
Investor & Analyst Day June 2, 2025

Disclaimer This presentation contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, express or implied statements regarding: the clinical development of RAP-219 for the treatment of refractory focal epilepsy, bipolar mania and diabetic peripheral neuropathic pain, including the initiation, timing, progress and results of our ongoing and planned clinical trials; expectations for the activity, tolerability, and commercial potential of RAP-219; the potential of Rapport's RAP technology platform; and expectations for Rapport’s uses of capital, expenses and financial results, including its cash runway through the end of 2026. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect Rapport’s business, operating results, financial condition and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to the company’s research and development activities; Rapport’s ability to execute on its strategy including obtaining the requisite regulatory approvals on the expected timeline, if at all; uncertainties relating to preclinical and clinical development activities; the company’s dependence on third parties to conduct clinical trials, manufacture its product candidates and develop and commercialize its product candidates, if approved; Rapport’s ability to attract, integrate and retain key personnel; risks related to the company’s financial condition and need for substantial additional funds in order to complete development activities and commercialize a product candidate, if approved; risks related to regulatory developments and approval processes of the U.S. Food and Drug Administration and comparable foreign regulatory authorities; risks related to establishing and maintaining Rapport’s intellectual property protections; and risks related to the competitive landscape for Rapport’s product candidates; as well as other risks described in “Risk Factors,” in the company’s Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q, as well as discussions of potential risks, uncertainties, and other important factors in Rapport’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Rapport’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Rapport expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations or any changes in events, conditions or circumstances on which any such statement is based, except as required by law, and claims the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.

Agenda 01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda

Leadership with track record of innovation and expertise Reid Huber, Ph.D. Director Partner, Third Rock Ventures; CEO, Merida Biosciences James Healy, M.D., Ph.D. Director Managing Partner, Sofinnova Investments Wendy Young, Ph.D. Director Former Head of Small Molecule Drug Discovery, Genentech Steve Paul, M.D. Founder and Board ChairPartner, Third Rock Ventures John Maraganore, Ph.D. Director Former Founding CEO, Alnylam 1 Employee director. Robert Perez Director Operating Partner, General Atlantic, Former CEO, Cubist Pharmaceuticals Founder and Chairman, Life Science Cares Raymond Sanchez, M.D. Director Senior Advisor, Bain Life Sciences; Former CMO, Cerevel Therapeutics Paul Silva Director Former Chief Accounting Officer, Vertex Pharmaceuticals Management Team Board of Directors

Company builders with industry-proven leadership Differentiated pharmacology we believe promotes high selectivity and specificity Potential to transform the treatment of neurological and psychiatric disorders with differentiated profile Strong Foundation & Differentiated Precision Approach Small molecule precision medicines for patients with neurological and psychiatric disorders TARP8: transmembrane AMPA regulatory protein gamma-8; AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. 1 Includes cash, cash equivalents, and short-term investments, excluding restricted cash. Robust Clinical & Discovery Pipeline Potential first-in-class programs leveraging receptor associated protein (RAP) science RAP-219 forebrain restricted TARP8 AMPAR modulator–significant opportunity in initial indication in focal epilepsy Medicinal chemistry-enabled discovery portfolio with potential in additional indications Topline Phase 2a Results in refractory focal epilepsy expected in Q3 25 Initiate Phase 2a in bipolar mania in Q3 25 with topline results in 1H 27 Finalizing plans for Phase 2a in diabetic peripheral neuropathic pain RAP-219: Pipeline-in-a-Product with Multiple Catalysts Well Financed $285.4 million as ofMarch 31, 20251 Cash runway expected to fund operations through end of 2026

