UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended March 31, 2024
OR
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
FOR THE TRANSITION PERIOD FROM TO
Commission File Number 001-41250
DIH HOLDING US, INC.
(Exact name of Registrant as specified in its Charter)
Delaware |
98-1624542 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
77 Accord Park Drive; Suite D-1 Norwell, MA |
02061 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (617) 871-2101
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Class A Common Stock |
|
DHAI |
|
The Nasdaq Stock Market LLC |
Warrants |
|
DHAIW |
|
The Nasdaq Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. YES ☐ NO ☒
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. YES ☐ NO ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YES ☒ NO ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit such files). YES ☒ NO ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YES ☐ NO ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on the Nasdaq Stock Market on September 30, 2023, was $60,871,668.
The number of shares of Registrant’s Common Stock outstanding as of June 30,2024 was 40,544,935
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement for the 2024 Annual Meeting of Stockholders to be filed with the U.S. Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K are incorporated by reference in Part III of this Annual Report on Form 10-K.
Table of Contents
|
|
Page |
PART I |
|
|
Item 1. |
1 |
|
Item 1A. |
12 |
|
Item 1B. |
32 |
|
Item 1C. |
32 |
|
Item 2. |
34 |
|
Item 3. |
34 |
|
Item 4. |
34 |
|
|
|
|
PART II |
|
|
Item 5. |
35 |
|
Item 6. |
35 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
36 |
Item 7A. |
45 |
|
Item 8. |
46 |
|
Item 9. |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
80 |
Item 9A. |
81 |
|
Item 9B. |
82 |
|
Item 9C. |
Disclosure Regarding Foreign Jurisdictions that Prevent Inspections |
82 |
|
|
|
PART III |
|
|
Item 10. |
83 |
|
Item 11. |
83 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
83 |
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
83 |
Item 14. |
83 |
|
|
|
|
PART IV |
|
|
Item 15. |
84 |
|
Item 16. |
84 |
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (“Annual Report”) contains forward-looking statements within the meaning of the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, or the Private Securities Litigation Reform Act of 1995. Investors are cautioned that such forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management and involve risks and uncertainties. Forward-looking statements include statements regarding our plans, strategies, objectives, expectations and intentions, which are subject to change at any time at our discretion. Forward-looking statements include our assessment from time to time of our competitive position, the industry environment, potential growth opportunities, the effects of regulation and events outside of our control, such as natural disasters, wars or health epidemics. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “hopes,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions.
Forward-looking statements are merely predictions and therefore inherently subject to uncertainties and other factors which could cause the actual results to differ materially from the forward-looking statement. These uncertainties and other factors include, among other things:
● |
unexpected technical and marketing difficulties inherent in major research and product development efforts; |
|
|
● |
our ability to remain a market innovator, to create new market opportunities, and/or to expand into new markets; |
|
|
● |
the potential need for changes in our long-term strategy in response to future developments; |
|
|
● |
our ability to attract and retain skilled employees; |
|
|
● |
our ability to raise sufficient capital to support our operations and fund our growth initiatives; |
|
|
● |
unexpected changes in significant operating expenses, including components and raw materials; |
|
|
● |
any disruptions or threatened disruptions to our relations with our resellers, suppliers, customers and employees, including shortages in components for our products; |
|
|
● |
changes in the supply, demand and/or prices for our products; |
|
|
● |
the complexities and uncertainty of obtaining and conducting international business, including export compliance and other reporting and compliance requirements; |
|
|
● |
the impact of potential security and cyber threats or the risk of unauthorized access to our, our customers’ and/or our suppliers’ information and systems; |
|
|
● |
changes in the regulatory environment and the consequences to our financial position, business and reputation that could result from failing to comply with such regulatory requirements; |
|
|
● |
our ability to continue to successfully integrate acquired companies into our operations, including the ability to timely and sufficiently integrate international operations into our ongoing business and compliance programs; |
|
|
● |
failure to develop new products or integrate new technology into current products; |
|
|
● |
unfavorable results in legal proceedings to which we may be subject; |
|
|
● |
failure to establish and maintain effective internal control over financial reporting; and |
|
|
ii
● |
general economic and business conditions in the United States and elsewhere in the world, including the impact of inflation. |
You should refer to Part I, Item 1A “Risk Factors” of this Annual Report on Form 10-K for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of the risks, uncertainties and assumptions described under “Risk Factors” and elsewhere, we cannot assure you that the forward-looking statements in this Annual Report on Form 10-K will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance, or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this report to conform these statements to new information, actual results or changes in our expectations, except as required by law.
The forward-looking statements contained in this report are based on our current expectations and beliefs concerning future developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, those factors described under the heading “Risk Factors.” Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
iii
PART I
Item 1. Business.
Overview
DIH Holding US, Inc., a Delaware corporation and its consolidated subsidiaries are referred to in this Form 10-K as "we," “our,” “us,” the “Company,” or “DIH.” DIH is a global provider of advanced robotic devices used in physical rehabilitation, which incorporate visual stimulation in an interactive manner to enable clinical research and intensive functional rehabilitation and training in patients with walking impairments, reduced balance and/or impaired arm and hand functions. We strive to serve the rehabilitation market by providing a broad array of devices and services focused on the customer and patient recovery. DIH stands for our vision to “Deliver Inspiration & Health” to improve the daily lives of millions of people with disabilities and functional impairments.
DIH offers innovative, robotic-enabled rehabilitation devices in an interactive environment. These solutions allow for intensive rehabilitation across the spectrum of patient specific levels of care, while also tracking patients’ progress and providing a network of collaboration and encouragement. DIH is dedicated to restoring mobility and enhancing human performance through a broad array of devices that can enable the transformation of rehabilitation care at our customers. Our revenue is concentrated in Europe, Middle East and Africa (“EMEA”) and Americas, with the remaining revenue in Asia Pacific (“APAC”).
Corporate History
DIH Technology Ltd. (“DIH Cayman”) was founded in 2014 by Chief Executive Officer and Chairman, Jason Chen, with the belief that synergies could be created by integrating the niche players in the rehabilitation therapy and research markets to build a global leading growth platform. As part of this strategy, in April 2015, DIH Hong Kong, a wholly owned subsidiary of DIH Cayman, acquired Motek ForceLink B.V. and its subsidiaries (together “Motek" or "Motek Group"), a Netherlands-based technology leader in sophisticated VR-enabled movement platforms that set the standards for human movement research and treatment; and in September 2016 acquired Hocoma AG (“Hocoma”), a Switzerland-based global market leader in the development, manufacturing and marketing of robotic and sensor-based devices for functional movement therapy.
Subsequently, DIH Hong Kong organized Motek ForceLink and Hocoma under a global management framework, with the purpose of building a scalable global business blending the technical, product and market strengths of those two firms to create a scalable and fully aligned global growth and operational bases that can be leveraged for rapid growth.
Prior to the reverse recapitalization with Aurora Technology Acquisition Corp. (“ATAK”) and the reorganization, DIH Cayman owned 100% of DIH Hong Kong and DIH Hong Kong owned 100% of various operating entities including the manufacturing entities Hocoma AG and Motek Group. Hocoma AG was the sole owner of five commercial selling entities located in the United States, Chile, Slovenia, Germany, and Singapore. The commercial entities had exclusive rights to distribute the goods produced by Hocoma AG and Motek Group. While the business under DIH Cayman historically functioned together, they maintained largely independent management teams and did not rely on corporate or other support functions from DIH Cayman.
On October 6, 2020, Hocoma AG created a new wholly owned subsidiary, DIH US Corp, a Delaware entity. The purpose of DIH US Corp was to own 100% of the commercial selling entities. On May 31, 2021, Hocoma AG completed the share transfer of commercial entities to DIH US Corp.
On June 2, 2021, DIH Cayman formed a wholly owned subsidiary, DIH Holding US Inc., a Nevada Corporation (“Legacy DIH” or “DIH Nevada”). This entity was established to serve as a US-based holding company, to which assets could be transferred, setting the foundation for the future of the Company, which would eventually engage in the Business Combination with ATAK.
On June 21, 2021, Hocoma AG formed another wholly owned subsidiary, Hocoma Medical GmbH. The purpose of Hocoma Medical GmbH was to transfer the net assets of Hocoma AG, excluding intellectual property and non-transferable debt, and then sell the entity and its assets to DIH Nevada, for inclusion in the foundation of the future Company.
1
On July 1, 2021, DIH Cayman completed a series of reorganization steps to transfer DIH US Corp and its subsidiaries from Hocoma AG to DIH Nevada, effectively creating the Company. Hocoma AG entered into the following transactions:
However, on July 1, 2021, the former shareholders of Hocoma AG applied for and were granted an ex-parte preliminary injunctions by a Swiss district court. The injunctions prohibited Hocoma AG to transfer any business or assets to Hocoma Medical, and as well as the sale of Hocoma Medical from DIH Hong Kong to the Company. Consequently, Hocoma AG and its shareholders challenged these preliminary injunctions through their Swiss counsels at Homburger. On January 12, 2024, the court revoked the preliminary injunctions granted on July 1, 2021. Therefore, the injunctions no longer have any legal effect on the contribution of the business/assets of Hocoma AG to Hocoma Medical and the transfer of the ownership of Hocoma Medical GmbH to the Company. Hocoma Medical GmbH, including the business/assets transferred by Hocoma AG, became a wholly-owned subsidiary of DIH Nevada as of July 1, 2021.
DIH Cayman intended to transfer Hocoma AG (remaining assets and liabilities) and Motek Group to DIH Nevada pursuant to the Business Combination Agreement. However, DIH Cayman was subject to a lien in Hong Kong related to DIH China, a company formed in the People’s Republic of China (“DIH China”) and a wholly owned subsidiary of DIH Hong Kong. The lien was filed on July 31, 2021 on the immediate parent company of Hocoma AG and Motek Group and prevented the transfer of Hocoma AG and Motek Group. This matter is currently under review by local authorities and DIH Cayman is working to facilitate the completion of the intended transfer.
While the Company’s businesses have historically functioned together with the other businesses controlled by DIH Cayman, the Company’s businesses are largely isolated and not co-dependent on corporate or other support functions. DIH Hong Kong is a wholly-owned subsidiary of DIH Cayman and the Company was a wholly-owned subsidiary of DIH Cayman prior to closing of the Business Combination.
In October 2022, DIH Nevada acquired the SafeGait 360 and SafeGait Active smart mobility trainer systems from Gorbel, an innovative United States-based developer and manufacturer of smart material handling and fall protection equipment. The SafeGait acquisition was accounted for as an asset acquisition based on an evaluation of the U.S. GAAP guidance for business combinations.
Organization structure immediately prior to the Business Combination
Immediately before closing of the Business Combination, DIH Nevada was a wholly owned subsidiary of DIH Cayman. DIH Nevada held 100% ownership of DIH US Corp, which in turn owned the commercial entities. Additionally, DIH Nevada held 100% ownership of Hocoma Medical GmbH, which contained the net assets transferred from Hocoma AG.
DIH maintained exclusive distributor agreements with Motek Group for its advanced human movement research and rehabilitation products and services designed to support efficient functional movement therapy within specified territories. Under the distribution agreements, Motek supplied the products and services to the Company at the prices detailed in the agreement, with the Company entitled to a distributor margin.
Business Combination
On February 7, 2024 (the “Closing Date”), Aurora Technology Acquisition Corp. a Cayman Island exempted company which migrated and domesticated as a Delaware corporation (“ATAK”), Aurora Technology Merger Sub, a Nevada corporation and a direct, wholly-owned subsidiary of ATAK (“Merger Sub”) and DIH Nevada consummated a previously announced business combination pursuant to a business agreement dated as of February 26, 2023 (as amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement,” and the transactions contemplated thereby, the “Business Combination”) following the receipt of the required approval by ATAK’s and DIH Nevada’s shareholders and the fulfillment or waiver of other customary closing conditions.
2
In connection with the Closing, ATAK migrated and changed its domestication to become a Delaware corporation and changed its name to “DIH Holding US, Inc.” The Amended and Restated Certificate of Incorporation of DIH authorizes one class of common stock as Class A Common Stock ("Common Stock").
The historical financial results presented in the registration statements were prepared on a combined basis including Legacy DIH, Hocoma AG and Motek Group pursuant to the Business Combination Agreement for the intended Reorganization (as such term is defined in the Business Combination Agreement). Due to the lien on DIH Hong Kong related to DIH China, the Reorganization could not be completed as defined by the Business Combination Agreement, meaning that Motek Group and Hocoma AG ownership could not be transferred to the Company prior to the Closing.
In connection with the Closing of the Business Combination and in accordance with the terms of the Business Combination Agreement, ATAK agreed to waive the closing condition that the Reorganization be completed prior to Closing. The Company agreed to use its best efforts to complete the Reorganization as defined in the Business Combination Agreement as soon as possible thereafter. The Reorganization has not been completed as of the filing date of this Annual Report on Form 10-K. In this Annual Report on Form 10-K, the Company has recast historical financial statements on a consolidated basis including only operations from Legacy DIH. Hocoma AG and the Motek Group remained with DIH Hong Kong and are excluded from the consolidation of the Company.
Upon closing of the Business Combination with ATAK, the Company owns 100% of DIH US Corp, which in turn owns the commercial entities. Additionally, the Company owns 100% ownership of Hocoma Medical GmbH, which contains the net assets transferred from Hocoma AG. The Company maintains an exclusive distribution agreement with Motek Group as of the date of this Annual Report on Form 10-K. DIH Cayman owns approximately 34.7% shares of common stock of the Company, including earn-out shares held in escrow account. Jason Chen, the Company's Chief Executive Officer and Chairman of the Board of Directors does not own any shares of DIH directly but may be deemed to have indirect ownership of DIH through his ownership of approximately 42% of the outstanding shares of DIH Cayman.
The Company’s organizational chart as of the date of this report is as follows:
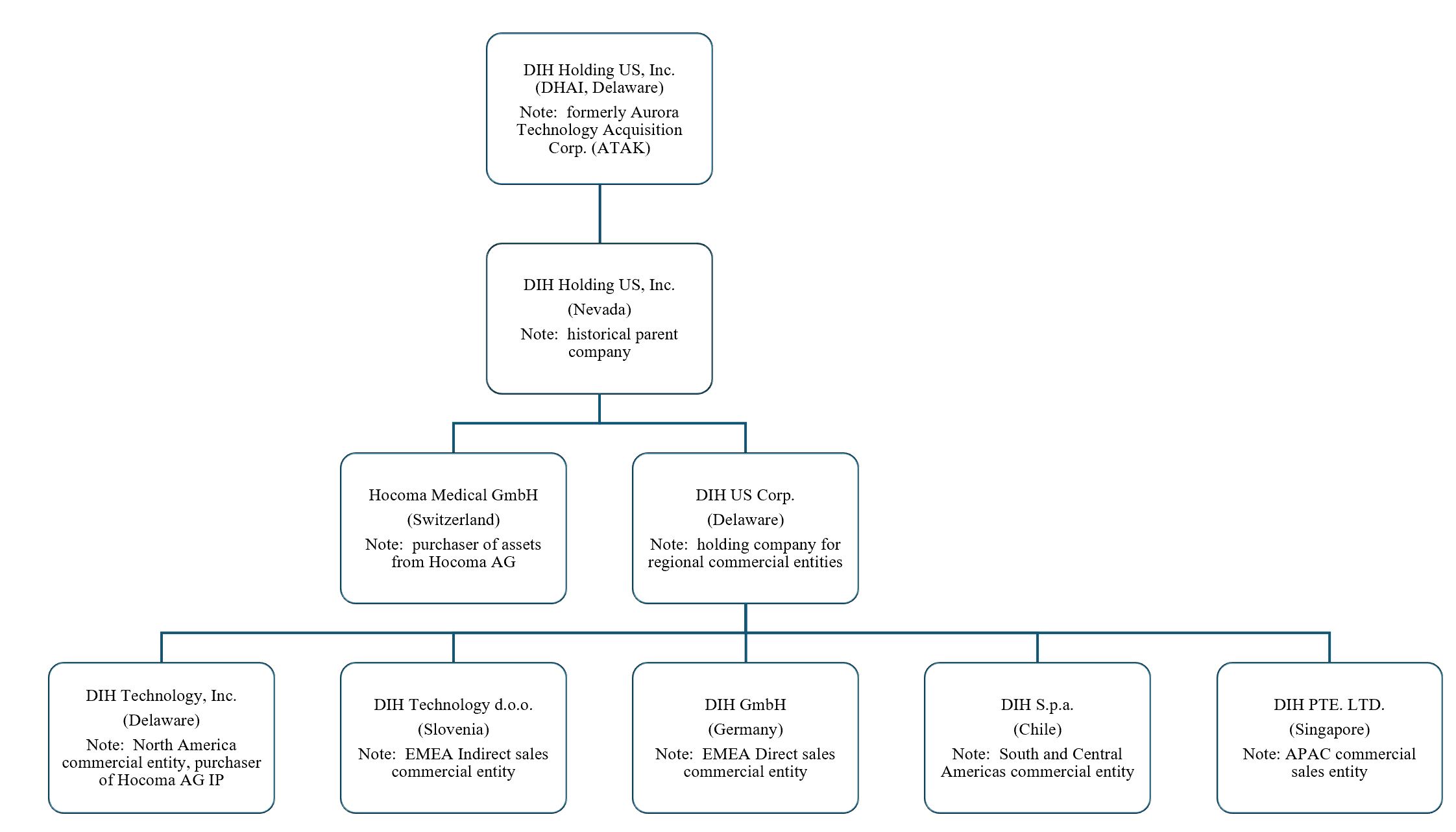
3
Industry and Market Overview
Market Opportunity
The market for robotic devices for rehabilitation and human performance enhancement is rapidly growing. As populations age and the consequent demand for healthcare services increases, we expect there will be a growing need for innovative solutions that can help individuals recover from injuries and optimize their physical abilities. Additionally, there is a growing interest in the use of technology to enhance human performance, whether in sports or in everyday life.
DIH’s target market is composed of three major sub-markets:
|
■ |
Advanced Research Facilities (“ARFs”): which include advanced human performance labs or rehabilitation/biomedical research centers at universities and academic hospitals); |
|
■ |
Inpatient rehabilitation facilities (“IRFs”) which include free standing rehabilitation hospitals and rehabilitation units in acute care hospitals); |
|
■ |
Outpatient rehabilitation facilities (“ORFs”) which include outpatient rehabilitation clinics, skilled nursing or long-term care facilities). We are currently focused on the North American and European markets to accelerate market penetration, while seeking early-stage opportunities in other international markets for future expansion |
For the ARF (or Research Market), our products enable thought-provoking and sophisticated simulation and evaluation of general human performance, and specifically focuses on dynamic gait and balance-focused movement research, through our industry-leading interactive VR platform. The platform is empowered by motion capture hardware, couple with advanced human body modeling software, that creates real time visualization of the active participant. The integration of this technology with advanced robotics and other smart systems expands imaginative research interests. Most of the top 50 global leading research centers in human performance and rehabilitation have adopted our technologies as the key base of their research exploration. We believe that by integrating leading products in biomechanical research we sourced through Motek (with which we have an exclusive distribution agreement) with our advanced robotics and other AI-based innovative products we could leverage clinical results with data insights to help transform protocols and processes in the industry related to human performance and movement disorders leading to solid growth potential in this important market segment in the next 5-10 years.
For IRFs (or Hospital Market), our products enable intensive functional training in patients with walking impairments, reduced balance and/or impaired arm and hand functions. Functional training is the backbone of rehabilitation and aims to restore lost abilities and enhance performance through learning mechanisms and neuroplasticity, and thereby increase independence in daily living and quality of life. Intensive rehabilitative therapy induces stronger and faster functional recovery. Our advanced rehabilitation robots enable intensive therapy, even in high acuity patients, by providing physical assistance and mobilization to patients as needed and relieving therapists from manual workload. Gamified exercises and feedback motivating patients during movement therapy, interactive reports and data integration further enhance the clinical value of our advanced rehabilitation solutions.
For the vast ORFs (or Clinical market), we enable modern specialty rehabilitation care models that differentiate and deliver high value with better and consistent outcomes due to the 3i intervention approach empowered by our technology. By blending technology with innovative care models, such modern specialty ORFs can deliver superior values to patients and their therapists by enabling one therapist to treat multiple patients with better outcomes due to the intensive, interactive, and integral approach enabled by our smart solutions. By leveraging the treatment protocol established by our advanced robots and movements platform, we are re-configuring our solutions through modularization and further acquisitions to exploit this vast and diverse market.
Our Strategy
Physical disabilities and impairments represent significant global challenges, due to the rapid aging, increasingly severe chronic diseases, and prevailing traumatic injuries from accidents and wars. According to the Company’s internal analysis, each year, approximately 20 million people suffer from new disabilities, and it is estimated that over 300 million people are currently suffering from some form of functional impairments or disabilities globally. Those functional impairments or disabilities may ultimately result in multiple functional health problems, including cognitive, physical, emotional and spiritual issues, imposing severe burdens to health systems and significant costs to society.
4
Adding to this, the number of people aged 65 and older globally is currently approximately 1 billion, and is estimated to grow to over 1.7 billion by 2040. Approximately 87% of elders suffer from chronic diseases, and over 25% are exposed to additional disability risks according to the Company’s estimate.
According to an online article dated October 14, 2022 by Grandview Research, the global rehabilitation care market is estimated to be over $100 billion and is extremely reliant upon manual therapies, with therapists’ payroll costing more than $50 billion. We believe that such a manually dominant approach not only imposes a huge labor burden to therapists, but also may result in inconsistent outcomes due to a lack of consistent intensity, integration, standardization and optimization throughout the weeks or months of long intervention processes. Unlike a machine which can be calibrated thereby producing consistent therapy, we believe that manual therapy is likely to vary therapist to therapist or even patient to patient. Measurements of progress may also be subjective, varying from therapist to therapist which may result in a patient requiring a longer period of therapy to achieve the desired results.
The rapidly aging and increased chronic-suffering population trend will generate more demand for high quality rehabilitation care, while reducing the supply of therapists, thus adding increasing pressure and tension to the current model.
We believe the way out of such a undesirable and increasingly high pressure state is to transform the rehabilitation care model through integrated solutions empowered by our advanced technologies. The core benefits we strive to deliver to customers from our core products and solutions include:
1) Enhancing customers’ strategic positioning as leading rehabilitation facilities to attract higher paying patient groups by enabling them to attract and treat high severity and acuity patient groups, especially neurological patients;
2) Reducing total therapy costs by enabling the therapist to concurrently treat multiple patients, improve therapy outcome with the same time of stay or reduce time of stay without losing clinical efficacy;
3) Providing streamlined intervention processes with data insights and potential network effects;
4) Enabling replication and franchise established treatment protocols and best practices across chains of rehabilitation facilities;
5) Reducing total health system costs.
To build on our unique position as a global robotic and VR-enabled smart technologies and solution provider to the rehabilitation market, our strategic plan is to continue to expand our leadership through sustaining innovation, selective acquisitions, with continued focus on delivering superior value to our customers, partners, patients and other stakeholders.
Our strategic focus is on the following three areas:
|
● |
Leveraging our strengths in technologies and core products to continuously expand our market leadership, drive market penetration and accelerate growth by building intensive market penetration capability in strategic markets in the United States and Europe, enriching our product offering with innovative financing solution to accelerate customers adoption, and sustain our product and technology leadership with continued innovation and integration efforts. |
|
|
|
|
● |
Leveraging our market leadership and global platform and infrastructure and consolidating the fragmented marketplace to drive standardization and economy of scale and scope. The breadth and depth of our business model and the scale advantage enables us, not only to sustain our market leadership, but also empowers us to act as active consolidators in the highly fragmented rehabilitation market. By complementing our organic innovation and core product leadership, DIH envisions executing 2-3 acquisition with the goal to acquire proven products and technologies from sub-optimal regional players to exploit global synergies and to accelerate the growth of DIH’s integrated solution offerings. |
5
|
|
|
|
● |
Leveraging our thought-provoking industry influence backed by leading brands and products, passionate people and organizational capability; DIH strives to develop transformative Total Solutions that will fundamentally enhance the therapy and business model of our customers and enable industry-wide transformation which is expected to eventually benefits millions of people, from therapists to patients. |
Core Product Overview
DIH offers innovative, robotic-enabled devices in an augmented and interactive environment. These devices focus on restoring different functional impairment issues, while using software thereby tracking patients’ progress and providing a network of collaboration and encouragement.
We currently offer 17 robotic rehabilitation and VR-based movement systems within three major product categories through the hospital, clinical and research markets. Our objective is to establish ourselves as a product and technology leader in each of the three categories, that correspond to three key functional impact issues, i.e. 1) upper extremity devices for arm and hand functional improvement; 2) lower extremity devices for gait and balance intervention; and 3) full body integrated intervention for strength and endurance enhancement. Through software networks, we aim not only to maximize the benefits from each of the devices itself, but also to deliver multi-dimensional clinical, economic, process and administrative benefits to therapists, patients and management by connecting and integrating these various devices into cohesive and integrated caring processes and models, enabling transformative change in therapies and business models.

Upper Extremity Product Categories
To address differing clinical and economic needs, while providing consistent therapeutic interventions with similar treatment concepts and protocols, we have developed three different device models, ArmeoPower, ArmeoSpring, and ArmeoSenso. All follow the same modular Armeo Therapy Concept, that covers the “Continuum of Rehabilitation” with one software platform throughout the different stages of rehabilitation; from the early stage where the patient is very weak and needs sophisticated power-assisted dynamic intervention to help rewire the neural pattern in a safe environment which ArmeoPower provides, to self-initiated interactive ArmeoSpring which follows a similar treatment protocol of ArmeoPower for patients who have gained certain muscle power and need to transition from controlled patterns to an open environment.
6
ArmeoSenso is for patients to apply what they learned from those self-initiated but still structurally controlled movement patterns to completely open movement environments, further expanding the patient transfer skills. The economic costs of devices, and the ratio of one therapist for multiple patients also improves dramatically, thus allowing service providers and health systems to gain significant benefits of learning curves, i.e. the learning patient picks up from early acute expensive interventions, which will be increasingly beneficial for later stages, generating a win-win, both economically and clinically.
ArmeoPower is the backbone robot within our Upper Extremity portfolio; it has been specifically designed for arm and hand therapy in an early stage of rehabilitation. It enables patients with even severe motor impairments to perform exercises with a high number of repetitions. It assists the patient’s arm on an “as needed” basis to enable the patient to successfully reach the goal of the exercise. The robotic arm assistance can be adapted to the individual’s needs and the changing abilities of each patient – from full assistance for patients with very little activity to no assistance at all for more advanced patients. Such adjustable robotic assistance while exercising, enables and motivates patients to actively participate in their training, while providing weight support to enable extensive training. ArmeoPower supports 1D (joint-specific), 2D and 3D movements, with extensive game-emerged Augmented Performance Feedback ("APF") exercises simulating tasks and activities essential for daily living, while enhancing strength and range of motion. Immediate performance feedback motivates patients and helps to improve their motor abilities. It improves efficiency of the therapy session by reducing the therapist’s physical effort and the need for continuous therapeutic guidance. Moreover, it enables therapists to make better use of their clinical know-how and expertise, by focusing on the optimal exercise planning, instead of manually delivering many repetitions.
ArmeoPower precisely records how patients perform during their therapy sessions. Standardized Assessment Tools evaluate a patient’s motor functions such as joint range of motion and forces. The results can be used to analyze and document the patient’s state and therapy progress. Results can then be shared with the patient and other clinicians. ManovoPower as an add-on module for ArmeoPower enables hand opening and closing exercises.
ArmeoSpring is targeted for less severe patients; it provides self-initiated repetitive arm and hand therapy in an extensive workspace. By providing arm weight support, it encourages the patients to achieve a higher number of arm and hand movements based on specific therapy goals. It also allows simultaneous arm and hand training in an extensive workspace. This enables patients to practice the movements important for their therapy progress. ArmeoSpring also supports 1D (joint-specific), 2D and 3D movements. An extensive library of motivating game-like APF exercises has been designed to train strength and range of motion needed for activities of daily living. Immediate performance feedback motivates patients and helps to improve their motor abilities. The ArmeoSpring enables therapists to deliver higher training efficiency (more hours per day) due to self-directed therapy. Furthermore, self-directed therapy enables patients to reach an even higher therapy intensity through extra training during after-hours and weekends.
Lower Extremity Product Categories
Similar to the Armeo Therapy Concept for arm and hand, we have also developed 3+1 Robotics + VR devices to address the different clinical and economic needs of patients across different stages of the patient journey, while providing consistent therapeutic interventions with similar treatment concepts and protocols. The Erigo Robot is designed for patients right after ICU who have none or very weak muscle power, with the goal to speed up the circulation and initiate early mobilization and prepare patients for intensive therapy, while preventing or reducing secondary further impairment. LokoMat is designed to provide maximum intensive therapy to rewire the broken neuro pathway to restricted functional capabilities through Neuroplasticity effect. Andago is designed to assist patients in walking in a real environment to maximize patient transfer skills after the patient’s functional pattern has been rewired by LokoMat. C-Mill is designed to enhance the patient’s adaptability, coordination and balancing skills in a challenging and integrative environment.
Erigo is uniquely designed to provide therapy intervention to the most severe patient even at a high acute and critical post-ICU stage. It uniquely combines gradual verticalization, leg mobilization, and intensive sensorimotor stimulation through cyclic leg loading.
7
The main benefits include:
|
■ |
Early and safe mobilization even in acute care |
|
■ |
Cardiovascular stabilization |
|
■ |
Improved orthostatic tolerance using the Erigo functional stimulation. |
|
■ |
Helping to reduce patient’s length of stay, improving efficiency and outcome |
Lokomat provides robot-assisted therapy that enables effective and intensive training to increase the strength of muscles and the range of motion of joints in order to improve walking. The physiological movement of the lower extremities is ensured by the individually adjustable patient interface. Additionally, the hip and knee joint angles can be adjusted during training to the patient’s specific needs. During rehabilitation, patients need to be challenged. Therapists can help patients reach their goals by setting the training parameters according to their performance. Lokomat motivates patients to reach their goals with various game-like exercises. This Augmented Performance Feedback, or APF, maximizes the effect of Lokomat training. Lokomat allows therapists to focus on the patient and the actual therapy. It enhances staff efficiency and safety, leading to higher training intensity, more treatments per therapist, and consistent, superior patient care.
Lokomat is available in two models, LokomatNanos and LokomatPro, and has other modules such as for pediatric use available. To date, we have installed over 1,085 Lokomat systems in over 650 facilities worldwide.
Andago is designed to assist patients in walking naturally which consequently triggers continuous physiological afferent input, due to its built-in dynamic support. With its robotics smart control system, it enables patient to walk seamlessly and freely due to its robotic system. Andago bridges the gap between treadmill-based gait training and free overground walking. No dedicated space is needed as it can be used flexibly in different spaces. Its intuitive workflow allows for a quick and easy therapy start and simple integration into clinical routine. The display of key training results and export of data via USB enables training progress documentation for clinical decision-making and for health insurance providers. No infrastructure modification, meaning flexible use from room to room.
