UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 25, 2025
Tenax Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
Delaware |
|
001-34600 |
|
26-2593535 |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
101 Glen Lennox Drive, Suite 300
Chapel Hill, North Carolina 27517
(Address of principal executive offices) (Zip Code)
919-855-2100
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, $0.0001 par value per share |
TENX |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR 230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR 240.12b-2).
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
DOCPROPERTY iManageFooter \* MERGEFORMAT 033329.0042-10011016v1
Item 2.02 |
Results of Operations and Financial Condition. |
On March 25, 2025, Tenax Therapeutics, Inc. (the “Company”) issued a press release announcing its financial results for the fourth quarter and full year ended December 31, 2024. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein in its entirety by reference.
The information in this Item 2.02 (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended or the Exchange Act, except as expressly set forth by specific reference in such a filing.
|
Item 9.01 |
Financial Statements and Exhibits. |
|
(d) |
Exhibits |
Exhibit No. |
|
Description |
|
|
|
99.1 |
|
Press release dated March 25, 2025. |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
DOCPROPERTY iManageFooter \* MERGEFORMAT 033329.0042-10011016v1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: March 25, 2025 |
Tenax Therapeutics, Inc. |
|
|
|
By: /s/ Christopher T. Giordano |
|
Christopher T. Giordano |
|
President and Chief Executive Officer |
DOCPROPERTY iManageFooter \* MERGEFORMAT 033329.0042-10011016v1
EXHIBIT 99.1

Tenax Therapeutics Reports Fourth Quarter and Full Year 2024 Financial Results and Provides Corporate Update
Successfully Completed Private Placements with Aggregate Gross Proceeds of Approximately $125 Million to Support Advancement of Two Registrational Studies for TNX-103 in PH-HFpEF and Fund Operations through 2027
Phase 3 LEVEL Study Expansion Increases Statistical Power; Enrollment Completion Targeted Around Year-End 2025, with Topline Data Expected Middle of 2026
Initiation of Second Phase 3 Study, LEVEL-2, Expected This Year
CHAPEL HILL, N.C., March 25, 2025 (GLOBE NEWSWIRE) -- Tenax Therapeutics, Inc. (Nasdaq: TENX) (“Tenax” or Tenax Therapeutics” or the “Company”), a Phase 3, development-stage pharmaceutical company using clinical insights to develop novel cardiopulmonary therapies, today reported financial results for the year ended December 31, 2024 and provided an update on its recent corporate progress.
“The past year has been transformational for Tenax Therapeutics in our quest to advance TNX-103, our oral levosimendan drug candidate, and bring meaningful clinical benefit to patients suffering from PH-HFpEF. With the continued support of investors, we are now well-positioned to expand our investment in TNX-103 and accelerate development timelines, paving the way for an earlier potential regulatory filing,” said Chris Giordano, President and Chief Executive Officer of Tenax Therapeutics. “We remain committed to a lean cost structure and responsible capital stewardship, ensuring our current funding sustains Tenax well beyond topline LEVEL data readout. We believe TNX-103 has the potential to improve the quality of life of patients living with PH-HFpEF, and expect to share topline data from LEVEL in the middle of 2026.”