Advancing precision therapeutics aimed at solving long-standing challenges in neuromedicine Drugs interact with receptors that are ubiquitous in the brain and body Drugs not designed with precision for disease-specific neuroanatomic sites / receptors Drug interactions and adverse events lead to noncompliance and discontinuation Drug discovery with conventional approaches (lacking RAPs) can miss high potential, previously unexplored targets RAPs are components of the broader neuronal receptor complexes and play critical roles in regulating receptor assembly and function RAPs serve as unique binding sites targetable by novel pharmacophores designed for increased selectivity, providing neuroanatomical specificity RAPs can enable differentiated pharmacology and potentially provide favorable efficacy, safety, and administration profiles RAPs can “unlock” drug targets previously inaccessible to study in vitro, allowing for potentially first-in-class drug discovery programs Conventional CNS drug discovery The potential of RAPs

Precision neuroscience pipeline with opportunity to address large market opportunities 1 IND is currently on clinical hold with the FDA; 2 nicotinic acetylcholine receptor. Note: We have conducted four Phase 1 trials supportive of multiple RAP-219 indications. Category Program Discovery Preclinical Phase 1 Phase 2 Phase 3 Next Expected Milestone RAP-219 TARPγ8 AMPAR Programs Refractory Focal Epilepsy Topline Results September 2025 AMPA modulator Bipolar Mania Trial Initiation Q3 2025 Topline Results 1H 2027 Diabetic Peripheral Neuropathic Pain Trial Initiation1 nAChR2 Discovery Programs α6 Chronic Pain Development Candidate α9α10 Hearing Disorders Development Candidate Strong intellectual property with worldwide rights to all programs Trial Underway; Enrollment Complete

RAP-219 enables “pipeline-in-a-product” opportunity Internal Market Research, 2023 1 Diagnosed prevalence; 2 True prevalence (diagnosed prevalence divided by the diagnosis rate); 3 Diagnosed prevalence across diabetic peripheral neuropathy (~2.8 million), post-herpetic neuralgia (~1.8 million); trigeminal neuralgia (~1.0 million). Potency data and metabolic profile support RAP-219’s development as a long acting injectable, which could improve compliance and outcomes Focal epilepsy in U.S. patients1 Peripheral neuropathic pain in U.S. patients3 Bipolar disorder in U.S. patients2 ~1.8M ~7.2M ~5.6M TARP8 is a preclinically and clinically validated target for epilepsy Once daily (QD) dosing Differentiated tolerability profile No observed drug-drug interactions (DDI) Compelling data supporting potential in bipolar disorder and peripheral neuropathic pain

Unmet need in epilepsy highlights limitations of current antiseizure medications 1 Refractory patients are those who continue to experience recurring seizures despite taking two or more ASMs; 2 Source: Epilepsy Foundation. Limitations of antiseizure medications (ASMs) 3.0M U.S. Epilepsy Patients (ages 18+) 60% Focal Epilepsy 1.8M Focal Epilepsy Patients 30-40% % Drug-resistant1 Limited Efficacy: Despite over 20 FDA approved ASMs, 30-40% of patients are still drug-resistant1 Tolerability Issues: Especially CNS side-effects, such as sedation, ataxia, and cognitive problems Potential for Serious Adverse Events: Such as severe cutaneous reactions, serious hematological disorders, and hepatic failure Risk of Breakthrough Seizures: Missing doses of seizure medicine is the most common cause of breakthrough seizures due to the short half-life of many antiseizure medications2 Complicated Administration: Long titration, drug-drug interactions, and lab monitoring

