UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 OR 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 2, 2024
KINTARA THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
Nevada |
|
001-37823 |
|
99-0360497 |
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer Identification No.) |
|
|
|
|
|
9920 Pacific Heights Blvd, Suite 150 San Diego, CA |
|
|
|
92121 |
(Address of principal executive office) |
|
|
|
(Zip Code) |
Registrant’s telephone number, including area code: (858) 350-4364
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
|
|
☒ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock |
|
KTRA |
|
The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01 Entry into a Material Definitive Agreement.
Agreement and Plan of Merger
On April 2, 2024, Kintara Therapeutics, Inc. (“Kintara”), Kayak Mergeco, Inc., a wholly-owned subsidiary of Kintara incorporated in the State of Delaware (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (“TuHURA”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) pursuant to which Merger Sub will merge with and into TuHURA, with TuHURA surviving the merger and becoming a direct, wholly-owned subsidiary of Kintara (the “Merger”). The Merger is expected to be completed in the third quarter of 2024.
Subject to the terms and conditions of the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (i) each then-outstanding share of TuHURA common stock, par value $0.001 per share (the “TuHURA Common Stock”) (other than any shares held in treasury and Dissenting Shares (as defined in the Merger Agreement)) will be converted into shares of Kintara common stock, par value $0.001 per share (the “Kintara Common Stock”) equal to the Exchange Ratio, as such term is defined in the Merger Agreement and described below, (ii) each then-outstanding TuHURA stock option will be assumed and converted into an option to purchase shares of Kintara Common Stock, subject to certain adjustments as set forth in the Merger Agreement, and (iii) each then-outstanding warrant to purchase shares of TuHURA Common Stock (the “TuHURA Warrants”) will be assumed and converted into and exchangeable for a warrant of like tenor entitling the holder to purchase shares of Kintara Common Stock, subject to certain adjustments as set forth in the Merger Agreement. In addition to the foregoing, the Merger Agreement provides that, at the closing of the Merger, the corporate name of Kintara will be changed to “TuHURA Biosciences, Inc.”
On a pro forma basis, TuHURA equityholders would own approximately 97.15% of the combined company on an “as converted” to Kintara Common Stock basis (or 94.55% of the combined company after giving effect to the issuance of the CVR Shares (as defined below)) and Kintara equityholders as of immediately prior to the Effective Time would own approximately 2.85% of the combined company (or 5.45% of the combined company after giving effect to the issuance of the CVR Shares) (excluding in each such case the effect of out-of-the-money options and warrants of Kintara that will remain outstanding after the Merger). The Exchange Ratio will be equal to the quotient obtained by dividing (a) the Company Merger Shares by (b) the Company Outstanding Shares, as those terms are defined and further described in the Merger Agreement, which has the effect and purpose of determining the number of shares to be issued to TuHURA stockholders (or issuable to TuHURA option and warrant holders in respect of such options and warrants) based on the relative valuations and fully-diluted shares of each of Kintara and TuHURA as of immediately prior to the closing of the Merger. For purposes of calculating the Exchange Ratio, (i) shares of Kintara Common Stock underlying Kintara stock options and warrants outstanding as of immediately prior to the closing of the Merger with an exercise price per share of greater than or equal to $0.20 (subject to adjustment pursuant to the Merger Agreement) will be disregarded, (ii) all shares of Kintara preferred stock, par value $0.001 per share (“Kintara Preferred Stock”) will be deemed outstanding on an “as converted” to Kintara Common Stock basis and (iii) all shares of TuHURA Common Stock underlying TuHURA stock options and warrants will be deemed to be outstanding.
In connection with the Merger, Kintara will seek the approval of its stockholders to, among other things, (a) approve the Merger Agreement and the transactions contemplated thereby, (b) if deemed necessary by the parties, amend Kintara’s articles of incorporation (x) to increase the number of authorized shares of Kintara Common Stock and/or (y) to effect a reverse stock split of Kintara Common Stock, (c) elect the post-closing directors of Kintara as contemplated by the Merger Agreement, (d) approve the reincorporation of Kintara from the State of Delaware to the State of Nevada and (e) approve a new equity compensation plan in a form approved by Kintara and TuHURA ((a), (b) and (c), collectively, the “Required Kintara Stockholder Proposals” and (a), (b), (c), (d) and (e), collectively, the “Kintara Stockholder Matters”).
Each of Kintara and TuHURA has agreed to customary representations, warranties and covenants in the Merger Agreement, including, among others, covenants relating to (1) obtaining the requisite approval of its respective stockholders, (2) non-solicitation of alternative acquisition proposals, (3) the conduct of its respective business during the period between the signing of the Merger Agreement and the closing of the Merger and (4) Kintara filing with the U.S. Securities and Exchange Commission (the “SEC”) and causing to become effective a registration statement to register the shares of Kintara Common Stock to be issued in connection with the Merger (the “Registration Statement”).
Consummation of the Merger is subject to certain closing conditions, including, among other things, (1) approval by the requisite Kintara stockholders of the Required Kintara Stockholder Proposals, (2) adoption and approval by the requisite TuHURA stockholders of the Merger Agreement and the transactions contemplated thereby, (3) the effectiveness of the Registration Statement, (4) the Parent Closing Net Cash (as defined in the Merger Agreement) being no less than $750,000 if the Effective Time is on or before June 30, 2024; $625,000 if the Effective Time is between July 1, 2024 and July 31, 2024; 500,000 if the Effective Time is between August 1, 2024 and August 31, 2024; or $0 if the Effective Time is on or after September 1, 2024, (5) the holders of at least 50% of TuHURA’s outstanding shares of common stock on an “as converted” basis (which includes the outstanding shares of TuHURA’s common stock and preferred stock) executing Lock-Up Agreements (as defined below), and (6) TuHURA receiving an aggregate amount of cash no less than $20 million from the offering of its convertible notes. Each party’s obligation to consummate the Merger is also subject to other specified customary conditions, including regarding the accuracy of the representations and warranties of the other party, subject to the applicable materiality standard, and the performance in all material respects by the other party of its obligations under the Merger Agreement required to be performed on or prior to the date of the closing of the Merger.
The Merger Agreement contains certain termination rights for both Kintara and TuHURA, including the right to terminate the Merger Agreement in the event of a triggering event tied to an adverse recommendation change or an acquisition proposal. In addition, either Kintara or TuHURA may terminate the Merger Agreement if the Merger is not consummated on or before November 1, 2024 (the “End Date”), provided that the End Date may be extended by either party for up to sixty (60) days in the event that the SEC has not declared effective the Registration Statement by the date which is sixty (60) days prior to the End Date. Upon termination of the Merger Agreement under specified circumstances, Kintara may be required to pay TuHURA a termination fee of $1 million or reimburse TuHURA’s expenses up to a maximum of $750,000, and TuHURA may be required to pay Kintara a termination fee of $1 million or reimburse TuHURA’s expenses up to a maximum of $750,000.
At the Effective Time, the board of directors of Kintara (the “Board”) is expected to consist of five members, four of whom will be designated by TuHURA and one of whom will be designated by Kintara. At the Effective Time, the officers of TuHURA as of immediately prior to the Effective Time will become the officers of Kintara.
Certain Agreements Related to the Merger
Support Agreements
Concurrently with the execution of the Merger Agreement, (i) certain stockholders of TuHURA have entered into support agreements with Kintara and TuHURA to vote all of their shares of capital stock of TuHURA in favor of the adoption and approval of the Merger Agreement and the transactions contemplated thereby (the “TuHURA Support Agreement”) and (ii) officers and directors of Kintara (solely in their respective capacities as Kintara stockholders) have entered into support agreements with Kintara and TuHURA to vote all of their shares of capital stock of Kintara in favor of the Kintara Stockholder Matters and against any alternative acquisition proposals (the “Kintara Support Agreement”).
Lock-Up Agreements
Concurrently with the execution of the Merger Agreement, certain stockholders of TuHURA and Robert Hoffman, the Chief Executive Officer of Kintara, have entered into lock-up agreements (the “Lock-Up Agreement”), pursuant to which, subject to specified exceptions, they have agreed not to transfer their shares of Kintara Common Stock during the 180-day period following the closing of the Merger. In addition, under the Merger Agreement, additional stockholders of TuHURA, representing no less than 50% of TuHURA’s outstanding shares of common stock on an “as converted” basis (which includes the outstanding shares of TuHURA’s common stock and preferred stock) executing Lock-Up Agreements prior to the closing of the Merger.
Contingent Value Rights Agreement
At or prior to the Effective Time, Kintara will enter into a Contingent Value Rights Agreement (the “CVR Agreement”) with Mountain Share Transfer Inc. (“Rights Agent”), pursuant to which Kintara Common Stock holders, Kintara Series C Preferred Stock holders and Kintara Common Stock warrant holders, in each case, as of record as of the close of business on the business day immediately prior to the Effective Time, will receive one contingent value right (each, a “CVR”) for each outstanding share of Kintara Common Stock held by such stockholder (or, in the case of the warrants and holders of Series C Kintara Preferred Stock, each share of Kintara Common Stock for which such warrant is exercisable or which such Series C Kintara Preferred Stock is settable into as of such date). Each CVR shall entitle the holder thereof to receive its portion of 53,897,125 shares of Kintara Common Stock (the “CVR Shares”) if Kintara (i) enrolls a minimum of ten cutaneous metastic breast cancer patients in a study to determine whether a dose of REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (ii) such patients enrolled in the study complete eight weeks of follow-up, in each case, on or before December 31, 2025 (the “Milestone”).
The payment date for the CVR Shares will be within 10 business days after the rights agent receives the CVR Shares as the payment for achievement of the Milestone. In the event that the Milestone is not achieved, holders of the CVRs will not receive any CVR Shares pursuant to the CVR Agreement. There can be no assurances that any holders of CVRs will receive any CVR Shares with respect thereto.
The CVRs are not transferable, except in certain limited circumstances as will be provided in the CVR Agreement, will not be certificated or evidenced by any instrument and will not be listed for trading on any exchange.
The preceding summaries do not purport to be complete and are qualified in their entirety by reference to the Merger Agreement, the form of Kintara Support Agreement, the form of TuHURA Support Agreement, the form of Lock-Up Agreement and the form of Contingent Value Rights Agreement, which are filed as Exhibits 2.1, 10.1, 10.2, 10.3 and 10.4, respectively, and which are incorporated herein by reference. The Merger Agreement has been attached as an exhibit to this Current Report on Form 8-K to provide investors and securityholders with information regarding its terms. It is not intended to provide any other factual information about TuHURA or Kintara or to modify or supplement any factual disclosures about Kintara in its public reports filed with the SEC. The Merger Agreement includes representations, warranties and covenants of TuHURA, Kintara and Merger Sub made solely for the purpose of the Merger Agreement and solely for the benefit of the parties thereto in connection with the negotiated terms of the Merger Agreement. Investors should not rely on the representations, warranties and covenants in the Merger Agreement or any descriptions thereof as characterizations of the actual state of facts or conditions of TuHURA, Kintara or any of their respective affiliates. Moreover, certain of those representations and warranties may not be accurate or complete as of any specified date, may be subject to a contractual standard of materiality different from those generally applicable to SEC filings or may have been used for purposes of allocating risk among the parties to the Merger Agreement, rather than establishing matters of fact.
Item 5.02. Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
Executive Bonus
On April 2, 2024, the Board approved a one-time special bonus to Mr. Hoffman in the amount of $327,030 for his service as Kintara’s Chief Executive Officer.
Director Fees
As previously disclosed, on November 20, 2023, the Board agreed to defer payments of fees earned by directors for serving on the Board. On April 2, 2024, the Board agreed to (i) resume the payment of fees earned by directors for serving on the Board and (ii) pay an aggregate of $93,000 in accrued fees to such directors.
Item 7.01. Regulation FD Disclosure.
On April 3, 2024 Kintara and TuHURA issued a joint press release announcing the execution of the Merger Agreement. Following the publication of the press release, Kintara and TuHURA will host a joint conference call at 8:30 a.m. (Eastern Time) on April 3, 2024, via webcast, to discuss the proposed Merger. In addition, Kintara and TuHURA provided supplemental information regarding the Merger in a joint investor presentation. Copies of the press release, the conference call transcript and the investor presentation are attached hereto as Exhibit 99.1, Exhibit 99.2, and Exhibit 99.3, respectively. The press release, conference call transcript, the investor presentation, and the information set forth therein shall not be deemed to be filed for purposes of Section 18 of the Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise be subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act.
Item 8.01. Other Events.
In connection with the proposed Merger, TuHURA has a $31 million subscribed financing of convertible notes. Pursuant to their terms, the convertible notes will automatically convert into shares of TuHURA common stock prior to the Effective Time and be exchanged for Kintara Common Stock pursuant to the Exchange Ratio.
Forward-Looking Statements
This Current Report on Form 8-K and the press release attached hereto as Exhibit 99.1 contain forward-looking statements based upon Kintara’s and TuHURA’s current expectations. This communication contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are identified by terminology such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,” “project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. These statements are only predictions. Kintara and TuHURA have based these forward-looking statements largely on their then-current expectations and projections about future events, as well as the beliefs and assumptions of management. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond each of Kintara’s and TuHURA’s control, and actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to: (i) the risk that the conditions to the closing or consummation of the proposed Merger are not satisfied, including the failure to obtain stockholder approval for the proposed Merger; (ii) uncertainties as to the timing of the consummation of the proposed Merger and the ability of each of Kintara and TuHURA to consummate the transactions contemplated by the proposed Merger; (iii) risks related to Kintara’s and TuHURA’s ability to correctly estimate their respective operating expenses and expenses associated with the proposed Merger, as applicable, as well as uncertainties regarding the impact any delay in the closing would have on the anticipated cash resources of the resulting combined company upon closing and other events and unanticipated spending and costs that could reduce the combined company’s cash resources; (iv) the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the proposed Merger by either Kintara or TuHURA; (v) the effect of the announcement or pendency of the proposed Merger on Kintara’s or TuHURA’s business relationships, operating results and business generally; (vi) costs related to the proposed Merger; (vii) the outcome of any legal proceedings that may be instituted against Kintara, TuHURA, or any of their respective directors or officers related to the Merger Agreement or the transactions contemplated thereby; (vii) the ability of Kintara or TuHURA to protect their respective intellectual property rights; (viii) competitive responses to the proposed Merger; (ix) unexpected costs, charges or expenses resulting from the proposed Merger; (x) whether the combined business of TuHURA and Kintara will be successful; (xi) legislative, regulatory, political and economic developments; and (xii) additional risks described in the “Risk Factors” section of Kintara’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023 filed with the SEC. Additional assumptions, risks and uncertainties are described in detail in Kintara’s registration statements, reports and other filings with the SEC, which are available on Kintara’s website, and at www.sec.gov. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Neither Kintara nor TuHURA can assure you that the events and circumstances reflected in the forward-looking statements will be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. The forward-looking statements made in this communication relate only to events as of the date on which the statements are made. Except as required by applicable law or regulation, Kintara and TuHURA undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made or to reflect the occurrence of unanticipated events. Investors should not assume that any lack of update to a previously issued “forward-looking statement” constitutes a reaffirmation of that statement.
Additional Information about the Proposed Merger and Where to Find It
This Current Report on Form 8-K does not constitute an offer to buy or sell or the solicitation of an offer to buy or sell any securities or a solicitation of any vote or approval. This Current Report on Form 8-K relates to the proposed Merger of Kintara and TuHURA. In connection with the proposed Merger, Kintara will file a Registration Statement on Form S-4, which will include a document that serves as a prospectus and proxy statement of Kintara (the “proxy statement/prospectus”), and Kintara will file other documents regarding the proposed Merger with the SEC. No offering of securities shall be made, except by means of a prospectus meeting the requirements of Section 10 of the Securities Act. INVESTORS AND SECURITY HOLDERS ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS AND OTHER RELEVANT DOCUMENTS FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY, WHEN THEY BECOME AVAILABLE, BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION THAT STOCKHOLDERS SHOULD CONSIDER BEFORE MAKING ANY DECISION REGARDING THE PROPOSED MERGER. A definitive proxy statement/prospectus will be sent to Kintara’s stockholders. Investors and security holders will be able to obtain these documents (when available) free of charge from the SEC’s website at www.sec.gov. In addition, investors and stockholders should note that Kintara communicates with investors and the public using its website (www.kintara.com), the investor relations website (https://www.kintara.com/investors) where anyone will be able to obtain free copies of the proxy statement/prospectus and other documents filed by Kintara with the SEC, and stockholders are urged to read the proxy statement/prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the proposed Merger.
Participants in the Solicitation
Kintara, TuHURA and their respective directors and executive officers and other members of management and employees and certain of their respective significant stockholders may be deemed to be participants in the solicitation of proxies from Kintara and TuHURA stockholders in respect of the proposed Merger. Information about Kintara’s directors and executive officers is available in Kintara’s proxy statement, which was filed with the SEC on September 11, 2023 for the 2023 Annual Meeting of Stockholders, Kintara’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023, which was filed with the SEC on September 18, 2023. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the proxy solicitation and a description of their direct and indirect interests, by security holding or otherwise, will be contained in the proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the proposed Merger when they become available. Investors should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these documents from the SEC and Kintara as indicated above.
No Offer or Solicitation
This Current Report on Form 8-K shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits:
Exhibit No. |
|
Description |
|
|
|
2.1* |
|
|
10.1 |
|
|
10.2 |
|
|
10.3 |
|
|
10.4 |
|
|
99.1 |
|
Press release of Kintara Therapeutics, Inc. issued April 3, 2024 |
99.2 |
|
|
99.3 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
*Schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. Kintara hereby undertakes to furnish copies of any of the omitted schedules upon request by the SEC.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
KINTARA THERAPEUTICS, INC. |
||
|
|
|
||
Date: April 3, 2024 |
|
By: |
|
/s/ Robert E. Hoffman |
|
|
|
|
Name: Robert E. Hoffman |
|
|
|
|
Title: Chief Executive Officer |
Exhibit 2.1
Execution Version
AGREEMENT AND PLAN OF MERGER
by and among
KINTARA THERAPEUTICS, INC.,
KAYAK MERGECO, INC.
and
TUHURA BIOSCIENCES, INC.
Dated as of April 2, 2024
TABLE OF CONTENTS
Page
ARTICLE I CERTAIN GOVERNANCE MATTERS |
3 |
Section 1.1 Parent Matters |
3 |
Section 1.2 Surviving Company Matters |
3 |
ARTICLE II THE MERGER |
4 |
Section 2.1 Incorporation of Merger Sub |
4 |
Section 2.2 The Merger |
4 |
Section 2.3 Closing |
4 |
Section 2.4 Effective Time |
4 |
Section 2.5 Effects of the Merger |
4 |
ARTICLE III EFFECT ON THE CAPITAL STOCK OF THE CONSTITUENT COMPANIES; EXCHANGE OF CERTIFICATES |
5 |
Section 3.1 Conversion of Capital Stock |
5 |
Section 3.2 Treatment of Options and Warrants |
7 |
Section 3.3 Exchange and Payment |
8 |
Section 3.4 Withholding Rights |
10 |
Section 3.5 Dissenters Rights |
10 |
Section 3.6 Contingent Value Right |
10 |
Section 3.7 Second Merger |
11 |
ARTICLE IV REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
11 |
Section 4.1 Organization, Standing and Power |
12 |
Section 4.2 Capital Stock |
12 |
Section 4.3 Subsidiaries |
14 |
Section 4.4 Authority |
14 |
Section 4.5 No Conflict; Consents and Approvals |
15 |
Section 4.6 Financial Statements |
16 |
Section 4.7 No Undisclosed Liabilities |
17 |
Section 4.8 Absence of Certain Changes or Events |
17 |
Section 4.9 Litigation |
18 |
Section 4.10 Compliance with Laws |
18 |
Section 4.11 Health Care Regulatory Matters |
18 |
Section 4.12 Benefit Plans |
21 |
Section 4.13 Labor and Employment Matters |
23 |
Section 4.14 Environmental Matters |
25 |
Section 4.15 Taxes |
26 |
Section 4.16 Contracts |
28 |
Section 4.17 Insurance |
29 |
-i-
Section 4.18 Properties |
29 |
Section 4.19 Intellectual Property |
30 |
Section 4.20 Takeover Law |
32 |
Section 4.21 No Rights Plan |
32 |
Section 4.22 Related Party Transactions |
32 |
Section 4.23 Certain Payments |
32 |
Section 4.24 Brokers |
33 |
Section 4.25 No Other Representations and Warranties |
33 |
ARTICLE V REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB |
33 |
Section 5.1 Organization, Standing and Power |
33 |
Section 5.2 Capital Stock |
34 |
Section 5.3 Subsidiaries |
35 |
Section 5.4 Authority |
36 |
Section 5.5 No Conflict; Consents and Approvals |
36 |
Section 5.6 SEC Reports; Financial Statements |
37 |
Section 5.7 No Undisclosed Liabilities |
39 |
Section 5.8 Absence of Certain Changes or Events |
39 |
Section 5.9 Litigation |
40 |
Section 5.10 Compliance with Laws |
40 |
Section 5.11 Health Care Regulatory Matters |
40 |
Section 5.12 Benefit Plans |
42 |
Section 5.13 Labor and Employment Matters |
44 |
Section 5.14 Environmental Matters |
46 |
Section 5.15 Taxes |
47 |
Section 5.16 Contracts |
49 |
Section 5.17 Insurance |
49 |
Section 5.18 Properties |
50 |
Section 5.19 Intellectual Property |
50 |
Section 5.20 Related Party Transactions |
52 |
Section 5.21 Certain Payments |
52 |
Section 5.22 Brokers |
52 |
Section 5.23 Opinion of Financial Advisor |
52 |
Section 5.24 Merger Sub |
53 |
Section 5.25 No Other Representations or Warranties |
53 |
ARTICLE VI COVENANTS |
53 |
Section 6.1 Operation of Parent’s Business |
53 |
Section 6.2 Operation of the Company’s Business |
55 |
Section 6.3 Access and Investigation |
57 |
Section 6.4 No Solicitation |
58 |
Section 6.5 Notification of Certain Matters |
59 |
Section 6.6 Registration Statement; Proxy Statement |
60 |
Section 6.7 Company Stockholder Written Consent |
62 |
-ii-
Section 6.8 Stockholders’ Meeting |
64 |
Section 6.9 Efforts; Transaction Litigation |
66 |
Section 6.10 Indemnification, Exculpation and Insurance |
67 |
Section 6.11 Disclosure |
68 |
Section 6.12 Listing |
69 |
Section 6.13 Section 16 Matters |
69 |
Section 6.14 Employee Matters |
69 |
Section 6.15 Takeover Law |
70 |
Section 6.16 Tax Matters |
70 |
Section 6.17 Calculation of the Exchange Ratio |
71 |
Section 6.18 Obligations of Merger Sub |
72 |
Section 6.19 Officers and Directors |
72 |
Section 6.20 Termination of Certain Agreements and Rights |
72 |
Section 6.21 Allocation Certificate; Net Cash Schedule |
72 |
Section 6.22 Parent SEC Documents |
73 |
Section 6.23 Legends |
73 |
Section 6.24 Reincorporation Covenants |
73 |
ARTICLE VII CONDITIONS PRECEDENT |
73 |
Section 7.1 Conditions Precedent to Each Party’s Obligation to Effect the Merger |
73 |
Section 7.2 Additional Conditions Precedent to Obligations of Parent and Merger Sub |
74 |
Section 7.3 Additional Conditions Precedent to Obligation of the Company |
76 |
ARTICLE VIII TERMINATION |
77 |
Section 8.1 Termination |
77 |
Section 8.2 Effect of Termination |
79 |
Section 8.3 Expenses; Termination Fees |
79 |
ARTICLE IX GENERAL PROVISIONS |
82 |
Section 9.1 Non-survival of Representations and Warranties |
82 |
Section 9.2 Amendment or Supplement |
82 |
Section 9.3 Waiver |
82 |
Section 9.4 Notices |
82 |
Section 9.5 Certain Definitions |
83 |
Section 9.6 Interpretation |
89 |
Section 9.7 Entire Agreement |
89 |
Section 9.8 No Third Party Beneficiaries |
89 |
Section 9.9 Governing Law |
90 |
Section 9.10 Submission to Jurisdiction |
90 |
Section 9.11 Assignment; Successors |
90 |
Section 9.12 Other Remedies; Specific Performance |
91 |
Section 9.13 Currency |
91 |
-iii-
Section 9.14 Further Assurances |
91 |
Section 9.15 Severability |
91 |
Section 9.16 Waiver of Jury Trial |
91 |
Section 9.17 Counterparts |
91 |
Section 9.18 Facsimile or .pdf Signature |
92 |
Section 9.19 No Presumption Against Drafting Party |
92 |
EXHIBITS
Exhibit A-1 Form of Parent Support Agreement
Exhibit A-2 Form of Company Support Agreement
Exhibit B Form of Lock-Up Agreement
Exhibit C Illustrative Calculation of Exchange Ratio
Exhibit D Form of CVR Agreement
-iv-
INDEX OF DEFINED TERMS
Definition Section
2024 Incentive Plan |
6.8(a) |
AAA |
6.18(e) |
Acceptable Confidentiality Agreement |
9.5(a) |
Accounting Firm |
6.18(e) |
Acquisition Inquiry |
9.5(b) |
Acquisition Proposal |
9.5(c) |
Acquisition Transaction |
9.5(d) |
Action |
4.9 |
Affiliate |
9.5(e) |
Aggregate Valuation |
3.1(a)(i) |
Agreement |
Preamble |
Allocation Certificate |
6.22 |
Anticipated Closing Date |
9.5(f) |
Assumed Option |
3.2(a) |
Book-Entry Shares |
3.3(d) |
Business Day |
9.5(g) |
Cash and Cash Equivalents |
9.5(h) |
Closing |
2.3 |
Closing Date |
2.3 |
COBRA |
4.12(c)(iv) |
Code |
Recitals |
Committed Funds |
3.1(a)(v) |
Company |
Preamble |
Company Allocation Percentage |
3.1(a)(ii) |
Company Audited S-4 Financial Statements |
6.6(e) |
Company Balance Sheet |
9.5(i) |
Company Board |
4.4(b) |
Company Board Adverse Recommendation Change |
6.7(d) |
Company Board Recommendation |
6.7(c) |
Company Bylaws |
4.1(b) |
Company Capitalization Representations |
9.5(i) |
Company Charter |
4.1(b) |
Company Common Stock |
Recitals |
Company Contract |
9.5(k) |
Company Convertible Notes |
3.1(a)(iv) |
Company Disclosure Letter |
Article IV |
Company Equity Plan |
3.2(a) |
Company Financial Statements |
4.6(a) |
Company Fundamental Representations |
9.5(j) |
Company Interim S-4 Financial Statements |
6.6(e) |
Company Merger Shares |
3.1(a)(iii) |
Company Option |
3.2(a) |
v
Company Outstanding Shares |
Section 3.1(a)(iv) |
Company Owned IP |
9.5(k), 9.5(t) |
Company Plans |
4.12(a) |
Company Preferred Stock |
4.2(a) |
Company Products |
4.11(c) |
Company Real Estate Leases |
4.18(c) |
Company Registered IP |
4.19(a) |
Company Required S-4 Information |
6.6(d) |
Company S-4 Financial Statements |
6.6(e) |
Company Stock Awards |
4.2(b) |
Company Stockholder Approval |
Recitals |
Company Stockholder Written Consent |
6.7(a) |
Company Termination Fee |
8.3(b) |
Company Triggering Event |
9.5(l) |
Company Valuation |
3.1(a)(v) |
Company Warrant |
3.2(b) |
Confidential Agreement |
9.5(m) |
Contract |
4.5(a)(iii) |
control |
9.5(n) |
Covered Person |
5.2(d) |
CVR |
3.6(a) |
CVR Agreement |
3.6(a) |
CVR Distribution |
3.6(a) |
D&O Indemnified Parties |
6.10(a) |
DGCL |
Recitals |
Dispute Notice |
6.18(b) |
Disqualifying Event |
5.2(d) |
Dissenting Shares |
3.5 |
Effective Time |
2.4 |
End Date |
8.1(b) |
Environmental Law |
4.14(b) |
ERISA |
4.12(a) |
Exchange Act |
4.5(b) |
Exchange Agent |
3.3(a) |
Exchange Fund |
3.3(a) |
Exchange Ratio |
3.1(a) |
Exchange Ratio Statement |
6.18(a) |
Excluded Shares |
3.1(c) |
FDA |
4.11(c) |
FDA Ethics Policy |
4.11(i) |
FDCA |
4.11(d) |
Form S-4 |
6.6(a) |
GAAP |
4.6(a) |
Governmental Entity |
4.5(b) |
Hazardous Substance |
4.14(c) |
Health Care Laws |
9.5(o) |
-vi-
Intellectual Property |
9.5(p) |
Intended Tax Treatment |
Recitals |
IRS |
4.12(a) |
IT Systems |
4.19(g) |
Key Employee |
9.5(q) |
knowledge |
9.5(r) |
Law |
4.5(a)(iv) |
Liability |
4.7 |
Liens |
4.5(a)(i) |
Lock-Up Agreements |
Recitals |
Material Adverse Effect |
4.1(a) |
Material Contracts |
4.16(a) |
Measurement Date |
5.2(a) |
Merger |
Recitals |
Merger Consideration |
3.1(a) |
Merger Filings |
2.4 |
Merger Sub |
Preamble |
Nasdaq |
9.5(s) |
Nasdaq Listing Application |
6.12 |
Net Cash Calculation |
6.8 |
Net Cash Schedule |
6.8 |
Notice Period |
6.7(d) |
Ordinary Course Agreement |
4.15(g) |
Ordinary Course of Business |
9.5(u) |
Parent |
Preamble |
Parent Allocation Percentage |
3.1(a)(vi) |
Parent Board |
5.4(b) |
Parent Board Adverse Recommendation Change |
6.8(c) |
Parent Board Recommendation |
6.8(b) |
Parent Bylaws |
1.1(b) |
Parent Capitalization Representations |
9.5(w) |
Parent Charter |
1.1(a) |
Parent Closing Cash |
9.5(v) |
Parent Common Stock |
Recitals |
Parent Disclosure Letter |
Article V |
Parent Fundament Representations |
9.5(y) |
Parent IT Systems |
5.19(d) |
Parent Material Adverse Effect |
5.1(a) |
Parent Material Contracts |
5.16(a) |
Parent Notice Period |
6.8(c) |
Parent Options |
3.1(a)(vii) |
Parent Outstanding Shares |
3.1(a)(vii) |
Parent Owned IP |
9.5(w) |
Parent Plans |
5.12(a) |
Parent Preferred Stock |
5.2(a) |
Parent Products |
5.11(c) |
-vii-
Parent Real Estate Leases |
5.18(c) |
Parent Registered IP |
5.19(a) |
Parent RSUs |
3.1(a)(vii) |
Parent SEC Documents |
5.6(a) |
Parent Stockholder Approval |
9.5(x) |
Parent Stockholder Matters |
6.8(a) |
Parent Stockholder Meeting |
6.8(a) |
Parent Termination Fee |
8.3(f) |
Parent Triggering Event |
9.5(y) |
Parent Valuation |
3.1(a)(viii) |
Parent Warrants |
3.1(a)(vii) |
PBGC |
4.12(c)(iii) |
Pension Plan |
4.12(b) |
Permits |
4.1 |
Permitted Alternative Agreement |
9.5(z) |
Permitted Liens |
4.18(a) |
Person |
9.5(aa) |
Personal Information |
4.19(h) |
Post-Closing Parent Shares |
3.1(a)(ix) |
Pre-Closing Period |
6.1(a) |
Privacy Laws |
4.19(h) |
Proxy Statement |
6.6(a) |
Registration Statement |
6.6(a) |
Reincorporation |
1.1(a) |
Replacement Warrant |
3.2(b) |
Representative |
9.5(bb) |
Required Parent Stockholder Proposals |
6.8(a) |
Response Date |
6.18(b) |
Rights Agent |
3.6(b) |
Safety Notices |
4.11(g) |
Sarbanes-Oxley Act |
5.6(a) |
SEC |
9.5(cc) |
SEC Documents |
6.23 |
Securities Act |
4.5(b) |
Stockholder Notice |
6.7(b) |
Subsequent Transaction |
9.5(dd) |
Subsidiary |
9.5(ee) |
Superior Offer |
9.5(ff) |
Surviving Company |
2.2 |
Takeover Laws |
4.2 |
Tax Action |
4.15(d) |
Tax Return |
9.5(gg) |
Taxes |
9.5(hh) |
Trade Secrets |
9.5(p) |
Transaction Expenses |
9.5(ii) |
Transaction Litigation |
6.9(b) |
-viii-
WARN Act |
4.13(d) |
-ix-
AGREEMENT AND PLAN OF MERGER
THIS AGREEMENT AND PLAN OF MERGER (this “Agreement”), dated as of April 2, 2024, by and among Kintara Therapeutics, Inc., a Nevada corporation (which shall reincorporate as a Delaware corporation in connection with the consummation of the transactions contemplated hereby) (“Parent”), Kayak Mergeco, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (the “Company”).
RECITALS
WHEREAS, prior to the consummation of the Merger (as defined below), Parent shall continue out of the State of Nevada and into the State of Delaware so as to re-domicile as and become a Delaware corporation;
WHEREAS, Parent and the Company intend to effect a merger of Merger Sub with and into the Company (the “Merger”) in accordance with this Agreement and the General Corporation Law of the State of Delaware (the “DGCL”). Upon consummation of the Merger, Merger Sub will cease to exist and the Company will become a wholly owned subsidiary of Parent;
WHEREAS, for United States federal income tax purposes, it is intended that (i) the Merger (together with the Second Merger (as defined below) if the Second Merger is required by Section 3.7) shall qualify as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”), and (ii) the reincorporation of Parent from Nevada to Delaware shall be treated as a separate “reorganization” of Parent under Section 368(a)(1)(F) of the Code (the “Intended Tax Treatment“) and (iii) this Agreement shall constitute a “plan of reorganization” within the meaning of Treasury Regulations Sections 1.368-2(g) and 1.368-3(a) with respect to each reorganization;
WHEREAS, the Board of Directors of the Company has deemed it advisable and in the best interests of the Company and its stockholders that the Company engage in the Merger and the transactions contemplated by this Agreement, including the Reincorporation (as defined below) and, if required by Section 3.7, the Second Merger;
WHEREAS, the Board of Directors of the Company has unanimously approved this Agreement and the Merger, with the Company continuing as the Surviving Company (as defined below), after the Effective Time (as defined below), pursuant to which each share of common stock, par value $0.001 per share, of the Company (the “Company Common Stock”) shall be converted into the right to receive a number of shares of common stock, par value $0.001 per share, of Parent (the “Parent Common Stock”) equal to the Exchange Ratio (as defined below), upon the terms and subject to the conditions set forth in this Agreement;
WHEREAS, Merger Sub is a newly incorporated Delaware corporation that is wholly owned by Parent, and has been formed for the sole purpose of effecting the Merger;
WHEREAS, the respective Boards of Directors of Parent and Merger Sub have each unanimously approved this Agreement and the Merger; WHEREAS, Parent, Merger Sub and the Company each desire to make certain representations, warranties, covenants and agreements in connection with the Merger and also to prescribe certain conditions to the Merger as specified herein;
WHEREAS, concurrently with the execution and delivery of this Agreement and as a condition and inducement to the Company’s willingness to enter into this Agreement, the officers and directors of Parent have entered into Parent Support Agreements, dated as of the date of this Agreement, in the form attached hereto as Exhibit A-1, pursuant to which such holders have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of capital stock of Parent in favor of the Parent Stockholder Matters;
WHEREAS, concurrently with the execution and delivery of this Agreement and as a condition and inducement to Parent’s willingness to enter into this Agreement, the officers, directors and stockholders of the Company listed on Section A-1 of the Company Disclosure Letter which represents at least fifty percent (50%) of the outstanding shares of Company Common Stock on an “as-converted” basis (which includes the outstanding shares of Company Common Stock and Company Preferred Stock), have entered into Company Support Agreements, dated as of the date of this Agreement, in the form attached hereto as Exhibit A-2, pursuant to which such holders have, subject to the terms and conditions set forth therein, agreed to vote all of their shares of capital stock of the Company in favor of the adoption of this Agreement and the transactions contemplated thereby;
WHEREAS, concurrently with the execution and delivery of this Agreement and as a condition and inducement to each of Parent and the Company’s willingness to enter into this Agreement, the chief executive officer of Parent, solely in his capacity as a stockholder of Parent, and all of the officers, all of the directors and the stockholders of the of the Company listed on Section A-2 of the Company Disclosure Letter, each solely in their capacity as stockholders of the Company, will execute lock-up agreements in the form attached hereto as Exhibit B (the “Lock-Up Agreements”); and
WHEREAS, no later than the second (2nd) Business Day after the Registration Statement is declared effective under the Securities Act, the holders of shares of capital stock of the Company sufficient to adopt and approve this Agreement and the transactions contemplated hereby as required under the DGCL and the Company’s Certificate of Incorporation and Bylaws, including (i) the holders of at least a majority of the outstanding shares of Company Common Stock, (ii) the holders of at least a majority of the outstanding shares of Company Preferred Stock, voting on an aggregate basis and (iii) the holders of at least a majority of the outstanding shares of each series of Company Preferred Stock, voting as each individual series, will execute and deliver an action by written consent adopting this Agreement and approving the transactions contemplated hereby, in form and substance reasonably acceptable to Parent (the “Company Stockholder Approval”).
AGREEMENT
NOW, THEREFORE, in consideration of the premises, and of the representations, warranties, covenants and agreements contained herein, and intending to be legally bound hereby, the parties hereto hereby agree as follows:
-2-
ARTICLE I
CERTAIN GOVERNANCE MATTERS
Section 1.1 Parent Matters.
(a) Parent Reincorporation and Articles of Incorporation. Before the Effective Time and after the receipt of the Parent Stockholder Approval, Parent shall continue out of the State of Nevada and into the State of Delaware so as to re-domicile as and become a Delaware corporation pursuant to the applicable provisions of the Nevada Business Corporation Act and the DGCL (the “Reincorporation”). The Certificate of Incorporation of Parent after the Reincorporation (the “Parent Charter”) shall be in the form and substance reasonably agreed to by Parent and the Company prior to the Effective Time. As of the Effective Time, the Certificate of Incorporation of Parent shall be identical to the Parent Charter immediately prior to the Effective Time, until thereafter amended in accordance with its terms and as provided by applicable Law; provided, however, that at the Effective Time, subject to obtaining the Parent Stockholder Approval, Parent shall file an amendment to its Parent Charter to (i) change the name of Parent to “TuHURA Biosciences, Inc.”, (ii) effect the Nasdaq Reverse Split and/or increase the number of authorized shares of Parent Common Stock (to the extent applicable and necessary) and (iii) make such other changes as are mutually agreeable to Parent and the Company.
(b) Parent Bylaws. As of the Effective Time, the Bylaws of Parent (the “Parent Bylaws”) shall be identical to the Bylaws of Parent immediately prior to the Effective Time, until thereafter amended in accordance with their terms and as provided by applicable Law; provided, however, that the Parent Bylaws shall include such changes as are mutually agreeable to Parent and the Company.
(c) Parent Board of Directors. The parties shall take all action necessary (including, to the extent necessary, procuring the resignation or removal of any directors on the Parent Board immediately prior to the Effective Time) so that, as of the Effective Time, the number of directors that comprise the full Parent Board shall be five (5), with four (4) individuals to be designated by the Company and one (1) individual to be designated by Parent, with such designations to be made by the respective party so entitled no later than twenty (20) days after the date hereof.
(d) Parent Officers. The officers of the Company immediately prior to the Effective Time shall be the officers of Parent immediately after the Effective Time.
Section 1.2 Surviving Company Matters.
(a) Surviving Company Certificate of Incorporation. At the Effective Time, the Certificate of Incorporation of the Surviving Company shall be amended to read in its entirety as the Certificate of Incorporation of Merger Sub (except that references to the name of Merger Sub shall be replaced by references to the name of the Surviving Company), until thereafter amended in accordance with applicable Law.
(b) Surviving Company Bylaws. At the Effective Time, the Bylaws of the Surviving Company shall be amended to read in their entirety as the Bylaws of Merger Sub (except that references to the name of Merger Sub shall be replaced by references to the name of the Surviving Company), until thereafter amended in accordance with applicable Law.
-3-
(c) Surviving Company Directors and Officers. The directors and officers of the Company immediately prior to the Effective Time shall the be directors and officers of the Surviving Company immediately after the Effective Time, each to hold office in accordance with the Certificate of Incorporation and Bylaws of the Surviving Company.
ARTICLE II
THE MERGER
Section 2.1 Incorporation of Merger Sub. Parent has caused Merger Sub to be incorporated under the laws of the State of Delaware.
Section 2.2 The Merger. Upon the terms and subject to the conditions set forth in this Agreement and in accordance with the DGCL, at the Effective Time, Merger Sub shall be merged with and into the Company. Following the Merger, the separate corporate existence of Merger Sub shall cease, and the Company shall continue as the surviving corporation in the Merger (the “Surviving Company”) and a wholly owned subsidiary of Parent.
Section 2.3 Closing. The closing of the Merger (the “Closing”) shall take place as promptly as practicable (but in no event later than the second Business Day following the satisfaction or waiver of the last to be satisfied or waived of the conditions set forth in ARTICLE VII, other than those conditions that by their nature are to be satisfied at the Closing, but subject to the satisfaction or waiver of each of such conditions), or at such other date, time or place as agreed to in writing by Parent and the Company, remotely by electronic exchange of documents. The date on which the Closing occurs is referred to in this Agreement as the “Closing Date.”
Section 2.4 Effective Time. Upon the terms and subject to the provisions of this Agreement, at the Closing, the parties shall cause a certificate of merger meeting the applicable requirements of the DGCL (the “Merger Filing”) relating to the Merger to be properly executed and filed with the Secretary of State of the State of Delaware in accordance with the terms and conditions of the DGCL and in such form as is reasonably satisfactory to both Parent and the Company. The Merger shall become effective at the later of the times of acceptance of the Merger Filing with the Secretary of State of the State of Delaware in accordance with the DGCL or at such later time which the parties hereto shall have agreed and designated in the Merger Filing as the effective time of the Merger (the “Effective Time” ).
Section 2.5 Effects of the Merger. At and after the Effective Time, the Merger shall have the effects set forth in this Agreement and in the relevant provisions of the DGCL. Without limiting the generality of the foregoing, and subject thereto, at the Effective Time, all the property, rights, privileges, powers and franchises of the Company and Merger Sub shall vest in the Surviving Company, and all debts, liabilities and duties of the Company and Merger Sub shall become the debts, liabilities and duties of the Surviving Company.
-4-
ARTICLE III
EFFECT ON THE CAPITAL STOCK OF THE CONSTITUENT COMPANIES; EXCHANGE OF CERTIFICATES
Section 3.1 Conversion of Capital Stock. At the Effective Time, by virtue of the Merger and without any action on the part of Parent, Merger Sub, the Company or the holders of any shares of capital stock of the Parent, Merger Sub or the Company:
(a) Each share of Company Common Stock issued and outstanding immediately prior to the Effective Time (other than any Excluded Shares or Dissenting Shares) shall thereupon be converted into and become exchangeable for a number of shares of Parent Common Stock equal to the Exchange Ratio (the “Merger Consideration”). As of the Effective Time, all such shares of Company Common Stock shall no longer be outstanding and shall automatically be cancelled and shall cease to exist, and shall thereafter only represent the right to receive the Merger Consideration, any dividends or other distributions payable pursuant to Section 3.3(c), without interest. For the avoidance of doubt, as of the Effective Time, no shares of the capital stock of the Company shall be outstanding other than the Company Common Stock. For purposes of this Agreement, the “Exchange Ratio” shall mean, subject to Section 3.1(e), the ratio (rounded to four decimal places) equal to the quotient obtained by dividing (a) the Company Merger Shares by (b) the Company Outstanding Shares, in which:
(i) “Aggregate Valuation” means the sum of (A) the Company Valuation, plus (B) the Parent Valuation.
(ii) “Company Allocation Percentage” means the quotient (rounded to four decimal places) determined by dividing (A) the Company Valuation by (B) the Aggregate Valuation.
(iii) “Company Merger Shares” means the product determined by multiplying (A) the Post-Closing Parent Shares by (B) the Company Allocation Percentage.
(iv) “Company Outstanding Shares” means, subject to Section 3.1(e), the total number of shares of Company Common Stock outstanding immediately prior to the Effective Time expressed on a fully diluted and as-converted-to-Company Common Stock basis, assuming, without limitation or duplication, (A) the exercise of all Company Options (as defined below) and Company Warrants (as defined below) outstanding as of immediately prior to the Effective Time; (B) the conversion of all convertible promissory notes of the Company (the “Company Convertible Notes”) into Company Common Stock; and (C) the issuance of shares of Company Common Stock in respect of all other options, warrants or rights to receive such shares that will be outstanding immediately after the Effective Time.
(v) “Company Valuation” means $190,753,000; provided, however, that if the Company receives (x) less than $30,753,000 (the “Committed Funds”) before the Effective Time in their convertible note financing, the “Company Valuation” will be reduced by the difference between the Committed Funds and those funds actually received by the Company in connection with the convertible note financing or (y) more than the Committed Funds before the Effective Time in their convertible note financing, the “Company Valuation” will be increased by the difference between those funds actually received by the Company in connection with the convertible note financing and the Committed Funds.
-5-
(vi) “Parent Allocation Percentage” the quotient (rounded to four decimal places) determined by dividing (A) the Parent Valuation by (B) the Aggregate Valuation.
(vii) “Parent Outstanding Shares” means, subject to Section 3.1(e), the sum of (i) total number of shares of Parent Common Stock and Parent Preferred Stock (including, for the avoidance of doubt, any shares of Parent Common Stock or Parent Preferred Stock payable as dividends on any Parent Preferred Stock or to be paid on such shares regardless of whether or not such dividend would accrue or be payable prior to or after the Effective Time) outstanding immediately prior to the Effective Time expressed on a fully diluted and as-converted-to-Parent Common Stock basis, and assuming the exercise of options to purchase shares of Parent Common Stock (the “Parent Options”) and warrants to acquire shares of Parent Common Stock (the “Parent Warrants”) and the settlement of restricted stock units that can be settled in shares of Parent Common Stock (the “Parent RSUs”) (using the treasury stock method), and other derivative rights of Parent, plus (ii) the number of shares of Parent Common Stock that is included in the CVR Payment Amount (as defined in the CVR Agreement) as of the date of the CVR Agreement and without regard to whether the Milestone (as defined in the CVR Agreement) is achieved. Notwithstanding any of the foregoing, any Parent Options and Parent Warrants with an exercise price equal to, or greater than, $0.20 per share (subject to Section 3.1(e)) shall not be included in the total number of shares of Parent Common Stock outstanding for purposes of determining the Parent Outstanding Shares.
(viii) “Parent Valuation” means $11,000,000.
(ix) “Post-Closing Parent Shares” means the quotient determined by dividing (A) the Parent Outstanding Shares by (B) the Parent Allocation Percentage.
For the avoidance of doubt and for illustrative purposes only, a sample “Exchange Ratio” calculation is attached hereto as Exhibit C.
(b) At the Effective Time, each share of Parent Common Stock issued and outstanding immediately prior to the Effective Time shall remain outstanding. Immediately following the Effective Time, shares of Parent Common Stock, if any, owned by the Surviving Company shall be surrendered to Parent without payment therefor.
(c) Each share of Company Common Stock held in the treasury of the Company or owned, directly or indirectly, by Parent or Merger Sub immediately prior to the Effective Time (collectively, “Excluded Shares”) shall automatically be cancelled and shall cease to exist, and no consideration shall be delivered in exchange therefor.
(d) Each share of common stock, par value $0.001 per share, of Merger Sub issued and outstanding immediately prior to the Effective Time shall be converted into and become one validly issued, fully paid and nonassessable share of common stock, par value $0.001 per share, of the Surviving Company.
-6-
(e) For purposes of calculating the Company Outstanding Shares and Parent Outstanding Shares, the calculation of the Exchange Ratio shall be adjusted to reflect fully the appropriate effect of any stock split, split-up, reverse stock split (including the Nasdaq Reverse Split to the extent such split has not previously been taken into account in calculating the Exchange Ratio), stock dividend or distribution of securities convertible into Company Common Stock or Parent Common Stock, reorganization, recapitalization, reclassification or other like change with respect to the Company Common Stock or Parent Common Stock having a record date occurring on or after the date of this Agreement and prior to the Effective Time; provided, that nothing in this Section 3.1(e) shall be construed to permit the Company or Parent to take any action with respect to its securities that is prohibited by the terms of this Agreement.
Section 3.2 Treatment of Options and Warrants.
(a) At the Effective Time, each outstanding option (each, a “Company Option”) to purchase shares of Company Common Stock granted under the Company’s Amended and Restated Equity Incentive Plan adopted on January 13, 2019 (the “Company Equity Plan”), whether vested or unvested, that is outstanding immediately prior to the Effective Time shall, at the Effective Time, cease to represent a right to acquire shares of Company Common Stock and shall be assumed and converted, at the Effective Time, into an option to purchase shares of Parent Common Stock (an “Assumed Option”), on the same terms and conditions (including any forfeiture and post-termination exercise provisions, but not taking into account any accelerated vesting provided for in the Company Equity Plan or in the related award document by reason of the transactions contemplated hereby) as were applicable to such Company Option as of immediately prior to the Effective Time; provided, however, that (i) the number of shares of Parent Common Stock subject to each such Assumed Option shall be equal to the number of shares of Company Common Stock subject to each Company Option immediately prior to the Effective Time multiplied by the Exchange Ratio, (ii) the exercise price per share of such Assumed Option shall be the exercise price per share of such Option immediately prior to the Effective Time divided by the Exchange Ratio, and (iii) from and after the Effective Time, (x) references to the “Company” under the Company Equity Plan shall be deemed to refer to Parent, (y) references to the “Board” under the Company Equity Plan shall be deemed to refer to the Parent Board, and (z) the committee that administers the Company Equity Plan shall be a committee established by the Parent Board. In all other material respects, the Assumed Options shall continue to be governed by the terms of the Company Equity Plan from and after the Effective Time, subject to such additional modifications as the Parent Board (or a committee appointed by the Parent Board) deems appropriate to reflect the Merger. Notwithstanding the foregoing, the exercise price and the number of shares of Parent Common Stock subject to each such Assumed Option shall be subject to such adjustments as are necessary in order to avoid the imposition of any additional Taxes under Section 409A of the Code (and regulations issued by the IRS thereunder) or, in the case of any Assumed Option to which Section 422 of the Code applies as of the Effective Time, in order to satisfy the requirements of Section 424(a) of the Code (and regulations issued by the IRS thereunder).
(b) Each warrant entitling the holder to purchase shares of Company Common Stock (each, a “Company Warrant”) issued and outstanding immediately prior to the Effective Time shall thereupon be converted into and become exchangeable for a warrant of like tenor entitling the holder to purchase shares of Parent Common Stock (each, a “Replacement Warrant”)
-7-
that complies with and satisfies the applicable terms and conditions of the applicable warrant agreement between the Company and the holder of the Company Warrant and providing that such Replacement Warrant shall be exercisable for a number of shares of Parent Common Stock equal to the product of (i) the number of shares of Company Common Stock that would have been issuable upon exercise of the Company Warrant and (ii) the Exchange Ratio, and such Replacement Warrant shall have an exercise price per share equal to: (i) the exercise price per share of Company Common Stock otherwise purchasable pursuant to such Company Warrant, divided by (ii) the Exchange Ratio.
(c) Prior to the Effective Time, the Company shall take all action necessary for the adjustment of the Company Options and Company Warrants under this Section 3.2 (including, but not limited to, with respect to the Company Options, the waiver of any provisions providing for accelerated vesting by reason of the transactions contemplated hereby). The Company shall ensure that, as of the Effective Time, no holder of a Company Option (or former holder of a Company Option) or a participant in the Company Equity Plan or any holder of a Company Warrant (or former holder of a Company Warrant) shall have any rights thereunder to acquire, or other rights in respect of, the capital stock of the Company, the Surviving Company or any of their Subsidiaries, or any other equity interest therein.
