UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2024
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to .
Commission File Number: 001-36332
ALDEYRA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
Delaware |
|
20-1968197 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
131 Hartwell Avenue, Suite 320 |
|
|
Lexington, MA |
|
02421 |
(Address of principal executive offices) |
|
(Zip Code) |
(781) 761-4904
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, $0.001 par value per share |
ALDX |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer a smaller reporting company or an emerging growth company. See the definitions of the “large accelerated filer,” “accelerated filer,” “non-accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of July 29, 2024, there were 59,421,551 shares of the registrant’s common stock issued and outstanding.
Aldeyra Therapeutics, Inc.
Quarterly Report on Form 10-Q
For the Quarter Ended June 30, 2024
INDEX
|
Page |
|
PART I – FINANCIAL INFORMATION |
||
ITEM 1. |
3 |
|
|
Consolidated Balance Sheets at June 30, 2024 (Unaudited) and December 31, 2023 |
3 |
|
4 |
|
|
5 |
|
|
6 |
|
|
Consolidated Statements of Cash Flows for the six months ended June 30, 2024 and 2023 (Unaudited) |
8 |
|
Notes to Condensed Consolidated Financial Statements (Unaudited) |
9 |
ITEM 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
20 |
ITEM 3. |
28 |
|
ITEM 4. |
28 |
|
PART II – OTHER INFORMATION |
|
|
ITEM 1. |
29 |
|
ITEM 1A. |
29 |
|
ITEM 2. |
76 |
|
ITEM 3. |
76 |
|
ITEM 4. |
76 |
|
ITEM 5. |
76 |
|
ITEM 6. |
77 |
|
78 |
||
2
Part I – FINANCIAL INFORMATION
Item 1. Condensed Consolidated Financial Statements.
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED BALANCE SHEETS
|
|
June 30, |
|
|
|
|
||
|
|
2024 |
|
|
December 31, |
|
||
|
|
(unaudited) |
|
|
2023 |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
31,029,723 |
|
|
$ |
142,823,016 |
|
Cash equivalent - reverse repurchase agreements |
|
|
40,000,000 |
|
|
|
— |
|
Marketable securities |
|
|
49,301,960 |
|
|
|
— |
|
Prepaid expenses and other current assets |
|
|
5,317,836 |
|
|
|
4,987,317 |
|
Total current assets |
|
|
125,649,519 |
|
|
|
147,810,333 |
|
Right-of-use assets |
|
|
391,595 |
|
|
|
510,814 |
|
Fixed assets, net |
|
|
2,720 |
|
|
|
5,764 |
|
Total assets |
|
$ |
126,043,834 |
|
|
$ |
148,326,911 |
|
|
|
|
|
|
|
|
||
LIABILITIES AND STOCKHOLDERS' EQUITY |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
254,434 |
|
|
$ |
1,338,057 |
|
Accrued expenses |
|
|
5,842,802 |
|
|
|
5,536,464 |
|
Current portion of debt |
|
|
15,242,327 |
|
|
|
15,146,546 |
|
Operating lease liabilities |
|
|
255,039 |
|
|
|
239,183 |
|
Deferred collaboration revenue |
|
|
6,000,000 |
|
|
|
— |
|
Total current liabilities |
|
|
27,594,602 |
|
|
|
22,260,250 |
|
Deferred collaboration revenue, long-term |
|
|
— |
|
|
|
6,000,000 |
|
Operating lease liabilities, long-term |
|
|
138,893 |
|
|
|
271,631 |
|
Total liabilities |
|
|
27,733,495 |
|
|
|
28,531,881 |
|
Commitments and contingencies (Notes 3, 9, & 14) |
|
|
|
|
|
|
||
Stockholders' equity: |
|
|
|
|
|
|
||
Preferred stock, $0.001 par value, 15,000,000 shares authorized, none |
|
|
— |
|
|
|
— |
|
Common stock, voting, $0.001 par value; 150,000,000 authorized and |
|
|
59,415 |
|
|
|
59,196 |
|
Additional paid-in capital |
|
|
517,449,424 |
|
|
|
513,994,982 |
|
Accumulated other comprehensive loss |
|
|
(9,658 |
) |
|
|
— |
|
Accumulated deficit |
|
|
(419,188,842 |
) |
|
|
(394,259,148 |
) |
Total stockholders’ equity |
|
|
98,310,339 |
|
|
|
119,795,030 |
|
Total liabilities and stockholders’ equity |
|
$ |
126,043,834 |
|
|
$ |
148,326,911 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
3
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS (Unaudited)
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
$ |
14,969,743 |
|
|
$ |
6,962,907 |
|
|
$ |
21,153,251 |
|
|
$ |
18,198,767 |
|
General and administrative |
|
|
3,038,064 |
|
|
|
3,379,750 |
|
|
|
6,248,420 |
|
|
|
8,947,167 |
|
Loss from operations |
|
|
(18,007,807 |
) |
|
|
(10,342,657 |
) |
|
|
(27,401,671 |
) |
|
|
(27,145,934 |
) |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest income |
|
|
1,637,836 |
|
|
|
1,882,800 |
|
|
|
3,448,105 |
|
|
|
3,561,685 |
|
Interest expense |
|
|
(477,601 |
) |
|
|
(527,141 |
) |
|
|
(976,128 |
) |
|
|
(1,018,428 |
) |
Total other income, net |
|
|
1,160,235 |
|
|
|
1,355,659 |
|
|
|
2,471,977 |
|
|
|
2,543,257 |
|
Net loss |
|
$ |
(16,847,572 |
) |
|
$ |
(8,986,998 |
) |
|
$ |
(24,929,694 |
) |
|
$ |
(24,602,677 |
) |
Net loss per share - basic and diluted |
|
$ |
(0.28 |
) |
|
$ |
(0.15 |
) |
|
$ |
(0.42 |
) |
|
$ |
(0.42 |
) |
Weighted average common shares outstanding - basic and diluted |
|
|
59,414,489 |
|
|
|
58,791,920 |
|
|
|
59,414,489 |
|
|
|
58,791,762 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
4
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS (Unaudited)
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
||||
Net loss |
|
$ |
(16,847,572 |
) |
|
$ |
(8,986,998 |
) |
|
$ |
(24,929,694 |
) |
|
$ |
(24,602,677 |
) |
Other comprehensive (loss) income: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net unrealized (loss) on marketable securities |
|
|
(6,629 |
) |
|
|
— |
|
|
|
(9,658 |
) |
|
|
— |
|
Reclassification of losses to net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
103,938 |
|
Total other comprehensive (loss) income |
|
$ |
(6,629 |
) |
|
$ |
— |
|
|
$ |
(9,658 |
) |
|
$ |
103,938 |
|
Comprehensive loss |
|
$ |
(16,854,201 |
) |
|
$ |
(8,986,998 |
) |
|
$ |
(24,939,352 |
) |
|
$ |
(24,498,739 |
) |
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
5
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (Unaudited)
|
|
Stockholders' Equity |
|
|||||||||||||||||||||
|
|
Common Stock |
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
Additional |
|
|
Other |
|
|
Accumulated |
|
|
Total |
|
||||||
Balance, December 31, 2023 |
|
|
59,195,951 |
|
|
$ |
59,196 |
|
|
$ |
513,994,982 |
|
|
$ |
— |
|
|
$ |
(394,259,148 |
) |
|
$ |
119,795,030 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
3,436,470 |
|
|
|
— |
|
|
|
— |
|
|
|
3,436,470 |
|
Issuance of common stock, employee |
|
|
6,097 |
|
|
|
7 |
|
|
|
18,184 |
|
|
|
— |
|
|
|
— |
|
|
|
18,191 |
|
Issuance of common stock, vested |
|
|
212,441 |
|
|
|
212 |
|
|
|
(212 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(9,658 |
) |
|
|
— |
|
|
|
(9,658 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(24,929,694 |
) |
|
|
(24,929,694 |
) |
Balance, June 30, 2024 |
|
|
59,414,489 |
|
|
$ |
59,415 |
|
|
$ |
517,449,424 |
|
|
$ |
(9,658 |
) |
|
$ |
(419,188,842 |
) |
|
$ |
98,310,339 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance, December 31, 2022 |
|
|
58,560,078 |
|
|
$ |
58,560 |
|
|
$ |
507,770,045 |
|
|
$ |
(103,938 |
) |
|
$ |
(356,716,638 |
) |
|
$ |
151,008,029 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
3,316,058 |
|
|
|
— |
|
|
|
— |
|
|
|
3,316,058 |
|
Issuance of common stock, exercise |
|
|
9,604 |
|
|
|
9 |
|
|
|
5,283 |
|
|
|
— |
|
|
|
— |
|
|
|
5,292 |
|
Issuance of common stock, employee |
|
|
16,272 |
|
|
|
17 |
|
|
|
52,542 |
|
|
|
— |
|
|
|
— |
|
|
|
52,559 |
|
Issuance of common stock, vested |
|
|
215,253 |
|
|
|
215 |
|
|
|
(215 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Other comprehensive income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
103,938 |
|
|
|
— |
|
|
|
103,938 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(24,602,677 |
) |
|
|
(24,602,677 |
) |
Balance, June 30, 2023 |
|
|
58,801,207 |
|
|
$ |
58,801 |
|
|
$ |
511,143,713 |
|
|
$ |
— |
|
|
$ |
(381,319,315 |
) |
|
$ |
129,883,199 |
|
6
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (Unaudited)
|
|
Stockholders' Equity |
|
|||||||||||||||||||||
|
|
Common Stock |
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
Additional |
|
|
Other |
|
|
Accumulated |
|
|
Total |
|
||||||
Balance, March 31, 2024 |
|
|
59,414,489 |
|
|
$ |
59,415 |
|
|
$ |
515,704,325 |
|
|
$ |
(3,029 |
) |
|
$ |
(402,341,270 |
) |
|
$ |
113,419,441 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
1,745,099 |
|
|
|
— |
|
|
|
— |
|
|
|
1,745,099 |
|
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(6,629 |
) |
|
|
— |
|
|
|
(6,629 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(16,847,572 |
) |
|
|
(16,847,572 |
) |
Balance, June 30, 2024 |
|
|
59,414,489 |
|
|
$ |
59,415 |
|
|
$ |
517,449,424 |
|
|
$ |
(9,658 |
) |
|
$ |
(419,188,842 |
) |
|
$ |
98,310,339 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance, March 31, 2023 |
|
|
58,791,603 |
|
|
$ |
58,792 |
|
|
$ |
509,516,738 |
|
|
$ |
— |
|
|
$ |
(372,332,317 |
) |
|
$ |
137,243,213 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
1,621,692 |
|
|
|
— |
|
|
|
— |
|
|
|
1,621,692 |
|
Issuance of common stock, exercise |
|
|
9,604 |
|
|
|
9 |
|
|
|
5,283 |
|
|
|
— |
|
|
|
— |
|
|
|
5,292 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(8,986,998 |
) |
|
|
(8,986,998 |
) |
Balance, June 30, 2023 |
|
|
58,801,207 |
|
|
$ |
58,801 |
|
|
$ |
511,143,713 |
|
|
$ |
— |
|
|
$ |
(381,319,315 |
) |
|
$ |
129,883,199 |
|
7
ALDEYRA THERAPEUTICS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS (Unaudited)
|
|
Six Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(24,929,694 |
) |
|
$ |
(24,602,677 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Stock-based compensation |
|
|
3,484,366 |
|
|
|
5,763,896 |
|
Non-cash interest expense |
|
|
95,781 |
|
|
|
187,501 |
|
Net amortization of premium on marketable securities |
|
|
(697,119 |
) |
|
|
(14,542 |
) |
Depreciation and amortization expense |
|
|
122,263 |
|
|
|
131,312 |
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
(330,519 |
) |
|
|
2,918,672 |
|
Accounts payable |
|
|
(1,083,623 |
) |
|
|
137,191 |
|
Accrued expenses and other liabilities |
|
|
141,560 |
|
|
|
(7,323,007 |
) |
Net cash used in operating activities |
|
|
(23,196,985 |
) |
|
|
(22,801,654 |
) |
CASH FLOWS FROM INVESTING ACTIVITIES: |
|
|
|
|
|
|
||
Purchases of marketable securities |
|
|
(60,614,499 |
) |
|
|
— |
|
Maturities of marketable securities |
|
|
12,000,000 |
|
|
|
30,000,000 |
|
Net cash (used in) provided by investing activities |
|
|
(48,614,499 |
) |
|
|
30,000,000 |
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
||
Proceeds from exercise of stock options |
|
|
- |
|
|
|
5,292 |
|
Proceeds from employee stock purchase plan |
|
|
18,191 |
|
|
|
52,559 |
|
Net cash provided by financing activities |
|
|
18,191 |
|
|
|
57,851 |
|
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS |
|
|
(71,793,293 |
) |
|
|
7,256,197 |
|
CASH AND CASH EQUIVALENTS, BEGINNING OF PERIOD |
|
|
142,823,016 |
|
|
|
144,419,364 |
|
CASH AND CASH EQUIVALENTS, END OF PERIOD |
|
$ |
71,029,723 |
|
|
$ |
151,675,561 |
|
|
|
|
|
|
|
|
||
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
||
Cash paid during the period for interest |
|
$ |
884,500 |
|
|
$ |
823,521 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
8
ALDEYRA THERAPEUTICS, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited)
Aldeyra Therapeutics, Inc., together with its wholly-owned subsidiaries (the “Company” or “Aldeyra”), a Delaware corporation, is a clinical-stage biotechnology company devoted to discovering innovative therapies designed to treat immune-mediated and metabolic diseases.
The Company’s principal activities to date include research and development activities along with related general business planning, including raising capital.
The accompanying interim condensed consolidated financial statements and related disclosures are unaudited and have been prepared in accordance with U.S. generally accepted accounting principles (GAAP) for interim financial information and the instructions to Form 10-Q and Regulation S-X. Accordingly, they do not include all the information and footnotes required by GAAP for complete financial statements and should be read in conjunction with the Company’s audited consolidated financial statements and related notes included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the Securities and Exchange Commission on March 7, 2024 (2023 Annual Report).
The financial information as of June 30, 2024, and the three and six months ended June 30, 2024 and 2023, respectively, is unaudited. In the opinion of management, all adjustments, consisting only of normal recurring adjustments considered necessary for the fair presentation of financial position, results of operations, and cash flows at the dates and for the periods presented, have been included. The balance sheet data as of December 31, 2023 was derived from audited consolidated financial statements. The results of the Company’s operations for any interim periods are not necessarily indicative of the results that may be expected for any other interim period or for a full fiscal year.
Based on its current operating plan, and excluding any potential licensing and product revenue, the Company believes that its cash, cash equivalents and marketable securities will be sufficient to fund the Company's currently projected operating expenses and debt obligations for at least the next 12 months from the date the financial statements are issued. The Company has based its projections of operating capital requirements on its current operating plan, which includes several assumptions that may prove to be incorrect, and the Company may use all of its available capital resources sooner than the Company expects. The Company will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources, in order to carry out all of the Company’s planned research and development activities and regulatory activities; commence or continue ongoing commercialization activities, including manufacturing, sales, marketing and distribution, for any of its product candidates for which the Company may receive marketing approval; or conduct any substantial, additional development requirements requested by the FDA. Additional funding may not be available to the Company on acceptable terms, or at all. If the Company is unable to secure additional funding, it could be forced to delay, reduce, or eliminate its research and development programs and its reproxalap commercialization efforts, whether alone or with others.
Curtailment of operations would cause significant delays in the Company’s efforts to develop and introduce its products to market, which is critical to the realization of its business plan and the future operations of the Company.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions, including fair value estimates for investments that affect the reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements, and the reported amounts of expenses during the reporting periods. The Company’s management evaluates its estimates and assumptions on an ongoing basis. Management’s most significant estimates in the Company’s condensed consolidated financial statements include, but are not limited to, deferred and accrued research and development costs, stock-based compensation, and accounting for income taxes and related valuation allowance. Although these estimates and assumptions are based on the Company’s knowledge of current events and actions it may undertake in the future, actual results may ultimately materially differ from these estimates and assumptions.
9
Summary of Significant Accounting Policies
There were no changes to significant accounting policies during the six months ended June 30, 2024, as compared to those identified in the 2023 Annual Report.
Recent Accounting Pronouncements
In November 2023, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures (ASU 2023-07). ASU 2023-07 is intended to improve reportable segment disclosure requirements, primarily through additional disclosures about significant segment expenses, including for single reportable segment entities. The standard is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024, with early adoption permitted. The amendments should be applied retrospectively to all prior periods presented in the financial statements. The Company is evaluating the disclosure requirements related to this new standard. However, given the Company has one reportable segment, this policy is not expected to have a material impact on the Company's consolidated financial statements.
In December 2023, the FASB issued ASU No. 2023-09, Improvements to Income Tax Disclosures (ASU 2023-09). ASU 2023-09 requires more detailed income tax disclosures. The guidance requires entities to disclose disaggregated information about their effective tax rate reconciliation as well as expanded information on income taxes paid by jurisdiction. The disclosure requirements will be applied on a prospective basis, with the option to apply them retrospectively. The standard is effective for fiscal years beginning after December 15, 2024, with early adoption permitted. The Company is evaluating the disclosure requirements related to this new standard.
On January 28, 2019 (Closing Date), the Company acquired Helio Vision, Inc. (Helio) and thereby obtained rights to ADX-2191 pursuant to an Agreement and Plan of Merger dated as of January 24, 2019 (the Merger Agreement). As a result of the acquisition, the Company issued an aggregate of 1,407,006 shares of common stock to the former securityholders and an advisor of Helio, including 246,562 shares issued in January 2021, pursuant to the terms of the acquisition agreement. In addition, the Company, subject to the conditions of the acquisition agreement, is contingently obligated to make additional payments to the former securityholders of Helio as follows: (a) $10.0 million of common stock following approval by the FDA of a new drug application (NDA) for the prevention and/or treatment of proliferative vitreoretinopathy or a substantially similar label prior to the 10th anniversary of the Closing Date; and (b) $2.5 million of common stock following FDA approval of an NDA for an indication (other than proliferative vitreoretinopathy or a substantially similar label) prior to the 12th anniversary of the Closing Date (the shares of common stock issuable pursuant to the preceding clauses (a) and (b) are referred to herein as the Milestone Shares), provided that in no event shall the Company be obligated to issue more than an aggregate of 5,248,885 shares of common stock in connection with the Helio acquisition. If the Company ceases to use commercially reasonably efforts to develop and obtain regulatory approval for ADX-2191, subject to the terms and conditions of the Merger Agreement, ADX-2191 and related intellectual property rights may revert back to an entity designated by the representative of the former Helio stockholders. Additionally, in the event of certain change of control or divestitures by the Company, certain former convertible noteholders of Helio will be entitled to a tax gross-up payment in an amount not to exceed $1.0 million in the aggregate.
The Company determined that liability accounting is not required for the Milestone Shares under FASB ASC Topic 480, Distinguishing Liabilities from Equity (ASC 480). The Company also determined that the Milestone Shares meet the scope exception as a derivative under FASB ASC Topic 815, Derivatives and Hedging (ASC 815), from inception of the Milestone Shares through June 30, 2024. Accordingly, the Milestone Shares are evaluated under FASB ASC Topic 450, Contingencies (ASC 450) and the Company will record a liability related to the Milestone Shares if the milestones are achieved, and the obligation to issue the Milestone Shares becomes probable. At such time, the Company will record the cost of the Milestone Shares issued to the Helio founders as a compensation expense and to the other former securityholders of Helio as an in-process research and development expense if there is no alternative future use. No milestones related to the remaining Milestone Shares are considered probable of being achieved as of June 30, 2024.
10
For the three and six months ended June 30, 2024 and 2023, diluted weighted average common shares outstanding is equal to basic weighted average common shares due to the Company’s net loss position.
The following potentially dilutive securities outstanding have been excluded from the computation of diluted weighted-average shares outstanding, because such securities had an antidilutive impact:
|
|
For the Three and Six Months Ended June 30, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Options to purchase common stock |
|
|
8,033,741 |
|
|
|
6,567,153 |
|
Nonvested restricted stock units |
|
|
732,056 |
|
|
|
1,208,100 |
|
Total of common stock equivalents |
|
|
8,765,797 |
|
|
|
7,775,253 |
|
At June 30, 2024, cash, cash equivalents, and marketable securities were comprised of:
|
|
Carrying |
|
|
Unrecognized |
|
|
Unrecognized |
|
|
Estimated |
|
|
Cash and Cash |
|
|
Current |
|
||||||
Cash |
|
$ |
3,434,204 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
3,434,204 |
|
|
$ |
3,434,204 |
|
|
$ |
— |
|
Money market funds |
|
|
27,595,519 |
|
|
|
— |
|
|
|
— |
|
|
|
27,595,519 |
|
|
|
27,595,519 |
|
|
|
— |
|
Reverse repurchase agreements |
|
|
40,000,000 |
|
|
|
— |
|
|
|
— |
|
|
|
40,000,000 |
|
|
|
40,000,000 |
|
|
|
— |
|
Total cash and cash equivalents |
|
$ |
71,029,723 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
71,029,723 |
|
|
$ |
71,029,723 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
U.S. government agency securities |
|
|
49,311,618 |
|
|
|
486 |
|
|
|
(10,144 |
) |
|
|
49,301,960 |
|
|
|
— |
|
|
|
49,301,960 |
|
Available for sale marketable securities (1) |
|
|
49,311,618 |
|
|
|
486 |
|
|
|
(10,144 |
) |
|
|
49,301,960 |
|
|
|
— |
|
|
|
49,301,960 |
|
Total cash, cash equivalents, and current marketable securities |
|
|
|
|
|
|
|
|
|
|
|
|
|
$ |
71,029,723 |
|
|
$ |
49,301,960 |
|
||||
The contractual maturities of all available for sale securities were less than one year at June 30, 2024.
At December 31, 2023, cash, and cash equivalents were comprised of:
|
|
Carrying |
|
|
Estimated |
|
|
Cash and Cash |
|
|
|||
Cash |
|
$ |
128,510,451 |
|
|
$ |
128,510,451 |
|
|
$ |
128,510,451 |
|
|
Money market funds |
|
|
14,312,565 |
|
|
|
14,312,565 |
|
|
|
14,312,565 |
|
|
Total cash and cash equivalents |
|
$ |
142,823,016 |
|
|
$ |
142,823,016 |
|
|
$ |
142,823,016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
There were no marketable securities held at December 31, 2023.
11
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value are performed in a manner to maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820, Fair Value Measurements, establishes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 – Quoted prices in active markets that are accessible at the market date for identical unrestricted assets or liabilities.
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs for which all significant inputs are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The following table presents information about the Company’s assets measured at fair value at June 30, 2024 and December 31, 2023:
|
|
June 30, 2024 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds (a) |
|
$ |
27,595,519 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
27,595,519 |
|
U.S. government agency securities (b) |
|
|
49,301,960 |
|
|
|
— |
|
|
|
— |
|
|
|
49,301,960 |
|
Reverse repurchase agreements (c) |
|
|
— |
|
|
|
40,000,000 |
|
|
|
— |
|
|
|
40,000,000 |
|
Total assets at fair value |
|
$ |
76,897,479 |
|
|
$ |
40,000,000 |
|
|
$ |
— |
|
|
$ |
116,897,479 |
|
|
|
December 31, 2023 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds (a) |
|
$ |
14,312,565 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
14,312,565 |
|
Total assets at fair value |
|
$ |
14,312,565 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
14,312,565 |
|
There were no liabilities measured at fair value at June 30, 2024 or December 31, 2023.
Financial instruments including clinical trial prepayments to contract research organizations and accounts payable are carried in the condensed consolidated financial statements at amounts that approximate their fair value based on the short maturities of those instruments. The carrying amount of the Company’s term loan under the Hercules Credit Facility (as defined in Note 9) approximates market rates currently available to the Company.
12
Prepaid expenses and other current assets at June 30, 2024 and December 31, 2023 were:
|
|
June 30, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Deferred research and development expenses, and deposits |
|
$ |
3,859,927 |
|
|
$ |
4,463,783 |
|
Prepaid insurance expenses |
|
|
1,036,223 |
|
|
|
340,388 |
|
Miscellaneous prepaid expenses and other current assets |
|
|
421,686 |
|
|
|
183,146 |
|
Total prepaid expenses and other current assets |
|
$ |
5,317,836 |
|
|
$ |
4,987,317 |
|
Accrued expenses at June 30, 2024 and December 31, 2023 were:
|
|
June 30, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Accrued compensation |
|
$ |
2,571,463 |
|
|
$ |
3,087,937 |
|
Accrued research and development expenses |
|
|
2,411,867 |
|
|
|
1,687,327 |
|
Accrued other expenses |
|
|
859,472 |
|
|
|
761,200 |
|
Total accrued expenses |
|
$ |
5,842,802 |
|
|
$ |
5,536,464 |
|
The Company’s current and long-term debt obligation consists of amounts the Company is obligated to repay under its credit facility with Hercules Capital, Inc. (Hercules). In March 2019, the Company entered into a Loan and Security Agreement (Loan and Security Agreement or Hercules Credit Facility) with Hercules and several banks and other financial institutions or entities, from time-to-time parties thereto (collectively, referred to herein as Lender), providing for a term loan of up to $60.0 million, subject to the satisfaction of certain conditions contained therein, that is secured by a lien covering all of the Company’s assets, other than the Company’s intellectual property. The Loan and Security Agreement provided for (i) an initial term loan advance of up to $5.0 million at the Company’s option, which expired unutilized on April 15, 2019; (ii) three additional term loan advances of up to $15.0 million each, at the Company’s option, available to the Company upon the occurrence of certain pre-specified funding conditions prior to September 30, 2019 (2019 Tranche), March 31, 2020 (2020 Tranche), and March 31, 2021 (2021 Tranche); and (iii) a final additional term loan advance (Fourth Loan Tranche) of up to $10.0 million prior to December 31, 2021, at the Company’s option, subject to approval by the Lender’s investment committee. The 2019 Tranche was drawn down in full by the Company in September 2019 and the 2020 Tranche and 2021 Tranche expired unutilized prior to the Company satisfying the funding conditions for such tranche. On April 20, 2021, the Company entered into the First Amendment to the Loan and Security Agreement (First Amendment). The First Amendment, among other things, (i) increased the Fourth Loan Tranche from $10.0 million to $20.0 million and extended the deadline for drawing down the Fourth Loan Tranche to July 1, 2022; (ii) lowered the variable per annum rate of interest on borrowings under the Loan and Security Agreement from the greater of (a) 9.10% and (b) the prime rate (as reported in the Wall Street Journal or any successor publication thereto) plus 3.10% to the greater of (x) the Prime Rate (as defined therein) plus 3.10% or (y) 8.60%; (iii) extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are required from May 1, 2021 to July 1, 2022; and (iv) following the satisfaction of certain conditions, which conditions were satisfied in April 2021, further extended the expiration of the interest-only period and the deadline for drawing down the Fourth Loan Tranche to May 1, 2023. Repayment of the aggregate outstanding principal balance of the term loan, in monthly installments, commences upon expiration of the interest-only period and continues through October 1, 2023 (Maturity Date). The First Amendment was determined to be a modification in accordance with FASB ASC Topic 470, Debt and did not result in extinguishment.
