
© 2025 CLEARPOINT NEURO WHEN YOUR PATH IS UNCLEAR, WE POINT THE WAY. Nasdaq: CLPT May 2025

DISCLAIMER This presentation and discussion contain forward-looking statements within the context of the federal securities laws, including the Company’s expectation for revenues, gross margin, the adequacy of cash and cash equivalent balances to support operations and meet future obligations, the future market of its products and services, and other performance and results. These forward-looking statements are based on management’s current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: macroeconomic and inflationary conditions; regulatory and policy uncertainty due to presidential administration change; the introduction of or changes in tariffs, sanctions, or trade barriers; changes in monetary policy; geopolitical trends, such as protectionism and economic nationalism; future revenue from sales of the Company’s products and services; the Company’s ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company’s products and services in their delivery of therapies; the Company’s ability to maintain its current relationships with biologics and drug delivery partners or enter into new relationships with such partners; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company’s ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of new products and the new products of its biologics and drug delivery partners. More detailed information on these and additional factors that could affect the Company’s actual results are described in the “Risk Factors” section of the Company’s Annual Report on Form 10-K for the year ended December 31, 2024,which has been filed with the Securities and Exchange Commission, and the Company’s Quarterly Report on Form 10-Q for the three months ended March 31, 2025, which the company intends to file with the Securities and Exchange Commission on or before May 15, 2025. The Company does not assume any obligation to update these forward-looking statements. © 2025 CLEARPOINT NEURO

OUR COMPANY We Enable Cell, Gene and Device Therapies by Offering Precise Navigation to the Brain and Spine Our Unique Platform Includes Both Proven Clinical Products Used by Neurosurgeons, and Drug Development Services Used by BioPharma Partners © 2025 CLEARPOINT NEURO
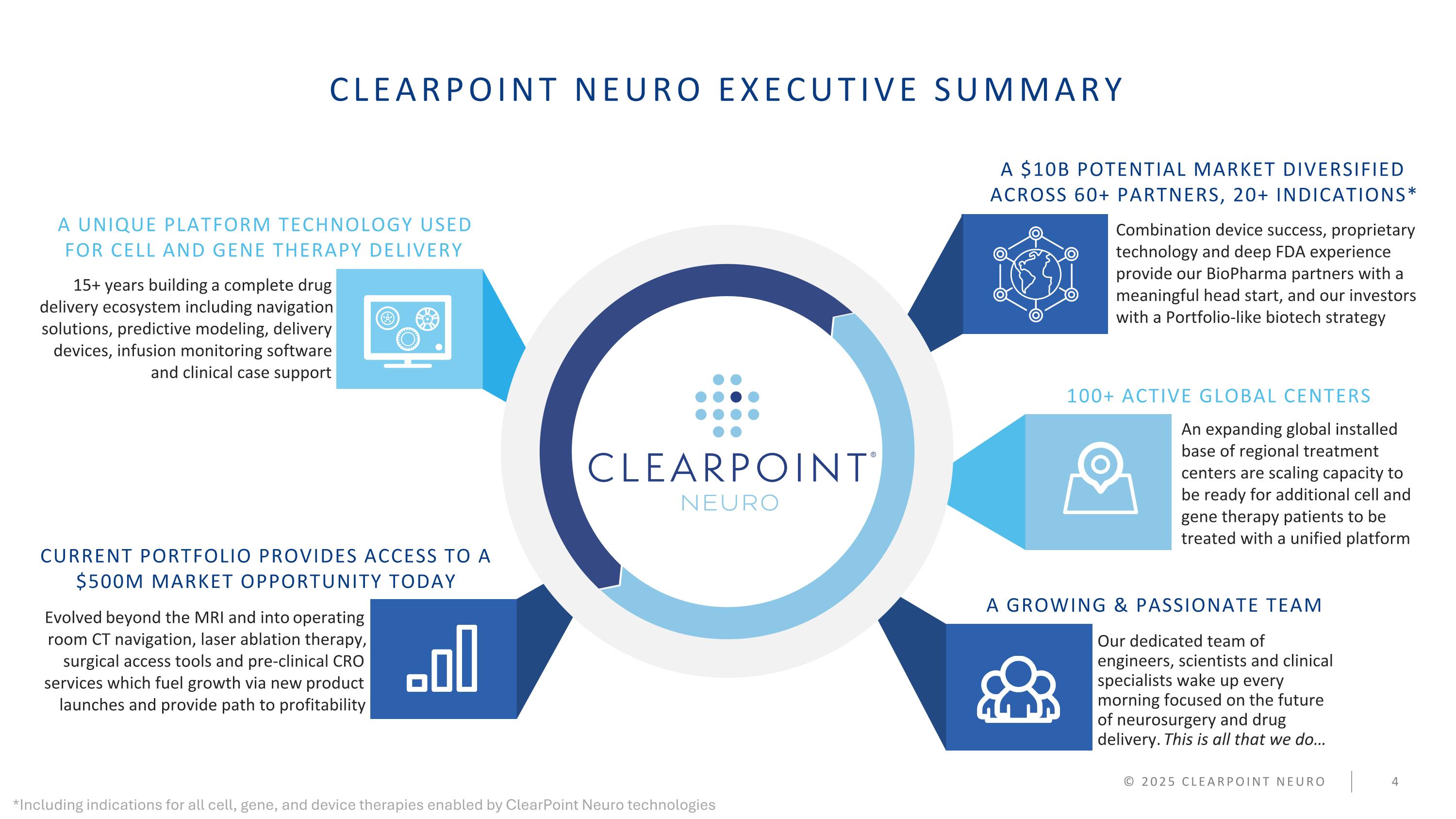
CLEARPOINT NEURO EXECUTIVE SUMMARY 15+ years building a complete drug delivery ecosystem including navigation solutions, predictive modeling, delivery devices, infusion monitoring software and clinical case support Combination device success, proprietary technology and deep FDA experience provide our BioPharma partners with a meaningful head start, and our investors with a Portfolio-like biotech strategy An expanding global installed base of regional treatment centers are scaling capacity to be ready for additional cell and gene therapy patients to be treated with a unified platform Evolved beyond the MRI and into operating room CT navigation, laser ablation therapy, surgical access tools and pre-clinical CRO services which fuel growth via new product launches and provide path to profitability Our dedicated team of engineers, scientists and clinical specialists wake up every morning focused on the future of neurosurgery and drug delivery. This is all that we do… A UNIQUE PLATFORM TECHNOLOGY USED FOR CELL AND GENE THERAPY DELIVERY CURRENT PORTFOLIO PROVIDES ACCESS TO A $500M MARKET OPPORTUNITY TODAY 100+ ACTIVE GLOBAL CENTERS A GROWING & PASSIONATE TEAM © 2025 CLEARPOINT NEURO A $10B POTENTIAL MARKET DIVERSIFIED ACROSS 60+ PARTNERS, 20+ INDICATIONS* *Including indications for all cell, gene, and device therapies enabled by ClearPoint Neuro technologies

Neurological diseases cost Americans nearly $800 Billion annually. The only way to decrease these costs is to improve treatment. Despite some available treatments, very few of these patients undergo a direct surgical intervention to improve their quality of life… © 2025 CLEARPOINT NEURO More than 30 Million People in the U.S. are estimated to suffer from severe and debilitating neurological disorders; Parkinson’s Disease (1,000,000) Essential Tremor (7,000,000) Epilepsy (2,900,000) Huntington’s Disease (41,000) Rare Childhood Genetic Disorders (25,000) Dementia and Alzheimer’s Disease (6,900,000) Tumor and Glioblastoma (280,000) Severe OCD (1,000,000) Treatment Resistant Depression (2,900,000) ALS and Spinal Cord Injury (300,000) Stroke Rehabilitation (7,000,000) Neuropathic Pain (2,000,000) The Future of Cell and Gene Therapy is Not Coming… It is HERE TODAY Our Company Sources on file at ClearPoint Neuro