(d) As soon as practicable following the Effective Time, Parent shall use its reasonable best efforts to file a registration statement on Form S-8 (or any successor form, or if Form S-8 is not available, other appropriate forms) with respect to the shares of Parent Common Stock, on an as-converted basis, subject to the Assumed Options.
Section 3.3 Exchange and Payment.
(a) Promptly after the Effective Time, Parent shall cause a bank or trust company designated by Parent (the “Exchange Agent”) to issue and send to each holder of shares of Company Common Stock, other than with respect to Excluded Shares or Dissenting Shares, (1) that number of whole shares of Parent Common Stock to which such holder of shares of Company Common Stock shall have become entitled pursuant to the provisions of Section 3.1(a) (which shall be in book-entry form unless a physical certificate is requested), and (2) any dividends or other distributions payable pursuant to Section 3.3(c). No interest will be paid or accrued on any unpaid dividends and distributions, if any, payable to holders of shares of Company Common Stock. Each share of Company Common Stock shall be deemed after the Effective Time to represent only the right to receive the Merger Consideration payable in respect thereof, any dividends or other distributions payable pursuant to Section 3.3(c). All book-entry shares of Parent Common Stock, certificates representing shares of Parent Common Stock, dividends, distributions and cash deposited with the Exchange Agent are hereinafter referred to as the “Exchange Fund.”
(b) If payment of the Merger Consideration is to be made to a Person other than the Person in whose name the share of Company Common Stock is registered, it shall be a condition of payment that such share of Company Common Stock shall be properly transferred and that the Person requesting such payment shall have paid any transfer and other Taxes required by reason of the payment of the Merger Consideration to a Person other than the registered holder of such share of Company Common Stock or shall have established to the satisfaction of Parent that such Tax is not applicable.
-8-
(c) Notwithstanding anything in the foregoing to the contrary, other than the CVRs (as defined below), holders of shares of Company Common Stock who are entitled to receive shares of Parent Common Stock under this ARTICLE III shall be paid (A) at the time of payment of such Parent Common Stock by the Exchange Agent under Section 3.3(a), the amount of dividends or other distributions with a record date after the Effective Time theretofore paid with respect to such whole shares of Parent Common Stock, and (B) at the appropriate payment date, the amount of dividends or other distributions with a record date after the Effective Time but prior to the time of such payment by the Exchange Agent under Section 3.3(a) and a payment date subsequent to the time of such payment by the Exchange Agent under Section 3.3(a) payable with respect to such whole shares of Parent Common Stock.
(d) The Merger Consideration, any dividends or other distributions payable pursuant to Section 3.3(c) in accordance with the terms of this ARTICLE III shall be deemed to have been issued and paid in full satisfaction of all rights pertaining to the shares of Company Common Stock. At the Effective Time, the stock transfer books of the Company shall be closed and there shall be no further registration of transfers of the shares of Company Common Stock that were outstanding immediately prior to the Effective Time. If, after the Effective Time, transfer is sought for uncertificated shares of Company Common Stock represented by book entry (“Book-Entry Shares”), such Book-Entry Shares shall be cancelled and exchanged as provided in this ARTICLE III.
(e) Fractional shares of Parent Common Stock otherwise issuable upon consummation of the Merger shall be rounded up or down to the nearest whole share. Any fractional shares of Parent Common Stock a holder of shares of Company Common Stock upon the conversion of shares of Company Common Stock would otherwise be entitled to receive shall be aggregated together first and prior to eliminating fractional shares.
(f) Any portion of the Exchange Fund that remains undistributed or unallocated to the holders of Book-Entry Shares six months after the Effective Time shall be delivered to the Surviving Company, upon demand, and any remaining holders of Book-Entry Shares (except to the extent representing Excluded Shares or Dissenting Shares) shall thereafter look only to the Surviving Company, as general creditors thereof, for payment of the Merger Consideration, any unpaid dividends or other distributions payable pursuant to Section 3.3(c) (subject to abandoned property, escheat or other similar laws), without interest.
(g) None of Parent, the Surviving Company, the Exchange Agent or any other Person shall be liable to any Person in respect of shares of Parent Common Stock, dividends or other distributions with respect thereto properly delivered to a public official pursuant to any applicable abandoned property, escheat or similar Law. If any Book-Entry Shares shall not have been allocated their Merger Consideration prior to two (2) years after the Effective Time (or immediately prior to such earlier date on which the related Merger Consideration (and all dividends or other distributions with respect to shares of Parent Common Stock) would otherwise escheat to or become the property of any Governmental Entity), any such Merger Consideration (and such dividends, distributions and cash) in respect thereof shall, to the extent permitted by applicable Law, become the property of the Surviving Company, free and clear of all claims or interest of any Person previously entitled thereto.
-9-
(h) The Exchange Agent shall invest any cash included in the Exchange Fund as directed by Parent on a daily basis. Any interest and other income resulting from such investments shall be paid to Parent.
Section 3.4 Withholding Rights. Parent, Merger Sub, the Surviving Company and the Exchange Agent shall each be entitled to deduct and withhold, or cause to be deducted and withheld, from any amounts payable under this Agreement or the CVR Agreement such amounts as Parent, Merger Sub, the Surviving Company or the Exchange Agent reasonably determines it is required to deduct and withheld under the Code, or any provision of state, local or foreign Tax Law. To the extent that amounts are so deducted and withheld and are remitted to the applicable Taxing authority, such amounts shall be treated for all purposes of this Agreement as having been paid to the Person in respect of whom such deduction and withholding was made.
Section 3.5 Dissenters Rights. Notwithstanding anything in this Agreement to the contrary, each share of the Company Common Stock (other than Excluded Shares) outstanding immediately prior to the Effective Time and held by a holder who is entitled to demand and has properly demanded appraisal for such shares of the Company Common Stock in accordance with Section 262 of the DGCL (“Dissenting Shares”), shall not be converted into or be exchangeable for the right to receive a portion of the Merger Consideration unless and until such holder fails to perfect or withdraws or otherwise loses such holder’s right to appraisal and payment under the DGCL. If, after the Effective Time, any such holder fails to perfect or withdraws or loses such holder’s right to appraisal, such Dissenting Shares shall thereupon be treated as if they had been converted as of the Effective Time into the right to receive the portion of the Merger Consideration, if any, to which such holder is entitled pursuant to Section 3.1(a), without interest. The Company shall give Parent (a) prompt notice of any demands received by the Company for appraisal of any shares of the Company Common Stock issued and outstanding immediately prior to the Effective Time, attempted written withdrawals of such demands, and any other instruments served pursuant to the DGCL and received by the Company relating to stockholders’ rights to appraisal with respect to the Merger and (b) the opportunity to participate in all negotiations and proceedings with respect to any exercise of such appraisal rights under the DGCL. The Company shall not, except with the prior written consent of Parent, which shall not be unreasonably withheld, conditioned or delayed, voluntarily make any payment with respect to any demands for payment of fair value for capital stock of the Company, offer to settle or settle any such demands or approve any withdrawal of any such demands.
Section 3.6 Contingent Value Right.
(a) Prior to the Effective Time, the Board of Directors of Parent shall declare a distribution (the “CVR Distribution”) to the holders of Parent Common Stock, the holders of Parent Warrants and the holders of Series C Parent Preferred Stock that are entitled to the CVR Distribution, in each case, of record as of immediately prior to the Effective Time of the right to receive, less applicable withholding taxes (or less CVRs with a value, in the determination of Parent, equal to the applicable withholding taxes), one contingent value right (each, a “CVR”) for each outstanding share of Parent Common Stock held by such stockholder as of such date (or, in the case of Parent Warrants and holders of Series C Parent Preferred Stock that are entitled to the CVR Distribution, each share of Parent Common Stock for which such Parent Warrant is exercisable or which such Series C Parent Preferred Stock is convertible into), with each such CVR representing the right to receive contingent payments upon the occurrence of certain events set forth in, and subject to and in accordance with the terms and conditions of, the Contingent Value Rights Agreement in the form attached hereto as Exhibit D (the “CVR Agreement”).
-10-
The record date for the CVR Distribution shall be the close of business on the Business Day immediately prior to the Closing Date (or such other date before the Closing Date so that the CVR Distribution will be made to stockholders, holders of Parent Warrants and holders of Series C Parent Preferred Stock immediately prior to the Effective Time) and the payment date for which shall be three (3) Business Days after the Effective Time; provided that the payment of such distribution may be conditioned upon the occurrence of the Effective Time (and, for the avoidance of doubt, Parent Stockholder Approval).
(b) Parent and a rights agent to be appointed by Parent (the “Rights Agent”) shall, at or prior to the Effective Time, duly authorize, execute and deliver the CVR Agreement, in the form attached hereto as Exhibit D, subject to any reasonable revisions to the CVR Agreement that are requested by such Rights Agent and are reasonably acceptable to Parent and the Company. Parent agrees to pay the fees and expenses of the Rights Agent in connection with this Agreement and as agreed upon in writing and to reimburse the Rights Agent for all reasonable, documented and necessary out-of-pocket- expenses incurred by the Rights Agent in connection with the administration by the Rights Agent of its duties hereunder and thereunder.
Section 3.7 Second Merger. If the demands for appraisal of shares of the Company Common Stock in accordance with Section 262 of the DGCL in connection with the Merger are such that, if such demands were perfected and the related appraisal rights were not withdrawn or lost, the Merger would fail to qualify as a “reorganization” described in Section 368(a)(2)(E) of the Code, Parent shall, as promptly as practical following the Merger, and as part of an integrated transaction with the Merger for federal income tax purposes, cause the Company to be merged with and into a limited liability company directly wholly-owned by Parent that is formed specifically for purposes of such merger and is treated for federal income tax purposes as an entity whose separate existence from Parent is disregarded, with such limited liability company surviving such merger (such merger, the “Second Merger”); provided that the Second Merger will not be required if, prior to the Second Merger, sufficient demands for appraisal fail to be perfected or related appraisal rights are withdrawn or lost so that the Merger would qualify as a “reorganization” described in Section 368(a)(2)(E) of the Code.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
Except as set forth in the corresponding section or subsection of the disclosure letter delivered by the Company to Parent immediately prior to the execution of this Agreement (the “Company Disclosure Letter”), the Company represents and warrants to Parent and Merger Sub as follows:
Section 4.1 Organization, Standing and Power.
-11-
(a) Each of the Company and its Subsidiaries (i) is an entity duly organized, validly existing and in good standing under the Laws of the jurisdiction of its organization, (ii) has all requisite corporate or similar power and authority to own, lease and operate its properties and to carry on its business as now being conducted and (iii) is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the nature of its business or the ownership, leasing or operation of its properties makes such qualification or licensing necessary, except in the case of this clause (iii), where the failure to be so qualified or licensed or in good standing, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect. For purposes of this Agreement, “Material Adverse Effect” means any event, change, circumstance, occurrence, effect or state of facts that (A) is or would reasonably be expected to be materially adverse to the business, assets, liabilities, financial condition, or results of operations of the Company and its Subsidiaries, taken as a whole or (B) materially impairs the ability of the Company to consummate, the Merger or any of the other transactions contemplated by this Agreement; provided, however, that in the case of clause (A) only, Material Adverse Effect shall not include any event, change, circumstance, occurrence, effect or state of facts to the extent resulting from (1) changes or conditions generally affecting the industries in which the Company operates, or the economy or the financial, debt, banking, capital, credit or securities markets, in the United States, including effects on such industries, economy or markets resulting from any regulatory and political conditions or developments in general, (2) the outbreak or escalation of war or acts of terrorism or any natural disasters, acts of God or comparable events, epidemic, pandemic or disease outbreak (including the COVID-19 virus) or any worsening of the foregoing, or any declaration of martial law, quarantine or similar directive, policy or guidance or Law or other action by any Governmental Entity in response thereto, (3) changes in Law or GAAP, or the interpretation or enforcement thereof, (4) the public announcement of this Agreement, or (5) any specific action taken (or omitted to be taken) by the Company that is required by this Agreement or actions or omissions taken with Parent’s consent; provided, that, with respect to clauses (1), (2) and (3), the impact of such event, change, circumstance, occurrence, effect or state of facts is not disproportionately adverse to the Company and its Subsidiaries, taken as a whole, as compared to other participants in the industries in which the Company operates.
(b) The Company has previously made available to Parent true and complete copies of the Company’s Certificate of Incorporation (the “Company Charter”) and Bylaws (the “Company Bylaws”), in each case as amended to the date of this Agreement, and each as so delivered is in full force and effect. The Company is not in violation of any provision of the Company Charter or Company Bylaws.
Section 4.2 Capital Stock.
(a) As of the date of this Agreement, (i) the authorized capital stock of the Company consists of 300,000,000 shares of Company Common Stock and 150,000,000 shares of preferred stock, par value $0.0001 per share (the “Company Preferred Stock”), (ii) 68,074,466 shares of Company Common Stock (excluding treasury shares) are issued and outstanding, (iii) no shares of Company Common Stock are held by the Company in its treasury, (iv) 80,561,243 shares of Company Preferred Stock (excluding treasury shares) are issued and outstanding, of which (a) 33,186,955 are designated Series A Preferred Stock, (b) 22,221,257 are designated Series A-1 Preferred Stock, and (c) 25,153,031 are designated Series B Preferred Stock, (v) no shares of Company Preferred Stock are held by the Company in its treasury, (vi) 20,000,000 shares of Company Common Stock are reserved for issuance pursuant to the Company Equity Plan (of which 19,883,834 shares are subject to outstanding Company Options), (vii) 45,185,556 Company Warrants are issued and outstanding, and (viii) 45,185,556 shares of Company Common Stock were reserved for issuance pursuant to the Company Warrants.
-12-
All outstanding shares of capital stock of the Company are, and all shares reserved for issuance will be, when issued, duly authorized, validly issued, fully paid and nonassessable and not subject to any preemptive rights. Neither the Company nor any of its Subsidiaries has outstanding any bonds, debentures, notes or other obligations having the right to vote (or convertible into, or exchangeable or exercisable for, securities having the right to vote) with the stockholders of the Company or such Subsidiary on any matter. Except as set forth above in this Section 4.2(a), there are no outstanding (A) shares of capital stock or other voting securities or equity interests of the Company, (B) securities of the Company or any of its Subsidiaries convertible into or exchangeable or exercisable for shares of capital stock of the Company or other voting securities or equity interests of the Company or its Subsidiaries, (C) stock appreciation rights, “phantom” stock rights, performance units, interests in or rights to the ownership or earnings of the Company or any of its Subsidiaries or other equity equivalent or equity-based awards or rights, (D) subscriptions, options, warrants, calls, commitments, Contracts or other rights to acquire from the Company or its Subsidiaries, or obligations of the Company or any of its Subsidiaries to issue, any shares of capital stock of the Company or any of its Subsidiaries, voting securities, equity interests or securities convertible into or exchangeable or exercisable for capital stock or other voting securities or equity interests of the Company or its Subsidiaries or rights or interests described in the preceding clause (C), or (E) obligations of the Company or any of its Subsidiaries to repurchase, redeem or otherwise acquire any such securities or to issue, grant, deliver or sell, or cause to be issued, granted, delivered or sold, any such securities. There are no stockholder agreements, voting trusts or other agreements or understandings to which the Company is a party or of which the Company has knowledge with respect to the holding, voting, registration, redemption, repurchase or disposition of, or that restricts the transfer of, any capital stock or other voting securities or equity interests of the Company.
(b) Section 4.2(b) of the Company Disclosure Letter sets forth a true and complete list of all holders, as of the date hereof, of outstanding Company Options, and other similar rights to purchase or receive shares of Company Common Stock or similar rights granted under the Company Equity Plan or otherwise (collectively, “Company Stock Awards”), indicating as applicable, with respect to each Company Stock Award then outstanding, the type of award granted, the number of shares of Company Common Stock subject to such Company Stock Award, the name of the plan under which such Company Stock Award was granted, the date of grant, exercise or purchase price, vesting schedule, payment schedule (if different from the vesting schedule) and expiration thereof, and whether (and to what extent) the vesting of such Company Stock Award will be accelerated or otherwise adjusted in any way or any other terms will be triggered or otherwise adjusted in any way by the consummation of the Merger and the other transactions contemplated by this Agreement or by the termination of employment or engagement or change in position of any holder thereof following or in connection with the Merger. Each Company Option intended to qualify as an “incentive stock option” under Section 422 of the Code so qualifies (without reference to the applicable $100,000 limitation) and the exercise price of each Company Option is no less than the fair market value of a share of Company Common Stock as determined on the date of grant of such Company Option. The Company has made available to Parent a true and complete copy of the Company Equity Plan and the forms of all award agreements evidencing outstanding Company Stock Awards. The Company does not sponsor, maintain or administer any employee or director stock option, stock purchase or equity compensation plan or arrangement other than the Company Equity Plan. The Company is under no obligation to issue shares of Company Common Stock pursuant to any employee or director stock option, stock purchase or equity compensation plan or arrangement other than the Company Equity Plan.
-13-
Section 4.2(b) of the Company Disclosure Letter sets forth the following information with respect to each Company Convertible Note: (i) the name of the holder, (ii) the issue date, (iii) the principal amount, (iv) the interest rate, (v) the maturity date and (vi) the number, class and series of Company Capital Stock into which such Company Convertible Note shall convert in connection with the Closing.
(c) All outstanding Company Common Stock, Company Preferred Stock, Company Options, Company Warrants and Company Convertible Notes and other securities of the Company have been issued and granted in material compliance with (i) the Company Charter and the Company Bylaws and all applicable securities Laws and other applicable Law and (ii) all requirements set forth in applicable Contracts.
Section 4.3 Subsidiaries. Section 4.3 of the Company Disclosure Letter sets forth each Subsidiary of the Company. All of the issued and outstanding shares of capital stock of each Subsidiary of the Company have been duly authorized and are validly issued, fully paid, and non-assessable. The Company holds of record and beneficially all of the outstanding shares of each Subsidiary of the Company, free and clear of any restrictions on transfer (other than restrictions under the Securities Act and state securities Laws). Other than the Subsidiaries listed on Section 4.3 of the Company Disclosure Letter, neither the Company nor any of its Subsidiaries owns, directly or indirectly, any equity, membership interest, partnership interest, joint venture interest, or other equity or voting interest in, or any interest convertible into, exercisable or exchangeable for any of the foregoing, nor is it under any current or prospective obligation to form or participate in, provide funds to, make any loan, capital contribution, guarantee, credit enhancement or other investment in, or assume any liability or obligation of, any Person. No Subsidiary of the Company is in violation of its organizational documents.
Section 4.4 Authority.
(a) The Company has all necessary corporate power and authority to execute, deliver and perform its obligations under this Agreement and to consummate the Merger and the other transactions contemplated hereby. The execution, delivery and performance of this Agreement by the Company and the consummation by the Company of the Merger and the other transactions contemplated hereby have been duly authorized by all necessary corporate action on the part of the Company and no other corporate proceedings on the part of the Company are necessary to approve this Agreement or to consummate the Merger and the other transactions contemplated hereby, subject, in the case of the consummation of the Merger, to the Company Stockholder Approval. This Agreement has been duly executed and delivered by the Company and, assuming the due authorization, execution and delivery by Parent and Merger Sub, constitutes a valid and binding obligation of the Company, enforceable against the Company in accordance with its terms (except to the extent that enforceability may be limited by applicable bankruptcy, insolvency, moratorium, reorganization or similar Laws affecting the enforcement of creditors’ rights generally or by general principles of equity).
-14-
(b) The Board of Directors of the Company (the “Company Board”), at a meeting duly called and held at which all directors of the Company were present, duly and unanimously adopted resolutions (i) determining that the terms of this Agreement, the Merger and the other transactions contemplated hereby are fair to and in the best interests of the Company and its stockholders, (ii) approving and declaring advisable this Agreement and the transactions contemplated hereby, including the Merger, (iii) directing that this Agreement be submitted to the stockholders of the Company for adoption and (iv) resolving to recommend that the Company’s stockholders vote in favor of the adoption of this Agreement and the transactions contemplated by this Agreement, including the Merger, which resolutions have not been subsequently rescinded, modified or withdrawn in any way.
(c) The Company Stockholder Approval is the only vote of the holders of any class or series of the Company’s capital stock or other securities required in connection with the consummation of the Merger and the other transactions contemplated hereby. Other than the Company Stockholder Approval, no vote of the holders of any class or series of the Company’s capital stock or other securities is required in connection with the consummation of any of the transactions contemplated hereby to be consummated by the Company other than the Merger.
Section 4.5 No Conflict; Consents and Approvals.
(a) Subject to the obtaining the Company Stockholder Approval, the execution, delivery and performance of this Agreement by the Company does not, and the consummation of the Merger and the other transactions contemplated hereby and compliance by the Company with the provisions hereof will not, conflict with, or result in any violation or breach of, or default (with or without notice or lapse of time, or both):
(i) result in the creation of any pledge, claim, lien, charge, option, right of first refusal, encumbrance or security interest of any kind or nature whatsoever (including any limitation on voting, sale, transfer or other disposition or exercise of any other attribute of ownership) (collectively, “Liens”) in or upon any of the properties, assets or rights of the Company or any of its Subsidiaries;
(ii) contravene, conflict with or result in a violation of any of the provisions of the Company Charter or Company Bylaws;
(iii) conflict with, or result in the violation or breach of, or default under, or give rise to a right of, or result in, termination, cancellation, modification or acceleration of any obligation or any increased, additional, accelerated or guaranteed rights or entitlements under, or require any consent, waiver or approval of any Person pursuant to, any provisions of, any material bond, debenture, note, mortgage, indenture, guarantee, license, lease, purchase or sale order or other contract, commitment, agreement, instrument, obligation, arrangement, understanding, undertaking, permit, concession or franchise, whether oral or written (each, including all amendments thereto, a “Contract”) to which the Company or any of its Subsidiaries is a party or by which the Company, its Subsidiaries or any of their respective properties or assets may be bound, except in the case of any nonmaterial breach, default, penalty or modification; or
(iv) subject to the governmental filings and other matters referred to in Section 4.5(b), contravene, conflict with or result in a material violation of, any federal, state, local or foreign law (including common law), statute, ordinance, rule, code, regulation, order, judgment, injunction, decree or other legally enforceable requirement (“Law”) applicable to the Company or its Subsidiaries or by which the Company, its Subsidiaries or any of their respective properties or assets may be bound, except as would not reasonably be expected to be material to the Company, its Subsidiaries or any of their respective properties or assets.
-15-
(b) No consent, approval, order or authorization of, or registration, declaration, filing with or notice to, any federal, state, local or foreign government or subdivision thereof or any other governmental, administrative, judicial, arbitral, legislative, executive, regulatory or self-regulatory authority, instrumentality, agency, commission or body (each, a “Governmental Entity”) is required by or with respect to the Company in connection with the execution, delivery and performance of this Agreement by the Company or the consummation by the Company of the Merger and the other transactions contemplated hereby or compliance with the provisions hereof, except for (i) the filing with the SEC of such reports under Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as may be required in connection with this Agreement and the transactions contemplated hereby, (ii) such other filings and reports as may be required pursuant to the applicable requirements of the Securities Act of 1933, as amended (the “Securities Act”), the Exchange Act and any other applicable state or federal securities, takeover and “blue sky” laws, (iii) the filing of the Merger Filing with the Secretary of State of the State of Delaware as required by the DGCL and (iv) such other consents, approvals, orders, authorizations, registrations, declarations, filings or notices the failure of which to be obtained or made, individually or in the aggregate, would not reasonably be expected to be material to the Company, its Subsidiaries or any of their respective properties or assets.
Section 4.6 Financial Statements.
(a) True and complete copies of the audited consolidated balance sheet of the Company as at December 31, 2023 and December 31, 2022, and the related audited statements of income, retained earnings, stockholders’ equity and changes in financial position of the Company and its Subsidiaries, together with all related notes and schedules thereto, accompanied by the reports thereon of the Company’s independent auditors (collectively referred to as the “Company Financial Statements”) are attached hereto as Section 4.6(a) of the Company Disclosure Letter. The Company Financial Statements (i) are correct and complete in all material respects and have been prepared in accordance with the books and records of the Company and its Subsidiaries; (ii) have been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”) applied on a consistent basis throughout the periods indicated (except as may be indicated in the notes thereto) and (iii) fairly present, in all material respects, the financial position, results of operations and cash flows of the Company and its Subsidiaries as at the respective dates thereof and for the respective periods indicated therein, except as otherwise noted therein.
(b) The books of account and financial records of the Company and its Subsidiaries are true and correct and have been prepared and are maintained in accordance with sound accounting practice.
(c) The Company maintains a system of internal accounting controls designed to provide reasonable assurance that: (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of the financial statements of the Company in conformity with GAAP and to maintain accountability of the Company’s and its Subsidiaries’ assets, (iii) access to the Company’s and its Subsidiaries’ assets is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for the Company’s and its Subsidiaries’ assets is compared with the existing assets at regular intervals and appropriate action is taken with respect to any differences.
-16-
The Company maintains internal control over financial reporting that provides reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP.
(d) Since January 1, 2022, there have been no formal internal investigations regarding financial reporting or accounting policies and practices discussed with, reviewed by or initiated at the direction of the chief executive officer or chief financial officer of the Company, the Company Board or any committee thereof. Since January 1, 2022, neither the Company nor its independent auditors have identified (i) any significant deficiency or material weakness in the design or operation of the system of internal accounting controls utilized by the Company, (ii) any fraud, whether or not material, that involves the Company, the Company’s management or other employees who have a role in the preparation of financial statements or the internal accounting controls utilized by the Company or (iii) any claim or allegation regarding any of the foregoing.
Section 4.7 No Undisclosed Liabilities. Neither the Company nor any of its Subsidiaries has any liabilities, indebtedness, obligations or expense of any of any nature, whether accrued, absolute, contingent, matured or otherwise, known or unknown, whether due or to become due and whether or not required to be recorded or reflected on a balance sheet under GAAP (each a “Liability”), except (a) to the extent accrued or reserved against in the Company Financial Statements, (b) for Liabilities incurred in the Ordinary Course of Business since December 31, 2023 that are not material to the Company and its Subsidiaries, taken as a whole, (c) Liabilities incurred in connection with the transactions contemplated hereby or (d) Liabilities which would not, individually or in the aggregate, reasonably be expected to be material the Company or any of its Subsidiaries.
Section 4.8 Absence of Certain Changes or Events. Except as set forth in Section 4.8 of the Company Disclosure Letter, since December 31, 2023 through the date of this Agreement, (i) except in connection with the execution of this Agreement and the consummation of the transactions contemplated hereby, the Company and its Subsidiaries have conducted their respective business only in the Ordinary Course of Business; (ii) there has not been any Material Adverse Effect; and (iii) neither the Company nor any of its Subsidiaries has taken any action or committed or agreed to take any action that would be prohibited by Section 6.2 (without giving effect to Schedule 6.2) if such action were taken on or before the date hereof without the consent of Parent.
Section 4.9 Litigation. There is no action, suit, claim, arbitration, investigation, inquiry, grievance, proceeding (including any civil, criminal, administrative, investigative or appellate proceeding), hearing, audit, examination or investigation commenced, brought, conducted or heard by or before, or otherwise involving, any court or other Governmental Entity or any arbitrator or arbitration panel (each, an “Action”) (or basis therefor) pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, or any of their respective material properties or assets, or any present or former officer, director or employee of the Company or any of its Subsidiaries in such individual’s capacity as such. Neither the Company nor any of its Subsidiaries nor any of their respective properties or assets is subject to any outstanding judgment, order, injunction, rule or decree of any Governmental Entity.
-17-
There is no Action pending or, to the knowledge of the Company, threatened seeking to prevent, hinder, modify, delay or challenge the Merger or any of the other transactions contemplated by this Agreement.
Section 4.10 Compliance with Laws. The Company and each of its Subsidiaries are and have been in compliance in all material respects with all Laws applicable to their businesses, operations, properties or assets, including laws relating to escheat and unclaimed property. None of the Company or any of its Subsidiaries has received, since January 1, 2021, a notice or other written communication alleging or relating to a possible material violation of any Law applicable to their businesses, operations, properties, assets or Company Products (as defined below). The Company and each of its Subsidiaries have in effect all material permits, licenses, variances, exemptions, applications, approvals, clearances, authorizations, registrations, formulary listings, consents, operating certificates, franchises, orders and approvals (collectively, “Permits”) of all Governmental Entities necessary or advisable for them to own, lease or operate their respective properties and assets and to carry on their respective businesses and operations as now conducted, and there has occurred no violation of, default (with or without notice or lapse of time or both) under or event giving to others any right of revocation, nonrenewal, adverse modification or cancellation of, with or without notice or lapse of time or both, any such Permit, nor would any such revocation, nonrenewal, adverse modification or cancellation result from the consummation of the transactions contemplated hereby.
Section 4.11 Health Care Regulatory Matters. Except as set forth in Section 4.11 of the Company Disclosure Letter:
(a) The Company and its Subsidiaries, and to the knowledge of the Company, each of their respective directors, officers, management employees, agents (while acting in such capacity), contract manufacturers, suppliers, and distributors are, and at all times prior hereto were, in material compliance with all Health Care Laws to the extent applicable to the Company, its Subsidiaries or any of their respective products or activities. To the knowledge of the Company, there are no facts or circumstances that reasonably would be expected to give rise to any material liability under any Health Care Laws.
(b) Neither the Company nor any of its Subsidiaries is party to any material corporate integrity agreements, monitoring agreements, consent decrees, settlement orders, or similar agreements with or imposed by any Governmental Entity.
(c) All applications, notifications, submissions, information, claims, reports and statistical analyses, and other data and conclusions derived therefrom, utilized as the basis for or submitted in connection with any and all requests for a Permit from the U.S. Food and Drug Administration (“FDA”) or other Governmental Entity relating to products that are regulated as drugs, medical devices, or other healthcare products under Health Care Laws, including drug and biological candidates, compounds or products being researched, tested, stored, developed, labeled, manufactured, packed and/or distributed by the Company or any of its Subsidiaries (“Company Products”), including, without limitation, investigational new drug applications, when submitted to the FDA or other Governmental Entity were true, complete and correct in all material respects as of the date of submission and any necessary or required updates, changes, corrections or modification to such applications, submissions, information and data have been submitted to the FDA or other Governmental Entity.
-18-
Neither the Company nor any of its Subsidiaries has knowledge of any facts or circumstances that would be reasonably likely to lead the revocation, suspension, limitation, or cancellation of a Permit required under Health Care Laws or of any application for marketing approval currently pending before the FDA or such other Governmental Entity.
(d) All preclinical studies and clinical trials conducted by or, to the knowledge of the Company, on behalf of the Company or any of its Subsidiaries have been, and if still pending are being, conducted in compliance with research protocols and all applicable Health Care Laws, including, but not limited to, the Federal Food, Drug and Cosmetic Act (“FDCA”) and its applicable implementing regulations at 21 C.F.R. Parts 50, 54, 56, 58, 312, 314, 320 and 814. No clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has been conducted using any clinical investigators who have been disqualified. No clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has been terminated or suspended prior to completion, and no clinical investigator that has participated or is participating in, or institutional review board that has or has had jurisdiction over, a clinical trial conducted by or on behalf of the Company or any of its Subsidiaries has placed a clinical hold order on, or otherwise terminated, delayed or suspended, such a clinical trial at a clinical research site based on an actual or alleged lack of safety or efficacy of any Company Product or a failure to conduct such clinical trial in compliance with applicable Health Care Laws.
(e) All manufacturing operations conducted by or, to the knowledge of the Company, for the benefit of the Company or any of its Subsidiaries have been and are being conducted in material compliance with all Permits under applicable Health Care Laws, all applicable provisions of the FDA’s current good manufacturing practice (cGMP) regulations for biological products at 21 C.F.R. Parts 600 and 610, the Quality System (QS) regulations at 21 C.F.R. Part 820 and all comparable foreign regulatory requirements of any Governmental Entity.
(f) Neither the Company nor any of its Subsidiaries has received any written communication that relates to an alleged violation or non-compliance with any Health Care Laws, including any notification of any pending or threatened claim, suit, proceeding, hearing, enforcement, investigation, arbitration, import detention or refusal, FDA Warning Letter or Untitled Letter, or any action by a Governmental Entity relating to any Health Care Laws. All Warning Letters, Form-483 observations, or comparable findings from other Governmental Entities listed in Section 4.11 of the Company Disclosure Letter have been resolved to the satisfaction of the applicable Governmental Entity.
(g) There have been no seizures, withdrawals, recalls, detentions or suspensions of manufacturing, testing or distribution relating to the Company Products required or requested by a Governmental Entity, or other notice of action relating to an alleged lack of safety, efficacy or regulatory compliance of the Company Products, or any adverse experiences relating to the Company Products that have been reported to FDA or other Governmental Entity (“Safety Notices”), and, to the knowledge of the Company, there are no facts or circumstances that reasonably would be expected to give rise to a Safety Notice. All Safety Notices listed in Section 4.11(g) of the Company Disclosure Letter have been resolved to the satisfaction of the applicable Governmental Entity.
-19-
(h) Except as set forth in Section 4.11(g) of the Company Disclosure Letter, there are no unresolved Safety Notices, and to the knowledge the Company, there are no facts that would be reasonably likely to result in a material Safety Notice with respect to the Company Products or a termination or suspension of developing and testing of any of the Company Products.
(i) Neither the Company or any of its Subsidiaries, nor, to the knowledge of the Company, any officer, employee, agent, or distributor of the Company or any of its Subsidiaries has made an untrue statement of a material fact or fraudulent or misleading statement to a Governmental Entity, failed to disclose a material fact required to be disclosed to a Governmental Entity, or committed an act, made a statement, or failed to make a statement that would reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” Final Policy set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto (the “FDA Ethics Policy”). None of the aforementioned is or has been under investigation resulting from any allegedly untrue, fraudulent, misleading, or false statement or omission, including data fraud, or had any action pending or threatened relating to the FDA Ethics Policy.
(j) All reports, documents, claims, Permits and notices required to be filed, maintained or furnished to the FDA or any Governmental Entity by the Company or any of its Subsidiaries have been so filed, maintained or furnished, except where failure to file, maintain or furnish such reports, documents, claims, Permits or notices have not had and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect. All such reports, documents, claims, Permits and notices were true and complete in all material respects on the date filed (or were corrected in or supplemented by a subsequent filing).
(k) Neither the Company or any of its Subsidiaries nor, to the knowledge of the Company, any officer, employee, agent, or distributor of the Company or any of its Subsidiaries has been convicted of any crime or engaged in any conduct that has resulted, or would reasonably be expected to result, in debarment under applicable Law, including, without limitation, 21 U.S.C. § 335a, or exclusion under 42 U.S.C. § 1320a-7, or any other statutory provision or similar Law. Neither the Company or any of its Subsidiaries nor, to the knowledge of the Company, any officer, employee, agent or distributor of the Company or any of its Subsidiaries, has been excluded from participation in any federal health care program or convicted of any crime or engaged in any conduct for which such Person could be excluded from participating in any federal health care program under Section 1128 of the Social Security Act of 1935, as amended, or any similar Health Care Law or program.
Section 4.12 Benefit Plans.
(a) Section 4.12(a) of the Company Disclosure Letter contains a true and complete list of each material “employee benefit plan” (within the meaning of section 3(3) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), whether or not subject to ERISA), “multiemployer plans” (within the meaning of ERISA section 3(37)), and all stock purchase, stock option, phantom stock or other equity-based plan, severance, employment,
-20-
collective bargaining, change-in-control, fringe benefit, bonus, incentive, deferred compensation, supplemental retirement, health, life, or disability insurance, dependent care and all other employee benefit and compensation plans, agreements, programs, policies or other arrangements, whether or not subject to ERISA (including any funding mechanism therefor now in effect or required in the future as a result of the transactions contemplated by this Agreement or otherwise), whether formal or informal, written or oral, legally binding or not, under which any current or former employee, director or consultant of the Company or any of its Subsidiaries (or any of their dependents) has any present or future right to compensation or benefits or the Company or any of its Subsidiaries sponsors or maintains, is making contributions to or has any present or future liability or obligation (contingent or otherwise) or with respect to which it is otherwise bound. All such plans, agreements, programs, policies and arrangements shall be collectively referred to as the “Company Plans.” The Company has provided or made available to Parent a current, accurate and complete copy of each material Company Plan, or if such Company Plan is not in written form, a written summary of all of the material terms of such Company Plan. With respect to each Company Plan, the Company has furnished or made available to Parent a current, accurate and complete copy of, to the extent applicable: (i) any related trust agreement or other funding instrument, (ii) the most recent determination letter of the Internal Revenue Service (the “IRS”), (iii) any summary plan description, summary of material modifications, and other similar material written communications (or a written description of any material oral communications) to the employees of the Company or any of its Subsidiaries concerning the extent of the benefits provided under a Company Plan and (iv) for the three most recent years and as applicable (A) the Form 5500 and attached schedules, (B) audited financial statements and (C) actuarial valuation reports.
(b) Neither the Company, its Subsidiaries or any member of their Controlled Group (defined as any organization which is a member of a controlled, affiliated or otherwise related group of entities within the meaning of Code Sections 414(b), (c), (m) or (o)) has ever sponsored, maintained, contributed to or been required to contribute to or incurred any liability (contingent or otherwise) with respect to: (i) a “multiemployer plan” (within the meaning of ERISA section 3(37)), (ii) an “employee pension benefit plan,” within the meaning of Section 3(2) of ERISA (“Pension Plan”) that is subject to Title IV of ERISA or Section 412 of the Code, (iii) a Pension Plan which is a “multiple employer plan” as defined in Section 413 of the Code, or (iv) a “funded welfare plan” within the meaning of Section 419 of the Code.
(c) With respect to the Company Plans:
(i) each Company Plan complies in all material respects with its terms and materially complies in form and in operation with the applicable provisions of ERISA and the Code and all other applicable legal requirements;
(ii) each Company Plan intended to be qualified under Section 401(a) of the Code has received a favorable determination, advisory and/or opinion letter, as applicable, from the IRS that it is so qualified and nothing has occurred to the knowledge of the Company since the date of such letter that would reasonably be expected to cause the loss of the sponsor’s ability to rely upon such letter, and nothing has occurred to the knowledge of the Company that would reasonably be expected to result in the loss of the qualified status of such Company Plan; (iii) there is no material Action (including any investigation, audit or other administrative proceeding) by the Department of Labor, the Pension Benefit Guaranty Corporation (the “PBGC”), the IRS or any other Governmental Entity or by any plan participant or beneficiary pending, or to the knowledge of the Company, threatened, relating to the Company Plans, any fiduciaries thereof with respect to their duties to the Company Plans or the assets of any of the trusts under any of the Company Plans (other than routine claims for benefits) nor, to the knowledge of the Company, are there facts or circumstances that exist that could reasonably give rise to any such actions;
-21-
(iv) none of the Company Plans currently provides, or reflects or represents any liability to provide post-termination or retiree welfare benefits to any person for any reason, except as may be required by Section 601, et seq. of ERISA and Section 4980B(b) of the Code or other applicable similar law regarding health care coverage continuation (collectively “COBRA”), and none of the Company, its Subsidiaries or any members of its Controlled Group has any liability to provide post-termination or retiree welfare benefits to any person or ever represented, promised or contracted to any employee or former employee of the Company or any of its Subsidiaries (either individually or to employees as a group) or any other person that such employee(s) or other person would be provided with post-termination or retiree welfare benefits, except to the extent required by statute or except with respect to a contractual obligation to reimburse any premiums such person may pay in order to obtain health coverage under COBRA;
(v) each Company Plan is subject exclusively to United States Law; and
(vi) the execution and delivery of this Agreement and the consummation of the Merger will not, either alone or in combination with any other event, (A) entitle any current or former employee, officer, director or consultant of the Company or any of its Subsidiaries to severance pay, unemployment compensation or any other similar termination payment, or (B) accelerate the time of payment or vesting, or increase the amount of or otherwise enhance any benefit due any such employee, officer, director or consultant.
(d) Neither the Company nor any of its Subsidiaries is a party to any agreement, contract, arrangement or plan (including any Company Plan) that may reasonably be expected to result, separately or in the aggregate, in connection with the transactions contemplated by this Agreement (either alone or in combination with any other events), in the payment of any “parachute payments” within the meaning of Section 280G of the Code. There is no agreement, plan or other arrangement to which the Company or any of its Subsidiaries is a party or by which the Company or any of its Subsidiaries is otherwise bound to compensate any person in respect of Taxes or other liabilities incurred with respect to Section 409A or 4999 of the Code.
(e) Each Company Plan that is a “nonqualified deferred compensation plan” within the meaning of Section 409A of the Code (or any comparable or similar provision of state, local, or foreign Law) complies in both form and operation in all material respects with the requirements of Section 409A of the Code (or any comparable or similar provision of state, local, or foreign Law) and all applicable IRS guidance issued with respect thereto (and has so complied for the entire period during which Section 409A of the Code has applied to such Company Plan) so that no amount paid or payable pursuant to any such Company Plan is subject to any additional Tax or interest under Section 409A of the Code (or any comparable or similar provision of state, local, or foreign Law).
-22-
Section 4.13 Labor and Employment Matters.
(a) The Company and its Subsidiaries are and for the past three (3) years have been in compliance in all material respects with all applicable Laws relating to labor and employment, including those relating to employment practices, terms and conditions of employment, collective bargaining, disability, immigration, health and safety, wages, hours and benefits, non-discrimination in employment, workers’ compensation, the collection and payment of withholding and/or payroll Taxes and similar Taxes, unemployment compensation, equal employment opportunity, discrimination, harassment, employee and contractor classification, information privacy and security, and continuation coverage with respect to group health plans. During the preceding three (3) years, there has not been, and as of the date of this Agreement there is not pending or, to the knowledge of the Company, threatened, any labor dispute, work stoppage, labor strike or lockout against the Company or any of its Subsidiaries by employees.
(b) No employee of the Company or any of its Subsidiaries is covered by an effective or pending collective bargaining agreement or similar labor agreement. To the knowledge of the Company, there has not been any activity on behalf of any labor union, labor organization or similar employee group to organize any employees of the Company or any of its Subsidiaries. There are no (i) unfair labor practice charges or complaints against the Company or any of its Subsidiaries pending before the National Labor Relations Board or any other labor relations tribunal or authority and to the knowledge of the Company no such representations, claims or petitions are threatened, (ii) representation claims or petitions pending before the National Labor Relations Board or any other labor relations tribunal or authority or (iii) grievances or pending arbitration proceedings against the Company or any of its Subsidiaries that arose out of or under any collective bargaining agreement.
(c) To the knowledge of the Company, no current employee or officer of the Company or any of its Subsidiaries intends, or is expected, to terminate his employment relationship with such entity in connection with or as a result of the transactions contemplated hereby.
(d) During the preceding three (3) years, (i) neither the Company nor any of its Subsidiaries has effectuated a “plant closing” (as defined in the Worker Adjustment Retraining and Notification Act of 1988, as amended (the “WARN Act”)) affecting any site of employment or one or more facilities or operating units within any site of employment or facility, (ii) there has not occurred a “mass layoff” (as defined in the WARN Act) in connection with the Company or any of its Subsidiaries affecting any site of employment or one or more facilities or operating units within any site of employment or facility and (iii) neither the Company nor any of its Subsidiaries has engaged in layoffs or employment terminations sufficient in number to trigger application of any similar state, local or foreign law. The Company and its Subsidiaries currently properly classify and for the past three (3) years have properly classified their respective employees as exempt or nonexempt in accordance with applicable overtime laws, and no person treated as an independent contractor or consultant by the Company or any of its Subsidiaries within the past three (3) years should have been properly classified as an employee under applicable Law.
-23-
(e) Except as set forth on Section 4.13(e) of the Company Disclosure Letter, with respect to any current or former employee, officer, consultant or other service provider of the Company or any of its Subsidiaries, there are no Actions against the Company or any of its Subsidiaries pending, or to the Company’s knowledge, threatened to be brought or filed, in connection with the employment or engagement of any current or former employee, officer, consultant or other service provider of the Company or any of its Subsidiaries, including, without limitation, any claim relating to employment discrimination, harassment, retaliation, equal pay, employment classification or any other employment related matter arising under applicable Laws, except where such action would not, individually or in the aggregate, result in the Company or any of its Subsidiaries incurring a material liability.
(f) Except as set forth on Section 4.13(f) of the Company Disclosure Letter, the execution of this Agreement and the consummation of the transactions set forth in or contemplated by this Agreement will not result in any breach or violation of, or cause any payment to be made under, any applicable Laws respecting labor and employment or any collective bargaining agreement to which the Company or any of its Subsidiaries is a party.
(g) Since January 1, 2022, (i) no allegations of workplace sexual harassment, discrimination or other misconduct have been made, initiated, filed or, to the knowledge of the Company, threatened against the Company or any of its Subsidiaries or any of their respective current or former directors, officers or senior level management employees, (ii) to the knowledge of the Company, no incidents of any such workplace sexual harassment, discrimination or other misconduct have occurred, and (iii) neither the Company nor any of its Subsidiaries has entered into any settlement agreement related to allegations of sexual harassment, discrimination or other misconduct by any of their directors, officers or employees described in clause (i) hereof or any independent contractor.
(h) The Company and its Subsidiaries are and have at all relevant times been in compliance in all material respects with (i) COVID-19 related Laws, standards, regulations, orders and guidance (including without limitation relating to business reopening), including those issued and enforced by the Occupational Safety and Health Administration, the Centers for Disease Control, the Equal Employment Opportunity Commission, and any other Governmental Entity; and (ii) the Families First Coronavirus Response Act (including with respect to eligibility for tax credits under such Act) and any other applicable COVID-19 related leave Law, whether state, local or otherwise.
Section 4.14 Environmental Matters.
-24-
(a) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect, (i) the Company and its Subsidiaries have conducted their respective businesses in compliance with all, and have not violated any, applicable Environmental Laws; (ii) the Company and its Subsidiaries have obtained all Permits of all Governmental Entities and any other Person that are required under any Environmental Law; (iii) there has been no release of any Hazardous Substance by the Company, any of its Subsidiaries or any other Person in any manner that has given or would reasonably be expected to give rise to any remedial or investigative obligation, corrective action requirement or liability of the Company or any of its Subsidiaries under applicable Environmental Laws; (iv) neither the Company nor any of its Subsidiaries has received any claims, notices, demand letters or requests for information (except for such claims, notices, demand letters or requests for information the subject matter of which has been resolved prior to the date of this Agreement) from any federal, state, local, foreign or provincial Governmental Entity or any other Person asserting that the Company or any of its Subsidiaries is in violation of, or liable under, any Environmental Law; (v) no Hazardous Substance has been disposed of, arranged to be disposed of, released or transported in violation of any applicable Environmental Law, or in a manner that has given rise to, or that would reasonably be expected to give rise to, any liability under any Environmental Law, in each case, on, at, under or from any current or former properties or facilities owned or operated by the Company or any of its Subsidiaries or as a result of any operations or activities of the Company or any of its Subsidiaries at any location and, to the knowledge of the Company, Hazardous Substances are not otherwise present at or about any such properties or facilities in amount or condition that has resulted in or would reasonably be expected to result in liability to the Company or any of its Subsidiaries under any Environmental Law; and (vi) neither the Company, any of its Subsidiaries nor any of their respective properties or facilities are subject to, or are threatened to become subject to, any liabilities relating to any suit, settlement, court order, administrative order, regulatory requirement, judgment or claim asserted or arising under any Environmental Law or any agreement relating to environmental liabilities.
(b) As used herein, “Environmental Law” means any Law relating to (i) the protection, preservation or restoration of the environment (including air, surface water, groundwater, drinking water supply, surface and subsurface soils and strata, wetlands, plant and animal life or any other natural resource) or (ii) the exposure to, or the use, storage, recycling, treatment, generation, transportation, processing, handling, labeling, production, release or disposal of Hazardous Substances.
(c) As used herein, “Hazardous Substance” means any substance listed, defined, designated, classified or regulated as a waste, pollutant or contaminant or as hazardous, toxic, radioactive or dangerous or any other term of similar import under any Environmental Law, including but not limited to petroleum.
Section 4.15 Taxes.
(a) Each of the Company and its Subsidiaries has (i) filed all income and other material Tax Returns required to be filed by or on behalf of it (taking into account any applicable extensions thereof) and all such Tax Returns are true, accurate and complete in all material respects; and (ii) paid in full (or caused to be timely paid in full) all material Taxes that are required to be paid by or with respect to it, whether or not such Taxes were shown as due on such Tax Returns.
(b) All material Taxes not yet due and payable by the Company or any of its Subsidiaries as of the date of the Company Balance Sheet have been, in all respects, properly accrued in accordance with GAAP on the Company Financial Statements, and such Company Financial Statements reflect an adequate reserve (in accordance with GAAP) for all material Taxes accrued but unpaid by the Company or any of its Subsidiaries through the date of such financial statements. Since the date of the Company Financial Statements, neither the Company nor any of its Subsidiaries has incurred, individually or in the aggregate, any material liability for Taxes outside the Ordinary Course of Business.
-25-
(c) Neither the Company nor any of its Subsidiaries has executed any waiver of any statute of limitations on, or extended the period for the assessment or collection of, any amount of Tax, in each case that has not since expired.
(d) No material audits or other investigations, proceedings, claims, assessments or examinations by any Governmental Entity (each, a “Tax Action”) with respect to Taxes or any Tax Return of the Company or any of its Subsidiaries are presently in progress or have been asserted, threatened or proposed in writing and to the knowledge of the Company, no such Tax Action is being contemplated. No deficiencies or claims for a material amount of Taxes have been claimed, proposed, assessed or asserted in writing against the Company or any of its Subsidiaries by a Governmental Entity, other than any such claim, proposal, assessment or assertion that has been satisfied by payment in full, settled with no further liability remaining on the part of the Company or any of its Subsidiaries or withdrawn.
(e) Subject to exceptions as would not be material, each of the Company and its Subsidiaries has timely withheld all Taxes required to have been withheld from payments made (or deemed made) to its employees, independent contractors, creditors, shareholders and other third parties and, to the extent required, such Taxes have been timely paid to the relevant Governmental Entity.
(f) Neither the Company nor any of its Subsidiaries has engaged in a “listed transaction” as set forth in Treasury Regulations § 1.6011-4(b)(2).
(g) Neither the Company nor any of its Subsidiaries (i) is a party to or bound by, or has any liability pursuant to, any Tax sharing, allocation, indemnification or similar agreement or obligation, other than any such agreement or obligation which is a customary commercial agreement obligation entered into in the Ordinary Course of Business with vendors, lessors, lenders or the like the primary purpose of which is unrelated to Taxes (each, an “Ordinary Course Agreement”); (ii) is or has been a member of a group (other than a group the common parent of which is the Company) filing a consolidated, combined, affiliated, unitary or similar income Tax Return; (iii) has any liability for the Taxes of any Person (other than the Company) pursuant to Treasury Regulations § 1.1502-6 (or any similar provision of state, local or non-United States Law) as a transferee or successor, by Contract or otherwise by operation of Law; or (iv) is or has been treated as a resident for any income Tax purpose, or as subject to Tax by virtue of having a permanent establishment, an office or fixed place of business, in any country other than the country in which it was or is organized.
(h) No private letter rulings, technical advice memoranda or similar material agreements or rulings have been requested, entered into or issued by any taxing authority with respect to the Company or any of its Subsidiaries which rulings remain in effect.
-26-
(i) Neither the Company nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of (i) a change in, or use of improper, method of accounting requested or initiated at or prior to the Closing (other than any such change required, or any such use treated as improper, as a result of the transactions provided for herein), (ii) a “closing agreement” as described in Section 7121 of the Code (or any similar provision of Law) executed at or prior to the Closing, (iii) an installment sale or open transaction disposition made at or prior to the Closing, (iv) any prepaid amount received or deferred revenue accrued at or prior to the Closing, other than in respect of such amounts reflected in the Company Balance Sheet, or received in the Ordinary Course of Business since the date of the Company Balance Sheet, (v) to the Company’s knowledge, an intercompany transaction or excess loss amount described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign income Tax Law) or (vi) an election under Section 965 of the Code or (vii) the application of Sections 951 or 951A of the Code with respect to income earned or recognized or payments received prior to the Closing.
(j) No non-U.S. Subsidiary of the Company (i) has recognized or is expected to recognize any material amount of “subpart F income” as defined in Section 952 of the Code or (ii) has recognized or is expected to recognize any material amount of income under Section 951A of the Code. No non-U.S. Subsidiary of the Company has recognized or is expected to recognize any material amount of income from the ownership or sale of any “United States real property interest” within the meaning of Section 897 of the Code.
