UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 31, 2025 |
Palvella Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
Nevada |
001-37471 |
30-0784346 |
||
(State or other jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
125 Strafford Ave, Suite 360 |
|
|||
Wayne, Pennsylvania |
|
19087 |
||
(Address of principal executive offices) |
|
(Zip Code) |
||
Registrant’s telephone number, including area code: (484) 253-1461 |
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.001 par value per share |
|
PVLA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On March 31, 2025, Palvella Therapeutics, Inc. (the “Company”) announced its financial results for the year ended December 31, 2024. A copy of the press release is being furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished pursuant to this Item 2.02, including Exhibit 99.1 attached hereto, is intended to be furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 7.01 Regulation FD Disclosure.
On March 31, 2025, the Company will hold its earnings call and use a slide presentation in conjunction with the earnings call. A copy of the presentation is furnished herewith as Exhibit 99.2, and incorporated herein by reference.
The information furnished pursuant to Item 7.01, including Exhibit 99.2, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities of that section, and shall not be deemed to be incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
Exhibit No. |
|
Description |
99.1 |
|
Press Release of Palvella Therapeutics, Inc., dated March 31, 2025* |
99.2 |
|
Earnings Call Presentation of Palvella Therapeutics, Inc., dated March 31, 2025* |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Furnished herewith
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Palvella Therapeutics, Inc. |
|
|
|
|
Date: |
March 31, 2025 |
By: |
/s/ Matthew Korenberg |
|
|
|
Matthew Korenberg |
|
|
|
Chief Financial Officer |
Exhibit 99.1

Palvella Therapeutics Reports Full Year 2024 Financial Results and Provides Corporate Update
Upon close of merger and $78.9mm concurrent private placement from a syndicate of leading healthcare-dedicated investors, completed transformation to a publicly traded rare disease biopharmaceutical company advancing a late clinical-stage pipeline and a platform for treating serious, rare genetic skin diseases
Top-line results from SELVA, a Phase 3 single-arm, baseline-controlled trial evaluating QTORIN™ 3.9% rapamycin anhydrous gel (QTORIN™ rapamycin) for the treatment of microcystic lymphatic malformations (microcystic LMs), on track for the first quarter of 2026
Top-line results from TOIVA, a Phase 2 single-arm, baseline-controlled trial evaluating QTORIN™ rapamycin for the treatment of cutaneous venous malformations (cutaneous VMs), on track for the fourth quarter of 2025
Planned QTORIN™ pipeline expansion in second half of 2025
Cash and cash equivalents of over $83 million as of December 31, 2024 expected to fund operations into the second half of 2027
Company to host conference call at 8:30 a.m. ET today
WAYNE, PA., March 31, 2025 (GLOBE NEWSWIRE) -- (Nasdaq: PVLA) Palvella Therapeutics, Inc. (Palvella or “the Company”), a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies to treat patients suffering from serious, rare genetic skin diseases for which there are no U.S. Food and Drug Administration (FDA)-approved therapies, reported financial results for the full year ending December 31, 2024 and provided a corporate update.
"2024 was marked by significant progress towards achieving our vision of becoming the leading rare disease biopharmaceutical company focused on serious, rare genetic skin diseases," said Wes Kaupinen, Founder and Chief Executive Officer of Palvella. "Upon the close of our merger and concurrent private placement in December 2024, we were able to rapidly advance QTORIN™ rapamycin, our lead product candidate from the QTORIN™ platform, into the Phase 3 SELVA study for the treatment of microcystic lymphatic malformations and the Phase 2 TOIVA study for the treatment of cutaneous venous malformations. Microcystic LMs and cutaneous VMs are both serious, rare, and chronically debilitating genetic diseases for which QTORIN™ rapamycin has the potential to be the first FDA-approved therapy and standard of care in the U.S.”
Mr. Kaupinen continued, “In addition to these two initial indications for QTORIN™ rapamycin, which we believe together could exceed $1 billion in U.S. peak annual sales, we plan to broaden our pipeline in 2025 by advancing new and existing QTORIN™ programs for additional serious, rare genetic skin diseases with no FDA-approved therapies."

Recent Research and Development Highlights
QTORIN™ rapamycin for the treatment of microcystic LMs
QTORIN™ rapamycin for the treatment of cutaneous VMs

QTORIN™ rapamycin and QTORIN™ platform expansion
Recent Corporate Highlights

Full Year 2024 Financial Results
Conference Call Details
Palvella will host a conference call and live audiovisual webcast to discuss the Company's full year 2024 financial results and provide a corporate update at 8:30 a.m. ET today. To access the live webcast of the call with slides please click here or visit the "Events & Presentations" section of Palvella’s website. To access the call by phone, please use this registration link, and you will be provided with dial in details. A replay of the webcast will be available approximately 2 hours after the conclusion of the call and archived for 90 days under the "Events & Presentations" section of the Company's website at www.palvellatx.com.
About Microcystic Lymphatic Malformations
Microcystic LMs are a rare, chronically debilitating genetic disease caused by dysregulation of the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway. The disease is characterized by malformed lymphatic vessels that protrude through the skin and persistently leak lymph fluid (lymphorrhea) and bleed, often leading to recurrent serious infections and cellulitis that can cause hospitalization.

