ROC
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-Q
(Mark One)
☒ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2024
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from |
|
to |
|
|
Commission File Number: 001-41827
MEI Pharma, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
DELAWARE |
|
51-0407811 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
9920 Pacific Heights Blvd., Suite 150, San Diego, CA 92121
(Address of principal executive offices) (Zip Code)
(858) 369-7100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.00000002 par value |
|
MEIP |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
Emerging growth company |
|
☐ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 8, 2024, the number of shares outstanding of the issuer’s common stock, $0.00000002 par value, was 6,662,857.
MEI PHARMA, INC.
Table of Contents
|
|
|
|
|
|
|
|
|
|
|
Page |
|
|
|
|
|
||||
PART I |
|
|
|
3 |
|
|
|
|
|
||||
Item 1. |
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
Condensed Consolidated Balance Sheets as of September 30, 2024 and June 30, 2024 (Unaudited) |
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
6 |
|
|
|
|
|
|
|
|
|
|
|
Notes to Condensed Consolidated Financial Statements (Unaudited) |
|
|
7 |
|
|
|
|
|
|
|
|
Item 2. |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
|
19 |
|
|
|
|
|
|
|
|
Item 3. |
|
|
|
27 |
|
|
|
|
|
|
|
|
|
Item 4. |
|
|
|
28 |
|
|
|
|
|
||||
PART II |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 1. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 1A. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 2. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 3. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 4. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 5. |
|
|
|
29 |
|
|
|
|
|
|
|
|
|
Item 6. |
|
|
|
30 |
|
|
|
|
|||||
|
|
32 |
|
|||
2
PART I FINANCIAL INFORMATION
Item 1. Condensed Consolidated Financial Statements
MEI PHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(In thousands, except par value data)
|
|
September 30, |
|
|
June 30, |
|
||
|
|
2024 |
|
|
2024 |
|
||
|
|
(Unaudited) |
|
|
|
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
6,952 |
|
|
$ |
3,705 |
|
Short-term investments |
|
|
19,953 |
|
|
|
34,640 |
|
Prepaid expenses and other current assets |
|
|
840 |
|
|
|
2,424 |
|
Total current assets |
|
|
27,745 |
|
|
|
40,769 |
|
Operating lease right-of-use asset |
|
|
— |
|
|
|
214 |
|
Property and equipment, net |
|
|
5 |
|
|
|
392 |
|
Total assets |
|
$ |
27,750 |
|
|
$ |
41,375 |
|
|
|
|
|
|
|
|
||
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
976 |
|
|
$ |
3,168 |
|
Accrued liabilities |
|
|
1,896 |
|
|
|
5,187 |
|
Total current liabilities |
|
|
2,872 |
|
|
|
8,355 |
|
Total liabilities |
|
|
2,872 |
|
|
|
8,355 |
|
|
|
|
|
|
|
|
||
Commitments and contingencies (Note 6) |
|
|
|
|
|
|
||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $0.01 par value; 100 shares authorized; none outstanding |
|
|
— |
|
|
|
— |
|
Common stock, $0.00000002 par value; 226,000 shares authorized; 6,663 |
|
|
— |
|
|
|
— |
|
Additional paid-in capital |
|
|
421,104 |
|
|
|
421,239 |
|
Accumulated deficit |
|
|
(396,226 |
) |
|
|
(388,219 |
) |
Total stockholders’ equity |
|
|
24,878 |
|
|
|
33,020 |
|
Total liabilities and stockholders’ equity |
|
$ |
27,750 |
|
|
$ |
41,375 |
|
See accompanying notes to condensed consolidated financial statements.
3
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
(Unaudited)
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Revenues: |
|
|
|
|
|
|
||
Revenue from customers |
|
$ |
— |
|
|
$ |
752 |
|
Revenue from collaboration agreements |
|
|
— |
|
|
|
64,545 |
|
Total revenues |
|
|
— |
|
|
|
65,297 |
|
|
|
|
|
|
|
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
|
3,163 |
|
|
|
3,485 |
|
General and administrative |
|
|
5,189 |
|
|
|
6,531 |
|
Total operating expenses |
|
|
8,352 |
|
|
|
10,016 |
|
(Loss) income from operations |
|
|
(8,352 |
) |
|
|
55,281 |
|
Other income (expense): |
|
|
|
|
|
|
||
Interest and dividend income |
|
|
355 |
|
|
|
1,094 |
|
Other expense, net |
|
|
(10 |
) |
|
|
(1 |
) |
Total other income, net |
|
|
345 |
|
|
|
1,093 |
|
Net (loss) income |
|
$ |
(8,007 |
) |
|
$ |
56,374 |
|
|
|
|
|
|
|
|
||
Net (loss) income per share - basic and diluted |
|
$ |
(1.20 |
) |
|
$ |
8.46 |
|
|
|
|
|
|
|
|
||
Weighted-average shares used in computing net (loss) income |
|
|
6,663 |
|
|
|
6,663 |
|
See accompanying notes to condensed consolidated financial statements.
4
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(In thousands)
(Unaudited)
|
|
Common |
|
|
Additional |
|
|
Accumulated |
|
|
Total |
|
||||
Balance at June 30, 2024 |
|
|
6,663 |
|
|
$ |
421,239 |
|
|
$ |
(388,219 |
) |
|
$ |
33,020 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
(8,007 |
) |
|
|
(8,007 |
) |
Share-based compensation |
|
|
— |
|
|
|
(135 |
) |
|
|
— |
|
|
|
(135 |
) |
Balance at September 30, 2024 |
|
|
6,663 |
|
|
$ |
421,104 |
|
|
$ |
(396,226 |
) |
|
$ |
24,878 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Common |
|
|
Additional |
|
|
Accumulated |
|
|
Total |
|
||||
Balance at June 30, 2023 |
|
|
6,663 |
|
|
$ |
430,621 |
|
|
$ |
(405,997 |
) |
|
$ |
24,624 |
|
Net income |
|
|
— |
|
|
|
— |
|
|
|
56,374 |
|
|
|
56,374 |
|
Share-based compensation |
|
|
— |
|
|
|
363 |
|
|
|
— |
|
|
|
363 |
|
Balance at September 30, 2023 |
|
|
6,663 |
|
|
$ |
430,984 |
|
|
$ |
(349,623 |
) |
|
$ |
81,361 |
|
See accompanying notes to condensed consolidated financial statements.
5
MEI PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
(Unaudited)
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net (loss) income |
|
$ |
(8,007 |
) |
|
$ |
56,374 |
|
Adjustments to reconcile net (loss) income to net cash used in operating activities: |
|
|
|
|
|
|
||
Share-based compensation |
|
|
(135 |
) |
|
|
363 |
|
Noncash lease expense |
|
|
214 |
|
|
|
372 |
|
Depreciation expense |
|
|
368 |
|
|
|
87 |
|
Loss on disposal of property and equipment |
|
|
9 |
|
|
|
— |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Unbilled receivables |
|
|
— |
|
|
|
85 |
|
Prepaid expenses and other current assets |
|
|
1,584 |
|
|
|
530 |
|
Accounts payable |
|
|
(2,192 |
) |
|
|
(2,921 |
) |
Accrued liabilities |
|
|
(3,291 |
) |
|
|
(8,172 |
) |
Deferred revenue |
|
|
— |
|
|
|
(64,862 |
) |
Operating lease liability |
|
|
— |
|
|
|
(347 |
) |
Net cash used in operating activities |
|
|
(11,450 |
) |
|
|
(18,491 |
) |
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchases of short-term investments |
|
|
— |
|
|
|
(24,197 |
) |
Proceeds from maturity of short-term investments |
|
|
14,687 |
|
|
|
29,154 |
|
Proceeds from sale of property and equipment |
|
|
10 |
|
|
|
— |
|
Net cash provided by investing activities |
|
|
14,697 |
|
|
|
4,957 |
|
|
|
|
|
|
|
|
||
Net increase (decrease) in cash and cash equivalents |
|
|
3,247 |
|
|
|
(13,534 |
) |
Cash and cash equivalents at beginning of the period |
|
|
3,705 |
|
|
|
16,906 |
|
Cash and cash equivalents at end of the period |
|
$ |
6,952 |
|
|
$ |
3,372 |
|
|
|
|
|
|
|
|
||
Noncash investing activities: |
|
|
|
|
|
|
||
Purchases of property and equipment included in accounts payable |
|
$ |
— |
|
|
$ |
7 |
|
See accompanying notes to condensed consolidated financial statements.
6
MEI PHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
1. Description of Business and Basis of Presentation
Description of Business
MEI Pharma, Inc. (Nasdaq: MEIP) is a pharmaceutical company that has been developing novel and differentiated cancer therapies. We built our pipeline by acquiring promising cancer agents and creating value in programs through development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development has been to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. Our pipeline includes voruciclib, an oral cyclin-dependent kinase 9 (CDK9) inhibitor, and prior to its sale (as discussed below), ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway.
Strategic Alternatives
On July 22, 2024, we announced that our Board of Directors (Board) had determined unanimously to begin the evaluation of our strategic alternatives, including potential transactions as well as an orderly wind down of operations, if appropriate, to maximize the value of our assets for our stockholders. We commenced a reduction-in-force (the 2024 RIF) beginning August 1, 2024, which will continue in stages as our operational and strategic direction evolves. We have discontinued the clinical development of voruciclib, while certain nonclinical activities related to MEI’s drug candidate assets will continue to be conducted by us. As part of the review of strategic alternatives, we may consider options such as out-licensing opportunities for existing programs and merger and acquisition opportunities.
Consistent with our intention to preserve cash, David M. Urso, our President and Chief Executive Officer, and Richard Ghalie, M.D., our Chief Medical Officer, stepped down effective August 1, 2024. Mr. Urso also left the Board at that date. We have entered into consulting agreements with both Mr. Urso and Dr. Ghalie under which they will remain available to assist us in our strategic efforts. Charles V. Baltic III, the Chairperson of the Board, also stepped down from the Board contemporaneously with the announcement on July 22, 2024. Our Board appointed Justin J. File, our current Chief Financial Officer, to assume the position of Acting Chief Executive Officer and has appointed Frederick W. Driscoll as Chairperson of the Board.
Basis of Presentation and Consolidation
The condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the U.S. (U.S. GAAP) for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X. Accordingly, the accompanying financial statements do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, the accompanying condensed consolidated financial statements reflect all adjustments (consisting of normal recurring adjustments) that are necessary for a fair statement of the financial position, results of operations and cash flows for the periods presented.
The accompanying unaudited condensed consolidated financial statements include the accounts of MEI Pharma, Inc. and our wholly owned subsidiary, Meadow Merger Sub, Inc. We have eliminated all intercompany accounts and transactions in consolidation.
The accompanying unaudited condensed consolidated financial statements for the quarterly period ended September 30, 2024 should be read in conjunction with the audited financial statements and notes thereto as of and for the fiscal year ended June 30, 2024, included in our Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on September 19, 2024 (2024 Annual Report). Interim results are not necessarily indicative of results for a full year.
The Company has evaluated subsequent events through the date the condensed consolidated financial statements were issued.
Liquidity
To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. We have accumulated losses of $396.2 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2024, we had $26.9 million in cash and cash equivalents and short-term investments. In connection with our July 2024 announcement regarding the evaluation of our strategic alternatives, we discontinued the clinical development of voruciclib, while certain nonclinical research and development activities continued. As a result, we continue to incur research and development expenses in connection with clinical trial closing costs and the completion of our nonclinical projects. We believe that our cash balance, including our short-term investments, will be sufficient to meet our obligations and fund operations for at least the next 12 months from the issuance of these condensed consolidated financial statements.
7
2. Summary of Significant Accounting Policies
There have been no material changes to our significant accounting policies from those described in the notes to our audited consolidated financial statements contained in the 2024 Annual Report.
Risks and Uncertainties
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates, including those related to the valuation of share-based awards, the discount rate used in estimating the present value of the right-of-use assets and lease liabilities, the useful lives of property and equipment, the recoverability of long-lived assets, clinical trial accruals, periods over which revenue should be recognized, relative stand-alone selling price, deferred income taxes and related valuation allowances, and the assessment of our ability to fund our operations for at least the next 12 months from the date of issuance of these condensed consolidated financial statements. We base our estimates on historical experience and other market-specific or other relevant assumptions that we believe to be reasonable under the circumstances. Estimates are assessed each reporting period and updated to reflect current information. As future events and their effects cannot be determined with precision, actual results may materially differ from those estimates or assumptions.
Short-term Investments
Short-term investments are marketable securities with maturities greater than three months but less than one year from date of purchase. As of September 30, 2024 and June 30, 2024, our short-term investments consisted of $20.0 million and $34.6 million, respectively, in United States government securities. The short-term investments held as of September 30, 2024 and June 30, 2024 are considered to be held to maturity and are carried at amortized cost. As of September 30, 2024 and June 30, 2024, gross unrealized gains and losses were immaterial.