On December 22, 2022, the Company entered into the Second Amendment to the Loan and Security Agreement (Second Amendment), which became effective as of December 31, 2022 (Second Amendment Effective Date). The Second Amendment, among other things, (i) extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are made from May 1, 2023 to May 1, 2024; (ii) extended the Maturity Date from October 1, 2023 to October 1, 2024; (iii) extended the availability of the Fourth Loan Tranche commitment of $20 million, which remained conditioned on approval by the Lenders’ investment committee, from May 1, 2023 to May 1, 2024; and (iv) amended the Prepayment Charge (as defined therein) to equal 0.75% of the amount prepaid during the 12-month period following the Second Amendment Effective Date, and 0% thereafter. In addition, a supplemental end of term charge of $292,500 (Supplemental End of Term Charge) shall be due on the earlier of (A) the Maturity Date, as amended, or (B) repayment of the aggregate amount of advances under the Loan and Security Agreement. The initial end of term charge of $1,042,500 (End of Term Charge) was paid on October 2, 2023.
13
The Second and Third Amendments were determined to be modifications in accordance with FASB ASC Topic 470, Debt and did not result in extinguishment.
On April 29, 2024, the Company entered into the Third Amendment to the Loan and Security Agreement (Third Amendment). The Third Amendment, among other things, extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are made from May 1, 2024 to October 1, 2024. On May 1, 2024, the Fourth Loan Tranche commitment expired unutilized.
In connection with the Hercules Credit Facility, the Company incurred a commitment charge of $25,000, transaction costs of $273,186, a fee of $375,000 upon closing, the End of Term Charge, which was paid in October 2023, and the Company will be required to pay the Supplemental End of Term Charge. The fees and transaction costs are amortized to interest expense from 2019 through the Maturity Date using the effective interest method. The End of Term Charge was amortized to interest expense from 2019 through October 2023, and the Supplemental End of Term Charge is amortized to interest expense from December 2022 through the Maturity Date, both using the effective interest method. The effective interest rate was 12.6% at June 30, 2024. At the Company’s option, the Company may elect to prepay all, but not less than all, of the outstanding term loan by paying the entire principal balance and all accrued and unpaid interest thereon plus all fees and other amounts due under the Loan and Security Agreement as of the date of such prepayment.
As of June 30, 2024, $15 million has been funded under the Loan and Security Agreement and no additional amounts were available to the Company for borrowing.
Long-term debt consisted of the following:
|
|
June 30, |
|
|
December 31, |
|
||
|
|
2024 |
|
|
2023 |
|
||
Term loan payable |
|
$ |
15,000,000 |
|
|
$ |
15,000,000 |
|
Supplemental end of term charge |
|
|
251,643 |
|
|
|
173,646 |
|
Unamortized debt issuance costs |
|
|
(9,316 |
) |
|
|
(27,100 |
) |
Less: current portion |
|
|
(15,242,327 |
) |
|
|
(15,146,546 |
) |
Total long-term debt |
|
$ |
— |
|
|
$ |
— |
|
Future principal payments, including the Supplemental End of Term Charge, are as follows for the years ending December 31:
2024 |
|
|
15,292,500 |
|
Total |
|
$ |
15,292,500 |
|
The Loan and Security Agreement also contains certain events of default, representations, warranties and non-financial covenants of the Company. As of June 30, 2024, the Company was in compliance with all covenants of the Hercules Credit Facility in all material respects. In addition, subject to the terms of the Loan and Security Agreement, the Company granted the Lender the right to purchase up to an aggregate of $2.0 million of the Company’s equity securities, or instruments exercisable for or convertible into equity securities, sold to investors in financings upon the same terms and conditions afforded to such other investors.
In March 2021, the Company entered into an Open Market Sales Agreement SM with Jefferies LLC (Jefferies), as sales agent (2021 Jefferies Sales Agreement), under which the Company had the ability to offer and sell, from time to time through Jefferies, shares of common stock providing for aggregate sales proceeds of up to $100.0 million. As of June 30, 2024, no shares of common stock may be sold under the 2021 Jefferies Sales Agreement, and no sales had previously been made pursuant to the 2021 Jefferies Sales Agreement.
No current or deferred tax provision expenses for federal and state income taxes have been recorded as the Company has incurred losses since inception for tax purposes. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
14
In assessing the realizability of net deferred taxes in accordance with Accounting Standards Codification (ASC) 740, Income Taxes (ASC 740), the Company considers whether it is more likely than not that some portion or all the deferred tax assets will not be realized. Based on the weight of available evidence, primarily the incurrence of net losses since inception, anticipated net losses in the near future, reversals of existing temporary differences, and expiration of various federal and state attributes, the Company does not consider it more likely than not that some or all of the net deferred taxes will be realized. Accordingly, a 100% valuation allowance has been applied against net deferred tax assets.
Under Section 382 and 383 of the Internal Revenue Code of 1986, as amended (Section 382 and 383), a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating losses (NOLs) and certain other tax assets (tax attributes) to offset future taxable income. In general, an ownership change occurs if the aggregate stock ownership of certain stockholders increases by more than 50 percentage points over such stockholders’ lowest percentage ownership during the testing period (generally three years). Transactions involving the Company’s common stock, within the testing period, even those outside the Company’s control, such as purchases or sales by investors, within the testing period could result in an ownership change. A limitation on the Company’s ability to utilize some or all its NOLs or credits could have a material adverse effect on the Company’s results of operations and cash flows. Prior to December 31, 2021, the Company believes it underwent four ownership changes. However, management believes that its aggregate Section 382 and 383 limitation (including the additional limitation for recognized “built-in-gains”) is sufficient so that no current impairment of its pre-ownership change tax attributes is required. The Company does not believe an ownership change has occurred from December 31, 2021, through June 30, 2024, based on a review of its equity history during that period. Any future ownership changes, including those resulting from the Company’s future financing activities, may cause its existing tax attributes to incur additional limitations.
As of June 30, 2024, the Company is subject to tax in the U.S. (Federal and Massachusetts). The Company is open to examination for the tax years ended December 31, 2023, 2022, 2021, and 2020. In addition, any loss years remain open to the extent that losses are available for carryover to future years.
The Company accounts for uncertain tax positions pursuant to ASC 740-10 which prescribes a recognition threshold and measurement process for financial statement recognition of uncertain tax positions taken or expected to be taken in a tax return. If the tax position meets this threshold, the benefit to be recognized is measured as the tax benefit having the highest likelihood of being realized upon ultimate settlement with the taxing authority. Accordingly, in the provision for income taxes, the Company recognizes interest accrued related to unrecognized tax benefits and penalties; however, management is currently unaware of any uncertain tax positions. As a result, the Company does not have any liabilities recorded including interest or penalties for uncertain tax positions.
The Inflation Reduction Act (IRA) was enacted on August 16, 2022. Based on review of the IRA, the Company does not expect any impact to its tax provision. In particular, the Company does not expect to pay Corporate Alternative Minimum Tax (CAMT) in the next few years based on its projected losses. The IRA introduces a 15% CAMT for corporations whose average annual adjusted financial statement income for any consecutive three-tax-year period preceding the tax year exceeds $1 billion starting in 2023.
The Company approved the 2013 Equity Incentive Plan in October 2013, which was amended in June 2016 and June 2018, (Amended 2013 Plan). The Amended 2013 Plan provided for the granting of stock options, restricted stock units (RSU), stock appreciation rights, and stock units to certain employees, members of the board of directors and consultants of the Company.
In May 2023, the Company's Board of Directors approved the 2023 Equity Incentive Plan (2023 Equity Plan) to replace the Amended 2013 Plan. On June 30, 2023, the Company's stockholders approved the 2023 Equity Plan at the Company's 2023 annual meeting of stockholders. Pursuant to the 2023 Equity Plan, the Company will not make any further grants under the Amended 2013 Plan following June 30, 2023, though awards previously granted under the Amended 2013 Plan will remain outstanding. The 2023 Equity Plan is effective for a period of ten years after June 30, 2023, and a total of 5,450,000 shares of the Company’s common stock, in addition to shares of the Company’s common stock that are subject to awards granted under the Amended 2013 Plan that are outstanding as of such date and that are subsequently forfeited, cancelled, or expire before being exercised or settled in full, are authorized for issuance under the 2023 Equity Plan. As of June 30, 2024, there were 3,542,805 shares of common stock available for grant under the 2023 Equity Plan.
In 2022 the Company granted cash awards under its Management Cash Incentive Plan, as amended (the Management Cash Incentive Plan). The Management Cash Incentive Plan provides its participants with the opportunity to earn cash incentive awards for the achievement of goals relating to the performance of the Company and was adopted in 2016.
15
The cash awards vest in four annual installments from the date of grant based on continued service and entitle the employees to receive a cash payment, on the earlier of (i) four years from the date of grant or (ii) a change of control, equal in value to the amount by which the then value of the Company’s common stock exceeds the base value. As of June 30, 2024, $0.2 million was accrued as compensation expense for vested cash awards. There was no unrecognized expense as of June 30, 2024.
In 2022, the Company granted performance cash settled bonus awards (CSBUs) under its Management Cash Incentive Plan. As the performance criteria had been met, the awards will vest in four annual installments from the date of grant based on continued service, and entitle the employees to receive a cash payment for each vested CSBU, on the earlier of (i) four years from the date of grant or (ii) a change of control, equal in value of the closing price per share of the Company's common stock on the Nasdaq Capital Market (Nasdaq) on the payment date. As of June 30, 2024, $1.7 million was accrued as compensation expense for CSBUs as the Performance Criteria was met in February 2023. There was no unrecognized expense as of June 30, 2024.
The Company recognizes stock-based compensation expense over the requisite service period. The Company's share-based awards are accounted for as equity instruments, except for cash awards and CSBUs, which are accounted for as liabilities. The amounts included in the consolidated statements of operations relating to stock-based compensation associated with the two equity incentive plans, cash awards, and CSBUs are as follows:
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
2024 |
|
|
2023 |
|
|
2024 |
|
|
2023 |
|
||||
Research and development expenses |
|
$ |
1,034,174 |
|
|
$ |
862,370 |
|
|
$ |
1,898,358 |
|
|
$ |
3,053,738 |
|
General and administrative expenses |
|
|
901,355 |
|
|
|
711,170 |
|
|
|
1,586,008 |
|
|
$ |
2,710,157 |
|
Total stock-based compensation expense |
|
$ |
1,935,529 |
|
|
$ |
1,573,540 |
|
|
$ |
3,484,366 |
|
|
$ |
5,763,895 |
|
Stock Options
The table below summarizes activity relating to stock options under the incentive plans for the six months ended June 30, 2024:
|
|
Number of |
|
|
Weighted |
|
|
Weighted |
|
|
Aggregate |
|
||||
Outstanding at December 31, 2023 |
|
|
5,868,816 |
|
|
$ |
6.40 |
|
|
|
6.44 |
|
|
$ |
3,996 |
|
Granted |
|
|
2,376,328 |
|
|
$ |
3.67 |
|
|
|
|
|
|
— |
|
|
Expired |
|
|
(179,897 |
) |
|
$ |
7.90 |
|
|
|
|
|
|
— |
|
|
Forfeited |
|
|
(31,506 |
) |
|
$ |
4.49 |
|
|
|
|
|
|
— |
|
|
Outstanding at June 30, 2024 |
|
|
8,033,741 |
|
|
$ |
5.57 |
|
|
|
7.19 |
|
|
$ |
— |
|
Exercisable at June 30, 2024 |
|
|
4,829,541 |
|
|
$ |
6.28 |
|
|
|
5.86 |
|
|
$ |
— |
|
As of June 30, 2024, unamortized stock-based compensation for stock options outstanding was $10.2 million and is expected to be recognized over a weighted average period of 2.66 years. Total unrecognized compensation cost will be adjusted for future forfeitures, if necessary.
Restricted Stock Units
The table below summarizes activity relating to restricted stock units (RSUs) for the six months ended June 30, 2024:
|
|
Number |
|
|
Weighted-Average Grant Date Fair Value |
|
||
Outstanding at December 31, 2023 |
|
|
944,497 |
|
|
$ |
5.30 |
|
Settled in common stock |
|
|
(212,441 |
) |
|
|
5.33 |
|
Outstanding at June 30, 2024 |
|
|
732,056 |
|
|
$ |
5.29 |
|
16
There were no RSUs granted during the six months ended June 30, 2024. The total grant date fair value of RSUs vested was $1.1 million for the six months ended June 30, 2024. As of June 30, 2024, the outstanding RSUs had unamortized stock-based compensation of $2.8 million with a weighted-average remaining recognition period of 2.07 years and an aggregate intrinsic value of $2.4 million.
Employee Stock Purchase Plan
At June 30, 2024, the Company had 2,928,226 shares available for issuance under the 2016 Employee Stock Purchase Plan (2016 ESPP). A summary of the weighted-average grant-date fair value, and total stock-based compensation expense recognized related to the 2016 ESPP are as follows:
|
Six Months Ended June 30, |
|
|||||
|
2024 |
|
|
2023 |
|
||
Weighted-average grant-date fair value per share |
$ |
1.44 |
|
|
$ |
2.70 |
|
Total stock-based compensation expense |
$ |
9,505 |
|
|
$ |
26,734 |
|
The Company currently leases an office used to conduct business. The Company regularly evaluates the renewal options and when they are reasonably certain of exercise, the Company includes the renewal period in its lease term. As the Company’s lease does not provide an implicit rate, the Company uses its incremental borrowing rate based on the information available at the lease commencement date in determining the present value of the lease payments. In November 2023, the Company entered into a lease amendment that extended the lease by 12 months through December 31, 2024 and contained two options to extend the term of the lease for an additional 12 months each. Each option shall be exercisable, if at all, by giving a nine month written notice to the landlord. In April 2024, the Company extended the option to extend the term of the lease for an additional 12 months into December 2025, which will have no impact on the balance sheet as the option to extend the term of the lease for an additional 12 months was accounted for as of December 31, 2023.
As of June 30, 2024, the Company maintained an unamortized Right-Of-Use asset with a corresponding operating lease liability of approximately $0.4 million based on the present value of the minimum rental payments in accordance with ASC Topic 842, Leases. The weighted average discount rate used for leases as of June 30, 2024 is 9.1%. The weighted average remaining lease term as of June 30, 2024 was 1.50 years. The operating lease expense for the six months ended June 30, 2024 was $140.3 thousand. Maturities and balance sheet presentation of the Company’s lease liabilities for all operating leases as of June 30, 2024 is as follows:
2024 remaining total lease payments |
|
$ |
423,132 |
|
Less: effect of discounting |
|
|
(29,200 |
) |
Present value of lease liabilities |
|
|
393,932 |
|
|
|
|
|
|
Current operating lease liabilities |
|
$ |
255,039 |
|
Non-current operating lease liabilities |
|
$ |
138,893 |
|
Total |
|
$ |
393,932 |
|
The Company’s gross future minimum payments under all non-cancelable operating leases as of June 30, 2024, are:
|
|
Total |
|
|
2024 |
|
|
2025 |
|
|
2026 |
|
|
2027 |
|
|||||
Operating lease obligations |
|
$ |
423,132 |
|
|
$ |
137,928 |
|
|
$ |
285,204 |
|
|
$ |
— |
|
|
$ |
— |
|
Guarantees and Indemnifications
As permitted under Delaware law, the Company indemnifies its officers and directors for certain events or occurrences while the officer or director is, or was, serving at the Company’s request in such capacity. The term of the indemnification is for the officer’s or director’s lifetime. Through June 30, 2024, the Company had not experienced any losses related to these indemnification obligations and no material claims were outstanding.
17
The Company does not expect significant claims related to these indemnification obligations, and consequently, concluded that the fair value of these obligations is negligible, and no related reserves were established.
In-License Agreements
MEEI Agreement
The Company is developing ADX-2191 pursuant to an Exclusive License Agreement with Massachusetts Eye and Ear Infirmary (MEEI) originally entered into in July 2016 between MEEI and Helio Vision, Inc., as amended, (MEEI Agreement). The Company assumed the MEEI Agreement in connection with its 2019 acquisition of Helio Vision.
Pursuant and subject to the MEEI Agreement, the Company obtained an exclusive, worldwide license from MEEI to develop and commercialize ADX-2191 under certain patents and patent applications, and other licenses to intellectual property (MEEI Patent Rights). The Company has agreed to use commercially reasonable efforts, to develop ADX-2191, and to meet certain specified effort and achievement benchmarks by certain dates.
In consideration for the rights licensed under the MEEI Agreement, Helio Vision issued MEEI a number of shares of its preferred stock and Helio Vision agreed to pay non-creditable non-refundable license maintenance fees to MEEI of $15,000 on each of the second and third anniversary of the MEEI Agreement, $25,000 on each of the fourth and fifth anniversary of the MEEI Agreement and $35,000 on the sixth and each subsequent anniversary of the MEEI Agreement during the term of such agreement. In addition, Helio Vision was obligated to make future sales-dependent milestone payments to MEEI of up to the low seven figures in the aggregate, as well as royalty payments to MEEI at a rate which, as a percentage of net sales, is in the low single digits for products that incorporate or use the MEEI Patent Rights in the United States and as a percentage in the low single digits for products that incorporate or use the MEEI Patent Rights outside the United States. The Company is also obligated under the MEEI Agreement to pay MEEI a percentage of certain sublicense revenue that it receives in connection with entering into any sublicensing arrangements with any third parties, at a percentage rate which tiers downward from low-double digits to mid-single digits based on the date of the sublicense. Following the Company’s acquisition of Helio Vision, the Company became obligated to make any future payments owed under the MEEI Agreement. There is no additional equity consideration issuable under the MEEI Agreement.
The MEEI Agreement will remain in effect until the expiration date of the last to expire patent licensed under the MEEI Agreement. The Company may terminate the MEEI Agreement with timely written notice to MEEI. MEEI has the right to terminate the MEEI Agreement if it, subject to certain specified cure periods, ceases all business operations with respect to licensed products, fails to pay amounts due under the MEEI Agreement, fail to comply with certain due diligence obligations, defaults in the Company's obligation to maintain insurance, one of the Company's officers is convicted of a felony relating to the manufacture, use, sale or importation of licensed products, the Company materially breaches any provisions of the MEEI Agreement or in the event of its insolvency or bankruptcy.
In the event of an early termination of the MEEI Agreement, all rights licensed and developed by the Company under the MEEI Agreement may revert back to MEEI. The Company has agreed to indemnify MEEI for certain claims that may arise under the MEEI Agreement.
Legal Proceedings
On July 31, 2023, a purported stockholder filed a putative class action lawsuit (the Securities Class Action) in the U.S. District Court for the District of Massachusetts, against the Company and certain current and former officers, captioned Juliana Paice v. Aldeyra Therapeutics, Inc., et al. (No. 23-cv-11737). On January 2, 2024, the lead plaintiff filed an amended complaint. The lawsuit alleges violations by the defendants of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and SEC Rule 10b-5. The plaintiff alleges that the defendants made false or misleading statements or failed to disclose certain information concerning (i) the New Drug Application (NDA) for and the prospects of ADX-2191 for the treatment of primary vitreoretinal lymphoma and (ii) the NDA for and the prospects of reproxalap for the treatment of dry eye disease. The lawsuit seeks, among other things, compensatory damages on behalf of herself and all persons and entities that purchased or otherwise acquired the Company's securities between January 7, 2021, and October 16, 2023, as well as attorneys’ fees and costs. On March 4, 2024, defendants filed a motion to dismiss the amended complaint, the plaintiff filed its opposition to the motion on April 18, 2024, and defendants filed a reply to plaintiff's opposition on May 20, 2024. Oral argument on the motion to dismiss was heard on July 24, 2024. The Company disputes the plaintiff's claims and intends to vigorously defend the suit. At this time, the Company cannot reasonably predict the outcome or estimate potential losses, if any, that could result from this matter.
18
On March 7, 2024, a purported stockholder of the Company filed a derivative complaint in the U.S. District Court for the District of Massachusetts, captioned Fawaz Al-Jaljouli v. Todd C. Brady, et al. (No. 24-cv-10585), against certain of the Company’s executive officers and directors, and naming the Company as a nominal defendant. The derivative complaint alleges, purportedly on behalf of the Company, breach of fiduciary duty and violations of Section 14(a) of the Securities Exchange Act of 1934. The claims are based on substantially identical allegations as the complaint in the Securities Class Action. The lawsuit seeks, among other things, an award of damages, certain changes to the Company’s corporate governance, and attorneys’ fees and costs. On June 24, 2024, the plaintiff voluntarily dismissed all claims without prejudice.
In addition, from time to time, the Company is subject to litigation and claims arising in the ordinary course of business but, except as stated above, the Company is not currently a party to any material legal proceedings and the Company is not aware of any pending or threatened legal proceedings against them that the Company believes could have a material adverse effect on the Company's business, operating results, cash flows, or financial condition.
AbbVie Option Agreement
On October 31, 2023 (the Option Agreement Effective Date), the Company entered into an exclusive option agreement (the Option Agreement) with AbbVie Inc. (AbbVie), pursuant to which the Company granted AbbVie an exclusive option (the Option) to obtain (a) a co-exclusive license in the United States to facilitate a collaboration with the Company to develop, manufacture and commercialize reproxalap in the United States, (b) an exclusive license to develop, manufacture and commercialize reproxalap outside the United States, (c) a right of first negotiation for compounds that are owned or otherwise controlled by the Company in the field of ophthalmology relating to treating conditions of the ocular surface, and (d) a right to review data for any other compounds that are owned or otherwise controlled by the Company in the fields of ophthalmology and immunology before such data is shared with any other third party (the Collaboration Agreement). AbbVie has paid the Company a non-refundable payment of $1 million in consideration of the Option (the Option Payment).
On December 21, 2023, pursuant to the Option Agreement, AbbVie extended the period during which it may exercise the Option (the Exercise Period Extension) by paying the Company a non-refundable payment of $5 million (the Option Extension Fee). As a result of the Exercise Period Extension, AbbVie may exercise the Option by delivering written notice to the Company at any time during the period following the Option Agreement Effective Date until the earlier of (a) the tenth (10th) business day after the date, if any, that the Company receives approval from the U.S. Food and Drug Administration of the NDA for reproxalap in dry eye disease (the FDA Decision) and (b) the date that is eighteen (18) months after the Option Agreement Effective Date. If the Collaboration Agreement is entered into, the Option Payment and the Option Extension Fee will be credited against the upfront cash payment payable by AbbVie.
Upon AbbVie’s delivery of the agreement execution notice and the parties entering into the Collaboration Agreement, AbbVie would pay the Company a $100 million upfront cash payment, less the Option Payment and the Option Extension Fee. In addition, the Company would be eligible to receive up to approximately $300 million in regulatory and commercial milestone payments, inclusive of a $100 million milestone payment payable if the FDA Decision is received prior to or after the execution of the Collaboration Agreement. In the United States, the Company would share profits and losses with AbbVie from the commercialization of reproxalap according to a split of 60% for AbbVie and 40% for the Company. Outside of the United States, the Company would be eligible to receive tiered royalties on net sales of reproxalap. As of August 1, 2024, AbbVie has not exercised the Option.
As of December 31, 2023, the Company had recognized zero collaboration revenue and $6.0 million of deferred collaboration revenue, long-term, related to the Option Agreement and Exercise Period Extension. During the three months ended June 30, 2024, the deferred collaboration revenue was moved from a long-term liability to a current liability on the Company’s balance sheet as a result of the Option expiring pursuant to the terms of the Option Agreement in less than one year if not exercised. The Company concluded, using ASC 606 by analogy for recognition considerations as the Option Agreement was not considered to be a vendor-customer relationship, that the transaction price is $6.0 million (the Transaction Price), and all other amounts are excluded from the Transaction Price as they relate to fees that can only be achieved subsequent to the exercise of the Option. The Transaction Price was allocated to the single unit of account, the Option to enter into a future Collaboration Agreement which is a material right, as the Option Extension Fee and the Option Payment are creditable against the upfront payments payable by AbbVie if the Collaboration Agreement is entered into. The Company concluded that all other performance obligations were immaterial promises in the context of the Option Agreement and did not represent additional units of account. The Company will begin to recognize revenue, if and, when the Option is exercised or when the Option expires.
19
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Cautionary Note Regarding Forward-Looking Statements
Various statements throughout this report are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this report regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management are forward-looking statements. These statements are subject to risks and uncertainties and are based on information currently available to our management. Words such as, but not limited to, “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “contemplates,” “predict,” “project,” “target,” “likely,” “potential,” “continue,” “ongoing,” “design,” “might,” “objective,” “will,” “would,” “should,” “could,” or the negative of these terms and similar expressions or words, identify forward-looking statements. These statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. The events and circumstances reflected in our forward-looking statements may not occur and actual results could differ materially from those projected in our forward-looking statements. Meaningful factors which could cause actual results to differ include, but are not limited to:
20
All written and verbal forward-looking statements attributable to us or any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section. We caution investors not to rely on the forward-looking statements we make or that are made on our behalf. We undertake no obligation, and specifically decline any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events, or otherwise. You are advised, however, to consult any further disclosures we make on related subjects in any annual, quarterly, or current reports that we may file with the Securities and Exchange Commission (SEC).