The natural history of microcystic LMs is persistent and progressive without spontaneous resolution, with symptoms generally worsening during life, including increases in the number and size of malformed vessels that lead to complications and lifetime morbidity. There are currently no FDA-approved treatments for the estimated more than 30,000 diagnosed patients with microcystic LMs in the United States.
About Cutaneous Venous Malformations
Cutaneous VMs are a rare genetic disease caused by mutations in genes that cause overactivation of the PI3K/mTOR signaling pathway, leading to dysfunctional veins within the skin. These malformations can cause substantial morbidity and functional impairment, significantly impact quality of life, and are associated with severe bleeding, ulceration, and other potential complications. An urgent need exists for an FDA-approved, targeted, localized therapy to treat cutaneous VMs. While published case studies and real-world evidence have provided preliminary evidence of clinical benefit from the off-label use of systemic mTOR inhibitors for venous malformations, there are currently no FDA-approved therapies for the estimated more than 75,000 diagnosed patients with cutaneous VMs in the U.S.
About Palvella Therapeutics
Founded and led by rare disease drug development veterans, Palvella Therapeutics, Inc. (Nasdaq: PVLA) is a clinical-stage biopharmaceutical company focused on developing and commercializing novel therapies to treat patients suffering from serious, rare genetic skin diseases for which there are no FDA-approved therapies. Palvella is developing a broad pipeline of product candidates based on its patented QTORIN™ platform, with an initial focus on serious, rare genetic skin diseases, many of which are lifelong in nature. Palvella’s lead product candidate, QTORIN 3.9% rapamycin anhydrous gel (QTORIN™ rapamycin), is currently being evaluated in the Phase 3 SELVA clinical trial in microcystic lymphatic malformations and the Phase 2 TOIVA clinical trial in cutaneous venous malformations. For more information, please visit www.palvellatx.com or follow Palvella on LinkedIn or X (formerly known as Twitter).
QTORIN™ rapamycin is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency for any indication.
Forward-Looking Statements
This press release contains forward-looking statements (including within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended (Securities Act)). These statements may discuss goals, intentions, and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the management of Palvella, as well as assumptions made by, and information currently available to, the management of Palvella.

Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions or the negative or plural of these words, or other similar expressions that are predictions or indicate future events or prospects, although not all forward-looking statements contain these words. Statements that are not historical facts are forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding the expected timing of the presentation of data from ongoing clinical trials, Palvella’s clinical development plans and related anticipated development milestones, Palvella’s cash and financial resources and expected cash runway, and the potential of, and expectations regarding, Palvella’s programs, including QTORIN™ rapamycin, and its research-stage opportunities, including its expected therapeutic potential and market opportunity. Forward-looking statements are based on current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the ability to raise additional capital to finance operations; the ability to advance product candidates through preclinical and clinical development; the ability to obtain regulatory approval for, and ultimately commercialize, Palvella’s product candidates, including QTORIN™ rapamycin; the outcome of early clinical trials for Palvella’s product candidates, including the ability of those trials to satisfy relevant governmental or regulatory requirements; the fact that data and results from clinical studies may not necessarily be indicative of future results; Palvella’s limited experience in designing clinical trials and lack of experience in conducting clinical trials; the ability to identify and pivot to other programs, product candidates, or indications that may be more profitable or successful than Palvella’s current product candidates; the substantial competition Palvella faces in discovering, developing, or commercializing products; the negative impacts of global events on operations, including ongoing and planned clinical trials and ongoing and planned preclinical studies; the ability to attract, hire, and retain skilled executive officers and employees; the ability of Palvella to protect its intellectual property and proprietary technologies; reliance on third parties, contract manufacturers, and contract research organizations; and the risks and uncertainties described in the filings made by Palvella with the Securities and Exchange Commission (SEC), including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that Palvella may face. Except as required by applicable law, Palvella does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

This press release contains hyperlinks to information that is not deemed to be incorporated by reference into this press release.
Contact Information
Investors
Wesley H. Kaupinen
Founder and CEO, Palvella Therapeutics
wes.kaupinen@palvellatx.com
Media
Marcy Nanus
Managing Partner, Trilon Advisors LLC
mnanus@trilonadvisors.com

PALVELLA THERAPEUTICS, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share amounts)
|
|
Year Ended December 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
$ |
8,151 |
|
|
$ |
8,793 |
|
General and administrative |
|
|
5,944 |
|
|
|
3,076 |
|
Total operating expenses |
|
|
14,095 |
|
|
|
11,869 |
|
Loss from operations |
|
|
(14,095 |
) |
|
|
(11,869 |
) |
Total other income (expense), net |
|
|
(3,339 |
) |
|
|
30,560 |
|
Net loss |
|
$ |
(17,434 |
) |
|
$ |
18,691 |
|
Less: Cumulative Series D preferred dividends |
|
|
— |
|
|
|
(776 |
) |
Net (loss) income attributable to common stockholders |
|
$ |
(17,434 |
) |
|
$ |
17,915 |
|
|
|
|
|
|
|
|
||
Net (loss) income per share: |
|
|
|
|
|
|
||
— Basic |
|
$ |
(7.83 |
) |
|
$ |
2.19 |
|
— Diluted |
|
$ |
(7.83 |
) |
|
$ |
2.17 |
|
Weighted-average number of common shares used in computing net (loss) income per share: |
|
|
|
|
|
|
||
— Basic |
|
|
2,225,934 |
|
|
|
1,770,167 |
|
— Diluted |
|
|
2,225,934 |
|
|
|
1,793,980 |
|
PALVELLA THERAPEUTICS, INC.
CONDENSED CONSOLIDATED BALANCE SHEET INFORMATION
(in thousands)
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
83,602 |
|
|
$ |
7,350 |
|
Other current assets |
|
|
4,632 |
|
|
|
198 |
|
Total current assets |
|
|
88,234 |
|
|
|
7,548 |
|
Total assets |
|
$ |
88,234 |
|
|
$ |
7,548 |
|
Liabilities, Convertible Preferred Stock and Stockholders' Equity (Deficit) |
|
|
|
|
|
|
||
Current liabilities |
|
$ |
12,038 |
|
|
$ |
2,360 |
|
Non-current liabilities |
|
|
13,589 |
|
|
|
9,068 |
|
Total liabilities |
|
|
25,627 |
|
|
|
11,428 |
|
Total convertible preferred stock |
|
|
— |
|
|
|
70,603 |
|
Total stockholders' equity (deficit) |
|
|
62,607 |
|
|
|
(74,483 |
) |
Total liabilities, convertible preferred stock and stockholders’ equity (deficit) |
|
$ |
88,234 |
|
|
$ |
7,548 |
|

First-in-disease therapies for patients with rare genetic skin diseases Full Year 2024 Financial Results & Corporate Update March 31, 2025