Revenue Recognition
Revenues from Customers
In accordance with ASC Topic 606, Revenue from Contracts with Customers (Topic 606), we recognized revenue when control of the promised goods or services was transferred to our customers, in an amount that reflects the consideration we expected to be entitled to in exchange for those goods or services. For enforceable contracts with our customers, we first identified the distinct performance obligations – or accounting units – within the contract. Performance obligations are commitments in a contract to transfer a distinct good or service to the customer.
Payments received under commercial arrangements, such as licensing technology rights, may include non-refundable fees at the inception of the arrangements, milestone payments for specific achievements designated in the agreements, and royalties on the sale of products. At the inception of arrangements that include milestone payments, we used judgment to evaluate whether the milestones were probable of being achieved, and we estimated the amount, if any, to include in the transaction price using the most likely method. If it were probable that a significant revenue reversal would not occur, the estimated amount was included in the transaction price. Milestone payments that were not within our or the licensee’s control, such as regulatory approvals, were not included in the transaction price until those approvals were received. At the end of each reporting period, we re-evaluated the probability of achievement of development milestones and any related constraint and, as necessary, we adjusted our estimate of the overall transaction price.
To the extent a contract included multiple promised deliverables, we applied judgment to determine whether promised deliverables were capable of being distinct and were distinct within the context of the contract. If these criteria were not met, the promised deliverables were accounted for as a combined performance obligation. For arrangements with multiple distinct performance obligations, we allocated variable consideration related to our 50-50 cost share for development services directly to the associated performance obligation and then allocated the remaining consideration among the performance obligations based on their relative stand-alone selling price.
When not directly observable, we typically estimated the stand-alone selling price for each distinct performance obligation. Variable consideration that related specifically to our efforts to satisfy specific performance obligations was allocated entirely to those performance obligations. Other components of the transaction price were allocated based on the relative stand-alone selling price, over which management has applied significant judgment. We developed assumptions that required judgment to determine the stand-alone selling price for license-related performance obligations, which may have included forecasted revenues, development timelines, reimbursement rates for personnel costs, discount rates and probabilities of technical, regulatory and commercial success. We estimated stand-alone selling price for research and development performance obligations by forecasting the expected costs of satisfying a performance obligation plus an appropriate margin.
8
In the case of a license that is a distinct performance obligation, we recognized revenue allocated to the license from non-refundable, up-front fees at the point in time when the license was transferred to the licensee and the licensee can use and benefit from the license. For licenses that are bundled with other distinct or combined obligations, we used judgment to assess the nature of the performance obligation to determine whether the performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. If the performance obligation is satisfied over time, we evaluated the measure of progress in each reporting period and, if necessary, adjusted the measure of performance and related revenue recognition.
The selection of the method to measure progress towards completion requires judgment and is based on the nature of the products or services to be provided. Revenue is recorded proportionally as costs are incurred. We generally used the cost-to-cost measure of progress because it best depicted the transfer of control to the customer which occurred as we incurred costs. Under the cost-to-cost measure of progress, the extent of progress towards completion was measured based on the ratio of costs incurred to date to the total estimated costs at completion of the performance obligation (an input method under Topic 606). We used judgment to estimate the total cost expected to complete the research and development performance obligations, which included subcontractors’ costs, labor, materials, other direct costs and an allocation of indirect costs. We evaluated these cost estimates and the progress each reporting period and, as necessary, we adjusted the measure of progress and related revenue recognition.
In connection with our, now terminated, April 2020 License, Development and Commercialization Agreement with Kyowa Kirin Co., Ltd. (KKC) (the KKC Commercialization Agreement) described in Note 7. License Agreements, we performed development services related to our 50-50 cost sharing arrangement for which revenue was recognized over time. Additionally, we performed services for KKC at their request, the costs of which were fully reimbursed to us. We recorded the reimbursement for such pass through services as revenue at 100% of reimbursed costs, as control of the additional services for KKC was transferred at the time we incurred such costs. The costs of these services were recognized in the condensed consolidated statements of operations as research and development expense.
During the three months ended September 30, 2024, we did not recognize any revenue associated with the KKC Commercialization Agreement. During the three months ended September 30, 2023, we recognized revenue associated with the KKC Commercialization Agreement as follows (in thousands):
Timing of Revenue Recognition: |
|
|
|
|
Services performed over time |
|
$ |
743 |
|
Pass through services at a point in time |
|
|
9 |
|
|
|
$ |
752 |
|
Contract Balances
Contract liabilities were included in deferred revenue and deferred revenue, long-term in our condensed consolidated balance sheets. Our contract liabilities accounted for under Topic 606 related to the amount of initial upfront consideration allocated to the development services performance obligations.
As of June 30, 2024 and September 30, 2024, we had no accounts receivable, unbilled receivable balances or contract liabilities. A reconciliation of the beginning and ending amount of contract liabilities as of June 30, 2024, which was primarily related to the combined performance obligation for the transfer of development services under the KKC Commercialization Agreement and was a separate performance obligation in our contracts pursuant to research plans under the agreements, was as follows:
|
|
June 30, 2024 |
|
|
Beginning balance |
|
$ |
317 |
|
Recognized as revenue: |
|
|
|
|
Revenue recognized upon satisfaction of performance obligations |
|
|
(317 |
) |
Ending balance |
|
$ |
— |
|
The timing of revenue recognition, invoicing and cash collections results in billed accounts receivable and unbilled receivables (contract assets) and deferred revenue (contract liabilities). We invoiced our customers in accordance with agreed-upon contractual terms, typically at periodic intervals or upon achievement of contractual milestones. Invoicing may have occurred subsequent to revenue recognition, resulting in unbilled receivables. Advanced payments from our customers before revenue was recognized resulted in contract liabilities.
9
Revenues from Collaborators
At contract inception, we assessed whether the collaboration arrangements were within the scope of ASC Topic 808 Collaborative Agreements (Topic 808), to determine whether such arrangements involved joint operating activities performed by parties that were both active participants in the activities and exposed to significant risks and rewards dependent on the commercial success of such activities. This assessment was performed based on the responsibilities of all parties in the arrangement. For collaboration arrangements within the scope of Topic 808 that contain multiple units of account, we first determined which units of account within the arrangement were within the scope of Topic 808 and which elements were within the scope of Topic 606. For units of account within collaboration arrangements that were accounted for pursuant to Topic 808, an appropriate recognition method was determined and applied consistently, by analogy to authoritative accounting literature. For elements of collaboration arrangements that were accounted for pursuant to Topic 606, we recognized revenue as discussed above. Consideration received that did not meet the requirements to satisfy Topic 606 revenue recognition criteria was recorded as deferred revenue and classified as either current or long-term deferred revenue based on our best estimate of when such amounts would be recognized.
Net (Loss) Income Per Share
Basic and diluted net (loss) income per share is computed using the weighted-average number of shares of common stock outstanding during the period, less any shares subject to repurchase or forfeiture. There were no shares of common stock subject to repurchase or forfeiture for the three months ended September 30, 2024 and 2023. Diluted net (loss) income per share is computed based on the sum of the weighted-average number of common shares and potentially dilutive common shares outstanding during the period determined using the treasury-stock and if-converted methods.
For purposes of the diluted net (loss) income per share calculation for the three months ended September 30, 2024 and 2023, potentially dilutive securities are excluded from the calculation of diluted net income per share because their weighted-average exercise prices were above our weighted-average share price as of September 30, 2024 and 2023, respectively, therefore, basic and diluted net loss per share were the same for the three months ended September 30, 2024 and 2023.
The following table presents potentially dilutive shares that have been excluded from the calculation of net (loss) income per share because of their anti-dilutive effect (in thousands):
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Stock options |
|
|
1,124 |
|
|
|
1,447 |
|
Warrants |
|
|
103 |
|
|
|
103 |
|
Total anti-dilutive shares |
|
|
1,227 |
|
|
|
1,550 |
|
Recent Accounting Pronouncement
Recently Issued
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board (FASB) or other standards setting bodies that are adopted as of the specified effective date. The Company believes the impact of recently issued standards and any issued but not yet effective standards will not have a material impact on its condensed consolidated financial statements upon adoption.
In November 2023, the FASB issued Accounting Standard Update (ASU) No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires a public entity to disclose significant segment expenses and other segment items on an annual and interim basis and provide in interim periods all disclosures about a reportable segment’s profit or loss and assets that are currently required annually. Additionally, it requires a public entity to disclose the title and position of the Chief Operating Decision Maker. This ASU does not change how a public entity identifies its operating segments, aggregates them, or applies the quantitative thresholds to determine its reportable segments. The new standard is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. A public entity should apply the amendments in this ASU retrospectively to all prior periods presented in the financial statements. We expect this ASU to only impact our disclosures with no impacts to our results of operations, cash flows and financial condition.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which focuses on the rate reconciliation and income taxes paid. ASU No. 2023-09 requires a public business entity (PBE) to disclose, on an annual basis, a tabular rate reconciliation using both percentages and currency amounts, broken out into specified categories with certain reconciling items further broken out by nature and jurisdiction to the extent those items exceed a specified threshold. In addition, all entities are required to disclose income taxes paid, net of refunds received disaggregated by federal, state/local, and foreign and by jurisdiction if the amount is at least 5% of total income tax payments, net of refunds received.
10
For PBEs, the new standard is effective for annual periods beginning after December 15, 2024, with early adoption permitted. An entity may apply the amendments in this ASU prospectively by providing the revised disclosures for the period ending December 31, 2025 and continuing to provide the pre-ASU disclosures for the prior periods, or may apply the amendments retrospectively by providing the revised disclosures for all periods presented. We expect this ASU to only impact our disclosures with no impacts to our results of operations, cash flows, and financial condition.
In November 2024, the FASB issued ASU No. 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40). The amendments in this update require disclosure, in the notes to the financial statements, of specific expense categories present within expense captions presented on the face of the income statement within continuing operations of PBE's. The amendments in this update are effective for annual periods beginning after December 15, 2026 and interim periods beginning after December 15, 2027. Early adoption is permitted. The amendments should be applied either prospectively to financial statements issued for reporting periods after the effective date of this ASU or retrospectively to any and all prior periods presented in the financial statements. We are currently evaluating this ASU to determine its impact on our disclosures.
3. Balance Sheet Details
Prepaid and Other Current Assets
Prepaid and other current assets consisted of the following, in thousands:
|
|
September 30, 2024 |
|
|
June 30, 2024 |
|
||
Prepaid clinical costs |
|
$ |
— |
|
|
$ |
1,050 |
|
Software licenses |
|
|
329 |
|
|
|
442 |
|
Security deposit |
|
|
263 |
|
|
|
263 |
|
Other |
|
|
248 |
|
|
|
669 |
|
Total prepaid and other current assets |
|
$ |
840 |
|
|
$ |
2,424 |
|
Property and Equipment
Property and equipment consisted of the following, in thousands:
|
|
September 30, 2024 |
|
|
June 30, 2024 |
|
||
Furniture and fixtures |
|
$ |
— |
|
|
$ |
1,000 |
|
Equipment |
|
|
7 |
|
|
|
187 |
|
Leasehold improvements |
|
|
— |
|
|
|
969 |
|
|
|
|
7 |
|
|
|
2,156 |
|
Less: accumulated depreciation (1) |
|
|
(2 |
) |
|
|
(1,764 |
) |
Property and equipment, net |
|
$ |
5 |
|
|
$ |
392 |
|
_____________
(1) Includes impairment charge of $0.5 million during fiscal year 2024, see below discussion on Impact of the Agreement (as discussed in Note 9. Leases).
Depreciation expense of property and equipment for the three months ended September 30, 2024 and 2023 are presented in the condensed consolidated statements of cash flows.
Impact of the Agreement (as discussed in Note 9. Leases)
As noted in Note 9. Leases, we agreed to sell our furniture and fixtures to the landlord for $1.00 on our lease termination date of September 30, 2024. We completed an evaluation of the impact of the Agreement, as defined in Note 9. Leases, on the carrying value of our property and equipment (Other Long-Lived Assets). This process included evaluating the remaining estimated useful lives, significant changes in the use and potential impairment charges related to the Other Long-Lived Assets. Based upon our evaluation, we recorded an impairment charge during our fiscal year ended June 30, 2024 of approximately $0.5 million for the furniture and fixtures sold to the landlord on September 30, 2024, which was included in the impairment of long-lived assets in the condensed consolidated statements of operations for the fiscal year ended June 30, 2024. We also changed our estimate of the remaining useful lives of our leasehold improvements resulting in an acceleration of depreciation of approximately $0.1 million during our fiscal year ended June 30, 2024.
