UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT of 1934
For the fiscal year ended December 31, 2023
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _________ to __________
Commission File Number 000-30833
BRUKER CORPORATION
(Exact name of registrant as specified in its charter)
Delaware |
04-3110160 |
(State or other jurisdiction of |
(I.R.S. Employer |
|
|
40 Manning Road, Billerica, MA |
01821 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code:
(978) 663-3660
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbols(s)
|
Name of each exchange on which registered
|
Common Stock |
BRKR |
Nasdaq Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☒ |
Accelerated filer |
☐ |
|
|
|
|
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
|
|
|
|
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant as of June 30, 2023 (the last business day of the registrant’s most recently completed second fiscal quarter) was $6,098,907,904 based on the reported last sale price on the Nasdaq Global Select Market. The number of shares of the registrant’s common stock outstanding as of February 25, 2024 was 137,671,143.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the information required by Part III of this report (Items 10, 11, 12, 13 and 14) are incorporated by reference from the registrant’s Definitive Proxy Statement on Schedule 14A for its 2024 Annual Meeting of Shareholders to be filed within 120 days of the close of the registrant’s fiscal year.
BRUKER CORPORATION
ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
|
|
Page |
Part I |
|
|
3 |
||
18 |
||
32 |
||
33 |
||
34 |
||
35 |
||
35 |
||
|
|
|
Part II |
|
|
36 |
||
37 |
||
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
38 |
|
49 |
||
51 |
||
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
109 |
|
109 |
||
109 |
||
Disclosure Regarding Foreign Jurisdictions That Prevent Inspections |
110 |
|
|
|
|
Part III |
|
|
111 |
||
111 |
||
Security Ownership of Certain Beneficial Owners and Management and Related Shareholder Matters |
111 |
|
Certain Relationships and Related Transactions, and Director Independence |
111 |
|
111 |
||
|
|
|
Part IV |
|
|
112 |
||
115 |
||
|
116 |
|
1
Any statements contained in this Annual Report on Form 10-K that are not statements of historical fact may be deemed to be forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. Without limiting the foregoing, the words “believe,” “anticipate,” “plan,” “expect,” “seek,” “may,” “will,” “intend,” “estimate,” “should,” and similar expressions are intended to identify forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding:
Any forward-looking statements contained herein are based on current expectations but are subject to a number of risks and uncertainties, and the Company’s actual results may differ significantly from the results discussed in the forward-looking statements. Factors that might cause such differences include, but are not limited to, continued volatility in the capital markets, the impact of increased interest rates, our ability to raise capital to support our operations on favorable terms, product development and market acceptance of our products, competition, rapidly changing technologies and product obsolescence, the effects of climate change, including natural disasters, catastrophic events and other events beyond our control, and those discussed in Part I, Item 1A of this Annual Report on Form 10-K under the heading “Risk Factors.” While we may elect to update forward-looking statements in the future, we specifically disclaim any obligation to do so, even if our estimates change, and readers should not rely on those forward-looking statements as representing our views as of any date subsequent to the date of the filing of this report. References to “we,” “us,” “our,” “management” or the “Company” refer to Bruker Corporation and, in some cases, its subsidiaries, as well as all predecessor entities.
Our principal executive offices are located at 40 Manning Road, Billerica, MA 01821, and our telephone number is (978) 663-3660. Information about Bruker Corporation is available at www.bruker.com. The information on our website is not incorporated by reference into and does not form a part of this report. All trademarks, trade names or copyrights referred to in this report are the property of their respective owners.
2
PART I
ITEM 1 BUSINESS
Our Business
We are a developer, manufacturer and distributor of high-performance scientific instruments and analytical and diagnostic solutions that enable our customers to explore life and materials at microscopic, molecular and cellular levels. Many of our products are used to detect, measure and visualize structural characteristics of chemical, biological and industrial material samples. Our products and solutions address the rapidly evolving needs of a diverse array of customers in life science research, pharmaceuticals, biotechnology, applied markets, cell biology, clinical research, microbiology, in-vitro diagnostics, nanotechnology and materials science research. Our technology platforms include magnetic resonance technologies, mass spectrometry technologies, gas and liquid chromatography, triple quadrupole mass spectrometry technologies, X-ray technologies, spark-optical emission spectroscopy, atomic force microscopy, stylus and optical metrology technology, fluorescence optical microscopy, and infrared and Raman molecular spectroscopy technologies. Our product portfolio also includes testing solutions used in microbiology and infectious disease diagnostics, including our MALDI Biotyper rapid pathogen identification platform and related test kits, DNA test strips and fluorescence-based polymerase chain reaction (PCR) technology for selected infectious disease applications. We develop, manufacture and distribute a range of field analytical systems for chemical, biological, radiological, nuclear and explosives, or CBRNE detection. We also develop, manufacture and market low temperature superconducting materials and devices based primarily on metallic low temperature superconductors. Our corporate headquarters are located in Billerica, Massachusetts. We maintain major technical and manufacturing centers in Europe, North America and Southeast Asia, and have sales offices located throughout the world.
Business Segments
We have four reportable segments, Bruker Scientific Instruments (BSI) BioSpin, BSI CALID, BSI Nano, and Bruker Energy & Supercon Technologies (BEST).
BSI BioSpin Segment
The BSI BioSpin Segment comprises the Bruker Magnetic Resonance, Applied Industrial and Clinical, Preclinical Imaging and Service and Lifecycle Support and Integrated Data Solution Divisions. BSI BioSpin designs, manufactures and distributes enabling life science tools based on magnetic resonance technology. Magnetic resonance is a natural phenomenon occurring when a molecule placed in a magnetic field emits a signature radio frequency. The signature radio frequency is characteristic of the particular molecule and provides a multitude of precise chemical and structural information. Depending on the intended application, we market and sell to our customers an NMR system or an EPR system (each as defined below). BSI BioSpin also manufactures and sells single and multiple modality systems using MRI, PET, SPECT, CT and MPI Technologies (each as defined below).
BSI BioSpin’s products, which have particular application in structural proteomics, drug discovery, pharmaceutical and biotechnology research and production, and the food and materials science fields, provide customers with the ability to determine the structure, dynamics, and function of specific molecules, such as proteins, and thus allows them to understand fundamental biological processes including the formation and progression of diseases. Furthermore, BSI BioSpin’s product enables the characterization of mixtures and also complex materials, rendering them high value-added tools for a variety of industrial applications.
BSI BioSpin also develops system agnostic software solutions. Software offerings include analytical instrumentation and data management support for life sciences and other industries to accelerate research, product development and process optimization.
The majority of BSI BioSpin’s customers are academic and government research facilities. Other customers include pharmaceutical and biotechnology companies; chemical, food and beverage, clinical and polymer companies; and nonprofit laboratories.
3
We continued to enable access to our high-performance GHz NMR technology. Four additional GHz NMR systems, representing the top end of our portfolio, were accepted by our customers (two 1.2 GHz, one 1.1 GHz, and one 1.0 GHz). This was supported by the launch of a unique single-story 1.0 GHz system that significantly reduces footprint, weight, and ceiling height requirements and cuts liquid helium consumption by two-thirds. In the applied markets, we made significant progress with our new solutions that are built on the benchtop Fourier NMR platform, such as reaction monitoring in chemical and pharmaceutical research as well as GxP solutions for quality control in manufacturing. In our clinical segment, we launched an NMR assay for Research Use Only for molecular phenomics research on blood samples from ‘Long COVID’ patients. In addition, the introduction of the new MRI portfolio for preclinical imaging markets eliminates the need for liquid helium or nitrogen refills while providing high field sensitivity and resolution for advanced MRI and PET/MR research.
BSI BioSpin Segment’s instruments are based on the following technology platforms:
NMR is a qualitative and quantitative analytical technique that is used to determine the molecular structure and purity of a sample. Molecules are placed in a magnetic field and give off a radio frequency signature that is recorded by a sensitive detector. Analysis software helps to determine the molecular structure of the sample. The NMR technique is used in academia, by pharmaceutical, biotechnology, food and beverage and clinical companies, and by other industrial users in life science and material science research.
EPR is a process of absorption of microwave radiation by paramagnetic ions or molecules with at least one unpaired electron that spins in the presence of a static magnetic field. EPR detects unpaired electrons unambiguously, whereas other techniques can only provide indirect evidence of their presence. In addition, EPR can identify the paramagnetic species that are detected, which present information on the molecular structure near the unpaired electron and give insight into dynamic processes such as molecular motions or fluidity. Our EPR instruments are used for a wide range of applications, including advanced materials research, materials analysis and quality control.
MRI is a process of creating an image from the manipulation of hydrogen atoms in a magnetic field. In the presence of an external magnetic field, atoms will align with or against the external magnetic field. Application of a radio frequency causes the atoms to jump between high and low energy states. MRI and magnetic resonance spectroscopy, or MRS, include many methods including diffusion-weighted, perfusion-weighted, molecular imaging and contrast-enhance. MRI offers high resolution morphologic information, as well as functional, metabolic or molecular information. Customers use our MRI systems in pharmaceutical research, including metabolomics, to study a number of diseases, including diabetes, neurology, oncology and cardiovascular disorders.
MPI is a process of creating an image from magnetic particles administered to the body of an animal. The magnetic particles are manipulated in a combination of oscillating magnetic fields exhibiting a field free zone. The response of the particles allows a real time 3D data set acquisition of the whole body of an animal, showing the contrast agent distributing in and flowing through the body. This imaging modality is used to detect cardiovascular disorders.
PET is a process of creating an image from positrons after administration of a positron emitting radionuclide to the body of an animal. Annihilation of the positron produces two photons which show an angle of 180° between them, distinguishing these photons from photons originating from other sources. The PET tracer enriches in certain regions of interest within the body and gains molecular information from the animal in vivo. This has widespread applications, most importantly for oncology, inflammation, neurology and cardiovascular disorders, as well as metabolic disease, drug discovery and bone disease.
SPECT uses a contrast agent containing radionuclides which directly emit single photons. The contrast agent enriches in certain parts of the body of an animal and generates images of the radionuclide distribution in the body. SPECT has widespread application in animal investigations in vivo, most importantly in oncology, neurology and cardiovascular disorders.
CT is a technology based on X-rays which are used to generate a complete 3D data set. The most important applications are tissue sample analysis or non-invasive in vivo animal imaging. CT offers the highest spatial resolution of all preclinical imaging modalities and is especially useful to generate morphological information about the object or animal under investigation. CT is being used in a wide range of preclinical investigations in the fields of bone-orthopedics, cardiology, pulmonology, oncology and metabolism among others.
4
The BSI BioSpin Segment also offers a range of services, product lifecycle support, scientific software and workflow solutions to customers who use BSI BioSpin products.
BSI CALID Segment
The BSI CALID Segment comprises the Bruker Life Sciences Mass Spectrometry, Bruker Applied Mass Spectrometry, Bruker Microbiology and Diagnostics and Bruker Optics Divisions.
The Bruker Life Sciences Mass Spectrometry primarily designs, manufactures and distributes life science mass spectrometry, or MS, instruments that can be integrated and used along with sample preparation or chromatography instruments to design an analytical workflow and mass spectrometry-based solutions including informatics software. Bruker Life Science Mass Spectrometry products are used in research, pharmaceutical and biotechnology development.
Bruker Applied Mass Spectrometry primarily designs solutions based on mass spectrometry for the food, environmental, forensics, clinical research, and industrial markets. Analytical areas such as toxicology, safety, authenticity, adulteration, quality control of starting and finished goods are amongst the applications covered and are used across industrial, government and academic institutes.
Mass spectrometers are sophisticated devices that measure the mass or weight of a molecule and can provide accurate information on the identity, quantity and primary structure of the molecule. Mass spectrometry-based solutions often combine advanced mass spectrometry instrumentation, automated sampling and sample preparation robots, reagent kits and other disposable products used in conducting tests, or assays, and bioinformatics software. We offer mass spectrometry systems and integrated solutions for applications in multiple existing and emerging life science markets and chemical and applied markets, including expression proteomics, clinical proteomics research, metabolic and peptide, lipid or glycan biomarker profiling, drug discovery and development, molecular diagnostics research and molecular and systems biology, as well as basic molecular medicine research.
The Bruker Microbiology and Diagnostics Division develops, manufactures and distributes innovative solutions for microbial identification, antibiotic resistance and susceptibility testing, polymerase chain reaction (PCR) based molecular diagnostic solutions for culture-free infectious disease diagnostics, as well as monoclonal antibodies and recombinant proteins as raw materials for diagnostic assays. Bruker Microbiology and Diagnostics solutions are used primarily in the human and veterinary clinical diagnostic, food microbiology and pharma microbiology settings.
Our MALDI Biotyper mass spectrometry solution and test kits, DNA test strips and fluorescence-based PCR technologies are designed for in-vitro diagnostic (IVD) use in clinical microbiology markets in certain configurations and certain countries, where regulatory approvals have been achieved. In addition to culture-based microbial identification with the MALDI Biotyper platform, the Genotype and Fluorotype molecular diagnostics (MDx) kits enable a culture-free detection and analysis of microbes and viruses directly from patient samples with a special focus on tuberculosis, HIV viral load, viral hepatitis and sexually transmitted diseases.
Molecular Diagnostics utilize PCR assays and systems to provide diagnostic solutions for a number of different disease states, including respiratory, mycobacteria (including tuberculosis), virology, safety of immunocompromised patients, sexually transmitted infections, gastroenteric diseases as well as other microbiology tests. Depending on the assay being used, the technology enables users to ascertain basic identification of a certain infection, distinguish infections which can cause similar symptoms and detect specific microbial resistance, all from a single sample. The GenoType portfolio has been established for over 30 years and has been successful in mycobacteria and tuberculosis detection, differentiation, and identification of antibiotic resistance markers. The portfolio now includes FluoroType®, using fluorescence-based real-time PCR technology, and more recently we have also developed LiquidArray® assays based on melt curve analysis for optimized asymmetrical PCR technology. LiquidArray® uses light-on-off probes, providing a powerful technology to identify a broad number of indicators for different infections or resistance markers from a single sample, providing greater depth of information. We are applying this approach to a new portfolio of syndromic panels for the diagnostics of sexually transmitted diseases and gastro-intestinal diseases. As a producer of extraction chemistry and instrumentation alongside integrated thermocyclers, software and a range of assays, Bruker brings complete diagnostic solutions to the Molecular Diagnostics market.
The Bruker Optics Division primarily designs, manufactures and distributes research, analytical and process analysis instruments and solutions based on infrared and Raman molecular spectroscopy and imaging technologies. These products are utilized in industry, government and academia for a wide range of applications and solutions for life science, pharmaceutical, food and agricultural analysis, quality control and process analysis applications. Infrared and Raman spectroscopies are widely used in both research and industry as simple, rapid, nondestructive and reliable techniques for applications ranging from basic sample identification and quality control to advanced research and also in remote sensing setups for environmental control. The Bruker Optics Division also utilizes Fourier transform and dispersive Raman measurement techniques on an extensive range of laboratory and process spectrometers. The Bruker Optics Division’s products are complemented by a wide range of sampling accessories and techniques, which include, among others, microanalysis and high-throughput screening to help users find suitable solutions to analyze their samples effectively.
5
Customers of our BSI CALID Segment include pharmaceutical, biotechnology and diagnostics companies, contract research organizations, academic institutions, medical schools, nonprofit or for-profit forensic laboratories, agriculture, food and beverage safety, environmental and clinical microbiology laboratories, hospitals, and government departments and agencies.