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda
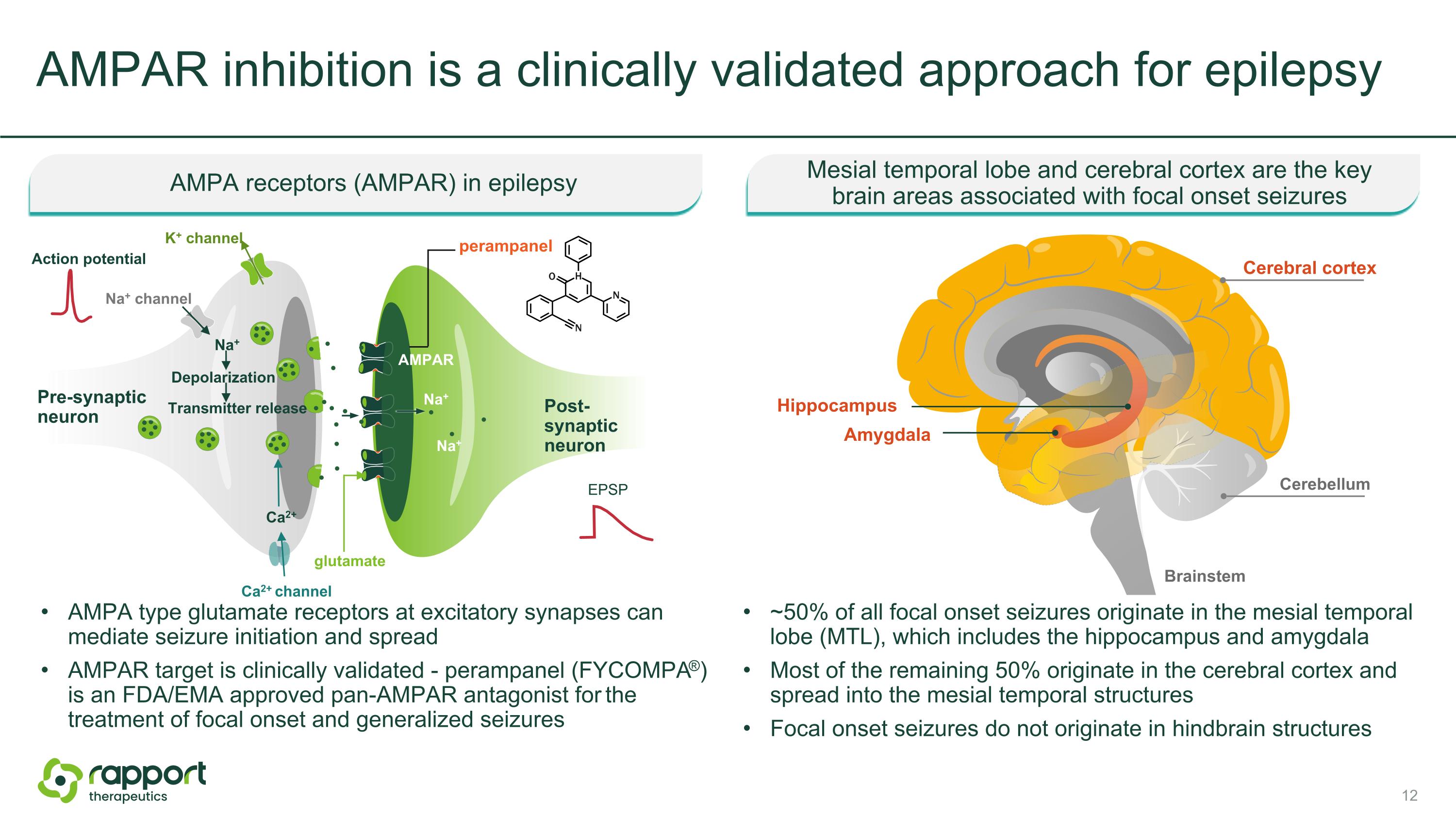
AMPAR inhibition is a clinically validated approach for epilepsy ~50% of all focal onset seizures originate in the mesial temporal lobe (MTL), which includes the hippocampus and amygdala Most of the remaining 50% originate in the cerebral cortex and spread into the mesial temporal structures Focal onset seizures do not originate in hindbrain structures AMPA type glutamate receptors at excitatory synapses can mediate seizure initiation and spread AMPAR target is clinically validated - perampanel (FYCOMPA®) is an FDA/EMA approved pan-AMPAR antagonist for the treatment of focal onset and generalized seizures AMPA receptors (AMPAR) in epilepsy Mesial temporal lobe and cerebral cortex are the keybrain areas associated with focal onset seizures Pre-synaptic neuron Post-synaptic neuron Na+ channel Action potential glutamate Ca2+ channel AMPAR Na+ Transmitter release Ca2+ Na+ perampanel EPSP K+ channel Depolarization Na+ Amygdala Cerebral cortex Brainstem Hippocampus Cerebellum

