Q1false--12-310001645460http://fasb.org/us-gaap/2024#LicenseMemberhttp://fasb.org/us-gaap/2024#LicenseMemberhttp://fasb.org/us-gaap/2024#LicenseMember0001645460cue:SiliconValleyBankMembercue:TrancheALoanMember2025-01-012025-03-3100016454602025-05-080001645460srt:MinimumMember2024-01-012024-03-310001645460cue:TwoThousandTwentyTwoPreFundedWarrantsMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2022-11-160001645460us-gaap:StockOptionMember2025-01-012025-03-310001645460us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001645460us-gaap:LeaseholdImprovementsMember2025-03-3100016454602023-12-310001645460cue:OppenheimerCoIncMembercue:TwoThousandTwentyFourCommonStockWarrantsMember2024-12-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMemberus-gaap:WarrantMember2024-09-262024-09-260001645460us-gaap:GeneralAndAdministrativeExpenseMember2024-01-012024-03-310001645460cue:EinsteinLicenseAgreementMember2025-01-012025-03-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMemberus-gaap:CommonStockMember2024-09-260001645460us-gaap:RetainedEarningsMember2025-01-012025-03-310001645460cue:OppenheimerCoIncMembercue:TwoThousandTwentyFourCommonStockWarrantsAndTwoThousandTwentyFourPre-FundedWarrantsMember2024-09-262024-09-260001645460cue:TwoThousandTwentyFourPre-FundedWarrantsMember2025-01-012025-03-310001645460cue:OppenheimerCoIncMembercue:TwoThousandTwentyFourCommonStockWarrantsMember2024-09-262024-09-260001645460us-gaap:FurnitureAndFixturesMember2024-12-310001645460us-gaap:CommonStockMember2024-03-3100016454602024-01-012024-03-310001645460cue:Agreement2Membercue:TransactionExpenseMember2025-01-012025-03-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMemberus-gaap:WarrantMember2024-09-260001645460us-gaap:SubsequentEventMember2025-04-140001645460cue:TwoThousandTwentyTwoPreFundedWarrantsMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2025-01-012025-03-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMembercue:TwoThousandTwentyFourPreFundedWarrantsMember2024-09-260001645460cue:OppenheimerCoIncMembercue:TwoThousandTwentyFourPre-FundedWarrantsMembersrt:MaximumMember2024-12-310001645460us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001645460us-gaap:StockOptionMembersrt:MaximumMember2025-01-012025-03-310001645460srt:MinimumMemberus-gaap:FurnitureAndFixturesMember2025-03-310001645460cue:OppenheimerCoIncMember2024-09-260001645460cue:OnoCollaborationAndOptionAgreementMember2025-01-012025-03-310001645460us-gaap:FairValueMeasurementsRecurringMember2024-12-310001645460cue:SiliconValleyBankMembercue:TermLoanAgreementMember2022-02-150001645460cue:JefferiesLlcMembercue:TransactionExpenseMember2025-03-310001645460cue:PreFundedWarrantsMemberus-gaap:SubsequentEventMember2025-04-140001645460cue:CommonStockOptionsMember2024-01-012024-03-310001645460us-gaap:SubsequentEventMembercue:UnderwritingAgreementMembercue:CommonStockWarrantsMember2025-04-1400016454602024-12-310001645460cue:OnoCollaborationAndOptionAgreementMember2024-12-310001645460us-gaap:AdditionalPaidInCapitalMember2024-03-310001645460us-gaap:CertificatesOfDepositMember2024-12-310001645460cue:LGChemLifeSciencesMembercue:CollaborationAgreementWithLGChemLifeSciencesMember2025-01-012025-03-310001645460us-gaap:SubsequentEventMembercue:CommonStockWarrantsMember2025-04-140001645460us-gaap:RetainedEarningsMember2024-12-310001645460cue:Agreement2Membercue:JefferiesLlcMembercue:TransactionExpenseMember2024-03-3100016454602024-03-310001645460srt:MaximumMember2024-01-012024-03-310001645460cue:JefferiesLlcMembersrt:MaximumMember2025-01-012025-03-310001645460us-gaap:AdditionalPaidInCapitalMember2024-01-012024-03-310001645460us-gaap:SubsequentEventMember2025-04-142025-04-140001645460us-gaap:FurnitureAndFixturesMembersrt:MaximumMember2025-03-310001645460srt:MinimumMember2025-01-012025-03-310001645460cue:SiliconValleyBankMembercue:TermLoanAgreementMember2025-03-310001645460us-gaap:FairValueMeasurementsRecurringMember2025-03-310001645460us-gaap:AdditionalPaidInCapitalMember2025-03-310001645460us-gaap:ResearchAndDevelopmentExpenseMember2025-01-012025-03-3100016454602025-03-310001645460cue:TwoThousandTwentyFourPre-FundedWarrantsMember2024-12-310001645460cue:Agreement2Membercue:TransactionExpenseMember2025-03-310001645460cue:FortyGOfficeAndAdditionalLaboratoryMember2025-03-310001645460us-gaap:CommonStockMember2025-03-310001645460cue:OnoCollaborationAndOptionAgreementMember2024-01-012024-03-310001645460us-gaap:ComputerEquipmentMember2025-03-310001645460cue:LicenseAgreementForPatentRightsMember2025-01-012025-03-310001645460us-gaap:EquipmentMember2024-12-310001645460us-gaap:RetainedEarningsMember2023-12-310001645460cue:BICollaborationAndLicenseAgreementMemberus-gaap:SubsequentEventMember2025-04-100001645460us-gaap:TrademarksMember2025-01-012025-03-310001645460srt:MaximumMember2025-01-012025-03-310001645460us-gaap:FurnitureAndFixturesMember2025-03-310001645460cue:OnNovemberSixteenTwoThousandTwentyTwoMember2024-01-012024-12-3100016454602025-01-012025-03-310001645460us-gaap:CertificatesOfDepositMember2025-03-310001645460us-gaap:LeaseholdImprovementsMember2024-12-310001645460cue:Agreement2Membercue:JefferiesLlcMembercue:TransactionExpenseMember2024-01-012024-03-310001645460us-gaap:AdditionalPaidInCapitalMember2024-12-310001645460cue:SiliconValleyBankMembersrt:MinimumMembercue:TermLoanAgreementMember2025-01-012025-03-310001645460us-gaap:WarrantMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2022-11-160001645460us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001645460us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMember2024-09-262024-09-260001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMemberus-gaap:WarrantMember2025-03-310001645460us-gaap:CommonStockMember2024-12-310001645460us-gaap:EquipmentMember2025-03-310001645460cue:PreFundedWarrantsMemberus-gaap:SubsequentEventMembercue:UnderwritingAgreementMember2025-04-140001645460cue:OppenheimerCoIncMember2024-09-262024-09-260001645460cue:LGChemLifeSciencesMembercue:CollaborationAgreementWithLGChemLifeSciencesMember2024-01-012024-03-310001645460cue:PreFundedWarrantsMemberus-gaap:SubsequentEventMember2025-04-142025-04-140001645460us-gaap:AccountingStandardsUpdate201602Member2025-03-310001645460cue:LaboratoryEquipmentMember2025-03-310001645460cue:TwoThousandTwentyTwoPreFundedWarrantsMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2022-11-162022-11-160001645460cue:SiliconValleyBankMembersrt:MaximumMembercue:TermLoanAgreementMember2025-01-012025-03-310001645460cue:PreFundedWarrantsMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2025-03-310001645460us-gaap:RetainedEarningsMember2025-03-310001645460us-gaap:RetainedEarningsMember2024-03-310001645460us-gaap:CommonStockMember2023-12-310001645460us-gaap:TrademarksMember2024-01-012024-03-310001645460cue:SiliconValleyBankMember2025-01-012025-03-310001645460us-gaap:WarrantMembercue:OnNovemberSixteenTwoThousandTwentyTwoMember2025-03-310001645460cue:PreFundedWarrantsMembercue:OnSeptemberTwentySixTwoThousandTwentyFourMember2024-03-310001645460cue:SiliconValleyBankMembercue:TermLoanAgreementMember2025-01-012025-03-3100016454602024-01-012024-12-310001645460cue:LicenseAgreementForPatentRightsMember2024-01-012024-03-310001645460cue:OppenheimerCoIncMember2024-12-310001645460us-gaap:CommonStockMemberus-gaap:SubsequentEventMember2025-04-140001645460us-gaap:CommonStockMember2024-01-012024-03-310001645460cue:OnSeptemberTwentySixTwoThousandTwentyFourMembercue:TwoThousandTwentyFourPreFundedWarrantsMember2024-09-262024-09-260001645460us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310001645460us-gaap:ResearchAndDevelopmentExpenseMember2024-01-012024-03-310001645460us-gaap:GeneralAndAdministrativeExpenseMember2025-01-012025-03-310001645460us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2025-03-310001645460cue:FortyGOfficeAndAdditionalLaboratoryMember2024-12-310001645460us-gaap:AdditionalPaidInCapitalMember2023-12-310001645460cue:PreFundedWarrantsMembercue:OnSeptemberTwentySixTwoThousandTwentyFourMember2024-09-260001645460cue:CommonStockOptionsMember2025-01-012025-03-310001645460us-gaap:RetainedEarningsMember2024-01-012024-03-310001645460us-gaap:WarrantMember2024-01-012024-03-310001645460us-gaap:ComputerEquipmentMember2024-12-310001645460us-gaap:WarrantMember2025-01-012025-03-310001645460us-gaap:AdditionalPaidInCapitalMember2025-01-012025-03-31xbrli:purexbrli:sharesiso4217:USD
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE QUARTERLY PERIOD ENDED MARCH 31, 2025
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE TRANSITION PERIOD FROM TO
Commission file number: 001-38327
Cue Biopharma, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
Delaware |
|
47-3324577 |
|
(State or other jurisdiction of
incorporation or organization)
|
|
(I.R.S. Employer
Identification No.)
|
|
|
|
|
40 Guest Street
Boston, Massachusetts
|
|
02135
|
(Address of principal executive offices) |
|
(Zip Code) |
(617) 949-2680
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, par value $0.001 per share |
CUE |
Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
Large accelerated filer |
|
|
Accelerated filer |
|
|
|
|
|
|
|
|
Non-accelerated filer |
|
☒ |
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes No
As of May 8, 2025, the registrant had 75,349,881 shares of Common Stock ($0.001 par value per share) outstanding.
CUE BIOPHARMA, INC.
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND INDUSTRY DATA
This Quarterly Report on Form 10-Q contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “goal,” “project,” “estimate,” “anticipate,” “strategy,” “future,” “likely” or other comparable terms. All statements, other than statements of historical fact, contained in this Quarterly Report on Form 10-Q, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans and objectives of management, are forward-looking statements.
The forward-looking statements in this Quarterly Report on Form 10-Q include, among other things, statements about:
•
the initiation, timing, progress and results of our ongoing and planned preclinical studies and any future clinical trials and our research and development programs;
•
our estimates regarding expenses, future revenue, capital requirements and need for additional financing;
•
our expectations regarding our ability to fund our projected operating requirements with our existing cash resources and the period in which we expect that such cash resources will enable us to fund such operating requirements;
•
our plans to develop our drug product candidates, including our intention to prioritize our autoimmune programs, including CUE-401 and the CUE-500 series (excluding CUE-501, which has been licensed to Boehringer Ingelheim International GmbH);
•
our plans to pursue third party support through partnerships and collaborations to further develop the CUE-100 series programs, including CUE-101 and CUE-102, as we have done for CUE-501;
•
the timing of and our ability to submit applications for, and to obtain and maintain regulatory approvals for, our drug product candidates;
•
the potential advantages of our drug product candidates;
•
the rate and degree of market acceptance and clinical utility of our drug product candidates, if approved;
•
our estimates regarding the potential market opportunity for our drug product candidates;
•
our commercialization, marketing and manufacturing capabilities and strategy;
•
our intellectual property position;
•
our ability to identify additional products, drug product candidates or technologies with significant commercial potential that are consistent with our commercial objectives;
•
the impact of government laws and regulations, general economic and market conditions, inflation, and the imposition of new or revised global trade tariffs;
•
our competitive position;
•
developments relating to our competitors and our industry;
•
our ability to continue as a going concern; and
•
our ability to maintain and establish collaborations or obtain additional funding.
Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include the factors discussed below under the headings “Risk Factor Summary,” and Part II. Item 1A. “Risk Factors,” and the risk factors detailed further in Part I. Item 1A., “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission on March 31, 2025.
This report includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties as well as our own estimates. All of the market data used in this report involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our drug product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions.
Any forward-looking statement made by us in this Quarterly Report on Form 10-Q is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
RISK FACTOR SUMMARY
Investment in our securities involves risk. You should carefully consider the following summary of what we believe to be the principal risks facing our business, in addition to the risks described more fully in Part II. Item 1A, “Risk Factors” in this Quarterly Report on Form 10-Q, and Part I. Item 1A, “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission on March 31, 2025 and other information included in this report. The risks and uncertainties described below are not the only risks and uncertainties we face. Additional risks and uncertainties not presently known to us or that we presently deem less significant may also impair our business operations.
If any of the following risks occurs, our business, financial condition and results of operations and future growth prospects could be materially and adversely affected, and the actual outcomes of matters as to which forward-looking statements are made in this report could be materially different from those anticipated in such forward-looking statements.
•
Our recurring losses from operations raise substantial doubt regarding our ability to continue as a going concern.
•
We are a clinical-stage biopharmaceutical company, have no history of generating commercial revenue, have a history of operating losses, and may never achieve or maintain profitability.
•
We currently do not have, and may never develop, any FDA-approved or commercialized products.
•
We are substantially dependent on the success of our drug product candidates, only two of which are currently being tested in clinical trials, and significant additional research and development and clinical testing will be required before we can potentially seek regulatory approval for or commercialize any of our drug product candidates.
•
We have limited experience in conducting clinical trials and no history of commercializing biologic products, which may make it difficult to evaluate the prospects for our future viability.
•
Success in preclinical studies or early clinical trials may not be indicative of results obtained in later trials.
•
We plan to continue to seek collaborations or strategic alliances. However, we may not be able to establish such relationships, and relationships we have established may not provide the expected benefits.
•
We may not be successful in our efforts to identify additional drug product candidates. Due to our limited resources and access to capital, we must prioritize the development of certain drug product candidates; these decisions may prove to be wrong and may adversely affect our business.
•
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively. Our competitors may be able to develop other compounds or drugs that are able to achieve similar or better results than our drug product candidates.
•
We rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to successfully complete development of, obtain regulatory approval for, or commercialize our drug product candidates and our business could be substantially harmed.
•
We rely completely on third parties to manufacture our preclinical and clinical drug supplies for our drug product candidates.
•
If we or our licensor(s) are unable to protect our or its intellectual property, then our financial condition, results of operations and the value of our technology and potential products could be adversely affected.
•
Even if we, or any collaborators we may have, obtain marketing approvals for any of our drug product candidates, the terms of approvals and ongoing regulation of our products could require the substantial expenditure of resources and may limit how we, or they, manufacture and market our products, which could materially impair our ability to generate revenue.
•
We will need substantial additional financing to support our growth and ongoing operations. Additional capital may be difficult to obtain, restrict our operations, require us to relinquish rights to our technologies or drug product candidates, encumber our assets and result in ongoing debt service cost, or result in additional dilution to our stockholders.
•
We have a loan agreement that requires us to meet certain operating covenants and place restrictions on our operating and financial flexibility.
PART I. FINANCIAL INFORMATION
Item 1. Financial Statements
Cue Biopharma, Inc.
Condensed Consolidated Balance Sheets
(Unaudited)
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
March 31,
2025 |
|
|
December 31,
2024 |
|
ASSETS |
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
$ |
13,136 |
|
|
$ |
22,459 |
|
Accounts receivable |
|
|
— |
|
|
|
945 |
|
Deposits, current portion |
|
|
912 |
|
|
|
929 |
|
Prepaid expenses and other current assets |
|
|
1,883 |
|
|
|
805 |
|
Total current assets |
|
|
15,931 |
|
|
|
25,138 |
|
Property and equipment, net |
|
|
523 |
|
|
|
471 |
|
Operating lease right-of-use asset |
|
|
3,589 |
|
|
|
4,370 |
|
Deposits |
|
|
1,956 |
|
|
|
1,955 |
|
Restricted cash |
|
|
153 |
|
|
|
152 |
|
Other long-term assets |
|
|
102 |
|
|
|
105 |
|
Total assets |
|
$ |
22,254 |
|
|
$ |
32,191 |
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
Accounts payable |
|
$ |
3,784 |
|
|
$ |
2,823 |
|
Accrued expenses |
|
|
4,230 |
|
|
|
2,908 |
|
Other payable |
|
|
535 |
|
|
|
— |
|
Research and development contract liability, current portion |
|
|
— |
|
|
|
85 |
|
Operating lease liabilities, current |
|
|
3,575 |
|
|
|
3,540 |
|
Current portion of long-term debt, net |
|
|
3,374 |
|
|
|
4,333 |
|
Total current liabilities |
|
|
15,498 |
|
|
|
13,689 |
|
Operating lease liabilities, non-current |
|
|
176 |
|
|
|
1,003 |
|
Total liabilities |
|
$ |
15,674 |
|
|
$ |
14,692 |
|
Commitments and contingencies (Note 11) |
|
|
|
|
|
|
Stockholders’ equity: |
|
|
|
|
|
|
Preferred stock, $0.001 par value; 10,000,000 shares authorized and 0 shares issued and outstanding at March 31, 2025 and December 31, 2024 |
|
|
— |
|
|
|
— |
|
Common stock, $0.001 par value; 200,000,000 shares authorized and 61,819,101 shares issued and outstanding at March 31, 2025 and December 31, 2024 |
|
|
62 |
|
|
|
62 |
|
Additional paid in capital |
|
|
360,639 |
|
|
|
359,301 |
|
Accumulated deficit |
|
|
(354,121 |
) |
|
|
(341,864 |
) |
Total stockholders’ equity |
|
|
6,580 |
|
|
|
17,499 |
|
Total liabilities and stockholders’ equity |
|
$ |
22,254 |
|
|
$ |
32,191 |
|
|
|
|
|
|
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
Cue Biopharma, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
March 31, |
|
|
|
|
2025 |
|
|
2024 |
|
|
Collaboration revenue |
|
$ |
421 |
|
|
$ |
1,717 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
General and administrative |
|
|
4,173 |
|
|
|
4,186 |
|
|
Research and development |
|
|
8,547 |
|
|
|
10,199 |
|
|
Total operating expenses |
|
|
12,720 |
|
|
|
14,385 |
|
|
Loss from operations |
|
|
(12,299 |
) |
|
|
(12,668 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
Interest income |
|
|
170 |
|
|
|
562 |
|
|
Interest expense |
|
|
(128 |
) |
|
|
(241 |
) |
|
Total other income, net |
|
|
42 |
|
|
|
321 |
|
|
Net loss |
|
$ |
(12,257 |
) |
|
$ |
(12,347 |
) |
|
Comprehensive loss |
|
$ |
(12,257 |
) |
|
$ |
(12,347 |
) |
|
Net loss per common share – basic and diluted |
|
$ |
(0.17 |
) |
|
$ |
(0.25 |
) |
|
Weighted average common shares outstanding – basic and diluted |
|
|
74,254,700 |
|
|
|
49,466,711 |
|
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
Cue Biopharma, Inc.
Condensed Consolidated Statements of Stockholders’ Equity
(Unaudited)
(in thousands, except share and per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
Additional |
|
|
|
|
|
Total |
|
|
|
Shares |
|
|
Par
Value |
|
|
Paid-in
Capital |
|
|
Accumulated
Deficit |
|
|
Stockholders’
Equity |
|
Balance, December 31, 2023 |
|
|
47,215,116 |
|
|
$ |
47 |
|
|
$ |
338,228 |
|
|
$ |
(301,190 |
) |
|
$ |
37,085 |
|
Issuance of common stock from ATM offering, net of sales agent commission and fees |
|
|
1,428,200 |
|
|
|
1 |
|
|
|
3,353 |
|
|
|
— |
|
|
|
3,354 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
1,946 |
|
|
|
— |
|
|
|
1,946 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(12,347 |
) |
|
|
(12,347 |
) |
Balance, March 31, 2024 |
|
|
48,643,316 |
|
|
$ |
48 |
|
|
$ |
343,527 |
|
|
$ |
(313,537 |
) |
|
$ |
30,038 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2024 |
|
|
61,819,101 |
|
|
$ |
62 |
|
|
$ |
359,301 |
|
|
$ |
(341,864 |
) |
|
$ |
17,499 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
1,338 |
|
|
|
— |
|
|
|
1,338 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(12,257 |
) |
|
|
(12,257 |
) |
Balance, March 31, 2025 |
|
|
61,819,101 |
|
|
$ |
62 |
|
|
$ |
360,639 |
|
|
$ |
(354,121 |
) |
|
$ |
6,580 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
Cue Biopharma, Inc.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
|
|
2025 |
|
|
2024 |
|
Cash flows from operating activities |
|
|
|
|
|
|
Net loss |
|
$ |
(12,257 |
) |
|
$ |
(12,347 |
) |
Adjustments to reconcile net loss to cash used in operating activities: |
|
|
|
|
|
|
Depreciation and amortization |
|
|
101 |
|
|
|
99 |
|
Stock-based compensation |
|
|
1,338 |
|
|
|
1,946 |
|
Decrease in the carrying amount of right-of-use-assets |
|
|
781 |
|
|
|
750 |
|
Amortization of debt issuance costs |
|
|
9 |
|
|
|
9 |
|
Accretion of final payment on term loans |
|
|
32 |
|
|
|
33 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
Accounts receivable |
|
|
945 |
|
|
|
298 |
|
Prepaid expenses and other current assets |
|
|
(1,078 |
) |
|
|
(998 |
) |
Deposits |
|
|
16 |
|
|
|
— |
|
Other payable |
|
|
535 |
|
|
|
— |
|
Accounts payable |
|
|
961 |
|
|
|
418 |
|
Accrued expenses |
|
|
1,322 |
|
|
|
1,071 |
|
Research and development contract liability |
|
|
(85 |
) |
|
|
(322 |
) |
Operating lease liability |
|
|
(792 |
) |
|
|
(741 |
) |
Net cash used in operating activities |
|
|
(8,172 |
) |
|
|
(9,784 |
) |
Cash flows from investing activities |
|
|
|
|
|
|
Purchases of property and equipment |
|
|
(150 |
) |
|
|
(55 |
) |
Net cash used in investing activities |
|
|
(150 |
) |
|
|
(55 |
) |
Cash flows from financing activities |
|
|
|
|
|
|
Proceeds from ATM offering, net of sales agent commission and fees |
|
|
— |
|
|
|
3,354 |
|
Repayment of term loans |
|
|
(1,000 |
) |
|
|
(1,000 |
) |
Net cash (used in) provided by financing activities |
|
|
(1,000 |
) |
|
|
2,354 |
|
Net decrease in cash, cash equivalents, and restricted cash |
|
|
(9,322 |
) |
|
|
(7,485 |
) |
Cash, cash equivalents, and restricted cash at beginning of period |
|
|
22,611 |
|
|
|
48,665 |
|
Cash, cash equivalents, and restricted cash at end of period |
|
$ |
13,289 |
|
|
$ |
41,180 |
|
Supplemental disclosures of non-cash investing and financing activities: |
|
|
|
|
|
|
Cash paid for interest |
|
$ |
90 |
|
|
$ |
209 |
|
The accompanying notes are an integral part of these condensed consolidated financial statements.
Cue Biopharma, Inc.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
1.
Organization and Basis of Presentation
Cue Biopharma, Inc. (the "Company") is a clinical-stage biopharmaceutical company developing a novel class of therapeutic biologics to selectively modulate disease-specific T cells directly within the patient’s body. The Company's vision is to translate nature's signals, or "cues", into protein therapeutics by generating a new class of T cell engagers for selective modulation of disease specific T cells. The Company’s corporate office and research facilities are located in Boston, Massachusetts.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The Company is in the clinical development and preclinical research and development stages and has incurred recurring losses and negative cash flows from operations since inception. As of March 31, 2025, the Company had cash and cash equivalents of $13.1 million. The future viability of the Company is dependent on its ability to raise additional capital to finance its operations and fund research and development costs in order to seek approval for commercialization of its drug product candidates. In April 2025, the Company received approximately $18.0 million in net proceeds from an underwritten public offering of shares of its common stock and warrants to purchase shares of its common stock. Also in April 2025, the Company entered into a Collaboration and License Agreement (the “BI Collaboration and License Agreement”) with Boehringer Ingelheim International GmbH (“BI”) pursuant to which the Company has received an upfront payment of approximately $12.0 million. For further information regarding these transactions, please refer to Note 13 Subsequent Events.
The Company continues to explore raising additional capital through a combination of equity offerings, collaborations, and other strategic alliances, and, depending on the availability and level of additional financings, potential cash expenditure reduction, there is no guarantee that the Company will be successful in these mitigation efforts. The Company’s failure to access additional capital as and when needed would have a negative impact on its financial condition and its ability to pursue its business strategies as this capital is necessary for the Company to perform the research and development activities required to develop and commercialize the Company’s drug product candidates in order to generate future revenue streams. The Company’s accumulated deficit, history of losses, negative cash flows from operations and future expected losses raises substantial doubt about the Company’s ability to continue as a going concern within one year of the issuance date of these financial statements.
2.
Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements as of March 31, 2025, and for the three months ended March 31, 2025 and 2024, have been prepared in accordance with the rules and regulations of the Securities and Exchange Commission (the “SEC”) and generally accepted accounting principles in the United States (“U.S. GAAP”) for financial information, which prescribes elimination of all significant intercompany accounts and transactions in the accounts of the Company and its wholly owned subsidiary, Cue Biopharma Securities Corp., which was incorporated in the Commonwealth of Massachusetts in December 2018. In the opinion of management, these financial statements reflect all adjustments which are necessary for a fair statement of the Company’s financial position and results of its operations, as of and for the periods presented. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S. GAAP have been condensed or omitted from this report, as is permitted by such rules and regulations. Accordingly, these financial statements should be read in conjunction with the financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, filed with the Securities and Exchange Commission on March 31, 2025.
Interim results for the three months ended March 31, 2025 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2025, or any future periods.
Public Offerings
In October 2021, the Company entered into an open market sale agreement (the “ATM Sales Agreement”) with Jefferies LLC ("Jefferies"), as agent, to sell shares of the Company’s common stock for aggregate gross proceeds of up to $80 million, from time to time, through an at-the-market equity offering program. The ATM Sales Agreement will terminate upon the earliest of (a) the sale of $80 million of shares of the Company’s common stock pursuant to the ATM Sales Agreement or (b) the termination of the ATM Sales Agreement by the Company or Jefferies.
The Company did not sell any shares during the three months ended March 31, 2025 under the ATM Sales Agreement. During the three months ended March 31, 2024, the Company sold 1,428,200 shares of common stock under the ATM Sales Agreement for proceeds of $3.4 million, net of commissions paid, but excluding transaction expenses. As of March 31, 2025, the Company had sold an aggregate of 9,072,231 shares of common stock under the ATM Sales Agreement for proceeds of $40.4 million, net of commissions paid, but excluding transaction expenses, since its inception.
On September 26, 2024, the Company entered into an underwriting agreement (the “2024 Underwriting Agreement”) with Oppenheimer & Co. Inc., as representative of the several underwriters named therein (collectively, the “2024 Underwriters”), relating to an underwritten public offering of (i) 11,564,401 shares (the “2024 Shares”) of the Company’s common stock, $0.001 par value per share, and accompanying common stock warrants (the “2024 Common Stock Warrants”) to purchase 2,891,100 shares of the Company’s common stock, and (ii) to certain investors in lieu of common stock, pre-funded warrants (the “2024 Pre-Funded Warrants,” and together with the 2024 Common Stock Warrants, the “2024 Warrants”) to purchase 12,435,599 shares of the Company’s common stock and accompanying 2024 Common Stock Warrants to purchase 3,108,900 shares of the Company’s common stock. All of the 2024 Shares and the 2024 Warrants were sold by the Company. Each 2024 Share was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.50, and each 2024 Pre-Funded Warrant was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.499, which is equal to the combined offering price per share of common stock and accompanying 2024 Common Stock Warrant less the $0.001 exercise price of each 2024 Pre-Funded Warrant. The Company received net proceeds from the offering of $10.8 million, after deducting underwriting discounts and commissions and offering expenses of $1.2 million, and excluding any proceeds that may be received from exercise of the 2024 Warrants. At March 31, 2025, the weighted average exercise price of the 2024 Warrants was $0.50 and the weighted average contractual life was 4.5 years.
Consolidation
The accompanying condensed consolidated financial statements include the Company and its wholly owned subsidiary, Cue Biopharma Securities Corp. The Company has eliminated all intercompany transactions.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates include estimates related to collaboration revenue, the accounting for potential liabilities and accrued expenses, the assumptions utilized in valuing stock-based compensation issued for services, the realization of deferred tax assets, and the useful life with respect to long-lived assets and intangibles. Actual results could differ from those estimates.
The Company maintains its cash balances with financial institutions in federally insured accounts and may periodically have cash balances in excess of insurance limits. The Company maintains its accounts with financial institutions with a high credit rating. The Company has not experienced any losses to date from the Company's deposits with these financial institutions and believes that it is not exposed to any significant credit risk on cash.
Cash and Cash Equivalents
The Company considers all highly liquid investments with a maturity of three months or less at the date of purchase to be cash equivalents. The Company invests available cash in money market funds.
Marketable Securities
Marketable securities consist of investments with original maturities greater than ninety days and less than one year from the date of the Company's condensed consolidated balance sheets. The Company classifies all of its investments as available-for-sale securities. Accordingly, these investments are recorded at fair value, which is based on quoted market prices. Unrealized gains and losses are recognized and determined on a specific identification basis and are included in comprehensive loss. Realized gains and losses are determined on a specific identification basis and are included in other income on the condensed consolidated statements of operations and comprehensive loss. Amortization and accretion of discounts and premiums is recorded in interest income. The Company did not invest in any marketable securities as of March 31, 2025 and December 31, 2024.
At March 31, 2025 and 2024, the Company invested available cash in money market funds.
The Company had $0.2 million in restricted cash deposited with a separate commercial bank to collateralize Company credit cards as of March 31, 2025 and December 31, 2024.
Property and Equipment
Property and equipment is recorded at cost. Major improvements are capitalized, while maintenance and repairs are charged to expense as incurred. Gains and losses from dispositions of property and equipment are included in income and expense when realized. Amortization of leasehold improvements is provided using the straight-line method over the shorter of the lease term or the useful life of the underlying assets. Depreciation of property and equipment is provided using the straight-line method over the following estimated useful lives:
|
|
|
Laboratory equipment |
|
5 years |
Computer equipment |
|
3 years |
Furniture and fixtures |
|
3-8 years |
The Company recognizes depreciation and amortization expense in general and administrative expenses and in research and development expenses in the Company’s condensed consolidated statements of operations and comprehensive loss, depending on how each category of property and equipment is utilized in the Company’s business activities.
Trademark
Trademark consists of the Company’s right, title and interest to the CUE BIOLOGICS Mark, and any derivative mark incorporating CUE, throughout the world, together with all associated goodwill and common law rights appurtenant thereto, including, but not limited to, any right, title and interest in any corporate name, company name, business, name, trade name, dba, domain name, or other source identifier incorporating CUE.
The Company has classified the trademark as a component of other long-term assets, having a useful life of 15 years. The Company evaluates the status of this intangible asset for amortization and impairment at each quarter end and year end reporting date. For each of the three months ended March 31, 2025 and 2024, the Company recorded approximately $3,000 in amortization expense on a straight-line basis.
Debt Issuance Costs
Debt issuance costs are deferred and presented as a reduction to long-term debt. Debt issuance costs are amortized using the effective interest rate method over the term of the loan. Amortization of deferred debt issuance costs are included in interest expense in the condensed consolidated statements of operations and comprehensive loss.
Revenue Recognition
The Company recognizes collaboration revenue under certain of the Company’s license and collaboration agreements that are within the scope of Accounting Standards Codification (“ASC”), Topic 606, Revenue from Contracts with Customers (“ASC 606”). The Company’s contracts with customers typically include promises related to licenses to intellectual property and research and development services. If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. Accordingly, the transaction price is generally comprised of a fixed fee due at contract inception and variable consideration in the form of milestone payments due upon the achievement of specified events and tiered royalties earned when customers recognize net sales of licensed products. The Company measures the transaction price based on the amount of consideration to which it expects to be entitled in exchange for transferring the promised goods and/or services to the customer. The Company utilizes the “expected value method” method to estimate the amount of variable consideration, to predict the amount of consideration to which it will be entitled for its one open contract. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
At the inception of each arrangement that includes development and regulatory milestone payments, the Company evaluates whether the associated event is considered probable of achievement and estimates the amount to be included in the transaction price using the expected value method.
Research and Development Expenses
Research and development expenses consist primarily of compensation costs, fees paid to consultants, outside service providers and organizations (including research institutes at universities), facility costs, and development and clinical trial costs with respect to the Company’s drug product candidates.
Research and development expenses incurred under contracts are expensed ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, or other information indicates that a different pattern of performance is more appropriate. Other research and development expenses are charged to operations as incurred.
Nonrefundable advance payments are recognized as an expense as the related services are performed. The Company evaluates whether it expects the services to be rendered at each quarter end and year end reporting date. If the Company does not expect the services to be rendered, the advance payment is charged to expense. Nonrefundable advance payments for research and development services are included in prepaid and other current assets on the Company's condensed consolidated balance sheets. To the extent that a nonrefundable advance payment is for contracted services to be performed within 12 months from the reporting date, such advance is included in current assets; otherwise, such advance is included in non-current assets.
The Company evaluates the status of its research and development agreements and contracts, and the carrying amount of the related assets and liabilities, at each quarter end and year end reporting date, and adjusts the carrying amounts and their classification on the Company's condensed consolidated balance sheets as appropriate.
The Company is the exclusive worldwide licensee of, and has patent applications pending for, numerous domestic and foreign patents. Due to the significant uncertainty associated with the successful development of one or more commercially viable drug product candidates based on the Company’s research efforts and any related patent applications, all patent costs, including patent-related legal fees, filing fees and other costs are charged to general and administrative expense as incurred.
Licensing Fees and Costs
Licensing fees and costs consist primarily of costs relating to the acquisition of the Company’s license agreement with the Albert Einstein College of Medicine, including related royalties, maintenance fees, milestone payments and product development costs. Licensing fees and costs are charged to research and development expense as incurred.
Long-Lived Assets
The Company reviews long-lived assets, consisting of property and equipment, for impairment when events or changes in circumstances indicate the carrying value of these assets may exceed their current fair values. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized for the amount by which the carrying amount of the asset exceeds the fair value of the assets. Assets to be disposed of are separately presented in the Company's condensed consolidated balance sheets and reported at the lower of the carrying amount or fair value less costs to sell and are no longer depreciated. The Company has not historically recorded any impairment to its long-lived assets. In the future, if events or market conditions affect the estimated fair value to the extent that a long-lived asset is impaired, the Company will adjust the carrying value of these long-lived assets in the period in which the impairment occurs.
The Company accounts for leases under ASC 842, Leases, which requires a lessee to record a right-of-use asset and a corresponding lease liability for most lease arrangements on the Company's condensed consolidated balance sheets. Under the standard, disclosure of key information about leasing arrangements to assist users of the financial statements with assessing the amount, timing and uncertainty of cash flows arising from leases are required.
Stock-Based Compensation
The Company periodically issues stock-based awards to officers, directors, employees, scientific and clinical advisory board members and consultants for services rendered. Such awards vest and expire according to terms established at the issuance date.
Stock-based compensation to officers, directors, employees, scientific and clinical advisory board members and consultants, including grants of employee stock options, is recognized in the financial statements based on their grant date fair values. Stock option grants, which are generally time-vested, are measured at the grant date fair value and charged to operations on a straight-line basis over the service period, which generally approximates the vesting term. The Company also grants performance-based awards periodically to officers of the Company. The Company recognizes compensation costs related to performance awards over the requisite service period if and when the Company concludes that it is probable that the performance condition will be achieved.
The fair value of stock options and restricted stock units is determined utilizing the Black-Scholes valuation model. This valuation model takes into account the exercise price of the award, as well as a variety of significant assumptions. The assumptions used to estimate the fair value of stock options include the expected term, the expected volatility of the Company's stock over the expected term, the risk-free interest rate over the expected term, and the Company's expected annual dividend yield. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant. The expected dividend yield is based on the current yield at the grant date; the Company has never declared or paid dividends and has no plans to do so for the foreseeable future. As permitted by Staff Accounting Bulletin No. 107, due to the Company’s limited trading history and option activity, management utilizes the simplified method to estimate the expected term of options at the date of grant. The exercise price is determined based on the fair value of the Company's common stock at the date of grant. The Company accounts for forfeitures as they occur.
The Company recognizes the fair value of stock-based compensation in general and administrative expenses and in research and development expenses in the Company’s condensed consolidated statements of operations and comprehensive loss, depending on the type of services provided by the recipient of the equity award.
Comprehensive Income (Loss)
Components of comprehensive income or loss, including net income or loss, are reported in the financial statements in the period in which they are recognized. Other comprehensive income or loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Net income (loss) and other comprehensive income (loss) are reported net of any related tax effect to arrive at comprehensive income (loss). Comprehensive income (loss) includes net income (loss) as well as changes in stockholders’ equity that result from transactions and economic events other than those with stockholders. There were no elements of other comprehensive income (loss) in the periods presented.
Earnings (Loss) Per Share
The Company’s computation of earnings (loss) per share (“EPS”) for the respective periods includes basic and diluted EPS. Basic EPS is measured as the income (loss) attributable to common stockholders divided by the weighted average number of common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares that would result from the exercise of outstanding stock options and warrants as if they had been exercised at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. Basic and diluted loss per common share is the same for all periods presented because all outstanding stock options and warrants are anti-dilutive.
Per ASC 260-10-45-13, shares issuable for little to no consideration should be included in the number of outstanding shares used for basic EPS. The Financial Accounting Standards Board (“FASB”) proposed that warrants or options exercisable for little to no cost (sometimes referred to as “penny warrants”) be included in the denominator of basic EPS (and therefore diluted EPS) once there were no further vesting conditions or contingencies associated with them. The Company included 12,435,599 and 1,531,440 pre-funded warrants in the denominator of basic EPS at March 31, 2025 and 2024, respectively.
At March 31, 2025 and 2024, the Company excluded the securities summarized below, which entitled the holders thereof to acquire shares of common stock, from its calculation of EPS, as their effect would have been anti-dilutive.
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
|
2025 |
|
2024 |
|
Common stock warrants |
|
|
15,151,906 |
|
|
9,188,406 |
|
Common stock options |
|
|
18,674,826 |
|
|
9,270,666 |
|
Total |
|
|
33,826,732 |
|
|
18,459,072 |
|
Fair Value of Financial Instruments
The authoritative guidance with respect to fair value established a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels and requires that assets and liabilities carried at fair value be classified and disclosed in one of three categories, as presented below.
Level 1. Observable inputs such as quoted prices in active markets for an identical asset or liability that the Company has the ability to access as of the measurement date. Financial assets and liabilities utilizing Level 1 inputs include active exchange-traded securities and exchange-based derivatives.
Level 2. Inputs, other than quoted prices included within Level 1, which are directly observable for the asset or liability or indirectly observable through corroboration with observable market data. Financial assets and liabilities utilizing Level 2 inputs include fixed income securities, non-exchange-based derivatives, mutual funds, and fair-value hedges.
