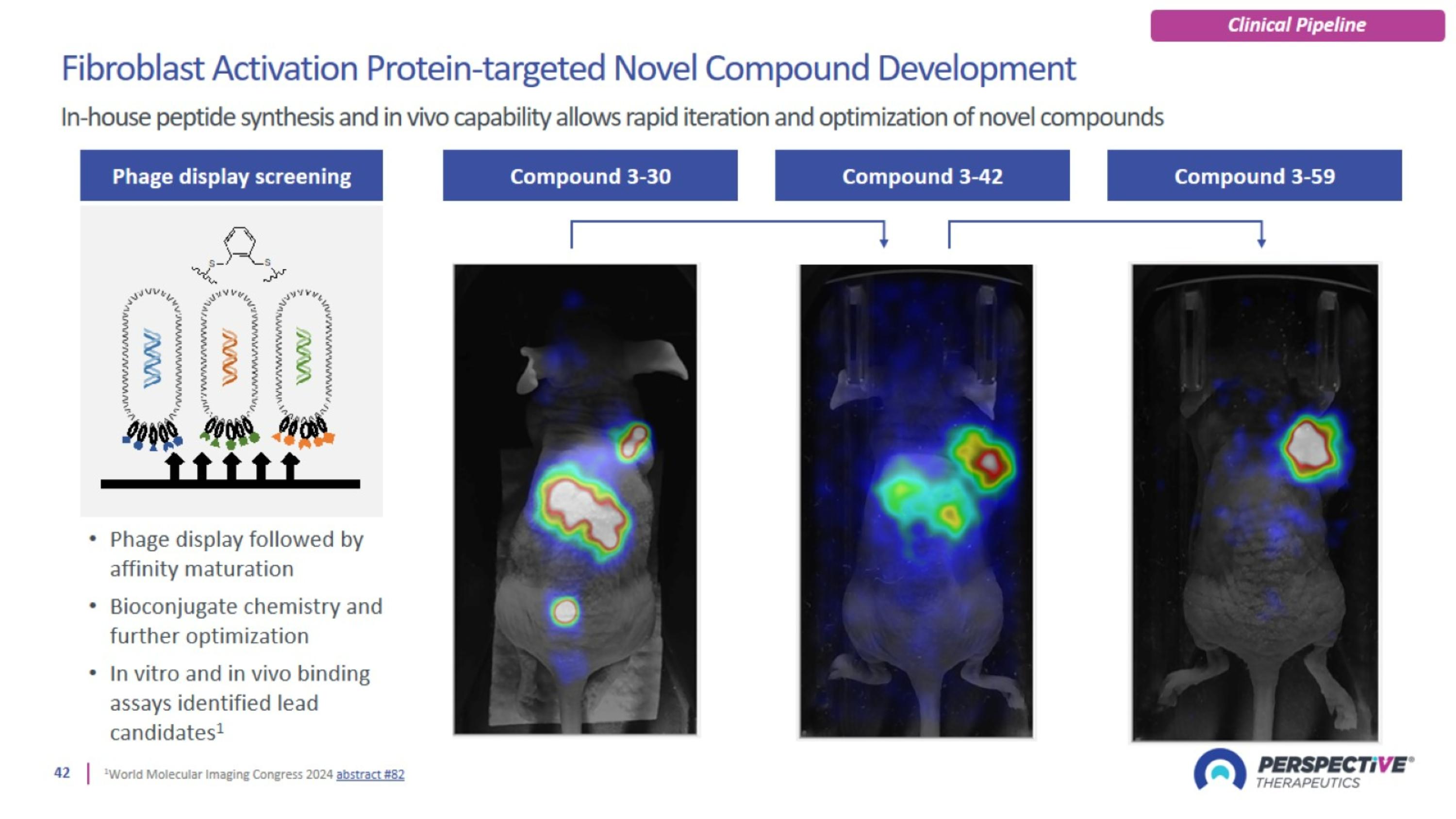
[212Pb]Pb-VMT-α-NET dosimetry in patients with advanced SSTR2 positive tumors in the VMT-α-NET-T101 trial Stephen A. Graves1, Thorvardur R. Halfdanarson2, Richard L. Wahl3, Lowell Anthony4, Lilja B. Solnes5, Sam H. Mehr6, Ian R. Marsh7, Lucia Baratto7, Stephen M. Keefe7, Markus Puhlmann7, Vikas Prasad3 1University of Iowa, 2Mayo Clinic, 3Washington University, 4University of Kentucky, 5Johns Hopkins, 6Nebraska Cancer Specialists, 7Perspective Therapeutics [212Pb]Pb-VMT-α-NET is a novel alpha-emitting radiopharmaceutical therapy (RPT) developed for the treatment of somatostatin receptor subtype 2 expressing (SSTR2) tumors. VMT-α-NET-T101 (NCT05636618) is a multi-center phase I/IIa trial of [212Pb]Pb-VMT-α-NET in PRRT-naïve patients with unresectable or metastatic SSTR2+ neuroendocrine tumors. As part of this investigation, a subset of patients were enrolled in a dosimetry sub-study utilizing pre-treatment SPECT imaging of [203Pb]Pb-VMT-α-NET. Here we present preliminary dosimetry analysis for [212Pb]Pb-VMT-α-NET based on theranostic imaging studies of [203Pb]Pb-VMT-α-NET. Background Study Design: VMT-α-NET-T101 VMT-α-NET Dosimetry The theranostic pairing of 203Pb and 212Pb provides a valuable tool for imaging, dosimetry, and treatment. [212Pb]VMT-α-NET dosimetry using [203Pb]VMT-α-NET as a theranostic imaging surrogate is feasible and should be considered as a valuable adjunct to this trial’s clinical data. The most appropriate RBE weighting may be radionuclide- and agent-specific. RBE=5 may not be applicable for every targeted-alpha particle RPT. Taken together, these clinical safety data and dosimetry analyses suggest that the reported estimated cumulative absorbed doses of radiation to organs of interest likely represent tolerable levels for [212Pb]VMT-α-NET. Ultimately, clinical safety data will drive and set these limits. Conclusions Patient eligibility was assessed based on approved DOTATATE PET imaging. A pre-therapy multi-timepoint imaging study using 6.0 mCi (222 MBq) of [203Pb]Pb-VMT-α-NET was acquired for dosimetry analysis. SPECT/CT images of [203Pb]Pb-VMT-α-NET were acquired at 1, 4, and 24 h following administration. Blood & urine samples were collected through 24 h post administration. The mean time between SPECT imaging and therapy was 9 ± 5 days. Standard amino acid co-administration was used for both [203Pb]Pb-VMT-α-NET and [212Pb]Pb-VMT-α-NET. Dosimetry sub-study patients (n = 6) were enrolled across 3 clinical sites. Dosimetry Sub-Study Time-Activity Curves Modeling Redistribution of 212Bi Quantitative SPECT Imaging of 203Pb Partial Volume Correction Safety & Efficacy Profile Pharmacokinetic model for bismuth in the human body. Reproduced from