(k) There are no liens for Taxes upon any of the assets of the Company or any of its Subsidiaries other than Liens for current Taxes and assessments not yet past due.
(l) Neither the Company nor any of its Subsidiaries has distributed stock of another Person or has had its stock distributed by another Person, in a transaction (or series of transactions) that was purported or intended to be governed in whole or in part by Sections 355 or 361 of the Code.
(m) Neither the Company nor any of its Subsidiaries has been a United States real property holding corporation, as defined in Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(n) No material claim has been made in writing by any Governmental Entity in a jurisdiction where the Company or any of its Subsidiaries does not currently file or has filed a Tax Return that the Company or any of its Subsidiaries is or may be subject to taxation by such jurisdiction.
(o) There are no outstanding shares of company stock issued in connection with the performance of services (within the meaning of Section 83 of the Code) that immediately prior to the Effective Time are subject to a substantial risk of forfeiture (as such terms are defined in Section 83 of the Code) or for which a valid election under Section 83(b) of the Code has not been made.
(p) To the Company’s knowledge, each of the Company and its Subsidiaries has not been, is not, and immediately prior to the Effective Time will not be, treated as an “investment company” within the meaning of Section 368(a)(2)(F) of the Code.
-27-
(q) Neither the Company nor any of its Subsidiaries has taken any action nor knows of any fact or circumstance that could reasonably be expected to prevent the Merger (together, if required by Section 3.7, with the Second Merger) from qualifying as a transaction qualifying for the Intended Tax Treatment.
(r) For purposes of this Section 4.15, where the context permits, each reference to the Company or any of its Subsidiaries shall include a reference to any person for whose Taxes the Company or any of its Subsidiaries is liable under applicable Law.
Section 4.16 Contracts.
(a) As of the date of this Agreement, there are no contracts that would constitute a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S‑K under the Securities Act), with respect to the Company or any of its Subsidiaries (assuming the Company was subject to the requirements of the Exchange Act), other than those contracts identified in Section 4.16 of the Company Disclosure Letter, which, for the avoidance of doubt, shall exclude any Company Plans (all such contracts, “Material Contracts”).
(b) As of the date of this Agreement, (i) each Company Material Contract is legal, valid and binding on the Company or its applicable Subsidiary and to the knowledge of the Company, each other party thereto, and is in full force and effect and enforceable in accordance with its terms; (ii) the Company or its applicable Subsidiary, and, to the knowledge of the Company, each other party thereto, has performed all material obligations required to be performed by it under each Material Company Contract and (iii) there is no material default under any Material Company Contract by the Company or its applicable Subsidiary or, to the knowledge of the Company, any other party thereto, and no event or condition has occurred that constitutes, or, after notice or lapse of time or both, would constitute, a material default on the part of the Company or its applicable Subsidiary or, to the knowledge of the Company, any other party thereto under any such Material Company Contract, nor has the Company or any of its Subsidiaries received any notice of any such material default, event or condition. The Company has made available to Parent true and complete copies of all Material Contracts, including all amendments thereto.
Section 4.17 Insurance. Each of the Company and its Subsidiaries is covered by valid and currently effective insurance policies issued in favor of the Company or one or more of its Subsidiaries that are customary and adequate for companies of similar size in the industries and locations in which the Company operates. Section 4.17 of the Company Disclosure Letter sets forth, as of the date hereof, a true and complete list of all material insurance policies issued in favor of the Company or any of its Subsidiaries, or pursuant to which the Company or any of its Subsidiaries is a named insured or otherwise a beneficiary, as well as any historic incurrence-based policies still in force. With respect to each such insurance policy, (a) such policy is in full force and effect and all premiums due thereon have been paid, (b) neither the Company nor any of its Subsidiaries is in breach or default, and has not taken any action or failed to take any action which (with or without notice or lapse of time, or both) would constitute such a breach or default, or would permit termination or modification of, any such policy and (c) to the knowledge of the Company, no insurer issuing any such policy has been declared insolvent or placed in receivership, conservatorship or liquidation. No notice of cancellation or termination has been received with respect to any such policy, nor will any such cancellation or termination result from the consummation of the transactions contemplated hereby.
-28-
Section 4.18 Properties.
(a) The Company or one of its Subsidiaries has good and valid title to, or in the case of leased property and leased tangible assets, a valid leasehold interest in, all of its real properties and tangible assets, free and clear of all Liens other than (i) Liens for current Taxes and assessments not yet past due or the amount or validity of which is being contested in good faith by appropriate proceedings, (ii) mechanics’, workmen’s, repairmen’s, warehousemen’s and carriers’ Liens arising in the Ordinary Course of Business of the Company and its Subsidiaries and (iii) Liens that have arisen in the Ordinary Course of Business that do not, individually or in the aggregate, materially impair the continued ownership, use and operation of the assets to which they relate in the business of the Company and its Subsidiaries as currently conducted (“Permitted Liens”). The tangible personal property currently used in the operation of the business of the Company and its Subsidiaries is in good working order (reasonable wear and tear excepted) and is suitable and adequate for the uses for which it is intended or is being used.
(b) Each of the Company and its Subsidiaries has complied with the terms of all leases to which it is a party in all material respects, and all such leases are in full force and effect. Each of the Company and its Subsidiaries enjoys peaceful and undisturbed possession under all such leases.
(c) Section 4.18(c) of the Company Disclosure Letter sets forth a true and complete list of (i) all real property owned by the Company or any of its Subsidiaries and (ii) all real property leased for the benefit of the Company or any of its Subsidiaries (the “Company Real Estate Leases” ).
(d) This Section 4.18 does not relate to intellectual property, which is the subject of Section 4.19.
Section 4.19 Intellectual Property.
(a) Section 4.19(a) of the Company Disclosure Letter sets forth a true and complete list of all (i) material patents and patent applications; (ii) material trademark registrations and applications and (iii) material copyright registrations and applications, in each case owned by the Company and its Subsidiaries (collectively, “Company Registered IP”) and a true and complete list of all domain names owned or exclusively licensed by the Company and its Subsidiaries. Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect, (A) all of the Company Registered IP is subsisting and, in the case of any Company Registered IP that is registered or issued and to the knowledge of the Company, valid and enforceable, (B) no Company Registered IP is involved in any interference, reissue, derivation, reexamination, opposition, cancellation or similar proceeding and, to the knowledge of the Company, no such action is threatened with respect to any of the Company Registered IP and (C) the Company or its Subsidiaries own exclusively, free and clear of any and all Liens (other than Permitted Liens), all Company Owned IP, including all Intellectual Property created on behalf of the Company or its Subsidiaries by employees or independent contractors.
-29-
(b) Section 4.19(b) of the Company Disclosure Letter accurately identifies (i) all contracts pursuant to which any material Intellectual Property licensed to the Company or any of its Subsidiaries (other than (A) any non-customized software that (1) is so licensed solely in executable or object code form pursuant to a nonexclusive, internal use software license and other Intellectual Property associated with such software and (2) is not incorporated into, or material to the development, manufacturing, or distribution of, any of the Company’s or any of its Subsidiaries’ products or services, (B) any Intellectual Property licensed on a nonexclusive basis ancillary to the purchase or use of equipment, reagents or other materials, (C) any confidential information provided under confidentiality agreements and (D) agreements between Company and its employees in Company’s standard form thereof), (ii) the corresponding contract pursuant to which such Intellectual Property is licensed to the Company or its Subsidiaries, as applicable and (iii) whether the license or licenses granted to the Company or its Subsidiaries, as applicable, are exclusive or nonexclusive.
(c) Section 4.19(c) of the Company Disclosure Letter accurately identifies each contract pursuant to which any Person has been granted any license or covenant not to sue under, or otherwise has received or acquired any right (whether or not currently exercisable) or interest in, any Company Registered IP (other than (i) any confidential information provided under confidentiality agreements and (ii) any Company Registered IP nonexclusively licensed to academic collaborators, suppliers or service providers for the sole purpose of enabling such academic collaborator, supplier or service providers to provide services for the Company’s and its Subsidiaries’ benefit).
(d) To the knowledge of Company, the Company Registered IP, together with the Intellectual Property licensed to Company pursuant to a contract identified on Section 4.19(b) of the Company Disclosure Letter or described in clauses (A) through (D) of Section 4.19(b)(i)), constitutes all Intellectual Property necessary for the Company and its Subsidiaries to conduct their respective businesses as currently conducted; provided, however, that the foregoing representation is not a representation with respect to noninfringement of Intellectual Property.
(e) The Company and its Subsidiaries have taken commercially reasonable measures to maintain the confidentiality of all information that constitutes or constituted a material Trade Secret of the Company or its Subsidiaries, including requiring all Persons having access thereto to execute written nondisclosure agreements or other binding obligations to maintain confidentiality of such information.
(f) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect, (i) to the knowledge of the Company, the conduct of the businesses of the Company and its Subsidiaries, including the manufacture, marketing, offering for sale, sale, importation, use or intended use or other disposal of any product as currently sold or under development by Company or its Subsidiaries, has not infringed, misappropriated or diluted, and does not infringe, misappropriate or dilute, any Intellectual Property of any Person, (ii) neither the Company nor any of its Subsidiaries has received any written notice or claim asserting or suggesting that any such infringement, misappropriation, or dilution is or may be occurring or has or may have occurred and (iii) to the knowledge of the Company, no Person is infringing, misappropriating, or diluting in any material respect any Company Registered IP.
-30-
(g) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect, (i) the Company and its Subsidiaries have taken commercially reasonable steps to protect the confidentiality and security of the computer and information technology systems used by the Company and its Subsidiaries (the “IT Systems”) and the information and transactions stored or contained therein or transmitted thereby, (ii) to the knowledge of the Company, during the past two (2) years, there has been no unauthorized or improper use, loss, access, transmittal, modification or corruption of any such information or data and (iii) during the past two (2) years, there have been no material failures, crashes, viruses, security breaches (including any unauthorized access to any personally identifiable information), affecting the IT Systems.
(h) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Material Adverse Effect, (i) to the knowledge of the Company, the Company and its Subsidiaries have at all times complied in all material respects with all applicable Laws relating to privacy, data protection, and the collection, retention, protection, and use of information that alone or in combination with other information can be used to identify an individual (“Personal Information”) collected, used, or held for use by the Company or any of its Subsidiaries (collectively, “Privacy Laws”), (ii) during the past two (2) years, no claims have been asserted or, to the knowledge of the Company, threatened in writing against the Company or any of its Subsidiaries alleging a violation of any Person’s privacy or Personal Information, (iii) neither this Agreement nor the consummation of the transactions contemplated hereby will breach or otherwise violate any Privacy Laws and (iv) the Company and its Subsidiaries have taken commercially reasonable steps to protect the Personal Information collected, used or held for use by the Company or its Subsidiaries against loss and unauthorized access, use, modification, disclosure or other misuse.
(i) To the knowledge of the Company, no government funding, facilities or resources of a university, college, other educational institution or research center or funding from third parties was used in the development of the Company Owned IP, to the knowledge of the Company, exclusively licensed to the Company or any of its Subsidiaries, and no Governmental Entity, university, college, other educational institution or research center has, to the knowledge of the Company, any claim or right in or to such Intellectual Property.
(j) Except as set forth on Section 4.19(j) of the Company Disclosure Letter, the execution, delivery and performance by the Company of this Agreement, and the consummation of the transactions contemplated hereby, will not result in the loss of, or give rise to any right of any third party to terminate or modify any of the Company’s or f its Subsidiaries’ rights or obligations under any agreement under which the Company or any of its Subsidiaries grants to any Person, or any Person grants to the Company or any of its Subsidiaries, a license or right under or with respect to any Intellectual Property that is material to any of the businesses of the Company or any of its Subsidiaries
Section 4.20 Takeover Law. The Company Board has taken all actions necessary to ensure that the Takeover Laws are, and will be, inapplicable to the execution, delivery and performance of this Agreement and the timely consummation of the Merger and the other transactions contemplated hereby and will not restrict, impair or delay the ability of Parent, after the Effective Time, to vote or otherwise exercise all rights as a stockholder of the Company.
-31-
“Takeover Laws” shall mean any “moratorium,” “control share acquisition,” “affiliated transactions,” “business combination,” “fair price” or other form of anti-takeover Laws of any jurisdiction or other applicable Laws that purport to limit or restrict mergers or business combinations or the ability to limit or restrict mergers or business combinations or the ability to acquire or to vote shares, including as set forth in Section 203 of the DGCL.
Section 4.21 No Rights Plan. There is no stockholder rights plan, “poison pill” anti-takeover plan or other similar device in effect to which the Company or any of its Subsidiaries is a party or is otherwise bound.
Section 4.22 Related Party Transactions. Except as set forth on Section 4.22 of the Company Disclosure Letter, since January 1, 2022 through the date of this Agreement, there have been no transactions, agreements, arrangements or understandings between the Company or any of its Subsidiaries, on the one hand (other than the Company’s Subsidiaries), and the Affiliates of the Company, on the other hand that would be required to be disclosed under Item 404 of Regulation S-K under the Securities Act (assuming the Company was subject to the requirements of the Exchange Act) or otherwise.
Section 4.23 Certain Payments. Neither the Company nor any of its Subsidiaries nor, to the knowledge of the Company or any of its respective Representatives acting on their behalf, (a) has used or is using any corporate funds for any illegal contributions, gifts, entertainment or other unlawful expenses relating to political activity, (b) has used or is using any corporate funds for any direct or indirect unlawful payments to any foreign or domestic governmental officials or employees, (c) has violated or is violating any provision of the Foreign Corrupt Practices Act of 1977 or any other applicable local or foreign anti-corruption or bribery Law, (d) has established or maintained, or is maintaining, any unlawful fund of corporate monies or other properties or (e) has made any bribe, unlawful rebate, payoff, influence payment, kickback or other unlawful payment of any nature.
Section 4.24 Brokers. No broker, investment banker, financial advisor or other Person, other than as set forth on Section 4.24 of the Company Disclosure Letter, the fees and expenses of which will be paid by the Company, or following the Effective Time, Parent is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated by this Agreement based upon arrangements made by or on behalf of the Company or any of its Affiliates. The Company has furnished to Parent a true and complete copy of any Contract between the Company or any of its Subsidiaries and any Person identified on Section 4.24 of the Company Disclosure Letter pursuant to which such Person could be entitled to any payment from the Company or any of its Subsidiaries, or, following the Effective Time, Parent, relating to the transactions contemplated hereby.
Section 4.25 No Other Representations and Warranties. The Company acknowledges and agrees that, except for the representations and warranties of Parent and Merger Sub set forth in ARTICLE V or in any certificate delivered by Parent or Merger Sub to the Company pursuant to this Agreement, neither the Company nor any other Person, is relying on any other representation or warranty of Parent or any other Person made outside of ARTICLE V or such certificate, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied.
-32-
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF PARENT AND MERGER SUB
Except (a) as disclosed in the Parent SEC Documents at least three Business Days prior to the date of this Agreement and that is reasonably apparent on the face of such disclosure to be applicable to the representation and warranty set forth herein (but (i) without giving effect to any amendment thereof filed with, or furnished to the SEC on or after the date hereof and (ii) excluding any disclosures contained or referenced therein under the captions “Risk Factors,” “Forward-Looking Statements,” “Quantitative and Qualitative Disclosures About Market Risk,” and any other disclosures contained or referenced therein of information, factors, or risks that are predictive, cautionary, or forward looking in nature); or (b) as set forth in the corresponding section or subsection of the disclosure letter delivered by Parent to the Company immediately prior to the execution of this Agreement (the “Parent Disclosure Letter”), each of Parent and Merger Sub represent and warrant to the Company as follows:
Section 5.1 Organization, Standing and Power.
(a) Each of Parent and its Subsidiaries (i) is a corporation duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation, (ii) has all requisite corporate power and authority to own, lease and operate its properties and to carry on its business as now being conducted and (iii) is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the nature of its business or the ownership, leasing or operation of its properties makes such qualification or licensing necessary, except in the case of this clause (iii), where the failure to be so qualified or licensed or in good standing, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect. For purposes of this Agreement, “Parent Material Adverse Effect” means any event, change, circumstance, occurrence, effect or state of facts that (A) is or would reasonably be expected to be materially adverse to the business, assets, liabilities, financial condition, or results of operations of Parent and its Subsidiaries, taken as a whole, or (B) materially impairs the ability of Parent or Merger Sub to consummate the Merger or any of the other transactions contemplated by this Agreement; provided, however, that in the case of clause (A) only, Parent Material Adverse Effect shall not include any event, change, circumstance, occurrence, effect or state of facts to the extent resulting from (1) changes or conditions generally affecting the industries in which Parent operates, or the economy or the financial, debt, banking, capital, credit or securities markets, in the United States, including effects on such industries, economy or markets resulting from any regulatory and political conditions or developments in general, (2) the outbreak or escalation of war or acts of terrorism or any natural disasters, acts of God or comparable events, epidemic, pandemic or disease outbreak (including the COVID-19 virus) or any worsening of the foregoing, or any declaration of martial law, quarantine or similar directive, policy or guidance or Law or other action by any Governmental Entity in response thereto, (3) changes in Law or GAAP, or the interpretation or enforcement thereof, (4) the public announcement of this Agreement or (5) any specific action taken (or omitted to be taken) by Parent that is required by this Agreement or actions or omissions taken with the Company’s consent; provided, that, with respect to clauses (1), (2) and (3), the impact of such event, change, circumstance, occurrence, effect or state of facts is not disproportionately adverse to Parent and its Subsidiaries, taken as a whole, as compared to other participants in the industries in which Parent operates.
-33-
(b) Parent has previously made available to the Company true and complete copies of the Parent Charter and Parent Bylaws and the organizational documents of Merger Sub, in each case, as amended to the date of this Agreement, and each as so delivered is in full force and effect. Neither Parent nor Merger Sub is in violation of any provision of the Parent Charter, Parent Bylaws or Certificate of Incorporation or Bylaws, respectively.
Section 5.2 Capital Stock.
(a) The authorized capital stock of Parent consists of 75,000,000 shares of Parent Common Stock and 5,000,000 shares of preferred stock, par value $0.001 per share (the “Parent Preferred Stock”). As of the close of business on March 29, 2024 (the “Measurement Date”), (i) 55,304,658 shares of Parent Common Stock (excluding treasury shares) were issued and outstanding, (ii) no shares of Parent Common Stock were held by Parent in its treasury, (iii) 292,198 shares of Parent Preferred Stock (excluding treasury shares) are issued and outstanding, of which (a) 278,530 are designated Series A Preferred Stock, (b) no shares of Series B Preferred Stock were outstanding, and (c) 10,925 are designated Series C-1 Preferred Stock, (d) 898 are designated Series C-2 Preferred Stock, (e) 1,845 are designated Series C-3 Preferred Stock, (iv) no shares of Parent Preferred Stock were held by Parent in its treasury, (v) 288,261 shares of Parent Common Stock were reserved for issuance pursuant to Parent’s Amended and Restated 2017 Omnibus Equity Incentive Plan, as amended (of which 222,459 shares were subject to outstanding Parent Options and of which 65,802 shares were subject to outstanding Parent RSUs), (vi) 692,922 Parent Warrants are issued and outstanding and (vii) 42,037 warrants to acquire shares of Parent Series C Preferred Stock are issued and outstanding. All outstanding shares of capital stock of Parent are, and all shares reserved for issuance will be, when issued, duly authorized, validly issued, fully paid and nonassessable and not subject to any preemptive rights. Except as set forth above in this Section 5.2(a), neither Parent nor any of its Subsidiaries has outstanding any bonds, debentures, notes or other obligations having the right to vote (or convertible into, or exchangeable or exercisable for, securities having the right to vote) with the stockholders of Parent or such Subsidiary on any matter. Except as set forth above in this Section 5.2(a) and except for changes since the close of business on the Measurement Date resulting from the exercise of any options as described above, as of the Measurement Date, there are no outstanding (A) shares of capital stock or other voting securities or equity interests of Parent, (B) securities of Parent or any of its Subsidiaries convertible into or exchangeable or exercisable for shares of capital stock of Parent or other voting securities or equity interests of Parent or its Subsidiaries, (C) stock appreciation rights, “phantom” stock rights, performance units, interests in or rights to the ownership or earnings of Parent or its Subsidiaries or other equity equivalent or equity-based awards or rights, (D) subscriptions, options, warrants, calls, commitments, Contracts or other rights to acquire from Parent or its Subsidiaries, or obligations of Parent or any of its Subsidiaries to issue, any shares of capital stock of Parent or any of its Subsidiaries, voting securities, equity interests or securities convertible into or exchangeable or exercisable for capital stock or other voting securities or equity interests of Parent or its Subsidiaries or rights or interests described in the preceding clause (C) or (E) obligations of Parent or any of its Subsidiaries to repurchase, redeem or otherwise acquire any such securities or to issue, grant, deliver or sell, or cause to be issued, granted, delivered or sold, any such securities.
-34-
(b) The authorized capital stock of Merger Sub consists of 100 shares of common stock, par value $0.001 per share, of which 100 shares are issued and outstanding, all of which shares are beneficially owned by Parent.
(c) The shares of Parent Common Stock to be issued pursuant to the Merger will be duly authorized, validly issued, fully paid and nonassessable and not subject to any preemptive rights.
(d) To the knowledge of Parent as of the date of this Agreement and as of the Closing, no “bad actor” disqualifying event described in Rule 506(d)(1)(i)-(viii) of the Securities Act (a “Disqualifying Event”) is applicable to Parent or, to Parent’s knowledge, any Covered Person, except for a Disqualifying Event as to which Rule 506(d)(2)(ii-iv) or (d)(3) of the Securities Act is applicable. “Covered Person” means, with respect to Parent as an “issuer” for purposes of Rule 506 promulgated under the Securities Act, any person listed in the first paragraph of Rule 506(d)(1).
(e) All outstanding Parent Common Stock, Parent Preferred Stock, Parent Options, Parent RSUs and Parent Warrants and other securities of Parent have been issued and granted in material compliance with (i) the Parent Charter and the Parent Bylaws and all applicable securities Laws and other applicable Law and (ii) all requirements set forth in applicable Contracts.
Section 5.3 Subsidiaries. Section 5.3 of the Parent Disclosure Letter sets forth each Subsidiary of Parent. All of the issued and outstanding shares of capital stock of each Subsidiary of Parent have been duly authorized and are validly issued, fully paid, and non-assessable. Parent holds of record and beneficially all of the outstanding shares of each Subsidiary of Parent, free and clear of any restrictions on transfer (other than restrictions under the Securities Act and state securities Laws). Other than the Subsidiaries listed on Section 5.3 of the Parent Disclosure Letter, neither Parent nor any of its Subsidiaries owns, directly or indirectly, any equity, membership interest, partnership interest, joint venture interest or other equity or voting interest in, or any interest convertible into, exercisable or exchangeable for any of the foregoing, nor is it under any current or prospective obligation to form or participate in, provide funds to, make any loan, capital contribution, guarantee, credit enhancement or other investment in, or assume any liability or obligation of, any Person. No Subsidiary of Parent is in violation of its organizational documents.
Section 5.4 Authority.
(a) Each of Parent and Merger Sub has all necessary corporate power and authority to execute, deliver and perform its obligations under this Agreement and to consummate the Merger and the other transactions contemplated hereby. The execution, delivery and performance of this Agreement by Parent and Merger Sub and the consummation by Parent and Merger Sub of the Merger and the other transactions contemplated hereby have been duly authorized by all necessary corporate action on the part of Parent and Merger Sub and no other corporate proceedings on the part of Parent or Merger Sub are necessary to approve this Agreement or to consummate the Merger and the other transactions contemplated hereby, subject to (i) the Parent Stockholder Approval and (ii) the approval of this Agreement by Parent as the sole stockholder of Merger Sub.
-35-
This Agreement has been duly executed and delivered by Parent and Merger Sub and, assuming the due authorization, execution and delivery by the Company, constitutes a valid and binding obligation of each of Parent and Merger Sub, enforceable against each of Parent and Merger Sub in accordance with its terms (except to the extent that enforceability may be limited by applicable bankruptcy, insolvency, moratorium, reorganization or similar Laws affecting the enforcement of creditors’ rights generally or by general principles of equity).
(b) The Board of Directors of Parent (the “Parent Board”), at a meeting duly called and held at which all directors of Parent were present, duly and unanimously adopted resolutions (i) determining that the terms of this Agreement, the Merger and the other transactions contemplated hereby are fair to and in the best interests of Parent and its stockholders, (ii) approving and declaring advisable this Agreement and the transactions contemplated hereby, including the Merger, and (iii) resolving to recommend, upon the terms and subject to the conditions of this Agreement, that the stockholders of Parent vote to approve the Parent Stockholder Matters, which resolutions have not been subsequently rescinded, modified or withdrawn in any way.
(c) The Parent Stockholder Approval is the only vote of the holders of any class or series of Parent capital stock or other securities required in connection with the consummation of the Merger and the other transactions contemplated hereby. Other than the Parent Stockholder Approval, no vote of the holders of any class or series of the Parent’s capital stock or other securities is required in connection with the consummation of any of the transactions contemplated hereby to be consummated by Parent.
Section 5.5 No Conflict; Consents and Approvals.
(a) Subject to obtaining the Parent Stockholder Approval, the execution, delivery and performance of this Agreement by each of Parent and Merger Sub does not, and the consummation of the Merger and the other transactions contemplated hereby and compliance by each of Parent and Merger Sub with the provisions hereof will not, conflict with, or result in any violation or breach of, or default (with or without notice or lapse of time, or both):
(i) result in the creation of any Lien in or upon any of the properties, assets or rights of Parent or any of its Subsidiaries;
(ii) contravene, conflict with or result in a violation of any of the provisions of the Parent Charter, the Parent Bylaws or any of the organizational documents of Merger Sub;
(iii) conflict with, or result in the violation or breach of, or default under, or give rise to a right of, or result in, termination, cancellation, modification or acceleration of any obligation or any increased, additional, accelerated or guaranteed rights or entitlements under, or require any consent, waiver or approval of any Person pursuant to, any provisions of, any material Contract to which Parent or any of its Subsidiaries is a party or by which Parent, its Subsidiaries or any of their respective properties or assets may be bound, except in the case of any nonmaterial breach, default, penalty or modification; or
(iv) subject to the governmental filings and other matters referred to in Section 5.5(b), contravene, conflict with or result in a material violation of, any Law applicable to Parent or its Subsidiaries or by which Parent, its Subsidiaries or any of their respective properties or assets may be bound, except as would not reasonably be expected to be material to Parent, its Subsidiaries or any of their respective properties or assets.
-36-
(b) Other than pursuant to the Securities Act, the Exchange Act, any similar state securities Laws or any rule or regulation of Nasdaq applicable to Parent or Merger Sub or by which Parent, Merger Sub or any of their respective properties or assets may be bound, no consent, approval, order or authorization of, or registration, declaration, filing with or notice to, any Governmental Entity is required by or with respect to Parent or Merger Sub in connection with the execution, delivery and performance of this Agreement by Parent or Merger Sub or the consummation by Parent or Merger Sub of the Merger and the other transactions contemplated hereby or compliance with the provisions hereof, except for such consents, approvals, orders, authorizations, registrations, declarations, filings or notices the failure of which to be obtained or made, individually or in the aggregate, would not reasonably be expected to be material to Parent, Merger Sub or any of their respective properties or assets.
(c) The Parent Board and the Merger Sub board have taken all actions necessary to ensure that the Takeover Laws are, and will be, inapplicable to the execution, delivery and performance of this Agreement and to the consummation of the transactions contemplated by this Agreement.
Section 5.6 SEC Reports; Financial Statements.
(a) Parent has filed with or furnished, as applicable, to the SEC on a timely basis true and complete copies of all forms, reports, schedules, statements and other documents required to be filed with or furnished, as applicable, to the SEC by Parent since July 1, 2022 (all such documents, together with all exhibits and schedules to the foregoing materials and all information incorporated therein by reference, the “Parent SEC Documents”). As of their respective filing dates (or, if amended or superseded by a filing prior to the date of this Agreement, then on the date of such filing), the Parent SEC Documents complied in all material respects with the applicable requirements of the Securities Act, the Exchange Act and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as the case may be, including, in each case, the rules and regulations promulgated thereunder, and none of the Parent SEC Documents contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading, in each case giving effect to any amendments thereto filed or furnished prior to the date that is three Business Days before the date of this Agreement.
(b) The financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents (i) have been prepared in a manner consistent with the books and records of Parent and its Subsidiary, (ii) have been prepared in accordance with GAAP (except, in the case of unaudited statements, as permitted by the SEC on Form 10‑Q under the Exchange Act) applied on a consistent basis during the periods involved (except as may be indicated in the notes thereto), (iii) comply as to form in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto and (iv) fairly present in all material respects the consolidated financial position of Parent and its Subsidiaries as of the dates thereof and their respective consolidated results of operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal and recurring year-end audit adjustments that were not, or are not expected to be, material in amount), all in accordance with GAAP, and complied in all material respects with the published rules and regulations promulgated by the SEC. Since July 1, 2023, Parent has not made any change in the accounting practices or policies applied in the preparation of its financial statements, except as required by GAAP, SEC rule or policy or applicable Law. The books and records of Parent and its Subsidiaries have been, and are being, maintained in all material respects in accordance with GAAP (to the extent applicable) and in accordance with applicable Law.
-37-
(c) Parent has established and maintains disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Such disclosure controls and procedures are designed to ensure that information relating to Parent, including its consolidated Subsidiaries, required to be disclosed in Parent’s periodic and current reports under the Exchange Act, is made known to Parent’s chief executive officer and its chief financial officer by others within those entities to allow timely decisions regarding required disclosures as required under the Exchange Act. The chief executive officer and chief financial officer of Parent have evaluated the effectiveness of Parent’s disclosure controls and procedures and, to the extent required by applicable Law, presented in any applicable Parent SEC Document that is a report on Form 10‑K or Form 10‑Q, or any amendment thereto, its conclusions about the effectiveness of the disclosure controls and procedures as of the end of the period covered by such report or amendment based on such evaluation.
(d) Parent and its Subsidiaries have established and maintain a system of internal control over financial reporting (as defined in Rules 13a‑15(f) and 15d‑15(f) under the Exchange Act) which is effective in providing reasonable assurance regarding the reliability of Parent’s financial reporting and the preparation of Parent’s financial statements for external purposes in accordance with GAAP. Parent has disclosed, based on its most recent evaluation of Parent’s internal control over financial reporting prior to the date hereof, to Parent’s auditors and audit committee (i) any significant deficiencies and material weaknesses in the design or operation of Parent’s internal control over financial reporting which are reasonably likely to adversely affect Parent’s ability to record, process, summarize and report financial information and (ii) any fraud, whether or not material, that involves management or other employees who have a significant role in Parent’s internal control over financial reporting. A true, correct and complete summary of any such disclosures made by management to Parent’s auditors and audit committee is set forth as Section 5.6(d) of Parent Disclosure Letter.
(e) Since July 1, 2023, (i) neither Parent nor any of its Subsidiaries nor, to the knowledge of the Company, any director, officer, employee, auditor, accountant or representative of the Parent or any of its Subsidiaries has received or otherwise had or obtained knowledge of any material complaint, allegation, assertion or claim, whether written or oral, regarding the accounting or auditing practices, procedures, methodologies or methods of Parent or any of its Subsidiaries or their respective internal accounting controls, including any material complaint, allegation, assertion or claim that Parent or any of its Subsidiaries has engaged in questionable accounting or auditing practices and (ii) no attorney representing Parent or any of its Subsidiaries, whether or not employed by Parent or any of its Subsidiaries, has reported evidence of a material violation of securities Laws, breach of fiduciary duty or similar violation by Parent or any of its Subsidiaries or any of their respective officers, directors, employees or agents to the Parent Board or any committee thereof or to any director or officer of Parent or any of its Subsidiaries.
-38-
(f) As of the date of this Agreement, there are no outstanding or unresolved comments in the comment letters received from the SEC staff with respect to the Parent SEC Documents. To the knowledge of Parent, none of the Parent SEC Documents is subject to ongoing review or outstanding SEC comment or investigation.
(g) Parent is in compliance in all material respects with (i) the provisions of the Sarbanes-Oxley Act and (ii) the rules and regulations of Nasdaq, in each case, that are applicable to Parent.
(h) No Subsidiary of Parent is required to file any form, report, schedule, statement or other document with the SEC.
Section 5.7 No Undisclosed Liabilities. Neither Parent nor any of its Subsidiaries has any Liability, except (a) to the extent accrued or reserved against in the unaudited consolidated balance sheet of Parent and its Subsidiaries as of December 31, 2023 included in the Quarterly Report on Form 10‑Q filed by Parent with the SEC on February 14, 2024 (without giving effect to any amendment thereto filed on or after the date hereof), (b) for liabilities and obligations incurred in the Ordinary Course of Business since December 31, 2023 that are not material to Parent and its Subsidiaries, taken as a whole, (c) Liabilities incurred in connection with the transactions contemplated hereby or (d) Liabilities which would not, individually or in the aggregate, reasonably be expected to be material to Parent or any of its Subsidiaries.
Section 5.8 Absence of Certain Changes or Events. Except as disclosed in the Parent SEC Documents, since December 31, 2023 through the date of this Agreement, (i) except in connection with the execution of this Agreement and the consummation of the transactions contemplated hereby, Parent and its Subsidiaries have conducted their respective business only in the Ordinary Course of Business; (ii) there has not been any Parent Material Adverse Effect and (iii) neither Parent nor any of its Subsidiaries have taken any action or committed or agreed to take any action that would be prohibited by Section 6.1(b) (without giving effect to Schedule 6.1) if such action were taken on or before the date hereof without the consent of the Company.
Section 5.9 Litigation. There is no Action (or basis therefor) pending or, to the knowledge of Parent, threatened against or affecting Parent or any of its Subsidiaries, or any of their respective material properties or assets, or any present or former officer, director or employee of Parent or any of its Subsidiaries in such individual’s capacity as such. Neither Parent nor any of its Subsidiaries nor any of their respective properties or assets is subject to any outstanding judgment, order, injunction, rule or decree of any Governmental Entity. There is no Action pending or, to the knowledge of Parent, threatened seeking to prevent, hinder, modify, delay or challenge the Merger or any of the other transactions contemplated by this Agreement.
Section 5.10 Compliance with Laws. Parent and each of its Subsidiaries are and have been in compliance in all material respects with all Laws applicable to their businesses, operations, properties or assets, including laws relating to escheat and unclaimed property. None of Parent or any of its Subsidiaries has received, since January 1, 2021, a notice or other written communication alleging or relating to a possible material violation of any Law applicable to their businesses, operations, properties, assets or Parent Products (as defined below).
-39-
Parent and each of its Subsidiaries have in effect all material Permits of all Governmental Entities necessary or advisable for them to own, lease or operate their properties and assets and to carry on their respective businesses and operations as now conducted, and there has occurred no violation of, default (with or without notice or lapse of time or both) under or event giving to others any right of revocation, non-renewal, adverse modification or cancellation of, with or without notice or lapse of time or both, any such Permit, nor would any such revocation, nonrenewal, adverse modification or cancellation result from the consummation of the transactions contemplated hereby.
Section 5.11 Health Care Regulatory Matters. Except as set forth in Section 5.11 of the Parent Disclosure Letter:
(a) Parent and its Subsidiaries, and to the knowledge of Parent, each of their respective directors, officers, management employees, agents (while acting in such capacity), contract manufacturers, suppliers and distributors are, and at all times prior hereto were, in material compliance with all health care laws to the extent applicable to Parent, its Subsidiaries or any of their respective products or activities, including, but not limited to the Health Care Laws, to the extent applicable to Parent. To the knowledge of Parent, there are no facts or circumstances that reasonably would be expected to give rise to any material liability under any Health Care Laws.
(b) Neither Parent nor any of its Subsidiaries is party to any material corporate integrity agreements, monitoring agreements, consent decrees, settlement orders or similar agreements with or imposed by any Governmental Entity.
(c) All applications, notifications, submissions, information, claims, reports and statistical analyses, and other data and conclusions derived therefrom, utilized as the basis for or submitted in connection with any and all requests for a Permit from the FDA or other Governmental Entity relating to products that are regulated as drugs, medical devices, or other healthcare products under Health Care Laws, including drug and biological candidates, medical devices, compounds or products being researched, tested, stored, developed, labeled, manufactured, packed, marketed, sold and/or distributed by Parent or any of its Subsidiaries (“Parent Products”), including, without limitation, investigational new drug applications and investigational device exemptions, when submitted to the FDA or other Governmental Entity were true, complete and correct in all material respects as of the date of submission and any necessary or required updates, changes, corrections or modification to such applications, submissions, information and data have been submitted to the FDA or other Governmental Entity. Neither Parent nor any of its Subsidiaries has knowledge of any facts or circumstances that would be reasonably likely to lead the revocation, suspension, limitation, or cancellation of a Permit required under Health Care Laws or any application for marketing approval currently pending before the FDA or such other Governmental Entity.
(d) All preclinical studies and clinical trials conducted by or, to the knowledge of Parent, on behalf of Parent or any of its Subsidiaries have been, and if still pending are being, conducted in compliance with research protocols and all applicable Health Care Laws, including, but not limited to, the FDCA and its applicable implementing regulations at 21 C.F.R. Parts 50, 54, 56, 58, 312, 314 and 812. No clinical trial conducted by or on behalf of Parent or any of its Subsidiaries has been conducted using any clinical investigators who have been disqualified.
-40-
No clinical trial conducted by or on behalf of the Parent or any of its Subsidiaries has been terminated or suspended prior to completion, and no clinical investigator that has participated or is participating in, or institutional review board that has or has had jurisdiction over, a clinical trial conducted by or on behalf of Parent or any of its Subsidiaries has placed a clinical hold order on, or otherwise terminated, delayed or suspended, such a clinical trial at a clinical research site based on an actual or alleged lack of safety or efficacy of any Parent Product or a failure to conduct such clinical trial in compliance with applicable Health Care Laws.
(e) All manufacturing operations conducted by or, to the knowledge of Parent, for the benefit of Parent or any of its Subsidiaries have been and are being conducted in material compliance with all Permits under applicable Health Care Laws, all applicable provisions of the FDA’s current good manufacturing practice (cGMP) regulations at 21 C.F.R. Parts 210-211 and Parts 600 and 610 and FDA’s Quality System (QS) regulations at 21 C.F.R. Part 820, and all comparable foreign regulatory requirements of any Governmental Entity.
(f) Neither Parent nor any of its Subsidiaries has received any written communication that relates to an alleged violation or noncompliance with any Health Care Laws, including any notification of any pending or threatened claim, suit, proceeding, hearing, enforcement, investigation, arbitration, import detention or refusal, FDA Warning Letter or Untitled Letter, or any action by a Governmental Entity relating to any Health Care Laws. All Warning Letters, Form-483 observations, or comparable findings from other Governmental Entities listed in Section 5.11 of the Parent Disclosure Letter have been resolved to the satisfaction of the applicable Governmental Entity.
(g) There have been no seizures, withdrawals, recalls, detentions or suspensions of manufacturing, testing, or distribution relating to the Parent Products required or requested by a Governmental Entity, or other Safety Notices, and, to the knowledge of Parent, there are no facts or circumstances that reasonably would be expected to give rise to a Safety Notice. All Safety Notices listed in Section 5.11(g) of the Parent Disclosure Letter have been resolved to the satisfaction of the applicable Governmental Entity.
(h) Except as set forth in Section 5.11(g) of the Parent Disclosure Letter, there are no unresolved Safety Notices, and to the knowledge Parent, there are no facts that would be reasonably likely to result in a material Safety Notice with respect to the Parent Products or a termination or suspension of developing and testing of any of the Parent Products.
(i) Neither Parent or any of its Subsidiaries, nor, to the knowledge of Parent, any officer, employee, agent or distributor of Parent or any of its Subsidiaries has made an untrue statement of a material fact or fraudulent or misleading statement to a Governmental Entity, failed to disclose a material fact required to be disclosed to a Governmental Entity, or committed an act, made a statement or failed to make a statement that would reasonably be expected to provide a basis for the FDA to invoke its FDA Ethics Policy. None of the aforementioned is or has been under investigation resulting from any allegedly untrue, fraudulent, misleading, or false statement or omission, including data fraud, or had any action pending or threatened relating to the FDA Ethics Policy.
(j) All reports, documents, claims, Permits and notices required to be filed, maintained or furnished to the FDA or any Governmental Entity by Parent or any of its Subsidiaries have been so filed, maintained or furnished, except where failure to file, maintain or furnish such reports, documents, claims, Permits or notices have not had and would not reasonably be expected to have, individually or in the aggregate, a Parent Material Adverse Effect.
-41-
All such reports, documents, claims, Permits and notices were true and complete in all material respects on the date filed (or were corrected in or supplemented by a subsequent filing).
(k) Neither Parent or any of its Subsidiaries nor, to the knowledge of Parent, any officer, employee, agent, or distributor of Parent or any of its Subsidiaries has been convicted of any crime or engaged in any conduct that has resulted, or would reasonably be expected to result, in debarment under applicable Law, including, without limitation, 21 U.S.C. § 335a, or exclusion under 42 U.S.C. § 1320a-7, or any other statutory provision or similar Law. Neither Parent or any of its Subsidiaries nor, to the knowledge of Parent, any officer, employee, agent or distributor of Parent or any of its Subsidiaries, has been excluded from participation in any federal health care program or convicted of any crime or engaged in any conduct for which such Person could be excluded from participating in any federal health care program under Section 1128 of the Social Security Act of 1935, as amended, or any similar Health Care Law or program.
Section 5.12 Benefit Plans.
(a) Section 5.12(a) of the Parent Disclosure Letter contains a true and complete list of each material “employee benefit plan” (within the meaning of section 3(3) of ERISA, whether or not subject to ERISA), “multiemployer plans” (within the meaning of ERISA section 3(37)), and all stock purchase, stock option, phantom stock or other equity-based plan, severance, employment, collective bargaining, change-in-control, fringe benefit, bonus, incentive, deferred compensation, supplemental retirement, health, life, or disability insurance, dependent care and all other employee benefit and compensation plans, agreements, programs, policies or other arrangements, whether or not subject to ERISA (including any funding mechanism therefor now in effect or required in the future as a result of the transactions contemplated by this Agreement or otherwise), whether formal or informal, written or oral, legally binding or not, under which any current or former employee, director or consultant of Parent or any of its Subsidiaries (or any of their dependents) has any present or future right to compensation or benefits or Parent or any of its Subsidiaries sponsors or maintains, is making contributions to or has any present or future liability or obligation (contingent or otherwise) or with respect to which it is otherwise bound. All such plans, agreements, programs, policies and arrangements shall be collectively referred to as the “Parent Plans.” Parent has provided or made available to the Company a current, accurate and complete copy of each material Parent Plan, or if such Parent Plan is not in written form, a written summary of all of the material terms of such Parent Plan. With respect to each Parent Plan, Parent has furnished or made available to the Company a current, accurate and complete copy of, to the extent applicable: (i) any related trust agreement or other funding instrument, (ii) the most recent determination letter of the IRS, (iii) any summary plan description, summary of material modifications, and other similar material written communications (or a written description of any material oral communications) to the employees of Parent or any of its Subsidiaries concerning the extent of the benefits provided under a Parent Plan, and (iv) for the three most recent years and as applicable (A) the Form 5500 and attached schedules, (B) audited financial statements and (C) actuarial valuation reports.
-42-
(b) Neither Parent, its Subsidiaries or any member of their Controlled Group (defined as any organization which is a member of a controlled, affiliated or otherwise related group of entities within the meaning of Code Sections 414(b), (c), (m) or (o)) has ever sponsored, maintained, contributed to or been required to contribute to or incurred any liability (contingent or otherwise) with respect to: (i) a “multiemployer plan” (within the meaning of ERISA section 3(37)), (ii) a Pension Plan that is subject to Title IV of ERISA or Section 412 of the Code, (iii) a Pension Plan which is a “multiple employer plan” as defined in Section 413 of the Code or (iv) a “funded welfare plan” within the meaning of Section 419 of the Code.
(c) With respect to the Parent Plans:
(i) each Parent Plan complies in all material respects with its terms and materially complies in form and in operation with the applicable provisions of ERISA and the Code and all other applicable legal requirements;
(ii) each Parent Plan intended to be qualified under Section 401(a) of the Code has received a favorable determination, advisory and/or opinion letter, as applicable, from the IRS that it is so qualified and nothing has occurred to the knowledge of Parent since the date of such letter that would reasonably be expected to cause the loss of the sponsor’s ability to rely upon such letter, and nothing has occurred to the knowledge of the Parent that would reasonably be expected to result in the loss of the qualified status of such Parent Plan;
(iii) there is no material Action (including any investigation, audit or other administrative proceeding) by the Department of Labor, the PBGC, the IRS or any other Governmental Entity or by any plan participant or beneficiary pending, or to the knowledge of Parent, threatened, relating to the Parent Plans, any fiduciaries thereof with respect to their duties to Parent Plans or the assets of any of the trusts under any of Parent Plans (other than routine claims for benefits) nor, to Parent’s knowledge, are there facts or circumstances that exist that could reasonably give rise to any such actions;
(iv) none of the Parent Plans currently provides, or reflects or represents any liability to provide post-termination or retiree welfare benefits to any person for any reason, except as may be required by COBRA or through the last day of the month in which an employee separates from service, and none of Parent, its Subsidiaries or any members of their Parent Controlled Group has any liability to provide post-termination or retiree welfare benefits to any person or ever represented, promised or contracted to any employee or former employee of Parent or any of its Subsidiaries (either individually or to employees as a group) or any other person that such employee(s) or other person would be provided with post-termination or retiree welfare benefits, except to the extent required by statute or except with respect to a contractual obligation to reimburse any premiums such person may pay in order to obtain health coverage under COBRA;
(v) each Parent Plan is subject exclusively to United States Law; and
(vi) the execution and delivery of this Agreement and the consummation of the Merger will not, either alone or in combination with any other event, (A) entitle any current or former employee, officer, director or consultant of Parent or any of its Subsidiaries to severance pay, unemployment compensation or any other similar termination payment, or (B) accelerate the time of payment or vesting, or increase the amount of or otherwise enhance any benefit due any such employee, officer, director or consultant.
-43-
(d) Neither Parent nor any of its Subsidiaries is a party to any agreement, contract, arrangement or plan (including any Parent Plan) that may reasonably be expected to result, separately or in the aggregate, in connection with the transactions contemplated by this Agreement (either alone or in combination with any other events), in the payment of any “parachute payments” within the meaning of Section 280G of the Code. There is no agreement, plan or other arrangement to which Parent or any of its Subsidiaries is a party or by which Parent or any of its Subsidiaries is otherwise bound to compensate any person in respect of Taxes or other liabilities incurred with respect to Section 409A or 4999 of the Code.
Section 5.13 Labor and Employment Matters.
(a) Parent and its Subsidiaries are and for the past three (3) years have been in compliance in all material respects with all applicable Laws relating to labor and employment, including those relating to employment practices, terms and conditions of employment, collective bargaining, disability, immigration, health and safety, wages, hours and benefits, non-discrimination in employment, workers’ compensation, the collection and payment of withholding and/or payroll Taxes and similar Taxes, unemployment compensation, equal employment opportunity, discrimination, harassment, employee and contractor classification, information privacy and security, and continuation coverage with respect to group health plans. During the preceding three years, there has not been, and as of the date of this Agreement there is not pending or, to the knowledge of Parent, threatened, any labor dispute, work stoppage, labor strike or lockout against Parent or any of its Subsidiaries by employees.
(b) No employee of Parent or any of its Subsidiaries is covered by an effective or pending collective bargaining agreement or similar labor agreement. To the knowledge of Parent, there has not been any activity on behalf of any labor union, labor organization or similar employee group to organize any employees of Parent or any of its Subsidiaries. There are no (i) unfair labor practice charges or complaints against Parent or any of its Subsidiaries pending before the National Labor Relations Board or any other labor relations tribunal or authority and to the knowledge of Parent no such representations, claims or petitions are threatened, (ii) representation claims or petitions pending before the National Labor Relations Board or any other labor relations tribunal or authority or (iii) grievances or pending arbitration proceedings against Parent or any of its Subsidiaries that arose out of or under any collective bargaining agreement.
(c) To the knowledge of Parent, no current employee or officer of Parent or any of its Subsidiaries intends, or is expected, to terminate his employment relationship with such entity in connection with or as a result of the transactions contemplated hereby.
(d) During the preceding three (3) years, (i) neither Parent nor any of its Subsidiaries has effectuated a “plant closing” (as defined in the WARN Act) affecting any site of employment or one or more facilities or operating units within any site of employment or facility, (ii) there has not occurred a “mass layoff” (as defined in the WARN Act) in connection with Parent or any of its Subsidiaries affecting any site of employment or one or more facilities or operating units within any site of employment or facility and (iii) neither Parent nor any of its Subsidiaries has engaged in layoffs or employment terminations sufficient in number to trigger application of any similar state, local or foreign law. Parent and its Subsidiaries currently properly classify and for the past three (3) years have properly classified their respective employees as exempt or nonexempt in accordance with applicable overtime laws and, to the knowledge of Parent, no person treated as an independent contractor or consultant by Parent or any of its Subsidiaries within the past three (3) years should have been properly classified as an employee under applicable Law.
-44-
(e) Except as set forth on Section 5.13(e) of the Parent Disclosure Letter, with respect to any current or former employee, officer, consultant or other service provider of Parent or any of its Subsidiaries, there are no Actions against Parent or any of its Subsidiaries pending, or to Parent’s knowledge, threatened to be brought or filed, in connection with the employment or engagement of any current or former employee, officer, consultant or other service provider of Parent or any of its Subsidiaries, including, without limitation, any claim relating to employment discrimination, harassment, retaliation, equal pay, employment classification or any other employment related matter arising under applicable Laws, except where such action would not, individually or in the aggregate, result in Parent or any of its Subsidiaries incurring a material liability.
(f) Except as set forth on Section 5.13(f) of the Parent Disclosure Letter, the execution of this Agreement and the consummation of the transactions set forth in or contemplated by this Agreement will not result in any breach or violation of, or cause any payment to be made under, any applicable Laws respecting labor and employment or any collective bargaining agreement to which Parent or any of its Subsidiaries is a party.
(g) Since January 1, 2022, (i) no allegations of workplace sexual harassment, discrimination or other misconduct have been made, initiated, filed or, to the knowledge of Parent, threatened against Parent or any of its Subsidiaries or any of their respective current or former directors, officers or senior level management employees, (ii) to the knowledge of Parent, no incidents of any such workplace sexual harassment, discrimination or other misconduct have occurred and (iii) neither Parent nor any of its Subsidiaries has entered into any settlement agreement related to allegations of sexual harassment, discrimination or other misconduct by any of their directors, officers or employees described in clause (i) hereof or any independent contractor.
(h) Parent and its Subsidiaries are and have at all relevant times been in compliance in all material respects with (i) COVID-19 related Laws, standards, regulations, orders and guidance (including without limitation relating to business reopening), including those issued and enforced by the Occupational Safety and Health Administration, the Centers for Disease Control, the Equal Employment Opportunity Commission, and any other Governmental Entity; and (ii) the Families First Coronavirus Response Act (including with respect to eligibility for tax credits under such Act) and any other applicable COVID-19 related leave Law, whether state, local or otherwise.
Section 5.14 Environmental Matters.
-45-
(a) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect, (i) Parent and its Subsidiaries have conducted their respective businesses in compliance with all, and have not violated any, applicable Environmental Laws; (ii) Parent and its Subsidiaries have obtained all Permits of all Governmental Entities and any other Person that are required under any Environmental Law; (iii) there has been no release of any Hazardous Substance by Parent or any of its Subsidiaries or any other Person in any manner that has given or would reasonably be expected to give rise to any remedial or investigative obligation, corrective action requirement or liability of Parent or any of its Subsidiaries under applicable Environmental Laws; (iv) neither Parent nor any of its Subsidiaries has received any claims, notices, demand letters or requests for information (except for such claims, notices, demand letters or requests for information the subject matter of which has been resolved prior to the date of this Agreement) from any federal, state, local, foreign or provincial Governmental Entity or any other Person asserting that Parent or any of its Subsidiaries is in violation of, or liable under, any Environmental Law; (v) no Hazardous Substance has been disposed of, arranged to be disposed of, released or transported in violation of any applicable Environmental Law, or in a manner that has given rise to, or that would reasonably be expected to give rise to, any liability under any Environmental Law, in each case, on, at, under or from any current or former properties or facilities owned or operated by Parent or any of its Subsidiaries or as a result of any operations or activities of Parent or any of its Subsidiaries at any location and, to the knowledge of Parent, Hazardous Substances are not otherwise present at or about any such properties or facilities in amount or condition that has resulted in or would reasonably be expected to result in liability to Parent or any of its Subsidiaries under any Environmental Law and (vi) neither Parent, any of its Subsidiaries nor any of their respective properties or facilities are subject to, or are threatened to become subject to, any liabilities relating to any suit, settlement, court order, administrative order, regulatory requirement, judgment or claim asserted or arising under any Environmental Law or any agreement relating to environmental liabilities.
