UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the Month of April 2024
(Commission File No. 001-41636)
Oculis Holding AG
(Translation of registrant’s name into English)
Bahnhofstrasse 7
CH-6300
Zug, Switzerland
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
INFORMATION CONTAINED IN THIS REPORT ON FORM 6-K
On April 29, 2024, Oculis Holding AG (the “Registrant”) published the invitation to its 2024 Annual General Meeting to be held on May 29, 2024 at Ochsen-Zug, Kolinplatz 11, CH-6300 Zug, Switzerland, at 3:30 p.m. CEST / 9:30 a.m. EDT. The press release, the Invitation to the 2024 Annual General Meeting, the Annual General Meeting Proxy Card, and the 2023 Annual Report of the Registrant are furnished hereto as Exhibits 99.1, 99.2, 99.3 and 99.4, respectively.
EXHIBIT INDEX
|
|
|
Exhibit |
|
Description |
|
99.1 99.2 99.3 99.4 |
|
Press Release dated April 29, 2024 Invitation to the 2024 Annual General Meeting of Shareholders |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
OCULIS HOLDING AG |
||
|
|
|
|
|||
Date: April 29, 2024 |
|
|
|
By: |
|
/s/ Sylvia Cheung |
|
|
|
|
|
|
Sylvia Cheung Chief Financial Officer |
Exhibit 99.1

Oculis Publishes Invitation to the Annual General Meeting
ZUG, Switzerland, April 29, 2024 – Oculis Holding AG (Nasdaq: OCS) (“Oculis”), today published the invitation to the 2024 Annual General Meeting, which will be held on May 29, 2024 at Ochsen-Zug, Kolinplatz 11, CH-6300 Zug, Switzerland, at 3:30 p.m. CEST / 9:30 a.m. EDT.
All information pertaining to the 2024 Annual General Meeting, including meeting materials, can be accessed on the Oculis website at https://investors.oculis.com/events-presentations.
About Oculis
Oculis is a global biopharmaceutical company (Nasdaq: OCS) purposefully driven to save sight and improve eye care. Oculis’ highly differentiated pipeline comprises multiple innovative product candidates in development. It includes OCS-01, a topical eye drop candidate for diabetic macular edema (DME) and for the treatment of inflammation and pain following cataract surgery; OCS-02 (licaminlimab), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and for non-infectious anterior uveitis; and OCS-05, a disease modifying candidate for acute optic neuritis (AON) and other neuro-ophthalmic disorders such as glaucoma, diabetic retinopathy, geographic atrophy, and neurotrophic keratitis. Headquartered in Switzerland and with operations in the U.S. and Iceland, Oculis’ goal is to deliver life-changing treatments to patients worldwide. The Company is led by an experienced management team with a successful track record and is supported by leading international healthcare investors.
For more information, please visit: www.oculis.com
Oculis Contacts
Ms. Sylvia Cheung, CFO
sylvia.cheung@oculis.com
Investor & Media Relations
LifeSci Advisors
Corey Davis, Ph.D.
cdavis@lifesciadvisors.com
1-212-915-2577
Exhibit 99.4

Oculis Holding AG
2023 Annual Report
Exhibit 99.4
Exhibit 99.4

1
Dear Shareholder,
2023 was Oculis’ first year as a public company. We had a milestone-rich year with undisputed operational and clinical success, and we are off to a strong start in 2024. It is only appropriate to take a moment to express my immense gratitude for your support along our journey, provide an update on some of these advancements throughout the last year and share our exciting outlook for 2024 and beyond.
In March 2023, we successfully listed on NASDAQ, and during the first half of 2023 we raised gross proceeds of approximately $146 million, which has supported the advancement of our differentiated pipeline comprising multiple innovative product candidates. The most advanced product candidate in our pipeline is OCS-01, a topical eye drop for diabetic macular edema (DME), treatment of inflammation and pain following ocular surgery, and cystoid macular edema (CME). Our second product candidate is OCS-02 (Licaminlimab), a topical biologic anti-TNFα eye drop candidate for dry eye disease (DED) and chronic and recurrent non-infectious anterior uveitis. Our third product candidate, OCS-05, is a potential disease modifying and neuroprotective candidate for acute optic neuritis (AON) and other neuro-ophthalmic disorders such as glaucoma, diabetic retinopathy, geographic atrophy, and neurotrophic keratitis.
Major pipeline advancement
We are thrilled to have positively delivered on our commitments in 2023, establishing ourselves favorably as we advance our late-stage clinical development programs. Following our successful listing on NASDAQ, we had two positive topline readouts for OCS-01: a Stage 1 Phase 3 readout for OCS-01 in DME, and a Stage 1 Phase 3 readout for OCS-01 in inflammation and pain following cataract surgery.
In May 2023, we announced positive topline results for Stage 1 of the Phase 3 DIAMOND trial of OCS-01 in DME. DME is a leading cause of vision impairment in working-age adults. Stage 1 of the Phase 3 DIAMOND trial met both primary and secondary endpoints. OCS-01 demonstrated robust statistically significant improvement in vision and reduction in retinal edema vs. vehicle, and was well-tolerated with no unexpected safety findings. Following the positive results from the DIAMOND trial, we initiated Stage 2 for our Phase 3 DIAMOND program, including first patient first visit for DIAMOND-1 in December 2023, and first patient first visit for DIAMOND-2 in February 2024. Data from both DIAMOND trials are intended to support our NDA submission to the Food and Drug Administration (FDA) for OCS-01 in DME. If approved, OCS-01 has the potential to become the first and only topical eye drop and non-invasive treatment option for DME.
In August 2023, we announced positive topline results from our Phase 3 OPTIMIZE-1 trial of OCS-01 for the treatment of inflammation and pain following cataract surgery. OPTIMIZE-1 met both primary endpoints demonstrating superior reduction in inflammation and pain following cataract surgery compared to vehicle. In December 2023, we initiated the second Phase 3 OPTIMIZE-2 trial of OCS-01 for the treatment of inflammation and pain following cataract surgery. The OPTIMIZE-2 topline readout is expected in the fourth quarter of 2024 and, if positive, would support an NDA submission to the FDA. Also in August 2023, the first patient was enrolled in LEOPARD, an investigator-initiated trial with OCS-01 eye drops for the treatment of CME. CME may occur as a complication of ocular conditions, including uveitis and ocular surgery, and is a leading cause of vision loss worldwide. The LEOPARD trial is administratively sponsored by the Global Ophthalmic Research Center (Los Altos, California). Enrollment is ongoing and topline results are expected in the first quarter of 2025.
Our second product candidate, OCS-02 (Licaminlimab), is currently being evaluated in the Phase 2b RELIEF trial to assess its potential as a topical anti-TNFα treatment for DED. While OCS-02 is intended to be developed for moderate to severe DED patients, we are advancing the development of OCS-02 in conjunction with the development of a potentially novel genetic biomarker intended to identify patients who may have a greater response to OCS-02 therapy. In November 2023, we initiated the Phase 2b RELIEF trial and completed enrollment in February 2024. The RELIEF topline readout is expected before the end of second quarter of 2024.
Furthermore, we continue to advance the Proof-of-Concept (PoC) ACUITY trial for OCS-05, a potential disease-modifying candidate for acute optic neuritis (AON). The trial evaluates the safety and tolerability of a once-daily intravenous infusion of OCS-05 compared to placebo for 5 days in addition to current standard of care and includes a follow up period of 6 months. The readout is expected in the fourth quarter of 2024. Concurrently, we plan to complete IND enabling activities in the U.S.
2
Conclusion
To conclude, 2023 was a pivotal and transformative year. The efficient use of capital coupled with our operational excellence, we have made significant progress on multiple fronts. Our core growth objectives are centered on advancing our product candidates, and we entered 2024 stronger and well positioned to continue to deliver strong value to our shareholders and patients. Building upon the momentum, we started 2024 with already achieving two clinical milestones as described above, as well as listing on Nasdaq Iceland Main Market with a concurrent $59 million Registered Direct financing in April. Additionally, as we continue to advance our clinical programs and start to prepare for our first potential launch in the U.S., we expanded our executive team with the appointment of Rebecca Weil as our Chief Commercial Officer, Virginia Dean as our Chief Human Resources Officer, and Snehal Shah as our President of Research & Development. In 2024 we anticipate readouts from: Phase 2b RELIEF trial for OCS-02 in DED, second Phase 3 OPTIMIZE-2 trial for OCS-01 for the treatment of inflammation and pain following cataract surgery, and PoC ACUITY trial for OCS-05 in AON. We look forward to providing updates to our valued shareholders on our progress throughout the year.
Sincerely,
Riad Sherif, M.D.
Chief Executive Officer
3

