
Corporate Presentation May 5, 2025

Forward Looking Statements & Safe Harbor Certain matters discussed in this presentation are “forward-looking statements”. The Company may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the commercial success of the Company’s SUNOSI®, AUVELITY®, and SYMBRAVO® products and the success of the Company’s efforts to obtain any additional indication(s) with respect to solriamfetol and/or AXS-05; the Company’s ability to maintain and expand payer coverage; the success, timing and cost of the Company’s ongoing clinical trials and anticipated clinical trials for the Company’s current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including the Company’s ability to fully fund the Company’s disclosed clinical trials, which assumes no material changes to the Company’s currently projected revenues or expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of the Company’s ongoing clinical trials, and/or data readouts, and the number or type of studies or nature of results necessary to support the filing of a new drug application (“NDA”) for any of the Company’s current product candidates; the Company’s ability to fund additional clinical trials to continue the advancement of the Company’s product candidates; the timing of and the Company’s ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, the Company’s product candidates, including statements regarding the timing of any NDA submission; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the success of the Company’s license agreements; the acceptance by the market of the Company’s products and product candidates, if approved; the Company’s anticipated capital requirements, including the amount of capital required for the commercialization of SUNOSI, AUVELITY, and SYMBRAVO and for the Company’s commercial launch of its other product candidates, if approved, and the potential impact on the Company’s anticipated cash runway; the Company’s ability to convert sales to recognized revenue and maintain a favorable gross to net sales; unforeseen circumstances or other disruptions to normal business operations arising from or related to domestic political climate, geo-political conflicts or a global pandemic and other factors, including general economic conditions and regulatory developments, not within the Company’s control. The factors discussed herein could cause actual results and developments to be materially different from those expressed in or implied by such statements. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstances. This presentation contains statements regarding the Company’s observations based upon the reported clinical data. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about the Company's industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. Axsome, AUVELITY, SUNOSI, SYMBRAVO, and MoSEIC, are trademarks or registered trademarks of Axsome Therapeutics, Inc. or its affiliates. Except as with respect to AUVELITY and SUNOSI for their approved indications, the development products referenced herein have not been approved by the FDA.

Our Mission Develop and deliver transformative medicines for the hundreds of millions of people impacted by central nervous system conditions
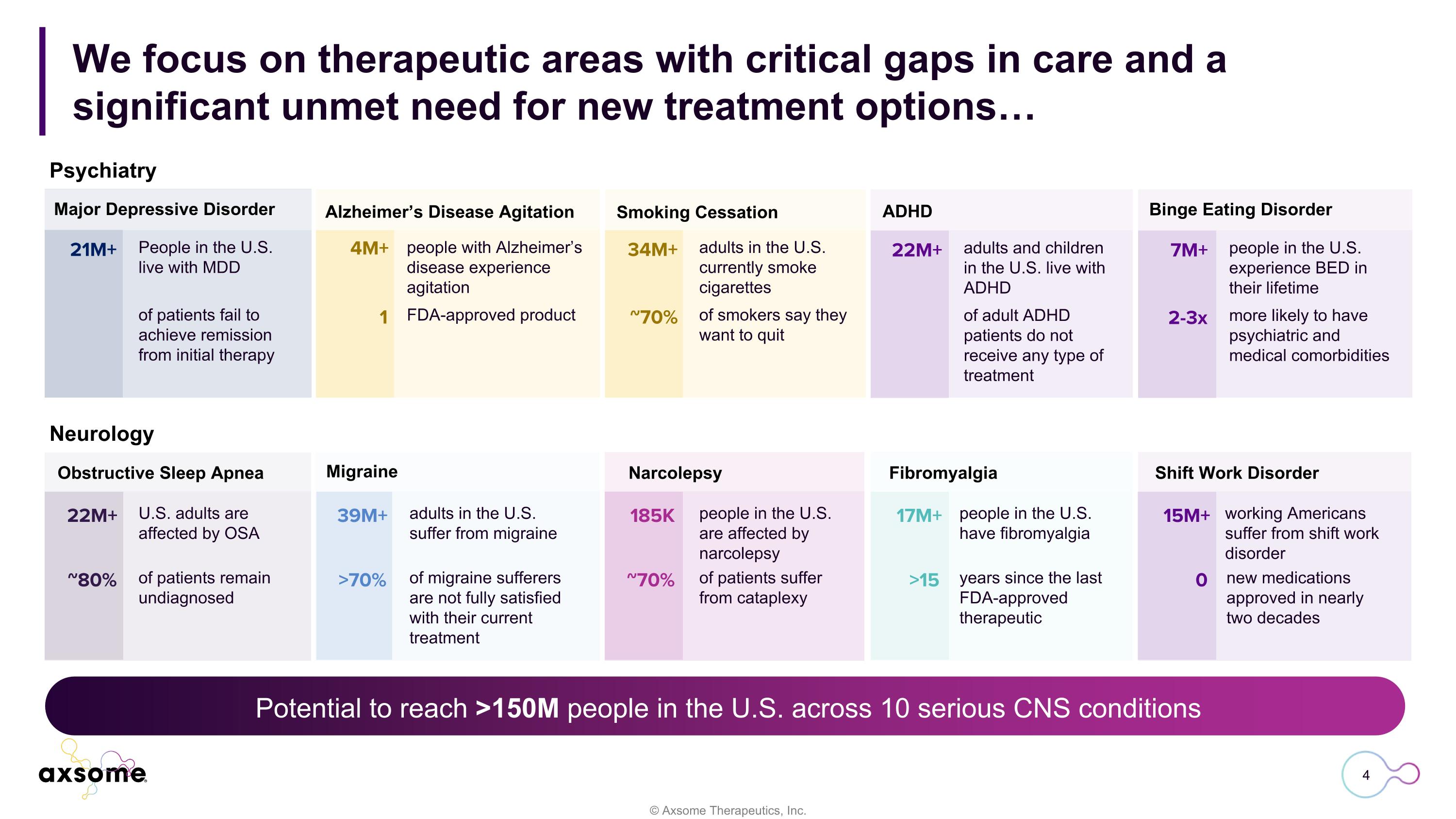
Potential to reach >150M people in the U.S. across 10 serious CNS conditions We focus on therapeutic areas with critical gaps in care and a significant unmet need for new treatment options… Major Depressive Disorder Alzheimer’s Disease Agitation Smoking Cessation ADHD Binge Eating Disorder Neurology Obstructive Sleep Apnea Migraine Narcolepsy Fibromyalgia Shift Work Disorder 21M+ ~ People in the U.S. live with MDD of patients fail to achieve remission from initial therapy 4M+ 1 people with Alzheimer’s disease experience agitation FDA-approved product 34M+ ~70% adults in the U.S. currently smoke cigarettes of smokers say they want to quit 22M+ ~ adults and children in the U.S. live with ADHD of adult ADHD patients do not receive any type of treatment 7M+ 2-3x people in the U.S. experience BED in their lifetime more likely to have psychiatric and medical comorbidities 22M+ ~80% U.S. adults are affected by OSA of patients remain undiagnosed 39M+ >70% adults in the U.S. suffer from migraine of migraine sufferers are not fully satisfied with their current treatment 185K ~70% people in the U.S. are affected by narcolepsy of patients suffer from cataplexy 17M+ >15 people in the U.S. have fibromyalgia years since the last FDA-approved therapeutic 15M+ 0 working Americans suffer from shift work disorder new medications approved in nearly two decades Psychiatry
…And lead in innovation to expand the therapeutic possibilities for CNS conditions Multi-mechanistic approaches Metabolic pharmacokinetic modulation Clinical trial innovation Molecular drug delivery First-in-class mechanisms of action

Well-positioned to deliver significant near- and long-term value to patients and shareholders through 2040s and beyond In-market innovations NDA-stage product candidates Phase 3 development programs ongoing Potential new product/indication launches through 2027 Highly prevalent and/or difficult-to-treat CNS conditions 3 3 4 7 10 + + + +
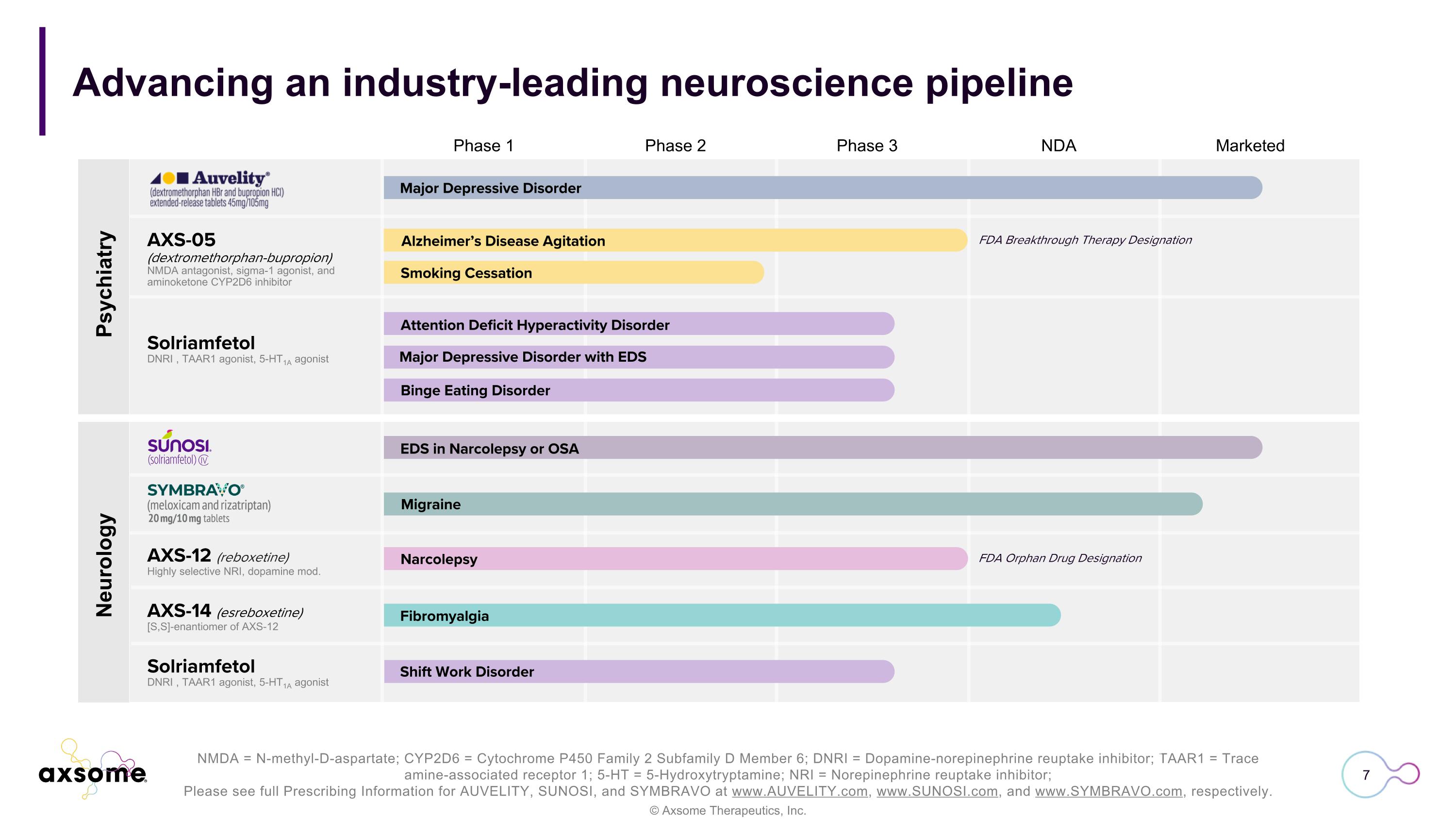
NMDA = N-methyl-D-aspartate; CYP2D6 = Cytochrome P450 Family 2 Subfamily D Member 6; DNRI = Dopamine-norepinephrine reuptake inhibitor; TAAR1 = Trace amine-associated receptor 1; 5-HT = 5-Hydroxytryptamine; NRI = Norepinephrine reuptake inhibitor; Please see full Prescribing Information for AUVELITY, SUNOSI, and SYMBRAVO at www.AUVELITY.com, www.SUNOSI.com, and www.SYMBRAVO.com, respectively. Phase 1 Phase 2 Phase 3 NDA Marketed Advancing an industry-leading neuroscience pipeline FDA Breakthrough Therapy Designation FDA Orphan Drug Designation Major Depressive Disorder Alzheimer’s Disease Agitation Smoking Cessation Attention Deficit Hyperactivity Disorder Binge Eating Disorder Major Depressive Disorder with EDS EDS in Narcolepsy or OSA Migraine Narcolepsy Fibromyalgia Shift Work Disorder Psychiatry Neurology AXS-05 (dextromethorphan-bupropion) NMDA antagonist, sigma-1 agonist, and aminoketone CYP2D6 inhibitor Solriamfetol DNRI , TAAR1 agonist, 5-HT1A agonist AXS-12 (reboxetine) Highly selective NRI, dopamine mod. AXS-14 (esreboxetine) [S,S]-enantiomer of AXS-12 Solriamfetol DNRI , TAAR1 agonist, 5-HT1A agonist