The Bruker LSMS Division has launched a number of new mass spectrometry-based solutions and additional workflows, including the timsTOF Ultra mass spectrometer, the evolution of our timsTOF SCP, enabling researchers to analyze proteins from even sub-single cell amounts allowing for increased throughput in immunopeptidomic workflows while also increasing linear dynamic range. The microGRID capability for the timsTOF flex and MALDI-2 platforms allowing 5 um spatial resolution for tissue imaging. In tissue, protein analysis using MALDI Imaging applications were enhanced by the launch of the MALDI Hi-Plex Immunohistochemistry (IHC) labels that can potentially allow for multiplexing hundreds of proteins in a single MALDI-TOF analysis. The photocleavable mass tag approach used in the HiPlex IHC technique are uniquely formulated for high-sensitivity MALDI mass spectrometry analysis. In 2022, Prolab AG was acquired, a Swiss-based, microfluidics components manufacturer whose components are an integral part of the nanoElute nanoflow LC system and PepSep, a nanocolumns manufacturer to augment the proteomics workflow for the timsTOF platform. This was further augmented by the 2022 acquisition of PreOmics, a company specializing in sample preparation with their iST-workflow for efficient and automatable sample clean-up as well as the beatbox enabling easy-to-use tissue lysis. The 2023 acquisition of the Swiss-based Biognosys AG further enhances the post-processing portfolio with their industry leading software Spectronaut for DIA data analysis as well as granting access to important quality control tools like the iRT Kits, rounding out the workflow options from sample preparation until final data processing and interpretation. In 2023 Fasmatech was acquired gaining an additional R&D site in Athens as well as access to the Omnitrap technology allowing research into addition fragmentation methods. Since its launch in 2016, the timsTOF has evolved into six variants, namely the Pro 2, the HT, the SCP, the Ultra, the fleX and the fleX MALDI-2 platforms which showcases the power of trapped ion mobility for life sciences applications such as multiomics, tissue imaging, single cell analysis and immunopeptidomics.
In 2023, the newly formed BAMS Division, launched its first dedicated solution based on the EVOQ DART TQ+ which focused on driving chromatography-free mass spectrometry as a faster and greener analytical innovation to move away from lengthy chromatographic methods. One of the initial key strategies centers on addressing the clinical and forensic toxicology market in the USA, aided by the acquisition of PinPoint with their ToxBox technology.
In our microbiology and molecular diagnostics markets, we introduced the MALDI Biotyper Sirius broadly into the market. In our molecular diagnostics portfolio, we launched two assays in the field of respiratory disease testing, primarily covering SARS-CoV 2 testing for the diagnosis of COVID-19 infection. The Fluorotyper-SARS-CoV 2 plus kit allows for a real-time PCR detection of the SARS-CoV 2 virus. It detects two viral genes in parallel as a mechanism for high sensitivity. The additional FluoroType® SARS-CoV-2/Flu/RSV assay is a multiplex real-time PCR kit that detects four viruses of clinical significance causing respiratory disease during the winter season: SARS-CoV 2, influenza A, influenza B and respiratory syncytial virus (RSV).
Our Bruker Optics division launched LUMOS II during 2020, a fully automated stand-alone FTIR imaging microscope. LUMOS II provides ultrafast FTIR imaging capabilities based on modern focal plane array (FPA) detector technology. The novel LUMOS II is designed to identify particles, to determine coatings and contamination, and to reveal the polymeric composition of plastics. During 2022, the Bruker Optics division launched HYPERION II with QCL microscopy (ILIM) as the leading IR microscope, which provides IR imaging down to the diffraction limit. HYPERION II ILIM combines FT-IR and Infrared Laser Imaging microscopy for the first time in a single device, offering all three measurement modes: transmission, reflection, and ATR. With Hyperion II ILIM users can access an IR microscope that combines FT-IR and QCL technology in one instrument. During 2023 the Bruker Optics division launched Mobile IR II, a mobile FT-IR spectrometer with laboratory performance, and Matrix-F II, a dedicated FT-NIR process spectrometer. Mobile IR II is one of the only portable FT-IR spectrometers with true lab-grade spectral performance. MATRIX-F II is the only FT-NIR spectrometer which can measure material in contact as well as contactless with just one instrument using light fiber technology. It allows direct measurements in process reactors and pipelines, leading to a better understanding and control of the process.
The BSI CALID Segment’s instruments are based on the following technology platforms:
6
MALDI-TOF mass spectrometers utilize an ionization process to analyze solid samples using a laser that combines high sample throughput with high mass range and sensitivity. Our MALDI-TOF mass spectrometers are particularly useful for applications in clinical diagnostics, environmental and taxonomical research and food processing and quality control. Specific applications include: oligonucleotide and synthetic polymer analysis; protein identification and quantification; peptide de novo sequencing; determination of post-translational modifications of proteins; interaction proteomics and protein function analysis; drug discovery and development; and fast body fluid and tissue peptide or protein biomarker detection. MALDI mass spectrometry allows users to classify and identify microorganisms quickly and reliably with minimal sample preparation efforts and life cycle costs. Our MALDI Biotyper solution, which serves the clinical microbiology market, enables identification, taxonomical classification or dereplication of microorganisms like bacteria, yeasts and fungi.
ESI-TOF mass spectrometers utilize an electrospray ionization process to analyze liquid samples. This ionization process, which does not dissociate the molecules, allows for rapid data acquisition and analysis of large biological molecules. ESI-TOF mass spectrometers are particularly useful for: identification, protein analysis and functional complex analysis in proteomics and protein function; molecular identification in metabolomics, natural product and drug metabolite analysis; combinatorial chemistry high throughput screening; and fast liquid chromatography mass spectrometry, or liquid chromatography mass spectrometry (LC-MS), in drug discovery and development.
MRMS systems utilize high-field superconducting magnets to offer the highest resolution, selectivity, and mass accuracy currently achievable in mass spectrometry. Our systems based on this technology often eliminate the need for time-consuming separation techniques in complex mixture analyses. In addition, our systems can fragment molecular ions to perform exact mass analysis on all fragments to determine molecular structure. MRMS systems are particularly useful for: the study of the structure and function of biomolecules, including proteins, DNA and natural products; complex mixture analysis including body fluids or combinatorial libraries; high-throughput proteomics and metabolomics; and top-down proteomics of intact proteins without the need for enzymatic digestion of the proteins prior to analysis. We offer next-generation hybrid MRMS systems that combine a traditional external quadrupole mass selector and hexapole collision cell with a high-performance MRMS for further ion dissociation, top-down proteomics tools and ultra high-resolution detection.
ITMS systems collect all ions simultaneously, which improves sensitivity relative to previous quadrupole mass spectrometers. Ion trap mass spectrometers are particularly useful for sequencing and identification based on peptide structural analysis, quantitative liquid chromatography mass spectrometry, identification of combinatorial libraries and generally enhancing the speed and efficiency of the drug discovery and development process.
GC-MS systems combine the features of gas chromatography and mass spectrometry to identify different substances within a test sample. The two components, used together, allow for a finer degree of substance identification than either system when used separately. The result is a quantitative analysis of the components and the mass spectrum of each component. Our GC-MS systems are available in triple quadrupole configurations and can be configured with a variety of options to suit a range of applications. Our GC-MS systems have applications in food and product safety, forensics, clinical and toxicology testing and environmental, pharmaceutical and chemical analysis.
LC-MS systems combine the separation features of liquid chromatography with the molecular identification features of mass spectrometry to separate, identify and quantify different substances within a test sample. As a complementary technique to GC-MS, which analyzes volatile compounds, LC-MS can be used to analyze a wide range of non-volatile compounds in complex samples. Our LC-MS systems are available in a wide range of configurations to suit a user’s specific needs. Although primarily used for life science applications, our LC-MS systems also have applications in food and product safety, forensics and clinical and toxicology testing, as well as environmental, pharmaceutical and chemical analysis.
FT-IR spectrometers utilize the mid- and far-infrared regions of the electromagnetic spectrum. Our FT-IR systems are commonly used for various quality control and materials research applications.
7
NIR spectrometers utilize the near-infrared region of the electromagnetic spectrum. Our NIR instruments are primarily used for quality and process control applications in the pharmaceutical, food and agriculture and chemical industries. The pharmaceutical industry is the leading user of NIR instruments, and applications include quality control, research and development and process analytical technology. The food and agricultural industry is the second largest user of NIR instrumentation, with an increasing demand for food, feed and beverage quality control.
PCR the innovative LiquidArray® technology optimizes asymmetrical multiplex PCR for creating excess single-stranded amplicons with detection by lights-on/-off probes that contain a quencher (lights-off) or both fluorophore and quencher (lights-on). During melting curve analysis, lights-on/-off probes detach from the amplicon at specific temperatures and as fluorescence is either emitted or suppressed, specific fluorescence signatures are generated by the unique FluoroCycler®XT thermocycler for the LiquidArray® multiplex PCR technology. The LiquidArray® technology supports multiplexed assays where a large number of targets is analyzed simultaneously from single samples. For example, the LiquidArray®-powered, WHO-endorsed FluoroType®MTDBR VER 2.0 assay detects more than 500 genotypes by the combined analysis of up to 45 different mutations in mycobacteria.
Raman spectroscopy provides information on molecular structure. The mechanism of Raman scattering is different from that of infrared absorption, in that Raman and IR spectra provide complementary information. Raman is useful for the identification of both organic and inorganic compounds and functional groups. It is a nondestructive technique and can be used for the analysis of both liquids and solids. Raman is well suited for use in the polymer and pharmaceutical industries, and has applications in the metals, electronics and semiconductors industries. The technique also has applications in life sciences, forensics and artwork authentication.
Additionally, the Bruker Detection product line offers a wide range of portable analytical and bioanalytical detection systems and related products for CBRNE detection. Our customers use these devices for nuclear, biological agent and chemical agent defense applications, anti-terrorism, law enforcement and process and facilities monitoring. Our CBRNE detection products use many of the same technology platforms as our life science products, as well as additional technologies, including infrared stand-off detection and ion mobility spectrometry, for handheld chemical detectors. We also provide integrated, comprehensive detection suites that include our multiple detection systems, consumables, training and simulators.
BSI Nano Segment
The BSI Nano Segment comprises the Bruker AXS, Bruker Nano Analytics, Bruker Nano Surfaces and Metrology divisions, Consolidated Fluorescence Microscopy, Canopy Biosciences and Acuity Business Units. The Bruker AXS Division designs, manufactures and distributes advanced X-ray instruments that use electromagnetic radiation with extremely short wavelengths to determine the characteristics of matter and the three-dimensional structure of molecules. This includes a product portfolio of instruments based on X-ray fluorescence spectroscopy (XRF), X-ray diffraction (XRD) and X-ray micro computed tomography (µCT), or X-ray microscopy, as well as spark optical emission spectroscopy systems (S-OES) used to analyze the concentration of elements in metallic samples.
The Bruker Nano Analytics Division manufactures and markets analytical tools for electron microscopes, including energy-dispersive X-ray spectrometers (EDS), electron backscatter diffraction systems (EBSD) and µCT accessories, as well as mobile and bench top micro X-ray fluorescence (µXRF), total reflection X-ray fluorescence spectrometers (TXRF) and handheld, portable and mobile X-ray fluorescence (HMP-XRF) spectrometry instruments.
The Bruker Nano Surfaces and Metrology Division’s products include atomic force microscopy instrumentation (AFM). Such instruments provide atomic or near atomic resolution of surface topography and nanoscale, mechanical, electrical and chemical information using nano scale probes. The Bruker Nano Surfaces and Metrology Division also provides non-contact nanometer resolution solution topography through white light interferometry and stylus profilometry. In addition, the division manufacturers and markets automated X-ray metrology, automated AFM defect-detection and photomask repair and cleaning equipment for semiconductor process control.
The Consolidated Fluorescence Microscopy Business Unit provides advanced optical fluorescence microscopy instruments with multi-photon, multipoint scanning confocal, miniature head-mount, 3D super-resolution, light-sheet modalities for studies in life science applications.
The Canopy Business Unit provides products and services to support the multi-omics needs of researchers in translational research, drug and biomarker discovery.
Customers of our BSI Nano Segment include academic institutions, governmental customers, nanotechnology companies, semiconductor companies, raw material manufacturers, industrial companies, biotechnology and pharmaceutical companies, and other businesses involved in materials analysis.
During 2023, we launched several new products including D6 Phaser, TI 990, Infinite Focus G6, and NanoMet.
8
In October 2023 we acquired PhenomeX Inc., a functional cell biology company renamed Bruker Cellular Analysis or “BCA” that provides single-cell biology research tools to deliver deep insights into cellular function and new perspectives on phenomes and genotype-to-phenotype.
The BSI Nano Segment systems are based on the following technology platforms:
XRD systems investigate polycrystalline samples or thin films with single wavelength X-rays. The atoms in the polycrystalline sample scatter the X-rays to create a unique diffraction pattern recorded by a detector. Computer software processes the pattern and produces a variety of information, including stress, texture, qualitative and quantitative phase composition, crystallite size, percent crystallinity and layer thickness, composition, defects and density of thin films and semiconductor material. Our XRD systems contribute to a reduction in the development cycles for new products in the catalyst, polymer, electronic, optical material and semiconductor industries. Customers also use our XRD systems in academic and government research, as well as in a variety of other fields, including forensics, art and archaeology.
XRF systems determine the elemental composition of a material and provide a full qualitative and quantitative analysis. Our XRF systems direct X-rays at a sample, and the atoms in the sample absorb the X-ray energy. The elements in the sample then emit X-rays that are characteristic for each element. The system collects the X-rays, and the software analyzes the resulting data to determine the elements that are present. Our XRF products provide automated solutions on a turn-key basis for industrial users that require automated, controlled production processes that reduce product and process cost, increase output and improve product quality. Our XRF products cover substantially all of the periodic table and can analyze solid, powder or liquid samples.
SC-XRD systems determine the three-dimensional structures of molecules in a chemical, mineral, or biological substance being analyzed. SC-XRD systems have the capability to determine structure in both small chemical molecules and larger biomolecules. SC-XRD systems direct an X-ray beam at a solid, single crystal sample. The atoms in the crystal sample scatter the X-rays to create a precise diffraction pattern recorded by an electronic detector. Software then reconstructs a model of the structure and provides the unique arrangement of the atoms in the sample. This information on the exact arrangement of atoms in the sample is a critical part of molecular analysis and can provide insight into a variety of areas, including how a protein functions or interacts with a second molecule. Our SC-XRD systems are designed for use in the life sciences industry, academic research and a variety of other applications.
µCT is X-ray imaging in 3D, by the same method used in hospital CT scans, but on a small scale with massively increased resolution. 3D microscopy allows users to image the internal structure of objects non-destructively on a very fine scale. Bruker µCT is available in a range of easy-to-use desktop instruments, which generate 3D images of the sample’s morphology and internal microstructure with resolution down to the sub-micron level. Our µCT systems are used for numerous applications in materials research and in the life sciences industry.
9
EDS systems analyze the chemical composition of materials under investigation in electron microscopes by utilizing the fact that atoms of different chemical elements, when exposed to the high energy electron beam generated by the microscope, irradiate X-rays of different characteristic energy. The evaluation of the energy spectrum collected by our spectrometer allows the determination of the qualitative and quantitative chemical sample composition at the current beam position. EDS systems allow for simultaneous analysis of all elements in the periodic table, beginning with atomic number 4 (beryllium). Our EDS systems are used for a range of applications, including nanotechnology and advanced materials research, as well as materials analysis and quality control. Customers for EDS systems include industrial customers, academia and government research facilities.
EBSD systems are used to perform quantitative microstructure analysis of crystalline samples in electron microscopes. The microscope’s electron beam strikes the tilted sample and diffracted electrons form a pattern on a fluorescent screen. This pattern is characteristic of the crystal structure and orientation of the sample region from which it was generated. It provides the absolute crystal orientation with sub-micron resolution. EBSD can be used to characterize materials with regard to crystal orientation, texture, stress, strain, and grain size. EBSD also allows the identification of crystalline phases and their distribution and is applied to many industries such as metals processing, aerospace, automotive, microelectronics and earth sciences.
S-OES instruments are used for analyzing metals. S-OES covers a broad range of applications for metals analysis from pure metals trace analysis to high alloyed grades and allows for analysis of a complete range of relevant elements simultaneously. S-OES instruments pass an electric spark onto a sample, which burns the surface of the sample and causes atoms to jump to a higher orbit. Our detectors quantify the light emitted by these atoms and help our customers to determine the elemental composition of the material. This technique is widely used in production control laboratories of foundries and steel mills.
CS/ONH carrier gas systems incorporate a furnace and infrared or thermal conductivity detection to analyze inorganic materials for the determination of carbon, sulfur, nitrogen, oxygen and hydrogen. Combustion and inert gas fusion analyzers are used for applications in metal production and processing, chemicals, ceramics and cement, coal processing, oil refining and semiconductors.