Business Update
4
Exhibit 99.4
1. Information on the Company
A. History and Development of the Company
We are a stock corporation (Aktiengesellschaft) that was incorporated under the laws of Switzerland on October 31, 2022. We are registered with the commercial register of the Canton of Zug under company registration number CHE-396.695.611. The mailing address of our principal executive office after the Acquisition Closing is Oculis Holding AG, Bahnhofstrasse 7, CH-6300, Zug, Switzerland. Neither our articles of association nor the operation of law limit our duration.
Certain additional information about the Company is included in "Item 1.B. Business Overview” of this Section "Business Update" and is incorporated herein by reference. The material terms of the Business Combination are described in Item 10 of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024. The Company is subject to certain of the informational filing requirements of the Exchange Act. Since the Company is a “foreign private issuer”, it is exempt from the rules and regulations under the Exchange Act prescribing the furnishing and content of proxy statements, and the officers, directors and principal shareholders of the Company are exempt from the reporting and “short-swing” profit recovery provisions contained in Section 16 of the Exchange Act with respect to their purchase and sale of Ordinary Shares. In addition, the Company is not required to file reports and financial statements with the SEC as frequently or as promptly as U.S. public companies whose securities are registered under the Exchange Act. However, the Company is required to file with the SEC an Annual Report on Form 20-F containing financial statements audited by an independent accounting firm. The SEC also maintains a website at http://www.sec.gov that contains reports and other information that the Company files with or furnishes electronically to the SEC.
Our telephone number is +41-41-711-9325 and our website is www.oculis.com.
B. Business Overview
Company Overview
We are a late clinical-stage biopharmaceutical company, based in Switzerland, with substantial expertise in therapeutics used to treat ocular diseases, engaged in the development of innovative drug candidates which embrace the potential to address large unmet medical needs for many eye-related conditions. Our focus is on advancing therapeutic candidates intended to treat significant and prevalent ophthalmic diseases which result in vision loss, blindness or reduced quality of life. Our mission is to improve the health and quality of life of patients around the world by developing medicines that save sight and improve eye care for patients. To realize this mission, we intend to become a global leader in ocular therapeutics.
Our pipeline currently includes three clinical-stage therapeutic candidates: OCS-01, OCS-02 (Licaminlimab) and OCS-05. Our lead product candidate, OCS-01, is currently being evaluated in two ongoing Phase 3 clinical programs: as a topical option for the treatment of DME, and as a once-daily steroid for the treatment of inflammation and pain following cataract surgery. Our second product candidate is OCS-02, currently being evaluated in a Phase 2b clinical trial to assess its potential as a topical anti-TNFα treatment for dry eye disease (“DED”) and potentially the use of a particular genotype to predict treatment response, which could be considered as a biomarker in a precision medicine approach. A second clinical trial for OCS-02, designed to evaluate its use as a potential treatment for non-infectious anterior uveitis, is expected to follow thereafter. Our third product candidate is OCS-05, a potential disease modifying neuroprotective agent against neurological damage with potential application in multiple indications, including glaucoma, dry age-related macular degeneration (“AMD”) and diabetic retinopathy (“DR”). We are conducting a Phase 2 Proof-of-Concept (“PoC”) trial evaluating OCS-05 as a potential treatment for acute optic neuritis (“AON”) for which there is currently no approved therapeutic treatment.
5
Summary of Our Product Candidates Portfolio
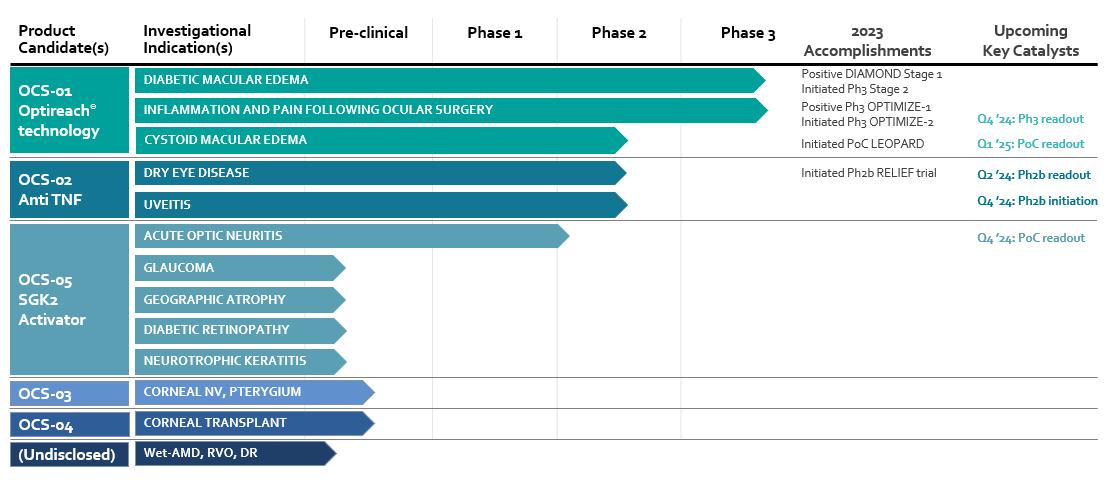
AMD, age-related macular degeneration; RVO, retinal vein occlusion; DR, diabetic retinopathy. OCS-01 is based on the OPTIREACH technology, OCS-02 is a single chain antibody fragment (ScFv) against TNF alpha and OCS-05 is a SGK-2 activator peptidomimetic small molecule with novel MoA targeting the activation of the trophic factor pathways. The Company’s additional earlier stage development candidates are discussed in the section under the header “Our clinical development candidates” below.
Utilizing our internal core competency in formulation discovery and drug development capabilities, together with extensive licensing, collaboration and acquisition activities, we have assembled a pipeline of attractive development candidates that include both late-stage clinical candidates as well as earlier stage preclinical initiatives. Our clinical candidate portfolio includes:
OCS-01
Our lead candidate is OCS-01, a novel, high concentration, topical Optireach formulation (ophthalmic suspension) of dexamethasone, designed to enhance drug penetration into both the anterior and posterior segments of the eye with enhanced persistence following topical application. We are evaluating OCS-01 for use as a topical eye drop for the treatment of DME, as a once-daily steroid treatment for inflammation and pain following ocular surgery, and in treating two forms of cystoid macular edema (“CME”). LEOPARD, an investigator-initiated trial (“IIT”) for the treatment of Uveitic Macular Edema (“UME”) or Post-Surgical Macular Edema (“PSME”), both forms of CME, is ongoing and the related readout is expected in the first quarter of 2025. Using our proprietary Optireach® technology, OCS-01 was designed to enhance drug penetration into both the anterior and posterior segments of the eye. This therapeutic approach is in contrast to currently available therapies, which require the use of more invasive treatments such as ocular implants or intravitreal injections to deliver medication to the retina. Furthermore, current treatment of DME often involves multiple intravitreal injections per year. Given the burden of therapy, FDA-approved therapeutics are not widely used for early disease intervention. It has been reported that 60% of DME patients are not treated 12 months after the diagnostic (IRIS data base June 23), despite the deterioration in visual acuity in 19.0% of untreated patients within two years. In addition, approximately 40.0% of patients treated with anti-VEGF intravitreal injections have an inadequate response at 12 weeks.
OCS-01 is a topical dexamethasone Optireach formulation which is designed to deliver therapeutic levels of drug to the retina via an eye drop, a route of administration for DME treatment that may enable earlier intervention and thereby significantly increase the proportion of patients being treated as well as increase the prescribing physician base by providing a treatment option to general ophthalmologists. An eye drop treatment could also provide a new treatment option for patients with inadequate response to the current invasive standard of care. We are currently not aware of the existence of any other eye drop treatment for DME which is in a similar or more advanced stage of active clinical development; however, we cannot guarantee that OCS-01 will receive regulatory approval. We have two ongoing Phase 3 clinical trials: (i) DME and (ii) for the treatment of inflammation and pain following ocular surgery. For DME, the first stage of the DIAMOND Phase 3 clinical trial met its objective of validating the induction and maintenance dosing regimen designed to optimize OCS-01 efficacy potential with robust statistical significance. For the treatment of inflammation and pain following cataract surgery, the first Phase 3 clinical trial, OPTIMIZE-1, met both hierarchical primary efficacy endpoints with statistical significance, the absence of inflammation at day 15 and the absence of pain at day 4. Following the positive DIAMOND trial outcome, we are advancing the planned OCS-01 development program for DME into DIAMOND Stage 2, which includes two global pivotal Phase 3 clinical trials, DIAMOND-1 and DIAMOND-2.
6
We announced first patient first visit in DIAMOND-1 and DIAMOND-2 in December 2023 and February 2024, respectively. Each trial is expected to enroll approximately 350 to 400 patients. Following the positive OPTIMZE-1 trial outcome, we are advancing the development program for inflammation and pain following cataract surgery into the second Phase 3 trial, OPTIMIZE-2. In December 2023, we also announced first patient first visit in OPTIMIZE-2. Data from the two OPTIMIZE trials are intended to support the our future NDA submission to the FDA.
In addition to the Phase 3 trials, we are conducting the LEOPARD trial, which is an IIT to investigate the safety and efficacy of OCS-01 in UME and PSME. LEOPARD is sponsored by Global Ophthalmic Research Center (GORC). This PoC trial’s data readout is expected in the first quarter of 2025.
The total U.S. prevalence of DME in 2023 is estimated at 3.0 million, with the diagnosed U.S. prevalence estimated at 1.8 million by the Decision Resources Group DME Landscape November 2020 report. The same report estimates that 0.9 million U.S. DME patients were treated with drugs in 2023, leaving 0.9 million U.S. patients diagnosed but untreated. These 0.9 million patients are a key addressable market segment for OCS-01. Additionally, OCS-01 is also intended to address the market segment of patients with inadequate response to anti-VEGF therapy. A study published in the American Journal of Ophthalmology in 2016 found that nearly 40.0% of patients treated with anti-VEGF therapy had inadequate responses at 12 weeks. By applying this figure to the number of treated U.S. patients, we estimate that inadequate response occurs in approximately 0.4 million patients. In total, we estimate that 1.3 million DME patients in the United States are addressable by OCS-01.
The Informa Meddevicetracker Ophthalmic Surgical Products Market 2017 report projected that ophthalmic surgeries are on the rise, mainly due to the aging population and lifestyle changes, and are expected to reach close to 10 million procedures per year in the U.S. alone by 2037. Cataract surgeries are the most prevalent procedures of all medical specialties with an estimated 5 million procedures in 2021 in the U.S. Ophthalmic surgeries cause the release of inflammatory factors and can be associated with ocular pain. Cataract surgery, even with a very small incision, creates inflammation in the cornea, anterior chamber and iris. Given our observations in Stage 1 of the DIAMOND Phase 3 trial that OCS-01 treatment led to improvements in visual acuity and macular thickness in patients with DME, we believe OCS-01, if approved for inflammation and pain following ocular surgery, may be of benefit to patients at risk of retinal complications following ocular surgery. An exploratory investigator initiated trial is ongoing in the U.S. to assess its benefits in treating CME. A pilot study demonstrated favorable effects of OCS-01 on eyes with non-infectious uveitic macular oedema and vitritis (Schulman et al, Acta Ophthalmologica 2015).
OCS-02 (Licaminlimab)
We are also advancing the clinical development of licaminlimab, or OCS-02, a next-generation biologic treatment for ocular inflammation, specifically as a treatment for DED and non-infectious chronic anterior uveitis. Differentiating OCS-02 is its use of a single chain antibody fragment specifically formulated for topical delivery in ophthalmology, TNF inhibitors are directed against the cytokine human tumor necrosis factor alpha (“TNFα”). Furthermore, the small size of the fragment enables the topical delivery of an anti-TNFα construct with increased concentrations and enhanced ocular tissue penetration. The anti-inflammatory and anti-necrotic properties of therapeutics inhibiting TNFα activity are well established with anti-TNF pharmaceuticals already approved as systemic treatments for ocular disease. While OCS-02 is intended to be developed for all comers, we are advancing the development of OCS-02 in conjunction with the development of a potentially novel genetic biomarker intended to identify patients who may have a greater response to OCS-02 therapy. Two Phase 2 clinical trials in patients with symptoms of DED were conducted (the first with the predecessor of OCS-02, and the second with OCS-02), as well as one Phase 2 clinical trial in acute anterior uveitis. Topical ocular administration of OCS-02 was associated with improvements in the global ocular discomfort score versus vehicle in patients with DED, and with reaching a pre-specified responder rate in patients with non-infectious anterior uveitis, as well as being well tolerated in all three studies. In February 2024, we completed enrollment in the Phase 2b RELIEF trial evaluating OCS-02 for the treatment of DED, with topline results expected in the second quarter of 2024. We plan to commence a Phase 2b trial for OCS-02 as a treatment for chronic anterior uveitis thereafter.
We estimate the segment of DED patients in the United States addressable by OCS-02 (patients with moderate or severe DED) to be approximately 10 million patients. This comprises an estimated 7 million patients with moderate DED and 3 million patients with severe DED (based on the rates of approximately 35.0% moderate and 14.0% severe patients as reported by the Dry Eye Products Market Report published in Market Scope 2023 of approximately 20.0 million diagnosed prevalent cases of DED in the U.S. as estimated for 2024 by Decision Resources Group Dry Eye Disease Landscape and Forecast, December 2020).
7
We also estimate OCS-02 could help address a medical need in patients suffering from either chronic or recurring non-infectious anterior uveitis. This addressable patient population is estimated to be approximately 170 thousand in the United States based on a prevalence rate of non-infectious uveitis of 121 per 100,000, applied to the U.S. population and the fact that anterior uveitis is the most prevalent form representing 81.0% of all cases, as found in a study published in the Journal of the American Medical Association Ophthalmology in 2016, and based on a prevalence of recurrent and chronic disease being estimated at 51.0%, as found in a study published in the Journal of the American Medical Association Ophthalmology in 2013.
OCS-05
Our third clinical candidate is OCS-05, a novel serum/glucocorticoid-regulated protein kinase 2 (“SGK2”) activator peptidomimetic small molecule, in development as a potential disease modifying neuroprotective agent against neurological damage to the optic nerve. We are initially developing OCS-05 as a potential therapeutic to treat AON, a rare disease with high unmet medical need, as currently there is no treatment which is approved by the FDA or European Commission for AON. OCS-05 has been granted Orphan Drug Designation by both the FDA and the European Commission for this indication. OCS-05 has been studied in preclinical studies suggesting efficacious neuroprotective and remyelinating activity, as well as in a UK Phase 1 clinical trial under the Medicines and Healthcare products Regulatory Agency (“MHRA”) in healthy volunteers in which OCS-05 was observed to be well tolerated. We are currently conducting a First-in-Patient clinical trial of OCS-05 in AON in France to test the candidate’s safety and tolerability, and we are currently conducting IND-enabling activities for OCS-05 in the United States. Should the clinical results of our AON trial prove sufficiently compelling, we intend to evaluate the promise of OCS-05 to treat other neuro-ophthalmic disorders such as geographic atrophy, glaucoma, diabetic retinopathy and neurotrophic keratitis.
Additional development initiatives
In addition to these six clinical development programs involving the three clinical candidates, we also are engaged in a number of earlier preclinical development initiatives, including:
Our Executive Management Team
We are led by an experienced management team, composed of individuals who have extensive backgrounds in drug discovery and development, clinical trial design and operations, regulatory affairs, business development and commercial and general management at both large pharmaceutical companies and emerging biopharmaceutical organizations. Collectively, our management team has a track record of advancing new drug candidates through regulatory approval and successful commercialization. The expertise of our management team is complemented by our board of directors, which includes many accomplished industry veterans with significant capabilities in guiding the success of emerging biopharmaceutical companies such as ours. Since our inception we have raised approximately CHF 239.2 million from leading North American, European and Asian life science venture capital investors including Brunnur Ventures (Brunnur vaxtarsjodur slhf.), BVCF Management (BEYEOTECH), EQT Life Sciences, Novartis Bioventures Ltd., LSP 7 Coöperatief, funds managed by Earlybird Capital, funds managed by Pivotal Partners, funds managed by Aberdeen (formerly Tekla Capital Management LLC), and VI Partners, among others. Please note that prospective investors should not rely on these named investors’ investment decisions, as each of such investor’s risk tolerance and investment strategy and goal may be different from those of other prospective investors.
Our Strategy
We intend to become a leader in developing therapeutics to address ocular diseases characterized by significant medical needs with large market opportunities. To accomplish this objective, we plan to focus on successful completion of our key strategic initiatives, which include:
8
Based on results achieved in the Stage 1 Phase 3 trial, we have progressed to the Stage 2 Phase 3 trials of OCS-01 in DME, DIAMOND-1 and DIAMOND-2, which are currently ongoing. We believe the use of OCS-01 formulated as a non-invasive, self-administered eye drop, could, if approved, promote a shift in the current treatment paradigm to allow earlier intervention and increase both the treated patient population and the prescribing physician base. In addition, OCS-01, if approved, could benefit patients who are diagnosed with DME and who have an inadequate response to anti-VEGF intravitreal injections.
Following positive results in the first Phase 3 trial, OPTIMIZE-1, we have initiated the second Phase 3 trial, OPTIMIZE-2, of OCS-01 in the treatment of inflammation and pain following ocular surgery, with first patient first visit achieved in December 2023. OCS-01 could be differentiated in the anterior segment by its potential ability to deliver therapeutic drug levels to the back of the eye. Topline results from OPTIMIZE-2 are expected in the fourth quarter of 2024. An investigator-initiated PoC trial is currently ongoing to explore further the potential of OCS-01 in treating edema in CME. We believe this potential benefit in CME, if supported by this study and validated by future studies, and if OCS-01 is approved, may enable us to achieve enhanced market access.
Based on results achieved in three Phase 2 clinical trials, we have advanced OCS-02 into a Phase 2b RELIEF clinical trial to assess its clinical benefit in treating DED. We intend to initiate a second Phase 2 trial for OCS-02 as a treatment for chronic anterior uveitis thereafter. OCS-02 is differentiated by its use of single-chain antibody fragment formulation technology, which enables the topical delivery of an anti-TNFα agent. We are advancing the development of OCS-02 in conjunction with further analysis of a potential novel genetic biomarker intended to identify patients who may demonstrate an enhanced response to OCS-02 therapy and believe this precision medicine approach may allow the candidate to deliver superior outcomes in this patient group, if approved.
The differentiated and novel mechanism of action of OCS-05, coupled with its potential disease modifying neuroprotective properties, suggests potential benefits across many of the more pervasive neurological pathologies of the eye including geographic atrophy, diabetic retinopathy, glaucoma and neurotrophic keratitis. We initially intend to assess the safety of OCS-05 as a treatment for AON and are currently evaluating OCS-05 in a First-in-Patient study called the ACUITY trial in France. There is currently no approved therapy for treatment of AON. OCS-05 has been granted Orphan Drug Designation by both the FDA and the European Commission. We believe that demonstration of therapeutic benefits in AON may provide compelling support for the exploration of OCS-05 in larger market opportunities.
We intend to complement our ongoing development programs by accessing additional innovative product candidates and technologies through in-licensing, strategic collaborations and acquisitions. We believe that the depth of our formulation discovery and drug development expertise specific to ocular therapeutics, coupled with the industry network of our executive management, board of directors and advisors, provide us with the differentiated set of capabilities necessary to identify and advance product candidates successfully in this therapeutic category.
We have retained rights globally to all of our indications, including our lead product candidate OCS-01, for the potential treatment of DME and inflammation and pain following ocular surgery; OCS-02 for the potential treatment of DED and non-infectious anterior uveitis; and OCS-05 as a neuroprotective agent. Given the potential to treat patients worldwide, we may opportunistically enter into strategic collaborations around certain product candidates, diseases or geographic regions.
Diseases and disorders of the eye
9
Numerous diseases and disorders, many of which represent significant medical needs, are associated with the human eye. Ocular diseases, which may result in visual impairment, blindness or reduced quality of life include retinal diseases such as DME, macular degeneration (including geographic atrophy), diabetic retinopathy, and retinal vein occlusion (“RVO”); disorders caused by swelling and inflammation such as DED, corneal keratitis and uveitis; and glaucoma, among other disease states. The global market for therapeutics used to treat eye disease is estimated to have exceeded $22 billion in 2020. We employ our substantial expertise in the development of therapeutics, in particular pharmaceuticals used to treat ocular diseases, to potentially address many eye-related conditions with high unmet medical needs. Our focus is on developing innovative drug candidates to address significant and growing ophthalmic diseases, which result in vision loss, blindness or reduced quality of life, for which there are currently limited treatment options.
Our clinical development candidates
Utilizing our internal formulation discovery and drug development capabilities, together with extensive licensing, collaboration and acquisition activities, we have assembled a pipeline of attractive development candidates that include both late-stage clinical candidates as well as earlier stage preclinical initiatives. Our clinical portfolio is made up of (i) OCS-01, currently in two ongoing Stage 2 Phase 3 clinical trials, one evaluating its use as a treatment for DME and the other assessing its utility to treat inflammation and pain following ocular surgery; (ii) OCS-02 (Licaminlimab), currently in one ongoing Phase 2b clinical trial evaluating its use as a potential treatment for DED and anticipated to enter a second Phase 2b trial evaluating its potential use as a therapy for the treatment of non-infectious anterior uveitis; and (iii) OCS-05, a novel neuroprotective agent with potential application in multiple indications, including glaucoma, GA and DR, which we are initially evaluating as a potential treatment for AON. A detailed assessment of each of these clinical candidates is contained in the descriptions provided below.
OCS-01
Key program highlights:
Our lead development candidate OCS-01 is a 1.5% suspension of the anti-inflammatory corticosteroid dexamethasone for use as a potential treatment for DME and for inflammation and pain following ocular surgery. In contrast to currently available formulations of dexamethasone, which require the use of more invasive treatments such as an implant or intravitreal injection to deliver the medication to the retina, differentiating OCS-01 is our use of the proprietary Optireach® technology, which enables the topical delivery, as an eye drop, of dexamethasone to the back of the eye for the treatment of diseases affecting the retina. OCS-01 is a topical dexamethasone formulation which we have observed in clinical trials to be capable of delivering therapeutic levels of drug to the retina via eye drop, a route of administration for DME treatment that may enable earlier treatment intervention and thereby significantly increase the proportion of patients being treated as well as increase the prescribing physician base by providing a treatment option to general ophthalmologists. We are currently not aware of the existence of any other eye drop treatment for DME which is in a similar or more advanced stage of active clinical development; however, we cannot guarantee that OCS-01 will receive regulatory approval.
10
During 2023, we reported data from two Phase 3 clinical trials in which we observed: in DME, a statistically significant improvement in BCVA and visual acuity; and in inflammation and pain following ocular surgery, a statistically significant increase in the proportion of subjects with absence of inflammation and pain under OCS-01 treatment versus vehicle. Following those results, we have initiated a Stage 2 OPTIMIZE Phase 3 trial for the treatment of inflammation and pain following ocular surgery and begun the first Stage 2 DIAMOND-1 Phase 3 trial for the treatment of DME.
Dexamethasone is a widely studied and well characterized pharmaceutical commonly used to treat a range of inflammatory conditions and is currently included on the World Health Organization’s List of Essential Medicines. It may be administered orally, by injection, or topically. Specific to ocular disorders, dexamethasone intravitreal implants have been approved by the FDA to treat DME, uveitis and macular edema caused by RVO. Dexamethasone is also used as an ophthalmic suspension for ocular inflammation though the required frequency of dosing in order to achieve a therapeutic effect often limits its utility.
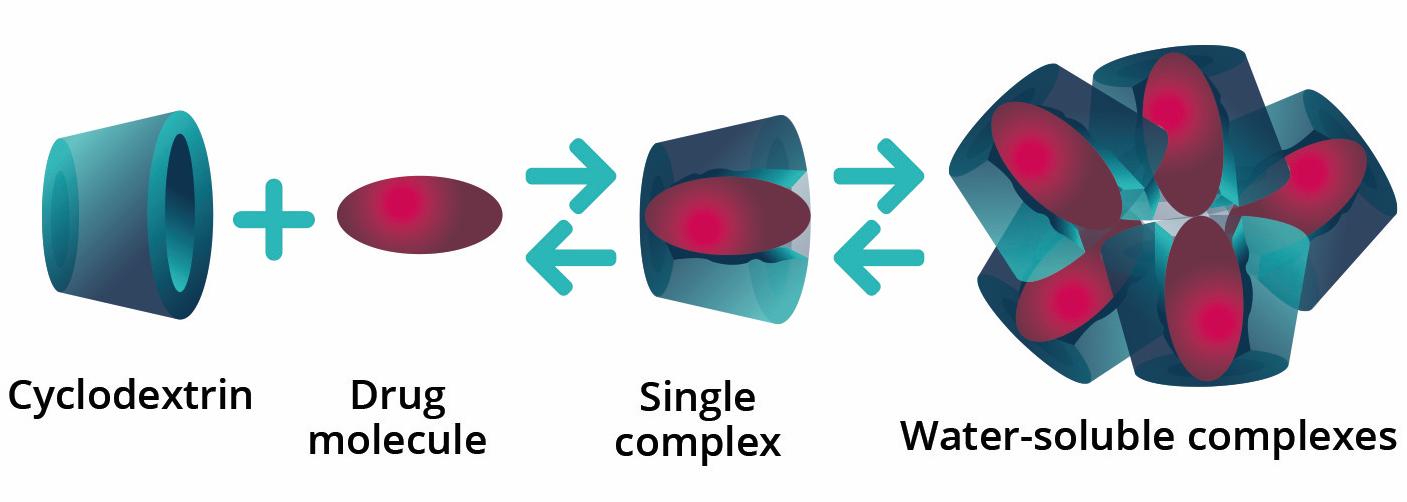
We are developing OCS-01 as a g cyclodextrin-based formulation of dexamethasone, using the Optireach® delivery technology, in order to enhance its residence time at the anterior segment and its penetration into the posterior segment of the eye following topical application. The increased drug residence time produced by the delivery vehicle, combined with enhanced drug penetration allows for increases in drug concentration of more than 15-fold over conventional dexamethasone. We are currently not aware of the existence of any other topically administered formulation of dexamethasone or other active pharmaceutical ingredient in development intended to deliver sustained therapeutic levels of drug to diseased tissue at the back of the eye.
The Optireach® technology enables the topical delivery of therapeutics to the back of the eye.
OCS-01 for DME
We are advancing OCS-01 as a treatment for DME, which is a complication of diabetes and is caused by the progressive growth of new blood vessels under the retina that leak fluid and lipids, leading to swelling of the macula, which can result in significant blurring of vision and contribute to the risk of blindness from DR. DME is strongly associated with uncontrolled blood sugar levels, high blood pressure and high cholesterol. An estimated 5.5% of diabetics worldwide are affected by the disease. It is a leading cause of blindness among the U.S. adult population. In the G7 countries (the United States, France, Germany, Italy, Spain, UK and Japan), the market for the treatment of DME is estimated to have totaled approximately $4.4 billion in 2024.
DME is estimated to impact 3.0 million people in the United States alone. Of those three million, we estimate that 1.3 million patients in the United States are addressable by OCS-01.
We are currently conducting the Stage 2 DIAMOND-1 and DIAMOND-2 Phase 3 trials in study sites in the United States.
Limitations of current treatments for DME
11
The DME disease onset may initially go unnoticed and as a result an estimated 42.0% of patients with DME may go undiagnosed. A study by the American Academy of Ophthalmology indicates that, among diagnosed patients, fewer than half are treated, with therapeutic intervention used most commonly in the one-third of patients who have moderate to severe visual impairment. Pharmacotherapy involves the invasive administration of a monoclonal antibody therapeutic targeting the vascular endothelial growth factor (“VEGF”) receptor to inhibit blood vessel growth. However, we estimate that approximately 40.0% of patients have an inadequate response to therapy after 12 weeks of anti-VEGF treatment, according to the results of a study published in the American Journal of Ophthalmology in 2016. Moreover, multiple intravitreal injections are required to maintain a therapeutic effect, which necessitates an increased treatment burden on patients, their caregivers and healthcare providers. Patients whose disease progresses while on anti-VEGF therapy may then receive a steroid implant, or laser photocoagulation of the retina.
Currently, physicians often do not treat patients who present with DME in its earlier stages of progression (patients with recent disease onset or mild visual impairment), a category that makes up approximately 67.0% of diagnosed patients with symptoms. We believe this decision to observe and not intervene is often driven by the significant burden current treatment options (laser photocoagulation, frequent intravitreal injections, intravitreal implants) place on the patient, as well as the expense and significant demands placed on healthcare resources. FDA approved therapeutics are not widely used for early disease intervention, despite the deterioration in visual acuity of approximately five letters, the equivalent of one line, or more in 19.0% of this observed/untreated patient population within two years.
OCS-01’s innovation and differentiation
OCS-01 is in development to be a topical treatment for DME, and we are currently not aware of the existence of any other eye drop treatment for DME which is in a similar or more advanced stage of active clinical development. In addition to this potential breakthrough advancement, we believe that an eye drop therapy would allow for an easy, accessible, low-burden, self-administered treatment for DME and would therefore significantly address the limitations of current, invasive therapies for DME. We expect that OCS-01, if approved, could address patients who are diagnosed with DME, with recent onset of disease or mild visual impairment and who are therefore currently observed and untreated, as well as patients who are diagnosed with DME and who have an inadequate response to anti-VEGF intravitreal injections. We estimate that both segments of patients combined totals 1.3 million in the United States alone.
OCS-01 has produced clinical trial results which support its continued development as a potential topical treatment for DME
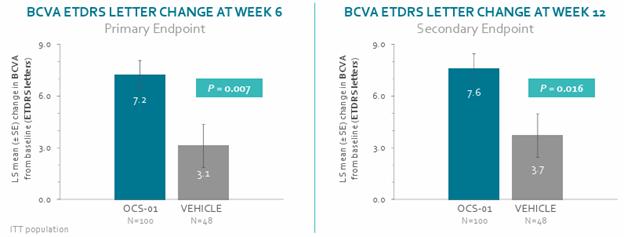
In Stage 1 of our DIAMOND Phase 3 clinical trial which evaluated the use of OCS-01 as a treatment for DME, patients who received OCS-01 demonstrated a statistically significant improvement from baseline in key measurements of therapeutic efficacy. In this randomized, double masked trial of 148 DME patients with 2:1 randomization (OCS-01 vs. vehicle), 100 of the trial participants self-administered OCS-01 eye drops six times per day for a six-week induction phase then three times per day for a subsequent 6-week maintenance phase, with 48 participants administered vehicle only. As noted in the graphic presented below, OCS-01 demonstrated improvement in mean BCVA “Early Treatment Diabetic Retinopathy Study” chart (BCVA ETDRS) score from baseline to Week 6 versus (vs) vehicle (OCS-01: 7.2 letters vs vehicle: 3.1 letters, p=0.007) demonstrating strong visual gain in the treatment arm. The effect was sustained to Week 12 with statistical significance (OCS-01: 7.6 letters vs vehicle 3.7 letters, p= 0.016).
12
OCS-01 generated improvements in both CMT and BCVA measurements.
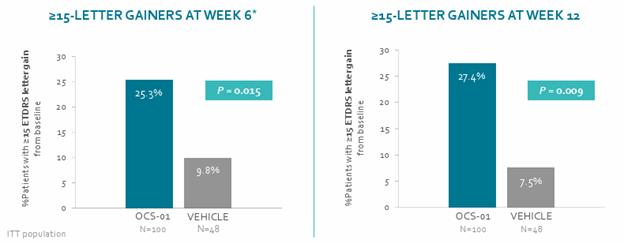
Furthermore, there was a higher percentage of patients in the OCS-01 group who achieved ≥15-letter improvement in BCVA from baseline vs vehicle at Week 6 (OCS-01: 25.3% vs vehicle: 9.8%, p=0.015), which was sustained to Week 12 (OCS-01: 27.4% vs vehicle 7.5%, p=0.009).
Improvements in both CMT and BCVA were greater among patients with lower baseline visual acuity.
A rapid reduction in retinal edema was observed in the OCS-01 treatment arm at week 2 of the study. The observed statistical significant treatment effect versus vehicle was preserved throughout the study.
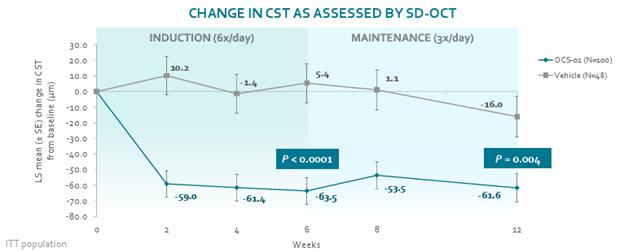
13
Treatment emergent adverse events (“TEAEs”) were noted in 70 of the 100 trial participants who received OCS-01, with the most prevalent AE being an increase in intraocular pressure (“IOP”), which was observed in 14 of the 100 patients in the active group. There was a small mean IOP increase, which was similar across induction and maintenance phase.
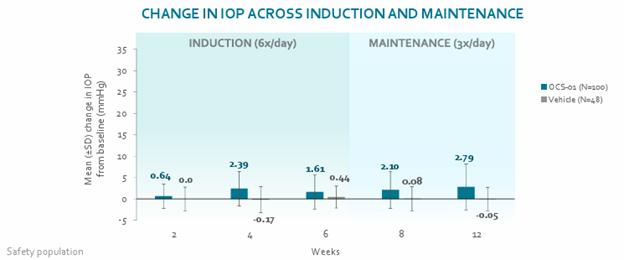
These findings of increased IOP were consistent with our expectations given glucocorticoids’ well-known ocular safety profiles, including the profile of an approved dexamethasone ocular implant. The findings were also consistent with current literature. Overall, the IOP effects observed in our trial were consistent with what is generally expected given established ophthalmic use of dexamethasone. Other AEs observed during clinical trials included diabetic and macular edema, which was noted more frequently in vehicle treated patients.
Except for increased IOP, AEs of a similar nature and number were noted among trial participants who received vehicle. The number of subjects with any ocular or non-ocular AEs leading to trial discontinuation was higher in the vehicle arm compared to the active arm. While OCS-01 may contribute to an accelerated onset of cataracts, no evidence of cataract formation was observed in the treatment arm up to 12 weeks.
The Stage 1 DIAMOND Phase 3 clinical trial results achieved with OCS-01 in treating DME follow outcomes achieved in the earlier Phase 2 study and two earlier small exploratory studies of DexNP (a previous formulation of OCS-01). In one of the studies, which was conducted in Japan, a 22-patient evaluation conducted in 2015 compared the use of a topically delivered g cyclodextrin-based formulation of dexamethasone to the posterior injection of 20 mg triamcinolone acetonide. Used at the time of the trial as an off-label treatment for DME, the gcyclodextrin-based formulation generated significant improvements in visual acuity and decreased macular thickness, comparable to the results achieved using triamcinolone acetonide. The results of this 2015 study confirmed similar findings achieved in another 19-person exploratory Japanese study conducted in 2011.
Phase 3 trial design for OCS-01
Our DIAMOND program includes two stages: Stage 1 has been completed, and in Stage 2, we are conducting two, 52-week pivotal Phase 3 trials, DIAMOND-1 and DIAMOND-2. We anticipate that each of these global Phase 3 trials will enroll between an estimated 350 and 400 subjects. The primary endpoint of these studies is the mean change from baseline in BCVA at 52 weeks. Key secondary endpoints include the mean change in macular thickness (“CST”), as assessed by spectral domain optical coherence tomography and the percentage of participants that exhibit ETDRS improvement of 15 letters or more from baseline. Key inclusion criteria are similar to those used in Stage 1 of the program. The Phase 3 clinical trial protocol was reviewed by the FDA during an End-of-Phase 2 meeting.
OCS-01 has the potential to expand the number of treated patients and prescribing physicians
OCS-01 was designed to address two sizeable treatment gaps among the DME patient population in early on-set and in severe segments. Furthermore, the delivery of the drug to the back of the eye and non-invasive self-administration are unique differentiators to currently available treatments. Addressing the two existing treatment gaps may allow for increased early disease intervention with expanded treatment of retinal edema due to reduced treatment burden and improved access to care. Success in demonstrating therapeutic efficacy to treat the earlier-stages of DME disease progression may promote the use of OCS-01, if approved, among those DME patients whose treatment is currently restricted to observation.
14
We believe that this potential expansion of the patient base to include earlier-stage DME patients may also increase the number of prescribing physicians, with general ophthalmologists, not just retina specialists, more likely to engage in disease management. If approved, OCS-01 may also be used as a non-invasive complement to currently approved therapeutic regimens, including anti-VEGF medications, potentially extending or enhancing the clinical benefit of those treatments particularly among those patients with more advanced diseases whose condition have not responded adequately to the current standard of care protocol.
OCS-01 for ocular surgery patients
There were approximately 6.8 million cataract, glaucoma, refractive, and vitrectomy surgical procedures performed in 2021 in the United States. Inflammation and pain remain an expected consequence of ocular surgery. While steroids have proven to be an effective treatment, compliance and potency are major issues with topical steroids dosed several times per day.
An estimated 30.0% of the patients who undergo cataract surgery are at an elevated risk for CME. Clinically significant CME occurs in up to 5.8% of cataract surgeries. Similar to DME, CME involves an accumulation of excess fluid in the macula which distorts central vision. CME is the most significant cause of postoperative vision loss among patients who undergo ocular surgery. Although the specific causes of CME are not well understood, comorbidities including diabetes and uveitis, among other factors, are believed to be significant contributors to disease emergence. In addition to developing OCS-01 to treat DME, we are also developing OCS-01 to treat inflammation and pain following from ocular surgery and conducting a PoC investigator initiated study to assess its potential in CME treatment. Prior to OCS-01’s commercial launch, if approved, we anticipate a PoC trial of OCS-01 (IIT) as a potential treatment for CME to be completed.
Limitations of current therapies for inflammation and pain post ocular surgery and OCS-01’s differentiation
Inexpensive steroids such as prednisone are currently widely prescribed after ocular surgery; however, since they are not formulated to reach the retina, their therapeutic benefit in treating or preventing complications related to CME has not been established. An investigator initiated PoC trial is currently ongoing to explore further the potential of OCS-01 in treating edema in CME. We believe this potential benefit in CME, if supported by this study and validated by future studies, and if OCS-01 is approved, may enable us to achieve enhanced market access.
OCS-01 has produced clinical trial results which support its continued development as a potential treatment for inflammation and pain post ocular surgery
During 2023, we conducted the Phase 3 OPTIMIZE-1 trial, which enrolled 241 subjects in a placebo-controlled, multi-center clinical trial in 25 sites across the United States, to assess the safety and efficacy of OCS-01, dosed once daily for 14 days, as a treatment for inflammation and pain following cataract surgery. After screening for an anterior chamber cell count of grade 2 or higher, an indication of intraocular inflammation, eligible trial participants were randomized into one of two cohorts, an active drug cohort administered OCS-01 once daily, and a cohort which received vehicle beginning one day after surgery for 15 consecutive days followed by a one-week observation period. The primary endpoints of the trial were the absence of anterior chamber cells at Day 15 and the absence of pain at Day 4. The key secondary endpoints were the absence of anterior chamber cells at Day 4 and 8, and the absence of pain at Days 2, 8, and 15.
The trial met both its hierarchical primary efficacy endpoints, the absence of inflammation at Day 15 and the absence of pain at Day 4, with robust statistical significance. A single daily application of OCS-01 was shown to reduce anterior chamber cells at Day 15 to zero in 57.2% of trial participants (p<0.0001), compared to 24.0% of subjects in the cohort that received vehicle alone. The elimination of pain at Day 4 was observed among 75.5% of subjects who received once-daily dosing of OCS-01 (p<0.0001), as compared to 52.0% in the vehicle only cohort.
OCS-01 was also well tolerated with a favorable safety profile. Overall, a higher number of ocular treatment emergent adverse events (TEAEs) were reported for the vehicle group (n=84) compared with the OCS-01 once-daily group (n=37). There was no meaningful difference in intraocular pressure (IOP) between treatment groups with a mean change from baseline to Day 15 of -0.90 mmHg in both the OCS-01 group and the vehicle group.
In December 2023, we announced first patient first visit in the second Phase 3 OPTIMIZE-2 trial. Data from both pivotal phase 3 trials, OPTIMIZE-1 (completed in 2023) and OPTIMIZE-2, are intended to support our future NDA submission to the FDA. OPTIMIZE-2 is identical in design compared with OPTIMIZE-1 (multi-center, randomized, double-masked, vehicle-controlled Phase 3 trial) and is evaluating OCS-01 for the treatment of inflammation and pain following cataract surgery.
15
Similar to the OPTIMIZE-1 trial, patients in the OPTIMIZE-2 trial are being treated with once-daily OCS-01 post-cataract surgery versus vehicle for 2 weeks. Primary endpoints are the absence of anterior chamber cells (inflammation) on Day 15 and absence of pain on Day 4. Topline data readout is expected in the fourth quarter of 2024.
OCS-02 (Licaminlimab)
Key Program Highlights:
We are developing OCS-02 (Licaminlimab) as a next-generation biologic treatment for both DED, and as a treatment for non-infectious anterior uveitis. OCS-02 is differentiated by its use of a single chain antibody fragment formulation directed against the cytokine human TNFα to enable the topical delivery of an anti-TNFα construct at increased concentrations. The anti-inflammatory and anti-necrotic/anti-apoptotic properties of therapeutics inhibiting TNFα activity are well established with anti-TNF pharmaceuticals already approved as systemic treatments for ocular disease. While OCS-02 is intended to be developed for all comers with DED, we are advancing the development of OCS-02 in conjunction with the development of a potentially novel genetic biomarker intended to identify patients who may have a greater response to OCS-02 therapy and believe this precision medicine approach may allow the candidate to deliver superior outcomes in these patients if approved. Two Phase 2 clinical trials in patients with symptoms of DED were conducted (the first with the predecessor of OCS-02, and the second with OCS-02), as well one Phase 2 clinical trial in acute anterior uveitis. Topical ocular administration of OCS-02 demonstrated improvements in the global ocular discomfort score versus vehicle in patients with DED, and with reaching a pre-specified responder rate in patients with non-infectious anterior uveitis, as well as being well tolerated in all three studies. In February 2024, we completed enrollment of the Phase 2b RELIEF study evaluating OCS-02 as a treatment for moderate-to-severe DED, with topline results anticipated in the second quarter of 2024. We plan to commence a second Phase 2 trial for OCS-02 as a treatment for chronic anterior uveitis thereafter.
TNFα performs important roles in the initiation and propagation of both normal and aberrant immune responses via mechanisms ranging from the stimulation of other cytokines to inflammatory cell recruitment to the alteration of vascular permeability. Inhibition of TNFα has demonstrated significant clinical benefit in the treatment of an array of diseases arising from dysfunctional immune system activity and anti TNFα therapeutics have become among the most widely prescribed biologics. Three anti-TNFα therapeutics (etanercept, sold under the brand name Enbrel®, infliximab, sold under the brand name Remicade®, and adalimumab, sold under the brand name Humira®), have each been studied for use in ocular disease. While the use of antagonists to TNFα have demonstrated favorable efficacy in the treatment of ocular inflammatory diseases, these drugs require intravenous infusion or subcutaneous injection and systemic anti-TNFα therapies are associated with a range of often serious adverse effects. Ocular diseases, such as DED and non-infectious anterior uveitis, involve a local TNFα driven inflammatory process which may not justify general, systemic TNFα-suppressive therapy. The novel design of OCS-02 embracing lower molecular weight single chain antibody fragment technology may enable it to be used in ocular disease as an eye drop for localized administration.
OCS-02 (Licaminlimab) for the treatment of DED
Keratoconjunctivitis sicca, also referred to as DED results from inflammation related to tear gland damage. DED is a multifactorial disease of the tears and ocular surface characterized by ocular surface inflammation and increased osmolarity of the tear film that results in ocular discomfort, visual disturbance and tear film instability. The etiology of DED can involve several deficiencies of the tear film, including the aqueous layer, the lipid layer, mucin layer or a combination of the three layers. The disease often presents as a complication of other diseases, prominently autoimmune disorders such as rheumatoid arthritis, diabetes and Sjogren’s syndrome, which may contribute to its manifestation. As such, DED may afflict individuals with differing severity of burning sensation, a feeling of dryness, and other symptoms of ocular discomfort. In severe cases, vision may be significantly impaired. Although the pathogenesis of DED includes a variety of causes, common consequences are a breakdown of corneal tear film with dehydration of the exposed outer corneal surfaces, ocular surface inflammation and subsequent damage to exposed tissues.
16
Increased concentration of pro-inflammatory cytokines, such as TNFα, in patient tears or conjunctival tissue has been demonstrated to correlate with disease severity.
In 2024, the U.S. DED patient population is estimated to be approximately 39.3 million people and is expected to rise to 41.3 million patients by 2029. The market for prescription medications to treat DED is forecasted to increase to $7.3 billion in the G7 countries (the United States, France, Germany, Italy, Spain, UK and Japan) by 2029 from $3.9 billion in 2019. We estimate the segment of DED patients in the United States addressable by OCS-02 (patients with moderate or severe DED) to be approximately 10 million patients.
Limitations of current therapies and potential for OCS-02 (Licaminlimab) in DED
The DED patient population is significantly underpenetrated with only an estimated 13.0% of diagnosed U.S. patients expected to receive prescription treatments in 2024. The vast majority of patients who do receive treatment are treated with anti-inflammatory drugs, yet among treated patients only 13.0% feel that their chronic dry eye disease is well managed. Approved topical treatments for DED include Restasis®, Cequa® and Vevye®, which are formulations of cyclosporine. These drugs act only to increase tear production and are not indicated to reduce DED symptoms. Further limiting cyclosporine’s therapeutic utility is a delayed onset of action necessitating a two- to three-month steroid bridge, and a stinging sensation on application in some patients. Topical steroids, including Eysuvis®, are also often used to treat DED but are contraindicated for long-term use because of their side effects including glaucoma and cataracts. Furthermore, other treatments available for DED include Xiidra® and recently launched Tyrvaya® and Miebo®.
OCS-02’s differentiation as a potential treatment for DED
Given the central role of ocular inflammation in sustaining the pathology of DED and the utility of anti-TNFα as a highly effective anti-inflammatory agent, we believe the localized application of OCS-02 as an anti-TNFα therapeutic, if approved, may provide a differentiated DED treatment approach, which may effectively reduce ocular discomfort, avoid undesirable features of current therapies (such as stinging sensation, delayed onset of action, or steroid-related side effects), and provide benefit for many patients who do not receive lasting relief from current therapies.
We estimate the segment of DED patients in the United States addressable by OCS-02 (patients with moderate or severe DED) to be approximately 10 million patients.
OCS-02 has produced clinical trial results which support its continued development as a potential treatment for DED
Novartis, from whom we have obtained certain exclusive, worldwide rights to develop and commercialize OCS-02 through a December 19, 2018 licensing agreement (please see the section entitled “Material Licenses, Partnerships and Collaborations” below), conducted a randomized, multi-center, double-masked, vehicle controlled Phase 2 clinical PoC trial designed to assess the safety and tolerability of OCS-02 and its efficacy in reducing DED symptoms. In the trial, patients were randomized on a 1:1 ratio into two cohorts. For a six-week period, the first trial cohort received a 60 mg/ml ophthalmic solution of OCS-02, while the second received vehicle. Participants in both cohorts self-administered one drop to each eye three times per day. The primary efficacy endpoint of the trial was improvement in the global ocular discomfort score as compared to vehicle. The global ocular discomfort score is a composite of discomfort frequency and severity as assessed by a visual analog scale using an electronic patient reported outcome. Improvement results in a reduction of the discomfort frequency or severity, or both, translating into a reduction of the resulting Global Ocular Discomfort Score as compared to baseline. A negative change from baseline indicates improvement. The secondary efficacy endpoint was an assessment of the number of patients that achieved more than 20 points improvement in the global ocular discomfort score. The data generated in this trial, consisting of 67 participants in the active group and 64 in the control group, are presented in the charts below.
17
OCS-02 generated statistically significant improvement in ocular discomfort as compared to vehicle.
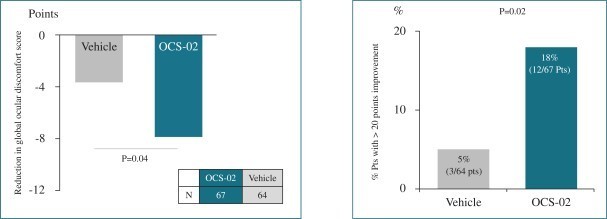
The trial met both primary and secondary endpoints. As is noted in the left chart above, administration of OCS-02 resulted in a statistically significant 7.9 mean point reduction in the global ocular discomfort score from baseline to treatment day 29 as compared to a 3.6 point mean reduction among patients that received vehicle only. In addition, as is noted in the right chart above, OCS-02 generated an improvement in the global ocular discomfort score of greater than 20 points in 12 of the 67 patients, or 18.0% of total trial participants. A similar level of response was achieved in only 5.0%, or three of the 64, patients included in the vehicle control group. The results of exploratory endpoints, which included physician graded conjunctival hyperemia, corneal staining, Meibomian gland assessment and tear film osmolarity, were similar across treatment groups. OCS-02 demonstrated a statistically significant improvement in the global ocular discomfort score compared to vehicle in patients with severe DED. It was well tolerated, with no increase in IOP and minimal systemic drug exposure.
Proprietary genetic biomarker may enable a precision medicine approach to DED
We conducted an exploratory pharmacogenetic analysis focused on the genes relevant to the TNF pathway and Sjogren’s syndrome among those 12 out of 86 patients who had the CC genotype gene variance or SNP. Among the gene variants analyzed, a correlation between one variant (rs1800693 CC genotype, “CC genotype”) in the TNFR1 gene, and a greater response (p<0.0001) to OCS-02 was observed at Day 29. The below figure shows individual patient profiles by study days for change from baseline global ocular discomfort score for participants with the CC genotype.
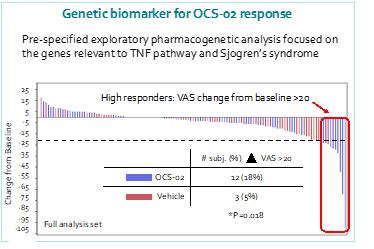
Patients with this variant displayed a significant reduction in inflammatory factors, including interleukin 1 beta (IL1B), interleukin 8 (IL8) and TNFα. This correlation is evidenced in the messenger RNA (“mRNA”) expression profiles of TNFα presented in the charts below which compared expression levels of patients with the various gene variants at Days 29 and 43 after dosing with either active drug candidate or vehicle. It was represented in 12 of 86 patients (14.0%) analyzed for the primary efficacy endpoint in this study, similar to the 13.0% of patients in the U.S. study.
18
A specific gene variant may enable biomarker based treatment
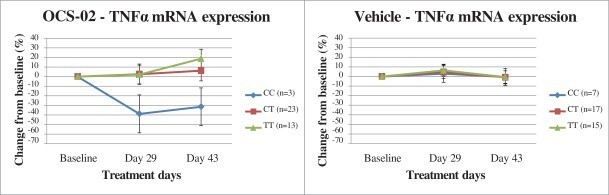
We believe that further validation of this genetic biomarker may enable us to identify a specific high response patient population which may allow us to enrich clinical trial enrollment and enhance our ability to evaluate the efficacy of OCS-02 in this indication and subpopulation. We intend to further evaluate the utility of this biomarker during our ongoing Phase 2b RELIEF trial of OCS-02.
Phase 2b trial design
In light of the results generated by OCS-02 in its Phase 2 PoC trials, we have advanced OCS-02 into an estimated 120 subject Phase 2b RELIEF clinical trial to evaluate the safety and efficacy of OCS-02 in treating the signs and symptoms of DED. This trial is randomized, multi-center, double masked and vehicle-controlled. Following initial screening, trial participants are randomized on a 1:1 basis into either the treatment cohort or the vehicle cohort and receive OCS-02 60mg/mL or vehicle three times daily for six weeks, followed by a two week follow up period. The efficacy measures and endpoints of the trial include a significant improvement in signs of DED, such as total corneal fluorescein staining, the percentage of patients with a 10 mm or greater increase in Schirmer’s test, as well as symptoms of DED such as global ocular discomfort compared to vehicle. Biomarker analyses (from impression cytology samples), as well as genotyping of subjects, are additional endpoints of the trial. Topline results are anticipated in the second quarter of 2024.
OCS-02 (Licaminlimab) for the treatment of non-infectious anterior uveitis
In addition to its potential use as a therapeutic to treat DED, we are also evaluating OCS-02 (Licaminlimab) for use as a treatment option for patients with non-infectious anterior uveitis, including patients with chronic or recurrent non-infectious anterior uveitis who would benefit from a steroid-sparing option.
Uveitis is a condition characterized by the inflammation of the uveal tract but can also cause the inflammation of nearby tissues, such as the retina, the optic nerve, and the vitreous humor. Uveitis is caused by inflammatory responses inside the eye in response to an attack from the body’s own immune system, infection, or trauma and injury to the eye. Uveitis is closely associated with various systemic diseases, including autoimmune disorders, and infectious diseases. However, a significant proportion of uveitis is idiopathic, with no identifiable cause for the disease. It primarily affects people between 20 and 60 years of age but can present at any age. If left untreated, uveitis can cause complications including macular edema, retina scarring, glaucoma, cataracts, optic nerve damage, retinal detachment and permanent vision loss. Uveitis, which can affect one or both eyes, accounts for between 10.0% to 20.0% of all cases of blindness in the United States and Europe, and causes approximately 30,000 new cases of blindness each year in the United States alone.
Loss of vision is correlated with the severity, frequency and duration of inflammatory episodes. Accordingly, the objective of treatment is fast and complete suppression of inflammation. Uveitis is categorized as either anterior, intermediate or posterior uveitis depending on the location of inflammation, or as panuveitis if present in multiple locations. Anterior uveitis is the most prevalent form of the disease and is associated with visual impairment. We estimate that that approximately 51.0% of patients in the United States who are diagnosed with anterior uveitis experience chronic or recurrent disease.
We estimate OCS-02 to address a patient segment of 170,000 patients with chronic or recurrent, anterior, non-infectious uveitis in the United States for 2024.
19
Limitations with the standard of care to treat anterior uveitis
The standard of care for uveitis is corticosteroids, which are administered as topical, intravitreal, periocular or oral depending on the location and severity of the disease. Active non-infectious uveitis is treated with topical corticosteroids. While topical corticosteroids have demonstrated clinical efficacy, their use is associated with a number of adverse ocular and systemic events. Topical ocular corticosteroid use is estimated to cause an increase in IOP of more than 15 mmHg among 4.0% and 6.0% of the general population and an increase of between 6 and 15 mmHg in up to one-third of users after daily application for four to six weeks. Persistent elevation in IOP may result in glaucoma, characterized by visual field loss and optic nerve damage, or the formation of cataracts. Incidence of cataract worsening or formation is related to total topical dose and duration. Based on multi-year studies with ocular corticosteroid implants, we estimate that approximately 31.0 to 47.0% more patients developed or experienced worsening of cataracts compared to control arms (sham implants or standard of care).
OCS-02 differentiated as a steroid-sparing treatment for anterior uveitis
Given the limitations related to longer-term steroid use in patients with recurrent or chronic uveitis, we believe OCS-02 has potential as a steroid-sparing treatment alternative. In November 2019, we commissioned a market research report which involved interviews with 14 key opinion leaders, high volume practitioners of uveitis treatment (ophthalmologists and uveitis specialists) and payer experts. The results suggested that physicians are likely to be receptive to prescribing a topical, non-steroidal treatment after initial administration of a topical corticosteroid that may both shorten the duration of topical steroid use and obviate the potential need to advance patients to oral steroids. If approved, OCS-02 may also be appropriate for patients who demonstrate an inability to tolerate steroid treatment.
OCS-02 Phase 2 clinical trial results support its continued development as a potential treatment for non-infectious anterior uveitis
Novartis also conducted a Phase 2 clinical PoC trial to evaluate the use of OCS-02 as a potential treatment for acute anterior uveitis (“AAU”). This trial was a randomized, multi-center, double-masked, active controlled evaluation to assess the safety, tolerability and efficacy of OCS-02 administered for up to 21 days in resolving ocular inflammation in the anterior chamber associated with AAU. A 60 mg/ml ophthalmic solution of OCS-02 was administered to trial participants in the OCS-02 cohort and topical dexamethasone administered to patients in the active-control cohort. Trial participants received a maximum of eight drops daily per treated eye for the first two weeks with dosing tapered for the following two-week period. Response to treatment was defined as a reduction from baseline of 2 or more anterior chamber cell grades.
35 patients completed the trial, with 25 patients in the OCS-02 cohort and 10 patients in the active control cohort. OCS-02 achieved the primary endpoint established for the trial, which was a responder rate in excess of 30.0%. Among the 25 participants that completed the trial and were treated with OCS-02, 14 patients, or 56.0%, demonstrated a response to OCS-02 treatment at Day 22, specified as the PoC treatment period for the trial. In the trial, OCS-02 was observed to be well tolerated. No increase in IOP related to OCS-02 was observed, and no systemic adverse safety signals were observed.
Planned Phase 2b trial evaluating OCS-02 as a treatment for non-infectious anterior uveitis
Given the encouraging results generated by OCS-02 in the Phase 2 clinical PoC trial, we intend to advance this clinical candidate into a Phase 2b trial for evaluation as a therapeutic for non-infectious chronic anterior uveitis with potential as a steroid-sparing alternative to the currently used drugs. Trial parameters to be incorporated into this clinical evaluation are in development. The commencement of this trial will follow after the Phase 2b DED trial.
OCS-05
Key Program Highlights:
20
In addition to development candidates intended to modulate inflammatory conditions associated with ocular disease pathologies, we are also advancing OCS-05, a small molecule in development as a potential disease modifying neuroprotective agent designed to address neurological damage to the optic nerve. We are initially developing OCS-05 as a potential therapeutic to treat AON. OCS-05 has been granted Orphan Drug Designation by both the FDA and the European Commission for this indication. OCS-05 has been studied in preclinical studies suggesting neuroprotective and remyelinating activity, as well as in a UK Phase 1 clinical trial (with 48 healthy volunteers) in which OCS-05 was well tolerated and showed pharmacokinetics (“PK”) with good correlation with its pre-clinical animal studies. We are currently studying OCS-05 in a PoC trial in AON in France, for which we anticipate topline data readout in the fourth quarter of 2024. Should the clinical results of our AON trials prove sufficiently compelling, we intend to evaluate OCS-05 to treat other more pervasive neurological pathologies of the eye such as geographic atrophy, neurotrophic keratitis and glaucoma. We obtained an exclusive license, worldwide to develop OCS-05 through a licensing agreement we entered into with Accure Therapeutics SL (“Accure”), dated as of January 29, 2022 (Please see the section entitled “—Material Licenses, Partnerships and Collaborations” below).
OCS-05 is a small molecule peptidomimetic that has a differentiated mechanism of action through the activation of SGK2 which is hypothesized as part of the neurotrophic factor signaling pathways that supports neuronal cell development, survival and repair, including oligodendrocyte precursor differentiation and myelination. Enzymes in the SGK2 family are recognized to regulate a range of fundamental cellular processes such as cellular proliferation and survival. SGK2 activation leads to an upregulation of signaling molecules forkhead box O3 (“FOXO3”), which reduces apoptosis, the downregulation of glycogen synthase kinase 3 beta (“GSK3B”), which improves anti-oxidation, and an upregulation of N-myc downstream-regulated gene 1 (“NDRG1”) involved in oligodendrocyte development and differentiation. The potential disease modulating activity of OCS-05 may distinguish it as a neuroprotective SGK2 activator.
OCS-05 was placed on a clinical hold by the FDA in 2016
Accure had conducted a limited set of animal regulatory toxicology studies in 2016 and submitted them to the FDA in an IND requesting the initiation of human testing. Upon review, the FDA found the data insufficient and asked for more animal toxicology data to be generated prior to human studies, thereby placing OCS-05 on the regulatory status of “clinical hold” pending the availability of the requested data. In response, Accure chose to withdraw the IND in 2017, rather than invest in further toxicology studies to address the FDA’s request, and pursue the development in the UK and France. Upon our license of OCS-05 from Accure in 2022, we reinstated the IND and are currently working on activities to enable a clinical trial under the IND in the U.S., including the necessary toxicology studies. Other health authorities where clinical studies have been proposed, including the UK and France, have authorized the initiation of clinical studies of selected doses and reinforced safety measures as in our European Phase 1 trial in AON.
OCS-05 for the treatment of acute optic neuritis
AON is an inflammation of the optic nerve that can cause the death of neurons, leading to vision impairment. A variety of infectious diseases, immune disorders, demyelinating disorders, non-inflammatory systemic disease or trauma can cause AON. AON is commonly associated with multiple sclerosis (“MS”) and shares similar physiopathology. AON is the presenting symptom of MS in 15.0-20.0% of patients and will impact over 50.0-65.0% of patients with MS at some time during their lifetime. However, the causes of AON are not always clear, as it can also arise in patients without MS.
The acute inflammation of the optic nerve causes the loss of myelin and oligodendrocytes, optic nerve conduction block and loss of vision. At the onset of AON, patients often suffer from ocular pain increasing with eye movement, associated with a variety of visual impairments. Deterioration of visual acuity, color vision or flashes of light are common. The loss of vision ranges considerably between patients from mild blurring to loss of perception of light. The condition tends to worsen over the first several days after the appearance of symptoms before starting to improve over the first two weeks. The recovery continues for as long as a year after onset. Even if high contrast visual acuity returns to near normal, patients often report that their vision has not completely recovered. There remains a persistent impairment of low contrast letter acuity and clinically meaningful reduction in vision-related quality of life.
When the inflammation recedes, remyelination often occurs but it is incomplete, the result of persistent demyelination and neuronal death. Without the myelin sheath which normally protects the axon, neurons located in demyelinated segments become fragile and prone to death. Thinning of the retinal neural fiber layer (“RNFL”), which is made up of unmyelinated axons originating from the retinal ganglion cell (“RGC”) bodies, indicates significant AON-induced axonal loss.
21
RNFL thinning, most pronounced three to six months after an acute AON event, along with thinning of the ganglion cell bodies layer, correlates with diminished scores of visual acuity and visual field sensitivity.
No therapeutic is currently approved that preserves vision and ganglion/retinal nerve integrity after an acute episode of AON. Medication intended to treat the inflammation and related symptoms can be administered just after AON onset and patients often receive high doses of corticosteroids for a few days to alleviate disabling vision-related symptoms caused by the inflammation. Corticosteroids have become the current standard of care, as the therapy acts to shorten the attack and accelerate recovery of acute visual symptoms. However, vision loss persists in 10.0% to 20.0% of patients despite administration of IOP lowering therapy. We believe a neuroprotective therapeutic, such as OCS-05, if approved, could prevent long term axonal loss may promote enhanced clinical outcomes.
OCS-05 demonstrated compelling neuroprotective qualities in an animal model of AON
In a rat model of AON, animals were segregated into four groups. The first group of healthy animals represented a sham control. Three additional groups received lysolecithin via injection into the optic nerve of study animals to induce inflammation and demyelination. Rats in group two received no treatment and served as a pathological control group. Groups three and four were administered OCS-05 once daily over a five-day period. Animals in group three received a 35 mg/kg dose of OCS-05 while animals in the fourth group received a dose of 70 mg/kg. The animals were sacrificed on the sixth day and assessed for a decline in RGC count.
As is noted in the results presented below, both groups of animals that received OCS-05 generated a statistically significant reduction in RGC loss when administered following the lysolecithin challenge, with rats administered the 35 mg/kg dose of OCS-05 demonstrating a 35.9% mean reduction of RGC loss. Animals in the higher dose treatment group who received a 70 mg/kg dose of OCS-05 displayed a more profound benefit from OCS-05 dosing, with RGC loss declining 64.0%.
RGC loss in animals treated with OCS-05 was significantly reduced in an animal model of AON.
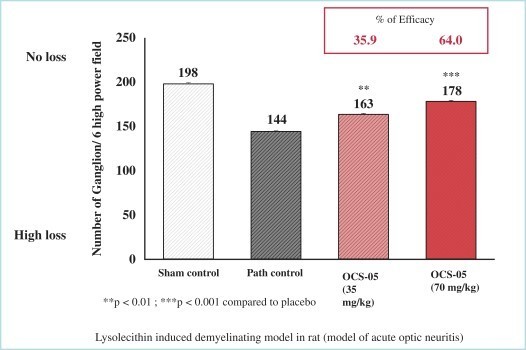
The reduction in RGC loss was also observed in a visual assessment of representative tissue samples collected from animals in three of the four study groups, the sham control group, the pathological control group and rats treated with the higher 70 mg/kg dose of OCS-05. As is depicted in the slides of the optic nerve presented below, normal ganglion cell density was observed in the evaluation of tissue taken from a healthy animal in the sham control group. In contrast, cell counts taken from samples of rats included the lysolecithin challenge group that made up the pathological control witnessed a prominent decrease. After completion of the five-day protocol, this decline was noted to have reversed, with rats who received the 70 mg/kg dose of OCS-05 observed to have retained a significantly higher number of ganglion cells. Similar results illustrating a reduction in axonal loss and demyelination, along with improvement in clinical function, have been achieved in animal models of AON.
22
OCS-05 was seen to bolster ganglion cell counts after lysolecithin challenge.
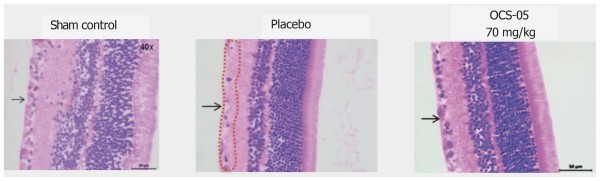
OCS-05 was well tolerated in a trial involving healthy volunteers
A randomized, double-masked, placebo controlled single-ascending dose and multiple-ascending dose trial was conducted in the United Kingdom to evaluate the safety, tolerability and PK and pharmacodynamics of OCS-05 dosing through the intravenous infusion of healthy volunteers with the drug candidate. This trial was designed to include four interlocking cohorts of eight adult subjects each to evaluate eight single ascending doses, with an additional two cohorts of eight adult subjects each included in the two multiple ascending dose trials. The single ascending dose cohorts were administered drug in doses ranging from .05 mg/kg to 3.2 mg/kg. The two cohorts in the multiple ascending dose trial received either a 2.4 mg/kg dose or a 3.0 mg/kg dose, once daily, for five consecutive days. In this trial, it was observed that OCS-05 was well tolerated with no serious AEs noted. Human PK data produced by this trial showed good correlation with data produced in animal studies of the compound. This trial was conducted under a clinical trial protocol approved by European regulatory authorities.
We are investigating OCS-05 as a treatment for AON in a First-in-Patient clinical trial
The results of prior clinical and preclinical trials of OCS-05 in promoting disease modifying effects, together with the safety and PK profile observed in this first-in-human clinical trial enabled us to advance the compound into a First-in-Patient clinical PoC trial. The Acute OptiC NeUrITis of DemYelinating Origin (“ACUITY”) trial, a randomized, double-masked, placebo controlled, multiple center trial, is a First-in-Patient trial enrolling patients diagnosed with AON within ten days of acute disease episode onset. The objective of this study is to assess the safety and tolerability of OCS-05 along with initial signs of efficacy. In addition to the trial’s primary safety endpoint, a key secondary endpoint will be the effect of OCS-05 on retinal layer thickness and other visual parameters in the affected eye. The study is currently being conducted in France under French regulatory guidance and we are anticipating topline data readout in the fourth quarter of 2024.
We believe that positive outcomes in this trial could support the compound’s possible development as a potential treatment in other ophthalmic conditions involving the posterior segment including glaucoma, geographic atrophy, DR as well as certain diseases of the anterior segment including corneal keratitis. The novel mechanism of action of OCS-05 may enable it to demonstrate benefit in treating these additional ocular conditions and may additionally allow its development in non-ocular neurological disorders involving neuronal inflammation such as MS.
In 2016, the OCS-05 development program was placed on clinical hold by the FDA related to the absence of no observed adverse effects levels (“NOAEL”), in prior preclinical studies conducted by the sponsor at that time. After we licensed the asset from Accure, our strategy has included plans to work with a CRO to complete the additional studies required to establish NOAEL, in order to enable our submission of an IND application with the FDA.
We are planning to investigate OCS-05’s potential as a treatment for Neurotrophic Keratitis and should the outcome of the AON trial be positive, we will also evaluate the potential as a treatment for neuro-ophthalmology diseases such as Glaucoma
Preclinical studies of OCS-05 in a model of glaucoma in Sprague rats showed results which support its potential to be developed as a treatment for glaucoma. In these two experiments, high intraocular pressure was induced in rats by injecting hypertonic saline solution into the episcleral vein of one eye of each rat, and then the rats were treated for six weeks. In one experiment, rats in the active group were treated with OCS-05 as an eye drop twice daily for six weeks, rats in the positive control group received nerve growth factor (“NGF”), and rats in the control group received placebo of saline 5.0% dimethyl sulfoxide (“DMSO”). In the other experiment, rats in the active group were treated with OCS-05 as an intravitreal injection once every two weeks, for six weeks, and rats in the control group received placebo of saline 5.0% DMSO.
23
Retinal ganglion cells (“RGCs”) count was measured via haematoxylin and eosin stain (“H & E”) histological quantification, and IOP was also measured.
Sprague rats displayed significant loss of RGCs one month after the induction of ocular hypertension. In animals treated with OCS-05, either as eye drops or through intravitreal injection, there were statistically significant increases in RGCs surviving compared with those that received the placebo. In the experiment which included a positive control of NGF, OCS-05 treatment showed a similar effect to that seen with NGF. In addition, IOP did not significantly decrease with administration of OCS-05. We believe this data suggests that OCS-05 may promote neuronal survival in this animal model of glaucoma via neuroprotection (and not by reversing the induced ocular hypertension).
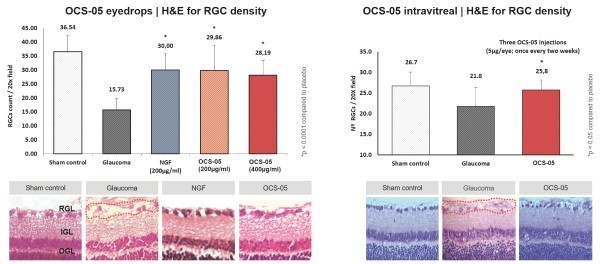
OCS-05 (eyedrops and intravitreal) prevents RGCs damage without reducing intraocular pressure
Given the results from these preclinical studies, we plan to further study OCS-05, and if results from our ACUITY trial in AON further support OCS-05’s potential as a neuroprotective compound, we may prepare for and initiate clinical development of OCS-05 in glaucoma. Glaucoma represents a large market, and we are not currently aware of the existence of any other compound in a similar or more advanced stage of development as a neuroprotective drug for glaucoma.
Additionally, we also plan to further study OCS-05 for its potential to enter clinical development as a treatment for neurotrophic keratitis (“NK”). NK is a rare eye disorder which results from damage or loss of function of nerves which innervate the cornea, which can lead to corneal perforation, corneal scarring, corneal melting, loss of vision, or loss of the eye. In 2018, the FDA approved the NGF drug cenergermin (“Oxervate”) to treat NK. However, Oxervate may be cost prohibitive for patients and payors, as ASCRS Eyeworld estimated in 2020 that Oxervate costs approximately $11,000 per week for an 8-week treatment course for NK.
Given that preclinical studies of OCS-05 have shown data suggesting that the OCS-05 could provide neuroprotective benefits, we believe it may have potential to treat the nerve impairment underlying NK. If results from our ACUITY trial in AON further support OCS-05’s potential as a neuroprotective compound, we may prepare for and initiate clinical development of OCS-05 in NK. We are currently not aware of the existence any other drugs except for Oxervate which are approved or in a similar or more advanced stage of development as a treatment for NK.
We are currently conducting formulation studies to develop a topical formulation of OCS-05 which can be used in further preclinical or in clinical development of OCS-05 in glaucoma or in NK.
24
Additional Development Initiatives
In addition to our six clinical development programs involving OCS-01, OCS-02 (Licaminlimab) and OCS-05, we also are engaged in a number of earlier preclinical development initiatives, including the evaluation of OCS-03 as a possible treatment for corneal neovascularization, a common disorder caused by the aberrant development of new blood vessels into the cornea and pterygium, a pink colored growth that originates in the conjunctiva. We are also assessing the preclinical candidate OCS-04, an innovative topical ophthalmic formulation project, preliminarily intended for corneal graft rejection prevention and possibly other inflammatory related conditions targeting the ocular surface.
Material Licenses, Partnerships and Collaborations
License Agreement with Novartis for OCS-02 (Licaminlimab)
Pursuant to a license agreement, dated as of December 19, 2018, as amended, by and between us and Novartis (the “Novartis Agreement”), we obtained an exclusive, royalty-bearing, sublicensable (subject to certain conditions), assignable (subject to certain conditions), worldwide license under certain patents, know-how and manufacturing platform technology to develop, manufacture and commercialize pharmaceutical, therapeutic or diagnostic products containing a specified single chain antibody fragment formulation as an active ingredient in the licensed field as defined in the Novartis Agreement. The license granted to us by Novartis includes sublicenses of rights granted to Novartis by certain third parties, and our license to such rights is expressly subject to the applicable terms and conditions of the agreements between Novartis and such third parties.
We are deemed the owner of any inventions that are (a) created solely by or on behalf of us pursuant to the Novartis Agreement and (b) severable from the licensed products, and grant Novartis a first right to negotiate a worldwide, royalty-bearing license under any patents directed at such inventions for purposes outside of the licensed field. We also grant Novartis a worldwide, non-exclusive, perpetual, irrevocable, royalty-free, fully paid-up license back under any patents owned by us that (i) cover inventions arising from the Novartis Agreement, the practice of which would infringe the patents licensed to us by Novartis, or (ii) otherwise incorporate Novartis’ proprietary information, in each case, for certain uses outside of the licensed field.
We made an upfront payment to Alcon of CHF 4.7 million ($4.7 million at the exchange rate at the time of payment) in cash and issued 401,709 ordinary shares (recast using the Exchange Ratio to reflect the impact of the BCA) for the residual between the fair value and the upfront payment. This was accounted for as a share-based payment transaction under IFRS 2. We originally entered into the Novartis Agreement with Alcon Research, Ltd. (“Alcon”), which subsequently assigned its rights and obligations under the Novartis Agreement to Novartis in connection with Alcon's spin-off from Novartis. As of December 31, 2023, we were obligated to pay Novartis additional up to CHF 81.6 million ($97.0 million at the December 31, 2023 exchange rate) in the aggregate upon the achievement of certain development, regulatory, sales and other milestones and tiered royalties ranging from a mid-single digit to a mid-teen percentage on net sales. In consideration for the exclusive sublicense from Novartis under certain third-party intellectual property rights, we are obligated to pay a low-single digit royalty on our net sales of the licensed product, however, such payments will be deducted from royalties payable to Novartis. Our royalty payment obligations are subject to certain reductions and expire with respect to any licensed product on a country-by-country basis upon the later of (a) the expiration of the last to expire valid claim of any licensed patent covering any such licensed product in such country; (b) the expiration of the period of data exclusivity in any country worldwide; or (c) twelve (12) years after first commercial sale of such licensed product in such country (“Royalty Term”).
Under the Novartis Agreement, we are obligated to use diligent efforts to develop, manufacture or have manufactured, and commercialize the licensed products in the licensed field worldwide. The Novartis Agreement will expire upon the last-to-expire Royalty Term. We may terminate the Novartis Agreement without cause at any time upon advance written notice to Novartis. Upon written notice to Novartis, we may terminate the Novartis Agreement for cause due to the following events: (a) an insolvency event occurs; (b) Novartis materially breaches its obligations under the Novartis Agreement and fails to cure such breach within a specified period of time; or (c) upon advance written notice for material scientific, technical or medical reasons or in case of a material adverse change that renders further continuation of the Novartis Agreement by us commercially unreasonable or otherwise not viable. Upon written notice to us, Novartis may terminate the Novartis Agreement for cause due to the following events: (i) we fail to pay any undisputed amount due under the Novartis Agreement and we fail to remedy such failure within a specified period of time; (ii) an insolvency event occurs; (iii) we materially breach our obligations under the Novartis Agreement and fail to cure such breach within a specified period of time; or (iv) following negative clinical trial results, we terminate development of the licensed product and do not pursue any further indications in the licensed field.
25
License Agreement with Accure for OCS-05
Pursuant to a license agreement, dated as of January 29, 2022, by and between us and Accure (the “Accure Agreement”), we obtained an exclusive, worldwide, sublicensable (subject to certain conditions) and transferable (subject to certain conditions) license under certain patents, know-how and inventory of Accure for any and all uses and purposes, including to perform research, development, manufacturing and commercialization activities in any manner and for any purpose. The licensed patents are co-owned by Accure with third parties who have reserved the right to use the licensed patents for education and research purposes pursuant to an inter-institutional agreement.
As of December 31, 2023, we have paid the full contractual non-refundable upfront fee of CHF 3.0 million and reimbursed costs in the amount of approximately CHF 0.5 million. As of December 31, 2023, we were obligated to pay Accure (a) up to CHF 94.3 million ($112.1 million at the December 31, 2023 exchange rate) in the aggregate upon the achievement of certain development, regulatory and sales milestones; (b) tiered royalties ranging from a mid-single digit to a low mid-teen percentage on net sales of licensed products; and (c) a percentage in the high teens on sublicensing revenues received any time after 36 months from the agreement effective date, and a higher percentage on sublicensing revenues received prior to such date, in all cases subject, in the case of this clause (c), to reduction for any amounts that were previously paid or are concurrently or later paid by us to Accure pursuant to our milestone payment obligations. Our royalty payment obligations are subject to certain reductions and expire on a licensed product-by-licensed product and country-by-country basis upon the later of (i) the expiration of the last valid claim of any licensed patent covering such licensed product in such country; (ii) the expiration of such licensed product’s Orphan Drug status, if any, in such country; or (iii) ten (10) years following the date of first commercial sale of such licensed product in such country (the “Payment Period”). Under the Accure Agreement, we are obligated to use commercially reasonable efforts to develop and seek regulatory approval for a licensed product in major countries of the territory as defined in the Accure Agreement.
The Accure Agreement will expire on a licensed product-by-licensed product and country-by-country basis upon the expiration of the applicable Payment Period with respect to such licensed product in such country. We may terminate the Accure Agreement in whole or in part at any time upon advance written notice (a) for documented reasonable scientific, regulatory, commercial reasons related to the licensed product without incurring any penalty or liability to Accure and (b) for no reason. Each party may terminate the Accure Agreement with immediate effect upon written notice to the other party (i) in the event such other party commits a material breach of its obligations under the Accure Agreement and fails to cure that breach within a specified period of time or (ii) with certain exceptions, upon such other party’s bankruptcy. Accure may terminate the Accure Agreement with immediate effect upon written notice to us if we file any action to invalidate any of the licensed patents or fail to maintain the licensed patents in major countries of the territory as defined in the Accure Agreement, or, subject to certain exceptions, if we fail to meet certain development obligations and are unable to agree upon modifications to the development plan with Accure.
Manufacturing Strategy
We oversee and manage third-party contract manufacturing organizations (“CMOs”), to support the development and manufacture of product candidates for our clinical trials, and, if any product candidates receive marketing approval, we expect to rely on such manufacturers to meet commercial demand. We expect this strategy will enable us to maintain a more efficient operating and cost infrastructure, avoiding dependence on our own manufacturing facility and equipment, while simultaneously enabling us to focus our expertise on the clinical development and future commercialization of our products, if approved. Currently, we rely on and have agreements with third-party contract manufacturers for developing and manufacturing API/drug substance/drug product for OCS-01, OCS-02 (Licaminlimab) and OCS-05, and we expect to enter into commercial supply agreements with such manufacturers prior to any potential approval. We continue to develop and improve the manufacturing processes for OCS-02 and OCS-05 and to address the requirements in these highly regulated markets. Improvement of manufacturing processes may involve transferring the development and manufacturing to another CMO, taking into account technical, quality and economic aspects.
Each of OCS-01, OCS-02 and OCS-05 is manufactured via conventional pharmaceutical processing procedures, employing commercially available excipients and packaging materials. The procedures and equipment employed for manufacture and analysis are consistent with standard pharmaceutical production, and are transferable to a range of manufacturing facilities, if needed.
Competition
We face substantial competition from multiple sources, including large and specialty pharmaceutical and biotechnology companies, academic research institutions and governmental agencies and public and private research institutions.
26
Our competitors compete with us on the level of the technologies employed, or on the level of development of product candidates. In addition, many small biotechnology companies have formed collaborations with large, established companies to (i) obtain support for their research, development and commercialization of products or (ii) combine several treatment approaches to develop longer lasting or more efficacious treatments that may potentially directly compete with our current or future product candidates. We anticipate that we will continue to face increasing competition as new therapies and combinations thereof, technologies, and data emerge within the treatment of ocular conditions.
In addition to the current standard of care treatments for patients with ocular diseases, numerous commercial and academic preclinical studies and clinical trials are being undertaken by a large number of parties to assess novel technologies and product candidates.
Several large pharmaceutical and biopharmaceutical companies that have commercialized, or are developing treatments for ocular diseases, compete with us. Companies that compete with us directly on the level of the development of product candidates targeting DME include Abbvie, Alimera Sciences, Bayer, Novartis, Regeneron and Roche, among others. Companies that have commercialized or are developing drug candidates to treat inflammation and pain associated with ocular surgery include Abbvie, Alcon, Bausch + Lomb and Teva Pharmaceuticals, among others; companies that compete with us in the area of DED include Abbvie, Alcon, Bausch + Lomb, Viatris and Sun Pharmaceuticals, among others. Companies engaged in the commercialization or development of therapeutics to treat uveitis include Abbvie and Bausch + Lomb, among others. We are also aware of an eye drop product candidate in clinical development by OcuTerra Therapeutics for the treatment of diabetic retinopathy and DME, an indication related to that for which we are developing OCS-01.
Many of our competitors, either alone or in combination with their respective strategic partners, have significantly greater financial resources and expertise in research and development, manufacturing, regulatory approval process and marketing than we do. Mergers and acquisition activity in the pharmaceutical, biopharmaceutical and biotechnology sector is likely to result in greater resource concentration among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through sizeable collaborative arrangements with established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites, patient registration for clinical trials and acquiring technologies complementary to, or necessary for, our programs.
Our commercial opportunities could be reduced or eliminated if one or more of our competitors develop and commercialize products that are safer, more effective, better tolerated, or of greater convenience or economic benefit than our proposed product offerings. Our competitors also may be in a position to obtain FDA or other regulatory approval for their products more rapidly, resulting in a stronger or dominant market position before we are able to enter the market. The key competitive factors affecting the success of all of our programs are likely to be product safety, efficacy, convenience and treatment cost.
Intellectual Property
Intellectual property is of vital importance in our field and in biotechnology generally. We seek to protect and enhance proprietary technology, inventions, and improvements that are commercially important to the development of our business by obtaining, maintaining, enforcing and defending intellectual property rights, including patent rights, whether owned or licensed from third parties. We will also seek to rely on regulatory protection afforded through inclusion in expedited development and review, data exclusivity, market exclusivity and patent term extensions where available.
We have sought patent protection in the United States and internationally related to our novel drug targets, composition of matter, formulations and other inventions and improvements that are central to our R&D efforts. For our product candidates, our strategy is to pursue patent protection covering compositions of matter, formulations and methods of use. In addition, we seek to identify additional means of obtaining patent protection, including specific therapeutic indications and dosing regimen-related claims, which may enhance commercial success. We also rely on trade secrets that may be important to the development of our business. Trade secrets are difficult to protect and provide us with only limited protection.
As of December 31, 2023 and currently, we own and exclusively in-licensed a patent portfolio that included 11 issued U.S. patents, five issued European patents validated in multiple jurisdictions, and 56 issued patents in other foreign jurisdictions, as well as twelve pending non-provisional U.S. patent applications, and 65 foreign pending patent applications, including eight pending European patent applications, and four pending Patent Cooperation Treaty ("PCT") applications related to our different product candidates, namely, OCS-01, OCS-02 (Licaminlimab), OCS-03, OCS-04 and OCS-05.
27
OCS-01
Regarding our OCS-01 product candidate, as of December 31, 2023 and currently, we own a patent family that consisted of three issued U.S. patents and one granted European patent validated in 12 jurisdictions (Belgium, France, Germany, Great Britain, Iceland, Ireland, Italy, the Netherlands, Poland, Spain, Switzerland, Turkey) with claims covering the composition including dexamethasone. These patents will expire in 2026, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of December 31, 2023 and currently, we own a second patent family that consisted of two issued U.S. patents, two pending non-provisional U.S. patent applications, one granted European patent validated in 41 jurisdictions (Albania, Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Germany, Great Britain, Greece, Finland, France, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Republic of Moldova, Monaco, Montenegro, Morocco, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey), sixteen issued patents in other foreign jurisdictions (Australia, China, Colombia, Eurasia, India, Japan, Mexico, South Africa (two patents),Taiwan (two patents), Ukraine, Hong Kong, Singapore, South Korea, Chili) and 14 pending foreign patent applications, including one pending European patent application, with claims covering the composition of matter of OCS-01. Patents (including any patents that issue from such patent applications) in this family will expire in 2037, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of December 31, 2023 and currently, we also own a patent family that consisted of six U.S. non-provisional patent applications and 21 additional foreign patent applications in other jurisdictions, including one European patent application, directed to specific formulations of OCS-01 and methods for stabilizing the composition for use as an eye drop. Patents, if issued from patent applications in this family, will expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
OCS-02 (Licaminlimab)
Regarding our OCS-02 (Licaminlimab) product candidate, as of December 31, 2023, we exclusively licensed from Novartis under the Novartis Agreement, in the licensed field as defined in the Novartis Agreement, one patent family that consisted of three issued U.S. patents and two granted European patents validated in 36 jurisdictions (Albania, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Great Britain, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Monaco, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey) and six jurisdictions (France, Germany, Great Britain, Italy, Spain, Switzerland), respectively, 22 issued patents in other foreign jurisdictions (Argentina, Australia, Brazil, Canada, Chile (two patents), China (two patents), India, Hong-Kong (two patents), GCC, Japan (two patents), Republic of Korea, Mexico (two patents), Philippines, Russia, South Africa, Taiwan, Ukraine) and two patent applications pending in other foreign jurisdictions, with claims covering composition of matter of OCS-02 or methods of use. Patents (including any patents that issue from such patent applications) will expire in 2031, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
In addition, as of December 31, 2023, we exclusively licensed from Novartis under the Novartis Agreement, in the licensed field as defined in the Novartis Agreement, one patent family directed on a biomarker for patient selection, that consists of one pending European and one U.S. patent application and four patent applications pending in Canada, China, Japan (two patent applications). Patents (including any patents that issue from such patent applications) will expire in 2037, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
In addition, as of December 31, 2023, we exclusively licensed from Novartis under the Novartis Agreement, in the licensed field as defined in the Novartis Agreement, six additional patent families covering composition of matter of OCS-02 or methods of use, which (including any patents that issue from patent applications in these families) will expire between 2023 and 2031, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
28
Under the terms of the Novartis Agreement, Novartis is responsible for the prosecution and maintenance of these six patent families.
OCS-03
As of December 31, 2023 and currently, we own a patent family that consists of one pending U.S. non provisional application and one pending European application as well as one pending Taiwanese application, with claims covering composition of matter of OCS-03 and its use. Patents (including any patents that issue from patent application) will expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
OCS-04
As of December 31, 2023 and currently, we own one pending PCT application as well as pending applications in Argentina and Taiwan, with claims covering composition of matter of OCS-04 and manufacturing processes. In order for any future patent applications to claim the benefit of such PCT application, they must be filed not later than 30 or 31 months (depending on the jurisdiction) after the earliest priority date of such PCT application. Patents, if issued from the patent applications claiming the benefit of such priority application, if issued, will expire in 2043, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
OCS-05
Regarding our OCS-05 product candidate, as of December 31, 2023, we exclusively licensed from Accure under the Accure Agreement a patent family that consisted of three issued U.S. patents and one granted European patent validated in 24 jurisdictions (Austria, Belgium, Croatia, Czech Republic, Denmark, Finland, France, Germany, Great Britain, Greece, Hungary, Ireland, Italy, Luxembourg, Malta, the Netherlands, Norway, Poland, Portugal, Slovenia, Spain, Sweden, Switzerland, Turkey), as well as 10 issued patents (Australia, Brazil, Canada, China, India, Israel, Japan, Republic of Korea, Mexico, Russia) in other foreign jurisdictions, with claims covering composition of matter of OCS-05. These patents (including any patents that issue from such patent applications) will expire in 2031, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of December 31, 2023, we also exclusively licensed from Accure under the Accure Agreement a patent family that consisted of one pending non-provisional U.S. patent application and 15 pending foreign patent applications, including one pending European patent application, directed to the method of use of the composition of OCS-05 in combination with active compounds. Patents, if issued from such patent applications, will expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of December 31, 2023, we also exclusively licensed from Accure under the Accure Agreement a patent family consisting of one pending non-provisional U.S. patent application and six pending foreign patent applications, including one pending European patent application, with claims directed to specific dosage regimen for administering the active pharmaceutical ingredient of OCS-05. Patents, if issued from such patent applications, will expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of December 31, 2023 and currently, we also own a priority European patent application with claims covering a manufacturing process of OCS-05 and OCS-05’s intermediate synthesis products. In order for any future patent applications to claim the benefit of such priority application, such future patent application must be filed no later than 12 months after the filing date of such priority application. Patents, if issued from the patent applications claiming the benefit of such priority application, will expire in 2042 or 2043, assuming a filing within the 12-month priority period, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
Our commercial success will depend in part on obtaining, maintaining, protecting and enforcing patent protection and trade secret protection of our current and future product candidates and the methods used to develop and manufacture them, as well as successfully defending any such patents against third-party challenges, enforcing such patents against third-party infringers, and operating without infringing on, misappropriating or otherwise violating the intellectual property or proprietary rights of others. Our ability to stop third parties from making, using, selling, offering to sell or importing our product candidates will depend on the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
29
We cannot be sure that patents will be issued with respect to any of our owned or in-licensed pending patent applications or with respect to any patent applications filed by us or our licensors in the future, nor can we be sure that any patents that may be granted to us or our licensors in the future will be commercially useful in protecting our product candidates, discovery programs and processes. For this and more comprehensive risks related to our intellectual property, please see the section entitled “Risk Factors—Risks Related to Our Intellectual Property.”
The terms of individual patents depend upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, including the United States, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office (“USPTO”), in examining and granting a patent, or may be shortened if a patent is terminally disclaimed over an earlier filed patent. In the United States, the term of a patent that covers an FDA-approved drug may also be eligible for extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the subject drug candidate is under regulatory review. U.S. patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent applicable to an approved drug may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. Similar provisions to extend the term of a patent that covers an approved drug are available in Europe and other foreign jurisdictions. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We plan to seek patent term extensions to any issued patents we may obtain in any jurisdiction where such patent term extensions are available, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment that such extensions should be granted, and if granted, the length of such extensions. For more information regarding the risks related to our intellectual property, see section entitled “Risk Factors—Risks Related to Our Intellectual Property" included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024.
We file U.S. non-provisional applications and PCT applications that claim the benefit of the priority date of earlier filed priority applications, when applicable. The PCT system allows a single application to be filed within 12 months of the original priority date of the patent application, and to designate all of the PCT member states in which national patent applications can later be pursued based on the international patent application filed under the PCT. The PCT searching authority performs a patentability search and issues a non-binding patentability opinion which can be used to evaluate the chances of success for the national applications in foreign countries prior to having to incur the filing fees. Although a PCT application is not issued as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications. At the end of the period of two and a half years from the first priority date of the patent application, separate patent applications can be pursued in any of the PCT member states either by direct national filing or, in some cases by filing through a regional patent organization, such as the European Patent Office. The PCT system delays expenses, allows a limited evaluation of the chances of success for national/regional patent applications and enables substantial savings where applications are abandoned within the first two and a half years of filing.
For all patent applications, we determine claiming strategy on a case-by-case basis. Advice of counsel and our business model and needs are always considered. We seek to file patents containing claims for protection of all useful applications of our proprietary technologies and any product candidates, as well as all new applications and/or uses we discover for existing technologies and product candidates, assuming these are strategically valuable. We continuously reassess the number and type of patent applications in our portfolio, as well as the pending and issued patent claims to pursue maximum coverage and value for our processes and compositions, given existing patent office rules and regulations. Further, claims may be narrowed during patent prosecution, to the extent allowed, to meet our intellectual property and business needs.
We recognize that the ability to obtain patent protection and the degree of such protection depends on a number of factors, including the extent of the prior art, the novelty and non-obviousness of the invention, and the ability to satisfy the enablement requirement of the patent laws. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted or further altered even after patent issuance. Consequently, we or our licensors may not obtain or maintain adequate patent protection for any of our future product candidates or for our Optireach® technology platform. We cannot predict whether the owned or in-licensed patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents we own or in-license will provide sufficient proprietary protection from competitors. Any patents that we own or in-license may be challenged, circumvented or invalidated by third parties.
30
The patent positions of biotechnology companies like ours are generally uncertain and involve complex legal, scientific and factual questions. Our commercial success will also depend in part on not infringing upon, misappropriating or otherwise violating the intellectual property or proprietary rights of third parties. Third-party patents could require us to alter our development or commercial strategies, or our product candidates or processes, obtain licenses or cease certain activities. Our breach of any license agreements or our failure to obtain a license to intellectual property or proprietary rights required to develop or commercialize our product candidates or future products may have a material adverse impact on us. If third parties prepare and file patent applications in the United States that also claim technology to which we have rights, we may have to participate in interference or derivation proceedings in the USPTO to determine priority of invention. For more information, please see the section entitled “Risk Factors—Risks Related to Intellectual Property" included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024.
In addition to patent protection, we also rely on trademark registration, trade secrets, know how, other proprietary information and continuing technological innovation to develop and maintain our competitive position. As of December 31, 2023, we owned four registered U.S. trademarks (three of which being fractions of international registrations), four international trademark registrations (either granted or still under examination in several countries), 11 registered foreign trademarks as well as two pending foreign trademark applications. We seek to protect and maintain the confidentiality of proprietary information to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Thus, we may not be able to meaningfully protect our trade secrets. It is our policy to require our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information concerning our business or financial affairs developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific circumstances. Our agreements with employees also provide that all inventions conceived by the employee in the course of employment with us or from the employee’s use of our confidential information are our exclusive property. However, such confidentiality agreements and invention assignment agreements can be breached and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our consultants, contractors or collaborators use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting trade secrets, know-how and inventions. For more information regarding the risks related to our intellectual property, please see the section entitled “Risk Factors—Risks Related to Intellectual Property" included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024.
When available to expand market exclusivity, our strategy is to obtain, or license additional intellectual property or proprietary rights related to current or contemplated development platforms, core elements of technology and/or clinical candidates.
Government Regulation
Government authorities in the United States at the federal state and local level, and other countries extensively regulate, among other things, the research, development, nonclinical and clinical testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, and export and import of products such as those we are developing. Generally, before a new drug or biologic can be marketed, considerable data must be generated, which demonstrate the product’s quality, safety, and efficacy. Such data must then be organized into a format specific for each regulatory authority, submitted for review and approved by the regulatory authority.
U.S. Drug and Biologic Development Process
In the United States, the FDA regulates drugs and biologics under the federal Food, Drug, and Cosmetic Act (“FDCA”), and its implementing regulations. Biologics are additionally subject to regulations under the Public Health Service Act. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, the approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties.
31
Any agency or judicial enforcement action could have a material adverse effect on us.
The process required by the FDA before a biopharmaceutical may be marketed in the United States generally involves the following:
Prior to beginning the first clinical trial with a product candidate in the United States, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an IND product to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, PK, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical study. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the study until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the clinical trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which may review data and endpoints at designated check points, make recommendations and/or halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical study results to public registries.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
32
Phase One: Phase 1 clinical trials are designed to test a new therapy in a small group of people for the first time to evaluate safety (e.g., to determine a safe dosage range and to identify adverse effects). It can include healthy participants or patients.
Phase Two: Phase 2 clinical trials are designed to study an investigational therapy in a larger group of people to determine efficacy and to further evaluate its safety. It is conducted in participants with the condition or disease under study and will determine common short-term adverse effects and risks.
Phase Three: Phase 3 clinical trials are designed to study the efficacy of the investigational therapy in large groups of patients by comparing the therapy to other standard or experimental therapies as well as to monitor adverse effects, and to collect information that will allow the therapy being studied to be used safely.
Post-approval clinical trials, sometimes referred to as Phase 4 studies, may be conducted after initial marketing approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. In certain instances, the FDA may mandate the performance of Phase 4 clinical trials as a condition of approval of an NDA or BLA.
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. In addition, some clinical trials are overseen by an independent group of qualified experts organized by the sponsor, known as a data safety monitoring board or committee. Depending on its charter, this group may determine whether a clinical trial may move forward at designated check points based on access to certain data from the clinical trial.
During the development of a new biopharmaceutical, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA or BLA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and the FDA to reach agreement on the next phase of development. Sponsors typically use the meetings at the end of the Phase 2 clinical trial to discuss Phase 2 clinical results and present plans for the pivotal Phase 3 clinical trials that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP regulations. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality, and purity of the final product. In addition, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
While the IND is active and before approval, progress reports summarizing the results of the clinical trials and nonclinical studies performed since the last progress report must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and investigators for serious and unexpected suspected AEs, findings from other studies suggesting a significant risk to humans exposed to the same or similar drugs, findings from animal or in vitro testing suggesting a significant risk to humans, and any clinically important increased incidence of a serious suspected adverse reaction compared to that listed in the protocol or investigator brochure.
NDA or BLA Review and Approval Process
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development nonclinical and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of substantial user fees, although a waiver of such fees may be obtained under certain limited circumstances. Additionally, no user fees are assessed on NDAs or BLAs for products designated as orphan drugs, unless the product also includes a non-orphan indication.
The FDA reviews an NDA or BLA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality, and purity. Under the Prescription Drug User Fee Act (PDUFA) guidelines, the FDA has a goal of ten months from the date of “filing” of a standard NDA or BLA for a new molecular entity to review and act on the submission.
33
This review typically takes 12 months from the date the NDA or BLA is submitted to FDA because the FDA has approximately two months to make a “filing” decision after the application is submitted. The FDA conducts a preliminary review of all NDAs or BLAs within the first 60 days after submission, before accepting them for filing, to determine whether they are sufficiently complete to permit substantive review The FDA may request additional information rather than accept an NDA or BLA for filing. In this event, the application must be resubmitted with the additional information. The resubmitted application is also subject to review before the FDA accepts it for filing.
The FDA may refer an application for a novel drug to an advisory committee. An advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA or BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP regulations and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCPs. If the FDA determines that the application, manufacturing process, or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates an NDA or BLA, it will issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the product with prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete, and the application will not be approved in its present form. A Complete Response Letter usually describes the specific deficiencies in the application identified by the FDA and may require additional clinical data, such as an additional pivotal Phase 3 clinical trial or other significant and time-consuming requirements related to clinical trials, nonclinical studies, or manufacturing. If a Complete Response Letter is issued, the sponsor must resubmit the application, addressing all of the deficiencies identified in the letter, or withdraw the application. Even if such data and information are submitted, the FDA may decide that the application does not satisfy the criteria for approval.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the application with a Risk Evaluation and Mitigation Strategy ("REMS") to ensure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a medicine and to enable patients to have continued access to such medicines by managing their safe use. It could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may offer conditional approval subject to, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may also require one or more Phase 4 post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could impact the timeline for regulatory approval or otherwise impact ongoing development programs.
Post-Approval Requirements
Any products manufactured or distributed by us pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. There are continuing, annual program fees for any marketed products. Drug manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP regulations, which impose certain procedural and documentation requirements upon us and our third-party manufacturers. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP regulations and impose reporting requirements upon us and any third-party manufacturers that we may decide to use.
34
Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain compliance with cGMP regulations and other aspects of regulatory compliance.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including AEs of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
The FDA closely regulates the marketing, labeling, advertising, and promotion of biopharmaceutical products. A company can make only those claims relating to safety and efficacy that are approved by the FDA and in accordance with the provisions of the approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising, and potential civil and criminal penalties. Physicians may prescribe, in their independent professional medical judgment, legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict manufacturer’s communications on the subject of off-label use of their products. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of off-label use and has enjoined companies from engaging in off-label promotion. The FDA and other regulatory agencies have also required that companies enter into consent decrees or permanent injunctions under which specified promotional conduct is changed or curtailed. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA-approved labelling.
Marketing Exclusivity
Market exclusivity provisions authorized under the FDCA can delay the submission and approval of certain marketing applications for products containing the same active ingredient. The FDCA permits patent term restoration of up to five years as compensation for a patent term lost during product development and FDA regulatory review process to the first applicant to obtain approval of an NDA for a new chemical entity in the United States. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not approve or even accept for review an abbreviated new drug application (“ANDA”) or an NDA submitted under Section 505(b)(2) (“505(b)(2) NDA”), submitted by another company for another drug based on the same active moiety, regardless of whether the drug is intended for the same indication as the original innovative drug or for another indication, where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement to one of the patents listed with the FDA by the innovator NDA holder.
The FDCA alternatively provides three years of marketing exclusivity for an NDA, or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example new indications, dosages, or strengths of an existing drug.
35
This three-year exclusivity covers only the modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from approving ANDAs or 505(b)(2) NDAs for drugs containing the active agent for the original indication or condition of use. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to any nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Pediatric exclusivity is another type of marketing exclusivity available in the United States. Pediatric exclusivity provides for an additional six months of marketing exclusivity attached to another period of exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA. The issuance of a written request does not require the sponsor to undertake the described clinical trials. In addition, orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain circumstances.
Section 505(b)(2) NDAs
A special type of NDA, commonly referred to as a Section 505(b)(2) NDA, enables the applicant in certain circumstances to rely, in part, on the FDA’s prior findings in approving a similar product or published literature in support of its application. A Section 505(b)(2) NDA may provide an alternate path to FDA approval for a new or improved formulation, a new route of administration, or a new use of a previously approved product. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. If the Section 505(b)(2) applicant can establish that reliance on the FDA’s prior findings of safety and/or effectiveness is scientifically appropriate, it may eliminate the need to conduct certain preclinical or clinical studies of the new product. The FDA may also require companies to perform additional studies or measurements to support the change from the approved product. The FDA may then approve the new product candidate for all, or some, of the indications for which the referenced product has been approved, as well as for any new indication sought by the Section 505(b)(2) applicant. If we choose to rely on the 505(b)(2) process to seek approval for OCS-01, there can be no assurance that the FDA will agree with our use of that pathway.
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s prior findings of safety or effectiveness for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the Orange Book to the same extent that an ANDA applicant would. Thus, approval of a Section 505(b)(2) NDA can be stalled until all the listed patents claiming the referenced product have expired, until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired, and, in the case of a Paragraph IV certification and subsequent patent infringement suit, until the earlier of 30 months, settlement of the lawsuit or a decision in the infringement case that is favorable to the Section 505(b)(2) applicant.
FDA Approval and Regulation of Companion Diagnostics
A therapeutic product may rely upon an in vitro companion diagnostic for use in selecting the patients that will be more likely to respond to that therapy. If the FDA determines that a companion diagnostic device is essential to the safe and effective use of a novel therapeutic product or indication, the FDA generally will not approve the therapeutic product or new therapeutic product indication if the companion diagnostic device is not approved or cleared for that indication. Approval or clearance of the companion diagnostic device will ensure that the device has been adequately evaluated and has adequate performance characteristics in the intended population. The review of in vitro companion diagnostics in conjunction with the review of our therapeutic product candidate OCS-02 (Licaminlimab) will, therefore, likely involve coordination of review by the FDA’s Center for Biologics Evaluation and Research and the FDA’s Center for Devices and Radiological Health.
Under the FDCA, in vitro diagnostics, including companion diagnostics, are regulated as medical devices. In the United States, the FDCA and its implementing regulations, and other federal and state statutes and regulations govern, among other things, medical device design and development, preclinical and clinical testing, premarket clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution, export and import, and post-market surveillance. Unless an exemption applies, diagnostic tests require marketing clearance or approval from the FDA prior to commercial distribution. The three primary types of FDA marketing authorization applicable to a medical device include premarket notification, also called 510(k) clearance, premarket approval (“PMA”), and de novo classification requests.
EU/Rest of World Regulation
36
Conduct of Clinical Trials in the EU
In addition to regulations in the United States, there are a variety of regulations in other jurisdictions governing, among other things, clinical trials, commercial sales and distribution of medicinal products. Even if FDA approval of a particular product is obtained, it must still obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials.
In the EU, the Clinical Trials Regulation (EU) No 536/2014 entered into application on January 31, 2022. The Regulation is intended to harmonize and streamline clinical trial authorizations, simplify adverse-event reporting procedures, improve the supervision of clinical trials and increase their transparency. Specifically, the new Regulation, which is directly applicable in all EU Member States, introduces a streamlined application procedure via a single entry point, the “EU portal”, the Clinical Trials Information System (“CTIS”); a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors. A harmonized procedure for the assessment of applications for clinical trials has been introduced and is divided into two parts. Part I is assessed by the competent authorities of a reference member state selected by the trial sponsor largely of the type of clinical trial, risk-benefit analysis, and compliance with technical requirements. This assessment is then submitted to the competent authorities of all the concerned member states in which the trial is to be conducted for their review. Part II is assessed separately by the competent authorities and ECs in each EU member state concerned. Individual EU Member States shall retain the power to authorize the conduct of clinical trials on their territory. The extent to which on-going clinical trials will be governed by the Clinical Trials Regulation will depend on the duration of the individual clinical trial. If a clinical trial continues for more than three years from January 31, 2022, the Clinical Trials Regulation will at that time begin to apply to the clinical trial.
Pathways to Obtain a Marketing Authorization in the EU
In the European Economic Area (“EEA”), which consists of the 27 Member States of the European Union, as well as Norway, Iceland and Liechtenstein, medicinal products can only be commercialized after a related marketing authorization has been granted. A company may submit a marketing authorization application (“MAA”) on the basis of the centralized, decentralized or mutual recognition procedure. Under the centralized procedure, MAAs are submitted to the EMA for scientific review by the EMA’s Committee for Medicinal Products for Human Use (“CHMP”). The CHMP issues an opinion concerning whether the quality, safety and efficacy of the product has been demonstrated. The opinion is considered by the European Commission which is responsible for granting a centralized marketing authorization in the form of a binding European Commission decision. If the application is approved, the European Commission C grants a single marketing authorization that is valid throughout the EEA. The centralized procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, advanced-therapy medicines such as gene-therapy, somatic cell-therapy or tissue-engineered medicines and medicinal products containing a new active substance indicated for the treatment of HIV, AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions and viral diseases. The centralized procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the European Union.
National marketing authorizations, which are issued by the competent authorities of EEA countries and only cover their respective territory, are available for products not falling within the mandatory scope of the centralized procedure. Where a product has already been authorized for marketing in an EEA country, this national marketing authorization can be recognized in another EEA country through the mutual recognition procedure. The mutual recognition procedure provides for the EEA countries selected by the applicant to mutually recognize a national marketing authorization that has already been granted by the competent authority of another EEA country, referred to as the Reference Member State (“RMS”). The decentralized procedure is used when the product in question has yet to be granted a marketing authorization in any EEA country. Under this procedure the applicant can select the EEA country that will act as the RMS. In both the mutual recognition and decentralized procedures, the RMS reviews the application and submits its assessment of the application to the EEA countries for which marketing authorizations are being sought, referred to as Concerned Member States.
Within 90 days of receiving the application and assessment report, each Concerned Member State must decide whether to recognize the RMS assessment or reject it on the basis of potential serious risk to public health. If the disputed points cannot be resolved, the matter is first referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralized Procedures for agreement.
37
If the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralized Procedures cannot reach an agreement, a referral is made to the EMA. The CHMP will provide an opinion that will form the basis of a decision to be issued by the European Commission that is binding on all EEA countries. If the application is successful during the decentralized or mutual recognition procedure, national marketing authorizations will be granted by the competent authorities in each of the EEA countries chosen by the applicant.
In principle, a marketing authorization has an initial validity of five years. The marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EEA country in which the original marketing authorization was granted. To support the application, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the eCTD (Common Technical Document) providing up to date data concerning the quality, safety and efficacy of the product, including all variations introduced since the marketing authorization was granted, at least nine months before the marketing authorization ceases to be valid. The European Commission or the competent authorities of the EEA countries may decide, on justified grounds relating to pharmacovigilance, to proceed with one further five year renewal period for the marketing authorization. Once subsequently definitively renewed, the marketing authorization shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (in case of centralized procedure) or on the market of the authorizing EEA country within three years after authorization ceases to be valid (the so-called sunset clause).
In the EU, conditional marketing authorizations may be granted in the centralized procedure for a limited number of medicinal products for human use in cases where the related clinical dataset is not yet complete. A conditional marketing authorization may be granted for a medicinal product, if (i) the risk-benefit balance of the product is positive, (ii) it is likely that the applicant will be in a position to provide the required comprehensive data after the authorization, (iii) the medicinal product fulfills unmet medical needs and (iv) the benefit to public health of the immediate availability on the market of the medicinal product outweighs the risk inherent in the fact that additional data are still required. The authorization is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once any pending studies are provided, the conditional marketing authorization can be converted into a traditional marketing authorization. However, if the conditions are not fulfilled within the timeframe set by the EMA, the marketing authorization will cease to be renewed.
A marketing authorization may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on the efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information, or when generating data may be contrary to generally accepted ethical principles. Like a conditional marketing authorization, a marketing authorization granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard marketing authorization. However, unlike the conditional marketing authorization, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data. Although the marketing authorization “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually and the marketing authorization is withdrawn in case the risk-benefit ratio is no longer favorable.
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines (“PRIME”), scheme, which provides incentives similar to the breakthrough therapy designation in the U.S. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that target unmet medical needs. It permits increased interaction and early dialogue with companies developing promising medicinal products, to optimize their product development plans and speed up their evaluation to help the product reach patients earlier than normal. Product developers that benefit from PRIME designation are potentially eligible for accelerated assessment of their MAA although this is not guaranteed. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
In addition to an MAA, various other requirements apply to the manufacturing and placing on the EU market of medicinal products. Manufacture of medicinal products in the EU requires a manufacturing authorization, and import of medicinal products into the EU requires a manufacturing authorization allowing for import. The manufacturing authorization holder must comply with various requirements set out in the applicable EU laws, regulations and guidance. These requirements include compliance with EU GMP standards when manufacturing medicinal products and APIs, including the manufacture of APIs outside of the EU with the intention to import the APIs into the Union. Similarly, the distribution of medicinal products within the EU is subject to compliance with the applicable EU laws, regulations and guidelines, including the requirement to hold appropriate authorizations for distribution granted by the competent authorities of the EU member states.
38
Marketing authorization holders and/or manufacturing and import authorization (MIA) holders and/or distribution authorization holders may be subject to civil, criminal or administrative sanctions, including suspension of manufacturing authorization, in case of non-compliance with the EU or EU member states’ requirements applicable to the manufacturing of medicinal products.
Data and Market Exclusivity
In the EU, innovative medicinal products that are subject to marketing authorization on the basis of a full dossier and do not fall within the scope of the concept of global marketing authorization qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. The concept of global marketing authorization prevents the same marketing authorization holder or members of the same group, or companies that have concluded tacit or explicit agreements concerning the marketing of the same medicinal product, from obtaining separate data and market exclusivity periods for medicinal products that contain the same active substance. Data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced. However, the generic product or biosimilar products cannot be marketed in the EU for a further two years thereafter. The overall ten-year period may be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
Pediatric Development
In the EU, Regulation (EC) No 1901/2006 provides that all MAAs for new medicinal products must include the results of trials conducted in the pediatric population, in compliance with a pediatric investigation plan (“PIP”), agreed with the EMA’s Pediatric Committee (“PDCO”). The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the medicinal product for which marketing authorization is being sought. The PDCO may grant a deferral of the obligation to implement some or all of the measures provided in the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults. Furthermore, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data are not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once the marketing authorization is obtained in all EU Member States and study results are included in the product information, even when negative, the product is eligible for a six-month extension to the Supplementary Protection Certificate or SPC if any is in effect at the time of authorization or, in the case of orphan medicinal products, a two-year extension of orphan market exclusivity. For other countries outside of the European Union, such as certain countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product approval, pricing and reimbursement vary from country to country. In all cases, the clinical trials are to be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
Orphan Medicinal Products
Regulation (EC) No. 141/2000, as implemented by Regulation (EC) No. 847/2000 provides that a medicinal product can be designated as an orphan medicinal product by the European Commission if its sponsor can establish that: (i) the product is intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions; (ii) either (a) such conditions affect not more than 5 in 10,000 persons in the EU when the application is made, or (b) the product without the benefits derived from orphan status, would not generate sufficient return in the EU to justify the necessary investment in developing the medicinal product; and (iii) there exists no satisfactory authorized method of diagnosis, prevention, or treatment of the condition that has been authorized in the EU, or even if such method exists, the product will be of significant benefit to those affected by that condition.
Orphan medicinal product designation entitles an applicant to incentives such fee reductions or fee waivers, protocol assistance, and access to the centralized marketing authorization procedure. Upon grant of a marketing authorization, orphan medicinal products are entitled to a ten-year period of market exclusivity for the approved therapeutic indication, which means that the EMA cannot accept another marketing authorization application, or grant a marketing authorization, or accept an application to extend a marketing authorization for a similar product for the same indication for a period of ten years. The period of market exclusivity is extended by two years for orphan medicinal products that have also complied with an agreed PIP. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications. Orphan medicinal product designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
39
The period of market exclusivity may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria on the basis of which it received orphan medicinal product destination, including where it can be demonstrated on the basis of available evidence that the original orphan medicinal product is sufficiently profitable not to justify maintenance of market exclusivity or where the prevalence of the condition has increased above the threshold. Additionally, a marketing authorization may be granted to a similar medicinal product with the same orphan indication during the 10 year period if: (i) if the applicant consents to a second original orphan medicinal product application; (ii) if the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities; or (iii) if the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior to the original orphan medicinal product. A company may voluntarily remove a product from the register of orphan products.
Post-Approval Requirements
Where a marketing authorization is granted in relation to a medicinal product in the EU, the holder of the marketing authorization is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products.
Similar to the United States, both marketing authorization holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission and/or the competent regulatory authorities of the individual EEA countries. The holder of a marketing authorization must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports (“PSURs”).
All new marketing authorization applications must include a risk management plan (“RMP”), describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the marketing authorization. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
In the EU, the advertising and promotion of medicinal products are subject to both EU and EEA countries laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals, misleading and comparative advertising and unfair commercial practices. Although general requirements for advertising and promotion of medicinal products are established under EU directives, the details are governed by regulations in each member state and can differ from one country to another. For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics (“SmPC”), as approved by the competent authorities in connection with a marketing authorization. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU.
Regulation of Companion Diagnostics in the EU
In the EU, despite the absence of a legal definition, companion diagnostics are deemed to be in vitro diagnostic medical devices and are governed by Directive 98/79/EC (“IVDD”). The IVDD currently regulates the placing on the market, the CE-marking, the essential requirements, the conformity assessment procedures, the registration obligations for manufacturers and devices as well as the vigilance procedure related to such products. In vitro diagnostic medical devices, including companion diagnostics, must comply with the requirements provided for in the IVDD, and with further requirements implemented at national level (as the case may be).
In vitro diagnostic medical devices (including companion diagnostics) are currently required to conform with the essential requirements of the IVDD. To demonstrate compliance with the essential requirements laid down in Annex I to the IVDD, the manufacturer must conduct a conformity assessment procedure.
For general in vitro diagnostic medical devices (i.e. all IVDs other than those covered by Annex II to the IVDD and IVDs for self-testing), the conformity assessment is performed through a self-assessment of the manufacturer without the intervention of a notified body which is an independent organization designated by the competent authorities of an EU member state to assess the conformity of devices before being placed on the market. The manufacturer must prepare an EC Declaration of Conformity confirming conformity of its products with the essential requirements laid down in the IVDD before placing the product on the EU market.
40
By contrast, the conformity assessment of in vitro diagnostic medical devices for self-testing or that are listed in Annex II (i.e. essentially moderate and high risk reagents and reagent products) to the IVDD requires the intervention of a notified body. Following successful completion of a conformity assessment procedure the notified body will issue a CE Certificate of Conformity. The device manufacturer may, after having completed remaining related procedures and obligations, affix the CE mark to its medical device after having prepared and signed a related EC Declaration of Conformity.
The regulation of companion diagnostics will be subject to further requirements once the in vitro diagnostic medical devices Regulation (No 2017/746), (“IVDR”), becomes applicable on May 26, 2022. The IVDR introduces a new classification system for companion diagnostics which are now specifically defined as diagnostic tests that support the safe and effective use of a specific medicinal product, by identifying patients that are suitable or unsuitable for treatment. Companion diagnostics will have to undergo a conformity assessment by a notified body. If the medicinal product has, or is in the process of, been authorized through the centralized procedure for the authorization of medicinal products, the notified body will, before it can issue a CE Certificate of Conformity, be required to seek a scientific opinion from the EMA on the suitability of the companion diagnostic for use in relation to the medicinal product concerned. For medicinal products that have or are in the process of authorization through any other route provided in EU legislation, the notified body must seek the opinion of the national competent authority of an EU Member State.
Other Healthcare Laws
Pharmaceutical manufacturers are subject to additional healthcare laws, regulation, and enforcement by the U.S. federal government and by authorities in the states and foreign jurisdictions in which they conduct their business. Such laws include, without limitation:
41
These laws and regulations are subject to change, which can increase the resources needed for compliance and delay drug approval or commercialization. Any action brought against us for violations of these laws or regulations, even successfully defended, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Also, we may be subject to private “qui tam” actions brought by individual whistleblowers on behalf of the federal or state governments. Actual or alleged violation of any such laws or regulations may lead to investigations and other claims and proceedings by regulatory authorities and in certain cases, private actors, and violation of any of such laws or any other governmental regulations that apply may result in penalties, including, without limitation, significant administrative, civil and criminal penalties, damages, fines, disgorgement, additional reporting obligations, and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, the curtailment or restructuring of operations, exclusion from participation in government healthcare programs and imprisonment.
The collection and use of personal health data in the EEA is governed by the General Data Protection Regulation ((EU) 2016/679), (“GDPR”), which became effective May 25, 2018. The GDPR applies to any company established in the EEA and to companies established outside the EEA that process personal data in connection with the offering of goods or services to data subjects in the EU or the monitoring of the behavior of data subjects in the EU. The GDPR enhances data protection obligations for controllers and processors of personal data, including stringent requirements relating to the consent of data subjects, expanded disclosures about how personal data is used, requirements to conduct privacy impact assessments for high-risk processing, limitations on retention of personal data and mandatory data breach notification and privacy by design requirements, and creates direct obligations on service providers acting as data processors. The GDPR also imposes strict rules on the transfer of personal data out of the EEA to countries that do not ensure the same level of protection, such as the United States. Failure to comply with the requirements of the GDPR and the related national data protection laws of the EEA countries may result in fines up to 20 million Euros or 4.0% of a company’s global annual revenues for the preceding financial year, whichever is higher. Moreover, the GDPR grants data subjects the right to claim compensation for damages resulting from infringement of the GDPR.
Coverage and Reimbursement
Sales of any pharmaceutical product depend, in part, on the extent to which such product will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance, and managed healthcare organizations, and the level of reimbursement for such product by third-party payors. Significant uncertainty exists as to the coverage and reimbursement status of any newly approved product.
42
Decisions regarding the extent of coverage and amount of reimbursement to be provided are made on a plan-by-plan basis. One third-party payor’s decision to cover a particular product does not ensure that other payors will also provide coverage for the product. As a result, the coverage determination process can require manufacturers to provide scientific details, information on cost-effectiveness, and clinical support for the use of a product to each payor separately. This can be a time-consuming process, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. In addition, third-party payors are increasingly reducing reimbursements for pharmaceutical products and related services. The U.S. government and state legislatures have continued implementing cost-containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Third-party payors are increasingly challenging the prices charged, examining the medical necessity and reviewing the cost-effectiveness of pharmaceutical products, in addition to questioning their safety and efficacy. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit sales of any product. Decreases in third-party reimbursement for any product or a decision by a third-party payor not to cover a product could reduce physician usage and patient demand for the product.
In international markets, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. Pharmaceutical products may face competition from lower-priced products in foreign countries that have placed price controls on pharmaceutical products and may also compete with imported foreign products. Furthermore, there is no assurance that a product will be considered medically reasonable and necessary for a specific indication, that it will be considered cost-effective by third-party payors, that an adequate level of reimbursement will be established even if coverage is available, or that the third-party payors’ reimbursement policies will not adversely affect the ability for manufacturers to sell products profitably.
Healthcare Reform
In the United States and certain foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. In March 2010, the ACA was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States. By way of example, the ACA increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1%; it required collection of rebates for drugs paid by Medicaid managed care organizations; imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell certain “branded prescription drugs” to specified federal government programs; it implemented a new methodology under which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; it expanded the eligibility criteria for Medicaid programs; it created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and it established a Center for Medicare Innovation at the Centers for Medicare & Medicaid Services ("CMS"), to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
Since its enactment, there have been executive, judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. Since January 2017, President Trump signed several Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the ACA have passed. For example, on June 17, 2021, the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the ACA will remain in effect in its current form. Further, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. In addition, on August 16, 2022, President Biden signed the Inflation Reduction Act of 2022 (“IRA”), into law, which among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025 and eliminates the “donut hole” under the Medicare Part D program beginning in 2025, by significantly lowering the beneficiary maximum out-of-pocket cost through a newly established manufacturer discount program.
43
It is possible that the ACA will be subject to judicial or Congressional challenges in the future. It is unclear how any such challenges or additional health reform measures of the Biden administration will impact the ACA.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2.0% per fiscal year, effective April 1, 2013, which, due to subsequent legislative amendments, will stay in effect until 2031 with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through March 31, 2021, unless additional congressional action is taken. Under current legislation, the actual reduction in Medicare payments will vary from 1.0% in 2022 to up to 4.0% in the final fiscal year of this sequester. On March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several types of providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to product pricing, to review the relationship between pricing and manufacturer patient programs, and to reform government program reimbursement methodologies for pharmaceutical products. For example, in July 2021, the Biden administration released an executive order, “Promoting Competition in the American Economy,” with multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, the U.S. Department of Health and Human Services (“HHS”), released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform and sets out a variety of potential legislative policies that Congress could pursue to advance these principles. In addition, the IRA, among other things: (i) allows HHS to negotiate the price of certain single-source drugs and biologics covered under Medicare, and subjects drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” under the law, and (ii) imposes rebates under Medicare Part B and Medicare Part D to penalize price increases that outpace inflation. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. These provisions will take effect progressively starting in 2023, although they may be subject to legal challenges.
In addition, individual states in the United States have also become increasingly active in implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, mechanisms to encourage importation from other countries and bulk purchasing. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference to pricing systems and publication of discounts and list prices.
The Health Technology Assessment (“HTA”) process, which is currently governed by the national laws of the individual EU Member States, is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. A new regulation adopted in December 2021 the HTA Regulation, is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and to provide the basis for cooperation at EU level for joint clinical assessments in these areas. The Regulation will apply from 2025 followed by a phased roll-out ending in 2028.
C. Organizational Structure
Upon consummation of the Business Combination on March 2, 2023, Merger Sub 1 merged with and into EBAC, with EBAC as the surviving company of the First Merger, and EBAC merged with and into Merger Sub 2, with Merger Sub 2 as the surviving company of the Second Merger and wholly owned subsidiary of the Company. During the third quarter of 2023, the Company gave effect in its financial statements to the impending dissolution of Merger Sub 2, which will be completed in April 2024. On July 6, 2023, Legacy Oculis merged with and into Oculis Operations GmbH (“Oculis Operations”), and the separate corporate existence of Legacy Oculis ceased. Oculis Operations is the surviving company and remains a wholly-owned subsidiary of Oculis Holding AG. The following diagram illustrates our corporate structure as of December 31, 2023.
44
D. Property, Plants and Equipment
We currently lease approximately 8,800 square feet of facilities for our operations, including 4,300 square feet of laboratory and office space in Iceland, with main activities of research, business and clinical development, 2,740 square feet of office space in Switzerland, with main activities of business and clinical development and 1,725 square feet of office space in the United States, with main activities being general and administrative in nature.
We believe these facilities will be adequate for the foreseeable future and that suitable additional or substitute space will be available as and when needed. We believe that these facilities are adequate to meet our current needs, but we are constantly evaluating our needs for expanding and or adding to our existing facilities.
45
Exhibit 99.4