C-Mill is a powerful tool that allows for more efficient rehabilitation. Besides objective assessment of balance and gait, the C-Mill provides a safe and comfortable training environment using a treadmill, augmented reality and VR. Using our technology, patients are able to train foot placement with the C-Mill, work through balance and dual-tasks with C-Mill VR or use C-Mill VR+ for early to late rehabilitation with body weight support. It is a complete, advanced gait-lab and training center on a compact space.
CAREN, “Computer Assisted Rehabilitation Environment”, is the most advanced and sophisticated VR-enabled real time movement platform, that targets all aspects of balance and locomotion with visualization of full body participation empowered by Human Body Modeling. CAREN provides researchers with the tools to efficiently study advanced human movement by collecting objective human performance data in real time and functionally challenging environments. CAREN enables the most versatile human movement research as a result of its dual-belt instrumented treadmill mounted on a 6 degree-of-freedom movable platform, motion capture system, immersive and interactive environments and dedicated real-time and offline software packages; the CAREN is the most advanced system for your human movement research, training, and assessment. We believe CAREN will enable pioneering research in many fields of application, such as: motor control and learning, dual-tasking and feedback, balance assessment and therapy, gait analysis and adaptability, real-time human body modeling, virtual reality and integrated smart systems like robot integration. We believe CAREN is considered as the world’s most advanced biomechanics lab.
GRAIL, “Gait Real-time Analysis Interactive Lab”, the total package solution for gait analysis training and research, employs an instrumented dual-belt treadmill and motion capture system combined with virtual reality and video cameras. GRAIL provides analysis and therapy in challenging conditions to improve gait, while real-time feedback enables analysis and training during the same session.
The Total Solution
DIH’s vision includes providing a Total Solution option for our customers and their patients. The Total Solution is a product package specifically designed for our customer and is aimed at maximizing the benefits of DIH's products and solutions to achieve optimal rehabilitation outcomes.
8
This offering includes DIH’s clinical integration approach, that emphasizes three key factors:
Customer Overview
Research Market
Due to the powerfulness of our technology platform and products, and the versatile applications they enable; there are six major customer groups that are actively employing our CAREN, GRAIL and MGAIT, etc. in their leading research efforts. Universities purchase them to build modern biomedical labs and initiate systemic training, research hospitals and military purchase them to assess and define innovative interventions to restore and enhance human functions and performance, scientific and technological corporations purchase them to establish an integrated testing foundation to evaluate new concepts and accelerate new product or intervention modalities; and athletic institutions purchase them to accelerate the recovery of athletes and enhance their core performance foundations.
Hospital Market
Hospital Markets, or Inpatient rehabilitation facilities (IRFs), include free standing rehabilitation hospitals and rehabilitation units in acute care hospitals.
Our products and solutions benefit both the rehabilitation units in acute care hospitals and free standing rehabilitation hospitals. Given our limited sales resources, our primary focused customer group are rehabilitation hospitals and acute care hospitals which have a high number of neurological patients.
Within rehabilitation hospitals, it can by further broken down by 1) academic or leading national rehabilitation hospitals, 2) new modern rehabilitation hospitals, 3) neurological patient focused rehabilitation hospitals, 4) leading regional rehabilitation hospital, 5) conventional or me-too rehabilitation hospitals. Our target markets are the first two groups. Our main objective is to increase our market penetration in those groups from an estimated 25% current penetration to 66% in focused countries.
Clinical Market
The Clinical Market, or outpatient rehabilitation facilities (ORFs), include outpatient rehabilitation clinics, skilled nursing or long-term care facilities (SNF and LTC).
Given there are hundreds of thousands of facilities in these massive and diverse markets and we have limited resources, our primary focus is on the modern outpatient rehabilitation clinics (M-Clinics) and top SNFs with a focus on neurological patients (SNF-N) in our target countries. Our products can provide strategic, clinical and operational value to the M-Clinics and SNF-N, as in the hospital market.
Manufacturing and Supply Chain
Our manufacturing and supply chain strategy is founded on a commitment to blending Swiss quality mindset with Dutch agility, utilizing lean manufacturing and supply chain practices, leveraging an the Oracle ERP system implemented, ensuring efficient order fulfillment to global markets, and delivering exceptional value and commitment to our customers and patients.
Manufacturing
We manufacture the Lokomat, Andago, Erigo, Armeo Power, Armeo Spring and Armeo Senso devices at Hocoma Medical GmbH in Switzerland ). The product line we distribute for hospitals and clinics, C-Mill, is manufactured at Motek Medical B.V. in The Netherlands together with all research products (RYSEN, M-GAIT, GRAIL and CAREN).
9
For the SafeGait 360 and Active product line that we acquired from Gorbel, those two products currently are only sold in the United States and are manufactured through our manufacturing facility in Leeds, Alabama.
Supply Chain
For standardized products (for hospitals and clinics) DIH conducts production planning based on the sales budget (yearly) and sales forecast (quarterly). To have the correct alignment between all stakeholders, there is a monthly standard operating procedures (“S&OP”) meeting in place. In this meeting, all relevant stakeholders are involved, such as planning, procurement, production, order fulfillment, sales, finance, operational engineering, service and product management. Additionally, we have the inputs from regulatory and quality as well. In the S&OP the forecast and the production/procurement planning for the quarters are set and the current fulfillment situation is monitored.
Our research products are generally fairly differentiated, which makes it difficult to manage supply chain dynamics far in advance. Many of the parts are completely customized, and inputs are only known during the project phase when the order has been received. Basic parts such as treadmills, drives and motors can be planned and procured accordingly. For these research projects, there is also an S&OP in place limited to the research group.
Facilities
Our executive offices are located at 77 Accord Drive, Suite D-1 Norwell, MA. We do not own any properties, rather we lease properties to meet our needs. Currently, we have a research and development and operational campus that we lease for Hocoma operation in Switzerland located at Industriestrasse 2 and 4a in 8604 Volketswil.
Beside the main campuses, we also lease five commercial offices space at the following locations to house the regional Sales & Marketing, Clinical Application & Training, Technical Services, Finance, Logistics, Administration and other local market support functions.
|
■ |
DIH Technology Inc. leases commercial office for the American team at 77 Accord Park Dr., Suite D-1, Norwell, MA 02061, United States |
|
■ |
DIH Technology d.o.o leases commercial office for EMEA Indirect sales team at Letališka 29a, 1000 Ljubljana, Slovenia |
|
■ |
DIH GmbH leases commercial office for the Direct Sales team in DACH region, at Konrad-Adenauer Strasse 13, 50996 Köln, Germany |
|
■ |
DIH Pte Ltd leases commercial office for APAC team at 67 Ubi Avenue 1, #06-17 Starhub Green, Singapore 408942 |
|
■ |
DIH SpA leases commercial office for LATAM team at Pdte. Kennedy Lateral 5488, Oficina 1402; Vitacura, Santiago, Chile |
Human Capital
As of April 30, 2024, we employed 192 employees, of which approximately 78 percent were outside the U.S. Our employees are the Company’s most valued asset and the driving force behind our success. For this reason, we aspire to be an employer that is known for cultivating a positive and welcoming work environment and one that fosters growth, provides a safe place to work, supports diversity and embraces inclusion.
Diversity, Equity, and Inclusion
We are committed to fostering, cultivating and preserving a culture of diversity, equity and inclusion (DE&I). We recognize that a diverse, extensive talent pool provides the best opportunity to acquire unique perspectives, experiences, ideas, and solutions to drive our business forward. We believe that diverse teams solving complex problems leads to the best business results. We promote diversity by developing policies, programs, and procedures that foster a work environment where differences are respected, and all employees are treated fairly.
Employee Health and Safety
10
During the fiscal year ending March 31, 2024, there have been no OSHA recordable or lost time injuries in the United States and zero injuries at our other global sites.
Intellectual Property
We have over 20 different trademark families registered, including our most prominent product family names such as Lokomat, Armeo, Andago, and RYSEN. These trademarks are registered in 18 strategically important countries, resulting in a total of 411 registrations. The latest registration was made in 2020, and the earliest in 2004.
Name/Description of Patent |
|
Status |
|
Owned or Licensed |
|
Type of patent protection |
|
Expiration Date |
|
Jurisdictions |
US8834169/Method and apparatus for automating arm and grasping movement training for rehabilitation of patients with motor impairment |
|
Issued |
|
Licensed |
|
Utility |
|
24.11.2030 |
|
US |
|
|
|
|
|
|
|
|
|
|
|
US8192331/Device for adjusting the prestress of an elastic means around a predetermined tension or position |
|
Issued |
|
Owned |
|
Utility |
|
10.09.2028 |
|
US, DE, FR, UK, IT, CH, CN, RU |
|
|
|
|
|
|
|
|
|
|
|
US9017271/System for Arm therapy |
|
Issued |
|
Licensed |
|
Utility |
|
10.02.2031 |
|
US, DE, FR |
|
|
|
|
|
|
|
|
|
|
|
US8924010 /Method to Control a Robot Device and Robot Device |
|
Issued |
|
Owned |
|
Utility |
|
06.10.2031 |
|
US, DE, FR, NL, CH, UK |
|
|
|
|
|
|
|
|
|
|
|
US9987511/Gait training apparatus |
|
Issued |
|
Owned |
|
Utility |
|
19.09.2034 |
|
US, DE, FR, UK, IT, CH, CN, PL, KR |
|
|
|
|
|
|
|
|
|
|
|
EP3095430/Gait training apparatus (Div) |
|
Issued |
|
Owned |
|
Utility |
|
09.11.2032 |
|
DE, FR, UK, CH |
|
|
|
|
|
|
|
|
|
|
|
US10780009/Apparatus for locomotion therapy |
|
Issued |
|
Owned |
|
Utility |
|
06.01.2037 |
|
US, DE, FR, UK, CH, CN, RU |
|
|
|
|
|
|
|
|
|
|
|
EP3100707/Apparatus for locomotion therapy (Div) |
|
Issued |
|
Owned |
|
Utility |
|
16.11.2032 |
|
DE, FR, UK, IT, CH TR, PL, CN |
|
|
|
|
|
|
|
|
|
|
|
US9808668/Apparatus for automated walking training |
|
Issued |
|
Owned |
|
Utility |
|
10.08.2034 |
|
US, DE, FR, UK, IT, CH, CN, PL, TR, NL, FI, ES |
|
|
|
|
|
|
|
|
|
|
|
EP3035901/ Hand motion exercising device |
|
Issued |
|
Owned |
|
Utility |
|
14.08.2034 |
|
DE, FR, UK, NL, SI, CH, CN |
|
|
|
|
|
|
|
|
|
|
|
US10349869/Method and system for an assessment of a movement of a limb-related point in a predetermined 3D space |
|
Issued |
|
Owned |
|
Utility |
|
16.02.2036 |
|
US, DE, FR, UK, CH, AU, IT, CN |
|
|
|
|
|
|
|
|
|
|
|
US10500122/Apparatus for gait training |
|
Issued, |
|
Owned |
|
Utility |
|
20.08.2037 |
|
US, DE, FR, UK, CH, CN, |
11
|
|
Pending for KR |
|
|
|
|
|
|
|
TR, NL, SE, ES, RU, KR |
|
|
|
|
|
|
|
|
|
|
|
US10925799/ Suspension device for balancing a weight |
|
Issued |
|
Owned |
|
Utility |
|
27.06.2037 |
|
US, AU, CH, DE, FR, UK, IT, NL, PL, CN, KR |
|
|
|
|
|
|
|
|
|
|
|
US-20230039187-A1/Leg Actuation Apparatus and Gait Rehabilitation Apparatus |
|
Pending |
|
Owned |
|
Utility |
|
|
|
US, IN, CN, RU, EP, KR |
|
|
|
|
|
|
|
|
|
|
|
US-2023-0039187-A1/User Attachment for Gait and Balance Rehabilitation Apparatus |
|
Pending |
|
Owned |
|
Utility |
|
|
|
US, CN, EP, KR |
|
|
|
|
|
|
|
|
|
|
|
DM/091 450/Wheeled walking frame |
|
Issued |
|
Owned |
|
Design |
|
08.06.2041 |
|
CH, EM, US, UK |
|
|
|
|
|
|
|
|
|
|
|
DM/221 948/ArmeoSpring Pro-Design |
|
Issued |
|
Owned |
|
Design |
|
01.07.2047 |
|
CH, EM, US, UK |
Item 1A. Risk Factors.
RISK FACTORS
You should carefully consider the risks and uncertainties described below, together with the other information in this Annual Report, including our financial statements and the related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before making an investment in our securities. We cannot assure you that any of the events discussed in the risk factors below will not occur. These risks could have a material and adverse impact on our business, results of operations, financial condition and growth prospects. If that were to happen, the trading price of our securities could decline. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations or financial condition. In this section, we first provide a summary of the principal risks and uncertainties we face and then provide a full set of risk factors and discuss them in greater detail.
Risks Related to Our Business and Our Industry
We have not fully completed our planned corporate reorganization
In connection with the Business Combination, we had anticipated completing a corporate reorganization in which, among other changes, Motekforce Link BV and its subsidiaries and Hocoma AG were to become wholly owned subsidiaries of DIH Holding US, Inc. The parties were unable to complete this corporate reorganization prior to the Business Combination and, as previously disclosed, the parties opted to close the Business Combination and waive the condition to close that this reorganization be completed. These entities are owned by DIH Technology, Inc., our largest stockholder.
The products produced by Motek remain a key part of our product line and we operate with Motek pursuant to the terms of an exclusive contract which obligates Motek to provide these products to us. While we do not believe this arrangement currently has a material adverse effect on our results of operations, there can be no assurance that Motek will not begin to sell its products to our competitors which would have an adverse impact on us.
There can be no assurance that the complete reorganization will be completed Our success is substantially dependent on our ability to continue to generate and grow revenue from the sales of our current key product lines, LokoMat, Erigo, Armeo, C-Mill and CAREN/Grail, which represent approximately 90% of our revenue.
We are substantially dependent on the commercial success of our current key product lines
12
Our success will depend on many factors including, but not limited to, our ability to:
|
● |
develop and execute our sales and marketing strategies and maintain and manage the necessary sales, marketing and other capabilities and infrastructure that are required to successfully commercialize our products; |
|
● |
achieve, maintain and grow market acceptance of, and demand for our current products; |
|
|
|
|
● |
establish or demonstrate in the medical community the safety and efficacy of our rehabilitation products and their potential advantages over in comparison to, existing competing products and devices and products currently in development; |
|
|
|
|
● |
offer our products at competitive prices as compared to alternative options, and our ability to achieve a suitable profit margin from the sales of our products; |
|
|
|
|
● |
comply with applicable legal and regulatory requirements, including medical device compliance; |
|
|
|
|
● |
maintain our distribution and supply arrangements with third parties; and |
|
|
|
|
● |
enforce our intellectual property rights related to current and future products, if any. |
If we do not achieve one or more of these factors, many of which are beyond our control, in a timely manner or at all, we may not be able to continue to generate and grow revenue from the sales of our current products, which may materially impact the success of our business.
We rely on sales from certain key products and markets, any disruptions to those products or markets due to change of market environment, regulatory requirements, or personal and sales practices, could generate adverse effects to our sales and business performance.
One of our key product lines, LokoMat accounts for more than 45% of our revenue; our other key products, Erigo, Armeo, C-Mill and CAREN/Grail collectively account for 55% of our revenues. In addition, approximately 80% of our revenue is concentrated in Europe, Middle East and Africa (“EMEA”) and Americas, with the remaining portion in Asia Pacific (“APAC”). Any disruptions to those key products and/or markets due to changes in market conditions, regulatory requirements, or personal and sales practices, could generate adverse effects to our sales and business performance.
Global, regional, and local economic weakness and uncertainty could adversely affect our demand for our products and services and our business and financial performance.
Our business and financial performance depends on worldwide economic conditions and the demand for our products and services in the markets in which we compete. Ongoing economic weakness, including an economic slowdown or recession, uncertainty in markets throughout the world and other adverse economic conditions, including inflation, changes in monetary policy and increased interest rates, may result in decreased demand for our products and services and increased expenses and difficulty in managing inventory levels and accurately forecasting revenue, gross margin, cash flows and expenses.
Prolonged or more severe economic weakness and uncertainty could also cause our expenses to vary materially from our expectations. Any financial turmoil affecting the banking system and financial markets or any significant financial services institution failures could negatively impact our treasury operations, as the financial condition of such parties may deteriorate rapidly and without notice.
War, geopolitical factors, and foreign exchange fluctuations could adverse effect the performance of our business.
Due to our significant presence in Europe, and emerging needs from South East Asia and the Middle East, war or geopolitical instability in those regions could adversely affect demand and supply chain disruptions from those regions; and foreign exchange, especially the Euro’s depreciation versus the US dollar would adversely depress our US dollar-denominated revenue and profitability We believe that an increasing percentage of our future revenue will come from international sales as we continue to expand our operations and develop opportunities in additional territories.
13
International sales are subject to a number of additional risks, including:
|
● |
difficulties in staffing and managing our foreign operations; |
|
|
|
|
● |
difficulties in penetrating markets in which our competitors’ products are more established; |
|
|
|
|
● |
reduced protection for intellectual property rights in some countries; |
|
|
|
|
● |
export restrictions, trade regulations and foreign tax laws; |
|
|
|
|
● |
fluctuating foreign currency exchange rates; |
|
|
|
|
● |
obtaining and maintaining foreign certification and compliance with other regulatory requirements; |
|
|
|
|
● |
customs clearance and shipping delays; and |
|
|
|
|
● |
political and economic instability. |
If one or more of these risks were realized, we could be required to dedicate significant resources to remedy the situation, and if we are unsuccessful at finding a solution, our revenue may decline.
Geopolitical risks associated with the ongoing conflict in Israel and Palestine could result in increased market volatility and uncertainty, which could negatively impact our business, financial condition, and results of operations.
The uncertain nature, scope, magnitude, and duration of hostilities stemming from recent events in Israel and Palestine have disrupted global markets and contributed to increased market volatility and uncertainty, which could have an adverse impact on macroeconomic and other factors that affect our business and supply chain. Any disruption in our supply chain could reduce our revenue and adversely impact our financial results. Such a disruption could occur as a result of any number of events, including, but not limited to, military conflicts, geopolitical developments, war or terrorism, including the ongoing conflict in Israel and Palestine, regional or global pandemics, and disruptions in utility and other services. Any inability to obtain adequate deliveries or any other circumstance that would require us to seek alternative sources of supply or to manufacture, assemble, and test such components internally could significantly delay our ability to ship our products, which could damage relationships with current and prospective customers and could harm our reputation and brand and could adversely affect our business, financial condition, and results of operations.
We may not have sufficient funds to meet certain future operating needs or capital requirements, which could impair our efforts to develop and commercialize existing and new products, and as a result, we may in the future consider one or more capital-raising transactions, including future equity or debt financings, strategic transactions, or borrowings which may also dilute our shareholders.
We may need to raise additional capital to fund our growth, working capital and strategic expansion. Given the turbulent global environment and volatile capital market, we may not be able to secure such financing in a timely manner and with favorable terms. Any such capital raise involving the sale of equity securities would result in dilution to our shareholders. If we cannot raise the required funds, or cannot raise them on terms acceptable to us or investors, we may be forced to curtail substantially our current operations and scale down our growth plan.
The market for robotics and VR-enabled smart rehabilitation systems are in the early growth stage, and important assumptions about the potential market for our current and future products may not be realized.
Although the market for robotics and VR-enabled “smart” rehabilitation systems has enjoyed increasing recognition from our customers, to date, the market is small. Significant market development efforts are still required to cross in order for us to enjoy accelerating growth.
14
As such, it is difficult to predict the future size and rate of growth of the market; and we cannot assure you that our estimate regarding our current products is achievable or that our estimate regarding future products profile will remain the same. If our estimates of our current or future addressable market are incorrect, our business may not develop as we expect, and the price of our securities may suffer.
Currently, most of our products are purchased by customers as capital equipment, funded by our customers’ own capital budgets, government grants, or charitable organizations’ donations. There is a risk that such grants or donations may not be secured timely or at all or capital budgets reduced; which could adversely impact our sales forecasts.
While we have seen significant interest in our products to support our growth plan, due to limited sales and clinician application personnel that are instrumental to our efforts to convert such interest into sales orders, at any quarter we can only focus on a fraction of the total sales opportunities. Accordingly, if there are delays or disruptions to potential customers’ budgeting processes due to customers’ internal capital budget limitations, delays in funding of government grants or charitable organizations’ donations, our sales opportunities may not be realized.
In the future, we may develop operational leasing or vendor-enabled financing to expand our growth beyond capital budget limitations, as part of our efforts to enrich and expanding our business models. There can be no assurance that we will have adequate working capital to do so after the Business Combination.
If we are unable to train customers on the safe and appropriate use of our products, we may be unable to achieve our expected growth.
It is critical to the success of our commercialization efforts to train a sufficient number of customers and provide them with adequate instruction in the safe and appropriate use of our products. This training process may take longer than expected and may therefore affect our ability to increase sales. Following completion of training, we rely on the trained customers to advocate the benefits of our products in the marketplace. Convincing our customers to dedicate the time and energy necessary for adequate training is challenging, and we cannot assure you that we will be successful in these efforts. If we cannot attract potential new customers to our education and training programs, we may be unable to achieve our expected growth. If our customers are not properly trained, they may misuse or ineffectively use our products. This may also result in, among other things, unsatisfactory patient outcomes, patient injury, negative publicity or lawsuits against us, any of which could have an adverse effect on our business and reputation.
If customers misuse our products, we may become subject to prohibitions on the sale or marketing of our products, significant fines, penalties, sanctions, or product liability claims, and our image and reputation within the industry and marketplace could be harmed.
Our customers may also misuse our devices, or our future products or use improper techniques, potentially leading to adverse results, side effects or injury, which may lead to product liability claims. If our current or future products are misused or used with improper techniques or are determined to cause or contribute to consumer harm, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend, result in sizable damage awards against us that may not be covered by insurance and subject us to negative publicity resulting in reduced sales of our products. Furthermore, the use of our current or future products for indications other than those cleared by the FDA may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and consumers. Any of these events could harm our business and results of operations and cause our stock price to decline.
If we are unable to educate clinicians on the safe, effective and appropriate use of our products, we may experience increased claims of product liability and may be unable to achieve our expected growth.
Certain of our products require the use of specialized techniques and/or product-specific knowledge. It is critical to the success of our business to broadly educate clinicians who use or desire to use our products in order to provide them with adequate instructions in the appropriate use of our products. It is also important that we educate our other customers and patients on the risks associated with our products. Failure to provide adequate training and education could result in, among other things, unsatisfactory patient outcomes, patient injury, negative publicity or increased product liability claims or lawsuits against us, any of which could have a material and adverse effect on our business and reputation.
15
We make extensive educational resources available to clinicians and our other customers in an effort to ensure that they have access to current treatment methodologies, are aware of the advantages and risks of our products, and are educated regarding the safe and appropriate use of our products. However, there can be no assurance that these resources will successfully prevent all negative events and if we fail to educate clinicians, our other customers and patients, they may make decisions or form conclusions regarding our products without full knowledge of the risks and benefits or may view our products negatively. In addition, claims against us may occur even if such claims are without merit and/or no product defect is present, due to, for example, improper surgical techniques, inappropriate use of our products, or other lack of awareness regarding the safe and effective use of our products. Any of these events could harm our business and results of operations.
As an emerging leader in a fragmented industry, we need time and efforts to develop talent, expertise, competencies, process and infrastructure; if we lose key employees or fail to replicate and leverage our sales, marketing, and training infrastructure, our growth would suffer adverse effects.
A key element of our long-term business strategy is the continued leveraging of our sales, marketing, clinical training and services infrastructure, through the training, retention, and motivation of skilled sales, marketing, clinical applications training, and services representatives with industry experience and knowledge. In order to continue growing our business efficiently, we need coordinate the development of our sales, marketing, clinical training and services infrastructure with the timing of market expansion, new product launch, regulatory approvals, limited resources consideration and other factors in various geographies. Managing and maintaining our sales and marketing infrastructure is expensive and time consuming, and an inability to leverage such an organization effectively, or in coordination with regulatory or other developments, could inhibit potential sales and the penetration and adoption of our products into both existing and new markets.
Newly hired sales representatives require training and take time to achieve full productivity. If we fail to train new hires adequately, or if we experience high turnover in our sales force in the future, we cannot be certain that new hires will become as productive as may be necessary to maintain or increase our sales. In addition, if we are not able to retain existing and recruit new trainers to our clinical staff, we may not be able to successfully train customers on the use of our sophisticated products, which could inhibit new sales and harm our reputation. If we are unable to expand our sales, marketing, and training capabilities, we may not be able to effectively commercialize our products, or enhance the strength of our brand, which could have a material adverse effect on our operating results.
The health benefits of our products have not yet been substantiated by long-term large randomized clinical data, which could limit sales of such products.
Although there have been numerous published research studies supporting the benefits of our products and users of our products have reported encouraging health benefits of our products, currently there is no large scale, randomized clinical trial establishing the long-term health benefits of our or competitors’ products due to the relatively small size of the applicable user population, and the fragmented application practice that we are still in the early stage to change through consolidation and integration. While many of the top rehabilitation hospitals have purchased some of our products, many potential conservative customers and healthcare providers may be slower to adopt or recommend our products.
Our success depends largely upon consumer satisfaction with the effectiveness of our products.
In order to generate repeat and referral business, consumers must be satisfied with the effectiveness of our products. If consumers are not satisfied with the benefits of our products, our reputation and future sales could suffer.
For certain of our products, we rely on sole source third parties to manufacture and supply certain raw materials. If these manufacturers are unable to supply these raw materials or products in a timely manner, or at all, we may be unable to meet customer demand, which would have a material adverse effect on our business.
We currently depend on sole source, third party manufacturers, to manufacture and supply certain raw materials and products. We cannot assure you that these manufacturers will be able to provide these raw materials, and products in quantities that are sufficient to meet demand in a timely manner, or at all, which could result in decreased revenues and loss of market share. There may be delays in the manufacturing process over which we have no control, including shortages of raw materials, labor disputes, backlogs and failure to meet FDA standards.
16
We are aware that certain of our sole source manufacturers also rely on sole source suppliers with respect to materials used in our products. We rely on our third-party manufacturers to maintain their manufacturing facilities in compliance with applicable international, FDA and other federal, state and/or local regulations including health, safety and environmental standards. If they fail to maintain compliance with critical regulations, they could be ordered to suspend, curtail or cease operations, which would have a material adverse impact on our business. Increases in the prices we pay our manufacturers, interruptions in our supply of raw materials or products, or lapses in quality, such as failures to meet our specifications and other regulatory requirements, could materially adversely affect our business. Any manufacturing defect or error discovered after our products have been produced and distributed could result in significant consequences, including costly recall procedures and damage to our reputation. Our ability to replace an existing manufacturer may be difficult, because the number of potential manufacturers is limited. If we do undertake to negotiate terms of supply with another manufacturer or other manufacturers, our relationships with our existing manufacturers could be harmed. Any interruption in the supply of raw materials or products, or the inability to obtain these raw materials or products from alternate sources in a timely manner, could impair our ability to meet the demands of our customers, which would have a material adverse effect on our business.
We utilize independent distributors who are free to market other products that compete with our products for sales.
While we have proportionally more influence on the independent distributors we are using to cover majority of the global markets due to our limited direct sales force, considering the fact that the rehabilitation technology market is very fragmented, we generally do not sign mutual exclusive distribution agreement with distributors. Consequently, our distribution partners could indirectly compete against our interests by promoting alternative technologies to prospective customers in lieu of ours. We believe that as we assemble more and integrated offering through our consolidation and integration strategy, the influence and motivation we may impose on our distribution partners to dedicate on selling and promoting our products and solution shall increase and such kind of competition risk would be better addressed.
To ensure credibility and enforce the effective genesis of our distributor management, we may terminate a distributor who has not demonstrated its best efforts and/or interests in selling and promoting our products and solutions, albeit such termination may adversely affect our sales performance in the market covered by such distributor.
Due to the nature of market fragmentation, our product and solution offerings may not always deliver the targeted sales amount, or may take longer than expected to establish itself in customers minds, and accepted by mainstream.
The fragmented market reflects both opportunity for consolidation and challenges of overcoming customers’ mindsets used to using alternative approaches as well as fragmented clinical practices. Change and acceptance of new idea and solution normally happens over time and in multiple wave-shaped phases instead of a straight line progression. Consequently, our new innovative product and solution offerings may not deliver the targeted sales amount or face uncertain time periods for customers to accept due to various dynamic factors that may influence the perceptions and consensus formation among prospective customers. Consequently, such judgments and self-reinforcing efforts may cause the actual results to deviate from our planned results for a sustained period, which may have adverse effect on our performance.
We may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances, business acquisitions or partnerships with third parties that may not result in the development of commercially viable products, the generation of significant future revenue, or consistent realization of deal economics.
In the ordinary course of our business, we may enter into collaborations, in-licensing arrangements, joint ventures, strategic alliances, business acquisitions, partnerships or other arrangements to develop our products and to pursue new geographic or product markets. Proposing, negotiating, and implementing collaborations, in-licensing arrangements, joint ventures, strategic alliances, or partnerships may be a lengthy and complex process.
We may not identify, secure, or complete any such transactions or arrangements in a timely manner, on a cost-effective basis, on acceptable terms or at all. We have limited institutional knowledge and experience with respect to these business development activities, and we may also not realize the anticipated benefits from some of those transactions or arrangements.
17
Additionally, as we pursue these arrangements and choose to pursue other collaborations, in-licensing arrangements, joint ventures, strategic alliances, or partnerships in the future, we may not be in a position to exercise sole decision-making authority regarding the transaction or arrangement. This could create the potential risk of creating impasses on decisions, and our collaborators may have economic or business interests or goals that are, or that may become, inconsistent with our business interests or goals. It is possible that conflicts may arise with our collaborators. Our collaborators or any future collaborators may act in their self-interest, which may be adverse to our best interest, and they may breach their obligations to us. Disputes between us and our collaborators or any future collaborators may result in litigation or arbitration which would increase our expenses and divert the attention of our management. Further, these transactions and arrangements are contractual in nature and may be terminated or dissolved under the terms of the applicable agreements. Our collaborators or any future collaborators may allege that we have breached our agreement with them, and accordingly seek to terminate such agreement, which could adversely affect our competitive business position and harm our business prospects.
Furthermore, due to the fragmentation nature and the fact that most acquisition targets are at sub-optimal immature organization stage with less than $10 million in revenue, the risk of integrating such organizations and products can also be higher than acquisitions and consolidations in a mature industry. Consequently, there are risks that some of those acquisitions may fail to deliver the expected deal economics and could have adverse effect on our financial condition and business results.
We may not successfully integrate newly acquired product lines into our business operations or realize the benefits of our partnerships with other companies, acquisitions of complementary products or technologies or other strategic alternatives.