NatComm 2022 13:734 GluA1 GluA2 TARP8 Western blot JCB 2003 161:805 STG γ-3 γ-4 γ-8 tubulin TARPγ8 clinical PET RAP-219 target/TARPγ8 is selectively expressed in brain regions associated with focal onset seizures TARPs regulate the trafficking, subcellular localization and gating of AMPA receptors High expression in the neocortex and mesial temporal lobe, where nearly all seizures originate Relatively low expression in cerebellum, thalamus and brainstem Cryogenic electron microscopy of GluA1/2 + TARPγ8 complex

RAP-219 possesses potentially optimal drug properties for an ASM Potency at TARPγ8 IC50 ~ 100 pM Selectivity vs. other TARPs, NMDAR >4,000X target affinity Selectivity vs. CEREP and kinase panels >10,000X target affinity PK Orally bioavailable | %F = 80-100 Brain penetration Brain/plasma ~70% DDI/CYP inhibition/CYP induction >10,000 X target affinity. Not CYP substrate Solubility/permeability BCS Class I Target receptor occupancy 50% RO at <50 μg/kg oral dose in rodent Preclinical safety NOAEL in top dose of IND enabling studies Non-sedating No effects at exposures 1000X EC50 NMDAR: N-methyl-D-aspartate receptor; DDI: drug-drug interactions; BCS: Biopharmaceutics Classification System; NOAEL: No Observed Adverse Effect Level.

Robust dose-dependent seizure protection observed in the gold standard pre-clinical model for refractory focal epilepsy Corneal Kindling Responders in Mice Robust, dose-dependent seizure protection observed at 50-70% receptor occupancy RAP-219 Plasma Concentration (ng/mL) Corneal Kindling Responders (%) % Corneal Kindling Responders

% Corneal Kindling Responders RAP-219 Plasma Concentration (ng/mL) Corneal Kindling and Rotarod Failures (%) % Rotarod Failures Highly potent with no rotarod failures observed at highest exposure Corneal Kindling Responders and Rotarod Failures in Mice The Rotarod Performance Test is considered a gold standard assay for assessing motor coordination and detecting common ASM side effects like sedation, somnolence, and ataxia

RAP-219 precision has the potential to significantly improve the therapeutic index Therapeutic Index = TD50 (toxic dose) on Rotarod/ED50 (effective dose) for efficacy. 1 Data on file, Rapport Therapeutics; https://panache.ninds.nih.gov/;2 Data based on published reports from different preclinical studies at different points in time, with differences in preclinical study design and subject population. As a result, cross-study comparisons cannot be made. No head-to-head studies have been conducted. RAP-2191 Levetiracetam Tiagabine Ezogabine Carbamazepine Valproate Lacosamide Clobazam Gabapentin Lamotrigine Topiramate Phenytoin Corneal kindling mouse model therapeutic index (TD50 rotarod/ED50 efficacy) for RAP-2191 and other ASMs2