Level 3. Unobservable inputs in which there is little or no market data for the asset or liability which requires the reporting entity to develop its own assumptions. Financial assets and liabilities utilizing Level 3 inputs include infrequently traded non-exchange-based derivatives and commingled investment funds and are measured using present value pricing models.
The Company determines the level in the fair value hierarchy within which each fair value measurement falls in its entirety, based on the lowest level input that is significant to the fair value measurement in its entirety. In determining the appropriate levels, the Company performs an analysis of the assets and liabilities at each reporting period end.
The Company had $12.6 million and $21.8 million in cash equivalents as of March 31, 2025 and December 31, 2024, respectively.
The carrying value of financial instruments (consisting of cash, a certificate of deposit, debt, accounts payable, accrued compensation and accrued expenses) is considered to be representative of their respective fair values due to the short-term nature of those instruments.
Recent Accounting Pronouncements
ASU 2023-07 - Segment Reporting
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (“ASU 2023-07”). ASU 2023-07 requires disclosure of incremental segment information on an annual and interim basis. The amendments also require companies with a single reportable segment to provide all disclosures required by these amendments and all existing segment disclosures in ASC 280, Segment Reporting. The amendments are effective for fiscal years beginning after December 15, 2023, and interim periods beginning after December 15, 2024. The Company adopted ASU 2023-07 effective December 31, 2024.
Operating segments are defined as components of an entity for which separate financial information is available and that is regularly reviewed by the Chief Operating Decision Maker (the “CODM”) in deciding how to allocate resources to an individual segment and in assessing performance. The Company's CODM is the chief executive officer.
The Company is in the development stage, has not yet earned revenue from product sales, and has incurred recurring losses and negative cash flows from operations since inception. The Company operates as a single reporting segment, focused on developing a novel class of therapeutic biologics to selectively modulate disease-specific T cells directly within the patient’s body. The CODM manages and allocates resources to the operations of the Company on a total company basis and therefore does not measure separate segment profit or loss. Managing and allocating resources on a total company basis enables the CODM to assess the overall level of resources available and how to best deploy these resources across functions and research and development programs that are in line with the Company’s long-term strategic corporate goals. Consistent with this decision-making process, the CODM uses consolidated financial information for purposes of evaluating performance, forecasting future period financial results, allocating resources and setting incentive targets. Operating expenses are used to monitor budget versus actual results. All the Company’s long-lived assets are held in the United States and all the Company’s revenues since inception have been earned from collaboration agreements as none of the Company's drug product candidates have yet been approved for commercial sale. The resources utilized for any specific purpose may vary significantly and will depend on a number of factors, including, but not limited to, the Company’s research and development activities and programs, clinical testing, regulatory approval, market conditions, and changes in or revisions to the Company’s business strategy and technology development plans.
ASU 2023-09 - Income Taxes
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures(“ASU 2023-09”). The guidance in ASU 2023-09 improves the transparency of income tax disclosures by greater disaggregation of information in the rate reconciliation and income taxes paid disaggregated by jurisdiction. The standard is effective for public companies for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact that the adoption of ASU 2023-09 may have on its consolidated financial statements.
Management does not believe that any other recently issued, but not yet effective, authoritative guidance, if currently adopted, would have a material impact on the Company’s financial statement presentation or disclosures.
The Company accounts for its financial assets and liabilities using fair value measurements. The authoritative accounting guidance defines fair value, establishes a framework for measuring fair value under U.S. GAAP and enhances disclosures about fair value measurements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date.
The following table presents information about the Company’s assets that are measured at fair value on a recurring basis as of March 31, 2025 and December 31, 2024, and indicates the level of the fair value hierarchy utilized to determine such fair value:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements as of March 31, 2025 |
|
|
|
(in thousands) |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Fair Value |
|
Cash equivalents |
|
$ |
12,613 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
12,613 |
|
Total |
|
$ |
12,613 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
12,613 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value Measurements as of December 31, 2024 |
|
|
|
(in thousands) |
|
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Fair Value |
|
Cash equivalents |
|
$ |
21,813 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
21,813 |
|
Total |
|
$ |
21,813 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
21,813 |
|
As of March 31, 2025, the Company had $12.6 million in cash equivalents. The Company measures the cash equivalents that are invested in money market funds using Level 1 inputs for identical securities. As of December 31, 2024, the Company had $21.8 million in cash equivalents. During the three months ended March 31, 2025, and the year ended December 31, 2024, there were no transfers between Level 2 and Level 3.
4.
Property and Equipment, Net
Property and equipment, net as of March 31, 2025 and December 31, 2024 consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
March 31,
2025 |
|
|
December 31,
2024 |
|
|
|
(in thousands) |
|
Laboratory equipment |
|
$ |
3,934 |
|
|
$ |
3,785 |
|
Furniture and fixtures |
|
|
68 |
|
|
|
68 |
|
Computer equipment |
|
|
180 |
|
|
|
180 |
|
Leasehold improvements |
|
|
118 |
|
|
|
118 |
|
Total property and equipment |
|
|
4,300 |
|
|
|
4,151 |
|
Less: accumulated depreciation |
|
|
(3,777 |
) |
|
|
(3,680 |
) |
Property and equipment, net |
|
$ |
523 |
|
|
$ |
471 |
|
Depreciation expense for the three months ended March 31, 2025 and 2024 was included in the condensed consolidated statements of operations and comprehensive loss as follows:
|
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
2025 |
|
|
2024 |
|
|
|
|
(in thousands) |
|
|
General and administrative |
|
$ |
4 |
|
|
$ |
4 |
|
|
Research and development |
|
|
93 |
|
|
|
92 |
|
|
Depreciation total |
|
$ |
97 |
|
|
$ |
96 |
|
|
5.
Loan with First Citizens Bank (formerly with Silicon Valley Bank)
On February 15, 2022 (the “Closing Date”), the Company entered into a Loan and Security Agreement (the “Loan Agreement”), with Silicon Valley Bank, as lender (“SVB”). The Company drew $10,000,000 in term loans under the Loan Agreement (the "Term Loans") on the Closing Date. The Loan Agreement was amended in April 2023 and October 2024.
The Term Loans bear interest at a floating rate per annum equal to the greater of (A) the prime rate (as published in the money rates section of The Wall Street Journal) plus 2.25% and (B) 5.50%. The Term Loans were interest only from the Closing Date through June 30, 2023, after which the Company is required to pay 30 equal monthly installments of principal. At March 31, 2025, the interest rate was 9.75% which is based on the prime rate plus 2.25%.
The Term Loans may be prepaid in full with payment of a 1.00% prepayment premium. Upon prepayment or repayment in full of the Term Loans, the Company will be required to pay a one-time final payment fee equal to 5.00% of the original principal amount of any funded Term Loans being repaid. This one-time final payment fee is recorded to interest expense using the effective interest method over the period of the Term Loans in the condensed consolidated statements of operations and comprehensive loss.
The Term Loans and related obligations under the Loan Agreement are secured by substantially all of the Company’s properties, rights and assets, except for its intellectual property which is subject to a negative pledge under the Loan Agreement.
The Loan Agreement, as amended, contains customary representations, warranties, events of default and covenants. In addition to the foregoing, the Company is required to have at all times on deposit in accounts of the Company maintained with SVB, unrestricted and unencumbered cash in an amount equal to the lesser of (i) 100% of the dollar value of the Company’s consolidated cash, in the aggregate, at all financial institutions and (ii) $20,000,000. On March 10, 2023, SVB was closed and the Federal Deposit Insurance Corporation (the “FDIC”) was appointed receiver for the bank. The FDIC created a successor bridge bank, and all deposits and loans of SVB were transferred to the bridge bank under a systemic risk exception approved by the United States Department of the Treasury, the Federal Reserve and the FDIC. On March 27, 2023, First Citizens Bank & Trust Company (“First Citizens Bank”), assumed all of SVB’s deposits and certain other liabilities and acquired substantially all of SVB’s loans and certain other assets from the FDIC. First Citizens Bank continues to hold the Company’s Term Loans under the same existing terms and covenants which were in place with SVB.
During the three months ended March 31, 2025 and 2024, the Company recognized interest expense related to the Term Loans of $0.1 million and $0.2 million, respectively. During both the three months ended March 31, 2025 and 2024, the Company recognized interest expense related to accretion of the final payment of less than $0.1 million. All outstanding principal and accrued and unpaid interest under the Term Loans and all other outstanding obligations with respect to the Term Loans are due and payable in full on December 1, 2025.
Debt Issuance Costs
Debt issuance costs are deferred and presented as a reduction to long-term debt. Debt issuance costs are amortized using the effective interest rate method over the term of the loan. Amortization of deferred debt issuance costs are included in interest expense in the condensed consolidated statements of operations and comprehensive loss.
The Company incurred $142,000 in debt issuance costs related to the Loan Agreement at its onset. For both the three months ended March 31, 2025 and 2024, the Company recorded $9,000 in amortization of debt issuance costs to interest expense in the condensed consolidated statements of operations and comprehensive loss. The Company recorded less than $0.1 million to short- and long-term debt issuance costs contra-liabilities as of March 31, 2025.
6. Accrued Expenses
Accrued expenses consist of the following:
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
December 31, |
|
(In thousands) |
|
2025 |
|
|
2024 |
|
Employee and board compensation |
|
$ |
2,610 |
|
|
$ |
1,812 |
|
Contract research services |
|
|
1,200 |
|
|
|
773 |
|
Contract manufacturing services |
|
|
267 |
|
|
|
9 |
|
Professional services |
|
|
153 |
|
|
|
314 |
|
Total |
|
$ |
4,230 |
|
|
$ |
2,908 |
|
7.
Einstein License Agreement
On January 14, 2015, the Company entered into a license agreement, as amended and restated on July 31, 2017 and as further amended on October 30, 2018, January 13, 2024, and April 10, 2025 (the “Einstein License”), with Albert Einstein College of Medicine (“Einstein”) for certain patent rights relating to the Company’s core technology platform for the engineering of biologics to control T cell activity, precision, immune-modulatory drug product candidates, and two supporting technologies that enable the discovery of costimulatory signaling molecules (ligands) and T cell targeting peptides.
Under the Einstein License, the Company holds an exclusive worldwide license, with the right to sublicense, import, make, have made, use, provide, offer to sell, and sell all products, processes and services that use the patents covered by the Einstein License, including certain technology received from Einstein relating thereto (the “Einstein Licensed Products”). Under the Einstein License, the Company is required to:
•
Pay royalties and amounts based on a certain percentage of proceeds, as defined in the Einstein License, from sales of Einstein Licensed Products and sublicense agreements.
•
Pay escalating annual maintenance fees, which are nonrefundable, but are creditable against the amount due to Einstein for royalties.
•
Make significant payments based upon the achievement of certain milestones, as defined in the Einstein License. Payments made upon achievement of milestones are nonrefundable and are not creditable against any other payment due to Einstein. At March 31, 2025, the Company had made aggregate payments totaling $1.2 million since inception with respect to achievement of these milestones.
•
Incur minimum product development costs until the first commercial sale of the first Einstein Licensed Product.
The Company was in compliance with its obligations under the Einstein License at March 31, 2025 and 2024.
The Einstein License expires upon the expiration of the Company’s last obligation to make royalty payments to Einstein which may be due with respect to certain Einstein Licensed Products, unless terminated earlier under the provisions thereof. The Einstein License includes certain termination provisions if the Company fails to meet its obligations thereunder.
Pursuant to the Einstein License, the Company issued to Einstein 671,572 shares of the Company’s common stock in connection with the consummation of the initial public offering of its common stock on December 27, 2017. Please refer to Note 13 Subsequent Events for information regarding an amendment to the Einstein License.
The Company accounts for license fees incurred in connection with the Einstein License in accordance with ASC 730, Research and Development. Please refer to Note 10 Collaboration Revenue.
8.
Stock-Based Compensation
Stock Option Valuation
For stock options requiring an assessment of value during the three months ended March 31, 2025 and 2024, the fair value of each stock option award was estimated using the Black-Scholes option-pricing model utilizing the following assumptions:
|
|
|
|
|
March 31, 2025 |
Risk-free interest rate |
|
4.05% - 4.46% |
Expected dividend yield |
|
0% |
Expected volatility |
|
86.46% - 87.69% |
Expected life |
|
5.50 to 6.25 years |
|
|
|
|
|
March 31, 2024 |
Risk-free interest rate |
|
3.83% - 4.28% |
Expected dividend yield |
|
0% |
Expected volatility |
|
66.27% - 109.86% |
Expected life |
|
5.50 to 8.91 years |
A summary of stock option activity for the three months ended March 31, 2025 is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
Shares |
|
|
Weighted
Average
Exercise
Price |
|
|
Weighted
Average
Remaining
Contractual
Life
(in Years) |
|
Stock options outstanding at December 31, 2024 |
|
|
10,471,724 |
|
|
$ |
5.61 |
|
|
|
7.27 |
|
Granted |
|
|
1,889,300 |
|
|
|
0.99 |
|
|
|
— |
|
Exercised |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Cancelled |
|
|
(22,563 |
) |
|
|
3.42 |
|
|
|
— |
|
Stock options outstanding at March 31, 2025 |
|
|
12,338,461 |
|
|
|
4.91 |
|
|
|
7.48 |
|
Stock options exercisable at March 31, 2025 |
|
|
5,982,995 |
|
|
$ |
8.41 |
|
|
|
5.63 |
|
The aggregate intrinsic value of exercisable but unexercised in-the-money stock options at March 31, 2025 was less than $0.1 million based on a weighted average exercise price of $8.41 per share. The aggregate intrinsic value of options is calculated as the difference of the market close price of $0.91 on March 31, 2025, and the weighted average exercise price of $8.41, with a weighted average remaining contractual term of 5.63 years.
Stock-based Compensation
Stock-based compensation for the three months ended March 31, 2025 and 2024 was included in the Company’s condensed consolidated statements of operations and comprehensive loss as follows:
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
March 31, |
|
(in thousands) |
|
2025 |
|
|
2024 |
|
General and administrative |
|
$ |
695 |
|
|
$ |
956 |
|
Research and development |
|
|
643 |
|
|
|
990 |
|
Total stock-based compensation |
|
$ |
1,338 |
|
|
$ |
1,946 |
|
As of March 31, 2025, total unrecognized stock-based compensation expense was $6.0 million, which is expected to be recognized as an operating expense in the Company’s condensed consolidated statements of operations and comprehensive loss over the weighted average remaining period of 2.15 years.
On September 26, 2024, the Company entered into the 2024 Underwriting Agreement with Oppenheimer & Co. Inc., as representative of the 2024 Underwriters, relating to an underwritten public offering of (i) 11,564,401 shares of the Company’s common stock, $0.001 par value per share, and accompanying common stock warrants to purchase 2,891,100 shares of common stock, and (ii) to certain investors in lieu of common stock, pre-funded warrants to purchase 12,435,599 shares of common stock and accompanying common stock warrants to purchase 3,108,900 shares of common stock.
All of the 2024 Shares and the 2024 Warrants were sold by the Company. Each 2024 Share was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.50, and each 2024 Pre-Funded Warrant was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.499, which is equal to the combined offering price per share of common stock and accompanying 2024 Common Stock Warrant less the $0.001 exercise price of each 2024 Pre-Funded Warrant. The Company received net proceeds from the offering of $10.8 million, after deducting underwriting discounts and commissions and offering expenses of $1.2 million, and excluding any proceeds that may be received from exercise of the 2024 Warrants. At March 31, 2025, 5,963,500 of the 2024 Common Stock Warrants and 12,435,599 of the 2024 Pre-Funded Warrants remained outstanding.
On November 16, 2022, the Company issued 9,188,406 warrants with an exercise price of $3.93 and a 5-year term (the “2022 Common Stock Warrants”), and 1,531,440 pre-funded warrants at a nominal exercise price of $0.0001 per share (the “2022 Pre-Funded Warrants”). The Company recorded cash received from 7,656,966 shares of common stock, 9,188,406 2022 Common Stock Warrants and 1,531,440 2022 Pre-Funded Warrants to additional paid in capital in the amount of $27.4 million, net of placement fees of $2.6 million during the year ended December 31, 2022. At March 31, 2025, 9,188,406 of the 2022 Common Stock Warrants and zero of the 2022 Pre-Funded Warrants remained outstanding.
Each tranche of warrants was evaluated under ASC 480, Distinguishing Liabilities from Equity, and ASC 815, Derivatives and Hedging, and the Company determined that equity classification was appropriate. The Company determined equity classification for both warrants and pre-funded warrants as they do not embody an obligation for the Company to repurchase its shares and permit the holders to receive a fixed number of shares of common stock upon exercise. Per ASC 815-40-25, the Company accounts for the warrants and pre-funded warrants as equity, as the Company does not provide the holder a fixed or guaranteed return.
The Company recognizes collaboration revenue under certain of the Company’s license or collaboration agreements that are within the scope of ASC 606. The Company’s contracts with customers typically include promises related to licenses to intellectual property and research and development services. If the license to the Company’s intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, the Company recognizes revenue from non-refundable, up-front fees allocated to the license when the license is transferred to the licensee and the licensee is able to use and benefit from the license. For licenses that are bundled with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and if, over time, the appropriate method of measuring progress for purposes of recognizing revenue from non-refundable, up-front fees. The Company’s contracts may include options to acquire additional goods and/or services.
The terms of the Company’s arrangements with customers typically include the payment of one or more of the following: (i) non-refundable, up-front payment, and pass through costs related to research activities, (ii) development, regulatory and commercial milestone payments, (iii) future options and (iv) royalties on net sales of licensed products. Accordingly, the transaction price is generally comprised of a fixed fee due at contract inception and variable consideration in the form of pass through costs and milestone payments due upon the achievement of specified events and tiered royalties earned when customers recognize net sales of licensed products. The Company measures the transaction price based on the amount of consideration to which it expects to be entitled in exchange for transferring the promised goods and/or services to the customer. The Company utilizes the “expected value method” method to estimate the amount of variable consideration, to predict the amount of consideration to which it will be entitled for its one open contract. Amounts of variable consideration are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Milestone payments that are not within the control of the Company or the licensee, such as those dependent upon receipt of regulatory approval, are not considered to be probable of achievement until the triggering event occurs. At the end of each reporting period, the Company reevaluates the probability of achievement of each milestone and any related constraint, and, if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment.
For arrangements that include sales-based royalties, including milestone payments based upon the achievement of a certain level of product sales, the Company recognizes revenue upon the later of: (i) when the related sales occur or (ii) when the performance obligation to which some or all of the payment has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any development, regulatory or commercial milestones or royalty revenue resulting from any of its collaboration arrangements. Consideration that would be received for optional goods and/or services is excluded from the transaction price at contract inception.
The Company allocates the transaction price to each performance obligation identified in the contract on a relative standalone selling price basis, when applicable. However, certain components of variable consideration are allocated specifically to one or more particular performance obligations in a contract to the extent both of the following criteria are met: (i) the terms of the payment relate specifically to the efforts to satisfy the performance obligation or transfer the distinct good or service and (ii) allocating the variable amount of consideration entirely to the performance obligation or the distinct good or service is consistent with the allocation objective of the standard whereby the amount allocated depicts the amount of consideration to which the entity expects to be entitled in exchange for transferring the promised goods or services. The Company develops assumptions that require judgment to determine the standalone selling price for each performance obligation identified in each contract. The key assumptions utilized in determining the standalone selling price for each performance obligation may include forecasted revenues, development timelines, estimated research and development costs, discount rates, likelihood of exercise and probabilities of technical and regulatory success.
Revenue is recognized based on the amount of the transaction price that is allocated to each respective performance obligation when or as the performance obligation is satisfied by transferring a promised good and/or service to the customer. For performance obligations that are satisfied over time, the Company recognizes revenue by measuring the progress toward complete satisfaction of the performance obligation using a single method of measuring progress which depicts the performance in transferring control of the associated goods and/or services to the customer. The Company uses input methods to measure progress toward the complete satisfaction of performance obligations satisfied over time. The Company evaluates the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenue and net loss in the period of adjustment. The Company measures progress toward satisfaction of the performance obligation over time as effort is expended.
Collaboration Agreement with LG Chem
On November 6, 2018, the Company entered into a Collaboration, License and Option Agreement (as amended from time to time, the “LG Chem Collaboration Agreement”), with LG Chem Ltd. (“LG Chem”) related to the development of the Company’s CUE-101 and CUE-102 Immuno-STATs focused in the field of oncology. Pursuant to the LG Chem Collaboration Agreement, the Company granted LG Chem an exclusive license to develop, manufacture and commercialize CUE-101, as well as CUE-102 Immuno-STATs that target T cells against two additional cancer antigens, in certain Asian countries (collectively, the “LG Chem Territory”).
Aside from the $6.8 million in milestone payments earned to date, the Company does not believe that any variable consideration should be included in the transaction price as of March 31, 2025. Such assessment considered the application of the constraint to ensure that estimates of variable consideration would be included in the transaction price only to the extent the Company had a high degree of confidence that revenue would not be reversed in a subsequent reporting period. The Company will re-evaluate the transaction price, including the estimated variable consideration included in the transaction price and all constrained amounts, in each reporting period and as other changes in circumstances occur. For the three months ended March 31, 2025, the Company did not recognize any revenue related to the LG Chem Collaboration Agreement. For the three months ended March 31, 2024, the Company recognized revenue of less than $0.1 million related to the LG Chem Collaboration Agreement. The Company did not record short or long-term research and development liabilities on its balance sheets dated March 31, 2025 and 2024, as the performance obligations have been met and completed. Research and development cost sharing provisions under the agreement expired on March 31, 2022, and thereafter, the Company recognized revenue on intellectual patent filing passthrough costs in the LG Chem Territory.
On March 11, 2025, the Company and LG Chem entered into the Ninth Amendment to the LG Chem Collaboration Agreement. As of the date of the amendment, the Company regained its rights to the LG Chem Territory for the CUE-101 program which had been licensed to LG Chem, and LG Chem terminated all of its rights to the same program. Pursuant to the Ninth Amendment, the Company agreed to make future payments to LG Chem, if and when, one or more potential scenarios related to the CUE-101 program occur up to a predetermined aggregate amount. LG Chem continues to maintain its interest and rights in the CUE-102 program, targeting Wilms’ tumor 1 protein expressing cancers, pursuant to the LG Chem Collaboration Agreement.
Collaboration and Option Agreement with Ono
In February 2023, the Company entered into a strategic collaboration agreement (the "Ono Collaboration and Option Agreement") with Ono Pharmaceutical Co., Ltd. ("Ono") to further develop CUE-401. In March 2025, the Company and Ono agreed to terminate the Ono Collaboration and Option Agreement effective as of March 6, 2025. At such time, the Ono Collaboration and Option Agreement had no further force or effect with the exception of certain customary provisions which are intended to survive termination and expiration of the Ono Collaboration and Option Agreement. The Company retained all rights to CUE-401.
Under the terms of the Ono Collaboration and Option Agreement, Ono paid the Company an upfront payment and agreed to fully fund all research and development activities related to CUE-401 through a specified option period of 24 months (the “Ono Research Term”). Per the agreement, as consideration for the research and development activities performed by the Company, Ono (i) made a one-time, non-refundable, non-creditable upfront payment of $3.0 million to the Company in March 2023 and (ii) agreed to reimburse the Company for all costs incurred in conducting research, including (a) pass through costs from third party contractors and (b) full-time employee salaries capped at $2.1 million in the first 18 months of the Ono Research Term. Subsequently, the Company and Ono agreed to increase this cap for full-time employee salaries to $3.1 million.
As of the date of this report, both Ono and the Company have satisfied all of their performance obligations and made all outstanding payments required under the agreement. For the three months ended March 31, 2025 and 2024, the Company recognized revenue of $0.4 million and $1.7 million, respectively, related to the Ono Collaboration and Option Agreement. The Company did not record short or long-term research and development liabilities on its balance sheet dated March 31, 2025, as the performance obligation has been met and completed. For the year ended December 31, 2024, the Company recorded short-term research and development liabilities on its consolidated balance sheets of $0.1 million.
11.
Commitments and Contingencies
Einstein License Agreement
In 2015, the Company entered into the Einstein License with Einstein for certain patent rights relating to the Company’s core technology platform for the engineering of biologics to control T cell activity, precision, immune-modulatory drug product candidates, and two supporting technologies that enable the discovery of costimulatory signaling molecules (ligands) and T cell targeting peptides. The Company entered into an amended and restated license agreement on July 31, 2017, as amended on October 2018, which modified certain obligations of the parties under the Einstein License. The Einstein License was further amended on January 13, 2024 and April 10, 2025.
For each of the three months ended March 31, 2025 and 2024, the Company incurred less than $0.1 million in fees payable to Einstein in relation to this license.
The Company’s remaining commitments with respect to the Einstein License are based on the attainment of future milestones. The aggregate amount of milestone payments made under the Einstein License may equal up to $1.85 million for each Einstein Licensed Product, and up to $1.85 million for each new indication of an Einstein Licensed Product. Additionally, the aggregate amount of one-time milestone payments based on cumulative sales of all Einstein Licensed Products may equal up to $5.75 million. The Company is also party to a service agreement with Einstein to support the Company’s ongoing research and development activities.
Collaboration Agreement with LG Chem
See discussion of the LG Chem Collaboration Agreement in Note 10.
Collaboration and Option Agreement with Ono
See discussion of the Ono Collaboration and Option Agreement in Note 10.
Contingencies
The Company accrues contingent liabilities to the extent that the liability is probable and estimable. There are no accruals for contingent liabilities in the Company’s condensed consolidated financial statements.
The Company may be subject to various legal proceedings from time to time as part of its business. As of March 31, 2025, the Company was not a party to any legal proceedings or threatened legal proceedings, the adverse outcome of which, individually or in the aggregate, would have a material adverse effect on its business, financial condition or results of operations.
On March 28, 2022, the Company entered into a License Agreement (the “License”) with MIL 40G, LLC (the “Licensor”), pursuant to which the Company leases approximately 13,000 square feet of office, research and development and laboratory space located at 40 Guest Street, Boston, Massachusetts 02135. The Company recognized a right of use asset of $9.1 million and an operating lease liability of $9.1 million which were recorded as of the Term Commencement Date (as defined below) related to the License. The term of the License commenced on April 15, 2022 (the “Term Commencement Date”) and expires on April 14, 2026. The License has a monthly rental rate of $225,760.
On May 3, 2022, the Company entered into the First Amendment to the License with the Licensor, pursuant to which the License was expanded to include an additional room effective July 15, 2022. On May 31, 2022, the Company entered into an operating lease for additional laboratory space at 40 Guest Street for the period from December 1, 2022, through December 1, 2024 (the “40G Additional Laboratory Lease”).
On November 20, 2024, the Company extended the term of the 40G Additional Laboratory Lease through July 14, 2026. The monthly rental rate is $61,519 through November 30, 2025 and $63,979 for the remainder of the term until July 14, 2026. During the year ended December 31, 2024, the Company recognized a right of use asset of $1.1 million and short-term and long-term operating lease liabilities of $0.7 million and $0.4 million, respectively, using a discount rate of 10%, which were recorded as of the term commencement date of the 40G Additional Laboratory Lease.
For the three months ended March 31, 2025, the Company recorded $0.1 million in interest expense to the lease liability.
At March 31, 2025, operating lease right-of-use assets totaled $3.6 million. Corresponding operating lease liabilities totaled $3.8 million, of which $3.6 million were recorded in current liabilities, and $0.2 million were recorded in long-term liabilities on the Company’s condensed consolidated balance sheets.
As of both March 31, 2025 and December 31, 2024, security deposits of $0.6 million related to the 40G Additional Laboratory Lease were included in deposits on the Company’s consolidated balance sheets.
Future minimum lease payments under these leases at March 31, 2025 are as follows:
|
|
|
|
|
(in thousands) |
|
2025 (remaining 9 months) |
$ |
2,676 |
|
2026 |
|
1,234 |
|
2027 |
|
— |
|
Total lease payments |
|
3,910 |
|
Less: imputed interest |
|
(159 |
) |
Present value of lease payments |
$ |
3,751 |
|
Rent expense of $0.9 million was included in the condensed consolidated statements of operations and comprehensive loss for both the three months ended March 31, 2025 and 2024.
The weighted average remaining lease term and discount rate related to the Company's leases were as follows:
|
|
|
|
|
|
|
|
|
March 31,
2025 |
|
|
December 31,
2024 |
|
Weighted average remaining lease term (years) |
|
1.10 |
|
|
|
1.35 |
|
Weighted average discount rate |
|
6.88 |
% |
|
|
6.85 |
% |
13. Subsequent Events
BI Collaboration and License Agreement
On April 10, 2025, the Company entered into the BI Collaboration and License Agreement to research, develop and commercialize differentiated B cell depletion molecules, including CUE-501.
Under the terms of the BI Collaboration and License Agreement, the Company and BI will conduct collaborative research focused on CUE-501 during a four-year period or, if earlier, the completion of activities under the research plans, or the BI Research Term. In addition to, or instead of, CUE-501, BI may elect, at its sole discretion, to include additional or alternative compounds targeted at B cell depletion. BI will have an exclusive, royalty-bearing, worldwide, sublicensable license, under the Company's applicable patents and know-how, to develop, manufacture and commercialize such compounds and their derivatives, or BI Licensed Products, for all uses, and BI shall be responsible for all further research, preclinical and clinical development, manufacturing, regulatory approvals, and commercialization of BI Licensed Products at its expense. During the BI Research Term, the Company is prohibited from developing or commercializing any molecule for applications in B cell depletion.
Pursuant to the terms of the BI Collaboration and License Agreement, the Company received an upfront payment of approximately $12.0 million and will be eligible to receive up to an aggregate of approximately $345.0 million in success-based research, development and commercial milestone payments, beginning with two preclinical development milestones, as well as royalty payments on net sales. The royalty payments will be subject to reduction due to patent expiration, payments made under certain licenses for third-party intellectual property and generic competition. During the BI Research Term, BI will also make research support payments to us.
The BI Collaboration and License Agreement will continue, on a product-by-product and country-by-country basis, until the expiration of the applicable royalty term, unless earlier terminated. BI has the right to terminate the BI Collaboration and License Agreement for any reason after a specified notice period. Each party has the right to terminate the BI Collaboration and License Agreement on account of the other party’s bankruptcy or material, uncured breach.
In connection with the Company's entry into the BI Collaboration and License Agreement, the Company entered into an amendment to the Company's Einstein License whereby Einstein consented to the Company's entry into the BI Collaboration and License Agreement and granted the Company the right to sublicense to BI. In addition, the Company and Einstein agreed to amend specified upstream payment obligations that may be owed to Einstein by the Company, solely in connection with the sublicense to BI.
Amendment to Einstein License Agreement
On April 10, 2025, the Company entered into an amendment to the Einstein License. Pursuant to the amendment, Einstein consented to the Company’s entry into the BI Collaboration and License Agreement and granted the Company the right to sublicense to BI. In addition, Einstein and the Company agreed to amend specified upstream payment obligations that may be owed to Einstein by the Company, solely in connection with the sublicense to BI.
Underwriting Agreement
On April 14, 2025, the Company entered into an underwriting agreement (the “2025 Underwriting Agreement”) with Oppenheimer & Co. Inc., as representative of the several underwriters named therein (collectively, the “2025 Underwriters”), relating to an underwritten public offering of (i) 13,530,780 shares (the “2025 Shares”) of the Company’s common stock, $0.001 par value per share, and accompanying common stock warrants (“2025 Common Stock Warrants”) to purchase 3,382,695 shares of the Company’s common stock, and (ii) to certain investors in lieu of common stock, pre-funded warrants (the “2025 Pre-Funded Warrants”) to purchase 11,469,216 shares of the Company’s common stock and accompanying 2025 Common Stock Warrants to purchase 2,867,304 shares of common stock. All of the 2025 Shares, the 2025 Pre-Funded Warrants and the 2025 Common Stock Warrants will be sold by the Company. Each 2025 Share is being offered and sold together with an accompanying 2025 Common Stock Warrant at a combined offering price of $0.79, and each 2025 Pre-Funded Warrant is being offered and sold together with an accompanying 2025 Common Stock Warrant at a combined offering price of $0.789, which is equal to the combined offering price per share of common stock and accompanying 2025 Common Stock Warrant less the $0.001 exercise price of each 2025 Pre-Funded Warrant. The 2025 Underwriters will purchase (i) each 2025 Share and accompanying 2025 Common Stock Warrant from the Company pursuant to the 2025 Underwriting Agreement at a combined price of $0.7426 and (ii) each 2025 Pre-Funded Warrant and accompanying 2025 Common Stock Warrant from the Company pursuant to the 2025 Underwriting Agreement at a combined price of $0.74166.
The Company received approximately $18.0 million in net proceeds from the offering, after deducting underwriting discounts and commissions and offering expenses, and excluding any proceeds that may be received from exercise of the 2025 Common Stock Warrants and the 2025 Pre-Funded Warrants.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations of Cue Biopharma, Inc. and its subsidiary (“Cue Biopharma”, “we”, “us”, “our” or the “Company”) should be read in conjunction with our financial statements and accompanying notes included in this Quarterly Report on Form 10-Q and the financial statements and accompanying notes thereto for the fiscal year ended December 31, 2024 and the related Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission, or SEC, on March 31, 2025, or the 2024 Annual Report.
Overview
We are a clinical-stage biopharmaceutical company developing a novel class of injectable therapeutics engineered to selectively engage and modulate disease-specific T cells for the treatment of autoimmune disease and cancer. Unlike conventional approaches that broadly activate the immune system, our Immuno-STAT™ platform is designed to selectively modulate disease-relevant T cells, enhancing efficacy while minimizing off-target effects. We believe our Immuno-STAT platform holds the promise of producing drug product candidates with the potential of establishing new standards of care in the treatment of autoimmune disease and cancer. Our programs include, but are not limited to, drug product candidates designed to:
•
CUE-400 series (Autoimmune Diseases): Induce and expand regulatory T cells, or Tregs, with a novel and unique mechanism to not only proliferate but also generate Tregs, with the potential of restoring immune balance (e.g., CUE-401 for autoimmune conditions).
•
CUE-500 series (Targeted Cell Depletion): Redirect anti-viral killer T cells to target and eliminate defined pathogenic cells (e.g., CUE-501 for autoimmune B cell depletion, which has been licensed to Boehringer Ingelheim International GmbH).
•
CUE-100 series (Oncology): Selectively activate and expand tumor-specific T cells (e.g., CUE-101 for HPV+ cancers and CUE-102 for Wilms’ tumor 1 protein, or WT1, expressing cancers).
We aim to leverage our differentiated platform to establish new standards of care, forge strategic partnerships, and accelerate clinical development.
Immuno-STAT Platform Pipeline of Assets for Restoration of Immune Balance
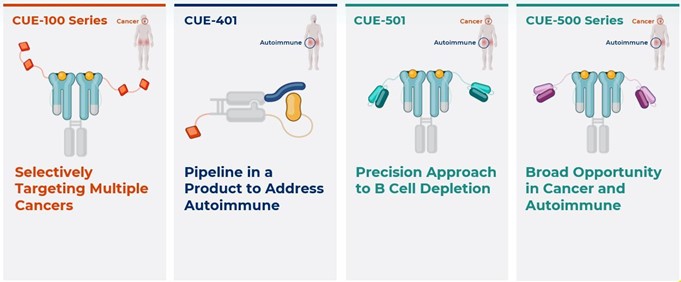
The Immuno-STAT framework is engineered to be highly flexible and modular, potentially enabling us to deploy the same or similar core functional elements to restore immune balance across diverse therapeutic approaches.
CUE-401
In autoimmune, we have developed novel approaches to restoring immune balance and health. CUE-401 is a novel bispecific composed of a masked, attenuated TGF-β with an interleukin 2, or IL-2, attenuated variant that together has demonstrated preclinically the ability to potently stimulate the generation and proliferation of Tregs. The masking of TGF-β enables the drug to have a biased, or conditional, binding to immune cells having both the IL-2 receptor, as well as the TGF-β receptors, enabling concurrent, conditional signaling. In autoimmune disease, Tregs are the master regulators of maintaining immune homeostasis, or balance, and health. Autoreactive T cells, referred to as Teff cells, are reactive against “self” proteins and foster inflammation and induce chronic tissue damage. Tregs are important to maintaining immune balance in that they possess the ability to dampen and control the Teff cells. CUE-401 has been specifically engineered and designed to foster Treg control by proliferating existing natural regulatory T cells, or nTregs, that develop in the thymus as well as inducing new Tregs, or iTregs, and reducing the number of inflammatory Teff cells, thereby restoring the essential Treg/Teff balance. As such, we believe that CUE-401 represents a novel approach to selectively induce and expand Tregs with the potential to provide superior therapeutic effects for the treatment for chronic autoimmune diseases.
We have conducted preclinical studies to characterize the pharmacokinetics, pharmacodynamics, and tolerability of CUE-401 across multiple animal species and provide insights towards translation to human clinical studies. These results, along with advances in the manufacturing of CUE-401, have derisked the development of this asset and identified a lead candidate molecule. Scale-up manufacturing and other IND-enabling studies for CUE-401 are ongoing. We are preparing to file an investigational new drug, or IND, application in the third quarter of 2026, with human proof-of-mechanism data anticipated in the first half of 2027, subject to sufficient funding. These early clinical trial results are anticipated to validate and further support the underlying mechanism of action representing a potential new standard of care of CUE-401 in multiple high-value autoimmune disease indications.
CUE-500 Series
In addition to CUE-401, we have also developed the CUE-500 series to enable T cell-mediated depletion of pathogenic cell types. We believe these biologics have the potential to achieve immune balance in autoimmune patients and are significantly differentiated from other competing approaches such as antibody drug conjugates, pan-T cell engagers, IL-2 muteins, TNFR2 agonists, and CAR-T therapies. The CUE-500 series represents a novel approach to selectively target disease-causing cells and redirect existing anti-viral memory T cells to targeted disease-causing cells and deplete these cells. CUE-501, for which we recently established a collaboration and license agreement with Boehringer Ingelheim International GmbH, or BI, is being developed to target and deplete autoimmune disease-causing B cells, in patients with autoimmune disease caused by autoreactive, pathogenic B cells. Targeted B cell depletion is widely recognized in the industry as a clinically validated and important approach for the treatment of B cell mediated autoimmune and inflammatory diseases. Due to its modularity, we believe that the CUE-500 series has therapeutic potential across multiple therapeutic areas. The mode of redirecting a defined population of already existing anti-viral T cells may apply to many pathogenic cell types readily addressed by swapping different cell-targeting antibody domains into the CUE-500 series framework.
We believe the preclinical data generated to date for the CUE-401 and the CUE-500 series demonstrates the intended mechanistic effect of these novel approaches for the potential treatment of autoimmune disease, and each represent potential breakthrough therapeutic opportunities for significant patient populations and potential near-term value creation opportunities for our shareholders as potential innovations providing transformative therapies to large, underserved patient populations.
CUE-100 Series
Historically, we primarily focused our resources on the development of our CUE-100 series for oncology, namely the CUE-101 and CUE-102 drug product candidates, which are representative of our approach to selectively activate targeted CD8+ T cells against cancer. Although we are prioritizing CUE-401 and the CUE-500 series, we are currently continuing to treat and monitor patients in a Phase 1b open-label expansion study investigating CUE-101 in the treatment of HPV+ recurrent metastatic, or R/M, head and neck squamous cell carcinoma, or HNSCC, in second line and beyond patients as a monotherapy. We are also conducting a Phase 1b clinical trial investigating CUE-101 in the treatment of HNSCC, as a first line therapy in combination with standard of care KEYTRUDA. We are also currently continuing to monitor patients in a Phase 1b clinical trial of CUE-102 as a monotherapy in late line R/M WT1+ colorectal, gastric, ovarian, and pancreatic cancer and are enabling an investigator sponsored trial to evaluate CUE-102 in recurrent glioblastoma, or GBM.
