UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2024
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-39387

Renalytix plc
(Exact name of Registrant as specified in its Charter)
England and Wales |
Not Applicable |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
2 Leman Street |
E1W 9US |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: +44 20 3139 2910
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
American Depositary Shares, each representing two ordinary shares, nominal value £0.0025 per share |
|
RNLX |
|
The Nasdaq Stock Market, LLC |
Ordinary shares, nominal value £0.0025 per share |
|
* |
|
The Nasdaq Stock Market, LLC* |
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
As of December 29, 2023, the aggregate market value of the Registrant’s ordinary shares, nominal value £0.0025 per share, held by non-affiliates of the Registrant, based on the closing price of the American Depositary Shares on the Nasdaq Global Market on December 29, 2023, was $14,024,875. The Registrant has no non-voting common equity.
As of September 16, 2024, there were 165,925,513 ordinary shares outstanding, which if all were held in ADS form would be represented by 82,912,756 American Depositary Shares, each representing two ordinary shares.
RENALYTIX PLC
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
|
|
Page |
|
|
|
PART I |
|
|
Item 1. |
||
Item 1A. |
||
Item 1B. |
||
Item 1C. |
||
Item 2. |
||
Item 3. |
||
Item 4. |
||
|
|
|
PART II |
|
|
Item 5. |
||
Item 6. |
||
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
Item 7A. |
||
Item 8. |
||
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
Item 9A. |
||
Item 9B. |
||
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
|
|
|
|
PART III |
|
|
Item 10. |
||
Item 11. |
||
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
|
Item 14. |
||
|
|
|
PART IV |
|
|
Item 15. |
||
Item 16. |
||
i
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the year ended June 30, 2024 (this “Annual Report”) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by the words “may,” “might,” “will,” “could,” “would,” “should,” “goal,” “target,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue” and “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. The forward-looking statements and opinions contained in this Annual Report are based upon information available to us as of the date of this Annual Report and, while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. Forward-looking statements include statements about:
ii
You should refer to the sections of this Annual Report titled “Item 1A. Risk Factors” and “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. Forward-looking statements speak only as of the date on which such statements are made. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law, applicable regulations or the rules of the Nasdaq Stock Market LLC.
You should read this Annual Report, the documents that we reference in this Annual Report and the documents we have filed as exhibits to this Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
iii
PART I
Item 1. Business.
Introductory Note
In this Annual Report, we use the terms “KidneyIntelX”, “KidneyIntelX Technology”, “KidneyIntelX Technology Platform” and “kidneyintelX.dkd.” When we refer to KidneyIntelX, we are referring to our diagnostic platform and any products developed based on this platform including our KidneyIntelX laboratory developed test offered as a testing service across the United States from our CLIA certified laboratories. When we refer to kidneyintelX.dkd, we are referring to the specific testing service from our KidneyIntelX technology platform or KidneyIntelX technology that has received De Novo marketing authorization from the U.S. Food and Drug Administration ("FDA") to assess risk of progressive kidney function decline in adults with type 2 diabetes and early-stage kidney disease. The FDA granted De Novo marketing authorization for kidneyintelX.dkd on June 29, 2023.
Overview
At Renalytix, we developed kidneyintelX.dkd, the first FDA authorized in-vitro prognostic test, comprising of blood biomarkers and an artificial intelligence-enabled algorithm, and used as an aid in assessment of the risk of progressive decline in kidney function. The test is designed to identify which patients are most at risk for significant sustained decline in kidney function in the early stages of disease. When used as intended, kidneyintelX.dkd can potentially support decisions regarding medical interventions early-on in the disease process, before major damage occurs and when therapies can be most effective thereby potentially avoiding kidney failure requiring long-term dialysis or kidney transplant. The current addressable market for kidneyintelX.dkd in the US is approximately 14 million patients with adult diabetes and diagnosed chronic kidney diseases. Globally the addressable market for kidneyintelX.dkd is estimated to be 260 million patients.
The kidneyintelX.dkd test received formal Medicare coverage in June of 2024 at $950 per test and has now received coverage by several major insurance plans including large state Blue Cross Blue Shield plans. It is expected that the majority of patients who meet the intended use for kidneyintelX.dkd in the US are over the age of 65 years and therefore are covered by the Medicare.
KidneyIntelX was included as a risk assessment tool for patients with early stage CKD in KDIGO (Kidney Disease, Improving Global Outcomes) 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (CKD), published in April 2024.
Chronic kidney disease is one of the largest and urgent medical needs, affecting an estimated 850 million people globally and responsible for an unsustainable societal cost burden. We believe an important part of the answer is preventative medicine and the ability to identify individuals with advancing chronic kidney disease early, where new drug therapies and clinical strategies have the optimal chance to stop uncontrolled disease progression.
Recent Developments
This past year has seen the achievement of milestones necessary for broad commercial expansion of the use of the KidneyIntelX technology in select regions of the United States with high rates of diabetes and kidney disease. Key milestones include launching the FDA De Novo marketing authorized test, kidneyintelX.dkd, inclusion of KidneyIntelX for clinical use in the KDIGO 2023 Clinical Practice Guideline for Evaluation and Management of Chronic Kidney Disease, securing long term Medicare coverage at $950 per test for kidneyintelX.dkd, an expansion of commercial insurance coverage at the Medicare national payment rate of $950 per test, and publication of real world utility and outcomes data.
During this past year, Management has completed a series of operational restructurings that has resulted in new sales leadership, refinement of commercial strategy with an emphasis on direct to doctor sales, and a shift away from research & development activities related to clinical study and regulatory processes to a largely commercial sales focus. With the de-risking of major regulatory, reimbursement, clinical data and guidelines inclusion milestones, Management believes all major elements are in place to support continued sequential annual revenue growth.
Management is now completing the restructuring of the cost structure of the company which has resulted in a year over year 60% reduction in cash-burn rate, and a year over year 50% reduction in overhead.
Collectively as a result of crossing major commercial milestones, achievements of cost reductions, recent success with revenue growth and addition a of major new network of healthcare providers in September 2024, management now believes the Company can achieve financial break-even status in two years.
1
The Company is expected to close an equity financing in October 2024 that will increase balance sheet cash by approximately $14 million. Management projects that this equity financing, to be completed largely with a group of recognized institutional investment concerns, should provide more than adequate resources to significantly grow testing adoption and revenues and is adequate to cover operating costs through to profitability.
On June 29, 2023, the FDA granted De Novo marketing authorization granted for kidneyintelX.dkd for the assessment of risk of progressive kidney function decline in adults with diabetes and early-stage kidney disease (also referred to as diabetic kidney disease ("DKD"). An estimated 14 million Americans adults currently fall within the FDA authorized indicated use population for kidneyintelX.dkd. In the reporting period, we successfully transitioned our customers to the FDA approved test from the prior laboratory developed test (LDT) version, including upgrading of Sales & Marketing materials, optimization of internal processes, streamlining of Electronic Health Record interfaces with health systems and inclusion of kidneyintelX.dkd in insurance payor contracts.
Further, KidneyIntelX has been included in the published Kidney Disease Improving Global Outcomes (KDIGO) 2023 Clinical Practice Guideline for Evaluation and Management of Chronic Kidney Disease. KDIGO guideline development follows an explicit process to translate global scientific evidence review and appraisal into practical recommendations for clinicians and patients. The final version of these kidney disease guidelines highlights the importance of risk assessment in the early stage of disease and includes KidneyIntelX as the only in-vitro diagnostic test indicated for this purpose.
Importantly, these significant milestones in the diagnostic product lifecycle would not be achievable without establishing a comprehensive, peer reviewed portfolio of data publications covering four key areas: 1) clinical outcomes, 2) clinical utility, 3) health economics, and 4) performance validations. Since Renalytix achieved its first large capital infusion from listing on the London Stock Exchange nearly five years ago, we have invested heavily in these core categories of proof in support of the KidneyIntelX technology and believe we have exceeded standards for delivering an extensive data portfolio necessary to support broad-scale clinical use and insurance reimbursement. Significantly, in the reporting period, Real World Evidence (“RWE”) after 12 months of follow-up in 2,569 patients with Type 2 diabetes and diabetic kidney disease (“DKD”) at a major U.S. health system, was published in the Primary Care and Community Health journal. Results in the diverse cohort, including 27% black patients, demonstrate that use of KidneyIntelX was associated with clinical actions that led to a significant slowing of chronic kidney disease progression and improved Type 2 diabetes control, most notably in the highest risk patients. Improved kidney health was evidenced by reduction in the rate of kidney function decline (eGFR slope) and diabetes control was evidenced by improved A1C levels.
Our commercial model is focused on expanding clinical use in a limited group of regions in the United States with high rates of adult diabetes, where we have established comprehensive insurance coverage and where there are large physician networks and health systems available to enable outreach to large groups of primary care physicians. In the period, the regional focus has been on New York, Florida and Texas whereby the above conditions have been established. As we continue to demonstrate revenue growth and adoption, we will continue to add regions where the kidneyintelX.dkd service provision can demonstrate attractive return-on-investment and revenue growth potential.
Kidney disease is a worldwide public health crisis, resulting in more deaths per year than breast or prostate cancer. The National Kidney Foundation (the "NKF"), estimates that one-third of adults in the United States are at risk of developing kidney disease. Moreover, the kidney disease crisis is continuing to grow along with the increased prevalence of contributing risk factors. One of the most significant risk factors for developing CKD is diabetes. It is estimated that there are approximately 14 million adults with DKD in the United States, and DKD is the most common cause of End Stage Kidney Disease (ESKD) in most developed countries. Obesity is believed to account for 80% to 85% of the risk of developing type 2 diabetes. The worldwide prevalence of obesity nearly tripled between 1975 and 2016. Further, according to a 2019 study from the Harvard T.H. Chan School of Public Health, by 2030, it is estimated that about half of the U.S. adult population will be classified as obese and about a quarter as severely obese. This significant projected increase in the prevalence of obesity is expected to continue to drive an increase in diabetes, CKD, DKD and ESKD.
Managing a CKD population of this scale and the associated healthcare spending presents a unique healthcare system challenge, requiring a solution that provides a clearer understanding of clinical risk which can inform specific guideline-driven clinical actions. The ability to predict which patients will experience progressive and sustained kidney function decline or kidney failure (requiring initiation of long-term dialysis or kidney transplant), is critical to changing patient outcomes and health economics. Other methods for risk stratification of patients with CKD lack sufficient precision in predicting progressive kidney function decline, especially at earlier stages of the disease. This can exacerbate the occurrence of unexpected and expensive clinical events. In fact, up to 38% of patients with CKD initiate dialysis with little or no prior clinical specialist consultation, and up to 63% of patients with CKD initiate dialysis in an unplanned fashion with a central venous catheter and/or during emergency hospitalization, which we refer to as “dialysis crash.” This highlights the need for risk assessment as reflected in the updated KDIGO guidelines and a mechanism to identify potential instances of rapidly progressing CKD before it becomes critical to the patient’s health and costly to healthcare systems.
2
KidneyIntelX technology addresses this challenge as a first-in-class, artificial intelligence-enabled prognostic test to help guide care management for adults with DKD by reporting three discrete risk levels (low, moderate, and high) within the following five year period. This timely information on patient risk for progressive decline in kidney function within five years, provides independent information from the current standard of care measures and can be readily deployed at the primary care level where the vast majority of patients with early-stage disease are being treated.
Early detection and intervention can result in health economic benefits in three key areas: (1) slowing progression to the next stage of DKD, (2) delaying or preventing progression to ESKD and the need for dialysis or kidney transplant and (3) avoiding dialysis crashes.
According to an independent review commissioned by Boston Healthcare Associates, based on the Medicare established price of $950 per reportable test, successful incorporation of the KidneyIntelX technology could generate a positive return for health insurers in 12-24 months and deliver a cost savings of up to $1.1 billion over five years per 100,000 patients with DKD, when considering these key areas of benefit.
In the reporting period, having crossed the significant technical, regulatory and reimbursement milestones, our executive team, sales and operations teams has been re-configured to reflect the transition to full commercialization of the FDA authorized kidneyintelX.dkd test.
Recent Clinical Publications and Presentations
A significant milestone was achieved with the publication of outcomes at 12 months following enrollment into the KidneyIntelX Real Word Evidence program. The publication titled “A Real-World Precision Medicine Program Including the KidneyIntelX Test Effectively Changes Management Decisions and Outcomes for Patients With Early-Stage Diabetic Kidney Disease” presented compelling data showing the multiple impacts early stage risk assessment by KidneyIntelX including risk based provider actions, therapy management and associated improvement in clinical outcomes.
Additional scientific, clinical and health economics data were published and/or presented in multiple scientific conferences including the American Society of Nephrology Kidney Week 2023, the National Kidney Foundation Spring Clinical Meeting 2024, American Diabetes Association 83rd Scientific Session in June 2024, and AMCP 2024.
Our strategy
Our goal is to lower healthcare costs and improve patient quality of life by transforming the paradigm for kidney disease risk assessment and clinical management through the use of the now FDA authorized kidneyintelX.dkd test. Core strategy elements to achieve this goal include the following:
3
We believe KidneyIntelX technology produces early, actionable prognosis that can support clinician intervention to slow the progression of kidney disease and potentially prevent decline to kidney failure and the need for long-term dialysis or kidney transplant. We have built a comprehensive body of published evidence through clinical validation studies and real-world data generation to demonstrate that accurate and early identification of patient risk to inform guidelines-based clinical care, can have a measurable positive impact on patient quality of life and significantly lower healthcare costs. By involving a broad range of expert clinical opinions, testing a growing number of patient samples, consulting closely with clinical society and patient advocacy organizations, partnering with healthcare systems and payors and developing a detailed understanding of the clinical practice environment, we believe successful use of KidneyIntelX technology will help ease suffering and improve outcomes for patients living with DKD.
Our competitive strengths
The KidneyIntelX platform has the following key strengths:
4
Industry background
Chronic kidney disease
Kidney disease is a worldwide public health crisis, resulting in more deaths per year than breast or prostate cancer. The International Society of Nephrology estimates that kidney disease affects over 850 million people worldwide. According to the Centers for Disease Control and Prevention (the "CDC") affects approximately 36 million people in the United States alone, and the National Kidney Foundation (the "NKF"), estimates that one third of adults in the United States are at risk of developing kidney disease.
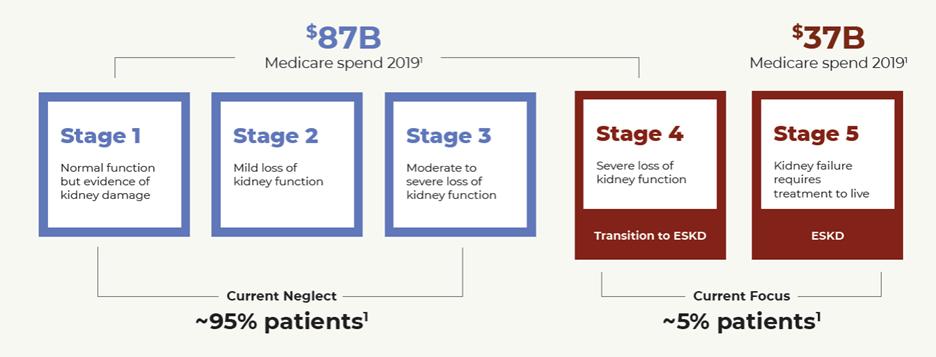
CKD, also called chronic kidney disease, is the loss of kidney function over time. Advanced kidney disease is generally not reversible. There are five stages of CKD, from mild kidney damage in Stage 1 to complete kidney failure in Stage 5. The stages of kidney disease are based on how well the kidneys can filter waste and extra fluid out of the blood, as measured by an individual’s estimated glomerular filtration rate ("eGFR"). The estimation of GFR is derived from a routine blood test for creatinine, a waste product in blood. When CKD reaches an advanced stage (e.g., Stage 4), dangerous levels of extra fluid, electrolytes and wastes can build up in the body. An eGFR of 60 mL/min/1.73m2 or more is considered normal function, but is classified as Stage 1 or 2 CKD if there is other evidence of kidney damage based a urinary albumin creatinine ratio ("uACR") of ≥ 30 mg/g. Albumin is a protein made by the liver that helps keep fluid in the bloodstream and albuminuria, or the presence of too much albumin in an individual’s urine, is a sign that the kidneys are damaged. As a patient’s disease progresses, the eGFR will decrease and uACR will typically increase. An eGFR of less than 15 mL/min/1.73m2 indicates a patient is in Stage 5, the last stage of CKD, which is kidney failure or ESKD. ESKD is fatal without long-term dialysis or a kidney transplant.
1United States Renal Data System. 2020 USRDS Annual Report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2020
Commonly referred to as a “silent disease,” CKD is often asymptomatic until approximately 70% to 80% of kidney function has been lost. According to the CDC, in the United States, nine out of ten adults with CKD are not aware they have the disease. In fact, up to 38% of patients with CKD initiate dialysis with little or no prior clinical specialist consultation, and up to 63% of patients with CKD initiate dialysis in an unplanned fashion with a central venous catheter and/or during emergency hospitalization, which we refer to as “dialysis crash.” This highlights the need for an early mechanism to identify potential instances of rapidly progressing CKD before it becomes critical to the patient’s health and costly to healthcare providers.
5
In 2018, more than 783,000 patients had ESKD with more than 554,000 requiring dialysis at least three times a week. More than 131,000 patients begin dialysis each year to treat ESKD. The incidence and prevalence rates of ESKD are projected to increase significantly as set forth in the figure below.
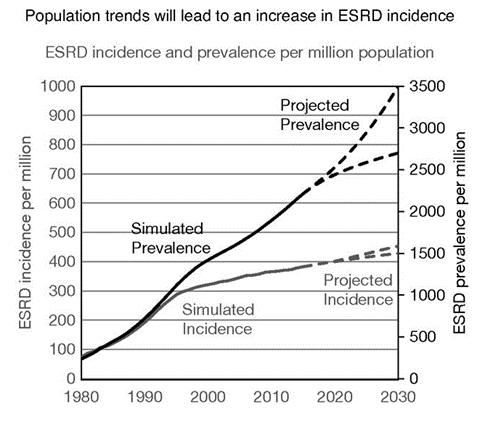
Figure. Projected Incidence and Prevalence of ESRD in the United States (McCullough, KP, et al. Journal of the American Society of Nephrology 30(1):p 127-135, 2019)
Once on dialysis, patients typically experience a five-year mortality rate of up to 70%, about the equivalent rate for brain cancer. As of July 2019, nearly 100,000 Americans were on the waiting list to receive a kidney transplant and 13 patients die in the United States while waiting for a kidney transplant every day.
Studies have shown that ancestry is a determining factor for kidney disease risk. According to the CDC, Americans of African ancestry are three times more likely to develop kidney disease than Caucasians. Since approximately 13% of the U.S. population is of African ancestry, this is a crucial population group that can benefit from advanced and ongoing risk assessment of kidney health.
Chronic kidney disease, obesity and diabetes
One of the most significant risk factors for developing CKD is type 2 diabetes, referred to as DKD. It is estimated that there are approximately 12.6 million adults with DKD in the United States. DKD is the most common cause of ESKD in most developed countries and accounts for approximately half of all patients who will experience kidney failure, or nearly 50,000 patients in the United States each year.
6
Further, the number of individuals with diabetes is growing. According to a study published in 2018, the number of adults in the United States diagnosed with diabetes is projected to nearly triple, reaching 60 million in 2060.
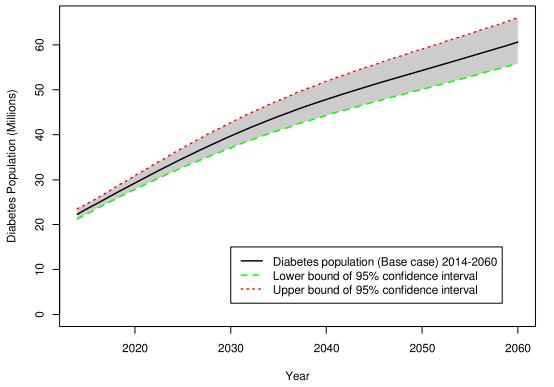
Figure. Projection of diagnosed diabetes prevalence in US adults (Lin et al. Population Health Metrics 2018 16:9)
The primary driver of type 2 diabetes is obesity, which is believed to account for 80% to 85% of the risk of developing type 2 diabetes. Recent research suggests that obese people are up to 80 times more likely to develop type 2 diabetes than those with a body mass index, or BMI of less than 22. According to the World Health Organization (the "WHO"), in 2016, more than 1.9 billion adults aged 18 years and older were overweight. Of these, over 650 million adults were obese. For adults, the WHO defines overweight as having a BMI greater than or equal to 25 and obesity as having a BMI greater than or equal to 30. The worldwide prevalence of obesity nearly tripled between 1975 and 2016. Further, according to a 2019 study from the Harvard T.H. Chan School of Public Health, by 2030, about half of the adult U.S. population will be obese and about a quarter will be severely obese, which is defined as having a BMI greater than 40 (or 100 pounds over an individual’s healthy body weight). This significant projected increase in the prevalence of obesity and severe obesity is expected to continue to drive an increase in diabetes, CKD, DKD and ESKD.
Significant healthcare system costs associated with CKD
According to the United States Renal Data System’s 2019 Annual Data Report (USRDS), Medicare spends over $120 billion per year, or over 20% of its total budget, on the treatment of CKD, including approximately $36 billion for the treatment of patients with ESKD. Treatment for kidney disease consumes 6.7% of the total Medicare budget to care for less than 1% of the covered population. In the United States, hemodialysis costs approximately $90,000 per patient per year and a kidney transplant costs approximately $260,000, with annual follow-up costs averaging approximately $40,000. According to the NKF, more than two million people worldwide are treated with dialysis or kidney transplants, making CKD a global public health crisis.
7
Current risk classification paradigm and limitations
The KDIGO classification system is the standard clinical assessment to predict risk for progression of CKD, including DKD. The KDIGO classification system uses cut-offs of two continuous biologic variables, eGFR and uACR, to group patients into risk strata. There are six strata for eGFR and three categories of albuminuria. Patients are then categorized into four categories of risk: low risk (green), moderately increased risk (yellow), high risk (orange) and very high risk (red) as presented below.
CKD staging based on
Kidney disease improving global outcomes (KDIGO) guidelines
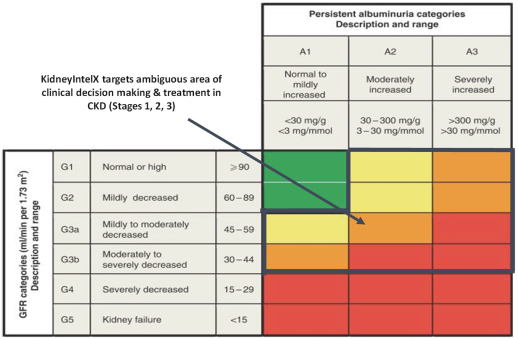
While we believe the KDIGO guidelines set an important baseline of classification and represent a core component for clinical management of CKD, problems arise with its real-world application. First, the KDIGO classification boundaries represent approximations, which stratify patients into easy to remember categories. As a result, however, patients at the extremes of risk strata, with widely differing risk for clinical outcomes, can be grouped into one risk category.
For example, patients with uACR 30 mg/mmol (milligram albumin per millimole creatinine) or 400 mg/mmol are both classified as A3 albuminuria. Further, a patient with an eGFR of 43 and one with an eGFR of 31 are both classified at G3b. In both cases, these patients have very different risk of disease outcomes.
Second, there are biologic differences within the KDIGO classification system that are not recognized, and there are dichotomies created that are not biologically or prognostically heterogeneous. For example, eGFR of 46 versus 44 crosses G3a to G3b and places someone in a different KDIGO risk category, as does a UACR of 29 vs. 32 mg/mmol. In other words, the KDIGO classification system imposes cutoffs of risk strata despite the fact that the underlying biologic variables are continuous. As a result, the KDIGO classification system has been shown in practice to lack sufficient precision to predict who will experience rapid and sustained progression in kidney function decline, especially in earlier stages of DKD (Stages 1 through 3).
Further, lack of ability to accurately predict which patients with CKD are at high risk of progression has led to strained clinician resources, inadequate referrals to clinical specialists and suboptimal treatment of DKD resulting in significant patient suffering and diminished quality of life. Because kidney disease is so common and the current standard of care does not adequately risk stratify patients, primary care physicians or endocrinologists typically are caring for most people with non-dialysis dependent CKD and many high-risk patients are not referred to clinical specialists in a timely manner. Indeed, more than 90% of individuals with CKD are in stages 1-3b, where awareness of the disease is only approximately 10%. One of the reasons for the inertia for most patients with stages 1-3 CKD is the high patient burden and lack of available time do not allow these physicians to fully assess the vast amount of data from the EHR to enable proper risk stratification and treatment for these patients. For example, only around half of all eligible patients with DKD are on antagonists of the renin angiotensin aldosterone system, medications which are the standard of care, and less than 10% are on sodium-glucose transport protein 2 inhibitors, (SGLT2i), newer medications that have been shown to substantially slow kidney disease progression.
8
In addition, there is a lack of appropriate patient counseling on the progressive nature of the patient’s disease, leading to lack of compliance with treatment protocol and decreased awareness of kidney disease.
Moreover, in the United States, there is a limited number of nephrologists to handle the ever-increasing number of patients with CKD. According to the CDC, there are approximately 9,000 nephrologists in the United States, or one specialist to 1,666 patients. Targeted referral of patients who have been accurately identified as having a high risk of progression over the immediate period of the next 5 years, can help to assure clinical resources are utilized efficiently and effectively. There is a critical need for easily interpretable and accurate diagnostic and predictive tools for CKD and DKD, with seamless integration into clinical workflow.
In publication of the 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, KDIGO have recognized the clinical need to address the absence of accurate risk assessment for patients in earlier stages of disease in whom progression can still occur through inclusion of KidneyIntelX as a validated to provide a more personalized approach to treatment and care based on risk.
Market opportunity
Our goal is to improve quality of life and lower healthcare costs by transforming the paradigm for kidney disease risk assessment and clinical management. We believe the use of our KidneyIntelX technology and our now FDA authorized kidneyintelX.dkd test will continue to drive improved patient outcomes and significantly lower healthcare costs. It is estimated that there are approximately 14 million adults in the United States that meet the indicated use of our FDA authorized test.
Our technology platform solution
Overview
We designed the KidneyIntelX technology platform to enable risk prediction of progressive kidney function decline in patients with CKD. The platform employs an artificial intelligence-enabled algorithm that is capable of using diverse data inputs, including validated blood-based biomarkers from a patient blood draw, inherited genetics and personalized patient data from EHR systems, to generate a unique patient risk score. The unique patient risk score is then reported to the treating clinician through an interface that provides the reportable risk level to help inform guideline-driven clinical actions.
The kidneyintelX.dkd test is comprised of the following core elements:
(A) A multiplex electrochemiluminescence assay for the in vitro quantitative determination of tumor necrosis factor receptors 1 and 2 (TNFR-1, TNFR-2), and kidney injury molecule 1 (KIM-1) in human plasma (whole blood K2EDTA blood collection tube). The assay is designed for use with the MESO SECTOR® S 600 instrument to quantify the three biomarkers. The assay is performed by trained laboratory personnel at Renalytix using the assay components that includes the KidneyintelX.dkd 96-well plate, the detection antibody, calibrator, and controls along with the MesoScale Diagnostics diluents, blocker, wash buffer and read buffer.
(B) The kidneyintelX.dkd Portal, a cloud-based system that contains the software algorithm provides a kidneyintelX.dkd Level (High, Moderate, Low) by combining the biomarker results from the assay and patient- specific results for uACR, HbA1C, and BUN.
(C) A kidneyintelX.dkd Test Report containing the risk level and interpretation of the test result.
The kidneyintelX.dkd test is an in-vitro diagnostic performed by Renalytix laboratory and is for Prescription Use Only.
Advanced Prognostic Performance
To support FDA De Novo marketing authorization of kidneyintelX.dkd, clinical validation studies were performed to demonstrate prognostic performance of the test in representative patient populations. Training of the machine learning algorithm was performed in a cohort from the University of Pennsylvania Biobank (UPenn) and validation was completed in an external cohort from the BioMe biobank from Icahn School of Medicine at Mount Sinai.
9
Kaplan-Meier curves were plotted for each of the kidneyintelX.dkd levels to display the incidence of subjects with progressive decline in kidney function over time up to a maximum follow-up of 5 years. Progressive decline in kidney function was assessed according to estimated cumulative risk at each level as shown in the figure below, demonstrating excellent separation and stratification for progressive decline in kidney function between the low, moderate and high risk categories.
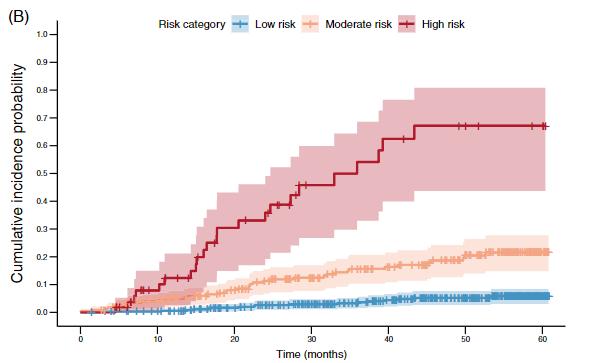
Figure. Cumulative incidence curves for progressive decline in kidney function. (Nadkarni, GN, Stapleton, S, Takale, D, et al. Derivation and independent validation of KidneyintelX.dkd: A prognostic test for the assessment of diabetic kidney disease progression. Diabetes Obes Metab. 2023; 1-9. doi:10.1111/dom.15273)
The cumulative incidence of progressive decline of kidney function decline was approximately 2/3rds in the high-risk group (67%, 95% CI 49% - 84%), and the rates of eGFR decline in the low risk group were comparable to that of normal physiologic aging.
Using Cox Proportional Hazard Ratios to compare risk across the levels, an18-fold increased risk for high vs. low risk levels, and 4-fold increased risk for moderate vs. low risk levels were observed. The test was shown to be robustly prognostic after adjustment for key demographics and clinical variables (adjusted HR for high vs. low 7.7 and 3.7 for moderate vs. low risk), with consistent performance across diverse subgroups of the intended use population.
The KidneyIntelX model
At the core of our approach is an artificial intelligence-enabled algorithm capable of synthesizing a set of current and diverse data inputs, such as biomarkers, EHR data, genomics, patient-generated digital data, environmental information, clinical utility, and actuarial and clinical compliance information.
Proprietary blood-based biomarkers
Blood-based biomarkers are typically genes or proteins that indicate the existence and severity of certain conditions (such as kidney disease) and can be measured from a simple blood sample. KidneyIntelX includes inputs from three specific blood-based biomarkers that have previously been examined in several academic and clinical study settings as reported in scientific publications. These publications support consistent associations of soluble Tumor Necrosis Factor Receptor (sTNFR) 1 and 2 and plasma Kidney Injury Molecule-1 (KIM-1), with reliable independent predictive signals for kidney disease progression in DKD patients. The concentration of these biomarkers are measured using a proprietary, analytically validated multiplex assay with excellent accuracy and precision.
We are exploring additional biomarkers from blood, urine and other biological samples for subsequent KidneyIntelX technology platform offerings that could support enhanced predictive performance and expanded indicated uses.
10
Seamless integration with electronic health record systems for test ordering and reporting results
KidneyIntelX is designed to interface with EHR systems in order to securely access the information required for each ordered test, which is then combined with biomarker data to generate the risk score and test report. The test result can then be reported directly to the ordering physician through the EHR system.
In this way, the treating physician can have all of the relevant information pertinent to the patient’s care delivered to them at the time of the clinical encounter and can trigger care pathways directly from the EHR interface, with the goal of driving a virtuous cycle in which patients and clinicians have increased visibility and awareness of changes in care management and patient behavior on kidney health.
All personal health information captured by the kidneyintelX.dkd application is at all times stored in secure Microsoft Azure-supported cloud infrastructure and is encrypted using Advanced Encryption Standard. All transfers of data and reports through firewalls of the health system are executed using secure transfer protocols in accordance with internationally accepted Transport Layer Security versions 1.2 and 1.3. Security components also include rigid authentication and authorization of all users, a continuous monitoring tool, intrusion detection system and periodic penetration testing to mitigate risks of cyber-attacks.
Our Key Agreements
Mount Sinai Health System
In May 2018, we entered into a license agreement (the "Mount Sinai Agreement") with the Icahn School of Medicine at Mount Sinai pursuant to which we obtained a worldwide, royalty-bearing, exclusive license under certain patents and a worldwide, royalty-bearing, non-exclusive license under certain know-how of Mount Sinai to develop and commercialize licensed products in connection with the application of artificial intelligence for the diagnosis of kidney disease. Pursuant to the terms of the Mount Sinai Agreement, we are obligated to use commercially reasonable efforts in connection with the development and commercialization of the licensed products, including in accordance with certain diligence milestones.
Under the terms of the Mount Sinai Agreement, we are obligated to pay Mount Sinai $1.5 million and $7.5 million in commercial milestone payments upon achieving worldwide net sales of KidneyIntelX of $50.0 million and $300.0 million, respectively. We are also obligated to pay Mount Sinai a 4% to 5% royalty on net sales of KidneyIntelX, subject to customary reductions. Royalties are payable on a product-by-product basis from first commercial sale of such product until the later of (1) expiration of the last valid claim of a licensed patent covering such product or (2) on a country-by-country basis, 12 years from first commercial sale of such product in such country. Moreover, we are obligated to pay Mount Sinai between 15% and 25% of any consideration received by us from a sublicensee.
Joslin Diabetes Center
In July 2017, EKF Diagnostics Holding Plc ("EKF") entered into a license agreement (the "Joslin Agreement") with the Joslin Diabetes Center, Inc. ("Joslin"). In October 2018, EKF assigned to us all of its rights, title, interest and benefit in the Joslin Agreement.
Pursuant to the Joslin Agreement and the related assignment from EKF, we obtained a worldwide, royalty- bearing, exclusive license under any patents and any related know-how of Joslin related to the patent application filed with respect to the use the TNFR1 and TNFR2 biomarkers for determining whether a patient has an increased risk of developing CKD or ESKD (the "Joslin IP") to make, have made, use, offer for sale and sell licensed products covered by claims in the Joslin IP, and to perform, practice offer for sale and sell certain licensed processes related to the Joslin IP. We are obligated to use commercially reasonable efforts in connection with the development and commercialization of the licensed products and licensed processes, including in accordance with a development plan.
Under the terms of the Joslin Agreement, we are obligated to pay Joslin certain milestone payments of up to $1.3 million in the aggregate based on specified commercial milestones as follows: $300,000 upon the achievement of total net sales of $2.0 million for any licensed products or licensed processes and $1.0 million upon the achievement of total net sales of $10.0 million for any licensed products or licensed processes. We are also obligated to pay Joslin a 5% royalty on net sales of any licensed products or licensed processes, subject to customary reductions. Moreover, we are obligated to pay Joslin 25% of any consideration received by us from a sublicensee.
In February 2024, we entered into an extended exclusive option agreement (the "Joslin Option Agreement") with Joslin for patent filings on certain additional novel biomarkers in kidney disease for development and deployment in the KidneyIntelX platform. We believe that these novel biomarkers have the potential to provide additional clinical utility for understanding early disease progression and risk of kidney failure, therapeutic response, and the mechanistic pathways of kidney disease beyond the inflammatory and tubular injury markers that are currently captured by KidneyIntelX.
11
Commercialization
We are deploying kidneyintelX.dkd to patient populations with DKD on a regional basis through direct to physician sales and in conjunction with health systems and provider networks. We believe that our core partnership with Mount Sinai Health System, a large integrated disease network in the New York metropolitan area, has demonstrated the value of our partnership model which includes a variety of supporting resources for providers, including Electronic Health Record integration for seamless ordering and reporting, eligible patient identification, direct customer service and specialist educational programs.
Reimbursement and regulatory developments
We have achieved the following reimbursement and regulatory milestones critical to broad-scale commercial adoption and utilization:
In June 2024 , Medicare issued a final Local Coverage Determination (“LCD”) effective for dates of service on or after August 1, 2024. The established Medicare price for kidneyintelX.dkd is $950 per test. A distinct CPT Codes (Common Procedural Terminology Codes) have been established for kidneyintelX.dkd and is published in CMS’ 2024 Clinical Lab Fee Schedule. The LCD was issued by National Government Services (“NGS”). NGS is a subsidiary of Elevance Health, Inc. (previously Anthem, Inc.), a Medicare Administrative Contractor responsible for claims processing for testing performed in the Company’s New York City laboratory.
We successfully passed the ISO-13485:2016 and ISO27001:2022 inspections and retain certifications to these International Standards for Quality Management and Information Security Management Systems.
Our commercial laboratory in New York City received a clinical laboratory permit from the New York State Department of Health to provide commercial testing of KidneyIntelX in 2020.
We retain certification of compliance to CMS Clinical Laboratory Improvement Amendments (CLIA) regulation and are accredited by the Collage of America Pathology (CAP).
Competition
We do not believe there is currently a directly competitive product with kidneyintelX.dkd. However, we may expect to face competition from clinical reference laboratories and diagnostics manufacturers in the future. These potential competitors could include large diagnostic laboratories such as Quest Diagnostics Inc. and Laboratory Corporation of America Holdings (LabCorp) and large diagnostics manufacturers such as ThermoFisher Scientific Inc., Danaher Corporation, Roche Holding AG, Abbott Laboratories, Bio-Rad Laboratories, Inc., Ortho Clinical Diagnostics NV and Siemens Healthineers AG, all of which have widespread brand recognition and market penetration and substantially greater financial, technical, research and development and selling and marketing capabilities than we do. None of these companies currently offer tests that are comparable to kidneyintelX.dkd, however several of these companies offer existing tests used in the kidney space, such as serum creatinine or Cystatin C which only provide information on the current status of kidney function through an estimation of eGFR.
We could also potentially face competition in the future from data analytics companies that have developed technology-based or artificial intelligence-based approaches to healthcare applications and medical devices and that currently or in the future may develop diagnostic or prognostic products focused on kidney disease. However, we know of no current competitive efforts that have achieved FDA approval and Medicare reimbursement – two key factors we believe would be required to establish a broad-scale use for clinical prognosis currently offered by kidneyintelX.dkd.
Principal competitive factors in our market include:
12
We believe we compete effectively based on these factors; however, we cannot assure you that we will continue to do so. Many of our competitors have longer operating histories, larger customer bases, greater brand recognition and market penetration, substantially greater financial, technological and research and development resources, extensive sales and marketing capabilities, and more experience dealing with third-party payors. As a result, they may be able to respond more quickly to changes in customer requirements and devote greater resources to the development, promotion and sale of their diagnostic tests. We may not be able to compete effectively against these organizations should they choose to enter the market for kidney disease prognostics.
Manufacturing, supply and operations
KidneyIntelX is an artificial intelligence-enabled in vitro prognostic testing solution that has been developed to be commercialized as a single-site in vitro diagnostic. We operate quality management systems at our laboratories in accordance with FDA QSR 21 CFR Part 820. Medical Device Manufacturers must establish current Good Manufacturing Practices to ensure marketed devices meet applicable regulatory, quality requirements meeting the specifications. Requirements are similar to ISO 13485 – Medical Device Quality Management System Requirements, to which we are also certified. We are both the Manufacturer of Record and the service provider for the testing solution.
Our laboratory in New York City, New York is located within a JLabs facility and was established for research, development and clinical testing. In June 2020, we announced that our commercial laboratory in New York City received a clinical laboratory permit from the New York State Department of Health to provide commercial testing of KidneyIntelX.
Meso Scale Diagnostics LLC based in Rockville, Maryland are our primary supplier of reagents and materials used in measurement of our proprietary biomarkers in our laboratories. We closely monitor inventory levels and quality control parameters to ensure continuity of supply and test performance.
Intellectual property
Intellectual property is of vital importance in our field and in diagnostics generally. We seek to protect and enhance proprietary technology, inventions, and improvements that are commercially important to the development of our business by seeking, maintaining, and defending patent rights, whether developed internally, acquired or licensed from third parties. We will also seek to rely on regulatory protection afforded through data exclusivity, market exclusivity and patent term extensions where available.
Our intellectual property estate is designed to provide multiple layers of protection, including: patent rights with claims directed to platform technologies, such as key biomarkers, and patent rights covering specific products, such as KidneyIntelX. We also rely on trade secrets that may be important to the development of our business.
We believe our current patent estate, together with our efforts to develop and patent next generation technologies, provides us with substantial intellectual property protection.
We have sought patent protection in the United States and internationally for our KidneyIntelX product. However, the area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties.
Our commercial success will depend in part on obtaining and maintaining patent protection and trade secret protection of our current and future diagnostic products and the methods used to develop and manufacture them, as well as successfully defending these patents against third-party challenges and operating without infringing on the proprietary rights of others. Our ability to stop third parties from making, using, selling, offering to sell or importing our diagnostic products depends on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
13
We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be commercially useful in protecting our diagnostic products, discovery programs and processes. For this and more comprehensive risks related to our intellectual property, see “Risk Factors—Risks Related to Our Intellectual Property.”
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, including the United States, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent or delays on the part of a patentee. For more information regarding the risks related to our intellectual property, see “Risk Factors—Risks Related to Our Intellectual Property.”
In some instances, we submit patent applications directly with the USPTO as provisional patent applications. Corresponding non-provisional patent applications must be filed not later than 12 months after the provisional application filing date. While we intend to timely file non-provisional patent applications relating to our provisional patent applications, we cannot predict whether any such patent applications will result in the issuance of patents that provide us with any competitive advantage.
We file U.S. non-provisional applications and Patent Cooperation Treaty ("PCT") applications that claim the benefit of the priority date of earlier filed provisional applications, when applicable. The PCT system allows a single application to be filed within 12 months of the original priority date of the patent application, and to designate all of the PCT member states in which national patent applications can later be pursued based on the international patent application filed under the PCT. The PCT searching authority performs a patentability search and issues a non-binding patentability opinion which can be used to evaluate the chances of success for the national applications in foreign countries prior to having to incur the filing fees. Although a PCT application does not issue as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications. At the end of the period of two and a half years from the first priority date of the patent application, separate patent applications can be pursued in any of the PCT member states either by direct national filing or, in some cases by filing through a regional patent organization, such as the European Patent Organization. The PCT system delays expenses, allows a limited evaluation of the chances of success for national/regional patent applications and enables substantial savings where applications are abandoned within the first two and a half years of filing.
For all patent applications, we determine claiming strategy on a case-by-case basis. Advice of counsel and our business model and needs are always considered. We file patents containing claims for protection of all useful applications of our proprietary technologies and any products, as well as all new applications and/or uses we discover for existing technologies and products, assuming these are strategically valuable. We continuously reassess the number and type of patent applications, as well as the pending and issued patent claims to ensure that maximum coverage and value are obtained for our processes, and compositions, given existing patent office rules and regulations. Further, claims may be modified during patent prosecution to meet our intellectual property and business needs.
We recognize that the ability to obtain patent protection and the degree of such protection depends on a number of factors, including the extent of the prior art, the novelty and non-obviousness of the invention, and the ability to satisfy the enablement requirement of the patent laws. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted or further altered even after patent issuance. Consequently, we may not obtain or maintain adequate patent protection for any of our future diagnostic products or for our technology platform. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
In addition to patent protection, we also rely on trademark registration, trade secrets, know how, other proprietary information and continuing technological innovation to develop and maintain our competitive position. We seek to protect and maintain the confidentiality of proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Thus, we may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information concerning our business or financial affairs developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. Our agreements with employees also provide that all inventions conceived by the employee in the course of employment with us or from the employee’s use of our confidential information are our exclusive property. However, such confidentiality agreements and invention assignment agreements can be breached and we may not have adequate remedies for any such breach.
14
In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting trade secrets, know-how and inventions. For more information regarding the risks related to our intellectual property, see “Risk Factors—Risks Related to Our Intellectual Property.”
The patent positions of companies like ours are generally uncertain and involve complex legal, scientific and factual questions. Our commercial success will also depend in part on not infringing upon the proprietary rights of third parties. It is uncertain whether the issuance of any third-party patent would require us to alter our development or commercial strategies, or our products or processes, obtain licenses or cease certain activities. Our breach of any license agreements or our failure to obtain a license to proprietary rights required to develop or commercialize our future products may have a material adverse impact on us. If third parties prepare and file patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference or derivation proceedings in the USPTO to determine priority of invention. For more information, see “Risk Factors—Risks Related to Our Intellectual Property.”
When available to expand market exclusivity, our strategy is to obtain, or license additional intellectual property related to current or contemplated development platforms, core elements of technology and/or clinical candidates.
In-licensed intellectual property
The KidneyIntelX diagnostic is covered by a published PCT application filed in December 2009 that has been in-licensed from Joslin. National phase applications from this PCT were filed in the United States and Europe. There are two issued United States patents, which will both expire in December 2029. The claims are directed to methods of determining whether a human subject has an increased risk of developing CKD or ESKD or both. There is an issued European patent, which will expire in December 2029. The claims are directed to methods of determining whether a human subject has an increased risk of developing early renal function decline. The European patent is regionally validated in Belgium, Denmark, France, Germany, Ireland, Italy, Netherlands and Spain, and additionally in Hong-Kong. There is also a pending divisional EP patent application.
In addition, the KidneyIntelX diagnostic is covered by a provisional patent application that have been in-licensed from the Mount Sinai School of Medicine.
Government regulation and product approval
Clinical laboratory framework
Clinical Laboratory Improvement Amendments of 1988
As a clinical reference laboratory, with locations in Utah and New York, we are required to hold certain federal, state and local licenses, certifications and permits to conduct our business. CMS regulates all non-research laboratory testing performed on humans in the United States through the CLIA. In total, CLIA covers approximately 260,000 laboratory entities. The Division of Clinical Laboratory Improvement & Quality, within the Quality, Safety & Oversight Group, under the Center for Clinical Standards and Quality ("CCSQ"), has the responsibility for implementing the CLIA program. Under CLIA, we are required to hold a certificate applicable to the type of laboratory tests we perform and to comply with standards applicable to our operations, including test processes, personnel, facilities administration, equipment maintenance, recordkeeping, quality systems and proficiency testing, which are intended to ensure, among other things, that clinical laboratory testing services are accurate, reliable and timely.
We maintain a CLIA Certificate of Compliance for our Utah and Florida laboratories that allows us to perform non-waived (moderate and/or high complexity) testing at those sites. In June 2020, we received CLIA certification for our New York laboratory through the New York State Department of Health.
In November 2023, the Company consolidated Utah lab operations and in February 2024, the Company further consolidated lab operations of the Company's Florida lab.
In addition, a laboratory that is certified as “high complexity” under CLIA may develop, manufacture, validate and use proprietary tests referred to as LDTs. CLIA requires analytical validation including accuracy, precision, specificity, sensitivity and establishment of a reference range for any LDT used in clinical testing. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
Penalties for non-compliance with CLIA requirements include a range of enforcement actions, including suspension, limitation or revocation of the laboratory’s CLIA certificate, as well as directed plan of correction, state on-site monitoring, civil monetary penalties, civil injunctive suit or criminal penalties.