Forward Looking Statements This presentation contains forward-looking statements of Palvella Therapeutics, Inc. (the Company”) within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this presentation include, but are not limited to, statements regarding the Company’s future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters the Company’s current and prospective product candidates, the Company's planned research and development activities, the Company's planned clinical trials, including timing of receipt of data from the same, the planned regulatory framework for the Company's product candidates, the strength of the Company's intellectual property portfolio, and projections of the Company’s future financial results and other metrics. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon current estimates and assumptions of the Company and its management and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this presentation. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: competition, the ability of the company to grow and manage growth, maintain relationships with suppliers and retain its management and key employees; the success, cost and timing of the Company’s product development activities, studies and clinical trials; changes in applicable laws or regulations; the possibility that the Company may be adversely affected by other economic, business or competitive factors; the Company’s estimates of expenses and profitability; the evolution of the markets in which the Company competes; the ability of the Company to implement its strategic initiatives and continue to innovate its existing products; and the ability of the Company to defend its intellectual property. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. Industry and Market Data The Company may from time to time provide estimates, projections and other information concerning its industry, the general business environment, and the markets for certain conditions, including estimates regarding the potential size of those markets and the estimated incidence and prevalence of certain medical conditions. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events, circumstances or numbers, including actual disease prevalence rates and market size, may differ materially from the information reflected in this presentation. Unless otherwise expressly stated, we obtained this industry, business information, market data, prevalence information and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data, and similar sources, in some cases applying our own assumptions and analysis that may, in the future, prove not to have been accurate. Trademarks This Presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM © or ® symbols, but the Company will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights.

Vision: Build the leading therapeutics company focused on rare genetic skin diseases Palvella debuted as a publicly listed company (NASDAQ:PVLA) in December 2024
What Sets Palvella Apart Our Mission is to Serve Patients with Rare Diseases First-in-Disease Therapies Exclusively focused on developing transformational therapies for rare diseases with no FDA approved treatments Rare Disease Expertise Team with expertise in rare disease drug development, including regulatory and patient interactions Proven track record building successful rare disease companies, including Insmed Capital Efficiency Disciplined approach to operating business with our investors’ capital top of mind Late-stage Pipeline and Platform Lead product candidate, QTORINTM rapamycin, in two ongoing studies: Phase 3 (microcystic LMs) and Phase 2 (cutaneous VMs) Versatile QTORINTM platform with potential across rare diseases
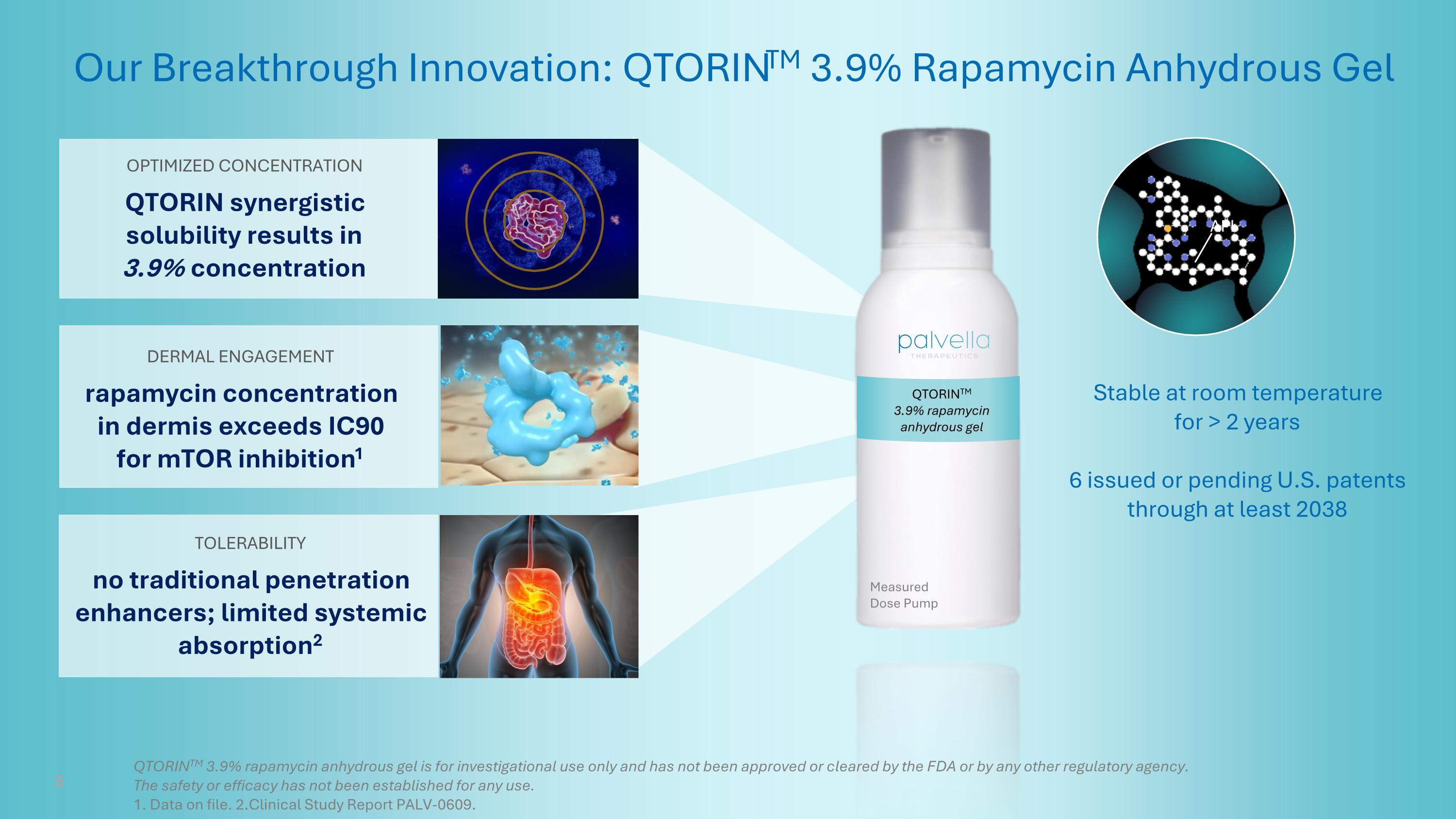
QTORINTM 3.9% rapamycin anhydrous gel API Our Breakthrough Innovation: QTORINTM 3.9% Rapamycin Anhydrous Gel DERMAL ENGAGEMENT rapamycin concentration in dermis exceeds IC90 for mTOR inhibition1 OPTIMIZED CONCENTRATION QTORIN synergistic solubility results in 3.9% concentration TOLERABILITY no traditional penetration enhancers; limited systemic absorption2 Measured Dose Pump Stable at room temperature for > 2 years 6 issued or pending U.S. patents through at least 2038 5 QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. 1. Data on file. 2.Clinical Study Report PALV-0609.
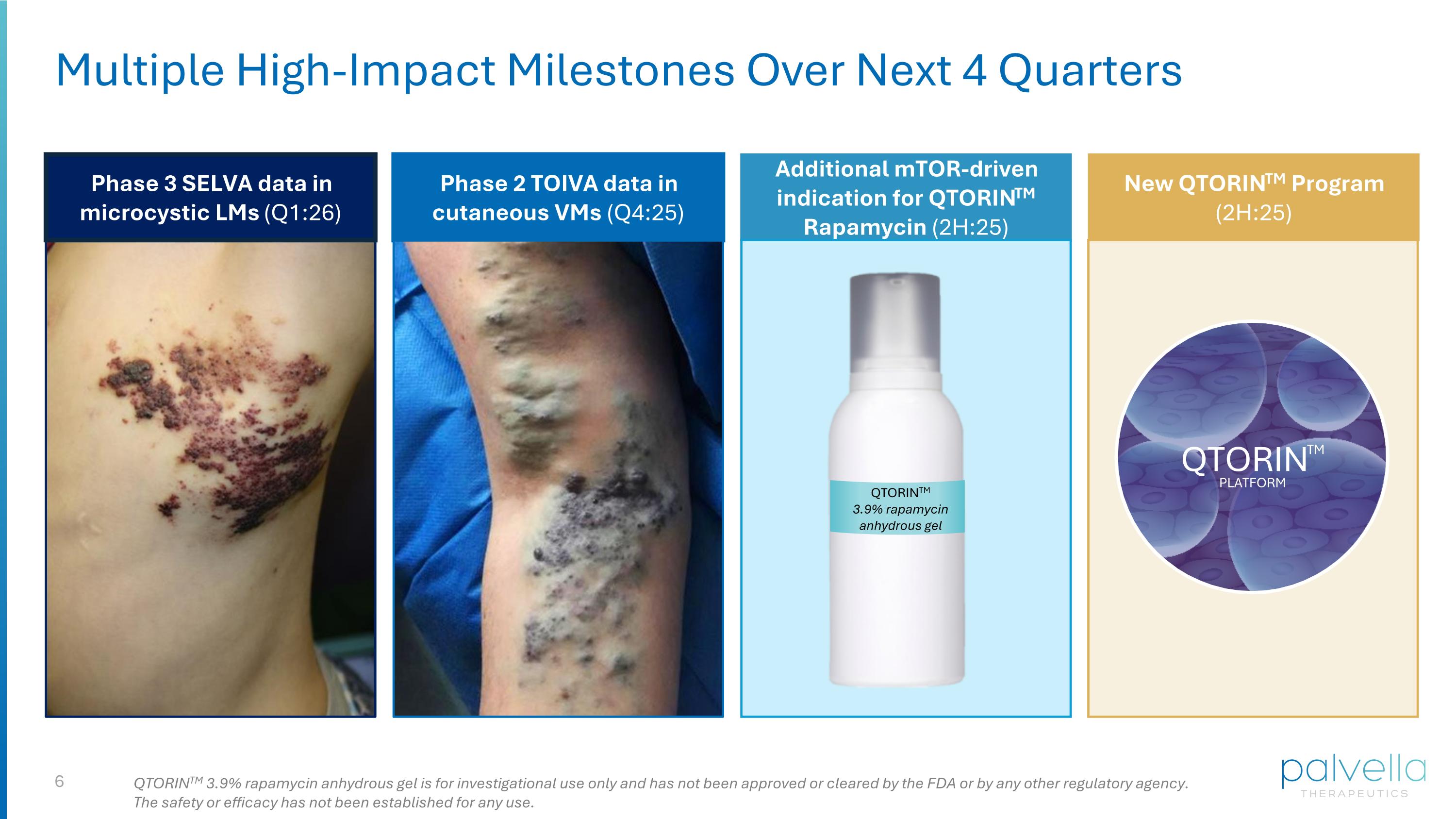
Multiple High-Impact Milestones Over Next 4 Quarters Phase 3 SELVA data in microcystic LMs (Q1:26) Phase 2 TOIVA data in cutaneous VMs (Q4:25) Additional mTOR-driven indication for QTORINTM Rapamycin (2H:25) New QTORINTM Program (2H:25) QTORINTM QTORINTM PLATFORM QTORINTM 3.9% rapamycin anhydrous gel QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use.