Plan of Operation
Our precision immunotherapies are in the development phase. We believe that our platforms have the potential for creating a diverse pipeline of promising drug product candidates addressing multiple medical indications. We also believe that our science is de-risked with clinical tolerability across a wide range of cancers from our Phase 1 trials of CUE-101 and CUE-102, with potential application in autoimmune disease. We intend to maximize the value and probability of commercialization of our Immuno-STAT drug product candidates by focusing on the development of CUE-401, for which we are preparing to file an IND application in the third quarter of 2026, while also establishing collaborations across our pipeline, such as our recent strategic collaboration and license agreement with BI for the development of CUE-501 described below and in Note 13 to the condensed consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q.
As a development-stage company, the majority of our business activities to date have been, and our planned future activities will be, devoted to furthering research and development of our drug product candidates.
Since a fundamental element of our corporate development strategy is to establish strategic partnerships with leading pharmaceutical or biotechnology organizations that will allow us to more fully exploit the potential of our technology platforms in the areas of oncology and autoimmune disease and accelerate and expand our pipelines, we are actively seeking collaborations for our CUE-101 and CUE-102 assets.
Events that Raise Substantial Doubt About Our Ability to Continue as a Going Concern
We will need to raise additional capital to fund our future operations and remain as a going concern. We expect to finance our future cash needs through a combination of equity offerings, collaborations, and other strategic alliances. Volatility in capital markets and general economic conditions in the U.S. may be a significant obstacle to raising the required funds and, as a result, we may be unable to secure the necessary funding on acceptable terms. This raises substantial doubt about our ability to continue as a going concern. For a further discussion of factors that raise substantial doubt about our ability to continue as a going concern, please see “– Liquidity and Capital Resources – Funding Requirements” and Part II. Item 1A, “Risk Factors” herein.
Critical Accounting Estimates and Significant Judgments
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting principles in the United States, or U.S. GAAP. The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements, and the reported revenue and expenses during the reported periods. We evaluate these estimates and judgments, including those described below, on an ongoing basis. We base our estimates on historical experience, known trends and events, contractual milestones and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our condensed consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q, we believe that the estimates, assumptions and judgments involved in the accounting policies described in Management’s Discussion and Analysis of Financial Condition and Results of Operations in Item 7 of our 2024 Annual Report have the greatest potential impact on our financial statements, so we consider those estimates, assumptions and judgments to be our critical accounting policies and estimates. There were no material changes to our critical accounting policies and estimates during the three months ended March 31, 2025.
Recent Accounting Pronouncements and Adopted Standards
A discussion of recent accounting pronouncements is included in Note 2 to our condensed consolidated financial statements appearing elsewhere in this Quarterly Report on Form 10-Q.
Significant Contracts and Agreements Related to Research and Development Activities
Einstein License Agreement
On January 14, 2015, we entered into a license agreement, as amended and restated on July 31, 2017, and as further amended on October 30, 2018, January 13, 2024 and April 10, 2025, or the Einstein License, with Albert Einstein College of Medicine, or Einstein, for certain patent rights, or the Patents, relating to our core technology platform for the engineering of biologics to control T cell activity, precision, immune-modulatory drug product candidates, and two supporting technologies that enable the discovery of costimulatory signaling molecules (ligands) and T cell targeting peptides.
We hold an exclusive worldwide license, with the right to sublicense, import, make, have made, use, provide, offer to sell, and sell all products, processes and services that use the Patents, including certain technology received from Einstein related thereto, which we refer to as the Einstein Licensed Products. Under the Einstein License, we are required to:
•
Pay royalties and amounts based on a certain percentage of proceeds, as defined in the Einstein License, from sales of Einstein Licensed Products and sublicense agreements.
•
Pay escalating annual maintenance fees, which are non-refundable, but are creditable against the amount due to Einstein for royalties.
•
Make significant payments based upon the achievement of certain milestones, as defined in the Einstein License. As of March 31, 2025, two of these milestones had been achieved, as we had filed an IND application in 2019, and initiated the investigator sponsored Phase 1b neoadjuvant clinical trial for CUE-101 in 2021.
•
Incur minimum product development costs per year and meet certain diligence obligations until the first commercial sale of the first Einstein Licensed Product.
On April 10, 2025, we entered into an amendment to the Einstein License. Pursuant to the amendment, Einstein consented to our entry into the BI Collaboration and License Agreement and granted us the right to sublicense to BI. In addition, we and Einstein agreed to amend specified upstream payment obligations that may be owed to Einstein by us, solely in connection with the sublicense to BI.
As of March 31, 2025, we were in compliance with our obligations under the Einstein License.
We account for the costs incurred in connection with the Einstein License in accordance with Accounting Standards Codification 730, Research and Development. For both the three months ended March 31, 2025 and 2024, we incurred less than $0.1 million in fees payable to Einstein in relation to this license. Such costs are included in research and development costs in our condensed consolidated statements of operations and comprehensive loss.
Collaboration Agreement with LG Chem
On November 6, 2018, we entered into a Collaboration, License and Option Agreement, and as amended from time to time, or the LG Chem Collaboration Agreement, with LG Chem Ltd., or LG Chem, pertaining to the development of CUE-101 and CUE-102 Immuno-STATs focused in the field of oncology.
Pursuant to the LG Chem Collaboration Agreement, we granted LG Chem an exclusive license to develop, manufacture and commercialize CUE-101, as well as CUE-102 Immuno-STATs that target T cells against two additional cancer antigens in certain Asian countries, which we refer to collectively as the LG Chem Territory.
On March 11, 2025, we and LG Chem entered into the Ninth Amendment to the LG Chem Collaboration Agreement, or the Ninth Amendment. As of the date of the Ninth Amendment, we regained our rights to the LG Chem Territory for the CUE-101 program, which had been licensed to LG Chem, and LG Chem terminated all of its rights to the same program. Pursuant to the Ninth Amendment, we agreed to make future payments to LG Chem, if and when one or more potential scenarios related to the CUE-101 program occur, up to a predetermined aggregate amount. LG Chem continues to maintain its interest and rights in the CUE-102 program, targeting WT1 expressing cancers, pursuant to the LG Chem Collaboration Agreement.
We did not recognize any revenue related to the LG Chem Collaboration Agreement for the three months ended March 31, 2025 . For the three months ended March 31, 2024 we recognized revenue of less than $0.1 million related to the LG Chem Collaboration Agreement. As of March 31, 2025, we had recorded $20.0 million in collaboration revenue related to this agreement since the agreement was entered into. The majority of the research phase of the LG Chem Collaboration Agreement was completed by March 31, 2022.
Collaboration and Option Agreement with Ono
In February 2023, we entered into a strategic collaboration agreement, or the Ono Collaboration and Option Agreement, with Ono Pharmaceutical Co., Ltd., or Ono, to further develop CUE-401. In March 2025, we and Ono agreed to terminate the Ono Collaboration and Option Agreement, effective as of March 6, 2025. At such time, the Ono Collaboration and Option Agreement had no further force or effect with the exception of certain customary provisions which are intended to survive termination and expiration of the Ono Collaboration and Option Agreement. We retained all rights to CUE-401.
Under the terms of the Ono Collaboration and Option Agreement, Ono paid us an upfront payment and agreed to fully fund all research and development activities related to CUE-401 through a specified option period of 24 months, or the Ono Research Term. Per the agreement, as consideration for the research and development activities performed by us, Ono (i) made a one-time, non-refundable, non-creditable upfront payment of $3.0 million to us in March 2023, and (ii) agreed to reimburse us for all costs incurred in conducting research, including (a) pass through costs from third party contractors and (b) full time employee salaries capped at $2.1 million in the first 18 months of the Ono Research Term. Subsequently, we and Ono agreed to increase this cap for full-time employee salaries to $3.1 million.
For the three months ended March 31, 2025 and 2024, we recognized revenue of $0.4 million and $1.7 million, respectively, related to the Ono Collaboration and Option Agreement. We and Ono have satisfied all of our performance obligations and made all outstanding payments required under the agreement.
BI Collaboration and License Agreement
On April 10, 2025, we entered into a Collaboration and License Agreement with BI, or the BI Collaboration and License Agreement, to research, develop and commercialize differentiated B cell depletion molecules, including CUE-501.
Under the terms of the BI Collaboration and License Agreement, we and BI will conduct collaborative research focused on CUE-501 during a four-year period or, if earlier, the completion of activities under the research plans, or the BI Research Term. In addition to, or instead of, CUE-501, BI may elect, at its sole discretion, to include additional or alternative compounds targeted at B cell depletion. BI will have an exclusive, royalty-bearing, worldwide, sublicensable license, under our applicable patents and know-how, to develop, manufacture and commercialize such compounds and their derivatives, or BI Licensed Products, for all uses, and BI shall be responsible for all further research, preclinical and clinical development, manufacturing, regulatory approvals, and commercialization of BI Licensed Products at its expense. During the BI Research Term, we are prohibited from developing or commercializing any molecule for applications in B cell depletion.
Pursuant to the terms of the BI Collaboration and License Agreement, we received an upfront payment of approximately $12.0 million and will be eligible to receive up to an aggregate of approximately $345.0 million in success-based research, development and commercial milestone payments, beginning with two preclinical development milestones, as well as royalty payments on net sales. The royalty payments will be subject to reduction due to patent expiration, payments made under certain licenses for third-party intellectual property and generic competition. During the BI Research Term, BI will also make research support payments to us.
The BI Collaboration and License Agreement will continue, on a product-by-product and country-by-country basis, until the expiration of the applicable royalty term, unless earlier terminated. BI has the right to terminate the BI Collaboration and License Agreement for any reason after a specified notice period. Each party has the right to terminate the BI Collaboration and License Agreement on account of the other party’s bankruptcy or material, uncured breach. In connection with our entry into the BI Collaboration and License Agreement, we entered into an amendment to our Einstein License whereby Einstein consented to our entry into the BI Collaboration and License Agreement and granted us the right to sublicense to BI. In addition, we and Einstein agreed to amend specified upstream payment obligations that may be owed to Einstein by us, solely in connection with the sublicense to BI.
Components of Results of Operations
Collaboration Revenue
We have not yet generated commercial revenue from product sales. To date, we have generated revenue from collaboration agreements with Merck Sharp & Dohme Corp. (which terminated in December 2022), LG Chem, and Ono (which terminated in March 2025). We expect to begin to recognize revenue from the BI Collaboration and License Agreement in the second quarter of 2025.
Our collaboration revenue may vary from period to period depending on the progress of our work in connection with our collaboration agreements.
Research and Development Expenses
Research and development expenses consist primarily of compensation costs, fees paid to consultants, outside service providers and organizations (including research institutes at universities), facility costs, and development and clinical trial costs with respect to our drug product candidates. We utilize our employee and infrastructure resources across multiple research and development programs, and do not track these costs by project. We believe the attempted allocation of these costs by project would be arbitrary and not meaningful.
Research and development expenses incurred under contracts are expensed ratably over the life of the underlying contracts, unless the achievement of milestones, the completion of contracted work, or other information indicates that a different pattern of performance is more appropriate. Other research and development expenses are charged to operations as incurred.
Nonrefundable advance payments are recognized as an expense as the related services are performed. We evaluate whether we expect the services to be rendered at each quarter end and year end reporting date. If we do not expect the services to be rendered, the advance payment is recorded as expense. Nonrefundable advance payments for research and development services are included in prepaid and other current assets on the balance sheet. To the extent that a nonrefundable advance payment is for contracted services to be performed within 12 months from the reporting date, such advance is included in current assets; otherwise, such advance is included in non-current assets.
We evaluate the status of our research and development agreements and contracts, and the carrying amount of the related assets and liabilities, at each quarter end and year end reporting date, and adjust the carrying amounts and their classification on the balance sheet as appropriate.
The following table summarizes our research and development expenses by category for the three months ended March 31, 2025 and 2024 (in millions):
|
|
|
|
|
|
|
|
|
|
|
|
March 31, |
|
|
2025 |
|
|
2024 |
|
|
Employee compensation |
|
$ |
3.0 |
|
|
$ |
3.9 |
|
|
Clinical trial costs |
|
|
1.8 |
|
|
|
2.6 |
|
|
Facilities and overhead |
|
|
1.2 |
|
|
|
1.2 |
|
|
Contract manufacturing costs |
|
|
1.9 |
|
|
|
1.6 |
|
|
Lab costs |
|
|
0.4 |
|
|
|
0.7 |
|
|
Professional fees |
|
|
0.2 |
|
|
|
0.2 |
|
|
Total |
|
$ |
8.5 |
|
|
$ |
10.2 |
|
|
General and Administrative Expenses
General and administrative expenses consist of salaries and related expenses for executive, legal, finance, human resources, information technology and administrative personnel, as well as professional fees, insurance costs, and other general corporate expenses. We expect general and administrative expenses to remain consistent in future periods as we continue to incur expenses related to our operation as a public company, which requires our ongoing compliance with certain laws and regulations.
Interest Income
We earn interest income from cash invested in money market funds.
Interest Expense
We incur interest expense from borrowings under our Loan and Security Agreement, as amended, or the Loan Agreement, with Silicon Valley Bank, a division of First Citizens Bank & Trust Company, or SVB.
Results of Operations
Three Months Ended March 31, 2025 and 2024
Our condensed consolidated statements of operations and comprehensive loss for the three months ended March 31, 2025 and 2024, as discussed herein, are presented below in thousands.
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
March 31, |
|
|
|
|
2025 |
|
|
2024 |
|
|
Collaboration revenue |
|
$ |
421 |
|
|
$ |
1,717 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
General and administrative |
|
|
4,173 |
|
|
|
4,186 |
|
|
Research and development |
|
|
8,547 |
|
|
|
10,199 |
|
|
Total operating expenses |
|
|
12,720 |
|
|
|
14,385 |
|
|
Loss from operations |
|
|
(12,299 |
) |
|
|
(12,668 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
Interest income |
|
|
170 |
|
|
|
562 |
|
|
Interest expense |
|
|
(128 |
) |
|
|
(241 |
) |
|
Total other income, net |
|
|
42 |
|
|
|
321 |
|
|
Net loss |
|
$ |
(12,257 |
) |
|
$ |
(12,347 |
) |
|
Collaboration Revenue
Collaboration revenue decreased by $1.3 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. The decrease was due to more revenue earned during the three months ended March 31, 2024 from the Ono Collaboration and Option Agreement, which was terminated in March 2025.
General and Administrative Expenses
General and administrative expenses decreased by less than $0.1 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. The decrease was primarily due to a decrease in employee compensation, which includes stock-based compensation, offset by an increase in professional fees.
Research and Development Expenses
Research and development expenses decreased by $1.7 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. The decrease was primarily due to decreases in clinical trial costs for our CUE-101 and CUE-102 Phase 1 trials as activities shifted to patient survival monitoring, as well as decreases in employee compensation, which includes stock-based compensation.
Interest Income
Interest income decreased by $0.4 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. The decrease was due to lower interest earned on cash and cash equivalents.
Interest Expense
Interest expense decreased by $0.1 million for the three months ended March 31, 2025 compared to the three months ended March 31, 2024. This was due to a decrease in interest owed from borrowings under our Loan Agreement with SVB.
Liquidity and Capital Resources
We have financed our working capital requirements primarily through private and public offerings of equity securities, cash received from Merck Sharp & Dohme Corp., LG Chem, and Ono under the respective collaboration agreements, and borrowings under the Loan Agreement.
The amounts that we actually spend for any specific purpose may vary significantly and will depend on a number of factors, including, but not limited to, our research and development activities and programs, clinical testing, regulatory approval, market conditions, and changes in or revisions to our business strategy and technology development plans.
On May 9, 2023, we filed a registration statement on Form S-3, which was declared effective on May 26, 2023 (File No. 333-271786), to register for sale from time to time up to $300 million of our common stock, preferred stock, debt securities, warrants, subscription rights and/or units in one or more offerings.
In October 2021, we entered into an open market sale agreement, or the ATM Sales Agreement, with Jefferies LLC, or Jefferies, as agent, to sell shares of our common stock for aggregate gross proceeds of up to $80 million, from time to time, through an at-the-market equity offering program. The ATM Sales Agreement will terminate upon the earliest of (a) the sale of $80 million of shares of our common stock pursuant to the ATM Sales Agreement or (b) the termination of the ATM Sales Agreement by us or Jefferies. We did not sell any shares under the ATM Sales Agreement during the three months ended March 31, 2025. During the three months ended March 31, 2024, we sold 1,428,200 shares of common stock under the ATM Sales Agreement for proceeds of $3.4 million, net of commissions paid, but excluding transaction expenses. As of March 31, 2025, we sold an aggregate of 9,072,231 shares of common stock under the ATM Sales Agreement for proceeds of $40.4 million, net of commissions paid, but excluding transaction expenses, since its inception.
On February 15, 2022, we entered into the Loan Agreement, pursuant to which we have borrowed $10.0 million. The Loan Agreement was amended in April 2023 and October 2024. The term loans under the Loan Agreement, or the Term Loans, bear interest at a floating rate per annum equal to the greater of (A) the prime rate (as published in the money rates section of The Wall Street Journal) plus 2.25% and (B) 5.50%. On the first calendar day of each month, we will be required to make monthly interest payments and commencing on June 30, 2023, we began repayment of the Term Loans in (i) 30 consecutive installments of principal plus monthly payments of accrued interest if the additional term loans are not advanced and (ii) 24 months if the additional term loans are advanced. All outstanding principal and accrued and unpaid interest under the Term Loans and all other outstanding obligations with respect to the Term Loans are due and payable in full on December 1, 2025.
The Loan Agreement permits voluntary prepayment of all, but not less than all, of the Term Loans, subject to a prepayment premium except if the facility is refinanced with another First Citizens Bank facility. Such prepayment premium would be 1.00% of the principal amount of the Term Loans. Upon prepayment or repayment in full of the Term Loans, we will be required to pay a one-time final payment fee equal to 5.00% of the original principal amount of any funded Term Loans being repaid. The Loan Agreement, as amended, also requires us to have at all times on deposit in our accounts maintained with SVB, unrestricted and unencumbered cash in an amount equal to the lesser of (i) 100% of the dollar value of our consolidated cash, in the aggregate, at all financial institutions, and (ii) $20,000,000.
On March 10, 2023, SVB was closed and the Federal Deposit Insurance Company, or FDIC, was appointed receiver for the bank. The FDIC created a successor bridge bank, and all deposits of SVB were transferred to the bridge bank under a systemic risk exception approved by the U.S. Department of the Treasury, the Federal Reserve and the FDIC. On March 27, 2023, First Citizens Bank assumed all of SVB’s deposits and certain other liabilities and acquired substantially all of SVB’s loans and certain other assets from the FDIC. First Citizens Bank continues to hold our Term Loans under the same existing terms and covenants which were in place with SVB.
On September 26, 2024, we entered into an underwriting agreement, or the 2024 Underwriting Agreement, with Oppenheimer & Co. Inc., as representative of the several underwriters named therein, or, collectively, the 2024 Underwriters, relating to an underwritten public offering of (i) 11,564,401 shares, or the 2024 Shares, of our common stock, $0.001 par value per share, and accompanying common stock warrants, or the2024 Common Stock Warrants, to purchase 2,891,100 shares of our common stock, and (ii) to certain investors in lieu of common stock, pre-funded warrants, or the 2024 Pre-Funded Warrants, to purchase 12,435,599 shares of our common stock and accompanying 2024 Common Stock Warrants to purchase 3,108,900 shares of common stock. All of the 2024 Shares, the 2024 Pre-Funded Warrants and the 2024 Common Stock Warrants were sold by us. Each 2024 Share was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.50, and each 2024 Pre-Funded Warrant was offered and sold together with an accompanying 2024 Common Stock Warrant at a combined offering price of $0.499, which is equal to the combined offering price per share of common stock and accompanying 2024 Common Stock Warrant less the $0.001 exercise price of each 2024 Pre-Funded Warrant.
The 2024 Underwriters purchased (i) each 2024 Share and accompanying 2024 Common Stock Warrant from us pursuant to the 2024 Underwriting Agreement at a combined price of $0.47 and (ii) each 2024 Pre-Funded Warrant and accompanying 2024 Common Stock Warrant from us pursuant to the 2024 Underwriting Agreement at a combined price of $0.46906. We recorded net proceeds from the offering of $10.8 million, after deducting underwriting discounts and commissions and offering expenses of $1.1 million, and excluding any proceeds that may be received from exercise of the 2024 Common Stock Warrants and the 2024 Pre-Funded Warrants.
On April 14, 2025, we entered into an underwriting agreement, or the 2025 Underwriting Agreement, with Oppenheimer & Co. Inc., as representative of the several underwriters named therein, or, collectively, the 2025 Underwriters, relating to an underwritten public offering of (i) 13,530,780 shares, or the 2025 Shares, of our common stock, $0.001 par value per share, and accompanying common stock warrants, or the 2025 Common Stock Warrants to purchase 3,382,695 shares of our common stock, and (ii) to certain investors in lieu of common stock, pre-funded warrants, or the 2025 Pre-Funded Warrants, to purchase 11,469,216 shares of our common stock and accompanying 2025 Common Stock Warrants to purchase 2,867,304 shares of common stock. All of the 2025 Shares, the 2025 Pre-Funded Warrants and the 2025 Common Stock Warrants were sold by us. Each 2025 Share was offered and sold together with an accompanying 2025 Common Stock Warrant at a combined offering price of $0.79, and each 2025 Pre-Funded Warrant was offered and sold together with an accompanying 2025 Common Stock Warrant at a combined offering price of $0.789, which is equal to the combined offering price per share of common stock and accompanying 2025 Common Stock Warrant less the $0.001 exercise price of each 2025 Pre-Funded Warrant. The 2025 Underwriters purchased (i) each 2025 Share and accompanying 2025 Common Stock Warrant from us pursuant to the 2025 Underwriting Agreement at a combined price of $0.7426 and (ii) each 2025 Pre-Funded Warrant and accompanying 2025 Common Stock Warrant from us pursuant to the 2025 Underwriting Agreement at a combined price of $0.74166. We received net proceeds from the offering of approximately $18.0 million, after deducting underwriting discounts and commissions and offering expenses, and excluding any proceeds that may be received from exercise of the 2025 Common Stock Warrants and the 2025 Pre-Funded Warrants.
Cash Flows
Based on our current plans and forecasted expenses, we believe our existing cash and cash equivalents as of March 31, 2025, along with the approximately $18.0 million of net proceeds received from our offering in April 2025 and an upfront payment of approximately $12.0 million we received pursuant to the BI Collaboration and License Agreement, will enable us to fund our operations into the second quarter of 2026. However, we will need to raise substantial additional capital to fund our future operations and remain as a going concern. We expect to finance our future cash needs through a combination of equity offerings, collaborations, and other strategic alliances. Volatility in capital markets and general economic conditions in the United States may be a significant obstacle to raising the required funds and, as a result, we may be unable to secure the necessary funding on acceptable terms. This raises substantial doubt about our ability to continue as a going concern.
The following table summarizes our changes in cash, cash equivalents, and restricted cash for the three months ended March 31, 2025 and 2024 in thousands:
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended |
|
|
|
March 31, |
|
|
|
2025 |
|
|
2024 |
|
Net cash provided by (used in): |
|
|
|
|
|
|
Operating activities |
|
$ |
(8,172 |
) |
|
$ |
(9,784 |
) |
Investing activities |
|
|
(150 |
) |
|
|
(55 |
) |
Financing activities |
|
|
(1,000 |
) |
|
|
2,354 |
|
Net change in cash, cash equivalents, and restricted cash |
|
$ |
(9,322 |
) |
|
$ |
(7,485 |
) |
Operating Activities
Net cash used in operating activities totaled $8.2 million for the three months ended March 31, 2025 compared to $9.8 million for the three months ended March 31, 2024. The decrease of $1.6 million was primarily due to decreases in cash outflows from changes in stock-based compensation, offset by increases from changes in accounts receivable and accounts payable.
Investing Activities
Net cash used in investing activities totaled $0.2 million for the three months ended March 31, 2025 compared to $0.1 million during the three months ended March 31, 2024. The increase of $0.1 million in cash used was due to an increase in purchases of property and equipment during the three months ended March 31, 2025.
Financing Activities
Net cash used in financing activities totaled $1.0 million for the three months ended March 31, 2025 compared to net cash provided by financing activities of $2.4 million for the three months ended March 31, 2024. The change of $3.4 million was due to proceeds received from sales under our ATM Sales Agreement during the three months ended March 31, 2024.
Funding Requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we continue the research and development of our Immuno-STAT platform and continue ongoing and initiate new clinical trials of and seek marketing approval for our drug product candidates. In addition, we expect to incur additional costs associated with operating as a public company. Our expenses will also increase if, and as, we:
•
continue the preclinical development of CUE-401 and the CUE-500 series (excluding CUE-501, which has been licensed to BI);
•
continue to assess maturing clinical data of our CUE-100 series, including CUE-101 and CUE-102, which we have deprioritized;
•
leverage our programs, including our autoimmune programs, to advance our other drug product candidates into preclinical and clinical development;
•
seek regulatory approvals for any drug product candidates for which we successfully complete clinical trials;
•
seek to discover and develop additional drug product candidates;
•
establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any drug product candidates for which we may obtain marketing approval and intend to commercialize on our own or jointly;
•
expand our manufacturing, quality, operational, financial and management systems, including personnel to support these functions;
•
maintain, expand and protect our intellectual property portfolio;
•
acquire or in-license other drug product candidates and technologies; and
•
incur additional legal, accounting and other expenses in operating as a public company.
Under Accounting Standards Update, or ASU, 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40), or, ASC 205-40, we have the responsibility to evaluate whether conditions or events raise substantial doubt about our ability to meet our future financial obligations as they become due within one year after the date the financial statements are issued. Under ASC 205-40, this evaluation initially cannot take into consideration the potential mitigating effects of plans that have not been fully implemented as of the date the financial statements are issued. Since we currently believe that our existing cash and cash equivalents, as of March 31, 2025, and our current operating plans, which reflect the approximately $18.0 million of net proceeds received from our offering in April 2025 and an upfront payment of approximately $12.0 million we received pursuant to the BI Collaboration and License Agreement, will enable us to fund our operations into the second quarter of 2026, we have determined that this cash runway of less than 12 months from the date of issuance of our financial statements included in this Quarterly Report on Form 10-Q, along with our accumulated deficit, history of losses, and future expected losses meet the ASC 205-40 standard for raising substantial doubt about our ability to continue as a going concern within one year of the issuance date of our financial statements included in this Quarterly Report on Form 10-Q. While we have plans in place to mitigate this risk, which primarily consist of raising additional capital through a combination of equity offerings, collaborations, and other strategic alliances, and, depending on the availability and level of additional financings, and cash expenditure reduction, there is no guarantee that we will be successful in these mitigation efforts.
We will need to raise additional capital or incur additional indebtedness to continue to fund our operations in the near term. Our ability to raise additional funds will depend on financial, economic and market conditions, many of which are outside of our control, and we may be unable to raise financing when needed, or on terms favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, reduce or eliminate our product development or future commercialization efforts, or grant rights to develop and market drug product candidates that we would otherwise prefer to develop and market ourselves, which could adversely affect our business prospects, and we may be unable to continue our operations. Because of numerous risks and uncertainties associated with the research, development and commercialization of our drug product candidates, we are unable to estimate the exact amount of our working capital requirements. Factors that may affect our planned future capital requirements and accelerate our need for additional working capital include the following:
•
the progress, timing, scope and costs of our clinical trials, including the ability to timely enroll patients in our ongoing, planned and any future clinical trials;
•
our ability to secure third party support through partnerships and collaborations to further develop the CUE-100 series programs, including CUE-101 and CUE-102, as we have done for CUE-501;
•
the outcome, timing and cost of regulatory approvals by the FDA and other comparable regulatory authorities, including the potential that the FDA or other comparable regulatory authorities may require that we perform more studies than those that we currently expect;
•
the number and characteristics of drug product candidates that we may in-license and develop;
•
our ability to successfully commercialize our drug product candidates, if approved;
•
the amount of sales and other revenues from drug product candidates that we may commercialize, if any, including the selling prices for such potential products and the availability of adequate third-party reimbursement;
•
selling and marketing costs associated with our potential products, including the cost and timing of expanding our marketing and sales capabilities;
•
the terms and timing of any potential future collaborations, licensing or other arrangements that we may establish;
•
cash requirements of any future acquisitions and/or the development of other drug product candidates;
•
the costs of operating as a public company;
•
the cost and timing of completion of commercial-scale, outsourced manufacturing activities;
•
the time and cost necessary to respond to technological and market developments;
•
the impact of government laws and regulations, general economic and market conditions, inflation, and the imposition of new or revised global trade tariffs;
•
any disputes which may occur between us and our employees, collaborators, including Einstein, LG Chem and BI, or other prospective business partners; and
•
the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights.
A change in the outcome of any of these or other variables with respect to the development of any of our drug product candidates could significantly change the costs and timing associated with the development of that drug product candidate. Further, our operating plans may change in the future, and we may need additional funds to meet operational needs and capital requirements associated with such operating plans.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties and grants from organizations and foundations. If we raise additional funds by selling shares of our common stock or other equity-linked securities, the ownership interest of our current stockholders will be diluted. New investors may demand rights, preferences or privileges senior to those of existing holders of our common stock. If we issue debt securities, we may be required to grant security interests in our assets, could have substantial debt service obligations, and lenders may have a senior position (compared to stockholders) in any potential future bankruptcy or liquidation. We may seek to access the public or private capital markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or drug product candidates or to grant licenses on terms that may not be acceptable to us.
Additionally, corporate collaboration and licensing arrangements may require us to incur non-recurring and other charges, give up certain rights relating to our intellectual property and research and development activities, increase our near and long-term expenditures, issue securities that dilute our existing stockholders, issue debt which may require liens on our assets and which will increase our monthly expense obligations, or disrupt our management and business.
If we are unable to raise additional capital when needed, we may be required to curtail the development of our technology or materially curtail or reduce our operations. We could be forced to sell or dispose of our rights or assets. Any inability to raise adequate funds on commercially reasonable terms could have a material adverse effect on our business, results of operation and financial condition, including the possibility that a lack of funds could cause our business to fail, dissolve and liquidate with little or no return to investors.
Principal Commitments
There have been no material changes to our contractual obligations and commitments as described in Management’s Discussion and Analysis of Financial Condition and Results of Operations in Item 7 of our 2024 Annual Report. Additional information regarding the BI Collaboration and License Agreement and the amendment to our Einstein License may be found above and in Note 13 to our condensed consolidated financial statements in this Quarterly Report on Form 10-Q.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As a smaller reporting company, we are not required to provide the information required by this Item 3.
ITEM 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We are responsible for maintaining disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, or the Exchange Act. Disclosure controls and procedures are controls and other procedures designed to ensure that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Based on our management’s evaluation (with the participation of our principal executive officer and our principal financial officer) of our disclosure controls and procedures as required by Rule 13a-15 under the Exchange Act, our principal executive officer and our principal financial officer have concluded that our disclosure controls and procedures were effective as of March 31, 2025, the end of the period covered by this report.
Inherent Limitations on Effectiveness of Controls
Our management, including our principal executive officer and our principal financial officer, do not expect that our disclosure controls or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. The design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Further, because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of control effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting during the three months ended March 31, 2025 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
PART II. OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
We are not currently a party to any material legal proceedings.
ITEM 1A. RISK FACTORS
We operate in a rapidly changing environment that involves a number of risks that could materially affect our business, financial condition or future results, some of which are beyond our control. The occurrence of any of these risks could harm our business, financial condition, results of operations and/or growth prospects or cause our actual results to differ materially from those contained in forward-looking statements we have made in this report and those we may make from time to time. In evaluating us and our business, you should carefully consider the following risks, the information included in this Quarterly Report on Form 10-Q and in other documents we file with the SEC and the risk factors previously disclosed in Part I. Item 1A, “Risk Factors” of our 2024 Annual Report.
Our recurring losses from operations raise substantial doubt regarding our ability to continue as a going concern.
We have incurred significant losses since our inception and have never generated revenue or profit from product sales, and it is possible we will never generate revenue or profit from product sales. As of March 31, 2025, we had cash and cash equivalents of $13.1 million. Based on our current operating plans, which reflect the approximately $18.0 million of net proceeds received from our offering in April 2025 and an upfront payment of approximately $12.0 million we received pursuant to the BI Collaboration and License Agreement, we believe we will have sufficient funds to meet our obligations into the second quarter of 2026. However, we will need to raise substantial additional capital to fund our future operations. There can be no assurance that we will be able to obtain additional funding, including through a combination of equity offerings, collaborations, and other strategic alliances, or other sources on acceptable terms, if at all. To the extent that we raise additional capital through future equity offerings, the ownership interest of common stockholders will be diluted, which dilution may be significant. We cannot guarantee that we will be able to obtain any or sufficient additional funding or that such funding, if available, will be obtainable on terms satisfactory to us. In the event that we are unable to obtain any or sufficient additional funding, there can be no assurance that we will be able to continue as a going concern, and we will be forced to delay, reduce or discontinue our product development programs or consider other various strategic alternatives.
Moreover, these factors raise substantial doubt about our ability to continue as a going concern. Substantial doubt about our ability to continue as a going concern may materially and adversely affect the price per share of our common stock, and it may be more difficult for us to obtain financing. If existing or potential collaborators decline to do business with us or potential investors decline to participate in any future financings due to such concerns, our ability to increase our cash position may be limited. The perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations.
Changes in and uncertainty surrounding U.S. trade policy could have a material adverse impact on our business, financial condition and results of operations.
The Trump Administration has recently imposed a series of tariffs on U.S. trading partners. On April 2, 2025, the President issued an Executive Order announcing a “baseline” reciprocal tariff of 10% on all U.S. trading partners effective April 5, 2025, and higher individualized reciprocal tariffs on 57 countries (with certain product exemptions for pharmaceutical-related products, among others). Previously, the administration had imposed a 25% tariff on Canada and Mexico for goods not covered by the United States-Mexico-Canada Agreement, or USMCA, and tariffs equaling 20% on China. In response, several countries threatened retaliatory measures, including Canada and China, which then imposed retaliatory tariffs. Prior to when the country-specific reciprocal tariffs were scheduled to take effect, the administration delayed the effective date of such tariffs for all countries except China. The 10% baseline reciprocal tariff on all countries remains in effect, in addition to the tariffs on China (which were a minimum of 145% as of May 8, 2025 and Canada and Mexico (which were 25% as of May 8, 2025 for goods that are not covered by the USMCA).
Separately, on April 16, 2025, the U.S. Department of Commerce announced an investigation under Section 232 of the Trade Expansion Act of 1962 into imports of pharmaceuticals and pharmaceutical ingredients, including finished drug products, medical countermeasures, critical inputs such as active pharmaceutical ingredients, and key starting materials, and derivative products of those items. The investigation will examine the impact of these imports on U.S. national security culminating in a decision by the President whether to take action to remedy any identified threats, including by imposing additional tariffs.
The statute provides that the Commerce Department report must be completed within 270 days of initiation of the investigation and that the President must decide whether to act within 90 days of receiving the report.
As a result of changes in tariffs that have been announced and/or implemented, and the underlying uncertainty currently surrounding international trade, we could experience a negative impact to our costs of materials and production processes, and supply chain disruptions and delays as a result of any new tariff policies or trade restrictions. If we are unable to obtain necessary raw materials or product components in sufficient quantity and in a timely manner due to disruptions in the global supply chain caused by macroeconomic events and conditions, the development, testing and clinical trials of our product candidates may be delayed or infeasible, and regulatory approval or commercial launch of any resulting product may be delayed or not obtained, which could significantly harm our business. We cannot yet predict the effect of the recently imposed U.S. tariffs on imports, or the extent to which other countries will impose quotas, duties, tariffs, taxes or other similar restrictions upon imports or exports in the future, nor can we predict future trade policy or the terms of any renegotiated trade agreements and their impact on our business.
If we fail to comply with the continued listing requirements of Nasdaq, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
We are required to comply with the continued listing requirements of the Nasdaq Stock Market LLC, or Nasdaq, including, among other things, maintaining a minimum closing bid price of at least $1.00 per share, or the Minimum Bid Requirement, or shares of our common stock may be subject to delisting, which would have a material adverse effect on our business.
On May 12, 2025, we received a deficiency letter, or the Notice, from the Listing Qualifications Department, or the Staff, of Nasdaq indicating that we failed to comply with the Minimum Bid Requirement. The Notice has no immediate effect on the listing of our common stock. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have an initial period of 180 calendar days (which expires on November 10, 2025) to regain compliance with the Minimum Bid Requirement. To regain compliance, the closing bid price of our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180 calendar day period, at which time the Staff will provide written notification to us that we comply with the Minimum Bid Requirement, unless the Staff exercises its discretion to extend this ten-day period pursuant to Nasdaq Listing Rule 5810(c)(3)(H).
If we do not regain compliance with the Minimum Bid Requirement during the initial 180 calendar day period, we may be eligible for an additional 180 calendar day compliance period. To qualify, we will be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the Minimum Bid Requirement, and will need to provide written notice to the Staff of our intention to cure the deficiency during the additional compliance period by effecting a reverse stock split, if necessary.
However, if we do not regain compliance with the Minimum Bid Requirement by November 10, 2025 and it appears to the Staff that we will not be able to regain compliance with the Minimum Bid Requirement during the additional compliance period, or that we are otherwise not eligible for an additional compliance period at that time, the Staff will provide notice that our common stock will become subject to delisting. Upon receipt of such notice, under Nasdaq rules, we may appeal the Staff’s delisting determination to a Hearings Panel, or the Panel. However, if we do appeal the delisting determination by the Staff to the Panel, there can be no assurance that such appeal would be successful, or that we will be able to regain compliance with the Minimum Bid Requirement or maintain compliance with the other listing requirements.
We intend to actively monitor the closing bid price of our common stock and will evaluate available options to regain compliance with the Minimum Bid Requirement. However, there can be no assurance that we will be able to regain compliance with the Minimum Bid Requirement. This potential delisting, and any other potential delisting, of our common stock could have a material adverse effect on the market for, and liquidity and price of, our common stock and would adversely affect our ability to raise capital on terms acceptable to us, or at all. Delisting from Nasdaq could also have other negative results, including, without limitation, the potential loss of confidence by investors, customers and employees and fewer business development opportunities. Any delisting of our common stock from Nasdaq would also make it more difficult for our stockholders to sell their shares of our common stock in the public market.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
None.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
(a) Notice of Delisting or Failure to Satisfy a Continued Listing Rule or Standard; Transfer of Listing
On May 12, 2025, we received a deficiency letter, or the Notice, from the Listing Qualifications Department, or the Staff, of Nasdaq notifying us that, for the last 30 consecutive business days, the bid price for our common stock had closed below $1.00 per share, which is the minimum bid price required to maintain continued listing on The Nasdaq Capital Market under Nasdaq Listing Rule 5550(a)(2), or the Minimum Bid Requirement.
The Notice has no immediate effect on the listing of our common stock. In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have an initial period of 180 calendar days (which expires on November 10, 2025) to regain compliance with the Minimum Bid Requirement. To regain compliance, the closing bid price of our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180 calendar day period, at which time the Staff will provide written notification to us that we comply with the Minimum Bid Requirement, unless the Staff exercises its discretion to extend this ten-day period pursuant to Nasdaq Listing Rule 5810(c)(3)(H).