15
State laboratory licensing
In addition to federal certification requirements of laboratories under CLIA, CLIA provides that states may adopt laboratory regulations and licensure requirements that are more stringent than those under federal law. A number of states have implemented their own more stringent laboratory regulatory requirements. Such laws, among other things, establish standards for the day-to-day operation of a clinical laboratory, including the training and skills required of personnel and quality control. Five states require a separate out-of-state license before we can provide testing services for their residents: California, Maryland, New York, Pennsylvania and Rhode Island. We have received all five out-of-state licenses for our New York, Florida and Utah laboratories.
Federal oversight of laboratory developed tests
The laws and regulations governing the marketing of clinical laboratory testing and diagnostic products are evolving, extremely complex and, in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. Clinical laboratory tests are regulated under CLIA, as administered by CMS, as well as by applicable state laws. In addition, the Federal Food, Drug and Cosmetic Act ("FDCA") defines a medical device to include any instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory, intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals. Our in vitro testing products are considered by the FDA to be subject to regulation as medical devices. Among other things, pursuant to the FDCA and its implementing regulations, the FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, pre-market clearance or approval, marketing and promotion, and sales and distribution of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the export of medical devices manufactured in the United States to international markets.
Although the FDA has statutory authority to assure that medical devices are safe and effective for their intended uses, the FDA has generally exercised its enforcement discretion and not enforced applicable regulations with respect to in vitro diagnostics that are designed, manufactured, and used within a single laboratory for use only in that laboratory (i.e., LDTs).
Legislative and administrative proposals proposing to amend FDA’s oversight of LDTs have been introduced in recent years and we expect that new legislative and administrative proposals will continue to be introduced from time to time. It is possible that legislation could be enacted into law or regulations or guidance could be issued by the FDA which may result in new or increased regulatory requirements to develop and introduce new tests as LDTs.
Medical device regulatory framework
Pursuant to its authority under the FDCA, the FDA has jurisdiction over medical devices, which are defined to include, among other things, in vitro diagnostic devices. The FDA regulates, among other things, the research, design, development, preclinical and clinical testing, manufacturing, safety, effectiveness, packaging, labeling, storage, recordkeeping, pre-market clearance or approval, adverse event reporting, marketing, promotion, sales, distribution and import and export of medical devices. Unless an exemption applies, each new or significantly modified medical device we seek to commercially distribute in the United States could require a premarket notification to the FDA requesting permission for commercial distribution under Section 510(k) of the FDCA, also referred to as a 510(k) clearance, approval from the FDA of a premarket approval ("PMA") application, or a de novo request for classification ("de novo request"). The 510(k) clearance, PMA and de novo processes can be resource intensive, expensive, and lengthy, and require payment of significant user fees.
Device classification
Under the FDCA, medical devices are classified into one of three classes (Class I, Class II or Class III) depending on the degree of risk associated with each medical device and the extent of control needed to provide reasonable assurances with respect to safety and effectiveness.
Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be reasonably assured by adherence to General Controls for Medical Devices, which require compliance with the applicable portions of the FDA’s Quality System Regulation, facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. While some Class I devices also require premarket clearance by the FDA through the 510(k) premarket notification process described below, most Class I products are exempt from the premarket notification requirements.
16
Class II devices are those that are subject to the General Controls, as well as Special Controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These Special Controls can include performance standards, patient registries and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process.
Class III devices include devices deemed by the FDA to pose the greatest risk, such as life-supporting, life- sustaining devices, or implantable devices, in addition to those deemed novel and not substantially equivalent following the 510(k) process. The safety and effectiveness of Class III devices cannot be reasonably assured solely by the General Controls and Special Controls described above. Therefore, these devices are subject to the PMA application process, which is generally more costly and time-consuming than the 510(k) process. Through the PMA application process, the applicant must submit data and information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA typically includes, but is not limited to, extensive technical information regarding device design and development, preclinical and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device studies. The PMA application must provide valid scientific evidence that demonstrates to the FDA’s satisfaction a reasonable assurance of the safety and effectiveness of the device for its intended use.
The 510(k) clearance process
Under the 510(k) clearance process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent” to a legally marketed predicate device. A predicate device is a legally marketed device that is not subject to a PMA (i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device) and for which a PMA is not required), a device that has been reclassified from Class III to Class II or I, or a device that was previously found substantially equivalent through the 510(k) process. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence.
After a 510(k) premarket notification is submitted, the FDA determines whether to accept it for substantive review. If it lacks necessary information for substantive review, the FDA will refuse to accept the 510(k) premarket notification. If it is accepted for filing, the FDA begins a substantive review. By statute, the FDA is required to complete its review of a 510(k) notification within 90 days of receiving the 510(k) notification. As a practical matter, clearance often takes longer, and clearance is never assured. Although many 510(k) premarket notifications are cleared without clinical data, the FDA may require further information, including clinical data, to make a determination regarding substantial equivalence, which may significantly prolong the review process. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device.
If the FDA determines that the device is not “substantially equivalent” to a predicate device, or if the device is automatically classified into Class III, the device sponsor must then fulfill the much more rigorous premarketing requirements of the PMA approval process, or seek classification of the device through the de novo process. The de novo classification process is an alternate pathway to classify medical devices that are automatically classified into Class III but which are low to moderate risk. A manufacturer can submit a request for direct de novo review if the manufacturer is unable to identify an appropriate predicate device and the new device or new use of the device presents a moderate or low risk. De novo classification may also be available after receipt of a “not substantially equivalent” letter following submission of a 510(k) to FDA.
After a device receives 510(k) clearance or marketing authorization through the de novo classification process whereupon the device is classified into a classification regulation subject to 510(k), any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, could require a PMA application or new de novo request. The FDA requires each manufacturer to determine whether the proposed change requires a new submission in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. Many minor modifications are accomplished by an internal letter-to-file in which the manufacture documents its reasoning for why a change does not require premarket submission to the FDA. The letter-to-file is in lieu of submitting a new 510(k) to obtain clearance for such change. The FDA can always review these letters to file in an inspection. If the FDA disagrees with a manufacturer’s determination regarding whether a new premarket submission is required for the modification of an existing 510(k)-cleared device, the FDA can require the manufacturer to cease marketing and/or recall the modified device until marketing authorization is obtained. In addition, in these circumstances, the FDA can impose significant regulatory fines or penalties for failure to submit the requisite application(s).
17
The PMA approval process
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period of time. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA.
Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Prior to approval of a PMA, the FDA may conduct inspections of the clinical trial data and clinical trial sites, as well as inspections of the manufacturing facility and processes. Overall, the FDA review of a PMA application generally takes between one and three years, but may take significantly longer. The FDA can delay, limit or deny approval of a PMA application for many reasons, including:
If the FDA evaluation of a PMA is favorable, the FDA will issue either an approval letter, or an approvable letter, the latter of which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter authorizing commercial marketing of the device, subject to the conditions of approval and the limitations established in the approval letter. If the FDA’s evaluation of a PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the PMA or issue a not approvable letter. The FDA also may determine that additional tests or clinical trials are necessary, in which case the PMA approval may be delayed for several months or years while the trials are conducted and data is submitted in an amendment to the PMA, or the PMA is withdrawn and resubmitted when the data are available. The PMA process can be expensive, uncertain and lengthy and a number of devices for which the FDA approval has been sought by other companies have never been approved by the FDA for marketing.
New PMA applications or PMA supplements are required for modification to the manufacturing process, equipment or facility, quality control procedures, sterilization, packaging, expiration date, labeling, device specifications, ingredients, materials or design of a device that has been approved through the PMA process. PMA supplements often require submission of the same type of information as an initial PMA application, except that the supplement is limited to information needed to support any changes from the device covered by the approved PMA application and may or may not require as extensive technical or clinical data or the convening of an advisory panel, depending on the nature of the proposed change.
In approving a PMA application, as a condition of approval, the FDA may also require some form of post- approval study or post-market surveillance, whereby the applicant conducts a follow-up study or follows certain patient groups for a number of years and makes periodic reports to the FDA on the clinical status of those patients when necessary to protect the public health or to provide additional or longer term safety and effectiveness data for the device. The FDA may also approve a PMA application with other post-approval conditions intended to ensure the safety and effectiveness of the device, such as, among other things, restrictions on labeling, promotion, sale, distribution and use. New PMA applications or PMA supplements may also be required for modifications to any approved diagnostic tests, including modifications to our manufacturing processes, device labeling and device design, based on the findings of post-approval studies.
18
De novo Classification
Medical device types that the FDA has not previously classified as Class I, II, or III are automatically classified into Class III regardless of the level of risk they pose. To market low to moderate risk medical devices that are automatically placed into Class III due to the absence of a predicate device, a manufacturer may request a de novo classification of its medical device into Class I or Class II on the basis that the device presents low or moderate risk, rather than requiring the submission and approval of a PMA application. A medical device may be eligible for de novo classification if the manufacturer first submitted a 510(k) premarket notification and received a determination from the FDA that the device was not substantially equivalent or a manufacturer may request de novo classification directly without first submitting a 510(k) premarket notification to the FDA and receiving a not substantially equivalent determination. The FDA is required to classify the device within 120 calendar days following receipt of the de novo application, although in practice, the FDA’s review may take significantly longer. If the manufacturer seeks reclassification into Class II, the manufacturer must include a draft proposal for special controls that are necessary to provide a reasonable assurance of the safety and effectiveness of the medical device. In addition, the FDA may reject the de novo request for classification if it identifies a legally marketed predicate device that would be appropriate for a 510(k) or determines that the device is not low to moderate risk or that general controls would be inadequate to control the risks and special controls cannot be developed. In the event the FDA determines the data and information submitted demonstrate that general controls or general and special controls are adequate to provide reasonable assurance of safety and effectiveness, the FDA will grant the de novo request for classification. When the FDA grants a de novo request for classification, the device is granted marketing authorization and further can serve as a predicate for future devices of that type, through a 510(k) premarket notification.
The investigational device process
In the United States, absent certain limited exceptions, human clinical trials intended to support medical device clearance or approval require an investigational device exemption, ("IDE") application. Some types of studies deemed to present “non-significant risk” are deemed to have an approved IDE—without affirmative submission of an IDE application to the FDA—once certain requirements are addressed and Institutional Review Board ("IRB"), approval is obtained. If the device presents a “significant risk” to human health, as defined by the FDA, the sponsor must submit an IDE application to the FDA and obtain IDE approval prior to commencing the human clinical trials. The IDE application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. Generally, clinical trials for a significant risk device may begin once the IDE application is approved by the FDA and the study protocol and informed consent are approved by appropriate IRBs at the clinical trial sites. Submission of an IDE will not necessarily result in the ability to commence clinical trials, and although the FDA’s approval of an IDE allows clinical testing to go forward for a specified number of subjects, it does not bind the FDA to accept the results of the trial as sufficient to prove the product’s safety and efficacy, even if the trial meets its intended success criteria.
Such clinical trials must be conducted in accordance with the FDA’s IDE regulations that govern investigational device labeling, prohibit promotion and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. Clinical trials must further comply with good clinical practice regulations for IRB approval and for informed consent and other human subject protections. Required records and reports are subject to inspection by the FDA for any clinical trials subject to FDA oversight. The results of clinical testing may be unfavorable, or, even if the intended safety and efficacy success criteria are achieved, may not be considered sufficient for the FDA to grant marketing approval or clearance of a product. The commencement or completion of any clinical trial may be delayed or halted, or be inadequate to support approval of a PMA application or clearance of a 510(k) premarket notification, for numerous reasons.
Post-market regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
19
Device manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file, and complaint files. Manufacturers are subject to periodic scheduled or unscheduled inspections by the FDA. A failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, manufacturing operations and the recall or seizure of products. The discovery of previously unknown problems with products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that a manufacturer has failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, including the following:
U.S. federal and state health care laws
Federal and state physician self-referral prohibitions
We are subject to the federal physician self-referral prohibitions, commonly known as the Stark Law, and to comparable state laws. Together these restrictions generally prohibit us from billing a patient or governmental or private payor for certain designated health services, including clinical laboratory services, when the physician ordering the service, or a member of such physician’s immediate family, has a financial relationship, such as an ownership or investment interest in or compensation arrangement with us, unless the relationship meets an applicable exception to the prohibition. Several Stark Law exceptions are relevant to many common financial relationships involving clinical laboratories and referring physicians, including: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) space and equipment rental arrangements that satisfy certain requirements, and (4) personal services arrangements that satisfy certain requirements. The laboratory cannot submit claims to the Medicare Part B program for services furnished in violation of the Stark Law, and Medicaid reimbursements may be at risk as well.
Sanctions for a Stark Law violation include the following:
20
The Stark law is a strict liability statute, which means these prohibitions apply regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral. In addition, knowing violations of the Stark Law may also serve as the basis for liability under the federal False Claims Act (the "FCA"), which can result in additional civil and criminal penalties.
Federal and state anti-kickback laws
The federal Anti-Kickback Statute (the "AKS"), makes it a felony for a person or entity, including a clinical laboratory and a medical device manufacturer, to knowingly and willfully offer, pay, solicit or receive any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in order to induce business that is reimbursable under any federal healthcare program. A violation of the AKS may result in imprisonment, significant administrative and civil penalties and monetary fines and to exclude healthcare providers and others engaged in prohibited activities from Medicare, Medicaid and other federal healthcare programs. The government may also assert that a claim that includes items or services resulting from a violation of the AKS constitutes a false or fraudulent claim under the FCA, which is discussed in greater detail below. Additionally, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Although the AKS applies only to items and services reimbursable under any federal healthcare program, a number of states have passed statutes substantially similar to the AKS that apply to all payors. Penalties for violations of such state laws include imprisonment and significant monetary fines.
Federal and state law enforcement authorities scrutinize arrangements between healthcare providers and potential referral sources to ensure that the arrangements are not designed as a mechanism to induce patient care referrals or induce the purchase or prescribing of particular products or services. Generally, courts have taken a broad interpretation of the scope of the AKS, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals or purchases.
In addition to statutory exceptions to the AKS, regulations provide for a number of safe harbors. If an arrangement meets the provisions of an applicable exception or safe harbor, it is deemed not to violate the AKS. An arrangement must fully comply with each element of an applicable exception or safe harbor in order to qualify for protection.
Failure to meet the requirements of the safe harbor, however, does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances. On October 9, 2019, the Office of Inspector General of HHS ("OIG"), and CMS proposed further modifications to the federal AKS safe harbor protections for certain coordinated care and value-based arrangements among clinicians, providers and others. CMS also proposed multiple new exceptions and revisions to current exceptions for value-based arrangements under the Stark Law. It is unknown at this time which, if any, of these modifications will go into effect and what effect it will have on our business.
Corporate practice of medicine; fee splitting
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. Activities in addition to those directly related to the delivery of medical care also may be considered an element of the practice of medicine in many states. We may enter into services contracts with healthcare providers organizations pursuant to which we provide them with a range of services. These contractual relationships are subject to various state laws, including those of New York, Texas and California, that prohibit fee splitting or the practice of medicine by lay entities or persons and are intended to prevent unlicensed persons from interfering with or influencing the physician’s professional judgment. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, or fee-splitting, we could be required to restructure our contractual and other arrangements with certain physicians and other healthcare professions.
21
Some of these requirements may apply to us even if we do not have a physical presence in the state, based solely on our agreements with providers licensed in the state. However, regulatory authorities or other parties, including our providers, may assert that we are engaged in the corporate practice of medicine or that our contractual arrangements with our provider clients constitute unlawful fee splitting. In addition, violation of these laws may result in significant civil, criminal and administrative penalties, such as sanctions imposed against us and/or the professional through licensure proceedings, and exclusion from state and federal healthcare programs.
Other federal and state healthcare laws
In addition to the requirements discussed above, several other healthcare fraud and abuse laws could have an effect on our business. For example, provisions of the Social Security Act permit Medicare and Medicaid to exclude an entity that charges the federal healthcare programs substantially in excess of its usual charges for its services. The terms “usual charge” and “substantially in excess” are subject to varying interpretations.
The FCA prohibits, among other things, a person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval and from, making, using, or causing to be made or used, a false record or statement material to a false or fraudulent claim in order to secure payment or retaining an overpayment by the federal government. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud through whistleblower or qui tam actions. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government intervenes and is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the plaintiff will receive a percentage of the recovery. In addition, the improper retention of an overpayment for 60 days or more is also a basis for a FCA action, even if the claim was originally submitted appropriately. Penalties for FCA violations include fines for each false claim, plus up to three times the amount of damages sustained by the federal government. A FCA violation may provide the basis for exclusion from the federally funded healthcare programs. In addition, some states have adopted similar fraud, whistleblower and false claims provisions. The Social Security Act includes its own provisions that prohibit the filing of false claims or submitting false statements in order to obtain payment. The Social Security Act also includes civil monetary penalty provisions that impose penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. In addition, a person who offers or provides to a Medicare or Medicaid beneficiary any remuneration, including waivers of co-payments and deductible amounts (or any part thereof), that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of Medicare or Medicaid payable items or services may be liable under the civil monetary penalties statute. Moreover, in certain cases, providers who routinely waive copayments and deductibles for Medicare and Medicaid beneficiaries, for example, in connection with patient assistance programs, can also be held liable under the AKS and FCA. One of the statutory exceptions to the prohibition is non-routine, unadvertised waivers of copayments or deductible amounts based on individualized determinations of financial need or exhaustion of reasonable collection efforts. The OIG, emphasizes, however, that this exception should only be used occasionally to address special financial needs of a particular patient. Although this prohibition applies only to federal healthcare program beneficiaries, applicable state laws related to, among other things, unlawful schemes to defraud, excessive fees for services, tortious interference with patient contracts and statutory or common law fraud, may also be implicated for similar practices offered to patients covered by private third-party payors.
The federal Health Insurance Portability and Accountability Act of 1996 ("HIPAA"), created new federal criminal statutes that prohibit, among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the AKS, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Several states in which we operate have also adopted similar fraud and abuse laws as described above. The scope of these laws and the interpretations of them vary from state to state and are enforced by state courts and regulatory authorities, each with broad discretion. Some state fraud and abuse laws apply to items or services reimbursed by any third party payor, including commercial insurers, not just those reimbursed by a federally funded healthcare program. A determination of liability under such state fraud and abuse laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 ("HITECH") and their respective implementing regulations, also impose certain requirements on certain covered healthcare providers, health plans, and healthcare clearinghouses and their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information relating to the privacy, security and transmission of individually identifiable health information as well as their covered subcontractors.
22
HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions.
The Physician Payments Sunshine Act, enacted as part of the ACA, and its implementing regulations, also imposed annual reporting requirements on manufacturers of certain devices, drugs and biologics for payments available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals; as well as ownership and investment interests held by physicians and their immediate family members. Any failure to comply with these reporting requirements could result in significant fines and penalties. A determination that we have violated these laws and regulations, or a public announcement that we are being investigated for possible violations, could adversely affect our business, prospects, results of operations or financial condition.
The Eliminating Kickbacks in Recovery Act of 2018 ("EKRA") prohibits knowingly and willfully soliciting or receiving any remuneration (including any kickback, bribe or rebate) directly or indirectly, overtly or covertly, in cash or in kind, in return for referring a patient or patronage to a laboratory; or paying or offering any remuneration (including any kickback, bribe or rebate) directly or indirectly, overtly or covertly, in cash or in kind, to induce a referral of an individual to a laboratory or in exchange for an individual using the services of that laboratory. EKRA was enacted to help reduce opioid-related fraud and abuse. However, EKRA defines the term “laboratory” broadly and without reference to any connection to substance use disorder treatment. EKRA applies to all payors including commercial payors and government payors. Violations of EKRA are subject to significant fines and/or up to ten years in jail, separate and apart from existing AKS regulations and penalties. The law includes a limited number of exceptions, some of which closely align with corresponding AKS exceptions and safe harbors, and others that materially differ. Currently, there is no regulation interpreting or implementing EKRA, nor any guidance released by a federal agency regarding the scope of EKRA.
Federal consumer protection and unfair competition laws broadly regulate marketplace activities and activities that potentially harm consumers.
Finally, there are analogous state and foreign laws and regulations, such as state and foreign laws that require medical device companies to comply with the medical device industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state and foreign laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or product pricing; state and local laws that require the registration of medical device sales representatives; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from the business.
Efforts to ensure that our internal operations and business arrangements with third parties comply with applicable laws and regulations involve substantial costs. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the fraud and abuse laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal, civil and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, integrity oversight and reporting obligations, if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, diminished profits and future earnings, and the curtailment or restructuring of our operations. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Any action for violation of these laws, even if successfully defended, could cause a manufacturer to incur significant legal expenses and divert management’s attention from the operation of the business. Prohibitions or restrictions on sales or withdrawal of future marketed products could materially affect business in an adverse way.
23
International regulations
Many countries in which we may offer any of our testing products in the future have anti-kickback regulations prohibiting providers from offering, paying, soliciting or receiving remuneration, directly or indirectly, in order to induce business that is reimbursable under any national healthcare program. In situations involving physicians employed by state-funded institutions or national healthcare agencies, violation of a local anti-kickback law may also constitute a violation of the U.S. Foreign Corrupt Practices Act ("FCPA").
The FCPA prohibits any U.S. individual, business entity or employee of a U.S. business entity from offering or providing, directly or through a third-party, including any potential distributors we may rely on in certain markets, anything of value to a foreign government official with corrupt intent to influence an award or continuation of business or to gain an unfair advantage, whether or not such conduct violates local laws. In addition, it is illegal for a company that reports to the Securities and Exchange Commission (the "SEC") to have false or inaccurate books or records or to fail to maintain a system of internal accounting controls. We will also be required to maintain accurate information and control over sales and distributors’ activities that may fall within the purview of the FCPA, its books and records provisions and its anti-bribery provisions.
The standard of intent and knowledge in the foreign anti-bribery context is minimal; intent and knowledge often may be inferred from that fact that bribery took place. The accounting provisions do not require intent.
Violations of the FCPA’s anti-bribery provisions for corporations and other business entities are subject to a fine of up to $2.0 million and officers, directors, stockholders, employees, and agents are subject to a fine of up to $100,000 and imprisonment for up to five years. Other countries, including the United Kingdom and other OECD Anti-Bribery Convention members, have similar anti-corruption regulations, such as the United Kingdom Anti- Bribery Act.
When marketing our testing products outside of the United States, we may be subject to foreign regulatory requirements governing human clinical testing, prohibitions on the import of tissue necessary for us to perform our testing products or restrictions on the export of tissue imposed by countries outside of the United States or the import of tissue into the United States, and marketing approval. These requirements vary by jurisdiction, differ from those in the United States and may in some cases require us to perform additional preclinical or clinical testing. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required.
Privacy and security laws
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 ("HITECH"), and their respective implementing regulations, impose, among other things, requirements relating to the privacy, security and transmission of protected health information ("PHI"), on covered entities including certain healthcare providers, health plans, and health clearinghouses and their respective “business associates,” those independent contractors or agents of covered entities that perform services for covered entities that involve the creation, use, receipt, maintenance or disclosure of individually identifiable health information as well as their covered subcontractors. HIPAA also regulates standardization of data content, codes and formats used in certain healthcare transactions and standardization of identifiers for health plans and providers.
HITECH created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions. HIPAA also authorizes state attorneys general to file suit on behalf of their residents for violations. Courts are able to award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to file suit against us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI. In addition, HIPAA mandates that the Secretary of the Department of Health and Human Services ("HHS") conduct periodic compliance audits of HIPAA covered entities, such as us, and their business associates for compliance with the HIPAA privacy and security standards. It also tasks HHS with establishing a methodology whereby harmed individuals who were the victims of breaches of unsecured PHI may receive a percentage of the civil monetary penalty paid by the violator.
As a covered entity with downstream vendors and subcontractors and, in certain instances, as a business associate of other covered entities with whom we have entered into a business associate agreement, we have certain obligations under HIPAA regarding the use and disclosure of any PHI that may be provided to us. HIPAA and HITECH impose significant administrative, civil and criminal penalties against covered entities and business associates for noncompliance with privacy and security requirements.
24
Furthermore, we are, or may become, subject to various other data privacy and security obligations, including local and foreign laws, regulations, guidance, and industry standards related to data privacy, security, and protection. Such obligations may include, without limitation, the U.S. Federal Trade Commission Act, the U.S. Controlling the Assault of Non-Solicited Pornography And Marketing Act of 2003, the California Consumer Privacy Act of 2018 (“CCPA”), the European Union’s General Data Protection Regulation 2016/679 (“EU GDPR”), the EU GDPR as it forms part of United Kingdom (“UK”) law by virtue of section 3 of the European Union (Withdrawal) Act 2018 (“UK GDPR”), and the ePrivacy Directive. Several states within the United States have also enacted or proposed data privacy laws. For example, Virginia passed the Consumer Data Protection Act, and Colorado passed the Colorado Privacy Act.
The CCPA and EU GDPR are examples of the increasingly stringent and evolving regulatory frameworks related to personal data (including patient health information) processing that may increase our compliance obligations and exposure for any noncompliance. For example, the CCPA imposes obligations on covered businesses to provide specific disclosures related to a business’s collecting, using, and disclosing personal data and to respond to certain requests from California residents related to their personal data (for example, requests to know of the business’s personal data processing activities, to delete the individual’s personal data, and to opt out of certain personal data disclosures). Also, the CCPA provides for civil penalties and a private right of action for data breaches which may include an award of statutory damages. In addition, the California Privacy Rights Act of 2020 (“CPRA”), effective January 1, 2023, expands the CCPA, and, among other things, gives California residents the ability to limit use of certain sensitive personal data, expands the types of data breaches that are subject to the CCPA’s private right of action, and establishes a new California Privacy Protection Agency to implement and enforce the new law.
Furthermore, the EU GDPR imposes significant and complex compliance obligations on entities that are subject to the law. Such obligations may include limiting personal data processing to only what is necessary for specified, explicit, and legitimate purposes; requiring a legal basis for personal data processing; requiring the appointment of a data protection officer in certain circumstances; increasing transparency obligations to data subjects; requiring data protection impact assessments in certain circumstances; limiting the collection and retention of personal data; increasing rights for data subjects; formalizing a heightened and codified standard of data subject consents; requiring the implementation and maintenance of technical and organizational safeguards for personal data; and mandating notice of certain personal data breaches to the relevant supervisory authority(ies) and affected individuals.
See the section titled “Risks Related to Reimbursement and Regulation” for additional information about the laws and regulations to which we may become subject and about the risks to our business associated with such laws and regulations.
Healthcare reform
In March 2010, the Patient Protection and Affordable Care Act of 2010, as amended by the Healthcare and Education Reconciliation Act of 2010, collectively the ACA, was enacted in the United States. The ACA made a number of substantial changes to the way healthcare is financed both by governmental and private insurers. For example, the ACA also contains a number of provisions, including provisions governing enrollment in federal and state healthcare programs, reimbursement matters and fraud and abuse, which we expect will impact our industry and our operations in ways that we cannot currently predict. There have been executive, judicial and Congressional challenges to certain provisions of the ACA. For example, President Trump signed Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA. Concurrently, Congress considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, it has enacted laws that modify certain provisions of the ACA such as removing penalties, starting January 1, 2019, for not complying with the ACA’s individual mandate to carry health insurance, eliminating the implementation of certain ACA-mandated fees, and increasing the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D.
On June 17, 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. However, it is possible that the ACA will be subject to additional judicial or Congressional challenges in the future. In addition, the ACA has been subject to various health reform measures. For example, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which began on February 15, 2021 and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. On August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 ("IRA") into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The Inflation Reduction Act also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost through a newly established manufacturer discount program. It is unclear how any additional healthcare reform measures of the Biden administration will impact the ACA.
25
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect in April 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2032 unless additional Congressional action is taken. In January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. As part of the 2020 federal spending package, the ACA-required medical device manufacturer 2.3% sales tax has been eliminated, effective January 1, 2020.
Moreover, payment methodologies may be subject to changes in healthcare legislation and regulatory initiatives. For example, CMS may develop new payment and delivery models, such as bundled payment models. In addition, recently there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their commercial products, which has resulted in several Congressional inquiries and proposed and enacted state and federal legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for products. Further, based on a recent executive order, the Biden administration expressed its intent to pursue certain policy initiatives to reduce drug prices. At the state level, legislatures are increasingly passing legislation and implementing regulations designed to control pharmaceutical and medical device pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Corporate Information
The terms “Company”, “Renalytix”, “we”, “us”, or “our” refer to Renalytix plc and its subsidiaries. We were incorporated as a public limited company under the laws of England and Wales on March 15, 2018, with company number 11257655. Our principal executive offices in the United States are located at 1460 Broadway, New York, New York 10036, and our telephone number is +1 646 397 3970. Our registered office in the United Kingdom is located at 2 Leman Street, London, E1W 9US, United Kingdom, and the telephone number of our registered office is +44 20 3139 2910. Our agent for service of process in the United States is Renalytix AI, Inc., located at 1460 Broadway, New York, New York 10036.
Renalytix AI, Inc., a Delaware corporation, and Renalytix AI Limited, an Irish corporation, are our wholly owned subsidiaries. The SEC maintains an Internet site that contains reports, proxy information statements and other information regarding issuers that file electronically with the SEC. The address of that site is http://www.sec.gov. Our website address is www.renalytix.com, and we make available free of charge on our website (https://www.renalytix.com) our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other filings pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and amendments to such filings, as soon as reasonably practicable after each are electronically filed with, or furnished to, the SEC. The reference to our website is an inactive textual reference only and information contained in, or that can be accessed through, our website or any other website cited in this Annual Report is not part of this Annual Report.
Item 1A. Risk Factors.
Our business faces significant risks. You should carefully consider all of the information set forth in this annual report and in our other filings with the SEC, including the following risk factors which we face and which are faced by our industry. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. This annual report also contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in these forward-looking statements, as a result of certain factors including the risks described below and elsewhere in this annual report and our other SEC filings. See “Special Note Regarding Forward-Looking Statements” above.
Risk Factor Summary
Our business is subject to a number of risks and uncertainties, including those risks discussed at-length in the section below titled “Risk Factors.” These risks include, among others, the following:
26
27
Risks Related to Our Financial Condition and Capital Requirements
We have not generated material revenue, have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future.
Since inception, our operations have been primarily limited to developing clinical-grade, artificial intelligence-enabled in vitro diagnostics for kidney disease and investing in our technology platform. We are currently continuing to conduct clinical utility and other studies for KidneyIntelX to determine its clinical value and performance in different CKD populations and we expect to continue to conduct additional clinical studies for the foreseeable future. We have only recently begun to generate revenue from sales of KidneyIntelX and we cannot guarantee that our commercialization and partnership efforts will result in significant revenue to us in future periods. Consequently, any predictions about our future success or viability, or any evaluation of our business and prospects, may not be accurate.
We have incurred losses in each year since our inception. Our net losses for the fiscal years ended June 30, 2024 and 2023 were $33.5 million and $45.6 million, respectively. We have devoted most of our financial resources to research and development, including planning and conducting clinical validation and other studies for KidneyIntelX and evaluating its potential health economic impacts.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future, and these net losses may fluctuate significantly. We anticipate that our expenses will increase substantially as we conduct clinical utility and other studies for KidneyIntelX and continue its commercial launch, develop and refine our artificial intelligence technology platform, seek regulatory clearances or approvals for other products we develop, establish and maintain partnerships with healthcare systems, pursue our coverage and reimbursement strategy and continue to invest in our infrastructure to support our manufacturing and other activities.
28
Our future capital needs are uncertain, and our independent registered public accounting firm has expressed in its report on our audited financial statements for the fiscal year ended June 30, 2024, a substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is dependent on our ability to raise additional capital and our operations could be curtailed if we are unable to obtain the required additional funding when needed. We may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us.
Our financial statements for the fiscal years ended June 30, 2024 and 2023 included in this report have been prepared assuming we will continue to operate as a going concern. However, due to our recurring losses from operations, and working capital deficiency, there is substantial doubt about our ability to continue as a going concern. Because we expect to continue to experience negative cash flow, our ability to continue as a going concern is subject to our ability to obtain necessary funding from outside sources, including obtaining additional funding from offerings of our equity securities or debt, transactions involving product development, licensing or collaboration, or other forms of financing. Management intends to continue its efforts to contain costs and to raise additional capital until we can generate sufficient cash from commercial sales to support operations, if ever. If we are unable to obtain sufficient financing, we may be required to delay, scale back or discontinue one or more product development programs, curtail our commercialization activities and significantly reduce expenses or we may not be able to continue as a going concern. As a result, our independent registered public accounting firm has expressed in its auditors’ report on the financial statements included in this report a substantial doubt regarding our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from the outcome of the uncertainty regarding our ability to continue as a going concern. If we cannot continue as a going concern, our shareholders may lose their entire investment in our securities. Future reports from our independent registered public accounting firm may also contain statements expressing doubt about our ability to continue as a going concern.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
We are an artificial intelligence-enabled in vitro diagnostics company with a limited operating history. Our company was formed in March 2018. As an organization, we have limited experience in establishing and maintaining successful partnerships with healthcare systems, manufacturing KidneyIntelX at commercial scale, conducting sales and marketing activities necessary for successful commercialization and achieving major reimbursement milestones. We may encounter unforeseen expenses, difficulties, complications and delays in achieving our business objectives. Our very short history as an operating company makes any assessment of our future success or viability subject to significant uncertainty. If we do not address these risks successfully or are unable to transition at some point to a company capable of supporting commercial activities and maintaining partnerships with healthcare systems, then our business will suffer.
We will require substantial additional funding to commercialize and scale KidneyintelX.dkd, which may not be available to us on acceptable terms, or at all, and, if not so available, may require us to delay, curtail or discontinue our operations.
We expect our expenses to increase in connection with our ongoing activities, particularly as we conduct commercial sales at scale. We expect to incur significant commercialization expenses related to product manufacturing, marketing, sales and distribution. We will continue to incur additional costs associated with operating as a company that is both publicly traded in the United States and admitted to trading on AIM in the United Kingdom.
Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. On September 30, 2024, we announced our intention to raise approximately £11 million gross proceeds through a placing, a subscription and a retail offer of new ordinary shares of the Company at an issue price of £0.09 per new ordinary share to new and existing institutional and other investors. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, curtail or discontinue our research and development programs or any future commercialization efforts. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect, or our operating plan may change as a result of many factors unknown to us. These factors, among others, may necessitate that we seek additional capital sooner than currently planned. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements will depend on many factors, including:
29
Any efforts to secure additional financing may divert our management from their day-to-day activities, which may adversely affect our ability to continue development and commercialization of KidneyIntelX. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect our business, the holdings or the rights of our shareholders or holders of our ADSs, or the value of our ordinary shares or ADSs.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue our research and development programs relating to KidneyIntelX or any commercialization efforts, be unable to expand our operations, or be unable to otherwise capitalize on our business opportunities, as desired, which could harm our business and potentially cause us to discontinue operations.
Covenants under our Second Amended and Restated Bond Agreement and any future debt arrangements may result in the acceleration of outstanding indebtedness and limit the manner in which we operate.
The Second Amended and Restated Bond Agreement we entered into with CVI Investments, Inc. ("CVI") in March 2024 (the “Bond Agreement”) contains customary terms and covenants, as well as customary events of default, after which the bonds may be due and payable immediately, including defaults related to payment compliance, material inaccuracy of representations and warranties, covenant compliance, material adverse changes, bankruptcy and insolvency proceedings, judgments against the Company, and change of control or delisting events. For more information regarding the foregoing, see “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Critical Accounting Estimates – Convertible Notes” below.
In addition, the Bond Agreement contains, and any future indebtedness we incur may contain, various negative covenants that restrict or may restrict, among other things, our ability to:
30
As a result, we are limited in the manner in which we conduct our business and we may be unable to engage in favorable business activities, repurchase our ordinary shares or finance future operations or capital needs.
Servicing these bonds requires a significant amount of cash, and we may not have sufficient cash flow from our business to pay our substantial debt. Our ability to make scheduled payments of the principal of, to pay interest on or to refinance our indebtedness depends on our future performance, which is subject to economic, financial, competitive and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt or obtaining additional equity capital on terms that may be onerous or highly dilutive. If we are unable to make our quarterly installment payments in cash, we may be forced to issue a significant number of ordinary shares which could dilute existing shareholders. Our ability to refinance our indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on our debt obligations.
Risks Related to Our Business and Strategy
If we cannot continue to execute on our strategy to partner with healthcare systems to incorporate KidneyIntelX into their treatment regime and integrate their EHR systems with our technology, our revenue prospects could be significantly reduced.
We have only recently commercially launched KidneyIntelX. Partnerships with healthcare systems are a core part of our adoption and growth strategy.
Our ability to execute on this strategy could suffer if:
The strength of our partnerships will depend on many factors, including effectiveness of patient and clinician compliance, the effectiveness of our efforts to educate clinicians and healthcare systems on the implementation and use of KidneyIntelX and the effectiveness of our efforts to integrate KidneyIntelX into the clinical workflow and integrate with the healthcare system’s EHR systems for test ordering and reporting. The success of a partnership may also be dependent on factors that are beyond our control, such as healthcare system budgetary cuts, changes in key executive, administrative, IT and clinical personnel, changes in control or acquisitions and changes in the local regulatory environment.
If our partnership strategy is unsuccessful, we may need to change our commercialization strategy and significantly expand our direct sales force, which would involve significant time and expense and which may not be successful.
We may underestimate the timing and complexity of successfully integrating KidneyIntelX into the clinical guidelines of new healthcare systems with which we partner.
Integration of KidneyIntelX with healthcare providers’ clinical workflow is a core part of our adoption and growth strategy. Integrated partnerships are designed to allow KidneyIntelX to be deployed directly to patient populations and their treating clinicians in a cost-efficient and timely manner.
Each deployment and integration of KidneyIntelX in a new health system is complex and must be meticulously tailored to the specifics of the health system, including, among other factors:
31
Although we carefully study each potential partnership and expend significant time and resources to support the deployment of KidneyIntelX, we may underestimate the time, costs and complexity of integration, and our integration efforts may ultimately be unsuccessful.
Our ability to be profitable in the future will depend on our ability to successfully commercialize KidneyIntelX, and any other products we may develop in the future, to scale nationally in the United States.
Our ability to be profitable in the future will depend on our ability to commercially scale KidneyIntelX and any other products we may develop in the future in the United States, including:
If KidneyIntelX fails to demonstrate clinical utility, does not maintain regulatory approval or does not achieve market acceptance, we may never become profitable. Our net losses have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital. Because of the numerous risks and uncertainties associated with diagnostic product development and commercialization, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. The amount of future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues.
Guidelines and recommendations published by various organizations can reduce the use of our products.
Government agencies promulgate regulations and guidelines directly applicable to us and to our products. However, professional societies, practice management groups, insurance carriers, physicians, private health/science foundations and organizations involved in various diseases from time to time may also publish guidelines or recommendations to healthcare providers, administrators and payers, and patient communities. Recommendations by government agencies or those other groups/organizations may relate to such matters as usage and reimbursement of our products by government and private payers. Recommendations or guidelines that are followed by patients, healthcare providers and payers could result in decreased use of our products, and any recommendations or guidelines that result in decreased use or reimbursement of our products could materially and adversely affect our product sales, business and operating results.
Risks Related to Development of Our Products and Technology Platform
KidneyIntelX is based on novel artificial intelligence technologies that are rapidly evolving. Our artificial intelligence-enabled algorithms and other technologies depend on our ability to continue to build a substantial repository of kidney disease-related data and validate additional product designs.
KidneyIntelX is a first-in-class in vitro diagnostics platform that employs a proprietary artificial intelligence- enabled algorithm to combine diverse data inputs, including validated blood-based biomarkers, inherited genetics and personalized patient data from EHR systems to generate a unique patient risk score. This use of artificial intelligence-enabled algorithms that combine both biological markers of disease along with EHR systems is a novel approach to kidney disease patient risk stratification. This new category of medical device and the kidney disease clinical indication are rapidly evolving fields of specialty that include uncertainties in acceptance, utility and clinical practice. There is no guarantee that we have fully understood all the implications of introducing a novel technology such as KidneyIntelX into such a large and evolving field of medicine.
32
In addition, we must execute on our strategy to build a significant repository of kidney disease-related data to support the robustness and accuracy of KidneyIntelX and allow us to develop additional artificial intelligence- enabled applications. We believe that access to contemporary and historical patient data, combined with the ability to analytically and clinically validate study results in a quality-controlled framework, provides us with a robust, reproducible method for product development. Moreover, the depth, specificity and quality of data are of paramount importance to developing novel solutions such as KidneyIntelX that can demonstrate clinical utility across a range of practice specialties and patient demographics. These features are also central to our product strategy of demonstrating both short- and long-term impact on patient outcomes and health economics. If we are unable to continue to build our data repository, we may not be able to keep pace with rapidly evolving technology and improve the predictive capabilities and clinical utility of KidneyIntelX, and our business could be harmed.
KidneyintelX.dkd is subject to ongoing regulation and could be subject to post-marketing restrictions or withdrawal from the market.
KidneyintelX.dkd is subject to the FDA’s quality system regulation ("QSR"), labeling regulations, registration and listing, the Medical Device Reporting regulation which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur and the Reports of Corrections and Removals regulation, which requires manufacturers to report recalls and field actions to the FDA if initiated to reduce a risk to health posed by the device or to remedy a violation of the FDCA. The FDA enforces these requirements by inspection and market surveillance. If the FDA finds a violation, it can institute a wide variety of enforcement actions, ranging from an untitled or public warning letter to more severe sanctions such as fines, injunctions and civil penalties; recall or seizure of products; operating restrictions and partial suspension or total shutdown of production; refusing requests for 510(k) clearance or PMA approval of new products; withdrawing a marketing authorization already granted; and criminal prosecution.
Accordingly, we will continue to expend time, money and effort in all areas of regulatory compliance.
Modifications to our products may require new 510(k) clearances or PMAs or may require us to recall or cease marketing our products until clearances or approvals are obtained, which could harm our business, financial condition and results of operations.
In the United States, our KidneyintelX.dkd is marketed pursuant to de novo authorization issued by the FDA. Any modifications to the device that could significantly affect its safety or effectiveness, such as changes to the intended use or technological characteristics, may require new 510(k) clearances or PMAs or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. Based on FDA published guidelines, the FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplemental approval or clearance; however, the FDA can review a manufacturer’s decision. Any modification to an FDA-cleared device that could significantly affect its safety or efficacy or that would constitute a major change in its intended use would require a new 510(k) clearance, or if such modification put the device into Class III, possibly a PMA. We may not be able to obtain additional 510(k) clearances or PMAs for new products or for modifications to, or additional indications for, our products in a timely fashion, or at all. Delays in obtaining required future clearances or approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Due to our limited resources and access to capital, our strategic decisions with respect to the development of certain diagnostic products may affect the development or timing of our business prospects.
Because we have limited resources and access to capital to fund our operations, we must decide which diagnostic products to pursue and the amount of resources to allocate to each. As such, we are currently primarily focused on the development of KidneyIntelX.
Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular diagnostic and prognostic programs or potential new products may not lead to the development of viable commercial products and may divert resources away from more promising opportunities. We may not choose the right product or programs to develop, or may be required to collaborate with third parties to advance a particular product at terms that are less than optimal to us. If we make incorrect determinations regarding the market potential of our diagnostic products or misread trends in the diagnostics industry, our business prospects could be harmed.
33
Acquisitions or joint ventures we may pursue may be unsuccessful.
We may consider the acquisition of other products or businesses that either complement or expand our existing business, or may enter into joint ventures. Any future acquisitions or joint ventures we pursue may involve a number of risks, including some or all of the following:
Risks Related to Reimbursement and Regulation
Our commercial success could be compromised if we do not obtain and maintain coverage and adequate reimbursement from third-party payors for KidneyIntelX.
The commercial success of KidneyIntelX and any future products we may develop will depend on the extent to which our customers obtain and maintain coverage and adequate reimbursement from third-party payors, including government payors such as Medicare and Medicaid, managed care organizations and commercial payors.
There are three key components for reimbursement in the United States: (1) coding, (2) pricing and (3) coverage. “Coding” refers to distinct numeric and alphanumeric billing codes, including Current Procedural Terminology ("CPT"), codes that are used to report the provision of certain health care services, including laboratory services, to third-party payors. “Coverage” refers to decisions made by third-party payors as to whether or not to provide their members access to and pay for such health care services, and if so, what conditions, such as specific diagnoses and clinical indications, are covered.
We received a positive local or national Medicare coverage determination in June 2024. If we do not maintain a positive Medicare coverage determination, we could experience negative consequences including:
34
Coverage and reimbursement by a payor may depend on a number of factors, including a payor’s determination that our products are:
Accordingly, even though we received Medicare national pricing for KidneyIntelX set at $950 per reportable test result, we may not continue to be reimbursed at that rate. As we enter into partnerships and contracts with healthcare systems and third-party payors, we will establish a reimbursement rate through contractual negotiations.
In the United States, the principal decisions about reimbursement for new medical products are typically made by the CMS. CMS decides whether and to what extent a new product will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Because there is no uniform policy of coverage and reimbursement in the United States, each payor generally determines for its own enrollees or insured patients whether to cover or otherwise establish a policy to reimburse our diagnostic tests, and seeking payor approvals is a time-consuming and costly process. We cannot be certain that coverage for our current and our planned future products will be provided in the future by additional payors or that existing agreements, policy decisions or reimbursement levels will remain in place, remain adequate, or be fulfilled under existing terms and provisions.
If we cannot obtain and maintain coverage and adequate reimbursement from private and governmental payors such as Medicare and Medicaid for our current products or new products that we may develop in the future, demand for such products may decline or may not grow as we expect, which could limit our ability to generate revenue and have a material adverse effect on our financial condition, results of operations and cash flow. In order to secure coverage and reimbursement for our products that might be approved for sale, we may need to conduct expensive studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals. Additionally, companies may also need to provide discounts to purchasers, private health plans or government healthcare programs. Nonetheless, products may not be considered medically necessary or cost effective. Further, we may experience delays and interruptions in the receipt of payments from payors due to missing documentation and/or other issues, which could cause delay in collecting our revenue.
In addition, the coverage and reimbursement market is ever changing and we are not in control of how our competitors’ coverage and pricing strategies are established. Some of our competitors have widespread brand recognition and substantially greater financial and technical resources and development, production and marketing capabilities than we do. Others may develop lower-priced, less complex tests that payors and physicians could view as functionally equivalent to our products, which could force us to lower the list price of our tests and impact our operating margins and our ability to achieve and maintain profitability. Payors may compare our products to our competitors and utilize them as precedents, which may impact our coverage and/or reimbursement. In addition, technological innovations that result in the creation of enhanced diagnostic tools that are more effective than ours may enable other clinical laboratories, hospitals, physicians or medical providers to provide specialized diagnostic tests similar to ours in a more patient-friendly, efficient or cost-effective manner than is currently possible. If we cannot compete successfully against current or future competitors, we may be unable to increase or create market acceptance and sales of our products, which could prevent us from increasing or sustaining our revenue or achieving or sustaining profitability.