11
Accrued Liabilities
Accrued liabilities consisted of the following, in thousands:
|
|
|
|
|
|
|
||
|
|
September 30, 2024 |
|
|
June 30, 2024 |
|
||
Accrued pre-clinical and clinical trial expenses |
|
$ |
247 |
|
|
$ |
1,407 |
|
Accrued compensation and benefits(1) |
|
|
1,393 |
|
|
|
2,821 |
|
Accrued legal and professional services |
|
|
201 |
|
|
|
33 |
|
Accrued reimbursement to KKC |
|
|
— |
|
|
|
892 |
|
Other |
|
|
55 |
|
|
|
34 |
|
Total accrued liabilities |
|
$ |
1,896 |
|
|
$ |
5,187 |
|
(1) Includes employee termination benefits of approximately $1.0 million and $21,000 as of September 30, 2024 and June 30, 2024, respectively, as more fully described in Note 5. Employee Termination Benefits.
4. Fair Value Measurements
The carrying amounts of financial instruments such as cash equivalents, short-term investments and accounts payable approximate the related fair values due to the short-term maturities of these instruments. We invest our excess cash in financial instruments which are readily convertible into cash, such as money market funds and U.S. government securities. Cash equivalents and short-term investments are classified as Level 1 as defined by the fair value hierarchy. As of September 30, 2024 and June 30, 2024, we had no assets or liabilities measured on a recurring or non-recurring basis.
5. Employee Termination Benefits
In connection with our joint decision to discontinue development of zandelisib outside of Japan in December 2022, we announced a realignment of our clinical development efforts that streamlined our organization towards the continued clinical development of our two earlier clinical-stage assets, voruciclib and ME-344. As a result, our Board approved a staggered workforce reduction (the 2023 RIF), which was completed during fiscal year 2024.
In August 2024, in connection with our Strategic Alternatives announcement described in Note 1. Description of Business and Basis of Presentation, we commenced the 2024 RIF. Including contractual pro-rata fiscal year 2025 bonuses, we expect to incur charges not to exceed a total of $6.0 million in retention, severance and COBRA costs related to the termination of our employees due to our related wind down activities. The charges that we expect to incur in connection with the 2024 RIF are subject to a number of assumptions, and actual results may differ materially. We may also incur additional costs not currently contemplated due to events that may occur as a result of, or that are associated with, the 2024 RIF.
For the three months ended September 30, 2024, we recorded employee termination benefits of $1.3 million and $1.8 million within research and development and general and administrative expense, respectively. For the three months ended September 30, 2023, we recorded additional employee termination benefits of $28,000 within research and development expense.
The following table summarizes our activity related to employee benefits included in accrued liabilities (in thousands):
|
|
September 30, 2024 |
|
|
June 30, 2024 |
|
||
Beginning balance |
|
$ |
21 |
|
|
$ |
993 |
|
Increase in accrued restructuring |
|
|
3,137 |
|
|
|
556 |
|
Cash payments |
|
|
(2,203 |
) |
|
|
(1,528 |
) |
Ending Balance |
|
$ |
955 |
|
|
$ |
21 |
|
6. Commitments and Contingencies
We have contracted with various consultants and third parties to assist us in pre-clinical research and development and clinical trials work for our leading drug compounds. The contracts are terminable at any time but obligate us to reimburse the providers for any time or costs incurred through the date of termination. See the Sale of ME-344 discussion within Note 12. Subsequent Events for additional information regarding contracts associated with ME-344 assumed by the Purchaser. We also have employment agreements with certain of our current employees that provide for severance payments and accelerated vesting for share-based awards if their employment is terminated under specified circumstances.
12
Litigation
From time to time, we may be involved in various lawsuits, legal proceedings, or claims that arise in the ordinary course of business. Management believes there are no claims or actions pending against us as of September 30, 2024, which will have, individually or in the aggregate, a material adverse effect on its business, liquidity, financial position, or results of operations. Litigation, however, is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Indemnification
In accordance with our amended and restated certificate of incorporation and sixth amended and restated bylaws, we have indemnification obligations to our officers and directors for certain events or occurrences, subject to certain limits, while they are serving in such capacity. There have been no claims to date, and we have a directors and officers liability insurance policy that may enable it to recover a portion of any amounts paid for future claims.
Presage License Agreement
As discussed in Note 8. Other License Agreements, we are party to a license agreement with Presage Biosciences, Inc. (Presage) under which we may be required to make future payments upon the achievement of certain development, regulatory and commercial milestones, as well as potential future royalties based upon net sales. As of September 30, 2024, we had no accruals for potential future payments as achievement of the milestones had not been met.
7. License Agreements
Kyowa Kirin Co., Ltd. License, Development and Commercialization Agreement
In April 2020, we entered into the KKC Commercialization Agreement under which we granted to KKC a co-exclusive, sublicensable, payment-bearing license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in the U.S. (the U.S. License), and an exclusive (subject to certain retained rights to perform obligations under the KKC Commercialization Agreement), sublicensable, payment-bearing, license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in countries outside of the U.S. KKC granted to us a co-exclusive, sublicensable, license under certain patents and know-how controlled by KKC to develop and commercialize zandelisib for all human indications in the U.S., and a co-exclusive, sublicensable, royalty-free, fully paid license under certain patents and know-how controlled by KKC to perform our obligations in the Ex-U.S. under the KKC Commercialization Agreement. KKC paid us an initial nonrefundable payment of $100.0 million.
In July 2023, we entered into a termination agreement with KKC to mutually terminate the KKC Commercialization Agreement and all other related agreements between the parties (Termination Agreement) and jointly decided to discontinue zandelisib development in the U.S. Prior to the execution of the Termination Agreement, KKC was responsible for the development and commercialization of zandelisib in Ex-U.S. and, subject to certain exceptions, was solely responsible for all costs related thereto. We also provided to KKC certain drug supplies necessary for the development and commercialization of zandelisib in the Ex-U.S., with the understanding that KKC would have assumed responsibility for manufacturing for the Ex-U.S. as soon as practicable.
During fiscal year 2023, we updated our assessment of the total transaction price from the KKC Commercialization Agreement to reflect the upfront payment, milestone payments, estimated development cost-sharing, and deferred revenue. After announcing our plan to discontinue the global development of zandelisib outside of Japan, in December 2022, we decreased our estimate for variable consideration related to development cost sharing.
With the execution of the Termination Agreement, we regained full, global rights (subject to KKC's limited rights for compassionate use) and KKC has no further rights to develop, use or commercialize zandelisib in the U.S., nor do we have any remaining performance obligations with all consideration received from KKC being nonrefundable. Therefore, the remaining long-term deferred revenue as of June 30, 2023, of $64.5 million that was allocated to the U.S. License obligation accounted for under Topic 808 at inception of the KKC Commercialization Agreement was recognized as revenue from collaboration agreements in the three months ended September 30, 2023, utilizing contract termination analogous to guidance provided in Topic 606.
The $64.5 million transaction price allocated to the U.S. License obligation accounted for under Topic 808 was included as noncurrent deferred revenue as of June 30, 2023. As of June 30, 2023, we also had deferred revenue of approximately $0.3 million related to the transaction price allocated to the Development Services performance obligations and recognized the remaining revenue based on the proportional performance of these development activities. The KKC Agreement was terminated on July 14, 2023 and all remaining amounts of deferred revenue were recognized during the fiscal year ended June 30, 2024.
13
8. Other License Agreements
Presage License Agreement
In September 2017, we, as licensee, entered into a license agreement with Presage. Under the terms of the license agreement, Presage granted us exclusive worldwide rights to develop, manufacture and commercialize voruciclib, a clinical-stage, oral and selective CDK inhibitor, and related compounds. In exchange, we paid $2.9 million to Presage. With respect to the first indication, an incremental $2.0 million payment, due upon dosing of the first subject in the first registration trial, will be owed to Presage, for total payments of $4.9 million prior to receipt of marketing approval of the first indication in the U.S., EU or Japan. Additional potential payments of up to $179.0 million will be due upon the achievement of certain development, regulatory and commercial milestones. We will also pay mid-single digit tiered royalties on the net sales of any product successfully developed. As an alternative to milestone and royalty payments related to countries in which we sublicense product rights, we will pay to Presage a tiered percentage (which decreases as product development progresses) of amounts received from such sublicensees. During the three months ended September 30, 2024 and 2023, we made no payments under the Presage license agreement.
BeiGene Collaboration
In October 2018, we entered into a clinical collaboration with BeiGene, Ltd. (BeiGene) to evaluate the safety and efficacy of zandelisib in combination with BeiGene’s zanubrutinib (marketed as Brukinsa®), an inhibitor of Bruton’s tyrosine kinase, for the treatment of patients with B-cell malignancies. Under the terms of the clinical collaboration agreement, we amended our ongoing Phase 1b trial to include evaluation of zandelisib in combination with zanubrutinib in patients with B-cell malignancies. Study costs are being shared equally by the parties, and we agreed to supply zandelisib and BeiGene agreed to supply zanubrutinib. We record the costs reimbursed by BeiGene as a reduction of our research and development expenses. We retained full commercial rights for zandelisib and BeiGene retained full commercial rights for zanubrutinib. With the discontinuation of the zandelisib program outside of Japan, this clinical collaboration was terminated on September 28, 2023. The Company did not recognize any cost reimbursement reductions within research and development during the three months ended September 30, 2024. Cost reimbursements recorded as a reduction of research and development costs, in the condensed consolidated statements of operations, during the three months ended September 30, 2023 were approximately $0.1 million.
9. Leases
In July 2020, we entered into a lease agreement for approximately 32,800 square feet of office space in San Diego, California. The lease agreement was scheduled to expire in March 2028. We accounted for the lease agreement as an operating lease. The lease agreement contained an option to renew and extend the lease term, which was not included in the determination of the right-of-use (ROU) asset and operating lease liability, as it was not reasonably certain to be exercised. In July 2022, we amended the lease to extend the lease termination date from March 2028 to November 30, 2029 and to add an additional 12,300 square feet of office space adjacent to our current office in San Diego (the Amended Lease). Upon commencement of the Amended Lease, we recognized an additional ROU asset and a corresponding operating lease liability of $4.3 million. The Amended Lease includes variable non-lease components (e.g., common area maintenance, maintenance, etc.) that are not included in the ROU asset and operating lease liability and are reflected as an expense in the period incurred as a component of the lease cost.
Lease Termination
On June 18, 2024 (the Agreement Date), we entered into a lease termination agreement (Agreement) with our landlord pursuant to which the parties agreed to terminate the lease for our existing office space as of September 30, 2024. The original (as amended) scheduled expiration date was November 30, 2029. As consideration for the Agreement, we agreed to pay the landlord a termination fee of approximately $11.1 million (the Termination Fee) and to prepay the remaining rent due under the Agreement in the amount of approximately $0.2 million (the Remaining Rent) and sell all the furniture and fixtures to the landlord for $1.00 (see Property and Equipment within Note 3. Balance Sheet Details for further discussion on the impact of the Agreement on our property and equipment). We received our security deposit, which is classified as a component of prepaid and other current assets, from the landlord in October 2024.
The Agreement was accounted for as a lease modification of the original contract. As a result of the Agreement, we reduced both the remaining ROU asset and lease liability by approximately $22,000, resulting in no impact to our consolidated statements of operations for the fiscal year ended June 30, 2024. We reassessed the lease classification, as of the Agreement Date, noting the current classification as an operating lease remained appropriate. Both the Termination Fee and the Remaining Rent were paid prior to June 30, 2024. Subsequent to the payment of both the Termination Fee and the Remaining Rent, our lease liability was relieved and the balance was reduced to zero.
14
We incurred direct costs of approximately $0.2 million in connection with the Agreement which accordingly was recorded to the ROU assets as a direct cost of modifying the Agreement. As of the Agreement Date, we determined a triggering event, in accordance with ASC 360, had occurred and therefore completed an impairment analysis on its ROU asset resulting in an impairment charge of approximately $10.4 million being recorded in our consolidated statements of operations for the fiscal year ended June 30, 2024.
The total operating lease costs for the Amended Lease were as follows for the periods presented (in thousands):
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Operating lease cost |
|
$ |
214 |
|
|
$ |
608 |
|
Variable lease costs |
|
|
— |
|
|
|
12 |
|
Total lease costs included in operating expenses |
|
$ |
214 |
|
|
$ |
620 |
|
Supplemental cash flow information related to our operating leases was as follows for the periods presented (in thousands):
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Cash paid for amount included in the measurement |
|
|
|
|
|
|
||
Operating cash flows from operating leases |
|
$ |
— |
|
|
$ |
584 |
|
As of June 30, 2024, we had no remaining future minimum rental payments for our operating leases and the remaining ROU asset balance was $0.2 million. As of September 30, 2024, we had vacated the facility and the leased property reverted to the landlord. In addition, the ROU asset has been fully amortized. During fiscal year 2024, the ROU asset balance was increased by approximately $0.2 million related to direct costs associated with the Agreement. Additionally, the ROU asset balance was decreased by: (1) approximately $22,000 associated with our reassessment of the lease liability as of the Agreement Date and (2) $10.4 million associated with the impairment of the ROU asset, as discussed above.