Section 5.15 Taxes.
(a) Parent has (i) filed all income and other material Tax Returns required to be filed by or on behalf of it (taking into account any applicable extensions thereof) and all such Tax Returns are true, accurate and complete in all material respects; and (ii) paid in full (or caused to be timely paid in full) all material Taxes that are required to be paid by or with respect to it, whether or not such Taxes were shown as due on such Tax Returns.
(b) All material Taxes not yet due and payable by Parent as of the date of the balance sheet included in the financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents have been, in all respects, properly accrued in accordance with GAAP on the financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents, and such financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents reflect an adequate reserve (in accordance with GAAP) for all material Taxes accrued but unpaid by Parent through the date of such financial statements. Since the date of financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents, Parent has not incurred, individually or in the aggregate, any material liability for Taxes outside the Ordinary Course of Business.
(c) Parent has not executed any waiver of any statute of limitations on, or extended the period for the assessment or collection of, any amount of Tax, in each case that has not since expired.
-46-
(d) No material Tax Action with respect to Taxes or any Tax Return of Parent are presently in progress or have been asserted, threatened or proposed in writing and to the knowledge of Parent, no such Tax Action is being contemplated. No deficiencies or claims for a material amount of Taxes have been claimed, proposed, assessed or asserted in writing against Parent by a Governmental Entity, other than any such claim, proposal, assessment or assertion that has been satisfied by payment in full, settled with no further liability on the part of the Parent or withdrawn.
(e) Subject to exceptions as would not be material, the Parent has timely withheld all Taxes required to have been withheld from payments made (or deemed made) to its employees, independent contractors, creditors, shareholders and other third parties and, to the extent required, such Taxes have been timely paid to the relevant Governmental Entity.
(f) Parent has not engaged in a “listed transaction” as set forth in Treasury Regulations § 1.6011-4(b)(2).
(g) Parent (i) is not a party to or bound by, or has any liability pursuant to, any Tax sharing, allocation, indemnification or similar agreement or obligation other than any Ordinary Course Agreement; (ii) is not and has not been a member of a group (other than a group the common parent of which is Parent) filing a consolidated, combined, affiliated, unitary or similar income Tax Return; (iii) does not have any liability for the Taxes of any Person (other than Parent) pursuant to Treasury Regulations § 1.1502-6 (or any similar provision of state, local or non-United States Law) as a transferee or successor, by Contract, or otherwise by operation of Law; or (iv) is not and has not been treated as a resident for any income Tax purpose, or as subject to Tax by virtue of having a permanent establishment, an office or fixed place of business, in any country other than the country in which it was or is organized.
(h) No private letter rulings, technical advice memoranda, or similar material agreements or rulings have been requested, entered into or issued by any taxing authority with respect to Parent which rulings remain in effect.
(i) Parent will not be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of (i) a change in, or use of improper, method of accounting requested or initiated at or prior to the Closing (other than any such change required, or any such use treated as improper, as a result of the transactions provided for herein), (ii) a “closing agreement” as described in Section 7121 of the Code (or any similar provision of Law) executed at or prior to the Closing, (iii) an installment sale or open transaction disposition made at or prior to the Closing, (iv) any prepaid amount received or deferred revenue accrued at or prior to the Closing, other than in respect of such amounts reflected in the balance sheet included in the financial statements (including the related notes and schedules thereto) included (or incorporated by reference) in the Parent SEC Documents, or received in the Ordinary Course of Business since the date of such balance sheet, (v) to Parent’s knowledge, an intercompany transaction or excess loss amount described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of state, local or foreign income Tax Law) or (vi) an election under Section 965 of the Code, or (vii) the application of Sections 951 or 951A of the Code with respect to income earned or recognized or payments received prior to the Closing.
-47-
(j) No non-U.S. Subsidiary of the Parent (i) has recognized or is expected to recognize any material amount of “subpart F income” as defined in Section 952 of the Code, or (ii) has recognized or is expected to recognize any material amount of income under Section 951A of the Code. No non-U.S. Subsidiary of the Company has recognized or is expected to recognize any material amount of income from the ownership or sale of any “United States real property interest” within the meaning of Section 897 of the Code.
(k) There are no liens for Taxes upon any of the assets of Parent other than Liens for current Taxes and assessments not yet past due.
(l) Parent has not distributed stock of another Person or has had its stock distributed by another Person, in a transaction (or series of transactions) that was purported or intended to be governed in whole or in part by Sections 355 or 361 of the Code.
(m) Parent has not been a United States real property holding corporation, as defined in Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code.
(n) No material claim has been made in writing by any Governmental Entity in a jurisdiction where Parent does not currently file or has not filed a Tax Return that Parent is or may be subject to taxation by such jurisdiction
(o) There are no outstanding shares of company stock issued in connection with the performance of services (within the meaning of Section 83 of the Code) that immediately prior to the Effective Time are subject to a substantial risk of forfeiture (as such terms are defined in Section 83 of the Code) or for which a valid election under Section 83(b) of the Code has not been made.
(p) To Parent’s knowledge, Parent has not been, is not, and immediately prior to the Effective Time will not be, treated as an “investment company” within the meaning of Section 368(a)(2)(F) of the Code.
(q) Parent has not taken any action nor knows of any fact or circumstance that could reasonably be expected to prevent the Merger (together, if required by Section 3.7, with the Second Merger) from qualifying as a transaction qualifying for the Intended Tax Treatment under Section 368 of the Code.
(r) For purposes of this Section 5.15, where the context permits, each reference to Parent shall include a reference to any person for whose Taxes Parent is liable under applicable Law.
Section 5.16 Contracts.
(a) Except as set forth in the Parent SEC Documents publicly available prior to the date of this Agreement, neither Parent nor any of its Subsidiaries is a party to or is bound by any “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K under the Securities Act excluding, however, any Company Plans) (all such contracts, “Parent Material Contracts”).
-48-
(b) As of the date of this Agreement, (i) each Parent Material Contract is legal, valid and binding on Parent or its applicable Subsidiary and to the knowledge of Parent, each other party thereto, and is in full force and effect and enforceable in accordance with its terms; (ii) Parent or its applicable Subsidiary, and, to the knowledge of Parent, each other party thereto, has performed all material obligations required to be performed by it under each Parent Material Contract and (iii) there is no material default under any Parent Material Contract by Parent or its applicable Subsidiary or, to the knowledge of Parent, any other party thereto, and no event or condition has occurred that constitutes, or, after notice or lapse of time or both, would constitute, a material default on the part of Parent or its applicable Subsidiary or, to the knowledge of Parent, any other party thereto under any such Parent Material Contract, nor has Parent or any of its Subsidiaries received any notice of any such material default, event or condition. Parent has made available to the Company true and complete copies of all Parent Material Contracts, including all amendments thereto.
Section 5.17 Insurance. Each of Parent and its Subsidiaries is covered by valid and currently effective insurance policies issued in favor of Parent or one or more of its Subsidiaries that are customary and adequate for companies of similar size in the industries and locations in which Parent operates. Section 5.17 of the Parent Disclosure Letter sets forth, as of the date hereof, a true and complete list of all material insurance policies issued in favor of Parent or any of its Subsidiaries, or pursuant to which Parent or any of its Subsidiaries is a named insured or otherwise a beneficiary, as well as any historic incurrence-based policies still in force. With respect to each such insurance policy, (a) such policy is in full force and effect and all premiums due thereon have been paid, (b) neither Parent nor any of its Subsidiaries is in breach or default, and has not taken any action or failed to take any action which (with or without notice or lapse of time, or both) would constitute such a breach or default, or would permit termination or modification of, any such policy and (c) to the knowledge of Parent, no insurer issuing any such policy has been declared insolvent or placed in receivership, conservatorship or liquidation. No notice of cancellation or termination has been received with respect to any such policy, nor, except as set forth on Section 5.17 of the Parent Disclosure Letter, will any such cancellation or termination result from the consummation of the transactions contemplated hereby.
Section 5.18 Properties.
(a) Parent or one of its Subsidiaries has good and valid title to, or in the case of leased property and leased tangible assets, a valid leasehold interest in, all of its real properties and tangible assets, free and clear of all Liens other than Permitted Liens. The tangible personal property currently used in the operation of the business of Parent and its Subsidiaries is in good working order (reasonable wear and tear excepted), is suitable and adequate for the uses for which it is intended or is being used and, except as set forth on Section 5.18(a) of the Parent Disclosure Letter, is physically located at locations to which the Parent or one of its Subsidiaries has a valid, written lease.
(b) Each of Parent and its Subsidiaries has complied with the terms of all leases to which it is a party, in all material respects, and all such leases are in full force and effect. Each of Parent and its Subsidiaries enjoys peaceful and undisturbed possession under all such leases.
-49-
(c) Section 5.18(c) of the Parent Disclosure Letter sets forth a true and complete list of (i) all real property owned by Parent or any of its Subsidiaries and (ii) all real property leased for the benefit of Parent or any of its Subsidiaries (the “Parent Real Estate Leases”).
(d) This Section 5.18 does not relate to intellectual property, which is the subject of Section 5.19.
Section 5.19 Intellectual Property.
(a) Section 5.19(a) of the Parent Disclosure Letter sets forth a true and complete list of all (i) material patents and patent applications; (ii) material trademark registrations and applications; and (iii) material copyright registrations and applications, in each case owned by the Parent and its Subsidiaries (collectively, “Parent Registered IP”) and a true and complete list of all domain names owned or exclusively licensed by Parent and its Subsidiaries. Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect, (i) all of the Parent Registered IP is subsisting and, in the case of any Parent Registered IP that is registered or issued and to the knowledge of Parent, valid and enforceable, (ii) no Parent Registered IP is involved in any interference, reissue, derivation, reexamination, opposition, cancellation or similar proceeding and, to the knowledge of Parent, no such action is threatened with respect to any of the Parent Registered IP and (iii) Parent or its Subsidiaries own exclusively, free and clear of any and all Liens (other than Permitted Liens), all Parent Owned IP, including all Intellectual Property created on behalf of Parent or its Subsidiaries by employees or independent contractors.
(b) Parent and its Subsidiaries have taken commercially reasonable measures to maintain the confidentiality of all information that constitutes or constituted a material Trade Secret of Parent or its Subsidiaries, including requiring all Persons having access thereto to execute written non-disclosure agreements or other binding obligations to maintain confidentiality of such information.
(c) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect, (i) to the knowledge of Parent, the conduct of the businesses of Parent and its Subsidiaries, including the manufacture, marketing, offering for sale, sale, importation, use or intended use or other disposal of any product as currently sold or under development by Parent or its Subsidiaries, has not infringed, misappropriated or diluted, and does not infringe, misappropriate or dilute, any Intellectual Property of any Person, (ii) neither Parent nor any of its Subsidiaries has received any written notice or claim asserting or suggesting that any such infringement, misappropriation, or dilution is or may be occurring or has or may have occurred and (iii) to the knowledge of Parent, no Person is infringing, misappropriating, or diluting in any material respect any Parent Registered IP.
(d) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect, (i) Parent and its Subsidiaries have taken commercially reasonable steps to protect the confidentiality and security of the computer and information technology systems used by Parent and its Subsidiaries (the “Parent IT Systems”) and the information and transactions stored or contained therein or transmitted thereby, (ii) to the knowledge of Parent, during the past two (2) years, there has been no unauthorized or improper use, loss, access, transmittal, modification or corruption of any such information or data, and (iii) during the past two (2) years, there have been no material failures, crashes, viruses, security breaches (including any unauthorized access to any personally identifiable information), affecting the Parent IT Systems.
-50-
(e) Except as, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect, (i) to the knowledge of Parent, Parent and its Subsidiaries have at all times complied in all material respects with all applicable Privacy Laws, (ii) during the past two (2) years, no claims have been asserted or, to the knowledge of Parent, threatened in writing against Parent or any of its Subsidiaries alleging a violation of any Person’s privacy or Personal Information, (iii) neither this Agreement nor the consummation of the transactions contemplated hereby will breach or otherwise violate any Privacy Laws and (iv) Parent and its Subsidiaries have taken commercially reasonable steps to protect the Personal Information collected, used or held for use by Parent or its Subsidiaries against loss and unauthorized access, use, modification, disclosure or other misuse.
(f) To the knowledge of Parent and, except as set forth on Section 5.9(f) of the Parent Disclosure Letter, no government funding, facilities or resources of a university, college, other educational institution or research center or funding from third parties was used in the development of the Parent Owned IP, to the knowledge of Parent, exclusively licensed to Parent or any of its Subsidiaries, and no Governmental Entity, university, college, other educational institution or research center has, to the knowledge of Parent, any claim or right in or to such Intellectual Property. Except as set forth on Section 5.19(f) of the Parent Disclosure Letter, the execution, delivery and performance by Parent of this Agreement, and the consummation of the transactions contemplated hereby, will not result in the loss of, or give rise to any right of any third party to terminate or modify any of Parent’s or any Subsidiaries’ rights or obligations under any agreement under which Parent or any of its Subsidiaries grants to any Person, or any Person grants to Parent or any of its Subsidiaries, a license or right under or with respect to any Intellectual Property that is material to any of the businesses of Parent or any of its Subsidiaries.
Section 5.20 Related Party Transactions. Since July 1, 2021 through the date of this Agreement, there have been no transactions, agreements, arrangements or understandings between Parent or any of its Subsidiaries, on the one hand, and the Affiliates of Parent, on the other hand (other than Parent’s Subsidiaries) that would be required to be disclosed under Item 404 of Regulation S-K under the Securities Act and that have not been so disclosed in the Parent SEC Documents.
Section 5.21 Certain Payments.
(a) Neither Parent nor any of its Subsidiaries nor, to the knowledge of Parent or any of its respective Representatives acting on their behalf (a) has used or is using any corporate funds for any illegal contributions, gifts, entertainment or other unlawful expenses relating to political activity, (b) has used or is using any corporate funds for any direct or indirect unlawful payments to any foreign or domestic governmental officials or employees, (c) has violated or is violating any provision of the Foreign Corrupt Practices Act of 1977 or any other applicable local or foreign anti-corruption or bribery Law, (d) has established or maintained, or is maintaining, any unlawful fund of corporate monies or other properties or (e) has made any bribe, unlawful rebate, payoff, influence payment, kickback or other unlawful payment of any nature
-51-
Section 5.22 Brokers. No broker, investment banker, financial advisor or other Person, other than Ladenburg Thalmann & Co. and Lucid Capital Markets, LLC (formerly known as Americas Executions, LLC), the fees and expenses of which will be paid by Parent, is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the transactions contemplated by this Agreement based upon arrangements made by or on behalf of Parent or any of its Affiliates. Parent has furnished to Company a true and complete copy of any Contract (a) between the Parent and Ladenburg Thalmann & Co. pursuant to which Ladenburg Thalmann & Co. could be entitled to any payment from the Parent relating to the transactions contemplated hereby and (b) between the Parent and Lucid Capital Markets, LLC pursuant to which Lucid Capital Markets, LLC could be entitled to any payment from the Parent relating to the transactions contemplated hereby.
Section 5.23 Opinion of Financial Advisor. Parent Board has received the opinion of Lucid Capital Markets, LLC, dated the date of this Agreement, to the effect that, as of such date and based upon and subject to the qualifications, limitations, assumptions and other matters set forth therein, the Exchange Ratio, is fair, from a financial point of view, to the holders of Parent Common Stock, a signed true and complete copy of which opinion has been or will promptly be provided to the Company.
Section 5.24 Merger Sub. Merger Sub was formed solely for the purpose of engaging in the Merger and the other transactions contemplated hereby and has engaged in no business other than in connection with the transactions contemplated by this Agreement.
Section 5.25 No Other Representations or Warranties. Parent acknowledges and agrees that, except for the representations and warranties of the Company set forth in ARTICLE IV or in any certificate delivered by the Company to Parent pursuant to this Agreement, neither Parent nor any other Person, is relying on any other representation or warranty of the Company or any other Person made outside of ARTICLE IV or such certificate, including regarding the accuracy or completeness of any such other representations or warranties or the omission of any material information, whether express or implied.
ARTICLE VI
COVENANTS
Section 6.1 Operation of Parent’s Business.
(a) Except (i) as expressly contemplated or permitted by this Agreement, including the Nasdaq Reverse Split, (ii) as set forth on Section 6.1(a) of the Parent Disclosure Letter, (iii) as required by applicable Law or (iv) unless the Company shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during the period commencing on the date of this Agreement and continuing until the earlier to occur of the termination of this Agreement pursuant to ARTICLE VIII and the Effective Time (the “Pre-Closing Period”), Parent shall, and shall cause its Subsidiaries to, use commercially reasonable efforts to conduct its business and operations in the Ordinary Course of Business and in material compliance with all applicable Law and the requirements of all Contracts that constitute Parent Material Contracts.
-52-
(b) Except (i) as expressly contemplated or permitted by this Agreement, including the Nasdaq Reverse Split, (ii) as set forth in Section 6.1(b) of the Parent Disclosure Letter, (iii) as required by applicable Law or (iv) with the prior written consent of the Company (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, Parent shall not, nor shall it cause or permit any of Subsidiaries to, do any of the following:
(i) other than the CVR Distribution, declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of its capital stock or repurchase, redeem or otherwise reacquire any shares of its capital stock or other securities (except for repurchase or redemption of shares of Parent Common Stock from terminated employees, directors or consultants of Parent);
(ii) other than the CVR Distribution, sell, issue, grant, pledge or otherwise dispose of or encumber or authorize the issuance of: (A) any capital stock or other security (except for shares of Parent Common Stock issued upon the valid exercise of Parent Options or Parent Warrants, or the settlement of Parent RSUs, in each case as issued and outstanding as of the date of this Agreement or issued in accordance with this Section 6.1), (B) any option, warrant or right to acquire any capital stock or any other security or (C) any instrument convertible into or exchangeable for any capital stock or other security;
(iii) except as required to give effect to anything in contemplation of the Closing, amend any of its organizational documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the transactions contemplated by this Agreement;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Person or enter into a joint venture with any other Person;
(v) A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others or (D) other than the incurrence or payment of Transaction Expenses, make any capital expenditure or commitment in excess of $10,000;
(vi) (A) adopt, establish or enter into any employee plan, including, for avoidance of doubt, any equity awards plans, (B) cause or permit any employee plan to be amended other than as required by Law or in order to make amendments for the purposes of compliance with Section 409A of the Code, (C) pay any bonus or make any profit-sharing or similar payment to (except with respect to obligations in place on the date of this Agreement pursuant to any employee plan disclosed to the Company), or increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors, officers, employees or consultants, (D) increase the severance or change of control
-53-
benefits offered to any current or new employees, directors or consultants or (E) hire any officer, employee or consultant;
(vii) enter into any material transaction outside the Ordinary Course of Business;
(viii) acquire any material asset or sell, lease, license or otherwise irrevocably dispose of any of its assets or properties, or grant any Lien with respect to such assets or properties;
(ix) other than in the Ordinary Course of Business: (A) make, change or revoke any material Tax election; (B) file any amended income or other material Tax Return; (C) adopt or change any material accounting method in respect of Taxes; (D) enter into any material Tax closing agreement, settle any material Tax claim or assessment; (E) consent to any extension or waiver of the limitation period applicable to or relating to any material Tax claim or assessment or (F) surrender any material claim for refund;
(x) waive, settle or compromise any pending or threatened Action against Parent or any of its Subsidiaries, other than waivers, settlements or agreements (A) for an amount not in excess of $50,000 in the aggregate (excluding amounts to be paid under existing insurance policies or renewals thereof) and (B) that do not impose any material restrictions on the operations or businesses of Parent or its Subsidiaries, taken as a whole, or any equitable relief on, or the admission of wrongdoing by Parent or any of its Subsidiaries;
(xi) delay or fail to repay when due any material obligation, including accounts payable and accrued expenses;
(xii) forgive any loans to any Person, including its employees, officers, directors or Affiliate;
(xiii) other than, for the avoidance of doubt, obtaining “tail” insurance coverage in connection with the Closing, terminate or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
(xiv) (A) materially change pricing or royalties or other payments set or charged by Parent or any of its Subsidiaries to its customers or licensees or (B) agree to materially change pricing or royalties or other payments set or charged by Persons who have licensed Intellectual Property to Parent or any of its Subsidiaries;
(xv) enter into, amend or terminate any Parent Material Contract; or
(xvi) agree, resolve or commit to do any of the foregoing.
Nothing contained in this Agreement shall give the Company, directly or indirectly, the right to control or direct the operations of Parent prior to the Effective Time. Prior to the Effective Time, Parent shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations. Notwithstanding anything to the contrary set forth in this Agreement, no consent of the Company shall be required with respect to any matter set forth in this Section 6.1 or elsewhere in this Agreement to the extent that the requirement of such consent could violate any applicable Laws.
-54-
Section 6.2 Operation of the Company’s Business.
(a) Except (i) as expressly contemplated or permitted by this Agreement, (ii) as set forth in Section 6.2(a) of the Company Disclosure Letter, (iii) as required by applicable Law or (iv) unless Parent shall otherwise consent in writing (which consent shall not be unreasonably withheld, delayed or conditioned), during the Pre-Closing Period the Company shall, and shall cause its Subsidiaries to, use commercially reasonable efforts to conduct its business and operations in the Ordinary Course of Business and in material compliance with all applicable Law and the requirements of all Contracts that constitute Company Material Contracts.
(b) Except (i) as expressly contemplated or permitted by this Agreement, (ii) as set forth in Section 6.2(b) of the Company Disclosure Letter, (iii) as required by applicable Law or (iv) with the prior written consent of Parent (which consent shall not be unreasonably withheld, delayed or conditioned), at all times during the Pre-Closing Period, the Company shall not, nor shall it cause or permit any of its Subsidiaries to, do any of the following:
(i) declare, accrue, set aside or pay any dividend or make any other distribution in respect of any shares of capital stock; or repurchase, redeem or otherwise reacquire any shares of capital stock of the Company or other securities (except for repurchase or redemption of shares of Company Common Stock from terminated employees, directors or consultants of the Company);
(ii) except as required to give effect to anything in contemplation of the Closing, amend any of its or its Subsidiaries’ organizational documents, or effect or be a party to any merger, consolidation, share exchange, business combination, recapitalization, reclassification of shares, stock split, reverse stock split or similar transaction except, for the avoidance of doubt, the transactions contemplated by this Agreement;
(iii) sell, issue, grant, pledge or otherwise dispose of or encumber or authorize any of the foregoing actions with respect to: (A) any capital stock or other security of the Company or any of its Subsidiaries (except for shares of Company Common Stock issued upon the valid exercise of Company Options or Company Warrants issued and outstanding as of the date of this Agreement or issued in accordance with this Section 6.2), (B) any option, warrant or right to acquire any capital stock or any other security or (C) any instrument convertible into or exchangeable for any capital stock or other security of the Company or any of its Subsidiaries;
(iv) form any Subsidiary or acquire any equity interest or other interest in any other Person or enter into a joint venture with any other Person;
(v) (A) lend money to any Person, (B) incur or guarantee any indebtedness for borrowed money, (C) guarantee any debt securities of others or (D) make any capital expenditure or commitment in excess of $100,000;
(vi) other than in the Ordinary Course of Business: (A) adopt, establish or enter into any employee plan, including, for the avoidance of doubt, any equity awards plans,
-55-
(B) cause or permit any employee plan to be amended other than as required by law or in order to make amendments for the purposes of compliance with Section 409A of the Code, (C) pay any material bonus or make any material profit-sharing or similar payment to (except with respect to obligations in place on the date of this Agreement pursuant to any employee plan disclosed to Parent), or materially increase the amount of the wages, salary, commissions, fringe benefits or other compensation or remuneration payable to, any of its directors, officers or employees, (D) increase the severance or change of control benefits offered to any current or new employees, directors or consultants or (E) hire, engage or appoint any individual who may reasonably be deemed to be an “executive officer” as defined under the Exchange Act;
(vii) enter into any material transaction outside the Ordinary Course of Business;
(viii) acquire any material asset or sell, lease, license or otherwise irrevocably dispose of any of its assets or properties, or grant any Lien with respect to such assets or properties, except in the Ordinary Course of Business;
(ix) sell, assign, transfer, license, sublicense or otherwise dispose of any material Company Owned IP (other than pursuant to non-exclusive licenses in the Ordinary Course of Business);
(x) other than in the Ordinary Course of Business: (A) make, change or revoke any material Tax election; (B) file any amended income or other material Tax Return; (C) adopt or change any material accounting method in respect of Taxes; (D) enter into any material Tax closing agreement, settle any material Tax claim or assessment; (E) consent to any extension or waiver of the limitation period applicable to or relating to any material Tax claim or assessment or (F) surrender any material claim for refund;
(xi) waive, settle or compromise any pending or threatened Action against the Company, other than waivers, settlements or agreements (A) for an amount not in excess of $50,000 in the aggregate (excluding amounts to be paid under existing insurance policies or renewals thereof) and (B) that do not impose any material restrictions on the operations or businesses of the Company or any equitable relief on, or the admission of wrongdoing by the Company;
(xii) delay or fail to repay when due any material obligation, including accounts payable and accrued expenses, other than in the Ordinary Course of Business;
(xiii) forgive any loans to any Person, including its employees, officers, directors or Affiliate;
(xiv) other than, for the avoidance of doubt, obtaining “tail” insurance coverage in connection with the Closing, terminate or modify in any material respect, or fail to exercise renewal rights with respect to, any material insurance policy;
-56-
(xv) enter into, amend or terminate any Company Material Contract; (xvi) (A) materially change pricing or royalties or other payments set or charged by the Company or any of its Subsidiaries to its customers or licensees or (B) agree to materially change pricing or royalties or other payments set or charged by Persons who have licensed Intellectual Property to the Company or any of its Subsidiaries; or
(xvii) agree, resolve or commit to do any of the foregoing.
(c) Nothing contained in this Agreement shall give Parent, directly or indirectly, the right to control or direct the operations of the Company prior to the Effective Time. Prior to the Effective Time, the Company shall exercise, consistent with the terms and conditions of this Agreement, complete unilateral control and supervision over its business operations. Notwithstanding anything to the contrary set forth in this Agreement, no consent of the Parent shall be required with respect to any matter set forth in this Section 6.2 or elsewhere in this Agreement to the extent that the requirement of such consent could violate any applicable Laws.
Section 6.3 Access and Investigation.
(a) Subject to the terms of the Confidentiality Agreement, which the parties agree will continue in full force following the date of this Agreement, during the Pre-Closing Period (but, for the avoidance of doubt, at the Effective Time, the Confidentiality Agreement shall terminate and be of no further force or effect), upon reasonable advance notice, Parent, on the one hand, and the Company, on the other hand, shall and shall use commercially reasonable efforts to cause such party’s Representatives to: (i) provide the other party and such other party’s Representatives with reasonable access during normal business hours to such party’s Representatives, personnel, property and assets and to all existing books, records, Tax Returns, work papers and other documents and information relating to such party and its Subsidiaries, (ii) provide the other party and such other party’s Representatives with such copies of the existing books, records, Tax Returns, work papers, product data, and other documents and information relating to such party and its Subsidiaries, and with such additional financial, operating and other data and information regarding such party and its Subsidiaries as the other party may reasonably request, (iii) permit the other party’s officers and other employees to meet, upon reasonable notice and during normal business hours, with the chief financial officer and other officers and managers of such party responsible for such party’s financial statements and the internal controls of such party to discuss such matters as the other party may reasonably request and (iv) make available to the other party copies of any material notice, report or other document filed with or sent to or received from any Governmental Entity in connection with the transactions contemplated by this Agreement. Any investigation conducted by either Parent or the Company pursuant to this Section 6.3 shall be conducted in such manner as not to interfere unreasonably with the conduct of the business of the other party.
(b) Notwithstanding anything herein to the contrary in this Section 6.3, no access or examination contemplated by this Section 6.3 shall be permitted to the extent that it would require any party or its Subsidiaries to waive the attorney-client privilege or attorney work product privilege, or violate any applicable Law; provided, that such party or its Subsidiary shall use its commercially reasonable efforts to obtain any required consents for the disclosure of such information and take such other reasonable action (including, to the extent permitted, redacted versions of any such information or entering into a joint defense agreement or similar arrangement to avoid loss of attorney-client privilege) with respect to such information as is necessary to permit disclosure without jeopardizing such attorney-client privilege or violating applicable Law, as applicable.
-57-
Section 6.4 No Solicitation.
(a) Each of Parent and the Company agrees that, during the Pre-Closing Period, neither it nor any of its Subsidiaries shall, nor shall it or any of its Subsidiaries authorize any of its Representatives to, directly or indirectly: (i) solicit, initiate or knowingly encourage, induce or facilitate the communication, making, submission or announcement of any Acquisition Proposal or Acquisition Inquiry or take any action that could reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry, (ii) furnish any non-public information regarding such party to any Person in connection with or in response to an Acquisition Proposal or Acquisition Inquiry, (iii) engage in discussions (other than to inform any Person of the existence of the provisions of this Section 6.4) or negotiations with any Person with respect to any Acquisition Proposal or Acquisition Inquiry, (iv) approve, endorse or recommend any Acquisition Proposal (subject to Section 6.7 and Section 6.8), (v) execute or enter into any letter of intent or any Contract contemplating or otherwise relating to any Acquisition Transaction, (vi) take any action that would reasonably be expected to lead to an Acquisition Proposal or Acquisition Inquiry or (vii) publicly propose to do any of the following; provided, however, that, notwithstanding anything contained in this Section 6.4 and subject to compliance with this Section 6.4, prior to the approval of this Agreement by a party’s stockholders (i.e., the Company Stockholder Approval, in the case of the Company and its Subsidiaries, or the Parent Stockholder Approval in the case of Parent), such party may furnish non-public information regarding such party and its Subsidiaries to, and enter into discussions or negotiations with, any Person in response to a bona fide written Acquisition Proposal by such Person which such party’s board of directors determines in good faith, after consultation with such party’s financial advisors and outside legal counsel, constitutes, or is reasonably likely to result in, a Superior Offer (and is not withdrawn) if: (A) neither such party nor any Representative of such party shall have breached this Section 6.4 in any material respect to such Acquisition Proposal, (B) the board of directors of such party concludes in good faith based on the advice of outside legal counsel, that the failure to take such action would reasonably be expected to be inconsistent with the board of directors’ fiduciary duties under applicable Law, (C) as promptly as reasonably practicable prior to initially furnishing any such nonpublic information to, or entering into discussions with, such Person, such party gives the other party written notice of the identity of such Person and of such party’s intention to enter into discussions with, such Person, (D) such party receives from such Person an executed Acceptable Confidentiality Agreement and (E) as promptly as reasonably practicable prior to furnishing any such nonpublic information to such Person, such party furnishes such nonpublic information to the other party (to the extent such information has not been previously furnished by such party to the other party). Without limiting the generality of the foregoing, each party acknowledges and agrees that, in the event any Representative of such party takes any action that, if taken by such party, would constitute a breach of this Section 6.4 by such party, the taking of such action by such Representative shall be deemed to constitute a breach of this Section 6.4 by such party for purposes of this Agreement.
(b) If any party or any Representative of such party receives an Acquisition Proposal or Acquisition Inquiry at any time during the Pre-Closing Period, then such party shall promptly (and in no event less than twenty-four (24) hours after such party becomes aware of such Acquisition Proposal or Acquisition Inquiry) advise the other party orally and in writing of such Acquisition Proposal or Acquisition Inquiry (including the identity of the Person making or submitting such Acquisition Proposal or Acquisition Inquiry, and the terms thereof).
-58-
Such party shall keep the other party reasonably informed with respect to the status and terms of any such Acquisition Proposal or Acquisition Inquiry and any material modification or material proposed modification thereto.
(c) Each party shall immediately cease and cause to be terminated any existing discussions, negotiations and communications with any Person that relate to any Acquisition Proposal or Acquisition Inquiry that has not already been terminated as of the date of this Agreement.
Section 6.5 Notification of Certain Matters. During the Pre-Closing Period, each of the Company, on the one hand, and Parent, on the other hand, shall promptly notify the other (and, if in writing, furnish copies of) if any of the following occurs: (a) any notice or other communication is received from any Person alleging that the consent of such Person is or may be required in connection with any of the transactions contemplated by this Agreement, (b) any notice or other communication from any Governmental Entity in connection with the transactions contemplated by this Agreement, (c) any Action against or involving or otherwise affecting such party or its Subsidiaries is commenced, or, to the knowledge of such party, threatened against such party or, to the knowledge of such party, any director, officer or Key Employee of such party, (d) such party becomes aware of any inaccuracy in any representation or warranty made by such party in this Agreement or (e) the failure of such party to comply with any covenant or obligation of such party; in each case that could reasonably be expected to make the timely satisfaction of any of the conditions set forth in ARTICLE VII, as applicable, impossible or materially less likely. No such notice shall be deemed to supplement or amend the Company Disclosure Letter or the Parent Disclosure Letter for the purpose of (x) determining the accuracy of any of the representations and warranties made by the Company or the Parent, as applicable, in this Agreement or (y) determining whether any condition set forth in ARTICLE VII has been satisfied. Any failure by either party to provide notice pursuant to this Section 6.5 shall not be deemed to be a breach for purposes of Section 7.2(b) or Section 7.3(b), as applicable, unless such failure to provide such notice was knowing and intentional.
Section 6.6 Registration Statement; Proxy Statement.
(a) As promptly as practicable after the date of this Agreement, (i) Parent shall prepare and cause to be filed with the SEC a proxy statement relating to the Parent Stockholder Meeting to be held in connection with the Merger (together with any amendments thereof or supplements thereto, the “Proxy Statement”) and (ii) Parent, in cooperation with the Company, shall prepare and cause to be filed with the SEC a registration statement on Form S-4 (the “Form S-4”), in which the Proxy Statement shall be included as a part (the Proxy Statement and the Form S-4, collectively, the “Registration Statement”), in connection with the registration under the Securities Act of the shares of Parent Common Stock to be issued by virtue of the transactions contemplated hereby. Parent shall use its reasonable best efforts to (i) cause the Registration Statement to comply with the applicable rules and regulations promulgated by the SEC, (ii) cause the Registration Statement to become effective as promptly as practicable and (iii) respond as soon as practicable to any comments or requests of the SEC or its staff relating to the Registration Statement.
-59-
Each of the Company and Parent shall reasonably cooperate with the other party and furnish all information concerning itself and their Affiliates, as applicable, to the other parties that is required by Law to be included in the Registration Statement as the other parties may reasonably request in connection with such actions and the preparation of the Registration Statement and Proxy Statement.
(b) Parent covenants and agrees that the Registration Statement (and the letter to stockholders, notice of meeting and form of proxy included therewith) will (i) comply as to form in all material respects with the requirements of applicable federal securities Laws and Nevada law and (ii) not, at the time the Registration Statement or any amendment or supplement thereto is filed with the SEC or is first mailed to Parent stockholders, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The Company covenants and agrees that the information supplied by or on behalf of the Company to Parent for inclusion in the Registration Statement (including the Company Balance Sheet) will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make such information, in light of the circumstances under which they were made, not misleading. Notwithstanding the foregoing, neither party makes any covenant, representation or warranty with respect to statements made in the Registration Statement (and the letter to stockholders, notice of meeting and form of proxy included therewith), if any, based on information provided by the other party or any of its Representatives regarding such other party or its Affiliates for inclusion therein.
(c) Parent shall use reasonable best efforts to cause the Proxy Statement to be mailed to Parent’s stockholders as promptly as practicable after the Registration Statement is declared effective under the Securities Act. If at any time before the Effective Time, (i) Parent, Merger Sub or the Company (A) become aware of any event or information that, pursuant to the Securities Act or the Exchange Act, should be disclosed in an amendment or supplement to the Registration Statement, (B) receives notice of any SEC request for an amendment or supplement to the Registration Statement or for additional information related thereto, or (C) receives SEC comments on the Registration Statement or (ii) the information provided in the Registration Statement has become “stale” and new information should be disclosed in an amendment or supplement to the Registration Statement, as the case may be, then such party, as the case may be, shall promptly inform the other parties thereof and shall cooperate with such other parties in filing such amendment or supplement with the SEC (and, if appropriate, in mailing such amendment or supplement to the Parent stockholders) or otherwise addressing such SEC request or comments and each party and shall use their reasonable best efforts to cause any such amendment to become effective, if required. Parent shall promptly notify the Company if it receives oral or written notice thereof, (1) that the Registration Statement has become effective, (2) of the issuance of any stop order or suspension of the qualification or registration of the Parent Common Stock issuable in connection with the transactions contemplated by this Agreement for offering or sale in any jurisdiction, or (3) any order of the SEC related to the Registration Statement, and shall promptly provide to the Company copies of all written correspondence between it or any of its Representatives, on the one hand, and the SEC or staff of the SEC, on the other hand, with respect to the Registration Statement and all orders of the SEC relating to the Registration Statement.
-60-
(d) The Company shall provide, and cause its Representatives to provide, Parent and its Representatives, with all true, correct and complete information regarding the Company that is required by Law to be included in the Registration Statement or reasonably requested by Parent to be included in the Registration Statement (collectively, the “Company Required S-4 Information”). Without limiting the foregoing, the Company will use reasonable best efforts to cause to be delivered to Parent a consent letter of the Company’s independent accounting firm, dated no more than two (2) Business Days before the date on which the Registration Statement is filed with the SEC (and reasonably satisfactory in form and substance to Parent), that is customary in scope and substance for consent letters delivered by independent public accountants in connection with registration statements similar to the Registration Statement. The Company and its legal counsel shall be given reasonable opportunity to review and comment on the Registration Statement, including all amendments and supplements thereto, prior to the filing thereof with the SEC, and on the response to any comments of the SEC on the Registration Statement, prior to the filing thereof with the SEC. Parent may file the Registration Statement, or any amendment or supplement thereto, without the prior consent of the Company, provided that Parent has included the Company Required S-4 Information in the Registration Statement in substantially the same form as it was provided to Parent by the Company pursuant to this Section 6.6; provided, further, that if the prior consent of the Company is not obtained then, notwithstanding anything else herein, the Company makes no covenant or representation regarding the portion of such information supplied by or on behalf of the Company to Parent for inclusion in such Registration Statement that the Company reasonably identifies prior to such filing of the Registration Statement.
(e) As promptly as reasonably practicable following the date of this Agreement, the Company will use reasonable best efforts to furnish to Parent audited financial statements for each of its fiscal years required to be included in the Registration Statement (the “Company Audited S-4 Financial Statements”). During the Pre-Closing Period, within thirty (30) calendar days following the end of each three-month quarterly period and each fiscal year, the Company will use reasonable best efforts to furnish to Parent the unaudited interim financial statements that would be required to be included in the Registration Statement or any periodic report due prior to the Closing if the Company were subject to the periodic reporting requirements under the Securities Act or the Exchange Act (the “Company Interim S-4 Financial Statements” and together with the Company Audited S-4 Financial Statements, the “Company S-4 Financial Statements”). Each of the Company S-4 Financial Statements will be suitable for inclusion in the Registration Statement, if applicable, and prepared in accordance with GAAP as applied on a consistent basis during the periods involved (except in each case as described in the notes thereto) and on that basis will present fairly, in all material respects, the financial position and the results of operations, changes in stockholders’ equity and cash flows of the Company as of the dates of and for the periods referred to in the Company S-4 Financial Statements.
Section 6.7 Company Stockholder Written Consent.
-61-
(a) Promptly after the Registration Statement has been declared effective under the Securities Act, and in any event no later than two (2) Business Days thereafter, the Company shall obtain the approval by written consent from Company stockholders sufficient for the Company Stockholder Approval in lieu of a meeting pursuant to Section 228 of the DGCL, for purposes of (i) adopting and approving this Agreement and the transactions contemplated by this Agreement, in accordance with the Company’s Certificate of Incorporation and Sections 228 and 251 of the DGCL, (ii) acknowledging that the approval given thereby is irrevocable and that such stockholder is aware of its rights to demand appraisal for its shares pursuant to Section 262 of the DGCL, a copy of which will be attached thereto, and that such stockholder has received and read a copy of Section 262 of the DGCL and (iii) acknowledging that by its approval of the Merger it is not entitled to appraisal rights with respect to its shares in connection with the Merger and thereby waives any rights to receive payment of the fair value of its capital stock under the DGCL (the “Company Stockholder Written Consent”). Under no circumstances shall the Company assert that any other approval or consent is necessary by its stockholders to approve this Agreement and the transactions contemplated by this Agreement.
(b) Reasonably promptly following receipt of the Company Stockholder Approval, the Company shall prepare and mail a notice (the “Stockholder Notice”) to every stockholder of the Company that did not execute the Company Stockholder Written Consent. The Stockholder Notice shall (i) notify Company stockholders that the Company Board determined that the Merger is advisable in accordance with Section 251(b) of the DGCL and in the best interests of the stockholders of the Company and unanimously approved and adopted this Agreement, the Merger and the other transactions contemplated by this Agreement, (ii) provide the stockholders of the Company to whom it is sent with notice of the actions taken in the Company Stockholder Written Consent, including the adoption and approval of this Agreement, the Merger and the other transactions contemplated by this Agreement in accordance with Section 228(e) of the DGCL and the certificate of incorporation and bylaws of the Company and (iii) include a description of the appraisal rights of the Company’s stockholders available under Section 262 of the DGCL, along with such other information as is required thereunder and pursuant to applicable Law. All materials (including any amendments thereto) submitted to the stockholders of the Company in accordance with this Section 6.7(b) shall be subject to Parent’s advance review and reasonable approval. The Stockholder Notice shall include therewith a copy of Section 262 of the DGCL and shall be sufficient in form and substance to start the 20-day period during which a stockholder seeking to assert appraisal rights must demand appraisal of such stockholder’s capital stock of the Company as contemplated by Section 262(d)(2) of the DGCL.
(c) The Company agrees that, subject to Section 6.7(d): (i) the Company Board shall recommend that the Company’s stockholders vote to adopt and approve this Agreement and the transactions contemplated by this Agreement and shall use commercially reasonable efforts to solicit such approval within the time set forth in Section 6.7(a) (the recommendation of the Company Board that the Company’s stockholders vote to adopt and approve this Agreement being referred to as the “Company Board Recommendation”) and (ii) the Company Board Recommendation shall not be withdrawn or modified (and the Company Board shall not publicly propose to withdraw or modify the Company Board Recommendation) in a manner adverse to Parent, and no resolution by the Company Board or any committee thereof to withdraw or modify the Company Board Recommendation in a manner adverse to Parent or to adopt, approve or recommend (or publicly propose to adopt, approve or recommend) any Acquisition Proposal shall be adopted or proposed.
-62-
(d) Notwithstanding anything to the contrary contained in Section 6.7(c), and subject to compliance with Section 6.4 and Section 6.7, if at any time prior to approval and adoption of this Agreement by the Company Stockholder Approval, the Company receives a bona fide written Superior Offer, the Company Board may withhold, amend, withdraw or modify the Company Board Recommendation (or publicly propose to withhold, amend, withdraw or modify the Company Board Recommendation) in a manner adverse to Parent (collectively, a “Company Board Adverse Recommendation Change”) if, but only if, following the receipt of and on account of such Superior Offer, (i) the Company Board determines in good faith, based on the advice of its outside legal counsel, that the failure to withhold, amend, withdraw or modify such recommendation would reasonably be expected to be inconsistent with its fiduciary duties under applicable Law, (ii) the Company has during the Notice Period (as defined below), negotiated with Parent in good faith to make such adjustments to the terms and conditions of this Agreement so that such Acquisition Proposal ceases to constitute a Superior Offer and (iii) if after Parent shall have delivered to the Company a written offer to alter the terms or conditions of this Agreement during the Notice Period, the Company Board shall have determined in good faith, based on the advice of its outside legal counsel, that the failure to withhold, amend, withdraw or modify the Company Board Recommendation would reasonably be expected to be inconsistent with its fiduciary duties under applicable Law (after taking into account such alterations of the terms and conditions of this Agreement); provided that (x) Parent receives written notice from the Company confirming that the Company Board has determined to change its recommendation at least four (4) Business Days in advance of the Company Board Adverse Recommendation Change (the “Notice Period”), which notice shall include a description in reasonable detail of the reasons for such Company Board Adverse Recommendation Change, and written copies of any relevant proposed transaction agreements with any party making a potential Superior Offer, (y) during any Notice Period, Parent shall be entitled to deliver to the Company one or more counterproposals to such Acquisition Proposal and the Company will, and cause its Representatives to, negotiate with Parent in good faith (to the extent Parent desires to negotiate) to make such adjustments in the terms and conditions of this Agreement so that the applicable Acquisition Proposal ceases to constitute a Superior Offer and (z) in the event of any material amendment to any Superior Offer (including any revision in the amount, form or mix of consideration the Company’s stockholders would receive as a result of such potential Superior Offer), the Company shall be required to provide Parent with notice of such material amendment and the Notice Period shall be extended, if applicable, to ensure that at least three (3) Business Days remain in the Notice Period following such notification during which the parties shall comply again with the requirements of this Section 6.7(d) and the Company Board shall not make a Company Board Adverse Recommendation Change prior to the end of such Notice Period as so extended (it being understood that there may be multiple extensions).
(e) The Company’s obligation to solicit the consent of its stockholders to sign the Company Stockholder Written Consent in accordance with Section 6.7(a) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or other Acquisition Proposal, or by any Company Board Adverse Recommendation Change.
Section 6.8 Stockholders’ Meeting.
-63-
(a) As promptly as reasonably practicable after the effectiveness of the Registration Statement, Parent shall take all action necessary under applicable Law to call, give notice of and hold a meeting of the holders of Parent Common Stock (the “Parent Stockholder Meeting”) to consider and vote (i) to approve this Agreement and thereby approve the transactions contemplated by this Agreement; (ii) if deemed necessary by the parties, to amend Parent’s certificate of incorporation (x) to increase the number of authorized shares of Parent Common Stock and/or (y) to effect the Nasdaq Reverse Split; (iii) to elect the directors of Parent as contemplated by Section 1.1(c); (iv) to effect the Reincorporation and (v) to adopt a new equity compensation plan, in a form approved by the Company and Parent (the “2024 Incentive Plan”), which 2024 Incentive Plan will provide for new awards for a number of shares of Parent Common Stock as mutually agreed upon by Parent and the Company, and subject to approval by the Parent Board, (for avoidance of doubt, such number of shares shall be in addition to the number of shares of Parent Common Stock subject to outstanding Parent Options and Parent RSUs or subject to Company Options assumed by Parent as contemplated by Section 3.2(a)) (clauses (i), (ii) and (iii) collectively, the “Required Parent Stockholder Proposals”, and clauses (i), (ii), (iii), (iv) and (v) collectively, the “Parent Stockholder Matters”). The Parent Stockholder Meeting shall be held as promptly as practicable after the date that the Registration Statement is declared effective under the Securities Act, and in any event, no later than 45 calendar days after the effective date of the Registration Statement. Parent shall take reasonable measures to ensure that all proxies solicited in connection with the Parent Stockholder Meeting are solicited in compliance with all applicable Law. Notwithstanding anything to the contrary contained herein, if on the date of the Parent Stockholder Meeting, or a date preceding the date on which the Parent Stockholder Meeting is scheduled, Parent reasonably believes that (i) it will not receive proxies sufficient to obtain the Parent Stockholder Approval, whether or not a quorum would be present or (ii) it will not have sufficient shares of Parent Common Stock represented (whether in person or by proxy) to constitute a quorum necessary to conduct the business of the Parent Stockholder Meeting, Parent may postpone or adjourn, or make one or more successive postponements or adjournments of, the Parent Stockholder Meeting as long as the date of the Parent Stockholder Meeting is not postponed or adjourned more than an aggregate of thirty (30) calendar days in connection with any postponements or adjournments.
(b) Parent agrees that (i) the Parent Board shall recommend that the holders of Parent Common Stock vote to approve the Parent Stockholder Matters and shall use commercially reasonable efforts to solicit such approval within the timeframe set forth in Section 6.8 above and (ii) the Proxy Statement shall include a statement to the effect that the Parent Board recommends that Parent’s stockholders vote to approve the Parent Stockholder Matters (the recommendation of the Parent Board being referred to as the “Parent Board Recommendation”).
-64-
(c) Notwithstanding anything to the contrary contained in Section 6.8(b), and subject to compliance with Section 6.4 and Section 6.8, if at any time prior to the Parent Stockholder Approval, Parent receives a bona fide written Superior Offer, the Parent Board may withhold, amend, withdraw or modify the Parent Board Recommendation with respect to the Required Parent Stockholder Proposals (or publicly propose to withhold, amend, withdraw or modify the Parent Board Recommendation with respect to the Required Parent Stockholder Proposals) in a manner adverse to the Company (collectively, a “Parent Board Adverse Recommendation Change”) if, but only if, following the receipt of and on account of such Superior Offer, (i) the Parent Board determines in good faith, based on the advice of its outside legal counsel, that the failure to withhold, amend, withdraw or modify such recommendation would reasonably be expected to be inconsistent with its fiduciary duties under applicable Law, (ii) Parent has, and has caused its financial advisors and outside legal counsel to, during the Parent Notice Period (as defined below), negotiated with the Company in good faith to make such adjustments to the terms and conditions of this Agreement so that such Acquisition Proposal ceases to constitute a Superior Offer and (iii) if after the Company shall have delivered to the Company a written offer to alter the terms or conditions of this Agreement during the Parent Notice Period, the Parent Board shall have determined in good faith, based on the advice of its outside legal counsel, that the failure to withhold, amend, withdraw or modify the Parent Board Recommendation would reasonably be expected to be inconsistent with its fiduciary duties under applicable Law (after taking into account such alterations of the terms and conditions of this Agreement); provided that (x) the Company receives written notice from Parent confirming that the Parent Board has determined to change its recommendation at least four (4) Business Days in advance of the Parent Board Adverse Recommendation Change (the “Parent Notice Period”), which notice shall include a description in reasonable detail of the reasons for such Parent Board Adverse Recommendation Change, and written copies of any relevant proposed transaction agreements with any party making a potential Superior Offer, (y) during any Parent Notice Period, the Company shall be entitled to deliver to Parent one or more counterproposals to such Acquisition Proposal and Parent will, and cause its Representatives to, negotiate with the Company in good faith (to the extent the Company desires to negotiate) to make such adjustments in the terms and conditions of this Agreement so that the applicable Acquisition Proposal ceases to constitute a Superior Offer and (z) in the event of any material amendment to any Superior Offer (including any revision in the amount, form or mix of consideration the Parent’s stockholders would receive as a result of such potential Superior Offer), Parent shall be required to provide the Company with notice of such material amendment and the Parent Notice Period shall be extended, if applicable, to ensure that at least three (3) Business Days remain in the Parent Notice Period following such notification during which the parties shall comply again with the requirements of this Section 6.8(c) and the Parent Board shall not make a Parent Board Adverse Recommendation Change prior to the end of such Parent Notice Period as so extended (it being understood that there may be multiple extensions).
(d) Parent’s obligation to call, give notice of and hold the Parent Stockholder Meeting in accordance with Section 6.8(a) shall not be limited or otherwise affected by the commencement, disclosure, announcement or submission of any Superior Offer or Acquisition Proposal, or by any Parent Board Adverse Recommendation Change.