Historically we have acquired or gained the rights to our product lines through acquisitions and other strategic alternatives. As a result of these acquisitions, we have undergone substantial changes to our business and product offerings in a short period of time. Additionally, in the future, we may consider other opportunities to partner with or acquire other businesses, products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base or advance our business strategies.
Although we have previously been successful in integrating such products and technologies into our business and operations, there can be no assurances that we will continue to do so in the future. If we fail to successfully integrate collaborations, assets, products or technologies, or if we fail to successfully exploit acquired product or distribution rights, our business could be harmed. Furthermore, we may have to incur debt or issue equity securities in connection with proposed collaborations or to pay for any product acquisitions or investments, the issuance of which could be dilutive to our existing shareholders. Identifying, contemplating, negotiating or completing a collaboration or product acquisition and integrating an acquired product or technology could significantly divert management and employee time and resources.
Moreover, integrating new product lines with that of our own is a complex, costly and time-consuming process, which requires significant management attention and resources. The integration process may disrupt our existing operations and, if implemented ineffectively, would preclude realization of the full benefits that are expected. Our failure to meet the challenges involved in successfully integrating our acquisitions in order to realize the anticipated benefits may cause an interruption of, or a loss of momentum in, our operating activities and could adversely affect our results of operations. Potential difficulties, costs, and delays we may encounter as part of the integration process may include:
|
● |
distracting management from day-to-day operations; |
|
|
|
|
● |
an inability to achieve synergies as planned; |
|
|
|
|
● |
risks associated with the assumption of contingent or other liabilities; |
|
|
|
|
● |
adverse effects on existing business relationships with suppliers or customers; |
18
|
|
|
|
● |
inheriting and uncovering previously unknown issues, problems and costs from the acquired product lines; |
|
|
|
|
● |
uncertainties associated with entering new markets in which we have limited or no experience; |
|
|
|
|
● |
increased legal and accounting costs relating to the product line or compliance with regulatory matters; |
|
|
|
|
● |
delays between our expenditures to acquire new products, technologies or businesses and generating net sales from those acquired products, technologies or businesses; and |
|
|
|
|
● |
increased difficulties in managing our business due to increased personnel, increased data and information to analyze, and the potential addition of international locations. |
Any one or all of these factors may increase operating costs or lower anticipated financial performance. Many of these factors are also outside of our control. In addition, even if new product lines or businesses are integrated successfully, we may not realize the full benefits of the acquisition, including the synergies, cost savings or sales or growth opportunities that we expect or within the anticipated time frame. Additional unanticipated costs may be incurred in the integration of product lines or businesses. All of these factors could decrease or delay the expected accretive effect of the transaction, and negatively impact the price of our common stock. The failure to integrate any acquired product line or business successfully would have a material adverse effect on our business, financial condition and results of operations.
We may pursue acquisitions, which involve a number of risks, and if we are unable to address and resolve these risks successfully, such acquisitions could harm our business.
We may in the future acquire businesses, products or technologies to expand our offerings and capabilities, user base and business. We have evaluated, and expect to continue to evaluate, a wide array of potential strategic transactions; however, we have limited experience completing or integrating acquisitions. Any acquisition could be material to our financial condition and results of operations and any anticipated benefits from an acquisition may never materialize. In addition, the process of integrating acquired businesses, products or technologies may create unforeseen operating difficulties and expenditures. Acquisitions in international markets would involve additional risks, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries.
The process of integrating an acquired business, product or technology can create unforeseen operating difficulties, expenditures and other challenges such as:
|
● |
potentially increased regulatory and compliance requirements; |
|
|
|
|
● |
implementation or remediation of controls, procedures and policies at the acquired company;
|
|
● |
diversion of management time and focus from operation of its then-existing business to acquisition integration challenges; |
|
|
|
|
● |
coordination of product, sales, marketing and program and systems management functions; |
|
|
|
|
● |
transition of the acquired company’s users and providers onto our systems; |
|
|
|
|
● |
retention of employees from the acquired company; |
|
|
|
|
● |
integration of employees from the acquired company into our organization; |
|
|
|
|
● |
integration of the acquired company’s accounting, information management, human resources and other administrative systems and operations into our systems and operations; |
|
|
|
19
|
● |
liability for activities of the acquired company prior to the acquisition, including violations of law, commercial disputes and tax and other known and unknown liabilities; and |
|
|
|
|
● |
litigation or other claims in connection with the acquired company, including claims brought by terminated employees, providers, former stockholders or other third parties. |
We may not be able to address these risks successfully, or at all, without incurring significant costs, delays or other operational problems and if we were unable to address such risks successfully our business could be harmed.
We may have difficulty managing our growth which could limit our ability to increase sales and cash flow.
We anticipate experiencing significant growth in our operations and the number of our employees if our current and future products are successful. This growth will place significant demands on our management, as well as our financial and operational resources. In order to achieve our business objectives, we will need to grow our business. Continued growth would increase the challenges involved in:
|
● |
implementing appropriate operational and financial systems; |
|
|
|
|
● |
expanding our sales and marketing infrastructure and capabilities; |
|
|
|
|
● |
ensuring compliance with applicable FDA, and other regulatory requirements; |
|
|
|
|
● |
providing adequate training and supervision to maintain high quality standards; and |
|
|
|
|
● |
preserving our culture and values. |
Our growth will require us to continually develop and improve our operational, financial and other internal controls. If we cannot scale and manage our business appropriately, we will not realize our projected growth and our financial results could be adversely affected.
Risks Related to Government Regulation
We are subject to extensive and dynamic medical device regulation, which may impede or hinder the approval or sale of our products and, in some cases, may ultimately result in an inability to obtain approval of certain products or may result in the recall or seizure of previously approved products.
Our products, marketing, sales and development activities and manufacturing processes are subject to extensive and rigorous regulation by various regulatory agencies and governing bodies. Under the US Food, Drug and Cosmetic Act, medical devices must receive FDA clearance or approval or an exemption from such clearance or approval before they can be commercially marketed in the United States. In the European Union, we are required to comply with applicable medical device directives (including the Medical Devices Directive and the European Medical Device Regulation) and obtain CE Mark (European Conformity) certification in order to market medical devices. In addition, exported devices are subject to the regulatory requirements of each country to which the device is exported. Many countries require that product approvals be renewed or recertified on a regular basis, generally every four to five years. The renewal or recertification process requires that we evaluate any device changes and any new regulations or standards relevant to the device and conduct appropriate testing to document continued compliance. Where renewal or recertification applications are required, they may need to be renewed and/or approved in order to continue selling our products in those countries. There can be no assurance that we will receive the required approvals for new products or modifications to existing products on a timely basis or that any approval will not be subsequently withdrawn or conditioned upon extensive post-market study requirements.
The European Union regulatory bodies finalized a new Medical Device Regulation (“MDR”) in 2017, which replaced the existing directives and provided three years for transition and compliance. The MDR changes several aspects of the existing regulatory framework, such as updating clinical data requirements and introducing new ones, such as Unique Device Identification. We and those who will oversee compliance to the new MDR face uncertainties as the MDR is rolled out and enforced by the Commission and EEA Competent Authorities, creating risks in several areas, including the CE Marking process and data transparency, in the upcoming years.
20
Regulations regarding the development, manufacture and sale of medical devices are evolving and subject to future change. We cannot predict what impact, if any, those changes might have on our business. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations. Later discovery of previously unknown problems with a product or manufacturer could result in fines, delays or suspensions of regulatory clearances or approvals, seizures or recalls of products, physician advisories or other field actions, operating restrictions and/or criminal prosecution. We may also initiate field actions as a result of a failure to strictly comply with our internal quality policies. The failure to receive product approval clearance on a timely basis, suspensions of regulatory clearances, seizures or recalls of products, physician advisories or other field actions, or the withdrawal of product approval by regulatory authorities could have a material adverse effect on our business, financial condition or results of operations.
If we fail to obtain regulatory approvals in the United States or foreign jurisdictions for our products, or any future products, we will be unable to market our products in those jurisdictions.
In addition to regulations in the United States, we are subject to a variety of foreign regulations governing manufacturing, clinical trials, commercial sales and distribution of our future products. Whether or not we obtain FDA approval for a product, we must obtain approval of the product by the comparable regulatory authorities of foreign countries before commencing clinical trials or marketing in those countries. The approval procedures vary among countries and can involve additional clinical testing, or the time required to obtain approval may differ from that required to obtain FDA approval. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval.
Due to the fact that more than 95% of our revenue comes from health-regulated medical device products, if we do not obtain or maintain necessary regulatory clearances or approvals, or if clearances or approvals for future medical products or modifications to existing medical products are delayed or not issued, our commercial operations and sales targets would be adversely affected.
We operate under highly regulated global health markets and must register and maintain effectiveness and compliance of such registration, with each of our medical devices with every markets’ relevant authority either directly or through our agent or distributors. Any missing or failure to comply with such registrations may disrupt any sales activities in that particular market, and result in adverse effects.
We may be subject to adverse medical device reporting obligations, voluntary corrective actions or agency enforcement actions.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. Manufacturers may also, under their own initiative, recall a product if any material deficiency in a device is found or withdraw a product to improve device performance or for other reasons. The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of a perceived or actual unacceptable risk to health, component failures, malfunctions, manufacturing errors, design or labelling defects or other deficiencies and issues. Regulatory agencies in other countries have similar authority to recall devices because of material deficiencies or defects in design or manufacture that could endanger health. Any recall would divert management attention and financial resources and could cause the price of our stock to decline, expose us to product liability or other claims and harm our reputation with customers. Such events could impair our ability to produce our products in a cost-effective and timely manner in order to meet customer demands. A recall involving our silicone gel breast implants could be particularly harmful to our business, financial and operating results. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or similar foreign governmental authorities.
21
We may initiate voluntary recalls involving our products in the future that we determine do not require notification of the FDA or foreign governmental authorities. If the FDA or foreign governmental authorities disagree with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA or a foreign governmental authority could take enforcement action for failing to report the recalls when they were conducted.
In addition, under the FDA’s medical device reporting regulations, we are required to report to the FDA any incident in which our product may have caused or contributed to a death or serious injury or in which our product malfunctioned and, if the malfunction were to recur, would likely cause or contribute to death or serious injury. Repeated product malfunctions may result in a voluntary or involuntary product recall. We are also required to follow detailed record-keeping requirements for all self-initiated medical device corrections and removals, and to report such corrective and removal actions to the FDA if they are carried out in response to a risk to health and have not otherwise been reported under the medical device reporting regulations. Depending on the corrective action we take to address a product’s deficiencies or defects, the FDA may require, or we may decide, that we need to obtain new approvals or clearances for the device before marketing or distributing the corrected device. Seeking such approvals or clearances may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties, or civil or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales as well as face significant adverse publicity or regulatory consequences, which could harm our business, including our ability to market our products in the future.
Any adverse event involving our products, whether in the United States or abroad, could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection, mandatory recall or other enforcement action. Any corrective action, whether voluntary or involuntary, will likely oblige us to defend ourselves in resulting lawsuits, and will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
Legislative or regulatory healthcare reforms in the United States and other countries may make it more difficult and costly for us to obtain regulatory clearance or approval of any future product candidates and to produce, market, and distribute our products after clearance or approval is obtained.
Recent political, economic and regulatory influences are subjecting the health care industry to fundamental changes. Both the federal and state governments in the United States and foreign governments continue to propose and pass new legislation and regulations designed to contain or reduce the cost of health care, improve quality of care, and expand access to healthcare, among other purposes. Such legislation and regulations may result in decreased reimbursement for medical devices and/or the procedures in which they are used, which may further exacerbate industry-wide pressure to reduce the prices charged for medical devices. This could harm our ability to market and generate sales from our products.
In addition, regulations and guidance are often revised or reinterpreted by governmental agencies, including the FDA, CMS, and the Department of Health and Human Services Office of the Inspector General (“OIG”) and others, in ways that may significantly affect our business and our products. Any new regulations, revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of our products.
In the future there may continue to be additional proposals relating to the reform of the United States. healthcare system. Certain of these proposals could limit the prices we are able to charge for our products or the amount of reimbursement available for our products, and could limit the acceptance and availability of our products, any of which could have a material adverse effect on our business, results of operations and financial condition.
United States and foreign privacy and data protection laws and regulations may impose additional liabilities on us.
While we do not store patient data at our premises or DIH-managed data center, United States, federal and state privacy and data security laws and regulations regulate how we and our partners collect, use and share certain information. In addition to HIPAA, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
22
Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, the California Consumer Privacy Act, or CCPA, went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. The CCPA was recently amended by the California Privacy Rights Act or CPRA, expanding certain consumer rights such as the right to know. It remains unclear what, if any, additional modifications will be made to these laws by the California legislature or how these laws will be interpreted and enforced. The California Attorney General has issued clarifying regulations and initiating enforcement activity. The potential effects of the CCPA and CPRA are significant and may cause us to incur substantial costs and expenses to comply. The CCPA has prompted a wave of proposals for new federal and state privacy legislation, some of which may be more stringent than the CCPA, that, if passed, could increase our potential liability, increase our compliance costs, and adversely affect our business.
We may also be subject to or affected by foreign laws and regulations, including regulatory guidance, governing the collection, use, disclosure, security, transfer, and storage of personal data, such as information that we collect about customers and patients in connection with our operations abroad. The global legislative and regulatory landscape for privacy and data protection continues to evolve, and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future. This evolution may create uncertainty in our business, result in liability, or impose additional costs on us. The cost of compliance with these laws, regulations and standards is high and is likely to increase in the future.
For example, the European Union implemented the General Data Protection Regulation (“GDPR”) a broad data protection framework that expands the scope of European Union data protection law to include certain non-European Union entities that process the personal data of European Union residents, including clinical trial data. The GDPR increases our compliance burden with respect to data protection, including by mandating potentially burdensome documentation requirements and granting certain rights to individuals to control how we collect, use, disclose, retain and protect information about them. The processing of sensitive personal data, such as information about health conditions, leads to heightened compliance burdens under the GDPR and is a topic of active interest among European Union regulators. In addition, the GDPR provides for breach reporting requirements, more robust regulatory enforcement and fines of up to the greater of 20 million euros or 4% of annual global revenue. The GDPR increases our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional mechanisms to ensure compliance with the GDPR, which could divert management’s attention and increase our cost of doing business.
A data security breach or other privacy violation that compromises the confidentiality, integrity or availability of the personal information of our customers, clinical trials participants, collaborators or employees could harm our reputation, compel us to comply with United States. or international breach notification laws, subject us to mandatory corrective action, and otherwise subject us to liability under United States. or foreign laws and regulations. Data breaches or other security incidents could also compromise our trade secrets or other intellectual property. If we are unable to prevent such data security breaches and security incidents or implement satisfactory remedial measures, our operations could be disrupted, and we may suffer reputational harm, financial loss or other regulatory penalties. In addition, such events can be difficult to detect, and any delay in identifying them may lead to increased harm.
Finally, it is possible that these privacy laws may be interpreted and applied in a manner that is inconsistent with our practices. Any failure or perceived failure by us to comply with federal, state, or foreign laws or self-regulatory organization’s rules or regulations could result in an expense or liability to us.
Changes in law or regulation could make it more difficult and costly for DIH and its subsidiaries to manufacture, market and distribute its products or obtain or maintain regulatory approval of new or modified products.
The experience with the transition to the EU MDR showed how complex, time-consuming and expensive a change in Medical Device Legislation can be. Progression on innovations and new products could be significantly delayed during the work on compliance with new legislations.
23
We may fail to comply with regulations of the United States and foreign regulatory agencies which could delay, or prevent entirely, and the commercialization of our products.
Given the non-invasive and lower risk nature of rehabilitation products, similar to other rehabilitation technology providers, most of our products are in FDA risk class 1 and this class is not subject to mandatory scrutiny by the U.S. authorities. There is the possibility that, in the future, the FDA may not agree with our classification. We might have to register if disagreement arises, and consequently we would have to stop distributing the device in the U.S. Under such a scenario, possible alternatives registration pathways might be 510(k)s or PMAs, which amount to an increase in the registration time from six months to multiple years; result in significant suspension of our sales activity for products in question in the US.
In some instances, in our advertising and promotion, we may make claims regarding our product as compared to competing products, which may subject us to heightened regulatory scrutiny, enforcement risk, and litigation risks.
The FDA applies a heightened level of scrutiny to comparative claims when applying its statutory standards for advertising and promotion, including with regard to its requirement that promotional labelling be truthful and not misleading. There is potential for differing interpretations of whether certain communications are consistent with a product’s FDA-required labelling, and FDA will evaluate communications on a fact-specific basis.
In addition, making comparative claims may draw attention from our competitors. Where a company makes a claim in advertising or promotion that its product is superior to the product of a competitor (or that the competitor’s product is inferior), this creates a risk of a lawsuit by the competitor under federal and state false advertising or unfair and deceptive trade practices law, and possibly also state libel law. Such a suit may seek injunctive relief against further advertising, a court order directing corrective advertising, and compensatory and punitive damages where permitted by law.
Any such lawsuit or threat of lawsuit against us will likely oblige us to defend ourselves in court, and will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results. If any such lawsuit against us is successful, we would suffer additional losses of time and capital in taking any required corrective action and would suffer harm to our reputation, all of which would have an adverse effect on our business.
If we fail to obtain or maintain the necessary ISO 13485 certification or the certification according to (EU) 2017/745 (MDR), our commercial operations in the EU and some other countries will be harmed.
As the certifications according to ISO 13485 and (EU) 2017/745 constitute the legal basis for any commercial activity in the European Union and many other countries, these certifications and maintenance of such certifications is a vital task for us. Failure to certify will lead to a disruption of device sales not only in the European Union, but also in the United States and many other countries, as these usually consider a certification a prerequisite for any device registrations.
The majority of our products are classified as medical devices and are regulated by the FDA, the European Union and other governmental authorities both inside and outside of the United States. These agencies enforce laws and regulations that govern the development, testing, manufacturing, labeling, advertising, marketing and distribution, and market surveillance of our medical products. Our failure to comply with these complex laws and regulations could have a material adverse effect on our business, results of operations, financial condition and cash flows. Even after regulatory clearance or approval has been granted, a cleared or approved product and its manufacturer are subject to extensive regulatory requirements relating to manufacturing, labeling, packaging, adverse event reporting, storage, advertising and promotion for the product. If we fail to comply with the regulatory requirements of the FDA or other non-U.S. regulatory authorities, or if previously unknown problems with our products or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including restrictions on the products, manufacturers or manufacturing process; adverse inspectional observations (Form 483), warning letters, non-warning letters incorporating inspectional observations; civil or criminal penalties or fines; injunctions; product seizures, detentions or import bans; voluntary or mandatory product recalls and publicity requirements; suspension or withdrawal of regulatory clearances or approvals; total or partial suspension of production; imposition of restrictions on operations, including costly new manufacturing requirements; refusal to clear or approve pending applications or premarket notifications; and import and export
24
Modifications to our products may require re-registration, new 510(k) clearances or premarket approvals, or may require us to renew existing registrations in non-European Union countries.
Product modifications consisting either of changes to hardware or software or in expanding or restricting indications or contraindications can have an impact on the validity of our registrations. Thus, a product modification may lead to regulatory change projects, which will consume time and resources. A delay in marketing activities for the respective products may result. Many of these changes are beyond our control, as they are initiated by suppliers of components. Often those changes cannot be predicted, as their announcement happens on short notice, thus increasing the risk of business disruption.
The innovative development of our products may lead to the application of new laws, regulations, standards, etc. not considered until now.
Developing our products further in the direction of increasingly independent acting devices might bring those products into the scope of standards or regulations for robotic devices or artificial intelligence, or other similar areas. As this requires further competencies, resources and time, a potential delay or disruption of our commercial activities could result.
Any negative publicity concerning our products could harm our business and reputation and negatively impact our financial results.
The reactions of potential patients, physicians, the news media, legislative and regulatory bodies and others to information about complications or alleged complications of our products could result in negative publicity and could materially reduce market acceptance of our products. These reactions, or any investigations and potential resulting negative publicity, may have a material adverse effect on our business and reputation and negatively impact our financial condition, results of operations or the market price of our common stock. In addition, significant negative publicity could result in an increased number of product liability claims against us.
United States or European healthcare reform measures and other potential legislative initiatives could adversely affect our business.
Europe and the United States are our major markets, and any major healthcare reform that may change the health industry landscape or reimbursement environment, may have a significant impact on our sales performance and growth projects in the affected markets.
Any political changes in the United States or in Europe could result in significant changes in, and uncertainty with respect to, legislation, regulation, global trade, and government policy that could substantially impact our business and the medical device industry generally. The FDA and European Union Commission’s policies may also change, and additional government regulations may be issued that could prevent, limit, or delay regulatory approval of our future products, or impose more stringent product labeling and post-marketing testing and other requirements.
Risks Related to War in Ukraine and Israel and Palestine
The credit and financial markets have experienced extreme volatility and disruptions due to the current conflict between Ukraine and Russia. The conflict is expected to have further global economic consequences, including but not limited to the possibility of severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in inflation rates and uncertainty about economic and political stability. In addition, the United States and other countries have imposed sanctions on Russia which increases the risk that Russia, as a retaliatory action, may launch cyberattacks against the United States, its government, infrastructure and businesses. Any of the foregoing consequences, including those we cannot yet predict, may cause our business, financial condition, results of operations and the price of our Common Stock to be adversely affected.
Risks Related to Our Intellectual Property and Information Technology
25
We depend on computer and information systems we do not own or control and failures in our systems or a cybersecurity attack or breach of our IT systems or technology could significantly disrupt our business operations or result in sensitive information being compromised which would adversely affect our reputation and/or results of operations.
We have entered into agreements with third parties for hardware, software, telecommunications, and other information technology services in connection with the operation of our business. It is possible we or a third party that we rely on could incur interruptions from a loss of communications, hardware or software failures, a cybersecurity attack or a breach of our IT systems or technology, computer viruses or malware. Though most of those information systems and platforms are provided by well-established multinational firms like Oracle and Microsoft, any interruptions to our arrangements with third parties, to our computing and communications infrastructure, or to our information systems or any of those operated by a third party that we rely on could significantly disrupt our business operations.
In the current environment, there are numerous and evolving risks to cybersecurity and privacy, including criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error. A cyberattack of our systems or networks that impairs our information technology systems could disrupt our business operations and result in loss of service to customers, including technical support for our robotics and VR-enabled devices.
Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our products.
Our success depends in part on our ability to obtain and maintain protection for the intellectual property relating to or incorporated into our products. We seek to protect our intellectual property through a combination of patents, trademarks, confidentiality, and assignment agreements with our employees and certain of our contractors, as well as confidentiality agreements with certain of our consultants, scientific advisors, and other vendors and contractors. In addition, we rely on trade secrets law to protect our proprietary software and product candidates/products in development.
The patent position of robotic and VR-enabled inventions can be highly uncertain and involves many new and evolving complex legal, factual, and technical issues. Patent laws and interpretations of those laws are subject to change and any such changes may diminish the value of our patents or narrow the scope of our right to exclude others. In addition, we may fail to apply for or be unable to obtain patents necessary to protect our technology or products from copycats or fail to enforce our patents due to lack of information about the exact use of technology or processes by third parties. Also, we cannot be sure that any patents will be granted in a timely manner or at all with respect to any of our patent pending applications or that any patents that are granted will be adequate to exclude others for any significant period of time or at all. Given the foregoing and in order to continue reducing operational expenses in the future, we may invest fewer resources in filing and prosecuting new patents and on maintaining and enforcing various patents, especially in regions where we currently do not focus our market growth strategy.
Litigation to establish or challenge the validity of patents, or to defend against or assert against others infringement, unauthorized use, enforceability, or invalidity, can be lengthy and expensive and may result in our patents being invalidated or interpreted narrowly and restricting our ability to be granted new patents related to our pending patent applications. Even if we prevail, litigation may be time consuming, force us to incur significant costs, and could divert management’s attention from managing our business while any damages or other remedies awarded to us may not be valuable.
In addition, we seek to protect our trade secrets, know-how, and confidential information that is not patentable by entering into confidentiality and assignment agreements with our employees and certain of our contractors and confidentiality agreements with certain of our consultants, scientific advisors, and other vendors and contractors. However, we may fail to enter into the necessary agreements, and even if entered into, these agreements may be breached or otherwise fail to prevent disclosure, third-party infringement, or misappropriation of our proprietary information, may be limited as to their term and may not provide an adequate remedy in the event of unauthorized disclosure or use of proprietary information. Enforcing a claim that a third party illegally obtained or is using our trade secrets without authorization may be expensive and time consuming, and the outcome is unpredictable.
26
Some of our employees or consultants may own certain technology which they license to us for a set term. If these technologies are material to our business after the term of the license, our inability to use them could adversely affect our business and profitability.
We are not able to protect our intellectual property rights in all countries.
Filing, prosecuting, maintaining, and defending patents on each of our products in all countries throughout the world would be prohibitively expensive, and thus our intellectual property rights outside the United States and Europe are limited. In addition, the laws of some foreign countries, especially developing countries, such as China, do not protect intellectual property rights to the same extent as federal and state laws in the United States. It may not be possible to effectively enforce intellectual property rights in some countries at all or to the same extent as in the United States and other countries. Consequently, we are unable to prevent third parties from using our inventions in all countries, or from selling or importing products made using our inventions in the jurisdictions in which we do not have (or are unable to effectively enforce) patent protection. Copycats may use our technologies in jurisdictions where we have not obtained patent protection to develop, market or otherwise commercialize their own products, and we may be unable to prevent those copycats from importing those infringing products into territories where we have patent protection, but enforcement may not be as strong as in the United States. These products may compete with our products and our patents and other intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions.
We may be subject to patent infringement claims, especially for products acquired through acquisitions, which could result in substantial costs and liability and prevent us from commercializing such acquired products.
The medical device industry is characterized by competing intellectual property, given the existence of large number of patents, the rapid rate of new patent issuances, and the complexities of the technology involved; and patent infringement assessments require costly due diligence and extensive resources to cope with the complexity to assess infringement risks in a complex world of regulations and intellectual property filings. As a result, we may choose not to conduct extensive and expensive intellectual property due diligence, especially for small deal value; as a consequence, we might be vulnerable to certain unknown intellectual property infringement claims, especially related to products we acquired from others. Determining whether a product infringes a patent involves complex legal and factual issues and the outcome of patent litigation is often uncertain. Even though we have conducted research of issued patents, no assurance can be given that patents containing claims covering our products, technology or methods do not exist, have not been filed or could not be filed or issued. In addition, because patent applications can take years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware, and which may result in issued patents that our current or future products infringe.
Infringement actions and other intellectual property claims brought against us, whether with or without merit, may cause us to incur meaningful costs and could place a significant strain on our financial resources, divert the attention of management, and harm our reputation.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Some of our employees were previously employed at other medical device companies, including our competitors or potential competitors, and we may hire employees in the future that are so employed. We could in the future be subject to claims that these employees, or we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. If any of these technologies or features that are important to our products, this could prevent us from selling those products and could have a material adverse effect on our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and divert the attention of management.
Risks Related to Ownership of DIH Common Stock
27
Future sales of a substantial number of shares of DIH Common Stock by us or our large stockholders, certain of whom may have registration rights, or dilutive exercises of a substantial number of warrants by our warrant holders could adversely affect the market price of our Common Stock.
Sales by us or our stockholders of a substantial number of shares of DIH Common Stock in the public market following the Business Combination, or the perception that these sales might occur, could cause the market price of the DIH Common Stock to decline or could impair our ability to raise capital through a future sale of our equity securities. Additionally, dilutive exercises of a substantial number of warrants by our warrant-holders, or the perception that such exercises may occur, could put downward price on the market price of our Common Stock.
Future grants of shares of DIH Common Stock under our equity incentive plan to our employees, non-employee directors and consultants, or sales by these individuals in the public market, could result in substantial dilution, thus decreasing the value of your investment in DIH Common Stock. In addition, stockholders will experience dilution upon the exercise of outstanding warrants.
Shareholders approved an equity incentive plan which provides for the issuance of up to 4,300,000 additional shares of New DIH Common Stock. Additionally, to the extent registered on a Form S-8, shares of DIH Common Stock granted or issued under our equity incentive plans will, subject to vesting provisions and Rule 144 volume limitations applicable to our “affiliates,” be available for sale in the open market immediately upon registration. Further, as of March 31, 2024, there were 13,355,000 shares of DIH Common Stock underlying issued and outstanding warrants, which if exercised, could decrease the net tangible book value of our DIH Common Stock and cause dilution to our existing stockholders. Sales of a substantial number of the above-mentioned shares of DIH Common Stock in the public market could result in a significant decrease in the market price of the DIH Common Stock and have a material adverse effect on your investment.
If securities or industry analysts do not publish research or reports about DIH’s business, or if they issue an adverse opinion regarding its stock, its stock price and trading volume could decline.
The trading market for DIH Common Stock is influenced by the research and reports that industry or securities analysts publish about DIH or its business. DIH does not currently have and may never obtain research coverage by securities and industry analysts. Since DIH became public through a merger, securities analysts of major brokerage firms may not provide coverage of DIH since there is no incentive to brokerage firms to recommend the purchase of its common stock. If no or few securities or industry analysts commence coverage of DIH, the trading price for its stock would be negatively impacted. In the event DIH obtains securities or industry analyst coverage, if any of the analysts who cover it issues an adverse opinion regarding DIH, its business model, its intellectual property or its stock performance, or if its clinical trials and operating results fail to meet the expectations of analysts, its stock price would likely decline. If one or more of these analysts cease coverage of DIH or fail to publish reports on it regularly, DIH could lose visibility in the financial markets, which in turn could cause its stock price or trading volume to decline.
We are emerging growth company and a “smaller reporting company” and the reduced reporting requirements applicable to such companies may make our DIH Common Stock less attractive to investors.
DIH is an emerging growth company, as defined in the JOBS Act. For as long as DIH continues to be an emerging growth company, it may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory stockholder votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. DIH cannot predict if investors will find its common stock less attractive because DIH may rely on these exemptions. If some investors find DIH Common Stock less attractive as a result, there may be a less active trading market for DIH Common Stock and its stock price may be more volatile.
DIH will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following February 7, 2027 (the fifth anniversary of the closing of ATAK’s IPO), (b) in which it has total annual gross revenue of at least $1.235 billion, or (c) in which it is deemed to be a large accelerated filer, which requires the market value of its common stock that is held by non-affiliates to equal or exceed $700 million as of the last business day of the second fiscal quarter of such year, and (2) the date on which DIH has issued more than $1 billion in non-convertible debt during the prior three-year period.
28
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. DIH has opted to continue to take advantage of the benefits of the extended transition period, although it may decide to early adopt such new or revised accounting standards to the extent permitted by such standards. This may make it difficult or impossible to compare DIH’s financial results with the financial results of another public company that is either not an emerging growth company or is an emerging growth company that has chosen not to take advantage of the extended transition period exemptions because of the potential differences in accounting standards used.