TARPγ8 AMPAR NAMs active across a range of preclinical epilepsy models Preclinical epilepsy models are highly translatable, with probabilities of clinical success up to 70%, according to epileptologist Jacqueline French, MD Robust efficacy across a broad array of preclinical focal and generalized seizure models Activity not seen in maximal electroshock (MES) model, consistent with performance of levetiracetam and some other effective ASMs Potent activity in kindling model has been observed to predict efficacy in focal epilepsy * Used RAP-219; where not noted, used other TARPg8 NAM CNS & Neurological Disorders-Drug Targets (2017) 16:1099; J Pharmacol Exp Ther (2016) 357:394; J Amer Soc for Exper NeuroTherapeutics (2007) 4:12; Jackie French AES Presentation, Professor, Neurology, NYU Grossman School of Medicine; Director, The Epilepsy Study Consortium (TESC); Barker-Haliski, M. (2019) Expert Opinion on Drug Discovery, 14(10), 947–951. Chronic seizure models [like corneal kindling] offer the most etiologically relevant platform on which to accurately replicate clinical epilepsy” Barker-Haliski, Expert Opinion on Drug Discovery Model Corneal kindling – mouse* PTZ - mouse* Rotarod* Amygdala kindling – mouse Hippocampal kindling – mouse 6Hz stimulation – mouse Frings audiogenic seizure – mouse GAERS absence epilepsy – rat

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda

Phase 1 development: 100 healthy volunteers exposed to RAP-219 Single dose up to 3 mg and multiple doses up to 1.75 mg SAD-101 8/cohort (6 active: 2 placebo) 01 MAD-102 8/cohort (6 active: 2 placebo) 02 PET-103 03 MAD-104 8/cohort (6 active: 2 placebo) 04 N=36* 3.0 mg 2.0 mg 1.0 mg 0.5 mg 0.25 mg Food Effect N=16* 0.25 mg x 7d 0.5 mg x 7d 0.25 mg x 14d 0.75 mg x 5d 1.25 mg x 9d N=18* 0.5 mg x 2d 1.0 mg x 2d 1.75 mg x 24d 0.75 mg x 2d 1.25 mg x 2d 1.75 mg x 4d 0.75 mg x 3d 1.25 mg x 3d 1.75 mg x 2d N=30* 0.75 mg x 5d 1.25 mg x 23d 0.75 mg x 28d 0.5 mg x 28d 0.25 mg x 7d 0.5 mg x 7d 0.25 mg x 14d * Number of subjects receiving RAP-219. SAD: Single ascending dose; MAD: Multiple ascending dose; PET: Positron emission tomography.

TEAE SUMMARY All Grade 1 or 2 Tended to occur early in treatment and resolve without further action No SAEs or clinically significant laboratory, vital signs, or ECG abnormalities 3 discontinuations Safety and tolerability of RAP-219 in Phase 1 multiple dose studies Favorable profile in healthy volunteers Note: Includes studies 102, 103, 104; AE start date is after first dose of RAP-219. TEAE: Treatment emergent adverse event; SAE: Serious adverse event; ECG: Electrocardiogram. TEAEs (≥ 5% participants) Total(N=64) n (%) n grade 1/2 Placebo(N=16)n (%) Headache 12 (18.75%) 9/3 0 Dry mouth 5 (7.81%) 4/1 0 Brain fog 5 (7.81%) 5/0 1 (6.25%) Fatigue 5 (7.81%) 5/0 0 Sinus tachycardia 4 (6.25%) 4/0 0 Insomnia 4 (6.25%) 4/0 0 Decreased appetite 4 (6.25%) 4/0 1 (6.25%) Diarrhea 4 (6.25%) 4/0 0 Vision blurred 4 (6.25%) 3/1 0 Medical device site reaction 3 (4.69%) 0/3 1 (6.25%) Constipation 3 (4.69%) 3/0 1 (6.25%) Disturbance in attention 3 (4.69%) 3/0 0 Catheter site hematoma 3 (4.69%) 2/1 0 Back pain 3 (4.69%) 3/0 0 Abnormal dreams 3 (4.69%) 3/0 0 Bowel movement irregularity 3 (4.69%) 2/1 0

RAP-219 human PET study TARP8 highly expressed in neocortex and mesial temporal lobe High expression of TARP8 in the neocortex and mesial temporal lobe (including hippocampus and amygdala), where nearly all focal onset seizures originate and relatively low expression in cerebellum, thalamus, and brainstem supports a potentially favorable and differentiated benefit/risk profile Group-averaged parametric PET images (co-registered to MRI) following tracer administration in 16 subjects. Low expression