$16.5B peak sales potential driven by current commercial and late-stage assets AXS-05 AD Agitation $0.5-$1B AXS-14 Fibromyalgia AXS-12 Narcolepsy $0.5-$1B $1.5-$3B Solriamfetol MDD with EDS Solriamfetol ADHD $1-$3B $0.5-$1B Solriamfetol BED $0.3-$0.5B Solriamfetol SWD $0.5-$1B AXS-05 Smoking Cessation $1-$1.5B $0.3-$0.5B $1-$3B $0.5-$1B
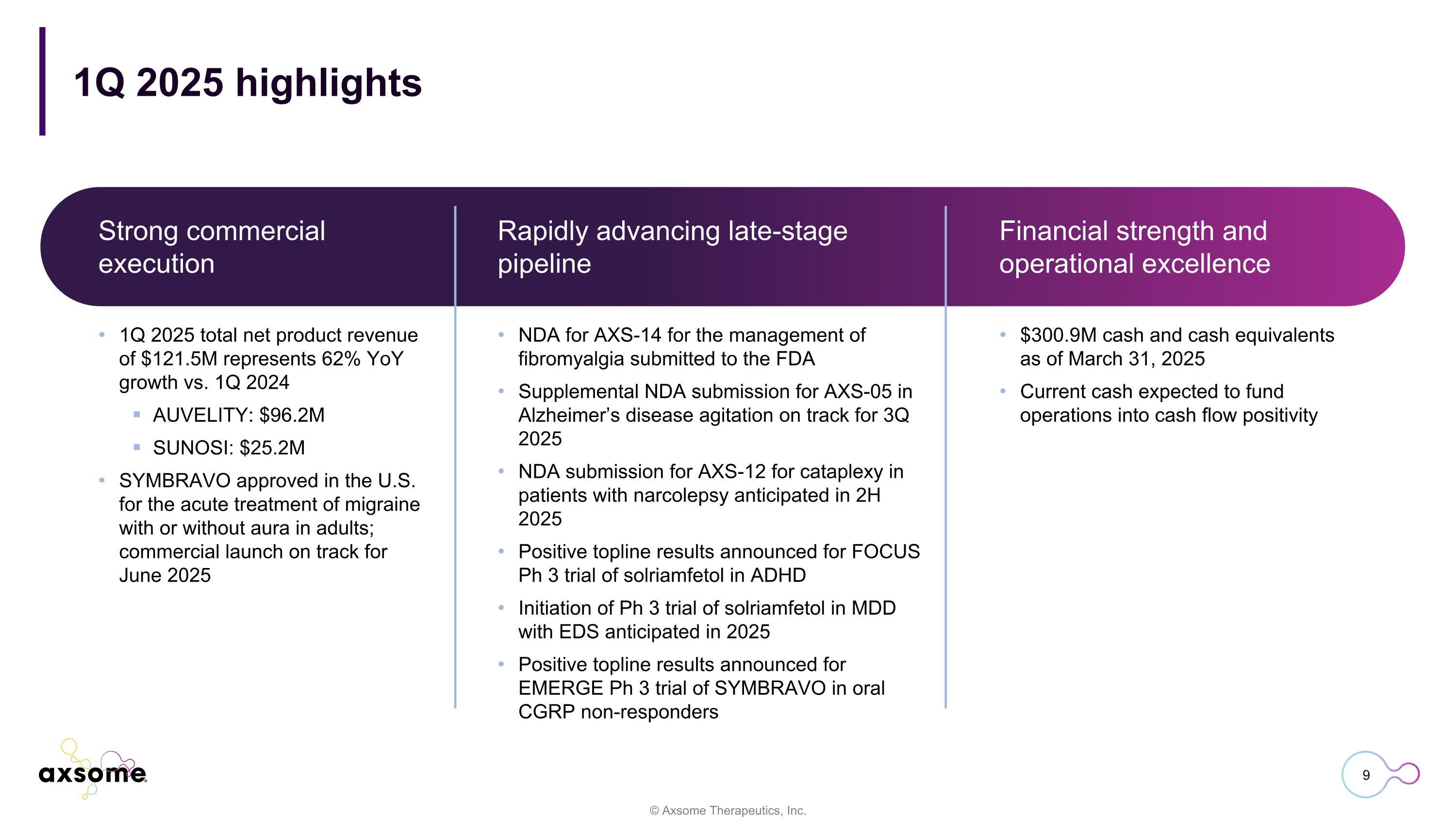
1Q 2025 highlights Rapidly advancing late-stage pipeline 1Q 2025 total net product revenue of $121.5M represents 62% YoY growth vs. 1Q 2024 AUVELITY: $96.2M SUNOSI: $25.2M SYMBRAVO approved in the U.S. for the acute treatment of migraine with or without aura in adults; commercial launch on track for June 2025 $300.9M cash and cash equivalents as of March 31, 2025 Current cash expected to fund operations into cash flow positivity NDA for AXS-14 for the management of fibromyalgia submitted to the FDA Supplemental NDA submission for AXS-05 in Alzheimer’s disease agitation on track for 3Q 2025 NDA submission for AXS-12 for cataplexy in patients with narcolepsy anticipated in 2H 2025 Positive topline results announced for FOCUS Ph 3 trial of solriamfetol in ADHD Initiation of Ph 3 trial of solriamfetol in MDD with EDS anticipated in 2025 Positive topline results announced for EMERGE Ph 3 trial of SYMBRAVO in oral CGRP non-responders Strong commercial execution Financial strength and operational excellence

Maintaining momentum into 2025 with catalyst-rich path ahead 2025 & 2026 Clinical Trial Topline Results Clinical Trial Initiations & Progress Updates Positive topline results from EMERGE Ph 3 trial of SYMBRAVO in oral CGRP non-responders (1Q 2025) Positive topline results from FOCUS Ph 3 trial of solriamfetol in ADHD in adults (1Q 2025) Topline results from PARADIGM Ph 3 trial of solriamfetol in MDD (1Q 2025) ENGAGE Ph 3 trial of solriamfetol in BED (2026) SUSTAIN Ph 3 trial of solriamfetol in SWD (2026) 2025 Initiate Ph 2/3 trial of AXS-05 in smoking cessation (2025) Initiate Ph 3 trial of solriamfetol in ADHD in pediatric patients (2025) Initiate Ph 3 trial of solriamfetol in MDD with EDS (2025) SYMBRAVO approved in the U.S. for the acute treatment of migraine with or without aura in adults (January 2025) Commercial launch of SYMBRAVO in the U.S. (June 2025) FDA filing acceptance decision for NDA for AXS-14 in fibromyalgia (2Q 2025) sNDA submission for AXS-05 in Alzheimer’s disease agitation (3Q 2025) NDA submission for AXS-12 in narcolepsy (2H 2025) Regulatory & Commercial
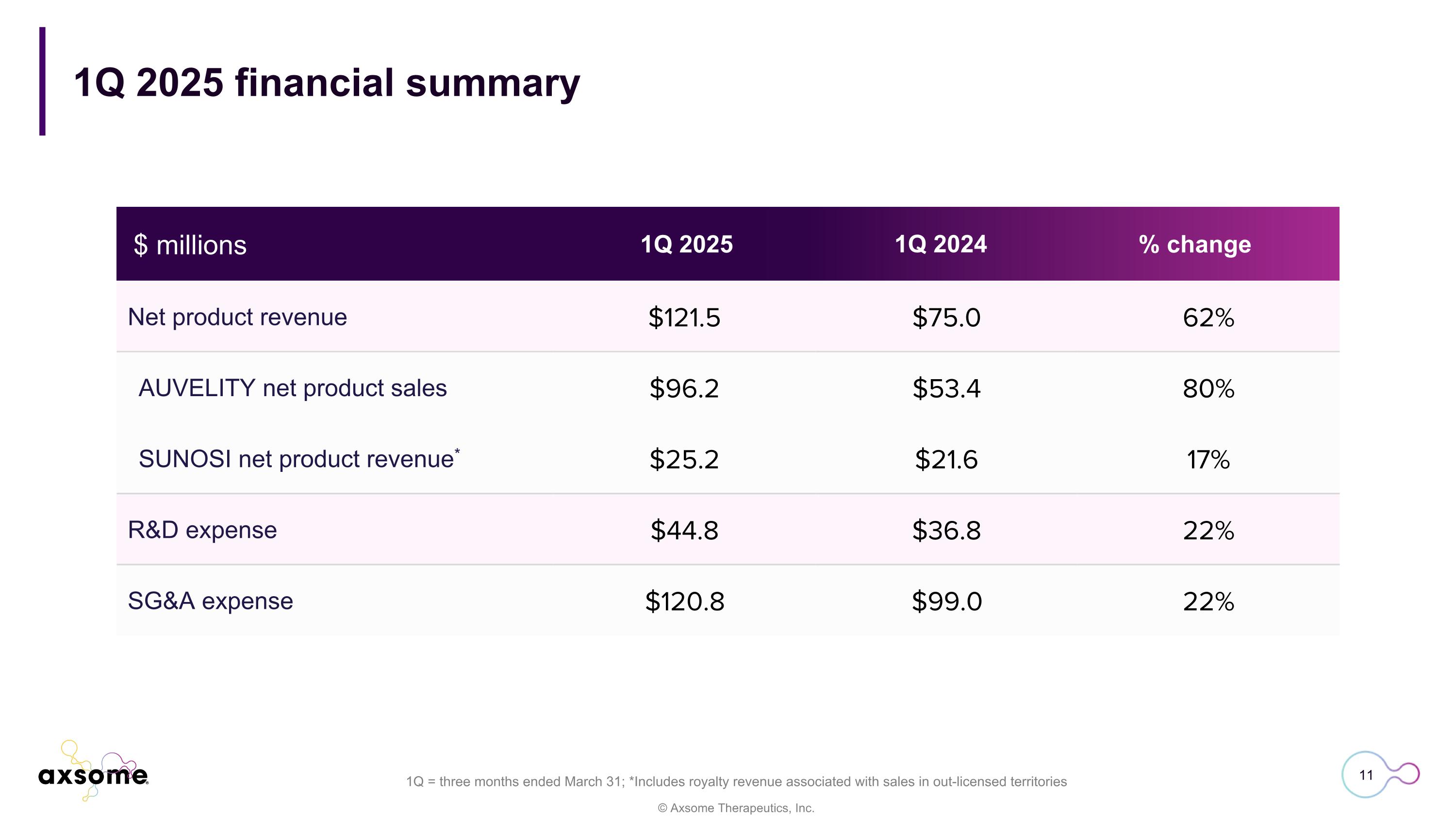
Net product revenue $121.5 $75.0 62% AUVELITY net product sales $96.2 $53.4 80% SUNOSI net product revenue* $25.2 $21.6 17% R&D expense $44.8 $36.8 22% SG&A expense $120.8 $99.0 22% 1Q 2025 financial summary 1Q = three months ended March 31; *Includes royalty revenue associated with sales in out-licensed territories 1Q 2025 1Q 2024 % change $ millions
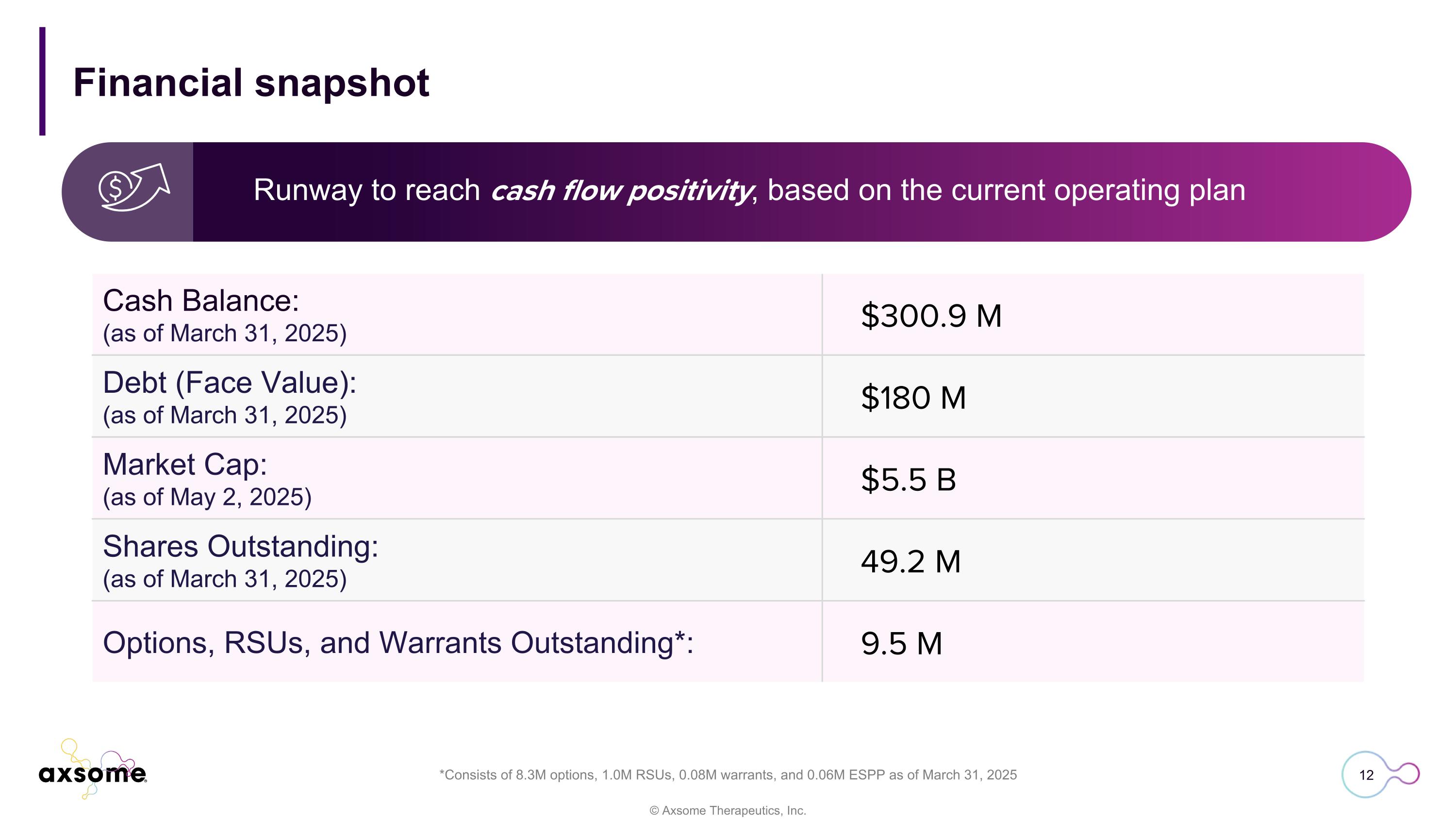
Cash Balance: (as of March 31, 2025) $300.9 M Debt (Face Value): (as of March 31, 2025) $180 M Market Cap: (as of May 2, 2025) $5.5 B Shares Outstanding: (as of March 31, 2025) 49.2 M Options, RSUs, and Warrants Outstanding*: 9.5 M Runway to reach cash flow positivity, based on the current operating plan Financial snapshot *Consists of 8.3M options, 1.0M RSUs, 0.08M warrants, and 0.06M ESPP as of March 31, 2025