We encourage you to read “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Risk Factors,” as well as our unaudited condensed consolidated financial statements contained in this quarterly report on Form 10-Q. We also encourage you to read our Annual Report on Form 10-K for the year ended December 31, 2023, which was filed with the SEC on March 7, 2024 (2023 Annual Report), and which contains a more complete discussion of the risks and uncertainties associated with our business. In addition to the risks described above and in our 2023 Annual Report, other unknown or unpredictable factors also could affect our results. Therefore, the information in this report should be read together with other reports and documents that we file with the SEC from time to time, including Forms 10-Q, 8-K, and 10-K, which may supplement, modify, supersede, or update those risk factors. There can be no assurance that the actual results or developments anticipated by us will be realized or, even if substantially realized, that our results will lead to the expected consequences to, or effects on us. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved.
Overview
Aldeyra Therapeutics, Inc., including its wholly-owned subsidiaries (we, us, or the Company), is a biotechnology company devoted to discovering innovative therapies designed to treat immune-mediated and metabolic diseases. Our approach is to develop pharmaceuticals that modulate protein systems, instead of directly inhibiting or activating single protein targets, with the goal of optimizing multiple pathways at once while minimizing toxicity. Our late-stage product candidates are reproxalap, a RASP modulator for the potential treatment of dry eye disease and allergic conjunctivitis, and ADX-2191, a novel formulation of intravitreal methotrexate for the potential treatment of retinitis pigmentosa. Our preclinical RASP platform includes ADX-248, ADX-743, ADX-631, and other product candidates in development for inflammatory and metabolic diseases. Our development pipeline, as of the date of filing of this quarterly report on Form 10-Q is illustrated below.
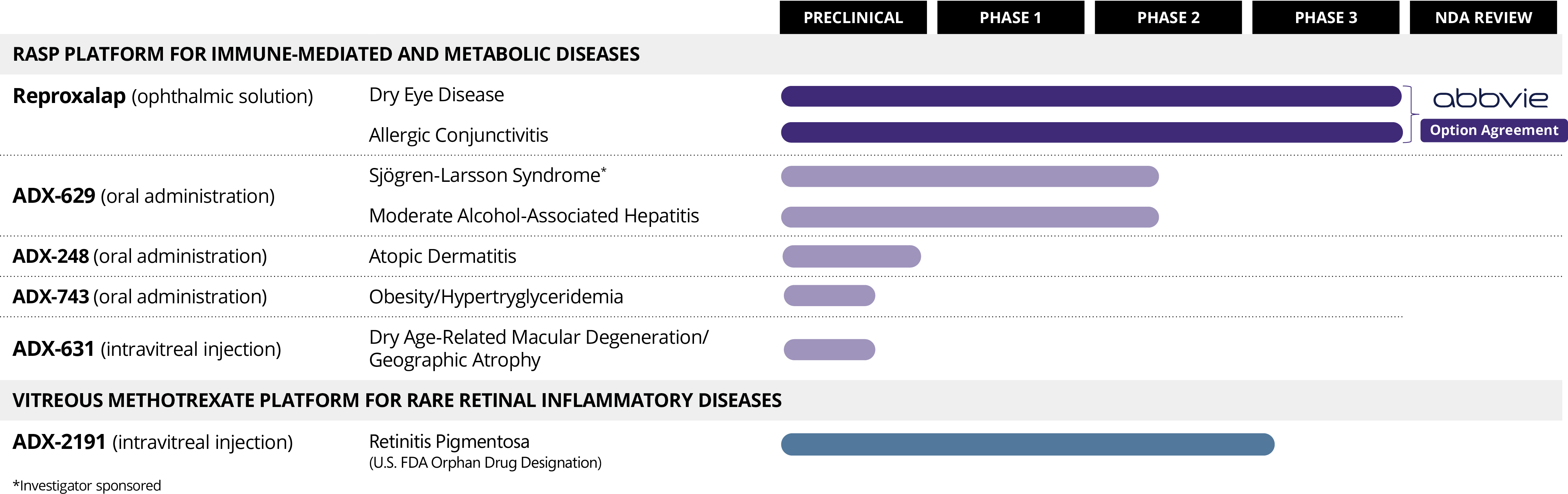
On October 31, 2023 (the Option Agreement Effective Date), we entered into an exclusive option agreement (the Option Agreement) with AbbVie Inc. (AbbVie), pursuant to which we granted AbbVie an exclusive option (the Option) to obtain (a) a co-exclusive license in the United States to facilitate a collaboration with us to develop, manufacture and commercialize reproxalap in the United States, (b) an exclusive license to develop, manufacture and commercialize reproxalap outside the United States, (c) a right of first negotiation for compounds that are owned or otherwise controlled by us in the field of ophthalmology relating to treating conditions of the ocular surface, and (d) a right to review data for any other compounds that are owned or otherwise controlled by us in the fields of ophthalmology and immunology before such data is shared with any other third party (the Collaboration Agreement).
21
AbbVie paid us a non-refundable payment of $1 million in consideration of the Option (the Option Payment).
On December 21, 2023, pursuant to the Option Agreement, AbbVie extended the period during which it may exercise the Option (the Exercise Period Extension) by paying us a non-refundable payment of $5 million (the Option Extension Fee). As a result of the Exercise Period Extension, AbbVie may exercise the Option by delivering written notice to us at any time during the period following the Option Agreement Effective Date until the earlier of (a) the tenth (10th) business day after the date, if any, that we receive approval from the U.S. Food and Drug Administration of the NDA for reproxalap in dry eye disease (the FDA Decision) and (b) the date that is eighteen (18) months after the Option Agreement Effective Date. If the Collaboration Agreement is entered into, the Option Payment and the Option Extension Fee will be credited against the upfront cash payment payable by AbbVie.
Upon AbbVie’s delivery of the agreement execution notice and the parties entering into the Collaboration Agreement, AbbVie would pay us a $100 million upfront cash payment, less the Option Payment and the Option Extension Fee. In addition, we would be eligible to receive up to approximately $300 million in regulatory and commercial milestone payments, inclusive of a $100 million milestone payment payable if the FDA Decision is received prior to or after the execution of the Collaboration Agreement. In the United States, we would share profits and losses with AbbVie from the commercialization of reproxalap according to a split of 60% for AbbVie and 40% for us. Outside of the United States, we would be eligible to receive tiered royalties on net sales of reproxalap. As of August 1, 2024, AbbVie has not exercised the Option.
All of our development plans and timelines are subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, funding, and other factors that could delay the initiation, completion, or reporting of clinical trials. Regulatory review timelines are flexible and subject to change based on the regulator’s workload and other potential review issues. The timing of ongoing clinical trials depends, in part, on the availability of clinical research facilities and staffing, and the ability to recruit patients.
As we continue to execute on our strategy of expanding our product candidate pipeline, we may license or acquire new immune-modulating approaches with novel therapeutic potential. In January 2019, for example, we acquired Helio Vision, Inc. (Helio) and thereby obtained rights to ADX-2191.
We have no products approved for sale in the United States or elsewhere. We will not receive any revenue from sales of our product candidates that we develop until we obtain regulatory approval. We intend to commercialize our products, if approved for sale, through collaborations. Although we may receive commercial and license revenue in the future, we have to date primarily funded our operations through the sale of our common stock, convertible preferred stock, convertible promissory notes, warrants, and borrowings under debt facilities. We will need to raise additional capital in the form of debt or equity or through partnerships to fund additional development of our product candidates, and we may in-license, acquire, or invest in complementary businesses or products. In addition, contingent on capital resources, we may augment, diminish, or otherwise modify the clinical development plan described herein.
In March 2021, we entered into an Open Market Sales Agreement SM with Jefferies LLC (Jefferies), as sales agent (2021 Jefferies Sales Agreement), under which we had the ability to offer and sell, from time to time through Jefferies, shares of common stock providing for aggregate sales proceeds of up to $100.0 million. As of June 30, 2024, no shares of common stock may be sold under the 2021 Jefferies Sales Agreement, and no sales had previously been made pursuant to the 2021 Jefferies Sales Agreement.
On January 28, 2019, we acquired Helio and thereby acquired rights to ADX-2191 pursuant to an Agreement and Plan of Merger dated as of January 24, 2019 (the Merger Agreement). As a result of the acquisition, we have issued an aggregate of 1,407,006 shares of common stock to the former securityholders and an advisor of Helio. Subject to the conditions of the acquisition agreement, we are contingently obligated to make additional payments to the former securityholders of Helio as follows: (a) $10.0 million of common stock following approval by the FDA of an NDA for the prevention and/or treatment of proliferative vitreoretinopathy or a substantially similar label prior to the 10th anniversary of the closing date; and (b) $2.5 million of common stock following FDA approval of an NDA for an indication (other than proliferative vitreoretinopathy or a substantially similar label) prior to the 12th anniversary of the closing date, provided that in no event shall we be obligated to issue more than 5,248,885 shares of common stock in the aggregate in connection with the acquisition. If we cease to use commercially reasonably efforts to develop and obtain regulatory approval for ADX-2191, subject to the terms and conditions of the Merger Agreement, ADX-2191 and related intellectual property rights may revert back to an entity designated by the representative of the former Helio stockholders. Additionally, in the event of certain change of control or divestitures by us, certain former convertible noteholders of Helio will be entitled to a tax gross-up payment in an amount not to exceed $1.0 million in the aggregate.
22
In March 2019, we entered into the Hercules Credit Facility, which provided for a term loan of up to $60.0 million, $15.0 million of which has been funded as of June 30, 2024. In April 2021, the Hercules Credit Facility was amended to, among other things, increase the amount which may become available for draw-down prior to May 2023, subject to the satisfaction of certain conditions contained therein, from $10.0 million to $20.0 million. In December 2022, the Hercules Credit Facility was further amended to, among other things, (i) extend the expiration of the period in which interest-only payments on borrowings from May 1, 2023 to May 1, 2024; (ii) extend the Maturity Date from October 1, 2023 to October 1, 2024; and (iii) extend the availability of the $20.0 million draw-down from May 2023 to May 2024, subject to the satisfaction of certain conditions contained therein. The Hercules Credit Facility contains customary affirmative and negative covenants and events of default. Affirmative covenants include, among others, covenants requiring us to maintain our legal existence and governmental approvals, deliver certain financial reports, and maintain insurance coverage. Negative covenants include, among others: restrictions on transferring any part of our business or intellectual property; incurring additional indebtedness; engaging in mergers or acquisitions; paying dividends or making other distributions; making investments; and creating other liens on our assets, in each case subject to customary exceptions. On April 29, 2024, the Hercules Credit Facility was further amended to, among other things, extend the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are made from May 1, 2024 to October 1, 2024. The Hercules Credit Facility, as amended, is described in Note 9 to the notes to the condensed consolidated financial statements contained in this Quarterly Report on Form 10-Q. As of June 30, 2024, $15.0 million was outstanding under the Hercules Credit Facility.
Research and development expenses
We expense all of our research and development expenses as they are incurred. Research and development costs that are paid in advance of performance are capitalized as a prepaid expense until incurred. Research and development expenses primarily include:
To date, substantially all of our research and development expenses have been incurred in connection with reproxalap and ADX-2191, as well as the proof of concept trials with ADX-629. We expect our research and development expenses to increase for the foreseeable future as we advance ADX-248, ADX-743, ADX-631, and other compounds through preclinical and clinical development. The process of conducting clinical trials necessary to obtain regulatory approval is costly and time consuming. We are unable to estimate with any certainty the costs we will incur in the continued development of our product candidates. Clinical development timelines, the probability of success, and development costs can differ materially from expectations. We may never succeed in achieving marketing approval for our product candidates. The costs of clinical trials may vary significantly over the life of a project owing to, but not limited to, the following:
23
Included in research and development are expenses associated with asset acquisitions. Assets purchased in an asset acquisition transaction are expensed as in-process research and development unless the assets acquired are deemed to have an alternative future use. Acquired in-process research and development payments are immediately expensed, and include upfront payments, as well as transaction fees and subsequent milestone payments. Development costs incurred after the asset acquisition are expensed as incurred.
We do not expect reproxalap or any of our other product candidates to be commercially available, if at all, before at least the first half of 2025.
General and administrative expenses
During the six months ended June 30, 2024 and 2023, our general and administrative expenses consisted primarily of employee-related expenses, including benefits and stock-based compensation for our full-time employees. Other general and administrative expenses include insurance premiums, consulting including pre-commercial costs, and professional fees for auditing, tax, investor relations, and legal services, including patent-related costs. We expect that general and administrative expenses will increase in the future as we expand our operating activities and continue to incur additional costs associated with being a publicly-traded company, including costs associated with maintaining compliance with exchange listing and SEC requirements. Increases in general and administrative expenses will likely include higher consulting costs, fees for commercializing our product candidates, legal fees, accounting fees, insurance premiums, and fees associated with investor relations.
Other income (expense)
Total other income (expense) consists primarily of interest income we earn on interest-bearing accounts and interest expense incurred on our outstanding debt.
Comprehensive loss
Comprehensive loss is defined as the change in equity during a period from transactions and other events and/or circumstances from non-owner sources. For the six months ended June 30, 2024, comprehensive loss is equal to our net loss of $24.9 million and our net unrealized loss on marketable securities of $9.7 thousand. For the six months ended June 30, 2023, comprehensive loss is equal to our net loss of $24.6 million and $0.1 million of losses on marketable securities reclassified to net loss.
Critical Accounting Estimates
The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of our financial statements, as well as the reported expenses during the reported periods. We evaluate these estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates under different assumptions or conditions. Our significant accounting policies are more fully described in the notes to our unaudited condensed consolidated financial statements in this Quarterly Report on Form 10-Q.
There were no material changes to our critical accounting estimates during the six months ended June 30, 2024, as compared to those described in Management’s Discussion and Analysis of Financial Condition and Results of Operations included in our 2023 Annual Report. It is important that the discussion of our operating results that follow be read in conjunction with the critical accounting policies disclosed in our 2023 Annual Report.
24
Results of Operations
We anticipate that our results of operations will fluctuate for the foreseeable future due to several factors, including the progress of our research and development efforts, the timing and outcome of clinical trials, and regulatory requirements. Our limited operating history makes predictions of future operations difficult or impossible. Since our inception, we have incurred significant losses.
Three months ended June 30, 2024 compared to three months ended June 30, 2023
Research and development expenses. Research and development expenses were $15.0 million for the three months ended June 30, 2024, compared to $7.0 million for the three months ended June 30, 2023. The increase of $8.0 million was primarily related to an increase in external clinical development costs and drug product manufacturing costs.
General and administrative expenses. General and administrative expenses were $3.0 million for the three months ended June 30, 2024, compared to $3.4 million for the three months ended June 30, 2023. The decrease of $0.4 million was primarily related to lower personnel costs, consulting expenditures, and legal costs.
Other income (expense). Total other income (expense), net, was $1.2 million and $1.4 million for the three months ended June 30, 2024 and 2023, respectively. The decrease in net other income of $0.2 million was due to lower interest income for the three months ended June 30, 2024, compared with the three months ended June 30, 2023.
Six months ended June 30, 2024 compared to six months ended June 30, 2023
Research and development expenses. Research and development expenses were $21.2 million for the six months ended June 30, 2024, compared to $18.2 million for the six months ended June 30, 2023. The increase of $3.0 million was primarily related to an increase in external clinical development costs and drug product manufacturing expenditures, offset by a decrease of $1.5 million in personnel costs.
General and administrative expenses. General and administrative expenses were $6.2 million for the six months ended six months ended June 30, 2024, compared to $8.9 million for the six months ended June 30, 2023. The decrease of $2.7 million was primarily related to lower personnel costs, consulting expenditures, and legal costs.
Other income (expense). Total other income (expense), net, was $2.5 million and $2.5 million for the six months ended June 30, 2024 and 2023, respectively, which reflects consistent interest income for the six months ended June 30, 2024 and 2023.
Liquidity and Capital Resources
We have funded our operations primarily from the sale of equity securities and convertible equity securities and borrowings under credit facilities. Since inception, we have incurred operating losses and negative cash flows from operating activities and have devoted substantially all our efforts to research and development. At June 30, 2024, we had total stockholders’ equity of approximately $98.3 million, and cash, cash equivalents, and marketable securities of $120.3 million. During the six months ended June 30, 2024, we had a net loss of approximately $24.9 million. We expect to generate operating losses for the foreseeable future.
In March 2021, we entered into the 2021 Jefferies Sales Agreement under which we had the ability to offer and sell, from time to time through Jefferies, shares of common stock providing for aggregate sales proceeds of up to $100.0 million. As of June 30, 2024, no shares of common stock may be sold under the 2021 Jefferies Sales Agreement, and no sales had previously been made pursuant to the 2021 Jefferies Sales Agreement.
In March 2019, we entered into the Hercules Credit Facility (the Loan and Security Agreement), pursuant to which a term loan of up to an aggregate principal amount of $60.0 million may be made available to us. The Loan and Security Agreement provides for (i) an initial term loan advance of up to $5.0 million at our option, which expired unutilized on April 15, 2019; (ii) three additional term loan advances of up to $15.0 million each, at our option, available to us upon the occurrence of certain funding conditions prior to September 30, 2019 (2019 Tranche), March 31, 2020 (2020 Tranche), and March 31, 2021 (2021 Tranche); and (iii) a final additional term loan advance (Fourth Loan Tranche) of up to $10.0 million prior to December 31, 2021, at our option, subject to approval by Lender’s investment committee. We drew down the 2019 Tranche in full in September 2019 and the 2020 Tranche and the 2021 Tranche expired unutilized prior to us satisfying the funding conditions for such tranche. On April 20, 2021, we entered into the First Amendment (First Amendment) to Loan and Security Agreement with Hercules. The First Amendment, among other things, (i) increased the Fourth Loan Tranche from $10.0 million to $20.0 million and extended the deadline for drawing down the Fourth Loan Tranche to July 1, 2022; (ii) lowered the variable per annum rate of interest on borrowings under the Loan and Security Agreement to the greater of (a) the Prime Rate plus 3.10% or (b) 8.60%; (iii) extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are required from May 1, 2021 to July 1, 2022; and (iv) following the satisfaction of certain conditions, which conditions were satisfied in April 2021, further extended the expiration of the interest-only period and the deadline for drawing down the Fourth Loan Tranche to May 1, 2023. On December 22, 2022, we entered into the Second Amendment (Second Amendment) to the Loan and Security Agreement with Hercules, which became effective as of December 31, 2022 (Second Amendment Effective Date).
25
The Second Amendment, among other things, (i) extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are made from May 1, 2023 to May 1, 2024; (ii) extended the Maturity Date from October 1, 2023 to October 1, 2024 (Maturity Date); (iii) extended the availability of the Fourth Loan Tranche commitment of $20 million, which remained conditioned on approval by the Lenders’ investment committee, from May 1, 2023 to May 1, 2024; and (iv) amended the Prepayment Charge (as defined therein) to equal 0.75% of the amount prepaid during the 12-month period following the Second Amendment Effective Date, and 0% thereafter. In addition, a supplemental end of term charge of $292,500 (Supplemental End of Term Charge) shall be due on the earlier of (A) the Maturity Date, as amended, or (B) repayment of the aggregate amount of advances under the Loan and Security Agreement. The initial end of term charge of $1,042,500 (End of Term Charge) was paid on October 2, 2023. On April 29, 2024, we entered into the Third Amendment (Third Amendment) to the Loan and Security Agreement with Hercules. The Third Amendment, among other things, extended the expiration of the period in which interest-only payments on borrowings under the Loan and Security Agreement are made from May 1, 2024 to October 1, 2024. On May 1, 2024, the Fourth Loan Tranche commitment expired unutilized.
The Loan and Security Agreement contains customary affirmative and negative covenants and events of default. Affirmative covenants include, among others, covenants requiring us to maintain our legal existence and governmental approvals, deliver certain financial reports, and maintain insurance coverage. Negative covenants include, among others: restrictions on transferring any part of our business or intellectual property; incurring additional indebtedness; engaging in mergers or acquisitions; paying dividends or making other distributions; making investments; and creating other liens on our assets, in each case subject to customary exceptions. As of June 30, 2024, $15.0 million was outstanding under the Hercules Credit Facility and no amounts remained available for borrowing.
On October 31, 2023 (the Option Agreement Effective Date), we entered into an exclusive option agreement (the Option Agreement) with AbbVie Inc. (AbbVie), pursuant to which we granted AbbVie an exclusive option (the Option) to obtain (a) a co-exclusive license in the United States to facilitate a collaboration with us to develop, manufacture and commercialize reproxalap in the United States, (b) an exclusive license to develop, manufacture and commercialize reproxalap outside the United States, (c) a right of first negotiation for compounds that are owned or otherwise controlled by us in the field of ophthalmology relating to treating conditions of the ocular surface, and (d) a right to review data for any other compounds that are owned or otherwise controlled by us in the fields of ophthalmology and immunology before such data is shared with any other third party (the Collaboration Agreement). AbbVie has paid us a non-refundable payment of $1 million in consideration of the Option (the Option Payment).
On December 21, 2023, pursuant to the Option Agreement, AbbVie extended the period during which it may exercise the Option (the Exercise Period Extension) by paying us a non-refundable payment of $5 million (the Option Extension Fee). As a result of the Exercise Period Extension, AbbVie may exercise the Option by delivering written notice to us at any time during the period following the Option Agreement Effective Date until the earlier of (a) the tenth (10th) business day after the date, if any, that we receive approval from the U.S. Food and Drug Administration of the NDA for reproxalap in dry eye disease (the FDA Decision) and (b) the date that is eighteen (18) months after the Option Agreement Effective Date. If the Collaboration Agreement is entered into, the Option Payment and the Option Extension Fee will be credited against the upfront cash payment payable by AbbVie.
AbbVie may exercise the Option by delivering the Option Exercise Notice to us at any time during the period following the Option Agreement Effective Date until the earlier of (a) the tenth (10th) business day after the FDA Decision Date and (b) the date that is eighteen (18) months after the Option Agreement Effective Date.
Upon AbbVie’s delivery of the agreement execution notice and the parties entering into the Collaboration Agreement, AbbVie would pay us a $100 million upfront cash payment, less the Option Payment and the Option Extension Fee. In addition, we would be eligible to receive up to approximately $300 million in regulatory, and commercial milestone payments, inclusive of a $100 million milestone payment payable if the FDA Decision is received prior to or after the execution of the Collaboration Agreement. In the United States, we would share profits and losses with AbbVie from the commercialization of reproxalap according to a split of 60% for AbbVie and 40% for us. Outside of the United States, we would be eligible to receive tiered royalties on net sales of reproxalap.
Based on our current operating plan and excluding any potential licensing and product revenue, we believe that our cash, cash equivalents and marketable securities as of June 30, 2024, will be sufficient to fund our currently projected operating expenses and debt obligations through 2026, including continued early and late-stage development of our product candidates. We base our projections of operating capital requirements on our current operating plan, which includes several assumptions that may prove to be incorrect, and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development, and commercialization (as applicable) of product candidates, we are unable to estimate the exact amount of our working capital requirements. We will need to secure additional funding in the future, from one or more equity or debt financings, collaborations, or other sources in order to carry out all of our planned research and development activities and regulatory activities; commence or continue ongoing commercialization, including manufacturing, sales, marketing and distribution for our product candidates; or conduct any substantial additional development requirements requested by the FDA. At this time, due to the risks inherent in the drug development process, we are unable to estimate with any certainty the costs we will incur in the continued clinical development of reproxalap, and our other product candidates.
26
Subsequent trials initiated at a later date will cost considerably more, depending on the results of our prior clinical trials, and feedback from the FDA or other third parties. Accordingly, we will continue to require substantial additional capital to continue our clinical development and potential commercialization activities. The amount and timing of our future funding requirements will depend on many factors, including but not limited to:
We may need or desire to obtain additional capital to finance our operations through debt, equity, or alternative financing arrangements. We may also seek capital through collaborations or partnerships with other companies. The issuance of debt could require us to grant additional liens on certain of our assets that may limit our flexibility. If we raise additional capital by issuing equity securities, the terms and prices for these financings may be much more favorable to the new investors than the terms obtained by our existing stockholders. These financings also may significantly dilute the ownership of our existing stockholders. We are in a period of economic uncertainty, inflation, and capital markets disruption, which has been significantly impacted by adverse developments affecting the financial services industry, including geopolitical instability due to, among other things, the continued hostilities in Ukraine and Israel and the surrounding areas. In addition, the disruption in the capital markets could make any financing more challenging, and there can be no assurance that we will be able to obtain such financing on commercially reasonable terms or at all. If we are unable to obtain additional financing, we may be required to reduce the scope of our future activities, which could harm our business, financial condition, and operating results. There can be no assurance that any additional financing required in the future will be available on acceptable terms, if at all.
We will continue to incur costs as a public company, including, but not limited to, costs and expenses for directors' fees; increased directors' and officers' insurance; investor relations fees; expenses for compliance with the Sarbanes-Oxley Act of 2002 and related to rules implemented by the SEC and Nasdaq, on which our common stock is listed; and various other costs. The Sarbanes-Oxley Act of 2002 requires that we maintain effective disclosure controls and procedures and internal controls.
Cash Flows
27
The following table summarizes our cash flows for the six months ended June 30, 2024 and 2023:
|
|
For the Six Months |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Net cash used in operating activities |
|
$ |
(23,196,985 |
) |
|
$ |
(22,801,654 |
) |
Net cash (used in) provided by investing activities |
|
|
(48,614,499 |
) |
|
|
30,000,000 |
|
Net cash provided by financing activities |
|
|
18,191 |
|
|
|
57,851 |
|
Net (decrease) increase in cash and cash equivalents |
|
$ |
(71,793,293 |
) |
|
$ |
7,256,197 |
|
Operating Activities. Net cash used in operating activities was $23.2 million for the six months ended June 30, 2024, compared to net cash used in operating activities of $22.8 million for the same period in 2023. The primary use of cash was to fund our operations. The increase in the amount of cash used in operating activities for the six months ended June 30, 2024 as compared to 2023 was principally due to an increase in net loss, primarily from research and development activities; changes in accrued expenses, due to the amount and timing of payments for research and development activities; and changes in prepayments, due to timing of payment and collection of a receivable; in addition to a decrease in stock compensation for the six months ended June 30, 2024 compared to the same period in 2023.