If we do not regain compliance with the Minimum Bid Requirement during the initial 180 calendar day period, we may be eligible for an additional 180 calendar day compliance period. To qualify, we will be required to meet the continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the Minimum Bid Requirement, and will need to provide written notice to the Staff of our intention to cure the deficiency during the additional compliance period by effecting a reverse stock split, if necessary.
However, if we do not regain compliance with the Minimum Bid Requirement by November 10, 2025 and it appears to the Staff that we will not be able to regain compliance with the Minimum Bid Requirement during the additional compliance period, or that we are otherwise not eligible for an additional compliance period at that time, the Staff will provide notice that our common stock will become subject to delisting. Upon receipt of such notice, under Nasdaq rules, we may appeal the Staff’s delisting determination to a Hearings Panel, or the Panel. However, if we do appeal the delisting determination by the Staff to the Panel, there can be no assurance that such appeal would be successful, or that we will be able to regain compliance with the Minimum Bid Requirement or maintain compliance with the other listing requirements.
We intend to actively monitor the closing bid price of our common stock and will evaluate available options to regain compliance with the Minimum Bid Requirement. However, there can be no assurance that we will be able to regain compliance with the Minimum Bid Requirement.
(c) Director and Officer Trading Arrangements
None of our directors or officers adopted or terminated a Rule 10b5-1 trading arrangement or a non-Rule 10b5-1 trading arrangement (as defined in Item 408(c) of Regulation S-K) during the quarterly period covered by this report.
ITEM 6. EXHIBITS
|
|
|
|
|
|
|
|
|
Incorporated by Reference |
|
Exhibit
Number
|
Exhibit Description |
Filed
Herewith
|
Form |
Exhibit |
Filing Date |
Registration/File No. |
3.1 |
Amended and Restated Certificate of Incorporation, as amended |
|
10-Q |
3.1 |
11/14/2024 |
001-38327 |
10.1# |
Ninth Amendment to Collaboration, License and Option Agreement, dated March 6, 2025, between the Registrant and LG CHEM LTD. |
|
10-K |
10.11 |
3/31/2025 |
001-38327
|
10.2# |
Collaboration and License Agreement between the Registrant and Boehringer Ingelheim International GmbH dated April 10, 2025 |
X
|
|
|
|
|
10.3# |
Third Amendment to the Amended and Restated License Agreement with Albert Einstein College of Medicine dated April 10, 2025 |
X
|
|
|
|
|
31.1 |
Certification Pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 |
X |
|
|
|
|
31.2 |
Certification Pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934 |
X |
|
|
|
|
32.1 |
Certification Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
X |
|
|
|
|
101.INS |
Inline eXtensible Business Reporting Language (XBRL) Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
X |
|
|
|
|
101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
X |
|
|
|
|
101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
X |
|
|
|
|
101.DEF |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
X |
|
|
|
|
101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
X |
|
|
|
|
101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
X |
|
|
|
|
104 |
The cover page from the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, has been formatted in Inline XBRL. |
X |
|
|
|
|
# Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
Cue Biopharma, Inc. |
|
|
|
|
|
|
|
|
|
|
Dated: May 12, 2025 |
|
By: |
|
/s/ Daniel R. Passeri |
|
|
|
|
|
|
|
|
|
Daniel R. Passeri
Chief Executive Officer
(Principal Executive Officer)
|
|
|
|
|
|
Dated: May 12, 2025 |
|
By: |
|
/s/ Kerri-Ann Millar |
|
|
|
|
|
|
|
|
|
Kerri-Ann Millar
Chief Financial Officer
(Principal Financial and Accounting Officer)
|
EX-3.1
2
cue-ex3_1.htm
EX-3.1
EX-3.1
Exhibit 3.1
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
CUE BIOPHARMA, INC.
The present name of the corporation is Cue Biopharma, Inc. The corporation was incorporated under the name “Imagen Biopharma, Inc.” by the filing of its original Certificate of Incorporation with the Secretary of State of the State of Delaware on December 31, 2014. This Amended and Restated Certificate of Incorporation of the corporation, which restates and integrates and also further amends the provisions of the corporation’s Certificate of Incorporation was duly adopted in accordance with the provisions of Sections 242 and 245 of the Delaware General Corporation Law and by the written (or electronic) consent of its stockholders in accordance with Section 228 of the Delaware General Corporation Law. The Certificate of Incorporation of the corporation is hereby amended, integrated and restated to read in its entirety as follows:
ARTICLE I
Identification
SECTION 1.01. Name. The name of the Corporation is “Cue Biopharma, Inc.” (the “Corporation”).
ARTICLE II
Purpose
SECTION 2.01. Purpose. The purpose for which the Corporation is organized is to engage in any lawful act or activity for which corporations may be organized under the Delaware General Corporation Law (“DGCL”).
ARTICLE III
Capital Stock
SECTION 3.01. Amount. The total number of shares which the Corporation has authority to issue is 60,000,000 shares, consisting of: 10,000,000 shares designated as Preferred Stock, par value of $0.001 per share (“Preferred Stock”), and 50,000,000 shares designated as Common Stock, par value of $0.001 per share (“Common Stock”).
SECTION 3.02. Preferred Stock. Shares of Preferred Stock may be issued from time to time in one or more series. The Board of Directors (or any committee to which it may duly delegate the authority granted in this Article III) is hereby empowered to authorize the issuance from time to time of shares of Preferred Stock in one or more series, for such consideration and for such corporate purposes as the Board of Directors (or such committee thereof) may from time to time determine, and by filing a certificate (a “Preferred Stock Designation”) pursuant to applicable law of the State of Delaware, as it presently exists or may hereafter be amended, to establish from time to time for each such series the number of shares to be included in each such series and to fix the designations, powers, rights, and preferences of the shares of each such series, and the qualifications, limitations, and restrictions thereof to the fullest extent now or hereafter permitted by this Amended and Restated Certificate of Incorporation and the laws of the State of Delaware, including, without limitation, voting rights (if any), dividend rights, dissolution rights, conversion rights, exchange rights, and redemption rights thereof, as shall be stated and expressed in a resolution or resolutions adopted by the Board of Directors (or such committee thereof) providing for the issuance of such series of Preferred Stock. Each series of Preferred Stock shall be distinctly designated.
SECTION 3.03. Common Stock.
(A) The holders of shares of Common Stock shall be entitled to one vote for each such share on each matter submitted to the stockholders on which the holders of shares of Common Stock are entitled to vote. Except as otherwise required by law or this Amended and Restated Certificate of Incorporation, and subject to the rights of the holders of Preferred Stock, at any annual or special meeting of the stockholders the holders of shares of Common Stock shall have the right to vote for the election of directors and on all other matters submitted to a vote of the stockholders; provided, however, that, except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to this Amended and Restated Certificate of Incorporation that relates solely to the terms, number of shares, powers, designations, preferences, or relative participating, optional, or other special rights (including, without limitation, voting rights), or to qualifications, limitations, or restrictions thereon, of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together with the holders of one more other such series, to vote thereon pursuant to this Amended and Restated Certificate of Incorporation (including, without limitation, by any Preferred Stock Designation or pursuant to the DGCL.
(B) Subject to the rights of the holders of Preferred Stock, the holders of shares of Common Stock shall be entitled to receive such dividends and other distributions (payable in cash, property, or capital stock of the Corporation) when, as and if declared thereon by the Board of Directors from time to time out of any assets or funds of the Corporation legally available therefor, and shall share equally on a per share basis in such dividends and distributions.
(C) In the event of any voluntary or involuntary liquidation, dissolution, or winding-up of the Corporation, after payment or provision for payment of the debts and other liabilities of the Corporation, and subject to the rights of the holders of Preferred Stock in respect thereof, the holders of shares of Common Stock shall be entitled to receive all the remaining assets of the Corporation available for distribution to its stockholders, ratably in proportion to the number of shares of Common Stock held by them.
ARTICLE IV
Directors
SECTION 4.01. Management of the Corporation. The business and affairs of the Corporation shall be managed by or under the direction of the Board of Directors of the Corporation.
SECTION 4.02. Number. The number of directors of the Corporation shall be determined exclusively by resolution adopted by a majority of the Whole Board. For purposes of this Amended and Restated Certificate of Incorporation, the term “Whole Board” means the total number of authorized directors whether or not there exist any vacancies in previously authorized directorships.
SECTION 4.03. Election of Directors. The directors shall be elected at the annual meeting of stockholders by such stockholders as have the right to vote on such election. Directors need not be stockholders of the Corporation. Unless required by the Bylaws, the election of the Board of Directors need not be by written ballot.
SECTION 4.04. Vacancies. Any vacancy in the Board of Directors, however occurring, including a vacancy resulting from an enlargement of the Board of Directors, may be filled only by vote of a majority of the directors then in office, even if less than a quorum, or by a sole remaining director.
SECTION 4.05. Amendment of the Bylaws by the Board. The Board of Directors of the Corporation is expressly authorized to adopt, amend or repeal the Bylaws of the Corporation.
ARTICLE V
Indemnification
SECTION 5.01. Right to Indemnification and Advancement. The Corporation shall indemnify (and advance expenses to) its officers and directors to the fullest extent permitted by the DGCL, as amended from time to time.
ARTICLE VI
Director Liability
SECTION 6.01. Waiver of Liability. A director of the Corporation shall not be personally liable either to the Corporation or to any of its stockholders for monetary damages for breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. Any amendment or modification or repeal of the foregoing sentence or of the DGCL shall not adversely affect any right or protection of a director of the Corporation hereunder in respect of any act or omission occurring prior to the time of such amendment, modification, or repeal. If the DGCL hereafter is amended to further eliminate or limit the liability of a director, then a director of the Corporation, in addition to the circumstances in which a director is not personally liable as set forth in the preceding sentence, shall not be liable to the fullest extent permitted by the amended DGCL.
ARTICLE VII
Registered Agent and Registered Office
SECTION 7.01. Registered Agent and Office. The name and street address of the registered agent at the Corporation’s registered office are:
National Registered Agents, Inc.
160 Greentree Drive, Suite 101
Dover, DE 19904
County of Kent
ARTICLE VIII
Quorum Requirement
SECTION 8.01. Quorum. The holders representing a majority of the combined voting power of the capital stock issued and outstanding and entitled to vote at a meeting, present in person or represented by proxy, shall constitute a quorum.
ARTICLE IX
Cumulative Voting
SECTION 9.01. No Cumulative Voting. No holder of any shares of any class of stock of the Corporation shall be entitled to cumulative voting rights in any circumstances.
ARTICLE X
Preemptive Rights
SECTION 10.01. No Preemptive Rights. No stockholder shall have any preemptive rights to acquire unissued shares of the Corporation or securities of the Corporation convertible into or carrying a right to subscribe to or acquire shares.
ARTICLE XI
Internal Corporate Claims
SECTION 11.01. Venue for Internal Corporate Claims. Unless the Corporation consents in writing to the selection of an alternative forum, the Court of Chancery in the State of Delaware shall be the sole and exclusive forum for all “internal corporate claims.” “Internal corporate claims” mean claims, including claims in the right of the Corporation, (i) that are based upon a violation of a duty by a current or former director or officer or stockholder in such capacity or (ii) as to which Title 8 of the Delaware Code confers jurisdiction upon the Court of Chancery, except for, as to each of (i) through (ii) above, any claim as to which the Court of Chancery determines that there is an indispensable party not subject to the jurisdiction of the Court of Chancery (and the indispensable party does not consent to the personal jurisdiction of the Court of Chancery within ten days following such determination), which is vested in the exclusive jurisdiction of a court or forum other than the Court of Chancery, or for which the Court of Chancery does not have subject matter jurisdiction. If any provision or provisions of this Article XI shall be held to be invalid, illegal or unenforceable as applied to any person or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability of such provisions in any other circumstance and of the remaining provisions of this Article XI (including, without limitation, each portion of any sentence of this Article XI containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) and the application of such provision to other persons or entities and circumstances shall not in any way be affected or impaired thereby.
ARTICLE XII
Supermajority Provisions
SECTION 12.01. Amendment of the Certificate of Incorporation by Stockholders. The Corporation reserves the right to amend, alter, change or repeal any provision contained in this Amended and Restated Certificate of Incorporation, in the manner now or hereafter prescribed by statute, and all rights conferred upon stockholders herein are granted subject to this reservation; provided, however, that, notwithstanding any other provision of the Amended and Restated Certificate of Incorporation or any provision of law that might otherwise permit a lesser vote or no vote, but in addition to any vote of the holders of any class or series of the stock of the Corporation required by law or by this Amended and Restated Certificate of Incorporation, the affirmative vote of the holders of at least sixty-six and two-thirds percent (66 2/3%) of the voting power of the outstanding shares of stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to amend or repeal, or adopt any provision of this Amended and Restated Certificate of Incorporation inconsistent with Articles IV, V, XI and XII.
SECTION 12.02. Amendments to Bylaws by Stockholders. The stockholders shall also have the power to adopt, amend or repeal the Bylaws of the Corporation; provided, however, that, in addition to any vote of the holders of any class or series of stock of the Corporation required by law or by this Amended and Restated Certificate of Incorporation, the amendment of the Bylaws by the Corporation’s stockholders shall require the affirmative vote of the holders of at least sixty-six and two-thirds percent (66 2/3%) of the voting power of all of the then outstanding shares of the capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class.
IN WITNESS WHEREOF, this Amended and Restated Certificate of Incorporation has been executed by a duly authorized officer of this corporation on this 21st day of December, 2017.
CUE BIOPHARMA, INC.
|
|
|
/s/ Daniel R. Passeri |
|
By:
Title:
|
Daniel R. Passeri
Chief Executive Officer
|
STATE OF DELAWARE
CERTIFICATE OF AMENDMENT
OF AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
CUE BIOPHARMA, INC., a corporation duly organized and existing under the General Corporation Law of the State of Delaware (the “Corporation”), does hereby certify that:
FIRST: Pursuant to Section 242 of the General Corporation Law of the State of Delaware, this Certificate of Amendment of Amended and Restated Certificate of Incorporation amends and restates Section 3.01 of this corporation’s Amended and Restated Certificate of Incorporation to read in its entirety as follows:
SECTION 3.01. Amount: The total number of shares which the Corporation has authority to issue is 110,000,000 shares, consisting of: 10,000,000 shares designated as Preferred Stock, par value of $0.001 per share (“Preferred Stock”), and 100,000,000 shares designated as Common Stock, par value of $0.001 per share (“Common Stock”).
SECOND: The foregoing amendment was duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware.
IN WITNESS WHEREOF, said corporation has caused this certificate to be signed this 15th day of July, 2020.
CUE BIOPHARMA, INC.
|
|
|
/s/ Daniel R. Passeri |
|
By:
Title:
|
Daniel R. Passeri
Chief Executive Officer
|
CERTIFICATE OF AMENDMENT
OF
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
CUE BIOPHARMA, INC.
Pursuant to Section 242 of the
General Corporation Law of the State of Delaware
Cue Biopharma, Inc. (the “Corporation”), a corporation organized and existing under and by virtue of the General Corporation Law of the State of Delaware, does hereby certify:
FIRST: That the Board of Directors of the Corporation has duly adopted resolutions in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware authorizing, declaring advisable and approving an amendment to the Amended and Restated Certificate of Incorporation of the Corporation to (i) increase the number of authorized shares of capital stock of the Corporation and (ii) increase the number of authorized shares of Common Stock of the Corporation. Thereafter, the stockholders of the Corporation duly adopted such amendment in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware.
SECOND: That the amendment to the Amended and Restated Certificate of Incorporation of the Corporation set forth in this Certificate of Amendment was duly adopted in accordance with the provisions of Section 242 of the General Corporation Law of the State of Delaware by the Board of Directors and stockholders of the Corporation.
THIRD: That, upon the effectiveness of this Certificate of Amendment, Section 3.01 of Article III of the Amended and Restated Certificate of Incorporation, as heretofore amended, is hereby amended and restated in its entirety as follows:
“SECTION 3.01. Amount: The total number of shares which the Corporation has authority to issue is 210,000,000 shares, consisting of: 10,000,000 shares designated as Preferred Stock, par value of $0.001 per share (“Preferred Stock”), and 200,000,000 shares designated as Common Stock, par value of $0.001 per share (“Common Stock”).”
IN WITNESS WHEREOF, this Certificate of Amendment of Amended and Restated Certificate of Incorporation has been executed by a duly authorized officer of the Corporation on this 8th day of October, 2024.
CUE BIOPHARMA, INC.
|
|
|
/s/ Daniel R. Passeri |
|
By:
Title:
|
Daniel R. Passeri
Chief Executive Officer
|
EX-10.2
3
cue-ex10_2.htm
BI COLLABORATION
EX-10.2
Exhibit 10.2
Certain identified information has been excluded from the exhibit because it is both (i) not material and (ii) is the type of information that the registrant treats as private or confidential. Double asterisks denote omissions.
COLLABORATION AND LICENSE AGREEMENT
BY AND BETWEEN
BOEHRINGER INGELHEIM INTERNATIONAL GMBH
AND
CUE BIOPHARMA, INC.
BI Contract No.: 926652
BI PO No.: 43116788
COLLABORATION AND LICENSE AGREEMENT
This COLLABORATION AND LICENSE AGREEMENT (the “Agreement”) is effective as of April 10, 2025 (the “Effective Date”) and is entered into by and between BOEHRINGER INGELHEIM INTERNATIONAL GMBH, a corporation organized and existing under the laws of Germany, having a business address at Binger Str. 173, 55216 Ingelheim am Rhein, Germany (“BI”), and Cue Biopharma, Inc., a corporation organized and existing under the laws of the State of Delaware, having its registered office at 40 Guest Street, Boston, MA 02135, USA (“CUE”). BI and CUE are referred to individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, BI is a research-based pharmaceutical company that is a member of the Boehringer Ingelheim group of companies, which group possesses expertise and resources relating to the research, development, manufacturing and marketing of pharmaceutical products;
WHEREAS, CUE is biopharma company focused on biologic therapies for oncology and immunology driven diseases;
WHEREAS, BI recognizes CUE’s expertise in the field of T cell-mediated B cell depletion and the Parties wish to enter into a research collaboration to research and develop molecules consisting of a B Cell Targeting Module, [**] (as each defined below) with the initial focus on achieving lead optimization of CUE’s CUE-501 (as defined below) as further described in the Research Program;
WHEREAS, BI desires to obtain an exclusive license from CUE to further research, develop and commercialize Compounds and Products in the Territory (each as defined below), and CUE is willing to grant such a license to BI on the terms and conditions set forth herein;
WHEREAS, this Agreement sets forth the terms and conditions of the exclusive license to BI under the CUE Technology, the duration and scope of the rights granted to each Party, the ownership of Intellectual Property related to and/or generated under this Agreement, the collaborative research and/or development activities with respect to Compounds such as, inter alia, the responsibilities and activities to be performed by each Party under the Research Plan(s) (as defined below), and the consideration payments owed by BI to CUE.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and for good and sufficient consideration, the sufficiency of which is acknowledged by the Parties, the Parties hereby agree as follows:
Unless specifically set forth to the contrary under this Agreement, the following terms, whether used in singular or plural form, shall have the respective meanings set forth below.
1.1
“Accounting Standards” means in the case of BI and its Affiliates, the maintenance of records and books of accounts prepared in accordance with German Generally Accepted Accounting Principles (German GAAP, according to the German commercial code (“Handelsgesetzbuch”)), consistently applied by BI and as audited by BI’s group auditor.
If the applied accounting standards change in the future, then “Accounting Standards” means the accounting standards then consistently applied by BI across its operations and as audited by BI’s group auditor. In the case of any Sublicensee, “Accounting Standards” means the maintenance of records and books of accounts in accordance with the generally accepted accounting principles as consistently applied by the Sublicensee in their consolidated financial statements (if available) or as applied in their individual financial statements, as agreed in the sublicense agreement between BI and the Sublicensee, each as at the relevant balance sheet date.
1.2
“Affiliate” means in view of a Party or a Third Party, any corporation, firm, limited liability company, partnership, or other entity, that directly or indirectly controls, or is controlled by, or is under common control with such Party or such Third Party. For the purpose of this definition only, “control” and, with correlative meanings, the terms “controlled by” and “under common control with”, means ownership, directly or indirectly, of fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign entity in a particular jurisdiction provided that such foreign entity has the power to direct or cause the direction of the management or policies of a corporation) or more of the shares of stock entitled to vote for the election of directors in the case of a corporation, or fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign entity in a particular jurisdiction provided that such foreign entity has the power to direct or cause the direction of the management or policies of any other type of entity) or more of the equity interests in the case of any other type of legal entity, or status as a general partner in any partnership, or any other arrangement whereby the respective Party or Third Party controls or has the right to control the board of directors or equivalent governing body of a corporation or other entity and has the power to direct or cause the direction of such corporation or other entity.
1.3
“Annual Net Sales” means all Net Sales in a given territory in a Calendar Year.
1.4
“Applicable Law” means all laws, statutes, ordinances, regulations, rules, orders, and other pronouncements having the effect of law of any Governmental Authority or court of competent jurisdiction that may be in effect from time to time during the Term of this Agreement and applicable to a Party or a Party’s activities hereunder, including for clarity such rules, regulations and other requirements of any Regulatory Authority.
1.5
“Background IP” means any Intellectual Property Controlled by a Party (i) prior to or on the Effective Date or (ii) after the Effective Date and created, generated or acquired independently by a Party outside the scope of a Party’s activities under this Agreement during the Term.
1.6
“B Cell” means any white blood cell of the B lymphocyte lineage.
1.7
“B Cell Depletion” means an approach that involves the targeted reduction or elimination of B lymphocytes.
1.8
“[**] Claim” means a claim in any [**] that (a) recites a [**], and (b) does not recite a [**].
1.9
“B Cell Targeting Module” means a B Cell targeting single-chain variable fragment of a molecule.
1.10
“BI Steered CUE Patents” means [**].
1.11
“Business Day” means any day other than Saturday, Sunday or any other day on which commercial banks in Boston, USA or Ingelheim, Germany (as applicable) are authorized or required by law to remain closed.
1.12
“Calendar Quarter” means a period of three calendar months ending on March 31, June 30, September 30 or December 31 in any Calendar Year, or part thereof during the first or last quarter of the Term.
1.13
“Calendar Year” means a period of twelve (12) months commencing on January 1 and ending on December 31, except that the first Calendar Year during the Term shall commence on the Effective Date and end on December 31, 2025, and the last Calendar Year during the Term shall commence on January 1 of the year in which the Term ends and end on the last day of the Term.
1.14
“Change of Control” means, with respect to a Party, (1) a merger or consolidation or similar transaction of such Party with a Third Party that results (i) in the voting securities of such Party outstanding immediately prior thereto, or any securities into which such voting securities have been converted or exchanged, ceasing to represent more than fifty percent (50%) of the combined voting power of the surviving entity or the parent of the surviving entity immediately after such merger or consolidation or (ii) in the members of the board of directors of such Party immediately prior to such transaction constituting less than a majority of the members of the board of directors of such Party immediately following such transaction, or (2) a transaction or series of related transactions in which a Third Party, together with its Affiliates, (i) becomes the beneficial owner of more than fifty percent (50%) of the combined voting power of the outstanding securities of such Party or (ii) has the power, directly or indirectly, to elect a majority of the members of the Party’s board of directors, or similar governing body, or (3) the sale or other transfer to a Third Party of all or substantially all of such Party’s business or assets to which this Agreement relates, but in each case (1) through (3), excluding any consolidation or merger effected exclusively to change the domicile of a Party, any transaction or series of transactions effected principally for а bona fide financing transaction, and a stock sale to underwriters in a public offering. Any acquiring or combining Third Party with respect to a Party, and any of such Third Party’s Affiliates (other than the acquired Party and its Affiliates as in existence prior to the applicable transaction) are referred to collectively herein as the “Acquirer”.
1.15
“Clinical Trial” means any experiment in which a drug or therapy is administered or dispensed to, or used involving, one or more human subjects (including but not limited to a Phase I Clinical Trial, a Phase II Clinical Trial, and a Phase III Clinical Trial).
1.16
“Collaboration Foreground IP” means all Intellectual Property that is conceived, reduced to practice, discovered, developed or otherwise made by or on behalf of a Party after the Effective Date in the performance of a Research Project (either alone or jointly with the other Party).
1.17
“Collaboration Foreground Patents” mean Patents within Collaboration Foreground “Combination Product” means a pharmaceutical formulation containing as its active ingredients both a Compound and one or more other therapeutically active ingredients that is not a Compound.
1.19
“Commercialization” means any and all activities directed to the commercialization of a pharmaceutical product (including any Product), including Manufacturing for commercial sale, marketing, detailing, promotion, market research, distributing, order processing, handling returns and recalls, booking sales, customer service, administering, and commercially selling such product, importing, exporting, and transporting such product for commercial sale, patenting activities, and seeking pricing and reimbursement approval of such product (if applicable) whether before or after Regulatory Approval has been obtained, as well all regulatory compliance with respect to the foregoing, but excluding, in each case, activities directed to Development. When used as a verb, “Commercialize” means to engage in Commercialization.
1.20
“Commercially Reasonable Efforts” means, with respect to BI’s activities relating to the Development or Commercialization of a Compound and Product, [**], as the case may be, [**], in each case [**]. “Commercially Reasonable Efforts” means with respect to all other activities under this Agreement, [**].
1.21
“Compound” means CUE-501, each New Compound, and each Differentiated Compound.
1.22
“Confidential Information” means all information, data or Know-How, whether technical or non-technical, that is disclosed, orally, electronically, visually or in writing, by one Party or its Affiliates (“Disclosing Party”) to the other Party or its Affiliates (“Receiving Party”) pursuant to this Agreement provided, however, that Confidential Information shall not include any such information, data or Know-How that:
a)
is published or generally known to the public through no fault or omission on the part of the Receiving Party;
b)
was known by the Receiving Party prior to its disclosure by the Disclosing Party to the Receiving Party and not subject to restriction, as evidenced by the Receiving Party’s written records;
c)
is disclosed (other than in connection with this Agreement) to the Receiving Party by a Third Party without breaching any confidentiality obligations towards another Third Party or towards the Disclosing Party; or
d)
is independently developed by the Receiving Party as demonstrated by its contemporaneous written records without the use of Confidential Information of the Disclosing Party.
Information that is confidential and consists of a combination of information shall not be deemed to be in the public domain if individual elements of such information are in the public domain, unless the specific combination of those elements is also in the public domain. The terms of this Agreement shall be deemed the Confidential Information of both Parties. Confidential Information of a Party shall include any information disclosed by a Third Party to such Party under obligations of confidentiality and the terms of any agreements between such Third Party and such Party.
1.23
“Confidentiality Agreement” means collectively the Mutual Confidential Disclosure Agreement executed by and between CUE and BI on [**], the Confidential Disclosure Agreement Unilateral executed by and between CUE and BI on [**], the Mutual Confidential Disclosure Agreement executed by and between CUE and BI on [**], and the Confidentiality Agreement executed by and between CUE and BI on [**].
1.24
“Confidentiality Period” means the period of the Term of this Agreement, as determined in Section 19.1 (“Term”), and a period of [**] thereafter.
1.25
“Control”, “Controls” or “Controlled by” means for any item of or right under any Intellectual Property as well as for any Material, the possession of (whether by ownership, license or other right of use, other than pursuant to this Agreement) the ability of a Party or its Affiliates to grant access to, or a license or sublicense of, such items or right as provided for under this Agreement without violating the terms of any agreement or other arrangement with any Third Party, without incurring any new financial obligations after the Effective Date to any Third Party (unless expressly agreed in writing to be assumed by the other Party and subject to Section 12.17), and without violation of any Applicable Law. Notwithstanding the foregoing or anything to the contrary herein, a Party will not be deemed to “Control” any item of or right under Intellectual Property or any Material that (a) prior to the consummation of a Change of Control of such Party, was owned or controlled by any Acquirer or (b) following the consummation of such Change of Control of such Party, that any Acquirer invents or acquires independently and outside of the activities under the Research Program or this Agreement and without accessing or practicing any Confidential Information of the other Party or any Foreground IP.
1.26
“Co-Packaged Product” means a single packaged product containing a Product and one or more other therapeutically or prophylactically active product(s) or Device(s) in a co-packaged form.
1.27
“Cover” means, with respect to (a) any claim of any Patent and (b) any compound, Invention, product, and/or process (including, without limitation any Compound and Product and/or the Manufacture thereof) in any jurisdiction, that such claim would be infringed (or if such claim is in a pending patent application, such claim would be infringed if it were issued), absent a license or ownership, by the research, discovery, Development, Commercialization, making, having made, use, sale, offer for sale, export or import of such compound, Invention, product and/or process (including, without limitation any Compound and Product and/or the Manufacture thereof) in such jurisdiction, considering the claims of Patent applications as if they have already been issued and are valid and enforceable.
1.28
“CUE Invention” means any Invention that is conceived or first reduced to practice in the performance of a Research Project by CUE’s or its Affiliates’ employees, or any other individuals or legal entities engaged by or on behalf of CUE or its Affiliates (either solely by or on behalf of CUE or its Affiliates or jointly with BI or its Affiliates).
1.29
“CUE Know-How” means all Know-How Controlled by CUE or any of its Affiliates on the Effective Date or during the Term of the Agreement that is necessary or reasonably useful to Exploit Compounds or Products in the Territory in the Field.
1.30
“CUE Patents” means (i) [**], and (ii) any additional Patents Controlled by CUE or any of its Affiliates on the Effective Date or during the Term of the Agreement having a claim Covering or having a disclosure sufficient to support a claim Covering any CUE Know-How, including but not limited to Patents that claim the method of manufacture thereof, formulations thereof, Combination Products and/or the intended use(s) thereof.
1.31
“CUE Steered CUE Patents” means all CUE Patents other than the BI Steered CUE Patents.
1.32
“CUE Technology” means CUE Know-How and CUE Patents. A brief non-exhaustive description of the Cue Technology existing as of the Effective Date is included in Schedule 1.32 (“Non-Exhaustive List of Cue Technology”).
1.33
“CUE-501” means (a) [**] B Cell Depletion molecule Controlled by CUE on the Effective Date and (b) all [**] Potential Collaboration Molecules that are (i) Controlled by CUE on the Effective Date or during the Term of the Agreement, (ii) discovered, identified or Developed by either Party, or together by both Parties in their performance of a Research Plan, or (iii) derived by or on behalf of BI from a molecule described in (i) or (ii) during the Term through the use of the CUE Technology.
1.34
“Default” means with respect to a Party, that such Party shall have failed to comply in any material respect with any material provision set forth in this Agreement, including a material breach of a material provision set forth in BI’s payment obligations under Article 12 (“PAYMENT TERMS”), the confidentiality obligations set forth under Article 10 (“CONFIDENTIALITY”), the publicity and publication provisions set forth under Article 11 (“PUBLICITY and PUBLICATIONS”) or the Representations, Warranties and Covenants set forth under Article 13 (“REPRESENTATION, WARRANTIES and COVENANTS”) of this Agreement or intentional or grossly negligent provision of materially incorrect CUE Deliverables.
1.35
“Development” means all activities that relate to obtaining, maintaining or expanding Regulatory Approval of a pharmaceutical product (including any Product), including: (a) the conduct of research activities other than the research activities carried out pursuant to the Research Plan (including drug discovery, identification, or synthesis) with respect to a Compound or Product; and (b) preclinical and clinical drug development activities and other development activities with respect to a Compound or Product, including Manufacturing of Compounds or Products for development purposes, test method development and stability testing, toxicology, formulation, process development, qualification and validation, quality assurance, quality control, the conduct of Clinical Trials, statistical analysis and report writing, the preparation and submission of regulatory materials with respect to the foregoing, and all other activities necessary or useful or otherwise requested or required by a Regulatory Authority or as a condition or in support of obtaining, maintaining or expanding a Regulatory Approval. When used as a verb, “Develop” means to engage in Development.
1.36
“Device” means any instrument, apparatus, machine, or digital product which is intended to be used in the Field (i) forming an integral part of a Product or (ii) co-packed and to be used together with a Product, such as prefilled syringes, prefilled pens or autoinjectors and any other kind of administration device.
1.37
“Differentiated Compound” means any Potential Collaboration Molecule that (a) includes the same B Cell Targeting Module as CUE-501 or a New Compound, but that has [**] and (b) BI elects to include as a Compound under this Agreement in accordance with Section 2.4. Differentiated Compound also includes any Potential Collaboration Molecule that contains the same three (3) Compound Modules as a Differentiated Compound designated by BI pursuant to Section 2.4 and that is (a) Controlled by CUE on the Selection Date, (b) discovered, identified or Developed by either Party, or together by both Parties in their performance of the Research Plan as amended for such Differentiated Compound or (c) is derived by or on behalf of BI from a molecule described in (a) or (b) during the Term through the use of the CUE Technology.
1.38
“Einstein College Upstream License” means the exclusive Amended and Restated License Agreement between CUE and Albert Einstein College of Medicine, Inc., dated July 31, 2017, as subsequently amended by that certain First Amendment to the Amended and Restated License Agreement dated October 30, 2018, by that certain Second Amendment to the Amended and Restated License Agreement dated January 13, 2024 as well as by the Boehringer Ingelheim Amendment to the Amended and Restated License Agreement dated March 10, 2025 and under which CUE is granted an exclusive sublicensable license under certain intellectual property, including without limitation the Patent titled [**] controlled by Albert Einstein College of Medicine, Inc., as further described in Appendix A of the Amended and Restated License Agreement with Albert Einstein College of Medicine, Inc. (the “Einstein College Upstream Patent”). The Einstein College Upstream License, as amended, is attached hereto under Schedule 1.38 (“Einstein College Upstream License”).
1.39
“EMA” means the European Medicines Agency and any successor agency thereto.
1.40
“Exploit” means to research, Develop, Manufacture, use, register, market, make, offer for sale, sell, distribute, export and import or otherwise Commercialize.
1.41
“External Costs” means (a) reasonable expenses paid to permitted subcontractors of CUE (or payable to such subcontractors and accrued in accordance with CUE’s Accounting Standards) for services of such subcontractors provided on behalf of CUE solely in the performance of CUE’s activities under the Research Program and (b) all amounts paid for materials or supplies solely used in connection with CUE’s performance of its activities under the Research Program that are not used in the course of CUE’s ordinary business, in each case, (a) and (b) as such services of CUE subcontractors and materials or supplies are specifically set forth on Schedule 1.41 (“External Costs”), as amended following mutual written agreement of the Parties.
1.42
“FDA” means the United States Food and Drug Administration and any successor agency thereto.
1.43
“Field” means all fields and uses, including but not limited to the use of a Product for the diagnosis, treatment, palliation or prevention of a disease or medical or aesthetic condition in humans or animals.
1.44
“First Commercial Sale” means, on a country-by-country and Product-by-Product basis, the first sale of such Product by or under the authority of BI, its Affiliates or Sublicensees in an arm’s length transaction of such Product to a Third Party other than a Sublicensee in such country in exchange for cash or contributions in kind for distribution, re-sale, end use, consumption or other use after Regulatory Approval for such Product has been granted in such country.
1.45
“Foreground IP” means all Intellectual Property that is conceived, reduced to practice, discovered, developed or otherwise made by or on behalf of a Party after the Effective Date and within the scope of this Agreement (either alone or jointly with the other Party). Foreground IP includes Collaboration Foreground IP and Post-Collaboration Foreground IP.
1.46
“Foreground Patents” means Patents relating to any Invention made by or on behalf of either or both Parties, which Invention is part of Foreground IP.
1.47
“FTE” means a full-time equivalent person-year based upon a total of [**] working hours per Calendar Year of scientific or technical work carried out by a duly qualified employee of CUE on or directly related to the work to be conducted under the Agreement. Overtime, and work on weekends, holidays and the like shall not be counted with any multiplier (e.g. time-and-a-half or double time) toward the number of hours that are used to calculate the FTE contribution. The portion of a FTE billable by CUE for one (1) individual during a given accounting period shall be determined by dividing the number of hours worked directly by said individual on the work to be conducted under the Agreement during such accounting period and the number of FTE hours applicable for such accounting period based on [**] working hours per Calendar Year.
1.48
“FTE Rate” means an annual rate of [**] US dollars (US $ [**]) per FTE. Such FTE Rate shall include, inter alia, all costs for standard laboratory supplies used in the ordinary course of CUE’s business, equipment maintenance costs, utilities, waste removal, travel expenses and a pro rata allocation of general and administrative expenses. Notwithstanding the foregoing, the FTE Rate shall exclude External Costs.
1.49
“Full Market Exclusivity” means a situation where no generic or biosimilar version of the Product can be marketed in the country (not even for other indications, with other formulations etc.) without infringing a Valid Claim of a CUE Patent, Collaboration Foreground Patent, or Assigned Patent in such country.
1.50
“Generic Competition” means and shall be deemed to exist in a particular country in the Territory with respect to a particular Product in a given Calendar Quarter if in such country during such Calendar Quarter one or more Generic Products (other than a Generic Product sold by or under the authority BI or its Affiliates or by a Sublicensee under a license granted, directly or indirectly, by BI or its Affiliates) in the aggregate account for [**] in such country, as measured by [**] or, if such data is not available, such other reliable data source as reasonably agreed upon by CUE and BI. If no data is commercially available, then the Parties shall agree upon a methodology for estimating the percentage [**] in such country.
1.51
“Generic Product” means, with respect to a particular Product and a particular country, (i) any pharmaceutical product (other than the Product, as applicable) that contains the same active ingredient(s), independent from its polymorphic form or its salt form, in a comparable quality and quantity as such Product, as applicable, irrespective of its pharmaceutical form and is approved under an Abbreviated New Drug Application (ANDA) or under 505(b)(2) of the United States Federal Food, Drug and Cosmetic Act or any similar abbreviated route of approval in such country, or (ii) any biologic medicinal product (other than the Product, as applicable) that is a biosimilar of such Product, and, if the Product is a component of a Combination Product, a biosimilar of the Combination Product, and is approved under a biological product licensure application submitted by any person under 42 U.S.C.
§ 262(k) or any similar abbreviated route of approval in such country.
1.52
“Governmental Authority” means any multi-national, national, federal, state, local, municipal, provincial, county, or other political subdivision, agency or other body, domestic or foreign or other government authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court, tribunal or other entity).
1.54
“Initiation” means, with respect to a Clinical Trial, the first dosing of the first human subject in such Clinical Trial.
1.55
“Intellectual Property” means all results and all rights in Inventions, Patents, priority rights, copyrights, design rights, trade dress, trade names, trademarks, service marks, Know-How, database rights, domain names and all other intellectual property rights (whether registered or unregistered) and all applications and rights to apply for any of them, anywhere in the world.
1.56
“Invention” means any new and useful process, method, utility, compound, composition of matter, formulation, machine, apparatus or article of manufacture, or any improvement thereof, or discovery or finding that (a) is conceived and/or first reduced to practice by either Party, or together by both Parties, and (b) is or may be patentable in at least one country in the Territory. When used as a verb, “Invent” means to conceive of or reduce to practice an Invention.
1.57
“Invoice” means an original or electronically signed invoice sent by CUE to BI with respect to any payment due hereunder, containing the information and meeting the requirements as set forth in Schedule 1.57 (“Requirement for Invoices”), which shall be modified in the event of a change in the Applicable Law.
1.58
“Know-How” means all technical information, know-how and data, in any tangible or intangible form and whether or not patentable, including Inventions, discoveries, trade secrets, specifications, instructions, processes, formulae, methods, practices, techniques, results, software, algorithms, technology, test data (including pharmacological, biological, chemical, biochemical, toxicological and clinical test data, analytical and quality control data and stability data), studies and procedures, and all technical information, know-how and data relating to compounds, formulations, compositions, products or to their manufacture, development, registration, use or commercialization or methods of assaying or testing them.