ICRP Report 137 6. Elementally Matched Theranostic Pair 212Bi Bismuth 60.6 m 208Tl Thallium 3.1 m 212Po Polonium 0.3 µs 208Pb Lead Stable 212Pb Lead 10.6 h β- 203Tl Thallium Stable 203Pb Lead 51.9 h 0.57 MeV β- (64%) 2.3 MeV α (36%) 6.1 MeV β- 1.8 MeV α 8.8 MeV ε γ 279 keV Creatinine (mg/dL) Study Week 0 24 48 64 8 16 32 72 56 40 5 4 3 2 0 1 Treatment-emergent adverse events (all patients treated, n = 42). Blood creatinine during follow-up for all patients treated (n = 42). Data cutoff 2025-04-30. Clinical data from this trial has been summarized as of 2025-04-30 Safety data are reported for all patients treated, n = 42 Efficacy data reported for patients enrolled for DLT-observation, n = 9 Safety data demonstrate a tolerable profile No DLTs observed among patients treated with [212Pb]VMT-α-NET No grade ≥ 4 AEs were reported No treatment-related discontinuations occurred No serious renal complications or myelosuppression was reported No dysphagia was reported Clinical activity was observed 7 of 9 DLT-observation patients were without progression after more than 1y of follow-up Investigator-assessed RECIST v1.1 objective responses were observed among 4 of 7 cohort two DLT-observation patients (three confirmed) [212Pb]Pb-VMT-α-NET Neuroendocrine tumors are heterogenous. >80% overexpress somatostatin receptor 2 (SSTR2). Despite the availability of [177Lu]Lu-DOTATATE, there remains a high unmet medical need for novel therapies. VMT-α-NET is a modified SSTR2 binding peptide with proprietary chelation technology for 203Pb, 212Pb, & 212Bi. PSC – A Proprietary Lead-Specific Chelator1 Designed specifically for Pb isotopes. Optimized for rapid renal clearance with neutralized formal charge. Improved radiolabeling, receptor binding, & internalization. Enhanced retention of 212Bi, reducing off-target toxicity. 212Pb2+ NET 0 PSC Molecular Weight: 1789.83 212Pb2+ DOTA 212Pb2+ NET 2+ NET 2- TCMC Generic Chelators Charged molecules can be more often reabsorbed through the kidneys.2 212Bi leaks from these chelators at up to 36%.3 Dose-finding phase Expansion phase Recommended Phase 2 Dose (RP2D) Expansion Cohort [212Pb]Pb-VMT--NET RP2D mCi x 4 Recruited Cohort 2 [212Pb]Pb-VMT--NET n = 7 / 5 mCi x 4 Cohort 3 [212Pb]Pb-VMT--NET n = 3 – 8 / 7.5 mCi x 4 Recruitment Complete Cohort 1 [212Pb]Pb-VMT--NET n = 2 / 2.5 mCi x 4 Cohort 4 [212Pb]Pb-VMT--NET n = 3 – 8 / 10 mCi x 4 Pre-agreed FDA interaction before dosing next cohort Expansion into non-NET indications (e.g., SCLC) also possible Intermediate doses or de-escalation possible for Cohort 2 - 4 Added slots: Total of up to 47 (Recruitment ongoing) Dose-finding Population Key Study Features Study Endpoints Advanced/Unresectable or metastatic NETs Progressive disease on prior