Commercial Highlights
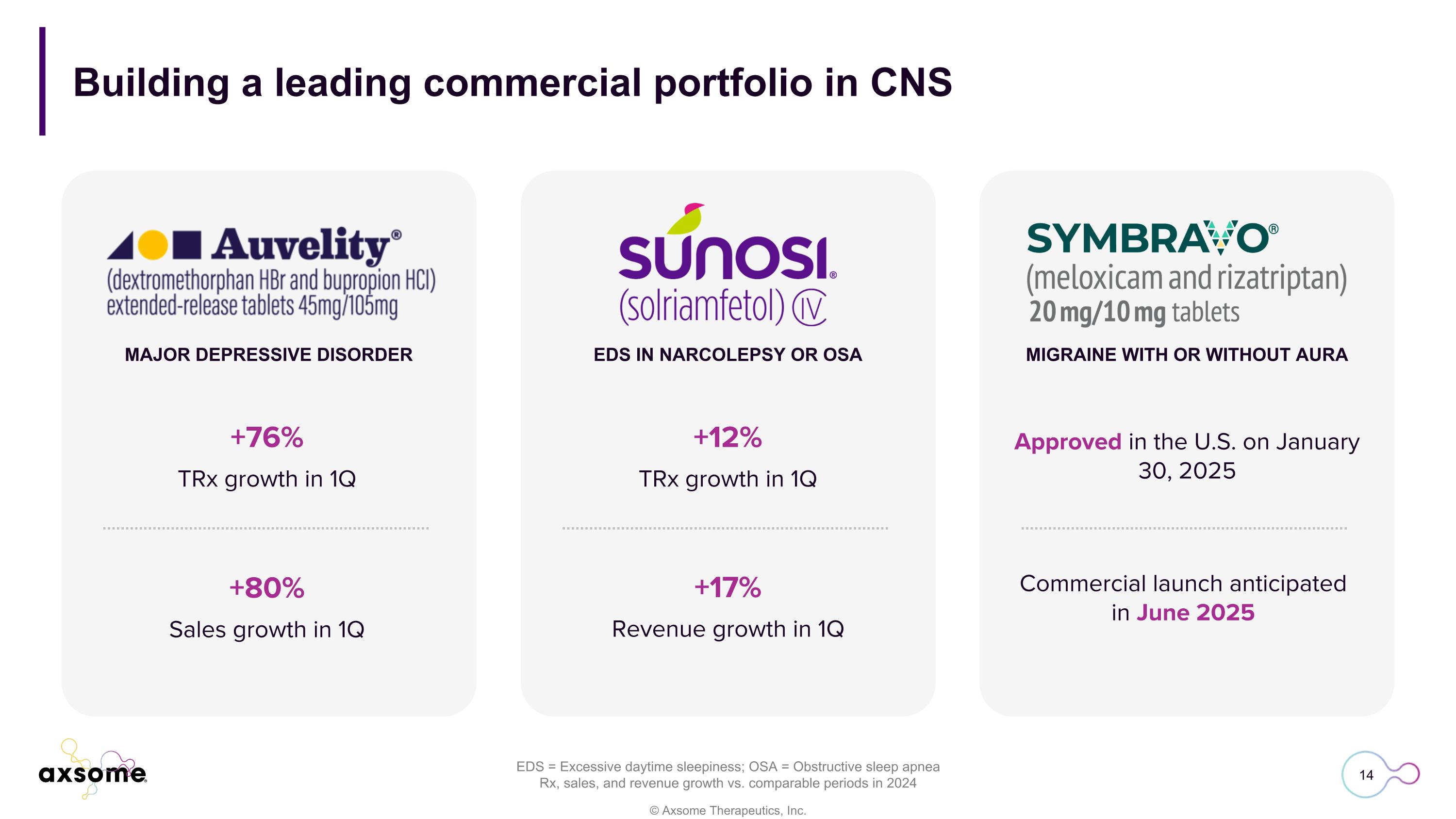
Building a leading commercial portfolio in CNS MAJOR DEPRESSIVE DISORDER EDS IN NARCOLEPSY OR OSA MIGRAINE WITH OR WITHOUT AURA Approved in the U.S. on January 30, 2025 EDS = Excessive daytime sleepiness; OSA = Obstructive sleep apnea Rx, sales, and revenue growth vs. comparable periods in 2024 +76% TRx growth in 1Q +80% Sales growth in 1Q +12% TRx growth in 1Q +17% Revenue growth in 1Q Commercial launch anticipated in June 2025
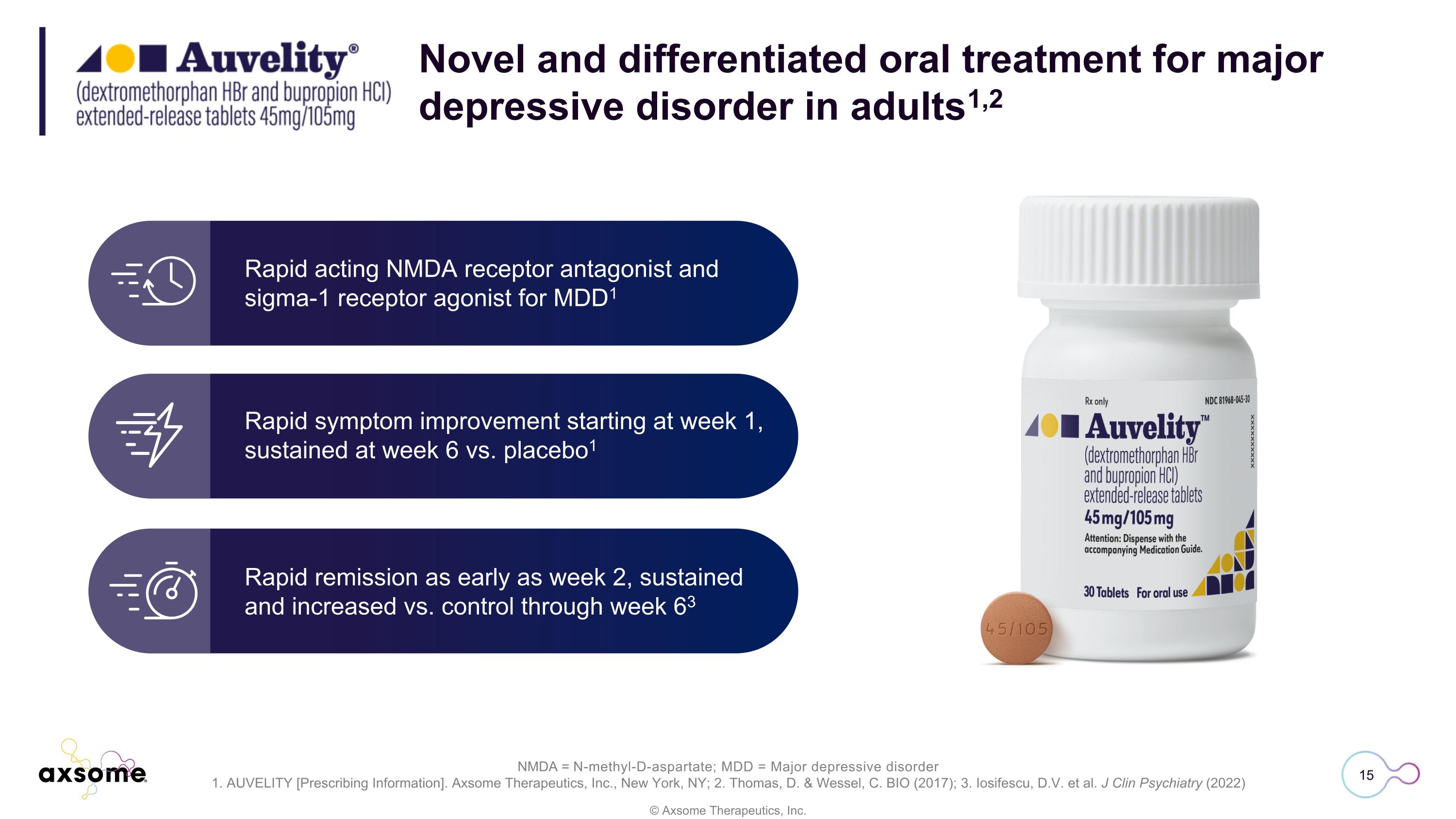
Rapid acting NMDA receptor antagonist and sigma-1 receptor agonist for MDD1 Rapid remission as early as week 2, sustained and increased vs. control through week 63 Rapid symptom improvement starting at week 1, sustained at week 6 vs. placebo1 Novel and differentiated oral treatment for major depressive disorder in adults1,2 NMDA = N-methyl-D-aspartate; MDD = Major depressive disorder 1. AUVELITY [Prescribing Information]. Axsome Therapeutics, Inc., New York, NY; 2. Thomas, D. & Wessel, C. BIO (2017); 3. Iosifescu, D.V. et al. J Clin Psychiatry (2022)
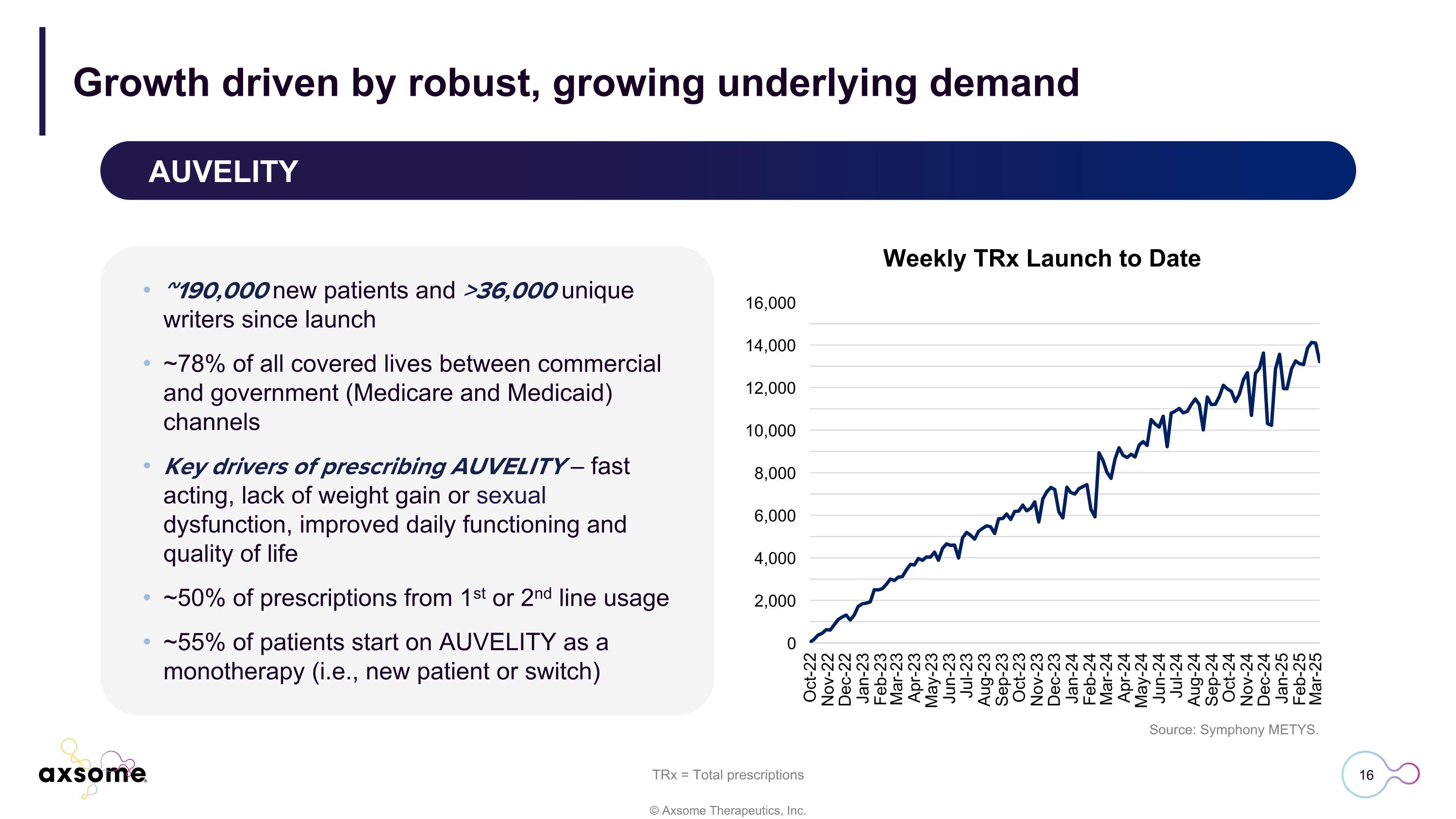
AUVELITY Growth driven by robust, growing underlying demand Source: Symphony METYS. Weekly TRx Launch to Date ~190,000 new patients and >36,000 unique writers since launch ~78% of all covered lives between commercial and government (Medicare and Medicaid) channels Key drivers of prescribing AUVELITY – fast acting, lack of weight gain or sexual dysfunction, improved daily functioning and quality of life ~50% of prescriptions from 1st or 2nd line usage ~55% of patients start on AUVELITY as a monotherapy (i.e., new patient or switch) TRx = Total prescriptions
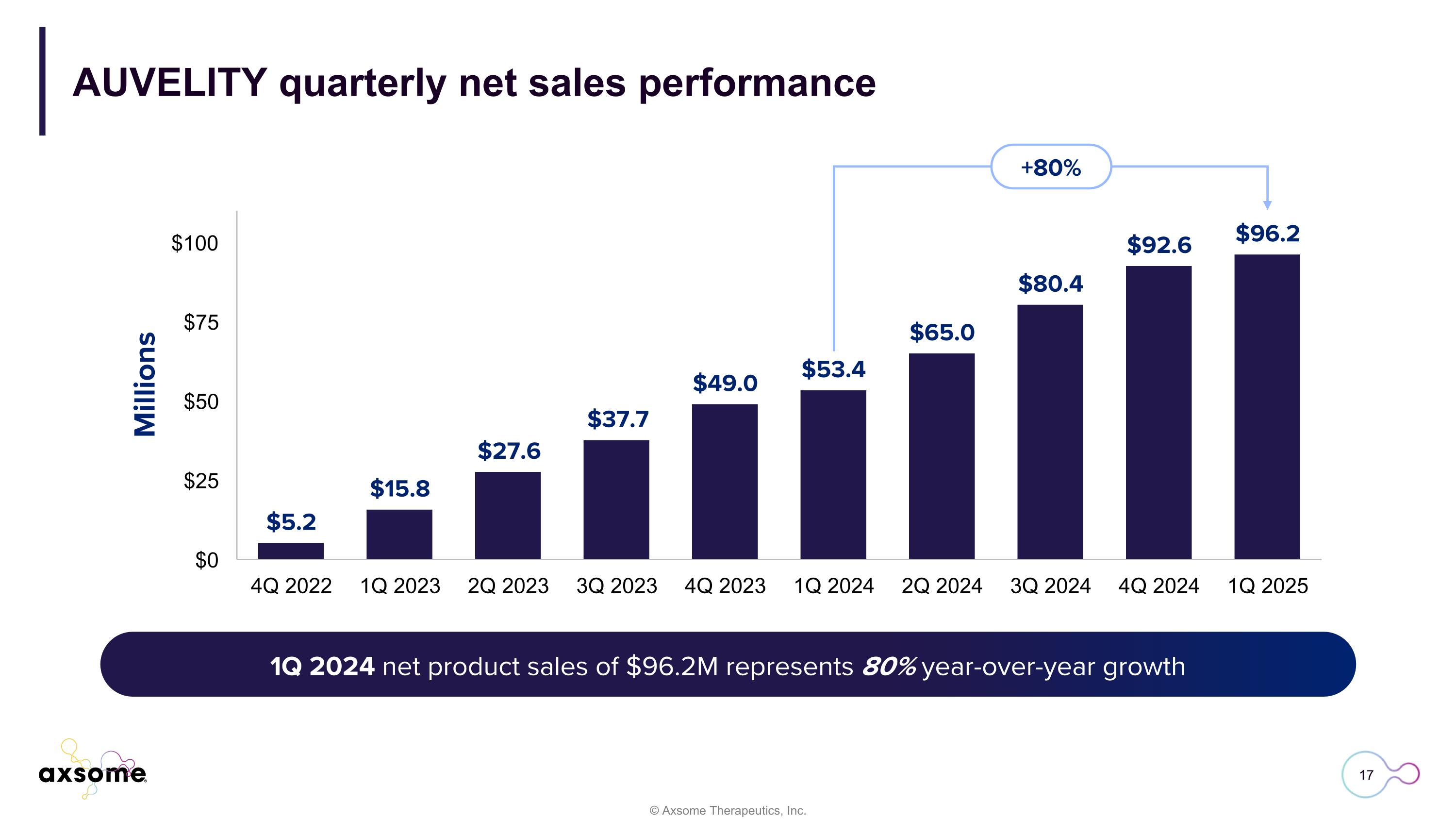
AUVELITY quarterly net sales performance 3Q 2024 net sales of $80.4M represents 113% year-over-year growth vs. 3Q 2023 1Q 2024 net product sales of $96.2M represents 80% year-over-year growth +80%