Financial Review
46
Exhibit 99.4
1. Operating and Financial Review and Prospects
Certain information called for by this Section "Operating and Financial Review and Prospects", including a discussion of the year ended December 31, 2021, as well a comparison of the year ended December 31, 2022 against the year ended December 31, 2021, has been reported previously in our Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on March 28, 2023 under "Item 5. Operating and Financial Review and Prospects”.
All amounts discussed are in Swiss francs, unless otherwise indicated.
Company Overview
We are a late clinical-stage biopharmaceutical company, based in Switzerland, with substantial expertise in therapeutics used to treat ocular diseases, engaged in the development of innovative drug candidates which embrace the potential to address large unmet medical needs for many eye-related conditions. Our focus is on advancing therapeutic candidates intended to treat significant and prevalent ophthalmic diseases which result in vision loss, blindness or reduced quality of life. Our mission is to improve the health and quality of life of patients around the world by developing medicines that save sight and improve eye care for patients. To realize this mission, we intend to become a global leader in ocular therapeutics.
Our pipeline currently includes three clinical-stage therapeutic candidates: OCS-01, OCS-02 (Licaminlimab) and OCS-05. Our lead product candidate, OCS-01, is currently being evaluated in two ongoing Phase 3 clinical programs: as a treatment for DME and as a once-daily topical for the treatment of inflammation and pain following cataract surgery. Our second product candidate is OCS-02, currently being evaluated in a Phase 2b clinical trial to assess its potential as a topical anti-TNFα treatment for DED and the potential use of a particular genotype to predict treatment response, which could be considered as a biomarker in a precision medicine approach. A second clinical trial for OCS-02, designed to evaluate its use as a potential treatment for non-infectious anterior uveitis, is expected to follow thereafter. Our third product candidate is OCS-05, a potential disease modifying neuroprotective agent against neurological damage with potential application in multiple indications, including glaucoma, dry AMD and DR. We are conducting a Phase 2 PoC trial evaluating OCS-05 as a potential treatment for AON for which there is currently no approved therapeutic treatment.
Numerous diseases and disorders, many of which represent significant medical needs, are associated with the human eye. The National Eye Institute, a part of the U.S. National Institutes of Health, estimates that in the United States, blindness or significant visual impairment impacts approximately seven million people, including those with vision loss resulting from retinal diseases such as DME, macular degeneration, DR, and retinal vein occlusion (“RVO”); disorders caused by swelling and inflammation such as DED, corneal keratitis and uveitis; and glaucoma, among other disease states. It is estimated that the global spending for ophthalmology therapeutics will reach $33 billion in 2027, according to an industry source.
To date, we have primarily financed our operations through the proceeds from share issuances and grants. We have no products approved for commercialization and have never generated any revenues from product sales. Pharmaceutical and biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. It may be several years, if ever, before we have a product candidate approved for commercialization, and we begin to generate revenue and royalties from product sales. We have also incurred significant operating losses. We incurred net losses of CHF 88.8 million for the year ended December 31, 2023, and an accumulated losses balance of CHF 199.8 million as of December 31, 2023.
Factors Affecting Our Performance
Business Environment
The biopharmaceutical industry is extremely competitive. We are subject to risks and uncertainties common to any clinical-stage biopharmaceutical company. These risks include, but are not limited to, the introduction of new products, therapies, standards of care or technological innovations, our ability to obtain, maintain, protect and enforce our licensed technology, data and other intellectual property and proprietary rights and compliance with extensive government regulation and oversight. Please see the section entitled “Risk Factors” included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024 for more information. We are also dependent upon the services of key personnel, including our Chief Executive Officer, executive team and other highly skilled employees. Demand for experienced personnel in the pharmaceutical and biotechnology industries is high and competition for talent is intense.
47
We face potential competition from many different sources, including pharmaceutical and biotechnology companies, academic institutions and governmental agencies as well as public and private research institutions. Many of our competitors are working to develop or have commercialized products similar to those we are developing and have considerable experience in undertaking clinical trials and in obtaining regulatory approval to market pharmaceutical products. Our competitors may also have significantly greater financial resources, established presence in the markets in which we hope to compete, expertise in research and development, manufacturing, preclinical and clinical testing, obtaining regulatory approvals and reimbursement and marketing approved products. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and registering patients for clinical trials, entering into agreements with CMOs for the manufacture of our product candidates, as well as in acquiring technologies complementary to, or necessary for, our programs.
Business combination with European Biotech Acquisition Corp ("EBAC")
On March 2, 2023, we consummated a business combination with EBAC (the “Business Combination”) pursuant to the Business Combination Agreement ("BCA") between Legacy Oculis and EBAC dated as of October 17, 2022. We received gross proceeds of CHF 97.6 million or $103.7 million comprising CHF 12.0 million or $12.8 million of cash held in EBAC’s trust account and CHF 85.6 million or $90.9 million from private placement (“PIPE”) investments and conversion of notes issued under Convertible Loan Agreements (“CLA”) into our Ordinary Shares. In connection with the Business Combination, Oculis was listed on the Nasdaq Global Market with the ticker symbol "OCS" for its ordinary shares and “OCSAW” for its public warrants.
Under the terms of the BCA, EBAC formed four new legal entities (i) Oculis, (ii) Oculis Merger Sub I Company ("Merger Sub 1"), (iii) Merger Sub 2, and (iv) Oculis Operations. After two consecutive mergers between Merger Sub 1 and EBAC, and EBAC and Merger Sub 2, EBAC and Merger Sub 1 ceased to exist and Merger Sub 2 was the surviving company. During the third quarter of 2023, we gave effect in our financial statements to the impending dissolution of Merger Sub 2, which will be completed in April 2024. As a result, the cumulative translation adjustments related to Merger Sub 2 previously reported as equity and recognized in other comprehensive income, were reclassified from equity to the Consolidated Statement of Loss for the year ended December 31, 2023. The resulting foreign exchange impact of such reclassification amounted to CHF 5.0 million for the year ended December 31, 2023.
As a result of the BCA and as of the acquisition closing date on March 2, 2023:
48
PIPE and CLA Financing
In connection with the BCA, EBAC entered into subscription agreements with the PIPE investors for an aggregate of 7,118,891 shares of EBAC Class A ordinary shares at CHF 9.42 or $10.00 per share for aggregate gross proceeds of CHF 67.1 million or $71.2 million.
In connection with the BCA, Legacy Oculis and the investor parties thereto entered into CLAs pursuant to which the investor lenders granted Legacy Oculis a right to receive an interest free convertible loan with certain conversion rights with substantially the same terms as the PIPE investors. Following the mergers, we assumed the CLAs and the lenders exercised their conversion rights in exchange for 1,967,000 ordinary shares at CHF 9.42 or $10.00 per share for aggregate gross proceeds of CHF 18.5 million or $19.7 million.
Together, the PIPE and CLA financing resulted in aggregate gross cash proceeds of CHF 85.6 million or $90.9 million to Oculis in exchange for 9,085,891 ordinary shares.
Merger and listing expense
The Business Combination is accounted for as a capital re-organization. As EBAC does not meet the definition of a business in accordance with IFRS 3 Business Combinations, the BCA is accounted for within the scope of IFRS 2 Share-based Payment.
The Business Combination is treated as the equivalent of the Company issuing shares for the net assets of EBAC as of the acquisition closing date, accompanied by a recapitalization. The net assets of EBAC are stated at historical cost, with no goodwill or other intangible assets recorded. Any excess of the fair value of the Company’s shares issued considering a fair value of CHF 10.54 or $11.19 per share (price of EBAC ordinary share at the closing date) over the fair value of EBAC’s identifiable net assets acquired represents compensation for the service of a stock exchange listing for its shares.
This expense was incurred in the first quarter of 2023 and amounted to CHF 34.9 million, which was expensed to the statement of loss as operating expenses, “Merger and listing expense”. The expense is non-recurring in nature and represents a share-based payment made in exchange for a listing service and does not lead to any cash outflows.
|
|
Per share value |
|
|
Shares |
|
|
March 2, 2023 |
|
|||
Fair value of equity consideration issued by the Company |
|
|
|
|
|
|
|
|
|
|||
EBAC public shareholders |
|
|
10.54 |
|
|
|
12,754,784 |
|
|
|
134,435 |
|
EBAC sponsor class B |
|
|
10.54 |
|
|
|
3,188,696 |
|
|
|
33,609 |
|
EBAC sponsor class A |
|
|
10.54 |
|
|
|
455,096 |
|
|
|
4,797 |
|
Redemptions of EBAC public shareholders |
|
|
10.54 |
|
|
|
(11,431,606 |
) |
|
|
(120,489 |
) |
Sponsors shares forfeiture |
|
|
10.54 |
|
|
|
(1,596,490 |
) |
|
|
(16,827 |
) |
Total consideration transferred |
|
|
|
|
|
3,370,480 |
|
|
|
35,525 |
|
|
Less net assets of EBAC |
|
|
|
|
|
|
|
|
(662 |
) |
||
Merger and listing expense |
|
|
|
|
|
|
|
|
34,863 |
|
||
|
|
March 2, 2023 |
|
|
|
|
(in CHF thousands) |
|
|
Net assets of EBAC |
|
|
|
|
Cash and cash equivalents |
|
|
11,547 |
|
Public & private warrants |
|
|
(2,136 |
) |
Deferred underwriting fee |
|
|
(3,108 |
) |
Accrued transaction costs |
|
|
(4,400 |
) |
Others |
|
|
(1,241 |
) |
Net assets of EBAC |
|
|
662 |
|
Capitalization
49
The following summarizes the actual ordinary shares issued and outstanding and the ownership interests of Oculis immediately after the Business Combination:
|
|
Shares |
|
|
% |
|
||
Issuance of ordinary shares to Legacy Oculis shareholders in connection with BCA (1) |
|
|
20,277,002 |
|
|
|
61.9 |
% |
Issuance of ordinary shares in connection with closing of the PIPE financing |
|
|
7,118,891 |
|
|
|
21.7 |
% |
Issuance of ordinary shares under CLA |
|
|
1,967,000 |
|
|
|
6.0 |
% |
Ordinary shares owned by sponsors |
|
|
2,047,302 |
|
|
|
6.3 |
% |
Ordinary shares owned by EBAC public shareholders |
|
|
1,323,178 |
|
|
|
4.1 |
% |
Total (2) |
|
|
32,733,373 |
|
|
|
100.0 |
% |
(1) As a result of the BCA, Oculis issued 20,277,002 ordinary shares to Legacy Oculis shareholders in exchange for:
(2) In addition to the shares already issued, the following contingently issuable shares were granted: 3,793,995 earnout shares, 369,737 earnout options, 1,762,949 shares of outstanding conversion options, 4,251,595 public warrants and 151,699 private warrants. The earnout shares are contingently forfeitable if the price targets are not achieved during the earnout period.
|
|
Legacy Oculis shares |
|
|
Exchange ratios |
|
|
Oculis ordinary shares issued |
|
|||
Ordinary shares |
|
|
3,406,771 |
|
|
|
|
|
|
|
||
Treasury shares cancelled |
|
|
(100,000 |
) |
|
|
|
|
|
|
||
Ordinary shares after cancellation of treasury shares |
|
|
3,306,771 |
|
|
1.1432 |
|
|
|
3,780,399 |
|
|
Preferred shares: |
|
|
|
|
|
|
|
|
|
|||
Series A |
|
|
1,623,793 |
|
|
|
1.1432 |
|
|
|
1,856,370 |
|
Series B1 |
|
|
2,486,188 |
|
|
|
1.4154 |
|
|
|
3,518,922 |
|
Series B2 T1 |
|
|
1,675,474 |
|
|
|
1.3900 |
|
|
|
2,328,872 |
|
Series B2 T2 |
|
|
426,378 |
|
|
|
1.3310 |
|
|
|
567,508 |
|
Series B2 T3 |
|
|
603,472 |
|
|
|
1.3142 |
|
|
|
793,082 |
|
Series C T1 |
|
|
5,337,777 |
|
|
|
1.2658 |
|
|
|
6,756,580 |
|
Series C T2 |
|
|
362,036 |
|
|
|
1.2205 |
|
|
|
441,854 |
|
Series C T3 |
|
|
197,745 |
|
|
|
1.1804 |
|
|
|
233,415 |
|
Total preferred shares |
|
|
12,712,863 |
|
|
|
1.2976 |
|
|
|
16,496,603 |
|
|
|
|
|
|
|
|
|
|
|
|||
Total |
|
|
16,019,634 |
|
|
|
|
|
|
20,277,002 |
|
|
Earnout consideration
As a result of the BCA, Legacy Oculis preferred, ordinary and option holders (collectively “equity holders”) received consideration in the form of 3,793,995 earnout shares and 369,737 earnout options with an exercise price of CHF 0.01.
The earnout consideration is subject to forfeiture in the event of a failure to achieve the price targets during the earnout period defined as follows: (i) 1,500,000, (ii) 1,500,000 and (iii) 1,000,000 earned based on the achievement of post-acquisition closing share price targets of $15.00, $20.00 and $25.00, respectively, in each case, for any 20 trading days within any consecutive 30 trading day period commencing after the acquisition closing date and ending on or prior to March 2, 2028 (the “earnout period”). A given share price target described above will also be deemed to be achieved if there is a change of control, as defined in the BCA, during the earnout period.
Public offering of ordinary shares
On May 31, 2023, we entered into an underwriting agreement with BofA Securities Inc. and SVB Securities, LLC, as representatives of several underwriters, and on June 5, 2023, closed the issuance and sale in a public offering of 3,500,000 ordinary shares at a public offering price of CHF 10.45 or $11.50 per share, for total gross proceeds of CHF 36.6 million or $40.3 million before deducting underwriting discounts, commissions and offering expenses.
50
In addition, we granted the underwriters an option to purchase additional ordinary shares which was partially exercised on June 13, 2023 for 154,234 ordinary shares and resulted in gross proceeds of CHF 1.6 million or $1.7 million before deducting underwriting discounts, commissions and offering expenses. After giving issuance to these additional shares, we sold a total of 3,654,234 Ordinary Shares in the offering for aggregate gross proceeds of CHF 38.2 million or $42.0 million, before deducting underwriting discounts, commissions and offering expenses. The unexercised portion of the underwriters’ options to purchase additional Ordinary Shares expired on June 30, 2023.
We intend to use the net proceeds from this offering, together with its existing resources, to advance our development programs, in particular Diabetic Macular Edema and for other ophthalmic indications, and for working capital and general corporate purposes.
Licensing agreement with Accure Therapeutics
Since our inception, we have entered into strategic collaboration agreements with a range of partners covering a number of our product candidates in our strategy to diversify our product portfolio and become a global ophthalmology company.
On January 29, 2022, Legacy Oculis entered into a License Agreement with Accure for the exclusive global licensing of its OCS-05 technology. Under this agreement, Oculis licensed a small molecule in development as a potential disease modifying neuroprotective agent designed to address neurological damage to the optic nerve.
As of December 31, 2023, we have paid the full contractual non-refundable upfront fee and reimbursed costs of CHF 3.5 million increasing our licenses intangible asset up to CHF 12.2 million as of December 31, 2023. We have not reached any milestones or royalties thresholds according to the agreement. If all predefined milestones will be reached, we will be obligated to pay up to CHF 94.3 million ($112.1 million ). In case of a commercialization, sublicense revenues will be subject to further royalty payments.
Components of Results of Operations
Revenue
We have not generated any revenue from the sale of products since our inception and do not expect to generate any revenue from the sale of products in the near future. If our development efforts for our product candidates are successful and result in regulatory approval or if we enter into collaboration or licensing agreements with third parties, we may generate revenue in the future from a combination of product sales or payments from such collaboration or licensing agreements. However, there can be no assurance as to when we will generate such revenue, if at all.
Grant Income
Grant income reflects reimbursement of research and development expenses and income from certain research projects managed by Icelandic governmental institutions. We maintain a subsidiary in Iceland that provides research and development for our product candidates. Certain expenses qualify for incentives from the Icelandic government in the form of tax credits or cash reimbursements. We do not anticipate generating significant grant income in the future.
Operating Expenses
Research and development expenses
Research and development expenses consist primarily of costs incurred in connection with the research and development of our product candidates and programs. We expense research and development costs and the cost of acquired intangible assets used in research and development activities as incurred. Research and development expenditures are capitalized only if they meet the recognition criteria of IAS 38 (“Intangible Assets”) and in such cases are amortized over the useful economic life on a straight-line basis. These expenses include:
51
We historically did not track our research and development costs by project category, primarily because we use our employee and infrastructure resources across multiple research and development programs that we are advancing in parallel, and therefore do not allocate salaries, stock-based compensation, employee benefit expenses or other indirect costs related to our research and development to specific product candidates.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will increase substantially in connection with our planned clinical development activities in the near term and in the future. At this time, we cannot accurately estimate or know the nature, timing and costs of the efforts that will be necessary to complete the clinical development of any current or future product candidates.
Prior to 2023, we did not track our total research and development costs by project category, primarily because our clinical development costs may vary significantly based on factors such as:
52
The successful development and commercialization of product candidates is highly uncertain. This is due to the numerous risks and uncertainties associated with product development and commercialization, including the following:
A change in the outcome of any of these variables with respect to the development of our product candidates could significantly change the costs and timing associated with the development of that product candidate. We may never succeed in obtaining regulatory approval for any of our product candidates or programs.
General and administrative expenses
General and administrative expenses consist primarily of salaries and related costs for personnel in executive management, finance, corporate and business development, and administrative functions. General and administrative expenses also include legal fees relating to patent and corporate matters; professional fees for accounting, auditing, tax, and administrative consulting services; insurance costs; marketing and communications expenses; and other operating costs.
Beginning in 2022 and continuing in 2023, we incurred increased accounting, audit, legal and other professional services costs associated with the Business Combination and the associated transition from a private company to a public company. We anticipate that our general and administrative expenses will continue to increase in the future in relation with costs associated with being a public company such as increased costs for fees to members of the board of directors, increased employee-related expenses, increased director and officer insurance premiums, audit and legal fees, investor relations fees and expenses for compliance with public company reporting requirements under the Exchange Act and Nasdaq rules.
53
Finance income and Finance expense
Finance income consists primarily of interest income earned from our short-term financial assets.
Prior to March 2023, Finance expense consisted primarily of accrued interest costs associated with the preferred dividend payment of 6.0% to the holders of Legacy Oculis preferred Series B and C shares. The preferred Series B and C shares are classified as liabilities under IAS 32 and the associated accrued dividend is recognized as interest expense. All preferred shares were converted into Ordinary Shares upon consummation of the Business Combination on March 2, 2023.
Fair value adjustment on warrant liabilities
Fair value adjustment on warrant liabilities reflects the changes in fair value of our warrant instruments. The fair value is dependent on the change in the underlying market price of the warrants and the number of outstanding warrants at the reporting date. The market price of the warrants is in general directly correlated with the market price of our ordinary shares. Assuming the number of outstanding warrants remains constant, we would expect a fair value loss due to an increase in the market price of the warrants, and a fair value gain due to a decrease in the market price of the warrants.
Foreign currency exchange gain (loss)
Foreign currency exchange gains and losses consisted of currency exchange differences that arise from transaction denominated in currencies other than Swiss Francs.
Income tax expense
We are subject to corporate Swiss federal, cantonal and communal taxation, respectively, in Switzerland, Canton of Zug, and Commune of Zug, as well as in the Canton of Vaud, and Commune of Ecublens, near Lausanne. We are also subject to taxation in other jurisdictions in which we operate, in particular the United States, France, China and Iceland where our wholly owned subsidiaries are incorporated.
We are entitled, under Swiss laws, to carry forward any losses incurred for a period of seven years and can offset our losses carried forward against future taxes owed. As of December 31, 2023, we had tax loss carry-forwards totaling CHF 170.4 million. There is no certainty that we will make sufficient profits to be able to utilize tax loss carry-forwards in full and no deferred tax assets have been recognized in the financial statements.
54
A. Operating Results
The following table summarizes our results of operations for the periods presented:
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
In CHF thousands |
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Grant income |
|
883 |
|
|
|
912 |
|
|
|
(29 |
) |
|
|
(3.2 |
%) |
Operating income |
|
883 |
|
|
|
912 |
|
|
|
(29 |
) |
|
|
(3.2 |
%) |
Research and development expenses |
|
(29,247 |
) |
|
|
(22,224 |
) |
|
|
(7,023 |
) |
|
|
(31.6 |
%) |
General and administrative expenses |
|
(17,487 |
) |
|
|
(11,064 |
) |
|
|
(6,423 |
) |
|
|
(58.1 |
%) |
Merger and listing expense |
|
(34,863 |
) |
|
|
- |
|
|
|
(34,863 |
) |
|
|
(100.0 |
%) |
Operating expenses |
|
(81,597 |
) |
|
|
(33,288 |
) |
|
|
(48,309 |
) |
|
|
(145.1 |
%) |
Operating loss |
|
(80,714 |
) |
|
|
(32,376 |
) |
|
|
(48,338 |
) |
|
|
(149.3 |
%) |
Finance income |
|
1,429 |
|
|
|
126 |
|
|
|
1,303 |
|
|
|
1034.1 |
% |
Finance expense |
|
(1,315 |
) |
|
|
(6,442 |
) |
|
|
5,127 |
|
|
|
(79.6 |
%) |
Fair value adjustment on warrant liabilities |
|
(3,431 |
) |
|
|
- |
|
|
|
(3,431 |
) |
|
|
(100.0 |
%) |
Foreign currency exchange (loss) gain |
|
(4,664 |
) |
|
|
49 |
|
|
|
(4,713 |
) |
|
|
9618.4 |
% |
Finance result |
|
(7,981 |
) |
|
|
(6,267 |
) |
|
|
(1,714 |
) |
|
|
(27.3 |
%) |
Loss before tax for the period |
|
(88,695 |
) |
|
|
(38,643 |
) |
|
|
(50,052 |
) |
|
|
(129.5 |
%) |
Income tax expense |
|
(107 |
) |
|
|
(55 |
) |
|
|
(52 |
) |
|
|
(94.5 |
%) |
Loss for the period |
|
(88,802 |
) |
|
|
(38,698 |
) |
|
|
(50,104 |
) |
|
|
(129.5 |
%) |
Comparison of the Years Ended December 31, 2023 and 2022
Grant Income
Grant income for the years ended December 31, 2023 and 2022 was CHF 0.9 million for both years. The grant income is dependent upon the Icelandic government making such reimbursement available for research and development activities. While certain of our research and development expenses have historically qualified for reimbursement and we anticipate incurring a similar level of costs in the future, there is no assurance that the Icelandic government will continue with the tax reimbursement program.
Research and Development Expenses
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
In CHF thousands |
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Personnel expenses |
|
6,509 |
|
|
|
4,608 |
|
|
|
1,901 |
|
|
|
41.3 |
% |
Payroll |
|
4,796 |
|
|
|
4,313 |
|
|
|
483 |
|
|
|
11.2 |
% |
Share-based compensation |
|
1,713 |
|
|
|
295 |
|
|
|
1,418 |
|
|
|
480.7 |
% |
Operating expenses |
|
22,738 |
|
|
|
17,616 |
|
|
|
5,122 |
|
|
|
29.1 |
% |
External service providers |
|
22,256 |
|
|
|
17,205 |
|
|
|
5,051 |
|
|
|
29.4 |
% |
Other operating expenses |
|
258 |
|
|
|
184 |
|
|
|
74 |
|
|
|
40.2 |
% |
Depreciation of property and equipment |
|
106 |
|
|
|
111 |
|
|
|
(5 |
) |
|
|
(4.5 |
%) |
Depreciation of right-of-use assets |
|
118 |
|
|
|
116 |
|
|
|
2 |
|
|
|
1.7 |
% |
Total research and development expense |
|
29,247 |
|
|
|
22,224 |
|
|
|
7,023 |
|
|
|
31.6 |
% |
Research and development expenses were CHF 29.2 million for the year ended December 31, 2023 compared to CHF 22.2 million for the year ended December 31, 2022. The net increase of CHF 7.0 million, or 31.6%, was primarily due to an increase in external CRO expenses as a result of the completion and subsequent startup activities and of multiple OCS-01 clinical trials and the commencement of the OCS-02 DED Phase 2b clinical trial, as well as an increase in research and development personnel costs. The increase in development expenses reflects the OCS-01 DIAMOND Phase 3 clinical trials, OCS-01 OPTIMIZE Phase 3 clinical trials, OCS-01 LEOPARD investigator-initiated trial ("IIT"), OCS-02 (Licaminlimab) drug development and OCS-05 ACUITY proof-of-concept ("PoC") clinical trial for AON. We anticipate that our research and development expenses will continue to increase as we advance our planned clinical development programs.
|
For the year ended |
|
|
|
December 31, 2023 |
|
|
OCS-01 |
|
15,135 |
|
OCS-02 |
|
8,793 |
|
OCS-05 |
|
3,354 |
|
Other development projects |
|
1,965 |
|
Total |
|
29,247 |
|
55
Prior to 2023, we did not track our total research and development costs by project category. For the year ended December 31, 2023, research and development expenses were primarily driven by our OCS-01 DME DIAMOND Phase 3 Stages 1 and 2 clinical trials, OCS-01 OPTIMIZE Phase 3 clinical trial for inflammation and pain following cataract surgery, the OCS-02 Phase 2b DED clinical and drug development, and OCS-05 ACUITY PoC clinical trial for AON.
General and Administrative Expenses (excluding Merger and Listing Expense)
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
In CHF thousands |
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Personnel expenses |
|
7,029 |
|
|
|
4,449 |
|
|
|
2,580 |
|
|
|
58.0 |
% |
Payroll |
|
5,134 |
|
|
|
3,939 |
|
|
|
1,195 |
|
|
|
30.3 |
% |
Share-based compensation |
|
1,895 |
|
|
|
510 |
|
|
|
1,385 |
|
|
|
271.6 |
% |
Operating expenses |
|
10,458 |
|
|
|
6,615 |
|
|
|
3,843 |
|
|
|
58.1 |
% |
External service providers |
|
7,695 |
|
|
|
2,294 |
|
|
|
5,401 |
|
|
|
235.4 |
% |
Other operating expenses |
|
2,700 |
|
|
|
4,249 |
|
|
|
(1,549 |
) |
|
|
(36.5 |
%) |
Depreciation of property and equipment |
|
19 |
|
|
|
20 |
|
|
|
(1 |
) |
|
|
(5.0 |
%) |
Depreciation of right-of-use assets |
|
44 |
|
|
|
52 |
|
|
|
(8 |
) |
|
|
(15.4 |
%) |
Total |
|
17,487 |
|
|
|
11,064 |
|
|
|
6,423 |
|
|
|
58.1 |
% |
General and administrative expenses (excluding merger and listing expense) were CHF 17.5 million for the year ended December 31, 2023, compared to CHF 11.1 million for the year ended December 31, 2022. The increase of CHF 6.4 million, or 58.1%, was primarily due to non-capitalized financing transaction costs, public liability insurances, as well as personnel-related expenses. These expenses were largely attributable to the Business Combination, Nasdaq listing and operating as a public company.
Merger and listing Expense
|
For the years ended December 31, |
|
|
|
|
|
|
||||||
In CHF thousands |
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|||
Merger and listing expense |
|
(34,863 |
) |
|
|
- |
|
|
|
(34,863 |
) |
|
- |
We incurred a non-recurring merger and listing expense of CHF 34.9 million in the year ended December 31, 2023 in connection with the Business Combination. The Business Combination was accounted for as a share-based payment transaction involving the transfer of shares in Oculis for the net assets of EBAC. This expense represented one-time non-cash compensation for a stock exchange listing service equal to the excess of the fair value of the shares transferred compared to the fair value of the net assets.
Finance Income and Finance Expense
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Finance income |
|
1,429 |
|
|
|
126 |
|
|
|
1,303 |
|
|
|
1034.1 |
% |
Finance expense |
|
(1,315 |
) |
|
|
(6,442 |
) |
|
|
5,127 |
|
|
|
(79.6 |
%) |
Finance income was CHF 1.4 million for the year ended December 31, 2023 compared to CHF 0.1 million for the year ended December 31, 2022. The increase of CHF 1.3 million was due to interest on short-term financial assets recorded during the year ended December 31, 2023. Finance expense was CHF 1.3 million for the year ended December 31, 2023, compared to CHF 6.4 million for the year ended December 31, 2022. The decrease of CHF 5.1 million was primarily due to two months of interest expense accrued during 2023 compared to twelve months of interest expense accrued for the comparative period in 2022, related to Legacy Oculis’ preferred Series B and C shares, which were converted into ordinary shares on March 2, 2023 under the BCA.
56
Fair Value Adjustment on Warrant Liabilities
|
For the years ended December 31, |
|
|
|
|
|
|
||||||
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|||
Fair value adjustment on warrant liabilities |
|
(3,431 |
) |
|
|
- |
|
|
|
(3,431 |
) |
|
- |
We incurred a fair value loss of CHF 3.4 million for the year ended December 31, 2023 primarily due to an increase in the market price of the warrants assumed by Oculis from March 2, 2023 to December 31, 2023.
Foreign Currency Exchange (Loss) Gain
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Foreign currency exchange (loss) gain |
|
(4,664 |
) |
|
|
49 |
|
|
|
(4,713 |
) |
|
|
(9618.4 |
%) |
Foreign currency exchange loss was CHF 4.7 million for the year ended December 31, 2023, compared to a gain of CHF 49 thousand for the year ended December 31, 2022. For the year ended December 31, 2023, the unfavorable currency exchange was mainly due to the fluctuation of U.S. dollar against the Swiss Franc producing a foreign exchange loss over the year related to our U.S. dollar denominated cash balances, as well as a loss on the revaluation of the U.S dollar denominated Series C long-term financial debt (former preferred shares) from January to March 2023. The Series C long-term financial debt was fully converted to ordinary shares pursuant to the Business Combination in March 2023. For the year ended December 31, 2022, favorable currency exchange was mainly due to revaluation of U.S. dollar producing foreign exchange gains over the year related to our cash balances, offset by the full year 2022 revaluation of the Series C long-term debt.
Comparison of Years Ended December 31, 2022 and 2021
For a discussion of the financial results and condition for the fiscal year ended December 31, 2021, please refer to “Item 5. Operating and Financial Review and Prospects” for a comparison of years ended December 31, 2022 and 2021” of our Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC on March 28, 2023.
B. Liquidity and Capital Resources
Overview
Since our inception, we have incurred significant operating losses. We have not yet commercialized any products and we do not expect to generate revenue from sales of products for several years, if at all. As of December 31, 2023, we have funded our operations primarily with CHF 103.4 million of proceeds from the sale of our preferred stock, CHF 97.6 million of gross proceeds from the Business Combination, PIPE Financing and conversion of CLA and CHF 38.2 million of gross proceeds from the sale of our ordinary shares in the Public Offering. As of December 31, 2023 and 2022, we had cash, cash equivalents and short-term investments of CHF 91.7 million and CHF 19.8 million, respectively. We had accumulated losses of CHF 199.8 million and CHF 111.0 million as of December 31, 2023 and 2022, respectively.
We expect to incur additional operating losses in the near future and our operating expenses will increase as we continue to expand our organization through in-licensing, strategic collaboration and acquisition, and invest in the development of our product candidates through additional research and development activities and clinical trials. See “Risk Factors—Risks related to development and regulatory approval of our investigational therapies.” We will continue to incur additional costs associated with operating as a public company, including expenses related to legal, accounting, financial reporting and regulatory matters, maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums and investor relations.
Based on our current operating plan, we believe that our existing cash, cash equivalents and short-term financial assets will be sufficient to fund our operations and capital expenses through at least the next twelve months from the date that the Annual Report on Form 20-F for the year ended December 31, 2023 was filed with the SEC. We have based our estimate on assumptions that may prove to be wrong, and we may use our available capital resources sooner than we currently expect. We may require additional capital resources due to underestimation of the nature, timing and costs of the efforts that will be necessary to complete the development of our product candidates. We may also need to raise additional funds more quickly if we choose to expand our development activities, our portfolio or if we consider acquisitions or other strategic transactions, including licensing transactions.
57
For more information regarding these risk and factors that could influence our future capital requirements and the timing thereof, please see the section entitled “Risk Factors" included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024.
Future Funding Requirements
Product development is very expensive and involves a high degree of risk. Only a small number of research and development programs result in the commercialization of a product. We will not generate revenue from product sales unless and until we successfully complete clinical development and are able to obtain regulatory approval for and successfully commercialize the product candidates we are currently developing or that we may develop. We currently do not have any product candidates approved for commercial sale.
Our product candidates, currently under development or that we may develop, will require significant additional research and development efforts, including extensive clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel infrastructure and extensive compliance and reporting capabilities. There can be no assurance that our research and development activities will be successfully completed, that adequate protection for our licensed or developed technology will be obtained and maintained, that products developed will obtain necessary regulatory approval or that any approved products will be commercially viable.
If we obtain regulatory approval for one or more of our product candidates, we expect to incur significant expenses related to developing our commercialization capabilities to support product sales, marketing and distribution activities, either alone or in collaboration with others. Further, as discussed further below, we expect to incur additional costs associated with operating as a public company. As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy.
Until such time, if ever, we can generate substantial product revenue, we may finance our operations through a combination of private or public equity offerings, debt financings, collaborations, strategic alliances, marketing, distribution or licensing arrangements or through other sources of financing. Adequate capital may not be available to us when needed or on acceptable terms. To the extent that we raise additional capital through the sale of private or public equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of Ordinary Shares. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures.
Debt financing would also result in fixed payment obligations. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our research, product development or future commercialization efforts, grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves, obtain funds through arrangement with collaborators on terms unfavorable to us or pursue merger or acquisition strategies, all of which could adversely affect the holdings or the rights of our shareholders. Please see the section entitled “Risk Factors—Risks related to our business, financial condition, capital requirements, or financial operations” included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024 for additional risks associated with our substantial capital requirements.
We expect our expenses to increase substantially in connection with our ongoing activities, particularly as we advance the preclinical activities, manufacturing and clinical development of our product candidates. In addition, we have incurred additional costs associated with the Business Combination and will continue to incur additional costs associated with operating as a public company, including significant legal, accounting, investor relations and other expenses that we did not incur or incremental to operating a private company. Our expenses will also increase as we:
58
See the section titled “Risk Factors” included in Item 3.D. of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024 for additional risks associated with our substantial capital requirements.
Material Cash Requirements for Known Contractual Obligations and Commitments
We have certain payment obligations under various license and collaboration agreements. Under these agreements, we are required to pay non-refundable, upfront license fees, predefined development and commercial milestone payments and royalties on net sales of licensed products.
License Agreement with Novartis for OCS-02 (Licaminlimab)
Pursuant to a license agreement, dated as of December 19, 2018, as amended, by and between us and Novartis (the “Novartis Agreement”), we obtained an exclusive, royalty-bearing, sublicensable (subject to certain conditions), assignable (subject to certain conditions), worldwide license under certain patents, know-how and manufacturing platform technology to develop, manufacture and commercialize pharmaceutical, therapeutic or diagnostic products containing a specified single chain antibody fragment formulation as an active ingredient in the licensed field as defined in the Novartis Agreement. The license granted to us by Novartis includes sublicenses of rights granted to Novartis by certain third parties, and our license to such rights is expressly subject to the applicable terms and conditions of the agreements between Novartis and such third parties.
We originally entered into the Novartis Agreement with Alcon Research, Ltd. (“Alcon”), which subsequently assigned its rights and obligations under the Novartis Agreement to Novartis in connection with its spin-off from Novartis.
We are deemed the owner of any inventions that are (a) created solely by or on behalf of us pursuant to the Novartis Agreement and (b) severable from the licensed products, and grant Novartis a first right to negotiate a worldwide, royalty-bearing license under any patents directed at such inventions for purposes outside of the licensed field. We also grant Novartis a worldwide, non-exclusive, perpetual, irrevocable, royalty-free, fully paid-up license back under any patents owned by us that (i) cover inventions arising from the Novartis Agreement, the practice of which would infringe the patents licensed to us by Novartis, or (ii) otherwise incorporate Novartis’ proprietary information, in each case, for certain uses outside of the licensed field.
59
We paid in full the contractual non-refundable upfront payment to Alcon of CHF 4.7 million ($4.7 million at the exchange rate at the time of payment) in cash and issued 401,709 ordinary shares (recast subsequent to the BCA) for the residual between the fair value and the upfront payment. This was accounted for as a share-based payment transaction under IFRS 2. As of December 31, 2023, we were obligated to pay Novartis additional up to CHF 81.6 million ($97.0 million at the December 31, 2023 exchange rate) in the aggregate upon the achievement of certain development, regulatory, sales and other milestones and tiered royalties ranging from a mid-single digit to a low mid-teen percentage on net sales. In consideration for the exclusive sublicense from Novartis under certain third-party intellectual property rights, we are obligated to pay a low-single digit royalty on our net sales of the licensed product, however, such payments will be deducted from royalties payable to Novartis. Our royalty payment obligations are subject to certain reductions and expire with respect to any licensed product on a country-by-country basis upon the later of (a) the expiration of the last to expire valid claim of any licensed patent covering any such licensed product in such country; (b) the expiration of the period of data exclusivity in any country worldwide; or (c) twelve (12) years after first commercial sale of such licensed product in such country (“Royalty Term”).
Under the Novartis Agreement, we are obligated to use diligent efforts to develop, manufacture or have manufactured, and commercialize the licensed products in the licensed field worldwide. The Novartis Agreement will expire upon the last-to-expire Royalty Term. We may terminate the Novartis Agreement without cause at any time upon advance written notice to Novartis. Upon written notice to Novartis, we may terminate the Novartis Agreement for cause due to the following events: (a) an insolvency event occurs; (b) Novartis materially breaches its obligations under the Novartis Agreement and fails to cure such breach within a specified period of time; or (c) upon advance written notice for material scientific, technical or medical reasons or in case of a material adverse change that renders further continuation of the Novartis Agreement by us commercially unreasonable or otherwise not viable. Upon written notice to us, Novartis may terminate the Novartis Agreement for cause due to the following events: (i) we fail to pay any undisputed amount due under the Novartis Agreement and we fail to remedy such failure within a specified period of time; (ii) an insolvency event occurs; or (iii) we materially breach our obligations under the Novartis Agreement and fail to cure such breach within a specified period of time; or (iv) following negative clinical trial results, we terminate development of the licensed product and do not pursue any further indications in the licensed field.
License Agreement with Accure for OCS-05
Pursuant to a license agreement, dated as of January 29, 2022, by and between us and Accure (the “Accure Agreement”), we obtained an exclusive, worldwide, sublicensable (subject to certain conditions) and transferable (subject to certain conditions) license under certain patents, know-how and inventory of Accure for any and all uses and purposes, including to perform research, development, manufacturing and commercialization activities in any manner and for any purpose. The licensed patents are co-owned by Accure with third parties who have reserved the right to use the licensed patents for education and research purposes pursuant to an inter-institutional agreement.
As of December 31, 2023, we had paid the full contractual non-refundable upfront fee of CHF 3.0 million and reimbursed costs in the amount of approximately CHF 0.5 million. As of December 31, 2023, we were obligated to pay Accure (a) up to CHF 94.3 million ($112.1 million at the December 31, 2023 exchange rate) in the aggregate upon the achievement of certain development, regulatory and sales milestones; (b) tiered royalties ranging from a mid-single digit to a low mid-teen percentage on net sales of licensed products; and (c) high teens on sublicensing revenues received any time after 36 months from the agreement effective date, and a higher percentage on sublicensing revenues received prior to such date, in all cases subject to reduction for any amount that were previously paid or are concurrently or later paid by us to Accure pursuant to our milestone payment obligations and such amounts received from a sublicensee will be deduced from amounts owned to Accure. Our royalty payment obligations are subject to certain reductions and expire on a licensed product-by-licensed product and country-by-country basis upon the later of (i) the expiration of the last valid claim of any licensed patent covering such licensed product in such country; (ii) the expiration of such licensed product’s Orphan Drug status, if any, in such country; or (iii) ten (10) years following the date of first commercial sale of such licensed product in such country (the “Payment Period”).
Under the Accure Agreement, we are obligated to use commercially reasonable efforts to develop and seek regulatory approval for a licensed product in major countries of the territory as defined in the Accure Agreement.
The Accure Agreement will expire on a licensed product-by-licensed product and country-by-country basis upon the expiration of the applicable Payment Period with respect to such licensed product in such country. We may terminate the Accure Agreement in whole or in part at any time upon advance written notice (a) for documented reasonable scientific, regulatory, commercial reasons related to the licensed product without incurring any penalty or liability to Accure and (b) for no reason. Each party may terminate the Accure Agreement with immediate effect upon written notice to the other party (i) in the event such other party commits a material breach of its obligations under the Accure Agreement and fails to cure that breach within a specified period of time or (ii) with certain exceptions, upon such other party’s bankruptcy.
60
Accure may terminate the Accure Agreement with immediate effect upon written notice to us if we file any action to invalidate any of the licensed patents or fail to maintain the licensed patents in major countries of the territory as defined in the Accure Agreement, or, subject to certain exceptions, if we fail to meet certain development obligations and are unable to agree upon modifications to the development plan with Accure.
Other Commitments
The majority of our near term cash needs relate to our clinical and Chemistry, Manufacturing and Controls ("CMC") projects. We have conducted research and development programs through collaborative programs that include, among others, arrangements with universities, CROs and clinical research sites. As of December 31, 2023, commitments for external research projects totaled CHF 50.5 million, with CHF 23.6 million due within one year and CHF 26.9 million due between one and five years.
In addition, we enter into agreements in the normal course of business with CROs for clinical trials and with vendors for preclinical studies, manufacturing services, and other services and products for operating purposes, which are generally cancelable upon written notice.
We have entered into three real estate lease agreements for lab and office facilities. At December 31, 2023, these lease agreements have aggregate lease liabilities of CHF 0.2 million due within one year and CHF 0.4 million due in more than one year.
Refer to Notes 10 and 19 to our audited consolidated financial statements included elsewhere in this 2023 Annual Report of Oculis Holding AG ("Annual Report") for further details on our obligations and timing of expected future payments.
Cash Flows
The following table summarizes our sources and uses of cash and cash equivalents for each of the periods presented:
|
For the years ended December 31, |
|
|
|
|
|
|
|
|||||||
|
2023 |
|
|
2022 |
|
|
Change |
|
|
% Change |
|
||||
Net cash outflow from operating activities |
|
(53,845 |
) |
|
|
(25,074 |
) |
|
|
(28,771 |
) |
|
|
114.7 |
% |
Net cash outflow from investing activities |
|
(54,211 |
) |
|
|
(3,548 |
) |
|
|
(50,663 |
) |
|
|
1427.9 |
% |
Net cash inflow from financing activities |
|
129,672 |
|
|
|
1,714 |
|
|
|
127,958 |
|
|
|
7465.5 |
% |
Increase/(Decrease) in cash and cash equivalents |
|
21,616 |
|
|
|
(26,909 |
) |
|
|
48,525 |
|
|
|
180.3 |
% |
Operating Activities
For the year ended December 31, 2023, operating activities used CHF 53.8 million of cash, primarily consisting of a loss before tax of CHF 88.7 million and a decrease in net working capital of CHF 13.0 million, partially offset by non-cash adjustments of CHF 46.8 million. Changes in net working capital were driven by an CHF 11.5 million increase in accrued expenses and other payables and a CHF 5.6 million increase in other current assets, partially offset by a CHF 3.7 million increase in trade payables. Non-cash charges primarily consisted of a non-recurring CHF 34.9 million of listing service expenses in connection with the Business Combination, CHF 3.5 million of foreign exchange transactions impacting net financial result, CHF 3.6 million of share-based compensation expense and CHF 3.4 million related to the fair value adjustment on warrant liabilities.
For the year ended December 31, 2022, operating activities used CHF 25.1 million of cash, primarily consisting of a net loss before tax of CHF 38.6 million partially offset by a decrease in net working capital of CHF 6.0 million and non-cash adjustments of CHF 7.6 million. Changes in net working capital were driven by a CHF 7.9 million increase in accrued expenses and other payables and trade payables partly offset by CHF 1.8 million increase in other current assets. Non-cash charges primarily consisted of CHF 6.3 million from interest expense accrued on preferred Series B and C shares, CHF 0.8 million of share-based compensation expense and CHF 0.6 million from non-realized foreign exchange differences.
Investing Activities
For the years ended December 31, 2023 and 2022, investing activities used CHF 54.2 million and CHF 3.5 million, respectively. For the year ended December 31, 2023, CHF 54.2 million was used for the purchase of short term financial assets. For the year ended December 31, 2022, CHF 3.5 million was related to the license agreement with Accure for the exclusive global licensing of OCS-05 technology that was capitalized as an intangible asset.
Financing Activities
61
For the year ended December 31, 2023, net cash provided by financing activities was CHF 129.7 million, which relates primarily to the closing of the Business Combination, the PIPE Financing, the conversion of the CLAs and the Public Offering.
For the year ended December 31, 2022, net cash provided by financing activities was CHF 1.7 million, which primarily consisted of proceeds from the issuance of preferred Series C shares, classified as liabilities, net of transaction costs.
For a discussion of our cash flows for the year ended December 31,2021, see “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources” in our Annual Report on Form 20-F filed with the SEC on March 28, 2023.
C. Research and Development, Patents and Licenses, etc.
Full details of our research and development activities and expenditures are given in "Item 1.B. Information on the Company - Business Overview" of the Section "Business Update" and in "Item 1. Operating and Financial Review and Prospects" of this Section "Financial Review" of this Annual Report.
D. Trend Information
Other than as described elsewhere in this Annual Report, we are not aware of any trends, uncertainties, demands, commitments or events that are reasonably likely to have a material adverse effect on our revenue, income from continuing operations, profitability, liquidity or capital resources, or that would cause our reported financial information not necessarily to be indicative of future operating results or financial condition.
E. Critical Accounting Estimates
We prepared our consolidated financial statements in accordance with IFRS Accounting Standards as issued by the IASB. Refer to Note 3 and 4 to our audited consolidated financial statements included elsewhere in this Annual Report for further details on the most significant accounting policies applied in the preparation of our consolidated financial statements and our critical accounting estimates and judgments.
62
Exhibit 99.4

