance, or achievements to differ materially from the results expressed or implied in the forward-looking statements. Such risks and factors include, but are not limited to, the following:
•
negative operating cash flow;
•
liquidity and future financing risk;
•
the financial position of the Company and its potential need for additional liquidity and capital in the future;
•
the Company may experience going concern risk;
•
the Company may fail to remediate a material weakness;
•
the success of any of the Company's current or future strategic alliances;
•
the Company may become involved in regulatory or agency proceedings, investigations and audits;
•
the Company may be subject to litigation in the ordinary course of its business;
•
the ability of the Company to obtain, protect and enforce patents on its technology and products;
•
risks associated with applicable regulatory processes;
•
the ability of the Company to achieve publicly announced milestones;
•
the effectiveness of the Company's business development and marketing strategies;
•
the competitive conditions of the industry in which the Company operates;
•
market perception of smaller companies;
•
the Company cannot assure the production of new and innovative processes, procedures or innovative approaches to antibody production or new antibodies;
•
the ability of the Company to manage growth;
•
the selection and integration of acquired businesses and technologies;
•
the Company may lose clients;
•
any reduction in demand;
•
any reduction or delay in government funding of research and development ("R&D");
•
costs of being a public company in the United States;
•
the Company may fail to meet the delivery and performance requirements set forth in client contracts;
•
the Company may become subject to patent and other intellectual property litigation;
•
the Company's dependence upon key personnel;
•
the Company may not achieve sufficient brand awareness;
•
the Company's directors and officers may have interests which conflict with those of the Company; the outsourcing trend in non-clinical discovery stages of drug discovery;
•
the Company's products, services and expertise may become obsolete or uneconomical;
•
the effect of global economic conditions;
•
the Company has a limited number of suppliers;
•
the Company may become subject to liability for risks against which it cannot insure;
•
clients may restrict the Company's use of scientific information;
•
the Company may experience failures of its laboratory facilities;
•
any contamination in animal populations;
•
any unauthorized access into information systems;
•
prospective investors' ability to enforce civil liabilities;
•
the Company's status as a foreign private issuer;
•
exposure to foreign exchange rates;
•
the effects of future sales or issuances of equity securities or debt securities;
•
the market price of the common shares of the Company (the “Common Shares”) may experience volatility;
•
the Company's failure to meet the continued listing requirements of The Nasdaq Capital Market ("Nasdaq"), particularly the minimum bid price requirements within the second extension period;
•
the anticipated use of proceeds from this offering, if any;
•
the Company has not declared or paid any dividends on the Common Shares and does not intend to do so in the foreseeable future; and
•
a liquid market for the Common Shares may not develop.
Although the Company has attempted to identify important risks and factors that could cause actual actions, events, or results to differ materially from those described in forward-looking statements, there may be other factors and risks that cause actions, events or results not to be as anticipated, estimated or intended. Further, any forward-looking statements are made as of the date of the Annual Report or the documents incorporated by reference herein and therein, as applicable. Other than as required by applicable securities laws, the Company assumes no obligation to update or revise them to reflect new events or circumstances. New factors emerge from time to time, and it is not possible for management to predict all such factors and to assess in advance the impact of each such factor on the Company's business or the extent to which any factor, or combination of factors, may cause actual realities to differ materially from those contained in any forward-looking statement. Accordingly, readers should not place undue reliance on forward-looking statements contained in this Annual Report or the documents incorporated by reference herein and therein. All forward-looking statements disclosed in this Annual Report and the documents incorporated by reference herein and therein are qualified by this cautionary statement.
STATUS AS AN EMERGING GROWTH COMPANY
We are an “emerging growth company” as defined in Section 3(a) of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”) by the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”), and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. We will continue to qualify as an "emerging growth company" until the earliest to occur of: (a) the last day of the fiscal year during which we had total annual gross revenues of U.S.$1,235,000,000 (as such amount is indexed for inflation every 5 years by the United States Securities and Exchange Commission (“SEC”)) or more; (b) the last day of our fiscal year following the fifth anniversary of the date of the first sale of equity securities pursuant to an effective registration statement under the United States Securities Act of 1933, as amended (the “Securities Act”); (c) the date on which we have, during the previous 3-year period, issued more than U.S.$1,000,000,000 in non-convertible debt; or (d) the date on which we are deemed to be a “large accelerated filer”, as defined in Exchange Act Rule 12b-2.
Generally, a registrant that registers any class of its securities under Section 12 of the Exchange Act is required to include in the second and all subsequent annual reports filed by it under the Exchange Act a management report on internal control over financial reporting and, subject to an exemption available to registrants that are neither an “accelerated filer” or a “large accelerated filer” (as those terms are defined in Exchange Act Rule 12b-2), an auditor attestation report on management’s assessment of internal control over financial reporting. However, for so long as we continue to qualify as an emerging growth company, we will be exempt from the requirement to include an auditor attestation report on management’s assessment of internal controls over financial reporting in its annual reports filed under the Exchange Act, even if we were to qualify as an “accelerated filer”. In addition, Section 103(a)(3) of the Sarbanes-Oxley Act of 2002 has been amended by the JOBS Act to provide that, among other things, auditors of an emerging growth company are exempt from any rules of the Public Company Accounting Oversight Board requiring a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the company.
FOREIGN PRIVATEISSUER FILINGS
We are considered a “foreign private issuer” pursuant to Rule 405 promulgated under the Securities Act. In our capacity as a foreign private issuer, we are exempt from certain rules under the Exchange Act that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales of our shares. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information. For as long as we are a “foreign private issuer” we intend to file our annual financial statements on Form 20-F and furnish our quarterly financial statements on Form 6-K to the SEC for so long as we are subject to the reporting requirements of Section 13(g) or 15(d) of the Exchange Act. However, the information we file or furnish may not be the same as the information that is required in annual and quarterly reports on Form 10-K or Form 10-Q for United States domestic issuers. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a domestic issuer.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We are required to determine our status as a foreign private issuer on an annual basis at the end of our second fiscal quarter. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by United States residents and any of the following three circumstances applies: (1) the majority of our executive officers or directors are United States citizens or residents; (2) more than 50% of our assets are located in the United States; or (3) our business is administered principally in the United States. If we lose our “foreign private issuer status” we would be required to comply with Exchange Act reporting and other requirements applicable to United States domestic issuers, which are more detailed and extensive than the requirement for foreign private issuers.
NON-IFRS MEASURES
The information presented in this Annual Report includes certain measures that are not recognized under IFRS and do not have a standardized meaning prescribed by IFRS. They are therefore unlikely to be comparable to similar measures presented by other companies. The Company uses non-IFRS measures, including “adjusted EBITDA” and “adjusted operating expenses” as additional information to complement IFRS measures by providing further understanding of the Company’s results of operations from management’s perspective. Management believes that these measures provide useful information in that they may exclude amounts that are not indicative of the Company’s core operating results and ongoing operations and provide a more consistent basis for comparison between periods.
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
B.
Capitalization and Indebtedness
Not applicable.
C.
Reasons for the Offer and Use of Proceeds
Not applicable.
There are numerous and varied risks, known and unknown, that may prevent us from achieving our goals. The risks described below are not the only ones we will face. If any of these risks actually occur, our business, financial condition or results of operations may be materially and adversely affected. In that case, the trading price of our securities could decline and investors in such securities could lose all or part of their investment.
We currently have negative operating cash flows.
We have negative cash flow from operating activities and have historically incurred net losses. There is no assurance that we will generate sufficient revenues in the near future. To the extent that we have negative operating cash flows in future periods, we may need to deploy a portion of our existing working capital to fund such negative cash flows. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favorable to us as those previously obtained, or at all. If we are unable to obtain additional financing from outside sources and eventually generate enough revenues, we may be forced to sell a portion or all of our assets, curtail or discontinue our operations. If any of these events happen, investors may lose all or part of their investment.
We may have difficulties in managing our liquidity risk, which may adversely affect our financial and operating performance and limit our growth.
Although we are a going concern, we do not have cash reserves to fund all our operations for one year, and strategic future growth and expansion plans. We have historically incurred net losses. There is no assurance that sufficient revenues will be generated in the near future. To the extent that we have negative operating cash flows in future periods, we may need to deploy a portion of our existing working capital to fund such negative cash flows. We may need to raise additional funds through issuances of Common Shares or through loan financing. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favorable to us as those previously obtained, or at all. If we are unable to obtain additional financing from outside sources and eventually generate enough revenues, we may be forced to sell a portion or all of our assets or curtail or discontinue our operations.
We have additional needs for liquidity and capital which may have an adverse impact on our business.
We are an AI-driven biopharmaceutical discovery and development company focused on creating safer and more efficacious novel therapeutic antibodies. IPA does not seek regulatory approval of its early-stage candidates, but instead, aims to out-license its assets prior to clinical trial research. We have not generated substantial revenues from collaboration and licensing agreements to date, and have incurred significant research, development and other expenses related to ongoing operations. As a result, we have not been profitable and have incurred operating losses in every reporting period since inception and have a significant accumulated deficit. Operating costs are expected to increase in the near term as we continue to build our AI-driven software development, namely LENSai, and the Company expects that this will continue until either subscription-based payments of our future product sales, partnership fees, licensing fees, milestone payments or royalty payments are sufficient to generate revenues to fund continuing operations. We are unable to predict the extent of any future losses or when our business will become profitable, if ever. Even if we achieve profitability, we may not be able to sustain or increase profitability on an ongoing basis.
We may experience difficulties managing our resources to fund operations for one year, impacting our growth and business.
Although the Company is a going concern, the Company does not have cash reserves to fund all its operations for one year, and strategic future growth and expansion plans. The Company has historically incurred net losses. There is no assurance that sufficient revenues will be generated in the near future. To the extent that the Company has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Company may need to raise additional funds through issuances of Common Shares or through loan financing. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favorable to the Company as those previously obtained, or at all. If the Company is unable to obtain additional financing from outside sources and eventually generate enough revenues, the Company may be forced to sell a portion or all of the Company's assets or curtail or discontinue the Company's operations.
We may fail to remediate a material weakness that could affect our financial reporting.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. Management concluded that we did not have sufficient resources to assist us in identifying, evaluating and addressing complex technical accounting issues that affect our consolidated financial statements on a timely basis. The remediation measures intended to correct the material weakness in internal controls may be insufficient to remediate the material weakness and could impact financial reporting.
We have and will continue to enter into strategic alliances which may have an adverse impact on our business.
We currently have, and may in the future enter into, strategic alliances with third parties that we believe will complement or augment our existing business. Our ability to enter into strategic alliances is dependent upon, and may be limited by, the availability of suitable candidates and capital. In addition, strategic alliances could present unforeseen integration obstacles or costs, may not enhance our business, and may involve risks that could adversely affect us, including significant amounts of management time that may be diverted from operations in order to pursue and complete such transactions or maintain such strategic alliances. Future strategic alliances could result in the incurrence of additional debt, costs and contingent liabilities, and there can be no assurance that future strategic alliances will achieve, or that our existing strategic alliances will continue to achieve, the expected benefits to our business or that we will be able to consummate future strategic alliances on satisfactory terms, or at all. Any of the foregoing could have a material adverse effect on our business, financial condition and results of operation.
We may not be able to enter into collaboration agreements on terms favorable to us or at all. Furthermore, some of those agreements may give substantial responsibility over our drug candidates to the collaborator.
If we enter into collaboration agreements for one or more of our drug candidates, the success of such drug candidates will depend in great part upon our collaborators’ success in promoting them as superior to other treatment alternatives. We believe that our drug candidates may be proven to offer disease treatment with notable advantages over other drugs. However, there can be no assurance that we will be able to prove these advantages or that the advantages will be sufficient to support the successful commercialization of our drug candidates.
We may become subject to litigation, regulatory or agency proceedings, investigations and audits.
Our business requires compliance with many laws and regulations. Failure to comply with these laws and regulations could subject us to regulatory or agency proceedings or investigations and could also lead to damage awards, fines and penalties. We may become involved in a number of government or agency proceedings, investigations and audits. The outcome of any regulatory or agency proceedings, investigations, audits, and other contingencies could harm our reputation, require us to take, or refrain from taking, actions that could harm our operations or require us to pay substantial amounts of money, harming our financial condition. There can be no assurance that any pending or future regulatory or agency proceedings, investigations and audits will not result in substantial costs or a diversion of management’s attention and resources or have a material adverse impact on our business, financial condition and results of operations.
We carry litigation risk.
We may become party to litigation from time to time in the ordinary course of business including, but not limited to, in connection with our operations or pursuant to the terms of any of our commercial agreements, which could adversely affect our business. Should any litigation in which we become involved be decided against us, such a decision could adversely affect our ability to continue operating and the value of our securities and could use significant resources. Even if we are involved in litigation and win, litigation can redirect a significant amount of our resources, including the time and attention of management and available working capital. Litigation may also create a negative perception of our brand.
Protecting and defending our intellectual property claims may have a material adverse effect on our business.
Our success will depend on our ability to obtain, protect and enforce patents on our technology and products. Any patents that we may own or license in the future may not afford meaningful protection for our technology and products. Our efforts to enforce and maintain our intellectual property rights may not be successful and may result in substantial costs and diversion of management time. In addition, others may challenge patents we may obtain in the future and, as a result, these patents could be narrowed, invalidated or rendered unenforceable or we may be forced to stop using the technology covered by these patents or to license the technology from third parties. In addition, current and future patent applications on which we depend may not result in the issuance of patents. Even if our rights are valid, enforceable and broad in scope, competitors may develop products based on similar technology that is not covered by our patents. Further, since there is a substantial backlog of patent applications at the various patent offices, the approval or rejection of our competitors’ patent applications may take several years.
In addition to patent protection, we also rely on copyright and trademark protection, trade secrets, know-how, continuing technological innovation and licensing opportunities. In an effort to maintain the confidentiality and ownership of our trade secrets and proprietary information, we require our employees, consultants and advisors to execute confidentiality and proprietary information agreements. However, these agreements may not provide us with adequate protection against improper use or disclosure of confidential information and there may not be adequate remedies in the event of unauthorized use or disclosure. Furthermore, like many companies in our industry, we may from time to time hire scientific personnel formerly employed by other companies involved in one or more areas similar to the activities we conduct. In some situations, our confidentiality and proprietary information agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants or advisors have prior employment or consulting relationships. Although we require our employees and consultants to maintain the confidentiality of all confidential information of previous employers, we or these individuals may be subject to allegations of trade secret misappropriation or other similar claims as a result of their prior affiliations. Finally, others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets. Our failure to protect our proprietary information and techniques may inhibit or limit our ability to exclude certain competitors from the market and execute our business strategies.
The announcements we make are forward-looking and are based on best estimates of management, which may not be updated or revised as a result of new information or future events.
From time to time, we may announce the timing of certain events which are expected to occur, such as the anticipated timing of results from partnerships or out-licensing events. These statements are forward-looking and are based on the best estimates of management at the time. However, the actual timing of such events may differ significantly from what has been publicly disclosed. The timing of events such as the initiation or completion of a transaction, may ultimately vary from what is publicly disclosed. These variations in timing may occur as a result of different events, including the nature of the results obtained during research, delays from partners, or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business plan, financial condition or operating results, and the trading price of the Common Shares.
Our business development and marketing strategies alter our future growth and profitability.
Our future growth and profitability will depend on the effectiveness and efficiency of our national and international business development and marketing and sales strategy, including our ability to (i) grow our brand recognition for our services internationally; (ii) determine appropriate business development, marketing and sales strategies and (iii) maintain acceptable operating margins on such costs.
There can be no assurance that business development, marketing and sales costs will result in revenues for our business in the future or will generate awareness of our products and services. In addition, no assurance can be given that we will be able to manage our business development, marketing and sales costs on a cost-effective basis.
If we are unable to compete effectively, our business, financial condition and results of operations would be materially and adversely affected.
Although we believe that there are only a limited number of full-service, biologics, CRO firms, we may face intense competition in selling our products and services. Some competitors may have marketing, financial, development and personnel resources which exceed our own. As a result of this competition, we may be unable to maintain our operations or develop them as currently proposed on terms we consider acceptable or at all. Increased competition by larger, better-financed competitors with geographic advantages could materially and adversely affect our business, financial condition and results of operations. To remain competitive, we believe that we must effectively and economically provide: (i) products and services that satisfy client demands, (ii) superior client service, (iii) high levels of quality and reliability, and (iv) dependable and efficient distribution networks. Increased competition may require us to reduce prices or increase spending on sales and marketing and client support, which may have a material adverse effect on our financial condition and results of operations. Any decrease in the quality of our products or level of service to clients or any occurrence of a price war among our competitors may adversely affect the business and results of operations.
Client reach, service and on-time delivery will continue to be a hallmark of our ability to compete with other market players. Further, the
acquisitions translate to spreading our footprint on two continents. In addition, we have deployed a sales team tasked with continually sourcing and providing market intelligence as part of our activities.
We may have difficulty raising funds due to the market perception of smaller companies.
Market perception of smaller companies may change, potentially affecting the value of investors’ holdings and our ability to raise further funds through the issuance of further Common Shares or otherwise. The share price of smaller publicly traded companies can be highly volatile. The value of the Common Shares may go down as well as up and, in particular, the share price may be subject to sudden and large falls in value given the restricted marketability of the Common Shares, results of operations, changes in earnings estimates or changes in general market, economic and political conditions.
Our employment of scientific staff does not guarantee success in research and product development.
We are an AI-driven biotherapeutic research, technology and scientifically robust life science company that discovers and develops customized and novel antibodies by generating proprietary and patented processes, procedures and innovative approaches to antibody discovery, development, and production. We have been engaged in these activities for over 60 collective years and have had several assets enter the clinical successfully. Continued investment in retaining key scientific staff, as well as an ongoing commitment in R&D activities, will continue to be a cornerstone in our development of new services, processes, and competitive advantages such as Rapid Prime, B cell Select, DeepDisplay and our methods for the production of complex proteins and antibodies. We realize that such research and product development activities endeavor, but cannot assure, the production of new and innovative processes, procedures or innovative approaches to antibody production or new antibodies. Furthermore, if we do not achieve sufficient market acceptance of our expansion of our commercialization of our products and services, it will be difficult for us to achieve consistent profitability. Our marketing and sales approach and external sales personnel continue to introduce a steady stream of new clients.
Growth may cause pressure on our management and systems.
We may be subject to growth-related risks including pressure on our internal systems and controls. Our ability to manage growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and manage our employee base. Our inability to deal with this growth could have a material adverse impact on our business, operations and prospects. We may experience growth in the number of our employees and the scope of our operating and financial systems, resulting in increased responsibilities for our personnel, the hiring of additional personnel and, in general, higher levels of operating expenses. In order to manage our current operations and any future growth effectively, we will also need to continue to implement and improve our operational, financial and management information systems and to hire, train, motivate, manage and retain employees. There can be no assurance that we will be able to manage such growth effectively, that our management, personnel or systems will be adequate to support our operations or that we will be able to achieve the increased levels of revenue commensurate with the increased levels of operating expenses associated with this growth.
We are subject to risks associated with selection and integration of acquired businesses and technologies.
We have expanded our business through acquisitions. We may plan to continue to acquire businesses and technologies and form strategic alliances. However, businesses and technologies may not be available on terms and conditions we find acceptable. Thus, we risk spending time and money investigating and negotiating with potential acquisition or alliance partners, but not completing transactions. Acquisitions and alliances involve numerous risks which may include:
•
difficulties in achieving business and financial success;
•
difficulties and expenses incurred in assimilating and integrating operations, services, products, technologies or pre-existing relationships with our clients, distributors and suppliers;
•
challenges with developing and operating new businesses, including those that are materially different from our existing businesses and that may require the development or acquisition of new internal capabilities and expertise;
•
potential losses resulting from undiscovered liabilities of acquired companies that are not covered by the indemnification we may obtain from the seller or the insurance acquired in connection with the transaction;
•
the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies;
•
diversion of management’s attention from other business concerns;
•
a more expansive regulatory environment; acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our Common Shares to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing shareholders;
•
differences in foreign business practices, customs and importation regulations, language and other cultural barriers in connection with the acquisition of foreign companies;
•
new technologies and products may be developed that cause businesses or assets we acquire to become less valuable; and
•
disagreements or disputes with prior owners of an acquired business, technology, service or product that may result in litigation expenses and diversion of our management’s attention.
If an acquired business, technology or an alliance does not meet expectations, our results of operations may be adversely affected.
Some of the same risks exist when we decide to sell a business, site or product line. In addition, divestitures could involve additional risks, including the following:
•
difficulties in the separation of operations, services, products, and personnel;
•
diversion of management’s attention from other business concerns; and
•
the need to agree to retain or assume certain current or future liabilities in order to complete the divestiture.
We continually evaluate the performance and strategic fit of our businesses (including specific product lines and service offerings) to determine whether any divestitures are appropriate. Any divestitures may result in significant write-offs, including those related to goodwill and other intangible assets and which could have an adverse effect on our results of operations and financial condition. In addition, we may encounter difficulty in finding buyers or alternative exit strategies at acceptable prices and terms, and in a timely manner. We may not be successful in managing these or any other significant risks that we encounter in divesting a business, site or product line or service offering and, as a result, may not achieve some or all of the expected benefits of the divestiture.
Our business may be disrupted based on our clients’ ability to terminate their contracts.
Our clients may terminate their contracts with it upon 30 to 90 days’ notice for a number of reasons or, in some cases, for no reason. Although our clients are currently comprised of a number of small and larger pharma entities, we are making a strategic shift to increase the number of larger pharma and biotech clients, including the size of each service contract. If any one of our major clients cancels our contract with us, our revenue may decrease.
Our business could be harmed if there is a reduction in demand.
Our business could be adversely affected by any significant decrease in drug R&D expenditures by pharmaceutical and biotechnology companies, as well as by academic institutions, government laboratories or private foundations. Similarly, economic factors and industry trends that affect our clients in these industries also affect their R&D budgets and, consequentially, our business as well.
Our clients include researchers at pharmaceutical and biotechnology companies. Our ability to continue to grow and win new business is dependent in large part upon the ability and willingness of the pharmaceutical and biotechnology industries to continue to spend on molecules in the non-clinical phases of R&D and to outsource the products and services we provide. Furthermore, our clients (particularly larger biopharmaceutical companies) continue to search for ways to maximize the return on their investments with a focus on lowering R&D costs per drug candidate. Fluctuations in the expenditure amounts in each phase of the R&D budgets of these researchers and their organizations could have a significant effect on the demand for our products and services. R&D budgets fluctuate due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities, general economic conditions, institutional budgetary policies and the impact of government regulations, including potential drug pricing legislation. Available funding for biotechnology clients in particular may be affected by the capital markets, investment objectives of venture capital investors and priorities of biopharmaceutical industry sponsors.
A reduction or delay in government funding of R&D may significantly and adversely affect our future revenue.
A small portion of revenue is derived from clients at academic institutions and research laboratories whose funding is partially dependent on both the level and timing of funding from government sources in Canada, such as Canadian National Research Council’s Innovation Research Assistance Program, and the United States, such as the United States’ National Institutes of Health, and international agencies, which can be difficult to forecast. Government funding of R&D is subject to the political process, which is inherently fluid and unpredictable. Our revenue may be adversely affected if our clients delay purchases as a result of uncertainties surrounding the approval of government budget proposals, included reduced allocations to government agencies that fund R&D activities. Government proposals to reduce or eliminate budgetary deficits have sometimes included reduced allocations to government agencies that fund R&D activities, or such funding may not be directed towards projects and studies that require the use of our products and services, both of which could adversely affect our business and financial results.
As a public company in the United States, we have increased costs and disruptions to the regular operations of our business.
As a public company in the United States, we incur additional legal, accounting, reporting and other expenses that we would not incur as a public company solely listed in Canada. The additional demands associated with being a United States public company may disrupt regular operations of business by diverting the attention of some of our senior management team away from revenue-producing activities to additional management and administrative oversight, adversely affecting our ability to attract and complete business opportunities and increasing the difficulty in both retaining professionals and managing and growing our business. Any of these effects could harm our business, results of operations and financial condition. In general, the United States tends to be more litigious than Canada and being a public company in the United States may make it more likely that we are subjected, from time to time, to the types of lawsuits that affect public companies in the United States.
Our revenue streams are contingent on the delivery and performance requirements in client contracts.
In order to maintain our current client relationships and to meet the performance and delivery requirements in our client contracts, we must be able to provide products and services at appropriate levels and with acceptable quality and at an acceptable cost. Our ability to deliver the products and provide the services we offer to our clients is limited by many factors, including the difficulty of the processes associated with our products and services, the lack of predictability in the scientific process and the shortage of qualified scientific personnel. In particular, a large portion of our revenue depends on producing biologics and the current rate at which we are producing them. Some of our clients can influence when we will deliver products and perform services under their contracts. If we are unable to meet our contractual commitments, it may delay or lose revenue, lose clients or fail to expand our existing relationships.
We may infringe intellectual property rights of third parties.
The drug research and development industry has a history of patent and other intellectual property litigation and these lawsuits will likely continue. Because we produce and provide many different products and services in this industry, we face potential patent infringement suits by companies that control patents for similar products and services. In order to protect or enforce our intellectual property rights, we may have to initiate legal proceedings against third parties. In addition, others may sue us for infringing their intellectual property rights or we may initiate a lawsuit seeking a declaration from a court that we do not infringe the proprietary rights of others. The patent positions of pharmaceutical, biotechnology and drug discovery companies are generally uncertain and involve complex legal and factual questions. No consistent policy has emerged from the United States Patent and Trademark Office or the courts regarding the breadth of claims allowed or the degree of protection afforded under patents like those for which we have applied. Legal proceedings relating to intellectual property would be expensive, take significant time and divert management’s attention from other business concerns, whether we win or lose. The cost of such litigation could affect our profitability.
Further, if we do not prevail in an infringement lawsuit brought against us, we might have to pay substantial damages, including treble damages, and we could be required to stop the infringing activity or obtain a license to use the patented technology. Any required license may not be available to us on acceptable terms, or at all. In addition, some licenses may be nonexclusive, and therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license or is unable to design around a patent, we may be unable to sell some of our products or services.
Our success depends on management and key personnel.
Our success will depend on our directors’ and officers’ ability to develop our business and manage operations, and on our ability to attract and retain the Chief Executive Officer, management team and other key technical, sales, public relations and marketing staff or consultants to operate and grow the business. The loss of any key person or the inability to find and retain new key persons could have a material adverse effect on our business. Competition for experienced scientists is intense. We compete with pharmaceutical and biotechnology companies, including our clients and collaborators, medicinal chemistry outsourcing companies, contract research companies, and academic and research institutions to recruit scientists. Our inability to hire additional qualified personnel may also require an increase in the workload for both existing and new personnel. We may not be successful in attracting new scientists or management or in retaining or motivating our existing personnel. The shortage of experienced scientists, and other factors, may lead to increased recruiting, relocation and compensation costs for such scientists, which may exceed our expectations. These increased costs may reduce our profit margins or make hiring new scientists impracticable.
If we are unable to create brand awareness, our business may be harmed.
Our expansion of products and services depends on increasing brand awareness with respect to our products and services. There is no assurance that we will be able to achieve sufficient brand awareness. In addition, we must successfully develop a larger market for our services in order to increase the sales of our services. If we are not able to successfully develop a market for our services, then such failure will have a material adverse effect on our business, financial condition and operating results. We are currently investing in our brand awareness through a rebranding project due to commence in the second quarter of fiscal year 2026.
Our directors, officers or members of management may have conflicts of interest.
Certain directors and officers are also involved as advisors for other companies. Situations may arise in connection with potential acquisitions or opportunities where the other interests of these directors and officers conflict with or diverge from our interests. In accordance with the BCBCA, directors who have a material interest in any person who is a party to a material contract or a proposed material contract are required, subject to certain exceptions, to disclose that interest and generally abstain from voting on any resolution to approve the contract.
In addition, the directors and the officers are required to act honestly and in good faith with a view to our best interests. However, in conflict of interest situations, our directors and officers may owe the same duty to another company and will need to balance their competing interests with their duties to us. Circumstances (including with respect to future corporate opportunities) may arise that may be resolved in a manner that is unfavorable to us.
Our industry follows an outsourcing trend in non-clinical discovery stages of drug discovery.
Over the past decade, pharmaceutical and biotechnology companies have generally increased their outsourcing of non-clinical research support activities, such as antibody discovery. While many industry analysts expect the outsourcing trend to continue to increase for the next several years (although with different growth rates for different phases of drug discovery and development), decreases in such outsourcing may result in a diminished growth rate in the sales of any one or more of our service lines and may adversely affect our financial condition and results of operations.
Our industry has a high level of competition and a rapid rate of obsolescence.
The pharmaceutical and biotechnology industries are characterized by rapid and continuous technological innovation. We compete with companies around the world that are engaged in the development and production of products and services, including pharmaceutical companies, biotechnology companies, and contract research companies. Academic institutions, governmental agencies and other research organizations also are conducting research and developing technologies in areas in which we provide services, either on our own or through collaborative efforts. Our pharmaceutical and biotechnology company clients have internal departments that provide products and services that directly compete with our products and services. Many of our competitors offer a broader range of products and services and have greater access to financial, technical, scientific, business development, recruiting and other resources than we do, and some of our competitors may also operate with a lower cost structure. We anticipate that we will face increased competition in the future as we expand our operations and our products and services and as new companies enter the market and advanced technologies become available. Our products, services and expertise may become obsolete or uneconomical due to technological advances or entirely different approaches developed by us, our clients or one or more of our competitors. For example, advances in databases and molecular modeling tools that predict how effectively compounds will treat a targeted disease may render some of our technologies obsolete. While we plan to develop technologies that will give us a competitive advantage, we may not be able to develop the technologies necessary for us to successfully compete in the future. Additionally, the existing approaches of our competitors or new approaches or technologies developed by our competitors may be more effective than those we develop. We may not be able to compete successfully with existing or future competitors.
Other competitive factors could force us to lower prices or could result in reduced sales. In addition, new products developed by others could emerge as competitors to our drug candidates. If we are not able to compete effectively against current and future competitors, our business will not grow and our financial condition and operations will suffer.
Global economic turmoil and regional economic conditions in the United States could adversely affect our business.
Global economic instability and geopolitical tensions, including the imposition of tariffs and other trade barriers, could have an adverse effect on our business and results of operations. Market disruptions have included extreme volatility in securities prices, as well as severely diminished liquidity and credit availability. The economic crisis may adversely affect us in a variety of ways. Access to lines of credit or the capital markets may be severely restricted, which may preclude us from raising funds required for operations and to fund continued development. It may be more difficult for us to complete strategic transactions with third parties. The financial and credit market turmoil could also negatively impact suppliers, clients and banks with whom we do business. Trade barriers or tariffs may be imposed on a temporary or permanent basis. Such developments could decrease our ability to source, produce and distribute our products or obtain financing and could expose us to a risk that one of our suppliers, clients or banks will be unable to meet their obligations under agreements with us.
We are dependent on our limited number of suppliers.
We currently purchase animals and certain key components of biological and chemical materials that we use in our products and services from a limited number of outside sources. Our reliance on suppliers exposes us to risks, including: (i) the possibility that one or more of our suppliers could terminate their services at any time without penalty; (ii) the potential inability of our suppliers to obtain required materials; (iii) the potential delays and expenses of seeking alternative sources of supply; and (iv) reduced control over pricing, quality and timely delivery due to the difficulties in switching to alternative suppliers.
Consequently, if materials from our suppliers are delayed or interrupted for any reason, we may not be able to deliver our products and perform our services on a timely basis or in a cost-efficient manner.
Our insurance policies may be inadequate to fully protect us from material judgments and expenses.
We may become subject to liability for risks against which we cannot insure or against which we may elect not to insure due to the high cost of insurance premiums or other factors. The payment of any such liabilities would reduce the funds available for our usual business activities. Payment of liabilities for which we do not carry insurance may have a material adverse effect on our financial position and operations.
We restrict use of scientific information which may limit our ability to improve the efficiency of the drug discovery services we provide.
Our ability to improve the efficiency of the AI-powered biologic CRO services we provide by, among other things, developing an effective database designed to predict how chemical compounds interact with a targeted disease-related protein, depends in part on our generation and use of information that is not proprietary to our clients and that we derive from performing these services. However, our clients may not allow us to use this information with other clients, such as the general interaction between types of chemistries and types of drug targets that we generate when performing drug discovery services for our clients. Without the ability to use this information, we may not be able to develop a database, which may limit our ability to improve the efficiency of the drug discovery services we provide.
Our operations could suffer if there is a failure of laboratory facilities.
Our operations could suffer as a result of a failure of our laboratory facilities. Our business will be dependent upon a laboratory infrastructure to produce products and services. Our systems and operations are vulnerable to damage and interruption from fires, earthquakes, telecommunications failures, and other events. Any such errors or inadequacies in the software that may be encountered could adversely affect operations, and such errors may be expensive or difficult to correct in a timely manner.
Further, many of our operations are comprised of complex mechanical systems that are subject to periodic failure, including aging fatigue. Such failures are unpredictable, and while we have made significant capital expenditures designed to create redundancy within these mechanical systems, strengthened biosecurity, improved operating procedures to protect against contaminations, and replaced impaired systems and equipment in advance of such events, failures and/or contaminations may still occur.
The production of monoclonal and polyclonal antibodies requires state of the art laboratory facilities and the success of these laboratory services depends on the recruitment and retention of highly qualified technical staff to maintain the level and quality of standard of our products and services expected from clients. There is no assurance that we will be able to expand and operate such state of the art laboratory services and recruit and retain qualified staff.
We produce and supply antibodies and there is no guarantee that such production will be successful and produce the desired results. As a result, we continue to be exposed to potential liability that may exceed any insurance coverage that we may obtain in the future. As a result, we may incur significant liability exposure, which may exceed any insurance coverage that we may obtain in the future. Even if we elect to purchase such insurance in the future, we may not be able to maintain adequate levels of insurance at reasonable cost and/or on reasonable terms. Excessive insurance costs or uninsured claims may increase our operating loss and affect our financial condition.
Contaminations in animal populations may have an adverse impact on our business operations.
Animals that we use must be free of certain infectious agents, such as certain viruses and bacteria, because the presence of these contaminants can distort or compromise the quality of research results and could adversely impact animal health. The presence of these infectious agents in our animal facility and certain service operations could disrupt our animal service businesses, harm our reputation and result in decreased sales.
Contaminations are unanticipated and difficult to predict and could adversely impact our financial results. If they occur, contaminations typically require cleaning up, renovating, disinfecting, retesting and restarting production or services. Such clean-ups result in inventory loss, clean-up and start-up costs, and reduced sales as a result of lost client orders and potentially credits for prior shipments. Contaminations also expose us to risks that clients will request compensation for damages in excess of our contractual indemnification requirements.
We will be reliant on information technology systems and may be subject to damaging cyber-attacks.
We operate large and complex information systems that contain significant amounts of client data. As a routine element of our business, we collect, analyze and retain substantial amounts of data pertaining to the non-clinical research we conduct for our clients. Unauthorized third parties could attempt to gain entry to such information systems to steal data or disrupt the systems. We have taken measures to protect them from intrusion.
Our contracts with our clients typically contain provisions that require us to keep confidential the information generated from the research conducted. In the event the confidentiality of such information is compromised, whether by unauthorized access or other breaches, we could be exposed to significant harm, including termination of customer contracts, damage to our customer relationships, damage to our reputation and potential legal claims from customers, employees and other parties. In addition, we may face investigations by government regulators and agencies as a result of a breach.
Further, we are required to comply with data privacy and security laws in many jurisdictions. For example, we are required to comply with the European Union General Data Protection Regulation (“GDPR”), which became effective on May 25, 2018 and imposes heightened obligations and enhanced penalties for non-compliance (including up to four percent (4%) of global revenue). The cost of compliance, and the potential for fines and penalties for non-compliance, with GDPR may have a significant adverse effect on our business and operations. Also, the California legislature passed the California Consumer Privacy Act (“CCPA”), which became effective January 1, 2020. The CCPA creates new transparency requirements and grants California residents several new rights with regard to their personal information. Failure to comply with the CCPA may result in, among other things, significant civil penalties and injunctive relief, or potential statutory or actual damages. We have made changes to, and investments in, our business practices and will continue to monitor developments and make appropriate changes to help attain compliance with these evolving and complex regulations.
It may not be possible for United States investors to enforce actions against us, and our directors and officers.
We are organized under the laws of the Province of British Columbia, with our registered place of business in Canada, some of our directors and officers reside outside the United States and the majority of our assets and all or a substantial portion of the assets of these persons may be located outside the United States. Consequently, it may be difficult for investors who reside in the United States to effect service of process in the United States upon us or upon such persons who are not residents of the United States, or to realize upon judgments of courts of the United States predicated upon the civil liability provisions of the United States federal securities laws.
Our status as a Foreign Private Issuer under United States securities laws.
We are a “foreign private issuer”, under applicable U.S. federal securities laws, and are, therefore, not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, we do not file the same reports that a U.S. domestic issuer would file with the SEC, although we are required to file with or furnish to the SEC the continuous disclosure documents that we are required to file in Canada under Canadian securities laws. In addition, our officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions of Section 16 of the Exchange Act. Therefore, our shareholders may not know on as timely a basis when our officers, directors and principal shareholders purchase or sell Common Shares, as the reporting periods under the corresponding Canadian insider reporting requirements are longer.
As a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements. We are also exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While we comply with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, these requirements differ from those under the Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, we may not be required under the Exchange Act to file annual and quarterly reports with the SEC as promptly as U.S. domestic companies whose securities are registered under the Exchange Act.
In addition, as a foreign private issuer, we have the option to follow certain Canadian corporate governance practices, except to the extent that such laws would be contrary to U.S. securities laws, and provided that we disclose the requirements we are not following and describe the Canadian practices we follow instead. We may in the future elect to follow home country practices in Canada with regard to certain corporate governance matters. As a result, our shareholders may not have the same protections afforded to shareholders of U.S. domestic companies that are subject to all corporate governance requirements.
We may we lose our Foreign Private Issuer status, which would alter our reporting requirements.
We may lose our status as a foreign private issuer if, as of the last business day of our second fiscal quarter for any year, more than 50% of our outstanding voting securities (as determined under Rule 405 of the Securities Act) are directly or indirectly held of record by residents of the United States. The regulatory and compliance costs under U.S. federal securities laws as a U.S. domestic issuer may be significantly more than the costs incurred as a Canadian foreign private issuer eligible to use the MJDS. If we are not a foreign private issuer, we would not be eligible to use the MJDS or other foreign issuer forms and would be required to file periodic and current reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. These increased costs may have a material adverse effect on our business, financial condition or results of operations.
We are incorporated in Canada and therefore are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq corporate governance listing standards; these practices may afford less protection to shareholders than they would enjoy if we complied fully with the Nasdaq corporate governance listing standards.
As we are incorporated in Canada and listed on Nasdaq, we are subject to the Nasdaq corporate governance listing standards. However, Nasdaq rules permit a foreign private issuer to follow the corporate governance practices of an issuer’s home country. Certain corporate governance practices in Canada, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards for U.S. domestic issuers. We have relied on home country practices with respect to our corporate governance.
We may fail to meet the continued listing requirements of Nasdaq which could result in a delisting of our securities.
If we fail to satisfy the continued listing requirements of Nasdaq, such as minimum bid price requirements, Nasdaq may take steps to delist our Common Shares. Such a delisting would have a materially adverse effect on the price of our outstanding securities, impair the ability to sell or purchase our Common Shares or securities convertible or exercisable into Common Shares when persons wish to do so, and materially and adversely affect our ability to raise capital or pursue strategic restructuring, refinancing or other transactions on acceptable terms, or at all.
To maintain the listing of our Common Shares on Nasdaq, we must satisfy minimum financial and other continued listing requirements and standards, including those related to the price of our Common Shares. Pursuant to the requirements of Nasdaq, if the closing bid price of a company's stock falls below US$1.00 per share for 30 consecutive business days (the "Minimum Bid Requirement"), Nasdaq will notify the company that it is no longer in compliance with the Nasdaq listing qualifications. If a company is not in compliance with the Minimum Bid Requirement, the company will have 180 calendar days to regain compliance. On August 19, 2024, we initially received notice from Nasdaq that we were no longer in compliance with the Minimum Bid Requirement (the "Initial Nasdaq Non-Compliance Notice"). On July 13, 2025, Nasdaq notified the Company that it has determined that for the last 10 consecutive business days, from June 26, 2025 to July 10, 2025, the closing bid price of the Company’s common stock has been at $1.00 per share or greater. Accordingly, the Company has regained compliance with Listing Rule 5550(a)(2), and this matter is now closed.
We are an emerging growth company and rely on exemptions from certain disclosure requirements which may make our Common Shares less attractive to investors.
We are an “emerging growth company” as defined in section 3(a) of the Exchange Act (as amended by the JOBS Act, enacted on April 5, 2012), and we will continue to qualify as an emerging growth company until the earliest to occur of: (a) the last day of the fiscal year during which we have total annual gross revenues of U.S.$1,235,000,000 (as such amount is indexed for inflation every five years by the SEC) or more; (b) the last day of our fiscal year following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration statement under the Securities Act; (c) the date on which we have, during the previous three year period, issued more than U.S.$1,000,000,000 in non-convertible debt; and (d) the date on which we are deemed to be a “large accelerated filer”, as defined in Rule 12b-2 under the Exchange Act. We will qualify as a large accelerated filer (and would cease to be an emerging growth company) at such time when on the last business day of our second fiscal quarter of such year the aggregate worldwide market value of our common equity held by non-affiliates will be U.S.$700,000,000 or more.
For so long as we remain an emerging growth company, we are permitted to and intend to rely upon exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the JOBS Act. We take advantage of some, but not all, of the available exemptions available to emerging growth companies. We cannot predict whether investors will find the Common Shares less attractive because we rely upon certain of these exemptions. If some investors find the Common Shares less attractive as a result, there may be a less active trading market for the Common Shares and the Common Share price may be more volatile. On the other hand, if we no longer qualify as an emerging growth company, we would be required to divert additional management time and attention from our development and other business activities and incur increased legal and financial costs to comply with the additional associated reporting requirements, which could negatively impact our business, financial condition and results of operations.
We may be a “passive foreign investment company” for U.S. federal income tax purposes, which could result in adverse U.S. federal income tax consequences for U.S. Holders.
We believe that we were not a “passive foreign investment company” (“PFIC”) for our tax year ended April 30, 2025, and we have not yet made a determination regarding our potential classification as a PFIC for our current tax year. While we do not intend to become a PFIC for our current tax year or in the future, based on cash raised in one or more offerings and current business plans and financial expectations, we may be a PFIC for our current tax year and may be a PFIC in the future. Our PFIC classification for our current or future tax years may depend on, among other things, how quickly we may raise cash pursuant to one or more offerings, the manner in which, and how quickly, we utilize our cash on hand and the cash proceeds received from any such offerings, as well as on changes in the market value of our Common Shares. Whether we are a PFIC for any taxable year will also depend on the composition of our income and the composition, nature and value of our assets from time to time (including the value of our goodwill, which may be determined by reference to the value of our Common Shares, which could fluctuate). If we are a PFIC for any year during a U.S. Holder’s (as defined below under the heading “Certain Material United States Federal Income Tax Considerations”) holding period of Common Shares, then such U.S. Holder generally will be required to treat any gain realized upon a disposition of the Common Shares or any so-called excess distribution received on its Common Shares as ordinary income, and to pay an interest charge on a portion of such gain or distribution.
In certain circumstances, the sum of the tax and the interest charge may exceed the total amount of proceeds realized on the disposition, or the amount of excess distribution received, by the U.S. Holder. Subject to certain limitations, these tax consequences may be mitigated if a U.S. Holder makes a timely and effective QEF Election (as defined below under the heading “Certain Material U.S. Federal Income Tax Considerations”) with respect to the Common Shares or a Mark-to-Market Election (as defined below under the heading “Certain Material United States Federal Income Tax Considerations”) with respect to the Common Shares. U.S. Holders should be aware that there can be no assurances that we will satisfy the record keeping requirements that apply to a QEF (as defined below under the heading “Certain Material United States Federal Income Tax Considerations”), or that we will supply U.S. Holders with information that such U.S. Holders are required to report under the QEF rules, in the event that we are a PFIC. Thus, U.S. Holders may not be able to make a QEF Election with respect to their Common Shares. A U.S. Holder who makes a Mark-to-Market Election generally must include as ordinary income each year the excess of the fair market value of the Common Shares over the U.S. Holder’s tax basis therein. Each potential investor who is a U.S. Holder should review the discussion below under the heading “Certain Material United States Federal Income Tax Considerations — Passive Foreign Investment Company Rules” in its entirety and should consult its own tax advisor regarding the tax consequences of the PFIC rules and the acquisition, ownership, and disposition of the Common Shares.
Currency fluctuations may have a material effect on us.
We may conduct business with clients, distributors, suppliers, other service providers and affiliates in currencies other than Canadian Dollars. Therefore, our business could be adversely affected by fluctuations in domestic or foreign currencies.
We may require additional capital which may result in dilution to existing shareholders.
We may sell additional equity securities in subsequent offerings (including through the sale of securities convertible into equity securities) and may issue additional equity securities to finance operations, acquisitions or other projects. We cannot predict the size of future issuances of equity securities or the size and terms of future issuances of debt instruments or other securities convertible into equity securities or the effect, if any, that future issuances and sales of securities will have on the market price of the Common Shares. Any transaction involving the issuance of previously authorized but unissued Common Shares, or securities convertible into Common Shares, would result in dilution, possibly substantial, to securityholders. Exercises of presently outstanding share options may also result in dilution to security holders.
Our board of directors (“Board”) has the authority to authorize certain offers and sales of additional securities without the vote of, or prior notice to, shareholders. Based on the need for additional capital to fund expected expenditures and growth, we expect that we will issue additional securities to provide such capital. Such additional issuances may involve the issuance of a significant number of Common Shares at prices less than the current market price for the Common Shares.
Sales of substantial amounts of securities, or the availability of such securities for sale, could adversely affect the prevailing market prices for securities and dilute investors’ earnings per share. A decline in the market prices of the securities could impair our ability to raise additional capital through the sale of securities should we desire to do so. Sales of Common Shares by shareholders might also make it more difficult for us to sell equity securities at a time and price that it may deem to be appropriate.
The market price of our securities may be volatile.
An investment in our securities is highly speculative. The market prices for the securities of pharmaceutical companies, including ours, have historically been highly volatile. The market has from time to time experienced significant price and volume fluctuations that are unrelated to the financial performance or prospects of any particular company. In addition, because of the nature of our business, certain factors such as announcements, competition from new therapeutic products or technological innovations, governmental regulations, fluctuations in operating results, results of clinical trials, public concern regarding the safety of drugs generally, general market conditions, developments in patent and proprietary rights, our financial condition or results of operations as reflected in our quarterly and annual financial statements, operating performance and the performance of competitors and other similar companies, changes in earnings estimates or recommendations by research analysts who track our securities or securities of other companies in the life sciences sector, general market conditions, announcements relating to litigation, the arrival or departure of key personnel and the factors listed under the heading “Risk Factors” can have an adverse impact on the market price of the Common Shares.
Any negative change in the public’s perception of our prospects could cause the price of our securities, including the price of the Common Shares, to decrease dramatically. Furthermore, any negative change in the public’s perception of the prospects of life sciences companies in general could depress the price of our securities, including the price of the Common Shares, regardless of our financial and operating results. In the past, following declines in the market price of a company’s securities, securities class-action litigation often has been instituted against said company. Litigation of this type, if instituted, could result in substantial costs and a diversion of our management’s attention and resources.
Our discretion in use of proceeds is based on a number of factors that may change from what we planned and disclosed previously.
We will have broad discretion over the use of proceeds from an offering of our securities. Because of the number and variability of factors that will determine our use of such proceeds, our ultimate use might vary substantially from any planned use disclosed us. Investors and security holders may not agree with how we allocate or spend the proceeds from an offering of securities. We may pursue acquisitions, collaborations or other opportunities that do not result in an increase in the market value of our securities, including the market value of the Common Shares, and that may increase our losses.
We have never paid dividends to our common shareholders.
No dividends on the Common Shares have been paid by us to date. We do not intend to declare or pay any cash dividends in the foreseeable future. Payment of any future dividends will be at the discretion of the Board, after taking into account a multitude of factors appropriate in the circumstances, including our operating results, financial condition and current and anticipated cash needs.
Our Common Shares may be illiquid.
Our shareholders may be unable to sell significant quantities of Common Shares into the public trading markets without a significant reduction in the price of their Common Shares, or at all. There can be no assurance that there will be sufficient liquidity of our Common Shares on the trading market, and that we will continue to meet the listing requirements of the Nasdaq.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
Name, Address and Incorporation
The Company was incorporated under the Business Corporations Act (British Columbia) (the “BCBCA”) on September 2, 2016. On December 21, 2016, the Company changed its name to “ImmunoPrecise Antibodies Ltd. ”The address of the Company’s head office is Industrious 823 Congress Ave Suite 300 Austin, Texas 78701. The registered and records office of the Company is located at 19th Floor, 885 West Georgia Street, Vancouver, British Columbia V6B0M3V6C 3H4, Canada.
Our business activities are carried on by our wholly owned subsidiaries, see Item 4.C. - Organizational Structure.
Our Common Shares are listed and posted for trading on Nasdaq under the symbol “IPA.”
The SEC maintains an internet site at http://www.sec.gov/edgar that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Our internet site is https://www.ipatherapeutics.com; our telephone number is 250-483-0308.
Events in the Development of the Business
Over the last three years, the Company has focused on growing its service and product offerings and revenues through organic growth as well as acquisitions, as set out below.
Fiscal Year Ended 2023
Key additions and changes to the board and management team
In August 2022, Ms. Lisa Helbling retired as Chief Financial Officer ("CFO"). The Board appointed Mr. Brad McConn as CFO effective August 6, 2022.
In November 2022, Dr. Anna Pettersson notified the Company she had accepted a position with another company, and due to a potential conflict of interest did not stand for re-election. In December 2022, the Board appointed Ms. Lisa Helbling as director upon conclusion of the annual general meeting.
Strategic Partnerships
On October 12, 2022, the Company’s subsidiary, Talem entered into a multi-target license agreement with OmniAb, Inc. (“OmniAb”), a subsidiary of Ligand Pharmaceuticals Incorporated (the “OmniAb License Agreement”). The agreement builds upon Talem’s extensive antibody development expertise and its access to LENSai in silico software technology to further the development and commercialization of OmniChicken-derived antibody panels against B7H3, CD38 and TIM3, which are immuno-oncology targets. Under the terms of the agreement, Talem will oversee the development and optimization of the antibodies for each program. OmniAb and Talem will share downstream economics upon potential out-licensing or commercialization of the programs.
On November 30, 2022, the Company’s subsidiary, BioStrand entered into a research collaboration and license agreement with BriaCell Therapeutics Corp. (“BriaCell”) (the “BriaCell Therapeutics License Agreement”). Under the terms of the agreement, BioStrand and BriaCell will collaborate on the design, discovery, and development of anti-cancer antibodies. Upon successful antibody discovery, BioStrand will receive an upfront payment of U.S.$500,000, and will be eligible to receive future success-based development milestones, including those for the submission of Investigational New Drugs, clinical milestone payments, and commercial royalties on net sales of products.
On March 15, 2023, the Company’s subsidiary, Talem, an independently operating subsidiary of IPA, and Libera Bio S.L., signed a collaboration agreement to jointly address intracellular targets (the “Libera Bio Agreement”).
On March 30, 2023, the Company’s subsidiary, Talem entered into a research collaboration and exclusive option license agreement with Xyphos Biosciences, Inc. (a wholly owned subsidiary of Astellas Pharma Inc., “Astellas”) (the “Astellas Research Collaboration and License Option Agreement”). Under the terms of the agreement, the companies agreed to jointly conduct research activities to identify and optimize proprietary LENSai in silico generated antibodies, targeting an undisclosed target in the tumor microenvironment (TME), as potential therapeutic development candidates. Targeting this molecule has the potential to markedly enhance anti-tumor immunity with other Astellas therapies including chimeric antigen receptor-based (CAR) technologies. Astellas has the exclusive option to license any development candidates generated as part of the collaboration.
Fiscal Year Ended 2024
Funding
Public Offering
On July 11, 2023, the Company filed a U.S.$300 million shelf registration statement on Form F-3 (File No. 333-273197) (the “Registration Statement”) with the SEC, under which the Company may offer for sale, from time to time, either separately or together in any combination, equity, debt, or other securities described in the Registration Statement through the 36-month expiration period. The Registration Statement was declared effective by the SEC on July 14, 2023.
On December 5, 2023, the Company entered into an underwriting agreement with The Benchmark Company LLC, (the “Underwriting Agreement”) and closed a U.S.$1.265 million underwritten public offering of 1,265,000 Common Shares, including 165,000 Common Shares issued pursuant to the full exercise by the underwriter of its over-allotment option. The public offering price for each common share, before the underwriter’s discount and commissions, was U.S.$1.00. All of the securities in the underwritten public offering were sold by the Company. The Company intends to use the net proceeds from the proposed offering for R&D; capital expenditures, including expansion of existing laboratory facilities; and working capital and general corporate purposes. The Benchmark Company acted as the sole Book-Running Manager and R.F. Lafferty acted as Co-Manager for the offering.
ATM Offering
On August 15, 2023, the Company and Jefferies LLC entered into an Open Market Sale Agreement (the “Open Market Sale Agreement”) relating to the sale of Common Shares having an aggregate offering price of up to U.S.$60,000,000. The Open Market Sale Agreement was terminated on February 13, 2024.
On February 23, 2024, the Company and Clear Street LLC (“Clear Street”) entered into an At-The-Market Offering Agreement (the “Clear Street ATM Agreement”). Under the terms of the Clear Street ATM Agreement, the Company was entitled, at its discretion and from time-to-time during the term of the Clear Street ATM Agreement, to sell, through Clear Street, acting as sole sales agent, Common Shares having an aggregate gross sales price of up to U.S.$60 million. In fiscal 2024, 629,240 common shares were sold under the ATM with proceeds net of commissions of $1.8 million. From May 1, 2024 through July 26, 2024, 357,760 common shares were sold under the ATM with proceeds net of commissions of $0.5 million.
Key additions and changes to the board and management team
On August 9, 2023, the Board adopted a majority voting policy (the “Majority Voting Policy”) based on its belief that each of its directors should carry the confidence and support of the Company’s shareholders and its commitment to upholding high standards incorporate governance. Under the Majority Voting Policy, any director who receives more "withheld" votes than "for" votes will be required to tender his or her resignation to the Board. Absent extraordinary circumstances, the Board is expected to accept such resignation.
On September 5, 2023, the Company announced changes to the composition of the Board. Mr. Gregory S. Smith resigned as a director of the Company. Messrs. Barry A. Springer, Dirk Witters and Chris Buyse were appointed to the Board of the Company.
On September 19, 2023, the Company announced that Mr. Brad McConn had resigned as Chief Financial Officer, effective September 29, 2023. Ms. Kristin Taylor, MBA, CPA (Inactive), was named as interim Chief Financial Officer and on June 16, 2024, she was appointed Chief Financial Officer.
On October 2, 2023, the Board appointed Mr. Chris Buyse as the Chairman of the Remuneration and Nomination Committee.
On November 15, 2023, the Board appointed Mr. Mitch Levine as the Chairman of the Board.
On November 15, 2023, the Board appointed Mr. Dirk Witters as the Chairman of the Audit Committee
On January 12, 2024, the Board appointed Mr. Mitch Levine as the Chairman of the Corporate Governance Committee
Product Line
On June 6, 2023, the Company introduced an AI-driven rapid therapeutic screening platform, the result of a collaboration between IPA Canada and its subsidiary, BioStrand. This solution aims to expedite the early stages of drug discovery by enabling the early elimination of less promising therapeutic candidates, thereby reducing time, cost, and the risk of failure during later stage discovery.
On October 23, 2023, BioStrand’s integrated platform, designed to enhance customers' drug discovery and development, began its limited release through a phased rollout strategy. The Company charges a fee-for-service with a planned roll-up to a Software as a Service.
On October 25, 2023, the Company’s subsidiary, BioStrand, commercially launched its state-of-the-art Retrieval Augmented Generation (RAG)-based Large Language Model (LLM) platform. This pioneering platform integrates with the Company’s patented HYFT technology and LENSai platform, which aims to ensure accuracy, interpretability, and data-centric design in generative AI tools.
New Processes
On May 30, 2023, BioStrand solved the Information Integration Dilemma (“IID”) by developing technology that enables their patented HYFT Technology to encapsulate and unify diverse data modalities - including syntactical (sequence) data, 3D structural data, unstructured scientific information (e.g., scientific literature), and more - into a singular, integrated framework. This breakthrough approach facilitates efficient data fusion, enabling a comprehensive analysis and interpretation of complex biological data.
On June 13, 2023, BioStrand’s IID solution announced a new use case, providing a unified framework that encapsulates and integrates diverse data modalities, including syntactical (sequence) data, 3D structural data, unstructured scientific information, and more.
On November 13, 2023, BioStrand published a preprint of its white paper titled, "New Paradigm for Biological Sequence Retrieval Inspired by Natural Language Processing and Database Research" on bioRχiv. The publication delves into the intricacies of one of BioStrand’s applications based on its patented HYFT-based methodology, a novel and proprietary approach to biological sequence retrieval, and its clear advantages over the gold standard algorithm, Basic Local Alignment Search Tool “BLAST”. By detailing their innovative approach and its potential implications for the scientific community, BioStrand aims to foster collaboration and drive innovation in the realm of bioinformatics.
On March 7, 2024, the Company announced the development of a Foundation AI Model that represents an advancement in life sciences research and development, combining the strengths of Large Language Models through an advanced stacking technique with BioStrand's patented HYFT Technology.
Strategic Partnerships
On March 28, 2024, InterSystems, a creative data technology provider dedicated to helping customers solve critical scalability, interoperability, and speed challenges, together with the Company announced a collaboration that integrates the new vector search capability of the InterSystems IRIS® data platform with IPA's subsidiary BioStrand's LENSai platform. This innovative integration marries the precision of Vector Search, which enables efficient and accurate retrieval of relevant information from massive datasets using vector embeddings, with the depth of analysis provided by LENSai Universal Foundation AI Model and BioStrand's patented HYFT Technology. The result is a platform that offers capabilities in accessing, analyzing, and leveraging complex biological data for drug discovery, understanding disease mechanisms, and beyond.
Fiscal Year Ended 2025
Recent Developments
On September 26, 2024, the Company announced the clinical progress achieved with rabbit monoclonal antibodies designed and developed using IPA’s proprietary B Cell Select® platform for the clinical-stage company, OncoResponse Inc.
On October 2, 2024, the Company and Biotheus Inc. (“Biotheus”), jointly announced entering into a Material Transfer and Evaluation Agreement (“MTEA”) pertaining to a Talem therapeutic antibody asset for the development of a bispecific therapy against solid tumors, under which Biotheus will obtain the rights to further evaluate the suitability of Talem’s Artificial Intelligence (AI)-enhanced TATX-20 lead candidate for the development of novel bispecific antibodies for the treatment of hypoxicsolid tumors. Under the MTEA, Biotheus will receive a specialized antibody asset from Talem Therapeutics, a subsidiary of IPA.
On October 28, 2024, the Company announced its contribution and advancements in anti-aging research with Mayo Clinic Study.
On November 13, 2024, the Company announced a breakthrough in its primary cancer research initiatives through pioneering high-impact antibody development for next-generation Antibody-Drug Conjugates (“ADC”) therapies.
On December 23, 2024, the Company announced insider share purchases, with CEO Dr. Jennifer Bath and BioStrand co-founders Dirk Van Hyfte and Ingrid Brands collectively acquiring a total of 763,120 Common Shares on the open market for an aggregate amount of USD $306,000.
On January 17, 2025, the Company announced the launch of its AI-powered pipeline of both optimized and new therapeutics, set to transform therapeutic development by empowering drug discovery with AI and first-principles innovation.
On January 22, 2025, the Company announced it developed a new class of GLP-1 therapies entirely through artificial intelligence, designed to enhance efficacy, safety, therapy longevity, and patient satisfaction in diabetes treatment.
On January 27, 2025, the Company announced the completion of its previously disclosed “at-the-market” equity offering program along side the full conversion of its outstanding debenture with Yorkville, significantly enhancing the Company’s capital structure.
On February 24, 2025, the Company announced the appointment of Kamil Isaev to the Board and Joseph Scheffler as Interim Chief Financial Officer, along with the departure of director Chris Buyse. On February 26, 2025, the Company announced a strategic collaboration with RIBOPRO, a pioneering technology provider specializing in mRNA and lipid nanoparticle (LNP) technologies, to revolutionize the discovery and development of therapeutic antibodies by integrating RIBOPRO’s advanced mRNA-based antigen expression expertise with IPA’s in silico and wet-lab antibody discovery capabilities.
On March 13, 2025, the Company announced a strategic partnership with a leading biotechnology company to advance the discovery and development of ADCs and bispecific antibodies for the treatment of cancer, focusing on leveraging contract research expertise while integrating IPA’s proprietary B-cell Select™ platform and artificial intelligence-driven discovery capabilities to enhance the efficiency and precision of therapeutic development.
On May 12, 2025, the Company announced new benchmarking results that validate the accuracy and utility of its in silico epitope mapping application, part of the LENSai™ platform, with a direct comparison to gold-standard wet-lab methods.
On May 21, 2025, the Company announced it engaged CORE IR, a strategic investor and public relations firm, to support the Company’s ongoing investor relations and communications initiatives, with CORE IR specializing in working with emerging and established growth companies to enhance investor awareness, strengthen shareholder engagement, and broaden outreach to various institutional and retail audiences.
On June 5, 2025, the Company announced the discovery of a highly conserved epitope across all four dengue virus serotypes using its proprietary LENSai™ platform powered by their patented HYFT® technology.
On June 12, 2025, the Company announced compelling in vitro results demonstrating that its artificial intelligence-designed GLP-1receptor agonist (GLP-1RA) peptide sequences achieve comparable or superior receptor activation to Semaglutide, a benchmark GLP-1 therapy and one of the most commercially successful drugs in the world. The in vitro analysis was conducted by an independent third party, further strengthening the objectivity and reliability of the findings.
On June 24, 2025, the Company announced that advancements in the universal dengue vaccine, confirming safety, immune activation and structural stability using its LENSai™ platform powered by patented HYFT® Technology
Securities Purchase Agreement
On July 16, 2024 the Company announced that it entered into a securities purchase agreement (the “Securities Purchase Agreement”) with YA II PN, Ltd., an investment fund managed by Yorkville Advisors Global, LP (“Yorkville”), under which the Company agreed to sell and issue to Yorkville U.S.$3.0 million aggregate principal amount of convertible debentures (the “Convertible Debentures”) in two tranches and at a purchase price of 95% of the aggregate principal amount.
The Convertible Debentures were convertible into common shares of the Company (the “Common Shares”). The sale and issue of the first tranche consisted of U.S.$2.0 million principal amount of Convertible Debentures and was completed on July 16, 2024 (the “First Closing”). The sale and issue of the second tranche consisted of U.S.$1.0 million principal amount of Convertible Debentures and closed on August 16, 2024. In connection with the offering, the Company and Yorkville entered into a customary Registration Rights Agreement pursuant to which the Company provided certain registration rights to Yorkville under the Securities Act.
On August 19, 2024, the Company announced the ability to engineer in silico antibodies to elusive tumor protein entirely through computer simulations using patented LENSai technology.
On August 23, 2024, the Company announced that it received written notification from Nasdaq, indicating that the Company is not in compliance with the minimum bid price requirement set forth in the Nasdaq Rule 5450(a)(1) based on the closing bid price of the Company's common shares being less than US$1.00 per share for the 30 consecutive business days from July 5, 2024 to August 15,2024.
New Processes
On June 10, 2024, BioStrand has introduced an advanced API (Application Programming Interface) within their groundbreaking software for AI-driven drug discovery. This API allows seamless integration with existing research workflows, providing enhanced capabilities for data analysis, molecular modeling, and predictive analytics. Its customizable interface ensures that researchers can tailor the API functionalities to meet specific project requirements, thus accelerating the drug discovery process with increased accuracy and efficiency.
Key additions and changes to the board and management team
On December 31, 2024 the Company announced that Ms. Kristin Taylor had resigned as Chief Financial Officer, effective January 16, 2025. Mr. Joseph Scheffler, MBA, was named as interim Chief Financial Officer on February 24, 2025.
On February 24, 2025 the Company announced changes to the composition of the Board. Mr. Chris Buyse resigned as a director of the Company. Kamil Isaev was appointed to the Board of the Company.
Principal Capital Expenditures and Divestitures
We made the following capital expenditures over the last three financial years.
Fiscal Year Ended 2023
The Company made equipment purchases of $1.5 million during the year ended April 30, 2023.
The acquisition of U-Protein Express B.V. ("UPE") and ModiQuest Research B.V. ("MQR"), now collectively named IPA Europe, has deepened the Company’s technological competence, and expanded its capabilities for partners worldwide. The team from MQR in Oss brings extensive expertise in various areas, including in vitro antibody phage library generation, antibody characterization, optimization, and engineering. The UPE team in Utrecht specializes in the production of complex proteins and antibodies, supporting numerous programs across various sectors using their proprietary expression platform rPEx®.
Fiscal Year Ended 2024
The Company made equipment purchases of $1.4 million during the year ended April 30, 2024.
On March 20, 2024, the Company acquired the LSA® instrument platform from Carterra®, a leading provider of high-throughput large and small molecule screening and characterization solutions. This instrument allows for high throughput surface plasmon resonance-based antibody characterizations thereby increasing the Company's capacity in performing various label-free protein interaction analyses including kinetics, epitope binning, quantitation, epitope mapping, and blocking/neutralization assays.
During fiscal 2024 we began expansion of our lab site at 3204 – 4464 Markham Street, Victoria, British Columbia V8Z 7X8 with an expected completion date prior to the end of early 2026. This will be funded through a combination of leasehold improvement credits from the landlord, internal funding and potentially proceeds from a financing. We have no material equipment capital expenditures underway.
Fiscal Year Ended 2025
The following capital expenditures, which began in 2024, remained in progress in 2025, with a completion date of fiscal year 2026: lab expansion at – 4464 Markham Street, Victoria, British Columbia V8Z 7X8.
B. Business Overview
The Company is a leading AI-driven biotherapeutic research and technology firm, distinguished by its proficiency in both in silico and wet lab methodologies. At the intersection of systems biology, multi-omics modeling, and complex artificial intelligence systems, the company has carved out a unique space within the field. The core of the company's operations encompasses a diverse suite of proprietary technologies that aid in the exploration, discovery, and development of novel drugs and biologics.
Integrated within ImmunoPrecise's wet lab infrastructure is a diverse array of in silico technologies. As an end-to-end service provider of antibody discovery and development, IPA’s state-of-the-art computational methodologies allow the Company to perform detailed and comprehensive evaluations across various stages of biologic discovery and development.
The synergy between ImmunoPrecise's in silico analyses and wet lab technologies enhances the efficacy of the workflow, thereby offering a unique value proposition to its partners aimed at reducing the time, cost and risk associated with therapeutic antibody discovery and development. This strategic integration underscores ImmunoPrecise's commitment to innovative solutions, driving not only operational efficiency but also pioneering advancements in the industry.
The Company believes that its experience, innovation, technologies, scientific rigor, and focus on producing quality products, provide a unique experience in one-stop service offerings, and assist the Company in its aim to reduce the time required for, and the inherent risk associated with, conventional multi-vendor product development.
The Company has achieved organic revenue growth through market penetration and service diversification in the biologics, CRO space, as well as accretive growth through strategic expansion of its operations in Europe, by acquiring and integrating innovative technologies, and through investments in R&D.
Products and Services
The breadth of services provided by ImmunoPrecise unfolds sequentially in alignment with the process of antibody discovery and development. Starting from the in silico arena, the company utilizes custom antigen modeling, target analysis using Natural Language Processing, and the patented HYFTTM analysis to lay the groundwork for the subsequent experimental phases.
As the projects transition into the wet lab phase, ImmunoPrecise's capabilities diversify, offering an array of services such as design and manufacturing, B cell sorting incorporating IPA's proprietary Function First B Cell screening and sequencing, and the production and screening of custom, immune, and proprietary naïve phage display libraries. IPA's wet lab antibody discovery technologies are compatible with in-depth mining of antibody repertoires by next generation sequencing and computational analysis. The Company's hybridoma discovery and production services, enhanced by multiplexed high-throughput screening and single clone-picking, complement the expertise it possesses with transgenic animals and multi-species antibody discovery.
The Company then steps into antibody characterization studies, which encompass affinity measurements, epitope landscape profiling, functional assays, and in silico analyses including immunogenicity, three-dimensional modeling, relative affinity rankings, molecular docking, and off-target analyses. Additional services include the creation of bi-specifics, single domain (such as VHH and VNAR (shark)) antibodies, recombinant cloning, protein and antibody production and downstream processing, stable cell line generation, antibody engineering, optimization including humanization, and cryopreservation and cryostorage.
ImmunoPrecise's wholly owned subsidiaries, IPA Canada and IPA Europe, have received recognition as approved Contract Research Organizations ("CRO") for top-tier transgenic animal platforms producing antibodies with human antigen binding domains, along with protein manufacturing. The subsidiaries also form a critical component of the Company's R&D investments, promoting the development of proprietary technologies like B cell Select® and DeepDisplayTM platforms, applicable across a wide array of species and strains, including transgenic animals.
Moreover, in the past two years, the Company has gained increasing recognition as a rising leader in the biologics CRO space, with a focus on organic growth through market penetration and service diversification, as well as strategic expansion with platform and process integration. Furthermore, end-to-end services have been leveraged through acquisition, enabling a steady foundation for future growth.
In fiscal 2025, the Company’s CRO services accounted for 100% (2024: 100%) of the Company’s revenue.
The Company’s wholly owned subsidiaries, IPA Canada and IPA Europe, have both been designated as approved CROs for the world’s leading, transgenic animal platform producing human antibodies, and exercised an advantage in optimizing services for various transgenic animal vendors. The Company made strategic investments in R&D activities to develop proprietary technologies enabling the application of their B cell Select™ and DeepDisplay™ platforms to address a range of transgenic animal species and strains and efficiently deliver fully-human, clinically relevant antibodies to its clients.
The Company’s key CRO services are set forth in detail below:
|
|
|
Service |
|
Details |
B cell SelectTM |
|
In 2018, the Company built on its decade of experience in single B cell interrogation to offer B cell services in both North America and Europe on species agnostic platforms, including the use of transgenic, humanized animals. These services are offered for a broad range of therapeutically relevant protein families, including GPCRs and other challenging, membrane-spanning proteins. The Company’s B cell Select™ platforms enable antibody screening directly from B cells, facilitating the analysis of a more diverse set of antibodies, and for faster, deeper screening compared to traditional technologies. By adding a high throughput, label-free Octet HTX biosensor (under the tradenames FortéBio, Sartorius) at IPA Canada, the Company uses a state-of-the-art high throughput platform that facilitates the rapid characterization and development of lead antibody candidates and addresses the need for increased speed and sample throughput when characterizing large panels of therapeutic antibody candidates, which are generated with its B cell or library-based platforms. |
|
|
|
Phage Display |
|
The Company’s phage display services are based on building custom immune libraries from multiple species, including transgenic animals, or, alternatively, the selection of antigen-specific, recombinant antibody fragments from its proprietary human or llama phage libraries. The proprietary libraries have been made from human auto-immune (diseased) patients and naïve (healthy donors) scFv (single chain fragment variable) repertoires, as well as from naïve llama (VHH) repertoires. Custom immune libraries are prepared from blood, spleen, lymph nodes, and bone marrow of immunized animals and aim to capture the entire immune repertoire for panning, rescue, and identification of unique antibodies with pre-specified characteristics. |
|
|
|
DeepDisplayTM |
|
A powerful new technology utilizing a combination of transgenic animal platforms, like e.g., Ligand’s OmniAb®, and IPA’s custom phage display antibody selection. |
|
|
|
AbthenaTM Bispecifics |
|
The Company’s bispecific Abthena™ technology complements its diverse discovery process, integrating seamlessly with the Artemis™ Intelligence Metadata (AIM)™ capabilities, to enable rapid turnaround on additional algorithmic outputs in therapeutic antibody optimization, stability, affinity, and manufacturability. |
|
|
|
LucinaTechTM Humanization |
|
The Company provides a robust and efficient antibody humanization service, which consistently retains affinity and specificity levels. The approach is based on state-of-the-art in silico antibody modeling to identify essential framework and CDR residues for grafting onto a human antibody framework. |
|
|
|
Affinity Maturation |
|
Antibody affinity is important in therapeutic and diagnostic applications. The Company’s affinity maturation service can improve antibody affinities. The Company applies different strategies to increase the affinity of the antibody, including gene shuffling and random mutagenesis. |
|
|
|
Immunization, hybridoma, sequencing |
|
The Company offers antibody development services including a variety of immunization methods: Rapid Prime™ immunization, DNA immunization (NonaVac™), cell-based immunization (ModiVacc™), electro-fusion and hybridoma generation using semi-solid media and clone picking, as well as high throughput, multiplexed screening methods. With ImmunoProtect™, the DNA sequence of the antibody is determined and can be used to express the antibody recombinantly. |
|
|
|
rPExTM protein manufacturing |
|
The Company provides large-scale production of recombinant mammalian proteins and antibodies for research and non-clinical applications. With a track record of successfully producing difficult-to-express proteins and antibodies (e.g., Fc-fusion proteins and bispecific antibodies), the Company offers gram scale production with low endotoxin levels. |
|
|
|
Cell line development |
|
Using its proprietary vectors, the Company offers stable cell line development services (non GMP) of target proteins or antibodies adapted to specific growth conditions and media. |
Therapeutic Discovery Program
While CRO services are the mainstay of the Company, IPA has worked continuously on building an IP estate and portfolio of proprietary methods and physical assets through collaborations, acquisitions and in-licensing. The Company has strategically invested in the development and licensing of antibody discovery platforms and related IP assets. The onboarding of existing assets with regard to equipment, technologies, IP and licenses within the Company’s European Union operations has been compounded by active R&D at all operational sites, including the ongoing development of new service offerings, but more notably, internal discovery programs focused on novel, therapeutic antibodies, primarily in the field of immuno-oncology.
The Company formed Talem, based in Cambridge, Massachusetts, to support its internal and partnered therapeutic discovery programs. Talem offers strategic partnerships with pharma and biotech companies and is the only company to offer these services as a partnership in OmniAb® transgenic animals using their own license. The depth and speed of IPA’s offerings enables Talem to customize each program and leverages the Company’s expertise and technologies in the antibody discovery.
Principal Markets
Our total revenues by category of activity and geographic market for each of the last three financial years were as follows:
At April 30, 2025, 2024 and 2023, the Company has one reportable segment, being antibody production and related services.