therapy PRRT naive FDA approved SSTR2 PET/CT avid disease Bayesian mTPI-2 design based on iterative toxicity probability monitoring Dosimetry to be assessed during screening period for cohorts 1 & 2 using [203Pb]Pb-VMT--NET Primary: to measure incidence of DLTs following a single administration of [212Pb] Pb-VMT--NET in order to determine the MTD and/or MFD, and RP2D Secondary / exploratory ORR, DOR, PFS, OS by RECIST v1.1 Using dosimetry, estimate selected organ and whole body absorbed radiation doses for [212Pb]Pb-VMT--NET Trial Parameters (NCT05636618) 79 mm 200 mm 200 mm 250 ml Nalgene bottle References Diagnostic Therapeutic Li M, Baumhover NJ, Liu D, et al. Pharmaceutics. 2023. Paquet F, Bailey MR, Leggett RW, et al. ICRP Publication 137. Ann ICRP. 2017. ICRP Publication 103. Ann ICRP. 2007 Bolch WE, Eckerman KF, Sgouros G, et al. MIRD Pamphlet 21. J Nucl Med. 2009. [203Pb]Pb-VMT-α-NET [212Pb]Pb-VMT-α-NET [68Ga]Ga- / [64Cu]Cu-DOTATATE PET/CT Imaging SPECT/CT Imaging (7-14 d) (<6 months) (8 wks) (8 wks) (8 wks) 203Pb SPECT images were acquired using a triple energy window centered on the 279 keV photopeak (20%) with upper and lower scatter windows (10%). SPECT reconstruction was performed using CT-based attenuation correction and a window-based scatter correction. SPECT sensitivity factors (cps/MBq) were derived from phantom calibration studies4. A 100 ml reference standard was included in each patient scan for quality assurance. Phantom-based SPECT Calibration 1 h 4 h 24 h 4 h [203Pb]Pb-VMT-α-NET Cylindrical solid water phantom (Graves Phantom) used for quantitative 203Pb SPECT calibration studies. The bottle ROI is morphologically expanded by 10 mm in all dimensions to capture activity spill out when determining a sensitivity factor. SPECT/CT imaging of [203Pb]Pb-VMT-α-NET in a representative patient (103-105). Maximum intensity projections (MIPs) of SPECT images at each timepoint (above) alongside transaxial and coronal slices of the liver at 4 h (below), showing tumor-specific uptake and retention. Regions of interest were delineated for the kidneys, liver, spleen, whole body, and tumors based on anatomic and functional imaging. Time-activity curves (TACs) were generated for [212Pb]Pb-VMT-α-NET, accounting for the differences in physical decay between 203Pb (t1/2 = 51.9 h) and 212Pb (t1/2 = 10.6 hours). TACs were fit to either mono-exponential or bi-exponential functions, depending on goodness of fit. Mono-exponential Bi-exponential Time activity curves in units of percent injected activity of [212Pb]Pb-VMT-α-NET for select normal tissues and lesions in a representative patient (103-101). Dosimetry analysis for [212Pb]Pb-VMT-α-NET was performed using the MIRD methodology with OLINDA v2.2.3 and is