Corporate Governance
63
1. Directors, Senior Management and Employees
The following table sets forth the current executive officers and directors of Oculis as of the filing date. Unless otherwise noted, the business address of each of our directors and executive officers is EPFL Innovation Park, Bat D 3e Route J.A. Colladon, CH 1015, Lausanne, Switzerland.
Name |
|
Age |
|
Title |
Executive Officers |
|
|
|
|
Riad Sherif, M.D. |
|
56 |
|
Chief Executive Officer and Director |
Sylvia Cheung |
|
49 |
|
Chief Financial Officer |
Páll Ragnar Jóhannesson |
|
43 |
|
Chief Business Officer |
Non-Employee Directors |
|
|
|
|
Anthony Rosenberg |
|
71 |
|
Chairman of the Board of Directors |
Christina Ackermann |
|
59 |
|
Director |
Lionel Carnot |
|
56 |
|
Director |
Pravin Dugel, M.D. |
|
60 |
|
Director |
Martijn Kleijwegt |
|
69 |
|
Director |
Geraldine O'Keeffe |
|
58 |
|
Director |
Executive Officers
Riad Sherif, M.D., 56, has served as the Chief Executive Officer and Director of Oculis since December 2017. Previously, from June 2016 to September 2017, Dr. Sherif served as Entrepreneur in Residence at the Novartis Venture Fund. Before that, Dr. Sherif served as the President of Europe, Middle East and Africa of Alcon, Inc. from March 2014 to May 2016. Prior to that, from January 2002 to April 2014, Dr. Sherif held roles of increasing responsibility at Novartis AG, including as the Global Sales Head in the Transplant and Infectious Disease unit, as the Head for Latin America in transplant and infectious disease, as the President of the Novartis Vaccines and Diagnostics Division for Latin America and where he co-founded Synergium a leading biotech company, and most recently as the President of Novartis Pharmaceuticals, Canada. Prior to Novartis, Dr. Sherif worked for several pharmaceutical companies, holding positions of increasing seniority, mainly in marketing and general management with international scope. Dr. Sherif currently serves as a member of the board of directors of Revenio Group corporation. Dr. Sherif previously served as the Vice Chairman for the Innovative Medicine Canada Association, as the Chairman of In-Vivo Montreal, and as the Chairman of the Board Ophthalmic Surgery and Vision Care of Eucomed. Dr. Sherif is a Medical Doctor by training, and holds an MBA from IMD Business School and a Specialized Master’s Degree in Medical Management from ESCP.
Sylvia Cheung, 49, has served as the Chief Financial Officer of Oculis since September 2020. Prior to that, from October 2005 to August 2020, Ms. Cheung held executive positions at Anika Therapeutics, Inc., a publicly-traded joint preservation company. Most recently, from April 2013 to August 2020, Ms. Cheung served as the Chief Financial Officer of Anika Therapeutics, Inc. Previously, from 2000 to 2005, Ms. Cheung held a series of financial management positions of increasing responsibility at Transkaryotic Therapies, Inc., which was acquired by Shire Pharmaceuticals in 2005. Before that, from 1995 to 2000, Ms. Cheung served as a Senior Associate at PricewaterhouseCoopers. Ms. Cheung holds a Bachelor of Business Administration degree in Accounting from the University of Massachusetts in Amherst, an MBA from Boston University, and is a Certified Public Accountant in Massachusetts.
Páll Ragnar Jóhannesson, 43, has served as the Chief Business Officer of Oculis since January 2023. Previously, from September 2020 to January 2023, Mr. Jóhannesson served as the Chief Strategy Officer of Oculis. Previously, from January 2018 to September 2020, Mr. Jóhannesson served as the Chief Financial Officer of Oculis. Additionally, Mr. Jóhannesson has served as the Managing Director of Oculis Iceland ehf. since May 2015. Prior to that, from February 2012 to April 2015, Mr. Jóhannesson held a series of corporate finance positions of increasing responsibility at Straumur Investment Bank, and most recently, from September 2013 to April 2015, Mr. Jóhannesson served as the Managing Director, Corporate Finance. Before that, from January 2009 to November 2011, Mr. Jóhannesson served as a Director, Corporate Finance at Íslandsbanki and its predecessor Glitnir Bank. Mr. Jóhannesson holds a B.Sc. in Industrial Engineering from the University of Iceland, an M.Phil in Management Science from the University of Cambridge, and was certified as securities broker in Iceland.
64
Non-Employee Directors
Anthony Rosenberg, 71, has served as Chairman of the board of directors of Oculis since April 2018. Since April 2015, Mr. Rosenberg has served as the Chief Executive Officer of TR Advisory Services GmbH. Additionally, from April 2015 to April 2020, Mr. Rosenberg served as a Managing Director of MPM Capital. Prior to that, from 2005 to 2012, Mr. Rosenberg held a series of business development and licensing positions of increasing seniority at Novartis, and most recently, from 2012 to 2015, Mr. Rosenberg served as the Corporate Head of M&A and Licensing at Novartis International AG. Mr. Rosenberg currently serves on the boards of directors of Argenx BV and Cullinan Oncology. Mr. Rosenberg previously served on the boards of directors of SiO2 Materials Science, TriNetX and Radius Health, Inc. Mr. Rosenberg holds a B.Sc. (Hons) from the University of Leicester and a M.Sc. in Physiology from the University of London.
Christina Ackermann, 59, has served as a member of the board of directors of Oculis since March 2023. From January 2022 to May 2023, Ms. Ackermann served as Executive Vice President, General Counsel & President of Ophthalmic Pharmaceuticals at Bausch + Lomb. Ms. Ackermann joined Bausch Health as Executive Vice President, General Counsel, in August 2016. Prior to Bausch Health, Ms. Ackermann was part of the Novartis group of companies for 14 years, most recently serving as Senior Vice President, General Counsel for Alcon, where she was responsible for the legal, intellectual property and compliance functions, in addition to Trade Compliance Function, Enterprise Risk Management and Diversity & Inclusion. Previously, she served as Global Head, Legal and General Counsel at Sandoz, the generics division of Novartis, from 2007 to 2012. She joined Novartis Pharma in 2002 as Head, Legal Technical Operations and Ophthalmics, and assumed the role of Head Legal General Medicine in July 2005. Before Novartis, Ms. Ackermann served in Associate General Counsel roles with Bristol Myers Squibb and DuPont Pharmaceuticals, as well as in private practice, where she focused on securities, and mergers & acquisitions. From August 2021 to March 2023, Ms. Ackermann has served on the board of directors of Graybug Vision. Since September 2023, Ms. Ackermann also serves on the board of directors of Verona Pharma, where she is a member of the Audit Committee. Ms. Ackermann holds a LL.B in law from Queen’s University in Ontario, Canada and a post graduate degree in EU competition law from King’s College in London, England.
Lionel Carnot, 56, has served as a member of the board of directors of Oculis since December 2017. Since March 2012, Mr. Carnot has served as the Partner of Earlybird Venture Capital. Additionally, since 2005, Mr. Carnot has served as the Managing Director of Bay City Capital LLC. Prior to that, from 2000 to 2005, Mr. Carnot served as an Associate of The Pritzker Organization, LLC. Before that, from 1999 to 2000, Mr. Carnot served as a Principal of Oracle Partners. Prior to that, from 1997 to 1998, Mr. Carnot served as a Senior Associate of Booz Allen and Hamilton. Before that, from 1995 to 1997, Mr. Carnot served as a Product Manager of Eli Lilly & Co. Prior to that, from 1991 to 1994, Mr. Carnot served as a Senior Consultant of Accenture. Before that, from 1989 to 1991, Mr. Carnot served as a sales and marketing professional at Rhone-Poulenc. Mr. Carnot currently serves on the board of directors of iSTAR Medical, iQone Healthcare Group, and Priothera. Mr. Carnot previously served on the board of directors of Atlantic Therapeutics, Merus, Interleukin Genetics, Madrigal Pharmaceuticals Inc., Nabsys, Bioseek, Pathway Diagnostics, and Reliant Pharmaceuticals. Mr. Carnot holds an MBA with Distinction from INSEAD and a M.Sc. in Molecular Biology from the University of Geneva.
Pravin Dugel, M.D., 60, has served as a member of the board of directors of Oculis since March 2023. Mr. Dugel served as the President of Iveric Bio from May 2021 to October 2023. He joined as Executive Vice President in April 2020 and was promoted to President of the Company in May 2021. Dr. Dugel was previously Managing Partner, Retinal Consultants of Arizona and the Retinal Research Institute; Clinical Professor, USC Eye Institute, Keck School of Medicine, University of Southern California; and Founding Member, Spectra Eye Institute in Sun City, Arizona. Dr. Dugel has authored more than 200 papers, 35 book chapters and has been invited to lecture at several marquis medical meetings and to serve as a visiting professor at universities worldwide, including in Japan, India, China, Malaysia, Egypt, the United Kingdom, France, Germany, Austria, Italy, Poland, Denmark, Norway, Czechoslovakia, Canada and Australia. Dr. Dugel is internationally recognized as a major clinical researcher and has been a principal investigator in over 100 multicenter clinical trials. His research and educational contributions earned him the prestigious Senior Honor Award from the American Academy of Ophthalmology (AAO). He has been elected and previously served as the Retina Subspecialty Day Board Chairman for the American Academy of Ophthalmology Annual Meeting, as a member of the Board of Directors of the largest retina society in the United States, the American Society of Retina Specialists (ASRS), and the largest retina society in Europe, EURETINA. Dr. Dugel graduated from Columbia University in New York City. He then attended UCLA School of Medicine where he obtained his M.D. He completed his residency in ophthalmology at the USC Eye Institute, Keck School of Medicine and completed his medical retina fellowship at the Bascom Palmer Eye Institute and his surgical retina fellowship at the USC Eye Institute, where he was elected to serve on the faculty as the Resident Director.
65
Martijn Kleijwegt, 69, has served as a member of the board of directors of Oculis since March 2023. Previously, he served as a member and the Chairman of the EBAC Board from EBAC’s inception in January 2021 to March 2023. Mr. Kleijwegt founded LSP in 1998 and is currently a partner at EQT Life Sciences (f/k/a Life Science Partners). Mr. Kleijwegt has over 30 years of hands-on finance and investment experience. Mr. Kleijwegt currently serves on the boards of Vico Therapeutics and A-M Pharma. Mr. Kleijwegt has a master’s degree in Economics from Amsterdam University.
Geraldine O’Keeffe, 58, has served as a member of the board of directors of Oculis since March 2023. Ms. O'Keeffe joined LSP in 2008. She became a Partner of the firm in 2010. Ms. O’Keeffe’s prime focus and responsibility within LSP is to invest in listed securities. Prior to joining LSP, she held the position of Senior Healthcare Analyst at Fortis Investment Banking. In that position, she researched a wide range of innovative life sciences companies, both in Europe and the US. Before joining the financial community, she worked within the life sciences industry for a number of years, gaining first-hand product development experience in a commercial setting. Prior to working in the industry, she lectured in Biomedical Sciences for several years at the Dublin Institute of Technology. Since July 2022, Ms. O’Keeffe serves on the board of directors of T-Knife Therapeutics, where she is a member of the Audit Committee. Ms. O’Keeffe has a Bachelor’s degree in Biochemistry and Microbiology from University College Cork and a Master’s degree in Biotechnology from University College Galway. She also conducted post-graduate research, inter alia at the prestigious Max Planck Institute for Biophysical Chemistry in Göttingen, Germany. In addition, Ms. O’Keeffe is also a graduate of The Dublin School of Business.
Diversity of the Board of Directors
The table below provides certain information regarding the diversity of our board of directors as of the filing date of this Annual Report. Our board diversity matrix for our prior fiscal year can be found in our Annual Report on Form 20-F for the year ended December 31, 2022, filed with the SEC on March 28, 2023.
Board Diversity Matrix |
||||
Country of Principal Executive Offices |
Switzerland |
|||
Foreign Private Issuer |
Yes |
|||
Disclosure Prohibited under Home Country Law |
No |
|||
Total Number of Directors |
7 |
|||
|
Female |
Male |
Non-Binary |
Did Not |
Part I: Gender Identity |
||||
Directors |
2 |
5 |
0 |
0 |
Part II: Demographic Background |
||||
Underrepresented Individual in Home Country Jurisdiction |
0 |
|||
LGBTQ+ |
0 |
|||
Did Not Disclose Demographic Background |
0 |
|||
Family Relationships
There are no family relationships among any of our executive officers or directors.
Corporate Governance
We structured our corporate governance in a manner we believe closely aligns our interests with those of our shareholders. Notable features of this corporate governance include:
66
Non-Classified Board of Directors
In accordance with our articles of association, our board of directors is not divided into classes of directors. The directors were appointed until the end of the general meeting of shareholders called to approve our annual accounts for the 2024 financial year.
Compensation of Executive Officers
Historically, our executive compensation program has reflected our innovative growth and development-oriented corporate culture. To date, the compensation of our Chief Executive Officer and our other executive officers has consisted of a combination of base salary, bonuses and long-term incentive compensation in the form of restricted common stock awards and/or stock options. Our executive officers who are full-time employees, like all other full-time employees, are participants in applicable retirement plans in the jurisdiction in which they reside. We evaluate our compensation values and philosophy and compensation plans and arrangements as circumstances merit. We review executive compensation periodically with input from a third-party compensation consultant. As part of this review process, the board of directors and the remuneration committee apply our values and philosophy, while considering the compensation levels needed to ensure our executive compensation program remains competitive with our peers. In connection with our executive compensation program, we also review whether we are meeting our retention objectives and the potential cost of replacing a key employee.
We use base salaries to recognize the experience, skills, knowledge and responsibilities required of all our executive officers. Base salaries are reviewed annually, typically in connection with our annual performance review process, and adjusted from time to time to align salaries with market levels after taking into account individual responsibilities, performance and experience. In addition, our executives are entitled to annual cash bonuses for their performance over the fiscal year, based on goals established by our board of directors. Furthermore, we have a formal process with respect to the grant of equity incentive awards to our employees, including our executive officers. We believe that equity incentive awards provide our employees with a strong link to our long-term performance, create an ownership culture and help to align the interests of our employees, including our executive officers, and our stockholders. In addition, we believe that equity incentive awards with time-based vesting features promote employee retention because this feature incentivizes our employees, including our executive officers, to remain in our employment during the vesting period.
Adoption of Clawback Policy
In October 2023, in accordance with Rule 10D-1 promulgated under the Exchange Act and Nasdaq Listing Rule 5608, we adopted an Incentive Compensation Recoupment Policy which was filed with the SEC on March 19, 2024 as Exhibit 97.1 of the Annual Report on Form 20-F for the year ended December 31, 2023.
Compensation of Directors
Our board of directors adopted a board of directors’ compensation policy that is designed to enable us to attract and retain, on a long-term basis, highly qualified non-employee directors. As of the filing date, we pay each eligible director who is not an employee of the Company annual cash retainers, as set forth below.
|
|
Annual |
||||
Board of Directors |
|
|
$ 45,200 |
|
|
|
Board of Directors Chair |
|
|
$ 84,750 |
|
|
|
Audit Committee Chair |
|
|
$ 22,600 |
|
|
|
Audit Committee Member |
|
|
$ 11,300 |
|
|
|
Remuneration Committee Chair |
|
|
$ 13,560 |
|
|
|
Remuneration Committee Member |
|
|
$ 6,780 |
|
|
|
Nomination and Governance Committee Chair |
|
|
$ 10,170 |
|
|
|
Nomination and Governance Committee Member |
|
|
$ 5,085 |
|
|
|
67
In addition, each eligible director elected or appointed to our board of directors is eligible to participate in the Stock Option and Incentive Plan Regulation 2023 of the Company (the “2023 Plan”), subject to its terms and conditions as approved and amended by our board of directors from time to time. Upon joining Oculis, we issue to eligible directors a one-time equity incentive award in the form of stock option or similar awards under the 2023 Plan or other equity incentive plans then in effect, at an estimated equity value of $240,000. The exact number of options to be granted and the vesting schedule shall be determined by the board of directors in the grant notice in its free discretion and only such grant notice shall have legal effect. We will also issue to eligible directors an annual equity incentive award in the form of stock option or similar awards under the 2023 Plan or other equity incentive plans then in effect, at an estimated equity value of $120,000, generally granted on the date of our annual general meeting.
The eligible directors are not eligible to any benefits other than those set out in the directors compensation policy, unless our board of directors decides otherwise. We reimburse all reasonable expenses in accordance with the terms and conditions of our travel and expense policy then in effect.
Compensation of Directors and Executive Officers
For the year ended December 31, 2023, the aggregate compensation earned by the members of our board of directors and our executive officers for services in all capacities was CHF 8.1 million.
For the year ended December 31, 2023, fees, salaries and other short-term employee benefits earned by the members of our board of directors and our executive officers was CHF 2.1 million.
The amount contributed by us to provide post-employment benefits to executive officers amounted to a total of CHF 0.2 million for the year ended December 31, 2023.
During the year ended December 31, 2023, 1,029,765 options, excluding earnout options, to purchase registered ordinary shares were granted to members of our board of directors and our executive officers for a total fair value of CHF 4.8 million.
See Note 13 to our audited consolidated financial statements included elsewhere in this Annual Report for further details regarding the share options, SARs and restricted stock, including the exercise price and the expiration date. The above compensation amounts may differ from those reported in our Swiss compensation report because the Swiss compensation report covers the period from March 2, 2023 through December 31, 2023, rather than the full fiscal year.
Risk Oversight
The board of directors is responsible for overseeing our risk management process. The board of directors focuses on our general risk management strategy, the most significant risks, and oversees the implementation of risk mitigation strategies by management. The audit committee is also responsible for discussing our policies with respect to risk assessment and risk management. The board of directors believes its administration of its risk oversight function has not negatively affected the board of directors’ leadership structure.
Code of Business Conduct and Ethics
Our board of directors adopted a Code of Business Conduct and Ethics applicable to the directors, executive officers and employees that complies with the rules and regulations of Nasdaq and the SEC. The Code of Business Conduct and Ethics is available on our website. In addition, we posted on the Corporate Governance section of our website all disclosures that are required by law or Nasdaq listing standards concerning any amendments to, or waivers from, any provision of the Code of Business Conduct and Ethics. The reference to our website address in this Annual Report does not include or incorporate by reference the information on our website into this Annual Report.
Stock Option and Incentive Plan Regulation 2023
The Stock Option and Incentive Plan Regulation 2023 (the “2023 Plan”) was approved by our board of directors on the Acquisition Closing Date and provides for the grant of options, restricted stock awards or units or stock appreciation rights to acquire the Ordinary Shares.
The purpose of the 2023 Plan is to attract and retain highly qualified personnel and to provide key employees with additional incentive to increase their efforts on behalf and in the best interest of us and our subsidiaries by giving them the opportunity to acquire a proprietary interest in us as an incentive for them to remain in the service of us. The terms of the 2023 Plan are described in more detail below.
68
The 2023 Plan shall be administered by a plan administrator (one or several persons) elected by our board of directors from time to time. The plan administrator acts within the guidelines set and approved by our board of directors or a committee thereof and is authorized to, among others, determine (i) which eligible persons are to receive awards under the 2023 Plan, (ii) the time or times when such options or rights grants are to be made, (iii) the nature of the shares and the number of awards covered by each such grant, (iv) the time or times at which each option or stock appreciation right is to become exercisable, (v) the vesting conditions applicable to the options or rights, (vi) the maximum term for which the options or rights are to remain outstanding, and (vii) any terms and conditions of any restricted stock award, in each case, subject to the guidelines set and approved by our board of directors or a committee thereof. Persons eligible to participate in our 2023 Plan are employees, members of the board of directors and consultants of Oculis or a subsidiary. The plan administrator determines within the guidelines set and approved by our board of directors or a committee which eligible persons are to receive rights to acquire options under the 2023 Plan.
The 2023 Plan provides for up to 7,835,544 registered shares corresponding to 16.0% of the Ordinary Shares on a fully diluted basis at the Acquisition Closing Date. In the event registered shares that otherwise would have been issuable under the 2023 Plan are withheld by us in payment of the exercise price or withholding obligations, such shares shall remain available for issuance under the 2023 Plan. In the event that an outstanding award expires or is cancelled, forfeited or terminated for any reason, the shares allocable to the unexercised or unsettled portion shall remain available for issuance under the 2023 Plan.
A participant may only exercise an option or stock appreciation right to the extent that the option or stock appreciation right has vested and has not lapsed under the 2023 Plan. Unless otherwise determined by our board of directors at the grant date or set forth in the grant notice, an option or an award in the form of a restricted stock unit or stock appreciation right granted under the 2023 Plan typically vests as to 25.0% of the award at the end of the first year following the vesting start date, with the remaining 75.0% of the award vesting monthly over the 3 years after the first year following the vesting start date. Any restricted stock may not be transferred or pledged. Such restriction expires with the expiration of any repurchase right for the restricted stock. The 2023 Plan provides provisions that govern the exercise of any awards held by the participant at the time the legal relationship forming the basis of the service is coming to an end. Generally, any award not vested shall immediately lapse at the time a notice of termination has been received (regardless of which party gives notice) or at the end of the term in case of a board member. If indicated in the grant notice or otherwise resolved by the board of directors, upon the occurrence of a “Corporate Transaction” (as defined in the 2023 Plan ), all options and awards in the form of a restricted stock unit or stock appreciation rights (i) shall fully vest and (ii) in the case of options and stock appreciation rights must be immediately exercised, except if such options or awards in the form of a restricted stock unit or stock appreciation rights are repurchased by Oculis or a third party designated by Oculis for a cash consideration equivalent to the economic value applicable to such option or stock appreciation right under the 2023 Plan.
Our board of directors has complete and exclusive power and authority to amend or modify the 2023 Plan in any or all respects. Such amendment or modification shall be communicated in appropriate form as an amendment of the 2023 Plan. Unless such change is required to comply with applicable law, listing requirements, accounting rules or tax requirements, no such amendment or modification shall, without the consent of the concerned participant, adversely affect materially his/her rights and obligations under the 2023 Plan.
Composition of Our Board of Directors
Our board of directors is currently composed of seven members. In accordance with our articles of association, the board of directors is not divided into classes of directors. The directors were appointed until the end of the general meeting of shareholders called to approve our annual accounts for the 2024 financial year.
Six of seven directors are independent as defined in Nasdaq listing standards and applicable SEC rules and our board of directors has an independent audit committee, a nomination and governance committee, and a remuneration committee.
Committees of our Board of Directors
Our board of directors has three standing committees: an audit committee, a remuneration committee, and a nominating and nomination and governance committee. The board has adopted written charters that are available to shareholders on our website at https://investors.oculis.com/corporate-governance. The reference to our website address in this Annual Report does not include or incorporate by reference the information on our website into this Annual Report.
69
Audit Committee
The audit committee consists of Lionel Carnot, Geraldine O’Keeffe and Christina Ackermann. The audit committee assists the board of directors in overseeing our accounting and financial reporting processes and the audits of our financial statements. Mr. Carnot serves as chairperson of the audit committee. In addition, the audit committee is responsible for the appointment, compensation, retention and oversight of the work of our independent registered public accounting firm. Our board of directors has determined that Mr. Carnot, Ms. O’Keeffe and Ms. Ackermann satisfy the “independence” requirements set forth in Rule 10A-3 under the Exchange Act and Mr. Carnot qualifies as an “audit committee financial expert,” as such term is defined in the rules of the SEC.
Each of the members of our audit committee qualify as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to audit committee membership. In addition, all of the audit committee members meet the requirements for financial literacy under applicable SEC and Nasdaq rules and at least one of the audit committee members qualifies as an “audit committee financial expert,” as such term is defined in Item 407(d) of Regulation S-K. The audit committee is governed by a charter that complies with applicable Nasdaq rules, which charter is posted on our website. We have adopted an audit committee charter, which details the principal functions of the audit committee, including:
70
Remuneration Committee
The remuneration committee consists of Christina Ackermann, Pravin Dugel and Lionel Carnot. The remuneration committee assists the board of directors in determining compensation for our executive officers and our directors. Ms. Ackermann serves as chairperson of the remuneration committee.
As of the first day of trading after the Business Combination, we were subject to the Swiss provisions regarding compensations for listed companies under the Swiss Code of Obligations, which require Swiss corporations listed on a stock exchange to establish a remuneration committee. In accordance with the Swiss Code of Obligations, the members of our remuneration committee must be elected by our general meeting of shareholders and the aggregate amount of compensation of each of our directors and our executive committee must also be approved by our general meeting of shareholders, in each case commencing with our first annual general meeting of shareholders as a public company. On March 2, 2023 the general meeting of shareholders approved the compensation packages for the board of directors and the executive committee until the general meeting of shareholders to be held in 2024. Our board of directors appointed Ms. Ackermann as the chair of the remuneration committee and will fill any vacancies on the remuneration committee until completion of the next annual general meeting of shareholders.
Each of the members of our remuneration committee qualifies as independent directors according to the rules and regulations of the SEC and Nasdaq with respect to remuneration committee membership, including the heightened independence standards for members of a remuneration committee. The remuneration committee is governed by a charter that is posted on our website. We have adopted a remuneration committee charter, which details the principal functions of the remuneration committee, including:
71
Nomination and Governance Committee
The nomination and governance committee consists of Dr. Pravin Dugel, Geraldine O’Keeffe and Martijn Kleijwegt. The nomination and governance committee assists our board of directors in identifying individuals qualified to become our directors consistent with criteria established by us and in developing our code of business conduct and ethics. Dr. Dugel serves as chairperson of the nomination and governance committee. The nomination and governance committee is governed by a charter that is posted on our website. We have adopted a nomination and governance committee charter, which details the principal functions of the nomination and governance committee, including:
72
As of December 31, 2023, we had 36 employees. Our headcount for R&D was 18, and our headcount for G&A was 18. Our employees include 16 executive leadership, administrative, and development personnel based in Switzerland; 8 executive leadership, administrative, and research personnel based in Iceland; 7 executives and administrators based in the United States; 5 management, research and administrative personnel based in France, UK and China. Pursuant to local laws, our employees in Iceland and France are represented by a labor union or covered under a collective bargaining agreement. We consider our relationship with our employees to be good.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
For information regarding the share ownership of our directors and executive officers, see "Item 2.A Major Shareholders” and "Item 1.B Compensation” of this Section "Corporate Governance" of this Annual Report for a discussion of the 2023 Plan.
Not applicable.
The following table sets forth information regarding the beneficial ownership of Ordinary Shares as of December 31, 2023:
Except as otherwise noted herein, the number and percentage of Ordinary Shares beneficially owned is determined in accordance with Rule 13d-3 of the Exchange Act, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rule, beneficial ownership includes any Ordinary Shares as to which the holder has sole or shared voting power or investment power and also any Ordinary Shares which the holder has the right to acquire within 60 days of the Closing Date through the exercise of any option, warrant or any other right.
We have based percentage ownership on 36,649,705 Ordinary Shares outstanding as of December 31, 2023. The table below does not include earn-out shares which are issued and contingently forfeitable and are not deemed to be outstanding.
73
Name and Address of Beneficial Owners |
|
Number of Shares |
|
|
% Ownership |
|
||
Directors and Executive Officers (1) |
|
|
|
|
|
|
||
Riad Sherif(2) |
|
|
881,895 |
|
|
|
2.4 |
% |
Sylvia Cheung(3) |
|
|
201,067 |
|
|
* |
|
|
Páll Ragnar Jóhannesson(4) |
|
|
528,413 |
|
|
|
1.4 |
% |
Christina Ackermann |
|
|
11,718 |
|
|
* |
|
|
Lionel Carnot |
|
|
— |
|
|
|
— |
|
Pravin Dugel(5) |
|
|
23,819 |
|
|
* |
|
|
Martijn Kleijwegt(6) |
|
|
1,997,302 |
|
|
|
5.4 |
% |
Geraldine O'Keeffe |
|
|
— |
|
|
|
— |
|
Anthony Rosenberg(7) |
|
|
116,257 |
|
|
* |
|
|
All officers and directors as a group (9 individuals) |
|
|
|
|
|
|
||
Five Percent Holders of the Company |
|
|
|
|
|
|
||
LSP 7 Coöperatief U.A.(8) |
|
|
5,327,362 |
|
|
|
14.5 |
% |
Brunnur vaxtarsjóður slhf. (9) |
|
|
2,335,841 |
|
|
|
6.4 |
% |
BVCF Management (BEYEOTECH) (10) |
|
|
2,070,020 |
|
|
|
5.6 |
% |
Funds managed by Pivotal Partners(11) |
|
|
1,898,502 |
|
|
|
5.2 |
% |
* Indicates beneficial ownership of less than 1.0% of the total ordinary shares outstanding.
74
Significant Changes in Percentage Ownership
In March 2023, we experienced significant changes in the percentage ownership held by major shareholders as a result of the Business Combination.
Voting Rights
The voting rights of the principal shareholders do not differ from the voting rights of other shareholders.
Shareholders in the United States
As of February 15, 2023, to the best of our knowledge 40,503,780 of our outstanding ordinary shares, including earnout shares, were held by 104 shareholders of record in the United States. The actual number of holders is greater than these numbers of record holders, and includes beneficial owners whose ordinary shares are held in street name by brokers and other nominees. This number of holders of record also does not include holders whose shares may be held in trust by other entities.
Policies and Procedures for Related Person Transactions
Our board of directors has adopted a written related person transaction policy that sets forth certain policies and procedures for the review and approval or ratification of transactions involving us in which a related person has or will have a direct or indirect material interest, as determined by the audit committee of the board of directors. A “Related Person” for purposes of the policy means: (i) enterprises that directly or indirectly through one or more intermediaries, control or are controlled by, or are under common control with, us; (ii) associates (defined as, unconsolidated enterprises in which we have a Significant Influence or which has Significant Influence over us); (iii) individuals owning, directly or indirectly, an interest in the voting power of us that gives them Significant Influence over us, and close members of any such individual’s family; (iv) key management personnel (i.e., having authority and responsibility for planning, directing and controlling our activities), including directors and close members of such individuals’ families; and (v) enterprises in which a substantial interest in the voting power is owned, directly or indirectly, by any person described in (iii) or (iv) above or over which such a person is able to exercise Significant Influence, including enterprises owned by our directors or major shareholders and enterprises that have a member of key management in common with us.
75
“Significant Influence” for purposes of the policy means the power to participate in the financial and operating policy decisions of an enterprise but is less than control over those policies, provided that shareholders beneficially owning a 10.0% or more interest in the voting power of the enterprise concerned are presumed to have a significant influence on such enterprise.
Pursuant to the policy, each executive director, nominee for the position of executive director, and executive officer shall promptly notify the designated contact of any transaction involving us and a Related Person. The designated contact will present any new Related Person transactions, and proposed transactions involving Related Persons, to the audit committee of the board of directors at its next occurring regular meeting. If the audit committee determines that the Related Person involved has a direct or indirect material interest in the transaction, and therefore that the transaction is a related party transaction, the audit committee shall consider all relevant facts and circumstances, including the commercial reasonableness of the terms, the benefit and perceived benefit, or lack thereof, to Oculis, opportunity costs of alternate transactions, the materiality and character of the Related Person’s direct or indirect interest, and the actual or apparent conflict of interest of the Related Person. The audit committee will not approve or ratify a Related Person transaction unless it shall have determined that, upon consideration of all relevant information, the transaction is in, or not inconsistent with, our best interests . On an annual basis, the audit committee shall review previously approved Related Person transactions, under the standard described above, to determine whether such transactions should continue. If after the review described above, the audit committee determines not to approve or ratify a Related Person transaction (whether such transaction is being reviewed for the first time or has previously been approved and is being reviewed), the transaction will not be entered into or continued.
Agreements with our Executive Officers and Directors
Aside from standard employment agreements, there are no transactions between the Company and its directors and executive officers. The remuneration of the directors and executive officers (excluding non-executive directors), who are the key management personnel, is described in the section entitled “Compensation.”
Indemnification Agreements
The articles of association provide that we will indemnify our directors and officers to the fullest extent permitted by Swiss law, subject to certain exceptions contained in our articles of association.
In connection with the Business Combination, we also entered into indemnification agreements with each of our directors and executive officers. The indemnification agreements provide the indemnities with contractual rights to indemnification, and expense advancement and reimbursement, to the fullest extent permitted under Swiss law, subject to certain exceptions contained in those agreements.
Not applicable.
3. Financial Information
A. Consolidated Statements and Other Financial Information
Our consolidated financial statements are appended to this Annual Report.
Legal Proceedings
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
B. Significant Changes
Please see Note 23 Subsequent Events, included in the audited consolidated financial statements starting included elsewhere in this Annual Report. Other than the events included in this note, no significant changes have occurred.
76