RAP-219 dose-exposure-RO relationship Human PET RO data support lower dose levels, potentially increasing dose flexibility Preclinical models demonstrated maximal seizure protection at 50-70% RO (green band) Human target RO (50-70%) achieved at doses/exposures lower than predicted from animal models (as low as 0.25 mg), and lower than the dose being used in Phase 2a FOS trial, potentially affording greater flexibility in dose selection Phase 2a dose (green line) achieves target RO within 5 days and > 80% RO by day 28 Simulated RO based on Phase 1 PK and PET RO data RO: Receptor occupancy. *Trough values calculated based on non-parametric superposition using data from the 101 and 102 studies 85% RO 80% RO 70% RO 60% RO 50% RO RAP-219 Trough* Concentration (ng/mL) 0.75 mg x 5 days, 1.25 mg/day x 23 days 0.75 mg x 28 days 0.25 mg x 28 days

RAP-219 summary Phase 1 results provide strong support for further development of RAP-219 Generally well tolerated No SAEs; relatively low incidence of TEAEs; none greater than Grade 2 Consistent with target biology, no sedation observed Precision targeting of areas where focal onset seizures originate PET results confirm TARP8 expressed in neocortex and mesial temporal lobe with relatively low expression in cerebellum, thalamus, and brainstem Doses as low as 0.25 mg predicted to achieve clinically efficacious exposures, based on human dose-exposure-RO PET results May afford flexibility in dosing to optimize tolerability and potential benefit Excellent pharmaceutical properties supporting development Once-a-day dosing; minimal or no DDIs; no CYP interactions observed CYP: Cytochrome P450.

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda

Phase 2a proof-of-concept trial in FOS patients with implanted RNS system Two probes implanted into regions of the brain known to have epileptiform activity Probes continuously detect and record epileptiform activity, including long episodes (LEs) & stimulate the region to attenuate seizure activity FDA-approved device for patients with refractory FOS Approx. 6,500 RNS-treated patients in the U.S.1 1 Source: NeuroPace, January 2025 Neuropace RNS System®

RNS measures and stores episode starts and long episodes in real time, all the time EEG RNS Progression of epileptiform activity to clinical seizures Seizure Diary Clinical Seizures Electrographic Seizures Increased amplitude, spread, and persist Epileptiformdischarges Clinical Seizures Long Episodes / Electrographic Seizures Episode Starts

RNS system provides objective measure of epileptiform activity and early signal of clinical response to ASMs Epilepsy & Behavior. 2018; 83: 192-200; Epilepsia. 2020; 61:138-148. “It could be argued that long episodes are an even better therapeutic target than reported clinical seizures.” “Changes in long episode rates had the strongest correlation with changes in clinical seizure rates. These data suggest that these measures may provide an objective assessment of cortical excitability and response to AEDs.”

Rationale for selecting LEs as primary endpoint Well-established relationship between long episode and clinical seizure responder rates 1 Skarpaas et al., Epilepsy & Behavior 83 (2018); 2 Quraishi et al., Epilepsia 61 (2020); 3 Gammaitoni et al, American Epilepsy Society (AES) 2024 Annual Meeting, Poster #1.494. Skarpaas (2018)1: significant correlation exists between reduction in long episodes and clinical seizure frequency Quraishi (2020)2: ASMs resulting in a ≥ 20% decrease in long episodes were clinical efficacious (≥ 50% reduction in seizures) Gammaitoni (2024)3: ASMs resulting in a ≥ 30% reduction in LEs were associated with a ≥ 50% reduction in clinical seizures N=45 from three highly effective ASMs ASMs initiated: CLB (n=15), LEV (n=4), LCM (n=26)