1.59
“Knowledge” means [**].
1.60
“Manufacture” or “Manufacturing” means to make, produce, manufacture, process, fill, finish, package, label, perform quality control and quality assurance testing, release, ship, or store a compound or product or any component thereof.
1.61
“Major Market Country” means each of [**].
1.62
“Material” means any reagent, assays, tool, cell line, protein, antibody, virus, chemical compound, product, sample or other substance or tangible material, as well as any derivatives or modifications thereof.
1.63
“Net Sales” means, with respect to a certain time period, the gross invoiced sales charged for Product(s) sold by or for BI, its Affiliates and Sublicensees in arm’s length transactions to Third Parties (but not including sales of Product(s) between BI, its Affiliates, or their respective Sublicensees) during such time period, less the total of the following charges or expenses as determined in accordance with the relevant Accounting Standards, consistently applied across all products sold by BI, in each case without duplication:
[**].
BI, its Affiliates and Sublicensees shall not [**] on the Product in order to [**], such that the Product would [**]. All defined deductions in this section shall be consistent with BI’s audited financial statements.
[**] shall not be included in Net Sales. [**] shall not be included in Net Sales either.
Upon any sale or other disposal of any Product that should be included in Net Sales for any consideration other than an exclusively monetary consideration on bona fide arm’s length terms, then for purposes of calculating the Net Sales under this Agreement, such Product shall be deemed to be [**] during the applicable reporting period [**] for such Product in the country in which such sale or other disposal occurred when such Product is [**]. In the event no [**] is available for the Product [**] in such country during the applicable reporting period, then such Product shall be deemed to be [**] at the [**] during the applicable reporting period [**] for such Product in all countries in which such sale or other disposal occurred when such Product is [**] (provided, however, that if such Product is not [**] in any country, then BI shall calculate in good faith [**] for the Product, [**] as are then [**] to [**] then being [**] and having [**]; provided, however, that if [**], the Parties shall [**].
In the event a Product is sold as a Combination Product or Co-Packaged Product, Net Sales of the Combination Product or Co-Packaged Product will be calculated as follows, in each case on a country-by-country and Calendar Quarter-by-Calendar Quarter basis:
a)
If the Combination Product and/or Co-Packaged Product, the Product and the other product are sold separately, Net Sales of the Product portion of Combination Products and Co-Packaged Products will be calculated by multiplying the total Net Sales of the Combination Product or Co-Packaged Product by the fraction A/(A+B), where A is [**], and B is [**].
b)
If the Combination Product and/or the Co-Packaged Product and the Product are sold separately, but the [**] cannot be determined, Net Sales of the Combination Product or the Co-Packaged Product shall be equal to the Net Sales of the Combination Product or Co-Packaged Product multiplied by the fraction A/C wherein A is [**] and C is [**].
c)
If the Combination Product and/or the Co-Packaged Product and the Other Actives are sold separately, but the [**] cannot be determined, Net Sales of the Combination Product and/or Co-Packaged Product shall be equal to the Net Sales of the Combination Product and/or Co-Packaged Product multiplied by the following formula: one (1) minus B/C wherein B is [**] and C is [**].
d)
If the Combination Product or Co-Packaged Product are sold but the [**] can be determined, Net Sales of the Combination Product or Co-Packaged Product shall be equal to Net Sales of the Combination Product or Co-Packaged Product multiplied by [**].
The [**] for such [**] contained in the Combination Product or Co-Packaged Product shall be calculated for each country in the Territory in such Calendar Quarter by [**], as published by IQVIA or another mutually agreed independent source.
In the initial Calendar Year during which a Combination Product or Co-Packaged Product is sold, a forecasted [**] shall be used for the Product, Other Active(s), Combination Product and Co-Packaged Product. Any over- or underpayment due to a difference between forecasted and actual [**] shall be paid or credited in the second royalty payment of the following Calendar Year. In the following Calendar Year, the [**] of the previous Calendar Year shall apply from the second royalty payment on.
1.64
“New Compound” means a Potential Collaboration Molecule directed to a B Cell Depletion target other than [**] that BI includes as a Compound under this Agreement in accordance with Section 2.3.
1.65
“[**] Claim” means a claim in any [**] that (i) recites a [**], and (ii) does not recite a [**].
1.66
“[**] Targeting Module” means an antibody or binding fragment of an antibody (e.g., a single-chain variable fragment of a molecule) that binds one or more cells other than [**].
1.67
“Notify” means to provide formal written notice (“Notice”) according to Section 21.8.
1.68
“Patent” means any and all (i) patents, (ii) patent applications, including all provisional and non-provisional applications, foreign patent application, patent cooperation treaty (PCT) applications, substitutions, continuations, continuations-in-part, divisions and renewals, and all patents granted thereon, (iii) all patents-of-addition, reissues, re-examinations and extensions or restorations by existing or future extension or restoration mechanisms, including supplementary protection certificates or equivalents thereof such as patent term extensions, (iv) inventor’s certificates or letters patent, (v) utility models, (vi) design patents, or (vii) any other substantially equivalent form of government-issued right substantially similar to any of the foregoing described in subsections (i)-(vi) above.
1.69
“Phase I Clinical Trial” means a human clinical trial conducted in any country that meets the requirements of 21 CFR §312.21(a), (as amended (or its successor regulation)) or its foreign equivalent. By way of example and not limitation, a Phase I Clinical Trial is usually [**] to assess [**] of an investigational drug, and the emphasis in Phase I Clinical Trials is usually on [**] and it is typically [**]. For clarity, a Phase I Clinical Trial may also [**].
1.70
“Phase II Clinical Trial” means a human clinical trial conducted in any country that meets the requirements of 21 CFR §312.21(b), (as amended (or its successor regulation)) or its foreign equivalent. By way of example and not limitation, a Phase II Clinical Trial is usually [**] to assess [**] about an investigational drug, along with [**].
For clarity, a Phase II Clinical Trial may also [**].
1.71
“Phase III Clinical Trial” means a human clinical trial conducted in any country that meets the requirements of 21 CFR §312.21(c), (as amended (or its successor regulation)) or its foreign equivalent. By way of example and not limitation, a Phase III Clinical Trial is [**] to gather [**] of the drug and, along with [**], to provide [**]. For clarity, a Phase III Clinical Trial may also [**].
1.72
“PMDA” means the Japanese Pharmaceuticals and Medical Devices Agency and any successor agency thereof.
1.73
“Post-Collaboration Foreground IP” means all Foreground IP other than Collaboration Foreground IP.
1.74
“Potential Collaboration Molecule” means a B Cell Depletion molecule that contains a B Cell Targeting Module, [**] (each of these three variable modules a “Compound Module”).
1.75
“Product” means any pharmaceutical product in finished dosage form containing at least one Compound. For clarity, Product includes Combination Product and Co-Packaged Product.
1.76
“Public Official” means any officer or employee of a local or foreign government or any department, agency, political party, institution, or instrumentality thereof (including officers and employees of government controlled entities), or of a public international organization as well as any person acting in an official capacity for or on behalf of any such government, department, agency, institution or instrumentality, or for or on behalf of any such public international organization as well healthcare professionals, working in healthcare institutions, in which the central, regional or local government owns an interest or has control or which are paid partly or as a whole by the government.
1.77
“Regulatory Approval” means both: (a) the technical, medical and scientific licenses, registrations, authorizations and approvals (including approvals of marketing authorization applications and variation approvals), and (b) any necessary pricing and/or reimbursement authorizations and approvals, of any Regulatory Authority necessary for the distribution, marketing, promotion, offer for sale, use, import, export or sale of such pharmaceutical product in such country.
1.78
“Regulatory Authority” means any (i) Governmental Authority, notified bodies or other organization in a country or region that regulates the manufacture or sale of pharmaceutical or medicinal products or medical devices, including the FDA, PMDA and the EMA, and any successors thereto and (ii) any other relevant bodies authorized by Applicable Law to review or otherwise exercise oversight over marketing authorization applications, other regulatory filings or regulatory approvals.
1.79
“Regulatory Exclusivity” means, with respect to a particular Product in a country in the Territory, any period of regulatory data protection, market exclusivity or similar regulatory protection afforded by a Regulatory Authority in such country, that prevents such Regulatory Authority from accepting or approving any and all applications by a Third Party to market a generic or biosimilar version of such Product and/or prevents such Regulatory Authority from authorizing any Third Party to market any and all generic or biosimilar version of such Product; for the sake of clarity, such Regulatory Exclusivity shall be directly attributable to the Compound incorporated in such Product and the Regulatory Exclusivity period in a particular country shall end no later than the first marketing authorization or approval of a Generic Product by a Regulatory Authority in such country.
It is understood and agreed by the Parties that Regulatory Exclusivity shall not include any exclusivity conferred by any Patent.
1.80
“Research Plan” means any research plan agreed by the Parties and outlining the activities performed by or on behalf of CUE and/or BI as set forth in Article 2 of this Agreement. The Research Plans include the Initial Research Plan covering the collaborative research for CUE-501 attached hereto as Schedule 1.80 (“Initial Research Plan”) (and its amendments) as well as any additional Research Plan (and amendments thereof) covering the collaborative research on New Compounds or Differentiated Compounds (if any).
1.81
“Research Program Start Date” means the earlier of either (a) the date when either Party begins to perform an activity under the Initial Research Plan, or (b) the project kickoff meeting for such Initial Research Plan, in each case (a) and (b) such date to be documented in the minutes of the JSC meeting immediately following such first research activities or the project kickoff meeting.
1.82
“Research Term” means the period beginning on the Research Program Start Date and ending upon the earlier of (a) completion of the activities under the Research Plans (as amended) and (b) the fourth (4th) anniversary of the Research Program Start Date, unless this Agreement is terminated earlier. Any extension of the Research Term will require the mutual agreement of the Parties.
1.83
“Results” means all materials, information, know-how, data, documents, measurement results, Inventions, software and other intellectual property generated and/or first reduced to practice or writing in the performance of the Research Program.
1.84
“Selection Date” means, with respect to a given Differentiated Compound, the date that BI Notifies CUE of BI’s election to include such Differentiated Compound as a Compound under this Agreement in accordance with Section 2.4.
1.85
“Senior Executives” means: (i) in the case of CUE, its Chief Executive Officer; and (ii) in the case of BI, depending on the actual status of the relevant Product(s), the senior-most executive vice-president responsible for research, development, medicine or marketing and sales, or, in each case, such individual’s nominated designee who is a member of BI’s senior management with appropriate decision making authority.
1.86
“Start of Development” or “SoD” means the event where BI selects a Compound that meets [**].
1.87
“Start of Lead Optimization” or “SoLO” means the event where BI selects a Compound from lead discovery for further optimization [**].
1.88
“Sublicensees” means with respect to BI a Third Party to whom BI (or its Affiliate or another of its Sublicensees), subject to Section 5.6, grants a license or sublicense under any rights granted to BI under this Agreement to Develop or Commercialize Compounds or Products in the Territory.
For clarity, Affiliates working for or on behalf of BI and Third Party Collaborators will be deemed to practice such rights on behalf of BI and are not considered Sublicensees under this Agreement; provided that any breach by an Affiliate or a Third Party Collaborator of any term or condition of this Agreement will be deemed a direct breach by BI of such term or condition.
1.90
“Taxes” means all forms of preliminary or finally imposed domestic and foreign taxes, fees, levies, duties and other assessments or charges of whatever kind (including but not limited to sales, use, excise, stamp, transfer, property, value added, goods and services, withholding and franchise taxes) together with any interest, penalties, fine or additions payable thereon.
1.91
“Territory” means any and all countries in the world.
1.92
“Third Party” means an entity other than (i) BI or any of its Affiliates, or (ii) CUE or any of its Affiliates.
1.93
“Third Party Collaborations” means collaborations and service provider relationships between BI and Third Party Collaborators pursuant to which such Third Party Collaborators in collaboration with BI or its Affiliates, or on behalf of BI or its Affiliates, conduct Development and/or Commercialization activities in connection with the Compounds or Products, provided that (a) no rights under this Agreement are granted to such Third Party Collaborators save for the rights strictly necessary to conduct such Development and/or Commercialization activities in collaboration or on behalf of BI or its Affiliates, as applicable, and (b) Third Party Collaborations expressly exclude any collaboration that involves granting or otherwise permitting such Third Party Collaborator the right to offer for sale and/or sell a Compound or a Product solely on their own behalf. For clarity, any sales of a Product by a Third Party Collaborator on behalf of BI will be accounted for in Net Sales under this Agreement.
1.94
“Third Party Collaborators” means Third Party collaborators and subcontractors of BI involved in conducting Development and/or Commercialization activities relating to the Compounds or Products pursuant to Third Party Collaborations, including without limitation CMOs or CROs.
1.95
“Valid Claim” means (a) a claim of an issued Patent that has not expired or has not been abandoned, or has not been revoked, held invalid or unenforceable by a patent office, court or other governmental agency of competent jurisdiction in a final and non-appealable judgment (or judgment from which no appeal was taken within the allowable time period), or (b) a claim within a Patent application which application has not been pending for more than [**] from the date of its priority filing date provided such claim has not been irretrievably revoked, irretrievably cancelled, irretrievably withdrawn, held invalid or abandoned by a patent office, court or other governmental agency of competent jurisdiction in a final and non-appealable judgment (or judgment from which no appeal was taken within the allowable time period), or finally determined to be unallowable in a decision from which an appeal cannot or can no longer be taken. For clarity, a claim of an issued Patent that ceased to be a Valid Claim before it issued because it had been pending too long, but subsequently issues and is otherwise described by clause (a), shall again be considered to be a Valid Claim once it issues. The same principle shall apply in similar circumstances such as if, for example (but without limitation), a final rejection of a claim is overcome.
1.96
“VAT” means value added tax and similar indirect taxes (including goods and service tax).
The following Capitalized Terms have the meaning that is given in the Section indicated in the table below:
|
|
Term |
Section |
Acquirer |
1.14 |
Additional Cure Period |
19.2.1 |
Agreement |
Preamble |
Alliance Manager |
7.6 |
Assigned Patents |
9.1.4 |
Auditor |
12.10.5 |
Bankruptcy Code |
9.7 |
BI |
Preamble |
BI Auditors |
12.16 |
BI Enforced Patent |
9.5.1 |
BI Provided Materials |
2.7.1 |
Clinical Development Milestone Event / Payment |
12.6 |
Collaboration Request Period |
2.4.1 |
Commercial Milestone Event / Payment |
12.8 |
Competing Infringement |
9.5.1 |
Competing Product |
9.5.1 |
Compound Module |
1.74 |
CUE |
Preamble |
[**] |
9.2.1 a) |
[**] |
9.2.1 a) |
[**] |
9.2.1 b) |
|
|
CUE Deliverables |
2.6 |
CUE Developers |
13.3.3 |
CUE Enforced Patent |
9.5.5 |
CUE Indemnitees |
18.2 |
CUE Know-How |
1.24 |
CUE Patents |
1.25 |
CUE Provided Materials |
2.7.1 |
CUE Technology |
1.26 |
Damages |
18.1 |
Data Package |
6.1 |
Default Cure Period |
19.2.1 |
Default Notice |
19.2.1 |
Defaulting Party |
19.2.1 |
Disclosing Party |
1.22 |
Dispute |
20.1 |
Effective Date |
Preamble |
Einstein College Upstream Patent |
1.37 |
Exclusivity Period |
4.2 |
ICC |
20.2 |
Indemnified Party |
18.2 |
Indemnifying Party |
18.2 |
Joint Steering Committee or JSC |
7.1 |
Material Provider |
2.7 |
Material Receiver |
2.7 |
New Compound Proposal |
2.3 |
New Compound Selection Fee |
12.2 |
|
|
Non-Defaulting Party |
19.2 |
Party / Parties |
Preamble |
Patenting Activities |
9.2 |
Preclinical Milestone Event / Payment |
12.5 |
Program Director |
7.5 |
Proposed New Target |
2.3 |
Provided Materials |
2.7 |
Publication |
11.2.1 |
Receiving Party |
1.22 |
Regulatory Milestone Event / Payment |
12.7 |
Request |
20.2 |
Research Program |
2.1 |
Research Project |
2.1 |
Research Support Payments |
12.2 |
Royalty |
12.10.1 |
Royalty Term |
12.10.2 |
Rules |
20.2 |
Successful New Compound Inclusion |
2.3 |
Term |
19.1 |
Terminated Product |
12.9 |
Third Party Claim |
18.2 |
[**] |
9.2.1 b) |
[**] |
9.2.1 b) |
2.1
Collaboration. During the Research Term and subject to the oversight and review of the JSC, BI and CUE shall conduct the activities assigned to them in the Research Plans in accordance with the terms of this Agreement and all Applicable Laws (the activities under each Research Plan, each a “Research Project” and altogether, the “Research Program”).
Each Party (itself or by permitted subcontracting) shall use scientific, technical and other personnel who are sufficiently qualified and have requisite skills to perform the activities assigned to it and shall use Commercially Reasonable Efforts to conduct its respective activities under the Research Program, including in accordance with the timelines outlined in each Research Plan. Each Party’s obligation to perform any specific activity under a given Research Plan shall be excused and tolled for any period during which it is prevented from performing such obligation by the other Party’s failure to perform one or more of its obligations under this Agreement. Further, CUE will not be required to provide any FTE’s, incur any expenses, or perform any activities assigned to it under any Research Plan to the extent that doing so would exceed the Research Funding stipulated in Section 12.2, unless mutually agreed by the Parties in writing.
2.2
Initial Research. The Parties will collaborate to develop CUE-501 by performing the activities outlined in the initial Research Plan attached hereto as Schedule 1.80 (“Initial Research Plan”) and with the objective of achieving Start of Lead Optimization of CUE-501 (“Initial Research Plan”).
2.3
Inclusion of New Compounds. In addition to, or instead of CUE-501 to the extent BI elects to terminate its rights to CUE-501 pursuant to Section 19.3, at any time during the Term of the Agreement, BI may elect, at its sole discretion (but subject to the terms and conditions of this Agreement, including the provisions of this Section 2.3), to include under this Agreement additional or alternative Compounds that are New Compounds.
If BI desires to add one or more New Compounds:
(i) at any time during the Research Term, then BI will Notify CUE of its election referencing this Section 2.3 and the New Compound will then be successfully included under the Agreement upon receipt by CUE of such Notice;
(ii) after the Research Term, then BI shall Notify CUE of its proposed election referencing this Section 2.3 (each a “New Compound Proposal”) and request CUE to confirm availability of the involved new B cell depletion target (each a “Proposed New Target”). Within [**] of receipt of the New Compound Proposal, CUE shall Notify BI whether or not such Proposed New Target is “available” (as defined below) to be licensed to BI. The New Compound is successfully included under this Agreement upon either receipt by BI of Notice from CUE that the Proposed New Target is available to be licensed to BI or failure of CUE to provide truthful Notice regarding availability of the Proposed New Target within [**] (except in cases where CUE has granted exclusive rights with respect to the Proposed New Target to a Third Party prior to its receipt of the applicable New Compound Proposal from BI);
(in each case, (i) and (ii) a “Successful New Compound Inclusion”).
The Parties agree that any Proposed New Target shall be considered “available” unless CUE (i) has granted rights or is contractually obligated to grant rights with respect to such Proposed New Target to any Third Party as demonstrated by an executed agreement (redacted / anonymized, if needed) or other appropriate evidence, or (ii) is not willing to make the Proposed New Target available to BI because it has either (a) initiated discussions with a Third Party for such a grant, as demonstrated by a non-binding term sheet representing terms pursuant to which CUE would grant such right, or (b) initiated any internal development or commercialization program with respect to any product directed to such Proposed New Target, as demonstrated by appropriate evidence.
2.4
Effects of Successful New Compound Inclusion
Upon each Successful New Compound Inclusion,
(a)
the B Cell depletion target of the respective New Compound shall be designated a Target under this Agreement, the exclusivity obligations of CUE pursuant to Section 4.2 shall apply, and the diligence obligations of BI pursuant to Section 3.2 shall apply; and
(b)
BI may (at its sole discretion) request CUE to collaborate on such New Compound within [**] following Successful New Compound Inclusion (the “Collaboration Request Period”) and if CUE agrees, the Parties will mutually agree to a separate Research Plan and associated research support payments to CUE for each such New Compound, if necessary, by way of an amendment of this Agreement.
2.4.2
Payment of New Compound Selection Fee
In each case of Successful New Compound Inclusion (but subject to Section 2.4.3),
(i) if BI has requested CUE within the Collaboration Request Period to collaborate on such New Compound,
(a) upon mutual agreement of the Parties of a new Research Plan for such New Compound or
(b) in case CUE rejects to collaborate or the Parties cannot agree on a new Research Plan and if BI does not drop the New Compound again in accordance with Section 2.4.3,
or (ii) if BI has not requested CUE within the Collaboration Request Period to collaborate or if BI informs CUE at an earlier point in time in writing to advance the New Compound without CUE, upon lapse of the Collaboration Request Period or upon such information by BI to CUE, as applicable.
BI will pay CUE the New Compound Selection Fee pursuant to Section 12.3 following receipt of the corresponding Invoice.
2.4.3
Dropping of New Compound
In case BI has successfully included a New Compound and requested CUE to collaborate on such New Compound, but CUE either declines the collaboration or the Parties are unable to agree on a new Research Plan including, if applicable, associated research support payments for such New Compound, BI may choose to drop such New Compound within [**] following definite and written refusal by CUE to collaborate or CUE’s definite failure to agree on a such Research Plan (as applicable). In case BI drops a New Compound during such [**] time period, the New Compound shall no longer be considered a Compound and the respective B Cell depletion target of such New Compound shall no longer be considered a Target under this Agreement and the effects of Successful New Compound Inclusion no longer apply to such New Compound including that the New Compound Selection Fee will not become due. For clarity, in case BI fails to drop a New Compound during such [**] time period, such New Compound shall be deemed a Compound and the effects of Successful New Compound Inclusion will apply to such Compound and BI will pay CUE the New Compound Selection Fee pursuant to Section 12.3 following receipt of the corresponding Invoice.
2.5
Inclusion of Differentiated Compounds. At any time during the Term of the Agreement, BI may elect, at its sole discretion, to Develop one or more Differentiated Compounds with respect to a given Target. BI may do so by Notifying CUE of its election and the Differentiated Compound is officially included under the Agreement upon receipt by CUE of such Notice. BI can freely decide if it wants to perform research on any elected Differentiated Compound without CUE’s involvement, or it may request that CUE participates in such research pursuant to a mutually agreeable Research Plan and, if necessary, after agreement on associated research support payments to CUE, if necessary by way of an amendment of this Agreement.
2.6
CUE Deliverables. CUE shall provide to BI: (a) all Results, and (b) all reports, documents and generated Materials described as deliverables in each Research Plan (both (a) and (b) the “CUE Deliverables”). The CUE Deliverables shall be provided within [**] of completion of the respective work package in the respective Research Plan, unless otherwise outlined in such Research Plan, or, in case of an earlier termination of this Agreement or any Target and relevant Compounds and Products, within [**] of the effective date of such termination. All reports shall be provided in sufficient detail and must comply with industry standards and data integrity guidelines ensuring that all experimental procedures and results are documented in a thorough and traceable manner and CUE shall make reasonable modifications to any reports consistent with the foregoing at BI’s request. The respective reports as outlined in each Research Plan shall be in the form of and meet the requirements of Schedule 2.6 (“Requirements for Reports”). On BI’s request all or a portion of all Materials generated under the Research Program shall be provided by CUE to BI.
2.7.1
Each Party (in such capacity, the “Material Provider”) shall provide the other Party (in such capacity, the “Material Receiver”) those of its Materials required by the Research Plan to be provided by such Party using Schedule 2.7 (“Material Transfer Record Form”), if any, for the conduct of the Research Program (such Materials provided to the other Party, the “Provided Materials” and if Controlled and provided by or on behalf of BI or generated in the performance of the Parties’ obligations under a Research Plan, the “BI Provided Materials”, if Controlled and provided by or on behalf of CUE, the “CUE Provided Materials”). For clarity, the provision of such Material, including the Compound produced by CUE for the activities under the Initial Research Plan, will be [**] for the Material Receiver, however, BI will [**] for (a) [**] and (b) [**], in each case within [**] following BI’s [**] therefor.
2.7.2
Delivery of Materials is subject to Article 15 (“CUSTOMS”).
2.7.3
Any BI Provided Materials shall be employed by CUE solely in the conduct of the Research Program according to the Research Plan. BI Provided Materials, or any portion thereof, shall only be provided to those employees or BI approved subcontractors of CUE who are directly involved in conducting the Research Program and have a need for such BI Provided Materials pertaining to the performance of the Research Program. CUE Provided Materials may only be used by BI in accordance with the License Technology license in Section 5.1, for the avoidance of doubt, including the transfer to Affiliates, Sublicensees and Third Party Collaborators subject to the terms and conditions of this Agreement.
2.7.4
All Provided Materials are provided “as-is”. The Material Provider does not represent, warrant or guarantee that the Provided Materials are merchantable or satisfactory for any particular purpose and the Material Provider hereby disclaims all warranties, express or implied, to such effect. Information and data relating to Provided Materials provided hereunder do not certify, validate, imply or represent that any Provided Materials are fit for any use.
2.7.5
Except as expressly outlined in the Research Plan or (regarding CUE Provided Materials) necessary or reasonably useful for BI to Exploit Compounds or Products, the Material Receiver shall not (a) reverse engineer, disassemble, decompile, analyze, sequence or otherwise determine the structure of any of the Provided Materials, nor attempt to do so, directly or indirectly, through the use of a Third Party; (b) use the Provided Materials in connection with any Third Party materials; or (c) modify the Provided Materials in any manner inconsistent with the Research Plan.
2.7.6
Except in cases where this Agreement or any other written agreement among the Parties provides for a transfer of ownership of Provided Materials to the Material Receiver, the Material Receiver, at the Material Provider’s request and expense, shall cooperate with the Material Provider in securing and filing any necessary statements or documents to preserve and evidence the Material Provider’s ownership of and security interest in the Provided Materials in any jurisdiction in which the Material Receiver uses the Provided Materials as reasonably requested by the Material Provider.
2.7.7
The Material Receiver shall ensure that any and all Provided Materials are securely stored.
2.7.8
Within [**] of completion of the Research Program or, earlier, upon BI’s request, CUE shall return to BI, or dispose of, and shall cause its Affiliates and permitted subcontractors to so return or dispose of, all quantities of unused BI Provided Materials, as instructed by BI in writing, by fax or e-mail, at BI’s cost, and confirm, in writing, by fax or e-mail, such disposition to BI, if applicable.
2.7.9
Within [**] of the expiration or termination of this Agreement, BI shall return to CUE, or dispose of, and shall cause its Affiliates, Sublicensees and Third Party Collaborators to so return or dispose of, all quantities of unused CUE Provided Materials, as instructed by CUE in writing, by fax or e-mail, at CUE’s cost, and confirm, in writing, by fax or e-mail, such disposition to CUE, if applicable.
3.
DEVELOPMENT AND COMMERCIALIZATION OF PRODUCTS; DILIGENCE
3.1
Development/Commercialization. After the activities under the Research Plan for a given Compound is completed (or earlier if requested by BI), BI has the exclusive right and sole responsibility for conducting, either itself or through one or more Affiliates or Sublicensees, the Development and Commercialization of any Compound(s) and/or Product(s) in the Territory for use in the Field in accordance with the requirements of this Agreement.
3.2
BI Diligence. BI shall use (and shall cause its Affiliates and Sublicensees to use) Commercially Reasonable Efforts to (a) [**] Compound or Product, as applicable, [**] and (b) [**]. Notwithstanding the foregoing, BI shall be permitted to [**].
3.3
Reports. Until the [**] in the Field in the Territory BI shall keep CUE reasonably informed as to the progress of its Development activities in respect of such Product by providing to CUE, on [**] basis a high-level summary in English describing the progress of the Development activities. CUE shall keep such reports confidential in accordance with Article 10 (Confidentiality).
4.1
Research Term Exclusivity. During the Research Term, CUE shall not, and will procure that its Affiliates shall not, independently work on or assist any Third Party in working on any molecule for applications in B Cell Depletion.
4.2
Target Exclusivity. In addition, during the Exclusivity Period for each Target, CUE shall not, and will procure that its Affiliates shall not, independently work on or assist any Third Party in working on any molecule for B Cell Depletion that is directed to such Target. “Exclusivity Period” means the period beginning (a) with respect to [**], on the Effective Date and (b) with respect to another Target, upon Successful New Compound Inclusion and ending in each case (a) and (b) upon the earlier of (i) expiration or termination of this Agreement in its entirety or with respect to such Target and (ii) [**].
4.3
Impact on Acquirers. The applicability of Section 4.1 and Section 4.2 to Acquirers of CUE will be determined in accordance with Section 8.1.
5.1
CUE Technology License. Subject to the terms and conditions of this Agreement, CUE hereby grants to BI an exclusive (even as to CUE and its Affiliates), royalty-bearing, transferable, sub-licensable in multiple tiers (in accordance with Section 5.6) license during the Term under all CUE Technology to Exploit Compounds and/or Products in the Field in the Territory. Notwithstanding any provision of this Agreement to the contrary, any sublicense granted by CUE to BI pursuant to this Section 5.1 with respect to CUE Technology Controlled by CUE by virtue of the Einstein College Upstream License shall be exclusive as to B Cell Depletion and subject to the specific provisions contained in Schedule 5.8 (“Einstein College Upstream License Provisions”). For the avoidance of doubt, the license set forth in this Section 5.1 does not extend to any active pharmaceutical ingredient other than a Compound contained in a Product and, subject to the exclusivity obligations described in Section 4, CUE shall retain all rights under the CUE Technology to research, Develop, Commercialize and otherwise Exploit any compound or product in any field and territory other than a Compound or Product in the Field in the Territory.
5.2
Research Program License.
BI shall grant and hereby grants to CUE, a worldwide, royalty-free, fully paid-up, cost-free, non-exclusive license to use its Background IP, Collaboration Foreground IP and the CUE Technology (to the extent exclusively licensed to BI pursuant to Section 5.1), in each case that are necessary or reasonably useful, for the purpose of carrying out or preparing its activities or obligations under the Research Program. CUE shall not grant any sublicense under the license set forth in this Section 5.2 without the prior written consent of BI.
5.3
Collaboration Foreground IP License. Subject to the terms and conditions of this Agreement (including CUE’s exclusivity obligations outlined in Section 4), BI grants to CUE a non-exclusive, perpetual, irrevocable, fully-paid-up, sublicensable through multiple tiers, worldwide license under any Collaboration Foreground IP for all purposes other than the Development, Commercialization or other Exploitation of Compound or Product.
5.4
Assigned Patent License. Upon assignment of any Assigned Patents from CUE to BI pursuant to Section 9.1.4 or Section 9.2.1 b) (iii) and subject to the terms and conditions of this Agreement, BI grants to CUE an exclusive, perpetual, irrevocable, fully paid-up, sublicensable through multiple tiers, worldwide license under the Assigned Patents for all purposes other than the Development, Commercialization or other Exploitation of any Compound or Product.
5.5
No Implied Licenses. Except as specifically set forth in this Agreement, neither Party shall acquire any license or any other right or interest, by implication or otherwise, in any Confidential Information or other Know-How disclosed to it under this Agreement or under any Intellectual Property owned or Controlled by the other Party or its Affiliates.
5.6
Sublicensing. BI shall have the right to grant sublicenses with respect to the rights licensed to BI under this Agreement without the prior consent of CUE, provided that each sublicense granted by BI to a sublicensee (a) shall be in a written agreement, (b) shall be subject and subordinate to the terms and conditions of this Agreement, and (c) shall contain terms and conditions consistent with those in this Agreement, including with respect to confidentiality and diligence, and shall not in any way diminish, reduce or eliminate any of BI’s obligations or CUE’s rights under this Agreement. BI shall enforce compliance by each of its Sublicensees with the applicable sublicense agreement and shall be and remain responsible for all acts or omissions of its Sublicensees. Any breach by a Sublicensee of any term or condition of this Agreement shall be deemed a direct breach by BI of such term or condition. BI shall provide CUE with a true and complete copy of each sublicense agreement within [**] after it becomes effective, subject to BI’s right to redact any confidential or proprietary information contained therein that is not necessary for CUE to determine compliance with this Agreement. BI shall be free to grant sublicenses with respect to the rights licensed to BI under this Agreement to its Affiliates and Third Party Collaborators, and the conditions of this Section 5.6 (“Sublicensing”) shall not apply to such Affiliates or Third Party Collaborations; provided that any breach by an Affiliate or a Third Party Collaborator of any term or condition of this Agreement will be deemed a direct breach by BI of such term or condition.
5.7
Survival of Sublicenses. Any such sublicense granted by BI to a Sublicensee that contains terms and conditions materially consistent with those in this Agreement shall survive [**] of the licenses under this Agreement, provided that within [**] of such [**] the Sublicensee requests in writing to [**] that [**] terms and conditions applicable to the CUE Technology [**] agreement between BI and Sublicensee.
CUE herewith agrees to [**] provided that (i) CUE does not have any obligations [**] pursuant to this Agreement and (ii) CUE’s rights thereunder to [**] pursuant to this Agreement. If CUE and the Sublicensee enter into such CUE/Sublicensee Agreement, then Section 19.4.1 e) and f) (“Effects of Termination”) of this Agreement will not apply to BI and CUE.
5.8
Einstein College Upstream License. To the extent the license granted to BI under Section 5.1 includes rights to any Patents or Know-How controlled by Albert Einstein College of Medicine, Inc., the Parties hereby agree to comply with the provisions contained in Schedule 5.8 (“Einstein College Upstream License Provisions”). All capitalized terms used in Schedule 5.8 but not defined therein shall have the meaning set forth in the Einstein College Upstream License.
6.
DATA AND MATERIAL TRANSFER; REGULATORY FILINGS
6.1
Technology Data Package Transfer. During the first [**] after the Effective Date (or such extended period upon mutual agreement of the Parties), CUE shall furnish to BI a data package (“Data Package”) that includes the items set forth in Schedule 6.1 (“Technology Data Package Transfer”). During the Research Term, CUE shall transfer to BI any newly generated CUE Technology (if any) or any additional CUE Technology that was not included in the initial Data Package, in each case which are necessary or reasonably useful for BI to Develop, Commercialize or otherwise Exploit of Compounds and Products in accordance with the terms of this Agreement, on not less than a [**] basis. Within [**] after the conclusion of the Research Term, CUE shall transfer to BI relevant details of CUE Technology not yet provided during the Research Term as set forth above or (if applicable) as CUE Deliverables. At any time within [**] following the end of the Research Term, CUE shall provide to BI upon BI’s request any further details to the CUE Technology which has not been furnished to BI. For clarity, under this Section 6.1, CUE shall only be obligated to transfer to BI CUE Technology that is necessary or reasonably useful for BI to Develop, Commercialize or otherwise Exploit Compounds and Products in accordance with the terms of this Agreement.
6.2
Technical Assistance. CUE shall cause the personnel it employs to (a) answer [**] questions received from BI regarding the transferred CUE Technology within [**] after BI’s receipt of any portion of CUE Technology, and (b) at BI’s [**] request, [**] cooperate with and assist BI with understanding and using the CUE Technology in exercise of BI’s rights under Section 5.1, including without limitation the CUE Know-How provided by CUE to BI under this Agreement. Such cooperation and assistance shall include, without limitation, providing BI with [**] by teleconference or in person to CUE personnel who are [**] for such purpose, and/or who were [**] of Compounds. BI shall be responsible for ensuring that its personnel who receive such assistance are appropriately qualified and experienced for such purpose.
6.3
Regulatory Filings. Following the Effective Date of this Agreement, BI shall control, at its sole cost and expense, the preparation and filing of all regulatory submissions and applications with Regulatory Authorities as may be required for the further Development and Commercialization of Compounds or Products. CUE shall provide to BI reasonable support and assistance in connection therewith as may be requested from time to time by BI. For the avoidance of doubt, BI or its designated Affiliate shall be the title holder of any regulatory authorizations.
6.4
Material Transfer and Supply. CUE shall provide to BI promptly following the end of the Research Term [**] of the Compound [**].
7.1
Implementation of Joint Steering Committee. The Research Program shall be conducted under the direction of a joint steering committee (“JSC”). Within [**] after the Effective Date of this Agreement, the Parties shall establish the JSC comprised of up to [**] representatives of BI and up to [**] representatives of CUE. Each Party may replace its representatives at any time upon prior written information, by email or otherwise, to the other Party. In addition, each Party may invite non-voting employees and consultants or scientific advisors reasonably acceptable to the other Party upon prior written information, by email or otherwise, to the other Party and subject to (i) the prior consent of the other Party (not to be unreasonably withheld) and (ii) a written non-conflict of interest statement of the invited individuals other than BI employees, to attend the meetings of the JSC, subject to appropriate written confidentiality undertakings substantially consistent with each Party’s obligations under this Agreement. The JSC will meet in person (whenever possible, alternating between each Party’s site or otherwise at a mutually agreed location) or by videoconference at least [**] within a Calendar Year, unless otherwise mutually agreed by the Parties. Meeting dates will be defined after mutual agreement by both Parties. Each Party shall bear its own costs related to the attendance of such meetings by its representatives, including but not limited to its own travel and lodging expenses. The JSC will disband after [**].
7.2
Responsibilities. The JSC shall oversee the conduct of the collaboration by steering and monitoring the Research Program. Within such scope the JSC shall:
(i)
review the efforts and the progress of the Parties in the conduct of the Research Program, set or extend non-binding timelines for CUE Deliverables and confirm completeness of the CUE Deliverables and approve completion of the research activities under the Research Plans;
(ii)
make decisions regarding the Research Program;
(iii)
review and approve any newly proposed Research Plans and proposed amendments to any existing Research Plans;
(iv)
oversee and make strategic decisions regarding the conduct of the Research Program;
(v)
reallocate FTE and other resources dedicated to the Research Program, subject however to budget considerations of the Parties and only as permitted under this Agreement;
(vi)
address such other scientific matters relating to the activities of the Research Program as either Party may bring before the JSC.
Except for the mandate to amend the Research Plan pursuant to Section 7.2 (iii), the JSC shall not have the power to amend the terms of, or waive compliance with, this Agreement. Furthermore, the JSC shall not have the power to exercise its authority with respect to any of the matters described in Section 7.3 (a)-(e). For avoidance of doubt, the approval of any newly proposed Research Plan and any amendments to any existing Research Plan by the JSC must be [**]. During the Research Term, on not less than a [**] basis, CUE shall provide the JSC with a written summary of any updates to the CUE Technology that are applicable to an existing Compound or Product that is/are then known to CUE.
7.3
Decision Making Authority. A quorum for a meeting of the JSC shall require the presence of at least one representative for each Party. The JSC shall endeavor to reach decisions by consensus. Each Party, through its representative members of the JSC, shall collectively have one vote for decision making purposes. In the event the JSC is unable to reach an unanimous decision on any matter, the matter shall promptly be submitted to the Senior Executives of the Parties or his or her designee for discussion in good faith based on the goals of the Research Program. If the Senior Executives cannot agree within [**] of the matter having been referred to them, [**] shall have the final casting vote on such matter. Notwithstanding any provision of this Agreement to the contrary, neither the JSC nor a Party through exercise of its final decision-making authority, shall have the authority to: (a) decide on any matter which may have a negative impact on the Research Support Payments (including payment timing) due under this Agreement, (b) require [**] costs which are [**], (c) approve budget-related aspects of any new or amended Research Plan(s), (d) amend, modify or waive compliance with this Agreement or otherwise determine any such matter in a manner inconsistent with the express terms of this Agreement, or (e) determine whether or not a breach of this Agreement has occurred. For further clarity, the issues to be formally decided by the JSC are only those specific issues that are expressly provided in this Agreement to be decided by the JSC. Notwithstanding any provision of this Agreement to the contrary, to the extent [**] exercises its final decision-making authority on any matter that was opposed by [**] or that [**] did not expressly agree to, and the exercise of such final decision-making authority by [**] causes [**] to Default, such Default shall not be deemed a Default under the Agreement and shall be excused.