Report of the Statutory Auditor to the General Meeting
on the Consolidated Financial Statements 2023
77
Report of the statutory auditor
to the General Meeting of Oculis Holding AG
Zug
Report on the audit of the consolidated financial statements
Opinion
We have audited the consolidated financial statements of Oculis Holding AG and its subsidiaries (the Group), which comprise the consolidated statement of financial position as of December 31, 2023, and the consolidated statement of loss, the consolidated statement of comprehensive loss, the consolidated statement of changes in equity, the consolidated statement of cash flows for the year then ended, and notes to the consolidated financial statements, including material accounting policy information.
In our opinion, the consolidated financial statements (pages 86 to 121) give a true and fair view of the consolidated financial position of the Group as of December 31, 2023 and its consolidated financial performance and its consolidated cash flows for the year then ended in accordance with IFRS Accounting Standards and comply with Swiss law.
Basis for opinion
We conducted our audit in accordance with Swiss law, International Standards on Auditing (ISAs) and Swiss Standards on Auditing (SA-CH). Our responsibilities under those provisions and standards are further described in the 'Auditor’s responsibilities for the audit of the consolidated financial statements' section of our report. We are independent of the Group in accordance with the provisions of Swiss law and the requirements of the Swiss audit profession, as well as the International Code of Ethics for Professional Accountants (including International Independence Standards) issued by the International Ethics Standards Board for Accountants (IESBA Code), and we have fulfilled our other ethical responsibilities in accordance with these requirements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Our audit approach
Overview |
Overall Group materiality: CHF 2,580 thousand |
|
We concluded full scope audit work at 3 entities, which addressed over 95% of Group’s total operating expenses. In addition, specified procedures were performed on a further 3 entities representing a further 3% of the Group’s total operating expenses. |
|
As key audit matter the following area of focus has been identified: Accounting Impact of the Capital Reorganization |
Materiality
The scope of our audit was influenced by our application of materiality. Our audit opinion aims to provide reasonable assurance that the consolidated financial statements are free from material misstatement. Misstatements may arise due to fraud or error.
78
They are considered material if, individually or in aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of the consolidated financial statements.
Based on our professional judgement, we determined certain quantitative thresholds for materiality, including the overall Group materiality for the consolidated financial statements as a whole as set out in the table below. These, together with qualitative considerations, helped us to determine the scope of our audit and the nature, timing and extent of our audit procedures and to evaluate the effect of misstatements, both individually and in aggregate, on the consolidated financial statements as a whole.
Overall Group materiality |
CHF 2,580 thousand |
Benchmark applied |
Adjusted loss before tax |
Rationale for the materiality benchmark applied |
We chose adjusted loss before tax as the benchmark, to be aligned with the common practice in the U.S. for clinical stage life science companies while considering non-recurring items related to the capital reorganization. In addition, in our view, the applied benchmark is aligned with investors and Audit Committee expectations. |
We agreed with the Audit Committee that we would report to them misstatements above CHF 258 thousand identified during our audit as well as any misstatements below that amount which, in our view, warranted reporting for qualitative reasons.
Audit scope
We tailored the scope of our audit in order to perform sufficient work to enable us to provide an opinion on the consolidated financial statements as a whole, taking into account the structure of the Group, the accounting processes and controls, and the industry in which the Group operates.
Oculis is a global biopharmaceutical company purposefully driven to save sight and improve eye care. Headquartered in Switzerland, the Group also has operations in the U.S., Iceland, France and Hong-Kong.
The Group’s financial statements are a consolidation of 7 reporting units. We identified 3 reporting units that, in our view, required a full scope audit due to their size or risk characteristics. Specified procedures were also carried out at a further 3 reporting entities to give appropriate coverage of material balances. The majority of the audit procedures was performed by the Group auditor out of Switzerland.
Key audit matters
Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the consolidated financial statements of the current period. These matters were addressed in the context of our audit of the consolidated financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters.
Accounting Impact of the Capital Reorganization
Key audit matter |
|
How our audit addressed the key audit matter |
As described in Notes 2, 4, 5, 7C, 13, 15, 16 and 18 to the consolidated financial statements, as of March 2, 2023, a capital reorganization took place within the Group as a result of the merger with European Biotech Acquisition Corp. (“EBAC”), and resulted in the listing of Oculis Holding AG on the NASDAQ. The accounting treatment for the capital reorganization entailed a high degree of complexity including the impact related to the issuance of both ordinary shares to EBAC and Legacy Oculis (formerly Oculis SA) stockholders as well as contingently issuable shares. Despite EBAC being the legal acquirer, Legacy Oculis was determined to be the accounting acquirer for financial reporting purposes. As a result, Oculis incurred |
|
Addressing the matter involved performing procedures and evaluating audit evidence. These procedures included, among others: -
obtaining a detailed understanding of the transaction through inquiries with management and review of management’s reorganization step-plan and how this was effectuated through the associated accounting entries;
|
79
|
merger and listing expense of CHF 34,863 thousand corresponding with charges associated with the capital reorganization, which included non-cash issuance charge representing the difference in the fair value of equity in instruments held by EBAC stockholders over the fair value of identifiable net assets of EBAC. Also, the transaction was accounted for a capital reorganization. Legacy Oculis and EBAC incurred costs directly related to the capital reorganization (“Transaction costs”) of CHF 4,821 thousand associated with equity issuance, which qualify for capitalization and are accounted for as a deduction of share premium. To capture costs associated with the new equity, the Group allocated non-directly attributable capitalizable transaction costs to the various transaction components at the percentages of 38% and 62% for new shares and old shares, respectively. The principal considerations for our determination that performing procedures relating to the accounting impact of the capital reorganization is a key audit matter are the significant complexities and judgements of the capital reorganization that required a high degree of IFRS technical knowledge. This in turn led to a high degree of audit effort in applying procedures relating to the accounting impact of the capital reorganization to the consolidated financial statements. |
|
-
tracing the details of the accounting entries to the underlying agreements and cash movements as applicable;
-
we assessed, with the support of financial reporting specialists (i) the accounting treatment under IFRS of the impact of the capital reorganization, (ii) the accounting treatment of the non-cash issuance costs and (iii) the accounting treatment of the capitalizable transaction costs.
On the basis of the procedures performed, we consider that the significant judgements applied and conclusions drawn by management with respect to the Accounting Impact of the Capital Reorganization were reasonable. |
Other information
The Board of Directors is responsible for the other information. The other information comprises the information included in the annual report, but does not include the financial statements, the consolidated financial statements, the compensation report and our auditor’s reports thereon.
Our opinion on the consolidated financial statements does not cover the other information and we do not express any form of assurance conclusion thereon.
In connection with our audit of the consolidated financial statements, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the consolidated financial statements or our knowledge obtained in the audit, or otherwise appears to be materially misstated.
If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard.
Board of Directors' responsibilities for the consolidated financial statements
The Board of Directors is responsible for the preparation of consolidated financial statements that give a true and fair view in accordance with IFRS Accounting Standards and the provisions of Swiss law, and for such internal control as the Board of Directors determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the consolidated financial statements, the Board of Directors is responsible for assessing the Group's ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the Board of Directors either intends to liquidate the Group or to cease operations, or has no realistic alternative but to do so.
Auditor’s responsibilities for the audit of the consolidated financial statements
Our objectives are to obtain reasonable assurance about whether the consolidated financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Swiss law, ISAs and SA-CH will always detect a material misstatement when it exists.
80
Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these consolidated financial statements.
As part of an audit in accordance with Swiss law, ISAs and SA-CH, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
We communicate with the Board of Directors or its relevant committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide the Board of Directors or its relevant committee with a statement that we have complied with relevant ethical requirements regarding independence, and communicate with them regarding all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, actions taken to eliminate threats or safeguards applied.
From the matters communicated with the Board of Directors or its relevant committee, we determine those matters that were of most significance in the audit of the consolidated financial statements of the current period and are therefore the key audit matters. We describe these matters in our auditor’s report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, we determine that a matter should not be communicated in our report because the adverse consequences of doing so would reasonably be expected to outweigh the public interest benefits of such communication.
Report on other legal and regulatory requirements
In accordance with article 728a para. 1 item 3 CO and PS-CH 890, we confirm the existence of an internal control system that has been designed, pursuant to the instructions of the Board of Directors, for the preparation of the consolidated financial statements.
We recommend that the consolidated financial statements submitted to you be approved.
81
PricewaterhouseCoopers SA
Michael Foley |
Alex Fuhrer |
|
Licensed audit expert Auditor in charge |
Licensed audit expert |
Lausanne, March 19, 2024
82
83

IFRS Consolidated Financial Statements as of and for the year ended December 31, 2023
83

