UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 01, 2024 |
Organovo Holdings, Inc.
(Exact name of Registrant as Specified in Its Charter)
Delaware |
001-35996 |
27-1488943 |
||
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
11555 Sorrento Valley Rd Suite 100 |
|
|||
San Diego, California |
|
92121 |
||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: (858) 224-1000 |
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
Common Stock, $0.001 par value |
|
ONVO |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 Regulation FD Disclosure.
Organovo Holdings, Inc. (the “Company”) is furnishing a corporate presentation, attached as Exhibit 99.1 to this Current Report on Form 8-K (the “Corporate Presentation”), which the Company intends to post on the Company’s website. The Corporate Presentation is current as of May 1, 2024, and the Company disclaims any obligation to update this material in the future.
The information in this Item 7.01, including the Corporate Presentation attached hereto as Exhibit 99.1, is being furnished under Item 7.01 of Form 8-K and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, and it shall not be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
|
|
|
Number |
|
Description |
|
|
|
99.1 |
|
|
|
|
|
104 |
|
Cover Page Interactive Data File, formatted in Inline Extensible Business Reporting Language (iXBRL). |
|
|
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Organovo Holdings, Inc. |
|
|
|
|
Date: |
May 1, 2024 |
By: |
/s/ Keith Murphy |
|
|
|
Name: Keith Murphy |

INVESTOR PRESENTATION May 2024 NASDAQ: ONVO Exhibit 99.1

Certain statements contained in this presentation or in other documents of Organovo Holdings, Inc. (the “Company” or “Organovo”) and of any of its affiliates, along with certain statements that may be made by management of the Company orally in presenting this material, are or may be considered “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as "estimate," "expect," "intend," "believe," "plan," "anticipate," “potential,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition of the Company. Organovo cautions that these statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties. Market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such market size estimates will prove accurate. Future actions, future performance and/or future results may differ from those set forth in the forward-looking statements. Because actual actions, performance and results are affected by potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward-looking statement. The Company assumes no obligation to update forward-looking statements for any reason after the date of this Presentation. Investors are advised to consult further disclosures that the Company makes or has made regarding such risks, contingencies and uncertainties in the Company’s most recent periodic reports filed with the Securities and Exchange Commission, including the Annual Report on Form 10-K for the year ended March 31, 2023, subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K that the Company has filed or may file with the Securities and Exchange Commission, including the risk factors set forth in those filings. In presenting this material or responding to inquiries in connection with a presentation, management may refer to results, projections or performance measures that are not prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) as reported in the Company’s SEC filings. These results, projections or performance measures are non-GAAP measures and are not intended to replace or substitute for results measured under GAAP and are supplemental to GAAP reported results. This Presentation does not constitute an offer to sell or a solicitation of an offer to buy securities in any potential transaction, nor shall there be any offer, solicitation, or sale of any such securities in any jurisdiction, or to whom any person, where such offer, solicitation, or sale would be unlawful. Forward Looking Statements