based on multi-timepoint SPECT imaging of [203Pb]Pb-VMT-α-NET. Results are reported both in terms of physical abs. dose and RBE-weighted dose (RBE = 5). Dose calculations for [212Pb]Pb-VMT-α-NET account for all daughters in the decay chain (weighted by branching ratio), partial volume effects, and redistribution of progeny 212Bi. Bone marrow dosimetry was estimated using decay corrected blood activity concentration measurements from [203Pb]Pb-VMT-α-NET. With a half-life of 61 minutes, the redistribution of progeny 212Bi following 212Pb beta decay can describe the fate of all 212Pb daughters. Redistribution of progeny 212Bi was modeled using a whole-body pharmacokinetic model based on transfer coefficients published in ICRP 137. For [212Pb]Pb-VMT-α-NET, utilizing PSC, the release fraction (RF) of 212Bi is taken to be 5%.5 Relative Biological Effectiveness RBE is defined as the ratio of a dose of low-LET reference radiation (e.g. x-rays) to a dose of high-LET radiation that produce the same biological effect.7 Experimentally reported RBE values range from 1 to 8 for cell killing, in vivo, and differ based on the biological endpoint, reference radiation, and alpha particle energy.8 In the absence of any clinical data to support a specific RBE value for [212Pb]Pb-VMT-α-NET, it is generally conventional to conservatively assume an RBE weighting factor of 5 for all alpha-particle dose contributions. Normal Tissues %/RF PSC (RF = 5%) DOTA (RF = 36%) Kidneys +0.79% +3.94% +28.35% Spleen -0.18% -0.91% -6.55% Liver +0.46% +2.28% +16.44% Red Marrow -0.50% -2.48% -17.82% Summary of the impact of 212Bi redistribution on the TIAC for select organs. The limited spatial resolution of SPECT causes partial volume effects that result in underestimation of activity in ROIs, particularly for smaller ROIs. A dual contouring approach was used to correct for partial volume effects in the kidney and lesion ROI analysis. Contours were first delineated on the CT images to determine the volume of the ROI. A second set of ROIs was then generated to capture the apparent activity of the ROI in the 4 h SPECT image, accounting for spill out activity and organ displacement between SPECT and CT acquisitions. For the kidney, functional ROIs were generated by morphologically expanding the anatomic volume by 5 mm in all dimensions. The average recovery coefficients were 0.72 ± 0.05 for the kidneys and 0.44 ± 0.12 for the lesions. The study team deeply thanks patients and their families for participating in this clinical trial. This study is a multicenter clinical trial with enrollment from 11 clinical trial sites, and many thanks are extended to investigators and site personnel for their incredible partnership in this work. Acknowledgements ROI