(e) Nothing contained in this Agreement shall prohibit Parent or the Parent Board from (i) complying with Rules 14d-9 and 14e-2(a) promulgated under the Exchange Act, (ii) issuing a “stop, look and listen” communication or similar communication of the type contemplated by Section 14d-9(f) under the Exchange Act or (iii) otherwise making any disclosure to Parent’s stockholders; provided, however, that in the case of the foregoing clause (iii) the Parent Board determines in good faith, after consultation with its outside legal counsel, that failure to make such disclosure could be reasonably likely to be inconsistent with applicable Law, including its fiduciary duties under applicable Law; provided, further, that any such disclosures (other than a “stop, look and listen” communication or similar communication of the type contemplated by Section 14d-9(f) under the Exchange Act) shall be deemed to be a change of the Parent Board Recommendation unless the Parent Board expressly publicly reaffirms the Parent Board Recommendation (i) in such communication or (ii) within three (3) Business Days after being requested in writing to do so by the Company.
-65-
Section 6.9 Efforts; Transaction Litigation.
(a) The parties shall use reasonable best efforts to promptly take, or cause to be taken, all actions, and to do, or cause to be done, and to assist and cooperate with the other parties hereto in doing, all things necessary, property or advisable to consummate the transactions contemplated by this Agreement. Without limiting the generality of the foregoing, each party: (i) shall make all necessary filings, registrations, declaration and other submissions (if any) and give all notices (if any) required to be made and given by such party in connection with the transactions contemplated by this Agreement, (ii) shall use commercially reasonable efforts to obtain all necessary and advisable actions or non-actions, waivers and consents, (if any) (pursuant to any applicable Law or Contract, or otherwise) by such party in connection with the transactions contemplated by this Agreement or for such Contract to remain in full force and effect, (iii) shall use reasonable best efforts to lift any injunction prohibiting, or any other legal bar to, the transactions contemplated by this Agreement and (iv) shall use reasonable best efforts to satisfy the conditions precedent to the consummation of the transactions contemplated by this Agreement.
(b) Without limiting the generality of the foregoing, prior to the Closing, Parent shall give the Company prompt written notice of any litigation against Parent and/or its directors relating to this Agreement or the transactions contemplated by this Agreement (“Transaction Litigation”) (including by providing copies of all pleadings with respect thereto) and keep Company reasonably informed with respect to the status thereof. Parent shall conduct and control the settlement and defense of any Transaction Litigation; provided that prior to the Closing no such settlement shall be agreed to without the prior written consent of the Company (such consent not to be unreasonably withheld, conditioned or delayed); provided further that any settlement or other resolution of any Transaction Litigation that names one or more of the directors of Parent as a defendant and commenced prior to Closing and agreed to by Parent after the Closing shall be approved in advance by the Parent appointed Board member (such approval not to be unreasonably withheld, conditioned or delayed). Without limiting the foregoing, prior to the Closing, Parent shall give the Company the opportunity to consult with Parent in connection with the defense and settlement of any Transaction Litigation.
Section 6.10 Indemnification, Exculpation and Insurance.
(a) From the Effective Time through the sixth anniversary of the date on which the Effective Time occurs, each of Parent and the Surviving Company, jointly and severally, shall indemnify and hold harmless each person who is now, or has been at any time prior to the date hereof, or who becomes prior to the Effective Time, a director or officer of Parent or the Company or either of its Subsidiary, respectively (the “D&O Indemnified Parties”), against all claims, losses, liabilities, damages, judgments, fines and reasonable fees, costs and expenses, including attorneys’ fees and disbursements, incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to the fact that the D&O Indemnified Party is or was a director or officer of Parent, the Company or either of its Subsidiaries, whether asserted or claimed prior to, at or after the Effective Time, or the enforcement of such D&O Indemnified Parties’ rights under this Section 6.10, in each case, to the fullest extent permitted under applicable Law. Each D&O Indemnified Party will be entitled to advancement of expenses incurred in the defense of any such claim, action, suit, proceeding or investigation from each of Parent and the Surviving Company, jointly and severally, upon receipt by Parent or the Surviving Company from the D&O Indemnified Party of a request therefor; provided, that any such person to whom expenses are advanced provides an undertaking to Parent, to the extent then required by the applicable Law, to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
-66-
(b) The provisions of the Parent Charter and Parent Bylaws with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers of Parent that are set forth in the Parent Charter and Parent Bylaws shall not be amended, modified or repealed for a period of six years from the Effective Time in a manner that would adversely affect the rights thereunder of individuals who, at or prior to the Effective Time, were officers or directors of Parent, unless such modification is required by applicable Law. The Certificate of Incorporation and Bylaws of the Surviving Company shall contain, and Parent shall cause the Certificate of Incorporation and Bylaws of the Surviving Company to so contain, provisions no less favorable with respect to indemnification, advancement of expenses and exculpation of present and former directors and officers as those set forth in the Parent Charter and Parent Bylaws.
(c) From and after the Effective Time, (i) the Surviving Company shall fulfill and honor in all respects the obligations of the Company to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Company’s Certificate of Incorporation and Bylaws and pursuant to any indemnification agreements between the Company and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time and (ii) Parent shall fulfill and honor in all respects the obligations of Parent to its D&O Indemnified Parties as of immediately prior to the Closing pursuant to any indemnification provisions under the Parent Charter and Parent Bylaws and pursuant to any indemnification agreements between Parent and such D&O Indemnified Parties, with respect to claims arising out of matters occurring at or prior to the Effective Time.
(d) From and after the Effective Time, Parent shall maintain directors’ and officers’ liability insurance policies, with an effective date as of the Closing Date, on commercially available terms and conditions and with coverage limits customary for U.S. public companies similarly situated to Parent. In addition, Parent shall purchase, prior to the Effective Time, a six (6) year prepaid “D&O tail policy” for the non-cancelable extension of the directors’ and officers’ liability coverage of Parent’s existing directors’ and officers’ insurance policies for a claims reporting or discovery period of at least six (6) years from and after the Effective Time with respect to any claim related to any period of time at or prior to the Effective Time with terms, conditions, retentions and limits of liability that are no less favorable than the coverage provided under Parent’s existing policies as of the date of this Agreement, except that Parent will not commit or spend on such “D&O tail policy” annual premiums in excess of 250% of the annual premiums paid by Parent in its last full fiscal year prior to the date hereof for Parent’s current policies of directors’ and officers’ liability insurance and fiduciary liability insurance, and if such premiums for such “D&O tail policy” would exceed 250% of such annual premium, then Parent shall purchase policies that provide the maximum coverage available at an annual premium equal to 250% of such annual premium. The Company shall in good faith cooperate with Parent prior to the Effective Time with respect to the procurement of such “D&O tail policy.”
-67-
(e) From and after the Effective Time, Parent shall pay all expenses, including reasonable attorneys’ fees, that are incurred by the persons referred to in this Section 6.10 in connection with their enforcement of the rights provided to such persons in this Section 6.10.
(f) The provisions of this Section 6.10 are intended to be in addition to the rights otherwise available to the current and former officers and directors of Parent and the Company by Law, charter, statute, bylaw or agreement, and shall operate for the benefit of, and shall be enforceable by, each of the D&O Indemnified Parties, their heirs and their Representatives.
(g) In the event Parent or the Surviving Company or any of their respective successors or assigns (i) consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or (ii) transfers all or substantially all of its properties and assets to any Person, then, and in each such case, proper provision shall be made so that the successors and assigns of Parent or the Surviving Company, as the case may be, shall succeed to the obligations set forth in this Section 6.10. Parent shall cause the Surviving Company to perform all of the obligations of the Surviving Company under this Section 6.10.
Section 6.11 Disclosure. The parties shall use their commercially reasonable efforts to agree to the text of the initial press release and Parent’s Form 8-K announcing the execution and delivery of this Agreement. Without limiting any party’s obligations under the Confidentiality Agreement, no party shall, and no party shall permit any of its Subsidiaries or any of its Representatives to, issue any further press release(s) or otherwise make any public statement, announcement or disclosure regarding the transactions contemplated by this Agreement unless: (a) the other party shall have approved such press release or disclosure in writing, such approval not to be unreasonably conditioned, withheld or delayed; or (b) such party shall have determined in good faith, upon the advice of outside legal counsel, that such disclosure is required by applicable Law and, to the extent practicable, before such press release or disclosure is issued or made, such party advises the other party of, and consults with the other party regarding, the text of such press release or disclosure; provided, however, that (1) Parent may, without such consultation or consent, make any public statement, announcement or disclosure as may be required pursuant to applicable Laws, including securities Laws or stock exchange regulations and (2) each of the Company and Parent may, without such consultation or consent, make any public statement in response to specific questions by the press, analysts, investors or those attending industry conferences or financial analyst conference calls and make internal announcements to employees, so long as any such statements are consistent with previous press releases, public disclosures or public statements made by the Company or Parent in compliance with this Section 6.11. Notwithstanding the foregoing, a party need not consult with any other parties in connection with such portion of any press release, public statement or filing to be issued or made pursuant to Section 6.7(d) or pursuant to Section 6.8(e).
Section 6.12 Listing. At or prior to the Effective Time, Parent shall use its commercially reasonable efforts to (a) maintain its listing on Nasdaq until the Effective Time and to obtain approval of the listing of the combined corporation on Nasdaq, (b) to the extent required by the rules and regulations of Nasdaq, prepare and submit to Nasdaq a notification form for the listing of the shares of Parent Common Stock to be issued in connection with the transactions contemplated by this Agreement and to cause such shares to be approved for listing (subject to official notice of issuance); (c) prepare and timely submit to Nasdaq a notification form for the Nasdaq Reverse Split (if required) and to submit a copy of the amendment to the Parent Charter effecting the Nasdaq Reverse Split, certified by the Secretary of State of the State of Delaware, to Nasdaq on the Closing Date; and (d) to the extent required by Nasdaq Marketplace Rule 5110, assist the Company in preparing and filing an initial listing application for the Parent Common Stock on Nasdaq (the “Nasdaq Listing Application”) and to cause such Nasdaq Listing Application to be conditionally approved prior to the Effective Time.
-68-
Each of Parent and the Company will reasonably promptly inform the other party of all verbal or written communications between Nasdaq and such party or its representatives. The parties will use commercially reasonable efforts to coordinate with respect to compliance with Nasdaq rules and regulations. The party not filing the Nasdaq Listing Application will cooperate with the other party as reasonably requested by such filing party with respect to the Nasdaq Listing Application and promptly furnish to such filing party all information concerning itself and its members that may be required or reasonably requested in connection with any action contemplated by this Section 6.12. All Nasdaq fees associated with any action contemplated by this Section 6.12 shall be borne by the Company.
Section 6.13 Section 16 Matters. Prior to the Effective Time, each of Parent and the Company shall take all such steps as may be necessary or appropriate to cause the acquisitions of Parent Common Stock (including derivative securities with respect to such Parent Common Stock) resulting from the transactions contemplated by this Agreement by each individual who will become subject to such reporting requirements with respect to Parent to be exempt under Rule 16b‑3 promulgated under the Exchange Act.
Section 6.14 Employee Matters. At the Effective Time, Parent shall assume the employment agreements for each of the employees of the Company set forth on Section 6.14 of the Company Disclosure Letter and the Company shall cause each such employee to waive any change of control or severance benefits that would otherwise arise solely by virtue of the consummation of the Merger (alone or in combination with any other event).
Section 6.15 Takeover Law. If any Takeover Law is or may become applicable to the Merger and the transactions contemplated hereby, each of the Company, the Company Board, Parent and the Parent Board, as applicable, shall grant such approvals and take such actions as are necessary so that the Merger and the transactions contemplated hereby may be consummated as promptly as practicable on the terms contemplated by this Agreement and otherwise act to render such Takeover Law inapplicable to the Merger and the transactions contemplated hereby.
Section 6.16 Tax Matters.
(a) Each of Parent and the Company will (and will cause its Affiliates to) (i) use all reasonable best efforts to cause the Merger (together, if required by Section 3.7, with the Second Merger) to constitute as a transaction qualifying for the Intended Tax Treatment and (ii) not take any action not required by this Agreement or the CVR Agreement or fail to take any action required by this Agreement or the CVR Agreement that could reasonably be expected to prevent or impede the Merger (together, if required by Section 3.7, with the Second Merger) from qualifying as a transaction qualifying for the Intended Tax Treatment. Except as otherwise required by applicable law, Parent shall not file (or cause its Affiliates, including the Company, to file) any U.S.
-69-
federal, state or local Tax Return after the Closing Date in a manner that is inconsistent with the treatment of the Merger (together, if required by Section 3.7, with the Second Merger) as a transaction qualifying for the Intended Tax Treatment for U.S. federal, state income and other relevant Tax purposes, and shall not take any inconsistent position during the course of any audit, litigation or other proceeding with respect to Taxes, in each case, unless otherwise required by a determination within the meaning of Section 1313(a) of the Code or a corresponding event with respect to state or local income Tax law.
(b) All transfer, documentary, sales, use, stamp, registration, excise, recording, registration value added and other such similar Taxes and fees (including any penalties and interest) that become payable in connection with or by reason of the execution of this Agreement and the transactions contemplated hereby shall be borne and paid by the Company. Unless otherwise required by applicable law, the Company shall timely file any Tax Return or other document with respect to such Taxes or fees (and the Parent shall reasonably cooperate with respect thereto as necessary). The Parent shall timely file any Tax Return or other document with respect to such Taxes or fees that it is required to file under applicable law (and the Company shall reasonably cooperate with respect thereto as necessary).
(c) On the Closing Date, the Company shall provide Parent with a certificate on behalf of the Company, prepared in a manner consistent and in accordance with the requirements of Treasury Regulations § 1.897-2(g), (h) and 1.1445-2(c)(3), certifying that no interest in the Company is, or has been during the relevant period specified in Section 897(c)(1)(A)(ii) of the Code, a “U.S. real property interest” within the meaning of Section 897(c) of the Code, and a form of notice to the Internal Revenue Service prepared in accordance with the provisions of Treasury Regulations § 1.897-2(h)(2), which notice Parent shall be authorized to submit to the Internal Revenue Service
Section 6.17 Calculation of the Exchange Ratio.
(a) No later than five (5) Business Days before the Anticipated Closing Date, Parent will deliver to the Company Parent’s determination of the Exchange Ratio (the “Exchange Ratio Statement”); provided, that, the Company shall cooperate with Parent and provide information to Parent to the extent necessary to allow Parent to calculate the Exchange Ratio.
(b) No later than three (3) Business Days after delivery of the Exchange Ratio Statement (the last day of such period, the “Response Date”), the Company shall have the right to dispute any part of the Exchange Ratio Statement by delivering a written notice to that effect to Parent (a “Dispute Notice”). Any Dispute Notice shall identify in reasonable detail and to the extent known the nature and amounts of any proposed revisions to the Exchange Ratio Statement and will be accompanied by reasonably detailed materials supporting the basis for such revisions.
(c) If, on or prior to the Response Date, the Company notifies Parent in writing that it has no objections to the Exchange Ratio Statement or, if on the Response Date, the Company fails to deliver a Dispute Notice as provided in Section 6.18(b), then the Exchange Ratio as set forth in the Exchange Ratio Statement shall be deemed to have been finally determined for purposes of this Agreement and to represent the Exchange Ratio for purposes of this Agreement.
-70-
(d) If the Company delivers a Dispute Notice on or prior to the Response Date, then Representatives of Parent and the Company shall promptly meet and attempt in good faith to resolve the disputed item(s) and negotiate an agreed-upon determination of the Exchange Ratio, which, if agreed, shall be deemed to have been finally determined for purposes of this Agreement and to represent the Exchange Ratio for purposes of this Agreement.
(e) If Representatives of Parent and the Company are unable to negotiate an agreed-upon determination of the Exchange Ratio pursuant to Section 6.18(d) within three (3) Business Days after delivery of the Dispute Notice (or such other period as Parent and the Company may mutually agree upon), then any remaining disagreements as to the calculation of the Exchange Ratio shall be referred to an independent auditor of recognized national standing jointly selected by Parent and the Company. If the parties are unable to select an independent auditor within five (5) days, then either Parent or the Company may thereafter request that the American Arbitration Association (“AAA”) make such selection (either the independent auditor jointly selected by both parties or such independent auditor selected by the AAA, the “Accounting Firm”). Parent and the Company shall promptly deliver to the Accounting Firm the work papers and back-up materials used in preparing the Exchange Ratio Statement and the Dispute Notice, and Parent and the Company shall use commercially reasonable efforts to cause the Accounting Firm to make its determination within five (5) Business Days of accepting its selection. Parent and the Company shall be afforded the opportunity to present to the Accounting Firm any material related to the unresolved disputes and to discuss the issues with the Accounting Firm; provided, however, that no such presentation or discussion shall occur without the presence of a Representative of each of Parent and the Company. The determination of the Accounting Firm shall be limited to the disagreements submitted to the Accounting Firm. The determination of the amount of the Exchange Ratio made by the Accounting Firm shall be made in writing delivered to each of Parent and the Company, shall be final and binding on Parent and the Company and shall (absent manifest error) be deemed to have been finally determined for purposes of this Agreement and to represent the Exchange Ratio for purposes of this Agreement. The parties shall delay the Closing until the resolution of the matters described in this Section 6.18(e). The fees and expenses of the Accounting Firm shall be allocated between Parent and the Company in the same proportion that the disputed portion of the Exchange Ratio that was unsuccessfully disputed by such party (as finally determined by the Accounting Firm) bears to the total disputed portion of the Exchange Ratio. If this Section 6.18(e) applies as to the determination of the Exchange Ratio, upon resolution of the matter in accordance with this Section 6.18(e), the parties shall not be required to determine the Exchange Ratio again even though the Closing may occur later than the Anticipated Closing Date.
Section 6.18 Obligations of Merger Sub. Parent will take all action necessary to cause Merger Sub to perform its obligations under this Agreement and to consummate the Merger on the terms and conditions set forth in this Agreement.
Section 6.19 Officers and Directors. Until successors are duly elected or appointed and qualified in accordance with applicable Law, the parties shall use commercially reasonable efforts and take all necessary action so that the Persons contemplated herein are elected or appointed, as applicable, to the positions of officers of Parent and officers and directors of the Surviving Company, as set forth herein, to serve in such positions effective as of the Effective Time. If any such Person is unable or unwilling to serve as officer of Parent or an officer or director of the Surviving Company, the party appointing such Person shall designate a successor.
-71-
The parties shall use reasonable best efforts to have each of the Persons that will serve as directors and executive officers of the Parent following the Closing to execute and deliver a Lock-Up Agreement prior to Closing.
Section 6.20 Termination of Certain Agreements and Rights. Except as set forth on Section 6.21 of the Company Disclosure Letter, the Company shall cause any stockholder agreements, voting agreements, registration rights agreements, co-sale agreements, loan agreements, promissory notes and any other similar Contracts with future obligations or contingent liabilities on the part of the Company or any of its Subsidiaries (or Parent, from and after the Closing) between the Company and any holders of capital stock of the Company (or any officer or director of the Company), including any such Contract granting any Person investor rights, rights of first refusal, registration rights or director registration rights, to be terminated immediately prior to the Effective Time, without any liability being imposed on the part of Parent or the Surviving Company.
Section 6.21 Allocation Certificate; Net Cash Schedule. The Company will prepare and deliver to Parent prior to the Closing a certificate signed by the Company’s Chief Executive Officer in a form reasonably acceptable to Parent setting forth (as of immediately prior to the Effective Time) (a) (i) the authorized capital stock of the Company, (ii) the number of shares of Company Common Stock (excluding treasury shares) issued and outstanding, (iii) the number of shares of Company Common Stock held by the Company in its treasury, (iv) the number of shares of Company Common Stock reserved for issuance pursuant to the Company Equity Plan (and the number of shares that are subject to outstanding Company Options), (v) the number of Company Warrants issued and outstanding and (vi) the number of shares of Company Common Stock reserved for issuance pursuant to the Company Warrants and (b)(i) each holder of capital stock of the Company, (ii) such holder’s name and address, (iii) the number or percentage and type of capital stock of the Company held as of the Closing Date for each such holder and (iv) the number of shares of Parent Common Stock to be issued to such holder pursuant to this Agreement in respect of the capital stock of the Company held by such holder as of immediately prior to the Effective Time (the “Allocation Certificate”). Parent will prepare and deliver to the Company prior to the Closing a schedule (the “Net Cash Schedule”) setting forth, in reasonable detail, Parent’s good faith, estimated calculation of the Parent Closing Net Cash, including each component thereof (the “Net Cash Calculation” as of the close of business on the last Business Day prior to the Closing Date) prepared and certified by Parent’s principal financial or accounting officer. Parent shall make available to the Company, as requested by the Company, the work papers and back-up materials used or useful in preparing the Net Cash Schedule.
Section 6.22 Parent SEC Documents. From the date of this Agreement to the Effective Time, Parent shall timely file with the SEC all registration statements, proxy statements, certifications, reports, schedules, exhibits, forms and other documents required to be filed by Parent with the SEC required to be filed by it under the Exchange Act or the Securities Act (“SEC Documents”). As of its filing date, or if amended after the date of this Agreement, as of the date of the last such amendment, each SEC Document filed by Parent with the SEC (a) shall comply in all material respects with the applicable requirements of the Exchange Act and the Securities Act, and (b) shall not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading.
-72-
Section 6.23 Legends. Parent shall be entitled to place appropriate legends on the book entries and/or certificates evidencing any shares of Parent Common Stock to be received in the Merger by equityholders of the Company who may be considered “affiliates” of Parent for purposes of Rules 144 and 145 under the Securities Act reflecting the restrictions set forth in Rules 144 and 145 and to issue appropriate stop transfer instructions to the transfer agent for Parent Common Stock.
Section 6.24 Reincorporation Covenants. At least seven (7) Business Days prior to the initiation of the Reincorporation, Company will prepare and deliver to Parent the Parent Charter for review and comment and Parent shall provide each other agreement, document, instrument, form and/or certificate to be executed or delivered in connection with the Reincorporation to the Company for review and comment prior to any intended effective date of the Reincorporation and Parent and the Company shall accept any reasonable comments provided by the other.
ARTICLE VII
CONDITIONS PRECEDENT
Section 7.1 Conditions Precedent to Each Party’s Obligation to Effect the Merger. The obligation of each party to effect the Merger and otherwise consummate the transactions contemplated by this Agreement at the Closing is subject to the satisfaction, or, to the extent permitted by applicable Law, the written waiver by each of the parties, at or prior to the Closing, of each of the following conditions:
(a) Effectiveness of Registration Statement. The Registration Statement shall have become effective in accordance with the provisions of the Securities Act, and shall not be subject to any stop order or proceeding (or threatened proceeding by the SEC) seeking a stop order with respect to the Registration Statement that has not been withdrawn. Any material state securities Laws applicable to the issuance of the shares of Parent Common Stock in connection with the transactions contemplated by this Agreement shall have been complied with, to the extent able to be complied with prior to the Effective Time, and no stop order (or similar order) shall have been issued or threatened in writing in respect of such shares of Parent Common Stock by any applicable state securities commissioner or court of competent jurisdiction.
(b) Stockholder Approval. (i) the Company shall have obtained the Company Stockholder Approval and (ii) Parent shall have obtained the Parent Stockholder Approval.
(c) No Injunctions or Legal Restraints; Illegality. No temporary restraining order, preliminary or permanent injunction or other judgment, order or decree issued by any court of competent jurisdiction or other legal restraint or prohibition shall be in effect, and no Law shall have been enacted, entered, promulgated, enforced or deemed applicable by any Governmental Entity that, in any such case, prohibits or makes illegal the consummation of the Merger and the transactions contemplated by this Agreement.
(d) Nasdaq Listing. The approval of the Nasdaq Listing Application and the approval of the listing of the additional shares of Parent Common Stock on Nasdaq shall have been obtained and the shares of Parent Common Stock to be issued in the transactions contemplated by this Agreement and pursuant to this Agreement shall have been approved for listing (subject to official notice of issuance) on Nasdaq.
-73-
(e) Lock-Up Agreement. Each Lock-Up Agreement shall be in full force and effect in accordance with the terms thereof as of the Closing.
(f) CVR Agreement. The CVR Agreement shall be in full force and effect in accordance with the terms thereof as of the Closing.
Section 7.2 Additional Conditions Precedent to Obligations of Parent and Merger Sub. The obligations of Parent and Merger Sub to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by Parent, at or prior to the Closing, of each of the following additional conditions:
(a) Accuracy of Representations. The Company Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The Company Capitalization Representations shall have been true and correct in all respects as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on and as of such date, except, in each case, (x) for such inaccuracies which are de minimis, individually or in the aggregate, (y) for those representations and warranties which address matters only as of a particular date (which representations and warranties shall have been true and correct, subject to the qualifications as set forth in the preceding clause (x), as of such particular date) or (z) for such inaccuracies that are taken into account in the calculation of the Company Outstanding Shares and the Exchange Ratio. The representations and warranties of the Company contained in this Agreement (other than the Company Fundamental Representations and the Company Capitalization Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date except (a) in each case, or in the aggregate, where the failure to be so true and correct would not reasonably be expected to have a Material Adverse Effect (without giving effect to any references therein to any Material Adverse Effect or other materiality qualifications) and (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Company Disclosure Letter made or purported to have been made after the date of this Agreement shall be disregarded).
(b) Performance of Covenants. The Company shall have performed or complied with in all material respects all agreements and covenants required to be performed or complied with by it under this Agreement at or prior to the Effective Time.
-74-
(c) Documents. Parent shall have received the following documents, each of which shall be in full force and effect:
(i) a certificate executed by the Chief Executive Officer or Chief Financial Officer of the Company certifying (i) that the conditions set forth in Section 7.2(a), (b), (d) and (e) have been duly satisfied and (ii) that the information (other than emails and addresses) set forth in the Allocation Certificate delivered by the Company in accordance with Section 6.20 is true and accurate in all respects as of the Closing Date;
(ii) a certificate pursuant to Treasury Regulations Sections 1.1445-2(c) and 1.897-2(h), together with a form of notice to the IRS in accordance with the requirements of Treasury Regulations Section 1.897-2(h), in each case, in form and substance reasonably acceptable to Parent; and
(iii) the Allocation Certificate.
(d) No Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Material Adverse Effect.
(e) Company Stockholder Written Consent. The Company Stockholder Written Consent executed by the stockholders of the Company shall be in full force and effect.
(f) Company Lock-Up Agreements. Stockholders of the Company representing no less than 50% of the outstanding shares of Company Common Stock on an “as-converted” basis (which includes the outstanding shares of Company Common Stock and Company Preferred Stock) as of immediately prior to the Effective Time have executed and delivered to Parent Lock-Up Agreements
(g) Committed Funding. The Company shall have received an aggregate amount of cash no less than $20,000,000 from the offering of its Company Convertible Notes.
Section 7.3 Additional Conditions Precedent to Obligation of the Company. The obligations of the Company to effect the Merger and otherwise consummate the transactions to be consummated at the Closing are subject to the satisfaction or the written waiver by the Company, at or prior to the Closing, of each of the following additional conditions:
(a) Accuracy of Representations. Each of the Parent Fundamental Representations shall have been true and correct in all material respects as of the date of this Agreement and shall be true and correct in all material respects on and as of the Closing Date with the same force and effect as if made on and as of such date (except to the extent such representations and warranties are specifically made as of a particular date, in which case such representations and warranties shall be true and correct in all material respects as of such date). The Parent Capitalization Representations shall have been true and correct in all respects as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on and as of such date, except, in each case, (w) for such inaccuracies which are de minimis, individually or in the aggregate, (x) for those representations and warranties which address matters only as of a particular date (which representations and warranties shall have been true and correct, subject to the qualifications as set forth in the preceding clause (w), as of such particular date) or (y) for such inaccuracies that are taken into account in the calculation of the Parent Outstanding Shares and the Exchange Ratio. The representations and warranties of Parent and Merger Sub contained in this Agreement (other than the Parent Fundamental Representations and the Parent Capitalization Representations) shall have been true and correct as of the date of this Agreement and shall be true and correct on and as of the Closing Date with the same force and effect as if made on the Closing Date except (a) in each case, or in the aggregate, where the failure to be true and correct would not reasonably be expected to have a Parent Material Adverse Effect (without giving effect to any references therein to any Parent Material Adverse Effect or other materiality qualifications) and (b) for those representations and warranties which address matters only as of a particular date (which representations shall have been true and correct, subject to the qualifications as set forth in the preceding clause (a), as of such particular date) (it being understood that, for purposes of determining the accuracy of such representations and warranties, any update of or modification to the Parent Disclosure Letter made or purported to have been made after the date of this Agreement shall be disregarded).
-75-
(b) Performance of Covenants. Parent and Merger Sub shall have performed or complied with in all material respects all of their agreements and covenants required to be performed or complied with by each of them under this Agreement at or prior to the Effective Time.
(c) Documents. The Company shall have received the following documents, each of which shall be in full force and effect:
(i) a certificate executed by an executive officer of Parent certifying that the conditions set forth in Section 7.3(a), (b), (d) and (e) have been duly satisfied;
(ii) written resignations in forms reasonably satisfactory to the Company, dated as of the Closing Date and effective as of the Closing executed by the officers and directors of Parent who are not to continue as officers or directors of Parent pursuant to Section 6.20 hereof;
(iii) the Exchange Ratio Statement; and
(iv) the Net Cash Schedule.
(d) No Parent Material Adverse Effect. Since the date of this Agreement, there shall not have occurred any Parent Material Adverse Effect.
(e) Parent Closing Net Cash. The Parent Closing Net Cash shall not be less than as set forth on Annex I of this Agreement.
(f) Parent Contracts. Notice terminating the Parent Contracts listed on Section 7.3(f) of the Parent Disclosure Letter shall be sent to the applicable counterparty no later than five (5) Business Days prior to the Closing Date.
-76-
ARTICLE VIII
TERMINATION
Section 8.1 Termination. This Agreement may be terminated prior to the Effective Time (whether before or after adoption of this Agreement by the Company’s stockholders and whether before or after approval of the Parent Stockholder Matters by Parent’s stockholders, unless otherwise specified below):
(a) by mutual written consent of Parent and the Company;
(b) by either Parent or the Company if the Merger shall not have been consummated by November 1, 2024 (subject to possible extension as provided in this Section 8.1(b), the “End Date”); provided, however, that the right to terminate this Agreement under this Section 8.1(b) shall not be available to the Company or Parent if such party’s (or in the case of Parent, Merger Sub) action or failure to act has been a principal cause of the failure of the Merger to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement, provided further, however, that, in the event that the SEC has not declared effective under the Securities Act the Registration Statement by the date which is sixty (60) days prior to the End Date, then either the Company or Parent shall be entitled to extend the End Date for an additional sixty (60) days;
(c) by either Parent or the Company if a court of competent jurisdiction or other Governmental Entity shall have issued a final and nonappealable order, or shall have taken any other action, having the effect of permanently restraining, enjoining or otherwise prohibiting the transactions contemplated by this Agreement;
(d) by Parent if the Company Stockholder Approval shall not have been delivered on or prior to the second (2nd) Business Day after the Registration Statement is declared effective under the Securities Act; provided, however, that once the Company Stockholder Approval has been obtained, Parent may not terminate this Agreement pursuant to this Section 8.1(d);
(e) by either Parent or the Company if (i) the Parent Stockholder Meeting (including any adjournments and postponements thereof) shall have been held and completed and Parent’s stockholders shall have taken a final vote on the Parent Stockholder Matters and (ii) the Parent Stockholder Approval shall not have been obtained at the Parent Stockholder Meeting (or at any adjournment or postponement thereof); provided, however, that the right to terminate this Agreement under this Section 8.1(e) shall not be available to Parent where the failure to obtain the Parent Stockholder Approval shall have been caused by the action or failure to act of Parent and such action or failure to act constitutes a material breach by Parent of this Agreement;
(f) by the Company (at any time prior to the Parent Stockholder Approval) if a Parent Triggering Event shall have occurred;
-77-
(g) by Parent (at any time prior to the adoption of this Agreement and the approval of the transactions contemplated by this Agreement by the Company Stockholder Approval) if a Company Triggering Event shall have occurred; (h) by the Company, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by Parent or Merger Sub or if any representation or warranty of Parent or Merger Sub shall have become inaccurate, in either case, such that the conditions set forth in Section 7.3(a) or Section 7.3(b) would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided, that the Company is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided further, that if such inaccuracy in Parent’s or Merger Sub’s representations and warranties or breach by Parent or Merger Sub is curable by Parent or Merger Sub, then this Agreement shall not terminate pursuant to this Section 8.1(h) as a result of such particular breach or inaccuracy until the earlier of (i) the expiration of a thirty-(30) day period commencing upon delivery of written notice from the Company to Parent or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to this Section 8.1(h) and (ii) Parent or Merger Sub (as applicable) ceasing to exercise commercially reasonable efforts to cure such breach following delivery of written notice from the Company to Parent or Merger Sub of such breach or inaccuracy and its intention to terminate pursuant to this Section 8.1(h) (it being understood that this Agreement shall not terminate pursuant to this Section 8.1(h) as a result of such particular breach or inaccuracy if such breach by Parent or Merger Sub is cured prior to such termination becoming effective);
(i) by Parent, upon a breach of any representation, warranty, covenant or agreement set forth in this Agreement by the Company or if any representation or warranty of the Company shall have become inaccurate, in either case, such that the conditions set forth in Section 7.2(a) or Section 7.2(b) would not be satisfied as of the time of such breach or as of the time such representation or warranty shall have become inaccurate; provided that Parent is not then in material breach of any representation, warranty, covenant or agreement under this Agreement; provided, further, that if such inaccuracy in the Company’s representations and warranties or breach by the Company is curable by the Company then this Agreement shall not terminate pursuant to this Section 8.1(i) as a result of such particular breach or inaccuracy until the earlier of (i) the expiration of a thirty-(30) day period commencing upon delivery of written notice from Parent to the Company of such breach or inaccuracy and its intention to terminate pursuant to this Section 8.1(i) and (ii) the Company ceasing to exercise commercially reasonable efforts to cure such breach following delivery of written notice from Parent to the Company of such breach or inaccuracy and its intention to terminate pursuant to this Section 8.1(i) (it being understood that this Agreement shall not terminate pursuant to this Section 8.1(i) as a result of such particular breach or inaccuracy if such breach by the Company is cured prior to such termination becoming effective); or
(j) by Parent (at any time prior to the Parent Stockholder Approval) and following compliance with all of the requirements set forth in the proviso to this Section 8.1(j), upon the Parent Board authorizing Parent to enter into a Permitted Alternative Agreement; provided, however, that Parent shall not enter into any Permitted Alternative Agreement unless: (i) the Company shall have received written notice from Parent of Parent’s intention to enter into such Permitted Alternative Agreement at least four (4) Business Days in advance, with such notice describing in reasonable detail the reasons for such intention as well as the material terms and conditions of such Permitted Alternative Agreement, including the identity of the counterparty together with copies of the then current draft of such Permitted Alternative Agreement and any other related principal transaction documents, (ii) Parent shall have complied in all material respects with its obligations under Section 6.4 and Section 6.8, (iii) the Parent Board shall have determined in good faith, after consultation with its outside legal counsel, that the failure to enter into such Permitted Alternative Agreement would reasonably be expected to be inconsistent with its fiduciary obligations under applicable Law and (iv) Parent shall concurrently pay to the Company the Company Termination Fee in accordance with Section 8.3(c).
-78-
The party desiring to terminate this Agreement pursuant to this Section 8.1 (other than pursuant to Section 8.1(a)) shall give a notice of such termination to the other party specifying the provisions hereof pursuant to which such termination is made and the basis therefor described in reasonable detail.
Section 8.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 8.1, this Agreement shall be of no further force or effect; provided, however, that (a) this Section 8.2, Section 8.3 and ARTICLE IX (and the related definitions of the defined terms in such Article) shall survive the termination of this Agreement and shall remain in full force and effect and (b) the termination of this Agreement and the provisions of Section 8.3 shall not relieve any party of any liability for fraud or for any willful and material breach of any representation, warranty, covenant, obligation or other provision contained in this Agreement.
Section 8.3 Expenses; Termination Fees.
(a) Except as set forth in this Section 8.3 and Section 6.12 all fees and expenses incurred in connection with this Agreement and the transactions contemplated by this Agreement shall be paid by the party incurring such expenses, whether or not the Merger is consummated.
(b) If (i) at any time after the date of this Agreement and prior to the Parent Stockholder Meeting, an Acquisition Proposal with respect to Parent shall have been publicly announced, disclosed or otherwise communicated to the Parent Board (and shall not have been withdrawn) and (ii) this Agreement is terminated by the Company pursuant to Section 8.1(f), Parent shall pay to the Company, within ten (10) Business Days after termination, a nonrefundable fee in an amount equal to $1,000,000 (the “Company Termination Fee”).
(c) If (i) at any time after the date of this Agreement and prior to the Parent Stockholder Meeting, an Acquisition Proposal with respect to Parent shall have been publicly announced, disclosed or otherwise communicated to the Parent Board (and shall not have been withdrawn), (ii) this Agreement is terminated by Parent or the Company pursuant to Section 8.1(e), and (iii) within twelve (12) months after the date of such termination, Parent enters into a definitive agreement with respect to a Subsequent Transaction or consummates a Subsequent Transaction, then Parent shall pay to the Company, within ten (10) Business Days after consummation of a Subsequent Transaction, the Company Termination Fee.
(d) If this Agreement is terminated by the Company pursuant to Section 8.1(e) (when at the time this Agreement is terminated, the Company had the right to terminate this Agreement pursuant to Section 8.1(f)), then Parent shall pay to the Company within ten (10) Business Days after such termination, the Company Termination Fee.
-79-
(e) If this Agreement is terminated by Parent pursuant to Section 8.1(j), then Parent shall pay to the Company, concurrent with such termination, the Company Termination Fee.
(f) If (i) at any time after the date of this Agreement and before obtaining the Company Stockholder Approval, an Acquisition Proposal with respect to the Company shall have been publicly announced, disclosed or otherwise communicated to the Company Board (and shall have not been withdrawn) and (i) this Agreement is terminated by Parent pursuant to Section 8.1(g), then the Company shall pay to Parent, within ten (10) Business Days after termination, a nonrefundable fee in an amount equal to $1,000,000 (the “Parent Termination Fee”).
(g) If (i) at any time after the date of this Agreement and prior to obtaining the Company Stockholder Approval, an Acquisition Proposal with respect to the Company shall have been publicly announced, disclosed or otherwise communicated to the Company Board (and shall not have been withdrawn), (ii) this Agreement is terminated by Parent pursuant to Section 8.1(d) and (iii) within twelve (12) months after the date of such termination, the Company enters into a definitive agreement with respect to a Subsequent Transaction or consummates a Subsequent Transaction, then the Company shall pay to Parent, within ten (10) Business Days after consummation of a Subsequent Transaction, the Parent Termination Fee.
(h) If this Agreement is terminated by the Company pursuant to Section 8.1(h), Parent shall reimburse the Company for all reasonable out-of-pocket fees and expenses incurred by the Company in connection with this Agreement and the transactions contemplated by this Agreement, up to a maximum of $750,000 by wire transfer of same-day funds within ten (10) Business Days following the date on which the Company submits to Parent true and correct copies of reasonable documentation supporting such expenses.
(i) If this Agreement is terminated by Parent pursuant to Section 8.1(i), the Company shall reimburse Parent for all reasonable out-of-pocket fees and expenses incurred by Parent in connection with this Agreement and the transactions contemplated by this Agreement, up to a maximum of $750,000, by wire transfer of same-day funds within ten (10) Business Days following the date on which Parent submits to the Company true and correct copies of reasonable documentation supporting such expenses.
(j) If either party fails to pay when due any amount payable by it under this Section 8.3, then (i) such party shall reimburse the other party for reasonable costs and expenses (including reasonable fees and disbursements of counsel) incurred in connection with the collection of such overdue amount and the enforcement by the other party of its rights under this Section 8.3 and (ii) such party shall pay to the other party interest on such overdue amount (for the period commencing as of the date such overdue amount was originally required to be paid and ending on the date such overdue amount is actually paid to the other party in full) at a rate per annum equal to the “prime rate” (as announced by Bank of America or any successor thereto) in effect on the date such overdue amount was originally required to be paid plus three percent.
(k) The parties agree that, subject to Section 8.2, the payment of the fees and expenses set forth in this Section 8.3 shall, in the circumstances in which it is owed in accordance with the terms of this Agreement, shall be the sole and exclusive remedy of each party following a termination of this Agreement under the circumstances described in this Section 8.3, it being understood that in no event shall either Parent or the Company be required to pay the individual fees or damages payable pursuant to this Section 8.3 on more than one occasion.
-80-
Subject to Section 8.2, following the payment of the fees and expenses set forth in this Section 8.3 by a party, (i) such party shall have no further liability to the other party in connection with or arising out of this Agreement or the termination thereof, any breach of this Agreement by the other party giving rise to such termination, or the failure of the transactions contemplated by this Agreement to be consummated, (ii) no other party or their respective Affiliates shall be entitled to bring or maintain any other claim, action or proceeding against such party or seek to obtain any recovery, judgment or damages of any kind against such party (or any partner, member, stockholder, director, officer, employee, Subsidiary, Affiliate, agent or other Representative of such party) in connection with or arising out of this Agreement or the termination thereof, any breach by such party giving rise to such termination or the failure of the transactions contemplated by this Agreement to be consummated and (iii) all other parties and their respective Affiliates shall be precluded from any other remedy against such party and its Affiliates, at law or in equity or otherwise, in connection with or arising out of this Agreement or the termination thereof, any breach by such party giving rise to such termination or the failure of the transactions contemplated by this Agreement to be consummated. Each of the parties acknowledges that (x) the agreements contained in this Section 8.3 are an integral part of the transactions contemplated by this Agreement, (y) without these agreements, the parties would not enter into this Agreement and (z) any amount payable pursuant to this Section 8.3 is not a penalty, but rather is liquidated damages in a reasonable amount that will compensate the parties in the circumstances in which such amount is payable.
ARTICLE IX
GENERAL PROVISIONS
Section 9.1 Non-survival of Representations and Warranties. None of the representations, warranties, covenants or agreements in this Agreement or in any instrument delivered pursuant to this Agreement shall survive the Effective Time, other than those covenants or agreements of the parties which by their terms apply, or are to be performed in whole or in part, after the Effective Time.
Section 9.2 Amendment or Supplement. This Agreement may be amended, modified or supplemented by the parties by action taken or authorized by their respective Boards of Directors at any time, whether before or after Company Stockholder Approval or the Parent Stockholder Approval has been obtained; provided, however, that after the Company Stockholder Approval or the Parent Stockholder Approval has been obtained, no amendment shall be made that pursuant to applicable Law requires further approval or adoption by the stockholders of the Company or Parent, as applicable, without such further approval or adoption. This Agreement may not be amended, modified or supplemented in any manner, whether by course of conduct or otherwise, except by an instrument in writing specifically designated as an amendment hereto, signed on behalf of each of the parties in interest at the time of the amendment.
Section 9.3 Waiver. The parties may, by action taken or authorized by their respective Boards of Directors, to the extent permitted by applicable Law, waive compliance with any of the agreements or conditions of the other parties contained herein; provided, however, that after the Company Stockholder Approval or the Parent Stockholder Approval has been obtained, no waiver may be made that pursuant to applicable Law requires further approval or adoption by the stockholders of the Company or Parent, as applicable, without such further approval or adoption.
-81-
Any agreement on the part of a party to any such waiver shall be valid only if set forth in a written instrument executed and delivered by a duly authorized officer on behalf of such party. No failure or delay of any party in exercising any right or remedy hereunder shall operate as a waiver thereof, nor shall any single or partial exercise of any such right or power, or any abandonment or discontinuance of steps to enforce such right or power, or any course of conduct, preclude any other or further exercise thereof or the exercise of any other right or power. The rights and remedies of the parties hereunder are cumulative and are not exclusive of any rights or remedies which they would otherwise have hereunder.
Section 9.4 Notices. All notices and other communications hereunder shall be in writing and shall be deemed duly given (a) on the date of delivery if delivered personally, or if by e‑mail, upon written confirmation of receipt by e‑mail or otherwise, (b) on the first Business Day following the date of dispatch if delivered utilizing a next-day service by a recognized next-day courier or (c) on the earlier of confirmed receipt or the fifth Business Day following the date of mailing if delivered by registered or certified mail, return receipt requested, postage prepaid. All notices hereunder shall be delivered to the addresses set forth below, or pursuant to such other instructions as may be designated in writing by the party to receive such notice:
(i) if to Parent or Merger Sub, to:
Kintara Therapeutics, Inc.
9920 Pacific Heights Blvd, Suite 150
San Diego, CA 92121
Attention: Robert Hoffman
Email: rhoffman@kintara.com
with a copy (which shall not constitute notice) to:
Lowenstein Sandler LLP
1251 Avenue of the Americas
New York, NY 10020
Attention: Steven M. Skolnick, Esq.
Email: sskolnick@lowenstein.com
(ii) if to Company or the Surviving Company, to:
TuHURA Biosciences, Inc.
10500 University Center Drive
Suite 110
Tampa, FL 3361
Attention: Dan Dearborn, Chief Financial Officer
E-mail:
-82-
with a copy (which shall not constitute notice) to:
Foley & Lardner LLP
100 North Tampa Street
Suite 2700
Tampa, FL 33602-5810
Attention: Curt P. Creely, Esq.
Garrett F. Bishop, Esq.
Email: ccreely@foley.com
gbishop@foley.com
Section 9.5 Certain Definitions. For purposes of this Agreement:
(a) “Acceptable Confidentiality Agreement” means a confidentiality agreement containing terms not materially less restrictive in the aggregate to the counterparty thereto than the terms of the Confidentiality Agreement, except such confidentiality agreement need not contain any standstill, non-solicitation or no hire provisions. Notwithstanding the foregoing, a Person who has previously entered into a confidentiality agreement with Parent relating to a potential Acquisition Proposal on terms that are not materially less restrictive than the Confidentiality Agreement with respect to the scope of coverage and restrictions on disclosure and use shall not be required to enter into a new or revised confidentiality agreement, and such existing confidentiality agreement shall be deemed to be an Acceptable Confidentiality Agreement.
(b) “Acquisition Inquiry” means, with respect to the Company or Parent, an inquiry, indication of interest or request for information (other than an inquiry, indication of interest or request for information made or submitted by Parent, on the one hand, or the Company, on the other hand, to the other party) that could reasonably be expected to lead to an Acquisition Proposal.
(c) “Acquisition Proposal” means, with respect to the Company or Parent, any offer or proposal, whether written or oral (other than an offer or proposal made or submitted by or on behalf of Parent or any of its Affiliates, on the one hand, or by or on behalf of the Company or any of its Affiliates, on the other hand, to the other party) contemplating or otherwise relating to any Acquisition Transaction with such party.
(d) “Acquisition Transaction”means any transaction or series of related transactions involving:
(i) any merger, consolidation, amalgamation, share exchange, business combination, issuance of securities, acquisition of securities, reorganization, recapitalization, tender offer, exchange offer or other similar transaction: (i) in which a party is a constituent Person, (ii) in which a Person or “group” (as defined in the Exchange Act and the rules promulgated thereunder) of Persons directly or indirectly acquires beneficial or record ownership of securities representing more than 20% of the outstanding securities of any class of voting securities of a party or any of its Subsidiaries or (iii) in which a party or any of its Subsidiaries issues securities representing more than 20% of the outstanding securities of any class of voting securities of such party or any of its Subsidiaries; or
-83-
(ii) any sale, lease, exchange, transfer, license, acquisition or disposition of any business or businesses or assets that constitute or account for 20% or more of the consolidated book value or the fair market value of the assets of a party and its Subsidiaries, taken as a whole.
(e) “Affiliate” of any Person means any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such first Person.
(f) “Anticipated Closing Date”means the anticipated Closing Date, as agreed upon by Parent and the Company.
(g) “Business Day” means any day other than a Saturday, a Sunday or a day on which banks in the State of New York or the State of Delaware are authorized or required by applicable Law to be closed.
(h) “Cash and Cash Equivalents“ means all (a) cash and cash equivalents, (b) marketable securities and (c) short-term investments, in each case determined in accordance with GAAP, and excluding restricted cash, if any.
(i) “Company Balance Sheet” means the audited consolidated balance sheet of the Company as at December 31, 2023, together with all related notes and schedules thereto.
(j) “Company Capitalization Representations” means the representations and warranties of the Company set forth in Sections 4.2(a) and 4.2(b).
(k) “Company Contract” means any Contract: (a) to which the Company is a Party, (b) by which the Company is or may become bound or under which the Company or any of its Subsidiaries has, or may become subject to, any obligation or (c) under which the Company has or may acquire any right or interest.
(l) “Company Fundamental Representations” means the representations and warranties of the Company set forth in Sections 4.1(a), 4.1(b), 4.4 and 4.24.
(m) “Company Owned IP” means all Intellectual Property owned by the Company or any of its Subsidiaries in whole or in part.
(n) “Company Triggering Event” shall be deemed to have occurred if: (a) the Company Board shall have made a Company Board Adverse Recommendation Change; (b) the Company Board or any committee thereof shall have publicly approved, endorsed or recommended any Acquisition Proposal; or (c) the Company shall have entered into any letter of intent or similar document or any Contract relating to any Acquisition Proposal.
(o) “Confidentiality Agreement” means that certain Mutual Non-Disclosure Agreement by and between Parent and the Company, dated as of February 22, 2024.
-84-
(p) “control” (including the terms “controlled,” “controlled by” and “under common control with”) means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
(q) “Health Care Laws” means the FDCA; the Public Health Service Act (42 U.S.C. § 201 et seq.), including the Clinical Laboratory Improvement Amendments of 1988 (42 U.S.C. § 263a); the Federal Trade Commission Act (15 U.S.C. § 41 et seq.); the Controlled Substances Act (21 U.S.C. § 801 et seq.); the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)); the civil monetary penalties law (42 U.S.C. § 1320a-7a); the civil False Claims Act (31 U.S.C. § 3729 et seq.); the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)); the Stark law (42 U.S.C. § 1395nn); the Criminal Health Care Fraud Statute (18 U.S.C. § 1347); the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.) as amended by the Health Information Technology for Economic and Clinical Health Act (42 U.S.C. § 17921 et seq.); the exclusion laws (42 U.S.C. § 1320a-7); Medicare (Title XVIII of the Social Security Act); Medicaid (Title XIX of the Social Security Act); and the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care and Education Reconciliation Act of 2010 (42 U.S.C. § 18001 et seq.); any regulations promulgated pursuant to such laws; and any other state, federal or ex-U.S. laws, accreditation standards, or regulations governing the manufacturing, development, testing, labeling, advertising, marketing or distribution of drugs or biological products, kickbacks, patient or program charges, recordkeeping, claims process, documentation requirements, medical necessity, referrals, the hiring of employees or acquisition of services or supplies from those who have been excluded from government health care programs, quality, safety, privacy, security, licensure, accreditation or any other aspect of providing health care, clinical laboratory or diagnostic products or services, to the extent applicable to the Company or any of its Subsidiaries.
(r) “Intellectual Property” means all intellectual property rights of any kind or nature in any jurisdiction throughout the world, including all of the following to the extent protected by applicable law: (i) trademarks or service marks (whether registered or unregistered), trade names, domain names, social media user names, social media addresses, logos, slogans, and trade dress, including applications to register any of the foregoing, together with the goodwill symbolized by any of the foregoing; (ii) patents, utility models and any similar or equivalent statutory rights with respect to the protection of inventions, and all applications for any of the foregoing, together with all re-issuances, continuations, continuations-in-part, divisionals, revisions, extensions and reexaminations thereof; (iii) copyrights (registered and unregistered) and applications for registration; (iv) trade secrets and customer lists, in each case to the extent any of the foregoing derives economic value (actual or potential) from not being generally known to other Persons who can obtain economic value from its disclosure or use, and other confidential information (“Trade Secrets”) and (v) any other proprietary or intellectual property rights of any kind or nature.
(s) “Key Employee” of Parent or the Company, as the case may be, means (i) any executive officer of such party or any of its Subsidiaries; and (ii) any employee of such party or any of its Subsidiaries that reports directly to the Board of Directors of such party or to an executive officer of such party or any of its Subsidiaries.
-85-
(t) “knowledge” of any party means (i) the actual knowledge of any executive officer of such party or other officer having primary responsibility for the relevant matter or (ii) any fact or matter which any such officer of such party could be expected to discover or otherwise become aware of in the course of conducting a reasonably comprehensive investigation, consistent with such officer’s title and responsibilities, concerning the existence of the relevant matter. With respect to any matters relating to Intellectual Property, such awareness or reasonable expectation to have knowledge does not require any such individual to conduct or have conducted or obtain or have obtained any freedom to operate opinions of counsel or any Intellectual Property rights clearance searches.