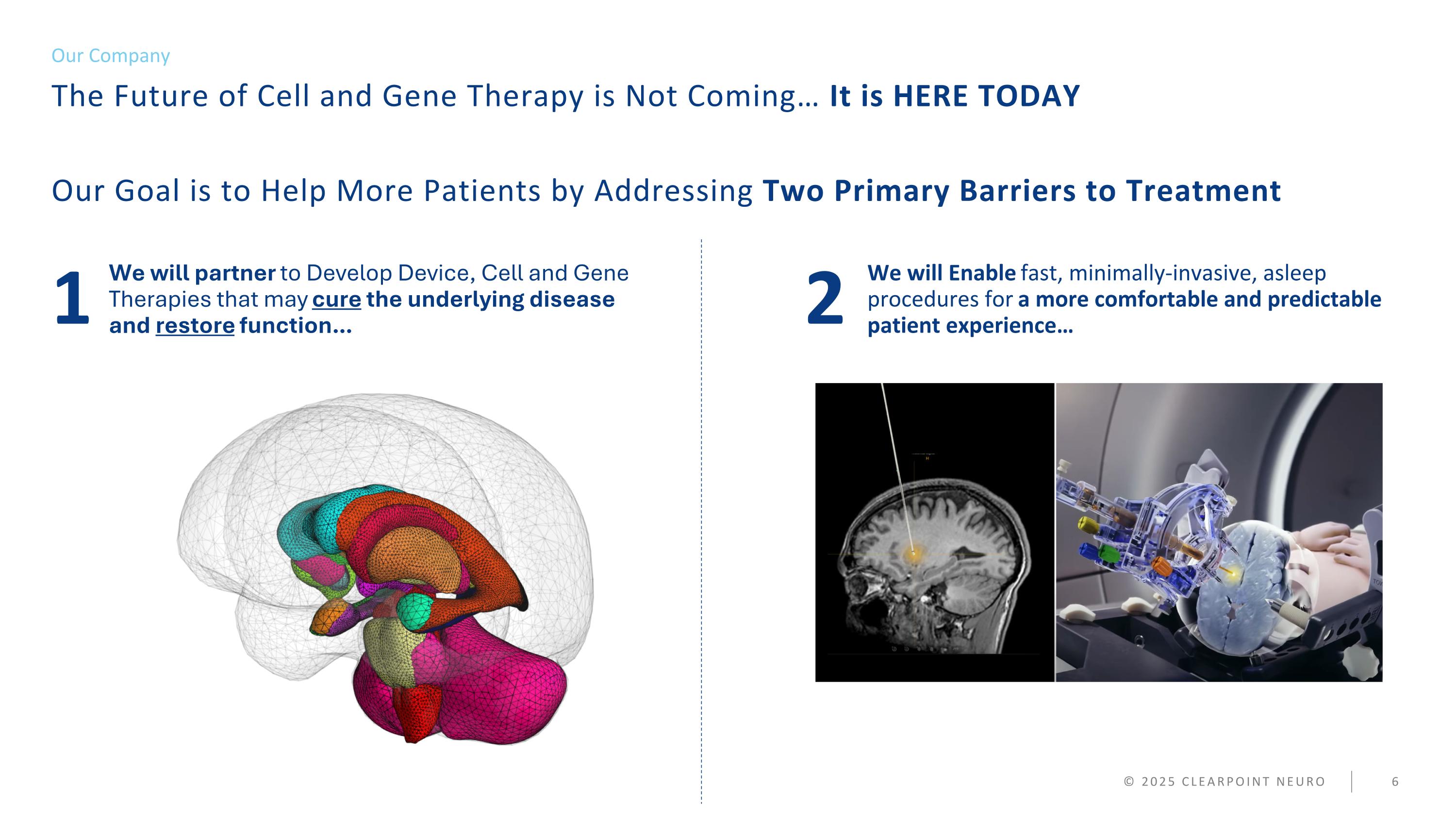
We will Enable fast, minimally-invasive, asleep procedures for a more comfortable and predictable patient experience… © 2025 CLEARPOINT NEURO We will partner to Develop Device, Cell and Gene Therapies that may cure the underlying disease and restore function… Our Goal is to Help More Patients by Addressing Two Primary Barriers to Treatment Our Company 1 2 The Future of Cell and Gene Therapy is Not Coming… It is HERE TODAY
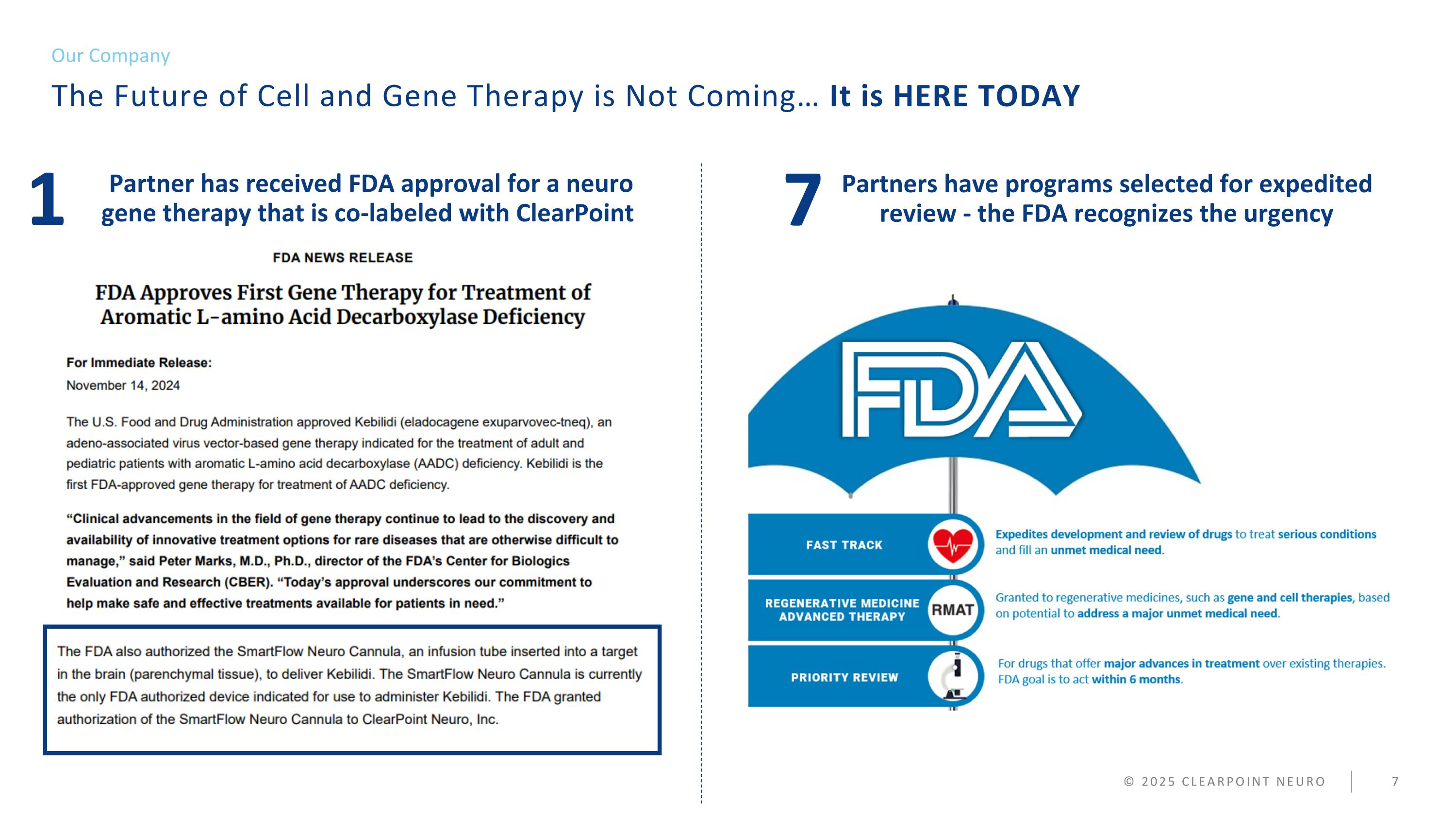
© 2025 CLEARPOINT NEURO Partners have programs selected for expedited review - the FDA recognizes the urgency The Future of Cell and Gene Therapy is Not Coming… It is HERE TODAY Our Company 7 Partner has received FDA approval for a neuro gene therapy that is co-labeled with ClearPoint 1
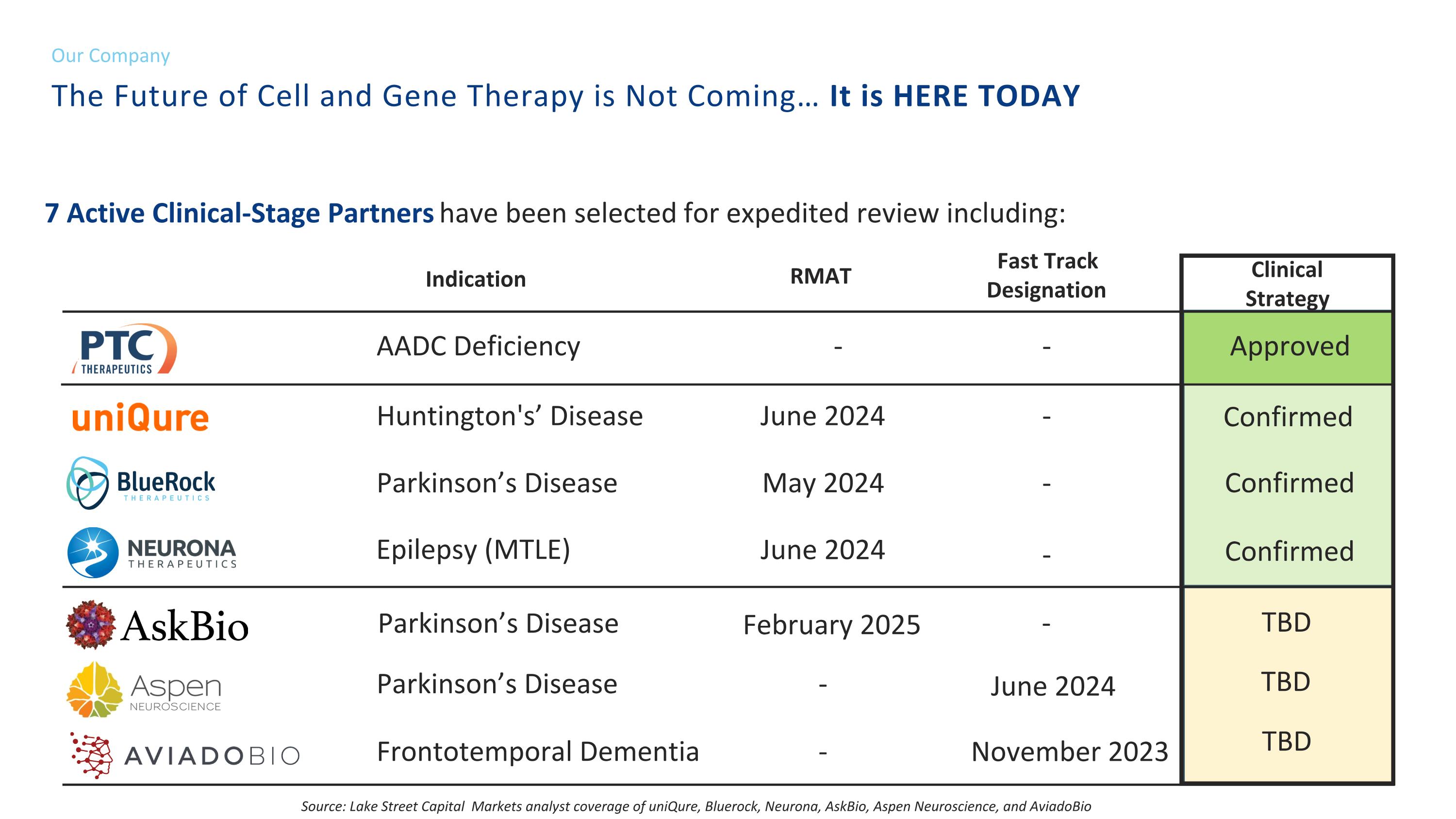
7 Active Clinical-Stage Partners have been selected for expedited review including: AADC Deficiency Huntington's’ Disease Parkinson’s Disease Epilepsy (MTLE) Parkinson’s Disease Parkinson’s Disease Frontotemporal Dementia RMAT - June 2024 May 2024 February 2025 June 2024 - - Indication Fast Track Designation - - - - June 2024 November 2023 Clinical Strategy Approved Confirmed Confirmed Confirmed Source: Lake Street Capital Markets analyst coverage of uniQure, Bluerock, Neurona, AskBio, Aspen Neuroscience, and AviadoBio - TBD TBD TBD The Future of Cell and Gene Therapy is Not Coming… It is HERE TODAY Our Company
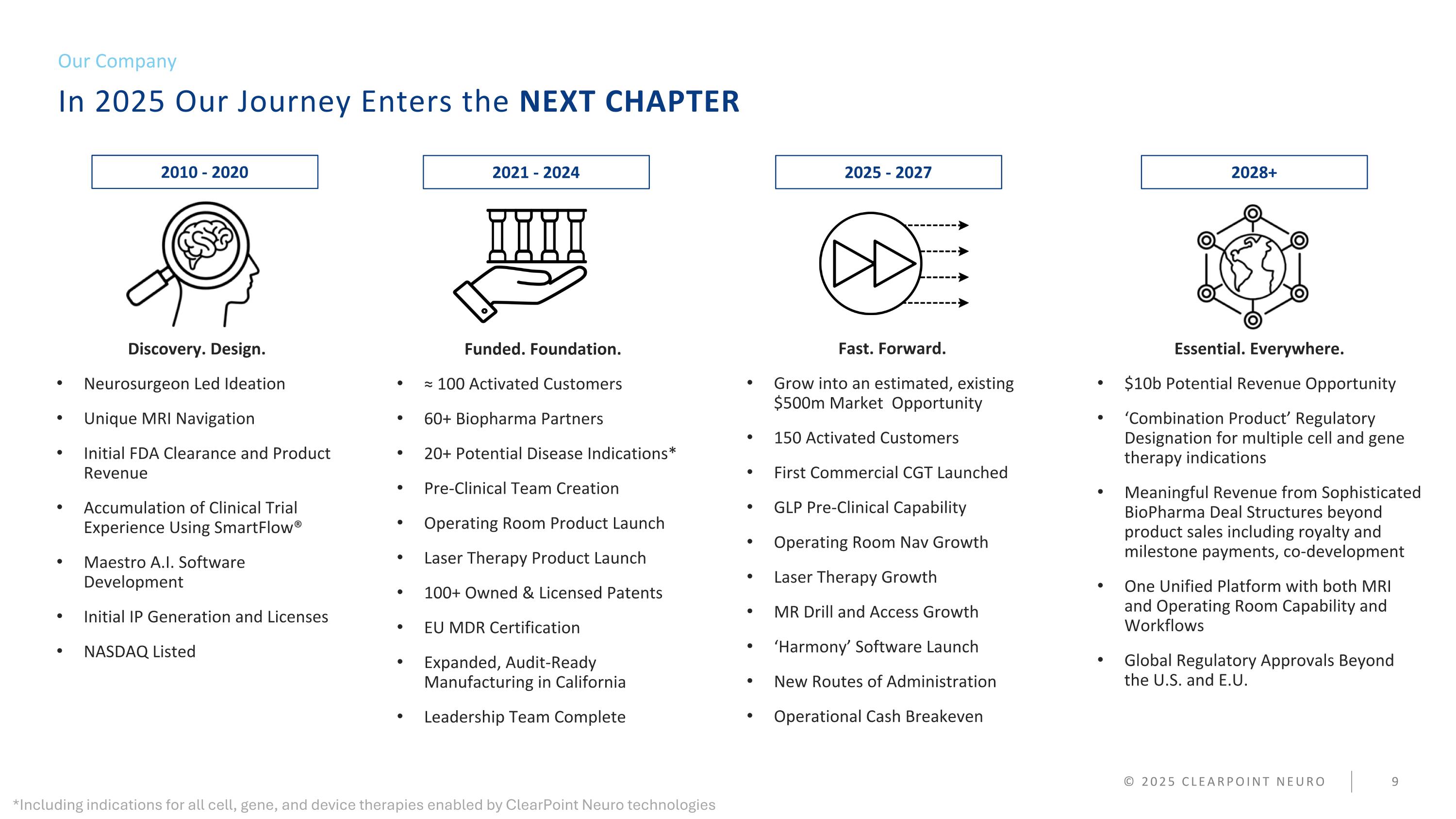
In 2025 Our Journey Enters the NEXT CHAPTER Our Company © 2025 CLEARPOINT NEURO Discovery. Design. Neurosurgeon Led Ideation Unique MRI Navigation Initial FDA Clearance and Product Revenue Accumulation of Clinical Trial Experience Using SmartFlow® Maestro A.I. Software Development Initial IP Generation and Licenses NASDAQ Listed Funded. Foundation. ≈ 100 Activated Customers 60+ Biopharma Partners 20+ Potential Disease Indications* Pre-Clinical Team Creation Operating Room Product Launch Laser Therapy Product Launch 100+ Owned & Licensed Patents EU MDR Certification Expanded, Audit-Ready Manufacturing in California Leadership Team Complete Fast. Forward. Grow into an estimated, existing $500m Market Opportunity 150 Activated Customers First Commercial CGT Launched GLP Pre-Clinical Capability Operating Room Nav Growth Laser Therapy Growth MR Drill and Access Growth ‘Harmony’ Software Launch New Routes of Administration Operational Cash Breakeven Essential. Everywhere. $10b Potential Revenue Opportunity ‘Combination Product’ Regulatory Designation for multiple cell and gene therapy indications Meaningful Revenue from Sophisticated BioPharma Deal Structures beyond product sales including royalty and milestone payments, co-development One Unified Platform with both MRI and Operating Room Capability and Workflows Global Regulatory Approvals Beyond the U.S. and E.U. 2010 - 2020 2021 - 2024 2025 - 2027 2028+ *Including indications for all cell, gene, and device therapies enabled by ClearPoint Neuro technologies
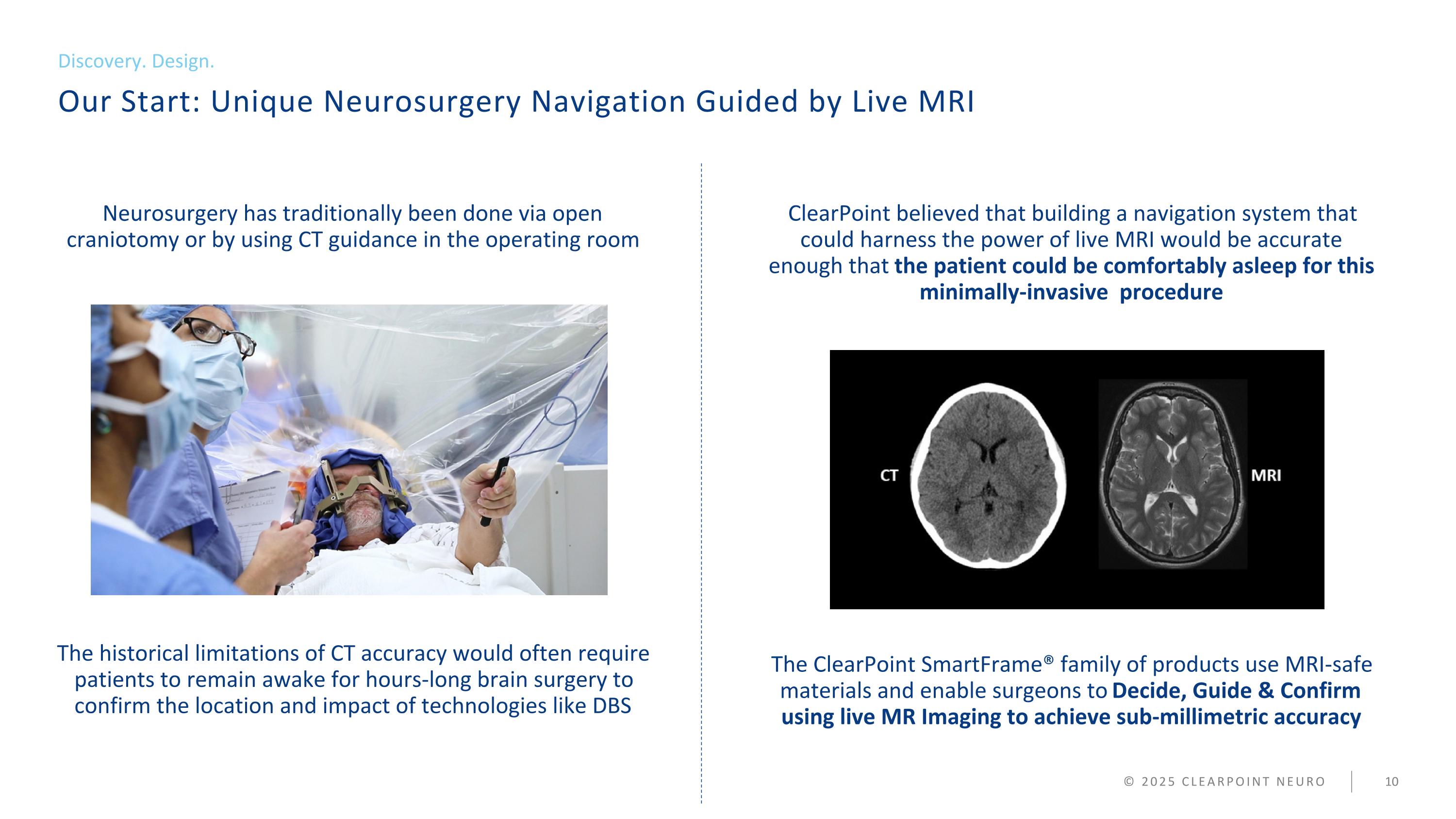
Our Start: Unique Neurosurgery Navigation Guided by Live MRI Discovery. Design. © 2025 CLEARPOINT NEURO Neurosurgery has traditionally been done via open craniotomy or by using CT guidance in the operating room The historical limitations of CT accuracy would often require patients to remain awake for hours-long brain surgery to confirm the location and impact of technologies like DBS ClearPoint believed that building a navigation system that could harness the power of live MRI would be accurate enough that the patient could be comfortably asleep for this minimally-invasive procedure The ClearPoint SmartFrame® family of products use MRI-safe materials and enable surgeons to Decide, Guide & Confirm using live MR Imaging to achieve sub-millimetric accuracy