10. Stockholders’ Equity
Equity Transactions
Shelf Registration Statement
We have a shelf registration statement (February 2024 Shelf Registration Statement) that permits us to sell, from time to time, up to $100.0 million of common stock, preferred stock, warrants, rights and units, subject to the "Baby Shelf Limitation" described below. The February 2024 Shelf Registration Statement was filed February 20, 2024 and declared effective February 28, 2024.
At-The-Market Equity Offering
On February 20, 2024, we entered into a capital on demand sales agreement with JonesTrading Institution Services LLC, pursuant to which we can offer and sell shares having an aggregate offering price of up to $25.0 million (the ATM Program). In no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million (Baby Shelf Limitation) As of January 2, 2024, the date used under applicable rules of the SEC to determine our public float at the commencement of the offering, one-third of our public float was equal to approximately $9.9 million. As of September 30, 2024, no shares have been issued and sold under our ATM Program.
Cooperation Agreement and Cash Dividend
On October 31, 2023, we announced our entry into a Cooperation Agreement with Anson Funds Management LP and Cable Car Capital LLC, which, among other non-financial related items provided for a capital return to stockholders in the form of a dividend in the amount of $1.75 per share of common stock that was declared on November 6, 2023 to stockholders of record at the close of business on November 17, 2023. The total dividend of $11.7 million was paid on December 6, 2023 and was recorded as a reduction of additional paid-in capital in the condensed consolidated statements of stockholders' equity, as we have an accumulated deficit, rather than retained earnings.
15
Rights Agreement
On October 1, 2023, our Board approved and adopted a rights agreement (Rights Agreement) by and between us and Computershare, Inc., as Rights Agent (as defined in the Rights Agreement). Pursuant to the Rights Agreement, the Board declared a dividend of one preferred share purchase right (each, a Right) for each outstanding share of our common stock, par value $0.00000002 (each a Common Share and collectively, the Common Shares). The Rights are distributable to stockholders of record as of the close of business on October 12, 2023. One Right also will be issued together with each Common Share issued by us after October 12, 2023, but before the Distribution Date, as defined in the Rights Agreement (or the earlier of the redemption or expiration of the Rights) and, in certain circumstances, after the Distribution Date. The Rights and the Rights Agreement expired at the close of business on September 30, 2024. No rights were redeemed or exchanged under the Rights Agreement.
Warrants
As of September 30, 2024, we have outstanding warrants to purchase 102,513 shares of our common stock issued to Torreya Partners LLC. The warrants are fully vested, exercisable at a price of $6.80 per share and expire in October 2027. No warrants were exercised as of September 30, 2024.
Description of Capital Stock
Our total authorized share capital is 226,100,000 shares consisting of 226,000,000 shares of common stock, $0.00000002 par value per share, and 100,000 shares of preferred stock, $0.01 par value per share.
Common Stock
The holders of common stock are entitled to one vote per share. In the event of a liquidation, dissolution or winding up of our affairs, holders of the common stock will be entitled to share ratably in all our assets that are remaining after payment of our liabilities and the liquidation preference of any outstanding shares of preferred stock. All outstanding shares of common stock are fully paid and non-assessable. The rights, preferences and privileges of holders of common stock are subject to any series of preferred stock that we have issued or that we may issue in the future. The holders of common stock have no preemptive rights and are not subject to future calls or assessments by us.
Preferred Stock
Our Board has the authority to issue up to 100,000 shares of preferred stock with a par value of $0.01 per share in one or more series and to fix the rights, preferences, privileges and restrictions in respect of that preferred stock, including dividend rights, dividend rates, conversion rights, voting rights, terms of redemption (including sinking fund provisions), redemption prices and liquidation preferences, and the number of shares constituting such series and the designation of any such series, without future vote or action by the stockholders. Therefore, the Board, without the approval of the stockholders, could authorize the issuance of preferred stock with voting, conversion and other rights that could affect the voting power, dividend and other rights of the holders of shares or that could have the effect of delaying, deferring or preventing a change of control. There were no shares of preferred stock outstanding as of September 30, 2024 and June 30, 2024.
11. Share-based Compensation
We use equity-based compensation programs to provide long-term performance incentives for our employees. These incentives consist primarily of stock options and RSUs. In December 2008, we adopted the MEI Pharma, Inc. 2008 Stock Omnibus Equity Compensation Plan (Omnibus Plan), as amended and restated from time-to-time, under which 1,850,739 shares of common stock are currently authorized for issuance. The Omnibus Plan provides for the grant of options and/or other stock-based or stock-denominated awards to our non-employee directors, officers, and employees. As of September 30, 2024, there were 691,915 shares available for future grant under the Omnibus Plan.
In May 2021, we adopted the 2021 Inducement Plan (Inducement Plan), under which 125,000 shares of common stock are authorized for issuance. On June 9, 2023, our Board approved an amendment and restatement of the Inducement Plan to increase the aggregate number of shares of common stock authorized for issuance by 92,000 shares. The Inducement Plan is intended to assist us in attracting and retaining selected individuals to serve as employees who are expected to contribute to our success, by providing an inducement for such individuals to enter into employment with us, and to achieve long-term objectives that will benefit our stockholders. As of September 30, 2024, there were 137,983 shares available for future grant under the Inducement Plan.
16
During the three months ended September 30, 2024 due to the 2024 RIF, as discussed in Note 5. Employee Termination Benefits, we reversed share-based compensation in excess of that recorded. Total share-based compensation for all stock awards consisted of the following for the periods presented (in thousands):
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Research and development |
|
$ |
(106 |
) |
|
$ |
(69 |
) |
General and administrative |
|
|
(29 |
) |
|
|
432 |
|
Total share-based compensation |
|
$ |
(135 |
) |
|
$ |
363 |
|
Stock Options
Stock options granted to employees vest 25% one year from the date of grant and ratably each month thereafter on the first day of the month following the one-year anniversary of the grant date for a period of 36 months and generally expire ten years from the date of grant. Stock options granted to directors vest ratably each month for a period of 12 months from the date of grant and generally expire ten years from the date of grant. Upon separation from the Company, without cause, our Board members have three years to exercise their vested stock options. As of September 30, 2024, there were 1,123,685 total options outstanding of which 1,044,668 were granted under the Omnibus Plan and 79,017 were granted under the Inducement Plan.
A summary of our stock option activity and related data follows:
|
|
Number of |
|
|
Weighted-Average |
|
|
Weighted-Average |
|
|
Aggregate |
|
||||
Outstanding at June 30, 2024 |
|
|
1,357,213 |
|
|
$ |
31.60 |
|
|
|
|
|
|
|
||
Forfeited |
|
|
(233,528 |
) |
|
$ |
21.85 |
|
|
|
|
|
|
|
||
Outstanding at September 30, 2024 |
|
|
1,123,685 |
|
|
$ |
33.62 |
|
|
|
6.6 |
|
|
$ |
— |
|
Vested and expected to vest at September 30, 2024 |
|
|
1,123,685 |
|
|
$ |
33.62 |
|
|
|
6.6 |
|
|
$ |
— |
|
As of September 30, 2024, the aggregate intrinsic value of outstanding options was calculated as the difference between the exercise price of the underlying options and the closing price of our common stock of $2.85 on that date.
Unrecognized compensation expense related to non-vested stock options totaled $0.3 million as of September 30, 2024. Such compensation expense is expected to be recognized over a weighted-average period of 1.52 years.
We use the Black-Scholes valuation model to estimate the grant date fair value of stock options. During the three months ended September 30, 2024, we did not grant any stock options. To calculate these fair values, the following weighted-average assumptions were used for the period presented:
|
|
For the Three Months Ended September 30, |
|
|
|
|
2023 |
|
|
Risk-free interest rate |
|
|
4.6 |
% |
Expected life (years) |
|
|
5.7 |
|
Volatility |
|
|
89.8 |
% |
Dividend yield |
|
|
— |
% |
Weighted-average grant date fair value |
|
$ |
5.27 |
|
17
12. Subsequent Events
Sale of ME-344
On October 22, 2024 (the Closing Date), MEI and Aardvark Therapeutics, Inc. (the Purchaser), entered into an Asset Purchase Agreement (the Asset Purchase Agreement), pursuant to which we sold to Purchaser our rights, title and interest in and to certain assets related to ME-344, including relevant intellectual property rights, technology and contracts. Pursuant to the Asset Purchase Agreement, the Purchaser paid us an initial payment of $0.5 million in cash plus a reimbursement amount of $55,000 at the closing of the transaction. The Purchaser may also make future milestone payments up to $62.0 million after the Closing Date, payable upon the achievement of certain regulatory and revenue milestones. The Purchaser also assumed certain of our liabilities after the Closing Date, including liabilities arising under the contracts transferred under the Asset Purchase Agreement.
18
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
This Quarterly Report on Form 10-Q (Quarterly Report) includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act) and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act). All statements other than statements of historical facts contained in this Quarterly Report, including statements regarding the future financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. The words “may,” “will,” “intend,” “plan,” “believe,” “anticipate,” “expect,” “estimate,” "predict," "potential," “continue,” "likely," or “opportunity,” the negative of these words or similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, without limitation, those described in Risk Factors in our 2024 Annual Report on Form 10-K (2024 Annual Report), as filed with the Securities and Exchange Commission (SEC) on September 19, 2024.
Unless the context requires otherwise, references in this Annual Report to “MEI Pharma,” “MEI,” “we,” “us” and “our” refer to MEI Pharma, Inc.
Overview
MEI Pharma, Inc. (Nasdaq: MEIP) is a pharmaceutical company that has been developing novel and differentiated cancer therapies. We built our pipeline by acquiring promising cancer agents and creating value in programs through clinical development, strategic partnerships, and out-licensing or commercialization, as appropriate. Our approach to oncology drug development has been to evaluate our drug candidates in combinations with standard-of-care therapies to overcome known resistance mechanisms and address clear medical needs to provide improved patient benefit. Our drug candidate pipeline includes voruciclib, an oral cyclin-dependent kinase 9 (CDK9) inhibitor, and prior to its sale (as discussed below) ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway.
Strategic Alternatives
On July 22, 2024, we announced that our Board of Directors (Board) had determined unanimously to begin the evaluation of our strategic alternatives, including potential transactions as well as an orderly wind down of operations, if appropriate, to maximize the value of our assets for our stockholders. We commenced a reduction-in-force (the 2024 RIF) beginning August 1, 2024, which will continue in stages as our operational and strategic direction evolves. We have discontinued the clinical development of voruciclib, while certain nonclinical activities related to MEI’s drug candidate assets will continue to be conducted by us. As part of the review of strategic alternatives, we may consider options such as out-licensing opportunities for existing programs and merger and acquisition opportunities.
Consistent with our intention to preserve cash, David M. Urso, our President and Chief Executive Officer, and Richard Ghalie, M.D., our Chief Medical Officer, stepped down effective August 1, 2024. Mr. Urso also left the Board at that date. We have entered into consulting agreements with both Mr. Urso and Dr. Ghalie under which they will remain available to assist us in our strategic efforts. Charles V. Baltic III, the Chairperson of the Board, also stepped down from the Board contemporaneously with the announcement on July 22, 2024. Our Board appointed Justin J. File, our current Chief Financial Officer, to assume the position of Acting Chief Executive Officer and has appointed Frederick W. Driscoll as Chairperson of the Board.
Sale of ME-344
On October 22, 2024 (the Closing Date), MEI and Aardvark Therapeutics, Inc. (the Purchaser), entered into an Asset Purchase Agreement (the Asset Purchase Agreement), pursuant to which we sold to Purchaser our rights, title and interest in and to certain assets related to ME-344, including relevant intellectual property rights, technology and contracts. Pursuant to the Asset Purchase Agreement, the Purchaser paid us an initial payment of $0.5 million in cash plus a reimbursement amount of $55,000 at the closing of the transaction. The Purchaser may also make future milestone payments up to $62.0 million after the Closing Date, payable upon the achievement of certain regulatory and revenue milestones. The Purchaser also assumed certain of our liabilities after the Closing Date, including liabilities arising under the contracts transferred under the Asset Purchase Agreement.
Clinical Development Programs
Our clinical-stage drug candidate pipeline includes voruciclib, an oral CDK9 inhibitor, and prior to its sale, ME-344, an intravenous small molecule mitochondrial inhibitor targeting the oxidative phosphorylation pathway in the mitochondria.