(u) “Nasdaq” means the Nasdaq Stock Market, LLC.
(v) “Nasdaq Reverse Split” means a reverse stock split of all outstanding shares of Parent Common Stock effected by Parent for the purpose of maintaining compliance with Nasdaq listing standards.
(w) “Ordinary Course of Business” means, in the case of each of the Company and Parent, such actions taken in the ordinary course of its normal operations and consistent with its past practices; provided, however, that to the extent such activity results in ongoing post-Closing obligations to Parent or Company, the terms of such activity shall be reasonably acceptable to Company.
(x) “Parent Capitalization Representations” means the representations and warranties of Parent and Merger Sub set forth in Sections 5.2(a) through 5.2(d).
(y) “Parent Closing Net Cash” means the amount, as of the Effective Time, without duplication, equal to (i) Parent’s Cash and Cash Equivalents determined, to the extent in accordance with GAAP, in a manner consistent with the manner in which such items were historically determined and in accordance with the financial statements (including any related notes) contained or incorporated by reference in the Parent SEC Documents and the Parent Balance Sheet, plus (ii) all prepaid expenses set forth on Section 9.5 of the Parent Disclosure Letter (that continue to be prepaid expenses in nature and amount as of the Effective Time), minus (iii) the sum of Parent’s consolidated short-term and long-term liabilities accrued as of the Closing Date to the extent in accordance with GAAP, which includes, for the avoidance of doubt, any amounts payable to Parent’s officers and directors for fees, expenses, and accrued bonuses and other liabilities, which as of the date hereof was equal to approximately $148,000 in the aggregate, minus (iv) Transaction Expenses, minus (v) to the extent payable in cash in connection with or at Closing, any and all liabilities incurred as a result of a change of control, including liabilities paid to third parties and to any employee (including change of control payments, retention payments, severance and other employee-related termination costs, or other payments) of Parent, the Company or any of their respective Subsidiaries and minus (vi) $50,000 to be reserved for future dividend payments to Series A Preferred Stock of Parent.
(z) “Parent Fundamental Representations” means the representations and warranties of Parent and Merger Sub set forth in Sections 5.1(a), Section 5.1(b), 5.4 and 5.22.
-86-
(aa) “Parent Owned IP” means all Intellectual Property owned by Parent or any of its Subsidiaries in whole or in part.
(bb) “Parent Stockholder Approval” means, approval by the holders of Parent Common Stock in accordance with Nevada law and the Parent Charter of the Required Parent Stockholder Proposals.
(cc) “Parent Triggering Event” shall be deemed to have occurred if: (a) Parent shall have failed to include in the Proxy Statement the Parent Board Recommendation, (b) the Parent Board or any committee thereof shall have made a Parent Board Adverse Recommendation Change or publicly approved, endorsed or recommended any Acquisition Proposal or (c) Parent shall have entered into any letter of intent or similar document or any Contract relating to any Acquisition Proposal (other than an Acceptable Confidentiality Agreement permitted pursuant to Section 6.4).
(dd) “Permitted Alternative Agreement” means a definitive agreement that contemplates or otherwise relates to an Acquisition Transaction that constitutes a Superior Offer.
(ee) “Person” means an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, including any Governmental Entity.
(ff) “Representative” means a party’s directors, officers, employees, investment bankers, financial advisors, attorneys, accountants or other advisors, agents or representatives.
(gg) “SEC” means the Securities and Exchange Commission.
(hh) “Subsequent Transaction” means any Acquisition Transaction (with all references to 20% in the definition of Acquisition Transaction being treated as references to 50% for these purposes).
(ii) “Subsidiary” means, with respect to any Person, any other Person of which stock or other equity interests having ordinary voting power to elect more than 50% of the board of directors or other governing body are owned, directly or indirectly, by such first Person.
(jj) “Superior Offer” means an unsolicited bona fide written Acquisition Proposal (with all references to 20% in the definition of Acquisition Transaction being treated as references to 50% for these purposes) that: (a) was not obtained or made as a direct or indirect result of a breach of (or in violation of) the Agreement, (b) is on terms and conditions that the Parent Board or the Company Board, as applicable, determines in good faith, based on such matters that it deems relevant (including the likelihood of consummation thereof and the financing terms thereof), as well as any written offer by the other party to the Agreement to amend the terms of the Agreement, and following consultation with its outside legal counsel and financial advisors, if any, are more favorable, from a financial point of view, to Parent’s stockholders or the Company’s stockholders, as applicable, than the terms of the transactions contemplated by this Agreement, (c) is not subject to any financing conditions (and if financing is required, such financing is then fully committed to the third party) and (d) is reasonably capable of being completed on the terms proposed without unreasonable delay.
-87-
(kk) “Tax Return” means any return, declaration, report, certificate, bill, election, claim for refund, information return, statement or other written information and any other document filed or supplied or required to be filed or supplied to any Governmental Entity or any other Person with respect to Taxes, including any schedule, attachment or supplement thereto, and including any amendment thereof.
(ll) “Taxes” means (i) all federal, state, local, foreign and other net income, gross income, gross receipts, sales, use, stock, ad valorem, transfer, transaction, franchise, profits, gains, registration, license, wages, lease, service, service use, employee and other withholding, social security, unemployment, welfare, disability, payroll, employment, excise, severance, stamp, occupation, workers’ compensation, premium, real property, personal property, windfall profits, net worth, capital, value-added, alternative or add-on minimum, customs duties, estimated and other taxes, fees, assessments, charges or levies of any kind whatsoever (whether imposed directly or through withholding and including taxes of any third party in respect of which a Person may have a duty to collect or withhold and remit and any amounts resulting from the failure to file any Tax Return), whether disputed or not, together with (ii) any interest and any penalties, additions to tax or additional amounts with respect thereto.
(mm) “Transaction Expenses” means the aggregate amount (without duplication) of all costs, fees and expenses incurred by Parent or any of its Subsidiaries (including Merger Sub), or for which Parent or any of its Subsidiaries are or may become liable in connection with the transactions contemplated hereby and the negotiation, preparation and execution of this Agreement or any other agreement, document, instrument, filing, certificate, schedule, exhibit, letter or other document prepared or executed in connection with the transactions contemplated hereby, including (a) any fees and expenses of legal counsel and accountants, the maximum amount of fees and expenses payable to financial advisors, investment bankers, brokers, consultants, tax advisors, transfer agents, proxy solicitor and other advisors of Parent and (b) any bonus, retention payments, severance, fundamental transaction, change-in-control payments or similar payment obligations (including payments with “single-trigger” provisions triggered at and as of the consummation of the transactions contemplated hereby) that become due or payable to any director, officer, employee, consultant, contract counterparty or equity holder in connection with the consummation of the transactions contemplated hereby, together with the employer’s share of any payroll Taxes associated therewith; provided, however, that Transaction Expenses shall specifically exclude the value of any settlement or judgment that is awarded post-Closing relating to stockholder litigation arising out of or in connection with the transactions contemplated by this Agreement.
Section 9.6 Interpretation. When a reference is made in this Agreement to a Section, Article, Exhibit or Schedule such reference shall be to a Section, Article, Exhibit or Schedule of this Agreement unless otherwise indicated. The table of contents and headings contained in this Agreement or in any Exhibit or Schedule are for convenience of reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. All words used in this Agreement will be construed to be of such gender or number as the circumstances require. Any capitalized terms used in any Exhibit or Schedule but not otherwise defined therein shall have the meaning as defined in this Agreement. All Exhibits and Schedules annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth herein. The word “including” and words of similar import when used in this Agreement will mean “including, without limitation,” unless otherwise specified. The words “hereof,” “herein” and “hereunder”
-88-
and words of similar import when used in this Agreement shall refer to the Agreement as a whole and not to any particular provision in this Agreement. The term “or” is not exclusive. The word “will” shall be construed to have the same meaning and effect as the word “shall.” References to days mean calendar days unless otherwise specified.
Section 9.7 Entire Agreement. This Agreement (including the Exhibits hereto), the Company Disclosure Letter, the Parent Disclosure Letter and the Confidentiality Agreement constitute the entire agreement, and supersede all prior written agreements, arrangements, communications and understandings and all prior and contemporaneous oral agreements, arrangements, communications and understandings among the parties with respect to the subject matter hereof and thereof.
Section 9.8 No Third Party Beneficiaries.
(a) Nothing in this Agreement, express or implied, is intended to or shall confer upon any Person other than the parties and their respective successors and permitted assigns any legal or equitable right, benefit or remedy of any nature under or by reason of this Agreement, except as provided in Section 6.10.
(b) The representations and warranties in this Agreement are the product of negotiations among the parties hereto and are for the sole benefit of the parties hereto. Any inaccuracies in such representations and warranties are subject to waiver by the parties hereto in accordance with Section 9.3 without notice or liability to any other Person. In some instances, the representations and warranties in this Agreement may represent an allocation among the parties hereto of risks associated with particular matters regardless of the knowledge of any of the parties hereto. Consequently, Persons other than the parties hereto may not rely upon the representations and warranties in this Agreement as characterizations of actual facts or circumstances as of the date of this Agreement or as of any other date.
Section 9.9 Governing Law. This Agreement and all disputes or controversies arising out of or relating to this Agreement or the transactions contemplated hereby shall be governed by, and construed in accordance with, the internal laws of the State of Delaware, without regard to the laws of any other jurisdiction that might be applied because of the conflicts of laws principles of the State of Delaware.
Section 9.10 Submission to Jurisdiction. Each of the parties irrevocably agrees that any legal action or proceeding arising out of or relating to this Agreement brought by any party or its Affiliates against any other party or its Affiliates shall be brought and determined in the Court of Chancery of the State of Delaware; provided, that if jurisdiction is not then available in the Court of Chancery of the State of Delaware, then any such legal action or proceeding may be brought in any federal court located in the State of Delaware or any other Delaware state court. Each of the parties hereby irrevocably submits to the jurisdiction of the aforesaid courts for itself and with respect to its property, generally and unconditionally, with regard to any such action or proceeding arising out of or relating to this Agreement and the transactions contemplated hereby. Each of the parties agrees not to commence any action, suit or proceeding relating thereto except in the courts described above in Delaware, other than actions in any court of competent jurisdiction to enforce any judgment, decree or award rendered by any such court in Delaware as described herein. Each of the parties further agrees that notice as provided herein shall constitute sufficient service of process and the parties further waive any argument that such service is insufficient.
-89-
Each of the parties hereby irrevocably and unconditionally waives, and agrees not to assert, by way of motion or as a defense, counterclaim or otherwise, in any action or proceeding arising out of or relating to this Agreement or the transactions contemplated hereby, (a) any claim that it is not personally subject to the jurisdiction of the courts in Delaware as described herein for any reason, (b) that it or its property is exempt or immune from jurisdiction of any such court or from any legal process commenced in such courts (whether through service of notice, attachment prior to judgment, attachment in aid of execution of judgment, execution of judgment or otherwise) and (c) that (i) the suit, action or proceeding in any such court is brought in an inconvenient forum, (ii) the venue of such suit, action or proceeding is improper or (iii) this Agreement, or the subject matter hereof, may not be enforced in or by such courts.
Section 9.11 Assignment; Successors. Neither this Agreement nor any of the rights, interests or obligations under this Agreement may be assigned or delegated, in whole or in part, by operation of law or otherwise, by any party without the prior written consent of the other parties, and any such assignment or delegation without such prior written consent shall be null and void. Subject to the preceding sentence, this Agreement will be binding upon, inure to the benefit of, and be enforceable by, the parties and their respective successors and assigns.
Section 9.12 Other Remedies; Specific Performance. The parties agree that irreparable damage would occur in the event that the parties hereto do not perform the provisions of this Agreement in accordance with its terms or otherwise breach such provisions. Accordingly, the parties acknowledge and agree that each party shall be entitled to an injunction, specific performance and other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof in the Court of Chancery of the State of Delaware; provided, that if jurisdiction is not then available in the Court of Chancery of the State of Delaware, then in any federal court located in the State of Delaware or any other Delaware state court, this being in addition to any other remedy to which such party is entitled at law or in equity; provided, further, for the avoidance of doubt, under no circumstance shall the Company or Parent be permitted or entitled to receive both a grant of specific performance that results in the consummation of the transactions contemplated by this Agreement and monetary damages, including, without limitation, any monetary damages in lieu of specific performance and/or the Parent Termination Fee or the Company Termination Fee, as applicable. Each of the parties hereby further waives (a) any defense in any action for specific performance that a remedy at law would be adequate and (b) any requirement under any law to post security as a prerequisite to obtaining equitable relief.
Section 9.13 Currency. All references to “dollars” or “$” or “US$” in this Agreement refer to United States dollars, which is the currency used for all purposes in this Agreement.
Section 9.14 Further Assurances. Each party agrees to cooperate fully with the other party and to execute and deliver such further documents, certificates, agreements and instruments and to take such other actions as may be reasonably requested by the other party to evidence or reflect the transactions contemplated by this Agreement and to carry out the intent and purposes of this Agreement.
-90-
Section 9.15 Severability. Whenever possible, each provision or portion of any provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable Law, but if any provision or portion of any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable Law or rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or portion of any provision in such jurisdiction, and this Agreement shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision or portion of any provision had never been contained herein.
Section 9.16 Waiver of Jury Trial. EACH OF THE PARTIES TO THIS AGREEMENT HEREBY IRREVOCABLY WAIVES ALL RIGHT TO A TRIAL BY JURY IN ANY ACTION, PROCEEDING OR COUNTERCLAIM ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY.
Section 9.17 Counterparts. This Agreement may be executed in two or more counterparts, all of which shall be considered one and the same instrument and shall become effective when one or more counterparts have been signed by each of the parties and delivered to the other party.
Section 9.18 Facsimile or .pdf Signature. This Agreement may be executed by facsimile or .pdf signature and a facsimile or .pdf signature shall constitute an original for all purposes.
Section 9.19 No Presumption Against Drafting Party. Each of Parent, Merger Sub and the Company acknowledges that each party to this Agreement has been represented by counsel in connection with this Agreement and the transactions contemplated by this Agreement. Accordingly, any rule of law or any legal decision that would require interpretation of any claimed ambiguities in this Agreement against the drafting party has no application and is expressly waived.
[The remainder of this page is intentionally left blank.]
-91-
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed as of the date first written above by their respective officers thereunto duly authorized.
KINTARA THERAPEUTICS, INC.
By: /s/ Robert Hoffman
Name: Robert Hoffman
Title: Chief Executive Officer
KAYAK MERGECO, INC.
By: /s/ Robert Hoffman
Name: Robert Hoffman
Title: President and Secretary
TUHURA BIOSCIENCES, INC.
By: /s/ James D. Bianco
Name: James D. Bianco
Title: Chief Executive Officer
[Signature Page to Agreement and Plan of Merger]
Annex I
If the Effective Time is:
i. On or before June 30, 2024, the Parent Closing Net Cash shall not be less than $750,000;
ii. Between July 1, 2024 and July 31, 2024, the Parent Closing Net Cash shall not be less than $625,000;
iii. Between August 1, 2024 and August 31, 2024, the Parent Closing Net Cash shall not be less than $500,000; or
iv. On or after September 1, 2024, the Parent Closing Net Cash shall not be less than $0.
Exhibit 10.1
FORM OF PARENT STOCKHOLDER SUPPORT AGREEMENT
KINTARA THERAPEUTICS, INC.
THIS SUPPORT AGREEMENT (this “Agreement”), dated as of April [●], 2024, is made by and among Kintara Therapeutics, Inc., a Nevada corporation (“Parent”), TuHURA Biosciences, Inc., a Delaware corporation (the “Company”), and the undersigned holder (“Stockholder”) of shares of capital stock (the “Shares”) of Parent.
WHEREAS, Parent, Kayak Mergeco, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and the Company, have entered into an Agreement and Plan of Merger, dated of even date herewith (the “Merger Agreement”), providing for the merger of Merger Sub with and into the Company, with the Company surviving the merger and becoming a wholly owned subsidiary of Parent (the “Merger”), upon the terms and subject to the conditions set forth in the Merger Agreement;
WHEREAS, Stockholder beneficially owns and has sole or shared voting power with respect to the number of Shares, and holds options to purchase shares of Parent Common Stock (“Parent Options”), restricted stock units to acquire shares of Parent Common Stock (“Parent Restricted Stock Units”), and/or such other rights to acquires shares of Parent Common Stock, as the case may be, in each case in the number of Shares indicated opposite Stockholder’s name on Schedule 1 attached hereto;
WHEREAS, as an inducement and a condition to the willingness of Parent, Merger Sub and the Company to enter into the Merger Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder has agreed to enter into and perform this Agreement; and
WHEREAS, all capitalized terms used in this Agreement without definition herein shall have the meanings ascribed to them in the Merger Agreement.
NOW, THEREFORE, in consideration of, and as a condition to, Parent, Merger Sub and the Company’s entering into the Merger Agreement and proceeding with the transactions contemplated thereby, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder, Parent and the Company agree as follows:
-2-
-3-
-4-
-5-
-6-
-7-
-8-
[Remainder of Page has Intentionally Been Left Blank]
-9-
EXECUTED as of the date first above written.
|
|
STOCKHOLDER
STOCKHOLDER NAME: |
|
|
|
Signature: ___________________________ |
Name (if an Entity): |
Title (if an Entity): |
[Signature Page to Support Agreement]
EXECUTED as of the date first above written.
|
KINTARA THERAPEUTICS, INC. |
|
|
By: ___________________________ |
Name: |
Title: |
|
|
[Signature Page to Support Agreement]
TuHURA BIOSCIENCES, INC. |
|
|
By: ___________________________ |
Name: |
Title: |
[Signature Page to Support Agreement]
SCHEDULE 1
|
|
|
Name of Stockholder |
|
|
Address: |
|
|
|
|
|
Attention: |
|
|
Email: |
|
|
|
|
|
PARENT SECURITIES HELD |
||
Shares: |
|
|
Parent Stock Options: |
|
|
Parent Restricted Stock Units: |
|
|
Shares underlying other rights (e.g., Parent Warrants): |
|
|
Exhibit 10.2
FORM OF COMPANY STOCKHOLDER SUPPORT AGREEMENT
TUHURA BIOSCIENCES, INC.
THIS SUPPORT AGREEMENT (this “Agreement”), dated as of April [●], 2024, is made by and among Kintara Therapeutics, Inc., a Nevada corporation (“Parent”), TuHURA Biosciences, Inc., a Delaware corporation (the “Company”), and the undersigned holder (“Stockholder”) of shares of capital stock (the “Shares”) of the Company.
WHEREAS, Parent, Kayak Mergeco, Inc., a Delaware corporation and a wholly owned subsidiary of Parent (“Merger Sub”), and the Company, have entered into an Agreement and Plan of Merger, dated of even date herewith (the “Merger Agreement”), providing for the merger of Merger Sub with and into the Company, with the Company surviving the merger and becoming a wholly owned subsidiary of Parent (the “Merger”), upon the terms and subject to the conditions set forth in the Merger Agreement;
WHEREAS, Stockholder beneficially owns and has sole or shared voting power with respect to the number of Shares, and holds options to purchase shares of Company Common Stock (“Company Options”), and/or such other rights to acquires shares of Company Common Stock, as the case may be, in each case in the number of Shares] indicated opposite Stockholder’s name on Schedule 1 attached hereto;
WHEREAS, as an inducement and a condition to the willingness of Parent, Merger Sub and the Company to enter into the Merger Agreement, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder has agreed to enter into and perform this Agreement; and
WHEREAS, all capitalized terms used in this Agreement without definition herein shall have the meanings ascribed to them in the Merger Agreement.
NOW, THEREFORE, in consideration of, and as a condition to, Parent, Merger Sub and the Company’s entering into the Merger Agreement and proceeding with the transactions contemplated thereby, and in consideration of the substantial expenses incurred and to be incurred by them in connection therewith, Stockholder, Parent and the Company agree as follows:
-2-
-3-
-4-
A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY
-5-
-6-
-7-
-8-
-9-
[Remainder of Page has Intentionally Been Left Blank]
-10-
EXECUTED as of the date first above written.
|
|
STOCKHOLDER
STOCKHOLDER NAME: |
|
|
|
Signature: ___________________________ |
Name (if an Entity): |
Title (if an Entity): |
[Signature Page to Support Agreement]
EXECUTED as of the date first above written.
|
|
TuHURA BIOSCIENCES, INC.
|
|
|
By: ___________________________ |
Name: |
Title: |
|
|
[Signature Page to Support Agreement]
|
|
KINTARA THERAPEUTICS, INC.
|
|
By: ___________________________ |
Name: |
Title: |
[Signature Page to Support Agreement]
SCHEDULE 1
|
|
|
Name of Stockholder |
|
|
Address: |
|
|
|
|
|
Attention: |
|
|
Email: |
|
|
|
|
|
COMPANY SECURITIES HELD |
||
Shares of Common Stock and Shares of Preferred Stock:
Company Stock Options:
Shares underlying other rights (e.g., Company Warrants):
Exhibit 10.3
FORM OF LOCK-UP AGREEMENT
April [●], 2024
Kintara Therapeutics, Inc. |
|
Ladies and Gentlemen:
The undersigned signatory of this lock-up agreement (this “Lock-Up Agreement”) understands that Kintara Therapeutics, Inc., a Nevada corporation (“Parent”), has entered into an Agreement and Plan of Merger, dated as of April 2, 2024 (as the same may be amended from time to time, the “Merger Agreement”) with Kayak Mergeco, Inc., a Delaware corporation and a wholly owned subsidiary of Parent, and TuHURA Biosciences, Inc., a Delaware corporation (the “Company”). Capitalized terms used but not otherwise defined herein shall have the respective meanings ascribed to such terms in the Merger Agreement.
provided that, in the case of any transfer or distribution pursuant to this clause (a), such transfer is not for value and each donee, heir, beneficiary or other transferee or distributee shall sign and deliver to Parent, a lock-up agreement in the form of this Lock-Up Agreement with respect to the shares of Parent Common Stock or such other securities that have been so transferred or distributed;
2
and provided, further, that, with respect to each of clauses (a), (b), (c), (d) and (e) above, no filing by any party (including any donor, donee, transferor, transferee, distributor or distributee) under Section 16 of the Exchange Act or other public announcement shall be required or shall be made voluntarily in connection with such transfer or disposition during the Restricted Period (other than (i) any exit filings or public announcements that may be required under applicable federal and state securities Laws or (ii) in respect of a required filing under the Exchange Act in connection with the exercise of an option to purchase Parent Common Stock or in connection with the net settlement of any restricted stock unit or other equity award that represents the right to receive in the future shares of Parent Common Stock settled in shares of Parent Common Stock that would otherwise expire during the Restricted Period, provided that reasonable notice shall be provided to Parent prior to any such filing).
THE SHARES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO AND MAY ONLY BE TRANSFERRED IN COMPLIANCE WITH A LOCK-UP AGREEMENT, A COPY OF WHICH IS ON FILE AT THE PRINCIPAL OFFICE OF THE COMPANY.
3
4
(Signature Page Follows)
5
|
Very truly yours, |
|
|
Print Name of Stockholder: |
[ ] |
|
|
|
|
|
|
|
Signature (for individuals): |
|
|
|
|
|
_____________________________ |
|
|
|
|
|
Signature (for entities): |
|
|
|
|
|
_____________________________ |
|
|
|
By: _________________________ |
|
Name: _______________________ |
|
Title: ________________________ |
|
|
|
|
[Signature Page to Lock-Up Agreement]
Accepted and Agreed |
|
by KINTARA THERAPEUTICS, INC. |
|
|
|
|
|
By: _________________________ |
|
Name: _______________________ |
|
Title: ________________________ |
|
|
|
|
|
[Signature Page to Lock-Up Agreement]
Accepted and Agreed |
|
By TUHURA BIOSCIENCES, INC. |
|
|
|
|
|
By: _________________________ |
|
Name: _______________________ |
|
Title: ________________________ |
|
[Signature Page to Lock-Up Agreement]
Exhibit 10.4
FORM OF CONTINGENT VALUE RIGHTS AGREEMENT
THIS CONTINGENT VALUE RIGHTS AGREEMENT, dated as of [●], 2024 (this
“Agreement”), is entered into by and between Kintara Therapeutics, a Nevada corporation corporation (“Parent”), Mountain Share Transfer, LLC, as Rights Agent (the “Rights Agent”) and Robert Hoffman, solely in his capacity as the initial representative, agent and attorney-in-fact of the Holders (the “Representative”).
RECITALS
WHEREAS, Parent, Kayak Mergeco, Inc., a Delaware corporation (“Merger Sub”), and TuHURA Biosciences, Inc., a Delaware corporation (the “Company”), have entered into an Agreement and Plan of Merger, dated as of April 2, 2024 (as it may be amended or supplemented from time to time pursuant to the terms thereof, the “Merger Agreement”), pursuant to which Merger Sub will merge with and into the Company (the “Merger”), with the Company surviving the Merger as the surviving corporation and a wholly-owned subsidiary of Parent. Capitalized terms used herein and not otherwise defined shall have the meaning ascribed to them in the Merger Agreement;
WHEREAS, pursuant to the Merger Agreement, and in accordance with the terms and conditions thereof, Parent has agreed to provide to the Holders (as defined herein) contingent value rights (the “CVRs” and, each individually, a “CVR”) as hereinafter described; and
WHEREAS, the Holders desire that the Representative (as defined herein) act as their agent for the purposes of accomplishing the intent and implementing the provisions of this Agreement and facilitating the consummation of the transactions contemplated hereby and performing the other services described in this Agreement.
NOW, THEREFORE, in consideration of the foregoing and the consummation of the transactions referred to above, Parent, Rights Agent, and Representative agree, for the equal and proportionate benefit of all Holders, as follows:
ARTICLE I
DEFINITIONS; CERTAIN RULES OF CONSTRUCTION
Section 1.1 Definitions. Capitalized terms used but not otherwise defined herein shall have the meanings ascribed to them in the Merger Agreement. As used in this Agreement, the following terms will have the following meanings:
“Acting Holders” means, at the time of determination, Holders of at least twenty-five percent (25%) of the outstanding CVRs as set forth in the CVR Register.
“Assignee” has the meaning set forth in Section 6.3.
“Code” shall mean the Internal Revenue Code of 1986, as amended, and the rules and regulations promulgated thereunder.
“CVR Payment” means the number of shares of Parent Common Stock equal to 53,897,125 shares of Parent Common Stock.
“CVR Payment Amount” means, with respect to the CVR Payment and each Holder, a number of shares of Parent Common Stock, rounded down, equal to the product of (i) the CVR Payment times (ii) the quotient of the total number of CVRs held by such Holder as reflected on the CVR Register divided by the total number of CVRs as reflected on the CVR Register. For the avoidance of doubt, no CVR Payment Amount shall be made with respect to any CVRs not provided to holders as a result of withholding tax imposed upon the provision of the CVRs, and the CVR Payment Amounts that would otherwise have been associated with such CVRs shall be retained by Parent.
“CVR Period” means the period beginning immediately following the Effective Time and ending on December 31, 2025.
“CVR Register” has the meaning set forth in Section 2.3(b). “Delaware Courts” has the meaning set forth in Section 6.6(b).
“Entity” means any corporation (including any non-profit corporation), general partnership, limited partnership, limited liability partnership, joint venture, estate, trust, company (including any company limited by shares, limited liability company or joint stock company), firm, society or other enterprise, association, organization or entity.
“Governmental Body” means any federal, state, local or foreign government or subdivision thereof or any other governmental, administrative, judicial, arbitral, legislative, executive, regulatory or self-regulatory authority, instrumentality, agency, commission or body.
“Holder” means, at the relevant time, a Person in whose name CVRs are registered in the CVR Register.
“Milestone” means, (1) the enrollment of a minimum of ten cutaneous metastic breast cancer patients in a study to determine whether a dose of REM-001 lower than 1.2 mg/kg elicits a treatment effect similar to that seen in prior studies of REM-001 at the 1.2 mg/kg dose and (2) the completion of eight weeks of follow-up for such patients, in each case, during the CVR Period.
“Milestone Notice” has the meaning set forth in Section 2.4(a).
“Milestone Payment Date” has the meaning set forth in Section 2.4(b).
“Officer’s Certificate” means a certificate signed by the chief executive officer of Parent, in his or her capacity as such an officer, and delivered to the Rights Agent and Representative.
-2-
“Parent Common Stock” means shares of common stock, par value $0.001 per share, of Parent.
“Permitted Transfer” means: a Transfer of CVRs (a) upon death of a Holder by will or intestacy; (b) pursuant to a court order; (c) by operation of law (including by consolidation or merger) or without consideration in connection with the dissolution, liquidation or termination of any corporation, limited liability company, partnership or other Person; or (d) in the case of CVRs held in book-entry or other similar nominee form, from a nominee to a beneficial owner and, if applicable, through an intermediary, in each case as permitted by the Depository Trust Company.
“Person” means any individual, Entity or Governmental Body.
“Review Request Period” has the meaning set forth in Section 4.7(a).
“Rights Agent” means the Rights Agent named in the first paragraph of this Agreement, until a successor Rights Agent will have become such pursuant to the applicable provisions of this Agreement, and thereafter “Rights Agent” will mean such successor Rights Agent.
“Tax” shall mean any tax (including any income tax, franchise tax, capital gains tax, gross receipts tax, value-added tax, surtax, estimated tax, unemployment tax, national health insurance tax, excise tax, premium, alternative or minimum tax, ad valorem tax, transfer tax, stamp tax, sales tax, use tax, property tax, business tax, withholding tax or payroll tax), levy, assessment, tariff, impost, imposition, duty (including any customs duty) or other tax or charge of any kind whatsoever, including any charge or amount (including any fine, penalty, interest or other additions thereto) related thereto, imposed, assessed or collected by or under the authority of any Governmental Body, including as a result of being or having been a member of an affiliated, consolidated, controlled, fiscal, combined, unitary or aggregate group or being a transferee of or successor to any Person or as a result of any express obligation to assume such Taxes or to indemnify any other Person.
“Transfer” means any transfer, pledge, hypothecation, encumbrance, assignment or other disposition (whether by sale, merger, consolidation, liquidation, dissolution, dividend, distribution or otherwise), the offer to make such a transfer or other disposition, and each contract, arrangement or understanding, whether or not in writing, to effect any of the foregoing.
Section 1.2 Rules of Construction. Except as otherwise explicitly specified to the contrary, (a) references to a Section means a Section of this Agreement unless another agreement is specified, (b) the word “including” (in its various forms) means “including without limitation”, (c) references to a particular statute or regulation include all rules and regulations thereunder and any successor statute, rules or regulation, in each case as amended or otherwise modified from time to time, (d) words in the singular or plural form include the plural and singular form, respectively, (e) references to a particular Person include such Person’s successors and assigns to the extent not prohibited by this Agreement and (f) all references to dollars or “$” refer to United States dollars.
-3-
ARTICLE II CONTINGENT VALUE RIGHTS
Section 2.1 CVRs.
Section 2.2 Nontransferable. The CVRs may not be sold, assigned, Transferred, pledged, encumbered or in any other manner transferred or disposed of, in whole or in part, other than through a Permitted Transfer. Any attempted Transfer, pledge, encumbrance or disposition of CVRs, in whole or in part, in violation of this Section 2.2 shall be void ab initio and of no effect.
Section 2.3 No Certificate; Registration; Registration of Transfer; Change of Address.
-4-
Section 2.4 Payment Procedures; Notices.
-5-
Section 2.5 No Voting, Dividends or Interest; No Equity or Ownership Interest in Parent.
-6-
Section 2.6 Ability to Abandon CVR. A Holder may at any time, at such Holder’s option, abandon all of such Holder’s remaining rights in a CVR by transferring such CVR to Parent or a Person nominated in writing by Parent (with written notice thereof from Parent to the Rights Agent) without consideration therefor, and such rights will be cancelled, with the Rights Agent being promptly notified in writing by Parent of such Transfer and cancellation. Nothing in this Agreement shall prohibit Parent or any of its Affiliates from offering to acquire or acquiring any CVRs for consideration from the Holders, in private transactions or otherwise, in its sole discretion. Any CVRs acquired by Parent or any of its Affiliates shall be automatically deemed extinguished and no longer outstanding for purposes of this Agreement.
Section 2.7 No Obligations of Parent. (A) Parent and its Affiliates shall have the power and right to control all aspects of their businesses and operations (and all of their assets and products), and subject to its compliance with the terms of this Agreement, Parent and its Affiliates may exercise or refrain from exercising such power and right as it may deem appropriate and in the best overall interests of Parent and its Affiliates and its and their stockholders, rather than the interest of the Holders, (B) subject to Section 4.2, none of Parent or any of its Affiliates shall have any obligation to own, operate, use, sell, transfer, convey, license, develop, commercialize or otherwise exploit in any particular manner any of their business or operations (or any of their assets or products) or to negotiate or enter into any agreement, including in order to obtain, maximize or expedite the completion of any Milestone, and (C) none of Parent or any of its Affiliates (or any directors, officer, employee, or other representative of the foregoing) owes any fiduciary duty or similar duty to any Holder in respect of the CVRs.
ARTICLE III THE RIGHTS AGENT
Section 3.1 Certain Duties and Responsibilities. The Rights Agent will not have any
liability for any actions taken or not taken in connection with this Agreement, except to the extent such liability arises as a result of the willful or intentional misconduct, bad faith, intentional breach, gross negligence or fraud of the Rights Agent or any of its Affiliates or its or their respective directors, officers, employees, agents, advisors, or other representatives. Notwithstanding anything in this Agreement to the contrary, in no event will the Rights Agent be liable for special, punitive, indirect, incidental or consequential loss or damages of any kind whatsoever (including, without limitation, lost profits), even if the Rights Agent has been advised of the likelihood of such loss or damages and regardless of the form of action.
-7-
Section 3.2 Certain Rights of Rights Agent. Parent hereby appoints the Rights Agent to act as rights agent for Parent in accordance with the express terms and conditions hereof and the Rights Agent undertakes to perform such duties and only such duties as are specifically set forth in this Agreement, and no implied covenants or obligations will be read into this Agreement against the Rights Agent. In addition:
-8-
Section 3.3 Resignation and Removal; Appointment of Successor.
Section 3.4 Acceptance of Appointment by Successor. Every successor Rights Agent appointed pursuant to Section 3.3(b) hereunder will execute, acknowledge and deliver to Parent and to the predecessor Rights Agent an instrument accepting such appointment and a counterpart of this Agreement, and thereupon such successor Rights Agent, without any further act, deed or conveyance, will become vested with all the rights, powers, trusts and duties of the predecessor Rights Agent. On request of Parent or the successor Rights Agent, the predecessor Rights Agent will execute and deliver an instrument transferring to the successor Rights Agent all the rights, powers and trusts of the predecessor Rights Agent, but such predecessor Rights Agent shall not be required to make any additional expenditure or assume any additional liability in connection with the foregoing, unless, if requested by Rights Agent, it has been furnished with assurances of repayment or indemnity satisfactory to it.
ARTICLE IV COVENANTS
-9-
Section 4.1 List of Holders. Parent will furnish or cause to be furnished to the Rights
Agent (with a copy to the Representative) in such form as Parent receives from Parent’s transfer agent (or other agent performing similar services for Parent), the names and addresses of the Holders within twenty (20) Business Days of the Effective Time.
Section 4.2 Efforts to Achieve Milestones. Parent shall, and shall cause its controlled Affiliates to, use commercially reasonable efforts to achieve the Milestone; provided, however, there is no guarantee that Parent will achieve the Milestone; provided, further, that for purposes hereof, commercially reasonable efforts shall not require Parent to expend monetary resources in excess of $700,000 after taking into account the amount Parent reasonably believes to be eligible for and will be reimbursed (or already reimbursed) under the Parent’s National Institute of Health grants under Federal Award Number 1R44CA281615-01 and Grant Number 1R41HL151235-01. Notwithstanding anything to the contrary herein, neither Parent nor any of its controlled Affiliates shall act in bad faith for purposes of avoiding achievement of the Milestone or the payment of any CVR Payment.
Section 4.3 Prohibited Actions. Prior to the occurrence of the Milestone, Parent shall not (i) grant any lien, security interest, pledge or similar interest in any portion of the CVR Payment and (ii) shall not permit its Affiliates to, grant, assign, transfer or otherwise convey any portion of the CVR Payment (including any option to obtain rights) to any third party.
ARTICLE V
AMENDMENTS
Section 5.1 Amendments without Consent of Holders.
-10-
Section 5.2 Amendments with Consent of Holders.
or
-11-
Section 5.3 Execution of Amendments. In executing any amendment permitted by this Article V, the Rights Agent will be entitled to receive, and will be fully protected in relying upon, an opinion of counsel selected by Parent stating that the execution of such amendment is authorized or permitted by this Agreement. The Rights Agent may, but is not obligated to, enter into any such amendment that affects the Rights Agent’s own rights, privileges, covenants or duties under this Agreement or otherwise, including any amendments pursuant to Section 5.1(a)(viii).
Section 5.4 Effect of Amendments. Upon the execution of any amendment under this Article V, this Agreement will be modified in accordance therewith, such amendment will form a part of this Agreement for all purposes and every Holder will be bound thereby.
ARTICLE VI
OTHER PROVISIONS OF GENERAL APPLICATION
Section 6.1 Notices to Rights Agent and Parent. Any notice or other communication required or permitted hereunder shall be in writing and shall be deemed given (a) on the date of delivery, if delivered in person, by FedEx or other internationally recognized overnight courier service or, by email (upon confirmation of receipt) prior to 5:00 p.m. in the time zone of the receiving party or on the next Business Day, if delivered after 5:00 p.m. in the time zone of the receiving party or (b) two (2) Business Days after being sent by registered or certified mail (postage prepaid, return receipt requested), as follows:
If to the Rights Agent, to it at:
Mountain Share Transfer, LLC
2030 Powers Ferry Road SE
Suite # 212
Atlanta, GA. 30339
Attention: Erik Nelson, President
Email: service@mountainsharetransfer.com
If to Parent, to it at:
Kintara Therapeutics, Inc.
9920 Pacific Heights Blvd, Suite 150
San Diego, CA 92121
Attention: [●]
Email: [●]
-12-
with a copy to:
Foley & Lardner LLP
100 North Tampa Street
Suite 2700
Tampa, FL 33602-5810
Attention: Curt P. Creely, Esq.
Garrett F. Bishop, Esq.
Email: ccreely@foley.com
gbishop@foley.com
If to Representative, to it at:
Robert Hoffman
[●]
Attention: [●]
Email: [●]
The Rights Agent or Parent may specify a different address or facsimile number by giving notice in accordance with this Section 6.1.
Section 6.2 Notice to Holders. Where this Agreement provides for notice to Holders, such notice will be sufficiently given (unless otherwise herein expressly provided) if in writing and mailed, first-class postage prepaid, to each Holder affected by such event, at the Holder’s address as it appears in the CVR Register, not later than the latest date, and not earlier than the earliest date, if any, prescribed for the giving of such notice. In any case where notice to Holders is given by mail, neither the failure to mail such notice, nor any defect in any notice so mailed, to any particular Holder will affect the sufficiency of such notice with respect to other Holders.
Section 6.3 Parent Successors and Assigns. Parent may assign any or all of its rights, interests and obligations hereunder to (a) in its sole discretion and without the consent of any other party, (i) any controlled Affiliate of Parent, but only for so long as it remains a controlled Affiliate of Parent, (ii) to any purchaser or licensee of substantial rights to the Product or (b) with the prior written consent of the Acting Holders, any other Person (any permitted assignee under clause (a) or (b), an “Assignee”), in each case provided that the Assignee agrees to assume and be bound by all of the terms of this Agreement. Any Assignee may thereafter assign any or all of its rights, interests and obligations hereunder in the same manner as Parent pursuant to the prior sentence. In connection with any assignment to an Assignee described in clause (a) above in this Section 6.3, Parent (and such other assignor, if applicable) shall agree to remain liable for the performance by each Assignee (and such other assignor, if applicable) of all obligations of Parent hereunder (provided that no assignor shall be obligated with respect to any amendment to the obligations hereunder effected following such assignee’s assignment). This Agreement will be binding upon, inure to the benefit of and be enforceable by Parent’s successors and each Assignee. Each of Parent’s successors and Assignees shall expressly assume by an instrument supplemental hereto, executed and delivered to the Rights Agent and Representative, the due and punctual payment of the CVRs and the due and punctual performance and observance of all of the covenants and obligations of this Agreement to be performed or observed by Parent.
-13-
Unless a successor assignee meets the requirements set forth in Section 3.3(b) and, as of the date of such assignment, is an Affiliate of the Rights Agent, the Rights Agent may not assign this Agreement without Parent’s written consent. Any attempted assignment of this Agreement or any such rights in violation of this Section 6.3 shall be void and of no effect.
Section 6.4 Benefits of Agreement. Nothing in this Agreement, express or implied, will give to any Person (other than the Rights Agent and its successors and assigns, the Representative and its successors and assigns, Parent, Parent’s successors and Assignees, the Holders and the Holders’ successors and assigns pursuant to a Permitted Transfer) any benefit or any legal or equitable right, remedy or claim under this Agreement or under any covenant or provision herein contained, all such covenants and provisions being for the sole benefit of the foregoing. The rights of Holders and their successors and assigns pursuant to Permitted Transfers are limited to those expressly provided in this Agreement and the Merger Agreement. Notwithstanding anything to the contrary contained herein, any Holder or Holder’s successor or assign pursuant to a Permitted Transfer may agree to renounce, in whole or in part, its rights under this Agreement by written notice to the Rights Agent and Parent, which notice, if given, shall be irrevocable.
Section 6.6 Governing Law; Jurisdiction; Waiver of Jury Trial.
-14-
(B) ACKNOWLEDGES THAT SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS CONTAINED IN THIS SECTION 6.6(C).
Section 6.7 Severability. If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this Agreement shall remain in full force and effect. Any provision of this Agreement held invalid or unenforceable only in part or degree shall remain in full force and effect to the extent not held invalid or unenforceable. The parties further agree to replace such invalid or unenforceable provision of this Agreement with a valid and enforceable provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable provision.
Section 6.8 Counterparts and Signature. This Agreement may be signed manually or by facsimile or other electronic transmission by the parties (including in .pdf, .tiff, .jpg or similar format), in any number of counterparts, each of which shall be an original, with the same effect as if the signatures thereto and hereto were upon the same instrument. This Agreement shall become effective when each party hereto shall have received a counterpart hereof signed by all of the other parties hereto. Until and unless each party has received a counterpart hereof signed by the other party hereto, this Agreement shall have no effect and no party shall have any right or obligation hereunder (whether by virtue of any other oral or written agreement or other communication).
Section 6.9 Termination. This Agreement will automatically terminate and of no force or effect, the parties hereto will have no liability hereunder (including the monies due and owing by Parent to Rights Agent) and no payments will be required to be made, upon the earliest to occur of (a) the expiration of the CVR Period, (b) the mailing by the Rights Agent to the address of each Holder as reflected in the CVR Register the full amount of all potential CVR Payment Amounts required to be paid under the terms of this Agreement or (c) the delivery of a written notice of termination duly executed by Parent and the Acting Holders.
Section 6.10 Entire Agreement. This Agreement (including the fee schedule referred to in Section 3.2(g)) and the Merger Agreement contain the entire understanding of the parties hereto with reference to the transactions and matters contemplated hereby and supersedes all prior agreements, written or oral, between the parties hereto.
Section 6.11 Legal Holiday. In the event that a Milestone Payment Date shall not be a Business Day, then, notwithstanding any provision of this Agreement to the contrary, any payment required to be made in respect of the CVRs on such date need not be made on such date, but may be made on the next succeeding Business Day with the same force and effect as if made on the Milestone Payment Date.
Section 6.12 Force Majeure. Notwithstanding anything to the contrary contained herein, the Rights Agent shall not be liable for any delays or failures in performance resulting from acts beyond its reasonable control including, without limitation, acts of God, terrorist acts, shortage of supply, breakdowns or malfunctions, interruptions or malfunctions of any utilities, communications, or computer facilities, or loss of data due to power failures or mechanical difficulties with information storage or retrieval systems, labor difficulties, war or civil unrest.
-15-
[Remainder of page intentionally left blank]
-16-
IN WITNESS WHEREOF, each of the parties has caused this Agreement to be executed on its behalf by its duly authorized officers as of the day and year first above written.
KINTARA THERAPEUTICS, INC.
By:_____________________
Name:
Title:
MOUNTAIN SHARE TRANSFER, LLC
By:_____________________
Name: Erik Nelson
Title: President
________________________
Robert Hoffman
[Signature Page to Contingent Value Rights Agreement]


Exhibit 99.1
Kintara Therapeutics and TuHURA Biosciences Enter into Definitive Merger Agreement
SAN DIEGO, CA & TAMPA, FL, April 3, 2024 – Kintara Therapeutics, Inc. (Nasdaq: KTRA) (“Kintara”), a biopharmaceutical company investigating REM-001 in an NIH-sponsored and funded open label study in cutaneous metastatic breast cancer, and TuHURA Biosciences, Inc. (“TuHURA”), a Phase 3 registration-stage immune-oncology company developing novel technologies to overcome resistance to cancer immunotherapy, today announced that they have entered into a definitive agreement for an all-stock transaction forming a company with expertise and resources to advance a risk diversified late-stage oncology pipeline. The combined company will focus on advancing TuHURA’s personalized cancer vaccine(s) and first-in-class bi-functional ADCS, two technologies that seek to overcome the major obstacles that limit the effectiveness of current immunotherapies in treating cancer. The combined company is expected to operate under the name “TuHURA Biosciences, Inc.” and to trade on The Nasdaq Capital Market (“Nasdaq”) under the ticker “HURA”. The transaction is expected to close in the third quarter of 2024.
Robert E. Hoffman, Kintara’s President and Chief Executive Officer, commented, “Following a thorough review and evaluation of opportunities to rebuild value for Kintara shareholders, we believe merging with TuHURA, a Phase 3 immuno-oncology company focused in two compelling areas of research, represents the best path forward for our stockholders and has the potential to deliver near and long-term value. Our board believes that the combined company will be well-positioned to develop powerful new therapies with the potential to overcome resistance to current immunotherapies, an area of significant unmet need.”
TuHURA’s technologies are being developed to overcome primary and acquired resistance-- two major obstacles to cancer immunotherapy’s ability to treat and cure cancer.
"With IFx-2.0 readying to enter a single Phase 3 registration trial, we believe this is the optimal time for TuHURA to transition from a private company to a public company. This proposed merger with Kintara lets us achieve that goal while combining the resources of the two companies. Coupled with a $31 million subscribed financing by TuHURA in connection with the merger agreement, and which is expected to fund the combined company’s operations and development programs through late 2025, we believe this merger will also allow our combined company the flexibility to focus our resources and efforts on advancing IFx-2.0 to market and our other drug candidates toward human clinical trials,” said Dr. James Bianco, President and Chief Executive Officer of TuHURA. “This transaction with Kintara serves as a significant next step in our continued commitment to patients to develop novel therapies to overcome the major obstacles that limit the effectiveness of immunotherapies to treat and cure cancer.”
About the Proposed Transaction, Management & Organization
Under the terms of the merger agreement, subject to stockholder approval, on a pro forma basis, post-merger Kintara equityholders are expected to collectively own up to approximately 2.85%, or approximately 5.45% including the shares underlying the contingent value rights (CVR) to be received by certain of Kintara’s equityholders as described below, of the common stock of post-merger combined company on a pro forma fully diluted basis. TuHURA equityholders are expected to collectively own approximately 97.15%, or 94.55% assuming the distribution of the CVR shares, of the common stock of combined company on a pro forma fully diluted basis.
Pre-merger Kintara common stockholders, certain warrant holders and certain preferred stockholders of record of Kintara will receive a CVR, entitling the holder to receive shares of Kintara common stock to be issued upon achievement of the CVR milestone. The Kintara CVR shares will be issued and distributed once 10 patients are enrolled and tracked in a study to determine whether a lower dose of REM-001 elicits a treatment effect similar to that seen in prior REM-001 studies. Once completed, the company will seek to out-license or identify an acquirer for the technology.
The merger agreement has been approved by the boards of directors of both companies and is subject to stockholder approval of both companies and other customary closing conditions. The proposed merger is expected to close in the third quarter of 2024.
Following the merger, the combined company will be headquartered in Tampa, Florida, and the executive officers are expected to be James Bianco, MD as President and Chief Executive Officer, and Dan Dearborn, CPA as Chief Financial Officer. The merger agreement provides that the board of directors of the combined company will be composed of five members, with four members initially designated by TuHURA and one member initially designated by Kintara.
Lucid Capital Markets, LLC is acting as the exclusive financial advisor and Lowenstein Sandler LLP is acting as legal counsel to Kintara. H.C. Wainwright & Co. is acting as the exclusive financial advisor and Foley & Lardner LLP is acting as legal counsel to TuHURA.
Conference Call & Webcast Details
The companies plan to hold a joint conference call and webcast today, April 3, 2024 at 8:30 AM ET to discuss the Merger details.
Interested participants and investors may access the conference call and webcast via the Investors section of the Kintara website at www.kintara.com and on TuHURA’s website, tuhurabio.com. A webcast replay will be available following the live event and will be accessible for 90 days.
About Kintara
Located in San Diego, California, Kintara is dedicated to the development of novel cancer therapies for patients with unmet medical needs. Kintara is developing therapeutics for clear unmet medical needs with reduced risk development programs. Kintara’s lead program is REM-001 Therapy for cutaneous metastatic breast cancer (CMBC).
Kintara has a proprietary, late-stage photodynamic therapy platform that holds promise as a localized cutaneous, or visceral, tumor treatment as well as in other potential indications. REM-001 Therapy, which consists of the laser light source, the light delivery device, and the REM-001 drug product, has been previously studied in four Phase 2/3 clinical trials in patients with CMBC who had previously received chemotherapy and/or failed radiation therapy. In CMBC, REM-001 has a clinical efficacy to date of 80% complete responses of CMBC evaluable lesions and an existing robust safety database of approximately 1,100 patients across multiple indications.
For more information, please visit www.kintara.com or follow us on X at @Kintara_Thera, Facebook and LinkedIn.
About TuHURA Biosciences, Inc.
TuHURA Biosciences is a Phase 3 registration-stage immuno-oncology company developing novel technologies to overcome resistance to cancer immunotherapy. TuHURA’s lead personalized cancer vaccine candidate, IFx-2.0, is designed to overcome primary resistance to checkpoint inhibitors. TuHURA is preparing to initiate a single randomized placebo-controlled Phase 3 registration trial of IFx-2.0 administered as an adjunctive therapy to Keytruda® (pembrolizumab) in first line treatment for advanced Merkel Cell Carcinoma.
In addition to its cancer vaccine product candidates, TuHURA is leveraging its Delta receptor technology to develop first-in-class bi-functional antibody drug conjugates (ADCs), targeting Myeloid Derived Suppressor Cells (MDSCs) to inhibit their immune suppressing effects on the tumor microenvironment to prevent T cell exhaustion and acquired resistance to checkpoint inhibitors and cellular therapies.
For more information, please visit tuhurabio.com and connect with TuHURA on Facebook, X, and LinkedIn.
No Offer or Solicitation
This communication is not intended to and shall not constitute an offer to buy or sell or the solicitation of an offer to buy or sell any securities, or a solicitation of any proxy, consent, authorization, vote or approval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made, except by means of a prospectus meeting the requirements of the U.S. Securities Act of 1933, as amended.
Additional Information About the Proposed Transaction for Investors and Shareholders
In connection with the proposed transaction between Kintara and TuHURA (the “Proposed Transaction”), Kintara intends to file relevant materials with the U.S. Securities and Exchange Commission (the “SEC”), including a registration statement on Form S-4 that will contain a proxy statement/prospectus of Kintara. This press release is not a substitute for the registration statement or for any other document that Kintara may file with the SEC in connection with the Proposed Transaction. KINTARA URGES INVESTORS AND STOCKHOLDERS TO READ THE REGISTRATION STATEMENT, PROXY STATEMENT/PROSPECTUS AND ANY OTHER RELEVANT DOCUMENTS THAT MAY BE FILED WITH THE SEC, AS WELL AS ANY AMENDMENTS OR SUPPLEMENTS TO THESE DOCUMENTS, CAREFULLY AND IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT KINTARA, TUHURA, THE PROPOSED TRANSACTION AND RELATED MATTERS. Investors and stockholders will be able to obtain free copies of the proxy statement/prospectus and other documents filed by Kintara with the SEC (when they become available) through the website maintained by the SEC at www.sec.gov. In addition, investors and stockholders should note that Kintara communicates with investors and the public using its website (www.kintara.com), the investor relations website (https://www.kintara.com/investors) where anyone will be able to obtain free copies of the proxy statement/prospectus and other documents filed by Kintara with the SEC, and stockholders are urged to read the proxy statement/prospectus and the other relevant materials when they become available before making any voting or investment decision with respect to the Proposed Transaction.