Our Start: Decide, Guide & Confirm Discovery. Design. © 2025 CLEARPOINT NEURO Three Primary Use Cases demonstrated the value of the ClearPoint MRI Navigation System: Functional Neurosurgeons could confidently place DBS electrodes with the patient comfortably asleep Neuro-Oncologists could perform entire Tumor Laser Ablations in one room instead of having to transport the patient from the OR to the MRI BioPharma researchers could confirm that cell and gene therapies are not only delivered to a precise location, but could also confirm proper coverage of the target structure before closing the patient
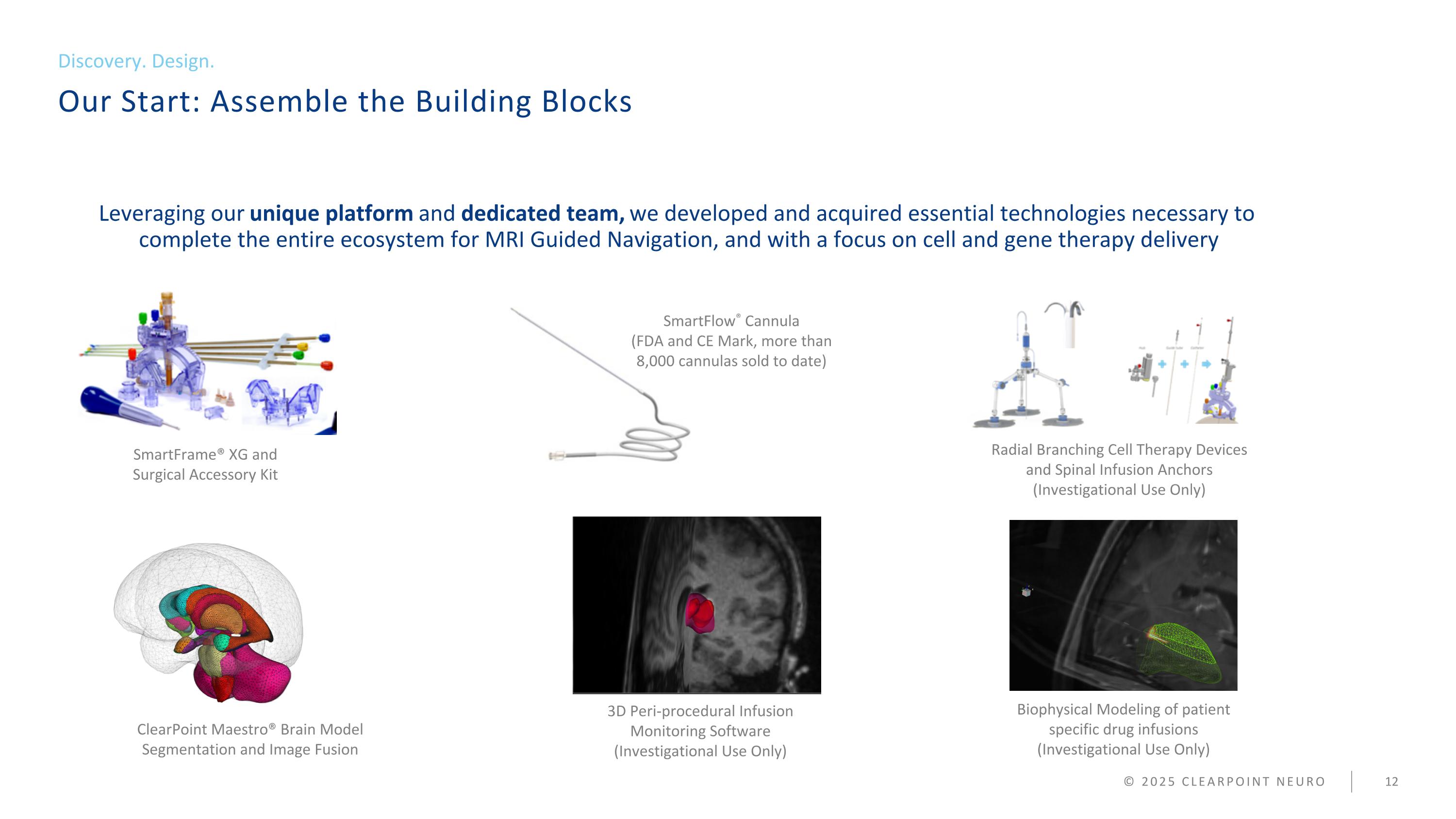
Our Start: Assemble the Building Blocks Discovery. Design. © 2025 CLEARPOINT NEURO Leveraging our unique platform and dedicated team, we developed and acquired essential technologies necessary to complete the entire ecosystem for MRI Guided Navigation, and with a focus on cell and gene therapy delivery SmartFlow® Cannula (FDA and CE Mark, more than 8,000 cannulas sold to date) Radial Branching Cell Therapy Devices and Spinal Infusion Anchors (Investigational Use Only) ClearPoint Maestro® Brain Model Segmentation and Image Fusion Biophysical Modeling of patient specific drug infusions (Investigational Use Only) SmartFrame® XG and Surgical Accessory Kit 3D Peri-procedural Infusion Monitoring Software (Investigational Use Only)
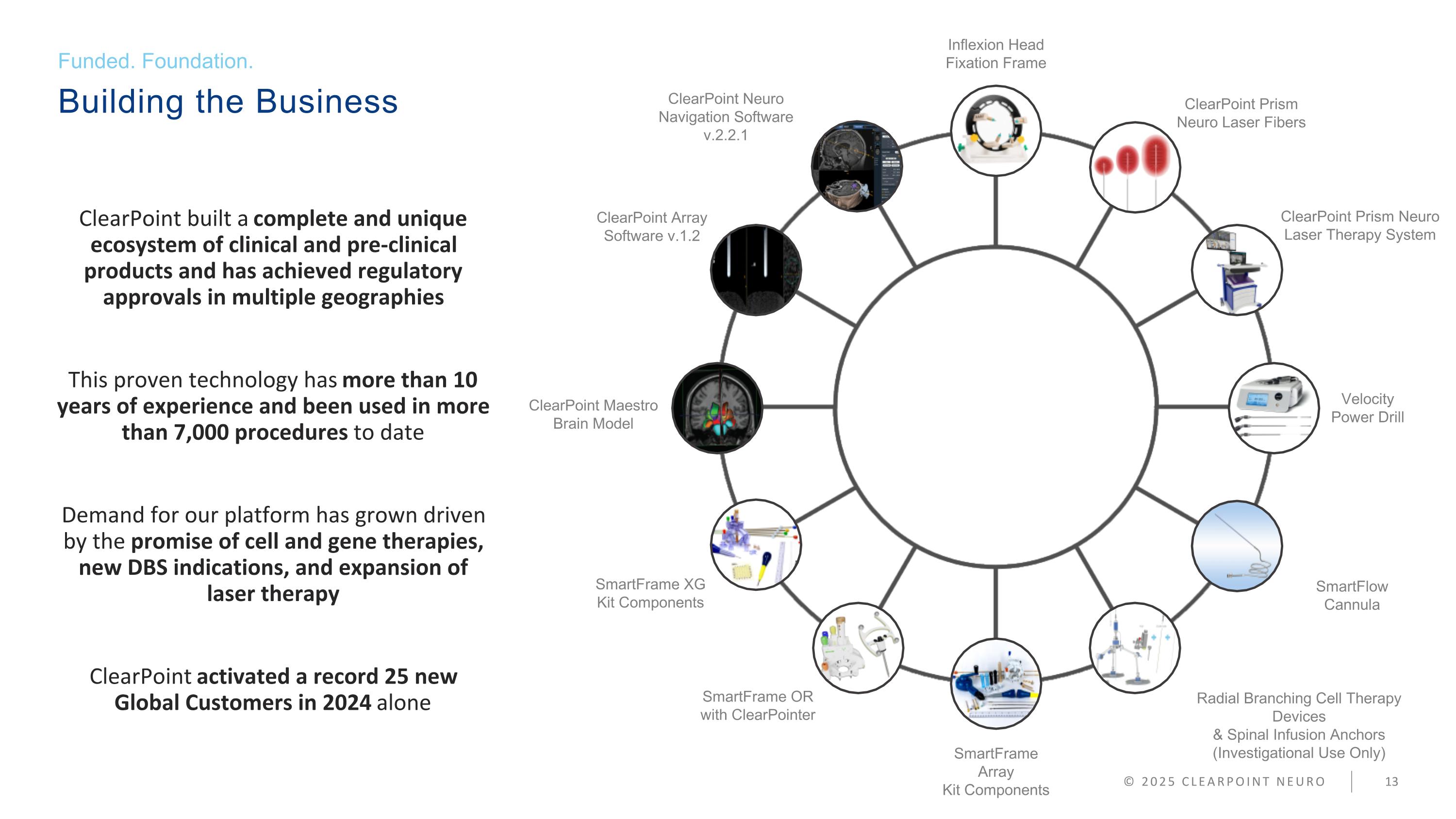
Building the Business Funded. Foundation. © 2025 CLEARPOINT NEURO ClearPoint Neuro Navigation Software v.2.2.1 ClearPoint Array Software v.1.2 ClearPoint Maestro Brain Model SmartFrame XG Kit Components SmartFrame OR with ClearPointer Inflexion Head Fixation Frame SmartFrame Array Kit Components ClearPoint Prism Neuro Laser Fibers ClearPoint Prism Neuro Laser Therapy System Velocity Power Drill SmartFlow Cannula Radial Branching Cell Therapy Devices & Spinal Infusion Anchors (Investigational Use Only) ClearPoint built a complete and unique ecosystem of clinical and pre-clinical products and has achieved regulatory approvals in multiple geographies This proven technology has more than 10 years of experience and been used in more than 7,000 procedures to date Demand for our platform has grown driven by the promise of cell and gene therapies, new DBS indications, and expansion of laser therapy ClearPoint activated a record 25 new Global Customers in 2024 alone

Building the Business: Our Four-Pillar Growth Strategy Remains Our Foundation Funded. Foundation. © 2025 CLEARPOINT NEURO BIOLOGICS & DRUG DELIVERY 1 NEURSURGERY NAVIGATION 2 VISUALASE LASER THERAPY & ACCESS 3 CLEARPOINT NAVIGATION IS COMPATIBLE WITH MAJOR DIAGNOSTIC AND INTRAOPERATIVE MRI AND CT SCANNERS GLOBAL SCALE 4 100+ GLOBAL CENTERS 60+ INDUSTRY & ACADEMIC PARTNERS

Banner Health Tucson Baptist Memorial Hospital-Memphis Barnes-Jewish Hospital Barrow Neurological Institute/St. Joseph's Hospital Benioff Children's Hospital Beth Israel Deaconess Boston Children's Hospital Brigham & Women's Hospital Brown University / Rhode Island Hospital Carilion Clinic Children's Hospital of Alabama Children’s Mercy Hospital Children's National Hospital CHOA Scottish Rite Cincinnati Children's Hospital Cincinnati Jewish Hospital Cleveland Clinic Hospital Cook Children's Hospital Corewell Health Dallas Presbyterian Hospital Dartmouth-Hitchcock Duke University Emory University Froedtert Hospital Hackensack University Medical Center Henry Ford Health Henry Ford West Bloomfield Hospital Hospital of University Pennsylvania Houston Methodist Hospital INOVA Fairfax JFK University Medical Center Johns Hopkins University Kaleida Health Kettering Health Loma Linda University Health Lucile Packard Children's Hospital Massachusetts General Hospital Mayo Clinic in Arizona Mayo Clinic in Florida MD Anderson Cancer Center MedStar Georgetown University Hospital Memorial Sloan-Kettering Cancer Center Methodist Hospital San Antonio Mt. Sinai West Nationwide Children's Northwestern Central DuPage Ochsner Medical Center Ohio State University Oregon Health & Science University Orlando Health