AFM systems provide atomic or near-atomic resolution of material surface topography using a nano-scale probe that is brought into light contact with the sample being investigated. In addition to presenting a surface image, AFM can also provide quantitative nano-scale measurements of feature sizes, material properties, electrical information, chemical properties and other sample characteristics. Our AFM systems are used for applications in academic and governmental materials and biological research and semiconductor, data storage hard drive, LED, battery, solar cells, polymers, and pharmaceutical product development and manufacturing.
FM products use fluorescence microscopy to determine the structure and composition of life science samples. Our products include two-photon microscopes, multipoint scanning confocal microscopes, miniature head-mounted microscopes, super-resolution microscopes, light-sheet microscopes, laser illumination sources, photoactivation, photostimulation and photoablation accessories and synchronization and analysis software. Two-photon microscopes allow imaging deep into tissues and cells and are used widely in neuroscience. Multipoint scanning confocal systems allow live cell imaging with rapid acquisition of images for structural and composition analysis. Miniature head-mount microscopes allow monitoring of animal brain activity during free-roaming, naturalistic behavior at cellular level. Super-resolution and single-molecule localization microscopy products allow imaging below the optical diffraction limit by an order of magnitude. Light-sheet based products allow fast 3D volume imaging with very low phototoxicity and photo-damage effects enabling live cell and large volume imaging.
SOM systems provide atomic or near-atomic two dimensional and three-dimensional surface resolution using white light interferometry, confocal optical and stylus profilometry methods. SOM profilers range from low-cost manual tools for single measurements to advanced, highly automated systems for production line quality assurance and quality control applications where the combination of throughput, repeatability and reproducibility is essential. SOM profilers support a range of applications in research, product development, tribology, quality control and failure analysis related to materials and machining in the automotive, orthopedic, ophthalmic, high brightness LED, semiconductor, data storage, optics and other markets.
TMT systems provide a platform for all types of common mechanical, friction, durability, scratch and indentation tests for a wide spectrum of materials. Tribology systems are utilized for both academic research of the fundamental material properties and industrial applications in the semiconductor, aerospace, petroleum, automotive and other industries.
NanoIR systems perform infrared (IR) spectroscopy at the nanoscale. Our systems use nanoprobe technology similar to what is used in our atomic force microscopes to deliver quantitative chemical information from the nanoscale to the sub-micron and macro scales. The NanoIR measurement gives the user varying physical and chemical properties with nanoscale spatial resolution in a diverse range of fields, including polymers, 2D materials, materials science, life science and the micro-electronics industry. Our systems allow nanoscale IR absorption spectroscopy with interpretable IR spectra that directly correlates to FTIR as well as the complementary technique of nanoscale s-SNOM. With our broadband sources, these systems allow broadband scientific spectroscopy.
10
Alicona systems combine the functionalities of a micro coordinate measurement machine (CMM) with those of a surface measurement system. These dimensional metrology systems are based on the pioneering development of optical focus-variation measurement algorithms and provide the noncontact measurement of form and roughness of complex, miniaturized geometries. These systems serve many quality assurance application areas requiring precision measurement and dimensional metrology, including aerospace, automotive, precision medical products, additive manufacturing, and micro precision manufacturing.
Canopy provides spatial profiling services and instruments which include both our CellScape instrument and ChipCytometry service for quantitative, high plex, targeted spatial proteomics in single cell and tissues. These technologies, along with Canopy’s more basic IHC and FISH services, allow researchers to elucidate gene and protein expression in a spatial context, which is useful for deep biological insight into gene expression and for the development of biomarkers. Canopy also provides transcriptional profiling services covering a variety of assays, including RNASeq and qPCR. Our multi-omic services provide data elucidating gene expression, signaling pathways, and differential expression trends on customer provided biological samples. These services generally incorporate a data analysis service as well and can be utilized with multiple types of samples from very early discovery research through clinical trials.
BCA provides customers with, among other offerings, Optofluidic platforms such as the Beacon and Beacon Select as well as Proteomic Barcoding platforms, such as the IsoLight System and the IsoSpark System. These platforms provide scientists with opportunities to perform cell biology research through experiments using our proprietary consumables.
BEST Segment
The BEST Segment designs, manufactures and distributes superconducting materials, primarily metallic low temperature superconductors, for use in magnetic resonance imaging, nuclear magnetic resonance, fusion energy research and other applications. Additionally, BEST develops, manufactures and markets sophisticated devices and complex tools based primarily on metallic low temperature superconductors that have applications in “big science” research, including radio frequency accelerator cavities and modules, power couplers and linear accelerators. BEST also manufactures and sells non-superconducting high technology tools, such as synchrotron and beamline instrumentation, principally to customers engaged in materials research and “big science” research projects.
Sales and Marketing
We maintain direct sales forces throughout North America, Europe, China, Japan, and elsewhere in the Asia Pacific region. We also utilize indirect sales channels to reach customers. We have various international distributors, independent sales representatives and various other representatives in parts of Asia, Latin America, Africa, the Middle East and Eastern Europe. These entities augment our direct sales force and provide coverage in areas where we do not have direct sales personnel. In addition, we have adopted a distribution business model in which we engage in strategic distribution alliances with other companies to address certain market segments. The sales cycle for our products is dependent on the size and complexity of the system and budgeting cycles of our customers. Our sales cycle is typically three to twenty-four months for academic and high-end research products and two weeks to six months for industrial products. The sales cycle of our low temperature superconducting materials is typically four to twelve months, with cycles of certain high-end materials exceeding one year. Sales of our high-end NMR and superconducting devices typically take more than one year and certain large, complex contracts can take more than two years to complete.
We have well-equipped applications and demonstration facilities and qualified application personnel who assist customers and provide product demonstrations in specific application areas. We maintain our primary demonstration facilities at our production facilities, as well as in other key market locations.
Seasonal Nature of Business
Historically, we have higher levels of revenue in the fourth quarter and lower levels of revenues in the first quarter of the year, which we believe is influenced by our customers’ budgeting cycles.
Major Customers
We have a broad and diversified customer base and we do not depend on any single customer. No single customer accounted for more than 10% of revenue in any of the last three fiscal years through December 31, 2023, nor more than 10% of accounts receivable as of December 31, 2023, and 2022.
11
Competition
Our existing products and solutions and any products and solutions that we develop in the future may compete in multiple, highly competitive markets. In addition, there has been a trend towards consolidation in our industries and some of our competitors have substantially greater financial, technical and marketing resources than we do. Our competitors may succeed in developing and offering products that could render our products or those of our strategic partners obsolete or noncompetitive. Our competitors may also have cost and price advantages based upon the value of their currencies compared with the U.S. Dollar or Euro. In addition, some of these competitors have significantly more experience in the life sciences, chemical and materials markets. Our ability to compete successfully will depend on our ability to develop proprietary products that reach our target markets in a timely manner and are technologically superior to and/or less expensive, or more cost effective, than products marketed by our competitors. Current competitors or other companies may possess or develop technologies and products that are more effective than ours. Our technologies and products may be rendered obsolete or uneconomical by technological advances or by entirely different approaches developed by one or more of our competitors.
We also compete with companies that provide analytical, or automation tools based on technologies other than those we offer. These technologies may prove to be more successful in meeting demands in the markets that our products and solutions are intended to serve. In addition, other companies may choose to enter our fields in the future. We believe that the principal competitive factors in our markets are technology-based applications expertise, product specifications, functionality, reliability, marketing expertise, distribution capability, proprietary patent portfolios and cost effectiveness.
BSI BioSpin Segment Competition
The BSI BioSpin Segment competes with companies that offer magnetic resonance spectrometers, mainly JEOL, QOne Instruments, Ciqtek, Magritek, Nanalysis and Oxford Instruments. In the field of preclinical imaging, BioSpin competes with PerkinElmer Inc., Mediso, Trifoil, MR Solutions and others.
BSI CALID Segment Competition
The BSI CALID Segment competes with a variety of companies that offer mass spectrometry-based and molecular spectrometry-based systems. BSI CALID’s competitors in the life science markets and chemical and applied markets include Danaher, Agilent, GE-Healthcare, Waters, Thermo Fisher Scientific, Shimadzu, Hitachi and JEOL. In the microbiology market, CALID competes with Biomerieux. In molecular diagnostics, CALID competes with a number of companies offering products for infectious disease diagnostics. CALID also competes with a variety of companies that offer molecular spectrometry-based systems, including Thermo Fisher Scientific, PerkinElmer, Agilent, Foss, ABB Bomem, Buchi, Shimadzu, Horiba, Rigaku and Jasco. CALID’s CBRNE detection customers are highly fragmented, and it competes with a number of companies in this area, of which the most significant competitor is Smiths Detection.
BSI Nano Segment Competition
The BSI Nano Segment competes with companies that offer analytical X-ray solutions, OES systems, AFM and SOM systems and optical fluorescence systems, primarily Rigaku, Oxford Instruments, Agilent, Thermo Fisher Scientific, Ametek’s Spectro and Edax divisions, PANalytical, Park Systems, Olympus, Nikon, Zeiss and Danaher’s Leica business.
BEST Segment Competition
BEST competes with Western Superconducting Technologies Co., Ltd. (WST), Luvata, and Jastec Co., Ltd. in low temperature superconducting materials. BEST further competes with Zanon, Mitsubishi Electric and AES in the development and supply of accelerator cavities, with Thales, Toshiba and CPI International in the development and supply of radio frequency couplers, with Mitsubishi Heavy Industries in the development and supply of superconducting accelerator modules and with AES and Thales for electron linear accelerators.
12
Manufacturing and Suppliers
Several of our manufacturing facilities are certified under ISO 9001:2008 and ISO 13485, international quality standards. We manufacture and test our magnetic resonance products at our facilities in Faellanden, Switzerland; Wissembourg, France; and Karlsruhe, Germany. We manufacture and test our preclinical imaging products at our facilities in Ettlingen and Leipzig, Germany; Wissembourg, France; Kontich, Belgium; and Faellanden, Switzerland. We manufacture and test our mass spectrometry products at our facilities in Bremen, Germany. We principally manufacture and test our molecular spectroscopy products, including CBRNE detection products, at our facilities in Ettlingen, Germany. We manufacture and test our X-ray, OES and AFM products at our facilities in Penang, Malaysia; Karlsruhe and Berlin, Germany; Santa Barbara, California, U.S.A.; and Migdal HaEmek, Israel. We manufacture and test the majority of our energy and superconducting products at our facilities in Hanau, Germany; Bergisch Gladbach, Germany; Perth, Scotland; and Carteret, New Jersey, U.S.A. Manufacturing processes at our facilities in Europe, Israel and California, U.S.A. include all phases of manufacturing, such as machining, fabrication, subassembly, system assembly, and final testing. Our other facilities primarily perform high-level assembly, system integration and final testing. We typically manufacture critical components in-house to ensure key competence and outsource to third party manufacturers non-critical components.
We purchase materials and components from various suppliers that are either standard products or built to our specifications. We obtain some of the components included in our products from a limited group of suppliers or from a single-source supplier for items such as charge coupled device area detectors, X-ray tubes, robotics, infrared optics and others. BEST has an ongoing collaboration and a joint technology development agreement with Allegheny Technologies Incorporated to advance state-of-the-art niobium-based superconductors, including those used in MRI magnets for the medical industry, and preclinical MRI magnets used in the life-science tools industry.
Research and Development
We commit substantial capital and resources to internal and collaborative research and development projects in order to provide innovative products and solutions to our customers. We conduct research primarily to enhance system performance and improve the reliability of existing products, and to develop revolutionary new products and solutions. Our research and development efforts are conducted for the relevant products within each of the operating segments, as well as in collaboration with others on areas such as microfluidics, automation and workflow management software. We have been the recipient of government grants from Germany and the United States for various projects related to early-stage research and development. We have generally retained, at a minimum, non-exclusive rights to any items or enhancements we develop under these grants. The German government requires that we use, and market technology developed under grants in order to retain our rights to the technology. We have also accepted some sponsored research contracts from private sources.
BSI BioSpin Segment Research and Development
The research and development performed in the BSI BioSpin Segment is primarily conducted at our facilities in Ettlingen, Germany; Faellanden, Switzerland and Wissembourg, France. The BSI BioSpin Segment maintains technical competencies in core magnetic resonance technologies and single- and multimodal imaging technologies and capabilities, including NMR, EPR, MRI, MPI, PET and CT. The most recent technological innovations included Bruker’s ultra-high-field class in the NMR and MRI product lines and the benchtop Fourier NMR platform. As part of our continuous development of systems and software, we have achieved major milestones in 2023 to make NMR and MRI technologies accessible to more labs, such as reduced siting requirements.
BSI CALID Segment Research and Development
The research and development performed in the BSI CALID Segment is primarily conducted at our facilities in Bremen and Ettlingen, Germany; Faellanden, Switzerland and Wissembourg, France. The BSI CALID Segment maintains technical competencies in core mass spectrometry technologies and capabilities, including: MALDI, ESI and EI/CI ion source, TOF, TOF/TOF, ion traps, MRMS, quadrupole and IMS analyzers and bioinformatics. Recent projects include the innovative timsTOF mass spectrometer for separation and analysis of unresolved compounds and conformations. The BSI CALID Segment also maintains technical competencies in core vibrational spectroscopy technologies and capabilities, including FT-IR, NIR and Raman.
BSI Nano Segment Research and Development
The research and development performed in the BSI Nano Segment is primarily conducted at our facilities in Karlsruhe, Berlin and Leipzig, Germany; Penang, Malaysia; Madison, Wisconsin, Eden Prairie, Minnesota, San Jose, Santa Barbara and Emeryville, California, and St. Louis, Missouri, U.S.A. The BSI Nano Segment maintains technical competencies in core X-ray technologies and capabilities, including detectors used to sense X-ray and X-ray diffraction patterns, X-ray sources and optics that generate and focus the X-rays, robotics and sample handling equipment that holds and manipulates the experimental material, and software that generates the structural data.
13
Recent projects include fluorescence microscopy with simultaneous, all-optical stimulation and imaging platforms for optogenetics neuroscience research and light sheet cell microscopy systems, which enable brain research and high-resolution live cell research and single-cell biology research tools to deliver deep insights into cellular function and new perspectives on phenomes and genotype-to-phenotype linkages. The BSI Nano Segment also has competencies in AFM technology, which involve sub-angstrom level position and motion control, as well as sub-pico newton force control. The BSI Nano Segment technologies also include 3D optical inference-based microscopy, stylus profilometry, tribology testing, nano-indentation, optical fluorescence two-photon microscopy, multipoint scanning microscopy, high-speed, 3D super-resolution florescence microscopy and spatial biology and single-cell targeted proteomics technologies. Recent innovations include elemental analyzer systems for advanced applications and research and simultaneous, all-optical stimulation and imaging platforms for neuroscience applications.
BEST Segment Research and Development
The research and development performed in the BEST Segment is primarily conducted at our facilities in Hanau and Bergisch Gladbach, Germany; and Carteret, New Jersey, U.S.A. BEST maintains technical competencies in the production and development of low and high temperature superconducting materials and devices.
Intellectual Property
Our intellectual property consists of patents, copyrights, trade secrets, know-how, and trademarks. Protection of our intellectual property is a strategic priority for our businesses because of the length of time and expense associated with bringing new products through the development process and to the marketplace. We have a substantial patent portfolio, and we intend to file additional patent applications as appropriate. We believe our owned and licensed patent portfolio provides us with a competitive advantage. This portfolio permits us to maintain access to a number of key technologies. We license our owned patent rights where appropriate. We intend to enforce our patent rights against infringers, if necessary. The patent positions of life sciences tools companies involve complex legal and factual questions. As a result, we cannot predict the enforceability of our patents with certainty. In addition, we are aware of the existence from time to time of patents in certain countries, which, if valid, could impair our ability to manufacture and sell products in these countries.
We also rely upon trade secrets, know-how, trademarks, copyright protection and licensing to develop and maintain our competitive position. We generally require the execution of confidentiality agreements by our employees, consultants, and other scientific advisors. These agreements provide that all confidential information made known during the course of a relationship with us will be held in confidence and used only for our benefit. In addition, these agreements provide that we own all inventions generated during the course of the relationship.