First and only wakefulness promoting agent proven to improve wakefulness through 9 hours1 Improvements in cognitive functioning vs. placebo demonstrated in clinical trials 90% of patients reported feeling better with SUNOSI 150 mg2 First and only DNRI approved for EDS associated with narcolepsy or OSA1 EDS = Excessive daytime sleepiness; OSA = Obstructive sleep apnea; DNRI = Dopamine-norepinephrine reuptake inhibitor 1. SUNOSI [Prescribing Information]. Axsome Therapeutics, Inc., New York, NY; 2. Schweitzer, P.K. et al. Am J Resp Crit Care Med. (2019)
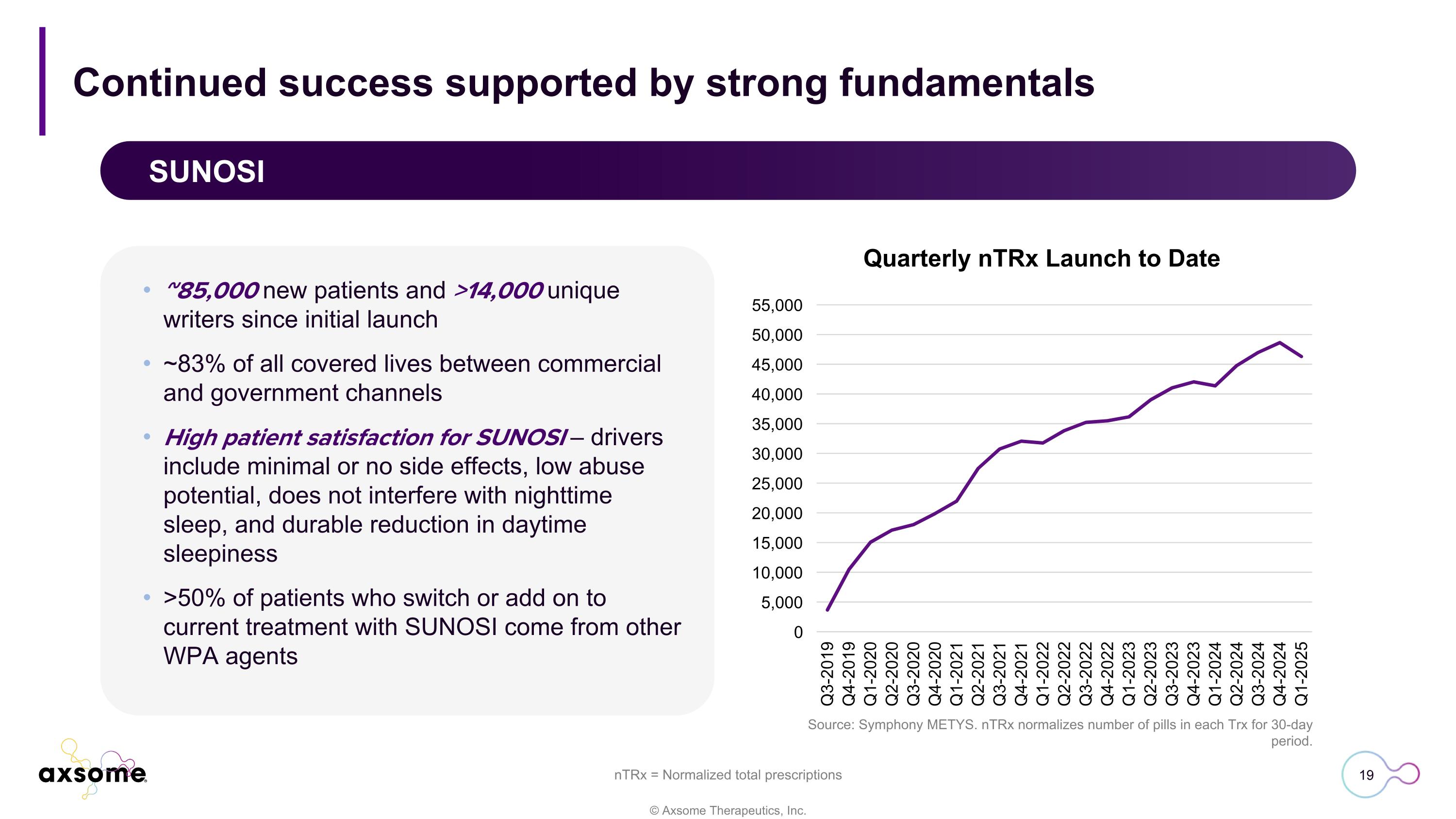
Continued success supported by strong fundamentals SUNOSI Quarterly nTRx Launch to Date Source: Symphony METYS. nTRx normalizes number of pills in each Trx for 30-day period. ~85,000 new patients and >14,000 unique writers since initial launch ~83% of all covered lives between commercial and government channels High patient satisfaction for SUNOSI – drivers include minimal or no side effects, low abuse potential, does not interfere with nighttime sleep, and durable reduction in daytime sleepiness >50% of patients who switch or add on to current treatment with SUNOSI come from other WPA agents nTRx = Normalized total prescriptions

SUNOSI quarterly net revenue performance 3Q 2024 net revenue of $24.4M represents 21% year-over-year growth vs. 3Q 2023 1Q 2025 net product revenue of $25.2M represents 17% year-over-year growth +17%
Single, oral dose provided rapid migraine pain freedom and return to normal functioning within 2 hours1 Harnesses Axsome’s rapid absorption technology to target multiple pathways underlying a migraine attack Superior efficacy demonstrated across a broad range of migraine severity (mild, moderate, severe)1 Novel, multi-mechanistic approach for the acute treatment of migraine1 1. SYMBRAVO [Prescribing Information]. Axsome Therapeutics, Inc., New York, NY
Positioning SYMBRAVO for immediate impact and long-term growth 1. Symphony Health Claims, New to Brand Patients (2022-2023); 2. Lipton et al. Neurology (2015) Launch readiness High patient dissatisfaction due to limited efficacy and/or burdensome side effects >80% of patients discontinue their acute migraine treatment in the first 12 months1 Inadequate acute treatment is associated with increased risk of progression to chronic migraine2 Unmet needs in migraine Launch preparations on track Initial launch strategy prioritizing headache centers and headache specialists Actively engaged with payers across channels to ensure patient access Positive feedback from healthcare providers highlight unique profile of SYMBRAVO Commercial availability anticipated in June 2025

