
Investor Presentation May 2025 ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Exhibit 99.2

Legal Disclaimers ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Various statements made in this presentation are forward-looking, within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, and are inherently subject to risks, uncertainties and potentially inaccurate assumptions. All statements that address activities, events or developments that we intend, expect, plan or believe may occur in the future, are forward-looking statements, including but not limited to statements regarding: our expectations regarding our clinical development and regulatory plans; our expected timing for the completion of clinical trial enrollment and the timing of the availability and release of clinical data; our expected cash runway; our optimism that DURAVYU has the potential to change the current treatment paradigm and revolutionize real-world outcomes for patients suffering from serious retinal diseases; our belief that DURAVYU has the potential to maintain a majority of patients with active disease with no supplemental anti-VEGF therapy for six months or longer; our expectations regarding our manufacturing capabilities; and our expectations regarding the timing and clinical development of our other product candidates, including EYP-2301. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, these risks and uncertainties include the timing, progress and results of the company’s clinical development activities, including DURAVYU; uncertainties and delays relating to communications with the U.S. Food and Drug Administration and the ability to obtain regulatory approval from FDA for the commercialization of DURAVYU; unanticipated costs and expenses; the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the risk that results of clinical trials may not be predictive of future results, and interim and preliminary data are subject to further analysis and may change as more data becomes available; unexpected safety or efficacy data observed during clinical trials; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for approval of the company’s product candidates; changes in the regulatory environment; disruptions at the FDA, including due to a reduction in the FDA’s workforce and/or inadequate funding for the FDA; changes in U.S. and international trade policies; changes in expected or existing competition; the success of current and future license agreements; our dependence on contract research organizations, and other outside vendors and service providers; product liability; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; delays, interruptions or failures in the manufacture and supply of our product candidates; the availability of and the need for additional financing; our ability to obtain additional funding to support our clinical development programs; uncertainties regarding the timing and results of the August 2022 subpoena from the U.S. Attorney’s Office for the District of Massachusetts; uncertainties regarding the FDA warning letter pertaining to our Watertown, MA manufacturing facility; and other factors described in our filings with the Securities and Exchange Commission (SEC). More detailed information on these and additional factors that could affect our actual results are described in our filings with the SEC, including our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, as revised or supplemented by its Quarterly Reports on Form 10-Q and other documents filed with the SEC. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