QTORINTM 3.9% RAPAMYCIN Microcystic Lymphatic Malformations FOR

Microcystic Lymphatic Malformations:Serious, Debilitating, and Lifelong Genetics & Pathophysiology: Somatic PIK3CA mutations cause hyperactivated PI3K/mTOR Lymphorrhea: Persistent discharge of lymphatic fluid through skin layers Deep infections: Cellulitis and other serious infections Proliferation of infiltrative lesions over time with no spontaneous resolution Leads to serious impact to quality of life and hospitalizations, with no FDA approved therapies Current options: surgeries, sclerotherapy (chemotherapy injections), laser therapy, off label oral and topical mTOR inhibitors > 30k patients ESTIMATED DIAGNOSED IN THE US1 Early onset: Present at birth and significant impact to adolescents 1. Primary prospective research conducted by Clarity Pharma and Sentero Pharma, claims analysis conducted by Trinity Life Sciences (June 2024). Includes microcystic LM and mixed LM (patients with both microcystic and macrocystic disease).
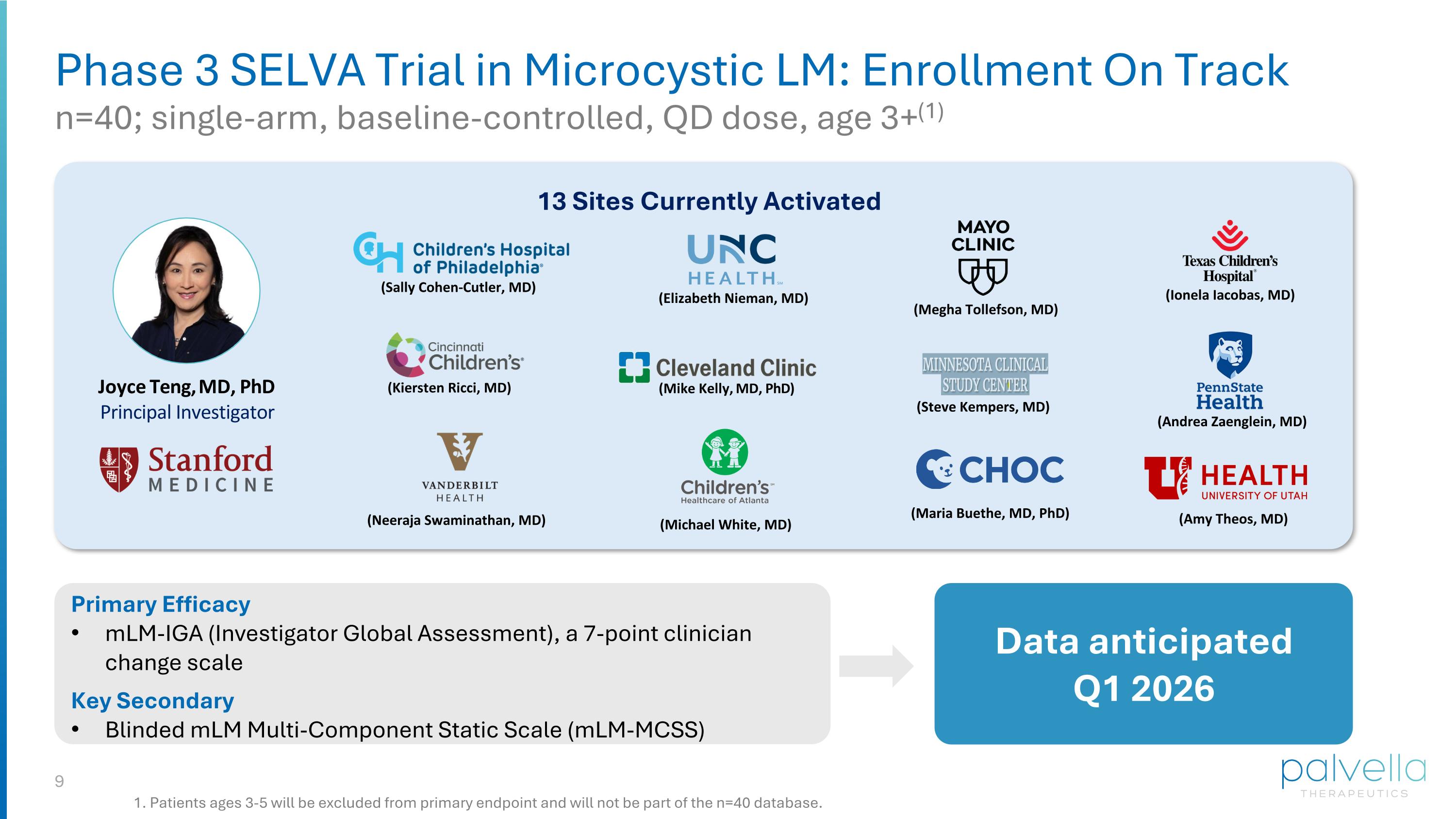
Phase 3 SELVA Trial in Microcystic LM: Enrollment On Track n=40; single-arm, baseline-controlled, QD dose, age 3+(1) (Mike Kelly, MD, PhD) (Kiersten Ricci, MD) (Megha Tollefson, MD) (Sally Cohen-Cutler, MD) (Elizabeth Nieman, MD) (Ionela Iacobas, MD) (Andrea Zaenglein, MD) (Steve Kempers, MD) Primary Efficacy mLM-IGA (Investigator Global Assessment), a 7-point clinician change scale Key Secondary Blinded mLM Multi-Component Static Scale (mLM-MCSS) Data anticipated Q1 2026 13 Sites Currently Activated Joyce Teng, MD, PhD Principal Investigator (Neeraja Swaminathan, MD) (Michael White, MD) (Maria Buethe, MD, PhD) (Amy Theos, MD) 1. Patients ages 3-5 will be excluded from primary endpoint and will not be part of the n=40 database.

* * * * * * Statistically significant across key clinician-assessed individual signs of microcystic LM at week 12 Height (p<0.0001) Leaking (p<0.005) Bleeding (p<0.05) Erythema (p<0.005) Hyperkeratosis (p<0.005) Phase 2: Clinically Meaningful, Statistically Significant Improvements Mean CGI-C: + 2.42 (p<0.0001) Clinician Global Impression of Change Patient Global Impression of Change Week 4 Week 8 Week 12 Week 4 Week 8 Week 12 Very Much Improved Much Improved Minimal Improvement No Change Minimally Worse Much Worse Very Much Worse n=12; QD dose * = p-value <0.0001

Week 12 Phase 2 Results: Visible Improvement Baseline Patient CGI-C: Very Much Improved (+3). QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use. Underarm Underarm

Week 12 Baseline Phase 2 Results: Visible Improvement Wrist Wrist Patient CGI-C: Very Much Improved (+3). QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use.
Phase 2 Study Results Published in Journal of Vascular Anomalies (JoVA) “Efficacy from this phase 2 study showed a robust clinical response as measured from both the clinicians’ and patients’ perspective. All 12 patients in the study demonstrated clinical and statistical improvements across a variety of endpoints, including remarkable visual improvement in disease symptoms from photographs of microcystic LM lesions. In addition, patient exit interviews that assessed baseline disease severity and changes in disease severity after treatment confirmed the results from this study.” QTORINTM 3.9% rapamycin anhydrous gel is for investigational use only and has not been approved or cleared by the FDA or by any other regulatory agency. The safety or efficacy has not been established for any use.