Recent Corporate and Clinical Highlights
Fourth Quarter and Full Year 2024 Financial Results
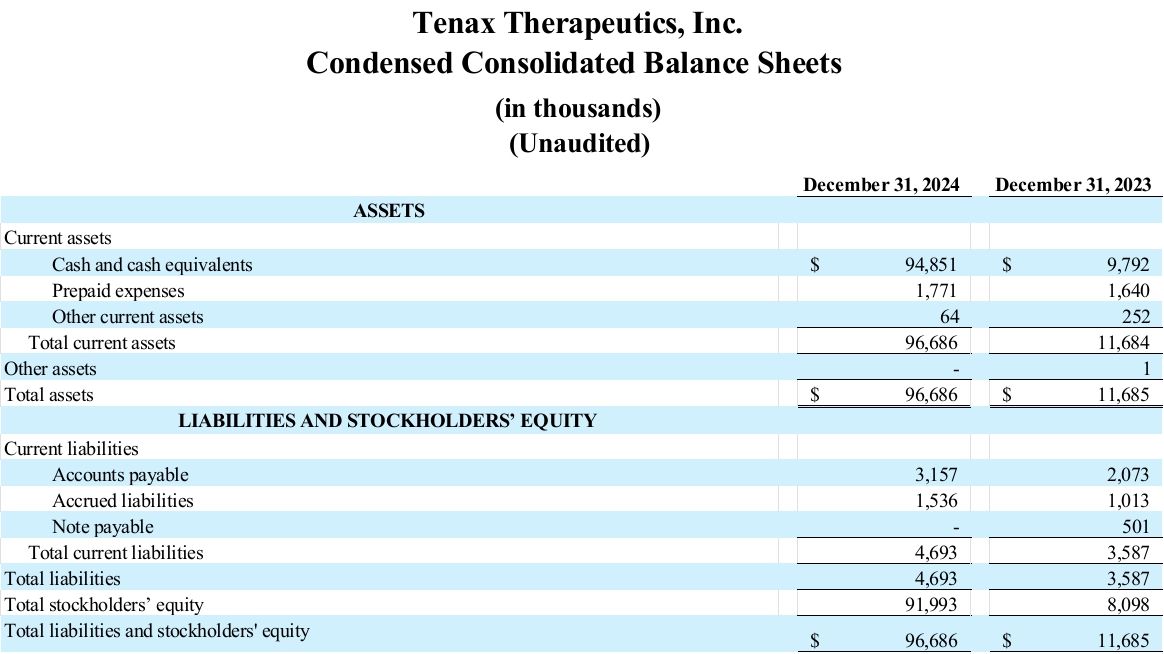
Cash position: Tenax Therapeutics reported cash and cash equivalents of $94.9 million as of December 31, 2024. In addition, in March 2025 the Company raised approximately $25 million in gross proceeds from a private placement financing. With the proceeds from the March offering, management believes that Tenax is now funded through 2027.
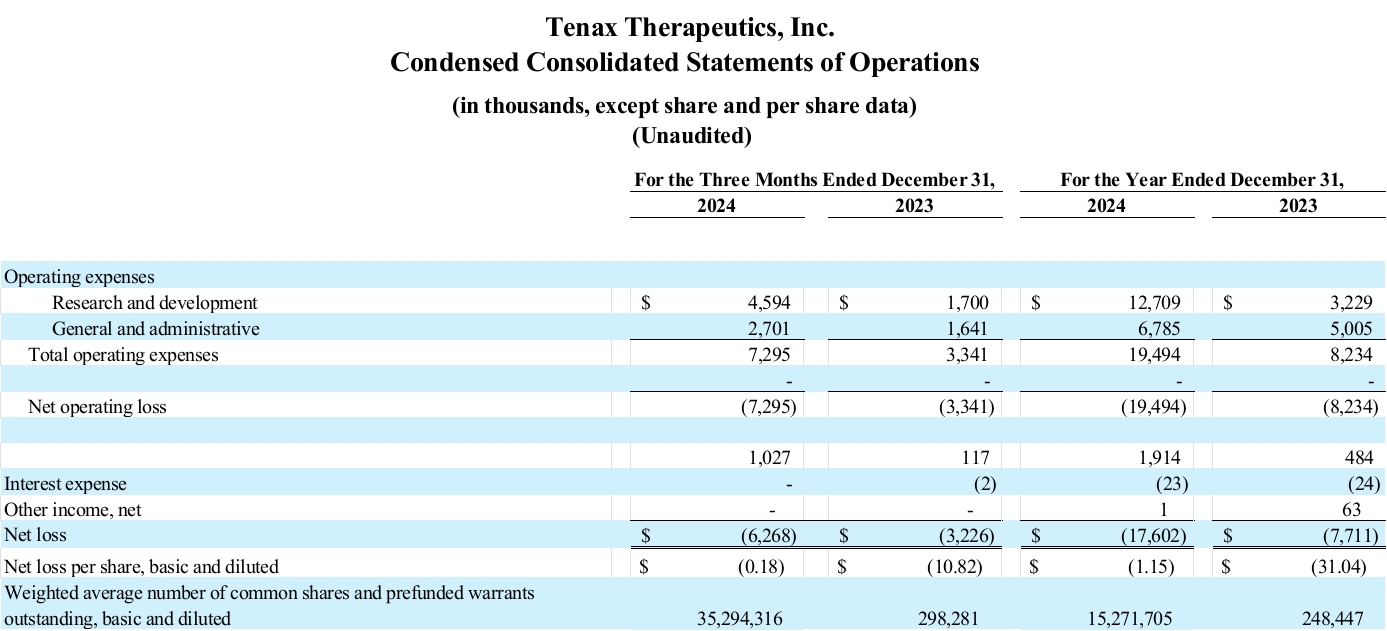
Research and development (R&D): R&D expenses for the fourth quarter of 2024 were $4.6 million, compared to $1.7 million for the fourth quarter of 2023. R&D expenses for the year ended December 31, 2024 were $12.7 million, compared to $3.2 million for the year ended December 31, 2023. The increase in both periods was primarily attributable to increased expenses associated with the Company’s Phase 3 LEVEL study for TNX-103, compared with costs for 2023, associated with the Phase 2 HELP open-label extension (OLE) study, as well as increased personnel costs (including some stock-based compensation) and regulatory consulting costs.