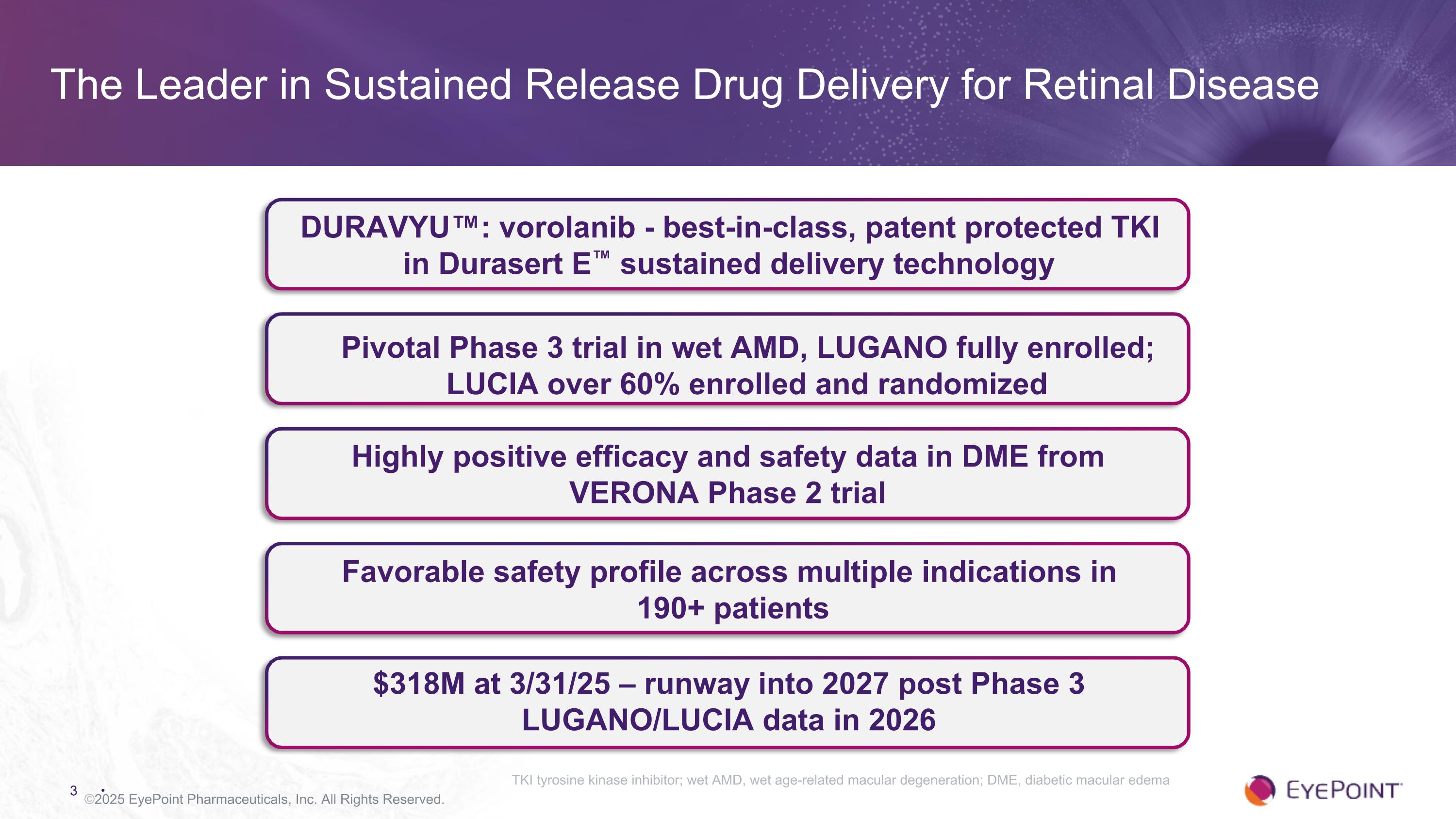
The Leader in Sustained Release Drug Delivery for Retinal Disease ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Highly positive efficacy and safety data in DME from VERONA Phase 2 trial $318M at 3/31/25 – runway into 2027 post Phase 3 LUGANO/LUCIA data in 2026 DURAVYU™: vorolanib - best-in-class, patent protected TKI in Durasert E™ sustained delivery technology Favorable safety profile across multiple indications in 190+ patients TKI tyrosine kinase inhibitor; wet AMD, wet age-related macular degeneration; DME, diabetic macular edema Pivotal Phase 3 trial in wet AMD, LUGANO fully enrolled; LUCIA over 60% enrolled and randomized
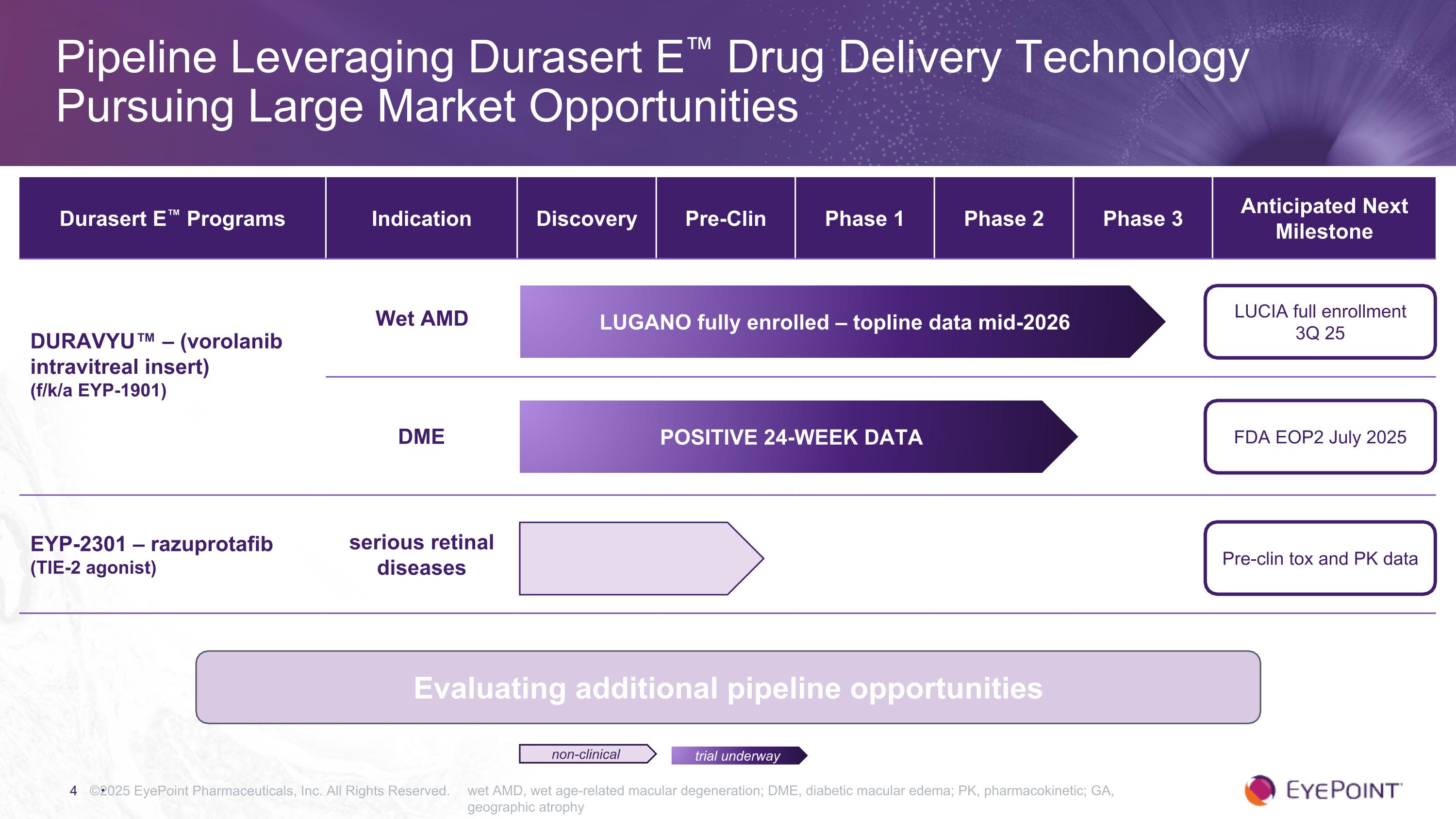
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. wet AMD, wet age-related macular degeneration; DME, diabetic macular edema; PK, pharmacokinetic; GA, geographic atrophy Pipeline Leveraging Durasert E™ Drug Delivery Technology Pursuing Large Market Opportunities trial underway non-clinical Durasert E™ Programs Indication Discovery Pre-Clin Phase 1 Phase 2 Phase 3 Anticipated Next Milestone DURAVYU™ – (vorolanib intravitreal insert) (f/k/a EYP-1901) Wet AMD DME EYP-2301 – razuprotafib (TIE-2 agonist) serious retinal diseases LUCIA full enrollment 3Q 25 FDA EOP2 July 2025 Pre-clin tox and PK data LUGANO fully enrolled – topline data mid-2026 Positive 24-Week Data Evaluating additional pipeline opportunities

Chronic disease treated with short acting anti-VEGFs places significant burden on physicians and patients 77% of Retina Specialists say improved durability is most important when choosing a treatment2. A delay in care/missed visit can result in vision loss A delay in treatment of only 5.34 weeks resulted in vision loss3 An increasing lifespan means significantly more injections in a patient’s lifetime Current anti-VEGF treatments are dosed on average every two months in the United States4 Many patients with wet AMD are chronically undertreated >80% of Retina Specialists say undertreatment is due to patient noncompliance, scheduling limitations or provider preference for less frequent dosing1 1. 2022 PAT Survey; 2. 2024 PAT Survey; 3. American Academy of Ophthalmology, The Effect of Delay in Care Among Patients Requiring Intravitreal Injections, Welin Song, BS et al; 4. NIH Current and Upcoming Anti-VEGF Therapies and Dosing Strategies for the treatment of wet AMD: a comparative review, Saira Khanna et al, Dec. 2019 There is a Significant Need for More Durable Therapies in Wet AMD 1 2 3 4
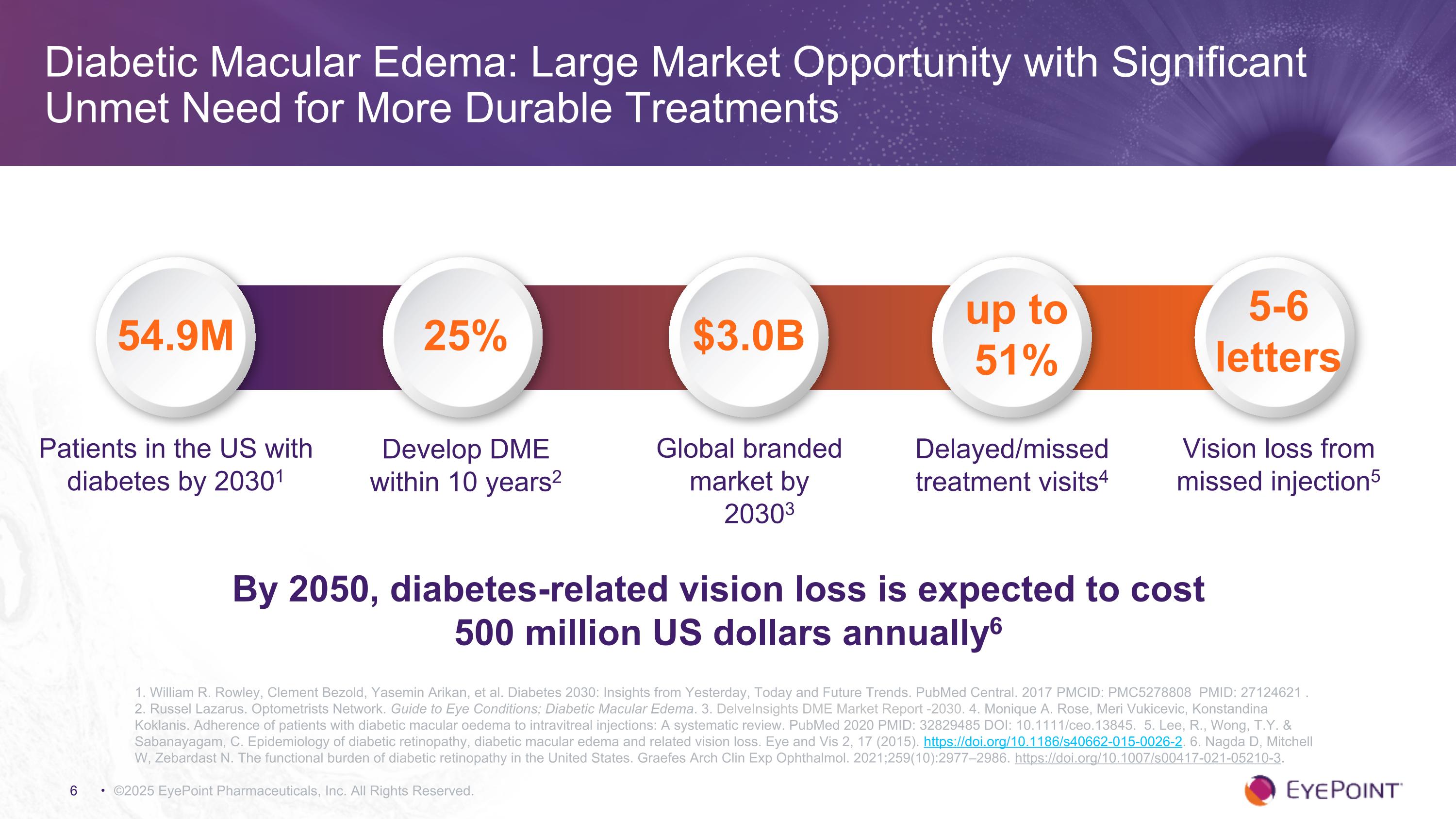