In some foreign countries, the proposed pricing for a product must be approved before it may be lawfully marketed. The requirements governing pricing vary widely from country to country. For example, the European Union (the "EU"), provides options for its member states to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost effectiveness of a particular product to the current standard of care. A Member State may approve a specific price for the product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for diagnostic products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the EU do not follow price structures of the United States and generally prices tend to be significantly lower.
The coverage and reimbursement market may be additionally impacted by future legislative changes. There are increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs which may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our products. Specifically, there have been several recent U.S. presidential executive orders.
35
Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug and medical device pricing, reduce the cost under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies. For example, based on a recent executive order, the Biden administration expressed its intent to pursue certain policy initiatives to reduce drug prices. We expect to experience pricing pressures in connection with the sale of any of our products due to the trend toward managed healthcare, the increasing influence of health maintenance organizations, cost containment initiatives and additional legislative changes.
Payors from whom we may receive reimbursement are able to withdraw or decrease the amount of reimbursement provided for KidneyIntelX or our other products at any time in the future.
Our commercial success depends on our ability to maintain coverage and adequate reimbursement from those payors that decide to cover and reimburse KidneyIntelX, whether marketed as an LDT or medical device, and our other products that we commercialize. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor. Payors could withdraw coverage and stop providing reimbursement for our commercialized products in the future or may reimburse our products only on a case-by-case basis. Managing reimbursement on a case-by-case basis is time consuming and contributes to an increase in the number of days it takes us to collect accounts receivable and increases our risk of non-payment. Negotiating reimbursement on a case-by-case basis also typically results in the provision of reimbursement at a significant discount to the list price of our commercialized products.
Further, even if we obtain written agreements regarding coverage and reimbursement with certain payors, these agreements are not guarantees of indefinite coverage in an adequate amount. For example, these agreements are typically terminable without cause by either party and are typically renewable annually, and the applicable payor could opt against renewal upon expiration. In addition, the terms of certain of our written arrangements may require us to seek pre-approval from the payor or put in place other controls and procedures prior to conducting a test for a customer. To the extent we fail to follow these requirements, we may fail to receive some or all of the reimbursement payments to which we are otherwise entitled. These payors must also conclude that our claim satisfies the applicable contractual criteria. In addition, our written agreements regarding reimbursement with payors may not guarantee us the receipt of reimbursement payments at what we believe to be the applicable contracted rate for each reimbursement claim that we submit to such payors. If payors withdraw coverage for KidneyIntelX or our other products, once commercialized, or reduce the reimbursement amounts for such products, our ability to generate revenue could be limited, which may have a material adverse effect on our financial condition, results of operations and cash flow.
Long payment cycles of Medicare, Medicaid and/or other third-party payors, or other payment delays, could hurt our cash flows and increase our need for working capital.
Medicare and Medicaid have complex billing and documentation requirements that we must satisfy in order to receive payment, and the programs can be expected to carefully audit and monitor our compliance with these requirements. We must also comply with numerous other laws applicable to billing and payment for healthcare services, including, for example, privacy laws. Failure to comply with these requirements may result in, among other things, non-payment, refunds, exclusion from government healthcare programs, and significant administrative, civil or criminal penalties, any of which may have a material adverse effect on our revenues and earnings. In addition, failure by third-party payors to properly process our payment claims in a timely manner could delay our receipt of payment for our products and services, which may have a material adverse effect on our cash flows.
Billing for our products is complex and requires substantial time and resources to collect payment.
Billing for clinical laboratory testing services is complex, time-consuming and expensive. With respect to our LDT products, including KidneyIntelX, while marketed as an LDT, we anticipate we, through a third party service provider, will be billing various payors, including Medicare, Medicaid, private insurance payors and patients, all of which have different billing requirements. The billing arrangements and applicable law differ, which complicates our compliance efforts. To the extent laws or contracts require us to bill patient co-payments or co-insurance, we must also comply with these requirements. We may also face increased risk in our collection efforts, including potential write-offs of accounts receivable and long collection cycles, which could adversely affect our business, results of operations and financial condition.
Several factors make the billing process complex, including:
36
Billing code changes can result in a risk of an error being made in the claim adjudication process. Claims adjudication errors can occur with claims submission, third-party transmission or in the processing of the claim by the payor. Claim adjudication errors may result in a delay in payment processing or a reduction in payment processing or a reduction in the amount of the payment we receive. The addition of billing codes will require changes to our billing process and financial reporting systems. Failure or delays in effecting these changes in external billing and internal systems and processes could negatively affect our collection rates, revenue and cost of collecting.
Additionally, our billing activities will require us to implement compliance procedures and oversight, train and monitor our employees, and undertake internal audits to evaluate compliance with applicable laws and regulations as well as internal compliance policies and procedures. If a payor denies a claim we may submit, we may challenge the reason, low payment amount or payment denials. Payors also conduct external audits to evaluate payments, which add further complexity to the billing process. If the payor makes an overpayment determination, there is a risk that we may be required to return all or some portion of prior payments we have received.
Additionally, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (the "ACA"), requires providers and suppliers to report and return any overpayments received from government payors under the Medicare and Medicaid programs within 60 days of identification. Failure to identify and return such overpayments exposes the provider or supplier to liability under federal false claims laws. These billing complexities, and the related uncertainty in obtaining payment for our products, could negatively affect our revenue and cash flow, our ability to achieve profitability, and the consistency and comparability of our results of operations.
We rely on third-party billing provider software, and an in-house billing function, to transmit claims to payors, and any delay in transmitting claims could have an adverse effect on our revenue.
While we manage the overall processing of claims, we rely on third-party billing provider software to transmit the actual claims to payors based on the specific payor billing format. The potential exists for us to experience delays in claims processing when third-party providers make changes to their invoicing systems. Additionally, coding for diagnostic assays may change, and such changes may cause short-term billing errors that may take significant time to resolve. If claims are not submitted to payors on a timely basis or are erroneously submitted, or if we are required to switch to a different software provider to handle claim submissions, we may experience delays in our ability to process these claims and receipt of payments from payors, or possibly denial of claims for lack of timely submission, which would have an adverse effect on our revenue and our business.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties or our inability to operate.
We are and will be subject to multiple different state and federal laws and regulations that require significant expense, expertise and professional support to remain within compliance. For example, we operate under CLIA, a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance and inspections. Under CLIA, we are required to hold a certificate applicable to the type of laboratory tests we perform and to comply with standards applicable to our operations, including test processes, personnel, facilities administration, equipment maintenance, recordkeeping, quality systems and proficiency testing, which are intended to ensure, among other things, that clinical laboratory testing services are accurate, reliable and timely.
37
We must maintain CLIA compliance and certification to be eligible to bill for clinical laboratory services provided to federal health care program beneficiaries. We have received CLIA Certificates of Compliance for our New York laboratory and to renew our CLIA certificates, we are subject to survey and inspection every two years to assess compliance with program standards. We also may be subject to additional unannounced inspections. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. In addition, a laboratory that is certified as “high complexity” under CLIA may develop, manufacture, validate and use LDTs. CLIA requires analytical validation including accuracy, precision, specificity, sensitivity and establishment of a reference range for any LDT used in clinical testing. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
Penalties for non-compliance with CLIA requirements include a range of enforcement actions, including suspension, limitation or revocation of the laboratory’s CLIA certificate, as well as directed plan of correction, state on-site monitoring, civil monetary penalties, civil injunctive suit or criminal penalties.
In addition to federal certification requirements of laboratories under CLIA, CLIA provides that states may adopt laboratory regulations and licensure requirements that are more stringent than those under federal law. A number of states have implemented their own more stringent laboratory regulatory requirements. Such laws, among other things, establish standards for the day-to-day operation of a clinical laboratory, including the training and skills required of personnel and quality control.
If we were to lose our CLIA certification, whether as a result of a revocation, suspension or limitation, we would no longer be able to offer our tests, which would limit our revenues and seriously harm our business. If we were to lose, or fail to obtain, a license in any other state where we are required to hold a license, we would not be able to test specimens from those states, which also could limit our revenues and seriously harm our business.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
We are or expect to become subject to broadly applicable health care laws, including fraud and abuse, transparency, and privacy and security laws, which are regulated and enforced by both the federal government and the states in which we conduct our business. These health care laws and regulations include, for example:
38
39
The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform, especially in light of the lack of applicable precedent and regulations. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time- and resource-consuming and can divert a company’s attention from the business.
Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations, including any of our partnerships with healthcare systems, are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including, among others, significant administrative, civil and criminal penalties, damages, fines, disgorgement, reputational harm, imprisonment, integrity oversight and reporting obligations, and exclusion from participation in government funded healthcare programs such as Medicare and Medicaid. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, may have a material adverse effect on our business, financial condition and results of operations.
The ACA substantially changed the way health care is financed by both governmental and private insurers. Among other things, the ACA required each certain medical device manufacturer to pay an excise tax equal to 2.3% (the "Medical Device Excise Tax"), of the price for which such manufacturer sells its medical devices that are listed with the FDA. However, this tax was permanently eliminated as part of the 2020 federal spending package, effective January 1, 2020. The ACA also includes provisions of importance that:
Although some of these provisions may negatively impact payment rates for clinical laboratory tests, the ACA also extends coverage to over 30 million previously uninsured people. Some of the provisions of the ACA have yet to be implemented, and there have been executive, judicial and Congressional challenges to certain aspects of the ACA. For example, President Trump signed executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress considered legislation that would repeal or repeal and replace all or part of the ACA.
While Congress has not passed repeal legislation, it has enacted laws that modify certain provisions of the ACA such as removing penalties effective January 1, 2019, for not complying with the ACA’s individual mandate to carry health insurance and eliminating the implementation of certain ACA-mandated fees. On June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. However, it is possible that the ACA will be subject to additional judicial or Congressional challenges in the future. In addition, the ACA has been subject to various health reform measures. For example, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which began on February 15, 2021 and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. On August 16, 2022, President Biden signed the IRA of 2022 ("IRA") into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The Inflation Reduction Act also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost through a newly established manufacturer discount program.
40
It is unclear how any additional healthcare reform measures of the Biden administration will impact the ACA. Other legislative changes have been proposed and adopted since the ACA was enacted. PAMA was signed to law, which, among other things, significantly altered the payment methodology under the CLFS. Under the law, issued in 2016 and the reporting period beginning in 2017 and every three years thereafter (or annually in the case of advanced diagnostic laboratory tests), applicable clinical laboratories must report laboratory test payment data for each Medicare- covered clinical diagnostic laboratory test that it furnishes during the specified time period. Reporting of payment data under PAMA for clinical diagnostic laboratory tests has been delayed on numerous occasions. The reported data must include the payment rate (reflecting all discounts, rebates, coupons and other price concessions) and the volume of each test that was paid by each private payor (including health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations). Effective January 1, 2018, the Medicare payment rate for each clinical diagnostic laboratory test is equal to the weighted median amount for the test from the most recent data collection period. The payment rate applies to laboratory tests furnished by a hospital laboratory if the test is separately paid under the hospital outpatient prospective payment system. Also, under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. For an existing test that is cleared or approved by the FDA and for which Medicare payment is made as of April 1, 2014, CMS is required to assign a unique billing code if one has not already been assigned by the agency. In addition to assigning the code, CMS is required to publicly report payment for the tests. Further, under PAMA, CMS is required to adopt temporary billing codes to identify new tests and new advanced diagnostic laboratory tests that have been cleared or approved by the FDA. We cannot determine at this time the full impact of PAMA on our business, financial condition and results of operations.
Additionally, the Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers and suppliers of up to 2% per fiscal year, starting in 2013, and, due to subsequent legislative amendments to the statute, will remain in effect until 2032 unless additional congressional action is taken. The full impact on our business of the sequester law is uncertain. In addition, the Middle-Class Tax Relief and Job Creation Act of 2012 (the "MCTRJCA"), mandated an additional change in Medicare reimbursement for clinical laboratory tests. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
Some of our laboratory assay business is subject to the Medicare Physician Fee Schedule. The Medicare Access and CHIP Reauthorization Act of 2015 ended the use of the statutory formula, also referred to as the Sustainable Growth Rate, for clinician payment and established a quality payment incentive program, also referred to as the Quality Payment Program. This program provides clinicians with two ways to participate, including through the Advanced Alternative Payment Models ("APMs"), and the Merit-based Incentive Payment System ("MIPS"). In November 2019, CMS issued a final rule finalizing the changes to the Quality Payment Program. At this time, it is unclear how the introduction of the Quality Payment Program will impact overall physician reimbursement under the Medicare program. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry, and changes to the reimbursement amounts paid by Medicare and other payors for our current assays, whether marketed as an LDT or a medical device, and our planned future assays, may reduce our profits, if any, and have a materially adverse effect on our business, financial condition, results of operations and cash flows. Moreover, Congress has proposed on several occasions to impose a 20% coinsurance payment requirement on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. In the event that Congress was to ever enact such legislation, the cost of billing and collecting for our products, once commercialized, could often exceed the amount actually received from the patient.
Our business activities may be subject to the Foreign Corrupt Practices Act and similar anti-bribery and anti- corruption laws.
Our business activities may be subject to the FCPA, and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate, including, in the U.K., the Bribery Act 2010. The FCPA generally prohibits offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. Recently, the SEC and Department of Justice have increased their FCPA enforcement activities with respect to biotechnology and medical device companies. There is no certainty that all of our employees, agents, contractors, or those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, the closing down of our facilities, requirements to obtain export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs, and prohibitions on the conduct of our business.
41
Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and financial condition.
We are subject to stringent and evolving laws, regulations, rules, contractual obligations, policies and other obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation (including class claims) and mass arbitration demands; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; and other adverse business consequences.
In the ordinary course of business, we collect, receive, store, process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, processing) personal data and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, data we collect about trial participants in connection with clinical trials, and sensitive third-party data (collectively, sensitive data). Our data processing activities subject us to numerous data privacy and security obligations, such as various laws, regulations, guidance, external and internal privacy and security policies, contractual obligations, and other obligations relating to data privacy and security.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws and regulations, including data breach notification laws, state and federal health information privacy laws, personal data privacy laws, and federal and state consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., wiretapping laws). In addition, we may obtain health data from third parties (including research institutions from which we obtain clinical trial data) that is subject to privacy and security requirements under HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 ("HITECH"), and their respective implementing regulations. Depending on the facts and circumstances, we could be subject to civil, criminal, and administrative penalties if we knowingly obtain, use, or disclose individually identifiable protected health information in a manner that is not authorized or permitted by HIPAA. In addition, the California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act of 2020 ("CPRA"), (collectively, the CCPA), applies to personal data of consumers, business representatives, and employees, and requires businesses to provide specific disclosures in privacy notices and honor requests of California residents to exercise certain privacy rights. The CCPA provides for civil penalties of up to $7,500 per violation and allows private litigants affected by certain data breaches to recover significant statutory damages. Further, the CPRA expands the CCPA’s requirements, including by adding a new right for individuals to correct their personal data and establishing a new regulatory agency to implement and enforce the law. Although the CCPA exempts some data processed in the context of clinical trials, the CCPA increases compliance costs and potential liability with respect to other personal data we maintain about California residents. Other states, such as Virginia, Colorado, Utah, and Connecticut have also passed comprehensive privacy laws, and similar laws are being considered in several other states, as well as at the federal and local levels. While these states, like the CCPA, also exempt some data processed in the context of clinical trials, these developments may further complicate compliance efforts, and increase legal risk and compliance costs for us and the third parties upon whom we rely.
Outside the United States, an increasing number of laws, regulations, and industry standards may govern data privacy and security. For example, the European Union’s General Data Protection Regulation (“EU GDPR”), the United Kingdom’s GDPR (“UK GDPR”), Brazil’s General Data Protection Law (Lei Geral de Proteção de Dados Pessoais, or “LGPD”) (Law No. 13,709/2018), and China’s Personal Information Protection Law (“PIPL”) impose strict requirements for processing personal data.
For example, under GDPR, companies may face temporary or definitive bans on data processing and other corrective actions; fines of up to 20 million Euros under the EU GDPR, 17.5 million pounds sterling under the UK GDPR or, in each case, 4% of annual global revenue, whichever is greater; or private litigation related to processing of personal data brought by classes of data subjects or consumer protection organizations authorized at law to represent their interests.
In addition, we may be unable to transfer personal data from Europe and other jurisdictions to the United States or other countries due to data localization requirements or limitations on cross-border data flows. Europe and other jurisdictions have enacted laws requiring data to be localized or limiting the transfer of personal data to other countries. In particular, the European Economic Area (EEA) and the United Kingdom (UK) have significantly restricted the transfer of personal data to the United States and other countries whose privacy laws it generally believes are inadequate. Other jurisdictions may adopt similarly stringent interpretations of their data localization and cross-border data transfer laws. Although there are currently various mechanisms that may be used to transfer personal data from the EEA and UK to the United States in compliance with law, such as the EEA and UK’s standard contractual clauses and the UK’s International Data Transfer Agreement / Addendum, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. If there is no lawful manner for us to transfer personal data from the EEA, the UK, or other jurisdictions to the United States, or if the requirements for a legally-compliant transfer are too onerous, we could face significant adverse consequences, including the interruption or degradation of our operations, the need to relocate part of or all of our business or data processing activities to other jurisdictions (such as Europe) at significant expense, increased exposure to regulatory actions, substantial fines and penalties, the inability to transfer data and work with partners, vendors and other third parties, and injunctions against our processing or transferring of personal data necessary to operate our business.
42
Additionally, companies that transfer personal data out of the EEA and UK to other jurisdictions, particularly to the United States, are subject to increased scrutiny from regulators, individual litigants, and activities groups. Some European regulators have ordered certain companies to suspend or permanently cease certain transfers of personal data out of Europe for allegedly violating the GDPR’s cross-border data transfer limitations.
Our employees and personnel use generative artificial intelligence (“AI”) technologies to perform their work, and the disclosure and use of personal information in generative AI technologies is subject to various privacy laws and other privacy obligations. Governments have passed and are likely to pass additional laws regulating generative AI. Our use of this technology could result in additional compliance costs, regulatory investigations and actions, and consumer lawsuits. If we are unable to use generative AI, it could make our business less efficient and result in competitive disadvantages. Several jurisdictions around the globe, including Europe and certain U.S. states, have proposed or enacted laws governing AI/ML. For example, European regulators have proposed a stringent AI regulation, and we expect other jurisdictions will adopt similar laws. Additionally, certain privacy laws extend rights to consumers (such as the right to delete certain personal data) and regulate automated decision making, which may be incompatible with our use of AI/ML. These obligations may make it harder for us to conduct our business using AI/ML, lead to regulatory fines or penalties, require us to change our business practices, retrain our AI/ML, or prevent or limit our use of AI/ML. For example, the FTC has required other companies to turn over (or disgorge) valuable insights or trainings generated through the use of AI/ML where they allege the company has violated privacy and consumer protection laws. If we cannot use AI/ML or that use is restricted, our business may be less efficient, or we may be at a competitive disadvantage.
In addition to data privacy and security laws, we are contractually subject to industry standards adopted by industry groups and may become subject to such obligations in the future. We are also bound by other contractual obligations related to data privacy and security, and our efforts to comply with such obligations may not be successful.
We publish privacy policies, marketing materials, and other statements, such as compliance with certain certifications or self-regulatory principles, regarding data privacy and security. If these policies, materials or statements are found to be deficient, lacking in transparency, deceptive, unfair, or misrepresentative of our practices, we may be subject to investigation, enforcement actions by regulators, or other adverse consequences.
Obligations related to data privacy and security are quickly changing, becoming increasingly stringent, and creating regulatory uncertainty. Additionally, these obligations may be subject to differing applications and interpretations, which may be inconsistent or conflict among jurisdictions. Preparing for and complying with these obligations requires us to devote significant resources and may necessitate changes to our services, information technologies, systems, and practices and to those of any third parties that process personal data on our behalf.
We may at times fail (or be perceived to have failed) in our efforts to comply with our data privacy and security obligations. Moreover, despite our efforts, our personnel or third parties on whom we rely upon may fail to comply with such obligations, which could negatively impact our business operations. If we or the third parties on which we rely fail, or are perceived to have failed, to address or comply with applicable data privacy and security obligations, we could face significant consequences, including but not limited to: government enforcement actions (e.g., investigations, fines, penalties, audits, inspections, and similar); litigation (including class-action claims) and mass arbitration demands; additional reporting requirements and/or oversight; bans on processing personal data; and orders to destroy or not use personal data. In particular, plaintiffs have become increasingly more active in bringing privacy-related claims against companies, including class claims and mass arbitration demands. Some of these claims allow for the recovery of statutory damages on a per violation basis, and, if viable, carry the potential for monumental statutory damages, depending on the volume of data and the number of violations. Any of these events could have a material adverse effect on our reputation, business, or financial condition, including but not limited to: loss of customers; inability to process personal data or to operate in certain jurisdictions; limited ability to develop or commercialize our products; expenditure of time and resources to defend any claim or inquiry; adverse publicity; or substantial changes to our business model or operations.
Our employees, principal investigators, consultants, professional service providers, manufacturers and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants, professional service providers, manufacturers and commercial partners. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and non-United States regulatory authorities, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and cause serious harm to our reputation.
43
We have implemented a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and our code of conduct and the other precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses, or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, which could have a significant impact on our business. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these actions or investigations.
If we use hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the use of hazardous chemicals and biohazardous waste, including chemical, biological agents and compounds, human blood and urine. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling, or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject on an ongoing basis to federal, state, and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste services. The cost of compliance with these laws and regulations may become significant and could negatively affect our business, financial condition and results of operations.
Risks Related to Our Reliance on Third Parties
We are highly reliant on our partnership with Mount Sinai, and our failure to maintain that relationship could negatively impact our business, reputation and strategic goals.
Mount Sinai is our initial launch partner for KidneyIntelX. To the extent that this partnership fails to produce the anticipated outcomes, our business and reputation could be harmed. Under the Mount Sinai Agreement, we and Mount Sinai agreed to conduct a clinical utility study. There can be no certainty that we will complete the clinical utility study with Mount Sinai or that the Mount Sinai Agreement will not be terminated early. If our partnership with Mount Sinai is terminated and if we have not yet established, or are unable to establish, significant partnerships with other healthcare systems, our business would be adversely affected.
In October 2018, we, Mount Sinai and NPLUS1 Singer Advisory LLP ("Singer"), entered into a Relationship Agreement ("the Relationship Agreement"), to regulate the terms of the relationship between the Company and Mount Sinai and to ensure that we can operate independently of Mount Sinai, pursuant to which, among other things, Mount Sinai has the right to appoint one member to our Board of Directors and Mount Sinai has agreed to not take any action intended to prevent our Board of Directors from operating independently of Mount Sinai. The Relationship Agreement is filed as an exhibit to this Annual Report on Form 10-K and is incorporated herein by reference, and the foregoing description of the Relationship Agreement is qualified in its entirety by reference thereto.
In September 2021, we and Mount Sinai announced scaled-up implementation of the KidneyIntelX early-stage risk assessment testing and care management program across primary care and specialty clinician networks under a real-world evidence development program for patients with DKD. There can be no guarantee that this scaled-up implementation will proceed on the timelines expected or result in the volume of tests expected.
We also license intellectual property from Mount Sinai. In May 2018, we entered into the Mount Sinai Agreement pursuant to which we obtained a worldwide, royalty-bearing, exclusive license under certain patents and a worldwide, royalty-bearing, non-exclusive license under certain know-how of Mount Sinai to develop and commercialize licensed products in connection with the application of artificial intelligence for the diagnosis of kidney disease. Pursuant to the terms of the Mount Sinai Agreement, we are obligated to use commercially reasonable efforts in connection with the development and commercialization of the licensed products, including in accordance with specified diligence milestones. If we fail to meet our obligations under the Mount Sinai Agreement or if the Mount Sinai Agreement is terminated for any reason, it could negatively impact our business and strategic goals.
Further, our collaborative research studies with Mount Sinai utilize the Mount Sinai BioMe biobank. BioMe, which is a biobank linked to longitudinal de-identified EHR data from consented participants, has allowed us to conduct rapid prospective validation of our platform using samples banked at “time zero” (i.e. time of sample collection), prior to the occurrence of progressive kidney function decline. If, for any reason, we are unable to continue our collaborative research studies that rely on the use of BioMe, and a comparable biobank is not available or a collaborative relationship has not been established, our ability to support the continued development and validation of our KidneyIntelX platform could be harmed.
44
If we do not continue to enter into partnerships with health systems at the rate and on the scale that we anticipate, or if these existing partnerships are terminated or do not result in the rate and quantity of KidneyIntelX testing that we anticipate, our business could be harmed.
We rely on a limited number of suppliers or, with respect to our multiplex biomarker assays, a single supplier, for the assay reagents and associated materials and may not be able to find replacements or immediately transition to alternative suppliers.
We have sourced and will continue to source components of our technology, including instruments and reagents and other laboratory materials, from third parties. The assay reagents and materials for the KidneyIntelX test are sourced from Meso Scale Diagnostics, LLC ("MSD"), and the assay is performed on the MSD instrument platform. The instruments used are not specific to KidneyIntelX; we purchase them directly from MSD as standard items along with a comprehensive service agreement. The multiplex assay plate (whereby three biomarkers—sTNFR1, sTNFR2 and KIM-1—are measured concurrently in a single well), diluents, calibrators, quality controls, detection antibodies and other assay materials were developed specifically for us under a master services agreement we entered into in 2018. In the event that this supply is interrupted, we believe the assay could be substantially reproduced through a combination of use of off-the-shelf materials provided by MSD and access to critical raw materials such as antibodies available from other manufacturers. Alternatively, the assay could be transferred to another technology platform, including those supplied by leading diagnostics manufacturers. However, either of these scenarios would require substantial development time, effort and extensive analytical and clinical validation and potentially new regulatory clearance.
If the supply of components we receive does not meet our quality control or performance standards, we may not be able to use the components, or if we use them not knowing that they are of inadequate quality, which occasionally occurs with respect to certain reagents, our tests may not work properly or at all, or they may provide erroneous results. As a result, we may be subject to significant delays caused by interruption in production or manufacturing or to lost revenue from such interruption or from spoiled tests. In addition, any natural or other disaster, including acts of war or terrorism, shipping embargoes, labor unrest or political instability or similar events at our third-party manufacturers’ facilities that cause a loss of manufacturing capacity would heighten the risks that we face.
In the event of any adverse developments with our suppliers, in particular for those products that are sole sourced, or if any of our suppliers modifies any of the components they supply to us, our ability to supply our products may be interrupted, and obtaining substitute components could be difficult or require us to re-design or re-validate our products. In addition, if we obtain FDA clearance, approval or authorization for any of our tests as an in vitro diagnostic, such issues with suppliers or the components that we source from suppliers could affect our commercialization efforts for such an in vitro diagnostic. Our failure to maintain a continued supply of components that meets our quality control requirements, or changes to or termination of our agreements or inability to renew our agreements with these parties or enter into new agreements with other suppliers, particularly in the case of sole suppliers, could result in the loss of access to important components of our tests and impact our test performance or affect our ability to perform our tests in a timely manner or at all, which could impair, delay or suspend our commercialization activities. Moreover, in the event that we transition to a new supplier from any of our sole suppliers, doing so could be time-consuming and expensive, may result in interruptions in our ability to supply our products to the market, could affect the performance of our tests or could require that we re-validate KidneyIntelX using replacement equipment and supplies, and should such a change be made following obtaining an FDA marketing authorization, may require a new submission, such as, for example, a new 510(k) and obtaining FDA clearance prior to implementation of the modified test, which could delay the performance of our tests and result in increased costs. Any of these occurrences could have a material adverse effect on our business, financial condition and results of operations.
If one or more of our laboratory facilities become damaged or inoperable, if we are required to vacate any of our laboratory facilities, or if we are delayed in obtaining or unable to obtain additional laboratory space or delayed in commencing operations in our laboratory facilities, our ability to manufacture our products, pursue our research and development efforts and fulfill our contractual obligations may be jeopardized.
We currently have our laboratory in New York. Our facility and equipment could be harmed or rendered inoperable by natural or man-made disasters, including war, fire, earthquake, power loss, communications or Internet failure or interruption, terrorism, or pandemic which may render it difficult or impossible for us to provide these services for some period of time. The inability to provide these services or to reduce the backlog of analyses that could develop if one or more of our laboratories become inoperable, for even a short period of time, may result in the loss of customers or harm to our reputation, and we may be unable to regain those customers or repair our reputation in the future. Furthermore, our facilities and the equipment we use to perform our research and development work could be unavailable or costly and time- consuming to repair or replace. It would be difficult, time-consuming, and expensive to rebuild any of our facilities or license or transfer our proprietary technology to a third party, particularly in light of the licensure and accreditation requirements for commercial laboratories like ours. We may be unable to negotiate commercially reasonable terms with such third parties. Adverse consequences resulting from an interruption of our overall laboratory operations could harm relationships with our customers and regulatory authorities, and our reputation, and could affect our ability to generate revenue.
45
We may also construct, acquire, or enter into relationships with third parties to procure additional laboratory space inside and outside the United States to support our existing and new services. If we are unable to obtain or are delayed in obtaining or establishing new laboratory space to support these commercialization and development efforts, or if our potential future ex-United States laboratory operations are harmed or are rendered inoperable, we could fail to meet certain contractual obligations and agreed upon timelines with certain of our partners or provide existing services and develop and launch new services in certain territories, which could result in harm to our business and reputation, and adversely affect our business, financial condition, and results of operations. As we continue to transition some of our services to new laboratories, we could experience disruptions in overall laboratory operations and could require adjustments to meet regulatory requirements, resulting in our inability to meet customer turnaround time expectations. Any delays in this transition could result in slower realization of laboratory efficiencies anticipated from operating an additional laboratory facility. Adverse consequences resulting from an interruption of our overall laboratory operations could harm relationships with our customers and regulators, and our reputation, and could affect our ability to generate revenue.
We carry insurance for damage to our property and laboratory and the disruption of our business, but this insurance may not cover all of the risks associated with damage to our property or laboratory or disruption to our business, may not provide coverage in amounts sufficient to cover our potential losses, may be challenged by insurers underwriting the coverage, and may not continue to be available to us on acceptable terms, if at all.
Risks Related to Our Business Operations and Industry
If we are unable to compete successfully with respect to our current or future products, we may be unable to increase or sustain our revenues or achieve profitability.
We face competition from clinical reference laboratories and diagnostics manufacturers, including large diagnostic laboratories such as Quest Diagnostics Inc. and Laboratory Corporation of America Holdings (LabCorp) and large diagnostics manufacturers such as ThermoFisher Scientific Inc., Danaher Corporation, Roche Holding AG, Abbott Laboratories, Bio-Rad Laboratories, Inc., Ortho Clinical Diagnostics NV and Siemens Healthineers AG, all of which have widespread brand recognition and market penetration and substantially greater financial, technical, research and development and selling and marketing capabilities than we do.
We also face competition from data analytics companies that have developed technology-based or artificial intelligence-based approaches to healthcare applications and medical devices and that currently or in the future may develop diagnostic or prognostic products focused on kidney disease.
Principal competitive factors in our market include:
While we believe we compete effectively based on these factors, our product is novel and market acceptance is untested at this time. Further, even if we are able to secure partnerships with additional healthcare systems, commercial and clinical acceptance rates are currently unknown. Many of our competitors and potential competitors have longer operating histories, larger customer bases, greater brand recognition and market penetration, substantially greater financial, technological and research and development resources and selling and marketing capabilities, and more experience dealing with third-party payors. As a result, they may be able to respond more quickly to changes in customer requirements, devote greater resources to the development, promotion and sale of their diagnostic tests. We may not be able to compete effectively against these organizations should they choose to enter the market for early stage kidney disease prognostics.
46
Medical advances may impact the diagnostics industry and prevent us from achieving increased market penetration and improved operating results.
The medical industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. During 2023 and into the second half of 2024, glucagon-like peptide 1 ("GLP-1s"), a class of drug indicated for diabetes and obesity, continued to gain popularity as a weight-loss drug. For example, on June 21, 2024, Eli Lilly announced that, following the release of results from its SURMOUNT phase 3 clinical trials, it has submitted its GLP-1 drug candidate, tirzepatide, for the potential treatment of chronic weight management to the FDA and plans to initiate submissions for other global regulatory agencies in the near term.
Increased popularity of GLP-1s, or similar treatments, may directly or indirectly lead to reductions in the prevalence of obesity, along with a resulting reduction in diabetes, CKD, DKD and ESKD. If GLP-1s are successful in reducing the prevalence of kidney disease in the population, demand for our products could be reduced.
Our long-term strategy depends in part on our ability to improve KidneyIntelX, through versioning, to keep pace with rapid advances in artificial intelligence, technology, medicine and science. If we experience delays or challenges in creating and deploying new versions of KidneyIntelX, our operating results and competitive position could be harmed.
The diagnostics industry is characterized by rapid technological changes, scientific breakthroughs, frequent new product and service introductions and enhancements, and evolving industry standards, all of which could make KidneyIntelX obsolete. Further, the field of artificial intelligence is rapidly advancing and we must ensure that we keep pace with these changes in our technology and algorithms in order to ensure that KidneyIntelX delivers accurate and clinically relevant results.
Our future success will depend on our ability to keep pace with the evolving needs of our customers and the evolution of our industry on a timely and cost-effective basis and to pursue new market opportunities that develop as a result of scientific and technological advances. In recent years, there have been numerous advances in technologies relating to life sciences research and the diagnosis and treatment of kidney disease. There have also been advances in technologies used to computationally analyze very large amounts of biologic information. If we do not update KidneyIntelX through the creation and deployment of new versions to reflect advances in artificial intelligence, new scientific knowledge about new disease diagnostics and therapies or the diseases we seek to target, KidneyIntelX could become obsolete.
If we lose, or cannot garner, the support of key thought leaders, it may be difficult to establish KidneyIntelX as a standard of care for patients at risk for kidney disease, which may limit our revenue growth and ability to achieve profitability.
We have established relationships with key thought leaders at premier medical institutions and networks. If these key thought leaders determine that KidneyIntelX is not clinically effective, that alternative technologies and products are more effective, or if they elect to use internally developed products, we could encounter significant difficulty validating our technology platform, driving adoption, and establishing KidneyIntelX as a standard of care, which would limit our revenue growth and our ability to achieve profitability.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We plan to grow our business operations initially in the United States. Any future growth could create strain on our organizational, administrative, and operational infrastructure, including laboratory operations, quality control, customer service, and sales force management. We may not be able to maintain the quality or expected turnaround times of our services or satisfy customer demand as it grows. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and managerial controls, as well as our reporting systems and procedures.
For example, we believe we have capacity at our facility in New York to process sufficient KidneyIntelX tests to meet projected demand in the near-term. However, our strategy is based on a model that assumes we will be successful in entering into partnerships with healthcare systems and third-party payors, which could result in large increases in demand for KidneyIntelX tests as these new partnerships are forged. It will be critical that we carefully manage our ability to scale as we seek new partnerships. If we fail to do so effectively, we may not be able to meet the demand of the partners we engage, we may fail to produce and process tests in a timely manner or may be forced to forego growth opportunities because we failed to adequately scale our business. Any of these could have a material adverse effect on our business.
47
We have material weakness in our controls and procedures.
We have conducted an evaluation of our internal control over financial reporting based on the framework in "Internal Control Integrated Framework" issued by the Committee of Sponsoring Organizations for the Treadway Commission ("COSO") and published in 2013, and subsequent guidance prepared by COSO specifically for smaller public companies. Based on that evaluation, management concluded that our internal control over financial reporting was not effective as of June 30, 2024 for the reasons discussed below:
Management identified the following material weakness in its assessment of the effectiveness of internal control over financial reporting as of June 30, 2024: an error in the mark-to-market adjustment to our convertible debt that had been elected under the fair value option, which resulted in insufficient expense recognition. The deficiency arose due to the high complexity and technical nature of the convertible debt instrument, which the accounting team lacked technical knowledge on this area.
The management of the Company believes that this material weakness will remain until such time that the Company has the resources to increase the number of personnel committed to the performance of its financial duties so that such weakness can be specifically addressed. This will include, but not limited to, the retention of outside consultants to review our controls and procedures.
A material weakness is a deficiency or a combination of deficiencies in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, is designed to prevent fraud. If our internal control over financial reporting or our disclosure controls and procedures are not effective, we may not be able to accurately report our financial results, prevent fraud, or file our periodic reports in a timely manner, which may cause investors to lose confidence in our reported financial information and may lead to a decline in our stock price. In addition, a material weakness will not be considered remediated until the applicable controls operate for a sufficient period of time and management has concluded, through testing, that these controls are designed and operating effectively.
Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. In addition, any testing by us, as and when required, conducted in connection with Section 404 of the Sarbanes-Oxley Act, or Section 404, or any subsequent testing by our independent registered public accounting firm, as and when required, may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. As a growing company, implementing and maintaining effective controls may require more resources, and we may encounter internal control integration difficulties. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
Adverse market and economic conditions may exacerbate certain risks associated with commercializing our products.
Future sales of our products will be dependent on purchasing decisions of and reimbursement from government health administration authorities, distributors and other organizations. As a result of adverse conditions affecting the global economy and credit and financial markets, including disruptions due to political instability, global pandemics and diseases or otherwise, these organizations may defer purchases, may be unable to satisfy their purchasing or reimbursement obligations, or may delay payment for any of our products.
Our business could be adversely affected by the effects of health pandemics or epidemics, in regions where we or third parties on which we rely have significant manufacturing facilities, concentrations of validation study sites or other business operations.
Our business could be adversely affected by such pandemics or epidemics in regions where we have concentrations of validation study sites or other business operations, and could cause significant disruption in the operations of third parties upon whom we rely. Health pandemics or epidemics, such as the COVID-19 pandemic, have in the past resulted, and could again in the future result, in quarantines, stay-at-home orders, remote work policies or other similar events that may disrupt businesses, delay our research and development programs and timelines, negatively impact productivity and increase risks associated with cybersecurity, the future magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations.
48
Moreover, such pandemics or epidemics, such as the COVID-19 pandemic, could materially affect our operations, including at our U.S. headquarters in New York and at our validation study sites, as well as the business or operations of our partner, Mount Sinai, and other third parties with whom we conduct business. Such pandemics or epidemics have in the past resulted, and may in the future result, in the imposition of orders and policies that could negatively impact productivity, disrupt our business and delay our clinical programs and timelines, the magnitude of which would depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. In addition, such orders or policies, such or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, related to COVID-19 or other infectious diseases could impact personnel at third-party manufacturing facilities in the United States and other countries, or the availability or cost of materials, which would disrupt our supply chain.
In addition, our validation studies and commercial launch plans or timelines have in the past, and may in the future, be affected by such pandemics or epidemics. Moreover, our ability to recruit and retain patients and site staff may be hindered, which would adversely affect our plans or timelines.
While the effect of COVID-19 has subsided and continues to subside, the full extent to which the COVID-19 pandemic may continue to impact our business, results of operations, and financial condition will depend on future developments that are uncertain and cannot be accurately predicted. We cannot assure you that these effects will remain reduced in the future, including due to potential new public health outbreaks. We could face further operational disruptions and incur additional expenses in connection with future public health outbreaks, including expenses associated with our health and safety protocols and processes, that could adversely affect our business and results of operations. To the extent future public health outbreaks adversely affect our business and financial results, they may also have the effect of heightening many of the other risks described in this “Risk Factors” section.
Our international operations may be adversely affected by actions taken by foreign governments or other forces or events over which we may have no control.
Our global operations are dependent upon products manufactured, purchased and sold around the world, including in countries with political and economic instability or uncertainty. This includes, for example, the current conflict between Russia and Ukraine, ongoing tensions across the Middle East, the adoption and expansion of trade restrictions, including the occurrence or escalation of a “trade war,” or other governmental action related to tariffs or trade agreements or policies among the governments of the United States, China and other countries and other global events. The global credit and financial markets have recently experienced extreme volatility and disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. Sanctions imposed by the United States, the United Kingdom and other countries in response to such conflicts, including the one in Ukraine, may also adversely impact the financial markets and the global economy, and any economic countermeasures by affected countries and others could exacerbate market and economic instability. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Furthermore, Brexit could cause disruptions to, and create uncertainty surrounding our business, including affecting our relationships with our existing and future customers, suppliers and associates, which could have an adverse effect on our business, financial results and operations. Effects of Brexit, which include changes in customs regulations, shortages of truck drivers in the U.K., and administrative burdens placed on transportation companies, have led to challenges and delays in moving inventory across U.K. or EU borders, and higher importation, freight and distribution costs. If such trends continue, we may experience further cost increases.
Some countries have greater political and economic volatility and greater vulnerability to infrastructure and labor disruptions than others. Our business could be negatively impacted by adverse fluctuations in freight costs, limitations on shipping and receiving capacity, and other disruptions in the transportation and shipping infrastructure at important geographic points of exit and entry for our products. Operating in different regions and countries exposes us to a number of risks, including:
49
The occurrence of one or more of these events may adversely affect our business, financial condition and results of operations.
We may experience inflationary pressures, which could increase our manufacturing costs and operating expenses and have a material adverse impact on our results of operations.
We continuously monitor the effects of inflationary factors, such as increases in our cost of goods sold and selling and operating expenses, which may adversely affect our results of operations. Specifically, we may experience inflationary pressure affecting the cost of the components for our products and in the wages that we pay our employees due to challenging labor market conditions. Competitive and regulatory conditions may restrict our ability to fully recover these costs through price increases. As a result, it may be difficult to fully offset the impact of persistent inflation. Our inability or failure to do so could have a material adverse effect on our business, financial condition and results of operations or cause us to need to obtain additional capital in future earlier than anticipated.
The loss or transition of any of our executive officers or our inability to attract and retain highly skilled scientists, clinicians, and salespeople could adversely affect our business.
Our success depends on the skills, experience, and performance of key members of our executive team. The individual and collective efforts of these individuals will be important as we continue to develop our artificial intelligence technology, develop and seek regulatory clearance for our products and prepare for commercialization. The loss or incapacity of key members of our executive team could adversely affect our operations if we experience difficulties in hiring qualified successors.
Our research and development programs and laboratory operations depend on our ability to attract and retain highly skilled scientists and technicians. We may not be able to attract or retain qualified scientists and technicians in the future due to the intense competition for qualified personnel among life science businesses. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. We may have difficulties locating, recruiting, or retaining qualified sales people. Recruitment and retention difficulties can limit our ability to support our research and development and sales programs, which could in turn have an adverse effect on our business, financial condition and results of operations.
We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations.
As we mature, we expect to expand our full-time employee base and to hire more scientists and technicians. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time toward managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional products or technologies. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenues could be reduced, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize products and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our future growth depends, in part, on our ability to penetrate international markets, where we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend on our ability to commercialize our products in the United States, United Kingdom, the European Union and other territories around the world. If we commercialize our products in international markets, we would be subject to additional risks and uncertainties, including:
50
Sales of our products outside the United States could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale, and use of our products could lead to the filing of product liability claims were someone to allege that our diagnostic tests identified inaccurate or incomplete information regarding the risk or likely severity of the patient’s kidney disease, the risk of rejection of a patient’s kidney transplant, or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of, or inappropriate reliance upon the information we provide in the ordinary course of our business activities. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
We maintain product and professional liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation or cause current partners to terminate existing agreements and potential partners to seek other partners, any of which could impact our results of operations.
If our information technology systems or data, or those of third parties upon which we rely, are or were compromised, we could experience adverse consequences resulting from such compromise, including but not limited to regulatory investigations or actions; litigation; fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; and other adverse consequences.
In the ordinary course of our business, we and the third parties upon which we rely process sensitive data, and, as a result, we and the third parties upon which we rely face a variety of evolving threats, including but not limited to ransomware attacks, which could cause security incidents. Cyber-attacks, malicious internet-based activity, online and offline fraud, and other similar activities threaten the confidentiality, integrity, and availability of our sensitive data and information technology systems, and those of the third parties upon which we rely. Such threats are prevalent and continue to rise, are increasingly difficult to detect, and come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists,” organized criminal threat actors, personnel (such as through theft or misuse), sophisticated nation states, and nation-state-supported actors.
51
Some actors now engage and are expected to continue to engage in cyber-attacks, including without limitation nation-state actors for geopolitical reasons and in conjunction with military conflicts and defense activities. During times of war and other major conflicts, we and the third parties upon which we rely, may be vulnerable to a heightened risk of these attacks, including retaliatory cyber-attacks, that could materially disrupt our systems and operations, supply chain, and ability to produce, sell and distribute our services. Further, the loss of clinical trial data from completed or ongoing clinical trials could result in delays in, or cancellations of, any regulatory approval or clearance efforts and significantly increase our costs to recover or reproduce the data, and subsequently commercialize the product. Additionally, theft of our intellectual property or proprietary business information could require substantial expenditures to remedy.
We and the third parties upon which we rely are subject to a variety of evolving threats, including but not limited to social-engineering attacks (including through deep fakes, which may be increasingly more difficult to identify as fake, and phishing attacks), malicious code (such as viruses and worms), malware (including as a result of advanced persistent threat intrusions), denial-of-service attacks (such as credential stuffing), credential harvesting, personnel misconduct or error, ransomware attacks, supply-chain attacks, software bugs, server malfunctions, software or hardware failures, loss of data or other information technology assets, adware, telecommunications failures, earthquakes, fires, floods, and other similar threats.
In particular, severe ransomware attacks are becoming increasingly prevalent and can lead to significant interruptions in our operations, loss of sensitive data and income, reputational harm, and diversion of funds. Extortion payments may alleviate the negative impact of a ransomware attack, but we may be unwilling or unable to make such payments due to, for example, applicable laws or regulations prohibiting such payments.
Remote work has become more common and has increased risks to our information technology systems and data, as more of our employees utilize network connections, computers, and devices outside our premises or network, including working at home, while in transit and in public locations. Additionally, future or past business transactions (such as acquisitions or integrations) could expose us to additional cybersecurity risks and vulnerabilities, as our systems could be negatively affected by vulnerabilities present in acquired or integrated entities’ systems and technologies. Furthermore, we may discover security issues that were not found during due diligence of such acquired or integrated entities, and it may be difficult to integrate companies into our information technology environment and security program.