7.4
Additional Communication. Regular telephone conferences will be scheduled as needed and appropriate in addition to the JSC meetings.
7.5
Program Director. Within [**] following the Effective Date, each Party shall name a program director (the “Program Director”). The respective Program Directors shall appoint, and provide written information, by email or otherwise of the election of scientific sub-team project members who will serve as the day-to-day contacts between the Parties with respect to the Research Program sub-teams and will be primarily responsible for facilitating the flow of information and otherwise promoting communication of the day-to-day work for the Research Program sub-teams. The Program Directors shall conduct regular telephone conferences every [**], or more frequently as deemed necessary or appropriate, to exchange informal information regarding the progress of the Research Program sub-team under the Research Plan.
7.6
Alliance Manager. Within [**] following the Effective Date, each Party shall appoint an alliance manager (“Alliance Manager”) to facilitate logistics, ensure compliance with the Agreement, support the JSC in its governance tasks, and work together to facilitate resolution (in accordance with the terms of this Agreement) of issues between the Parties that arise in connection with this Agreement.
The Alliance Managers shall aim to organize a kick-off meeting within [**] after the Effective Date to introduce relevant members of both Parties, including but not limited to Project Directors, team members and JSC members to the Research Program, clarify responsibilities and each Party’s expectations and outline key contractual aspects to ensure alignment among both Parties. The Alliance Managers shall be permanently invited guests to participate in JSC meetings. The Alliance Managers themselves shall communicate regularly, at a minimum, [**].
8.1
Change of Control of CUE. CUE shall promptly Notify BI of any Change of Control and shall disclose to BI the identity of the Acquirer, in each case, following the first public announcement by CUE of such Change of Control or the execution and closing of a definitive agreement relating to such Change of Control (if such disclosure is not prohibited under Applicable Law or by the terms of any written agreement between CUE and any Third Party). Following a Change of Control of CUE:
8.2
Change of Control of BI. In case of a Change of Control of BI, BI shall promptly inform CUE and shall disclose to CUE the identity of the Acquirer, in each case, following the first public announcement by BI of such Change of Control or the execution and closing of a definitive agreement relating to such Change of Control (if such disclosure is not prohibited under Applicable Law or by the terms of any written agreement between BI and any Third Party). The Parties shall convene a meeting of the executive representatives [**] after CUE’s receipt of such information from BI to discuss the plans and strategy of the Acquirer with respect to the Development and Commercialization of Compounds and Products.
9.1.1
Background IP. Subject to Section 9.1.4 (“Assignment and Transfer of Patents”) of this Agreement, this Agreement shall not affect the ownership of any Background IP.
9.1.2
Foreground IP. All Foreground IP shall be solely owned by BI and considered BI Confidential Information.
Subject to the license granted by BI to CUE in Section 5.3, CUE shall assign and transfer, and hereby assigns and transfers, to BI all of its right, title and interest in Foreground IP and ВI accepts such assignment and transfer in order to establish full and sole ownership of BI with respect to the Foreground IP.
9.1.3
Reporting of CUE Inventions. CUE shall disclose all CUE Inventions made during the Research Term promptly and fully to BI in writing. Such disclosure shall contain the names and contact details of all CUE inventors and a brief description of such CUE Invention.
9.1.4
Assignment and Transfer of Patents. If and to the extent so requested in writing by BI, upon [**] for any Product or any time thereafter, CUE shall assign and transfer, and hereby assigns and transfers, all rights, titles and interests in and to BI Steered CUE Patents and ВI accepts such assignment and transfer (hereinafter “Assigned Patents”) in order to establish full and sole ownership of BI with respect to the Assigned Patents. Upon assignment, such Assigned Patents shall be treated as Collaboration Foreground IP for all purposes under this Agreement with the exception of the determination of royalties and Royalty Term under Section 12.10 (“Royalties”) wherein they will continue to be treated as CUE Patents and such transfer to BI will not affect BI’s payment obligation of all milestones and royalties that become due to CUE under the terms of this Agreement. In order to effect the assignment and transfer, CUE will support BI, upon BI´s request, in executing all assignment documentation and providing any declaration which may be necessary to effect the assignment and full transfer of the Assigned Patents from CUE to BI. BI shall be responsible for and will bear the costs accruing for the assignment and transfer of the Assigned Patents, including the fees for notarization and legalization of the assignment documents, and for recording such assignment documents with the competent patent offices.
9.1.5
Joint Counsel for [**]. The Parties shall jointly select and jointly engage U.S. patent counsel, and such counsel shall have the power of attorney for prosecuting all U.S. [**] and be responsible for directing BI’s non-U.S. counsel for the prosecution of all [**] outside of the U.S.
9.2
Patent Prosecution/Maintenance.
a) Non-U.S. [**]. Upon execution of this Agreement, the Parties will work together to file divisional applications for the [**] in [**], such that at least [**] applications in the [**] will exist in each such country. BI will prepare claims that are limited to [**], and CUE will prepare the claims to [**] for the other application in each such country [**]. The Parties will mutually review and agree such claims and claim amendments. CUE will make every reasonable effort to enable BI to prosecute such [**] before each such patent office, and each such [**] shall become a BI Steered CUE Patent. It is understood that CUE may authorize the filing of additional [**] that have [**], and BI may authorize the filing of additional [**] that have only [**]. For the [**], the Parties will work together to [**]. If the Parties are successful [**], then the Parties shall [**].
b) U.S. [**].
[**].
c) Activities after Separation of [**]. For clarity, after the Parties have filed the [**] and the [**] the Parties will not make any filing with respect to any CUE Steered CUE Patents that includes a [**], and will not make any filing with respect to any BI Steered CUE Patents that include a [**]. Furthermore, the Parties will not pursue any generic claim which could Cover both a compound or product comprising a [**] and a compound or product comprising a [**].
9.2.2
CUE Patenting Activities. CUE shall have full responsibility for, and shall control the preparation, filing, prosecution and maintenance of all CUE Steered CUE Patents, including but not limited to [**] (hereinafter “CUE Patenting Activities”), in its own name and in its own discretion. CUE shall keep BI reasonably informed with respect to such CUE Patenting Activities. CUE shall provide BI with the opportunity to review and comment on all correspondence received from any patent office and all filings related to the CUE Patenting Activities, [**] prior to any filing deadline, which comments CUE will [**]. Notwithstanding the foregoing, BI shall (a) [**] and (b) [**] and filings of the [**] and such [**] shall be subject to [**]. Notwithstanding the foregoing, CUE shall have no obligation to BI relating to CUE Patenting Activities for any CUE Patent that is not [**].
a)
For clarity, the right to prosecute and maintain CUE Steered CUE Patents shall include, in Europe, the right to file a request for unitary effect for a European Patent and the right to decide on “opt-out” and “opt-in” with respect to the jurisdiction of the Unified Patent Court. For any granted European Patent that is a CUE Steered CUE Patent, CUE shall have the sole right to (i) decide in which countries to validate the granted European Patent, and (ii) decide whether to file a request for unitary effect for a European Patent and the right to decide on “opt-out” and “opt-in” with respect to the jurisdiction of the Unified Patent Court.
b)
CUE shall be solely responsible for all costs relating to any CUE Steered CUE Patent.
c)
If CUE decides to abandon or let lapse any CUE Steered CUE Patents, without the intention to file a continuing application or divisional application of such application in order to maintain a pending application in that family, (“CUE Dropped Patent”), then BI will be given a reasonable period of time to decide whether to take over the prosecution and maintenance therefore at its own cost and its own discretion. Upon written information provided by BI to CUE of such decision, CUE shall transfer responsibility (but not ownership) for continued prosecution and maintenance thereof to BI. CUE shall, at BI’s expense, fully cooperate with BI with respect to any such CUE Dropped Patent and execute all lawful papers and instruments, make all rightful oaths and declarations, and provide consultation and assistance as may be reasonably necessary.
9.2.3
BI Patenting Activities. BI shall have full responsibility for, and shall control the preparation, filing, prosecution and maintenance of all BI Steered CUE Patents and all Foreground Patents at BI’s sole expense, including but not limited to [**] (hereinafter “BI Patenting Activities” if and to the extent such activities relate to BI Steered CUE Patents or to Collaboration Foreground Patents; the BI Patenting Activities together with the CUE Patenting Activities, the “Patenting Activities”). CUE shall fully cooperate with BI with respect to any such BI Patenting Activities and execute all lawful papers and instruments, make all rightful oaths and declarations, and provide consultation and assistance as may be necessary in the BI Patenting Activities, provided that BI shall be responsible for any non-de minimis expense required to be incurred by CUE to comply with the obligations of this sentence. BI shall keep CUE reasonably informed with respect to such BI Patenting Activities. BI shall provide CUE with the opportunity to review and comment on all material correspondence received from any patent office and all material filings related to the BI Patenting Activities, [**] prior to any filing deadline, which comments BI will [**]. Notwithstanding any provision of this Agreement to the contrary, BI shall not make any filing with respect to any BI Steered CUE Patent which may reasonably be expected to have a material adverse impact on any other CUE Patent as CUE has informed BI of in writing.
a)
For the sake of clarity, the right to prosecute or maintain a Patent shall include, in Europe, the right to file a request for unitary effect for a European Patent and the right to decide on “opt-out” and “opt-in” with respect to the jurisdiction of the Unified Patent Court. Upon BI’s request, CUE shall promptly give its consent in writing and provide BI with the necessary mandate, or carry out the necessary actions itself, to effect the opt-out of a European Patent from the jurisdiction of the Unified Patent Court.
b)
If BI decides to abandon or let lapse any BI Steered CUE Patents or any Assigned Patents, without the intention to refile such Patent, (“BI Dropped Patent”), then CUE will be given a reasonable period of time to decide whether to take over the full ownership (if applicable), prosecution and maintenance of such Patent at its own costs and its own discretion. Upon written information provided by CUE to BI of such decision to take over the full ownership (if applicable), prosecution and maintenance of such Patent, BI shall assign ownership thereof to CUE (if applicable) and transfer responsibility for continued prosecution and maintenance thereof to CUE. For the sake of clarity, such BI Dropped Patent, if maintained, shall thereafter be considered a CUE Steered CUE Patent, the prosecution and maintenance thereof being governed by Section 9.2.2 herein.
9.3
Engaging Individuals. CUE shall ensure that all of its and its Affiliates’ employees and any other individuals and legal entities it or its Affiliates engage in the performance of the work under this Agreement will be obligated by Applicable Law, employment agreements or other agreements to validly assign all of their rights, title and interest in and to any CUE Invention and related Intellectual Property to CUE. If it is discovered at any time that any individual or legal entity performing work under this Agreement does not have such obligation, CUE will use reasonable efforts to ensure that an agreement providing for such obligation shall be signed promptly but not later than [**] after discovery, without prejudice to any other remedies of BI.
9.4
Patent Term Extensions. BI shall have full and exclusive right and discretion to determine and control all filings of requests for patent term extensions, supplementary protection certificates or equivalents thereto, in any country in the Territory, for any and all BI Steered CUE Patents and all Foreground Patents, or otherwise in connection with any Regulatory Approval of a Product (hereinafter “Patent Term Extensions”). All costs and expenses relating to the Patent Term Extensions shall be born solely by BI. Upon request by BI, CUE shall provide support, assistance, and all necessary documents in fully executed form if needed to BI for the purpose of supporting, filing, obtaining and maintaining Patent Term Extensions, at BI’s expense.
9.5.1
A Party shall promptly inform the other Party in writing if it becomes aware of any suspected, threatened or actual infringement of a Collaboration Foreground Patent or BI Steered CUE Patent (“BI Enforced Patent”) by a pharmaceutical product of a Third Party that contains a compound that binds to a Target (“Competing Product”), and shall promptly provide the other Party with any available evidence of such suspected, threatened or actual infringement (“Competing Infringement”).
9.5.2
BI shall have the first right, but not the obligation, to enforce or defend the BI Enforced Patents against Competing Infringement at BI’s sole discretion and at BI’s sole risk, cost and expense. Where BI desires to enforce or defend an BI Enforced Patent against Competing Infringement, but may not do so due to Applicable Law or regulation, or the requirements of a court of competent jurisdiction, then BI may request and CUE shall enforce or defend such BI Enforced Patent against such Competing Infringement, or join BI to the extent necessary in such proceedings, at BI’s sole risk, cost and expense, and CUE waives any right to refuse to enforce, defend or join such proceeding and waives any objection to the jurisdiction of the court in which such proceeding is brought. BI shall take the lead in the control and conduct of any such enforcement or defense under this Section 9.5.2. Upon reasonable request by BI, CUE shall assist BI in any enforcement or defense action under this Section 9.5.2 at BI’s sole cost and expense, except that CUE shall have the right to engage its own counsel and, to the extent CUE engages its own counsel, such costs and expenses shall be the responsibility of CUE.
9.5.3
Only in the event that BI does not intend to enforce or defend a BI Enforced Patent which constitutes a BI Steered CUE Patent against a Competing Infringement, then BI shall inform CUE of this intent [**], taking into consideration any deadline set by a court or other venue of competent jurisdiction, and, upon BI’s prior written consent, CUE will have the right but not the obligation to enforce or defend such BI Enforced Patent against such Competitive Infringement in CUE’s sole discretion and at CUE’s sole cost and expense; provided, however, [**].
The proceeds (e.g., damages or other compensation) of any enforcement or defense action of BI under this Section 9.5 shall be for the benefit of BI; provided, however, that any such proceeds actually received by BI shall, on a Product-by-Product basis, be deemed to be Net Sales of such Product, and provided further that BI shall be entitled to deduct, on a Product-by-Product basis, the costs and expenses actually borne by and not reimbursed to BI in relation to the enforcement and/or defense of the BI Enforced Patent against Competitive Infringement from the Net Sales of such Product (and where such costs and expenses exceed the proceeds, carry forward the respective non-deductible costs). The proceeds (e.g., damages or other compensation) of any enforcement or defense action of CUE under this Section 9.5 shall be for the benefit of CUE.
9.5.5
CUE shall promptly inform BI in writing if CUE becomes aware of any suspected, threatened or actual infringement of a CUE Steered CUE Patent (“CUE Enforced Patent”) by a Competing Product and shall within [**] provide BI with any available evidence of such suspected, threatened or actual infringement. CUE shall have the sole right, but not the obligation, to enforce or defend the CUE Enforced Patent against such Competing Product, at CUE’s sole discretion and at CUE’s sole risk, cost and expense. CUE shall provide BI with the opportunity to review and comment on all material correspondence and all material filings related to the enforcement or defence of CUE Enforced Patents against such Competing Product [**] which comments CUE will consider [**], with CUE having final decision-making authority with respect thereto. Notwithstanding any provision of this Agreement to the contrary, CUE shall have the sole and exclusive right, but not the obligation, to enforce or defend the CUE Enforced Patents with respect to all infringements other than infringements by a Competing Product.
9.5.6
Only in the event that CUE does not intend to enforce or defend a CUE Enforced Patent against infringement by a Competing Product, then CUE shall inform BI of this intent [**], taking into consideration any deadline set by a court or other venue of competent jurisdiction, and upon CUE’s written consent, BI will have the right but not the obligation to enforce or defend such CUE Enforced Patent against such Competing Product in BI’s sole discretion and at BI’s sole cost and expense; provided, however, [**].
9.5.7
Notwithstanding any provision of this Agreement to the contrary, to the extent a [**], CUE will retain the sole and exclusive right to enforce such [**] against any infringement, but may do so only upon BI’s prior written consent, which consent BI may withhold in BI’s sole discretion. CUE shall provide BI with the opportunity to review and comment on all material correspondence and all material filings related to the enforcement or defence of such [**] in a reasonable period of time.
9.5.8
The provisions of this Section 9.5 shall additionally apply in the case of any objection, opposition or challenge, by a Third Party, with respect to Collaboration Foreground Patents and BI Steered CUE Patents on the one side and CUE Steered CUE Patents on the other side, and such an opposed, challenged or objected-to (a) Collaboration Foreground Patent or BI Steered CUE Patent shall be considered a BI Enforced Patent and (b) CUE Steered CUE Patent shall be considered a CUE Enforced Patent, in each case for the purposes of interpreting this Section 9.5.
Objections, oppositions or challenges to a Patent under this Section 9.5 include, for example, nullity actions, declaratory judgment proceedings, inter partes reexamination proceedings, post grant review proceedings, patent interference proceedings, ex parte and inter parte reexam proceedings, and patent opposition proceedings in a court, patent office or other administrative authority of competent jurisdiction in any country within the Territory.
9.5.9
The Party enforcing or defending a Patent pursuant to this Section 9.5 shall have the right to settle any such dispute; provided that neither Party will have the right to settle any dispute under this Section 9.5 in a manner that (a) [**], or (b) [**], in either case (a) or (b), without the prior written consent of such other Party, not to be unreasonably conditioned, withheld or delayed. Additionally, BI shall not be entitled to settle any such dispute involving a BI Steered CUE Patent without the express written permission of CUE where such settlement involves any admission or other statement relating to infringement (or non-infringement), validity (or invalidity), or enforceability (or unenforceability) of the BI Steered CUE Patent.
9.6.1
Notice. If any action, suit or proceeding is brought against either Party or any Affiliate of either Party or any Sublicensee alleging the infringement or misappropriation of the Intellectual Property of a Third Party by reason of the Exploitation of any Compound or Product by or on behalf of BI, its Affiliates, Sublicensees or Third Party Collaborators (each, an “IP Action”), then such Party will Notify the other Party as promptly as possible following the receipt of service of process in such action, suit or proceeding, or the date such Party becomes aware that such action, suit or proceeding has been instituted, and the Parties, or their appropriate respective designees, will meet as soon as possible to discuss the overall strategy for defense of such matter.
9.6.2
Defense. BI will have the right, but not the obligation, to defend against any IP Action. CUE will cooperate with BI in all reasonable respects in any such IP Action at BI’s sole cost and expense. BI will promptly furnish CUE with a copy of each communication relating to the alleged infringement or misappropriation that is received by BI including all documents filed in any litigation. Unless otherwise set forth herein, BI will have the right to settle such litigation; provided BI will not have the right to settle any litigation under this Section 9.6.2 in a manner that (a) [**], or (b) [**], in either case ((a) or (b)), without the prior written consent of CUE, not to be unreasonably conditioned, withheld or delayed; provided, further that nothing in this Section 9.6.2 will [**]. Additionally, BI shall not be entitled to settle any such dispute without the express written permission of CUE where such settlement involves any admission or other statement relating to infringement (or non-infringement), validity (or invalidity), or enforceability (or unenforceability) of any CUE Patent.
9.7
Patent Listings. BI shall have the sole right and responsibility to make all filings relating to Compounds or Product with Regulatory Authorities throughout the Territory with respect to BI Steered CUE Patents and Foreground Patents, including as required or allowed in the United States in the FDA’s Orange Book, or other international equivalents, and to determine which Patents are to be listed therein.
At BI’s reasonable request, CUE shall (i) provide to BI all information necessary or reasonably useful to enable BI to make such filings with Regulatory Authorities in the Territory with respect to such Patents and (ii) cooperate with BI’s reasonable requests in connection therewith, including meeting any submission deadlines, in each case ((i) and (ii)), to the extent required or permitted by Applicable Law. For clarity, BI shall have no rights relating any filings described in this Section 9.7 with respect to CUE Steered CUE Patents.
9.8
Generic Competition. Notwithstanding the foregoing, if either Party (a) reasonably believes that a Third Party may be filing or preparing or seeking to file a generic or abridged application for Regulatory Approval for a Generic Product that refers or relies on regulatory documentation submitted by either Party to any Regulatory Authority, or (b) receives any notice that any Regulatory Authority had received or accepted an application for a Generic Product, or (c) receives any notification or other communication under 42 U.S.C. § 262(1)(2), § 262(1)(3)(B), § 262(I)(5)(A) or (B), or § 262(I)(8)(A) in connection with an application for licensure of a biological product under 42 U.S.C. § 262(k) for which a Product is the reference product (as defined under 42 U.S.C. § 262(j)(4)) or (d) receives any notice of certification regarding a CUE Patent or Foreground Patent pursuant to the U.S. “Drug Price Competition and Patent Term Restoration Act” of 1984 (21 United States Code §355(b)(2)(A)(iv) or (j)(2)(A)(vii)(IV)) (“ANDA Act”) claiming that any such Patents are invalid or unenforceable or claiming that any such Patents will not be infringed or (e) receives any equivalent or similar certification or notice in any other jurisdiction, it shall (i) promptly Notify the other Party in writing identifying the alleged applicant or potential applicant and furnishing the information upon which determination is based and (ii) provide the other Party with a copy of any such notice of certification within [**] of the date of receipt. The Parties’ rights and obligations with respect to any legal action as a result of such certification shall be as set forth in Sections 9.5.2, 9.5.3, 9.5.4, 9.5.5, 9.5.6 and 9.5.7, as applicable.
9.9
Section 365(n) of the Bankruptcy Code. All rights and licenses granted under or pursuant to any section of this Agreement are and will otherwise be deemed to be for purposes of Section 365(n) of the United States Bankruptcy Code (Title 11, U.S. Code), as amended (the “Bankruptcy Code”) or any comparable law outside the United States, licenses of rights to “intellectual property” as defined in Section 101(35A) of the Bankruptcy Code. The Parties will retain and may fully exercise all of their respective rights and elections under the Bankruptcy Code and any comparable Law outside the United States. Each Party agrees that the other Party, as licensee of such rights under this Agreement, will retain and may fully exercise all of its rights and elections under the Bankruptcy Code or any other provisions of Applicable Law outside the United States that provide similar protection for “intellectual property.” The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against a Party under the Bankruptcy Code or analogous provisions of Applicable Law outside the United States, the other Party will be entitled to a complete copy of (or complete access to, as appropriate) such intellectual property and all embodiments of such intellectual property, which, if not already in such other Party’s possession, will be promptly delivered to it upon such other Party’s written request thereof. Any agreement supplemental hereto will be deemed to be “agreements supplementary to” this Agreement for purposes of Section 365(n) of the Bankruptcy Code.
9.10
Registration of Exclusive License. CUE shall use [**] to register BI as exclusive licensee for any BI Steered CUE Patent in the respective patent registers in the Territory, at BI’s reasonable request. In the event of a termination of this Agreement for any reason, BI shall use [**] to delete such registration in the respective patent registers, and BI shall promptly cooperate and sign all documents that are provided by CUE to BI that are reasonably useful or necessary to delete such registration.
10.1
General. Each Party acknowledges that confidentiality and Know-How protection is of paramount importance for the other Party.
10.2
Non-disclosure and Non-Use Obligation. Each Party agrees, for the duration of the Confidentiality Period to:
10.2.1
hold in strict confidence all Confidential Information of the Disclosing Party which has been or will be made available to the Receiving Party and, subject to the provisions of this Agreement, not to disclose the Disclosing Party’s Confidential Information to any person or entity except to Receiving Party’s Affiliates, employees, consultants and other Third Parties who are required to receive such information to effectuate the purpose of this Agreement, provided that prior to any such disclosure, the Receiving Party shall have first imposed written confidentiality and non-use obligations on such entities or individuals materially equivalent to those imposed on the Receiving Party under this Agreement, however, the imposition of such measures shall not relieve the Receiving Party from its obligations hereunder; and
10.2.2
take reasonable precautions to protect the Disclosing Party’s Confidential Information including, without limitation, safeguarding it in a manner at least as secure as it uses to protect its own Confidential Information of like nature, but in any event in a manner not less than reasonable care; and
10.2.3
comply with all Applicable Laws applicable to the Confidential Information including, without limitation, any applicable restrictions on exports, re-exports, deemed exports or other transfers of information to other countries, entities or persons; and
10.2.4
not use the Disclosing Party’s Confidential Information in any way except solely for the purpose of exercising its rights and obligations under this Agreement; and
10.2.5
not input the Disclosing Party’s Confidential Information into any generative AI system or program or any other AI-based applications, including but not limited to machine learning models and natural language processing systems, without the prior written consent of the Disclosing Party, unless it is an internal, secure system or program.
10.3
Permitted Disclosures
Either Party may disclose Confidential Information disclosed to it by the other Party to the extent such disclosure is required by Applicable Law or for making applications or submissions to or otherwise dealing with a Regulatory Authority in connection with the Development, Manufacture or marketing of Products or for the filing, prosecution, maintenance and enforcement of Patents under, and in accordance with, this Agreement provided, however, that such Confidential Information shall be disclosed only to the extent reasonably necessary to comply with Applicable Law, to obtain authorizations or to file, prosecute, maintain or enforce Patents under this Agreement, and provided that no generative AI system or program is used for any such activities without the Disclosing Party’s prior written consent, unless it is an internal, secure system or program.
10.3.2
If a Party is required by Applicable Law to disclose Confidential Information that is subject to the non-disclosure provisions of Section 10.2, such Party shall promptly inform the other Party of the disclosure that is being sought in order to provide the other Party an opportunity to challenge or limit the disclosure obligations and the Party subject to disclosure obligations shall take into account any measures reasonably requested and comments made by the other Party in good faith. Unless Confidential Information is disclosed to the public according to Applicable Law, it shall remain otherwise subject to the confidentiality and non-use provisions of Section 10.2, and the Party disclosing Confidential Information pursuant to Applicable Law shall take all steps reasonably necessary, including obtaining an order of confidentiality, to ensure the continued confidential treatment of such Confidential Information.
10.3.3
A Party may disclose Confidential Information of the other Party, including the existence and terms of this Agreement (however excluding, as far as legally possible, any and all information and terms contained within the Agreement), or the Parties’ activities under this Agreement, to the extent such disclosure is reasonably necessary when a Party is or becomes obliged to disclose Confidential Information by Applicable Laws that apply to publicly traded companies, such as e.g. U.S. securities laws, rules and regulations published by the National Association of Securities Dealers Automated Quotations (NASDAQ) or other applicable stock exchanges, the U.S. Stock Exchange Regulations or the EU Market Abuse Regulation as reasonably determined by the legal counsel of such Party, provided that (i) in case of a prospectus and associated offering material, any disclosures in associated offering materials shall be non-sensitive and the level of information shall be consistent with customary disclosures made by such Party and (ii) whenever possible, the other Party shall be given adequate (commonly at least [**], but in any case not less than [**]) advance notice of any such disclosure to provide comments to the Disclosing Party, and the Disclosing Party shall apply reasonable efforts to reasonably consider such comments provided by such other Party on the proposed disclosure and to redact the information consistent with the intentions of this Agreement that information shall not be disclosed unless necessary to comply with Applicable Law or applicable other regulations or if the other Party provides its prior written consent. In any event, a Party shall notify the other Party of any disclosure made pursuant to this Section 10.3.3 promptly after such disclosure. In case either Party is obliged to publish the Agreement as a “material agreement” in accordance with the U.S. stock exchange regulations or similar regulations of another country (“SEC Filing”), the Agreement shall be redacted by the filing Party as far as legally possible, and the filing Party shall cooperate with the other Party reasonably in advance to such SEC Filing to enable the other Party to review and comment on the scope of such redaction.
10.3.4
BI may use and disclose CUE Confidential Information with respect to CUE Technology to the extent reasonably necessary for BI to practice its rights under Section 5.1 (“CUE Technology License”).
10.3.5
CUE may use and disclose BI Confidential Information with respect to Collaboration Foreground IP to the extent reasonably necessary for CUE to practice its rights under Section 5.3 (“Collaboration Foreground IP License”), provided that, CUE may not use and disclose any such BI Confidential Information that (a) was developed solely by BI or (b) that is relevant to B Cell Depletion.
10.3.6
Remedies. The Receiving Party recognizes that disclosure of the Disclosing Party’s Confidential Information may result in irreparable harm that cannot be calculated or fully compensated by monetary damages. Therefore, the Disclosing Party may, in addition to any other legal or equitable remedies, seek injunctive relief or specific performance with any court of competent jurisdiction against a breach or threatened breach of this Article 10 without the necessity of posting any bond or surety.
11.
PUBLICITY AND PUBLICATIONS
11.1
Publicity/ Use of Names; Press Releases. Subject to the provisions of Section 10.3 which shall apply accordingly to this Section 11.1, no disclosure of the existence, or the terms, of this Agreement may be made by either Party, and no Party shall use the name, trademark, trade name or logo of the other Party, its Affiliates or their respective employees in any publicity, promotion, news release or disclosure relating to this Agreement or its subject matter, without the prior express written permission of the other Party, except as may be required by Applicable Law or applicable stock exchange in which case Sections 10.3.2 and 10.3.3 apply accordingly. The Parties will issue a press release following the execution of this Agreement describing the nature of the collaboration between BI and CUE in the form as attached to this Agreement as Schedule 11 (“Publicity/ Use of Names; Press Releases”). Each Party consents that after any press release agreed between the Parties, including the initial press release pursuant to this Section 11 (“Publicity/ Use of Names; Press Releases”), has been issued, the Parties may make subsequent public disclosures disclosing the same content without having to again follow the procedures set forth herein; provided such information remains accurate as of such time. Each Party may withdraw such consent at its discretion by Notice to the other Party for future publications.
11.2
Scientific and Other Publications.
11.2.1
Subject to Section 10.3.5, CUE agrees to provide BI with the opportunity to review any proposed abstract, manuscript, scientific presentation (including any public verbal presentation) or any other publication (each a “Publication”) that relates to [**] at least [**] prior to its intended submission for publication. CUE shall not be entitled to publish or present such Publication without first obtaining BI’s prior written consent, such consent not to be unreasonably withheld.
In case BI consents to the Publication in general, CUE agrees to make any reasonable changes regarding the contents, or the timeline of the Publication as requested by BI. Notwithstanding the foregoing, if CUE is required by Applicable Law or applicable stock exchange regulations to publish such Publication, Sections 10.3.2 and 10.3.3 shall apply accordingly. With respect to any Publication published before the Effective Date or in accordance with this Section 11.2.1 during the Term, each Party may disclose to Third Parties the information contained in such Publication without the need for further notice to the other Party in accordance with this Section 11.2.1.
11.2.2
Without limiting the foregoing, no Publication by or on behalf of CUE shall include a [**].
11.2.3
During the Term, BI agrees to provide CUE with the opportunity to review any proposed Publication that relates to the activities performed under the Research Program at least [**] prior to its intended submission for publication. During the Research Term, BI may not publish any such Publication without the prior written consent of CUE which may not be unreasonably withheld. After the Research Term, BI is free to publish any Publication relating to the activities performed pursuant to this Agreement (for clarity, including if it relates to CUE Technology) or the Foreground IP at any time and without any restrictions. Unless such disclosure is permitted pursuant to Section 10.3, BI shall not be entitled to disclose in its Publications any Confidential Information of CUE without the prior written consent of CUE.
12.1
Upfront Payment. As partial consideration of the rights and license granted by CUE to BI pursuant to the terms of this Agreement, BI shall pay to CUE a one-time upfront payment of twelve million US dollars (USD 12,000,000.00). The Invoice for the upfront payment is attached as Schedule 12.1 (“Invoice for Upfront Payment”) to this Agreement. The upfront payment shall be due and payable within [**] following the Effective Date.
12.2
Research Support Payments. During the Research Term, BI will make the following payments to CUE: (a) FTE payments at the FTE Rate multiplied by a maximum amount of FTEs as agreed in each Research Plan to cover research expenses actually incurred for the respective Research Project and (b) reimbursement payments to cover fees charged by permitted subcontractors of CUE and other External Costs reasonably incurred by CUE in connection with the performance of its activities under the Research Program and in accordance with Schedule 1.41 (“External Costs”) ((a) and (b) collectively, the “Research Support Payments”), it being understood that for the activities under the Initial Research Plan for CUE-501 such Research Support Payments shall cover the provision of up to [**] FTEs at CUE working on the Research Project in accordance with the Initial Research Plan, as amended plus all reasonable External Costs. Notwithstanding any provision of this Agreement to the contrary, unless otherwise mutually agreed in advance by the Parties in writing, CUE will have no obligation to provide more than [**] FTEs in the aggregate to perform its activities under the Research Program at any given time during the Research Term. BI will pay such Research Support Payments on a Calendar Quarter basis for the work completed in the previous quarter, provided that the first and last Calendar Quarters of the Research Term shall be pro-rated. All Research Support Payments are subject to receipt by BI of a corresponding Invoice.
Together with such Invoice, CUE shall provide supporting documentation with respect to the previous Calendar Quarter certified by CUE’s [**] or any delegate for the purpose of verifying (i) the number and the calculation of FTEs to be charged to and paid by BI for the work performed by CUE in accordance with the respective Research Project, (ii) the actual performance of the CUE activities pursuant to the Research Program, including the achievement of the CUE Deliverables defined therein, and (iii) details of any External Costs.
12.3
New Compound Selection Fee. BI shall pay to CUE [**] US dollars (USD [**]) for each Successful New Compound Inclusion (the “New Compound Selection Fee”). The payments shall be due and payable within [**] after BI’s receipt of an Invoice of such amount from CUE.
12.4
Milestone Payments in general. BI shall inform CUE in writing within [**] after it becomes aware of the achievement of any milestone event for which a payment to CUE is required under Section 12.5 (“Preclinical Milestone Payments”) – Section 12.8 (“Commercial Milestone Payments”). CUE shall provide an Invoice to BI as soon as practicable after BI has informed CUE. Each milestone payment shall be due and payable to CUE within [**] after receipt of an Invoice from CUE.
12.5
Preclinical Milestone Payments. On a Target-by-Target basis, BI shall pay to CUE the following one-time preclinical milestone payments (each a “Preclinical Milestone Payment”) set forth below upon the first achievement (by BI or its Affiliates or Sublicensees) of a given preclinical milestone by a Compound or Product directed to a given Target (each a “Preclinical Milestone Event”). For clarity, each such milestone payment shall be due only once with respect to each Target and not on a Compound-by-Compound basis:
|
|
Preclinical Milestone Event |
Preclinical Milestone Payment |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
12.6
Clinical Development Milestone Payments. BI shall pay to CUE the one-time clinical development milestone payments (each a “Clinical Development Milestone Payment”) set forth below upon the first achievement (by BI or its Affiliates or Sublicensees) of a given clinical development milestone with respect to any Product (each a “Clinical Development Milestone Event”). For clarity, each such milestone payment shall be due only once and not on a Target-by-Target basis nor on a Product-by-Product basis (except for each first and second Product as set forth below).
|
|
Clinical Development Milestone Event |
Clinical Development Milestone Payment |
|
|
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
12.7
Regulatory Milestone Payments. On a Product-by-Product basis, BI shall pay to CUE each of the following one-time regulatory milestone payments (each a “Regulatory Milestone Payment”) for the first achievement (by BI or its Affiliates or Sublicensees) of the corresponding regulatory milestone set forth in the table below by a given Product (each a “Regulatory Milestone Event”):
|
|
Regulatory Milestone Event |
Regulatory Milestone Payment |
[**] |
$[**] |
[**] |
$[**] |
[**] |
$[**] |
12.8
Commercial Milestones. On a Product-by-Product basis, BI shall pay to CUE each of the following one-time commercial milestone payments (each a “Commercial Milestone Payment”) for the first achievement (by BI or its Affiliates or Sublicensees) of the corresponding commercial milestone set forth in the table below by a given Product (each a “Commercial Milestone Event”):
|
|
Commercial Milestone Event |
Commercial Milestone Payment |
First achievement of Annual Net Sales of a given Product in the Territory greater than [**] Euros |
$[**] |
First achievement of Annual Net Sales of a given Product in the Territory greater than [**] Euros |
$[**] |
First achievement of Annual Net Sales of a given Product in the Territory greater than [**] Euros |
$[**] |
Each Commercial Milestone Payment shall be paid together with the Royalty payments of the Calendar Quarter during which the milestone has been achieved.
12.9
Replacement Products. Notwithstanding the foregoing, and for clarity, if (i)
Development or Commercialization of a Product is terminated after any milestone payment set forth in Section 12.7 (“Regulatory Milestone Payments”) or Section 12.8 (“Commercial Milestone Payments”) above has been made with respect to such Product (“Terminated Product”), and (ii) a different Product with the same (but not identical) Compound Modules as the Terminated Product is selected to replace the Terminated Product (“Replacement Product”), then [**] upon achievement of the same milestone set forth in Section 12.7 and Section 12.8 by such Replacement Product for which CUE already received a milestone payment for the Terminated Product [**].
a)
Sales in the US. BI shall pay to CUE on a Calendar Quarter basis royalties (the “Royalty”) on Annual Net Sales generated in the US of each Product as set forth below by the corresponding amount of incremental aggregate annual Net Sales of such Product in the US:
|
|
Annual Net Sales in the US of a Product in a Calendar Year |
Royalty on Net Sales
|
On that portion below [**] Euros |
[**]% |
On that portion equal to or greater than [**] Euros and below [**] Euros |
[**]% |
On that portion equal to or greater than [**] Euros and below [**] Euros |
[**]% |
On that portion equal to or greater than [**] Euros |
[**]% |
b)
Sales outside the US. BI shall pay to CUE on a Calendar Quarter basis Royalties on Annual Net Sales generated outside the US of Products in the amount set forth below:
|
|
Annual Net Sales of a Product outside the US in a Calendar Year |
Royalty on Net Sales
|
[**] Annual Net Sales of a Product outside the US |
[**]% |
a)
Beginning of Royalty Term. BI’s Royalty obligations as set forth in Section 12.10.1 (“Amount”) shall begin, on a country-by-country and Product-by-Product basis, with the First Commercial Sale of such Product in such country.
b)
End of Royalty Term. BI’s Royalty obligations to CUE under this Section 12.10 (“Royalties”) shall expire on a country-by-country and Product-by-Product basis upon the later to occur of (i) [**] following the First Commercial Sale of such Product in such country, (ii) the expiration of all Valid Claims of the CUE Patents (which for clarity includes Assigned Patents) and Collaboration Foreground Patents in such country [**] in such country, or (iii) the expiration of Regulatory Exclusivity for such Product in such country (“Royalty Term”).
a)
Non-Patented Product. During the applicable Royalty Term on a Product-by-Product and country-by-country basis, if a Product [**] is not Covered by a Valid Claim of a CUE Patent (which for clarity includes any Assigned Patent) or, Collaboration Foreground Patent in such country [**], at the time of sale, then the royalty rate for such Product in such country shall be reduced by [**] percent ([**]%) of the applicable rate determined pursuant to Section 12.10.1 (“Amount”).
b)
Third Party Offset. During the applicable Royalty Term on a Product-by-Product and country-by-country basis, if BI, [**], is required to obtain a licence after the Effective Date from one or more Third Parties under [**], then the Royalty payments due under Section 12.10.1 (“Amount”) with respect to Net Sales in a Calendar Quarter for such Product in such country shall be reduced by [**] percent ([**]%) of the royalty amounts payable by BI to such Third Party for such licence to such Product in such country for as long as such Third Party license remains in effect.
c)
Generic Competition. The Royalty otherwise due under Section 12.10.1 (“Amount”) shall be reduced, on a country-by-country and Product-by-Product basis, in the event of Generic Competition in a particular country of the Territory to [**] per cent ([**]%) of the applicable Royalty rate specified in Section 12.10.1 (“Amount”) above in any Calendar Quarter in which there is Generic Competition for so long as the Generic Competition exists in such country.
d)
Limits on Deductions. In no event shall the cumulative effect of the deductions in Sections 12.10.3(a) (“Non-Patented Product”), 12.10.3(b) (“Third Party Offset”), and (c) (“Generic Competition”) reduce the Royalties to less than [**] percent ([**]%) of the amounts due (prior to application of any deduction set forth in this Section 12.10.3) in any given Calendar Quarter for a Product in such country pursuant to Section 12.10.1 (“Amount”).
e)
Multiple Royalties. No multiple Royalties shall be payable because a Product, its manufacture, use or sale is or shall be Covered by more than one Valid Claim of a patent included in the licensed rights hereunder.