Rolling NDA submission with potential for six-month priority review planned for 2026 Regulatory Overview: NDA Submission Planned for 20261 Breakthrough Therapy Designation Fast Track Designation Orphan Drug Designation Phase 3 study in microcystic LMs ongoing; data expected in Q1 2026 Seeking full FDA approval based on clinical endpoints utilized in prospective Phase 2 and Phase 3 studies Leveraging 505(b)(2) pathway and real-world clinical evidence of rapamycin 1. NDA submission planned if Phase 3 study meets primary endpoint.

FDA Orphan Products Grant Recipient: Announced November 2024Based on scientific and technical merit as determined by rare disease and regulatory experts Regulator and Funder? FDA’s Orphan Products Grants Program awards significant funding to help move promising treatments through clinical development By Sarah Wicks & James E. Valentine “We would not expect clinical trials to be funded if there was not a meaningful degree of alignment between the FDA review division on the trial design, particularly for later stage trials. Receiving a Clinical Trials Grant provides insight that the FDA review team likely considered the proposed study as being capable of providing acceptable data that could contribute to product approval.” Out of 51 grant applications received by the FDA Orphan Products Grants Program in fiscal year 2024, Palvella’s clinical trial was one of seven new clinical trials and only Phase 3 program that was awarded a grant (up to $2.6 million)

Microcystic LMs: Large U.S. Market Opportunity *Includes microcystic LM and mixed LM (patients with both microcystic and macrocystic manifestations) Study conducted in partnership with Trinity Life Sciences Estimated microcystic/mixed US prevalence* 79.9K 1. Physician Survey published 2022 MULTIPLE APPROACHES SUPPORT > 30K DIAGNOSED PATIENTS IN U.S. 2. Claims Analysis conducted 20241 Estimated microcystic/mixed US prevalence* 38K Additional claims analysis ongoing to support commercial launch strategy (annual incidence, patient concentration in centers of excellence) 1. Claims analysis conducted by Trinity Life Sciences (June 2024) based on a lookback period of 3 years.
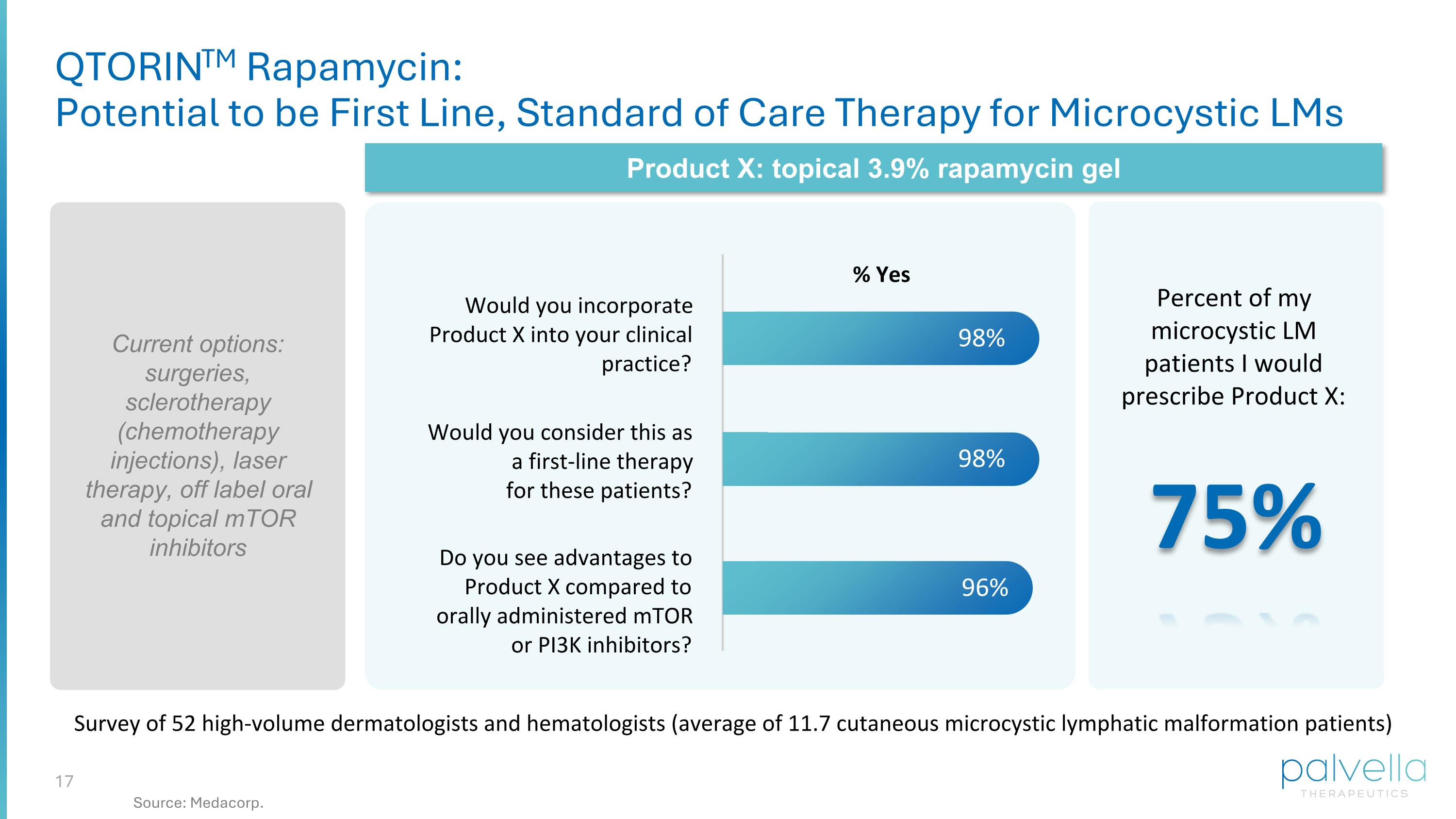
Would you incorporate Product X into your clinical practice? Would you consider this as a first-line therapy for these patients? Do you see advantages to Product X compared to orally administered mTOR or PI3K inhibitors? 98% 96% Survey of 52 high-volume dermatologists and hematologists (average of 11.7 cutaneous microcystic lymphatic malformation patients) % Yes Current options: surgeries, sclerotherapy (chemotherapy injections), laser therapy, off label oral and topical mTOR inhibitors Product X: topical 3.9% rapamycin gel Percent of my microcystic LM patients I would prescribe Product X: QTORINTM Rapamycin: Potential to be First Line, Standard of Care Therapy for Microcystic LMs 75% 98% Source: Medacorp.
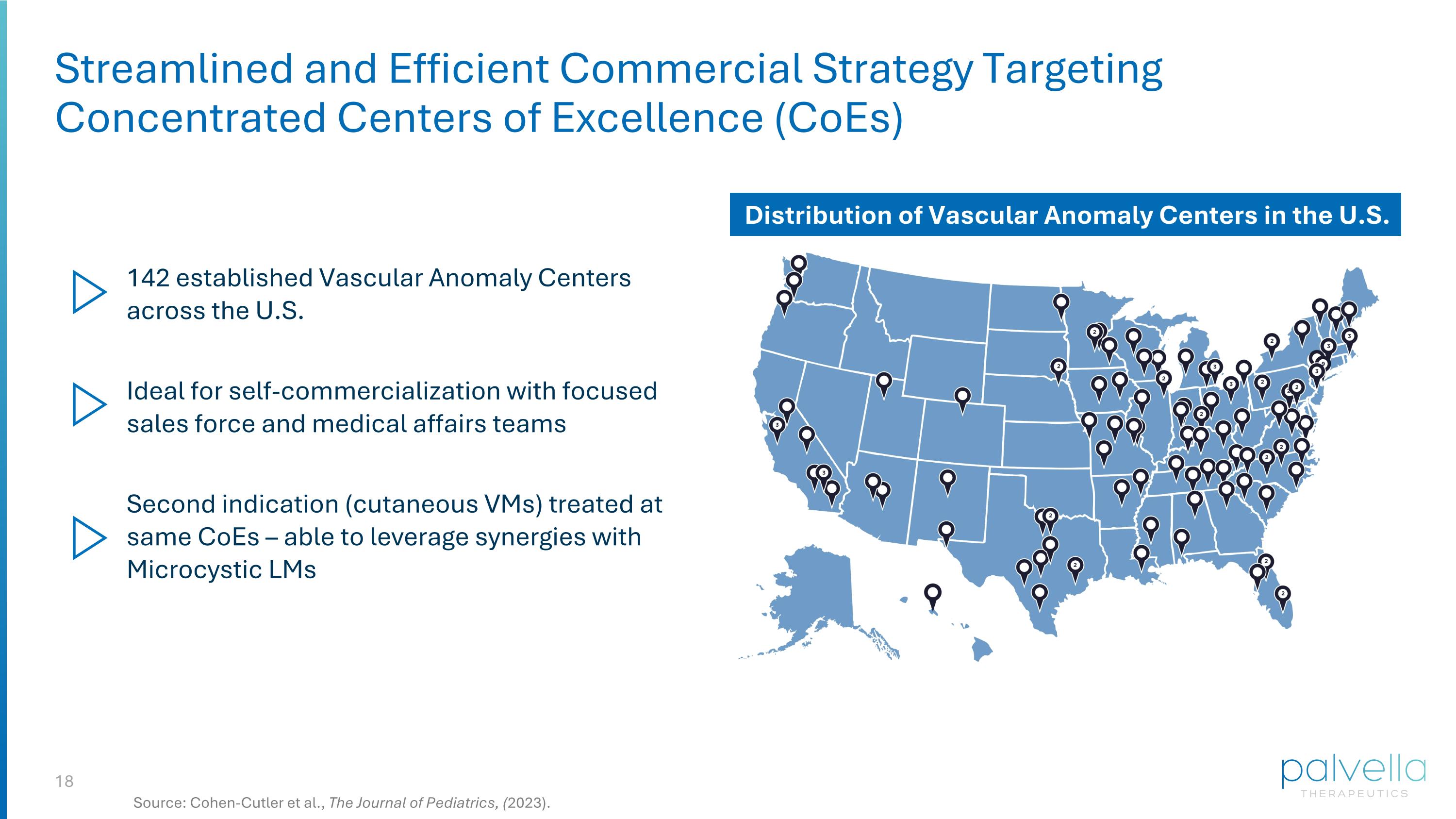
Streamlined and Efficient Commercial Strategy Targeting Concentrated Centers of Excellence (CoEs) 142 established Vascular Anomaly Centers across the U.S. Second indication (cutaneous VMs) treated at same CoEs – able to leverage synergies with Microcystic LMs Ideal for self-commercialization with focused sales force and medical affairs teams Distribution of Vascular Anomaly Centers in the U.S. Source: Cohen-Cutler et al., The Journal of Pediatrics, (2023).