Arnold Palmer Hospital for Children Prisma Health Riverside Methodist Hospital Rutgers/Robert Wood Johnson San Francisco VA Health Care System Southern Arizona VA Health Care System Stanford University Sunnyside Kaiser Permanente Tampa General Hospital Texas Children's Hospital University of Alabama at Birmingham University of California Los Angeles University of California San Diego University of California San Francisco University of Colorado University of Florida Jacksonville University of Kansas Medical Center University of Maryland Medical Center University of Michigan University of Minnesota University of North Carolina (UNC) Health University of Oklahoma Medical Center University of Utah University of Wisconsin USC Keck Hospital UT Southwestern Medical Center Wolfson Children’s Hospital Yale University Charité – Universitätsmedizin Berlin (Berlin, Germany) Fondazione I.R.C.C.S. Istituto Neurologico Carlo Besta (Milan, Italy) Great Ormond Street Hospital (London, UK) Hôpital Fondation Rothschild (Paris, France) Hospital Israelita Albert Einstein (São Paulo, Brazil) Hospital Santa Joana (Recife, Brazil) Mazowiecki Szpital Bródnowski (Warsaw, Poland) Meyer Children’s Hospital (Florence, Italy) Policlinico Umberto I (Rome, Italy) Rigshospitalet (Copenhagen, Denmark) Sahlgrenska Universitetssjukhuset (Gothenburg, Sweden) Skänes Universitetssjukhus Lund (Lund, Sweden) Santobono Children’s Hospital (Naples, Italy) Universitätsklinikum Tübingen (Tübingen, Germany) Universitätsklinikum Düsseldorf (Düsseldorf, Germany) Universitätsklinikum Freiburg (Freiburg, Germany) University Hospital of Wales (Cardiff, UK) Charles River Labs (Laval, Canada) Charles River Labs (Lyon, France) Charles River Labs (Mattawan, Michigan) Children's Hospital of Philadelphia Envol Biomedical (Florida) Labcorp (Madison, Wisconsin) Prisys Biotechnologies (Shanghai, China) TIDU GENOV - Institut du Cerveau (Paris, France) University of Pennsylvania Gene Therapy United States Centers International Centers Preclinical Centers 100+ GLOBAL CENTERS NOW ACTIVATED © 2025 CLEARPOINT NEURO

Building the Business Funded. Foundation. © 2025 CLEARPOINT NEURO We have invested in the Development, Quality and Supply infrastructure to build confidence for both Hospitals and BioPharma partners We are not a start-up company but an experienced and sophisticated medical device extension for any cell and gene therapy company ClearPoint assets available to our partners; HQ & Training Facility in Solana Beach Research Laboratory in San Diego Manufacturing Facility in Carlsbad ISO 13485 / MDSAP / EU MDR Certified QMS Significant and positive experience with BioPharma Audits, FDA and Global Notified Body inspections
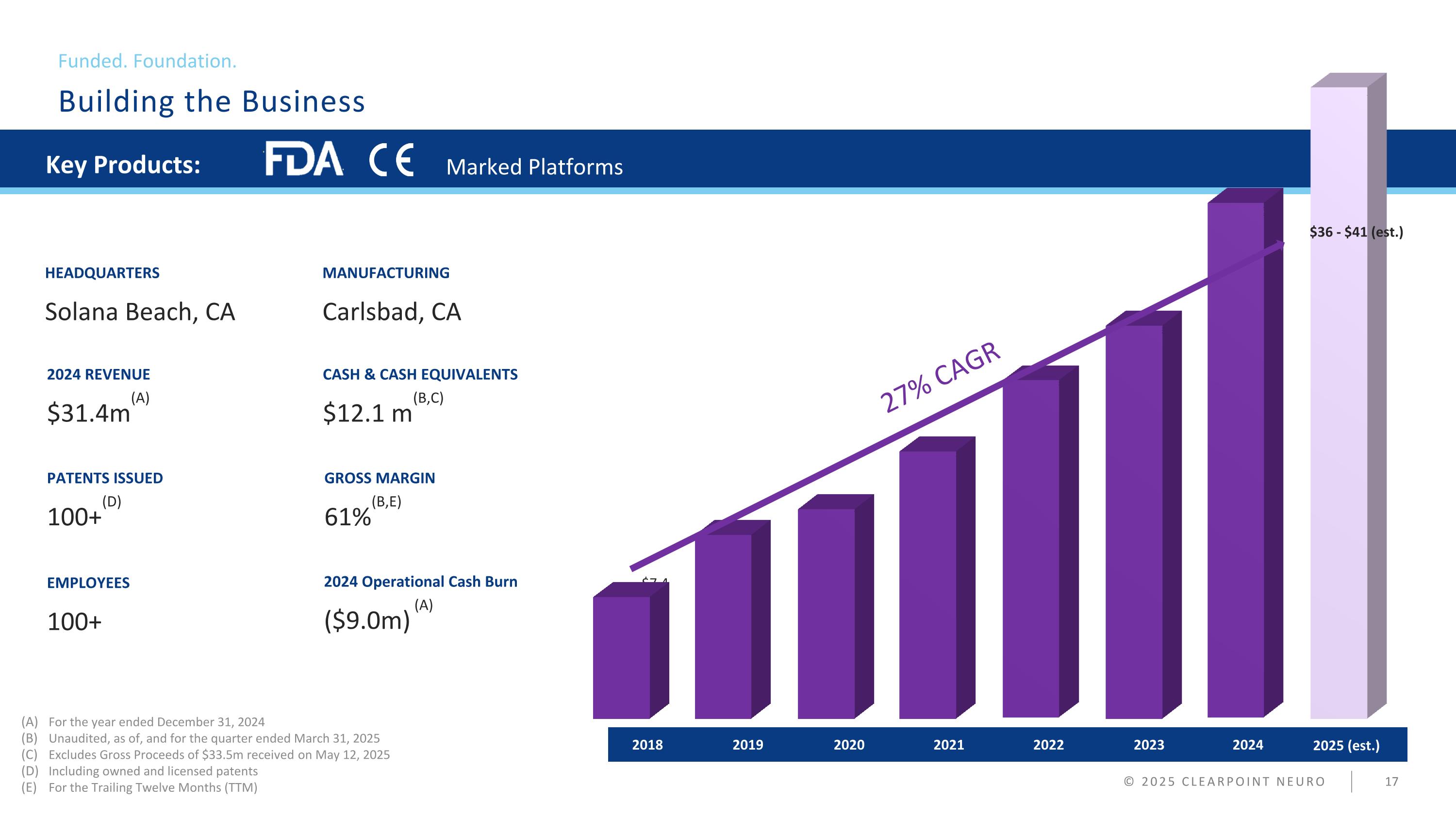
Building the Business Funded. Foundation. © 2025 CLEARPOINT NEURO Key Products: Marked Platforms EMPLOYEES 100+ 2024 REVENUE $31.4m(A) CASH & CASH EQUIVALENTS $12.1 m(B,C) HEADQUARTERS Solana Beach, CA GROSS MARGIN 61%(B,E) MANUFACTURING Carlsbad, CA For the year ended December 31, 2024 Unaudited, as of, and for the quarter ended March 31, 2025 Excludes Gross Proceeds of $33.5m received on May 12, 2025 Including owned and licensed patents For the Trailing Twelve Months (TTM) PATENTS ISSUED 100+(D) 2024 Operational Cash Burn ($9.0m) (A) Hostius polibu atus patiaedi, esct lorestimo conduces 2018 2019 2020 2021 2022 2023 2024 23.8% CAGR $7.4 $11.2 $12.8 $16.3 $20.6 $24.0 $31.4 27% CAGR 2025 (est.) $36 - $41 (est.)

Building the Business Funded. Foundation. © 2025 CLEARPOINT NEURO Joe Burnett President & Chief Executive Officer Danilo D’Alessandro Chief Financial Officer Jeremy Stigall Chief Business Officer Mazin Sabra Chief Operating Officer Ellisa Cholapranee General Counsel Megan Faulkenberry Vice President of Quality Mary McNamara-Cullinane Vice President of Regulatory Affairs Rob Korn Vice President U.S. Commercial Sales Lyubomir Zagorchev, PhD Vice President of Clinical Science & Applications Ernesto Salegio, PhD Vice President of Translational & Pre-Clinical Research EXECUTIVE LEADERSHIP TEAM Experienced leadership team with decades of leadership in medical devices, pharmaceuticals, and clinical research.
