UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 28, 2024
CANDEL THERAPEUTICS, INC.
(Exact name of Registrant as Specified in Its Charter)
Delaware |
001-40629 |
52-2214851 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
117 Kendrick St., Suite 450 Needham, MA |
|
02494 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (617) 916-5445
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, $0.01 par value per share |
|
CADL |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On March 28, 2024, Candel Therapeutics, Inc. announced its financial results for the quarter and year ended December 31, 2023. The full text of the press release issued in connection with the announcement is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in this Current Report on Form 8-K (including Exhibit 99.1 attached hereto) is furnished herewith and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit Number |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
Candel Therapeutics, Inc. |
|
|
|
|
|
Date: March 28, 2024 |
|
By: |
/s/ Paul Peter Tak |
|
|
|
Paul Peter Tak, M.D., Ph.D., FMedSci |
|
|
|
President and Chief Executive Officer |
Exhibit 99.1

Candel Therapeutics Reports Fourth Quarter and Full Year 2023 Financial Results and Recent Corporate Highlights
NEEDHAM, Mass., March 28, 2024 (GLOBE NEWSWIRE) — Candel Therapeutics, Inc. (Candel or the Company) (Nasdaq: CADL), a clinical stage biopharmaceutical company focused on developing multimodal biological immunotherapies to help patients fight cancer, today reported financial results for the fourth quarter and year ended December 31, 2023, and provided a corporate update.
“2023 was a significant year for Candel with two additional FDA Fast Track Designations for CAN-2409 for non-small cell lung cancer and borderline resectable pancreatic cancer, and most recently Candel received FDA Fast Track Designation for CAN-3110 for recurrent high-grade glioma in February of 2024. We are also excited that initial results in CAN-3110’s ongoing phase 1b clinical trial were published in the high-impact scientific journal, Nature,” said Paul Peter Tak, MD PhD FMedSci, President and CEO of Candel. “Further, initial patient data showed improved survival after experimental treatment with Candel’s investigational viral multimodal immunotherapies, as compared to standard of care, in non-small cell lung cancer (CAN-2409), pancreatic cancer (CAN-2409), and high-grade glioma (CAN-3110).”
Dr. Tak continued, “In 2024, we are expecting six data readouts across our three platforms, which include novel clinical and biomarker data in lung cancer, pancreatic cancer, and brain cancer, and a potentially registrational phase 3 clinical trial in prostate cancer. We look forward to sharing additional updates in the year ahead.”
Fourth Quarter 2023 & Recent Highlights
Anticipated Milestones
Financial Results for the Year and Fourth Quarter Ended December 31, 2023
Research and Development Service Revenue, related party: Research and development service revenue, related party, for each of the quarter and full year ended December 31, 2023 was $0, as compared to $31,000 and $125,000 for the quarter and full year ended December 31, 2022.
Research and Development Expenses: Research and development expenses were $7.3 million for the fourth quarter of 2023 compared to $5.0 million for the fourth quarter of 2022, and $24.5 million for the full year 2023 compared to $20.8 million for the full year 2022. The increase was primarily due to manufacturing and regulatory activities in support of the Company’s CAN-2409 programs, stock compensation costs, and impairment of fixed assets. Research and development expenses included non-cash stock compensation expense of $0.5 million and $1.3 million for the fourth quarter and full year of 2023, respectively, as compared to a non-cash stock compensation expense of $0.2 million and $0.8 million for the fourth quarter and full year of 2022.
General and Administrative Expenses: General and administrative expenses were $3.1 million for the fourth quarter of 2023 compared to $3.2 million for the fourth quarter of 2022, and $13.9 million for the full year 2023 compared to $14.1 million for the full year 2022. The decrease was primarily due to lower insurance and recruiting costs, which were partially offset by increased employee-related expenses. General and administrative expenses included non-cash stock compensation expense of $0.5 million and $1.7 million for the fourth quarter and full year of 2023, respectively, as compared to a non-cash stock compensation expense of $0.4 million and $1.5 million for the fourth quarter and full year of 2022.
Net Loss: Net loss for the fourth quarter of 2023 was $11.1 million compared to a net loss of $5.1 million for the fourth quarter of 2022, and included net other expense of $0.8 million and net other income $3.0 million, respectively. The change from net other income in the fourth quarter of 2022 to net other expense in the fourth quarter of 2023 was primarily related to the change in the fair value of the Company’s warrant liability. Net loss for the full year 2023 was $37.9 million compared to a net loss of $18.8 million for the full year 2022, and included net other income of $0.5 million and $15.9 million, respectively, related primarily to the change in the fair value of the Company’s warrant liability.