Government Contracts
We are a party to various government contracts. Under some of these government contracts, the government may receive license or similar rights to intellectual property developed under the contract. However, under the government contracts we enter, we generally receive at least non-exclusive rights to any items or technologies we develop. Although we transact business with various government agencies, we believe that no government contract is of such magnitude that a renegotiation of profits or termination of the contract or subcontracts at the election of the government would have a material adverse effect on our financial results.
Government Regulation
We are required to comply with federal, state, and local environmental protection regulations. We do not expect this compliance to have a significant impact on our capital spending, earnings or competitive position.
Our products are subject to the U.S. Food and Drug Administration’s, or the FDA’s, requirements for electronic radiation emitting products, which include requirements related to record-keeping and reporting; labeling; notification; product repairs, replacements and refunds; importation; and performance standards. For example, prior to introducing a product in the United States, our Bruker AXS subsidiary provides notice to the FDA in the form of a Radiation Safety Initial Product Abbreviated Report, which provides identification information and operating characteristics of the product. If the FDA finds that the report is complete, it provides approval in the form of what is known as an accession number. Bruker AXS may not market a product until it has received an accession number. In addition, Bruker AXS submits an annual report to the FDA that includes the radiation safety history of all products it sells in the United States. Bruker AXS is required to report to the FDA incidents of accidental exposure to radiation arising from the manufacture, testing, or use of any of its products. Bruker AXS also reports installations of its products to state government regulatory agencies responsible for the regulation of radiation emitting devices. For sales in Germany, Bruker AXS registers each product with the local authorities. In some countries where Bruker AXS sells systems, Bruker AXS uses the certificate that Bruker AXS obtained from the federal authorities in Germany to assist it in obtaining a license, registration or certificate, as applicable, from the country or its relevant authorities in which the sale occurs.
14
Our Bruker AXS subsidiary possesses low-level radiation materials licenses from the local radiation safety authority, Gewerbeaufsichtsamt Karlsruhe, for its facility in Karlsruhe, Germany, and from the local radiation safety authority, Kanagawa Prefecture, for its facility in Yokohama, Japan, as well as from various other countries in which it sells its products. Our Bruker Daltonics subsidiary possesses low-level radiation licenses for facilities in Billerica, Massachusetts and Leipzig, Germany. The U.S. Nuclear Regulatory Commission also has regulations concerning the exposure of our employees to radiation.
Certain of our clinical products are subject to regulation as medical devices in the United States by the FDA and by similar regulatory bodies in other countries where such products are sold. The regulatory requirements imposed by the FDA and other regulatory bodies govern a wide variety of product-related activities, from quality management, design and development to labeling, manufacturing, promotion, sales, and distribution. As such, we continually invest in our manufacturing infrastructure to gain and maintain certifications and registrations necessary for the relevant level of regulatory clearance. We also are required to maintain processes and systems for medical device product submissions. For example, our MALDI Biotyper CA system is subject to regulation by the FDA as a medical device and requires FDA premarket review and clearance via the 510(k) premarket notification process and our IVD-CE Certified MALDI BioTyper system is subject to regulation in the European Union under the provisions of Directive 98/79/EC and Regulation (EU) 2017/746. In addition, certain product changes, including changes to the product indications or label claims, could trigger the requirement for a new 510(k) or other FDA or foreign regulatory premarket submission. The process of preparing a premarket submission to, and obtaining marketing approval, authorization, or clearance from the FDA and comparable foreign regulatory authorities (including notified bodies in the EU) for new products, or for enhancements or modifications to existing products, could take a significant amount of time, require the expenditure of substantial financial and other resources, and require rigorous and expensive pre-clinical and clinical testing. Additionally, the FDA or comparable foreign regulatory authorities could impose limitations on the indications for use of our products. Should we pursue an FDA or comparable foreign regulatory authority clearance, authorization, or approval for a new device or device modification, we cannot be certain that we will receive required clearance, authorization, or approval on a timely basis or at all. The failure to receive clearance, authorization, or approval for significant new products or modifications to existing products on a timely basis or at all could have a material, adverse effect on our financial condition and results of operations.
Both before and after a medical device product is commercially released, we have ongoing responsibilities under FDA and foreign regulations. For example, we are required to comply with the FDA’s Quality System Regulation, which sets forth the good manufacturing requirements for medical devices. These include requirements related to design controls, production and process controls, process validation, purchasing controls, supplier oversight, complaint handling and investigation, corrective and preventative actions, and record-keeping. In addition, the FDA’s medical device reporting regulation requires us to provide information to the FDA whenever we become aware that there is evidence that reasonably suggests that a device may have caused or contributed to a death or serious injury or, that a malfunction occurred which would be likely to cause or contribute to a death or serious injury upon recurrence. We are also required to report to the FDA if we initiate a device removal (recall) or correction to reduce a risk to health posed by the device or to remedy a violation which may present a risk to health. The FDA and comparable foreign regulatory authorities also regulate the promotion and marketing of medical devices and require that manufacturers only make promotional claims or statements that are consistent with the indications and labeling cleared, authorized, or approved by the FDA or other regulatory authorities. The FDA and comparable foreign regulatory authorities may take enforcement action against us, should the FDA determine we have engaged in “off-label” promotion or other violative marketing activities.
The European Union Directive 98/97/EC (IVDD) was replaced by the Regulation (EU) 2017/746 of April 5, 2017, on in vitro diagnostic medical devices (IVDR). The IVDR became applicable in May 2022. The regime changes significantly with the IVDR. In comparison to the IVDD, the IVDR requires, among other things, more clinical evidence to demonstrate the claimed benefits and safety of the device in relation to its stated purpose, stricter classification and CE-marking requirements and ongoing post-market follow-up to ensure conformity. The IVDR also requires new databases to be set up to track which devices are CE marked and to register clinical studies and post-market monitoring. In addition, tracing is enhanced by a Unique Device Identification (UDI) System and through requirements on other economic operators in the supply chain. Our products that are currently approved under the IVDD, and not already placed on the market or put into service, must be recertified under the IVDR.
Backlog
Our backlog consists of firm orders under non-cancelable purchase orders received from customers. Total remaining performance obligations as of December 31, 2023, and 2022 was approximately $2,226.7 million and $2,258.8 million, respectively. The modest decrease in our backlog in 2023 compared to 2022 was primarily driven by lower BEST order bookings as of December 31, 2023. We generally experience varying revenue levels in the first three quarters of the year, while our fourth quarter revenues have historically been stronger than the rest of the year. As a result, backlog on any particular date can be indicative of our short-term revenue performance but is not necessarily a reliable indicator of long-term revenue performance.
15
Human Capital
We are committed to enabling scientists to make breakthrough discoveries and develop new applications that improve the quality of human life. Our employees are a critical component of that mission. We endeavor to attract, develop and retain top talent by offering our employees a challenging but rewarding work experience, as well as competitive compensation and benefits. Further, we strive to create a work environment that promotes integrity, respect and trust among our employees.
As of December 31, 2023, and 2022, we had 9,707 and 8,525 full-time employees worldwide, respectively, of which 1,792 and 1,410, respectively, were located in the United States. Our senior leadership team is 80% male and 20% female.
The table below provides our employees by functional area.
|
|
Number of Employees |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Production and distribution |
|
|
4,469 |
|
|
|
3,990 |
|
Selling and marketing |
|
|
2,313 |
|
|
|
2,070 |
|
General and administrative |
|
|
1,174 |
|
|
|
985 |
|
Research and development |
|
|
1,751 |
|
|
|
1,480 |
|
Total |
|
|
9,707 |
|
|
|
8,525 |
|
Diversity, Talent Retention and Development
Bruker has initiatives and programs to attract, develop and retain our talent tailored to specific employee populations and geographies, including leadership development programs, technical training, and other skill-based training.
Several leadership development programs are in place for global groups and functions where competency-based performance assessments are completed annually to identify employees who qualify as high performers and high potentials for advancement into key leadership positions. These individuals are provided with additional skill, mentoring, and project opportunities to strengthen their capabilities for career advancement as future successors across the organization.
Bruker has established a customized development program for talent providing them with an opportunity to develop their leadership skills over a period of 12 months by attending different modules, which are run in a hybrid format. Each participant is supported by a mentor during the program to facilitate their learning and advance their development.
In 2023, Bruker further expanded its online learning platform designed to support employee development. Bruker identified areas of growth opportunity through increased education and created specific training paths to support employee technical skill development. Learning modules were also integrated into quarterly training sessions to facilitate learning and knowledge sharing among users.
A global performance management process promotes regular feedback and coaching by managers to develop employees. Throughout the year, managers and employees engage in annual objective setting, mid-year reviews of performance as well as a year-end performance evaluation. Managers and employees participate in career goal conversations throughout the year. These discussions are critical tools that reinforce the alignment of employee activities and career goals throughout the year and provide employees opportunities to grow. Several talent assessment tools are used to support this dialogue and achieve career development goals.
Bruker continues to focus on employer branding initiatives to further improve visibility and attractiveness for potential candidates. In 2023 this included participation in local, regional and international career events providing an opportunity to engage with candidates about the innovative and exciting opportunities Bruker offers. Enhancing partnership with organizations including colleges and universities also increases awareness of Bruker.
Bruker is focused on promoting diversity across our organization. Bruker employees come from diverse backgrounds all over the world and are united by a shared purpose—innovation with integrity. The work performed every day at Bruker inspires and impacts global scientific research—and diverse and dynamic teams inspire each other to achieve their full potential. Cross-functional teams collaborate and enable the creation of new ideas by actively identifying and recruiting talent with diverse professional experiences, skills and backgrounds including from diverse gender, racial and ethnic backgrounds.
Several initiatives support increasing diversity and inclusion at Bruker. These programs support Bruker’s desire for a diverse workforce and support the talent attraction and development of all employees at Bruker, regardless of their gender. In the context of celebrating diversity across Bruker, employees engage in initiatives which support all genders in the workplace. Bruker male and female employees play active roles in these initiatives, which exhibit our commitment to inclusion. Germany’s nationwide Girls’ Day continues to be popular among girls ages 10 and older to support their career and educational aspirations and accomplishments. Additionally, Bruker participated in several Women’s Day celebration events across the globe, including a gathering of Bruker women in China. Events such as these provide opportunities for connection among colleagues and foster a sense of togetherness which supports employees of all genders in the workplace.
16
A program called Coffee Buddies has been popular in recent years at Bruker in Germany and has begun to expand to other countries. These programs offer positive connections for new hires, establishing internal networks across functions for employees who may not otherwise have opportunity to interact.
Events and connection opportunities are important to foster a diverse and inclusive environment, but training is also highly important. In 2023, Bruker talent acquisition professionals participated in an Unconscious Bias course which increases self-awareness of personal biases. This training program is highly important to support fairness in the recruitment, evaluation and decision making processes that drive talent acquisition and the development of employees.
Employee Health and Safety
Ensuring the safety and wellbeing of our employees is a top priority for Bruker. We accomplish this through compliance with applicable laws and regulations regarding workplace safety, including recognition and control of workplace hazards, the elimination of unsafe manufacturing and workplace practices, tracking injury and illness rates, utilizing a global travel health program and maintaining detailed emergency and disaster recovery plans. We are committed to reducing safety risks across business units and at our corporate and manufacturing sites worldwide. The following are examples of initiatives and programs designed with employee health and safety in mind:
Available Information
We maintain a website at www.bruker.com. We make available on our website documents describing our corporate governance and our Code of Conduct. We are not including the information contained on our website as a part of, or incorporating it by reference into, this Annual Report on Form 10-K. We make available free of charge through our website our annual reports on Form 10-K, our proxy statements, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to these reports filed with or furnished to the SEC pursuant to Sections 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. The SEC also maintains a website site that contains periodic reports, proxy and information statements and other information regarding our filings at www.sec.gov.
17
ITEM 1A RISK FACTORS
The following risk factors should be considered in conjunction with the other information included in this Annual Report on Form 10-K. This report may include forward-looking statements that involve risks and uncertainties. In addition to those risk factors discussed elsewhere in this report, we identify the following risk factors, which could affect our actual results and cause actual results to differ materially from those in the forward-looking statements.
Risk Factor Summary
Our business is subject to numerous risks and uncertainties, including those described in Item 1A “Risk Factors.” These risks include, but are not limited to, the following:
18
Risks Related to Our Business and Industry
Supply chain issues, including increasing demand for certain components used in our products and production delays, has and could continue result in significant additional costs and manufacturing inefficiencies, which could adversely impact our revenue, increase our manufacturing costs and have a material adverse effect on our operating results.
We have experienced supply chain interruptions as a result of general global economic conditions, the war in Ukraine, ongoing conflicts between the United States and China, geopolitical tensions, a tight labor market and other factors, including natural events and disasters. Various factors, including increased demand for certain components and production delays, are contributing to shortages of certain components used in our products including microelectronic components and increased difficulties in our ability to obtain a consistent supply of materials at stable pricing levels. Supply shortages and longer lead times for components used in our products, including limited source components, have and can result in significant additional costs and inefficiencies in manufacturing. A shortage of key components has in the past and may in the future cause a significant disruption to our production activities, which could have a substantial adverse effect on our financial condition or results of operations. If we are unsuccessful in resolving any such component shortages in a timely manner, we could experience a significant adverse impact on the timing of our revenue, a possible loss of revenue, or an increase in manufacturing costs, any of which could have a material adverse impact on our operating results.
Unfavorable economic or political conditions in the countries in which we operate may have an adverse impact on our business results or financial condition.
Our businesses and results of operations are affected by international, national and regional economic and political conditions. Our businesses or financial results may be adversely impacted by unfavorable changes in economic or political conditions in the countries and markets in which we operate, including, among others, adverse changes in interest rates or tax rates, volatility in financial and commodity markets, contraction in the availability of credit in the marketplace, geopolitical tensions and changes in capital spending patterns.
Our revenue from U.S. operations represented approximately 26% and 28% of total consolidated revenue for fiscal 2023 and 2022, respectively. Our revenue from operations in Europe represented approximately 33% of total consolidated revenue for fiscal years 2023 and 2022 respectively. Our revenue from operations in the Asia Pacific region represented approximately 33% and 32% of total consolidated revenue in each of the corresponding periods. Economic factors that could adversely influence demand for our products include the impact of geopolitical tensions and any related sanctions implemented, continued uncertainty about global economic conditions, including as a result of the pandemic, leading to ongoing reductions in investment, changes in government spending levels and/or priorities, the size and availability of government budgets, customers’ and suppliers’ access to credit and other macroeconomic factors affecting government, academic or industrial spending behavior. Slower economic growth or a deterioration in economic conditions could result in a decrease in government funding for scientific research, a delay in orders from current or potential customers or a reduction in purchases of our products.
We cannot predict how changes in economic conditions or political instability will affect our customers and suppliers or how any negative impact on our customers and suppliers might adversely impact our business results or financial condition.
19
Adverse global economic conditions, and geopolitical tensions, including in the Middle East, China and other regions, and other conditions that impact our increasingly global operations could have a negative effect on our business, results of operations and financial condition and liquidity.
As a global company, our performance is affected by global economic conditions as well as geopolitical tensions and other conditions with global reach. In recent years, concerns about the global economic outlook have adversely affected market and business conditions in general. Macroeconomic weakness and uncertainty make it more difficult for us to manage our operations and accurately forecast revenue, gross margin and expenses. Geopolitical tensions, including the conflict between Russia and Ukraine and related economic sanctions, the conflict in Israel, Palestine and surrounding areas, the possible expansion of such conflicts and potential geopolitical consequences, the ongoing tensions between the United States and China, tariff and trade policy changes, and increasing potential of conflict involving countries in Asia that are significant to the Company's supply chain operations, such as Taiwan and China, have resulted in increasing global tensions and create uncertainty for global commerce. As a result of the adverse economic impacts resulting from the conflict between Russia and Ukraine, such as increased prices for and a reduced supply of key metals used in our products, the Company has ceased its Russian operations. Sustained or worsening of global economic conditions and increasing geopolitical tensions may increase our cost of doing business, materially disrupt our supply chain operations, cause our customers to reduce or delay spending and intensify pricing pressures. We have recently experienced an increase in inflationary pressures in many of the jurisdictions in which we operate. We have and may continue to offset the effect of these inflationary pressures by increasing the prices of our products to customers. In 2023, however, our price increases did not fully offset the effects of inflation costs and may not offset any effects experienced during 2024. Any or all of these factors could negatively affect demand for our products and our business, financial condition and result of operations.