In addition, our reliance on third-party service providers could introduce new cybersecurity risks and vulnerabilities, including supply-chain attacks, and other threats to our business operations. We rely on third-party service providers and technologies to operate critical business systems to process sensitive data in a variety of contexts, including, without limitation, cloud-based infrastructure, data center facilities, encryption and authentication technology, employee email, content delivery to customers, and other functions. We also rely on third-party service providers to provide other products, services, parts, or otherwise to operate our business. Our ability to monitor these third parties’ information security practices is limited, and these third parties may not have adequate information security measures in place. If our third-party service providers experience a security incident or other interruption, we could experience adverse consequences. While we may be entitled to damages if our third-party service providers fail to satisfy their privacy or security-related obligations to us, any award may be insufficient to cover our damages, or we may be unable to recover such award. In addition, supply-chain attacks have increased in frequency and severity, and we cannot guarantee that third parties’ infrastructure in our supply chain or our third-party partners’ supply chains have not been compromised.
Any of the previously identified or similar threats could cause a security incident or other interruption that could result in unauthorized, unlawful, or accidental acquisition, modification, destruction, loss, alteration, encryption, disclosure of, or access to our sensitive data or our information technology systems, or those of the third parties upon whom we rely. A security incident or other interruption could disrupt our ability (and that of third parties upon whom we rely) to provide our services.
We may expend significant resources or modify our business activities to try to protect against security incidents. Additionally, certain data privacy and security obligations may require us to implement and maintain specific security measures or industry-standard or reasonable security measures to protect our information technology systems and sensitive data.
While we have implemented security measures designed to protect against security incidents, there can be no assurance that these measures will be effective. We take steps to detect and remediate vulnerabilities, but we may not be able to detect and remediate all vulnerabilities because the threats and techniques used to exploit the vulnerability change frequently and are often sophisticated in nature. Therefore, such vulnerabilities could be exploited but may not be detected until after a security incident has occurred. These vulnerabilities pose material risks to our business. Further, we may experience delays in developing and deploying remedial measures designed to address any such identified vulnerabilities.
Applicable data privacy and security obligations may require us to notify relevant stakeholders of security incidents. Such disclosures are costly, and the disclosure or the failure to comply with such requirements could lead to adverse consequences.
52
If we (or a third party upon whom we rely) experience a security incident or are perceived to have experienced a security incident, we may experience adverse consequences, such as government enforcement actions (for example, investigations, fines, penalties, audits, and inspections); additional reporting requirements and/or oversight; restrictions on processing sensitive data (including personal data); litigation (including class claims); indemnification obligations; negative publicity; reputational harm; monetary fund diversions; interruptions in our operations (including availability of data); financial loss; and other similar harms. Security incidents and attendant consequences may cause customers to stop using our services, deter new customers from using our services, and negatively impact our ability to grow and operate our business. Likewise, we rely on third parties to conduct clinical trials, and similar incidents relating to their information technology systems or data could also have a material adverse effect on our business.
Our contracts may not contain limitations of liability, and even where they do, there can be no assurance that limitations of liability in our contracts are sufficient to protect us from liabilities, damages, or claims related to our data privacy and security obligations. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or to mitigate liabilities arising out of our privacy and security practices, that such coverage will continue to be available on commercially reasonable terms or at all, or that such coverage will pay future claims.
In addition to experiencing a security incident, third parties may gather, collect, or infer sensitive information about us from public sources, data brokers, or other means that reveals competitively sensitive details about our organization and could be used to undermine our competitive advantage or market position.
Furthermore, any sensitive information (including confidential, competitive, proprietary, or personal data) that we input into a third-party generative AI/ML platform could be leaked or disclosed to others, including if sensitive information is used to train the third parties’ AI/ML model. Additionally, where an AI/ML model ingests personal data and makes connections using such data, those technologies may reveal other personal or sensitive information generated by the model. Moreover, AI/ML models may create flawed, incomplete, or inaccurate outputs, some of which may appear correct. This may happen if the inputs that the model relied on were inaccurate, incomplete or flawed (including if a bad actor “poisons” the AI/ML with bad inputs or logic), or if the logic of the AI/ML is flawed (a so-called “hallucination”). We may use AI/ML outputs to make certain decisions. Due to these potential inaccuracies or flaws, the model could be biased and could lead us to make decisions that could bias certain individuals (or classes of individuals), and adversely impact their rights, employment, and ability to obtain certain pricing, products, services, or benefits, including exposure to reputational and competitive harm, customer loss, and legal liability.
Changes in U.S. tax law could adversely affect our business and could differ materially from the financial statements provided herein.
The rules dealing with U.S. federal, state and local income taxation are constantly under review by the Internal Revenue Service, the U.S. Treasury Department and other governmental bodies. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our ADSs or ordinary shares. In recent years, many such changes have been made and changes are likely to continue to occur in the future. Future changes in tax laws could have a material adverse effect on our business, cash flow, financial condition or results of operations. We urge investors to consult with their legal and tax advisers regarding the implication of potential changes in tax laws on an investment in our ADSs or ordinary shares.
Our ability to use our U.S. net operating loss carryforwards and certain other U.S. tax attributes may be limited.
As of June 30, 2024, we had U.S. federal net operating loss carryforwards of approximately $116.8 million and U.S. state and local net operating loss carryforwards of approximately $202.3 million due to prior period losses. Under the Tax Cuts and Jobs Act of 2017 as modified by the Coronavirus Aid, Relief, and Economic Security (CARES) Act, (collectively, the "Tax Acts"), U.S. federal net operating losses incurred in taxable years beginning after December 31, 2017 may be carried forward indefinitely, but the deductibility of such federal net operating losses may be limited to 80% of our taxable income in taxable years beginning after December 31, 2020. It is uncertain if and to what extent various states will conform to the Tax Acts. In addition, under Sections 382 and 383 of the U.S. Internal Revenue Code of 1986, as amended, (the "Code"), if a corporation undergoes an “ownership change” (generally defined as a greater than 50 percentage-point cumulative change (by value) in the equity ownership of certain shareholders over a rolling three-year period), the corporation’s ability to use its pre-change net operating losses and other pre-change tax attributes to offset its post-change taxable income or taxes may be limited. We have not completed an analysis to determine whether any such limitations have been already triggered. We may also experience ownership changes as a result of shifts in our share ownership, some of which are outside our control. Therefore, as a result of ownership changes with respect to our ordinary shares, our ability to use our current net operating losses and other pre-change tax attributes to offset post-change taxable income or taxes could be subject to limitation. We will be unable to use our net operating losses if we do not attain profitability sufficient to offset our available net operating losses prior to their expiration.
53
We may be unable to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments or benefit from favorable U.K. tax legislation.
As a U.K. resident trading entity, we are subject to U.K. corporate taxation. Due to the nature of our business, we have generated losses since inception. As of June 30, 2024, we had cumulative carryforward tax losses of approximately $38.4 million in the UK. Subject to any relevant utilization criteria and restrictions (including those that limit the percentage of profits that can be reduced by carried forward losses and those that can restrict the use of carried forward losses where there is a change of ownership of more than half the ordinary shares of the company and a major change in the nature, conduct or scale of the trade), we expect these to be eligible for carry forward and utilization against future operating profits.
As a company that carries out extensive research and development ("R&D") activities, we seek to benefit from the U.K. R&D tax relief programs, being the Small and Medium-sized Enterprises R&D tax relief program (the "SME Program"), and, for certain specific categories of expenditure, the Research and Development Expenditure Credit program (the "RDEC Program"). The SME Program may be particularly beneficial to us, as under such program the trading losses that arise from our qualifying R&D activities can be surrendered for a cash rebate of up to 33.35% of qualifying R&D expenditure incurred prior to April 1, 2023, and up to 18.6% of qualifying expenditure incurred thereafter (unless we qualify as an “R&D-intensive SME” for an accounting period (broadly, a loss making SME whose qualifying R&D expenditure for an accounting period represents 40% or more of its total expenditure for that accounting period), in which case the cash rebate that may be claimed will be 26.97% of qualifying expenditure). Further, amendments to the U.K. R&D tax credit regime have been proposed that may (unless limited exceptions apply) introduce restrictions on the tax relief that can be claimed for expenditure incurred on sub-contracted R&D activities or externally provided workers, where such sub-contracted activities are not carried out in the U.K. or such workers are not subject to U.K. payroll taxes. These amendments are expected to take effect from April 1, 2024. In addition, the U.K. Government is currently considering a proposal to merge the SME Program and the RDEC Program into a single scheme with effect from April 2024; if such proposal is implemented in the manner provided in recently-published draft legislation, and we do not qualify as an R&D-intensive SME, we will either cease to be able to claim cash rebates in respect of our R&D activities, or only be able to receive such cash rebates at a significantly lower rate than at present. These and other potential changes to the U.K. R&D tax relief programs may mean we no longer qualify or have a material impact on the extent to which we can make claims or benefit from them.
We may benefit in the future from the United Kingdom’s “patent box” regime, which allows certain profits attributable to revenues from patented products (and other qualifying income) to be taxed at an effective rate of 10% by giving an additional tax deduction. We are the exclusive licensee or owner of one patent and several patent applications which, if issued, would cover our product candidates, and accordingly, future upfront fees, milestone fees, product revenues and royalties could be eligible for this deduction. When taken in combination with the enhanced relief available on our R&D expenditures, we expect a long-term rate of corporation tax lower than the statutory rate to apply to us. If, however, there are unexpected adverse changes to the U.K. R&D tax relief programs or the “patent box” regime, or for any reason we are unable to qualify for such advantageous tax legislation, or we are unable to use net operating loss and tax credit carryforwards and certain built-in losses to reduce future tax payments then our business, results of operations and financial condition may be adversely affected. This may impact our ongoing requirement for investment and the time frames within which additional investment is required.
Future changes to tax laws could materially adversely affect our company and reduce net returns to our shareholders.
The tax treatment of the company is, and our ADSs and ordinary shares are, subject to changes in tax laws, regulations and treaties, or the interpretation thereof, tax policy initiatives and reforms under consideration and the practices of tax authorities in jurisdictions in which we operate, as well as tax policy initiatives and reforms related to the Organization for Economic Co-Operation and Development’s, (the "OECD"), Base Erosion and Profit Shifting ("BEPS"), Project, the European Commission’s state aid investigations and other initiatives. Such changes may include (but are not limited to) the taxation of operating income, investment income, dividends received or (in the specific context of withholding tax) dividends paid, or the stamp duty or stamp duty reserve tax treatment of our ADSs or ordinary shares. We are unable to predict what tax reform may be proposed or enacted in the future or what effect such changes would have on our business, but such changes, to the extent they are brought into tax legislation, regulations, policies or practices, could affect our financial position and overall or effective tax rates in the future in countries where we have operations, reduce post-tax returns to our shareholders, and increase the complexity, burden and cost of tax compliance.
54
Tax authorities may disagree with our positions and conclusions regarding certain tax positions, or may apply existing rules in an unforeseen manner, resulting in unanticipated costs, taxes or non-realization of expected benefits.
A tax authority may disagree with tax positions that we have taken, which could result in increased tax liabilities. For example, His Majesty’s Revenue & Customs, ("HMRC"), the U.S. Internal Revenue Service or another tax authority could challenge our allocation of income by tax jurisdiction and the amounts paid between our affiliated companies pursuant to our intercompany arrangements and transfer pricing policies, including amounts paid with respect to our intellectual property development. Similarly, a tax authority could assert that we are subject to tax in a jurisdiction where we believe we have not established a taxable connection, often referred to as a ‘‘permanent establishment’’ under international tax treaties, and such an assertion, if successful, could increase our expected tax liability in one or more jurisdictions.
A tax authority could also disagree with our analysis of the tax treatment of the FractalDx spin-off, for ourselves and/or for our shareholders. A tax authority may take the position that material tax liabilities, interest and penalties are payable by us, in which case we expect that we might contest such assessment. Contesting such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase our anticipated effective tax rate, where applicable, or result in other liabilities.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain sufficient patent protection for our products, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to commercialize our products successfully may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary products. If we do not adequately protect our intellectual property, competitors may be able to erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability. To protect our proprietary position, we file patent applications in the United States and abroad related to our novel products that are important to our business. The patent application and approval process is expensive and time-consuming. We may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner.
Our ability to obtain patent protection for our products is uncertain due to a number of factors, including:
55
Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until patent issues from such applications. Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, our patents or pending patent applications may be challenged in the courts or patent offices in the United States and abroad. For example, we may be subject to a third party preissuance submission of prior art to the U.S. Patent and Trademark Office, (the "USPTO"), or become involved in post-grant review procedures, oppositions, derivations, reexaminations, inter partes review or interference proceedings, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. In addition, given the amount of time required for the development, testing and regulatory review of new products, patents protecting such products might expire before or shortly after such products are commercialized.
Obtaining and maintaining a patent portfolio entails significant expense and resources. Part of the expense includes periodic maintenance fees, renewal fees, annuity fees, various other governmental fees on patents and/or applications due in several stages over the lifetime of patents and/or applications, as well as the cost associated with complying with numerous procedural provisions during the patent application process. We may not choose to pursue or maintain protection for particular inventions. In addition, there are situations in which failure to make certain payments or noncompliance with certain requirements in the patent process can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. If we choose to forgo patent protection or allow a patent application or patent to lapse purposefully or inadvertently, our competitive position could suffer.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner. Our competitors may also seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend or assert our patents, or both, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or other agency with jurisdiction may find our patents invalid or unenforceable, or that our competitors are competing in a non-infringing manner. Thus, even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives.
Legal actions to enforce our patent rights can be expensive and may involve the diversion of significant management time. In addition, these legal actions could be unsuccessful and could also result in the invalidation of our patents or a finding that they are unenforceable. We may or may not choose to pursue litigation or other actions against those that have infringed or are currently infringing our patent rights, or used them without authorization, due to the associated expense and time commitment of monitoring these activities. If we fail to protect or to enforce our intellectual property rights successfully, our competitive position could suffer, which could harm our results of operations.
Even if we have or obtain patents covering our products or compositions, we may still be prevented from making, using, selling, offering for sale, or importing our products or technologies because of the patent rights of others. Others may have filed, and in the future may file, patent applications covering compositions or products that are similar or identical to ours. These filings could materially affect our ability to develop or sell our products. Because patent applications can take many years to issue and are not published for a period of time after filing, there may be currently pending applications unknown to us that may later result in issued patents that our products or compositions may infringe. These patent applications may have priority over patent applications filed by us.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful and issued patents covering our products could be found invalid or unenforceable if challenged in court.
If we initiate legal proceedings against a third party to enforce a patent covering one of our products or technologies, the defendant could counterclaim that the patent covering one of our products or technologies is invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and unenforceability of an asserted patent or patents are commonplace. Grounds for a validity challenge include alleged failures to meet any of several statutory requirements, including lack of novelty, obviousness, written description or non-enablement. Grounds for unenforceability assertions include allegations that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post- grant review and/or inter partes review and equivalent proceedings in foreign jurisdictions, such as, opposition proceedings. Such proceedings could result in revocation or amendment of our patents in such a way that they no longer cover our products or competitive products. Similarly, we may initiate proceedings before the Patent Trial and Appeal Board (the "PTAB") of the USPTO, such as post grant review ("PGR"), derivation, or inter partes review, against patents granted to third parties.
56
Even if we establish infringement, the court may decide not to grant an injunction against further infringing activity and instead award only monetary damages, which may or may not be an adequate remedy. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation in the United States, there is a risk that some of our confidential information could be compromised by disclosure during litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our ADSs or ordinary shares. Moreover, there can be no assurance that we will have sufficient financial or other resources to file and pursue such infringement claims in the federal courts, which typically last for years before they are concluded. Even if we ultimately prevail in such claims, the monetary cost of such litigation and the diversion of the attention of our management and scientific personnel could outweigh any benefit we receive as a result of the proceedings.
Diagnostic patents and patent applications involve highly complex legal and factual questions, which, if determined adversely to us, could negatively impact our patent position.
The patent positions of diagnostic companies can be highly uncertain and involve complex legal and factual questions. The interpretation and breadth of claims allowed in some patents covering our diagnostic products may be uncertain and difficult to determine, and are often affected materially by the facts and circumstances that pertain to the patented compositions and the related patent claims. The standards of the USPTO are evolving and could change in the future. Consequently, we cannot predict the issuance and scope of patents with certainty. Patents, if issued, may be challenged, invalidated or circumvented. U.S. patents and patent applications may also be subject to derivation or interference proceedings, and U.S. patents may be subject to reexamination proceedings, post-grant review and/or inter partes review in the USPTO. Foreign patents may be subject also to opposition or comparable proceedings in the corresponding foreign patent office, which could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such interference, reexamination, post-grant review, inter partes review and opposition proceedings may be costly. Accordingly, rights under any issued patents may not provide us with sufficient protection against competitive products or processes.
In addition, changes in or different interpretations of patent laws in the United States and foreign countries may permit others to use our discoveries or to develop and commercialize our technology and products without providing any compensation to us, or may limit the number of patents or claims we can obtain. The laws of some countries do not protect intellectual property rights to the same extent as U.S. laws and those countries may lack adequate rules and procedures for defending our intellectual property rights.
If we fail to obtain and maintain patent protection and trade secret protection for our products, we could lose our competitive advantage and competition we face would increase, reducing any potential revenues and adversely affecting our ability to attain or maintain profitability.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from developing or commercializing our products.
Our commercial success depends, in part, on our ability to develop, manufacture, market and sell our products and use our technologies without infringing the intellectual property and other proprietary rights of third parties. If any third-party patents or patent applications are found to cover our products or their methods of use, we may not be free to manufacture or market our products as planned without obtaining a license, which may not be available on commercially reasonable terms, or at all.
There is a substantial amount of intellectual property litigation in the biotechnology and pharmaceutical diagnostic industries, and we may become party to, or threatened with, litigation or other adversarial proceedings regarding intellectual property rights with respect to our products, including interference proceedings before the USPTO. Third parties may assert infringement claims against us based on existing or future intellectual property rights. The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The biotechnology and pharmaceutical diagnostic industries have produced a significant number of patents, and it may not always be clear to industry participants, including us, which patents cover various types of products or methods of use. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we were sued for patent infringement, we would need to demonstrate that our products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid or unenforceable, and we may not be able to do this. Proving invalidity is difficult. For example, in the United States, proving invalidity requires a showing of clear and convincing evidence to overcome the presumption of validity enjoyed by issued patents. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could significantly harm our business and operating results. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
If we are found to infringe a third party’s intellectual property rights, we could be forced, including by court order, to cease developing, manufacturing or commercializing the infringing product. Alternatively, we may be required to obtain a license from such third party in order to use the infringing technology and continue developing, manufacturing or marketing the infringing product.
57
However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, we could, in certain circumstances, be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our products or force us to cease some of our business operations, which could materially harm our business. Claims may also be made that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Developments in patent law in the United States and in other jurisdictions could have a negative impact on our business.
From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress, the USPTO or similar foreign authorities may change the standards of patentability and any such changes could have a negative impact on our business. In addition, the Leahy-Smith America Invents Act (the "America Invents Act"), which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process. In certain areas, these changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. The USPTO has developed new regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions, became effective on March 16, 2013. Substantive changes to patent law associated with the America Invents Act, or any subsequent U.S. legislation regarding patents, may affect our ability to obtain patents, and if obtained, to enforce or defend them.
Furthermore, recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances for diagnostic method claims and “gene patents” (see, two landmark Supreme Court cases, Mayo Collaborative v. Prometheus Laboratories (“Prometheus”), and Association for Molecular Pathology v. Myriad Genetics (“Myriad”)).
In view of the Supreme Court decisions in Prometheus, Myriad, and Alice Corp. Pty. Ltd. v. CLS Bank Int’l, as well as other federal appellate cases, we cannot guarantee that our efforts to seek patent protection for our tools and biomarkers will be successful.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to patent protection, because we operate in the highly technical field of molecular diagnostics, we rely in part on trade secret protection in order to protect our proprietary technology and processes. However, trade secrets are difficult to protect. We have entered into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors. These agreements generally require that the other party keep confidential and not disclose to third parties all confidential information developed by the party or made known to the party by us during the course of the party’s relationship with us. These agreements also generally provide that inventions conceived by the party in the course of rendering services to us will be our exclusively licensed property. However, these agreements may not be honored and may not effectively license intellectual property rights to us.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive, and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets. Trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
We will not seek to protect our intellectual property rights in all jurisdictions throughout the world and we may not be able to adequately enforce our intellectual property rights even in the jurisdictions where we seek protection.
Filing, prosecuting and defending patents on our products in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States and Europe could be less extensive than those in the United States and Europe, assuming that patent rights are obtained in the United States. Competitors may use our technologies in jurisdictions where we do not pursue and obtain patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States and Europe. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties from so competing.
58
In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as the federal and state laws in the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly in developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology or biopharmaceutical diagnostics. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties for certain products. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s samples, we may be limited in our ability to capitalize on the market potential of these inventions. In addition, we may face claims by third parties that our agreements with employees, contractors, or consultants obligating them to assign intellectual property to us are ineffective, or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such inventions. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property, or may lose our exclusive rights in that intellectual property. Either outcome could have an adverse impact on our business.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other biotechnology or diagnostics companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, and no such claims against us are currently pending, we may be subject to claims that we or our employees, consultants or independent contractors have used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
A dispute concerning the infringement or misappropriation of our proprietary rights or the proprietary rights of others could be time-consuming and costly, and an unfavorable outcome could harm our business.
There is significant litigation in the biopharmaceutical and diagnostics industry regarding patent and other intellectual property rights. While we are not currently subject to any pending intellectual property litigation, and are not aware of any such threatened litigation, we may be exposed to future litigation by third parties based on claims that our products, technologies or activities infringe the intellectual property rights of others. If our development activities are found to infringe any such patents, we may have to pay significant damages or seek licenses to such patents. A patentee could prevent us from using the patented diagnostic. We may need to resort to litigation to enforce a patent issued to us, to protect our trade secrets, or to determine the scope and validity of third-party proprietary rights. From time to time, we may hire scientific personnel or consultants formerly employed by other companies involved in one or more areas similar to the activities conducted by us. Either we or these individuals may be subject to allegations of trade secret misappropriation or other similar claims as a result of prior affiliations.
59
If we become involved in litigation, it could consume a substantial portion of our managerial and financial resources, regardless of whether we win or lose. We may not be able to afford the costs of litigation. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a negative impact on our cash position. Any legal action against us or our collaborators could lead to:
Any of these outcomes could hurt our cash position and financial condition and our ability to develop and commercialize our products.
If our trademarks and trade names are not adequately protected, we may not be able to build name recognition in our markets of interest.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we will need to build name recognition by potential partners or customers in our markets of interest. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively.
Risks Related to Ownership of Our ADSs and Ordinary Shares and Our Status as a U.S. Listed Company
The trading price of our ADSs and our ordinary shares may be volatile, and you could lose all or part of your investment.
The trading price of our ADSs and our ordinary shares has fluctuated, and is likely to continue to fluctuate, substantially in response to various factors, some of which are beyond our control, including limited trading volume. The stock market in general, and the market for diagnostics companies in particular, have experienced extreme volatility that has often been unrelated to the operating performance of these companies. As a result of this volatility, investors may not be able to sell their ADSs or ordinary shares at or above the price paid for the ADSs or ordinary shares, respectively. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this annual report, these factors include:
60
These and other market and industry factors may cause the market price and demand for our ADSs and ordinary shares to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from selling their ADSs or ordinary shares at or above the price paid for the ADSs or ordinary shares, respectively, and may otherwise negatively affect the liquidity of our ADSs and our ordinary shares.
Some companies that have experienced volatility in the trading price of their shares have been the subject of securities class action litigation. Any lawsuit to which we are a party, with or without merit, may result in an unfavorable judgment. We also may decide to settle lawsuits on unfavorable terms.
Any such negative outcome could result in payments of substantial damages or fines, damage to our reputation or adverse changes to our business practices. Defending against litigation is costly and time-consuming, and could divert our management’s attention and our resources. Furthermore, during the course of litigation, there could be negative public announcements of the results of hearings, motions or other interim proceedings or developments, which could have a negative effect on the market price of our ADSs and our ordinary shares.
The sale of a substantial number of our total outstanding ADSs or ordinary shares could cause the market price of our ADSs and ordinary shares to drop significantly, even if our business is doing well.
Sales of a substantial number of our ordinary shares or ADSs in the public market could occur at any time. If our shareholders sell, or the market perceives that our shareholders intend to sell, substantial amounts of our ordinary shares or ADSs in the public market, the market price of our ADSs and ordinary shares could decline significantly.
We had 154,368,191 ordinary shares outstanding as of June 30, 2024. Sales of a substantial number of such ADSs or ordinary shares or the perception that such sales may occur could cause the market price of our ADSs and/or ordinary shares to fall or make it more difficult for purchasers of ADSs to sell their ADSs at a time and price that they deem appropriate.
We have announced the delisting of our ADSs from trading on the Nasdaq Capital Market, which could limit investors' ability to make transactions in our ADSs and subject us to additional trading restrictions.
Currently, our ADSs are publicly traded on the Nasdaq Capital Market (“Nasdaq”). However, we intend to delist our ADSs from Nasdaq effective on or about October 7, 2024, and intend for our ADSs to become quoted on the OTC Markets’ OTCQX tier effective on or about October 8, 2024. No assurance can be given that the trading prices of our ADSs on the OTCQX will be indicative of the prices if our ADSs were traded on Nasdaq.
On December 22, 2023, we received two written notices from the Listing Qualifications Department of Nasdaq notifying us that (i) because the closing bid price for the ADSs was below $1.00 per ADS for at least 30 consecutive business days, we did not meet the $1.00 per ADS minimum bid price requirement (the “Minimum Bid Price Requirement”) and (ii) we were not in compliance with the requirement to maintain a minimum market value of listed securities (the “MVLS”) of $50,000,000 for continued listing on The Nasdaq Global Market (the “MVLS Requirement”). On June 21, 2024, at the end of the 180 day period to regain compliance with the Minimum Bid Price Requirement and the MVLS Requirement, we received written notice from Nasdaq notifying us that the Nasdaq staff determined that we did not regain compliance within the compliance period. As a result, we engaged in an appeal of the delisting determination before a Nasdaq Hearings Panel (the "Panel"), during which we presented a strategic plan to regain compliance with the applicable Nasdaq listing requirements. On August 23, 2024, we received a written notice (the “Notice”) from the Panel informing us that we now have until October 25, 2024 (the “Second Compliance Period”) to regain compliance with the Minimum Bid Price Requirement and other applicable Nasdaq listing requirements. In addition, as set forth in the Notice, we and Nasdaq determined that we are better suited to have our securities traded on the Nasdaq Capital Market; accordingly, trading of our ADSs was transferred to the Nasdaq Capital Market effective August 27, 2024. As a result of this transfer, and in lieu of satisfaction of the MVLS Requirement, (i) we must demonstrate compliance with the $1.00 per ADS minimum bid price requirement, (ii) we must achieve and demonstrate long-term compliance with the $2,500,000 minimum stockholders’ equity requirement set forth in Nasdaq Listing Rule 5550(b)(1), and (iii) we must provide the Panel with income projections for the next 12 months. There can be no assurance that the Company will achieve compliance during the Second Compliance Period.
61
If the Company's ADSs are delisted, whether as contemplated in the paragraph above or as a result of our failure to achieve compliance by the end of the Second Compliance Period, it could be more difficult to buy or sell the Company's ADSs or to obtain accurate quotations, and the price of the Company's ADSs could suffer a material decline. Delisting could also impair the Company's ability to raise capital.
As of the date of this Annual Report on Form 10-K, the Company's ADSs are listed on Nasdaq. As a result of the contemplated actions discussed above, we may face significant material adverse consequences, including:
The dual listing of ordinary shares and ADSs is costly to maintain and may adversely affect the liquidity and value of our ordinary shares and ADSs.
Our ordinary shares trade on AIM and, as previously disclosed, we intend to delist our ADSs from Nasdaq effective on or about October 7, 2024, and intend for our ADSs to become quoted on the OTC Markets’ OTCQX tier effective on or about October 8, 2024. Trading of our ordinary shares on AIM and our ADSs on Nasdaq, as of the date hereof, and OTCQX, in the future, will likely continue to generate additional costs, including increased legal, accounting, investor relations and other expenses, in addition to the costs associated with the additional reporting requirements described elsewhere in this annual report. We cannot predict the effect of this dual listing on the value of our ADSs and our ordinary shares. However, the dual listing of ADSs and ordinary shares may dilute the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for our ADSs. The price of our ADSs could also be adversely affected by trading in ordinary shares on AIM.
Our ADSs may be traded infrequently and in low volumes, so you may be unable to sell your ADSs at or near the quoted bid prices if you need to sell.
Unless our ADSs are listed on a national securities exchange, they will only be traded on OTCQX. In that market, however, the ADSs may trade infrequently and in low volumes, meaning that the number of persons interested in purchasing our ADSs at or near bid prices at any given time may be relatively small or non-existent. An investor may find it difficult to obtain accurate quotations as to the market value of our ADSs or to sell his or her ADSs at or near bid prices or at all. In addition, if we fail to meet the criteria set forth in SEC reporting regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our ADSs, which may further affect the liquidity of the ADSs. This would also make it more difficult for us to raise capital.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our ADSs and ordinary shares less attractive to investors.
We are an “emerging growth company” as defined in the SEC’s rules and regulations and we will remain an emerging growth company until the earlier to occur of (a) June 30, 2026, (b) the last day of the fiscal year (1) in which we have total annual gross revenues of at least $1.235 billion or (2) in which we are deemed to be a “large accelerated filer” under the rules of the SEC, which means the market value of our ordinary shares and ADSs that are held by non-affiliates exceeds $700.0 million as of the prior December 31, or (c) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
62
We may choose to take advantage of some, but not all, of the available exemptions. We have taken advantage of reduced reporting burdens in this annual report. In particular, we have not included all of the executive compensation information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our ADSs less attractive if we rely on certain or all of these exemptions. If some investors find our ADSs less attractive as a result, there may be a less active trading market for our ADSs and our ADS price may be more volatile.
In addition, the JOBS Act provides that an emerging growth company may take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and, as a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” if the market value of our ordinary shares and ADSs held by non-affiliates is below $250 million (or $700 million if our annual revenue is less than $100 million) as of December 31 in any given year, which would allow us to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in our periodic reports and, when required, our proxy statements.
We will continue to incur significant increased costs as a result of operating as a company that is both publicly traded in the United States and admitted to trading on AIM in the United Kingdom, and our executive officers and other personnel will continue to be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public reporting company trading in the United States, and particularly after we no longer qualify as an emerging growth company, we have begun to, and will continue to, incur significant legal, accounting and other expenses that we did not incur previously. The Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the requirements of OTCQX and other applicable securities rules and regulations impose various requirements on non-U.S. reporting public companies, including the establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our executive officers and other personnel must devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified senior management personnel or members for our Board of Directors.
In addition, these rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
To prepare for compliance with Section 404, once we no longer qualify as an emerging growth company, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404. We identified a material weakness in our internal controls over financial reporting. If we are unable to develop and maintain an effective system of internal controls and procedures required by Section 404(a) of the Sarbanes-Oxley Act of 2002 (the "Sarbanes-Oxley Act"), we may not be able to accurately report our financial results in a timely manner. If we fail to fully remediate any of our past and current material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Further, having ADSs trading in the U.S. and being an English public company with ordinary shares admitted to trading on AIM, impacts the disclosure of information and requires compliance with two sets of applicable rules. From time to time, this may result in uncertainty regarding compliance matters and result in higher costs necessitated by legal analysis of dual legal regimes, ongoing revisions to disclosure and adherence to heightened governance practices. As a result of the enhanced disclosure requirements of the U.S. securities laws, business and financial information that we report is broadly disseminated and highly visible to investors, which we believe may increase the likelihood of threatened or actual litigation, including by competitors and other third parties, which could, even if unsuccessful, divert financial resources and the attention of our management from our operations.
63
Securities traded on AIM may carry a higher risk than securities traded on other exchanges, which may impact the value of your investment.
Our ordinary shares are currently traded on AIM. Investment in equities traded on AIM is sometimes perceived to carry a higher risk than an investment in equities quoted on exchanges with more stringent listing requirements, such as the main market of the London Stock Exchange, New York Stock Exchange or Nasdaq. This is because AIM is less heavily regulated, imposes less stringent corporate governance and ongoing reporting requirements than those other exchanges. In addition, AIM requires only half-yearly, rather than quarterly, financial reporting. You should be aware that the value of our ordinary shares may be influenced by many factors, some of which may be specific to us and some of which may affect AIM companies generally, including the depth and liquidity of the market, our performance, a large or small volume of trading in our ordinary shares, legislative changes and general economic, political or regulatory conditions, and that the prices may be volatile and subject to extensive fluctuations. Therefore, the market price of our ordinary shares, the ADSs, or the ordinary shares underlying the ADSs, may not reflect the underlying value of our company.
Fluctuations in the exchange rate between the U.S. dollar and the pound sterling may increase the risk of holding ADSs and ordinary shares.
The share price of our ordinary shares is quoted on AIM in pounds sterling, while our ADSs trade on OTCQX in U.S. dollars. Fluctuations in the exchange rate between the U.S. dollar and the pound sterling may result in differences between the value of our ADSs and the value of our ordinary shares, which may result in heavy trading by investors seeking to exploit such differences. In addition, as a result of fluctuations in the exchange rate between the U.S. dollar and the pound sterling, the U.S. dollar equivalent of the proceeds that a holder of the ADSs would receive upon the sale in the United Kingdom of any ordinary shares withdrawn from the depositary, and the U.S. dollar equivalent of any cash dividends paid in pounds sterling on ordinary shares represented by the ADSs, could also decline.
If equity research analysts do not publish research or reports, or publish unfavorable research or reports, about us, our business or our market, the price and trading volume of our ADSs and ordinary shares could decline.
The trading market for our ADSs and ordinary shares is influenced in part by the research and reports that equity research analysts publish about us and our business. If no or few equity research analysts cover our company, the trading price for our ADSs and ordinary shares would be negatively impacted. We do not have any control over the analysts or the content and opinions included in their reports. The price of our ADSs and ordinary shares could decline if one or more equity research analysts downgrade our ADSs or ordinary shares or issue other unfavorable commentary or research about us. If one or more equity research analysts ceases coverage of us or fails to publish reports on us regularly, demand for our ADSs and ordinary shares could decrease, which in turn could cause the trading price or trading volume of our ADSs and ordinary shares to decline.
We have broad discretion in the use of proceeds from our global offering and private placements and may invest or spend the proceeds in ways with which you do not agree and in ways that may not increase the value of your investment.
Our management has broad discretion in the application of our cash including the net proceeds from the global offering we completed in July 2020 and the private placements of securities we completed in April 2022, February 2023, March 2024, and April 2024, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our ADSs or ordinary shares. The failure by our management to apply these funds effectively could result in financial losses that could have a negative impact on our business, cause the price of our ADSs or ordinary shares to decline and delay the development and commercialization of our products. Pending their use, we may invest our cash, in a manner that does not produce income or that loses value.
Raising additional capital may cause dilution to holders of our ADSs or ordinary shares or may restrict our operations.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting verification studies, commercialization efforts, expanded research and development activities and costs associated with operating a public company. For example, on September 30, 2024, we announced our intention to raise approximately £11 million gross proceeds through a placing, a subscription and a retail offer of new ordinary shares of the Company at an issue price of £0.09 per new ordinary share to new and existing institutional and other investors. Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through any or a combination of securities offerings, debt financings, collaborations, agreements, strategic alliances and marketing, distribution or licensing arrangements with third parties. If we raise capital through securities offerings, such sales may also result in material dilution to our existing shareholders, and new investors could gain rights, preferences and privileges senior to the holders of our ADSs or ordinary shares.
64
To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of ADSs or ordinary shares. Debt financing and preferred equity financing, if available, could result in fixed payment obligations, and we may be required to accept terms that restrict our ability to incur additional indebtedness, force us to maintain specified liquidity or other ratios or restrict our ability to pay dividends or make acquisitions.
Raising additional capital through any of these or other means could adversely affect our business and the holdings or rights of our security holders, and may cause the market price of our ADSs or ordinary shares to decline.
Holders of our ADSs have fewer rights than our shareholders and must act through the depositary to exercise their rights.
Holders of our ADSs do not have the same rights as shareholders who hold our ordinary shares directly and may only exercise their voting rights with respect to the underlying ordinary shares in accordance with the provisions of the deposit agreement. Holders of the ADSs will appoint the depositary or its nominee as their representative to exercise the voting rights attaching to the ordinary shares represented by the ADSs. When a general meeting is convened, if you hold ADSs, you may not receive sufficient notice of a shareholders’ meeting to permit you to withdraw the ordinary shares underlying your ADSs to allow you to vote with respect to any specific matter. We will make all commercially reasonable efforts to cause the depositary to extend voting rights to holders of ADSs in a timely manner, but we cannot assure purchasers of ADSs that they will receive voting materials in time to instruct the depositary to vote, and it is possible that they, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote. Furthermore, the depositary will not be liable for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, purchasers of ADSs may not be able to exercise their right to vote and they may lack recourse if their ADSs are not voted as they request. In addition, in their capacity as ADS holders, they will not be able to call a shareholders’ meeting.
The depositary for our ADSs is entitled to charge holders fees for various services, including annual service fees.
The depositary for our ADSs is entitled to charge holders fees for various services including for the issuance of ADSs upon deposit of ordinary shares, cancellation of ADSs, distributions of cash dividends or other cash distributions, distributions of ADSs pursuant to share dividends or other free share distributions, distributions of securities other than ADSs and annual service fees. In the case of ADSs issued by the depositary into The Depository Trust Company ("DTC"), the fees will be charged by the DTC participant to the account of the applicable beneficial owner in accordance with the procedures and practices of the DTC participant as in effect at the time. The depositary for our ADSs will not generally be responsible for any United Kingdom stamp duty or stamp duty reserve tax arising upon the issuance or transfer of ADSs.
Purchasers of ADSs may be subject to limitations on the transfer of ADSs and the withdrawal of the underlying ordinary shares.
ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when the depositary determines such action is necessary or advisable pursuant to the deposit agreement. The depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary thinks it is necessary or advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason, subject to certain rights to cancel ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting, or because we are paying a dividend on our ordinary shares or similar corporate actions.
In addition, purchasers of ADSs may not be able to cancel their ADSs and withdraw the underlying ordinary shares when they owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to the ADSs or to the withdrawal of our ordinary shares or other deposited securities.
65
ADS holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable results to the plaintiff(s) in any such action.
The deposit agreement governing our ADSs provides that owners and holders of ADSs irrevocably waive the right to a trial by jury in any legal proceeding arising out of or relating to the deposit agreement or the ADSs, including claims under U.S. federal securities laws, against us or the depositary to the fullest extent permitted by applicable law. If this jury trial waiver provision is prohibited by applicable law, an action could nevertheless proceed under the terms of the deposit agreement with a jury trial. Although we are not aware of a specific federal decision that addresses the enforceability of a jury trial waiver in the context of U.S. federal securities laws, it is our understanding that jury trial waivers are generally enforceable. Moreover, insofar as the deposit agreement is governed by the laws of the State of New York, New York laws similarly recognize the validity of jury trial waivers in appropriate circumstances. In determining whether to enforce a jury trial waiver provision, New York courts and federal courts will consider whether the visibility of the jury trial waiver provision within the agreement is sufficiently prominent such that a party has knowingly waived any right to trial by jury. We believe that this is the case with respect to the deposit agreement and the ADSs.
In addition, New York courts will not enforce a jury trial waiver provision in order to bar a viable setoff or counterclaim of fraud or one which is based upon a creditor’s negligence in failing to liquidate collateral upon a guarantor’s demand, or in the case of an intentional tort claim (as opposed to a contract dispute). No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with any provision of U.S. federal securities laws and the rules and regulations promulgated thereunder.
If any owner or holder of our ADSs brings a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under U.S. federal securities laws, such owner or holder may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us or the depositary. If a lawsuit is brought against us or the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different results than a trial by jury would have had, including results that could be less favorable to the plaintiff(s) in any such action, depending on, among other things, the nature of the claims, the judge or justice hearing such claims, and the venue of the hearing.
Concentration of ownership of our ordinary shares (including ordinary shares represented by ADSs) among our executive officers, directors and principal shareholders may prevent new investors from influencing significant corporate decisions and matters submitted to shareholders for approval.
Our executive officers, directors and current beneficial owners of 5% or more of our ordinary shares and their respective affiliates, in the aggregate, beneficially owned approximately 47.6% of our outstanding ordinary shares, based on the number of ordinary shares outstanding as of June 30, 2024. As a result, depending on the level of attendance at our general meetings of shareholders, these persons, acting together, would be able to significantly influence all matters requiring approval by our shareholders, including the election, re-election and removal of directors, any merger, scheme of arrangement, or sale of all or substantially all of our assets, or other significant corporate transactions, and amendments to our articles of association. In addition, these persons, acting together, may have the ability to control the management and affairs of our company. Accordingly, this concentration of ownership may harm the market price of our ADSs and ordinary shares by:
In addition, some of these persons or entities may have interests different than yours. For example, because some of these shareholders may have purchased their shares at prices substantially below the price at which you purchased your shares and may have held their shares for a longer period, they may be more interested in selling our company to an acquirer than other investors, or they may want us to pursue strategies that deviate from the interests of other shareholders.
66
Because we do not anticipate paying any cash dividends on ordinary shares (including ordinary shares represented by ADSs) in the foreseeable future, capital appreciation, if any, will be your sole source of gains and you may never receive a return on your investment.
You should not rely on an investment in our ADSs or ordinary shares to provide dividend income. Under current English law, a company’s accumulated realized profits must exceed its accumulated realized losses (on a non-consolidated basis) before dividends can be paid. Therefore, we must have distributable profits before issuing a dividend. We have never declared or paid a dividend on our ordinary shares in the past, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, capital appreciation, if any, on our ADSs or ordinary shares will be your sole source of gains for the foreseeable future.
Purchasers of ADSs may not receive distributions on our ordinary shares represented by the ADSs or any value for them if it is illegal or impractical to make them available to holders of ADSs.
Although we do not have any present plans to declare or pay any dividends, in the event we declare and pay any dividend, the depositary for the ADSs has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. Purchasers of ADSs will receive these distributions in proportion to the number of our ordinary shares their ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to register under U.S. securities laws any offering of ADSs, ordinary shares or other securities received through such distributions. We also have no obligation to take any other action to permit distribution on the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that purchasers of ADSs may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to them. These restrictions may have an adverse effect on the value of your ADSs.
Your right to participate in any future rights offerings may be limited, which may cause dilution to your holdings.
Under English law, shareholders usually have preemptive rights to subscribe on a pro rata basis in the issuance of new shares for cash. The exercise of preemptive rights by certain shareholders not resident in the United Kingdom may be restricted by applicable law or practice in the United Kingdom and overseas jurisdictions. We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to shareholders in the United States unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Also, under the deposit agreement, the depositary bank will not make rights available to ADS holders unless either both the rights and any related securities are registered under the Securities Act, or the distribution of them to ADS holders is exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. If the depositary does not distribute the rights, it may, under the deposit agreement, either sell them, if possible, or allow them to lapse. Accordingly, ADS holders may be unable to participate in our rights offerings and may experience dilution in their holdings. We are also permitted under English law to disapply preemptive rights (subject to the approval of our shareholders by special resolution or the inclusion in our articles of association of a power to disapply such rights) and thereby exclude certain shareholders, such as overseas shareholders, from participating in a rights offering (usually to avoid a breach of local securities laws).
If we are a passive foreign investment company, now or in the future, there could be adverse U.S. federal income tax consequences to U.S. Holders.
Under the Code, we will be a passive foreign investment company, ("PFIC"), for any taxable year in which (1) 75% or more of our gross income consists of passive income or (2) 50% or more of the average quarterly value of our assets consists of assets that produce, or are held for the production of, passive income. For purposes of these tests, passive income generally includes dividends, interest, gains from the sale or exchange of investment property and certain rents and royalties. In addition, for purposes of the above calculations, a non-U.S. corporation that directly or indirectly owns at least 25% by value of the shares of another non-U.S. corporation is treated as if it held its proportionate share of the assets and received directly its proportionate share of the income of such other non-U.S. corporation. If we are a PFIC for any taxable year during which a U.S. Holder holds our ADSs, the U.S. Holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under U.S. federal income tax laws and regulations.
67
We have not yet completed a final analysis to determine whether we were a PFIC for our taxable year ended June 30, 2024, but we currently believe based on the information available that we were a PFIC for our taxable year ended June 30, 2024. U.S. Holders should consult with their tax advisors regarding the implications of owning stock in a PFIC. The determination of whether we are a PFIC is a fact-intensive determination made on an annual basis and the applicable law is subject to varying interpretation. In particular, the characterization of our assets as active or passive may depend in part on our current and intended future business plans, which are subject to change. In addition, for our current and future taxable years, the total value of our assets for PFIC testing purposes may be determined in part by reference to the market price of our ordinary shares or ADSs from time to time, which may fluctuate considerably. Under the income test, our status as a PFIC depends on the composition of our income which will depend on the transactions we enter into in the future and our corporate structure. The composition of our income and assets is also affected by how, and how quickly, we spend the cash we raise in any offering. We cannot provide any assurances regarding our PFIC status. Because of the uncertainties involved in establishing our PFIC status, our U.S. tax counsel expresses no opinion regarding our PFIC status.
If we are a PFIC, U.S. holders of our ADSs would be subject to adverse U.S. federal income tax consequences, such as ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements under U.S. federal income tax laws and regulations.
Each U.S. Holder is strongly urged to consult its tax advisor regarding these issues.
A “U.S. Holder” is a holder of our common stock who, for U.S. federal income tax purposes: is an individual who is a citizen or resident of the United States; a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; an estate the income of which is subject to U.S. federal income taxation regardless of its source; or a trust (a) that is subject to the primary supervision of a court within the United States and the control of one or more United States persons as described in Section 7701(a)(30) of the Code, or (b) that has a valid election in effect under applicable Treasury regulations to be treated as a United States person.
If a United States person is treated as owning at least 10% of our ordinary shares, such United States person may be subject to adverse U.S. federal income tax consequences.
For U.S. federal income tax purposes, if a United States person is treated as owning (directly, indirectly or constructively) 10% or more of our stock by vote or value, such United States person will be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group (if any). Because our group includes at least one U.S. subsidiary, any non-U.S. subsidiaries we were to form or acquire in the future will be treated as controlled foreign corporations.
A United States shareholder of a controlled foreign corporation will be required to annually report and include in its U.S. federal taxable income its pro rata share (if any) of “subpart F income,” “global intangible low-taxed income” and investments in U.S. property by the controlled foreign corporation, regardless of whether such corporation makes any distributions of such income. Special rules, however, apply to United States persons that are partnerships or other pass-through entities for U.S. federal income tax purposes. Certain deductions and credits for foreign income taxes paid or accrued by the controlled foreign corporation may be claimed by a corporate United States shareholder, but may not be claimed by an individual United States shareholder.