12.10.4
Reports and payments. Within [**] following the end of each Calendar Quarter, BI shall submit to CUE a written report of Net Sales of Products sold by or on behalf of BI, its Affiliates and Sublicensees during a Calendar Quarter in each country of the Territory in sufficient detail to permit confirmation of the accuracy of Royalty payments paid.
BI shall pay to CUE, within [**] of receipt of CUE’s Invoice, all Royalties payable by BI. If applicable, by separate statement, BI will provide a confirmation on a Product-by-Product and country-by-country basis including:
(a) Net Sales of Products (including calculation of Net Sales of Combination Products or Co-Packaged Products) in the currency for which such Products were sold, and, if the currency of sale was not EUR, also in EUR;
(b) an accounting of deductions taken in the calculation of Net Sales;
(c) details of any royalty offsets as defined in the Agreement, and in the case of an offset based on Generic Competition, the relevant market share data used and the source of such data;
(d) the applicable exchange rate to convert from each country’s currency to EUR; BI shall use the monthly average exchange rates used by BI throughout its regular accounting system for the Calendar Quarter in which such Products are sold;
(e) the Royalty Payments payable in US dollars.
12.10.5
Financial Audit. BI shall keep (and shall cause its Affiliates and Sublicensees to keep) complete and accurate records pertaining to the sale or other disposition of Products in sufficient detail and in accordance with its Accounting Standards to permit CUE to confirm the accuracy of all Royalty payments reported, for at least [**] following the end of the Calendar Year to which they pertain. CUE shall have the right to cause an internationally recognized independent, certified public accountant reasonably acceptable to BI (the “Auditor”) to audit such records solely to confirm Net Sales and Royalty payments for a period covering not more than the preceding [**], provided that such audits may not be performed more than [**] and [**]. Such audits may be exercised during normal business hours upon reasonable prior written information to BI. The Auditor will execute a reasonable written confidentiality agreement with BI and will disclose to CUE only such information as is reasonably necessary to provide CUE with information regarding any actual or potential discrepancies between amounts reported and actually paid and amounts payable under this Agreement. The report of the Auditor will include the methodology and calculations used to determine the results, will be delivered to BI and CUE at the same time, and will be final [**] after delivery to both Parties, it being understood that BI will have the right during such [**] period to discuss the report with the Auditor. In the event the Parties are not in alignment after such [**] period, either Party may refer this matter for resolution in accordance with the defined dispute resolution procedure set forth in Article 20 (“DISPUTE RESOLUTION”) within [**] of the last Business Day in the draft audit report review period. CUE shall bear the full cost of such audit unless the report of the Auditor discloses an underpayment by BI of more than [**] percent ([**]%) of the amount due [**], in which case BI shall bear the full cost of such audit. BI shall pay the amount of any underpayment disclosed in the undisputed Auditor’s report, together with interest thereon to CUE within [**] after delivery to the Parties of the final Auditor’s report.
If such final Auditor’s report discloses an overpayment by BI of the amounts payable hereunder, [**] following the audit in question. Upon the expiration of [**] following the end of any Calendar Year, [**].
12.10.6
Currency Conversion. All Royalties shall be payable in full in USD. Any sales of Products incurred in a currency other than EUR shall be converted to the EUR equivalent using a rate of exchange that corresponds to the rate consistently applied by BI or any of its Affiliates in accordance with Accounting Standards. If such Party is not required to perform such currency conversion for its Accounting Standards reporting with respect to the applicable period, then for such period such Party shall convert its amounts received and expenses incurred into EUR using exchange rates published by the European Central Bank (ECB), Frankfurt, Germany. For exchange rates not published by ECB an alternative source will be agreed between the Parties. Any Royalty shall be calculated based upon the EUR equivalent calculated in accordance with the foregoing.
12.11
Payment Terms; Currency. BI shall pay all amounts payable under this Agreement as stated in the respective sections, upon delivery to BI of an Invoice for such amounts by CUE. All payments to be made by BI to CUE under this Agreement shall be made in USD and may be paid by bank wire transfer in immediately available funds to such bank account as may be designated by CUE from time to time.
12.12
Taxes in general. All payments connected with this Agreement shall be inclusive of any Taxes except as otherwise set forth in this Agreement, in particular in Section 12.13 (“VAT”). Each Party shall be responsible for and shall bear, pay or set-off its own Taxes assessed by a tax or other authority.
12.13
VAT. All payments connected with this Agreement are expressed to be exclusive of VAT. VAT shall be added to the payments due if legally applicable.
12.14
Withholding Tax. If Applicable Law requires BI and/or its Affiliates to withhold any Taxes, BI and/or its Affiliates shall subtract such withholding from the payments to be made in connection with this Agreement and submit such withholding to the competent tax authority on account of CUE. BI and/or its Affiliates shall deliver to CUE proof of payment on CUE’s request to enable CUE to either apply for a refund of such Taxes withheld or to receive a tax credit against home-country Taxes. The Parties shall cooperate and exercise reasonable efforts to minimize any such Taxes required to be withheld and obtain any exemption or reduction in withholding afforded by a tax treaty. Any effect of currency conversion, in calculating the withholding or any reimbursement of Taxes, shall be to the benefit or burden of CUE as tax-payer and are not refundable or taken by BI and/or its Affiliates.
12.15
Interest on Late Payments. If BI fails to pay any payment due under this Agreement as provided herein on or before the date such payment is due, then such late payment will bear interest, to the extent permitted by Applicable Law, at an annual rate of [**] which applied on the due date effective for the first date on which payment was delinquent and calculated for the exact number of days in the interest period based on a year of three hundred sixty (360) days (actual/360). If the [**] is no longer published, the Parties will agree upon another internationally recognized rate which has historically been substantially equivalent to the [**] and utilize such rate retroactively to such time as the rate was no longer available.
12.16
Record Retention; Audits. The Research Support Payments will be used solely for the purpose of supporting the Research Program consistent with the budget defined in the Research Plan and in Schedule 1.41 (“External Costs”). CUE will maintain financial and compliance records with respect to the Research Program under this Agreement. Such records will be adequate to determine whether the Research Program has been conducted and funding has been used in compliance with this Agreement and Applicable Laws. During the term of the Research Program, and up to [**] following the Research Program, BI reserves the right to audit CUE’s records with respect to the performance of this Agreement and the Research Program. At all reasonable times after reasonable notice (by e-mail or otherwise), CUE shall provide BI’s representatives, designees, auditors and regulators, as BI may designate from time to time (“BI Auditors”), access (i) to inspect [**] for the Research Program; (ii) to review and examine [**] performance of the Research Program; (iii) to review and examine [**] performance of the Research Program; (iv) to permit BI Auditors to [**] under the Research Program; and (v) to perform other audits and reviews of the Research Program necessary to enable BI to meet Applicable Laws; provided that such audit may not be performed more than [**]. The BI Auditors will execute a reasonable written confidentiality agreement with CUE and will disclose to BI only such information as is reasonably necessary to provide BI with information regarding any actual or potential discrepancies between amounts reported and actually paid and amounts payable as well as any actual or potential non-compliance with this Agreement or Applicable Law, in each case in the performance of the Research Program. Up to [**] following the Research Program, CUE must obtain prior written approval from BI prior to destroying any essential documents of the Research Program.
12.17
Upstream Licenses Payment Obligations. CUE shall be solely responsible to pay all consideration owed to its licensors under the Einstein College Upstream License. In case of BI becoming the direct licensee to any such Einstein College Upstream License on account of CUE’s uncured material breach or termination or consent to a termination agreement of such Einstein College Upstream License or with CUE’s prior written consent, all payments due to CUE under this Agreement, including royalties, will be made after deduction of the royalty and milestone amounts paid by BI under such Einstein College Upstream License. For clarification, this shall include any payments that became due before BI becoming the direct licensee to such Einstein College Upstream License in case such payment obligations have not been fulfilled by CUE and are being passed on to BI as the direct licensee.
13.
REPRESENTATIONS, WARRANTIES AND COVENANTS
13.1
CUE’s Representations. CUE represents and warrants to BI that as of the Effective Date:
13.1.1
CUE and its Affiliates are validly incorporated, existing and solvent under the Applicable Laws of the jurisdiction of their incorporation.
13.1.2
This Agreement does not violate any Applicable Law, has been duly executed and is legally binding and enforceable against CUE in accordance with its terms and the execution, delivery and performance of this Agreement by it has been duly authorized by all necessary corporate action.
13.1.3
CUE has the full corporate power, right, title and authority to enter into this Agreement, conduct the Research Program, grant the licences, disclose all Know-How to BI and transfer the Materials to BI as required by this Agreement.
13.1.4
There are no inconsistencies between this Agreement and the Loan and Security Agreement between CUE and the Silicon Valley Bank, dated February 15, 2022, and CUE has received any necessary permit or consent of the Silicon Valley Bank to enter into this Agreement.
13.1.5
CUE has not previously assigned, licensed or otherwise encumbered its right in the CUE Technology or entered into any other agreement that is inconsistent with the rights granted to BI or CUE’s obligations under this Agreement.
13.1.6
CUE has ensured that all existing Inventions, made by any individuals or other legal entities engaged in the generation of the CUE Technology, have been duly transferred to CUE or to the licensor of the Einstein College Upstream Patent, as applicable.
13.1.7
All CUE Technology that has been generated under any agreements between CUE and any Third Party (except the Einstein College Upstream Patents) is solely Controlled by CUE.
13.1.8
Schedule 1.32 (“Non-Exhaustive List of CUE Technology”) is correct and includes a complete list of all CUE Patents as of the Effective Date.
13.1.9
The CUE Patents listed on Schedule 1.32 (“Non-Exhaustive List of CUE Technology”) are in force or pending and have not been abandoned and all applicable renewal or other official fees have been paid.
13.1.10
CUE is not in breach with any requirements, and has complied with all its obligations under the Einstein College Upstream License, including but not limited to any payment obligations and due diligence requirements.
13.1.11
To CUE’s Knowledge, (a) there are no Third Party Intellectual Property rights that would be infringed by the use by BI or its Affiliates and Sublicensees of the CUE Technology as contemplated under the Initial Research Plan, even in the absence of a research privilege or any other research exemption, and (b) CUE has disclosed to BI any information available regarding any potential violation of any Third Party Intellectual Property by the commercial use of BI of Compound and/or Products.
13.1.12
To CUE’s Knowledge, no Intellectual Property other than CUE Technology is needed to conduct the initial Research Project in the manner contemplated under the Initial Research Plan.
13.1.13
To CUE’s Knowledge, the Materials and methods employed by or on behalf of CUE or provided by or on behalf of CUE to BI to perform the activities assigned to it under the Initial Research Plan [**].
13.1.14
To CUE’s Knowledge, there are no claims, judgments or settlements pending with respect to the CUE Technology and CUE has not received notice that any such claims, judgments or settlements are threatened.
13.1.15
To CUE’s Knowledge, CUE has furnished or made available to BI [**].
13.1.16
No additional information has been deposited in the data room after March 15, 2025.
13.1.17
CUE (i) has never been debarred or subject to debarment or has received notice from the FDA of an intent to debar or has been convicted of а crime for which an entity or person could be debarred under 21 U.S.C. §335а; or (ii) has never been under indictment for a crime for which a person or entity could be debarred under 21 U.S.С. §335а. If, during the Term, CUE has reason to believe that it or any of its employees, officers, independent contractors, consultants or agents rendering services relating to the Compounds or Products: (a) is or will be debarred or convicted of a crime for which a person or entity could be debarred under 21 U.S.C. §335а; or (b) is or will bе under indictment for a crime for which a person or entity could be debarred under 21 U.S.C. §335а, then CUE shall immediately Notify BI of same in writing.
13.1.18
In case animal experiments were conducted for the generation of CUE Technology, CUE obtained any and all necessary registrations, licenses, approvals and permits from governmental and other authorities and complied with all Applicable Laws governing the procurement, provision, care, welfare, treatment, and use of animals in research.
13.2
BI Representations. BI represents and warrants to CUE that as of the Effective Date:
13.2.1
BI is validly incorporated, existing and solvent under the Applicable Laws of the state of its incorporation.
13.2.2
This Agreement has been duly executed and is legally binding and enforceable against BI in accordance with its terms and the execution, delivery and performance of this Agreement by it has been duly authorized by all necessary corporate action.
13.2.3
BI has the full corporate power, right, title and authority to enter into this Agreement and carry out the provisions hereof, including to conduct the Research Program and transfer the Materials to CUE as required under this Agreement.
13.2.4
BI has not previously entered into any other agreement that is inconsistent with its obligations under this Agreement.
13.2.5
BI has never been (i) debarred or subject to debarment or has received notice from the FDA of an intent to debar or has been convicted of а crime for which an entity or person could be debarred under 21 U.S.C. §335а; or (ii) indictment for a crime for which a person or entity could be debarred under 21 U.S.С. §335а. If, during the Term, BI has reason to believe that BI its Affiliates, its Sublicensees, or any of their respective employees, officers, independent contractors, consultants, or agents rendering services relating to the Compounds or Products: (a) is or will be debarred or convicted of a crime for which a person or entity could be debarred under 21 U.S.C.
§335а; or (b) is or will bе under indictment for a crime for which a person or entity could be debarred under 21 U.S.C. §335а, then BI shall immediately Notify Cue of same in writing.
13.3.1
CUE shall not, during the Term as determined in Section 19.1 (“Term”), (i) assign, license or otherwise encumber any rights in the CUE Technology in a manner that violates any rights granted to BI under this Agreement or (ii) enter into any agreement that violates any rights granted to BI, or prevents CUE from performing its obligations, under this Agreement.
13.3.2
CUE shall comply with all requirements and obligations under the Einstein College Upstream License, including but not limited to any payment obligations and due diligence requirements.
13.3.3
CUE will not terminate the Einstein College Upstream License nor agree to any amendment of the Einstein College Upstream License that changes the specific stipulations of the Parties and Albert Einstein College of Medicine, Inc. in view of this Agreement or that has an adverse effect on the rights or obligations of BI regarding its sublicense under the Einstein College Upstream License without BI’s prior written consent.
13.3.4
CUE shall ensure that all right, title and interest in any CUE Inventions made by its and its Affiliates’ employees, and of any other individuals and legal entities engaged by or on behalf of CUE excluding BI and its Affiliates (however, including Inventions made jointly with BI) (collectively, “CUE Developers”) in the generation of the CUE Technology and Collaboration Foreground IP in connection with this Agreement will be duly transferred to CUE.
13.3.5
To the extent CUE is responsible for prosecution and maintenance of CUE Patents pursuant to Section 9.2, CUE shall ensure compliance with all Applicable Law in the continued prosecution and maintenance of such CUE Patents, including but not limited to the payment of appropriate fees (to the extent necessary, for a large entity) before the US Patent and Trademark Office and any other patent office or authority outside the United States, as applicable.
13.3.6
CUE shall ensure that all CUE Developers that perform any activities under any Research Plan shall be advised of their ongoing duty of confidentiality and cooperation, including in case they resign from their employment with CUE.
13.3.7
CUE shall not during the Term grant to any Third Party any license or other rights to practice under any CUE Steered CUE Patent in a manner that violates any rights granted to BI under this Agreement.
13.3.8
CUE and its Affiliates will not (i) emрlоу or use any Third Party that employs any person debarred by the FDA (or subject to similar sanction of the EMA or other Regulatory Authority) or (ii) еmрlоу any person that is the subject of an FDA debarment investigation or proceeding (or similar proceeding of the EMA or other Regulatory Authority), in each of (i)-(ii), in the conduct of its activities under this Agreement.
13.4.1
BI shall not, during the Term of this Agreement, enter into any agreement that is inconsistent with CUE’s rights or BI’s obligations under this Agreement.
13.4.2
To the extent BI is responsible, pursuant to Section 9.2, for prosecution and maintenance of BI Steered CUE Patents, BI shall ensure compliance with all Applicable Law in the continued prosecution and maintenance of such BI Steered CUE Patents, including but not limited to the payment of appropriate fees before the US Patent and Trademark Office and any other patent office or authority outside the United States, as applicable.
13.4.3
BI, its Affiliates and its Sublicensees will not (a) emрlоу or use any Third Party that employs any person debarred by the FDA (or subject to similar sanction of the EMA or other Regulatory Authority) or (b) еmрlоу any person that is the subject of an FDA debarment investigation or proceeding (or similar proceeding of the EMA or other Regulatory Authority), in each of (i)-(ii), in the conduct of their activities under the Research Program.
13.5
DISCLAIMER. EXCEPT FOR THE EXPRESS REPRESENTATIONS AND WARRANTIES SET FORTH HEREIN, NEITHER PARTY MAKES ANY REPRESENTATIONS OR GRANTS ANY WARRANTIES, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND ЕАСH PARTY SPECIFICALLY DISCLAIMS ANY OTHER WARRANTIES, WHETHER WRITTEN OR ORAL, OR EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF QUALITY, MERCHANTABILITY, OR FITNESS FOR A PARTICULAR USE OR PURPOSE OR ANY WARRANTY AS ТО THE VALIDITY OR ENFORCEABILITY OF ANY PATENTS OR ТНЕ NON-INFRINGEMENT OF ANY INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES.
14.1
The Parties acknowledge that any products, goods, software, technology (specific technical information necessary for the development, production or use of a product) and technical services provided by either Party under this Agreement (hereinafter “Items”) may be subject to international, EU, U.S. or other applicable trade compliance and/or export control laws and regulations (hereinafter “Laws”) restricting exports, re-exports, transfer or disclosures, regardless of the mode of provision. The Parties shall comply with all such Laws.
14.2
If any Item is subject to any restriction or license requirement under the Laws, the Parties shall inform each other in writing about these restrictions accordingly. Upon request, the Parties shall cooperate with each other by providing information and other assistance necessary for the classification, export documentation, license determination, export licensing etc. of Items provided under this Agreement.
14.3
The Parties confirm that they are neither a “Sanctioned Party” in terms of UN, U.S., EU or any national “Sanctioned Party List” nor Controlled by a “Sanctioned Party”. The Parties Notify each other without delay in case of any changes of this status.
15.1
Shipment of Compounds: CUE shall not ship any Material or Compound to BI without the prior written request of BI. BI’s request will outline the specific amount as agreed between the Parties. Material shall be delivered in accordance with CIP/CIF (INCOTERM 2020). Destination address for the shipment of materials shall be set out below, and the Parties shall inform each other about any change of the shipping address.
|
|
From BI to CUE |
40 Guest Street, Boston, MA 02135, USA |
From CUE to BI |
[**], Boehringer Ingelheim Pharmaceuticals Inc, 39 Briar Ridge Rd, Danbury, CT 06810, USA |
15.2
CUE’s obligation to collaborate for customs valuation purpose BI is legally bound to determine a customs value for any shipment from a third country entering the European Customs Union. If applicable, CUE must provide respective information (e.g., total amount synthesized) to BI within [**] of request. Scope of information is to be defined between CUE and BI at the request of either Party and will be documented in written form.
15.3
Approved exporter (or comparable status): If there is a reciprocal preferential trade agreement in place between sending country and European Union (as listed at Negotiations and agreements - Trade - European Commission (europa.eu), CUE shall confirm, if applicable, that the Material or Compound being under this Agreement originates from the United States and meets the rules of origin for preferential trade with the EU. CUE undertakes to make available to BI any additional documents required by the relevant customs authorities to prove this. CUE shall evaluate in alliance with BI if becoming an approved exporter (or a comparable status, e.g. registered exporter [REX]) in such country is viable and economically reasonable.
16.
ANIMAL WELFARE AND USE
16.1
CUE represents, warrants and covenants to BI that:
16.1.1
It has obtained and will, during the Term, maintain in full force and effect any and all registrations, licenses, approvals and permits from governmental and other authorities and has notified all authorities necessary for the conduct of animal experiments as it may be required to maintain to conduct the activities contemplated hereunder as required under Applicable Law and it will comply with all laws, regulations and guidelines for the handling, treatment, welfare and ethical treatment of animals in research and that it has truthfully completed the Animal Welfare Questionnaire attached hereto as Schedule 16.1.1 (“Animal Welfare Questionnaire”);
16.1.2
it will at all times during the Term maintain, adopt and adhere to generally accepted professional standards governing the procurement, provision, care, welfare, treatment, and use of research animals, including during actual research and experimentation, and shall ensure that all personnel involved in any such activities are qualified by training and experience to perform same in accordance with such standards.
16.1.3
to the extent CUE has the right to do so, it will permit BI to inspect and audit its animal care, welfare, housing and use facilities and related records upon request to ensure compliance with this Article and shall cooperate with, facilitate and otherwise support BI in any such inspection and audit at its own cost and expense.
16.2
The Parties acknowledge and agree that all laboratory animals used, procured or bred by CUE under this Agreement are not owned by BI.
17.1
The additional provisions contained in Schedule 17 (“Use of Human Biospecimens”) shall apply to the use of any Human Biospecimens by or on behalf of CUE, its Affiliates or their subcontractors under this Agreement.
18.
INDEMNIFICATION AND LIMITATION OF LIABILITY
18.1
Indemnification by CUE. CUE shall indemnify, defend, and hold harmless BI, and its Affiliates, and their respective officers, directors, employees, licensors, Sublicensees and their respective successors, heirs and assigns and representatives (the “BI Indemnitees”), from and against any and all damages, losses, suits, proceedings, liabilities, costs (including without limitation reasonable legal expenses, costs of litigation and reasonable attorney’s fees) or judgments, whether for money or equitable relief, of any kind (“Damages”) resulting from Third Party (including CUE employees) claims or actions, to the extent arising out of or relating to, directly or indirectly: (i) the negligence, recklessness or wrongful intentional acts or omissions of CUE, its Affiliates, subcontractors and/or licensors and its or their respective officers, directors, employees in connection with CUE’s performance of its obligations or exercise of its rights under this Agreement, (ii) any breach by CUE of any obligation, representation, warranty or covenant set forth in this Agreement, or (iii) the failure to comply with any Applicable Laws by CUE, its Affiliates, or any of its subcontractors or licensors, in each case (i), (ii) and (iii), except in any such case for Damages to the extent reasonably attributable to any BI Indemnitee with respect to any matter for which BI is liable to indemnify CUE pursuant to Section 18.2 (“Indemnification by BI”).
18.2
Indemnification by BI. BI shall indemnify, defend, and hold harmless CUE and its Affiliates, and its respective officers, directors, employees, licensors, and their respective successors, heirs and assigns and representatives (the “CUE Indemnitees”), from and against any and all Damages resulting from Third Party (including BI employees) claims or actions, to the extent arising out of or relating to, directly or indirectly: (i) the negligence, recklessness or wrongful intentional acts or omissions of BI or its Affiliates, subcontractors, Sublicensees and its or their respective directors, officers and employees in connection with BI’s performance of its obligations or exercise of its rights under this Agreement, (ii) any breach by BI of any obligation, representation, warranty or covenant in this Agreement, (iii) the failure to comply with Applicable Laws by BI or any of its Affiliates or Sublicensees, or (iv) the Development, Commercialization or other Exploitation of any Compound, Product, or component thereof by or on behalf of BI or its Affiliates or Sublicensees, including product liability claims, in each case (i), (ii) (iii) and (iv), except in any such case for Damages to the extent reasonably attributable to any CUE Indemnitee with respect to any matter for which CUE is liable to indemnify BI pursuant to Section 18.1 (“Indemnification by CUE”).
18.3
Notification; Assumption of Defence; Cooperation and Assistance.
18.3.1
In the event that a Party seeks indemnification hereunder with respect to a Third Party claim (a “Third Party Claim”), the Party seeking indemnification (the “Indemnified Party”) shall promptly Notify the other Party (the “Indemnifying Party”) of any Third Party Claim in respect of which it intends to claim indemnification under this Article 18.
18.3.2
Any failure to provide the Indemnifying Party with any such Notice will not relieve the Indemnifying Party from any liability that it may have to the Indemnified Party under this Article 18 except to the extent that the ability of the Indemnifying Party to defend such claim is materially prejudiced by the Indemnified Party’s failure to give such Notice.
18.3.3
If the Indemnifying Party assumes such defence, the Indemnified Party will have the right to participate in the defence thereof and to employ counsel, at its own expense, separate from the counsel employed by the Indemnifying Party.
18.3.4
If the Indemnifying Party does not assume control of the defence of a Third Party Claim within [**] after the receipt by the Indemnifying Party of the Notice required pursuant to this Section 18.3 (“Notification; Assumption of Defence; Cooperation and Assistance”), the Indemnified Party will have the right to defend such claim in such manner as it may deem appropriate at the reasonable cost and expense of the Indemnifying Party.
18.3.5
The Indemnified Party shall cooperate as may be reasonably requested in order to ensure the proper and adequate defence of any action, claim or liability covered by this indemnification.
18.3.6
The Indemnifying Party may not settle or otherwise dispose of any Third Party Claim without the prior written consent of the Indemnified Party, such consent not to be unreasonably withheld, unless such settlement includes only the payment of monetary damages (which are fully paid by the Indemnifying Party), does not impose any injunctive or equitable relief upon the Indemnified Party, does not require any admission or acknowledgment of liability or fault of the Indemnified Party and contains an unconditional release of the Indemnified Party in respect of such Third Party Claim.
18.3.7
The Indemnified Party may not settle or otherwise dispose of any Third Party Claim for which the Indemnifying Party may be liable for damages under this Agreement without the prior written consent of the Indemnifying Party.
18.4
LIMITATION OF LIABILITY.
NOTWITHSTANDING ANYTHING IN THIS AGREEMENT TO THE CONTRARY, NEITHER PARTY SHALL BE LIABLE TO THE OTHER WITH RESPECT TO ANY SUBJECT MATTER ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT, WHETHER UNDER ANY CONTRACT, NEGLIGENCE, STRICT LIABILITY OR OTHER LEGAL THEORY, FOR ANY INDIRECT, INCIDENTAL, SPECIAL, EXEMPLARY, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES (INCLUDING, WITHOUT LIMITATION, LOST PROFITS, LOSS OF USE, DAMAGE TO GOODWILL, OR LOSS OF BUSINESS); PROVIDED, HOWEVER, THAT THE FOREGOING SHALL NOT APPLY TO A PARTY’S INDEMNIFICATION OBLIGATIONS UNDER SECTION 18.1 or 18.2 ABOVE OR TO ANY BREACH BY A PARTY OF SECTION 3.2 (“BI DILIGENCE”), ARTICLE 10 (“CONFIDENTIALITY”), ARTICLE 12 (“PAYMENT TERMS”) OR ARTICLE 13 (“REPRESENTATION, WARRANTIES AND COVENANTS”), THE WILLFUL BREACH, WILLFUL MISCONDUCT, OR GROSS NEGLIGENCE BY A PARTY OR TO ANY LIABILITY FOR DEATH OR PERSONAL INJURY OR OTHER LIABILITY WHICH CANNOT BE LIMITED OR EXCLUDED UNDER APPLICABLE LAW.
19.1
Term. This Agreement shall become effective upon the Effective Date and, if not otherwise terminated earlier pursuant to this Article 19 (“TERM & TERMINATION”) or Section 21.7 (“Anti-Bribery/Anti-Corruption”), shall continue in full force and effect until the expiration of the last to expire Royalty Term (such period, the “Term”). Once the Royalty Term for a particular Product in a particular country has ended, BI’s licenses under this Agreement for such Product in such country (except with respect to any rights under the Einstein College Upstream License that CUE sublicenses to BI) shall be fully paid-up, perpetual, sublicensable without restriction, transferable without restriction, irrevocable and royalty-free and BI’s payment obligations under this Agreement for such Product in such country shall expire.
19.2
Termination for Cause. This Agreement may be terminated at any time during the term of this Agreement by either Party:
19.2.1
Upon Default by the other Party (the “Defaulting Party”) which Default remains uncured for [**] (the “Default Cure Period”), each measured from the date the Defaulting Party receives Notice provided by the other Party (the “Non-Defaulting Party”) of such Default (the “Default Notice”). The Default Notice shall identify the Default, the intent to so terminate this Agreement if the Default remains uncured during the Default Cure Period and the actions or conduct that it considers would be an acceptable cure of such Default. In case the Defaulting Party disputes the Default under this Section 19.2.1, then the issue of whether the Non-Defaulting Party may properly terminate this Agreement on expiration of the Default Cure Period shall be resolved in accordance with Article 20 (“DISPUTE RESOLUTION”). If as a result of such dispute resolution proceeding, it is determined that the alleged Defaulting Party committed a Default and does not cure such Default within [**] after the date of such judgment (the “Additional Cure Period”), then such termination shall be effective as of the expiration of the Additional Cure Period. If the Parties dispute whether such Default was so cured, either Party alone may request the same dispute resolution panel to determine whether it was so cured, and the Parties shall cooperate to allow such determination to be made within [**] after such request by either Party.
Such dispute resolution proceeding does not suspend any obligations of either Party hereunder, and each Party shall use reasonable efforts to mitigate any damage. If as a result of such dispute resolution proceeding it is determined that the alleged Defaulting Party did not commit such Default (or such Default was cured in accordance with this Section 19.2.1, the Additional Cure Period), then no termination shall be effective, and this Agreement shall continue in full force and effect. Notwithstanding the foregoing, [**].
19.2.2
To the extent permitted by Applicable Laws upon the filing or institution of bankruptcy, reorganization, liquidation or receivership proceedings, or upon an assignment of a substantial portion of the assets for the benefit of creditors by the other Party provided, however, that in the case of any involuntary bankruptcy proceeding such right to terminate shall only become effective if the Party consents to the involuntary bankruptcy or such proceeding is not dismissed within [**] after the filing thereof.
19.2.3
Except to the extent unenforceable under Applicable Law, CUE will have the right to terminate this Agreement upon Notice to BI if BI or any of its Affiliates or Sublicensees takes any action, directly or indirectly, to challenge the validity or enforceability of any Patent included in the CUE Technology (the foregoing activities, an “IP Challenge”), provided BI or its Affiliate or Sublicensee (as applicable) does not withdraw such IP Challenge within [**] after receipt of such Notice.
19.3
Termination by BI at Will. BI shall have the right to terminate this Agreement as to any Target (including all Compounds and Products directed to such Target) under this Agreement and/or terminate this Agreement as a whole at any time at will by giving Notice to CUE. Such termination shall take effect [**] after the date on which the Notice is given.
19.4
Effects of Termination.
19.4.1
Effects of Termination by CUE for Cause or by BI at Will. Upon termination of the Agreement by CUE pursuant to Section 19.2 (“Termination for Cause”), or upon termination of the Agreement or any Target and relevant Compounds and Products by BI according to Section 19.3 (“Termination by BI at Will”):
a)
Except for the perpetual licenses granted by BI to CUE under Section 5.3 and Section 5.4, all licenses granted by a Party to the other Party under this Agreement and in the case of Target termination, all licenses granted by a Party to the other Party with regard to the terminated Target and relevant Compounds and Products shall immediately terminate and any Patent originally Controlled or co-owned by CUE and thereafter assigned to BI pursuant to Section 9.1.4 and still existing at the time of termination shall be, and hereby is, effective as of the date of termination, assigned back to CUE.
b)
If such termination occurs prior to the expiration of the Research Term, (i) in case of termination of this Agreement as a whole, CUE and BI shall as soon as possible wind down and terminate further activities under the Research Program; (ii) in case of termination of a Target only, CUE and BI shall as soon as possible wind down and terminate further activities under any Research Plan relating to such Target and relevant Compounds and Products.
c)
Except as reasonably necessary to exercise any surviving right or obligation hereunder, (i) CUE shall promptly return to BI or destroy all Confidential Information of BI and, in accordance with Section 2.7.8 all BI Provided Materials and (ii) BI shall promptly return to CUE or destroy all Confidential Information of CUE and, in accordance with Section 2.7.8 all CUE Provided Materials; in case of Target termination such obligations shall apply to Confidential Information and Materials that relate solely to such terminated Target and relevant Compounds and Products.
d)
In case of termination of a Target only, the diligence obligations of BI pursuant to Section 3.2 and the exclusivity obligations of CUE pursuant to Section 4.2, in each case with respect to such Target and relevant Compounds and Products shall immediately terminate.
e)
To the extent requested by CUE within [**] following termination, BI shall, at its sole discretion, either elect to (i) enter into a license agreement whereby BI grants to CUE a non-exclusive, worldwide, royalty-bearing, sublicensable license, which license shall if requested by CUE be exclusive with respect to the Compound(s) and Product(s), under BI’s rights to the Collaboration Foreground IP that is reasonably necessary to further develop any Compounds and/or Product(s) as such exists as of date of termination, or (ii) to the extent possible, assign such rights to Collaboration Foreground IP to CUE; always provided that any rights related to any proprietary compound or product Controlled by BI or a Third Party included in any Combination Product(s) and/or any rights related to (a) any BI manufacturing technology or (b) proprietary Device Controlled by BI or a Third Party used in conjunction with a Product shall be excluded, and in each case (i) and (ii) under terms and conditions to be negotiated in good faith, including without limitation commercially reasonable consideration for such license or assignment; in case of Target termination the Collaboration Foreground IP to be licensed or assigned in accordance with the foregoing shall be limited to the Collaboration Foreground IP generated during the Research Term with respect to such terminated Target and relevant Compounds and Products.
f)
In case BI licenses or assigns the rights to Collaboration Foreground IP as set forth in Section 19.4.1 e), BI shall, to the extent requested by CUE, [**], provided that [**] and subject to [**]. Notwithstanding the foregoing, BI shall not be under an obligation to provide any such data if this is not permitted by Applicable Law, including without limitation, applicable data protection laws and regulations.
19.4.2
Effects of Termination by BI for Cause. Upon the termination of this Agreement by BI pursuant to Section 19.2 (“Termination for Cause”) [**] will [**] BI Termination [**].
19.4.3
[**] BI may in its discretion [**] and render Notice to CUE accordingly, [**]. For the avoidance of doubt, if BI [**], unless this Agreement [**] terminated by BI or CUE pursuant to Section 19.2 (“Termination for Cause”) or by BI pursuant to 19.2.3 (“Termination by BI at Will”), subject to the conditions set forth in this Section 19.4.3:
a)
BI shall [**], subject to [**]; however:
[**].
b)
Any [**] and, if applicable, all [**] will be [**] this Agreement.
c)
For the avoidance of doubt, in case BI [**] set forth in this Section 19.4.3 with regard to [**] this Agreement [**] BI may [**].
19.5
Rights Accruing Prior to Expiration or Termination. Expiration or termination of this Agreement shall not relieve the Parties of any obligation (including any payment obligation) accruing prior to such expiration or termination. Any expiration or termination of this Agreement shall be without prejudice to the rights of either Party against the other accrued or accruing under this Agreement prior to expiration or termination, including the obligation to pay for any amounts that accrued prior to the effective date of such expiration or termination.
19.6
Survival. The following provisions shall expressly survive any expiration or termination of this Agreement: Articles 1 (“DEFINITIONS”), 10 (“CONFIDENTIALITY”), 11 (“PUBLICITY AND PUBLICATIONS”), 15 (“CUSTOMS”), 18 (“INDEMNIFICATION AND LIMITATION OF LIABILITY”), and 20 (“DISPUTE RESOLUTION”) and Sections 2.6 (“CUE Deliverables”), 2.7.8, 2.7.9, 5.3 (“Collaboration Foreground IP License”), 5.4 (“Assigned Patent License”), 5.5 (“No Implied Licenses”), 5.7 (“Survival of Sublicenses”), 9.1.1 (“Background IP”), 9.1.2 (“Foreground IP”), 9.1.3 (“Reporting of CUE Inventions”), 12.2 (“Research Support Payments”) - 12.15 (“Interest on Late Payments) (in each case solely with respect to amounts that become payable prior to the effective date of such expiration or termination), 12.16 (“Record Retention; Audits”) (solely for the time period set forth therein), 12.17 (“Upstream Licenses Payment Obligations”), 13.3.4 (solely in view of Collaboration Foreground IP), 13.3.6, 13.5 (“Disclaimer”), 19.1 (“Term”) (solely with respect to the last sentence), 19.4.1 (“Effects of Termination by CUE for Cause or by BI at Will”), 19.4.2 (“Effects of Termination by BI for Cause”), 19.5 (“Rights Accruing Prior to Termination or Expiration”), 21.1 (“Affiliates”), 21.3 (“Assignment”) - 21.6 (“Waiver”), 21.8 (“Notices”), 21.10 (“Governing Law”) - 21.13 (“Independent Contractors”), 21.15 (“Third Party Beneficiaries”) - 21.18 (“Counterparts; Electronic Delivery”), and this Section 19.6 (“Survival”).
20.1
Dispute Resolution. Any claim or dispute arising out of or in connection with or relating to this Agreement that cannot be resolved otherwise, including but not limited to any question regarding its existence, validity or termination (each a “Dispute”) shall be referred for resolution to the respective Senior Executives of each Party or their duly authorized respective designees with equivalent decision-making authority with respect to matters under this Agreement.
The Senior Executives shall attempt in good faith to resolve such Dispute by unanimous consent.
20.2
If the Senior Executives cannot resolve such Dispute within [**] of the matter being referred to them and, in case and to the extent a Dispute concerns a matter for which [**] has final decision-making authority pursuant to Section 7.3, if [**] does not exercise such final casting vote, either Party may file for such Dispute to be finally settled by binding arbitration under the arbitration rules of the International Chamber of Commerce (the “ICC”) at the time the arbitration case is filed (the “Rules”), except as otherwise provided in this Article 20. Either Party may initiate arbitration of an unresolved Dispute by submitting a request for Arbitration (“Request”) to the arbitration institute, which Request shall specify in reasonable detail the nature of the unresolved Dispute. Such arbitration shall be subject to the following:
20.3
The arbitration shall be conducted by a tribunal of three persons, one nominated by the initiating Party in the Request, the second nominated by the other Party within [**] after receipt of the Request, and the third, who shall act as chairperson, nominated jointly by the arbitrators within [**] of the nomination of the second arbitrator. If any arbitrator is not nominated within these time periods, the ICC shall appoint such arbitrator. No arbitrator shall be an employee, director or shareholder of either Party or any of their Affiliates but each shall have experience in the pharmaceutical industry. The chairperson shall [**].
20.4
The arbitrators shall have the power to decide all questions of arbitrability pertaining to the Dispute to the extent permitted by Applicable Law. The scope of authority of the arbitrators should be limited to the strict determination of fact and application of law.
20.5
The seat and venue of the arbitration proceedings shall be New York, USA. The arbitration proceedings and all pleadings and written evidence shall be in the English language. Any written evidence originally in another language shall be submitted in English translation accompanied by the original or a true copy thereof. Each Party agrees to use reasonable efforts to make all of its current employees available to testify or provide written statements, if reasonably necessary.