Oculis Holding AG
Consolidated Financial Statements
84
85
Oculis Holding AG, Zug
Consolidated Statements of Financial Position
(in CHF thousands)
|
|
|
|
As of December 31, |
|
|
As of December 31, |
|
||
|
|
Note |
|
2023 |
|
|
2022 |
|
||
ASSETS |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Non-current assets |
|
|
|
|
|
|
|
|
||
Property and equipment, net |
|
8 |
|
|
288 |
|
|
|
365 |
|
Intangible assets |
|
9 |
|
|
12,206 |
|
|
|
12,206 |
|
Right-of-use assets |
|
10 |
|
|
755 |
|
|
|
758 |
|
Other non-current assets |
|
|
|
|
89 |
|
|
|
74 |
|
Total non-current assets |
|
|
|
|
13,338 |
|
|
|
13,403 |
|
|
|
|
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
|
|
||
Other current assets |
|
11 |
|
|
8,488 |
|
|
|
2,959 |
|
Accrued income |
|
11 |
|
|
876 |
|
|
|
912 |
|
Short-term financial assets |
|
14 |
|
|
53,324 |
|
|
|
- |
|
Cash and cash equivalents |
|
14 |
|
|
38,327 |
|
|
|
19,786 |
|
Total current assets |
|
|
|
|
101,015 |
|
|
|
23,657 |
|
|
|
|
|
|
|
|
|
|
||
TOTAL ASSETS |
|
|
|
|
114,353 |
|
|
|
37,060 |
|
|
|
|
|
|
|
|
|
|
||
EQUITY AND LIABILITIES |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||
Shareholders' equity |
|
|
|
|
|
|
|
|
||
Share capital |
|
16 |
|
|
366 |
|
|
|
39 |
|
Share premium |
|
16 |
|
|
288,162 |
|
|
|
10,742 |
|
Reserve for share-based payment |
|
13 |
|
|
6,379 |
|
|
|
2,771 |
|
Actuarial loss on post-employment benefit obligations |
|
12 |
|
|
(1,072 |
) |
|
|
(264 |
) |
Treasury shares |
|
16 |
|
|
- |
|
|
|
(1 |
) |
Cumulative translation adjustments |
|
|
|
|
(327 |
) |
|
|
(300 |
) |
Accumulated losses |
|
|
|
|
(199,780 |
) |
|
|
(110,978 |
) |
Total equity |
|
|
|
|
93,728 |
|
|
|
(97,991 |
) |
|
|
|
|
|
|
|
|
|
||
Non-current liabilities |
|
|
|
|
|
|
|
|
||
Long-term lease liabilities |
|
10 |
|
|
431 |
|
|
|
491 |
|
Long-term financial debt |
|
15 |
|
|
- |
|
|
|
122,449 |
|
Long-term payables |
|
|
|
|
378 |
|
|
|
- |
|
Defined benefit pension liabilities |
|
12 |
|
|
728 |
|
|
|
91 |
|
Total non-current liabilities |
|
|
|
|
1,537 |
|
|
|
123,031 |
|
|
|
|
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
|
|
||
Trade payables |
|
|
|
|
7,596 |
|
|
|
3,867 |
|
Accrued expenses and other payables |
|
17 |
|
|
5,948 |
|
|
|
8,011 |
|
Short-term lease liabilities |
|
10 |
|
|
174 |
|
|
|
142 |
|
Warrant liabilities |
|
18 |
|
|
5,370 |
|
|
|
- |
|
Total current liabilities |
|
|
|
|
19,088 |
|
|
|
12,020 |
|
|
|
|
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
20,625 |
|
|
|
135,051 |
|
|
|
|
|
|
|
|
|
|
||
TOTAL EQUITY AND LIABILITIES |
|
|
|
|
114,353 |
|
|
|
37,060 |
|
The accompanying notes form an integral part of the consolidated financial statements.
86
Oculis Holding AG, Zug
Consolidated Statements of Loss
(in CHF thousands, except loss per share data)
|
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
Note |
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Grant income |
|
7. (A) / 11 |
|
|
883 |
|
|
|
912 |
|
|
|
960 |
|
Operating income |
|
|
|
|
883 |
|
|
|
912 |
|
|
|
960 |
|
Research and development expenses |
|
7. (B) |
|
|
(29,247 |
) |
|
|
(22,224 |
) |
|
|
(9,568 |
) |
General and administrative expenses |
|
7. (B) |
|
|
(17,487 |
) |
|
|
(11,064 |
) |
|
|
(4,624 |
) |
Merger and listing expense |
|
7. (B) |
|
|
(34,863 |
) |
|
|
- |
|
|
|
- |
|
Operating expenses |
|
|
|
|
(81,597 |
) |
|
|
(33,288 |
) |
|
|
(14,192 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Operating loss |
|
|
|
|
(80,714 |
) |
|
|
(32,376 |
) |
|
|
(13,232 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Finance income |
|
7. (C) |
|
|
1,429 |
|
|
|
126 |
|
|
|
21 |
|
Finance expense |
|
7. (C) |
|
|
(1,315 |
) |
|
|
(6,442 |
) |
|
|
(5,120 |
) |
Fair value adjustment on warrant liabilities |
|
7. (C) / 18 |
|
|
(3,431 |
) |
|
|
- |
|
|
|
- |
|
Foreign currency exchange (loss) gain |
|
7. (C) |
|
|
(4,664 |
) |
|
|
49 |
|
|
|
(193 |
) |
Finance result |
|
|
|
|
(7,981 |
) |
|
|
(6,267 |
) |
|
|
(5,292 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Loss before tax for the period |
|
|
|
|
(88,695 |
) |
|
|
(38,643 |
) |
|
|
(18,524 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Income tax expense |
|
7. (D) |
|
|
(107 |
) |
|
|
(55 |
) |
|
|
(27 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Loss for the period |
|
|
|
|
(88,802 |
) |
|
|
(38,698 |
) |
|
|
(18,552 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|||
Basic and diluted loss attributable to equity holders |
|
22 |
|
|
(2.97 |
) |
|
|
(11.32 |
) |
|
|
(5.84 |
) |
The accompanying notes form an integral part of the consolidated financial statements.
87
Oculis Holding AG, Zug
Consolidated Statements of Comprehensive Loss
(in CHF thousands)
|
|
|
|
For the years ended December 31, |
|
|||||||||
|
Note |
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Loss for the period |
|
|
|
|
(88,802 |
) |
|
|
(38,698 |
) |
|
|
(18,552 |
) |
Other comprehensive loss |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
Items that will not be reclassified to profit or loss: |
|
|
|
|
|
|
|
|
|
|
|
|||
Actuarial gains/(losses) of defined benefit plans |
12 |
|
|
|
(808 |
) |
|
|
744 |
|
|
|
88 |
|
Items that may be reclassified subsequently to profit or loss: |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign currency translation differences |
2. (D) |
|
|
|
(5,005 |
) |
|
|
3 |
|
|
|
(28 |
) |
Foreign currency translation differences recycling |
5 |
|
|
|
4,978 |
|
|
|
- |
|
|
|
- |
|
Other comprehensive profit/(loss) for the period |
|
|
|
|
(835 |
) |
|
|
747 |
|
|
|
60 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Total comprehensive loss for the period |
|
|
|
|
(89,637 |
) |
|
|
(37,951 |
) |
|
|
(18,492 |
) |
The accompanying notes form an integral part of the consolidated financial statements.
88
Oculis Holding AG, Zug
Consolidated Statements of Changes in Equity
(in CHF thousands, except share numbers)
|
|
Legacy Oculis share capital |
|
Legacy Oculis treasury shares |
|
|
Oculis share capital |
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
Note |
|
Shares |
|
Share capital |
|
Shares |
|
Treasury shares |
|
|
Shares |
|
Share capital |
|
Share premium |
|
Reserve for share-based payment |
|
Cumulative translation adjustment |
|
Actuarial loss on post-employment benefit obligations |
|
Accumulated losses |
|
Total |
Balance as of December 31, 2020 (as previously reported) |
|
|
|
2,967,155 |
|
297 |
|
(100,000) |
|
(100) |
|
|
- |
|
- |
|
9,609 |
|
1,640 |
|
(275) |
|
(1,096) |
|
(53,728) |
|
(43,654) |
Retroactive application of the recapitalization due to the business combination |
|
5 / 2 (B) / 16 |
|
424,985 |
|
(263) |
|
(14,323) |
|
99 |
|
|
- |
|
- |
|
164 |
|
- |
|
- |
|
- |
|
- |
|
- |
Balance as of January 1, (effect of the recapitalization) |
|
|
|
3,392,140 |
|
34 |
|
(114,323) |
|
(1) |
|
|
- |
|
- |
|
9,773 |
|
1,640 |
|
(275) |
|
(1,096) |
|
(53,728) |
|
(43,654) |
Loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(18,552) |
|
(18,552) |
Other comprehensive profit/(loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actuarial gain on post-employment benefit obligations |
|
4. (C) / 12 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
88 |
|
- |
|
88 |
Foreign currency translation differences |
|
2. (D) |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
(28) |
|
- |
|
- |
|
(28) |
Total comprehensive loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
(28) |
|
88 |
|
(18,552) |
|
(18,492) |
Share-based compensation expense |
|
13 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
328 |
|
- |
|
- |
|
- |
|
328 |
Restricted shares awards |
|
|
|
441,419 |
|
4 |
|
- |
|
- |
|
|
- |
|
- |
|
872 |
|
- |
|
- |
|
- |
|
- |
|
876 |
Transaction costs |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
(12) |
|
- |
|
- |
|
- |
|
- |
|
(12) |
Balance as of December 31, 2021 (effect of the recapitalization) |
|
|
|
3,833,559 |
|
38 |
|
(114,323) |
|
(1) |
|
|
- |
|
- |
|
10,632 |
|
1,967 |
|
(303) |
|
(1,008) |
|
(72,280) |
|
(60,955) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2021 (as previously reported) |
|
|
|
3,353,271 |
|
335 |
|
(100,000) |
|
(100) |
|
|
- |
|
- |
|
10,434 |
|
1,967 |
|
(303) |
|
(1,008) |
|
(72,280) |
|
(60,955) |
Retroactive application of the recapitalization due to the business combination |
|
5 / 2 (B) / 16 |
|
480,288 |
|
(297) |
|
(14,323) |
|
99 |
|
|
- |
|
- |
|
198 |
|
- |
|
- |
|
- |
|
- |
|
- |
Balance as of January 1, 2022 (effect of the recapitalization) |
|
|
|
3,833,559 |
|
38 |
|
(114,323) |
|
(1) |
|
|
- |
|
- |
|
10,632 |
|
1,967 |
|
(303) |
|
(1,008) |
|
(72,280) |
|
(60,955) |
Loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(38,698) |
|
(38,698) |
Other comprehensive profit/(loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actuarial gain on post-employment benefit obligations |
|
4. (C) / 12 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
744 |
|
- |
|
744 |
Foreign currency translation differences |
|
2. (D) |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
3 |
|
- |
|
- |
|
3 |
Total comprehensive loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
3 |
|
744 |
|
(38,698) |
|
(37,951) |
Share-based compensation expense |
|
13 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
804 |
|
- |
|
- |
|
- |
|
804 |
Transaction costs |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
(9) |
|
- |
|
- |
|
- |
|
- |
|
(9) |
Stock option exercised |
|
13 |
|
61,163 |
|
1 |
|
- |
|
- |
|
|
- |
|
- |
|
119 |
|
- |
|
- |
|
- |
|
- |
|
120 |
Balance as of December 31, 2022 (effect of the recapitalization) |
|
|
|
3,894,722 |
|
39 |
|
(114,323) |
|
(1) |
|
|
- |
|
- |
|
10,742 |
|
2,771 |
|
(300) |
|
(264) |
|
(110,978) |
|
(97,991) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2022 (as previously reported) |
|
|
|
3,406,771 |
|
340 |
|
(100,000) |
|
(100) |
|
|
- |
|
- |
|
10,540 |
|
2,771 |
|
(300) |
|
(264) |
|
(110,978) |
|
(97,991) |
Retroactive application of the recapitalization due to the business combination |
|
5 / 2 (B) / 16 |
|
487,951 |
|
(301) |
|
(14,323) |
|
99 |
|
|
- |
|
- |
|
202 |
|
- |
|
- |
|
- |
|
- |
|
- |
Balance as of January 1, 2023 (effect of the recapitalization) |
|
|
|
3,894,722 |
|
39 |
|
(114,323) |
|
(1) |
|
|
- |
|
- |
|
10,742 |
|
2,771 |
|
(300) |
|
(264) |
|
(110,978) |
|
(97,991) |
Loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
(88,802) |
|
(88,802) |
Other comprehensive profit/(loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Actuarial loss on post-employment benefit obligations |
|
4. (C) / 12 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
(808) |
|
- |
|
(808) |
Foreign currency translation differences |
|
2. (D) |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
(5,005) |
|
- |
|
- |
|
(5,005) |
Foreign currency translation differences recycling |
|
5 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
4,978 |
|
- |
|
- |
|
4,978 |
Total comprehensive loss for the period |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
- |
|
(27) |
|
(808) |
|
(88,802) |
|
(89,637) |
Share-based compensation expense |
|
13 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
- |
|
3,608 |
|
- |
|
- |
|
- |
|
3,608 |
Conversion of Legacy Oculis ordinary shares and treasury shares into Oculis ordinary shares |
|
5 / 16 |
|
(3,894,722) |
|
(39) |
|
114,323 |
|
1 |
|
|
3,780,399 |
|
38 |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
Conversion of Legacy Oculis long-term financial debt into Oculis ordinary shares |
|
5 / 15 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
16,496,603 |
|
165 |
|
124,637 |
|
- |
|
- |
|
- |
|
- |
|
124,802 |
Issuance of ordinary shares to PIPE investors |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
7,118,891 |
|
71 |
|
66,983 |
|
- |
|
- |
|
- |
|
- |
|
67,054 |
Issuance of ordinary shares under CLA |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
1,967,000 |
|
20 |
|
18,348 |
|
- |
|
- |
|
- |
|
- |
|
18,368 |
Issuance of ordinary shares to EBAC shareholders |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
3,370,480 |
|
33 |
|
35,492 |
|
- |
|
- |
|
- |
|
- |
|
35,525 |
Transaction costs related to the business combination |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
(4,821) |
|
- |
|
- |
|
- |
|
- |
|
(4,821) |
Proceeds from sale of shares in public offering |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
3,654,234 |
|
36 |
|
38,143 |
|
- |
|
- |
|
- |
|
- |
|
38,179 |
Transaction costs related to the public offering |
|
5 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
- |
|
- |
|
(3,361) |
|
- |
|
- |
|
- |
|
- |
|
(3,361) |
Stock option exercised |
|
13 / 16 |
|
- |
|
- |
|
- |
|
- |
|
|
112,942 |
|
1 |
|
273 |
|
- |
|
- |
|
- |
|
- |
|
274 |
Issuance of shares in connection with warrant exercises |
|
16 / 18 |
|
- |
|
- |
|
- |
|
- |
|
|
149,156 |
|
2 |
|
1,726 |
|
- |
|
- |
|
- |
|
- |
|
1,728 |
Balance as of December 31, 2023 |
|
|
|
- |
|
- |
|
- |
|
- |
|
|
36,649,705 |
|
366 |
|
288,162 |
|
6,379 |
|
(327) |
|
(1,072) |
|
(199,780) |
|
93,728 |
The accompanying notes form an integral part of the consolidated financial statements.
89
Oculis Holding AG, Zug
Consolidated Statements of Cash Flows
(in CHF thousands)
|
|
|
|
For the years ended December 31, |
|
|||||||||
|
|
Note |
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Loss before tax for the period |
|
|
|
|
(88,695 |
) |
|
|
(38,643 |
) |
|
|
(18,524 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Non-cash adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|||
- Financial result |
|
|
|
|
3,454 |
|
|
|
(500 |
) |
|
|
53 |
|
- Depreciation of property and equipment |
|
8 |
|
|
125 |
|
|
|
132 |
|
|
|
88 |
|
- Depreciation of right-of-use assets |
|
10 |
|
|
162 |
|
|
|
167 |
|
|
|
147 |
|
- Share-based compensation expense |
|
13 |
|
|
3,608 |
|
|
|
804 |
|
|
|
328 |
|
- Payroll expenses related to restricted stock |
|
13 / 16 |
|
|
- |
|
|
|
- |
|
|
|
876 |
|
- Interest expense on Series B and C preferred shares |
|
15 / 7.(C) |
|
|
1,266 |
|
|
|
6,343 |
|
|
|
4,996 |
|
- Interest on lease liabilities |
|
10 |
|
|
42 |
|
|
|
45 |
|
|
|
49 |
|
- Post-employment benefits |
|
12 |
|
|
(171 |
) |
|
|
(9 |
) |
|
|
(139 |
) |
- Non-realized foreign exchange differences |
|
15 / 7.(C) |
|
|
(30 |
) |
|
|
583 |
|
|
|
(792 |
) |
- Fair value adjustment on warrant liabilities |
|
18 |
|
|
3,431 |
|
|
|
- |
|
|
|
- |
|
- Merger and listing expense |
|
5 |
|
|
34,863 |
|
|
|
- |
|
|
|
- |
|
Working capital adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|||
- De/(Increase) in other current assets |
|
11 |
|
|
(5,556 |
) |
|
|
(1,796 |
) |
|
|
(731 |
) |
- De/(Increase) in accrued income |
|
11 |
|
|
36 |
|
|
|
(152 |
) |
|
|
233 |
|
- Changes in receivables/payables from/to related parties |
|
|
|
|
- |
|
|
|
- |
|
|
|
29 |
|
- (De)/Increase in trade payables |
|
|
|
|
3,729 |
|
|
|
3,043 |
|
|
|
30 |
|
- (De)/Increase in accrued expenses and other payables |
|
17 |
|
|
(11,549 |
) |
|
|
4,903 |
|
|
|
(352 |
) |
- (De)/Increase in other operating assets/liabilities |
|
|
|
|
(29 |
) |
|
|
- |
|
|
|
- |
|
- (De)/Increase in long-term payables |
|
|
|
|
378 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Interest received |
|
|
|
|
1,238 |
|
|
|
126 |
|
|
|
- |
|
Interest paid on lease liabilities |
|
|
|
|
(46 |
) |
|
|
(100 |
) |
|
|
(116 |
) |
Taxes paid |
|
|
|
|
(101 |
) |
|
|
(20 |
) |
|
|
- |
|
Net cash outflow from operating activities |
|
|
|
|
(53,845 |
) |
|
|
(25,074 |
) |
|
|
(13,825 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Payment for purchase of property and equipment, net |
|
8 |
|
|
(48 |
) |
|
|
(65 |
) |
|
|
(28 |
) |
Payment for short-term financial assets, net |
|
14 |
|
|
(54,163 |
) |
|
|
- |
|
|
|
- |
|
Payment for purchase of intangible assets |
|
9 |
|
|
- |
|
|
|
(3,483 |
) |
|
|
- |
|
Net cash outflow from investing activities |
|
|
|
|
(54,211 |
) |
|
|
(3,548 |
) |
|
|
(28 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|||
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|||
Proceeds from the shares issued to PIPE investors |
|
5 |
|
|
67,054 |
|
|
|
- |
|
|
|
- |
|
Proceeds from the shares issued to CLA investors |
|
5 |
|
|
18,368 |
|
|
|
- |
|
|
|
- |
|
Proceeds from EBAC non-redeemed shareholders |
|
5 |
|
|
12,014 |
|
|
|
- |
|
|
|
- |
|
Transaction costs related to the business combination |
|
5 |
|
|
(4,607 |
) |
|
|
(214 |
) |
|
|
- |
|
Proceeds from sale of shares in public offering |
|
5 |
|
|
38,179 |
|
|
|
- |
|
|
|
- |
|
Transactions costs related to equity issuance in public offering |
|
5 |
|
|
(2,983 |
) |
|
|
- |
|
|
|
- |
|
Proceeds from exercises of warrants |
|
18 |
|
|
1,531 |
|
|
|
- |
|
|
|
- |
|
Proceeds from stock options exercised |
|
13 / 16 |
|
|
274 |
|
|
|
120 |
|
|
|
- |
|
Proceeds from issuance of preferred shares, classified as liabilities |
|
15 |
|
|
- |
|
|
|
2,030 |
|
|
|
56,096 |
|
Transaction costs for issuance of preferred shares, classified as liabilities/capital increase |
|
|
|
|
- |
|
|
|
(63 |
) |
|
|
(804 |
) |
Principal payment of lease obligations |
|
10 |
|
|
(158 |
) |
|
|
(159 |
) |
|
|
(98 |
) |
Net cash inflow from financing activities |
|
|
|
|
129,672 |
|
|
|
1,714 |
|
|
|
55,194 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Increase/(Decrease) in cash and cash equivalents |
|
|
|
|
21,616 |
|
|
|
(26,909 |
) |
|
|
41,341 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Cash and cash equivalents, beginning of period |
|
14 |
|
|
19,786 |
|
|
|
46,277 |
|
|
|
4,952 |
|
Effect of foreign exchange rate changes |
|
|
|
|
(3,075 |
) |
|
|
418 |
|
|
|
(16 |
) |
Cash and cash equivalents, end of period |
|
14 |
|
|
38,327 |
|
|
|
19,786 |
|
|
|
46,277 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Net cash and cash equivalents variation |
|
|
|
|
21,616 |
|
|
|
(26,909 |
) |
|
|
41,341 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Supplemental Non-Cash Financing Information |
|
|
|
|
|
|
|
|
|
|
|
|||
Transaction costs recorded in accrued expenses and other payables/trade payables |
|
|
|
|
378 |
|
|
|
356 |
|
|
|
- |
|
The accompanying notes form an integral part of the consolidated financial statements.
90
Oculis Holding AG, Zug
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Oculis Holding AG ("Oculis" or "the Company") is a stock corporation ("Aktiengesellschaft") with its registered office at Bahnhofstrasse 7, CH-6300, Zug, Switzerland. It was incorporated under the laws of Switzerland on October 31, 2022.
The Company controls six wholly-owned subsidiaries: Oculis Operations GmbH ("Oculis Operations") with its registered office in Lausanne, Switzerland, which was incorporated in Zug, Switzerland on December 27, 2022, Oculis ehf ("Oculis Iceland"), which was incorporated in Reykjavik, Iceland on October 28, 2003, Oculis France Sàrl ("Oculis France") which was incorporated in Paris, France on March 27, 2020, Oculis US, Inc. ("Oculis US") with its registered office in Newton MA, USA, which was incorporated in Delaware, USA, on May 26, 2020, Oculis HK, Limited ("Oculis HK") which was incorporated in Hong Kong, China on June 1, 2021 and Oculis Merger Sub II Company ("Merger Sub 2") which was incorporated in the Cayman Islands on January 3, 2023 and is pending dissolution which will be completed in April 2024. The Company and its wholly-owned subsidiaries form the Oculis Group (the "Group"). Prior to the Business Combination (as defined in Note 5), Oculis SA ("Legacy Oculis"), which was incorporated in Lausanne, Switzerland on December 11, 2017, and its wholly-owned subsidiaries Oculis Iceland, Oculis France, Oculis U.S. and Oculis HK formed the Oculis group. On July 6, 2023, Legacy Oculis merged with and into Oculis Operations, and the separate corporate existence of Legacy Oculis ceased. Oculis Operations is the surviving company and remains a wholly-owned subsidiary of Oculis.
The purpose of the Company is the research, study, development, manufacture, promotion, sale and marketing of pharmaceutical products and substances as well as the purchase, sale and exploitation of intellectual property rights, such as patents and licenses, in the field of ophthalmology. As a global biopharmaceutical company, Oculis is developing treatments to save sight and improve eye care with breakthrough innovations. The Company’s differentiated pipeline includes candidates for topical retinal treatments, topical biologics and disease modifying treatments.
The consolidated financial statements of Oculis as of and for the year ended December 31, 2023, were approved and authorized for issue by the Company's Board of Directors on March 15, 2024.
The Group's accounts are prepared on a going concern basis. To date, the Group has financed its cash requirements primarily from share issuances, as well as government research and development grants. The recent business combination with European Biotech Acquisition Corp. (“EBAC”) and the listing in NASDAQ early in March 2023 raised additional funding to secure business continuity as explained under note 5. The Board of Directors believes that the Group has the ability to meet its financial obligations for at least the next 12 months.
The Company is a late clinical stage company and is exposed to all the risks inherent to establishing a business. Inherent to the Company’s business are various risks and uncertainties, including the substantial uncertainty as to whether current projects will succeed. The Company’s success may depend in part upon its ability to (i) establish and maintain a strong patent position and protection, (ii) enter into collaborations with partners in the biotech and pharmaceutical industry, (iii) successfully move its product candidates through clinical development, and (iv) attract and retain key personnel. The Company’s success is subject to its ability to be able to raise capital to support its operations. To date, the Company has financed its cash requirements primarily through the sale of its preferred stock, proceeds from the Business Combination, PIPE Financing and conversion of CLA and the sale of its common stock. Shareholders should note that the long-term viability of the Company is dependent on its ability to raise additional capital to finance its future operations. The Company will continue to evaluate additional funding through public or private financings, debt financing or collaboration agreements. The Company cannot be certain that additional funding will be available on acceptable terms, or at all. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to (i) significantly delay, scale back or discontinue the development of one or more product candidates; (ii) seek collaborators for product candidates at an earlier stage than otherwise would be desirable and on terms that are less favorable than might otherwise be available; or (iii) relinquish or otherwise dispose of rights to product candidates that the Company would otherwise seek to develop itself, on unfavorable terms.
91
The consolidated financial statements of Oculis are prepared in accordance with IFRS Accounting Standards ("IFRS") as issued by the International Accounting Standards Board (“IASB”).
Prior to consummation of the Business Combination on March 2, 2023, the audited consolidated financial statements as of and for the year ended December 31, 2022 were issued for Legacy Oculis and its subsidiaries. Legacy Oculis became a wholly-owned subsidiary of the Company as a result of the Business Combination. In accordance with the BCA and described in Note 5, Oculis issued 3,780,399 ordinary shares to Legacy Oculis shareholders in exchange for 3,306,771 Legacy Oculis ordinary shares (after cancellation of 100,000 Legacy Oculis treasury shares) at the Exchange Ratio. The number of ordinary shares, and the number of ordinary shares within the loss per share held by the shareholders prior to the Business Combination have been adjusted by the Exchange Ratio to reflect the equivalent number of ordinary shares in the Company.
Reclassifications: given the immateriality of amounts recorded in financial assets and deferred income tax assets as of December 31, 2023 and 2022, these line items have been aggregated into Other non-current assets in the Consolidated Statements of Financial Position presented herein.
The policies set out below are consistently applied to all the years presented. The consolidated financial statements have been prepared under the historical cost convention, unless stated otherwise in the accounting policies in Note 3.
The totals are calculated with the original unit amounts, which could lead to rounding differences. These differences in thousands of units are not changed in order to keep the accuracy of the original data.
The consolidated financial statements of the Group are expressed in CHF, which is the Company's functional and presentation currency. The functional currency of the Company's subsidiaries is the local currency except for Oculis Iceland whose functional currency is CHF.
Assets and liabilities of foreign operations are translated into CHF at the rate of exchange prevailing at the reporting date and their statements of profit or loss are translated at yearly average exchange rates. The exchange differences arising on translation for consolidation are recognized in other comprehensive income.
The principal accounting policies adopted in the preparation of these financial statements are set out below. The policies set out below are consistently applied to all the years presented, unless otherwise stated.
The Company presents assets and liabilities in the balance sheet based on current/non-current classification. The Company classifies all amounts to be realized or settled within 12 months after the reporting period to be current and all other amounts to be non-current.
Foreign currency transactions are translated into the functional currency, Swiss Francs (CHF), using prevailing exchange rates at the dates of the transactions. Monetary assets and liabilities denominated in foreign currencies are translated into CHF at rates of exchange prevailing at reporting date. Any gains or losses from these translations are included in the statements of loss in the period in which they arise.
Oculis has six wholly owned subsidiaries, including Oculis Operations, Oculis Iceland, Oculis France, Oculis US, Oculis HK and Merger Sub 2. The Company's consolidated financial statements present the aggregate of the six Group entities, after elimination of intra-group transactions, balances, investments and capital.
92
The Company is managed and operated as one business. A single management team that reports to the Chief Executive Officer comprehensively manages the entire business and accordingly, has one reporting segment.
The Company has locations in five countries: Switzerland, Iceland, France, USA and Hong Kong. An analysis of non-current assets by geographic region is presented in Note 6.
All leases are accounted for by recognizing a right-of-use asset and a lease liability except for leases of low value assets and leases with a duration of 12 months or less.
Lease liabilities are measured at the present value of the expected contractual payments due to the lessor over the lease term, with the discount rate determined by reference to the rate inherent in the lease unless this is not readily determinable, in which case the Group’s incremental borrowing rate on commencement date of the lease is used. Variable lease payments are only included in the measurement of the lease liability if they depend on an index or rate and remain unchanged throughout the lease term. Other variable lease payments are expensed.
On initial recognition, the carrying value of the lease liability also includes amounts expected to be payable under any residual value guarantee, and the exercise price of any purchase option granted in favor of the group if it is reasonably certain to assess that option.
Right-of-use assets are initially measured at the amount of the lease liability, reduced for any lease incentives received, and increased for lease payments made at or before commencement of the lease and initial direct costs incurred.
Subsequent to the initial measurement, lease liabilities increase as a result of interest charged at a constant rate on the balance outstanding and are reduced for lease payments made. Right-of-use assets are depreciated on a straight-line basis over the remaining expected term of the lease or over the remaining economic life of the asset if this is judged to be shorter than the lease term.
When the Company revises its estimate of the term of any lease, it adjusts the carrying amount of the lease liability to reflect the expected payments over the revised term, which are discounted using a revised discount rate. The carrying value of lease liabilities is similarly revised if the variable future lease payments dependent on a rate or index is revised. In both cases, an equivalent adjustment is made to the carrying value of the right-of-use asset, with the revised carrying amount being amortized over the remaining lease term. If the carrying amount of the right-of-use asset is adjusted to zero, any further reduction is recognized in profit or loss.
Grant income is recognized where there is reasonable assurance that the grant will be received and all attaching conditions will be complied with, and in the year when the related expenses are incurred.
Taxes reported in the consolidated statements of loss include current and deferred taxes on profit. Taxes on income are accrued in the same periods as the revenues and expenses to which they relate.
Deferred tax is the tax attributable to the temporary differences that appear when taxation authorities recognize and measure assets and liabilities with rules that differ from those of the consolidated accounts. Deferred income tax is calculated using the liability method and determined using tax rates and laws that have been enacted or substantively enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realized, or the deferred income tax liability is settled. Any changes to the tax rates are recognized in the consolidated statements of loss unless related to items directly recognized in equity or other comprehensive loss.
Deferred income tax is recognized on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. Deferred income tax assets are recognized only to the extent that it is probable that future taxable profit will be available against which the temporary differences or the unused tax losses can be utilized. Deferred income tax assets from tax credit carry forwards are recognized to the extent that the national tax authority confirms the eligibility of such a claim and that the realization of the related tax benefit through future taxable profits is probable. Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income tax assets and liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities where there is an intention to settle the balances on a net basis.
93
The Company presents basic earnings / (loss) per share for each period in the financial statements. The earnings (loss) per share is calculated by dividing the earnings / (loss) of the period by the weighted average number of shares outstanding during the period. Diluted earnings per share, applicable in case of positive result, reflect the potential dilution that could occur if dilutive securities such as warrants or share options were exercised into common shares.
Judgment was required in determining the classification of the preferred shares issued by the Company as either equity or liabilities. The preferred shareholders hold certain preference rights that include preferential distribution of proceeds in the case of liquidity events as defined in the shareholder agreements. Under IAS 32 the Company classified the Preferred Shares as liabilities. This applied to Series A, B and C shares as per Note 15.
The Company considers all highly liquid investments with an original maturity of less than 3 months at the date of purchase to be cash equivalents. Cash and cash equivalents are recorded at cost, which approximates fair value.
Short-term financial assets consist of fixed term bank deposits with maturities between three and six months. Short-term financial assets are held in order to collect contractual cash flows made of payments of principal and interests.
Short-term financial assets are measured at amortized cost (approximates fair value) and are subsequently measured using the effective interest method. This method allocates interest income over the relevant period by applying the effective interest rate to the carrying amount of the asset. Gains and losses are recognized in the consolidated statements of loss when the asset is derecognized, modified or impaired.
The Company measures certain financial assets and liabilities at fair value on a recurring basis, including warrants. Fair value is the price the Company would receive to sell an asset or pay to transfer a liability in an orderly transaction with a market participant at the measurement date. The Company uses a three-level hierarchy that prioritizes fair value measurements based on the types of inputs used, as follows:
There was no change in the valuation techniques applied to financial instruments during all periods presented. There were no transfers between levels 1, 2 or 3 for recurring fair value measurements during the year. The Group recognizes transfers into and out of fair value hierarchy levels at the end of the reporting period.
All property and equipment are shown at cost, less subsequent depreciation and impairment. Cost includes expenditures that are directly attributable to the acquisition of the items. Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably.
Depreciation is calculated on a straight-line basis over the useful life, according to the following schedule:
Category |
Useful life in years |
Laboratory equipment |
5 - 7 |
Laboratory fixtures and fittings |
10 |
Office - IT tools |
2 - 3 |
Office furniture and equipment |
5 |
94
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at each balance sheet date. An asset’s carrying amount is impaired immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount. Gains and losses on disposal or retirement of tangible fixed assets are determined by comparing the net proceeds received with the carrying amounts and are included in the consolidated statements of loss.
The Company recognizes the warrant instruments as liabilities at fair value and adjusts the instruments to fair value at each reporting period (refer to Note 18). Any change in fair value is recognized in the Company’s consolidated statements of loss. The fair value of the public warrants traded in active markets is based on the quoted market prices at the end of the reporting period for such warrants. Since the private placement warrants have identical terms to the public warrants, the Company determined that the fair value of each private placement warrant is equivalent to that of each public warrant. Public warrant instruments are included in Level 1 and private warrants in Level 2 in the fair value hierarchy. Warrants were classified as short-term liabilities given the Company cannot defer the settlement for at least 12 months.
Research expenditure is recognized in expense in the year in which it is incurred. Internal development expenditure is capitalized only if it meets the recognition criteria of IAS 38 “Intangible Assets”. Where regulatory and other uncertainties are such that the criteria are not met, which is almost invariably the case prior to approval of the drug by the relevant regulatory authority, the expenditure is recognized in the consolidated statements of loss. Where, however, recognition criteria are met, internal development expenditure is capitalized and amortized on a straight-line basis over its useful economic life. The amortization of the licenses will start when the market approval is obtained.
Licenses acquired are capitalized as intangible assets at historical cost and amortized over their useful lives, which are determined on a basis of the expected pattern of consumption of the expected future economic benefits embodied in the licenses and which therefore commence only once the necessary regulatory and marketing approval has been received. These licenses are tested for impairment in the last quarter of each financial period, or when there is any indication for impairment.
Amortization of capitalized licenses is charged to research and development expenses.
Impairment of capitalized licenses is charged to research and development expenses.
Assets that are subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable.
An impairment loss is recognized for the amount by which the asset's carrying amount exceeds its recoverable amount. The recoverable amount is the higher of the asset's fair value less costs of disposal and value-in-use.
For the purpose of impairment testing, assets are grouped together into the smallest group of assets that generate cash inflows from continuing use that are largely independent of the cash flows of other assets ("cash-generating units"). Impairment losses are recognized in the consolidated statements of loss. Prior impairments of non-financial assets are reviewed for possible reversal of the impairment at each reporting date.
The principal financial instruments used by the Company are as follows:
95
These financial instruments are carried at amortized cost.
Due to their short-term nature, the carrying value of cash and cash equivalents, short-term financial assets, other current assets, excluding prepaid expenses, accrued income, trade payables, accrued expenses and other payables approximates their fair value. For details of the fair value hierarchy, valuation techniques, and significant unobservable inputs related to determining the fair value of long-term financial debt, refer to Note 21.
The carrying amount of other receivables/current assets is reduced through the use of an allowance account, and the amount of the loss is recognized in the consolidated statements of loss. Subsequent recoveries of amounts previously written off are credited to the consolidated statements of loss.
Grant income reflects reimbursement of research and development expenses and income from certain research projects managed by Icelandic governmental institutions. Certain expenses qualify for incentives from the Icelandic government in the form of tax credits or cash reimbursements.
Short-term financial assets consist of fixed term bank deposits with maturities between three and six months. Short-term financial assets are held in order to collect contractual cash flows made of payments of principal and interests.
Cash and cash equivalents include cash on hand and highly liquid investments with original maturities of three months or less. These investments are readily convertible to known amounts of cash.
Trade payables are amounts due to third parties in the ordinary course of business. Trade payables are non-interest bearing and are normally settled on 45-day terms.
Accrued expenses and other payables are amounts provided for / due to third parties in the ordinary course of business. Accrued expenses and other payables are non-interest bearing.
Lease liabilities are measured at the present value of the expected contractual payments due to the lessor over the lease term, with the discount rate determined by reference to the rate inherent in the lease unless this is not readily determinable, in which case the Group’s incremental borrowing rate on commencement date of the lease is used.
Long-term financial debt exclusively results from the issuance of preferred shares that qualify as financial liabilities under IAS 32. Long-term financial debt is carried at amortized cost, plus the accrued interest/preferred dividend payments that are due by the Group under certain conditions. Refer to Note 15 for further information.
96
The Company operates a defined benefit pension plan for its Swiss-based employees, which is held in a multi-employer fund. The pension plan is funded by payments from employees and from the Company. The Company’s contributions to the defined contribution plans are charged to the consolidated statements of loss in the year to which they relate.
The liability recognized in the balance sheet in respect of defined benefit pension plans is the present value of the defined benefit obligation at the balance sheet date less the fair value of plan assets and the possible effect of the asset ceiling, together with adjustments for unrecognized past-service costs. The defined benefit obligation is calculated annually by independent actuaries using the projected unit credit method.
When the Company has a surplus in the defined benefit pension plans, it measures the net defined benefit asset at the lower of:
The Company does not expect any refunds or contribution reductions in case of a surplus in the defined benefit pension plan calculated per IAS 19, therefore no assets would be recognized in the Consolidated Statements of Financial Position.
The present value of the defined benefit obligation is determined by discounting the estimated future cash outflows using interest rates of high-quality corporate bonds that are denominated in the currency in which the benefits will be paid, and that have terms to maturity approximating to the terms of the related pension liability.
Actuarial gains and losses arising from experience adjustments and changes in actuarial assumptions are charged or credited to equity in other comprehensive income in the period in which they arise.
Past-service costs are recognized immediately in income, unless the changes to the pension plan are conditional on the employees remaining in service for a specified period of time (the vesting period). In this case, the past-service costs are amortized on a straight-line basis over the vesting period.
The Company operates an equity-settled, share-based compensation plan, under which the entity receives services from employees as consideration for equity instruments (e.g. options) of the Company. The fair value of the awards granted in exchange of the employee services received is recognized as an expense.
Non-market vesting conditions are included in assumptions about the number of options that are expected to vest. The total expense is recognized over the vesting period, which is the period over which all of the specified vesting conditions are to be satisfied. At the end of each reporting period, the entity revises its estimates of the number of options that are expected to vest based on the non-market vesting conditions. It recognizes the impact of the revision to original estimates, if any, in the consolidated statements of loss, with a corresponding adjustment to equity.
When the options are exercised, the Company issues new shares. The proceeds received net of any directly attributable transaction costs are credited to share capital (nominal value) and share premium when the options are exercised.
The Company recognizes the earnout consideration as a share-based contingent consideration within the scope of IFRS 2, and therefore equity classified as the earnout consideration ultimately settles in ordinary shares. The Company has determined that the fair value of the earnout shares should be accounted for as a component of the deemed cost of the listing services upon consummation of the Business Combination. The fair value of total consideration transferred included in the calculation of the IFRS 2 share listing service expense will not be subsequently adjusted regardless of whether the price target is achieved or not. The earnout options granted to employees were determined to be compensation for the dilution to their previously held Legacy Oculis equity instruments.
97
No additional compensation charge is recognized under IFRS 2 because no additional fair value was granted as a result of the earnout options.
The Company capitalizes transaction costs within Other current assets in the Company’s consolidated balance sheet when costs are directly attributable to new equity financing instrument (including business combination related transactions) when it is highly probable that the financing transaction will take place in the future. If and when the Company completes the transaction, capitalized transaction costs will be offset against the proceeds and will be recorded as a reduction of share premium within the Company’s consolidated balance sheet. If the Company determines that it is not highly probable that the transaction will be completed, the Company will write-off capitalized transaction costs incurred during that respective quarter in the consolidated statement of loss.
There are no new IFRS standards, amendments or interpretations that are mandatory as of January 1, 2023 that are relevant to the Company. Additionally, the Company has not adopted any standard, interpretation or amendment that has been issued but is not yet effective. Such standards are not currently expected to have a material impact on the entity in the current or future reporting periods and on foreseeable future transactions.
The Group’s principal accounting policies are set out in Note 3 of the Group’s consolidated financial statements and conform to IFRS Accounting Standards. Significant judgments and estimates are used in the preparation of the consolidated financial statements which, to the extent that actual outcomes and results may differ from these assumptions and estimates, could affect the accounting in the areas described in this section.
The Group assesses whether there are any indicators of impairment for all licenses at each reporting date, which refers exclusively to the licenses of two specific product candidates: OCS-02 (Licaminlimab) and OCS-05. Given the stage of Oculis’ development activities and the importance of both products in Oculis’ portfolio, the impairment test is performed first on the basis of a fair value model for the entire Company using a market approach, and second on the basis of the continued development feasibility of the relevant product candidate. Refer to Note 9.
Deferred income tax assets are recognized for all unused tax losses only to the extent that it is probable that taxable profits will be available against which the losses can be utilized. Judgment is required from management to determine the amount of tax asset that can be recognized, based on forecasts and tax planning strategies. Given the uncertainty in the realization of future taxable profits, no deferred tax asset on unused tax losses has been recognized as of December 31, 2023, 2022 and 2021. Refer to Note 7 (D).
The present value of the pension obligations depends on several factors that are determined on an actuarial basis using a number of assumptions. The assumptions used in determining the net cost (income) for pensions include the discount rate. Any changes in these assumptions will impact the carrying amount of pension obligations. The independent actuary of the Group uses statistical based assumptions covering future withdrawals of participants from the plan and estimates on life expectancy. The actuarial assumptions used may differ materially from actual results due to changes in market and economic conditions, higher or lower withdrawal rates or longer or shorter life spans of participants. These differences could have a significant impact on the amount of pension income or expenses recognized in future periods.
The Group determines the appropriate discount rate at the end of each year. This is the interest rate used to determine the present value of estimated future cash outflows expected to be required to settle the pension obligations. In determining the appropriate discount rate, the Group considers the interest rates of high-quality corporate bonds that are denominated in the currency in which the benefits will be paid, and that have terms to maturity approximating the terms of the related pension liability. Other key assumptions for pension obligations are based in part on current market conditions. Refer to Note 12.
98
(D) Share-based compensation
Stock options granted are valued using the Black-Scholes option-pricing model (see Note 13). This valuation model as well as parameters used such as expected volatility and expected term of the stock options are partially based on management’s estimates. The expected volatility is estimated using historical stock volatilities of comparable peer public companies within the Company's industry. The expected term represents the period that share-based awards are expected to be outstanding. The Company classifies its share-based payments as equity-classified awards as they are settled in ordinary shares. The Company measures equity-classified awards at their grant date fair value using a Black-Scholes option pricing model and does not subsequently remeasure them. Compensation costs related to equity-classified awards are equal to the fair value of the award at grant-date amortized over the vesting period of the award using the graded method. The Company reclassifies a portion of vested awards to reserve for share-based payment as the awards vest. The proceeds received net of any directly attributable transaction costs are credited to share capital (nominal value) and share premium when the options are exercised.
In relation to the Business Combination, the following critical estimates and judgments were made:
Despite EBAC being the legal acquirer, Legacy Oculis was determined to be the accounting acquirer for financial reporting purposes. This determination is primarily based on the fact that subsequent to the Business Combination, i) the shareholders of Legacy Oculis have a majority of the voting interest in the combined company; ii) Legacy Oculis’ operations comprise all of the ongoing operations of the combined company; and iii) Legacy Oculis’ management comprise all of the senior management of the combined company.
EBAC was a Special Purpose Acquisition Company and therefore does not meet the definition of a business under IFRS 3 as it has no operations and the related BCA cannot be treated as a business combination. The Business Combination was accounted for as a continuation of Legacy Oculis financial statements with a deemed issuance of shares by the Company accompanied by a recapitalization of the Company’s equity. The excess of fair value of the shares deemed issued by the Company over EBAC’s identifiable net assets has been recorded as share-based payment expense in accordance with IFRS 2 and represents a public listing service received by the Company.
Legacy Oculis and EBAC incurred costs such as legal, accounting, auditing, printer fees and other professional fees directly related to the Business Combination (“Transaction costs”). Transaction costs directly associated with equity issuance qualify for capitalization and are accounted for as a deduction of share premium. To capture costs associated with the new equity, the Company allocated capitalizable transaction costs to the various transaction components (equity issuance and listing) at the percentages of 38.0% and 62.0% for new shares and old shares, respectively.
Business combination with European Biotech Acquisition Corp (“EBAC”)
On March 2, 2023, the Company consummated a business combination with EBAC (the “Business Combination”) pursuant to the Business Combination Agreement ("BCA") between Legacy Oculis and EBAC dated as of October 17, 2022. The Company received gross proceeds of CHF 97.6 million or $103.7 million comprising CHF 12.0 million or $12.8 million of cash held in EBAC’s trust account and CHF 85.6 million or $90.9 million from private placement ("PIPE") investments and conversion of notes issued under Convertible Loan Agreements (“CLA”) into Oculis' ordinary shares. In connection with the Business Combination, Oculis was listed on the Nasdaq Global Market with the ticker symbol "OCS" for its ordinary shares and “OCSAW” for its public warrants.
Under the terms of the BCA, EBAC formed four new legal entities (i) Oculis, (ii) Oculis Merger Sub I Company ("Merger Sub 1"), (iii) Merger Sub 2, and (iv) Oculis Operations. After two consecutive mergers between Merger Sub 1 and EBAC, and EBAC and Merger Sub 2, EBAC and Merger Sub 1 ceased to exist and Merger Sub 2 was the surviving company. During the third quarter of 2023, the Company gave effect in its financial statements to the impending dissolution of Merger Sub 2, which is expected to be completed in the second quarter of 2024.
99
As a result, the cumulative translation adjustments related to Merger Sub 2 previously reported as equity and recognized in other comprehensive income, were reclassified from equity to the Consolidated Statements of Loss for the year ended December 31, 2023. The resulting foreign exchange impact of such reclassification amounted to CHF 5.0 million for the year ended December 31, 2023.
As a result of the BCA and as of the acquisition closing date on March 2, 2023:
PIPE and CLA financing
In connection with the BCA, EBAC entered into subscription agreements with the PIPE investors for an aggregate of 7,118,891 shares of EBAC Class A ordinary shares at CHF 9.42 or $10.00 per share for aggregate gross proceeds of CHF 67.1 million or $71.2 million.
In connection with the BCA, Legacy Oculis and the investor parties thereto entered into CLAs pursuant to which the investor lenders granted Legacy Oculis a right to receive an interest free convertible loan with certain conversion rights with substantially the same terms as the PIPE investors. Following the mergers, Oculis assumed the CLAs and the lenders exercised their conversion rights in exchange for 1,967,000 ordinary shares at CHF 9.42 or $10.00 per share for aggregate gross proceeds of CHF 18.5 million or $19.7 million.
Together, the PIPE and CLA financing resulted in aggregate gross cash proceeds of CHF 85.6 million or $90.9 million to Oculis in exchange for 9,085,891 ordinary shares.
Merger and listing expense
The Business Combination is accounted for as a capital re-organization. As EBAC does not meet the definition of a business in accordance with IFRS 3 Business Combinations, the BCA is accounted for within the scope of IFRS 2 Share-based Payment.
The Business Combination is treated as the equivalent of the Company issuing shares for the net assets of EBAC as of the acquisition closing date, accompanied by a recapitalization. The net assets of EBAC are stated at historical cost, with no goodwill or other intangible assets recorded.
100
Any excess of the fair value of the Company’s shares issued considering a fair value of CHF 10.54 or $11.19 per share (price of EBAC ordinary share at the closing date) over the value of EBAC’s identifiable net assets acquired represents compensation for the service of a stock exchange listing for its shares.
This expense was incurred in the first quarter of 2023 and amounted to CHF 34.9 million, which was expensed to the statement of loss as operating expenses, “Merger and listing expense”. The expense is non-recurring in nature and represents a share-based payment made in exchange for a listing service and does not lead to any cash outflows.
|
|
Per share value |
|
|
Shares |
|
|
March 2, 2023 |
|
|||
Fair value of equity consideration issued by the Company |
|
|
|
|
|
|
|
|
|
|||
EBAC public shareholders |
|
|
10.54 |
|
|
|
12,754,784 |
|
|
|
134,435 |
|
EBAC sponsor class B |
|
|
10.54 |
|
|
|
3,188,696 |
|
|
|
33,609 |
|
EBAC sponsor class A |
|
|
10.54 |
|
|
|
455,096 |
|
|
|
4,797 |
|
Redemptions of EBAC public shareholders |
|
|
10.54 |
|
|
|
(11,431,606 |
) |
|
|
(120,489 |
) |
Sponsors shares forfeiture |
|
|
10.54 |
|
|
|
(1,596,490 |
) |
|
|
(16,827 |
) |
Total consideration transferred |
|
|
|
|
|
3,370,480 |
|
|
|
35,525 |
|
|
Less net assets of EBAC |
|
|
|
|
|
|
|
|
(662 |
) |
||
Merger and listing expense |
|
|
|
|
|
|
|
|
34,863 |
|
||
|
|
March 2, 2023 |
|
|
|
|
(in CHF thousands) |
|
|
Net assets of EBAC |
|
|
|
|
Cash and cash equivalents |
|
|
11,547 |
|
Public & private warrants |
|
|
(2,136 |
) |
Deferred underwriting fee |
|
|
(3,108 |
) |
Accrued transaction costs |
|
|
(4,400 |
) |
Others |
|
|
(1,241 |
) |
Net assets of EBAC |
|
|
662 |
|
Capitalization
The following summarizes the actual ordinary shares issued and outstanding and the ownership interests of Oculis immediately after the Business Combination:
|
|
Shares |
|
|
% |
|
||
Issuance of ordinary shares to Legacy Oculis shareholders in connection with BCA (1) |
|
|
20,277,002 |
|
|
|
61.9 |
% |
Issuance of ordinary shares in connection with closing of the PIPE financing |
|
|
7,118,891 |
|
|
|
21.7 |
% |
Issuance of ordinary shares under CLA |
|
|
1,967,000 |
|
|
|
6.0 |
% |
Ordinary shares owned by sponsors |
|
|
2,047,302 |
|
|
|
6.3 |
% |
Ordinary shares owned by EBAC public shareholders |
|
|
1,323,178 |
|
|
|
4.1 |
% |
Total (2) |
|
|
32,733,373 |
|
|
|
100.0 |
% |
101
|
|
Legacy Oculis shares |
|
|
Exchange ratios |
|
|
Oculis ordinary shares issued |
|
|||
Ordinary shares |
|
|
3,406,771 |
|
|
|
|
|
|
|
||
Treasury shares cancelled |
|
|
(100,000 |
) |
|
|
|
|
|
|
||
Ordinary shares after cancellation of treasury shares |
|
|
3,306,771 |
|
|
1.1432 |
|
|
|
3,780,399 |
|
|
Preferred shares: |
|
|
|
|
|
|
|
|
|
|||
Series A |
|
|
1,623,793 |
|
|
|
1.1432 |
|
|
|
1,856,370 |
|
Series B1 |
|
|
2,486,188 |
|
|
|
1.4154 |
|
|
|
3,518,922 |
|
Series B2 T1 |
|
|
1,675,474 |
|
|
|
1.3900 |
|
|
|
2,328,872 |
|
Series B2 T2 |
|
|
426,378 |
|
|
|
1.3310 |
|
|
|
567,508 |
|
Series B2 T3 |
|
|
603,472 |
|
|
|
1.3142 |
|
|
|
793,082 |
|
Series C T1 |
|
|
5,337,777 |
|
|
|
1.2658 |
|
|
|
6,756,580 |
|
Series C T2 |
|
|
362,036 |
|
|
|
1.2205 |
|
|
|
441,854 |
|
Series C T3 |
|
|
197,745 |
|
|
|
1.1804 |
|
|
|
233,415 |
|
Total preferred shares |
|
|
12,712,863 |
|
|
|
1.2976 |
|
|
|
16,496,603 |
|
|
|
|
|
|
|
|
|
|
|
|||
Total |
|
|
16,019,634 |
|
|
|
|
|
|
20,277,002 |
|
|
Earnout consideration
As a result of the BCA, Legacy Oculis preferred, ordinary and option holders (collectively “equity holders”) received consideration in the form of 3,793,995 earnout shares and 369,737 earnout options with an exercise price of CHF 0.01.
The earnout consideration is subject to forfeiture in the event of a failure to achieve the price targets during the earnout period defined as follows: (i) 1,500,000, (ii) 1,500,000 and (iii) 1,000,000 earned based on the achievement of post-acquisition closing share price targets of Oculis of $15.00, $20.00 and $25.00, respectively, in each case, for any 20 trading days within any consecutive 30 trading day period commencing after the acquisition closing date and ending on or prior to March 2, 2028 (the “Earnout period”). A given share price target described above will also be deemed to be achieved if there is a change of control, as defined in the BCA, transaction of Oculis during the earnout period.
Public offering of ordinary shares
On May 31, 2023, the Company entered into an underwriting agreement with BofA Securities Inc. and SVB Securities, LLC, as representatives of several underwriters, and on June 5, 2023, closed the issuance and sale in a public offering of 3,500,000 ordinary shares at a public offering price of CHF 10.45 or $11.50 per share, for total gross proceeds of CHF 36.6 million or $40.3 million before deducting underwriting discounts, commissions and offering expenses.
In addition, the Company granted the underwriters an option to purchase additional ordinary shares which was partially exercised on June 13, 2023, leading to an additional purchase of 154,234 ordinary shares and gross proceeds of CHF 1.6 million or $1.7 million before deducting underwriting discounts, commissions and offering expenses. After giving issuance to these additional shares, Oculis sold a total of 3,654,234 ordinary shares in the offering for aggregate gross proceeds of CHF 38.2 million or $42.0 million, before deducting underwriting discounts, commissions and offering expenses. All of the underwriters' unexercised options to purchase additional shares expired on June 30, 2023.
The Company intends to use the net proceeds from this offering, together with its existing resources, to advance its development programs in particular Diabetic Macular Edema and for other ophthalmic indications, and for working capital and general corporate purposes.
102
The Company is managed and operated as one business. A single management team that reports to the Chief Executive Officer comprehensively manages the entire business and accordingly, the Company has one reportable segment.
The table below provides the carrying amount of certain non-current assets, by geographic area:
in CHF thousands |
|
Switzerland |
|
Iceland |
|
Others |
|
Total |
||||||||
|
|
As of December 31, 2023 |
|
As of December 31, 2022 |
|
As of December 31, 2023 |
|
As of December 31, 2022 |
|
As of December 31, 2023 |
|
As of December 31, 2022 |
|
As of December 31, 2023 |
|
As of December 31, 2022 |
Intangible assets |
|
12,206 |
|
12,206 |
|
- |
|
- |
|
- |
|
- |
|
12,206 |
|
12,206 |
Property and equipment, net |
|
17 |
|
24 |
|
253 |
|
338 |
|
18 |
|
3 |
|
288 |
|
365 |
Right-of-use assets |
|
- |
|
- |
|
687 |
|
758 |
|
68 |
|
- |
|
755 |
|
758 |
Total |
|
12,223 |
|
12,230 |
|
940 |
|
1,096 |
|
86 |
|
3 |
|
13,249 |
|
13,329 |
Grant income reflects reimbursement of research and development expenses and income from certain research projects managed by Icelandic governmental institutions. Certain expenses qualify for incentives from the Icelandic government in the form of tax credits or cash reimbursements. Icelandic government grant income for the year ended December 31, 2023, is CHF 0.9 million compared to CHF 0.9 million and CHF 1.0 million for the same periods in 2022 and 2021, respectively. Refer to Note 11.
The tables below show the breakdown of the Total operating expenses by category:
in CHF thousands |
|
For the years ended December 31, |
||||||||||||||||
|
|
Research and development expenses |
|
General and administrative expenses |
|
Total operating expenses |
||||||||||||
|
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
|
2023 |
|
2022 |
|
2021 |
Personnel expense |
|
6,509 |
|
4,608 |
|
4,407 |
|
7,029 |
|
4,449 |
|
2,416 |
|
13,538 |
|
9,056 |
|
6,823 |
Payroll |
|
4,796 |
|
4,313 |
|
4,189 |
|
5,134 |
|
3,939 |
|
2,306 |
|
9,930 |
|
8,252 |
|
6,495 |
Share-based compensation expense |
|
1,713 |
|
295 |
|
218 |
|
1,895 |
|
510 |
|
110 |
|
3,608 |
|
804 |
|
328 |
Operating expenses |
|
22,738 |
|
17,616 |
|
5,161 |
|
10,458 |
|
6,615 |
|
2,208 |
|
68,059 |
|
24,231 |
|
7,369 |
External service providers |
|
22,256 |
|
17,205 |
|
4,786 |
|
7,695 |
|
2,294 |
|
1,681 |
|
29,951 |
|
19,499 |
|
6,467 |
Other operating expenses |
|
258 |
|
184 |
|
189 |
|
2,700 |
|
4,249 |
|
478 |
|
2,958 |
|
4,433 |
|
667 |
Depreciation of property and equipment |
|
106 |
|
111 |
|
78 |
|
19 |
|
20 |
|
10 |
|
125 |
|
132 |
|
88 |
Depreciation of right-of-use assets |
|
118 |
|
116 |
|
108 |
|
44 |
|
52 |
|
39 |
|
162 |
|
167 |
|
147 |
Merger and listing expense(1) |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
34,863 |
|
- |
|
- |
Total |
|
29,247 |
|
22,224 |
|
9,568 |
|
17,487 |
|
11,064 |
|
4,624 |
|
81,597 |
|
33,288 |
|
14,192 |
(1) Merger and listing expense is presented separately from research and development or general and administrative expenses on the consolidated statements of loss. The item relates to the BCA and is non-recurring in nature, representing a share-based payment made in exchange for a listing service.
in CHF thousands |
For the years ended December 31, |
|
|||||||||
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Finance income |
|
1,429 |
|
|
|
126 |
|
|
|
21 |
|
Finance expense |
|
(1,315 |
) |
|
|
(6,442 |
) |
|
|
(5,120 |
) |
Fair value adjustment on warrant liabilities |
|
(3,431 |
) |
|
|
- |
|
|
|
- |
|
Foreign currency exchange gain (loss) |
|
(4,664 |
) |
|
|
49 |
|
|
|
(193 |
) |
Finance result |
|
(7,981 |
) |
|
|
(6,267 |
) |
|
|
(5,292 |
) |
103
Finance result in 2022 and 2021 represented mainly interest expense related to the preferred dividend owed to the holders of Legacy Oculis preferred Series B and C shares (refer to Note 15). Preferred Series B and C shares qualified as liabilities under IAS 32 and the related accrued dividends as interest expense. The preferred Series B and C shares were fully converted to ordinary shares at the closing of the Business Combination on March 2, 2023 (refer to Note 5).
Finance income consists primarily of interest income earned from the Company's short-term financial assets.
Refer to Note 18 for further discussions of the fair value gain/(loss) on warrant liabilities. The foreign currency translation differences recycling is related to the Merger Sub 2 entity and its impending dissolution, discussed further in Note 5.
Financial result as presented in the statements of cash flows is comprised of interest and the foreign exchange effect on cash and financial assets, net.
|
For the years ended December 31, |
|
||||||||
in CHF thousands |
2023 |
|
|
2022 |
|
2021 |
|
|||
Current income tax expense |
|
(127 |
) |
|
|
(90 |
) |
|
(22 |
) |
Deferred tax income (expense) |
|
20 |
|
|
|
35 |
|
|
(5 |
) |
Total tax expense |
|
(107 |
) |
|
|
(55 |
) |
|
(27 |
) |
The Group's expected tax expense for each year is based on the applicable tax rate in each individual jurisdiction, which ranged between approximately 8.3% and 25.0% for 2023, 2022 and 2021 in the tax jurisdictions in which the Group operates. The weighted average tax rates applicable to the profits of the consolidated entities were 12.7%, 13.9% and 13.6% for the years 2023, 2022 and 2021, respectively. This decrease is due to changes in the mix of the taxable results and the changes in tax rates of the individual group companies. The tax on the Group's profit / (loss) before tax differs from the statutory amount that would arise using the weighted average applicable tax rate as follows:
|
For the years ended December 31, |
|
||||||||
in CHF thousands |
2023 |
|
|
2022 |
|
2021 |
|
|||
Groups average expected tax rate |
|
12.7 |
% |
|
|
13.9 |
% |
|
13.6 |
% |
Accounting loss before income tax |
|
(88,695 |
) |
|
|
(38,643 |
) |
|
(18,524 |
) |
Taxes at weighted average income tax |
|
11,294 |
|
|
|
5,380 |
|
|
2,521 |
|
Effect of unrecorded tax losses |
|
(10,520 |
) |
|
|
(4,468 |
) |
|
(1,869 |
) |
Effect of non-deductible expenses |
|
(6,041 |
) |
|
|
(968 |
) |
|
(679 |
) |
Effect of non-taxable income |
|
5,103 |
|
|
|
- |
|
|
- |
|
Effect of other items |
|
57 |
|
|
|
- |
|
|
- |
|
Total tax expense |
|
(107 |
) |
|
|
(55 |
) |
|
(27 |
) |
As of December 31, 2023, 2022 and 2021, the Group has tax losses which arose mainly in Switzerland that are available for offset against future taxable profits of the company until expiration. Deferred tax assets have not been recognized in respect of these losses in Switzerland as it is not probable that future taxable profit will be available against which the unused tax losses can be utilized. Given the amount of tax losses has not been yet validated by the Tax Authorities, there could be potentially still be subject to material adjustments.
This does not affect the management assumption on the going concern hypothesis of the Group. Below is the maturity of the Group reportable losses:
|
As of December 31, |
|
|||||
in CHF thousands |
2023 |
|
|
2022 |
|
||
2025 |
|
16,733 |
|
|
|
16,733 |
|
2026 |
|
13,113 |
|
|
|
13,113 |
|
2027 |
|
12,437 |
|
|
|
12,437 |
|
2028 |
|
14,865 |
|
|
|
14,865 |
|
2029 |
|
31,786 |
|
|
|
31,790 |
|
2030 |
|
81,509 |
|
|
|
- |
|
Total |
|
170,443 |
|
|
|
88,938 |
|
104
The Group did not recognize the following temporary differences:
|
As of December 31, |
|
|||||
in CHF thousands |
2023 |
|
|
2022 |
|
||
Pension |
|
728 |
|
|
|
91 |
|
Tax losses in Switzerland |
|
170,443 |
|
|
|
88,938 |
|
Leasing |
|
(150 |
) |
|
|
(125 |
) |
Intangible asset |
|
(4,025 |
) |
|
|
(4,025 |
) |
Total |
|
166,996 |
|
|
|
84,879 |
|
As of December 31, 2023 and 2022 the Company had recognized deferred tax assets of CHF 44 thousand and CHF 24 thousand, respectively, and no deferred tax liabilities.
The following tables present the movements in the book values of property and equipment, net:
in CHF thousands |
Lab - equipment |
|
|
Lab - fixtures and fittings |
|
|
Office equipment & hardware |
|
|
Total |
|
||||
Acquisition cost: |
|
|
|
|
|
|
|
|
|
|
|
||||
Balance as of December 31, 2021 |
|
555 |
|
|
|
195 |
|
|
|
101 |
|
|
|
851 |
|
Acquisitions |
|
45 |
|
|
|
- |
|
|
|
20 |
|
|
|
65 |
|
Balance as of December 31, 2022 |
|
600 |
|
|
|
195 |
|
|
|
121 |
|
|
|
916 |
|
Acquisitions |
|
18 |
|
|
|
- |
|
|
|
30 |
|
|
|
48 |
|
Balance as of December 31, 2023 |
|
618 |
|
|
|
195 |
|
|
|
151 |
|
|
|
964 |
|
|
Lab - equipment |
|
|
Lab - fixtures and fittings |
|
|
Office equipment & IT tools |
|
|
Total |
|
||||
Accumulated depreciation: |
|
|
|
|
|
|
|
|
|
|
|
||||
Balance as of December 31, 2021 |
|
(305 |
) |
|
|
(59 |
) |
|
|
(55 |
) |
|
|
(420 |
) |
Depreciation expense |
|
(70 |
) |
|
|
(28 |
) |
|
|
(34 |
) |
|
|
(132 |
) |
Balance as of December 31, 2022 |
|
(375 |
) |
|
|
(87 |
) |
|
|
(89 |
) |
|
|
(551 |
) |
Depreciation expense |
|
(89 |
) |
|
|
(19 |
) |
|
|
(17 |
) |
|
|
(125 |
) |
Balance as of December 31, 2023 |
|
(464 |
) |
|
|
(106 |
) |
|
|
(106 |
) |
|
|
(676 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Carrying amount: |
|
|
|
|
|
|
|
|
|
|
|
||||
Balance as of December 31, 2022 |
|
225 |
|
|
|
108 |
|
|
|
32 |
|
|
|
365 |
|
Balance as of December 31, 2023 |
|
154 |
|
|
|
89 |
|
|
|
45 |
|
|
|
288 |
|
The following tables summarize the movement of intangibles assets:
in CHF thousands |
Licenses |
|
|
Total |
|
||
Acquisition cost: |
|
|
|
|
|
||
Balance as of December 31, 2021 |
|
8,724 |
|
|
|
8,724 |
|
Additions |
|
3,482 |
|
|
|
3,482 |
|
Balance as of December 31, 2022 |
|
12,206 |
|
|
|
12,206 |
|
Additions |
|
- |
|
|
|
- |
|
Balance as of December 31, 2023 |
|
12,206 |
|
|
|
12,206 |
|
|
|
|
|
|
|
||
Carrying amount: |
|
|
|
|
|
||
As of December 31, 2022 |
|
12,206 |
|
|
|
12,206 |
|
As of December 31, 2023 |
|
12,206 |
|
|
|
12,206 |
|
105
Intangible assets as of December 31, 2023 and 2022 were CHF 12.2 million and represent licenses purchased under license agreements with Novartis and Accure. Intangible assets as of December 31, 2021 were CHF 8.7 million and represented licenses purchased under a license agreement with Novartis. The Novartis license agreement was dated as of December 19, 2018 between Legacy Oculis and Novartis and relates to a novel topical anti-TNFα antibody, renamed OCS-02 (Licaminlimab), for ophthalmic indications. The license agreement between Legacy Oculis and Accure, dated as of January 29, 2022, relates to the exclusive global licensing of its OCS-05 (formerly ACT-01) technology. This license agreement contained an upfront payment of CHF 3.0 million and reimbursement of development related costs of CHF 0.5 million. The Company intends to advance the development of OCS-05 with a focus on multiple ophthalmology neuroprotective applications.
The products candidates related to the capitalized intangible assets are not yet available for use. The amortization of the licenses will start when the market approval is obtained.
Oculis performs an assessment of its licenses in the context of its annual impairment test. Given the stage of Oculis’ development activities and the importance of the relevant product candidates, OCS-02 (Licaminlimab) and OCS-05, in Oculis’ portfolio, the impairment test is performed first on the basis of a fair value model for the entire Company using a market approach and second on the basis of the continued development feasibility of both candidates.
Oculis performs its annual impairment tests on its entire portfolio of research and development assets, by deriving the fair value from an observable valuation for the entire Company determined via its stock market price quoted in Nasdaq as per the reporting date. The fair value of the asset portfolio is derived by deducting the carrying value of tangible assets and the remaining assets, which consist primarily of short-term financial assets and cash and cash equivalents, from the Company valuation.
OCS-02 and OCS-05, are additionally tested for impairment by assessing their probability of success. Assessments include reviews of the following indicators, and if the candidate fails any of these indicators the entire balance is written off:
In 2023, 2022 and 2021, reviews of all these indicators for OCS-02 and OCS-05 (in 2023 and 2022) was positive. No impairment losses were recognized in 2023, 2022 and 2021.
The following table presents the right-of-use assets, which are related to our Iceland and U.S. facilities:
in CHF thousands |
Right-of-use assets |
|
||||
|
2023 |
|
2022 |
|
||
Balance as of January 1, |
|
758 |
|
|
855 |
|
Indexation for the period |
|
47 |
|
|
70 |
|
New lease |
|
118 |
|
|
- |
|
FX revaluation |
|
(6 |
) |
|
- |
|
Depreciation charge for the period |
|
(162 |
) |
|
(167 |
) |
Balance as of December 31, |
|
755 |
|
|
758 |
|
106
There are no variable lease payments which are not included in the measurement of lease obligations. Expected extension options have been included in the measurement of lease liabilities.
The following table presents the lease obligations:
in CHF thousands |
Lease liabilities |
|
||||
|
2023 |
|
2022 |
|
||
Balance as of January 1, |
|
(633 |
) |
|
(770 |
) |
New lease |
|
(118 |
) |
|
- |
|
FX revaluation |
|
35 |
|
|
48 |
|
Indexation for the period |
|
(47 |
) |
|
(70 |
) |
Interest expense for the period |
|
(42 |
) |
|
(45 |
) |
Lease payments for the period |
|
200 |
|
|
204 |
|
Balance as of December 31, |
|
(605 |
) |
|
(633 |
) |
in CHF thousands |
As of December 31, 2023 |
|
As of December 31, 2022 |
|
||
Current |
|
(174 |
) |
|
(142 |
) |
Non-current |
|
(431 |
) |
|
(491 |
) |
Total |
|
(605 |
) |
|
(633 |
) |
The table below shows the breakdown of the Other current assets by category:
in CHF thousands |
|
As of December 31, 2023 |
|
|
As of December 31, 2022 |
|
||
Prepaid clinical and technical development expenses |
|
|
6,748 |
|
|
|
1,586 |
|
Prepaid general and administrative expenses |
|
|
1,412 |
|
|
|
1,208 |
|
VAT receivable |
|
|
328 |
|
|
|
165 |
|
Total |
|
|
8,488 |
|
|
|
2,959 |
|
The increase in prepaid clinical and technical development expenses as of December 31, 2023 compared to prior year relates to the commencement of significant clinical trials in the fourth quarter of 2023.
The table below shows the movement of the accrued income for the years ended December 31, 2023 and 2022:
in CHF thousands |
|
2023 |
|
|
2022 |
|
||
Balance as of January 1, |
|
|
912 |
|
|
|
760 |
|
Accrued income recognized during the period |
|
|
883 |
|
|
|
912 |
|
Payments received during the period |
|
|
(915 |
) |
|
|
(726 |
) |
Foreign exchange revaluation |
|
|
(4 |
) |
|
|
(34 |
) |
Balance as of December 31, |
|
|
876 |
|
|
|
912 |
|
Accrued income is generated by incentives for research and development offered by the Icelandic government in the form of tax credits for innovation companies. The aid in Iceland is granted as a reimbursement of paid income tax or paid out in cash when the tax credit is higher than the calculated income tax. The tax credit is subject to companies having a research project approved as eligible for tax credit by the Icelandic Centre for Research (Rannís).
The Company’s Swiss pension plan is classified as a defined benefit plan under IFRS Accounting Standards. Employees of the Icelandic, French, Hong Kong and American subsidiaries are covered by local post-retirement defined contribution plans.
Pension costs are charged to the consolidated statements of loss when incurred. Iceland pension expenses of CHF 0.1 million were recorded in 2023, 2022 and 2021.
107
Pension costs are charged to the consolidated statements of loss when incurred. In 2023, pension costs amounted to CHF 41 thousand, CHF 42 thousand in 2022 and CHF 47 thousand in 2021.
The U.S. entity adopted a 401(k) defined contribution plan effective December 1, 2020. Accrued employer contribution was CHF 48 thousand for 2023. There were no employer contributions made in 2022 and 2021.
Pension costs are charged to the consolidated statements of loss when incurred. CHF 3 thousand and CHF 4 thousand were recorded related to Hong Kong pension expenses in 2023 and 2022. The subsidiary in Hong Kong did not employ any personnel in 2021. Consequently, there was no pension expense in 2021.
The Company’s Swiss entity is affiliated to a collective foundation administrating the pension plans of various unrelated employers that qualifies as defined benefit plan under IAS 19. For employees in Switzerland, the pension fund provides post-employment, death-in-service and disability benefits in accordance with the Swiss Federal Law on Occupational Retirement, Survivor’s and Disability Pension Plans which specifies the minimum benefits that are to be provided.
The pension plan of the Company’s Swiss entity is fully segregated from the ones of other participating employers. The collective foundation has reinsured all risks with an insurance company. The most senior governing body of the collective foundation is the Board of Trustees. All governing and administration bodies have an obligation to act in the interests of the plan beneficiaries.
The retirement benefits are based on the accumulated retirement capital, which is made of the yearly contributions towards the old age risk by both employer and employee and the interest thereon until retirement. The employee contributions are determined based on the insured salary, depending on the age, staff level and saving amount of the beneficiary. The interest rate is determined annually by the governing body of the collective plan in accordance with the legal framework, which defines the minimum interest rates.
If an employee leaves the pension plan before reaching retirement age, the law provides for the transfer of the vested benefits to a new pension plan. These vested benefits comprise the employee and the employer contributions plus interest, the money originally brought into the pension plan by the beneficiary and an additional legally stipulated amount. On reaching retirement age, the plan beneficiary may decide whether to withdraw the benefits in the form of an annuity or (entirely or partly) as a lump-sum payment. The annuity is calculated by multiplying the balance of the retirement capital with the applicable conversion rate.
All actuarial risks of the plan, e.g. old age, invalidity and death-in-service or investment, are fully covered by insurance. However, the collective foundation is able to withdraw from the contract with the Company at any time, in which case the Company would be required to join another pension plan. In addition, the risk premiums may be adjusted by the insurance company periodically.
The Company's Swiss pension plan is fully reinsured with Swiss Life (“Swiss Life Business Protect”), therefore the plan assets are 100% covered by an insurance contract. The insurance company bearing the investment risk is also making these investments on behalf of the collective foundation. As a result, the assets of the plan consist of a receivable from the insurance police.
The assets are invested by the pension plan, to which many companies contribute, in a diversified portfolio that respects the requirements of the Swiss Law. The insurance policy has been treated as a qualifying insurance policy and therefore the pension assets are presented as one asset and are not desegregated and presented in classes that distinguish the nature and risks of those assets.
108
The following tables summarize the components of net benefit expense recognized in the consolidated statements of loss, amounts recognized in the balance sheet and gains/(losses) recognized in other comprehensive loss.
in CHF thousands |
|
|
|
|
|
|
|
For the years ended December 31, |
|
|||||
Actuarial gains / (losses) recognized in other comprehensive loss: |
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
On plan assets |
|
|
|
|
|
|
|
|
(70 |
) |
|
|
26 |
|
On obligation |
|
|
|
|
|
|
|
|
(738 |
) |
|
|
718 |
|
Total |
|
|
|
|
|
|
|
|
(808 |
) |
|
|
744 |
|
in CHF thousands |
|
|
|
|
|
|
|
For the years ended December 31, |
|
|||||
Net benefit expense (recognized in personnel costs): |
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
Current service cost |
|
|
|
|
|
|
|
|
(391 |
) |
|
|
(446 |
) |
Interest cost on benefit obligation |
|
|
|
|
|
|
|
|
(149 |
) |
|
|
(31 |
) |
Interest income |
|
|
|
|
|
|
|
|
147 |
|
|
|
26 |
|
Impact of plan changes |
|
|
|
|
|
|
|
|
- |
|
|
|
37 |
|
Administration cost |
|
|
|
|
|
|
|
|
(7 |
) |
|
|
(6 |
) |
Net benefit income / (expense) |
|
|
|
|
|
|
|
|
(400 |
) |
|
|
(420 |
) |
in CHF thousands |
|
|
|
|
|
|
|
As of December 31, |
|
|||||
Benefit asset / (liability) |
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
Defined benefit obligation |
|
|
|
|
|
|
|
|
(9,930 |
) |
|
|
(6,494 |
) |
Fair value of plan assets |
|
|
|
|
|
|
|
|
9,202 |
|
|
|
6,403 |
|
Net benefit asset / (liability) |
|
|
|
|
|
|
|
|
(728 |
) |
|
|
(91 |
) |
The impact of plan changes relates mainly to the changes of applicable rates for converting mandatory savings when employees retire (see also below).
Changes in the present value of the defined benefit obligation are as follows:
|
|
|
|
|
|
|
|
As of December 31, |
|
|||||
in CHF thousands |
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
Defined benefit obligation at January 1, |
|
|
|
|
|
|
|
|
(6,494 |
) |
|
|
(5,666 |
) |
Interest cost |
|
|
|
|
|
|
|
|
(149 |
) |
|
|
(31 |
) |
Current service cost |
|
|
|
|
|
|
|
|
(391 |
) |
|
|
(446 |
) |
Administrative expenses |
|
|
|
|
|
|
|
|
(7 |
) |
|
|
(6 |
) |
Contributions paid by participants |
|
|
|
|
|
|
|
|
(3,710 |
) |
|
|
(1,686 |
) |
Employees' contributions |
|
|
|
|
|
|
|
|
(247 |
) |
|
|
(185 |
) |
Benefits paid from plan assets |
|
|
|
|
|
|
|
|
1,806 |
|
|
|
770 |
|
Impact of plan changes |
|
|
|
|
|
|
|
|
- |
|
|
|
37 |
|
Actuarial gains / (losses) |
|
|
|
|
|
|
|
|
(738 |
) |
|
|
718 |
|
Defined benefit obligation at December 31, |
|
|
|
|
|
|
|
|
(9,930 |
) |
|
|
(6,494 |
) |
Changes in the fair value of plan assets are as follows:
|
|
|
|
|
|
|
|
As of December 31, |
|
|||||
in CHF thousands |
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
Fair value of plan assets at January 1, |
|
|
|
|
|
|
|
|
6,403 |
|
|
|
4,821 |
|
Expected return |
|
|
|
|
|
|
|
|
147 |
|
|
|
26 |
|
Contributions by employer |
|
|
|
|
|
|
|
|
571 |
|
|
|
429 |
|
Employees' contributions |
|
|
|
|
|
|
|
|
247 |
|
|
|
185 |
|
Benefits paid from plan assets |
|
|
|
|
|
|
|
|
(1,806 |
) |
|
|
(770 |
) |
Contributions paid by participants |
|
|
|
|
|
|
|
|
3,710 |
|
|
|
1,686 |
|
Actuarial gains / (losses) |
|
|
|
|
|
|
|
|
(70 |
) |
|
|
26 |
|
Fair value of plan assets at December 31, |
|
|
|
|
|
|
|
|
9,202 |
|
|
|
6,403 |
|
The Group expects to contribute CHF 0.6 million to its defined benefit pension plan in 2024. The average duration of the plan was 14.7 years and 14.0 years as of December 31, 2023 and 2022, respectively.
109
The principal assumptions used in determining pension benefit obligations for the Group's plan are shown below:
|
|
|
|
|
|
|
|
As of December 31, |
|
|||||
|
|
|
|
|
|
|
|
|
2023 |
|
|
|
2022 |
|
Discount rate |
|
|
|
|
|
|
|
|
1.45 |
% |
|
|
2.30 |
% |
Future salary increases |
|
|
|
|
|
|
|
|
1.20 |
% |
|
|
1.20 |
% |
Future pensions increases |
|
|
|
|
|
|
|
|
0.00 |
% |
|
|
0.00 |
% |
Retirement age |
|
|
|
|
|
|
|
M65/F64 |
|
|
M65/F64 |
|
||
Demographic assumptions |
|
|
|
|
|
|
|
BVG 2020 GT |
|
|
BVG 2020 GT |
|
||
In regard to the underlying estimates for the calculation of the defined benefit pension liabilities the Company updated, among other minor updates, the discount rate assumption to 1.45%, 2.30% and 0.35% as of December 31, 2023, 2022 and 2021, respectively. All the actuarial assumption changes resulted in an actuarial loss of defined benefit pension liabilities of CHF 0.8 million. The net result is an increase of defined benefit pension liabilities of CHF 0.1 million as of December 31, 2022 to CHF 0.7 million as of December 31, 2023. Other assumptions for defined benefit pension liabilities remain unchanged.
In 2023, the guaranteed interest to be credited to employees' savings was 1.00% (same as in 2022) for mandatory retirement savings, and 0.25% for supplementary retirement savings. Given current Swiss interest environment, the Company updated the estimated interest to be credited to employees’ savings up to 1.45%. The applicable rate for converting mandatory savings at age 65 for male and 64 for female employees retiring in 2023 was 6.20% and will be reduced to 5.90% for male and female for 2024 and 5.68% for women and 5.65% for men for 2025 and further reductions are expected in subsequent years. The rate for converting supplementary savings to an annuity remains stable at 4.49% for years 2023, 2024, 2025 and subsequent years for male employees and 4.54% in 2023, 2024 and 2025 and subsequent years for female employees.
Sensitivity analysis
A quantitative sensitivity analysis for significant assumptions as of December 31, 2023 and 2022 is shown below:
in CHF thousands |
Discount rate |
|
|
Future salary |
|
|
Mortality |
|
||||||||||||
Assumptions as of December 31, 2023 |
+0.25% |
|
-0.25% |
|
|
+0.50% |
|
-0.50% |
|
|
+1 year |
|
-1 year |
|
||||||
Potential defined benefit obligation |
|
(9,582 |
) |
|
(10,317 |
) |
|
|
(9,980 |
) |
|
(9,880 |
) |
|
|
(10,039 |
) |
|
(9,811 |
) |
Decrease/(increase) from actual defined benefit obligation |
|
348 |
|
|
(387 |
) |
|
|
(50 |
) |
|
50 |
|
|
|
(109 |
) |
|
119 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Assumptions as of December 31, 2022 |
+0.25% |
|
-0.25% |
|
|
+0.50% |
|
-0.50% |
|
|
+1 year |
|
-1 year |
|
||||||
Potential defined benefit obligation |
|
(6,274 |
) |
|
(6,741 |
) |
|
|
(6,527 |
) |
|
(6,462 |
) |
|
|
(6,553 |
) |
|
(6,429 |
) |
Decrease/(increase) from actual defined benefit obligation |
|
221 |
|
|
(247 |
) |
|
|
(32 |
) |
|
32 |
|
|
|
(58 |
) |
|
65 |
|
The sensitivity analysis above is subject to limitations and has been determined based on a method that extrapolates the impact on net defined benefit obligation as a result of reasonable changes in key assumptions occurring at the end of the reporting period.
On March 2, 2023, the Company adopted a new Stock Option and Incentive Plan Regulation 2023 ("2023 Plan") which allows for the grant of equity incentives, including share-based options, stock appreciation rights ("SARs"), restricted shares and other awards. The 2023 Plan lays out the details for the equity incentives for talent acquisition and retention purposes.
Each grant of share-based options made under the 2023 Plan entitles the grantee to acquire ordinary shares from the Company with payment of the exercise price in cash. In the case of SARs, the intention of the Company is settling in equity. For each grant of share-based options or SARs, the Company issues a grant notice, which details the terms of the options or SARs, including number of shares, exercise price, vesting conditions and expiration date. Options granted under the 2023 Plan vest over periods ranging from one to four years and expire one day before the tenth anniversary of the grant date. The specific terms of each grant are set by the Board of Directors.
The 2023 Plan reflects the revised capital structure of the Company following completion of the Business Combination. As a result, all option holders holding options under the prior Stock Option and Incentive Plan Regulation 2018 (”2018 Plan”) prior to the close of the Business Combination exchanged their options held in Legacy Oculis for newly issued options to purchase ordinary shares of Oculis (“Converted Options”) and additional earnout options.
110
The Converted Options continue to be subject to substantially the same terms and conditions except that the number of ordinary shares of Oculis issuable and related exercise prices were adjusted by the Exchange Ratio with all other terms remaining unchanged. The comparative fair value calculation of options using the Black-Scholes model before and after the merger concluded there was no significant change in value. The exchange of equity awards under the 2018 Plan for equity awards under the 2023 Plan was determined to be a modification in accordance with IFRS 2 – Share-based payment. The Group will continue to record the related expense per the original valuation and vesting period without incremental charges.
Option awards and SARs
Each share-based option or SAR granted under the 2023 Plan entitles the grantee to acquire common shares from the Company with cash payment of the exercise price. For each grant of share-based options or SARs, the Company provides a grant notice which details the terms of the option, including exercise price, vesting conditions and expiration date. The terms of each grant are set by the Board of Directors.
The fair value of option awards and SARs is determined using the Black-Scholes option-pricing model. The weighted average grant date fair value of options and SARs granted during the year ended December 31, 2023 was CHF 5.24 or $5.83 per share. The weighted average grant date fair value of options granted during the year ended December 31, 2022 was CHF 1.66 or $1.74 per share. The weighted average grant date fair value of options granted during the year ended December 31, 2021 was CHF 1.26 or $1.38 per share.
The Black-Scholes fair value of SARs was determined using assumptions that were not materially different from those used to value options. The following assumptions were used in the Black-Scholes option-pricing model for determining the fair value of options and SARs granted during the years indicated:
|
|
For the years ended December 31, |
||||
|
|
2023 |
|
2022 |
|
2021 |
Weighted average share price at the date of grant (1) |
|
USD 8.30 (CHF 7.46) |
|
USD 3.57 (CHF 3.41) |
|
USD 2.95 (CHF 2.70) |
Range of expected volatilities (%) (2) |
|
68.7-83.8 |
|
96.3 |
|
82.1 |
Expected term (years) (3) |
|
6.25 |
|
2.50 |
|
2.50 |
Range of risk-free interest rates (%) (1)(4) |
|
3.5-4.8 |
|
0.7 |
|
0.0 |
Dividend yield (%) |
|
0.0 |
|
0.0 |
|
0.0 |
(1) Following the NASDAQ listing in 2023, the equity award exercise price is now denominated in USD and the applicable risk-free interest rate has been adjusted accordingly.
(2) The expected volatility was derived from the historical stock volatilities of comparable peer public companies within the Company's industry.
(3) The expected term represents the period that share-based awards are expected to be outstanding.
(4) The risk-free interest rate in 2023 is based on the U.S. Treasury yield curve in effect at the measurement date with maturities approximately equal to the expected term. Prior to 2023, the risk-free interest rate was based on Switzerland Short-Term Government Bonds with maturities approximately equal to the expected term.
The following table summarizes the Company’s stock option and SAR activity under the 2023 Plan for the following periods:
111
|
|
Number of options (1) |
|
|
Weighted average exercise price (1) (CHF) |
|
|
Range of expiration dates |
||
Outstanding as of January 1, 2021 |
|
|
1,116,045 |
|
|
|
1.96 |
|
|
2026-2029 |
Granted |
|
|
341,794 |
|
|
|
2.36 |
|
|
2030 |
Forfeited |
|
|
(168,749 |
) |
|
|
2.09 |
|
|
2027-2028 |
Outstanding as of December 31, 2021 |
|
|
1,289,090 |
|
|
|
2.05 |
|
|
2026-2030 |
Exercisable at December 31, 2021 |
|
|
759,324 |
|
|
|
1.90 |
|
|
2026-2030 |
Outstanding as of January 1, 2022 |
|
|
1,289,090 |
|
|
|
2.05 |
|
|
2026-2030 |
Granted |
|
|
629,295 |
|
|
|
2.98 |
|
|
2031 |
Forfeited |
|
|
(94,273 |
) |
|
|
2.35 |
|
|
2023-2030 |
Exercised |
|
|
(61,163 |
) |
|
|
1.85 |
|
|
2026-2027 |
Outstanding as of December 31, 2022 |
|
|
1,762,949 |
|
|
|
2.39 |
|
|
2027-2031 |
Exercisable at December 31, 2022 |
|
|
819,603 |
|
|
|
1.97 |
|
|
2027-2031 |
Outstanding as of January 1, 2023 |
|
|
1,762,949 |
|
|
|
2.39 |
|
|
2027-2031 |
Options granted(2) |
|
|
1,614,000 |
|
|
|
7.49 |
|
|
2033 |
SARs granted |
|
|
134,765 |
|
|
|
7.27 |
|
|
2033 |
Earnout options granted |
|
|
369,737 |
|
|
|
0.01 |
|
|
2028 |
Forfeited(2)(3) |
|
|
(302,299 |
) |
|
|
2.62 |
|
|
2033 |
Exercised(3) |
|
|
(112,942 |
) |
|
|
2.43 |
|
|
2028-2032 |
Outstanding as of December 31, 2023 |
|
|
3,466,210 |
|
|
|
4.50 |
|
|
2027-2033 |
Exercisable at December 31, 2023 |
|
|
1,164,513 |
|
|
|
2.21 |
|
|
2028-2033 |
(1) Retroactive application of the recapitalization effect due to the BCA for activity prior to March 2, 2023, the Exchange Ratio was applied to the number of options and the weighted average exercise price was divided by the same exchange ratio.
(2) Pursuant to the BCA, all outstanding and unexercised options to purchase Legacy Oculis ordinary shares were assumed by Oculis and each option was replaced by an option to purchase ordinary shares of Oculis (the “Converted Options”). The exchange of Legacy Oculis 2018 Plan options for converted 2023 Plan options is not reflected in the table above. Refer to Note 5 - Business Combination and Financing Activities for further details.
(3) Forfeited amount includes earnout options forfeited during the year ended December 31, 2023. No SARs have been exercised or forfeited during the year ended December 31, 2023.
Excluding earnout options, which have an exercise price of CHF 0.01, options outstanding as of December 31, 2023 have exercise prices ranging from CHF 1.84 to CHF 11.66. The weighted average remaining contractual life of options and SARs outstanding as of December 31, 2023 was eight years. The weighted average contractual life of options outstanding as of December 31, 2022 was seven years.
Restricted Stock Awards
Each restricted stock award granted under the 2018 Plan was immediately exercised and the expense was recorded at grant date in full. The Company is holding call options to repurchase shares diminishing ratably on a monthly basis over three years from grant date. For each restricted stock award granted, the Company issues a grant notice, which details the terms of the grant, including the number of awards, repurchase right start date and expiration date. The terms of each grant are set by the Board of Directors. Restricted shares are granted and expensed at fair value. No restricted stock awards were granted under the 2023 Plan during the years ended December 31, 2023 and 2022.
The number and weighted average exercise prices of restricted stock awards outstanding under the 2023 Plan are as follows (recast after applying the Exchange Ratio to reflect the impact of the BCA):
|
|
Number of Restricted Stock Awards |
|
|
Weighted Average Exercise Price (CHF) |
|
||
Issued and exercised as of January 1, 2021 |
|
|
745,512 |
|
|
|
1.58 |
|
Granted and exercised during 2021 |
|
|
441,419 |
|
|
|
1.99 |
|
Issued and exercised as of December 31, 2021 |
|
|
1,186,931 |
|
|
|
1.73 |
|
Not subject to repurchase at December 31, 2021 |
|
|
710,338 |
|
|
|
1.59 |
|
Issued and exercised as of January 1, 2022 |
|
|
1,186,931 |
|
|
|
1.73 |
|
Issued and exercised as of December 31, 2022 |
|
|
1,186,931 |
|
|
|
1.73 |
|
Not subject to repurchase at December 31, 2022 |
|
|
934,044 |
|
|
|
1.66 |
|
Issued and exercised as of January 1, 2023 |
|
|
1,186,931 |
|
|
|
1.73 |
|
Issued and exercised as of December 31, 2023 |
|
|
1,186,931 |
|
|
|
1.73 |
|
Not subject to repurchase at December 31, 2023 |
|
|
1,088,838 |
|
|
|
1.71 |
|
Share-based compensation expenses
112
The total expense recognized in the consolidated statements of loss for share options granted amounted to CHF 3.6 million for the year ended December 31, 2023, CHF 0.8 million for the year ended December 31, 2022, and CHF 0.3 million for the year ended December 31, 2021. No expense was recognized during the years ended December 31, 2023 or 2022 related to restricted stock awards. For the year ended December 31, 2021 the Company recognized CHF 1.0 million of expense related to restricted stock awards. The reserve for share-based payment increased from CHF 2.0 million as of December 31, 2021 to CHF 2.8 million as of December 31, 2022, and to CHF 6.4 million as of December 31, 2023.
Cash and cash equivalents consist primarily of cash balances held at banks and in the currencies:
in CHF thousands |
|
Cash and cash equivalents |
|
|
Short-term financial assets |
|
||||||||||
by currency |
|
As of December 31, 2023 |
|
|
As of |
|
|
As of December 31, 2023 |
|
|
As of |
|
||||
Swiss Franc |
|
|
19,144 |
|
|
|
7,216 |
|
|
|
33,532 |
|
|
|
- |
|
US Dollar |
|
|
16,610 |
|
|
|
9,741 |
|
|
|
15,148 |
|
|
|
- |
|
Euro |
|
|
2,020 |
|
|
|
2,350 |
|
|
|
4,644 |
|
|
|
- |
|
Iceland Krona |
|
|
542 |
|
|
|
383 |
|
|
|
- |
|
|
|
- |
|
Other |
|
|
11 |
|
|
|
96 |
|
|
|
- |
|
|
|
- |
|
Total |
|
|
38,327 |
|
|
|
19,786 |
|
|
|
53,324 |
|
|
|
- |
|
Short-term financial assets consist of fixed term bank deposits with maturities between three and six months.
As of December 31, 2022, Legacy Oculis had 12,712,863 preferred shares for a nominal amount of CHF 1.4 million. These shares were divided into 1,623,793 registered "A Series" shares of CHF 0.10 each, 5,191,512 registered "B Series" of CHF 0.10 each, 5,699,813 registered "C1a Series" shares (denominated in USD) of CHF 0.10 each and 197,745 registered "C1b Series" shares (denominated in USD) of CHF 0.50 each.
All preferred shares had a liquidation preference corresponding to their respective initial purchase price. Furthermore, the "B Series" and "C Series" shares included a preferred dividend payment of 6.0% (as a compounded interest) and the corresponding deemed interest expense of CHF 1.3 million was accrued for the period from January 1 to March 2, 2023. The cumulative interest expense accrued up to December 31, 2022 amounted to CHF 17.0 million. The nominal amounts (for "A, B and C Series") and the accrued preferred dividend resulted in a long-term debt of CHF 124.8 million as of March 2, 2023.
On March 2, 2023, at closing of the Business Combination, all preferred shares of Legacy Oculis were converted into ordinary shares of Oculis at the effective exchange ratios determined in accordance with the BCA and giving effect to the accumulated preferred dividends (refer to Note 5). The movement of the long-term financial debt is shown below:
in CHF thousands |
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
Series A shares |
|
|
Series B shares |
|
|
Series C shares |
|
|
Total |
|
||||
Balance as of December 31, 2021 |
|
|
8,179 |
|
|
|
48,569 |
|
|
|
56,754 |
|
|
|
113,502 |
|
Issuance of shares |
|
|
- |
|
|
|
- |
|
|
|
2,030 |
|
|
|
2,030 |
|
Transaction costs |
|
|
- |
|
|
|
- |
|
|
|
(54 |
) |
|
|
(54 |
) |
Interest |
|
|
- |
|
|
|
2,797 |
|
|
|
3,546 |
|
|
|
6,343 |
|
FX revaluation |
|
|
- |
|
|
|
- |
|
|
|
628 |
|
|
|
628 |
|
Balance as of December 31, 2022 |
|
|
8,179 |
|
|
|
51,366 |
|
|
|
62,904 |
|
|
|
122,449 |
|
Interest |
|
|
- |
|
|
|
519 |
|
|
|
747 |
|
|
|
1,266 |
|
FX revaluation |
|
|
- |
|
|
|
- |
|
|
|
1,087 |
|
|
|
1,087 |
|
Conversion of Legacy Oculis preferred shares into Oculis ordinary shares |
|
|
(8,179 |
) |
|
|
(51,885 |
) |
|
|
(64,738 |
) |
|
|
(124,802 |
) |
Balance as of December 31, 2023 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
113
As a result of the Business Combination, the Company has retroactively restated the number of shares as of December 31, 2020, 2021 and 2022 to give effect to the Exchange Ratio under the BCA as explained in Note 5:
|
|
Number of shares |
|
|
In CHF thousands |
|
|||||||||||||||||||||||
|
|
Legacy Oculis ordinary shares |
|
|
Legacy Oculis restricted share awards |
|
|
Legacy Oculis treasury shares |
|
|
|
Oculis ordinary shares (1) |
|
|
Share capital (2) |
|
|
Treasury shares (2) |
|
|
Share premium |
|
|||||||
Balance as of December 31, 2020 (effect of the recapitalization) |
|
|
2,646,629 |
|
|
|
745,511 |
|
|
|
(114,323 |
) |
|
|
|
- |
|
|
|
34 |
|
|
|
(1 |
) |
|
|
9,773 |
|
Restricted shares awards |
|
|
- |
|
|
|
441,419 |
|
|
|
- |
|
|
|
|
- |
|
|
|
4 |
|
|
|
- |
|
|
|
872 |
|
Transaction costs |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(12 |
) |
Balance as of December 31, 2021 (effect of the recapitalization) |
|
|
2,646,629 |
|
|
|
1,186,930 |
|
|
|
(114,323 |
) |
|
|
|
- |
|
|
|
38 |
|
|
|
(1 |
) |
|
|
10,632 |
|
Stock option exercised |
|
|
61,163 |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
1 |
|
|
|
- |
|
|
|
119 |
|
Transaction costs |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(9 |
) |
Balance as of December 31, 2022 (effect of the recapitalization) |
|
|
2,707,792 |
|
|
|
1,186,930 |
|
|
|
(114,323 |
) |
|
|
|
- |
|
|
|
39 |
|
|
|
(1 |
) |
|
|
10,742 |
|
Reorganization following Business Combination |
|
|
(2,707,792 |
) |
|
|
(1,186,930 |
) |
|
|
114,323 |
|
|
|
|
- |
|
|
|
(39 |
) |
|
|
1 |
|
|
|
- |
|
Conversion of Legacy Oculis ordinary shares and treasury shares into Oculis ordinary shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
3,780,399 |
|
|
|
38 |
|
|
|
- |
|
|
|
- |
|
Conversion of Legacy Oculis long-term financial debt into Oculis ordinary shares |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
16,496,603 |
|
|
|
165 |
|
|
|
- |
|
|
|
124,637 |
|
Issuance of ordinary shares to PIPE investors |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
7,118,891 |
|
|
|
71 |
|
|
|
- |
|
|
|
66,983 |
|
Issuance of ordinary shares under CLA |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
1,967,000 |
|
|
|
20 |
|
|
|
- |
|
|
|
18,348 |
|
Issuance of ordinary shares to sponsor |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
2,047,302 |
|
|
|
20 |
|
|
|
- |
|
|
|
34,843 |
|
Issuance of ordinary shares to non-redeemed shareholders |
|
|
|
|
|
|
|
|
|
|
|
|
1,323,178 |
|
|
|
13 |
|
|
|
- |
|
|
|
12,001 |
|
|||
Reorganization |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(11,352 |
) |
Transaction costs related to the business combination |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(4,821 |
) |
Proceeds from sale of shares in public offering |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
3,654,234 |
|
|
|
36 |
|
|
|
- |
|
|
|
38,143 |
|
Transaction costs related to the public offering |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
(3,361 |
) |
Stock option exercised |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
112,942 |
|
|
|
1 |
|
|
|
- |
|
|
|
273 |
|
Issuance of shares in connection with warrant exercises |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
149,156 |
|
|
|
2 |
|
|
|
- |
|
|
|
1,726 |
|
Balance as of December 31, 2023 |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
|
36,649,705 |
|
|
|
366 |
|
|
|
- |
|
|
|
288,162 |
|
(1) Fully paid-in registered shares with a par value of CHF 0.01
(2) Recasted Legacy Oculis shares through the date of the Business Combination
The conditional capital at December 31, 2023 amounted to a maximum of CHF 176,089.41 split into 17,608,941 ordinary shares, in connection with the potential future issuances of:
CHF 50,000.00 through the issuance of a maximum of 5,000,000 fully paid-up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of convertible rights and/or option rights or warrants, new bonds and similar debt instruments.
114
CHF 78,355.44 through the issuance of a maximum of 7,835,544 fully paid-up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of option rights or other equity-linked instruments granted to any employee, consultant or member of the Board of Directors of Oculis.
As of December 31, 2023, 112,942 options have been exercised and associated ordinary shares have been issued using the conditional share capital for employee benefit plans (refer to Note 13). These shares were not registered yet in the commercial register as of December 31, 2023.
CHF 44,032.94 through the issuance of a maximum of 4,403,294 fully paid up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of warrants.
As of December 31, 2023, 149,156 warrants have been exercised and associated ordinary shares have been issued using the conditional share capital for EBAC public and private warrants (refer to Note 18). These shares were not registered yet in the commercial register as of balance sheet date.
CHF 3,701.03 through the issuance of a maximum of 370,103 fully paid up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of option rights or other equity-linked instruments granted to any employee, consultant or member of the Board of Directors of Oculis.
The Group cancelled 100,000 treasury shares effective March 2, 2023 as a result of the Business Combination. No treasury shares were outstanding as of December 31, 2023.
(D) Capital band
The Company has a capital band between CHF 365,273.68 (lower limit) and CHF 543,684.52 (upper limit). The Company may effect an increase of the Company’s share capital in a maximum amount of CHF 178,410.84 by issuing up to 17,841,084 ordinary shares with a par value of CHF 0.01 each out of the Company’s capital band. The Board of Directors is authorized to increase the share capital to the upper limit or decrease the share capital to the lower limit at any time and as often as required until March 2, 2028. In Q2 2023, 3,654,234 shares were issued from this capital band.
in CHF thousands |
As of December 31, 2023 |
|
As of December 31, 2022 |
|
||
Trade payables |
|
(7,596 |
) |
|
(3,867 |
) |
Total |
|
(7,596 |
) |
|
(3,867 |
) |
The increase in trade payables as of December 31, 2023 compared to prior year relates to the commencement of several clinical trials in the fourth quarter of 2023 requiring upfront invoicing by vendors.
The table below shows the breakdown of the Accrued expenses and other payables by category:
in CHF thousands |
|
As of December 31, 2023 |
|
|
As of December 31, 2022 |
|
||
Product development related expenses |
|
|
2,801 |
|
|
|
4,805 |
|
Personnel related expenses |
|
|
2,301 |
|
|
|
2,249 |
|
General and administration related expenses |
|
|
765 |
|
|
|
957 |
|
Other payables |
|
|
81 |
|
|
|
- |
|
Total |
|
|
5,948 |
|
|
|
8,011 |
|
115
Pursuant to the BCA and the Warrant Assignment and Assumption Agreement executed in connection with the BCA, the Company has assumed 4,251,595 EBAC public warrants and 151,699 EBAC private warrants from EBAC, and issued 4,403,294 warrants as of March 2, 2023 with substantially the same terms. Each warrant entitles the registered holder to purchase one ordinary share at a price of CHF 9.68 or $11.50 per share, subject to certain adjustments, exercisable at any time commencing 30 days after the acquisition closing date, provided that the Company has an effective registration statement under the Securities Act covering the issuance of the ordinary shares issuable upon exercise of the warrants. This registration statement was filed with the SEC and declared effective on May 1, 2023. The warrants will expire on March 2, 2028.
As of March 2, 2023, the Company recognized the warrant liabilities at fair value of CHF 2.1 million. For the year ended December 31, 2023, the Company recognized a fair value loss in the Statement of Loss of CHF 3.4 million leading to an increase of the warrant liability up to CHF 5.4 million as of December 31, 2023. The exercise of 149,156 public warrants at a price of CHF 10.26 (average value of effective rate) or $11.50 per share for the year period ended December 31, 2023 resulted in a reduction of CHF 0.2 million to the liability, an additional CHF 1.5 million of cash and an increase of CHF 1.7 million in shareholder's equity (refer to Note 16).
The movement of the warrant liability is illustrated below:
in CHF thousands (except share number of warrants) |
|
Warrant liabilities |
|
|
Share number of outstanding public and private warrants |
|
||
Balance as of January 1, 2023 |
|
|
- |
|
|
|
- |
|
Issuance of assumed warrants from EBAC |
|
|
2,136 |
|
|
|
4,403,294 |
|
Fair value loss on warrant liability |
|
|
3,431 |
|
|
|
- |
|
Exercise of public and private warrants |
|
|
(197 |
) |
|
|
(149,198 |
) |
Balance as of December 31, 2023 |
|
|
5,370 |
|
|
|
4,254,096 |
|
The number of exercised warrants abovementioned of 149,198 warrants includes 149,156 EBAC warrants exercised in 2023 and an additional number of 42 EBAC warrants that are still formally part of the Company's conditional share capital, although they will not become exercisable because of the fractional conversion rate and rounding methodology applied when converting the initial warrants from EBAC into the Company's warrants.
Commitments related to Novartis license agreement
In December 2018, Oculis entered into an agreement with Novartis, under which Oculis licensed a novel topical anti-TNFα antibody, now named as Licaminlimab, or OCS-02, for ophthalmic indications. As consideration for the licenses, Oculis is obligated to pay non-refundable, upfront license fees, predefined development and commercial milestone payments and royalties on net sales of licensed products. Royalties range from high one digit to low teens, based on sales thresholds. As of December 31, 2019, Oculis has paid in full the contractual non-refundable upfront fee of CHF 4.7 million. Oculis has not reached any milestones or royalties thresholds according to the agreement. If all predefined milestones will be reached, Oculis will be obligated to pay additional CHF 81.6 million or $97.0 million. Oculis expects to reach the first milestone payment of CHF 4.2 million or $5.0 million in 2028. Royalties are based on net sales of licensed products, depending on the sales volumes reached.
Commitments related to Accure license agreement
On January 29, 2022, the Company entered into a License Agreement with Accure for the exclusive global licensing of its OCS-05 technology. Under this agreement, Oculis licensed a novel neuroprotective drug candidate, now renamed as OCS-05, for ophthalmic and other indications (refer to Note 9). As consideration for the licenses, Oculis is obligated to pay non-refundable, upfront license fees, predefined development and commercial milestone payments and royalties on net sales of licensed products. Royalties range from one digit to low teens, based on sales thresholds. As of December 31, 2023, Oculis has paid the full contractual non-refundable upfront fee of CHF 3.0 million and reimbursed costs in the amount of CHF 0.5 million. Oculis has not reached any milestones or royalties thresholds according to the agreement. If all predefined milestones will be reached, Oculis will be obligated to pay additional CHF 94.3 million or $112.1 million. In case of a commercialization, sublicense revenues will be subject to further royalty payments. The initial potential milestones under the agreement are IND approval by the U.S. FDA for the intravenous formulation of OCS-05 and completion of the first PoC clinical trial for AON for a combined amount of CHF 1.0 million or $1.2 million.
116
These milestones are estimated to be reached toward the end of 2024 or beginning of 2025.
Commitments related to Rennes University Collaboration Research agreement
On January 31, 2022, the Company entered into a collaboration research agreement with the Rennes University and CNRS in France. This agreement is for the research of Antisense Oligonucleotide (ASO) to modulate gene expressions. As consideration for the research performed by Rennes University and CNRS, Oculis is obligated to pay a non-refundable cost contribution of CHF 0.2 million or EUR 0.2 million. As of December 31, 2023, Oculis paid a contractual non-refundable cost contribution of CHF 0.1 million or EUR 0.1 million. Following completion of the research services, the parties shall sign a commercial agreement based on predefined development and commercial milestone payments and royalties on net sales of licensed products as defined in the collaboration research agreement. Oculis has not reached any milestones or royalties thresholds. If the commercial agreement was signed by the parties and development and commercial milestone payments were reached, Oculis would be obligated to pay additional CHF 6.5 million or EUR 7.0 million and royalties ranging from low to mid-single digit percentage on net sales. In case of sublicense revenues, Oculis shall be subject to further royalty payments.
Research and development commitments
The Group conducts product research and development programs through collaborative projects that include, among others, arrangements with universities, contract research organizations and clinical research sites. Oculis has contractual arrangements with these organizations. As of December 31, 2023, commitments for external research projects amounted to CHF 50.5 million, compared to CHF 13.1 million as of December 31, 2022, as detailed in the schedule below. The increase in commitments year over year is primarily due to the initiation of clinical trials in the last quarter of 2023.
in CHF thousands |
|
As of December 31, 2023 |
|
|
As of December 31, 2022 |
|
||
Within one year |
|
|
23,625 |
|
|
|
12,145 |
|
Between one and five years |
|
|
26,867 |
|
|
|
978 |
|
Total |
|
|
50,492 |
|
|
|
13,123 |
|
Key management, including the Board of Directors and the executive management team, compensation expenses were:
in CHF thousands |
|
For the years ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Salaries, cash compensation and other short-term benefits |
|
|
3,067 |
|
|
|
3,506 |
|
|
|
3,071 |
|
Payroll expenses related to restricted share |
|
|
- |
|
|
|
- |
|
|
|
951 |
|
Pension expense |
|
|
320 |
|
|
|
227 |
|
|
|
264 |
|
Share-based compensation expense |
|
|
2,543 |
|
|
|
535 |
|
|
|
251 |
|
Total |
|
|
5,930 |
|
|
|
4,268 |
|
|
|
4,537 |
|
Salaries, cash compensation and other short-term benefits include social security and board member fees.
The number of individuals reported as key management was reduced from 7 to 6 for the year ended December 31, 2023 as compared to the year ended December 31, 2022. The number of individuals reported for the Board of Directors increased from 1 to 3 for the year ended December 31, 2023 as compared to the year ended December 31, 2022.
117
Categories of financial instruments:
As indicated in Note 3, all financial assets and liabilities are shown at amortized cost, except for warrant liabilities that are held at fair value. The following table shows the carrying amounts of financial assets and liabilities:
in CHF thousands |
|
|
||||
Financial assets |
As of December 31, 2023 |
|
As of December 31, 2022 |
|
||
Financial assets - non-current |
|
45 |
|
|
50 |
|
Other current assets, excluding prepaids |
|
328 |
|
|
166 |
|
Accrued income |
|
876 |
|
|
912 |
|
Short-term financial assets |
|
53,324 |
|
|
- |
|
Cash and cash equivalents |
|
38,327 |
|
|
19,786 |
|
Total |
|
92,900 |
|
|
20,914 |
|
in CHF thousands |
|
|
||||
Financial liabilities |
As of December 31, 2023 |
|
As of December 31, 2022 |
|
||
Trade payables |
|
7,596 |
|
|
3,867 |
|
Accrued expenses and other payables |
|
5,948 |
|
|
8,011 |
|
Lease liabilities |
|
605 |
|
|
633 |
|
Warrant liabilities |
|
5,370 |
|
|
- |
|
Long-term financial debt related to preferred shares/accrued dividend |
|
- |
|
|
122,449 |
|
Total |
|
19,519 |
|
|
134,960 |
|
Below is the net debt table of liabilities from financing activities:
in CHF thousands |
Preferred shares |
|
Leasing |
|
Warrant liabilities |
|
Total |
|
||||
Net debt as of December 31, 2021 |
|
(113,502 |
) |
|
(770 |
) |
|
- |
|
|
(114,272 |
) |
Cashflows |
|
(2,030 |
) |
|
204 |
|
|
- |
|
|
(1,825 |
) |
Interest calculated on Series B & C shares |
|
(6,343 |
) |
|
- |
|
|
- |
|
|
(6,343 |
) |
Transaction costs related to 2021 |
|
54 |
|
|
- |
|
|
- |
|
|
54 |
|
Interest calculated on leases |
|
- |
|
|
(45 |
) |
|
- |
|
|
(45 |
) |
Indexation for the period |
|
- |
|
|
(70 |
) |
|
- |
|
|
(70 |
) |
FX revaluation |
|
(628 |
) |
|
48 |
|
|
- |
|
|
(580 |
) |
Net debt as of December 31, 2022 |
|
(122,449 |
) |
|
(633 |
) |
|
- |
|
|
(123,082 |
) |
Cashflows |
|
- |
|
|
200 |
|
|
- |
|
|
200 |
|
Interest calculated on Series B & C shares |
|
(1,266 |
) |
|
- |
|
|
- |
|
|
(1,266 |
) |
Issuance of warrants |
|
- |
|
|
- |
|
|
(2,136 |
) |
|
(2,136 |
) |
Fair value (gain)/loss on warrant liability |
|
- |
|
|
- |
|
|
(3,431 |
) |
|
(3,431 |
) |
Exercise of public and private warrants |
|
- |
|
|
- |
|
|
197 |
|
|
197 |
|
Addition of US lease |
|
- |
|
|
(118 |
) |
|
- |
|
|
(118 |
) |
Interest calculated on leases |
|
- |
|
|
(42 |
) |
|
- |
|
|
(42 |
) |
Indexation for the period |
|
- |
|
|
(47 |
) |
|
- |
|
|
(47 |
) |
FX revaluation |
|
(1,087 |
) |
|
35 |
|
|
- |
|
|
(1,052 |
) |
Conversion of Legacy Oculis preferred shares into Oculis ordinary shares |
|
124,802 |
|
|
- |
|
|
- |
|
|
124,802 |
|
Net debt as of December 31, 2023 |
|
- |
|
|
(605 |
) |
|
(5,370 |
) |
|
(5,975 |
) |
Fair values
Due to their short-term nature, the carrying value of cash and cash equivalents, short-term financial assets, other current assets, excluding prepaids, accrued income, trade payables and accrued expenses and other payables approximates their fair value.
The warrant liabilities are measured at fair value on a recurring basis, refer to Note 3.
Legacy Oculis preferred shares, presented as long-term financial debt as described in Note 15, had been converted into Oculis ordinary shares. The conversion occurred in March 2023. As of December 31, 2022, the fair value of preferred shares was determined from similar or identical instruments issued by the Company.
118
This level 2 value resulted in a fair value of CHF 115.7 million compared to a book value of CHF 122.4 million.
Risk assessment
Since 2018 the Company implemented an Internal Control System (ICS), which includes a risk assessment. The ultimate responsibility of the risk management is of the Board of Directors and a yearly review takes place during one of the Board of Directors meetings.
Market risk
Market risk is the risk that changes in market prices, such as foreign exchange rates, interest rates and equity prices will affect the Company’s income or the value of its holdings of financial instruments. The objective of market risk management is to manage and control market risk exposures within acceptable parameters, while optimizing the return.
As of December 31, 2023, if the listed price of the warrants had moved by 5% with all other variables held constant, the net loss for the period would have been lower/higher by CHF 0.3 million. As of December 31, 2022, the Company did not hold any warrant liabilities.
Foreign currency risks
Since 2020, Oculis has established a presence in the U.S., France and Hong Kong with local currencies in U.S. Dollar (USD), Euro (EUR) and Hong Kong Dollar (HKD), respectively. In 2023, foreign currency risks primarily relate to cash and cash equivalents, short term financial assets, prepaid expenses, trade payables and accrued expenses denominated in U.S. Dollar and Euro, with immaterial amounts recorded in ISK and HKD.
The following table demonstrates the impact of a possible change in USD and EUR against CHF in regard to monetary assets and liabilities denominated in local functional currencies, as well as the impact of foreign currency risk on the Company's consolidated net loss:
|
As of December 31, / For the years ended |
|
|||||||||||
in CHF thousands |
2023 |
|
|
2022 |
|
||||||||
Change in rate |
Net exposure in CHF |
|
Impact on loss |
|
|
Net exposure in CHF |
|
Impact on loss |
|
||||
+5.0% USD |
|
21,667 |
|
|
1,083 |
|
|
|
9 577 |
|
|
479 |
|
-5.0% USD |
|
21,667 |
|
|
(1,083 |
) |
|
|
9 577 |
|
|
(479 |
) |
+5.0% EUR |
|
4,049 |
|
|
202 |
|
|
|
2,176 |
|
|
109 |
|
-5.0% EUR |
|
4,049 |
|
|
(202 |
) |
|
|
2,176 |
|
|
(109 |
) |
Interest rate risk
The Company’s long-term financial debt, which resulted from the issuance of preferred shares as indicated in Note 15, bore a deemed interest resulting from the preferred dividend, due under certain circumstances, at a fixed rate of 6.0% per year until their conversion on March 2, 2023 in connection with the Business Combination. The other financial instruments of the Group are not bearing interest and are therefore not subject to interest rate risk.
Hedging activities
There are no hedging activities within the Group.
Credit risk
As of December 31, 2023, the maximum exposure is the carrying amount of the Company’s cash, cash equivalents and short-term financial assets are mainly held with two financial institutions, each with a high credit rating of A+ assigned by international credit-rating agencies. Management focuses on diversification strategies and monitors counterparties’ ratings to minimize exposure.
Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting the obligations associated with its financial liabilities that are settled by delivering cash or another financial asset. Liquidity management is performed by Group finance based on cash flow forecasts which are prepared on a rolling basis and focuses mainly on ensuring that the Group has sufficient cash to meet its operational needs.
119
The Group’s liquidity needs have been historically satisfied through the issuance of preferred shares, the Business Combination, PIPE and CLA financings, and the follow-on offering, discussed further in Note 5.
All of the Company’s financial instruments, except long-term financial debt and the long-term portion of the lease liabilities are due within one year.
in CHF thousands |
As of December 31, 2023 |
|
Less than one year |
|
Over |
|
|
As of December 31, 2022 |
|
Less than one year |
|
Over |
|
||||||
Trade payables |
|
7,596 |
|
|
7,596 |
|
|
- |
|
|
|
3,867 |
|
|
3,867 |
|
|
|
|
Accrued expenses and other payables |
|
5,948 |
|
|
5,948 |
|
|
- |
|
|
|
8,011 |
|
|
8,011 |
|
|
- |
|
Long-term financial debt |
|
- |
|
|
- |
|
|
- |
|
|
|
170,988 |
|
|
- |
|
|
170,988 |
|
Lease liability |
|
681 |
|
|
210 |
|
|
471 |
|
|
|
743 |
|
|
149 |
|
|
594 |
|
Total |
|
14,225 |
|
|
13,754 |
|
|
471 |
|
|
|
183,609 |
|
|
12,027 |
|
|
171,582 |
|
Long-term financial debt as of December 31, 2022 resulted from the issuance of preferred shares as indicated in Note 15.
Capital management
Since its incorporation, the Group has primarily funded its operations through capital increases, and at the current development stage, the Group frequently raises new funds to finance its projects. Refer to Notes 15 and 16 for further details.
As a result of the Business Combination, the Company has retroactively restated the weighted average number of outstanding shares prior to March 2, 2023 to give effect to the Exchange Ratio. The following table sets forth the loss per share calculations for the years ended December 31, 2023, 2022 and 2021.
|
For the years ended December 31, |
|
|||||||||
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Net loss for the period attributable to Oculis shareholders - in CHF thousands |
|
(88,802 |
) |
|
|
(38,698 |
) |
|
|
(18,552 |
) |
|
|
|
|
|
|
|
|
|
|||
Weighted-average number of shares used to compute loss per share basic and diluted for the periods ended December 31, 2022 and December 31, 2021, Legacy Oculis ordinary shares |
|
- |
|
|
|
2,989,434 |
|
|
|
2,777,589 |
|
Exchange Ratio |
|
- |
|
|
|
1.1432 |
|
|
|
1.1432 |
|
Weighted-average number of shares used to compute basic and diluted loss per share for the periods ended December 31, 2022 and December 31, 2021, Legacy Oculis ordinary shares (as restated) |
|
- |
|
|
|
3,417,521 |
|
|
|
3,175,340 |
|
Weighted-average number of shares used to compute basic and diluted loss per share for the period ended December 31, 2023, Oculis ordinary shares |
|
29,899,651 |
|
|
|
- |
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
|||
Basic and diluted net loss per share for the period, ordinary shares |
|
(2.97 |
) |
|
|
(11.32 |
) |
|
|
(5.84 |
) |
Since the Company has a loss for all periods presented, basic net loss per share is the same as diluted net loss per share. Potentially dilutive securities that were not included in the diluted loss per share calculations because they would be anti-dilutive were as follows:
The number of potentially dilutive securities prior to the Business Combination have been adjusted by the Exchange Ratio to reflect the equivalent number in the Company.
120
|
As of December 31, 2023 |
|
|
As of December 31, 2022 |
|
|
As of December 31, 2021 |
|
|||
Share options issued and outstanding |
|
3,096,473 |
|
|
|
1,762,949 |
|
|
|
1,289,090 |
|
Earnout options |
|
369,737 |
|
|
|
- |
|
|
|
- |
|
Share and earnout options issued and outstanding |
|
3,466,210 |
|
|
|
1,762,949 |
|
|
|
1,289,090 |
|
Restricted shares subject to repurchase |
|
98,094 |
|
|
|
252,880 |
|
|
|
476,581 |
|
Earnout shares |
|
3,793,995 |
|
|
|
- |
|
|
|
- |
|
Public warrants |
|
4,102,397 |
|
|
|
- |
|
|
|
- |
|
Private warrants |
|
151,699 |
|
|
|
- |
|
|
|
- |
|
Total |
|
11,612,395 |
|
|
|
2,015,829 |
|
|
|
1,765,671 |
|
There are no material subsequent events to report and no events out of the ordinary course of business.
121