We derive a significant portion of our revenue from international sales and are subject to the operational risks of doing business in foreign countries.
International sales account, and are expected to continue to account, for a significant portion of our total revenues. Our revenue from non-U.S. operations represented approximately 74% and 72% of our total consolidated revenue for fiscal 2023 and 2022, respectively. Our international operations are, and will continue to be, subject to a variety of risks associated with conducting business internationally, many of which are beyond our control. These risks, which may adversely affect our ability to achieve and maintain profitability and our ability to sell our products internationally, include:
The United States has implemented tariffs on certain imported goods. These additional tariffs could include items imported by us from China or other countries. In addition, China has imposed tariffs on a wide range of American products in retaliation for these new American tariffs. As a result, there is a concern that the imposition of additional tariffs by the United States could result in the adoption of additional tariffs by China and other countries as well. Any resulting trade war could negatively impact the global market for scientific instruments and could have a significant adverse effect on our business. The imposition of tariffs on items imported by us from China or other countries could increase our costs and could result in lowering our gross margin on products sold. Conversely, any imposition by China of tariffs on items that we export to China could adversely impact our customers’ ability to purchase our products and our competitive position in China or increase our costs, which could have a material adverse effect on our business and results of operations.
20
We must also comply with the European Union General Data Protection Regulation (GDPR) and other similar regulations in other countries, including the UK Data Protection Act 2018. These laws include strong protections for individual privacy rights of residents of the European Economic Area (“EEA”) and UK. GDPR purports to apply extraterritorially such that it can apply to businesses that are not established within the EEA, but that process personal data of individuals located within the European Union in connection with the offering of goods and services within the EEA. There are significant fines associated with non-compliance. In 2020, the Court of Justice of the European Union invalidated the EU-US Privacy Shield Framework, a key mechanism for transfers of personal data from the European Union to the United States and altered the international data transfer under GDPR. Even though Bruker did not rely on the EU-US Privacy Shield for its transfers to the United States, Bruker has conducted a transfer impact assessment to understand the risks of its EU-US persona data transfers and implemented the new EU Standard Contractual Clauses (including the UK addendum). More recently, the European Commission and United States have agreed on a new EU-US Transatlantic Data Privacy Framework that may stabilize rules for transfers of personal data from the EU to the United States. However, ongoing litigation and challenges relating to such transfers could cause disruption of data transfers and have a material adverse effect on our business.
While the impact of these factors is difficult to predict, any one or more of these factors could adversely affect our operations in the future.
Our competitive position and reported financial results may be adversely affected when we exchange foreign currency received from international sales into U.S. Dollars and by fluctuations in currency exchange rates.
A significant portion of our business is conducted in currencies other than the U.S. Dollar, which is our reporting currency. As a result, currency fluctuations among the U.S. Dollar and the currencies in which we do business could cause the price of our products to be more or less competitive than our principal competitors’ products. Currency fluctuations will increase or decrease our cost structure relative to those of our competitors, which could lessen the demand for our products and affect our competitive position. From time to time, we enter into certain hedging transactions and/or option and foreign currency exchange contracts which are intended to offset some of the market risk associated with our sales denominated in foreign currencies. We cannot predict the effectiveness of these transactions or their impact upon our future operating results, and from time to time they may negatively affect our quarterly earnings.
In addition to the foreign currency exposure associated with differences between where our products are manufactured and sold by us and our competitors, our exposure to currency exchange rate fluctuations results from the currency translation exposure associated with the preparation of our consolidated financial statements, as well as from the exposure associated with transactions of our subsidiaries that are denominated in a currency other than the respective subsidiary’s functional currency. While our financial results are reported in U.S. Dollars, the financial statements of many of our subsidiaries outside the U.S. are prepared using the local currency as the functional currency. During consolidation, these results are translated into U.S. Dollars by applying appropriate exchange rates. As a result, fluctuations in the exchange rate of the U.S. Dollar relative to the local currencies in which our foreign subsidiaries report could cause significant fluctuations in our reported results. Moreover, as exchange rates vary, revenue and other operating results may differ materially from our expectations. The effects of changes in currency exchange rates decreased our 2023 revenue by approximately $11.2 million, or 0.4%, decreased our 2022 revenue by approximately $168.0 million, or 6.9%, and increased our 2021 revenue by approximately $43.3 million, or 2.2%. Adjustments resulting from financial statement translations are included as a separate component of shareholders' equity. We recorded net gains from currency translation adjustments of $15.1 million in the year ended December 31, 2023, and net losses of $22.0 million in the year ended December 31, 2022.
Additionally, to the extent monetary assets and liabilities, including cash and debt, are held in a different currency than the reporting subsidiary’s functional currency, fluctuations in currency exchange rates may have a significant impact on our reported financial results, and may lead to increased earnings volatility. We may record significant gains or losses related to both the translation of assets and liabilities held by our subsidiaries into local currencies and the re-measurement of inter-company receivables and loan balances.
21
Goodwill, intangible assets and other long-lived assets are subject to impairment which could negatively impact our operating results.
We have recorded goodwill, intangible assets and other long-lived assets that must be periodically evaluated for potential impairment. We assess the realizability of the reported goodwill, intangible assets and other long-lived assets annually, as well as whenever events or changes in circumstances indicate that the assets may be impaired. These events or circumstances generally include operating losses or a significant decline in the earnings associated with the reporting unit these assets are reported within. A decline in our stock price and market capitalization may also cause us to consider whether goodwill, intangible assets and other long-lived assets may require an impairment assessment. Our ability to realize the value of these assets will depend on the future cash flows of the reporting unit in addition to how well we integrate the businesses we acquire. In connection with certain restructuring activities during fiscal 2023, the Company performed impairment assessments of its long-lived assets comparing the carrying values to the sum of their undiscounted future cash flows. Based on the results of these analyses, the Company determined there were no impairments to intangibles assets and goodwill for the years ended December 31, 2023, 2022, and 2021. However, the Company recorded an impairment loss for certain right of use assets as disclosed in Note 16 to our consolidated financial statements included in Item 8 of this Annual Report on Form 10-K.
If our products fail to achieve and sustain sufficient market acceptance across their broad intended range of applications, we will not generate expected revenue.
Our business strategy depends on our ability to successfully commercialize a broad range of products based on our technology platforms, including magnetic resonance technology, pre-clinical imaging technology, mass spectrometry technology, X-ray technology, atomic force microscopy technology, ChipCytometry technology, stylus and optical metrology technology, fluorescence microscopy technology, infrared technology and superconducting magnet technologies for use in a variety of life science, chemistry and materials analysis applications. Some of our products have only recently been commercially launched and have achieved only limited sales to date. The commercial success of our products depends on obtaining and expanding market acceptance by a diverse array of industrial, academic, clinical, pharmaceutical, biotechnology, applied, medical research and governmental customers around the world. We may fail to achieve or sustain substantial market acceptance for our products across the full range of our intended applications or in one or more of our principal intended applications. Any such failure could decrease our sales and revenue. To succeed, we must convince substantial numbers of potential customers to invest in new systems or replace their existing techniques with techniques employing our systems. Limited funding available for capital acquisitions by our customers, as well as our customers’ own internal purchasing approval policies, could hinder market acceptance of our products. Our intended customers may be reluctant to make the substantial capital investment generally needed to acquire our products or to incur the training and other costs involved with replacing their existing systems with our products. We also may not be able to convince our intended customers that our systems are an attractive and cost-effective alternative to other technologies and systems for the acquisition, analysis and management of molecular, cellular and microscopic information. Because of these and other factors, our products may fail to gain or sustain market acceptance.
Our products compete in markets that are subject to rapid technological change, and one or more of the technologies underlying our products could be made obsolete by new technology.
The market for discovery and analysis tools is characterized by rapid technological change and frequent new product introductions. Rapidly changing technology could make some or all of our product lines obsolete unless we are able to continually improve our existing products and develop new products. Because substantially all of our products are based on our technology platforms, including magnetic resonance technology, mass spectrometry technology, X-ray technology, atomic force microscopy technology, fluorescence microscopy technology, ChipCytometry technology, stylus and optical metrology technology and infrared technology, we are particularly vulnerable to any technological advances that would make these techniques obsolete as the basis for analytical systems in any of our markets. To meet the evolving needs of our customers, we must rapidly and continually enhance our current and planned products and services and develop and introduce new products and services. In addition, our product lines are based on complex technologies that are subject to rapid change as new technologies are developed and introduced in the marketplace. We may have difficulty in keeping abreast of the rapid changes affecting each of the different markets we serve or intend to serve. If we fail to develop and introduce products in a timely manner in response to changing technology, market demands or the requirements of our customers, our product sales may decline, and we could experience significant losses.
We face substantial competition. If we fail to compete effectively, it could harm our business results and materially impact the value of our company.
We face substantial competition in our industries, and we expect that competition in all of our markets will increase further. Currently, our principal competition comes from established companies providing products using existing technologies that perform many of the same functions for which we market our products. A number of our competitors have expanded their market share in recent years through business combinations.
22
Other companies also may choose to enter our fields in the future. Our competitors may develop or market products that are more effective or commercially attractive than our current or future products or that may render our products obsolete. Competition has in the past subjected, and is likely in the future to subject, our products to pricing pressure. Certain competitors have more experience in the market and substantially greater financial, operational, marketing and technical resources than we do, which could give them a competitive advantage in areas such as research and development, production, marketing and distribution. Our ability to compete successfully will depend, in part, on our ability to develop proprietary products that reach the market in a timely manner and are technologically superior to, less expensive than, or more cost-effective than, other currently marketed products.
If investment in life and material science research spending declines, our ability to generate revenue may suffer.
We are dependent, both directly and indirectly, upon general investment in life science research, particularly in the research and development budgets of the pharmaceutical and biotechnology industries, and in material science research as well as upon the financial condition and funding priorities of various governments and government agencies. Since our inception, both we and our academic collaborators and customers have benefited from various governmental contracts and research grants. Whether we or our academic collaborators will continue to be able to attract these grants depends not only on the quality of our products, but also on general spending patterns of public institutions.
Any reduction in the capital resources or government funding of our customers could reduce our sales and impede our ability to generate revenue.
A significant portion of our sales are capital purchases by our customers. The spending policies of our customers could have a significant effect on the demand for our products. These policies are based on a wide variety of factors, including the resources available to make purchases, the spending priorities among various types of equipment, policies regarding spending during recessionary periods and changes in the political climate.
Any changes in capital spending or changes in the capital budgets of our customers could significantly reduce demand for our products. The capital resources of our life science and other corporate customers may be limited by the availability of equity or debt financing. Any significant decline in research and development expenditures by our life science and material science customers could significantly decrease our sales. In addition, a substantial portion of our sales are to non-profit and government entities, which are dependent on government support for scientific research. Any decline in this support could decrease the ability of these customers to purchase our products.
Disruptions at any of our manufacturing facilities could adversely affect our business.
We have manufacturing facilities located in the United States, Europe, Israel and Malaysia. Many of our products are developed and manufactured at single locations, with limited alternate facilities. If we experience any significant disruption of those facilities for any reason, such as war or other geopolitical conflicts, strikes or other labor unrest, power interruptions, fire, earthquakes, or other events beyond our control, we may be unable to manufacture the relevant products at previous levels or at all. A reduction or interruption in manufacturing could harm our customer relationships, impede our ability to generate revenues from our backlog or obtain new orders and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
If employees were to engage in a strike or other work stoppage or interruption, our business, results of operations, financial condition and liquidity could be materially adversely affected.
Many of our employees are represented by workers’ councils and labor unions in certain jurisdictions, primarily in Germany and France. If disputes with these employees arise, or if our workers engage in a strike or other work stoppage or interruption, we could experience a significant disruption of, or inefficiencies in, our operations or incur higher labor costs, which could have a material adverse effect on our business, results of operations, financial condition and liquidity.
In addition to the risks applicable to our life science and materials analysis products, our CBRNE detection products are subject to a number of additional risks, including lengthy product development and contract negotiation periods and certain risks inherent in long-term government contracts.
Our CBRNE detection products are subject to many of the same risks associated with our life science products, including vulnerability to rapid technological change, dependence on mass spectrometry and other technologies and substantial competition. In addition, our CBRNE detection products and certain FT-IR products are generally sold to government agencies under long-term contracts. These contracts generally involve lengthy pre-contract negotiations and product development. We may be required to devote substantial working capital and other resources prior to obtaining product orders. As a result, we may incur substantial costs before we recognize revenue from these products.
23
Moreover, in return for larger, longer-term contracts, our customers for these products often demand more stringent acceptance criteria. These criteria may also cause delays in our ability to recognize revenue from sales of these products. Furthermore, we may not be able to accurately predict in advance our costs to fulfill our obligations under these long-term contracts. If we fail to accurately predict our costs, due to inflation or other factors, we could incur significant losses. Also, the presence or absence of such contracts may cause substantial variation in our results of operations between fiscal periods and, as a result, our results of operations for any given fiscal period may not be predictive of our results for subsequent fiscal periods. The resulting uncertainty may have an adverse impact on our stock price.
We rely on information technology to support our operations and reporting environments. A security failure of that technology, including with respect to cybersecurity, could impact our ability to operate our businesses effectively, adversely affect our financial results, damage our reputation and expose us to potential liability or litigation.
We use information systems to carry out our operations and maintain our business records. Some systems are internally managed, and some are maintained by third-party service providers. Our ability to conduct business could be materially and adversely affected if these systems or resources are compromised, damaged or fail. This could be a result of a cyber-incident, social engineering scam, hacking, phishing attempts, malware, natural disaster, hardware or software corruption, failure or error, telecommunications system failure, service provider error or failure, intentional or unintentional personnel actions or other disruption.
In the ordinary course of business, we collect and store sensitive data, including intellectual property, other proprietary information and personally identifiable information. Despite our security measures, our information technology and infrastructure may be vulnerable to cyber-attacks by hackers, including intrusions designed to access and exfiltrate information and to disrupt and lock-up access to systems for the purpose of demanding ransom payments, or breached due to employee error, malfeasance, or other disruptions. Further, a breach of our security systems or infrastructure, or those of our customers, suppliers and other business partners, could result in the disclosure, misuse, corruption or loss of confidential information, including intellectual property, personally identifiable information and other critical data of the Company and our employees, customers suppliers and other business partners. If this data is compromised, destroyed or inappropriately disclosed, it could have a material adverse effect, including damage to our reputation, and our relationships with our employees, customers, suppliers and other business partners, decrease the value of our investments in research, development and engineering, disrupt our manufacturing processes, result in significant expenses to address and resolve the issues, fines or litigation or other proceedings by affected individuals, customers, suppliers, business partners or regulatory authorities.
Our debt may adversely affect our cash flows and may restrict our investment opportunities or limit our activities.
As of December 31, 2023, we had outstanding an aggregate principal amount of debt totaling approximately $1,282.8 million. We also had the ability to borrow an additional $599.6 million available under our existing credit facility. Most of our outstanding debt is in the United States and there are substantial cash requirements in the United States to service debt interest obligations, fund operations, capital expenditures and our declared dividends and finance potential acquisitions or share repurchases. Our ability to satisfy our debt obligations and meet our other liquidity needs depends on our future operating performance and on economic, financial, competitive and other factors beyond our control. Our business may not generate sufficient cash flow to meet our debt obligations or provide sufficient funds for our other objectives. If we are unable to service our debt or obtain additional financing, we may be forced to delay minority acquisitions, capital expenditures or research and development expenditures or suspend our dividend payments and share repurchases. We may not be able to obtain additional financing on terms acceptable to us or at all. Furthermore, a majority of our cash, cash equivalents and short-term investments is generated from foreign operations, with $398.4 million, or 81.6% held by foreign subsidiaries as of December 31, 2023. We may incur certain tax consequences relocating cash from our foreign operations to the United States. Our financial condition and results of operations could be adversely impacted if we are unable to maintain a sufficient level of cash flow in the United States to address our funding requirements through cash from operations and timely repatriation of cash from overseas or other sources obtained at an acceptable cost.