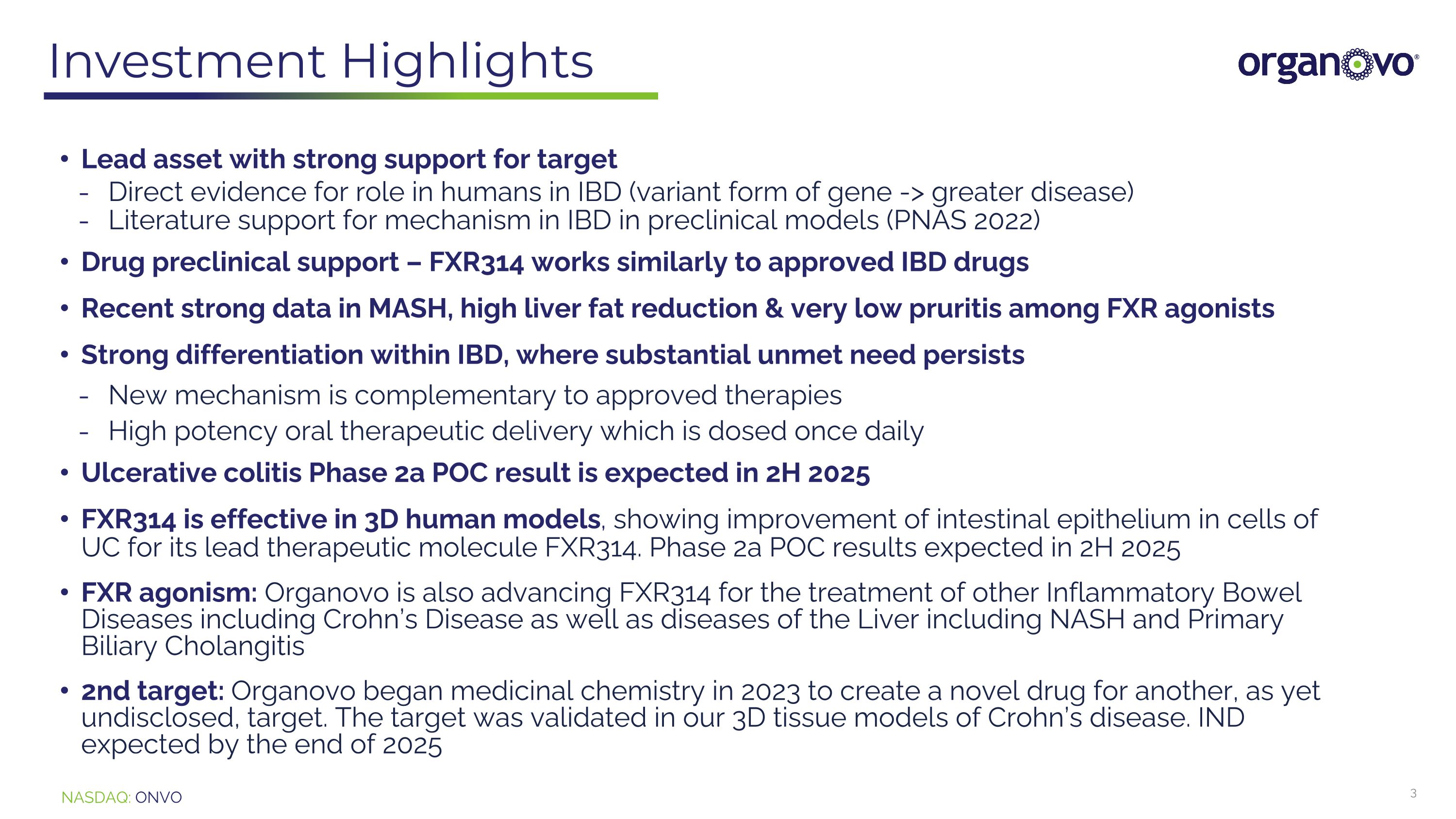
Investment Highlights Lead asset with strong support for target Direct evidence for role in humans in IBD (variant form of gene -> greater disease) Literature support for mechanism in IBD in preclinical models (PNAS 2022) Drug preclinical support – FXR314 works similarly to approved IBD drugs Recent strong data in MASH, high liver fat reduction & very low pruritis among FXR agonists Strong differentiation within IBD, where substantial unmet need persists New mechanism is complementary to approved therapies High potency oral therapeutic delivery which is dosed once daily Ulcerative colitis Phase 2a POC result is expected in 2H 2025 FXR314 is effective in 3D human models, showing improvement of intestinal epithelium in cells of UC for its lead therapeutic molecule FXR314. Phase 2a POC results expected in 2H 2025 FXR agonism: Organovo is also advancing FXR314 for the treatment of other Inflammatory Bowel Diseases including Crohn’s Disease as well as diseases of the Liver including NASH and Primary Biliary Cholangitis 2nd target: Organovo began medicinal chemistry in 2023 to create a novel drug for another, as yet undisclosed, target. The target was validated in our 3D tissue models of Crohn’s disease. IND expected by the end of 2025
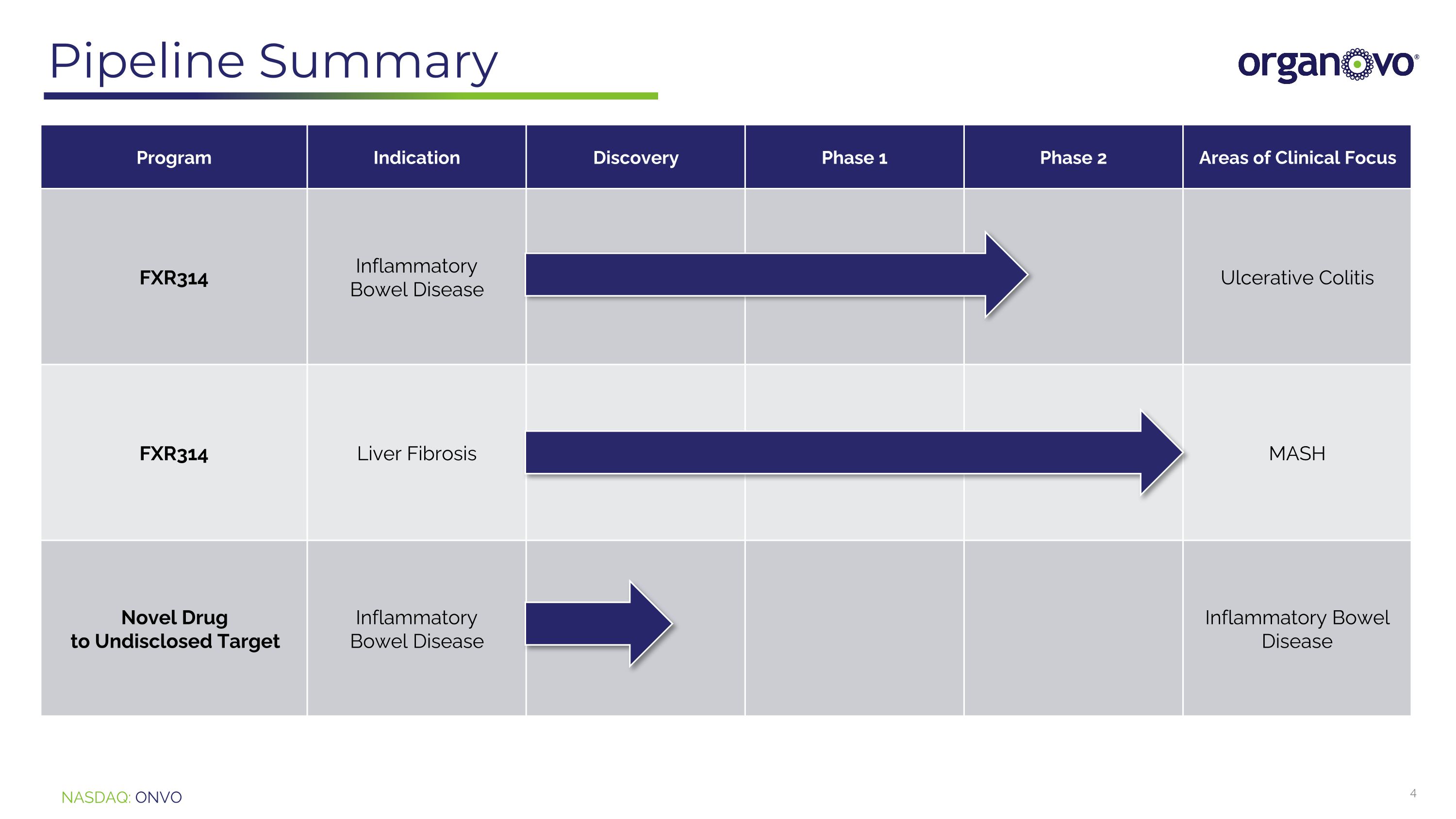
Pipeline Summary Program Indication Discovery Phase 1 Phase 2 Areas of Clinical Focus FXR314 Inflammatory Bowel Disease Ulcerative Colitis FXR314 Liver Fibrosis MASH Novel Drug to Undisclosed Target Inflammatory Bowel Disease Inflammatory Bowel Disease
FXR314 in Liver Disease April 2024 MASH Results
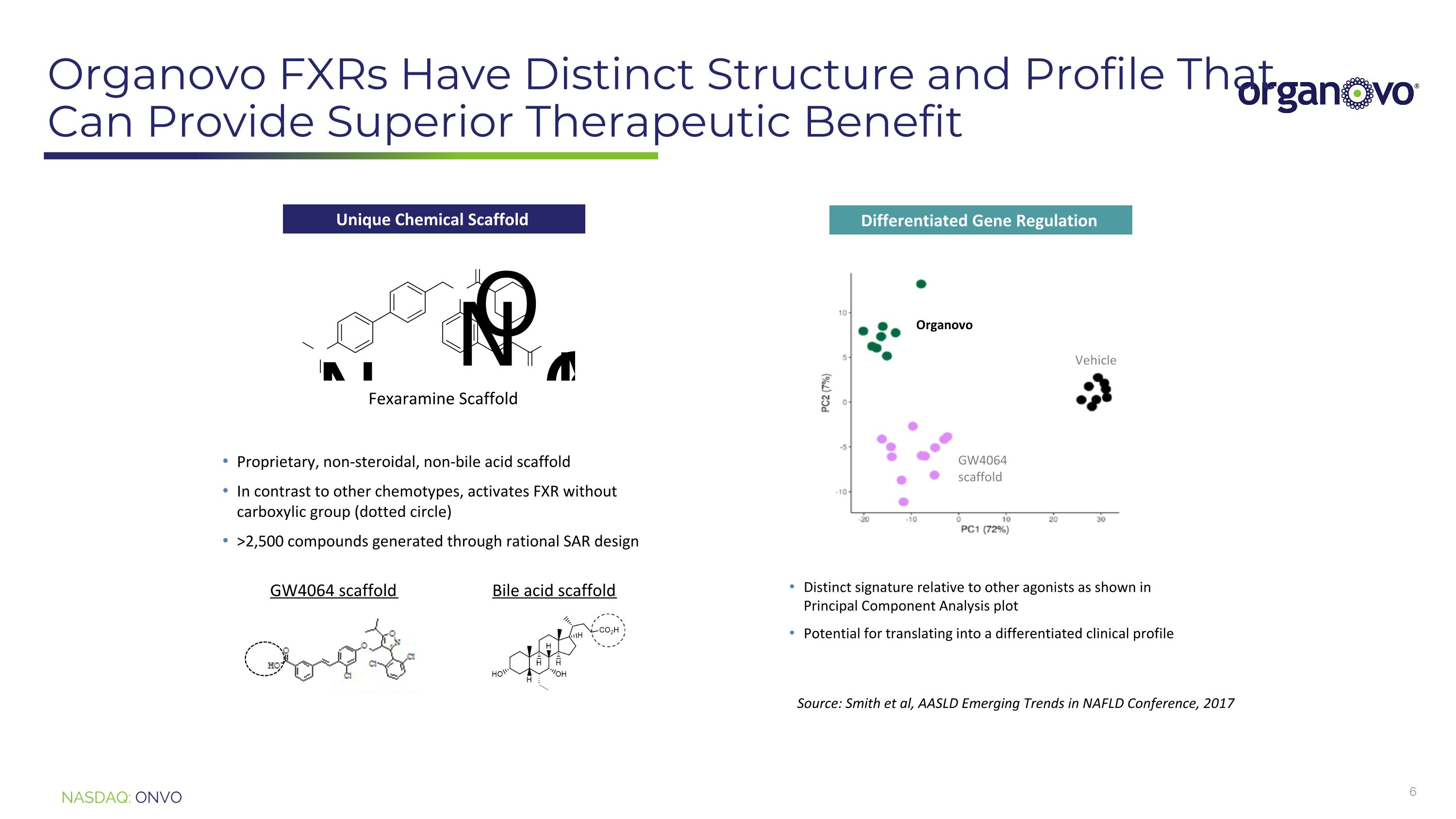