Report of the Statutory Auditor to the General Meeting
on the Financial Statements 2023
122
Report of the statutory auditor
to the General Meeting of Oculis Holding AG
Zug
Report on the audit of the financial statements
Opinion
We have audited the financial statements of Oculis Holding AG (the Company), which comprise the balance sheet as of December 31, 2023, and the profit and loss statement for the period from October 31, 2022 to December 31, 2023, and notes to the financial statements, including a summary of significant accounting policies.
In our opinion, the financial statements (pages 130 - 139) comply with Swiss law and the Company’s articles of incorporation.
Basis for opinion
We conducted our audit in accordance with Swiss law and Swiss Standards on Auditing (SA-CH). Our responsibilities under those provisions and standards are further described in the 'Auditor’s responsibilities for the audit of the financial statements' section of our report. We are independent of the Company in accordance with the provisions of Swiss law and the requirements of the Swiss audit profession, and we have fulfilled our other ethical responsibilities in accordance with these requirements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Our audit approach
Overview |
Overall materiality: CHF 3,000 thousand |
|
We tailored the scope of our audit in order to perform sufficient work to enable us to provide an opinion on the financial statements as a whole, taking into account the structure of the Company, the accounting processes and controls, and the industry in which the Company operates. |
|
As key audit matter the following area of focus has been identified: Accounting impact related to the De-SPAC transaction |
Materiality
The scope of our audit was influenced by our application of materiality. Our audit opinion aims to provide reasonable assurance that the financial statements are free from material misstatement. Misstatements may arise due to fraud or error.

123
They are considered material if, individually or in aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of the financial statements.
Based on our professional judgement, we determined certain quantitative thresholds for materiality, including the overall materiality for the financial statements as a whole as set out in the table below. These, together with qualitative considerations, helped us to determine the scope of our audit and the nature, timing and extent of our audit procedures and to evaluate the effect of misstatements, both individually and in aggregate, on the financial statements as a whole.
Overall materiality |
CHF 3,000 thousand |
Benchmark applied |
Total assets |
Rationale for the materiality benchmark applied |
We chose total assets as the benchmark, because, in our view, it is the benchmark against which the performance of the Company, which has limited operating activities and which mainly holds investments in subsidiaries and intra-group loans, is most commonly measured, and it is a generally accepted benchmark for holding companies. |
We agreed with the Audit Committee that we would report to them misstatements above CHF 300 thousand identified during our audit as well as any misstatements below that amount which, in our view, warranted reporting for qualitative reasons.
Audit scope
We designed our audit by determining materiality and assessing the risks of material misstatement in the financial statements. In particular, we considered where subjective judgements were made; for example, in respect of significant accounting estimates that involved making assumptions and considering future events that are inherently uncertain. As in all of our audits, we also addressed the risk of management override of internal controls, including among other matters consideration of whether there was evidence of bias that represented a risk of material misstatement due to fraud.
Key audit matters
Key audit matters are those matters that, in our professional judgement, were of most significance in our audit of the financial statements of the current period. These matters were addressed in the context of our audit of the financial statements as a whole, and in forming our opinion thereon, and we do not provide a separate opinion on these matters.
Accounting impact related to the De-SPAC transaction
Key audit matter |
|
How our audit addressed the key audit matter |
|
As described in Notes 2, 3, 5 and 8 to the financial statements, as of March 2, 2023, a capital reorganization took place within the Company as a result of the merger with European Biotech Acquisition Corp. (“EBAC”), and resulted in the listing of Oculis Holding AG on the NASDAQ. Management concluded that the contribution of EBAC and Oculis SA (“Legacy Oculis”) into Oculis Holding AG is recognized at Fair Market Value for CHF 289,877 thousand. Besides, earnout shares issued to Legacy Oculis’ shareholders are recognized as an increase of the share capital of the Company for CHF 38 thousand. Lastly, management assessed that the dividend income of CHF 69,251 thousand received by the Company from Oculis |
|
Addressing the matter involved performing procedures and evaluating audit evidence. These procedures included, among others: -
obtaining a detailed understanding of the transaction through review of management’s reorganization step-plan and how this was effectuated through the associated accounting entries;
-
tracing the details of the accounting entries to the underlying agreements and cash movements as applicable; and review of the accounting treatment under Swiss Code of Obligations;
|

124
|
Merger Sub II should be recorded as an extraordinary income. The principal considerations for our determination that performing procedures relating to the accounting impact related to the De-SPAC transaction is a key audit matter are the significant complexities of the overall transaction that required a high degree of Swiss Code of Obligations knowledge. This in turn led to a high degree of audit effort in applying procedures relating to the accounting impact of : (i) the contributions of EBAC and Oculis SA into the Company followed by the merger between Oculis SA and Oculis Operations Sàrl, (ii) the issuance of warrants, earn-outs and ESOP 2023 replacement stock-options by the Company and (iii) the accounting for dividend payment received by the Company from Oculis Merger Sub II. |
|
-
we assessed, with the support of financial reporting specialists (i) the accounting treatment of the contributions of EBAC and Oculis SA into the Company followed by the merger between Oculis SA and Oculis Operations Sàrl, (ii) the accounting treatment of the issuance of warrants, earn-outs and replacement stock-options by the Company and (iii) the accounting for dividend payment received by the Company from Oculis Merger Sub II.
On the basis of the procedures performed, we consider that the conclusions drawn by management regarding the accounting impact related to the De-SPAC transaction were reasonable. |
Other information
The Board of Directors is responsible for the other information. The other information comprises the information included in the annual report, but does not include the financial statements, the consolidated financial statements, the compensation report and our auditor’s reports thereon.
Our opinion on the financial statements does not cover the other information and we do not express any form of assurance conclusion thereon.
In connection with our audit of the financial statements, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the financial statements or our knowledge obtained in the audit, or otherwise appears to be materially misstated.
If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard.
Board of Directors' responsibilities for the financial statements
The Board of Directors is responsible for the preparation of financial statements in accordance with the provisions of Swiss law and the Company’s articles of incorporation, and for such internal control as the Board of Directors determines is necessary to enable the preparation of financial statements that are free from material misstatement, whether due to fraud or error.
In preparing the financial statements, the Board of Directors is responsible for assessing the Company's ability to continue as a going concern, disclosing, as applicable, matters related to going concern and using the going concern basis of accounting unless the Board of Directors either intends to liquidate the Company or to cease operations, or has no realistic alternative but to do so.
Auditor’s responsibilities for the audit of the financial statements
Our objectives are to obtain reasonable assurance about whether the financial statements as a whole are free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Swiss law and SA-CH will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these financial statements.

125
As part of an audit in accordance with Swiss law and SA-CH, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
We communicate with the Board of Directors or its relevant committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide the Board of Directors or its relevant committee with a statement that we have complied with relevant ethical requirements regarding independence, and communicate with them regarding all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, actions taken to eliminate threats or safeguards applied.
From the matters communicated with the Board of Directors or its relevant committee, we determine those matters that were of most significance in the audit of the financial statements of the current period and are therefore the key audit matters. We describe these matters in our auditor’s report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, we determine that a matter should not be communicated in our report because the adverse consequences of doing so would reasonably be expected to outweigh the public interest benefits of such communication.
Report on other legal and regulatory requirements
In accordance with article 728a para. 1 item 3 CO and PS-CH 890, we confirm the existence of an internal control system that has been designed, pursuant to the instructions of the Board of Directors, for the preparation of the financial statements.
We further confirm that the proposed carry forward of the accumulated losses complies with Swiss law and the Company’s articles of incorporation. We recommend that the financial statements submitted to you be approved.