19
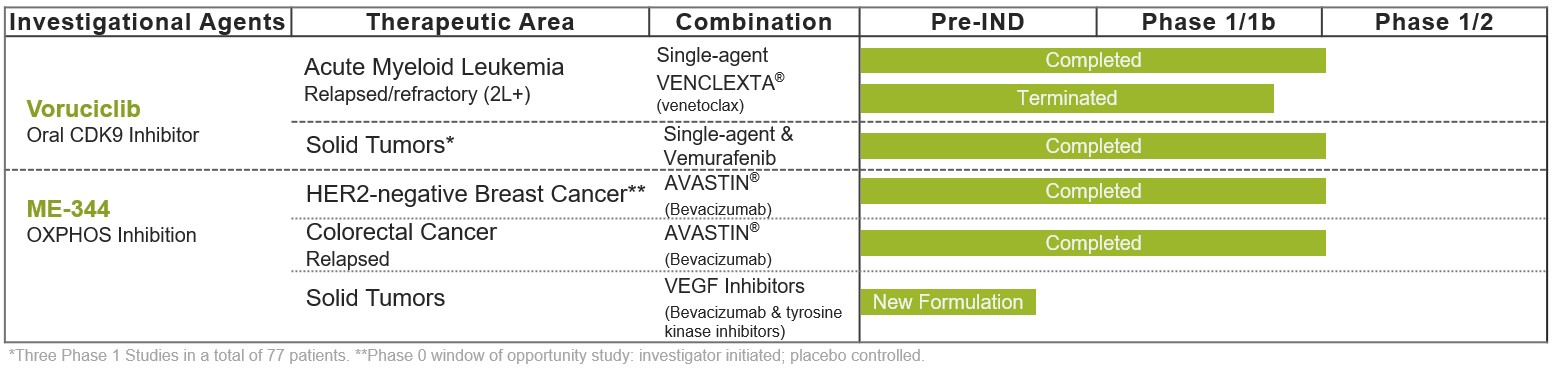
Voruciclib: Potent Orally Administered CDK9 Inhibitor in Phase 1 Studies
Following the announcement of the exploration of strategic alternatives on July 22, 2024, all ongoing clinical trial efforts for voruciclib have ceased, while certain nonclinical activities have continued. Voruciclib is a selective orally administered CDK9 inhibitor. Voruciclib recently completed a Phase 1 trial evaluating dose and schedule in patients with acute myeloid leukemia (AML) in combination with the B-cell lymphoma 2 (BCL-2) inhibitor venetoclax (marketed as Venclexta®). Voruciclib is also being evaluated in pre-clinical studies to explore potential activity in various solid tumor cancers including in combination with therapies that target the RAS signaling pathway, such as KRAS inhibitors.
Voruciclib Scientific Overview: Cell Cycle Signaling
CDK9 has important functions in cell cycle regulation, including the modulation of two therapeutic targets in cancer:
Directly inhibiting MCL1 and MYC has historically been difficult, but CDK9 is a promising approach to indirectly target these oncogenes.
Voruciclib: Inhibition of MCL1
CDK9 is a known transcriptional regulator of MCL1. Over expression of MCL1 is frequently observed in many tumor types and is closely associated with tumorigenesis, poor prognosis and drug resistance. In AML, MCL1 is upregulated in about half of patients with relapsed and refractory (R/R) disease and is associated with poor prognosis in these patients. Also important, high levels of MCL1 expression are associated with resistance to venetoclax.
In pre-clinical studies, voruciclib shows dose-dependent suppression of MCL1; in December 2017, a study of voruciclib published in the journal Nature Scientific Reports reported that the combination of voruciclib plus the BCL-2 inhibitor venetoclax was capable of inhibiting two master regulators of cell survival, MCL-1 and BCL-2, and achieved synergistic antitumor effect in an aggressive subset of DLBCL cells.
In a peer reviewed manuscript published in 2020, it was reported that the inhibition of CDK9 by voruciclib synergistically enhances cell death induced by the BCL-2 inhibitor venetoclax in preclinical models of AML. The data demonstrated that voruciclib synergizes with venetoclax to induce programmed cell death, or apoptosis, in both AML cell lines and primary patient samples. It was also demonstrated that voruciclib downregulates MCL1, which is relevant for the synergy between voruciclib and venetoclax, and further that voruciclib downregulates MYC, which also contributes to the synergies with venetoclax.
Subsequently, and consistent with the reported pre-clinical studies, data from an ongoing Phase 1 study evaluating voruciclib as a single agent and in combination with venetoclax in patients with relapsed or refractory (R/R) AML have also demonstrated the anticipated decreases in Mcl-1 protein.
The research suggests that voruciclib is potentially an attractive therapeutic agent for treating cancers in combination with venetoclax or other BCL-2 inhibitors, to address potential resistance associated with MCL1, and is supportive of our ongoing clinical evaluation of voruciclib in AML.
20
Voruciclib: Inhibition of MYC
Many cancers are associated with over expression of MYC, a transcription factor regulating cell proliferation and growth. CDK9 is a known regulator of MYC transcription and a modulator of MYC protein phosphorylation. Data reported at the American Association for Cancer Research (AACR) Annual Meeting 2021 in preclinical models demonstrated that voruciclib:
The research presented suggests that voruciclib could be an attractive therapeutic agent for both hematological cancers, as well as solid tumors, dependent on the activity of MYC.
Clinical Programs
In a recent Phase 1 clinical trial, we evaluated the dose and schedule of voruciclib in combination with venetoclax, a BCL-2 inhibitor, in patients with R/R AML. The trial started with the evaluation of dose and schedule of voruciclib as a monotherapy in patients with relapsed and refractory B-cell malignancies and AML after failure of prior standard therapies to determine the safety, preliminary efficacy and maximum tolerated dose. The primary objectives of the study were to determine the safety and biologic effective dose of voruciclib monotherapy or voruciclib in combination with venetoclax. Secondary objectives of the study included assessing the preliminary efficacy, pharmacokinetics, pharmacodynamics, and biomarkers of voruciclib monotherapy or voruciclib in combination with venetoclax.
As we reported in a poster presented at the American Society of Hematology (ASH) Annual Meeting in December 2023, the voruciclib monotherapy dose escalation/expansion stage of the study enrolled a total of 40 patients and is complete. The majority of patients (n=21) had AML and the remaining patients (n=19) had B-cell malignancies. Of the 40 patients enrolled, the first 16 were dosed daily continuously at 50 and 100 mg and the following 24 patients were dosed on an intermittent schedule (14 consecutive days on therapy in a 28-day cycle) at 100, 150 and 200 mg. All patients were heavily pre-treated with a median of three prior therapies (range 1-9), and five patients had prior hematopoietic stem cell transplant. Voruciclib at doses up to 200 mg administered on 14 consecutive days in a 28-day cycle (Cohort 2) was well tolerated with no dose limiting toxicities (DLT) reported. The most common adverse events (≥20% of patients) were diarrhea, nausea, anemia and fatigue. The large majority of adverse events were Grade 1-2; of note, the only Grade 3-4 adverse events in Cohort 2 were diarrhea (n=1) and anemia (n=5). Pharmacokinetics were dose proportional and a mean half-life of approximately 24 hours supports once daily dosing.
On the intermittent dosing schedule selected for further development, no DLTs were observed, there were no Grade 3 or higher drug related toxicities, and dose escalation was stopped at 200 mg before reaching the maximum tolerated dose because plasma concentrations reached levels considered sufficient for target inhibition. In the 21 patients enrolled with AML, one patient at 100 mg achieved a morphologic leukemia-free state and nine patients had disease stabilization, which lasted at least three months in two patients. In the 19 patients enrolled with B-cell malignancies, four patients had stable disease with a decrease in tumor size. Initial results from correlative studies assessing myeloid leukemia cell differentiation protein (Mcl-1) and RNA Pol II phosphorylation on Ser2 (RNA Pol II p-S2) demonstrated reduction in expression consistent with the anticipated on-target pharmacodynamic effect of voruciclib on Mcl-1 and RNA Pol II p-S2.
The next stage of the study evaluated seven voruciclib dose levels from 50 mg every other day to 300 mg daily for 14 consecutive days in a 28-day cycle in combination with standard dose venetoclax in patients with R/R AML. A total of 41 patients with R/R AML, median age 67 years (range 34-89), enrolled in this dose escalation stage of the study evaluating voruciclib in combination with venetoclax. These patients were generally heavily pretreated; the median number of prior therapies was 2 (range 1-7), and 18 (44%) patients had ≥3 prior lines. Almost all patients (39/41) were treated with venetoclax in an earlier line of therapy. Additionally, 30 (73%) patients were noted as being in an adverse 2017 ELN Risk Category due to adverse cytogenetics and molecular mutations.
Of the 32 patients administered voruciclib at doses ≥ 100 mg in combination with venetoclax 10 (31%) achieved disease control. Three patients achieved a response, including two patients that achieved a complete response with incomplete hematologic recovery (CRi) and one patient that achieved a morphologic leukemia-free state (MLFS), in each case having received venetoclax in an earlier line of treatment. Responses lasted 6 months in one patient, 9 months and ongoing in the second patient, and the third patient was referred to stem cell transplant. Further, an additional 7 patients had stable disease which lasted more than 90 days and 13 had stable disease < 3 months.
21
In the 28 patients administered voruciclib in combination with venetoclax and with blood samples available for analysis, initial results from correlative biomarker assay studies demonstrated anticipated decreases of Mcl-1, including a greater decrease in Mcl-1 in responding patients. This supports our hypothesis that voruciclib, as an inhibitor or CDK9, regulates Mcl-1 and therefore may address the upregulation of MCL1 associated with venetoclax. Additional evidence of anti-leukemic activity was also demonstrated including decreases in bone marrow blast counts post voruciclib/venetoclax administration versus pre drug administration in ~50% (11/21) of evaluable patients.
Voruciclib at doses up to 300 mg administered on 14 consecutive days in a 28-day cycle in combination with standard dose venetoclax was well tolerated with no dose limiting toxicities observed. The maximum tolerated dose of voruciclib administered on this schedule with venetoclax has not been established. There were no discontinuations due to drug-related adverse events and no evidence of overlapping toxicity has been observed to date. The most common (≥5% of patients) grade 3 adverse events were myelosuppression associated with AML. Only 1 patient was observed as having a non-hematologic grade 3 drug-related adverse event (diarrhea).
Before ending the study, three patients were administered 150 mg voruciclib over 21 consecutive days in a 28-day cycle in combination with venetoclax to increase dose intensity and potentially optimize patient response based upon the rebound of peripheral blast counts in 44% (8/18) of the patients between Day 14 and Day 28 when voruciclib was stopped while continuing venetoclax.
Voruciclib was also previously evaluated in more than 70 patients with solid tumors in multiple Phase 1 studies. The totality of the clinical data, along with data from pre-clinical studies, suggests voruciclib’s ability to inhibit its molecular target at a projected dose as low as 150 mg daily. In one clinical study, voruciclib was evaluated in combination with vemurafenib (marketed as Zelboraf®) in nine patients with BRAF mutated advanced/ inoperable malignant melanoma. All three BRAF/MEK naive patients achieved a response: two partial responses and one complete response. In this study voruciclib was dosed at 150 mg daily plus vemurafenib 720 mg or 960 mg twice daily in 28-day cycles. The most common adverse events were fatigue, constipation, diarrhea, arthralgia and headache. One instance of grade 3 fatigue was dose limiting and no serious adverse events related to voruciclib were reported. Other clinical studies evaluated voruciclib at doses up to 850 mg in patients with solid tumors, demonstrating additional evidence of potential biologic activity and an adverse event profile generally consistent with other drugs in its class.
ME-344: Mitochondrial Inhibitor with Combinatorial Potential
Nonclinical activities related to ME-344 continued following the announcement of the exploration of strategic alternatives on July 22, 2024 and ended with the closing of its sale, as discussed under Sale of ME-344, elsewhere in this Quarterly Report on Form 10-Q. MEI-344 is a novel drug candidate that inhibits mitochondrial OXPHOS, a fundamental metabolic pathway involved in the production of adenosine triphosphate (ATP) in the mitochondria. ATP provides energy to drive many metabolic cell processes, including division, proliferation, and growth. By disrupting the production of ATP, ME-344 has been shown to induce cancer cell death in nonclinical models and was associated with antitumor activity in clinical studies. ME-344 has also demonstrated clinical activity in multiple clinical studies in combinations, including with bevacizumab (Avastin®).
Recently, we were advancing ME-344 via development of a new formulation with the goal of increasing biological activity, improving patient convenience of administration and increasing commercial opportunity. We believe a new formulation represents the optimal approach to pursue the potential of the program after observing encouraging data in two clinical studies evaluating the prior ME-344 formulation in combination with bevacizumab (Avastin®).
ME-344 Scientific Overview: Cancer Metabolism
Energy supplied in the form of ATP fuels tumor metabolism supporting cell division and growth. Accordingly, tumor cells often display a high metabolic rate to support tumor cell survival and proliferation. This heightened metabolism requires a continual supply of energy in the form of ATP.
Anti-angiogenics, such as the vascular endothelial growth factor (VEGF) inhibitor bevacizumab, have the potential to normalize vasculature and decrease reliance on glycolysis for ATP. The resulting reduction in glycolysis may trigger an increased dependence on mitochondrial ATP production for energy to support continued tumor proliferation. In such cases of tumor plasticity, the combination of ME-344 and bevacizumab may induce metabolic synthetic lethality, providing a novel therapeutic strategy. Specifically, leveraging the ability of antiangiogenics like bevacizumab to reduce glycolysis and force tumor cells to switch to mitochondrial respiration via OXPHOS, which is inhibited by ME-344, may reduce access to ATP needed for cell division and growth in tumors.