The Company’s revenues are allocated to geographic regions for the year ended April 30, 2025, 2024 and 2023 as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Revenue by Region
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
United States of America |
|
|
12,614 |
|
|
|
12,556 |
|
|
|
9,365 |
|
Europe |
|
|
10,178 |
|
|
|
10,867 |
|
|
|
9,450 |
|
Canada |
|
|
234 |
|
|
|
389 |
|
|
|
618 |
|
Australia |
|
|
896 |
|
|
|
482 |
|
|
|
630 |
|
Other |
|
|
598 |
|
|
|
224 |
|
|
|
602 |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
The Company’s revenues are allocated according to revenue types for the year ended April 30, 2025, 2024 and 2023 as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Revenue Allocation
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Project revenue |
|
|
22,175 |
|
|
|
22,235 |
|
|
|
18,677 |
|
Product sales revenue |
|
|
2,107 |
|
|
|
2,035 |
|
|
|
1,747 |
|
Cryostorage revenue |
|
|
238 |
|
|
|
248 |
|
|
|
241 |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
Market for Products
Market Segment and Geographic Areas
The market for therapeutic antibodies, including monoclonal, biospecific and antibody drug conjugates according to February 2024 Research and Markets publication, is expected to generate U.S.$428 billion by the end of 2029, up from U.S.$211 billion in 2022. During the forest period, 2024-2029, Global Therapeutic Antibody is expected to expand at a compounded annual growth rate ("CAGR") of 11.2%. Growth drivers in the antibody market are as follows:
•
Increasing R&D expenditures in the life science sector and in the therapeutics industry
•
Emergence of innovative, facilitating platforms
•
Growing demand for revolutionary therapies for major diseases as populations age and life expectancies increase
•
Growing emphasis on antibody development at CROs
•
Increasing applications in the environmental sectors
•
Biopharmaceuticals is the fastest growing pharma sector. This market is mainly dominated by large pharmaceutical companies, like AbbVie, Novartis, Roche and Johnson & Johnson. Companies are currently sponsoring clinical studies for more than 570 monoclonal antibodies (mAbs). Of these, approximately 90% are early-stage studies designed to assess safety (Phase I) or safety and preliminary efficacy (Phase I/II or Phase II) in patient populations.
According to Marketandmarkets.com, the global immunoassay market was worth U.S.$35 billion in 2023 and is anticipated to grow with a CAGR of 5.9% to U.S. $46 billion by 2028.
In recent years, the number of monoclonal antibody drugs approved for commercialization has proliferated, with the 100thmonoclonal antibody approved by the United States Food and Drug Administration (“FDA”) as of May 2021 (Nature Reviews Drug Discovery) and further 17 investigational antibody therapeutics in regulatory review in either the United States or Europe as of June 2021 according to AntibodySociety.org.
The current antibody and protein-related market is in excess of U.S.$16 billion in 2022 and expected to reach U.S. $42 billion and growing a CAGR of approximately 13% annually, according to GrandViewResearch.com.
Prior to the acquisitions of UPE and IPA Europe, the Company focused on serving primarily the diagnostic antibody market in North America. Since such acquisitions, the Company has redirected most of its focus to the therapeutic antibody market and delivering an expanded portfolio of products and services to customers in Europe, a broader segment of North America and the rest of the world.
Specialized Skill and Knowledge
The Company’s qualified staff of research and development scientists have experience in biotechnology and the pharmaceutical sector, academic research and government. The Company brings 30 years of experience in the production of antibodies and has a strong reputation for the delivery of a high standard of quality and professional antibody services and products.
Further, the Company has an in-house research staff, including a number of research scientists with MSc and a cadre of technical staff, innovating proprietary Rapid Prime immunization, single step cloning using semi- solid media for HAT selection of hybridomas, and B cell selection and screening.
Competitive Conditions
The Company competes primarily against other full-service CROs as well as services provided by in-house research and development, or R&D, departments of biopharmaceutical companies. The Company’s major CRO competitors include Abveris Inc., Genovac GmbH (formerly part of Aldevron LLC), Antibody Solutions, Genscript Biotech Corp, Lake Pharma Inc. (now part of Curia Inc.), and several specialty and regional CROs.
Competitive factors in the industry in which the Company operates include, but are not limited to, experience within specific therapeutic areas, quality of staff and services, reliability, range of provided services, ability to recruit principal investigators and patients into studies expeditiously, ability to organize and manage large-scale, global clinical trials, global presence with strategically located facilities, speed to completion, price and overall value. The Company believes it competes effectively with its competitors across these factors, particularly due to its full-service operating model, its therapeutic expertise, its global platform and its experienced and committed management team. However, some of the Company’s competitors have greater financial resources and a wider range of service offerings over a greater geographic area than the Company, which could put the Company at a competitive disadvantage with respect to these competitors. Many are also well known for niche specialties such as antibody development against glycosylated peptides or specific chemical modifications, specialties that the Company also houses, but is not yet well known for, which could put the Company at a competitive disadvantage with respect to these competitors.
Many competitors offer custom antibody production services in addition to large catalogues of antibodies available for sale through their websites. Over the years a number of competitors have been acquired and merged into larger companies, particularly larger laboratory facilities.
The R&D antibodies market is highly fragmented and served by numerous small suppliers of a similar size and scale to the Company, and no single company appears to dominate the market.
Regulatory Environment
The development, testing, manufacturing, labeling, storage and approval of antibody and therapeutic products are subject to regulation by various government authorities in Canada and in Europe. Companies in the pharmaceutical and biotechnology industries, such as the Company’s clients, that carry out clinical trials are subject to stringent regulations. These regulations apply to the Company’s clients and are generally applicable to the Company when it provides services to its clients. Consequently, the Company must comply with relevant laws and regulations in the conduct of its business. The Company is in compliance with all Canadian and European regulations regarding the on-going operation of its laboratory facilities and delivery of all its products and services.
Seasonality
Sales of the Company’s products and services have not been subject to seasonality fluctuations.
Marketing Plans and Strategies
Market Acceptance
The Company has a long-standing acceptance of its customized antibodies and protein production services in the market. The Company believes that the market acceptance of its products will continue as it organically grows its business, optimizes its laboratory, new sales and marketing capacity and production process to support long-term growth.
Further, the Company is one of the few approved CROs for multiple transgenic animal providers on the market, enabling the faster development of therapeutic antibodies. Among 28 human antibodies approved by the FDA between 2002 and 2019, 19 were animal derived and nine were generated by phage display.
Proprietary Protection
The Company has initiated the protection of new innovation in its product pipeline and has trademarked its HYFT®, LENSai®, B cell Select®, rPEx®, Rapid Prime®, DeepDisplay™, NonaVac®, Abthena®, Artemis®, LucinaTec®, and ImmunoProtect® technologies. Currently, the Company has filed patent applications related to its proprietary HYFT technology (3 patent families), and to protect its PolyTope SARS-CoV-2 , TATX-200 (TrkB-CD3 bispecifics), TATX-024 (CD3), and TATX-112 (TrkB) intellectual property. Its IP strategy has been to protect its intellectual property primarily through a combination of trade secrets and copyright. See also “Risk Factors”.
The Company continues to develop new products such as novel biotherapeutics in a broad range of indications. New screening methodologies, screening services and data mining methodologies may also provide an expansion and new commercial opportunities for the Company.
Changes to Contracts
The Company uses a standard Master Services Agreement (“MSA”) with all customers for custom monoclonal and polyclonal antibodies and peptide production and does not anticipate any changes in its MSA. The Company has a standard form of contract for its other services and anticipates development of a standard license agreement to take advantage of new licensing opportunities.
Foreign Operations
The Company currently conducts business activities in Canada and a significant portion of the Company’s business activities depend on foreign operations in the Netherlands and the United States. The Company distributes and offers its products and services globally. Significant portions of our revenues are from global sales. In fiscal 2025, 51% of our revenues came from sales to the United States, 42% from Europe and 6% to countries other than Canada.
Locations of Operations
IPA is a global operation with a presence in Utrecht and Oss in the Netherlands, Diepenbeek in Belgium, Victoria, British Columbia, in Canada and Fargo, North Dakota and Austin Texas in the United States. This broad reach enables IPA to tap into thriving locations that strongly support the life sciences industry and the development of artificial intelligence.
The Company's leadership, spanning North America and Europe, holds global responsibility for financial and accounting oversight, sales and marketing, investor relations, and information technology. An enterprise resource management system aids in automating marketing and sales, enhancing customer relationship management, and simplifying accounting, financial reporting, and project management tasks.
The principal executive office is in Austin, Texas and the Company’s base office in Canada is in Victoria, British Columbia. The base for U.S. operations is in Fargo, North Dakota. IPA Canada operates from Victoria, British Columbia (Canada), performing custom antibody generation since its inception. The Company has recently completed the expansion of its vivarium in Victoria while simultaneously intensifying its capabilities in measuring protein binding kinetics and high-throughput label-free protein-protein interactions and further developing and improving technologies such as its B cell Select® platform.
The acquisition of U-Protein Express B.V. ("UPE") and ModiQuest Research B.V. ("MQR"), now collectively named IPA Europe, has deepened the Company’s technological competence, and expanded its capabilities for partners worldwide. The team from MQR in Oss brings extensive expertise in various areas, including in vitro antibody phage library generation, antibody characterization, optimization, and engineering. The UPE team in Utrecht specializes in the production of complex proteins and antibodies, supporting numerous programs across various sectors using their proprietary expression platform rPEx®.
On April 14, 2022, the Company successfully acquired BioStrand BV, BioKey BV, and BioClue BV, a group of innovative artificial intelligence entities based in Belgium. These entities are leaders in the field of multi-omics and in silico biotechnology, specializing in the intricate task of identifying unique biological fingerprints within proteins, RNA, and DNA across multiple information layers, giving rise to unprecedented insights into biological molecules, including intricate relationships between protein structure and function. They have constructed a comprehensive knowledge base of these distinctive biological markers, which serves as a significant tool for their comparison and processing. This strategic acquisition further bolsters the Company’s standing in the rapidly advancing fields of multi-omics and in silico antibody discovery and development.
The Company continues to broaden its intellectual property portfolio in additional, meaningful ways, including internal R&D, acquisitions, and collaborations. There is also an emphasis on therapeutic antibody asset development in areas such as oncology, inflammation, neurodegenerative diseases, autoimmunity, and atherosclerosis.
C. Organizational Structure
The following chart sets out the Company’s intercorporate relationships with its subsidiaries, along with the jurisdiction in which such subsidiaries were formed. All of the Company’s subsidiaries are wholly owned by the Company.
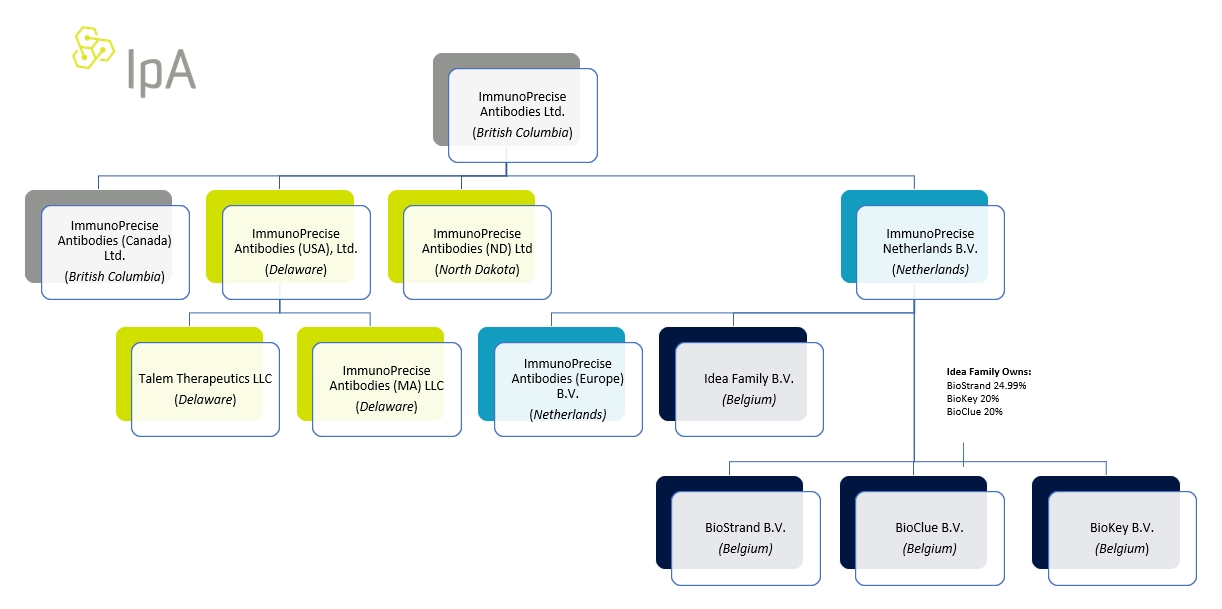
ImmunoPrecise Antibodies (Canada), Ltd.
On May 9, 1995, we incorporated ImmunoPrecise Antibodies (Canada), Ltd. a direct wholly owned subsidiary, under the laws of British Columbia.
ImmunoPrecise Antibodies (USA), Ltd.
On September 11, 2019, we incorporated ImmunoPrecise Antibodies (USA), Ltd. a direct wholly owned subsidiary, under the laws of Delaware.
Talem Therapeutics LLC
On January 18, 2019, Talem Therapeutics LLC was incorporated under the laws of Delaware. Talem Therapeutics LLC is an indirect wholly-owned subsidiary.
ImmunoPrecise Antibodies (MA), LLC
On January 18, 2019, we formed ImmunoPrecise Antibodies (MA), LLC an indirect wholly owned subsidiary, under the laws of Delaware.
ImmunoPrecise Antibodies (ND), Ltd.
On May 25, 2018, we incorporated ImmunoPrecise Antibodies (ND), Ltd. a direct wholly owned subsidiary, under the laws of North Dakota.
ImmunoPrecise Netherlands B.V.
On January 25, 2005, we incorporated ImmunoPrecise Netherlands B.V. a direct wholly owned subsidiary, under the laws of the Netherlands.
ImmunoPrecise Antibodies (Europe) B.V.
On April 5, 2018, we acquired ImmunoPrecise Antibodies (Europe), B.V. an indirect wholly owned subsidiary.
Idea Family B.V.
On April 14, 2022, Idea Family B.V. was acquired. Idea Family B.V. is an indirect wholly owned subsidiary.
BioStrand B.V.
On April 14, 2022, BioStrand B.V. was acquired.
BioKey B.V.
On April 14, 2022, BioKey B.V. was acquired.
BioClue B.V.
On April 14, 2022, BioClue B.V. was acquired.
D. Property, Plants and Equipment
We do not own any real estate property. We operate from leased premises in five different locations, as detailed in the following table:
|
|
|
|
Location |
Area
(approx.) |
Premise Use |
Expiry Date |
Agoralaan Abis, 3590 Diepenbeek
Belgium |
104 sq m |
Artificial intelligence research and development, including for in silico antibody discovery and development |
Monthly |
4837 Amber Valley Parkway, Suite 11
Fargo, ND 58104, USA |
200 sq ft |
U.S. head office |
Monthly |
Pivot Park – building OP, Kloosterstraat 95349 AB Oss, The Netherlands |
1,142 sq m |
Preclinical antibody drug discovery and development lab facility |
December 31, 2028 |
Uppsalalaan 17, 10th Floor, 3584 CT Utrecht, The Netherlands |
1,164 sq m |
Production site for complex proteins and antibodies |
March 31, 2032 |
3204-4464 Markham St. Victoria, BC V8Z 7X8 Canada |
6,210 sq ft |
Global head office, preclinical antibody drug discovery and development lab facility |
December 31, 2033 |
Industrious 823 Congress Avenue, Suite 300, Austin TX 78701 USA |
200 sq ft
|
Principal Executive Office |
Monthly
|
Not applicable.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following Operating and Financial Review and Prospects section is intended to help the reader understand the factors that have affected the Company’s financial condition and results of operations for the historical period covered by the financial statements and management’s assessment of factors and trends which are anticipated to have a material effect on the Company’s financial condition and results in future periods. This section is provided as a supplement to, and should be read in conjunction with, our Consolidated Financial Statements and the other financial information contained elsewhere in this document. Our Consolidated Financial Statements have been prepared in accordance with International Reporting Standards as issued by the International Accounting Standards Board (“IFRS”). Our discussion contains forward-looking statements based on current expectations that involve risks and uncertainties, such as our plans, objectives and intentions. Our actual results may differ from those indicated in such forward-looking statements.
A. Operating Results
Overview
During Fiscal 2025, we had some significant highlights to our operating results:
•
Achieved record breaking revenue of $24.5 million in fiscal year 2025.
•
Delivered highest-ever fourth quarter revenue of $7.0 million
•
Reported record fourth quarter Adjusted EBITDA of ($0.3) million, reflecting improved operating efficiency
•
Achieved fourth quarter gross margin of 64%, representing strongest margin performance since Q3 of Fiscal Year 2021
•
BioStrand segment grew over 180% in Fiscal year 2025 and had gross margins approaching 90%
•
BioStrand currently represents over 5% of total annual revenue this year, up from less than 2% in Fiscal Year 2024
Selected Annual Information
The following selected financial data has been extracted from the audited Fiscal 2025 financial statements (expressed in Canadian Dollars).
The following is a summary of certain selected financial information of the Company for the years ended April 30, 2025, 2024, and 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands except loss per share) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Revenue |
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
Cost of sales |
|
|
(10,972 |
) |
|
|
(12,465 |
) |
|
|
(9,102 |
) |
Expenses |
|
|
(47,108 |
) |
|
|
(41,177 |
) |
|
|
39,966 |
|
Net loss |
|
|
(30,234 |
) |
|
|
(26,115 |
) |
|
|
(26,560 |
) |
Total assets |
|
|
44,441 |
|
|
|
59,988 |
|
|
|
77,813 |
|
Total liabilities |
|
|
(20,815 |
) |
|
|
(24,310 |
) |
|
|
(20,010 |
) |
Loss per share |
|
|
(0.91 |
) |
|
|
(1.02 |
) |
|
|
(1.07 |
) |
Comparison of the years ended April 30, 2025 and April 30, 2024
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
|
Change
% |
|
Project revenue |
|
|
22,175 |
|
|
|
22,235 |
|
|
|
(60 |
) |
|
|
-0.3 |
% |
Product sales revenue |
|
|
2,107 |
|
|
|
2,035 |
|
|
|
72 |
|
|
|
3.5 |
% |
Cryostorage revenue |
|
|
238 |
|
|
|
248 |
|
|
|
(10 |
) |
|
|
-4.0 |
% |
Total revenue |
|
|
24,520 |
|
|
|
24,518 |
|
|
|
2 |
|
|
|
0.0 |
% |
Revenue for the year ended April 30, 2025 was $24.5 million, compared to $24.5 million for the year ended April 30, 2024.
Gross Profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
|
Change
% |
|
Gross profit |
|
|
13,548 |
|
|
|
12,053 |
|
|
|
1,495 |
|
|
|
12.4 |
% |
Gross profit margin |
|
|
55.3 |
% |
|
|
49.2 |
% |
|
|
|
|
|
|
Gross profit totaled $13.5 million during the year ended April 30, 2025, an increase of 12.4% compared to the year ended April 30, 2024. Gross profit margin increased to 55.3% from 49.2% during the prior year driven by a higher proportion of revenue generated from the high-margin BioStrand segment and reduced salaries and lab supplies.
Research and development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
|
Change
% |
|
Research and development |
|
|
4,943 |
|
|
|
4,043 |
|
|
|
900 |
|
|
|
22.3 |
% |
During the year ended April 30, 2025, R&D expenses increased to $4.9 million from $4.0 million compared to the year ended April 30, 2024 reflecting increased investment in R&D activities within the BioStrand segment.
Sales and marketing
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
|
Change
% |
|
Sales and marketing |
|
|
4,298 |
|
|
|
3,543 |
|
|
|
755 |
|
|
|
21.3 |
% |
Sales and marketing expenses totaled $4.3 million during the year ended April 30, 2025, compared to $3.5 million during the year ended April 30, 2024 due to increases related to our marketing strategy and social media expenses.
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
|
Change
% |
|
General and administrative |
|
|
14,735 |
|
|
|
15,592 |
|
|
|
(857 |
) |
|
|
-5.5 |
% |
During the year ended April 30, 2025, general and administrative expenses totaled $14.7 million, a decrease of $0.9 million as compared to the year ended April 30, 2024, due to a reduction in compensation and consulting expenses.
Other Income/ Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
Change
$ |
|
Change
% |
|
Accretion |
|
|
(10 |
) |
|
|
(19 |
) |
|
|
9 |
|
|
(47.4 |
)% |
Grant and subsidy income |
|
|
180 |
|
|
|
331 |
|
|
|
(151 |
) |
|
(45.6 |
)% |
Interest and other (expense) income |
|
|
(283 |
) |
|
|
23 |
|
|
|
(306 |
) |
|
(1330.4 |
)% |
Unrealized foreign exchange (loss) gain |
|
|
(594 |
) |
|
|
86 |
|
|
|
(680 |
) |
|
(790.7 |
)% |
Total other (expense) income |
|
|
(707 |
) |
|
|
421 |
|
|
|
(1,128 |
) |
|
(267.9 |
)% |
The Company recorded other loss of $0.7 million during the year ended April 30, 2025, a decrease from other income of $0.4 million during the year ended April 30, 2024 due to favorable exchange rates.
Comparison of the years ended April 30, 2024 and April 30, 2023
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
|
Change
% |
|
Project revenue |
|
|
22,235 |
|
|
|
18,677 |
|
|
|
3,558 |
|
|
|
19.1 |
% |
Product sales revenue |
|
|
2,035 |
|
|
|
1,747 |
|
|
|
288 |
|
|
|
16.5 |
% |
Cryostorage revenue |
|
|
248 |
|
|
|
241 |
|
|
|
7 |
|
|
|
2.9 |
% |
Total revenue |
|
|
24,518 |
|
|
|
20,665 |
|
|
|
3,853 |
|
|
|
18.6 |
% |
Revenue for the year ended April 30, 2024 was $24.5 million, compared to $20.7 million for the year ended April 30, 2023. This increase of $3.9 million was primarily driven by project revenue growth on discovery projects as well as protein manufacturing services.
Gross Profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
|
Change
% |
|
Gross profit |
|
|
12,053 |
|
|
|
11,563 |
|
|
|
490 |
|
|
|
4.2 |
% |
Gross profit margin |
|
|
49.2 |
% |
|
|
56.0 |
% |
|
|
|
|
|
|
Gross profit totaled $12.1 million during the year ended April 30, 2024, an increase of 4.2% compared to the year ended April 30, 2023. Gross profit margin decreased to 49.2% from 56.0% during the prior year reflecting increased costs due to expansion as well as inflationary pressures.
Research and development
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
|
Change
% |
|
Research and development |
|
|
4,043 |
|
|
|
14,101 |
|
|
|
(10,058 |
) |
|
|
-71.3 |
% |
During the year ended April 30, 2024, R&D expenses decreased to $4.0 million from $14.1 million compared to the year ended April 30, 2023. Expenditures were reduced as research activities wrapped up on Talem’s PolyTope® antibody combination therapy, and primarily represent R&D efforts at our BioStrand subsidiary.
Sales and marketing
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
|
Change
% |
|
Sales and marketing |
|
|
3,543 |
|
|
|
3,608 |
|
|
|
(65 |
) |
|
|
(1.8 |
)% |
Sales and marketing expenses totaled $3.5 million during the year ended April 30, 2024, compared to $3.6 million during the year ended April 30, 2023, representing a slight decrease as the Company focused on cost reduction efforts while still supporting revenue growth.
General and administrative
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
|
Change
% |
|
General and administrative |
|
|
15,592 |
|
|
|
15,383 |
|
|
|
209 |
|
|
|
1.4 |
% |
During the year ended April 30, 2024, general and administrative expenses totaled $15.6 million, a slight increase compared to the year ended April 30, 2023. This represents the Company's focus on keeping expenses flat during continued revenue growth and conserving cash to fund R&D efforts within its BioStrand subsidiary.
Other Income/ Expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
|
|
|
|
|
(in thousands) |
|
2024
$ |
|
|
2023
$ |
|
|
Change
$ |
|
Change
% |
|
Accretion |
|
|
(19 |
) |
|
|
(30 |
) |
|
|
11 |
|
|
(36.7 |
)% |
Grant and subsidy income |
|
|
331 |
|
|
|
332 |
|
|
|
(1 |
) |
|
(0.3 |
)% |
Interest and other income |
|
|
23 |
|
|
|
122 |
|
|
|
(99 |
) |
|
(81.1 |
)% |
Unrealized foreign exchange gain |
|
|
86 |
|
|
|
227 |
|
|
|
(141 |
) |
|
(62.1 |
)% |
Total other income |
|
|
421 |
|
|
|
651 |
|
|
|
(230 |
) |
|
(35.3 |
)% |
The Company recorded other income of $0.4 million during the year ended April 30, 2024, a slight decrease from other income of $0.7 million during the year ended April 30, 2023.
Non-IFRS Measures
The following are non-IFRS measures and investors are cautioned not to place undue reliance on them and are urged to read all IFRS accounting disclosures present in the consolidated financial statements and accompanying notes for the year ended April 30, 2025.
The Company uses certain non-IFRS financial measures as supplemental indicators of its financial and operating performance. These non-IFRS financial measures are adjusted EBITDA and adjusted operating expenses. The Company believes these supplementary financial measures reflect the Company’s ongoing business in a manner that allows for meaningful period-to-period comparisons and analysis of trends in its business. These non-IFRS measures do not have any standardized meaning prescribed under IFRS and are therefore unlikely to be comparable to similar measures presented by other companies.
The Company defines adjusted EBITDA as operating earnings before interest, accretion, taxes, depreciation, amortization, share-based compensation, foreign exchange gain/loss, and asset impairment charges. Adjusted EBITDA is presented on a basis consistent with the Company’s internal management reports. The Company discloses adjusted EBITDA to capture the profitability of its business before the impact of items not considered in management’s evaluation of operating unit performance. The most directly comparable IFRS measure to adjusted EBITDA is net loss.
The Company defines adjusted operating expenses as operating expenses before taxes, interest, share-based compensation, depreciation, amortization, accretion, foreign exchange loss, and asset impairment charges. Adjusted operating expenses are presented on a basis consistent with the Company’s internal management reports. The Company discloses adjusted operating expenses to capture the true operational costs by excluding one-time charges and non-recurring expenses, thereby providing a clearer picture of the ongoing financial performance. The most directly comparable IFRS measure to adjusted operating expenses is operating expenses.
The non-IFRS measures are reconciled to reported IFRS figures in the tables below:
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
Net loss |
|
|
(30,234 |
) |
|
|
(26,115 |
) |
Income taxes |
|
|
(4,033 |
) |
|
|
(2,588 |
) |
Amortization and depreciation |
|
|
5,119 |
|
|
|
5,735 |
|
Accretion |
|
|
10 |
|
|
|
19 |
|
Asset impairment charge |
|
|
21,184 |
|
|
|
15,031 |
|
Foreign exchange realized gain (loss) |
|
|
(5 |
) |
|
|
142 |
|
Interest expense |
|
|
948 |
|
|
|
849 |
|
Interest and other income |
|
|
283 |
|
|
|
(23 |
) |
Unrealized foreign exchange loss (gain) |
|
|
594 |
|
|
|
(86 |
) |
Share-based expense |
|
|
445 |
|
|
|
1,535 |
|
Adjusted EBITDA |
|
|
(5,689 |
) |
|
|
(5,501 |
) |
|
|
|
|
|
|
|
|
|
|
|
Year Ended
April 30, |
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
Operating expenses |
|
|
(47,108 |
) |
|
|
(41,177 |
) |
Amortization and depreciation |
|
|
2,078 |
|
|
|
3,273 |
|
Asset impairment charge |
|
|
21,184 |
|
|
|
15,031 |
|
Foreign exchange gain (loss) |
|
|
(5 |
) |
|
|
142 |
|
Interest expense |
|
|
948 |
|
|
|
849 |
|
Share-based expense |
|
|
445 |
|
|
|
1,535 |
|
Adjusted Operating Expenses |
|
|
(22,458 |
) |
|
|
(20,347 |
) |
B. Liquidity and Capital Resources
The Company’s objectives when managing capital are to ensure sufficient liquidity for operations and adequate funding for growth and capital expenditures while maintaining an efficient balance between debt and equity. The capital structure of the Company consists of shareholders’ equity.
The Company adjusts its capital structure upon approval from its Board, considering economic conditions and the Company’s working capital requirements. The Company is not subject to any externally imposed capital requirements.
On July 16, 2024, YA II PN, Ltd., an investment fund managed by Yorkville, entered into a securities purchase agreement under which the Company agreed to sell and issue to Yorkville “the Convertible Debentures" in two tranches and at a purchase price of 95% of the aggregate principal amount. In connection with the offering, the Company and Yorkville entered into a customary registration rights agreement pursuant to which the Company agreed to provide certain registration rights to Yorkville under the U.S. Securities Act of 1933, as amended.
As of April 30, 2025, the Company completed the full conversion of the debenture with Yorkville.
As of April 30, 2025, the Company held cash of $10.8 million (April 30, 2024 – $3.5 million). During the year ended April 30, 2025, the cash used in operating activities was $6.4 million. As part of the investing activities, the Company made property and equipment purchases of $0.8 million. As part of the financing activities, the Company incurred lease payments of $1.6 million.
The consideration paid for the acquisition of BioStrand includes a contingent earnout payment based on the profitability of BioStrand over a 7-year period, which shall not exceed in total €12.0 million. As of April 30, 2025, no amount has been earned or paid on the Company's contingent earnout related to the BioStrand acquisition.
Although the Company is presented as a going concern, the Company does not have cash reserves to fund all its operations for one year, and strategic future growth and expansion plans. The Company has historically incurred net losses. There is no assurance that sufficient revenues will be generated in the near future. To the extent that the Company has negative operating cash flows in future periods, it may need to deploy a portion of its existing working capital to fund such negative cash flows. The Company may need to raise additional funds through issuances of Common Shares and/or through debt financing. There is no assurance that additional capital or other types of financing will be available if needed or that these financings will be on terms at least as favorable to the Company as those previously obtained, or at all. If the Company is unable to obtain additional financing from outside sources and eventually generate enough revenues, the Company may be forced to sell a portion or all of the Company's assets or curtail or discontinue the Company's operations.
On December 8, 2023, the Company closed an underwritten public offering of 1,265,000 Common Shares, including 165,000 Common Shares issued pursuant to the full exercise by the underwriter of its over-allotment option. The public offering price for each Common Share, before the underwriter’s discount and commissions, was U.S.$1.00. The Company intends to use the estimated net proceeds of approximately $1.1 million from the offering for R&D; capital expenditures, including expansion of existing laboratory facilities; and working capital and general corporate purposes. The Common Shares were offered and sold pursuant to its Registration Statement.
On February 23, 2024, the Company entered into the Clear Street ATM Agreement. Under the terms of the Clear Street ATM Agreement, the Company was entitled, at its discretion and from time-to-time during the term of the ATM Agreement, to sell, through Clear Street LLC, acting as sole sales agent, Common Shares of the Company having an aggregate gross sales price of up to U.S. $60 million.
Financing Activities
Fiscal Year Ended 2023 Transactions
During the year ended April 30, 2023, the Company issued 263,537 Common Shares pursuant to the exercise of stock options for total gross proceeds of $0.7 million. A value of $0.8 million was transferred from contributed surplus to share capital as a result.
During the year ended April 30, 2023, the Company issued 309,877 Common Shares with a value of $1.3 million pursuant to the conversion of $1.4 million principal balance of convertible debenture.
Fiscal Year Ended 2024 Transactions
During the year ended April 30, 2024, the Company issued 1,265,000 Common Shares, including 165,000 Common Shares issued pursuant to the full exercise by the underwriter of its over-allotment option. The public offering price for each Common Share, before the underwriter’s discount and commissions, was U.S.$1.00, raising U.S.$1,265,000.
During the year ended April 30, 2024, the Company established an at-the-market equity offering facility ("ATM Facility") and entered into the Clear Street ATM Agreement. The Company is entitled, at its discretion and from time-to-time during the term of the Clear Street ATM Agreement, to sell Common Shares through Clear Street. On February 23, 2024, in connection with the ATM Facility, the Company filed a prospectus supplement permitting the sales of Common Shares having an aggregate gross sales price of up to U.S. $60.0 million. In fiscal 2024, 629,240 common shares were sold under the ATM with proceeds net of commissions of $1.8 million.
Fiscal Year Ended 2025 Transactions
During the year ended April 30, 2025, the Company issued 13,315,850 Common Shares under the ATM Facility with proceeds net of commissions of $12.2 million.
On July 16, 2024, the Company entered into a securities purchase agreement (the "Securities Purchase Agreement") with YA II PN, Ltd., an investment fund managed by Yorkville Advisors Global, LP ("Yorkville"), under which the Company agreed to sell and issue to Yorkville U.S,$3.0 million aggregate principal amount of convertible debentures (the "Convertible Debentures") in two tranches and at a purchase price of 95% of the aggregate principal amount (the “July 2024 Offering”).
The Convertible Debentures are convertible into Common Shares. The sale and issue of the first tranche consists of U.S.$2.0 million principal amount of Convertible Debentures and was completed on July 16, 2024 (the "First Closing"). The sale and issue of the second tranche consists of U.S.$1.0 million principal amount of Convertible Debentures and is expected to close on or about the date the initial registration statement of the Company filed pursuant to the Registration Rights Agreement (as defined below) has first been declared effective by the SEC.
Each Convertible Debenture will be an unsecured obligation of the Company and will be wholly and unconditionally guaranteed by certain of the Company's subsidiaries. The Convertible Debentures will incur interest at a rate of 8.0% per annum. The outstanding principal amount of and accrued and unpaid interest, if any, on, the Convertible Debentures must be paid by the Company in cash when the same becomes due and payable under the terms of the Convertible Debentures at their stated maturity, upon their redemption or otherwise. The Convertible Debentures are redeemable at any time provided that the volume-weighted average price ("VWAP") for the Common Shares is less than U.S.$1.16, at a redemption price equal to the principal amount, plus accrued and unpaid interest on the principal amount to be redeemed, plus a 10% premium. If at any time on and after November 1, 2024, the daily VWAP for the Common Shares is less than U.S.$0.20 for five trading days during a period of seven consecutive trading days or a default with respect to the registration statement has occurred, the Company shall be required to make monthly installments payments on the Convertible Debentures in an amount equal to U.S.$300,000 principal amount, plus accrued and unpaid interest on the outstanding principal amount, plus a 10% premium.
Subject to certain limitations contained within the Securities Purchase Agreement and the Convertible Debentures, holders of the Convertible Debentures will be entitled to convert the principal amount of, and accrued and unpaid interest, if any, on each Convertible Debenture, in whole or in part, from time to time, into a number of Common Shares at a conversion price equal to the lower of (i) U.S.$1.16 per Common Share, or (ii) 95% of the lowest daily VWAP for the Common Shares during the 10 consecutive trading days immediately preceding the conversion date or other date of determination (the "Market Price"), but which Market Price shall not be lower than U.S.$0.20. The conversion price is subject to anti-dilution adjustments pursuant to the terms and conditions of the Securities Purchase Agreement and the Convertible Debentures. During any consecutive 30-day period, the holders of the Convertible Debentures may not, without the prior written consent of the Company, convert more than U.S.$300,000 in principal amount of Convertible Notes during any 30-day period if the conversion price is less than U.S.$1.16, provided, however, that the foregoing limitation shall not apply during the occurrence and during the continuance of an event of default under the Convertible Debentures.
The Company and Yorkville entered into a customary Registration Rights Agreement on July 16, 2024 (the "Registration Rights Agreement"), pursuant to which the Company has agreed to provide certain registration rights to Yorkville under the Securities Act.
Furthermore, in connection with the July 2024 Offering and pursuant to the Securities Purchase Agreement, ImmunoPrecise Antibodies (Canada), Ltd., ImmunoPrecise Antibodies (Europe) BV and BioStrand B.V. (together, the “Guarantors”), entered into a global guaranty agreement on July 16, 2024 (the “Global Guaranty Agreement”) in favor of Yorkville, under which the Guarantors guaranteed to Yorkville the payment when due and the performance, of all liabilities, agreements and other obligations of the Company to Yorkville contained in the Securities Purchase Agreement and Convertible Debentures.
As of April 30, 2025, the Company completed the full conversion of the debenture with Yorkville
Capital Expenditures
The Company made property and equipment purchases of $0.8 million during the year ended April, 2025 (2024 - $1.4 million).
Contractual Obligations and Commitments
The consideration paid for the acquisition of BioStrand includes a contingent earnout payment based on the profitability of BioStrand over a 7-year period, which shall not exceed in total €12.0 million. As of April 30, 2025, no amount has been earned or paid on the Company's contingent earnout related to the BioStrand acquisition.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements that have or are reasonably likely to have, a current or future effect on our results of operations, financial condition, revenues or expenses, liquidity, capital expenditures or capital resources.
C. Research and Development, Patents and Licenses, etc.
See Item 5.A. – Operating Results – Selected Annual Information for a description of our R&D activities during the last three fiscal years.
See Item 4.B. – Business Overview – Proprietary Protection for a listing of patents and product development in progress.
D. Trend Information
See Item 5.A. – Operating Results – Seasonality for trend information.
E. Critical Accounting Estimates.
Not Applicable.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth the name of each of our directors and executive officers, as well as such individual’s place of residence, position with us, principal business activities performed outside those with us and period of service as a director (if applicable).
Directors and Executive Officers
|
|
|
|
Name |
Position With ImmunoPrecise Antibodies Ltd. |
Principal occupation, business, or employment and, if not a previously elected Director, occupation, business, or employment during the past 5 years |
Director/Officer Since |
|
Dr. Jennifer L. Bath
North Dakota, United States
|
CEO, President and Director |
CEO, President of the Company; Global Director of Aldevron, LLC from July 2015 to February 2018, a company that provides plasmid deoxyribonucleic acid (DNA), messenger ribonucleic acid (mRNA), and recombinant proteins for biopharma clients. |
February 2018(1) |
|
Kamil Isaev (2) (5)
Oregon, United States
|
Director |
30 years of expertise in AI, semiconductor technologies, and global R&D operations venture
Leadership roles at Intel, Dell EMC, Align Technology
Partner at ABRT VC; providing investors with data-driven insights to identify high-potential opportunities
|
February 2025 |
|
Dirk Witters (3) (4)
Beveren, Belgium
|
Chair of the Board and Director |
Founder of Conanti Consult BV, an advisory boutique since 2019.
Advisor to the founder of New Rhein Healthcare Investors, a private equity investment firm, from July 2019 to March 2020.
Director Program Management Office, Sustainable Finance for KBC Group, from December 2018 to June 2019.
President of the board of BioStrand BV from June 2020 to April 2022.
|
September 2023 |
|
Joseph Scheffler
Florida, United States
|
Interim CFO |
N/A |
February 2025 |
|
Dr. Ilse Roodink
Loenen, Netherlands
|
CSO |
N/A |
July 2021 |
|
Kari Graber
North Dakota, United States
|
Vice President of Commercial Services |
N/A |
November 2021(6) |
Notes:
(1)
Dr. Bath, who has been CEO and President since February 2018 and was appointed Director in May 2018.
(2)
Member of the Audit and Risk Committee.
(3)
Member and Chair of the Audit and Risk Committee
(4)
Member of the Compensation, Nomination and Governance Committee.
(5)
Member and Chair of the Compensation, Nomination and Governance Committee
(6)
Ms. Graber has been working for the Company since May 2018.
The following are brief biographies of our directors and executive officers.
Dr. Jennifer Bath
Dr. Bath is the CEO and President of IPA. She obtained a Ph.D. in Cellular and Molecular Biology, specializing in immunology and biochemistry with a focus on discovering and validating biologics for the prevention and treatment of neglected tropical diseases. Dr. Bath held a tenured position as an Associate Professor of Cellular and Molecular Biology, while concurrently serving as the Founder and Executive Director of the Concordia Global Vaccine Institute. She also served many years as a strategic growth and business operations advisor for global pharma, biotech, and government.
Dr. Bath has held executive roles in both biotechnology and contract research organizations, with her most recent post on the executive team at Aldevron, LLC. There, she headed the global sales and client relations teams, and defined business strategies by applying knowledge based on the science, technology, and market. In addition, she served as a key technical specialist, converting challenges for pharmaceutical and biotechnology clients into operational initiatives.
Dirk Witters
Mr. Witters founded Conanti Consult BV in 2019, an advisory boutique, where he, among other things, advises on the execution of acquisitions and capital raising assignments. Prior to that, Mr. Witters also served as an advisor to the founder of New Rhein Healthcare Investors, a private equity investment firm from July 2019 to March 2020. In addition, Mr. Witters was the Director Program Management Office, Sustainable Finance for KBC Group from December 2018 to June 2019; and the president of the Board of BioStrand BV from June 2020 to April 2022.
Kamil Isaev
Mr. Isaev has over 30 years of expertise in AI, semiconductor technologies, and global R&D operations, and has held leadership roles at Intel, Dell EMC, Align Technology, and ABRT VC. Mr. Isaev currently serves as a Venture Partner at ABRT VC, where he leads the ABRT AI Lab and the VC Score project, developing AI-powered evaluation models to assess and rank AI startups, providing investors with data-driven insights to identify high-potential opportunities. His role at ABRT VC is focused on bridging cutting-edge AI research with commercialization strategies, helping AI-driven companies refine their go-to-market approach and maximize scalability. .Mr. Isaev is also a member of IEEE, a guest lecturer at leading universities, and a frequent speaker at AI and semiconductor industry conferences. He holds an MSc and PhD in Physics (Plasma Physics and Plasma Chemistry) from Moscow State University and has authored over 30 scientific publications in plasma physics and semiconductor technology.
Joseph Scheffler
Mr. Scheffler serves as the interim CFO for IPA and is responsible for overseeing the financial strategy and reporting to ensure compliance with regulations, optimizing capital allocation to support growth, and providing financial leadership. He previously worked for Ernst & Young and has held executive positions of CFO, Vice President of Finance and Corporate Treasurer. His background includes 30 years of experience and blends publicly traded company operations with successful startup ventures. He holds a BS degree in Accounting and an MBA, with concentrations in Finance and Strategic Management, from the Loyola University of Chicago.
Dr. Ilse Roodink
Dr. Roodink serves as CSO of IPA, supporting the company’s global research and development teams. Prior to her appointment as CSO, she held different scientific positions at the Company’s Dutch facility in Oss from 2013 until 2021. In her last role as Scientific Director of IPA Europe, Dr. Roodink was overseeing contract research project execution and management and actively involved in the integration of innovative technologies supporting antibody characterization and engineering. Following its establishment in 2019, Dr. Roodink has served as Chairwoman of Talem Therapeutics’ Scientific Advisory Committee, leading the development of Talem’s pipeline assets. Dr. Roodink graduated from Radboud University of Nijmegen, the Netherlands with a Master’s degree in Biomedical Health Sciences and a Ph.D. in Medical Sciences. Her work, resulting in several peer-reviewed publications, focused on platform development to facilitate the discovery of antibodies specifically recognizing native tumor targets.
Kari Graber
Ms. Graber serves as the Vice President of Commercial Services for IPA and is responsible for the overall leadership and implementation of the Project Management program throughout IPA’s global family of companies. She has over 20 years of experience in developing, implementing and directing laboratory operations, quality assurance, regulatory compliance, and supply chain management programs for various food manufacturers, and spent five years as Sales and Technical Director for a pasteurization/sterilization technology and equipment supplier. Prior to joining IPA, Ms. Graber served at Aldevron LLC, where she held a client relations management role for their antibody services platform. She holds a Bachelor of Science in Food Science & Technology and a Minor in Microbiology.
B. Compensation
Compensation for Fiscal 2025
The aggregate amount of compensation paid during the year ended April 30, 2025, directly and indirectly, including directors’ fees, to our named executive officers and directors in their capacity as such, was $2.9 million (Fiscal 2024: $3.2 million).
This discussion describes our compensation program for each person who acted as President and CEO, ICFO and the three most highly-compensated executive officers (or three most highly-compensated individuals acting in a similar capacity), other than the CEO and the ICFO, whose total compensation was more than CAD $150,000 in our last fiscal year and who was performing a policy-making function in respect of the Company (each a “NEO” and collectively the “NEOs”). This section addresses our philosophy and objectives and provides a review of the process that the Board follows in deciding how to compensate the NEOs. This section also provides discussion and analysis of the Board’s specific decisions about the compensation of the NEOs for the fiscal year ended April 30, 2025. We had seven (7) NEOs during the fiscal year ended April 30, 2025, namely: Dr. Jennifer Bath, CEO; Joseph Scheffler, ICFO; Kristin Taylor, former CFO; Brad McConn, former CFO; Dr. Ilse Roodink, CSO; Dr. Barry Duplantis, former Vice President of Client Relations; and Kari Graber, Vice President of Commercial Services.
Summary Compensation Table
The following table provides a summary of the compensation paid by the Company to each NEO of the Company for the financial years ended April 30, 2025, 2024, and 2023. All cash payments in the table below are made in U.S. dollars, except for Dr. Roodink’s and Dr. Duplantis’, which are made in Euros and Canadian dollars, respectively. All amounts listed are in Canadian dollars, translated using the average daily exchange rate on the last day of the period provided by the Bank of Canada. The average daily exchange rates on the relevant date as reported by the Bank of Canada are:
|
|
Bank of Canada USD/CAD Average Daily Exchange Rate |
April 30, 2025 |
1.3812 |
April 30, 2024 |
1.3746 |
April 30, 2023 |
1.3578 |
Bank of Canada EUR/CAD Average Daily Exchange Rate |
April 30, 2025 |
1.5687 |
April 30, 2024 |
1.4695 |
April 30, 2023 |
1.3578 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-equity incentive plan compensation(1)
($) |
|
|
|
|
|
|
|
Name and principal position |
Year |
Salary
($) |
|
Share-based
awards
($) |
|
Option-based
awards
($) |
|
Annual
incentive
plans |
|
Long-term
incentive
plans |
|
Pension
value
($) |
|
All other
compensation
($) |
|
Total
compensation
($) |
|
Dr. Jennifer L. Bath(2) |
2025 |
|
877,062 |
|
|
— |
|
|
280,800 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
1,157,862 |
|
CEO, |
2024 |
|
731,989 |
|
|
— |
|
|
— |
|
|
273,353 |
|
|
— |
|
|
— |
|
|
— |
|
|
1,005,342 |
|
President, and
Director |
2023 |
|
713,558 |
|
|
— |
|
|
1,069,310 |
|
|
678,031 |
|
|
— |
|
|
— |
|
|
— |
|
|
2,460,899 |
|
Joseph Scheffler(3)
Interim CFO |
2025 |
|
146,929 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
146,929 |
|
Brad
McConn(4) |
2025 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Former CFO |
2024 |
|
188,613 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
188,613 |
|
|
2023 |
|
400,462 |
|
|
— |
|
|
213,540 |
|
|
167,274 |
|
|
— |
|
|
— |
|
|
— |
|
|
781,276 |
|
Dr. Ilse
Roodink |
2025 |
|
372,924 |
|
|
— |
|
|
109,200 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
482,124 |
|
CSO |
2024 |
|
276,930 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
276,930 |
|
|
2023 |
|
298,419 |
|
|
— |
|
|
142,360 |
|
|
78,297 |
|
|
— |
|
|
— |
|
|
— |
|
|
519,076 |
|
Dr. Barry
Duplantis |
2025 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
VP of Client
Relations |
2024 |
|
176,392 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
176,392 |
|
|
2023 |
|
287,879 |
|
|
— |
|
|
177,950 |
|
|
155,106 |
|
|
— |
|
|
— |
|
|
— |
|
|
620,935 |
|
Kari Graber |
2025 |
|
331,488 |
|
|
— |
|
|
62,400 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
393,888 |
|
VP of Commercial |
2024 |
|
273,599 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
273,599 |
|
Services |
2023 |
|
262,395 |
|
|
— |
|
|
177,950 |
|
|
76,312 |
|
|
— |
|
|
— |
|
|
— |
|
|
516,657 |
|
Kristin Taylor(3) |
2025 |
|
259,103 |
|
|
— |
|
|
212,958 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
472,061 |
|
Former CFO |
2024 |
|
533,477 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
533,477 |
|
Notes:
(1)
Non-equity incentive plan compensation includes bonuses earned during the financial year and payable as of the year-end date. Cash payments are made upon approval by the Board in the fiscal quarter following year-end.
(2)
Dr. Bath received no compensation in her capacity as director of the Company.
(3)
Ms. Taylor was acting as CFO until January 16, 2025. Mr. Scheffler was appointed as interim CFO on February 1, 2025, and was employed through a consulting firm. His salary represents compensation paid to the consulting firm.
(4)
Mr. McConn was acting as CFO of the Company until September 29, 2023.
The Company uses the Black-Scholes option pricing model to calculate the fair value of stock options on their grant date. The Company applies this methodology to value the stock options as accurately as possible using observable market inputs. The assumptions used in the model and the resulting fair value for each issuance is shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Black-Scholes model inputs |
|
Optionee |
Year |
Fair value of
option
($) |
|
Number of
options
awarded |
|
Fair value of
award
($) |
|
Common
share price
on grant date
($) |
|
|
Exercise price
($) |
|
|
Expected life
(years) |
|
Risk-free
rate |
|
Dr. Jennifer L. Bath |
2025 |
|
1.040 |
|
|
270,000 |
|
|
280,800 |
|
|
0.860 |
|
|
|
0.860 |
|
|
|
10.00 |
|
|
2.88 |
% |
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2023 |
|
3.559 |
|
|
300,452 |
|
|
1,069,310 |
|
|
4.100 |
|
(1) |
|
4.100 |
|
(1) |
|
5.00 |
|
|
3.57 |
% |
Kristin Taylor(2) |
2025 |
|
1.040 |
|
|
204,767 |
|
|
212,958 |
|
|
0.860 |
|
|
|
0.860 |
|
|
|
— |
|
|
— |
|
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
Brad McConn(3) |
2025 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2023 |
|
3.559 |
|
|
60,000 |
|
|
213,540 |
|
|
4.100 |
|
(1) |
|
4.100 |
|
(1) |
|
5.00 |
|
|
3.57 |
% |
Dr. Barry Duplantis |
2025 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2023 |
|
3.559 |
|
|
50,000 |
|
|
177,950 |
|
|
4.100 |
|
(1) |
|
4.100 |
|
(1) |
|
5.00 |
|
|
3.57 |
% |
Dr. Ilse Roodink |
2025 |
|
1.040 |
|
|
105,000 |
|
|
109,200 |
|
|
0.860 |
|
|
|
0.860 |
|
|
|
10.00 |
|
|
2.88 |
% |
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2023 |
|
3.559 |
|
|
40,000 |
|
|
142,360 |
|
|
4.100 |
|
(1) |
|
4.100 |
|
(1) |
|
5.00 |
|
|
3.57 |
% |
Kari Graber |
2025 |
|
1.040 |
|
|
60,000 |
|
|
62,400 |
|
|
0.860 |
|
|
|
0.860 |
|
|
|
10.00 |
|
|
2.88 |
% |
|
2024 |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
— |
|
|
2023 |
|
3.559 |
|
|
50,000 |
|
|
177,950 |
|
|
4.100 |
|
(1) |
|
4.100 |
|
(1) |
|
5.00 |
|
|
3.57 |
% |
Notes:
(2)
Ms. Taylor resigned effective January 16, 2025.
(3)
Mr. McConn resigned effective September 29, 2023.
Outstanding Equity Awards at April 30, 2024
The following table sets forth information concerning all the outstanding equity awards held by each NEO as at April 30, 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option-based awards |
|
|
Share-based awards |
|
Name |
Issuance
date |
|
Number of
securities
underlying
unexercised
options
(#) |
|
Option
exercise
price
($) |
|
|
Option
expiration
date |
|
Value of
unexercised
in-the-money
options
($) |
|
Number of
shares or
units of
shares that
have not
vested
(#) |
|
Market or
payout
value of
share-based
awards that
have not
vested
($) |
|
Market or
payout
value of
vested share-
based
awards not
paid out or
distributed
($) |
|
Dr. Jennifer L. |
09/01/2020 |
|
|
210,000 |
|
|
8.500 |
|
|
09/01/2025 |
|
|
1,254,750 |
|
|
— |
|
|
— |
|
|
1,254,750 |
|
Bath |
01/07/2022 |
|
|
120,000 |
|
|
7.940 |
|
|
01/07/2027 |
|
|
594,720 |
|
|
— |
|
|
— |
|
|
594,720 |
|
|
02/19/2023 |
|
|
300,452 |
|
|
4.100 |
|
(1) |
02/19/2028 |
|
|
1,069,310 |
|
|
— |
|
|
— |
|
|
1,069,309 |
|
|
8/3/2025 |
|
|
270,000 |
|
|
0.860 |
|
(1) |
8/3/2034 |
|
|
280,800 |
|
|
270,000 |
|
|
280,800 |
|
|
— |
|
Kristin Taylor |
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Joseph Scheffler |
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Dr. Ilse |
01/06/2021 |
|
|
15,000 |
|
|
20.300 |
|
|
01/06/2026 |
|
|
175,545 |
|
|
— |
|
|
— |
|
|
175,545 |
|
Roodink |
01/07/2022 |
|
|
50,000 |
|
|
7.940 |
|
|
01/07/2027 |
|
|
247,800 |
|
|
— |
|
|
— |
|
|
247,800 |
|
|
02/19/2023 |
|
|
40,000 |
|
|
4.100 |
|
(1) |
02/19/2028 |
|
|
142,360 |
|
|
— |
|
|
— |
|
|
142,360 |
|
|
8/3/2025 |
|
|
105,000 |
|
|
0.860 |
|
(1) |
8/3/2034 |
|
|
109,200 |
|
|
105,000 |
|
|
109,200 |
|
|
— |
|
Dr. Barry Duplantis |
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Kari Graber |
09/01/2020 |
|
|
10,000 |
|
|
8.500 |
|
|
09/01/2025 |
|
|
59,750 |
|
|
— |
|
|
— |
|
|
59,750 |
|
|
02/19/2023 |
|
|
50,000 |
|
|
4.100 |
|
(1) |
02/19/2028 |
|
|
177,950 |
|
|
— |
|
|
— |
|
|
177,950 |
|
|
8/3/2025 |
|
|
60,000 |
|
|
0.860 |
|
(1) |
8/3/2034 |
|
|
62,400 |
|
|
60,000 |
|
|
62,400 |
|
|
— |
|
Note:
Value Vested or Earned During the Year
The following table shows the incentive plan awards value vested or earned for each NEO for the fiscal year ended April 30, 2025:
|
|
|
|
|
|
|
|
|
|
Name |
Option-based awards –
Value vested during the year
($) |
|
Share-based awards –
Value vested during the year
($) |
|
Non-equity incentive plan
compensation – Value
earned during the year
($) |
|
Dr. Jennifer L. Bath |
|
237,623 |
|
|
— |
|
|
— |
|
Kristin Taylor |
|
18,344 |
|
|
— |
|
|
— |
|
Joseph Scheffler |
|
— |
|
|
— |
|
|
— |
|
Dr. Ilse Roodink |
|
31,635 |
|
|
— |
|
|
— |
|
Dr. Barry Duplantis |
|
— |
|
|
— |
|
|
— |
|
Kari Graber |
|
39,543 |
|
|
— |
|
|
— |
|
Director Compensation Table
The following table provides a summary of compensation paid by the Company to each director of the Company for the financial year ended April 30, 2025. Cash payments are made in U.S. dollars, translated using the USD/CAD average daily exchange rate on April 30, 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name(1) |
Fees earned
($) |
|
Share-
based awards
($) |
|
Option-
based awards
($) |
|
Non-equity
incentive plan
compensation
($) |
|
Pension
value
($) |
|
All other
compensation
($) |
|
Total
($) |
|
Mitch Levine(2) |
|
77,798 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
77,798 |
|
Kamil Isaev |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Chris Buyse(4) |
|
83,126 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
83,126 |
|
Dirk Witters |
|
94,619 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
94,619 |
|
Barry Springer(3) |
|
62,239 |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
62,239 |
|
Notes:
(1)
The compensation of Dr. Jennifer L. Bath, a director and the CEO and President of the Company, is set out in the summary compensation table above. Dr. Bath did not receive any compensation for her role as a director of the Company.
(2)
Ceased to be a board member November 14, 2024 after not standing for reelection.
(3)
Ceased to be board member January 22, 2025.
(4)
Ceased to be a board member February 16, 2025.
Directors of the Company are paid a base annual retainer for various positions, detailed below:
|
|
|
|
|
Position |
|
Additional Annual Compensation
(U.S. $) |
|
Chair/Lead Independent Director |
|
|
65,000 |
|
Independent Director, on at least one Committee |
|
|
45,000 |
|
Independent Director, if not on at least one Committee |
|
|
40,000 |
|
Annual compensation is provided for the year beginning at the Annual General Meeting of Shareholders, and payments are made quarterly in arrears. Fees earned in the Director Compensation Table reflect cash compensation during the fiscal year ended April 30, 2025.
Director Outstanding Share-based Awards and Option-based Awards
The following table of compensation securities provides a summary of all compensation securities outstanding to each director as of April 30, 2025.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Option-based awards |
|
|
Share-based awards |
|
Name |
Issuance
date |
|
Number of
securities
underlying
unexercised
options
(#) |
|
Option
exercise
price
($) |
|
|
Option
expiration
date |
|
Value of
unexercised
in-the-
money
options
($) |
|
|
Number of
shares or
units of
shares that
have not
vested
(#) |
|
Market or
payout value
of share-
based
awards
that have
not vested
($) |
|
Market or
payout value
of vested
share-based
awards not
paid out or
distributed
($) |
|
Dirk Witters |
1/19/2024 |
|
|
60,000 |
|
|
1.480 |
|
(1) |
1/19/2029 |
|
|
86,175 |
|
|
|
21,111 |
|
|
31,244 |
|
|
54,931 |
|
Dr. Jennifer L. Bath |
09/01/2020 |
|
|
210,000 |
|
|
8.500 |
|
|
09/01/2025 |
|
|
1,254,750 |
|
|
|
— |
|
|
— |
|
|
1,254,750 |
|
|
01/07/2022 |
|
|
120,000 |
|
|
7.940 |
|
|
01/07/2027 |
|
|
594,720 |
|
|
|
— |
|
|
— |
|
|
594,720 |
|
|
02/19/2023 |
|
|
300,452 |
|
|
4.100 |
|
(1) |
02/19/2028 |
|
|
1,069,309 |
|
|
|
— |
|
|
— |
|
|
1,069,309 |
|
|
8/3/2025 |
|
|
270,000 |
|
|
0.860 |
|
(1) |
8/3/2034 |
|
|
232,200 |
|
|
|
270,000 |
|
|
232,200 |
|
|
|
Kamil Isaev |
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
— |
|
|
|
— |
|
|
— |
|
|
— |
|
Note:
Employment, Consulting and Management Agreements
Dr. Jennifer L. Bath
Dr. Jennifer L. Bath entered into an executive employment agreement with the Company on February 7, 2018, pursuant to which Dr. Bath is paid U.S.$350,000 per annum for providing services as CEO of the Company. The Company will pay Dr. Bath a guaranteed annual bonus of U.S.$150,000 and a U.S.$200,000 annual bonus payable upon achievement of performance targets mutually agreed to with the Board. In the event of termination without cause, Dr. Bath will be entitled to the equivalent of 12 months’ salary. During 2022, the Board approved an adjustment to Dr. Bath’s base salary to U.S.$535,080 per annum and annual bonus of 100% of base salary with U.S.$200,000 guaranteed. During fiscal year 2025, the Board approved an adjustment to Dr. Bath’s base salary to U.S.$635,000 per annum and annual bonus of 70% of base salary.
Kari Graber
Ms. Graber entered into an executive employment agreement with the Company on July 1, 2019 (the “Kari Graber Employment Agreement”) pursuant to which Ms. Graber is paid U.S.$135,200 per annum for providing services as VP of Clients Relations and Project Management of the Company. The Company will pay Ms. Graber an annual bonus payable upon achievement of targets mutually agreed to with the Chief Business Officer. During 2022, the Board approved an adjustment to Ms. Graber’s base salary to U.S.$200,000 per annum and an annual bonus of 30% of base salary. During fiscal year 2025, the Board approved an adjustment to Ms. Graber’s base salary to U.S.$240,000 per annum and an annual bonus of 30% of base salary.
Dr. Ilse Roodink
Dr. Ilse Roodink entered into an executive employment agreement with the Company on July 1, 2021, which became effective on July 1, 2021, pursuant to which Dr. Roodink is paid €143,400 per annum for providing services as CSO of the Company. The Company will pay Dr. Roodink an annual bonus payable upon achievement of targets mutually agreed to with the CEO. During 2022, the Board approved an adjustment to Dr. Roodink’s base salary to U.S.$240,115 and an annual bonus of 40% of base salary. During fiscal year 2025, the Board approved an adjustment to Dr. Roodink’s base salary to U.S.$270,000 per annum and an annual bonus of 40% of base salary.
Joseph Scheffler
Mr. Scheffler was appointed Interim CFO on February 1, 2025, as the Company engaged a professional services consulting firm. Under the terms of the engagement, the consulting firm supplied an experienced finance executive who fulfilled the responsibilities of the CFO on an interim basis. As a result of this arrangement, the Company incurred consulting expenses of approximately U.S.$108,738 in fiscal year 2025, which are included in general and administrative expenses.
Termination and Change of Control Benefits
Dr. Jennifer L. Bath
The Company has entered into a change of control agreement (the “Change of Control Agreement”) with Dr. Jennifer L. Bath, which provides for payments in the event of a change of control of the Company. The term “Change of Control” is defined as meaning that a person or group of persons acting jointly or in concert acquires, beneficially or otherwise (whether by purchase, exchange, amalgamation, merger, consolidation, or otherwise), directly or indirectly, in one transaction or in a series of related transactions, (a) Control of the Company (as defined below), or (b) all or substantially all of the assets of the Company. The term “Control” is defined as meaning the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of the Company through the ownership of more than 50% of the voting securities.
If certain circumstances occur within 18 months following a change of control, the Change of Control Agreement provides for payments to be made to Dr. Bath. These circumstances include: (a) the assignment to Dr. Bath of any duties which are materially inconsistent, in an adverse respect, with her position, authority, status, duties, or responsibilities prior to the Change of Control, other than the assignment of duties related to the transition to a person who gains control of the Company or who acquires all or substantially all of the assets of the Company pursuant to the Change of Control (a “Successor”) that are reasonably commensurate with Dr. Bath’s position; (b) the removal or elimination of one or more of Dr. Bath’s duties, responsibilities, or functions that were material to her position, authority, status, duties or responsibilities prior to the Change of Control; (c) a reduction in Dr. Bath’s base salary or annual bonus compensation opportunity; (d) a requirement that Dr. Bath relocate to or be based at a location which is 50 kilometres or more from the location where she was based immediately prior to the Change of Control; (e) the failure to continue Dr. Bath’s participation in substantially all of the insured group benefit plans (or substantially equivalent successor plans, programs, or policies) as were in effect for Dr. Bath immediately prior to the Change of Control, including medical, dental, life, and other benefits plans, but excluding short and long term disability coverage and out of country medical coverage (“Benefit Plans”); and (f) any other change in the terms and conditions of Dr. Bath’s employment that would constitute a constructive dismissal at common law (each such circumstance, a “Triggering Event”).
In the event that Dr. Bath’s employment with the Company is terminated: (a) by Dr. Bath within three months after a Triggering Event where just cause for the Company to terminate Dr. Bath’s employment does not exist; or (b) by the Company within 12 months of a Change of Control where just cause does not exist and other than (i) in response to a resignation by Dr. Bath that is not a resignation set out in (a) above; and (ii) where a Successor offers to employ or engage Dr. Bath immediately following a Change of Control on terms and conditions that, on the whole, are at least as favourable to Dr. Bath as she enjoyed immediately prior to the Change of Control, excluding the terms of the Change of Control Agreement (either such termination, an “Involuntary Termination”), then the Change of Control Agreement entitles Dr. Bath to receive: (v) an amount equal to 24 months of her salary; (w) an amount equal to twice the amount of her guaranteed bonus; (x) an amount equal to twice the amount of the discretionary bonus paid to her for the last full bonus year; (y) her guaranteed bonus for the year in which the Involuntary Termination occurred, prorated based on the number of days worked in the year; and (z) a discretionary bonus for the year in which the Involuntary Termination occurred, calculated based on the discretionary bonus paid to her for the last full bonus year, pro-rated based on the number of days worked in the year.
In addition, if an Involuntary Termination occurs, Dr. Bath’s rights and entitlements upon termination under any incentive plans will be determined by the terms and conditions of such incentive plans, and the Company will continue to provide Dr. Bath with coverage under Benefit Plans for a period of 24 months following such Involuntary Termination, subject to the terms of such Benefit Plans and the consent of the applicable carrier. For any portion of such 24 month period during which the Company is unable to continue to provide coverage under a Benefit Plan due to the applicable carrier’s refusal or the terms of such Benefit Plan, the Company will pay to Dr. Bath compensation equal to the cost to the Company of the Benefit Plan coverage that is not maintained, provided that Dr. Bath does not become entitled to participate in substantially similar benefits through another benefits provider.
The following table sets forth an estimated aggregate amount that Dr. Bath would have been entitled to receive pursuant to the Change of Control Agreement (assuming the continuation of coverage under all applicable Benefits Plans) if an Involuntary Termination had occurred on April 30, 2025:
|
|
|
|
|
|
|
|
|
Change of control compensation based on
salary, guaranteed bonus, and
discretionary bonus
($) |
|
Entitlements under
incentive plans |
|
Total
($) |
|
|
706,064 |
|
|
— |
|
|
706,064 |
|
Kari Graber
The Board has approved an amendment to the employment agreement with Ms. Graber, whereby if Ms. Graber’s employment with the Company is terminated without cause or she resigns for good reason, and such termination or resignation is not in connection with a Change in Control, she will receive severance pay equivalent to six (6) months of her base salary and continuation of health insurance coverage (COBRA) for six (6) months.
Additionally, if Ms. Graber’s employment with the Company is terminated without cause or she resigns for good reason within twelve (12) months following a Change in Control (double trigger), she will receive severance pay equivalent to six (6) months of her base salary, payment of her target bonus for the year of termination, equivalent to one half (0.5) times the target bonus, continuation of health insurance coverage (COBRA) for six (6) months, and full acceleration of all outstanding equity awards.
Except as disclosed in this Annual Report, no other NEO is entitled to any other benefits upon termination of their employment or a change of control of the Company.
Pension
The Company does not provide any pension benefits for directors or executive officers.
C. Board Practices
Each of our directors will hold office until the next annual general meeting of our shareholders or until his or her office is earlier vacated, in accordance with our Articles of Incorporation (the “Articles”) and the BCBCA. Each of our officers serves at the pleasure of our Board. Please also refer to Directors and Senior Management above for further details regarding the periods of service of each of our current directors and officers.
As of April 30, 2025, we did not have any service contracts with any of our independent directors.
Board Nomination
The identification of potential candidates for nomination as our directors is carried out by all directors, who are encouraged to participate in the identification and recruitment of new directors. Potential candidates are primarily identified through referrals and business contacts.
Audit Committee Disclosure
The Audit Committee’s Charter
Our directors have adopted a Charter for the Audit Committee, which sets out the Audit Committee’s mandate, organization, powers and responsibilities. The full text of our Audit Committee Charter is available on request from us.
Composition of the Audit Committee
The members of the Audit Committee are Dirk Witters (Chair) and Kamil Isaev. All members are independent (as determined under Exchange Act Rule 10A-3 and Rule 5605(a)(2) of The Nasdaq Stock Market Rules and as defined in National Instrument 52-110 - Audit Committees (“NI 52-110”) adopted by the Canadian Securities Administrators), and all members are financially literate (as defined in NI 52-110). [The Audit Committee meets regularly on at least a quarterly basis. The members of the Audit Committee do not have fixed terms and are appointed and replaced from time to time by resolution of the Board.]
The Board has determined that Dirk Witters and Kamil Isaev each qualify as a financial expert (as defined in Item 407(d)(5)(ii) of Regulation S-K under the Exchange Act) and Rule 5605(c)(2)(A) of The Nasdaq Stock Market Rules; and (ii) is independent (as determined under Exchange Act Rule 10A-3 and Rule 5605(a)(2) of The Nasdaq Stock Market Rules).
Relevant Education and Experience
All of the Audit Committee members [Dirk Witters and Kamil Isaev] are senior-level professionals with experience in financial matters; each has a broad understanding of accounting principles used to prepare financial statements and varied experience as to general application of such accounting principles.
For further relevant education and experience of Messrs. Dirk Witters and Kamil Isaev refer to their respective biographies. See Item 6.A – Directors and Senior Management - Directors, Senior Management and Employees.
Audit Committee Oversight
At no time during this past fiscal year have any recommendations by the Audit Committee respecting the appointment and/or compensation of our external auditors not been adopted by the Board.
Pre-Approval Policies and Procedures
Under its charter, the Audit Committee is required to pre-approve all non-audit services to be performed by the external auditors in relation to us, together with approval of the engagement letter for such non-audit services and estimated fees thereof. The pre-approval process for non-audit services will also involve a consideration of the potential impact of such services on the independence of the external auditors.
Remuneration and Nomination Committee Disclosure
The Remuneration and Nomination Committee’s Charter
Our directors have adopted a Charter for the Remuneration and Nomination Committee, which sets out the Remuneration and Nomination Committee’s mandate, organization, powers and responsibilities. The full text of our Remuneration and Nomination Charter is available on request from us.
Composition of the Remuneration and Nomination Committee
The members of the Remuneration and Nomination Committee are Dirk Witters (Chair) and Kamil Isaev, all of whom are independent directors.
D. Employees
The following table sets forth the number of employees we had at the end of each fiscal period:
|
|
|
|
|
|
|
|
|
|
Fiscal Year Ended |
Full Time |
|
Part Time |
|
Total |
|
April 30, 2023 |
|
82 |
|
|
20 |
|
|
102 |
|
April 30, 2024 |
|
72 |
|
|
29 |
|
|
101 |
|
April 30, 2025 |
|
81 |
|
|
21 |
|
|
102 |
|
None of our employees are members in a labor union.
E. Share Ownership
As of July 25, 2025, the NEOs named in this Annual Report as well as our current directors and executive officers, as a group, beneficially owned a total of 509,610 Common Shares, representing beneficial ownership of 1% of the Common Shares.
The table below sets forth the number of Common Shares beneficially owned by the NEOs named in this Annual Report as well as our directors and executive officers as of July 25, 2025. The persons listed below are deemed to be the beneficial owners of Common Shares underlying options that are exercisable within 60 days from the above date, including “out-of-the money” options. The percentages shown below are based on 46,154,118 outstanding Common Shares as of July 25, 2025.
Shareholdings of Directors and Executive Officers
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Name of Beneficial Owner |
Common
Shares Held |
|
Exercisable
Options |
|
Convertible
RSUs |
|
Exercisable
Warrants |
|
Number of
Common
Shares
Beneficially
Owned |
|
Percent of
Outstanding
Common
Shares |
|
Dr. Jennifer Bath |
|
498,118 |
|
|
630,452 |
|
|
— |
|
|
— |
|
|
498,118 |
|
|
1.08 |
% |
Joseph Scheffler |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
0.00 |
% |
Dr. Ilse Roodink |
|
9,542 |
|
|
105,000 |
|
|
— |
|
|
— |
|
|
9,542 |
|
|
0.02 |
% |
Kamil Isaev |
|
— |
|
|
— |
|
|
2,556 |
|
|
— |
|
|
— |
|
|
0.00 |
% |
Kari Graber |
|
700 |
|
|
60,000 |
|
|
— |
|
|
— |
|
|
— |
|
|
0.00 |
% |
Jon Lieber |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
0.00 |
% |
Dirk Witters |
|
1,950 |
|
|
38,889 |
|
|
— |
|
|
— |
|
|
1,950 |
|
|
0.00 |
% |
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
The Company has adopted an incentive compensation recovery policy effective October 2, 2023 (“Incentive Compensation Recovery Policy”) as required by Nasdaq listing rules and pursuant to Rule 10D-1 of the Exchange Act. The Incentive Compensation Recovery Policy is filed as Exhibit 97.1 to this Annual Report. At no time during or after the fiscal year ended April 30, 2025 (as of the date of this Annual Report), was the Company required to prepare an accounting restatement that required recovery of erroneously awarded compensation pursuant to the Incentive Compensation Recovery Policy and, as of April 30, 2025, there was no outstanding balance of erroneously awarded compensation to be recovered from the application of the Incentive Compensation Recovery Policy to a prior restatement.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
To our best knowledge, the following are our only shareholders that beneficially own, directly or indirectly, or exercise control over, shares carrying more than 5% of the outstanding voting rights attached to our Common Shares as of July 25, 2025.
|
|
|
|
|
|
|
|
|
Name of Shareholder |
|
Number of
Common
Shares |
|
|
Percentage of
Common
Shares |
|
Charmquark TWEE |
|
|
1,828,365 |
|
|
|
3.96 |
% |
Charmquark EEN |
|
|
1,828,365 |
|
|
|
3.96 |
% |
|
|
|
|
|
|
|
On April 14, 2022, the Company completed the acquisition of control over BioStrand BV, BioKey BV, and BioClue BV (hereinafter collectively referred to as “BioStrand”), a group of Belgian biotech entities and pioneers in the field of bioinformatics and biotechnology, through its wholly owned subsidiary ImmunoPrecise Netherlands BV. The Company paid a consideration of approximately €20 million to the vendors, consisting of an aggregate of 4,077,774 Common Shares, resulting in Charmquark TWEE and Charmquark EEN each carrying more than 5% of our outstanding Common Shares, and a cash payment of approximately €3,734,500. The consideration also includes a contingent earnout payment based on the profitability of BioStrand over a 7-year period, which shall not exceed in total €12 million. As of December 31, 2024, each of Charmquark TWEE and Charmquark EEN own approximately 3.96% of our outstanding Common Shares, and as a group 7.92%, as reported in the Amendment No. 2 to Schedule 13G filed by Charmquark TWEE and Charmquark EEN with the SEC on January 24, 2025.
We are a publicly owned company, and our Common Shares are owned by Canadian residents, United States residents, and residents of other countries. To our knowledge, we are not directly owned or controlled by another corporation, any foreign government or any other natural or legal person(s), whether severally or jointly. We are not aware of any arrangement, the operation of which may result in a change of control of us.
B. Related Party Transactions
To our knowledge, none of our directors or executive officers, nor any of our subsidiaries or insiders, nor any of our shareholders owning more than 10% of our voting shares, and no person with ties to any of the aforementioned, nor any member of the same group, has had or expects to have an interest in any transactions concluded since the beginning of our fiscal year ended April 30, 2023 that has had or could have a material impact on us, or in any projected transactions, except as described below.