Participants in the Solicitation
Kintara, TuHURA and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies from stockholders in connection with the Proposed Transaction. Information about Kintara’s directors and executive officers including a description of their interests in Kintara is included in Kintara’s most recent Annual Report on Form 10-K, including any information incorporated therein by reference, as filed with the SEC. Additional information regarding these persons and their interests in the transaction will be included in the proxy statement/prospectus relating to the Proposed Transaction when it is filed with the SEC. These documents can be obtained free of charge from the sources indicated above.
Forward-Looking Statements
This news release contains forward-looking statements that are not historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and other future conditions. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,” “project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. Examples of such forward-looking statements include but are not limited to express or implied statements regarding Kintara’s or TuHURA’s management team’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, statements regarding: the Proposed Transaction and the expected effects, perceived benefits or opportunities and related timing with respect thereto, expectations regarding clinical trials and research and development programs, in particular with respect to TuHURA’s IFx-Hu2.0 product candidate and its TME modulators development program, and any developments or results in connection therewith; the anticipated timing of the results from those studies and trials; expectations regarding the use of capital resources, including the net proceeds from the financing that closed in connection with the signing of the definitive agreement, and the time period over which the combined company’s capital resources will be sufficient to fund its anticipated operations; and the expected trading of the combined company’s stock on the Nasdaq Capital Market. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. You are cautioned that such statements are not guarantees of future performance and that actual results or developments may differ materially from those set forth in these forward-looking statements. Factors that could cause actual results to differ materially from these forward-looking statements include: the risk that the conditions to the closing or consummation of the Proposed Transaction are not satisfied, including the failure to obtain stockholder approval for the Proposed Transaction; uncertainties as to the timing of the consummation of the Proposed Transaction and the ability of each of Kintara and TuHURA to consummate the transactions contemplated by the Proposed Transaction; risks related to Kintara’s and TuHURA’s ability to correctly estimate their respective operating expenses and expenses associated with the Proposed Transaction, as applicable, as well as uncertainties regarding the impact any delay in the closing would have on the anticipated cash resources of the resulting combined company upon closing and other events and unanticipated spending and costs that could reduce the combined company’s cash resources; the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the Proposed Transaction by either company; the effect of the announcement or pendency of the Proposed Transaction on Kintara’s or TuHURA’s business relationships, operating results and business generally; costs related to the merger; the outcome of any legal proceedings that may be instituted against Kintara, TuHURA, or any of their respective directors or officers related to the merger agreement or the transactions contemplated thereby; the ability of Kintara or TuHURA to protect their respective intellectual property rights; competitive responses to the Proposed Transaction; unexpected costs, charges or expenses resulting from the Proposed Transaction; whether the combined business of TuHURA and Kintara will be successful; legislative, regulatory, political and economic developments; and additional risks described in the “Risk Factors” section of Kintara’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023 filed with the SEC. Additional assumptions, risks and uncertainties are described in detail in our registration statements, reports and other filings with the SEC, which are available on Kintara’s website, and at www.sec.gov.
You are cautioned that such statements are not guarantees of future performance and that our actual results may differ materially from those set forth in the forward-looking statements. The forward-looking statements and other information contained in this news release are made as of the date hereof and Kintara does not undertake any obligation to update publicly or revise any forward-looking statements or information, whether as a result of new information, future events or otherwise, unless so required by applicable securities laws. Nothing herein shall constitute an offer to sell or the solicitation of an offer to buy any securities.
Investor Contacts:
Kintara Therapeutics, Inc.
Robert E. Hoffman
Kintara Therapeutics
rhoffman@kintara.com
TuHURA Biosciences, Inc.
Jenene Thomas
JTC Team, LLC
tuhura@jtcir.com
Exhibit 99.2
Project Kayak Conference Call Script
Slide 1 (Operator)
Hello and welcome to the Kintara and TuHURA conference call and webcast. As a brief reminder, all participants are currently in a listen-only mode. If anyone requires operator assistance during the event, please press star zero on your telephone keypad.
Slide 2 (Operator)
At this time, I'd like to remind our listeners that, except for statements of historical fact, the remarks on today's conference call may include forward-looking statements within the meaning of the securities laws. Forward-looking statements are based on current expectations, projections and interpretations that involve a number of risks and uncertainties that could cause actual results to differ materially from those anticipated by Kintara. These risks and uncertainties are described in our registration statements, reports and other filings with the Securities and Exchange Commission which are available on our website at kintara.com and sec.gov, as well as in the safe harbor statement included with today’s press release. You are cautioned that such statements are not guarantees of future performance and that our actual results may differ materially from those set forth in the forward-looking statements. Kintara does not undertake any obligation to update publicly or revise any forward-looking statements or information, whether as a result of new information, future events or otherwise.
1
Joining me on today’s call are Robert Hoffman, President, Chief Executive Officer and Chief Financial Officer of Kintara and Dr. James Bianco, Chief Executive Officer of TuHURA Biosciences.
I’d now like to turn the call over to Robert Hoffman, Kintara’s President, Chief Executive Officer and Chief Financial Officer. Please proceed.
Slide 3 (Robert)
Thank you, (Operator’s name) and thank you everyone for joining us today. We are very pleased to have announced earlier today the proposed merger of Kintara and TuHURA and are looking forward to walking you through the exciting value proposition that we believe provides increased value for stakeholders of both companies.
Slide 4 (Robert)
We believe this merger would provide Kintara stockholders with a risk diversified development pipeline and transform the company into a Phase 3 clinical stage company.
TuHURA’s Immune Fx, or “IFx”, personalized cancer vaccines are designed to overcome tumor intrinsic factors, which allow tumors to escape recognition and attack by the patient’s immune system. It is designed to prime the activation of an innate immune response which can target and destroy cancer cells.
The lead product candidate, IFx-2.0, is being developed under the FDA’s accelerated approval pathway for the treatment of an aggressive form of skin cancer, called Merkel Cell Carcinoma. TuHURA expects to initiate
2
a Phase 3 registration study in the second half of this year. In an ongoing Phase 1b study, IFx-2.0 demonstrated the ability to produce durable systemic anti-tumor responses in 80% of patients who initially failed therapy with checkpoint inhibitors.
In addition to its cancer vaccine technology, TuHURA is also developing Bi-functional antibody drug conjugates or ADCs designed to target the myeloid derived suppressor cells and modulate the tumor microenvironment to overcome factors outside the biology of the tumor that leads to acquired resistance, causing cancer immunotherapy to stop working. Myeloid Derived Suppressor Cells play a central role in acquired resistance by creating a highly immune suppressing microenvironment in which the tumor can live and escape immune attack.
So, as you can see, a combination with TuHURA would transform the Company into a Phase 3-ready company with two innovative technology platforms that have the ability to fuel a growing pipeline for years to come. With the proposed transaction that we announced today, I believe the combined company would be well positioned to make a true difference in the lives of patients suffering from cancer, while also delivering near- and long-term value to our shareholders.
Slide 5 (Robert)
I would like to summarize the key details of the transaction. Under the terms of the merger agreement, subject to stockholder approval, on a pro forma basis, post-merger Kintara equity holders are expected to collectively own up to approximately 5.45% (including the shares underlying the contingent value rights (CVR) to be received by certain of Kintara’s equityholders) and TuHURA equityholders are expected to
3
collectively own approximately 94.55% with the CVR, of the common stock of Kintara on a pro forma fully diluted basis.
As part of the 5.45% pro forma ownership, pre-merger Kintara stockholders, certain warrant holders and certain preferred stockholders of record will receive a CVR. Holders of the CVR will be entitled to receive additional shares of common stock of Kintara in connection with Kintara’s ongoing NIH-sponsored and funded open label 15-patient study of REM-001 in patients with cutaneous metastatic breast cancer (“CMBC”). The additional shares of Kintara through the CVR will be earned and distributed if the study shows an acceptable benefit risk profile. Once completed the company expects to seek to out-license or identify an acquirer for the technology.
In connection with the transaction, TuHURA has a $31 million subscribed financing of convertible notes that will convert into common equity at the closing of the merger, and that financing is included in the pro forma ownership percentages I just mentioned.
Following the merger, the combined company, TuHURA Biosciences will be headquartered in Tampa, Florida, and the executive officers are expected to be Dr. Bianco as Chief Executive Officer, and Dan Dearborn, CPA as Chief Financial Officer. The merger agreement provides that the board of directors of the combined company will be composed of five members, with four members initially designated by TuHURA and one member initially designated by Kintara.
4
With that, I will now turn the call over to Dr. Jim Bianco to provide additional background and details on TuHURA’s technologies, pipeline, and strategy. Jim?
Slide 6 (Jim)
Thank you, Robert, I could not be more excited about this merger.
Slide 7 (Jim)
As Robert mentioned, we're a Phase 3 registration stage company with novel technologies targeting two compelling areas in the immuno-oncology space – specifically personalized cancer vaccines and Antibody drug conjugates or ADCs. We have built a pipeline of drug candidates spanning preclinical development for our bi-functional antibody drug conjugates, to IND-enabling studies for our intravenous tumor targeted mRNA cancer vaccine, to our Phase 3 registration trial for our pDNA vaccine in Merkel cell carcinoma, providing the Company with what we believe is a well-balanced - risk diversified pipeline.
Slide 8 (Jim)
Our IFx-2.0 cancer vaccine is entering a phase 3 trial as adjunctive therapy with Keytruda, Merck’s checkpoint inhibitor, with a goal of increasing Keytruda’s response rate.
We believe allowing checkpoint inhibitors to work better in more patients is an attractive high value commercial opportunity for the following reasons.
5
Checkpoint inhibitors have clearly revolutionized the treatment of cancer, in some cases replacing chemotherapy as a new standard of care. They are currently approved in more than 20 cancer types with 9 checkpoint inhibitors on the market and several more in development. Merck’s checkpoint inhibitor, Keytruda, dominates this market with sales far exceeding the others on a run rate in 2024 of $30 billion in sales – which represents almost 40% of Merck’s total annual sales. Importantly Keytruda comes off patent in 2028, which may be relevant to our Phase 3 trial design we will discuss shortly.
When checkpoint inhibitors work, they typically produce long lasting remissions. Unfortunately, they only work in 20% to 30% of patients.
Increasing the response rate to checkpoint inhibitors represents an attractive commercial opportunity.
Slide 9 (Jim)
Let me tell you how our personalized cancer vaccine, IFx-2.0, works to overcome the main reason checkpoint inhibitors fail – a process termed primary resistance.
Slide 10 (Jim)
In order for a checkpoint inhibitor to work your tumor must appear foreign to your immune system resulting in activation of cancer fighting T cells. However the majority of tumors don’t look foreign to your immune system.
Therefore, the primary goal of any cancer vaccine is to make your tumor look foreign and activate an immune response so checkpoint inhibitors The way IFx-2.0 does this is by harnessing the power of our innate immune system to activate an immune response.
6
can work where they otherwise would fail. That is what IFx-2.0 does, it makes a patient’s tumor appear foreign and activates an immune response allowing checkpoint inhibitors to work.
IFx-2.0 is a pDNA, which when injected into a tumor cell, encodes for the production of a highly immunogenic bacterial protein which becomes expressed on the surface of a tumor cell. So, even if your tumor didn’t look foreign to your immune system– it now will look like a bacterium which will activate an immune response against the tumor.
Pathogens, like viruses, and bacteria are made up of proteins that have a specific molecular fingerprint, or patterns called “motifs.” These molecular patterns are recognized by pattern recognition receptors on our immune cells that are present at birth – these immune cells are called antigen presenting cells or APCs, which are part of our innate immune system.
These receptors have been conserved and pre-programmed through evolution to recognize these specific patterns as the body’s first line of defense against pathogens like bacteria which will naturally activate an innate immune response.
Activated APCs will recognize the bacterial protein as being foreign and digest the entire tumor cell and package all of the tumor foreign proteins or neoantigens that are present inside the tumor cell and present them to newly produced T cells and B cells. Now your T and B cells are educated to these tumor foreign antigens and will, in the case of B cells,
7
produce tumor specific antibodies. These antibodies along with tumor specific T cells will seek out, attack and destroy the patient’s tumor.
It is in this setting that checkpoint inhibitors can work to amplify the tumor killing ability of T cells.
Slide 11 (Jim)
To test the ability of IFx-2.0 to prime an innate immune response we conducted a Phase 1b trial among patients with an aggressive and often lethal form of skin cancer called Merkel cell carcinoma and another skin cancer called Squamous cell carcinoma.
This trial was designed to test different doses and schedules of IFx-2.0 to determine the best dose and schedule to maximize activation of an immune response.
In the right panel you can see there were 3 cohorts with 3 patients in each cohort. IFx-2.0 was administered weekly for 1, 2 or 3 weeks. The study demonstrated that IFx-2.0 could be safely administered weekly for 3 weeks. It also demonstrated that a patient’s immune response was more robust at 3 weekly dose level – which is the dosing schedule for subsequent studies.
Following the safety portion of the protocol, the investigator rechallenged most patients with a checkpoint inhibitor. Based on those results the study opened an expansion phase to enroll an additional 11 patients.
8
June. The preliminary trial results for all 20 patients were submitted for presentation to ASCO this year.
The results in the first 9 patients were reported at the ASCO meeting last I am going to present the data that was reported last year at the ASCO meeting on 5 of the 6 patients with advanced Merkel cell and 2 of the 3 patients with advanced Squamous cell that received first line therapy with Keytruda or a Keytruda like checkpoint inhibitor and no subsequent therapy before receiving IFx-2.0.
Following IFx-2.0, each of these 7 patients were then rechallenged with Keytruda or a Keytruda-like drug after having failed them.
Slide 12 (Jim)
This slide shows the chronology of these patients’ treatment. Patients 1-5 noted on the lefthand side were the Merkel cell patients and 6 and 7 Squamous cell.
Each of these patients received first line therapy with Keytruda or a Keytruda-like checkpoint inhibitor. This is shown by the dark purple bars PD stands for progressive disease.
Each patient rapidly progressed while receiving a checkpoint inhibitor as their first line treatment. Once they progressed, they stopped receiving the checkpoint inhibitor and entered our the IFx-2.0, Phase 1b trial.
The yellow bar shows when IFx-2.0 was administered into a cancerous skin lesion or lymph node. Following IFx-2.0 evaluation for safety they then received the same class of checkpoint inhibitor they initially failed.
4 of the 5 Merkel patients and 1 of the 2 Squamous patients achieved a durable anti-tumor response ranging from a partial response to pathologic complete response or complete response.
9
We also observed the ability to overcome checkpoint inhibitor resistance in a separate phase 1 study in advanced refractory melanoma where 3 of 4 patients achieved a response. Those data are being published later this year in Cancer Immunology, the journal of the American Association for Cancer Research or AACR.
Slide 13 (Jim)
I thought it would be helpful to show you the degree of clinical responses that were observed following IFx and rechallenge with checkpoint inhibitor.
In the left-hand panel, this is a patient with what is termed in-transit metastasis. All of those nodules are metastatic deposits of Merkel cell carcinoma. Despite receiving 3 months of avelumab, a Keytruda-like checkpoint inhibitor, these lesions continued to grow and spread. The patient then received IFx-2.0 weekly for 3 weeks. In the upper right-hand corner of the panel on the left, is a sharpie mark where IFx was administered to Merkel lesions once weekly for 3 weeks. This patient was put back on a checkpoint inhibitor, in this case Keytruda. You can see marked necrosis and tumor regression in middle panel with clearing of the lesion in right hand panel. This patient’s PR lasted 33 months.
Slide 14 (Jim)
Here is another example. This is a CT scan of a patient’s thigh. The blue arrow points to large solitary tumor mass.
Despite receiving 3 months of Keytruda the tumor continued to grow at which point the patient received IFx-2.0.
IFx was injected once weekly x 2 weeks into the tumor mass.
10
Following IFx the patient was retreated with Keytruda. The panel on the right shows marked reduction in the size of the lesion classifying it as a PR. Upon surgical removal, pathology revealed no evidence of tumor and the response was reclassified from radiographic PR to pathologic CR.
This patient’s response is ongoing for more than 23 months.
Slide 15 (Jim)
This is a CT scan of a patient’s abdomen. A is anterior or front of the abdomen and P is posterior. The blue arrows point to subdermal metastatic deposits and the blue lines in the center of the scan represent measurements of 2 large retroperitoneal tumors.
Like the other cases, this patient’s cancer progresses through 2 months of Keytruda at which point the drug was discontinued.
IFx was then injected into the subdermal lesions shown by the blue arrows. Following IFx they were then rechallenged with a Keytruda like drug.
The 2 subdermal lesions disappeared and the large masses in the abdomen shrunk by 80%. This partial response is ongoing at 20 months.
Slide 16 (Jim)
Let me provide a little background on Merkel cell. This aggressive form of skin cancer is caused by a virus called polyoma virus, with approximately 2,500 patients diagnosed each year in the US.
In 2018 the checkpoint inhibitor Keytruda received accelerated approval as first line therapy for advanced or metastatic Merkel cell.
11
The approval was based on a single arm study showing Keytruda could produce an overall response rate of 56% with 20% of the responses being CR and 36% PR.
The panel on the right shows if you achieved a CR almost 75% of those patients were still in CR at 2 years, and almost 50% if you achieved a PR.
While these data are encouraging, there still exists a major unmet medical need as there are no effective or approved therapies for the 45% of patients who don’t respond to Keytruda.
Slide 17 (Jim)
Given our data demonstrating IFx may provide durable responses in patients who failed checkpoint inhibitor therapy when they are re-treated with a checkpoint inhibitor, we approached the FDA focusing on the potential to conduct a single registration trial utilizing the FDA’s accelerated approval pathway, a process that worked successfully for 3 other drug candidates I developed at my prior company.
Working with both the FDA’s Office of Cellular, Tissue and Gene Therapy and Oncology Center of Excellence the FDA agreed to our conducting a single randomized placebo-controlled trial in patients with advanced or metastatic Merkel cell who are checkpoint inhibitor naïve under the FDA’s accelerated approval pathway.
We were encouraged by FDA to conduct the trial in first line – the rationale was driven by the results demonstrating the potential to “salvage” patients who are failing first line checkpoint inhibitor therapy. Treating with IFx prior to failure may allow more patients to respond to front line Keytruda than what is typically observed with Keytruda alone.
12
In this Phase 3 trial, patients will receive Keytruda, the front-line standard of care, along with placebo injections compared to Keytruda and adjunctive therapy with IFx-2.0 once weekly for 3 weeks. The primary endpoint of the study is ORR.
The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and fill an unmet medical need based on a surrogate endpoint like Overall Response
ORR is utilized in accelerated approval trials since response rates can be evaluated at 3 months and confirmed at 6 months at which point, if positive, a drug candidate can be submitted for regulatory approval consideration. Endpoints like PFS and OS may take 4 to 5 years to evaluate results before potential submission for regulatory approval.
We expect to begin this phase 3 trial in the 2nd half of this year with a 12-month projected patient enrollment followed by 6 months to have top line data analyzing the ORR primary endpoint.
We also expect to conduct this trial under a Special Protocol Assessment Agreement, or SPA, with the FDA.
Slide 18 (Jim)
Unlike Fast Track or Orphan Drug Designation the Accelerated Approval Pathway is not granted frequently by FDA, in fact last year the FDA posted that there were more than 12,000 ongoing oncology phase 3 trials ongoing while only 54 trials being conducted under the Accelerated Approval Pathway. Most of these trials are conducted by large biotech or pharma companies.
13
Slide 19 (Jim)
We recognize Merkel cell is a relatively small indication with about 2,000 patients receiving front line therapy with Keytruda. However, since the biologic mechanism of how tumors don’t respond to checkpoint inhibitors is similar irrespective of the type of cancer, then IFx may have the ability to overcome resistance to checkpoint inhibitors across a number of cancers which occur at a high incidence, like Breast or Ovarian cancers. We anticipate starting what is called a basket trial across several cancers like ovarian and trpile negative breast cancer among patient’s whose tumors exhibit primary resistance to checkpoint inhibitor therapy later this year. This could lead to the potential expansion of the potential commercial application for IFx-2.0
Slide 20 (Jim)
I have been how IFx-2.0 uses intratumoral injection of our pDNA to prime an innate immune response. For tumors that are not accessible to intratumoral injection, like blood related cancers such as leukemia or lymphoma, we are developing IFx-3.0.
Slide 21 (Jim)
We believe IFx-3.0 would be the first intravenously administered, tumor targeted cancer vaccine. We plan on investigating its first application in aggressive non-Hodgkins lymphoma.
We are developing what is termed an antibody fragment, or scFv that targets CD22. CD22 is a receptor that is highly over expressed on the surface of malignant B cell cancers like aggressive lymphoma.
14
We plan on linking that antibody fragment to a lipid nanoparticle containing our mRNA which also encodes for the same immunogenic bacterial protein we see with IFx-2.0.
Aggressive lymphoma is often a widespread disease which infiltrates the bone marrow, lymph nodes, and spleen. Even the blood has a high percentage of circulating malignant lymphoma cells.