We obtained initial clinical data on this approach in a completed investigator-initiated, multi-center, randomized, controlled, window of opportunity clinical trial evaluating ME-344 in combination with bevacizumab that enrolled a total of 42 patients with human epidermal growth factor receptor 2 (HER2) negative breast cancer. Further clinical support for the combination of ME-344 in combination with bevacizumab was reported in April 2024 from a Phase 1b study of patients with relapsed metastatic colorectal cancer (mCRC) after failure of standard therapies. This study demonstrated clinical activity, including an effect on progression free survival in a cohort of 23 patients.
22
An earlier Phase 1 clinical study evaluating ME-344 as a single-agent in patients with refractory solid tumors also demonstrated anti-tumor activity, further validating the potential of mitochondrial inhibition as a promising therapeutic modality.
Clinical Program
ME-344 has been evaluated pre-clinically and clinically as a single agent and in combination with anti-angiogenics such as bevacizumab. When evaluated as a single agent, ME-344 demonstrated evidence of activity against refractory solid tumors in a Phase 1b trial, and in pre-clinical studies tumor cells treated with ME-344 resulted in a rapid loss of ATP and cancer cell death. In addition to single agent activity, ME-344 has also demonstrated significant potential in combination with anti-angiogenic therapeutics.
Pre-clinical studies have shown that one outcome of anti-angiogenics is a reduced rate of glycolysis in tumors as a mechanism to slow tumor growth. However, when faced with reduced glycolysis and reduced ATP production, tumor metabolism was able to shift to mitochondrial metabolism for energy production to support continued tumor proliferation. In such cases of tumor plasticity in the presence of treatment with anti-angiogenics, contemporaneously targeting the mitochondria as an alternative metabolic source of ATP with ME-344 may open an important development opportunity.
Support for this combinatorial use of ME-344 was first published in the June 2016 edition of Cell Reports; pre-clinical data from a collaboration with the Spanish National Cancer Research Centre in Madrid demonstrated mitochondria-specific effects of ME-344 in cancer cells, including substantially enhanced anti-tumor activity when combined with agents that inhibit the activity of VEGF. These data demonstrating the potential anti-cancer effects of combining ME-344 with a VEGF inhibitor due to an inhibition of both mitochondrial and glycolytic metabolism provided a basis for commencement of an investigator-initiated trial of ME-344 in combination with bevacizumab in HER2 negative breast cancer patients.
Results published in the November 2019 issue of Clinical Cancer Research from a multi-center, investigator-initiated, randomized, controlled, clinical trial that evaluated the combination of ME-344 and bevacizumab in 42 women with early HER2-negative breast cancer provided evidence for the combinatorial use of ME-344 with anti-angiogenic therapeutics.
The primary objective of the trial was to show proof of ME-344 biologic activity as measured by reductions in the nuclear protein Ki67 (expression of which is strongly associated with tumor cell proliferation and growth) from days 0 to 28 compared to the control group who received bevacizumab alone. Secondary objectives included determining whether ME-344 biologic activity correlates with vascular normalization. The data demonstrated significant biologic activity in the ME-344 treatment group:
Treatment was generally well tolerated; three grade 3 adverse events of high blood pressure were reported, two in the ME-344 arm and one in the bevacizumab monotherapy arm.
Building on the clinical study evaluating patients with breast cancer, a Phase 1b study evaluating ME-344 in combination with bevacizumab in patients with relapsed metastatic colorectal cancer (mCRC) after failure of standard therapies was initiated. The study was designed to evaluate ME-344 plus bevacizumab in up to two cohorts of approximately 20 patients each. The option to enroll the second cohort was conditioned upon Cohort 1 reaching a predetermined non-progression threshold of at least 20% at four months. Patients in the study were treated until disease progression or intolerability. The primary endpoint of the study was 16-week progression free survival (PFS), and secondary endpoints included overall PFS, duration of response, overall survival and safety.
ME-344 was administered once weekly on Days 1, 8 and 15 combined with bevacizumab on Days 1 and 15 of each 28-day cycle. Cohort 1 enrolled a total of 23 patients with relapsed mCRC. Patients were generally heavily pretreated; the median number of prior lines of therapy was 4 (range 1-8), 18 (78%) patients had ≥3 prior lines, and all patients had previously received bevacizumab and standard chemotherapy. As reported in April 2024, the combination was generally well tolerated with no overlapping toxicities observed. Two patients (9%) discontinued therapy due to an adverse event: fatigue considered related to study drugs and sepsis considered unrelated. The most common (≥10% of patients) drug-related adverse events (all grades/grade ≥3) were fatigue in 8 (35%) / 3 (13%) patients and abdominal pain in 3 (13%) / 2 (9%) patients.
It was further reported that in the first cohort, 5 of 20 (25%) evaluable patients completed 16 weeks of therapy without evidence of disease progression, exceeding the 20% predetermined threshold as set forth in the Clinical Study Protocol to proceed to Cohort 2. The median PFS was 1.9 months, the 4-month PFS rate was 31.2%, and the median overall survival was 6.7 months with 15 patients censored at the time of analysis.
23
Nine (45%) of the 20 evaluable patients had stable disease. Although Cohort 1 exceeded the predetermined PFS threshold, we decided not to initiate enrollment in a second cohort.
Results from an earlier, first-in-human, single-agent Phase 1 clinical trial of ME-344 in patients with refractory solid tumors were published in the April 1, 2015 edition of Cancer. The results indicated that eight of 21 evaluable patients (38%) treated with ME-344 achieved stable disease or better, including five who experienced progression-free survival that was at least twice the duration of their last prior treatment before entry into the trial. In addition, one of these patients, a heavily pre-treated patient with small cell lung cancer, achieved a confirmed partial response and remained on study for two years. ME-344 was generally well tolerated at doses equal to or less than 10 mg/kg delivered on a weekly schedule for extended durations. Treatment-related adverse events included nausea, dizziness and fatigue. Dose-limiting toxicities were observed at both the 15 mg/kg and 20 mg/kg dose levels, consisting primarily of grade 3 peripheral neuropathy.
Through October 22, 2024, we were pursuing ME-344 via development of a new formulation to advance our novel approach to inducing synthetic lethality in tumors in combination with VEGF inhibitors such as bevacizumab (Avastin®). We had already initiated research and development activity of the new formulation, with the goal of increasing biological activity, improving patient convenience of administration and increasing commercial opportunity.
Also, through October 22, 2024, we were advancing ME-344 in combination with the anti-angiogenic antibody bevacizumab in a Phase 1b study evaluating patients with relapsed colorectal cancer. The study enrolled patients with progressive disease after failure of standard therapies with patients treated until disease progression or intolerance. The primary objective was progression free survival. Secondary endpoints included overall response rate, duration of response, overall survival and safety. Additionally, ME-344 may also have clinical potential against hematological malignancies. At the AACR Annual Meeting 2022, a poster presentation reported results from preclinical studies exploring the ability of ME-344 to enhance the activity of venetoclax against AML. Data from the in vitro and in vivo preclinical studies evaluating the combination of ME-344 with venetoclax in standard-of-care-resistant AML cell lines and relapsed or refractory AML patient samples suggest that ME-344, both alone and in combination with venetoclax, inhibits purine biosynthesis, suppresses oxidative phosphorylation, induces apoptosis and decreases MCL-1, which together target metabolic vulnerabilities of AML cells. The data demonstrated that ME-344 and venetoclax prolong survival in MV4-11 and MV4-11/AraC-R-derived xenograft AML models. The poster concluded that ME-344 enhances venetoclax activity against AML cells including resistant AML.
Zandelisib: PI3Kδ Inhibitor Overview
Zandelisib is an oral, once-daily, selective PI3Kδ inhibitor that we were jointly developing with Kyowa Kirin Co., Ltd. (KKC) under a global license, development and commercialization agreement entered into in April 2020.
In March 2022, we and KKC jointly decided to discontinue global development of zandelisib for indolent forms of non-Hodgkin lymphoma outside of Japan. The discontinuation of zandelisib development outside of Japan was a business decision based on the most recent regulatory guidance from the FDA and was not related to the zandelisib clinical data generated to date. After making the joint decision to terminate development outside of Japan, we and KKC began closing all ongoing zandelisib clinical studies outside of Japan, including the Phase 3 COASTAL trial, the Phase 2 TIDAL trial, and the Phase 2 CORAL trial.
Subsequently, in May 2023, KKC decided to discontinue development of zandelisib in Japan. The discontinuation of zandelisib in Japan was a business decision by KKC based on the most recent regulatory guidance from the Pharmaceuticals and Medical Devices Agency in Japan and was not related to the zandelisib clinical data generated to date.
KKC License, Development and Commercialization Agreement
In April 2020, we entered into the April 2020, License, Development and Commercialization Agreement with KKC (the KKC Commercialization Agreement) under which we granted to KKC a co-exclusive, sublicensable, payment-bearing license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in the U.S. (the U.S. License), and an exclusive (subject to certain retained rights to perform obligations under the KKC Commercialization Agreement), sublicensable, payment- bearing, license under certain patents and know-how controlled by us to develop and commercialize zandelisib and any pharmaceutical product containing zandelisib for all human indications in countries outside of the U.S. (the Ex-U.S. and the Ex-U.S. License). Also, under the KKC Commercialization Agreement, we were granted a co-exclusive, sublicensable, license under certain patents and know-how controlled by KKC to develop and commercialize zandelisib for all human indications in the U.S., and a co-exclusive, sublicensable, royalty-free, fully paid license under certain patents and know-how controlled by KKC to perform our obligations in the Ex-U.S. and were paid an initial non-refundable payment of $100.0 million. Additionally, in Japan, the KKC Commercialization Agreement included potential regulatory and commercialization milestone payments plus royalties on net sales of zandelisib in Japan, which are tiered beginning in the teens. Prior to the execution of the Termination Agreement as discussed below, KKC was responsible for the development and commercialization of zandelisib in the Ex-U.S. and, subject to certain exceptions, solely responsible for all costs related thereto. We also provided to KKC certain drug supplies necessary for the development and commercialization of zandelisib in the Ex-U.S., with the understanding that KKC would have assumed responsibility for manufacturing for the Ex-U.S.
24
as soon as practicable.
On July 14, 2023, we entered into a Termination Agreement with KKC (the Termination Agreement) to mutually terminate the KKC Commercialization Agreement and all other related agreements between the parties. Pursuant to the Termination Agreement:
As of June 30, 2023, we had $64.9 million of aggregate deferred revenue associated with the KKC Commercialization Agreement, of which $64.5 million was allocated to the U.S. License and $0.3 million was allocated to the Development Services performance obligations which were recognized based on the proportional performance of these development activities through wind-down of the associated trials. As further discussed in Note 7. License Agreements, in connection with the execution of the Termination Agreement during the three months ended September 30, 2023, we recognized the $64.5 million of noncash long-term deferred revenue associated with the U.S. License as well as the remaining $0.3 million noncash deferred revenue associated with the completion of the underlying proportional performance activities. As of September 30, 2023, all deferred revenue associated with the KKC Commercialization Agreement had been recognized.
Results of Operations
Comparison of Three Months Ended September 30, 2024 and 2023
The following table summarizes certain components of our results of operations (in thousands):
|
|
For the Fiscal Three Months Ended September 30, |
|
|
|
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Revenues |
|
$ |
— |
|
|
$ |
65,297 |
|
|
$ |
(65,297 |
) |
|
|
(100.0 |
)% |
Research and development |
|
|
3,163 |
|
|
|
3,485 |
|
|
|
(322 |
) |
|
|
(9.2 |
)% |
General and administrative |
|
|
5,189 |
|
|
|
6,531 |
|
|
|
(1,342 |
) |
|
|
(20.5 |
)% |
Other income, net |
|
|
345 |
|
|
|
1,093 |
|
|
|
(748 |
) |
|
|
(68.4 |
)% |
Revenue: During the three months ended September 30, 2024, we recognized no revenue due to all deferred revenue associated with the KKC Commercialization Agreement having been recognized in fiscal year 2024, upon entry into the Termination Agreement. During the three months ended September 30, 2023, we recognized revenue of $65.3 million from the KKC Commercialization Agreement.