General and administrative (G&A): G&A expenses for the fourth quarter of 2024 were $2.7 million, compared to $1.6 million for the fourth quarter of 2023. G&A expenses for the year ended December 31, 2024 were $6.8 million, compared to $5.0 million for the year ended December 31, 2023. The increase in both periods is primarily a result of stock-based compensation from options grants made during the fourth quarter of 2024.
Net loss: Tenax Therapeutics reported a net loss of $6.3 million for the fourth quarter of 2024, compared to a net loss of $3.2 million for fourth quarter of 2023. Tenax Therapeutics reported a net loss of $17.6 million for the year ended December 31, 2024, compared to a net loss of $7.7 million for the year ended December 31, 2023.
About Levosimendan (TNX-101, TNX-102, TNX-103)
Levosimendan is a novel, first-in-class K-ATP activator/calcium sensitizer currently being evaluated to treat pulmonary hypertension (PH) with heart failure with preserved ejection fraction (PH-HFpEF). Levosimendan was first developed for intravenous use in hospitalized patients with acutely decompensated heart failure, and it has received market authorization in 60 countries in this indication, although it is not available in the United States or Canada. Tenax’s Phase 2 HELP study, including its open-label extension stage, demonstrated the potential of IV levosimendan (TNX-101) and TNX-103 to bring durable improvements in exercise capacity and quality of life, as well as other clinical assessments, in patients with PH-HFpEF. TNX-103 (oral levosimendan) is currently being evaluated in LEVEL, a Phase 3, double-blind, randomized, placebo-controlled clinical trial in patients with PH-HFpEF.
About Tenax Therapeutics
Tenax Therapeutics, Inc. is a Phase 3, development-stage pharmaceutical company using clinical insights to develop novel cardiopulmonary therapies. The Company owns global rights to develop and commercialize levosimendan, which it is developing for the treatment of PH-HFpEF, the most prevalent form of pulmonary hypertension globally, for which no product has been approved to date. For more information, visit www.tenaxthera.com. Tenax Therapeutics’ common stock is listed on The Nasdaq Stock Market LLC under the symbol “TENX”.
Caution Regarding Forward-Looking Statements
Except for historical information, all of the statements, expectations and assumptions contained in this press release are forward-looking statements. These forward-looking statements may include information concerning possible or projected future business operations. Actual results might differ materially from those explicit or implicit in the forward-looking statements. Important factors that could cause actual results to differ materially include: risks of our clinical trials, including, but not limited to, the timing, delays, costs, design, initiation, enrollment, and results of such trials; any delays in regulatory review and approval of product candidates in development; risks related to our business strategy, including the prioritization and development of product candidates; reliance on third parties, including Orion Corporation, our manufacturers and CROs; risks regarding the formulation, production, marketing, customer acceptance and clinical utility of our product candidates; our estimates regarding the potential market opportunity for our product candidates; the potential advantages of our product candidates; risks associated with our cash needs; our ability to maintain our culture and recruit, integrate and retain qualified personnel and advisors, including on our Board of Directors; our competitive position; intellectual property risks; volatility and uncertainty in the global economy and financial markets in light of the possibility of pandemics, global financial and geopolitical uncertainties, including in the Middle East and the Russian invasion of and war against the country of Ukraine; changes in legal, regulatory and legislative environments in the markets in which we operate and the impact of these changes on our ability to obtain regulatory approval for our products; and other risks and uncertainties set forth from time to time in our SEC filings. Tenax Therapeutics assumes no obligation and does not intend to update these forward-looking statements except as required by law.
Contact:
Investor and Media:
Merrill Barrett
Argot Partners
tenax@argotpartners.com