Additionally, DIH is a “smaller reporting company” as defined in Item 10(f) of Regulation S-K, which allows us to take advantage of certain scaled disclosure requirements available specifically to smaller reporting companies. For example, we may continue to use reduced compensation disclosure obligations, and we will not be obligated to follow the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act. We will remain a smaller reporting company until the last day of the fiscal year in which we have at least $100 million in revenue and at least $700 million in aggregate market value of our shares held by non-affiliated persons and entities (known as “public float”), or, alternatively, if our revenue exceeds $100 million, until the last day of the fiscal year in which our public float was at least $250.0 million (in each case, with respect to public float, as measured as of the last business day of the second quarter of such fiscal year). For the year ended March 31, 2024, DIH recorded revenue of approximately $64 million.
We cannot predict or otherwise determine if investors will find our securities less attractive as a result of our reliance on exemptions as a smaller reporting company and/or “non-accelerated filer.” If some investors find our securities less attractive as a result, there may be a less active trading market for our Common Stock and the price of our Common Stock may be more volatile.
The price of our Common Stock may be volatile, and you may lose all or part of your investment.
The market price of our Common Stock is volatile and may fluctuate substantially as a result of many factors. In addition, because the warrants are exercisable into shares of our Common Stock, volatility, or a reduction in the market price of our Common Stock could have an adverse effect on the trading price of the warrants. Factors which may cause fluctuations in the price of our Common Stock include, but are not limited to:
|
● |
actual or anticipated fluctuations in our growth rate or results of operations or those of our competitors; |
|
|
|
|
● |
customer acceptance of our products; |
|
|
|
|
● |
announcements by us or our competitors of new products or services, commercial relationships, acquisitions, or expansion plans; |
|
|
|
|
● |
announcements by us or our competitors of other material developments; |
|
|
|
|
● |
our involvement in litigation; |
|
|
|
|
● |
changes in government regulation applicable to us and our products; |
|
|
|
|
● |
sales, or the anticipation of sales, of our Common Stock, warrants and debt securities by us, or sales of our Common Stock by our insiders or other shareholders, including upon expiration of contractual lock-up agreements; |
|
|
|
|
● |
developments with respect to intellectual property rights; |
|
|
|
|
● |
competition from existing or new technologies and products; |
|
|
|
|
● |
changes in key personnel; |
29
|
|
|
|
● |
the trading volume of the Common Stock; |
|
|
|
|
● |
changes in the estimation of the future size and growth rate of our markets; |
|
|
|
|
● |
changes in our quarterly or annual forecasts with respect to operating results and financial conditions; |
|
|
|
|
● |
general economic and market conditions and |
|
|
|
|
● |
Announcements regarding business acquisitions. |
In addition, the stock markets have experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of our Common Stock, regardless of our operating performance. Technical factors in the public trading market for Common Stock may produce price movements that may or may not comport with macroeconomic, industry or DIH-specific fundamentals, including, without limitation, the sentiment of retail investors (including as may be expressed on financial trading and other social media sites), the amount and status of short interest in our securities, access to margin debt, trading in options and other derivatives on our Common Stock and any related hedging or other technical trading factors. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we become involved in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
General Risks
Exchange rate fluctuations between the U.S. dollar, the Euro and the Swiss Franc may negatively affect our revenue and earnings.
The U.S. dollar is our functional and reporting currency. However, more than 50% of our sales orders come from Europe in euros; and we pay a significant portion of our expenses in euro and Swiss Francs; and we expect this to continue. As a result, we are exposed to exchange rate risks that may materially and adversely affect our financial results. Accordingly, any depreciation of the euro relative to the U.S. dollar would adversely impact our revenue, and any appreciation of Swiss Franc against U.S. dollar will adversely impact net loss or net income, if any.
Our operations also could be adversely affected if we are unable to effectively hedge against currency fluctuations in the future.
We are subject to certain regulatory regimes that may affect the way that we conduct business internationally, and our failure to comply with applicable laws and regulations could materially adversely affect our reputation and result in penalties and increased costs.
We are subject to a complex system of laws and regulations related to international trade, including economic sanctions and export control laws and regulations. We also depend on our distributors and agents for compliance and adherence to local laws and regulations in the markets in which they operate. Significant political or regulatory developments in the jurisdictions in which we sell our products, such as those stemming from the presidential administration in the United States or the U.K.’s exit from the E.U. (known as “Brexit”), are difficult to predict and may have a material adverse effect on us. For example, in the United States, the Trump administration-imposed tariffs on imports from China, Mexico, Canada, and other countries, and expressed support for greater restrictions on free trade and increase tariffs on goods imported into the United States. Changes in U.S. political, regulatory, and economic conditions or in its policies governing international trade and foreign manufacturing and investment in the United States could adversely affect our sales in the United States.
We are also subject to the U.S. Foreign Corrupt Practices Act and may be subject to similar worldwide anti-bribery laws that generally prohibit companies and their intermediaries from making improper payments to government officials for the purpose of obtaining or retaining business. Despite our compliance and training programs, we cannot be certain that our procedures will be sufficient to ensure consistent compliance with all applicable international trade and anti-corruption laws, or that our employees or channel partners will strictly follow all policies and requirements to which we subject them.
30
Any alleged or actual violations of these laws may subject us to government scrutiny, investigation, debarment, and civil and criminal penalties, which may have an adverse effect on our results of operations, financial condition and reputation.
If there are significant disruptions in our information technology systems, our business, financial condition and operating results could be adversely affected.
The efficient operation of our business depends on our information technology systems like Oracle’s ERP and Microsoft 360 Office Platforms. We rely on our information technology systems to effectively manage sales and marketing data, accounting and financial functions, inventory management, product development tasks, research and development data, customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters, terrorist attacks, attacks by computer viruses or hackers, power losses, and computer system or data network failures. In addition, our data management application is hosted by a third-party service provider whose security and information technology systems are subject to similar risks, and our products’ systems contain software which could be subject to computer virus or hacker attacks or other failures.
The failure of our or our service providers’ information technology systems or our products’ software to perform as we anticipate or our failure to effectively implement new information technology systems could disrupt our entire operation or adversely affect our software products and could result in decreased sales, increased overhead costs, and product shortages, all of which could have a material adverse effect on our reputation, business, financial condition, and operating results.
If we fail to properly manage our anticipated growth, our business could suffer.
Our growth and product expansion has placed, and we expect that it will continue to place, a significant strain on our management team and on our financial resources. Failure to manage our growth effectively could cause us to misallocate management or financial resources, and result in losses or weaknesses in our infrastructure, which could materially adversely affect our business. Additionally, our anticipated growth will increase the demands placed on our suppliers, resulting in an increased need for us to manage our suppliers and monitor for quality assurance. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our business objectives.
We are highly dependent on the knowledge and skills of our global leadership team, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to continue to lead in this fragmented industry depends upon our ability to attract, develop and retain highly qualified managerial, scientific, sales and medical personnel. We are highly dependent on our global leadership team and have benefited substantially from the leadership and performance of our global leadership team. The loss of the services of any of our executive officers and other key global leadership team member, and our inability to find suitable replacements could result in delays in product development and harm the smooth operation of our business.
DIH’s management team has limited experience managing a public company.
Members of our management team have limited experience managing a publicly traded company, interacting with public company investors, and complying with the increasingly complex laws pertaining to public companies. We may not successfully or efficiently manage our transition to being a public company that is subject to significant regulatory oversight and reporting obligations under the federal securities laws and the continuous scrutiny of securities analysts and investors. These new obligations and constituents will require significant attention from our senior management and could divert their attention away from the day-to-day management of our business, which could harm our business, results of operations, and financial condition.
We have identified material weaknesses in our internal control over financial reporting. These material weaknesses could continue to adversely affect our ability to report our results of operations and financial condition accurately and in a timely manner .
31
Our management is responsible for establishing and maintaining adequate internal control over financial reporting designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP. Our management is likewise required to evaluate the effectiveness of our internal controls and to disclose any changes and material weaknesses identified through such evaluation in those internal controls. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
We identified a material weakness in our internal control over financial reporting with respect to our accounting personnel. Specifically, the Company concluded that it had limited accounting personnel and other resources with which to address its internal control over financial reporting in accordance with requirements applicable to public companies. Historically, the Company has not retained a sufficient number of professionals with an appropriate level of accounting knowledge, training and experience to appropriately analyze, record and disclose accounting matters under U. S. GAAP.
Any failure to maintain such internal control could adversely impact our ability to report our financial position and results from operations on a timely and accurate basis. If our financial statements are not accurate, investors may not have a complete understanding of our operations. Likewise, if our financial statements are not filed on a timely basis, we could be subject to sanctions or investigations by Nasdaq or any other exchange on which our Common Stock are listed, the SEC or other regulatory authorities. In either case, there could result a material adverse effect on our business. Ineffective internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of the Common Stock.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 1C. Cybersecurity.
Cybersecurity Risk Management and Strategy
We have developed and implemented a cybersecurity risk management program intended to protect the confidentiality, integrity, and availability of our critical systems and information.
We design and assess our program based on the National Institute of Standards and Technology Cybersecurity Framework Special Publication 800-53, 800-61, rev 2 (“NIST CSF). This does not imply that we meet any particular technical standards, specifications, or requirements. We use the NIST CSF as a guide to help us identify, assess, and manage cybersecurity risks relevant to our business.
Our cybersecurity risk management program is integrated into our overall enterprise risk management program and shares common methodologies, reporting channels, and governance processes that apply across the enterprise risk management program to other legal, compliance, strategic, operational, and financial risk areas.
Our cybersecurity risk management program includes the following:
|
● |
risk assessments designed to help identify material cybersecurity risks to our critical systems, information, products, services, and our broader enterprise IT environment; |
|
|
|
|
● |
a security team principally responsible for managing (1) our cybersecurity risk assessment processes, (2) our security controls, and (3) our response to cybersecurity incidents; |
|
|
|
|
● |
the use of external service providers, where appropriate, to assess, test, or otherwise assist with aspects of our security controls; |
|
|
|
|
● |
cybersecurity awareness training of our employees, incident response personnel, and senior management; and |
|
|
|
|
● |
a cybersecurity incident response plan that includes procedures for responding to cybersecurity incidents. |
32
There can be no assurance that our cybersecurity risk management program and processes, including our policies, controls or procedures, will be fully implemented, complied with or effective in protecting our systems and information.
We have not identified risks from known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected or are reasonably likely to materially affect us, including our operations, business strategy, results of operations, or financial condition
Cybersecurity Governance
Our Board considers cybersecurity risks as part of its risk oversight function of cybersecurity and other information technology risks.
The Chief Financial Officer oversees our information security programs, including cybersecurity initiatives, and is integrated into our Cybersecurity Incident response process. We assess and prioritize risks based on potential impact, implement technical controls, and monitor third-party vendors’ security practices.
The Audit Committee oversees management’s implementation of our cybersecurity risk management program and receives updates on the cybersecurity risk management program from management at least annually. In addition, management updates the Audit Committee regarding any material or significant cybersecurity incidents, as well as incidents with lesser impact potential as necessary. The Audit Committee oversees cybersecurity risk management activities, supported by Company management, the Board of Directors, and external consultants.
The Audit Committee reports to the full Board annually regarding cybersecurity. The full Board also receives annual briefings from external experts on cybersecurity as part of the Board’s continuing education on topics that impact public companies.
Ongoing Risks
We have not experienced any material cybersecurity incidents. We have not identified risks from known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected us, including our operations, business strategy, results of operations, or financial condition.
Risk Management and Strategy
The Company recognizes the critical importance of cybersecurity in safeguarding sensitive information, maintaining operational resilience, and protecting stakeholders’ interests. This cybersecurity policy is designed to establish a comprehensive framework for identifying, assessing, mitigating, and responding to cybersecurity risks across the organization.
The Company is in the process of establishing a cybersecurity policy which will implement protocols to evaluate, recognize, and address significant risks, including those posed by cybersecurity threats. This strategy encompasses the utilization of standard traffic monitoring tools, educating personnel to identify and report abnormal activities, and partnering with reputable service providers capable of upholding security standards equivalent to or exceeding our own.
These measures are to be seamlessly integrated into our broader operational risk management framework aimed at minimizing exposure to unnecessary risks across our operations. For cybersecurity, we collaborate with expert consultants and third-party service providers to implement industry-standard strategies aimed at identifying and mitigating potential threats or vulnerabilities within our systems. Additionally, the policy will have a comprehensive cyber crisis response plan to manage high severity security incidents, ensuring efficient coordination across the organization.
Cybersecurity threats haven’t significantly impacted our operations, and we don’t anticipate such risks materially affecting our business, strategy, financial condition, or results of operations. However, given the escalating sophistication of cyber threats, our preventive measures may not always suffice.
33
Despite well-designed controls, we acknowledge the inability to foresee all security breaches, including those stemming from third-party misuse of AI technologies, and the potential challenges in implementing timely preventive measures.
Item 2. Properties.
Our executive offices are located at 77 Accord Drive, Suite D-1 Norwell, MA. We do not own any properties, instead we lease properties to meet our needs. Currently, we have one main R&D and Operational campus that we lease for Hocoma Medical operation in Switzerland. The leased property is located at Industriestrasse 2 and 4a in 8604 Volketswil. Beside the main campuses, we also lease five commercial offices space at the following locations to house the regional Sales & Marketing, Clinical Application & Training, Technical Services, Finance, Logistics, Administration and other local market support functions.
|
■ |
DIH Technology Inc. leases commercial office for American team at 77 Accord Park Dr., Suite D-1, Norwell, MA 02061, United States |
|
■ |
DIH Technology d.o.o leases commercial office for EMEA Indirect sales team at Letališka 29a, 1000 Ljubljana, Slovenia |
|
■ |
DIH GmbH leases commercial office for the Direct Sales team in DACH region, at Konrad-Adenauer Strasse 13, 50996 Köln, Germany |
|
■ |
DIH Pte Ltd leases commercial office for APAC team at 67 Ubi Avenue 1, #06-17 Starhub Green, Singapore 408942 |
|
■ |
DIH SpA leases commercial office for LATAM team at Pdte. Kennedy Lateral 5488, Oficina 1402; Vitacura, Santiago, Chile |
Item 3. Legal Proceedings.
There is no material litigation, arbitration or governmental proceeding currently pending against us or any members of our management team in their capacity as such, and we and the members of our management team have not been subject to any such proceeding in the 12 months preceding the date of this Annual Report.
Item 4. Mine Safety Disclosures.
Not applicable.
34
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our Common Stock is listed on the Nasdaq Global Market under the symbol “DHAI,” and our Public Warrants are listed on the Nasdaq Capital Market under the symbol “DHAIW.”
Holders of Record
As of the date of May 31, 2024, there were 119 holders of record of our Common Stock, and 2 holders of record of our Public Warrants.
Dividend Policy
We have not paid any cash dividends on shares of our Common Stock to date and do not intend to pay cash dividends. The payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial condition. The payment of any dividends will be within the discretion of our board of directors. It is the present intention of our board of directors to retain all earnings, if any, for use in our business operations and, accordingly, our board of directors does not anticipate declaring any dividends in the foreseeable future.
Recent Sales of Unregistered Securities; Use of Proceeds from Registered Securities
In connection with the consummation of the Business Combination, DIH issued 229,796 shares of its Common Stock to Maxim Group LLC and to other vendors as partial payment of expenses owed.
On June 6, 2024, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with the purchasers named therein (the “Purchasers”), pursuant to which the Company sold on June 7, 2024, in a private placement, an aggregate of $3,300,000 in principal amount of 8% Original Issue Discount Senior Secured Convertible Debentures (the “Debentures”), initially convertible into an aggregate of 660,000 shares of the Company’s Common Stock, par value $0.0001 (the “Common Stock”) at a conversion price of $5.00 (the “Conversion Price”). The Debentures have an aggregate face value of $3,300,000 and were issued with an original issue discount of $300,000. In connection with the purchase of the Debentures, each Purchaser received warrants to purchase shares of Common Stock (the “Warrants”) equal to 50% of such Purchaser’s Conversion Shares or an aggregate of 330,000 Warrants. Each Warrant has an exercise price of $5.00 and a five year term.
The Debentures and the Warrants were sold pursuant to an exemption from registration under the Securities Act of 1933, as amended (the “Securities Act”), available under Section 4(a)(2) and Rule 506(b) of Regulation D promulgated thereunder. The Conversion Shares and the Warrant Shares will be issued pursuant to the same exemption or pursuant to the exemption provided by Section 3(a)(9) of the Securities Act. Accordingly, the securities issued in the private placement may not be offered or sold in the United States except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act and such applicable state securities laws.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 6. [Reserved]
35
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS OF DIH
The following discussion and analysis should be read together with the historical audited annual consolidated financial statements and related notes that are included elsewhere in this Form 10-K. The following discussion may contain forward-looking statements. Actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to these differences include those factors discussed below and elsewhere in this Form 10-K, particularly in sections therein entitled “Cautionary Note Concerning Forward Looking Statements” and “Risk Factors.” .
Our fiscal year ends on March 31. “Fiscal 2024” and “fiscal 2023” refer to the year ended March 31, 2024 and 2023, respectively.
Overview
DIH is a global solution provider in blending innovative robotic and VR technologies with clinical integration and insights. DIH has a focused portfolio of rehabilitation solutions, which includes both technology and products designed for the hospital, clinic, and research markets.
In fiscal 2024, DIH generated revenue of $64.5 million compared to $54.1 million in fiscal 2023.
DIH’s net loss for fiscal 2024 was $8.4 million, compared to $1.0 million in fiscal 2023. The net loss increased $7.4 million which was primarily driven by total transaction costs of $7.1 million to close the Business Combination with ATAK, which included a non-cash financial advisory fee of $3.5 million paid with 700,000 shares of Common Stock. The increase in net loss was also driven by an increase in the cost of goods sold, which was largely due to higher device sales volume, direct cost inflation, and an increase in the inventory reserve for slow moving parts, as well as, elevated costs related to professional service and IT costs related to audit, legal and other professional services to close the business combination discussed in more detail below. The increase in costs and expenses is offset by an increase in revenue as the Company is emerging from the COVID-19 pandemic period that depressed global sales volume due to social distancing measures, and the current year was free of additional non-recurring expenditures for the European Union Medical Device Regulation (EU MDR) and other large scale projects.
Recent Developments
Business Combination
On February 7, 2024, ATAK, Aurora Technology Merger Sub (“Merger Sub”) and DIH Nevada consummated a previously announced business combination pursuant to the Business Agreement dated as of February 26, 2023 following the receipt of the required approval by ATAK’s and DIH Nevada’s shareholders and the fulfillment or waiver of other customary closing conditions. ATAK agreed to waive the closing condition that the Reorganization be completed prior to Closing. As a result, at Closing of the Business Combination, the Company includes Hocoma Medical that holds assets transferred from Hocoma AG as well as other commercial entities controlled by the Company. Whereas, Hocoma AG and Motekforce Link BV and its subsidiaries were excluded. The Company agreed to use its best efforts to complete the intended Reorganization to transfer Hocoma AG and Motek to the Company as soon as possible thereafter.
In the interim, DIH continues its historical relationship with Motek as an exclusive distributor of the advanced human movement research and rehabilitation products and services designed to support efficient functional movement therapy within specified territories. DIH also intends to continue making periodic payments on notes payable to Hocoma AG, which arose from Hocoma AG transferring assets to the Company.
Upon closing of the Business Combination, the Company received cash held in trust account of $899 thousand. In connection with the Closing of the Business Combination, ATAK migrated and changed its domestication to become a Delaware corporation and changed its name to “DIH Holding US, Inc.” Legacy DIH stockholders received shares of Common Stock of DIH, as more fully described in the section in the proxy statement/prospectus entitled “The Business Combination Agreement.”
36
The historical financial results presented in the registration statements were prepared on a combined basis including Legacy DIH, Hocoma AG and Motek Group pursuant to the Business Combination Agreement for the intended Reorganization. In this Annual Report on Form 10-K, the Company has recast historical financial statements on a consolidated basis including operations from Legacy DIH excluding Hocoma AG and the Motek Group that remained with DIH Hong Kong. The Merger was accounted for as a reverse recapitalization, in accordance with GAAP. Under this method of accounting, ATAK was treated as the “acquired” company for financial reporting purposes. Accordingly, the Business Combination was treated as the equivalent of DIH Nevada issuing stock for the net assets of ATAK, accompanied by a recapitalization. The net assets of ATAK were stated at historical cost, with no goodwill or other intangible assets recorded. Operations prior to the Business Combination were those of Legacy DIH and its subsidiaries, excluding Hocoma AG and Motek Group.
As a consequence of the Business Combination, the Company became the successor to an SEC-registered company, which requires DIH to hire additional personnel and implement procedures and processes to address public company regulatory requirements and customary practices. DIH expects to incur additional annual expenses as a public company for, among other things, directors’ and officers’ liability insurance, director fees and additional internal and external accounting and legal and administrative resources, including increased audit and legal fees.
Key Factors Affecting the DIH’s Operating Results
DIH believes that its future success and financial performance depend on a number of factors that present significant opportunities for its business, but also pose risks and challenges, including those discussed below and in the Section of this Form 10-K entitled “Item 1a. Risk Factors.”
Supply Chain and Inflation
The global supply chain and logistics challenges continue to impact DIH and the industry. As a result of these challenges, DIH has experienced cost increases for freight and logistics, raw materials and purchased components as well as increased manufacturing conversion costs. These supply chain disruptions have not materially affected DIH’s business outlook and goals or its operating results, including its sales, revenue, or liquidity or capital resources and DIH has not implemented any mitigation efforts to date as a result. However, DIH cannot predict the impact to it of any future or prolonged supply chain disruptions or any mitigation efforts it may take going forward. For example as a result of these supply chain disruptions, DIH may be required to extend the overall shipment and installation timeline. In addition, DIH may consider additional or alternative third-party manufacturers and logistics providers, suppliers, vendors or distributors. Such mitigation efforts may result in cost increases and any attempts to offset such increases with price increases may result in reduced sales, increased customer dissatisfaction, or otherwise harm DIH’s reputation. Further, if DIH were to elect to transition or add manufacturers or logistics providers, suppliers, vendors or distributors, it may result in temporary or additional delays in shipments of products or risks related to consistent product quality or reliability. This in turn may limit DIH’s ability to fulfill customer sales orders and DIH may be unable to satisfy all of the demand for its products. DIH may in the future also purchase components further in advance, which in return can result in less capital being allocated to other activities such as marketing and other business needs. DIH cannot quantify the impact of such disruptions at this time or predict the impact of any mitigation efforts DIH may take in response to supply chain disruptions on its business, financial condition, and results of operations.
Input cost inflation historically has not been a material factor to our gross margin; however, beginning at the end of fiscal 2022 DIH began to experience increases in raw material and components costs due to inflation effects, which are expected to continue to remain at elevated levels for at least the near term.
Foreign Currency Fluctuations
DIH’s business operates in three different functional currencies (Euro, Swiss Franc, Singapore Dollar). DIH’s reporting currency is the U.S. Dollar. DIH’s results are affected by fluctuations in currency exchange rates that give rise to translational exchange rate risks. The extent of such fluctuations is determined in part by global economic conditions and macro-economic trends. Movements in exchange rates have a direct impact on DIH’s reported revenues. Generally, the impact on operating income or loss associated with exchange rate changes on reported revenues is partially offset from exchange rate impacts on operating expenses denominated in the same functional currencies.
37
As foreign currency exchange rates change, translation of the statements of operations of DIH’s international businesses into U.S. dollars may affect year-over-year comparability of DIH’s operating results.
EU MDR Implementation Costs
Changes in law or regulation could make it more difficult and costly for DIH and its subsidiaries to manufacture, market and distribute its products or obtain or maintain regulatory approval of new or modified products. DIH’s experience with the transition to the EU MDR, which it began in 2019, showed how complex, time-consuming and expensive a change in Medical Device Legislation can be. The EU MDR replaced the existing European Medical Devices Directive (MDD) and Active Implantable Medical Device Directive (AIMDD) regulatory frameworks, and manufacturers of medical devices were required to comply with EU MDR beginning in May 2021 for new product registrations and by May 2024 for medical devices which have a valid CE Certificate to the prior Directives (issued before May 2021). Updates to the legislative text of the EU MDR were adopted by the European Parliament and are currently being reviewed for adoption by the Council of the European Union, including an extension of the transitional period to 2027 for class IIb and III and 2028 for class I and IIa medical devices which have a valid CE Certificate to the prior Directives (issued before May 2021).
Macroeconomic Uncertainties on Future Operations
DIH’s operations are exposed to and impacted by various global macroeconomic factors. DIH faces continuing market and operating challenges across the globe due to, among other factors, impact of conflict in Ukraine, conditions related to the COVID-19 pandemic, supply chain disruption, higher interest rates and inflationary pressures. Continued evolution of these conditions could lead to economic slowdowns.
Basis of Presentation
Refer to Note 2 of the Notes to Annual Consolidated Financial Statements for a discussion of the underlying basis used to prepare the consolidated financial statements.
Components of Results of Operations
Revenue
DIH generates revenue from the sale of medical rehabilitation devices and technology. DIH’s primary customers include healthcare systems, clinics, third-party healthcare providers, distributors, and other institutions, including governmental healthcare programs and group purchasing organizations. Shipping and handling costs charged to customers are included in net sales. DIH expects revenue to increase sequentially in future periods as it expects the demand for its products to expand in represented markets.
Cost of Sales
Cost of sales primarily consists of direct materials, supplies, in-bound freight and labor-related costs, including salaries and benefits for our manufacturing personnel, technical support team, our professional consulting personnel and our training teams. Cost of sales also includes allocated overhead costs, including facilities costs, depreciation of manufacturing-related equipment and facilities and other direct costs. DIH expects cost of sales to increase in absolute dollars in future periods as it expects orders for its products to continue to grow and expects cost of sales per unit to decrease as leverage improves behind expected growth.
Selling, General and Administrative Expense
Selling, general and administrative expense primarily consists of personnel related expenses for DIH’s corporate, executive, finance and other administrative functions, expenses for outside professional services, including legal, audit and advisory services as well as expenses for facilities, depreciation, amortization, and marketing costs. Personnel-related expenses consist of salaries and benefits.
38
DIH expects selling, general and administrative expenses to increase for the foreseeable future as it scales headcount, expands hiring of engineers and designers, continues to invest in development of technology in order to drive the growth of the business, and as a result of operating as a public company, including compliance with the rules and regulations of the SEC, legal, audit, additional insurance expenses, investor relations activities and other administrative and professional services.
Research and Development
Research and development primarily consists of research, engineering, and technical activities to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include pre-approval regulatory and clinical trial expenses.
DIH expects research and development costs to increase as it continues to invest in product design and technology to drive the growth of the business.
Interest Expense
Interest expense primarily consists of interest expense associated with related party notes payable and bank charges.
Other Income (Expense), Net
Other income (expense), net primarily consists of the non-service components of net periodic defined benefit plan income (costs) and certain non-recurring costs in connection with the Business Combination.
Income Tax Expense
The income tax provision (benefit) consists of an estimate for U.S. federal, state and foreign income taxes based on enacted rates, as adjusted for allowable credits, deductions, uncertain tax positions, changes in deferred tax assets and liabilities and changes in the tax law.
Results of Operations
|
|
Year ended March 31, |
|
|
|
|
|
|
|
|||||||
(in thousands, except percentages) |
|
2024 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Revenue |
|
$ |
64,473 |
|
|
$ |
54,059 |
|
|
$ |
10,414 |
|
|
|
19.3 |
% |
Costs of sales |
|
|
34,702 |
|
|
|
23,474 |
|
|
|
11,228 |
|
|
|
47.8 |
% |
Gross Profit |
|
|
29,771 |
|
|
|
30,585 |
|
|
|
(814 |
) |
|
|
(2.7 |
)% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Selling, general and administrative expense |
|
|
25,776 |
|
|
|
22,957 |
|
|
|
2,819 |
|
|
|
12.3 |
% |
Research and development |
|
|
6,609 |
|
|
|
6,919 |
|
|
|
(310 |
) |
|
|
(4.5 |
)% |
Total operating expenses |
|
|
32,385 |
|
|
|
29,876 |
|
|
|
2,509 |
|
|
|
8.4 |
% |
Operating loss |
|
|
(2,614 |
) |
|
|
709 |
|
|
|
(3,323 |
) |
|
|
(468.7 |
)% |
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest expense |
|
|
(693 |
) |
|
|
(277 |
) |
|
|
(416 |
) |
|
|
150.2 |
% |
Other income (expense), net |
|
|
(3,890 |
) |
|
|
572 |
|
|
|
(4,462 |
) |
|
|
(780.1 |
)% |
Total other income (expense) |
|
|
(4,583 |
) |
|
|
295 |
|
|
|
(4,878 |
) |
|
|
(1653.6 |
)% |
Profit (loss) before income taxes |
|
|
(7,197 |
) |
|
|
1,004 |
|
|
|
(8,201 |
) |
|
|
(816.8 |
)% |
Income tax expense |
|
|
1,246 |
|
|
|
2,018 |
|
|
|
(772 |
) |
|
|
(38.3 |
)% |
Net loss |
|
$ |
(8,443 |
) |
|
$ |
(1,014 |
) |
|
$ |
(7,429 |
) |
|
|
732.6 |
% |
Revenue
The following table presents net revenue by major source for the year ended March 31, 2024 and 2023:
39
|
|
Year ended March 31, |
|
|
|
|
|
|
|
|||||||
(in thousands, except percentages) |
|
2024 |
|
|
2023 |
|
|
$ Change |
|
|
% Change |
|
||||
Devices |
|
$ |
51,125 |
|
|
$ |
43,452 |
|
|
$ |
7,673 |
|
|
|
17.7 |
% |
Services |
|
|
11,105 |
|
|
|
9,292 |
|
|
|
1,813 |
|
|
|
19.5 |
% |
Other |
|
|
2,243 |
|
|
|
1,315 |
|
|
|
928 |
|
|
|
70.6 |
% |
|
|
$ |
64,473 |
|
|
$ |
54,059 |
|
|
$ |
10,414 |
|
|
|
19.3 |
% |
Revenue for the year ended March 31, 2024 increased by $10.4 million, or 19.3%, to $64.5 million from $54.1 million for the year ended March 31, 2023. The overall increase was primarily due to an increase in devices sold of $7.7 million, or 17.7%. The increase in devices revenue was driven by higher sales volume in Europe, the Americas and Asia. Services revenue represented an increase of $1.8 million, up 19.5% compared to the prior period. Other revenues represented an increase of $0.9 million, up 70.6% compared to the prior period.
Changes in foreign currency exchange rates had a favorable impact on our net sales for the year ended March 31, 2024, resulting in an increase of approximately $1.7 million. This was mainly driven by fluctuations in Euro valuations throughout the period.
Cost of Sales
Cost of sales for the year ended March 31, 2024 increased by $11.2 million, or 47.8%, to $34.7 million from $23.5 million for the year ended March 31, 2023. The Cost of Goods for device sales increased by $7.3 million, which directly correlated to the increase in device sales with an incremental cost of $4.4 million for the additional volume and inflationary cost increases on direct costs of goods of approximately $2.2 million. The additional increase in cost of sales is mainly driven by an increase of $0.6 million in inventory reserve for slow moving parts and increased overhead and services parts costs of $3.9 million. The impact due to foreign currency translation losses resulted in an increase of approximately $0.1 million.