Organovo FXRs Have Distinct Structure and Profile That Can Provide Superior Therapeutic Benefit Unique Chemical Scaffold Differentiated Gene Regulation Fexaramine Scaffold Proprietary, non-steroidal, non-bile acid scaffold In contrast to other chemotypes, activates FXR without carboxylic group (dotted circle) >2,500 compounds generated through rational SAR design GW4064 scaffold Distinct signature relative to other agonists as shown in Principal Component Analysis plot Potential for translating into a differentiated clinical profile Vehicle Organovo GW4064 scaffold Bile acid scaffold Source: Smith et al, AASLD Emerging Trends in NAFLD Conference, 2017
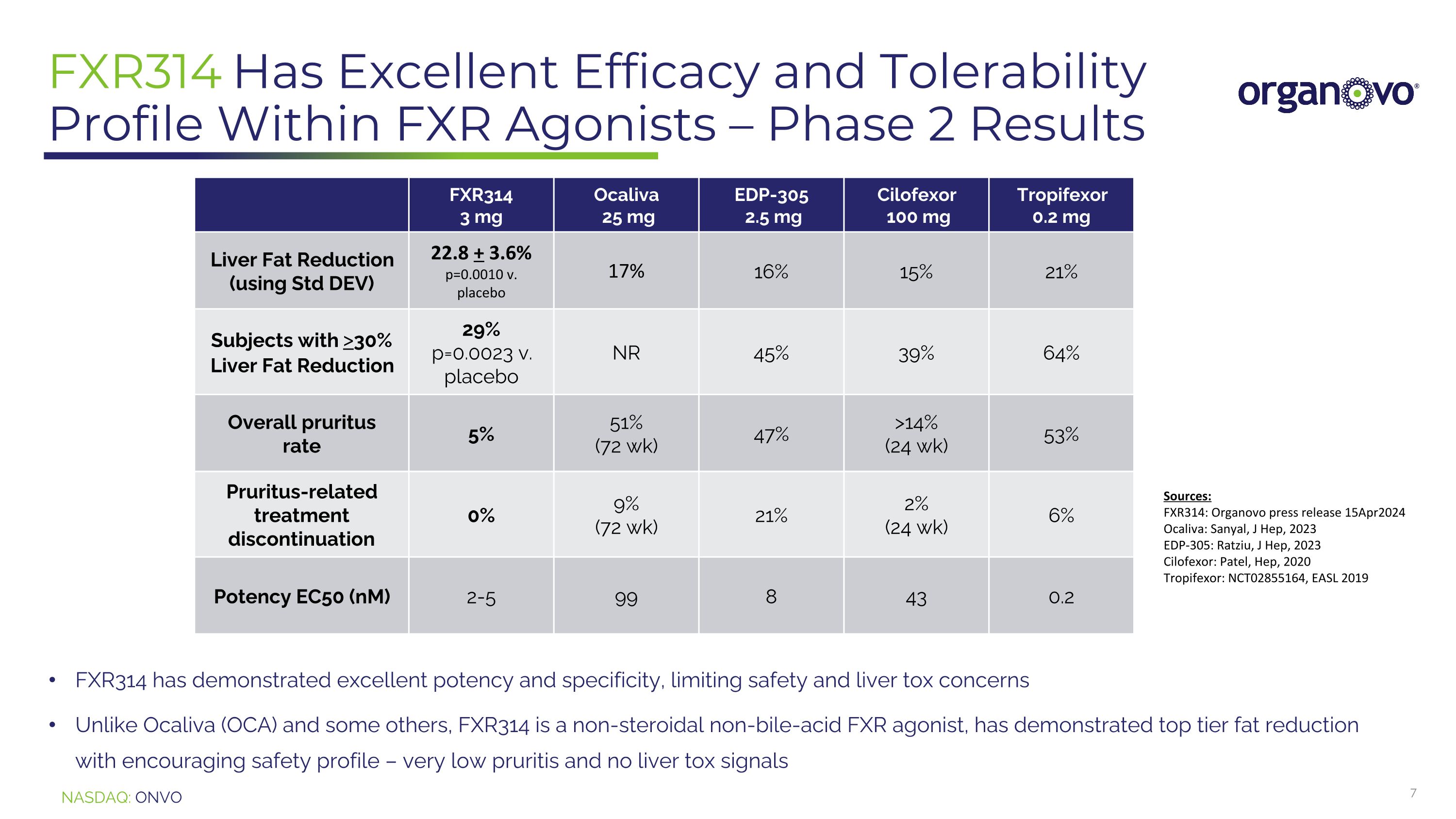
FXR314 Has Excellent Efficacy and Tolerability Profile Within FXR Agonists – Phase 2 Results FXR314 3 mg Ocaliva 25 mg EDP-305 2.5 mg Cilofexor 100 mg Tropifexor 0.2 mg Liver Fat Reduction (using Std DEV) 22.8 + 3.6% p=0.0010 v. placebo 17% 16% 15% 21% Subjects with >30% Liver Fat Reduction 29% p=0.0023 v. placebo NR 45% 39% 64% Overall pruritus rate 5% 51% (72 wk) 47% >14% (24 wk) 53% Pruritus-related treatment discontinuation 0% 9% (72 wk) 21% 2% (24 wk) 6% Potency EC50 (nM) 2-5 99 8 43 0.2 FXR314 has demonstrated excellent potency and specificity, limiting safety and liver tox concerns Unlike Ocaliva (OCA) and some others, FXR314 is a non-steroidal non-bile-acid FXR agonist, has demonstrated top tier fat reduction with encouraging safety profile – very low pruritis and no liver tox signals Sources: FXR314: Organovo press release 15Apr2024 Ocaliva: Sanyal, J Hep, 2023 EDP-305: Ratziu, J Hep, 2023 Cilofexor: Patel, Hep, 2020 Tropifexor: NCT02855164, EASL 2019
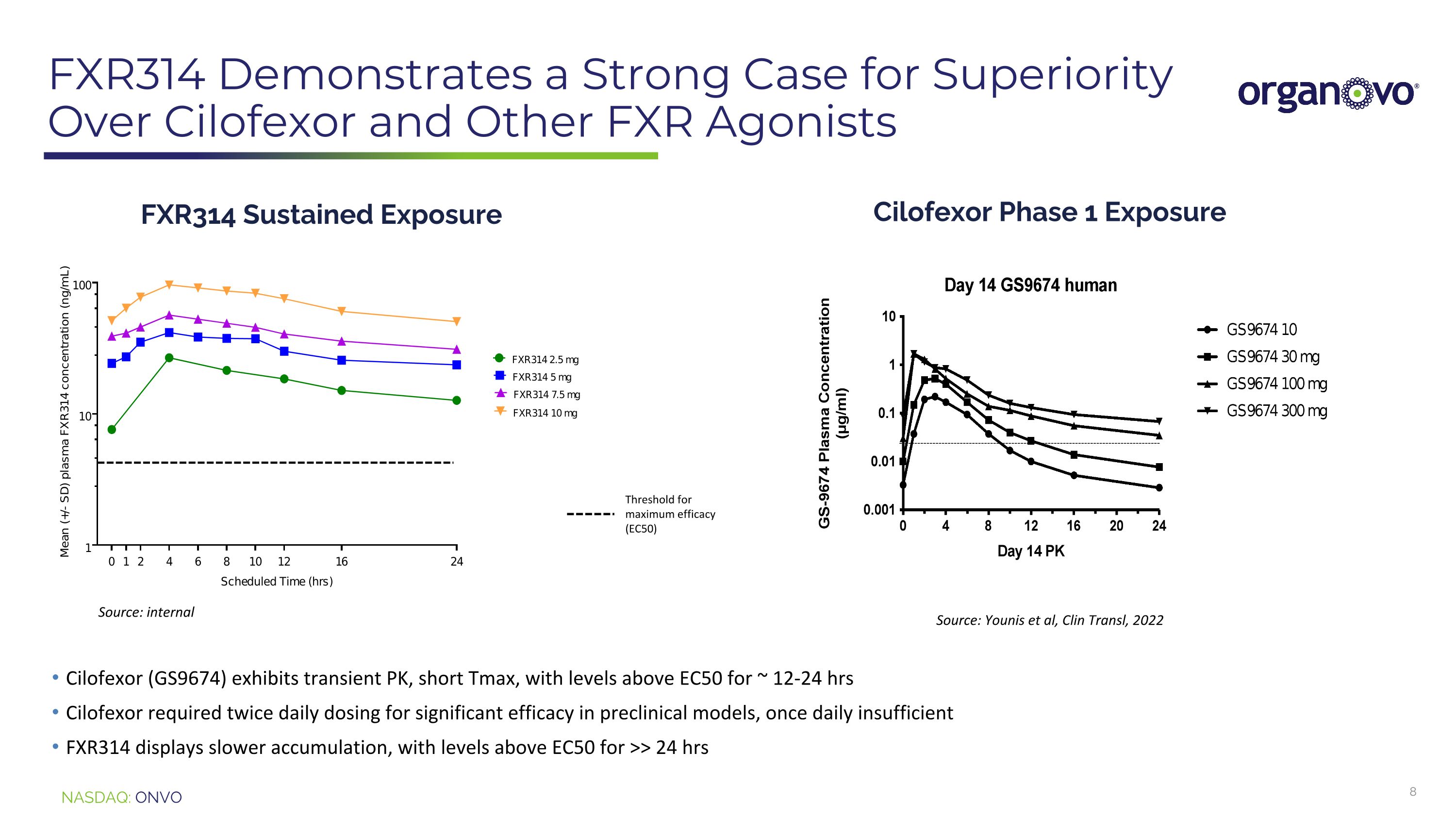
FXR314 Demonstrates a Strong Case for SuperiorityOver Cilofexor and Other FXR Agonists Cilofexor (GS9674) exhibits transient PK, short Tmax, with levels above EC50 for ~ 12-24 hrs Cilofexor required twice daily dosing for significant efficacy in preclinical models, once daily insufficient FXR314 displays slower accumulation, with levels above EC50 for >> 24 hrs FXR314 Sustained Exposure Threshold for maximum efficacy (EC50) Cilofexor Phase 1 Exposure Source: internal Source: Younis et al, Clin Transl, 2022