Diabetic Macular Edema: Large Market Opportunity with Significant Unmet Need for More Durable Treatments ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 1. William R. Rowley, Clement Bezold, Yasemin Arikan, et al. Diabetes 2030: Insights from Yesterday, Today and Future Trends. PubMed Central. 2017 PMCID: PMC5278808 PMID: 27124621 . 2. Russel Lazarus. Optometrists Network. Guide to Eye Conditions; Diabetic Macular Edema. 3. DelveInsights DME Market Report -2030. 4. Monique A. Rose, Meri Vukicevic, Konstandina Koklanis. Adherence of patients with diabetic macular oedema to intravitreal injections: A systematic review. PubMed 2020 PMID: 32829485 DOI: 10.1111/ceo.13845. 5. Lee, R., Wong, T.Y. & Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and Vis 2, 17 (2015). https://doi.org/10.1186/s40662-015-0026-2. 6. Nagda D, Mitchell W, Zebardast N. The functional burden of diabetic retinopathy in the United States. Graefes Arch Clin Exp Ophthalmol. 2021;259(10):2977–2986. https://doi.org/10.1007/s00417-021-05210-3. Patients in the US with diabetes by 20301 Global branded market by 20303 Delayed/missed treatment visits4 54.9M 25% up to 51% $3.0B Develop DME within 10 years2 Vision loss from missed injection5 5-6 letters By 2050, diabetes-related vision loss is expected to cost 500 million US dollars annually6

IVT, intravitreal DURASERT E™ TECHNOLOGY Sustained-Release Drug Delivery Standard in-office IVT injection Continuous dosing Zero-order kinetics drug release Solid injectable insert Drug formulated within a bioerodible matrix Designed to release drug load before matrix fully erodes Favorable safety profile across multiple indications No non-erodible polyamide coating from legacy Durasert technology ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Insert is 1/5000 of Vitreous Volume
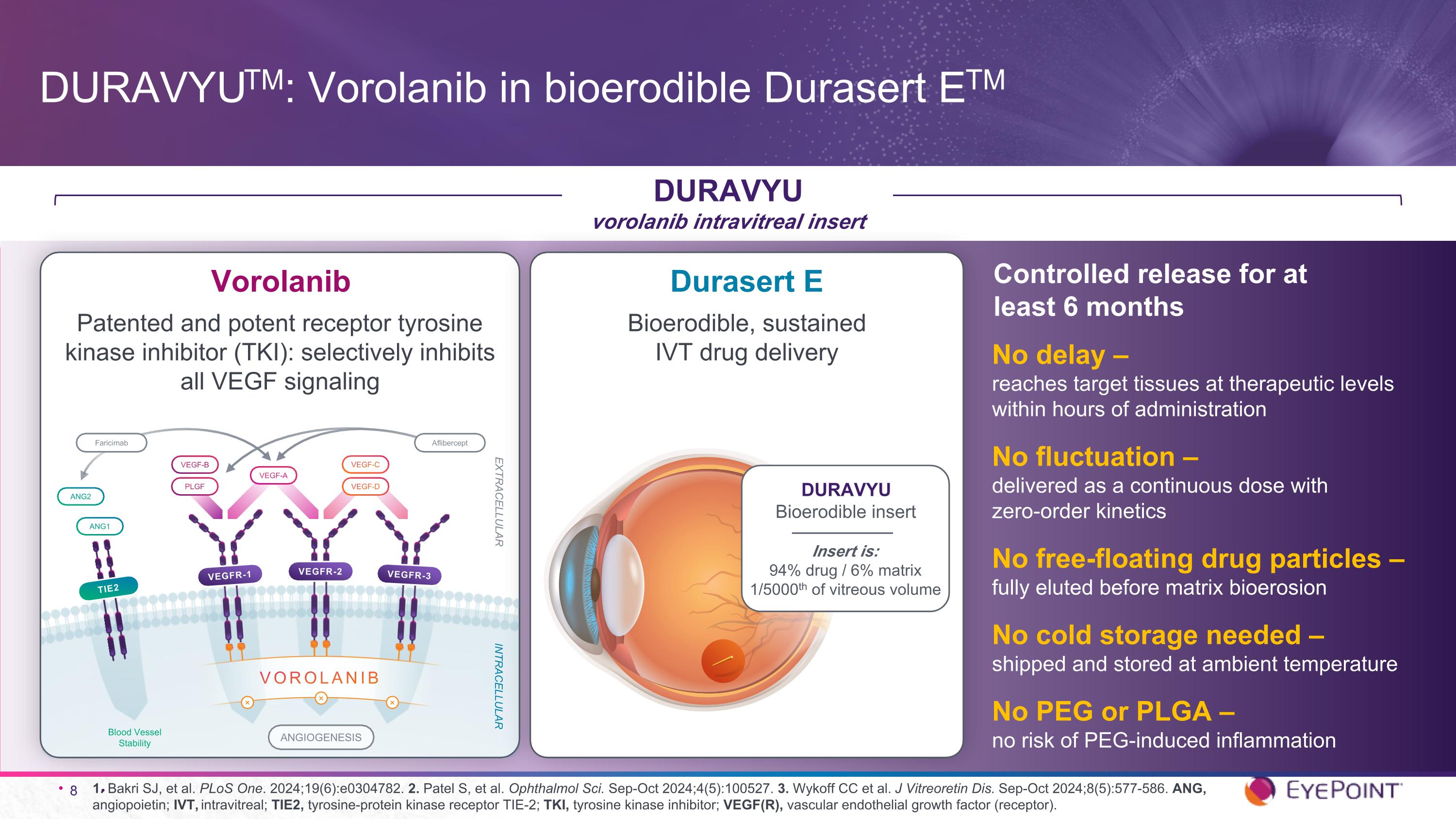
1. Bakri SJ, et al. PLoS One. 2024;19(6):e0304782. 2. Patel S, et al. Ophthalmol Sci. Sep-Oct 2024;4(5):100527. 3. Wykoff CC et al. J Vitreoretin Dis. Sep-Oct 2024;8(5):577-586. ANG, angiopoietin; IVT, intravitreal; TIE2, tyrosine-protein kinase receptor TIE-2; TKI, tyrosine kinase inhibitor; VEGF(R), vascular endothelial growth factor (receptor). DURAVYUTM: Vorolanib in bioerodible Durasert ETM DURAVYU vorolanib intravitreal insert No delay – reaches target tissues at therapeutic levels within hours of administration No fluctuation – delivered as a continuous dose with zero-order kinetics No free-floating drug particles – fully eluted before matrix bioerosion No cold storage needed – shipped and stored at ambient temperature No PEG or PLGA – no risk of PEG-induced inflammation Controlled release for at least 6 months ANGIOGENESIS TIE2 ANG2 ANG1 INTRACELLULAR EXTRACELLULAR Blood Vessel Stability VEGF-A VEGF-D VEGF-C PLGF VEGF-B VEGFR-1 VEGFR-2 VEGFR-3 ⨯ VOROLANIB ⨯ ⨯ Aflibercept Faricimab Vorolanib Patented and potent receptor tyrosine kinase inhibitor (TKI): selectively inhibits all VEGF signaling Durasert E Bioerodible, sustained IVT drug delivery DURAVYU Bioerodible insert Insert is: 94% drug / 6% matrix 1/5000th of vitreous volume
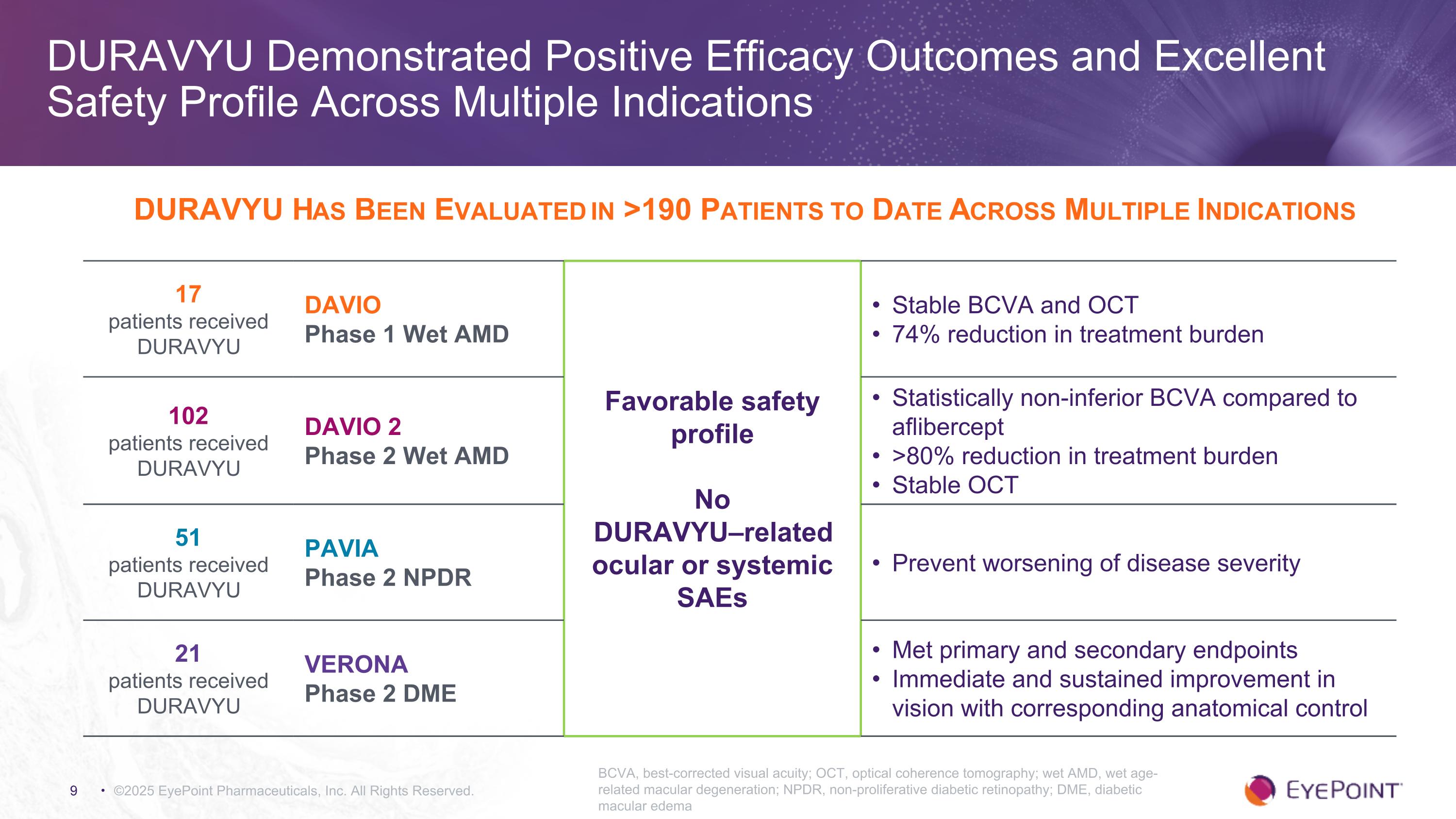