Development Pipeline

AXS-05 modulates the function of neurotransmitters implicated in Alzheimer’s disease (glutamate, sigma-1, norepinephrine, and dopamine)1-4 In Alzheimer’s disease, insoluble Aβ production and accumulation triggers secondary steps leading to synaptic loss and neuronal cell death1,2 Reductions in certain neurotransmitters are thought to contribute to cognitive and behavioral symptoms including agitation and aggression1-4 Brain regions implicated in AD agitation4 AXS-05 pharmacological actions5,6 Potentially first-in-class, best-in-class treatment for Alzheimer’s disease agitation AXS-05 (dextromethorphan-bupropion) 1. Cummings, J.L. N Engl J Med. (2004); 2. Querfurth, H.W. & LaFerla, F.M. N Engl J Med. (2010); 3. Porsteinsson, A.P. & Antonsdottir, I.M. Expert Opin Pharmacother. (2017); 4. Rosenberg, P.B., Nowrangi, M.A., & Lyketsos, C.G. Mol Aspects Med. (2015); 5. Stahl, S.M. CNS Spectr. (2019); 6. Cheng, W. et al. Mol Med Rep. (2015)
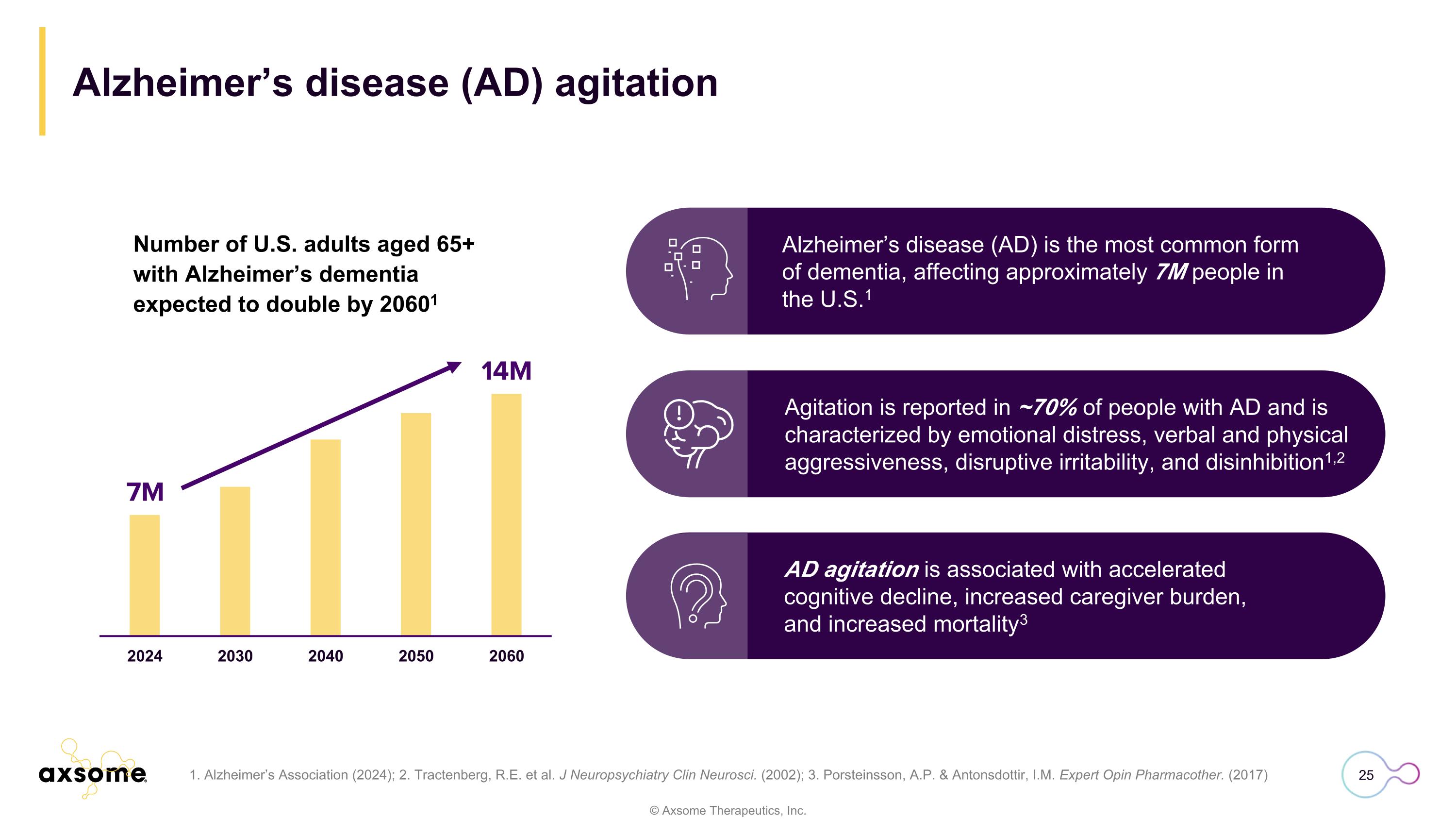
Number of U.S. adults aged 65+ with Alzheimer’s dementia expected to double by 20601 Alzheimer’s disease (AD) agitation Alzheimer’s disease (AD) is the most common form of dementia, affecting approximately 7M people in the U.S.1 AD agitation is associated with accelerated cognitive decline, increased caregiver burden, and increased mortality3 Agitation is reported in ~70% of people with AD and is characterized by emotional distress, verbal and physical aggressiveness, disruptive irritability, and disinhibition1,2 1. Alzheimer’s Association (2024); 2. Tractenberg, R.E. et al. J Neuropsychiatry Clin Neurosci. (2002); 3. Porsteinsson, A.P. & Antonsdottir, I.M. Expert Opin Pharmacother. (2017)
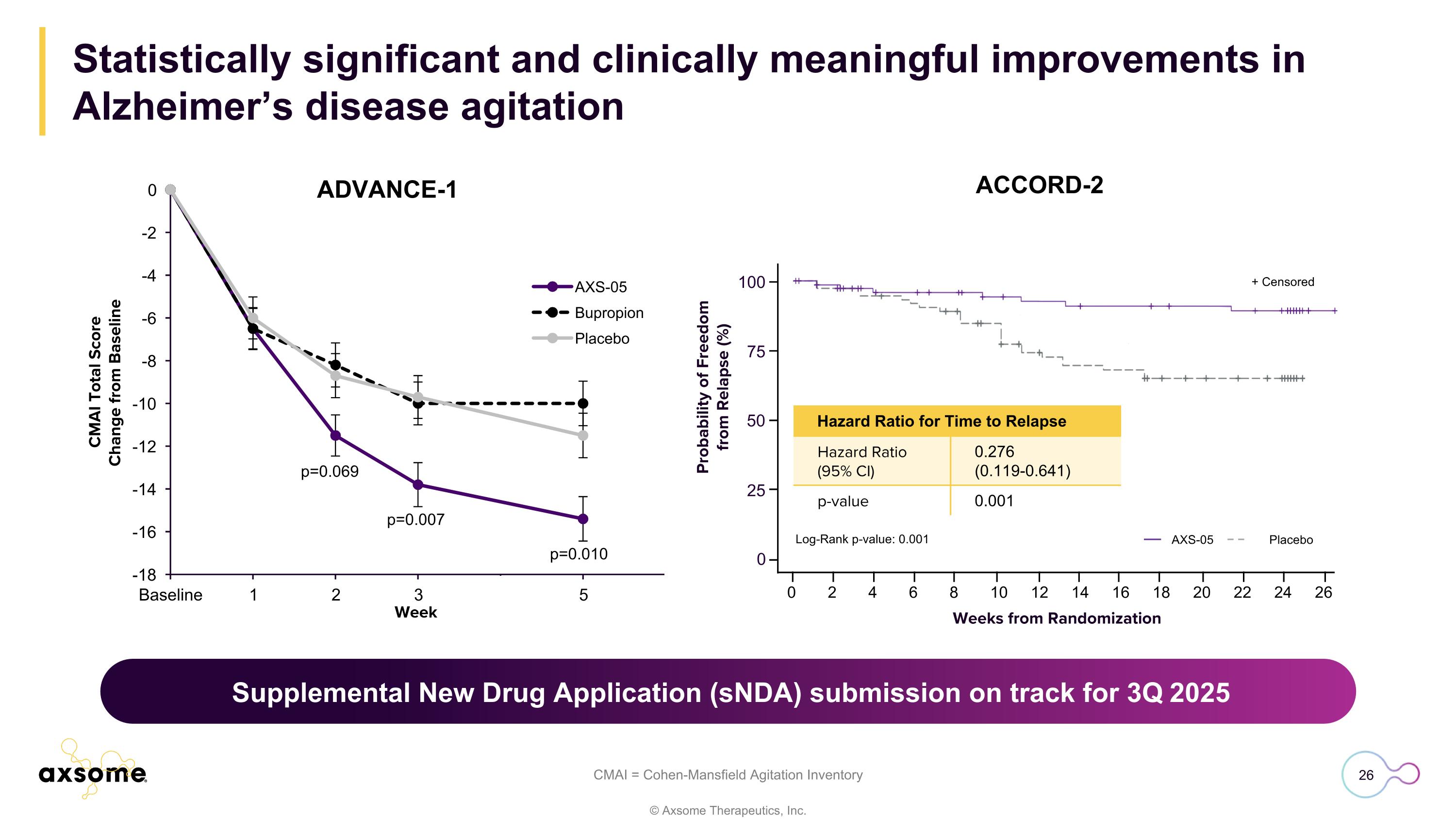
Statistically significant and clinically meaningful improvements in Alzheimer’s disease agitation CMAI = Cohen-Mansfield Agitation Inventory p=0.069 p=0.007 p=0.010 0 Weeks from Randomization Probability of Freedom from Relapse (%) ACCORD-2 100 75 50 25 0 2 4 6 8 10 12 14 16 18 20 22 24 26 + Censored Log-Rank p-value: 0.001 AXS-05 Placebo Hazard Ratio for Time to Relapse Hazard Ratio (95% CI) 0.276 (0.119-0.641) p-value 0.001 Supplemental New Drug Application (sNDA) submission anticipated 3Q 2025 Supplemental New Drug Application (sNDA) submission on track for 3Q 2025

70% of smokers want to quit2 Only 3-5% who attempt to quit without assistance are successful for 6-12 months2 Smoking cessation Single largest cause of preventable disease and death in the U.S., accounting for nearly 1 in 5 deaths1 Associated with over $300 billion in annual costs in the U.S.1 ~34M adults in the U.S. smoke cigarettes, ~50% of whom live with a smoking-related disease1 1. U.S. Department of Health and Human Services (2020); 2. Hughes J.R. et al. Addiction (2004)
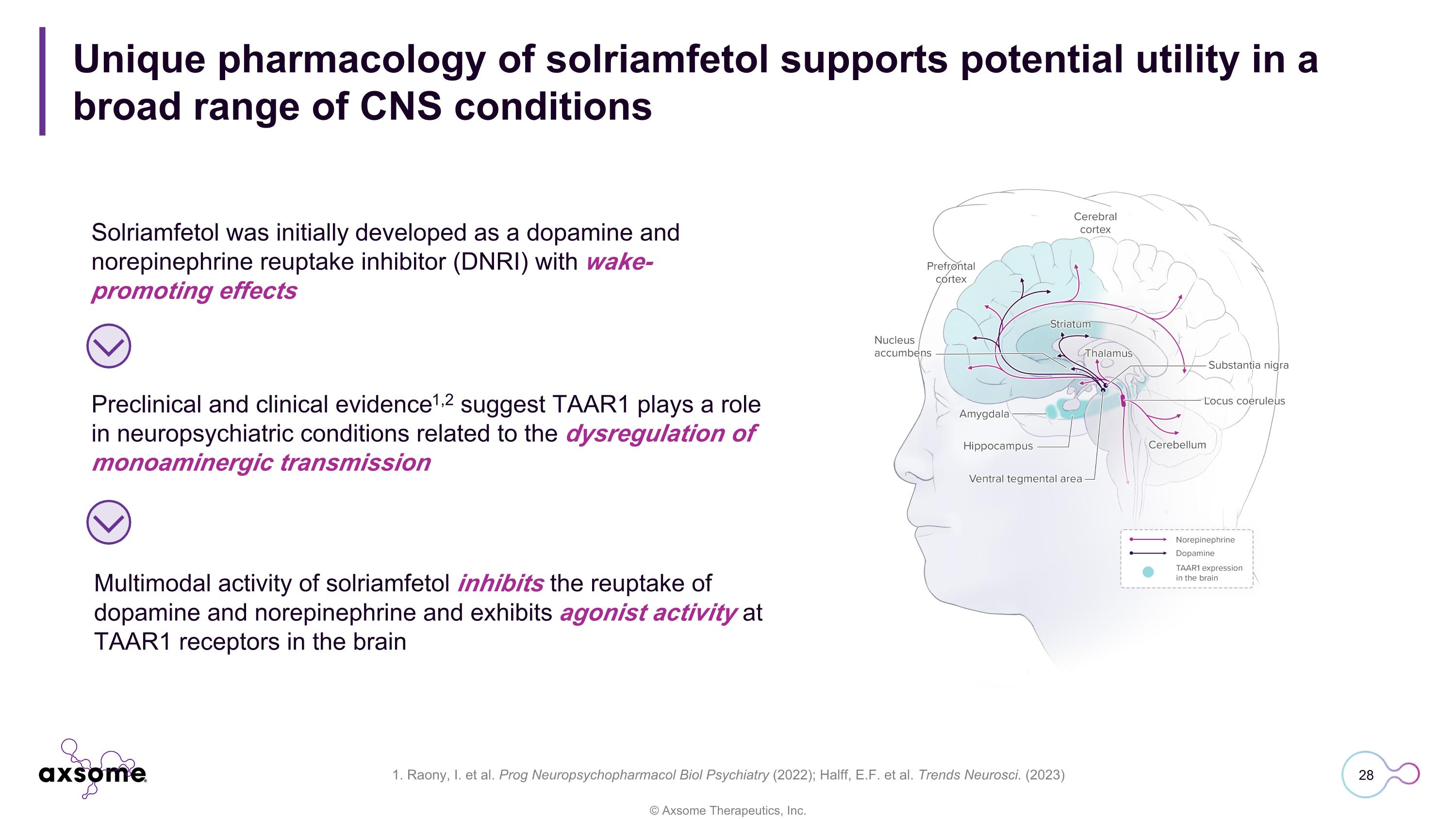
Unique pharmacology of solriamfetol supports potential utility in a broad range of CNS conditions 1. Raony, I. et al. Prog Neuropsychopharmacol Biol Psychiatry (2022); Halff, E.F. et al. Trends Neurosci. (2023) Solriamfetol was initially developed as a dopamine and norepinephrine reuptake inhibitor (DNRI) with wake-promoting effects Preclinical and clinical evidence1,2 suggest TAAR1 plays a role in neuropsychiatric conditions related to the dysregulation of monoaminergic transmission Multimodal activity of solriamfetol inhibits the reuptake of dopamine and norepinephrine and exhibits agonist activity at TAAR1 receptors in the brain