FXR314 in Inflammatory Bowel Disease
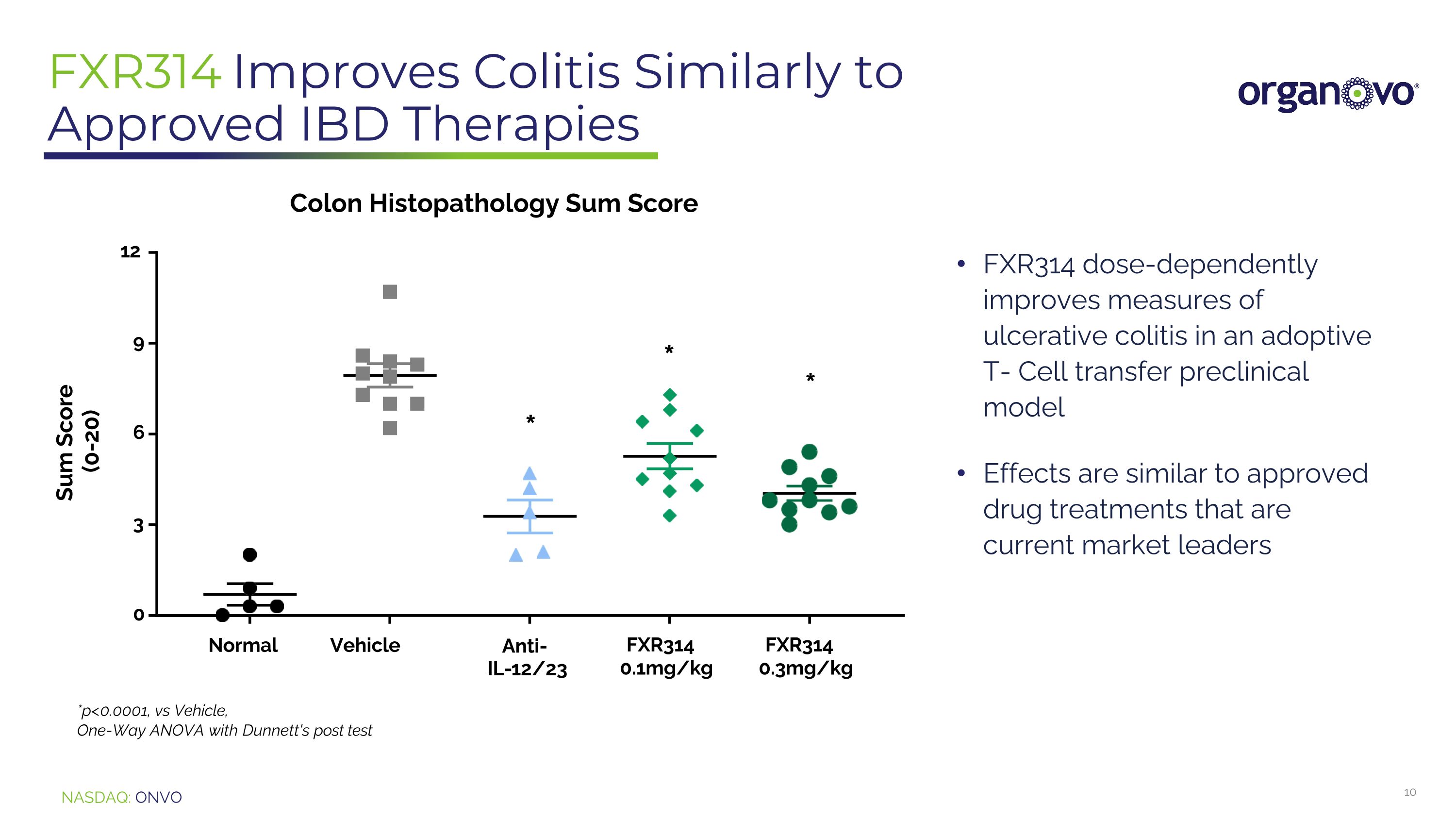
FXR314 Improves Colitis Similarly to Approved IBD Therapies 0 3 6 9 12 Colon Histopathology Sum Score Sum Score (0-20) Normal Vehicle Anti- IL-12/23 FXR314 0.3mg/kg *p<0.0001, vs Vehicle, One-Way ANOVA with Dunnett's post test FXR314 0.1mg/kg * * * FXR314 dose-dependently improves measures of ulcerative colitis in an adoptive T- Cell transfer preclinical model Effects are similar to approved drug treatments that are current market leaders
FXR314 Phase 2a Trial in Ulcerative Colitis Phase 2a RCT Study to Demonstrate POC Target enrollment of 75 patients, 2:1 drug:placebo FXR314 6mg vs. placebo 12 weeks of treatment, oral once daily Diagnosis of moderate to severe UC > 3 months prior to screening defined by clinical and endoscopic evidence, supported by histopathology report Primary objective: improvement in UC severity and symptoms via the modified Mayo score (mMS). Secondary objectives include: to evaluate the safety and tolerability of FXR314 in subjects with moderate to severe UC Enrollment expected to begin 3Q 2024 Study readout expected 2H 2025
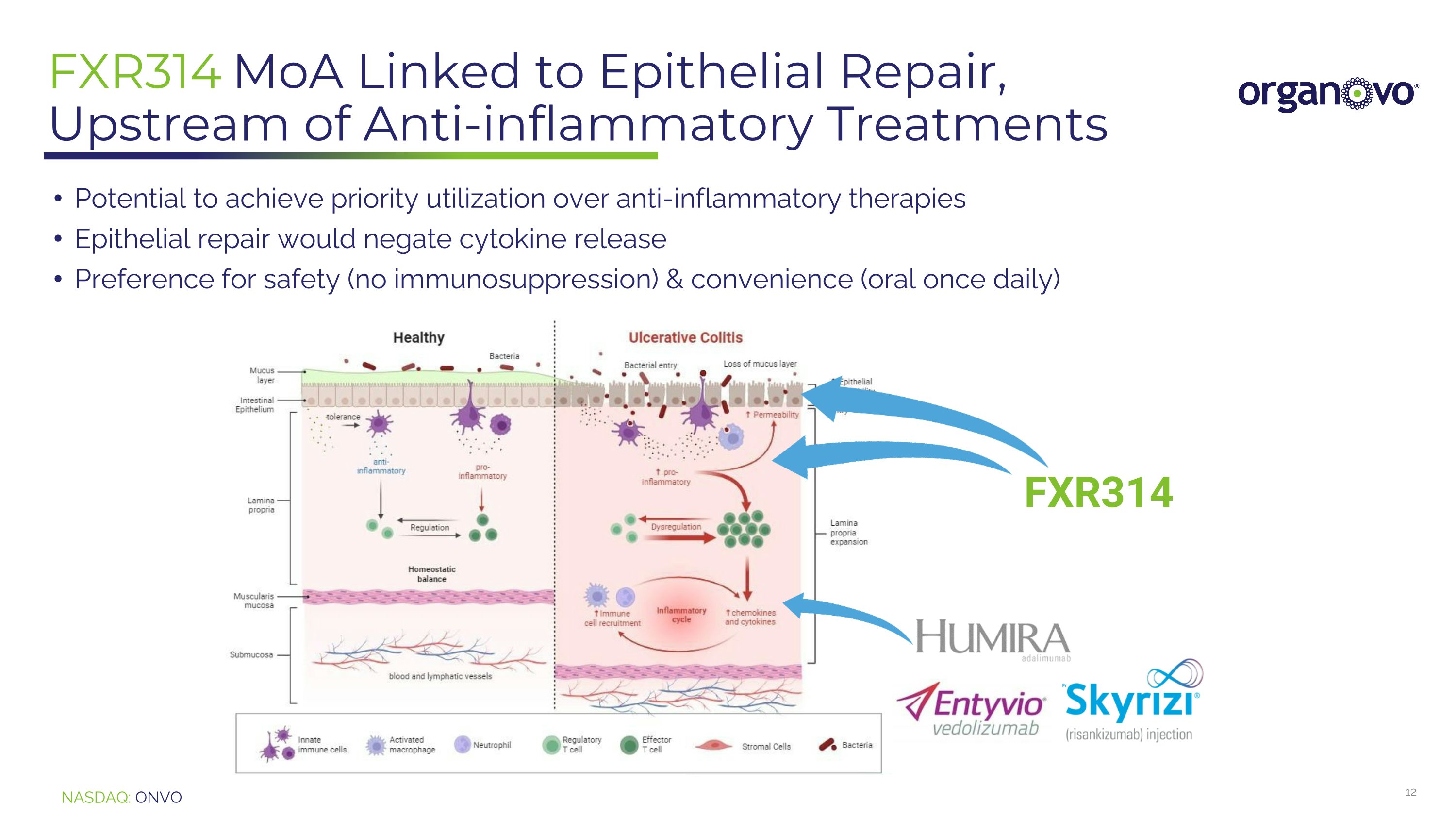
FXR314 MoA Linked to Epithelial Repair, Upstream of Anti-inflammatory Treatments FXR314 Potential to achieve priority utilization over anti-inflammatory therapies Epithelial repair would negate cytokine release Preference for safety (no immunosuppression) & convenience (oral once daily)