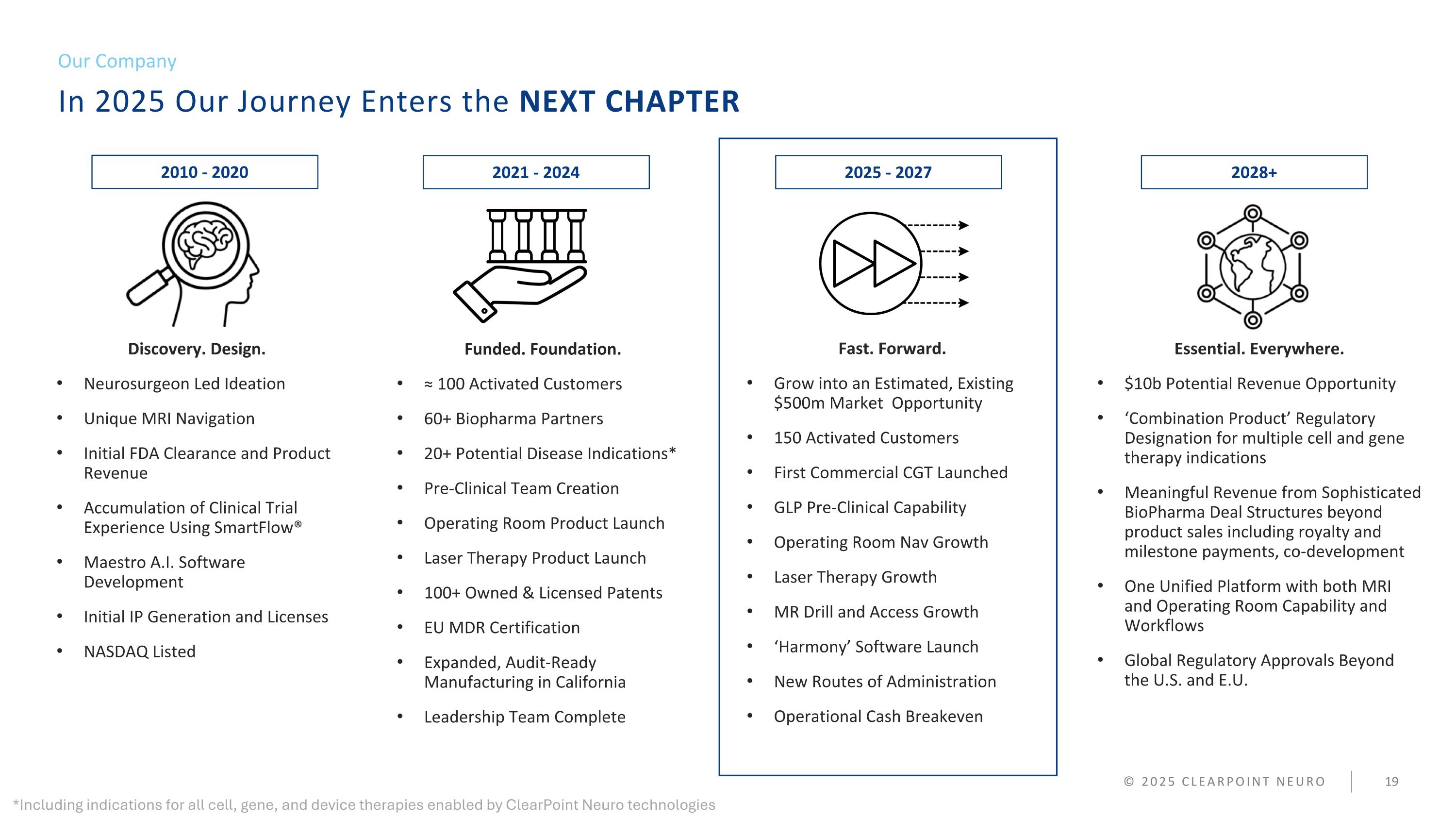
In 2025 Our Journey Enters the NEXT CHAPTER Our Company © 2025 CLEARPOINT NEURO Discovery. Design. Neurosurgeon Led Ideation Unique MRI Navigation Initial FDA Clearance and Product Revenue Accumulation of Clinical Trial Experience Using SmartFlow® Maestro A.I. Software Development Initial IP Generation and Licenses NASDAQ Listed Funded. Foundation. ≈ 100 Activated Customers 60+ Biopharma Partners 20+ Potential Disease Indications* Pre-Clinical Team Creation Operating Room Product Launch Laser Therapy Product Launch 100+ Owned & Licensed Patents EU MDR Certification Expanded, Audit-Ready Manufacturing in California Leadership Team Complete Fast. Forward. Grow into an Estimated, Existing $500m Market Opportunity 150 Activated Customers First Commercial CGT Launched GLP Pre-Clinical Capability Operating Room Nav Growth Laser Therapy Growth MR Drill and Access Growth ‘Harmony’ Software Launch New Routes of Administration Operational Cash Breakeven Essential. Everywhere. $10b Potential Revenue Opportunity ‘Combination Product’ Regulatory Designation for multiple cell and gene therapy indications Meaningful Revenue from Sophisticated BioPharma Deal Structures beyond product sales including royalty and milestone payments, co-development One Unified Platform with both MRI and Operating Room Capability and Workflows Global Regulatory Approvals Beyond the U.S. and E.U. 2010 - 2020 2021 - 2024 2025 - 2027 2028+ *Including indications for all cell, gene, and device therapies enabled by ClearPoint Neuro technologies
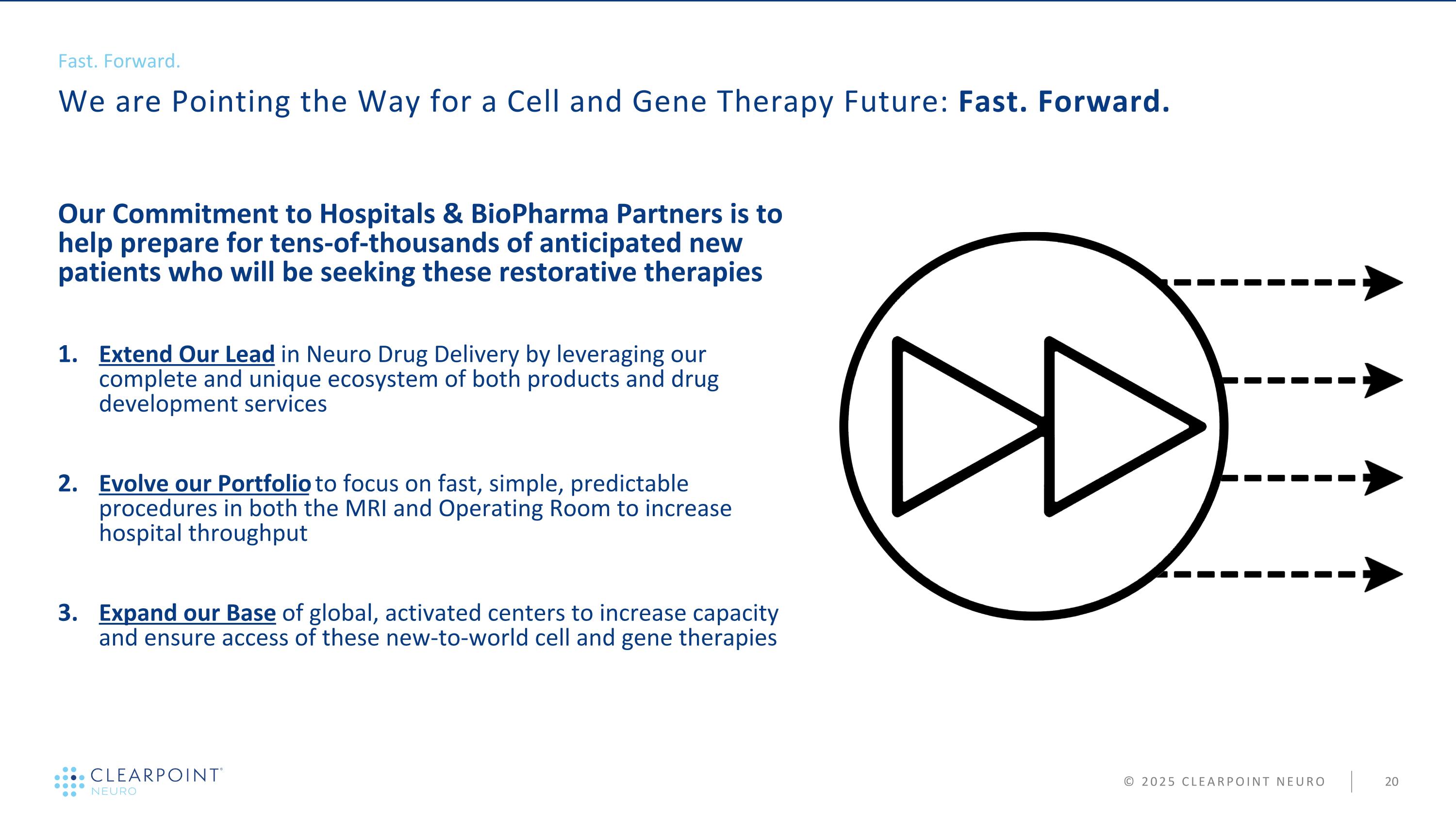
We are Pointing the Way for a Cell and Gene Therapy Future: Fast. Forward. Fast. Forward. Our Commitment to Hospitals & BioPharma Partners is to help prepare for tens-of-thousands of anticipated new patients who will be seeking these restorative therapies Extend Our Lead in Neuro Drug Delivery by leveraging our complete and unique ecosystem of both products and drug development services Evolve our Portfolio to focus on fast, simple, predictable procedures in both the MRI and Operating Room to increase hospital throughput Expand our Base of global, activated centers to increase capacity and ensure access of these new-to-world cell and gene therapies © 2025 CLEARPOINT NEURO
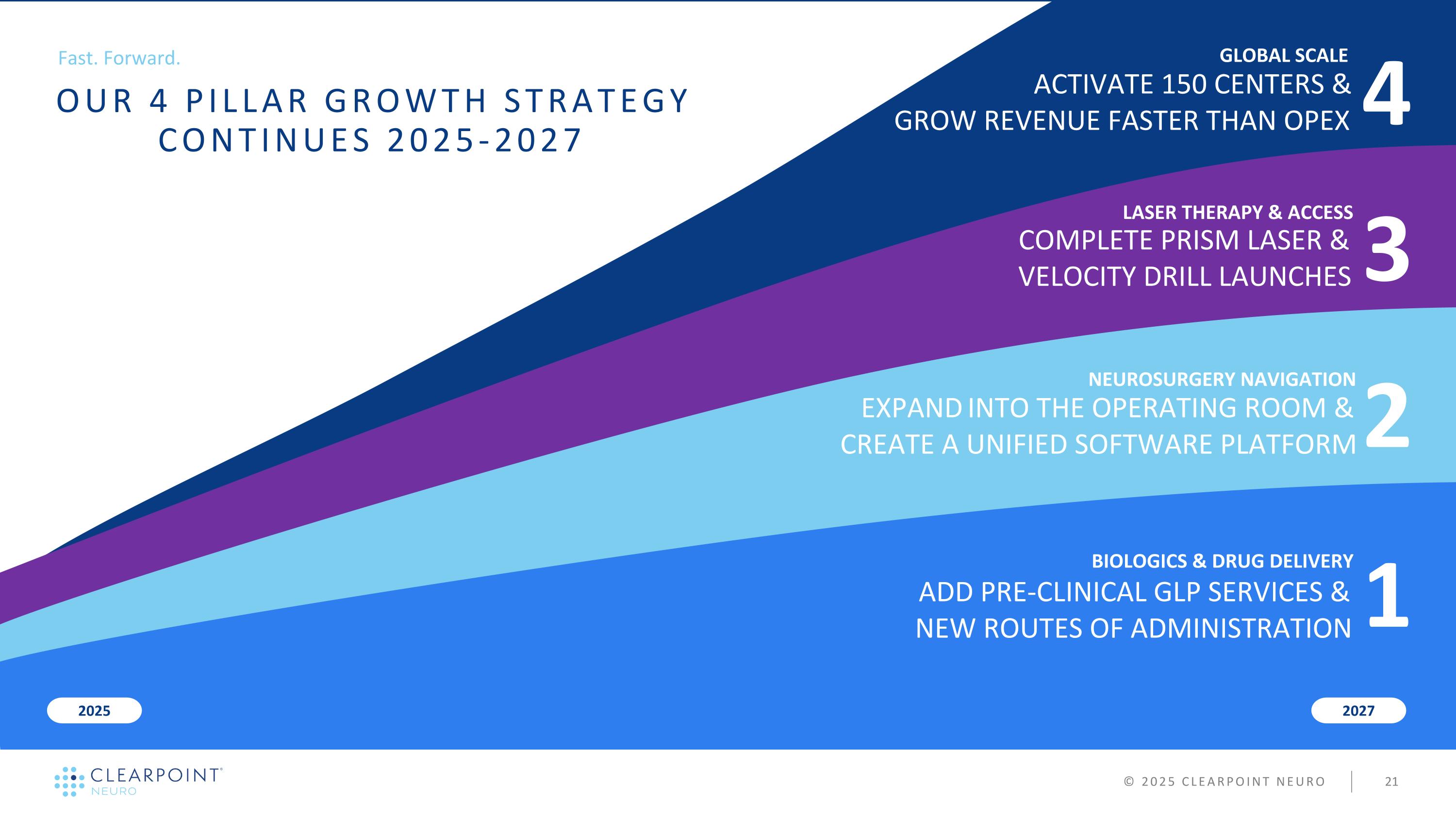
3 LASER THERAPY & ACCESS 2 NEUROSURGERY NAVIGATION BIOLOGICS & DRUG DELIVERY 2027 2025 COMPLETE PRISM LASER & VELOCITY DRILL LAUNCHES 4 GLOBAL SCALE ACTIVATE 150 CENTERS & GROW REVENUE FASTER THAN OPEX © 2025 CLEARPOINT NEURO 1 ADD PRE-CLINICAL GLP SERVICES & NEW ROUTES OF ADMINISTRATION EXPAND INTO THE OPERATING ROOM & CREATE A UNIFIED SOFTWARE PLATFORM Fast. Forward. OUR 4 PILLAR GROWTH STRATEGY CONTINUES 2025-2027
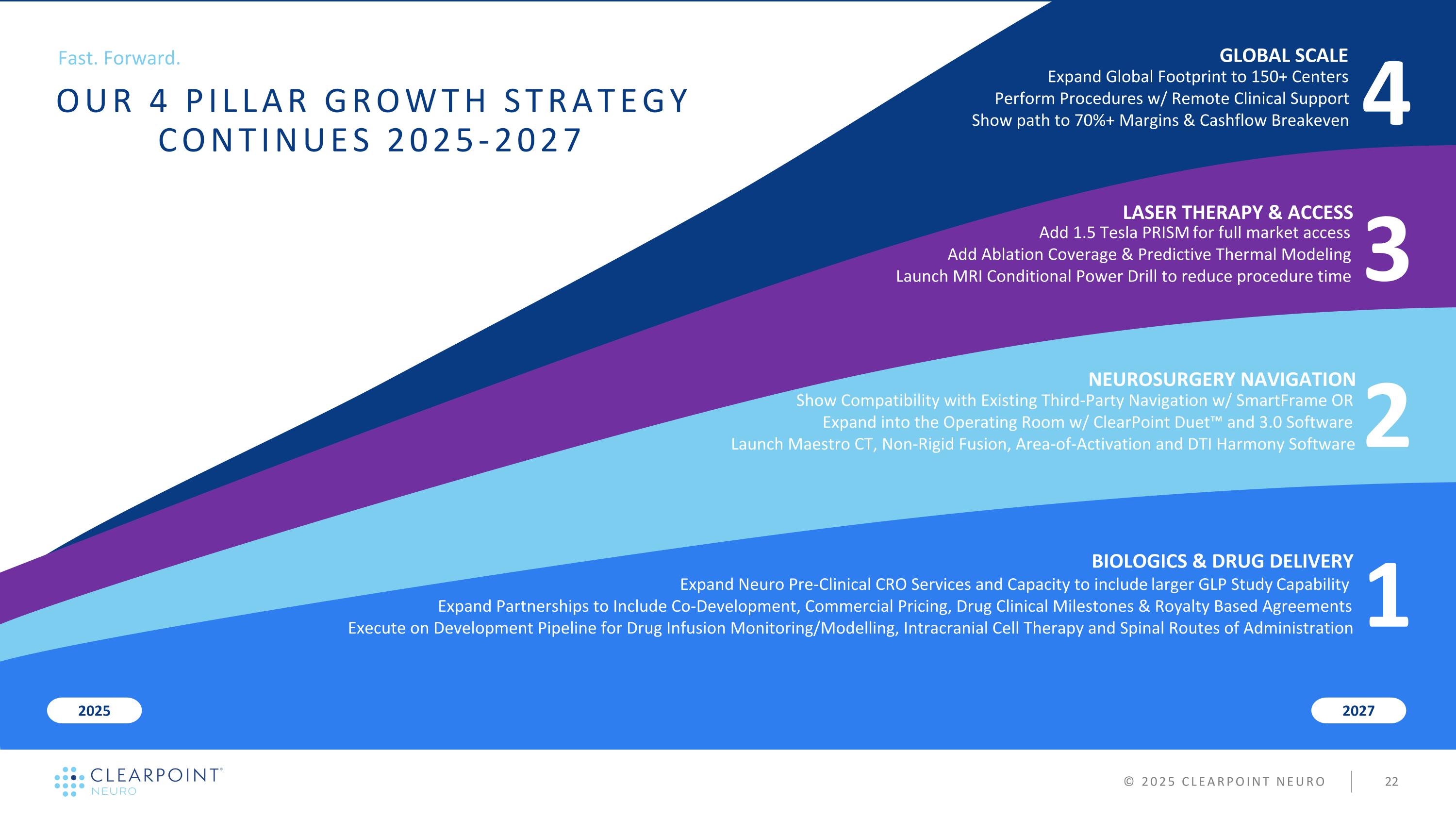