Investing Activities. Net cash used in investing activities was $48.6 million for the six months ended June 30, 2024, and $30.0 million provided by investing activities for the six months ended June 30, 2023. Net cash used in investing activities related to purchases of marketable securities for the six months ended June 30, 2024. Net cash provided by investing activities related to maturities of marketable securities for the six months ended June 30, 2023.
Financing Activities. Net cash provided by financing activities was $18.2 thousand for the six months ended June 30, 2024, compared to $57.9 thousand for the six months ended June 30, 2023. The net cash provided by financing activities for the six months ended June 30, 2024 and 2023 consisted of stock purchases under the employee stock purchase plan.
Item 3. Quantitative and Qualitative Disclosures about Market Risk.
Because we are allowed to comply with the disclosure obligations applicable to a "smaller reporting company," as defined by Rule 12b-2 of the Exchange Act, with respect to this Quarterly Report on Form 10-Q, we are not required to provide the information required by this Item.
Item 4. Controls and Procedures.
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our Disclosure Committee and management, including our Chief Executive Officer and Interim Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (Exchange Act)) as of the end of the period covered by this report. Based on our management’s evaluation (with the participation of our Chief Executive Officer and our Interim Chief Financial Officer), as of the end of the period covered by this report, our Chief Executive Officer and our Interim Chief Financial Officer have concluded that our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) during the three months ended June 30, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
28
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
On July 31, 2023, a purported stockholder filed a putative class action lawsuit (the Securities Class Action) in the U.S. District Court for the District of Massachusetts, against us and certain current and former officers, captioned Juliana Paice v. Aldeyra Therapeutics, Inc., et al. (No. 23-cv-11737). On January 2, 2024, the lead plaintiff filed an amended complaint. The lawsuit alleges violations by the defendants of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 and SEC Rule 10b-5. The plaintiff alleges that the defendants made false or misleading statements or failed to disclose certain information concerning (i) the NDA for and the prospects of ADX-2191 for the treatment of primary vitreoretinal lymphoma and (ii) the NDA for and the prospects of reproxalap for the treatment of dry eye disease. The lawsuit seeks, among other things, compensatory damages on behalf of herself and all persons and entities that purchased or otherwise acquired our securities between January 7, 2021, and October 16, 2023, as well as attorneys’ fees and costs. On March 4, 2024, defendants filed a motion to dismiss the amended complaint, the plaintiff filed its opposition to the motion on April 18, 2024, and defendants filed a reply to plaintiff’s opposition on May 20, 2024. Oral argument on the motion to dismiss was heard on July 24, 2024. We dispute the plaintiff's claims and intend to vigorously defend the suit. At this time, we cannot reasonably predict the outcome or estimate potential losses, if any, that could result from this matter.
On March 7, 2024, a purported stockholder of the Company filed a derivative complaint in the U.S. District Court for the District of Massachusetts, captioned Fawaz Al-Jaljouli v. Todd C. Brady, et al. (No. 24-cv-10585), against certain of the Company’s executive officers and directors, and naming the Company as a nominal defendant. The derivative complaint alleges, purportedly on behalf of the Company, breach of fiduciary duty and violations of Section 14(a) of the Securities Exchange Act of 1934. The claims are based on substantially identical allegations as the complaint in the Securities Class Action. The lawsuit seeks, among other things, an award of damages and restitution in favor of us, certain changes to our corporate governance, and attorneys’ fees and costs. On June 24, 2024, the plaintiff voluntarily dismissed all claims without prejudice.
In addition, from time to time, we are subject to litigation and claims arising in the ordinary course of business but, except as stated above, we are not currently a party to any material legal proceedings and we are not aware of any pending or threatened legal proceedings against us that we believe could have a material adverse effect on our business, operating results, cash flows or financial condition.
ITEM 1A. Risk Factors.
Our business is subject to numerous risks. You should carefully consider the risks described below together with the other information set forth in this quarterly report on Form 10-Q, which could materially affect our business, financial condition, and future results. The risks described below are not the only risks facing our company. Risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, prospects, financial condition, and operating results.
Summary of Risks Related to our Business
Below is a summary of the principal factors that make an investment in our common stock speculative or risky. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below and should be carefully considered, together with other information in this quarterly report on Form 10-Q and our other filings with the Securities and Exchange Commission before making investment decisions regarding our common stock.
29
Risks Related to the Potential Development and Commercialization of Reproxalap and our Product Candidates
Our business is dependent in large part on the successful commercialization of reproxalap, if approved. If we are unable to successfully obtain marketing approval for reproxalap or experience significant delays in doing so, or if, after obtaining marketing approvals, we or our strategic partners fail to successfully commercialize these product candidates, our business will be materially harmed.
We are dependent in large part on regulatory approval and successful commercialization of reproxalap for our future business success. There is a significant risk that we will fail to successfully obtain marketing approval and/or we or our partners will fail to successfully commercialize reproxalap. Of the large number of drugs in development in the pharmaceutical industry, only a small percentage result in the submission of an NDA to the FDA, and even fewer are approved for commercialization.
Prior to and following potential NDA approval, we will invest a significant portion of our time and financial resources on the commercialization of reproxalap. We cannot accurately predict when or if reproxalap will receive marketing approval. Our ability to generate product revenues will depend on our obtaining marketing approval for, and commercializing reproxalap alone or with others. The future regulatory and commercial success of reproxalap and our other product candidates is subject to a number of risks, including the following:
30
Furthermore, even if we do receive regulatory approval to market reproxalap or any of our other product candidates, any such approval may be subject to limitations on the indicated uses for which we may market the product. Accordingly, even if we are able to obtain the requisite financing to commercialize our product candidates or continue to fund our development programs, we cannot assure that reproxalap will be successfully commercialized, or our other product candidates will be successfully developed or commercialized. If we are unable to obtain regulatory approval for or, if approved, we or any of our future partners are unable to successfully commercialize reproxalap, and our other product candidates, we may not be able to generate sufficient revenue to continue our business.
To generate revenue, we will depend on FDA approval and successful commercialization of reproxalap. Our success in obtaining regulatory approval of reproxalap from the FDA depends on our ability to address the issues raised by the FDA in the reproxalap Complete Response Letter, and address any issues the FDA may raise in the future. If we are unable to successfully obtain FDA approval, or FDA approval is delayed or limited, our ability to generate revenue will be significantly delayed.
Our ability to generate revenue will depend on the successful development, regulatory approval and commercialization of reproxalap. We submitted an NDA for reproxalap for the treatment of the signs and symptoms of dry eye disease in December 2022. In February 2023, the FDA accepted the reproxalap NDA for filing and set a PDUFA date of November 23, 2023. On November 27, 2023, we announced that we had received a Complete Response Letter from the FDA (the reproxalap Complete Response Letter). In the reproxalap Complete Response Letter, the FDA stated that the NDA did not demonstrate “efficacy in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well-controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. On November 16, 2023, prior to receiving the reproxalap Complete Response Letter, we submitted to the FDA a Special Protocol Assessment (SPA) for a dry eye disease chamber crossover clinical trial (the proposed trial), which could potentially result in data acceptable for FDA review towards a potential NDA resubmission for reproxalap for the treatment of the signs and symptoms of dry eye disease. A SPA is an advanced declaration from the FDA that a planned trial’s design, clinical endpoints, and statistical analyses could potentially result in data acceptable for FDA review towards approval for the proposed indication. Based on SPA feedback received from the FDA in December 2023, we have amended the design and protocol of the proposed trial.
We cannot be certain that the design, conduct, and analysis of the results of the proposed trial will be sufficient to establish the effectiveness of reproxalap for treatment of dry eye disease to the FDA’s satisfaction, and therefore allow us to resubmit or receive approval of an NDA for reproxalap.
31
As part of the potential NDA resubmission, the FDA could require additional studies or clinical trials, and the submission of the results of those studies or clinical trials before a potential NDA resubmission will be reconsidered, which would require us to expend more resources than we planned or that are available to us, and could substantially delay acceptance and/or approval, if any, of a potential NDA resubmission. Any such requirement would increase our costs and delay approval and commercialization of reproxalap for the treatment of dry eye disease and would have a material adverse effect on our business and financial condition. Additionally, the FDA has substantial discretion in the approval process and may disagree with our interpretation of or the sufficiency of the data from our clinical trials. Clinical trial results frequently are susceptible to varying interpretations and regulatory authorities may disagree on what are appropriate methods for analyzing data, which may delay, limit or prevent regulatory approvals. There can be no assurance that a potential NDA resubmission to the FDA will be accepted or approved in a timely manner or at all. If marketing approval for reproxalap is delayed, limited or denied, our ability to market reproxalap, and our ability to generate product sales, would be adversely affected. Even if reproxalap is approved for the treatment of dry eye disease, the FDA may limit use to certain patient populations, include extensive warnings on the product labeling, or require costly ongoing requirements for post-marketing clinical studies and surveillance or other risk management measures to monitor the safety or efficacy of reproxalap.
Any regulatory approval of reproxalap, once obtained, may be withdrawn. Ultimately, the failure to obtain and maintain regulatory approvals would prevent reproxalap from being marketed and would have a material adverse effect on our business.
If the Option is not exercised by AbbVie and we remain responsible for funding further development and commercialization of reproxalap, we may be unable to raise the additional capital required to further develop and commercialize reproxalap or enter into a collaboration agreement with another pharmaceutical company with equivalent or comparable terms, or at all.
If the exclusive option (the Option) to enter into the Co-Development, Co-Commercialization and License Agreement (the Collaboration Agreement) is not exercised by AbbVie Inc. (AbbVie), pursuant to the exclusive option agreement with AbbVie, we will be responsible for funding further development and commercialization of reproxalap, and may be unable to raise the additional capital required to further develop and commercialize reproxalap or enter into a collaboration agreement with another pharmaceutical company with equivalent or comparable terms, or at all. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs and reproxalap commercialization efforts.
If we are required to continue the development and commercialization of reproxalap on our own, we may need to build its marketing, sales, distribution, managerial and other non-technical capabilities to commercialize reproxalap or make arrangements with third parties to perform these services. The establishment and development of our own sales force or the establishment of a contract sales force to market reproxalap would be expensive and time-consuming and could delay any commercial launch. Moreover, we cannot be certain that we will be able to successfully develop this capability. We would have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. We would also face competition in its search for third parties to assist it with the sales and marketing efforts of reproxalap.
If the Option is exercised and the Collaboration Agreement is executed, then pursuant to the terms of the Collaboration Agreement, we would work closely with AbbVie to execute a commercialization plan for reproxalap in the United States, and this commercialization plan may never achieve its desired outcomes.
Pursuant to the terms of the Collaboration Agreement, we would work with AbbVie to execute a joint commercialization plan for reproxalap in the United States, and execute upon the commercialization plan with the intention to optimize the commercial potential of reproxalap. If this collaboration is not successful, then our business, financial condition, and results of operations could be adversely affected.
If we fail to develop and commercialize other product candidates, we may be unable to grow our business.
As part of our growth strategy, we plan to evaluate the development and commercialization of other therapies related to immune-mediated and metabolic diseases. We will evaluate internal opportunities from our compound libraries, and also may choose to continue to in-license or acquire other product candidates, as well as commercial products, to treat patients suffering from immune-mediated and metabolic disorders with high unmet medical needs and limited treatment options. These other product candidates will require additional, time-consuming development efforts prior to commercial sale, including preclinical studies, clinical trials, and approval by the FDA and/or applicable foreign regulatory authorities. In-licensed product candidates may have been unsuccessfully developed by others in indications similar to those that we may pursue. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development, including the possibility that the product candidate will not be shown to be sufficiently safe and/or effective for approval by regulatory authorities. For example, in June 2023, we received a Complete Response Letter from the FDA regarding our NDA for ADX-2191 for the treatment of primary vitreoretinal lymphoma (the ADX-2191 Complete Response Letter). The ADX-2191 Complete Response Letter stated that there was a “lack of substantial evidence of effectiveness” due to “a lack of adequate and well-controlled investigations” in the literature-based NDA submission. In light of the FDA’s ADX-2191 Complete Response Letter, we halted pre-commercial activities related to ADX-2191 for the treatment of primary vitreoretinal lymphoma.
32
In January 2024 we de-prioritized the previously announced programs of ADX-629 in chronic cough and idiopathic nephrotic syndrome due to regulatory and trial feasibility challenges, respectively. Additionally, in the three months ended March 31, 2024, we deprioritized ADX-2191 for the treatment of proliferative vitreoretinopathy and primary vitreoretinal lymphoma due to the requirement from the FDA to run clinical trials that we did not deem to be feasible. If marketing approval for our other product candidates is delayed, limited or denied, our ability to market the product candidate, and our ability to generate product sales, would be adversely affected. Such a delay could occur because a competitor product is approved before our product and secures patent protection, market exclusivity, or both, and thereby precludes our product approval for a number of years. It is also possible that additional studies or clinical trials may not suffice to make our application approvable. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, adequately priced, successfully commercialized, or widely accepted in the marketplace or be more effective than other commercially available alternatives.
Any termination or suspension of, or delays in the commencement or completion of, our clinical trials could result in increased costs to us, delay or limit our ability to generate revenue, and adversely affect our commercial prospects.
Delays in the commencement or completion of our ongoing or planned clinical trials for our product candidates could significantly affect our product development costs and timeline. We do not know whether future trials will begin on time or be completed on schedule, if at all. The commencement and completion of clinical trials can be delayed for a number of reasons, including delays related to:
Product development costs will increase if we have delays in testing or approval of our product candidates or if we need to perform more, larger, or longer clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur and we or our partners may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit our clinical trial protocols to IRBs for reexamination, which may impact the costs, timing, or successful completion of a clinical trial. If we experience delays in completion of, or if we, the FDA, or other regulatory authorities, the IRB, other reviewing entities, or any of our clinical trial sites suspend or terminate any of our clinical trials, the commercial prospects for a product candidate may be harmed and our ability to generate product revenues, if any, will be delayed.
33
In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial of regulatory approval of a product candidate. Further, if one or more clinical trials are delayed, our competitors may be able to bring products to market before we do, and the commercial viability of our product candidates could be significantly reduced.
Reproxalap and our other product candidates are subject to extensive regulation, compliance with which is costly and time consuming, and such regulation may cause unanticipated delays, or prevent the receipt of the required approvals to commercialize our product candidates.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing, and distribution of our product candidates are subject to extensive regulation by the FDA in the United States and by comparable authorities in foreign markets. In the United States, we are not permitted to market our product candidates until we receive regulatory approval from the FDA. The process of obtaining regulatory approval is expensive and time-consuming, and can vary substantially based upon the type, complexity, and novelty of the products involved, as well as the target indication, and patient population. Approval policies or regulations may change, and the FDA has substantial discretion in the drug approval process, including the ability to delay, limit, or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval, and subsequent commercial success is uncertain and not guaranteed.
Reproxalap and our other product candidates, and the activities associated with development and commercialization, including testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale, and distribution, are subject to extensive regulation by the FDA and other regulatory agencies in the United States and by comparable authorities in other jurisdictions.
Our ongoing research and development activities and planned clinical development and commercialization for our product candidates may be delayed, modified, or ceased for a variety of reasons, including:
34
The FDA or comparable foreign regulatory authorities can delay, limit, or deny approval of a product candidate for many reasons, including but not limited to:
With respect to foreign markets, approval procedures vary among countries and, in addition to the aforementioned risks, can involve additional product testing, administrative review periods, and agreements with pricing authorities. In addition, events raising questions about the safety of certain marketed pharmaceuticals may result in increased cautiousness by the FDA and comparable foreign regulatory authorities in reviewing new drugs based on safety, efficacy, or other regulatory considerations and may result in significant delays in obtaining regulatory approvals. Any delay in obtaining, or inability to obtain, applicable regulatory approvals would prevent us or any of our future development partners from commercializing our product candidates. Moreover, we cannot predict healthcare reform initiatives, including potential reductions in federal funding or insurance coverage, that may be adopted in the future and whether or not any such reforms would have an adverse effect on our business and our ability to obtain regulatory approval for our current or future product candidates. There are evolving legal requirements that will continue to affect our business.
Because the Company has no experience in commercializing pharmaceutical products, there is a limited amount of information about us upon which to evaluate our product candidates and business prospects.
We have not yet demonstrated an ability to successfully overcome many of the pre-commercial and commercial risks and uncertainties frequently encountered by companies in new and rapidly evolving fields, particularly in the biopharmaceutical area. For example, to execute our business plan we will need to successfully:
35
If we are unsuccessful in accomplishing these objectives, we may not be able to develop product candidates, raise capital, expand our business, or continue our operations. Further, even if we are successful in clinical trials of product candidates, we may choose to place further development or commercialization on hold given perceived marketing challenges or the relative differences in commercial attractiveness within our portfolio.
The results of preclinical studies and earlier clinical trials are not always predictive of future results. Any product candidate we or any of our future development partners advance into clinical trials may not have favorable results in later clinical trials, if any, or receive regulatory approval.
Drug development has inherent risk. We or any of our future development partners will be required to demonstrate through adequate and well-controlled clinical trials that our product candidates are safe and effective, with a favorable benefit-risk profile, for use in their target indications before we can seek regulatory approvals for their commercial sale. Drug development is a long, expensive, and uncertain process, and delay or failure can occur at any stage of development, including after commencement of any of our clinical trials. Any negative results or perceived negative results in clinical trials for one indication may have an adverse effect on our ability to develop and potentially commercialize reproxalap or our other product candidates for the treatment of another indication. In addition, as product candidates proceed through development, the trial designs may often be different and may need to evolve and change from phase to phase or within the same phase or same trial, as is the case for adaptive trials; the vehicles or controls may be modified from trial to trial; and the product formulations or manufacturing process may differ due to the need to test product candidate samples that can be manufactured on a commercial scale. Success in run-in cohorts, earlier clinical trials, or clinical trials focused on a different indication does not mean that later clinical trials will be successful because product candidates in later-stage clinical trials may fail to demonstrate sufficient safety or efficacy despite having progressed through other phases of clinical testing. In addition, discussions with regulatory bodies, such as the FDA, may lead to changes in trial designs or programs. Companies frequently suffer significant setbacks in advanced clinical trials, even after run-in cohorts or earlier clinical trials have shown promising results. For example, the results of the TRANQUILITY Trial of reproxalap in dry eye disease did not reflect the results of the TRANQUILITY run-in cohort. Moreover, only a small percentage of drugs under development result in the submission of an NDA to the FDA and even fewer are approved for commercialization.
Because we are developing novel product candidates for the treatment of diseases in a manner which there is little clinical drug development experience and, in some cases, are designing adaptive trials or using new endpoints or methodologies, the regulatory pathways for approval are not well defined, and, as a result, there is greater risk that our clinical trials will not result in our desired outcomes or require additional trials.
Our clinical focus is on the development of new products for immune-mediated and metabolic diseases. We performed an adaptive trial in proliferative vitreoretinopathy, the GUARD trial, and may do so with other indications in the future. In an adaptive trial, the initial parts of the trial are not designed to be pivotal or definitive. Rather, the initial parts of adaptive trials are expected to provide data to guide subsequent parts of the trial, which could require design changes, including but not limited to, different endpoints. In addition, following the initial parts of adaptive trials, we may, among other things, decide to continue to the subsequent parts of the trial, conclude the trial based on its success or failure in such initial parts, or discuss the trial results and regulatory pathway with regulatory authorities prior to determining next steps with respect to the trial and development program. As such, the likelihood of success in our late-stage clinical programs cannot necessarily be predicted.
We could also face challenges in designing clinical trials and obtaining regulatory approval of our product candidates due to the lack of historical clinical trial experience for novel classes of therapeutics. Thus, it is difficult to determine whether regulatory agencies will be receptive to the approval of our product candidates, and to predict the time and costs associated with obtaining regulatory approvals.
36
The clinical trial requirements of the FDA and other regulatory agencies and the criteria regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty, and intended use and market of the potential products. The regulatory approval process for novel product candidates such as ours can be more expensive and require more time and trial data than for other, better known, or more extensively studied classes of product candidates. In addition, it is possible that, as regulatory bodies gain more familiarity with our type of product candidates by reviewing competitor candidates, those agencies could impose new conditions on our product candidates that we did not expect. Any inability to design clinical trials with protocols, methodology, and endpoints acceptable to applicable regulatory authorities, and to obtain regulatory approvals for our product candidates, would have an adverse impact on our business, prospects, financial condition, and results of operations.
Because some of our product candidates are, to our knowledge, new chemical entities, it is difficult to predict the time and cost of development and our ability to successfully complete clinical development of these product candidates and obtain the necessary regulatory approvals for commercialization.
Some of our product candidates are, to our knowledge, new chemical entities, and unexpected problems related to new technologies may arise that can cause us to delay, suspend, or terminate our development efforts. As a result, short and long-term safety, as well as prospects for efficacy, are not fully understood and are difficult to predict. Regulatory approvals of new product candidates can be more expensive and take longer than approvals for well-characterized or more extensively studied pharmaceutical product candidates. Following discussions with the FDA and experts in the field, we may determine that it is not cost effective for us to develop one or more of our products in certain indications or we may decide to cease development in that area or seek a strategic partner.
We may not be able to qualify for or obtain various designations from regulators that would have the potential to expedite the review process of one or more of our product candidates, and even if we do receive one or more of such designations there is no guarantee that they will ultimately expedite the process, or aid in our obtaining marketing approval or provide market exclusivity.
There exist several designations that we can apply for from the FDA and other regulators that would provide us with various combinations of the potential for expedited regulatory review, certain financial incentives as well as the potential for post-approval exclusivity for a period of time. These designations include but are not limited to orphan drug designation, breakthrough therapy designation, accelerated approval, fast track status, and priority review for our product candidates. We may seek one or more of these designations for our current and future product candidates. For example, ADX-2191 has received orphan designation for the treatment of retinitis pigmentosa. There can be no assurance that any of our other product candidates will qualify for any of these designations. There can also be no assurance that any of our product candidates, that do qualify for these designations, will be granted such designations or that the FDA will not revoke such a designation it grants at a later date. Further, there can be no assurance that any of our product candidates that are granted such designations will ever benefit from such designations or that the FDA would not withdraw such designations once granted. Were we to receive a designation that promised a period of market exclusivity, such as orphan drug exclusivity, such exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition. Further, with respect to orphan drug status, even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior if it is shown to be safer, more effective, or makes a major contribution to patient care.
To preserve trial integrity, clinical data from the initial parts of adaptive clinical trials may not be disclosed.
Adaptive clinical trials are often performed such that the initial parts of the trial are used to determine sample size and endpoints for subsequent, possibly pivotal parts of the trial. Results from the initial parts of adaptive trials are therefore not designed to be pivotal or definitive, and, in some cases, detailed trial data may not be disclosed so as not to positively or negatively bias investigators or patients involved in subsequent parts of the trial. Further, the initial parts of adaptive trials may be performed in part to assess biomarkers or surrogate markers that may require substantial time to generate, analyze, and interpret. Thus, disclosure of clinical results from the initial parts of adaptive trials may also be delayed due to the time required for biomarker or surrogate marker assessment.
We may find it difficult to enroll patients in our clinical trials or identify patients during commercialization (if our products are approved by regulatory agencies) for product candidates addressing orphan or rare diseases.
As part of our business strategy, we have and continue to evaluate the development and commercialization of product candidates for the treatment of orphan and other rare diseases, including Sjögren-Larsson and retinitis pigmentosa. We may not be able to initiate or continue clinical trials if we are unable to locate a sufficient number of eligible patients willing and able to participate in the clinical trials required by the FDA or other non-United States regulatory agencies.
37
In addition, if others develop products for the treatment of similar diseases, we would potentially compete with them for the enrollment in rare patient populations, which may adversely impact the rate of patient enrollment in and the timely completion of our current and planned clinical trials. Any negative results or perceived negative results in clinical trials of our product candidates may make it difficult or impossible to recruit or retain patients in other clinical trials of the same product candidate. Insufficient patient enrollment may be a function of other factors, including the size and nature of the patient population, the nature of the protocol, the proximity of patients to clinical sites, the timing and magnitude of disease symptom presentation, the availability of effective treatments for the relevant disease, and the eligibility criteria for the clinical trial. Our inability to identify and enroll a sufficient number of eligible patients for any of our current or future clinical trials would result in significant delays or may require us to abandon one or more clinical trials or development program. Public health epidemics or pandemics and the response thereto may have an impact on our ability to enroll and retain patients in our clinical trials. For instance, patient enrollment in our GUARD trial of ADX-2191 in proliferative vitreoretinopathy was negatively impacted as a result of limited clinical trial staffing at trial sites and some patients electing to delay surgery. Delays in patient enrollment in the future as a result of these and other factors may result in increased costs or may affect the timing or outcome of our clinical trials, which could prevent us from completing these trials and adversely affect our ability to advance the development of our product candidates. For instance, in rare diseases such as proliferative vitreoretinopathy and idiopathic nephrotic syndrome, lack of availability of, or difficulty recruiting or retaining a sufficient number of, patients may make it difficult or cost-prohibitive to sufficiently power our clinical trials, which may not enable us to continue development and seek regulatory approval for the applicable product candidate. Further, if our products are approved by regulatory agencies, we may not be able to identify sufficient number of patients to generate significant revenues.
Any product candidate we or any of our future development partners advance into clinical trials may cause unacceptable adverse events or have other properties that may delay or prevent its regulatory approval or commercialization or limit its commercial potential.
Unacceptable adverse events caused by any of our product candidates that we or others advance into clinical trials could cause us or regulatory authorities to interrupt, delay, or halt clinical trials, or impose a clinical hold, potentially resulting in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications and markets. This in turn could prevent us from completing development or commercializing the affected product candidate and generating revenue from its sale.
We continue to develop our product candidates for the treatment of the indications for which we intend to seek approval, and we currently do not know the full extent of adverse events that will be observed in subjects that receive any of our product candidates. If any of our product candidates cause unacceptable adverse events in clinical trials, which may be larger or longer than those previously conducted, we may not be able to obtain regulatory approval or commercialize such product candidate.
Even if we obtain marketing approval for reproxalap or any other product candidate, it could be subject to restrictions or withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any are approved.