Phase 2a proof-of-concept trial in focal epilepsy patients KOL-guided, innovative study using validated biomarker of clinical seizures Medically refractory focal epilepsy RNS probe implanted in seizure onset zone within MTL ≥ 15 months before screening Stable RNS System settings and therapies ≥ 16 LEs during 8-week retrospective review period ≥ 1 clinical seizure reported during 8-week retrospective review period > 50% concordance between long episodes and electrographic seizures RAP-219 washout RNS data collection, ongoing clinical seizure diary collection 0.75 mg x 5 days then 1.25 mg Baseline (no treatment) 4-week pre-treatment period 8-week open-label treatment period 8-week follow-up period End of Treatment End of Trial Start of Treatment Start of Enrollment Key Endpoints Long episode reduction proportion of patients with ≥30% reduction compared with baseline* median percent change from baseline Clinical seizure reduction proportion of patients with ≥50% reduction compared with pre-treatment baseline median percent change from baseline Percent change in estimated electrographic seizure frequency and additional iEEG biomarkers Key Entry Criteria * Baseline defined as 4-week average number of LEs over 4-week pretreatment, the 4-week screening, and the 4–week retrospective review periods.

Preliminary baseline characteristics of Phase 2a trial patients Seizure frequency consistent with patients enrolled in registrational FOS trials Note: Preliminary baseline characteristics reflect the first 14 patients enrolled (those with final baseline data available as of May 20, 2025) from an unlocked and ongoing study database. Median (1st to 4th quartile range) CS frequency per 28 days in 4-week prospective baseline 10 (4.25-18.25) LE frequency per 28 days in 12-week baseline 51 (21-194) Concordance between LEs and electrographic seizures (rated by an independent reviewer) 92% (71-96) Concomitant antiseizure medications 3 Baseline Characteristics from First 14 Patients Enrolled Median (range) Age 37 (20-61) Sex (n) 7F/7M Age at first seizure 19 (0.5-31) Years since RNS implantation 4.4 (1.4-10.2)

Agenda 01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay

Fireside Chat: KOL Perspective Jacqueline French, M.D. Professor of Neurology, NYU Langone Jeff Sevigny, M.D. Chief Medical Officer

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda

RAP-219 Phase 2a trial in patients with focal epilepsy Goal: Generate data from ≥20 participants showing convincing reductions in long episodes and clinical seizures to support advancement to registrational studies

RNS electrographic biomarker data is a highly objective complement to clinical seizure diary data Each patient serves as case study with 12 weeks of baseline data, detailed pharmacodynamic measures of onset of effect, and depth of response Depth of response and responder proportion can offer strong evidence of activity to support advancement to registrational trials As noted earlier, responder is defined by a ≥30% reduction in LE seizure frequency, consistent with a clinically meaningful effect Example highly effective medication starts of a marketed ASM* Episode Starts Long Episodes Before medication start Titration Maintenance *RNS data with introduction of currently marketed ASM; not RAP-219 data.

Responder rates in registrational trials of approved and pipeline ASMs ~25-55% of patients achieve a clinically meaningful ≥50% clinical seizure reduction in registrational trials for approved therapies Responder rates in this range result in statistically significant placebo-adjusted median percent changes Target responder rate set at ≥40% to align with clinical relevance and support advancement to pivotal trials Sources: Fig 1: (1) Chung et.al. Neurology 2020;94:e2311-e2322 (2) Krauss et.al. Lancet Neurol 2020;19:38-48, (3) French, et.al. XTOLE AES poster t we 1.419 December 2021, (4) (5) (6) Product package inserts. Non-Placebo Adjusted Proportion of 50% CS Responders in Registrational Trials of Approved and Pipeline Programs

Key endpoints and anticipated topline data Outcome Topline Data Long Episode Primary Endpoint Analysis LE frequency reduction responder analysis (% of patients that demonstrate ≥30% reduction in LEs compared with baseline)* Median percent change in LE frequency on treatment compared with baseline Clinical Seizure Key Secondary Endpoint Analysis Proportion of patients achieving ≥50% reduction in clinical seizures compared with baseline Median percent change in clinical seizure frequency compared with baseline TEAE Incidence and Grade *Power determinations were based on this outcome measure.