Selling, General and Administrative Expense
Selling, general and administrative expense for the year ended March 31, 2024 increased by $2.8 million, or 12.3%, to $25.7 million from $23.0 million for the year ended March 31, 2023. The increase was primarily due to an increase in professional service costs of $1.5 million related to audit, legal and other professional services in preparation for the business combination and becoming a publicly listed company, and investment in finance capacity in preparation for public company reporting obligations. The increase was also attributable to personnel related expense primarily due to an $1 million increase in pension expense resulting from changes in market yields. The increase was partially offset by a decrease in credit loss provisions.
Research and Development
Research and development costs for the year ended March 31, 2024 decreased by $0.3 million, or 4.5%, to $6.6 million from $6.9 million for the year ended March 31, 2023. The decrease was primarily due to a decrease in the research and development material purchase and external consulting of $0.2 million and charges pertaining to the Gorbel acquisition of $0.4 million, that are not recurring in the current period. The decrease was offset by an increase in personnel expenses of $0.3 million.
Interest Expense
Interest expense for the year ended March 31, 2024 increased by $416 thousand, or 150.2% in relates to interests on Related Party Notes and an increase in temporary bank charge.
Other Income (Expense), Net
Other income (expense), net for the year ended March 31, 2024 was $3.9 million of expense compared to $0.6 million of income for the year ended March 31, 2023. The change was primarily driven by a $3.5 million financial advisory fee paid with 700,000 shares of Common Stock in connection with closing of the Business Combination as well as realized foreign exchange losses during the period.
Income Tax Expense
40
Income tax expense for the year ended March 31, 2024 decreased by $0.8 million to $1.2 million. The change was primarily driven by changes in the net results of the underlying subsidiaries across jurisdictions. The tax expense for March 31, 2024 and March 31, 2023 is driven by pre-tax book income in certain jurisdictions while the benefit from pre-tax losses in other jurisdiction may not be realizable.
Liquidity and Capital Resources
As of March 31 2024 and 2023, DIH’s cash and cash equivalents amounted to $3.2 million and $3.2 million, respectively. DIH’s sources of liquidity have been predominantly from proceeds received from product sales and services provided. DIH’s sources of liquidity have enabled DIH to expand its installation base, capacity and grow its sales personnel to expand capabilities and enter new markets. For the year ended March 31, 2024 and 2023, the Company has not used proceeds from external financing to support its operation and growth.
DIH’s operating losses began in fiscal 2020 and continued through the year ended March 31, 2024. DIH’s historical operating losses resulted in an accumulated deficit of $(35.2) million as of March 31, 2024. Operating losses were mainly driven by decreased sales during the COVID-19 pandemic due to social distancing measures that affected demand for rehabilitation services, increased expenditures in connection with its implementation of a new financial system (Oracle) and increased compliance costs associated with the EU MDR. Additionally, DIH had elevated costs related to efforts of adopting to public company standards. During the year ended March 31, 2024, DIH had positive cash flows from operating activities for $5.2 million and operating loss for $2.6 million. DIH anticipates achieving positive cash flow in the future. This transition towards profitability is attributable to DIH's ongoing efforts to streamline its organizational structure and cost management enabled by digitization investments such as the Oracle system implementation, alongside anticipated revenue growth.
DIH’s gross revenue has increased by 19.3%, from $54.1 million to $64.5 million for the year ended March 31, 2024 and 2023, respectively. DIH plans to continue to fund its growth through cash flows from operations and future debt and equity financings. Management expects that its cash and cash equivalents, together with cash provided by our operating activities and proceeds from future debt and equity financings, will be sufficient to fund its operating expenses and capital expenditures requirements for at least the next 12 months.
In connection with the transfer of Hocoma AG's business and assets to DIH, we incurred three related party notes payable to Hocoma AG as further discussed in Note 13 of the Notes to Consolidated Financial Statements. The three Related Party Notes amounting to $10.47 million, $7.80 million and $1.57 million reflect transferring the assets, equity ownership in subsidiaries and IP rights it held to Legacy DIH. Each of the Related Party Notes Payable is due on June 30, 2026 with interest rate of 1.25%. The Company has made periodic payment on Related Party Notes payable with proceeds from its operations. The remaining balance on the Related Party Notes payable is $11.5 million and $17.3 million as of March 31, 2024 and 2023, respectively. We expect to continue our growth and generate sufficient proceeds in payments of Related Party Notes payable.
DIH’s other material contractual operating cash commitments at March 31, 2024 relate to leases and employee benefit plans. DIH’s employee benefit plans are discussed further in Note 14 of the Notes to Consolidated Financial Statements. DIH’s long-term lease obligations and future payments are discussed further in Note 17 of the Notes to Consolidated Financial Statements.
Cash Flows
The following table summarizes DIH’s cash flow activities for the periods presented:
|
|
Year ended March 31, |
|
|||||
(in thousands) |
|
2024 |
|
|
2023 |
|
||
Net cash (used in) / provided by operating activities |
|
$ |
5,192 |
|
|
$ |
5,501 |
|
Net cash (used in) / provided by investing activities |
|
|
(202 |
) |
|
|
(145 |
) |
Net cash (used in) / provided by financing activities |
|
|
(4,945 |
) |
|
|
(4,053 |
) |
Effect of currency translation on cash, cash equivalents and restricted cash |
|
|
5 |
|
|
|
(61 |
) |
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
$ |
50 |
|
|
$ |
1,242 |
|
Net Cash Provided by / (Used in) Operating Activities
41
Net cash provided by operating activities for the year ended March 31, 2024 was in line with that for the year ended March 31, 2023 primarily driven by:
This decrease in working capital was partially offset by increase of $2.2 million in advanced payments from customer for the year ended March 31, 2024 compared to the year ended March 31, 2023 primarily due to the timing of the order received. Many customers prepay a portion of each order, which supports the operations of the company in the production of the goods. We also observed a total increase of $5.2 million in accounts receivable and accounts payable as we are managing our working capital. Working capital was impacted by favorable changes in due from and due to related parties balances driven by the Company’s purchase from the Motek Group and change in balance from Hocoma AG.
Net Cash (Used in) / Provided by Investing Activities
Net cash used in investing activities is consistent between the year ended March 31, 2024 compared to that for the year ended March 31, 2023. The cash used in investing activities primarily includes purchase of property and equipment. DIH expects to fund future cash flows used in investing activities with cash flow generated by operations.
Net Cash (Used in) / Provided by Financing Activities
Net cash used in financing activities increased by $0.8 million to $4.9 million for the year ended March 31, 2024 compared to $4.1 million for the year ended March 31, 2023. The increase was primarily due to increase of $1.8 million in payments on related party notes payable resulting from the asset transfer from Hocoma AG to the Company offset by proceeds received upon closing of the Business Combination.
Critical Accounting Policies and Estimates
DIH’s financial statements are based on the selection and application of significant accounting policies, which require management to make significant estimates and assumptions. Management believes that the following are some of the more critical judgment areas in the application of accounting policies that currently affect DIH’s financial condition and results of operations.
Revenue Recognition
Sales are recognized as the performance obligations to deliver products or services are satisfied and are recorded based on the amount of consideration DIH expects to receive in exchange for satisfying the performance obligations. DIH’s sales are recognized primarily when it transfers control to the customer, which can be on the date of shipment of the product, the date of receipt of the product by the customer or upon completion of any required product installation service depending on the terms of the sales contracts and product shipping terms.
42
The sales amount of warranties are deferred and recognized as revenue on a straight-line basis over the warranty period.
We provide a variety of products and services to our customers. Most of our contracts consist of a single, distinct performance obligation or promise to transfer goods or services to a customer. For contracts that include multiple performance obligations, we allocate the total transaction price to each performance obligation using our best estimate of the standalone selling price of each identified performance obligation.
Deferred revenue primarily represents service contracts and equipment maintenance, for which consideration is received in advance of when service for the device or equipment is provided, and a smaller component of product shipments where a residual installation service is to be completed. Revenue related to services contracts and equipment maintenance is recognized over the service period as time elapses. Revenues related to products containing an installation clause, are recognized once the item is confirmed installed.
Employee Benefit Plans
DIH has defined contribution plans or benefit pension plans covering substantially all of its employees. We recognize a liability for the underfunded status of the single employer defined benefit plans. Actuarial gains or losses and prior service costs or credits are recorded within other comprehensive income (loss). The determination of our obligation and related expense for our sponsored pensions is dependent, in part, on management’s selection of certain actuarial assumptions in calculating these amounts.
The actuarial assumptions used for the defined benefit plans are based on the economic conditions prevailing in the jurisdiction in which they are offered. Changes in the defined benefit obligation are most sensitive to changes in the discount rate. The discount rate is based on the yield of high-quality corporate bonds quoted in an active market in the currency of the respective plan. A decrease in the discount curve increases the defined benefit obligation. DIH regularly reviews the actuarial assumptions used in calculating the defined benefit obligation to determine their continuing relevance. We utilized weighted discount rates of 1.50% and 2.10% for our pension plan expenses for fiscal 2024 and fiscal 2023, respectively.
Sensitivity to changes in the discount rate used in the calculation of our pension plan liabilities is illustrated below (dollars in millions).
|
|
Percentage |
|
Projected |
|
Service |
Discount rate |
|
+/-1.00% |
|
$(1.6) / 2.1 |
|
$(0.2) / 0.2 |
Income Taxes
DIH accounts for income taxes in accordance with Accounting Standards Codification Topic 740, Income Taxes (Topic 740). Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and other loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. DIH reviews its deferred income tax asset valuation allowances on a quarterly basis or whenever events or changes in circumstances indicate that a review is required. In determining the requirement for a valuation allowance, the historical and projected financial results of the legal entity or combined group recording the net deferred income tax asset is considered, along with any positive or negative evidence including tax law changes. Since future financial results and tax law may differ from previous estimates, periodic adjustments to DIH’s valuation allowances may be necessary. DIH has generated operating losses in each of the years presented.
DIH is subject to income taxes in the U.S. and numerous foreign jurisdictions These tax laws and regulations are complex and significant judgment is required in determining DIH’s worldwide provision for income taxes and recording the related deferred tax assets and liabilities.
43
In the ordinary course of DIH’s business, there are transactions and calculations where the ultimate tax determination is uncertain. Accruals for unrecognized tax benefits are provided for in accordance with the requirements of Topic 740. An unrecognized tax benefit represents the difference between the recognition of benefits related to items for income tax reporting purposes and financial reporting purposes. DIH’s tax returns are subject to regular review and audit by US and non-US tax authorities. Although the outcome of tax audits is always uncertain, DIH believes that it has appropriate support for the positions taken on its tax returns and that its annual tax provision includes amounts sufficient to pay any assessments. Nonetheless, the amounts ultimately paid, if any, upon resolution of the issues raised by the taxing authorities may differ materially from the amounts accrued for each year and would be the obligation of Parent. DIH accrues interest and penalties related to uncertain tax positions as a component of income tax expense.
Refer to Note 15 of the Notes to Annual Consolidated Financial Statements for further discussion regarding DIH’s income taxes.
Emerging Growth Company Status
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is either not an emerging growth company or an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
New Accounting Standards Not Yet Adopted
Other than the recent accounting pronouncements disclosed in DIH’s Annual Consolidated Financial Statements, there have been no new accounting pronouncements or changes in accounting pronouncements during the year ended March 31, 2024 that are significant or potentially significant to DIH.
44
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
As a “smaller reporting company” as defined by Item 10 of Regulation S-K, DIH is not required to provide this information.
45
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Report of Independent Registered Public Accounting Firm (BDO AG, Zurich, Switzerland, PCAOB ID # 5988) |
47 |
48 |
|
49 |
|
50 |
|
51 |
|
52 |
|
53 |
46
|
Phone +41 44 444 35 55 |
BDO Ltd |
Report of Independent Registered Public Accounting Firm
Stockholders and Board of Directors
DIH Holding US, Inc.
Norwell, MA
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of DIH Holding US, Inc. (the “Company”) as of March 31, 2024 and 2023, the related consolidated statements of operations, comprehensive loss, stockholders’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at March 31, 2024 and 2023, and the results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Zurich, July 15, 2024
BDO AG
/s/ Christoph Tschumi /s/ Philipp Kegele
Christoph Tschumi Philipp Kegele
We have served as the Company's auditor since 2022
47
DIH HOLDING US, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
|
|
March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
3,225 |
|
|
$ |
3,175 |
|
Accounts receivable, net of allowances of $667 and $1,683, respectively |
|
|
5,197 |
|
|
|
5,998 |
|
Inventories, net |
|
|
7,830 |
|
|
|
4,850 |
|
Due from related party |
|
|
5,688 |
|
|
|
6,383 |
|
Other current assets |
|
|
5,116 |
|
|
|
4,855 |
|
Total current assets |
|
|
27,056 |
|
|
|
25,261 |
|
Property, and equipment, net |
|
|
530 |
|
|
|
742 |
|
Capitalized software, net |
|
|
2,131 |
|
|
|
2,019 |
|
Other intangible assets, net |
|
|
380 |
|
|
|
380 |
|
Operating lease, right-of-use assets, net |
|
|
4,466 |
|
|
|
2,604 |
|
Other tax assets |
|
|
267 |
|
|
|
1 |
|
Other assets |
|
|
905 |
|
|
|
772 |
|
Total assets |
|
$ |
35,735 |
|
|
$ |
31,779 |
|
Liabilities and Deficit |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
4,305 |
|
|
$ |
2,190 |
|
Employee compensation |
|
|
2,664 |
|
|
|
3,163 |
|
Due to related party |
|
|
10,192 |
|
|
|
6,841 |
|
Current portion of deferred revenue |
|
|
5,211 |
|
|
|
7,714 |
|
Manufacturing warranty obligation |
|
|
513 |
|
|
|
973 |
|
Current portion of long-term operating lease |
|
|
1,572 |
|
|
|
1,005 |
|
Advance payments from customers |
|
|
10,562 |
|
|
|
6,255 |
|
Accrued expenses and other current liabilities |
|
|
9,935 |
|
|
|
8,631 |
|
Total current liabilities |
|
|
44,954 |
|
|
|
36,772 |
|
Notes payable - related party |
|
|
11,457 |
|
|
|
17,301 |
|
Non-current deferred revenues |
|
|
4,670 |
|
|
|
2,282 |
|
Long-term operating lease |
|
|
2,917 |
|
|
|
1,621 |
|
Deferred tax liabilities |
|
|
112 |
|
|
|
110 |
|
Other non-current liabilities |
|
|
4,171 |
|
|
|
2,647 |
|
Total liabilities |
|
$ |
68,281 |
|
|
$ |
60,733 |
|
Commitments and contingencies (Note 16) |
|
|
|
|
|
|
||
Deficit: |
|
|
|
|
|
|
||
Preferred Stock, $0.00001 par value; 10,000,000 shares authorized; no shares issued and outstanding at March 31, 2024; no shares authorized, issued and outstanding at March 31, 2023 |
|
|
— |
|
|
|
— |
|
Common stock, $0.0001 par value; 100,000,000 shares authorized; 34,544,935 shares issued and outstanding at March 31, 2024; 25,000,000 shares authorized, issued and outstanding at March 31, 2023 |
|
|
3 |
|
|
|
2 |
|
Additional paid-in-capital |
|
|
2,613 |
|
|
|
(1,898 |
) |
Accumulated deficit |
|
|
(35,212 |
) |
|
|
(26,769 |
) |
Accumulated other comprehensive income (loss) |
|
|
50 |
|
|
|
(289 |
) |
Total deficit |
|
$ |
(32,546 |
) |
|
$ |
(28,954 |
) |
Total liabilities and deficit |
|
$ |
35,735 |
|
|
$ |
31,779 |
|
The accompanying notes are an integral part of these consolidated financial statements.
48
DIH HOLDING US, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share data)
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Revenue |
|
$ |
64,473 |
|
|
$ |
54,059 |
|
Cost of sales |
|
|
34,702 |
|
|
|
23,474 |
|
Gross profit |
|
|
29,771 |
|
|
|
30,585 |
|
Operating expenses: |
|
|
|
|
|
|
||
Selling, general, and administrative expense |
|
|
25,776 |
|
|
|
22,957 |
|
Research and development |
|
|
6,609 |
|
|
|
6,919 |
|
Total operating expenses |
|
|
32,385 |
|
|
|
29,876 |
|
Operating income (loss) |
|
|
(2,614 |
) |
|
|
709 |
|
Other income (expense): |
|
|
|
|
|
|
||
Interest (expense) |
|
|
(693 |
) |
|
|
(277 |
) |
Other income (expense), net |
|
|
(3,890 |
) |
|
|
572 |
|
Total other income (expense) |
|
|
(4,583 |
) |
|
|
295 |
|
Income (loss) before income taxes |
|
|
(7,197 |
) |
|
|
1,004 |
|
Income tax expense |
|
|
1,246 |
|
|
|
2,018 |
|
Net loss |
|
$ |
(8,443 |
) |
|
$ |
(1,014 |
) |
|
|
|
|
|
|
|
||
Net loss per share, basic and diluted |
|
$ |
(0.32 |
) |
|
$ |
(0.04 |
) |
Weighted-average shares outstanding, basic and diluted |
|
|
26,382 |
|
|
|
25,000 |
|
The accompanying notes are an integral part of these consolidated financial statements.
49
DIH HOLDING US, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(in thousands)
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
2023 |
|
|||
Net loss |
|
$ |
(8,443 |
) |
|
$ |
(1,014 |
) |
Other comprehensive (loss) income, net of tax |
|
|
|
|
|
|
||
Foreign currency translation adjustments, net of tax of $0 and $0 |
|
|
1,455 |
|
|
|
(503 |
) |
Pension liability adjustments, net of tax of $0 and $0 |
|
|
(1,116 |
) |
|
|
(421 |
) |
Other comprehensive (loss) income |
|
|
339 |
|
|
|
(924 |
) |
Comprehensive loss |
|
$ |
(8,104 |
) |
|
$ |
(1,938 |
) |
See accompanying notes to the consolidated financial statements.
50
DIH HOLDING US, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(8,443 |
) |
|
$ |
(1,014 |
) |
Adjustments to reconcile net loss to net cash provided by operating activities: |
|
|
|
|
|
|
||
Depreciation and amortization |
|
|
302 |
|
|
|
66 |
|
Provision for credit losses |
|
|
(1,016 |
) |
|
|
669 |
|
Allowance for inventory obsolescence |
|
|
617 |
|
|
|
(1,639 |
) |
Noncash business combination expense |
|
|
3,514 |
|
|
|
- |
|
Pension contributions |
|
|
(530 |
) |
|
|
(569 |
) |
Pension (income) expense |
|
|
(75 |
) |
|
|
(400 |
) |
Foreign exchange (gain) loss |
|
|
376 |
|
|
|
(584 |
) |
Noncash lease expense |
|
|
1,590 |
|
|
|
1,423 |
|
Noncash interest expense |
|
|
28 |
|
|
|
19 |
|
Change in manufacturing warranty obligation estimate |
|
|
(626 |
) |
|
|
— |
|
Deferred and other noncash income tax expense |
|
|
(304 |
) |
|
|
58 |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
||
Accounts receivable |
|
|
1,853 |
|
|
|
(514 |
) |
Inventories |
|
|
(3,259 |
) |
|
|
518 |
|
Due from related parties |
|
|
1,018 |
|
|
|
(969 |
) |
Due to related parties |
|
|
3,337 |
|
|
|
2,471 |
|
Other assets |
|
|
(229 |
) |
|
|
(1,805 |
) |
Operating lease liabilities |
|
|
(1,782 |
) |
|
|
(1,448 |
) |
Accounts payable |
|
|
2,920 |
|
|
|
38 |
|
Employee compensation |
|
|
(551 |
) |
|
|
(151 |
) |
Other liabilities |
|
|
970 |
|
|
|
(96 |
) |
Deferred revenue |
|
|
(90 |
) |
|
|
4,059 |
|
Manufacturing warranty obligation |
|
|
163 |
|
|
|
160 |
|
Advance payments from customers |
|
|
4,338 |
|
|
|
2,083 |
|
Accrued expense and other current liabilities |
|
|
1,071 |
|
|
|
3,126 |
|
Net cash provided by operating activities |
|
|
5,192 |
|
|
|
5,501 |
|
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
(202 |
) |
|
|
(145 |
) |
Net cash used in investing activities |
|
|
(202 |
) |
|
|
(145 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
||
Proceeds from reverse recapitalization |
|
|
899 |
|
|
|
— |
|
Payments on related party notes payable |
|
|
(5,844 |
) |
|
|
(4,053 |
) |
Net cash used in financing activities |
|
|
(4,945 |
) |
|
|
(4,053 |
) |
Effect of currency translation on cash and cash equivalents |
|
|
5 |
|
|
|
(61 |
) |
Net increase in cash, and cash equivalents, and restricted cash |
|
|
50 |
|
|
|
1,242 |
|
Cash, and cash equivalents, and restricted cash - beginning of year |
|
|
3,175 |
|
|
|
1,933 |
|
Cash, and cash equivalents, and restricted cash - end of year |
|
$ |
3,225 |
|
|
$ |
3,175 |
|
Cash and cash equivalents - end of year |
|
$ |
3,225 |
|
|
$ |
3,175 |
|
Restricted cash - end of year |
|
|
— |
|
|
|
— |
|
Total cash, and cash equivalents, and restricted cash - end of year |
|
$ |
3,225 |
|
|
$ |
3,175 |
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
||
Interest paid |
|
$ |
665 |
|
|
$ |
258 |
|
Income tax paid |
|
$ |
— |
|
|
$ |
210 |
|
Supplemental disclosure of non-cash investing and financing activity: |
|
|
|
|
|
|
||
Accrued liability related to asset acquisition |
|
$ |
— |
|
|
$ |
533 |
|
Accounts payable settled through escrow account upon reverse recapitalization |
|
$ |
1,439 |
|
|
$ |
— |
|
The accompanying notes are an integral part of these consolidated financial statements.
51
DIH HOLDING US, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
(in thousands, except share data)
|
Common Stock |
|
|
|
|
|
|
|
|
|
||||||||
|
Shares(1) |
|
Amount |
|
Additional Paid-In Capital |
|
Accumulated Deficit |
|
Accumulated Other Comprehensive Income (Loss) |
|
Total Equity (Deficit) |
|
||||||
Balance, March 31, 2022 |
|
25,000,000 |
|
$ |
2 |
|
$ |
(1,776 |
) |
$ |
(25,755 |
) |
$ |
635 |
|
$ |
(26,894 |
) |
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(1,014 |
) |
|
— |
|
|
(1,014 |
) |
Other comprehensive loss, net of tax |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(924 |
) |
|
(924 |
) |
Net transactions with DIH Cayman |
|
— |
|
|
— |
|
|
(122 |
) |
|
— |
|
|
— |
|
|
(122 |
) |
Balance, March 31, 2023 |
|
25,000,000 |
|
$ |
2 |
|
$ |
(1,898 |
) |
$ |
(26,769 |
) |
$ |
(289 |
) |
$ |
(28,954 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Net loss |
|
— |
|
|
— |
|
|
— |
|
|
(8,443 |
) |
|
— |
|
|
(8,443 |
) |
Issuance of common stock upon reverse recapitalization |
|
9,544,935 |
|
|
1 |
|
|
4,511 |
|
|
— |
|
|
— |
|
|
4,512 |
|
Other comprehensive income, net of tax |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
339 |
|
|
339 |
|
Balance, March 31, 2024 |
|
34,544,935 |
|
$ |
3 |
|
$ |
2,613 |
|
$ |
(35,212 |
) |
$ |
50 |
|
$ |
(32,546 |
) |
(1). All outstanding share and per-share amounts have been restated to reflect the reverse recapitalization as established in the Business Combination Agreement as described in Note 1.
The accompanying notes are an integral part of these consolidated financial statements.
52
DIH HOLDING US, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Description of Business
DIH Holding US, Inc. and its consolidated subsidiaries (the “Company” or “DIH”) (formerly known as Aurora Technology Acquisition Corp. a Cayman Island exempted company which migrated and domesticated as a Delaware corporation, “ATAK”) , is a global solution provider in blending innovative robotic and virtual reality (“VR”) technologies with clinical integration and insights. Built through the mergers of global-leading niche technologies, DIH is positioning itself as a transformative total smart solutions provider and consolidator in a largely fragmented and manual-labor-driven industry. The Company’s fiscal year ends on March 31.
Merger / Business Combination with Aurora Tech Acquisition Corp.
On February 7, 2024 (the "Closing Date"), ATAK, Aurora Technology Merger Sub (“Merger Sub”) and DIH Holding US, Inc., a Nevada corporation (“Legacy DIH” or "DIH Nevada") consummated a previously announced business combination pursuant to a business agreement dated as of February 26, 2023 (as amended, supplemented or otherwise modified from time to time, the “Business Combination Agreement,” and the transactions contemplated thereby, the “Business Combination”) following the receipt of the required approval by ATAK’s and DIH (Nevada)’s shareholders and the fulfillment or waiver of other customary closing conditions. Upon closing of the Business Combination, Legacy DIH received cash held in trust account of $899. Legacy DIH historically existed and functioned as part of the business of DIH Technology Ltd. (“DIH Cayman”). At Closing of the Business Combination, the Company owns 100% of DIH US Corp, which in turn owns the commercial entities. Additionally, the Company owns 100% ownership of Hocoma Medical GmbH, which contains the net assets transferred from Hocoma AG. Whereas, Hocoma AG and Motekforce Link BV and its subsidiaries ("Motek Group") that remained with the DIH Cayman were excluded as discussed in Note 13 to the Consolidated Financial Statements. The Company agreed to use its best efforts to complete the reorganization as defined in the Business Combination Agreement as soon as possible thereafter. The reorganization has not been completed as of the date the financial statements were issued.
In connection with the Closing of the Business Combination, (a) ATAK migrated and changed its domestication to become a Delaware corporation and changed its name to “DIH Holding US, Inc.” (b) each issued and outstanding ATAK Class A Ordinary Share was converted, on a one-for-one basis, into one share of DIH Class A Common Stock; (c) each issued and outstanding Class B Ordinary Share was converted, on a one-for-one basis, into one share of Domesticated Class B Common Stock; (d) each issued and outstanding ATAK Public Warrant, ATAK Private Warrant and ATAK Right was converted, on a one-for-one basis, into a DIH Public Warrant, DIH Private Warrant and DIH Right, respectively; and (e) the governing documents of ATAK were replaced by governing documents for the Delaware corporation. The Amended and Restated Certificate of Incorporation authorizes one class of common stock as Class A Common Stock ("Common Stock").
On the Closing date, (a) Stockholders of Legacy DIH received $250,000,000 in aggregate consideration (the “Aggregate Base Consideration”) in the form of newly-issued shares of DIH Common Stock, calculated based on a price of $10.00 per share; (b) DIH’s financial advisor received 700,000 shares of DIH Common Stock valued at the closing price of $5.02 as payment for the financial advisory fee due to it; (c) the 20,200,000 outstanding DIH Rights were converted into 2,020,000 shares of DIH Common Stock; (d) each outstanding share of DIH Class B Common Stock was converted into a share of DIH Common Stock. (e) in connection with the closing of the Business combination, additional 532,796 shares were issued to various ATAK service providers, including ATAK's underwriter, for services rendered in related to the transaction. The shares were issued as partial payments to those providers, whereas certain service providers forewent all or partial receipt of Common Stock.
In addition to the Aggregate Base Consideration, Legacy DIH stockholders as of the effective date of the merger may be entitled to receive up to 6,000,000 Earnout Shares, as additional consideration upon satisfaction of the following milestones, during the period beginning on the Closing Date and expiring on the fifth anniversary of the closing date (the “Earnout Period”):
53
The Earnout Founder Shares are accounted for as equity-classified equity instruments and recorded in additional paid-in capital as part of the Business Combination.
On February 8, 2024, the Company entered into a subscription agreement with OrbiMed, an existing shareholder of DIH Cayman. Pursuant to the agreement, the Company will issue 150,000 shares of Common Stock at a purchase price of $10.00 per share for aggregate purchase price of $1.5 million together with warrants to purchase an additional 300,000 shares of DIH Common Stock with an exercise price of $10.00. The transaction is not closed as of the date the financial statements were issued.
The Business Combination was accounted for as a reverse recapitalization, in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under this method of accounting, ATAK was treated as the acquired company and Legacy DIH was treated as the acquirer for financial reporting purposes. The net assets of ATAK were stated at carrying value, with no goodwill or other intangible assets recorded. The consolidated and combined assets, liabilities and results of operations prior to the Business Combination are those of Legacy DIH and the assets, liabilities and results of operations of ATAK were consolidated with Legacy DIH beginning on the Closing Date. The shares and net loss per common share prior to the Business Combination have been retrospectively restated as shares reflecting the 25.0 million shares issued to the Legacy DIH shareholders pursuant to the Business Combination Agreement. Legacy DIH was determined to be the accounting acquirer based on evaluation of the following facts and circumstance:
Liquidity and Capital Resources
As of March 31, 2024, the Company had $3,225 in cash and cash equivalents. The Company’s sources of liquidity have been predominantly from proceeds received from product sales and services provided. The Company’s sources of liquidity have enabled the Company to expand the installation base and grow its market share.
The Company’s net losses began in 2020 and continued through the twelve months ended March 31, 2024. The Company’s historical operating losses resulted in an accumulated deficit of $35.2 million as of March 31, 2024. Operating losses were mainly driven by decreased sales during the COVID-19 pandemic due to social distancing measures that affected demand for rehabilitation services, increased expenditures in connection with its implementation of a new financial system (Oracle) and increased compliance costs associated with the European Union Medical Device Regulation (EU MDR). Additionally, DIH had elevated costs related to efforts of adopting to public company standards. During the year ended March 31, 2024, the Company had positive cash flows from operating activities and negative operating results. The Company continues to take steps to streamline its organization and cost structure as well as improve future revenue growth.
54
The Company’s gross revenue has increased by 19.3%, from $54,059 to $64,473, for the year ended March 31, 2023 and 2024, respectively. The Company plans to continue to fund its growth through cash flows from operations and future debt and equity financing. The Company believes that its current cash and cash equivalents, together with cash provided by operating activities will provide adequate liquidity through one year from the date that these consolidated financial statements are issued.
The Company has three notes payable to a related party which are included in "Notes payable - related party". Each note is due on June 30, 2026 with an interest rate of 1.25% as further discussed in Note 13 to the Consolidated Financial Statements. The Company has made periodic payments on the principal and interests on the notes payable historically.
The Company’s future liquidity needs may vary materially from those currently planned and will depend on many factors, including the more aggressive and expansive growth plan, or for any unforeseen reductions in demand.