Additionally, the agreements governing our debt require that we maintain certain financial ratios related to maximum leverage and minimum interest coverage and contain affirmative and negative covenants, including among others, timely provision of audited consolidated financial statements, restrictions on liens, indebtedness of the Company and its subsidiaries, asset sales, dividends and transactions with affiliates. Our ability to comply with these financial restrictions and covenants is dependent on our operations and performance, which is subject to prevailing economic conditions and other factors, including factors that are beyond our control such as foreign currency translation rates and interest rates. Our failure to comply with any of these restrictions or covenants may result in an event of default under the applicable debt instrument, which could permit acceleration of the debt under the facility and require us to prepay the debt before its scheduled due date.
24
Changes in our effective income tax rate could adversely affect our results of operations.
We are subject to income taxes in both the United States and various foreign jurisdictions and our domestic and international tax liabilities are largely dependent upon the distribution of income among these different jurisdictions. Various factors may have favorable or unfavorable effects on our effective income tax rate. These factors include interpretations of existing tax laws, the accounting for stock options and other share-based compensation, changes in tax laws and rates, future levels of research and development spending, changes in accounting standards, changes in the mix of earnings in the various tax jurisdictions in which we operate, the outcome of examinations by the U.S. Internal Revenue Service and other tax authorities, the accuracy of our estimates for unrecognized tax benefits and realization of deferred tax assets and changes in overall levels of pre-tax earnings. A change in tax laws, treaties or regulations, or their interpretation, of any country in which we operate could result in a higher tax rate on our earnings, which could result in a significant negative impact on our earnings and cash flow from operations. For example, on August 16, 2022, the President of the United States signed into law the Inflation Reduction Act of 2022 (the “IRA”), a tax and spending package that introduces several tax-related provisions, including a 15% corporate alternative minimum tax (“CAMT”) on certain large corporations and a 1% excise tax on certain corporate stock repurchases. The IRA provisions, which became effective for the Company beginning on January 1, 2023, did not have a material impact on the Company during the year ended December 31, 2023.
In December 2021, the Organization for Economic Co-operation and Development (OECD)/G20 Inclusive Framework, agreed to implement a global minimum tax regime for multinationals known as ‘Pillar Two’. The OECD has released the Pillar Two model rules (the Global Anti-Base Erosion Proposal, or ‘GloBE’) to reform international corporate taxation. The Pillar Two model rules provide guidance for a global minimum tax. This guidance lays out a common approach for adopting the global minimum tax and enacting local legislation codifying the provisions that all 142 countries in the Inclusive Framework agreed to by consensus. The EU member states have agreed to adopt these rules in two stages with the first component effective on January 1, 2024, while the second component will be effective January 1, 2025. Non-EU countries have enacted or are expected to enact legislation on a similar timeline. Certain countries in which we operate have already enacted legislation to adopt the Pillar Two framework, while several other countries are expected to also implement similar legislation with varying effective dates in the future. When and how this framework is adopted or enacted by the various countries in which we do business will increase tax complexity and may increase uncertainty and adversely affect our provision for income taxes in the U.S. and non-U.S. jurisdictions.
We also assess the impact of various international tax reform proposals and modifications to existing tax treaties in all jurisdictions where we have operations that could result in a material impact on our income taxes. However, if such proposals were enacted, or if modifications were made to certain existing treaties, the consequences could have a materially adverse impact on us, including increasing our tax burden, increasing costs of our tax compliance or otherwise adversely affecting our financial condition, results of operations and cash flows.
Various international tax risks could adversely affect our earnings and cash flows.
We are subject to international tax risks. We could be subject to double taxation on income related to operations in certain countries that do not have tax treaties with the country of the trading partner. In addition, we may have a higher effective income tax rate than that of other companies in our industry if losses incurred by one operating company are not available to offset the income of an operating company located in another country. Also, distributions of earnings and other payments received from our subsidiaries may be subject to withholding taxes imposed by the countries where they are operating or are incorporated. If these foreign countries do not have income tax treaties with the United States or the countries where our subsidiaries are incorporated, we could be subject to high rates of withholding taxes on these distributions and payments. Additionally, the amount of the credit that we may claim against our U.S. federal income tax for foreign income taxes paid or accrued is subject to many limitations which may significantly restrict our ability to claim a credit for all of the foreign taxes we pay.
The unpredictability and fluctuation of our quarterly results may adversely affect the trading price of our common stock.
Our revenues and results of operations have in the past and will in the future vary from quarter to quarter due to a number of factors, many of which are outside our control and any of which may cause our stock price to fluctuate. The primary factors that may affect us include the following:
25
We can experience quarter-to-quarter fluctuations in our operating results as a result of various factors, some of which are outside our control, such as:
These factors have in the past affected the amount and timing of revenue recognized on sales of our products and receipt of related payments and will likely continue to do so in the future. Accordingly, our operating results in any particular quarter may not necessarily be an indication of any future quarter’s operating performance.
Historically we have higher levels of revenue in the fourth quarter of the year compared to the first, second and third quarters, which we believe is primarily the result of our customers’ budgeting cycles. Quarter-to-quarter comparisons of our results of operations should not be relied upon as an indication of our future performance. It is likely that in some future quarters, our results of operations may be below the expectations of public market analysts and investors. In this event, the price of our common stock may fall.
The ownership of our shares is highly concentrated, which could cause or exacerbate volatility in our share price as well as have significant influence over us.
As of February 6, 2024, Laukien family members, including our Chairman, President and Chief Executive Officer (“CEO”) Frank Laukien and his brother, Joerg Laukien, owned, in the aggregate, approximately 33% of our outstanding common stock. We may also repurchase shares in the future, which could further increase the concentration of our share ownership. Because of this reduced liquidity, the trading of relatively small quantities of shares by our shareholders could disproportionately influence the price of those shares in either direction. The price for our shares could, for example, decline precipitously if a large number of our shares were sold on the market without commensurate demand, as compared to a company with greater trading liquidity that could better absorb those sales without adverse impact on its share price. These shareholders may also exercise substantial influence over all matters requiring shareholder approval, including the election of directors and approval of significant corporate transactions. This could have the effect of delaying or preventing a change in control of our company and will make some transactions difficult to accomplish without the support of these shareholders.
The loss of key personnel or an inability to attract and retain additional personnel could affect our ability to successfully grow our business.
We are highly dependent upon the continued service and performance of our CEO and other members of senior management and key finance, technical, scientific and production personnel, any of whom may cease their employment with us at any time with minimal advance notice. Because the expertise of these individuals is highly specific and takes years to develop, we face intense competition for these individuals from many other companies. The loss of one or more of our key employees may significantly delay or prevent the achievement of our business objectives, and our failure to attract and retain suitably qualified individuals or to adequately plan for succession could have an adverse effect on our ability to implement our business plan.
26
Dividends on our common stock could be reduced or eliminated in the future.
In recent years, we have paid dividends on our common stock. In February 2024, we announced that our Board of Directors (“Board”) had declared a quarterly dividend of $0.05 per share that will be payable in March 2024. There is no guarantee that such dividends will continue indefinitely. In the future, our Board may determine to reduce or eliminate our common stock dividend in order to fund investments for growth, repurchase shares or conserve capital resources.
Risks Related to Our Dependence on Third Parties
If we lose our strategic partners, our marketing and sales efforts could be impaired.
A substantial portion of our sales of selected products consists of sales to third parties who incorporate our products into their systems. These third parties are responsible for the marketing and sales of their systems. We have little or no control over their marketing and sales activities or how they use their resources. Our present or future strategic partners may or may not purchase sufficient quantities of products from us or perform appropriate marketing and sales activities. In addition, if we are unable to maintain our relationships with strategic partners, our businesses may suffer. Failures by our present or future strategic partners, or our inability to maintain existing or enter into new arrangements with strategic partners for product distribution, could materially impede the growth of our businesses and our ability to generate sufficient revenue and profits.
We face risks related to sales through distributors and other third parties that we do not control, which could harm our business.
We sell some products through third party agents, including distributors and value-added resellers. This exposes us to various risks, including competitive pressure, concentration of sales volumes, credit risks, and compliance risks. We may rely on one or a few key distributors for a product or market, and the loss of these distributors could reduce our revenue and net earnings. Distributors may also face financial difficulties, including bankruptcy, which could harm our collection of accounts receivable. Risks related to our use of distributors may reduce sales, increase expenses, and weaken our competitive position. Moreover, violations of the FCPA or similar anti-bribery laws by distributors or other third-party agents could materially and adversely impact our business, reputation and results of operations.
Dependence on contract manufacturing may adversely affect our ability to bring products to market and damage our reputation.
As part of our efforts to streamline our operations and reduce our operating costs, we outsource certain aspects of our manufacturing processes and continue to evaluate additional outsourcing. If our contract manufacturers fail to perform their obligations in a timely manner or at satisfactory quality levels, our ability to bring products to market and our reputation could suffer. For example, during a market upturn, our contract manufacturers may be unable to meet our demand requirements, which may preclude us from fulfilling our customers’ orders on a timely basis. The ability of these manufacturers to perform is largely outside our control. Additionally, changing or replacing our contract manufacturers could cause disruptions or delays. Problems with outsourced manufacturing could result in lower revenues and unexecuted efficiencies, and adversely affect our financial condition and results of operations.
Our operations are dependent upon a limited number of suppliers and contract manufacturers.
We currently purchase components used in our products from a limited number of outside suppliers. Our reliance on a limited number of suppliers could result in time delays associated with redesigning a product due to an inability to obtain an adequate supply of required components and reduced control over pricing, quality and timely delivery. Any of these factors could adversely affect our revenues and profitability. In particular, our X-ray microanalysis business, which manufactures and sells accessories for electron microscopes, is partially dependent on cooperation from larger manufacturers of electron microscopes. Additionally, our elemental analysis business purchases certain optical detectors from a single supplier, PerkinElmer, Inc., the sole supplier of these detector components. BSI CALID purchases detectors and power supplies from sole or limited source suppliers and its focal plane array detectors from a single supplier, Lockheed Martin Corporation. Similarly, BSI BioSpin obtains various components from sole or limited source suppliers and BEST obtains various raw materials and uses key production equipment from sole or limited source suppliers or contract manufacturers. There are limited, if any, available alternatives to these suppliers. The existence of shortages of these components or the failure of delivery with regard to these components could have a material adverse effect upon our revenues and margins. In addition, price increases from these suppliers or contract manufacturers could have a material adverse effect upon our gross margins.
Because of the scarcity of some components, we may be unable to obtain an adequate supply of components, or we may be required to pay higher prices or to purchase components of lesser quality. Any delay or interruption in the supply of these or other components could impair our ability to manufacture and deliver our products, harm our reputation and cause a reduction in our revenues. In addition, any increase in the cost of the components that we use in our products could make our products less competitive and decrease our gross profits.
27
We may not be able to obtain sufficient quantities of required components on the same or substantially the same terms. Additionally, consolidation among our suppliers could result in other sole source suppliers for us in the future. Other events that could affect our ability to source materials, manufacture or distribute our products include fire, natural disaster or extreme weather or a pandemic and the impact of those events on our and our suppliers’ and contract manufacturers’ operations.
Supply shortages and increasing prices of raw materials could adversely affect our gross profit.
The last few years have seen periodic supply shortages and sharp increases in the prices for various raw materials, in part due to high demand from developing countries, which have been exacerbated by the war between Russia and Ukraine, on-going conflicts between the United States and China and other geopolitical tensions. We rely on some of these materials for the production of our products. For example, in our superconducting magnet production, both for the horizontal and vertical magnet series, we rely on the availability of copper, steel and other metallic raw materials as well as niobium titanium for the production of traditional low-temperature superconducting magnets, wires and devices. Higher prices for these commodities will increase the production cost of superconducting wires and superconducting magnets and may adversely affect gross profits.
The prices of copper and certain other raw materials used for superconductors have increased significantly over the last decade. Since copper is a main constituent of low temperature superconductors, this may affect the price of superconducting wire. This type of increase would have an immediate effect on the production costs of superconducting magnets and may negatively affect the profit margins for those products.
In order to operate superconducting magnets, we and our customers rely on liquid helium. Helium is controlled by the Federal Helium Reserve and is subject to price changes. Shortages of liquid helium associated with federal price controls or depleted natural reserves could drive increases in helium pricing and have an adverse impact on producing and operating our superconducting magnets, which may negatively impact the profit margins of those products.
Risks Related to Our Intellectual Property Rights
Our success depends on our ability to operate without infringing or misappropriating the proprietary rights of others.
Our commercial success depends on avoiding the infringement of other parties’ patents and proprietary rights as well as avoiding the breach of any licenses relating to our technologies and products. Given that there may be patents of which we are unaware, particularly in the United States where patent applications are confidential, avoidance of patent infringement may be difficult. Various third parties hold patents which may relate to our technology, and we may be found in the future to infringe these or other patents or proprietary rights of third parties, either with products we are currently marketing or developing or with new products which we may develop in the future. If a third-party holding rights under a patent successfully asserts an infringement claim with respect to any of our current or future products, we may be prevented from manufacturing or marketing our infringing product in the country or countries covered by the patent we infringe, unless we can obtain a license from the patent holder. We may not be able to obtain such a license on commercially reasonable terms, if at all, especially if the patent holder is a competitor. In addition, even if we can obtain a license, it may be non-exclusive, which will permit others to practice the same technology licensed to us. We also may be required to pay substantial damages to the patent holder in the event of infringement. Under some circumstances in the United States these damages could include damages equal to triple the actual damages the patent holder incurs. If we have supplied infringing products to third parties for marketing by them or licensed third parties to manufacture, use or market infringing products, we may be obligated to indemnify these third parties for any damages they may be required to pay to the patent holder and for any losses the third parties may sustain themselves as the result of lost sales or license payments they are required to make to the patent holder. Any successful infringement action brought against us may also adversely affect marketing of the infringing product in other markets not covered by the infringement action, as well as our marketing of other products based on similar technology. Furthermore, we will suffer adverse consequences from a successful infringement action against us even if the action is subsequently reversed on appeal, nullified through another action or resolved by settlement with the patent holder. The damages or other remedies awarded, if any, may be significant. As a result, any successful infringement action against us may harm our business.
28
If we are unable to effectively protect our intellectual property, third parties may use our technology, which would impair our ability to compete in our markets.
Our continued success will depend in significant part on our ability to obtain and maintain meaningful patent protection for our products throughout the world. We rely on patents to protect a significant part of our intellectual property and to enhance our competitive position. However, our presently pending or future patent applications may not issue as patents, and any patent previously issued to us may be challenged, invalidated, held unenforceable or circumvented. Furthermore, the claims in patents which have been issued, or which may be issued to us in the future, may not be sufficiently broad to prevent third parties from producing competing products similar to our products. In addition, the laws of various foreign countries in which we compete may not protect our intellectual property to the same extent as do the laws of the United States. Failure to obtain adequate patent protection for our proprietary technology could materially impair our ability to be commercially competitive.
In addition to patent protection, we also rely on the protection of trade secrets, know-how and confidential and proprietary information. To maintain the confidentiality of trade secrets and proprietary information, we generally seek to enter into confidentiality agreements with our employees, consultants and strategic partners upon the commencement of a relationship with us. However, we may not obtain these agreements in all circumstances. In the event of unauthorized use or disclosure of this information, these agreements, even if obtained, may not provide meaningful protection for our trade secrets or other confidential information. In addition, adequate remedies may not exist in the event of unauthorized use or disclosure of this information. The loss or exposure of our trade secrets and other proprietary information would impair our competitive advantages and could have a material adverse effect on our operating results, financial condition and future growth prospects. Furthermore, others may have, or may in the future independently develop, substantially similar or superior know-how and technology.
We may be involved in lawsuits to protect or enforce our patents that are brought by us which could be expensive and time consuming and, if determined adversely, could adversely affect our patent position.
In order to protect or enforce our patent rights, we may initiate patent litigation against third parties, and we may be similarly sued by others. We may also become subject to interference proceedings conducted in the patent and trademark offices of various countries to determine the priority of inventions. The defense and prosecution, if necessary, of intellectual property suits, interference proceedings and related legal and administrative proceedings is costly and diverts our technical and management personnel from their normal responsibilities. We may not prevail in any of these suits. An adverse determination of any litigation or defense proceedings could put our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments in the litigation. If securities analysts or investors perceive these results to be negative, it could have a substantial negative effect on the trading price of our common stock.