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU Demonstrated Positive Efficacy Outcomes and Excellent Safety Profile Across Multiple Indications BCVA, best-corrected visual acuity; OCT, optical coherence tomography; wet AMD, wet age-related macular degeneration; NPDR, non-proliferative diabetic retinopathy; DME, diabetic macular edema DURAVYU Has Been Evaluated in >190 Patients to Date Across Multiple Indications 17 patients received DURAVYU DAVIO Phase 1 Wet AMD Favorable safety profile No DURAVYU–related ocular or systemic SAEs Stable BCVA and OCT 74% reduction in treatment burden 102 patients received DURAVYU DAVIO 2 Phase 2 Wet AMD Statistically non-inferior BCVA compared to aflibercept >80% reduction in treatment burden Stable OCT 51 patients received DURAVYU PAVIA Phase 2 NPDR Prevent worsening of disease severity 21 patients received DURAVYU VERONA Phase 2 DME Met primary and secondary endpoints Immediate and sustained improvement in vision with corresponding anatomical control

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. LUGANO/LUCIAPhase 3 Pivotal Trials in wet AMD NON-INFERIORITY VERSUS AN AFLIBERCEPT CONTROL
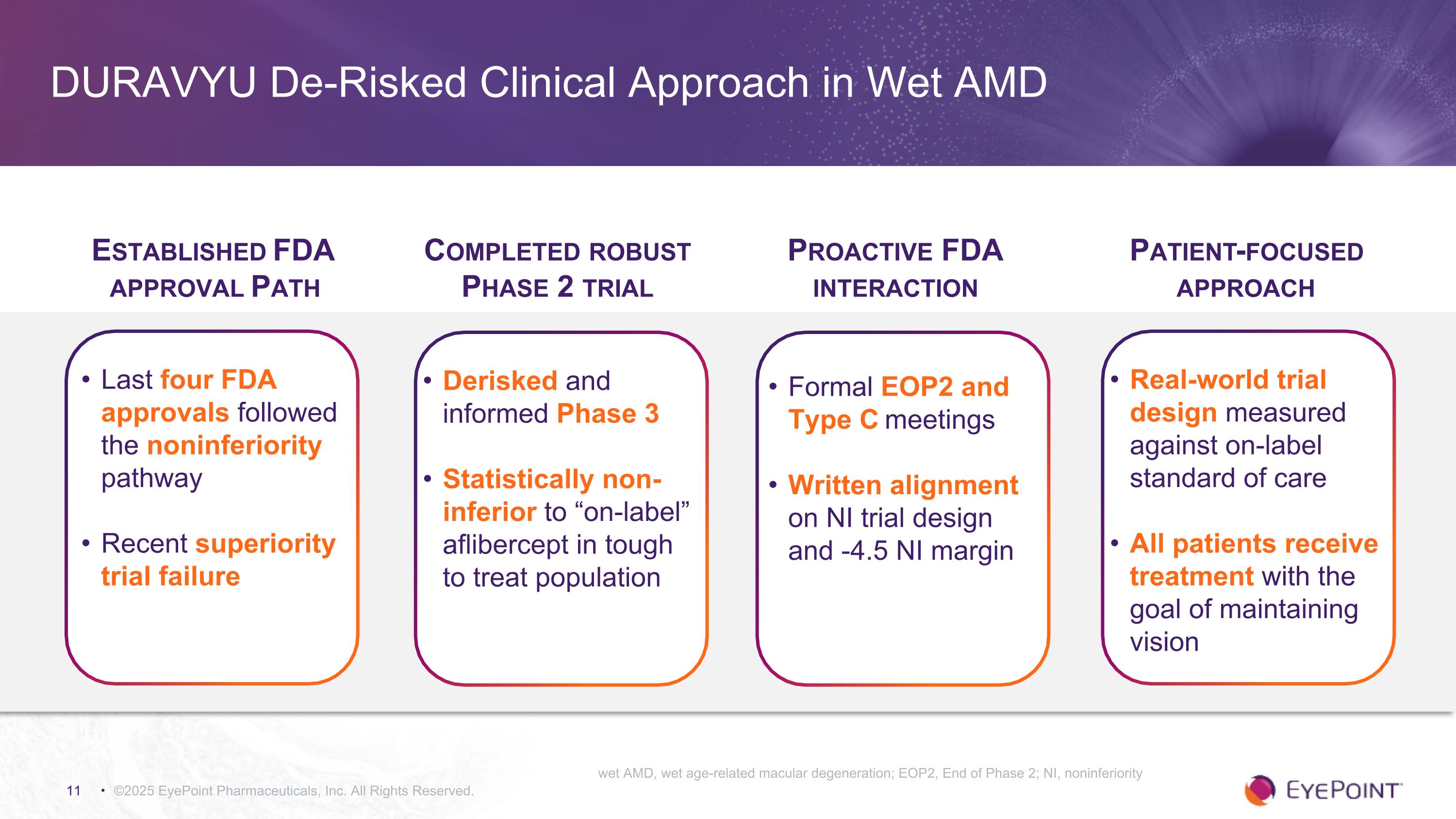
DURAVYU De-Risked Clinical Approach in Wet AMD ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Established FDA approval Path Last four FDA approvals followed the noninferiority pathway Recent superiority trial failure Completed robust Phase 2 trial Derisked and informed Phase 3 Statistically non-inferior to “on-label” aflibercept in tough to treat population Proactive FDA interaction Formal EOP2 and Type C meetings Written alignment on NI trial design and -4.5 NI margin Patient-focused approach Real-world trial design measured against on-label standard of care All patients receive treatment with the goal of maintaining vision wet AMD, wet age-related macular degeneration; EOP2, End of Phase 2; NI, noninferiority
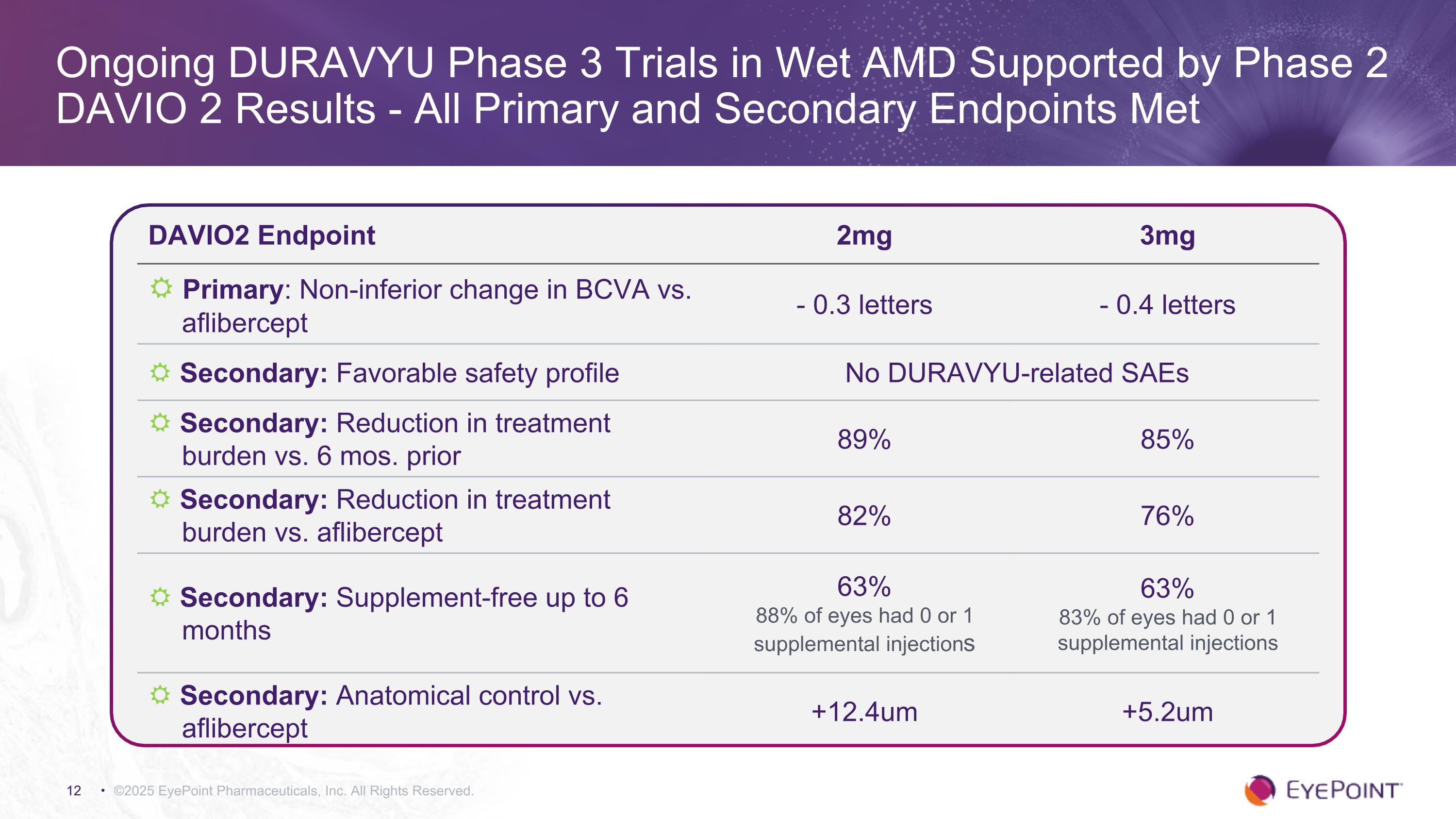
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Ongoing DURAVYU Phase 3 Trials in Wet AMD Supported by Phase 2 DAVIO 2 Results - All Primary and Secondary Endpoints Met DAVIO2 Endpoint 2mg 3mg R Primary: Non-inferior change in BCVA vs. aflibercept - 0.3 letters - 0.4 letters R Secondary: Favorable safety profile No DURAVYU-related SAEs R Secondary: Reduction in treatment burden vs. 6 mos. prior 89% 85% R Secondary: Reduction in treatment burden vs. aflibercept 82% 76% R Secondary: Supplement-free up to 6 months 63% 88% of eyes had 0 or 1 supplemental injections 63% 83% of eyes had 0 or 1 supplemental injections R Secondary: Anatomical control vs. aflibercept +12.4um +5.2um
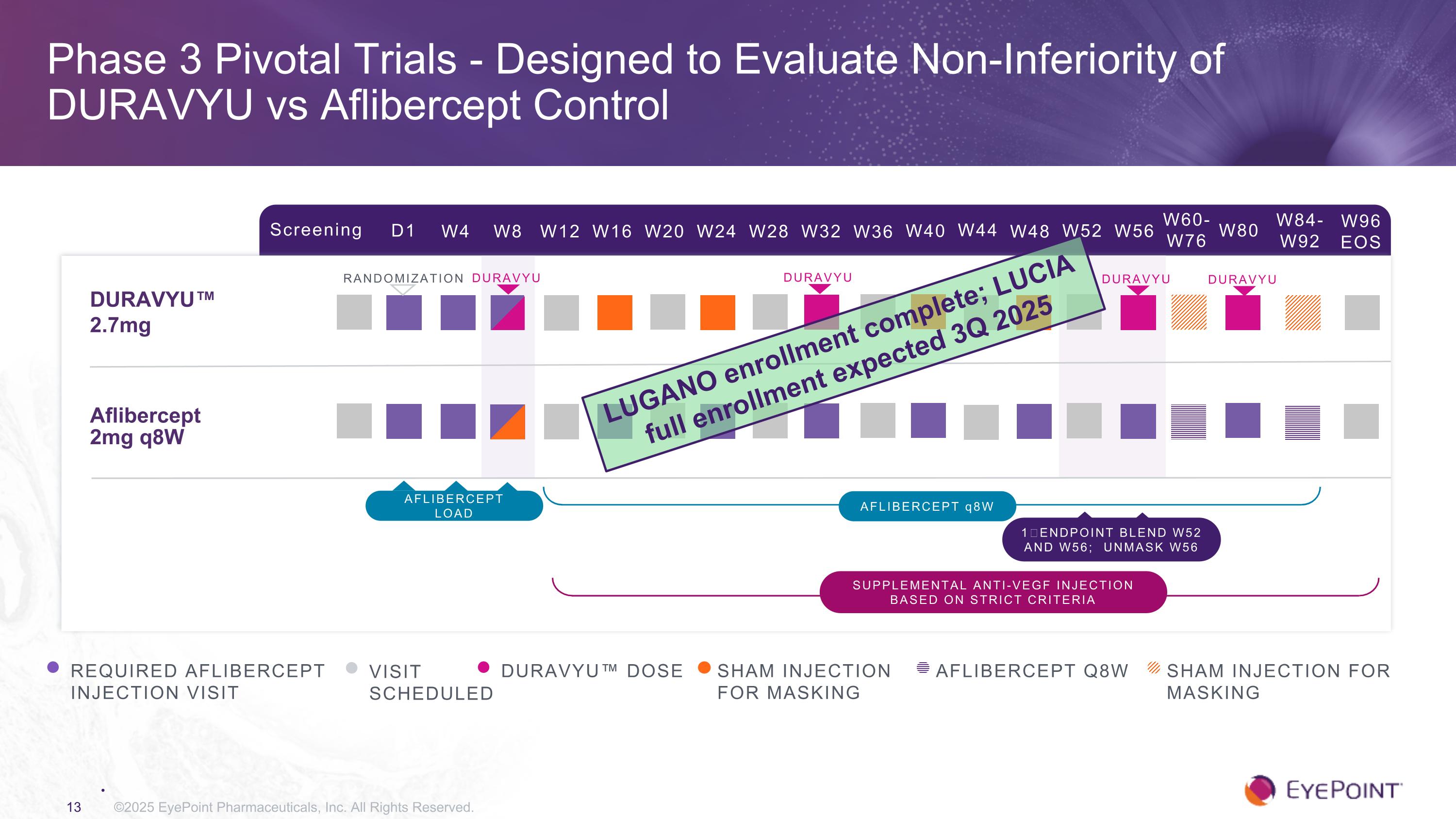
Phase 3 Pivotal Trials - Designed to Evaluate Non-Inferiority of DURAVYU vs Aflibercept Control ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Screening D1 W4 W8 W12 W16 W24 W32 W84-W92 W20 W28 DURAVYU™ 2.7mg Aflibercept 2mg q8W RANDOMIZATION REQUIRED AFLIBERCEPT INJECTION VISIT VISIT SCHEDULED DURAVYU™ DOSE AFLIBERCEPT q8W 1⁰ ENDPOINT BLEND W52 AND W56; UNMASK W56 SHAM INJECTION FOR MASKING W36 W40 W44 W48 W52 W56 W60-W76 W80 W96 EOS AFLIBERCEPT Q8W Sham injection For Masking Supplemental anti-VEGF injection based on strict criteria AFLIBERCEPT load DURAVYU DURAVYU DURAVYU DURAVYU LUGANO enrollment complete; LUCIA full enrollment expected 3Q 2025
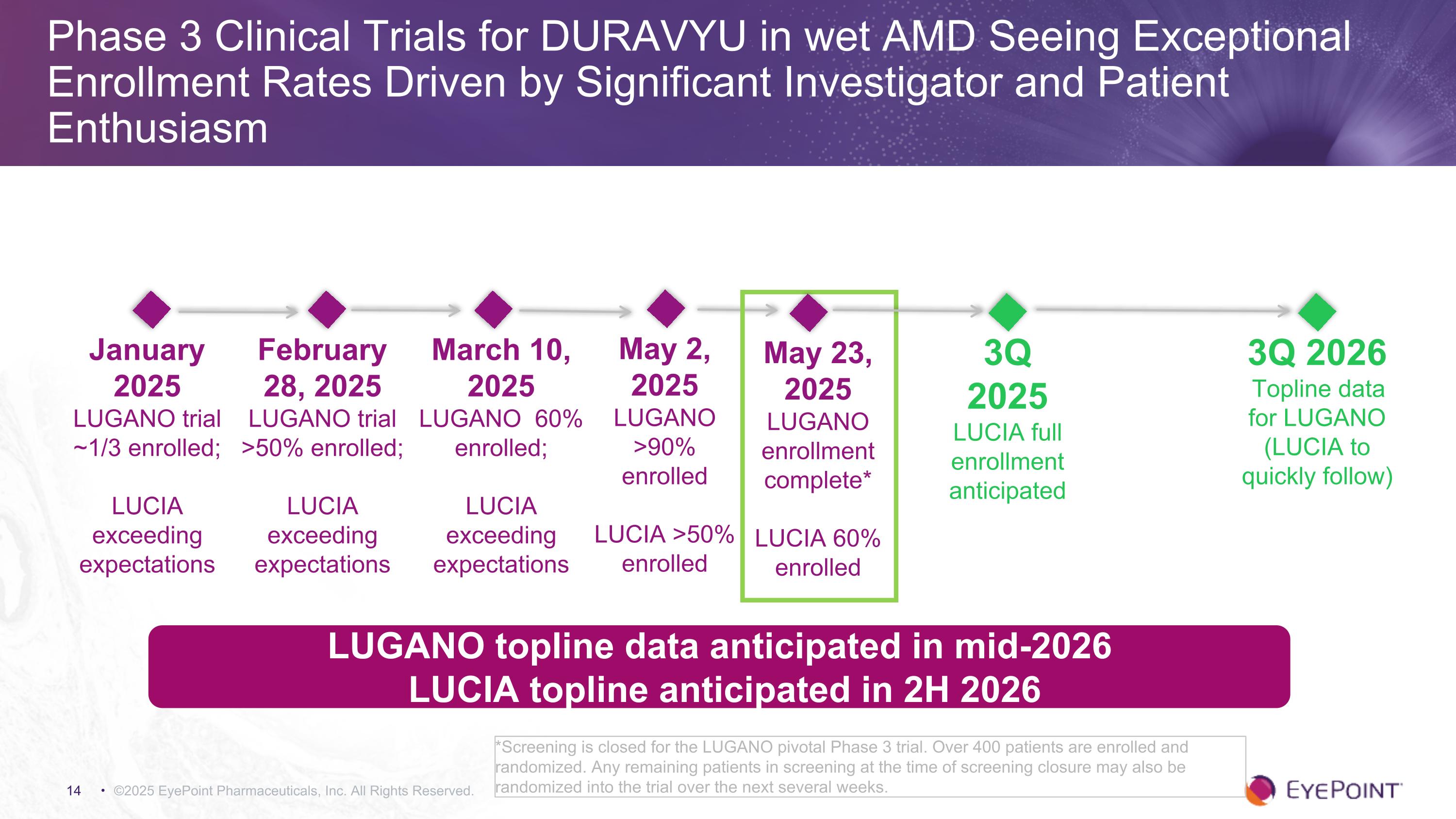