3 LASER THERAPY & ACCESS 2 NEUROSURGERY NAVIGATION BIOLOGICS & DRUG DELIVERY 2027 2025 Add 1.5 Tesla PRISM for full market access Add Ablation Coverage & Predictive Thermal Modeling Launch MRI Conditional Power Drill to reduce procedure time 4 GLOBAL SCALE Expand Global Footprint to 150+ Centers Perform Procedures w/ Remote Clinical Support Show path to 70%+ Margins & Cashflow Breakeven © 2025 CLEARPOINT NEURO 1 Expand Neuro Pre-Clinical CRO Services and Capacity to include larger GLP Study Capability Expand Partnerships to Include Co-Development, Commercial Pricing, Drug Clinical Milestones & Royalty Based Agreements Execute on Development Pipeline for Drug Infusion Monitoring/Modelling, Intracranial Cell Therapy and Spinal Routes of Administration Show Compatibility with Existing Third-Party Navigation w/ SmartFrame OR Expand into the Operating Room w/ ClearPoint Duet™ and 3.0 Software Launch Maestro CT, Non-Rigid Fusion, Area-of-Activation and DTI Harmony Software Fast. Forward. OUR 4 PILLAR GROWTH STRATEGY CONTINUES 2025-2027
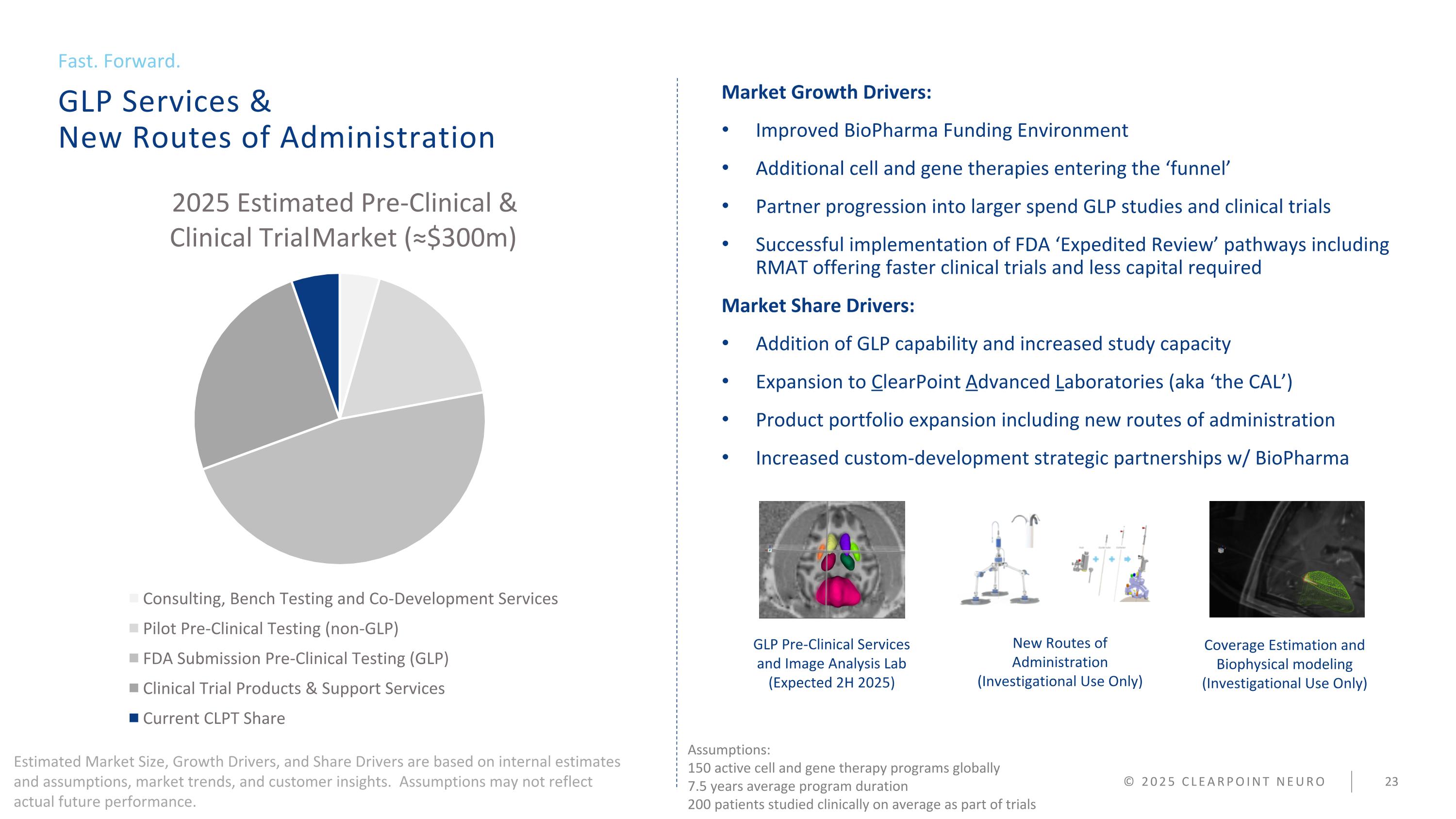
GLP Services & New Routes of Administration Fast. Forward. Market Growth Drivers: Improved BioPharma Funding Environment Additional cell and gene therapies entering the ‘funnel’ Partner progression into larger spend GLP studies and clinical trials Successful implementation of FDA ‘Expedited Review’ pathways including RMAT offering faster clinical trials and less capital required Market Share Drivers: Addition of GLP capability and increased study capacity Expansion to ClearPoint Advanced Laboratories (aka ‘the CAL’) Product portfolio expansion including new routes of administration Increased custom-development strategic partnerships w/ BioPharma © 2025 CLEARPOINT NEURO GLP Pre-Clinical Services and Image Analysis Lab (Expected 2H 2025) New Routes of Administration (Investigational Use Only) Coverage Estimation and Biophysical modeling (Investigational Use Only) Assumptions: 150 active cell and gene therapy programs globally 7.5 years average program duration 200 patients studied clinically on average as part of trials Estimated Market Size, Growth Drivers, and Share Drivers are based on internal estimates and assumptions, market trends, and customer insights. Assumptions may not reflect actual future performance.
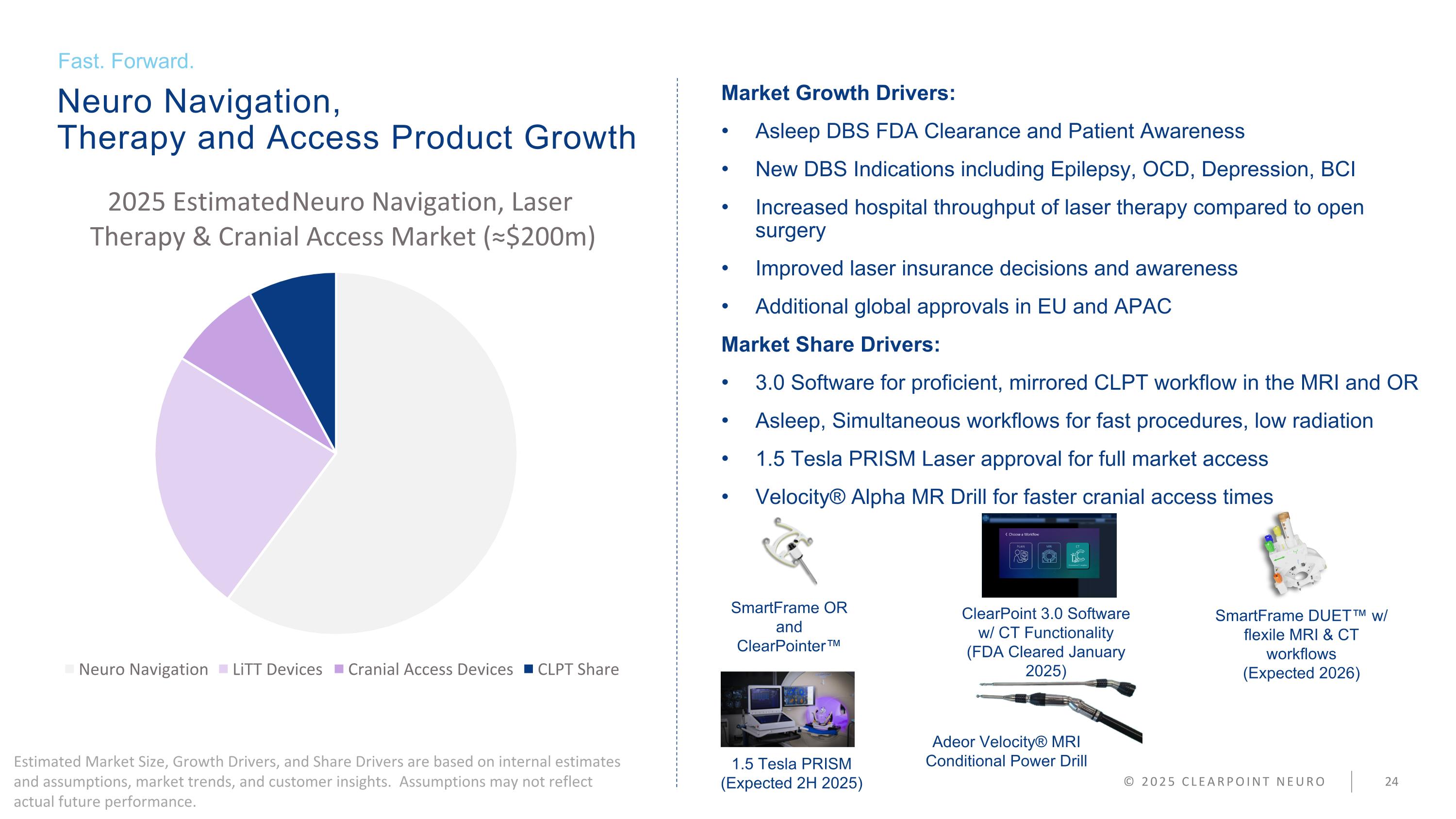
Market Growth Drivers: Asleep DBS FDA Clearance and Patient Awareness New DBS Indications including Epilepsy, OCD, Depression, BCI Increased hospital throughput of laser therapy compared to open surgery Improved laser insurance decisions and awareness Additional global approvals in EU and APAC Market Share Drivers: 3.0 Software for proficient, mirrored CLPT workflow in the MRI and OR Asleep, Simultaneous workflows for fast procedures, low radiation 1.5 Tesla PRISM Laser approval for full market access Velocity® Alpha MR Drill for faster cranial access times Neuro Navigation, Therapy and Access Product Growth Fast. Forward. © 2025 CLEARPOINT NEURO SmartFrame OR and ClearPointer™ ClearPoint 3.0 Software w/ CT Functionality (FDA Cleared January 2025) SmartFrame DUET™ w/ flexile MRI & CT workflows (Expected 2026) 1.5 Tesla PRISM (Expected 2H 2025) Adeor Velocity® MRI Conditional Power Drill Estimated Market Size, Growth Drivers, and Share Drivers are based on internal estimates and assumptions, market trends, and customer insights. Assumptions may not reflect actual future performance.