Targets for anticipated topline data Outcome RAP-219 Target Topline Data Long Episode Primary Endpoint Analysis LE frequency reduction responder analysis (% of patients that demonstrate ≥30% reduction in LEs compared with baseline)* ≥40% Median percent change in LE frequency on treatment compared with baseline ~30% Clinical Seizure Key Secondary Endpoint Analysis Proportion of patients achieving ≥50% reduction in clinical seizures compared with baseline ≥40% Median percent change in clinical seizure frequency compared with baseline ~50% TEAE Incidence and Grade *Power determinations were based on this outcome measure.

01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi, Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay Agenda

Pipeline targets large market opportunities True Prevalence Bipolar disorder in U.S. patients2 ~7.2M Peripheral neuropathic pain in U.S. patients3 ~5.6M 1 Diagnosed prevalence; 2 True adult prevalence (diagnosed prevalence divided by the diagnosis rate); 3 Diagnosed prevalence across diabetic peripheral neuropathy (~2.8 million), post-herpetic neuralgia (~1.8 million), and trigeminal neuralgia (~1.0 million). Focal epilepsy in U.S. patients1 ~1.8M Market Size 4%

Cash runway and anticipated catalysts Cash balance of $285.4mm1 (as of 3/31/25) supports Rapport through end of 2026 1Cash, cash equivalents and short-term investments, excluding restricted cash. Sept 2025 RAP-219 Phase 2a trial in focal epilepsy topline results expected Q3 2025 RAP-219 Phase 2a trial in bipolar mania expected to begin 2025 Plan for POC trial in diabetic peripheral neuropathic pain finalized 1H 2027 RAP-219 Phase 2a trial in bipolar mania topline results expected

Questions & Answers

Agenda 01 Welcome and Introductions Abe Ceesay, Chief Executive Officer 02 RAP-219: Program Overview David Bredt, M.D., Ph.D., Founder, Chief Scientific Officer 03 RAP-219: Phase 1 Data | RAP-219: Phase 2a Focal Epilepsy Trial Overview Jeff Sevigny, M.D., Chief Medical Officer 04 Fireside Chat: KOL Perspective Jacqueline French, M.D., Professor of Neurology, NYU Langone | Jeff Sevigny, M.D. 05 RAP-219: Phase 2a FOS Trial: Framing Future Data William Motley, M.D., RAP-219 Program Leader 06 Financial Update Troy Ignelzi– Chief Financial Officer 07 Questions and Answers 08 Closing Remarks Abe Ceesay

Rapport Therapeutics: investment summary RAP-219 precision designed to maximize efficacy while avoiding typical ASM adverse events Highly expressed in forebrain regions associated with seizures and minimal expression in hindbrain areas potentially associated with AEs Phase 2a FOS trial design improves probability of success for future pivotal trials Objective and translatable biomarker used as more reliable measure than patient-reported diaries Trial patients are representative of registration and commercial target patient population Majority of epilepsy opinion leaders believe design is superior to other proof-of-concept designs1 Validated across preclinical and Phase 1 studies Efficacy in highly predictive seizure models Potential to significantly improve therapeutic index Generally well tolerated across four Phase 1 trials with 100 subjects dosed (no TEAEs above Grade 2) Differentiated profile promotes broad potential use QD dosing and rapid achievement of target RO associated with maximal seizure protection Minimal drug-drug interactions and not observed to interact with CYP enzymes Long half life minimizes daily drug fluctuations and may reduce risk of breakthrough seizures Potency and physicochemical properties enable potential as first long-acting injectable ASM 1Third-party market research, March 2025

Thank you














