Fiscal Year Ended 2025
The share purchase agreement related to the acquisition of BioStrand includes contingent earnout payments based on 20% of the EBITDA of BioStrand, as defined in the share purchase agreement, over a 7-year period, which shall not exceed in total €12.0 million. The Company has determined these payments relate to post-acquisition services because they are contingent on the employment of two key employees and will be expensed in the period earned. As of April 30, 2025, no amount has been earned or paid on the Company's contingent earnout related to the BioStrand acquisition.
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
Financial Statements
This Annual Report contains the Company’s our audited consolidated financial statements as at and for the year ended April 30, 2025, the year ended April 30, 2024, and the year ended April 30, 2023. The audit reports of Grant Thornton LLP are included therein.
Legal Proceedings and Regulatory Actions
As of the end of financial year ended April 30, 2025, the Company is not aware of: (a) any legal proceedings to which it is a party, or by which any of its property is subject, which would be material to it and are not aware of any such proceedings being contemplated; (b) any penalties or sanctions imposed against the Company by a court relating to securities legislation or by a securities regulatory authority, or any other penalties or sanctions imposed by a court or regulatory body against it that would likely be considered important to a reasonable investor making an investment decision; and (c) any settlement agreements that it has entered into before a court relating to securities legislation or with a securities regulatory authority.
Dividend Policy
The Company has not paid any dividends. The Company intends to retain its earnings, if any, to finance the future growth and development of its business and does not expect to pay dividends or to make any other distributions in the foreseeable future. Payment of dividends in the future is dependent upon the earnings and financial condition of the Company and other factors which the Board may deem appropriate at the time.
There are no restrictions in the constating documents of the Company, and it is not currently expected that there will exist such restriction elsewhere, which could prevent the Company from paying dividends.
B. Significant Changes
Except as otherwise disclosed in this Annual Report, there have been no significant changes in our financial condition since the most recent audited consolidated financial statements for the year ended April 30, 2025.
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
Our Common Shares are listed and posted for trading on Nasdaq under the symbol “IPA”.
B. Plan of Distribution
Not applicable.
C. Markets
See Item 9.A. – The Offer and Listing - Offer and Listing Details.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
The description of the Articles of the Company (the “Articles”) and the BCBCA below are subject to and qualified in their entirety by reference to the applicable provisions of the Articles and the BCBCA.
Incorporation
See Item 4.A. – Name, Address and Incorporation.
Objects and Purposes
The Articles do not contain a limitation on the objects and purposes of the Company.
Directors
Article 17 of the Articles deals with a directors’ disclosable interest (as defined in the BCBCA) in contracts or transactions into which the Company has entered or proposes to enter. Article 17.2 provides that a director who holds such a disclosable interest is not entitled to vote on any directors’ resolution to approve such contract or transaction, unless all the directors have a disclosable interest in that contract or transaction, in which case any or all of those directors may vote on such resolution.
Pursuant to the BCBCA, a director holds a disclosable interest in a contract or transaction if (a) the contract or transaction is material to the Company, (b) the Company has entered, or proposes to enter, into the contract or transaction, (c) either the director has a material interest in the contract or transaction or the director is a director or senior officer of, or has a material interest in, a person who has a material interest in the contract or transaction and (d) the interest is known by the director or reasonably ought to have been known. Pursuant to the BCBCA, a director does not have a disclosable interest in a number of prescribed situations, including without limitation in respect of a contract or transaction merely because the contract or transaction relates to the remuneration of the director in that person’s capacity as a director of the Company.
The directors may act notwithstanding any vacancy in the Board, but if the Company has fewer directors in office than the number set pursuant to the Articles as the quorum of directors, the directors may only act for the purpose of appointing directors up to that number or of summoning a meeting of shareholders for the purpose of filling any vacancies on the Board or, subject to the BCBCA, for any other purpose. The quorum necessary for the transaction of the business of the directors may be set by the directors and, if not so set, is deemed to be set at two directors or, if the number of directors is set at one, is deemed to be set at one director, and that director may constitute a meeting.
Article 8 of the Articles deals with the borrowing powers of the Company. The Company, if authorized by the directors, may: (i) borrow money in the manner and amount, on the security, from the sources and on the terms and conditions that they consider appropriate; (ii) issue bonds, debentures and other debt obligations either outright or as security for any liability or obligation of the Company or any other person and at such discounts or premiums and on such other terms as they consider appropriate; (iii) guarantee the repayment of money by any other person or the performance of any obligation of any other person; and (iv) mortgage, charge, whether by way of specific or floating charge, grant a security interest in, or give other security on, the whole or any part of the present and future assets and undertaking of the Company.
Qualifications of Directors
The Articles do not specify a retirement age for directors. Directors are not required to own any Common Shares of the Company.
Section 124 of the BCBCA provides that an individual is not qualified to become or act as a director of a company if that individual is:
1.
under the age of 18 years;
2.
found by a court, in Canada or elsewhere, to be incapable of managing the individual’s own affairs, unless a court, in Canada or elsewhere, subsequently finds otherwise;
3.
a person in respect of whom a certificate of incapability is issued under the Adult Guardianship Act, unless the certificate is subsequently cancelled under section 37 (4) of that Act,
4.
an undischarged bankrupt; or
5.
convicted in or out of the Province of British Columbia of an offence in connection with the promotion, formation or management of a corporation or unincorporated business, or of an offence involving fraud, unless:
a.
the court orders otherwise;
b.
5 years have elapsed since the last to occur of:
i.
the expiration of the period set for suspension of the passing of sentence without a sentence having been passed;
ii.
the imposition of a fine;
iii.
the conclusion of the term of any imprisonment; and
iv.
the conclusion of the term of any probation imposed; or
c.
a pardon was granted or issued, or a record suspension ordered, under the Criminal Records Act (Canada) and the pardon or record suspension, as the case may be, has not been revoked or ceased to have effect.
A director who ceases to be qualified to act as a director of the Company must promptly resign.
Section 120 of the BCBCA provides that every company must have at least one director, and a public company must have at least three directors.
Rights, Preference and Restrictions
Holders of Common Shares are entitled to receive notice of any meeting of shareholders of the Company, to attend and to cast one vote per share at such meetings. Holders of Common Shares are also entitled to receive on a pro rata basis such dividends, if any, as and when declared by the Board at its discretion from funds legally available therefor and upon the liquidation, dissolution, or winding up of the Company are entitled to receive on a pro rata basis, the net assets of the Company after payment of debts and other liabilities, in each case subject to the rights, privileges, restrictions, and conditions attaching to any other series or class of shares ranking senior in priority. Common Shares do not carry any pre-emptive, subscription, redemption, conversion rights, sinking fund provisions, liability to further capital calls by the Company, or provisions discriminating against any existing or prospective holder of Common Shares as a result of such shareholder owning a substantial number of Common Shares.
The rights of shareholders of the Company may be altered only with the approval of the holders of two thirds or more of the Common Shares voted at a meeting of the Company’s shareholders called and held in accordance with the Articles and applicable law.
Shareholder Meetings
The BCBCA provides that: (i) a general meeting of shareholders must be held in the Province of British Columbia, unless otherwise provided in the Company’s Articles or as approved by ordinary resolution of shareholders; (ii) the Company must hold an annual general meeting of shareholders not later than 15 months after the last preceding annual general meeting and once in every calendar year; (iii) for the purpose of determining shareholders entitled to receive notice of or vote at a meeting of shareholders, the directors may set a date as the record date for that determination, provided that such date shall not precede by more than 2 months (or, in the case of a general meeting requisitioned by shareholders under the BCBCA, by more than 4 months) or be less than 21 days before the date on which the meeting is to be held; (iv) a quorum for the transaction of business at a meeting of shareholders of the Company is the quorum established by the Articles (Article 11.3 of the Articles provides that the quorum for the transaction of business at a meeting of shareholders is two shareholders who are present in person or represented by proxy); (v) the holders of not less than 5% of the issued shares entitled to vote at a meeting may requisition the directors to call a meeting of shareholders for the purpose of transacting any business that may be transacted at a general meeting; and (vi) the Court may, on its own motion or on the application of the Company, upon the application of a director or the application of a shareholder entitled to vote at the meeting: (a) order that a meeting of shareholders be called, held and conducted in a manner that the Court considers appropriate; and (b) give directions it considers necessary as to the call, holding and conduct of the meeting.
Advance Notice Policy
On July 12, 2017 the Board adopted an advance notice policy (the “Advance Notice Policy”), which was approved by the Company’s shareholders on August 30, 2017. The purpose of the Advance Notice Policy is to provide a clear process for the shareholders, directors and management of the Company to follow when nominating directors of the Company. The Advance Notice Policy is meant to ensure that shareholders receive adequate notice of director nominations and sufficient information regarding all director nominees and to allow shareholders to register an informed vote after having been afforded reasonable time for appropriate deliberation.
The Advance Notice Policy, among other things, includes a provision that requires advance notice to the Company in certain circumstances where nominations of persons for election to the Board are made by shareholders of the Company. This Advance Notice Policy also sets a deadline by which director nominations must be submitted to the Company prior to any annual general or special meeting of the shareholders of the Company and also sets out the required information that must be included in the notice to the Company. No person will be eligible for election as a director of the Company unless nominated in accordance with the Advance Notice Policy.
In the case of an annual meeting of shareholders, notice to the Company must be made not less than 30 nor more than 65 days prior to the date of the annual meeting; provided, however, that, in the event that the annual meeting is to be held on a date that is less than 50days after the date on which the first public announcement of the date of the annual meeting was made, notice may be made not later than the close of business on the 10th day following such public announcement.
In the case of a special meeting of shareholders (which is not also an annual meeting), notice to the Company must be made not later than the close of business on the 15th day following the day on which the first public announcement of the date of the special meeting was made.
Majority Voting Policy
The Board believes that each of its members should carry the confidence and support of the Company’s shareholders and, accordingly, has adopted, effective August 9, 2023, the Majority Voting Policy for the election of directors in respect of uncontested elections. An “uncontested election” means an election where the number of nominees for director is equal to the number of directors authorized to be elected upon such election as determined by the Board. The Majority Voting Policy provides that, in an uncontested election of directors, if the number of shares “withheld” for any nominee exceeds the number of shares voted “for” the nominee, then he or she shall, immediately tender his or her written resignation to the Board. Such resignation will not be effective until accepted by the Board. Under the Majority Voting Policy, the Board will consider such offer of resignation and shall make a determination whether or not to accept or reject the resignation no later than 90 days following the date of the applicable shareholders’ meeting and shall accept the resignation absent exceptional circumstances. The Board will promptly announce its decision via press release. If the Board determines not to accept the resignation, the press release must fully state the reasons for its decision. No director who is required to tender his or her resignation shall participate in any meeting of the Board at which the resignation is considered. If a resignation is accepted by the Board, and subject to any corporate law restrictions, the Board may leave any resulting vacancy unfilled until the Company’s next annual general meeting, or may appoint a new director to fill the vacancy, or may call a special meeting of shareholders at which there will be presented a new candidate to fill the vacant position.
Limitations on Ownership of Securities
Except as provided in the Investment Canada Act, there are no limitations specific to the rights of non-Canadians to hold or vote the Common Shares under the laws of Canada or the Province of British Columbia or in the Company’s constating documents.
Change in Control
The Company has adopted a shareholder rights plan (the “Rights Plan”). Under the Rights Plan, the Company has issued one right (a “Right”) in respect of each Common Share or other security which entitles the holder to vote generally in the election of directors (“Voting Share”). The Rights will separate from the Voting Shares and will generally only be exercisable ten trading days after a person has acquired, or commences to acquire, 20% or more of the Voting Shares, other than by acquisition pursuant to: a voting share reduction (generally, a repurchase or redemption of shares by the Company which has the effect of increasing the person’s or company’s percentage ownership of the Company); a takeover bid permitted by the Rights Plan (a “Permitted Bid” or a “Competing Permitted Bid”); an exempt acquisition (an acquisition in respect of which the Board has waived the application of the Rights Plan or an acquisition made pursuant to a shareholder-approved transaction such as an amalgamation or arrangement or an acquisition made as an intermediate step in a larger transaction where the acquiring party has then distributed the shares out to its security holders); and a pro rata acquisition (generally, the acquisition of shares pursuant to a rights offering, public offering or private placement to the extent necessary to prevent dilution of the person’s or company’s shareholding). The acquisition by any person (an “Acquiring Person”) of more than 20% of the Voting Shares, other than by way of a voting share reduction, a Permitted Bid, a Competing Permitted Bid, an exempt acquisition, and a pro rata acquisition is referred to as a “Flip-in Event”. Any Rights held by an Acquiring Person will become void upon the occurrence of a Flip-in Event. Ten trading days after the occurrence of the Flip-in Event, each Right (other than those held by the Acquiring Person), will permit the purchase of shares at a 50% discount in accordance with the terms of the Rights Plan.
Ownership Threshold
There are no provisions in the Company’s constating documents or under applicable corporate law requiring share ownership to be disclosed. Securities legislation in Canada requires that shareholder ownership (as well as ownership of an interest in, or right or obligation associated with, a related financial instrument of a security of the Company) must be disclosed once a person beneficially owns or has control or direction over, directly or indirectly, securities of a reporting issuer carrying more than 10% of the voting rights attached to all the reporting issuer’s outstanding voting securities. This threshold is higher than the 5% threshold under U.S. securities legislation at which stockholders must report their share ownership.
Changes to Capital
There are no conditions imposed by the Articles governing changes in the capital where such conditions are more significant than under the BCBCA for as long as the Company is a public company. Otherwise, Section 26.3 of the Articles provides that no share or designated security may be sold, transferred or otherwise disposed of without the consent of the directors and the directors are not required to give any reason for refusing to consent to any such sale, transfer or other disposition.
Description of Capital Structure
The Company’s authorized share structure consists of an unlimited number of Common Shares without par value. All of the Common Shares are of the same class and the Company does not own any of its Common Shares.
C. Material Contracts
Except for contracts entered into in the ordinary course of business, the only material contracts the Company or a subsidiary is a party to as of the date of this Annual Report are the following:
•
Global Guaranty Agreement
•
Material Transfer and Evaluation Agreement
Global Guarantee Agreement
See Item 5.B – Liquidity and Capital Resources – Financing Activities – Fiscal Year Ended 2024 Transactions
Material Transfer and Evaluation Agreement
See Item 4.A – Events in the Development of the Business – Fiscal Year Ended 2025
D. Exchange Controls
Canada has no system of exchange controls. There are no Canadian governmental laws, decrees, or regulations relating to restrictions on the repatriation of capital or earnings of the Company to non-resident investors. There are no laws in Canada or exchange control restrictions affecting the remittance of dividends or other payments made by the Company in the ordinary course to non-resident holders of the Common Shares by virtue of their ownership of such Common Shares, except as discussed below in Item 10.E. - Certain Material United States Federal Income Tax Considerations and Certain Canadian Federal Income Tax Consequences.
There are no limitations under the laws of Canada or in the organizing documents of the Company on the right of foreigners to hold or vote securities of the Company, except that the Investment Canada Act may require prior review and approval by the Minister of Innovation, Science and Economic Development of an acquisition of “control” of the Company by a “non-Canadian,” where applicable thresholds are exceeded. The acquisition of one-third or more of the voting shares of the Company would give rise a rebuttable presumption of an acquisition of control, and the acquisition of more than fifty percent of the voting shares of the Company would be deemed to be an acquisition of control. In addition, the Investment Canada Act provides the Canadian government with broad discretionary powers in relation to national security to review and potentially prohibit, condition or require the divestiture of, any investment in the Company by a non-Canadian, including non-control level investments. “Non-Canadian” generally means an individual who is neither a Canadian citizen nor a permanent resident of Canada within the meaning of the Immigration and Refugee Protection Act (Canada) who has been ordinarily resident in Canada for not more than one year after the time at which he or she first became eligible to apply for Canadian citizenship, or a corporation, partnership, trust or joint venture that is ultimately controlled by non-Canadians.
E. Taxation
Certain Material United States Federal Income Tax Considerations
The following is a general summary of certain material U.S. federal income tax considerations applicable to a U.S. Holder (as defined below) arising from and relating to the acquisition, ownership and disposition of Common Shares. This summary is for general information purposes only and does not purport to be a complete analysis or listing of all potential U.S. federal income tax considerations that may apply to a U.S. Holder arising from or relating to the acquisition, ownership and disposition of Common Shares. In addition, this summary does not take into account the individual facts and circumstances of any particular U.S. Holder that may affect the U.S. federal income tax consequences to such U.S. Holder, including, without limitation, specific tax consequences to a U.S. Holder under an applicable income tax treaty. Accordingly, this summary is not intended to be, and should not be construed as, legal or U.S. federal income tax advice with respect to any particular U.S. Holder. This summary does not address the U.S. federal alternative minimum tax, U.S. federal net investment income tax, U.S. federal estate and gift tax, U.S. state and local tax, and non-U.S. tax consequences to U.S. Holders of the acquisition, ownership and disposition of Common Shares. In addition, except as specifically set forth below, this summary does not discuss applicable income tax reporting requirements. Each prospective U.S. Holder should consult its own tax advisors regarding the U.S. federal, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
No legal opinion from legal counsel or ruling from the Internal Revenue Service (the “IRS”) has been requested, or will be obtained, regarding the U.S. federal income tax considerations applicable to a U.S. Holder arising from or relating to the acquisition, ownership and disposition of Common Shares. This summary is not binding on the IRS, and the IRS is not precluded from taking a position that is different from, or contrary to, the positions taken in this summary. In addition, because the authorities on which this summary is based are subject to various interpretations, the IRS and the U.S. courts could disagree with one or more of the conclusions described in this summary.
Authorities
This summary is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations (whether final, temporary, or proposed) promulgated thereunder, published rulings of the IRS, published administrative positions of the IRS, the current provisions of the Convention Between Canada and the United States of America with respect to Taxes on Income and on Capital of 1980, as amended (the “Canada-U.S. Tax Treaty”), and U.S. court decisions that are applicable, and, in each case, as in effect and available, as of the date of this document. Any of the authorities on which this summary is based could be changed in a material and adverse manner at any time, and any such change could be applied on a retroactive or prospective basis, which could affect the U.S. federal income tax considerations described in this summary. Except as provided herein, this summary does not discuss the potential effects, whether adverse or beneficial, of any proposed legislation that, if enacted, could be applied on a retroactive or prospective basis.
U.S. Holders
For purposes of this summary, the term “U.S. Holder” means a beneficial owner of Common Shares that is for U.S. federal income tax purposes:
•
an individual who is a citizen or resident of the United States;
•
a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia;
•
an estate whose income is subject to U.S. federal income taxation regardless of its source; or
•
a trust that (1) is subject to the primary supervision of a court within the U.S. and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable Treasury Regulations to be treated as a U.S. person.
Non-U.S. Holders
For purposes of this summary, a “non-U.S. Holder” is a beneficial owner of Common Shares that is not a U.S. Holder or an entity or arrangement classified as a partnership for U.S. federal income tax purposes. This summary does not address the U.S. federal, state or local tax consequences to non-U.S. Holders arising from or relating to the acquisition, ownership and disposition of Common Shares. Accordingly, a non-U.S. Holder should consult its own tax advisors regarding the U.S. federal, state or local and non-U.S. tax consequences (including the potential application of and operation of any income tax treaties) relating to the acquisition, ownership and disposition of Common Shares.
U.S. Holders Subject to Special U.S. Federal Income Tax Rules Not Addressed
This summary does not address the U.S. federal income tax considerations applicable to U.S. Holders that are subject to special provisions under the Code, including, but not limited to U.S. Holders that: (a) are tax-exempt organizations, qualified retirement plans, individual retirement accounts, or other tax-deferred accounts; (b) are banks, financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; (c) are broker-dealers, dealers, or traders in securities or currencies that elect to apply a mark-to-market accounting method; (d) have a “functional currency” other than the U.S. dollar; (e) own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other integrated transaction; (f) acquire Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; (g) hold Common Shares other than as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment purposes); (h) are subject to the alternative minimum tax; (i) are subject to special tax accounting rules with respect to the Common Shares; (j) are partnerships or other “pass-through” entities (and partners or other owners thereof); (k) are S corporations (and shareholders thereof); (l) are U.S. expatriates or former long-term residents of the United States subject to Section 877 or 877A of the Code; (m) hold Common Shares in connection with a trade or business, permanent establishment, or fixed base outside the United States; or (n) own or have owned or will own (directly, indirectly, or by attribution) 10% or more of the total combined voting power or value of our outstanding shares. U.S. Holders that are subject to special provisions under the Code, including, but not limited to, U.S. Holders described immediately above, should consult their own tax advisors regarding the U.S. federal, U.S. state and local, and non-U.S. tax consequences relating to the acquisition, ownership and disposition of Common Shares.
If an entity or arrangement that is classified as a partnership (or other “pass-through” entity) for U.S. federal income tax purposes holds Common Shares, the U.S. federal income tax consequences to such entity or arrangement and the partners (or other owners or participants) of such entity or arrangement generally will depend on the activities of the entity or arrangement and the status of such partners (or owners or participants). This summary does not address the tax consequences to any such partner (or owner or participant). Partners (or other owners or participants) of entities or arrangements that are classified as partnerships or as “pass-through” entities for U.S. federal income tax purposes should consult their own tax advisors regarding the U.S. federal income tax consequences arising from and relating to the acquisition, ownership and disposition of Common Shares.
Passive Foreign Investment Company Rules
If we were to constitute a “passive foreign investment company” (“PFIC”) for any year during a U.S. Holder’s holding period, then certain potentially adverse rules would affect the U.S. federal income tax consequences to a U.S. Holder resulting from the acquisition, ownership and disposition of Common Shares. We believe that we were not a PFIC for our tax year ended April 30, 2025, and we have not yet made a determination regarding our potential classification as a PFIC for our tax year. While we do not intend to become a PFIC for our current tax year or in the future, based on the cash raised in one or more offerings and current business plans and financial expectations, we may be a PFIC for our current tax year and may be a PFIC in the future. Our PFIC classification for our current or future tax years may depend on, among other things, how quickly we may raise cash pursuant to one or more offerings, the manner in which, and how quickly, we utilize our cash on hand and the cash proceeds received from any such offerings, as well as on changes in the market value of our Common Shares. Whether we are a PFIC for any taxable year will also depend on the composition of our income and the composition, nature and value of our assets from time to time (including the value of our goodwill, which may be determined by reference to the value of our Common Shares, which could fluctuate). No opinion of legal counsel or ruling from the IRS concerning our status as a PFIC has been obtained or is currently planned to be requested. The determination of whether any corporation was, or will be, a PFIC for a tax year depends, in part, on the application of complex U.S. federal income tax rules, which are subject to differing interpretations. In addition, whether any corporation will be a PFIC for any tax year depends on the assets and income of such corporation over the course of each such tax year and, as a result, cannot be predicted with certainty as of the date of this document. Accordingly, there can be no assurance that the IRS will not challenge any determination made by us (or any of our non-U.S. subsidiaries) concerning our (or its) PFIC status. Each U.S. Holder should consult its own tax advisors regarding our PFIC status of the PFIC status of each of our non-U.S. subsidiaries.
In any year in which we are classified as a PFIC, a U.S. Holder will be required to file an annual report with the IRS containing such information as Treasury Regulations and/or other IRS guidance may require. In addition to penalties, a failure to satisfy such reporting requirements may result in an extension of the time period during which the IRS can assess a tax. U.S. Holders should consult their own tax advisors regarding the requirements of filing such information returns under these rules, including the requirement to file an IRS Form 8621 annually.
We generally will be a PFIC if, for a tax year, (a) 75% or more of our gross income in such tax year is passive income (the “PFIC income test”) or (b) 50% or more of the value of our assets either produce passive income or are held for the production of passive income, based on the quarterly average of the fair market value of such assets (the “PFIC asset test”). “Gross income” generally includes all sales revenues less the cost of goods sold, plus income from investments and from incidental or outside operations or sources, and “passive income” generally includes, for example, dividends, interest, certain rents and royalties, certain gains from the sale of stock and securities, and certain gains from commodities transactions.
For purposes of the PFIC income test and PFIC asset test described above, if we own, directly or indirectly, 25% or more of the total value of the outstanding shares of another corporation, we will be treated as if we (a) held a proportionate share of the assets of such other corporation and (b) received directly a proportionate share of the income of such other corporation. In addition, for purposes of the PFIC income test and PFIC asset test described above, and assuming certain other requirements are met, “passive income” does not include certain interest, dividends, rents, or royalties that are received or accrued by us from certain “related persons” (as defined in Section 954(d)(3) of the Code) also organized in Canada, to the extent such items are properly allocable to the income of such related person that is not passive income. Passive assets generally include cash and assets readily convertible into cash.
Under certain attribution rules, if we are a PFIC, U.S. Holders will generally be deemed to own their proportionate share of our direct or indirect equity interest in any company that is also a PFIC (a “Subsidiary PFIC”), and will generally be subject to U.S. federal income tax as described below under “Default PFIC Rules Under Section 1291 of the Code” on their proportionate share of (a) any “excess distributions,” as described below, on the stock of a Subsidiary PFIC and (b) a disposition or deemed disposition of the stock of a Subsidiary PFIC by us or another Subsidiary PFIC, both as if such U.S. Holders directly held the shares of such Subsidiary PFIC. In addition, U.S. Holders may be subject to U.S. federal income tax on any indirect gain realized on the stock of a Subsidiary PFIC on the sale or disposition of Common Shares. Accordingly, U.S. Holders should be aware that they could be subject to tax under the PFIC rules even if no distributions are received and no redemptions or other dispositions of Common Shares are made.
Default PFIC Rules Under Section 1291 of the Code
If we are a PFIC for any tax year during which a U.S. Holder owns Common Shares, the U.S. federal income tax consequences to such U.S. Holder of the acquisition, ownership, and disposition of Common Shares will depend on whether such U.S. Holder makes a "qualified electing fund" or "QEF" election (a "QEF Election") to treat us as a QEF or makes a mark-to-market election under Section 1296 of the Code (a "Mark-to-Market Election") with respect to the Common Shares. A U.S. Holder that does not make either a QEF Election or a Mark-to-Market Election (a "Non-Electing U.S. Holder") will be taxable as described below.
A Non-Electing U.S. Holder will be subject to the rules of Section 1291 of the Code (described below) with respect to: (a) any gain recognized on the sale or other taxable disposition of Common Shares; and (b) any “excess distribution” received on the Common Shares.
A distribution generally will be an “excess distribution” to the extent that such distribution (together with all other distributions received in the current tax year) exceeds 125% of the average distributions received during the three preceding tax years (or during a U.S. Holder’s holding period for the Common Shares, if shorter).
Under Section 1291 of the Code, any gain recognized on the sale or other taxable disposition of Common Shares of a PFIC (including an indirect disposition of the stock of any Subsidiary PFIC), and any “excess distribution” received on Common Shares or a distribution by a Subsidiary PFIC to its shareholder that is deemed to be received by a U.S. Holder, must be ratably allocated to each day in a Non-Electing U.S. Holder’s holding period for the Common Shares. The amount of any such gain or excess distribution allocated to the tax year of disposition or distribution of the excess distribution and to years before the entity became a PFIC, if any, would be taxed as ordinary income (and not eligible for certain preferential tax rates, as discussed below). The amounts allocated to any other tax year would be subject to U.S. federal income tax at the highest tax rate applicable to ordinary income in each such year, and an interest charge would be imposed on the tax liability for each such year, calculated as if such tax liability had been due in each such year. A Non-Electing U.S. Holder that is not a corporation must treat any such interest paid as “personal interest,” which is not deductible.
If we are a PFIC for any tax year during which a Non-Electing U.S. Holder holds Common Shares, we will continue to be treated as a PFIC with respect to such Non-Electing U.S. Holder, regardless of whether we cease to be a PFIC in one or more subsequent tax years. If we cease to be a PFIC, a Non-Electing U.S. Holder may terminate this deemed PFIC status with respect to Common Shares by electing to recognize gain (which will be taxed under the rules of Section 1291 of the Code discussed above), but not loss, as if such Common Shares were sold on the last day of the last tax year for which we were a PFIC.
QEF Election
A U.S. Holder that makes a timely and effective QEF Election for the first tax year in which the holding period of its Common Shares begins generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to its Common Shares. However, a U.S. Holder that makes a timely and effective QEF Election will be subject to U.S. federal income tax on such U.S. Holder’s pro rata share of (a) our net capital gain, which will be taxed as long-term capital gain to such U.S. Holder, and (b) our ordinary earnings, which will be taxed as ordinary income to such U.S. Holder. Generally, “net capital gain” is the excess of (i) net long-term capital gain over (ii) net short-term capital loss, and “ordinary earnings” are the excess of (x) “earnings and profits” over (y) net capital gain. A U.S. Holder that makes a QEF Election will be subject to U.S. federal income tax on such amounts for each tax year in which we are a PFIC, regardless of whether such amounts are actually distributed to such U.S. Holder by us. However, for any tax year in which we are a PFIC and have no net income or gain, U.S. Holders that have made a QEF Election would not have any income inclusions as a result of the QEF Election. If a U.S. Holder that made a QEF Election has an income inclusion, such a U.S. Holder may, subject to certain limitations, elect to defer payment of current U.S. federal income tax on such amounts, subject to an interest charge. If such U.S. Holder is not a corporation, any such interest paid will be treated as “personal interest,” which is not deductible.
A U.S. Holder that makes a timely and effective QEF Election with respect to us generally (a) may receive a tax-free distribution from us to the extent that such distribution represents our “earnings and profits” that were previously included in income by the U.S. Holder because of such QEF Election and (b) will adjust such U.S. Holder’s tax basis in the Common Shares to reflect the amount included in income or allowed as a tax-free distribution because of such QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of Common Shares.
The procedure for making a QEF Election, and the U.S. federal income tax consequences of making a QEF Election, will depend on whether such QEF Election is timely. A QEF Election will be treated as “timely” for purposes of avoiding the default PFIC rules discussed above if such QEF Election is made for the first year in the U.S. Holder’s holding period for the Common Shares in which we are a PFIC. A U.S. Holder may make a timely QEF Election by filing the appropriate QEF Election documents at the time such U.S. Holder files a U.S. federal income tax return for such year.
A QEF Election will apply to the tax year for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. If a U.S. Holder makes a QEF Election and, in a subsequent tax year, we cease to be a PFIC, the QEF Election will remain in effect (although it will not be applicable) during those tax years in which we are not a PFIC. Accordingly, if we become a PFIC in another subsequent tax year, the QEF Election will be effective and the U.S. Holder will be subject to the QEF rules described above during any subsequent tax year in which we qualify as a PFIC.
U.S. Holders should be aware that there can be no assurances that we will satisfy the record keeping requirements that apply to a QEF, or that we will supply U.S. Holders with information that such U.S. Holders are required to report under the QEF rules, in the event that we are a PFIC. Thus, U.S. Holders may not be able to make a QEF Election with respect to their Common Shares. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a QEF Election with respect to us and any Subsidiary PFIC.
A U.S. Holder makes a QEF Election by attaching a completed IRS Form 8621, including a PFIC Annual Information Statement, to a timely filed United States federal income tax return. However, if we do not provide the required information with regard to us or any of our Subsidiary PFICs, U.S. Holders will not be able to make a QEF Election for such entity and will continue to be subject to the rules of Section 1291 of the Code discussed above that apply to Non-Electing U.S. Holders with respect to the taxation of gains and excess distributions.
Mark-to-Market Election
A U.S. Holder may make a Mark-to-Market Election with respect to Common Shares only if the Common Shares are marketable stock. The Common Shares generally will be “marketable stock” if the Common Shares are regularly traded on (a) a national securities exchange that is registered with the SEC, (b) the national market system established pursuant to section 11A of the Exchange Act, or (c) a foreign securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such foreign exchange has trading volume, listing, financial disclosure, and surveillance requirements, and meets other requirements and the laws of the country in which such foreign exchange is located, together with the rules of such foreign exchange, ensure that such requirements are actually enforced and (ii) the rules of such foreign exchange effectively promote active trading of listed stocks. If such stock is traded on such a qualified exchange or other market, such stock generally will be “regularly traded” for any calendar year during which such stock is traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Each U.S. Holder should consult its own tax advisor in this matter.
A U.S. Holder that makes a Mark-to-Market Election with respect to its Common Shares generally will not be subject to the rules of Section 1291 of the Code discussed above with respect to such Common Shares. However, if a U.S. Holder does not make a Mark-to-Market Election beginning in the first tax year of such U.S. Holder’s holding period for the Common Shares for which we are a PFIC and such U.S. Holder has not made a timely QEF Election, the rules of Section 1291 of the Code discussed above will apply to certain dispositions of, and distributions on, the Common Shares.
A U.S. Holder that makes a Mark-to-Market Election will include in ordinary income, for each tax year in which we are a PFIC, an amount equal to the excess, if any, of (a) the fair market value of the Common Shares as of the close of such tax year over (b) such U.S. Holder’s adjusted tax basis in such Common Shares. A U.S. Holder that makes a Mark-to-Market Election will be allowed a deduction in an amount equal to the excess, if any, of (a) such U.S. Holder’s adjusted tax basis in the Common Shares, over (b) the fair market value of such Common Shares (but only to the extent of the net amount of previously included income as a result of the Mark-to-Market Election for prior tax years).
A U.S. Holder that makes a Mark-to-Market Election generally also will adjust such U.S. Holder’s tax basis in the Common Shares to reflect the amount included in gross income or allowed as a deduction because of such Mark-to-Market Election. In addition, upon a sale or other taxable disposition of Common Shares, a U.S. Holder that makes a Mark-to-Market Election will recognize ordinary income or ordinary loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of such Mark-to-Market Election for prior tax years over (b) the amount allowed as a deduction because of such Mark-to-Market Election for prior tax years). Losses that exceed this limitation are subject to the rules generally applicable to losses provided in the Code and Treasury Regulations.
A U.S. Holder makes a Mark-to-Market Election by attaching a completed IRS Form 8621 to a timely filed United States federal income tax return. A Mark-to-Market Election applies to the tax year in which such Mark-to-Market Election is made and to each subsequent tax year, unless the Common Shares cease to be “marketable stock” or the IRS consents to revocation of such election. Each U.S. Holder should consult its own tax advisors regarding the availability of, and procedure for making, a Mark-to-Market Election.
Although a U.S. Holder may be eligible to make a Mark-to-Market Election with respect to the Common Shares, no such election may be made with respect to the stock of any Subsidiary PFIC that a U.S. Holder is treated as owning, because such stock is not marketable. Hence, the Mark-to-Market Election will not be effective to avoid the application of the default rules of Section 1291 of the Code described above with respect to deemed dispositions of Subsidiary PFIC stock or distributions from a Subsidiary PFIC to its shareholder.
Other PFIC Rules
Under Section 1291(f) of the Code, the IRS has issued proposed Treasury Regulations that, subject to certain exceptions, would cause a U.S. Holder that had not made a timely QEF Election to recognize gain (but not loss) upon certain transfers of Common Shares that would otherwise be tax-deferred (e.g., gifts and exchanges pursuant to corporate reorganizations). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which the Common Shares are transferred.
If finalized in their current form, the proposed Treasury Regulations applicable to PFICs would be effective for transactions occurring on or after April 1, 1992. Because the proposed Treasury Regulations have not yet been adopted in final form, they are not currently effective, and there is no assurance that they will be adopted in the form and with the effective date proposed. Nevertheless, the IRS has announced that, in the absence of final Treasury Regulations, taxpayers may apply reasonable interpretations of the Code provisions applicable to PFICs and that it considers the rules set forth in the proposed Treasury Regulations to be reasonable interpretations of those Code provisions.
The PFIC rules are complex, and the implementation of certain aspects of the PFIC rules requires the issuance of Treasury Regulations which in many instances have not been promulgated and which, when promulgated, may have retroactive effect. U.S. Holders should consult their own tax advisors about the potential applicability of the proposed Treasury Regulations.
Certain additional adverse rules may apply with respect to a U.S. Holder if we are a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example, under Section 1298(b)(6) of the Code, a U.S. Holder that uses Common Shares as security for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such Common Shares.
In addition, a U.S. Holder who acquires Common Shares from a decedent will not receive a “step up” in tax basis of such Common Shares to fair market value unless such decedent had a timely and effective QEF Election in place.
Special rules also apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to such special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. The rules relating to distributions by a PFIC and their eligibility for the foreign tax credit are complicated, and a U.S. Holder should consult with its own tax advisors regarding the availability of the foreign tax credit with respect to distributions by a PFIC.
The PFIC rules are complex, and each U.S. Holder should consult its own tax advisors regarding the PFIC rules (including the availability and advisability of making a QEF Election or Mark-to-Market Election) and how the PFIC rules may affect the U.S. federal income tax consequences of the acquisition, ownership, and disposition of Common Shares.
Certain additional adverse rules may apply with respect to a U.S. Holder if we are a PFIC, regardless of whether the U.S. Holder makes a QEF Election. These rules include special rules that apply to the amount of foreign tax credit that a U.S. Holder may claim on a distribution from a PFIC. Subject to these special rules, foreign taxes paid with respect to any distribution in respect of stock in a PFIC are generally eligible for the foreign tax credit. U.S. Holders are urged to consult their own tax advisors regarding the potential application of the PFIC rules to the ownership and disposition of Common Shares, and the availability of certain U.S. tax elections under the PFIC rules.
General Rules Applicable to the Ownership and Disposition of Common Shares
The following discussion is subject, in its entirety, to the rules described above under the heading “Passive Foreign Investment Company Rules”.
Distributions on Common Shares
A U.S. Holder that receives a distribution, including a constructive distribution, with respect to a Common Share (including any constructive distribution) will be required to include the amount of such distribution in gross income as a dividend (without reduction for any Canadian income tax withheld from such distribution) to the extent of our current and accumulated “earnings and profits”, as computed for U.S. federal income tax purposes. A dividend generally will be taxed to a U.S. Holder at ordinary income tax rates if we are a PFIC for the tax year of such distribution or were a PFIC for the preceding tax year. To the extent that a distribution exceeds our current and accumulated “earnings and profits”, such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s adjusted tax basis in the Common Shares and thereafter as gain from the sale or exchange of such Common Shares. (See “Sale or Other Taxable Disposition of Common Shares” below). However, we do not intend to maintain the calculations of our earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder therefore should assume that any distribution by us with respect to the Common Shares will constitute dividend income. Dividends received on Common Shares by corporate U.S. Holders generally will not be eligible for the “dividends received deduction” generally applicable to corporations. Subject to applicable limitations and provided we are eligible for the benefits of the Canada-U.S. Tax Treaty or the Common Shares are readily tradable on a United States securities market, dividends paid by us to non-corporate U.S. Holders, including individuals, in respect of Common Shares generally will be eligible for the preferential tax rates applicable to long-term capital gains for dividends, provided certain holding period and other conditions are satisfied, including that we not be classified as a PFIC in the tax year of distribution or in the preceding tax year. The dividend rules are complex, and each U.S. Holder should consult its own tax advisors regarding the application of such rules.
Sale or Other Taxable Disposition of Common Shares
Upon the sale or other taxable disposition of Common Shares, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the U.S. dollar value of cash received plus the fair market value of any property received and such U.S. Holder’s tax basis in such Common Shares sold or otherwise disposed of. Gain or loss recognized on such sale or other taxable disposition generally will be long-term capital gain or loss if, at the time of the sale or other taxable disposition, the Common Shares have been held for more than one year.
Preferential tax rates may apply to long-term capital gain of a U.S. Holder that is an individual, estate, or trust. There are currently no preferential tax rates for long-term capital gain of a U.S. Holder that is a corporation. Deductions for capital losses are subject to significant limitations under the Code.
Additional Considerations
Receipt of Foreign Currency
The amount of any distribution paid to a U.S. Holder in foreign currency, or on the sale, exchange or other taxable disposition of Common Shares generally will be equal to the U.S. dollar value of such foreign currency based on the exchange rate applicable on the date of receipt or, if applicable, the date of settlement if the Common Shares are traded on an established securities market (regardless of whether such foreign currency is converted into U.S. dollars at that time). A U.S. Holder will have a tax basis in the foreign currency equal to its U.S. dollar value on the date of receipt. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Different rules apply to U.S. Holders who use the accrual method of tax accounting. Each U.S. Holder should consult its own U.S. tax advisors regarding the U.S. federal income tax consequences of receiving, owning, and disposing of foreign currency.
Foreign Tax Credit
Dividends paid on the Common Shares (including any constructive distributions) will be treated as foreign-source income, and generally will be treated as “passive category income” or “general category income” for U.S. foreign tax credit purposes. Any gain or loss recognized on a sale or other disposition of Common Shares generally will be United States source gain or loss. Certain U.S. Holders that are eligible for the benefits of Canada-U.S. Tax Treaty may elect to treat such gain or loss as Canadian source gain or loss for U.S. foreign tax credit purposes. The Code applies various complex limitations on the amount of foreign taxes that may be claimed as a credit by U.S. taxpayers. In addition, Treasury Regulations that apply to foreign taxes paid or accrued (the “Foreign Tax Credit Regulations”) impose additional requirements for Canadian withholding taxes to be eligible for a foreign tax credit, and there can be no assurance that those requirements will be satisfied. The Treasury Department has released guidance temporarily pausing the application of certain of the Foreign Tax Credit Regulations.
Subject to the PFIC rules and the Foreign Tax Credit Regulations, each as discussed above, a U.S. Holder that pays (whether directly or through withholding) Canadian income tax with respect to dividends paid on the Common Shares (including any constructive distributions) generally will be entitled, at the election of such U.S. Holder, to receive either a deduction or a credit for such Canadian income tax. Generally, a credit will reduce a U.S. Holder's U.S. federal income tax liability on a dollar-for-dollar basis, whereas a deduction will reduce a U.S. Holder's income that is subject to U.S. federal income tax. This election is made on a year-by-year basis and applies to all foreign taxes paid (whether directly or through withholding) by a U.S. Holder during a year. The foreign tax credit rules are complex and involve the application of rules that depend on a U.S. Holder’s particular circumstances. Accordingly, each U.S. Holder should consult its own U.S. tax advisor regarding the foreign tax credit rules.
Backup Withholding and Information Reporting
Under U.S. federal income tax law and Treasury Regulations, certain categories of U.S. Holders must file information returns with respect to their investment in, or involvement in, a foreign corporation. For example, U.S. return disclosure obligations (and related penalties) are imposed on individuals who are U.S. Holders that hold certain specified foreign financial assets in excess of certain threshold amounts. The definition of specified foreign financial assets includes not only financial accounts maintained in foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a non-U.S. person, any financial instrument or contract held for investment that has an issuer or counterparty other than a U.S. person and any interest in a non-U.S. entity. U.S. Holders may be subject to these reporting requirements unless their Common Shares are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. U.S. Holders should consult their own tax advisors regarding the requirements of filing information returns, including the requirement to file an IRS Form 8938.
Payments made within the U.S. or by a U.S. payor or U.S. middleman, of dividends on, and proceeds arising from the sale or other taxable disposition of Common Shares will generally be subject to information reporting and backup withholding tax (currently at a rate of 24%) if a U.S. Holder (a) fails to furnish such U.S. Holder’s correct U.S. taxpayer identification number (generally on IRS Form W-9), (b) furnishes an incorrect U.S. taxpayer identification number, (c) is notified by the IRS that such U.S. Holder has previously failed to properly report items subject to backup withholding tax, or (d) fails to certify, under penalty of perjury, that such U.S. Holder has furnished its correct U.S. taxpayer identification number and that the IRS has not notified such U.S. Holder that it is subject to backup withholding tax. However, certain exempt persons generally are excluded from these information reporting and backup withholding rules. Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding tax rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner.
The discussion of reporting requirements set forth above is not intended to constitute a complete description of all reporting requirements that may apply to a U.S. Holder. A failure to satisfy certain reporting requirements may result in an extension of the time period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting requirement. Each U.S. Holder should consult its own tax advisors regarding the information reporting and backup withholding rules.
THE ABOVE SUMMARY IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSIDERATIONS APPLICABLE TO U.S. HOLDERS WITH RESPECT TO THE ACQUISITION, OWNERSHIP AND DISPOSITION OF COMMON SHARES. U.S. HOLDERS SHOULD CONSULT THEIR OWN TAX ADVISORS AS TO THE TAX CONSIDERATIONS APPLICABLE TO THEM IN LIGHT OF THEIR OWN PARTICULAR CIRCUMSTANCES
Certain Canadian Federal Income Tax Consequences
The following summary describes the principal Canadian federal income tax considerations under the Income Tax Act(Canada) and the regulations thereunder (collectively, the “Tax Act”) generally applicable to a holder who acquires or holds as beneficial owner Common Shares, and who, for purposes of the Tax Act and at all relevant times: (i) is not, and is not deemed to be, resident in Canada, (ii) holds the Common Shares as capital property, (iii) deals at arm’s length with, and is not affiliated with, the Company, (iv) does not use or hold and will not be deemed to use or hold, the Common Shares in a business carried on in Canada, (a “Non-Resident Holder”). Special rules, which are not discussed in this summary, may apply to a Non-Resident Holder that is an “authorized foreign bank” within the meaning of the Tax Act or an insurer carrying on an insurance business in Canada and elsewhere. Such Non-Resident Holders should consult their own tax advisors.
This summary is not applicable to a Non-Resident Holder (i) that is a “financial institution”, as defined in the Tax Act for the purposes of the mark-to-market rules in the Tax Act, (ii) that is a “specified financial institution”, as defined in the Tax Act, (iii) an interest in which is a “tax shelter investment” as defined in the Tax Act, (iv) that has elected to determine its Canadian tax results in a “functional currency” other than the Canadian dollar, (v) that has entered into or will enter into a “derivative forward agreement” or a “synthetic disposition arrangement” with respect to the Common Shares, (vi) that receives dividends on Common Shares under or as part of a “dividend rental arrangement”, as defined in the Tax Act, (vii) that is a “foreign affiliate” (as defined in the Tax Act) of a taxpayer resident in Canada, or (viii) that is exempt from tax under Part I of the Tax Act. Such Non-Resident Holders should consult their own tax advisors with respect to an investment in Common Shares.
This summary is based upon the current provisions of the Tax Act, all specific proposals to amend the Tax Act that have been publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”), the Canada-United States Tax Convention (1980) (the “Treaty”), as amended, and an understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (the “CRA”) published in writing and made publicly available before the date hereof. This summary assumes the Proposed Amendments will be enacted in the form proposed; however, no assurance can be given that the Proposed Amendments will be enacted in their current form, or at all. This summary is not exhaustive of all possible Canadian federal income tax considerations and, except for the Proposed Amendments, does not take into account or anticipate any changes in the law or any changes in the CRA’s administrative policies or assessing practices, whether by legislative, governmental or judicial action or decision, nor does it take into account or anticipate any other federal or any provincial, territorial or foreign tax considerations, which may differ significantly from those discussed herein.
This summary is of a general nature only and is not, and is not intended to be, nor should it be construed to be, legal or tax advice to any prospective purchaser or holder of the Common Shares. This summary does not address the deductibility of interest on any funds borrowed by a Non-Resident Holder to purchase Common Shares. Prospective purchasers or holders of Common Shares should consult their own tax advisors having regard to their particular circumstances.
Currency Conversion
Generally, for purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of Common Shares must be converted into Canadian dollars based on the exchange rates as determined in accordance with the Tax Act. The amounts subject to withholding tax and any capital gains or capital losses realized by a Non-Resident Holder may be affected by fluctuations in the Canadian-U.S. dollar or other applicable exchange rate.
Dividends
Dividends paid or credited (or deemed to be paid or credited) on Common Shares to a Non-Resident Holder by the Company are subject to Canadian withholding tax under Part XIII of the Tax Act at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Resident Holder is entitled under any applicable income tax treaty or convention.
For example, the rate of withholding tax on dividends paid or credited (or deemed to be paid or credited) to a Non-Resident Holder that is the beneficial owner of the dividend, is resident in the United States for purposes of the Treaty, and is fully entitled to the benefits of the Treaty, is generally reduced to 15% (or 5% in the case of a company and which owns at least 10% of the voting stock of the Company) of the gross amount of the dividend. The Multilateral Convention to Implement Tax Treaty Related Measures to Prevent Base Erosion and Profit Shifting (the “MLI”) of which Canada is a signatory, affects many of Canada’s bilateral tax treaties(but not the Treaty), including the ability to claim benefits thereunder. Non-Resident Holders are urged to consult their own tax advisors to determine their entitlement to relief under an applicable income tax treaty.
Dispositions
A Non-Resident Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of Common Shares, nor will capital losses arising therefrom be recognized under the Tax Act, unless the Common Shares constitute “taxable Canadian property” (as defined in the Tax Act) of the Non-Resident Holder and the Non-Resident Holder is not entitled to relief under an applicable income tax treaty or convention (including as a result of the application of the MLI).
Provided the Common Shares are listed on a “designated stock exchange,” as defined in the Tax Act (which currently includes the Nasdaq), at the time of disposition, the Common Shares will generally not constitute taxable Canadian property of a Non-Resident Holder at that time, unless at any time during the 60-month period immediately preceding the disposition the following two conditions are satisfied concurrently: (i) one or any combination of (a) the Non-Resident Holder, (b) persons with whom the Non-Resident Holder did not deal at “arm’s length” (for purposes of the Tax Act) and (c) partnerships in which the Non-Resident Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, owned 25% or more of issued shares of any class or series of the capital stock of the Company; and (ii) more than 50% of the fair market value of the Common Shares of the Company was derived directly or indirectly from one or any combination of: (a) real or immovable property situated in Canada, (b) “Canadian resource property” (as defined in the Tax Act), (c) “timber resource property” (as defined in the Tax Act) and (d) options in respect of, or interests in, or for civil law rights in, property described in any of the foregoing paragraphs (a) to (c), whether or not such property exists. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, Common Shares may be deemed to be taxable Canadian property to a Non-Resident Holder. Non-Resident Holders whose Common Shares may constitute taxable Canadian property should consult their own tax advisor.
F. Dividends and Paying Agents
Not applicable
G. Statement by Experts
Not applicable.
H. Documents on Display
Any statement in this Annual Report about any of our contracts or other documents is not necessarily complete. If the contract or document is filed as an exhibit to this Annual Report, the contract or document is deemed to modify the description contained in this Annual Report. Readers must review the exhibits themselves for a complete description of the contract or document.
We are subject to the informational requirements of the Exchange Act and file reports and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC at http://www.sec.gov.edgar.
We are required to file reports and other information with the securities commissions in Canada. You are invited to read and copy any reports, statements or other information, other than confidential filings, that we file with the provincial securities commissions. These filings are also electronically available from SEDAR+, the Canadian equivalent of EDGAR.
Copies of our material contracts are kept at our registered office.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders ITEM 11.
Not applicable.
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to a number of financial risks arising through the normal course of business, including credit risk, currency risk, and liquidity risk. Refer to Note [16] of our audited consolidated financial statements for Fiscal 2025 (for the years ended April 30, 2025, 2024, and 2023).
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not Applicable.
|
|
D. |
American Depository Receipts |
The Company does not have securities registered as American Depository Receipts.
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
There has not been a material default in the payment of principal, interest, a sinking or purchase fund installment, or any other material default not cured within thirty days, relating to indebtedness of the Company or any of its significant subsidiaries. There are no payments of dividends by the Company in arrears, nor has there been any other material delinquency relating to any class of preference shares of the Company.
A. If there has been:
1. a material default in the payment of principal, interest, a sinking or purchase fund installment, or
2. any other material default not cured within 30 days, relating to indebtedness of you or any of your significant subsidiaries, and if the amount of the indebtedness exceeds 5% of your total assets on a consolidated basis, identify the indebtedness and state the nature of the default. If the default falls under paragraph A.1 above, state the amount of the default and the total arrearage on the date you file this report.
B. If the payment of dividends is in arrears or there has been any other material delinquency not cured within 30 days, relating to:
1. any class of your preferred stock which is registered or ranks prior to any class of registered securities, or
2. any class of preferred stock of your significant subsidiaries, state the title of the class and the nature of the arrearage or delinquency. If the payment of dividends is in arrears, state the amount of this arrearage and the total arrearage on the date you file this report.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
A. to D.
None.
E. Use of Proceeds
Not applicable.
ITEM 15. CONTROLS AND PROCEDURES
A. Disclosure Controls and Procedures
The Chief Executive Officer (“CEO”) and the interim Chief Financial Officer (“ICFO”) have designed disclosure controls and procedures, or have caused them to be designed under their supervision. Such procedures are designed to ensure that material information relating to the Company and its consolidated subsidiaries is made known to the CEO and ICFO by others within the Company, and such disclosure controls and procedures were established in order to provide reasonable assurance that:
•
material information relating to the Company is made known to the CEO and ICFO by others, particularly during the period in which the interim and annual filings are being prepared; and
•
information required to be disclosed by the Company in its annual filings, interim filings or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in securities legislation.
Our management, with the participation of our CEO and ICFO, have evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the United States Securities and Exchange Act of 1934, as amended, or the Exchange Act), as of the period ended April 30, 2025, the end of the period covered by this Annual Report on Form 20-F. Based on such evaluation, our CEO and ICFO have identified and concluded that, as of such date, our disclosure controls and procedures were not effective because of a material weakness in our internal control over financial reporting as described below. As of April 30, 2025, this remains unremediated.
Material Weakness
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis.
Management identified the following material weakness in internal controls over financial reporting during the three-month period ended January 31, 2025, which continues to exist at April 30, 2025:
Management concluded that we did not have sufficient resources to assist in identifying, evaluating and addressing complex technical accounting issues that affect our consolidated financial statements on a timely basis.
Ongoing Remediation Efforts to Address the Identified Material Weakness
Management, with oversight from the Audit Committee of our Board of Directors, is taking steps to remediate the control deficiencies which resulted in the material weakness described above by designing and implementing remediation measures intended to address the material weakness identified, by implementing subject matter expert reviews to our internal control over financial reporting. The remediation measures intended to correct the material weakness includes engaging with expert and subject matter consultants on such complex accounting issues that may arise, as well as providing additional in-house training to personnel to support internal controls over financial reporting. With the additional measures, we intend to enhance our technical accounting expertise within the Company to better identify and address complex technical accounting issues if and when they arise.
As we continue to evaluate and work to improve our internal control over financial reporting, management may determine to take additional measures to strengthen controls or to modify the remediation plan described above. When operational, we believe the controls we have designed or plan to design will remediate the control deficiency that has led to the material weakness that we have identified. The material weakness will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
B. Management’s Annual Report on Internal Control Over Financial Reporting
The Company’s management has employed a framework consistent with Exchange Act Rule 13a-15(c), to evaluate the Company’s internal control over financial reporting described below. A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements. It should be noted that a control system, no matter how well conceived or operated, can only provide reasonable, not absolute, assurance that the objectives of the control system are met. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies and procedures may deteriorate.
Management of the Company, including the CEO and ICFO, is responsible for establishing and maintaining adequate internal control over financial reporting, and has used the framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013) (COSO) to evaluate the effectiveness of our controls for the period covered by this Annual Report. Based on this evaluation, management concluded that our internal controls over financial reporting were not effectively designed as at April 30, 2025 and did not provide a reasonable assurance of the reliability of our financial reporting and preparation of financial statements.
The Company’s management, including the CEO and ICFO, believe that disclosure controls and procedures and internal control over financial reporting, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, they cannot provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been prevented or detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by unauthorized override of the controls. The design of any control system also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed.
Due to its inherent limitations, internal controls over financial reporting and disclosure may not prevent or detect all misstatements. Management will continue to monitor the effectiveness of its internal control over financial reporting and disclosure controls and procedures and may make modifications from time to time as considered necessary.
C. Attestation Report of Registered Public Accounting Firm
In accordance with the JOBS Act enacted on April 5, 2012, the Registrant qualifies as an “emerging growth company”, which entitles the Registrant to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. Specifically, the JOBS Act defers the requirement to have the Registrant’s independent auditor assess the Registrant’s internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act.
As such, the Registrant is exempt from the requirement to include an auditor attestation report in this Annual Report, and will continue to be exempt from such requirement, for so long as the Registrant remains an emerging growth company, which may be for as long as five years following its initial registration in the United States.
D. Changes in Internal Controls Over Financial Reporting
We are working towards implementing processes and procedures to address the material weakness noted above. Other than changes in personnel, there were no changes in our internal control over financial reporting identified in management’s evaluation pursuant to Rules 13a-15(e) and 15d-15(e) of the Exchange Act for the period ended April 30, 2025 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
The Board has determined that Dirk Witters (Chairman) and Kamil Isaev, each qualify as a financial expert (as defined in Item 407(d)(5)(ii) of Regulation S-K under the Exchange Act) and Rule 5605(c)(2)(A) of The Nasdaq Stock Market Rules; and (ii) is independent (as determined under Exchange Act Rule 10A-3 and Rule 5605(a)(2) of The Nasdaq Stock Market Rules).
The SEC has indicated that the designation or identification of a person as an audit committee financial expert does not make such person an “expert” for any purpose, impose any duties, obligations or liability on such person that are greater than those imposed on members of the audit committee and the Board who do not carry this designation or identification, or affect the duties, obligations or liability of any other member of the audit committee or Board.
ITEM 16B. CODE OF ETHICS
We adopted a Code of Ethics and Business Conduct applicable to all each of our directors, officers and employees, including our CEO, CFO, corporate controller and persons performing similar functions, which is a “code of ethics” as defined in section 406(c) of the Sarbanes-Oxley Act.
There were no amendments, or waivers granted in respect of, the Code of Ethics and Business Conduct during the fiscal year ended April 30, 2025. The Code of Ethics and Business Conduct is available at https://ir.ipatherapeutics.com/governance/governance-documents. Amendments to the Code of Ethics and Business Conduct and waivers, if any, for executive officers will be disclosed on the Company’s website. Except for the Code of Ethics and Business Conduct, no information contained on the Company’s website or any other site shall be incorporated by reference into this Annual Report or in the documents incorporated by reference herein or attached as exhibits hereto.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Aggregate fees paid and payable to our external auditor, Grant Thornton LLP (PCAOB ID 248) during the financial years ended April 30, 2025 and 2024 were as follows:
|
|
|
|
|
|
|
|
|
|
|
2025 Fee Amount ($) |
|
|
2024 Fee Amount ($) |
|
Audit Fees(1) |
|
$ |
486,534 |
|
|
$ |
375,470 |
|
Audit-Related Fees(2) |
|
$ |
37,118 |
|
|
$ |
20,553 |
|
Tax Fees(3) |
|
$ |
112,123 |
|
|
$ |
94,036 |
|
All Other Fees(4) |
|
$ |
4,316 |
|
|
$ |
306,473 |
|
Total: |
|
$ |
640,091 |
|
|
$ |
796,532 |
|
Notes:
(1)
“Audit fees” include fees rendered by the Company’s external auditor for professional services necessary to perform the annual audit, quarterly reviews of the Company’s financial statements, services rendered in connection with the filing of prospectuses in the United States and Canada, and review of documents filed with the SEC and consents and other services normally provided in connection with statutory and regulatory filings or engagements. This includes fees for the review of tax provisions and for accounting consultations on matters reflected in the financial statements.
(2)
“Audit-related fees” include fees for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements and that are not included in the “Audit Fees” category.
(3)
“Tax fees” include fees for professional services rendered by the Company’s external auditor for tax compliance, tax advice and tax planning.
(4)
“All other fees” include fees for products and services provided by the Company’s external auditor, other than services reported under the table headings “Audit Fees”, “Audit-Related Fees” or “Tax Fees”.
Pre-Approval Policies and Procedures
From time to time, management recommends to and request approval from the Audit Committee for audit and non-audit services to be provided by the Company’s independent registered public accounting firm. The Audit Committee satisfies the pre-approval requirement if: (a) the aggregate amount of all the non-audit services that were not pre-approved is reasonably expected to constitute no more than five per cent of the total amount of fees paid by the issuer and its subsidiary entities to the issuer’s external auditor during the financial year in which the services are provided; (b) the Company or the subsidiary of the Company, as the case may be, did not recognize the services as non-audit services at the time of the engagement; and (c) the services are promptly brought to the attention of the Audit Committee and approved by the Audit Committee or by one or more of its members to whom authority to grant such approvals has been delegated by the Audit Committee, prior to the completion of the audit.
The Audit Committee may delegate to one or more independent Members the authority to pre-approve non-audit services in satisfaction of the requirement. The pre-approval of non-audit services by any Member to whom authority has been delegated must be presented to the Audit Committee at its first scheduled meeting following such pre-approval.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN COMPANY’S CERTIFYING ACCOUNTANT
On June 13, 2025, the Company received notice of the resignation of Grant Thornton (“GT”) as auditor, effective with the completion of the Company's annual audit for the year ended April 30, 2025. Grant Thornton was approved as the Company's auditor in 2021.
The audit reports of Grant Thornton on the consolidated financial statements of the Company as of and for the fiscal years ended April 30, 2025 and April 30, 2024 and through the date of the filing (July 29, 2025) did not contain an adverse opinion or a disclaimer of opinion, nor were they qualified or modified as to audit scope or accounting principles, except for going concern uncertainty modification.
As more fully described in Item 15. Controls and Procedures, of this Form 20-F, we have identified a material weakness that existed as of April 30, 2025 related to insufficient resources to assist us in identifying, evaluating and addressing complex technical accounting issues. As a result of this material weakness, management concluded that our disclosure controls and procedures and internal controls over financial reporting were not effective as of April 30, 2025.
During the fiscal years ended April 30, 2025 and April 30, 2024 and through the date of filing (July 29, 2025), there has been no disagreements with Grant Thornton on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedures, which disagreement if not resolved to the satisfaction of Grant Thornton, would have caused it to make references to the subject matter of the disagreement in connection with its report.
The resignation of Grant Thornton, effective with completion of annual audit for the period ended April 30, 2025 and the selection of Davidson & Company, LLP ("Davidson"), as successor auditor, for the fiscal year of 2026, was considered and recommended by Audit Committee and the Board of Directors.
On July 29, 2025, the Board approved, on the recommendation by the Audit Committee, the appointment of Davidson & Company LLP (“Davidson”) as the Company’s new independent registered public accounting firm for the fiscal year ending April 30,2026 effective immediately. During the Company’s two most recent fiscal years ended April 30, 2025, and 2024 and the subsequent interim periods through July 29,2025, neither the Company nor anyone acting on its behalf consulted with Davidson with respect to: (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s consolidated financial statements, and Davidson did not provide either a written report or oral advice to the Company that Davidson concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing, or financial reporting issue, or (ii) (a) any matter that was either the subject of a disagreement (as defined in Item 16F(a)(2) of Form 20-F and the related instructions) or (b) a “reportable event” as described in Item 304(a)(1)(v) of Regulation S-K. The Company has authorized the Grant Thornton to respond fully to the inquiries of the successor accountant concerning the subject matter of the reportable event [see Item 16F(a)(iv)(C)].
ITEM 16G. CORPORATE GOVERNANCE
The Registrant is a “foreign private issuer” as defined in Rule 3b-4 under the Exchange Act and its common shares are listed on Nasdaq. Nasdaq Marketplace Rule 5615(a)(3) permits a foreign private issuer to follow its home country practices in lieu of certain requirements in the Nasdaq Listing Rules. A foreign private issuer that follows home country practices in lieu of certain corporate governance provisions of the Nasdaq Listing Rules must disclose each Nasdaq corporate governance requirement that it does not follow and include a brief statement of the home country practice the issuer follows in lieu of the Nasdaq corporate governance requirement(s), either on its website or in its annual filings with the Commission. A description of the significant ways in which the Registrant’s corporate governance practices differ from those followed by domestic companies pursuant to the applicable Nasdaq Listing Rules is disclosed on the Registrant’s website at www. ipatherapeutics.com under “Investor Relations/ Corporate Governance/ Nasdaq Statement of Corporate Governance Differences” and are also indicated below:
Meetings of Board of Directors: Rule 5605(b)(2) requires that “independent directors” must have regularly scheduled meetings at which only “independent directors” are present. In lieu of following Rule 5605(b)(2), the Company has elected to follow Canadian practices. The Company does not have mandated meetings of its independent directors. However, at each board meeting, the independent directors of the Company may meet without senior executives of the Company or any non-independent directors.
Quorum Requirements: Rule 5620(c) provides that the minimum quorum requirement for a meeting of shareholders is 33 1/3% of the outstanding common voting shares. In lieu of following Rule 5620(c), the Company has elected to follow Canadian practices consistent with the requirements of the BCBCA. Under the Company’s articles, quorum for the transaction of business at any meeting of shareholders is at least two shareholders.
Content of Audit Committee Charter: Rule 5605(c)(1) requires that the formal written audit committee charter of an issuer specify the audit committee’s responsibility for ensuring its receipt from the outside auditors of a formal written statement delineating all relationships between the auditor and the Company, actively engaging in a dialogue with the auditor with respect to any disclosed relationships or services that may impact the objectivity and independence of the auditor and for taking, or recommending that the full board take, appropriate action to oversee the independence of the outside auditor. In lieu of following Rule 5605(c)(1), the Company has elected to follow Canadian practices. The Charter of the Audit Committee provides for the Audit Committee’s responsibility to review and discuss, with the external auditor, all significant relationships that the external auditor and its affiliates have with the Company and its affiliates in order to determine the external auditor’s independence by requesting, receiving and reviewing, on a periodic basis, written or oral information from the external auditor delineating all relationships that may reasonably be thought to bear on the independence of the external auditor with respect to the Company.
Audit Committee Composition: Rule 5605(c)(2)(A) requires an Audit Committee of at least three members comprised solely of directors each of whom: (1) meets Nasdaq's definition of independence contained in Rule 5605(a)(2) (subject to the exception provided in Rule 5605(c)(2)(B) and the cure period provided in Rule 5605(c)(4)); (2) meets the requirements of SEC Rule10A-3(b)(1) (subject to exceptions provided in Rule 10A-3(c) and the cure period provided in Rule 5605(c)(4)); (3) has not participated in the preparation of the financial statements of the Company or any current subsidiary of the Company at any time during the past three years; and (4) is able to read and understand fundamental financial statements, including a company's balance sheet, income statement, and cash flow statement, as required by Rule 5605(c)(2). Additionally, the Company needs to have, at least one member of the Audit Committee who has past employment experience in finance or accounting, requisite professional certification in accounting, or any other comparable experience or background which results in the individual's financial sophistication, including being or having been a chief executive officer, chief financial officer or other senior officer with financial oversight responsibilities. In lieu of following Rule 5605(c)(2)(A), the Company has elected to follow Canadian practices consistent with the requirements of the BCBCA.
Remuneration and Nomination Committee Charter: Rule 5605(d)(1) requires the formal written compensation committee charter of an issuer to specify that the chief executive officer may not be present during voting or deliberations on his or her compensation. In lieu of following Rule 5605(d)(1), the Company has elected to follow Canadian practices. The Charter of the Remuneration and Nomination Committee of the Company provides the Chair of the Committee shall hold in camera sessions of the Committee, without management present, at each meeting, as determined necessary.
Rule 5605(d)(2) also requires the formal written compensation committee charter of an issuer to specify that the compensation committee may select, or receive advice from, a compensation consultant, legal counsel or other adviser to the compensation committee only after taking into consideration the specific factors enumerated in Rule 5605(d)(3)(D). In lieu of following Rule 5605(d)(2), the Company has elected to follow Canadian practices. The Charter of the Remuneration and Nomination Committee of the Company provides that the Remuneration and Nomination Committee can engage, at the expense of the Company, any external professional or other advisors which it determines necessary in order to carry out its duties, but does not specify the factors to be considered as required by Rule 5605(d)(3)(D).
Independent Director Oversight of Director Nominations: Rule 5605(e), requires that director nominees either be selected, or recommended for a board of directors' selection, either by: (i) independent directors constituting a majority of the board's independent directors in a vote in which only independent directors participate; or (ii) a nominees committee comprised solely of independent directors.
In lieu of following Rule 5605(e), the Company has elected to follow Canadian laws and regulations, which do not require independent director involvement in the selection of director nominees.
Shareholder Approval Requirements: Rule 5635(a) requires shareholder approval prior to the issuance of securities in connection with the acquisition of the stock or assets of another company in certain circumstances, including (1) where the common stock to be issued will have voting power equal to or in excess of 20% of the voting power outstanding before the issuance, or the number of shares to be issued will be equal to or in excess of 20% of the number of shares outstanding before the issuance; and (2) if any director, officer or substantial shareholder of the company has a 5% or greater interest (or such persons collectively have a 10% or greater interest), directly or indirectly, in the company or assets to be acquired or in the consideration to be paid, and the present or potential issuance of securities could result in an increase in outstanding common shares or voting power of 5% or more.
Rule 5635(c) requires shareholder approval of most equity compensation or purchase plans or arrangements and material amendments thereto (with a few limited exceptions), and this applies whether the securities issuable pursuant to such plan or arrangement are newly issued or bought over the open market.
Rule 5635(d) requires shareholder approval in order to enter into any transaction, other than a public offering, involving the sale, issuance or potential issuance of common shares (or securities convertible into or exercisable for common shares) equal to 20% or more of the outstanding share capital of a company or 20% or more of the voting power outstanding before the issuance for less than the greater of book or market value of the common shares.
In lieu of following Rule 5635(a), (c), and (d), the Company has elected to follow the applicable requirements of the BCBCA, which does not require shareholder approval for the issuance of securities or the approval of equity compensation plans.
Proxy Solicitations: Rule 5620(b) requires any listed company that is not a limited partnership to solicit proxies and provide proxy statements for all meetings of shareholders, and also provide copies of such proxy solicitation materials to Nasdaq. As a foreign private issuer, the Company’s equity securities are exempt from the proxy rules set forth in Sections 14(a), 14(b), 14(c) and 14(f) of the Exchange Act. The Company solicits proxies in accordance with applicable rules and regulations in Canada.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J – INSIDER TRADING POLICIES
We have adopted an insider trading policy, which governs the purchase, sale and other dispositions of our securities by our directors, officers and other employees. This policy is reasonably designed to promote compliance with applicable securities laws and regulations, including those that prohibit insider trading. A copy of our Insider Trading Policy is filed as an exhibit to this Annual Report on Form 20-F.
ITEM 16K – CYBERSECURITY
Risk Management and Strategy
As of the date of the filing of this Annual Report, the Company has information systems in place and has not suffered a “cybersecurity threat” (as defined in Item 106(a) of Regulation S-K) or “cybersecurity incident” (as defined in Item 106(a) of Regulation S-K). Moreover, the Company is aware of the evolution of cybersecurity risks and is taking proactive steps by keeping up to date our information systems and educating our personnel about these risks.
In order to mitigate these risks to a degree, the Company has an in-house IT Director and utilizes Software as a server (SaaS) to monitor and update the Company’s information systems.
The Company has implemented multiple measures to combat and reduce the risk of cybersecurity threats and cybersecurity incidents such as:
•
Engaging a IT Director in-house, who is available to respond immediately in the event of any cybersecurity threat or cybersecurity incident;
•
Developing internal System Use Policy and Information Security Policy reviewed by the CFO and IT Director enhancing the scrutiny of the emails received via a third-party security service provider to identify potential threats; and Implementing informal educational outreach programs including email reminders to educate staff about certain cybersecurity risks.
Governance
The IT Director monitors cybersecurity risks and potential incidents while following and periodically reviewing the System Use policy and Information Security policy recommending updates to the CFO where needed. The CEO advises the Board of any potential cybersecurity threat and the corresponding mitigation steps needed.
At the time of filing this Annual Report the Company does not have a subcommittee dedicated to cybersecurity. However, as the Company’s situation evolves, the Board will consider increased oversight to manage the risks from cybersecurity threats.
PART III
ITEM 17. FINANCIAL STATEMENTS
See Item 18 – Financial Statements.
ITEM 18. FINANCIAL STATEMENTS
The Consolidated Financial Statements and schedules appear on pages F-1 through F-36 of this Annual Report and are incorporated herein by reference. Our audited financial statements as prepared by our management and approved by the Board include:
ITEM 19. EXHIBITS
EXHIBIT INDEX
The following documents are being filed with the SEC as Exhibits to this Form 20-F:
Financial Statements
|
|
|
|
Description
|
|
Page |
|
|
|
Consolidated Financial Statements and Notes |
|
F-1- F-35 |
|
|
Exhibit |
|
No. Item |
Description of Exhibit |
1.1 |
Notice of Articles and Articles of Incorporation, (incorporated by reference to Exhibit 4.1 to the Company’s Form F-3 filed with the SEC on July 10, 2023) |
|
2.1
|
Description of securities registered under Section 12 of the Exchange Act |
2.2 |
Convertible Debenture between ImmunoPrecise Antibodies Ltd. and YA II PN, Ltd., dated July 16, 2024 (incorporated by reference to Exhibit 2.2 to the Company's 20-F filed with the SEC on July 29, 2024) |
4.1 |
Stock Option Plan (incorporated by reference to exhibit 99.1 of Form 6-K filed on April 21, 2023) |
4.2 |
Open Market Sales Agreement between ImmunoPrecise Antibodies Ltd. and Jefferies LLC, dated August 15, 2023 (incorporated by reference to Exhibit 10.1 to the Company’s Form 6-K filed with the SEC on August 15, 2023)
|
4.3 |
Underwriting Agreement between ImmunoPrecise Antibodies Ltd. and the Benchmark Company LLC, dated December 5, 2023 (incorporated by reference to Exhibit 10.1 to the Company’s Form 6-K filed with the SEC on December 5, 2023) |
4.4 |
Sales Agreement between ImmunoPrecise Antibodies Ltd. and Clear Street LLC, dated February 23, 2024 (incorporated by reference to Exhibit 10.1 to the Company’s Form 6-K filed with the SEC on February 23, 2024) |
4.5 |
Securities Purchase Agreement between ImmunoPrecise Antibodies Ltd. and YA II PN, Ltd., dated July 16, 2024 (incorporated by reference to Exhibit 4.5 to the Company's 20-F filed with the SEC on July 29, 2024) |
4.6 |
Registration Rights Agreement between ImmunoPrecise Antibodies Ltd. and YA II PN, Ltd., dated July 16, 2024 (incorporated by reference to Exhibit 4.6 to the Company's 20-F filed with the SEC on July 29, 2024) |
4.7 |
Global Guaranty Agreement between ImmunoPrecise Antibodies (Canada), Ltd., ImmunoPrecise Antibodies (Europe) BV, BioStrand B.V., and YA II PN, LTD., dated July 16, 2024 (incorporated by reference to Exhibit 4.7 to the Company's 20-F filed with the SEC on July 29, 2024) |
4.8 |
Material Transfer and Evaluation Agreement between ImmunoPrecise Antibodies Ltd. and Biotheus Inc., dated October 2, 2024 |
8.1 |
List of Subsidiaries of ImmunoPrecise Antibodies Ltd.
|
11.1 |
Insider Trading Policy
|
12.1 |
Certification of the Chief Executive Officer pursuant to rule 13a-14(a) of the Securities Exchange Act of 1934
|
12.2 |
Certification of the Chief Financial Officer pursuant to rule 13a-14(a) of the Securities Exchange Act of 1934
|
13.1 |
Certification of Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
13.2 |
Certification of Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
15.1 |
Consent of Grant Thornton LLP
|
|
16.1
|
Concurrence letter from Grant Thornton to IPA |
SIGNATURES
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
|
|
|
|
|
|
|
ImmunoPrecise Antibodies Ltd. |
|
|
|
|
|
Date: July 29, 2025 |
|
By: |
|
/s/ Jennifer Bath |
|
|
Name: |
|
Jennifer Bath |
|
|
Title: |
|
Chief Executive Officer |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
ImmunoPrecise Antibodies Ltd.