Solriamfetol Phase 3 development programs Topline data 2026 Topline data 2026 Complete Complete Substantial and statistically significant improvements in ADHD symptoms and disease severity Initiation of Phase 3 pediatric trial anticipated in 2025 Solriamfetol FOCUS Phase 3 (N=516) PARADIGM Phase 3 (EDS subgroup n=51) ENGAGE Phase 3 (N=450) SUSTAIN Phase 3 (N=450) Numerically greater improvements in depressive symptoms in prespecified subgroup of patients with severe EDS Initiation of Phase 3 trial in MDD with EDS in 2025 Efficacy and safety of solriamfetol vs. placebo in adults with binge eating disorder 12-week, double-blind, randomized, placebo-controlled, parallel group trial Efficacy and safety of solriamfetol vs. placebo in adults with shift work disorder 12-week, double-blind, randomized, placebo-controlled, parallel group trial ADHD MDD BED SWD ADHD = Attention deficit hyperactivity disorder; MDD = Major depressive disorder; BED = Binge eating disorder; SWD = Shift work disorder
Attention deficit hyperactivity disorder (ADHD) Chronic neurobiological and developmental disorder affecting an estimated ~22M people in the U.S.1, including ~7M children aged 3-17 years old2 Characterized by a persistent pattern of inattention and/or hyperactive-impulsive behaviors3 Associated with significant impairment in social, academic, and occupational functioning and development3 1. “Facts About ADHD in Adults.” CDC (2024); 2. “Data and Statistics on ADHD.” CDC (2024); 3. “Attention-Deficit/Hyperactivity Disorder.” NIMH (2024)
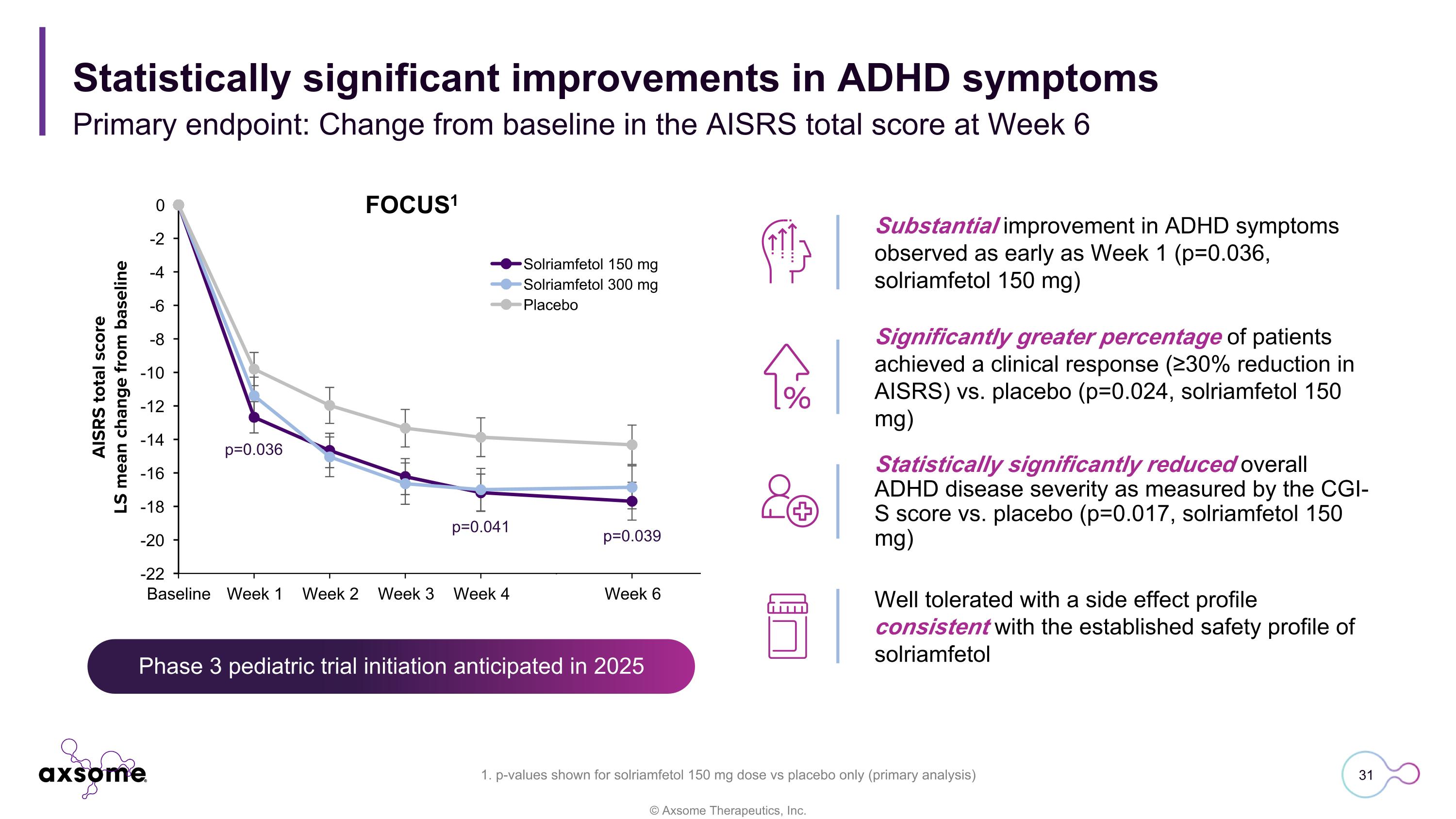
Phase 3 pediatric trial initiation anticipated in 2025 Statistically significant improvements in ADHD symptoms Primary endpoint: Change from baseline in the AISRS total score at Week 6 Substantial improvement in ADHD symptoms observed as early as Week 1 (p=0.036, solriamfetol 150 mg) Significantly greater percentage of patients achieved a clinical response (≥30% reduction in AISRS) vs. placebo (p=0.024, solriamfetol 150 mg) Statistically significantly reduced overall ADHD disease severity as measured by the CGI-S score vs. placebo (p=0.017, solriamfetol 150 mg) Well tolerated with a side effect profile consistent with the established safety profile of solriamfetol 1. p-values shown for solriamfetol 150 mg dose vs placebo only (primary analysis) p=0.036 p=0.041 p=0.039
Major depressive disorder (MDD) ~2/3 of patients experience inadequate response to first-line treatment3 1. “Major Depression.” NIMH (2023); 2. Hasin, D.S. et al. JAMA Psychiatry (2018); 3. Rush, A.J. et al. Am J Psychiatry (2006). 4. Hein et al. J Affect Disord. (2019). Serious and chronic mental health condition causing persistently low or depressed mood and a loss of interest or pleasure in daily activities, and may impair one’s sleep, appetite, ability to concentrate, and/or self-worth1 Approximately 50% of patients with MDD also experience excessive daytime sleepiness (EDS)4, for which there are no approved treatments One of the most common mental disorders in the U.S., impacting ~21M adults each year1,2
Unmet medical need associated with a 2- to 3-fold increased risk of psychiatric and medical comorbidities3 Binge eating disorder (BED) Solriamfetol’s dopamine, norepinephrine, and TAAR1 mechanisms appear relevant to the pathophysiology of BED4-6 ~7 million people in the U.S. have BED2 BED is 1.75x more common in women than in men2 Binge eating disorder (BED) is the most common eating disorder and is thought to involve issues with food reward processing, impulse control, and appetite regulation1,2 1. Kessler, R.M. et al. Neurosci Biobehav Rev. (2016); 2. Hudson, J.I. et al. Biol Psychiatry (2007); 3. McElroy, S.L. et al. J Clin Psychiatry (2020); 4. Giel, KL. et al. Nat Rev Dis Primer (2022); 5. Bello, N.T. & Hajnal, A. Pharmacol Biochem Behav. (2010); 6. Pruccoli et al. Int J Mol Sci. (2021)