In September 2019, Luxendo GmbH (“Luxendo”), a subsidiary of Bruker Corporation, was sued in Germany by Carl Zeiss Microscopy GmbH, a subsidiary of Carl Zeiss AG (“Zeiss”), for infringement of a registered German utility model. After the utility model was canceled by the German Patent and Trademark Office in 2021, Zeiss withdrew its infringement action at the end of 2022 and the proceedings were terminated. However, a parallel European patent application, a family member of the utility model, is still pending in the European Patent Office (“EPO”). The Company is closely monitoring progress in the granting procedure and does not believe that a grant is imminent. The Company is presently unable to predict the EPO's final decision on the patent application.
Risks Related to Legal, Regulatory and Compliance
Our manufacture and sale of products could lead to product liability claims for which we could have substantial liability.
The manufacture and sale of our products expose us to product liability claims if any of our products cause injury or are found otherwise unsuitable or defective as a consequence of product design, manufacturing, marketing, sale or customer use. In particular, if one of our CBRNE detection products malfunctions, this could lead to civilian or military casualties in a time of unrest, exposing us to increased potential for high-profile liability. If our CBRNE detection products malfunction by generating a false-positive to a potential threat, we could be exposed to liabilities associated with actions taken that otherwise would not have been required. Additionally, the nuclear magnetic resonance, research magnetic resonance imaging, Fourier transform mass spectrometry and certain electron paramagnetic resonance magnets of BSI BioSpin utilize high magnet fields and cryogenics to operate at approximately 4 Kelvin, the temperature of liquid helium. There is an inherent risk of potential product liability due to the existence of these high magnetic fields, associated stray fields outside the magnet, and the handling of the cryogens associated with superconducting magnets. In addition, our MALDI Biotyper product has an IVD-CE mark and U.S. FDA approval and is used for the identification of microorganisms. Misidentification or a false-negative of certain viruses, bacteria, yeasts or fungi could lead to inappropriate treatment for patients and could expose us to product liability claims.
29
A successful product liability claim brought against us in excess of, or outside the coverage of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations. We may not be able to maintain product liability insurance on acceptable terms, if at all, and insurance may not provide adequate coverage against potential liabilities.
We are subject to environmental laws and regulations, which may impose significant compliance or other costs on us.
Our manufacturing, product development and research and development operations and processes involve the controlled use of certain hazardous materials. In addition, we own and/or lease a number of facilities, some of which have been in operation for many decades, where we or others may have used substances or generated and disposed of wastes which are considered hazardous or may be considered hazardous in the future. We also have acquired various companies which historically may have used certain hazardous materials, and which may have owned and/or leased facilities at which hazardous materials have been used. For all of these reasons, we are subject to federal, state, foreign, and local laws and regulations governing the use, manufacture, storage, transportation, handling, treatment, remediation, and disposal of hazardous materials and certain waste products. We have potential liability under these laws and regulations with respect to the remediation of past contamination in certain of the facilities we now own or lease. Additionally, in the future our facilities and the disposal sites owned by others to which we send or sent waste, may be identified as contaminated and require remediation. Accordingly, we may become subject to additional compliance costs or environmental liabilities which may be significant and could materially harm our results of operations or financial condition.
Specifically, we use controlled hazardous and radioactive materials in our business and generate wastes that are regulated as hazardous wastes under U.S. federal, and Massachusetts, California, New Jersey, Washington and Wisconsin state environmental and atomic energy regulatory laws and under equivalent provisions of law in those and other jurisdictions in which our research and manufacturing facilities are located. Our use of these substances and materials is subject to stringent, and periodically changing, regulation that can impose costly compliance obligations on us and has the potential to adversely affect our manufacturing activities. The risk of accidental contamination or injury from these materials cannot be completely eliminated. If an accident with these substances occurs, we could be held liable for any damages that result, in addition to incurring clean-up costs and liabilities, which can be substantial. Additionally, an accident could damage our research and manufacturing facilities resulting in delays and increased costs.
We are subject to existing and potential additional regulation and government inquiry, which can impose burdens on our operations and narrow the markets for our products.
We are subject, both directly and indirectly, to the adverse impact of existing and potential future government regulation of our operations and markets. For example, the export of our products is subject to U.S. and non-U.S. export control, sanctions, customs, import and anti-boycott laws and regulations, including, as applicable, the International Traffic in Arms Regulations, the Export Administration Regulations and the sanctions laws, regulations and executive orders administered and enforced by the U.S. Department of the Treasury’s Office of Foreign Assets Control, and other laws and regulations adopted by the governments or agencies of other countries relating to the same subject matter as the U.S. laws and regulations described above.
The failure to satisfy export control criteria or obtain necessary clearances could delay or prevent shipment of products, which could adversely affect our revenues and profitability. Failure by the Company, our employees or others working on our behalf to comply with these laws and regulations could result in administrative, civil or criminal liabilities, including suspension, debarment from bidding for or performing government contracts, or suspension of our export privileges, which could have a material adverse effect on us. We frequently team with international subcontractors and suppliers who are also exposed to similar risks. In some cases, compliance with the laws and regulations of one country could violate the laws and regulations of another country. Violations of these laws and regulations could materially adversely affect our brand, international growth efforts and business.
In addition, as a result of our international operations, we are subject to compliance with various laws and regulations, including the FCPA and local anti-bribery laws in the jurisdictions in which we do business (including some higher risk countries according to the Transparency International Corruption Index), which generally prohibit companies and their intermediaries or agents from engaging in bribery or making improper payments to foreign officials or their agents. The FCPA also requires proper record keeping and characterization of such payments in our reports filed with the SEC. Despite maintaining policies and procedures that require our employees to comply with these laws and our standards of ethical conduct, we cannot ensure that these policies and procedures will always protect us from intentional, reckless or negligent acts committed by our employees or third-party agents.
Moreover, the life sciences industry, which is the market for our principal products, has historically been heavily regulated. Given the evolving nature of this industry, legislative bodies or regulatory authorities may adopt additional regulation that adversely affects our market opportunities. Our business is also directly affected by a wide variety of government regulations applicable to business enterprises generally and to companies operating in the life sciences industry in particular.
30
Our products are subject to the FDA’s requirements for electronic radiation emitting products, which include requirements related to record-keeping and reporting; labeling; notification; product repairs, replacements, and refunds; importation; and performance standards. In addition, our clinical products are subject to regulation as medical devices in the United States by the FDA and by similar regulatory bodies in other countries where such products are sold. These regulations govern a wide variety of product related activities, from quality management, design, development, and testing to labeling, manufacturing, complaint handling, reporting, promotion, sales and distribution. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through periodic inspections by the FDA and other regulatory authorities, which may result in written inspectional observations. The FDA and comparable foreign regulatory authorities also monitor product promotion and marketing materials and activities. If we or any of our suppliers or distributors fail to comply with FDA or other applicable regulatory requirements, or are perceived to potentially have failed to comply, we may face, among other things, warning letters; adverse publicity affecting both us and our customers; investigations or notices of non-compliance, fines, injunctions, and civil or criminal penalties; import or export restrictions; partial suspensions or total shutdown of production facilities or the imposition of operating restrictions; increased difficulty in obtaining required FDA clearances or approvals or foreign equivalents; seizures or recalls of our products or those of our customers; or the inability to sell such products. Any such FDA or comparable foreign regulatory actions could disrupt our business and operations, lead to significant remedial costs and have a material adverse impact on our financial position and results of operations. In addition, negative publicity and product liability claims resulting from any adverse regulatory action could have a material, adverse effect on our financial condition and results of operations. Further, the replacement of the European Union IVD Directive by the IVD Regulation (EU) 2017/746 in May 2022 has resulted in a stricter regime on manufacturers of IVDs and under transitional arrangements our products currently approved under the Directive must be recertified under the Regulation by May 2025.
We have been, are, and expect to be in the future, subject to inquiries from the government agencies that enforce these regulations, including the U.S. Department of State, the U.S. Department of Commerce, the U.S. FDA, the U.S. Internal Revenue Service, the U.S. Department of Labor, the U.S. Department of Homeland Security, the U.S. Department of Justice, the SEC, the Federal Trade Commission, U.S. Customs and Border Protection and the U.S. Department of Defense, among others, as well as from state or foreign governments and their departments and agencies. As a result, from time to time, the attention of our management and other resources may be diverted to attend to these inquiries. In addition, failure to comply with these regulations or obtain or maintain necessary permits and licenses could result in a variety of fines or other censures or an interruption in our business operations which may have a negative impact on our ability to generate revenues and could adversely affect our financial condition and results of operations.
Failure to maintain effective internal controls may cause a loss of investor confidence in the reliability of our financial statements or cause us to delay filing our periodic reports with the SEC and adversely affect our stock price.
The SEC, as directed by Section 404 of the Sarbanes-Oxley Act of 2002, adopted rules requiring public companies to include a report of management on internal control over financial reporting in their annual reports on Form 10-K that contain an assessment by management of the effectiveness of our internal control over financial reporting. In addition, our independent registered public accounting firm must attest to and report on the effectiveness of our internal control over financial reporting. Although we test our internal control over financial reporting in order to ensure compliance with the Section 404 requirements, our failure to maintain adequate internal controls over financial reporting could result in an adverse reaction in the financial marketplace due to a loss of investor confidence in the reliability of our financial statements or a delay in our ability to timely file our periodic reports with the SEC, which ultimately could negatively impact our stock price.
We operate as an entrepreneurial, decentralized company, which presents both benefits and certain risks. In particular, significant growth in a decentralized operating model may put strain on certain business group resources and our corporate functions, which could materially and adversely affect our business, financial condition and results of operations.
Decentralization necessarily places significant control and decision-making powers in the hands of local management, which presents certain risks, including the risk that we may be slower to detect or react to compliance-related matters and that “company-wide” business initiatives may be more challenging or costly to implement, and the risk of noncompliance or failures higher than they may be in a more centralized operating environment. In addition, key business group resources and our corporate functions, which are leanly staffed but responsible for supporting our decentralized operations, may also not be able to detect or resolve financial, operational, and compliance matters on a timely basis. Our failure to adapt our financial, operational and compliance controls and systems to effectively manage our decentralized business and comply with our obligations as a public company could materially and adversely affect our business, financial condition or results of operations.
31
General Risks Factors
If we are not able to successfully integrate the businesses we acquire through mergers, acquisitions or strategic alliances, we may not be able to realize all of the cost savings and other benefits that we expect to result from the transactions and our financial results may be different than expected.
Our strategy includes expanding our technology base and product offerings through selected mergers, acquisitions and strategic alliances. For example, from January 1, 2021, to December 31, 2023, we acquired 21 businesses to expand our technologies and product offerings.
Successful integration of the businesses we acquire involves a number of risks, including, among others, risks related to:
We may have difficulty developing, manufacturing and marketing the products of a newly acquired company or business in a way that enhances the performance of our combined businesses or product lines. As a result, we may not realize the value from expected synergies. Acquisitions have resulted, and may in the future result, in unexpected significant costs and expenses, including disputes over contingent consideration and complicated accounting for complex transaction structures. In the future, we may be required to record charges to earnings during the period if we determine there is an impairment of goodwill or intangible assets, up to the full amount of the remaining carrying value of the assets.
We generally assume the liabilities of businesses we acquire, which could include liability for an acquired business’ violation of law that occurred before we acquired it. We frequently acquire smaller, privately held companies that may not have the same culture of compliance or the same level of internal control of a larger, publicly traded company. Any failure to implement adequate training, controls, and monitoring of any acquired company could cause us to be liable for post-acquisition legal or accounting violations.
Other companies may have difficulty acquiring us, even if doing so would benefit our shareholders, due to provisions under our corporate charter and bylaws, as well as Delaware law.
Provisions in our restated certificate of incorporation, and our amended and restated bylaws, as well as Delaware law could make it more difficult for other companies to acquire us, even if doing so would benefit our shareholders. Our restated certificate of incorporation, and amended and restated bylaws contain the following provisions, among others, which may inhibit an acquisition of our company by a third party:
ITEM 1B UNRESOLVED STAFF COMMENTS The Board’s Audit Committee oversees risks relating to cybersecurity threats and the steps management takes to monitor and control such exposures.
Not applicable.
32
ITEM 1C CYBERSECURITY
The Company has instituted an Information Security Incident Response Plan (“IRP”) which provides a framework to assist the Company in responding to actual or potential cybersecurity incidents. Our IRP includes detailed response procedures to be followed in the event of a cybersecurity incident, which outline steps to be executed from detection to assessment to notification and recovery, including internal notifications to the Audit Committee as appropriate. These incidents may consist of any actual, threatened, suspected, or reported event or occurrence that may affect the confidentiality, integrity, or availability of Company systems or data, or of any such event affecting a third party that may affect Company systems or data. The objective of the IRP is to facilitate a timely and coordinated enterprise-level response to such incidents to mitigate impact on the Company and its employees, stockholders, customers, business partners, and other stakeholders. The Audit Committee receives regular reporting from senior officers (such as the Chief Information Security Officer and the Director of Risk Management & Insurance) on operational risk and the steps management has taken to monitor and control these risks. Such reporting includes updates on the Company’s IRP, the external threat environment, and the Company’s programs to address and mitigate the risks associated with the cybersecurity threat environment. The IRP and internal controls around cybersecurity are periodically evaluated by external experts and the results of those reviews are reported to the Audit Committee.
The Company has established a corporate-level global Information Security Incident Response Team (“ISIRT”), which provides a centralized, coordinated response to, and management of, cybersecurity incidents that may present significant risk to the Company’s operations, valuation, brand or reputation, employees, and customer or business relationships. The Company’s cybersecurity response team is comprised of multiple subject-matter experts, including information technology, cybersecurity and risk management members with a combined experience of well over 60 years. Core members of the ISIRT consist of the Vice President, Financial Operations and Project Management (“Financial Ops”); Senior Vice President, General Counsel, and Corporate Secretary (“General Counsel”); Chief Information Security Officer (“CISO”) who reports to the Chief Information Officer (“CIO”); Chief Privacy Officer (“CPO”); Vice President, Corporate Treasurer (“Treasury”); Director, Risk Management & Insurance (“Risk Management”); and Cyber Security Manager (“Information Security”). If a cybersecurity incident warrants activation of the ISIRT, the Company’s Financial Ops and the General Counsel will notify, as appropriate, the Company’s executive leadership and the Audit Committee. We also engage specialized third-party consultants to proactively support our cybersecurity efforts, which include but are not limited to, application and network security, information risk management, as well as business continuity and disaster recovery.
Cybersecurity incidents may occur at, or be reported to, any of the Company’s facilities worldwide. The Company has an IT Service Desk which acts as the single point of contact for cybersecurity incident reporting. Employees can notify the IT Service Desk of any event that they observe or is reported to them that may constitute a cybersecurity incident. Once notified, the IT Service Desk team conducts an initial classification and escalates, when needed, to the CISO and other members of ISIRT as per the Company’s IRP. Financial Ops, in consultation with the General Counsel, CPO and CISO, decide whether to activate the ISIRT in connection with any escalated incident. When activated, the ISIRT coordinates and directs all aspects of the response, including, as applicable, investigation, containment, business continuity and recovery, remediation, notifications, communications, and post-incident activities with executive leadership, including the CIO, and the Audit Committee and/or Board of Directors, as appropriate in the circumstances. As of December 31, 2023, no identified risk has required activation of the ISIRT.
In addition, our third-party service providers play a role in our risk management and strategy as well as with the investigation of cybersecurity incidents. Based upon the assessment of the type of incident and risk presented, the ISIRT engages outside counsel and/or external resources, such as forensic consultants, to conduct or assist with cybersecurity investigations in order to provide advice to the Company. The vendors we engage with are globally recognized companies with expertise in cybersecurity. We conduct due diligence before onboarding new vendors and maintain ongoing evaluations to ensure compliance with our security standards.
For a discussion of information technology rights that may materially impact us, see Item 1A “Risk Factors—We rely on information technology to support our operations and reporting environments. A security failure of that technology, including with respect to cybersecurity, could impact our ability to operate our businesses effectively, adversely affect our financial results, damage our reputation and expose us to potential liability or litigation.”