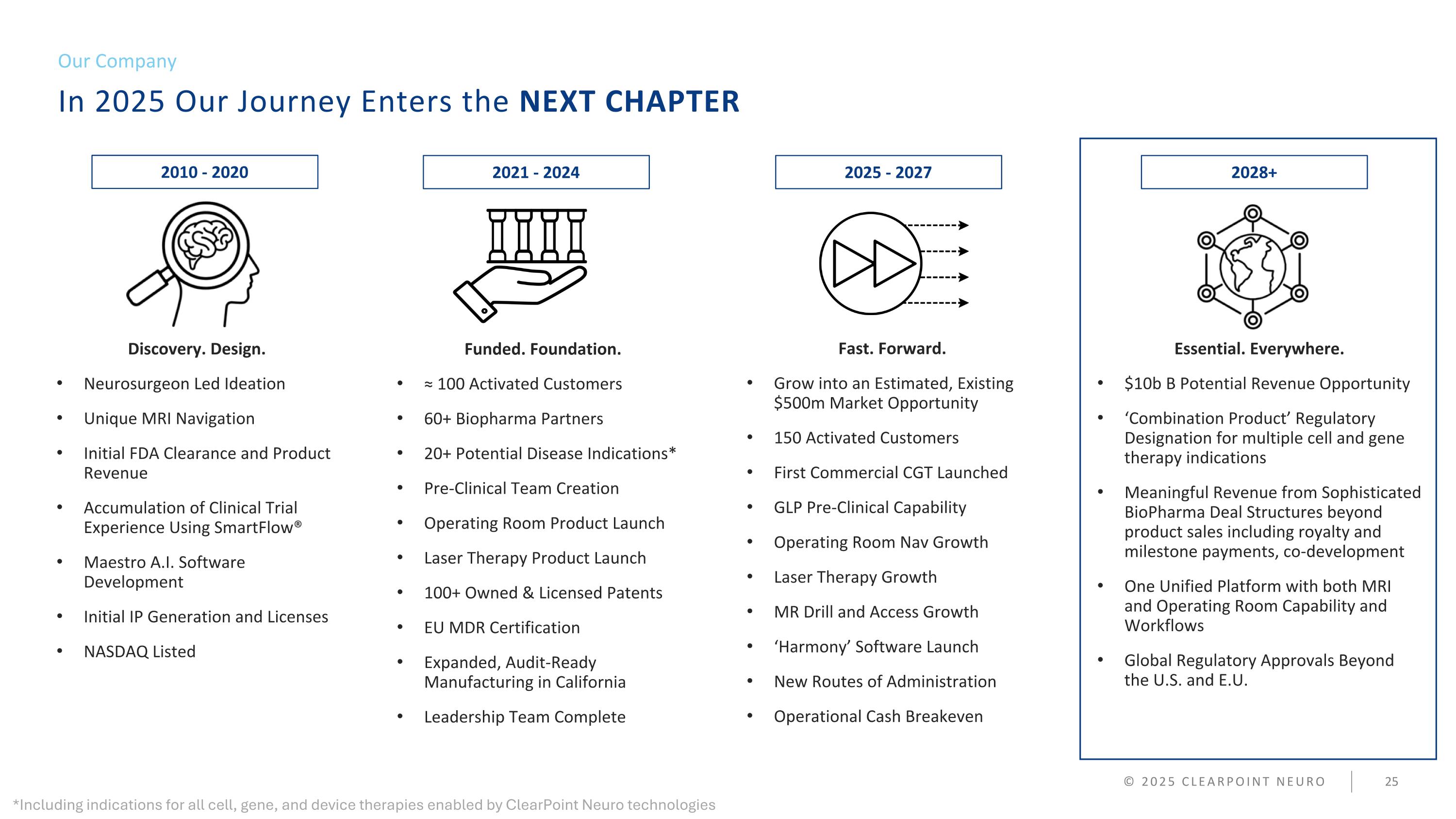
In 2025 Our Journey Enters the NEXT CHAPTER Our Company © 2025 CLEARPOINT NEURO Discovery. Design. Neurosurgeon Led Ideation Unique MRI Navigation Initial FDA Clearance and Product Revenue Accumulation of Clinical Trial Experience Using SmartFlow® Maestro A.I. Software Development Initial IP Generation and Licenses NASDAQ Listed Funded. Foundation. ≈ 100 Activated Customers 60+ Biopharma Partners 20+ Potential Disease Indications* Pre-Clinical Team Creation Operating Room Product Launch Laser Therapy Product Launch 100+ Owned & Licensed Patents EU MDR Certification Expanded, Audit-Ready Manufacturing in California Leadership Team Complete Fast. Forward. Grow into an Estimated, Existing $500m Market Opportunity 150 Activated Customers First Commercial CGT Launched GLP Pre-Clinical Capability Operating Room Nav Growth Laser Therapy Growth MR Drill and Access Growth ‘Harmony’ Software Launch New Routes of Administration Operational Cash Breakeven Essential. Everywhere. $10b B Potential Revenue Opportunity ‘Combination Product’ Regulatory Designation for multiple cell and gene therapy indications Meaningful Revenue from Sophisticated BioPharma Deal Structures beyond product sales including royalty and milestone payments, co-development One Unified Platform with both MRI and Operating Room Capability and Workflows Global Regulatory Approvals Beyond the U.S. and E.U. 2010 - 2020 2021 - 2024 2025 - 2027 2028+ *Including indications for all cell, gene, and device therapies enabled by ClearPoint Neuro technologies
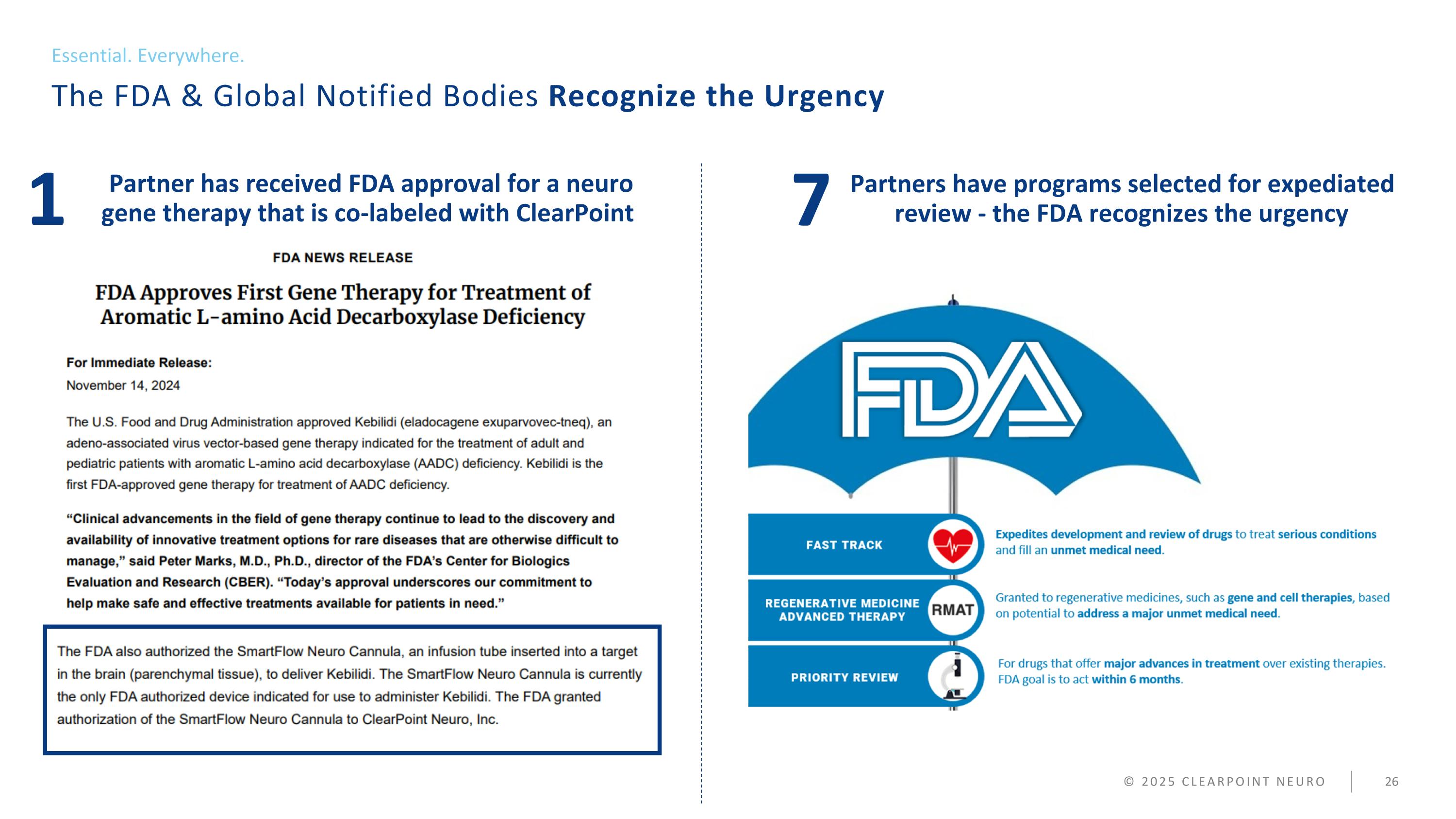
© 2025 CLEARPOINT NEURO Partners have programs selected for expediated review - the FDA recognizes the urgency The FDA & Global Notified Bodies Recognize the Urgency Essential. Everywhere. 7 Partner has received FDA approval for a neuro gene therapy that is co-labeled with ClearPoint 1
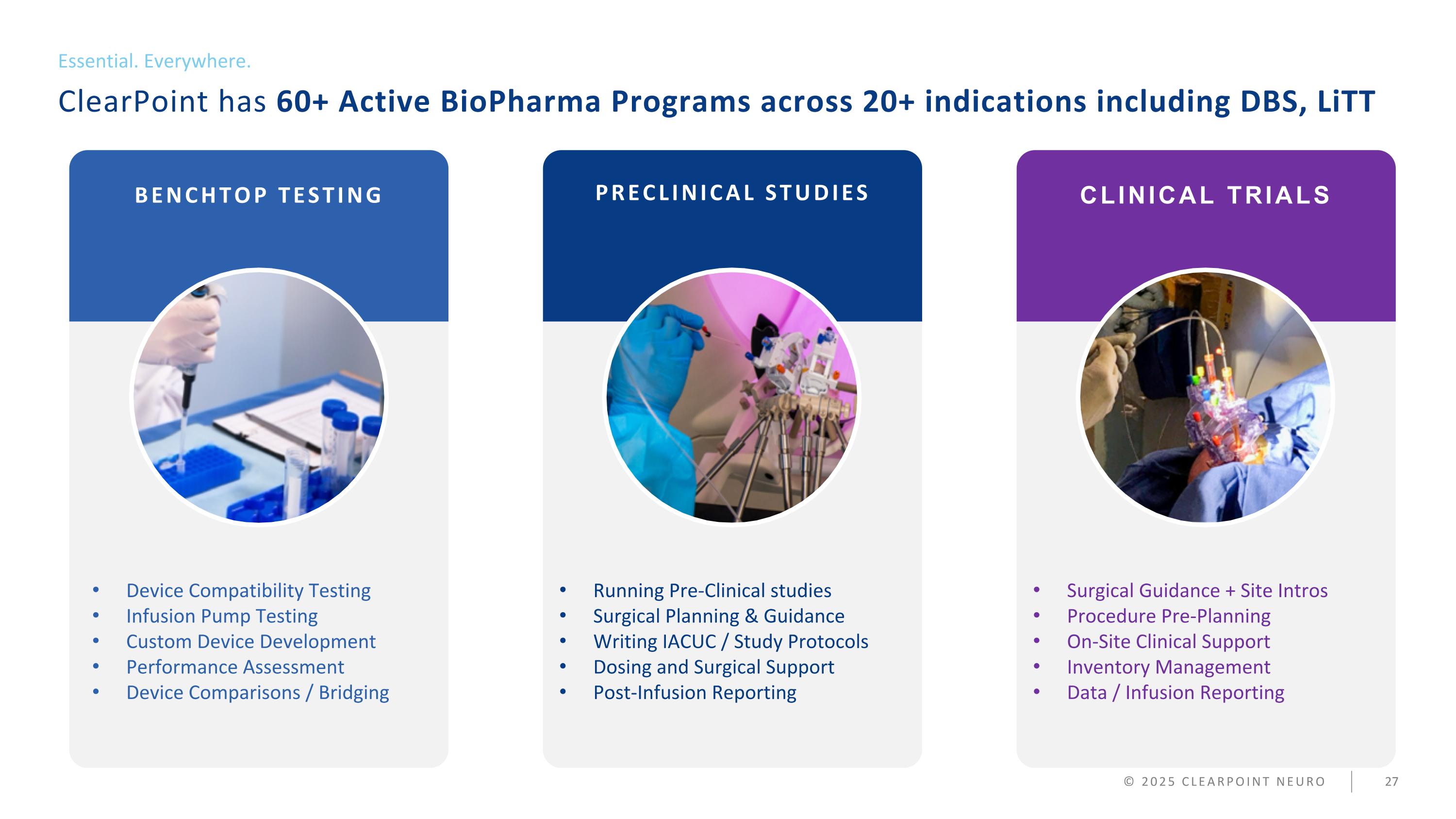
ClearPoint has 60+ Active BioPharma Programs across 20+ indications including DBS, LiTT Essential. Everywhere. © 2025 CLEARPOINT NEURO BENCHTOP TESTING PRECLINICAL STUDIES Device Compatibility Testing Infusion Pump Testing Custom Device Development Performance Assessment Device Comparisons / Bridging Running Pre-Clinical studies Surgical Planning & Guidance Writing IACUC / Study Protocols Dosing and Surgical Support Post-Infusion Reporting Surgical Guidance + Site Intros Procedure Pre-Planning On-Site Clinical Support Inventory Management Data / Infusion Reporting CLINICAL TRIALS
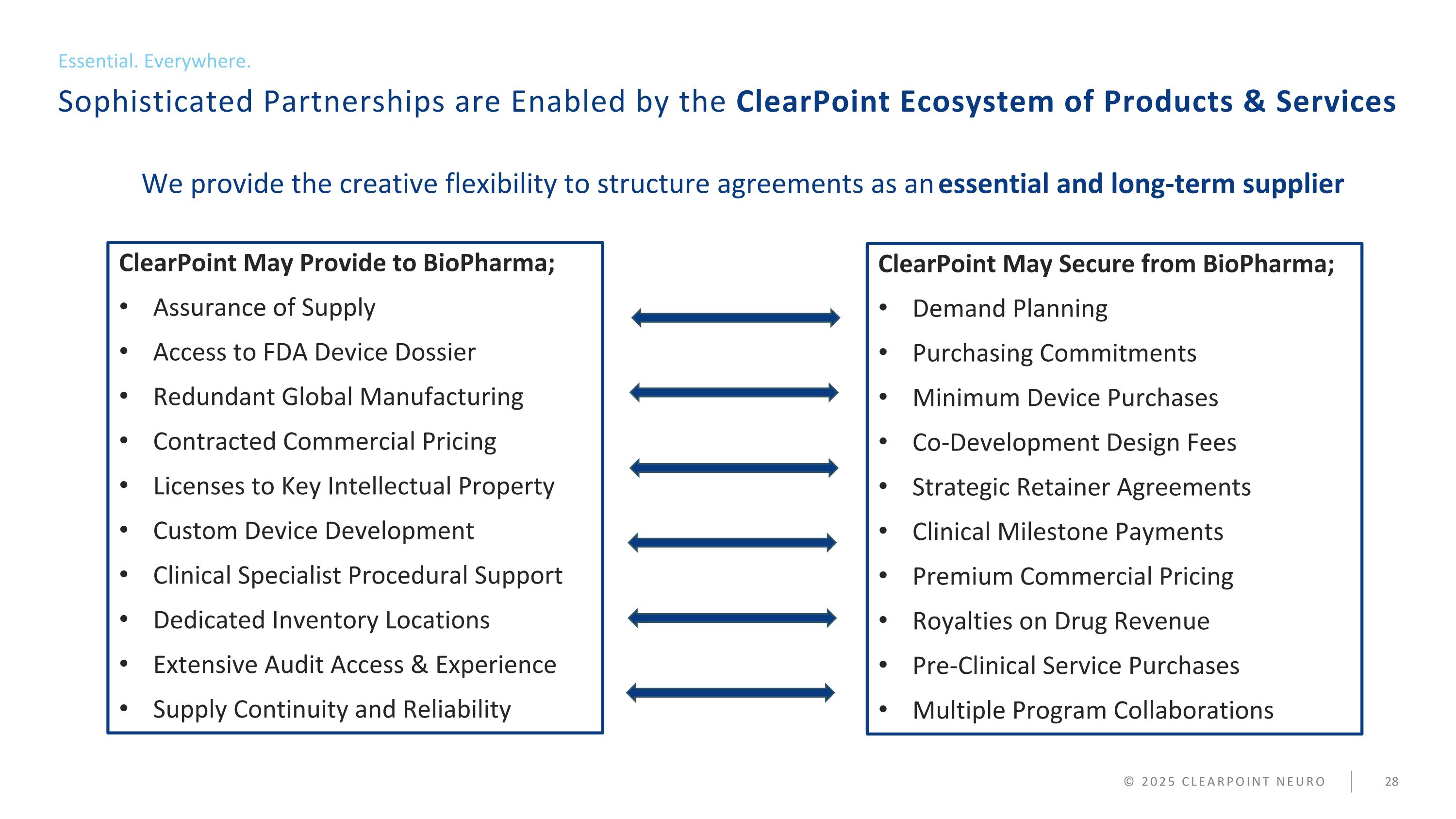
Sophisticated Partnerships are Enabled by the ClearPoint Ecosystem of Products & Services Essential. Everywhere. © 2025 CLEARPOINT NEURO ClearPoint May Provide to BioPharma; Assurance of Supply Access to FDA Device Dossier Redundant Global Manufacturing Contracted Commercial Pricing Licenses to Key Intellectual Property Custom Device Development Clinical Specialist Procedural Support Dedicated Inventory Locations Extensive Audit Access & Experience Supply Continuity and Reliability We provide the creative flexibility to structure agreements as an essential and long-term supplier ClearPoint May Secure from BioPharma; Demand Planning Purchasing Commitments Minimum Device Purchases Co-Development Design Fees Strategic Retainer Agreements Clinical Milestone Payments Premium Commercial Pricing Royalties on Drug Revenue Pre-Clinical Service Purchases Multiple Program Collaborations