Phase 3 Clinical Trials for DURAVYU in wet AMD Seeing Exceptional Enrollment Rates Driven by Significant Investigator and Patient Enthusiasm ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. *Screening is closed for the LUGANO pivotal Phase 3 trial. Over 400 patients are enrolled and randomized. Any remaining patients in screening at the time of screening closure may also be randomized into the trial over the next several weeks. 3Q 2025 LUCIA full enrollment anticipated LUGANO topline data anticipated in mid-2026 LUCIA topline anticipated in 2H 2026 January 2025 LUGANO trial ~1/3 enrolled; LUCIA exceeding expectations 3Q 2026 Topline data for LUGANO (LUCIA to quickly follow) February 28, 2025 LUGANO trial >50% enrolled; LUCIA exceeding expectations March 10, 2025 LUGANO 60% enrolled; LUCIA exceeding expectations May 2, 2025 LUGANO >90% enrolled LUCIA >50% enrolled May 23, 2025 LUGANO enrollment complete* LUCIA 60% enrolled

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 VERONA Clinical Trial in DME 24 Week results Primary and secondary endpoints achieved DME, diabetic macular edema
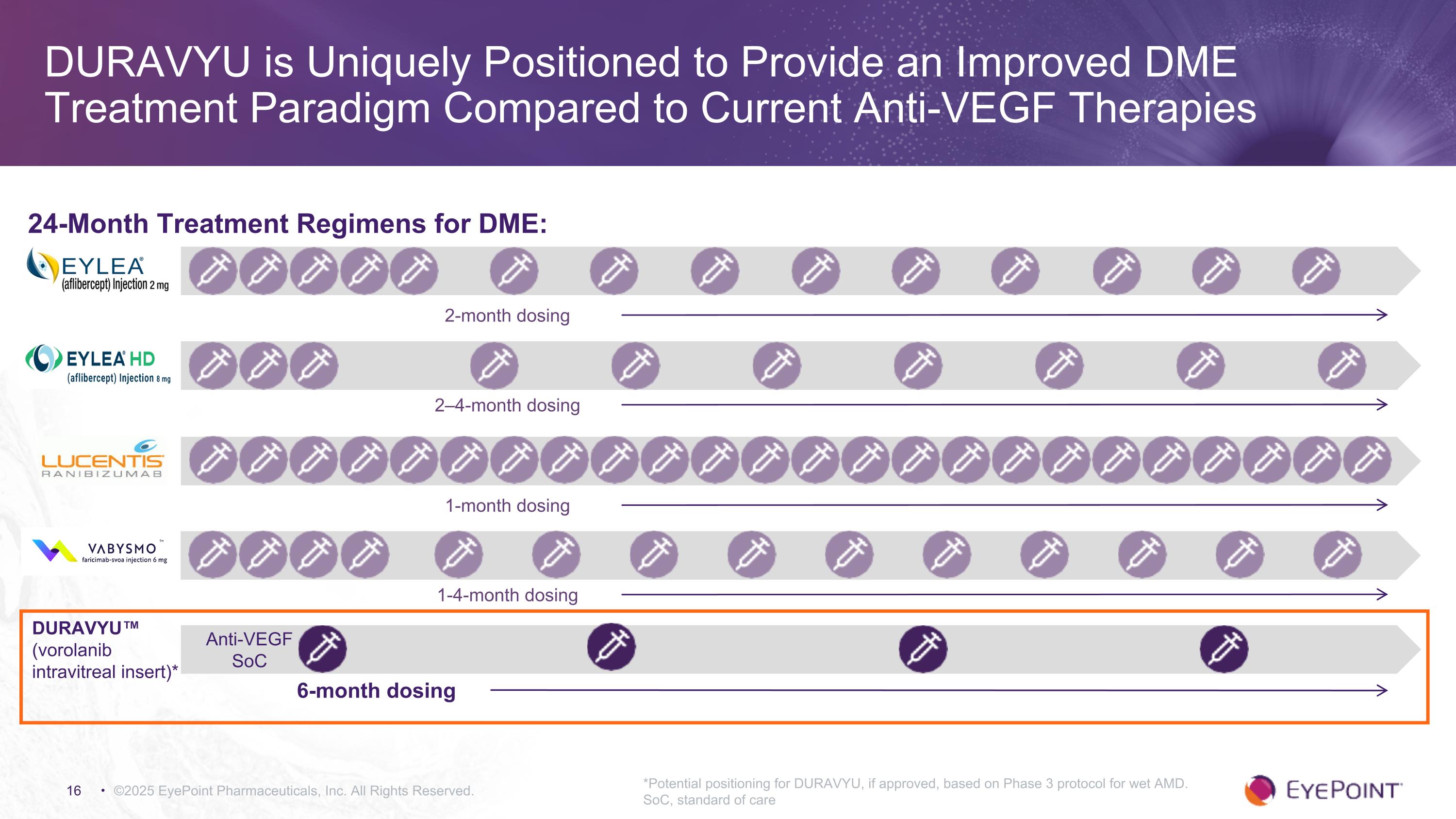
DURAVYU is Uniquely Positioned to Provide an Improved DME Treatment Paradigm Compared to Current Anti-VEGF Therapies ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. 24-Month Treatment Regimens for DME: 2–4-month dosing 1-4-month dosing Anti-VEGF SoC DURAVYU™ (vorolanib intravitreal insert)* 2-month dosing 1-month dosing 6-month dosing *Potential positioning for DURAVYU, if approved, based on Phase 3 protocol for wet AMD. SoC, standard of care
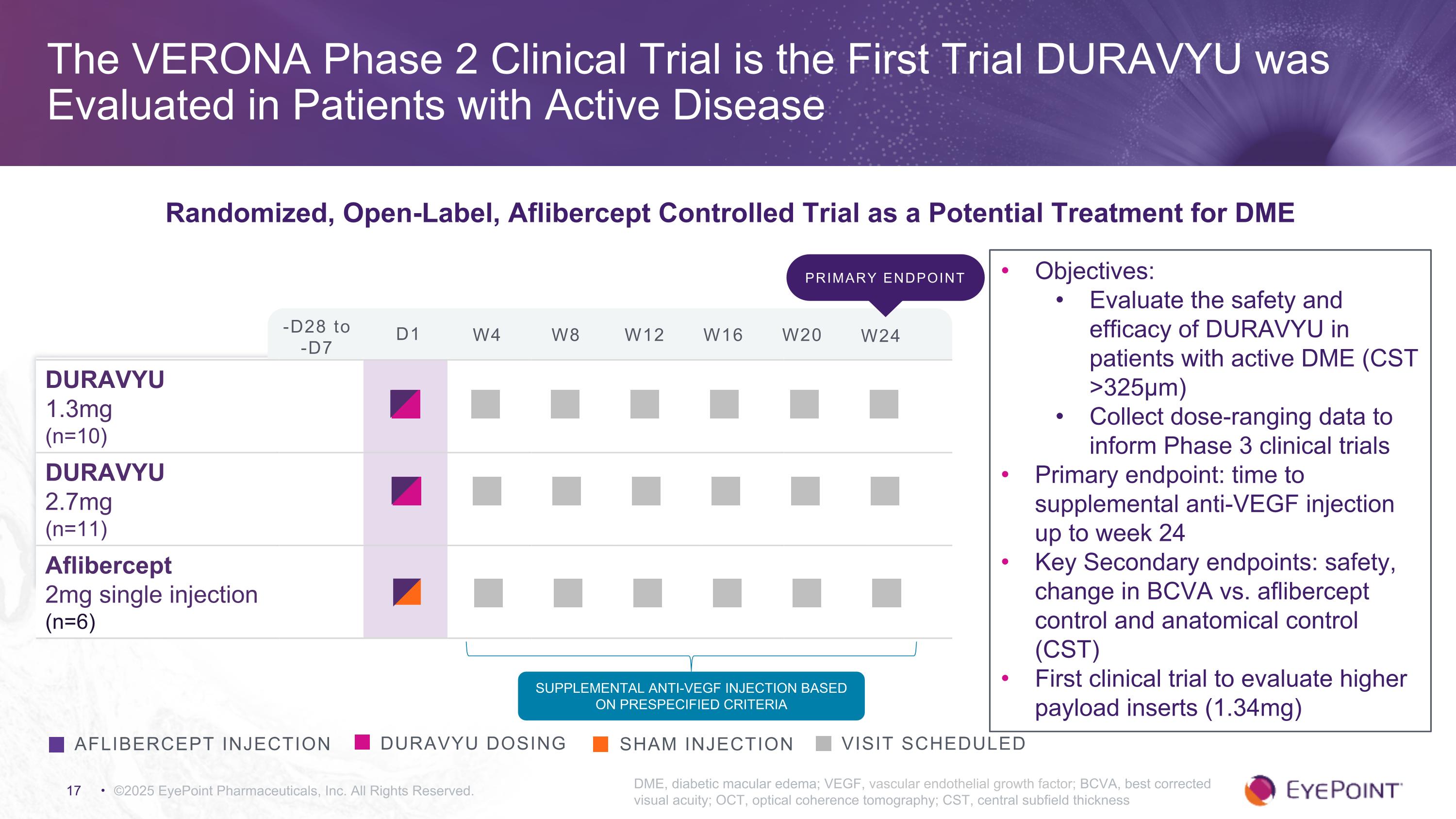
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DME, diabetic macular edema; VEGF, vascular endothelial growth factor; BCVA, best corrected visual acuity; OCT, optical coherence tomography; CST, central subfield thickness DURAVYU dosing Visit Scheduled aflibercept injection Sham injection DURAVYU 1.3mg (n=10) DURAVYU 2.7mg (n=11) Aflibercept 2mg single injection (n=6) Supplemental Anti-VEGF injection based on prespecified criteria Objectives: Evaluate the safety and efficacy of DURAVYU in patients with active DME (CST >325μm) Collect dose-ranging data to inform Phase 3 clinical trials Primary endpoint: time to supplemental anti-VEGF injection up to week 24 Key Secondary endpoints: safety, change in BCVA vs. aflibercept control and anatomical control (CST) First clinical trial to evaluate higher payload inserts (1.34mg) -D28 to -D7 D1 W4 W8 W12 W16 W20 W24 The VERONA Phase 2 Clinical Trial is the First Trial DURAVYU was Evaluated in Patients with Active Disease Randomized, Open-Label, Aflibercept Controlled Trial as a Potential Treatment for DME Primary endpoint
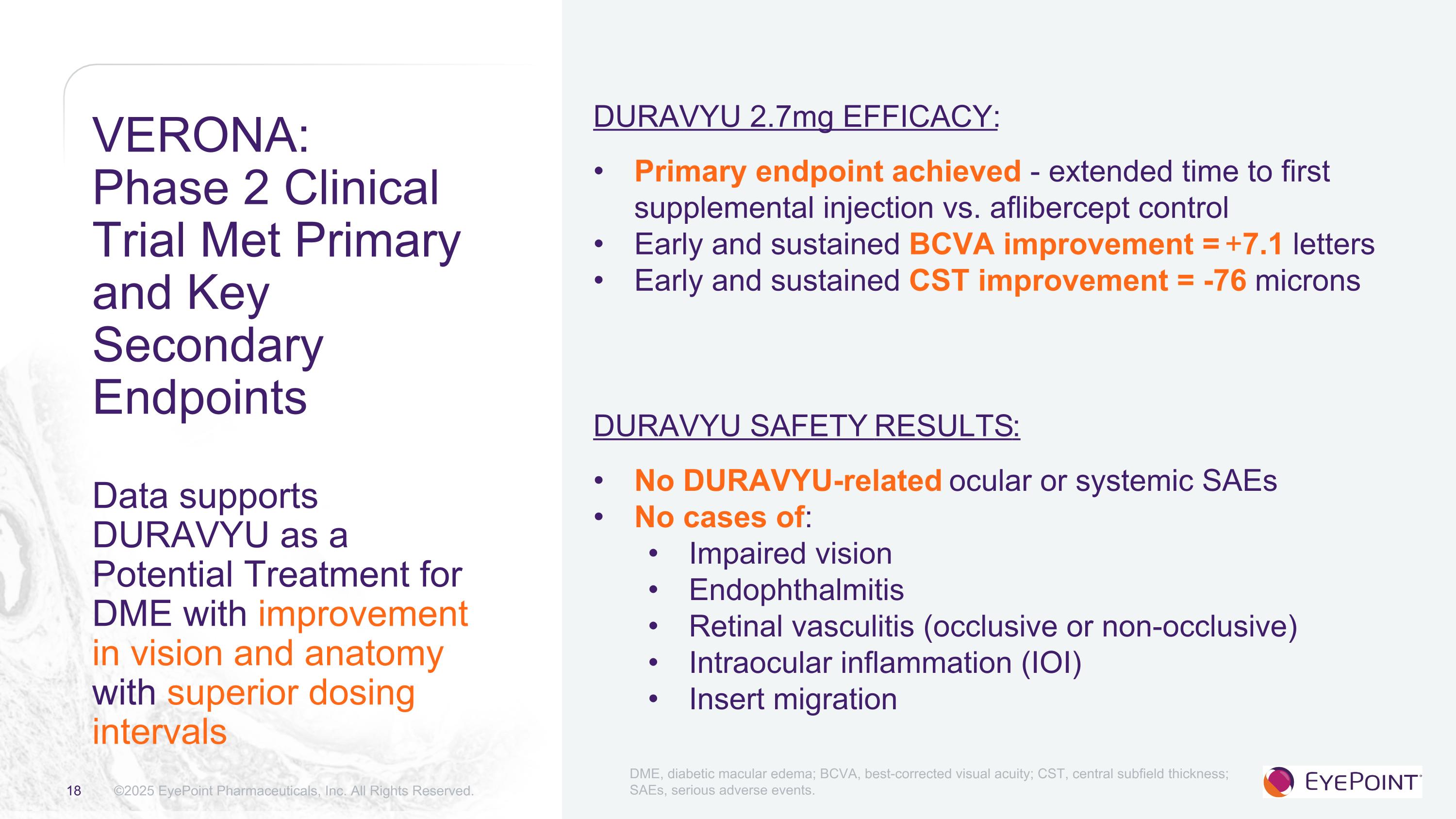
VERONA:Phase 2 Clinical Trial Met Primary and Key Secondary EndpointsData supports DURAVYU as a Potential Treatment for DME with improvement in vision and anatomy with superior dosing intervals ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7mg EFFICACY: Primary endpoint achieved - extended time to first supplemental injection vs. aflibercept control Early and sustained BCVA improvement = +7.1 letters Early and sustained CST improvement = -76 microns DURAVYU SAFETY Results: No DURAVYU-related ocular or systemic SAEs No cases of: Impaired vision Endophthalmitis Retinal vasculitis (occlusive or non-occlusive) Intraocular inflammation (IOI) Insert migration DME, diabetic macular edema; BCVA, best-corrected visual acuity; CST, central subfield thickness; SAEs, serious adverse events.