Research and Development: The following is a summary of our research and development expenses to supplement the more detailed discussion below (in thousands).
|
|
For the Three Months Ended September 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
zandelisib |
|
$ |
54 |
|
|
$ |
449 |
|
voruciclib |
|
|
717 |
|
|
|
(335 |
) |
ME-344 |
|
|
357 |
|
|
|
1,220 |
|
Other |
|
|
2,035 |
|
|
|
2,151 |
|
Total research and development expenses |
|
$ |
3,163 |
|
|
$ |
3,485 |
|
Research and development costs decreased by $0.3 million to $3.2 million for three months ended September 30, 2024 compared to $3.5 million for the three months ended September 30, 2023. Costs related to ME-344 decreased $0.9 million due to decreased manufacturing and clinical costs related to the Phase 1b study. Costs related to zandelisib decreased $0.4 million, primarily as a result of the discontinuation of the program during fiscal year 2023 and the completion of wind-down related activities. Costs related to voruciclib increased $1.1 million mainly due to greater recognized manufacturing and clinical costs in the Phase 1 study and costs associated with nonclinical studies. Other research and development costs decreased a net $0.1 million primarily due to reduced payroll related costs partially offset by increased expenses associated with our plan to explore strategic alternatives that was announced in July 2024.
25
General and Administrative: General and administrative expenses decreased by $1.3 million to $5.2 million for the three months ended September 30, 2024 compared to $6.5 million for the three months ended September 30, 2023. The decrease was primarily due to a $0.8 million decrease in legal costs and $0.6 in professional fees associated with various stockholder related items from the prior fiscal year with no current period recurrence, a $0.5 decrease in share-based compensation costs due to reversal of expense in excess of vested shares recognized upon execution of our 2024 RIF and a $0.4 million decrease in corporate overhead costs. These decreases were partially offset by a $0.9 million increase in expenses associated with the 2024 RIF partially offset by a decrease in payroll related costs.
Other Income, net: Other income, net, decreased by $0.8 million to $0.3 million for the three months ended September 30, 2024 compared to $1.1 million for the three months ended September 30, 2023. We received interest and dividend income of $0.4 million for the three months ended September 30, 2024 compared to $1.1 million for the three months ended September 30, 2023. The decrease in interest and dividend income was primarily due to lower average balances during the three months ended September 30, 2024 compared to the three months ended September 30, 2023.
Liquidity and Capital Resources
To date, we have obtained cash and funded our operations primarily through equity financings and license agreements. There can be no assurance that we will be able to continue to raise additional capital in the future. We have accumulated losses of $396.2 million since inception and expect to incur operating losses and generate negative cash flows from operations for the foreseeable future. As of September 30, 2024, we had $26.9 million in cash and cash equivalents, and short-term investments. On July 22, 2024, we announced that our Board had determined unanimously to begin the evaluation of our strategic alternatives, including potential transactions as well as an orderly wind down of operations, if appropriate, to maximize the value of our assets for our stockholders. In connection with the exploration of strategic alternatives, we initiated the 2024 RIF to reduce headcount on August 1, 2024 and discontinued the clinical development of voruciclib. As a result of this announcement, we expect our research and development expenses to decrease significantly as we discontinued our clinical research and development activities along with decreased general and administrative costs as we reduce headcount and other operational expenses that supported research and development activities. We will continue to incur research and development expenses in connection with clinical trial closing costs and the completion of certain ongoing nonclinical activities. We believe our cash balance, including our short-term investments, is sufficient to fund operations for at least the next 12 months.
Sources and Uses of Our Cash
Net cash used in operating activities for the three months ended September 30, 2024 of $11.5 million consisted of our net loss of $8.0 million and $3.9 million in changes in our operating assets partially offset by $0.5 million for noncash items. Net cash used in operating activities for the three months ended September 30, 2023 of $18.5 million consisted of our net income of $56.4 million and $0.8 million in noncash items offset by changes in our operating assets and liabilities of $75.7 million primarily due to recognition of $64.9 million in deferred revenue.
Net cash provided by investing activities for the three months ended September 30, 2024 was $14.7 million as compared to $5.0 million cash provided by investing activities for the three months ended September 30, 2023. The change in cash provided by investing activities was primarily due to more net short-term investment activity during the three months ended September 30, 2024.
Capital Resource Requirements
On June 18, 2024, we entered into a lease termination agreement (Agreement) with our landlord for our offices at 11455 El Camino Real, Suite 200 and Suite 250, San Diego, California. Under the Agreement, the lease terminated on September 30, 2024, rather than its scheduled expiration date of November 30, 2029. During the fiscal year ended June 30, 2024, we paid the landlord a termination fee totaling approximately $11.1 million in addition to prepaying the remaining rent under the Agreement in the amount of approximately $0.2 million. We have no further financial obligations under the Agreement.
As of September 30, 2024, we have the following potential purchase obligations for which the timing and/or likelihood of occurrence is unknown; however, if such claims arise in the future, they could have a material effect on our financial position, results of operations, and cash flows.
26
Our future capital requirements will depend on many factors, including:
Estimate Considerations Related to Macroeconomic Conditions and other Geopolitical Conditions
Due to recent disruptions in access to bank deposits and lending commitments associated with macroeconomic and geopolitical conditions, there has been uncertainty and disruption in the global economy and financial markets. We are not aware of any specific event or circumstance that would require an update to our estimates or judgments or a revision of the carrying value of our assets or liabilities as of September 30, 2024. While there was no material impact to our condensed consolidated financial statements as of and for the three months ended September 30, 2024, these estimates may change, as new events occur and additional information is obtained, which could materially impact our condensed consolidated financial statements in future reporting periods.
Critical Accounting Estimates
We describe our significant accounting policies in Note 2. Summary of Significant Accounting Policies, of the notes to the financial statements included in our 2024 Annual Report. We discuss our critical accounting estimates in Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, in our 2024 Annual Report. There have been no changes in our significant accounting policies or critical accounting estimates since June 30, 2024.
Recent Accounting Pronouncement
See Note 2. Summary of Significant Accounting Policies in the Notes to Condensed Consolidated Financial Statements in this Quarterly Report.
Item 3. Quantitative and Qualitative Disclosures about Market Risk
As a smaller reporting company, the Company is not required to provide the information otherwise required by this Item.
27
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures of a company that are designed to ensure that information required to be disclosed by the company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. As of the end of the period covered by this Quarterly Report, or September 30, 2024, our management, with the participation of our principal executive officer and principal financial officer, has evaluated the effectiveness of our disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based on such evaluation, our principal executive officer and principal financial officer have concluded that, as of such date, our disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting
Under the supervision and with the participation of our management, including our principal executive and principal financial officer, we carried out an evaluation of any potential changes in our internal control over financial reporting during the fiscal quarter ended September 30, 2024. There were no changes in our internal control over financial reporting (as defined in Rule 13a‑15(f) of the Exchange Act) that occurred during the fiscal quarter ended September 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations of Internal Controls
Our management does not expect that our disclosure controls and procedures or our internal controls over financial reporting will prevent or detect all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Due to the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
28
PART II OTHER INFORMATION
Item 1. Legal Proceedings
None.
Item 1A. Risk Factors
There have been no material changes in our risk factors from those included in our 2024 Annual Report
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None of our executive officers or directors have adopted, modified or terminated any contract, instruction, or written plan for the purchase or sale of the Company's securities that was intended to satisfy the affirmative defense conditions of Rule 10b5-1 (c) or any "non-Rule 10b5-1 trading arrangement" (as defined in Item 408(c) of Regulation S-K) during the three months ended September 30, 2024.
29
Item 6. Exhibits
Exhibit Index
|
|
|
|
Incorporated by Reference Herein |
||||||
|
Exhibit Number |
|
Description |
|
Schedule/ Form |
|
File No. |
|
Exhibit |
|
Filing Date |
2.1 |
|
|
8-K |
|
001-41827 |
|
2.1 |
|
October 25, 2022 |
|
3.1 |
|
|
8-K |
|
000-50484 |
|
3.1 |
|
April 14, 2023 |
|
3.2 |
|
Sixth Amended and Restated Bylaws of MEI Pharma, Inc. adopted as of December 18, 2023 |
|
8-K |
|
001-41827 |
|
3.1 |
|
December 22, 2023 |
3.3 |
|
Certificate of Designation of Series A Convertible Preferred Stock of Marshall Edwards, Inc. |
|
8-K |
|
000-50484 |
|
3.1 |
|
May 11, 2011 |
3.4 |
|
Certificate of Designation of Series B Preferred Stock of Marshall Edwards, Inc. |
|
8-K |
|
000-50484 |
|
10.1 |
|
March 18, 2011 |
3.5 |
|
Certification of Designation of Series A Junior Participating Preferred Stock |
|
8-K |
|
000-50484 |
|
3.1 |
|
October 3, 2023 |
4.1 |
|
|
S-1 |
|
333-109129 |
|
4.1 |
|
October 31, 2023 |
|
4.2 |
|
|
8-K |
|
000-50484 |
|
10.1 |
|
May 16, 2018 |
|
10.1+ |
|
Addendum to Employment Agreement between MEI Pharma, Inc. and Justin J. File, dated August 1, 2024 |
|
10-K |
|
000-50484 |
|
10.24 |
|
September 19, 2024 |
10.2* |
|
|
|
|
|
|
|
|
|
|
10.3* |
|
|
|
|
|
|
|
|
|
|
10.4* |
|
|
|
|
|
|
|
|
|
|
10.5* |
|
|
|
|
|
|
|
|
|
|
31.1* |
|
|
|
|
|
|
|
|
|
|
32.1** |
|
|
|
|
|
|
|
|
|
|
101INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
|
|
|
|
30
104 |
|
Cover Page Interactive Data File – the cover page interactive data file does not appear in the Interactive Data File because its XBRL tags are embedded within the XBRL document. |
|
|
|
|
|
|
|
|
* |
|
Filed herewith |
** |
|
Furnished herewith |
+ |
|
Portions of this exhibit have been omitted in accordance with Item 601(a)(6) and Item 601(b)(10) of Regulation S-K because such information (i) is not material and (ii) is the type that the registrant treats as private or confidential. |
31
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this Quarterly Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
MEI Pharma, Inc. |
|
|
|
/s/ Justin J. File |
|
Justin J. File Acting Chief Executive Officer, Chief Financial Officer and Secretary
|
|
|
|
Date: November 12, 2024 |
32
Exhibit 10.2
Separation and Release Agreement
This Separation and Release Agreement (“Agreement”) is made between you, Dr. Richard Ghalie, and MEI Pharma, Inc., and sets forth the terms of your separation from employment with MEI Pharma, Inc. and its affiliates (“MEI”). This Agreement will become effective upon the “Effective Date” as specified in Section 10(i), below. Once effective, this Agreement will be a legally binding document representing the entire agreement between you and MEI regarding the subjects it covers. Throughout this Agreement, the term “MEI” includes all of MEI’s affiliates and related entities, and their current and former trustees, officers, agents, employees, insurers and attorneys, and all other employee benefit plans and arrangements and their administrators, trustees and other fiduciaries, and all successors and assigns of all of the foregoing.
1
Exhibit 10.2
2
Exhibit 10.2
You specifically acknowledge that you are aware of and have been advised of the provisions of CALIFORNIA CIVIL CODE SECTION 1542, which provides as follows: A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
For the purpose of implementing a full and complete release, you hereby expressly waive all rights and benefits you may have under this section, as well as under any other statutes or common law principle of similar effect which provides any remedy of any kind, and acknowledge that the release set forth in this Agreement is intended to include the discharge of all claims which you do not know or suspect to exist at the time this Agreement is effective. You agree and acknowledge that this is a knowing and voluntary waiver. You are aware that you may hereafter discover claims or facts in addition to or different from those you now know or believe to exist with respect to the subject matter of this agreement which if you had known, may have affected your decision to sign this Agreement; however, you hereby settle and release all of the claims which you have or may have against MEI and the Released Parties including arising out of such additional or different facts.
You understand and acknowledge that you are hereby waiving and releasing any age claims or rights you may have under the ADEA, and that this waiver and release is knowing and voluntary. You understand and agree that this waiver and release does not apply to any rights or claims that may arise under the ADEA after the date you sign this Agreement. You understand and acknowledge that the consideration given for this waiver and release is in addition to anything of value to which you were already entitled. You further understand and acknowledge that you have been advised by this writing that: (a) you should consult with an attorney prior to executing this Agreement; (b) you have the periods described in Section 10 below to sign and revoke this Agreement.
3
Exhibit 10.2
Notwithstanding the foregoing, you are not waiving your right to (i) any vested benefits under a MEI benefit plan in which you participated, the rights to which are governed by the terms of the applicable plan documents and/or award agreement, (ii) claims for unemployment or workers’ compensation benefits, (iii) any medical claim incurred during your employment that is payable under applicable medical plans or a MEI-insured liability plan, (iv) claims arising after the date on which you sign this Agreement, (v) any rights to indemnification and defense under MEI’s bylaws and under directors and officers insurance with respect to your service as an employee or officer of MEI, or (vi) claims that are not otherwise waivable under applicable law.