Even if United States regulatory approval is obtained, the FDA may still impose significant restrictions on a product’s indicated uses or marketing or impose ongoing requirements for potentially costly and time-consuming post-approval studies or clinical trials, post-market surveillance, or other potential additional clinical trials. Following approval, if any, of reproxalap or any other product candidate, such candidate will also be subject to ongoing FDA requirements governing the labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping, and reporting of safety and other post-market information. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMP requirements, including those relating to quality control, quality assurance, and corresponding maintenance of records and documents. If we or a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated seriousness, severity, or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requesting recall or withdrawal of the product from the market or suspension of manufacturing.
If we or the manufacturing facilities for reproxalap or any other product candidate that may receive regulatory approval, if any, fail to comply with applicable regulatory requirements, a regulatory agency may:
38
The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue.
The FDA has the authority to require a risk evaluation and mitigation strategy (REMS) plan as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria, and requiring treated patients to enroll in a registry.
In addition, if reproxalap or any of our other product candidates is approved, its product labeling, advertising, and promotion would be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. If we receive marketing approval for a product candidate, physicians may nevertheless prescribe it to patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The government has also entered into consent decrees and Corporate Integrity Agreements under which specified promotional conduct is changed or curtailed.
Even if we receive regulatory approval for reproxalap or any other product candidate, we or are partners, if any, still may not be able to successfully commercialize, and the revenue that we generate from its sales, if any, could be limited.
Even if our product candidates receive regulatory approval, they may not gain market acceptance among physicians, patients, healthcare payors, or the medical community. Coverage and reimbursement of our product candidates by third-party payors, including government payors, is also generally necessary for commercial success. In addition, we or are partners, if any, may not be able to secure advantageous contracts with payors or price our products at the expected level or at levels that make successful commercialization viable. The pricing of our products will be subject to numerous factors, many of which are outside of our control, including the pricing of similar products. The degree of market acceptance of our product candidates will depend on a number of factors, including but not limited to:
39
In addition, because the active ingredient of ADX-2191 (methotrexate) is a generic drug, a generic manufacturer may be able to develop and market a competitive intravitreal formulation of methotrexate following expiration of commercial exclusivity mandated via certain orphan drug designations. Generic drug competition would have a material and adverse effect on the commercial potential of ADX-2191. Further, our ability to successfully commercialize ADX-2191, if approved, depends on a number of additional factors, including but not limited to, the level of enforcement by the FDA to ensure that compounded copies of commercially available FDA-approved products manufactured by compounding pharmacies, including compounded copies of ADX-2191, that may be in violation of the federal Drug Quality and Security Act (DQSA) and other relevant provisions of the United States Federal Food, Drug, and Cosmetic Act (FDCA), are not produced and dispensed to patients.
Moreover, we cannot predict what healthcare reform initiatives may be adopted in the future. Further federal and state legislative and regulatory developments are likely, and we expect that ongoing initiatives in the United States will increase pressure on drug pricing. Such reforms could have an adverse effect on the pricing of and anticipated revenues from our current or future product candidates for which we may obtain regulatory approval and may affect our overall financial condition and ability to develop product candidates.
If any product candidate is approved but does not achieve an adequate level of acceptance by physicians, hospitals, healthcare payors, or patients, we may not generate sufficient revenue from that product candidate and may not become or remain profitable. Our or our partners’ efforts to educate the medical community and third-party payors on the benefits of reproxalap or any of our other product candidates may require significant resources and may never be successful. In addition, our or our partners’ ability to successfully commercialize our product candidate will depend on our ability to manufacture our products, differentiate our products from competing products and defend the intellectual property of our products. Competitors with numerous approved products may be able to negotiate pricing and reimbursement that is substantially more advantageous than that which we will be able to negotiate.
Additionally, if any of our competitors’ products are approved and are unable to gain market acceptance for any reason, there could be a market perception that products such as reproxalap are not able to adequately meet an unmet medical need. If we or our partners, if any, are unable to demonstrate to physicians, hospitals, third-party payors, or patients that our products are better alternatives, we or our partners, if any, may not be able to gain market acceptance for our products at the levels we anticipate and our business may be materially harmed as a result.
If the market opportunities for reproxalap and our other product candidates are smaller than we believe they are, and if we are not able to successfully identify patients and achieve significant market share, our revenues may be adversely affected and our business may suffer.
We focus our research and product development on treatments for immune-mediated and metabolic diseases. Our estimated addressable markets and market opportunities for our product candidates are based on a variety of inputs, including data published by third parties, our own market insights and internal market intelligence, and internally generated data and assumptions. We have not independently verified any third-party information and cannot be assured of its accuracy or completeness. Our projections of both the number of people who have diseases in our target markets, as well as the subset of people with diseases who have the potential to benefit from treatment with our product candidates, are based on estimates that have been derived from a variety of sources, including scientific literature, surveys of clinics, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of diseases in our target markets. The number of patients may turn out to be lower or more difficult to identify than expected. In addition, our product candidates may not achieve commercial success due to market conditions or regulatory challenges.
Any of these factors may negatively affect our ability to generate revenues from sales of our product and our ability to achieve and maintain profitability, and as a consequence, our business may suffer. In addition, these inaccuracies or errors may cause us to misallocate capital and other critical business resources, which could harm our business.
40
Reimbursement may be limited or unavailable in certain market segments for our product candidates, which could make it difficult for us to sell our product candidates profitably.
Market acceptance and sales of our product candidates will depend significantly on the availability of adequate insurance coverage and reimbursement from third-party payors for any of our product candidates and may be affected by existing and future health care reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will pay for and establish reimbursement levels. The reimbursement levels may be significantly less than the currently anticipated pricing of our product candidates. As a result of negative trends in the general economy in the United States or other jurisdictions in which we may do business, government authorities or third-party payors may be unable to satisfy reimbursement obligations or may delay payment. Reimbursement by a third-party payor may depend upon a number of factors including the third-party payor’s determination that use of a product candidate is:
Obtaining coverage and reimbursement approval for a product candidate from a government or other third-party payor is a time-consuming and costly process that could require us to provide supporting scientific, clinical, and cost effectiveness data for the use of the applicable product candidate to the payor. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. We cannot be sure that coverage or adequate reimbursement will be available for any of our product candidates. Further, we cannot be sure that reimbursement amounts will not reduce the demand for, or the price of, our product candidates. If reimbursement is not available or is available only in limited levels, we may not be able to commercialize certain of our product candidates profitably, or at all, even if approved. In recent years, through legislative and regulatory actions, the federal government has made substantial changes to the United States healthcare system, including changes to the methods for, and amounts of, Medicare reimbursement. Many members of the United States Congress have attempted to repeal and replace the Patient Protection and Affordable Care Act (PPACA), but they have been unsuccessful in doing so as of the date of the filing of this report. We cannot predict the ultimate form or timing of any repeal or replacement of PPACA or the effect such repeal or replacement would have on our business. Regardless of the impact of repeal or replacement of PPACA on us, the government has shown significant interest in pursuing healthcare reform and reducing healthcare costs. These reforms could significantly reduce payments from Medicare and Medicaid over the next ten years. Reforms or other changes to these payment systems, including modifications to the conditions on qualification for payment, bundling of payments, or the imposition of enrollment limitations on new providers, may change the availability, methods, and rates of reimbursements from Medicare, private insurers, and other third-party payers for our current and future product candidates, if any, for which we are able to obtain regulatory approval. Some of these changes and proposed changes could result in reduced reimbursement rates for such product candidates, if approved, which would adversely affect our business strategy, operations, and financial results.
As a result of legislative proposals and the trend toward managed health care in the United States, third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drugs. Payors may also refuse to provide coverage of approved product candidates for medical indications other than those for which the FDA has granted market approvals. As a result, significant uncertainty exists as to whether and how much third-party payors will reimburse patients for use of newly approved drugs, which in turn could lower drug pricing. We expect to experience pricing pressures in connection with the sale of our product candidates due to the trend toward managed health care, the increasing influence of health maintenance organizations, larger companies contracting with payors to diminish reimbursement for competitive products, and additional legislative proposals as well as country, regional, or local healthcare budget limitations.
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products.
The process of manufacturing our products is complex, highly regulated, and subject to several risks, including:
41
In order to conduct clinical trials of our product candidates, we will need to manufacture them in large quantities. Quality issues may arise during scale-up activities. Our reliance on a limited number of Contract Manufacturing Organizations (CMOs), the complexity of drug manufacturing and the difficulty of scaling up a manufacturing process could cause the delay of clinical trials, regulatory submissions, required approvals or commercialization of our product candidates, and cause us to incur higher costs and prevent us from commercializing our product candidates successfully. Furthermore, if our CMOs fail to deliver the required commercial quality and quantities of materials on a timely basis and at commercially reasonable prices, and we are unable to secure one or more replacement CMOs capable of production in a timely manner at a substantially equivalent cost, then testing and clinical trials of that product candidate may be delayed or infeasible, and regulatory approval or commercial launch of any resulting product may be delayed or not obtained, which could significantly harm our business. In addition, failure of CMOs to comply with regulatory and quality requirements could delay manufacturing or the review of our marketing applications.
Any adverse developments affecting manufacturing operations for our products, including public health epidemics or pandemics or responses taken thereto, may result in shipment delays; inventory shortages; lot failures; product withdrawals, recalls, approvals; or other interruptions in the supply of our products. We may also have to account for inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts, or seek more costly manufacturing alternatives.
Issues with product quality could have a material adverse effect upon our business, subject us to regulatory actions and cause a loss of customer confidence in us or our products.
Our success depends upon the quality of our products. Quality controls, assurance, and management plays an essential role in meeting customer requirements, preventing defects, improving our product candidates and services, and assuring the safety and efficacy of our product candidates. Our future success depends on our ability to maintain and continuously improve our quality management program. A quality or safety issue may result in adverse inspection reports, warning letters, product recalls or seizures, monetary sanctions, injunctions to halt manufacture and distribution of products, civil or criminal sanctions, costly litigation, refusal of a government to grant approvals and licenses, restrictions on operations, or withdrawal of existing approvals and licenses. An inability to address a quality or safety issue in an effective and timely manner may also cause negative publicity, a loss of customer confidence in us or our future products, which may result in difficulty in successfully launching product candidates and the loss of sales, which could have a material adverse effect on our business, financial condition, and results of operations.
42
If our competitors develop treatments for the target indications of our product candidates that are approved more quickly than ours, marketed more successfully, or demonstrated to be safer or more effective than our product candidates, our commercial opportunity will be reduced or eliminated.
We operate in highly competitive segments of the biotechnology market. We face competition from many different sources, including commercial pharmaceutical and biotechnology enterprises, academic institutions, government agencies, and private and public research institutions. Our product candidates, if successfully developed and approved, will compete with established therapies (including generic and over-the-counter drugs) as well as with new treatments that may be introduced by our competitors. With the exception of proliferative vitreoretinopathy and retinitis pigmentosa, there are a variety of approved drugs and drug candidates in development for the indications that we intend to test. Current treatments that are used in the United States for dry eye disease include over the counter artificial tears, Restasis®, Xiidra®, Cequa®, Eysuvis®, Tyrvaya®, MieboTM, and Vevye®. In February 2022, the FDA approved the first generic version of Restasis®, which is now available for sale in the U.S. Many of our competitors have significantly greater financial, product candidate development, manufacturing, and marketing resources than we do. Large pharmaceutical and biotechnology companies have extensive experience in clinical testing and obtaining regulatory approval for drugs. In addition, universities and private and public research institutes could be in direct competition with us. We also may compete with these organizations to recruit management, scientists, and commercial and clinical development personnel. We will also face competition from these third parties in establishing clinical trial sites, registering subjects for clinical trials, and in identifying and in-licensing new product candidates. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
New developments, including the development of other pharmaceutical technologies and methods of treating disease, occur in the pharmaceutical and life sciences industries at a rapid pace. Developments by competitors may render our product candidates obsolete or noncompetitive. Other parties may discover and patent treatment approaches and compositions that are similar to or different from ours. Competition in drug development is intense. We anticipate that we will face intense and increasing competition as new treatments enter the market and advanced technologies become available.
Our future success depends on our or our partners’ ability to demonstrate and maintain a competitive advantage with respect to the design, development, and commercialization of reproxalap or our other product candidates. Inflammatory and metabolic diseases may be treated with a variety drugs, some of which are generic. Our potential competitors may be developing novel therapies that may be safer or more effective than our product candidates.
If we are unable to enter into agreements with third parties to market, sell, and distribute our product candidates, we may be unable to generate any revenues.
We have no experience as a Company in the sale, marketing or distribution of biopharmaceutical products. Although we currently plan to commercialize reproxalap through a collaboration with a third party, if reproxalap or any of our other product candidates ultimately receives regulatory approval and we remain responsible for the commercialization of such approved product, we may not be able to effectively market and distribute the product candidate. We will have to invest significant amounts of financial and management resources to develop and maintain internal sales, distribution, and marketing capabilities, some of which will be committed prior to any confirmation that the applicable product candidates will be approved.
We may not be successful in entering into arrangements with third parties to market and sell our product candidates or may be unable to do so on terms that are acceptable to us. Any of these third parties may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales and marketing capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
If the FDA or comparable foreign regulatory authorities approve generic versions of any of our product candidates that receive marketing approval, or such authorities do not grant our product candidates appropriate periods of data or market exclusivity before approving generic versions of our product candidates, the sales of our product candidates could be adversely affected.
Once an NDA is approved, the drug covered thereby becomes a “reference-listed drug” in the FDA’s publication, “Approved Drug Products with Therapeutic Equivalence Evaluations.” Manufacturers may seek marketing approval of generic versions of reference-listed drugs through submission of abbreviated new drug applications (ANDAs) in the United States. In support of an ANDA, a generic manufacturer need not conduct clinical trials demonstrating safety and efficacy. Rather, the applicant generally must show that its drug is pharmaceutically equivalent to the reference listed drug, in that it has the same active ingredient(s), dosage form, strength, route of administration and conditions of use or labeling as the reference-listed drug, and that the generic version is bioequivalent to the reference-listed drug, meaning it is absorbed in the body at the same rate and to the same extent. Generic drugs may be significantly less costly to bring to market than the reference-listed drug and companies that produce generic drugs are generally able to offer drug products at lower prices.
43
Thus, following the introduction of a generic drug, a significant percentage of the sales of any branded product or reference-listed drug is typically lost to the generic drug.
The FDA may not approve an ANDA for a generic drug until any applicable period of non-patent exclusivity for the reference-listed drug has expired. The FDCA provides a period of five years of non-patent exclusivity for a new drug containing a new chemical entity. During the exclusivity period, the FDA may not accept for review an ANDA or a 505(b)(2) NDA submitted by another company for another version of such product candidate where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity, enforceability or non-infringement. The FDCA also provides three years of marketing exclusivity for a 505(b)(1) NDA, 505(b)(2) NDA or supplement to an approved NDA if new clinical investigations other than bioavailability studies (e.g., investigations that support new indications, dosages, or strengths of an existing drug) were conducted or sponsored by the applicant and are deemed by the FDA to be essential to the approval of the application. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving competitor products for product candidates containing the original active agent for other conditions of use. Five-year and three-year exclusivity will not delay the submission or approval of a full 505(b)(1) NDA. Manufacturers may seek to launch these generic drugs following the expiration of the marketing exclusivity period, even if we still have patent protection for our drug.
In the EU and the UK, innovative medicinal products are authorized based on a full marketing authorization application (as opposed to an application for marketing authorization that relies on data in the marketing authorization dossier for another, previously approved medicinal product). Applications for marketing authorization for innovative medicinal products must contain the results of pharmaceutical tests, preclinical tests, and clinical trials conducted with the medicinal product for which marketing authorization is sought (and where applicable the result of the pediatric studies unless a waiver or a deferral has been obtained - as described further below). In the EU, these applications must be made pursuant to either Directive 2001/83/EC (for the decentralized procedure or the mutual recognition procedure) or Regulation 726/2004 (for the centralized procedure). In the UK, there are various procedures available under the new regulatory legal framework to pharmaceutical products, including the possibility of a recognized assessment conducted by the European authorities under certain circumstance or by applying directly to the UK regulatory authority (MHRA).
Where an applicant for a marketing authorization submits a full dossier containing its own pharmaceutical, pre-clinical tests and clinical trials data, and where the application does not fall within the "global marketing authorization" of an existing medicinal product, the applicant is entitled to eight years of regulatory data protection upon grant of the marketing authorization (the period starts to run from the first marketing authorization in the EU/ European Economic Area (EEA)). During this period, applicants for approval of generics or biosimilars cannot rely on data contained in the marketing authorization dossier submitted for the already authorized, or reference, medicinal product to support their application. After the expiration of the eight-year period of regulatory data protection, the reference medicinal product benefits from a further two-year period of marketing protection. During these two years of marketing protection, no generic or biosimilar medicinal product that relies upon the reference medicinal product’s dossier may be placed on the EU market, but a generic or biosimilar marketing authorization application can be submitted to the competent regulatory authorities in the EU Member States during this time. The two-year period of marketing protection can further be extended by one year if, during the first eight years of the grant of the first marketing authorization, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, even if a compound is considered to be a new active substance and the innovator is able to gain the period of regulatory data protection and marketing protection, provided that no other IP or regulatory exclusivities applied, another unrelated company could also apply for a marketing authorization and market another competing medicinal product for the same therapeutic indication if such company obtained its own marketing authorization based on a separate marketing authorization application based on a full self-standing scientific data package supporting the application. The period of regulatory data protection and marketing protection applies in the UK (running from the date of the first authorization in Great Britain).
In the EU, pursuant to Regulation 1901/2006, and in the UK pursuant to the Human Medicines Regulations 2012 (as amended), marketing authorization applications must include pediatric data based on pediatric investigation plans agreed with the EMA if the MAA concerns (i) a new active substance, or (ii) a new indication, pharmacological form, or route of administration (where the product is protected by a supplementary protection certificate or a patent qualifying for a supplementary certificate). Applicants may obtain waivers or deferrals to these requirements in certain circumstances (for example a waiver may be obtained if the condition only occurs in adult populations). Where required, pediatric studies must cover all sub-sets of the pediatric population for both existing and new indications, pharmacological forms and route of administrations. Limited further exclusions apply, including in relation to generic or biosimilar applications. Certain rewards may be available for completion of pediatric studies. For example, where MAAs include the results of all studies conducted in compliance with an agreed pediatric investigation plan, the holder of the patent or supplementary protection certificate may be entitled to a six-month extension to the supplementary protection certificate.
44
In order to obtain orphan designation in the EEA, the product must fulfill certain challenging criteria. Under Article 3 of Regulation (EC) 141/2000, a medicinal product may be designated as an orphan medicinal product if it meets the following criteria: (1) is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; and (2) either the prevalence of such condition must not be more than five in 10,000 persons in the EU when the application is made, or without the benefits derived from orphan status, it must be unlikely that the marketing of the medicine would generate sufficient return in the EU to justify the investment needed for its development; and (3) there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the EU or if such a method exists, the product will be of significant benefit to those affected by the condition, as defined in Regulation (EC) 847/2000.
Products receiving orphan designation in the EU may receive 10 years of orphan market exclusivity, which can be further extended by two years if pediatric studies have been conducted in accordance with an agreed pediatric investigational plan. Applications must first satisfy the orphan designation criteria and apply for orphan designation before making the application for marketing authorization. The applicant must then successfully maintain the orphan designation at the time of the marketing authorization application in order to qualify for 10 years of orphan market exclusivity. During this 10-year period, the competent authorities of the EU Member States and European Commission may not accept applications or grant marketing authorization for other similar medicinal products for the same orphan therapeutic indication. The protection afforded by orphan market exclusivity in the EU may, in some circumstances, be circumvented by competitor products which are demonstrated not to be "similar" or which are authorized for different therapeutic indications. There may be a risk that products may be prescribed "off-label" for the orphan therapeutic indication by healthcare professions in some EU Member States.
There are also three exceptions to the orphan market exclusivity principle. Marketing authorization may be granted to a similar medicinal product for the same orphan therapeutic indication if:
An orphan product can also obtain an additional two years of orphan market exclusivity in the EU if the marketing authorization application contains the results of all pediatric studies conducted in accordance with and agreed pediatric investigation plan. The 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation (e.g., the product is sufficiently profitable not to justify maintenance of market exclusivity).
The UK’s regulatory legal framework provides for similar periods of protection, namely regulatory data protection, marketing protection and market exclusivity.
It is important to note that the regulatory protection afforded to medicinal product such as data exclusivity, marketing protection, market exclusivity for orphan indications, and pediatric extension are currently under review at EU level. It is expected that the protection currently afforded in the EU will be reduced in the years to come.
Competition that our product candidates may face from generic versions of our product candidates could materially and adversely impact our future revenue, profitability, and cash flows and could substantially limit our ability to obtain a return on the investments we have made in those product candidates. Our future revenues, profitability, and cash flows could also be materially and adversely affected and our ability to obtain a return on the investments we have made in those product candidates may be substantially limited if our product candidates, if and when approved, are not afforded the appropriate periods of non-patent exclusivity.
The FDA’s ability to review and approve new products may be hindered by a variety of factors, including budget and funding levels; ability to hire and retain key personnel; and statutory, regulatory, and policy changes.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including budget and funding levels; ability to hire and retain key personnel; and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
The ability of the FDA and other government agencies to properly administer their functions is highly dependent on the levels of government funding and the ability to fill key leadership appointments, among various factors. Delays in filling or replacing key positions could significantly impact the ability of the FDA and other agencies to fulfill their functions, and could greatly impact healthcare and the pharmaceutical industry.
45
In December 2016, the 21st Century Cures Act was signed into law, and was designed to advance medical innovation and empower the FDA with the authority to directly hire positions related to drug and device development and review. In the past, the FDA was often unable to offer key leadership candidates (including scientists) competitive compensation packages as compared to those offered by private industry. The 21st Century Cures Act was designed to streamline the agency’s hiring process and enable the FDA to compete for leadership talent by expanding the narrow ranges that are provided in the existing compensation structures.
Disruptions at the FDA and other governmental agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our operating results and business.
Failure to obtain regulatory approval in foreign jurisdictions would prevent us from marketing and commercializing our products abroad and may limit our ability to generate revenue from product sales.
We intend to market and commercialize our product candidates internationally. To market and sell our product candidates in jurisdictions outside the United States, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, we must secure product reimbursement approvals before regulatory authorities will approve the product for sale in that country. Failure to obtain foreign regulatory approvals on a timely basis or non-compliance with foreign regulatory requirements could result in significant delays, difficulties, and costs for us and could delay or prevent the introduction of our product candidates in certain countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any jurisdiction, which would materially impair our ability to generate revenue.
The UK's exit from the EU continues to create political and economic uncertainty, particularly in the UK and the EU. The UK is now being treated as a "third country" by the EU and new UK legislation has taken effect. This means that some regulatory activities, such as batch testing and Qualified Person certification conducted in Great Britain is no longer recognized in the EU. However, the UK and EU have concluded a Trade and Cooperation Agreement (TCA), which has been approved by the UK Parliament, European Council and European Parliament and has limited the disruption to the supply of medicines, particularly by enabling tariff and quota-free trade between the UK and the EU (provided that the rules of origin requirements are met), and has streamlined some issues, for example by enabling mutual recognition of cGMP inspections and certificates. The regulatory framework for medicines that existed before the end of the transition period has also effectively been preserved in UK domestic legislation as "retained EU law." By retaining a snapshot of EU legislation at its core, the UK has prevented substantial divergence to the regulation of medicines (although divergence has appeared in some areas). However, some changes to the UK legislation have been immediately necessary, including the implementation of the Northern Ireland Protocol (NIP), pursuant to which, the EU pharmaceutical legal framework acquis continues to apply in Northern Ireland (subject to periodic consent of the Northern Ireland Legislative Assembly), and only products compliant with EU law can be placed in the Northern Ireland market - adding an extra layer of regulatory complexity. As companies now need to comply with a separate UK regulatory legal framework in order to commercialize medicinal products in Great Britain (namely, England, Wales and Scotland, as EU law continues to apply in Northern Ireland). The UK government is currently trying to renegotiate fundamental aspects of the NIP so this is an unpredictable area for companies in the near future. The TCA allows for future deviation from the current regulatory framework and it is not known if and/or when any deviations may occur, which may have an impact on development, manufacture, marketing authorization, commercial sales and distribution of pharmaceutical products. It is also important to note that obtaining a marketing authorization is not sufficient to gain effective access to the market in the EU and in the UK; companies still need to agree to a reimbursement price for the products and in some jurisdictions, such as the UK and Germany, a further positive recommendation from health technology on cost-effectiveness is required for the products to be actually prescribed and reimbursed by the respective national health systems (see below). If we fail to comply with the regulatory requirements in international markets and thus receive applicable marketing approvals, our target market will be reduced, our ability to realize the full market potential of our product candidates will be harmed and our business will be adversely affected. We may not obtain foreign regulatory approvals on a timely basis, if at all. Our failure to obtain approval of any of our product candidates by regulatory authorities in another country may significantly diminish the commercial prospects of that product candidate and our business prospects could decline.
46
Risks Related to our Financial Position and Capital Requirements
We have incurred significant operating losses since inception and we expect to incur significant losses over the next several years. We may never become profitable or, if achieved, be able to sustain profitability.
We have incurred significant operating losses since we were founded in 2004 and expect to incur significant losses for the next several years as we continue our clinical trial, development programs, and commercial activities for reproxalap and our other product candidates. Net loss for the three months ended June 30, 2024 and 2023 was approximately $16.8 million and $9.0 million, respectively. As of June 30, 2024, we had total stockholders’ equity of $98.3 million and an accumulated deficit of $419.2 million. Losses have resulted principally from costs incurred in our clinical trials, research and development programs and from general and administrative expenses. In the future, we intend to continue to conduct research and development, clinical testing, regulatory compliance activities, pre-commercial activities, and, if reproxalap or any of our other product candidates is approved and we do not enter into collaboration agreements with third parties, commercialization efforts, including sales and marketing activities, that, together with anticipated general and administrative expenses, will likely result in our incurring further significant losses for the next several years. Our net losses may fluctuate significantly from quarter to quarter and year to year.