More than 30 Million People in the U.S. alone suffer from severe and debilitating neurological disorders; Parkinson’s Disease (1.0m) Essential Tremor (7.0m) Epilepsy (2.9m) Huntington’s Disease (41k) Rare Childhood Genetic Disorders (25k) Dementia and Alzheimer’s Disease (6.9m) Tumor and Glioblastoma (280k) Severe OCD (1.0m) Treatment Resistant Depression (2.8m) ALS and Spinal Cord Injury (300k) Stroke Rehabilitation (7.0m) Neuropathic Pain (2.0m) ClearPoint is Diversified Across; 60+ BioPharma Partners 20+ Indications including device, cell and gene therapies Redundant Partners for multiple indications Many Partners with multiple programs Additional Device treatments including DBS, Laser, BCI Many ‘Shots on Goal’ with a path to operational cash breakeven; If just 1% of patients with diseases under expedited review are treated each year, at current ASP’s that would yield more than $250m in additional CLPT revenue © 2025 CLEARPOINT NEURO CLPT is like a Portfolio of BioPharma without drug development costs or binary outcomes Essential. Everywhere.
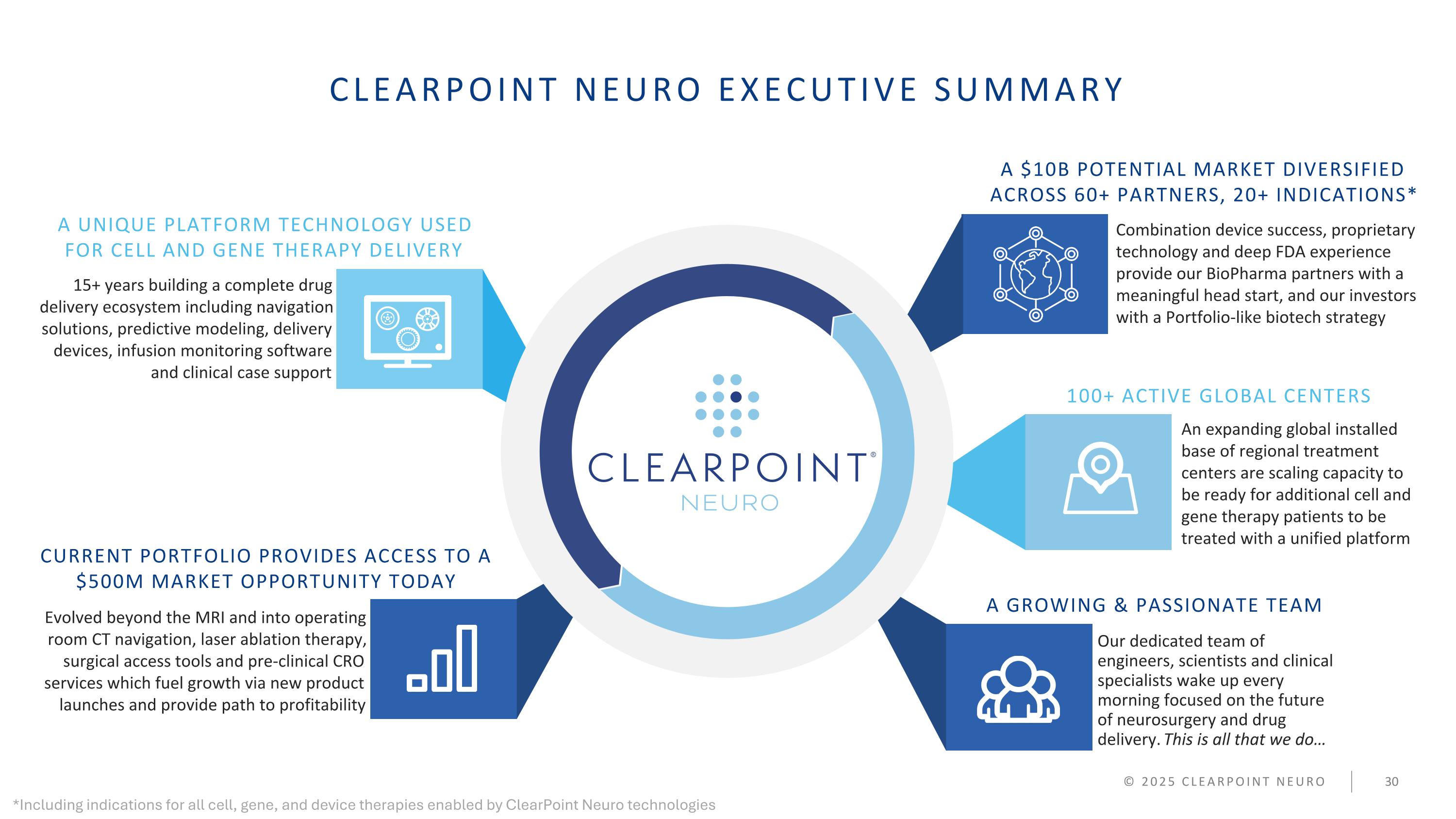
CLEARPOINT NEURO EXECUTIVE SUMMARY 15+ years building a complete drug delivery ecosystem including navigation solutions, predictive modeling, delivery devices, infusion monitoring software and clinical case support Combination device success, proprietary technology and deep FDA experience provide our BioPharma partners with a meaningful head start, and our investors with a Portfolio-like biotech strategy An expanding global installed base of regional treatment centers are scaling capacity to be ready for additional cell and gene therapy patients to be treated with a unified platform Evolved beyond the MRI and into operating room CT navigation, laser ablation therapy, surgical access tools and pre-clinical CRO services which fuel growth via new product launches and provide path to profitability Our dedicated team of engineers, scientists and clinical specialists wake up every morning focused on the future of neurosurgery and drug delivery. This is all that we do… A UNIQUE PLATFORM TECHNOLOGY USED FOR CELL AND GENE THERAPY DELIVERY CURRENT PORTFOLIO PROVIDES ACCESS TO A $500M MARKET OPPORTUNITY TODAY 100+ ACTIVE GLOBAL CENTERS A GROWING & PASSIONATE TEAM © 2025 CLEARPOINT NEURO A $10B POTENTIAL MARKET DIVERSIFIED ACROSS 60+ PARTNERS, 20+ INDICATIONS* *Including indications for all cell, gene, and device therapies enabled by ClearPoint Neuro technologies

Sources © 2025 CLEARPOINT NEURO Sources Alzheimer's Association 2024 Alzheimer's Disease Facts and Figures How Many People in the USA Have Essential Tremor? Deriving a Population Estimate Based on Epidemiological Data - PMC Parkinson's Disease: Challenges, Progress, and Promise | National Institute of Neurological Disorders and Stroke Epilepsy Facts and Stats | Epilepsy | CDC Prevalence of Huntington’s Disease in the US (954) | Neurology What is Friedreich's ataxia? - Friedreich's Ataxia Research Alliance Angelman syndrome | About the Disease | GARD Brain Tumor Facts https://pmc.ncbi.nlm.nih.gov/articles/PMC3250269/#Abs1 Amyotrophic lateral sclerosis estimated prevalence cases from 2022 to 2030, data from the National ALS Registry | National ALS Registry | CDC Spinal Cord Injury Prevalence In The U.S. | Reeve Foundation Abbott Initiates Clinical Study to Evaluate the Use of Its Deep Brain Stimulation System to Manage Severe Depression - Sep 4, 2024 The prevalence of neuropathic pain: Clinical evaluation compared with screening tools in a community population - PMC Deep brain stimulation for obsessive-compulsive disorder: A systematic review of worldwide experience after 20 years - PMC How common is OCD? Burden of Neurological Disorders Across the US From 1990-2017: A Global Burden of Disease Study | Dementia and Cognitive Impairment | JAMA Neurology | JAMA Network