QTORINTM 3.9% RAPAMYCIN Cutaneous Venous Malformations FOR

Cutaneous Venous Malformations:Serious, High Unmet Need Genetics & Pathophysiology: TIE2 or PIK3CA over-activating mutations; part of the PI3K/AKT/mTOR pathway Dysregulated growth of malformed veins impacting the skin Functional impairment Hemorrhaging Deformity as lesions continue to grow Leads to physical & functional impairment, psychological distress, with no FDA approved therapies Current options: laser treatment, off label systemic pharmacotherapies limited by toxicities > 75k patients ESTIMATED DIAGNOSED IN THE US1 1. Primary prospective research conducted by Clarity Pharma.

Rapamycin and the treatment of venous malformations Summary Targeted molecular therapies are becoming interesting additions to the management of vascular anomalies, and rapamycin is establishing itself as the gold standard for venous malformations. 1 2 “Rapamycin is the first targeted therapy that improves considerably the QoL of these patients” “Topical agents…could abolish the need for systemic treatments that have wider toxicity” Rapamycin recognized as a targeted molecular therapy for venous malformations Need for topical therapies Current unmet need for targeted, localized therapy for Cutaneous Venous Malformations VASCULAR BIOLOGY: EDITED BY M. LUISA IRUELA ARISPE Seront, Emmanuela,b; Van Damme, An b,c,; Boon, Laurence M.d,e; Vikkula, Miikkabde Real World Clinical Evidence Supporting Rapamycin’s Potential in VM Led to FDA Fast Track Designation for QTORINTM Rapamycin Summary Takeaways

“A sustained benefit of sirolimus was observed in 84% of TIE2-mutated and 83% of the PIK3CA-mutated patients.” Data Supporting Rapamycin Use in TIE2 and PIK3CA Mutations 1. Data on file. VENOUS MALFOMATION Pathophysiology Queisser 2021 TIE2 VENOUS MALFORMATION MODEL Preclinical Evidence Boscolo 2015 ORAL RAPAMYCIN VASE Clinical Study Seront 2023 20+ published papers support rapamycin’s clinical benefit in Venous Malformations (1) “Efficacy of rapamycin in this model and in patients in our clinical pilot study suggests rapamycin as the first molecular therapy for VMs.”