FXR314 Next Steps In Liver Fibrosis / MASH Drug Successful in Phase 2 Studies; Opportunity for Development Supportive Data for Superiority to Cilofexor, which is in Development in Combination in MASH Next Step: Preclinical evaluation of combination opportunities to complete in 2024 Likely combination therapy with GLP-1, especially oral Combination study with GLP-1 in preclinical setting will be run, with results expected by end of calendar 2024 Results will indicate potential dose and clinical phase 3 opportunity for MASH development Will engage in partnering discussions; regarding development of FXR314 in MASH as well

FXR314 Preclinical Support
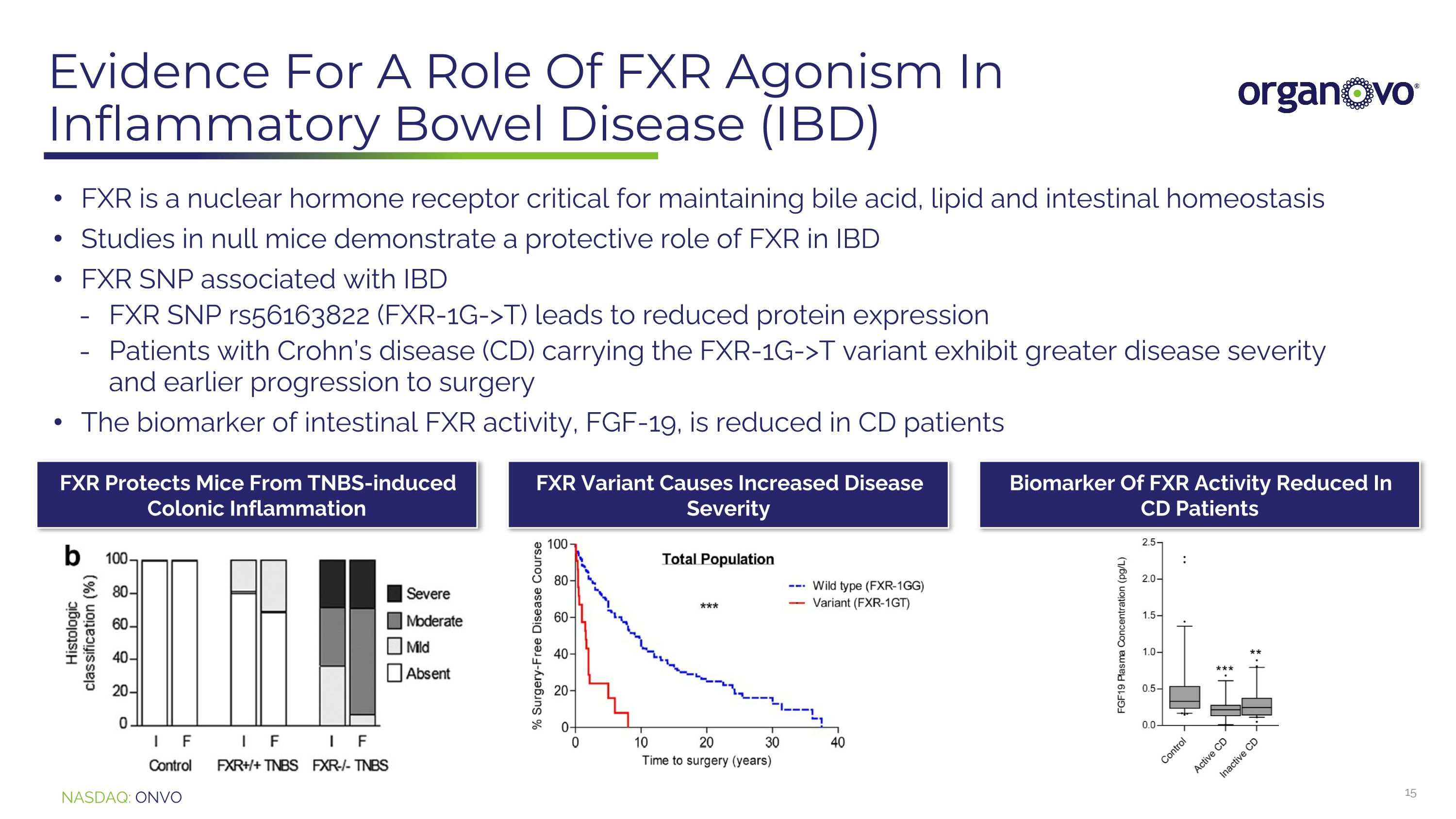
Evidence For A Role Of FXR Agonism In Inflammatory Bowel Disease (IBD) FXR is a nuclear hormone receptor critical for maintaining bile acid, lipid and intestinal homeostasis Studies in null mice demonstrate a protective role of FXR in IBD FXR SNP associated with IBD FXR SNP rs56163822 (FXR-1G->T) leads to reduced protein expression Patients with Crohn’s disease (CD) carrying the FXR-1G->T variant exhibit greater disease severity and earlier progression to surgery The biomarker of intestinal FXR activity, FGF-19, is reduced in CD patients FXR Protects Mice From TNBS-induced Colonic Inflammation FXR Variant Causes Increased Disease Severity Biomarker Of FXR Activity Reduced In CD Patients
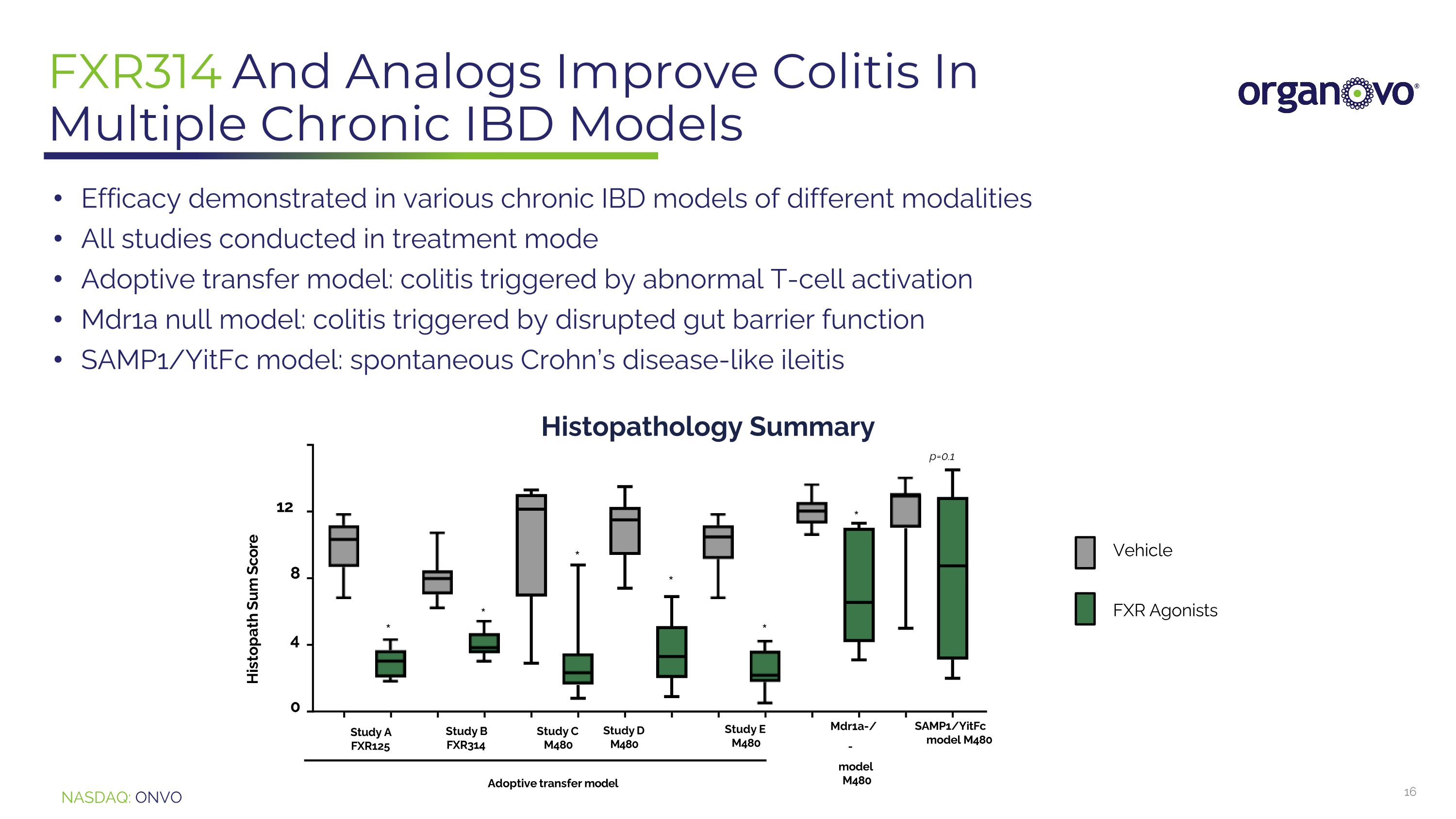
FXR314 And Analogs Improve Colitis In Multiple Chronic IBD Models Efficacy demonstrated in various chronic IBD models of different modalities All studies conducted in treatment mode Adoptive transfer model: colitis triggered by abnormal T-cell activation Mdr1a null model: colitis triggered by disrupted gut barrier function SAMP1/YitFc model: spontaneous Crohn’s disease-like ileitis 0 4 8 12 Histopath Sum Score Study A FXR125 Adoptive transfer model Mdr1a-/ - model M480 SAMP1/YitFcmodel M480 * * p=0.1 * * * * Study B FXR314 Study C Study D M480 M480 Study E M480 Vehicle FXR Agonists Histopathology Summary
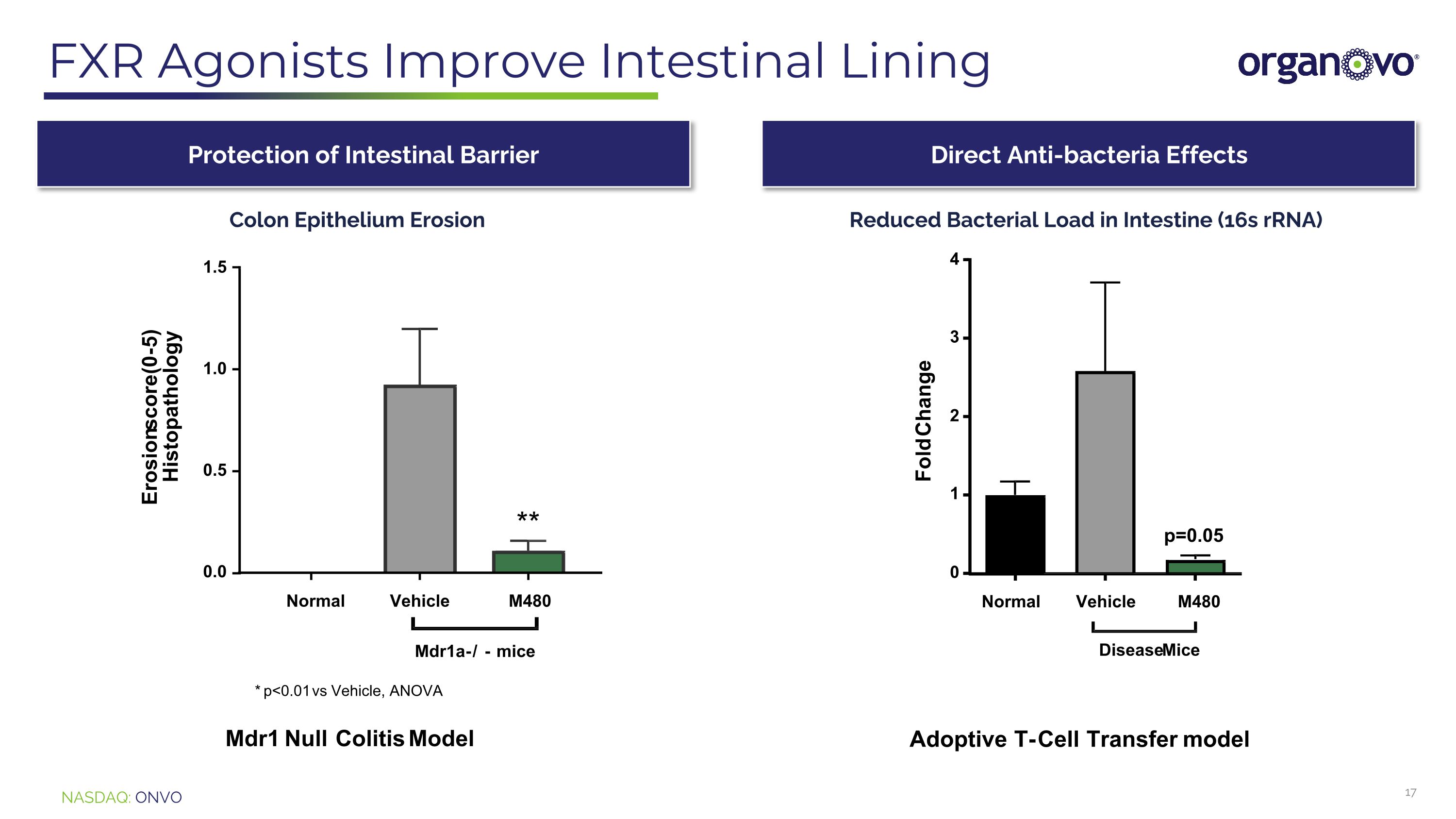