We cannot provide any assurance that we will furnish to any United States shareholder the information required to comply with the reporting and tax-paying obligations discussed applicable to a United States shareholder in respect of controlled foreign corporations. Failure to comply with such reporting obligations may subject a holder of our ordinary shares that is a United States shareholder to significant monetary penalties and may prevent the statute of limitations with respect to its U.S. federal income tax return for the year for which reporting was due from starting. Holders of our ordinary shares that are United States persons should consult their tax advisors regarding the potential application of these rules to their investment in our ordinary shares.
Risks Related to Investing in a U.K. Company
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
We are incorporated under English law. The rights of holders of ordinary shares and, therefore, certain of the rights of holders of our ADSs, are governed by English law, including the provisions of the U.K. Companies Act 2006 (the "Companies Act"), and by our articles of association. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations.
68
Protections found in provisions under the U.K. City Code on Takeovers and Mergers may delay or discourage a takeover attempt, including attempts that may be beneficial to holders of our ADSs and ordinary shares.
The U.K. City Code on Takeovers and Mergers (the "Takeover Code") applies, among other things, to an offer for a public company whose registered office is in the United Kingdom and whose securities are admitted to trading on a multilateral trading facility in the United Kingdom, which includes AIM. We are therefore currently subject to the Takeover Code.
The Takeover Code provides a framework within which takeovers of certain companies organized in the United Kingdom are regulated and conducted. The following is a brief summary of some of the most important rules of the Takeover Code:
69
As an English public company, certain capital structure decisions will require shareholder approval, which may limit our flexibility to manage our capital structure.
English law provides that a Board of Directors may only allot shares (or grant rights to subscribe for, or to convert any security into, shares) with the prior authorization of shareholders by ordinary resolution, being a resolution passed by a simple majority of votes cast, such authorization stating the aggregate nominal amount of shares that it covers and being valid for a maximum period of five years, each as specified in the articles of association or relevant shareholder resolution. In either case, this authorization would need to be renewed by our shareholders upon expiration (i.e., at least every five years). Typically, English public companies renew the authorization of their directors to allot shares on an annual basis at their annual general meeting. We have obtained authority from our shareholders to allot additional shares up to an aggregate nominal amount of 77,369.72 from June 8, 2023 (being the date of our general meeting) until the conclusion of our next annual general meeting or the close of business on September 8, 2024, whichever is earlier, which authorization will need to be renewed or replaced upon expiration (other than in the case of a preemptive offering).
English law also generally provides shareholders with preemptive rights when new shares are issued for cash. However, it is possible for the articles of association, or for shareholders to pass a special resolution at a general meeting, being a resolution passed by at least 75% of the votes cast, to disapply preemptive rights. Such a disapplication of preemptive rights may be for a maximum period of up to five years from the date of adoption of the articles of association, if the disapplication is contained in the articles of association, or from the date of the shareholder special resolution, if the disapplication is by shareholder special resolution, but not longer than the duration of the authority to allot shares to which the disapplication relates. In either case, this disapplication would need to be renewed by our shareholders upon its expiration (i.e., at least every five years). Typically, English public companies renew the disapplication of preemptive rights on an annual basis at their annual general meeting. We have obtained authority from our shareholders to disapply preemptive rights in respect of shares allotted under the authorization described in the paragraph above up to an aggregate nominal amount of £46,890.74 from June 8, 2023 (being the date of our general meeting) until the conclusion of our next annual general meeting, or the close of business on September 8, 2024, whichever is earlier, which disapplication will need to be renewed or replaced upon expiration.
English law also generally prohibits a public company from repurchasing its own shares without the prior approval of shareholders by ordinary resolution, being a resolution passed by a simple majority of votes cast, and other formalities. Such approval may be for a maximum period of up to five years.
Claims of U.S. civil liabilities may not be enforceable against us.
We are incorporated under English law. A substantial amount of our assets is located outside the United States. In addition, some of our executive officers and directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce judgments obtained in U.S. courts against them or us, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws.
The United States and the United Kingdom do not currently have a treaty providing for the reciprocal recognition and enforcement of judgments (other than arbitration awards) in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in England and Wales. In addition, uncertainty exists as to whether the English and Welsh courts would entertain original actions brought in England and Wales against us or our directors or executive officers predicated upon the securities laws of the United States or any state in the United States. Any final and conclusive monetary judgment for a definite sum obtained against us in U.S. courts would be treated by the courts of England and Wales as a cause of action in itself and sued upon as a debt so that no retrial of the issues would be necessary, provided that certain requirements are met consistent with English law and public policy. Whether these requirements are met in respect of a judgment based upon the civil liability provisions of the U.S. securities laws is an issue for the English court making such decision. If an English court gives judgment for the sum payable under a U.S. judgment, the English judgment will be enforceable by methods generally available for this purpose.
As a result, U.S. investors may not be able to enforce against us or our executive officers, Board of Directors or certain experts named herein who are residents of the United Kingdom or countries other than the United States any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws.
Our articles of association provide that the U.S. federal district courts are the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act.
Our articles of association provide that, unless we consent in writing to the selection of an alternative forum in the United States of America, the U.S. federal district courts are the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. There is uncertainty as to whether a court would enforce such provision, and the enforceability of similar choice of forum provisions in other companies’ constitutive documents has been challenged in legal proceedings.
70
If a court were to find the choice of forum provision contained in our articles of association to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our results of operations and financial condition.
This choice of forum provision may limit a shareholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits.
If we qualify as a foreign private issuer, we will be exempt from a number of rules under the U.S. securities laws and will be permitted to file less information with the SEC than a U.S. domestic public company, which may limit the information available to our shareholders.
We may qualify as a foreign private issuer, as such term is defined in Rule 405 under the Securities Act. As a foreign private issuer, we will not be subject to all of the disclosure requirements applicable to public companies organized within the United States. For example, we will be exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. As long as we are a foreign private issuer, we will not be required to obtain shareholder approval for certain dilutive events, such as the establishment or material amendment of certain equity-based compensation plans, we will not be required to provide detailed executive compensation disclosure in our periodic reports, and we will be exempt from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. In addition, our officers and directors will be exempt from the reporting and "short-swing" profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities.
If we qualify as a foreign private issuer, we intend to submit semi-annual interim consolidated financial data to the SEC under cover of the SEC's Form 6-K, as we will not be required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. domestic public companies, and will not be required to file quarterly reports on Form 10-Q or current reports on Form 8-K under the Exchange Act.
Also, as a foreign private issuer, we will be permitted to follow home country practice in lieu of certain Nasdaq or OTC corporate governance rules, including those that permit a lower quorum requirement and require listed companies to have a majority of independent directors (although all of the members of the audit committee must be independent under the Exchange Act) and independent director oversight of executive compensation, nomination of directors and corporate governance matters; have regularly scheduled executive sessions with only independent directors; and adopt and disclose a code of ethics for directors, officers and employee. Accordingly, our shareholders may not have the same protections afforded to shareholders of listed companies that are subject to all of the applicable corporate governance requirements.
The withdrawal of the United Kingdom from the EU may result in our having to obtain relevant regulatory clearances for our products for the United Kingdom and the rest of Europe separately.
We are not actively pursuing regulatory clearance and commercialization of our products outside of the United States at this time. Prior to the withdrawal of the United Kingdom from the EU, the United Kingdom benefited from, and we expected to be able to benefit from, the harmonization of certain regulatory requirements within the EU. Such regulatory requirements are no longer harmonized between the EU and Great Britain. As a result, any future efforts to market our products in both Great Britain and the EU may require us to complete separate regulatory processes, which may increase the time and cost associated with gaining relevant regulatory approvals in such markets depending on the arrangements in place between the United Kingdom and the EU at the relevant time.
Exchange rate fluctuations may adversely affect our results of operations and financial condition.
Owing to the international scope of our operations, fluctuations in exchange rates, particularly between the pound sterling and the U.S. dollar, may adversely affect us. Since the Brexit referendum in 2016, there has been a significant increase in the volatility of the exchange rate between the pound sterling and the U.S. dollar and an overall weakening of the pound sterling. Our business and the price of our ADSs may be affected by fluctuations in foreign exchange rates not only between the pound sterling and the U.S. dollar, but also the currencies of other countries, which may have a significant impact on our results of operations and cash flows from period to period. Currently, we do not have any exchange rate hedging arrangements in place.
71
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 1C. Cybersecurity
We rely on complex information technology systems and various software applications to operate our business. We have developed a comprehensive cybersecurity program as part of our ISO 27001 2022 certification designed to protect our systems and the confidentiality, integrity and availability of our data.
We have implemented processes that are intended to assess, identify, manage and reduce cybersecurity risks. We maintain a global incident response plan and disaster recovery management plan, each designed to protect against, identify, evaluate, respond to and recover from an incident. These plans anticipate an array of potential scenarios and provide for the assembly of a cybersecurity incident response team in the event of a cyber incident. The incident response team is a cross-functional group that may be composed of both company personnel and external service providers, and which is tailored to a particular incident so that individuals with appropriate experience and expertise are available.
We regularly conduct exercises to help ensure the plans’ effectiveness and our overall preparedness. We also have invested in tools and technologies to protect our and our patients', customers' and business partners' data and information technology, and we regularly monitor our information technology systems and infrastructure to identify and assess cybersecurity risks.
Identified issues are logged within the organization’s ticketing system, or managed by the change management policy. Automated monitoring and reporting mechanisms are in place wherever possible and appropriate. Vulnerability and penetration testing is performed by appropriately qualified internal personnel or hired specialist, in managed by the Supplier & Vendor Management Policy.
We rely in part on third parties (including assessors, consultants, advisors and others) in connection with our processes for assessing, identifying, managing and reducing cyber risks. In addition, we have implemented a cybersecurity awareness program designed to educate and train our entire employee network on how to identify and report cybersecurity threats.
We also provide specialized training for employees in specialized information technology roles. We take measures to regularly update and improve our cybersecurity program, including conducting independent program assessments, penetration testing and scanning of our systems for vulnerabilities.
We are certified to ISO27001, 2022 and have satisfied the requirements for Information security, cybersecurity and privacy protection — Information security management systems (ISMS).
Oversight of the ISMS is provided through the ISMS Board chaired by a qualified Chief Information Security Officer (CISO). With representation of the executive management team and board through the Chief Technology Officer, the ISMS collectively makes the primary strategic decision around information security and issues that may arise. All identified risks or incidents are addressed and evaluated by an Information Security Incident Response Team (ISIRT) who are responsible for notifying executive management, board and other internal or external stakeholders as deemed necessary.
With respect to third-party service providers, our information security program includes conducting due diligence of relevant service providers’ information security programs prior to onboarding.
Item 2. Properties.
We lease laboratory and office space in New York City, New York on short-term leases that automatically renew. The laboratory is use for KidneyIntelX testing and the office is used for corporate. Combined, our laboratory and office space in New York are approximately 1,000 square feet.
Item 3. Legal Proceedings.
From time to time, we may become involved in legal proceedings arising in the ordinary course of our business. We are not currently subject to any material legal proceedings.
Item 4. Mine Safety Disclosures.
Not applicable.
72
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our ADSs have been listed on the Nasdaq Global Market under the symbol “RNLX” since July 17, 2020. However, we intend to delist our ADSs from Nasdaq effective on or about October 7, 2024 and intend for our ADSs to become quoted on the OTC Markets’ OTCQX tier effective on or about October 8, 2024. Our ordinary shares have been listed on AIM, a market operated by the London Stock Exchange, under the symbol, “RENX,” since November 6, 2018.
Holders of Common Equity
As of September 24, 2024, there were approximately 690 holders of record of our ordinary shares and 79 holders of record of our ADSs. Because our ADSs are held by depositaries, brokers and other nominees, the number of beneficial holders of our shares is substantially larger than the number of stockholders of record.
Dividend Distribution Policy
Since our incorporation, we have not declared or paid any dividends on our issued share capital. We intend to retain any earnings for use in our business and do not currently intend to pay dividends on our ordinary shares. The declaration and payment of any future dividends will be at the discretion of our Board of Directors and will depend upon our results of operations, cash requirements, financial condition, contractual restrictions, any future debt agreements or applicable laws and other factors that our Board of Directors may deem relevant.
Under the laws of England and Wales, among other things, we may only pay dividends if we have sufficient distributable reserves (on a non-consolidated basis), which are our accumulated realized profits that have not been previously distributed or capitalized less our accumulated realized losses, so far as such losses have not been previously written off in a reduction or reorganization of capital.
On May 15, 2020, our shareholders approved at a general meeting the reduction of our share capital by the cancellation of our share premium account in its entirety in order to create realized profits, which was confirmed by the High Court in England and Wales on June 9, 2020. This was necessary to increase our distributable reserves to allow us to implement the distribution in specie for the FractalDx spin-off, which distribution was declared by our Board of Directors on July 7, 2020 and distributed on July 10, 2020.
Item 6. [Reserved]
73
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following management’s discussion and analysis of financial condition and results of operations describes the principal factors affecting our results of operations, liquidity, capital resources and contractual cash obligations. This discussion should be read in conjunction with “Item 1A. Risk Factors”, the accompanying audited consolidated financial statements and related notes thereto, as well as other cautionary statements and risks described elsewhere in this Annual Report on Form 10-K. For the discussion of the financial condition and results of operations for the year ended June 30, 2023 compared to the year ended June 30, 2022, please refer to the information contained under Management’s Discussion and Analysis of Financial Condition and Results of Operations and "Risk Factors" contained in the Annual Report on Form 10-K for the year ended June 30, 2023, which was filed with the SEC, on September 30, 2023.
Overview
Renalytix is focused on providing doctors around the world with a safe, reliable and effective tool to identify which patients are or are not in danger of losing significant kidney function and falling into kidney failure and may require long-term dialysis or a kidney transplant. Chronic kidney disease is one of the largest urgent medical needs, globally affecting an estimated 850 million people, and is responsible for an unsustainable and growing societal cost burden.
We believe an important part of the answer is preventative medicine and the ability to identify individuals with advancing chronic kidney disease early, where new drug therapies and clinical strategies have the optimal chance to stop uncontrolled disease progression.
At Renalytix, we developed kidneyintelX.dkd, the first U.S. Food and Drug Administration ("FDA") authorized in vitro prognostic test that uses an artificial intelligence-enabled algorithm to aid in assessment of the risk of progressive decline in kidney function. The test is designed to predict early in the progression of kidney disease who is at risk for significant sustained decline in kidney function. Prognostic tests, such as kidneyintelX.dkd, are not intended for diagnosing any disease or for monitoring disease progression or the effect of any therapeutic product. Rather, prognostic tests are intended to be used in conjunction with other clinical and diagnostic findings and consistent with professional standards of practice, including information obtained by alternative methods, and clinical evaluation, as appropriate. When used as intended, potential interventions can be considered early, ideally before major damage is done and when treatments can be most effective. KidneyintelX.dkd is part of a family of clinical tests being developed from the KidneyIntelX technology platform developed using technology licensed from the Icahn School of Medicine at Mount Sinai in New York, the Joslin Diabetes Center in Boston and under development through U.S. and international collaborations.
We are deploying KidneyIntelX to patient populations with DKD on a regional basis through partnerships with healthcare systems and insurance payors that provide coverage to those healthcare systems’ patients. In June 2024 , Medicare issued a final Local Coverage Determination (“LCD”) for the Company’s kidneyintelX.dkd testing and is effective for dates of service on or after August 1, 2024. The established Medicare price for kidneyintelX.dkd is $950 per test. Distinct CPT Codes (Common Procedural Terminology Codes) have been established for kidneyintelX.dkd and is published in CMS’ 2024 Clinical Lab Fee Schedule. The LCD specifies coverage for use of kidneyintelX.dkd for patients with diagnosed Type 2 diabetes and Stage 1-3b Chronic Kidney Disease is reasonable and necessary. The LCD was issued by National Government Services (“NGS”). NGS is a subsidiary of Elevance Health, Inc. (previously Anthem, Inc.), a Medicare Administrative Contractor responsible for claims processing for testing performed in the Company’s New York City laboratory.
Since our inception in March 2018, we have focused primarily on organizing and staffing our company, raising capital, developing the KidneyIntelX platform, conducting clinical validation studies for KidneyIntelX, establishing and protecting our intellectual property portfolio and commercial laboratory operations, pursuing regulatory approval and developing our reimbursement strategy. We have funded our operations primarily through equity and debt financings.
Macroeconomic Considerations
During fiscal year 2024, we continued to monitor the situation related to COVID-19 and may take actions that alter our business operations to the extent that may be required by federal, state or local authorities, or that we determine are in the best interests of our employees, partners and shareholders.
Unfavorable conditions in the economy in the United States and abroad may negatively affect the growth of our business and our results of operations. For example, macroeconomic events, including conflicts in Ukraine-Russia and Middle East, increased inflation rates and U.S. and U.K. interest rates, have led to economic uncertainty globally. The effect of macroeconomic conditions may not be fully reflected in our results of operations until future periods. If, however, economic uncertainty increases or the global economy worsens, our business, financial condition and results of operations may be harmed. For further discussion of the potential impacts of macroeconomic events on our business, financial condition, and operating results, see the section titled “Risk Factors.”
74
Our Key Agreements
Mount Sinai Health System
In May 2018, we entered into the Mount Sinai Agreement, with Mount Sinai, pursuant to which we obtained a worldwide, royalty-bearing, exclusive license under certain patents and a worldwide, royalty-bearing, non-exclusive license under certain know-how of Mount Sinai to develop and commercialize licensed products in connection with the application of artificial intelligence for the diagnosis of kidney disease. Pursuant to the terms of the Mount Sinai Agreement, we are obligated to use commercially reasonable efforts in connection with the development and commercialization of the licensed products, including in accordance with specified diligence milestones.
We paid Mount Sinai $10.0 million as an up-front payment upon entering into the Mount Sinai Agreement. Under the terms of the Mount Sinai Agreement, we are obligated to pay Mount Sinai $1.5 million and $7.5 million in commercial milestone payments upon achieving worldwide net sales of KidneyIntelX of $50.0 million and $300.0 million, respectively. We are also obligated to pay Mount Sinai a 4% to 5% royalty on net sales of KidneyIntelX, subject to customary reductions. Royalties are payable on a product-by-product basis from first commercial sale of such product until the later of (1) expiration of the last valid claim of a licensed patent covering such product or (2) on a country-by-country basis, 12 years from first commercial sale of such product in such country. Moreover, we are obligated to pay Mount Sinai between 15% and 25% of any consideration received by us from a sublicensee. The two provisional patent applications covering the KidneyIntelX diagnostic in-licensed under the Mount Sinai Agreement were filed in February 2020 and April 2020, respectively. If issued, these patents will expire in February 2041 and April 2041, respectively. Furthermore, we agreed to carry out and fund a clinical utility study for KidneyIntelX at a total estimated cost of $10.7 million.
The Mount Sinai Agreement expires on the later of the tenth anniversary of the execution of the agreement and expiration of the last remaining royalty term. We may terminate the Mount Sinai Agreement at any time upon 90 days’ prior written notice. Mount Sinai may terminate the agreement for our uncured material breach, our failure to meet certain diligence milestones, our insolvency, or in the event that we challenge the validity or enforceability of any licensed patent.
Joslin Diabetes Center
In July 2017, EKF entered into the Joslin Agreement with Joslin. In October 2018, we purchased all of EKF’s rights, title, interest and benefit in the Joslin Agreement in exchange for the issuance of 15.4 million of our ordinary shares.
Pursuant to the Joslin Agreement and the related assignment from EKF, we obtained a worldwide, royalty-bearing, exclusive license under any patents and any related know-how of Joslin related to the patent application filed with respect to the Joslin IP to make, have made, use, offer for sale and sell licensed products covered by claims in the Joslin IP, and to perform, practice offer for sale and sell certain licensed processes related to the Joslin IP. We are obligated to use commercially reasonable efforts in connection with the development and commercialization of the licensed products and licensed processes, including in accordance with a development plan.
Under the terms of the Joslin Agreement, we are obligated to pay Joslin aggregate commercial milestone payments of $0.3 million and $1.0 million in commercial milestone payments upon achieving worldwide net sales of licensed products and processes of $2.0 million and $10.0 million, respectively. We are also obligated to pay Joslin a 5% royalty on net sales of any licensed products or licensed processes, subject to customary reductions. Moreover, we are obligated to pay Joslin 25% of any consideration received by us from a sublicensee.
The Joslin Agreement initially expires on July 31, 2025, and is subject to an automatic five-year extension unless either party notifies the other party of its intent not to extend the agreement at least 180 days prior to initial expiration. Either party may terminate the Joslin Agreement earlier upon an uncured material breach of the agreement by the other party, the insolvency of the other party, or in the event the other party is unable to perform its obligations under the agreement for a specified period. Additionally, Joslin may terminate the agreement in the event that we cease developing or commercializing licensed products or processes, if we fail to maintain certain required insurance policies, and if we fail to pay patent expenses related to the licensed patents.
Components of Results of Operations
Revenues
During the fiscal years ended June 30, 2024 and 2023, we continued to deploy KidneyIntelX to patient populations with DKD, on a regional basis through partnerships with healthcare systems and insurance payors that provide coverage to those healthcare systems’ patients. If these strategic partners fail to meet their key contractual obligations or to purchase KidneyIntelX tests, it will likely have an adverse effect on us and our ability to achieve our commercial objectives, potentially including the attainment of sales volumes leading to profitability.
75
Cost of Revenue
During the fiscal years ended June 30, 2024 and 2023, cost of revenue consists of costs directly attributable to the KidneyIntelX testing and services rendered, including labor, lab consumables and sample collection costs directly related to revenue generating activities.
Research and Development Expenses
Research and development costs consist primarily of costs incurred in connection with the development of KidneyIntelX. We are currently continuing to conduct clinical utility and other studies for KidneyIntelX to determine clinical value and performance in different CKD populations. We expense research and development costs as incurred. Because we have limited resources and access to capital to fund our operations, we must decide which diagnostic product to pursue and the amount of resources to allocate to each. As such, we have been focused primarily on the development of KidneyIntelX and studies to further demonstrate the clinical utility of KidneyIntelX.
We incur both direct and indirect expenses related to our research and development programs. Direct expenses include third-party expenses related to our programs such as expenses for data science and artificial intelligence capabilities, consulting fees, lab supplies, assay development services and clinical validation costs. Indirect expenses include salaries and other personnel-related costs, including share-based compensation for personnel in research and development functions and rent.
At the end of the reporting period, we compare payments made to third-party service providers to the estimated progress toward completion of the research or development objectives. Such estimates are subject to change as additional information becomes available. Depending on the timing of payments to the service providers and the progress that we estimate to have been made as a result of the service provided, we may record net prepaid or accrued expense relating to these costs. Upfront milestone payments made to third parties who perform research and development services on our behalf are expensed as services are rendered.
The successful commercialization of KidneyIntelX is uncertain. This is due to the numerous risks and uncertainties associated with product development and commercialization, including:
A change in the outcome of any of these variables could result in a significant change in the costs and timing associated with our related development.
General and Administrative Expenses
General and administrative expenses consist principally of salaries and other personnel-related costs including share-based compensation; professional fees for accounting, auditing, tax and administrative consulting services; legal fees relating to patent and corporate matters; administrative travel expenses; insurance costs; marketing expenses and other operating costs. Additionally, general and administrative expenses include the cost of maintaining our admission to AIM and Nasdaq.
76
Impairment Loss on Property, Equipment and Other Long-lived Assets
On a quarterly basis, the respective carrying value of non-financial assets are assessed for impairment indicators and, if ultimately considered impaired, are adjusted and written down to their fair value, as estimated based on consideration of external market participant assumptions.
Equity Losses in Affiliate
Equity losses in affiliate represents the recognition of our proportionate share of loss from operations of Kantaro Biosciences LLC.
Foreign Currency Gain (Loss), net
Foreign currency gain (loss), net consists of foreign currency income (losses) due to exchange rate fluctuations on transactions denominated in a currency other than our functional currency.
Fair Value Adjustments to VericiDx Investment
In October 2020, the Company completed a spin off of VericiDx, a developer of advanced clinical diagnostics for organ transplant, and retained 9,831,681 ordinary shares of VericiDx. The Company accounts for the investment in VericiDx equity securities at fair value, with changes in fair value recognized in the consolidated statements of operations and comprehensive loss. In March 2024, the Company sold 750,000 ordinary shares of VericiDx for net proceeds of $0.1 million and a realized loss of $0.1 million. In May 2024, the Company sold 250,000 ordinary shares of VericiDx for net proceeds of $0.02 million and a realized loss of $0.04 million. As of June 30, 2024, the Company owns 8,831,682 shares of VericiDx.
Fair Value Adjustment on Convertible Notes
We elected to account for the bonds at fair value with qualifying changes in fair value recognized through the consolidated statements of operations and comprehensive loss until the notes are settled.
Other Income
Other income relates to interest income earned on our cash deposits, grant income earned for work performed under the Horizon Europe grant and other services provided to academic institutions or pharmaceutical companies.
77
Consolidated Results of Operations
|
|
Twelve Months Ended |
|
|
Change 2024 vs.2023 |
|
||||||||||
(in thousands, except share and per share data) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Revenue |
|
$ |
2,289 |
|
|
$ |
3,403 |
|
|
$ |
(1,114 |
) |
|
|
-33 |
% |
Cost of revenue |
|
|
2,133 |
|
|
|
2,683 |
|
|
|
(550 |
) |
|
|
-20 |
% |
Gross profit |
|
|
156 |
|
|
|
720 |
|
|
|
(564 |
) |
|
|
-78 |
% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
|
9,290 |
|
|
|
14,298 |
|
|
|
(5,008 |
) |
|
|
-35 |
% |
General and administrative |
|
|
19,751 |
|
|
|
28,662 |
|
|
|
(8,911 |
) |
|
|
-31 |
% |
Impairment loss on property, equipment and other long-lived assets |
|
|
723 |
|
|
|
— |
|
|
|
723 |
|
|
|
100 |
% |
Performance of contract liability to affiliate |
|
|
— |
|
|
|
(19 |
) |
|
|
19 |
|
|
|
-100 |
% |
Total operating expenses |
|
|
29,764 |
|
|
|
42,941 |
|
|
|
(13,177 |
) |
|
|
-31 |
% |
Loss from operations |
|
|
(29,608 |
) |
|
|
(42,221 |
) |
|
|
12,613 |
|
|
|
-30 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Equity in net losses earnings of affiliate |
|
|
— |
|
|
|
(9 |
) |
|
|
9 |
|
|
|
-100 |
% |
Foreign currency gain, net |
|
|
163 |
|
|
|
358 |
|
|
|
(195 |
) |
|
|
-54 |
% |
Fair value adjustment to VericiDx investment |
|
|
(505 |
) |
|
|
(1,282 |
) |
|
|
777 |
|
|
|
-61 |
% |
Fair value adjustment to convertible notes |
|
|
(3,751 |
) |
|
|
(3,107 |
) |
|
|
(644 |
) |
|
|
21 |
% |
Other income, net |
|
|
249 |
|
|
|
656 |
|
|
|
(407 |
) |
|
|
-62 |
% |
Net loss before income taxes |
|
|
(33,452 |
) |
|
|
(45,605 |
) |
|
|
12,153 |
|
|
|
-27 |
% |
Income tax expense |
|
|
(4 |
) |
|
|
(2 |
) |
|
|
(2 |
) |
|
|
70 |
% |
Net loss |
|
|
(33,456 |
) |
|
|
(45,607 |
) |
|
|
12,151 |
|
|
|
-27 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net loss per ordinary share—basic |
|
$ |
(0.31 |
) |
|
$ |
(0.55 |
) |
|
$ |
0.25 |
|
|
|
-44 |
% |
Net loss per ordinary share—diluted |
|
$ |
(0.31 |
) |
|
$ |
(0.55 |
) |
|
$ |
0.25 |
|
|
|
-44 |
% |
Weighted average ordinary shares—basic |
|
|
108,179,366 |
|
|
|
82,210,050 |
|
|
|
25,969,316 |
|
|
|
32 |
% |
Weighted average ordinary shares—diluted |
|
|
108,179,366 |
|
|
|
82,210,050 |
|
|
|
25,969,316 |
|
|
|
32 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Changes in the fair value of the convertible notes |
|
|
305 |
|
|
|
(337 |
) |
|
|
642 |
|
|
|
-191 |
% |
Foreign exchange translation adjustment |
|
|
(298 |
) |
|
|
(198 |
) |
|
|
(100 |
) |
|
|
51 |
% |
Comprehensive loss |
|
$ |
(33,449 |
) |
|
$ |
(46,142 |
) |
|
$ |
12,693 |
|
|
|
-28 |
% |
Comparison of years ended June 30, 2024 and 2023
Revenue
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Revenue |
|
$ |
2,289 |
|
|
$ |
3,403 |
|
|
$ |
(1,114 |
) |
|
|
-33 |
% |
During the year ended June 30, 2024, we recognized $2.1 million of revenue related to sales of KidneyIntelX and $0.14 million of revenue related to services performed for Eli Lilly and University Medical Center Gronigen. During the year ended June 30, 2023, we recognized $3.1 million revenue related to sales of KidneyIntelX and $0.3 million of revenue of pharmaceutical services revenue related to services performed for AstraZeneca and University Medical Center Gronigen. The $1.1 million decrease in revenue was primarily driven by an decrease in KidneyIntelX billable testing volumes due to the transition to a commercial billing structure under our arrangement with Mount Sinai.
78
Cost of Revenue
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Cost of revenue |
|
$ |
2,133 |
|
|
$ |
2,683 |
|
|
$ |
(550 |
) |
|
|
-20 |
% |
During the year ended June 30, 2024, we recognized cost of revenue of $2.1 million primarily attributable to KidneyIntelX testing, including labor, lab consumables and sample collection costs related to revenue generating activities. We recognized $2.7 million of cost of revenue for the year ended June 30, 2023. The $0.5 million decrease in cost of revenue was primarily driven by a decrease in KidneyIntelX testing volumes.
Research and Development Expenses
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Research and development expenses |
|
$ |
9,290 |
|
|
$ |
14,298 |
|
|
$ |
(5,008 |
) |
|
|
-35 |
% |
Research and development expenses decreased by $5.0 million from $14.3 million for the year ended June 30, 2023 to $9.3 million for year ended June 30, 2024. The decrease was attributable to a $2.7 million decrease in compensation and related benefits, $2.5 million decrease related to external R&D projects and studies with Mount Sinai and Wake Forest, $0.2 million decrease in lab supplies purchases, offset by $0.4 million increase related to consulting and professional fees and $0.1 million increase in other miscellaneous expenses.
General and Administrative Expenses
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
General and administrative |
|
$ |
19,751 |
|
|
$ |
28,662 |
|
|
$ |
(8,911 |
) |
|
|
-31 |
% |
General and administrative expenses decreased $8.9 million from $28.7 million for the year ended June 30, 2023 to $19.8 million for the year ended June 30, 2024. The decrease was driven by our previously announced cost cutting measures and was due to a $6.7 million decrease in compensation and related benefits, including share-based payments, due to decreased headcount, $1.3 million decrease in insurance costs, $0.6 million decrease in marketing and public relations, $0.5 million decrease in other operating expenses, and $0.4 million decrease in IT costs, offset by a $0.6 million increase in consulting and professional fees.
In connection with the delisting of our ADSs from Nasdaq effective on or about October 7, 2024, and our ADSs becoming quoted on the OTC Markets’ OTCQX tier effective on or about October 8, 2024, we anticipate that we will re-qualify as a foreign private issuer (“FPI”) at our next testing date for FPI status. As a foreign private issuer, we expect annual cost savings of up to $1.5 million from a reduction in regulatory filing costs, professional fees (audit, legal, and consulting), listing fees, insurance and other administrative costs.
Impairment Loss on Property, Equipment and Other Long-Lived Assets
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Impairment loss on property, equipment and other long-lived assets |
|
$ |
723 |
|
|
$ |
— |
|
|
$ |
723 |
|
|
|
100 |
% |
As part of our cost saving plan, we consolidated lab operations which resulted in a $0.6 million impairment of property and equipment at our Utah and Florida labs and a $0.1 million impairment of the Utah right-of-use asset for the year ended June 30, 2024. There was no impairment loss on property, equipment and other long-lived assets in the year ended June 30, 2023.
79
Foreign Currency Gain
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Foreign currency gain, net |
|
$ |
163 |
|
|
$ |
358 |
|
|
$ |
(195 |
) |
|
|
-54 |
% |
During the year ended June 30, 2024, we recognized a foreign currency gain of $0.2 million due to exchange rate fluctuations on transactions denominated in a currency other than our functional currency. During the year ended June 30, 2023, we recognized a foreign currency gain of $0.4 million due to exchange rate fluctuations on transactions denominated in a currency other than our functional currency and remeasurement of intercompany payables/receivables.
Fair Value Adjustments to VericiDx Investment
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Fair value adjustment to VericiDx investment |
|
$ |
(505 |
) |
|
$ |
(1,282 |
) |
|
$ |
777 |
|
|
|
-61 |
% |
We account for the investment in VericiDx equity securities at fair value, with changes in fair value recognized in the consolidated statement of operations and comprehensive loss. During the year ended June 30, 2024, we recorded a loss of $0.5 million to adjust the VericiDx investment to fair value. During the year ended June 30, 2023, we recorded a loss of $1.3 million to adjust the VericiDx investment to fair value.
Fair Value Adjustment on Convertible Notes
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Fair value adjustment to convertible notes |
|
$ |
(3,751 |
) |
|
$ |
(3,107 |
) |
|
$ |
(644 |
) |
|
|
21 |
% |
We elected to account for the bonds at fair value with qualifying changes in fair value recognized through the consolidated statements of operations and comprehensive loss until the notes are settled. This excludes fair value adjustments related to instrument-specific credit risk, which is recognized in Other Comprehensive Income (OCI). For the year ended June 30, 2024, we recorded a loss of $3.8 million to adjust the bonds to fair value. For the year ended June 30, 2023, we recorded a loss of $3.1 million to adjust the bonds to fair value. The change in fair value of the bond was driven by a decrease in term to maturity, increase in risk free rate and change in stock price.
Other Income
|
|
Twelve Months Ended |
|
|
Change 2024 vs. 2023 |
|
||||||||||
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
|
Change |
|
|
% |
|
||||
Other income, net |
|
$ |
249 |
|
|
$ |
656 |
|
|
$ |
(407 |
) |
|
|
-62 |
% |
During the year ended June 30, 2024, we realized $0.2 million of income which included $0.2 million of interest income earned on our cash deposits and $0.2 million of grant income, offset by $0.1 million of realized loss on the sale of VericiDx shares. During the year ended June 30, 2023, we realized $0.7 million of income related $0.2 million of income related to the dissolution of Kantaro, $0.3 million of income for refunds from Citibank, and $0.1 million interest income earned on our cash deposits.
Liquidity and Capital Resources
Since our inception, we have incurred net losses. We incurred net losses of $33.5 million and $45.6 million for the years ended June 30, 2024 and 2023, respectively. As of June 30, 2024, we had an accumulated deficit of $211.8 million.
On March 12, 2024, we entered into a Placing Agreement (the “Placing Agreement”) with Stifel Nicolaus Europe Limited (the “Bookrunner” or “Stifel”), pursuant to which we agreed to allot and issue new ordinary shares, nominal value £0.0025 per ordinary share (the “Placing Shares”) to certain investors (the “Placees”) in an unregistered offering (the “Private Placement”), up to an aggregate of 46,801,872 ordinary shares, in two tranches. We allotted and issued 19,986,031 Placing Shares at a placing price of £0.20 per Placing Share (the “First Tranche”), which closed on March 14, 2024. Subsequent to obtaining the required shareholder approval, we allotted and issued 26,815,841 Placing Shares at a placing price of £0.20 per Placing Share (the “Second Tranche”), which closed on April 24, 2024.
80
We received aggregate gross proceeds of approximately $12 million from the closing of the First Tranche and Second Tranche, before deducting fees and commissions to the Bookrunner and other offering expenses payable by us.
On April 5, 2024, we entered into a securities purchase agreement (the “DB Capital Purchase Agreement”) with an institutional investor pursuant to which we agreed to issue and sell in a registered direct offering (the “Registered Direct Offering”) 2,666,667 ordinary shares, nominal value £0.0025 per share. Pursuant to the DB Capital Purchase Agreement, we also granted the investor an option to purchase up to 7,811,696 additional ordinary shares at the offering price of $0.375 per share. The purchase price of each ordinary share is $0.375. On April 18, 2024, the investor partially exercised the option to purchase 1,333,334 ordinary shares. The gross proceeds to the Company from the Registered Direct Offering were approximately $1.5 million, before deducting offering expenses payable by the Company. The shares were offered by the Company pursuant to an effective shelf registration statement on Form S-3 (File No. 333-274733) that was filed with the SEC on September 28, 2023 and became effective on October 6, 2023, including the base prospectus contained therein, and a related prospectus supplement dated as of April 5, 2024 filed with the SEC.
On September 30, 2024, we announced our intention to raise approximately £11 million gross proceeds through a placing, a subscription and a retail offer of new ordinary shares of the Company at an issue price of £0.09 per new ordinary share to new and existing institutional and other investors. There can be no assurance that we will complete this fundraising or on the terms and in the timing expected.
October 2024 Proposed Debt Restructurings
The Company announced a proposed fundraising (the “Fundraise”) on September 30, 2024. In that announcement, the Company announced a proposed revision to the terms of its £8.7 million amortizing senior convertible bond (the “Convertible Bond”) held by a fund advised by Heights Capital Ireland LLC (the "Convertible Bond Investor"), subject to the closing of the fundraising which we expect to be on or around October 9, 2024. Under the proposed terms, the Convertible Bond would be repaid and restructured as follows (depending on the specific amount of capital committed in the financing): (i) £2.9 million of the Convertible Bond would be capitalized via issue to the Convertible Bond Investor of approximately 32.2 million ordinary shares (the “Heights Conversion Shares”), at the per share price (the “Issue Price”) being offered in the Fundraise; (ii) in the event that the Company were to raise more than £12.5 million (net of costs) through the Fundraise, the next £2.5 million raised in the Fundraise (above the £12.5 million (net of costs)) would be payable to the Convertible Bond Investor (such amount, the “Tranche 2 Payment”); and (iii) the balance of the Convertible Bond will be restructured as a new unsecured convertible bond (the “New Convertible Bond”). It is currently expected that the Heights Conversion Shares to be issued to the Convertible Bond Investor will represent 9.9% of the Company’s enlarged issued share capital. The Convertible Bond Investor will be subject to a 6-month lock-in and, in the event that the Tranche 2 Payment exceeds £2 million, the Convertible Bond Investor will agree to extend its lock-in period for a further 3 month period.
The New Convertible Bond will accrue interest at a rate of 5.5% per annum if paid in cash or 7.5% per annum if rolled into the principal amount, at the discretion of the Company. The New Convertible Bond will have a maturity date of July 31, 2029 and may not be converted before April 1, 2026 ,except in the event that the Company undertakes a further qualifying equity issuance in the future (which would exclude securities properly issued to employees and other staff of the Company for bona fide remuneration and incentivisation purposes). The New Convertible Bond can be redeemed as follows:
81
Additionally, an accounts payable balance with a professional adviser of approximately $850,000 (the “Advisor Accounts Payable Balance”) has been restructured such that 50% of the outstanding balance ($425,000) will convert to equity at the Issue Price. The remaining 50% will be repaid as follows: (i)
Additionally, other creditors with accounts payable having an aggregate value of approximately £650,000 have agreed to write-off their balances (the “Creditor Write-offs”).
The Company believes that the restructuring of the Convertible Bond and the Advisor Accounts Payable Balance, the creation of the New Convertible Bond and the Advisor Loan Note and the Creditor Write-offs, along with some ancillary debt restructuring, will substantially reduce the Company’s monthly cash burn and the Company estimates that this will remove more than 80% of the total forecasted cash obligations of the Company over the next 3 years (or approximately £485,000 per month).
The ordinary shares issued pursuant to the debt restructuring described in this section are expected to equate to approximately 36 million ordinary shares and are expected to be issued on or about October 30, 2024, subject to the passing of resolutions at the general meeting of the Company’s shareholders (the “General Meeting”), completion of the allotment and issue of the Non-EIS/VCT placing shares will take place after the General Meeting, and application to the London Stock Exchange for admission of such ordinary shares.
We expect to incur additional losses in the near future, and we expect our expenses to increase substantially in connection with our ongoing activities, particularly as we continue to commercialize and scale KidneyIntelX, as we conduct our ongoing and planned clinical utility and other studies for KidneyIntelX for its commercial launch, develop and refine our artificial intelligence technology platform, seek regulatory clearances or approvals for KidneyIntelX or any other product we develop, establish and maintain partnerships with healthcare systems, pursue our coverage and reimbursement strategy, and continue to invest in our infrastructure to support our manufacturing and other activities. In addition, we expect to incur additional costs associated with operating as a public company in the United States. The timing and amount of our operating expenditures will depend largely on:
To date, we have primarily financed our operations through equity and debt financings. As of June 30, 2024, we had cash of $4.7 million. We have incurred recurring losses and negative cash flows from operations since inception and had an accumulated deficit of $211.8 million as of June 30, 2024. We anticipate incurring additional losses until such time, if ever, that we can generate significant sales of KidneyIntelX or any future products currently in development. As a result of our losses and our projected cash needs, substantial doubt exists about our ability to continue as a going concern within 12 months after the date that the financial statements are issued.
82
Substantial additional capital will be necessary to fund our operations, expand our commercial activities and develop other potential diagnostic-related products. We plan to finance our cash needs through a combination of revenue from sales, securities offerings, debt financings, collaborations, strategic alliances and marketing, distribution or licensing arrangements with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our shareholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our shareholders.
Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or diagnostic products or grant licenses on terms that may not be favorable to us. Additional capital may not be available when needed, on reasonable terms, or at all, and our ability to raise additional capital may be adversely impacted by potential worsening global economic conditions and disruptions to and volatility in the credit and financial markets in the United States and worldwide. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, curtail or discontinue our product development or future commercialization efforts, or grant rights to develop and market products that we would otherwise prefer to develop and market ourselves.
Going Concern
We have limited sources of revenue to provide incoming cash flows to sustain our future operations. As outlined above, our ability to pursue our planned business activities is dependent upon our successful efforts to raise additional capital and the effectiveness of our cost-cutting and other capital preservation measures. Without additional financing, we expect our cash and cash equivalents as of June 30, 2024, combined with additional cost reduction options available, will be sufficient to fund our operating expenses and capital expenditure requirements into November 2024. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect or may fail in our efforts to enact additional cost reduction options. Furthermore, our operating plan may change, and we may need additional funds sooner than planned in order to meet operational needs and capital requirements for product development and commercialization.
On September 30, 2024, we announced a commitment from a number of existing and new investors to purchase equity securities from the Company in the aggregate gross amount of approximately £11 million. This equity financing is expected to close in two or more tranches beginning on October 9, 2024. This incremental equity capital is expected to fund the Company for at least the next 12 months. The funding has been committed but not yet received at the time of this filing.
These factors raise substantial doubt regarding our ability to continue as a going concern. Our consolidated financial statements have been prepared on a going concern basis, which implies that we will continue to realize our assets and discharge our liabilities in the normal course of business. Our financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Cash Flows
The following table shows a summary of our cash flows from operations for the periods indicated (in thousands):
|
|
Year ended June 30, |
|
|
Change 2024 vs.2023 |
|
|||||||||||||
(in thousands, except share and per share amounts) |
|
2024 |
|
|
|
2023 |
|
|
Change |
|
|
% |
|
||||||
Net cash used in operating activities |
|
$ |
|
(30,111 |
) |
|
|
$ |
|
(34,085 |
) |
|
|
3,974 |
|
|
|
-12 |
% |
Net cash used in investing activities |
|
|
|
(4 |
) |
|
|
|
|
— |
|
|
|
(4 |
) |
|
|
— |
|
Net cash provided by financial activities |
|
|
|
10,250 |
|
|
|
|
|
16,386 |
|
|
|
(6,136 |
) |
|
|
-37 |
% |
Effect of exchange rate changes on cash |
|
|
|
(137 |
) |
|
|
|
|
1,048 |
|
|
|
(1,185 |
) |
|
|
-113 |
% |
Net cash used in operating activities
During the year ended June 30, 2024, net cash used in operating activities was $30.1 million and was primarily attributable to our $33.5 million net loss as well as $6.9 million of noncash charges and $3.5 million net change in our operating assets and liabilities. Noncash charges were primarily related to $3.5 million fair value adjustment related to the bonds, $1.7 million in share-based compensation expense, $0.7 million of loss on write off of assets, $0.5 million fair value adjustment on our VericiDx investment, and $0.4 million of depreciation and amortization expense. The change in our operating assets and liabilities was primarily attributable to $4.4 million decrease in accrued expenses and other current liabilities, offset by $0.9 million increase in accounts receivables, prepaids and other current assets.
83
During the year ended June 30, 2023, net cash used in operating activities was $34.1 million and was primarily attributable to our $45.6 million net loss adjusted for $5.8 million of noncash charges and $5.7 million net change in our operating assets and liabilities. Noncash charges were primarily related to $2.9 million in share-based compensation expense, $2.0 million fair value adjustment related to the bonds, $1.3 million fair value adjustment on our VericiDx investment, $0.5 million of depreciation and amortization expense and $0.1 million of noncash lease expense, offset by $1.0 million in foreign exchange gains. The change in our operating assets and liabilities was primarily attributable to a $4.2 million increase in accrued expenses and other current liabilities and a $1.5 million increase in accounts receivables, prepaids and other current assets.
Net cash used in investing activities
During the year ended June 30, 2024, net cash used in investing activities was immaterial.
During the year ended June 30, 2023, no cash was used in investing activities.
Net cash provided by financing activities
During the year ended June 30, 2024, net cash provided by financing activities was $10.3 million and was primarily attributable to $13.5 million of gross proceeds from the issuance of ordinary shares in Private Placement, $0.1 million in proceeds from the issuance of ordinary shares under our employee stock purchase program, offset by $1.7 million in cash used to pay down the principal of the Bonds and $1.7 million in cash paid for offering costs related to the issuance of ordinary shares.
During the year ended June 30, 2023, net cash provided by financing activities was $16.4 million and was primarily attributable to $20.3 million of proceeds from the issuance of ordinary shares, offset by $1.0 million in offering costs. Additionally, we received $0.3 million in proceeds from the issuance of ordinary shares under our employee share purchase program. Net cash provided by financing activities was offset by $3.2 million for the repayment of convertible notes during the year.
Based upon the debt restructurings, Nasdaq delisting, OTCQX quotation and re-achieving foreign private issuer status discussed above, we are projecting that our over-all cash burn rate can be reduced to $0.75 million or less per month by the end of our fiscal year ending June 30, 2025.
Recent Accounting Pronouncements
See Note 3 to our consolidated financial statements found elsewhere in this report for a description of recent accounting pronouncements applicable to our financial statements.