Basis of Presentation
On February 7, 2024, the Company consummated the Business Combination and became a publicly-traded company and its financial statements are now presented on a consolidated basis. Prior to the Business Combination, the Company's historical financial statements were prepared on a combined basis derived from DIH Cayman in the registration statement.
In connection with the Closing of the Business Combination and in accordance with the terms of the Business Combination Agreement, ATAK agreed to waive the closing condition that the reorganization be completed prior to Closing. The Company has recast historical financial statements filed in the registration statements to exclude assets, liabilities and results of operations of entities that are not controlled by the Company as of March 31, 2024. Control exists when the Company has the power, directly and indirectly, to govern the financial and operating policies of an entity and be exposed to the variable returns from its activities. The financial statements for all periods presented, including historical periods prior to February 7, 2024, are now referred to as “Consolidated financial statements" and have been prepared in conformity with U.S. GAAP.
While the Company’s businesses have historically functioned together with the other businesses controlled by DIH Cayman, the Company’s businesses are largely isolated and not dependent on corporate or other support functions. DIH Cayman did not have significant corporate or operational activity and does not have shared services that it provides to its subsidiaries. The Company considered allocations from the DIH Cayman and its subsidiaries but they are insignificant because of the organizational structure such that the Company has been operating on a standalone basis historically.
As of March 31, 2023, legacy DIH and DIH International (“DIH Hong Kong”) were wholly owned subsidiaries of DIH Cayman. As of March 31, 2024, DIH Cayman remains the largest shareholder of the Company and continues to own 100% interest in DIH Hong Kong. Transactions with DIH Cayman, DIH Hong Kong and its subsidiaries are disclosed as related party transactions in Note 13.
All intercompany balances, transactions and profits are eliminated in consolidation.
Foreign Currency Reporting
The functional currency for the Company’s non-U.S. subsidiaries is their local currency. The assets and liabilities of foreign subsidiaries are translated into U.S. dollars using the exchange rate in effect as of the balance sheet date. Revenues and expenses are translated at the average exchange rates for each respective reporting period. Adjustments resulting from translating local currency financial statements into U.S. dollars are reflected in accumulated other comprehensive loss in equity (deficit).
Transactions denominated in currencies other than the functional currency are remeasured based on the exchange rates at the time of the transaction. Foreign currency gains and losses arising primarily from changes in exchange rates on foreign currency denominated intercompany transactions and balances between foreign locations are recorded in the consolidated statements of operations.
55
Realized and unrealized gains (losses) resulting from transactions conducted in foreign currencies for the years ended March 31, 2024 and 2023 were $(376) and $584, respectively.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenue and expenses during the reporting period. Significant estimates made by management in connection with the preparation of the accompanying consolidated financial statements include the useful lives of long-lived assets, inventory valuations, the allocation of transaction price among various performance obligations, valuation of securities, the allowance for credit losses, the fair value of financial assets, liabilities, actuarial valuation of pensions and realizability of deferred income tax asset or liabilities. Actual results could differ from those estimates.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to credit risk primarily consists of cash and cash equivalents and accounts receivable. The Company maintains its cash and cash equivalents with highly-rated financial institutions and limits the amount of credit exposure to any one entity. We believe we do not have any significant credit risk on our cash and cash equivalents. For accounts receivable, the Company is exposed to credit risk in the event of nonpayment by customers which is limited to the amounts recorded on the consolidated balance sheets. The risk associated with this concentration is mitigated by prepayment arrangement and our ongoing credit-review procedures and letters of credit or payment prior to shipment.
Major customers are defined as those individually comprising more than 10% of our trade accounts receivable or revenues. As of March 31, 2024, no customer represented more than 10% of total trade accounts receivables. As of March 31, 2023, one customer comprised 13.9% of total trade accounts receivables. For the year ended March 31, 2024, no customer comprised 10% of total revenue. For the year ended March 31, 2023, one customer comprised 12.0% of total revenue.
Revenue Recognition
Sales are recognized as the performance obligations to deliver products or services are satisfied and are recorded based on the amount of consideration the Company expects to receive in exchange for satisfying the performance obligations. The Company’s sales are recognized primarily when it transfers control to the customer, which can be on the date of shipment of the product, the date of receipt of the product by the customer or upon completion of any required product installation service depending on the terms of the sales contracts and product shipping terms. If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based upon a relative standalone selling price and recognizes the related revenue when or as control of each individual performance obligation is transferred to customers. The Company does not assess whether promised goods or services are performance obligations if they are immaterial in the context of the contract with the customer. Sales represent the amount of consideration the Company expects to receive from customers in exchange for transferring products and services. Net sales exclude sales tax, value added and other taxes the Company collects from customers. Sales for extended warranties are deferred and recognized as revenue on a straight-line basis over the warranty period. The Company extends terms of payment to its customers based on commercially reasonable terms for the markets of its customers, while also considering their credit quality. Shipping and handling costs charged to customers are included in net sales.
Certain of the Company’s products are sold through distributors and third-party sales representatives under standard agreements whereby distributors purchase products from the Company and resell them to customers. These arrangements do not provide stock rotation or price protection rights and do not contain extended payment terms. Rights of return are limited to repair or replacement of delivered products that are defective or fail to meet the Company’s published specifications. Provisions for these warranty costs are recognized in the same period that the related revenue is recorded similar to other assurance-type warranties.
56
Deferred revenue primarily represents service contracts and equipment maintenance, for which consideration is received in advance of when service for the device or equipment is provided. Revenue related to services contracts and equipment maintenance is recognized over the service period as time elapses. Revenues related to products containing an installation clause, are recognized once the item is confirmed installed. See Note 3 for further information on the Company’s deferred revenue balances and remaining performance obligations.
Revenues exclude any taxes that the Company collects from customers and remits to tax authorities. Amounts billed to the customer for shipping and handling are included in revenue, while the related shipping and handling costs are reflected in cost of sales in the period in which revenue is recognized. The Company has elected a practical expedient under ASC 606 that allows for shipping and handling activities that occur after the customer has obtained control of a good to be accounted for as a fulfillment cost. The Company does not adjust the promised amount of consideration for the effects of a significant financing component, if, at contract inception, the Company expects the period between the time when the Company transfers a promised good or service to the customer and the time when the customer pays for that good or service will be one year or less.
The Company exercises judgment in determining the timing of revenue by analyzing the point in time or the period over which the customer has the ability to direct the use of and obtain substantially all of the remaining benefits of the performance obligation. The Company primarily recognizes revenue from sales of products at the point in time that the customer obtains control, which is typically based upon the terms of delivery. The billing terms for these point-in time product contracts generally coincide with delivery to the customer and customer acceptance. When the Company receives customer advances, these are recognized as advance payments from customers in the consolidated balance sheet. The Company recognizes revenue from the sale of certain service contracts over time on a ratable basis consistent with the nature, timing and extent of services, which primarily relate to extended warranties. Our billing terms for these contracts vary and can occur in advance of or following the service period of service. The differences between the timing of our revenue recognized and customer billings (based on contractual terms) result in changes to our contract asset or contract liability positions.
Warranties
The Company generally provides warranties for its products from manufacturing defects on a limited basis for a period of one year after purchase, but also has extended warranties that are separately priced for periods of up to four years. During the term of the warranty, if the device fails to operate properly from defects in materials and workmanship, the Company will fix or replace the defective product. If the customer does not allow the required scheduled maintenance of the product during the extended warranty contract terms, the contract is canceled.
The company estimates the costs that it may incur under its warranty programs based on the number of units sold, historical and anticipated rates of warranty claims, and cost per claim, and records a liability equal to these estimated costs in cost of sales. The company assesses the adequacy of its recorded warranty liabilities on a quarterly basis and adjusts these amounts as necessary
A reconciliation of the changes in manufacturing warranty obligation is as follows:
|
|
Years Ended March 31, |
|
||||
|
|
2024 |
|
2023 |
|
||
Balance as of beginning of period |
|
$ |
973 |
|
$ |
836 |
|
Current-year provisions |
|
|
1,139 |
|
|
973 |
|
Reductions for settlements |
|
|
(973 |
) |
|
(836 |
) |
Adjustments related to changes in estimates |
|
|
(626 |
) |
|
- |
|
Balance as of end of period |
|
$ |
513 |
|
$ |
973 |
|
Cost of Sales
Cost of sales is comprised of direct materials and supplies consumed in the manufacture of products, as well as manufacturing labor, depreciation expense and direct overhead expense necessary to acquire and convert the purchased materials and supplies into finished product. Cost of sales also includes the cost to distribute products to customers, inbound freight costs, warehousing costs and other shipping and handling activity, excluding shipping and handling to customers.
57
Cost of service is comprised primarily of employee wages, benefits and related personnel expenses of our technical support team, our professional consulting personnel, and our training teams. It also includes costs related to travel and other associated expenses, as well as material and supplies consumed in providing services.
Selling, General and Administrative Expenses
Selling, general and administrative expense is comprised personnel related expenses for DIH’s sales and corporate functions and expenses for outside professional services as well as expenses for facilities, overhead, depreciation, amortization, and marketing costs.
Research and Development
Research and development costs are expensed when incurred except for production stage software research and development costs. Research and development costs include costs of research, engineering, and technical activities to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include pre-approval regulatory and clinical trial expenses.
Accounts Receivable, net
Accounts receivable, net in the accompanying consolidated balance sheets are presented net of allowances for credit losses. The Company performs evaluations of its customers’ financial condition and, generally, requires no collateral from its customers. The standard terms and conditions include provisions of prepayments of up to 100% of the contract value prior to shipping the product to the customer. The Company evaluates the collectability of its accounts receivable based upon several factors, including historical experience, the likelihood of payment from its customers, and any other known specific factors associated with its customers. Allowances are made based upon a specific review of aged invoices as well as a review of the overall quality and age of those invoices not specifically reviewed. Each period, the allowance for credit losses is adjusted through earnings to reflect expected credit losses over the remaining lives of the assets. Uncollectible accounts are written-off against the allowance when it is deemed that a customer account is uncollectible.
The decrease in Accounts Receivable related to the application of the Current Expected Credit Loss (CECL) methodology is primarily due to the more forward-looking and comprehensive approach to estimating credit losses under CECL compared to the previous incurred loss model.
The following table presents the allowance for credit loss and the changes therein:
Balance as of March 31, 2023 |
|
$ |
1,683 |
|
CECL implementation |
|
|
(547 |
) |
Recoveries |
|
|
(704 |
) |
Credit loss expense |
|
|
279 |
|
Write-offs |
|
|
(44 |
) |
Balance as of March 31, 2024 |
|
$ |
667 |
|
Fair Value Measurements
The Company uses any of three valuation approaches to measure fair value: the market approach, the income approach, and the cost approach in determining the appropriate valuation methodologies based on the nature of the asset or liability being measured and the reliability of the inputs used in arriving at fair value.
The Company’s financial instruments consist primarily of cash and cash equivalents, accounts receivable, accounts payable, long-term related party notes payable, accrued expenses and other current liabilities, and accrued employee benefits. The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and other current liabilities, and accrued employee benefits are representative of their respective fair values due to the short-term maturity of these instruments.
58
The Company's related party notes payable are due within two years and is classified as noncurrent in the consolidated balance sheet and the Company makes regular prepayments historically prior to the due date. Therefore the Company's related party notes payable's carrying value approximate the fair value due to the remaining duration.
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. These fair value measurements incorporate nonperformance risk (i.e., the risk that an obligation will not be fulfilled). In measuring fair value, the Company reflects the impact of credit risk on liabilities, as well as any collateral. The Company also considers the credit standing of counterparties in measuring the fair value of assets.
The Company follows the provisions of ASC 820, Fair Value Measurements (“ASC 820”) for non-financial assets and liabilities measured on a non-recurring basis such as on a potential impairment loss related to long-lived assets and assets and liabilities acquired in a business combination.
The framework for measuring fair value provides a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements).
The three levels of the valuation hierarchy are defined as follows:
A financial instrument’s categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability.
Cash and Cash Equivalents
The Company considers all highly liquid investments that are readily convertible into cash and have an original maturity of three months or less at the time of purchase to be cash equivalents.
Inventories, net
Inventories are stated at the lower of cost or net realizable value, with cost determined on a weighted average cost basis. The Company reduces the carrying value of inventories for items that are potentially excess, obsolete, or slow-moving based on changes in customer demand, technology developments, or other economic factors. These reserves are included within the raw materials and spare parts, work in process, and finished and semi-finished goods accounts.
Inventory costs for manufactured products consist primarily of direct labor and materials (including salary and fringe benefits, raw materials, and supplies) and indirect costs (including allocations of costs from departments that support manufacturing activities and facility allocations). The allocation of fixed production overhead costs is based on actual production levels, to the extent that they are within the range of the facility’s normal capacity. Inventory costs for products purchased for resale or manufactured under contract consist primarily of the purchase cost, freight-in charges, and indirect costs as appropriate.
59
The Company regularly evaluates its inventory to determine if the costs are appropriately recorded at the lower of cost or market value. Lower of cost or market value write-downs are recorded if the book value exceeds the estimated net realizable value of the inventory, based on recent sales prices at the time of the evaluation.
Property and Equipment, Net
Property and equipment are stated at cost and depreciated over the useful lives of the assets using the straight- line method except for leasehold improvements which are depreciated over the shorter of the useful life or the lease term. Useful lives by asset category are as follows:
|
Years |
Computer software and hardware |
3 years |
Machinery and equipment |
5-10 years |
Vehicles |
5 years |
Furniture and fixtures |
3-5 years |
Leasehold improvements |
Shorter of remaining lease term or estimated useful life |
Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. When assets are retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss are reflected in the accompanying consolidated statements of operations for the period.
Capitalized software, net
Software development costs are capitalized in accordance with ASC 350-40, Internal Use Software Accounting and Capitalization. Software development costs related to preliminary project activities and post-implementation and maintenance activities are expensed as incurred. Direct costs related to application development activities that are probable to result in additional functionality are capitalized. Capitalized software development costs are amortized using the straight-line amortization method over the estimated useful life of the applicable software, 5 years, from which the expected benefit will be derived.
Other intangible assets, net
Costs associated with the acquisition of patent and technology related intangibles are capitalized and amortized using the straight-line method over the estimated useful life of 10 years, from which the expected benefit will be derived.
Demonstration Units
The Company utilizes product demonstration units that are used to display the product’s capabilities and demonstrate how it works to potential customers or for other appropriate applications. The Company records and carries the cost of these demonstration units as either inventory or property and equipment depending on several factors including the nature of the product, length of time the units are in the field prior to being sold, and whether management’s intent is to sell the units. If the product demonstration units are classified as property and equipment, the balance will be carried net of accumulated depreciation.
Impairment of Long-Lived Assets, including intangible assets
Long-lived assets include acquired property and equipment, subject to amortization. The Company evaluates the recoverability of long-lived assets for possible impairment whenever events or changes in circumstances indicate that the related carrying amount may not be recoverable. Such events and changes may include significant changes in performance relative to expected operating results, significant changes in asset use, significant negative industry or economic trends, and changes in the Company’s business strategy. Recoverability is measured by a comparison of the carrying amount of an asset or asset group to the undiscounted future cash flows expected to be generated by the asset or asset group. When required, impairment losses on assets to be held and used are recognized based on the excess of the asset’s carrying amount over the fair value of the asset, while long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
60
Capitalized software costs and other intangible assets are tested for impairment whenever events or changes in circumstances that could impact recoverability occur.
For the years ended March 31, 2024 and 2023, the Company did not record any impairment losses.
Leases
The Company adopted the provisions of the Financial Accounting Standards Board ("FASB") Accounting Standards Codification ("ASC") 842 on April 1, 2021 using the modified retrospective approach and, as a result, did not restate prior periods. At the commencement of a contract, the Company determines if a contract meets the definition of a lease. A lease is a contract, or part of a contract, that conveys the right to control the use of identified property or equipment (an identified asset) for a period of time in exchange for consideration. The Company determines if the contract conveys the right to control the use of an identified asset for a period of time. The Company assesses throughout the period of use whether the Company has the following: (1) the right to obtain substantially all the economic benefits from use of the identified asset, and (2) the right to direct the use of the identified asset. This determination is reassessed if the terms of the contract are changed. Leases are classified as operating leases based on the terms of the lease agreement and certain characteristics of the identified asset. Right-of-use assets and lease liabilities are recognized at lease commencement date based on the present value of the minimum future lease payments. If the interest rate implicit in the Company’s leases is not readily determinable, in determining the weighted-average discount rate used to calculate the net present value of lease payments, the Company utilizes an estimate of its incremental borrowing rate.
The Company leases office space, vehicles and office equipment under operating leases. The Company has elected several practical expedients permitted under ASC 842. The Company has elected not to recognize right-of-use assets and liability for leases with a term of 12 months or less unless the lease includes an option to renew or purchase the underlying asset that are reasonably certain to be exercised. The Company has elected to account for lease and non-lease components as a single lease component for all of the Company's leases. The Company has elected to use hindsight relief in determining the lease term and assessing impairment of right-of-use assets when transitioning to ASC 842. The Company has elected to not re-evaluate existing or expired contracts containing a lease, the classification of leases, or the initial direct costs for any existing leases previously accounted for under ASC 840.
Most real estate leases contain clauses for renewal at the Company’s option with renewal terms that generally extend the lease term from six months to five years. Certain lease agreements contain options to purchase the leased property and options to terminate the lease. Payments to be made in option periods are recognized as part of the right-of-use lease assets and lease liabilities when it is reasonably certain that the option to extend the lease will be exercised or the option to terminate the lease will not be exercised or is not at the Company’s option. The Company determines whether the reasonably certain threshold is met by considering all relevant factors, including company-specific plans and economic outlook.
Contingencies
The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. Legal costs incurred in connection with loss contingencies are expensed as incurred.
Public and Private Placement Warrants
The Company assumed 20,200,000 warrants originally issued in ATAK's initial public offering (the “Public Warrants”) and 6,470,000 ATAK Private Placement Warrants. Each two warrants entitles the registered holder to purchase one share of Common Stock at a price of $11.50 per share, subject to adjustment
The Public Warrants are publicly traded and are exercisable for cash unless certain conditions occur, such as the failure to have an effective registration statement related to the shares issuable upon exercise or redemption by the Company under certain conditions, at which time the warrants may be cashless exercised at the option of the Company.
61
The Private Placement Warrants have terms and provisions that are identical to the Public Warrants except that the Private Placement Warrants holder can exercise their Private Placement Warrants for cash or on a cashless basis when the Company call the warrants for redemption at the option of private placement warrant holders and that the Private Placement Warrants were not transferable, assignable or salable until 30 days after the completion of the Business Combination.
The Company evaluated the Public and Private Placement Warrants under ASC 815-40, Derivatives and Hedging-Contracts in Entity’s Own Equity (“ASC 815-40”), and concluded they meet the criteria for equity classification as they are considered to be indexed to the Company’s own stock. Since the Public and Private Placement Warrants met the criteria for equity classification upon the consummation of the Business Combination, the Company recorded these warrants in additional paid-in capital as part of the Business Combination.
Segment Information
The Company operates in one operating and reportable segment. Operating segments are defined as components of an enterprise for which separate financial information is evaluated regularly by the chief operating decision maker (“CODM”), in deciding how to allocate resources and assess performance. The Company’s Chief Executive Officer is the Company’s CODM. The CODM reviews revenue at the geographic region level, and gross profit, operating income and expenses, and net income at the Company wide level to allocate resources and assess the Company’s overall performance. Accordingly, decision-making regarding the Company’s overall operating performance and allocation of Company resources is assessed on an aggregate basis.
Defined Benefit Plan
The Company sponsors defined a benefit pension plan (“pension plan”) for certain employees and retirees. The Company recognizes the funded status of its pension plan on the consolidated balance sheets based on the year-end measurements of plan assets and benefit obligations. When the fair value of plan assets is in excess of the plan benefit obligations, the amounts are reported in other current assets and other assets. When the fair value of plan benefit obligations is in excess of plan assets, the amounts are reported in accrued expenses and other long-term liabilities based on the amount by which the actuarial present value of benefits payable in the next twelve months included in the benefit obligation exceeds the fair value of plan assets.
Net periodic pension benefit cost/(income) is recorded in the consolidated statements of operations and includes service cost, interest cost, expected return on plan assets, amortization of prior service costs/(credits) and (gains) losses previously recognized as a component of other comprehensive income (loss) and amortization of the net transition asset remaining in accumulated other comprehensive income (loss). The service cost component of net benefit cost is recorded in selling, general and administrative in the consolidated statements of operations. The other components of net benefit cost are presented separately from service cost within other income (expense) in the consolidated statements of operations.
(Gains) losses and prior service costs/(credits) are recognized as a component of other comprehensive income (loss) in the consolidated statements of comprehensive loss as they arise. Those (gains) losses and prior service costs (credits) are subsequently recognized as a component of net periodic cost (income) pursuant to the recognition and amortization provisions of applicable accounting guidance. (Gains) losses arise as a result of differences between actual experience and assumptions or as a result of changes in actuarial assumptions. Prior service costs (credits) represent the cost of benefit changes attributable to prior service granted in plan amendments.
The measurement of benefit obligations and net periodic cost/(income) is based on estimates and assumptions approved by the company’s management. These valuations reflect the terms of the plans and use participant-specific information such as compensation, age, and years of service, as well as certain assumptions, including estimates of discount rates, expected return on plan assets, rate of compensation increases, interest crediting rates and mortality rates. See Note 14 for further information.
Acquisitions
In conjunction with each acquisition transaction, the Company determines if the acquisition meets the criteria to be accounted for as a business combination set forth in ASC 805, Business Combinations (“ASC 805”).
62
The Company evaluates the acquisition to assess whether or not the transaction should be accounted for as a business combination or asset acquisition by first applying a screen test to determine if substantially all of the fair value of the gross assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets. If the screen is met, the transaction is accounted for as an asset acquisition. If the screen is not met, further determination is required as to whether or not the Company has acquired inputs and processes that have the ability to create outputs which would meet the definition of a business.
If the transaction is determined not to be a business combination, it is accounted for as an asset acquisition. For asset acquisitions, the Company allocates the purchase price and other related costs incurred to the assets acquired and liabilities assumed based on recent independent appraisals and management judgment.
If the acquisition is determined to be a business combination, the Company records the fair value of acquired tangible assets and identified intangible assets and as well as any noncontrolling interest in accordance ASC 805. Any consideration paid in excess of the net fair value of the identifiable assets and liabilities acquired in a business combination is recorded to goodwill and acquisition-related costs are expensed as incurred.
In October 2022, DIH acquired the SafeGait 360 and SafeGait Active smart mobility trainer systems from Gorbel, an innovative United States-based developer and manufacturer of smart material handling and fall protection equipment. The SafeGait acquisition was accounted for as an asset acquisition based on an evaluation of the U.S. GAAP guidance for business combinations. The total cost of the asset acquisition was $0.8 million, of which $0.1 million was paid upon closing. The Company made subsequent payments of $0.2 million in the first quarter of the year ending March 31, 2024. These subsequent payments and the $0.5 million contingent consideration liability is presented within accrued expenses and other current liabilities in the consolidated balance sheet as of March 31, 2024. The Company determined that the contingent consideration was not subject to derivative accounting.
Income Taxes
Income taxes are accounted for under the asset-and-liability method. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities, as well as loss and tax credit carryforwards and their respective tax bases measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
A valuation allowance is established if, based upon the available evidence, it is more likely than not that some or all the deferred tax assets will not be realized. The Company considers all available evidence, both positive and negative, including historical levels of income, expectations and risks associated with estimates of future taxable income in assessing the need for a valuation allowance.
Deferred tax assets and deferred tax liabilities are presented as noncurrent in a classified balance sheet.
The Company’s tax positions are subject to income tax audits by multiple tax jurisdictions throughout the world. The Company recognizes the tax benefit of an uncertain tax position only if it is more likely than not the position will be sustainable upon examination by the taxing authority, including resolution of any related appeals or litigation processes. This evaluation is based on all available evidence and assumes that the tax authorities have full knowledge of all relevant information concerning the tax position. The tax benefit recognized is measured as the largest amount of benefit which is more likely than not (greater than 50% likely) to be realized upon ultimate settlement with the taxing authority. The Company recognizes interest accrued and penalties related to unrecognized tax benefits in income tax expense (benefit). The Company adjusts these reserves in accordance with the income tax guidance when facts and circumstances change, such as the closing of a tax audit or the refinement of an estimate. To the extent that the final tax outcome of these matters is different from the amounts recorded, such differences will affect the provision for income taxes in the period in which such determination is made and could have a material impact on the Company’s financial condition and operating results.
Under the Tax Cuts and Jobs Act, the Global Intangible Low-Taxed Income (“GILTI”) provisions impose a tax on foreign income in excess of a deemed return on tangible assets of foreign corporations. Under GAAP, companies are allowed to make an accounting policy election to either (i) account for GILTI as a period cost within income tax expense in the period in which it is incurred or (ii) account for GILTI in a company’s measurement of deferred taxes.
63
The Company elected to account for GILTI as a period cost.
Loss per share
Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding during the period. Diluted earnings (loss) per share is computed based on the sum of the weighted average number of common shares and potentially dilutive common shares outstanding during the period.
For periods prior to the closing of the Business Combination, basic and diluted income (loss) per share was calculated based on the 25.0 million shares issued to DIH Nevada's shareholders at the Closing Date.
Emerging Growth Company
The Company is an “emerging growth company,” as defined in Section 2(a) of the Securities Act, as modified by the JOBS Act, and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the independent registered public accounting firm attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is either not an emerging growth company or an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
Accounting Pronouncements Recently Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments ("ASC 326"). ASC 326 provides more decision-useful information about the expected credit losses on financial instruments, other commitments to extend credit held by a reporting entity at each reporting date, and requires the entity to estimate its credit losses as far as it can reasonably estimate. This update became effective for the Company on April 1, 2023. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In August 2020, the FASB issued ASU No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470- 20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current U.S. GAAP and simplifies the diluted earnings per share (“EPS”) calculation in certain areas. Under the new guidance there will be no separate accounting for embedded conversion features. It removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception. The amendments in this update are effective for the Company on April 1, 2024. Early adoption is permitted. We do not expect the adoption to have a material impact on our financial position or results of operations.
64
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. Update No. 2023-07 requires disclosure, on an annual and interim basis, of significant segment expenses that are regularly provided to the CODM and included within each reported measure of segment profit or loss in addition to disclosure of amounts for other segment items and a description of its composition. Update No. 2023-07 is effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. We are currently evaluating the impact of adopting ASU 2023-07.
In December 2023, the FASB issued ASU No. 2023-09 (“ASU 2023-09”), Income Taxes (Topic 740): Improvements to Income Tax Disclosures. ASU 2023-09addresses investor requests for more transparency about income tax information through improvements to income tax disclosures primarily related to the rate reconciliation and income taxes paid information. This update also includes certain other amendments to improve the effectiveness of income tax disclosures. The provisions of ASU 2023-09 are effective for annual periods beginning after December 15, 2024, with early adoption permitted. We are currently evaluating the impact of adopting ASU 2023-09.
The Company's revenues are derived from the sales of medical rehabilitation devices and technology services. The Company's primary customers include healthcare systems, clinics, third-party healthcare providers, distributors, and other institutions, including governmental healthcare programs and group purchasing organizations.
Disaggregation of Revenue
The Company disaggregates its revenue with customers by category and by geographic region based on customer location, see Note 4 for further information. The following represents the net revenue for the years ended March 31, 2024 and 2023, based on revenue category:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Devices |
|
$ |
51,125 |
|
|
$ |
43,452 |
|
Services |
|
|
11,105 |
|
|
|
9,292 |
|
Other |
|
|
2,243 |
|
|
|
1,315 |
|
Total revenue, net |
|
$ |
64,473 |
|
|
$ |
54,059 |
|
The revenue that is recognized at a point in time was primarily related to the revenues from devices and the revenue that is recognized over time was related to revenue from services. Other revenue primarily relates to freight and packaging on devices and recognized at a point in time.
Deferred Revenue and Remaining Performance Obligations
Deferred revenue as of March 31, 2024 and 2023 was $9,881 and $9,996, respectively. During the years ended March 31, 2024 and 2023, the Company recognized $7,405 and $5,358 of revenue that was included in deferred revenue as of March 31, 2023 and March 31, 2022, respectively. Remaining performance obligations include goods and services that have not yet been delivered or provided under existing, noncancelable contracts with minimum purchase commitments. As of March 31, 2024 and 2023, the aggregate amount of the contracted revenue allocated to unsatisfied performance obligations with an original duration of one year or more was approximately $4,670 and $2,698, respectively. As of March 31, 2024, the Company expects to recognize revenue on the majority of these remaining performance obligations over the next 2 years.
Advance Payments From Customers
The Company receives advance payments related to customers from their orders to support the operation of the company in the production of the goods. The Company recognizes these prepayments as a liability under “Advance payments from customers” on the consolidated balance sheets when they are received. Revenue associated with the advance payments is recognized when performance obligation is fulfilled. Advance payments from customers was $10.6 million and $6.3 million as of March 31, 2024 and 2023, respectively.
65
The following represents revenue attributed to geographic regions based on customer location:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Europe, Middle East and Africa (“EMEA”) |
|
$ |
36,002 |
|
|
$ |
31,454 |
|
Americas |
|
|
16,716 |
|
|
|
14,264 |
|
Asia Pacific (“APAC”) |
|
|
11,755 |
|
|
|
8,341 |
|
Total revenue |
|
$ |
64,473 |
|
|
$ |
54,059 |
|
Long-lived assets shown below include property and equipment, net. The following represents long-lived assets where they are physically located:
|
|
2024 |
|
|
2023 |
|
||
EMEA |
|
$ |
276 |
|
|
$ |
236 |
|
Americas |
|
|
206 |
|
|
|
390 |
|
APAC |
|
|
48 |
|
|
|
116 |
|
Total property and equipment, net |
|
$ |
530 |
|
|
$ |
742 |
|
Basic income (loss) per share is calculated by dividing net income (loss) by the weighted-average number of common shares outstanding during the period. Diluted income (loss) per share is computed based on the sum of the weighted average number of common shares and dilutive common shares outstanding during the period. As described in Note 1 - Business and Organization earnout shares issued in connection with the Business Combination are subject to vesting based on the volume weighted average trading prices (“VWAP”) of common shares during the earnout period. The earnout shares are excluded from the calculation of basic and diluted weighted-average number of common shares outstanding until vested. For periods prior to the Business Combination, basic and diluted loss per share was calculated based on the 25.0 million shares issued to Legacy DIH shareholders at the Closing Date. Potential shares of common stock are excluded from the computation of diluted net loss per share if their effect would have been anti-dilutive for the periods presented or if the issuance of shares is contingent upon events that did not occur by the end of the period.
As of March 31, 2024, there were 34,544,935 shares of Common Stock issued and outstanding, excluding earnout shares.