FXR Agonists Improve Intestinal Lining Protection of Intestinal Barrier Direct Anti-bacteria Effects Colon Epithelium Erosion Reduced Bacterial Load in Intestine (16s rRNA)

Market Opportunities For FXR314 13mm prevalent cases of ulcerative colitis globally in 2022 2.1mm in North America1 Global market size of $6.6 billion2 in 2021 Anticipated to reach $122 billion by 2032 Growing steadily at a CAGR of 6.0%2 Driven by the increasing prevalence of ulcerative colitis, and introduction of several new therapies NOTES: iHealthcareAnalyst, Inc., “Global Inflammatory Bowel Disease Market Landscape and Future Outlook” Future Market Insights, “Ulcerative Colitis Treatment Market Overview (2022 to 2032)”
Organovo’s 3D Human Inflammatory Bowel Disease Model And Path To FXR314
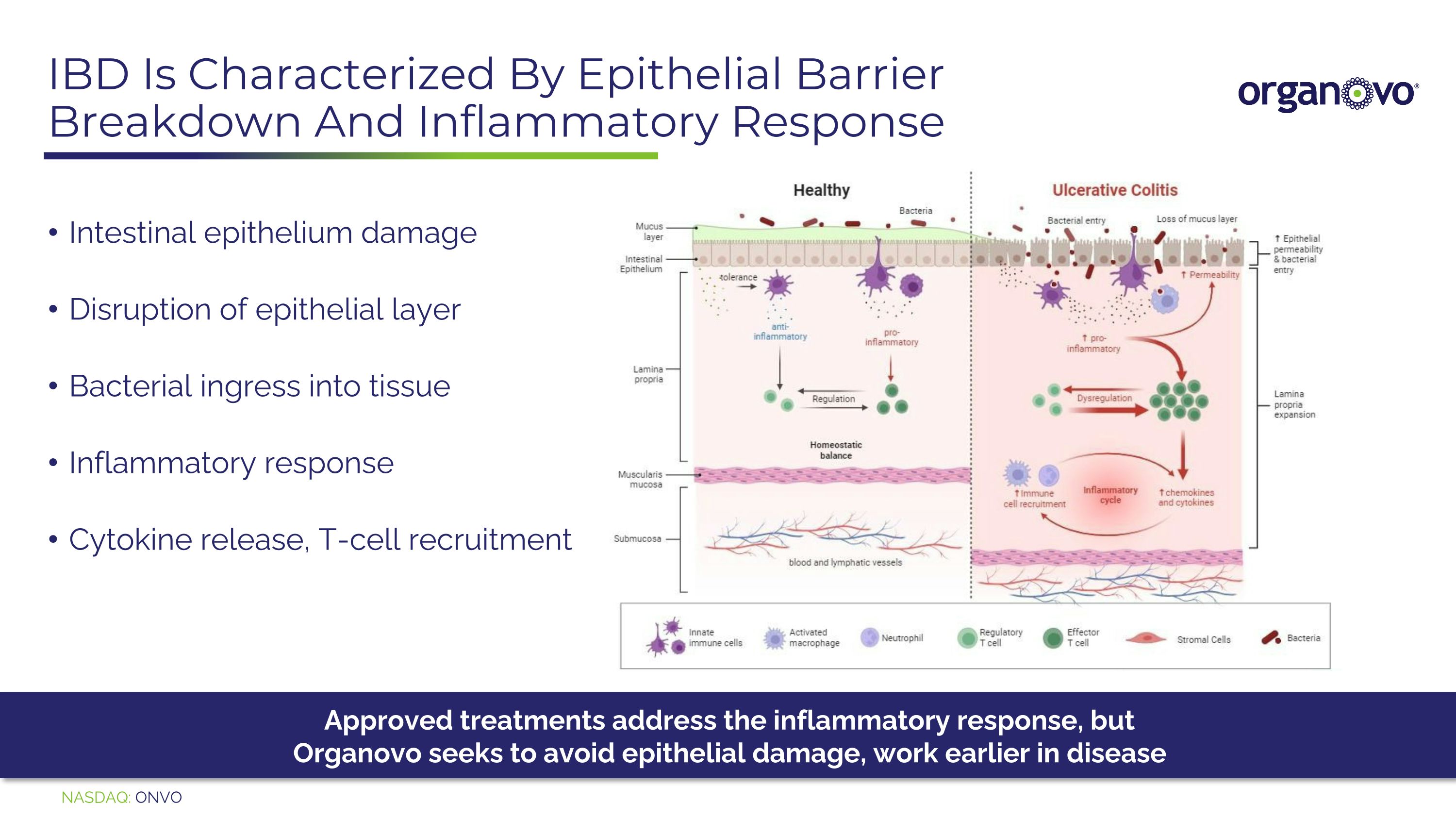
IBD Is Characterized By Epithelial Barrier Breakdown And Inflammatory Response Intestinal epithelium damage Disruption of epithelial layer Bacterial ingress into tissue Inflammatory response Cytokine release, T-cell recruitment Approved treatments address the inflammatory response, but Organovo seeks to avoid epithelial damage, work earlier in disease
Organovo 3D Disease Models Can Enable Better Clinical Outcomes Organovo creates exceptional 3D models using bioprinting and other 3D tissue technologies 3D tissue models created with cells taken directly from patients (UC or CD patients, biologic naïve or exposed, varying disease severity) Models can be used to test compounds, siRNA, etc. to validate targets or study drug effects Testing broad donor sets from a biobank allows us to understand population response to a drug or to target modulation
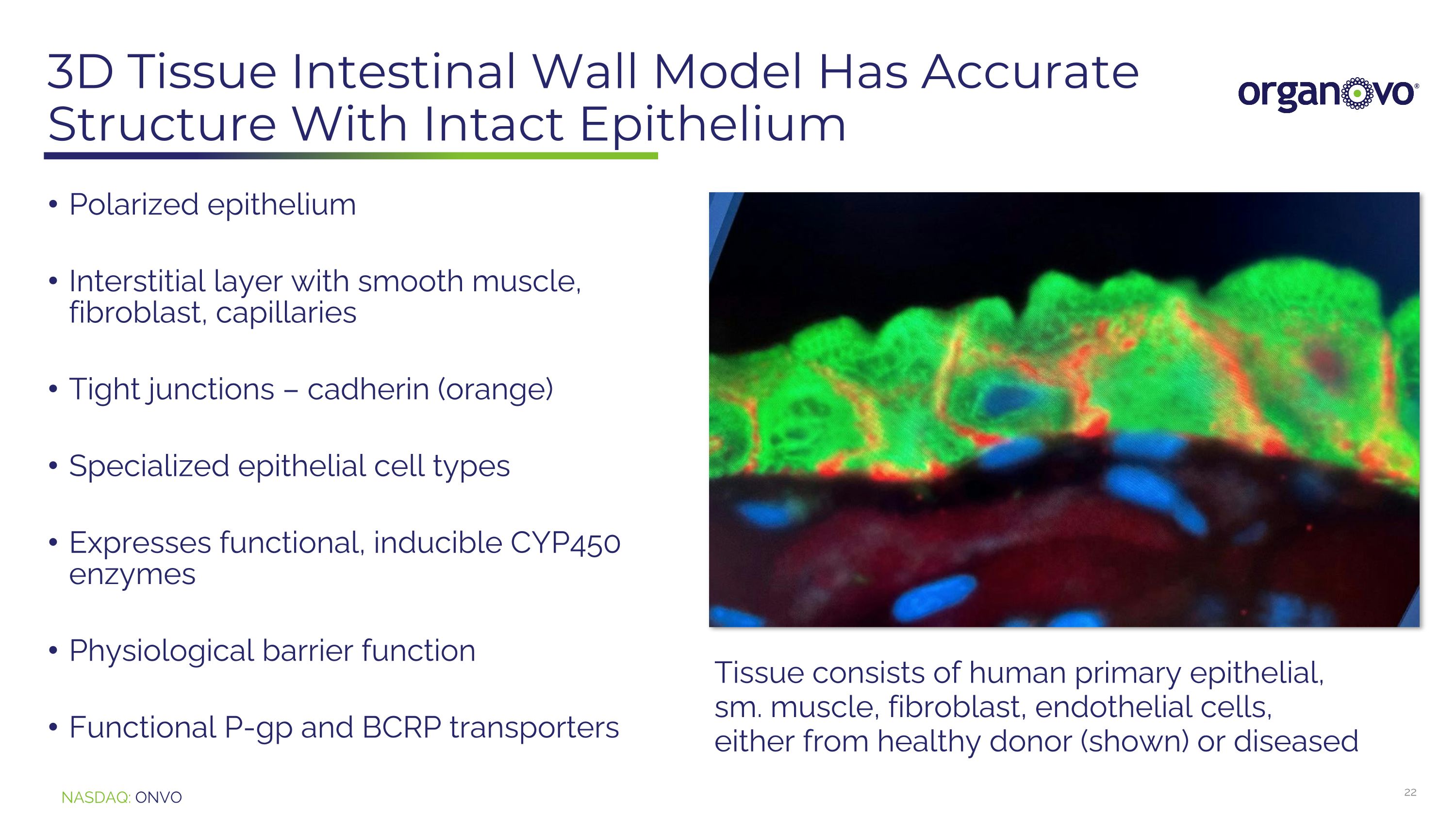
3D Tissue Intestinal Wall Model Has Accurate Structure With Intact Epithelium Polarized epithelium Interstitial layer with smooth muscle, fibroblast, capillaries Tight junctions – cadherin (orange) Specialized epithelial cell types Expresses functional, inducible CYP450 enzymes Physiological barrier function Functional P-gp and BCRP transporters Tissue consists of human primary epithelial, sm. muscle, fibroblast, endothelial cells, either from healthy donor (shown) or diseased