20.6
Each Party shall bear its own costs, expenses and attorneys’ fees incurred in connection with any arbitration. The costs of any arbitration, including administrative fees and fees of the arbitrator(s), shall be shared equally by the Parties to the dispute. However, the arbitral tribunal shall include in its award an allocation to any Party of costs, expenses and reasonable attorneys’ fees, including but not limited to any costs, fees and expenses imposed by the arbitrators or the ICC, as the arbitral tribunal shall deem reasonable. In making such allocation, the arbitral tribunal shall consider the relative success of the Parties on their claims, counterclaims and defences. The Parties agree that, any provision of Applicable Law notwithstanding, they will not request, and the arbitral tribunal shall have no authority to award, punitive or exemplary damages against any Party.
20.7
The decision and/or award rendered by the tribunal shall be written, final and non-appealable. Confirmation of, or judgment upon, any award rendered by the tribunal may be entered in any competent court or application may be made to any competent court for judicial acceptance of such an award and order for enforcement.
20.8
Any payment to be made by a Party pursuant to a decision of the tribunal shall be made in US Dollars, without any deductions made for tax obligations or any other deductions.
20.9
Neither Party shall be required to [**].
20.10
Interim Relief. Nothing in this Agreement shall limit the right of either Party to apply to the arbitration tribunal or any court of competent jurisdiction for any non-monetary interim relief or provisional remedy, including a temporary restraining order, preliminary injunction or other interim or conservatory relief that may be available under Applicable Law (including prior to or during the escalation of any Dispute pursuant to the escalation procedure set forth in Section 20.1). The arbitral tribunal shall have the authority to grant any provisional or interim remedy that would be available under the Rules or from a court of competent jurisdiction.
20.11
Confidentiality. Except to the extent necessary to confirm or obtain judgment on an award or decision or as may be required by Applicable Law, and except as may be otherwise required under Applicable Law, including without limitation stock market rules and regulations applicable to the Parties as listed companies, neither Party may, and the Parties shall instruct the arbitrators not to, disclose the existence, content, or results of a dispute without the prior written consent of the other Party.
20.12
Notwithstanding any provision of this Agreement to the contrary, any Dispute involving Patent infringement, validity or enforceability must not be pursued by way of arbitration as set forth in this Article 20, and may, at the choice of the Party seeking dispute resolution, instead be pursued by either Party in any court of competent jurisdiction.
21.1
Affiliates. BI may perform its obligations and exercise its rights hereunder personally or through one or more Affiliates, although BI shall nonetheless be solely responsible for the performance of its Affiliates. BI shall remain liable for any acts or omissions of its Affiliates, as if such acts and omissions were its own.
21.2
Subcontracting to Third Parties. CUE shall not be permitted to subcontract any of its obligations under the Research Program to any Third Party if not expressly agreed in writing by BI. Upon subcontracting of a Third Party agreed by BI, CUE shall in relation to BI be responsible for the acts and omissions of any subcontractors used to fulfill CUE’s obligations hereunder, as if such acts and omissions were its own. Notwithstanding any provision of this Agreement to the contrary, CUE’s obligation to perform any specific activity under the Research Program shall be [**] activities under the Research Program, provided [**].
21.3
Assignment. Subject to Sections 5.1, 5.3, 5.4, and 5.6, this Agreement may not be assigned or otherwise transferred, nor may any right or obligation under this Agreement, including but not limited to any license of Intellectual Property, be assigned or transferred, by either Party without the prior written consent of the other Party and any attempted assignment or transfer in violation of this Section 21.3 shall be null and void ab initio; provided, that each Party may, without such consent, assign this Agreement and its rights and obligations hereunder, including all of its licensed Intellectual Property rights hereunder, to an Affiliate or to a Third Party in connection with the sale of all or substantially all of its business or assets to which this Agreement relates, whether by merger, consolidation or other change in control transaction.
Subject to the foregoing, this Agreement will be [**].
21.4
Force Majeure. Neither Party shall be liable or deemed in default for failure to perform any duty or obligation (other than payment obligations hereunder, except if an event renders the processing of a payment for technical or other reasons infeasible) that such Party may have under this Agreement where such failure has been occasioned by any act of God, fire, pandemic, external strike, inevitable accidents, war, governmental acts, or any other cause outside the reasonable control of that Party, and occurring without its fault or negligence. The Party whose performance has so been interrupted shall give the other Party written information of the interruption and cause thereof, and shall use every reasonable means to resume full performance of this Agreement as soon as possible.
21.5
Severability. In the event that any clause or portion thereof in this Agreement is for any reason held to be invalid, illegal or unenforceable, the same shall not affect any other portion of this Agreement, as it is the intent of the Parties that this Agreement shall be construed in such fashion as to maintain its existence, validity and enforceability to the greatest extent possible. In any such event, this Agreement shall be construed as if such clause of portion thereof had never been contained in this Agreement, and there shall be deemed substituted therefore such provision as will most nearly carry out the intent of the Parties as expressed in this Agreement to the fullest extent permitted by Applicable Law unless doing so would have the effect of materially altering the right and obligations of the Parties.
21.6
Waiver. The failure of either Party to require performance by the other Party of any of that other Party’s obligations hereunder shall in no manner affect the right of such Party to enforce the same at a later time. No waiver by any Party of any condition, or of the breach of any provision, term, representation or warranty contained in this Agreement shall be deemed to be or construed as a further or continuing waiver of any such condition or breach, or of any other condition or of the breach of any other provision, term, representation, or warranty hereof. The remedies provided in this Agreement are not exclusive and the Party suffering from a breach or default of this Agreement may pursue all other remedies, both legal and equitable, alternatively or cumulatively.
21.7
Anti-Bribery/Anti-Corruption. Each Party represents and warrants that it, its owners, directors, officers, employees, sub-contractors and agents have acted and will act in full compliance with any applicable anti-bribery and anti-corruption laws and regulations, industry and professional codes of practice (including without limitation FCPA, UK Bribery Act, German Criminal Code, and any other international or local legislation, which may become applicable in connection to the Agreement) in connection with this Agreement and will not offer, promise, pay or arrange for payment or giving of a bribe or any benefit, advantage or anything of value to any Public Official, individual, entity or any other Third Party in exchange for an improper advantage in any form either directly or indirectly. Any violation of this Section 21.7 constitutes a material breach of this Agreement and will allow the other Party to terminate this Agreement with immediate effect.
21.8
Notices. Any Notices given under this Agreement shall be in writing (including original signature or signature by DocuSign, or by any other comparable electronic means).
Any Notice shall be deemed given (i) upon the date of personal delivery or by facsimile transmission or electronic mail (receipt verified), or (ii) one (1) Business Day after dispatch by overnight courier and if confirmed by delivery of the hardcopy original by overnight courier; or (iii) five (5) Business Days after dispatch of registered or certified mail (return receipt requested) and if confirmed by delivery of the hardcopy original by registered mail, in each case postage prepaid, provided that such date is a Business Day (otherwise on the next Business Day) and, in each of subsection (i), (ii) or (iii), above, to the Parties at the following addresses (or at such other address for a Party as shall be specified by like notice, provided, however, that notices of a change of address shall be effective only upon receipt thereof):
If to CUE:
Cue Biopharma, Inc.
Attn: Chief Executive Officer
40 Guest Street
Boston, MA 02135, USA
With a copy to:
Cue Biopharma, Inc.
40 Guest Street
Boston, Massachusetts 02135, United States of America
Attention: General Counsel
Email copy to [**]
If to BI:
Boehringer Ingelheim International GmbH
Attn: Transactions and Contract Management
Binger Strasse 173
55216 Ingelheim am Rhein
Germany
Fax: [**]
E-Mail: [**]
With a copy to:
Head of Corporate Legal and Compliance
(Address as above)
Fax: [**]
21.9
Insurance. During the Term of this Agreement, each of the Parties shall maintain insurance consistent with normal business practice and adequate to cover the risks under this Agreement in an amount and for a time period that are usual and customary for a pharmaceutical company of its size (or reasonable self-insurance sufficient to provide materially the same level and type of protection). Each Party shall provide to the other, upon request of the other Party from time to time during the Term of this Agreement, a certificate of insurance verifying the existence of such insurance.
However, it is understood that the maintenance of such insurance coverage will not relieve either Party of its other obligations under this Agreement.
21.10
Governing Law. This Agreement shall be construed in accordance with and governed exclusively by the laws of New York without regard to the United Nations Convention on Contracts for the International Sales of Goods (CISG) or the rules of conflict of law.
21.11
Entire Agreement; Amendments. This Agreement, together with its Schedules, represents the entire and integrated agreement between the Parties with respect to the subject matter herein and supersedes all prior and contemporaneous negotiations, representations or agreements, either written or oral, regarding the subject matter of this Agreement, including without limitation the Confidentiality Agreement. All confidential information disclosed by a Party or its Affiliates under a Confidentiality Agreement shall be deemed Confidential Information of such Party hereunder and subject to the terms of this Agreement. This Agreement may be amended, or any term hereof modified, only by a written instrument duly executed by authorized representatives of both Parties in accordance with Section 21.18. In the event of a conflict, inconsistency or discrepancy between the body of this Agreement and the Research Plan, the terms in the body of this Agreement shall prevail.
21.12
Headings; Interpretation. The captions to the Articles and Sections of this Agreement are not a part of this Agreement but are merely for convenience to assist in locating and reading the several Sections of this Agreement. Unless specified to the contrary, references to Articles, Sections and Schedules mean the Articles, Sections or Schedules to this Agreement and references to this Agreement include all Schedules hereto. Unless context otherwise clearly requires, whenever used in this Agreement: (i) the words “include” or “including” shall be construed as incorporating, also, “but not limited to” or “without limitation;” (ii) the word “day” or “year” means a calendar day or year unless otherwise specified; (iii) to “inform in writing” or to “provide written information” includes information by e-mail to the relevant contact person of the other Party involved in the subject matter; (iv) the words “hereof,” “herein,” “hereby” and derivative or similar words refer to this Agreement (including all Schedules); (v) the word “or” shall be construed as the inclusive meaning identified with the phrase “and/or;” (vi) provisions that require that a Party, the Parties or a committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise; (vii) words of any gender include the other gender; (viii) words using the singular or plural number also include the plural or singular number, respectively; (ix) the words “shall” and “will” have interchangeable meanings for purposes of this Agreement; (x) references to any specific law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement law, rule or regulation thereof; (xi) each accounting term not otherwise defined in this Agreement has the meaning assigned to it in accordance with Accounting Standards and any particular cost or expense shall be accounted for only once; and (xii) neither Party or its Affiliates shall be deemed to be acting “on behalf of” or “under authority of” the other Party.
21.13
Independent Contractors. It is expressly agreed that CUE and BI shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency.
Neither CUE nor BI shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other Party, without the prior written consent of the other Party.
21.14
Non-Employment. Each Party or where applicable, its subcontractors, shall at all times be and remain the sole employer of persons assigned to the performance of work by such Party hereunder and shall assume any and all obligations, responsibilities and risks to such employment and the possible termination thereof.
21.15
Third Party Beneficiaries. None of the provisions of this Agreement shall be for the benefit of or enforceable by any Third Party, including, without limitation, any creditor of either Party. No such Third Party shall obtain any right under any provision of this Agreement or shall by reasons of any such provision make any claim in respect of any debt, liability or obligation (or otherwise) against either Party.
21.16
Further Assurances. Each of CUE and BI agrees to duly execute and deliver, or cause to be duly executed and delivered, such further instruments and use reasonable efforts to do and cause to be done such further acts and things, including, without limitation, the filing of such additional assignments, agreements, documents and instruments, as the other Party may at any time and from time to time reasonably request in connection with this Agreement or to carry out more effectively the provisions and purposes of, or to better assure and confirm unto such other Party its rights and remedies under, this Agreement.
21.17
Cumulative Remedies. Except as otherwise expressly set forth in this Agreement, no remedy referred to in this Agreement is intended to be exclusive, but each shall be cumulative and in addition to any other remedy referred to in this Agreement or otherwise available under Applicable Law or in equity.
21.18
Counterparts; Electronic Delivery. This Agreement, and any amendments thereto, may be executed in counterparts with the same effect as if both Parties had signed the same document. All such counterparts shall be deemed an original, shall be construed together and shall constitute one and the same instrument. Signatures to this Agreement or its amendments, transmitted by email in “portable document format” (“.pdf”), via DocuSign, or by any other electronic means intended to preserve the original graphic and pictorial appearance of this Agreement, or its amendment, shall have the same effect as physical delivery of the paper document bearing original signature.
[the remainder of this page is intentionally blank]
IN WITNESS WHEREOF, the Parties have executed this Agreement in duplicate originals by their duly authorized representatives as of the date and year first above written.
BOEHRINGER INGELHEIM INTERNATIONAL GMBH
ppa. i.V.
By: /s/ Dr. Andreas Lenk_______ By: /s/ Dr. Scott DeWire_____
Name: Dr. Andreas Lenk Name: Dr. Scott DeWire
Title: Authorized Signatory Title: Authorized Signatory Schedule 1.32: Non-Exhaustive List of CUE Technology
Date: 10-Apr-2025 Date: 10-Apr-2025
CUE BIOPHARMA, INC.
By: /s/ Dan Passeri
Name: Dan Passeri
Title: President and Chief Executive Officer
Date: 10-Apr-2025
Schedules:
Schedule 1.38: Einstein College Upstream License
Schedule 1.41: External Costs
Schedule 1.57: Requirements for Invoices
Schedule 1.80: Initial Research Plan within the Research Program
Schedule 2.6: Requirements for Reports
Schedule 2.7: Material Transfer Record Form
Schedule 5.8: Einstein College Upstream License Provisions
Schedule 6.1: Technology Data Package Transfer
Schedule 6.4: Material Transfer and Supply
Schedule 11: Press Release
Schedule 12.1: Invoice for Upfront Payment
Schedule 16.1.1: Animal Welfare Questionnaire
Schedule 17: Use of Human Biospecimens Amended and Restated License Agreement between CUE and Albert Einstein College of Medicine, Inc., dated July 31, 2017:
Schedule 1.38
Einstein College Upstream License
(as amended)
Incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024
b)
First Amendment to the Amended and Restated License Agreement dated October 30, 2018:
Incorporated by reference to Exhibit 10.25 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024
c)
Second Amendment to the Amended and Restated License Agreement dated January 13, 2024:
Incorporated by reference to Exhibit 10.42 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024
d)
Boehringer Ingelheim Amendment to the Amended and Restated License Agreement dated March 10, 2025:
Incorporated by reference to Exhibit 10.3 to the Company’s
Quarterly Report on Form 10-Q for the fiscal period ended March 31, 2025
Schedule 1.41
External Costs
|
|
Year |
Anticipated External Costs |
Calendar Year 1 |
[**] |
Calendar Year 2 |
[**] |
Calendar Year 3 |
[**] |
Calendar Year 4 |
[**] |
Calendar Year 5 |
[**] |
Schedule 5.8
Einstein College Upstream License Provisions
Notwithstanding anything to the contrary in this Agreement:
1.
Consistent with Section 9.01 of the Einstein College Upstream License, BI shall not use the name of the Albert Einstein College of Medicine, Inc. (for purposes of this exhibit only, “Einstein”) without Einstein’s prior written consent, except if the use of such name is required by law, regulation, federal securities law, or judicial order, in which event BI will promptly inform Einstein, prior to any such required use. BI will not make any public announcement regarding the existence of the Einstein College Upstream License or the collaboration thereunder without obtaining the prior written consent of Einstein, except if such announcement is required by law, regulation, federal securities law or judicial order, in which event BI will promptly inform Einstein prior to any such required announcement.
2.
Consistent with Section 12.04 of the Einstein College Upstream License, BI agrees to indemnify Einstein and its current or former directors, governing board members, trustees, officers, faculty, medical and professional staff, employees, students and agents and their respective successors, heirs and assigns (Einstein and each such person being, solely for purposes of this Exhibit, the “Einstein Indemnified Parties”) for the cost of defense and for damages awarded and losses and liabilities incurred, if any, as a result of any Third Party claims, liabilities, suits or judgments based on or arising out of the research, development, marketing, manufacture, sale or provision of Licensed Products or Know-How Products (each as defined in the Einstein College Upstream License) by BI, its Affiliates and Sublicensees granted a sublicense under the Einstein College Upstream License, or otherwise related to the conduct of BI’s or its affiliates’ or sublicensees’ (direct and indirect) business, so long as such claims, liabilities, suits, or judgments are not solely attributable to grossly negligent or intentionally wrongful acts or omissions by the Einstein Indemnified Parties. This indemnity is conditioned upon Einstein’s obligation to: (i) advise BI of any claim or lawsuit, in writing promptly after Einstein or the Einstein Indemnified Party has received notice of said claim or lawsuit, (ii) assist BI and its representatives, at BI’s expense, in the investigation and defense of any lawsuit or claim for which indemnification is provided, and (iii) permit BI to control the defense of such claim or lawsuit for which indemnification is provided.
3.
Consistent with Section 12.05 of the Einstein College Upstream License, BI acknowledges that Einstein has not made:
(a) A warranty or representation that anything made or used by BI under any license or sublicense granted under the Einstein College Upstream License is or will be free from infringement of Patents, copyrights, and other rights of Third Parties; or
(b) A granting by implication, estoppel, or otherwise of any license, right or interest other than as expressly set forth in the Einstein College Upstream License.
4.
Consistent with Section 12.06 of the Einstein College Upstream License, BI acknowledges that, except as expressly set forth in the Einstein College Upstream License, EINSTEIN MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, STATUTE OR OTHERWISE, AND EINSTEIN SPECIFICALLY DISCLAIMS ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, OR WARRANTY OF NON-INFRINGEMENT.
IN ADDITION, EINSTEIN SHALL NOT BE LIABLE TO BI FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES, HOWEVER CAUSED, UNDER ANY THEORY OF LIABILITY AND WHETHER OR NOT EINSTEIN HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
5.
Consistent with Section 12.10 of the Einstein College Upstream License, BI and its Affiliates will observe and abide by the provisions of Section 12.10, as amended by the Boehringer Ingelheim Amendment to the Amended and Restated License Agreement, effective March 10, 2025 BI’s sublicensees (direct and indirect) shall also observe the provisions of Section 12.10, as amended.
EX-10.3
4
cue-ex10_3.htm
EINSTEIN AMENDMENT
EX-10.3
Exhibit 10.3
Certain identified information has been excluded from the exhibit because it is both (i) not material and (ii) is the type of information that the registrant treats as private or confidential. Double asterisks denote omissions.
BOEHRINGER INGELHEIM AMENDMENT TO THE AMENDED AND RESTATED
LICENSE AGREEMENT
This Boehringer Ingelheim Amendment to the Amended and Restated License Agreement (“BI Amendment”) is by and between Albert Einstein College of Medicine, a corporation organized and existing under the laws of the State of New York, having an office and place of business at 1300 Morris Park Avenue, Bronx, New York 10461 (“Licensor”) and Cue Biopharma, Inc., a corporation organized and existing under the laws of the State of Delaware, having an office and place of business at 40 Guest Street, Boston, MA 02135, USA (“Licensee”). The effective date of this BI Amendment is April 10, 2025 (“BI Amendment Effective Date”). Capitalized terms used herein but not otherwise defined shall have the meanings ascribed to them in the License (as defined below).
WHEREAS, Licensor and Licensee are parties to that certain Amended and Restated License Agreement entered into July 31, 2017, as subsequently amended by that certain First Amendment to the Amended and Restated License Agreement dated October 30, 2018 and that certain Second Amendment to the Amended and Restated License Agreement dated January 13, 2024 (collectively, as amended, the “License”);
WHEREAS, Licensee and Boehringer Ingelheim International GmbH, a corporation organized and existing under the laws of Germany (“BI”), desire to enter into a Collaboration and License Agreement in the form attached hereto as Exhibit A (the “BI Agreement”), pursuant to which, among other things, BI would receive a sublicense to certain rights licensed to Licensee under the License; and
WHEREAS, Licensor and Licensee wish to make the following changes to certain provisions of the License, solely with respect to the application of such provisions to the BI Agreement.
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, Licensor and Licensee hereby agree to amend the License as follows:
Present Amendments
1.
Licensor hereby consents to: (a) Licensee and BI entering into the BI Agreement, (b) the grant by Licensee to BI of a sublicense under the Agreement Patents and Know-How on the terms set forth in the BI Agreement, and (c) BI exercising Licensee’s rights under Section 4 of the License as granted to BI pursuant to Section 5.1 of the BI Agreement.
2.
Licensor and Licensee hereby acknowledge and agree that a Product (as defined in the BI Agreement) will only constitute a Licensed Product, Know-How Product or MHC Class II Product under the License to the extent such Product meets the definition of such terms in the License.
3.
As used in this BI Amendment, the term “Affiliate” means, with respect to BI, any corporation, firm, limited liability company, partnership, or other entity, that directly or indirectly controls, or is controlled by, or is under common control with BI. As used herein, “control” and, with correlative meanings, the terms “controlled by” and “under common control with”, means ownership, directly or indirectly, of fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign entity in a particular jurisdiction provided that such foreign entity has the power to direct or cause the direction of the management or policies of a corporation) or more of the shares of stock entitled to vote for the election of directors in the case of a corporation, or fifty percent (50%) (or such lesser percentage which is the maximum allowed to be owned by a foreign entity in a particular jurisdiction provided that such foreign entity has the power to direct or cause the direction of the management or policies of any other type of entity) or more of the equity interests in the case of any other type of legal entity, or status as a general partner in any partnership, or any other arrangement whereby BI controls or has the right to control the board of directors or equivalent governing body of a corporation or other entity and has the power to direct or cause the direction of such corporation or other entity.
4.
Licensor and Licensee further agree that the following Sections of the License (referenced in paragraphs 4-28 below) are amended solely with respect to their application of such provisions to the BI Agreement and that the License, other than as it relates to the BI Agreement, remains in full force and effect according to its terms and is not altered or amended by this BI Amendment as it relates to Licensee and any other Sublicensee.
5.
A new Section 1.21 is hereby added to the License, as follows:
“BI Agreement” shall mean that certain Collaboration and License Agreement between Licensee and Boehringer Ingelheim International GmbH, a corporation organized and existing under the laws of Germany (“BI”) effective as of April 10, 2025.
6.
A new Section 1.22 is hereby added to the License, as follows:
“BI Amendment” shall mean that certain Boehringer Ingelheim Amendment to this Agreement between Licensee and BI effective as of April 10, 2025.
7.
A new Section 1.23 is hereby added to the License, as follows:
“Commercially Reasonable Efforts” shall mean, with respect to BI’s activities relating to the development or commercialization of a Licensed Product, Know-How Product or MHC Class II Product, [**], as the case may be, [**], in each case [**].
8.
A new Section 1.24 is hereby added to the License, as follows:
“Major Market Country” means each of [**].
9.
Section 3.03 of the License is hereby deleted in its entirety and replaced by the following, with additions to the previous text shown in bold underlined text, and deletions shown in bold strikethrough:
As of and after the Original Effective Date, Licensee will pay the cost of preparing, filing, prosecuting, maintaining and resisting challenges to the validity of the Agreement Patents (as well as the cost of preparing, filing, prosecuting, maintaining and resisting challenges to the validity of corresponding applications in at least the United States, Europe (an EPO filing designating all member countries), Canada, Japan and Australia). As part of this obligation, Licensee will pay the cost of applying for an extension of the term of any patent included within Agreement Patents, if appropriate, under the Drug Price Competition and Patent Term Restoration Act of 1984 and/or European, Japanese and other foreign counterparts. Licensee will pay the cost of defending and/or prosecuting any interference, reexamination, reissue, opposition, cancellation and nullity proceedings involving Agreement Patents. In the event that Licensee and BI jointly elect not to pay to maintain, defend or prosecute any patent or patent application within the Agreement Patents, Licensee and BI shall jointly give Licensor thirty (30) days prior written notice of such election. Any patents or patent applications so elected shall at the end of the notice period cease to be considered Agreement Patents, and Licensor shall then be free, at its election, to abandon or maintain the prosecution of such patent application or issued patent or grant rights to such patent application or issued patent to third parties. eee s e s s
10.
Section 4.01 of the License is hereby deleted in its entirety and replaced by the following, with additions to the previous text shown in bold underlined text:
Subject to Section 2, Licensor hereby grants to Licensee and Affiliates a worldwide, exclusive license, with the right by Licensee only to grant sublicenses to unaffiliated third parties, under Licensor’s rights in the Agreement Patents and Know-How to research, develop, import and export, manufacture, make, have made, use, provide, register, market, distribute, offer to sell, and sell products, processes and services in the Field, namely, Licensed Products, Know How Products and MHC Class II Products. The terms of any sublicense agreement shall not contradict the terms of this Agreement and shall include (at least) the following provisions: prohibiting any use of Licensor’s name (consistent with Section 9.01), requiring indemnification of Licensor (consistent with Section 12.04), and requiring appropriate insurance (consistent with Section 12.10), and disclaiming any warranties or representations by Licensor (consistent with Sections 12.05 and 12.06).
Licensee shall provide Licensor with a full, unredacted and complete copy of any executed sublicense or amendment within thirty (30) days of execution thereof by Licensee, subject to BI’s right to redact any confidential or proprietary information contained therein that is not necessary for Licensor to determine compliance with this Agreement. Licensee may designate any such sublicense or amendment, in whole or in part, as Confidential Information. Notwithstanding the foregoing and other provisions in this Agreement, BI may grant further sublicenses under the rights sublicensed by Licensee to BI pursuant to Section 4.01 through multiple tiers to Affiliates and third parties, provided that any sublicenses granted by BI, through multiple tiers, comply with [**].
11.
Section 5.01 of the License is hereby amended as follows, with additions shown in bold underlined text:
Nothing herein contained shall preclude Licensor from making required reports or disclosures to the NIH or to any other philanthropic or governmental funding organization, provided, however, that no Confidential Information of Licensee or BI, nor use of Licensee’s or BI’s name is disclosed in the process, without prior approval from Licensee or BI, as applicable, unless such disclosure is required by applicable law.
12.
A new sentence is hereby added to the end of Section 5.03 of the License as follows:
Licensor’s obligations under this Section 5.03 shall extend to Confidential Information of BI, its Affiliates and its sublicensees, if any, as if it were Confidential Information of Licensee.
13.
Section 6.01(b) of the License is hereby deleted in its entirety and replaced with the following:
Licensee will pay to Licensor (i) [**] of the upfront payment that Licensee receives from BI pursuant to Section 12.1 of the BI Agreement and (ii) [**] of Net Proceeds that it receives thereafter under the BI Agreement.
14.
Section 6.01(c) of the License is hereby deleted in its entirety and replaced with the following:
Licensee will pay to Licensor [**] of the royalty amounts received by Licensee from BI pursuant to Section 12.10 of the BI Agreement for as long as BI pays royalties to Licensee pursuant to Section 12.10 of the BI Agreement.
15.
Section 6.04 is hereby deleted in its entirety from the License and replaced with “[Intentionally Omitted]”.
16.
In Section 7.03 of the License, “thirty (30)” is hereby replaced with “[**]”.
17.
Section 8.01 of the License is hereby amended as follows, with additions shown in bold underlined text:
Licensee or BI as permitted by Licensee pursuant to the BI Agreement shall have the right, in its sole discretion and its expense, to initiate legal proceedings on its behalf or in Licensor’s name, if necessary, against any infringer, or potential infringer, of an Agreement Patent who imports, makes, uses, sells or offers to sell products in the Field. Licensee (or BI as applicable) shall notify Licensor of its or BI’s intention to initiate such proceedings at least thirty (30) days prior to commencement thereof. Any settlement or recovery received from any such proceeding shall be divided [**] to Licensee (or BI as applicable) and [**] to Licensor after Licensee (or BI, as applicable) deducts from any such settlement or recovery its actual counsel fees and out-of-pocket expenses relating to any such legal proceeding. If Licensee or BI decides not to initiate legal proceedings against any such infringer, Licensee shall notify Licensor in writing of such decision, then Licensor shall have the right to initiate such legal proceedings. Any settlement or recovery received from any such proceeding initiated by Licensor shall be divided [**] to Licensor and [**] to Licensee or BI as applicable after Licensor deducts from any such settlement or recovery its actual counsel fees and out-of-pocket expenses relating to any such legal proceeding.
18.
Section 8.02 of the License is hereby amended as follows, with additions shown in bold underlined text:
In the event that either party (or BI) initiates or carries on legal proceedings to enforce any Agreement Patent against an alleged infringer, the other party/ the parties shall fully cooperate with and supply all assistance reasonably requested at the expense of the party (or BI) requesting such assistance. Further, the other party, at its expense, shall have the right to be represented by counsel of its choice in any such proceeding, However, if Licensee (or BI, as applicable) initiates legal proceedings in Licensor’s name, Licensee (or BI, as applicable) shall reimburse Licensor for any reasonable out of pocket counsel fees of Licensor associated with the legal proceedings. The party (or BI) who initiates or carries on the legal proceedings shall have the sole right to conduct such proceedings provided, however, that such party (or BI, as applicable) shall consult with the other party (i.e. with Licensor, in case BI carries on legal proceedings) to this Agreement prior to entering into any settlement thereof.
19.
Section 10.01 of the License is deleted in its entirety and replaced by the following:
Unless terminated earlier under other provisions hereof, this Agreement will expire with respect to BI and the BI Agreement upon expiration of BI’s royalty payment obligations under Section 12.10 of the BI Agreement. Upon expiration of this Agreement, Licensee’s, and if applicable, BI’s, licenses pursuant to Section 4.01 shall become fully paid-up, perpetual licenses. Upon termination or expiration of this Agreement for any reason as it relates to the BI Agreement, Section 1, 4.01 (to the extent set forth above), 5, 7, 8, 9, 10.01, 10.07 through 10.09, 11, 12.01 through 12.06, 12.14, 12.16 and 13 shall survive and all payment obligations under Sections 3 and 6.01-6.04 hereof accrued as of the termination date shall be paid by Licensee within thirty (30) days of such termination or expiration.
20.
In Section 10.06 of the License, “forty-five (45)” is hereby replaced with “[**]”.
21.
Section 10.07 of the License is hereby deleted in its entirety and replaced with the following:
Termination of this Agreement by Licensee or Licensor shall not prejudice the rights of either party accruing herein. Notwithstanding any provision herein to the contrary, no termination of this Agreement shall be construed as a termination of the BI Agreement (or any valid sublicense under the BI Agreement), and thereafter BI shall be considered a direct licensee of Licensor under this Agreement solely with respect to those rights under this Agreement sublicensed to BI pursuant to the BI Agreement and assume all of the rights and obligations of Licensee with respect thereto (such license agreement between BI and Licensor, the “BI Substitute License”) as from the date when the termination of this Agreement becomes effective between Licensor and Licensee (the “BI Substitute License Effective Date”), provided that (i) BI is not in material breach of the BI Agreement and (ii) BI agrees in writing to assume all applicable obligations of Licensee under this Agreement (as amended by the BI Amendment), unless agreed otherwise between Licensor and BI in writing.
22.
A new sentence is hereby added to the end of Section 10.08 of the License as follows:
Notwithstanding the foregoing, if BI is granted a sublicense by Licensee under Licensor’s Agreement Patents or Know-How in the Field, then the provisions of this Section 10.08 shall terminate and be of no further force or effect to the extent they relate to the BI Agreement.
23.
Section 10.09 of the License is hereby amended as follows, with additions shown in bold underlined text:
If Licensee terminates this Agreement pursuant to Section 10.02 or 10.03, or if Licensor terminates this Agreement pursuant to Sections 10.03, 10.04, 10.05 or 10.06 of this Agreement, Licensee shall submit a final Royalty Report to Licensor and any payments and patent costs due to Licensor hereunder as of the date of termination shall be payable within thirty (30) days of the date of termination. In addition, within ten (10) days of notice of such termination, and only if the BI Agreement is not in effect at the time of such termination, Licensee shall provide Licensor with a report showing the status of all Dependent Patents, including, without limitation, a list of all countries where Dependent Patents have been filed and a list of all actions which must be taken with respect to the Dependent Patents and relevant due dates.
24.
A new sentence is hereby added at the end of Section 12.10 of the License as follows:
Notwithstanding the foregoing in this Section 12.10, in the event the BI Agreement is in effect and BI is not in material breach thereof, BI shall be entitled to cover the risks set forth in this paragraph by maintaining insurance consistent with normal business practice and adequate to cover the risks under this Agreement in an amount and for a time period that are usual and customary for a pharmaceutical company of its size (or reasonable self-insurance sufficient to provide materially the same level and type of protection).
25.
Section 12.13 of the License is hereby deleted in its entirety and replaced with the following:
To the extent applicable under law or as otherwise may be required by the U.S. government, Licensee or BI, as applicable, shall comply with the applicable provisions of the Bayh-Dole Act, including the substantial manufacture requirement as required by 35 U.S. Code §204. The Parties understand that the Agreement Patents may claim a “subject invention” pursuant to Section 35 U.S.C. § 204. Upon Licensee’s or BI’s request, Licensor agrees to request, and reasonably cooperate with Licensee or BI, as applicable, to obtain a waiver of the substantial US manufacture requirement under Section 35 U.S.C. § 204, or to argue its inapplicability to any US governmental authority or other third party. Licensee will reimburse Licensor for costs and expenses incurred by Licensor in the performance of activities requested by Licensee or BI under this Section 12.13
26.
A new sentence is hereby added at the end of Section 12.15 of the License as follows:
Notwithstanding the foregoing, in the event the BI Agreement is in effect, in place of the foregoing due diligence requirements of this Section 12.15, BI shall use Commercially Reasonable Efforts to (i) [**] and (ii) [**]. Notwithstanding the foregoing, BI shall be permitted to [**] this provision.
27.
Licensor and Licensee acknowledge and agree that the following provisions of the License have, prior to the BI Amendment Effective Date, been fully performed and are of no further force or effect: (i) Section 6.02(a)-(d), (ii), 6.03(a), (iii) 6.03(c), (iv) Section 6.05, and (v) Section 6.06.
28.
Licensor and Licensee further agree that:
(a)
to the extent there is any inconsistency between this BI Amendment and the License, this BI Amendment shall control with respect to the rights and obligations of Licensee and Licensor solely as they relate to the sublicense granted to BI under the BI Agreement; and
(b)
the provisions of this BI Amendment shall not apply to any other sublicense granted by Licensee or to any other direct license that may arise with an entity other than BI pursuant to the provisions of Section 10.07 of the License. For all such other sublicenses and direct licenses, the original provisions of the License, as have and may in the future be amended in writing by Licensor and Licensee, remain in full force and effect and shall exclusively govern.
Contingent Amendments (applicable only if BI becomes a direct licensee of Licensor pursuant to Section 10.07 of the License)
29.
Licensor and Licensee further agree that if BI becomes a direct licensee of Licensor pursuant to Section 10.07 of the License as amended by the BI Amendment set forth in paragraphs 4-28 above (the “BI Present Amendment”), then (i) the BI Present Amendment is hereby further amended as set forth in paragraphs 29-35 below and (ii) to the extent there is any inconsistency between the BI Present Amendment and paragraphs 29-35 of this BI Amendment, the provisions of paragraphs 29-35 of this BI Amendment shall govern and control.
30.
Effective upon the BI Substitute License Effective Date, a new sentence is hereby added to the end of Section 1.08 of the BI Present Amendment:
In the event that a BI Substitute License pursuant to Section 10.07 of the BI Amendment becomes effective, effective upon the BI Substitute License Effective Date, BI shall substitute the definition and calculation of “Net Sales” under this Section 1.08 with the Net Sales definition (inclusive of interrelated terms (e.g., “Combination Products”) and concepts) set forth in the BI Agreement.
31.
Effective upon the BI Substitute License Effective Date, Section 6.01 of the BI Present Amendment is hereby deleted in its entirety and replaced by the following:
BI shall make the following payments to Licensor: (a) BI will pay to Licensor: (i) [**] of Net Sales on Licensed Products generated by or on behalf of BI in a country, provided, however, that this rate shall be reduced by [**] on a country-by-country basis, if a Competing Product (as defined in the BI Agreement) exists in such country. [**].
32.
Effective upon the BI Substitute License Effective Date, Sections 6.02 and 6.03 of the BI Present Amendment shall not be applicable and shall be deleted in their entirety.
33.
Effective upon the BI Substitute License Effective Date, a new sentence is hereby added to the end of Section 10.08 of the BI Present Amendment as follows:
Notwithstanding the foregoing, if BI becomes a direct licensee of Licensor’s Agreement Patents and Know-How in the Field pursuant to Section 10.07, then the provisions of this Section 10.08 shall terminate and be of no further force or effect.
34.
Effective upon the BI Substitute License Effective Date, a new sentence is hereby added to the end of Section 12.15 of the BI Present Amendment as follows:
Notwithstanding the foregoing, if BI becomes a direct licensee under this Agreement pursuant to Section 10.07, then, in place of the foregoing due diligence requirements of this Section 12.15, BI shall use Commercially Reasonable Efforts to (i) [**] and (ii) [**]. Notwithstanding the foregoing, BI shall be permitted to [**] this provision.
35.
Effective upon the BI Substitute License Effective Date, Section 12.16 of the BI Present Amendment shall not be applicable and shall be deleted in its entirety.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the Parties hereto have executed this BI Amendment as of the BI Amendment Effective Date.
ALBERT EINSTEIN COLLEGE OF MEDICINE
By: /s/ Janis Paradiso
Name: Janis Paradiso
Title: Director, Office of Biotechnology and Business Development I, Daniel R. Passeri, certify that:
CUE BIOPHARMA, INC.
By: /s/ Dan Passeri
Name: Dan Passeri
Title: President and Chief Executive Officer
EX-31.1
5
cue-ex31_1.htm
EX-31.1
EX-31.1
EXHIBIT 31.1
CERTIFICATION OF THE PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
1.
I have reviewed this Quarterly Report on Form 10-Q of Cue Biopharma, Inc.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
(a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
(b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: May 12, 2025
|
/s/ Daniel R. Passeri |
Name: Daniel R. Passeri |
Title: Chief Executive Officer |
(Principal Executive Officer) |
EX-31.2
6
cue-ex31_2.htm
EX-31.2
EX-31.2
EXHIBIT 31.2
CERTIFICATION OF THE PRINCIPAL FINANCIAL OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Kerri-Ann Millar, certify that:
1.
I have reviewed this Quarterly Report on Form 10-Q of Cue Biopharma, Inc.;
2.
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.
The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)
Evaluated the effectiveness of the registrant's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)
Disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.
The registrant's other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant's auditors and the audit committee of the registrant's board of directors (or persons performing the equivalent functions):
(a)
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant's ability to record, process, summarize and report financial information; and
(b)
Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal control over financial reporting.
Date: May 12, 2025
|
/s/ Kerri-Ann Millar |
Name: Kerri-Ann Millar |
|
Title: Chief Financial Officer
(Principal Financial Officer and Principal Accounting Officer)
|
EX-32.1
7
cue-ex32_1.htm
EX-32.1
EX-32.1
EXHIBIT 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350,
AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with this Quarterly Report on Form 10-Q of Cue Biopharma, Inc. (the “Company”) for the period ended March 31, 2025 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), we, Daniel R. Passeri, Chief Executive Officer of the Company, and Kerri-Ann Millar, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. § 1350, as adopted pursuant to § 906 of the Sarbanes-Oxley Act of 2002, to our knowledge that:
1.
The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
2.
The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
/s/ Daniel R. Passeri |
|
/s/ Kerri-Ann Millar |
Name: Daniel R. Passeri |
|
Name: Kerri-Ann Millar |
Title: Chief Executive Officer |
|
Title: Chief Financial Officer |
(Principal Executive Officer) |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
Date: May 12, 2025 |
|
Date: May 12, 2025 |