Computation of basic and diluted net loss per share for the years ended March 31, 2024 and 2023, is as follows (in thousands, except share and per share amounts):
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Net loss |
|
$ |
(8,443 |
) |
|
$ |
(1,014 |
) |
Weighted-average shares outstanding, basic and diluted |
|
|
26,382,190 |
|
|
|
25,000,000 |
|
Net loss per share – basic and diluted |
|
$ |
(0.32 |
) |
|
$ |
(0.04 |
) |
66
The following table outlines dilutive common share equivalents outstanding, which are excluded in the above diluted net loss per share calculation, as the effect of their inclusion would be anti-dilutive or the share equivalents were contingently issuable as of each period presented:
|
|
March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Earnout shares |
|
|
6,000,000 |
|
|
|
— |
|
Common Stock underlying Public Warrants |
|
|
10,100,000 |
|
|
|
— |
|
Common Stock underlying Private Placement Warrants |
|
|
3,235,000 |
|
|
|
— |
|
Total |
|
|
19,335,000 |
|
|
|
— |
|
As of March 31, 2024 and 2023, inventories, net, consisted of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Raw materials and spare parts |
|
$ |
3,882 |
|
|
$ |
4,619 |
|
Work in process |
|
|
4,769 |
|
|
|
1,105 |
|
Finished goods |
|
|
1,283 |
|
|
|
613 |
|
Less: reserves |
|
|
(2,104 |
) |
|
|
(1,487 |
) |
Total inventories, net |
|
$ |
7,830 |
|
|
$ |
4,850 |
|
Property and equipment, net as of March 31, 2024 and 2023 consisted of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Computer software and hardware |
|
$ |
849 |
|
|
$ |
802 |
|
Machinery and equipment |
|
|
807 |
|
|
|
661 |
|
Leasehold improvements |
|
|
1,357 |
|
|
|
1,249 |
|
Furniture and fixtures |
|
|
871 |
|
|
|
818 |
|
Vehicles |
|
|
70 |
|
|
|
55 |
|
Demonstration units |
|
|
222 |
|
|
|
466 |
|
Property and equipment |
|
|
4,176 |
|
|
|
4,051 |
|
Less: accumulated depreciation |
|
|
(3,646 |
) |
|
|
(3,309 |
) |
Property and equipment, net |
|
$ |
530 |
|
|
$ |
742 |
|
Depreciation expense totaled $302 and $66 for the years ended March 31, 2024 and 2023, respectively.
Capitalized software, net and other intangible assets, net as of March 31, 2024 and 2023 consisted of the following:
|
|
|
2024 |
|
|
2023 |
|
||||||||||||||||||
|
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net Carrying Amount |
|
|
Gross Carrying Amount |
|
|
Accumulated Amortization |
|
|
Net Carrying Amount |
|
||||||
Capitalized software |
$ |
2,131 |
|
|
$ |
— |
|
|
$ |
2,131 |
|
|
$ |
2,019 |
|
|
$ |
— |
|
|
$ |
2,019 |
|
||
Other intangible assets |
$ |
380 |
|
|
$ |
— |
|
|
$ |
380 |
|
|
$ |
380 |
|
|
$ |
— |
|
|
$ |
380 |
|
||
67
Other intangible assets include patent and technology related intangible assets of $380 acquired from the SafeGait asset acquisition discussed in Note 2, which represented non-cash investing activities for the year ended March 31, 2023. The weighted-average useful lives of these intangible assets are 10 years.
Capitalized software, net and other intangible assets, net are subject to amortization when they are available for their intended use. For the years ended March 31, 2024 and 2023, the Capitalized software, net and other intangible assets are not available for intended use and thus not amortized. The weighted-average useful life of capitalized software is 5 years.
Estimated annual amortization for intangible assets over the next five years are as follows:
|
|
|
2025 |
|
|
2026 |
|
|
2027 |
|
|
2028 |
|
|
2029 |
|
|||||
Estimated annual amortization |
$ |
90 |
|
|
$ |
464 |
|
|
$ |
464 |
|
|
$ |
464 |
|
|
$ |
464 |
|
||
Other current assets as of March 31, 2024 and 2023 consisted of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Deferred cost of sales |
|
$ |
3,754 |
|
|
$ |
3,505 |
|
Value added tax (“VAT”) receivable |
|
|
635 |
|
|
|
361 |
|
Advance payments |
|
|
414 |
|
|
|
726 |
|
Other current assets |
|
|
313 |
|
|
|
263 |
|
Total other current assets |
|
$ |
5,116 |
|
|
$ |
4,855 |
|
Accrued expenses and other current liabilities as of March 31, 2024 and 2023 consisted of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Taxes payable |
|
$ |
2,554 |
|
|
$ |
2,114 |
|
Other payables and current liabilities |
|
|
7,381 |
|
|
|
6,517 |
|
Total accrued expenses and other current liabilities |
|
$ |
9,935 |
|
|
$ |
8,631 |
|
Other non-current liabilities as of March 31, 2024 and 2023 consisted of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Provisions |
|
$ |
1,977 |
|
|
$ |
1,576 |
|
Pension liabilities |
|
|
2,194 |
|
|
|
1,071 |
|
Total other non-current liabilities |
|
$ |
4,171 |
|
|
$ |
2,647 |
|
68
Authorized and Outstanding Capital Stock
The authorized capital stock of the Company consists of 100,000,000 shares of Common Stock and 10,000,000 shares of preferred stock.
Common Stock
The Amended and Restated Certificate of Incorporation authorizes one class of common stock.
Holders of the Company’s common stock are entitled to one vote for each share held as of the record date for the determination of the shareholders entitled to vote on such matters, including the election and removal of directors, except as otherwise required by law. Under Delaware law, the right to vote cumulatively does not exist unless the certificate of incorporation specifically authorizes cumulative voting. The Company’s Amended and Restated Certificate of Incorporation does not authorize cumulative voting and provides that no shareholder is permitted to cumulate votes at any election of directors. Consequently, the holders of a majority of the outstanding shares of the Company’s common stock can elect all of the directors then standing for election, and the holders of the remaining shares are not able to elect any directors.
Subject to preferences that may apply to any shares of the Company’s preferred stock outstanding at the time, the holders of the Company’s common stock will be entitled to receive dividends out of funds legally available therefor if the Company’s board of directors, in its discretion, determines to authorize the issuance of dividends and then only at the times and in the amounts that the Company’s board of directors may determine. If the Company becomes subject to a liquidation, dissolution, or winding-up, the assets legally available for distribution to the Company’s shareholders would be distributable ratably among the holders of the Company’s common stock and any participating series of the Company’s preferred stock outstanding at that time, subject to prior satisfaction of all outstanding debt and liabilities and the preferential rights of, and the payment of any liquidation preferences on, any outstanding shares of the Company’s preferred stock.
Preferred Stock
Under the terms of our certificate of incorporation, our Board has the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the dividend, voting and other rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon, and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
The Company’s board of directors is able to authorize the issuance of the Company’s preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the Company’s common stock. The issuance of the Company’s preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring, or preventing a change in control of the Company and might adversely affect the market price of the Company’s common stock and the voting and other rights of the holders of the Company’s common stock. There are currently no plans to issue any shares of the Company’s preferred stock.
Earnout Shares
As described in Note 1 - Business and Organization earnout shares issued in connection with the Business Combination are subject to vesting based on the volume weighted average trading prices (“VWAP”) of common shares during the earnout period. If, upon the expiration of the Earnout Period, the vesting of any of the Earnout Shares has not occurred, then the applicable Earnout Shares that failed to vest shall terminate and no longer apply and the Company shall instruct the escrow agent to deliver the Earnout Shares applicable to such unachieved earnout triggers to the Company for cancellation.
Warrants
Each two warrants entitles the registered holder to purchase one share of Common Stock at a price of $11.50 per share, subject to adjustment as discussed below. Because the warrants may only be exercised for whole numbers of Common Stock, only an even number of warrants may be exercised at any given time by a warrant holder.
69
The warrants will expire five years after the completion of our initial business combination, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
Additionally, once the Public Warrants become exercisable, the Company can redeem the outstanding Public Warrants:
If the Company calls the Public Warrants for redemption as previously described, the Company has the option to require all holders that wish to exercise the Public Warrants to do so on a cashless basis.
Simultaneously with ATAK’s initial public offering, ATAK consummated a private placement of 6,470,000 Private Placement Warrants with ATAK’s sponsor. Each two Private Placement Warrants is exercisable for one share of common stock at a price of $11.50 per share, subject to adjustment. The Private Placement Warrants have terms and provisions that are identical to those of the Public Warrants except that the Private Placement Warrants holder can exercise their Private Placement Warrants for cash or on a cashless basis when the Company call the warrants for redemption at the option of private placement warrant holders the Private Placement Warrants were not transferable, assignable or salable until 30 days after the completion of the Business Combination.
Parties are considered related to the Company if the parties, directly or indirectly, through one or more intermediaries, control, are controlled by, or are under common control with the Company. Related parties also include principal owners of the Company, its management, members of the immediate families of principal owners of the Company and its management and other parties with which the Company may deal with if one party controls or can significantly influence the management or operating policies of the other to an extent that one of the transacting parties might be prevented from fully pursuing its own separate interests. The Company discloses all related party transactions.
Reorganization and Transaction with DIH Cayman and DIH Hong Kong
While the Company’s businesses have historically functioned together with the other businesses controlled by DIH Cayman, the Company’s businesses are largely isolated and not dependent on corporate or other support functions. DIH Hong Kong is a wholly-owned subsidiary of DIH Cayman and the Company was a wholly-owned subsidiary of DIH Cayman prior to closing of the Business Combination.
On July 1, 2021, DIH Cayman completed a series of reorganization steps to transfer DIH US Corp and its subsidiaries and Hocoma Medical GmbH from Hocoma AG to DIH Holding US Inc., Nevada, effectively creating the Company as explained in the Hocoma AG and share transfers section below. The reorganization was accounted for as a common control transaction and the assets contributed and liabilities assumed were recorded based on their historical carrying values.
Subsequent to the year ended March 31, 2022, the Company did not incur significant transactions with DIH Cayman or DIH Hong Kong. The balances recorded under "Due from relate party" and "Due to related party" are derived from historical transactions. The table below summarizes related party balances with DIH Hong Kong excluding Hocoma AG and Motek as of March 31, 2024 and 2023 On July 1, 2021, Hocoma AG entered into a series of agreements with the Company and its subsidiaries to transfer all business aspects of development and production of mechanical and electronic devices in the fields of medical technology and biotechnology to Hocoma Medical GmbH.
70
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Due from related party |
|
$ |
2,586 |
|
|
$ |
2,456 |
|
Due to related party |
|
$ |
1,470 |
|
|
$ |
1,311 |
|
Hocoma AG and share transfers
Between July 2021 and January 2024, Hocoma AG operated as a single entity, with all business operations conducted at Hocoma AG while all personnel, except for two employees managing the MDR certification, were employed by Hocoma Medical. The EU MDR 2017/745 came into effect in May 2021. All medical devices certified under the previous Medical Device Directive (MDD) must certify to the new requirements to ensure that they can continue to be sold in the European market. Hocoma AG holds the MDR certification, which cannot legally be transferred to Hocoma Medical. Upon the lifting of the injunction, management performed a retrospective separation of these entities to account for the original transactions reinstated by the court.
Transfer ownership of DIH US Corp to DIH Nevada:
Hocoma AG and DIH Nevada entered into a share purchase agreement effective on July 1, 2021, in which Hocoma AG agreed to sell all 10,000 shares of DIH US Corp and intercompany balances totaling $7.80 million between DIH US Corp and Hocoma AG to DIH Nevada. The purchase price was settled through a Note Agreement accruing interest at a rate of 1.25% annually (“Share Purchase Note”). The note has a term of 5 years, due on June 30, 2026, with prepayment allowed.
Contribution net assets to Hocoma Medical:
In a Contribution Agreement effective on July 1, 2021, Hocoma AG agreed to contribute its business to Hocoma Medical GmbH. The contributed business was valued at USD 10.47 million as amended where Hocoma Medical GmbH was a wholly owned subsidiary of Hocoma AG at the time. The Contribution Agreement explicitly excluded the intellectual property rights specified in the Contribution Agreement. Additionally, the assets excluded all 10,000 shares of DIH US Corporation and certain intercompany balances. The Agreement specifically excludes from these liabilities all indebtedness of Hocoma AG related to the contributed business as of the effective date, as well as any liability for taxes relating to the contributed business as of the effective date.
Transfer of ownership in Hocoma Medical to DIH Nevada:
Under a separate Share Purchase Agreement effective on July 1, 2021, Hocoma AG transferred all ownership in Hocoma Medical GmbH in the form of 200 membership interests to DIH Nevada for $10.47 million, based on the final valuation. The purchase price was settled through a Note Agreement with an interest rate of 1.25% (“Membership Interest Note”). The note was agreed for a term of 5 years, due on June 30, 2026, with prepayment allowed.
Transfer of intellectual property to DIH US Corp:
In a business/asset, share, and IP purchase agreement on July 12, 2021, which was amended on August 3, 2021 Hocoma AG transferred intellectual property rights as listed in the Annex to the agreement to DIH Technology Inc. (a wholly owned subsidiary of DIH US Corp) for $1.57 million through a note agreement. The note payable formalized in a note agreement effective July 1, 2021, with an interest rate of 1.25% (“IP Note”). The note was agreed for a term of 5 years, due on June 30, 2026, with prepayment allowed.
The Share Purchase Note, Membership Interest Note and IP Note together are referred to as "Related Party Notes".
Hocoma Medical GmbH has made periodically payments on the principal and interests of the Related Party Notes, resulting from the transfer of the business and assets above.
Additionally, the two employees who remained at Hocoma AG provided services for the business of Hocoma Medical. Historically, an immaterial premium was charged to the cost of the employees.
71
As of March 31, 2024 and 2023, the balances of Related Party Notes were $11,457 and $17,301, respectively included in Note payable - related party". The decrease resulted from the Company's payments of principal on Related Party Notes owed to Hocoma AG.
In addition to the Related Party Notes, as of March 31, 2024 and 2023, the Company recorded a related party balance of $(267) and $1,992, respectively, representing cash balances owed by Hocoma AG. As part of the transfer discussed above, the Company also recorded a long-term related party receivable for $324 as of March 31, 2024 and 2023, included in "Other assets".
Motek Group
The Company has entered into a distribution agreement with the Motek Group. The agreement, which has been historically in place, appoints the Company as the exclusive distributor of Motek's advanced human movement research and rehabilitation products and services designed to support efficient functional movement therapy within specified territories. Under the distribution agreement, Motek supplies the products and services to the Company at the prices detailed in the agreement, with the Company entitled to a distributor margin. Motek provides ongoing support and assistance, including training, marketing materials, and technical documentation to the Company.
For the years ended March 31, 2024 and 2023, the Company made purchases amounting to $13,599 and $11,869, respectively, from the Motek Group.
As part of these transactions, the Company made advance payments to Motek, included in "Due from related party," and also had trade payables, included in "Due to related party." The balances as of March 31, 2024 and 2023 are as follows:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Due from related party |
|
$ |
3,367 |
|
|
$ |
1,934 |
|
Due to related party |
|
$ |
8,667 |
|
|
$ |
5,530 |
|
Defined Contribution Plans
The Company sponsors a defined contribution plan in the United States. The Company’s obligation is limited to its contributions made in accordance with each plan document. Employer contributions to defined contribution plans are recognized as expense. Expenses related to the Company’s plans for the years ended March 31, 2024 and 2023 were $119 and $105, respectively.
Defined Benefit Plans
The Company has a Swiss defined benefit plans (the “Pension Plan”) covering substantially all the employees of Hocoma Medical GmbH in Switzerland. The Pension Plan exceed the minimum benefit requirements under Swiss pension law. The Swiss plans offer retirement, disability and survivor benefits and is governed by a Pension Foundation Board. The responsibilities of this board are defined by Swiss pension law and the plan rules.
The plans offer to members at the normal retirement age of 65 a choice between a lifetime pension and a partial or full lump sum payment. Participants can choose to draw early retirement benefits starting from the age of 58 but can also continue employment and remain active members of the plan until the age of 70. Employees can make additional purchases of benefits to fund early retirement benefits. The pension amount payable to a participant is calculated by applying a conversion rate to the accumulated balance of the participant’s retirement savings account at the retirement date. The balance is based on credited vested benefits transferred from previous employers, purchases of benefits, and the employee and employer contributions that have been made to the participant’s retirement savings account, as well as the interest accrued. The annual interest rate credited to participants is determined by the Pension Foundation Board at the end of each year.
72
Although the Swiss plans are based on a defined contribution promise under Swiss pension law, it is accounted for as a defined benefit plan under GAAP, primarily because of the obligation to accrue interest on the participants’ retirement savings accounts and the payment of lifetime pension benefits.
An actuarial valuation in accordance with Swiss pension law is performed regularly. Should an underfunded situation on this basis occur, the Pension Foundation Board is required to take the necessary measures to ensure that full funding can be expected to be restored within a maximum period of 10 years. If a Swiss plan were to become significantly underfunded on a Swiss pension law basis, additional employer and employee contributions could be required.
The investment strategy of the Swiss plan complies with Swiss pension law, including the rules and regulations relating to diversification of plan assets, and is derived from the risk budget defined by the Pension Foundation Board on the basis of regularly performed asset and liability management analyses. The Pension Foundation Board strives for a medium- and long-term balance between assets and liabilities.
Amounts recognized in the consolidated statements of operations for the years ended March 31, 2024 and 2023, in respect of the Pension Plan were as follows:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Current service cost |
|
$ |
655 |
|
|
$ |
678 |
|
Interest cost |
|
|
213 |
|
|
|
129 |
|
Expected return on plan assets |
|
|
(296 |
) |
|
|
(194 |
) |
Actuarial loss / (gain) recognized |
|
|
(161 |
) |
|
|
(179 |
) |
Actuarial loss / (gain) recognized because of settlement |
|
|
(341 |
) |
|
|
(699 |
) |
Amortization of prior service credit |
|
|
(145 |
) |
|
|
(135 |
) |
Net charge to statement of operations |
|
$ |
(75 |
) |
|
$ |
(400 |
) |
Details of the employee defined benefits obligations and plan assets in respect of the Pension Plan are as follows:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Change in present value of defined benefit obligation: |
|
|
|
|
|
|
||
Defined benefit obligation at the beginning of the year |
|
$ |
9,337 |
|
|
$ |
9,500 |
|
Interest on defined obligation |
|
213 |
|
|
129 |
|
||
Current service cost |
|
655 |
|
|
678 |
|
||
Contributions by plan participants |
|
444 |
|
|
476 |
|
||
Translation (gain) loss |
|
|
534 |
|
|
|
(20 |
) |
Benefits paid |
|
|
(289 |
) |
|
|
(1,095 |
) |
Actuarial loss arising on projected benefit obligation |
|
|
118 |
|
|
|
(331 |
) |
Defined benefit obligation at the end of the year |
|
$ |
11,012 |
|
|
$ |
9,337 |
|
Change in plan assets: |
|
|
|
|
|
|
||
Fair value of plan assets at the beginning of the year |
|
$ |
7,761 |
|
|
$ |
7,353 |
|
Actual return on plan assets |
|
|
(68 |
) |
|
|
457 |
|
Contributions by the employer |
|
530 |
|
|
569 |
|
||
Contributions by plan participants |
|
444 |
|
|
476 |
|
||
Benefits paid |
|
|
(289 |
) |
|
|
(1,095 |
) |
Translation loss |
|
|
440 |
|
|
|
1 |
|
Fair value of plan assets - at the end of the year |
|
$ |
8,818 |
|
|
$ |
7,761 |
|
Funded status at end of the year |
|
$ |
(2,194 |
) |
|
$ |
(1,576 |
) |
Amounts relating to these defined benefit plans with accumulated benefit obligations in excess of plan assets were as follows:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Accumulated benefit obligation |
|
$ |
10,686 |
|
|
$ |
9,049 |
|
Fair value of plan assets |
|
$ |
8,818 |
|
|
$ |
7,761 |
|
73
Amounts recognized in the Company’s consolidated balance sheet related to the present value of defined benefit obligations consist of the following:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Current liabilities |
|
|
— |
|
|
|
— |
|
Non-current liabilities |
|
|
2,194 |
|
|
|
1,576 |
|
Total recognized in the consolidated balance sheet |
|
$ |
2,194 |
|
|
$ |
1,576 |
|
Amounts recorded in accumulated other comprehensive income (loss) in respect of the pension plan consist of the following:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Net gain (loss) |
|
$ |
1,633 |
|
|
$ |
2,610 |
|
Prior service (cost) credit |
|
837 |
|
|
976 |
|
||
Total recorded in accumulated other comprehensive income |
|
$ |
2,470 |
|
|
$ |
3,586 |
|
Amortization of prior service (cost) credit is recorded in selling, general and administrative in the consolidated statements of operations.
The principal assumptions used for the purpose of actuarial valuation of the pension plan are as follows:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Discount rate |
|
|
1.50 |
% |
|
|
2.10 |
% |
Expected return on plan assets |
|
|
3.50 |
% |
|
|
3.50 |
% |
Expected rate of salary increase |
|
|
1.00 |
% |
|
|
1.00 |
% |
The actuarial assumptions used for the defined benefit plans are based on the economic conditions prevailing in the jurisdiction in which they are offered. Changes in the defined benefit obligation are most sensitive to changes in the discount rate. The discount rate is based on the yield of high-quality corporate bonds quoted in an active market in the currency of the respective plan. A decrease in the discount rate increases the defined benefit obligation. The Company regularly reviews the actuarial assumptions used in calculating the defined benefit obligation to determine their continuing relevance.
Investment Policy
It is the objective of the plan sponsor to maintain an adequate level of diversification to balance market risk, to prudently invest to preserve capital and to provide sufficient liquidity while maximizing earnings for near-term payments of benefits accrued under the plan and to pay plan administrative expenses. The assumption used for the expected long-term rate of return on plan assets is based on the long-term expected returns for the investment mix of assets currently in the portfolio. Historical return trends for the various asset classes in the class portfolio are combined with current and anticipated future market conditions to estimate the rate of return for each class. These rates are then adjusted for anticipated future inflation to determine estimated nominal rates of return for each class.
The table below represents the Company's pension plan’ weighted-average asset allocation as of March 31, 2024 and 2023 by asset category:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Equity securities |
|
|
36.58 |
% |
|
|
33.99 |
% |
Debt securities |
|
|
28.16 |
% |
|
|
26.43 |
% |
Other, primarily cash and cash equivalents, senior loans and mutual funds |
|
|
35.26 |
% |
|
|
39.58 |
% |
74
The table below presents the target allocation for each major asset category for the Company's pension plan for the years ended March 31, 2024 and 2023:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Equity securities |
|
|
34.00 |
% |
|
|
34.00 |
% |
Debt securities |
|
|
28.50 |
% |
|
|
28.00 |
% |
Other, primarily cash and cash equivalents, senior loans and mutual funds |
|
|
37.50 |
% |
|
|
38.00 |
% |
The following tables provides the fair value of plan assets held by the Company’s pension plan by asset category and by fair value hierarchy level:
|
|
As of March 31, 2024 |
|
|||||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||||
Cash and cash equivalents |
|
$ |
106 |
|
|
$ |
— |
|
|
|
$ |
— |
|
|
|
$ |
106 |
|
Equity securities |
|
|
3,316 |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
3,316 |
|
Debt securities |
|
|
2,310 |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
2,310 |
|
Real estate |
|
|
— |
|
|
|
1,851 |
|
|
|
|
— |
|
|
|
|
1,851 |
|
Non-traditional assets |
|
|
— |
|
|
|
1,235 |
|
|
|
|
— |
|
|
|
|
1,235 |
|
Total |
|
$ |
5,732 |
|
|
$ |
3,086 |
|
|
|
$ |
— |
|
|
|
$ |
8,818 |
|
|
|
As of March 31, 2023 |
|
|||||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||||
Cash and cash equivalents |
|
$ |
186 |
|
|
|
— |
|
|
$ |
|
— |
|
|
|
|
186 |
|
Equity securities |
|
|
2,763 |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
2,763 |
|
Debt securities |
|
|
1,987 |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
1,987 |
|
Real estate |
|
|
— |
|
|
|
1,855 |
|
|
|
|
— |
|
|
|
|
1,855 |
|
Non-traditional assets |
|
|
— |
|
|
|
970 |
|
|
|
|
— |
|
|
|
|
970 |
|
Total |
|
$ |
4,936 |
|
|
$ |
2,825 |
|
|
|
$ |
— |
|
|
|
$ |
7,761 |
|
For the year ending March 31, 2025, the Company expects to contribute $652 to its pension plan.
The following table presents expected pension plan payments over the next 10 years:
|
|
Amount |
|
|
Year Ending March 31, |
|
|
|
|
2025 |
|
$ |
43 |
|
2026 |
|
|
252 |
|
2027 |
|
|
81 |
|
2028 |
|
|
88 |
|
2029 |
|
|
95 |
|
2030-2034 |
|
|
1,059 |
|
The components of loss before income tax for the years ended March 31, 2024 and 2023 were as follows:
|
Year ended March 31, |
|
|||||
|
2024 |
|
|
2023 |
|
||
U.S. operations |
$ |
(11,652 |
) |
|
$ |
(4,806 |
) |
Non-U.S. operations |
|
4,455 |
|
|
|
5,810 |
|
Total loss before income taxes |
$ |
(7,197 |
) |
|
$ |
1,004 |
|
75
The provision for income taxes during the years ended March 31, 2024 and 2023 consists of the following:
|
Year ended March 31, |
|
|||||
|
2024 |
|
|
2023 |
|
||
Current: |
|
|
|
|
|
||
State |
$ |
— |
|
|
$ |
— |
|
Federal |
|
— |
|
|
|
— |
|
Foreign |
|
347 |
|
|
|
1,435 |
|
Deferred: |
|
— |
|
|
|
— |
|
State |
|
— |
|
|
|
— |
|
Federal |
|
1 |
|
|
|
58 |
|
Foreign |
|
|
|
|
|
||
|
|
|
|
|
|
||
Noncurrent: |
|
|
|
|
|
||
State |
|
— |
|
|
|
— |
|
Federal |
|
200 |
|
|
|
525 |
|
Foreign |
|
698 |
|
|
|
— |
|
Total |
$ |
1,246 |
|
|
$ |
2,018 |
|
A reconciliation of income tax expense computed at the statutory corporate income tax rate to the effective income tax rate for the years ended March 31, 2024 and 2023 is as follows:
|
Year ended March 31, |
|
|||||
|
2024 |
|
|
2023 |
|
||
Tax expense computed at federal statutory rate |
|
21.0 |
% |
|
|
21.0 |
% |
State tax |
|
6.4 |
% |
|
|
(12.7 |
)% |
Change in valuation allowance |
|
11.6 |
% |
|
|
80.0 |
% |
Foreign rate differential |
|
9.2 |
% |
|
|
(7.7 |
)% |
Non-deductible expenses |
|
(15.2 |
)% |
|
|
62.6 |
% |
Uncertain Tax Positions |
|
(53.5 |
)% |
|
|
52.3 |
% |
Other |
|
3.2 |
% |
|
|
5.5 |
% |
Total income tax expense |
|
(17.3 |
)% |
|
|
201.0 |
% |
76
The Company’s deferred tax position reflects the net tax effects of the temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax reporting. Significant components of the deferred tax assets and liabilities are as follows:
|
Year ended March 31, |
|
|||||
|
2024 |
|
|
2023 |
|
||
Deferred tax assets: |
|
|
|
|
|
||
Net operating loss carryforwards |
$ |
6,793 |
|
|
$ |
6,483 |
|
Pension |
|
422 |
|
|
|
323 |
|
Accrued expenses |
|
35 |
|
|
|
138 |
|
Section 163(j) interest expense carryforward |
|
84 |
|
|
|
165 |
|
Capitalized R&D |
|
332 |
|
|
|
689 |
|
GAAP to statutory adjustments |
|
741 |
|
|
|
686 |
|
Other |
|
217 |
|
|
|
152 |
|
Total gross deferred tax assets |
|
8,624 |
|
|
|
8,636 |
|
Less: valuation allowance |
|
(8,139 |
) |
|
|
(8,264 |
) |
Total deferred tax assets, net of valuation allowance |
$ |
485 |
|
|
$ |
372 |
|
Deferred tax liabilities: |
|
|
|
|
|
||
Depreciation |
$ |
6 |
|
|
$ |
7 |
|
GAAP to statutory adjustments |
|
418 |
|
|
|
424 |
|
Other |
|
173 |
|
|
|
51 |
|
Total gross deferred tax liabilities |
|
597 |
|
|
|
482 |
|
Net deferred tax liabilities |
$ |
112 |
|
|
$ |
110 |
|
The valuation allowance for deferred tax assets as of March 31, 2024 and 2023 primarily relates to net operating loss and interest deduction limitation carryforwards that, in the judgment of the Company, are not more-likely-than-not to be realized.
In assessing the realizability of deferred tax assets, the Company considers whether it is more-likely-than-not that some portion or all the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. The Company considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income, and tax-planning strategies in making this assessment. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible, the Company believes it is more-likely-than-not that it will realize the benefits of these deductible differences, net of the existing valuation allowances as of March 31, 2024 and 2023.
As of March 31, 2024 and 2023, the Company has tax effected net operating loss carryforwards in U.S. of $5,000 and $2,453, respectively, of which $761 will expire starting in 2035 and the remainder which can be carried forward indefinitely. As of March 31, 2024 and 2023, the Company has U.S. state tax effected net operating loss carryforwards of approximately $1,169 and $464 that, if unused, will expire starting in 2035. As of March 31, 2024 and 2023, the Company has other foreign tax effected net operating loss carryforwards of $912 and $4,946 of which the majority can be carried forward seven years.
The Company prepares its financial statements on a consolidated basis. Income tax expense is calculated in accordance with the local tax laws of each entity in its relevant jurisdiction on a separate company basis.
77
A reconciliation of beginning and ending amount of unrecognized tax liability is presented below:
|
Year ended March 31, |
|
|||||
|
2024 |
|
|
2023 |
|
||
Unrecognized Tax Liability – beginning balance |
$ |
— |
|
|
$ |
— |
|
Net Increases – tax positions in current year |
|
— |
|
|
|
— |
|
Net Increases – tax positions in prior year |
|
3,499 |
|
|
|
|
|
Total income tax expense |
$ |
3,499 |
|
|
|
— |
|
As of March 31, 2024 and March 31, 2023, the company had unrecognized tax benefits of $3,499, and $0, respectively, which related to tax positions that, if recognized, would affect the annual effective tax rate. The company recognized accrued interest and penalties in income tax expense. As of March 31, 2024 and March 31, 2023 accrued interest and penalties totaling to $159 thousand, and $0, respectively, is included in other long-term liabilities. The Company has identified potential penalty exposure in relation to specific information reporting requirements in the United States. Although the Company is trying to address these issues and pursue penalty abatement, it has recorded a long-term payable for the penalties, until potential relief is granted. As of March 31, 2024 and 2023, the recorded accrual balances stand at $1,200 and $1,000, respectively.