4
Exhibit 10.2
Please take notice that federal law provides criminal and civil immunity to federal and state claims for trade secret misappropriation to individuals who disclose a trade secret to their attorney, a court, or a government official in certain, confidential circumstances that are set forth at 18 U.S.C. §§ 1833(b)(1) and 1833(b)(2), related to the reporting or investigation of a suspected violation of the law, or in connection with a lawsuit for retaliation for reporting a suspected violation of the law.
MEI Pharma, Inc.
11455 El Camino Real, Suite 250
San Diego, CA 92130
Attn: Chief People Officer
5
Exhibit 10.2
6
Exhibit 10.2
[Signature Page Follows.]
7
Exhibit 10.2
IN WITNESS WHEREOF, and intending to be legally bound, the parties hereto agree to all terms of this Agreement as of the date of this signature.
Dr. Richard Ghalie |
|
MEI Pharma, Inc. |
||
|
|
|
||
Date: |
|
|
By: |
|
|
|
Title: |
|
|
|
|
Date: |
|
|
[Signature Page to Separation and Release Agreement]
Exhibit 10.2
Exhibit A
Pursuant to federal law, MEI hereby informs you that severance pay and benefits are being offered to eligible employees who will separate from employment with MEI in accordance with the following:
MEI Pharma, Inc.
11455 El Camino Real, Suite 250
San Diego, CA 92130
Attn: Chief People Officer
Any questions about this disclosure should be addressed to the Chief People Officer at the address listed above.
A-1
Exhibit 10.2
Exhibit A-1
Job Titles and Ages of All Active Employees in the Decisional Unit
Selected and Not Selected for Termination
Job Title |
Age |
Selected |
Not Selected |
|
|
|
|
Chief Executive Officer |
59 |
X |
|
Chief Financial Officer |
53 |
X |
|
Chief Medical Officer |
66 |
X |
|
Contracts Administrator & Executive Assistant |
64 |
X |
|
Director, Clinical Data Management |
53 |
X |
|
Director, Clinical Operations |
40 |
X |
|
Director, Drug & Safety Pharmacovigilance |
40 |
X |
|
Director, Info Security & Infrastructure |
40 |
X |
|
Director, Supply Chain Management |
40 |
X |
|
Executive Director, Project Management |
57 |
X |
|
Human Resources Administrator |
33 |
X |
|
IT Systems Analyst |
39 |
X |
|
Manager, Payroll |
33 |
X |
|
Process Manager, Clinical Operations |
63 |
X |
|
Senior Accountant |
63 |
X |
|
Senior Director, Finance |
39 |
X |
|
Senior Director, Quality Assurance |
63 |
X |
|
Senior Director, Technical & CMC Ops |
56 |
X |
|
Senior Manager, Quality Systems |
48 |
X |
|
Sr. Dir, Nonclinical & Translational Sciences |
59 |
X |
|
Staff Accountant |
57 |
X |
|
SVP, Chief People Officer |
69 |
X |
|
SVP, Corporate Affairs |
57 |
X |
|
SVP, Quality and Operations |
50 |
X |
|
Vice President, Portfolio Strategy & New Prod |
47 |
X |
|
VP, Clinical Operations |
64 |
X |
|
VP, Information Systems |
65 |
X |
|
A-2
Exhibit 10.2
Job Title |
Age |
Selected |
Not Selected |
VP, Legal Affairs |
52 |
X |
|
A-3
Exhibit 10.3
Separation and Release Agreement
This Separation and Release Agreement (“Agreement”) is made between you, David Urso, and MEI Pharma, Inc., and sets forth the terms of your separation from employment with MEI Pharma, Inc. and its affiliates (“MEI”). This Agreement will become effective upon the “Effective Date” as specified in Section 10(i), below. Once effective, this Agreement will be a legally binding document representing the entire agreement between you and MEI regarding the subjects it covers. Throughout this Agreement, the term “MEI” includes all of MEI’s affiliates and related entities, and their current and former trustees, officers, agents, employees, insurers and attorneys, and all other employee benefit plans and arrangements and their administrators, trustees and other fiduciaries, and all successors and assigns of all of the foregoing.
1
Exhibit 10.3
2
Exhibit 10.3
You specifically acknowledge that you are aware of and have been advised of the provisions of CALIFORNIA CIVIL CODE SECTION 1542, which provides as follows: A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
For the purpose of implementing a full and complete release, you hereby expressly waive all rights and benefits you may have under this section, as well as under any other statutes or common law principle of similar effect which provides any remedy of any kind, and acknowledge that the release set forth in this Agreement is intended to include the discharge of all claims which you do not know or suspect to exist at the time this Agreement is effective. You agree and acknowledge that this is a knowing and voluntary waiver. You are aware that you may hereafter discover claims or facts in addition to or different from those you now know or believe to exist with respect to the subject matter of this agreement which if you had known, may have affected your decision to sign this Agreement; however, you hereby settle and release all of the claims which you have or may have against MEI and the Released Parties including arising out of such additional or different facts.
You understand and acknowledge that you are hereby waiving and releasing any age claims or rights you may have under the ADEA, and that this waiver and release is knowing and voluntary. You understand and agree that this waiver and release does not apply to any rights or claims that may arise under the ADEA after the date you sign this Agreement. You understand and acknowledge that the consideration given for this waiver and release is in addition to anything of value to which you were already entitled. You further understand and acknowledge that you have been advised by this writing that: (a) you should consult with an attorney prior to executing this Agreement; (b) you have the periods described in Section 10 below to sign and revoke this Agreement.
3
Exhibit 10.3
Notwithstanding the foregoing, you are not waiving your right to (i) any vested benefits under a MEI benefit plan in which you participated, the rights to which are governed by the terms of the applicable plan documents and/or award agreement, (ii) claims for unemployment or workers’ compensation benefits, (iii) any medical claim incurred during your employment that is payable under applicable medical plans or a MEI-insured liability plan, (iv) claims arising after the date on which you sign this Agreement, (v) any rights to indemnification and defense under MEI’s bylaws and under directors and officers insurance with respect to your service as an employee or officer of MEI, or (vi) claims that are not otherwise waivable under applicable law.
4
Exhibit 10.3
Please take notice that federal law provides criminal and civil immunity to federal and state claims for trade secret misappropriation to individuals who disclose a trade secret to their attorney, a court, or a government official in certain, confidential circumstances that are set forth at 18 U.S.C. §§ 1833(b)(1) and 1833(b)(2), related to the reporting or investigation of a suspected violation of the law, or in connection with a lawsuit for retaliation for reporting a suspected violation of the law.
MEI Pharma, Inc.
11455 El Camino Real, Suite 250
San Diego, CA 92130
Attn: Chief People Officer
5
Exhibit 10.3
6
Exhibit 10.3
[Signature Page Follows.]
7
Exhibit 10.3
IN WITNESS WHEREOF, and intending to be legally bound, the parties hereto agree to all terms of this Agreement as of the date of this signature.
David Urso |
|
MEI Pharma, Inc. |
||
|
|
|
||
Date: |
|
|
By: |
|
|
|
Title: |
|
|
|
|
Date: |
|
|
[Signature Page to Separation and Release Agreement]
Exhibit 10.3
Exhibit A
Pursuant to federal law, MEI hereby informs you that severance pay and benefits are being offered to eligible employees who will separate from employment with MEI in accordance with the following:
MEI Pharma, Inc.
11455 El Camino Real, Suite 250
San Diego, CA 92130
Attn: Chief People Officer
Any questions about this disclosure should be addressed to the Chief People Officer at the address listed above.
A-1
Exhibit 10.3
Exhibit A-1
Job Titles and Ages of All Active Employees in the Decisional Unit
Selected and Not Selected for Termination
Job Title |
Age |
Selected |
Not Selected |
|
|
|
|
Chief Executive Officer |
59 |
X |
|
Chief Financial Officer |
53 |
X |
|
Chief Medical Officer |
66 |
X |
|
Contracts Administrator & Executive Assistant |
64 |
X |
|
Director, Clinical Data Management |
53 |
X |
|
Director, Clinical Operations |
40 |
X |
|
Director, Drug & Safety Pharmacovigilance |
40 |
X |
|
Director, Info Security & Infrastructure |
40 |
X |
|
Director, Supply Chain Management |
40 |
X |
|
Executive Director, Project Management |
57 |
X |
|
Human Resources Administrator |
33 |
X |
|
IT Systems Analyst |
39 |
X |
|
Manager, Payroll |
33 |
X |
|
Process Manager, Clinical Operations |
63 |
X |
|
Senior Accountant |
63 |
X |
|
Senior Director, Finance |
39 |
X |
|
Senior Director, Quality Assurance |
63 |
X |
|
Senior Director, Technical & CMC Ops |
56 |
X |
|
Senior Manager, Quality Systems |
48 |
X |
|
Sr. Dir, Nonclinical & Translational Sciences |
59 |
X |
|
Staff Accountant |
57 |
X |
|
SVP, Chief People Officer |
69 |
X |
|
SVP, Corporate Affairs |
57 |
X |
|
SVP, Quality and Operations |
50 |
X |
|
Vice President, Portfolio Strategy & New Prod |
47 |
X |
|
VP, Clinical Operations |
64 |
X |
|
VP, Information Systems |
65 |
X |
|
A-2
Exhibit 10.3
Job Title |
Age |
Selected |
Not Selected |
VP, Legal Affairs |
52 |
X |
|
A-3
Exhibit 10.4
CONSULTING SERVICES AGREEMENT
Amended September 13, 2024
THIS CONSULTING SERVICES AGREEMENT (this “Agreement”) is entered into as of August 2, 2024 by and between MEI Pharma, Inc. (the “Company”), and Dr. Richard Ghalie (“Consultant”).
AGREEMENT
WHEREAS, the Company wishes to obtain the services of Consultant, and Consultant wishes to provide such services, subject to the terms and condition of this Agreement.
NOW, THEREFORE, in consideration of the mutual promises hereinafter set forth, and intending to be legally bound hereby, the Company and Consultant hereby agree as follows:
Exhibit 10.4
Exhibit 10.4
Exhibit 10.4
Exhibit 10.4
Exhibit 10.4
[Signature Page Follows.]
Exhibit 10.4
IN WITNESS WHEREOF, the undersigned, intending to be legally bound, have duly executed this Agreement as of the date first above written.
MEI PHARMA, INC.
By: ___________________________
Name: Jay File
Title: Chief Financial Officer
Date:
CONSULTANT
_______________________________
Print Name: Dr. Richard Ghalie
Date:
Exhibit 10.4
Exhibit A
Description of Services; Compensation
Exhibit 10.5
CONSULTING SERVICES AGREEMENT
Amended September 13, 2024
THIS CONSULTING SERVICES AGREEMENT (this “Agreement”) is entered into as of August 2, 2024 by and between MEI Pharma, Inc. (the “Company”), and David Urso (“Consultant”).
AGREEMENT
WHEREAS, the Company wishes to obtain the services of Consultant, and Consultant wishes to provide such services, subject to the terms and condition of this Agreement.
NOW, THEREFORE, in consideration of the mutual promises hereinafter set forth, and intending to be legally bound hereby, the Company and Consultant hereby agree as follows:
Exhibit 10.5
Exhibit 10.5
Exhibit 10.5
Exhibit 10.5
Exhibit 10.5
[Signature Page Follows.]
Exhibit 10.5
IN WITNESS WHEREOF, the undersigned, intending to be legally bound, have duly executed this Agreement as of the date first above written.
MEI PHARMA, INC.
By: ___________________________
Name: Jay File
Title: Chief Financial Officer
Date:
CONSULTANT
_______________________________
Print Name: David Urso
Date:
Exhibit 10.5
Exhibit A
Description of Services; Compensation
Exhibit 31.1
CERTIFICATION
I, Justin J. File, certify that:
Date: November 12, 2024 |
|
|
|
/s/ Justin J. File |
|
|
|
|
Justin J. File Acting Chief Executive Officer, Chief Financial Officer and Secretary (Principal Executive Officer and Principal Financial Officer) |
Exhibit 32.1
CERTIFICATION
The undersigned hereby certifies, for the purposes of Section 1350 of Chapter 63 of Title 18 of the U.S. Code, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, in his capacity as Acting Chief Executive Officer and Chief Financial Officer of MEI Pharma, Inc. (“MEI Pharma”) that, to his knowledge, this Quarterly Report on Form 10-Q of MEI Pharma, for the quarter ended September 30, 2024, fully complies with the requirements of Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended, and that the information contained in such report fairly presents, in all material respects, the financial condition and results of operations of MEI Pharma.
Dated: November 12, 2024
|
/s/ Justin J. File |
|
Justin J. File |
|
Acting Chief Executive Officer, Chief Financial Officer and Secretary (Principal Executive and Principal Financial Officer) |
These certifications accompanying the report to which they relate, are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of MEI Pharma under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, except to the extent MEI Pharma specifically incorporates it by reference.
A signed original of this written statement required by Section 906 has been provided to MEI Pharma and will be retained by MEI Pharma and furnished to the Securities and Exchange Commission or its staff upon request.