Opinion on the financial statements
We have audited the accompanying consolidated balance sheets of Immuno Precise Antibodies Ltd. (a British Columbia limited company) and subsidiaries (the “Company”) as of April 30, 2025 and 2024, the related consolidated statements of comprehensive loss, changes in shareholders’ equity, and cash flows for each of the three years in the period ended April 30, 2025, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of April 30, 2025 and 2024, and the results of its operations and its cash flows for each of the three years in the period ended April 30, 2025, in conformity with Financial Reporting Standards as issued by the International Standards Board.
Going concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has incurred net operating losses since its inception, including a $30.2 million net loss for the year ended April 30, 2025, and as of that date, the Company had $10.8 million in cash on hand. Further, the Company believes it will need additional capital to finance its operations and strategic goals. These conditions, along with other matters as set forth in Note 1, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ GRANT THORNTON LLP
We have served as the Company’s auditor since 2021.
Houston, Texas
July 29, 2025
IMMUNOPRECISE ANTIBODIES LTD.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
(Expressed in Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Note |
|
April 30,
2025
$ |
|
|
April 30,
2024
$ |
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets |
|
|
|
|
|
|
|
|
Cash |
|
16 |
|
|
10,665 |
|
|
|
3,459 |
|
Amounts receivable, net |
|
16 |
|
|
4,115 |
|
|
|
3,790 |
|
Tax receivable |
|
|
|
|
143 |
|
|
|
414 |
|
Inventory |
|
17 |
|
|
2,095 |
|
|
|
2,139 |
|
Unbilled revenue |
|
|
|
|
548 |
|
|
|
277 |
|
Prepaid expenses |
|
|
|
|
1,188 |
|
|
|
1,408 |
|
|
|
|
|
|
18,754 |
|
|
|
11,487 |
|
Restricted cash |
|
|
|
|
126 |
|
|
|
86 |
|
Deposit on equipment |
|
|
|
|
502 |
|
|
|
475 |
|
Property and equipment |
|
7, 11 |
|
|
15,762 |
|
|
|
16,696 |
|
Intangible assets |
|
8 |
|
|
1,067 |
|
|
|
23,557 |
|
Goodwill |
|
9 |
|
|
8,230 |
|
|
|
7,687 |
|
Total assets |
|
|
|
|
44,441 |
|
|
|
59,988 |
|
LIABILITIES |
|
|
|
|
|
|
|
|
Current liabilities |
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities |
|
14, 16 |
|
|
5,283 |
|
|
|
4,372 |
|
Deferred revenue |
|
|
|
|
1,090 |
|
|
|
1,352 |
|
Tax payable |
|
|
|
|
475 |
|
|
|
553 |
|
Leases |
|
11 |
|
|
1,850 |
|
|
|
1,563 |
|
Deferred acquisition payments |
|
6 |
|
|
314 |
|
|
|
284 |
|
|
|
|
|
|
9,012 |
|
|
|
8,124 |
|
Leases |
|
11 |
|
|
11,553 |
|
|
|
12,118 |
|
Deferred income tax liability |
|
22 |
|
|
250 |
|
|
|
4,068 |
|
Total liabilities |
|
|
|
|
20,815 |
|
|
|
24,310 |
|
SHAREHOLDERS' EQUITY |
|
|
|
|
|
|
|
|
Share capital |
|
12 |
|
|
136,371 |
|
|
|
119,773 |
|
Contributed surplus |
|
12 |
|
|
12,833 |
|
|
|
12,388 |
|
Accumulated other comprehensive income |
|
|
|
|
3,216 |
|
|
|
2,077 |
|
Accumulated deficit |
|
|
|
|
(128,794 |
) |
|
|
(98,560 |
) |
|
|
|
|
|
23,626 |
|
|
|
35,678 |
|
Total liabilities and shareholders’ equity |
|
|
|
|
44,441 |
|
|
|
59,988 |
|
Nature of operations (Note 1)
Approved and authorized on behalf of the Board of Directors on July 29, 2025
“Kamil Isaev” Director “Dirk Witters” Director
IMMUNOPRECISE ANTIBODIES LTD.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Expressed in Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended
April 30, |
|
|
Year ended
April 30, |
|
|
Year ended
April 30, |
|
(in thousands, except share data) |
|
Note |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
REVENUE |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
COST OF SALES |
|
|
|
|
10,972 |
|
|
|
12,465 |
|
|
|
9,102 |
|
GROSS PROFIT |
|
|
|
|
13,548 |
|
|
|
12,053 |
|
|
|
11,563 |
|
EXPENSES |
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
4,943 |
|
|
|
4,043 |
|
|
|
14,101 |
|
Sales and marketing |
|
|
|
|
4,298 |
|
|
|
3,543 |
|
|
|
3,608 |
|
General and administrative |
|
|
|
|
14,735 |
|
|
|
15,592 |
|
|
|
15,383 |
|
Impairment of Goodwill |
|
9 |
|
|
— |
|
|
|
11,161 |
|
|
|
2,460 |
|
Impairment of Intangible assets |
|
8 |
|
|
21,184 |
|
|
|
3,870 |
|
|
|
— |
|
Amortization of Intangible assets |
|
8 |
|
|
1,948 |
|
|
|
2,968 |
|
|
|
4,414 |
|
|
|
|
|
|
47,108 |
|
|
|
41,177 |
|
|
|
39,966 |
|
Loss before other income (expenses) and income taxes |
|
|
|
|
(33,560 |
) |
|
|
(29,124 |
) |
|
|
(28,403 |
) |
OTHER INCOME (EXPENSES) |
|
|
|
|
|
|
|
|
|
|
|
Accretion |
|
6, 10 |
|
|
(10 |
) |
|
|
(19 |
) |
|
|
(30 |
) |
Grant and subsidy income |
|
19 |
|
|
180 |
|
|
|
331 |
|
|
|
332 |
|
Interest and other (expense) income |
|
|
|
|
(283 |
) |
|
|
23 |
|
|
|
122 |
|
Unrealized foreign exchange (loss) gain |
|
|
|
|
(594 |
) |
|
|
86 |
|
|
|
227 |
|
|
|
|
|
|
(707 |
) |
|
|
421 |
|
|
|
651 |
|
Loss before income taxes |
|
|
|
|
(34,267 |
) |
|
|
(28,703 |
) |
|
|
(27,752 |
) |
Income taxes |
|
22 |
|
|
4,033 |
|
|
|
2,588 |
|
|
|
1,192 |
|
NET LOSS FOR THE PERIOD |
|
|
|
|
(30,234 |
) |
|
|
(26,115 |
) |
|
|
(26,560 |
) |
OTHER COMPREHENSIVE INCOME (LOSS) |
|
Items that will be reclassified subsequently to loss |
|
Exchange difference on translating foreign operations |
|
|
1,139 |
|
|
|
(613 |
) |
|
|
5,104 |
|
COMPREHENSIVE LOSS FOR THE PERIOD |
|
|
(29,095 |
) |
|
|
(26,728 |
) |
|
|
(21,456 |
) |
LOSS PER SHARE – BASIC AND DILUTED |
|
|
(0.91 |
) |
|
|
(1.02 |
) |
|
|
(1.07 |
) |
WEIGHTED AVERAGE NUMBER OF SHARES OUTSTANDING |
|
|
|
|
33,385,499 |
|
|
|
25,635,526 |
|
|
|
24,897,185 |
|
IMMUNOPRECISE ANTIBODIES LTD.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS' EQUITY
(Expressed in Canadian dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands, except share data) |
|
Number of
Shares |
|
|
Share Capital
$ |
|
|
Contributed
Surplus
$ |
|
|
Accumulated
Other
Comprehensive
(Loss) Income
$ |
|
|
Accumulated
Deficit
$ |
|
|
Total
$ |
|
Balance, April 30, 2023 |
|
|
25,050,260 |
|
|
|
117,470 |
|
|
|
10,796 |
|
|
|
2,690 |
|
|
|
(72,445 |
) |
|
|
58,511 |
|
Share-based expense |
|
|
— |
|
|
|
— |
|
|
|
1,535 |
|
|
|
— |
|
|
|
— |
|
|
|
1,535 |
|
Shares issued pursuant to issuance of common shares |
|
|
1,894,240 |
|
|
|
2,303 |
|
|
|
57 |
|
|
|
— |
|
|
|
— |
|
|
|
2,360 |
|
Comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(613 |
) |
|
|
(26,115 |
) |
|
|
(26,728 |
) |
Balance, April 30, 2024 |
|
|
26,944,500 |
|
|
|
119,773 |
|
|
|
12,388 |
|
|
|
2,077 |
|
|
|
(98,560 |
) |
|
|
35,678 |
|
Shares issued pursuant to conversion of convertible debentures |
|
|
5,893,768 |
|
|
|
4,370 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
4,370 |
|
Shares issued pursuant to ATM |
|
|
13,315,850 |
|
|
|
12,228 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
12,228 |
|
Share-based expense |
|
|
— |
|
|
|
— |
|
|
|
445 |
|
|
|
— |
|
|
|
— |
|
|
|
445 |
|
Comprehensive loss for the period |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,139 |
|
|
|
(30,234 |
) |
|
|
(29,095 |
) |
Balance, April 30, 2025 |
|
|
46,154,118 |
|
|
|
136,371 |
|
|
|
12,833 |
|
|
|
3,216 |
|
|
|
(128,794 |
) |
|
|
23,626 |
|
IMMUNOPRECISE ANTIBODIES LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Note |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
|
Net loss for the period |
|
|
|
|
(30,234 |
) |
|
|
(26,115 |
) |
|
|
(26,560 |
) |
Items not affecting cash: |
|
|
|
|
|
|
|
|
|
|
|
Accretion |
|
6, 10 |
|
|
10 |
|
|
|
19 |
|
|
|
30 |
|
Amortization and depreciation |
|
7, 8, 11, 20 |
|
|
5,119 |
|
|
|
5,735 |
|
|
|
6,685 |
|
Asset impairment |
|
11 |
|
|
21,184 |
|
|
|
15,031 |
|
|
|
2,460 |
|
Deferred income taxes |
|
22 |
|
|
(3,935 |
) |
|
|
(1,773 |
) |
|
|
(926 |
) |
Foreign exchange |
|
|
|
|
622 |
|
|
|
15 |
|
|
|
(146 |
) |
Gain on investment |
|
|
|
|
(7 |
) |
|
|
(2 |
) |
|
|
(43 |
) |
Share-based expense |
|
10, 11 |
|
|
445 |
|
|
|
1,535 |
|
|
|
1,943 |
|
|
|
|
|
|
(6,796 |
) |
|
|
(5,555 |
) |
|
|
(16,557 |
) |
Changes in non-cash working capital related to operations: |
|
|
|
|
|
|
|
|
|
|
|
Amounts receivable |
|
16 |
|
|
(298 |
) |
|
|
(601 |
) |
|
|
(561 |
) |
Inventory |
|
17 |
|
|
138 |
|
|
|
(102 |
) |
|
|
(185 |
) |
Unbilled revenue |
|
|
|
|
(248 |
) |
|
|
360 |
|
|
|
21 |
|
Prepaid expenses |
|
|
|
|
261 |
|
|
|
624 |
|
|
|
90 |
|
Accounts payable and accrued liabilities |
|
14, 16 |
|
|
827 |
|
|
|
983 |
|
|
|
(1,665 |
) |
Sales and income taxes payable and receivable |
|
|
|
|
8 |
|
|
|
733 |
|
|
|
(896 |
) |
Deferred revenue |
|
|
|
|
(302 |
) |
|
|
374 |
|
|
|
(80 |
) |
Net cash used in operating activities |
|
|
|
|
(6,410 |
) |
|
|
(3,184 |
) |
|
|
(19,833 |
) |
Investing activities: |
|
|
|
|
|
|
|
|
|
|
|
Purchase of equipment |
|
7 |
|
|
(799 |
) |
|
|
(1,397 |
) |
|
|
(1,495 |
) |
Security deposit on leases |
|
|
|
|
— |
|
|
|
(141 |
) |
|
|
40 |
|
Deferred acquisition payments |
|
6 |
|
|
— |
|
|
|
(146 |
) |
|
|
(592 |
) |
Sale of QVQ Holdings BV shares |
|
|
|
|
— |
|
|
|
121 |
|
|
|
80 |
|
Net cash used in investing activities |
|
|
|
|
(799 |
) |
|
|
(1,563 |
) |
|
|
(1,967 |
) |
Financing activities: |
|
|
|
|
|
|
|
|
|
|
|
Proceeds from share issuance, net of transaction costs |
|
12 |
|
|
12,228 |
|
|
|
2,360 |
|
|
|
716 |
|
Proceeds from debenture |
|
10 |
|
|
4,242 |
|
|
|
— |
|
|
|
— |
|
Repayment of principal on leases |
|
11 |
|
|
(1,577 |
) |
|
|
(1,339 |
) |
|
|
(1,337 |
) |
Net cash provided by (used in) financing activities |
|
|
|
|
14,893 |
|
|
|
1,021 |
|
|
|
(621 |
) |
Increase (decrease) in cash during the period |
|
|
|
|
7,684 |
|
|
|
(3,726 |
) |
|
|
(22,421 |
) |
Foreign exchange |
|
|
|
|
(438 |
) |
|
|
(1,095 |
) |
|
|
740 |
|
Cash – beginning of the period |
|
|
|
|
3,545 |
|
|
|
8,366 |
|
|
|
30,047 |
|
Cash – end of the period |
|
|
|
|
10,791 |
|
|
|
3,545 |
|
|
|
8,366 |
|
Cash is comprised of: |
|
|
|
|
|
|
|
|
|
|
|
Cash |
|
|
|
|
10,665 |
|
|
|
3,459 |
|
|
|
8,280 |
|
Restricted cash |
|
|
|
|
126 |
|
|
|
86 |
|
|
|
86 |
|
|
|
|
|
|
10,791 |
|
|
|
3,545 |
|
|
|
8,366 |
|
Cash paid for interest |
|
|
|
|
— |
|
|
|
— |
|
|
|
263 |
|
Cash paid for income tax |
|
|
|
|
2 |
|
|
|
— |
|
|
|
591 |
|
Supplemental cash flow information (Note 21)
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
ImmunoPrecise Antibodies Ltd. (the "Company" or “IPA”) was incorporated under the laws of Alberta on November 22, 1983. The Company is listed on the NASDAQ Capital Market (“Nasdaq”) under the trading ticker symbol “IPA.” The Company is a supplier of custom antibody discovery services. The address of the Company's corporate office is Industrious 823 Congress Ave Suite 300 Austin, Texas 78701.
Going concern basis
The consolidated financial statements have been prepared on the basis of accounting principles applicable to a going concern. The Company has incurred net losses since its inception, including $30.2 million for the year ended April 30, 2025, and has accumulated a deficit of $128.8 million as of April 30, 2025. The Company had $10.8 million cash on hand as of April 30, 2025. The Company expects its cash on hand as of April 30, 2025 will be insufficient to fund the Company’s operations for at least one year from the date these financial statements are available to be issued. These conditions raise material uncertainties which cast significant doubt as to whether the Company will be able to continue as a going concern should it not be able to obtain financing necessary to fund its planned revenue growth and working capital requirements.
The Company will need to raise additional funds to finance its operations and strategic goals and there can be no assurances that sufficient funding, including adequate financing, will be available. The ability of the Company to arrange additional financing in the future depends in part on the prevailing capital market conditions and profitability of its operations. If the Company is unable to raise sufficient funds, reductions in expenditures will be required, and this may impact the future growth plans of the Company.
Nasdaq Deficiency Notice
On August 19, 2024, the Company first received written notification (the "Notification Letter") from The Nasdaq Stock Market LLC indicating that the Company is not in compliance with the minimum bid price requirement set forth in the Nasdaq Rule 5450(a)(1) based on the closing bid price of the Common Shares of IPA (the “Common Shares”) being less than U.S.$1.00 per share for the 30 consecutive business days (the “Minimum Bid Requirement”) from July 5, 2024 to August 15, 2024. The Company was given a 180-day compliance period, or until February 17, 2025, to regain compliance with the Minimum Bid Requirement.
The Company did not regain compliance during the first 180-calendar-day compliance period. However, on February 20, 2025, the Company transferred its securities to the Nasdaq Capital Market and was granted an additional 180-day compliance period, or until August 18, 2025, to regain compliance with the Minimum Bid Requirement.
The Notification Letter is only a notification of deficiency, it is not a notice of imminent delisting, and it has no current immediate effect on the listing or trading of the Common Shares on Nasdaq. Nasdaq’s determination is based on the Company meeting the continued listing requirement for market value of publicly held shares and all other applicable requirements for initial listing on the Nasdaq Capital Market, with the exception of the Minimum Bid Requirement.
On July 13, 2025, Nasdaq notified the Company that it has determined that for the last 10 consecutive business days, from June 26, 2025 to July 10, 2025, the closing bid price of the Company’s common stock has been at U.S.$1.00 per share or greater. Accordingly, the Company has regained compliance with Listing Rule 5550(a)(2), and this matter is now closed.
(a)
Statement of compliance
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standards Board ("IASB"), and include the significant accounting policies as described in Note 3.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Certain items have been reclassified in the prior year financial statements to conform to the presentation and classification used in the current year. These reclassifications had no effect on the Company's consolidated operating results, financial position or cash flows.
These consolidated financial statements were approved by the Board of Directors.
These consolidated financial statements have been prepared on the historical cost basis. In addition, these consolidated financial statements have been prepared using the accrual basis of accounting, except for cashflow information.
(c)
Basis of consolidation
These consolidated financial statements include the financial statements of the Company and the following subsidiaries which are wholly owned and subject to control by the Company:
|
|
|
|
|
|
|
Name of Subsidiary |
|
% Equity
Interest -
April 30, 2025,
2024 and 2023 |
|
Country of
Incorporation |
|
Functional Currency |
ImmunoPrecise Antibodies (Canada) Ltd. |
|
100% |
|
Canada |
|
Canadian dollar |
ImmunoPrecise Antibodies (USA) Ltd. ("IPA USA") |
|
100% |
|
USA |
|
US dollar |
ImmunoPrecise Antibodies (N.D.) Ltd. |
|
100% |
|
USA |
|
US dollar |
ImmunoPrecise Antibodies (MA) LLC |
|
100% |
|
USA |
|
US dollar |
Talem Therapeutics LLC ("Talem") |
|
100% |
|
USA |
|
US dollar |
ImmunoPrecise Netherlands B.V. |
|
100% |
|
Netherlands |
|
Euro |
ImmunoPrecise Antibodies (Europe) B.V. ("IPA Europe") |
|
100% |
|
Netherlands |
|
Euro |
BioStrand B.V. |
|
100% |
|
Belgium |
|
Euro |
Idea Family B.V. |
|
100% |
|
Belgium |
|
Euro |
BioKey B.V. |
|
100% |
|
Belgium |
|
Euro |
BioClue B.V. |
|
100% |
|
Belgium |
|
Euro |
Control is achieved when the Company is exposed, or has rights, to variable returns from its involvement with an entity and has the ability to affect those returns through its power over the investee. Subsidiaries are fully consolidated from the date on which control is obtained and continue to be consolidated until the date that such control ceases. Intercompany balances, transactions and unrealized intercompany gains and losses are eliminated upon consolidation.
(d)
Functional and presentation currency
The functional currency of a company is the currency of the primary economic environment in which the company operates. The presentation currency for a company is the currency in which the company chooses to present its financial statements. The presentation and functional currency of the Company is the Canadian dollar.
Foreign currency translation
Entities whose functional currencies differ from the presentation currency are translated into Canadian dollars as follows: assets and liabilities – at the closing rate as at the reporting date, and income and expenses – at the average rate of the period. All resulting changes are recognized in other comprehensive income as cumulative translation differences.
Foreign currency transactions
Transactions in foreign currencies are translated into the functional currency at exchange rates at the date of the transactions. Foreign currency monetary assets and liabilities are translated at the functional currency exchange rate at the reporting date. Non-monetary items that are measured in terms of historical cost in a foreign currency are translated using exchange rates as at the dates of the initial transactions.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Non-monetary items measured at fair value in a foreign currency are translated using the exchange rates at the date when the fair value is determined. All gains and losses on translation of these foreign currency transactions are included in profit or loss.
When the Company disposes of its entire interest in a foreign operation, or loses control, joint control, or significant influence over a foreign operation, the foreign currency gains or losses accumulated in other comprehensive income related to the foreign operation are recognized in profit or loss. If an entity disposes of part of an interest in a foreign operation which remains a subsidiary, a proportionate amount of foreign currency gains or losses accumulated in other comprehensive income related to the subsidiary are reallocated between controlling and non-controlling interests.
(e)
Correction of immaterial error
We corrected an immaterial error related to fiscal years 2023 and 2024. The adjustment related to the correction of the recognition of a deferred tax asset and resulting offset with the deferred income tax liability for fiscal years 2023 and 2024. The error had the impact of overstating the deferred tax liability and overstating the net loss in fiscal 2023 and 2024. Management evaluated the effect of the adjustment on previously issued interim and annual consolidated financial statements in accordance with IFRS guidelines and concluded that it was immaterial to the interim and annual periods. As a result, in accordance with IFRS, we corrected the comparative periods in our Consolidated Statements of Financial Position and Comprehensive Loss as of April 30, 2025.
The effects of this adjustment on the comparative periods in our Consolidated Statements of Financial Position and Comprehensive Loss as of April 30, 2025 are as follows:
|
|
|
|
|
|
|
|
|
|
|
Previously reported |
|
Adjustments |
|
As adjusted |
|
Balance sheet items:
(in thousands) |
4/30/2024 |
|
4/30/2024 |
|
4/30/2024 |
|
Deferred income tax liability |
|
5,825 |
|
|
(1,757 |
) |
|
4,068 |
|
Total liabilities |
|
26,067 |
|
|
(1,757 |
) |
|
24,310 |
|
Accumulated deficit |
|
(100,265 |
) |
|
1,705 |
|
|
(98,560 |
) |
Accumulated other comprehensive income |
|
2,025 |
|
|
52 |
|
|
2,077 |
|
Total shareholders' equity |
|
33,921 |
|
|
1,757 |
|
|
35,678 |
|
|
|
|
|
|
|
|
|
|
|
|
Previously reported (year) |
|
Adjustments |
|
As adjusted |
|
Income statement items:
(in thousands) |
4/30/2024 |
|
4/30/2024 |
|
4/30/2024 |
|
Income taxes |
|
1,526 |
|
|
1,062 |
|
|
2,588 |
|
Net loss for the period |
|
(27,177 |
) |
|
1,062 |
|
|
(26,115 |
) |
Exchange difference on translating foreign operations |
|
(600 |
) |
|
(13 |
) |
|
(613 |
) |
Comprehensive loss for the period |
|
(27,777 |
) |
|
1,049 |
|
|
(26,728 |
) |
Basic and diluted loss per share* |
|
(1.06 |
) |
|
0.04 |
|
|
(1.02 |
) |
* Because of the net loss, basic and diluted loss per share are the same given potential dilutive common shares are excluded from the computation as their effect would be anti-dilutive.
3.
SIGNIFICANT ACCOUNTING POLICIES
Business combinations
Acquisitions of businesses are accounted for using the acquisition model. The consideration transferred in a business combination is measured at fair value, which is calculated as the sum of the acquisition-date fair values of the assets transferred by the Company, liabilities incurred by the Company to the former owners of the acquiree and the equity interests issued by the Company in exchange for control of the acquiree.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Acquisition-related costs are recognized in profit or loss as incurred.
At the acquisition date, the identifiable assets acquired, and the liabilities assumed are recognized at their fair value at the acquisition date. Goodwill is measured as the excess of the sum of the consideration transferred, the amount of any non-controlling interests in the acquiree, and the fair value of the acquirer's previously held equity interest in the acquiree (if any) over the net of the acquisition-date amounts of the identifiable assets acquired and the liabilities assumed. If, after reassessment, the net of the acquisition-date amounts of the identifiable assets acquired and liabilities assumed exceeds the sum of the consideration transferred, the amount of any non-controlling interests in the acquiree and the fair value of the acquirer's previously held interest in the acquiree (if any), the excess is recognized immediately in profit or loss as a bargain purchase gain.
When the consideration transferred by the Company in a business combination includes assets or liabilities resulting from a contingent consideration arrangement, the contingent consideration is measured at its acquisition-date fair value and included as part of the consideration transferred in a business combination. Changes in the fair value of the contingent consideration that qualify as measurement period adjustments are adjusted retrospectively, with corresponding adjustments against goodwill. Measurement period adjustments are adjustments that arise from additional information obtained during the ‘measurement period’ (which cannot exceed one year from the acquisition date) about facts and circumstances that existed at the acquisition date.
Revenue recognition
The Company recognizes revenue from sale of antibodies and service agreements.
Sale of antibodies:
Revenue from sale of antibodies is recognized when the terms of a contract with a customer have been satisfied. This occurs when:
•
The control over the product has been transferred to the customer; and
•
The product is received by the customer or transfer of title to the customer occurs upon shipment.
Following delivery, the customer bears the risks of obsolescence and loss in relation to the goods. Revenue is recognized based on the price specified in the contract, net of estimated sales discounts and returns.
Contract revenue:
Revenues from contracted services are generally recognized as the performance obligations are satisfied over time, and the related expenditures are incurred pursuant to the terms of the agreement. Contract revenue is recognized over time based on the input method, specifically the hours incurred. Revenue is recognized as the work progresses, in proportion to the amount of labor hours expended on the contract. For contracts with no enforceable right to payment when the contract is incomplete, contract revenue is recognized when the customers are satisfied with the service at the end of the contract and control of the product has been transferred to the customer. We apply the practical expedient outlined in IFRS 15.121, which allows us not to disclose information about remaining performance obligations as our contract duration is less than one year and we have the right to invoice for performance to date. The following table summarizes revenue recognized over time versus at a point in time for the years ended April 30:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Timing of recognition
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Point-in-time |
|
|
22,175 |
|
|
|
22,235 |
|
|
|
18,677 |
|
Over time |
|
|
2,345 |
|
|
|
2,283 |
|
|
|
1,988 |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
Unbilled revenue and deferred revenue:
Amounts recognized as revenue in excess of billings are classified as unbilled revenue. Amounts received in advance of the performance of services are classified as deferred revenue.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Cost of sales:
Cost of sales includes materials, direct labor, and allocation of overhead including depreciation of lab equipment.
Cash and Cash Equivalents
Cash and cash equivalents in the statement of financial position comprise cash at banks and on hand and short-term deposits with an original maturity of three months or less, which are subject to an insignificant risk of changes in value and are readily convertible to known amounts of cash.
Recognition and Measurement
Cash and cash equivalents are initially recognized at fair value and subsequently measured at amortized cost using the effective interest method, less any impairment losses. Due to the short-term nature of these instruments, the carrying amount is considered to be the same as their fair value.
Restricted Cash
Restricted cash is classified separately from cash and cash equivalents. It represents amounts that are held in trust or escrow accounts or that are otherwise restricted as to withdrawal or usage. The nature and purpose of restrictions on cash balances are disclosed in the notes to the financial statements. Restricted cash is not considered a component of cash and cash equivalents for the purpose of the statement of cash flows.
Financial instruments
Recognition and Classification
The Company recognizes a financial asset or financial liability on the statement of financial position when it becomes party to the contractual provisions of the financial instrument.
The Company classifies its financial instruments in the following categories: at fair value through profit and loss (“FVTPL”), at fair value through other comprehensive income (loss) (“FVTOCI”) or at amortized cost. The Company determines the classification of financial assets at initial recognition. The classification of debt instruments is driven by the Company’s business model for managing the financial assets and their contractual cash flow characteristics.
Equity instruments that are held for trading are classified as FVTPL. For other equity instruments, on the day of acquisition the Company can make an irrevocable election (on an instrument-by-instrument basis) to designate them as at FVTOCI. Financial liabilities are measured at amortized cost, unless they are required to be measured at FVTPL (such as instruments held for trading or derivatives) or if the Company has opted to measure them at FVTPL.
|
|
|
|
|
Classification and measurement IFRS 9 |
Cash |
|
Amortized cost |
Amounts receivable |
|
Amortized cost |
Investment |
|
FVTPL |
Accounts payable and accrued liabilities |
|
Amortized cost |
Convertible Debentures |
|
Amortized cost |
Deferred acquisition payments |
|
Amortized cost |
Measurement
Financial assets and liabilities at FVTPL:
Financial assets and liabilities carried at FVTPL are initially recorded at fair value and transaction costs are expensed in profit or loss. Realized and unrealized gains and losses arising from changes in the fair value of the financial assets and liabilities held at FVTPL are included in profit or loss in the period in which they arise. Where management has opted to recognize a financial liability at FVTPL, any changes associated with the Company’s own credit risk will be recognized in other comprehensive income (loss).
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Financial assets at FVTOCI:
Elected investments in equity instruments at FVTOCI are initially recognized at fair value plus transaction costs. Subsequently they are measured at fair value, with gains and losses recognized in other comprehensive income (loss).
Financial assets and liabilities at amortized cost:
Financial assets and liabilities at amortized cost are initially recognized at fair value plus or minus transaction costs, respectively, and subsequently carried at amortized cost less any impairment.
Impairment of financial assets at amortized cost:
The Company recognizes a loss allowance for expected credit losses on financial assets that are measured at amortized cost. At each reporting date, the Company measures the loss allowance for the financial asset at an amount equal to the lifetime expected credit losses if the credit risk on the financial asset has increased significantly since initial recognition. If at the reporting date, the financial asset has not increased significantly since initial recognition, the Company measures the loss allowance for the financial asset at an amount equal to the twelve month expected credit losses.
Irrespective of the preceding policy, the Company always measures the loss allowance of trade receivables at an amount equal to the lifetime expected credit losses.
The Company shall recognize in profit or loss, as an impairment gain or loss, the amount of expected credit losses (or reversal) that is required to adjust the loss allowance at the reporting date to the amount that is required to be recognized.
Derecognition
Financial assets:
The Company derecognizes financial assets only when the contractual rights to cash flows from the financial assets expire, or when it transfers the financial assets and substantially all of the associated risks and rewards of ownership to another entity. Gains and losses on derecognition are generally recognized in profit or loss. However, gains and losses on derecognition of financial assets classified as FVTOCI remain within accumulated other comprehensive income (loss).
Financial liabilities:
The Company derecognizes financial liabilities only when its obligations under the financial liabilities are discharged, cancelled or expired. Generally, the difference between the carrying amount of the financial liability derecognized and the consideration paid and payable, including any non-cash assets, is recognized in profit or loss.
Government assistance
The Company periodically applies for financial assistance under available government incentive programs. Government assistance relating to capital expenditures is reflected as a reduction of the cost of such assets. Government assistance relating to research and development expenditures is recorded as a reduction of current year's expenses when the related expenditures are incurred.
Government grant
The Company periodically applies for financial assistance under available government incentive programs. The grant is recognized when there is reasonable assurance that the Company will comply with the conditions attached to them and the grants will be received. All funds received as part of the grant or subsidies are reflected in grant and subsidy income.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Inventory consists of supplies, parts and antibodies and is valued at the lower of weighted average cost and net realizable value. Costs include acquisition, freight and other directly attributable costs.
Equipment and leasehold improvements
Equipment and leasehold improvements are stated at cost, less accumulated depreciation and impairment losses. Depreciation is provided using the straight-line method over the following terms:
|
|
|
|
|
Asset |
|
Basis |
|
Term |
Lab equipment |
|
Straight line |
|
5 years |
Furniture and equipment |
|
Straight line |
|
5 years |
Computer hardware |
|
Straight line |
|
2 years |
Computer software |
|
Straight line |
|
1 year |
Building |
|
Straight line |
|
Remaining term of the property lease |
Automobile |
|
Straight line |
|
Remaining term of the automobile lease |
Leasehold improvements |
|
Straight line |
|
Shorter of useful life and remaining term of the lease plus the first renewal option |
The Company evaluates equipment and leasehold improvements for indications of impairment at the end of each reporting period. Impairment losses are immediately recognized in profit and loss.
Intangible assets
Intangible assets acquired separately are measured on initial recognition at cost. The cost of intangible assets acquired in a business combination is their fair value at the date of acquisition. Following initial recognition, intangible assets are carried at cost less any accumulated amortization and accumulated impairment losses. Internally generated intangibles, excluding capitalized development costs, are not capitalized and the related expenditure is reflected in profit or loss in the period in which the expenditure is incurred.
The useful lives of intangible assets are assessed as either finite or indefinite.
Intangible assets with finite lives are amortized over the useful economic life and assessed for impairment whenever there is an indication that the intangible asset may be impaired. The amortization period and the amortization method for an intangible asset with a finite useful life are reviewed at least at the end of each reporting period. Changes in the expected useful life or the expected pattern of consumption of future economic benefits embodied in the asset are considered to modify the amortization period or method, as appropriate, and are treated as changes in accounting estimates. Amortization for intangible assets with finite lives is provided over the following terms:
|
|
|
|
|
Asset |
|
Basis |
|
Term |
Internally generated development costs |
|
Straight line |
|
5 years |
Intellectual property |
|
Straight line |
|
10 - 15 years |
Proprietary processes |
|
Straight line |
|
5 years |
Certifications |
|
Straight line |
|
1 year |
Customer list |
|
Straight line |
|
2 years |
Intangible assets with indefinite useful lives are not amortized, but are tested for impairment annually, either individually or at the cash-generating unit ("CGU") level. The assessment of indefinite life is reviewed annually to determine whether the indefinite life continues to be supportable. If not, the change in useful life from indefinite to finite is made on a prospective basis.
Gains or losses arising from derecognition of an intangible asset are measured as the difference between the net disposal proceeds and the carrying amount of the asset and are recognized in profit or loss when the asset is derecognized.
During the fiscal year ended April 30, 2024, the Company recorded an impairment of intangible assets charge of $3.9 million related to the BioStrand CGU. See Note 9 for more information.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
During the fiscal year 2025, the Company recorded an impairment loss of $21.2 million for the BioStrand CGU. The loss was recorded as a reduction in the intangible assets in BioStrand. The primary factor for the impairment included a delay in expected cash flows of BioStrand due to the strategic plans and expected use of BioStrand's assets. The increased discount rate relates to additional forecast risk for the BioStrand CGU, as compared to fiscal year ended April 30, 2024.
Goodwill
Goodwill represents the excess of the purchase price in a business combination over the fair value of net tangible and intangible assets acquired. Goodwill is not subject to amortization and an impairment test is performed annually or as events occur that could indicate impairment.
Goodwill is reported at cost less any impairment. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (“CGU”). To test for impairment, goodwill is allocated to each of the Company’s CGUs, groups of CGUs, or an operating segment expected to benefit from the acquisition. Goodwill is tested by combining the carrying amounts of equipment and leasehold improvements, intangible assets and goodwill and comparing this to the recoverable amount. Fair value less costs of disposal is price to be received in an orderly transaction between market participants. Value in use is assessed using the present value of the expected future cash flows. Any excess of the carrying amount over the recoverable amount is recorded as impairment. Impairment charges, which are not tax affected, are recognized in profit or loss and are not reversed.
During the fiscal year ended April 30, 2023, the Company recorded an impairment charge of $2.5 million related to the BioStrand CGU. During the year ended April 30, 2024, the Company recorded an impairment of goodwill charge of $11.2 million related to the BioStrand CGU. No impairment was recorded against goodwill for BioStrand for the year ended April 30, 2025. See Note 9 for more information.
Impairment of long-lived assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by comparison of their carrying amount to the recoverable amount. The recoverable amount is the higher of the fair value less costs of disposal or the value in use. Value in use is determined by the present value of the future cash flows from the asset. If the recoverable amount is less than the carrying amount, then there is impairment. Where an impairment loss exists, the portion of the carrying amount exceeding the recoverable amount is recorded as an expense immediately. Assets that have been impaired in prior periods are tested for possible reversal of impairment whenever events or changes in circumstance indicate that the impairment has reversed. If the impairment has reversed, the carrying amount of the asset is increased to its recoverable amount but not beyond the carrying amount that would have been determined had no impairment loss been recognized for the asset in prior periods. The reversal is recognized in profit or loss immediately.
During the year ended April 30, 2024, the Company recorded an impairment of intangible assets charge of $3.9 million and an impairment of goodwill charge of $11.2 million related to the BioStrand CGU. The impairment losses were determined based on fair value less costs of disposal, considering discount rates, growth rates, and other relevant factors. See Note 8 and Note 9 for more information.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Income taxes
Income taxes are recognized in the statement of comprehensive loss, except where they relate to items recognized directly in equity, in which case the related taxes are recognized in equity.
Deferred tax assets and liabilities are recognized based on the difference between the tax and accounting values of assets and liabilities and are calculated using enacted or substantively enacted tax rates for the periods in which the differences are expected to reverse. The effect of tax rate changes is recognized in profit or loss or equity, as applicable, in the period of substantive enactment. Current taxes receivable or payable are estimated on taxable income for the current year at the statutory tax rates enacted or substantively enacted.
Deferred tax assets are recognized only to the extent that it is probable that future taxable profits of the relevant entity or group of entities, in a particular jurisdiction, will be available against which the assets can be utilized. As an exception, deferred tax assets and liabilities are not recognized if the temporary differences arise from the initial recognition of goodwill or an asset or liability in a transaction (other than in a business combination) that affects neither accounting profit nor taxable profit.
Investment tax credits (“ITCs”) are accounted for as a reduction in the cost of the expense when there is reasonable assurance that such credits will be realized. These ITCs are used to reduce current income taxes payable.
Leases
At inception of a contract, the Company assesses whether the contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration.
The liabilities for leases of right-of-use assets are recognized at the lease commencement date at the present value of the lease payments that are not paid at that date. The lease payments are discounted using the Company’s incremental borrowing rate. At the commencement date, a right-of-use asset is measured at cost, which is comprised of the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any decommissioning and restoration costs, less any lease incentives received.
Each lease payment is allocated between repayment of the lease principal and interest. Interest on the lease liability in each period during the lease term is allocated to produce a constant periodic rate of interest on the remaining balance of the lease liability. Except where the costs are included in the carrying amount of another asset, the Company recognizes in profit or loss (a) the interest on a lease liability and (b) variable lease payments not included in the measurement of a lease liability in the period in which the event or condition that triggers those payments occurs. The Company subsequently measures the right-of-use asset at cost less any accumulated depreciation and any accumulated impairment losses; and adjusted for any remeasurement of the lease liability. Right-of-use assets are depreciated over the shorter of the asset’s useful life or the lease term, except where the lease contains a bargain purchase option a right-of-use asset is depreciated over the asset’s useful life.
Research and development
Research and development cost is charged to the income statement in the period in which it is incurred. Property, plant and equipment used for research and development is capitalized and depreciated in accordance with the equipment and leasehold improvements policy.
Share capital
Equity instruments are contracts that give a residual interest in the net assets of the Company. The Company's common shares are classified as equity instruments.
Proceeds from unit placements are allocated between common shares and warrants issued based on the residual value method, with the common shares being valued first.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Costs directly identifiable with the raising of share capital financing are charged against share capital. Share issuance costs incurred in advance of share subscriptions are recorded as deferred assets. Share issuance costs related to uncompleted share subscriptions are charged to operations.
Share-based payments
Where equity-settled share options are awarded to employees, the fair value of the options at the date of grant is charged to profit or loss over the vesting period.
Where the terms and conditions of options are modified before they vest, the increase in the fair value of the options, measured immediately before and after the modification, is also charged to profit or loss over the remaining vesting period.
Where equity instruments are granted to non-employees, they are recorded at the fair value of the goods or services received in profit or loss, unless they are related to the issuance of shares. Amounts related to the issuance of shares are recorded as a reduction of share capital.
When the value of goods or services received in exchange for the share-based payment cannot be reliably estimated, the fair value is measured by use of a valuation model. The expected life used in the model is adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions, and behavioral considerations.
All equity-settled share-based payments are reflected in contributed surplus, until exercised. Upon exercise, shares are issued from treasury and the amount reflected in contributed surplus is credited to share capital, adjusted for any consideration paid.
Where a grant of options is cancelled or settled during the vesting period, excluding forfeitures when vesting conditions are not satisfied, the Company immediately accounts for the cancellation as an acceleration of vesting and recognizes the amount that otherwise would have been recognized for services received over the remainder of the vesting period. Any payment made to the employee on the cancellation is accounted for as the repurchase of an equity interest except to the extent the payment exceeds the fair value of the equity instrument granted, measured at the repurchase date. Any such excess is recognized as an expense.
Earnings (loss) per share
Basic earnings (loss) per share is calculated by dividing the net income (loss) available to common shareholders by the weighted average number of common shares outstanding during the period. Dilutive earnings per share reflect the potential dilution of securities that could share in the earnings of an entity. In periods where a net loss is incurred, potentially dilutive common shares are excluded from the loss per share calculation as the effect would be anti-dilutive and basic and diluted loss per common share is the same. In a profit year, under the treasury stock method, the weighted average number of common shares outstanding used for the calculation of diluted earnings per share assumes that the proceeds to be received on the exercise of dilutive stock options and warrants are used to repurchase common shares at the average price during the year.
4.
ADOPTION OF NEW ACCOUNTING STANDARDS
Standards adopted
Classification of Liabilities as Current or Non-Current (Amendments to IAS 1)
The amendments to IAS 1 provide a more general approach to the classification of liabilities based on the contractual arrangements in place at the reporting date.
These amendments are effective for reporting periods beginning on or after January 1, 2024, which is our fiscal year ending April 30, 2025. We adopted these amendments in our first fiscal quarter ending July 31, 2024 with no impact noted to our classification of liabilities.
Standards not yet adopted
IFRS 18
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The new requirements introduced in IFRS 18 will help to achieve comparability of the financial performance of similar entities, especially related to how ‘operating profit or loss’ is defined. The new disclosures required for some management-defined performance measures will also enhance transparency. The Company does not expect IFRS 18 to have a material impact on the Company's financial statements.
These amendments are effective for reporting periods beginning on or after January 1, 2027.
5.
CRITICAL ACCOUNTING ESTIMATES AND JUDGMENTS
The preparation of the consolidated financial statements in conformity with IFRS required estimates and judgments that affect the amounts reported in the financial statements. Actual results could differ from these estimates and judgments. Estimates are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the year in which the estimate is revised.
Judgments
Business combinations
Acquisitions of a business are accounted for as a business combination if the assets acquired and liabilities assumed constitute a business in accordance with IFRS 3. Judgment is required to determine if the transaction meets the definition of a business combination.
During the year ended April 30, 2022, the Company acquired all the issued and outstanding shares of Idea Family BV, BioStrand BV, BioKey BV, and BioClue BV (collectively “BioStrand”), as detailed in Note 6. Management concluded that BioStrand met the definition of a business and accounted for the transaction as a business combination.
The acquisition of BioStrand includes potential future earn-out payments dependent on the future profitability of the business. Judgment was required to determine whether the payments constitute an exchange for the business or are transactions separate from the business combination. The potential future earn-out payments to the selling shareholders of BioStrand will be accounted for separate from the business combination (see Note 18).
Impairments
For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows called a cash-generating unit (“CGU"). Management applies judgment to determine CGUs. Each asset or CGU is evaluated every reporting period to determine whether there are any indicators of impairment. If any such indicators exist, which is often judgment based, a formal estimate of recoverable amount is performed and an impairment charge is recognized to the extent that the carrying amount exceeds the recoverable amount.
The Company performs a goodwill impairment test annually and when circumstances indicate that the carrying value may not be recoverable. For the purposes of impairment testing, goodwill acquired through business combinations was allocated to three different CGUs, the Company’s Oss and Utrecht locations at IPA Europe, and BioStrand. The goodwill allocated to Oss and Utrecht was $3.3 million and $4.9 million, respectively, as of April 30, 2025. The goodwill allocated to Oss and Utrecht and BioStrand was $3.1 million, $4.6 million, respectively, as of April 30, 2024. The goodwill allocated to BioStrand was fully impaired as of April 30, 2024 and April 30, 2025 (see Note 9).
Estimates
Business combinations
At acquisition date, the identifiable assets acquired and liabilities assumed in a business combination are recognized at their fair value. Goodwill is measured as the excess of the consideration transferred over the net of the acquisition-date fair values of the identifiable assets acquired and liabilities assumed. Estimates are required to determine the fair value of assets acquired and liabilities assumed, and estimated fair values may vary from prices that would be achieved in an arm’s length transaction at the acquisition date (see Note 6).
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Impairment of long-lived assets
The recoverable amount of an asset or CGU of assets is measured at the higher of fair value less costs of disposal or value in use. These determinations and their individual assumptions require that management make a decision based on the best available information at each reporting period. The estimates and assumptions are subject to risk and uncertainty; hence, there is the possibility that changes in circumstances will alter these projections, which may impact the recoverable amount of the assets. In such circumstances, some or all of the carrying value of the assets may be further impaired or the impairment charge reversed with the impact recorded in profit or loss.
The recoverable amount of each CGU was based on value in use, determined by discounting the future cash flows to be generated from the continuing use of the CGU. The cash flows were projected over a five-year period for the Company's Utrecht and Oss locations, and a seven-year period for BioStrand. The projections are based on past experience and actual operating results.
The Company performed its annual goodwill impairment test for April 2025 and no CGUs were identified as requiring impairment. The values assigned to the key assumptions represented management’s assessment of future trends in the industry and were based on historical data from both internal and external sources. See Note 9 for details on the weighted average cost of capital used in the assessments of the three CGUs.
Useful life of intangible assets
Intangible assets are amortized based on estimated useful life less their estimated residual value. Significant assumptions are involved in the determination of useful life and residual values and no assurance can be given that the actual useful lives and residual values will not differ significantly from current assumptions. Actual useful life and residual values may vary depending on a number of factors including internal technical evaluation, attributes of the assets and experience with similar assets. Changes to these estimates may affect the carrying value of assets, net income (loss) and comprehensive income (loss) in future periods (see Note 8).
Share-based payments
Where equity-settled share options are awarded to employees, the fair value of the options at the date of grant is charged to profit and loss over the vesting period. The Company makes assumptions to determine the estimated forfeiture rate of the share options, and these estimates are reviewed at the end of each reporting period. Changes to these estimates may affect contributed surplus and net income (loss) (see Note 12).
6.
ACQUISITION OF BIOSTRAND
On April 13, 2022, the Company acquired all the issued and outstanding shares of BioStrand B.V., BioKey B.V., BioClue B.V. and Idea Family B.V. (collectively “BioStrand”) on terms as follows:
€2.7 million (CAD $3.7 million) was paid in cash on closing;
4,077,774 common shares of the Company were issued on closing;
Deferred cash payment of €0.5 million (CAD $0.7 million) to be paid 90 days subsequent to closing; and
Deferred cash payment of €0.5 million (CAD $0.6 million) to be paid over 3 years on the anniversary of the closing date.
BioStrand focuses on technology in the field of bioinformatics and biotechnology related to the identification of characteristic biological sequences in proteins, RNA and DNA, and their different information layers, the development of a knowledge base containing these characteristic biological sequences and information layers, and the use of this database to process biological sequences and compare processed biological sequences. The acquisition provides the Company with advanced omics capabilities to enhance its antibody discovery processes and offer multi-omics data analysis to its clients.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The transaction was accounted for as a business combination, as the operations of BioStrand meet the definition of a business. As the transaction was accounted for as a business combination, legal and consulting costs of $0.7 million and $0.1 million, respectively, were expensed during the year ended April 30, 2022. The goodwill resulting from the allocation of the purchase price to the total fair value of net assets will represent the sales growth potential and assembled workforce of BioStrand.
During the three months ended July 31, 2022, the Company recorded the right-of-use assets and lease liabilities in connection with building and vehicle leases at BioStrand. During the three months ended October 31, 2022, the Company adjusted goodwill upon the finalization of the deferred cash payment paid 90 days subsequent to closing. Both adjustments occurred during the measurement period and were applied retrospectively. The Company has allocated the purchase price as follows:
|
|
|
|
|
(in thousands) |
|
$ |
|
Cash |
|
|
4,985 |
|
Common shares of the Company |
|
|
29,126 |
|
Fair value of consideration |
|
|
34,111 |
|
|
|
|
|
Cash |
|
|
36 |
|
Amounts receivable |
|
|
80 |
|
Unbilled revenue |
|
|
8 |
|
Equipment and right-of-use assets |
|
|
247 |
|
Intellectual property (not deductible for tax purposes) |
|
|
28,459 |
|
Proprietary processes (not deductible for tax purposes) |
|
|
391 |
|
Goodwill (not deductible for tax purposes) |
|
|
12,658 |
|
Accounts payable and accrued liabilities assumed |
|
|
(342 |
) |
Deferred revenue |
|
|
9 |
|
Leases |
|
|
(223 |
) |
Deferred income tax liability |
|
|
(7,212 |
) |
|
|
|
34,111 |
|
The intellectual property assets are primarily comprised of acquired technology assets that are expected to have a useful life of 15 years.
The fair value of the 4,077,774 common shares issued ($29.1 million) was determined based on the Canadian dollar equivalent of the consideration required of €21.3 million pursuant to the share purchase agreement using the closing stock price at` the date of the acquisition. The common shares are subject to an escrow agreement and will be released to the vendors on the following schedule: 15% one year after closing, 20% two years after closing, and 65% three years after closing.
The deferred cash payments of €1.0 million was fair valued on the date of acquisition using a discounted cash flow model. The changes in the value of the subsequent payments during the years ended April 30, 2025, 2024 and 2023 are as follows:
|
|
|
|
|
(in thousands) |
|
$ |
|
Balance, April 30, 2023 |
|
|
717 |
|
Foreign exchange |
|
|
(11 |
) |
Accretion |
|
|
19 |
|
Working capital adjustment |
|
|
(294 |
) |
Deferred acquisition payment |
|
|
(146 |
) |
Balance, April 30, 2024 |
|
|
285 |
|
Foreign exchange |
|
|
19 |
|
Accretion |
|
|
10 |
|
Balance, April 30, 2025 |
|
|
314 |
|
Less: Current portion |
|
|
(314 |
) |
Non-current portion |
|
|
— |
|
The share purchase agreement related to the acquisition of BioStrand includes contingent earnout payments (see Note 18).
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The table below includes both property and equipment and right-of-use assets.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
Computer
Hardware
$ |
|
Furniture &
Equipment
$ |
|
Computer
Software
$ |
|
Building
$ |
|
Automobile
$ |
|
Leasehold
Improvements
$ |
|
Lab
Equipment
$ |
|
WIP - Leasehold
Improvements
$ |
|
Total
$ |
|
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
288 |
|
|
53 |
|
|
50 |
|
|
9,085 |
|
|
167 |
|
|
626 |
|
|
6,473 |
|
|
— |
|
|
16,742 |
|
Additions |
|
56 |
|
|
— |
|
|
— |
|
|
7,826 |
|
|
1 |
|
|
27 |
|
|
1,316 |
|
|
31 |
|
|
9,257 |
|
Disposals |
|
(111 |
) |
|
(31 |
) |
|
(49 |
) |
|
(1,634 |
) |
|
— |
|
|
(344 |
) |
|
(2,554 |
) |
|
— |
|
|
(4,723 |
) |
Foreign exchange |
|
(4 |
) |
|
(1 |
) |
|
(1 |
) |
|
(133 |
) |
|
(3 |
) |
|
(2 |
) |
|
(92 |
) |
|
— |
|
|
(236 |
) |
Balance, April 30, 2024 |
|
229 |
|
|
21 |
|
|
— |
|
|
15,144 |
|
|
165 |
|
|
307 |
|
|
5,143 |
|
|
31 |
|
|
21,040 |
|
Additions |
|
12 |
|
|
22 |
|
|
— |
|
|
210 |
|
|
207 |
|
|
20 |
|
|
812 |
|
|
79 |
|
|
1,362 |
|
Disposals |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(99 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(99 |
) |
Foreign exchange |
|
40 |
|
|
2 |
|
|
— |
|
|
820 |
|
|
18 |
|
|
9 |
|
|
300 |
|
|
— |
|
|
1,189 |
|
Balance, April 30, 2025 |
|
281 |
|
|
45 |
|
|
— |
|
|
16,174 |
|
|
291 |
|
|
336 |
|
|
6,255 |
|
|
110 |
|
|
23,492 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Depreciation: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
157 |
|
|
33 |
|
|
50 |
|
|
1,752 |
|
|
57 |
|
|
388 |
|
|
3,913 |
|
|
— |
|
|
6,350 |
|
Depreciation |
|
101 |
|
|
4 |
|
|
— |
|
|
1,723 |
|
|
56 |
|
|
58 |
|
|
849 |
|
|
— |
|
|
2,791 |
|
Disposals |
|
(110 |
) |
|
(31 |
) |
|
(49 |
) |
|
(1,606 |
) |
|
— |
|
|
(344 |
) |
|
(2,554 |
) |
|
— |
|
|
(4,694 |
) |
Foreign exchange |
|
(2 |
) |
|
— |
|
|
(1 |
) |
|
(38 |
) |
|
(1 |
) |
|
— |
|
|
(61 |
) |
|
— |
|
|
(103 |
) |
Balance, April 30, 2024 |
|
146 |
|
|
6 |
|
|
— |
|
|
1,831 |
|
|
112 |
|
|
102 |
|
|
2,147 |
|
|
— |
|
|
4,344 |
|
Depreciation |
|
66 |
|
|
8 |
|
|
— |
|
|
1,922 |
|
|
67 |
|
|
65 |
|
|
788 |
|
|
— |
|
|
2,916 |
|
Disposals |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(99 |
) |
|
— |
|
|
— |
|
|
— |
|
|
(99 |
) |
Foreign exchange |
|
11 |
|
|
— |
|
|
— |
|
|
158 |
|
|
7 |
|
|
3 |
|
|
390 |
|
|
— |
|
|
569 |
|
Balance, April 30, 2025 |
|
223 |
|
|
14 |
|
|
— |
|
|
3,911 |
|
|
87 |
|
|
170 |
|
|
3,325 |
|
|
— |
|
|
7,730 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Book Value: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
April 30, 2024 |
|
83 |
|
|
15 |
|
|
— |
|
|
13,313 |
|
|
53 |
|
|
205 |
|
|
2,996 |
|
|
31 |
|
|
16,696 |
|
April 30, 2025 |
|
58 |
|
|
31 |
|
|
— |
|
|
12,263 |
|
|
204 |
|
|
166 |
|
|
2,930 |
|
|
110 |
|
|
15,762 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Changes in the value of the intangible assets during the years ended April 30, 2025, 2024 and 2023 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Internally Generated
Development Costs
$ |
|
|
Intellectual
Property
$ |
|
|
Proprietary
Processes
$ |
|
|
Certifications
$ |
|
|
Customer List
$ |
|
|
Total
$ |
|
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
|
33 |
|
|
|
35,143 |
|
|
|
8,103 |
|
|
|
139 |
|
|
|
198 |
|
|
|
43,616 |
|
Impairments and disposals |
|
|
— |
|
|
|
(3,830 |
) |
|
|
(40 |
) |
|
|
— |
|
|
|
(193 |
) |
|
|
(4,063 |
) |
Foreign exchange |
|
|
— |
|
|
|
(595 |
) |
|
|
(136 |
) |
|
|
(3 |
) |
|
|
(5 |
) |
|
|
(739 |
) |
Balance, April 30, 2024 |
|
|
33 |
|
|
|
30,718 |
|
|
|
7,927 |
|
|
|
136 |
|
|
|
— |
|
|
|
38,814 |
|
Impairments and disposals |
|
|
— |
|
|
|
(21,184 |
) |
|
|
(156 |
) |
|
|
— |
|
|
|
— |
|
|
|
(21,340 |
) |
Foreign exchange |
|
|
— |
|
|
|
1,435 |
|
|
|
552 |
|
|
|
10 |
|
|
|
— |
|
|
|
1,997 |
|
Balance, April 30, 2025 |
|
|
33 |
|
|
|
10,969 |
|
|
|
8,323 |
|
|
|
146 |
|
|
|
— |
|
|
|
19,471 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Amortization: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
|
14 |
|
|
|
4,775 |
|
|
|
7,633 |
|
|
|
137 |
|
|
|
132 |
|
|
|
12,691 |
|
Amortization |
|
|
19 |
|
|
|
2,666 |
|
|
|
216 |
|
|
|
2 |
|
|
|
65 |
|
|
|
2,968 |
|
Disposals |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(193 |
) |
|
|
(193 |
) |
Foreign exchange |
|
|
— |
|
|
|
(75 |
) |
|
|
(127 |
) |
|
|
(3 |
) |
|
|
(4 |
) |
|
|
(209 |
) |
Balance, April 30, 2024 |
|
|
33 |
|
|
|
7,366 |
|
|
|
7,722 |
|
|
|
136 |
|
|
|
— |
|
|
|
15,257 |
|
Amortization |
|
|
— |
|
|
|
1,895 |
|
|
|
53 |
|
|
|
— |
|
|
|
— |
|
|
|
1,948 |
|
Foreign exchange |
|
|
— |
|
|
|
641 |
|
|
|
548 |
|
|
|
10 |
|
|
|
— |
|
|
|
1,199 |
|
Balance, April 30, 2025 |
|
|
33 |
|
|
|
9,902 |
|
|
|
8,323 |
|
|
|
146 |
|
|
|
— |
|
|
|
18,404 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Book Value: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
April 30, 2024 |
|
|
— |
|
|
|
23,352 |
|
|
|
205 |
|
|
|
— |
|
|
|
— |
|
|
|
23,557 |
|
April 30, 2025 |
|
|
— |
|
|
|
1,067 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,067 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
During the years ended April 30, 2024, the Company recorded an impairment loss of $3.9 million on impairment of intangible assets for the BioStrand CGU. The loss was recorded as a reduction in goodwill and the intangible assets. The primary factor for the impairment included a rise in the discount rate as compared to the prior year, along with a delay in expected cash flows in the forecast. The increased discount rate relates to increases in the forecast risk for the BioStrand CGU, increased economic risk, and increased global interest rates as compared to the prior year.
During the year ended April 30, 2025, the Company recorded an impairment loss of $21.2 million for the BioStrand CGU. The loss was recorded as a reduction in the intangible assets in BioStrand. The primary factor for the impairment included a delay in expected cash flows of BioStrand due to the strategic plans and expected use of BioStrand's assets. The increased discount rate relates to additional forecast risk for the BioStrand CGU, as compared to period ended April 30, 2024.
The goodwill was acquired as a result of the acquisitions of U-Protein, IPA Europe and BioStrand. The changes in the value of goodwill during the fiscal years ended April 30, 2025and 2024 are as follows:
|
|
|
|
|
(in thousands) |
|
$ |
|
Balance, April 30, 2023 |
|
|
19,171 |
|
Foreign exchange |
|
|
(323 |
) |
Asset impairment |
|
|
11,161 |
|
Balance, April 30, 2024 |
|
|
7,687 |
|
Foreign exchange |
|
|
543 |
|
Balance, April 30, 2025 |
|
|
8,230 |
|
Impairment testing
For annual impairment testing, goodwill is allocated to the following cash-generating units ("CGU"):
|
|
|
|
|
|
|
|
|
(in thousands) |
|
April 30,
2025
$ |
|
|
April 30,
2024
$ |
|
Oss |
|
|
3,272 |
|
|
|
3,056 |
|
Utrecht |
|
|
4,958 |
|
|
|
4,631 |
|
|
|
|
8,230 |
|
|
|
7,687 |
|
The recoverable amount of each CGU was based on value-in-use calculations and determined using a five-year forecast for Oss and Utrecht, followed by a terminal growth rate determined by management. The present value of the forecasted cash flows of each CGU is determined by applying a discount rate reflecting a current market assessment of the time value of money and risks specific to the CGU. The recoverable amount, growth rate assumptions and discount rates for each CGU as of April 30, 2025, 2024 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Recoverable amount |
|
Terminal growth rates |
|
Discount rates |
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
|
2025
$ |
|
|
2024
$ |
|
|
|
2025
$ |
|
|
2024
$ |
|
|
Oss |
|
|
7,039 |
|
|
|
6,723 |
|
|
|
|
2.0 |
% |
|
|
2.5 |
% |
|
|
|
22.0 |
% |
|
|
22.0 |
% |
|
Utrecht |
|
|
11,422 |
|
|
|
12,737 |
|
|
|
|
2.5 |
% |
|
|
2.5 |
% |
|
|
|
19.0 |
% |
|
|
19.0 |
% |
|
BioStrand |
|
|
— |
|
|
|
14,611 |
|
|
|
|
— |
|
|
|
2.5 |
% |
|
|
|
— |
|
|
|
45.0 |
% |
|
The terminal growth rates consider the average GDP growth rate of the Netherlands and Belgium. The discount rates reflect management’s assessment of market and specific risk of the CGU. Both the Oss and Utrecht CGUs operate in the same region and are included in the same operating segment of the Company. The cash flow forecasts include a key management assumption that future profit margins will remain stable and is based on previous performance of the CGU. The assumption for future profit margins is based on management’s review of the prior three years of performance of the CGU.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
During the year ended April 30, 2023, the Company recorded an impairment loss of $2.5 million for the BioStrand CGU. The loss was recorded as a reduction in goodwill. The primary factor for the impairment included a rise in the discount rate as compared to the prior year, along with a delay in expected cash flows in the forecast. The increased discount rate relates to increases in the forecast risk for the BioStrand CGU, increased economic risk, and increased global interest rates as compared to the prior year.
During the year ended April 30, 2024, the Company recorded an impairment loss of $11.2 million on impairment of goodwill. The loss was recorded as a reduction in goodwill and the intangible assets. The primary factor for the impairment included a rise in the discount rate as compared to the prior year, along with a delay in expected cash flows in the forecast. The increased discount rate relates to increases in the forecast risk for the BioStrand CGU, increased economic risk, and increased global interest rates as compared to the prior year.
10.
CONVERTIBLE DEBENTURES
On July 16, 2024 YA II PN, Ltd., an investment fund managed by Yorkville Advisors Global, LP (“Yorkville”), entered into a securities purchase agreement (the "Securities Purchase Agreement") under which the Company agreed to sell and issue to Yorkville U.S.$3.0 million aggregate principal amount of convertible debentures (the “Convertible Debentures”) in two tranches and at a purchase price of 95% of the aggregate principal amount.
The Convertible Debentures were convertible into Common Shares. The sale and issue of the first tranche consisted of U.S.$2.0 million principal amount of Convertible Debentures and was completed on July 16, 2024 with a maturity date of July 16, 2025. The sale and issue of the second tranche consisted of U.S.$1.0 million principal amount of Convertible Debentures and was completed on August 16, 2024, with a maturity date of July 16, 2025.
In connection with the offering, the Company and Yorkville entered into a customary registration rights agreement pursuant to which the Company agreed to provide certain registration rights to Yorkville under the U.S. Securities Act of 1933.
During the year ended April 30, 2025, the Company completed the complete conversions of both tranches.
The Company has leases for lab and office space, automobiles and one item of lab equipment. Each lease is reflected in the consolidated statement of financial position as a right-of-use asset and a lease liability. The Company classifies right-of-use assets in a consistent manner to its property and equipment. The following is a schedule of the Company’s future minimum lease payments related to the equipment and automobiles under finance lease and the office lease obligation:
|
|
|
|
|
(in thousands) |
|
$ |
|
2026 |
|
|
2,695 |
|
2027 |
|
|
2,692 |
|
2028 |
|
|
2,685 |
|
2029 |
|
|
2,366 |
|
2030 |
|
|
1,682 |
|
More than 5 years |
|
|
4,514 |
|
Total minimum lease payments |
|
|
16,634 |
|
Less: imputed interest |
|
|
(3,231 |
) |
Total present value of minimum lease payments |
|
|
13,403 |
|
Less: Current portion |
|
|
(1,850 |
) |
Non-current portion |
|
|
11,553 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Total cash outflow for leases during the year ended April 30, 2025 was $1.6 million (2024 - $1.3 and 2023 - $1.3 million).
The nature of the Company’s leases by type of right-of-use asset as of April 30, 2025 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Right-of-use asset type |
|
No. of
right-of-
use
assets
leased |
|
|
Range
of
remaining
term |
|
Average
remaining
lease term |
|
No. of
leases
with
extension
options |
|
|
No. of
leases
with
options to
purchase |
|
|
No. of
leases
with
variable
payments
linked to
an index |
|
|
No. of
leases
with
termination
options |
|
Lab and office facilities |
|
|
3 |
|
|
3.7 - 8.7 years |
|
6.6 years |
|
|
1 |
|
|
|
— |
|
|
|
3 |
|
|
|
3 |
|
Automobiles |
|
|
4 |
|
|
1.7 - 4.5 years |
|
3.7 years |
|
|
— |
|
|
|
— |
|
|
|
4 |
|
|
|
4 |
|
Lab equipment |
|
|
1 |
|
|
4.8 years |
|
4.8 years |
|
|
— |
|
|
|
1 |
|
|
|
1 |
|
|
|
1 |
|
Right-of-use assets
The Company reviews long-lived assets with finite useful lives for impairment whenever circumstances indicate that the carrying amount of the asset may not be recoverable.
During the year ended April 30, 2023, the Company recorded a right-of-use asset of $7.4 million upon commencement of a new lease at the Utrecht, the Netherlands location. The lease includes an initial term of five years, and a renewal option for an additional five years. The Company has determined that it is reasonably certain to exercise the renewal option.
During the year ended April 30, 2024, the Company recorded a right-of-use asset of $3.7 million upon commencement of a new lease at the Oss, the Netherlands location. The lease includes an initial term of five years, and a renewal option. The Company has determined that it is reasonably certain to exercise the renewal option.
During the year ended April 30, 2024, the Company recorded a right-of-use asset of $3.5 million upon commencement of a new lease at the Victoria, the Canadian location. The lease includes an initial term of ten years.
During the year ended April 30, 2024, the Company recorded a right-of-use asset of $3.5 million upon commencement of a new lease at the Victoria, the Canadian location. The lease includes an initial term of ten years.
During the year ended April 30, 2025, the Company recorded a right-of-use asset of $70 thousand upon commencement of a new car lease at the BioStrand, the Belgium location. The lease includes an initial term of five years.
During the year ended April 30, 2025, the Company recorded a right-of-use asset of $70 thousand upon commencement of a new car lease at the BioStrand, the Belgium location. The lease includes an initial term of five years.
During the year ended April 30, 2025, the Company recorded a right-of-use asset of $67 thousand upon commencement of a new car lease at the BioStrand, the Belgium location. The lease includes an initial term of five years.
During the year ended April 30, 2025, the Company recorded a right-of-use asset of $0.6 million upon commencement of a new lab equipment lease at the Victoria, the Canadian location. The lease includes an initial term of five years.
During the year ended April 30, 2025, the Company recorded a right-of-use asset of $0.2 million upon an adjustment to the lease at the Utrecht, the Netherlands location.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The changes in the value of right-of-use assets during the years ended April 30, 2025 and 2024 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
Building
$ |
|
|
Automobile
$ |
|
|
Lab Equipment
$ |
|
|
Total
$ |
|
Cost: |
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
|
9,085 |
|
|
|
167 |
|
|
|
— |
|
|
|
9,252 |
|
Additions |
|
|
7,826 |
|
|
|
1 |
|
|
|
— |
|
|
|
7,827 |
|
Disposals |
|
|
(1,634 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,634 |
) |
Foreign exchange |
|
|
(133 |
) |
|
|
(3 |
) |
|
|
— |
|
|
|
(136 |
) |
Balance, April 30, 2024 |
|
|
15,144 |
|
|
|
165 |
|
|
|
— |
|
|
|
15,309 |
|
Additions |
|
|
210 |
|
|
|
207 |
|
|
|
578 |
|
|
|
995 |
|
Disposals |
|
|
— |
|
|
|
(99 |
) |
|
|
— |
|
|
|
(99 |
) |
Foreign exchange |
|
|
820 |
|
|
|
18 |
|
|
|
— |
|
|
|
838 |
|
Balance, April 30, 2025 |
|
|
16,174 |
|
|
|
291 |
|
|
|
578 |
|
|
|
17,043 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Depreciation: |
|
|
|
|
|
|
|
|
|
|
|
|
Balance, April 30, 2023 |
|
|
1,752 |
|
|
|
57 |
|
|
|
— |
|
|
|
1,809 |
|
Depreciation |
|
|
1,723 |
|
|
|
56 |
|
|
|
— |
|
|
|
1,779 |
|
Disposals |
|
|
(1,606 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1,606 |
) |
Foreign exchange |
|
|
(38 |
) |
|
|
(1 |
) |
|
|
— |
|
|
|
(39 |
) |
Balance, April 30, 2024 |
|
|
1,831 |
|
|
|
112 |
|
|
|
— |
|
|
|
1,943 |
|
Depreciation |
|
|
1,922 |
|
|
|
67 |
|
|
|
13 |
|
|
|
2,002 |
|
Disposals |
|
|
— |
|
|
|
(99 |
) |
|
|
— |
|
|
|
(99 |
) |
Foreign exchange |
|
|
158 |
|
|
|
7 |
|
|
|
— |
|
|
|
165 |
|
Balance, April 30, 2025 |
|
|
3,911 |
|
|
|
87 |
|
|
|
13 |
|
|
|
4,011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Book Value: |
|
|
|
|
|
|
|
|
|
|
|
|
April 30, 2024 |
|
|
13,313 |
|
|
|
53 |
|
|
|
— |
|
|
|
13,366 |
|
April 30, 2025 |
|
|
12,263 |
|
|
|
204 |
|
|
|
565 |
|
|
|
13,032 |
|
Lease payments not recognized as a liability
The Company has elected not to recognize a lease liability for leases with an expected term of 12 months or less. Additionally, certain variable lease payments are not permitted to be recognized as lease liabilities and are recognized in profit and loss as incurred. The expense relating to payments not included in the measurement of the lease liability during the years ended April 30, 2025, 2024 and 2023 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Leases of low value assets |
|
|
21 |
|
|
|
7 |
|
|
|
40 |
|
Variable lease payments |
|
|
567 |
|
|
|
467 |
|
|
|
215 |
|
|
|
|
588 |
|
|
|
474 |
|
|
|
255 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Unlimited common shares without par value.
b)
Share capital transactions:
2023 Transactions
During the year ended April 30, 2023, the Company issued 263,537 common shares pursuant to the exercise of stock options for total gross proceeds of $0.7 million. A value of $0.8 million was transferred from contributed surplus to share capital as a result. The weighted average share price at the dates the stock options were exercised was U.S.$4.50.
During the year ended April 30, 2023, the Company issued 309,877 common shares with a value of $1.3 million pursuant to the conversion of $1.3 million principal balance of convertible debentures.
2024 Transactions
During the year ended April 30, 2024, the Company issued 1,265,000 common shares in an underwritten public offering, including 165,000 common shares issued pursuant to the full exercise by the underwriter of its over-allotment option. The public offering price for each common share, before the underwriter's discount and commissions, was U.S.$1.00.
During the year ended April 30, 2024, the Company established an at-the-market equity offering facility ("ATM Facility") with Clear Street LLC, replacing its previous ATM Facility with Jefferies LLC, which was terminated on February 1, 2024. An Open Market Sales Agreement ("ATM Agreement") was entered into with Clear Street LLC, as sole sales agent ("Agent") on February 23, 2024. The Company is entitled, at its discretion and from time-to-time during the term of the ATM Agreement, to sell, through the Agent common shares of the Company. On February 23, 2024, in connection with the ATM facility, the Company filed a prospectus supplement permitting the sales of common shares having an aggregate gross sales price of up to US$60.0 million. Sales of the common shares will be made in transactions that are deemed to be "at-the-market distributions" as defined in Rule 415(a)(4) of the United Securities Act of 1933, as amended, including, without limitation, sales made directly on Nasdaq or any other existing trading market for the common shares in the United States. Common shares will only be sold on the facilities of an exchange or market outside Canada to purchasers who the Company has no reason to believe are resident in Canada and, in all others cases, to purchasers who are not located or resident in Canada. The Company will determine, at its sole discretion, the date, minimum price and maximum number of common shares to be sold under the ATM Facility. The common shares will be distributed from time to time in negotiated transactions, at market prices prevailing at the time of sale, at prices relating to such prevailing market prices, and/or in any other manner permitted by applicable law. As such, the prices may vary between purchasers over time. The Company is not required to sell any common shares at any time during the term of the ATM Facility. In fiscal 2024, 629,240 common shares were sold under the ATM with proceeds net of commissions of $1.8 million.
2025 Transaction
During the year ended April 30, 2025, the Company issued 13,315,850 Common Shares under the ATM Facility with proceeds net of commissions of $12.2 million.
During the year ended April 30, 2025, the Company issued 5,893,768 common shares with a value of U.S.$3.0 million pursuant to the conversion of U.S.$3.0 million principal balance of convertible debentures.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The following table summarizes stock option awards during the years ended April 30, 2025, 2024 and 2023, including the fair value determined using the Black-Scholes option pricing model:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
grant date |
|
Stock
options
granted |
|
|
Exercisable price/option
$ |
|
|
Awarded to |
|
Share price
on grant
date
$ |
|
Dividend yield |
|
|
Expected volatility |
|
|
Risk-free rate |
|
|
Expected life |
|
Fair value |
May 15, 2022(2) |
|
|
80,000 |
|
|
|
5.79 |
|
|
Employees |
|
3.79 |
|
|
0 |
% |
|
|
77 |
% |
|
|
2.73 |
% |
|
5.0 years |
|
$0.3 million |
February 19, 2023(1) |
|
|
29,060 |
|
|
4.10(7) |
|
|
Directors |
|
4.10(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.57 |
% |
|
4.0 years |
|
$0.1 million |
February 19, 2023(2) |
|
|
609,452 |
|
|
4.10(7) |
|
|
Officers and employees |
|
4.10(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.57 |
% |
|
5.0 years |
|
$2.2 million |
January 19, 2024(6) |
|
|
240,000 |
|
|
1.48(7) |
|
|
Directors |
|
1.48(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.64 |
% |
|
5.0 years |
|
$0.4 million |
January 4, 2023(6) |
|
|
8,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$12 thousand |
January 23, 2023(6) |
|
|
8,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$12 thousand |
March 1, 2023(6) |
|
|
8,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$12 thousand |
March 15, 2023(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
April 2, 2023(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
May 8, 2023(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
May 23, 2023(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
June 11, 2023(6) |
|
|
8,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$12 thousand |
August 8, 2023(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
November 13, 2023(6) |
|
|
8,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$12 thousand |
January 1, 2024(6) |
|
|
12,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$18 thousand |
February 1, 2024(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
February 19, 2024(6) |
|
|
12,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$18 thousand |
February 20, 2024(6) |
|
|
4,000 |
|
|
1.47(7) |
|
|
Employees |
|
1.47(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$6 thousand |
August 3, 2024(6) |
|
|
799,767 |
|
|
0.86(7) |
|
|
Officers and employees |
|
0.86(7) |
|
|
0 |
% |
|
|
77 |
% |
|
|
3.68 |
% |
|
10 years |
|
$0.7 million |
(1)
Vesting conditions are as follows: one-quarter 3 months after grant date; one-quarter 6 months after grant date; one-quarter 9 months after grant date; and one-quarter 12 months after grant date.