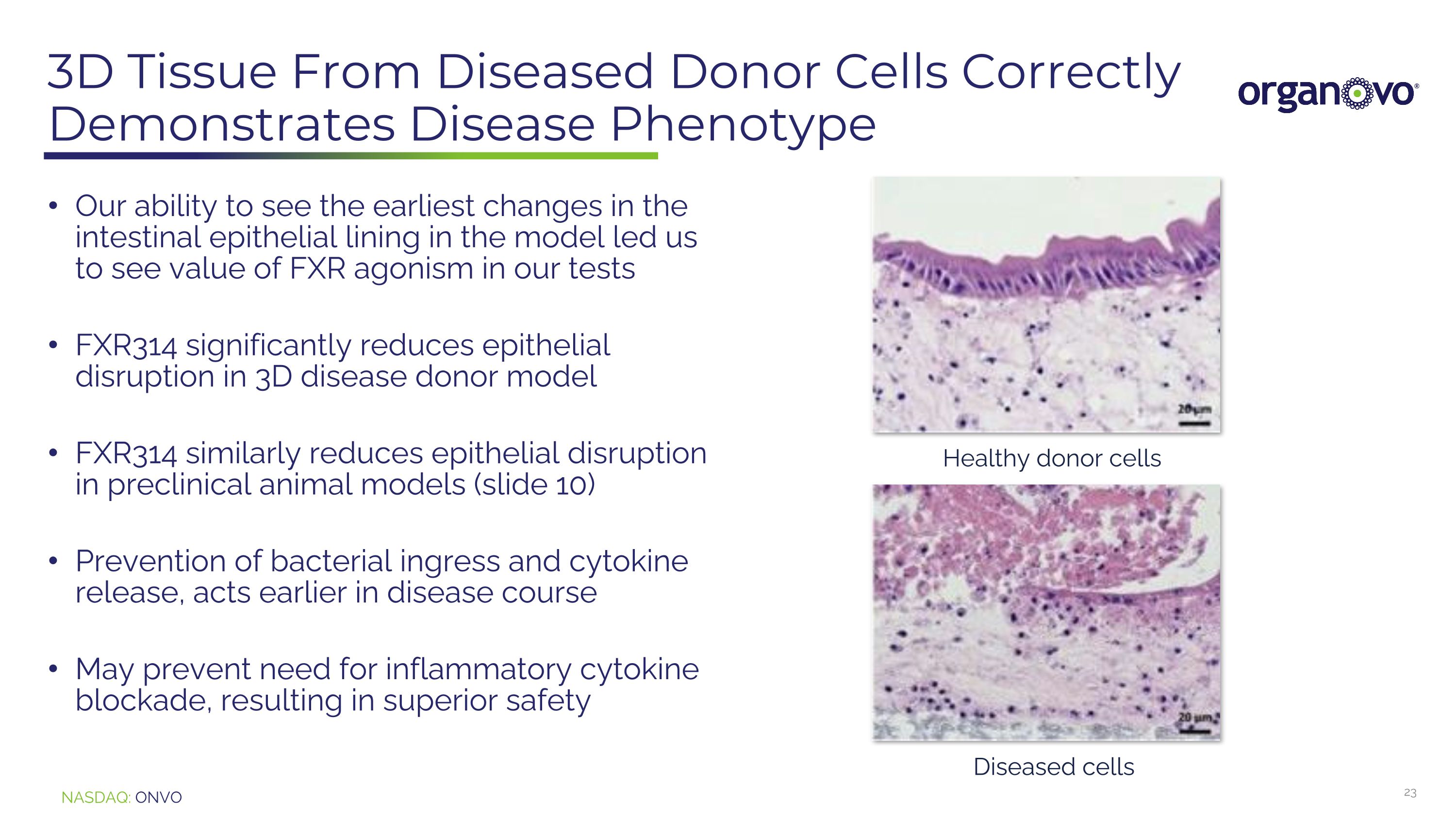
3D Tissue From Diseased Donor Cells Correctly Demonstrates Disease Phenotype Our ability to see the earliest changes in the intestinal epithelial lining in the model led us to see value of FXR agonism in our tests FXR314 significantly reduces epithelial disruption in 3D disease donor model FXR314 similarly reduces epithelial disruption in preclinical animal models (slide 10) Prevention of bacterial ingress and cytokine release, acts earlier in disease course May prevent need for inflammatory cytokine blockade, resulting in superior safety Healthy donor cells Diseased cells

Investment Summary Strong human genetics and preclinical support for target, FXR agonist Drug preclinical support – FXR314 works similarly to approved IBD drugs Strong differentiation within IBD, where substantial unmet need persists New mechanism is complementary to approved therapies High potency oral therapeutic delivery which is dosed once daily Ulcerative colitis Phase 2a POC result is expected in 2H 2025 FXR314 is effective in 3D human models, showing improvement of intestinal epithelium in cells of UC for its lead therapeutic molecule FXR314 FXR agonism: Organovo is also advancing FXR314 for the treatment of other Inflammatory Bowel Diseases including Crohn’s Disease as well as diseases of the Liver including NASH and Primary Biliary Cholangitis 2nd target: Organovo began medicinal chemistry in 2023 to create a novel drug for another, as yet undisclosed, target. The target was validated in our 3D tissue models of Crohn’s disease. IND expected by the end of 2025

CONTACT DETAILS Tom Hess, CFO thess@organovo.com INVESTOR & MEDIA RELATIONS CORE IRJustin Kulik ir@organovo.com NASDAQ: ONVO