Therefore administering IFx-3.0 via a vein into the bloodstream we have the potential to target much larger percentage of the tumor than we can achieve with local intratumoral injection with IFx-2.0. The result should be a more robust, widespread activation of the innate immune response.
We are currently examining different construct to select an optimized lead compound, targeting IND directed preclinical studies and first in human phase 1 testing by late 2025.
Slide 22 (Jim)
I'm going to close my part of the presentation discussing our novel bifunctional antibody drug conjugates or ADCs. There has been a flurry of large pharma acquisitions of next generation ADC companies. Those companies for the most part are developing ADCs focused on targeting tumor cells. Our ADC technology takes a truly novel direction, a paradigm shift by focusing on an important class of immune suppressing cells called myeloid derived suppressor cells or MDSCs that are key to why checkpoint inhibitors and cellular therapies like CAR-T stop working.
Slide 23 (Jim)
15
MDSCs are an important group of immune cells that are normally produced during pregnancy where they leave the bone marrow and populate the placenta.
These cells produce multiple substances that potently suppress both the innate and adaptive immune response.
In the setting of pregnancy, they create an immunologic sanctuary for the fetus. Given that 50% of the genetic makeup of the fetus is foreign to the mother’s immune system, this is nature’s way to prevent the fetus from being seen as foreign and attacked by the mother’s immune system.
In the setting of cancer, these cells are hijacked and populate the tissue that surrounds where the cancer lives, called the tumor microenvironment, providing the tumor an immunologic sanctuary to grow and evade immune attack by the patient’s immune system.
MDSCs are the primary culprit for causing what is termed acquired resistance which is why checkpoint inhibitors and cellular therapies stop working. These cells produce multiple immune suppressing soluble substances, cytokines even directly capable of inhibiting T cell activation and proliferation.
Over the past 5 years MDSCs have become a major target among pharma companies which are developing potential inhibitors of some of the individual immune suppressing substances produced by MDSCs as a way to overcome acquired resistance to immunotherapy.
Slide 24 (Jim)
16
Scientists at TuHURA and Moffitt Cancer Center discovered the presence of a well characterized Delta receptor that is highly overexpressed on tumor associated MDSCs.
What makes this receptor so important, it behaves as a master switch on MDSCs. Inhibition of the receptor prevents MDSCs from producing multiple of their immune suppressing substances and prevents their ability to leave the bone marrow and travel to the tumor or even prevent them from inhibiting T cell activation.
We are developing small molecule inhibitors of the Delta receptor to essentially shut off multiple ways the MDSCs cause immune suppression in the tumor microenvironment.
Utilizing our Delta receptor technology we have developed first-in-class bi-functional antibody drug conjugates. Unlike conventional ADCs, our ADCs are bi-functional – we use a small molecule drug to target the Delta receptor on MDSCs. The drug itself has a function, namely, to inhibit MDSC induced immunosuppression in the tumor microenvironment. Attached to that drug is an antibody payload, like Keytruda.
So, our bi-functional ADCs have the potential to remove the immunologic sanctuary that causes checkpoint inhibitors to stop working. At the same time, they localize an immune drug, like Keytruda where the tumor lives allowing checkpoint inhibitors potentially to keep on working. Localizing the checkpoint inhibitor in the tumor microenvironment may also decrease the severe toxicities seen in about 10% of patients receiving checkpoint inhibitors, by decreasing indiscriminate off target effects from checkpoint released T cells.
We are in the process of developing libraries of potent and selective inhibitors of the Delta receptor with the goal of having our bi-functional ADC for animal model testing later this year, with plans to advance to IND enabling studies a lead optimized compound by late 2025.
17
Slide 25 Jim to Robert
With that, I hope I was able to show why we are excited about the future of TuHURA and why we see this merger as being an important catalytic event for both Kintara and TuHURA stockholders.
I will now hand it back over to Robert.
Slide 26
Thank you, Jim. Before we close, I would like to touch on REM-001 and how it fits into our strategy going forward.
REM-001 is currently being assessed in an open-label study, evaluating safety and efficacy in 15 patients with cutaneous metastatic breast cancer. This study is funded principally by an SBIR Grant from the NIH and does not draw materially on the resources from the TuHURA financing mentioned earlier in the call. Given this, and the potential of this program, we intend to continue the study to completion and if successful, it will provide upside. Current Kintara shareholders will be provided with a Contingent Value Right under which they could receive additional shares of common stock as described earlier in this call. We will also look to the potential sale or partnering of this asset.
Slide 27
In closing, we could not be more excited about the proposed merger and the expected near-term transformation into a Phase 3 clinical stage company. With two innovative technology platforms targeting two of the most compelling areas in oncology, I believe this transaction is a
18
transformational event that is expected to propel this company forward and have the potential to drive significant value for all stockholders.
I will now turn the call back over to the Operator.
Operator
This concludes the Kintara and TuHURA conference call and webcast. We would like to thank you for attending today’s presentation and as a reminder, a webcast replay of today’s event will be accessible on Kintara/TuHURA websites. You may now disconnect.
19

Enter into Definitive Merger Agreement Phase 3 Clinical Stage Immuno-Oncology Company Addressing Major Obstacles to Overcoming Resistance to Cancer Immunotherapy

Forward-Looking Statements This presentation contains forward-looking statements that are not historical facts within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and other future conditions. In some cases you can identify these statements by forward-looking words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “could,” “should,” “would,” “project,” “plan,” “expect,” “goal,” “seek,” “future,” “likely” or the negative or plural of these words or similar expressions. Examples of such forward-looking statements include but are not limited to express or implied statements regarding Kintara’s or TuHURA’s management team’s expectations, hopes, beliefs, intentions or strategies regarding the future including, without limitation, statements regarding: the proposed merger transaction between Kintara and TUHURA (the “Proposed Transaction”) and the expected effects, perceived benefits or opportunities and related timing with respect thereto, expectations regarding clinical trials and research and development programs, in particular with respect to TuHURA’s IFx-Hu2.0 product candidate and its TME modulators development program, and any developments or results in connection therewith; the anticipated timing of the results from those studies and trials; expectations regarding the use of capital resources, including the net proceeds from the financing that closed in connection with the signing of the definitive agreement, and the time period over which the combined company’s capital resources will be sufficient to fund its anticipated operations; and the expected trading of the combined company’s stock on the Nasdaq Capital Market. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. You are cautioned that such statements are not guarantees of future performance and that actual results or developments may differ materially from those set forth in these forward-looking statements. Factors that could cause actual results to differ materially from these forward-looking statements include: the risk that the conditions to the closing or consummation of the Proposed Transaction are not satisfied, including the failure to obtain stockholder approval for the Proposed Transaction; uncertainties as to the timing of the consummation of the Proposed Transaction and the ability of each of Kintara and TuHURA to consummate the transactions contemplated by the Proposed Transaction; risks related to Kintara’s and TuHURA’s ability to correctly estimate their respective operating expenses and expenses associated with the Proposed Transaction, as applicable, as well as uncertainties regarding the impact any delay in the closing would have on the anticipated cash resources of the resulting combined company upon closing and other events and unanticipated spending and costs that could reduce the combined company’s cash resources; the occurrence of any event, change or other circumstance or condition that could give rise to the termination of the Proposed Transaction by either company; the effect of the announcement or pendency of the Proposed Transaction on Kintara’s or TuHURA’s business relationships, operating results and business generally; costs related to the merger; the outcome of any legal proceedings that may be instituted against Kintara, TuHURA, or any of their respective directors or officers related to the merger agreement or the transactions contemplated thereby; the ability of Kintara or TuHURA to protect their respective intellectual property rights; competitive responses to the Proposed Transaction; unexpected costs, charges or expenses resulting from the Proposed Transaction; whether the combined business of TuHURA and Kintara will be successful; legislative, regulatory, political and economic developments; and additional risks described in the “Risk Factors” section of Kintara’s Annual Report on Form 10-K for the fiscal year ended June 30, 2023 filed with the SEC. Additional assumptions, risks and uncertainties are described in detail in our registration statements, reports and other filings with the SEC, which are available on Kintara’s website, and at www.sec.gov. You are cautioned that such statements are not guarantees of future performance and that our actual results may differ materially from those set forth in the forward-looking statements. The forward-looking statements and other information contained in this presentation are made as of the date hereof and Kintara does not undertake any obligation to update publicly or revise any forward-looking statements or information, whether as a result of new information, future events or otherwise, unless so required by applicable securities laws. Nothing herein shall constitute an offer to sell or the solicitation of an offer to buy any securities.

President, CEO, CFO and Chairman of the Board Kintara Therapeutics Robert E. Hoffman
Focused on Bringing Novel Therapies to Treat and Cure Patients with Cancer Phase 3 Clinical Stage Company Advancing a Risk-Diversified Product Pipeline to Overcome Primary and Acquired Resistance to Cancer Immunotherapies Personalized Cancer Vaccines IFx Technology Harnesses the Power of the Innate Immune Response Single Phase 3 trial to initiate in 2H-2024 conducted under FDA’s Accelerated Approval Pathway. IFx-2.0, as adjunctive therapy with Keytruda® in 1st line treatment for advanced Merkel Cell Carcinoma Expanding use of checkpoint inhibitors is a commercially attractive opportunity Bi-Functional ADCs Delta Receptor Technology Targeting Myeloid Derived Suppressor Cells (MDSCs) First to identify novel Delta receptor high expression on tumor associated MDSCs Delta receptor represents a “master switch” controlling multiple MDSC pathways responsible for creating immune sanctuary where tumor lives Developing Delta specific small molecule inhibitors as core for the Company’s bi-functional antibody-drug conjugates (ADCs)

Transaction Highlights TEXT Combined company will operate under the name TuHURA Biosciences, Inc. and expects to trade on Nasdaq under the ticker HURA Structured as a stock-for-stock transaction whereby at closing, all of TuHURA’s outstanding equity interests expected to be exchanged for shares of Kintara common stock, warrants and options $31 million subscribed financing by TuHURA in connection with the merger agreement – combined company expected to have cash runway into late 2025 On a pro forma basis, post merger and including payment of CVR shares, Kintara equity holders are expected to collectively own approximately 5.45% and TuHURA equity holders expected to collectively own approximately 94.55%, respectively, of the common stock of the combined Company on a pro forma fully-diluted basis. Combined Company Merger Agreement Financing Ownership

James A Bianco, MD President and Chief Executive Officer TuHURA Biosciences
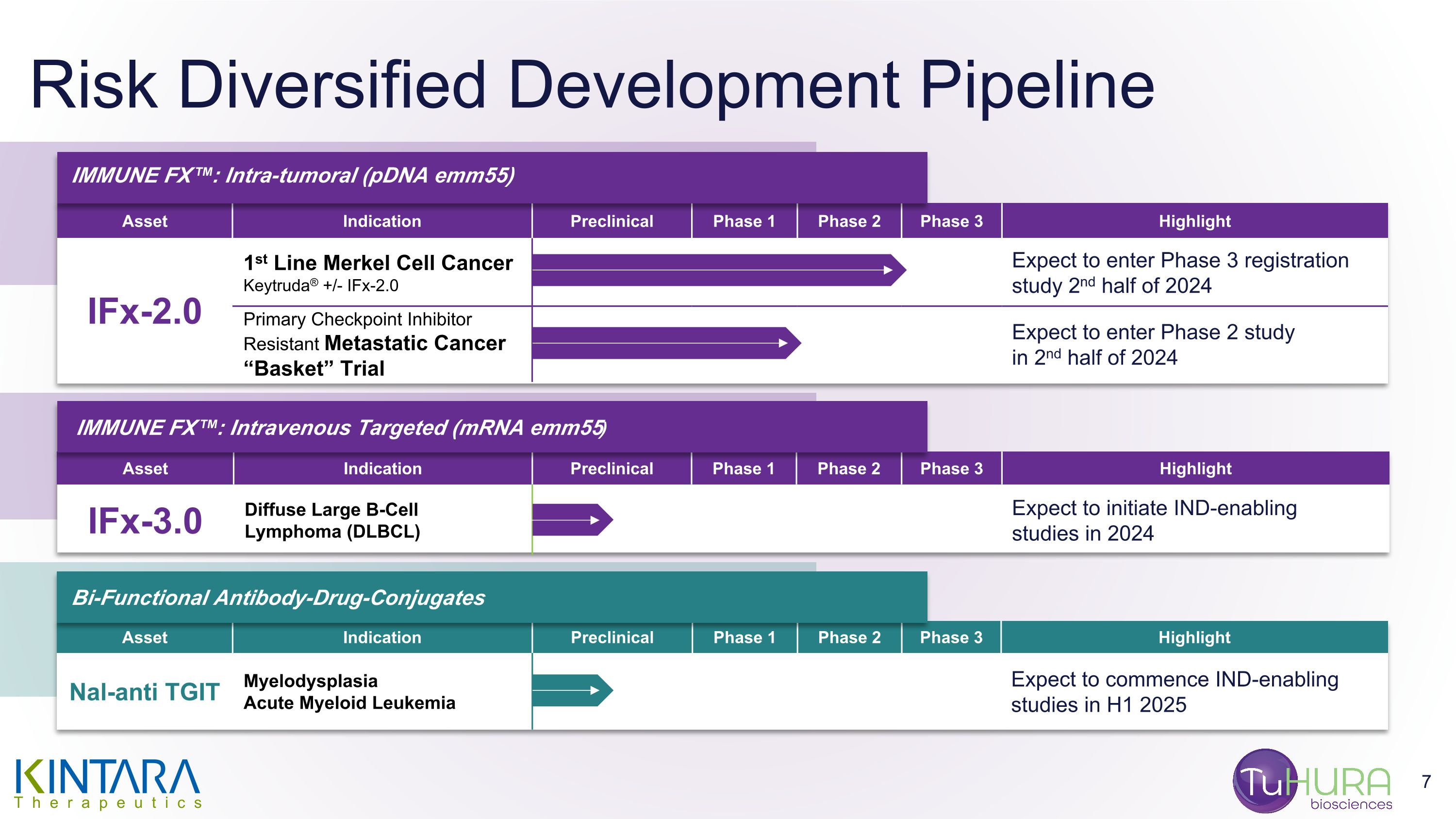
Risk Diversified Development Pipeline Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Highlight IFx-3.0 Diffuse Large B-Cell Lymphoma (DLBCL) Expect to initiate IND-enabling studies in 2024 Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Highlight Nal-anti TGIT MyelodysplasiaAcute Myeloid Leukemia Expect to commence IND-enabling studies in H1 2025 Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Highlight IFx-2.0 1st Line Merkel Cell Cancer Keytruda® +/- IFx-2.0 Expect to enter Phase 3 registration study 2nd half of 2024 IFx-Hu2.0 Primary Checkpoint Inhibitor Resistant Metastatic Cancer “Basket” Trial Expect to enter Phase 2 study in 2nd half of 2024 IMMUNE FX™: Intra-tumoral (pDNA emm55) IMMUNE FX™: Intravenous Targeted (mRNA emm55) Bi-Functional Antibody-Drug-Conjugates
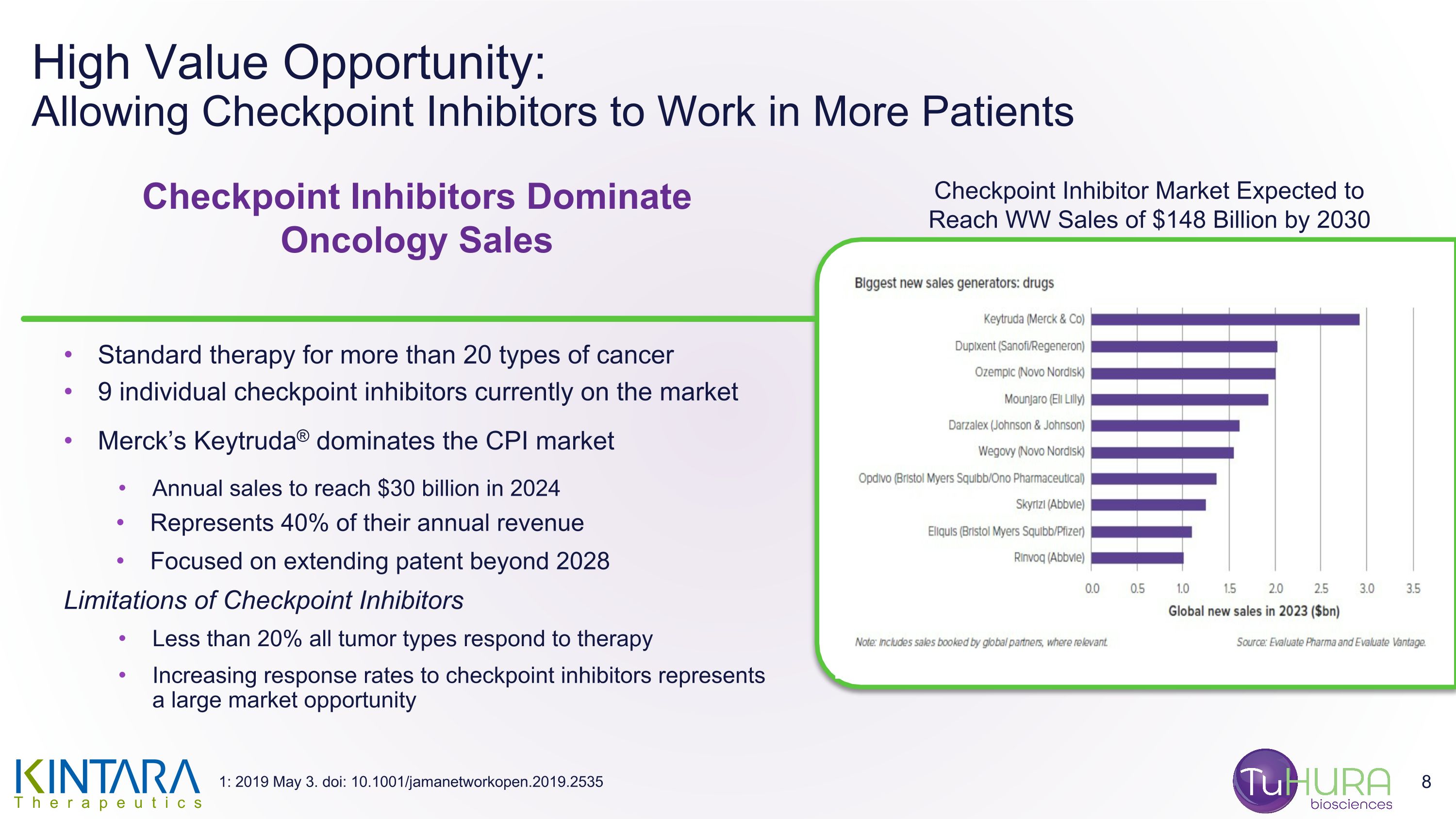
High Value Opportunity: Allowing Checkpoint Inhibitors to Work in More Patients Standard therapy for more than 20 types of cancer 9 individual checkpoint inhibitors currently on the market Merck’s Keytruda® dominates the CPI market Annual sales to reach $30 billion in 2024 Represents 40% of their annual revenue Focused on extending patent beyond 2028 Limitations of Checkpoint Inhibitors Less than 20% all tumor types respond to therapy Increasing response rates to checkpoint inhibitors represents a large market opportunity Checkpoint Inhibitors Dominate Oncology Sales Checkpoint Inhibitor Market Expected to Reach WW Sales of $148 Billion by 2030 1: 2019 May 3. doi: 10.1001/jamanetworkopen.2019.2535

Designed to Overcome Primary Resistance and Increase Response Rates to Checkpoint Inhibitor Therapy IFx-2.0 Personalized Cancer Vaccine
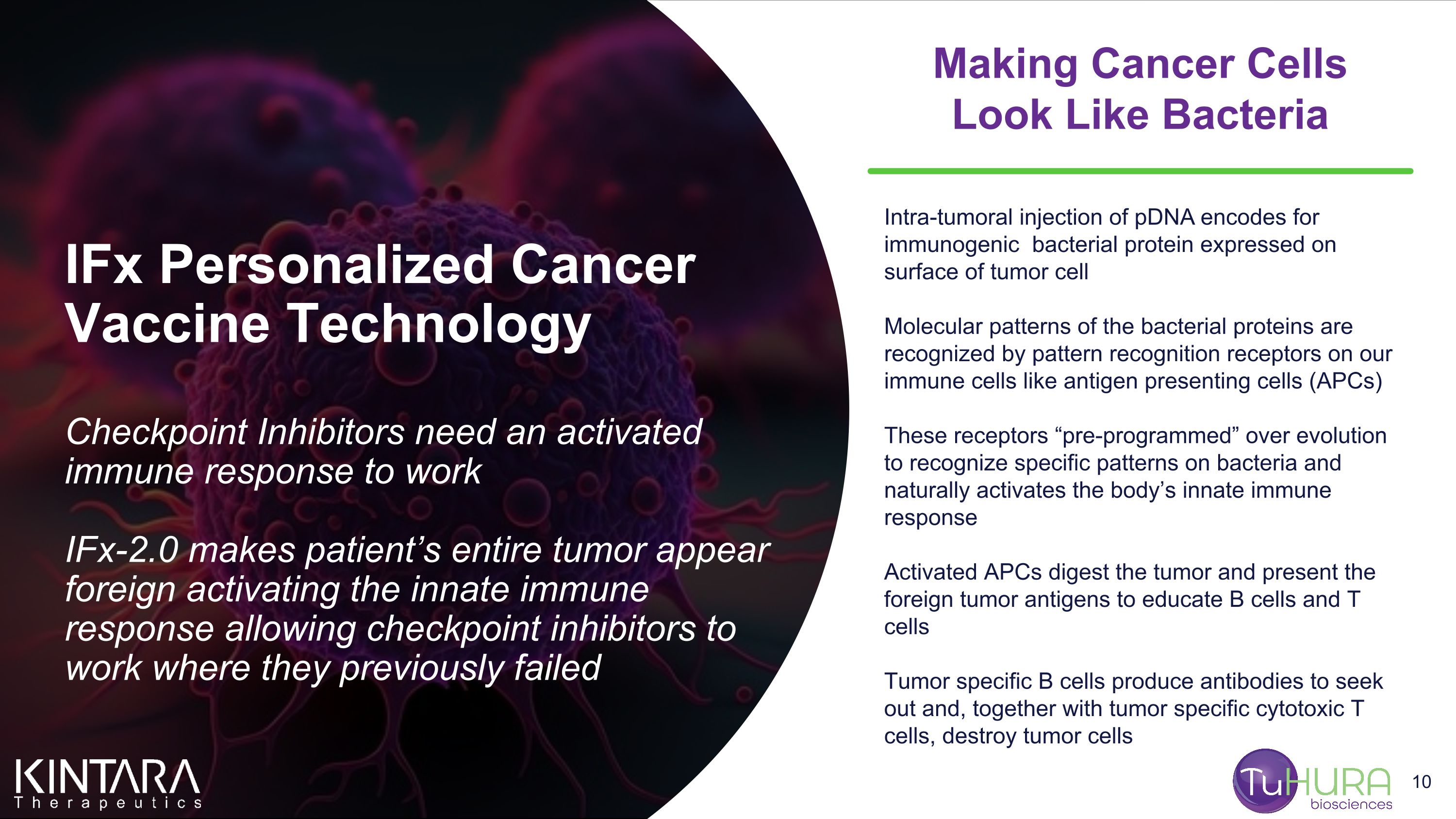
IFx Personalized Cancer Vaccine TechnologyCheckpoint Inhibitors need an activated immune response to work IFx-2.0 makes patient’s entire tumor appear foreign activating the innate immune response allowing checkpoint inhibitors to work where they previously failed Intra-tumoral injection of pDNA encodes for immunogenic bacterial protein expressed on surface of tumor cell Molecular patterns of the bacterial proteins are recognized by pattern recognition receptors on our immune cells like antigen presenting cells (APCs) These receptors “pre-programmed” over evolution to recognize specific patterns on bacteria and naturally activates the body’s innate immune response Activated APCs digest the tumor and present the foreign tumor antigens to educate B cells and T cells Tumor specific B cells produce antibodies to seek out and, together with tumor specific cytotoxic T cells, destroy tumor cells Making Cancer Cells Look Like Bacteria
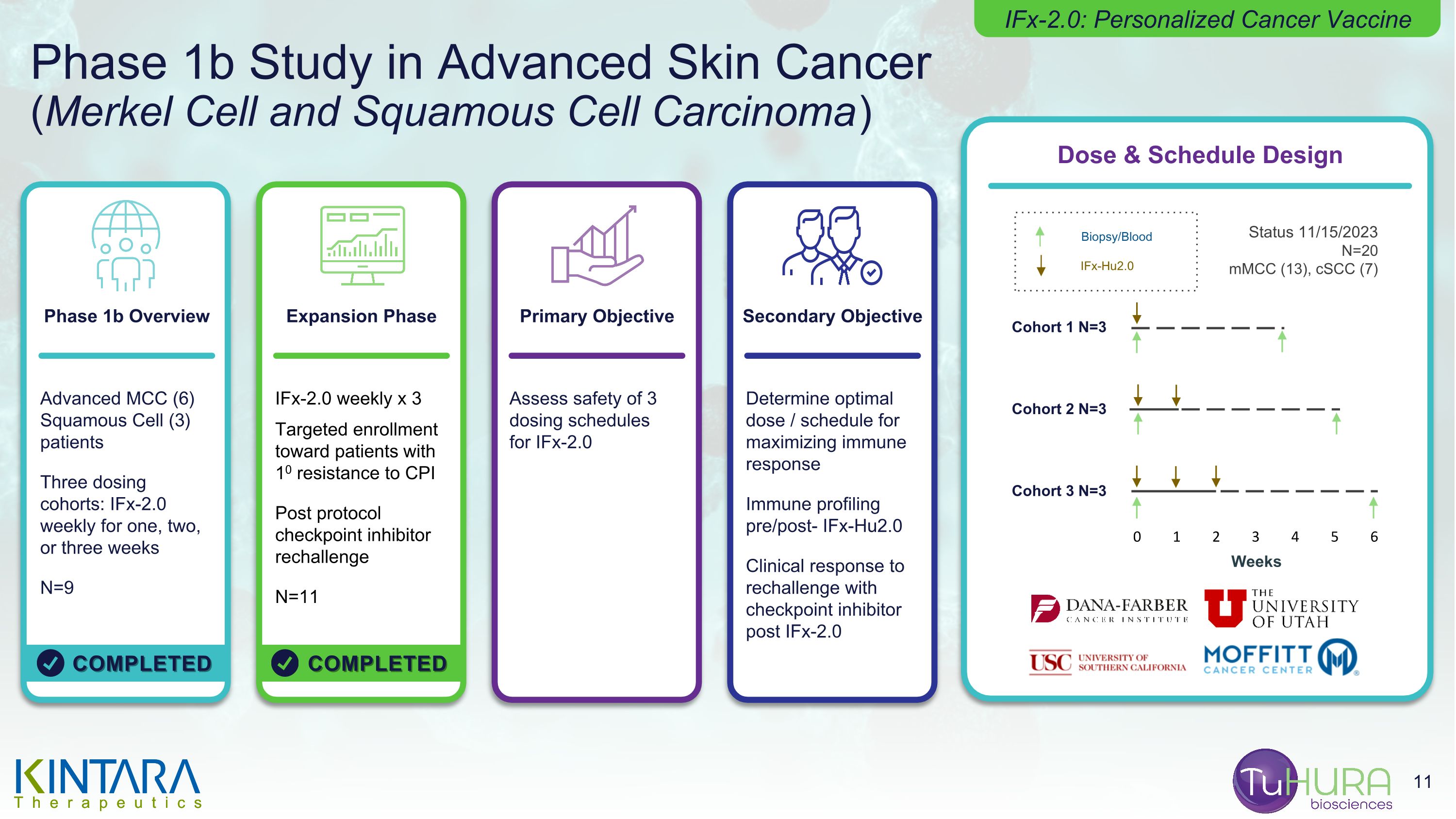
Phase 1b Study in Advanced Skin Cancer(Merkel Cell and Squamous Cell Carcinoma) IFx-2.0: Personalized Cancer Vaccine Advanced MCC (6) Squamous Cell (3) patients Three dosing cohorts: IFx-2.0 weekly for one, two, or three weeks N=9 IFx-2.0 weekly x 3 Targeted enrollment toward patients with 10 resistance to CPI Post protocol checkpoint inhibitor rechallenge N=11 Assess safety of 3 dosing schedules for IFx-2.0 Determine optimal dose / schedule for maximizing immune response Immune profiling pre/post- IFx-Hu2.0 Clinical response to rechallenge with checkpoint inhibitor post IFx-2.0 Primary Objective Secondary Objective Expansion Phase Phase 1b Overview COMPLETED COMPLETED IFx-Hu2.0 Biopsy/Blood Status 11/15/2023 N=20 mMCC (13), cSCC (7) Cohort 1 N=3 Cohort 2 N=3 Cohort 3 N=3 Weeks 0 1 2 3 4 5 6 Dose & Schedule Design
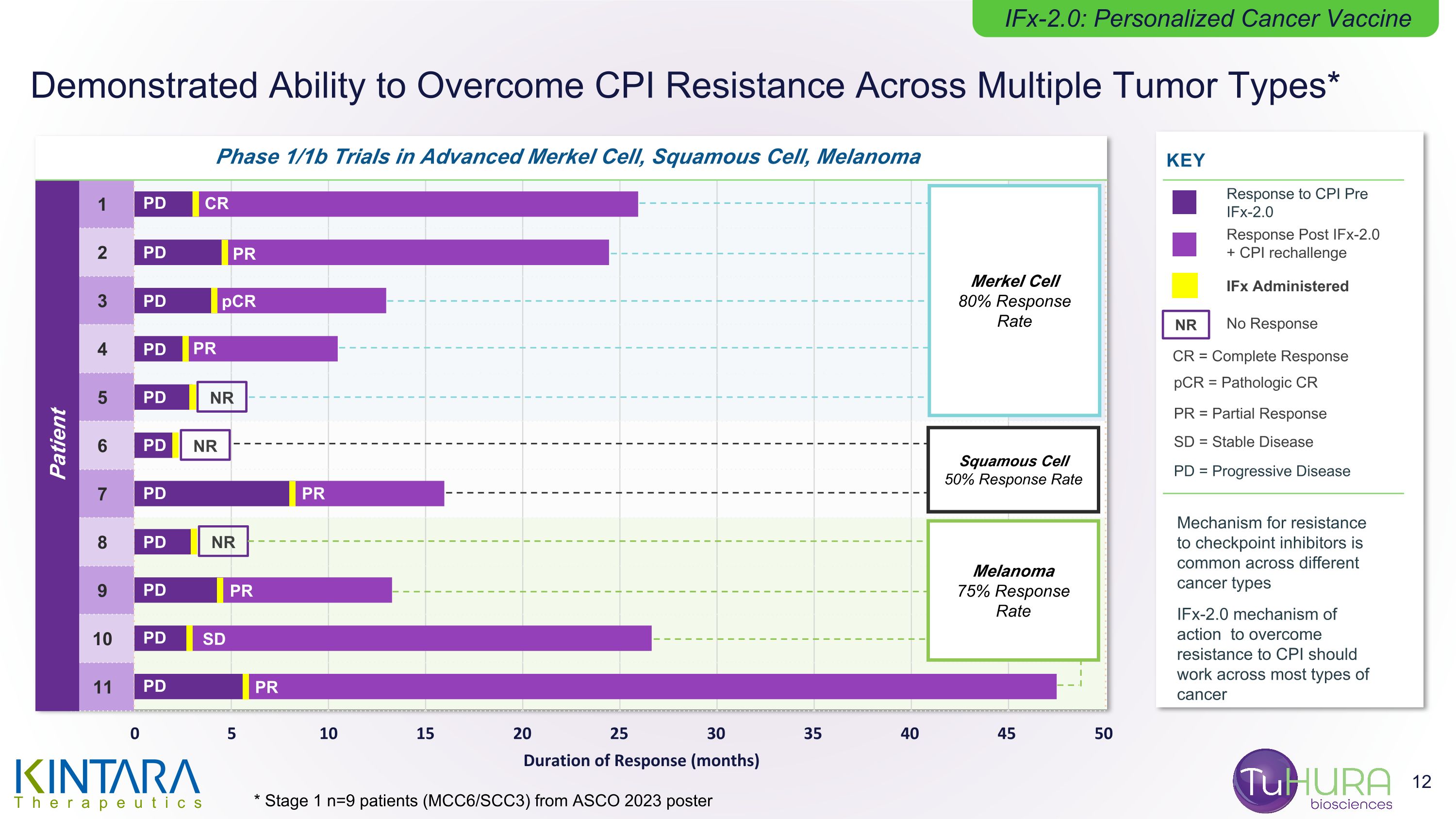
Demonstrated Ability to Overcome CPI Resistance Across Multiple Tumor Types* IFx-2.0: Personalized Cancer Vaccine 1 2 3 4 5 6 7 8 9 10 11 Phase 1/1b Trials in Advanced Merkel Cell, Squamous Cell, Melanoma Patient KEY NR NR NR PD PD PD PD PD PD PD PD PD PD PD CR PR pCR PR PR PR SD PR Merkel Cell80% Response Rate Squamous Cell 50% Response Rate Melanoma75% Response Rate Response Post IFx-2.0 + CPI rechallenge Response to CPI Pre IFx-2.0 No Response NR SD = Stable Disease PR = Partial Response CR = Complete Response pCR = Pathologic CR PD = Progressive Disease Mechanism for resistance to checkpoint inhibitors is common across different cancer types IFx-2.0 mechanism of action to overcome resistance to CPI should work across most types of cancer * Stage 1 n=9 patients (MCC6/SCC3) from ASCO 2023 poster IFx Administered
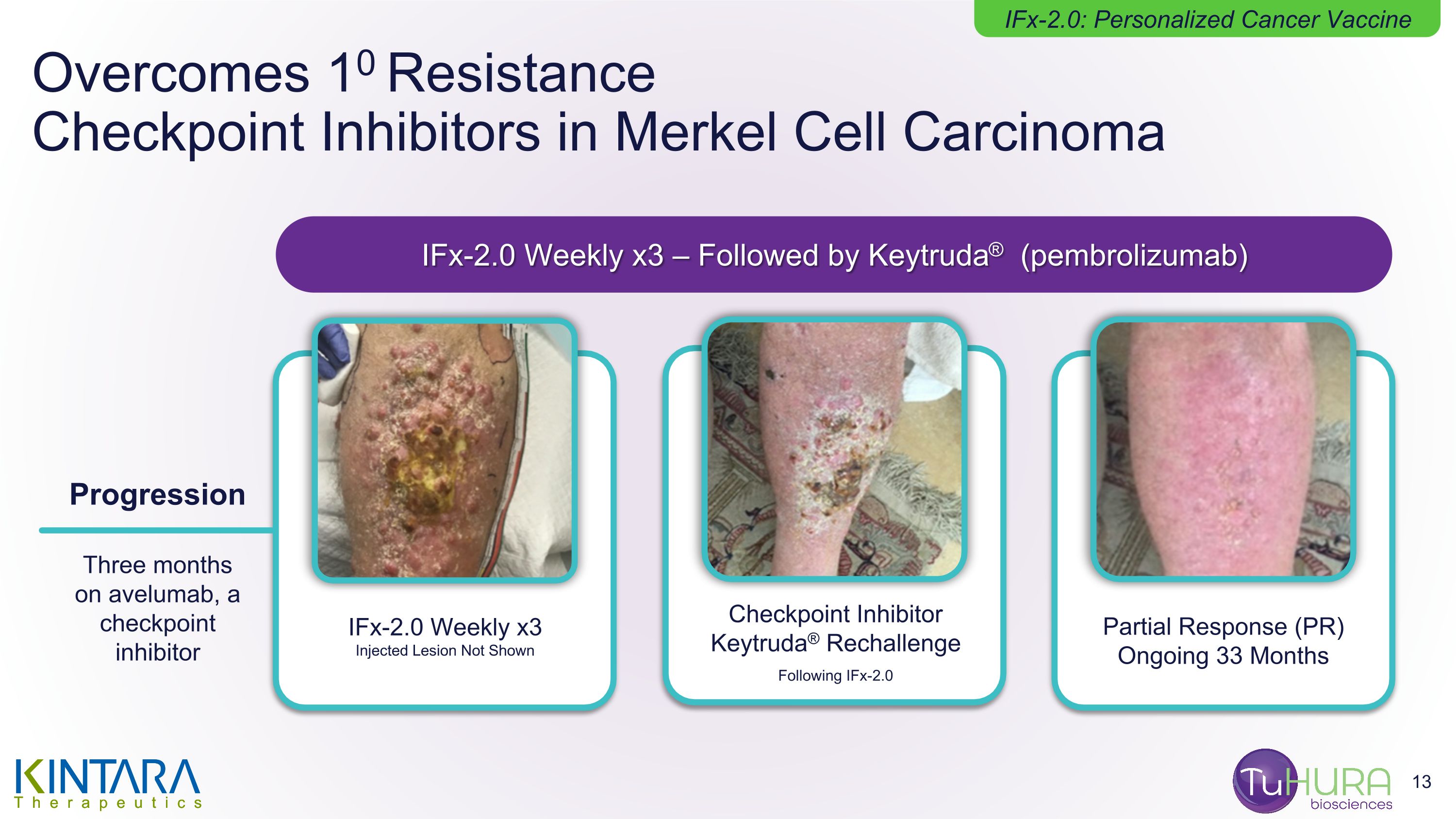
Overcomes 10 Resistance Checkpoint Inhibitors in Merkel Cell Carcinoma Progression Three months on avelumab, a checkpoint inhibitor IFx-2.0 Weekly x3 Injected Lesion Not Shown Checkpoint Inhibitor Keytruda® Rechallenge Following IFx-2.0 Partial Response (PR) Ongoing 33 Months IFx-2.0 Weekly x3 – Followed by Keytruda® (pembrolizumab) IFx-2.0: Personalized Cancer Vaccine
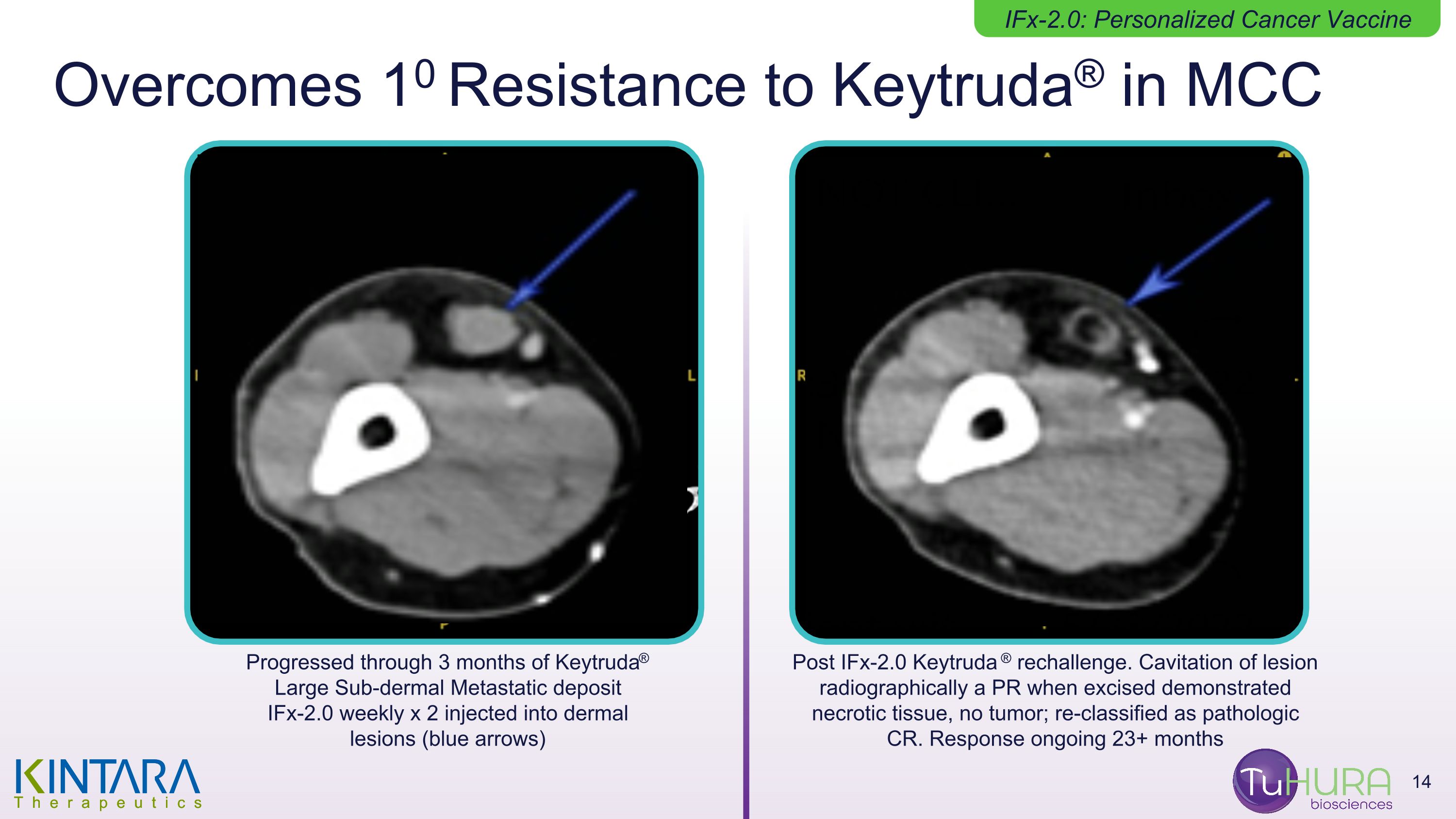
Overcomes 10 Resistance to Keytruda® in MCC Progressed through 3 months of Keytruda® Large Sub-dermal Metastatic deposit IFx-2.0 weekly x 2 injected into dermal lesions (blue arrows) Post IFx-2.0 Keytruda ® rechallenge. Cavitation of lesion radiographically a PR when excised demonstrated necrotic tissue, no tumor; re-classified as pathologic CR. Response ongoing 23+ months IFx-2.0: Personalized Cancer Vaccine
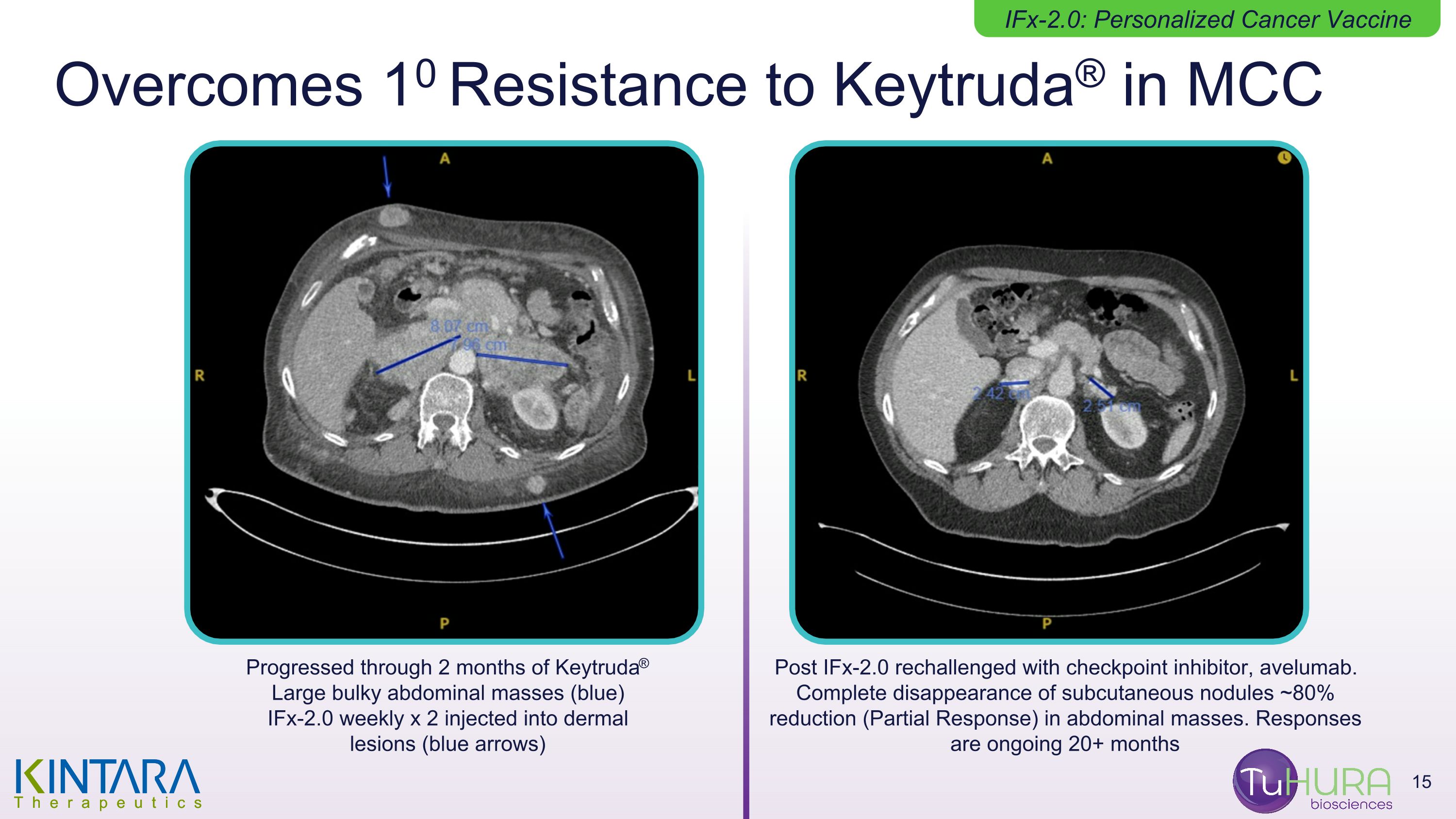
Progressed through 2 months of Keytruda® Large bulky abdominal masses (blue) IFx-2.0 weekly x 2 injected into dermal lesions (blue arrows) Post IFx-2.0 rechallenged with checkpoint inhibitor, avelumab. Complete disappearance of subcutaneous nodules ~80% reduction (Partial Response) in abdominal masses. Responses are ongoing 20+ months Overcomes 10 Resistance to Keytruda® in MCC IFx-2.0: Personalized Cancer Vaccine
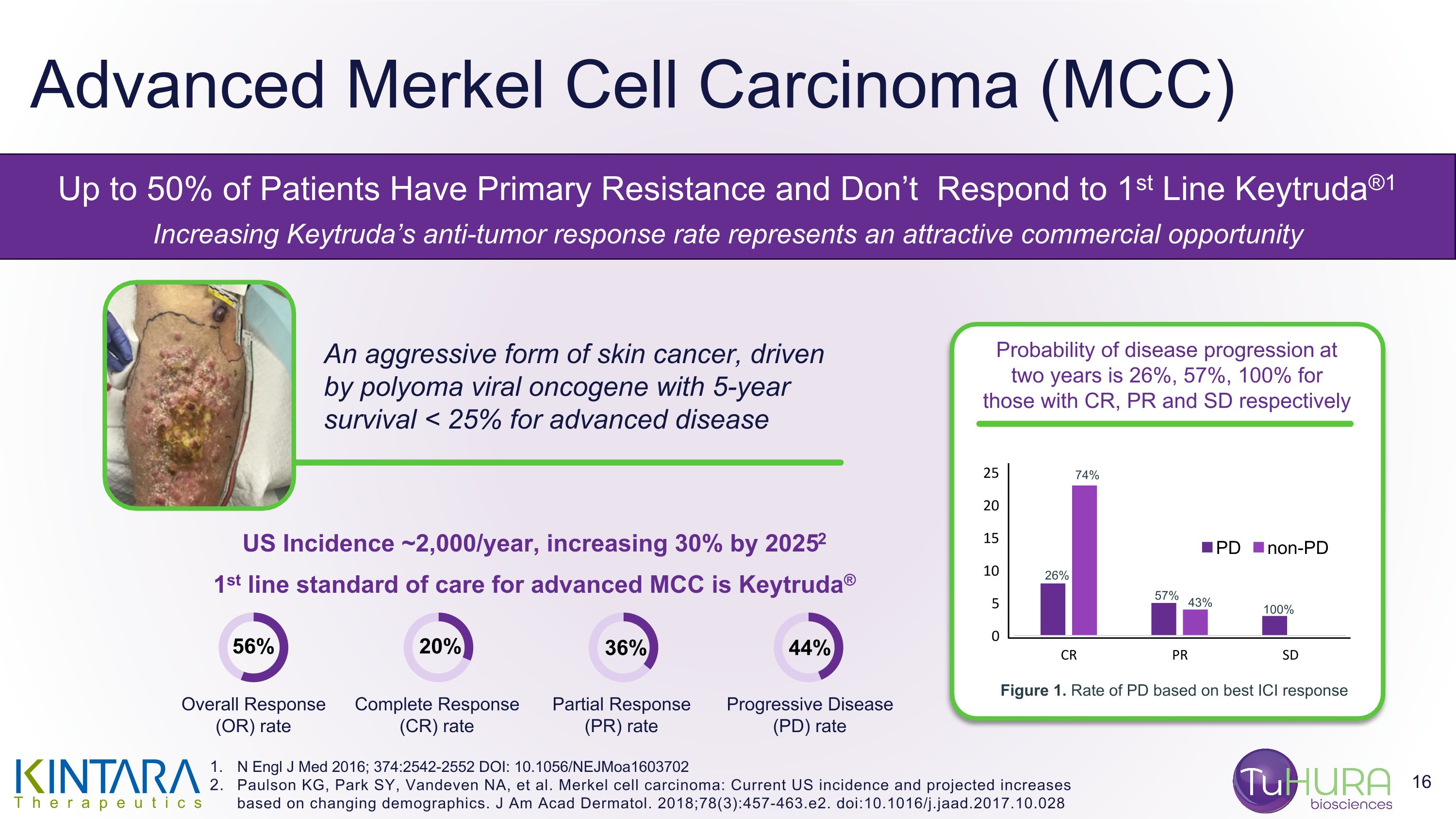
Advanced Merkel Cell Carcinoma (MCC) Up to 50% of Patients Have Primary Resistance and Don’t Respond to 1st Line Keytruda®1 Increasing Keytruda’s anti-tumor response rate represents an attractive commercial opportunity An aggressive form of skin cancer, driven by polyoma viral oncogene with 5-year survival < 25% for advanced disease US Incidence ~2,000/year, increasing 30% by 20252 1st line standard of care for advanced MCC is Keytruda® Probability of disease progression at two years is 26%, 57%, 100% for those with CR, PR and SD respectively 26% 74% 57% 43% 100% Figure 1. Rate of PD based on best ICI response 56% Overall Response (OR) rate Complete Response (CR) rate Partial Response (PR) rate Progressive Disease (PD) rate 20% 36% 44% N Engl J Med 2016; 374:2542-2552 DOI: 10.1056/NEJMoa1603702 Paulson KG, Park SY, Vandeven NA, et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J Am Acad Dermatol. 2018;78(3):457-463.e2. doi:10.1016/j.jaad.2017.10.028
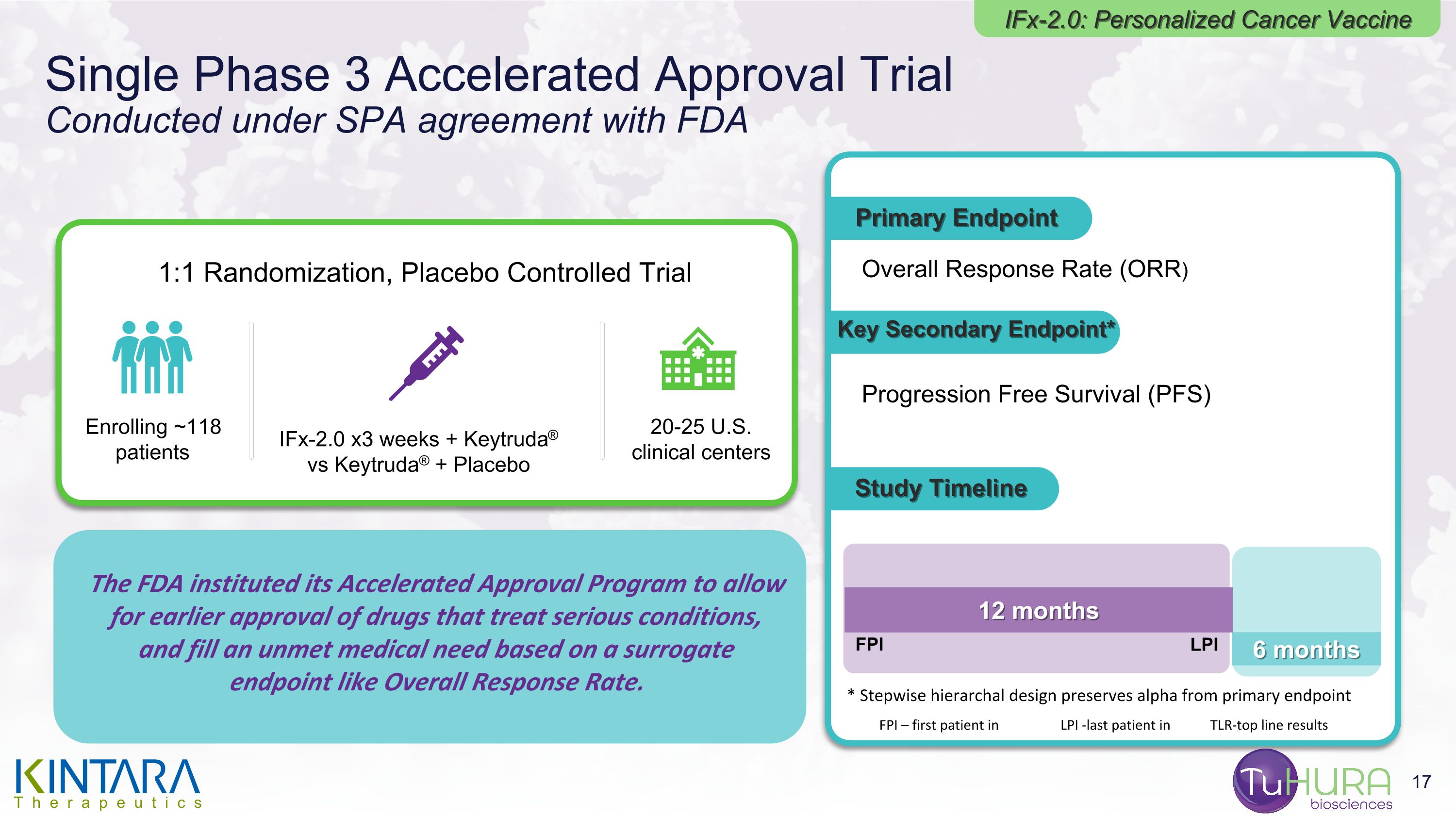
Single Phase 3 Accelerated Approval Trial Conducted under SPA agreement with FDA IFx-2.0: Personalized Cancer Vaccine 1:1 Randomization, Placebo Controlled Trial Enrolling ~118 patients IFx-2.0 x3 weeks + Keytruda® vs Keytruda® + Placebo 20-25 U.S. clinical centers Primary Endpoint Overall Response Rate (ORR) Key Secondary Endpoint* Progression Free Survival (PFS) Study Timeline 6 months TLR 12 months FPI LPI FPI – first patient in LPI -last patient in TLR-top line results The FDA instituted its Accelerated Approval Program to allow for earlier approval of drugs that treat serious conditions, and fill an unmet medical need based on a surrogate endpoint like Overall Response Rate. * Stepwise hierarchal design preserves alpha from primary endpoint
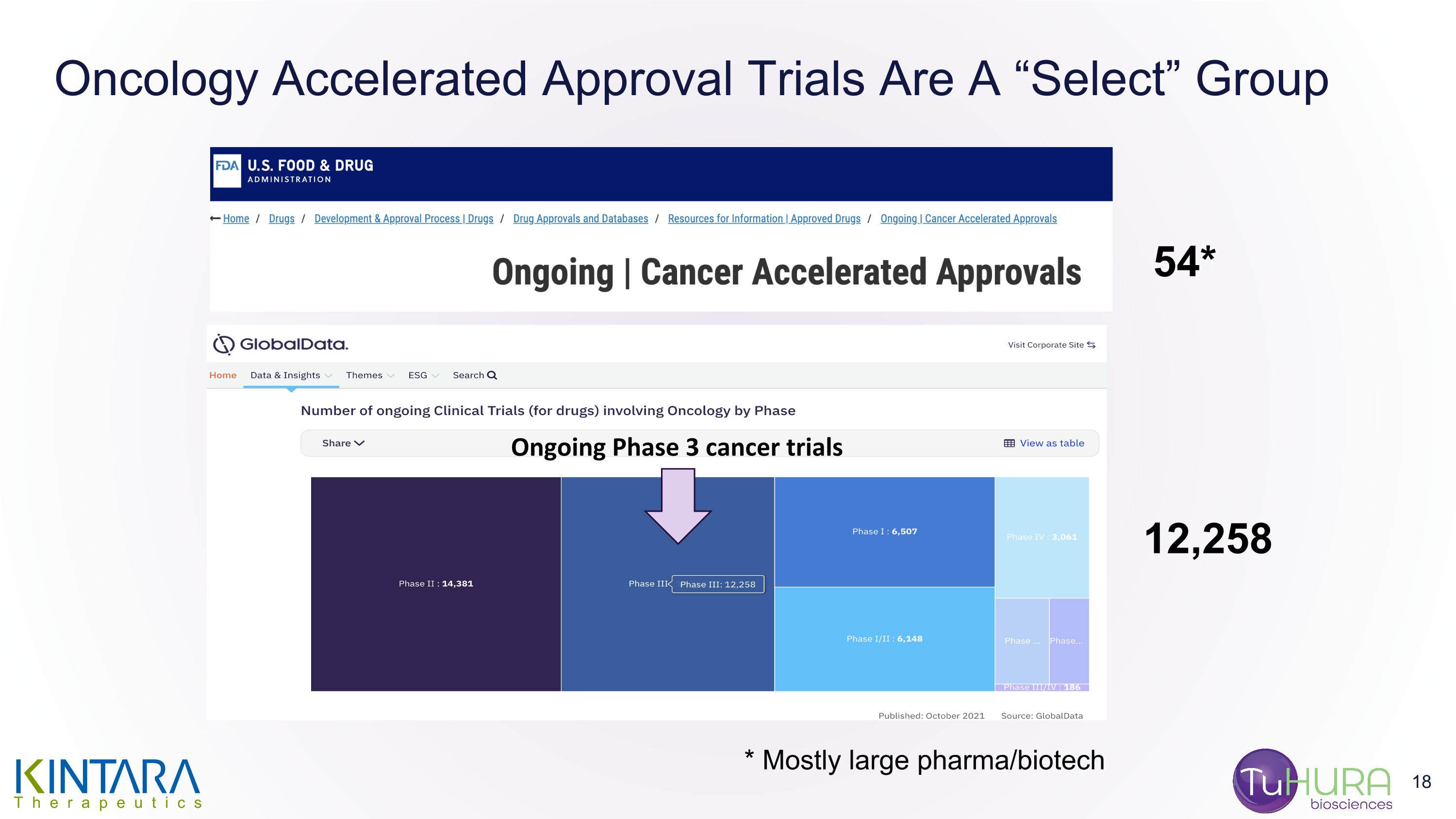
Oncology Accelerated Approval Trials Are A “Select” Group 54* 12,258 Ongoing Phase 3 cancer trials * Mostly large pharma/biotech
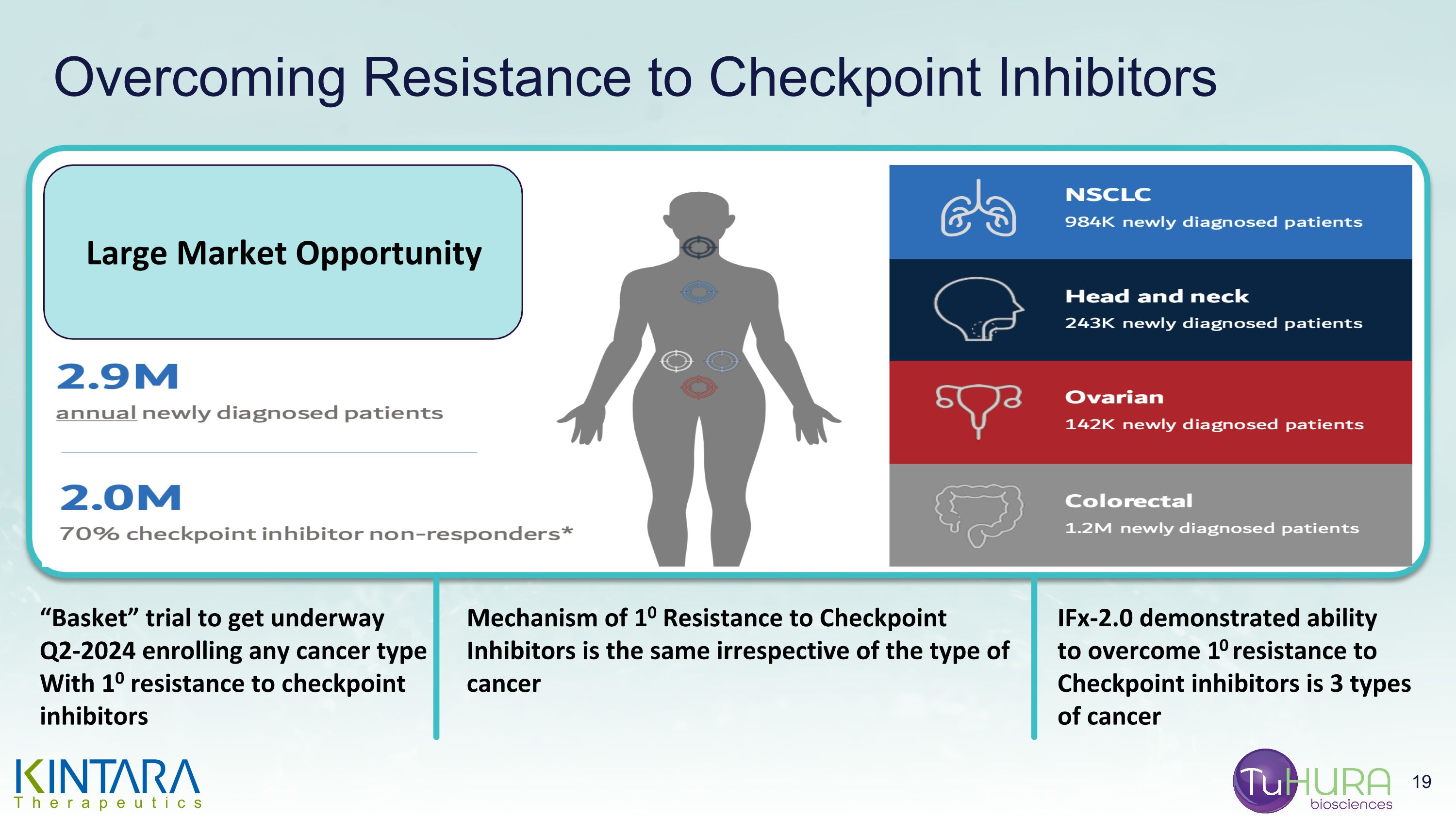
Overcoming Resistance to Checkpoint Inhibitors Large Market Opportunity Mechanism of 10 Resistance to Checkpoint Inhibitors is the same irrespective of the type of cancer IFx-2.0 demonstrated ability to overcome 10 resistance to Checkpoint inhibitors is 3 types of cancer “Basket” trial to get underway Q2-2024 enrolling any cancer type With 10 resistance to checkpoint inhibitors

IFx-3.0 – Tumor Targeted mRNA Personalized Cancer Vaccine Designed to Overcome Primary Resistance to Checkpoint Inhibitors and Cellular Therapies
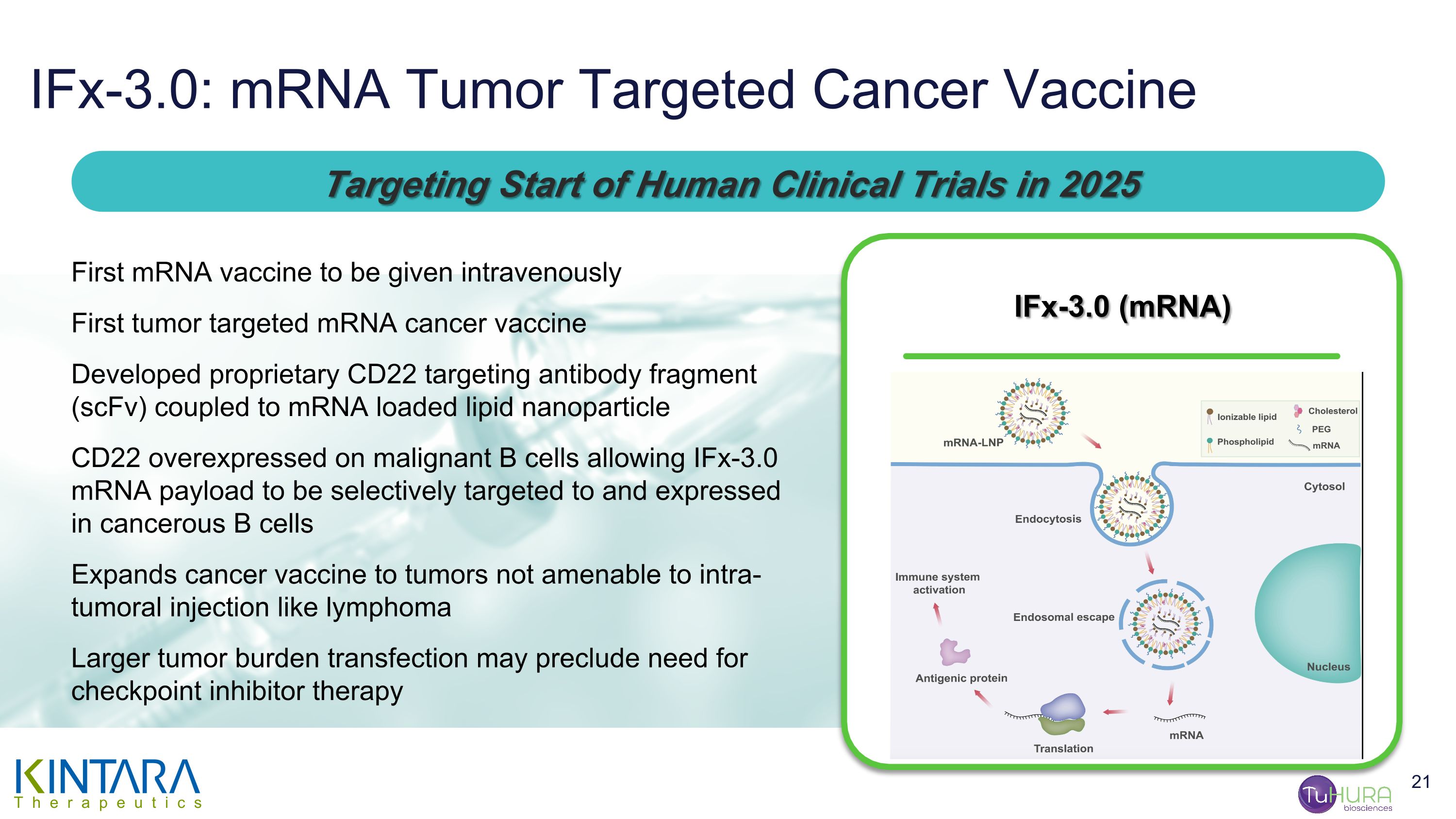
IFx-3.0: mRNA Tumor Targeted Cancer Vaccine Targeting Start of Human Clinical Trials in 2025 IFx-3.0 (mRNA) First mRNA vaccine to be given intravenously First tumor targeted mRNA cancer vaccine Developed proprietary CD22 targeting antibody fragment (scFv) coupled to mRNA loaded lipid nanoparticle CD22 overexpressed on malignant B cells allowing IFx-3.0 mRNA payload to be selectively targeted to and expressed in cancerous B cells Expands cancer vaccine to tumors not amenable to intra-tumoral injection like lymphoma Larger tumor burden transfection may preclude need for checkpoint inhibitor therapy

Bi-Functional ADC Inhibitors of MDSC Immune Suppression Designed to Overcome Acquired Resistance to Checkpoint Inhibitors and Cellular Therapies
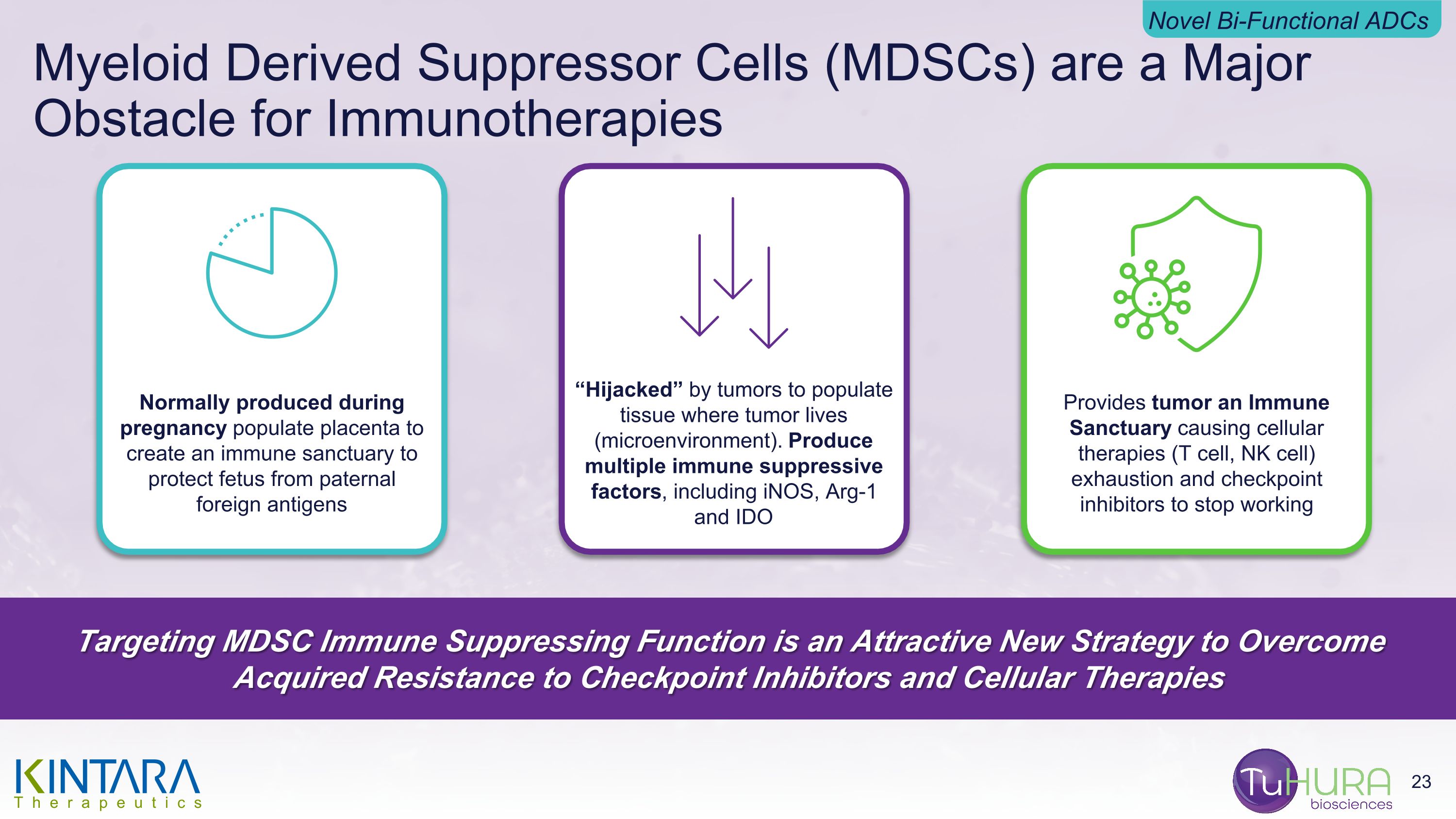
Myeloid Derived Suppressor Cells (MDSCs) are a Major Obstacle for Immunotherapies Normally produced during pregnancy populate placenta to create an immune sanctuary to protect fetus from paternal foreign antigens “Hijacked” by tumors to populate tissue where tumor lives (microenvironment). Produce multiple immune suppressive factors, including iNOS, Arg-1 and IDO Provides tumor an Immune Sanctuary causing cellular therapies (T cell, NK cell) exhaustion and checkpoint inhibitors to stop working Targeting MDSC Immune Suppressing Function is an Attractive New Strategy to Overcome Acquired Resistance to Checkpoint Inhibitors and Cellular Therapies Novel Bi-Functional ADCs
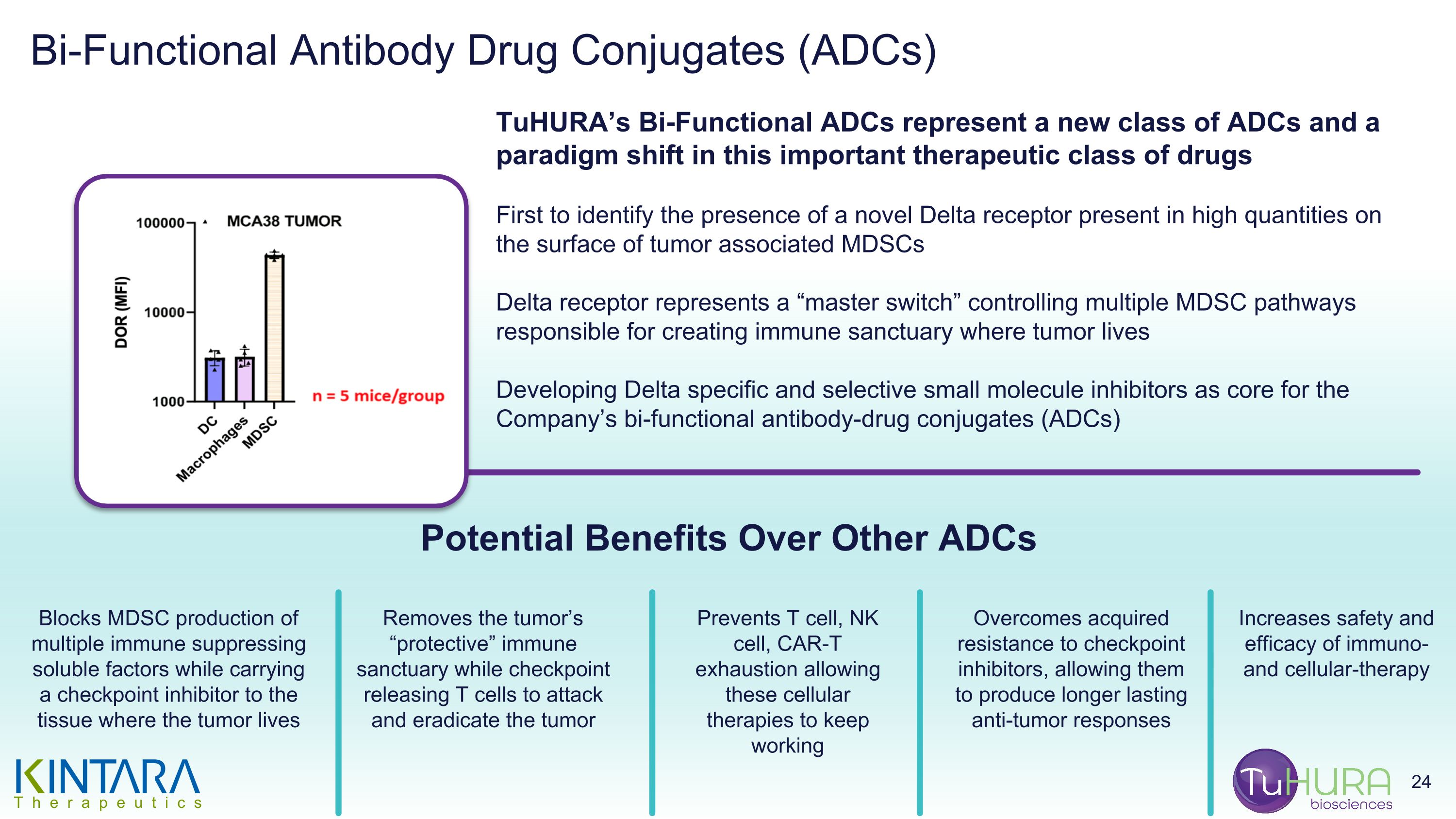
Bi-Functional Antibody Drug Conjugates (ADCs) Blocks MDSC production of multiple immune suppressing soluble factors while carrying a checkpoint inhibitor to the tissue where the tumor lives Removes the tumor’s “protective” immune sanctuary while checkpoint releasing T cells to attack and eradicate the tumor Prevents T cell, NK cell, CAR-T exhaustion allowing these cellular therapies to keep working Overcomes acquired resistance to checkpoint inhibitors, allowing them to produce longer lasting anti-tumor responses Increases safety and efficacy of immuno- and cellular-therapy Potential Benefits Over Other ADCs TuHURA’s Bi-Functional ADCs represent a new class of ADCs and a paradigm shift in this important therapeutic class of drugs First to identify the presence of a novel Delta receptor present in high quantities on the surface of tumor associated MDSCs Delta receptor represents a “master switch” controlling multiple MDSC pathways responsible for creating immune sanctuary where tumor lives Developing Delta specific and selective small molecule inhibitors as core for the Company’s bi-functional antibody-drug conjugates (ADCs)

President, CEO, CFO and Chairman of the Board Kintara Therapeutics Robert E. Hoffman
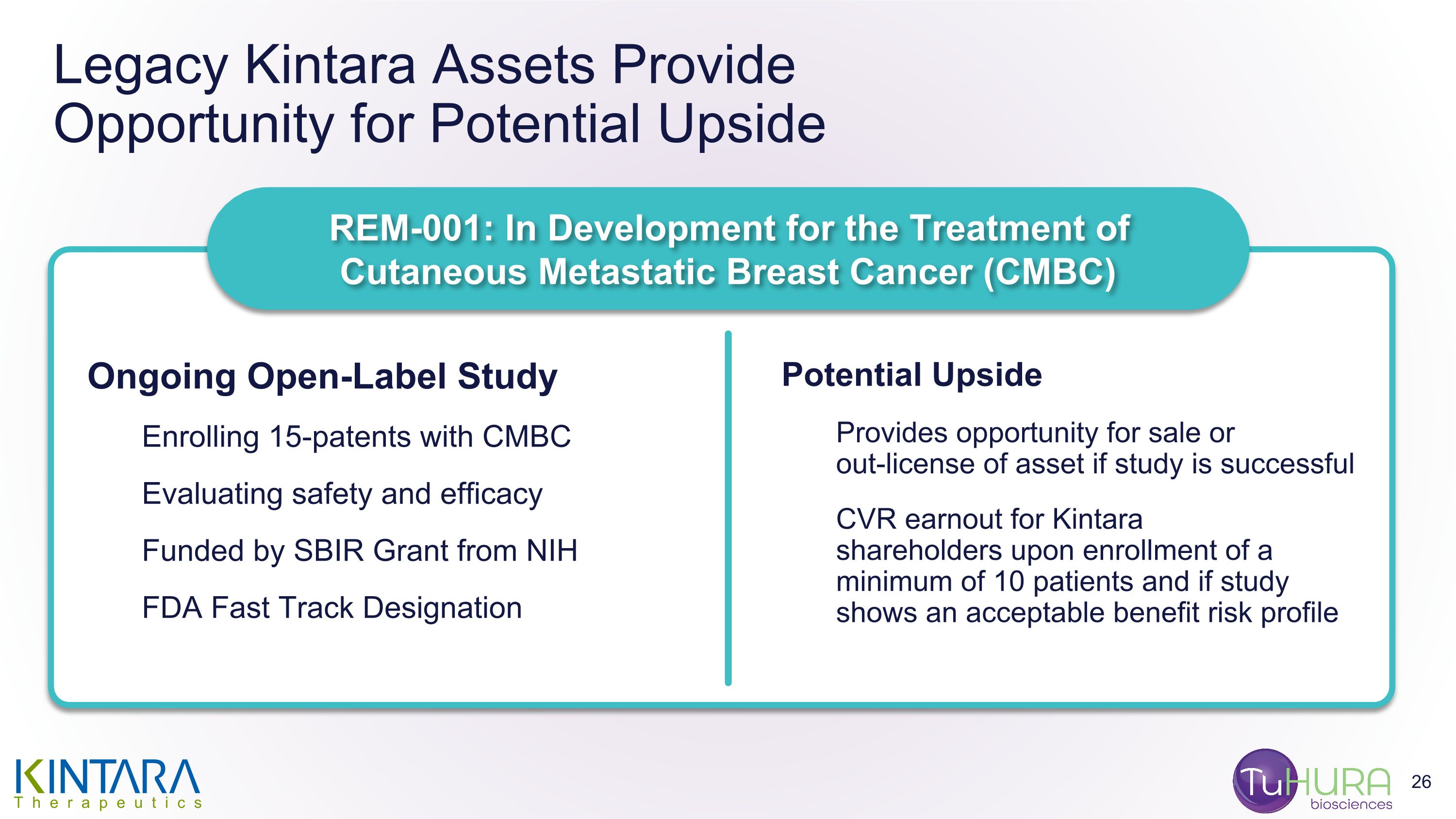
Legacy Kintara Assets Provide Opportunity for Potential Upside REM-001: In Development for the Treatment of Cutaneous Metastatic Breast Cancer (CMBC) Ongoing Open-Label Study Enrolling 15-patents with CMBC Evaluating safety and efficacy Funded by SBIR Grant from NIH FDA Fast Track Designation Potential Upside Provides opportunity for sale orout-license of asset if study is successful CVR earnout for Kintara shareholders upon enrollment of a minimum of 10 patients and if study shows an acceptable benefit risk profile
Among select group of accelerated approval Phase 3 oncology companies under SPA with FDA for novel trial design removing need for confirmatory trial Phase 3 results in Merkel Cell Carcinoma expected to be translatable to majority of cancers where checkpoint inhibitors don’t work Overcoming resistance to Keytruda® provides an attractive commercial opportunity Two Innovative Technology Platforms with Ability to Target Multiple High Value Oncology Indications Personalized Cancer Vaccines Bi-Functional ADC Inhibitors of MDSCs First to identify Delta receptor overexpressed on MDSCs Delta selective inhibitors remove MDSC immune suppression on tumor microenvironment Novel first-in-class bi-functional ADCs Sufficient Capital Expected to Fund Operations Through Mid-2025 Single Phase 3 trial for accelerated approval Attractive timeline and costs to Phase 3 trial results and potential FDA approval Basket trial could expand use to multiple tumor types IFx-3.0 represents next generation of tumor targeted cancer vaccines Summary

Enter into Definitive Merger Agreement Phase 3 Clinical Stage Immuno-Oncology Company Addressing Major Obstacles to Overcoming Resistance to Cancer Immunotherapy