Dose Coeff. (Gy/mCi) mean SD min max Kidneys 0.40 0.10 0.23 0.48 Spleen 0.22 0.12 0.11 0.43 Liver 0.11 0.04 0.07 0.16 Red Marrow 0.019 0.006 0.012 0.026 Lesions 1.08 0.61 0.36 2.83 Dose coefficient for [212Pb]Pb-VMT-α-NET without RBE-weighting and no redistribution of progeny 212Bi. ROI Dose Coeff., (Gy/mCi, RBE = 5) mean SD min max Kidneys 1.88 0.46 1.07 2.29 Spleen 0.99 0.56 0.50 1.92 Liver 0.52 0.18 0.32 0.73 Red Marrow 0.084 0.047 0.040 0.170 Lesions 5.00 2.82 1.68 13.07 RBE-weighted dose coefficient for [212Pb]Pb-VMT-α-NET assuming RBE=5 and accounting for 212Bi redistribution. ROI Cumulative Abs. Dose (Gy) 4 x 2.5 mCi 4 x 5.0 mCi mean SD mean SD Kidneys 4.0 1.0 8.0 1.9 Spleen 2.2 1.2 4.4 2.5 Liver 1.1 0.4 2.3 0.8 Red Marrow 0.19 0.06 0.38 0.1 Tumors 10.8 6.1 21.6 12.2 Cumulative absorbed dose for a full course of treatment with [212Pb]Pb-VMT-α-NET, without RBE weighting and no redistribution of 212Bi. Cumulative RBE-weighted dose for a full course of treatment with [212Pb]Pb-VMT-α-NET, assuming RBE=5 and accounting for redistribution of 212Bi. ROI Cumulative Abs. Dose (Gy, RBE=5) 4 x 2.5 mCi 4 x 5.0 mCi mean SD mean SD Kidneys 18.8 4.6 37.7 9.2 Spleen 9.9 5.6 19.7 11.2 Liver 5.2 1.8 10.5 3.5 Red Marrow 0.8 0.47 1.7 0.9 Tumors 50.0 28.2 100.0 56.5 PSC Patent Published, US12128115B2 Vegt E, de Jong M, Wetzels JF, et al. J Nucl Med. 2010. Mirzadeh S, Kumar K, Gansow OA. Radiochimica Acta. 1993. Graves S, Merrick M, Tiwari A, Sunderland J. J Nucl Med. 2021. Spider plot of tumor change over time by patient (all patients enrolled for DLT-observation, n = 9). Data cutoff 2025-04-30. Cohort 1 [212Pb]VMT--NET 2.5mCi Cohort 2 [212Pb]VMT--NET 5.0mCi Percent change in sum of diameters from baseline Preferred Term Grade 1-2 Grade 3 Total Cohort 1, 2.5 mCi n = 2, (n) Cohort 2, 5 mCi n = 40, (n) Cohort 1, 2.5 mCi n = 2, (n) Cohort 2, 5 mCi n = 40, (n) n = 42 [n (%)] Summary of TEAEs per patient 2 37 – 10 39 (93) Fatigue 2 23 – – 25 (60) Alopecia 2 20 – – 22 (52) Nausea 1 17 – – 18 (43) Diarrhea 2 9 – 3 14 (33) Anemia 2 9 – – 11 (26) Lymphocyte count decreased 1 7 – 3 11 (26) Abdominal pain 1 9 – – 10 (24) AST Increased 1 6 – – 7 (17) Blood creatinine increased – 7 – – 7 (17) ALT increased – 6 – – 6 (14) Hyperglycemia – 6 – – 6 (14) Hypocalcemia – 6 – – 6 (14) Back pain – 5 – – 5 (12) Headache 1 4 – – 5 (12) Hypertension – 3 – 2 5 (12) Vomiting – 4 – 1 5 (12) Hypokalemia – 1 – 1 2 (5) Presyncope – 1 – 1 2 (5) Cardiac failure – – – 1 1 (2) Syncope – – – 1 1 (2) The longer half-life of Pb-203 (51.9 h) allows for improved characterization of the biodistribution of 212Pb (10.6 h) at late timepoints. Representative example of the dual contouring approach for the kidney with anatomic (blue) and functional (green) ROIs.