JOBS Act Transition Period
In April 2012, the JOBS Act was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, for complying with new or revised accounting standards. An emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the extended transition period for complying with new or revised accounting standards and, as a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
We have evaluated the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we have chosen to rely on certain of these exemptions, including without limitation exemptions to the requirements for (1) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (2) complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an emerging growth company until the earlier to occur of (a) the last day of the fiscal year (1) following the fifth anniversary of the completion of our U.S. IPO, (2) in which we have total annual gross revenues of at least $1.235 billion or (3) in which we are deemed to be a “large accelerated filer” under the rules of the SEC, which means the market value of our ordinary shares and ADSs that are held by non-affiliates exceeds $700.0 million as of the prior December 31, or (b) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period.
84
Critical Accounting Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses and share-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 3 to our consolidated financial statements included elsewhere in this report, we believe the following accounting policies are the most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the development of KidneyIntelX. We expense research and development costs as incurred.
At the end of the reporting period, we compare payments made to third-party service providers to the estimated progress toward completion of the research or development objectives. Such estimates are subject to change as additional information becomes available. Depending on the timing of payments to the service providers and the progress that we estimate has been made as a result of the service provided, we may record a prepaid expense or accrued liability relating to these costs. Upfront milestone payments made to third parties who perform research and development services on our behalf are expensed as services are rendered. Contingent development or regulatory milestone payments are recognized upon the related resolution of such contingencies.
We make estimates of our accrued expenses as of each consolidated balance sheet date in our consolidated financial statements based on facts and circumstances known at that time. If the actual timing of the performance of services or the level of effort varies from the estimate, we will adjust the accrual accordingly. Nonrefundable advance payments for goods and services, including fees for process development or manufacturing and distribution of clinical supplies that will be used in future research and development activities, are deferred and recognized as expense in the period that the related goods are consumed or services are performed.
Share-based Compensation
We measure equity classified share-based awards granted to employees and nonemployees based on the estimated fair value on the date of grant and recognize compensation expense of those awards over the requisite service period, which is the vesting period of the respective award. We account for forfeitures as they occur. For share-based awards with service-based vesting conditions, we recognize compensation expense on a straight-line basis over the service period. The fair value of each stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model, which requires inputs based on certain subjective assumptions, including the expected stock price volatility, the expected term of the option, the risk-free interest rate for a period that approximates the expected term of the option, and our expected dividend yield. We were a privately-held organization prior to November 2018 and have been a publicly-traded company for a limited period of time and therefore lack company-specific historical and implied volatility information for our shares. Accordingly, we estimate our expected share price volatility based on the historical volatility of publicly-traded peer companies and expect to continue to do so until such time as we have adequate historical data regarding the volatility of our own traded share price. The expected term of our stock options has been determined utilizing the “simplified” method for awards that qualify as “plain-vanilla” options. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is none based on the fact that we have never paid cash dividends on ordinary shares and do not expect to pay any cash dividends in the foreseeable future.
We classify share-based compensation expense in our consolidated statement of operations and comprehensive loss in the same manner in which the award recipient’s payroll costs are classified or in which the award recipient’s service payments are classified.
85
Convertible Notes
In April 2022, we issued amortizing senior convertible bonds with a principal amount of $21.2 million in amortizing senior convertible bonds due in April 2027 (the “Bonds”) to CVI Investments, Inc. (the “Convertible Bond Investor”). The Bonds were issued at 85% par value with total net proceeds of $18.0 million and accrue interest at an annual rate of 5.5%, payable quarterly in arrears, in cash or ADSs valued at the ADS Settlement Price at our option. The principal and interest payments are due in equal quarterly installments starting in July 2022. The Bonds contain various conversion and redemption features. The initial conversion price for the Convertible Bonds of $8.70 has been set at a 20 percent premium to the Reference ADS Price. The Conversion Price may reset down at 12, 24 and 36 months, depending on share price performance, the Bonds have a hard floor in the conversion price of $7.25. As a result of the February 2023 private placement and pursuant to conditions of the bond agreement, the conversion price was adjusted to $8.2508 (previously $8.70) and the floor price was adjusted to $6.8757 (previously $7.25). Further, pursuant to conditions of the agreement, effective April 7, 2023, the conversion price was adjusted from $8.2508 to $7.7924. Between amortization dates, Convertible Bond Investor retains the right to advance future amortization payments, provided that (a) there shall be no amortization advancements during the first 12 months, (b) no more than two amortization advancements may occur in any 12-month period, and (c) no more than one amortization advancement may occur in any 3-month period. On March 28, 2024, the Company entered into a second amendment and restatement agreement with the Convertible Bond Investor, which amended the terms of the Company’s existing bond agreement, dated March 31, 2022. The Company performed an analysis and determined that the financial impact was immaterial as the amended and restated agreement was not substantially different than the previous agreement.
The Convertible Bond Investor is also permitted to defer up to two amortization payments to a subsequent amortization date. We retain the option to repay any deferred amortization in cash at 100 percent of the nominal amount. In July 2022, we made a cash amortization payment of $1.4 million, which consisted of $1.1 million of principal and $0.3 million of interest. In October 2022, the Convertible Bond Investor deferred the October amortization payment to maturity of the bond and we made an interest payment of $0.3 million. In January 2023, we made a cash amortization payment of $1.4 million, which consisted of $1.1 million of principal and $0.3 million of interest. In April 2023, we made a cash amortization payment of $1.4 million, which consisted of $1.1 million of principal and $0.3 million of interest. In July 2023, we made a cash amortization payment of $1.4 million, which consisted of $1.1 million of principal and $0.3 million of interest. Also in July 2023, the Convertible Bond Investor exercised its right to advance an amortization payment and we made an accelerated repayment of $1.1 million through the issuance of 526,211 ADSs representing 1,052,422 ordinary shares. In October 2023, we made an amortization payment of $1.3 million, which consisted of $1.1 million of principal and $0.2 million of interest, through the issuance of 2,335,388 ordinary shares in the form of 150,000 ordinary shares and 1,092,694 ADSs. In December 2023, we made an amortization payment of $1.3 million, which consisted of $1.1 million of principal and $0.2 million of interest, through the issuance of 2,500,000 ordinary shares and a cash payment of $0.6 million. On April 11, 2024, we issued 3,636,162 ordinary shares in the form of 1,818,081 ADSs to the Convertible Bond Investor (the “April Repayment”), which settled the principal and interest amounts due under the bonds on April 7, 2024. After settlement of the Repayment, the principal remaining under the Bonds was reduced by $1.06 million to $12.72 million. The Shares were issued without registration in reliance upon the exemption provided in Section 3(a)(9) of the Securities Act.
The Bond Agreement contains a negative pledge covenant that provides that for so long as the principal amount outstanding under the Bonds is equal to or exceeds U.S.$3,000,000, the Company shall not, and will procure that none of its subsidiaries, will, create or permit to subsist any Security Interest (as defined in the Bond Agreement), other than a Permitted Security Interest (as defined in the Bond Agreement), upon the whole or any part of its present or future undertaking, assets or revenues (including any uncalled capital) to secure any Financial Indebtedness (as defined in the Bond Agreement) or to secure any Financial Indebtedness Guarantee (as defined in the Bond Agreement), without at the same time or prior thereto securing the obligations of the Company under the Bonds and the Bond Agreement equally and rateably therewith or providing such other security, guarantees and/or other arrangements for the benefit of holders of the Bonds as may be approved by all of the holders of the Bonds. The Bond Agreement also contains a covenant regarding the incurrence of indebtedness which provides that for so long as the principal amount outstanding under the Bonds is equal to or exceeds U.S. $5,000,000, the Company shall not, and shall procure that its subsidiaries shall not, at any time permit to create, incur, assume or otherwise become liable in respect of any Financial Indebtedness, contingently or otherwise. The Bond also contains negative covenants regarding, among other things, the issuance or paying up any securities, modifying the rights attaching to the ordinary shares; issuing any share capital with rights which are more favorable than the rights attaching to the ordinary shares; modify securities already issued, or grant securities, below a consideration floor; grant or issue securities that could not be legally issued as fully paid; not reduce its share capital, share premium account, or any uncalled liability in respect thereof, or any non-distributable reserves; certain third party offers made to shareholders; and ADSs and the ADS facility.
We elected the fair value option to account for the bonds as we believe the fair value option provides users of the consolidated financial statements with greater ability to estimate the outcome of future events as facts and circumstances change, particularly with respect to changes in the fair value of the ordinary shares underlying the conversion option. The fair value of the Notes is determined using a scenario-based analysis that estimates the fair value based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to the noteholders. For each reporting period, changes in the fair value of the notes are recognized through other income (expense) with the portion of the change that results from a change in the instrument-specific credit risk recorded separately in OCI for each reporting period.
86
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company and not required to provide this information.
Item 8. Financial Statements and Supplementary Data.
The financial statements and supplementary financial information required by this Item 8 are included in our Consolidated Financial Statements and the Notes to Consolidated Financial Statements and are set forth in the pages indicated in Part IV, Item 15(a)(1) and 15(a)(2) of this Report and are incorporated herein by reference.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
New Independent Registered Public Accounting Firm
As part of the ongoing process to reduce cost of operations and optimize third-party support services for Renalytix plc (the “Company”), the Audit Committee (the “Committee”) of the Board of Directors of the Company recently conducted a selection process to determine the Company's independent registered public accounting firm for the fiscal year ended June 30, 2024. The Committee invited several public accounting firms to participate in the process and on June 19, 2024, the Committee approved the engagement of CohnReznick LLP (“CohnReznick”) to serve as our independent registered public accounting firm for the fiscal year ended June 30, 2024 with such appointment to be effective as of June 19, 2024.
During the Company’s two most recent fiscal years ended June 30, 2023 and 2022, as well as the subsequent interim periods through the date of this Current Report, neither the Company nor anyone acting on its behalf consulted with CohnReznick regarding any of the matters described in Items 304(a)(2)(i) and (ii) of Regulation S-K.
Previous Independent Registered Public Accounting Firm
On June 21, 2024, Ernst & Young LLP (“Ernst & Young”) was informed that the Committee approved Ernst & Young’s dismissal as the Company’s independent registered public accounting firm with such dismissal to be effective as of June 18, 2024.
Ernst & Young’s reports on the Company's consolidated financial statements for the fiscal years ended June 30, 2023, 2022 and 2021 did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principles, except for an explanatory paragraph regarding the entity’s ability to continue as a going concern included in Ernst & Young’s report on the Company's consolidated financial statements as of and for the year ended June 30, 2023.
During the Company’s fiscal years ended June 30, 2023, 2022 and 2021, and the subsequent interim period through the date of this Current Report: (i) there were no disagreements between the Company and Ernst & Young on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to Ernst & Young’s satisfaction, would have caused Ernst & Young to make reference to the subject matter of the disagreements in connection with its reports on the consolidated financial statements for such years and (ii) there were no reportable events as defined in Item 304(a)(1)(v) of Regulation S-K except for a material weakness in the design of the Company’s internal control over financial reporting reported in Item 15 on Form 20-F for the fiscal year end June 30, 2022 related to stock-based compensation, which was remediated as of June 30, 2023.
We have provided Ernst & Young with a copy of the foregoing disclosures and have requested that Ernst & Young furnish us with a letter addressed to the United States Securities and Exchange Commission stating that it agrees with the above disclosures. Attached as Exhibit 16.1 is a copy of that letter, dated June 24, 2024.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, that are designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed in our reports filed or submitted under the Exchange Act is accumulated and communicated to management, including our chief executive officer (principal executive officer) and interim chief financial officer (principal financial officer), as appropriate, to allow timely decisions regarding required disclosure.
87
Our principal executive officer and principal financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) as of June 30, 2024, have concluded that our disclosure controls and procedures were not effective due to the material weakness in internal control over financial reporting described below.
Management’s Annual Report on Internal Control Over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Management assessed the effectiveness of internal control over financial reporting as of June 30, 2024 based on the framework in “Internal Control—Integrated Framework” (2013 framework) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on that assessment, management has concluded that, as of June 30, 2024, the Company’s internal control over financial reporting was not effective due to the material weaknesses described below.
We have identified a material weakness in our internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim consolidated financial statements will not be prevented or detected on a timely basis. The material weakness is related to an error in the mark-to-market adjustment to our convertible debt that had been elected under the fair value option, which resulted in insufficient expense recognition.
This error resulted from the incorrect application of a mark-to-market adjustment for the convertible debt. The deficiency arose due to the high complexity and technical nature of the convertible debt instrument. We did not design and maintain effective controls over the accounting and disclosure for convertible debt. The control deficiency arose due to the lack of technical expertise.
This material weakness resulted in adjustments to expense and equity which were recorded prior to the issuance of the consolidated financial statements as of June 30, 2024.
Attestation Report of the Registered Public Accounting Firm
This annual report does not include an attestation report of the company’s registered public accounting firm. For so long as we qualify as an “emerging growth company” as defined under the JOBS Act, our independent registered accounting firm is not required to issue an attestation report on our internal control over financial reporting.
Remediation Measures for Material Weaknesses in Internal Control over Financial Reporting
We have taken measures to remediate the material weaknesses existing as of June 30, 2024 including engaging a third-party advisory firm to help navigate the complexity of the issue. Our team now has enough knowledge to make these adjustments going forward. In addition, we will implement additional layers of review on these transactions.
We are making progress toward the effectiveness of our internal control over financial reporting and disclosure controls and procedures. The measures that we are taking are subject to continued testing, ongoing senior management review, as well as audit committee oversight. We will not be able to conclude whether the measures we are taking will fully remediate these material weaknesses in our internal control over financial reporting until we have completed our remediation efforts and subsequent evaluation of their effectiveness. We may also conclude that additional measures may be required to remediate the material weaknesses in our internal control over financial reporting, which may necessitate additional implementation and evaluation time.
We will continue to assess the effectiveness of our internal control over financial reporting and take steps to remediate the known material weaknesses expeditiously.
Changes in Internal Control Over Financial Reporting
There have been no change in our internal control over financial reporting during the fiscal year ended June 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
88
Item 9B. Other Information
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
None.
89
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
BOARD OF DIRECTORS
The following table sets forth information with respect to our current directors, including their ages as of September 30, 2024. There are no family relationships among any of our directors.
Name |
|
Age |
|
Position(s) |
James McCullough |
|
56 |
|
Chief Executive Officer and Chairman of the Board of Directors |
Fergus Fleming |
|
57 |
|
Chief Technology Officer and Director |
Catherine Coste |
|
58 |
|
Non-Executive Director |
Daniel Levangie |
|
74 |
|
Non-Executive Director |
Erik Lium, Ph.D. |
|
56 |
|
Non-Executive Director |
Christopher Mills |
|
71 |
|
Non-Executive Director |
James McCullough has served as our co-founder and Chief Executive Officer since our inception. Mr. McCullough has leadership experience building emerging technology companies in both the public and private sectors with specific expertise in the life-sciences industry. From 2008 to 2014, he served as chief executive officer of Exosome Diagnostics Inc., a venture backed personalized medicine company developing non-invasive liquid biopsy diagnostics in cancer that was acquired by Bio-Techne Corporation in 2018. From 2018 to 2021, Mr. McCullough also served as a managing partner of Renwick Capital, LLC, a managing consulting firm specializing in assisting emerging healthcare technology companies with strategic planning and business execution. He received his B.A. from Boston University and an MBA from Columbia Business School.
Fergus Fleming has served as our Chief Technology Officer and as a member of our Board since our inception. Since June 2013, Mr. Fleming has served as Managing Director of FF Consulting Limited, where he has provided product development and commercialization support to medical devices and diagnostics companies. While working at FF Consulting Limited, Mr. Fleming has served as Head of Business Development for Oncomark Limited from November 2016 to October 2018, and served in a number of roles at EKF Diagnostics plc. Mr. Fleming has over 30 years of experience in the life sciences sector, including leadership positions with Baxter Healthcare, Boston Scientific and Trinity Biotech plc. Mr. Fleming received a degree in Science from University College Galway, Ireland.
Catherine Coste has served as a member of our Board of Directors since June 2023. Ms. Coste retired from Deloitte and Touche LLP in September 2020, where she was a senior partner and served as one of Deloitte’s life sciences industry executive leaders. She spent 32 years at Deloitte in both corporate and professional services positions leading global finance, internal audit and operations teams. Ms. Coste has served as a director of both Minerva Surgical, Inc. since February 2021, where she serves as Chair of the Audit Committee and as member of the Compensation Committee, and Biomerica, Inc. since August 2020, where she is Chair of the Audit Committee, and serves on the Compensation Committee and the Nominating and Corporate Governance Committee. Ms. Coste also has extensive experience in Sarbanes-Oxley compliance, corporate risk analysis and management, cyber risk assessment, fraud prevention, IT systems analysis and upgrades, internal controls, and corporate governance. Ms. Coste is a Certified Public Accountant. Ms. Coste earned her B.A. in business administration, accounting, from California State University, Hayward.
Daniel J. Levangie has served as a member of our Board of Directors since August 2021. Mr. Levangie is the co-founder of, and has served as manager of, ATON Partners, a private investment firm, since 2013 and as president and CEO of CereVasc, LLC, a medical device company, since September 2018. He has served on the Board of Directors of Exact Sciences Corporation (NASDAQ: EXAS) since 2010. From 2013 through 2017, Mr. Levangie served as president of Insulet Drug Delivery Systems and served as a lead director of Insulet Corporation. Prior to that, Mr. Levangie was chief executive officer of Dune Medical Devices, Inc. and co-founder and managing partner of Constitution Medical Investors, Inc., a Boston-based private investment and product development firm acquired by Roche Diagnostics Corporation in 2013, and held executive management positions with Cytyc Corporation (“Cytyc”) including executive vice president and chief operating officer, chief executive officer and president until the acquisition of Cytyc by Hologic, Inc. in 2007. He served on the board of Hologic from 2007 to 2009. Mr. Levangie received a B.S. in Pharmacy from Northeastern University.
90
Erik Lium, Ph.D. has served as a member of our Board of Directors since November 2018. Since March 2014, Dr. Lium has served in various roles at Mount Sinai, where he is currently the president of Mount Sinai Innovation Partners, and the executive vice president and chief commercial innovation officer of the Mount Sinai Health System. In addition to his service on the board Dr. Lium represents Mount Sinai on several private company boards and previously served as a member of the investment review committee for the Accelerate NY Seed Fund. Prior to joining Mount Sinai, Dr. Lium served in a number of role at the University of California, San Francisco (“UCSF”), including as the Assistant Vice Chancellor of Innovation, Technology & Alliances, Assistant Vice Chancellor of Research and principal investigator for the Bay Area National Science Foundation I-Corps node. Additionally, prior to its acquisition in 2004, Dr. Lium served as President of LabVelocity Inc. Mr. Lium pursued postdoctoral research at UCSF in the laboratory of J. Michael Bishop, M.D., earned a Ph.D. from the Integrated Program in Cellular, Molecular and Biophysical Studies at Columbia University in the laboratory of Dr. Saul J. Silverstein, and holds a B.S. in Biology from Gonzaga University.
Christopher Mills has served as a member of our Board of Directors since our inception. Mr. Mills founded Harwood Capital Management in 2011, a successor company to its former parent company, J.O. Hambro Capital Management, which Mr. Mills co-founded in 1993. Mr. Mills is Chief Executive Officer and Investment Manager of North Atlantic Smaller Companies Investment Trust plc and Chairman and Chief Executive Officer of Harwood Capital Management Ltd. Mr. Mills currently serves on the board of a number of public companies, including EKF Diagnostics plc, Sureserve Group plc, Augean plc and MJ Gleeson plc. Mr. Mills received a B.A. in Business Studies from Guildhall University.
There are no family relationships between any of our executive officers or directors, nor are there any arrangements or understandings with major shareholders, customers, suppliers or others, pursuant to which any executive officer or director was selected as such.
EXECUTIVE OFFICERS
The following table sets forth certain information with respect to our current executive officers, including their ages as of September 30, 2024. There are no family relationships among any of our executive officers.
Name |
|
Age |
|
Position |
James McCullough |
|
56 |
|
Chief Executive Officer and Director |
Fergus Fleming |
|
57 |
|
Chief Technology Officer and Director |
Howard Doran |
|
63 |
|
President |
Michael J. Donovan |
|
70 |
|
Chief Medical Officer |
Joel Jung |
|
66 |
|
Interim Chief Financial Officer |
The biography of Mr. McCullough and Mr. Fleming is set forth above under “Board of Directors.”
Michael J. Donovan, Ph.D., M.D. has served as our Chief Medical Officer since our inception. Since November 2011, Donovan has also served as a Professor of Experimental Pathology and Director of the Biorepository and Pathology core at the Icahn School of Medicine at Mount Sinai. In addition to an academic career at Harvard Medical School and Boston Children’s Hospital, Dr. Donovan has over 20 years’ experience in the biotechnology industry, serving in various senior management roles at Millennium Pharmaceuticals and Incyte Pharmaceuticals. He most recently served as Chief Clinical Officer of Vigilant Biosciences, Inc., Chief Medical Officer of MetaStat, Inc. and Chief Medical Officer of Exosome Diagnostics, Inc. Dr. Donovan received a B.S. in Zoology, an M.S. in Endocrinology and a Ph.D. in Cell and Developmental Biology from Rutgers University. He received his M.D. from the University of Medicine and Dentistry of New Jersey.
Howard Doran was appointed President in April 2024 after previously serving as Chief Business Officer of Renalytix since September 1, 2023. Prior to joining Renalytix, Mr. Doran served as LipoScience, Inc's President and Chief Executive Officer until successful completion of LabCorp’s acquisition of the company in November 2014. Preceding LipoScience, Mr. Doran was President and Chief Operating Officer of Constitution Medical, Inc., an early-stage in vitro diagnostics company and developer of the Bloodhound Fully Integrated Hematology System, which was acquired by Roche Diagnostics, Inc. in July 2013. Prior to Constitution Medical, Mr. Doran was a member of the senior executive team of Hologic, Inc. and served as President of Hologic's Global Diagnostics business. Mr. Doran joined the senior management team of Hologic in 2007 at the time of Hologic's acquisition of Cytyc Corporation, where he had been serving as Senior Vice President and Business Unit Director of Cytyc’s $500 million in vitro diagnostics business. From 1997 through 2007, he was a key member of the management team of Cytyc Corporation during Cytyc’s dramatic growth, serving in a number of senior commercial roles of increasing responsibility including physician sales and marketing, managed care initiatives and laboratory sales and marketing. Mr. Doran holds a Bachelor of Science degree in Management from West Chester University of Pennsylvania.
91
Joel Jung was appointed Interim Chief Financial Officer in May 2024 and previously, Mr. Jung served as Chief Financial Officer at Minerva Surgical, Inc. from July 2020 to February 2024. Mr. Jung served as a financial consultant to several life sciences companies from October 2018 to July 2020. From October 2018 to June 2019, Mr. Jung held various positions at uBiome, Inc., including as Chief Financial Officer from March 2019 to June 2019. Prior to that, Mr. Jung served as the Chief Financial Officer for four companies including Counsyl, Inc. (acquired by Myriad Genetics, Inc.), Bionano Genomics, Inc., AgraQuest, Inc. (acquired by Bayer CropScience), and Celera Corporation. Mr. Jung holds a B.S. Degree in Aeronautical Engineering from Purdue University and an M.B.A. from the Haas School of Business at the University of California, Berkeley.
CORPORATE GOVERNANCE
Audit committee
Our audit committee consists of Catherine Coste, Erik Lium and Daniel Levangie and assists the Board of Directors in overseeing our accounting and financial reporting processes and the audits of our financial statements. The audit committee consists exclusively of members of our board who are financially literate, and each of Ms. Coste and Mr. Levangie is considered an “audit committee financial expert” as defined by applicable SEC rules and has the requisite financial sophistication as defined under the applicable Nasdaq rules and regulations. The board made a qualitative assessment of each of Mr. Levangie’s and Ms. Coste’s level of knowledge and experience based on a number of factors, including their formal education and experience. Our board has determined that all of the members of the audit committee satisfy the “independence” requirements set forth in Rule 10A-3 under the Exchange Act.
Code of Ethics
The Company has adopted a Code of Conduct that applies to all officers, directors and employees including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. The Code of Conduct is available on the Company’s website at www.renalytix.com, under “Governance.” The Company intends to disclose on our website any amendments to, or waivers from, the Code of Conduct that are required to be disclosed pursuant to the disclosure requirements of Item 5.05 of Form 8-K within four business days following the date of the amendment or waiver.
Insider Trading Policies; Hedging and Pledging Prohibition
The Company has adopted insider trading policies and procedures governing the purchase, sale and/or other disposition of our securities by directors, officers and employees that are reasonably designed to promote compliance with insider trading laws, rules and regulations and applicable listing standards of Nasdaq.
Item 11. Executive Compensation.
EXECUTIVE COMPENSATION
Our named executive officers, or NEOs, for the fiscal year ended June 30, 2024, consisting of our principal executive officer, the next two most highly compensated executive officers serving as of June 30, 2024 and two additional individuals form whom disclosure would have been required but for the fact that the individual was not serving as an executive officer as of June 30, 2024 , were:
92
Summary Compensation Table
The following table presents all of the compensation awarded to or earned by or paid to our named executive officers for the fiscal years ended June 30, 2024 and 2023.
Name and Principal |
|
Year |
|
Salary |
|
Option |
|
Non-Equity |
|
All Other |
|
Total |
James McCullough |
|
2024 |
|
509,585 |
|
241,805 |
|
— |
|
28,720 |
(2) |
780,110 |
Chief Executive Officer and Director |
|
2023 |
|
601,271 |
|
— |
|
405,675 |
|
29,375 |
(3) |
1,036,321 |
Fergus Fleming |
|
2024 |
|
310,498 |
|
88,751 |
|
— |
|
26,453 |
(4) |
425,702 |
Chief Technology Officer and Director |
|
|
|
|
|
|
|
|
|
|
|
|
Michael J. Donovan |
|
2024 |
|
328,450 |
|
21,560 |
|
— |
|
30,004 |
(5) |
380,014 |
Chief Medical Officer |
|
|
|
|
|
|
|
|
|
|
|
|
Thomas McLain |
|
2024 |
|
420,649 |
|
257,850 |
|
— |
|
83,182 |
(7) |
761,681 |
Prior President (6) |
|
2023 |
|
450,271 |
|
— |
|
202,500 |
|
9,930 |
(8) |
662,701 |
O. James Sterling |
|
2024 |
|
345,365 |
|
109,377 |
|
— |
|
12,463 |
(10) |
467,205 |
Prior Chief Financial Officer (9) |
|
2023 |
|
426,251 |
|
— |
|
174,735 |
|
22,691 |
(11) |
623,677 |
Narrative to the Summary Compensation Table
We review compensation annually for all employees, including our executives. In setting executive base salaries and bonuses and granting equity incentive awards, we consider compensation for comparable positions in the market, the historical compensation levels of our executives, individual performance as compared to our expectations and objectives, our desire to motivate our employees to achieve short- and long-term results that are in the best interests of our stockholders and a long-term commitment to our company. We do not target a specific competitive position or a specific mix of compensation among base salary, bonus or long-term incentives.
93
The remuneration committee of our board determines our executives’ compensation. Our remuneration committee typically reviews and discusses management’s proposed compensation with the Chief Executive Officer for all executives other than the Chief Executive Officer, which is recommended by our board. Based on those discussions and its discretion, the remuneration committee then recommends the compensation for each executive officer. Our remuneration committee, without members of management present, discusses and approves (or recommends to the Board for determination and approval) the compensation of our executive officers. In 2023, the remuneration committee retained Aon plc., a compensation consulting firm, to evaluate and make recommendations with respect to our executive compensation program.
The table below sets out, for each element of pay, a summary of how remuneration of executive officers is structured and how it supports the Company’s strategy.
Purpose and Link to Strategy |
Operation |
Maximum Opportunity |
Performance Metrics |
Base Salary |
|||
To attract, retain and motivate executive officers of the highest caliber who are capable of delivering the Company’s strategic objectives, reflecting the individual’s experience and role within the Company. Base salary is designed to provide an appropriate level of fixed income to avoid an over-reliance on variable pay elements that could encourage excessive risk taking. |
Salaries are normally reviewed annually, and changes are generally effective from 1 October- The annual salary review of the executive officers takes into consideration a number of factors, including: •
scope of the individual’s responsibilities;
•
abilities, experience and performance of the individual;
•
business performance;
•
salary increases awarded to the overall employee population;
•
market competitiveness and US and UK market practice; and
•
the underlying rate of inflation.
|
Executive officer level salaries are determined considering industry benchmarking data. There is no prescribed maximum annual salary or salary increase. Base salary increases are awarded at the discretion of the Committee; however, the Committee is guided by the general increase for the broader employee population but may decide to award a lower increase for Executive officers or exceed this to recognize, for example, an increase in the scale, scope or responsibility of the role and/or take account relevant market movements. salary increases will normally executive officer level salaries are approved by the Board in line with corporate performance and are consistent with positions held. |
No formal metrics, although any increases take account of Company performance and the individual performance of the executive officer. |
Benefits |
|||
Benefits in kind offered to executive officers are provided on a market- competitive basis, to assist with their recruitment and retention. |
The Company aims to offer benefits that are in line with the executive officers’ local market and those offered to the wider workforce. |
There is no defined maximum value for benefits, but the Committee will consider the aggregate value of any such benefits when determining what should be offered. |
Not performance related. |
Pension / Retirement Benefits |
|||
The Company aims to provide a contribution towards life in retirement. |
Depending on their location and comparable benefits offered to local employees, executive officers may be eligible to receive employer contributions to a defined contribution pension scheme or a cash supplement in lieu of such contributions, or a mixture of both. |
The maximum employer pension contribution or cash in lieu amount will be a percentage of annual base salary aligned with that provided to other senior executives in the executive officer’s location. |
Not performance related. |
94
Purpose and Link to Strategy |
Operation |
Maximum Opportunity |
Performance Metrics |
Annual Bonus |
|||
An annual bonus rewards the achievement of objectives that support the Company’s corporate goals and delivery of the business strategy |
Bonuses are determined based on objectives that are agreed with the Committee, and the Board, at the start of each financial year although the Committee retains the discretion to amend objectives during the year if it considers that objectives are no longer appropriate. Different performance measures and weightings may be used each year, as agreed with the Committee, to take into account changes in the business strategy. Bonuses are normally paid in cash (but may be paid in the form of an equity award, at the discretion of the Committee). |
Executive officer level bonuses are approved by the Board in line with corporate performance and are consistent with positions held. |
Performance measures are determined by the Committee each year and may vary to ensure that they promote the Company’s business strategy and shareholder value. The annual bonus will be based on corporate measures, including, but not limited to, financial and/or strategic measures. Bonus measures are reviewed at least annually and the Committee has the discretion to change the measures or to introduce new measures when it deems appropriate. |
Equity Incentive Plan (“EIP”) |
|||
To attract, motivate, retain and reward for long-term, sustainable performance linked to corporate strategy and provide alignment with shareholders’ interests. |
Equity awards granted to executive officers may take the form of options, restricted shares, performance share units, restricted share units, or other forms of awards granted in accordance with the discretionary EIP that may be in place from time to time. The executive officers received a grant under the EIP’s predecessor plan upon listing on AIM and it is intended that top-up awards shall be issued under the EIP from time to time in the discretion of the Committee.
|
There is no maximum opportunity for equity incentives. However, the Committee will generally assess the position at similar sized comparative companies prior to making any award to ensure that any awards are aligned to the market. |
Vesting of equity awards is generally subject to continued employment and may also be subject to the achievement of performance conditions aligned with the Company’s strategic plan. Measures, their weightings and the period over which performance is tested will be determined by the Committee. The Committee will select the most appropriate form of EIP for awards each year and/or each individual grant. Vesting of equity awards may be accelerated in part or in full in connection with certain corporate events such as a change of control. |
All employee equity plans |
|||
Encourages employee share ownership and therefore increases alignment of interests with shareholders. |
The Company may, from time to time, operate tax- advantaged share plans for which executive officers would be eligible on the same basis as all other eligible employees. |
Within the limits of the relevant legislation. |
Not performance related. |
Annual Performance Bonuses
Each of our executive officers is eligible to receive performance bonus under our annual incentive compensation program. The Committee of the Board determined that while Management made progress in key areas in fiscal year 2024 growing the business, the Company did not achieve 100% of its annual corporate objectives, and therefore no bonuses for company executives will be paid. This outcome was based on achievements versus goals in the following key areas: overachievement in the area of technology/innovation, partial achievement in executive team performance, insurance reimbursement and governance, inclusion, and operations, and underachievement of the revenue target.
95
Retirement Benefits and Other Compensation
Health and Welfare and Retirement Benefits
All of our current named executive officers are eligible to participate in our employee benefit plans, including our medical, dental, vision, disability and life insurance plans, in each case on the same basis as all of our other employees.
401(k) Plan
The Company maintains a defined contribution 401(k) retirement plan which covers all U.S. employees. Employees are eligible after three months of service. Under the 401(k) plan, participating employees may make contributions in an amount up to the limit set by the Internal Revenue Service on an annual basis. The Company has a safe harbor plan and makes contributions to employee accounts of 5% of compensation and increased it to 6% effective January 2024 (as defined by the plan). The Company paid $0.1 million and $0.1 million in contributions for the years ended June 30, 2024 and 2023, respectively.
Outstanding Equity Awards at Fiscal Year End
The following table presents information regarding outstanding stock options held by our named executive officers as of June 30, 2024. None of our named executive officers held restricted stock or other stock awards as of June 30, 2024.
|
|
Option Awards |
||||||||||||||||
Name |
|
Total number of granted options |
|
|
Number of securities underlying unexercised options |
|
|
Equity incentive plan awards: Number of securities underlying unexercised unearned options (#) |
|
|
Option exercise price |
|
|
Option |
||||
James McCullough |
|
|
875,017 |
|
|
|
291,672 |
|
|
|
583,345 |
|
(3) |
$ |
1.30 |
|
(5) |
7/6/2033 |
Fergus Fleming |
|
|
538,161 |
|
|
|
538,161 |
|
|
— |
|
|
$ |
1.53 |
|
(6) |
11/1/2028 |
|
|
|
|
352,902 |
|
|
|
117,636 |
|
|
|
235,266 |
|
(3) |
$ |
1.30 |
|
(5) |
7/6/2033 |
Michael Donovan |
|
|
269,081 |
|
|
|
269,081 |
|
|
— |
|
|
$ |
1.53 |
|
|
11/1/2028 |
|
|
|
|
85,731 |
|
|
|
28,576 |
|
|
|
57,155 |
|
(3) |
$ |
1.30 |
|
(5) |
7/6/2033 |
Thomas McLain |
|
|
538,161 |
|
|
|
538,161 |
|
|
— |
|
|
$ |
2.55 |
|
(7) |
5/31/2029 |
|
|
|
|
200,000 |
|
|
|
200,000 |
|
|
— |
|
|
$ |
6.95 |
|
(8) |
8/28/2030 |
|
|
|
|
500,000 |
|
|
|
500,000 |
|
|
— |
|
|
$ |
11.70 |
|
(9) |
4/15/2031 |
|
|
|
|
394,486 |
|
|
|
230,118 |
|
|
|
164,368 |
|
(4) |
$ |
1.30 |
|
(5) |
7/6/2033 |
|
|
|
200,000 |
|
|
|
116,669 |
|
|
|
83,331 |
|
(4) |
$ |
1.49 |
|
(10) |
7/10/2033 |
O. James Sterling |
|
|
575,160 |
|
|
|
143,790 |
|
|
|
431,370 |
|
(4) |
$ |
1.30 |
|
(5) |
7/6/2033 |
96
Employment Agreements; Potential Payments Upon Termination or Change in Control
The material terms of the severance agreements we have entered into with our NEOs are summarized below.
Employment Agreement of James McCullough
James McCullough, our Chief Executive Officer, is employed by Renalytix AI, Inc., our wholly owned U.S. subsidiary, and entered into an employment agreement with Renalytix AI, Inc. in November 2018. Mr. McCullough also entered into a separate appointment letter with us in October 2018, which governs the terms of his appointment as a director. He receives no compensation or benefits for his service as a director above those that are provided under the employment agreement.
Pursuant to the terms of the employment agreement, Mr. McCullough is entitled to annual base salary, initially $350,000, which is subject to annual review by our remuneration committee and to a minimum annual increase of 3%. Our remuneration committee approved an increase to Mr. McCullough’s annual base salary to $601,000. Under the terms of the employment agreement, Mr. McCullough is also: (1) eligible for an annual cash bonus in the sole discretion of the remuneration committee; (2) entitled to participate on the same basis as similarly situated employees in our benefit plans in effect from time to time during his employment; and (3) entitled to five weeks’ holiday per annum.
Mr. McCullough is employed at-will. If his employment is terminated by us without “Cause,” as defined in the employment agreement, and in circumstances constituting a “separation from service,” as defined in the U.S. Treasury Regulation Section 1.409A-1(h), or by Mr. McCullough with “Good Reason,” as defined in the employment agreement, Mr. McCullough is entitled to be paid his salary and benefits in the usual way up to his termination date and, provided he complies with certain conditions including execution of a release, is entitled to receive the following severance benefits:
In the event that Mr. McCullough’s employment is terminated by us due to his death or “Disability,” as defined in the employment agreement, he is entitled to receive any accrued but unpaid bonus in relation to any prior year’s employment, together with a pro rata bonus in respect of the portion of the then current year worked.
Mr. McCullough has also entered into an employee confidential information and invention assignment agreement with Renalytix AI, Inc., which governs matters related to confidentiality, intellectual property and post- termination covenants. Mr. McCullough is subject to confidentiality obligations which remain in place following termination of employment, and to non-solicitation and non-compete restrictive covenants for a period of 12 months post-termination of his employment.
Employment Agreement of Fergus Fleming
Fergus Fleming, our Chief Technology Officer, is employed by Renalytix AI plc, our wholly owned U.K. subsidiary, and entered into a director's service agreement with Renalytix AI plc in November 2018. Mr. Fleming also entered into a separate appointment letter with us in October 2018, which governs the terms of his appointment as a director. He receives no compensation or benefits for his service as a director above those that are provided under the employment agreement.
Pursuant to the terms of the directors service agreement, Mr. Fleming is entitled to annual base salary, initially £200,000, which is subject to annual review by our remuneration committee. Our remuneration committee approved an increase to Mr. Fleming’s annual base salary to €252,000. Under the terms of the directors service agreement, Mr. Fleming is also: (1) entitled to become a member of any pension scheme operated by his employer, pursuant to which such employer will contribute 5% of the basic salary to the scheme each year that Mr. Fleming remains employed under the directors service agreement, (2) entitled to a car allowance, (3) entitled to participate, at his employer’s expense, in its private medical expenses insurance scheme, (3) entitled to participate in an annual employee bonus scheme, and (4) entitled to 25 working days paid holiday per annum and receive holiday pay between Christmas and New Year.
97
Mr. Fleming’s employment is terminable upon 12 months’ prior written notice. If his employment is terminated by us for certain specified reasons, we may terminate the directors service agreement with immediate effect without payment of compensation.
The directors service agreement also includes provisions which govern matters related to confidentiality, intellectual property and post- termination covenants. Mr. Fleming is subject to confidentiality obligations which remain in place following termination of employment, and to non-solicitation and non-compete restrictive covenants for a period of 12 months post-termination of his employment.
Employment Agreement of Michael J. Donovan
Michael J. Donovan, our Chief Medical Officer, is employed by Renalytix AI, Inc., our wholly owned U.S. subsidiary, and entered into an employment agreement with Renalytix AI, Inc. in December 2020.
Pursuant to the terms of the employment agreement, Mr. Donovan is entitled to annual base salary, initially $304,000, for 80% of his full-time work hours. Under the terms of the employment agreement, Mr. Donovan is also: (1) eligible for an annual bonus subject to the approval of the board of directors and (2) entitled to participate on the same basis as similarly situated employees in our benefit plans in effect from time to time during his employment. Mr. Donovan is employed at-will. If his employment is terminated by us without “Cause,” as defined in the employment agreement, Mr. Donovan is entitled to a severance payment equal to six months of his then-current base salary.
Mr. Donovan has also entered into an employee confidential information and invention assignment agreement with Renalytix AI, Inc., which governs matters related to confidentiality, intellectual property and post-termination covenants. Mr. Donovan is subject to confidentiality obligations which remain in place following termination of employment, and to non-solicitation and non-compete restrictive covenants for a period of 12 months post-termination of his employment.
Severance Agreement with Thomas McLain
Thomas McLain, who previously served as our President until April 30, 2024 (the “McLain Separation Date”), entered into a severance agreement, dated April 17, 2024 (the “McLain Severance Agreement”), with Renalytix AI, Inc., our wholly owned U.S. subsidiary. Pursuant to the McLain Severance Agreement, Mr. McLain received (i) his base salary for a period of six months following the McLain Separation Date (the “Severance Period”); (ii) contributions to the cost of health care continuation under the COBRA benefits until the earliest of (a) April, 2025, (b) the date Mr. McLain becomes eligible for substantially equivalent health insurance coverage in connection with new employment or self-employment, or (c) the date that Mr. McLain ceases to be eligible for COBRA continuation coverage for any reason including plan termination; (iii) a pro rata portion of his annual bonus for the fiscal year ending June 30, 2024, (iv) with respect to those stock options that are already vested as of the McLain Separation Date, the ability to exercise those stock options through the original term of the applicable stock option and (v) accelerated vesting of a portion of Mr. McLain's outstanding stock options equal to the number of stock options that would have vested if he had continued to be employed by the Company for 12 months following the McLain Separation Date, which will become vested as of the McLain Separation Date. Mr. McLain’s receipt of these benefits is contingent upon Mr. McLain's continued compliance with ongoing obligations provided for under the McLain Severance Agreement, including non-disparagement obligations and a general release of claims.
Employment Agreement with O. James Sterling
O. James Sterling, who previously served as our Chief Financial Officer until May 24, 2024, was employed by Renalytix AI, Inc., our wholly owned U.S. subsidiary, and entered into an employment agreement with Renalytix AI, Inc. in October 2018. Pursuant to the terms of the employment agreement, Mr. Sterling was entitled to annual base salary, initially $275,000, which is subject to annual review by our remuneration committee and to a minimum annual increase of 3%. Our remuneration committee approved an increase to Mr. Sterling’s annual base salary to $423,600. Under the terms of the employment agreement, Mr. Sterling is also: (1) eligible for an annual cash bonus in the sole discretion of the remuneration committee and (2) entitled to five weeks’ holiday per annum.
Mr. Sterling was employed at-will. On May 24, 2024, Mr. Sterling tendered his resignation from his position as Chief Financial Officer of the Company. Mr. Sterling remained with the Company through a transition period ending June 10, 2024.
Mr. Sterling is also party to an employee confidential information and invention assignment agreement with Renalytix AI, Inc., which governs matters related to confidentiality, intellectual property and post-termination covenants. Mr. Sterling is subject to confidentiality obligations which remain in place following termination of employment, and to non-solicitation and non-compete restrictive covenants for a period of 12 months post-termination of his employment.
98
Director Compensation
The table below sets out, for each element of pay, a summary of how remuneration of non-executive directors is structured and how it supports the Company’s strategy.
Purpose and Link to Strategy |
Operation |
Maximum Opportunity |
Performance Metrics |
Cash fees and benefits |
|||
Set at a level that is sufficient to attract and retain high caliber non-executives who contribute to the business. |
The Chair and the Non-Executive Directors receive fees paid in cash. Fees are paid and reviewed annually.
Non-Executive Directors ordinarily do not participate in any pension, bonus or performance-based share incentive plans. Travel, accommodation and other business-related expenses incurred in carrying out the role as well as fees for tax advice associated with completion of international tax returns will be paid by the Company including, if relevant, any gross-up for tax.
Tax equalization and/or relocation benefits may be provided to Non-Executive Directors who are required to relocate or become tax resident in a new jurisdiction. |
When reviewing fee levels and benefits, account is taken of market movements in the fees and benefits of Non-Executive Directors, Board Committee responsibilities and ongoing time commitments.
Actual fee levels are disclosed in the annual Directors’ Remuneration Report for the relevant financial year.
|
Not performance related. |
Equity-based awards |
|||
To facilitate share ownership and provide alignment with shareholders. |
Non-Executive Directors may receive equity awards under any equity incentive plan operated by the Company from time to time which permits their participation with careful consideration being given to ensuring their independence. Non-Executive Directors may receive an initial equity award upon appointment or election. Initial equity awards will normally vest over a specified period of time, subject generally to continued service. Vesting of equity awards may be accelerated in part or in full in connection with certain corporate events such as a change of control. In addition, Non-Executive Directors may be granted an equity award each year which may vest in full upon grant or over time subject to continued service. If |
There is no maximum number of equity incentive awards that may be awarded to individuals each year. However, when reviewing award levels, account is taken of market movements in equity incentive awards, Board committee responsibilities, ongoing time commitments and the general economic environment. |
Non-executive directors do not participate in performance based equity incentives. |
99
Purpose and Link to Strategy |
Operation |
Maximum Opportunity |
Performance Metrics |
|
a new Non- Executive Director joins the Board following the date of grant of this annual grant in any calendar year, such Non- Executive Director may be granted a pro rata portion of the next annual grant to reflect his or her service during the relevant part of the relevant year. |
|
|
We have historically provided our non-employee directors with an annual cash retainer of $14,096, which is the same for each non-employee director regardless of leadership position or committee membership.
2024 Director Compensation Table
The following table sets forth information regarding the compensation earned for service on our Board during the year ended June 30, 2024 by our non-employee directors. James McCullough, our Chief Executive Officer, and Fergus Fleming, our Chief Technology Officer, serve on the Board but do not receive any compensation for service as a director. Information about compensation for Messrs. McCullough and Fleming during the year ended June 30, 2024 is set forth above under “Summary Compensation Table.”
Name |
|
Fees Earned |
|
|
Option |
|
All Other |
|
Total ($) |
|
||
Catherine Coste |
|
|
68,145 |
|
|
— |
|
— |
|
|
68,145 |
|
Daniel J. Levangie |
|
|
65,019 |
|
|
— |
|
— |
|
|
65,019 |
|
Erik Lium, Ph.D. (1) |
|
— |
|
|
— |
|
— |
|
— |
|
||
Chirag R. Parikh, Ph.D., M.D. (2) |
|
|
11,357 |
|
|
— |
|
— |
|
|
11,357 |
|
Timothy Scannell (3) |
|
|
14,385 |
|
|
— |
|
— |
|
|
14,385 |
|
Christopher Mills |
|
— |
|
|
— |
|
— |
|
— |
|
||
The following table provides information regarding the aggregate number of option awards granted to our non-employee directors that were outstanding as of June 30, 2024:
Name |
|
|
|
|
Daniel J. Levangie |
|
|
135,000 |
|
Erik Lium, Ph.D. (1) |
|
|
204,501 |
|
Christopher Mills |
|
— |
|
|
Chirag R. Parikh, Ph.D., M.D. (2) |
|
|
115,724 |
|
Timothy Scannell (3) |
|
|
40,000 |
|
Catherine Coste |
|
|
285,000 |
|
In addition, each of the non-executive directors is entitled to be reimbursed for reasonable and properly documented expenses incurred in performing their duties as a director.