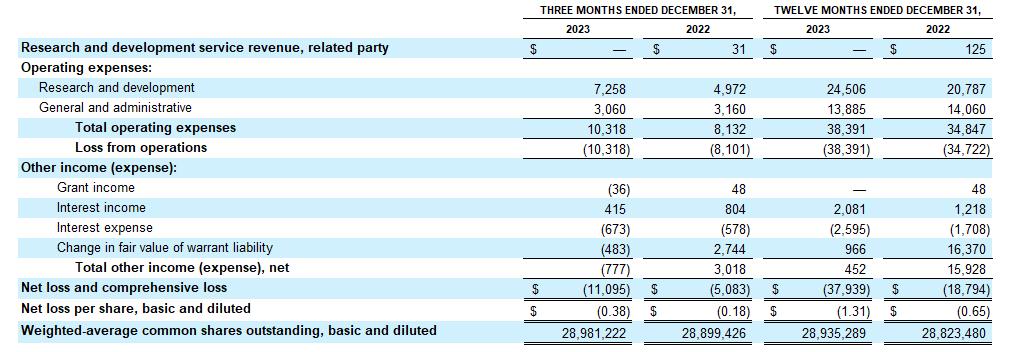
Cash Position: Cash and cash equivalents as of December 31, 2023 were $35.4 million, as compared to $70.1 million as of December 31, 2022. Based on current plans and assumptions, the Company expects that its existing cash and cash equivalents will be sufficient to fund its current operating plan into the fourth quarter of 2024.
About Candel Therapeutics
Candel is a clinical stage biopharmaceutical company focused on developing off-the-shelf multimodal biological immunotherapies that elicit an individualized, systemic anti-tumor immune response to help patients fight cancer. Candel has established two clinical stage multimodal biological immunotherapy platforms based on novel, genetically modified adenovirus and herpes simplex virus (HSV) gene constructs, respectively. CAN-2409 is the lead product candidate from the adenovirus platform and is currently in ongoing clinical trials in non-small cell lung cancer (NSCLC) (phase 2), borderline resectable pancreatic cancer (phase 2), and localized, non-metastatic prostate cancer (phase 2 and phase 3). CAN-3110 is the lead product candidate from the HSV platform and is currently in an ongoing investigator-sponsored phase 1 clinical trial in recurrent high-grade glioma (HGG). Finally, Candel’s enLIGHTEN™ Discovery Platform is a systematic, iterative HSV-based discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors.
For more information about Candel, visit: www.candeltx.com
Forward-Looking Statements
This press release includes certain disclosures that contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding the timing and advancement of development programs, including the timing and availability of additional data, key data readout milestones, expectations regarding the therapeutic benefit of the Company’s programs, including the potential for its programs to extend patient survival; and expectations regarding cash runway and expenditures. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties related to the timing and advancement of development programs; the Company’s ability to continue as a going concern; expectations regarding the therapeutic benefit of the Company’s programs; that final data from the Company’s pre-clinical studies and completed clinical trials may differ materially from reported interim data from ongoing studies and trials; the Company’s ability to efficiently discover and develop product candidates; the Company’s ability to obtain and maintain regulatory approval of product candidates; the Company’s ability to maintain its intellectual property; the implementation of the Company’s business model, including strategic plans for the Company’s business and product candidates, and other risks identified in the Company’s filings with the U.S.
Securities and Exchange Commission (SEC), including the Company’s most recent Annual Report on Form 10-K filed with the SEC, and subsequent filings with the SEC. The Company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. The Company disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent the Company’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
Investor Contact
Theodore Jenkins
VP Investor Relations and Business Development
Candel Therapeutics, Inc. tjenkins@candeltx.com
Media Contact Aljanae Reynolds Director Wheelhouse Life Science Advisors areynolds@wheelhouselsa.com (in thousands, except share and per share amounts)
Candel Therapeutics, Inc.
Consolidated Statements of Operations
(Unaudited)
Candel Therapeutics, Inc.
Consolidated Balance Sheet Data
(in thousands)