126
PricewaterhouseCoopers SA
Michael Foley |
Alex Fuhrer |
|
Licensed audit expert Auditor in charge |
Licensed audit expert |
Lausanne, March 19, 2024

127

Statutory Financial Statements of Oculis Holding AG for the period October 31, 2022 - December 31, 2023
128

Statutory Financial Statements
Oculis Holding AG
for the period October 31, 2022 - December 31, 2023
129
Oculis Holding AG, Zug
Table of Contents
FINANCIAL STATEMENTS |
|
|
|
|
|
|
|
APPROPRIATION OF AVAILABLE EARNINGS |
|
Appropriation of available earnings and reserves of Oculis Holding AG |
|
130
Oculis Holding AG, Zug
Balance Sheet
(in CHF thousands)
|
|
|
As of December 31, |
|
|
As of October 31, |
|
||
Assets |
Note |
|
2023 |
|
|
2022 |
|
||
|
|
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
464 |
|
|
|
100 |
|
Other current receivables |
|
|
|
2,145 |
|
|
|
- |
|
From third parties |
|
|
|
175 |
|
|
|
- |
|
From group subsidiaries |
|
|
|
1,970 |
|
|
|
- |
|
Prepaid expenses |
|
|
|
571 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Total current assets |
|
|
|
3,180 |
|
|
|
100 |
|
|
|
|
|
|
|
|
|
||
Non-current assets |
|
|
|
|
|
|
|
||
Loans to group subsidiaries |
6 |
|
|
115,033 |
|
|
|
- |
|
Investments |
7 |
|
|
191,067 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Total non-current assets |
|
|
|
306,100 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Total assets |
|
|
|
309,280 |
|
|
|
100 |
|
|
|
|
As of December 31, |
|
|
As of October 31, |
|
||
Liabilities and shareholders' equity |
Note |
|
2023 |
|
|
2022 |
|
||
|
|
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
|
||
Trade payables |
|
|
|
1,959 |
|
|
|
- |
|
To third parties |
|
|
|
29 |
|
|
|
- |
|
To group subsidiaries |
|
|
|
1,930 |
|
|
|
- |
|
Other short-term liabilities |
|
|
|
7 |
|
|
|
- |
|
Accrued expenses |
|
|
|
506 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
2,472 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Non-current liabilities |
|
|
|
|
|
|
|
||
Other long-term liabilities due to third parties |
|
|
|
378 |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Total non-current liabilities |
|
|
|
378 |
|
|
|
- |
|
|
|
|
|
|
|
|
|
||
Shareholders' equity |
|
|
|
|
|
|
|
||
Share capital |
8 |
|
|
404 |
|
|
|
100 |
|
Statutory capital reserves |
|
|
|
347,424 |
|
|
|
- |
|
Reserves from capital contribution |
8 |
|
|
237,187 |
|
|
|
- |
|
Other statutory capital reserves |
8 |
|
|
110,237 |
|
|
|
- |
|
Loss of the period |
|
|
|
(41,398 |
) |
|
|
- |
|
|
|
|
|
|
|
|
|
||
Total shareholders' equity |
|
|
|
306,430 |
|
|
|
100 |
|
|
|
|
|
|
|
|
|
||
Total liabilities and shareholders' equity |
|
|
|
309,280 |
|
|
|
100 |
|
The accompanying notes form an integral part of the financial statements.
131
Oculis Holding AG, Zug
Profit and loss statement for the period October 31, 2022 - December 31, 2023
(in CHF thousands)
|
|
Note |
|
From October 31, 2022 to December 31, 2023 |
|
|
|
|
|
|
|
|
|
Other operating expenses |
|
|
|
|
(9,311 |
) |
Operating expenses |
|
|
|
|
(9,311 |
) |
|
|
|
|
|
|
|
Operating loss |
|
|
|
|
(9,311 |
) |
|
|
|
|
|
|
|
Financial income |
|
4 |
|
|
1,930 |
|
Financial expense |
|
4 |
|
|
(4,458 |
) |
Loss before extraordinary items |
|
|
|
|
(11,839 |
) |
|
|
|
|
|
|
|
Extraordinary income |
|
5.1 |
|
|
69,251 |
|
Extraordinary expense |
|
5.2 |
|
|
(98,810 |
) |
|
|
|
|
|
|
|
Loss before taxes |
|
|
|
|
(41,398 |
) |
|
|
|
|
|
|
|
Direct taxes |
|
|
|
|
- |
|
|
|
|
|
|
|
|
Loss of the period |
|
|
|
|
(41,398 |
) |
The accompanying notes form an integral part of the financial statements.
132
Oculis Holding AG, Zug
NOTES TO THE STATUTORY FINANCIAL STATEMENTS
1. GENERAL INFORMATION
Oculis Holding AG (the "Company" or "Oculis") is a stock corporation (Aktiengesellschaft) with its registered office at Bahnhofstrasse 7, CH-6300, Zug, Switzerland. It was incorporated under the laws of Switzerland in accordance with article 620 et seq. of the Swiss Code of Obligations ("SCO") and registered as of October 31, 2022.
As of December 31, 2023, the Company controls directly or indirectly six wholly-owned subsidiaries:
The Company and its wholly-owned subsidiaries form the Oculis Group (the "Group"). Prior to the Business Combination (as defined in Note 2), Oculis SA ("Legacy Oculis"), which was incorporated in Lausanne Switzerland on December 11, 2017, and its wholly-owned subsidiaries Oculis Iceland, Oculis France, Oculis US and Oculis HK formed the Oculis group. On July 6, 2023, Legacy Oculis merged with and into Oculis Operations, and the separate corporate existence of Legacy Oculis ceased. Oculis Operations is the surviving company and remains a wholly-owned subsidiary of Oculis.
The purpose of the Company is the research, study, development, manufacture, promotion, sale and marketing of biopharmaceutical products and substances as well as the purchase, sale and exploitation of intellectual property rights, such as patents and licenses, in the field of ophthalmology. As a global biopharmaceutical company, Oculis is developing treatments to save sight and improve eye care with breakthrough innovations. The Company’s differentiated pipeline includes candidates for topical retinal treatments, topical biologics and disease modifying treatments.
2. SIGNIFICANT EVENTS IN THE CURRENT REPORTING PERIOD
Business combination with European Biotech Acquisition Corp (“EBAC”)
On March 2, 2023, the Company consummated a business combination with EBAC (the “Business Combination”) pursuant to the Business Combination Agreement ("BCA") between Legacy Oculis and EBAC dated as of October 17, 2022. The Company received gross proceeds of CHF 97.6 million or $103.7 million comprising CHF 12.0 million or $12.8 million of cash held in EBAC’s trust account and CHF 85.6 million or $90.9 million from private placement ("PIPE") investments and conversion of notes issued under Convertible Loan Agreements (“CLA”) into Oculis' ordinary shares. In connection with the Business Combination, Oculis was listed on the Nasdaq Global Market with the ticker symbol "OCS" for its ordinary shares and “OCSAW” for its public warrants.
133
Public offering of ordinary shares
On May 31, 2023, the Company entered into an underwriting agreement with BofA Securities Inc. and SVB Securities, LLC, as representatives of several underwriters, and on June 5, 2023, closed the issuance and sale in a public offering of 3,500,000 ordinary shares at a public offering price of CHF 10.45 or $11.50 per share, for total gross proceeds of CHF 36.6 million or $40.3 million before deducting underwriting discounts, commissions and offering expenses.
In addition, the Company granted the underwriters an option to purchase additional ordinary shares which was partially exercised on June 13, 2023, leading to an additional purchase of 154,234 ordinary shares and gross proceeds of CHF 1.6 million or $1.7 million before deducting underwriting discounts, commissions and offering expenses. After giving issuance to these additional shares, Oculis sold a total of 3,654,234 ordinary shares in the offering for aggregate gross proceeds of CHF 38.2 million or $42.0 million, before deducting underwriting discounts, commissions and offering expenses. All of the underwriters' unexercised options to purchase additional shares expired on June 30, 2023.
3. BASIS OF PREPARATION AND ACCOUNTING POLICIES
Basis of preparation
The statutory Financial Statements of Oculis, with registered office in Zug, Switzerland, were prepared according to the principles of the Swiss Law on Accounting and Financial Reporting (32nd title of the Swiss Code of Obligations). Where not prescribed by law, the significant accounting and valuation principles applied are described below. The Financial Statements are established from company incorporation on October 31, 2022 to the reporting date as of December 31, 2023.
Oculis is presenting its Consolidated Financial Statements according to IFRS ("IFRS Accounting Standards"). As a result, Oculis has applied the exemption included in art. 961d of the Swiss Code of Obligations and has not included additional disclosures and a cash flow statement in its statutory Financial Statements.
Going concern
Oculis accounts are prepared on a going concern basis. To date, the Group has financed its cash requirements primarily from share issuances, as well as government research and development grants. The recent Business Combination with EBAC and the listing in NASDAQ early in March 2023 raised funding to secure business continuity as explained under Note 2. In May and June 2023, the Company raised additional capital via a public offering. The Board of Directors believes that the Group has the ability to meet its financial obligations for at least the next 12 months.
Cash and cash equivalents
Cash and cash equivalents are valued at nominal value.
Investments
Investments are initially recognized at cost, assessed annually for impairment triggers, and adjusted to their recoverable amount as needed.
Loans to group subsidiaries
Short and long term loans to Oculis Group subsidiaries are valued at nominal value under consideration of any impairment if needed.
Foreign currency
The Company's books are expressed in Swiss Francs (CHF). During the year, transactions denominated in foreign currencies are converted into Swiss Francs at the rate in effect at the transaction date. At year-end, assets and liabilities denominated in foreign currencies are converted into Swiss Francs using the year-end exchange rates.
134
Realized and unrealized exchange gains and losses are recorded net as financial income or financial expenses.
Warrants
Liabilities related to warrants are recorded at nominal value. Given warrants have no nominal or intrinsic value, these are not recognized in the statutory Financial Statements. When holders of warrants exercise their rights to purchase Oculis shares, this transaction does not lead to any outflows from Oculis.
Earnout consideration
As a result of the BCA, Legacy Oculis preferred, ordinary and option holders (collectively “equity holders”) received consideration in the form of 3,793,995 earnout shares and 369,737 earnout options with an exercise price of CHF 0.01.
The earnout consideration is subject to forfeiture in the event of a failure to achieve the price targets during the earnout period defined as follows: (i) 1,500,000, (ii) 1,500,000 and (iii) 1,000,000 earned based on the achievement of post-acquisition closing share price targets of Oculis of CHF 12.62 or $15.00, CHF 16.83 or $20.00 and CHF 21.04 or $25.00, respectively, in each case, for any 20 trading days within any consecutive 30 trading day period commencing after the acquisition closing date and ending on or prior to March 2, 2028 (the “Earnout period”). A given share price target described above will also be deemed to be achieved if there is a change of control, as defined in the BCA, transaction of Oculis during the earnout period.
The earnout shares have been registered in the Registry of Commerce and are included in the number of outstanding shares as of December 31, 2023. The earnout shares are recorded at nominal value. Upon meeting the criteria, Oculis will not further increase its reserve from capital contribution.
4. FINANCIAL INCOME AND EXPENSE
Foreign exchange gain / (losses) reported into financial income and expenses are presented net per currency.
(in CHF thousands) |
|
From October 31, 2022 to December 31, 2023 |
|
|||||
|
|
Income |
|
|
Expenses |
|
||
Interest |
|
|
1,930 |
|
|
|
(339 |
) |
Net foreign exchange gain / (loss) |
|
|
- |
|
|
|
(4,119 |
) |
Total |
|
|
1,930 |
|
|
|
(4,458 |
) |
5. EXTRAORDINARY INCOME AND EXPENSE
(in CHF thousands) |
|
From October 31, 2022 to December 31, 2023 |
|
|||||
|
|
Income |
|
|
Expenses |
|
||
Dividend from Merger Sub II (Note 5.1) |
|
|
69,251 |
|
|
|
- |
|
Impairment of Merger Sub II Financial investment (Note 5.2) |
|
|
- |
|
|
|
(98,810 |
) |
Total Extraordinary income / (expense) |
|
|
69,251 |
|
|
|
(98,810 |
) |
5.1 Intra-group loan and dividend payment from Merger Sub II
Following the Business Combination and gross proceeds raised through the trust merger and PIPE financing by Merger Sub II (former EBAC), Oculis entered into a loan with its wholly owned subsidiary on March 3, 2023 in the amount of CHF 69.5 million for the purpose of developing Oculis business activities.
135
In connection with the ongoing process of dissolution of Merger Sub II, the Board of Directors of Merger Sub II and Oculis approved a dividend in favor of the shareholder Oculis in an amount of CHF 69.3 million (the "Dividend"), whereby such dividend was made effective as August 9, 2023, by way of a set-off declaration dated August 9, 2023, as further clarified on February 13, 2024. The payment of the Dividend was satisfied by offsetting the balance of the loan of CHF 69.3 million (initial loan of CHF 69.5 million minus CHF 0.2 million resulting from payments which Oculis has made on behalf of Merger Sub II). The loan is considered to have been repaid in full and there are no amounts outstanding under the Loan Agreement. Oculis recognized an extraordinary income of CHF 69.3 million in its Statement of loss.
5.2 Impairment of financial investment Merger Sub II
As per the contribution agreement signed between Oculis and EBAC on March 2, 2023, the transfer price of the contribution in kind of Merger Sub II (former EBAC) in exchange of 10,489,371 shares of Oculis amounted to CHF 98.8 million or $104.9 million. Following the Dividend payment and offset of the intra-group loan, the intrinsic value of the Merger Sub II entity was nil given all the cash raised during the Business Combination was transferred to Oculis. As a result, Oculis Management recognized the full impairment of its financial investment leading to an extraordinary expense of CHF 98.8 million.
6. LOAN TO GROUP SUBSIDIARIES
The following table presents the intra-group loan between Oculis and its subsidiary Oculis Operations as of December 31, 2023:
Original Borrower |
|
Start date |
|
Repayment |
|
USD |
|
|
EUR |
|
|
CHF |
|
|
Total CHF |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Oculis Operations GmbH |
|
March, 2023 |
|
December, 2027 |
|
|
30,772 |
|
|
|
8,815 |
|
|
|
80,950 |
|
|
|
115,033 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total Intra-group loans |
|
|
|
|
|
|
30,772 |
|
|
|
8,815 |
|
|
|
80,950 |
|
|
|
115,033 |
|
These loans were made to support the Group's clinical and business development activities and bears interest using the rate published by the Swiss federal Tax Administration for CHF, USD and Euro denominated loans to shareholders and intercompany entities.
7. INVESTMENTS
As of December 31, 2023, the Company had six direct and indirect subsidiaries. The following table describes the principal subsidiaries, the countries of incorporation and the percentage of ownership and voting interest held by the Company.
|
|
|
|
Share in Capital |
|
|
||
Company |
|
Domicile |
|
% of capital and vote |
|
Direct/indirect |
|
Main activities |
Oculis Operations GmbH |
|
Switzerland |
|
100% |
|
Direct |
|
Business and clinical development |
Oculis Merger Sub II Company |
|
Cayman Islands |
|
100% |
|
Direct |
|
Financing |
Oculis ehf |
|
Iceland |
|
100% |
|
Indirect |
|
Research, business and clinical development |
Oculis France Sarl |
|
France |
|
100% |
|
Indirect |
|
Research, business and clinical development |
Oculis US Inc |
|
USA |
|
100% |
|
Indirect |
|
Business and clinical development |
Oculis HK, Limited |
|
Hong Kong |
|
100% |
|
Indirect |
|
Business and clinical development |
136
8. SHARE CAPITAL AND STATUTORY CAPITAL RESERVES
Share capital
As of December 31, 2023, the Company had a share capital of CHF 404,437.00. The Company's share capital consists of 40,443,700 shares with a nominal value of CHF 0.01, of which, 262,098 new shares will be registered in the commercial register in the first quarter of 2024 for 2023 share activities.
In CHF thousands, except for the number of shares |
|
Shares |
|
Share capital |
|
Reserve from capital contribution |
|
Other capital reserves |
|
Share capital & Statutory capital reserves |
October 31, 2022 |
|
|
|
|
|
|
|
|
|
|
Incorporation of the Company |
|
10,000,000 |
|
100 |
|
- |
|
- |
|
100 |
Issuance new shares |
|
25,682,186 |
|
257 |
|
- |
|
- |
|
257 |
Cancellation initial shares |
|
(35,682,186) |
|
(357) |
|
- |
|
- |
|
(357) |
In connection with BCA |
|
|
|
|
|
|
|
|
|
|
Contribution of Legacy Oculis into Oculis Holding |
|
20,277,002 |
|
203 |
|
112,380 |
|
78,426 |
|
191,009 |
Convertible Loan Agreement |
|
1,967,000 |
|
20 |
|
18,348 |
|
|
|
18,368 |
Issuance of earnout shares to Legacy Oculis shareholders |
|
3,793,995 |
|
38 |
|
- |
|
- |
|
38 |
Contribution of EBAC into Oculis Holding |
|
10,489,371 |
|
105 |
|
66,894 |
|
31,811 |
|
98,810 |
Public offering / Follow-on financing |
|
3,654,234 |
|
36 |
|
37,767 |
|
- |
|
37,803 |
Shares issued for exercise of options |
|
112,942 |
|
1 |
|
273 |
|
- |
|
274 |
Shares issued for exercise of warrants |
|
149,156 |
|
1 |
|
1,525 |
|
- |
|
1,526 |
December 31, 2023 |
|
40,443,700 |
|
404 |
|
237,187 |
|
110,237 |
|
347,828 |
Contribution of Legacy Oculis into Oculis
As per contribution agreement signed on the account of Legacy Oculis shareholders and Oculis on March 2, 2023, the transfer price of the contribution in kind of shares in Legacy Oculis in exchange of 20,277,002 shares in Oculis amounted to CHF 191.0 million, considering a price per share of CHF 9.42 or $10.00 and CHF 38.0 thousands as par value of the granted earn-outs to former Legacy Oculis shareholders.
Convertible Loan Agreement
In connection with the BCA, Legacy Oculis and the investor parties thereto entered into CLAs pursuant to which the investor lenders granted Legacy Oculis a right to receive an interest free convertible loan. Following the Business Combination, Oculis assumed the CLAs and the lenders exercised their conversion rights in exchange for 1,967,000 ordinary shares at CHF 9.42 or $10.00 per share for aggregate gross proceeds of CHF 18.4 million or $19.7 million.
Earnout shares
As a result of the BCA, Legacy Oculis “equity holders” received consideration in the form of 3,793,995 earnout shares and 369,737 earnout options with an exercise price of CHF 0.01.
Contribution of EBAC into Oculis
As per contribution agreement signed on the account of EBAC shareholders and Oculis on March 2, 2023, the transfer price of the contribution in kind of shares in EBAC in exchange of 10,489,371 shares in Oculis amounted to CHF 98.8 million, considering a price per share of CHF 9.42 or $10.00.
Public follow-on financing
On June 5 and 13, 2023, the Company closed the issuance and sale in a public offering of 3,654,234 ordinary shares at a public offering price of CHF 10.45 or $11.50 per share, for total gross proceeds of CHF 38.2 million, before deducting underwriting discounts, commissions and offering expenses (refer to Note 2). This capital increase was made using the capital band.
137
Reserves from capital contribution
As of December 31, 2023, the reserves from capital contribution amounted to CHF 237.2 million. The Swiss Federal Tax administration has not yet confirmed the amount of reserves from capital contributions for 2023 in the sense of art. 20 para. 3 of the Federal Act on Direct Federal Taxation.
Capital band
The Company has a capital band between CHF 365,273.68 (lower limit) and CHF 543,684.52 (upper limit). The Company may effect an increase of the Company’s share capital in a maximum amount of CHF 178,410.84 by issuing up to 17,841,084 ordinary shares with a par value of CHF 0.01 each out of the Company’s capital band. The Board of Directors is authorized to increase the share capital up to the upper limit or decrease the share capital up to the lower limit at any time and as often as required until March 2, 2028. In Q2 2023, 3,654,234 shares were issued from this capital band.
Conditional share capital
The conditional capital at December 31, 2023 amounts to a maximum of CHF 176,089.41 split into 17,608,941 ordinary shares, in connection with the potential future issuances of:
CHF 50,000.00 through the issuance of a maximum of 5,000,000 fully paid up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of convertible rights and/or option rights or warrants, new bonds and similar debt instruments.
CHF 78,355.44 through the issuance of a maximum of 7,835,544 fully paid up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of option rights or other equity-linked instruments granted to any employee, consultant or member of the Board of Directors of the Group.
As of December 31, 2023, 112,942 options have been exercised and associated ordinary shares have been issued using the conditional share capital for employee benefit plans. These shares were not registered yet in the commercial register as of December 31, 2023.
CHF 44,032.94 through the issuance of a maximum of 4,403,294 fully paid up registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of warrants.
As of December 31, 2023, 149,156 warrants have been exercised and associated ordinary shares have been issued using the conditional share capital for EBAC warrants. These shares were not registered yet in the commercial register as of December 31, 2023.
CHF 3,701.03 through the issuance of a maximum of 370,103 fully paidup registered shares, each with a par value of CHF 0.01 (ordinary shares), in connection with the exercise of option rights or other equity-linked instruments granted to any employee, consultant or member of the Board of Directors of the Group.
9. WARRANTS
Pursuant to the BCA and the Warrant Assignment and Assumption Agreement executed in connection with the BCA, the Company has assumed 4,251,595 EBAC public warrants and 151,699 EBAC private warrants from EBAC, and issued 4,403,294 warrants as of March 2, 2023 with substantially the same terms. Each warrant entitles the registered holder to purchase one ordinary share at a price of CHF 10.52 or $11.50 per share, subject to certain adjustments, exercisable at any time commencing 30 days after the acquisition closing date, provided that the Company has an effective registration statement under the Securities Act covering the issuance of the ordinary shares issuable upon exercise of the warrants.
138
This registration statement was filed with the SEC and declared effective on May 1, 2023. The warrants will expire on March 2, 2028.
The movement of the number of public and private warrants is illustrated below:
|
|
|
|
Number of outstanding public and private warrants |
|
|
Balance as of October 31, 2022 |
|
|
|
|
- |
|
Issuance of warrants |
|
|
4,403,294 |
|
||
Exercise of public and private warrants |
|
|
(149,198 |
) |
||
Balance as of December 31, 2023 |
|
|
|
|
4,254,096 |
|
The number of exercised warrants abovementioned of 149,198 warrants includes 149,156 EBAC warrants exercised in 2023 and an additional number of 42 EBAC warrants that are still formally part of the Company's conditional share capital, although they will not become exercisable because of the fractional conversion rate and rounding methodology applied when converting the initial warrants from EBAC into the Company's warrants.
10. DECLARATION OF FULL TIME EQUIVALENT (FTE) EMPLOYEES
The Company had no employees during the period.
11. SHARES AND OPTIONS ON SHARES GRANTED TO EXECUTIVE OFFICERS, DIRECTORS AND EMPLOYEES
The following table presents information on the allocation of shares and equity awards to executive officers, directors and employees in accordance with Article 959c, paragraph 2, number 11 of the Swiss Code of Obligations (CO) for the period October 31, 2022 through December 31, 2023.
Shares and earnout shares values are based on the Company's closing share price of USD 11.23 (CHF 9.45). Options, stock appreciation rights ("SARs") and earnout options are recognized at fair value at grant date. The fair value of the Company's options, SARs and earnout options is determined using the Black-Scholes Model.
The following table summarizes equity awards granted from October 31, 2022 to December 31, 2023:
139
|
|
|
|
Shares / Earnout shares |
|
|
Options / Earnout options / SARs |
|
||||||||||
|
|
|
|
Number |
|
|
Fair value in CHF |
|
|
Number |
|
|
Fair value in CHF |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Issued to executive officers and directors |
|
|
270,828 |
|
|
|
2,559,398 |
|
|
|
109,802 |
|
|
|
1,027,274 |
|
||
Issued to employees |
|
|
- |
|
|
|
- |
|
|
|
242,001 |
|
|
|
2,264,089 |
|
||
Issued to consultants of the Company |
|
|
- |
|
|
|
- |
|
|
|
17,934 |
|
|
|
167,785 |
|
||
Total earnout consideration |
|
|
|
|
270,828 |
|
|
|
2,559,398 |
|
|
|
369,737 |
|
|
|
3,459,148 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Issued to executive officers and directors |
|
|
- |
|
|
|
- |
|
|
|
1,029,765 |
|
|
|
4,737,328 |
|
||
Issued to employees |
|
|
- |
|
|
|
- |
|
|
|
647,000 |
|
|
|
4,090,610 |
|
||
Issued to consultants of the Company |
|
|
- |
|
|
|
- |
|
|
|
72,000 |
|
|
|
331,229 |
|
||
Total other equity compensation |
|
|
|
|
- |
|
|
|
- |
|
|
|
1,748,765 |
|
|
|
9,159,167 |
|
Total |
|
|
270,828 |
|
|
|
2,559,398 |
|
|
|
2,118,502 |
|
|
|
12,618,315 |
|
||
12. CONTINGENT LIABILITIES
The Company has no contingent liabilities as of December 31, 2023.
140
Appropriation of available earnings and reserves of Oculis Holding AG
(in CHF thousands) |
|
|
|
|
|
|
From October 31, 2022 to December 31, 2023 |
|
|
Retained earnings carried forward |
|
|
|
|
Balance at the beginning of the period |
|
- |
|
|
Loss of the year |
|
|
(41,398 |
) |
Loss available to the ordinary general meeting |
|
|
(41,398 |
) |
|
|
|
|
|
Motion of the Board of Directors on the proposed carry forward of the accumulated losses |
|
|
|
|
Loss available to the ordinary general meeting |
|
|
(41,398 |
) |
Balance to be carried forward |
|
|
(41,398 |
) |
141

Report of the Statutory Auditor to the General Meeting on the Compensation Report 2023
142
Report of the statutory auditor
to the General Meeting of Oculis Holding AG
Zug
Report on the audit of the compensation report
Opinion
We have audited the compensation report of Oculis Holding AG (the Company) for the period from March 2, 2023 to December 31, 2023. The audit was limited to the information pursuant to article 734a-734f CO in the tables 2.c., 3.c., 4 and 5 and the information in sections 2.b. and 4 of the compensation report.
In our opinion, the information pursuant to article 734a-734f CO in the compensation report (pages 147 to 156) complies with Swiss law and the Company’s articles of incorporation.
Basis for opinion
We conducted our audit in accordance with Swiss law and Swiss Standards on Auditing (SA-CH). Our responsibilities under those provisions and standards are further described in the 'Auditor’s responsibilities for the audit of the compensation report' section of our report. We are independent of the Company in accordance with the provisions of Swiss law and the requirements of the Swiss audit profession, and we have fulfilled our other ethical responsibilities in accordance with these requirements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion.
Other information
The Board of Directors is responsible for the other information. The other information comprises the information included in the annual report, but does not include the tables 2.c., 3.c., 4 and 5 and the information in sections 2.b. and 4 in the compensation report, the consolidated financial statements, the financial statements and our auditor’s reports thereon.
Our opinion on the compensation report does not cover the other information and we do not express any form of assurance conclusion thereon.
In connection with our audit of the compensation report, our responsibility is to read the other information and, in doing so, consider whether the other information is materially inconsistent with the audited financial information in the compensation report or our knowledge obtained in the audit, or otherwise appears to be materially misstated.
If, based on the work we have performed, we conclude that there is a material misstatement of this other information, we are required to report that fact. We have nothing to report in this regard.
Board of Directors' responsibilities for the compensation report
The Board of Directors is responsible for the preparation of a compensation report in accordance with the provisions of Swiss law and the Company's articles of incorporation, and for such internal control as the Board of Directors determines is necessary to enable the preparation of a compensation report that is free from material misstatement, whether due to fraud or error. It is also responsible for designing the remuneration system and defining individual remuneration packages.
Auditor’s responsibilities for the audit of the compensation report
Our objectives are to obtain reasonable assurance about whether the information pursuant to article 734a-734f CO is free from material misstatement, whether due to fraud or error, and to issue an auditor’s report that includes our opinion. Reasonable assurance is a high level of assurance, but is not a guarantee that an audit conducted in accordance with Swiss law and SA-CH will always detect a material misstatement when it exists. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of this compensation report.
143
As part of an audit in accordance with Swiss law and SA-CH, we exercise professional judgement and maintain professional scepticism throughout the audit. We also:
We communicate with the Board of Directors or its relevant committee regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant deficiencies in internal control that we identify during our audit.
We also provide the Board of Directors or its relevant committee with a statement that we have complied with relevant ethical requirements regarding independence, and communicate with them all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, actions taken to eliminate threats or safeguards applied.
PricewaterhouseCoopers SA
Michael Foley |
Alex Fuhrer |
|
Licensed audit expert Auditor in charge |
Licensed audit expert |
Lausanne, March 19, 2024
144

Compensation Report 2023 of Oculis Holding AG
145

Compensation Report 2023 of
Oculis Holding AG
146
Compensation Report 2023
to the Shareholders’ Meeting of Oculis Holding AG
This compensation report (the “Compensation Report”) of OCULIS HOLDING AG (the “Company”) has been prepared in accordance with the Swiss Code of Obligations (“SCO”).
On March 2, 2023, after successfully closing the business combination between European Biotech Acquisition Corp. (“EBAC”) and Oculis SA (“Legacy Oculis”), the Company became listed on the Nasdaq Global Select Market (“Nasdaq”) effective March 3, 2023. The Compensation Report refers to the period from March 2, 2023 through December 31, 2023.
Unless otherwise indicated or the context otherwise requires, all references in the Compensation Report to the “Company”, “we”, “our”, “us” or similar terms refer to the Company and its consolidated subsidiaries.
Principles of the Compensation of the Board of Directors and the Executive Committee
Pursuant to Swiss law, the aggregate amount of compensation of the board of directors of the Company (the “Board of Directors”) and the persons whom the Board of Directors has entrusted with the management of the Company (the “Executive Committee”) shall be submitted to the annual general meeting of shareholders of the Company (the “AGM”) for a binding vote.
In the Compensation Report, the aggregate amounts of compensation, loans, and other forms of indebtedness to the Board of Directors and the Executive Committee respectively are disclosed, as well as the specific amount for each member of the Board of Directors and for the highest-paid member of the Executive Committee, specifying the name and function of each of these persons.
As a Swiss company listed on Nasdaq, we are prohibited from granting certain forms of compensation to members of the Board of Directors and the Executive Committee, such as:
Compensation to members of the Board of Directors and the Executive Committee for activities in Subsidiaries is prohibited, if (i) the compensation would be prohibited if it were paid directly by the Company, (ii) the Articles do not provide for it, or (iii) the compensation has not been approved by the AGM.
Each year, at the AGM, shareholders will vote on the proposals of the Board of Directors with respect to:
147
The Board of Directors may submit for approval to the AGM deviating, additional or conditional proposals relating to the maximum aggregate amount or maximum partial amounts for the same or different periods or specific compensation components.
If the AGM does not approve a proposal of the Board of Directors, the Board of Directors shall determine, considering all relevant factors, the respective (maximum) aggregate amount or (maximum) partial amounts, and submit the amount(s) so determined for approval to a new AGM or an extraordinary general meeting of shareholders of the Company or a binding vote.
The Company or Subsidiaries, on a go-forward basis, may pay or grant compensation prior to approval by the AGM, subject to subsequent approval.
Members of the Board of Directors may be paid in fixed basic remuneration, fixed committee fee for work in committee(s) of the Board of Directors, lump sum compensation for expenses, and in equity instruments of the Company. Members of the Executive Committee may be paid fixed remuneration payable in cash and equity compensation in the form of a performance-related remuneration payable in cash and shares or equity-linked instruments in the Company, depending on the continued service to the Company and performance of the Company as well as the individual. Performance is measured based on the achievement of pre-determined targets in a given year. The Board of Directors determines annually at the beginning of each calendar year the decisive targets and their weighting upon proposal by the remuneration committee of the Board of Directors (the “Remuneration Committee”).
Compensation may be paid or granted in the form of cash, shares, options, or other equity-linked instruments. The Board of Directors, upon proposal of the Remuneration Committee, allocates the members of the Executive Committee and the Board of Directors a fixed number of shares, options or other equity-linked instruments and the Board of Directors or, to the extent delegated to it, the Remuneration Committee shall determine grant, vesting, exercise, and forfeiture conditions.
Method of Determining Compensation
Role and Powers of the Remuneration Committee
The Remuneration Committee consists of at least two members, who will be (re-)elected at the AGM for a period until the following AGM. The Board of Directors appoints the chair of the Remuneration Committee and fills any vacancies until the following AGM.
The Remuneration Committee supports our Board of Directors in establishing and reviewing the compensation and benefits strategy and guidelines as well as in preparing the proposals to the AGM regarding the compensation of the members of the Board of Directors and the Executive Committee. The Remuneration Committee may submit proposals to the Board of Directors on other compensation-related matters.
The Remuneration Committee has the responsibility to, among other things:
148
Compensation of the Board of Directors
As per the Articles, the compensation of the non-executive members of the Board of Directors comprises the following elements: a fixed basic remuneration, a fixed committee fee for service in a committee of the Board of Directors, a lump sum compensation for expenses, and a number of options, shares or other equity-linked instruments in the Company. Total compensation shall take into account the position and level of responsibility of the relevant member of the Board of Directors. The Company deducts from the payments the applicable withholding tax and social security contributions.
As per the Articles, compensation may be paid in the form of cash, shares, options or other equity-linked instruments. The Board of Directors or, to the extent delegated to it, the Remuneration Committee, shall determine grant, vesting, exercise and forfeiture conditions. In particular, they may provide for continuation, acceleration, or removal of vesting, exercise and forfeiture conditions, for payment or grant of compensation based upon assumed target achievement, or for forfeiture, in each case in the event of pre-determined events such as a change-of-control or termination of an employment or mandate agreement.
Compensation of the Members of the Executive Committee
As per the Articles, the compensation of the members of the Executive Committee may consist of fixed and variable compensation elements. Fixed compensation comprises a fixed remuneration payable in cash. Variable compensation comprises a performance-related remuneration which depends on the Company's business success and Executive Committee individual’s performance or achievement of pre-determined targets during a calendar or fiscal year. Total compensation takes into account the position and level of responsibility of the recipient.
As per the Articles, compensation may be paid in the form of cash, shares, options, or other equity-linked instruments. Short-term compensation is in the form of base salary and target bonus, which are cash based, whereas long-term compensation are equity based. The Board of Directors or, to the extent delegated to it, the Remuneration Committee, shall determine grant, vesting, exercise, and forfeiture conditions. In particular, they may provide for continuation, acceleration, or removal of vesting, exercise, restriction and forfeiture conditions, for payment or grant of compensation based upon assumed target achievement, or for forfeiture, in each case in the event of pre-determined events such as a change-of- control or termination of an employment or mandate agreement.
Elements of Compensation for 2023
We believe that our overall compensation packages for members of the Executive Committee are market-competitive, given the importance of attracting, motivating, and retaining persons with the necessary skills, expertise and character. For 2023, the overall compensation consisted of base salary, cash bonus and equity incentive awards under the Company’s 2023 stock option and incentive plan regulation.
149
Base Salary
Per the results of external benchmarking conducted in 2023, we believe that our base salaries are in line with market practice. The base salary levels are based on the experience, skills, knowledge and responsibilities required for the relevant positions. Base salary and cash bonus are reviewed annually by the Remuneration Committee, taking into account individual responsibilities, performance and experience, as well as the results of the external benchmarking. The Remuneration Committee reviews and recommends for approval by the Board of Directors the annual base salary of the Chief Executive Officer, and, in consultation with the Chief Executive Officer, of the other members of the Executive Committee.
Bonus
The members of the Executive Committee are entitled to annual cash bonuses for their performance over the fiscal year, based on goals established by our Board of Directors. Annual cash bonuses may be earned by members of our Executive Committee based on achievement of individual performance objectives, and Company performance objectives which are approved by the Board of Directors each year. The bonus program is intended to strengthen the connection between individual compensation and Company success, reinforce our pay-for-performance philosophy by awarding higher bonuses to higher performing executives and help ensure that our compensation is competitive. Under the terms of the performance bonus program, the Remuneration Committee reviews and recommends for approval by the Board of Directors the annual cash bonus of the Chief Executive Officer, and, in consultation with the Chief Executive Officer, of the other members of the Executive Committee based on the achieved objectives.
Each member of the Executive Committee is eligible to receive an annual cash bonus under the program calculated by multiplying its base salary by a target percentage value assigned to it or to its position by the Remuneration Committee. The Remuneration Committee recommends for approval by the Board of Directors, and, in consultation with the Chief Executive Officer, of the other members of the Executive Committee if the annual cash bonus is to be paid at target, under target or above target. Under certain circumstances, new members of the Executive Committee may receive replacement awards to compensate them for amounts forgone in connection with their change of employment.
Stock Option and Incentive Plan Regulation
In connection with the Company’s Nasdaq listing in March 2023, the Company adopted the Stock Option and Incentive Plan Regulation 2023 (the “Stock Option and Incentive Plan Regulation 2023” or “2023 Plan”) which provides for the grant of options, restricted stock awards or units or stock appreciation rights to acquire shares of the Company. The purpose of the 2023 Plan is to attract and retain highly qualified personnel and to provide key employees with additional incentive to increase their efforts on behalf and in the best interest of the Company and its Subsidiaries by giving them the opportunity to acquire a proprietary interest in the Company as an incentive for them to remain in the service of the Company. The 2023 Plan is the sole means for the Company to grant new equity incentive awards.
Plan Administration. The Plan is administered by a plan administrator (one or several persons) elected by our Board of Directors from time to time. The plan administrator acts within the guidelines set and approved by our Board of Directors or the Remuneration Committee and is authorized to, among others, (i) determine the eligible persons who may receive equity awards under the 2023 Plan, (ii) determine the allocation of awards to all eligible participants, (iii) determine the exercise price and the term of each equity award, and (iv) establish such rules and regulations deemed to be appropriate and proper for the administration of the 2023 Plan, in each case, subject to the guidelines set and approved by our Board of Directors or the Remuneration Committee.
Eligible Participants. Persons eligible to participate in our 2023 Plan are members of our Board of Directors, and employees and consultants of the Company or a Subsidiary. The plan administrator determines within the guidelines set and approved by our Board of Directors or Remuneration Committee which eligible persons are to receive rights to acquire options under the 2023 Plan.
Awards. Equity incentive awards under the 2023 Plan may be granted in the form of options, restricted stock awards or rights, either in the form of restricted stock units (RSU) or in the form of stock appreciation rights (SAR) (“Award(s)”). The total number of Awards reserved and available for grant and issuance pursuant to the 2023 Plan will be Awards for 7'835'544 registered shares. Awards, if granted, have an exercise price determined by the plan administrator within the guidelines set and approved by the Board of Directors or the Remuneration Committee.
150
For options, the exercise price becomes immediately due upon exercise of the option, and shall be payable to the Company. For SARs, the exercise price shall be deducted from the contractual claim of the eligible participant.
Vesting. The vesting conditions for Awards pursuant the 2023 Plan are set forth in the applicable grant notices. Unless otherwise determined by our Board of Directors at the grant date or set forth in the grant notice, Awards granted under the 2023 Plan typically vest as to 25% of the Award at the end of the first year following the vesting start date, with the remaining 75% of the Award vesting monthly ratably over the 3 years after the first year following the vesting start date.
Earnout consideration. On March 2, 2023, the Company consummated a business combination ("Business Combination") pursuant to the Business Combination Agreement ("BCA") between Legacy Oculis and EBAC dated as of October 17, 2022. As a result of the BCA, Legacy Oculis preferred, ordinary and option holders received consideration in the form of 3,793,995 earnout shares and 369,737 earnout options with an exercise price of CHF 0.01.
The earnout consideration is subject to forfeiture in the event of a failure to achieve the price targets during the earnout period defined as follows: (i) 1,500,000, (ii) 1,500,000 and (iii) 1,000,000 earned based on the achievement of post-acquisition closing share price targets of Oculis of $15.00, $20.00 and $25.00, respectively, in each case, for any 20 trading days within any consecutive 30 trading day period commencing after the acquisition closing date and ending on or prior to March 2, 2028 (the “Earnout Period”). A given share price target described above will also be deemed to be achieved if there is a change of control, as defined in the BCA, transaction of Oculis during the Earnout Period.
In relation to the shares and equity awards disclosed below, any earn-out options and earnout shares have not been included in Section 2.c. (Board Compensation Amounts) and Section 3.c. (Executive Committee Compensation Amounts). Earn-out options and earnout shares are specifically tied to the Business Combination and are thus excluded from consideration as compensation. However, earnout options have been included in Section 4. (Equity and Equity-Linked Instruments Held by Members of the Board of Directors and the Executive Committee).
Termination of Service and Corporate Transaction
Generally, in the event of a participant’s termination of service, any Award not vested upon receipt of a notice of termination of the relevant legal relationship shall immediately lapse. Any option exercisable at the time a notice of termination has been received (regardless of which party gives notice) and outstanding at the time the legal relationship forming the basis of the service ends shall remain exercisable within three months post-termination of the service relationship unless the plan administrator provides for an exemption, provided that such period shall in no event end later than the original expiry date of the option. Should the participant's service be terminated for cause, then all outstanding Awards (whether vested or not), held by the participant shall terminate immediately and cease to be outstanding.
If indicated in the grant notice or otherwise resolved by the Board of Directors, in the event of any Corporate Transaction (as defined in the 2023 Plan), all Awards (i) shall fully vest and (ii) in the case of options and SARs must be immediately exercised, except if such Awards are repurchased by the Company or a third party designated by the Company for a cash consideration equivalent to the economic value applicable to such Award under the 2023 Plan. If indicated in the grant notice, in the event of any Corporate Transaction, the repurchase right for any restricted stock shall expire and such restricted stock shall become unrestricted shares. At the consummation of the Corporate Transaction, all vested Awards shall be exercised and/or settled and shall cease to be outstanding. The Company shall be entitled to terminate any unvested Awards and thereby shall compensate a participant for the economic value of such Awards.
Amendment. The Board of Directors shall have complete and exclusive power and authority to amend or modify the 2023 Plan in any or all respects. Unless such change is required to comply with applicable law, listing requirements, accounting rules or tax requirements, no such amendment or modification shall, without the consent of the concerned participant, adversely affect its rights and obligations under the 2023 Plan.
Pensions and other post-employment benefit plans
151
We maintain post-employment benefit plans that provide our employees with an opportunity to save for retirement on a tax advantaged basis. The Company’s Swiss entity is affiliated to a collective foundation administrating the pension plans of various unrelated employers. In addition, a customary Swiss pension plan is in place for eligible employees, in compliance with the requirements of applicable laws. The Company’s Icelandic entity makes contributions to pension funds selected by our employees according to applicable laws. For the Company’s U.S. entity, we have adopted a 401(k) defined contribution plan. For the Company’s entities in France and Hong Kong, we have adopted relevant local pension plans.
Social Charges
The Company pays social security contributions as required by applicable law. The Company also pays certain non-mandatory benefits under local social security plans.
Employment Agreements
We have entered into employment agreements with all the members of our Executive Committee. Each of these agreements provides for a base salary and annual cash bonus opportunity, equity eligibility participation, as well as participation in certain pension and welfare benefit plans. These agreements generally require advance notice of termination, and in some cases provide for gardening leave (paid leave). Members of our Executive Committee have agreed to covenants not to compete against us or solicit our employees or customers during and post-employment for a specified period following termination. We may be required to pay some members of our Executive Committee compensation for their covenant not to compete with us following termination for some period.
Our Board of Directors is composed of seven members (each a “Director”). Each Director is elected for a one-year term. Riad Sherif was appointed as a Director at the incorporation of the Company on October 31, 2022. All other current Directors were appointed at our Extraordinary Meeting of the Shareholders on March 2, 2023 to serve until our 2024 AGM.
The Company is a foreign private issuer listed on Nasdaq and subject to the rules of the SEC. We rely on Swiss home country governance requirements and certain exemptions thereunder rather than on the Nasdaq corporate governance requirements. The majority of our Directors are independent directors. There are no family relationships among any members of our Board of Directors or the Executive Committee.
Board of Directors
Name |
Role(s) |
Year Appointed |
Christina Ackermann |
Director |
2023 |
Lionel Carnot |
Director |
2023 |
Pravin Dugel, M.D. |
Director |
2023 |
Martijn Kleijwegt |
Director |
2023 |
Geraldine O’Keeffe |
Director |
2023 |
Anthony Rosenberg |
Chairman of the Board of Directors |
2023 |
Riad Sherif, M.D. |
Director and Chief Executive Officer |
2022 |
Board Committees
Name |
Audit Committee |
Remuneration Committee |
Nomination and Governance Committee |
Christina Ackermann |
Member |
Chairperson |
- |
Lionel Carnot |
Chairperson |
Member |
- |
Pravin Dugel |
- |
Member |
Chairperson |
Martijn Kleijwegt |
- |
- |
Member |
Geraldine O’Keeffe |
Member |
- |
Member |
152
Members of the Board of Directors who are not employees of the Company (including any of its affiliates) (“Eligible Director”) are paid an annual retainer reflecting the specific role and responsibility as well as the expected average time involved with the function. Such annual retainers have been established in line with market practice and represent the fee paid for being a member of the Board of Directors or Board Committee and the additional fee paid to the chair of the Board of Directors or Board Committee.
The following amounts were approved in USD and converted to CHF at the average USD/CHF rate in 2023 of 0.89851:
(amounts in thousands) |
Chair |
Member |
Board of Directors |
USD 84.75 (CHF 76.15) |
USD 45.20 (CHF 40.61) |
Audit Committee |
USD 22.60 (CHF 20.31) |
USD 11.30 (CHF 10.15) |
Remuneration Committee |
USD 13.56 (CHF 12.18) |
USD 6.78 (CHF 6.09) |
Nomination and Governance Committee |
USD 10.17 (CHF 9.14) |
USD 5.085 (CHF 4.57) |
In addition to the fixed compensation described above, each Eligible Director is eligible to participate in the 2023 Plan, subject to its terms and conditions as approved and amended by the Board of Directors from time to time. Upon joining the Company, the Company issues to Eligible Directors a one-time equity incentive Award under the 2023 Plan or other equity incentive plans then in effect, at an estimated equity value of USD 240,000. The one-time equity incentive Award of Eligible Directors joining the Company is typically subject to a quarterly vesting of three years. The exact number of Awards to be granted is determined by the Company in the grant notice in its free discretion and only such grant notice has legal effect. The Company will also issue to Eligible Directors for any subsequent year an annual equity incentive award in the form of stock option or similar awards under the 2023 Plan or other equity incentive plans then in effect, at an estimated equity value of USD 120,000 subject to a quarterly vesting of one year (generally the next AGM). Above amounts exclude the social charges.
The Eligible Directors are not eligible to any benefits other than those set out in the Board of Directors Compensation Policy of the Company, unless our Board of Directors decides otherwise. The Company reimburses all reasonable expenses in accordance with the terms and conditions of the Company’s travel and expense policy then in effect.
In the period from March 2, 2023 through December 31, 2023, the compensation of the members of the Board of Directors was as follows (converted from other currencies as applicable at the average prevailing exchange rate over the reporting period):
Amounts in CHF(1)
Name |
Role |
Gross cash compensation |
|
Employer social contributions(2) |
|
Total cash |
|
Equity FMV (3) |
|
||||
Christina Ackermann(4) |
Director |
|
55,460 |
|
|
5,097 |
|
|
60,556 |
|
|
249,945 |
|
Lionel Carnot(5) |
Director |
- |
|
- |
|
- |
|
- |
|
||||
Pravin Dugel, M.D.(4) |
Director |
|
49,464 |
|
|
4,546 |
|
|
54,010 |
|
|
249,945 |
|
Martijn Kleijwegt(5) |
Director |
- |
|
- |
|
- |
|
- |
|
||||
Geraldine O’Keeffe(5) |
Director |
- |
|
- |
|
- |
|
- |
|
||||
Anthony Rosenberg |
Chairman of the Board of Directors |
|
66,199 |
|
|
5,739 |
|
|
71,938 |
|
|
138,861 |
|
Riad Sherif, M.D.(6) |
Director and Chief Executive Officer |
- |
|
- |
|
- |
|
- |
|
||||
(1) The Equity FMV amounts in USD were converted to CHF at the USD/CHF rate at grant date.
(2) Includes social security contributions as required by applicable laws for the period March 2023 through December 2023.
(3) Amounts represent the aggregate grant date fair value of stock options granted to our non-employee Directors during 2023 at the date of grant, computed in accordance with IFRS 2. Assumptions used in the calculation of these amounts are included in Note 13 to our financial statements included in our Annual Report on Form 20-F for the year ended December 31, 2023. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the non-employee Directors. This equity FMV excludes social contributions that will be reported at the time when equity awards are exercised.
(4) Christina Ackermann and Pravin Dugel, M.D. received a one-time equity incentive award upon joining the Board in March 2023.
(5) Lionel Carnot, Martijn Kleijwegt and Geraldine O’Keeffe did not receive any compensation for their services on the Board of Directors due to policy requirements of their employers which are investors in the Company.
153
(6) As a member of the Executive Committee, Riad Sherif, M.D. does not receive any compensation for service on the Board of Directors. Compensation for Riad Sherif, M.D. is included in Section 3.c below.
No loans were extended to members of the Board of Directors or outstanding during the period from March 2, 2023 through December 31, 2023. No payments to former members of the Board of Directors in connection with their former role or that are not at arm’s length were made during and with respect to such period, and no severance payments to any member or former member of the Board of Directors were made during and with respect to such period in accordance with the SCO. No payments to related parties of members of the Board of Directors were made during such period.
As of December 31, 2023, our Executive Committee consisted of the following three members:
Name |
Position |
Riad Sherif, M.D. |
Chief Executive Officer and Director |
Sylvia Cheung |
Chief Financial Officer |
Páll Ragnar Jóhannesson |
Chief Business Officer |
Members of the Executive Committee receive compensation consisting of a base salary, annual cash bonus, social benefits and equity incentive awards granted under the 2023 Plan, as well as certain other benefits.
From March 2, 2023 through December 31, 2023, the fixed and variable compensation earned by the members of the Executive Committee was as follows (amounts in CHF converted from other currencies as applicable at the average prevailing exchange rate over the reporting period):
Name and Position |
Salary |
Bonus(1) |
Pension (employer)(2) |
Employer social contributions(3) |
Total |
Equity FMV(4) |
Riad Sherif, M.D. |
410,474 |
225,761 |
116,178 |
62,179 |
814,592 |
2,938,637 |
Total Executive Committee Compensation |
994,971 |
446,064 |
176,331 |
123,956 |
1,741,322 |
4,242,064 |
(1) Includes the earned or accrued bonus included in our financial statements for the period March 2023 through December 2023 payable in 2024.
(2) Includes Company contributions to benefit plans and life insurance premiums for the period March 2023 through December 2023.
(3) Includes social security contributions as required by applicable laws for the period March 2023 through December 2023.
(4) Amounts represent the aggregate grant date fair value of stock options granted to our Executive Committee members during 2023 at the date of grant, computed in accordance with IFRS 2. Assumptions used in the calculation of these amounts are included in Note 12 to our financial statements included in our Annual Report for the year ended December 31, 2023. These amounts do not necessarily correspond to the actual value recognized or that may be recognized by the Executive Committee members. This equity FMV excludes social contributions that will be reported at the time when equity awards are exercised.
No loans were extended to members of the Executive Committee or outstanding during the period from March 2, 2023 through December 31, 2023. No payments to former members of the Executive Committee in connection with their former role or that are not at arm's length were made during and with respect to such period, and no severance payments to members of the Executive Committee or former members of the Executive Committee were made during and with respect to such period in accordance with the SCO. No payments to related parties of members of the Executive Committee were made during such period.
154
Equity and Equity-Linked Instruments Held by Members of the Board of Directors (1)
The members of the Board of Directors and their related parties, if any, held the following equity and equity-linked instruments as of December 31, 2023:
Name(1) |
Role |
Ordinary shares(2) |
|
Earnout shares(3) |
|
Option / SARs |
|
Earnout Options |
|
Vested awards |
|
|||||
Christina Ackermann |
Director |
- |
|
- |
|
|
52,734 |
|
- |
|
|
11,718 |
|
|||
Lionel Carnot |
Director |
- |
|
- |
|
- |
|
- |
|
- |
|
|||||
Pravin Dugel, M.D. |
Director |
- |
|
- |
|
|
64,874 |
|
|
2,545 |
|
|
23,742 |
|
||
Martijn Kleijwegt |
Director |
|
1,997,302 |
|
- |
|
- |
|
- |
|
- |
|
||||
Geraldine O’Keeffe |
Director |
- |
|
- |
|
- |
|
- |
|
- |
|
|||||
Anthony Rosenberg |
Chairman of the Board of Directors |
|
96,670 |
|
|
20,276 |
|
|
33,491 |
|
|
879 |
|
|
19,412 |
|
Total |
|
|
2,093,972 |
|
|
20,276 |
|
|
151,099 |
|
|
3,424 |
|
|
54,872 |
|
(1) Excludes Riad Sherif, M.D. whose holdings are listed in the Executive Committee table.
(2) Aggregate number of share ownership at December 31, 2023.
(3) Aggregate number of earnout share awards outstanding at December 31, 2023
(4) Aggregate number of option/SARs awards outstanding at December 31, 2023.
(5) Aggregate number of earnout option awards outstanding at December 31, 2023.
(6) Equity securities this Director will have the right to acquire, or to acquire “voting power” and/or “investment power” as of December 31, 2023.
Equity and Equity-Linked Instruments Held by Members of the Executive Committee
The members of the Executive Committee and their related parties, if any, held the following equity and equity-linked instruments as of December 31, 2023:
Name |
Role |
Ordinary shares(1) |
|
Earnout shares(2) |
|
Option awards shares(3) |
|
Earnout Options(4) |
|
Vested awards shares(5) |
|
|||||
Riad Sherif, M.D. |
Chief Executive Officer |
|
878,486 |
|
|
184,264 |
|
|
627,116 |
|
|
1,492 |
|
|
3,113 |
|
Sylvia Cheung |
Chief Financial Officer |
|
66,808 |
|
|
14,013 |
|
|
370,356 |
|
|
38,878 |
|
|
126,536 |
|
Páll Ragnar Jóhannesson |
Chief Business Officer |
|
249,224 |
|
|
52,275 |
|
|
404,701 |
|
|
66,008 |
|
|
276,120 |
|
Total |
|
|
1,194,518 |
|
|
250,552 |
|
|
1,402,173 |
|
|
106,378 |
|
|
405,769 |
|
(1) Aggregate number of share ownership outstanding at December 31, 2023..
(2) Aggregate number of earnout share awards outstanding at December 31, 2023
(3) Aggregate number of option awards outstanding at December 31, 2023.
(4) Aggregate number of earnout option awards outstanding at December 31, 2023.
(5) Equity securities this executive officer will have the right to acquire, or to acquire “voting power” and/or “investment power” as of December 31, 2023.
5. Mandates outside the Company
According to article 39 of the Articles, limitations apply to mandates outside the Company for members of the Board of Directors and the Executive Committee. The following external mandates are subject to these limitations and are therefore presented in the Compensation Report.
155
|
Members of the Board of Directors(1) ______________________________________ Christina Ackermann Verona Pharma, Inc., UK(2) -
Member of the Board
-
Member of the Audit Committee
______________________________________ Lionel Carnot IQONE HEALTHCARE SWITZERLAND SA, Switzerland -
Member of the Board
iSTAR Medical SA, Belgium -
Member of the Board
-
Member of Audit Committee
Priothera Ltd., Ireland -
Member of the Board
-
Member of Audit Committee
______________________________________ Pravin Dugel, M.D. -
|
______________________________________ Martijn Kleijwegt VICO Therapeutics B.V., Netherlands -
Member of the Board
AM-Pharma B.V., Netherlands -
Member of the Board
______________________________________ Geraldine O’Keeffe T-Knife Therapeutics, Germany -
Member of the Board
-
Member of the Audit Committee
______________________________________ Anthony Rosenberg Cullinan Oncology Inc., US(2) -
Board Chair
-
Member of the Compensation Committee
-
Member of the Audit Committee
-
Member of the Board
-
Member of the Audit Committee
Argenx BV, Belgium(2)
|
|
Members of the Executive Committee ______________________________________ Riad Sherif, M.D. Revenio Group Oyi, Finland(2) -
Member of the Board
______________________________________ Sylvia Cheung - |
_________________________________ Páll Ragnar Jóhannesson Sjónarhóll fjárfestingar ehf., Iceland -
Board Chair
|
156

Forward Looking Statements
157
SPECIAL NOTE REGARDINGFORWARD-LOOKING STATEMENTS
This Annual Report contains or may contain forward-looking statements, that involve significant risks and uncertainties. All statements other than statements of historical facts are forward-looking statements. These forward-looking statements include information about our possible or assumed future results of operations or our performance. Words such as “may,” “might,” “will,” “could,” “would,” “should,” “expects,” “intends,” “plans,” “believes,” “anticipates,” “estimates,” “potential,” “continue,” “ongoing,” “targets”, “possible,” “project,” and “predict” and variations of such words and similar expressions are intended to identify the forward-looking statements. Forward-looking statements in this Annual Report may include, for example, statements about:
These forward-looking statements are based on information available as of the date of this Annual Report, and current expectations, forecasts and assumptions, and involve a number of judgments, risks and uncertainties. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date, and we do not undertake any obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, you should not place undue reliance on these forward-looking statements in deciding to invest in our securities.
158
As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. You should refer to the section titled “Item 3.D Risk Factors” of the Annual Report on Form 20-F for the year ended December 31, 2023 filed with the SEC on March 19, 2024 for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Annual Report.
159