Evaluating solriamfetol as a potential treatment for BED Solriamfetol (150 mg) Solriamfetol (300 mg) Placebo 1:1:1 R Screening (4 weeks) Double-blind Phase (12 weeks) Follow-up (1 week) Baseline ENGAGE Phase 3 Trial N=450 Key eligibility criteria 18-55 years of age with diagnosis of BED (DSM-5) Primary endpoint Change from baseline in days with binge eating episodes Solriafemtol’s dopamine, norepinephrine, and TAAR1 mechanisms appear relevant to the pathophysiology of BED1-3 Topline results from the ENGAGE Phase 3 trial of solriamfetol in binge eating disorder anticipated in 2026 TAAR1 = Trace amine-associated receptor 1 1. Giel, K.E. et al. Nat Rev Dis Primers (2022); 2. Bello, N.T. & Hajnal, A. Pharmacol Biochem Behav. (2010); 3. Pruccoli et al. Int J Mol Sci. (2021)
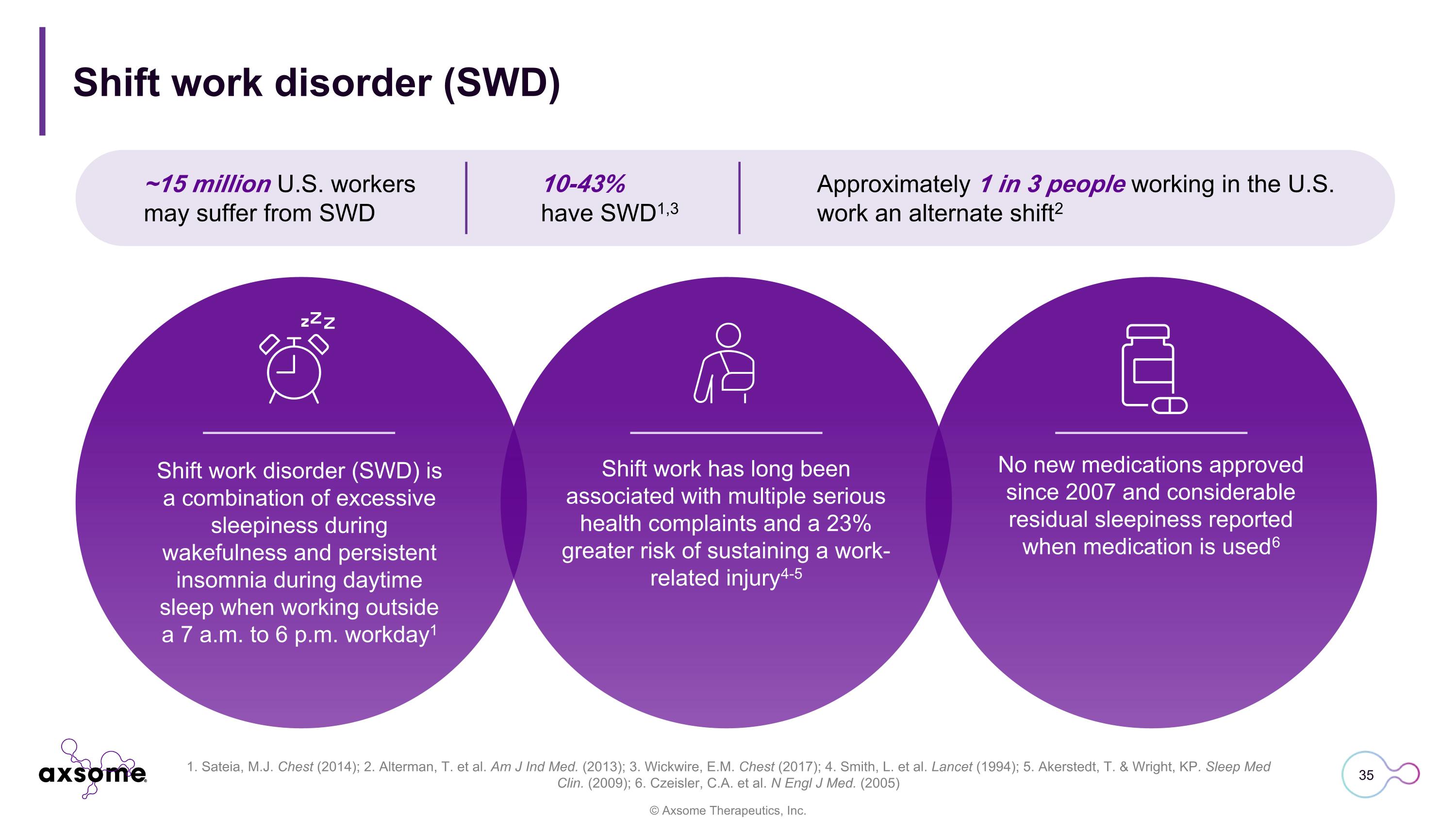
Shift work has long been associated with multiple serious health complaints and a 23% greater risk of sustaining a work-related injury4-5 Shift work disorder (SWD) No new medications approved since 2007 and considerable residual sleepiness reported when medication is used6 ~15 million U.S. workers may suffer from SWD Approximately 1 in 3 people working in the U.S. work an alternate shift2 10-43% have SWD1,3 Shift work disorder (SWD) is a combination of excessive sleepiness during wakefulness and persistent insomnia during daytime sleep when working outside a 7 a.m. to 6 p.m. workday1 1. Sateia, M.J. Chest (2014); 2. Alterman, T. et al. Am J Ind Med. (2013); 3. Wickwire, E.M. Chest (2017); 4. Smith, L. et al. Lancet (1994); 5. Akerstedt, T. & Wright, KP. Sleep Med Clin. (2009); 6. Czeisler, C.A. et al. N Engl J Med. (2005)
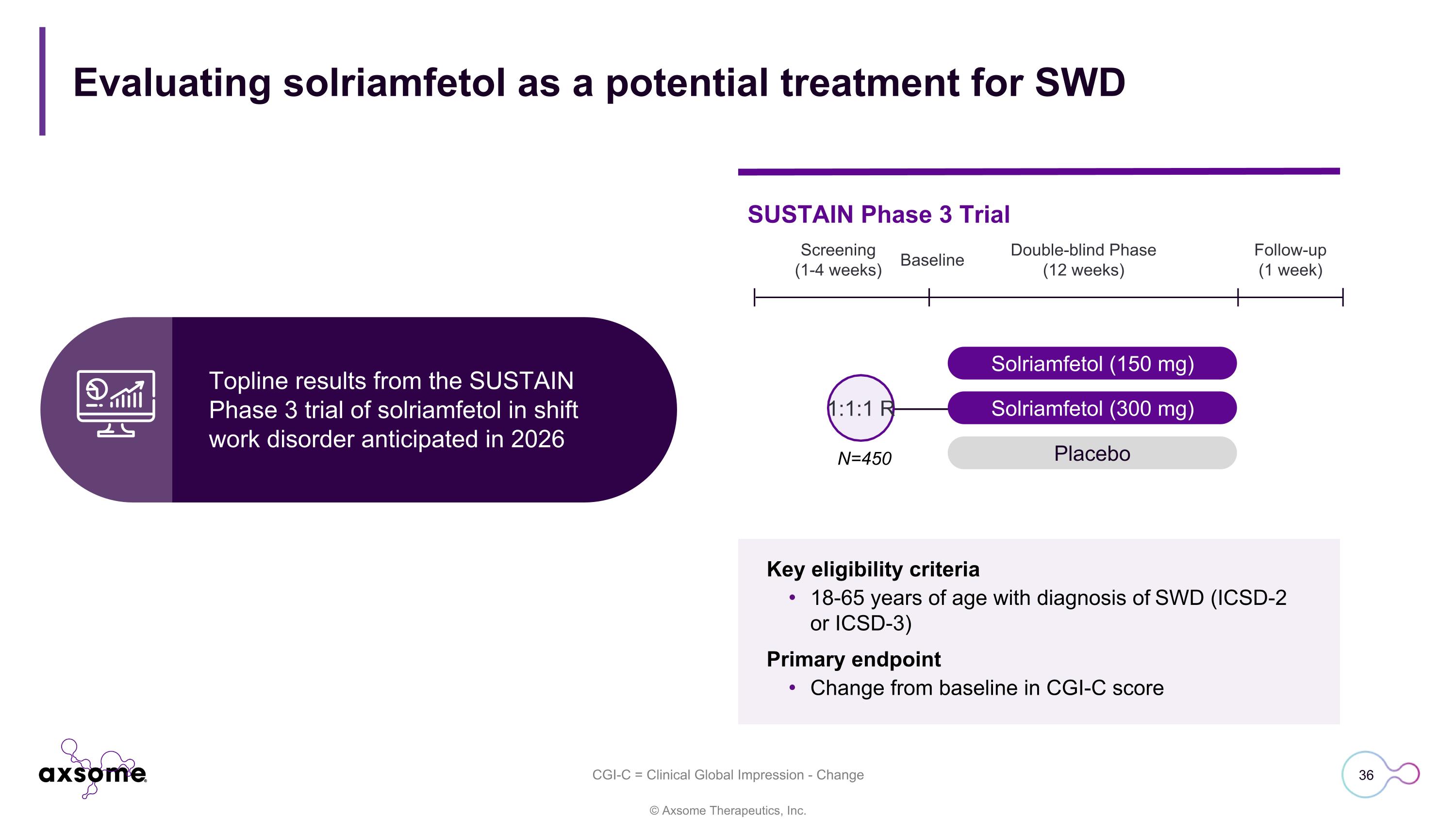
Evaluating solriamfetol as a potential treatment for SWD Solriamfetol (150 mg) Solriamfetol (300 mg) Placebo 1:1:1 R Screening (1-4 weeks) Double-blind Phase (12 weeks) Follow-up (1 week) Baseline SUSTAIN Phase 3 Trial N=450 Key eligibility criteria 18-65 years of age with diagnosis of SWD (ICSD-2 or ICSD-3) Primary endpoint Change from baseline in CGI-C score Topline results from the SUSTAIN Phase 3 trial of solriamfetol in shift work disorder anticipated in 2026 CGI-C = Clinical Global Impression - Change
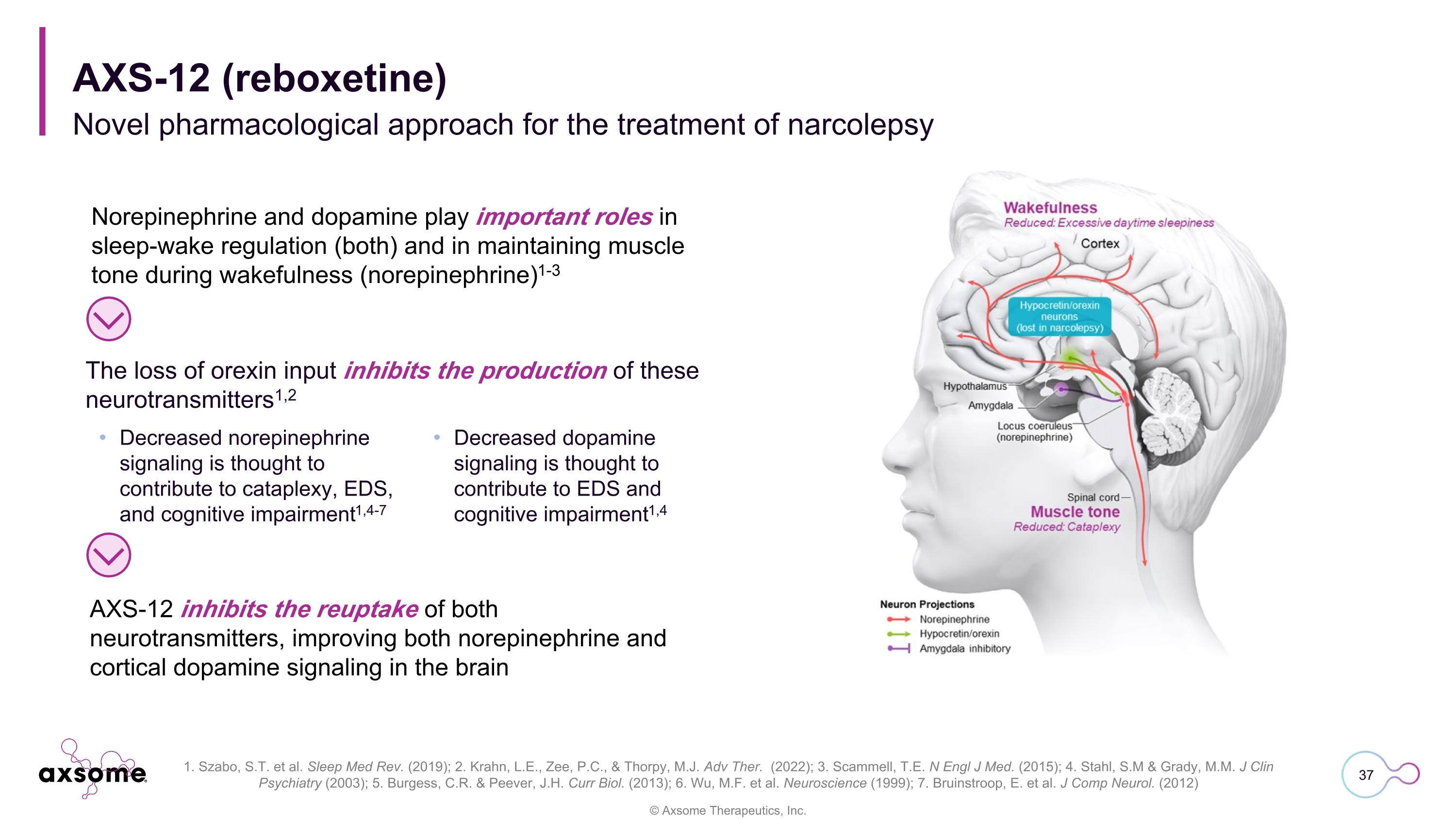
Norepinephrine and dopamine play important roles in sleep-wake regulation (both) and in maintaining muscle tone during wakefulness (norepinephrine)1-3 AXS-12 inhibits the reuptake of both neurotransmitters, improving both norepinephrine and cortical dopamine signaling in the brain The loss of orexin input inhibits the production of these neurotransmitters1,2 Decreased norepinephrine signaling is thought to contribute to cataplexy, EDS, and cognitive impairment1,4-7 Decreased dopamine signaling is thought to contribute to EDS and cognitive impairment1,4 AXS-12 (reboxetine) Novel pharmacological approach for the treatment of narcolepsy 1. Szabo, S.T. et al. Sleep Med Rev. (2019); 2. Krahn, L.E., Zee, P.C., & Thorpy, M.J. Adv Ther. (2022); 3. Scammell, T.E. N Engl J Med. (2015); 4. Stahl, S.M & Grady, M.M. J Clin Psychiatry (2003); 5. Burgess, C.R. & Peever, J.H. Curr Biol. (2013); 6. Wu, M.F. et al. Neuroscience (1999); 7. Bruinstroop, E. et al. J Comp Neurol. (2012)
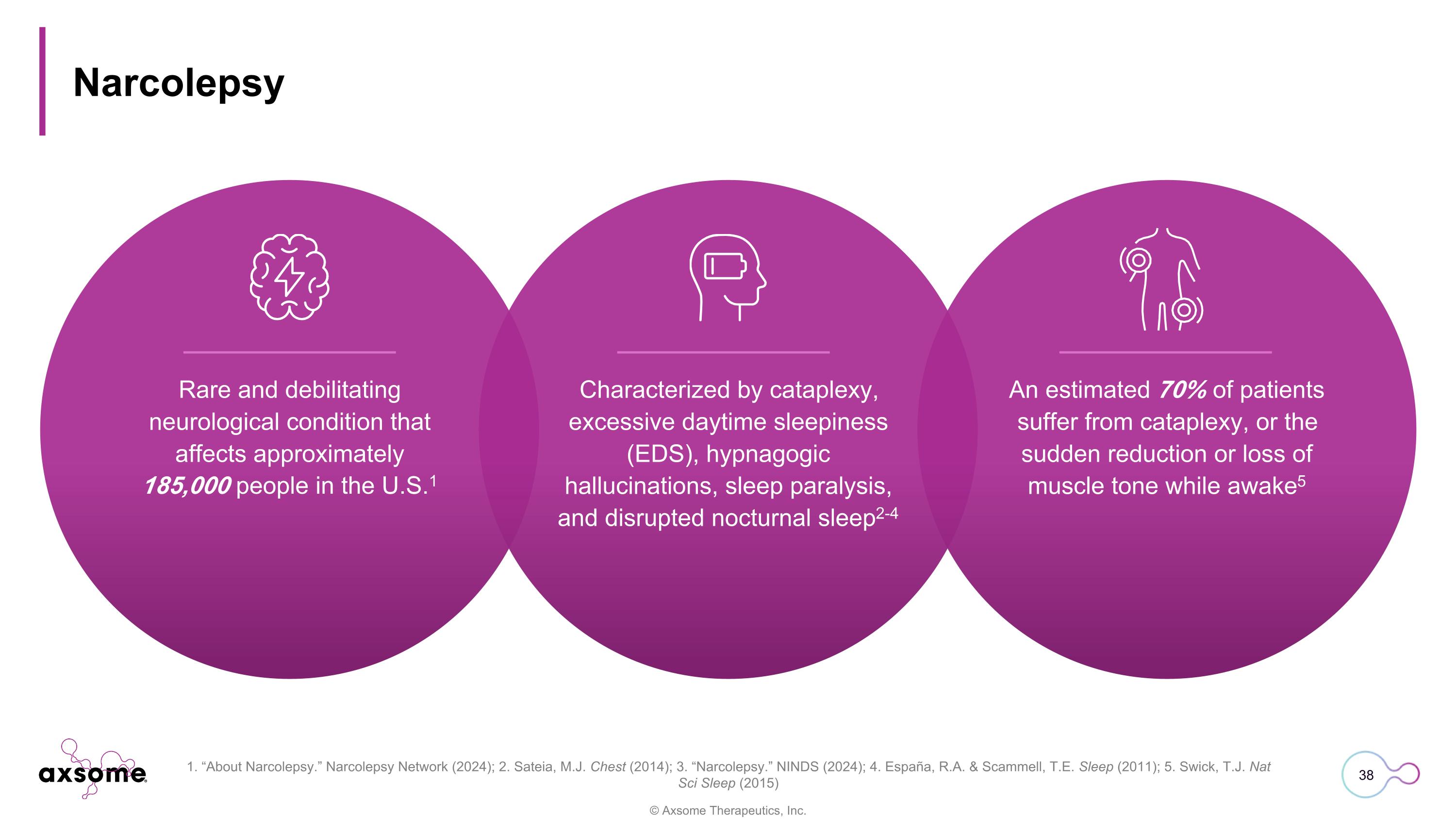
Narcolepsy Rare and debilitating neurological condition that affects approximately 185,000 people in the U.S.1 Characterized by cataplexy, excessive daytime sleepiness (EDS), hypnagogic hallucinations, sleep paralysis, and disrupted nocturnal sleep2-4 An estimated 70% of patients suffer from cataplexy, or the sudden reduction or loss of muscle tone while awake5 1. “About Narcolepsy.” Narcolepsy Network (2024); 2. Sateia, M.J. Chest (2014); 3. “Narcolepsy.” NINDS (2024); 4. España, R.A. & Scammell, T.E. Sleep (2011); 5. Swick, T.J. Nat Sci Sleep (2015)
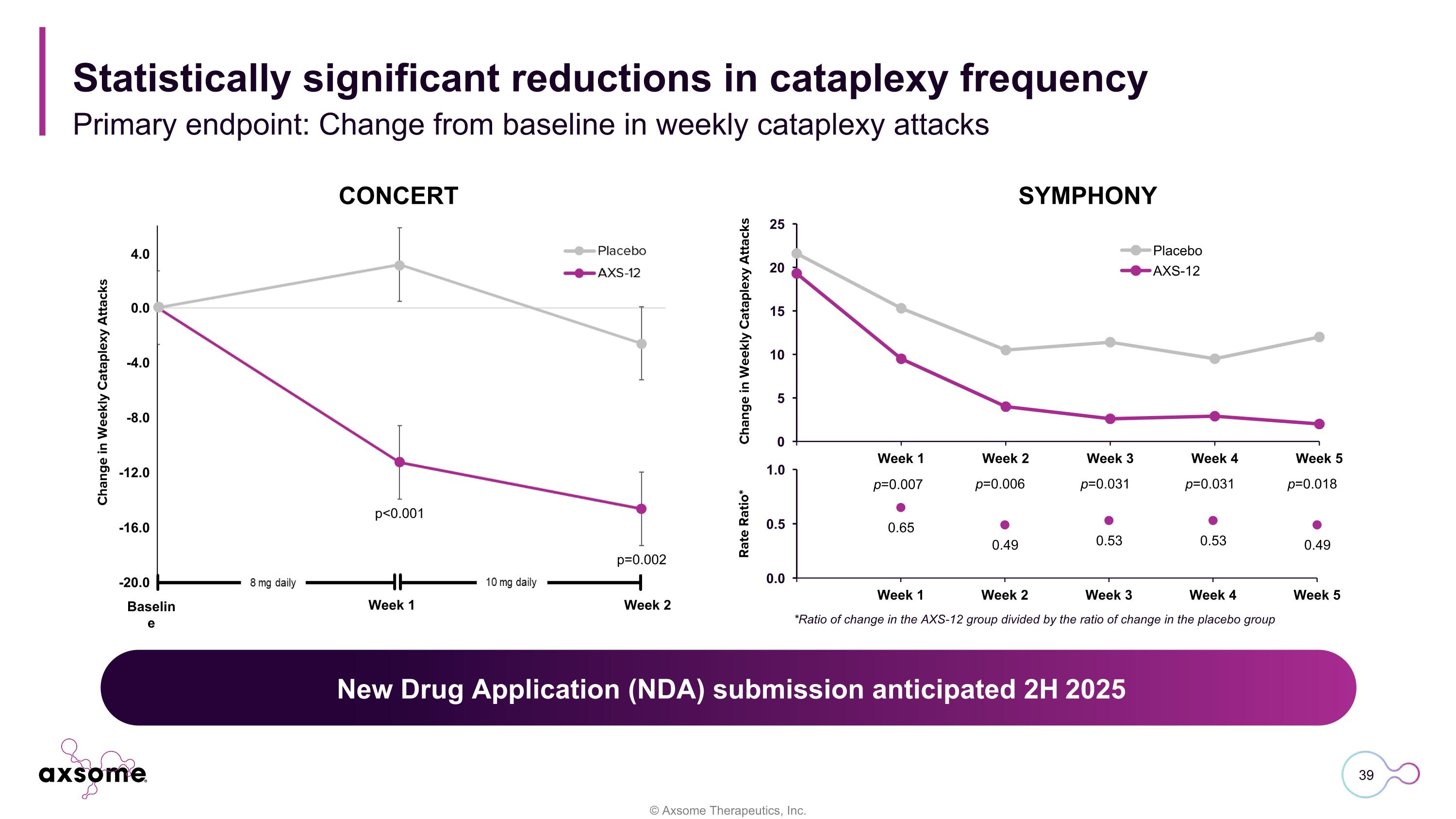
Rate Ratio* p=0.007 p=0.006 p=0.031 p=0.031 p=0.018 *Ratio of change in the AXS-12 group divided by the ratio of change in the placebo group SYMPHONY Primary endpoint: Change from baseline in weekly cataplexy attacks Statistically significant reductions in cataplexy frequency CONCERT p<0.001 p=0.002 Baseline Week 1 Week 2 4.0 0.0 -4.0 -8.0 -12.0 -16.0 -20.0 Change in Weekly Cataplexy Attacks Change in Weekly Cataplexy Attacks New Drug Application (NDA) submission anticipated 2H 2025 New Drug Application (NDA) submission anticipated 2H 2025