We anticipate that our expenses will increase substantially as compared to prior periods as we prepare for commercializing of reproxalap alone or with others, if approved, and continue development of ADX-2191, ADX-248, ADX-743, ADX-631, and other product candidates, and as a result of increased headcount, including management personnel to support our clinical, manufacturing and commercialization activities, expanded infrastructure, increased legal, compliance, accounting and investor and public relations expenses associated with being a public company, and increased insurance premiums, among other factors. Our license agreement with Massachusetts Eye and Ear Infirmary, or MEEI, under which we license certain of our patent rights and a significant portion of the technology for ADX-2191, imposes royalty and other financial obligations on us, and we may enter into additional licensing and funding arrangements with third parties that may impose milestone payment, royalty, insurance and other obligations on us.
Our expenses will also increase if and as we:
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve profitability. Our expenses will increase from what we anticipate if:
47
Our ability to become and remain profitable depends on our ability to generate revenue. We currently generate no revenue from sales, and we may never be able to commercialize reproxalap or our other product candidates. We do not currently have the required approvals to market any of our product candidates and we may never receive them. We do not expect to generate revenue from sales of our product candidates that is sufficient to achieve profitability, excluding any upfront licensing fees we may receive, unless and until we obtain marketing approval for and commercialize one or more of our product candidates. We do not expect to commercialize reproxalap alone or with others or any of our other product candidates before at least the first half of 2025, if ever. Achieving profitability will require us or our partners, if any, to be successful in a range of challenging activities, including:
We may never succeed in these activities and may never generate revenue that is sufficient to achieve profitability. Because of the numerous risks and uncertainties associated with developing and commercializing our product candidates, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product offerings or even continue our operations.
We will require substantial additional financing, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our product development, other operations or commercialization efforts.
The development and commercialization of biopharmaceutical products is capital intensive. We expect to devote substantial financial resources to our ongoing and planned activities, particularly as we seek marketing approval and prepare for commercialization of reproxalap alone or with others, and continue the development of our product candidates through preclinical and clinical development, including multiple ongoing and planned clinical trials for our product candidates. We expect our expenses to increase in connection with our ongoing activities, particularly as we prepare for commercializing reproxalap alone or with others, if approved, and we continue the research and development of, and, if successful, seek marketing approval for, our product candidates.
We currently plan to commercialize reproxalap through a collaboration with a third party. If we do obtain marketing approval for reproxalap and are not able to establish a suitable collaboration for the commercialization of reproxalap, or any other product candidate that we develop, we expect to incur significant additional commercialization expenses related to product sales, marketing, distribution and manufacturing. We may also need to raise additional funds sooner if we choose to pursue additional indications for our product candidates or otherwise expand more rapidly than we presently anticipate. Furthermore, we expect to continue to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed on attractive terms, if at all, we will be forced to delay, reduce, or eliminate certain of our clinical development plans, research and development programs or future commercialization efforts. In addition, there can be no assurance that we will be able to obtain such financing on commercially reasonable terms or at all. The development process for our product candidates is highly uncertain, and we cannot estimate with certainty the actual amounts necessary to successfully complete the development, regulatory approval, and commercialization of our product candidates for which we remain responsible for the commercialization of. Our operating plans may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than expected, through public or private equity, debt financings, or other sources.
48
The amount and timing of any expenditure needed to implement our development and commercialization programs will depend on numerous factors, including:
Some of these factors are outside of our control. Our existing capital resources are not sufficient to enable us to fund the commercialization of reproxalap and completion of our clinical trials and remaining development through commercial introduction for our product candidates. We expect that we will need to raise substantial additional funds in the near future.
We have not sold any products, and we do not expect to sell or derive revenue from any product sales for the foreseeable future. We may seek additional funding through collaboration agreements and public or private financings, including debt financings. The state of the global economy and market instability has made the business climate volatile and more costly. Uncertain economic conditions, uncertainty as to the general direction of the macroeconomic environment, and the price of our common stock, are beyond our control and may make any necessary debt or equity financing more difficult, more costly, and more dilutive. For example, the capital and credit markets may be adversely affected by the ongoing conflicts in Ukraine and Israel and the surrounding areas, the possibility of wider regional or global conflicts, and global sanctions imposed in response thereto. A severe or prolonged economic downturn, such as a global financial crisis, could affect our ability to raise additional capital. Additional funding may not be available to us on acceptable terms, or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders or be excessively dilutive. In addition, the issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay, reduce or discontinue our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates or curtail, delay, or discontinue one or more of our preclinical studies, clinical trials or other research or development programs. We may also be unable to expand our operations or otherwise capitalize on our business opportunities, may need to restructure our organization, or may be required to relinquish rights to our product candidates or other technologies, or otherwise agree to terms unfavorable to us. Any of these occurrences could materially affect our business, financial condition, and results of operations.
Our quarterly operating results may fluctuate significantly.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
49
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially. We believe that quarterly comparisons of our financial results are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Raising additional capital may cause dilution to stockholders, restrict our operations, or require us to relinquish rights to its technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, licensing arrangements, and marketing and distribution arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting its ability to take specific actions, such as incurring additional debt, making capital expenditures, or declaring dividends.
If we raise additional funds through collaborations, strategic alliances, licensing arrangements or marketing and distribution arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs, or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce, or terminate its product development or future commercialization efforts or grant rights to develop and market products or product candidates that we would otherwise prefer to develop and market on our own.
We may allocate our cash, cash equivalents, and marketable securities in ways that you or other stockholders may not approve.
Our management has broad discretion in the application of our cash, cash equivalents, and marketable securities. Because of the number and variability of factors that will determine our use of our cash, cash equivalents, and marketable securities, management’s ultimate use of cash, cash equivalents, and marketable securities may vary substantially from the currently intended use. Our management might not apply our cash, cash equivalents, or marketable securities in ways that ultimately increase the value of your investment. We expect to use our cash, cash equivalents, and marketable securities to: fund our planned clinical trials of a number of product candidates; continue to fund the potential NDA resubmission and approval process for reproxalap, including conducting any additional clinical trials or other activities that the FDA may require for approval of reproxalap; develop other molecules that relate to immune-mediated disease; pursue regulatory approval for our product candidates; service our debt obligations; and provide working capital and capital for other general corporate purposes. The failure by our management to apply these funds effectively could harm our business. We may invest our cash, cash equivalents, or marketable securities in short-term, investment-grade, interest-bearing securities, which may not yield a favorable return to our stockholders. If we do not invest or apply our cash, cash equivalents, or marketable securities in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
50
The terms of our secured debt facility require us to meet certain operating covenants and place restrictions on our operating and financial flexibility. If we raise additional capital through debt financing, the terms of any new debt could further restrict our ability to operate our business.
In March 2019, we entered into a credit facility with Hercules Capital, which was subsequently amended in April 2021, December 2022, and April 2024, that is secured by a lien covering all of our assets, other than our intellectual property. The loan agreement contains customary affirmative and negative covenants and events of default. Affirmative covenants include, among others, covenants requiring us to maintain our legal existence and governmental approvals, deliver certain financial reports, and maintain insurance coverage. Negative covenants include, among others: restrictions on transferring any part of our business or intellectual property; incurring additional indebtedness; engaging in mergers or acquisitions; paying dividends or making other distributions; making investments; and creating other liens on our assets, in each case subject to customary exceptions. If we raise any additional debt financing, the terms of such additional debt could further restrict our operating and financial flexibility. These restrictions may include, among other things, limitations on borrowing and specific restrictions on the use of our assets, as well as prohibitions on our ability to create liens, pay dividends, redeem capital stock, or make investments. If we default under the terms of the Hercules Credit Facility or any future debt facility, the lender may accelerate all of our repayment obligations and take control of our pledged assets, potentially requiring us to renegotiate our agreement on terms less favorable to us or to immediately cease operations. Further, if we are liquidated, the lender’s right to repayment would be senior to the rights of the holders of our common stock. The lender could declare a default upon the occurrence of any event that they interpret as a material adverse effect as defined under the loan agreement. Any declaration by the lender of an event of default could significantly harm our business and prospects and could cause the price of our common stock to decline. If we raise any additional debt financing, the terms of such additional debt could further restrict our operating and financial flexibility.
Our ability to use net operating loss carryforwards and tax credit carryforwards to offset future taxable income may be limited as a result of transactions involving our common stock.
In general, under Section 382 and 383 of the Internal Revenue Code of 1986, as amended, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating losses (NOLs) and certain other tax assets (tax attributes) to offset future taxable income. In general, an ownership change occurs if the aggregate stock ownership of certain stockholders increases by more than 50 percentage points over such stockholders’ lowest percentage ownership during the testing period (generally three years). Transactions involving our common stock within the testing period, even those outside our control, such as purchases or sales by investors, could result in an ownership change. A limitation on our ability to utilize some or all of our NOLs or credits could have a material adverse effect on our results of operations and cash flows. We believe, prior to December 31, 2021, that four ownership changes occurred since inception. Management believes that its aggregate Section 382 and 383 limitation (including the additional limitation for recognized "built-in gains") is sufficient so that no current impairment of its pre-ownership change tax attributes is required. We believe there were no ownership changes from December 31, 2021 through June 30, 2024, based on a review of our equity history during that period. Any future ownership changes, including those resulting from our recent or future financing activities, may cause our existing tax attributes to have additional limitations. However, subject to annual limitations, Federal NOLs generated in years 2018 and beyond will have an indefinite carryforward period and will not expire. Future changes in federal and state tax laws pertaining to NOL carryforwards may also cause limitations or restrictions from us claiming such NOLs. If the NOL carryforwards become unavailable to us or are fully utilized, our future taxable income will not be shielded from federal and state income taxation absent certain U.S. federal and state tax credits, and the funds otherwise available for general corporate purposes would be reduced.
Governments may impose price controls, which may adversely affect our future profitability.
We intend to seek approval to market our product candidates in both the United States and in foreign jurisdictions. If we obtain approval to market our product candidates in the United States, we will be subject to the Inflation Reduction Act of 2022 (IRA), which, among other things, will allow Department of Health and Human Services (HHS) to negotiate the selling price of certain drugs and biologics that Centers for Medicare & Medicaid Services (CMS) reimburses under Medicare Part B and Part D. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our product candidates. In some foreign countries, particularly in the EU, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. To obtain reimbursement or pricing approval in some countries, we, or our collaborators, may be required to conduct a clinical trial that compares the cost-effectiveness of our drug to other available therapies. Furthermore, in some European countries, the authorities conduct a Health Technology Appraisal to assess the cost-effectiveness of the product, which may significantly impact effective access to the market. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
51
Our operations could be subject to business disruptions such as earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, public health epidemics, regional or larger scale conflicts or geo-political actions, war or other military conflict (including an escalation of the conflicts in Ukraine and Israel and the surrounding areas), trade policies, sanctions, treaties and tariffs and other natural or man-made disasters or other business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition, and increase our costs and expenses. We rely on third-party manufacturers to produce reproxalap and our other product candidates. Our ability to obtain clinical and commercial supplies of reproxalap or our other product candidates could be disrupted, if the operations of these suppliers are affected by these or other business disruptions.
We are in a period of economic uncertainty and capital markets disruption, which has been significantly impacted by geopolitical instability due to the ongoing conflicts in Ukraine and Israel and the surrounding areas. Our business, financial condition, and results of operations may be materially adversely affected by the negative impact on the global economy and capital markets resulting from the conflicts in Ukraine and Israel or any other geopolitical tensions.
U.S. and global markets are experiencing volatility and disruption following the escalation of geopolitical tensions and the conflicts in Ukraine and Israel and the surrounding areas. In February 2022, a full-scale military invasion of Ukraine by Russian troops began. Although the length and impact of the ongoing military conflict is highly unpredictable, the conflict in Ukraine has led to market disruptions, including significant volatility in commodity prices, credit, and capital markets, as well as supply chain disruptions.
Additionally, various of Russia’s actions have led to sanctions and other penalties being levied by the U.S., the European Union, and other countries, as well as other public and private actors and companies, against Russia and certain other geographic areas, including agreement to remove certain Russian financial institutions from the SWIFT payment system and restrictions on imports of Russian oil, liquefied natural gas, and coal. Additional potential sanctions and penalties have also been proposed and/or threatened. Russian military actions and the resulting sanctions could further adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets, potentially making it more difficult for us to obtain additional funds.
In addition, in October 2023, Hamas terrorists infiltrated Israel’s southern border from the Gaza Strip and conducted a series of attacks on civilian and military targets. Hamas also launched extensive rocket attacks on Israeli population and industrial centers located along Israel’s border with the Gaza Strip and in other areas within the State of Israel. Following the attack, Israel’s security cabinet declared war against Hamas and initiated a military campaign Hamas. Significant clashes between Israel and Hezbollah in Lebanon have also occurred and may escalate in the future into a greater regional conflict.
Any of the above-mentioned factors could affect our business, prospects, financial condition, and operating results. The extent and duration of the military action, sanctions, and resulting market disruptions are impossible to predict, but could be substantial. Any such disruptions may also magnify the impact of other risks described in this annual report.
We maintain our cash at financial institutions, often in balances that exceed federally-insured limits. Adverse developments affecting financial institutions, companies in the financial services industry or the financial services industry generally, such as actual events or concerns involving liquidity, defaults or non-performance, could adversely affect our operations and liquidity.
Actual events involving limited liquidity, defaults, non-performance, or other adverse developments that affect financial institutions or other companies in the financial services industry or the financial services industry generally, or concerns or rumors about any events of these kinds, have in the past and may in the future lead to market-wide liquidity problems. The majority of our cash is held in accounts at U.S. banking institutions that we believe are of high quality. Cash held in depository accounts may exceed the $250,000 Federal Deposit Insurance Corporation (FDIC) insurance limits. If such banking institutions were to fail, we could lose all or a portion of those amounts held in excess of such insurance limits. Concerns regarding the U.S. or international financial systems, including bank failures and bailouts, and their potential broader effects and potential systemic risk on the banking sector generally, may adversely affect our access to capital. Any decline in available funding or access to our cash and liquidity resources could, among other risks, limit our ability to meet our capital needs and fund future growth or fulfill our other obligations, or result in breaches of our financial and/or contractual obligations. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our business, financial condition and results of operations.
Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail.
We may maintain cash balances at third-party financial institutions in excess of the FDIC insurance limit. A failure of a depository institution to return these deposits, or if a depository institution is subject to other adverse conditions in the financial or credit markets, could further impact access to our invested cash or cash equivalents and could adversely impact our operating liquidity and financial performance.
52
If we engage in an acquisition, reorganization, or business combination, we will incur a variety of risks that could adversely affect our business operations or our stockholders.
From time to time, we have entered into, and we will continue to consider in the future, strategic business initiatives intended to further the development of our business. These initiatives may include acquiring businesses, technologies, or products, or entering into a business combination with another company. For example, in January 2019 we acquired Helio Vision, Inc. and obtained the rights to ADX-2191, a vitreous-compatible methotrexate formulation for intraocular injection. Any acquisitions we undertake will likely be accompanied by business risks which may include, among other things:
These factors could harm our business, results of operations, or financial condition.
In addition to the risks commonly encountered in the acquisition of a business or assets as described above, we may also experience risks relating to the challenges and costs of closing a transaction. The risks described above may be exacerbated as a result of managing multiple acquisitions at once.
Risks Related to our Reliance on Third Parties
We rely and will continue to rely on outsourcing arrangements for many of our activities, including clinical development, commercial readiness preparations, and supply of reproxalap and our other product candidates.
As of June 30, 2024, we had only 10 full-time employees and, as a result, we rely, and expect to continue to rely, on outsourcing arrangements for a significant portion of our activities, including clinical research, data collection and analysis, manufacturing, commercial readiness preparations, financial reporting and accounting, and human resources, as well as for certain functions required of publicly traded companies. We may have limited control over third parties and we cannot guarantee that any third-party will perform its obligations in an effective and timely manner.
In addition, during challenging and uncertain economic environments, in tight credit markets and during public health epidemics, and with the continued hostilities in Ukraine and Israel and the surrounding areas, there may be a disruption or delay in the performance of our third-party contractors, suppliers, or partners. If such third parties are unable to satisfy their commitments to us, our business and results of operations would be adversely affected.
53
We rely on third parties to conduct our clinical trials. If any third-party does not meet our deadlines or otherwise conduct the trials as required and in accordance with regulations, our clinical development programs could be delayed or unsuccessful and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates when expected, or at all.
We do not have the ability to conduct all aspects of our preclinical testing or clinical trials ourselves. We are dependent on third parties to conduct the clinical trials for our product candidates and, therefore, the timing of the initiation and completion of these trials is controlled by such third parties and may occur on substantially different timing from our estimates. Specifically, we use CROs to conduct our clinical trials and we also rely on medical institutions, clinical investigators, and consultants to conduct our trials in accordance with our clinical protocols and regulatory requirements. Our CROs, investigators, and other third parties play a significant role in the conduct of these trials and subsequent collection and analysis of data.
There is no guarantee that CROs, investigators, or other third parties on which we rely for administration and conduct of our clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fails to meet expected deadlines, fails to adhere to our clinical protocols, or otherwise performs in a substandard manner, our clinical trials may be extended, delayed, or terminated. If any of our clinical trial sites terminates for any reason, we may experience the loss of follow-up information on subjects enrolled in our ongoing clinical trials unless we are able to transfer those subjects to another qualified clinical trial site. In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time, and may receive cash or equity compensation in connection with such services. Any worsening of the global business and economic environment may have the effect of heightening or exacerbating these risks.
Some of our product candidates may be studied in clinical trials co-sponsored by organizations or agencies other than us, or in investigator-initiated clinical trials, which means we have minimal or no control over the conduct of such trials.
We currently anticipate that part of our strategy for pursuing the wide range of indications potentially addressed by our product candidates will involve investigator-initiated clinical trials. Investigator-initiated clinical trials pose similar risks as those set forth elsewhere in this “Risk Factor” section relating to our internal clinical trials. While investigator-initiated trials may provide us with clinical data that can inform our future development strategy, we generally have less control over the conduct and design of the trials. Because we are not the sponsors of investigator-initiated trials, we do not control the protocols, administration, or conduct of the trials, including follow-up with patients and ongoing collection of data after treatment. As a result, we are subject to risks associated with the way investigator-initiated trials are conducted. In particular, we may be named in lawsuits that would lead to increased costs associated with legal defense. Additional risks include difficulties or delays in communicating with investigators or administrators, procedural delays and other timing issues, and difficulties or differences in interpreting data. Third-party investigators may design clinical trials with clinical endpoints that are more difficult to achieve, or in other ways that increase the risk of negative clinical trial results compared to clinical trials that we may design on our own. Negative results in investigator-initiated clinical trials could have a material adverse effect on our prospects and the perception of our product candidates. As a result, our lack of control over the conduct and timing of, and communications with the FDA regarding, investigator-sponsored trials expose us to additional risks and uncertainties, many of which are outside our control, and the occurrence of which could adversely affect the commercial prospects for our product candidates.
We rely completely on third parties to supply drug substance and manufacture drug product for our clinical trials and preclinical studies. We intend to rely on other third parties to produce commercial supplies of product candidates, and our dependence on third parties could adversely impact our business.
We are completely dependent on third-party suppliers of the drug substance and drug product for our product candidates. If third-party suppliers do not supply sufficient quantities of materials to us on a timely basis and in accordance with applicable specifications and other regulatory requirements, there could be a significant interruption of our supplies, which would adversely affect clinical development and commercialization. Furthermore, if any of our contract manufacturers cannot successfully manufacture material that conforms to our specifications within regulatory requirements, we will not be able to secure and/or maintain regulatory approval, if any, for our product candidates.
We also rely on our contract manufacturers to purchase from third-party suppliers the materials necessary to produce our product candidates for our anticipated clinical trials. We do not have any control over the process or timing of the acquisition of raw materials by our contract manufacturers. Moreover, we currently do not have agreements in place for the commercial production of these raw materials. Any significant delay in the supply of a product candidate or the raw material components thereof for an ongoing clinical trial, including as a result of the continued hostilities in Ukraine and Israel and the surrounding areas, could considerably delay completion of that clinical trial, product candidate testing, and potential regulatory approval of that product candidate.
We do not expect to have the resources or capacity to commercially manufacture any of our proposed product candidates if approved and will likely continue to be dependent on third-party manufacturers. Our dependence on third parties to manufacture and supply clinical trial materials and any approved product candidates may adversely affect our ability to develop and commercialize our product candidates on a timely basis.
54
We may not be successful in establishing and maintaining development, commercial, or other strategic partnerships, which could adversely affect our ability to develop and commercialize product candidates.
We have in the past chosen, and may in the future choose, to enter into development or other strategic partnerships, including collaborations with major biotechnology or pharmaceutical companies. For example, we currently plan to commercialize reproxalap through a collaboration with a third party. We face significant competition in seeking appropriate partners and the negotiation process is time consuming and complex. Moreover, we may not be successful in our efforts to establish other development partnerships or other alternative arrangements for any of our product candidates or programs because our research and development pipeline may be insufficient, our product candidates or programs may be deemed to be at too early a stage of development for collaborative effort, and/or third parties may not view our product candidates or programs as having the requisite commercial or technical potential. Even if we are successful in our efforts to establish development or commercial partnerships, the terms that we agree upon may not be favorable to us and we may not be able to maintain such partnerships if, for example, development or approval of a product candidate is delayed or sales of an approved product candidate are below expectations. Any delay in entering into development partnership agreements or collaborations related to our product candidates could delay the development and commercialization of our product candidates and reduce competitiveness, if approved.
Moreover, if we fail to maintain partnerships related to our product candidates:
We may not realize the benefits of our current or future strategic alliances.
We have in the past, and may in the future, form strategic alliances, create joint ventures or collaborations, or enter into licensing arrangements with third parties that we believe will complement or augment our existing business, including the continued development or commercialization of reproxalap or our other product candidates. We currently plan to commercialize reproxalap through a collaboration with a third party. Research, development, regulatory and commercialization activities undertaken by our partners, if any, pose similar risks as those set forth elsewhere in this “Risk Factor” section relating to our research, development, regulatory and commercialization activities. Strategic alliances may require us to incur non-recurring and other charges, increase our near- and long-term expenditures, issue securities that dilute our existing stockholders, or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for reproxalap or our other product candidates because third parties may view the risk of development failure as too significant or the commercial opportunity for our product candidate as too limited. We cannot be certain that, following a strategic transaction or license, we will achieve the revenues or specific net income that justifies such transaction.
Our internal computer systems, or those of our development partners, third-party clinical research organizations, or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs.
Despite the implementation of security measures, our internal computer systems and those of our current and any future CROs and other contractors, consultants, and collaborators are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war or other military conflict, including as a result of the continued hostilities in Ukraine and Israel and the surrounding areas, and telecommunication and electrical failures. While to our knowledge we have not experienced any such material system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our product candidates and conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business.
55
To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidate could be delayed.
We rely on email and other messaging services in connection with our operations. We may be targeted by parties using fraudulent spoofing and phishing emails to misappropriate passwords, payment information, or other personal information, or to introduce viruses through Trojan horse programs or otherwise through our networks, computers, smartphones, tablets, or other devices. Despite our efforts to mitigate the effectiveness of such malicious email campaigns through a variety of control and non-electronic checks, spoofing and phishing may damage our business and increase our costs. These risks may be heightened as a result of remote working arrangements. In addition, due to the political uncertainty involving the continued hostilities in Ukraine and Israel and the surrounding areas, there is an increased likelihood that escalation of tensions could result in cyberattacks that could either directly or indirectly impact our operations. Any of these events or circumstances could materially adversely affect our business, financial condition, and operating results.
Risks Relating to Our Intellectual Property
Our success depends on our and our licensors' ability to protect our intellectual property and our proprietary technologies.
Our commercial success depends in part on our ability to obtain and maintain patent protection and trade secret protection for our product candidates, proprietary technologies, and the use of our product candidates or proprietary technologies as well as our ability to operate without infringing upon the proprietary rights of others. There can be no assurance that our patent applications or those of our licensors will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technology, nor can there be any assurance that the patents issued will not be infringed, designed around, or invalidated by third parties. Even issued patents may later be found unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. This failure to properly protect the intellectual property rights relating to these product candidates could have a material adverse effect on our financial condition and results of operations.
Composition-of-matter patents on the active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any method of use. While we have issued composition-of-matter patents in the United States and other countries for reproxalap, and other product candidates, we cannot be certain that the claims in our patent applications covering composition-of-matter of early stage candidates will be considered patentable by the United States Patent and Trademark Office (USPTO) and courts in the United States or by the patent offices and courts in foreign countries, nor can we be certain that the claims in our issued composition-of-matter patents will not be found invalid or unenforceable if challenged. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products off-label. Although off-label prescriptions may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute. In addition, there are possibly treatment compositions and methods that we have not conceived of or attempted to patent, and other parties may discover and patent approaches and compositions that are similar to or different from ours.
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our future development partners will be successful in protecting our product candidates by obtaining and defending patents. These risks and uncertainties include the following:
56
In addition, we rely on the protection of our trade secrets and proprietary know-how. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants, and advisors, third parties may still obtain this information or may come upon this or similar information independently. If any of these events occurs or if we otherwise lose protection for our trade secrets or proprietary know-how, the value of our trade secrets or proprietary know-how may be greatly reduced.
Claims by third parties that we infringe their proprietary rights may result in liability for damages or prevent or delay our developmental and commercialization efforts.
The biotechnology industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by commercialization of reproxalap or our other product candidates. In addition, identification of third-party patent rights that may be relevant to our technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases, and the difficulty in assessing the meaning of patent claims. Any claims of patent infringement asserted by third parties would be time consuming and could likely:
Although no third-party has asserted a claim of patent infringement against us, others may hold proprietary rights that could prevent reproxalap or our other product candidates from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to our product candidate or processes could subject us to potential liability for damages and require us to obtain a license to continue to manufacture or market reproxalap or our other product candidates. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign our product candidate or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing reproxalap or our other product candidates, which could harm our business, financial condition, and operating results.
Any such claims against us could also be deemed to constitute an event of default under the loan and security agreement. In the case of a continuing event of default under the loan, Hercules could, among other remedies, elect to declare all amounts outstanding to be immediately due and payable and terminate all commitments to extend further credit. In the event we do not or are not able to repay the obligations at the time a default occurred, Hercules may elect to commence and prosecute bankruptcy and/or other insolvency proceedings, or proceed against the collateral granted to Hercules under the loan.
Our issued patents could be found invalid or unenforceable if challenged in court.