The Company is subject to taxation in Switzerland, the U.S., and other jurisdictions of its foreign subsidiaries. As of March 31, 2024, tax years 2020, 2021, and 2022 are subject to examination by the tax authorities in the U.S. The Company is not currently under examination by tax authorities in any jurisdiction.
From time to time, the Company may be involved in lawsuits, claims, investigations, and proceedings, consisting of intellectual property, commercial, employment, and other matters, which arise in the ordinary course of business. In accordance with ASC 450, Contingencies, the Company makes a provision for a liability when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
The Company is not presently a party to any litigation the outcome of which, it believes, if determined adversely to the Company, would individually or taken together, have a material adverse effect on the Company’s business, operating results, cash flows or financial condition. The Company has determined that the existence of a material loss is neither probable nor reasonably possible.
The Company leases office space (real estate), vehicles and office equipment under operating leases. The Company did not have any finance leases as of March 31, 2024 and 2023.
Right-of-use lease assets and lease liabilities that are reported in the Company’s consolidated balance sheet as of March 31, 2024 and 2023 are as follows:
|
|
As of March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Operating lease, right-of-use assets, net |
|
$ |
4,466 |
|
|
$ |
2,604 |
|
|
|
|
|
|
|
|
||
Current portion of long-term operating lease |
|
|
1,572 |
|
|
|
1,005 |
|
Long-term operating lease |
|
|
2,917 |
|
|
|
1,621 |
|
Total operating lease liabilities |
|
$ |
4,489 |
|
|
$ |
2,626 |
|
78
Lease expense for lease payments is recognized on a straight-line basis over the lease term. The expense is presented within Selling, general, and administrative expense. The components of lease expense related to the Company’s lease for the years ended March 31, 2024 and 2023 were:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Fixed operating lease costs |
|
$ |
1,766 |
|
|
$ |
1,604 |
|
Short-term lease costs |
|
|
13 |
|
|
|
— |
|
Total lease cost |
|
$ |
1,779 |
|
|
$ |
1,604 |
|
Supplemental cash flow information related to leases was as follows:
|
|
Years Ended March 31, |
|
|||||
|
|
2024 |
|
|
2023 |
|
||
Operating cash flows included in the measurement of lease liabilities |
|
$ |
(1,786 |
) |
|
$ |
(1,659 |
) |
Non-cash lease activity related to right-of-use assets obtained in exchange for new operating lease liabilities |
|
|
406 |
|
|
|
128 |
|
Other non-cash changes to ROU assets due to reassessment of the lease term |
|
|
2,946 |
|
|
— |
|
|
The weighted average remaining lease term and discount rate for the Company’s operating leases as of March 31, 2024 and 2023 were:
|
|
2024 |
|
|
2023 |
|
||
Weighted-average remaining lease term (in years) |
|
|
2.63 |
|
|
|
2.77 |
|
Weighted-average discount rate |
|
|
4.00 |
% |
|
|
4.00 |
% |
Lease duration was determined utilizing renewal options that the Company is reasonably certain to execute.
As of March 31, 2024, maturities of operating lease liabilities for each of the following five years ending March 31 and a total thereafter were as follows:
|
|
Operating Leases |
|
|
|
2025 |
|
$ |
1,717 |
|
|
2026 |
|
|
1,181 |
|
|
2027 |
|
|
899 |
|
|
2028 |
|
|
893 |
|
|
2029 |
|
|
111 |
|
|
Thereafter |
|
|
— |
|
|
Total lease payments |
|
|
4,801 |
|
|
Less: imputed interest |
|
|
(312 |
) |
|
Total lease liability |
|
$ |
4,489 |
|
|
The changes in accumulated other comprehensive income (loss) by component are summarized below:
79
|
Foreign Currency Translation |
|
Defined Benefit Plan Items |
|
Total Accumulated Other Comprehensive (Loss) Income |
|
|||
Balance at March 31, 2022 |
$ |
(3,372 |
) |
$ |
4,007 |
|
$ |
635 |
|
Other comprehensive income (loss) before reclassifications |
|
(503 |
) |
|
592 |
|
|
89 |
|
Reclassifications to statements of earnings |
|
— |
|
|
(1,013 |
) |
|
(1,013 |
) |
Total other comprehensive loss |
|
(503 |
) |
|
(421 |
) |
|
(924 |
) |
Balance, March 31, 2023 |
|
(3,875 |
) |
|
3,586 |
|
|
(289 |
) |
Other comprehensive income (loss) before reclassifications |
|
1,455 |
|
|
(469 |
) |
|
986 |
|
Reclassifications to statements of earnings |
|
— |
|
|
(647 |
) |
|
(647 |
) |
Total other comprehensive income (loss) |
|
1,455 |
|
|
(1,116 |
) |
|
339 |
|
Balance, March 31, 2024 |
$ |
(2,420 |
) |
$ |
2,470 |
|
$ |
50 |
|
On June 6, 2024, the Company entered into a Securities Purchase Agreement, pursuant to which the Company issued $3.3 million in principal amount of 8% Original Issue Discount Senior Secured Convertible Debentures (the “Debentures”). The Debentures were issued with an original issue discount of $300 thousand, resulting in gross proceeds of approximately $3 million and net proceeds of approximately $2.5 million after deducting estimated offering expenses.
The Debentures are convertible into an aggregate of 660,000 shares of the Company’s Common Stock at a conversion price of $5.00 per share, subject to adjustment. The Debentures mature on December 7, 2025, and bear interest at a rate of 8% per annum, payable monthly beginning one year from the issuance date.
Provided that no event of default has occurred or is continuing, and at least 33% of the principal amount of the Debentures has either previously been repaid or converted in accordance with the terms of the Debenture, the Company may elect, by notice to the holder of the Debentures, to extend the Maturity Date by six months upon the payment of six months’ interest on the then-outstanding principal amount.
The Debentures are secured by substantially all of the assets of the Company and its domestic subsidiaries, excluding certain specified assets. Additionally, the Company’s domestic subsidiaries have provided an unconditional guarantee of the Debentures. In connection with the issuance of the Debentures, the Company also issued warrants to purchase an aggregate of 330,000 shares of common stock at an exercise price of $5.00 per share, with a five-year term.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
On March 12, 2024, the Audit Committee of the Board of Directors dismissed Marcum LLP (“Marcum”) as the Company’s independent registered public accounting firm. Marcum had served as the Company’s independent registered public accounting firm from May 2, 2022 through March 12, 2024. Marcum continued to provide certain services to the Company in connection with the completion of the audit of the financial statements of ATAK for year ended December 31, 2023. Marcum had served as the Company’s independent registered public accounting firm up to the completion of the Company’s Business Combination.
Marcum’s audit reports on the Company’s financial statements as of and for the year ended December 31, 2022 did not contain an adverse opinion or a disclaimer of opinion and were not qualified or modified as to uncertainty, audit scope or accounting principles, other than an explanatory paragraph regarding the substantial doubt about the Company’s ability to continue as a going concern.
80
During the fiscal year ended December 31, 2022 and the subsequent interim period through March 12, 2024: (1) there were no “disagreements” (as defined in Item 304(a)(1)(iv) of Regulation S-K) with Marcum on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Marcum, would have caused Marcum to make reference to the subject matter of such disagreements in connection with its reports on the financial statements for such periods and (2) there were no “reportable events” (as defined in Item 304(a)(1)(v) of Regulation S-K), except for the disclosure of the material weakness in the Company’s internal control over financial reporting as disclosed in Part II, Item 9A of the Company’s Annual Report on Form 10-K for the year ended December 31, 2022
On March 12, 2024, the Audit Committee engaged BDO AG (“BDO”) as its new independent registered public accounting firm. The Company has authorized Marcum to respond fully to the inquiries of BDO, as the successor independent registered accounting firm.
BDO had served as independent registered public accounting firm for DIH Holding US, Inc., a Nevada corporation up through the completion of the Business Combination. During the two most recent fiscal years and the subsequent interim period through March 12, 2024, the Company did not consult with BDO with respect to (i) the application of accounting principles to a specified transaction, either completed or proposed, the type of audit opinion that might be rendered on the Company’s financial statements, and neither a written report nor oral advice was provided to the Company that BDO concluded was an important factor considered by the Company in reaching a decision as to any accounting, auditing or financial reporting issue, or (ii) any matter that was either the subject of a disagreement (as that term is defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions to Item 304 of Regulation S-K) or a reportable event (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Disclosure controls and procedures are designed to ensure that information required to be disclosed by us in our Exchange Act reports is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial and accounting officer or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of our management, including our Chief Executive Officer and the Company’s Chief Financial Officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d- 15(e) under the Exchange Act) as of the end of the fiscal year ended March 31, 2024. Based on this evaluation, our principal executive officer and principal financial and accounting officer have concluded that during the period covered by this report, our disclosure controls and procedures were not effective due to material weaknesses in our internal controls over financial reporting. Specifically, the Company concluded that it had limited accounting personnel and other resources with which to address its internal control over financial reporting in accordance with requirements applicable to public companies. In light of the material weakness, we performed additional analysis as deemed necessary to ensure that our financial statements were prepared in accordance with U.S. generally accepted accounting principles. Accordingly, management believes that the financial statements included in this Annual Report present fairly in all material respects our financial position, results of operations and cash flows for the periods presented.
Inherent Limitations on Effectiveness of Controls
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Management’s Report on Internal Controls Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America.
81
Our internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles in the United States of America, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
Any system of internal control, no matter how well designed, has inherent limitations, including the possibility that a control can be circumvented or overridden and misstatements due to error or fraud may occur and not be detected in a timely manner. Also, because of changes in conditions, internal control effectiveness may vary over time. Accordingly, even an effective system of internal control will provide only reasonable assurance with respect to financial statement preparation.
Management assessed the effectiveness of our internal control over financial reporting as of March 31, 2024. In making this assessment, management used the criteria set forth in 2013 by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in “Internal Control-Integrated Framework.” Based on management’s assessment using the COSO criteria, management has concluded that our internal control over financial reporting was not effective as of March 31, 2024 as a result of the material weaknesses in internal control over financial reporting described above.
This Annual Report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management’s report in this Annual Report.
Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered public accounting firm due to an exemption provided by the JOBS Act for “emerging growth companies” and our status as a non-accelerated filer under the Exchange Act.
Changes in Internal Control Over Financial Reporting
There have been no changes in our internal control over financial reporting during the last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
Insider Trading Plan
During the year ended March 31, 2024, no director or officer of the Company adopted or terminated a “Rule 10-b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408(a) of Regulation S-K Item 10.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not Applicable.
82
PART III
Directors, Executive Officers and Corporate Governance.
Information required by this item is incorporated herein by reference to the section entitled “Proposal 1 Election of Directors,” “Corporate Governance,” “Executive Officers” and, “Delinquent Section 16(a) Reports.” in the Proxy Statement.
Code of Ethics
We have adopted a written code of business conduct and ethics, which applies to all of our directors, officers and employees, including our principal executive officer, our principal financial officer, our principal accounting officer, and other persons performing similar finance functions. Our Code of Business Conduct and Ethics is available on our investor relations website https://ir.dih.com/ in the “Corporate Governance” section under “Governance Documents.” We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding amendment to, or waiver from, a provision of our Code of Business Conduct and Ethics, as well as Nasdaq’s requirement to disclose waivers with respect to directors and executive officers, by posting such information on our website at the address and location specified above.
Item 11. Executive Compensation.
The disclosure required by this Item is incorporated by reference to the sections entitled “Executive Compensation” and “Director Compensation” in our Definitive Proxy Statement for our 2024 Annual Meeting of Stockholders (the “Proxy Statement”) and is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required by this item is incorporated herein by reference to the section entitled “Beneficial Ownership Information” in the Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information required by this item is incorporated by reference to the sections entitled “Related Party Transactions” and “Corporate Governance” in the Proxy Statement
Item 14. Principal Accounting Fees and Services.
Information required by this item is incorporated by reference to the section entitled “Proposal 3: Ratification of Independent Registered Public Accounting Form - Principal Accounting Fees and Services” in the Proxy Statement.
83
PART IV
Item 15. Exhibits, Financial Statement Schedules.
The following documents are filed as part of this report
Item 16. Form 10-K Summary
Not applicable.
84
Exhibit Index
|
Exhibit Number |
|
Description |
2.1 |
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation of DIH Holding US, Inc. |
3.2 |
|
|
4.1 |
|
|
4.2 |
|
|
4.3 |
|
|
4.4 |
|
|
10.1 |
|
|
10.2 |
|
|
10.3 |
|
|
10.4 |
|
|
10.5 |
|
|
10.6 |
|
|
10.7 |
|
|
10.8 |
|
|
10.9* |
|
|
14 |
|
|
19 |
|
|
21* |
|
|
24* |
|
|
31.1* |
|
|
31.2* |
|
|
32.1* |
|
|
32.2* |
|
|
97 |
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema With Embedded Linkbase Documents |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
85
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
DIH HOLDING US, INC. |
|
|
|
|
|
Date: July 15, 2024 |
|
By: |
/s/ Jason Chen |
|
|
|
Name: Jason Chen |
|
|
|
Title: Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
|
Name |
|
Title |
|
Date |
|
|
|
|
|
|
By |
/s/ Jason Chen |
|
Chief Executive Officer and Chairman of the Board |
|
July 15, 2024 |
|
Jason Chen |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
By |
/s/ Lynden Bass |
|
Chief Financial Officer and Director |
|
July 15, 2024 |
|
Lynden Bass |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
By |
/s/ Dr. Patrick Bruno |
|
Chief Marketing Officer and Director |
|
July 15, 2024 |
|
Dr. Patrick Bruno |
|
|
|
|
|
|
|
|
|
|
By |
/s/ Max Baucus |
|
Director |
|
July 15, 2024 |
|
Max Baucus |
|
|
|
|
|
|
|
|
|
|
By |
/s/ F. Samuel Eberts III |
|
Director |
|
July 15, 2024 |
|
F. Samuel Eberts III |
|
|
|
|
|
|
|
|
|
|
By |
/s/ Ken Ludlum |
|
Director |
|
July 15, 2024 |
|
Ken Ludlum |
|
|
|
|
|
|
|
|
|
|
By |
/s/ Cathryn Chen |
|
Director |
|
July 15, 2024 |
|
Cathryn Chen |
|
|
|
|
|
|
|
|
|
|
86
EX10.9
SUBSCRIPTION AGREEMENT
DIH Holding US, Inc.
77 Accord Park Drive; Suite D-1 Norwell, MA 02061
Ladies and Gentlemen:
The undersigned (the "Investor") has agreed to purchase shares (the "Shares") of Class A common stock, par value $0.0001 per share (the "Common Stock"), of DIH Holding US, Inc., a Delaware corporation ("DIH"), for a purchase price of$ 10.00 per share (the "Per Share Purchase Price"). The aggregate purchase price to be paid by the Investor for the subscribed Shares (as set forth on the signature page hereto) is referred to herein as the "Subscription Amount." By executing this agreement (this "Subscription Agreement"), the Investor is agreeing to purchase an aggregate amount of 150,000 Shares, at a per share price equal to the Per Share Purchase Price for an aggregate subscription price ofUS$1,500,000.
In connection therewith, and in consideration of the foregoing and the mutual representations, warranties and covenants, and subject to the conditions, set forth herein, and intending to be legally bound hereby, the Investor and DIH agree as follows:
all respects), at and as of the Closing Date (except for these representations and warranties that speak as of a specified earlier date, which shall be so true and correct in all material respects (other than representations and warranties that are qualified as to materiality, which representations and warranties shall be true and correct in all respects as of such specified earlier date)), and consummation of the Closing shall constitute a reaffirmation by the Investor of each of the representations and warranties of the Investor contained in this Subscription Agreement as of the Closing Date or such earlier date, as applicable; and
DIH public warrants, 6,470,000 DIH private placement warrants and 40,544,936 shares of Class A Common Stock of
DIH are issued and outstanding.
U.S. Foreign Corrupt Practices Act of 1977 (as amended), the UK Bribery Act 2010, and any similar law that prohibits bribery or corruption.
pursuant to a private sale effected under Section 4(a)(7) of the Securities Act, and in each of cases (i) and (ii) in accordance with any applicable securities laws of the states and other jurisdictions of the United States, and that any certificates representing the Shares offered hereby shall contain a restrictive legend to such effect, which legend shall be subject to removal as set forth herein.
The Investor understands and agrees that the Shares offered hereby will be subject to transfer restrictions described herein, and, as a result, the Investor may not be able to readily offer, resell, transfer, pledge or otherwise dispose of the Shares and may be required to bear the financial risk of an investment in the Shares for an indefinite period of time. The Investor acknowledges and agrees that the Shares offered hereby will not immediately be eligible for offer, resale, transfer, pledge or disposition pursuant to Rule 144 promulgated under the Securities Act until at least one year from the date that DIH files a Current Report on Form 8-K that includes the "Form 10" information required under applicable SEC rules and regulations. The Investor understands that it has been advised to consult legal counsel prior to making any offer, resale, transfer, pledge or disposition of any of the Shares. By making the representations herein, the Investor does not agree to hold any of the Shares offered hereby for any minimum or other specific term and reserves the right to assign, transfer or otherwise dispose of any of the Shares at any time in accordance with or pursuant to a registration statement or an exemption under the Securities Act.
on the first date on which the Investor can sell all of its Registrable Securities under Rule 144 of the Securities Act without volume or manner of sale limitations and without the requirement DIH to be in compliance with the current public information required under Rule 144(c)(2) (or Rule l 44(i)(2), if applicable).
Shares (the "Transfer Agent") of a book-entry account or certificate representing Shares issued with a restrictive legend, deliver or cause the Transfer Agent to deliver to the Investor a book-entry account or certificate representing such Shares or, at the request of the Investor, deliver or cause to be delivered the Shares to the Investor by crediting the account of the Investor's prime broker with DTC through its Deposit/Withdrawal at Custodian (DWAC) system, in each case, free from all restrictive and other legends and stop transfer instructions (or similar notations).
(90) calendar days, in each case in any three hundred sixty (360) day period and (ii) DIH shall use its best efforts to make such Registration Statement available for the sale by the Investor of such securities as soon as practicable thereafter. If so directed by DIH, the Investor will destroy all copies of the prospectus covering the Shares in the Investor's possession; provided, however, that this obligation to deliver or destroy all copies of the prospectus covering the Shares shall not apply (i) to the extent the Investor is required to retain a copy of such prospectus (A) in order to comply with applicable legal or regulatory requirements or (B) in accordance with a bona fide pre-existing document retention policy or (ii) to copies stored electronically on archival servers as a result of automatic data back-up. Notwithstanding anything to the contrary, DIH shall cause its transfer agent to deliver unlegended Shares to a transferee of an Investor in connection with any sale of Shares with respect to which an Investor has entered into a contract for sale, prior to such Investor's receipt of the notice of a Suspension Event and for which such Investor has not yet settled.
period that the Registration Statement is effective or if as a result of a Suspension Event the Registration Statement or related prospectus contains any untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made (in the case of the prospectus) not misleading, the Investor agrees that it will promptly discontinue offers and sales of the Shares under the Registration Statement (excluding, for the avoidance of doubt, sales conducted pursuant to Rule 144 or the consummation of any sale pursuant to a contract entered into, or order placed, by the Investor prior to receipt of notice described in this sentence) until the Investor receives copies of a supplemental or amended prospectus (which DIH agrees to promptly prepare) that corrects the misstatement( s) or omission(s) referred to above and receives notice that any post-effective amendment has become effective or unless otherwise notified by DIH that it may resume such offers and sales (which notice shall not contain any material non-public information and which notice shall not be subject to any duty of confidentiality). Upon the occurrence of any event contemplated in clauses (i) through (v) above, except for such times as DIH is permitted hereunder to suspend, and has suspended, the use of a prospectus forming part of a Registration Statement, DIH shall use its best efforts to as soon as reasonably practicable prepare a post-effective amendment to such Registration Statement or a supplement to the related prospectus, or file any other required document so that, as thereafter delivered to purchasers of the Shares included therein, such prospectus will not include any untrue statement of a material fact or omit to state any material fact necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading. The Investor may deliver written notice (an "Opt-Out Notice") to DIH requesting that the Investor not receive notices from DIH otherwise required by this Section 8(h); provided, however, that the Investor may later revoke any such Opt-Out Notice in writing. Following receipt of an Opt-Out Notice from the Investor (unless subsequently revoked), (i) DIH shall not deliver any such notices to the Investor and the Investor shall no longer be entitled to the rights associated with any such notice and (ii) the Investor will notify DIH in writing at least two (2) business days in advance of each intended use of an effective Registration Statement, and if a notice of a Suspension Event (as defined below) was previously delivered (or would have been delivered but for the provisions of this Section 8(h)) and the related suspension period remains in effect, DIH will so notify Investor, within one (1) business day of the Investor's notification to DIH, by delivering to the Investor a copy of such previous notice of Suspension Event, and thereafter will provide the Investor with the related notice of the conclusion of such Suspension Event promptly following its availability.
threat or assertion of any proceeding arising from or in connection with the transactions contemplated by this Section of which DIH is aware. Such indemnity shall remain in full force and effect regardless of any investigation made by
or on behalf of an indemnified party and shall survive the transfer of the Shares by the Investor.
dollars in immediately available funds to the account specified by Investor, without any deduction for or on account of any tax withholding, charges or set-off.
SUBSCRIPTION AGREEMENT OR IN SUCH OTHER MANNER AS MAY BE PERMITTED BY LAW SHALL BE VALID AND SUFFICIENT SERVICE THEREOF.
EACH PARTY ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS SUBSCRIPTION AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES, AND THEREFORE EACH SUCH PARTY HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THIS SUBSCRIPTION AGREEMENT OR THE TRANSACTIONS CONTEMPLATED BY THIS SUBSCRIPTION AGREEMENT. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER; (II) SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THE FOREGOING WAIVER; (III) SUCH PARTY MAKES THE FOREGOING WAIVER VOLUNTARILY AND (IV) SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS SUBSCRIPTION AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIYER AND CERTIFICATIONS IN THIS SECTION l l(m).
All communications sent to DIH shall be sent to:
DIH Holding US, Inc.
77 Accord Park Drive; Suite D-1 Norwell, MA 02061
Attention: Jason Chen, Chief Executive Officer With a copy (which shall not constitute notice) to:
Loeb & Loeb LLP 345 Park Avenue New York, NY 10154
Attention: Mitchell Nussbaum, Esq.
All communications sent to the Investor shall be sent to:
c/o OrbiMed Advisors LLC Attention: General Counsel
601 Lexington Avenue, 54th Floor New York, NY 10022
Email: legal@orbimed.com
Each of the parties hereto shall pay all of its own expenses in connection with this Subscription Agreement and the transactions contemplated hereby.
employees, agents or representatives has provided to the Investor or any of the Investor's affiliates, attorneys, agents or representatives at any time prior to the filing of the Disclosure Document. As of such filing of the Disclosure Document, the Investor and the Investor's affiliates, attorneys, agents or representatives shall not be in possession of any material, non-public information received from DIH or any of its officers, directors, employees, agents or representatives and the Investor shall no longer be subject to any confidentiality or similar obligations under any then current agreement, whether written or oral, with DIH or any of its affiliates in connection this Subscription Agreement. DIH understands and confirms that the Investor and the Investor's affiliates, attorneys, agents or representatives will rely on the foregoing representations and covenants in effecting transactions of securities in DIH. Notwithstanding the foregoing, DIH shall not publicly disclose the name of the Investor or any affiliate or investment adviser of the Investor to any person, or include the name of the Investor or any affiliate or investment adviser of the Investor (i) in any press release or marketing materials without the prior written consent (including by e-mail) of the Investor (which consent shall not be unreasonably withheld or conditioned), or (ii) or in any filing with the SEC or any regulatory agency or trading market, without the prior written consent (including by e-mail) of the Investor (which consent shall not be unreasonably withheld or conditioned), except as required by the federal securities laws, rules or regulations, and to the extent such disclosure is required by other laws, rules or regulations, at the request of the staff of the SEC or regulatory agency or under the regulations of any national securities exchange on which DIH's securities are listed for trading, in which case DIH shall provide the Investor with prior written notice (including by e-mail) of such disclosure, and shall reasonably consult with the Investor regarding such disclosure.
[SIGNATURE PAGES FOLLOW]
IN WITNESS WHEREOF DIR has accepted this Subscription Agreement as of the date set forth below.
DIR HOLDING US, INC.
By /s/ Jason Chen
Name: Jason Chen
Title: Chief Executive Officer
The undersigned is signing for the purpose of confirming its obligation under Section 3(c)(vi) of this Subscription Agreement:
ATAC SPONSOR, LLC
Date: February 8, 2024
By: _ Name:
Title:
DocuSign Envelope ID: F4BD5FD8-3CB0-4BC9-9962-C9C91A24D5F4
IN WITNESS WHEREOF, DIH has accepted this Subscription Agreement as of the date set forth below.
DIH HOLDING US, INC.
By: _ Name: Jason Chen By: /s/ Zachary Wang Name: Zachary Wang
Title: Chief Executive Officer
The undersigned is signing for the purpose of confirming its obligation under Section 3(c)(vi) of this Subscription Agreement:
ATAC SPONSOR, LLC
Title: Manager
Date: February 8, 2024
IN WITNESS WHEREOF, the lnvestor bas executed or caused this Subscription Agreement to be executed by its duly authorized representative as of the date set fo1th below.
ORBIMED ASIA PAR1NERS 11, L.P. Domicile: Cayman Islands By: OrbiMed Asia GP II, L.P., its General Partner
By: OrbiMed Advisors I1 Limited, its General Partner
By: /s/ David G. Wang
Name: David G. Wang
Title: Director
Date: Februruy 2024
Investor’s EIN: 98-1125517
Business & Mailing Address:
c/o OrbiMed Advisors LLC Attention: General Counsel
601 Lexington Avenue, 54th Floor New York, NY I 0022
Email: Legal@OrbiMed.com Phone: +l (212) 739-6400
Number of Shares subscribed for: 150 000
Aggregate Subscription Amount: $1,500,000 U D Price Per Share: $ 10
22
SCHEDULE A
ELIGIBILITY REPRESENTATIONS OF THE INVESTOR
(Please check the applicable subparagraphs):
□x We are a "qualified institutional buyer" (as defined in Rule 144A under the Securities Act (a "QIB")).
**OR**
(Please check the applicable subparagraphs):
Rule 50l(a), in relevant part, states that an "accredited investor" shall mean any person who comes within any of the below listed categories, or who the issuer reasonably believes comes within any of the below listed categories, at the time of the sale of the securities to that person. The Investor has indicated, by marking and initialing the appropriate box below, the provision(s) below which apply to the Investor and under which the Investor accordingly qualifies as an "accredited investor."
□ Any plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions for the benefit of its employees, if such plan has total assets in excess of$5,000,000;
□ Any employee benefit plan, within the meaning of the Employee Retirement Income Security Act of 1974, if a bank, insurance company, or registered investment adviser makes the investment decisions, or if the plan has total assets in excess of$5,000,000;
□X Any organization described in Section 50l(c)(3) of the Internal Revenue Code, corporation, limited liability company, similar business trust, or partnership, not formed for the specific purpose of acquiring the securities offered, with total assets in excess of $5,000,000;
□ Any entity in which all of the equity owners are accredited investors meeting one or more of the above tests.
**AND**
□X We or the investment adviser that has been delegated decision-making authority over our account are an "institutional account" (as defined in FINRA RULE 4512(c)).
This page should be completed by the Investor and constitutes a part of the Subscription Agreement.
EXHIBIT 21
Subsidiaries of DIH Holding US, Inc
Legal Entities |
State or Country of Incorporation or Organization |
|
|
DIH Holding US, Inc. |
Delaware |
DIH Holding US, Inc. |
Nevada |
DIH US Corp. |
Delaware |
DIH Technology, Inc. |
Delaware |
DIH PTE. LTD. |
Singapore |
DIH Technology d.o.o. |
Slovenia |
DIH S.p.a. |
Chile |
DIH GmbH |
Germany |
Hocoma Medical GmbH |
Switzerland |
Exhibit 24.1
POWER OF ATTORNEY
Each person whose signature appears below hereby constitutes and appoints Jason Chen and Lyndon Bass, and each of them, any of whom may act without joinder of the other, the individual’s true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for the person and in his or her name, place and stead, in any and all capacities, to sign the Annual Report on Form 10-K and any or all amendments thereto, and all other documents in connection therewith to be filed with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact as agents or any of them, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof. This Power of Attorney may be signed in any number of counterparts, each of which shall constitute an original and all of which, taken together, shall constitute one Power of Attorney.
|
|
|
|
|
|
|
|
|
Signature |
|
Title |
|
Date |
|
By |
|
/s/Jason Chen |
|
Chief Executive Officer and Chairman of the Board |
|
June 24, 2024 |
|
|
Jason Chen |
|
(Principal Executive Officer) |
|
|
|
By |
|
/s/Lynden Bass |
|
Chief Financial Officer and Director |
|
June 24, 2024 |
|
|
Lynden Bass |
|
(Principal Financial and Accounting Officer) |
|
|
|
By |
|
/s/Dr. Patrick Bruno |
|
Chief Marketing Officer and Director |
|
June 24, 2024 |
|
|
Dr. Patrick Bruno |
|
|
|
|
|
By |
|
/s/Max Baucus |
|
Director |
|
June 24, 2024 |
|
|
Max Baucus |
|
|
|
|
|
By |
|
/s/F. Samuel Eberts II |
|
Director |
|
June 24, 2024 |
|
|
F. Samuel Eberts III |
|
|
|
|
|
By |
|
/s/Ken Ludlum |
|
Director |
|
June 24, 2024 |
|
|
Ken Ludlum |
|
|
|
|
|
By |
|
/s/Cathryn Chen |
|
Director |
|
June 24, 2024 |
|
|
Cathryn Chen |
|
|
|
|
DOCPROPERTY "DocID" \* MERGEFORMAT 239006436.1
243449-10002
Exhibit 31.1
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Jason Chen, certify that:
Date: July 15, 2024 |
|
By: |
/s/ Jason Chen |
|
|
|
Jason Chen |
|
|
|
Chief Executive Officer |
Exhibit 31.2
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Lynden Bass, certify that:
Date: July 15, 2024 |
|
By: |
/s/ Lynden Bass |
|
|
|
Lynden Bass |
|
|
|
Chief Financial Officer |
Exhibit 32.1
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of DIH Holding US, Inc. (the “Company”) on Form 10-K for the year ended March 31, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Jason Chen, Chief Executive Officer (Principal Executive Officer) of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
Date: July 15, 2024 |
|
By: |
/s/ Jason Chen |
|
|
|
Jason Chen |
|
|
|
Chief Executive Officer |
Exhibit 32.2
CERTIFICATION PURSUANT TO
18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of DIH Holding US, Inc. (the “Company”) on Form 10-K for the year ended March 31, 2024, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), I, Lynden Bass, Chief Financial Officer (Principal Financial Officer) of the Company, certify, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that:
Date: July 15, 2024 |
|
By: |
/s/ Lynden Bass |
|
|
|
Lynden Bass |
|
|
|
Chief Financial Officer |