Fibromyalgia pain is thought to be partially caused by dysregulated signaling in the descending analgesic system Norepinephrine, one of the key neurotransmitters in this pathway, has predominantly pain-inhibitory effects AXS-14 is a more potent and selective enantiomer of racemic reboxetine that inhibits the reuptake of norepinephrine, resulting in increased norepinephrine activity and decreased pain signaling AXS-14 (esreboxetine) Novel pharmacological approach for the management of fibromyalgia (FM) Adapted from Siracusa, R. et al. Int. J. Mol. Sci. (2021)

Fibromyalgia (FM) Chronic and debilitating neurological syndrome impacting ~17M people in the U.S.1 Characterized by widespread musculoskeletal pain, fatigue, disturbed sleep, depression, and cognitive impairment2 Limited treatment option with only 3 approved agents of variable and/or inadequate efficacy, with no novel therapeutics in over 15 years 1. Vincent et al. Arthritis Care Res (Hoboken) (2013); 2. Bair, M.J. & Krebs, E.E. Ann Intern Med. (2020)

Positive clinical data demonstrate statistically significant improvements in symptoms of fibromyalgia ~1,000 individuals with fibromyalgia dosed with esreboxetine across Phase 2 and Phase 3 clinical trials for up to 14 weeks Statistically significant and clinically meaningful reductions in pain scores, overall symptom severity, and improvements in patient-reported global functioning and fatigue p<0.001 p<0.001 p=0.025 Phase 3 Efficacy Results (N=1,122) p<0.001 p<0.001 p=0.023
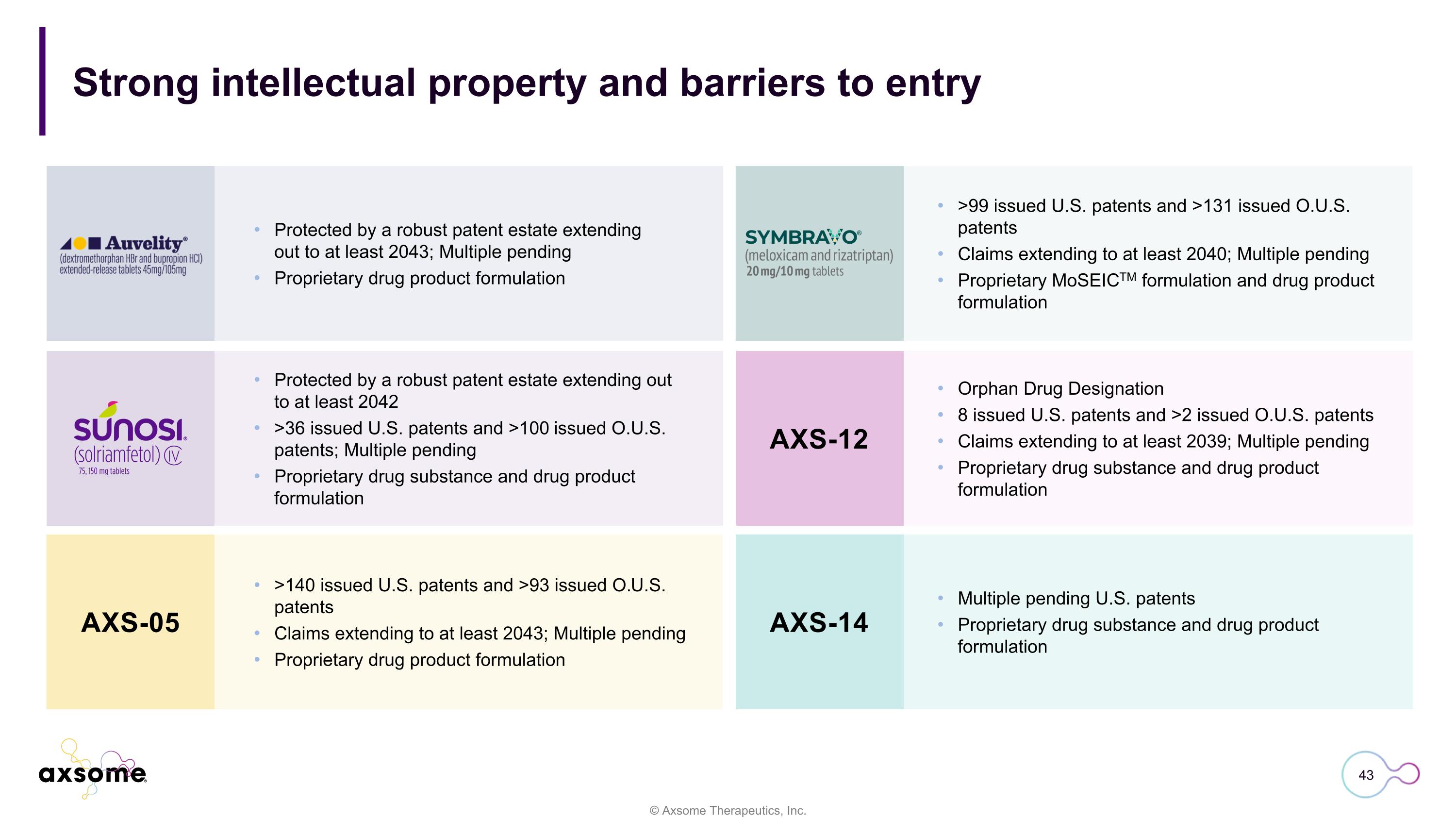
Strong intellectual property and barriers to entry Protected by a robust patent estate extending out to at least 2043; Multiple pending Proprietary drug product formulation >99 issued U.S. patents and >131 issued O.U.S. patents Claims extending to at least 2040; Multiple pending Proprietary MoSEICTM formulation and drug product formulation Protected by a robust patent estate extending out to at least 2042 >36 issued U.S. patents and >100 issued O.U.S. patents; Multiple pending Proprietary drug substance and drug product formulation Orphan Drug Designation 8 issued U.S. patents and >2 issued O.U.S. patents Claims extending to at least 2039; Multiple pending Proprietary drug substance and drug product formulation >140 issued U.S. patents and >93 issued O.U.S. patents Claims extending to at least 2043; Multiple pending Proprietary drug product formulation Multiple pending U.S. patents Proprietary drug substance and drug product formulation AXS-05 AXS-12 AXS-14

Leadership team Roger Jeffs, PhDCEO, Liquidia CorporationFormer President, Co-CEO, Director United Therapeutics Corp. Prior positions at Amgen and Burroughs Wellcome Herriot Tabuteau, MDFounder & CEO Management Board of Directors Nick Pizzie, CPA, MBAChief Financial Officer Mark Jacobson, MAChief Operating Officer Hunter Murdock, JDGeneral Counsel Ari MaizelChief Commercial Officer Mark Saad CEO, NuLids, LLCFormer COO of the Global Healthcare Group at UBS Mark Coleman, MDMedical Director, National Spine and Pain CentersDiplomat of the American Board of Anesthesiology Susan Mahony, PhD Former SVP of Eli Lilly and President Lilly Oncology Prior positions at BMS, Amgen and Schering-Plough Herriot Tabuteau, MD Chairman

Thank you













