If we or any of our future development partners were to initiate legal proceedings against a third-party to enforce a patent covering one of our product candidates, or one of our future product candidates, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement during prosecution. Third parties may also raise similar claims before the USPTO, even outside the context of litigation.
57
The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on such product candidate. Such a loss of patent protection would have a material adverse impact on our business.
We may fail to comply with any of our obligations under existing or future agreements pursuant to which we license or acquire rights or technology, which could result in the loss of rights or technology that are material to our business.
We are a party to technology licenses, including an in-license agreement for ADX-2191, and we may enter into additional licenses in the future. Such licenses do, and may in the future, impose commercial, contingent payment, royalty, insurance, indemnification, and other obligations on us. If we fail to comply with these obligations, the licensor may have the right to terminate the license, in which event we could lose valuable rights under our collaboration agreements and our ability to develop product candidates could be impaired. Additionally, should such a license agreement be terminated for any reason, there may be a limited number of replacement licensors, and a significant amount of time may be required to transition to a replacement licensor.
Our rights to develop and commercialize our in-license program are each subject in part to the terms and conditions of a third-party license, pursuant to which we have acquired exclusive rights and other intellectual property. Our rights with respect to the intellectual property to develop and commercialize the in-license program may terminate, in whole or in part, if we fail to meet certain milestones contained in each of our license agreements relating to their development and commercialization. We may also lose our rights to develop and commercialize either in-license agreement if we fail to pay required milestones or royalties. In the event of an early termination of our license agreement, all rights licensed and developed by us under this agreement may be extinguished, which may have an adverse effect on our business and results of operations.
On January 28, 2019, we acquired Helio and thereby acquired rights to ADX-2191 pursuant to the Merger Agreement. We agreed to use commercially reasonably efforts to develop and obtain regulatory approval for ADX-2191. ADX-2191 is currently being developed for the potential treatment of retinitis pigmentosa. In the three months ended March 31, 2024, we deprioritized ADX-2191 for the treatment of proliferative vitreoretinopathy and primary vitreoretinal lymphoma due to the requirement from the FDA to run clinical trials that we did not deem to be feasible. If we cease to use commercially reasonably efforts to develop and obtain regulatory approval for ADX-2191, subject to the terms and conditions of the Merger Agreement, ADX-2191 and related intellectual property rights may revert back to an entity designated by the representative of the former Helio stockholders.
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees, consultants, or agents have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is common in the biotechnology and pharmaceutical industry, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants and our employees were previously employed at, or may have previously provided or may be currently providing consulting services to, other biotechnology or pharmaceutical companies including our competitors or potential competitors. We may become subject to claims that our company or an employee, consultant, or agent inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team.
If we do not obtain protection under the Hatch-Waxman Amendments by extending the patent terms and obtaining data exclusivity for our product candidate, our business may be materially harmed.
Depending upon the timing, duration, and specifics of FDA marketing approval of reproxalap or other product candidates, one or more of our United States patents may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, we may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents, or otherwise failing to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our revenue could be reduced, possibly materially.
58
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest, and our business may be adversely affected. Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights, or other intellectual property may be ineffective and could result in substantial costs and diversion of resources, and could adversely impact our financial condition or results of operations.
Changes in United States patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves technological and legal complexity. Therefore, obtaining and enforcing biotechnology patents is costly, time consuming, and inherently uncertain. In addition, Congress may pass patent reform legislation. The Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available or weakening the rights of patent owners. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the United States Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents, or to enforce our existing patents and patents we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
While we have issued composition-of-matter patents covering reproxalap and certain of our other product candidates in the United States and other countries, filing, prosecuting, and defending patents on reproxalap and our other product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States may be less extensive and of significantly shorter duration than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and, further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the United States. These products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license. Furthermore, the growing use of generative AI presents an increased risk of unintentional and/or unauthorized disclosure or use of our intellectual property rights.
59
We and the third parties with whom we work are increasingly utilizing social media tools as a means of communication both internally and externally, and noncompliance with applicable requirements, policies, or contracts due to social media use or negative posts or comments could have an adverse effect on our business.
Social media is increasingly being used to communicate about our product candidates and clinical development programs, and we may intend to utilize appropriate social media in connection with our commercialization efforts following approval of any product candidates. Social media practices in the biopharmaceutical industry continue to evolve and regulations and regulatory guidance relating to such use are evolving and not always clear. In addition, our employees or third parties with whom we contract or may contract, such as CROs, may knowingly or inadvertently make use of social media in ways that may not comply with legal or contractual requirements, which may give rise to liability, lead to the loss of trade secrets or other intellectual property. Additionally, such use of social media by our employees or third parties with whom we contract or may contract may result in public exposure of personal information of our employees, clinical trial patients and others or information regarding any product candidates or clinical trials along with the potential for litigation related to off-label marketing or other prohibited activities. For example, clinical trial patients may use social media channels to comment on their experience in an ongoing blinded clinical trial or to report an alleged adverse event. When such disclosures occur, there is a risk that trial enrollment may be adversely impacted, that we may fail to monitor and comply with applicable adverse event reporting obligations or that we may not be able to defend our business or the public’s legitimate interests in the face of the political and market pressures generated by social media due to restrictions on what we may say about any product candidate.
There is also a risk of inappropriate disclosure of sensitive information or negative or inaccurate posts or comments about us on any social networking website. Furthermore, negative posts or comments about us or any of our product candidates on social media could seriously damage our reputation, brand image or goodwill. If any of these events were to occur or we otherwise fail to comply with applicable regulations, we could incur liability, face regulatory actions, or incur other harm to its business.
Risks Related to Employee Matters and Managing Growth
We are highly dependent on the services of our senior management team and certain key consultants.
As a company with a limited number of personnel, we are highly dependent on the development, regulatory, commercial, and financial expertise of our senior management team comprised of: Todd C. Brady, M.D., Ph.D., our President and Chief Executive Officer, and Stephen G. Machatha, Ph.D., our Chief Development Officer, as well as certain other employees. In addition, we rely on the services of a number of key consultants, including IP, pharmacokinetic, chemistry, toxicology, drug development, and financial and accounting consultants. Leadership transitions can be inherently difficult to manage and may cause disruption within our company. The loss of key individuals or the services of future members of our management team could delay or prevent the further development of our product candidates and, if we are not successful in finding suitable replacements, could harm our business.
If we fail to attract and retain senior management, we may be unable to successfully develop or commercialize our product candidates.
Our success depends on our continued ability to attract, retain, and motivate highly qualified management and scientific personnel, and we may not be able to do so in the future due to intense competition among biotechnology and pharmaceutical companies, universities, and research organizations for qualified personnel. If we are unable to attract and retain the necessary personnel, we may experience significant impediments to our ability to implement our business strategy.
In August 2024, we announced that Bruce Greenberg, our Senior Vice President of Finance and Interim Chief Financial Officer, will step down from his positions with the company effective August 31, 2024. Following his departure we expect to outsource the principal financial and principal accounting officer roles through the engagement of a third-party provider of strategic and operational finance and accounting services. Mr. Greenberg has agreed to continue to serve as Interim Chief Financial Officer until such time and we expect to enter into a consulting agreement with Mr. Greenberg pursuant to which Mr. Greenberg is expected to provide consulting services to the company through November 30, 2024 to help support the successful transfer of responsibilities, but we may experience difficulties or delays during the transition. This or other changes in our senior management may be disruptive to our business, and, if we are unable to manage an orderly transition, our business may be adversely affected.
60
Our future performance will depend, in part, on our ability to successfully integrate newly hired executive officers into our management team and our ability to develop an effective working relationship among senior management. Our failure to integrate these individuals and create effective working relationships among them and other members of management could result in inefficiencies in the development and commercialization of our product candidates, adversely affecting future regulatory approvals, sales of our product candidates, and results of our operations.
In order to commercialize our product candidates for those we remain responsible for the commercialization of, we will need to substantially grow the size of our organization. We may encounter difficulties in managing our growth and expanding our operations successfully.
As of June 30, 2024, we only had 10 full-time employees. We currently plan to commercialize reproxalap through a collaboration with a third party. However, if we are not able to establish a suitable collaboration, we may need to grow our organization to continue development and pursue the potential commercialization of reproxalap. In addition, we expect that we will need to grow our organization to continue development and pursue the potential commercialization of our other product candidates, as well as function as a public company. As we seek to advance reproxalap, alone or with others, and other product candidates towards potential commercialization, increase the number of ongoing product development programs, and advance our future product candidates through preclinical studies and clinical trials, we will need to expand our financial, development, regulatory, manufacturing, marketing, and sales capabilities, or contract with third parties to provide these capabilities for us. As our operations expand, we expect that we will need to manage additional relationships with various strategic partners, suppliers, and other third parties. Future growth will impose significant added responsibilities on members of management and require us to retain additional internal capabilities. Our future financial performance and our ability to commercialize our product candidates and to compete effectively will depend, in part, on our ability to manage any future growth effectively. To that end, we must be able to manage our development efforts and clinical trials effectively and hire, train, and integrate additional management, clinical and regulatory, financial, administrative and sales, and marketing personnel. We may not be able to accomplish these tasks, and our failure to so accomplish could prevent us from successfully growing our company.
Risks Related to Other Legal or Regulatory Matters
Our business is subject to political, economic, legal, and social risks, which could adversely affect our business.
There are significant regulatory, economic and legal barriers in markets in the United States and outside the United States that we must overcome. We may be subject to the burden of complying with a wide variety of national and local laws, including multiple and possibly overlapping and conflicting laws. We also may experience difficulties adapting to new cultures, business customs, and legal systems. Any sales and operations would be subject to political, economic, and social uncertainties including, among others:
61
Changes in United States social, political, regulatory, and economic conditions or in laws and policies governing foreign trade, manufacturing, development, and investment, and any negative sentiments towards the United States as a result of such changes, could adversely affect our business. Concerns over economic recession, interest rate increases and inflation, supply chain delays and disruptions, policy priorities of the U.S. presidential administration, trade wars, unemployment, or prolonged government shutdown may contribute to increased volatility and diminished expectations for the economy and markets. Recent and upcoming presidential and congressional elections in the United States could also result in significant changes in, and uncertainty with respect to, legislation, regulation, and government policy that may impact the biopharmaceutical industry in the United States. Any such impacts may have a negative impact on the United States economies and on our business, financial condition, and results of operations. Additionally, concern over geopolitical issues may also contribute to prolonged market volatility and instability. For example, continued hostilities in Ukraine and Israel and the surrounding areas, could lead to disruption, instability, and volatility in global markets and industries. The U.S. government and other governments in jurisdictions have imposed severe economic sanctions and export controls against Russia and Russian interests, have removed Russia from the Society for Worldwide Interbank Financial Telecommunication payment (SWIFT) system, and have threatened additional sanctions and controls. The impact of these measures, as well as potential responses to them by Russia, is unknown.
Any changes related to these and other factors could adversely affect any business operations that we conduct outside the United States.
Security breaches, cyberattacks, loss of data, and other disruptions impacting our information technology systems or those of our third-party collaborators, service providers, contractors or consultants could compromise the privacy, security, integrity or confidentiality of sensitive information related to our business or prevent us from accessing critical information and expose us to adverse consequences, including but not limited to regulatory investigations or actions, litigation, and significant fines and penalties, which could adversely affect our business, financial condition, and reputation.
In the ordinary course of our business, we and our current or future third-party collaborators, service providers, contractors, and consultants collect, may store and transmit sensitive data, including legally protected health information, personal data (also referred to as personal information or personally identifiable information under certain data privacy laws) about patients and employees, intellectual property, and our proprietary business and financial information (collectively, sensitive information). We manage and maintain data, including sensitive information, utilizing a combination of on-site systems, managed data center systems, and cloud-based data center systems. We face a number of risks related to our protection of, and our third-party collaborators’, service providers’, contractors’, and consultants’ protection of, this sensitive information, including loss of access, inappropriate disclosure and inappropriate or unauthorized access, as well as risks associated with our ability to identify and audit such events.
The secure processing, storage, maintenance, and transmission of sensitive information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure, and those of our third-party collaborators, service providers, contractors, and consultants, may be vulnerable to breakdown or other damage or interruption from service interruptions, system malfunctions, natural disasters, terrorism, war and telecommunications and electrical failures, as well as from cyberattacks by malicious third parties (including the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering, and other means to affect service reliability and threaten the confidentiality, integrity and availability of information) or viruses or otherwise breached due to employee or third-party error, malfeasance, or other activities. These risks may be heightened as a result of remote working arrangements.
While we are not aware of any such attack, breach or system failure, we cannot guarantee that our data protection efforts and our investment in information technology, or those of our third-party collaborators, service providers, contractors, and consultants will prevent significant breakdowns, data leakages, and breaches in the relevant systems or other cyber incidents. If such event were to occur and cause interruptions in our operations, our networks could be compromised and the sensitive information we store on those networks could be accessed by unauthorized parties, publicly disclosed, lost, or stolen. Any such unauthorized access, disclosure or other loss of information, or the perception that any of these has occurred, could result in legal claims or proceedings, liability under federal, state, and international laws that protect the privacy of personal data, including but not limited to private lawsuits or class actions under the California Consumer Privacy Act, as amended by the California Privacy Rights Act of 2020 (CPRA), and regulatory penalties, which could result in significant legal or financial exposure. In addition, we may be subject to state laws requiring notification of affected individuals and state regulators in the event of a breach of personal data, which is a broader class of information than the health information protected by the Health Insurance Portability and Accountability Act (HIPAA). Unauthorized access, loss, or dissemination of sensitive information could also disrupt our ability to conduct research and development activities; collect, process, and prepare company financial information; provide information about our product candidates and other patient and physician education or outreach efforts through our website; manage the administrative aspects of our business; or prevent damage to our reputation, any of which could adversely affect our business.
62
We are subject to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies, and other obligations related to data privacy and security. Our actual or perceived failure to comply with such obligations could lead to regulatory investigations or actions; litigation; significant fines and penalties; disruptions of our business operations; reputational harm; loss of revenue or profits; loss of customers or sales; and other adverse business consequences.
In the ordinary course of our business, we process, generate, use, transfer, disclose, make accessible, protect, secure, dispose of, transmit, and share (collectively, process) personal data (also referred to as personal information or personally identifiable information under certain data privacy laws) and other sensitive information, including proprietary and confidential business data, trade secrets, intellectual property, sensitive third-party data, and patient information. Our data processing activities may subject us to numerous data privacy and security obligations, such as various federal, state, and foreign laws, regulations, guidance, industry standards, external and internal privacy and security policies, contracts, and other obligations that govern the processing of personal data by us and on our behalf. We strive to comply with applicable data privacy and security obligations to the extent possible. However, it is possible that these obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict with other rules and/or our practices. Any failure or perceived failure by us to comply with applicable privacy and data security laws and regulations, our privacy policies, or our privacy-related obligations to third parties, or any compromise of security that results in the unauthorized access, release or transfer of personal data or other sensitive information, may result in governmental enforcement actions and fines or orders requiring that we change our practices, private litigation (including class action lawsuits), or public statements against us by consumer advocacy groups or others and could cause a loss of trust in us, which could result in significant legal or financial exposure and reputational damage that could potentially have an adverse effect on our business.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, and consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act). For example, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), imposes specific requirements relating to the privacy, security, and transmission of individually identifiable health information. In addition, we may be subject to various state data privacy and security laws and regulations, including the California Consumer Privacy Act, as amended by the CPRA, which, among other things, requires covered “businesses” to provide specific disclosures to California consumers concerning the collection, sale, and sharing of their personal data, and gives such consumers the right to opt-out of certain sales of personal information. The CPRA provides for civil penalties for violations, as well as a private right of action for certain security breaches that may increase the likelihood of, and the risks associated with, security breach litigation. Additionally, the CPRA created a new state agency to oversee implementation and enforcement efforts, potentially resulting in further uncertainty and requiring us to incur additional costs and expenses in an effort to comply. Many of the CPRA’s provisions became effective on January 1, 2023. Several states in the U.S. have proposed or enacted laws that contain obligations similar to the CPRA that have taken effect or will take effect in coming years. The U.S. federal government also is contemplating federal privacy legislation. The effects of recently proposed or enacted legislation potentially are far-reaching and could increase our potential liability, increase our compliance costs, and adversely affect our business.
Developments in Europe have created compliance uncertainty regarding the processing of personal data from Europe. For example, the European Union’s General Data Protection Regulation (EU GDPR), the United Kingdom’s GDPR (UK GDPR), and the Swiss Federal Act on Data Protection extend the geographical scope of European data protection laws to non-European entities and impose strict requirements for processing personal data. For example, under the EU GDPR and/or the UK GDPR, government regulators may impose temporary or definitive bans on data processing, as well as possible fines of up to 4% of global annual turnover for the preceding financial year or €20 million, whichever is higher, for the most serious infringements. This exposes us to two parallel sets of regulations, each of which potentially authorizes similar fines and other potentially divergent enforcement actions for certain violations. Further, individuals or consumer protection organizations authorized at law to represent their interests may initiate litigation related to the processing of individuals’ personal data.
63
In the ordinary course of our business, we may transfer personal data from Europe and other jurisdictions to the United States or other countries. The EU GDPR and UK GDPR prohibit the transfer of personal data to countries outside of the EEA, or the UK including the United States, that have not been deemed adequate by the European Commission or by the UK data protection regulator, respectively. Switzerland has adopted similar restrictions. Although there are legal mechanisms that allow for the transfer of personal data from the EEA, UK, and Switzerland to the United States, these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States. For example, legal developments in the EU have created complexity and uncertainty regarding such transfers and data protection authorities from the different EU Member States may interpret the EU GDPR differently. Additionally, guidance on implementation and compliance practices are often updated or otherwise revised, which adds to the complexity of processing personal data in the EU. These transfer mechanisms have also been subject to various legal challenges. In particular, on July 16, 2020, the Court of Justice of the European Union, in the case of Data Protection Commissioner v. Facebook Ireland Limited, Maximillian Schrems (Case C-311/18) (Schrems II), invalidated the EU-U.S. Privacy Shield Program for transfers of personal data from the EU to the U.S., and added further uncertainty and complexity to the use of standard contractual clauses as a compliance mechanism for transfers of personal data outside the EU.
If there is no lawful manner for us to transfer personal data from the EEA, UK, or Switzerland to the United States, or if the requirements for a legally-compliant transfer are too onerous, we could face significant adverse consequences, including the interruption or degradation of our operations, the need to relocate part or all of our business or data processing activities to other jurisdictions at significant expense, increased exposure to regulatory actions, substantial fines and penalties, the inability to transfer data and work with partners, vendors and other third-parties, which could limit our ability to conduct clinical trial activities in Europe or elsewhere, and injunctions against our processing or transferring of personal data necessary to operate our business.
In addition to the EU, UK, and Switzerland, a growing number of other global jurisdictions are considering or have passed legislation implementing data protection requirements or requiring local storage and processing of data or similar requirements that could increase the cost and complexity of our business. Some of these laws, such as the General Data Protection Law in Brazil, or the Act on the Protection of Personal Information in Japan, impose similar obligations as those under the EU GDPR and UK GDPR. Others, such as those in Russia, India, and China, could potentially impose more stringent obligations, including data localization requirements. If we are unable to meet these evolving legal requirements or if we violate or are perceived to violate any laws, regulations, or other obligations relating to privacy, data protection, or information security, we may experience harm to our reputation and become subject to investigations, claims, and other remedies, which could expose us to significant fines, penalties, and other damages, all of which would harm our business.
Current and future legislation may increase the difficulty and cost for us to obtain regulatory and marketing approval of and commercialize our product candidates, alone or with others, and may affect the prices we may obtain.
In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding healthcare systems that could prevent or delay marketing approval for our product candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval. The pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by legislative initiatives. Current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any FDA approved product.
Healthcare reform measures that may be adopted in the future, may result in reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies, and additional downward pressure on the price that we receive for any approved product and/or the level of reimbursement physicians receive for administering any approved product we might bring to market. Reductions in reimbursement levels may negatively impact the prices we receive or the frequency with which our products are prescribed or administered. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
64
To date, there have been several recent U.S. congressional inquiries and proposed and enacted state and federal legislation and regulation designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient support programs, reduce the costs of drugs under Medicare, and reform government program reimbursement methodologies for drug products. For example, included in the Consolidated Appropriations Act, 2021 were several drug price reporting and transparency measures, such as a new requirement for certain Medicare plans to develop tools to display Medicare Part D prescription drug benefit information in real time and for group and health insurance issuers to report information on pharmacy benefit and drug costs to the Secretaries of the HHS, the Department of Labor, and the Treasury. Additionally, both Congress and the Biden administration have each indicated willingness to continue to seek new legislative and/or administrative measures to address prescription drug costs. For example, on July 9, 2021, President Biden issued an Executive Order to promote competition in the U.S. economy that included several initiatives addressing prescription drugs. Among other provisions, the Executive Order stated that the Biden administration will “support aggressive legislative reforms that would lower prescription drug prices, including by allowing Medicare to negotiate drug prices, by imposing inflation caps, and through other related reforms.” In response to the Executive Order, on September 9, 2021, the HHS issued a Comprehensive Plan for Addressing High Drug Prices that identified potential legislative policies and administrative tools that Congress and the agency can pursue in order to make drug prices more affordable and equitable, improve and promote competition throughout the prescription drug industry, and foster scientific innovation. Congress has also continued to conduct inquiries into the prescription drug industry’s pricing practices.
These initiatives recently culminated in the enactment of the IRA, in August 2022, which, among other things, will allow HHS to negotiate the selling price of certain drugs and biologics that CMS reimburses under Medicare Part B and Part D, although this will only apply to high-expenditure single-source drugs that have been approved for at least 7 years (11 years for biologics). The negotiated prices, which will first become effective in 2026, will be capped at a statutory ceiling price representing a significant discount from average prices to wholesalers and direct purchasers. The law will also, beginning in October 2023, penalize drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation. In addition, the law eliminates the “donut hole” under Medicare Part D beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost through a newly established manufacturer discount program. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties. These provisions will take effect progressively starting in 2023, although they may be subject to legal challenges. Thus, although it is unclear how the IRA will be implemented, the IRA will likely have a significant impact on our business and the pharmaceutical industry as a whole.
At the state level, individual states are increasingly aggressive in passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing. These include legislation and regulations regarding price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, legislative action designed to encourage importation from other countries and bulk purchasing. In addition, regional health care authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other health care programs. These measures could reduce the ultimate demand for our products, if approved, or put pressure on our product pricing.
Legislative and regulatory proposals have also been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance, or interpretations will be changed, or what the impact of such changes on the potential approval and marketing approvals of our product candidates, if any, may be. Increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
The continuing efforts of the government, insurance companies, managed care organizations, and other payors of healthcare services to contain or reduce costs of health care may adversely affect:
65
Our operations and relationships with actual and potential customers, providers, and third-party payors will be subject to applicable anti-kickback, fraud and abuse, and other healthcare laws and regulations, which could expose us to penalties including criminal sanctions, civil penalties, exclusions from government programs, contractual damages, and reputational harm, and could diminish our future profits and earnings.
the level of taxes that we are required to pay; Our arrangements with third-party payors, physicians, and other potential customers will subject us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any product candidates for which we obtain marketing approval.
Applicable U.S. federal and state healthcare laws and regulations include the following:
66
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations, or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties; damages; fines; imprisonment; exclusion of product candidates from government-funded healthcare programs, such as Medicare and Medicaid; disgorgement; contractual damages; reputational harm; diminished profits and future earnings; and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may also be subject to criminal, civil, or administrative sanctions, including exclusions from government-funded healthcare programs. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action for an alleged or suspected violation can cause us to incur significant legal expenses and divert management’s attention from the operation of the business, even if such action is successfully defended.
Providing benefits or advantages to induce or reward improper performance generally to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order, or use of medicinal products is prohibited in the EU. The provision of benefits or advantages to induce or reward improper performance is governed by the national anti-bribery laws of EU Member States, and in respect of the U.K., the Bribery Act 2010. Infringement of these laws may result in substantial fines and imprisonment. EU Directive 2001/83/EC, which is the EU Directive governing medicinal products for human use, provides that, where medicinal products are being promoted to healthcare professionals, no gifts, pecuniary advantages, or benefits in kind may be supplied, offered or promised to such individuals unless they are inexpensive and relevant to the practice of medicine or pharmacy. This provision was transposed into the Human Medicines Regulations 2012 and as such remains applicable in the UK.
Payments made to physicians in certain EU Member States must be publicly disclosed. In addition, agreements with healthcare professionals must often be the subject of prior notification and approval by the healthcare professional’s employer, his or her competent professional organization, and/or the regulatory authorities of individual EU Member States. These requirements are set out in national laws, industry codes, or professional codes of conduct, applicable in the EU Member States and in the UK. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines, or imprisonment.
If we market products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties.
In addition to FDA restrictions on the marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include false claims statutes and anti-kickback statutes. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, or causing to be made, a false statement to get a false claim paid. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, or receiving remuneration to induce, or in return for, purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers, and formula managers on the other.
67
Although there are several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing, or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
Over the past few years, several pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants, and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicaid for non-covered, off-label uses; and submitting inflated best price information to the Medicaid Rebate Program to reduce liability for Medicaid rebates. Most states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines, and imprisonment.
Inadequate funding for the FDA, the SEC, and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner, or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels; ability to hire and retain key personnel and accept the payment of user fees; and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of the SEC and other government agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA and the SEC, have had to furlough critical FDA, SEC, and other government employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Further, in our operations as a public company, future government shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of reproxalap or our other product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize our product candidates. For example, we may be sued if reproxalap or our other product candidates allegedly cause injury or are found to be otherwise unsuitable during product testing, manufacturing, marketing, or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product candidate, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts.
If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
68
We maintain product liability insurance with $5.0 million in coverage. We anticipate that we will need to increase our insurance coverage if we commercialize any product candidate. Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of reproxalap or our other product candidates. Although we will maintain such insurance, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies will also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
We and our development partners, third-party manufacturers, and suppliers use biological materials and may use hazardous materials, and any claims relating to improper handling, storage, or disposal of these materials could be time consuming or costly.