100
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The following table sets forth certain information regarding the ownership of the Company’s ordinary shares (and our ADSs, each of which represents 2 ordinary shares) as of September 20, 2024 by: (i) each director; (ii) each of the executive officers named in the Summary Compensation Table; (iii) all current executive officers and directors of the Company as a group; and (iv) all those known by the Company to be beneficial owners of more than five percent of its ordinary shares.
We have determined beneficial ownership in accordance with the rules and regulations of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Except as indicated by the footnotes below, we believe, based on information furnished to us, that the persons and entities named in the table below have sole voting and sole investment power with respect to all shares that they beneficially own, subject to applicable community property laws.
This table is based upon information supplied by officers, directors and principal stockholders. Applicable percentage ownership is based on 165,925,513 ordinary shares, including ordinary shares in the form of ADSs, outstanding as of September 20, 2024. In computing the number of shares beneficially owned by a person and the percentage ownership of such person, we include all shares subject to options held by the person that are currently exercisable, or would be exercisable or would vest based on service-based vesting conditions as of November 19, 2024, which is 60 days after September 20, 2024. However, except as described above, we do not deem such shares outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the address for each beneficial owner listed in the table below is c/o Renalytix plc 2 Leman Street, London, United Kingdom.
Name of Beneficial Owner |
|
Number of Shares Beneficially Owned (1) |
|
|
Percentage of Shares Beneficially Owned |
|
|
||
5% Stockholders: |
|
|
|
|
|
|
|
||
Icahn School Of Medicine At Mount Sinai (2) |
|
|
23,979,726 |
|
|
|
14.5 |
|
% |
Jefferson River Capital LLC (3) |
|
|
8,533,280 |
|
|
|
5.1 |
|
% |
Polar Capital (4) |
|
|
13,866,061 |
|
|
|
8.4 |
|
% |
Pentwater Capital Management LP (5) |
|
|
8,095,187 |
|
|
|
4.9 |
|
% |
Nathaniel Philip Victor James Rothschild (6) |
|
|
5,788,078 |
|
|
|
3.5 |
|
% |
Directors and Named Executive Officers: |
|
|
|
|
|
|
|
||
James McCullough (7) |
|
|
3,038,058 |
|
|
|
1.8 |
|
% |
Fergus Fleming (8) |
|
|
1,225,278 |
|
|
|
0.7 |
|
% |
Michael Donovan (9) |
|
|
297,657 |
|
|
* |
|
|
|
Catherine Coste (10) |
|
|
63,333 |
|
|
* |
|
|
|
Daniel Levangie (11) |
|
|
30,000 |
|
|
* |
|
|
|
Erik Lium, Ph.D. |
|
— |
|
|
* |
|
|
||
Christopher Mills (12) |
|
|
14,072,500 |
|
|
|
8.5 |
|
% |
All directors and current executive officers as a group (7 persons) (13) |
|
|
18,726,826 |
|
|
|
11.0 |
|
% |
* Represents beneficial ownership of less than 1%.
101
Equity Compensation Plan Information
The following table provides certain information with respect to all of the Company’s equity compensation plans in effect as of June 30, 2024.
Plan Category |
|
(a) Number of securities |
|
|
(b) Weighted-average |
|
|
(c) Number of securities |
|
|||
Equity compensation plans approved |
|
|
7,451,626 |
|
|
$ |
3.07 |
|
|
|
21,020,692 |
|
Equity compensation plans not |
|
— |
|
|
— |
|
|
— |
|
|||
Total |
|
|
7,451,626 |
|
|
$ |
3.07 |
|
|
|
21,020,692 |
|
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Transactions With Related Persons
Related-Person Transactions Policy and Procedures
We have adopted a policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the approval or ratification of our Board or our Audit Committee. Any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock or any member of the immediate family of any of the foregoing persons, in which the amount involved exceeds $120,000 and such person would have a direct or indirect interest, must be presented to our Board or our Audit Committee for review, consideration and approval. In approving or rejecting any such proposal, our Board or our Audit Committee is to consider the material facts of the transaction, including whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
102
The related person transactions policy also covers related party transactions under the AIM Rules for Companies published by the London Stock Exchange, or the AIM Rules, which contains a different definition of a related party to the definition of a related person set out above for U.S. purposes. The AIM Rules require that any transaction with a related party (pursuant to the definition in the AIM Rules) that exceeds 5% in any of the class tests set out in the AIM Rules, taking into account certain provisions relating to aggregation of transactions, should be announced without delay as soon as the terms of the transaction are agreed, and that the announcement should include certain specified information including a statement that our directors (with the exception of any director who is involved in the transaction as a related party) consider, having consulted with our nominated adviser for AIM, that the terms of the transaction are fair and reasonable insofar as our stockholders are concerned.
Certain Related-Person Transactions
Except as described below, there have been no transactions since July 1, 2021 to which we have been a participant in which the amount involved exceeded or will exceed $120,000, and in which any of our directors, executive officers or holders of more than five percent of our capital stock, or any members of their immediate family, had or will have a direct or indirect material interest, other than compensation arrangements that are described under “Executive Compensation” and “Director Compensation.” For a description of severance and change in control arrangements that we have entered into with some of our executive officers, see “Executive Compensation—Potential Payments upon Termination or Change in Control.”
2023 Placement
In February 2023, we issued and sold an aggregate of 3,699,910 ordinary shares at a price of £0.90 per ordinary share and 7,511,525 American Depositary Shares ("ADSs"), at a price of $2.17 per ADS. The private placement generated gross cash proceeds of $20.3 million. The following table summarizes the number of ordinary shares purchased by related persons.
Stockholder |
|
Ordinary Shares |
|
|
Subscription Price ($) |
|
|
Gross Proceeds to Company ($) |
|
|||
Icahn School of Medicine at Mount Sinai(1) |
|
|
2,764,978 |
|
|
|
3,000,001.13 |
|
|
|
3,000,001.13 |
|
Oryx(2) |
|
|
32,794 |
|
|
|
35,417.52 |
|
|
|
35,417.52 |
|
NASCIT(2) |
|
|
313,581 |
|
|
|
338,667.48 |
|
|
|
338,667.48 |
|
2024 Placement
On March 12, 2024, the Company entered into the Placing Agreement with Stifel, pursuant to which the Company agreed to allot and issue the Placing Shares to the Placees in the Private Placement, up to an aggregate of 46,801,872 ordinary shares. On March 12, 2024, the Company announced that it successfully placed 46,801,872 ordinary shares with both UK and U.S. institutional investors, at a price of £0.20 per ordinary share, raising aggregate gross proceeds of approximately $12 million for the Company (see Note 12).
ISMMS subscribed for a total 9,360,374 ordinary shares at £0.20 per ordinary share in the Private Placement.
Christopher Mills, Non-Executive Chairman, and his related parties subscribed for a total of 4,000,000 ordinary shares at £0.20 per ordinary share in the Private Placement.
Relationship Agreement, by and among Renalytix plc, the Icahn School of Medicine at Mount Sinai and NPLUS1 Singer Advisory LLP
On October 30, 2018, we, Mount Sinai and NPLUS1 Singer Advisory LLP, or Singer, entered into a Relationship Agreement, or the Relationship Agreement, to regulate the terms of the relationship between us and Mount Sinai and to ensure that we can operate independently of Mount Sinai, pursuant to which, among other things, Mount Sinai has the right to appoint one member to our Board of Directors and Mount Sinai has agreed to not take any action intended to prevent our Board of Directors from operating independently of Mount Sinai.
103
The Relationship Agreement was effective from November 6, 2018, the date of admission of our ordinary shares to trading on AIM.
Pursuant to the Relationship Agreement and for so long as (i) Mount Sinai shall hold as beneficial owner 5% by nominal value of our issued ordinary shares and (ii) our ordinary shares are admitted to trading on AIM, Mount Sinai agreed among other things, to:
The Relationship Agreement provides that any respective dispute between us and Mount Sinai relating to our management, the operation of the Board of Directors or any transaction, agreement or arrangement with Mount Sinai shall be passed to, and dealt with on our behalf by, a committee comprising only of independent directors following consultation with Singer.
The obligations of the parties under the Relationship Agreement shall automatically terminate upon:
Pursuant to the Relationship Agreement, we agreed to appoint a representative designated by Mount Sinai to the Board of Directors as a non-executive director, and further the right to appoint a board observer. In connection therewith, Mount Sinai appointed Erik Lium to the Board of Directors. Mount Sinai’s right to maintain a representative on the Board of Directors and the right to appoint an observer at Board of Director meetings shall continue for so long as Mount Sinai continues to beneficially hold not less than 5% by nominal value of our issued ordinary shares.
The Relationship Agreement is filed with this Annual Report on Form 10-K as Exhibit 10.16 and is incorporated herein by reference, and the foregoing description of the Relationship Agreement is qualified in its entirety by reference thereto.
Indemnity Agreements
In July 2020, we entered into deeds of indemnity with each of our directors and executive officers in connection with the listing of our ADSs on Nasdaq. The deeds of indemnity and our articles of association require us to indemnify our directors and executive officers to the fullest extent permitted by law.
Independence of The Board of Directors
As required under the Nasdaq Stock Market (“Nasdaq”) listing standards, a majority of the members of a listed company’s Board of Directors must qualify as “independent,” as affirmatively determined by the Board of Directors. The Board consults with the Company’s counsel to ensure that the Board’s determinations are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of Nasdaq, as in effect from time to time.
Consistent with these considerations, after review of all relevant identified transactions or relationships between each director, or any of his or her family members, and the Company, its senior management and its independent auditors, the Board has affirmatively determined that the following four directors are independent directors within the meaning of the applicable Nasdaq listing standards: Catherine Coste, Daniel Levangie, Erik Lium, and Christopher Mills. In making this determination, the Board found that none of these directors or nominees for director had a material or other disqualifying relationship with the Company. James McCullough and Fergus Fleming are not independent based on their employment with the Company as executive officers.
104
Item 14. Principal Accounting Fees and Services.
The following table represents aggregate fees billed to the Company for the fiscal years ended June 30, 2024 and 2023 by CohnReznick and Ernst & Young LLP, Iselin, New Jersey (PCAOB ID: 42), respectively, the Company’s principal accountant.
|
|
Fiscal Year Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
|
|
(in thousands) |
|
|||||
Audit Fees |
|
$ |
564 |
|
|
$ |
465 |
|
Audit-Related Fees |
|
— |
|
|
— |
|
||
Tax Fees |
|
— |
|
|
— |
|
||
All Other Fees |
|
— |
|
|
— |
|
||
Total Fees |
|
$ |
564 |
|
|
$ |
465 |
|
Audit fees consist of fees billed for professional services provided in connection with the audit of our annual financial statements, the review of our quarterly financial statements, and audit services that are normally provided by the independent registered public accounting firm in connection with regulatory filings.
All fees described above were pre-approved by the Audit Committee.
Pre-Approval Policies and Procedures
The Audit Committee has adopted a policy and procedures for the pre-approval of audit and non-audit services rendered by the Company’s independent registered public accounting firm, CohnReznick and Ernst & Young LLP, as applicable. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services and tax services up to specified amounts. Pre-approval may also be given as part of the Audit Committee’s approval of the scope of the engagement of the independent auditor or on an individual, explicit, case-by-case basis before the independent auditor is engaged to provide each service. The pre-approval of services may be delegated to one or more of the Audit Committee’s members, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
The Audit Committee has determined that the rendering of services other than audit services by CohnReznick and Ernst & Young LLP, as applicable, is compatible with maintaining the principal accountant’s independence for the applicable period.
105
PART IV
Item 15. Exhibits, Financial Statement Schedules.
The report of our independent registered public accounting firm and Consolidated Financial Statements listed in the Index to Consolidated Financial Statements herein are filed as part of this Annual Report on Form 10-K.
There are no financial statement schedules filed as part of this this Annual Report on Form 10-K, since the required information is included in the financial statements, including the notes thereto, included in “Item 8. Financial Statements and Supplementary Data” or the circumstances requiring inclusion of such schedules are not present.
We have filed, or incorporated by reference into this Annual Report on Form 10-K, the exhibits set forth on the accompanying Exhibit Index immediately preceding the Index to Consolidated Financial Statements.
|
|
Incorporation by Reference |
|||
Exhibit No. |
Description |
Schedule/ |
File Number |
Exhibit |
File Date |
|
|
|
|
|
|
1.1 |
At The Market Offering Agreement by and between Renalytix plc and H.C. Wainwright & Co. LLC |
8-K |
001-39387 |
1.1 |
5/15/2024 |
3.1 |
10-Q |
001-39387 |
3.1 |
2/14/2024 |
|
4.1 |
|
|
|
|
|
4.2 |
F-1/A |
333-239414 |
4.1 |
7/13/2020 |
|
4.3 |
Form of American Depositary Receipt (included in Exhibit 4.1) |
F-1/A |
333-239414 |
4.1 |
7/13/2020 |
4.4 |
20-F |
001-39387 |
4.3 |
10/28/2020 |
|
10.1+ |
Renalytix plc Share Option Plan for Employees with Non-Employee Sub-Plan and U.S. Sub-Plan |
F-1 |
333-239414 |
10.1 |
6/24/2020 |
10.2†# |
F-1 |
333-239414 |
10.2 |
6/24/2020 |
|
10.3†# |
F-1 |
333-239414 |
10.3 |
6/24/2020 |
|
10.4+ |
F-1 |
333-239414 |
10.6 |
6/24/2020 |
|
10.5+ |
F-1 |
333-239414 |
10.7 |
6/24/2020 |
|
10.6* |
10-K |
001-39387 |
10.6 |
9/28/2023 |
|
10.7* |
10-K |
001-39387 |
10.7 |
9/28/2023 |
|
10.8* |
10-K |
001-39387 |
10.8 |
9/28/2023 |
|
10.9* |
10-K |
001-39387 |
10.9 |
9/28/2023 |
|
10.10* |
10-K |
001-39387 |
10.10 |
9/28/2023 |
|
10.11+ |
Form of Amended Deed of Indemnity between registrant and each of its directors |
F-1 |
333-239414 |
10.8 |
6/24/2020 |
10.12+ |
Form of Deed of Indemnity between registrant and each of its executive officers |
F-1 |
333-239414 |
10.9 |
6/24/2020 |
10.13 |
F-1 |
333-239414 |
10.1 |
6/24/2020 |
|
106
|
|
Incorporation by Reference |
|||
Exhibit No. |
Description |
Schedule/ |
File Number |
Exhibit |
File Date |
10.14 |
6-K |
001-39387 |
99.4 |
2/8/2023 |
|
10.15 |
20-F |
001-39387 |
10.11 |
10/31/2022 |
|
10.16* |
10-K |
001-39387 |
10.16 |
9/28/2023 |
|
10.17+ |
10-Q |
001-39387 |
10.2 |
11/14/2023 |
|
10.18 |
Placing Agreement, dated March 12, 2024, between Renalytix plc and Stifel Nicolaus Europe Limited |
8-K |
001-39387 |
10.1 |
3/13/2024 |
10.19 |
Bond Amendment, dated March 28, 2024, between Renalytix plc and CVI Investments, Inc. |
8-K |
001-39387 |
10.1 |
3/29/2024 |
10.20 |
8-K |
001-39387 |
10.1 |
4/9/2024 |
|
10.21 |
Separation Agreement, dated April 17, 2024, between Renalytix AI, Inc. and Tom McLain |
8-K |
001-39387 |
10.2 |
4/23/2024 |
10.22 |
8-K |
001-39387 |
10.1 |
4/23/2024 |
|
10.23+ |
Consulting Agreement, dated as of June 7, 2024, between Renalytix AI, Inc. and Joel R. Jung |
8-K |
001-39387 |
10.1 |
6/12/2024 |
21.1* |
|
|
|
|
|
23.1* |
Consent of Ernst & Young LLP, independent registered public accounting firm (PCAOB ID No. 42) |
|
|
|
|
23.2* |
Consent of CohnReznick LLP, independent registered public accounting firm |
|
|
|
|
24.1* |
|
|
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
|
32.1** |
|
|
|
|
|
101.INS* |
Inline XBRL Instance Document |
|
|
|
|
101.SCH* |
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
|
|
|
|
104 |
Cover Page Interactive Data File (formatted as inline XBRL and contained in Exhibit 101) |
|
|
|
|
* Filed herewith.
** Furnished herewith.
+ Indicates a management contract or any compensatory plan, contract or arrangement.
† Certain portions of this exhibit will be omitted because they are not material and would likely cause competitive harm to the registrant if disclosed.
# Certain exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant hereby undertakes to furnish supplementally a copy of any omitted exhibit or schedule upon request by the Securities and Exchange Commission.
Item 16. Form 10-K Summary
Not applicable.
107
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
RENALYTIX PLC |
|
|
|
|
|
|
|
By: |
/s/ James McCullough |
|
|
Name: |
James McCullough |
|
|
Title: |
Chief Executive Officer |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints James McCullough and Joel R. Jung, each acting alone, his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-facts and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitutes or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Name |
|
Title |
|
Date |
|
|
|
|
|
/s/ James McCullough |
|
Chief Executive Officer and Chairman of the Board |
|
September 30, 2024 |
James McCullough |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Joel R. Jung |
|
Interim Chief Financial Officer |
|
September 30, 2024 |
Joel R. Jung |
|
(Principal Financial Officer and Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Fergus Fleming |
|
Chief Technology Officer and Director |
|
September 30, 2024 |
Fergus Fleming |
|
|
|
|
|
|
|
|
|
/s/ Catherine Coste |
|
Director |
|
September 30, 2024 |
Catherine Coste |
|
|
|
|
|
|
|
|
|
/s/ Daniel J. Levangie |
|
Director |
|
September 30, 2024 |
Daniel J. Levangie |
|
|
|
|
|
|
|
|
|
/s/ Erik Lium |
|
Director |
|
September 30, 2024 |
Erik Lium, Ph.D. |
|
|
|
|
|
|
|
|
|
/s/ Christopher Mills |
|
Director |
|
September 30, 2024 |
Christopher Mills |
|
|
|
|
|
|
|
|
|
108
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of independent registered public accounting firm (PCAOB ID: 596) |
F-2 |
|
|
F-4 |
|
|
|
Consolidated statements of operations and comprehensive loss |
F-5 |
|
|
F-6 |
|
|
|
F-7 |
|
|
|
F-8 |
F-1
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of
Renalytix plc
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Renalytix plc (the “Company”) as of June 30, 2024, and the related consolidated statements of operations and comprehensive loss, shareholders’ equity (deficit), and cash flows for the year ended June 30, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of June 30, 2024, and the results of its operations and its cash flows for the year ended June 30, 2024, in conformity with accounting principles generally accepted in the United States of America.
The Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the entity will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the entity has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the entity’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the entity's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ CohnReznick LLP
We have served as the Company's auditor since June 2024.
New York, New York
September 30, 2024
F-2
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of Renalytix plc
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Renalytix plc (the Company) as of June 30, 2023, the related consolidated statements of operations and comprehensive loss, shareholders’ equity, and cash flows for the year in the period ended June 30, 2023, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at June 30, 2023, and the results of its operations and its cash flows for the year in the period ended June 30, 2023, in conformity with U.S. generally accepted accounting principles.
The Company's Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has suffered recurring losses and negative cash flows from operations, expects to incur additional losses and require substantial additional capital to fund its operations, and has stated that substantial doubt exists about the Company’s ability to continue as a going concern. Management's evaluation of the events and conditions and management’s plans regarding these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor from 2021 to 2024.
Iselin, New Jersey
September 28, 2023
F-3
RENALYTIX PLC
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data) |
|
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Assets |
|
|
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
$ |
4,680 |
|
|
$ |
24,682 |
|
Accounts receivable |
|
|
|
|
722 |
|
|
|
776 |
|
Prepaid expenses and other current assets |
|
|
|
|
716 |
|
|
|
1,424 |
|
Total current assets |
|
|
|
|
6,118 |
|
|
|
26,882 |
|
Property and equipment, net |
|
|
|
|
216 |
|
|
|
1,027 |
|
Right-of-use asset |
|
|
|
|
— |
|
|
|
159 |
|
Investment in VericiDx |
|
|
|
|
698 |
|
|
|
1,460 |
|
Other Assets |
|
|
|
|
940 |
|
|
|
1,101 |
|
Total assets |
|
|
|
$ |
7,972 |
|
|
$ |
30,629 |
|
|
|
|
|
|
|
|
|
|
||
Liabilities and Shareholders’ Equity (Deficit) |
|
|
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
|
|
||
Accounts payable |
|
|
|
$ |
1,590 |
|
|
$ |
1,485 |
|
Accounts payable – related party |
|
|
|
|
1,018 |
|
|
|
1,451 |
|
Accrued expenses and other current liabilities |
|
|
|
|
3,354 |
|
|
|
6,644 |
|
Accrued expenses – related party |
|
|
|
|
1,329 |
|
|
|
1,963 |
|
Current lease liability |
|
|
|
|
45 |
|
|
|
130 |
|
Convertible notes-current |
|
|
|
|
4,159 |
|
|
|
4,463 |
|
Total current liabilities |
|
|
|
|
11,495 |
|
|
|
16,136 |
|
Convertible notes-noncurrent |
|
|
|
|
4,331 |
|
|
|
7,485 |
|
Noncurrent lease liability |
|
|
|
|
— |
|
|
|
41 |
|
Total liabilities |
|
|
|
|
15,826 |
|
|
|
23,662 |
|
|
|
|
|
|
|
|
|
|
||
Commitments and contingencies (Note 10) |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Shareholders’ equity (deficit): |
|
|
|
|
|
|
|
|
||
Ordinary shares, £0.0025 par value per share: 161,842,057 shares |
|
|
|
|
478 |
|
|
|
286 |
|
Additional paid-in capital |
|
|
|
|
204,893 |
|
|
|
186,456 |
|
Accumulated other comprehensive loss |
|
|
|
|
(1,443 |
) |
|
|
(1,450 |
) |
Accumulated deficit |
|
|
|
|
(211,782 |
) |
|
|
(178,325 |
) |
Total shareholders’ equity (deficit) |
|
|
|
|
(7,854 |
) |
|
|
6,967 |
|
Total liabilities and shareholders’ equity (deficit) |
|
|
|
$ |
7,972 |
|
|
$ |
30,629 |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-4
RENALYTIX PLC
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
|
|
Twelve Months Ended |
|
|
Twelve Months Ended |
|
||
(in thousands, except share and per share data) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Revenue |
|
$ |
2,289 |
|
|
$ |
3,403 |
|
Cost of revenue |
|
|
2,133 |
|
|
|
2,683 |
|
Gross profit |
|
|
156 |
|
|
|
720 |
|
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
|
9,290 |
|
|
|
14,298 |
|
General and administrative |
|
|
19,751 |
|
|
|
28,662 |
|
Impairment loss on property, equipment and other long-lived assets |
|
|
723 |
|
|
|
— |
|
Performance of contract liability to affiliate |
|
|
— |
|
|
|
(19 |
) |
Total operating expenses |
|
|
29,764 |
|
|
|
42,941 |
|
Loss from operations |
|
|
(29,608 |
) |
|
|
(42,221 |
) |
|
|
|
|
|
|
|
||
Equity in net losses of affiliate |
|
|
— |
|
|
|
(9 |
) |
Foreign currency gain, net |
|
|
163 |
|
|
|
358 |
|
Fair value adjustment to VericiDx investment |
|
|
(505 |
) |
|
|
(1,282 |
) |
Fair value adjustment to convertible notes |
|
|
(3,751 |
) |
|
|
(3,107 |
) |
Other income, net |
|
|
249 |
|
|
|
656 |
|
Net loss before income taxes |
|
|
(33,452 |
) |
|
|
(45,605 |
) |
Income tax expense |
|
|
(4 |
) |
|
|
(2 |
) |
Net loss |
|
|
(33,456 |
) |
|
|
(45,607 |
) |
|
|
|
|
|
|
|
||
Net loss per ordinary share—basic |
|
$ |
(0.31 |
) |
|
$ |
(0.55 |
) |
Net loss per ordinary share—diluted |
|
$ |
(0.31 |
) |
|
$ |
(0.55 |
) |
Weighted average ordinary shares—basic |
|
|
108,179,366 |
|
|
|
82,210,050 |
|
Weighted average ordinary shares—diluted |
|
|
108,179,366 |
|
|
|
82,210,050 |
|
|
|
|
|
|
|
|
||
Other comprehensive income (loss): |
|
|
|
|
|
|
||
Changes in the fair value of the convertible notes |
|
|
305 |
|
|
|
(337 |
) |
Foreign exchange translation adjustment |
|
|
(298 |
) |
|
|
(198 |
) |
Comprehensive loss |
|
$ |
(33,449 |
) |
|
$ |
(46,142 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
RENALYTIX PLC
|
|
Ordinary shares |
|
|
Additional |
|
|
Accumulated |
|
|
Accumulated |
|
|
Total |
|
|||||||||
(in thousands, except share data) |
|
Shares |
|
|
Amount |
|
|
capital |
|
|
loss |
|
|
deficit |
|
|
equity (deficit) |
|
||||||
Balance at June 30, 2022 |
|
|
74,760,432 |
|
|
$ |
228 |
|
|
$ |
164,012 |
|
|
$ |
(915 |
) |
|
$ |
(132,718 |
) |
|
$ |
30,607 |
|
Shares issued under the Securities Purchase Agreement |
|
|
18,722,960 |
|
|
|
57 |
|
|
|
19,248 |
|
|
|
— |
|
|
|
— |
|
|
|
19,305 |
|
Shares issued under the employee share purchase plan |
|
|
298,086 |
|
|
|
1 |
|
|
|
260 |
|
|
|
— |
|
|
|
— |
|
|
|
261 |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
2,936 |
|
|
|
— |
|
|
|
— |
|
|
|
2,936 |
|
Changes in the fair value of the convertible notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(337 |
) |
|
|
— |
|
|
|
(337 |
) |
Currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(198 |
) |
|
|
— |
|
|
|
(198 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
(45,607 |
) |
|
|
(45,607 |
) |
|
Balance at June 30, 2023 |
|
|
93,781,478 |
|
|
|
286 |
|
|
|
186,456 |
|
|
|
(1,450 |
) |
|
|
(178,325 |
) |
|
|
6,967 |
|
Shares issued under the Securities Purchase Agreement, net of offering costs |
|
|
50,801,873 |
|
|
|
161 |
|
|
|
11,656 |
|
|
|
— |
|
|
|
— |
|
|
|
11,817 |
|
Shares issued for repayment of convertible bond |
|
|
9,523,972 |
|
|
|
30 |
|
|
|
4,978 |
|
|
|
— |
|
|
|
— |
|
|
|
5,008 |
|
Vesting of RSUs |
|
|
185,540 |
|
|
|
1 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1 |
|
Shares issued under the employee share purchase plan |
|
|
75,328 |
|
|
|
— |
|
|
|
93 |
|
|
|
— |
|
|
|
— |
|
|
|
93 |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
1,710 |
|
|
|
— |
|
|
|
— |
|
|
|
1,710 |
|
Changes in the fair value of the convertible notes |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
305 |
|
|
|
— |
|
|
|
305 |
|
Currency translation adjustments |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(298 |
) |
|
|
— |
|
|
|
(298 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(33,456 |
) |
|
|
(33,456 |
) |
Balance at June 30, 2024 |
|
|
154,368,191 |
|
|
$ |
478 |
|
|
$ |
204,893 |
|
|
$ |
(1,443 |
) |
|
$ |
(211,782 |
) |
|
$ |
(7,854 |
) |
The accompanying notes are an integral part of these consolidated financial statements.
F-6
RENALYTIX PLC
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands) |
|
Year Ended |
|
|
Year Ended |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(33,456 |
) |
|
$ |
(45,607 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
364 |
|
|
|
508 |
|
Impairment loss on property, equipment and other long-lived assets |
|
|
723 |
|
|
|
— |
|
Stock-based compensation |
|
|
1,710 |
|
|
|
2,932 |
|
Equity in losses of affiliate |
|
|
— |
|
|
|
9 |
|
Reduction of Kantaro liability |
|
|
— |
|
|
|
(55 |
) |
Fair value adjustment to VericiDx investment |
|
|
505 |
|
|
|
1,282 |
|
Unrealized foreign exchange gain |
|
|
— |
|
|
|
(1,008 |
) |
Realized loss on sale of ordinary shares in VericiDx |
|
|
135 |
|
|
|
— |
|
Realized foreign exchange gain |
|
|
(132 |
) |
|
|
— |
|
Fair value adjustment to convertible debt, net interest paid |
|
|
3,502 |
|
|
|
1,999 |
|
Non cash lease expense |
|
|
67 |
|
|
|
106 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable |
|
|
54 |
|
|
|
125 |
|
Prepaid expenses and other current assets |
|
|
798 |
|
|
|
1,299 |
|
Receivable from affiliates |
|
|
— |
|
|
|
75 |
|
Accounts payable |
|
|
106 |
|
|
|
80 |
|
Accounts payable – related party |
|
|
(433 |
) |
|
|
368 |
|
Accrued expenses and other current liabilities |
|
|
(3,419 |
) |
|
|
3,397 |
|
Accrued expenses – related party |
|
|
(635 |
) |
|
|
451 |
|
Deferred revenue |
|
|
— |
|
|
|
(46 |
) |
Net cash used in operating activities |
|
|
(30,111 |
) |
|
|
(34,085 |
) |
|
|
|
|
|
|
|
||
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchase of equipment |
|
|
(4 |
) |
|
|
— |
|
Net cash used in investing activities |
|
|
(4 |
) |
|
|
— |
|
|
|
|
|
|
|
|
||
Cash flows from financing activities: |
|
|
|
|
|
|
||
Payment of convertible notes principal |
|
|
(1,660 |
) |
|
|
(3,180 |
) |
Proceeds from issuance of ordinary shares in Private Placement |
|
|
13,533 |
|
|
|
20,296 |
|
Payment of offering costs |
|
|
(1,716 |
) |
|
|
(991 |
) |
Proceeds from the issuance of ordinary shares under employee share |
|
|
93 |
|
|
|
261 |
|
Net cash provided by financing activities |
|
|
10,250 |
|
|
|
16,386 |
|
Effect of exchange rate changes on cash |
|
|
(137 |
) |
|
|
1,048 |
|
Net decrease in cash and cash equivalents |
|
|
(20,002 |
) |
|
|
(16,651 |
) |
Cash and cash equivalents, beginning of year |
|
|
24,682 |
|
|
|
41,333 |
|
Cash and cash equivalents, end of year |
|
$ |
4,680 |
|
|
$ |
24,682 |
|
Supplemental noncash investing and financing activities: |
|
|
|
|
|
|
||
Noncash lease liabilities arising from obtaining right-of-use assets |
|
$ |
— |
|
|
$ |
265 |
|
Cash paid for interest on convertible debt |
|
$ |
249 |
|
|
$ |
— |
|
Issuance of shares for debt repayment |
|
$ |
(5,008 |
) |
|
$ |
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
F-7
RENALYTIX PLC
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business and risks
Renalytix PLC and its wholly-owned subsidiaries, the “Company” or "Renalytix," is an artificial intelligence-enabled in vitro diagnostics company, focused on optimizing clinical management of kidney disease to drive improved patient outcomes and significantly lower healthcare costs. KidneyIntelX, the Company’s first-in-class diagnostic platform, employs a proprietary artificial intelligence-enabled algorithm that combines diverse data inputs, including validated blood-based biomarkers, inherited genetics and personalized patient data from EHR systems, to generate a unique patient risk score. Additionally, the Company plans to pursue collaborations with pharmaceutical companies and make ‘Pharmaceutical Services Revenue’ a core part of the business going forward with the goal of improving guideline-based standard-of-care for optimal utilization of existing and novel therapeutics using the KidneyIntelX testing platform and proprietary care management software.
Since inception in March 2018, the Company has focused primarily on organizing and staffing the Company, raising capital, developing the KidneyIntelX platform, conducting clinical validation studies for KidneyIntelX, establishing and protecting its intellectual property portfolio and commercial laboratory operations, pursuing regulatory clearance and developing a reimbursement strategy. The Company has funded its operations primarily through equity and debt financings.
The Company is subject to risks and uncertainties common to early-stage companies in the diagnostics industry, including, but not limited to, ability to secure additional capital to fund operations, compliance with governmental regulations, development by competitors of new technological innovations, dependence on key personnel and protection of proprietary technology. To achieve widespread usage, KidneyIntelX and additional diagnostic products currently under development will require extensive clinical testing and validation prior to regulatory approval and commercialization. These efforts require significant amounts of additional capital, adequate personnel, and infrastructure and extensive compliance-reporting capabilities.
2. Liquidity and Going Concern
The Company has incurred recurring losses and negative cash flows from operations since inception and had an accumulated deficit of $211.8 million as of June 30, 2024. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant sales of KidneyIntelX or any future products currently in development.
As a result of the Company's losses and its projected cash needs, substantial doubt exists about the Company’s ability to continue as a going concern. Substantial additional capital will be necessary to fund the Company's operations, expand its commercial activities and develop other potential diagnostic related products. The Company plans to seek additional funding through public or private equity offerings, debt financings, other collaborations, strategic alliances and licensing arrangements. The Company may not be able to obtain financing on acceptable terms, or at all, and the Company may not be able to enter into strategic alliances or other arrangements on favorable terms, or at all. The terms of any financing may adversely affect the holdings or the rights of the Company’s shareholders. If the Company is unable to obtain funding it could be required to delay, curtail or discontinue research and development programs, product portfolio expansion or future commercialization efforts, which could adversely affect its business prospect.
The Company’s ability to continue as a going concern is contingent upon successful execution of management’s intended plan over the next 12 months to improve the Company’s liquidity and profitability, which includes, without limitation:
The consolidated financial statements do not include any adjustments that may result from the outcome of this going concern uncertainty.
F-8
3. Basis of presentation and summary of significant accounting policies
The accompanying consolidated financial statements have been prepared in conformity with generally accepted accounting principles in the United States (“U.S. GAAP”). Any reference in these notes to applicable guidance is meant to refer to U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”).
Principles of consolidation
The consolidated financial statements include the accounts of Renalytix plc and its wholly-owned subsidiaries. All inter-company balances and transactions have been eliminated in consolidation. The Company accounts for investments in which it has significant influence, but not a controlling financial interest, using the equity method of accounting.
Use of estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. Actual results could differ from those estimates. Due to the uncertainty of factors surrounding the estimates or judgments used in the preparation of the consolidated financial statements, actual results may materially vary from these estimates.
Estimates and assumptions are periodically reviewed, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined to be necessary. Significant areas that require management’s estimate include the assumptions used in determining the fair value of share-based awards, determining the fair value of the bonds, recording the prepaid/accrual and associated expense for research and development activities performed for the Company by third parties and determining useful lives of property and equipment and capitalized software.
Segment information
The Company manages its operations as a single operating segment for the purposes of assessing performance and making operating decisions. The Company’s singular focus is to make significant improvements in kidney disease diagnosis and prognosis, clinical care, patient stratification for drug clinical trials, and drug target discovery.
Foreign currency
The Company’s consolidated financial statements are presented in U.S. dollars, the reporting currency of the Company. The functional currency of Renalytix plc and Renalytix AI Limited is GB Pounds. The functional currency of Renalytix AI, Inc. is the U.S. dollar. Assets and liabilities of Renalytix plc and Renalytix AI Limited are translated at the rate of exchange at period-end, while the consolidated statements of operations and comprehensive loss are translated at the weighted average exchange rates in effect during the reporting period. The net effect of these translation adjustments is shown as a component of accumulated other comprehensive loss. Transaction gains and losses resulting from exchange rate changes on transactions denominated in currencies other than the functional currency are included in income in the period in which the change occurs and reported in the consolidated statements of operations and comprehensive loss.
Concentrations of credit risk and major customers
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents and accounts receivable balances. Periodically, the Company maintains deposits in accredited financial institutions in excess of federally insured limits. The Company deposits its cash and cash equivalents in financial institutions that it believes have high credit quality and are not exposed to any unusual credit risk beyond the normal credit risk associated with commercial banking relationships and has not experienced any losses on such accounts.
The Company’s accounts receivable related to testing services are derived from revenue earned from customers located in the U.S. For the year ended June 30, 2024, approximately 69% of all receivables related to KidneyIntelX testing revenue related to two customers and the remaining 31% of receivables were due from other third-party payors. For the year ended June 30, 2023, approximately 70% of all receivables related to KidneyIntelX testing revenue related to one customer, approximately 19% of receivables were due from other party payors and approximately 11% of receivables outstanding related to pharmaceutical services performed for one customer. The Company performs initial and ongoing credit reviews on its customers, which involve consideration of the customers’ financial information, their location, and other factors to assess the customers’ ability to pay and reserved for $0.1 million of receivables for the year ended June 30, 2024.
F-9
Fair value of financial instruments
At June 30, 2024 and 2023, the Company’s financial instruments included accounts receivable, prepaid expenses and other current assets, accounts payable, accrued expenses and other current liabilities. The carrying amounts of these assets and liabilities approximates fair value due to their short-term nature. The convertible notes are recorded at their estimated fair value.
Fair value option
Under the Fair Value Option Subsections of ASC subtopic 825-10, Financial Instruments – Overall, the Company has the irrevocable option to report most financial assets and financial liabilities at fair value on an instrument-by-instrument basis, with changes in fair value reported in earnings (see Note 5). The Company has elected to measure and record the convertible notes at their estimated fair value.
Cash and cash equivalents
The Company considers all highly-liquid investments purchased with an original maturity of 90 days or less to be cash equivalents. As of June 30, 2024 and 2023, the Company had a cash balance of $4.7 million and $24.7 million, respectively.
Accounts receivable
Accounts receivable are recorded at the invoice amount and are non-interest bearing. The Company considers receivables past due based on the contractual payment terms. The Company reserves specific receivables if collectability is no longer reasonably assured. Estimates for credit losses are determined based on existing contractual obligations, historical payment patterns, and individual customer circumstances. The Company reserved for $0.1 million and $0.1 million of receivables as of June 30, 2024 and 2023, respectively.
Property, equipment and other long-lived assets
Property and equipment are recorded at cost. Depreciation is determined using the straight-line method over the estimated useful lives ranging from three to ten years. Expenditures for maintenance and repairs are expensed as incurred while renewals and betterments are capitalized. When property and equipment are sold or otherwise disposed of, the cost and related accumulated depreciation are eliminated from the accounts and any resulting gain or loss is reflected in operations. In November 2023, the Company consolidated lab operations, which resulted in a $0.3 million impairment of property and equipment at the Company's Utah lab. In February 2024, the Company performed a recoverability assessment and determined the entire $0.1 million right-of-use asset related to the Utah lease to be impaired; in addition, the Company further consolidated lab operations, which resulted in a $0.3 million impairment of property and equipment at the Company's Florida lab. The Company recorded a $0.7 million impairment of property, equipment and other long-lived assets for the year ended June 30, 2024. There was no impairment loss on property, equipment and other long-lived assets in year ended June 30, 2023.
Performance of contract liability to affiliate
In May 2020, the Company and the Icahn School of Medicine at Mount Sinai entered into an operating agreement (“Kantaro Operating Agreement”) to form a joint venture, Kantaro Biosciences LLC (“Kantaro”), for the purpose of developing and commercializing laboratory tests for the detection of antibodies against SARS-CoV-2 originally developed by Mount Sinai. Kantaro has a fiscal year end of December 31st. Kantaro has partnered with Bio-Techne Corporation to develop and launch the new test which is designed for use in any authorized clinical testing laboratory without the need for proprietary equipment. On December 31, 2022, the members and managers of Kantaro decided that it was in the best interest of Kantaro to wind up the Kantaro business. As part of the termination agreement, the members agreed that Renalytix has no further liability to perform services on behalf of Kantaro. During the years ended June 30, 2024 and 2023, the Company recognized $0 and $0.01 million, respectively, related to the performance of the contract liability with Kantaro. This represents the allocation of costs for performing services on behalf of Kantaro.
Investments
VericiDx plc
The Company accounts for its ownership of VericiDx securities at fair value in accordance with ASC 321, Investments-Equity Securities, with changes in fair value recorded in earnings as the fair value of VericiDx's ordinary shares are readily determinable via the London Stock Exchange. Based on closing stock price of VericiDx, the fair value of the investment in VericiDx was $0.7 million and $1.5 million at June 30, 2024 and 2023, respectively.
F-10
In March 2024, the Company sold 750,000 ordinary shares of VericiDx for net proceeds of $0.1 million and a realized loss of $0.1 million. In May 2024, the Company sold 250,000 ordinary shares of VericiDx for net proceeds of $0.02 million and a realized loss of $0.04 million. The Company did not sell any shares during the year ended June 30, 2023. During the years ended June 30, 2024 and 2023, the Company recorded a decrease in fair value of $0.5 million and $1.3 million, respectively, in the consolidated statements of operations and comprehensive loss. The Company owned 3.7% and 5.8% of the ordinary shares of VericiDx at June 30, 2024 and 2023, respectively.
Impairment assessment
The Company evaluates its investments that are in unrealized loss positions, if any, and equity method investments for other-than-temporary impairment on a quarterly basis (see Note 5). Such evaluation involves a variety of considerations, including assessments of the risks and uncertainties associated with general economic conditions and distinct conditions affecting specific issuers or investees. Factors considered by the Company include (i) the length of time and the extent to which an investment’s fair value has been below its cost; (ii) the financial condition, credit worthiness, and near-term prospects of the issuer; (iii) the length of time to maturity; (iv) future economic conditions and market forecasts; (v) the Company’s intent and ability to retain its investment for a period of time sufficient to allow for recovery of market value; (vi) an assessment of whether it is more likely than not that the Company will be required to sell its investment before recovery of market value; and (vii) whether events or changes in circumstances indicate that the investment’s carrying amount might not be recoverable.
Software development costs
The Company follows the provisions of ASC 985, Software, which requires software development costs for software marketed externally to be expensed as incurred until the establishment of technological feasibility, at which time those costs are capitalized until the software is available for general release and are amortized over its estimated useful life of ten years. For the years ended June 30, 2024 and 2023, there was no capitalization of research and development expenses related to software development to record. Technological feasibility is established upon the completion of a working model that has been validated.
Revenue recognition
Pursuant to ASC 606, Revenue from Contracts with Customers, the Company recognizes revenue when a customer obtains control of promised goods or services. The Company records the amount of revenue that reflects the consideration that it expects to receive in exchange for those goods or services. The Company applies the following five-step model in order to determine this amount: (i) identification of the promised goods or services in the contract; (ii) determination of whether the promised goods or services are performance obligations, including whether they are distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation.
The Company only applies the five-step model to contracts when it is probable that it will collect the consideration to which it is entitled in exchange for the goods or services that it transfers to the customer. Once a contract is determined to be within the scope of ASC 606 at contract inception, the Company reviews the contract to determine which performance obligations it must deliver and which of these performance obligations are distinct. Certain contracts have options for the customer to acquire additional services. The Company evaluates these options to determine if a material right exists. If, after that evaluation, the Company determines a material right does exist, it assigns value to the material right based upon the renewal option approach. The Company recognizes as revenue the amount of the transaction price that is allocated to each performance obligation when that performance obligation is satisfied or as it is satisfied. The Company uses the present right to payment principle and customer acceptance as indicators to determine the transfer of control to the customer occurs at a point in time. Sales tax and other similar taxes are excluded from revenues.
Cost of revenue
Cost of revenue consists of costs directly attributable to the services rendered, including labor, rent, lab consumables, depreciation, amortization and sample collection costs directly related to revenue generating activities.
Research and development expenses
Research and development costs consist primarily of internal and external labor costs incurred in connection with the development of KidneyIntelX as well as expenses related to studies and clinical trials to further the clinical value, performance and utility of KidneyIntelX. Research and development costs are expensed as incurred.
F-11
Share-based compensation
The Company measures equity classified share-based awards granted to employees and nonemployees based on the estimated fair value on the date of grant and recognizes compensation expense of those awards over the requisite service period, which is the vesting period of the respective award. The Company accounts for forfeitures as they occur. For share-based awards with service-based vesting conditions, the Company recognizes compensation expense on a straight-line basis over the service period. The fair value of each stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model, which requires inputs based on certain subjective assumptions, including the expected stock price volatility, the expected term of the option, the risk-free interest rate for a period that approximates the expected term of the option, and the Company’s expected dividend yield. The Company was a privately-held organization prior to November 2018 and has been a publicly-traded company for a limited period of time and therefore lacks company-specific historical and implied volatility information for its shares. Accordingly, the Company estimates its expected share price volatility based on the historical volatility of publicly-traded peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded share price. The expected term of the Company’s stock options has been determined utilizing the “simplified” method for awards that qualify as “plain-vanilla” options. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. Expected dividend yield is none based on the fact that the Company has never paid cash dividends on ordinary shares and does not expect to pay any cash dividends in the foreseeable future.
The Company classifies share-based compensation expense in its consolidated statement of operations and comprehensive loss in the same manner in which the award recipient’s payroll costs are classified or in which the award recipient’s service payments are classified.
Income taxes
Income taxes are accounted for under the asset and liability method as required by FASB ASC Topic 740, Income Taxes ("ASC 740"). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A reduction in the carrying value of the deferred tax assets is required when it is not more likely than not that such deferred tax assets are realizable.
FASB ASC Subtopic 740-10, Accounting for Uncertainty of Income Taxes (ASC "740-10"), defines the criterion an individual tax position must meet for any part of the benefit of the tax position to be recognized in financial statements prepared in conformity with U.S. GAAP. The Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not such tax position will be sustained on examination by the taxing authorities, based solely on the technical merits of the respective tax position. The tax benefits recognized in the financial statements from such a tax position should be measured based on the largest benefit having a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority. In accordance with disclosure requirements of ASC 740-10, the Company’s policy on income statement classification of interest and penalties related to income tax obligations is to include such items as part of income tax expense.
Comprehensive loss
Comprehensive loss includes net loss as well as other changes in shareholders’ equity that result from transactions and economic events other than those with shareholders. For the periods presented, changes in shareholders’ equity include foreign currency translation as well as changes in fair value of the convertible note due to changes in instrument-specific credit risk. The change in instrument-specific credit risk was calculated as the change in the risk yield from the convertible debt issuance date to the valuation date. The instrument-specific credit risk at issuance date was calibrated such that the fair value of the convertible bond was equal to the issue price as of the issuance date. The risk yield was adjusted to reflect the change in credit spreads between the issuance date and the valuation date.
Net loss per ordinary share
Basic net loss per ordinary share is computed by dividing net loss by the weighted average number of ordinary shares outstanding during each period. Diluted net loss per ordinary share includes the effect, if any, from the potential exercise or conversion of securities, such as options and convertible debt which would result in the issuance of incremental ordinary shares.