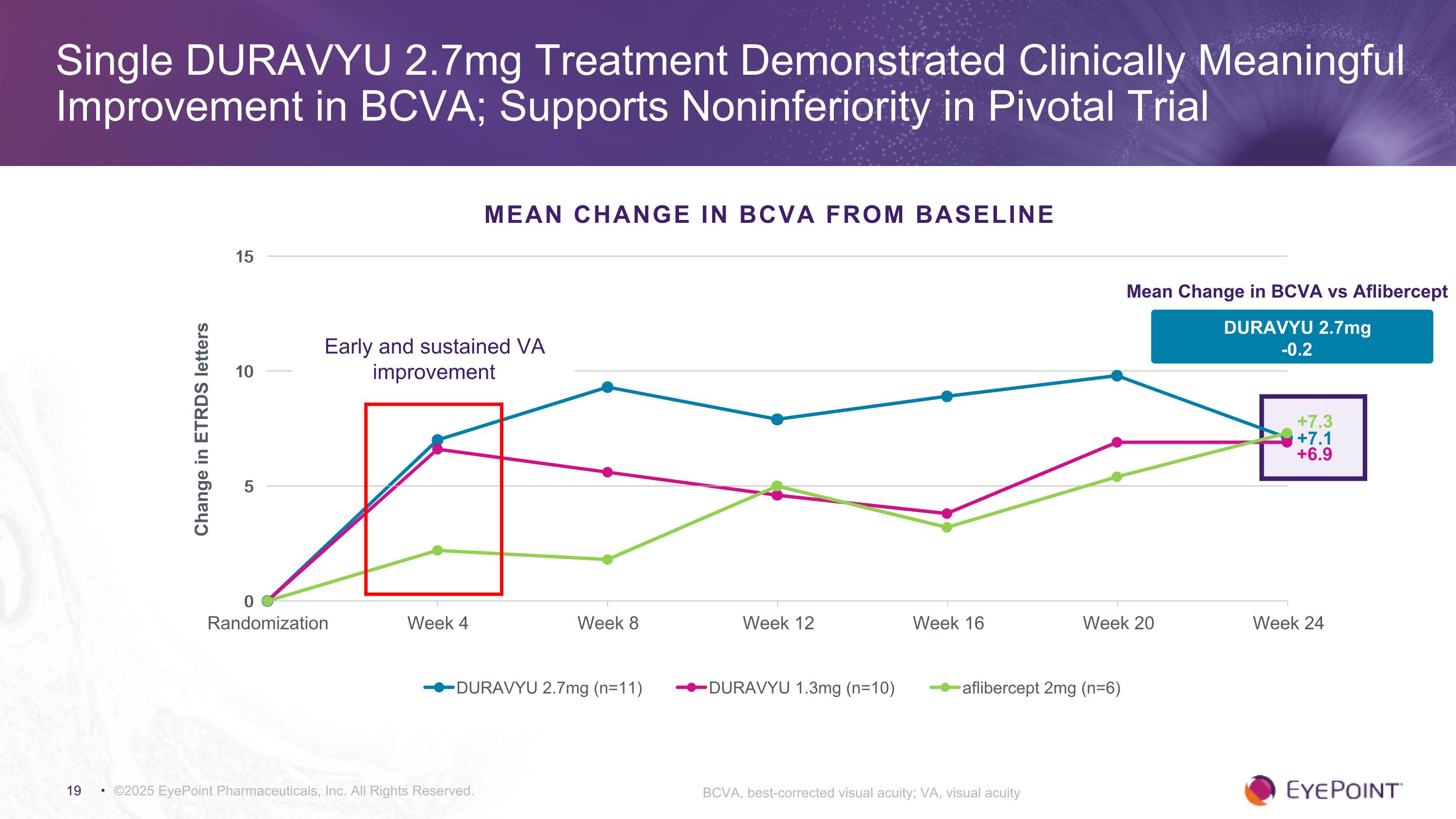
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Single DURAVYU 2.7mg Treatment Demonstrated Clinically Meaningful Improvement in BCVA; Supports Noninferiority in Pivotal Trial BCVA, best-corrected visual acuity; VA, visual acuity MEAN CHANGE IN BCVA FROM BASELINE +7.1 +7.3 DURAVYU 2.7mg -0.2 Mean Change in BCVA vs Aflibercept +6.9 Early and sustained VA improvement
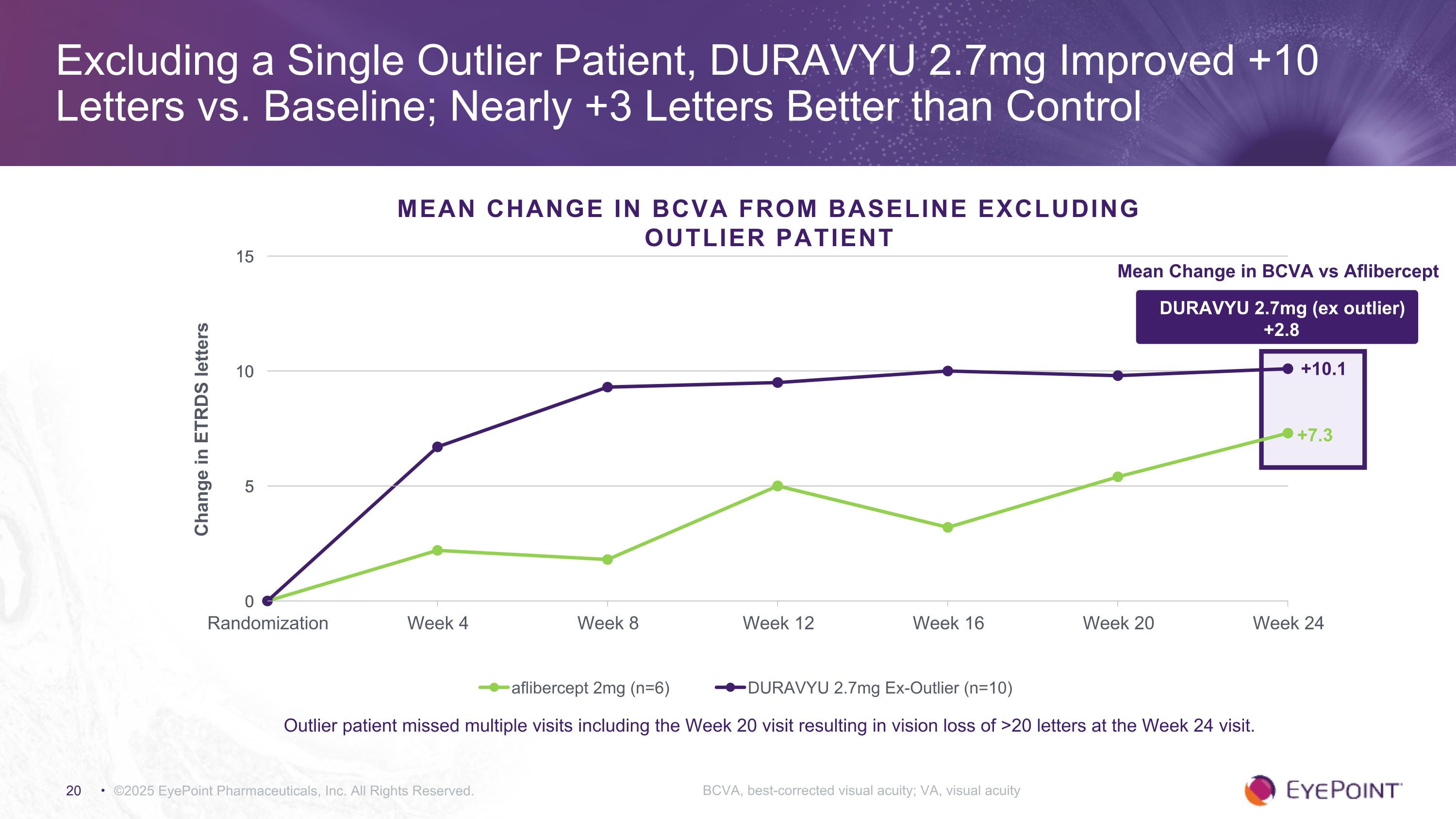
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Excluding a Single Outlier Patient, DURAVYU 2.7mg Improved +10 Letters vs. Baseline; Nearly +3 Letters Better than Control BCVA, best-corrected visual acuity; VA, visual acuity MEAN CHANGE IN BCVA FROM BASELINE Excluding Outlier Patient +10.1 +7.3 DURAVYU 2.7mg (ex outlier) +2.8 Mean Change in BCVA vs Aflibercept Outlier patient missed multiple visits including the Week 20 visit resulting in vision loss of >20 letters at the Week 24 visit.