We and our development partners, third-party manufacturers, and suppliers may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations and the operations of our development partner, third-party manufacturers, and suppliers also produce hazardous waste products. Federal, state, and local laws and regulations govern the use, generation, manufacture, storage, handling, and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage and our property, casualty, and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended.
We and any of our future development partners will be required to report to regulatory authorities if any of our approved products cause or contribute to adverse medical events, and any failure to do so would result in sanctions that would materially harm our business.
If we and any of our future development partners are successful in commercializing our products, the FDA and foreign regulatory authorities will require that we and any of our future development partners report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We and any of our future development partners may fail to report adverse events we become aware of within the prescribed timeframe or to perform inadequate investigations of their causes. We and any of our future development partners may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we and any of our future development partners fail to comply with our reporting obligations, the FDA or a foreign regulatory authority could take enforcement action including the issuance of a Warning Letter, the requirement of a labeling change, the initiation of a criminal prosecution, the imposition of civil monetary penalties, the seizure of our products, or delay in approval or clearance of future products.
We are subject to anti-corruption laws, as well as export control laws, customs laws, sanctions laws, and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, or other remedial measures and legal expenses, any of which could adversely affect our business, results of operations and financial condition.
Our operations are subject to anti-corruption laws, including the Foreign Corrupt Practices Act (FCPA), the Bribery Act and other anticorruption laws that apply in countries where we do business and may do business in the future. The FCPA, the Bribery Act, and other laws generally prohibit us, our officers, and our employees, and intermediaries from bribing, being bribed, or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. We may in the future operate in jurisdictions that pose a high risk of potential FCPA or Bribery Act violations, and we may participate in collaborations and relationships with third parties whose actions could potentially subject us to liability under the FCPA, the Bribery Act, or local anti-corruption laws. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
We also are subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United States, UK, and authorities in the EU, including applicable export control regulations, economic sanctions on countries and persons, customs requirements, and currency exchange regulations, which we collectively refer to as Trade Control Laws.
69
There is no assurance that we will be completely effective in ensuring our compliance with all applicable anti-corruption laws, including the FCPA, the Bribery Act, or other legal requirements including Trade Control Laws. If we are not in compliance with the FCPA, the Bribery Act, and other anti-corruption laws or Trade Control Laws, we may be subject to criminal and civil penalties, legal expenses, disgorgement, and other sanctions and remedial measures, which could have an adverse impact on our business, financial condition, results of operations, or liquidity. The SEC also may suspend or bar issuers from trading securities on U.S. exchanges for violations of the FCPA’s accounting provisions. Likewise, any investigation of any potential violations of the FCPA; the Bribery Act; or other anti-corruption laws or Trade Control Laws by U.S., U.K., or other authorities also could have an adverse impact on our reputation, our business, results of operations, or financial condition.
Our employees, independent contractors, vendors, principal investigators, contract research organizations (CROs), and consultants may engage in misconduct or other improper activities, including noncompliance with regulatory standards, regulatory requirements, and insider trading.
We are exposed to the risk that our employees, independent contractors, vendors, principal investigators, CROs and consultants may engage in fraudulent conduct or other illegal activity. Misconduct by these parties could include:
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations govern a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. While we have adopted a code of conduct and implemented other internal controls applicable to all our employees, it is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective. Additionally, we are subject to the risk that a person could allege fraud or other misconduct, even if none occurred. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business or cause reputational harm, including the imposition of civil, criminal and administrative penalties, and damages; possible exclusion from participation in Medicare, Medicaid, and other federal healthcare programs; and diminished profits and future earnings.
In addition, during the course of our operations, our directors, executives, employees, consultants, and other third parties may have access to material nonpublic information regarding our business, our results of operations, or potential transactions we are considering. We may not be able to prevent trading in our common stock on the basis of, or while having access to, material nonpublic information. If any such person was to be investigated or an action were to be brought against them for insider trading, it could have a negative impact on our reputation and our stock price. Such a claim, with or without merit, could also result in substantial expenditures of time and money, and divert attention of our management team from other tasks important to the success of our business.
We are subject to litigation risks.
From time to time, we may become involved in various litigation matters and claims, including regulatory proceedings, administrative proceedings, governmental investigations, and contract disputes. We may face potential claims or liability for, among other things, breach of contract, defamation, libel, fraud, or negligence. We may also face employment-related litigation, including claims of age discrimination, sexual harassment, gender discrimination, immigration violations, or other local, state, and federal labor law violations. Because of the uncertain nature of litigation and insurance coverage decisions, the outcome of such actions and proceedings cannot be predicted with certainty and an unfavorable resolution of one or more of them could have a material adverse effect on our business, financial condition, results of operations, cash flows, and the trading price of our securities. In addition, legal fees and costs associated with prosecuting and defending litigation matters could have a material adverse effect on our business, financial condition, results of operations, and the trading price of our securities.
We are, and could in the future be, subject to securities class action litigation.
In the past, securities class action litigation has often been brought against companies, including us, following a decline in the market price of its securities. The risk of securities class action litigation is especially relevant for us because biotechnology and pharmaceutical companies have experienced significant stock price volatility in recent years.
70
Such litigation could cause us to incur substantial costs and a diversion of management’s attention and resources, which could harm our business. For further information about specific litigation and proceedings, see the section titled “Legal Proceedings” contained in Part II, Item 1, and Note 14 of our Notes to Consolidated Financial Statements (unaudited) of this report.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for all categories of risk that our business may encounter. Some of the policies we currently maintain include general liability, product and clinical trial liability, workers’ compensation, and directors’ and officers’ insurance. We do not know, however, if we will be able to maintain existing insurance with adequate levels of coverage. Any significant, uninsured liability may require us to pay substantial amounts, which would adversely affect our working capital and results of operations.
U.S. federal income tax reform could adversely affect us.
New legislation or regulation which could affect our tax burden could be enacted by any governmental authority. We cannot predict the timing or extent of such tax-related developments which could have a negative impact on our financial results. Additionally, we use our best judgment in attempting to quantify and reserve for these tax obligations. However, a challenge by a taxing authority, our ability to utilize tax benefits such as carryforwards or tax credits, or a deviation from other tax-related assumptions could have a material adverse effect on our business, results of operations, or financial conditions.
Risks Related to Our Common Stock
In the absence of an active trading market for our common stock, investors may not be able to resell their shares at or above the price at which they purchased them.
In the absence of an active trading market for our common stock, investors may not be able to sell their common stock at or above the price they paid or at the time that they would like to sell. In addition, an inactive market may impair our ability to raise capital by selling shares and may impair our ability to acquire other companies or technologies by using our shares as consideration, which, in turn, could harm our business.
The trading price of the shares of our common stock has been and is likely to continue to be highly volatile, and purchasers of our common stock could incur substantial losses.
Our stock price has been and will likely continue to be volatile for the foreseeable future. The stock market in general and the market for biotechnology companies in particular have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their common stock at or above the price they paid. The market price for our common stock may be influenced by many factors, including:
71
Concerns over economic recession, interest rate increases and inflation, adverse developments affecting financial services industry, supply chain delays and disruptions, policy priorities of the U.S. presidential administration, upcoming presidential and congressional elections in the United States, trade wars, unemployment, or prolonged government shutdown may contribute to increased volatility and diminished expectations for the economy and markets. Additionally, concern over geopolitical issues may also contribute to prolonged market volatility and instability. For example, the continued hostilities in Ukraine and Israel and the surrounding areas, could lead to disruption, instability and volatility in global markets and industries. In connection with the hostilities between Russia and Ukraine the U.S. government and other governments and jurisdictions have imposed severe economic sanctions and export controls against Russia and Russian interests, have removed Russia from the SWIFT system, and have threatened additional sanctions and controls. The impact of these measures, as well as potential responses to them by Russia, is unknown.
In addition, in the past, stockholders have initiated class action lawsuits against biotechnology and pharmaceutical companies following periods of volatility in the market prices of these companies’ stock. Such litigation, if instituted against us, could cause us to incur substantial costs and divert management’s attention and resources, which could have a material adverse effect on our business, financial condition, and results of operations.
Our failure to meet the continued listing requirements of The Nasdaq Capital Market could result in a delisting of our common stock.
If we fail to satisfy the continued listing requirements of The Nasdaq Capital Market (Nasdaq), such as the corporate governance requirements or the minimum closing bid price requirement, Nasdaq may take steps to de-list our common stock. Such a delisting would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we would expect to take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the Nasdaq minimum bid price requirement, or prevent future non-compliance with Nasdaq’s listing requirements.
Because a small number of our existing stockholders own a substantial percentage of our outstanding common stock, your ability to influence corporate matters will be limited.
As of June 30, 2024, our executive officers, directors, and greater than 5% stockholders, in the aggregate, own approximately 39% of our outstanding common stock. As a result, such persons, acting together, may have the ability to control our management and business affairs and substantially all matters submitted to our stockholders for approval, including the election and removal of directors and approval of any significant transaction. This concentration of ownership may have the effect of delaying, deferring, or preventing a change in control, impeding a merger, consolidation, takeover, or other business combination involving us, or discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our business, even if such a transaction would benefit other stockholders.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not retain a listing on Nasdaq and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement.
72
These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
We do not intend to pay dividends on our common stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividend on our common stock, and do not currently intend to do so for the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation, and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. In addition, the Hercules Credit Facility currently prohibits, and any future debt financing arrangements may contain terms prohibiting or limiting the amount of, dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in the value of our common stock. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased shares.
A substantial number of shares of our common stock could be sold into the public market in the near future, which could depress our stock price.
Sales of substantial amounts of our common stock in the public market could reduce the prevailing market prices for our common stock. Substantially all of our outstanding common stock is eligible for sale as is common stock issuable under vested and exercisable stock options and upon settlement of vested RSUs. If our existing stockholders sell a large number of shares of our common stock, or the public market perceives that existing stockholders might sell shares of common stock, the market price of our common stock could decline significantly. Existing stockholder sales might also make it more difficult for us to sell additional equity securities at a time and price that we deem appropriate.
We are a smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are a smaller reporting company under Rule 12b-2 of the Securities Exchange Act of 1934. For as long as we continue to be a smaller reporting company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies, including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on smaller reporting company exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
We are incurring significant increased costs and demands upon management as a result of operating as a public company.
As a public company, and particularly if and after we cease to be a “smaller reporting company,” we incur significant legal, accounting, and other expenses that we did not incur as a private company. We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which require, among other things, that we file with the Securities and Exchange Commission, or the SEC, annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and Nasdaq to implement provisions of the Sarbanes-Oxley Act, imposes significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, in 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation related provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Stockholder activism, the current political environment, and the current high level of government intervention and regulatory reform may result in substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to continue to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. If public company rules and regulations divert the attention of our management and personnel from other business concerns, our business, financial condition, and results of operations could be adversely affected. Increased costs associated with public company expenses will increase our net loss. For example, public company rules and regulations make it more difficult and more expensive for us to obtain director and officer liability insurance, the cost of which has continued to rise in recent years, and thus we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements, the impact of which could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers.
73
If we fail to maintain proper and effective internal control over financial reporting in the future, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results, investors’ views of us and, as a result, the value of our common stock.
Pursuant to Section 404 of the Sarbanes-Oxley Act, our management is required to report upon the effectiveness of our internal control over financial reporting. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing, and possible remediation. To continue to comply with the requirements of being a reporting company under the Exchange Act, we will be required to continue to upgrade and maintain our systems including information technology; implement and maintain additional financial and management controls, reporting systems, and procedures; and hire additional accounting and finance staff. Furthermore, we rely on third-parties, including software and system providers, for ensuring our reporting obligations and effective internal controls, and to the extent these third parties fail to provide adequate service including as a result of any inability to scale to handle our growth and the imposition of these increased reporting and internal controls and procedures, we could incur material costs for upgrading or switching systems and our business could be materially affected.
However, as a smaller reporting company and a non-accelerated filer, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 for as long as we are not deemed an “accelerated filer” or “large accelerated filer.”
If we are unable to establish and maintain effective internal controls it could have a material adverse effect on our business, financial condition, results of operations or cash flows.
As we grow, we plan to hire additional personnel and engage in external temporary resources and may implement, document, and modify policies and procedures to maintain effective internal controls. However, we may identify deficiencies and weaknesses or fail to remediate previously identified deficiencies in our internal controls. If material weaknesses or deficiencies in our internal controls exist and go undetected or unremediated, our financial statements could contain material misstatements that, when discovered in the future, could cause us to fail to meet our future reporting obligations and cause the price of our common stock to decline. In addition, we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
If securities or industry analysts do not continue to publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us, our business, our market, or our competitors. If one or more of the analysts who covers us downgrades our stock or publish unfavorable research or reports about our business, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include:
74
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which limits the ability of stockholders owning in excess of 15% of our outstanding voting stock to merge or combine with us. Although we believe these provisions collectively provide for an opportunity to obtain greater value for stockholders by requiring potential acquirors to negotiate with our board of directors, the provisions would apply even if an offer rejected by our board were considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Our restated certificate of incorporation and amended and restated bylaws provide that the Court of Chancery of the State of Delaware and the federal district courts of the United States will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
Our restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on our behalf, any action asserting a breach of fiduciary duty, any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws or any action asserting a claim against us that is governed by the internal affairs doctrine. This provision would not apply to claims brought to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Our amended and restated bylaws further provide that the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. These choices of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees and may discourage these types of lawsuits. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation or bylaws has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive-forum provisions, and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. If a court were to find the exclusive-forum provision contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business.
Our business could be negatively affected as a result of the actions of activist stockholders.
Proxy contests have been waged against many companies in the biotechnology industry over the last few years. We may be particularly vulnerable to activist stockholders due to fluctuations in our stock price. If faced with a proxy contest or other type of stockholder activism, we may not be able to respond successfully to the contest or dispute, which would be disruptive to our business. Even if we are successful, our business could be adversely affected by a proxy contest or stockholder dispute involving us or our partners because:
These actions could cause our stock price to experience periods of volatility.
75
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
Interim Chief Financial Officer Transition
On August 1, 2024, we announced that Bruce Greenberg, our Senior Vice President of Finance and Interim Chief Financial Officer, will step down from his positions with the company effective August 31, 2024. Following his departure, we expect to outsource the principal financial and principal accounting officer roles through the engagement of a third-party provider of strategic and operational finance and accounting services. Mr. Greenberg has agreed to continue to serve as Interim Chief Financial Officer until such time and we expect to enter into a consulting agreement with Mr. Greenberg pursuant to which Mr. Greenberg is expected to provide consulting services to the company through November 30, 2024 to help support the successful transfer of responsibilities. Mr. Greenberg’s separation will be treated as an “Involuntary Termination” within the meaning of that certain offer letter, dated November 26, 2019, between the Company and Mr. Greenberg (which agreement has previously been filed with the SEC). Mr. Greenberg’s departure is not the result of any disagreement with the Company or the Board on any matter relating to the Company’s operations, policies or practices.
76
Item 6. Exhibits.
|
Exhibit Number |
|
Description |
|
|
|
3.1 |
|
|
|
|
|
3.2 |
|
|
10.1* |
|
|
|
|
|
10.2* |
|
|
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
|
32.1 |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
The certification attached as Exhibit 32.1 that accompanies this quarterly report on Form 10-Q is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Aldeyra Therapeutics, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this quarterly report on Form 10-Q, irrespective of any general incorporation language contained in such filing.
77
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
Aldeyra Therapeutics, Inc. |
|
|
|
[August 1, 2024] |
|
/s/ Todd C. Brady, M.D., Ph.D. |
|
|
Todd C. Brady, M.D., Ph.D. |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
|
|
|
|
|
Aldeyra Therapeutics, Inc. |
|
|
|
[August 1, 2024] |
|
/s/ Bruce Greenberg |
|
|
Bruce Greenberg |
|
|
Senior Vice President of Finance, Interim Chief Financial Officer |
|
|
(Principal Financial Officer and Principal Accounting Officer) |
78
Exhibit 10.1
Execution Version
THIRD AMENDMENT TO LOAN AND SECURITY AGREEMENT
THIS THIRD AMENDMENT TO LOAN AND SECURITY AGREEMENT (this "Amendment"), dated as of April 29, 2024 (the "Third Amendment Effective Date"), is entered into by and among ALDEYRA THERAPEUTICS INC., a Delaware corporation ("Aldeyra"), Helio Vision, LLC, a Delaware limited liability company, and each of Aldeyra's Qualified Subsidiaries (hereinafter collectively referred to as the "Borrower"), the several banks and other financial institutions or entities from time to time parties to the Loan Agreement (as defined below) (collectively, referred to as "Lender") and HERCULES CAPITAL, INC., a Maryland corporation, in its capacity as administrative agent and collateral agent for itself and Lender (in such capacity, "Agent").
Borrower, Lender and Agent are parties to that certain Loan and Security Agreement, dated as of March 25, 2019 (the "Original Loan Agreement"; the Original Loan Agreement, as amended by the First Amendment to Loan and Security Agreement dated April 20, 2021 and the Second Amendment to Loan and Security Agreement dated December 22, 2022, the "Existing Loan Agreement"; and the Existing Loan Agreement, as amended by this Amendment and as further amended, restated, supplemented or otherwise modified from time to time, the "Loan Agreement"). As of the Third Amendment Effective Date, the Term Loan principal balance outstanding is Fifteen Million Dollars ($15,000,000). Borrower has requested that Agent and Lender agree to certain amendments to the Loan Agreement. Agent and Lender have agreed to such request, subject to the terms and conditions hereof.
Accordingly, the parties hereto agree as follows:
SECTION 1 Definitions; Interpretation.
SECTION 2 Amendments to the Loan Agreement.
"Third Amendment Effective Date" means April 29, 2024.
"(d) Payment. Borrower will pay interest on each Term Loan Advance on the first Business Day of each month, beginning the month after the Advance Date. The entire Term Loan principal balance and all accrued but unpaid interest hereunder, shall be due and payable on Term Loan Maturity Date. Borrower shall make all payments under this Agreement without setoff, recoupment or deduction and regardless of any counterclaim or defense. Lender will initiate debit entries to the Borrower's account as authorized on the ACH Authorization (i) on each payment date of all periodic obligations payable to Lender under each Term Advance and (ii) reasonable and documented out-of-pocket legal fees and costs incurred by Agent or Lender in connection with Section 11.11 of this Agreement; provided that, with respect to clause (i) above, in the event that Lender or Agent informs Borrower that Lender will not initiate a debit entry to Borrower's account for a certain amount of the periodic obligations due on a specific payment date, Borrower shall pay to Lender such amount of periodic obligations in full in immediately available funds on such payment date; provided, further, that, with respect to clause (i) above, if Lender or Agent informs Borrower that Lender will not initiate a debit entry as described above later than the date that is three (3) Business Days prior to such payment date, Borrower shall pay to Lender such amount of periodic obligations in full in immediately available funds on the date that is three (3) Business Days after the date on which Lender or Agent notifies Borrower of such; provided, further, that, with respect to clause (ii) above, in the event that Lender or Agent informs Borrower that Lender will not initiate a debit entry to Borrower's account for certain amount of such reasonable and documented out-of-pocket legal fees and costs incurred by Agent or Lender, Borrower shall pay to Lender such amount in full in immediately available funds within three (3) Business Days after the date on which Lender or Agent notifies Borrower of such."
"(a) If to Agent:
HERCULES CAPITAL, INC.
Legal Department
Attention: Chief Legal Officer, Lake McGuire, Michael Dutra and Michael Bowden
1 North B Street, Suite 2000
San Mateo, CA 94401
email: legal@htgc.com; lmcguire@htgc.com; mdutra@htgc.com and mbowden@htgc.com
Telephone: 650-289-3060
"(b) If to Lender:
HERCULES CAPITAL, INC.
Legal Department
Attention: Chief Legal Officer, Lake McGuire, Michael Dutra and Michael Bowden
l North B Street, Suite 2000
San Mateo, CA 94401
email: legal@htgc.com; lmcguire@htgc.com; mdutra@htgc.com and mbowden@htgc.com
Telephone: 650-289-3060"
SECTION 3 Conditions of Effectiveness. The effectiveness of Section 2 of this Amendment shall be subject to the satisfaction of each of the following conditions precedent:
SECTION 4 Representations and Warranties.
2
To induce Agent and Lender to enter into this Amendment, each Borrower hereby confirms, as of the date hereof and the Third Amendment Effective Date, that (a) the representations and warranties made by it in Section 5 of the Loan Agreement and in the other Loan Documents are true and correct in all material respects; provided, however, that such materiality qualifier shall not be applicable to any representations and warranties that already are qualified or modified by materiality in the text thereof provided, further, that to the extent such representations and warranties by their terms expressly relate only to a prior date such representations and warranties shall be true and correct as of such prior date; (b) there has not been and there does not exist a Material Adverse Effect; (c) [reserved]; (d) Agent has and shall continue to have valid, enforceable and perfected first-priority liens, subject only to Permitted Liens, on and security interests in the Collateral and all other collateral heretofore granted by Borrower to Agent, pursuant to the Loan Documents or otherwise granted to or held by Agent; (e) the agreements and obligations of Borrower contained in the Loan Documents and in this Amendment constitute the legal, valid and binding obligations of Borrower, enforceable against Borrower in accordance with their respective terms, except as the enforceability thereof may be limited by bankruptcy, insolvency or other similar laws of general application affecting the enforcement of creditors' rights or by the application of general principles of equity; (f) the execution, delivery and performance of this Amendment by Borrower will not violate any law, rule, regulation, order, contractual obligation or organizational document of Borrower and will not result in, or require, the creation or imposition of any lien, claim or encumbrance of any kind on any of its properties or revenues; and (g) no Event of Default has occurred and is continuing.
SECTION 5 Miscellaneous.
3
U) Counterparts. This Amendment may be executed in any number of counterparts and by different parties on separate counterparts, each of which, when executed and delivered, is an original, and all taken together, constitute one Amendment. Delivery of an executed counterpart of a signature page of this Amendment by facsimile, portable document format (.pdf) or other electronic transmission will be as effective as delivery of a manually executed counterpart hereof.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
4
IN WITNESS WHEREOF, the parties hereto have duly executed this Amendment, as of the date first above
written.
BORROWER: |
|
|
|
|
|
ALDEYRA THERAPEUTICS, INC. |
|
|
|
|
|
Signature: |
/S/ Bruce Greenberg |
Print Name: |
Bruce Greenberg |
|
|
|
|
Title: |
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
HELIO VISION, LLC |
|
|
|
|
|
Signature: |
/S/ Bruce Greenberg |
|
|
|
|
Print Name: |
Bruce Greenberg |
|
|
|
|
|
|
Title: |
Chief Financial Officer |
[SIGNATURES CONTINUE ON THE NEXT PAGE]
[Signature Page to ThirdAmendment to Loan and Security Agreement]
AGENT: |
|
|
|
HERCULES CAPITAL, INC. |
|
|
|
Signature: |
/S/ Prentis Robinson III |
|
|
Print Name: |
Prentis Robinson III |
|
|
Title: |
Associate General Counsel |
|
|
|
|
LENDER: |
|
|
|
HERCULES CAPITAL, me. |
|
|
|
Signature: |
/S/ Prentis Robinson III |
|
|
Print Name: |
Prentis Robinson III |
|
|
Title: |
Associate General Counsel |
[Signature Page to ThirdAmendment to Loan and Security Agreement]
Exhibit 10.2
FIFTH AMENDMENT TO LEASE AGREEMENT
THIS FIFTH AMENDMENT TO LEASE AGREEMENT is made and entered into on April 29, 2024, by and between 131 Hartwell LLC, a Massachusetts limited
liability company ("Landlord") and Aldeyra Therapeutics, Inc. a Delaware corporation ("Tenant").
RECITALS:
AGREEMENT:
Lease Year |
Annual Base Rent |
Monthly Base Rent |
Per Sq. Ft. Rent |
1/1/2025 - 12/31/2025 |
$285,205.50 |
$23,767.13 |
$30.50 |
In all other matters and respects the Lease is ratified and affirmed.
IN WITNESS WHEREOF, the parties set their hands and seals to this Fifth Amendment to Lease Agreement to be executed the day and year first above written.
LANDLORD
131 Hartwell LLC
By: /s/ Charles P. Minasian
Its: Charles P. Minasian Managing Member
TENANT
Aldeyra Therapeutics, Inc.
By: /s/ Bruce M. Greenberg
Its: Bruce Greenberg Senior Vice President of Finance, Interim Chief Financial Officer I, Todd C. Brady, certify that:
Exhibit 31.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
August 1, 2024 |
|
/s/ Todd C. Brady, M.D., Ph.D. |
|
|
Todd C. Brady, M.D., Ph.D. |
|
|
Chief Executive Officer |
|
|
(Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER AND PRINCIPAL ACCOUNTING OFFICER
PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Bruce Greenberg, certify that:
August 1, 2024 |
|
/s/ Bruce Greenberg |
|
|
Bruce Greenberg |
|
|
Senior Vice President of Finance, Interim Chief Financial Officer |
|
|
(Principal Financial Officer and Principal Accounting Officer) |
Exhibit 32.1
CERTIFICATION
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
(Subsections (a) and (b) of Section 1350, Chapter 63 of Title 18, United States Code)
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), each of the undersigned officers of Aldeyra Therapeutics, Inc. (the “Company”), does hereby certify, to the best of such officer’s knowledge, that:
The Quarterly Report on Form 10-Q for the quarter ended June 30, 2024 (the “Form 10-Q”) of the Company fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and the information contained in the Form 10-Q fairly presents, in all material respects, the financial condition and results of operations of the Company.
August 1, 2024 |
|
/s/ Todd C. Brady, M.D., Ph.D. |
|
|
Todd C. Brady, M.D., Ph.D. |
|
|
Chief Executive Officer (Principal Executive Officer) |
August 1, 2024 |
|
/s/ Bruce Greenberg |
|
|
Bruce Greenberg |
|
|
Senior Vice President of Finance, Interim Chief Financial Officer (Principal Financial and Principal Accounting Officer) |
A signed original of this written statement required by Section 906 has been provided to the Company and will be retained by the Company and furnished to the Securities and Exchange Commission (SEC) or its staff upon request. This certification “accompanies” the Form 10-Q to which it relates, is not deemed filed with the SEC and is not to be incorporated by reference into any filing of the Company under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-Q), irrespective of any general incorporation language contained in such filing.