F-12
The dilutive effect of convertible securities is calculated using the if-converted method. Under the if-converted method, interest charges applicable to the convertible debt as well as nondiscretionary adjustments which include any expenses or charges that are determined based on the income (loss) for the period are added back to net income. The convertible debt is assumed to have been converted at the beginning of the period (or at time of issuance, if later). For the year ended June 30, 2024, under the if-converted method, the add back of nondiscretionary adjustments and inclusion of potentially converted shares would be anti-dilutive.
Emerging growth company
The Company is an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”). Under the JOBS Act, companies have extended transition periods available for complying with new or revised accounting standards. The Company has elected to avail itself of this exemption and, therefore, while the Company is an emerging growth company, it will not be subject to new or revised accounting standards at the same time that they become applicable to other public emerging growth companies that have not elected to avail themselves of this exemption.
Recently issued accounting pronouncements
In June 2016, the FASB issued ASU 2016-13, Financial Instruments—Credit Losses: Measurement of Credit Losses on Financial Instruments, which requires measurement and recognition of expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. This is different from the current guidance as this will require immediate recognition of estimated credit losses expected to occur over the remaining life of many financial assets. The Company implemented ASU 2016-13 in the fiscal year beginning July 1, 2023 and evaluated the impact of ASU 2016-13 and it did not have a material impact on the consolidated financial statements.
In August 2020, the FASB issued ASU 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging - Contracts in Entity’s Own Equity (Subtopic 815-40), Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”). ASU 2020-06 eliminates two of the three models in ASC 470-20 that require issuers to separately account for embedded conversion features and eliminates some of the requirements for equity classification in ASC 815-40-25 for contracts in an entity’s own equity. The guidance also requires entities to use the if-converted method for all convertible instruments in the diluted earnings per share calculation and generally requires them to include the effect of potential share settlement for instruments that may be settled in cash or shares. It is effective for annual periods beginning after December 15, 2023, and interim periods therein. The Company evaluated the effect ASU 2020-06 and it is not expected to have a material impact on the consolidated financial statements.
In November 2023, the Financial Accounting Standards Board (the “FASB”) issued Accounting Standards Update (“ASU”) No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (“ASU 2023-07”). ASU 2023-07 expands public entities’ segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. All disclosure requirements under ASU2023-07 are also required for public entities with a single reportable segment. The guidance is effective for the fiscal year ending June 30, 2025, and subsequent interim periods, with early adoption permitted. The Company is currently evaluating the impact of adopting this standard on its consolidated financial statements and disclosures.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which provides for improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. This guidance is effective for annual periods beginning after December 15, 2024, and the adoption of this standard is not anticipated to have a significant impact on the Company’s consolidated financial statements other than adding new disclosures, which the Company is currently evaluating.
4. Revenue
Testing services revenue
Each individual test is a performance obligation that is satisfied at a point in time upon completion of the testing process (when results are reported), which is when control passes to the customer and revenue is recognized. During the years ended June 30, 2024 and 2023, the Company recognized $2.1 million and $3.1 million, respectively, of testing services revenue. Sales tax and other similar taxes are excluded from revenue.
F-13
Pharmaceutical services revenue
Pharmaceutical services revenue is generated from the provision of analytical services to customers. Contracts with customers generally include an initial upfront payment and additional payments upon achieving performance milestones. The Company uses the present right to payment principle and customer acceptance as indicators to determine the transfer of control to the customer, which may occur at a point in time or over time depending on the individual contract terms. Sales tax and other similar taxes are excluded from revenue.
During the years ended June 30, 2024 and 2023, the Company recognized $0.14 million and $0.3 million, respectively, of pharmaceutical services revenue where performance obligations are satisfied at a point in time.
Deferred revenue
Deferred revenue represents the allocated transaction price to the material right which will be recognized as revenue when the renewal options are exercised which is expected to occur over the next six months.
The following table summarizes the changes in deferred revenue:
(in thousands) |
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Balance, beginning of period |
|
$ |
— |
|
|
$ |
46 |
|
Deferral of revenue |
|
|
— |
|
|
|
— |
|
Revenue recognized |
|
|
— |
|
|
|
(46 |
) |
Balance, end of period |
|
$ |
— |
|
|
$ |
— |
|
5. Fair value measurements and the fair value option
Assets and liabilities recorded at fair value on a recurring basis in the consolidated balance sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or an exit price that would be paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The authoritative guidance on fair value measurements establishes a three-tier fair value hierarchy for disclosure of fair value measurements as follows:
This hierarchy requires the use of observable market data when available and to minimize the use of unobservable inputs when determining fair value. The following fair value hierarchy table presents information about the Company’s assets measured at fair value on a recurring basis:
|
|
Fair value measurement at |
|
|||||||||
|
|
reporting date using |
|
|||||||||
(in thousands) |
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|||
June 30, 2024 |
|
|
|
|
|
|
|
|
|
|||
Assets: |
|
|
|
|
|
|
|
|
|
|||
Equity Securities |
|
$ |
698 |
|
|
$ |
— |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|||
Convertible notes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
8,490 |
|
June 30, 2023 |
|
|
|
|
|
|
|
|
|
|||
Assets: |
|
|
|
|
|
|
|
|
|
|||
Equity Securities |
|
$ |
1,460 |
|
|
$ |
— |
|
|
$ |
— |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|||
Convertible notes |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,948 |
|
F-14
The Company accounts for its ownership of VericiDx securities at fair value in accordance with ASC 321, Investments-Equity Securities, with changes in fair value recorded in earnings as the fair value of VericiDx's ordinary shares is readily determinable via the London Stock Exchange. As of June 30, 2024 and 2023, the Company owns 8,831,682 and 9,831,681 shares of VericiDx, respectively. Based on closing stock price of VericiDx, the fair value of the investment in VericiDx was $0.7 million and $1.5 million at June 30, 2024 and 2023, respectively.
As further described in Note 8, in April 2022 the Company issued convertible promissory notes (the “Notes”) to various investors. The fair value option, as prescribed by ASC 815, Derivatives and Hedging, was elected and applied in connection with the preparation of these consolidated financial statements. The fair value of the Notes is determined using a scenario-based analysis that estimates the fair value based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to the noteholders.
The Company adjusts the carrying value of the Notes to their estimated fair value at each reporting date, with qualifying increases or decreases in the fair value recorded as change in fair value of convertible promissory notes in the statements of operations and comprehensive loss. Changes in the fair value resulting from changes in the instrument-specific credit risk are presented separately in other comprehensive income.
(in thousands) |
|
June 30, 2024 |
|
|
Balance at the beginning of the year |
|
$ |
11,948 |
|
Change due to payment of principal and interest |
|
|
(6,161 |
) |
Fair value adjustments |
|
|
(305 |
) |
Change in credit risk |
|
|
2,972 |
|
FX Impact |
|
|
36 |
|
Balance at June 30, 2024 |
|
$ |
8,490 |
|
Non-financial assets and liabilities
The Company’s non-financial assets, which primarily consist of property and equipment, are not required to be measured at fair value on a recurring basis, and instead are reported at carrying value in its consolidated balance sheets. However, on a periodic basis or whenever events or changes in circumstances indicate that they may not be fully recoverable, the respective carrying values of non-financial assets are assessed for impairment and, if ultimately considered impaired, are adjusted and written down to their fair value, as estimated based on consideration of external market participant assumptions.
6. Property and equipment, net and intangibles
Property and equipment consists of the following:
(in thousands) |
|
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Lab equipment |
|
|
|
$ |
388 |
|
|
$ |
1,142 |
|
Office equipment |
|
|
|
|
127 |
|
|
|
124 |
|
Office furniture |
|
|
|
|
— |
|
|
|
35 |
|
Leasehold improvements |
|
|
|
|
— |
|
|
|
576 |
|
Total |
|
|
|
|
515 |
|
|
|
1,877 |
|
Less accumulated depreciation |
|
|
|
|
(299 |
) |
|
|
(850 |
) |
|
|
|
|
$ |
216 |
|
|
$ |
1,027 |
|
Depreciation expense was $0.2 million and $0.3 million for the years ended June 30, 2024 and 2023, respectively.
Software consists of:
(in thousands) |
|
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Software |
|
|
|
$ |
1,527 |
|
|
$ |
1,526 |
|
Less accumulated amortization |
|
|
|
|
(658 |
) |
|
|
(476 |
) |
|
|
|
|
$ |
869 |
|
|
$ |
1,050 |
|
F-15
As of June 30, 2024 and 2023, there was $0.9 million and $1.1 million, respectively, of unamortized costs of software development and purchased software. Amortization expense related to capitalized software development costs was $0.1 million and $0.2 million for the years ended June 30, 2024 and 2023, respectively, and was expensed within cost of revenue in the consolidated statements of operations and comprehensive loss.
As of June 30, 2024, the expected amortization expense for the next five years and thereafter is as follows:
(in thousands) |
|
|
|
|
2025 |
|
$ |
181 |
|
2026 |
|
|
142 |
|
2027 |
|
|
126 |
|
2028 |
|
|
126 |
|
Thereafter |
|
|
294 |
|
|
|
$ |
869 |
|
7. Accrued expenses and other current liabilities
Accrued expenses and other current liabilities consisted of the following:
(in thousands) |
|
|
|
June 30, 2024 |
|
|
June 30, 2023 |
|
||
Consulting and professional fees |
|
|
|
$ |
1,109 |
|
|
$ |
442 |
|
Research and development |
|
|
|
|
892 |
|
|
|
1,657 |
|
Payroll and related benefits |
|
|
|
|
388 |
|
|
|
3,866 |
|
License and royalty expense |
|
|
|
|
787 |
|
|
|
669 |
|
Other |
|
|
|
|
178 |
|
|
|
10 |
|
|
|
|
|
$ |
3,354 |
|
|
$ |
6,644 |
|
8. Convertible Notes
In April 2022, the Company issued amortizing senior convertible bonds with a principal amount of $21.2 million in amortizing senior convertible bonds due in April 2027 (the "Bonds") to CVI Investments, Inc. (the "Convertible Bond Investor"). The Bonds were issued at 85% par value with total net proceeds of $18.0 million and accrue interest at an annual rate of 5.5%, payable quarterly in arrears, in cash or American Depositary Shares ("ADSs") valued at the ADS Settlement Price at the option of the Company. The principal and interest payments are due in equal quarterly installments starting in July 2022. The Bonds contain various conversion and redemption features. The initial conversion price for the Bonds of $8.70 has been set at a 20 percent premium to the Reference ADS Price. The Conversion Price may reset down at 12, 24 and 36 months, depending on share price performance, and the Bonds have a hard floor in the conversion price of $7.25. As a result of the February 2023 private placement and pursuant to conditions of the bond agreement, the conversion price was adjusted to $8.2508 (previously $8.70) and the floor price was adjusted to $6.8757 (previously $7.25). Further, pursuant to conditions of the agreement, effective April 7, 2023, the conversion price was adjusted from $8.2508 to $7.7924. Between amortization dates, the Convertible Bond Investor retains the right to advance future amortization payments, provided that (a) there shall be no amortization advancements during the first 12 months, (b) no more than two amortization advancements may occur in any 12-month period, and (c) no more than one amortization advancement may occur in any 3-month period. On March 28, 2024, the Company entered into a second amendment and restatement agreement with the Convertible Bond Investor, which amended the terms of the Company’s existing bond agreement, dated March 31, 2022. The Bond Agreement Amendment amends the existing bond agreement to, among other things:
F-16
The Company performed an analysis and determined that the financial impact was immaterial as the amended and restated agreement was not substantially different than the previous agreement.
The Convertible Bond Investor is also permitted to defer up to two amortization payments to a subsequent amortization date. The Company retains the option to repay any deferred amortization in cash at 100 percent of the nominal amount. In July 2023, the Company made a cash amortization payment of $1.4 million, which consisted of $1.1 million of principal and $0.3 million of interest. Also in July 2023, the Convertible Bond Investor exercised its right to advance an amortization payment and the Company made an accelerated repayment of $1.1 million through the issuance of 526,211 ADSs. In October 2023, the Company made an amortization payment of $1.3 million, which consisted of $1.1 million of principal and $0.2 million of interest, through the issuance of 2,335,388 ordinary shares in the form of 150,000 ordinary shares and 1,092,694 ADSs. In December 2023, the Company made an amortization payment of $1.3 million, which consisted of $1.1 million of principal and $0.2 million of interest, through the issuance of 2,500,000 ordinary shares and a cash payment of $0.6 million. In April 2024, the Company made an amortization payment of $1.3 million, which consisted of $1.1 million of principal and $0.2 million of interest, through the issuance of 3,636,162 ordinary shares. As of June 30, 2024 and 2023, $12.7 million and $18.0 million, respectively, of principal was outstanding.
On issuance, the Company elected to account for the Bonds at fair value in accordance with ASC 815, Derivatives and Hedging, with qualifying changes in fair value being recognized through the statements of operations and comprehensive loss until the Bonds are settled. Changes in fair value related to instrument-specific credit risk are recognized through comprehensive loss until the Bonds are settled. The fair value of the bonds is determined using a scenario-based analysis that estimates the fair value based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to the noteholders. Significant assumptions used in the fair value analysis include the volatility rate, risk-free rate, dividend yield and risky yield. The fair value of the Bonds was determined to be $16.9 million on issuance, which is the principal amount of the Bonds. As of June 30, 2024, the fair value of the Bonds was determined to be $8.5 million. During the year ended June 30, 2024, the Company recognized a $0.3 million increase in fair value of the Notes related to the instrument-specific credit risk in comprehensive loss and a decrease in fair value related to noninstrument-specific credit risk of $3.8 million as loss in the consolidated statement of operations and comprehensive loss, respectively. The Company recognized a decrease in fair value of the Notes related to the instrument-specific credit risk of $0.3 million in the comprehensive loss and a decrease in fair value related to noninstrument-specific credit risk of $3.1 million as a loss in the consolidated statement of operations and comprehensive during the year ended June 30, 2023.
9. Leases
The Company leases certain office space and laboratory space. At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present. The Company does not recognize right-of-use assets or lease liabilities for leases determined to have a term of 12 months or less. Many of the Company's leases contain variable non-lease components such as maintenance, taxes, insurance, and similar costs for the spaces it occupies.
Variable executory costs, as it relates to net leases, are excluded from the calculation of the lease liability. Variable executory costs include costs relating to utilities, repairs, maintenance, insurance, common area expenses, and taxes paid for the leased asset during its economic life.
Upon adoption of ASC 842, the Company elected the package of practical expedients and the hindsight practical expedient but did not elect the easement practical expedient which is not applicable to the Company as the Company does not have any ground leases. In accordance with the package of practical expedients, the Company has not reassessed any of its existing or expired contracts or any other agreements that were previously concluded to not contain a lease for the following practical expedient guidance: (1) whether the arrangement is or contains a lease, (2) lease classification and (3) whether previously capitalized costs continue to qualify as initial direct costs.
The Company leased lab space in Salt Lake City, UT, under a five-year lease, the term of which commenced in November 2019. The Company has measured its right-of-use assets and lease liabilities based on lease terms ending in October 2024. Due to the Company consolidating its operations and moving out of the space, the Company performed a recoverability assessment and determined the entire $0.1 million right-of-use asset to be impaired and recorded a loss within the impairment loss on property, equipment, and other long-lived assets line of the statement of operations and comprehensive loss for the year ended June 30, 2024.
F-17
The Company leased lab space in New York City, NY, under an initial three-month lease, the term of which commenced in February 2019. The Company has classified this lease as a short-term lease as the Company concluded that the noncancelable terms of this lease was less than one year at the commencement and none of the Company's renewals or amendments were for additional noncancelable terms greater than one year.
The Company leased lab space in St. Petersburg, FL, under an initial one-year term, the term of which commenced in January 2022. The Company has classified this lease as a short-term lease as the Company concluded that the noncancelable terms of this lease was less than one year at the commencement and none of the Company's renewals or amendments were for additional noncancelable terms greater than one year. The St. Petersburg, FL, lease expired in the quarter ended March 31, 2024.
The Company leased office space in New York City, NY, under an initial month-to-month term, the term of which commenced in June 2018. The lease did not have termination or formal renewal options; however, the Company can renew their office space if they are still needed and are still available at the end of the term. The Company has classified this lease as a short-term lease as the Company concluded that the noncancelable terms of this lease was less than one year at the commencement and none of the Company's renewals or amendments were for additional noncancelable terms greater than one year.
The Company identified and assessed the following significant assumptions in recognizing its right-of-use assets and corresponding lease liabilities during the adoption of ASC 842:
As the Company's leases do not provide an implicit rate, it concluded that a 10.0% IBR, the approximate midpoint between the average commercial real estate loans during 2022, is an appropriate discount rate to use for the Utah lease, which was the only lease existing as of the adoption date.
The following table shows the lease balance sheet classification of leases for the year ended June 30, 2024:
(in thousands) |
|
June 30, 2024 |
|
|
Assets |
|
|
|
|
Operating lease right-of-use assets, net of accumulated amortization |
|
$ |
— |
|
Liabilities |
|
|
|
|
Current |
|
$ |
45 |
|
Operating lease liabilities, current |
|
|
|
|
Non-current |
|
|
|
|
Operating lease liabilities, non-current |
|
|
— |
|
Total lease liabilities |
|
$ |
45 |
|
The following table shows the lease costs for the year ended June 30, 2024:
Lease costs (in thousands) |
Statement of operations classification |
June 30, 2024 |
|
|
Operating lease costs |
Operating expenses: research and development |
$ |
74 |
|
Short-term lease costs |
Operating expenses: research and development |
|
81 |
|
Short-term lease costs |
Operating expenses: general and administrative |
|
102 |
|
Short-term lease costs |
Cost of goods sold |
|
353 |
|
Right-of-use asset |
Operating expenses: impairment loss |
|
92 |
|
Total lease costs |
|
$ |
702 |
|
Other information |
June 30, 2024 |
|
|
Cash paid for amounts included in the measurement of lease liabilities (in thousands) |
$ |
74 |
|
Remaining lease term - operating leases (in years) |
|
0.3 |
|
Discount rate - operating leases |
|
10 |
% |
F-18
The future minimum payments for noncancelable leases with terms in excess of one year as of June 30, 2024 are payable as follows:
(in thousands) |
|
|
|
|
2024 |
|
$ |
46 |
|
2025 |
|
|
— |
|
2026 |
|
|
— |
|
Total minimum lease payments |
|
|
46 |
|
Less amounts representing interest |
|
|
(1 |
) |
Present value of lease liabilities |
|
$ |
45 |
|
The Company recognized rent expense of $0.6 million and $0.7 million during the years ended June 30, 2024 and 2023, respectively, related to all leases. Rent expense is included within cost of revenue, research and development and general and administrative expenses in the consolidated statements of operations and comprehensive loss.
10. Commitments and contingencies
Leases
Lease payments under operating leases as of June 30, 2024 and information about the Company’s lease arrangements are disclosed in Note 9, "Leases".
Employment agreements
The Company has entered into employment agreements with certain key executives providing for compensation and severance in certain circumstances, as set forth in the agreements.
Retirement plans
The Company maintains a defined contribution 401(k) retirement plan which covers all U.S. employees. Employees are eligible after three months of service. Under the 401(k) plan, participating employees may make contributions in an amount up to the limit set by the Internal Revenue Service on an annual basis. The Company has a safe harbor plan and makes contributions to employee accounts of 5% of compensation and increases it to 6% effective January 2024 (as defined by the plan). The Company paid $0.5 million and $0.4 million in contributions for the years ended June 30, 2024 and 2023, respectively.
Legal proceedings
The Company is not a party to any litigation and does not have contingency reserves established for any litigation liabilities. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies.
11. License and services agreements
Mount Sinai license and sponsored research agreements
On May 30, 2018, the Company entered into an exclusive license agreement (the “ISMMS License Agreement”) and on March 7, 2019, a sponsored research agreement (the “ISMMS SRA”) with Mount Sinai. Under the terms of the ISMMS License Agreement, ISMMS granted the Company (i) an exclusive, sublicensable license to use certain patent rights covering specific inventions concerning the utilization of biomarkers guided artificial intelligence techniques for detecting kidney functional decline (the “ISMMS Technology”), (ii) a non-exclusive license under unregistered licensed copyrights and licensed know-how and (iii) an exclusive option to obtain licensed technology conceived after May 30, 2018. The Company is obligated to pay Mount Sinai $1.5 million and $7.5 million in commercial milestone payments upon achieving worldwide net sales of KidneyIntelX of $50.0 million and $300.0 million, respectively. The Company is also obligated to pay Mount Sinai a 4% to 5% royalty on net sales of KidneyIntelX, subject to customary reductions. Royalties are payable on a product-by-product basis from first commercial sale of such product until the later of (1) expiration of the last valid claim of a licensed patent covering such product or (2) on a country-by-country basis, 12 years from first commercial sale of such product in such country. Moreover, the Company is obligated to pay Mount Sinai between 15% and 25% of any consideration received from a sublicensee.
F-19
As part of the ISMMS SRA, the Company has agreed to fund several research projects to further develop the ISMMS Technology. The Company incurred expenses of $2.9 million and $2.6 million related to the ISMMS SRA for the years ended June 30, 2024 and 2023, respectively.
Mount Sinai Clinical Trial agreement
In July 2021, the Company entered into a Clinical Trial Agreement (the "CTA") with ISMMS. Under the CTA, ISMMS will undertake a sponsored clinical trial entitled, “A prospective decision impact trial of KidneyIntelX in patients with Type 2 diabetes and existing chronic kidney disease”. The clinical trial is to be conducted at ISMMS with Renalytix agreeing to pay ISMMS in accordance with the agreed-upon budget. The clinical trial is expected to last up to four years with a total estimated budget of $3.2 million. As of June 30, 2024, amounts due to ISMMS under the CTA totaled $0.04 million and $0.6 million was expensed during the year ended June 30, 2024. As of June 30, 2023, amounts due to ISMMS under the CTA totaled $0.8 million and $0.5 million was expensed during the year ended June 30, 2023.
Joslin Diabetes Center agreement
In October 2018, the Company purchased a worldwide exclusive license agreement (the “Joslin Agreement”) with the Joslin Diabetes Center, Inc. (“Joslin”) that was previously entered into with EKF Diagnostics Holding Plc (“EKF”), a related party, in July 2017. The license agreement provides the Company with the right to develop and commercialize licensed products covering a novel methodology of diagnosing and predicting kidney disease using certain biomarkers (the “Joslin Diabetes Technology”).
Under the terms of the Joslin Agreement, the Company is obligated to pay Joslin aggregate commercial milestone payments of $0.3 million and $1.0 million upon achieving worldwide net sales of licensed products and processes of $2.0 million and $10.0 million, respectively. The Company is also obligated to pay Joslin a 5% royalty on net sales of any licensed products or licensed processes, subject to customary reductions. Moreover, the Company is obligated to pay Joslin 25% of any consideration received from a sublicensee. The Company accrued $0.3 million related to achievement of the first sales milestone and accrued $0.4 million of royalties due to Joslin as of June 30, 2024, which were recorded as cost of revenue within the statement of operations and comprehensive loss. The Company accrued $0.3 million related to achievement of the first sales milestone and accrued $0.3 million of royalties due to Joslin for the year ended June 30, 2023.
The Joslin Agreement initially expires on July 31, 2025 and is subject to an automatic five-year extension unless either party notifies the other party of its intent not to extend the agreement at least 180 days prior to initial expiration. Either party may terminate the Joslin Agreement earlier upon an uncured material breach of the agreement by the other party, the insolvency of the other party, or in the event the other party is unable to perform its obligations under the agreement for a specified period. Additionally, Joslin may terminate the agreement in the event that the Company ceases developing or commercializing licensed products or processes, if the Company fails to maintain certain required insurance policies, and if the Company fails to pay patent expenses related to the licensed patents.
Wake Forest/Atrium Health
In May 2021, the Company entered into a partnership with Atrium Health, Wake Forest Baptist Health and Wake Forest School of Medicine to implement an advanced clinical care model to improve kidney health and reduce kidney disease progression and kidney failure. Through these partnerships, KidneyIntelX access will be enabled to primary care physicians, endocrinologists, nephrologists and care teams in 37 hospitals and more than 1,350 care locations across the Carolinas and Georgia. Additionally, the Company entered into a five-year clinical trial agreement with Wake Forest University Health Sciences to evaluate the clinical impact of KidneyIntelX on the management of patients with type 2 diabetes (T2D) and diabetic (chronic) kidney disease (stage 1-3). The total estimated cost of the clinical trial is $6.9 million. To date the Company has incurred $3.9 million in expenses and provided over 2,350 reportable patient results in the Atrium Wake Forest system across over 150 providers. As of June 30, 2024, the Company accrued for $0.7 million of expense related to the clinical trial agreement and amounts due to Wake Forest/Atrium Health under the clinical trial agreement totaled $1.3 million and $0.6 million were expensed during the year ended June 30, 2024. As of June 30, 2023, the Company accrued for $1.1 million of expense related to the clinical trial agreement and amounts due to Wake Forest/Atrium Health under the clinical trial agreement totaled $3.3 million and $2.1 million were expensed during the year ended June 30, 2023.
12. Shareholders’ equity
Ordinary shares
As of June 30, 2024, the Company had 161,842,057 ordinary shares authorized on a fully diluted basis. Each share entitles the holder to one vote on all matters submitted to a vote of the Company’s shareholders. Ordinary shareholders are entitled to receive dividends as may be declared by the Board of Directors. From inception through June 30, 2024, no cash dividends have been declared or paid.
F-20
Private Placement
On March 12, 2024, the Company entered into a Placing Agreement (the “Placing Agreement”) with Stifel Nicolaus Europe Limited (the “Bookrunner” or “Stifel”), pursuant to which the Company agreed to allot and issue new ordinary shares (the “Placing Shares”) to certain investors (the “Placees”) in an unregistered offering (the “Private Placement”), up to an aggregate of 46,801,872 ordinary shares. On March 12, 2024, the Company announced that it successfully placed 46,801,872 ordinary shares with both UK and U.S. institutional investors, at a price of £0.20 per ordinary share, raising aggregate gross proceeds of approximately $12 million for the Company.
The Private Placement consisted of two tranches. The Company agreed to allot and issue in the first tranche of the Private Placement 19,986,031 Placing Shares at a placing price of £0.20 per Placing Share (the “First Tranche”). The First Tranche closed on March 14, 2024 raising aggregate gross proceeds of approximately $5 million for the Company.
In addition, the Company agreed to allot and issue in the second tranche of the Private Placement 26,815,841 Placing Shares at a placing price of £0.20 per Placing Share (the “Second Tranche”). The closing of the Second Tranche of the Private Placement was conditioned upon receipt of Shareholder Approval (as defined below) (the “Second Closing Trigger”).
Pursuant to the Placing Agreement, the Company agreed to hold a meeting of its shareholders (the “General Meeting”) to seek approval to give the Company’s directors authority to allot and issue the Placing Shares to be issued and sold in the Second Tranche of the Private Placement, to disapply statutory pre-emption rights in respect of such authority, and to seek approval under the Nasdaq rules (collectively, “Shareholder Approval”). The General Meeting was held on April 22, 2024, during which the Company received the requisite Shareholder Approval. The Second Tranche closed on April 24, 2024. Refer to footnote 15 for more information about the Private Placement.
On April 5, 2024, we entered into a securities purchase agreement (the “DB Capital Purchase Agreement”) with an institutional investor pursuant to which we agreed to issue and sell in a registered direct offering (the “Registered Direct Offering”) 2,666,667 ordinary shares, nominal value £0.0025 per share. Pursuant to the DB Capital Purchase Agreement, we also granted the investor an option to purchase up to 7,811,696 additional ordinary shares at the offering price of $0.375 per share. The purchase price of each ordinary share is $0.375. On April 18, 2024, the investor partially exercised the option to purchase 1,333,334 ordinary shares. The gross proceeds to the Company from the Registered Direct Offering were approximately $1.5 million, before deducting offering expenses payable by the Company. The shares were offered by the Company pursuant to an effective shelf registration statement on Form S-3 (File No. 333-274733) that was filed with the SEC on September 28, 2023 and became effective on October 6, 2023, including the base prospectus contained therein, and a related prospectus supplement dated as of April 5, 2024 filed with the SEC.
13. Share-based compensation
Equity Incentive Plan
In November 2018, Company established the Renalytix AI plc Share Option Plan (the “2018 Share Option Plan”) and a U.S. Sub-Plan and Non-Employee Sub-Plan. In July 2020, the Company's Board of Directors adopted and the Company's shareholders approved the 2020 Equity Incentive Plan (the “EIP”), which superseded the 2018 Share Option Plan. The equity incentive plan provides for the Company to grant options, restricted share awards and other share-based awards to employees, directors and consultants of the Company. As of June 30, 2024, there were 17,331,289 shares available for future issuance under the EIP.
The EIP is administered by the Board of Directors. The exercise prices, vesting and other restrictions are determined at their discretion, except that all options granted have exercise prices equal to the fair value of the underlying ordinary shares on the date of the grant and the term of stock option may not be greater than ten years from the grant date.
With respect to the options outstanding as of June 30, 2024:
F-21
If options remain unexercised after the date one day before the tenth anniversary of grant, the options expire. On termination of employment, any options that remain unexercised are either forfeited immediately or after a delayed expiration period, depending on the circumstances of termination. Upon the exercise of awards, new ordinary shares are issued by the Company.
The Company recorded share-based compensation expense in the following expense categories in the consolidated statements of operations for the years ended June 30, 2024 and 2023 :
|
|
Twelve Months Ended June 30, |
|
|||||
(in thousands) |
|
2024 |
|
|
2023 |
|
||
Research and development |
|
$ |
312 |
|
|
$ |
312 |
|
General and administrative |
|
|
1,390 |
|
|
|
2,612 |
|
Cost of revenue |
|
|
8 |
|
|
|
12 |
|
|
|
$ |
1,710 |
|
|
$ |
2,936 |
|
The fair value of options is estimated using the Black-Scholes option-pricing model, which takes into account inputs such as the exercise price, the value of the underlying ordinary shares at the grant date, expected term, expected volatility, risk-free interest rate and dividend yield. The fair value of each grant of options during the years ended June 30, 2024 and 2023 were determined using the methods and assumptions discussed below.
For the years ended June 30, 2024 and 2023, the grant date fair value of all option grants was estimated at the time of grant using the Black-Scholes option-pricing model using the following weighted average assumptions:
|
|
Twelve Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Expected term (in years) |
|
|
6.3 |
|
|
|
6.1 |
|
Expected volatility |
|
|
75.0 |
% |
|
|
66.9 |
% |
Risk-free rate |
|
|
4.3 |
% |
|
|
3.2 |
% |
Dividend yield |
|
|
— |
% |
|
|
— |
% |
The weighted average fair value of the options granted during the years ended June 30, 2024 and 2023 was $0.76 and $1.19 per share, respectively.
F-22
The following table summarizes the stock option granted to employees and non-employees for the year ended June 30, 2024:
|
|
Number of |
|
|
Weighted- |
|
|
Weighted- |
|
|||
Outstanding at June 30, 2023 |
|
|
4,968,576 |
|
|
$ |
4.50 |
|
|
|
6.7 |
|
Granted |
|
|
3,640,296 |
|
|
$ |
1.04 |
|
|
|
|
|
Exercised |
|
|
— |
|
|
$ |
- |
|
|
|
|
|
Forfeited |
|
|
(1,133,443 |
) |
|
$ |
2.83 |
|
|
|
|
|
Expired |
|
|
(1,563 |
) |
|
$ |
6.64 |
|
|
|
|
|
Outstanding at June 30, 2024 |
|
|
7,473,866 |
|
|
$ |
3.06 |
|
|
|
7.0 |
|
Exercisable at June 30, 2024 |
|
|
5,249,671 |
|
|
$ |
3.84 |
|
|
|
6.1 |
|
Vested and expected to vest at June 30, 2024 |
|
|
7,473,866 |
|
|
$ |
3.06 |
|
|
|
7.0 |
|
As of June 30, 2024, there was $1.45 million in unrecognized compensation cost related to unvested options that will be recognized as expense over a weighted average period of 1.73 years. The aggregate intrinsic value of options outstanding and options exercisable at each of June 30, 2024 and 2023 was nil.
Employee Share Purchase Plan
The Company’s 2020 Employee Share Purchase Plan (the “ESPP”) became effective on August 17, 2020. The ESPP authorizes the issuance of up to 850,000 shares of the Company’s common stock. The number of shares of the Company’s common stock that may be issued pursuant to rights granted under the ESPP shall automatically increase on January 1st of each year, commencing on January 1, 2021 and continuing for 10 years, in an amount equal to the lesser of one percent of the total number of shares of the Company’s common stock outstanding on December 31st of the preceding calendar year, and 2,000,000 ordinary shares, subject to the discretion of the Board of Directors or remuneration committee to determine a lesser number of shares shall be added for such year.
Under the ESPP, eligible employees can purchase the Company’s common stock through accumulated payroll deductions at such times as are established by the Board of Directors or remuneration committee. Eligible employees may purchase the Company’s common stock at 85% of the lower of the fair market value of the Company’s common stock on the first day of the offering period or on the purchase date. Eligible employees may contribute up to 15% of their eligible compensation. Under the ESPP, a participant may not purchase more than $25,000 worth of the Company’s common stock for each calendar year in which such rights are outstanding. During the years ended June 30, 2024 and 2023, 75,328 and 298,086 shares were purchased under the ESPP, respectively.
In accordance with the guidance in ASC 718-50, Compensation – Stock Compensation, the ability to purchase shares of the Company’s common stock at 85% of the lower of the price on the first day of the offering period or the last day of the offering period (i.e., the purchase date) represents an option and, therefore, the ESPP is a compensatory plan under this guidance. Accordingly, share-based compensation expense is determined based on the option’s grant-date fair value as estimated by applying the Black Scholes option-pricing model and is recognized over the withholding period. The Company recognized share-based compensation expense of $0.01 million and $0.07 million in general and administrative expense and $0.01 million and $0.03 million in research and development expense during the years ended June 30, 2024 and 2023, respectively, related to the ESPP.
Restricted Stock Units
Activity for restricted stock units for the year ended June 30, 2024 is as follows:
|
|
Number of |
|
|
Weighted- |
|
||
Non-vested balance at June 30, 2023 |
|
|
40,340 |
|
|
$ |
1.72 |
|
Granted |
|
|
— |
|
|
$ |
- |
|
Vested |
|
|
(21,290 |
) |
|
$ |
2.24 |
|
Forfeited |
|
|
(11,120 |
) |
|
$ |
1.69 |
|
Non-vested balance at June 30, 2024 |
|
|
7,930 |
|
|
$ |
1.33 |
|
The total fair value of restricted stock units vested during the year ended June 30, 2024 was $0.1 million. Restricted stock units vest upon the achievement of time-based service requirements.
F-23
At June 30, 2024, total unrecognized compensation expense related to non-vested restricted stock units was approximately $0.003 million. Unrecognized compensation expense relating to restricted stock units that are deemed probably of vesting is expected to be recognized over a weighted-average period of approximately 0.23 years.
14. Income taxes
Loss from operations before income taxes was comprised of the following (in thousands):
|
|
Twelve Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
United Kingdom and other |
|
$ |
(12,501 |
) |
|
$ |
(712 |
) |
United States |
|
|
(20,951 |
) |
|
|
(44,894 |
) |
|
|
$ |
(33,452 |
) |
|
$ |
(45,606 |
) |
Due to the pretax losses reported in both the United Kingdom and United States for all periods since inception there is no income tax expense or benefit but for an immaterial amount of Ireland income tax expense for the period ended June 30, 2024.
A reconciliation of income tax benefit from continuing operations as reflected in the financial statements is as follows:
|
|
Twelve Months Ended June 30, |
|
|
|||||
|
|
2024 |
|
|
2023 |
|
|
||
U.K tax benefit at statutory rate |
|
|
(25.0 |
) |
% |
|
(20.5 |
) |
% |
State taxes, net of federal benefit |
|
|
(4.4 |
) |
|
|
(10.4 |
) |
|
Permanent differences |
|
|
0.7 |
|
|
|
0.9 |
|
|
Research and development |
|
|
— |
|
|
|
— |
|
|
Change in valuation allowance |
|
|
28.4 |
|
|
|
31.2 |
|
|
Foreign rate differential |
|
|
3.9 |
|
|
|
(1.1 |
) |
|
Deferred only |
|
|
(4.3 |
) |
|
|
— |
|
|
Rate change |
|
|
0.9 |
|
|
|
— |
|
|
Other |
|
|
(0.2 |
) |
|
|
(0.1 |
) |
|
Effective tax rate |
|
|
0.0 |
|
% |
|
0.0 |
|
% |
The principal components of the Company’s deferred tax assets and liabilities were as follows (in thousands):
|
|
Twelve Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating losses |
|
|
45,962 |
|
|
$ |
37,740 |
|
Capitalized research and development costs |
|
|
3,907 |
|
|
|
2,826 |
|
Research and development licenses |
|
|
2,352 |
|
|
|
2,814 |
|
Share-based compensation |
|
|
2,758 |
|
|
|
1,327 |
|
Accrued expenses |
|
46 |
|
|
|
964 |
|
|
Mark-to-market securities |
|
384 |
|
|
|
256 |
|
|
Lease liabilities |
|
14 |
|
|
|
55 |
|
|
Other |
|
97 |
|
|
|
73 |
|
|
Valuation allowances |
|
|
(55,438 |
) |
|
|
(45,602 |
) |
Total deferred tax assets |
|
|
82 |
|
|
|
453 |
|
Deferred tax liabilities: |
|
|
|
|
|
|
||
Depreciation |
|
|
(66 |
) |
|
|
(386 |
) |
Mark-to-market securities |
|
— |
|
|
|
— |
|
|
Right-of-use assets |
|
— |
|
|
|
(51 |
) |
|
Unrealized foreign exchange loss |
|
|
(16 |
) |
|
|
(16 |
) |
Total deferred tax liabilities |
|
|
(82 |
) |
|
|
(453 |
) |
Net deferred tax |
|
$ |
— |
|
|
$ |
— |
|
The Company does not have unrecognized tax benefits as of June 30, 2024 and 2023. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense.
F-24
The Company’s net operating loss carryforwards (“NOL”) for U.K., U.S. federal and U.S. state income tax purposes consisted of the following (in thousands):
|
|
Twelve Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
United Kingdom |
|
$ |
38,384 |
|
|
$ |
26,569 |
|
U.S. Federal |
|
|
116,768 |
|
|
|
98,555 |
|
U.S. State and Local |
|
|
202,300 |
|
|
|
177,306 |
|
The UK and federal net operating loss carryforwards have no expiration dates. The amount of UK annual profits that can be relieved by losses carried forward is limited to 50%, in excess of a threshold amount of £5 million of profits. Under the Tax Cuts and Jobs Act of 2017 ("TCJA") as modified by the Coronavirus Aid, Relief, and Economic Security (CARES) Act, or collectively, the Tax Acts, U.S. federal net operating losses incurred for taxable years beginning after December 31, 2017 may be carried forward indefinitely, but the deductibility of such federal net operating losses may be limited to 80% of taxable income in taxable years beginning after December 31, 2020. The federal NOL amount will carry forward indefinitely. Certain state net operating loss carryforwards begin to expire in 2038. The Company recorded a valuation allowance on the deferred tax assets as of June 30, 2024 and 2023 because of the uncertainty of their realization. The valuation allowance increased by $9.8 million for the year ended June 30, 2024 and $14.5 million for the year ended June 30, 2023.
Utilization of the net operating losses and general business tax credits carryforwards may be subject to a substantial limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if changes in ownership of the company have occurred previously or will occur in the future. Ownership changes may limit the amount of net operating losses and general business tax credits carryforwards that can be utilized annually to offset future taxable income and tax, respectively. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of 5-percent shareholders in the stock of a corporation by more than 50 percentage points over a three-year period. If the Company experiences a Section 382 ownership change, the tax benefits related to the NOL carry forwards may be further limited or lost. The Company may also experience ownership changes as a result of shifts in share ownership, some of which are outside its control. Therefore, as a result of ownership changes with respect to ordinary shares, the ability to use current net operating losses and other pre-change tax attributes to offset post-change taxable income or taxes could be subject to limitation. The Company has not undertaken a Section 382 study.
For tax years beginning on or after January 1, 2022, the TCJA eliminates the option to currently deduct research and development expenses and requires taxpayers to capitalize and amortize them over five years for research activities performed in the United States and 15 years for research activities performed outside the United States pursuant to IRC Section 174.
The Company files income tax returns in the United Kingdom, Ireland, the U.S. federal jurisdiction and various state jurisdictions. The Company's 2018 through 2023 tax years remain subject to examination. Carryforward attributes from prior years may be adjusted upon examination by tax authorities if they are used in an open period.
15. Related-party transactions
EKF Diagnostic Holdings
During the years ended June 30, 2024 and 2023, the Company incurred expenses of $0 and $0.1 million, respectively, related to employees of EKF who provided services to Renalytix.
Icahn School of Medicine at Mount Sinai
In May 2018, the Company secured its cornerstone license agreement with ISMMS for research and clinical study work and intended commercialization by the Company (see Note 11). As part of the collaboration, ISMMS became a shareholder in the Company and has subsequently made equity investments both in the Company’s IPO on AIM in November 2018, the subsequent sale of ordinary shares in July 2019 and the Company’s IPO on Nasdaq in July 2020, and private placements in April 2022 and February 2023. As of June 30, 2024 and 2023, amounts due to ISMMS totaled $2.3 million and $3.4 million, respectively. During the years ended June 30, 2024 and 2023, the Company incurred expenses of $3.9 million and $3.3 million, respectively, related to its obligations under the ISMMS license agreement.
Private Placement
On March 12, 2024, the Company entered into the Placing Agreement with Stifel, pursuant to which the Company agreed to allot and issue the Placing Shares to the Placees in the Private Placement, up to an aggregate of 46,801,872 ordinary shares.
F-25
On March 12, 2024, the Company announced that it successfully placed 46,801,872 ordinary shares with both UK and U.S. institutional investors, at a price of £0.20 per ordinary share, raising aggregate gross proceeds of approximately $12 million for the Company (see Note 12).
ISMMS subscribed for a total 9,360,374 ordinary shares at £0.20 per ordinary share in the Private Placement.
Christopher Mills, Non-Executive Chairman, and his related parties subscribed for a total of 4,000,000 ordinary shares at £0.20 per ordinary share in the Private Placement.
16. Net loss per ordinary share
Basic net loss per ordinary share is computed by dividing net loss by the weighted average number of ordinary shares outstanding during each period. Diluted net loss per ordinary share includes the effect, if any, from the potential exercise or conversion of securities, such as options which would result in the issuance of incremental ordinary shares. Potentially dilutive securities outstanding as of June 30, 2024 and 2023 have been excluded from the computation of diluted weighted average shares outstanding as they would be anti-dilutive. Therefore, the weighted average number of shares used to calculate both basic and diluted net loss per share are the same.
The following potentially dilutive securities have been excluded from the computation of diluted weighted-average shares of ordinary shares outstanding as they would be anti-dilutive:
|
|
Twelve Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Stock options to purchase ordinary shares |
|
|
7,473,866 |
|
|
|
4,968,576 |
|
Restricted stock units |
|
|
7,930 |
|
|
|
40,340 |
|
Conversion of convertible note |
|
|
3,264,719 |
|
|
|
5,441,198 |
|
|
|
|
10,746,515 |
|
|
|
10,450,114 |
|
17. Subsequent Events
On July 15, 2024, the Company announced the repayment of $1.06 million of the principal amount of the Company's convertible bond and the interest for the period through the issuance of 2,275,000 ordinary shares and 4,641,161 ADSs. 11,557,322 new ordinary shares of £0.0025 each in the capital of the Company will be issued to settle including conversion of 4,641,161 ADSs (9,282,322 ordinary shares with each ADS representing two ordinary shares). After settlement of the repayment, the principal remaining under the convertible bond will be reduced by $1.06 million to $11.66 million.
F-26
EXHIBIT 21.1
SUBSIDIARIES OF THE REGISTRANT
Name |
|
Jurisdiction |
Renalytix AI, Inc. |
|
United States |
|
|
|
Renalytix AI Limited |
|
Ireland |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the Registration Statements on Form S-8 (File Nos. 333-248741 and 333-274732) pertaining to the Share Option Plan for Employees with Non-Employee Sub-Plan and U.S. Sub-Plan, 2020 Employee Share Purchase Plan, and 2020 Equity Incentive Plan of Renalytix plc and Registration Statements on Form S-3 (File Nos. 333-265280, 333-271579, 333-274733, 333-278765 and 333-279966) of Renalytix plc of our report dated September 28, 2023, with respect to the consolidated financial statements of Renalytix plc included in this Annual Report (Form 10-K) for the year ended June 30, 2023.
/s/ Ernst & Young LLP
Iselin, New Jersey
September 30, 2024
Exhibit 23.2
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in the Registration Statement on Form S-8 (File No. 333-248741) and Form S-8 (File No. 333-274732) pertaining to the Share Option Plan for Employees with Non-Employee Sub-Plan and U.S. Sub-Plan, 2020 Employee Share Purchase Plan, and 2020 Equity Incentive Plan of Renalytix plc, and Forms S-3 (File Nos. 333-265280, 333-271579, 333-274733, 333-278765 and 333-279966) of our report dated September 30, 2024, with respect to the consolidated financial statements of Renalytix plc included in this Annual Report (Form 10-K) for the year ended June 30, 2024, which report includes an explanatory paragraph relating to Renalytix plc’s ability to continue as a going concern.
/s/ CohnReznick LLP
New York, New York
September 30, 2024
Exhibit 31.1
CERTIFICATIONS
I, James McCullough, certify that:
1. I have reviewed this Annual Report on Form 10-K of Renalytix plc;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: September 30, 2024
/s/ James McCullough______
James McCullough
Chief Executive Officer
Exhibit 31.2
CERTIFICATIONS
I, Joel R. Jung, certify that:
1. I have reviewed this Annual Report on Form 10-K of Renalytix plc.
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: September 30, 2024 /s/ Joel R. Jung_______ Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C.
Joel R. Jung
Interim Chief Financial Officer
EXHIBIT 32.1
CERTIFICATION BY THE PRINCIPAL EXECUTIVE OFFICER AND PRINCIPAL FINANCIAL OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE
SARBANES-OXLEY ACT OF 2002
§1350), James McCullough, Chief Executive Officer of Renalytix plc (the “Company”), and Joel R. Jung, Interim Chief Financial Officer of the Company, each hereby certifies that, to the best of his knowledge:
Date: September 30, 2024
/s/ James McCullough |
Name: James McCullough |
Title: Chief Executive Officer |
(Principal Executive Officer) |
/s/ Joel R. Jung |
Name: Joel R. Jung |
Title: Interim Chief Financial Officer |
(Principal Financial Officer) |
This certification accompanies the Report to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Renalytix plc under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Report), irrespective of any general incorporation language contained in such filing.