(2)
Vesting conditions are as follows: one-third 6 months after grant date; one-third 12 months after grant date; and one-third 18 months after grant date.
(3)
Vesting conditions are as follows: one-third one year after grant date; one-third two years after grand date; and one-third three years after grant date.
(4)
Vesting conditions are as follows: one-third 2 months after grant date; one-third 4 months after grant date; and one-third 6 months after grant date.
(5)
Vesting conditions are as follows: one-half 3 months after grant date; one-half 6 months after grant date.
(6)
Vesting conditions are as follows: one-fourth one year from hire date; one thirty-sixth each month after hire date.
Expected volatility of options granted is based on the historical volatility of the company from January 1, 2019 to the option grant date.
During the year ended April 30, 2025, the Company has recorded $0.4 million (2024 - $1.5 and 2023 - $1.9 million) of share-based payments expense.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The changes in the stock options for the years ended April 30, 2025, 2024 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
options
# |
|
|
Weighted
average
exercise price
$ |
|
|
Weighted
average life
remaining
(years) |
|
Balance, April 30, 2023 (outstanding) |
|
|
1,884,428 |
|
|
|
8.03 |
|
|
|
3.27 |
|
Granted |
|
|
332,000 |
|
|
|
2.02 |
|
|
|
— |
|
Expired |
|
|
(577,335 |
) |
|
|
7.15 |
|
|
|
— |
|
Forfeited |
|
|
(117,726 |
) |
|
|
4.15 |
|
|
|
— |
|
Balance, April 30, 2024 (outstanding) |
|
|
1,521,367 |
|
|
|
7.17 |
|
|
|
3.47 |
|
Granted |
|
|
799,767 |
|
|
|
1.22 |
|
|
|
— |
|
Expired |
|
|
(159,021 |
) |
|
|
3.80 |
|
|
|
— |
|
Forfeited |
|
|
(234,188 |
) |
|
|
1.10 |
|
|
|
— |
|
Balance, April 30, 2025 (outstanding) |
|
|
1,927,925 |
|
|
|
5.69 |
|
|
|
4.45 |
|
Unvested |
|
|
(661,194 |
) |
|
|
1.30 |
|
|
|
9.02 |
|
Exercisable, April 30, 2025 |
|
|
1,266,731 |
|
|
|
7.98 |
|
|
|
2.06 |
|
Details of the options outstanding as at April 30, 2025 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Expiry Date |
|
Exercise
price $ |
|
|
Remaining
life (year) |
|
|
Options
outstanding |
|
|
Unvested |
|
|
Exercisable |
|
September 1, 2025 |
|
|
8.50 |
|
|
|
0.34 |
|
|
|
220,000 |
|
|
|
— |
|
|
|
220,000 |
|
January 6, 2026 |
|
|
20.30 |
|
|
|
0.69 |
|
|
|
138,000 |
|
|
|
— |
|
|
|
138,000 |
|
January 2, 2026 |
|
|
6.89 |
|
|
|
0.68 |
|
|
|
5,650 |
|
|
|
- |
|
|
|
5,650 |
|
January 7, 2027 |
|
|
7.94 |
|
|
|
1.69 |
|
|
|
235,000 |
|
|
|
— |
|
|
|
235,000 |
|
January 13, 2027 |
|
|
8.30 |
|
|
|
1.71 |
|
|
|
16,000 |
|
|
|
- |
|
|
|
16,000 |
|
May 15, 2027 |
|
|
5.79 |
|
|
|
2.04 |
|
|
|
64,000 |
|
|
|
- |
|
|
|
64,000 |
|
February 19, 2027(2) |
|
|
5.81 |
|
|
|
1.81 |
|
|
|
7,265 |
|
|
|
- |
|
|
|
7,265 |
|
February 19, 2028(2) |
|
|
5.81 |
|
|
|
2.81 |
|
|
|
475,452 |
|
|
|
- |
|
|
|
475,452 |
|
January 19, 2034(3) |
|
|
2.10 |
|
|
|
3.73 |
|
|
|
95,558 |
|
|
|
21,111 |
|
|
|
74,447 |
|
January 4, 2033(4) |
|
|
2.08 |
|
|
|
7.69 |
|
|
|
8,000 |
|
|
|
3,667 |
|
|
|
4,333 |
|
January 23, 2033(4) |
|
|
2.08 |
|
|
|
7.74 |
|
|
|
8,000 |
|
|
|
3,667 |
|
|
|
4,333 |
|
March 1, 2033(4) |
|
|
2.08 |
|
|
|
7.84 |
|
|
|
8,000 |
|
|
|
4,000 |
|
|
|
4,000 |
|
April 2, 2033(4) |
|
|
2.08 |
|
|
|
7.93 |
|
|
|
4,000 |
|
|
|
2,083 |
|
|
|
1,917 |
|
May 8, 2033(4) |
|
|
2.08 |
|
|
|
8.03 |
|
|
|
4,000 |
|
|
|
2,167 |
|
|
|
1,833 |
|
June 11, 2033(4) |
|
|
2.08 |
|
|
|
8.12 |
|
|
|
8,000 |
|
|
|
4,500 |
|
|
|
3,500 |
|
August 8, 2033(4) |
|
|
2.08 |
|
|
|
8.28 |
|
|
|
4,000 |
|
|
|
2,417 |
|
|
|
1,583 |
|
November 13, 2033(4) |
|
|
2.08 |
|
|
|
8.55 |
|
|
|
8,000 |
|
|
|
5,333 |
|
|
|
2,667 |
|
January 1, 2034(4) |
|
|
2.08 |
|
|
|
8.68 |
|
|
|
12,000 |
|
|
|
8,500 |
|
|
|
3,500 |
|
February 1, 2034(4) |
|
|
2.08 |
|
|
|
8.76 |
|
|
|
4,000 |
|
|
|
2,917 |
|
|
|
1,083 |
|
February 19, 2034(4) |
|
|
2.08 |
|
|
|
8.81 |
|
|
|
8,000 |
|
|
|
5,833 |
|
|
|
2,167 |
|
August 2, 2034(4) |
|
|
1.22 |
|
|
|
9.26 |
|
|
|
595,000 |
|
|
|
595,000 |
|
|
|
— |
|
|
|
|
5.69 |
|
|
|
4.45 |
|
|
|
1,927,925 |
|
|
|
661,194 |
|
|
|
1,266,731 |
|
(1)
Exercise price of U.S.$7.72. The figure in the table above is translated at the April 30, 2025 rate.
(2)
Exercise price of U.S.$4.10. The figure in the table above is translated at the April 30, 2025 rate.
(3)
Exercise price of U.S.$1.48. The figure in the table above is translated at the April 30, 2025 rate.
(4)
Exercise price of U.S.$1.47. The figure in the table above is translated at the April 30, 2025 rate.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The changes in the finder’s warrants for the years ended April 30, 2025, 2024 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
warrants
# |
|
|
Weighted average
exercise price
$ |
|
|
Weighted average life
remaining (years) |
|
Balance, April 30, 2023 |
|
|
130,111 |
|
|
|
22.77 |
|
|
|
2.77 |
|
Issued |
|
|
56,650 |
|
|
|
1.37 |
|
|
|
4.61 |
|
Balance, April 30, 2024 |
|
|
186,761 |
|
|
|
16.44 |
|
|
|
2.62 |
|
Balance, April 30, 2025 |
|
|
186,761 |
|
|
|
17.02 |
|
|
|
1.62 |
|
Details of the finder’s warrants outstanding as at April 30, 2025 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Expiry Date |
|
Exercise price
$ |
|
|
Remaining life
(year) |
|
|
Warrants
outstanding |
|
February 3, 2026(1) |
|
|
23.81 |
|
|
|
0.76 |
|
|
|
130,111 |
|
December 8, 2028(2) |
|
|
1.42 |
|
|
|
3.61 |
|
|
|
56,650 |
|
(1)
Exercise price of U.S.$16.81. The figure in the table above is translated at the April 30, 2025 rate.
(2)
Exercise price of U.S.$1.00. The figure in the table above is translated at the April 30, 2025 rate.
The following table summarizes the activity related to the Company's RSUs for the year ended April 30, 2025. For purposes of this table, vested RSUs represent the shares for which the service condition had been fulfilled as of April 30, 2025:
|
|
|
|
|
|
|
|
|
|
|
Number of
Restricted Stock Units
# |
|
|
Weighted
average
grant date fair value
$ |
|
Balance, April 30, 2024 |
|
|
— |
|
|
|
— |
|
Granted |
|
|
46,000 |
|
|
|
0.42 |
|
Balance, April 30, 2025 (outstanding) |
|
|
46,000 |
|
|
|
0.42 |
|
Unvested |
|
|
(39,771 |
) |
|
|
0.42 |
|
Vested and outstanding, April 30, 2025 |
|
|
6,229 |
|
|
|
0.42 |
|
Expenses recognized for employee benefits for the years ended April 30, 2025, 2024 and 2023 are detailed below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Wages, salaries |
|
|
10,069 |
|
|
|
10,733 |
|
|
|
10,433 |
|
Employee benefits |
|
|
947 |
|
|
|
938 |
|
|
|
926 |
|
Payroll taxes |
|
|
772 |
|
|
|
774 |
|
|
|
939 |
|
Severance |
|
|
— |
|
|
|
60 |
|
|
|
194 |
|
Share-based expense |
|
|
445 |
|
|
|
1,535 |
|
|
|
1,943 |
|
|
|
|
12,233 |
|
|
|
14,040 |
|
|
|
14,435 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
14.
RELATED PARTY TRANSACTIONS
Key management personnel are those persons having authority and responsibility for planning, directing and controlling the activities of the Company. Key management consists of Dr. Jennifer Bath, President and CEO; Joseph Scheffler, Interim CFO; Kristin Taylor, former CFO; Brad McConn, former CFO; Dr. Stefan Lang, former Chief Business Officer; Dr. Ilse Roodink, Chief Scientific Officer; Lisa Helbling, former Director and Chief Financial Officer, Dr. Barry Duplantis, former Vice President of Client Relations; Dr. Yasmina Abdiche, former Chief Scientific Officer; and Directors of the Company. During the years ended April 30, 2025, 2024 and 2023, the compensation for key management is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Salaries and other short-term benefits |
|
|
3,828 |
|
|
|
2,454 |
|
|
|
2,632 |
|
Severance (included in salaries) |
|
|
— |
|
|
|
60 |
|
|
|
183 |
|
Share-based expense |
|
|
386 |
|
|
|
928 |
|
|
|
986 |
|
Director compensation (included in salaries) |
|
|
275 |
|
|
|
343 |
|
|
|
335 |
|
|
|
|
4,489 |
|
|
|
3,785 |
|
|
|
4,136 |
|
At April 30, 2025, included in accounts payable and accrued liabilities is nil (April 30, 2024 - $0.3 million and 2023 - $0.9 million) due to related parties. The amounts payable are non-interest bearing and unsecured.
These transactions are in the normal course of operations and are measured at the exchange amount, which is the amount of consideration established and agreed to by the related parties, unless otherwise noted.
The Company’s objectives when managing capital are to ensure sufficient liquidity for operations and adequate funding for growth and capital expenditures while maintaining an efficient balance between debt and equity. As of April 30, 2025 the capital structure of the Company consists of shareholders’ equity of $23.6 million.
The Company makes adjustments to its capital structure upon approval from its Board of Directors, in light of economic conditions and the Company’s working capital requirements. There were no changes in the Company’s approach to capital management during the year. The Company is not subject to any externally imposed capital requirements.
The Company’s financial instruments include cash, amounts receivable, restricted cash, investment, accounts payable and accrued liabilities, debentures, loans payable, leases and deferred acquisition payments.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy establishes three levels to classify the inputs to valuation techniques used to measure fair value, by reference to the reliability of the inputs used to estimate the fair values.
Level 1 – applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2 – applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3 – applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The fair value of investment is determined based on “Level 2” inputs as its value under the equity method was the best approximation of its fair value. As at April 30, 2025, the Company believes the carrying values of cash, amounts receivable, restricted cash, accounts payable and accrued liabilities, leases and deferred acquisition payments approximate their fair values because of their nature and relatively short maturity dates or durations.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Concentration of risk:
Concentrations of credit risk
Credit risk relates to cash, restricted cash and amounts receivable and arises from the possibility that counterparty to an instrument may fail to perform. At April 30, 2025, all of the Company’s cash was held with tier one banks. Details of amounts receivable and allowance for credit losses as of April 30, 2025, 2024 and 2023 are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Amounts receivable |
|
|
4,165 |
|
|
|
3,819 |
|
|
|
3,280 |
|
Allowance for credit losses |
|
|
(50 |
) |
|
|
(29 |
) |
|
|
(33 |
) |
Amounts receivable, net |
|
|
4,115 |
|
|
|
3,790 |
|
|
|
3,247 |
|
Currency risk
The Company operates in the US and Europe which gives rise to exposure to market risks from changes in foreign currency values. Most significantly, the Company is exposed to potential currency fluctuations between US and Canadian dollars, which was translated at 1.3812 at April 30, 2025, and the Euro and Canadian dollar, which was translated at 1.5687 at April 30, 2025. Fluctuations in the exchange rate could impact profitability.
At April 30, 2025, the Company is exposed to currency risk through the following assets and liabilities denominated in US dollars and Euros:
|
|
|
|
|
|
|
|
|
|
|
Euros |
|
|
US Dollars |
|
(in thousands) |
|
(€) |
|
|
(U.S.$) |
|
Cash |
|
|
1,237 |
|
|
|
5,207 |
|
Amounts receivable |
|
|
1,993 |
|
|
|
732 |
|
|
|
|
3,230 |
|
|
|
5,939 |
|
|
|
|
|
|
|
|
Accounts payable and accrued liabilities |
|
|
(1,819 |
) |
|
|
(1,872 |
) |
Deferred acquisition payments |
|
|
(193 |
) |
|
|
— |
|
Leases |
|
|
(6,315 |
) |
|
|
— |
|
|
|
|
(8,327 |
) |
|
|
(1,872 |
) |
|
|
|
|
|
|
|
Net |
|
|
(5,097 |
) |
|
|
4,067 |
|
Liquidity risk
The Company’s approach to managing its obligations is to maintain sufficient resources to meet its obligations when due without undue risk to the Company. The Company monitors its cash requirements on an ongoing basis to ensure that there are sufficient resources for operations as well as to fund anticipated leasing, capital and development expenditures. In addition, the Company manages its cash to meet its obligations and to fund general and administrative costs.
Contractual cash flow requirements as of April 30, 2025 were as follows:
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
< 1
year |
|
|
1 - 2
years |
|
|
2 - 5
years |
|
|
>5
years |
|
|
Total |
|
(in thousands) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
Accounts payable and accrued liabilities |
|
|
5,283 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5,283 |
|
Leases |
|
|
2,695 |
|
|
|
2,692 |
|
|
|
6,733 |
|
|
|
4,514 |
|
|
|
16,634 |
|
Total |
|
|
7,978 |
|
|
|
2,692 |
|
|
|
6,733 |
|
|
|
4,514 |
|
|
|
21,917 |
|
Inventories as of April 30, 2025 and 2024 consist of the following:
|
|
|
|
|
|
|
|
|
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
Supplies and parts |
|
|
1,714 |
|
|
|
1,734 |
|
Antibodies |
|
|
194 |
|
|
|
190 |
|
Work in process |
|
|
187 |
|
|
|
215 |
|
|
|
|
2,095 |
|
|
|
2,139 |
|
For the years ended April 30, 2025, and 2024, inventory write-offs amounted to nil. These write-offs were primarily due to obsolescence and changes in market conditions affecting the net realizable value of the inventory.
The share purchase agreement related to the acquisition of BioStrand includes contingent earnout payments based on 20% of the EBITDA of BioStrand, as defined in the share purchase agreement, over a 7-year period, which shall not exceed in total €12.0 million. The Company has determined these payments relate to post-acquisition services because they are contingent on the employment of two key employees and will be expensed in the period earned. As of April 30, 2025, the Company's unpaid maximum commitment related to the BioStrand earnout is €12.0 million.
19.
GRANT AND SUBSIDY INCOME
During May 2022, the Company received a €0.5 million round of grant funding from VLAIO (Flanders Innovation & Entrepreneurship), the research fund of the Flemish regional government in Belgium. The Company received the first disbursement of €0.2 million during the three months ended July 31, 2022. During the year ended April 30, 2025, the Company recorded €0.1 million in grant income related to this funding compared to the €0.2 million recorded in year ended April 30, 2024.
20.
SEGMENTED INFORMATION AND ECONOMIC DEPENDENCE
At April 30, 2025, 2024 and 2023, the Company has one reportable segment, being antibody production and related services.
The Company’s revenues are allocated to geographic regions for the year ended April 30, 2025, 2024 and 2023 as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Revenue by Region
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
United States of America |
|
|
12,614 |
|
|
|
12,556 |
|
|
|
9,365 |
|
Europe |
|
|
10,178 |
|
|
|
10,867 |
|
|
|
9,450 |
|
Canada |
|
|
234 |
|
|
|
389 |
|
|
|
618 |
|
Australia |
|
|
896 |
|
|
|
482 |
|
|
|
630 |
|
Other |
|
|
598 |
|
|
|
224 |
|
|
|
602 |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
The Company’s revenues are allocated according to revenue types for the year ended April 30, 2025, 2024 and 2023 as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Revenue Allocation
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
Project revenue |
|
|
22,175 |
|
|
|
22,235 |
|
|
|
18,677 |
|
Product sales revenue |
|
|
2,107 |
|
|
|
2,035 |
|
|
|
1,747 |
|
Cryostorage revenue |
|
|
238 |
|
|
|
248 |
|
|
|
241 |
|
|
|
|
24,520 |
|
|
|
24,518 |
|
|
|
20,665 |
|
As of April 30, 2025, all deferred revenue is expected to be recognized over the next twelve months.
The Company’s non-current assets are allocated to geographic regions as of April 30, 2025, 2024 and 2023 as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current Assets
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
North America - Corporate |
|
|
80 |
|
|
|
80 |
|
|
|
89 |
|
North America |
|
|
4,167 |
|
|
|
4,138 |
|
|
|
1,025 |
|
Belgium |
|
|
268 |
|
|
|
22,261 |
|
|
|
40,406 |
|
Netherlands |
|
|
21,172 |
|
|
|
22,022 |
|
|
|
19,501 |
|
|
|
|
25,687 |
|
|
|
48,501 |
|
|
|
61,021 |
|
Geographic segmentation of the Company’s net income (loss) for the year ended April 30, 2025, 2024 and 2023 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Net Income (Loss) by Region
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
North America - Corporate |
|
|
(8,142 |
) |
|
|
(7,846 |
) |
|
|
(8,422 |
) |
North America |
|
|
699 |
|
|
|
(449 |
) |
|
|
(12,601 |
) |
Belgium |
|
|
(23,908 |
) |
|
|
(19,009 |
) |
|
|
(7,024 |
) |
Netherlands |
|
|
1,117 |
|
|
|
1,189 |
|
|
|
1,487 |
|
|
|
|
(30,234 |
) |
|
|
(26,115 |
) |
|
|
(26,560 |
) |
Geographic segmentation of the interest and accretion, and amortization and depreciation for the year ended April 30, 2025, 2024 and 2023 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Interest and accretion
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
North America - Corporate |
|
|
39 |
|
|
|
4 |
|
|
|
39 |
|
North America |
|
|
223 |
|
|
|
231 |
|
|
|
19 |
|
Belgium |
|
|
— |
|
|
|
— |
|
|
|
20 |
|
Netherlands |
|
|
697 |
|
|
|
619 |
|
|
|
318 |
|
|
|
|
959 |
|
|
|
854 |
|
|
|
396 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years ended
April 30, |
|
Amortization and depreciation
(in thousands) |
|
2025
$ |
|
|
2024
$ |
|
|
2023
$ |
|
North America - Corporate |
|
|
5 |
|
|
|
11 |
|
|
|
14 |
|
North America |
|
|
672 |
|
|
|
687 |
|
|
|
720 |
|
Belgium |
|
|
1,612 |
|
|
|
2,422 |
|
|
|
2,543 |
|
Netherlands |
|
|
2,830 |
|
|
|
2,615 |
|
|
|
3,408 |
|
|
|
|
5,119 |
|
|
|
5,735 |
|
|
|
6,685 |
|
21.
SUPPLEMENTAL CASH FLOW INFORMATION
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash investing and financing transactions
(in thousands) |
|
April 30,
2025
$ |
|
|
April 30,
2024
$ |
|
|
April 30,
2023
$ |
|
Acquisition of building and equipment by lease |
|
|
995 |
|
|
|
7,826 |
|
|
|
7,593 |
|
Settlement of convertible debentures |
|
|
4,242 |
|
|
|
— |
|
|
|
1,315 |
|
The following changes in liabilities arose from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash changes |
|
|
|
|
(in thousands) |
|
April 30,
2024
$ |
|
|
Cash Flows
$ |
|
|
Acquisition
$ |
|
|
Debt forgiven
/ Settlement
/ Disposal
$ |
|
|
Accretion
$ |
|
|
Foreign
exchange
movements
and change
in estimates
$ |
|
|
April 30,
2025
$ |
|
Deferred acquisition payments |
|
#REF! |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
10 |
|
|
#REF! |
|
|
#REF! |
|
Convertible debentures |
|
|
— |
|
|
|
— |
|
|
|
4,242 |
|
|
|
(4,242 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Leases |
|
|
13,681 |
|
|
|
(1,577 |
) |
|
|
996 |
|
|
|
(99 |
) |
|
|
— |
|
|
|
402 |
|
|
|
13,403 |
|
Total |
|
#REF! |
|
|
|
(1,577 |
) |
|
|
5,238 |
|
|
|
(4,341 |
) |
|
|
10 |
|
|
#REF! |
|
|
#REF! |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash changes |
|
|
|
|
(in thousands) |
|
April 30,
2023
$ |
|
|
Cash Flows
$ |
|
|
Acquisition
$ |
|
|
Settlement
/ Disposal
$ |
|
|
Accretion
$ |
|
|
Foreign
exchange
movements
and change
in estimates
$ |
|
|
April 30,
2024
$ |
|
Deferred acquisition payments |
|
|
649 |
|
|
|
(146 |
) |
|
|
— |
|
|
|
(294 |
) |
|
|
19 |
|
|
#REF! |
|
|
#REF! |
|
Leases |
|
|
7,267 |
|
|
|
(1,339 |
) |
|
|
7,593 |
|
|
|
— |
|
|
|
— |
|
|
|
160 |
|
|
|
13,681 |
|
Total |
|
|
7,916 |
|
|
|
(1,485 |
) |
|
|
7,593 |
|
|
|
(294 |
) |
|
|
19 |
|
|
#REF! |
|
|
#REF! |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-cash changes |
|
|
|
|
(in thousands) |
|
April 30,
2022
$ |
|
|
Cash Flows
$ |
|
|
Acquisition
$ |
|
|
Settlement
/ Disposal
$ |
|
|
Accretion
$ |
|
|
Foreign
exchange
movements
and change
in estimates
$ |
|
|
April 30,
2023
$ |
|
Deferred acquisition payments |
|
|
1,237 |
|
|
|
(592 |
) |
|
|
— |
|
|
|
— |
|
|
|
27 |
|
|
|
(23 |
) |
|
|
649 |
|
Convertible debentures |
|
|
1,312 |
|
|
|
— |
|
|
|
— |
|
|
|
(1,315 |
) |
|
|
3 |
|
|
|
— |
|
|
|
— |
|
Leases |
|
|
1,455 |
|
|
|
(1,337 |
) |
|
|
7,593 |
|
|
|
— |
|
|
|
— |
|
|
|
(444 |
) |
|
|
7,267 |
|
Total |
|
|
4,004 |
|
|
|
(1,929 |
) |
|
|
7,593 |
|
|
|
(1,315 |
) |
|
|
30 |
|
|
|
(467 |
) |
|
|
7,916 |
|
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
Income tax expense differs from the amount that would be computed by applying the federal and provincial statutory tax rates of (2025 – 27%, 2024 – 27%, and 2023 – 27%) to the earnings before income taxes. The reasons for the differences and related tax effects are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
2023 |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Loss before income taxes |
|
|
(34,267 |
) |
|
|
(28,703 |
) |
|
|
(27,752 |
) |
|
|
|
|
|
|
|
|
|
|
Income taxes on earnings before income taxes, at above statutory rate |
|
|
(9,252 |
) |
|
|
(7,750 |
) |
|
|
(7,493 |
) |
Increase (decrease) in taxes resulting from: |
|
|
|
|
|
|
|
|
|
Nondeductible expenses |
|
|
8 |
|
|
|
1 |
|
|
|
8 |
|
Estimated SR&ED ITC |
|
|
(181 |
) |
|
|
(166 |
) |
|
|
(198 |
) |
Effects of tax rate change and foreign exchange |
|
|
— |
|
|
|
— |
|
|
|
209 |
|
Deferred tax liability |
|
|
(3,871 |
) |
|
|
(1,062 |
) |
|
|
— |
|
Tax rate difference by jurisdiction |
|
|
479 |
|
|
|
588 |
|
|
|
948 |
|
Tax benefits not recognized |
|
|
3,183 |
|
|
|
3,072 |
|
|
|
4,885 |
|
Impairment loss |
|
|
5,720 |
|
|
|
2,790 |
|
|
|
602 |
|
Prior year tax assessments and adjustments |
|
|
(197 |
) |
|
|
(183 |
) |
|
|
(420 |
) |
Other |
|
|
78 |
|
|
|
122 |
|
|
|
267 |
|
Income taxes |
|
|
(4,033 |
) |
|
|
(2,588 |
) |
|
|
(1,192 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
2023 |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Current income taxes |
|
|
185 |
|
|
|
352 |
|
|
|
(242 |
) |
Deferred income taxes |
|
|
(4,218 |
) |
|
|
(2,940 |
) |
|
|
(950 |
) |
Income taxes |
|
|
(4,033 |
) |
|
|
(2,588 |
) |
|
|
(1,192 |
) |
Temporary differences give rise to the following deferred income tax assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
2023 |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Other tax pools |
|
|
— |
|
|
|
— |
|
|
|
31 |
|
Capital assets net of lease liabilities |
|
|
217 |
|
|
|
16 |
|
|
|
(61 |
) |
Inventory and Intangible assets |
|
|
(467 |
) |
|
|
(4,084 |
) |
|
|
(7,631 |
) |
Recognized deferred income tax liabilities |
|
|
(250 |
) |
|
|
(4,068 |
) |
|
|
(7,661 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2025 |
|
|
2024 |
|
|
2023 |
|
|
|
$ |
|
|
$ |
|
|
$ |
|
Non-capital losses carried forward (expire from 2027 to 2040) |
|
|
12,945 |
|
|
|
13,424 |
|
|
|
9,930 |
|
Capital losses carried forward |
|
|
295 |
|
|
|
295 |
|
|
|
295 |
|
Capital assets net of lease liabilities |
|
|
— |
|
|
|
— |
|
|
|
20 |
|
Financing costs |
|
|
199 |
|
|
|
402 |
|
|
|
746 |
|
Less: unrecognized deferred income tax asset |
|
|
(13,439 |
) |
|
|
(14,121 |
) |
|
|
(10,991 |
) |
Unrecognized deferred income tax liabilities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
On July 4, 2025, tax legislation known as the One Big Beautiful Bill Act ("OBBBA") was enacted in the United States. OBBBA modifies certain international tax provisions such as the tax on Global Intangible Low Taxed Income ("GILTI") and renames GILTI as Net CFC Tested Income ("NCTI"). The Company records NCTI taxes on a deferred basis. The Company is currently evaluating the impact of U.S. tax law changes introduced by OBBBA on our consolidated financial statements. A quantitative estimate of the specific financial impacts cannot be reasonably determined at this time due to the complexity of the changes in OBBBA and the need for further analysis.
IMMUNOPRECISE ANTIBODIES LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended April 30, 2025, 2024 and 2023
(Expressed in Canadian Dollars)
On July 7, 2025 the Company announced the appointment of Jon Lieber to its Board of Directors, effective immediately.
Mr. Lieber brings over 30 years of financial and strategic leadership across the biotechnology and life sciences sectors, with deep expertise in capital markets, investor relations, and corporate development. He currently serves as Chief Financial Officer at Rallybio, a clinical-stage biotechnology company developing therapies for severe and rare diseases. He also brings valuable experience in Nasdaq governance, having served as both a senior executive and board member of publicly traded companies.
EX-2.1
2
ipa-ex2_1.htm
EX-2.1
EX-2.1
Exhibit 2.1
As of the date of the Annual Report on Form 20-F (the “Annual Report”) of which this Exhibit 2.1 is a part, ImmunoPrecise Antibodies Ltd. (the “Company”) has only one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended: the Company’s common shares (the “Common Shares”).
Description of Common Shares
The following description of the Common Shares is a summary and does not purport to be complete. It is subject to and qualified in its entirety by reference to the Company’s articles and notice of articles, each as amended, (collectively, the “Articles”) which are incorporated by reference as an Exhibit to the Annual Report of which this Exhibit 2.1 is a part.
Basic Rights of the Common Shares
We are authorized to issue an unlimited number of Common Shares, without par value. As of the date of the Annual Report there are 46,154,118 Common Shares issued and outstanding.
Each holder of Common Shares is entitled to receive notice of and to attend all meetings of shareholders of the Company, except meetings at which only holders of other classes or series of shares entitled to attend, and at all such meetings are entitled to one vote in respect of each Common Share held by such holder. The holders of Common Shares are entitled to receive dividends if and when declared by the board of directors of the Company, and in the event of any liquidation, dissolution or winding-up of the Company or other distribution of the assets of the Company among its shareholders for the purpose of winding-up its affairs, the holders of Common Shares are entitled, subject to the rights of holders of shares of any class ranking prior to the Common Shares, to receive the remaining property or assets of the Company.
Pre-emptive Rights
The Common Shares do not have pre-emptive rights to purchase additional Common Shares.
Transferability of Common Shares
The Articles do not impose restrictions on the transfer of Common Shares by a shareholder provided we remain a public company.
Change of Control restrictions for the Common Shares
There are no provisions in the Articles that would have the effect of preventing a change in control of the Company. The Company has adopted a shareholder rights plan which imposes restrictions on certain potential change of control transactions. See Item 10.B – Memorandum and Articles of Association of the Annual Report on Form 20-F of which this Exhibit 2.1 is a part for further details on the shareholder rights plan.
Action(s) to change Rights attaching to the Common Shares
Provisions as to the modification, amendment or variation of shareholder rights for holders of the Common Shares are contained in the Business Corporations Act (British Columbia) (the “BCBCA”). The BCBCA requires a “special resolution” of shareholders for specific corporate actions, including certain alterations of the Company’s share capital, with such “special resolution” requiring an affirmative two-thirds vote of shareholders (rather than a simple majority) for passage. No right or special right attached to any of the Company’s issued shares may be prejudiced or interfered with unless the shareholders holding shares of such class or series of shares to which the right or special right is attached consent by a separate “special resolution” of those shareholders.
Ownership disclosure threshold for the Common Shares
The Articles do not have any specific threshold requiring disclosure of ownership by holders of Common Shares. However, Canadian securities laws require disclosure of shareholder ownership by any shareholder owning more than 10% of the outstanding Common Shares.
EX-2.2
3
ipa-ex2_2.htm
EX-2.2
EX-2.2
Exhibit 2.2
THE SECURITIES REPRESENTED BY THIS CERTIFICATE AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE HAVE BEEN ACQUIRED SOLELY FOR INVESTMENT PURPOSES AND NOT WITH A VIEW TOWARD RESALE AND MAY ONLY BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (A) TO THE CORPORATION, (B) OUTSIDE THE UNITED STATES IN COMPLIANCE WITH RULE 904 OF REGULATION S UNDER THE SECURITIES ACT AND IN COMPLIANCE WITH LOCAL LAWS AND REGULATIONS, (C) IN COMPLIANCE WITH THE EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT PROVIDED BY RULE 144 THEREUNDER, IF AVAILABLE, AND IN COMPLIANCE WITH ANY APPLICABLE STATE SECURITIES OR “BLUE SKY” LAWS, (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, OR (E) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT AND, IN THE CASE OF SUBPARAGRAPH (C) OR (D), THE SELLER FURNISHES TO THE CORPORATION AN OPINION OF COUNSEL OF RECOGNIZED STANDING OR SUCH OTHER EVIDENCE AS THE CORPORATION MAY REQUIRE IN FORM AND SUBSTANCE REASONABLY SATISFACTORY TO THE CORPORATION TO SUCH EFFECT. NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.
IMMUNOPRECISE ANTIBODIES LTD.
Convertible Debenture
|
Principal Amount: $2,000,000 |
Debenture Issuance Date: July 16, 2024
Debenture Number: IPA-1
FOR VALUE RECEIVED, IMMUNOPRECISE ANTIBODIES LTD., a British Columbia Corporation (the "Company"), hereby promises to pay to the order of YA II PN, Ltd., or its registered assigns (the "Holder") the amount set out above as the principal amount (as reduced pursuant to the terms hereof pursuant to redemption, conversion or otherwise, the "Principal") when due, whether upon the Maturity Date (as defined below), acceleration, redemption or otherwise (in each case in accordance with the terms hereof) and to pay interest ("Interest") on any outstanding Principal at the applicable Interest Rate from the date set out above as the Debenture Issuance Date (the "Issuance Date") until the same becomes due and payable, whether upon the Maturity Date or acceleration, conversion, redemption or otherwise (in each case in accordance with the terms hereof). This Convertible Debenture (including all debentures issued in exchange, transfer or replacement hereof, this "Debenture") was originally issued pursuant to the Securities Purchase Agreement dated as of July 16, 2024, as it may be amended from time to time (the “Securities Purchase Agreement”) between the Company and the Buyers listed on the Schedule of Buyers attached thereto. Certain capitalized terms used herein are defined in Section (14).
(a)
Maturity Date. On the Maturity Date, the Company shall pay to the Holder an amount in cash representing all outstanding Principal, accrued and unpaid Interest, and any other amounts outstanding pursuant to the terms of this Debenture. The "Maturity Date" shall be July 16, 2025. Other than as specifically permitted by this Debenture, the Company may not prepay or redeem any portion of the outstanding Principal and accrued and unpaid Interest.
(b)
Interest Rate and Payment of Interest. Interest shall accrue on the outstanding Principal balance hereof at an annual rate equal to 8.00% (“Interest Rate”), which Interest Rate shall increase to an annual rate of 18.00% upon the occurrence of an Event of Default (for so long as such event remains uncured). Interest shall be calculated based on a 365-day year and the actual number of days elapsed, to the extent permitted by applicable law.
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(c)
Payment Dates. Whenever any payment or other obligation hereunder shall be due on a day other than a Business Day, such payment shall be made on the next succeeding Business Day.
(a)
Monthly Payments. If, any time on or after November 1, 2024, and from time to time thereafter, an Amortization Event occurs, then the Company shall make monthly payments beginning on the 7th Trading Day after the Amortization Event Date and continuing on the same day of each successive Calendar Month. Each monthly payment shall be in an amount equal to the sum of (i) $300,000 of Principal (in the aggregate among this Debenture and all Other Debentures), or the outstanding Principal, if the Principal (in the aggregate among this Debenture and all Other Debentures) is less than such amount (the “Amortization Principal Amount”), plus (ii) the Payment Premium (as defined below) in respect of such Amortization Principal Amount, and (iii) accrued and unpaid interest hereunder as of each payment date. The obligation of the Company to make monthly prepayments related to a Amortization Event shall cease (with respect to any payment that has not yet come due) if any time after the Amortization Event Date (A) in the event of a Floor Price Event, on the date that is the seventh (7th) consecutive Trading Day that the daily VWAP is greater than 110% of the Floor Price then in effect, or (B) in the event of a Registration Event, the condition or event causing the Registration Event has been cured or the Holder is able to resell the Common Shares issuable upon conversion of this Note in accordance with Rule 144 under the Securities Act, unless a subsequent Amortization Event occurs. If this Debenture and any Other Debentures are held by more than one holder, then the Amortization Principal Amount and Payment Premium in respect of such Amortization Principal Amount shall be allocated to each holder based on each holder’s pro-rata portion of the total outstanding Principal amount outstanding on this Debenture and all Other Debentures. If this Debenture and all Other Debentures are held by one holder, then such holder shall decide the allocation of payments between this Debenture and all Other Debentures in its sole discretion.
(b)
The Company shall have the right, but not the obligation, to redeem (“Optional Redemption”) early in cash a portion or all amounts outstanding under this Debenture at the Redemption Amount (as defined below) as described in this Section; provided that the Company provides the Holder with at least ten (10) Trading Days’ prior written notice (each, a “Redemption Notice”) of its desire to exercise an Optional Redemption, which Redemption Notice (i) shall be delivered to the Holder after the closing of regular trading hours on a Trading Day, and (ii) may only be given if the VWAP on the date such Redemption Notice is delivered is less than the Fixed Price. Each Redemption Notice shall be irrevocable and shall specify the outstanding Principal amount of the Debentures to be redeemed and the Redemption Amount. The “Redemption Amount” shall be an amount equal to the outstanding Principal balance being redeemed by the Company, plus the Payment Premium in respect of such Principal amount, plus all accrued and unpaid Interest on such Principal amount to be redeemed as of such redemption date. After receipt of a Redemption Notice, the Holder shall have ten (10) Trading Days (beginning with the Trading Day immediately following the date of such Redemption Notice) to elect to convert all or any portion of the outstanding Principal of the Debenture plus all accrued and unpaid Interest on such Principal amount, if any, plus the Payment Premium, if any, in respect of such Principal. On the eleventh (11th) Trading Day after the applicable Redemption Notice, the Company shall deliver to the Holder the Redemption Amount with respect to the Principal amount redeemed to the extent not converted and otherwise after giving effect to conversions or other payments made during the ten (10) Trading Day period.
(a)
An “Event of Default”, wherever used herein, means any one of the following events (whatever the reason and whether it shall be voluntary or involuntary or effected by operation of law or pursuant to any judgment, decree or order of any court, or any order, rule or regulation of any administrative or governmental body):
(i)
The Company's failure to pay to the Holder any amount of Principal after such payment is due, or any Redemption Amount. Payment Premium, Interest, or other amounts when and as due under this Debenture or any other Transaction Document and such failure continues for a period of five (5) Business Days;
(ii)
The Company or any Subsidiary of the Company shall commence, or there shall be commenced against the Company or any Subsidiary of the Company, any proceeding under any applicable bankruptcy or insolvency laws as now or hereafter in effect or any successor thereto, or the Company or any Subsidiary of the Company commences any other proceeding under any reorganization, arrangement, adjustment of debt, relief of debtors, dissolution, insolvency or liquidation or similar law of any jurisdiction whether now or hereafter in effect relating to the Company or any Subsidiary of the Company any such bankruptcy, insolvency or other proceeding which remains undismissed for a period of sixty one (61) days; or the Company or any Subsidiary of the Company is adjudicated insolvent or bankrupt; or any order of relief or other order approving any such case or proceeding is entered; or the Company or any Subsidiary of the Company suffers any appointment of any custodian, private or court appointed receiver or the like for it or all or substantially all of its property which continues undischarged or unstayed for a period of sixty one (61) days; or the Company or any Subsidiary of the Company makes a general assignment of all or substantially all of its assets for the benefit of creditors; or the Company or any Subsidiary of the Company shall fail to pay, or shall state in writing in a judicial, regulatory, or administrative proceeding or filing that it is unable to pay, its debts generally as they become due;;
2
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(iii)
The Company or any Subsidiary of the Company shall default in any of its obligations under any note, debenture, or any mortgage, credit agreement or other facility, indenture agreement, factoring agreement or other instrument under which there may be issued, or by which there may be secured or evidenced any indebtedness for borrowed money or money due under any long term leasing or factoring arrangement of the Company or any Subsidiary of the Company in an amount exceeding $100,000, whether such indebtedness now exists or shall hereafter be created and such default shall result in such indebtedness becoming or being declared due and payable before its stated maturity and such acceleration shall not have been rescinded or annulled or such default shall not have been cured, or such indebtedness is not paid or discharged, as the case may be, within fifteen (15) calendar days;
(iv)
a final judgment or judgments for the payment of money aggregating in excess of $100,000 are rendered against the Company and/or any of its Subsidiaries and which judgments are not, within thirty (30) days after the entry thereof, bonded, discharged, settled or stayed pending appeal, or are not discharged within thirty (30) days after the expiration of such stay; provided, however, any judgment which is covered by insurance or an indemnity from a credit worthy party shall not be included in calculating the $100,000 amount set forth above;
(v)
The Common Shares shall cease to be quoted or listed for trading, as applicable, on any Principal Market for a period of ten (10) consecutive Trading Days;
(vi)
The Company or any Subsidiary of the Company shall be a party to any Change of Control Transaction (as defined in Section (14)) unless in connection with such Change of Control Transaction this Debenture is redeemed under Section (2)(b);
(vii)
The Company's (A) failure to deliver the required number of Common Shares to the Holder within two (2) Trading Days after the applicable Share Delivery Date or (B) notice, written or oral, to any holder of the Debenture, including by way of public announcement, at any time, of its intention not to comply with a request for conversion of any Debenture into Common Shares that is tendered in accordance with the provisions of the Debenture, other than pursuant to Section (4)(c);
(viii)
The Company shall fail for any reason to deliver the payment in cash pursuant to a Buy-In (as defined herein) within five (5) Business Days after such payment is due;
(ix)
The Company’s failure to timely file with the Commission any Periodic Report on or before the due date of such filing as established by the Commission, it being understood, for the avoidance of doubt, that due date includes any permitted filing deadline extension under Rule 12b-25 under the Exchange Act, if such failure is not cured within five (5) Business Days;
(x)
Any material representation or warranty made or deemed to be made by or on behalf of the Company in or in connection with any Transaction Document, or any waiver hereunder or thereunder, shall prove to have been incorrect in any material respect (or, in the case of any such representation or warranty already qualified by materiality, such representation or warranty shall prove to have been incorrect) when made or deemed made;
(xi)
Any Transaction Document, at any time after its execution and delivery and for any reason other than as expressly permitted hereunder or thereunder, ceases to be in full force and effect; or the Company or any other Person contests in writing the validity or enforceability of any provision of any Transaction Document; or the Company denies in writing that it has any or further liability or obligation under any Transaction Document, or purports in writing to revoke, terminate (other than in line with the relevant termination provisions) or rescind any Transaction Document;
(xii)
The Company uses the proceeds of the issuance of this Debenture, whether directly or indirectly, and whether immediately, incidentally or ultimately, to purchase or carry margin stock (within the meaning of Regulations T, U and X of the Federal Reserve Board, as in effect from time to time and all official rulings and interpretations thereunder or thereof), or to extend credit to others for the purpose of purchasing or carrying margin stock or to refund indebtedness originally incurred for such purpose;
3
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(xiii)
Any Event of Default (as defined in the Other Debentures or in any Transaction Document other than this Debenture) occurs with respect to any Other Debentures; or
(xiv)
The Company shall fail to observe or perform any material covenant, agreement or warranty contained in, or otherwise commit any material breach or default of any provision of this Debenture (except as may be covered by Section (3)(a)(i) through (3)(a)(xiii) hereof) or any other Transaction Document in each case which is not cured or remedied within ten (10) Business Days of receipt by the Company of written notice of such breach or default from the Holder.
(b)
During the time that any portion of this Debenture is outstanding, if any Event of Default has occurred and is continuing, the full unpaid Principal amount of this Debenture, together with interest and other amounts owing in respect thereof, to the date of acceleration shall become at the Holder's election given by notice pursuant to Section (7), immediately due and payable in cash; provided that, in the case of any event with respect to the Company described in Section (3)(a)(ii), the full unpaid Principal amount of this Debenture, together with accrued and unpaid interest and other amounts owing in respect thereof to the date of acceleration, shall automatically become due and payable. Furthermore, in addition to any other remedies, the Holder shall have the right (but not the obligation) to convert, at the Conversion Price, on one or more occasions all or part of the Conversion Amount in accordance with Section (4) and subject to the limitations in Section (4)(c) at any time after (x) an Event of Default or (y) the Maturity Date, provided that this Debenture remains outstanding, at the Conversion Price. The Holder need not provide and the Company hereby waives any presentment, demand, protest or other notice of any kind, (other than required notice of conversion) and the Holder may immediately enforce any and all of its rights and remedies hereunder and all other remedies available to it under applicable law. Such declaration may be rescinded and annulled by the Holder in writing at any time prior to payment hereunder. No such rescission or annulment shall affect any subsequent Event of Default or impair any right consequent thereon. For the purposes hereof, an Event of Default relating to default in payment is “continuing” if it has not been waived, and an Event of Default relating to circumstances other than a default in payment is “continuing” if it has not been cured or waived.
(4)
CONVERSION OF DEBENTURE. This Debenture shall be convertible into Common Shares, on the terms and conditions set forth in this Section (4).
(a)
Conversion Right. Subject to the limitations of Section (4)(c), at any time or times on or after the Issuance Date, the Holder shall be entitled to convert any portion of the outstanding and unpaid Conversion Amount (as defined below) into fully paid and nonassessable Common Shares in accordance with Section (4)(b), at the Conversion Price (as defined below). The number of Common Shares issuable upon conversion of any Conversion Amount pursuant to this Section (4)(a) shall be determined by dividing (x) such Conversion Amount by (y) the Conversion Price. The Company shall not issue any fraction of a Common Share upon any conversion. All calculations under this Section (4) shall be rounded to the nearest $0.0001. If the issuance would result in the issuance of a fraction of a Common Share, the Company shall round such fraction of a Common Share down to the nearest whole share. The Company shall pay any and all transfer, stamp and similar taxes that may be payable with respect to the issuance and delivery of Common Shares upon conversion of any Conversion Amount, unless such taxes are due because the Holder requests such Common Shares to be issued in a name other than the Holder’s name, in which case the Holder shall pay such taxes.
(b)
Mechanics of Conversion.
(i)
Optional Conversion. To convert any Conversion Amount into Common Shares on any date (a "Conversion Date"), the Holder shall (A) transmit by email, for receipt on or prior to 11:59 p.m., New York time, on such date, a copy of an executed notice of conversion in the form attached hereto as Exhibit I (the "Conversion Notice") to the Company and (B) if required by Section (4)(b)(iii), surrender this Debenture to a nationally recognized overnight delivery service for delivery to the Company (or an indemnification undertaking reasonably satisfactory to the Company with respect to this Debenture in the case of its loss, theft or destruction). On or before the second (2nd) Business Day following the date of receipt of a Conversion Notice (the "Share Delivery Date"), the Company shall (X) if legends are not required to be placed on certificates or the book-entry position of the Common Shares and provided that the Company’s transfer agent is participating in the Depository Trust Company's ("DTC") Fast Automated Securities Transfer Program, instruct such transfer agent to credit such aggregate number of Common Shares to which the Holder shall be entitled to the Holder's or its designee's balance account with DTC through its Deposit Withdrawal Agent Commission system or (Y) if the Company’s transfer agent is not participating in the DTC Fast Automated Securities Transfer Program, or if restrictive legends are required to be placed on certificates or book-entry positions of the Common Shares, issue and deliver to the address as specified in the Conversion Notice, a certificate or book-entry position, registered in the name of the Holder or its designee, for the number of Common Shares to which the Holder shall be entitled.
4
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
If this Debenture is physically surrendered for conversion and the outstanding Principal of this Debenture is greater than the Principal portion of the Conversion Amount being converted, then the Company shall as soon as practicable and in no event later than three (3) Business Days after receipt of this Debenture and at its own expense, issue and deliver to the holder a new Debenture representing the outstanding Principal not converted. The Person or Persons entitled to receive the Common Shares issuable upon a conversion of this Debenture shall be treated for all purposes as the record holder or holders of such Common Shares upon the transmission of a Conversion Notice.
(ii)
Company's Failure to Timely Convert. If the Company shall fail, for any reason or for no reason, on or prior to the applicable Share Delivery Date to issue and deliver a certificate to the Holder or credit the Holder's balance account with DTC for the number of Common Shares to which the Holder is entitled upon such Holder's conversion of any Conversion Amount (a "Conversion Failure"), and if on or after such Share Delivery Date the Holder purchases (in an open market transaction or otherwise) Common Shares to deliver in satisfaction of a bona fide sale by the Holder to an unaffiliated third party of Common Shares issuable upon such conversion that the Holder anticipated receiving from the Company (a "Buy-In"), then the Company shall, within three (3) Business Days after the Holder's request and in the Holder's discretion, either (i) pay cash to the Holder in an amount equal to the Holder's total purchase price (including brokerage commissions and other out of pocket expenses, if any) for the Common Shares so purchased (the “Buy-In Price”), at which point the Company's obligation to issue and deliver such Common Shares shall terminate, or (ii) promptly honor its obligation to deliver to the Holder such Common Shares and pay cash to the Holder in an amount equal to the excess (if any) of the Buy-In Price over the product of (A) such number of Common Shares, times (B) the Closing Price on the Conversion Date.
(iii)
Book-Entry. Notwithstanding anything to the contrary set forth herein, upon conversion of any portion of this Debenture in accordance with the terms hereof, the Holder shall not be required to physically surrender this Debenture to the Company unless (A) the full Conversion Amount represented by this Debenture is being converted or (B) the Holder has provided the Company with prior written notice (which notice may be included in a Conversion Notice) requesting reissuance of this Debenture upon physical surrender of this Debenture. The Holder and the Company shall maintain records showing the Principal and Interest converted and the dates of such conversions or shall use such other method, reasonably satisfactory to the Holder and the Company, so as not to require physical surrender of this Debenture upon any partial conversion.
(c)
Limitations on Conversions.
(i)
Beneficial Ownership. The Holder shall not have the right to convert any portion of this Debenture to the extent that after giving effect to such conversion, the Holder, together with any affiliate thereof, would beneficially own (as determined in accordance with Section 13(d) of the Exchange Act and the rules promulgated thereunder) in excess of 4.99% of the number of Common Shares outstanding immediately after giving effect to such conversion. The Holder shall have the authority and obligation to determine whether the restriction contained in this Section will limit any particular conversion hereunder and to the extent that the Holder determines that the limitation contained in this Section applies, the determination of which portion of the Principal amount of this Debenture (taking into account the conversion of accrued Interest on such Principal amount) is convertible shall be the responsibility and obligation of the Holder. The provisions of this Section may be waived by a holder (but only as to itself and not to any other holder) upon not less than 65 days prior notice to the Company. Other holders shall be unaffected by any such waiver.
(ii)
Other Conversion Limitations. During any consecutive 30-day period, the Holder agrees that it shall not convert, together with its affiliates, more than an aggregate amount of Principal equal to $300,000 if the Conversion Price is less than the Fixed Price, provided, however, that the foregoing limitation in this Section 4(c)(ii) shall not apply upon the occurrence and during the continuance of an Event of Default. This limitation may be waived with the written consent of the Company.
(i)
All calculations under this Section (4) shall be rounded to the nearest $0.0001 or whole share.
5
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
The Company covenants that it will at all times authorize for issuance such number of Common Shares not less than the maximum number of Common Shares issuable upon conversion of this Debenture and the Other Debentures (assuming for purposes hereof that (x) this Debenture and such Other Debentures are convertible at the Floor Price as of the date of determination, (y) any such conversion shall not take into account any limitations on the conversion of the Debenture or Other Debentures set forth herein or therein (the “Required Reserve Amount”), provided that at no time shall the number of Common Shares authorized pursuant to this Section (4)(d)(ii) be reduced other than proportionally with respect to all Common Shares in connection with any conversion (other than pursuant to the conversion of this Debenture and the Other Debentures in accordance with their terms) and/or cancellation, or reverse stock split. The Company covenants that, upon issuance in accordance with conversion of this Debenture in accordance with its terms, the Common Shares, when issued, will be validly issued, fully paid and nonassessable.
(iii)
Nothing herein shall limit a Holder's right to pursue actual damages or declare an Event of Default pursuant to Section (3) herein for the Company’s failure to deliver certificates or book-entry for Common Shares upon conversion within the period specified herein and such Holder shall have the right to pursue all remedies available to it at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief, in each case without the need to post a bond or provide other security. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
(iv)
Legal Opinions. The Company is obligated to cause its legal counsel to deliver legal opinions to the Company’s transfer agent in connection with any legend removal upon the expiration of any holding period or other requirement for which the Underlying Shares may bear legends restricting the transfer thereof. To the extent such opinions are not provided (either timely or at all), then, in addition to being an Event of Default in accordance with Section 3(a)(xiv), the Company agrees to reimburse the Holder for all reasonable and documented out of pocket legal costs incurred by the Holder in connection with any legal opinions paid for by the Holder in connection with sale or transfer of Underlying Shares. The Holder shall notify the Company of any such costs it incurs that are referred to in this section from time to time and all amounts owed hereunder shall be paid by the Company with reasonable promptness.
(5)
Adjustments to Conversion Price
(a)
Adjustment of Conversion Price upon Subdivision or Combination of Common Shares. If the Company, at any time while this Debenture is outstanding, shall (a) pay a stock dividend or otherwise make a distribution or distributions on all or substantially all of its Common Shares or any other equity or equity equivalent securities payable in Common Shares (excluding any interest payments or equivalents thereto that are paid in Common Shares and, for the avoidance of doubt, excluding conversions, exercises or exchanges of warrants and other securities into or for Common Shares), (b) subdivide outstanding Common Shares into a larger number of shares, (c) combine (including by way of reverse stock split or consolidation) outstanding Common Shares into a smaller number of shares, or (d) issue by reclassification of Common Shares any shares of capital stock of the Company, then each of the Fixed Price and the Floor Price shall be multiplied by a fraction of which the numerator shall be the number of Common Shares (excluding treasury shares, if any) outstanding before such event and of which the denominator shall be the number of Common Shares outstanding after such event. Any adjustment made pursuant to this Section shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b)
Adjustment of Conversion Price upon Issuance of Common Shares. If the Company, at any time while this Debenture is outstanding, issues or sells any Common Shares or Convertible Securities (other than shares issued or sold by the Company in connection with any Excluded Securities), for a consideration per share (the “New Issuance Price”) less than a price equal to the Fixed Price in effect immediately prior to such issue or sale (such price the "Applicable Price") (the foregoing a "Dilutive Issuance"), then immediately after such Dilutive Issuance the Fixed Price then in effect shall be reduced to an amount equal to the New Issuance Price. For the purposes hereof, if the Company in any manner issues or sells any Convertible Securities (other than shares issued or sold by the Company in connection with any Excluded Securities) and the lowest price per share for which one Common Share is issuable upon such conversion or exchange or exercise thereof is less than the Applicable Price, then such Common Share shall be deemed to be outstanding and to have been issued and sold by the Company at the time of the issuance or sale of such Convertible Securities for such price per share. No further adjustment of the Conversion Price shall be made upon the actual issuance of such Common Share upon conversion or exchange or exercise of such Convertible Securities.
(c)
Adjustment of Conversion Price upon Issuance of Pursuant to the Clear Street ATM. If the Company issues any Common Shares pursuant to the Clear Street ATM during the period starting on the date of the Securities Purchase Agreement and ending on the date of the effectiveness of the initial Registration Statement filed pursuant to the Registration Rights Agreement, then the Fixed Price shall be subject to an adjustment (downwards only) to a price equal 120% of the VWAP on the Trading Day immediately prior to the date of effectiveness of the Registration Statement.
6
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(d)
Other Events. If any event occurs of the type contemplated by the provisions of this Section (5) but not expressly provided for by such provisions (including, without limitation, the granting of stock appreciation rights, phantom stock rights or other rights with equity features, or issuing Convertible Securities with a variable conversion formula that is more favorable than this Debenture), then the Company's Board of Directors will make an appropriate adjustment in the Conversion Price so as to protect the rights of the Holder under this Debenture; provided that no such adjustment will increase the Conversion Price as otherwise determined pursuant to this Section (5). If the Company issues any Convertible Securities with a variable conversion formula that is more favorable than this Debenture, then at the option of the Holder, the Market Price formula shall be changed to match that of the new Convertible Securities.
(e)
Other Corporate Events. In addition to and not in substitution for any other rights hereunder, prior to the consummation of any Fundamental Transaction pursuant to which holders of Common Shares are entitled to receive securities or other assets with respect to or in exchange for Common Shares (a "Corporate Event"), the Company shall make appropriate provision to ensure that the Holder will thereafter have the right to receive upon a conversion of this Debenture, at the Holder's option, (i) in addition to the Common Shares receivable upon such conversion, such securities or other assets to which the Holder would have been entitled with respect to such Common Shares had such Common Shares been held by the Holder upon the consummation of such Corporate Event (without taking into account any limitations or restrictions on the convertibility of this Debenture) or (ii) in lieu of the Common Shares otherwise receivable upon such conversion, such securities or other assets received by the holders of Common Shares in connection with the consummation of such Corporate Event in such amounts as the Holder would have been entitled to receive had this Debenture initially been issued with conversion rights for the form of such consideration (as opposed to Common Shares) at a conversion rate for such consideration commensurate with the Conversion Price. The provisions of this Section shall apply similarly and equally to successive Corporate Events and shall be applied without regard to any limitations on the conversion or redemption of this Debenture.
(f)
Whenever the Conversion Price is adjusted pursuant to Section (5) hereof, the Company shall promptly provide the Holder with a written notice setting forth the Conversion Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
(g)
In case of any (1) merger or consolidation of the Company with or into another Person, or (2) sale by the Company of all or substantially all the assets of the Company in one or a series of related transactions, a Holder shall have the right to (A) exercise any then applicable rights under Section (5)(b), (B) convert the aggregate amount of this Debenture then outstanding into the shares of stock and other securities, cash and property receivable upon or deemed to be held by holders of Common Shares following such merger, consolidation or sale, and such Holder shall be entitled upon such event or series of related events to receive such amount of securities, cash and property as the Common Shares into which such aggregate Principal amount of this Debenture could have been converted immediately prior to such merger, consolidation or sales would have been entitled, or (C) in the case of a merger or consolidation, require the surviving entity to issue to the Holder a convertible debenture with a Principal amount equal to the aggregate Principal amount of this Debenture then held by such Holder, plus all accrued and unpaid Interest and other amounts owing thereon, which such newly issued convertible debenture shall have terms identical (including with respect to conversion) to the terms of this Debenture, and shall be entitled to all of the rights and privileges of the Holder of this Debenture set forth herein and the agreements pursuant to which this Debenture was issued. In the case of clause (C), the conversion price applicable for the newly issued convertible debentures shall be based upon the amount of securities, cash and property that each Common Share would receive in such transaction and the Conversion Price in effect immediately prior to the effectiveness or closing date for such transaction. The terms of any such merger, sale or consolidation shall include such terms so as to continue to give the Holder the right to receive the securities, cash and property set forth in this Section upon any conversion or redemption following such event. This provision shall similarly apply to successive such events.
(6)
REISSUANCE OF THIS DEBENTURE.
(a)
Transfer. If this Debenture is to be transferred, the Holder shall surrender this Debenture to the Company, whereupon the Company will forthwith issue and deliver upon the order of the Holder a new Debenture (in accordance with Section (6)(d)), registered in the name of the registered transferee or assignee, representing the outstanding Principal being transferred by the Holder (along with any accrued and unpaid Interest thereof) and, if less than the entire outstanding Principal is being transferred, a new Debenture (in accordance with Section (6)(d)) to the Holder representing the outstanding Principal not being transferred. The Holder and any assignee, by acceptance of this Debenture, acknowledge and agree that, by reason of the provisions of Section (4)(b)(iii) following conversion or redemption of any portion of this Debenture, the outstanding Principal represented by this Debenture may be less than the Principal stated on the face of this Debenture.
7
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
The Debentures and the Common Shares issuable on the conversion thereof have not been registered under the Securities Act or the securities laws of any state of the United States; accordingly, the Debentures and the Common Shares issuable on the conversion thereof are (or will be when issued) “restricted securities” as defined under Rule 144(a)(3) under the Securities Act and the certificates representing such securities shall bear the restrictive legends in substantially the following form: THE SECURITIES REPRESENTED BY THIS CERTIFICATE [AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE] HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES [AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE] HAVE BEEN ACQUIRED SOLELY FOR INVESTMENT PURPOSES AND NOT WITH A VIEW TOWARD RESALE AND MAY ONLY BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (A) TO THE CORPORATION, (B) OUTSIDE THE UNITED STATES IN COMPLIANCE WITH RULE 904 OF REGULATION S UNDER THE SECURITIES ACT AND IN COMPLIANCE WITH LOCAL LAWS AND REGULATIONS, (C) IN COMPLIANCE WITH THE EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT PROVIDED BY RULE 144 THEREUNDER, IF AVAILABLE, AND IN COMPLIANCE WITH ANY APPLICABLE STATE SECURITIES OR “BLUE SKY” LAWS, (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, OR (E) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT AND, IN THE CASE OF SUBPARAGRAPH (C) OR (D), THE SELLER FURNISHES TO THE CORPORATION AN OPINION OF COUNSEL OF RECOGNIZED STANDING OR SUCH OTHER EVIDENCE AS THE CORPORATION MAY REQUIRE IN FORM AND SUBSTANCE REASONABLY SATISFACTORY TO THE CORPORATION TO SUCH EFFECT. NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.Certificates evidencing the Common Shares issuable on the conversion of the Debentures shall not contain any legend (including the legend set forth above), (i) while a registration statement covering the resale of such security is effective under the Securities Act, (ii) following any sale of such Common Shares issuable on the conversion of the Debentures pursuant to Rule 144, (iii) if such Common Shares issuable on the conversion of the Debentures are eligible for sale under Rule 144, or (iv) if such legend is not required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the SEC). This Debenture may only be transferred, upon compliance with the conditions prescribed in Exhibit II. (b) Lost, Stolen or Mutilated Debenture. Upon receipt by the Company of evidence reasonably satisfactory to the Company of the loss, theft, destruction or mutilation of this Debenture, and, in the case of loss, theft or destruction, of any indemnification undertaking by the Holder to the Company in customary form and, in the case of mutilation, upon surrender and cancellation of this Debenture, the Company shall execute and deliver to the Holder a new Debenture (in accordance with Section (6)(d)) representing the outstanding Principal. (c) Debenture Exchangeable for Different Denominations. This Debenture is exchangeable, upon the surrender hereof by the Holder at the principal office of the Company, for a new Debenture or Debentures (in accordance with Section (6)(d)) representing in the aggregate the outstanding Principal of this Debenture, and each such new Debenture will represent such portion of such outstanding Principal as is designated by the Holder at the time of such surrender. (d) Issuance of New Debentures. Whenever the Company is required to issue a new Debenture pursuant to the terms of this Debenture, such new Debenture (i) shall be of like tenor with this Debenture, (ii) shall represent, as indicated on the face of such new Debenture, the Principal remaining outstanding (or in the case of a new Debenture being issued pursuant to Section (6)(a) or Section (6)(c), the Principal designated by the Holder which, when added to the Principal represented by the other new Debentures issued in connection with such issuance, does not exceed the Principal remaining outstanding under this Debenture immediately prior to such issuance of new Debentures), (iii) shall have an issuance date, as indicated on the face of such new Debenture, which is the same as the Issuance Date of this Debenture, (iv) shall have the same rights and conditions as this Debenture, and (v) shall represent accrued and unpaid Interest from the Issuance Date. (7) NOTICES. Any notices, consents, waivers or other communications required or permitted to be given under the terms hereof must be in writing by letter and email and will be deemed to have been delivered: upon the later of (A) either (i) receipt, when delivered personally or (ii) one (1) Business Day after deposit with an overnight courier service with next day delivery specified, in each case, properly addressed to the party to receive the same and (B) receipt, when sent by electronic mail. The addresses and email addresses for such communications shall be:
8
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
|
|
If to the Company, to: |
ImmunoPrecise Antibodies Ltd. |
|
[Redacted: Personal information] |
|
|
|
|
|
|
|
with a copy (which shall not constitute notice) to:
|
Dorsey & Whitney LLP
[Redacted: Personal information]
|
|
If to the Holder:
|
YA II PN, Ltd
|
|
[Redacted: Personal information] |
|
|
|
|
|
|
|
|
or at such other address and/or email and/or to the attention of such other person as the recipient party has specified by written notice given to each other party three (3) Business Days prior to the effectiveness of such change. Written confirmation of receipt (i) given by the recipient of such notice, consent, waiver or other communication, (ii) electronically generated by the sender’s email service provider containing the time, date, recipient email address or (iii) provided by a nationally recognized overnight delivery service, shall be rebuttable evidence of personal service, receipt by facsimile or receipt from a nationally recognized overnight delivery service in accordance with clause (i), (ii) or (iii) above, respectively.
(8)
NO IMPAIRMENT. Except as expressly provided herein, no provision of this Debenture shall alter or impair the obligations of the Company, which are absolute and unconditional, to pay the Principal of, Interest and other charges (if any) on, this Debenture at the time, place, and rate, and in the currency, herein prescribed. This Debenture is a direct obligation of the Company. As long as this Debenture is outstanding, the Company shall not , without the consent of the Holder, (i) amend its notice of articles, articles or other charter documents so as to adversely affect any rights of the Holder; (ii) repay, repurchase or offer to repay, repurchase or otherwise acquire Common Shares or other equity securities (other than Excluded Securities); or (iii) enter into any agreement with respect to any of the foregoing;.
(9)
This Debenture shall not entitle the Holder to any of the rights of a shareholder of the Company, including without limitation, the right to vote, to receive dividends and other distributions, or to receive any notice of, or to attend, meetings of shareholders or any other proceedings of the Company, unless and to the extent converted into Common Shares in accordance with the terms hereof.
(10)
CHOICE OF LAW; VENUE; WAIVER OF JURY TRIAL
(a)
Governing Law. This Debenture and the rights and obligations of the Parties hereunder shall, in all respects, be governed by, and construed in accordance with, the laws (excluding the principles of conflict of laws) of the State of New York (the “Governing Jurisdiction”) (including Section 5-1401 and Section 5-1402 of the General Obligations Law of the State of New York), including all matters of construction, validity and performance.
(b)
Jurisdiction; Venue; Service.
(i)
The Company hereby irrevocably consents to the non-exclusive personal jurisdiction of the state courts of the Governing Jurisdiction and, if a basis for federal jurisdiction exists, the non-exclusive personal jurisdiction of any United States District Court for the Governing Jurisdiction.
(ii)
The Company agrees that venue shall be proper in any court of the Governing Jurisdiction selected by the Holder or, if a basis for federal jurisdiction exists, in any United States District Court in the Governing Jurisdiction. The Company waives any right to object to the maintenance of any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or in tort or otherwise, in any of the state or federal courts of the Governing Jurisdiction on the basis of improper venue or inconvenience of forum.
9
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(iii)
Any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or tort or otherwise, brought by the Company against the Holder arising out of or based upon this Debenture or any matter relating to this Debenture, or any other Transaction Document, or any contemplated transaction, shall be brought in a court only in the Governing Jurisdiction. The Company shall not file any counterclaim against the Holder in any suit, claim, action, litigation or proceeding brought by the Holder against the Company in a jurisdiction outside of the Governing Jurisdiction nlesss under the rules of the court in which the Holder brought such suit, claim, action, litigation or proceeding the counterclaim is mandatory, and not permissive, and would be considered waived unless filed as a counterclaim in the suit, claim, action, litigation or proceeding instituted by the Holder against the Company. The Company agrees that any forum outside the Governing Jurisdiction is an inconvenient forum and that any suit, claim, action, litigation or proceeding brought by the Company against the Holder in any court outside the Governing Jurisdiction should be dismissed or transferred to a court located in the Governing Jurisdiction. Furthermore, the Company irrevocably and unconditionally agrees that it will not bring or commence any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or in tort or otherwise, against the Holder arising out of or based upon this Debenture or any matter relating to this Debenture, or any other Transaction Document, or any contemplated transaction, in any forum other than the courts of the State of New York sitting in New York County, and the United States District Court of the Southern District of New York, and any appellate court from any thereof, and each of the parties hereto irrevocably and unconditionally submits to the jurisdiction of such courts and agrees that all claims in respect of any such suit, claim, action, litigation or proceeding may be heard and determined in such New York State Court or, to the fullest extent permitted by applicable law, in such federal court. The Company and the Holder agree that a final judgment in any such suit, claim, action, litigation or proceeding shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law.
(iv)
The Company and the Holder irrevocably consent to the service of process out of any of the aforementioned courts in any such suit, claim, action, litigation or proceeding by the mailing of copies thereof by registered or certified mail postage prepaid, to it at the address provided for notices in this Debenture, such service to become effective thirty (30) days after the date of mailing.
(v)
Nothing herein shall affect the right of the Holder to serve process in any other manner permitted by law or to commence legal proceedings or to otherwise proceed against the Company or any other Person in the Governing Jurisdiction or in any other jurisdiction.
(c)
THE PARTIES MUTUALLY WAIVE ALL RIGHT TO TRIAL BY JURY OF ALL CLAIMS OF ANY KIND ARISING OUT OF OR BASED UPON THIS DEBENTURE OR ANY MATTER RELATING TO THIS DEBENTURE, OR ANY OTHER TRANSACTION DOCUMENT, OR ANY CONTEMPLATED TRANSACTION. THE PARTIES ACKNOWLEDGE THAT THIS IS A WAIVER OF A LEGAL RIGHT AND THAT THE PARTIES EACH MAKE THIS WAIVER VOLUNTARILY AND KNOWINGLY AFTER CONSULTATION WITH COUNSEL OF THEIR RESPECTIVE CHOICE. THE PARTIES AGREE THAT ALL SUCH CLAIMS SHALL BE TRIED BEFORE A JUDGE OF A COURT HAVING JURISDICTION, WITHOUT A JURY.
(11)
If the Company fails to strictly comply with the terms of this Debenture, then the Company shall reimburse the Holder promptly for all reasonable and documented fees, costs and expenses, including, without limitation, reasonable and documented attorneys’ fees and expenses incurred by the Holder in any action in connection with this Debenture, including, without limitation, those incurred: (i) during any workout, attempted workout, and/or in connection with the rendering of legal advice as to the Holder’s rights, remedies and obligations, (ii) collecting any sums which become due to the Holder, (iii) defending or prosecuting any proceeding or any counterclaim to any proceeding or appeal; or (iv) the protection, preservation or enforcement of any rights or remedies of the Holder.
(12)
Any waiver by the Holder of a breach of any provision of this Debenture shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this Debenture. The failure of the Holder to insist upon strict adherence to any term of this Debenture on one or more occasions shall not be considered a waiver or deprive that party of the right thereafter to insist upon strict adherence to that term or any other term of this Debenture. Any waiver must be in writing.
10
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(13)
If any provision of this Debenture is invalid, illegal or unenforceable, the balance of this Debenture shall remain in effect, and if any provision is inapplicable to any person or circumstance, it shall nevertheless remain applicable to all other persons and circumstances. If it shall be found that any Interest or other amount deemed Interest due hereunder shall violate applicable laws governing usury, the applicable rate of interest due hereunder shall automatically be lowered to equal the maximum permitted rate of interest. The Company covenants (to the extent that it may lawfully do so) that it shall not at any time insist upon, plead, or in any manner whatsoever claim or take the benefit or advantage of, any stay, extension or usury law or other law which would prohibit or forgive the Company from paying all or any portion of the Principal of or Interest on this Debenture as contemplated herein, wherever enacted, now or at any time hereafter in force, or which may affect the covenants or the performance of this Debenture, and the Company (to the extent it may lawfully do so) hereby expressly waives all benefits or advantage of any such law, and covenants that it will not, by resort to any such law, hinder, delay or impeded the execution of any power herein granted to the Holder, but will suffer and permit the execution of every such as though no such law has been enacted.
(14)
CERTAIN DEFINITIONS. For purposes of this Debenture, the following terms shall have the following meanings:
(a)
Amortization Event” shall mean (i) the daily VWAP is less than the Floor Price then in effect for five Trading Days during a period of seven consecutive Trading Days (a “Floor Price Event”), or (ii) a Registration Default has occurred (the last such day of each such occurrence, a “Amortization Event Date”).
(b)
“Amortization Principal Amount” shall have the meaning set forth in Section (2)(a).
(c)
“Applicable Price” shall have the meaning set forth in Section (5)(b).
(d)
“Approved Stock Plan” means any employee benefit plan or share incentive plan which has been approved by the Board of Directors of the Company, pursuant to which the Company’s securities may be issued to any employee, officer or director for services provided to the Company.
(e)
“Bloomberg” means Bloomberg Financial Markets (or if not available, a similar service provider of national recognized standing).
(f)
“Business Day” means any day except Saturday, Sunday and any day which shall be a federal legal holiday in the United States or British Columbia, Canada or a day on which banking institutions in the State of New York or the Province of British Columbia are authorized or required by law or other government action to close.
(g)
“Buy-In” shall have the meaning set forth in (4)(b)(ii).
(h)
“Buy-In Price” shall have the meaning set forth in (4)(b)(ii).
(i)
“Calendar Month” means one of the twelve months of the year.
(j)
“Change of Control Transaction” means the occurrence of (a) an acquisition after the date hereof by an individual or legal entity or “group” (as described in Rule 13d-5(b)(1) promulgated under the Exchange Act) of effective control (whether through legal or beneficial ownership of capital stock of the Company, by contract or otherwise) of in excess of fifty percent (50%) of the voting power of the Company (except that the acquisition of voting securities by the Holder or any other current holder of convertible securities of the Company shall not constitute a Change of Control Transaction for purposes hereof), (b) a replacement at one time of more than one-half of the members of the board of directors of the Company (other than as due to the death or disability of a member of the board of directors) which is not approved by a majority of those individuals who are members of the board of directors on the date hereof (or by those individuals who are serving as members of the board of directors on any date whose nomination to the board of directors was approved by a majority of the members of the board of directors who are members on the date hereof), (c) the sale of all or substantially all of the asets of the Company in one or a series of related transaction with or into another entity, (d) the merger or consolidation of the Company in one or a series of related transactions with or into another entity and, after giving effect to such transaction, the shareholders of the Company immediately prior to such transaction own less than 50% of the aggregate voting power of the Company or the successor entity of such transaction, or (d) the execution by the Company of an agreement to which the Company is a party or by which it is bound, providing for any of the events set forth above in (a), (b) or (c).
11
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
No transfer to a wholly-owned Subsidiary shall be deemed a Change of Control Transaction under this provision.
(k)
“Clear Street ATM” means the sales agreement entered into with Clear Street LLC, dated February 23, 2024.