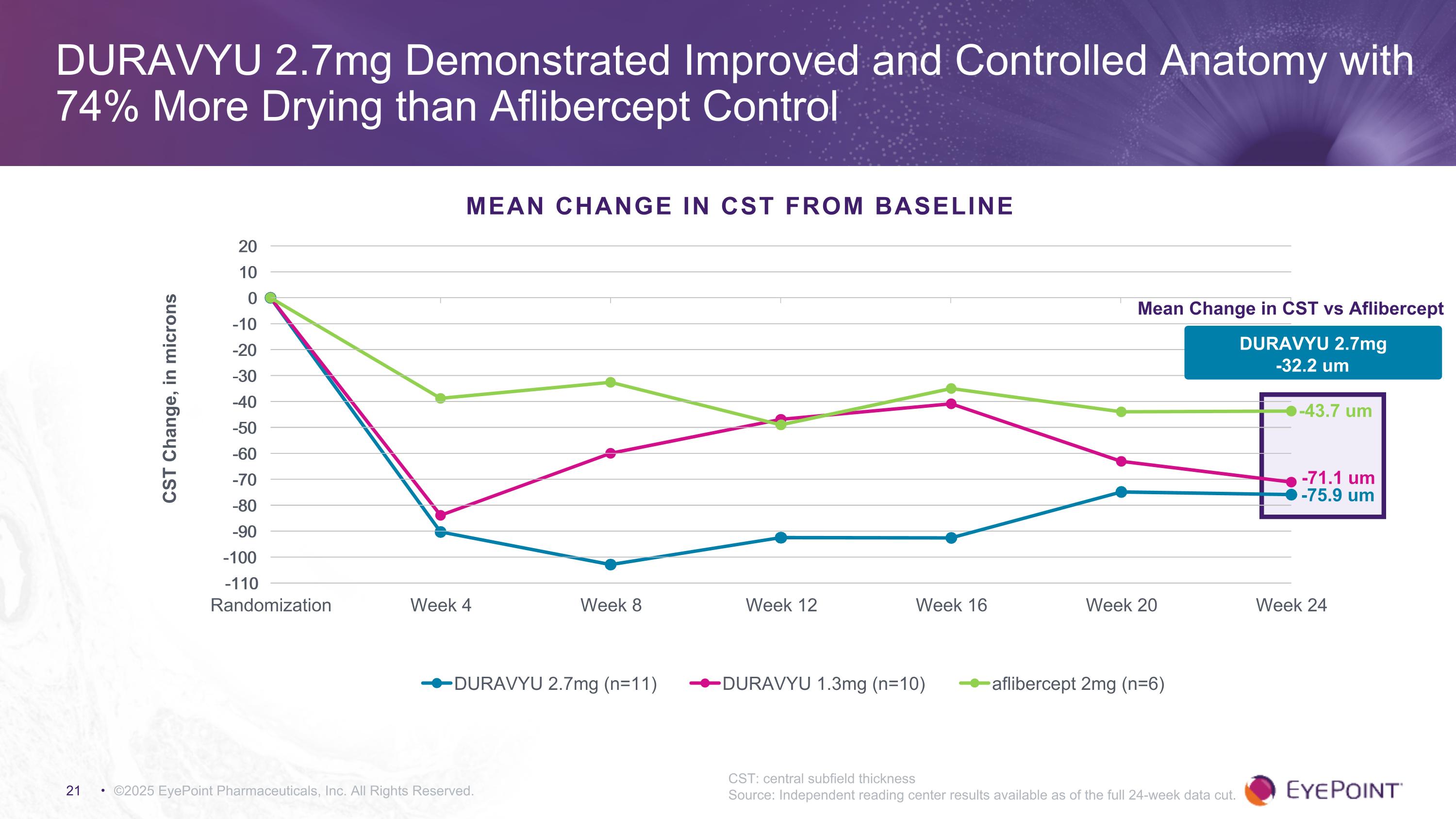
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DURAVYU 2.7mg Demonstrated Improved and Controlled Anatomy with 74% More Drying than Aflibercept Control CST: central subfield thickness Source: Independent reading center results available as of the full 24-week data cut. MEAN CHANGE IN CST FROM BASELINE -75.9 um -43.7 um Mean Change in CST vs Aflibercept -71.1 um DURAVYU 2.7mg -32.2 um

©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Phase 2 VERONA Clinical Trial in DME Sub-Group Analysis Of Patients Supplement-free at 24 Weeks DME, diabetic macular edema
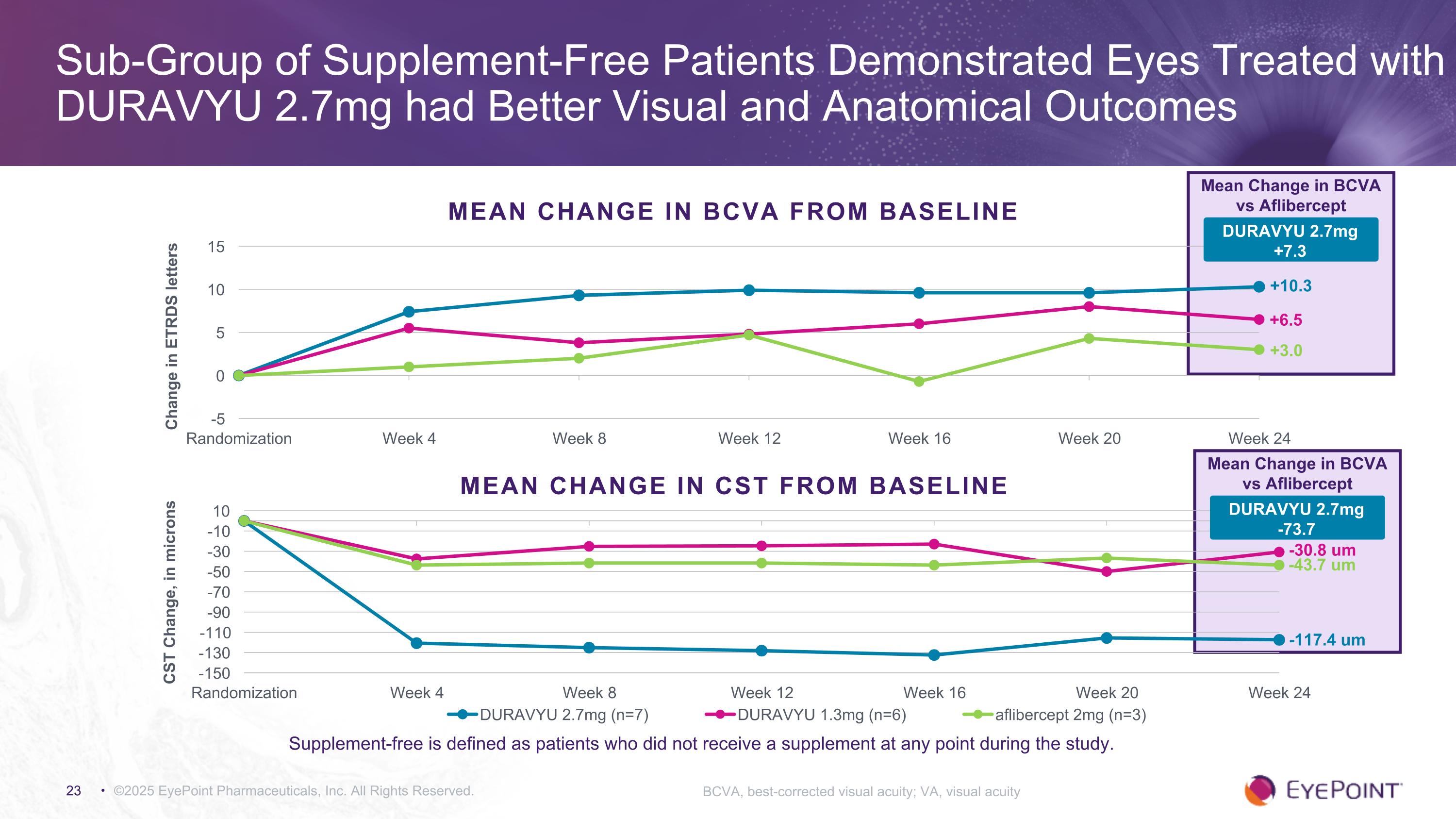
©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Sub-Group of Supplement-Free Patients Demonstrated Eyes Treated with DURAVYU 2.7mg had Better Visual and Anatomical Outcomes BCVA, best-corrected visual acuity; VA, visual acuity MEAN CHANGE IN BCVA FROM BASELINE +10.3 +3.0 Mean Change in BCVA vs Aflibercept +6.5 DURAVYU 2.7mg +7.3 Supplement-free is defined as patients who did not receive a supplement at any point during the study. MEAN CHANGE IN CST FROM BASELINE Mean Change in BCVA vs Aflibercept DURAVYU 2.7mg -73.7 -117.4 um -43.7 um -30.8 um
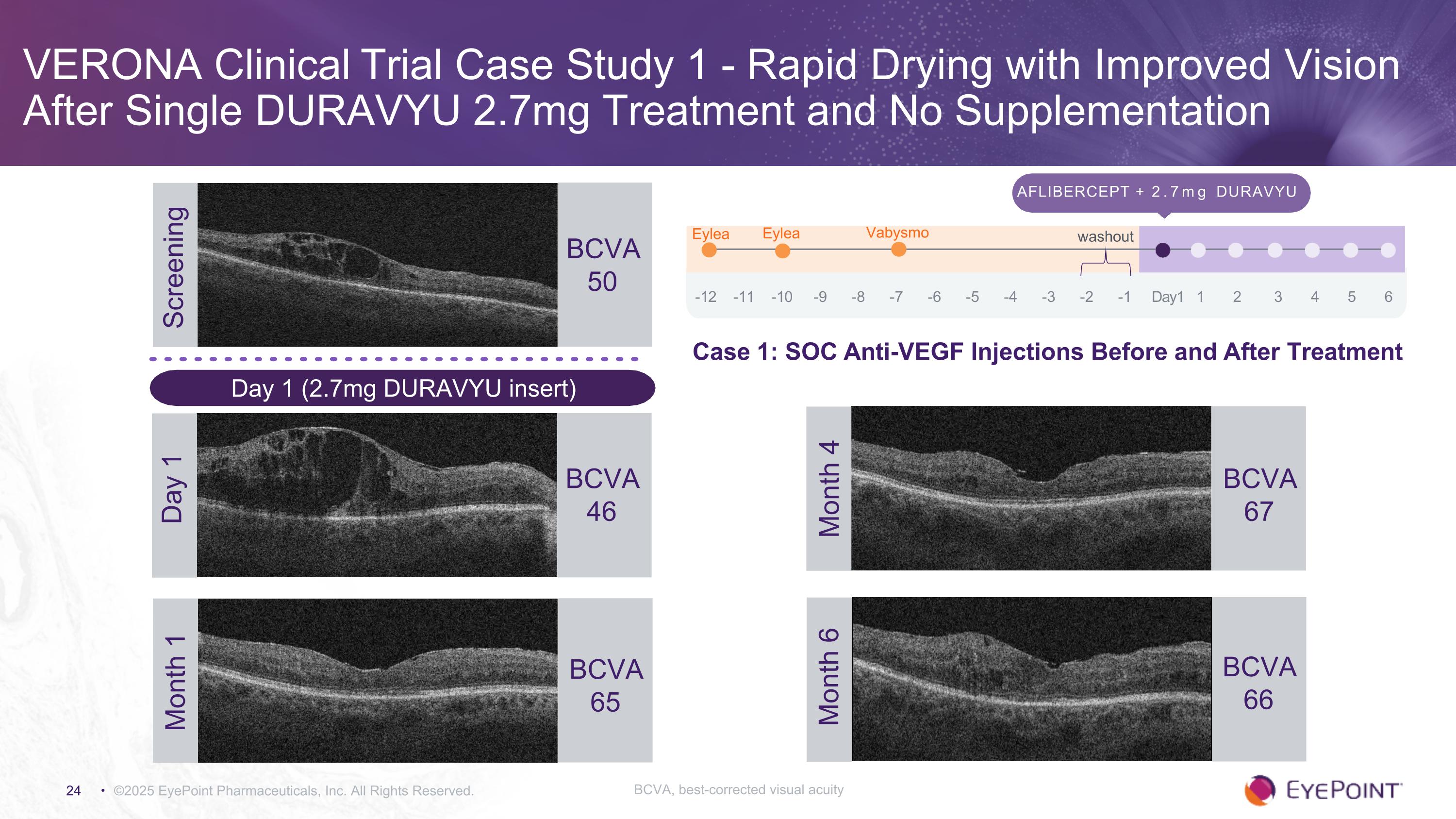
VERONA Clinical Trial Case Study 1 - Rapid Drying with Improved Vision After Single DURAVYU 2.7mg Treatment and No Supplementation ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Screening BCVA 50 Aflibercept + 2.7mg DURAVYU -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 Day1 1 2 3 4 5 6 Eylea Eylea Vabysmo washout BCVA 46 Day 1 BCVA 67 Month 4 BCVA 66 Month 6 Month 1 BCVA 65 Day 1 (2.7mg DURAVYU insert) Case 1: SOC Anti-VEGF Injections Before and After Treatment BCVA, best-corrected visual acuity
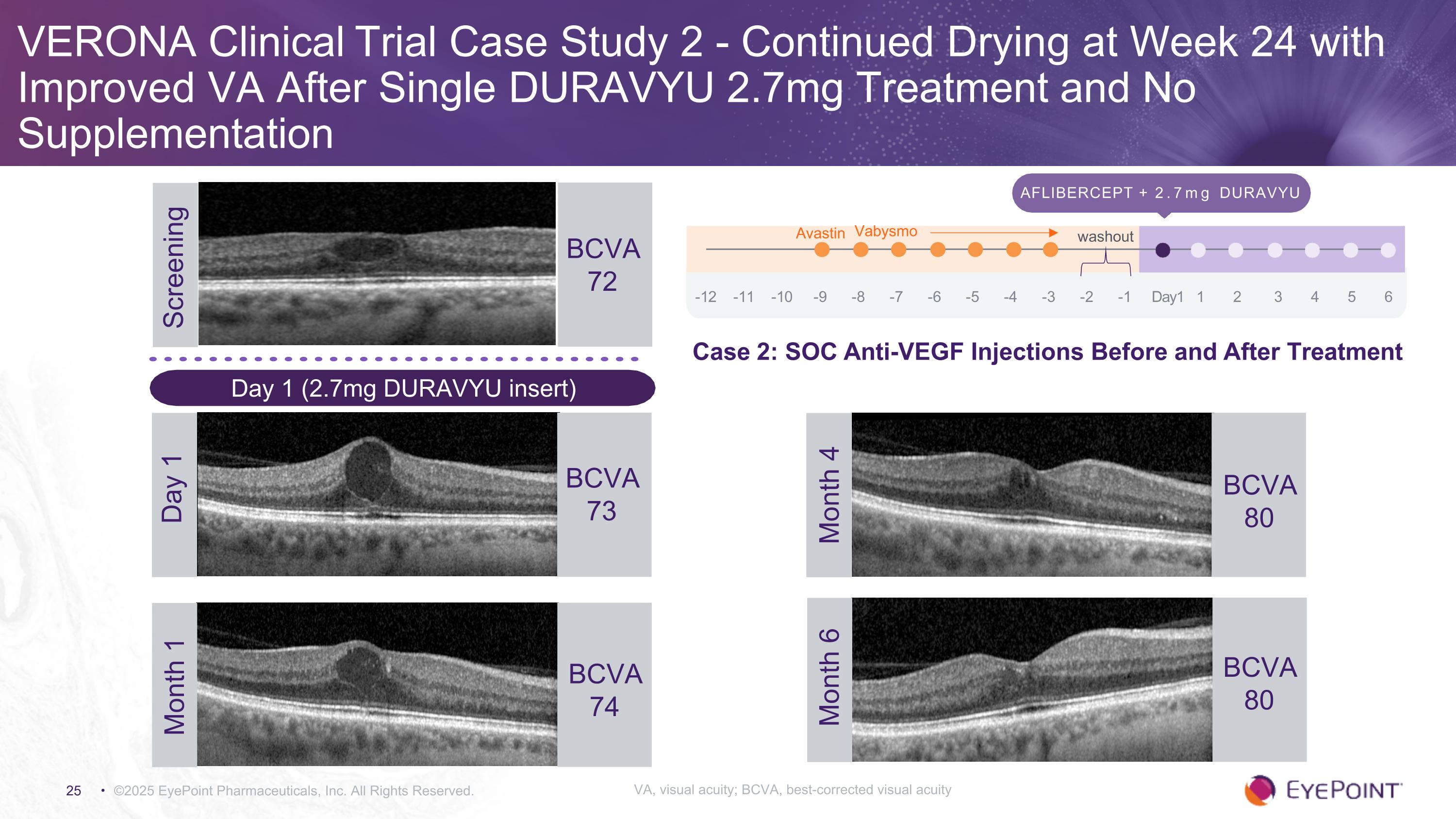
VERONA Clinical Trial Case Study 2 - Continued Drying at Week 24 with Improved VA After Single DURAVYU 2.7mg Treatment and No Supplementation ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Screening BCVA 72 Aflibercept + 2.7mg DURAVYU -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 Day1 1 2 3 4 5 6 Avastin Vabysmo washout BCVA 73 Day 1 BCVA 80 Month 4 BCVA 80 Month 6 Month 1 BCVA 74 Day 1 (2.7mg DURAVYU insert) Case 2: SOC Anti-VEGF Injections Before and After Treatment VA, visual acuity; BCVA, best-corrected visual acuity