Cutaneous Venous Malformations Phase 2 TOIVA Study n=~15; QD dose Safety Safety and tolerability Efficacy Cutaneous venous malformation – investigators’ global assessment (7-point clinician change scale) Cutaneous venous malformation - multicomponent static scale Other clinician and patient-reported outcomes Single arm, QTORINTM rapamycin treatment (QD) Efficacy Evaluation Period 12 weeks Screening / Baseline Visit Exit Interview with participant Data anticipated Q4 2025 Study objectives: evaluate safety and tolerability (incl. determining systemic concentration of rapamycin) and evaluate efficacy across multiple endpoints (no statistical hierarchy) Entry Interview with participant Treatment Extension Period 12 weeks = photography
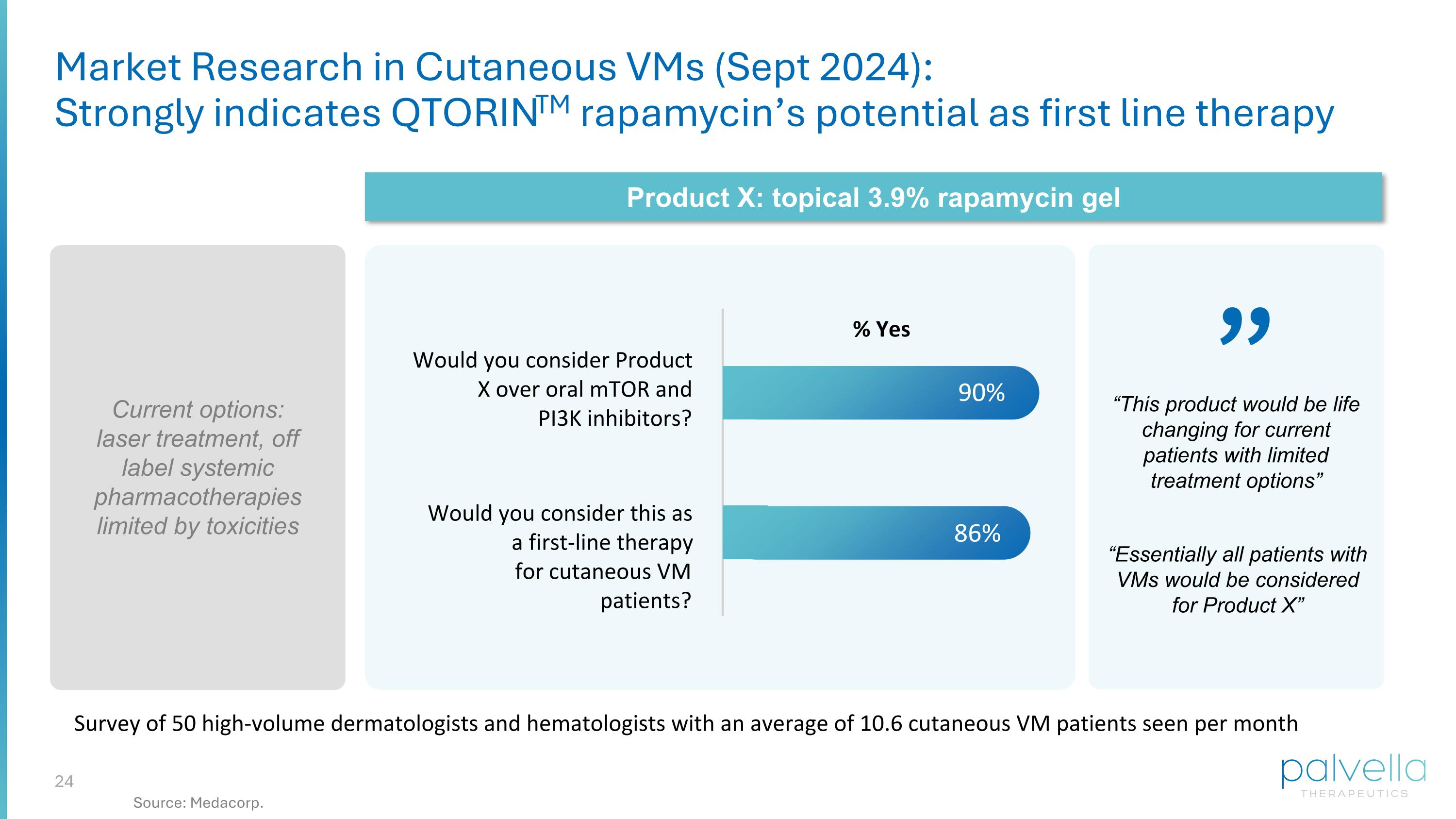
Would you consider Product X over oral mTOR and PI3K inhibitors? Would you consider this as a first-line therapy for cutaneous VM patients? 90% 86% Survey of 50 high-volume dermatologists and hematologists with an average of 10.6 cutaneous VM patients seen per month % Yes Current options: laser treatment, off label systemic pharmacotherapies limited by toxicities Product X: topical 3.9% rapamycin gel Market Research in Cutaneous VMs (Sept 2024): Strongly indicates QTORINTM rapamycin’s potential as first line therapy ” “Essentially all patients with VMs would be considered for Product X” “This product would be life changing for current patients with limited treatment options” Source: Medacorp.

Financial Overview

2024 Financial Highlights and 2025 Outlook $83.6 million Cash at 12/31/2024 2+ years Runway into 2H 2027 $14.1 million R&D + G&A spend in 2024 >$55 million Projected cash at year end

Q&A Striving to be first for rare disease patients