(l)
“Closing Price” means the price per share in the last reported trade of the Common Shares on a Principal Market or on the exchange which the Common Shares are then listed as quoted by Bloomberg.
(m)
“Commission” means the Securities and Exchange Commission.
(n)
“Common Shares” means the common shares in the capital of the Company and shares of any other class into which such shares may hereafter be changed or reclassified.
(o)
“Conversion Amount” means the portion of the Principal and accrued and unpaid Interest hereunder to be converted, redeemed or otherwise with respect to which this determination is being made.
(p)
“Conversion Price” means, as of any Conversion Date (as defined below) or other date of determination the lower of (i) $1.16 per share of Common Shares (the “Fixed Price”), or (ii) 95% of the lowest daily VWAP for the Common Shares during the 10 consecutive Trading Days immediately preceding the Conversion Date or other date of determination (the “Market Price”), but which Market Price shall not be lower than the Floor Price then in effect. The Conversion Price shall be adjusted from time to time pursuant to the other terms and conditions of this Debenture.
(q)
“Convertible Securities” means any stock or securities (other than Options) directly or indirectly convertible into or exercisable or exchangeable for Common Shares.
(r)
“Dilutive Issuance” shall have the meaning set forth in Section (5)(b).
(s)
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
(t)
“Excluded Securities” means any Common Shares issued or issuable or deemed to be issued by the Company: (i) under any Approved Stock Plan, (ii) pursuant to the Securities Purchase Agreement (including the Debentures and Other Debentures and the shares of Common Shares issued in connection with this Debenture and any of the Other Debentures); (iii) upon conversion, exercise or exchange of any Options or Convertible Securities which are outstanding on the day immediately preceding the date of the Securities Purchase Agreement; provided, that such issuance of Common Shares upon exercise of such Options or Convertible Securities is made pursuant to the terms of such Options or Convertible Securities in effect on such date and such Options or Convertible Securities are not amended, modified or changed on or after such date, (iv) pursuant to any Permitted ATM Sales as set forth in the Securities Purchase Agreement, or (v) upon a stock split, reverse stock split, distribution of bonus shares, combination or other recapitalization events.
(u)
“Floor Price” solely with respect to the Market Price, shall mean $0.20per Common Share.
(v)
“Fundamental Transaction” means any of the following: (1) the Company effects any merger or consolidation of the Company with or into another Person and the Company is the non-surviving company (other than a merger or consolidation with a wholly owned Subsidiary of the Company for the purpose of redomiciling the Company), (2) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (3) any take-over bid, tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Shares are permitted to tender or exchange their shares for other securities, cash or property, or (4) the Company effects any reclassification of the Common Shares or any compulsory share exchange pursuant to which the Common Shares are effectively converted into or exchanged for other securities, cash or property.
(w)
"New Issuance Price" shall have the meaning set forth in Section (5)(b).
12
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(x)
“Optional Redemption” shall have the meaning set forth in (2)(b).
(y)
“Options” means any rights, warrants or options to subscribe for or purchase Common Shares or Convertible Securities.
(z)
“Other Debentures” means any other debentures issued pursuant to the Securities Purchase Agreement and any other debentures, notes, or other instruments issued in exchange, replacement, or modification of the foregoing.
(aa)
“Payment Premium” means 10%.
(bb)
“Periodic Reports” shall mean all of the Company’s reports required to be filed by the Company with the Commission under applicable laws and regulations (including, without limitation, Regulation S-K), including annual reports, quarterly reports, and current reports, for so long as any amounts are outstanding under this Debenture; provided that all such Periodic Reports shall include, when filed, all information, financial statements, audit reports (when applicable) and other information required to be included in such Periodic Reports in compliance with all applicable laws and regulations.
(cc)
“Person” means a corporation, an association, a partnership, organization, a business, an individual, a government or political subdivision thereof or a governmental agency.
(dd)
“Principal Market” means the Nasdaq Global Market; provided however, that in the event the Company’s Common Shares are ever listed or traded on any of the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, or the Nasdaq Capital Market, or such successor thereto, the “Principal Market” shall mean that market on which the Common Shares are then listed or traded.
(ee)
“Redemption Amount” shall have the meaning set forth in (2)(b).
(ff)
“Redemption Notice” shall have the meaning set forth in (2)(b).
(gg)
“Registration Default” means any of the following: (i) a Registration Statement is not declared effective on or prior to the date that is ten Trading Days following its Effectiveness Deadline (as defined in the Registration Rights Agreement), or (ii) on any day after the effectiveness of a Registration Statement subject to Allowable Grace Periods (as defined in the Registration Rights Agreement), sales of all of the Registrable Securities (as defined in the Registration Rights Agreement) required to be included on such Registration Statement (after giving effect to any reduction for Cut Back Securities (as defined in the Registration Rights Agreement) cannot be made pursuant to such Registration Statement (including, without limitation, because of a failure to keep such Registration Statement effective, a failure to disclose such information as is necessary for sales to be made pursuant to such Registration Statement, or by reason of a stop order) or the prospectus contained therein is not available for use for any reason (a “Maintenance Failure”), which Maintenance Failure is not cured within 10 Trading Days.
(hh)
“Registration Rights Agreement” has the meaning given such term in the Securities Purchase Agreement.
(ii)
“Registration Statement” has the meaning given such term in the Registration Rights Agreement.
(jj)
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
(kk)
“Subsidiary means any subsidiary of the Company listed in Schedule III of the Securities Purchase Agreement, and any subsidiary that after the date of this Agreement becomes “significant subsidiary” of the Company (as defined in Rule 1-02(w) of Regulation S-X under the Exchange Act).
(ll)
“Trading Day” means a day on which the Common Shares are quoted or traded on a Principal Market on which the Common Shares are then quoted or listed; provided, that in the event that the Common Shares are not listed or quoted, then Trading Day shall mean a Business Day.
13
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
(mm)
“Transaction Document” has the meaning given such term in the Securities Purchase Agreement.
(nn)
“Underlying Shares” means the Common Shares issuable upon conversion of this Debenture in accordance with the terms hereof.
(oo)
“Underlying Shares Registration Statement” means a registration statement meeting the requirements set forth in the Registration Rights Agreement, covering among other things the resale of the Underlying Shares and naming the Holder as a “selling stockholder” thereunder.
(pp)
"VWAP" means, for any security as of any date, the daily dollar volume-weighted average price for such security on the Principal Market during regular trading hours as reported by Bloomberg through its “Historical Prices – Px Table with Average Daily Volume” functions.
[Signature Page Follows]
14
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
IN WITNESS WHEREOF, the Company has caused this Convertible Debenture to be duly executed by a duly authorized officer as of the date set forth above.
|
|
|
COMPANY: |
|
IMMUNOPRECISE ANTIBODIES LTD. |
|
|
|
By: (signed) “Jennifer Bath” |
|
Name: Jennifer Bath |
|
Title: Chief Executive Officer |
|
|
DOCPROPERTY "DocID" \* MERGEFORMAT
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
EXHIBIT I
CONVERSION NOTICE
(To be executed by the Holder in order to Convert the Debenture)
TO: IMMUNOPRECISE ANTIBODIES LTD.
Via Email:
The undersigned hereby irrevocably elects to convert a portion of the outstanding and unpaid Conversion Amount of Debenture No. IPA-1 into Common Shares of IMMUNOPRECISE ANTIBODIES LTD., according to the conditions stated therein, as of the Conversion Date written below.
|
|
|
|
|
Conversion Date: |
|
Principal Amount to be Converted: |
|
Accrued Interest to be Converted: |
|
Total Conversion Amount to be converted: |
|
Fixed Price: |
|
Market Price: |
|
Applicable Conversion Price: |
|
Number of Common Shares to be issued: |
|
|
|
Please issue the Common Shares in the following name and deliver them to the following account: |
Issue to: |
|
Broker DTC Participant Code: |
|
Account Number: |
|
|
|
Authorized Signature: |
|
Name: |
|
Title: |
|
|
|
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
EXHIBIT II
FORM OF TRANSFER
FOR VALUE RECEIVED, the undersigned transferor hereby sells, assigns and transfers unto
of the Debentures registered in the name of the undersigned transferor represented by the attached Debenture Certificate.
The undersigned hereby represents, warrants and certifies that the transfer is being made in accordance with a transaction that does not require registration under the United States Securities Act of 1933, as amended (the “Securities Act”) or any applicable state securities laws, and the undersigned has furnished to the Company an opinion of counsel of recognized standing or other evidence of exemption, in form and substance reasonably satisfactory to the Company to such effect.
DATED this day of , .
Signature of Holder
(Transferor)
Print name of Holder
Address
NOTE: The signature on this transfer form must correspond with the name as recorded on the face of the Debenture Certificate in every particular without alteration or enlargement or any change whatsoever or this transfer form must be signed by a duly authorized trustee, executor, administrator, curator, guardian, attorney of the Holder or a duly authorized signing officer in the case of a corporation. If this transfer form is signed by any of the foregoing, or any person acting in a fiduciary or representative capacity, the Debenture Certificate must be accompanied by evidence of authority to sign.
This transfer form must be accompanied by an opinion of counsel or other evidence (which must be reasonably satisfactory to the Company), to the effect that the transfer is exempt from the registration requirements of the Securities Act and the securities laws of all applicable states of the United States.
DOCPROPERTY "CUS_DocIDChunk0" 4855-3816-2126\9
EX-4.5
4
ipa-ex4_5.htm
EX-4.5
EX-4.5
Exhibit 4.5
SECURITIES PURCHASE AGREEMENT
THIS SECURITIES PURCHASE AGREEMENT (this “Agreement”), dated as of July 16, 2024, is between IMMUNOPRECISE ANTIBODIES LTD., a British Columbia corporation, with principal executive offices located at 3204-4464 Markham Street, Victoria, BC V8Z 7X8 (the “Company”), and each of the investors listed on the Schedule of Buyers attached as Schedule I hereto (individually, a “Buyer” and collectively the “Buyers”).
WITNESSETH
WHEREAS, the Company and each Buyer desire to enter into this transaction for the Company to sell and the Buyers to purchase the Convertible Debentures (as defined below) pursuant to an exemption from registration pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”) and/or Rule 506 of Regulation D (“Regulation D”) promulgated by the U.S. Securities and Exchange Commission (the “SEC”) thereunder;
WHEREAS, the parties desire that, upon the terms and subject to the conditions contained herein, the Company shall issue and sell to the Buyer, as provided herein, and the Buyer shall purchase convertible debentures in the form attached hereto as “Exhibit A” (the “Convertible Debentures”) in the aggregate principal amount of up to $3,000,000 (the “Subscription Amount”), which shall be convertible into common shares in the capital of the Company (the “Common Shares”) (as converted, the “Conversion Shares”), of which $2,000,000 shall be purchased upon the signing this Agreement (the “First Closing”) and $1,000,000 shall be purchased on or about the date the initial Registration Statement (as defined in the Registration Rights Agreement (as defined below)) has first been declared effective by the SEC (the “Second Closing”) (individually referred to as a “Closing” and collectively referred to as the “Closings”), at a purchase price equal to 95% of the Subscription Amount (the “Purchase Price”) in the respective amounts set forth opposite each Buyer name on Schedule I to this Agreement;
WHEREAS, on or before the First Closing Date (as defined in Section 1(c) below), the parties hereto are executing and delivering a Registration Rights Agreement (the “Registration Rights Agreement”) pursuant to which the Company has agreed to provide certain registration rights under the Securities Act and the rules and regulations promulgated thereunder, and applicable state securities laws;
WHEREAS, contemporaneously with the execution and delivery of this Agreement, the Company is delivering Irrevocable Transfer Agent Instructions (the “Irrevocable Transfer Agent Instructions”) to its transfer agent in the form attached hereto as “Exhibit B;”
WHEREAS, on or before the First Closing Date, ImmunoPrecise Antibodies (Canada), Ltd., ImmunoPrecise Antibodies (Europe) BV and BioStrand B.V. shall enter into a global guaranty agreement (the “Global Guaranty”) in favor of the Buyer;
WHEREAS, the Company has engaged Clear Street LLC as its exclusive placement agent (the “Placement Agent”) for the offering of the Securities (as defined below); and
WHEREAS, the Convertible Debentures and the Conversion Shares are collectively referred to herein as the “Securities.”
AGREEMENT
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and each Buyer hereby agree as follows:
1.
PURCHASE AND SALE OF CONVERTIBLE DEBENTURES.
(a)
Purchase of Convertible Debentures. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below, the Company shall issue and sell to each Buyer, and each Buyer severally, but not jointly, agrees to purchase from the Company at each Closing, Convertible Debentures with principal amount corresponding to the Subscription Amount set forth opposite each Buyer’s name on Schedule I attached hereto.
(b)
Closing Dates. Each Closing shall occur remotely by conference call and electronic delivery of documentation.
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
The date and time of each Closing shall be as follows: (i) the First Closing shall be 10:00 a.m., New York time, on the first Business Day on which the conditions to the Closing set forth in Sections 6 and 7 below are satisfied or waived (or such other date as is mutually agreed to by the Company and each Buyer) (the “First Closing Date”) and (ii) the Second Closing shall be 10:00 a.m., New York time, on the first Business Day after the Registration Statement is first declared effective by the SEC, provided the conditions to the Closing set forth in Sections 6 and 7 below are satisfied or waived (or such other date as is mutually agreed to by the Company and each Buyer) (the “Second Closing Date” and with the First Closing Date, the “Closing Dates”). As used herein “Business Day” means any day other than a Saturday, Sunday or other day on which commercial banks in New York, New York or Victoria, British Columbia are authorized or required by law to remain closed.
(c)
Form of Payment; Deliveries. Subject to the satisfaction (or waiver) of the terms and conditions of this Agreement, on each Closing Date, (i) the Buyers shall deliver to the Company, in immediately available funds to a bank account designated in writing by the Company, the Purchase Price for the Convertible Debentures to be issued and sold to such Buyer at such Closing, minus any fees or expenses to be paid directly from the proceeds of such Closing as set forth herein, and (ii) the Company shall deliver to each Buyer, Convertible Debentures which such Buyer is purchasing at such Closing with a principal amount corresponding with the Subscription Amount set forth opposite each Buyer’s name on Schedule of Buyers attached as Schedule I hereto, duly executed on behalf of the Company.
(d)
Home Country Practice. Prior to the date hereof, the Company has taken all actions required pursuant to Nasdaq Rule 5615(a)(3) to duly and validly rely on the exemption for foreign private issuers from applicable rules and regulations of the Nasdaq by adopting the home country practice (the “Home Country Practice”) in connection with the transactions contemplated hereunder (including an exemption from any Nasdaq rules that would otherwise require seeking shareholder approval in respect of such transactions). The Company may issue the relevant Conversion Shares upon conversion of any outstanding Convertible Debentures without regard to the limitations imposed by Nasdaq Rule 5635(d). So long as any Convertible Debentures are outstanding, the Company shall comply with the Home Country Practice rules and shall not take any action to change its Home Country Practice or become subject to Nasdaq Rule 5635(d) with respect to transactions contemplated herein. The Company’s practices in connection with the transactions contemplated hereunder are not prohibited by its home country’s laws.
2.
BUYER’S REPRESENTATIONS AND WARRANTIES.
Each Buyer, severally and not jointly, represents and warrants to the Company with respect to only itself that, as of the date hereof and as of each Closing Date:
(a)
Investment Purpose. The Buyer is acquiring the Securities as principal for its own account for investment purposes and not with a view towards, or for resale in connection with, the public sale or distribution thereof, except pursuant to sales registered under or exempt from the registration requirements of the Securities Act; provided, however, that by making the representations herein, such Buyer does not agree, or make any representation or warranty, to hold any of the Securities for any minimum or other specific term and reserves the right to dispose of the Securities at any time in accordance with, or pursuant to, a registration statement covering such Securities or an available exemption under the Securities Act. Such Buyer does not presently have any agreement or understanding, directly or indirectly, with any Person (as defined below) to distribute any of the Securities in violation of applicable securities laws. As used herein, “Person” means a corporation, a limited liability company, an association, a partnership, an organization, a business, an individual, a governmental or political subdivision thereof or a governmental agency.
(b)
Accredited Investor Status. The Buyer is an “Accredited Investor” as that term is defined in Rule 501(a)(3) of Regulation D.
(c)
No Resident of Canada Status. The Buyer is not a resident in any province or territory of Canada and acknowledges that (i) no Canadian securities commission or similar regulatory authority has reviewed or passed on the merits of Securities; (ii) there is no government or other insurance covering the Securities; and (iii) the Company hereby advises the Buyer that the Company is relying on an exemption from the requirements to provide the Buyer with a prospectus under the Securities Act (British Columbia) and other applicable securities laws (“Canadian Securities Laws”) and, as a consequence of acquiring the Securities pursuant to this exemption, certain protections, rights and remedies provided by Canadian Securities Laws, including statutory rights of rescission or damages, will not be available to the Buyer.
2
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
The Purchase Price which will be advanced by the Buyer to the Company hereunder will not represent proceeds of crime for the purposes of the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada) (the “PCMLA”) and the Buyer acknowledges that the Company may in the future be required by law to disclose the Buyer’s name and other information relating to this Agreement and the Buyer’s subscription hereunder, on a confidential basis, pursuant to the PCMLA; and to the best of its knowledge (i) none of the subscription funds to be provided by the Buyer (A) have been or will be derived from or related to any activity that is deemed criminal under the laws of Canada, the United States of America, or any other jurisdiction, or (B) are being tendered on behalf of a person or entity who has not been identified to the Buyer, and (ii) it shall promptly notify the Company if the Buyer discovers that any of such representations ceases to be true, and to provide the Company with appropriate information in connection therewith.
(e)
Reliance on Exemptions. The Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of such Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of such Buyer to acquire the Securities.
(f)
Information. The Buyer and its advisors (and its counsel), if any, have been furnished with all materials relating to the business, finances and operations of the Company and information the Buyer deemed material to making an informed investment decision regarding its purchase of the Securities, which have been requested by such Buyer, including, without limitation, documents and information concerning the Company’s draft report on Form 20-F (the “Draft 20-F Information”), which was provided to the Buyer confidentially pursuant to and in accordance with the terms and conditions of the non-disclosure agreement entered into on July 8, 2024, between the Company and Yorkville Advisors Global, LP, (the “Yorkville NDA”). The Buyer and its advisors, if any, have been afforded the opportunity to ask questions of the Company and its management. Neither such inquiries nor any other due diligence investigations conducted by such Buyer or its advisors, if any, or its representatives shall modify, amend or affect such Buyer’s right to rely on the Company’s representations and warranties contained in Section 3 below. The Buyer understands that its investment in the Securities involves a high degree of risk. The Buyer has sought such accounting, legal and tax advice, as it has considered necessary to make an informed investment decision with respect to its acquisition of the Securities. The Buyer acknowledges that the Draft 20-F Information may contain material, non-public and other information with respect to the Company that will not be publicly disclosed until the Company files its Report on Form 20-F with the SEC. The Buyer acknowledges and agrees that the Company does not make and has not made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 3.
(g)
Transfer or Resale. The Buyer understands that: (i) the Securities have not been registered under the Securities Act or any state securities laws, and may not be offered for sale, sold, assigned or transferred unless (A) subsequently registered thereunder, (B) such Buyer shall have delivered to the Company an opinion of counsel, in a generally acceptable form, to the effect that such Securities to be sold, assigned or transferred may be sold, assigned or transferred pursuant to an exemption from such registration requirements, or (C) such Buyer provides the Company with reasonable assurances (in the form of seller and broker representation letters) that such Securities can be sold, assigned or transferred pursuant to Rule 144 promulgated under the Securities Act, as amended (or a successor rule thereto) (collectively, “Rule 144”), in each case following the applicable holding period set forth therein, or (d) such Buyer shall have delivered to the Company and the Company’s Transfer Agent and Rule 904 declaration in a form agreed to by the Company and the Company’s Transfer Agent. Acting reasonably; and (ii) any sale of the Securities made in reliance on Rule 144 may be made only in accordance with the terms of Rule 144 and further, if Rule 144 is not applicable, any resale of the Securities under circumstances in which the seller (or the Person through whom the sale is made) may be deemed to be an underwriter (as that term is defined in the Securities Act) may require compliance with some other exemption under the Securities Act or the rules and regulations of the SEC thereunder. Notwithstanding the foregoing, the Securities may be pledged in connection with a bona fide margin account or other loan or financing arrangement secured by the Securities and such pledge of Securities shall not be deemed to be a transfer, sale or assignment of the Securities hereunder, and no Buyer effecting a pledge of Securities shall be required to provide the Company with any notice thereof or otherwise make any delivery to the Company pursuant to this Agreement or any other Transaction Document, including, without limitation, this Section 2(g).
(h)
Legends. The Buyer agrees to the imprinting, so long as its required by this Section 2(h), of a restrictive legend on the Securities in substantially the following form:
THE SECURITIES REPRESENTED BY THIS CERTIFICATE [AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE] HAVE NOT BEEN REGISTERED UNDER THE UNITED STATES SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR APPLICABLE STATE SECURITIES LAWS.
3
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
THE SECURITIES [AND THOSE SECURITIES INTO WHICH THEY ARE CONVERTIBLE] HAVE BEEN ACQUIRED SOLELY FOR INVESTMENT PURPOSES AND NOT WITH A VIEW TOWARD RESALE AND MAY ONLY BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (A) TO THE CORPORATION, (B) OUTSIDE THE UNITED STATES IN COMPLIANCE WITH RULE 904 OF REGULATION S UNDER THE SECURITIES ACT AND IN COMPLIANCE WITH LOCAL LAWS AND REGULATIONS, (C) IN COMPLIANCE WITH THE EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT PROVIDED BY RULE 144 THEREUNDER, IF AVAILABLE, AND IN COMPLIANCE WITH ANY APPLICABLE STATE SECURITIES OR “BLUE SKY” LAWS, (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, OR (E) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT AND, IN THE CASE OF SUBPARAGRAPH (C) OR (D), THE SELLER FURNISHES TO THE CORPORATION AN OPINION OF COUNSEL OF RECOGNIZED STANDING OR SUCH OTHER EVIDENCE AS THE CORPORATION MAY REQUIRE IN FORM AND SUBSTANCE REASONABLY SATISFACTORY TO THE CORPORATION TO SUCH EFFECT. NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.
Certificates evidencing the Conversion Shares shall not contain any legend (including the legend set forth above), (i) while a registration statement covering the resale of such security is effective under the Securities Act, (ii) following any sale of such Conversion Shares pursuant to Rule 144, (iii) if such Conversion Shares are eligible for sale under Rule 144, or (iv) if such legend is not required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the SEC). If a legend is not required pursuant to the foregoing, the Company shall no later than two (2) Trading Days (or such earlier date as required pursuant to the Exchange Act (as defined below) or other applicable law, rule or regulation for the settlement of a trade initiated on the date such Buyer delivers such legended certificate representing such securities to the Company) following the delivery by a Buyer to the Company or the transfer agent (with notice to the Company) of a legended certificate representing such securities (endorsed or with stock powers attached, and otherwise in form necessary to affect the reissuance and/or transfer, if applicable), together with any other deliveries from such Buyer as may be required above in this Section 2(h), as directed by such Buyer, either: (A) provided that the Company’s transfer agent is participating in the DTC Fast Automated Securities Transfer Program, credit the aggregate number of shares of Common Shares to which such Buyer shall be entitled to such Buyer’s or its designee’s balance account with DTC through its Deposit/Withdrawal at Custodian system or (B) if the Company’s transfer agent is not participating in the DTC Fast Automated Securities Transfer Program, issue and deliver (via reputable overnight courier) to such Buyer, a certificate representing such securities that is free from all restrictive and other legends, registered in the name of such Buyer or its designee. The Company shall be responsible for any transfer agent fees or DTC fees with respect to any issuance of Securities or the removal of any legends with respect to any Securities in accordance herewith. The Buyer agrees that the removal of a restrictive legend from certificates representing Securities as set forth in this Section 2(h) is predicated upon the Company’s reliance that the Buyer will sell any Securities pursuant to either the registration requirements of the Securities Act, including any applicable prospectus delivery requirements, or an exemption therefrom, and that if Securities are sold pursuant to a registration statement, they will be sold in compliance with the plan of distribution set forth therein.
(i)
Organization; Authority. Such Buyer is an entity duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization with the requisite power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents to which it is a party and otherwise to carry out its obligations hereunder and thereunder.
(j)
Authorization, Enforcement. The Transaction Documents to which each such Buyer is a party have been duly and validly authorized, executed and delivered on behalf of such Buyer and shall constitute the legal, valid and binding obligations of such Buyer enforceable against such Buyer in accordance with their terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors' rights and remedies.
(k)
No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the consummation by such Buyer of the transactions contemplated hereby will not (i) result in a violation of the organizational documents of such Buyer, (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which such Buyer is a party or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Buyer, except, in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which could not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Buyer to perform its obligations hereunder.
4
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(l)
Certain Trading Activities. The Buyer has not directly or indirectly, nor has any Person acting on behalf of or pursuant to any understanding with the Buyer, engaged in any transactions in the securities of the Company (including, without limitation, any Short Sales (as defined below) involving the Company's securities) during the period commencing as of the time that the Buyer first contacted the Company or the Company's agents regarding the specific investment in the Company contemplated by this Agreement and ending immediately prior to the execution of this Agreement by such Buyer.
(m)
No General Solicitation. The Buyer is not purchasing or acquiring the Securities as a result of any general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer or sale of the Securities.
(n)
Not an Affiliate. The Buyer is not (i) an officer or director of the Company or any of its Subsidiaries, (ii) an “affiliate” (as defined in Rule 144) of the Company or any of its Subsidiaries or (iii) a “beneficial owner” of more than 10% of the Common Shares (as defined for purposes of Rule 13d3 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)).
(o)
Independent Investment Decision. Such Buyer has independently evaluated the merits of its decision to purchase Securities pursuant to the Transaction Documents. Such Buyer understands that nothing in this agreement or any other materials presented by or on behalf of the Company to the Buyer in connection with the purchase of the Securities constitutes legal, tax or investment advice. Such Buyer has consulted such legal, tax and investment advisors as it, in its sole discretion, has deemed necessary or appropriate in connection with its purchase of the Securities. Such Buyer understands that the Placement Agent has acted solely as the agent of the Company in this placement of the Securities and such Buyer has not relied on the business or legal advice of the Placement Agent or any of its agents, counsel or affiliates in making its investment decision hereunder, and confirms that none of such Persons has made any representations or warranties to such Buyer in connection with the transactions contemplated by the Transaction Documents.
3.
REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
Except as set forth (i) under the corresponding section of the disclosure schedule (dated as of the date of this Agreement) delivered to the Buyer by the Company on the date of this Agreement (the “Disclosure Schedule”) which Disclosure Schedule shall be deemed a part hereof and to qualify any representation or warranty otherwise made herein to the extent of such disclosure, or (ii) in the SEC Documents (as defined below) that are available on the SEC’s website through the EDGAR system at least one (1) Business Day prior to the date of this Agreement (unless the context provides otherwise), or the Company hereby makes the representations and warranties set forth below to each Buyer:
(a)
Organization and Qualification. The Company and each of its Subsidiaries are entities formed, existing and in good standing under the laws of the jurisdiction in which they are formed, and have the corporate power and authority to own their properties and to carry on their business. The Company and each of its Subsidiaries is duly qualified as a foreign entity to do business and is in good standing in every jurisdiction in which its ownership of property or the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not reasonably be expected to have a Material Adverse Effect (as defined below). As used in this Agreement, “Material Adverse Effect” means any material adverse effect on (i) the business, properties, assets, liabilities, operations (including results thereof), condition (financial or otherwise) or prospects of the Company and its Subsidiaries, taken as a whole, (ii) the transactions contemplated hereby or in any of the other Transaction Documents or any other agreements or instruments to be entered into by the Company in connection herewith or therewith or (iii) the authority or ability of the Company to perform any of its obligations under any of the Transaction Documents. “Subsidiaries” means any subsidiary of the Company listed in Schedule II attached hereto, and any subsidiary that after the date of this Agreement becomes “significant subsidiary” of the Company (as defined in Rule 1-02(w) of Regulation S-X under the Exchange Act), and each of the foregoing, is individually referred to herein as a “Subsidiary.”
(b)
Authorization; Enforcement; Validity. The Company has the requisite corporate power and authority to enter into and perform its obligations under this Agreement and the other Transaction Documents and to issue the Securities in accordance with the terms hereof and thereof. The execution and delivery of this Agreement and the other Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Convertible Debentures, the reservation for issuance and issuance of the Conversion Shares issuable upon conversion of the Convertible Debentures), have been duly authorized by the Company's board of directors and no further filing, consent or authorization is required by the Company, its board of directors or its shareholders or other governmental body.
5
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
This Agreement has been, and the other Transaction Documents to which the Company is a party will be prior to the Closing, duly executed and delivered by the Company, and each constitutes the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with its respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors' rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. “Transaction Documents” means, collectively, this Agreement, the Registration Rights Agreement, the Convertible Debentures, the Global Guaranty, the Irrevocable Transfer Agent Instructions, and each of the other agreements and instruments entered into by the Company or delivered by the Company in connection with the transactions contemplated hereby and thereby, as may be amended from time to time.
(c)
Issuance of Securities. The issuance of the Securities has been duly authorized and, upon issuance and payment in accordance with the terms of the Transaction Documents the Securities shall be validly issued, fully paid and nonassessable and free from all preemptive or similar rights, mortgages, defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively “Liens”) with respect to the issuance thereof. As of each Closing Date, the Company shall have reserved no less than the Required Reserve Amount (as defined herein). Upon issuance or conversion in accordance with the Convertible Debentures, the Conversion Shares, when issued, will be validly issued, fully paid and nonassessable and free from all preemptive or similar rights or Liens with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of Common Shares.
(d)
No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Convertible Debentures, the Conversion Shares, and the reservation for issuance of the Conversion Shares) will not (i) result in a violation of the Articles (as defined below), certificate of formation, memorandum of association, articles of association, bylaws or other organizational documents of the Company or any of its Subsidiaries, or any capital stock or other securities of the Company or any of its Subsidiaries, (ii) conflict with, or constitute a default under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including, without limitation, U.S. federal and state securities laws and regulations, the securities laws of the jurisdictions of the Company's incorporation and the rules and regulations of the Nasdaq Global Market (the “Principal Market,” provided however, that in the event the Company’s Common Shares are ever listed or traded on any of the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, or the Nasdaq Capital Market, or such successor thereto, the “Principal Market” shall mean that market on which the Common Shares are then listed or traded) and including all applicable laws, rules and regulations of the jurisdiction of incorporation of the Company) applicable to the Company or any of its Subsidiaries or by which any property or asset of the Company or any of its Subsidiaries is bound or affected, except in the case of (ii) and (iii) for any conflict, default, right or violation that would not be reasonably be expected to result in a Material Adverse Effect.
(e)
Consents. The Company is not required to obtain any consent from, authorization or order of, or make any filing or registration with (other than any filings as may be required by any federal or state securities agencies and any filings as may be required by the Principal Market), any Governmental Entity (as defined below) or any regulatory or selfregulatory agency or any other Person in order for it to execute, deliver or perform any of its obligations under or contemplated by the Transaction Documents, in each case, in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company or any Subsidiary is required to obtain pursuant to the preceding sentence have been or will be obtained or effected on or prior to each Closing Date, and neither the Company nor any of its Subsidiaries are aware of any facts or circumstances which might prevent the Company or any of its Subsidiaries from obtaining or effecting any of the registration, application or filings contemplated by the Transaction Documents. The Company is not in violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which could reasonably lead to delisting or suspension of the Common Shares in the foreseeable future. The Company has notified the Principal Market of the issuance of all of the Securities hereunder, which does not require obtaining the approval of the shareholders of the Company or any other Person or Governmental Entity, and the Principal Market has completed its review of the related Listing of Additional Shares form. “Governmental Entity” means any nation, state, county, city, town, village, district, or other political jurisdiction of any nature, federal, state, local, municipal, foreign, or other government, governmental or quasigovernmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal), multinational organization or body; or body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature or instrumentality of any of the foregoing, including any entity or enterprise owned or controlled by a government or a public international organization or any of the foregoing.
6
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(f)
Acknowledgment Regarding Buyer's Purchase of Securities. The Company acknowledges and agrees that each Buyer is acting solely in the capacity of an arm's length purchaser with respect to the Transaction Documents and the transactions contemplated hereby and thereby and that no Buyer is (i) an officer or director of the Company or any of its Subsidiaries, (ii) to its knowledge, an "affiliate" (as defined in Rule 144 promulgated under the Securities Act (or a successor rule thereto) (collectively, “Rule 144”)) of the Company or any of its Subsidiaries or (iii) to its knowledge, a “beneficial owner” of more than 10% of the Common Shares (as defined for purposes of Rule 13d3 of the Exchange Act). The Company further acknowledges that no Buyer (nor any affiliate of any Buyer) is acting as a financial advisor or fiduciary of the Company or any of its Subsidiaries (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby, and any advice given by a Buyer or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby is merely incidental to such Buyer's purchase of the Securities. The Company further represents to each Buyer that the Company's decision to enter into the Transaction Documents to which it is a party has been based solely on the independent evaluation by the Company and its representatives.
(g)
No Integrated Offering. None of the Company, its Subsidiaries or any of their affiliates, nor any Person acting on their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to require approval of shareholders of the Company under any applicable shareholder approval provisions, including, without limitation, under the rules and regulations of any exchange or automated quotation system on which any of the securities of the Company are listed or designated for quotation. None of the Company, its Subsidiaries, their affiliates nor any Person acting on their behalf will take any action or steps that would cause the offering of any of the Securities to be integrated with other offerings of securities of the Company.
(h)
Dilutive Effect. The Company understands and acknowledges that the number of Conversion Shares will increase in certain circumstances. The Company further acknowledges its obligation to issue the Conversion Shares upon conversion of the Convertible Debentures in accordance with the terms thereof is, absolute and unconditional regardless of the dilutive effect that such issuance may have on the ownership interests of other shareholders of the Company.
(i)
Application of Takeover Protections; Rights Agreement. The Company and its board of directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, interested shareholder, business combination, poison pill (including, without limitation, any distribution under a rights agreement), shareholder rights plan or other similar antitakeover provision under the Articles or other organizational documents or the laws of the jurisdiction of its incorporation or otherwise which is or could become applicable to any Buyer as a result of the transactions contemplated by this Agreement, including, without limitation, the Company's issuance of the Securities and any Buyer's ownership of the Securities.
(j)
SEC Documents; Financial Statements. During the two (2) years prior to the date hereof, the Company has timely filed all reports, schedules, forms, proxy statements, statements and other documents required to be filed by it with the SEC pursuant to the reporting requirements of the Exchange Act (all of the foregoing filed prior to the date hereof and all exhibits and appendices included therein and financial statements, notes and schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”). The Company has delivered or has made available to the Buyers or their respective representatives true, correct and complete copies of each of the SEC Documents not available on the EDGAR system. As of their respective dates, the SEC Documents complied in all material respects with the requirements of the Exchange Act or the Securities Act, as applicable and none of the SEC Documents, at the time they were filed with the SEC (subject to amendments thereto), contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto as in effect as of the time of filing. Such financial statements have been prepared in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board (“IFRS”), consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal yearend audit adjustments which will not be material, either individually or in the aggregate). The reserves, if any, established by the Company or the lack of reserves, if applicable, are reasonable based upon facts and circumstances known by the Company on the date hereof and there are no loss contingencies that are required to be accrued by the Statement of Financial Accounting Standard No.
7
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
5 of the Financial Accounting Standards Board which are not provided for by the Company in its financial statements or otherwise. No other information provided by or on behalf of the Company to any of the Buyers which is not included in the SEC Documents (including, without limitation, information referred to in Section 2(d) or in the Disclosure Schedule to this Agreement) contains any untrue statement of a material fact or omits to state any material fact necessary in order to make the statements therein not misleading, in the light of the circumstance under which they are or were made. The Company is not currently contemplating to amend or restate any of the financial statements (including, without limitation, any notes or any letter of the independent accountants of the Company with respect thereto) included in the SEC Documents (the “Financial Statements”), nor is the Company currently aware of facts or circumstances which would require the Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financials Statements to be in compliance with IRFS and the rules and regulations of the SEC. The Company has not been informed by its independent accountants that they recommend that the Company amend or restate any of the Financial Statements or that there is any need for the Company to amend or restate any of the Financial Statements.
(k)
Absence of Certain Changes. Since the date of the Company's most recent audited financial statements contained in a Form 20-F, there has been no Material Adverse Effect, nor any event or occurrence specifically affecting the Company or its Subsidiaries that would be reasonably expected to result in a Material Adverse Effect. Since the date of the Company's most recent audited financial statements contained in a Form 20-F, neither the Company nor any of its Subsidiaries has (i) declared or paid any dividends, (ii) sold any material assets, individually or in the aggregate, outside of the ordinary course of business or (iii) made any material capital expenditures, individually or in the aggregate, outside of the ordinary course of business. Neither the Company nor any of its Subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy, insolvency, reorganization, receivership, liquidation or winding up, nor does the Company or any Subsidiary have any knowledge or reason to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or any actual knowledge of any fact which would reasonably lead a creditor to do so. The Company and its Subsidiaries, individually and on a consolidated basis, are not as of the date hereof, and after giving effect to the transactions contemplated hereby to occur at the Closing, will not be Insolvent (as defined below). For purposes of this Section 3(k), “Insolvent” means, (i) with respect to the Company and its Subsidiaries, on a consolidated basis, (A) the present fair saleable value of the Company’s and its Subsidiaries’ assets is less than the amount required to pay the Company’s and its Subsidiaries’ total Indebtedness (as defined below), (B) the Company and its Subsidiaries are unable to pay their debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company and its Subsidiaries intend to incur or believe that they will incur debts that would be beyond their ability to pay as such debts mature; or (ii) with respect to the Company and each Subsidiary, individually, (A) the present fair saleable value of the Company’s or such Subsidiary’s (as the case may be) assets is less than the amount required to pay its respective total Indebtedness, (B) the Company or such Subsidiary (as the case may be) is unable to pay its respective debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company or such Subsidiary (as the case may be) intends to incur or believes that it will incur debts that would be beyond its respective ability to pay as such debts mature. Neither the Company nor any of its Subsidiaries has engaged in any business or in any transaction, and is not about to engage in any business or in any transaction, for which the Company’s or such Subsidiary’s remaining assets constitute unreasonably small capital with which to conduct the business in which it is engaged as such business is now conducted and is proposed to be conducted.
(l)
No Undisclosed Events, Liabilities, Developments or Circumstances. No event, liability, development or circumstance has occurred or exists, or is reasonably expected to exist or occur specific to the Company, any of its Subsidiaries or any of their respective businesses, properties, liabilities, prospects, operations (including results thereof) or condition (financial or otherwise), that (i) would be required to be disclosed by the Company under applicable securities laws on a registration statement filed with the SEC relating to an issuance and sale by the Company of its Common Shares, (ii) could have a material adverse effect on any Buyer’s investment hereunder or (iii) would reasonably be expected to have a Material Adverse Effect, and which in each of (i) through (iii), has not been publicly announced or otherwise disclosed in the Disclosure Schedule or the Draft 20-F Information.
(m)
Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term under its Articles, any certificate of designation, preferences or rights of any other outstanding series of preferred stock of the Company or any of its Subsidiaries or their organizational charter, certificate of formation, memorandum of association, articles of association, Articles or certificate of incorporation or bylaws, respectively. Neither the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in violation of any of the foregoing, except in all cases for violations which would not reasonably be expected to have a Material Adverse Effect. Without limiting the generality of the foregoing, other than as disclosed in the Company’s SEC Documents that are available on the SEC’s website through the EDGAR system at least one (1) Business Day prior to the applicable Closing Date, the Company is not in violation of any of the rules, regulations or requirements of the Principal Market and has no knowledge of any facts or circumstances that could reasonably lead to delisting or suspension of trading of the Common Shares by the Principal Market in the foreseeable future.
8
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
During the one year prior to the date hereof, (i) the Common Shares have been listed or designated for quotation on the Principal Market, (ii) trading in the Common Shares has not been suspended by the SEC or the Principal Market and (iii) the Company has received no communication, written or oral, from the SEC or the Principal Market regarding the suspension or delisting of the Common Shares from the Principal Market, which has not been publicly disclosed. The Company and each of its Subsidiaries possess all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to conduct their respective businesses, except where the failure to possess such certificates, authorizations or permits would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, and neither the Company nor any of its Subsidiaries has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit. There is no agreement, commitment, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any of its Subsidiaries is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in the aggregate, which have not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its Subsidiaries.
(n)
Foreign Corrupt Practices. Neither the Company nor any of its Subsidiaries nor any director, officer, agent, employee, nor any other Person acting for or on behalf of the Company or any of its Subsidiaries (individually and collectively, a “Company Affiliate”) have violated the U.S. Foreign Corrupt Practices Act (the “FCPA) or any other applicable antibribery or anti corruption laws, nor has any Company Affiliate offered, paid, promised to pay, or authorized the payment of any money, or offered, given, promised to give, or authorized the giving of anything of value, to any officer, employee or any other Person acting in an official capacity for any Governmental Entity to any political party or official thereof or to any candidate for political office (individually and collectively, a “Government Official”) or to any Person under circumstances where such Company Affiliate knew or was aware of a high probability that all or a portion of such money or thing of value would be offered, given or promised, directly or indirectly, to any Government Official, for the purpose, in violation of applicable law, of: (i) (A) influencing any act or decision of such Government Official in his/her official capacity, (B) inducing such Government Official to do or omit to do any act in violation of his/her lawful duty, (C) securing any improper advantage, or (D) inducing such Government Official to influence or affect any act or decision of any Governmental Entity, or (ii) assisting the Company or its Subsidiaries in obtaining or retaining business for or with, or directing business to, the Company or its Subsidiaries.
(o)
Equity Capitalization.
(i)
Authorized and Outstanding Capital Stock. As of the date hereof, the authorized capital of the Company consists of unlimited common shares without par value, of which 27,302,260 are outstanding as of the date of this Agreement.
(ii)
Valid Issuance; Available Shares. All of such outstanding shares are duly authorized and have been validly issued and are fully paid and nonassessable. Set forth in a Disclosure Schedule to this Agreement is the number Common Shares that are (A) reserved for issuance pursuant to Convertible Securities (as defined below) (other than the Convertible Debentures) and (B) that are, as of the date hereof, owned by Persons who are “affiliates” (as defined in Rule 405 of the Securities Act and calculated based on the assumption that only officers, directors and holders of at least 10% of the Company’s issued and outstanding Common Shares are “affiliates” without conceding that any such Persons are “affiliates” for purposes of federal securities laws) of the Company or any of its Subsidiaries. To the Company’s knowledge, unless otherwise set forth in the Disclosure Schedule or SEC Documents, no Person owns 10% or more of the Company’s issued and outstanding Common Shares (calculated based on the assumption that all Convertible Securities (as defined below), whether or not presently exercisable or convertible, have been fully exercised or converted (as the case may be) taking account of any limitations on exercise or conversion (including “blockers”) contained therein without conceding that such identified Person is a 10% shareholder for purposes of federal securities laws). “Convertible Securities” means any capital stock or other security of the Company or any of its Subsidiaries that is at any time and under any circumstances directly or indirectly convertible into, exercisable or exchangeable for, or which otherwise entitles the holder thereof to acquire, any capital stock or other security of the Company (including, without limitation, Common Shares) or any of its Subsidiaries.
(iii)
Existing Securities; Obligations.
9
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
Except as disclosed in the SEC Documents: (A) none of the Company's or any Subsidiary's shares, interests or capital stock is subject to preemptive rights or any other similar rights or Liens suffered or permitted by the Company or any Subsidiary; (B) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to issue additional shares, interests or capital stock of the Company or any of its Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries; (C) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any of their securities under the Securities Act (except pursuant to this Agreement); (D) there are no outstanding securities or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security of the Company or any of its Subsidiaries; (E) there are no securities or instruments containing antidilution or similar provisions that will be triggered by the issuance of the Securities; and (G) neither the Company nor any Subsidiary has entered into any Variable Rate Transaction.
(iv)
Organizational Documents. The Company has furnished to the Buyers or filed on EDGAR true, correct and complete copies of the Company's Articles and Notice of Articles, as amended and as in effect on the date hereof (collectively, the “Articles”), and the terms of all convertible securities and the material rights of the holders thereof in respect thereto.
(p)
Indebtedness and Other Contracts. Other than as set forth in a Disclosure Schedule to this Agreement, neither the Company nor any of its Subsidiaries, (i) has any outstanding debt securities, notes, credit agreements, credit facilities or other agreements, documents or instruments evidencing Indebtedness of the Company or any of its Subsidiaries or by which the Company or any of its Subsidiaries is or may become bound, (ii) is a party to any contract, agreement or instrument, the violation of which, or default under which, by the other party(ies) to such contract, agreement or instrument could reasonably be expected to result in a Material Adverse Effect, (iii) has any financing statements securing obligations in any amounts filed in connection with the Company or any of its Subsidiaries; (iv) is in violation of any term of, or in default under, any contract, agreement or instrument relating to any Indebtedness, except where such violations and defaults would not result, individually or in the aggregate, in a Material Adverse Effect, (v) other than the sales agreement entered into with Clear Street LLC, dated February 23, 2024 (the “Clear Street ATM”), is a party to any Variable Rate Transaction, or (vi) is a party to any contract, agreement or instrument relating to any Indebtedness, the performance of which, in the judgment of the Company’s officers, has or is expected to have a Material Adverse Effect. Neither the Company nor any of its Subsidiaries have any liabilities or obligations required to be disclosed in the SEC Documents which are not so disclosed in the SEC Documents, other than those incurred in the ordinary course of the Company’s or its Subsidiaries’ respective businesses and which, individually or in the aggregate, do not or could not have a Material Adverse Effect. For purposes of this Agreement: (x) “Indebtedness” of any Person means, without duplication (A) all indebtedness for borrowed money, (B) all obligations issued, undertaken or assumed as the deferred purchase price of property or services (including, without limitation, “capital leases” in accordance with IFRS) (other than trade payables entered into in the ordinary course of business consistent with past practice), (C) all reimbursement or payment obligations with respect to letters of credit, surety bonds and other similar instruments, (D) all obligations evidenced by notes, bonds, debentures or similar instruments, including obligations so evidenced incurred in connection with the acquisition of property, assets or businesses, (E) all indebtedness created or arising under any conditional sale or other title retention agreement, or incurred as financing, in either case with respect to any property or assets acquired with the proceeds of such indebtedness (even though the rights and remedies of the seller or bank under such agreement in the event of default are limited to repossession or sale of such property), (F) all monetary obligations under any leasing or similar arrangement which, in connection with IFRS, consistently applied for the periods covered thereby, is classified as a capital lease, (G) all indebtedness referred to in clauses (A) through (F) above secured by (or for which the holder of such Indebtedness has an existing right, contingent or otherwise, to be secured by) any Lien upon or in any property or assets (including accounts and contract rights) owned by any Person, even though the Person which owns such assets or property has not assumed or become liable for the payment of such indebtedness, and (H) all Contingent Obligations in respect of indebtedness or obligations of others of the kinds referred to in clauses (A) through (G) above; and (y) “Contingent Obligation” means, as to any Person, any direct or indirect liability, contingent or otherwise, of that Person with respect to any Indebtedness, lease, dividend or other obligation of another Person if the primary purpose or intent of the Person incurring such liability, or the primary effect thereof, is to provide assurance to the obligee of such liability that such liability will be paid or discharged, or that any agreements relating thereto will be complied with, or that the holders of such liability will be protected (in whole or in part) against loss with respect thereto.
10
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(q)
Litigation. Other than as set forth in a Disclosure Schedule to this Agreement, there is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any court, public board, other Governmental Entity, selfregulatory organization or body pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, the Common Shares or any of the Company's or its Subsidiaries' officers or directors, whether of a civil or criminal nature or otherwise, in their capacities as such, which would reasonably be expected to result in a Material Adverse Effect. After reasonable inquiry of its employees, the Company is not aware of any event which might result in or form the basis for any such action, suit, arbitration, investigation, inquiry or other proceeding. Without limitation of the foregoing, there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any current or former director or officer of the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries is the subject of any order, writ, judgment, injunction, decree, determination or award of any Governmental Entity that would reasonably be expected to result in a Material Adverse Effect.
(r)
Intellectual Property Rights. The Company and its Subsidiaries own or possess adequate rights or licenses to use all trademarks, trade names, service marks, service mark registrations, service names, original works of authorship, patents, patent rights, copyrights, inventions, licenses, approvals, governmental authorizations, trade secrets and other intellectual property rights and all applications and registrations therefor (“Intellectual Property Rights”) necessary to conduct their respective businesses as now conducted and presently proposed to be conducted. Each of the patents owned by the Company or any of its Subsidiaries is set forth in a Disclosure Schedule to this Agreement. Except as set forth in such Disclosure Schedule, none of the Company’s Intellectual Property Rights have expired or terminated or have been abandoned or are expected to expire or terminate or are expected to be abandoned, within three years from the date of this Agreement. The Company does not have any knowledge of any infringement by the Company or its Subsidiaries of Intellectual Property Rights of others. There is no claim, action or proceeding being made or brought, or to the knowledge of the Company or any of its Subsidiaries, being threatened, against the Company or any of its Subsidiaries regarding its Intellectual Property Rights. Neither the Company nor any of its Subsidiaries is aware of any facts or circumstances which might give rise to any of the foregoing infringements or claims, actions or proceedings. The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy, confidentiality and value of all of their Intellectual Property Rights.
(s)
Environmental Laws. Except, in each case, as would not be reasonably anticipated to have a Material Adverse Effect, the Company and the Subsidiaries (a) are in compliance with any and all applicable laws relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants or contaminants, (b) have received and hold all material permits, licenses or other approvals required of them under all such laws to conduct their respective businesses and (c) are in compliance with all material terms and conditions of any such permit, license or approval.
(t)
Tax Status. The Company and each of its Subsidiaries (i) has timely made or filed all foreign, federal and state income and all other tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has timely paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations, except those being contested in good faith and (iii) has set aside on its books provision reasonably adequate for the payment of all taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid taxes in any material amount claimed in writing to be due by the taxing authority of any jurisdiction.
(u)
Internal Accounting and Disclosure Controls. The Company and each of its Subsidiaries maintains internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the Exchange Act) that is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, including that (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with IFRS and to maintain asset and liability accountability, (iii) access to assets or incurrence of liabilities is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for assets and liabilities is compared with the existing assets and liabilities at reasonable intervals and appropriate action is taken with respect to any difference. The Company maintains disclosure controls and procedures (as such term is defined in Rule 13a-15(e) under the Exchange Act) that are designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms of the SEC, including, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act, as applicable, is accumulated and communicated to the Company’s management, including its principal executive officer or officers and its principal financial officer or officers, as appropriate, to allow timely decisions regarding required disclosure.
11
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
Neither the Company nor any of its Subsidiaries has received any notice or correspondence from any accountant, Governmental Entity or other Person relating to any potential material weakness or significant deficiency in any part of the internal controls over financial reporting of the Company or any of its Subsidiaries, except as disclosed in the SEC Documents that are available on the SEC’s website through the EDGAR system at least (1) Business Day prior to the applicable Closing Date.
(v)
Investment Company Status. The Company is not, and upon consummation of the sale of the Securities will not be, an “investment company,” an affiliate of an “investment company,” a company controlled by an “investment company” or an “affiliated person” of, or “promoter” or “principal underwriter” for, an “investment company” as such terms are defined in the Investment Company Act of 1940, as amended.
(w)
Insurance. The Company and each of its Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as management of the Company believes to be prudent and customary in the businesses in which the Company and its Subsidiaries are engaged. Neither the Company nor any such Subsidiary has been refused any insurance coverage sought or applied for, and neither the Company nor any such Subsidiary has any reason to believe that it will be unable to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not have a Material Adverse Effect.
(x)
Manipulation of Price. Neither the Company nor any of its Subsidiaries has, and, to the knowledge of the Company, no Person acting on their behalf has, directly or indirectly, (i) taken any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company or any of its Subsidiaries to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company or any of its Subsidiaries.
(y)
Registration Eligibility. The Company is eligible to register the resale of the Conversion Shares by the Buyer using Form F3 promulgated under the Securities Act.
(aa)
Sanctions Matters. Neither the Company nor any of its Subsidiaries or, to the knowledge of the Company, any director, officer or controlled affiliate of the Company or any director or officer of any Subsidiary, is a Person that is, or is owned or controlled by a Person that is (i) the subject of any sanctions administered or enforced by the U.S. Department of Treasury’s Office of Foreign Asset Control (“OFAC”), the United Nations Security Council, the European Union, Her Majesty’s Treasury, or other relevant sanctions authorities, including, without limitation, designation on OFAC’s Specially Designated Nationals and Blocked Persons List or OFAC’s Foreign Sanctions Evaders List or other relevant sanctions authority (collectively, “Sanctions”), or (ii) located, organized or resident in a country or territory that is the subject of Sanctions that broadly prohibit dealings with that country or territory (including, without limitation, the Crimea, Zaporizhzhia and Kherson regions, the Donetsk People’s Republic and Luhansk People’s Republic in Ukraine, Cuba, Iran, North Korea, Russia, Sudan and Syria (the “Sanctioned Countries”)). Neither the Company nor any of its Subsidiaries nor any director, officer or controlled affiliate of the Company or any of its Subsidiaries, has ever had funds blocked by a United States bank or financial institution, temporarily or otherwise, as a result of OFAC concerns.
(bb)
Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided any of the Buyers or their agents or counsel with any information that constitutes or could reasonably be expected to constitute material, nonpublic information concerning the Company or any of its Subsidiaries, other than the Draft 20-F Information and the existence of the transactions contemplated by this Agreement and the other Transaction Documents. The Company understands and confirms that each of the Buyers will rely on the foregoing representations in effecting transactions in securities of the Company. All disclosures provided to the Buyers regarding the Company and its Subsidiaries, their businesses and the transactions contemplated hereby, including the schedules to this Agreement, and the Draft 20-F Information, furnished by or on behalf of the Company or any of its Subsidiaries, taken as a whole, are true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. All of the written information furnished after the date hereof by or on behalf of the Company or any of its Subsidiaries to each Buyer pursuant to or in connection with this Agreement and the other Transaction Documents, taken as a whole, will be true and correct in all material respects as of the date on which such information is so provided and will not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading.
12
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
No event or circumstance has occurred or information exists with respect to the Company or any of its Subsidiaries or its or their business, properties, liabilities, prospects, operations (including results thereof) or conditions (financial or otherwise), which, under applicable law, rule or regulation, requires public disclosure at or before the date hereof or announcement by the Company but which has not been so publicly disclosed. All financial projections and forecasts that have been prepared by or on behalf of the Company or any of its Subsidiaries and made available to the Buyers have been prepared in good faith based upon reasonable assumptions and represented, at the time each such financial projection or forecast was delivered to each Buyer, the Company's best estimate of future financial performance (it being recognized that such financial projections or forecasts are not to be viewed as facts and that the actual results during the period or periods covered by any such financial projections or forecasts may differ from the projected or forecasted results). The Company acknowledges and agrees that no Buyer makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 2.
(cc)
No General Solicitation. Neither the Company, nor any of its affiliates, nor any Person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D under the Securities Act) in connection with the offer or sale of the Securities.
(dd)
Private Placement. Assuming the accuracy of the Buyers’ representations and warranties set forth in Section 2, no registration under the Securities Act is required for the offer and sale of the Securities by the Company to the Buyers as contemplated hereby. The issuance and sale of the Securities hereunder does not contravene the rules and regulations of the Principal Market.
(ee)
No Disqualification Events. With respect to Securities to be offered and sold hereunder in reliance on Rule 506(b) under the Securities Act (“Regulation D Securities”), none of the Company, any of its predecessors, any affiliated issuer, any director, executive officer, other officer of the Company participating in the offering contemplated hereby, any beneficial owner of 20% or more of the Company’s outstanding voting equity securities, calculated on the basis of voting power, nor any promoter (as that term is defined in Rule 405 under the Securities Act) connected with the Company in any capacity at the time of sale (each, an “Issuer Covered Person” and, together, “Issuer Covered Persons”) is subject to any of the “Bad Actor” disqualifications described in Rule 506(d)(1)(i) to (viii) under the Securities Act (a “Disqualification Event”), except for a Disqualification Event covered by Rule 506(d)(2) or (d)(3). The Company has exercised reasonable care to determine whether any Issuer Covered Person is subject to a Disqualification Event. The Company has complied, to the extent applicable, with its disclosure obligations under Rule 506(e), and has furnished to the Buyers a copy of any disclosures provided thereunder.
(ff)
Other Covered Persons. The Company is not aware of any Person that has been or will be paid (directly or indirectly) remuneration for solicitation of Buyers or potential purchasers in connection with the sale of any Regulation D Securities.
(gg)
No Disagreements with Accountants and Lawyers. There are no material disagreements of any kind presently existing, or reasonably anticipated by the Company to arise, between the Company and the accountants and lawyers formerly or presently employed by the Company and the Company is current with respect to any fees owed to its accountants and lawyers which could affect the Company’s ability to perform any of its obligations under any of the Transaction Documents. In addition, on or prior to the date hereof, the Company had discussions with its accountants about its financial statements previously filed with the SEC. Based on those discussions, the Company has no reason to believe that it will need to restate any such financial statements or any part thereof.
(a)
Form D and Blue Sky. The Company shall file a Form D with respect to the Securities as required under Regulation D and to provide a copy thereof to each Buyer promptly after such filing. The Company shall, on or before the Closing Date, take such action as the Company shall reasonably determine is necessary in order to obtain an exemption for, or to, qualify the Securities for sale to the Buyers at the Closing pursuant to this Agreement under applicable securities or “Blue Sky” laws of the states of the United States (or to obtain an exemption from such qualification), and shall provide evidence of any such action so taken to the Buyers on or prior to the Closing Date. Without limiting any other obligation of the Company under this Agreement, the Company shall timely make all filings and reports relating to the offer and sale of the Securities required under all applicable securities laws (including, without limitation, all applicable federal securities laws and all applicable “Blue Sky” laws), and the Company shall comply with all applicable foreign, federal, state and local laws, statutes, rules, regulations and the like relating to the offering and sale of the Securities to the Buyers.
13
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(a)
Reporting Status. For the period beginning on the date hereof, and ending 6 months after the date on which all the Convertible Debentures are no longer outstanding (the “Reporting Period”), the Company shall use commercially reasonable efforts to file on a timely basis all reports required to be filed with the SEC pursuant to the Exchange Act, and the Company shall not terminate its status as an issuer required to file reports under the Exchange Act even if the Exchange Act or the rules and regulations thereunder would no longer require or otherwise permit such termination.
(b)
Use of Proceeds. Neither the Company nor any Subsidiary will, directly or indirectly, use the proceeds of the transactions contemplated herein to repay any loans to any executives or employees of the Company or to make any payments in respect of any related party debt. Neither the Company nor any of its Subsidiaries will, directly or indirectly, use the proceeds from the transactions contemplated herein, or lend, contribute or otherwise make available such proceeds to any subsidiary, joint venture partner or other Person (a) for the purpose of funding or facilitating any activities or business of or with any Person or in any country or territory that, at the time of such funding or facilitation, is the subject of Sanctions or is a Sanctioned Country, or (b) in any other manner that will result in a violation of Sanctions or Applicable Laws by any Person (including any Person participating in the transactions contemplated by this Agreement, whether as underwriter, advisor, investor or otherwise). For the past five years, neither the Company nor any of its Subsidiaries has engaged in, and is now not engaged in, any dealings or transactions with any Person, or in any country or territory, that at the time of the dealing or transaction is or was the subject of Sanctions or was a Sanctioned Country. The Company shall not, without the prior written consent of the Buyer, loan, invest, transfer or “downstream” any cash proceeds, or assets or property acquired with cash proceeds from the issuance and sale of the Convertible Debentures to any Subsidiary, unless the Buyer and the Subsidiary enter into a guarantee in the form of the Global Guaranty.
(c)
Listing. To the extent applicable, the Company shall promptly secure the listing or designation for quotation (as the case may be) of all of the Underlying Securities (as defined below) on the Principal Market, subject to official notice of issuance, and shall use reasonable efforts to maintain such listing or designation for quotation (as the case may be) of all Underlying Securities from time to time issuable under the terms of the Transaction Documents on such Principal Market for the Reporting Period. The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 4(d). “Underlying Securities” means the (i) the Conversion Shares, and (ii) any common shares of the Company issued or issuable with respect to the Conversion Shares or, including, without limitation, (1) as a result of any stock split, stock dividend, recapitalization, exchange or similar event or otherwise and (2) shares of capital stock of the Company into which the Common Shares are converted or exchanged without regard to any limitations on conversion of the Convertible Debentures.
(d)
Fees. The Company shall pay to the Buyer a one-time due diligence and structuring fee of $25,000, which the Buyer acknowledges was previously received.
(e)
Pledge of Securities. Notwithstanding anything to the contrary contained in this Agreement, the Company acknowledges and agrees that, subject to compliance with applicable federal and state securities laws, the Securities may be pledged by a Buyer in connection with a bona fide margin agreement or other loan or financing arrangement that is secured by the Securities. The Company hereby agrees to execute and deliver such documentation as a pledgee of the Securities may reasonably request in connection with a pledge of the Securities to such pledgee by a Buyer.
(f)
Disclosure of Transactions and Other Material Information.
(i)
Disclosure of Transactions. The Company shall, on or before the first Business Day after the date of this Agreement, file with the SEC a current report of foreign issuer on Form 6K describing all the material terms of the transactions contemplated by the Transaction Documents in the form required by the Exchange Act and attaching all the material Transaction Documents (including, required exhibits, the “Current Report”). From and after the filing of the Current Report and the Company’s Annual Report on Form 20-F for the year ended April 30, 2024 (the “2024 Form 20-F”), the Company shall have publicly disclosed all material, nonpublic information (if any) provided to any of the Buyers by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by the Transaction Documents, including all information provided in accordance with the terms and conditions of the Yorkville NDA. In addition, effective upon the filing of the Current Report and the 2024 Form 20-F, the Company acknowledges and agrees that any and all confidentiality or similar obligations with respect to the transactions contemplated by the Transaction Documents under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, including without limitation the Yorkville NDA, shall terminate.
14
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(ii)
Limitations on Disclosure. The Company shall not, and the Company shall cause each of its Subsidiaries and each of its and their respective officers, directors, employees and agents not to, provide any Buyer with any material, nonpublic information regarding the Company or any of its Subsidiaries from and after the date hereof without first obtaining the express prior written consent of such Buyer (which may be granted or withheld in such Buyer's sole discretion). In the event of a breach of any of the foregoing covenants or any of the covenants or agreements contained in any other Transaction Document, by the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees and agents (as determined in the reasonable good faith judgment of such Buyer), in addition to any other remedy provided herein or in the Transaction Documents, such Buyer shall have the right to make a public disclosure, in the form of a press release, public advertisement or otherwise, of such breach or such material, non-public information, as applicable, without the prior approval by the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees or agents. No Buyer shall have any liability to the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees, affiliates, shareholders or agents, for any such disclosure. To the extent that the Company delivers any material, non-public information to a Buyer without such Buyer’s consent, the Company hereby covenants and agrees that such Buyer shall not have any duty of confidentiality with respect to, or a duty not to trade on the basis of, such material, non-public information. Subject to the foregoing, neither the Company, its Subsidiaries nor any Buyer shall issue any press releases or any other public statements with respect to the transactions contemplated hereby; provided, however, the Company shall be entitled, without the prior approval of any Buyer, to make any press release or other public disclosure with respect to such transactions (i) in substantial conformity with the 6-K Filing and contemporaneously therewith and (ii) as is required by applicable law and regulations (provided that in the case of clause (i) each Buyer shall be consulted by the Company in connection with any such press release or other public disclosure prior to its release). Without the prior written consent of the applicable Buyer (which may be granted or withheld in such Buyer’s sole discretion), the Company shall not (and shall cause each of its Subsidiaries and affiliates to not) disclose the name of such Buyer in any filing, announcement, release or otherwise. Notwithstanding anything contained in this Agreement to the contrary and without implication that the contrary would otherwise be true, the Company expressly acknowledges and agrees that no Buyer shall have (unless expressly agreed to by a particular Buyer after the date hereof in a written definitive and binding agreement executed by the Company and such particular Buyer, including without limitation, the Yorkville NDA (it being understood and agreed that no Buyer may bind any other Buyer with respect thereto)), any duty of confidentiality with respect to, or a duty not to trade on the basis of, any material, non-public information regarding the Company or any of its Subsidiaries.
(iii)
Other Confidential Information. Disclosure Failures. In addition to other remedies set forth in this Section 4(g), and without limiting anything set forth in any other Transaction Document, at any time after the Closing Date if the Company, any of its Subsidiaries, or any of their respective officers, directors, employees or agents, provides any Buyer with material non-public information, not including the Draft 20-F Information, relating to the Company or any of its Subsidiaries (each, the “Confidential Information”), the Company shall, on or prior to the applicable Required Disclosure Date (as defined below), publicly disclose such Confidential Information on a Current Report on Form 6-K or otherwise (each, a “Disclosure”). From and after such Disclosure, the Company shall have disclosed all Confidential Information provided to such Buyer by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents. In addition, effective upon such Disclosure, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, shall terminate. “Required Disclosure Date” means (x) if such Buyer authorized the delivery of such Confidential Information, either (I) if the Company and such Buyer have mutually agreed upon a date (as evidenced by an e-mail or other writing) of Disclosure of such Confidential Information, such agreed upon date or (II) otherwise, the seventh (7th) calendar day after the date such Buyer first received any Confidential Information or (y) if such Buyer did not authorize the delivery of such Confidential Information, the first (1st) Business Day after such Buyer’s receipt of such Confidential Information.
(g)
Reservation of Shares. So long as any of the Convertible Debentures remain outstanding, the Company shall have reserved and authorized, and shall have instructed its transfer agent to irrevocably reserve, the maximum number of shares of Common Shares issuable upon (i) conversion of all Convertible Debentures (assuming for purposes hereof that (x) such Convertible Debentures are convertible at the Floor Price (as defined therein) as of the date of determination and (y) any such conversion shall not take into account any limitations on the conversion of the Convertible Debentures set forth therein) (the “Required Reserve Amount”); provided that at no time shall the number of Common Shares reserved pursuant to this Section be reduced other than proportionally in connection with any conversion and/or redemption, or reverse stock split..
(h)
Conduct of Business. The business of the Company and its Subsidiaries shall not be conducted in violation of any law, ordinance or regulation of any Governmental Entity, except where such violations would not reasonably be expected to result, either individually or in the aggregate, in a Material Adverse Effect.
15
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(i)
(i) Except as expressly set forth below, the Buyer covenants that from and after the date hereof through and ending when no Convertible Debentures remain outstanding (the “Restricted Period”), no Buyer or any of its officers, or any entity managed or controlled by the Buyer (collectively, the “Restricted Persons” and each of the foregoing is referred to herein as a “Restricted Person”) shall, directly or indirectly, engage in any “short sale” (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of the Common Shares, either for its own principal account or for the principal account of any other Restricted Person. Notwithstanding the foregoing, it is expressly understood and agreed that nothing contained herein shall (without implication that the contrary would otherwise be true) prohibit any Restricted Person during the Restricted Period from: (1) selling “long” (as defined under Rule 200 promulgated under Regulation SHO) Common Shares; or (2) selling a number of Common Shares equal to the number of Underlying Shares that such Restricted Person is entitled to receive, but has not yet received from the Company or the transfer agent, upon the completion of a pending conversion of the Convertible Debentures for which a valid Conversion Notice (as defined in the Convertible Debentures) has been submitted to the Company pursuant to Section 4(b) of the Convertible Debentures.
(j)
Trading Information. Upon the Company’s request, the Buyer agrees to provide the Company with daily trading reports setting forth the number and average sales prices of Conversion Shares sold the Buyer during each Trading Day.
(k)
Prohibited Transactions. From the date hereof until all of the Convertible Debentures have been repaid or converted into Common Shares, the Company agrees to not directly or indirectly enter into any contract, agreement or other item that would restrict or prohibit any of the Company’s obligations to the Buyer under the Transaction Documents, including, without limitation, any payments required by the Company to the Buyer upon a Trigger Event (as defined in the Convertible Debentures).
(l)
From the date hereof until all the Convertible Debentures have been repaid, without the prior written consent of the Buyer, the Company shall not, and shall not permit any of its subsidiaries (whether or not a subsidiary on the date hereof) to, directly or indirectly (i) amend its charter documents, including, without limitation, its certificate of incorporation and bylaws, in any manner that materially and adversely affects any rights of the holders of the Convertible Debentures, (ii) make any payments in respect of any related party debt, or (iii) other than Permitted ATM Sales, enter into, agree to enter into, or effect any Variable Rate Transaction other than with the Buyer. Notwithstanding the foregoing, the Company shall be permitted to execute sales pursuant to the Clear Street ATM (“Permitted ATM Sales”): (A) at any time prior to the effective date of the initial Registration Statement filed pursuant to the Registration Rights Agreement, (B) at any time during the effectiveness of a Registration Statement filed pursuant to the Registration Rights Agreement, provided that the market value of the Common Shares remaining available to be resold by the Buyer is greater than 150% of the principal balance of the Convertible Debentures then outstanding (C) in an amount not to exceed 5% of the daily volume on the Principal Market if, at the time of the delivery of an issuance notice, the market price of the Common Shares on the Principal Market is greater than the Fixed Price (as defined in the Convertible Debenture) but less than $2.00, and in an amount not to exceed 10% of the daily volume on the Principal Market if, at the time of the delivery of an issuance notice, the market price of the Common Shares on the Principal Market is greater than the Fixed Price and greater than or equal to $2.00, or (D) if 50% of the proceeds of any sales shall be used to repay a portion of the outstanding balance under the Convertible Debentures if $300,000 of Convertible Debentures has been converted during the trailing consecutive 30 day period.
“Variable Rate Transaction” shall mean a transaction in which the Company (i) issues or sells any equity, warrants, or debt securities that are convertible into, exchangeable or exercisable for, or include the right to receive additional Common Shares either (A) at a conversion price, exercise price, exchange rate or other price that is based upon and/or varies with the trading prices of or quotations for the Common Shares at any time after the initial issuance of such security, or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such security or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the Common Shares (including, without limitation, any “full ratchet” or “weighted average” anti-dilution provisions, but not including any standard anti-dilution protection for any reorganization, recapitalization, non-cash dividend, stock split or other similar transaction), (ii) enters into or effects any agreement, including but not limited to an “equity line of credit,” “ATM agreement” or other continuous offering or similar offering of Common Shares, or (iii) enters into or effects any transaction in which the Company issues or sells any equity, warrants, or debt securities at an implied discount (taking into account all the securities issuable in such offering, including the right to receive additional Common Shares) to the market price of the Common Shares at the time of the offering in excess of 35%.
5.
REGISTER; TRANSFER AGENT INSTRUCTIONS; LEGEND.
(a)
Register. The Company shall maintain at its principal executive offices or with the Transfer Agent (or at such other office or agency of the Company as it may designate by notice to each holder of Securities), a register for the Convertible Debentures in which the Company shall record the name and address of the Person in whose name the Convertible Debentures have been issued (including the name and address of each transferee), the amount of Convertible Debentures held by such Person.
16
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
The Company shall keep the register open and available at all times during business hours for inspection of any Buyer or its legal representatives. (b) Transfer Restrictions. The Securities may only be disposed of in compliance with state and federal securities laws. In connection with any transfer of Securities other than pursuant to an effective registration statement or Rule 144, to the Company or to an Affiliate of a Buyer or in connection with a pledge as contemplated herein, the Company may require the transferor thereof to provide to the Company an opinion of counsel selected by the transferor and reasonably acceptable to the Company, the form and substance of which opinion shall be reasonably satisfactory to the Company, to the effect that such transfer does not require registration of such transferred Securities under the Securities Act. As a condition of transfer, any such transferee shall agree in writing to be bound by the terms of this Agreement and shall have the rights and obligations of a Buyer under this Agreement. (c) Conversion and Exercise Procedures. The form of Conversion Notice included in the Convertible Debentures set forth the totality of the procedures required of the Buyers in order to convert the Convertible Debentures. Except as provided in Section 2(f) and Section 5(b), no additional legal opinion, other information or instructions shall be required of the Buyers to convert their Convertible Debentures. The Company shall honor conversions of the Convertible Debentures and shall deliver the Conversion Shares in accordance with the terms, conditions and time periods set forth in the Convertible Debentures. 6. CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL. The obligation of the Company hereunder to issue and sell the Convertible Debentures to each Buyer at each Closing is subject to the satisfaction, at or before each Closing Date, of each of the following conditions, provided that these conditions are for the Company's sole benefit and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof: (a) Such Buyer shall have executed each of the Transaction Documents to which it is a party and delivered the same to the Company. (b) Such Buyer and each other Buyer shall have delivered to the Company the Purchase Price (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(d), if any) for the Convertible Debentures being purchased by such Buyer at the Closing by wire transfer of immediately available funds in accordance with a letter, duly executed by an officer of the Company, setting forth the wire amounts of each Buyer and the wire transfer instructions of the Company (the “Closing Statement”). (c) The representations and warranties of such Buyer shall be true and correct in all material respects as of the date when made and as of each Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date), and such Buyer shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such Buyer at or prior to such Closing Date. 7. CONDITIONS TO EACH BUYER'S OBLIGATION TO PURCHASE. The obligation of each Buyer hereunder to purchase its Convertible Debentures at each Closing is subject to the satisfaction, at or before each Closing Date, of each of the following conditions, provided that these conditions are for each Buyer's sole benefit and may be waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof: (a) The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party and the Company shall have duly executed and delivered to such Buyer a Convertible Debenture with a principal amount corresponding to the Subscription Amount set forth opposite such Buyer’s name on the Schedule of Buyers attached as Schedule I for the Closing. (b) Such Buyer shall have received the opinion of counsel to the Company, dated as of the First Closing Date, in the form reasonably acceptable to such Buyer. (c) The Company shall have delivered to each Buyer copies of its and each Subsidiaries certified copies of its charter, as well as any shareholder or operating agreements by or among the shareholders or members of any of the Company’s Subsidiaries.
17
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(d)
The Company shall have delivered to such Buyer a certificate evidencing the incorporation and good standing of the Company as of a date within ten (10) days of the Closing Date.
(e)
Each and every representation and warranty of the Company shall be true and correct in all material respects (other than representations and warranties qualified by materiality, which shall be true and correct in all respects) as of the date when made and as of each Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date) and the Company shall have performed, satisfied and complied in all respects with the covenants, agreements and conditions set forth in each Transaction Document required to be performed, satisfied or complied with by the Company at or prior to each Closing Date.
(f)
The Common Shares (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been suspended, as of each Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the SEC or the Principal Market have been threatened, as of each Closing Date, either (I) in writing by the SEC or the Principal Market or (II) by receiving a notification from the Principal Market of falling below the minimum maintenance requirements of the Principal Market that is not subject to a cure period.