VERONA: Phase 2 Clinical Trial Summary ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. Primary endpoint met for both DURAVYU doses Demonstrated immediate and clinically meaningful results without a full load of aflibercept Significant improvement in vision and anatomic results paired with a meaningful reduction in treatment burden Sub-group analysis of supplement-free eyes demonstrated results are driven by treatment with DURAVYU and not by supplemental injections Continued favorable safety and tolerability EOP2 meeting scheduled with FDA in July 2025 to solidify plans for pivotal program

State-Of-The-Art cGMP Manufacturing Facility to Support DURAVYU through Phase 3 and Global Commercial Production ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. cGMP, current good manufacturing practices; FDA, Federal Drug Administration; EMA, European Medicines Agency; cGMP, current good manufacturing practice Located in Northbridge, MA Built to US FDA and EU EMA standards DURAVYU registration batches in-motion to support future NDA filing Built to EYPT specifications by landlord preserving upfront cash investment 40,000sf+ commercial manufacturing site
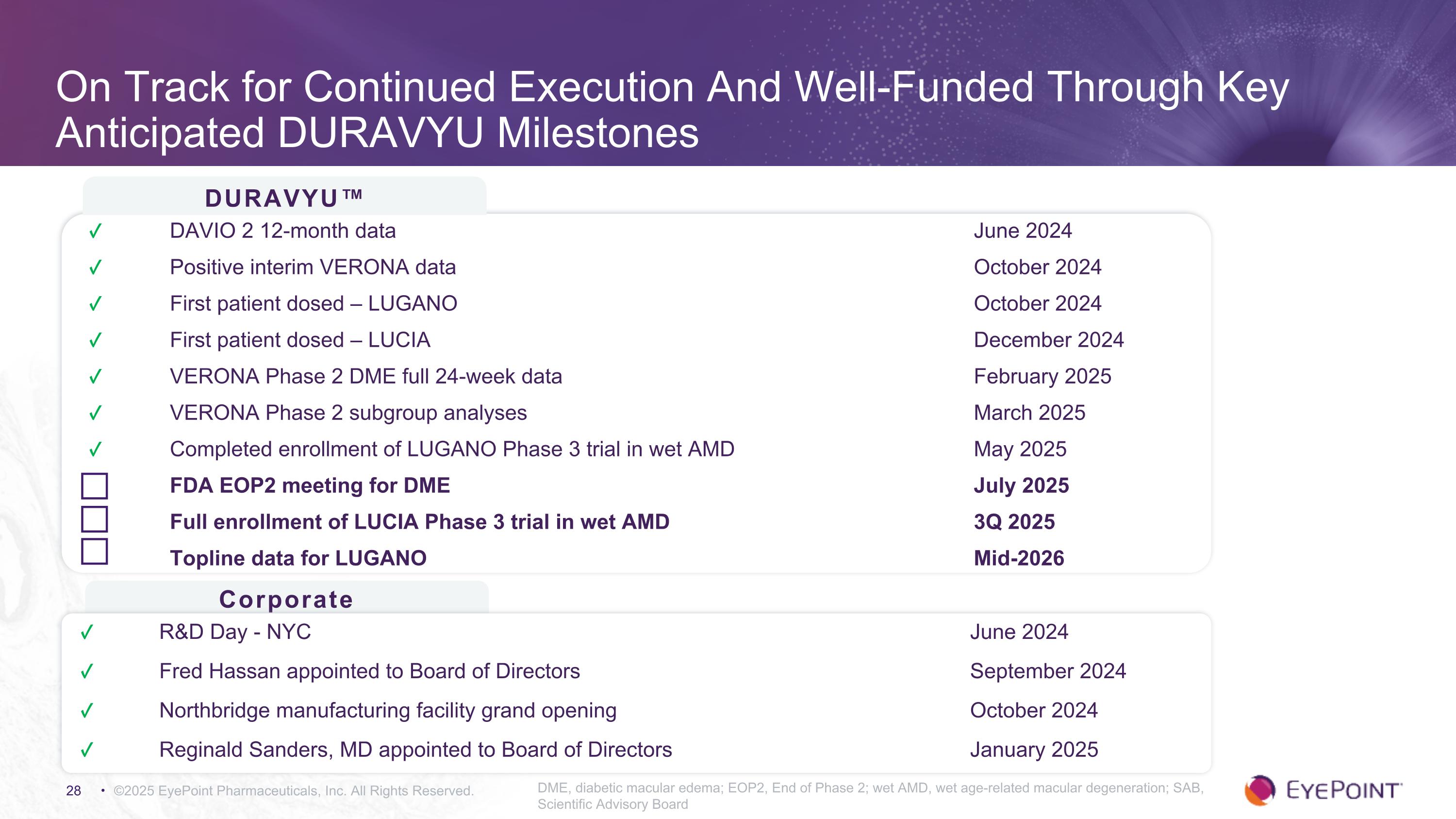
On Track for Continued Execution And Well-Funded Through Key Anticipated DURAVYU Milestones ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved. DME, diabetic macular edema; EOP2, End of Phase 2; wet AMD, wet age-related macular degeneration; SAB, Scientific Advisory Board DURAVYU™ Corporate ✓ DAVIO 2 12-month data June 2024 ✓ Positive interim VERONA data October 2024 ✓ First patient dosed – LUGANO October 2024 ✓ First patient dosed – LUCIA December 2024 ✓ VERONA Phase 2 DME full 24-week data February 2025 ✓ VERONA Phase 2 subgroup analyses March 2025 ✓ Completed enrollment of LUGANO Phase 3 trial in wet AMD May 2025 FDA EOP2 meeting for DME July 2025 Full enrollment of LUCIA Phase 3 trial in wet AMD 3Q 2025 Topline data for LUGANO Mid-2026 ✓ R&D Day - NYC June 2024 ✓ Fred Hassan appointed to Board of Directors September 2024 ✓ Northbridge manufacturing facility grand opening October 2024 ✓ Reginald Sanders, MD appointed to Board of Directors January 2025

Investor Presentation May 2025 ©2025 EyePoint Pharmaceuticals, Inc. All Rights Reserved.





