(g)
The Company shall have obtained all governmental, regulatory or third-party consents and approvals, if any, necessary for the sale of the Securities, including without limitation, those required by the Principal Market, if any.
(h)
No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated by the Transaction Documents.
(i)
Since the date of execution of this Agreement, no event or series of events shall have occurred that has resulted in or would reasonably be expected to result in a Material Adverse Effect, or an Event of Default (as defined in the Convertible Debentures).
(j)
The Company shall have notified the Principal Market of the intention to list or designate for quotation (as the case may be) the maximum number of Conversion Shares issuable pursuant to the Convertible Debentures to be issued at the Closing.
(k)
Such Buyer shall have received the Closing Statement.
(l)
From the date hereof to the applicable Closing Date, trading in the Common Shares shall not have been suspended by the SEC or the Principal Market (except for any suspension of trading of limited duration agreed to by the Company, which suspension shall be terminated prior to the Closing).
(m)
The board of directors of the Company has approved the transactions contemplated by the Transaction Documents; said approval has not been amended, rescinded or materially modified and remains in full force and effect as of such Closing, and a true, correct and complete copy of such resolutions duly adopted by the board of directors of the Company shall have been provided to the Buyers.
(n)
The Company shall have delivered to the Buyer a compliance certificate executed by an executive officer of the Company certifying that Company has complied with all of the conditions precedent to the applicable Closing set forth herein and which may be relied upon by the Buyer as evidence of satisfaction of such conditions without any obligation to independently verify.
(o)
The Company and its Subsidiaries shall have delivered to such Buyer such other documents, instruments or certificates relating to the transactions contemplated by this Agreement as such Buyer or its counsel may reasonably request.
(p)
Solely with respect to the Second Closing, the Company shall have filed the 2024 Form 20-F with the SEC in accordance with the rules and regulations for filing thereof and the Registration Statement shall be effective in accordance with the provisions set forth in the Registration Rights Agreement, including the effectiveness deadline set forth therein.
18
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
In the event that the First Closing shall not have occurred with respect to a Buyer within five (5) days of the date hereof, then such Buyer shall have the right to terminate its obligations under this Agreement with respect to itself at any time on or after the close of business on such date without liability of such Buyer to any other party; provided, however, (i) the right to terminate this Agreement under this Section 8 shall not be available to such Buyer if the failure of the transactions contemplated by this Agreement to have been consummated by such date is the result of such Buyer's breach of this Agreement and (ii) the abandonment of the sale and purchase of the Convertible Debentures shall be applicable only to such Buyer providing such written notice, provided further that no such termination shall affect any obligation of the Company under this Agreement to reimburse such Buyer for the expenses described herein. Nothing contained in this Section 8 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents.
(a)
Governing Law. This Agreement and the rights and obligations of the parties hereunder shall, in all respects, be governed by, and construed in accordance with, the laws (excluding the principles of conflict of laws) of the State of New York (including Section 5-1401 and Section 5-1402 of the General Obligations Law of the State of New York), including all matters of construction, validity and performance.
(b)
Jurisdiction; Venue; Service.
(i)
The Company hereby irrevocably consents to the non-exclusive personal jurisdiction of the state courts of the State of New York (the “Governing Jurisdiction”) and, if a basis for federal jurisdiction exists, the non-exclusive personal jurisdiction of any United States District Court for the Governing Jurisdiction.
(ii)
The Company agrees that venue shall be proper in any court of the Governing Jurisdiction selected by the Buyer or, if a basis for federal jurisdiction exists, in any United States District Court in the Governing Jurisdiction. The Company waives any right to object to the maintenance of any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or in tort or otherwise, in any of the state or federal courts of the Governing Jurisdiction on the basis of improper venue or inconvenience of forum.
(iii)
Any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or tort or otherwise, brought by the Company against the Buyer arising out of or based upon this Agreement or any matter relating to this Agreement, or any other Transaction Document, or any contemplated transaction, shall be brought in a court only in the Governing Jurisdiction. The Company shall not file any counterclaim against the Buyer in any suit, claim, action, litigation or proceeding brought by the Buyer against the Company in a jurisdiction outside of the Governing Jurisdiction unless under the rules of the court in which the Buyer brought such suit, claim, action, litigation or proceeding the counterclaim is mandatory, and not permissive, and would be considered waived unless filed as a counterclaim in the suit, claim, action, litigation or proceeding instituted by the Buyer against the Company. The Company agrees that any forum outside the Governing Jurisdiction is an inconvenient forum and that any suit, claim, action, litigation or proceeding brought by the Company against the Buyer in any court outside the Governing Jurisdiction should be dismissed or transferred to a court located in the Governing Jurisdiction. Furthermore, the Company irrevocably and unconditionally agrees that it will not bring or commence any suit, claim, action, litigation or proceeding of any kind or description, whether in law or equity, whether in contract or in tort or otherwise, against the Buyer arising out of or based upon this Agreement or any matter relating to this Agreement, or any other Transaction Document, or any contemplated transaction, in any forum other than the courts of the State of New York sitting in New York County, and the United States District Court of the Southern District of New York, and any appellate court from any thereof, and each of the parties hereto irrevocably and unconditionally submits to the jurisdiction of such courts and agrees that all claims in respect of any such suit, claim, action, litigation or proceeding may be heard and determined in such New York State Court or, to the fullest extent permitted by applicable law, in such federal court. The Company and the Buyer agree that a final judgment in any such suit, claim, action, litigation or proceeding shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law.
(iv)
The Company and the Buyer irrevocably consent to the service of process out of any of the aforementioned courts in any such suit, claim, action, litigation or proceeding by the mailing of copies thereof by registered or certified mail postage prepaid, to it at the address provided for notices in this Agreement, such service to become effective thirty (30) days after the date of mailing.
19
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
(v)
Nothing herein shall affect the right of the Buyer to serve process in any other manner permitted by law or to commence legal proceedings or to otherwise proceed against the Company or any other Person in the Governing Jurisdiction or in any other jurisdiction.
(c)
THE PARTIES MUTUALLY WAIVE ALL RIGHT TO TRIAL BY JURY OF ALL CLAIMS OF ANY KIND ARISING OUT OF OR BASED UPON THIS AGREEMENT OR ANY MATTER RELATING TO THIS AGREEMENT, OR ANY OTHER TRANSACTION DOCUMENT, OR ANY CONTEMPLATED TRANSACTION. THE PARTIES ACKNOWLEDGE THAT THIS IS A WAIVER OF A LEGAL RIGHT AND THAT THE PARTIES EACH MAKE THIS WAIVER VOLUNTARILY AND KNOWINGLY AFTER CONSULTATION WITH COUNSEL OF THEIR RESPECTIVE CHOICE. THE PARTIES AGREE THAT ALL SUCH CLAIMS SHALL BE TRIED BEFORE A JUDGE OF A COURT HAVING JURISDICTION, WITHOUT A JURY.
(d)
Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. In the event that any signature is delivered by by an email which contains a portable document format (.pdf) file of an executed signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such signature page were an original thereof.
(e)
Headings; Gender. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation of, this Agreement. Unless the context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine, neuter, singular and plural forms thereof. The terms "including," "includes," "include" and words of like import shall be construed broadly as if followed by the words "without limitation." The terms "herein," "hereunder," "hereof" and words of like import refer to this entire Agreement instead of just the provision in which they are found.
(f)
Entire Agreement, Amendments. This Agreement supersedes all other prior oral or written agreements between the Buyer, the Company, their affiliates and persons acting on their behalf with respect to the matters discussed herein, and this Agreement and the instruments referenced herein contain the entire understanding of the parties with respect to the matters covered herein and therein and, except as specifically set forth herein or therein, neither the Company nor any Buyer makes any representation, warranty, covenant or undertaking with respect to such matters. No provision of this Agreement may be amended other than by an instrument in writing signed by the party to be charged with enforcement. As a material inducement for each Buyer to enter into this Agreement, the Company expressly acknowledges and agrees that (x) no due diligence or other investigation or inquiry conducted by a Buyer, any of its advisors or any of its representatives shall affect such Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception to any of, the Company’s representations and warranties contained in this Agreement or any other Transaction Document and (y) unless a provision of this Agreement or any other Transaction Document is expressly preceded by the phrase “except as disclosed in the SEC Documents,” nothing contained in any of the SEC Documents shall affect such Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception to any of, the Company’s representations and warranties contained in this Agreement or any other Transaction Document.
(g)
Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing by letter and email and will be deemed to have been delivered: upon the later of (A) either (i) receipt, when delivered personally or (ii) one (1) Business Day after deposit with an overnight courier service with next day delivery specified, in each case, properly addressed to the party to receive the same and (B) receipt, when sent by electronic mail. The addresses and email addresses for such communications shall be:
|
|
If to the Company, to: |
IMMUNOPRECISE ANTIBODIES LTD. |
|
[Redacted: personal information]
|
With Copy to: |
Dorsey & Whitney LLP
[Redacted: personal information]
|
20
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
|
|
|
|
If to a Buyer, to its address and email address set forth on the Schedule of Buyers, with copies to such Buyer's representatives as set forth on the Schedule of Buyers, |
|
|
|
|
With copy to: |
[Redacted: personal information] |
|
|
or to such other address, email address and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) electronically generated by the sender's e-mail service provider containing the time, date, recipient e-mail address or (C) provided by an overnight courier service shall be rebuttable evidence of personal service, receipt by facsimile or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
(h)
Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and assigns, including any purchasers of any of the Convertible Debentures (but excluding any purchasers of Underlying Securities, unless pursuant to a written assignment by such Buyer). The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Buyers. In connection with any transfer of any or all of its Securities, a Buyer may assign all, or a portion, of its rights and obligations hereunder in connection with such Securities without the consent of the Company, in which event such assignee shall be deemed to be a Buyer hereunder with respect to such transferred Securities.
(i)
In consideration of each Buyer's execution and delivery of the Transaction Documents and acquiring the Securities thereunder and in addition to all of the Company's other obligations under the Transaction Documents, the Company shall defend, protect, indemnify and hold harmless each Buyer and each holder of any Securities and all of their stockholders, partners, members, officers, directors, employees and direct or indirect investors and any of the foregoing Persons' agents or other representatives (including, without limitation, those retained in connection with the transactions contemplated by this Agreement) (collectively, the “Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and reasonable and documented expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is sought), and including reasonable attorneys' fees and disbursements (the “Indemnified Liabilities”), incurred by any Indemnitee as a result of, or arising out of, or relating to (i) any material misrepresentation or material breach of any representation or warranty made by the Company in any of the Transaction Documents, (ii) any material breach of any material covenant, material agreement or material obligation of the Company or any Subsidiary contained in any of the Transaction Documents or (iii) any cause of action, suit, proceeding or claim brought or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company or any Subsidiary) or which otherwise involves such Indemnitee that arises out of or results from (A) the execution, delivery, performance or enforcement of any of the Transaction Documents (except to the extent any Indemnified Liabilities are determined by a court of competent jurisdiction, not subject to further appeal, to have resulted from the bad faith or gross negligence of an Indemnitee with respect to a Buyer), (B) any transaction financed or to be financed in whole or in part, directly or indirectly, with the proceeds of the issuance of the Securities, or (C) any disclosure properly made to such Buyer pursuant to Section 4(g), or (D) the status of such Buyer or holder of the Securities either as an investor in the Company pursuant to the transactions contemplated by the Transaction Documents or as a party to this Agreement (including, without limitation, as a party in interest or otherwise in any action or proceeding for injunctive or other equitable relief). To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law.
(ii)
Promptly after receipt by an Indemnitee under this Section 9(i) of notice of the commencement of any action or proceeding (including any governmental action or proceeding) involving an Indemnified Liability, such Indemnitee shall, if a claim in respect thereof is to be made against the Company under this Section 9(i), deliver to the Company a written notice of the commencement thereof, and the Company shall have the right to participate in, and, to the extent the Company so desires, to assume control of the defense thereof with counsel mutually reasonably satisfactory to the Company and the Indemnitee; provided, however, that an Indemnitee shall have the right to retain its own counsel with the fees and expenses of such counsel to be paid by the Company if: (A) the Company has agreed in writing to pay such fees and expenses; (B) the Company shall have failed promptly to assume the defense of such Indemnified Liability and to employ counsel reasonably satisfactory to such Indemnitee in any such Indemnified Liability; or (C) the named parties to any such Indemnified Liability (including any impleaded parties) include both such Indemnitee and the Company, and such Indemnitee shall have been advised by counsel that a conflict of interest is likely to exist if the same counsel were to represent such Indemnitee and the Company (in which case, if such Indemnitee notifies the Company in writing that it elects to employ separate counsel at the expense of the Company, then the Company shall not have the right to assume the defense thereof and such counsel shall be at the expense of the Company), provided further, that in the case of clause (C) above the Company shall not be responsible for the reasonable fees and expenses of more than one (1) separate legal counsel for the Indemnitees.
21
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
The Indemnitee shall reasonably cooperate with the Company in connection with any negotiation or defense of any such action or Indemnified Liability by the Company and shall furnish to the Company all information reasonably available to the Indemnitee which relates to such action or Indemnified Liability. The Company shall keep the Indemnitee reasonably apprised at all times as to the status of the defense or any settlement negotiations with respect thereto. The Company shall not be liable for any settlement of any action, claim or proceeding effected without its prior written consent, provided, however, that the Company shall not unreasonably withhold, delay or condition its consent. The Company shall not, without the prior written consent of the Indemnitee, consent to entry of any judgment or enter into any settlement or other compromise which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such Indemnitee of a release from all liability in respect to such Indemnified Liability or litigation, and such settlement shall not include any admission as to fault on the part of the Indemnitee. Following indemnification as provided for hereunder, the Company shall be subrogated to all rights of the Indemnitee with respect to all third parties, firms or corporations relating to the matter for which indemnification has been made. The failure to deliver written notice to the Company within a reasonable time of the commencement of any such action shall not relieve the Company of any liability to the Indemnitee under this Section 9(i), except to the extent that the Company is materially and adversely prejudiced in its ability to defend such action.
(iii)
The indemnification required by this Section 9(i) shall be made by periodic payments of the amount thereof during the course of the investigation or defense, within ten (10) days after bills supporting the Indemnified Liabilities are received by the Company.
(iv)
The indemnity agreement contained herein shall be in addition to (A) any cause of action or similar right of the Indemnitee against the Company or others, and (B) any liabilities the Company may be subject to pursuant to the law.
(j)
No Strict Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent, and no rules of strict construction will be applied against any party.
[REMAINDER PAGE INTENTIONALLY LEFT BLANK]
22
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
|
COMPANY:
|
|
IMMUNOPRECISE ANTIBODIES LTD. |
|
|
|
By: (signed) “Jennifer Bath” |
|
Name: Jennifer Bath |
|
Title: Chief Executive Officer |
|
|
23
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
|
|
|
BUYER:
|
|
YA II PN, LTD. |
|
|
|
By: Yorkville Advisors Global, LP |
|
Its: Investment Manager |
|
|
|
By: Yorkville Advisors Global II, LLC |
|
Its: General Partner |
|
|
|
By: (signed) “Matt Beckman” |
|
Name: Matt Beckman |
|
Title: Member |
24
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
LIST OF EXHIBITS:
EXHIBIT A: FORM OF CONVERTIBLE DEBENTURES
EXHIBIT B: FORM OF IRREVOCABLE TRANSFER AGENT INSTRUCTIONS
25
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
EXHIBIT A
FORM OF CONVERTIBLE DEBENTURES
26
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
EXHIBIT B
FORM OF IRREVOCABLE TRANSFER AGENT INSTRUCTIONS
COMPANY LETTERHEAD
[______], 2024
TA INFO
XXXX
XXXX
XXXX
Ladies and Gentlemen:
IMMUNOPRECISE ANTIBODIES LTD., a British Columbia corporation (the “Company”) and YA II PN, LTD. (the “Investor”) have entered into a Securities Purchase Agreement dated as of _____________, 2024 (the “Agreement”), providing for the issuance of Convertible Debentures in the aggregate principal amount of $3,000,000 (the “Debentures”) convertible into common in the Capital of the Company (“Common Stock”).
A copy of the form of Debentures is attached hereto. You should familiarize yourself with your issuance and delivery obligations, as Transfer Agent, contained therein. The shares to be issued are to be registered in the names of the registered holder of the securities submitted for conversion.
You are hereby irrevocably authorized and instructed to reserve a sufficient number of Common Shares of the Company for issuance upon full conversion of the Debentures in accordance with the terms thereof. The amount of Common Shares so reserved is shall initially be ________________ shares, as may be increased by the Company in accordance with the Agreement.
The ability to convert the Debentures in a timely manner is a material obligation of the Company pursuant to such securities. Your firm is hereby irrevocably authorized and instructed to issue Common Shares of the Company (without any restrictive legend) to the Investors without any further action or confirmation by the Company: (A) upon your receipt from any Investor of: (i) a notice of conversion (“Conversion Notice”) executed by the Investor; and (ii) an opinion of counsel of the Company or the Investor, in form, substance and scope customary for opinions of counsel in comparable transactions (and satisfactory to the transfer agent), to the effect that the Common Shares of the Company issued to such Investor pursuant to the Conversion Notice are not “restricted securities” as defined in Rule 144 and should be issued to such Investor without any restrictive legend; and (B) the number of shares to be issued is less than 4.99% of the total issued Common Shares of the Company.
The Company hereby requests that your firm act immediately, without delay and without the need for any action or confirmation by the Company with respect to the issuance of Common Shares pursuant to any Conversion Notices received from any Investor.
The Company shall indemnify you and your officers, directors, principals, partners, agents and representatives, and hold each of them harmless from and against any and all loss, liability, damage, claim or expense (including the reasonable fees and disbursements of its attorneys) incurred by or asserted against you or any of them arising out of or in connection with the instructions set forth herein, the performance of your duties hereunder and otherwise in respect hereof, including the costs and expenses of defending yourself or themselves against any claim or liability hereunder, except that the Company shall not be liable hereunder as to matters in respect of which it is determined that you have acted with gross negligence or in bad faith. You shall have no liability to the Company in respect to any action taken or any failure to act in respect of this if such action was taken or omitted to be taken in good faith, and you shall be entitled to rely in this regard on the advice of counsel.
The Board of Directors of the Company has approved the foregoing (irrevocable instructions) and does hereby extend the Company’s irrevocable agreement to indemnify your firm for all loss, liability or expense in carrying out the authority and direction herein contained on the terms herein set forth.
The Company agrees that in the event that the Transfer Agent resigns as the Company’s transfer agent, the Company shall engage a suitable replacement transfer agent that will agree to serve as transfer agent for the Company and be bound by the terms and conditions of these Irrevocable Instructions within three (3) business days.
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
The Investors are intended to be and are third party beneficiaries hereof, and no amendment or modification to the instructions set forth herein may be made without the consent of each such Investor.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
Very truly yours,
IMMUNOPRECISE ANTIBODIES LTD.
By: ______________________
Name:
Title: CEO
Acknowledged and Agreed:
YA II PN, Ltd.
By: ______________________________
Name:
Title:
Date
Acknowledged and Agreed:
[TRANSFER AGENT]
By: ______________________________
Name:
Title:
Date:
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
SCHEDULE I
SCHEDULE OF BUYERS
|
|
|
|
(a) |
|
(b) |
(c) |
Buyer |
|
Subscription Amount of Convertible Debentures |
Purchase Price (95% of Subscription Amount) |
|
|
|
|
YA II PN, Ltd. |
|
|
|
[Redacted: personal information] |
First Closing: |
$2,000,000.00 |
$1,900,000.00 |
|
Second Closing |
$1,000,000.00 |
$950,000.00 |
|
|
|
|
|
|
|
|
Aggregate: |
$3,000,000.00 |
$2,850,000.00 |
|
|
|
|
|
|
|
Legal Representative’s Address and E-Mail Address |
|
[Redacted: personal information] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
SCHEDULE II
LIST OF MATERIAL SUBSIDIARIES
|
|
|
|
Entity Name |
State of Incorporation |
Address |
Purpose |
ImmunoPrecise Antibodies (Canada) Ltd. |
British Columbia |
[Redacted] |
CRO operations, antibody discovery |
ImmunoPrecise Antibodies (Europe) BV |
Netherlands |
[Redacted] |
CRO operations, antibody discovery and development |
BioStrand B.V. |
Belgium |
[Redacted] |
LENSai IP |
DOCPROPERTY "CUS_DocIDChunk0" 4875-8576-3790\9
EX-4.6
5
ipa-ex4_6.htm
EX-4.6
EX-4.6
Exhibit 4.6
REGISTRATION RIGHTS AGREEMENT
THIS REGISTRATION RIGHTS AGREEMENT (this “Agreement”), dated as of July 16, 2024 is made by and between YA II PN, LTD., a Cayman Islands exempt limited partnership (the “Investor”), and IMMUNOPRECISE ANTIBODIES LTD., a British Columbia corporation (the “Company”). The Investor and the Company may be referred to herein individually as a “Party” and collectively as the “Parties.”
WITNESSETH
WHEREAS:
A. In connection with the Securities Purchase Agreement by and among the parties hereto of even date herewith (the “Securities Purchase Agreement”), the Company has agreed, upon the terms and subject to the conditions of the Securities Purchase Agreement, to issue and sell to the Investor up to $3,000,000 in aggregate principal amount of convertible debentures (the “Convertible Debentures”), which shall be convertible into common shares in the capital of the Company (the “Common Shares”) (as converted, the “Conversion Shares”). Capitalized terms not defined herein shall have the meaning ascribed to them in the Securities Purchase Agreement.
B. Pursuant to the terms of, and in consideration for the Investor entering into, and to induce the Investor to execute and deliver the Securities Purchase Agreement, the Company has agreed to provide certain registration rights under the Securities Act of 1933, as amended, and the rules and regulations thereunder, or any similar successor statute (collectively, the “Securities Act”), and applicable state securities laws and other rights as provided for herein.
AGREEMENT
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and the Investor hereby agree as follows:
Capitalized terms used herein and not otherwise defined herein shall have the respective meanings set forth in the Securities Purchase Agreement. As used in this Agreement, the following terms shall have the following meanings:
(a)
“Effective Date” means the date that the applicable Registration Statement has been declared effective by the SEC.
(b)
“Effectiveness Deadline” means, (i) with respect to the initial Registration Statement required to be filed pursuant to Section 2(b), either (A) the 45th calendar day following the date hereof, if such Registration Statement is not subject to review by the SEC, or (B) the 90th calendar day following the date hereof, if such Registration Statement is subject to review by the SEC, and (ii) with respect to any additional Registration Statements that may be required to be filed by the Company pursuant to this Agreement, the earlier of (A) the 75th calendar day following the date on which the Company was required to file such additional Registration Statement and (B) the fifth Business Day after the date the Company is notified (orally or in writing, whichever is earlier) by the SEC that such Registration Statement will not be reviewed or will not be subject to further review.
(c)
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
(d)
“Filing Deadline” means, (i) with respect to the initial Registration Statement required to be filed pursuant to Section 2(a), the 21st calendar day following the date hereof and (ii) with respect to any additional Registration Statements that may be required to be filed by the Company pursuant to this Agreement, the date on which the Company was required to file such additional Registration Statement pursuant to the terms of this Agreement.
(e)
“Person” means a corporation, a limited liability company, an association, a partnership, an organization, a business, an individual, a governmental or political subdivision thereof or a governmental agency.
(f)
“Prospectus” means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effective registration statement in reliance upon Rule 430A promulgated under the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to the Prospectus, including post‑effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in such Prospectus.
(g)
“Registrable Securities” means all of (i) the Common Shares issuable upon conversion of the Convertible Debentures, (ii) the additional shares issuable in connection with any anti-dilution provisions of the Convertible Debentures (without giving effect to any limitations on exercise set forth in the Convertible Debentures, as applicable) and (ii) any Common Shares issued or issuable with respect to any shares described in subsections (i) and (ii) above by way of any stock split, stock dividend or other distribution, recapitalization or similar event or otherwise (in each case without giving effect to any limitations on exercise set forth in the Convertible Debentures, as applicable).
(h)
“Registration Statement” means any registration statement of the Company filed pursuant to this Agreement, including the Prospectus, amendments and supplements to such registration statement or Prospectus, including post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference in such registration statement.
(i)
“Required Registration Amount” means (i) with respect to the initial Registration Statement at least 15,000,000 Common Shares issued or to be issued upon conversion of the Convertible Debentures, and (ii) with respect to subsequent Registration Statements such number of Common Shares as requested by the Investor not to exceed the maximum number of Common Shares issuable upon conversion of all Convertible Debentures then outstanding (assuming for purposes hereof that (x) such Convertible Debentures are convertible at the Floor Price (as defined therein) in effect as of the date of determination, and (y) any such conversion shall not take into account any limitations on the conversion of the Convertible Debentures set forth therein), in each case subject to any cutback set forth in Section 2(d).
(j)
“Rule 144” means Rule 144 under the Securities Act or any successor rule thereto.
(k)
“Rule 415” means Rule 415 promulgated by the SEC pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the SEC having substantially the same purpose and effect as such Rule.
(l)
“SEC” means the Securities and Exchange Commission or any other federal agency administering the Securities Act and the Exchange Act at the time.
(m)
“Securities Act” shall have the meaning set forth in the Recitals above.
(n)
“SEC Guidance” means (i) any publicly-available written or oral guidance of the SEC staff, or any comments, requirements or requests of the SEC staff and (ii) the Securities Act.
(a)
Registration Period. The Company’s registration obligations set forth in this Section 2 including its obligations to file Registration Statements, obtain effectiveness of Registration Statements, and maintain the continuous effectiveness of any Registration Statement that has been declared effective shall begin on the date hereof and continue until all the Registrable Securities have been sold or may be sold without any restrictions pursuant to Rule 144, as determined by the counsel to the Company pursuant to a written opinion letter to such effect, addressed and reasonably acceptable to the Company’s transfer agent (the “Registration Period”).
(b)
Mandatory Registration. Subject to the terms and conditions of this Agreement, the Company shall (i) on or prior to the Filing Deadline, prepare and file with the SEC an initial Registration Statement on Form F-3 (or, if the Company is not then eligible, on Form F-1) or any successor form thereto covering the resale by the Investor of Registrable Securities, and (ii) on or prior to the 30th calendar day following receipt of each written notice by the Investor (a “Demand Notice”) delivered pursuant to the terms hereof, prepare and file an additional Registration Statement covering the resale by the Investor of Registrable Securities not covered by the initial Registration Statement. Each Registration Statement prepared pursuant hereto shall register for resale at least the number of Common Shares equal to the Required Registration Amount as of date the Registration Statement is initially filed with the SEC. Each Registration Statement shall contain “Selling Stockholders” and “Plan of Distribution” sections. The Company shall use its best efforts to have each Registration Statement declared effective by the SEC as soon as practicable, but in no event later than the Effectiveness Deadline. By 9:30 am, New York time on the Business Day following the date of effectiveness, the Company shall file with the SEC in accordance with Rule 424 under the Securities Act the final Prospectus to be used in connection with sales pursuant to such Registration Statement. Prior to the filing of the Registration Statement with the SEC, the Company shall furnish a draft of the Registration Statement to the Investor for their review and comment. The Investor shall furnish comments on the Registration Statement to the Company within 24 hours of the receipt thereof from the Company. For the purposes hereof, the Investor shall be entitled to deliver a Demand Notice to the Company at any time during the Registration Period if at such time (i) no Registration Statement is then in effect which the Investor may use to resell Registrable Securities, or (ii) a Registration Statement is effective, but the holder has resold substantially all of the Common Shares registered on such Registration Statement. In addition, the Investor may deliver a Demand Notice to the Company at any time during the Registration Period during which (i) the Company does not have a class of securities listed, or approved for listing, on a national securities exchange registered pursuant to Section 6 of the Exchange Act, or (ii) Rule 144, as amended, would not allow the “tacking” of the holding period of the Convertible Debenture onto the holding period of the Conversion Shares issuable upon conversion thereof.
(c)
Amendments and Supplements. During the Registration Period, subject to Allowable Grace Periods (as defined in Section 2(g) below), the Company shall (i) promptly prepare and file with the SEC such amendments (including post-effective amendments) and supplements to a Registration Statement and the Prospectus used in connection with a Registration Statement, which Prospectus is to be filed pursuant to Rule 424 promulgated under the Securities Act, as may be necessary to keep such Registration Statement effective at all times during the Registration Period, (ii) prepare and file with the SEC additional Registration Statements in order to register for resale under the Securities Act all of the Registrable Securities in accordance with the terms of this Agreement; (iii) cause the related Prospectus to be amended or supplemented by any required Prospectus supplement (subject to the terms of this Agreement), and as so supplemented or amended to be filed pursuant to Rule 424; (iv) respond as promptly as reasonably possible to any comments received from the SEC with respect to a Registration Statement or any amendment thereto and as promptly as reasonably possible provide the Investor true and complete copies of all correspondence from and to the SEC relating to a Registration Statement (provided that the Company may excise any information contained therein which would constitute material non-public information as to any Investor which has not executed a confidentiality agreement with the Company); and (v) comply with the provisions of the Securities Act with respect to the disposition of all Registrable Securities of the Company covered by such Registration Statement until such time as all of such Registrable Securities shall have been disposed of in accordance with the intended methods of disposition by the seller or sellers thereof as set forth in such Registration Statement. In the case of amendments and supplements to a Registration Statement which are required to be filed pursuant to this Agreement (including pursuant to this Section 2(c)) by reason of the Company’s filing a report on Form 20-F, or Form 6-K or any analogous report under the Securities Exchange Act, the Company shall incorporate such report by reference into the Registration Statement, if applicable, or shall file such amendments or supplements with the SEC on the same day on which the Exchange Act report is filed which created the requirement for the Company to amend or supplement the Registration Statement.
(d)
Reduction of Registrable Securities Included in a Registration Statement. Notwithstanding anything contained herein, in the event that the SEC requires the Company to reduce the number of Registrable Securities to be included in a Registration Statement in order to allow the Company to rely on Rule 415 with respect to a Registration Statement, then the Company shall be obligated to include in such Registration Statement (which may be a subsequent Registration Statement if the Company needs to withdraw a Registration Statement and refile a new Registration Statement in order to rely on Rule 415) only such limited portion of the Registrable Securities as the SEC shall permit. Any Registrable Securities that are excluded in accordance with the foregoing terms are hereinafter referred to as “Cut Back Securities.” To the extent Cut Back Securities exist, promptly following such time as may be permitted by the SEC, the Company shall be required to file a Registration Statement covering the resale of the Cut Back Securities (subject also to the terms of this Section) and shall use its best efforts to cause such Registration Statement to be declared effective as promptly as practicable thereafter, but in no event later than the Effectiveness Deadline. Notwithstanding the foregoing to the contrary, the Company shall be obligated to use diligent efforts to advocate with the SEC for the registration of all of the Registrable Securities in accordance with the SEC Guidance, including without limitation, Compliance and Disclosure Interpretation 612.09.
Unless otherwise directed in writing by a holder as to its Registrable Securities, the number of Registrable Securities to be registered on such Registration Statement will be reduced as follows: (i) first, the Company shall reduce or eliminate any securities to be included other than Registrable Securities; and (ii) second, the Company shall reduce Registrable Securities on a pro rata basis based on the total number of Registrable Securities held by such holders (or as otherwise expressly directed by the SEC).
(e)
Piggy-Back Registrations. If at any time there is not an effective Registration Statement covering all of the Registrable Securities and the Company proposes to register the offer and sale of any Common Shares under the Securities Act (other than a registration (i) pursuant to a Registration Statement on Form S-8 ((or other registration solely relating to an offering or sale to employees or directors of the Company pursuant to any employee stock plan or other employee benefit arrangement), (ii) pursuant to a Registration Statement on Form S-4 (or similar form that relates to a transaction subject to Rule 145 under the Securities Act or any successor rule thereto), or (iii) in connection with any dividend or distribution reinvestment or similar plan), whether for its own account or for the account of one or more stockholders of the Company and the form of Registration Statement to be used may be used for any registration of Registrable Securities, the Company shall give prompt written notice (in any event no later than five days prior to the filing of such Registration Statement) to the holders of Registrable Securities of its intention to effect such a registration and, shall include in such registration all Registrable Securities with respect to which the Company has received written requests for inclusion from the holders of Registrable Securities; provided, however, that, the Company shall not be required to register any Registrable Securities pursuant to this Section 2(e) that have been sold or may be sold without any restrictions pursuant to Rule 144, as determined by the counsel to the Company pursuant to a written opinion letter to such effect, addressed and acceptable to the Company’s transfer agent.
(f)
Allowable Grace Period. Notwithstanding anything to the contrary contained herein, upon the advice of Company counsel in the form of a written opinion, at any time after the effective date of a particular Registration Statement, the Company may, upon written notice to the Investor, suspend the Investor’s use of any Prospectus (in which event the Investor shall discontinue sales of any Registrable Securities pursuant to such Registration Statement contemplated by this Agreement, but shall settle any previously made sales of Registrable Securities) if the Company (x) is pursuing an acquisition, merger, tender offer, reorganization, disposition or other similar transaction and the Company determines in good faith that (A) the Company’s ability to pursue or consummate such a transaction would be materially adversely affected by any required disclosure of such transaction in such Registration Statement or other registration statement or (B) such transaction renders the Company unable to comply with SEC requirements, in each case under circumstances that would make it impractical or inadvisable to cause any Registration Statement (or such filings) to be used by the Investor or to promptly amend or supplement any Registration Statement contemplated by this Agreement on a post effective basis, as applicable, or (y) has experienced some other material non-public event the disclosure of which at such time, in the good faith judgment of the Company, would materially adversely affect the Company (each, an “Allowable Grace Period”); provided, however, that in no event shall the Investor be suspended from selling Registrable Securities pursuant to any Registration Statement for a period that exceeds twenty (20) consecutive calendar days or an aggregate of thirty (30) calendar days in any 365-day period. Upon disclosure of such information or the termination of the condition described above, the Company shall provide prompt notice, but in any event within one Business Day of such disclosure or termination, to the Investor and shall promptly terminate any suspension of sales it has put into effect and shall take such other reasonable actions to permit registered sales of Registrable Securities as contemplated in this Agreement.
(a)
The Company shall, not less than three Business Days prior to the filing of each Registration Statement and not less than one Business Day prior to the filing of any related amendments and supplements to all Registration Statements (except for annual reports on Form 20-F, supplements and amendments to update the Registration Statement solely for information reflected in the Company’s annual reports on Form 20-F, or current reports on Form 6-K), furnish to each Investor copies of all such documents proposed to be filed, which documents (other than those incorporated or deemed to be incorporated by reference) will be subject to the reasonable and prompt review of such Investor, The Company shall not file a Registration Statement or any such Prospectus or any amendments or supplements thereto to which the Investor shall reasonably object in good faith; provided that, the Company is notified of such objection in writing no later than two (2) Business Days after the Investors have been so furnished copies of a Registration Statement.
(b)
The Company shall furnish to each Investor whose Registrable Securities are included in any Registration Statement, without charge, (i) an electronic copy of such Registration Statement as declared effective by the SEC and any amendment(s) thereto, including financial statements and schedules, all documents incorporated therein by reference, all exhibits and each preliminary prospectus, (ii) an electronic of the final prospectus included in such Registration Statement and all amendments and supplements thereto (or such other number of copies as such Investor may reasonably request) and (iii) such other documents, which are not publicly available through EDGAR, as such Investor may reasonably request from time to time in order to facilitate the disposition of the Registrable Securities owned by such Investor.
(c)
The Company shall use its best efforts to (i) register and qualify the Registrable Securities covered by a Registration Statement under such other securities or “blue sky” laws of such jurisdictions in the United States as the Investor reasonably requests, (ii) prepare and file in those jurisdictions, such amendments (including post-effective amendments) and supplements to such registrations and qualifications as may be necessary to maintain the effectiveness thereof during the Registration Period, (iii) take such other actions as may be necessary to maintain such registrations and qualifications in effect at all times during the Registration Period, and (iv) take all other actions reasonably necessary or advisable to qualify the Registrable Securities for sale in such jurisdictions; provided, however, that the Company shall not be required in connection therewith or as a condition thereto to (w) make any change to its articles of incorporation or by-laws, (x) qualify to do business in any jurisdiction where it would not otherwise be required to qualify but for this Section 3(c), (y) subject itself to general taxation in any such jurisdiction, or (z) file a general consent to service of process in any such jurisdiction. The Company shall promptly notify each Investor who holds Registrable Securities of the receipt by the Company of any notification with respect to the suspension of the registration or qualification of any of the Registrable Securities for sale under the securities or “blue sky” laws of any jurisdiction in the United States or its receipt of actual notice of the initiation or threat of any proceeding for such purpose.
(d)
At any time prior to the end of the Registration Period, as promptly as practicable after becoming aware of such event or development, the Company shall notify each Investor in writing of the happening of any event as a result of which the Prospectus included in a Registration Statement, as then in effect, includes an untrue statement of a material fact or omission to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading (provided that in no event shall such notice contain any material, nonpublic information), and promptly prepare a supplement or amendment to such Registration Statement to correct such untrue statement or omission, and deliver ten (10) copies of such supplement or amendment to the Investor. The Company shall also promptly notify each Investor in writing (i) when a Prospectus or any Prospectus supplement or post-effective amendment has been filed, and when a Registration Statement or any post-effective amendment has become effective (notification of such effectiveness shall be delivered to the Investor by facsimile on the same day of such effectiveness), (ii) of any request by the SEC for amendments or supplements to a Registration Statement or related prospectus or related information, and (iii) of the Company’s reasonable determination that a post-effective amendment to a Registration Statement would be appropriate. The Company shall respond as promptly as reasonably practicable to any comments received from the SEC with respect to a Registration Statement or any amendment thereto.
(e)
The Company shall use its best efforts to prevent the issuance of any stop order or other suspension of effectiveness of a Registration Statement, or the suspension of the qualification of any of the Registrable Securities for sale in any jurisdiction within the United States of America and, if such an order or suspension is issued, to obtain the withdrawal of such order or suspension at the earliest possible moment and to notify each Investor who holds Registrable Securities being sold of the issuance of such order and the resolution thereof or its receipt of actual notice of the initiation or threat of any proceeding for such purpose.
(f)
The Company shall hold in confidence and not make any disclosure of information concerning the Investor provided to the Company unless (i) disclosure of such information is necessary to comply with federal or state securities laws, (ii) the disclosure of such information is necessary to avoid or correct a misstatement or omission in any Registration Statement, (iii) the release of such information is ordered pursuant to a subpoena or other final, non-appealable order from a court or governmental body of competent jurisdiction, or (iv) such information has been made generally available to the public other than by disclosure in violation of this Agreement or any other agreement. The Company agrees that it shall, upon learning that disclosure of such information concerning the Investor is sought in or by a court or governmental body of competent jurisdiction or through other means, give prompt written notice to the Investor and allow the Investor, at the Investor’s expense, to undertake appropriate action to prevent disclosure of, or to obtain a protective order for, such information.
(g)
The Company shall use its best efforts to cause all the Registrable Securities to be listed on each securities exchange on which the Common Shares are then listed. The Company shall pay all fees and expenses in connection with satisfying its obligation under this Section 3(g).
(h)
The Company shall cooperate with the holders of the Registrable Securities to facilitate the timely preparation and delivery of certificates representing the Registrable Securities to be sold pursuant to such Registration Statement or Rule 144 free of any restrictive legends and representing such number of Common Shares and registered in such names as the holders of the Registrable Securities may reasonably request a reasonable period of time prior to sales of Registrable Securities pursuant to such Registration Statement or Rule; provided, that the Company may satisfy its obligations hereunder without issuing physical stock certificates through the use of The Depository Trust Company's Direct Registration System.
(i)
The Company shall use its best efforts to cause the Registrable Securities to be registered with or approved by such other governmental agencies or authorities as may be necessary to consummate the disposition of such Registrable Securities.
(j)
The Company shall otherwise use its best efforts to comply with all applicable rules and regulations of the SEC in connection with any registration hereunder.
(k)
Within one Business Day after a Registration Statement which covers Registrable Securities is declared effective by the SEC, the Company shall deliver, and shall cause legal counsel for the Company to deliver, to the transfer agent for such Registrable Securities (with copies to the Investor whose Registrable Securities are included in such Registration Statement) confirmation that such Registration Statement has been declared effective by the SEC.
(l)
The Company shall take all other reasonable actions necessary to expedite and facilitate disposition by each Investor of Registrable Securities pursuant to a Registration Statement.
4.
OBLIGATIONS OF THE INVESTOR.
(a)
The Investor agrees that, upon receipt of any notice from the Company of the happening of any event of the kind described in Section 2(g) the Investor will immediately discontinue disposition of Registrable Securities pursuant to any Registration Statement covering such Registrable Securities until the Investor’s receipt of the copies of the supplemented or amended prospectus contemplated by Section 2(g) or receipt of notice that no supplement or amendment is required. Notwithstanding anything to the contrary, subject to compliance with the securities laws, the Company shall cause its transfer agent to deliver unlegended certificates for Common Shares to a transferee of an Investor in accordance with the terms of the Securities Purchase Agreement in connection with any sale of Registrable Securities with respect to which an Investor has entered into a contract for sale prior to the Investor’s receipt of a notice from the Company of the happening of any event of the kind described in Section 2(g) and for which the Investor has not yet settled.
(b)
The Investor covenants and agrees that it will comply with the prospectus delivery requirements of the Securities Act as applicable to it or an exemption therefrom in connection with sales of Registrable Securities pursuant to the Registration Statement.
(c)
The Investor agrees to furnish to the Company a completed questionnaire (the “Selling Securityholder Questionnaire”) in the form attached to this Agreement as Annex A, on a date that is not less than two Business Days prior to the Filing Deadline. The delivery of such Selling Securityholder Questionnaire shall be a condition precedent to the Company’s obligation to file the Registration Statement hereunder.
(d)
The Investor agrees to cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of any amendments and supplements to the Registration Statement.
5.
EXPENSES OF REGISTRATION.
Each party shall bear its own fees and expenses related to the transactions contemplated by this Agreements. For the avoidance of doubt, all expenses incurred by the Company in complying with its obligations pursuant to this Agreement and in connection with the registration and disposition of Registrable Securities shall be paid by the Company, including, without limitation, all registration, listing and qualifications fees, printers expenses, and fees and expenses of the Company’s counsel and accountants (except legal fees of Investor’s counsel associated with the review of the Registration Statement). The Investor shall pay any sales or brokerage commissions and fees and expenses of counsel for, and other expenses of, the Investor incurred in connection with registration of Registrable Securities.
With respect to Registrable Securities which are included in a Registration Statement under this Agreement:
(a)
To the fullest extent permitted by law, the Company shall, and hereby does, indemnify, hold harmless and defend the Investor, the directors, officers, partners, employees, agents, representatives of, and each Person, if any, who controls any Investor within the meaning of the Securities Act or the Exchange Act (each, an “Indemnified Person”), against any losses, claims, damages, liabilities, judgments, fines, penalties, charges, costs, reasonable attorneys’ fees, amounts paid in settlement or expenses, joint or several (collectively, “Claims”) incurred in investigating, preparing or defending any action, claim, suit, inquiry, proceeding, investigation or appeal taken from the foregoing by or before any court or governmental, administrative or other regulatory agency, body or the SEC, whether pending or threatened, whether or not an indemnified party is or may be a party thereto (“Indemnified Damages”), to which any of them may become subject insofar as such Claims (or actions or proceedings, whether commenced or threatened, in respect thereof) arise out of or are based upon: (i) any untrue statement or alleged untrue statement of a material fact in a Registration Statement or any post-effective amendment thereto or in any filing made in connection with the qualification of the offering under the securities or other “blue sky” laws of any jurisdiction in which Registrable Securities are offered (“Blue Sky Filing”), or the omission or alleged omission to state a material fact required to be stated therein or necessary to make the statements therein not misleading; (ii) any untrue statement or alleged untrue statement of a material fact contained in any final prospectus (as amended or supplemented, if the Company files any amendment thereof or supplement thereto with the SEC) or the omission or alleged omission to state therein any material fact necessary to make the statements made therein, in light of the circumstances under which the statements therein were made, not misleading; or (iii) any violation or alleged violation by the Company of the Securities Act, the Exchange Act, any other law, including, without limitation, any state securities law, or any rule or regulation there under relating to the offer or sale of the Registrable Securities pursuant to a Registration Statement (the matters in the foregoing clauses (i) through (iii) being, collectively, “Violations”). The Company shall reimburse the Investor and each such controlling person promptly as such expenses are incurred and are due and payable, for any legal fees or disbursements or other reasonable expenses incurred by them in connection with investigating or defending any such Claim. Notwithstanding anything to the contrary contained herein, the indemnification agreement contained in this Section 6(a): (x) shall not apply to a Claim by an Indemnified Person arising out of or based upon a Violation which occurs in reliance upon and in conformity with information furnished in writing to the Company by such Indemnified Person expressly for use in connection with the preparation of the Registration Statement or any such amendment thereof or supplement thereto; (y) shall not be available to the extent such Claim is based on a failure of the Investor to deliver or to cause to be delivered the prospectus made available by the Company, if such prospectus was timely made available by the Company pursuant to Section 3(c); and (z) shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of the Company, which consent shall not be unreasonably withheld. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of the Indemnified Person.
(b)
In connection with a Registration Statement, the Investor agrees to indemnify, hold harmless and defend, to the same extent and in the same manner as is set forth in Section 6(a), the Company, each of its directors, each of its officers, employees, representatives, or agents and each Person, if any, who controls the Company within the meaning of the Securities Act or the Exchange Act (each an “Indemnified Party”), against any Claim or Indemnified Damages to which any of them may become subject, under the Securities Act, the Exchange Act or otherwise, insofar as such Claim or Indemnified Damages arise out of or is based upon any Violation, in each case to the extent, and only to the extent, that such Violation occurs in reliance upon and in conformity with written information furnished to the Company by such Investor expressly for use in connection with such Registration Statement; and, subject to Section 6(d), such Investor will reimburse any legal or other expenses reasonably incurred by them in connection with investigating or defending any such Claim; provided, however, that the indemnity agreement contained in this Section 6(b) and the agreement with respect to contribution contained in Section 7 shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the prior written consent of such Investor, which consent shall not be unreasonably withheld; provided, further, however, that the Investor shall be liable under this Section 6(b) for only that amount of a Claim or Indemnified Damages as does not exceed the net proceeds to such Investor as a result of the sale of Registrable Securities pursuant to such Registration Statement. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of such Indemnified Party. Notwithstanding anything to the contrary contained herein, the indemnification agreement contained in this Section 6(b) with respect to any prospectus shall not inure to the benefit of any Indemnified Party if the untrue statement or omission of material fact contained in the prospectus was corrected and such new prospectus was delivered to each Investor prior to such Investor’s use of the prospectus to which the Claim relates.
(c)
Promptly after receipt by an Indemnified Person or Indemnified Party under this Section 6 of notice of the commencement of any action or proceeding (including any governmental action or proceeding) involving a Claim, such Indemnified Person or Indemnified Party shall, if a Claim in respect thereof is to be made against any indemnifying party under this Section 6, deliver to the indemnifying party a written notice of the commencement thereof, and the indemnifying party shall have the right to participate in, and, to the extent the indemnifying party so desires, jointly with any other indemnifying party similarly noticed, to assume control of the defense thereof with counsel mutually satisfactory to the indemnifying party and the Indemnified Person or the Indemnified Party, as the case may be; provided, however, that an Indemnified Person or Indemnified Party shall have the right to retain its own counsel with the fees and expenses of not more than one (1) counsel for such Indemnified Person or Indemnified Party to be paid by the indemnifying party, if, in the reasonable opinion of counsel retained by the indemnifying party, the representation by such counsel of the Indemnified Person or Indemnified Party and the indemnifying party would be inappropriate due to actual or potential differing interests between such Indemnified Person or Indemnified Party and any other party represented by such counsel in such proceeding.
The Indemnified Party or Indemnified Person shall cooperate fully with the indemnifying party in connection with any negotiation or defense of any such action or claim by the indemnifying party and shall furnish to the indemnifying party all information reasonably available to the Indemnified Party or Indemnified Person which relates to such action or claim. The indemnifying party shall keep the Indemnified Party or Indemnified Person fully apprised at all times as to the status of the defense or any settlement negotiations with respect thereto. No indemnifying party shall be liable for any settlement of any action, claim or proceeding effected without its prior written consent; provided, however, that the indemnifying party shall not unreasonably withhold, delay or condition its consent. No indemnifying party shall, without the prior written consent of the Indemnified Party or Indemnified Person, consent to entry of any judgment or enter into any settlement or other compromise which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such Indemnified Party or Indemnified Person of a release from all liability in respect to such claim or litigation. Following indemnification as provided for hereunder, the indemnifying party shall be subrogated to all rights of the Indemnified Party or Indemnified Person with respect to all third parties, firms or corporations relating to the matter for which indemnification has been made. The failure to deliver written notice to the indemnifying party within a reasonable time of the commencement of any such action shall not relieve such indemnifying party of any liability to the Indemnified Person or Indemnified Party under this Section 6, except to the extent that the indemnifying party is prejudiced in its ability to defend such action.
(d)
The indemnification required by this Section 6 shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and when bills are received or Indemnified Damages are incurred.
(e)
The indemnity agreements contained herein shall be in addition to (i) any cause of action or similar right of the Indemnified Party or Indemnified Person against the indemnifying party or others, and (ii) any liabilities the indemnifying party may be subject to pursuant to the law.
To the extent any indemnification by an indemnifying party is prohibited or limited by law, the indemnifying party agrees to make the maximum contribution with respect to any amounts for which it would otherwise be liable under Section 6 to the fullest extent permitted by law; provided, however, that: (i) no seller of Registrable Securities guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any seller of Registrable Securities who was not guilty of fraudulent misrepresentation; and (ii) contribution by any seller of Registrable Securities shall be limited in amount to the net amount of proceeds received by such seller from the sale of such Registrable Securities.
8.
REPORTS UNDER THE EXCHANGE ACT.
With a view to making available to the Investor the benefits of Rule 144 promulgated under the Securities Act or any similar rule or regulation of the SEC that may at any time permit the Investor to sell securities of the Company to the public without registration, and as a material inducement to the Investor’s purchase of the Convertible Debentures, the Company represents, warrants, and covenants to the following:
(a)
The Company is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months prior to the date hereof (or for such shorter period that the issuer was required to file such reports), other than Form 8-K reports.
(b)
During the Registration Period, the Company shall use its commercially reasonable efforts to file with the SEC in a timely manner all required reports under Section 13 or 15(d) of the Exchange Act (it being understood that nothing herein shall limit the Company’s obligations under the Securities Purchase Agreement) and such reports shall conform to the requirement of the Exchange Act and the SEC for filing thereunder.
(c)
The Company shall furnish to the Investor so long as such Investor owns Registrable Securities, promptly upon request, (i) a written statement by the Company that it has complied with the reporting requirements of Rule 144, (ii) a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company, and (iii) such other information as may be reasonably requested to permit the Investor to sell such securities pursuant to Rule 144 without registration.
9.
AMENDMENT OF REGISTRATION RIGHTS.
Provisions of this Agreement may be amended and the observance thereof may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and Investor. Any amendment or waiver effected in accordance with this Section 9 shall be binding upon the Investor and the Company. No such amendment shall be effective to the extent that it applies to fewer than all of the holders of the Registrable Securities. No consideration shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of any of this Agreement unless the same consideration also is offered to all of the parties to this Agreement.
(a)
A Person is deemed to be a holder of Registrable Securities whenever such Person owns or is deemed to own of record such Registrable Securities or owns the right to receive the Registrable Securities. If the Company receives conflicting instructions, notices or elections from two or more Persons with respect to the same Registrable Securities, the Company shall act upon the basis of instructions, notice or election received from the registered owner of such Registrable Securities.
(b)
The Company shall not file any other registration statements on Form S-3, Form S-1, or otherwise until the initial Registration Statement required hereunder is declared effective by the SEC, provided that this Section 10(b) shall not prohibit the Company from filing amendments to registration statements already filed. The Company shall not include any other securities on a Registration Statement unless otherwise agreed by the Investor.
(c)
Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered pursuant to the notice provisions of the Securities Purchase Agreement or to such other address and/or electronic mail address and/or to the attention of such other person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) electronically generated by the sender’s email service provider containing the time, date, and recipient email or (C) provided by a courier or overnight courier service shall be rebuttable evidence of personal service, receipt by facsimile or receipt from a nationally recognized overnight delivery service in accordance with this section.
(d)
Failure of any party to exercise any right or remedy under this Agreement or otherwise, or delay by a party in exercising such right or remedy, shall not operate as a waiver thereof.
(e)
The laws of the State of New York shall govern all issues concerning the relative rights of the Company and the Investors as its stockholders. All other questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of New York or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of New York. Each party hereby irrevocably submits to the non-exclusive jurisdiction of the Supreme Court of the State of New York, sitting in New York County, New York and federal courts for the Southern District of New York sitting New York, New York, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. If any provision of this Agreement shall be invalid or unenforceable in any jurisdiction, such invalidity or unenforceability shall not affect the validity or enforceability of the remainder of this Agreement in that jurisdiction or the validity or enforceability of any provision of this Agreement in any other jurisdiction. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION HEREWITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
(f)
This Agreement and the rights, duties and obligations of the Investor hereunder may only be assigned upon the transfer of a Convertible Debenture or the Conversion Shares issued pursuant to a Convertible Debenture pursuant to the terms and restrictions on transfer set forth in the Securities Purchase Agreement and the applicable Convertible Debenture. This Agreement and the provisions hereof shall be binding upon and shall inure to the benefit of each of the parties and its successors and the permitted assigns of the parties. No assignment by any party hereto of such party’s rights, duties and obligations hereunder shall be binding upon or obligate the Company unless and until the Company shall have received (A) written notice of such assignment and (B) the written agreement of the assignee, in a form reasonably satisfactory to the Company, to be bound by the terms and provisions of this Agreement (which may be accomplished by an addendum or certificate of joinder to this Agreement).
(g)
The headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof.
(h)
This Agreement may be executed in identical counterparts, both which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. Facsimile or other electronically scanned and delivered signatures (including any electronic signature covered by the U.S. federal ESIGN Act of 2000, Uniform Electronic Transactions Act, the Electronic Signatures and Records Act or other applicable law, e.g., www.docusign.com), including by e-mail attachment, shall be deemed to have been duly and validly delivered and be valid and effective for all purposes of this Agreement.
(i)
Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as the other party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(j)
The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent and no rules of strict construction will be applied against any party.
(k)
This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
IN WITNESS WHEREOF, the Investor and the Company have caused their signature page to this Registration Rights Agreement to be duly executed as of the date first above written.
|
|
|
COMPANY: |
|
IMMUNOPRECISE ANTIBODIES LTD. |
|
|
|
By: (signed) “Jennifer Bath” |
|
Name: Jennifer Bath |
|
Title: Chief Executive Officer |
|
|
|
|
|
|
|
|
|
INVESTOR: |
|
YA II PN, Ltd. |
|
|
|
By: Yorkville Advisors Global, LP |
|
Its: Investment Manager |
|
By: Yorkville Advisors Global II, LLC
Its: General Partner
|
|
By: (signed) “Matt Beckman” |
|
Name: Matt Beckman |
|
Title: Member |
QUESTIONNAIRE
(a)
Full Legal Name of Selling Securityholder
(b)
Full Legal Name of Registered Holder (if not the same as (a) above) through which Registrable Securities are held:
(c)
Full Legal Name of Natural Control Person (which means a natural person who directly or indirectly alone or with others has power to vote or dispose of the securities covered by this Questionnaire):
2.
Address for Notices to Selling Securityholder:
Telephone:
Fax:
Contact Person:
E-Mail:
(a)
Are you a broker-dealer?
Yes ☐ No ☐
(b)
If “yes” to Section 3(a), did you receive your Registrable Securities as compensation for investment banking services to the Company?
Yes ☐ No ☐
Note: If “no” to Section 3(b), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement.
Yes ☐ No ☐
(d)
Are you an affiliate of a broker-dealer? If you are an affiliate of a broker-dealer, do you certify that you purchased the Registrable Securities in the ordinary course of business, and at the time of the purchase of the Registrable Securities to be resold, you had no agreements or understandings, directly or indirectly, with any person to distribute the Registrable Securities?
Yes ☐ No ☐
Note: If “no” to Section 3(d), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement.
4.
Beneficial Ownership of Securities of the Company Owned by the Selling Securityholder.
Except as set forth below in this Item 4, the undersigned is not the beneficial or registered owner of any securities of the Company other than the securities issuable pursuant to the Subscription Agreement.
(a)
Type and Amount of other securities beneficially owned by the Selling Securityholder:
5.
Relationships with the Company:
Except as set forth below, neither the undersigned nor any of its affiliates, officers, directors or principal equity holders (owners of 5% of more of the equity securities of the undersigned) has held any position or office or has had any other material relationship with the Company (or its predecessors or affiliates) during the past three years.
State any exceptions here:
The undersigned agrees to promptly notify the Company of any material inaccuracies or changes in the information provided herein that may occur subsequent to the date hereof at any time while the Registration Statement remains effective; provided, that the undersigned shall not be required to notify the Company of any changes to the number of securities held or owned by the undersigned or its affiliates.
By signing below, the undersigned consents to the disclosure of the information contained herein in its answers to Items 1 through 5 and the inclusion of such information in the Registration Statement and the related prospectus and any amendments or supplements thereto. The undersigned understands that such information will be relied upon by the Company in connection with the preparation or amendment of the Registration Statement and the related prospectus and any amendments or supplements thereto.
IN WITNESS WHEREOF the undersigned, by authority duly given, has caused this Notice and Questionnaire to be executed and delivered either in person or by its duly authorized agent.
Date: Beneficial
Owner:
By:
Name:
Title:
PLEASE EMAIL A .PDF COPY OF THE COMPLETED AND EXECUTED NOTICE AND QUESTIONNAIRE TO:
Dorsey & Whitney LLP
Attention: [Redacted: personal information]
E-mail: [Redacted: personal information]
EX-4.7
6
ipa-ex4_7.htm
EX-4.7
EX-4.7
Exhibit 4.7
GLOBAL GUARANTY AGREEMENT
This Guaranty is made as of July 16, 2024 by ImmunoPrecise Antibodies (Canada), Ltd., British Columbia corporation (“ImmunoCanada”), ImmunoPrecise Antibodies (Europe) BV, a company incorporated in the Netherlands (“ImmunoDutch”), and BioStrand B.V., a company incorporated in Belgium ((“BioStrand”) and collectively with ImmunoCanada and ImmunoDutch, the “Guarantors”) in favor of YA II PN, LTD. (“YA II” or the “Creditor”), with respect to all obligations of ImmunoPrecise Antibodies Ltd. a British Columbia corporation (the “Debtor”) owed to the Creditor.
RECITALS
WHEREAS, the Creditor and the Debtor have entered into a Securities Purchase Agreement (the “Agreement”) on July 16, 2024 pursuant to which the Creditor shall provide loans to the Debtor, to be evidenced by convertible debentures (the “Convertible Debentures”) to be issued by the Debtor to the Creditor, in the amount of up to $3 million;
WHEREAS, it is a condition precedent to the Creditor’s obligation to provide the loan to the Debtor that each Guarantor guarantees all of the Debtor’s obligations under the Agreement, the Convertible Debentures issued thereunder, and all other instruments, agreements or other items executed or delivered (collectively, the “Transaction Documents”) by the Debtor to the Creditor in connection with or related to the Agreement. The Creditor is only willing to enter into the Agreement and provide loans to the Creditor if each Guarantor agrees to execute and deliver to the Creditor this Guaranty; and
WHEREAS, the Guarantors are, or will be at the time of issuance of the Convertible Debentures, wholly owned, or majority owned subsidiaries of the Creditor and will benefit, directly or indirectly, from the Debtor entering into the Agreement, the issuance of the Convertible Debentures, and other Transaction Documents and extensions of credit the Creditor will make to Debtor;
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, each Guarantor covenants and agrees as follows:
1. Guaranty of Payment and Performance. Each Guarantor, jointly and severally, hereby guarantees to the Creditor the full, prompt and unconditional payment when due (whether at maturity, by acceleration or otherwise), and the performance, of all liabilities, agreements and other obligations of the Debtor to the Creditor contained in the Convertible Debentures and the Transaction Documents (all the foregoing, collectively, the “Obligations”). This Guaranty is an absolute, unconditional and continuing guaranty of the full and punctual payment and performance of the Obligations and not of their collectability only and is in no way conditioned upon any requirement that the Creditor first attempt to collect or require the performance of any of the Obligations from the Debtor or resort to any security or other means of obtaining their payment. Should the Debtor default in the payment or performance of any of the Obligations, the obligations of the Guarantors hereunder shall become immediately due and payable to the Creditor, without demand or notice of any nature, all of which are expressly waived by the Guarantor.
2. Limited Guaranty. The liability of the Guarantor hereunder shall be limited to the amount of the Obligations due to the Creditor.
3. Waivers by Guarantor; Creditor’s Freedom to Act. The Guarantor hereby agrees that the Obligations will be paid and performed strictly in accordance with their terms regardless of any law, regulation or order now or hereafter in effect in any jurisdiction affecting any of such terms or the rights of the Creditor with respect thereto. The Guarantor waives presentment, demand, protest, notice of acceptance, notice of Obligations incurred and all other notices of any kind, all defenses that may be available by virtue of any valuation, stay, moratorium law or other similar law now or hereafter in effect, any right to require the marshalling of assets of the Debtor, and all suretyship defenses generally. Without limiting the generality of the foregoing, the Guarantor agrees to the provisions of any instrument evidencing, securing or otherwise executed in connection with any Obligation and agrees that the obligations of the Guarantor hereunder shall not be released or discharged, in whole or in part, or otherwise affected by (i) the failure of the Creditor to assert any claim or demand or to enforce any right or remedy against the Debtor; (ii) any extensions or renewals of, or alteration of the terms of, any Obligation or any portion thereof unless entered into by the Creditor; (iii) any rescissions, waivers, amendments or modifications of any of the terms or provisions of any agreement evidencing, securing or otherwise executed in connection with any Obligation unless entered into by the Creditor; (iv) the substitution or release of any entity primarily or secondarily liable for any Obligation; (v) the adequacy of any rights the Creditor may have against any collateral or other means of obtaining payment or performance of the Obligations; (vi) the impairment of any collateral securing the Obligations, including without limitation the failure to perfect or preserve any rights the Creditor might have in such collateral or the substitution, exchange, surrender, release, loss or destruction of any such collateral; (vii) failure to obtain or maintain a right of contribution for the benefit of the Guarantor; (viii) errors or omissions in connection with the Creditor’s administration of the Obligations (except behavior constituting bad faith); or (ix) any other act or omission that might in any manner or to any extent vary the risk of any Guarantor or otherwise operate as a release or discharge of any Guarantor, all of which may be done without notice to any Guarantor.
4. Unenforceability of Obligations Against Debtor. If for any reason the Debtor is under no legal obligation to discharge or perform any of the Obligations, or if any of the Obligations have become irrecoverable from the Debtor by operation of law or for any other reason, this Guaranty shall nevertheless be binding on the Guarantors to the same extent as if the Guarantors at all times had been the principal obligors on all such Obligations. In the event that acceleration of the time for payment of the Obligations is stayed upon the insolvency, bankruptcy or reorganization of the Debtor, or for any other reason, all such amounts otherwise subject to acceleration under the terms of any agreement evidencing, securing or otherwise executed in connection with any Obligation shall be immediately due and payable by the Guarantors.
5. Subrogation; Subordination. Until the payment and performance in full of all Obligations, the Guarantors shall not exercise any rights against the Debtor arising as a result of payment by the Guarantors hereunder, by way of subrogation or otherwise, and will not prove any claim in competition with the Creditor in respect of any payment hereunder in bankruptcy or insolvency proceedings of any nature; the Guarantors will not claim any set‑off or counterclaim against the Debtor in respect of any liability of the Guarantors to the Debtor; and the Guarantors waive any benefit of and any right to participate in any collateral that may be held by the Creditor. The payment of any amounts due with respect to any indebtedness of the Debtor now or hereafter held by the Guarantor is hereby subordinated to the prior payment in full of the Obligations. The Guarantor agrees that after the occurrence of any default in the payment or performance of the Obligations, the Guarantors will not demand, sue for or otherwise attempt to collect any such indebtedness of the Debtor to the Guarantors until the Obligations shall have been paid or performed in full. If, notwithstanding the foregoing sentence, the Guarantors shall collect, enforce or receive any amounts in respect of such indebtedness, such amounts shall be collected, enforced and received by the Guarantor as trustee for the Creditor and be paid over to the Creditor on account of the Obligations without affecting in any manner the liability of the Guarantors under the other provisions of this Guaranty.
7. Termination; Reinstatement. This Guaranty is irrevocable and shall continue until such time as the Obligations have been indefeasibly paid or performed in full. This Guaranty shall be reinstated if at any time any payment made or value received with respect to an Obligation is rescinded or must otherwise be returned by the Creditor upon the insolvency, bankruptcy or reorganization of the Debtor, or otherwise, all as though such payment had not been made or value received.
8. Successors and Assigns. This Guaranty shall be binding upon each Guarantor, its successors and assigns, and shall inure to the benefit of and be enforceable by the Creditor and the Creditor’s shareholders, officers, directors, agents, successors and assigns.
9. Amendments and Waivers. No amendment or waiver of any provision of this Guaranty nor consent to any departure by the Guarantor therefrom shall be effective unless the same shall be in writing and signed by the Creditor. No failure on the part of the Creditor to exercise, and no delay in exercising, any right hereunder shall operate as a waiver thereof; nor shall any single or partial exercise of any right hereunder preclude any other or further exercise thereof or the exercise of any other right.
10. Notices. All notices and other communications called for hereunder to the Creditor or the Debtor shall be made in writing as provided in the Agreement. All notices and other communications called for hereunder to the Guarantors shall be made in writing as provided on Schedule I attached hereto or as the Guarantors may otherwise notify the Creditor.
11. Governing Law; Consent to Jurisdiction. This Guaranty is intended to take effect as a sealed instrument and shall be governed by, and construed in accordance with, the laws of the State of New York (excluding the laws applicable to conflicts or choice of law). The Guarantor agrees that any suit for the enforcement of this Guaranty may be brought in the courts of the State of New York, New York County and consents to the non‑exclusive jurisdiction of such court and to service of process in any such suit’s being made upon any Guarantor by mail at the address set forth at the head of this Guaranty. The Guarantor hereby waives any objection that it may now or hereafter have to the venue of any such suit or any such court or that such suit was brought in an inconvenient court.
[Rest of page intentionally left blank. Signature page follows.]
IN WITNESS WHEREOF, each Guarantor has caused this Guaranty to be executed and delivered as a sealed instrument as of the date appearing on page one.
ImmunoPrecise Antibodies (Canada), Ltd.
By: (signed) “Jennifer Bath”
Name: Jennifer Bath
Title: Director
ImmunoPrecise Antibodies (Europe) BV
By: (signed) “Jennifer Bath”
Name: Jennifer Bath
Title: Director
BioStrand B.V.
By: (signed) “Jennifer Bath”
Name: Jennifer Bath
Title: Director
Schedule I
The Guarantors
ImmunoPrecise Antibodies (Canada), Ltd.
[Address redacted]
ImmunoPrecise Antibodies (Europe) BV
[Address redacted]
BioStrand B.V.
[Address redacted]
EX-4.8
7
ipa-ex4_8.htm
EX-4.8
EX-4.8
MATERIAL TRANSFER AND EVALUATION AGREEMENT
THIS MATERIAL TRANSFER AND EVALUATION AGREEMENT (the “MTE Agreement,” and
together with its Appendices, the “MTEA”) is made as of the date of the last signature below (the “Effective Date”) by and between Biotheus, Inc. with an address at 10B, Building 4, No 1 Keji 7th Road, Xiang Zhou District, Tangjiawan Town, Zhuhai City, Guangdong Province, China (“Biotheus”), acting in its own name and on behalf of its Affiliates (listed in Appendix 2), and Talem Therapeutics LLC, a US company being a subsidiary of ImmunoPrecise Antibodies with an address at 4837 Amber Valley Parkway, Suite 11, Fargo, ND 58104 USA (“Talem”), acting in its own name and on behalf of its Affiliates (listed in Appendix 3). Each a “Party” and together the “Parties”.
1.
Background. Talem has developed an antagonistic antibody against CAXII (carbonic anhydrase XII) and antibodies that bind CAXII including binding sequences for in-vivo applications and is developing products for treatment of solid tumors. Biotheus has binders and antibodies including bi- specific antibodies to target relevant tumor targets for tumor types like CAIX (carbonic anhydrase 9). Both Parties are interested to use their proprietary binders in collaborative development programs.
In addition to that, Talem has its own capacities to characterize, develop, derisk and study these kind of products and Biotheus has an organization being capable of developing these antibody and antibody- like molecules throughout a biologics development process for enabling Biotheus to file IND (and similar regulatory filings) to start and proceed with clinical development of these molecules with the aim to achieve market authorization anywhere in the world as medicinal products.
Biotheus and Talem desire to evaluate the development of several bi-specific antibodies for treatment of (preferably hypoxic) solid tumors. The Parties hereby agree to conduct the Study and the Evaluation pursuant to the Work Plan(s) and the other terms and conditions as defined below and set forth herein. Any material change to the terms of this MTEA (including, without limitation, the Work Plan(s)) shall be documented in a written amendment to this MTEA that is signed by authorized representatives of each Party.
2.1
“Affiliates” means any corporation or other business entity that controls, is under common control with, or is controlled by Biotheus or Talem, and set forth in Appendix 2 and 3. As used in this definition, “control” of a person means the beneficial ownership (either directly or indirectly) of more than fifty per cent (50%) of the total voting power of the shares or securities then outstanding normally entitled to vote in elections of the board of directors or other managing authority of such person.
2.2
“Background Technology” means Biotheus Background Technology and/or Talem Background Technology, as applicable.
2.3
“Biotheus Background Technology” means any Intellectual Property relating to the use, creation, development, manufacture and method of delivery of Biotheus Material, which (a) is owned or controlled by Biotheus and (b) exists as of and/or was conceived prior to the Effective Date, or (c) is developed or obtained by Biotheus independently of this MTEA without the use of Talem’s Confidential Information, Talem Background Technology and/or Talem-Owned Improvements, and all Intellectual Property Rights in the foregoing. For purposes of clarity, Biotheus Background Technology includes Biotheus Material and Biotheus’ Confidential Information.
1
2.4
“Biotheus Material” means materials including relevant Biotheus’ antibody and antibody- like molecules being engineered with an antagonistic or agonistic binder and Biotheus’ Confidential Information (e.g. nucleic acid and/or amino acid sequences) provided to Talem developed by Biotheus or under control of Biotheus, and related documentation, solely for the purpose of Talem fulfilling its obligations under the Study and the Evaluation, as further detailed in the Work Plan(s).
2.5
“Biotheus-Owned Improvements” means any Improvements made by either Party that relate solely to Biotheus Background Technology, provided that such Biotheus-Owned Improvements do not contain and/or require the use of any Talem’s Confidential Information or Talem Material and do not relate to, cover or otherwise include, all or a portion of Talem Background Technology.
2.6
“CDA” means the Confidential Disclosure Agreement signed by the Parties hereto and valid from 6th of February 2024, attached hereto as Appendix 5.
2.7
“Confidential Information” has the meaning set out in the CDA; for the avoidance of doubt, all information disclosed by either Party to the other under this MTEA and all documents and information generated from the performance of this MTEA shall be deemed Confidential Information and be governed by the provisons of the MTEA and the CDA.
2.8
“Evaluation” means the evaluation (in accordance with the Work Plan(s) as attached hereto as Appendix 1) and summarization of the Study Results to determine the suitability of developing and commercializing the Products pursuant to a further collaboration between the Parties.
2.9
“Evaluation Results” means any and all results of the Evaluation, and any and all data and/or information achieved, obtained, and/or generated as a result of executing the Evaluation, and any and all Intellectual Property Rights therein and/or in relation thereto.
2.10
“Foreground Technology” means any and all Intellectual Property and Intellectual Property Rights arising out of, or in connection with, the performance of the Study and the Evaluation, other than (a) Biotheus Background Technology or (b) Talem Background Technology or (c) Biotheus-Owned Improvements or (d) Talem-Owned Improvements, and all related Intellectual Property Rights. For purposes of clarity, Foreground Technology includes, but is not limited to, any Intellectual Property and/or Intellectual Property Rights solely related to the Products and Results that are not a Biotheus-Owned Improvement or a Talem-Owned Improvement..
2.11
“Improvement” means any improvement, enhancement or modification arising in the performance of the Study and/or the Evaluation, whether patentable or not, to or of the Background Technology.
2.12
“Intellectual Property” means the embodiment of any Intellectual Property Rights.
2.13
“Intellectual Property Rights” means any and all intellectual property rights including (without limitation) the following, and all rights in, arising out of, or associated therewith (in each case, registered or not): patents, know-how, copyrights, trademarks, service marks, utility models, design patents and certificates of invention and other intellectual property or proprietary rights in any inventions, techniques, know-how, confidential information, trade secrets, or discoveries, whether or not patentable; any and all registrations of, or applications to register, or any rights to register or apply to register, any of the foregoing; any and all applications, provisional applications, divisional, continuations, continuations-in-part, converted provisionals and continued prosecution applications relating thereto as well as and all extensions or restorations by existing or future extension or restoration mechanism of any of the foregoing where registered; and/or any similar or analogous rights anywhere in the world.
2.14
“Material” means either Biotheus Material or Talem Material.
2.15
“Products” means any and all medicinal products made, conceived of or developed as part of the Study that incorporate a binding sequence or a sequence of an antibody or antibody-like molecule owned by Talem and/or Biotheus.
2.16
“Results” means the Study Results and the Evaluation Results.
2.17
“Study” means a work package of activities including (without limitation) the characterization, technical evaluation, in vitro and in vivo assessment and other activities set forth in the Work Plan(s) to develop the Products, to be performed by the Parties under the terms and conditions of this MTEA.
2.18
“Study Results” means any and all results of a Study, and any and all experimental data (including raw data and analyzed data) and/or any associated information and/or methods that are achieved, obtained, and/or generated as a result of executing a Study, and any and all Intellectual Property Rights therein and/or in relation thereto.
2.19
“Talem Background Technology” means any Intellectual Property relating to the use, creation, development, manufacture and method of delivery of Talem Material, which (a) is owned or controlled by Talem and (b) exists as of and/or was conceived prior to the Effective Date, or (c) is developed or obtained by Talem independently of this MTEA without the use of Biotheus’ Confidential Information, Biotheus Background Technology and/or Biotheus- Owned Improvements, and all Intellectual Property Rights in the foregoing. For purposes of clarity, Talem Background Technology includes Talem Material and Talem’s Confidential Information.
2.20
“Talem Material” means materials including relevant Talem antibody and antibody-like molecules being engineered with an antagonistic or agonistic binder and Talem’s Confidential Information (e.g. nucleic acid and/or amino acid sequences) provided to Biotheus developed by Talem or under control of Talem, and related documentation, solely for the purpose of Biotheus fulfilling its obligations under the Study and the Evaluation, as further detailed in the Work Plan(s).
2.21
“Talem-Owned Improvements” means any Improvements made by either Party that relate solely to Talem Background Technology provided that such Talem-Owned Improvements do not contain and/or require the use of any Biotheus’ Confidential Information or Biotheus Material and do not relate to, cover or otherwise include, all or a portion of Biotheus Background Technology.
2.22
“Work Plan” means one or more work plan(s) set out in Appendix 1.
3.
Confidentiality and Restrictions on Use.
The terms of the CDA shall continue and are hereby incorporated herein and shall govern disclosure made under this MTEA . The Background Property and Materials of each Party are Confidential Information of such Party.
4.
Obligations, Results and Purpose.
4.1
Obligations of the Parties. The Parties shall use their commercially reasonable efforts to perform, or have performed by their subcontractors, and complete the Study and the Evaluation as described and in accordance with the timeline set forth in the Work Plan(s). In addition, each Party shall use its commercially reasonable efforts to perform or have performed by its subcontractors as described in, and in accordance with the timeline set forth in, the Work Plan(s) attached hereto as Appendix 1. In the event one, or both of the Parties, subcontracts its obligations to subcontractor(s), each Party represents and warrants that the subcontractor (including but not limited to those listed in Appendix 4 and/or any Affiliates) will perform the services and duties in a manner consistent with the terms, conditions and obligations of this MTEA, and the subcontracting Party remains liable for the performance of such subcontractor. Talem shall prepare and deliver to Biotheus the Talem Material solely to enable Biotheus (and/or Biotheus’ subcontractors, including its Affiliates) to execute its obligations under the Work Plan(s) (including transferring to subcontractors and Affiliates). Biotheus shall not transfer the Talem Material or the Products to any third party (other than its subcontractors, including its Affiliates) without the prior express written consent of Talem. Biotheus shall prepare and deliver to Talem the Biotheus Material solely to enable Talem (and/or Talem’s subcontractors, including its Affiliates) to execute its obligations under the Work Plan(s) (including transferring to subcontractors and Affiliates). Talem shall not transfer the Biotheus Material or the Products to any third party (other than its subcontractors, including its Affiliates) without the prior express written consent of Biotheus. Neither Party will transfer the Products to any third party without the prior express written consent of the other Party, and each Party hereby consents to such transfer of the Products to the other Party’s respective Affiliates and the subcontractors listed in Appendix 2, 3 and Appendix 4. Each Party will promptly disclose in writing to the other Party any Foreground Technology it makes or discovers in the course of the Study, or the Evaluation. Talem will promptly disclose in writing to Biotheus any Biotheus-Owned-Improvements it makes or discovers in the course of the Evaluation and Biotheus will promptly disclose in writing to Talem any Talem-Owned- Improvements it makes or discovers in the course of the Evaluation. For the avoidance of doubt, each Party must obtain the other Party’s prior express written approval of any subcontractor proposed by such Party, except that the Parties agree that their respective Affiliates and the subcontractors listed in Appendix 2, 3 and Appendix 4 are pre-approved to be subcontractors under this MTEA.
4.2
Use of Material. Each of the Biotheus Material, the Talem Material, and the Products are intended exclusively for the Study and the Evaluation as described in the Work Plan(s), and is not intended nor are they authorized by either Party for for use in humans. Each Party will ensure that the other Party’s respective Material and/or the Products are handled by trained laboratory personnel only and with the same degree of care the Party’s own respective Material is, and/or the Products are, handled. Each Party will use the other Party’s respective Material and/or the Products in compliance with all applicable laws and regulations.
4.3
Results and Purpose. Nothing in this MTEA will obligate either Party to pursue a further collaboration or any other research, development, manufacturing, or commercial agreement. The Parties acknowledge that the Study and Evaluation are experimental in nature and, as such, that any interpretations and conclusions are uncertain. Neither Party makes any representations or warranties regarding the outcome or correctness of the Results, the Study, or the Evaluation.
Except as set forth in Section 7.3and 7.4, neither Party shall publish, present or otherwise publicly disclose the Results, the Study, the Evaluation or other information relating to the existence or activities performed under this MTEA without the express written consent of the other Party.
5.
Parties’ Representatives. In connection with this MTEA, the contact person for Biotheus will be the Senior Director of BD (currently Alex Wang) and the CSO (currently Andy Tsun), or such other individual designated by Biotheus. The contact person for Talem will be Senior Director Product Development (currently Jetta JE Bijlsma ), or such other individual designated by Talem.
6.
Background Technology. It is acknowledged by each Party that, as between the Parties: (a) the Biotheus Background Technology and (b) the Talem Background Technology are each the respective Party’s solely and exclusively owned, separate property, and such property rights thereto are not affected, transferred or assigned by this MTEA. Except as expressly stated in this Agreement, Talem will not, under this MTEA, acquire any right, title or interest to the Biotheus Background Technology. Except as expressly stated in this Agreement, Biotheus will not, under this MTEA, acquire any right, title or interest to the Talem Background Technology. Subject to the terms of this MTEA, Biotheus is granted by Talem a non-exclusive license to use Talem Background Technology and Talem Materials solely to perform the Study and the Evaluation. Subject to the terms of this MTEA, Talem is granted by Biotheus a non-exclusive license to use Biotheus Background Technology and Biotheus Materials solely to perform the Study and the Evaluation. Upon completion of the Study and the Evaluation, (i) each Party shall destroy or return to the other Party, at such other Party’s direction, any unused or reusable Products or other Party’s Materials and cease to use the other Party’s Background Technology. Notwithstanding the foregoing, if the Parties agree to pursue a further collaboration, each Party may retain the other Party’s Materials after completion of the Study and the Evaluation, provided that (i) it shall destroy or return to the other Party such other Party’s Material if the Parties do not enter into a collaboration agreement for clinical co-development of the Products (the “Definitive Agreement”) within six(6) months after completion of the Evaluation or at any time upon written request of the other Party; (ii) it shall only retain the other Party’s Materials as may be necessary for the conduct of work contemplated in the Definitive Agreement.
7.
Foreground Technology and Improvements
7.1
Improvements. Any Talem-Owned Improvements will be the sole and exclusive property of Talem, without regard to inventorship. Biotheus hereby assigns and transfers (including by way of present assignment and transfer of future rights) to Talem any and all ownership and Intellectual Property Rights it may have in any such Talem-Owned Improvements. Any Biotheus-Owned Improvements will be the sole and exclusive property of Biotheus without regard to inventorship. Talem hereby assigns and transfers (including by way of present assignment and transfer of future rights) to Biotheus any and all ownership and Intellectual Property Rights it may have in any such Biotheus-Owned Improvements. Each Party shall, at the reasonable request of the other Party owing the Improvement and at its expense, do all things (including, without limitation, making declarations and oaths) and execute all documents that may be necessary to give effect to the ownership rights in this Section 7.1.
7.2
Foreground Technology. Each Party shall disclose to the other Party all Results generated by each Party as part of the Study and Evaluation. Subject to the terms of this Section 7.2, all right, title and interest in and to the Foreground Technology will be jointly owned by Biotheus and Talem without regard to inventorship.
Each Party shall, and hereby does, assign and shall cause its representatives involved in the Study and Evaluation to assign, without requirement of additional consideration, an undivided joint ownership interest in all such right, title, and interest in and to the Foreground Technology as is necessary to fully effect the undivided joint ownership thereof by the Parties. Prior to filing any patent applications claiming Foreground Technology, the Parties shall enter into good faith discussions in order to enter into a Definitive Agreement, or another appropriate written agreement, executed by both Parties that defines the Parties’ respective activities and responsibilities for the preparation, filing, prosecution, maintenance and enforcement of such patent applications and any resulting patent rights. Save as otherwise explicitly provided in a Definitive Agreement,all costs of such preparation, filing, prosecution, maintenance, and enforcement the “Patent Cost” shall be shared equally by the Parties. In the event that one of the Parties (the “Waiving Party”) determines in its sole discretion to abandon, cease prosecution of or otherwise not file or maintain such patent in any jurisdiction, then the Waiving Party will provide the other Party written notice of such determination at least thirty (30) days before any deadline for taking action to avoid abandonment (or other loss of rights) and will choose (i) at its own expense, to provide such other Party with the right and all necessary signatures, assignments and other documents, to prepare, file, prosecute and maintain such patents in such jurisdiction at such other Party’s own expense, in such other Party’s own name and for such other Party’s sole benefits and profits, or (ii) continue to share half of the Patent Cost in such jurisdiction and allow such other Party to prepare, file, prosecute and/or maintain such patents in such jurisdiction in the name of both Parties and for their joint benefits and profits. Until the Parties enter into a Definitive Agreement or another appropriate written agreement as outlined above, the Parties shall not file any patent application claiming the Foreground Technology. .
7.3
Results as Confidential Information. . The Forground Technology and Results shall be considered the joint Confidential Information of both Parties and neither Party will publish or disclose the Foreground Technology for any purpose without the prior express written consent of the other Party, other than (a) to conduct the Study and the Evaluation; (b) to support patent applications relating to Biotheus Background Technology or Talem Background Technology, as applicable, provided that such applications do not claim Study and Evaluation Results on the any of the bsAb CA9 and CA12 molecules or Confidential Information or Materials of the other Party; (c) to support patent applications relating to jointly-owned Foreground Technology; or (d) in discussions conducted with current or prospective collaborators or potential investors, provided that (1) in the case of Talem, Talem shall not during the term of this MTEA (as stated below) and six (6) months following completion of the Evaluation, hereafter share or disclose the Results to any business or entity which, to the best of Talem’s
knowledge having made reasonable enquiries, is developing bi-specifics targeting identical targets used under this MTEA, (2) in the case of Biotheus, Biotheus shall not during the term of this MTEA (as stated below) and six (6) months following completion of the Evaluation,thereafter share or disclose the Results to any business or entity which, to the best of Biotheus’ knowledge having made reasonable enquiries, is developing bi-specifics targeting identical targets used under this MTEA, (3) in such discussions no Confidential Information of the other Party (other than the Results themselves) is transferred or disclosed; and (4) the Party making such disclosure of Results causes each such current or prospective collaborator or potential investor to be bound in writing to treat such Results as Confidential Information of Biotheus or Talem, as applicable, and to comply with confidentiality obligations covering such Results at least as restrictive as those covering Confidential Information under this MTEA. Except as permitted under and in accordance with Section 7.2, neither Party shall seek any patent or other form of Intellectual Property Rights protection based on the Results or the other Party’s Confidential Information.
In no event shall this MTEA prevent either Party from collaborating with third parties.
7.4
Scientific Disclosures/Publications. Except as permitted under Section 7.3(a) to (d), or as required by applicable law or stock exchange regulations, Biotheus and Talem shall not disclose or publish (including, without limitation, in any press releases, journal publications, and/or scientific presentations) the Results or any part of them (including without limitation, the existence, or content, of patent applications that have not been published by the relevant patent office) without the prior written consent of the other Party, which consent shall not be unreasonably withheld, conditioned or delayed. A request for such consent must be submitted in writing at least thirty (30) days prior to submitting such Results, in whole or in part, for purposes of publication or disclosure. Upon the written request of the non-disclosing Party, the disclosing Party will extend such initial thirty (30) day period for an additional period as reasonably needed to permit the filing of one or more patent applications directed to the subject matter of the intended publication or disclosure. If either Party so publishes or discloses any Results, such Party will give the other Party the option of receiving a mutually agreeable acknowledgement in such publication or disclosure for its contribution to the Evaluation.
8.
Collaboration. Within six (6) months following completion of the Evaluation, at the latest, the Parties shall discuss in good faith whether the Results justify further collaboration, which shall be governed by the Definitive Agreement to be negotiated. Such collaboration would be subject to customary due diligence. Unless otherwise agreed upon between the parties, the Definitive Agreement will define the form of a research, product development, cross-license and revenue- sharing agreement with a presumptive sharing of costs and benefit on a 50/50 basis. Before concluding the Defintive Agreement, neither Party shall develop, commercialize, assign, license or otherwise dispose of or exploit the Foreground Technology without first obtaining the written approval from the other Party.
9.
Term. This MTEA will commence on the Effective Date and will remain in full force and effect for twenty four (24) months of the Effective Date, unless extended by mutual written agreement of the Parties or terminated in accordance with this Section 10.
This MTEA may be earlier terminated by either Party without cause upon thirty (30) days written notice.
For the avoidance of doubt, notwithstanding anything to the contrary in the CDA, the term of the CDA shall be prolonged such that the CDA will terminate the same time as this MTEA. If the MTEA is early terminated pursuant to this Section 10, the CDA will also terminate automatically. Upon the termination of the CDA, each Party’s oblgiations under the CDA will survive the termination and will be binding upon such Party, its heirs, successors, and assigns for a period of ten (10) years thereafter. Notwithstanding the foregoing, as set out in the CDA, for Confidential Information that classifies as a trade secret under applicable law, confidentiality obligations under the CDA shall survive until such Confidential Information is no longer a trade secret through no action or inaction of the Receiving Party.
10.
Termination and Survival. Upon expiration or termination of this MTEA, and without prejudice to the exceptions set forth in Sections 6 and 7.1, (a) Talem will destroy any remaining Biotheus Material in its possession, (b) Biotheus will destroy any Talem Material in its possession, (c) each Receiving Party will promptly return all the Disclosing Party’s Confidential Information, and (d) the terms and obligations of Sections 2, 3, 4.2, 4.3, and 6-8, 10, 11, 13, 14, 20, 23-26, and 28 will survive.
11.
Representations and Warranties.
11.1
Each Party represents and warrants to the other Party that it has the right to enter into this MTEA and to perform its obligations under this MTEA (including, without limitation, to grant the licence of the Party’s Background Technology to the other Party) without conflict with, or prejudice to, any other obligations to, or infringement of the rights of, any third parties, and it shall not, during the term of this MTEA, enter into any contract or accept any obligation inconsistent or incompatible with its obligations under this MTEA.
11.2
Any Biotheus Background Technology, Talem Background Technology or Material delivered pursuant to this MTEA are experimental in nature and may have hazardous properties. EXCEPT AS MAY BE EXPRESSLY SET FORTH IN THIS MTEA, TO THE FULLEST EXTENT PERMISSIBLE BY APPLICABLE LAW, NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY OF ANY KIND WITH RESPECT TO ANY MATERIALS OR TECHNOLOGY SUPPLIED BY IT TO THE OTHER PARTY HEREUNDER; EACH PARTY ACKNOWLEDGES AND AGREES THAT ALL MATERIALS OR TECHNOLOGY ARE PROVIDED “AS IS” AND TO THE FULLEST EXTENT PERMISSIBLE BY APPLICABLE LAW, WITHOUT ANY WARRANTY, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY WARRANTY OF MERCHANTABILITY, TITLE, FITNESS FOR A PARTICULAR PURPOSE OR NON- INFRINGEMENT.
12.
Indemnification. Each Party (the “Indemnifying Party”) will indemnify the other Party (the “Indemnified Party”, which includes such other Party’s subcontractors and Affiliates) from any third party claims or liability to the extent resulting from (a) use of the Results or the Foreground Technology generated by the Indemnifying Party, its Affiliates, subcontractors and each of its and their respective Representatives, or (b) the misappropriation of any trade secret or an infringement of any patent or other Intellectual Property Right of any third party arising out of the Indemnifying Party’s Background Technology, (c) the negligence, recklessness or willful misconduct of the Indemnifying Party, its Affiliates, subcontractors and each of its and their respective Representatives, in the conduct of this MTEA, or (d) the breach by the Indemnifying Party, its Affiliates, subcontractors and each of its and their respective Representatives, of any of the Indemnifying Party’s representations, warranties, or obligations set forth in this MTEA, except in each case to the extent such claims or liability result from the Indemnified Party’s gross negligence, willful misconduct or breach of this MTEA. The Indemnified Party must promptly notify the Indemnifying Party of a covered claim, must tender to the Indemnifying Party (and/or its insurer) full authority to defend or settle the claim, and must reasonably cooperate with the defense or settlement at the Indemnifying Party’s expense. If the Indemnifying Party does not elect to assume control of the defense or settlement of a claim, the Indemnified Party shall have the right, at the expense of the Indemnifying Party, upon at least ten (10) business days’ prior written notice to the Indemnifying Party of its intent to do so, to undertake the defense or settlement of such claim for the account of the Indemnifying Party (with counsel reasonably selected by the Indemnified Party and approved by the Indemnifying Party, such approval not to be unreasonably withheld, conditioned or delayed); provided that the Indemnified Party shall keep the Indemnifying Party apprised of all material developments with respect to such claim and promptly provide the Indemnifying Party with copies of all correspondence and documents exchanged by the Indemnified Party and the opposing party(ies) to such claim. The Indemnified Party shall not settle any claim hereunder without the prior written consent of the Indemnifying Party, which consent will not be unreasonably withheld, conditioned or delayed.
13.
Notices. All notices must be written and sent to the address identified in this MTEA or a subsequent notice. All notices must be given (a) by personal delivery, with receipt acknowledged, (b) by prepaid certified or registered mail, return receipt requested, (c) by prepaid recognized delivery service or (d) by email (with confirmation of receipt).
Notices will be effective upon receipt. Notices to Biotheus must be marked “Attention: CEO and Legal Department.” Notices to Talem must be marked “Attention: CEO and Legal Department”.
14.
Assignment. This MTEA may not be transferred or assigned, in whole or in part, by either Party, without the prior written consent of the other Party. However, either Party may transfer or assign the MTEA, in whole or in part, to an affiliate, or in connection with a merger, consolidation, or a sale or transfer of all or substantially all of the assets to which this MTEA relates. In the event of such transfer or assignment, the other Party agrees that it shall release the transferring/assigning Party from any and all of such transferring/assigning Party’s obligations arising following the date of such transfer and/or assignment (except the transferring/assigning Party’s confidentiality obligations specified in Section 3 in this MTEA), provided that the relevant transferee/assignee agrees to assume any and all such obligations in writing.
15.
Entire Agreement. This MTEA constitutes the entire and only agreement between the Parties relating to the subject matter hereof, and all prior negotiations, representations, agreements and understanding of the Parties on the subject matter are superseded by the MTEA.
16.
Conflict. In the event of a conflict between the MTE Agreement, on the one hand, and Appendix 1, 2, 3 or 4 on the other hand, the terms of the MTE Agreement shall govern.
17.
No Modification. This MTEA may be changed only in writing signed by an authorized representative(s) of each Party.
18.
Independent Contractors. It is expressly agreed that the relationship between the Talem and Biotheus created by this MTEA shall be one of independent contractors, and neither Party shall have the power or authority to bind or obligate the other Party except as expressly set forth in the MTEA.
19.
Use of Name. No right, express or implied, is granted to either Party by this MTEA to use in any manner any trademark or trade name of the other Party without the prior written consent of the owning Party, except to the extent that the reference to a Party’s name is permitted by another provision of the MTEA. Neither Party shall make, place or disseminate any advertising, public relations, promotional material or any material of any kind using the name of the other Party and/or any subsidiary or affiliate of the other Party or using their trademarks, without the prior written approval of the other Party.
20.
Compliance. Each Party shall comply with all applicable laws in connection with this MTEA. Each Party shall comply, upon reasonable notice from the other Party, with all governmental requests directed to either Party regarding the subject matter of this MTEA and provide all information and assistance necessary to comply with such governmental requests.
21.
Force Majeure. Either Party shall be excused from delays in performing or from its failure to perform hereunder to the extent that such delays or failures result from causes beyond the reasonable control of such Party; provided that, in order to be excused from delay or failure to perform, such Party must act diligently to remedy the cause of such delay or failure.
22.
Severability; Reformation. Any of the provisions of this MTEA which are determined to be invalid or unenforceable in any jurisdiction will be ineffective to the extent of such invalidity or unenforceability in such jurisdiction, without rendering invalid or unenforceable the remaining provisions hereof and without affecting the validity or enforceability of any of the other terms of this MTEA in such jurisdiction, or the terms of this MTEA in any other jurisdiction. The Parties will substitute for the invalid or unenforceable provision a valid and enforceable provision that conforms as nearly as possible with the original intent of the Parties.
23.
Governing Law and Dispute Resolution. This MTEA and any dispute, controversy or claim arising out of, or relating to, this Agreement, including (without limitation), from the formation, existence, validity, interpretation, performance, breach, or termination hereof, or any dispute regarding non- contractual obligations arising out of, or relating to, any of them or this Agreement (“Dispute”) will be governed by and construed and enforced in accordance with the laws of the Singapore, without giving effect to any conflicts of laws principles that require the application of the law of a different state. Any disputes under this Agreement may be submitted to Singapore International Arbitration Centre (SIAC) for arbitration pursuant to its then effective rules. The venue of the arbitration shall be in Singapore. Any proceeding must be conducted in English language.
24.
Waiver. No waiver of any term, provision or condition of this MTEA in any one or more instances will be deemed to be or construed as a further or continuing waiver of any other term, provision or condition of this MTEA. Any such waiver must be evidenced by an instrument in writing executed by an officer authorized to execute waivers.
25.
Counterparts. This MTEA may be executed in any number of counterparts, each of which will be deemed to be an original and all of which together will constitute one and the same instrument. The Parties agree that execution of this MTEA by industry standard electronic signature software and/or by exchanging executed signature pages in .pdf format via e-mail shall have the same legal force and effect as the exchange of original signatures, and that in any proceeding arising under or related to this MTEA, each Party hereby waives any right to raise any defence or waiver based upon execution of this MTEA by means of such electronic signatures or maintenance of the executed MTEA electronically.
26.
Headings. This MTEA contains headings only for convenience and the headings do not constitute or form a part of this MTEA and should not be used in the construction of this MTEA.
IN WITNESS WHEREOF, the Parties each have caused this MTEA to be executed by their duly respective authorized representative as of the Effective Date.
Biotheus, Inc. By Print Name Andy Tsun Title: CSO Background: Talem and Biotheus are intending to develop products for treatment of solid tumors.
Talem Therapeutics LLC
By
Print Name: Jennifer Bath
Title CEO
CEO
APPENDIX 1
The following Work Plan(s) shall describe the design and testing of monoclonal, bi-specific or multispecific antibody molecules for application with humans in the agreed disease area(s).
Both Parties consider to evaluate a number of different antibodies including bi-specific antibodies and antibody-like molecules and a priority as follows:
Anti-CAIX x anti-CAXII bsAb for application of treatment of patients with hypoxic solid tumor in addition to that certain construct for comparison studies like a “naked” Fc construct and Fab binders and isotype control bsAbs shall be provided as defined below.
[the remainder of this page is intentionally left blank]
WORK PLAN No. 1
Co-development of A Bispecific Antibody Using Biotheus’ CAIX Binder and Talems’s CAXII Binder
1.
Design and expression of constructs
1+1 and 2+2 format
and
1.
CAIX: vHH sequence optimized (IP application has been filed) in a heterodimerized bsAb
2.
CAXII: Fab sequences in a heterodimerized bsAb
B.
Additional constructs for comparison (Controls I)
1.
Comparable Constructs from the repertoire of Biotheus with no reactivity in mice (e.g. C-terminally fused 4-1BB non cross-reactive with mice)
2.
Comparable molecules (1+2 and 2+2) that contain either the CAIX or the CAXII arm together with isotype control (IC) (for instance NistMab or MQR.1 molecule)
. EG CAXIIxNistMab
1.
CA9 (parental) x CA12 1+1 form
2.
CA9 (affinity Opi) x CA12 1+1 form
3.
CA9 (parental) x CA12 2+2 form
4.
CA9 (affinity Opi) x CA12 2+2 form
10.
CA12 (non-blocker) mAb
11.
CA9 (parental) x CA12 (non-blocker) 1+1 form
12.
CA9 (affinity Opi) x CA12 (non-blocker) 1+1 form
13.
CA9 (parental) x CA12 (non-blocker) 2+2 form
14.
CA9 (affinity Opi) x CA12 (non-blocker) 2+2 form Neg Ctrtl 1+ 1 IC_CAXII
18.
Neg Ctrtl 2+2 IC_CAXII
Constructs one to fifteen will be generated first and binding and internalization will be tested. Subsequently, based on the results the partners will then decide a short list of molecules for the preparation of ADCs.
i.
Biotheus – based on Talems sequence (to be communicated after signing this MTEA), Biotheus can construct the 1+1 and 2+2 bi-specific and perform characterization, in- vitro and in-vivo testing.
ii.
Talem – shall receive characterized and verified samples from bi-specific candidates and perform CAXII inhibition assay , in silico immunogenicity, developability and liability analysis before production of the molecules to potentially optimize the sequences
2.
Candidate Characterization
a.
Biotheus – KD measurement, cell binding transfected and (ovarian ) cancer cell lines (single positive vs double positive cell), internalization, in vitro killing of ovarian cancer cells grown as spheroids, in vivo xenograft model using ovarian cancer cell line (all test article will be prepared at DAR 4 for comparison during first round)
b.
Talem – CA12 functional blocking, shall receive characterized and verified samples from bi-specific candidates and perform CAXII inhibition assay , in silico immunogenicity, developability and liability analysis before production of the molecules to potentially optimize the sequences
…..
c.
Both parties : Decision making to pick the leading candidate to take into NHP pre-tox test
The follow-on activities like a) in-vivo efficacy study in disease model in mice (and/or other animal) studies, b) NHP PK and Safety Assessment, c) Manufacturing CHO Cell Generation, d) CMC Process Development and Characterization, e) GLP NHP Study for IND, f) IND Application and f) Clinical Development shall be performed under a pure Licensing or a Cross-Licensing and Co-Development Agreement (CLCDA) or similar agreement.
APPENDIX 2
Company Structure and Affiliates of Biotheus
Company Name: Biotheus
Company Address: Sertus Chambers, Governors Square, Suite # 5-204, 23 Lime Tree Bay Avenue, P.O. Box 2547, Grand Cayman, KY1-1104, Cayman Islands
AFFILIATES controlled by “Biotheus”:
Affiliate Name: Biotheus (Hong Kong) Ltd.
Affiliate Address: Level 54, Hopewell Centre, 183 Queen's Road East, Hong Kong Control rate: 100%
AFFILIATES controlled by “Biotheus (Hong Kong) Ltd.”:
Affiliate Name: Biotheus, Inc.
Affiliate Address: 10B, Building 4, No 1 Keji 7th Road, Xiang Zhou District, Tangjiawan Town, Zhuhai City, Guangdong Province, China post code:519080
Control rate: 100%
Affiliate Name: Biotheus (Suzhou) co., ltd.
Control rate: 70%
Affiliate Address: 3/F Room 309, Block D, 398 Ruoshui Road, Suzhou Industrial Park, Suzhou City, Jiangsu Province, China post code: 215123 Company Structure and Affiliates of Talem Company Name: ImmunoPrecise Antibodies LTD. (mother company) Company Address: 3204-4464 Markham Street Victoria, British Columbia V8Z 7X8, Canada AFFILIATES under common control by IPA: Affiliate Name: Talem Therapeutics LLC Affiliate Address: 4837 Amber Valley Parkway, Suite 11, Fargo, ND 58104 USA USA Control rate: 100% ImmunoPrecise Antibodies (USA), Ltd. 4837 Amber Valley Parkway Suite 11 Fargo, ND 58104 Control rate: 100% ImmunoPrecise Antibodies (Europe) B.V. (Oss) Kloosterstraat 9 RE21, 5349 AB Oss, The Netherlands Control rate: 100% ImmunoPrecise Antibodies (Europe) B.V. (Utrecht) Utrecht Science Park, Accelerator Building, 10th floor, Uppsalalaan 17, 3584 CT Utrecht, The Netherlands. Control rate: 100% BioStrand BV Abis, Agoralaan, 3590 Diepenbeek, Belgium Control rate: 100%
Pre-approved subcontractors
Biotheus
Biotheus (Suzhou) co., ltd. Talem Confidential Disclosure Agreement placed between the parties with the effective date of 6th of February, 2024.
&->Talem
MUTUAL CONFIDENTIALITY AGREEMENT
This Mutual Confidentiality Agreement (this "Agreement") is made as of 6th of February, 2024 (the "Effective Date") between Talem Tberapeutics LLC, a US corporation and Talem Affiliates (as defined hereunder) (Talem Therapeutics LLC and Talem Affiliates are referred to collectively as "Talem"), and Biotheus (Hoog Koog) Ltd., a Rong Kong corporation and Biotheus Affiliates (Biotheus (Hong Kong) Ltd. and Biotheus AffiJiates are referred to collectively as "COMPANY"). Talem and COMPANY are hereinafter referred to individually as a "Party" and collectively as the "Parties". For the purpose ofthis Agreement, the Talem Affiliates are BioStrand BV, ImmunoPrecise Antibodies (Europe) B.V., and ImrnunoPrecise Antibodies (Canada) Ltd. Por the purpose of this Agreement, the Biotheus Affiliates are Biotheus, Ine. and Biotheus (Suzhou) co., ltd.
RECITALS
Talem and COMPANY are interested in exploring a potential business cooperation (the "Cooperation"). In the course oftheir discussions, the Parties may exchange information related to their respective businesses, including without limitation, certain Confidential Information (as hereinafter defined).
The purpose of this Agreement is to provide for the proper safeguarding of such Con:fidential Information during the Parties' discussions and for return or destruction of such information by each Party that received it after the Disclosing Party serve a written notice to the Receiving Party.
A Party receiving information hereunder may be referred to herein as a "Receiving Party" and a Party and its Affiliates disclosing information hereunder may be referred to as a "Disclosing Party."
AGREEMENT
In consideration of the preceding, the Parties agree as follows:
1.
Confidential Information and Ownersbip Rights. For purposes of this Agreement, the term "Confidential Information" means all confidential or proprietary information and materials concerning the Disclosing Party and its affiliates, or their respective businesses and properties that is disclosed to the Receiving Party or any ofits Representatives (as defined herein), in whatever form, whether written or oral, electronic or visual, and whether or not it is marked as confidential, in connection with evaluating, entering into or perforrning the Cooperation, including, without limitation, any and all trade secrets, ideas, innovations, accounting and financial information, technica! information, information regarding products, services, pricing, business and operating methods, procedures, processes, policies, know-how, fonnulae, recipes, marketing, research, development, :;ales, costs, improvements, inventions, patents, copyrights, trademarks, trade names and any other intellectual property and applications therefore, business records, employee data, strategie plans, promotional data, business relationships, referral sources, and lists and information regarding customers, vendors and suppliers. Confidential Information also means and includes the status, nature, terms and conditions of any discussions and negotiations between the Parties with respect to the Cooperation.
The Disclosing Party has the exclusive rights to all the Confidential Information throughout the world, whether such rights currently exist or are recognized in the future, and in all media and languages, whether now or subsequently existing, including but not limited to: (a) all technica! data or other written or oral disclosures concerning any Confidential Information; (b) all know-how, techniques or processes conceming the use, marketing and development of the Confidential Information; (c) all future modifications to or improvements of Confidential Information; (d) all derivative works based on any Confidential lnforrnat.ioÄ_, and/or infonnation derived from the Confidential lnfonnation; and (e) all rights to exploit Confidential Information commercially.
@JTalem
Confidential Information does not include information which:
(xxvii)
was already known to the Receiving Party prior to disclosure by the Disclosing Party unless held under an obligation ofnondisclosure;
(xxviii)
is or becomes publicly available through no fault of the Receiving Party;
(xxix)
is rightfully received by the Receiving Party from third parties, without restriction; and/or
(xxx)
is independently developed by the Receiving Party without reference to, or reliance upon, the Confidential Information as provable by written evidense.
"Representatives" means, as to each Party, (i) the affiliates ofthat Party and (ii) the equity bolders, director.-, officers, employees, agents, advisors or other representatives of that Party and that Party's affiliates.
"Affiliate" shall mean, with respect to any Party, any corporation, partnership, limited liability company or other legal or business entity which, directly or indirectly, controls, is controlled by, or is under common control with, the specified Party. For purposes ofthis definition, the term "control" as applied to any Party or entity, means the possession, directly or indirectly, of the power to direct or cause the direction of the management ofthat Party or entity, whether through ownership of more than fifty percent (50%) of the voting securities ofsuch entity, by contract, or otherwise.
2.
Acknowledgments. The Receiving Party acknowledges and agrees that: (a) all Confidential Information is confidential, not generally known in the industry, is protectable under applicable intellectual property laws and may constitutes "Trade Secrets" of the Disclosing Party pursuant to applicable laws); (b) the Disclosing Party may have invested a significant amount of time and money to develop and will continue to invest significant sums to maintain the Confidential Infonnation; (c) the Disclosing Party has instituted procedures to protect the confidential ity of the Confidential Information and the Disclosing Party derives substantial economie benefit from the fact that the Confidential InfoilTlation is confidential; (d) the Disclosing Party's competitors would obtain unfair economie and competitive advantages if Confidential Infonnation was disclosed or used in competition with Disclosing Party, and any such disclosure or use would result in irreparable and continuing injury to Disclosing Party; and (e) although some Confïdential Information may bear a copyright legend, said legend in no way reduces the Trade Secret (if applicable), proprietary and/or confidential status, as the case may be, of the Confidential Information so marked.
3.
Coofidentiality. The Receiving Party agrees that, whether or not it engages in the Cooperation with the Disclosing Party, the Receiving Party will: (a) hold the Contidential Information in trust solely for the Disclosing Party's benefit and use; (b) not directly or indirectly sell, alienate, transfer, assign, disclose or divulge Con:fidential Information to any third party without the Disclosing Party's prior written consent; (c) not directly or indirectly use Confidential Information other than for the purpose of Cooperation, (d) not in any way utilize or exploit any Confidential Information commercially. The Receiving Party will cause all of its Representatives to whom Confidential Information is disclosed to comply with the terms of this Agreement. In the event that the Receiving Party is required by law or Iegal process to disclose Confidential Information, it may do so without being deemed to be in breach ofthis Agreement; provided that it promptly notities the Disclosing Party thereof and cooperates with the Disclosing Party on any reasonable measure to resist such legal process, to limit the Confidential Information required to be disclosed or to obtain confidential treatment for the Confidential Infonnation
required to be disclosed. lf the Receiving Party is unable to inform the Disclosing Party before Confidential Information is disclosed pursuant to this Section 3, it shall, to the extent permitted by law, inform the Disclosing Party of the fuJJ circumstances of the disclosure and the information that has been disclosed as soon as reasonably practicable after such disclosure has been made.
,/ 4-'

4.
Iniunctive and Otber Relief. The Receiving Party acknowledges that if it breaches any of its obligations under this Agreement, it will cause damage of an irreparable and continuing nature to the Disclosing Party, for which money damages alone will not provide adequate relief. Therefore, in addition to all appropriate money damages, the Disclosing Party is entitled to obtain injunctive relief (including but not limited to a temporary restraining order) to prohibit the Receiving Party's continuing breach of the terms of this Agreement.
5.
Title Protection. The Receiving Party wil! not utilize reverse engineering, disassembly or any other means to develop a product, database, list, processor business plan based on of the Confidential Information.
6.
Term. Tuis Agreement shall remain in effect fora term oftwo (2) years after the Effective Date, except that the obligations of the Receiving Party to destroy or return Confidential Information to the Disclosing Party shall survive until ful:filled. All confidentiality obligations under this Agreement shall remain binding on both Parties for a period of ten (10) years after the expiration or termination of this Agreement. Notwithstanding, for Confidential Information that classifies as a trade secret under applicable law, such obligations shall survive until such Confidential lnfonnation is no Jonger a trade secret through no action or inaction of the Receiving Party. Tennination of this Agreement shall not affect any rights or liabilities which may have accrued prior to the date of termination.
7.
Indemnification. The Receiving Party will hold harmless the Disclosing Party, its affiliates, and their respective equity holders, directors, officers, members, managers, employees, successors, agents, advisors and assigns (collectively the "Indemnitees") from any liabilities and expenses, including but not limited to reasonable attomeys' and accountants' fees, investigation costs, travel costs, transcript costs, disbursements, settlement amounts, judgments, fines or penalties, which any Indemnitees incur in connection with any claims, actions, suits or proceedings (whether civil, criminal, administrative or investigative, including all associated appeals) in which it is determined by a court of competent jurisdiction that Receiving Party breached its confidentiality and related obligations set forth in this Agreement.
8.
Return or Destruction of Data - No Use. If the Receiving Party does not enter into the Cooperation with the Disclosing Party, upon the written request of the Disclosing Party, the Receiving Party immediately will, and will cause its Representatives to, return or destroy the Disclosing Party, or in the event that any materials contain the Receiving Party's analysis of the Confidential Information the Disclosing Party may elect to destroy, all the Confidential Information and all notes, data, reference material, software, memoranda, programs, documents, records, copies of any of the foregoing and all other information which contains the Confident ia] Information. The Receiving Party and its Representatives will not retain any copies or abstracts of the foregoing items in any media, and acknowledges that this Agreement does not grant the Receiving Party any use or other rights in the Confidential Information, the Receiving Party's sole right being to review the Confidential Information for consideration of entering into the Cooperation with the Disclosing Party. Notwithstanding the foregoing, the Receiving Party and its Representatives may retain copies of the Confidential lnfonnation in order to comply with applicable law, regulation, professional standards or reasonable business practice and internal document retention policies.
9.
No Representations or Warranties. The Receiving Party understands that the Disclosing Party has not made any representation or warranty as to: (a) the accuracy, completeness or performance of the Confidential lnfonnation; (b) that the Confidential Infonnation is free from error; (c) whether the Confidential Infonnation or any component thereof will now or in the future be ready for release or commercial use; nor (d) the overalJ potential perfonnance and outcome of the Cooperation between the Disclosing Party and the Receiving Party.
The Receiving Party specifically waives and the Disclosing Party speciftcally disclaims all representations or warranties concerning the Disclosing Party's Confidential Information, whether express or implied, oral or written, arisi ng by trade usage or otherwise. The Disclosing Party shall have no liability for use of the Confidential Information by the Receiving Party nor for any claims of third parties howsoever arising from the use or possession of any Confidential Information by the Receiving Party or as a result of the Receiving Party's reliance on the accuracy and completeness of the Confidential Information.
10.
No License; No Contract. Each party acknowledges that the Conftdential Information may comprise valuable trade secrets and proprietary information belonging to the other. The Disclosing Party retains all of its rights, title, and interest in and to the Conftdential Information it discloses. Nothing in this Agreement shall be construed as an express or implied grant to either party of any right or license under any present or future proprietary or intellectual property right of the other party, except solely as may be required to carry out the purpose. Neither this Agreement nor any disclosure ofConfidential lnfonnation pursuant to this Agreement will be construed in any manner to create any legal obligation of either Party to enter into, or to commence or continue discussions or negotiations relating to, the Cooperation.
11.
Complete Understanding. This Agreement constitutes the complete understanding between the Parties. No alteration or modification of any of this Agreement's provisions will be valid unless made in writing and signed by both Parties.
12.
Assignment; Successors and Assignees. Tuis Agreement will be binding upon, inure to the benefit of and be enforceable by the Parties and their permitted successors and perrnitted assigns. Neither Party may assign this Agreement, or any rights or obligations under this Agreement, without the prior written consent of the other Party; and any attempted assignment without such consent will be void and without legal effect.
13.
Governing Law; Jurisdiction. The laws of Singapore (other than those pertaining to conflicts of law) wil! govern this Agreement. Any dispute arising in connection with or out of the perfonnance or the interpretation of this Agreement which the parties cannot settle amicably within thircy
(30) days shall be referred to and !inally resolved by arbitration in Singapore in accordance with the Arbitration Ru1es of the Singapore International Arbitration Centre ("SJAC Rules") for the time being in force. The language of the arbitration shall be English. The costs of arbitration, attomeys' foes and costs for expert and other witnesses, as well as the fees of the arbitrators shall be borne by the losing party.
14.
Amendment; Waiver. The provisions ofthis Agreement may not be amended or waived unless such amendment or waiver is set forth in a writing signed by both Parties. No failure or delay by either Party in exercising any right, power or privilege under this Agreement will operate as a waiver of such right, power or privilege.
1 S. Misçellaneous. This Agreement may be executed in any number of counterparts, each of which will be an original, but all ofwhich together will constitute one instrument Thîs Agreement, to the extent signed and delivered by means of a facsimile machine or other form of electronic transmission, will be treated in all manner and respects as an original agreement or instrument and will be considered to have the same binding legal effect as if it were the original signed version thereof delivered in person. �, IN WITNESS WHEREOF, the Parties have executed this Mutual Confidentiality Agreement as of the date first written above.
[SIGNATURE PAGE FOLLOWS]
�Talem
COMPANY:
Biotheus (Hong Kong) Ltd., a Hong Kong corporation
,
Name: R?J,ancl. Meise!, Ph.D. Title: VP ofBD
Date: 06.02.2024
TALEM:
Talem Therapeutics LLC, a US corporation
By:
Name:
Title:
Date:
[Signature Page to Mutual Confidentiality Agreement -
Talem Therapeulics LLC &
Bio/heus]
EX-8.1
9
ipa-ex8_1.htm
EX-8.1
EX-8.1
Exhibit 8.1
List of Subsidiaries of ImmunoPrecise Antibodies Ltd.
|
|
Entity Legal Name |
Jurisdiction of Incorporation |
ImmunoPrecise Antibodies (Canada), Ltd. |
British Columbia |
ImmunoPrecise Antibodies (USA), Ltd. |
Delaware |
Talem Therapeutics LLC |
Delaware |
ImmunoPrecise Antibodies (MA), LLC |
Delaware |
ImmunoPrecise Antibodies (ND), Ltd. |
North Dakota |
ImmunoPrecise Netherlands B.V. |
Netherlands |
ImmunoPrecise Antibodies (Europe) B.V. |
Netherlands |
Idea Family B.V. |
Belgium |
BioStrand B.V. |
Belgium |
BioKey B.V. |
Belgium |
BioClue B.V. |
Belgium |
EX-11.1
10
ipa-ex11_1.htm
EX-11.1
EX-11.1
DISCLOSURE, CONFIDENTIALITY & TRADING POLICY
The Policy:
This policy establishes procedures which are designed to: (i) permit the disclosure of information about IMMUNOPRECISE ANTIBODIES LTD. (the “Company”) to the public in an informative, timely and broadly disseminated manner; (ii) ensure that non- publicly disclosed information of the Company remains confidential; and, (iii) ensure that trading of the Company’s securities by directors, officers, employees, consultants and certain other persons related to the Company remains in compliance with applicable securities laws.
These procedures are consistent with sound disclosure practices of National Policy 51-201 and the Exchange’s rules.
This policy has been reviewed and approved by the directors of the Company on ____________, 2018.
Definitions Used in this Policy:
Certain defined terms used in this policy are set out in Schedule “A”.
Terms of this Policy:
PART I
DISCLOSURE
The Company will publicly disclose Material Information immediately upon it becoming apparent that the information is material, as defined pursuant to applicable laws, except in circumstances where, in the opinion of Directors of the Company, immediate release of the information would be unduly detrimental to the interest of the Company and where, in such an event, the Company complies with any confidential filing obligations and maintains confidentiality of the information. Examples of which would be detrimental to the interest of the Company may be found in section 11 hereof.
Information is material if it would reasonably be expected to result in a significant change in the market price or value of any of the Company’s securities or if the information would be considered important by investors making decisions to buy or sell securities of the Company.
Developments, whether actual or proposed, which are likely to give rise to material information with respect to the Company and its business and thus to require prompt disclosure may include, but are not limited to those events listed on Schedule “B”.
All public disclosure by the Company of Material Information pursuant to this policy must be made by way of press release, disseminated through a widely circulated newswire service company.
In order to maintain consistent and accurate disclosure about the Company, the following principles should generally be followed:
(a)
No selective disclosure. Previously undisclosed information may not be disclosed to selected persons. If there is disclosure, it must be made widely by way of a press release.
(b)
Disclosure must be updated if earlier disclosure has become misleading as a result of intervening events.
(c)
Unfavourable information must be disclosed as promptly and completely as favourable information.
(d)
Half truths are misleading. Disclosure must include any information without which the rest of the disclosure would be misleading.
(e)
If Material Information is to be announced at a conference, at a shareholders’ meeting, a press conference or other forum, its announcement must be coordinated with an advance on current general public announcement by a press release containing the relevant information.
The Company will maintain a routine procedure for all corporate communications. For Material Information the procedure consists of drafting a press release, circulating it for review to the directors of the Company, to confirm the accuracy of the information contained in the disclosure, alerting the Exchange and IIROC and disseminating the release through a national wire service (with respect to material announcements or announcements involving financial results). The Company may also use other distribution channels so as to effect broad dissemination to the public. With the exception of Material Changes requiring immediate disclosure, news releases will be released outside of market hours whenever possible.
The Company recognizes that posting information to its website will not, by itself, ordinarily satisfy the “generally disclosed” requirement of securities legislation. However, the Company will post to its website press releases disclosing Material Information and shall provide a link to SEDAR, for access to all material documents regarding the Company.
4.
Forward-Looking Information
Subject to the approval and disclosure procedures provided elsewhere in this policy, the Company may provide limited forward-looking information to enable shareholders and the investment community to better evaluate the Company and its strategy, prospects and opportunities. The Company will ensure that such statements are identified as forward-looking. Moreover, such statements will be accompanied by meaningful cautionary statements identifying important factors that could cause actual results to differ materially from those projected in the statements and a description of the factors or assumptions that were used in making the forward-looking statements.
As required under applicable laws, the Company will update forward-looking statements which continue to be material or which change materially over time.
5.
Correction of Selective or Inaccurate Disclosure
If previously Undisclosed Material Information has been inadvertently disclosed to an analyst or any other person or if Material Information that has been disclosed previously is revealed to be inaccurate or incomplete, the Undisclosed Material Information or the information required to correct any inaccuracy in previously disclosed Material Information must be publicly disclosed immediately by way of press release. The Exchange should be contacted and, as need be, a halt in trading in the Company securities should be requested pending the issuance of the press release. Pending the public release of the Material Information, the parties who have knowledge of the information should be advised that the information is material and has not been generally disclosed.
Rumors can cause unusual market activity. The Company will respond consistently to market rumors in the following manner: “it is our policy not to comment on market rumors or speculation”. If market activity indicates that trading is being unduly influenced by rumors, the Exchange may request, or the Company may determine, that a clarifying statement be made through a press release. A trading halt may be instituted or requested pending an announcement by the Company. If the rumor is true, either in whole or in part, immediate disclosure of Undisclosed Material Information will generally be required. The determination to make disclosure will be made by the Information Officer and, if necessary, by the directors.
7.
Contact with Significant Investors, Analysts and Others; Analyst Reports
The Company recognizes that meetings with significant investors, analysts and other market participants are an important element of the Company’s investor relations program. The Company will meet with investors, analysts and other market participants on an individual or small group basis (including participating in industry conferences) as needed and will initiate contacts or respond to calls in a timely, consistent and accurate fashion in accordance with the requirements of this policy. The Company recognizes, however, that private meetings carry with them the risk of inadvertent selective disclosure.
In the event that analyst reports are prepared with respect to the activities and prospects of the Company, the Directors of the Company should avoid getting involved in the content of an analyst’s report, except to correct factual errors. Confirmation of or attempting to influence an analyst’s opinions or conclusions may be considered to be selective disclosure by the Company. “No comment” is an acceptable answer to questions that cannot be answered without violating the rule against selective disclosure. With regard to responding to financial models or drafts of analyst’s reports, it is the Company’s policy to review, on request, the model or report for publicly disclosed factual content only and to give guidance only when assumptions have been made on the basis of incorrect public data. It is imperative that the control of this process be centralized through the Directors of the Company. The Company should confirm in writing that its review has been limited to publicly available factual information and detail what information (if any) has been provided. The Company will not confirm, or attempt to influence, an analyst’s opinions or conclusions and will not express comfort with an analyst’s model or earnings estimate. Meetings with analysts may include general discussions regarding the Company’s prospects, business environment, management philosophy and long-term strategy but should avoid discussions regarding non-publicly disclosed Material Information.
The Company may provide copies of analyst reports to persons outside of the Company. However, the Company will not post such reports on its website but may provide information on how to access these reports.
The Company will consider including in its regular periodic disclosures (such as its quarterly and annual management’s discussion and analysis disclosure) details about topics of interest to analysts, investors and other market participants as a means of providing more information to the marketplace generally and limiting its “selective disclosure” risks.
8.
Notification of Market Surveillance
When the Exchange is open for trading, advance notice of a press release announcing Material Information must be provided to the market surveillance department (or similar department) of IIROC and the Exchange to determine if a halt in trading is necessary to provide time for the market to digest the news. When a press release announcing Material Information is issued outside of trading hours, the market surveillance department of IIROC should be notified before the opening of the market. Copies of all press releases should be supplied to the market surveillance department of IIROC and to the relevant securities regulators immediately.
The Information Officer and directors of the Company will maintain, or cause to be maintained, a file containing all public information about the Company. This includes news releases, brokerage research reports, if any, reports in the press and notes, if any, from meetings with analysts, significant investors and other market participants.
10.
Electronic Communications; the Company Website
This policy also applies to electronic communications, including the Company’s website or social media posts. Accordingly, the Directors of the Company are also responsible for electronic communication of Material Information.
Disclosure on the Company’s website or by social media posts alone does not constitute adequate disclosure of information that is considered Undisclosed Material Information. Any disclosure of Material Information on the website will be preceded by the issuance of a press release.
In order to ensure that no Undisclosed Material Information is inadvertently disclosed, directors and officers of the Company may not participate in Internet chat rooms or newsgroup discussions on matters pertaining to the Company’s activities or its securities. Directors and officers who encounter a discussion pertaining to the Company should advise the Information Officer promptly, so that discussion may be monitored, if determined appropriate.
The Company will not host or link to chat rooms, bulletin boards or news groups; however, the Company may link to analyst reports on the Company on its website.
PART II
CONFIDENTIALITY
11.
When Information May Be Kept Confidential
Where the immediate disclosure of Material Information would be unduly detrimental to the interest of the Company, its disclosure may be delayed and kept confidential temporarily. Keeping information confidential can only be justified where the potential harm to the Company or to investors caused by immediate disclosure may reasonably be considered to outweigh the undesirable consequences of delaying disclosure and where confidentiality of the information is maintained.
Examples of circumstances in which disclosure might be unduly detrimental to the interests of the Company include: (a) where the release of information would prejudice the ability of the Company to pursue specific and limited objectives or to complete a transaction that is underway; (b) where the disclosure of the information would provide competitors with confidential information that would be of significant benefit to them or would undermine the competitive position of the Company; and (c) where the disclosure of information concerning the status of ongoing negotiations would prejudice the successful completion of those negotiations.
All decisions to keep Material Information confidential must be made by the Information Officer or, if necessary, by the directors of the Company. In such circumstances, the Company will comply with any obligation to make a confidential filing with applicable securities regulators and maintain confidentiality of the information.
12.
Access to Confidential Information
Employees will be given access to confidential information on an “as needed” basis only and must not disclose that information to anyone except with the prior approval of a director or officer of the Company and where such disclosure is in the necessary course of business (e.g., discussions with the Company’s bankers or advisers where the disclosure of the confidential information is necessary and the persons receiving it understand that it to be kept confidential).
Other circumstances where disclosure may be considered in the “necessary course of business” may include communications with: (i) vendors, suppliers or strategic partners; (ii) employees, officers and directors; (iii) lenders, legal counsel, auditors, financial advisors and underwriters; (iv) parties to negotiations; (v) labour unions and industry associations; (vi) government agencies in non-governmental regulators; and (vii) credit rating agencies. Selective disclosure of Material Information to an analyst, institutional investor or other market professional is not generally considered in the “necessary course of business”.
Employees must not discuss confidential information in situations where they may be overheard or participate in discussions regarding decisions by others about investments in the Company.
13.
Disclosure of Confidential Information
In the event that confidential information, or rumors respecting the same, is divulged in any manner (other than in the necessary course of business), the Company is required to make an immediate announcement on the matter. IIROC and the Exchange must be notified of the announcement in advance in the usual manner.
14.
Disclosure of Information to Outsiders
Before a meeting with other parties at which Undisclosed Material Information of the Company may be discussed in compliance with this policy, the other parties should be told that they must not divulge that information to anyone else, other than in the necessary course of business, and that they may not trade in the Company’s securities until after the information is publicly disclosed and a reasonable period of time for its dissemination has passed. In such circumstances, the feasibility of having such parties enter into a confidentiality agreement with the Company should be considered.
PART III
TRADING POLICY
This policy of the Company prescribes rules for directors, officers and employees of the Company with respect to trading in securities of the Company by them when there is Undisclosed Material Information. Strict adherence to this policy will promote investor confidence in securities of the Company by assuring to the investing community that persons who have access to Undisclosed Material Information will not make use of it by trading in securities of the Company before the information has been disclosed and properly disseminated.
In accordance with the prohibition set forth by applicable securities laws, no directors, officers or employees may trade in the securities of the Company when they are aware of Undisclosed Material Information, regardless of whether or not a specified Blackout Period has been imposed. Persons possessing such Undisclosed Material Information may trade during a window only after one business day following the widespread public news release of the Undisclosed Material Information.
In addition, directors, officers or employees are prohibited from informing, or “tipping”, anyone else about that information, or informing anyone else about an imposed Blackout Period. Anonymous disclosure of information is also prohibited.
This prohibition extends to other securities whose price or value may reasonably be expected to be affected by changes in the price of the Company’s securities and includes the granting or exercise of stock options.
Rapid buying and selling by directors, officers or employees of the Company’s securities is strongly discouraged because of the possible perception of trading on Undisclosed Material Information.
17.
Blackout Periods and Trading Windows
The Company will use reasonable efforts to notify Directors, officers, employees and other Restricted Persons when a general Blackout Period is in effect. However, it is the obligation of every Director, officer, employee and other Restricted Person to ensure, prior to affecting a trade, that a Blackout Period is not in effect or such person is not otherwise restricted from trading in securities of the Company. In the event that a Director, officer, employee and other Restricted Person is unsure whether they may trade in securities of the Company, they should contact the Information Officer to determine if a general Blackout Period is in effect or if the Director, officer, employee and other Restricted Person is in possession of Undisclosed Material Information.
Provided that no other Blackout Period is in effect, Restricted Persons may trade in securities of the Company only during the period beginning after the close of business one day following widespread public release of quarterly or year-end financial results and ending at the close of trading on the earlier to occur of the fifth day preceding a meeting of the board of directors of the Company or the Audit Committee to approve any distribution or earnings press release or any financial statements reflecting the Company's operating results. However, such trading windows may be modified from time to time.
18.
Undisclosed Material Information of Other Companies
Where directors, officers or employees become aware of Undisclosed Material Information concerning another public company, they may not trade in the securities of that company until the information is publicly disclosed and a reasonable period of time for its dissemination has passed. Generally, a “reasonable period of time” will be one business day; however, it may be shorter or longer depending upon the particular market following of that other company. An Information Officer should be consulted to determine what would be a “reasonable period of time” in the circumstances.
Restricted Persons are prohibited from trading whenever there are Pending Material Developments, even if they are unaware of the details of the same. In the circumstances where there are Pending Material Developments with respect to the Company, a communication will be sent to all Restricted Persons, as well as to other Employees, if it is determined appropriate, informing them of the Blackout Period with respect to such Pending Material Development at which time they shall cease trading until further notice. No reason for the trading restriction will be provided.
Transactions that may be necessarily justifiable for independent reasons (as in a family emergency situation) are no exceptions to the restrictions set forth in this policy.
The Information Officer will make the determination as to when a pending transaction would constitute a Pending Material Development. As guidance, a Blackout Period must at least commence once negotiations on a proposed transaction have progressed to a point where it reasonably could be expected that the market price of the Company’s securities would materially change if the status of the transaction were publicly disclosed.
20.
Insider Trading Reports
Pursuant to the National Statement 55-104, (“reporting insiders”) are required to file insider trading reports within 5 days of a change in their ownership position in any securities of the Company. This includes the grant of options or other convertible securities to such persons or the exercise by them of such options or convertible securities. Such persons are also required to file on SEDI an “initial” insider report upon such person becoming an insider (an initial report is not required, however, when a person becomes an insider if he/she has no direct or indirect beneficial ownership, control or direction over securities of the Company).
If a person falls into one of these categories, that person likely will be required to file insider trading reports in other provinces and should consult the Information Officer as soon as possible whenever the individual trades securities to confirm his/her statutory obligations.
All Restricted Persons must notify the Information Officer prior to entering into any transaction in the securities of the Company to obtain its consent before completing such transaction. A Restricted Person may not trade in the securities of the Company without such consent.
21.
Short Sales, Puts, Calls and Options
No Employee or Restricted Person shall sell the securities of the Company short or buy puts underlying the Company’s securities.
When Employees or other Restricted Persons violate this policy, it causes harm to the reputation to the Company and undermines investors’ confidence in the Company. As a result, the Company may take its own disciplinary actions, which could result in termination of employment or implementation of a probationary period. The Company is also entitled to pursue legal remedies through the courts. If appropriate, the Company will also report the matter to the appropriate regulatory authorities.
The prohibition against trading on (or informing others with respect to) Undisclosed Material Information as set forth in Canadian securities legislation can be enforced by securities regulators through a wide range of penalties, including: (a) fines and penal sanctions; (b) civil actions for damages; (c) an accounting to the Company for any benefit or advantage received; and (d) administrative sanctions by securities commissions, such as cease trade orders and removal of exemptions.
23.
Policy Review and Oversight
The Information Officer shall have overall responsibility for developing and implementing this policy, monitoring the effectiveness of and compliance with this policy and informing the Company’s directors, officers and employees about the policy.
The Company will review this policy annually to ensure that it is achieving its purpose and remains current based on the activities of the Company at the time of review. Based on the results of the review, the policy may be revised accordingly. The Chairman of the Compensation Committee shall be responsible for initiating the annual review. Any changes to this policy shall be approved by the board of directors.
SCHEDULE “A”
DEFINITIONS
“Blackout Period” means the period during which Employees and other Restricted Persons are prohibited from trading in the Company’s securities;
“Directors of the Company” means the individuals who are responsible for communicating with analysts, the news media and investors and ensuring that other Employees do not communicate confidential information about the Company;
“Employees” means all individuals currently employed by the Company and its subsidiaries who may become aware of Undisclosed Material Information;
“IIROC” means the Investment Industry Regulatory Organization of Canada;
“Information Officer” means the Chief Executive Officer or, as an alternative, the Chief Financial Officer of the Company;
“Material Change” means a change in the business, operations or capital of the Company that would reasonably be expected to have a significant effect on the market price or value of any of the securities of the Company and includes a decision to implement the change by the directors of the Company or by senior management of the Company who believe that confirmation of the decision by the directors is probable;
“Material Fact” means a fact that significantly affects or would reasonably be expected to have a Significant effect on the market price or value of the Company’s securities;
“Material Information” means any Material Fact or Material Change;
“Pending Material Developments” means a proposed transaction of the Company that would constitute Material Information, however, a decision to proceed with the transaction has not been made by the directors or by senior management, although there is an expectation of concurrence from the directors;
“Restricted Persons” means:
(a)
directors and officers of the Company and its subsidiaries; and
(b)
Employees of the Company and its subsidiaries; and
(c)
a person employed by the Company and its subsidiaries or retained by it on a professional or consulting basis; and
(d)
affiliates or associates of the Company and its subsidiaries; and
(e)
a person proposing to become a party to a reorganization, amalgamation, merger, or similar business relationship with the Company and its subsidiaries; and
(f)
a person who receives specific confidential information from a person previously described.
“Undisclosed Material Information” means Material Information pertaining to the Company that has not been publicly disclosed or information that has been publicly disclosed, but a reasonable period of time for its dissemination has not passed.
SCHEDULE “B”
EXAMPLES OF POTENTIALLY MATERIAL INFORMATION
The following are examples of the types of events or information which may be material to the Company. This list is not exhaustive and is based on the examples provided by National Policy 51-201 and in the policies of the Exchange.
Changes in Corporate Structure
• changes in share ownership that may affect control of the Company
•
major reorganizations, amalgamations, or mergers
• take-over bids, issuer bids, or insider bids
Changes in Capital Structure
• public or private sale of additional securities
• planned repurchases or redemptions of securities
•
planned splits of common shares or offerings or warrants or rights to buy shares
• any share consolidation, share exchange, or stock dividend
• changes in the Company’s dividend payments or policies
•
the possible initiation of a proxy fight
• material modifications to rights of security holders
Changes in Financial Results
• a significant increase or decrease in near-term earnings prospects
•
unexpected changes in the financial results for any periods
• shifts in financial circumstances, such as cash flow reductions, major asset write-offs or write- downs
• changes in the value or composition of the Company’s assets
•
any material change in the Company’s accounting policy
Changes in Business and Operations
• any development that affects the Company’s resources, services or markets
•
a significant change in capital investment plans or corporate objectives
• labour disputes or disputes with major suppliers or clients
• significant new contracts, beyond the normal course of business; or services • losses of significant contracts or business
•
changes to the Board of Directors or executive management, including the departure of the Company’s CEO, COO, Chairman or CFO (or persons in equivalent positions).
•
the commencement of, or developments in, material legal proceedings or regulatory matters
•
waivers of corporate ethics and conduct rules for officers, directors, and other key employees
•
any notice that reliance on a prior audit is no longer permissible
•
de-listing of the Company’s securities or their movement from one quotation system or exchange to another
Acquisitions and Dispositions
• significant acquisitions or dispositions of assets, property or joint venture interests
•
acquisitions of other companies including a take-over bid for, or merger with, another company
Changes in Credit Arrangements
•
the borrowing or lending of a significant amount of money, outside the normal course of business
•
any mortgaging or encumbering of the Company’s assets, outside the normal course of business
• defaults under debt obligations, agreements to restructure debt, or planned enforcement procedures by a bank or any other creditors
• changes in rating agency decisions, if any
•
significant new credit arrangements
Other
• any other developments relating to the business and affairs of the Company that would reasonably be expected to significantly affect the market price or value of any of the Company’s securities or that would reasonably be expected to have a significant influence on a reasonable investor’s investment decisions I, Jennifer Bath, certify that:
EX-12.1
11
ipa-ex12_1.htm
EX-12.1
EX-12.1
Exhibit 12.1
CERTIFICATION
1.
I have reviewed this annual report on Form 20-F of ImmunoPrecise Antibodies Ltd.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the issuer as of, and for, the periods presented in this report;
4.
The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the issuer and have:
a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the issuer, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)
Evaluated the effectiveness of the issuer’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)
Disclosed in this report any change in the issuer’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the issuer’s internal control over financial reporting; and
5.
The issuer’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the issuer’s auditor and the audit committee of the issuer’s board of directors (or persons performing the equivalent functions):
a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the issuer’s ability to record, process, summarize and report financial information; and
b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the issuer’s internal control over financial reporting.
|
|
|
|
|
|
Date: July 29, 2025 |
By: |
/s/ Jennifer Bath
|
|
|
Jennifer Bath |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
EX-12.2
12
ipa-ex12_2.htm
EX-12.2
EX-12.2
Exhibit 12.2
CERTIFICATION
I, Joseph Scheffler, certify that:
1.
I have reviewed this annual report on Form 20-F of ImmunoPrecise Antibodies Ltd.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the issuer as of, and for, the periods presented in this report;
4.
The issuer’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the issuer and have:
a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the issuer, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)
Evaluated the effectiveness of the issuer’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)
Disclosed in this report any change in the issuer’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the issuer’s internal control over financial reporting; and
5.
The issuer’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the issuer’s auditor and the audit committee of the issuer’s board of directors (or persons performing the equivalent functions):
a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the issuer’s ability to record, process, summarize and report financial information; and
b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the issuer’s internal control over financial reporting.
|
|
|
Date: July 29, 2025 |
By: |
/s/ Joseph Scheffler
|
|
|
Joseph Scheffler |
|
|
Interim Chief Financial Officer |
|
|
(Principal Financial and Accounting Officer) |
EX-13.1
13
ipa-ex13_1.htm
EX-13.1
EX-13.1
Exhibit 13.1
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of ImmunoPrecise Antibodies Ltd. (the “Company”) on Form 20-F for the period ended April 30, 2025 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Jennifer Bath, Chief Executive Officer of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)
The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
July 29, 2025 |
|
/s/ Jennifer Bath
|
|
|
Jennifer Bath |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
A signed original of this written statement required by Section 906 has been provided to ImmunoPrecise Antibodies Ltd. and will be retained by ImmunoPrecise Antibodies Ltd. and furnished to the Securities and Exchange Commission or its staff upon request.
EX-13.2
14
ipa-ex13_2.htm
EX-13.2
EX-13.2
Exhibit 13.2
CERTIFICATION PURSUANT TO
18 U.S.C. §1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of ImmunoPrecise Antibodies Ltd. (the “Company”) on Form 20-F for the period ended April 30, 2025 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Joseph Scheffler, Interim Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. §1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)
The information contained in this Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
July 29, 2025 |
|
/s/ Kristin Taylor
|
|
|
Joseph Scheffler |
|
|
Interim Chief Financial Officer |
|
|
(Principal Financial and Accounting Officer) |
A signed original of this written statement required by Section 906 has been provided to ImmunoPrecise Antibodies Ltd. and will be retained by ImmunoPrecise Antibodies Ltd. and furnished to the Securities and Exchange Commission or its staff upon request.
EX-15.1
15
ipa-ex15_1.htm
EX-15.1
EX-15.1
Exhibit 15.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We have issued our report dated July 29, 2025, with respect to the consolidated financial statements included in this Annual Report on Form 20-F of ImmunoPrecise Antibodies Ltd., as filed with the United States Securities and Exchange Commission, for the year ended April 30, 2025. We also consent to the incorporation by reference of our report in the Registration Statement of ImmunoPrecise Antibodies Ltd. on Form F-3 (File No. 333-273197) and Form S-8 (File No. 333-256730).
/s/ GRANT THORNTON LLP
Houston, Texas
July 29, 2025
EX-16.1
16
ipa-ex16_1.htm
EX-16.1
EX-16.1
Exhibit 16.1
July 29, 2025
U.S. Securities and Exchange Commission
Office of the Chief Accountant
100 F Street, NE
Washington, DC 20549
Re: ImmunoPrecise Antibodies Ltd.
File No. 001-39530
Dear Sir or Madam:
We have read Item 16F of Form 20-F of ImmunoPrecise Antibodies Ltd. dated July 29, 2025, and agree with the statements concerning our Firm contained therein.
Very truly yours,
/s/ GRANT THORNTON LLP
EX-97.1
17
ipa-ex97_1.htm
EX-97.1
EX-97.1
Exhibit 97.1
IMMUNOPRECISE ANTIBODIES LTD.
INCENTIVE COMPENSATION RECOVERY POLICY
The Board of Directors of ImmunoPrecise Antibodies Ltd. (the “Company”) believes that it is in the best interests of the Company and its shareholders to create and maintain a culture that emphasizes integrity and accountability and that reinforces the Company's compensation philosophy. The Board has therefore adopted this policy, which provides for the recovery of erroneously awarded incentive compensation in the event that the Company is required to prepare an accounting restatement due to material noncompliance of the Company with any financial reporting requirements under the federal securities laws (the “Policy”). This Policy is designed to comply with Section 10D of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), related rules and the listing standards of the Nasdaq Capital Market or any other securities exchange or marketplace on which the Company’s shares are listed or quoted in the future (the “Exchange”).
This Policy shall be administered by the Board or, if so designated by the Board,the Remuneration and Nomination Committee (the “Committee”), in which case, all references herein to the Board shall be deemed references to the Committee. Any determinations made by the Board shall be final and binding on all affected individuals.
This Policy applies to the Company’s current and former executive officers,as determined by the Board in accordance with Section 10D of the Exchange Act and the Listing Standards, and such other senior executives or employees who may from time to time be deemed subject to the Policy by the Committee (“Covered Executives”). The following are examples of persons who may be deemed executive officers:
•
Chief Executive Officer;
•
Chief Financial Officer or principal financial officer;
•
Chief Scientific Officer;
•
Principal accounting officer or controller;
•
Any vice president in charge of a principal business unit, division or function, such as sales administration or finance;
•
Any other officer who performs a policy-making function; and
•
Any other person (such as an executive officer of a subsidiary or parent entity)who performs similar policy-making functions for the company.
This Policy covers Incentive Compensation received by a person after beginning service as a Covered Executive and who served as a Covered Executive at any time during the performance period for that Incentive Compensation.
4.
Recovery: Accounting Restatement.
In the event the Company is required to prepare an Accounting Restatement, the Company will recover reasonably promptly any excess Incentive Compensation received by any Covered Executive during the three completed fiscal years immediately preceding the date on which the Company is required to prepare an Accounting Restatement, including transition periods resulting from a change in the Company’s fiscal year as provided in Rule 10D-1of the Exchange Act and Exchange listing standards. Incentive Compensation is deemed “received” in the Company’s fiscal period during which the Financial Reporting Measure specified in the Incentive Compensation award is attained, even if the payment or grant of the Incentive Compensation occurs after the end of that period.
a.
Definition of Accounting Restatement.
For purposes of this Policy, an “Accounting Restatement” means the Company is required to prepare an accounting restatement of its financial statements filed with the Securities and Exchange Commission (the “SEC”) due to the Company’s material noncompliance with any financial reporting requirements under the federal securities laws (including any required accounting restatement to correct an error in previously issued financial statements that is material to the previously issued financial statements, or that would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period).
The determination of the time when the Company is “required” to prepare an Accounting Restatement shall be made in accordance with applicable SEC rules and Exchange listing standards.
An Accounting Restatement does not include situations in which financial statement changes did not result from material non-compliance with financial reporting requirements, such as, but not limited to retrospective: (i) application of a change in accounting principles; (ii) revision to reportable segment information due to a change in the structure of the Company’s internal organization; (iii) reclassification due to a discontinued operation; (iv) application of a change in reporting entity, such as from a reorganization of entities under common control;
(v) adjustment to provision amounts in connection with a prior business combination; and (vi) revision for stock splits,stock dividends, reverse stock splits or other changes in capital structure.
b.
Definition of Incentive Compensation.
For purposes of this Policy, “Incentive Compensation” means any compensation that is granted, earned, or vested based wholly or in part upon the attainment of a Financial Reporting Measure” (as defined in paragraph (b) below), including, for example, bonuses or awards under the Company’s short and long-term incentive plans, grants and awards under the Company’s equity incentive plans, and contributions of such bonuses or awards to the Company’s deferred compensation plans or other employee benefit plans. Incentive Compensation does not include awards which are granted, earned and vested without regard to attainment of Financial Reporting Measures, such as certain time-vesting awards, discretionary awards and awards based wholly on subjective standards, strategic measures or operational measures.
c.
Financial Reporting Measures.
“Financial Reporting Measures” are those that are determined and presented in accordance with the accounting principles used in preparing the Company’s financial statements (including non-GAAP financial measures) and any measures derived wholly or in part from such Financial Reporting Measures. For the avoidance of doubt, Financial Reporting Measures include stock price and total shareholder return. A measure need not be presented within the financial statements or included in a filing with the SEC to constitute a Financial Reporting Measure for purposes of this Policy.
d.
Excess Incentive Compensation: Amount Subject to Recovery.
The amount(s) to be recovered from the Covered Executive will be the amount(s) by which the Covered Executive’s Incentive Compensation for the relevant period(s) exceeded the amount(s) that the Covered Executive otherwise would have received had such Incentive Compensation been determined based on the restated amounts contained in the Accounting Restatement. All amounts shall be computed without regard to taxes paid.
For Incentive Compensation based on Financial Reporting Measures such as stock price or total shareholder return, where the amount of excess compensation is not subject to mathematical recalculation directly from the information in an Accounting Restatement, the Board will calculate the amount to be reimbursed based on a reasonable estimate of the effect of the Accounting Restatement on such Financial Reporting Measure upon which the Incentive Compensation was received. The Company will maintain documentation of that reasonable estimate and will provide such documentation to the applicable Exchange.
The Board will determine, in its sole discretion, the method(s) for recovering reasonably promptly excess Incentive Compensation hereunder. Such methods may include, without limitation:
a.
requiring reimbursement of Incentive Compensation previously paid;
(ii) forfeiting any Incentive Compensation contribution made under the Company’s deferred compensation plans;
(iii) offsetting the recovered amount from any compensation that the Covered Executive may earn or be awarded in the future;
(iv) taking any other remedial and recovery action permitted by law, as determined by the Board; or
(v) some combination of the foregoing.
5.
No Indemnification [or Advance].
Subject to applicable law, the Company shall not indemnify, including by paying or reimbursing for premiums for any insurance policy covering any potential losses, any Covered Executives against the loss of any erroneously awarded Incentive Compensation.
The Board is authorized to interpret and construe this Policy and to make all determinations necessary, appropriate or advisable for the administration of this Policy. It is intended that this Policy be interpreted in a manner that is consistent with the requirements of Section 10D of the Exchange Act and any applicable rules or listing standards adopted by the SEC or any Exchange.
The effective date of this Policy is October 2, 2023 (the “Effective Date”). This Policy applies to Incentive Compensation received by Covered Executives on or after the Effective Date that results from attainment of a Financial Reporting Measure based on or derived from financial information for any fiscal period ending on or after the Effective Date. In addition, this Policy is intended to be and will be incorporated as an essential term and condition of any Incentive Compensation agreement, plan or program that the Company establishes or maintains on or after the Effective Date.
8.
Amendment and Termination.
The Board may amend this Policy from time to time in its discretion, and shall amend this Policy as it deems necessary to reflect changes in regulations adopted by the SEC under Section 10D of the Exchange Act and to comply with any rules or listing standards adopted by an Exchange.
The Board intends that this Policy will be applied to the fullest extent of the law. Upon receipt of this Policy, each Covered Executive is required to complete the Receipt and Acknowledgement attached as Schedule A to this Policy. The Board may require that any employment agreement or similar agreement relating to Incentive Compensation received on or after the Effective Date shall, as a condition to the grant of any benefit thereunder, require a Covered Executive to agree to abide by the terms of this Policy. Any right of recovery under this Policy is in addition to, and not in lieu
of, any (i) other remedies or rights of compensation recovery that may be available to the Company pursuant to the terms of any similar policy in any employment agreement, or similar agreement relating to Incentive Compensation, unless any such agreement expressly prohibits such right of recovery, and (ii) any other legal remedies available to the Company.The provisions of this Policy are in addition to (and not in lieu of) any rights to repayment the Company may have under Section 304 of the Sarbanes-Oxley Act of 2002 and other applicable laws.
The Company shall recover any excess Incentive Compensation in accordance with this Policy, except to the extent that certain conditions are met and the Board has determined that such recovery would be impracticable, all in accordance with Rule 10D-1 of the Exchange Act and Nasdaq Listing Rule 5608 or the listing standards of any other Exchange.
This Policy shall be binding upon and enforceable against all Covered Executives and their beneficiaries, heirs, executors, administrators or other legal representatives

 CEO
CEO