33
ITEM 2 PROPERTIES
We believe that our existing principal facilities are well maintained and in good operating condition and that they are adequate for our foreseeable business needs.
In addition to the principal facilities noted below, we lease additional facilities for sales, applications and service support in various countries throughout the world including Australia, Austria, Belgium, Brazil, China, Czech Republic, France, Germany, Hong Kong, India, Israel, Italy, Japan, Kenya, Malaysia, Mexico, Netherlands, Norway, Poland, Portugal, Russia, Singapore, South Africa, South Korea, Spain, Sweden, Switzerland, Taiwan, Thailand, Turkey, the United Kingdom and the United States. If we should require additional or alternative facilities, we believe that such facilities can be obtained on short notice at competitive rates.
The location and general character of our principal properties are as follows:
Location |
|
Principal Use |
|
Approximate |
|
|
Relationship |
|
Principal Facilities Used in Current Operations for BSI BioSpin: |
|
|
|
|
|
|||
Ettlingen, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
360,000 |
|
|
Owned |
Faellanden, Switzerland |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
381,000 |
|
|
Owned |
|
Wissembourg, France |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
189,000 |
|
|
Owned |
Principal Facilities Used in Current Operations for BSI CALID: |
|
|
|
|
|
|||
Bremen, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
298,000 |
|
|
Owned |
Ettlingen, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
182,000 |
|
|
Owned |
Faellanden, Switzerland |
|
Manufacturing, Research and Development, Marketing, Sales and Administrative |
|
|
14,600 |
|
|
Leased |
Nehren, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
89,000 |
|
|
Owned/ |
Principal Facilities Used in Current Operations for BSI Nano: |
|
|
|
|
|
|||
Karlsruhe, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
145,000 |
|
|
Owned |
Berlin, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
243,000 |
|
|
Owned |
Santa Barbara, CA, U.S.A. |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
100,000 |
|
|
Owned |
Graz, Austria |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
30,000 |
|
|
Leased |
Penang, Malaysia |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
100,000 |
|
|
Leased |
Migdal HaEmek, Israel |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
22,000 |
|
|
Leased |
Del Ray Beach, FL, U.S.A. |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
30,100 |
|
|
Leased |
Emeryville, CA, U.S.A. |
|
Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
84,500 |
|
|
Leased |
Principal Facilities Used in Current Operations for BEST: |
|
|
|
|
|
|||
Perth, Scotland |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
47,000 |
|
|
Owned |
Hanau, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
138,000 |
|
|
Owned |
Bergisch Gladbach, Germany |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
85,000 |
|
|
Owned |
|
Carteret, NJ, U.S.A. |
|
Manufacturing, Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
115,000 |
|
|
Leased |
Shared Principal Facilities: |
|
|
|
|
|
|||
Billerica, MA, U.S.A. |
|
Research and Development, Application and Demonstration, Marketing, Sales and Administrative |
|
|
200,000 |
|
|
Owned |
34
ITEM 3 LEGAL PROCEEDINGS
We are involved in lawsuits, claims, and proceedings, including, but not limited to, patent and commercial matters, which arise in the ordinary course of business. There are no such matters pending that we currently believe are reasonably likely to have a material impact on our business or to our consolidated financial statements.
Details on recent legal matters can be found in Note 18 to our consolidated financial statements included in Item 8 of this Annual Report on Form 10-K.
In addition, we are subject to regulation by national, state and local government agencies in the United States and other countries in which we operate. From time to time, we are the subject of governmental investigations often involving regulatory, marketing and other business practices. These governmental investigations may result in the commencement of civil and criminal proceedings, fines, penalties and administrative remedies which could have a material adverse effect on our financial position, results of operations and/or liquidity.
ITEM 4 MINE SAFETY DISCLOSURES ITEM 5 MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Not applicable.
35
PART II
Market Prices
Our common stock is traded on the Nasdaq Global Select Market under the symbol “BRKR.”
As of February 6, 2024, there were approximately 67 holders of record of our common stock. This number does not include individual beneficial owners of shares held in nominee name or within clearinghouse positions of brokerage firms and banks.
Dividends
In February 2016, we announced the establishment of a dividend policy and the declaration by our Board of Directors of an initial quarterly cash dividend in the amount of $0.04 per share of our issued and outstanding common stock. Beginning in 2022, we increased the quarterly cash dividend to our shareholders in the amount of $0.05 per share of our issued and outstanding common stock and in February 2024 announced that our Board had approved a quarterly dividend for the first quarter of 2024 of $0.05 per share, payable in March. Future dividend payments, if any, are subject to approval of our Board of Directors.
Stock Price Performance Graph
The graph below compares Bruker Corporation’s annual percentage change in cumulative total return on common shares over the past five years with the cumulative total return of companies comprising the Nasdaq US Benchmark TR Index and the SIC Code 3826 Laboratory Analytical Instruments Index. This presentation assumes that $100 was invested in shares of the relevant issuers on December 31, 2018, and that dividends received were immediately invested in additional shares. The graph plots the value of the initial $100 investment at one-year intervals for the fiscal years shown.
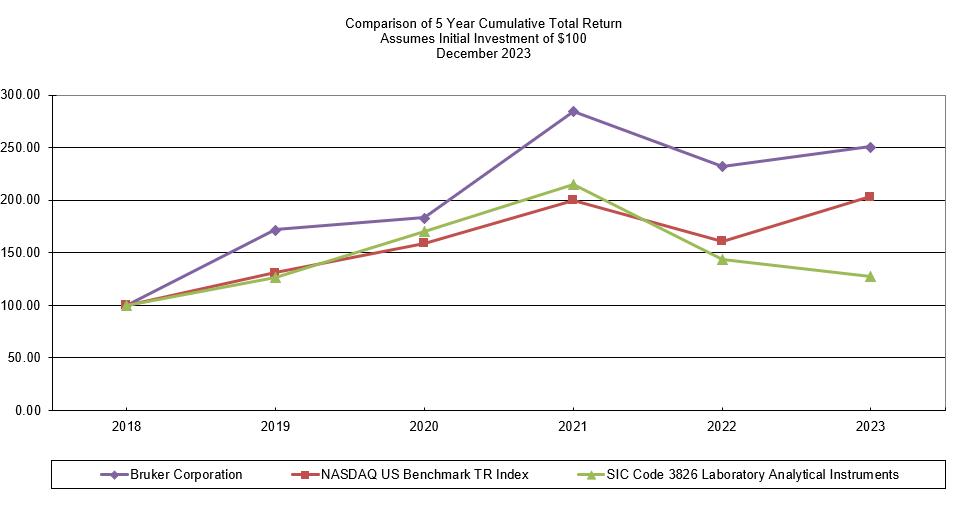
Cumulative Total Return Index for: |
|
2018 |
|
|
2019 |
|
|
2020 |
|
|
2021 |
|
|
2022 |
|
|
2023 |
|
||||||
Bruker Corporation |
|
$ |
87.18 |
|
|
$ |
149.82 |
|
|
$ |
159.67 |
|
|
$ |
248.06 |
|
|
$ |
202.70 |
|
|
$ |
250.70 |
|
Nasdaq US Benchmark TR Index |
|
|
94.56 |
|
|
|
124.03 |
|
|
|
150.41 |
|
|
|
189.36 |
|
|
|
152.00 |
|
|
|
203.23 |
|
SIC Code 3826 Laboratory Analytical Instruments |
|
|
108.61 |
|
|
|
137.43 |
|
|
|
184.99 |
|
|
|
228.85 |
|
|
|
152.79 |
|
|
|
127.67 |
|
The data for this performance graph was compiled by Zack’s Investment Research, Inc. and is used with its permission.
36
Issuer Purchases of Securities
The following table provides information about purchases made by or on behalf of the Company or any “affiliated purchaser,” as defined in Rule 10b-18(a)(3) under the Exchange Act, during the quarter ended December 31, 2023, of shares of our common stock.
Period |
|
Total Number of |
|
|
Average Price |
|
|
Total Number of |
|
|
Maximum Number (or |
|
||||
October 1—October 31, 2023 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
420,271,418 |
|
November 1—November 30, 2023 |
|
|
848,818 |
|
|
|
58.90 |
|
|
|
848,818 |
|
|
|
370,278,694 |
|
December 1—December 31, 2023 |
|
|
4,840 |
|
|
|
73.63 |
|
|
|
4,840 |
|
|
|
369,922,324 |
|
|
|
|
853,658 |
|
|
$ |
58.98 |
|
|
|
853,658 |
|
|
$ |
369,922,324 |
|
In May 2021, the Company’s Board of Directors approved a share repurchase program (the “2021 Repurchase Program”) authorizing the purchase of the Company’s common stock of up to $500.0 million, in amounts, at prices, and at such times as management deems appropriate, subject to market conditions, legal requirements and other considerations. We purchased a total of 4,215,094 shares at an aggregate cost of $264.7 million under the 2021 Repurchase Program during the year ended December 31, 2022. During the year ended December 31, 2023, we purchased a total of 315,318 shares at an aggregate cost of $22.2 million under the 2021 Repurchase Program. Authorization for the remaining $94.4 million on the 2021 Repurchase Program expired in May 2023.
In May 2023, our Board of Directors approved the “2023 Repurchase Program” authorizing the purchase of up to $500.0 million of our common stock over a two-year period, in amounts, at prices, and at such times as we deem appropriate, subject to market conditions, legal requirements and other consideration. We purchased a total of 2,097,119 shares of common stock with an aggregate cost of approximately $130.1 million under the 2023 Repurchase Program during the year ended December 31, 2023. The Company currently intends to fund future share repurchases from cash on hand, future cash flows from operations and available borrowings under our revolving credit facility.
ITEM 6 RESERVED
37
ITEM 7 MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and notes to those statements, appearing elsewhere in this report. This report contains forward-looking statements reflecting our current expectations that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. The cautionary statements made in this report should be read as applying to all related forward-looking statements wherever they appear in this report. Our actual results may differ materially from those indicated in the forward-looking statements due to a number of factors, including those discussed in Item 1A, Risk Factors and elsewhere in this report.
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A, describes the principal factors affecting the results of our operations, financial condition and changes in financial condition, as well as our critical accounting policies and estimates. Our MD&A is organized as follows:
Non-GAAP Measures
Although our consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”), we believe describing revenue and expenses, excluding the effects of foreign currency, acquisitions and divestitures, as well as certain other charges, net, provides meaningful supplemental information regarding our performance. We rely internally on certain measures that are not calculated according to GAAP. These measures are organic revenue, free cash flow, non-GAAP gross profit margin and non-GAAP operating margin. Our management believes that these financial measures provide relevant and useful information that is widely used by equity analysts, investors and competitors in our industry, as well as by our management, in assessing both consolidated and business unit performance.
We define the term organic revenue as GAAP revenue excluding the effect of foreign currency translation changes and the effect of acquisitions and divestitures. We define the term non-GAAP gross profit margin as GAAP gross profit margin with certain non-GAAP measures excluded and non-GAAP operating margin as GAAP operating margin with certain non-GAAP measures excluded. These non-GAAP measures exclude costs related to restructuring actions, acquisition and related integration expenses, amortization of acquired intangible assets, costs associated with our global information technology transition initiatives, and other non-operational costs and we believe these are useful measures to evaluate our continuing business.
We define free cash flow as GAAP net cash provided by operating activities less additions to property, plant, and equipment. We believe free cash flow is a useful measure to evaluate our business as it indicates the amount of cash generated after additions to property, plant, and equipment which is available for, among other things, investments in our business, acquisitions, share repurchases, dividends and repayment of debt.
We regularly use these non-GAAP financial measures internally to understand, manage, and evaluate our business results and make operating decisions. We also measure our employees and compensate them, in part, based on such non-GAAP measures and use this information for our planning and forecasting activities. These measures may also be useful to investors in evaluating the underlying operating performance of our business. The presentation of these non-GAAP financial measures is not intended to be a substitute for, or superior to, the financial information prepared and presented in accordance with GAAP and may be different from non-GAAP financial measures used by other companies, and therefore, may not be comparable among companies.
38
OVERVIEW
We are a developer, manufacturer and distributor of high-performance scientific instruments and analytical and diagnostic solutions that enable our customers to explore life and materials at microscopic, molecular and cellular levels. Our corporate headquarters are located in Billerica, Massachusetts. We maintain major technical and manufacturing centers in Europe, Asia and North America and we have commercial offices located throughout the world. Bruker is organized into four reportable segments: the BSI BioSpin Segment, the BSI CALID Segment, the BSI Nano Segment and the Bruker Energy & Supercon Technologies (BEST) Segment.
Revenue for the year ended December 31, 2023, increased by $433.8 million, or 17.1%, to $2,964.5 million, compared to $2,530.7 million for the comparable period in 2022. Included in revenue was an increase of approximately $11.2 million from favorable foreign exchange rate movements, and an increase of $56.2 million from acquisitions. Excluding the effects of foreign exchange rate movements and our recent acquisitions, our organic revenue, a non-GAAP measure, increased $366.4 million, or 14.5%. Revenue increases were driven by strong demand for our differentiated high-value scientific instruments and life science solutions in addition to pricing benefits compared to the same period in 2022.
Our gross profit margin decreased to 51.0% for the year ended December 31, 2023, as compared to 51.6% in the same period in 2022, primarily as result of the unfavorable impact of foreign exchange rate movements offset by modest improvement from mix, pricing, and acquisitions compared to 2022.
The income tax provision for the years ended December 31, 2023, and December 31, 2022 was $117.7 million and $116.4 million, respectively, representing effective tax rates of 21.6% and 28.1%, respectively. The decrease in our effective tax rate for the year ended December 31, 2023, compared to 2022, was primarily due to the non-taxable nature of the gain on bargain purchase from the acquisition of PhenomeX.
Diluted earnings per share for the year ended December 31, 2023, was $2.90, an increase of $0.91, compared to $1.99 per share in the same period in 2022. The increase in diluted earnings per share was primarily driven by higher net income including the bargain purchase gain associated with the acquisition of PhenomeX, and reduced diluted outstanding shares resulting from our share repurchase activity during the year.
The following table presents a reconciliation from net cash provided by operating activities, which is the most directly comparable GAAP operating financial measure, to free cash flow as used by management (in millions):
|
|
Year Ended |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net cash provided by operating activities |
|
$ |
350.1 |
|
|
$ |
274.4 |
|
Less: purchases of property, plant and equipment |
|
|
(106.9 |
) |
|
|
(129.2 |
) |
Free cash flow |
|
$ |
243.2 |
|
|
$ |
145.2 |
|
For the year ended December 31, 2023, our free cash flow was $98.0 million higher than the same period in 2022, primarily due to improved working capital performance and lower capital expenditures.
The following table presents a reconciliation from gross profit and gross profit margin, which are the most directly comparable GAAP operating performance measures, to non-GAAP gross profit and non-GAAP gross profit margin as used by management (dollars in millions):
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
2023 |
|
|
2022 |
|
||||||||||
Gross profit |
|
$ |
1,513.3 |
|
|
|
51.0 |
% |
|
$ |
1,305.7 |
|
|
|
51.6 |
% |
Non-GAAP adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Restructuring costs |
|
|
3.5 |
|
|
|
0.1 |
% |
|
|
0.9 |
|
|
|
— |
|
Acquisition-related costs |
|
|
2.5 |
|
|
|
0.1 |
% |
|
|
0.4 |
|
|
|
— |
|
Purchased intangible amortization |
|
|
24.3 |
|
|
|
0.9 |
% |
|
|
18.3 |
|
|
|
0.8 |
% |
Other costs |
|
|
4.0 |
|
|
|
0.1 |
% |
|
|
4.8 |
|
|
|
0.2 |
% |
Non-GAAP gross profit |
|
$ |
1,547.6 |
|
|
|
52.2 |
% |
|
$ |
1,330.1 |
|
|
|
52.6 |
% |
Our non-GAAP gross profit margin was 52.2% and 52.6% in the years ended December 31, 2023, and 2022, respectively remaining fairly consistent year over year.
39
The following table presents a reconciliation from operating income and operating margin, which are the most directly comparable GAAP operating performance measures, to non-GAAP operating income and non-GAAP operating margin as used by management (in millions):
|
|
Year Ended December 31, |
|
|||||||||||||
|
|
2023 |
|
|
2022 |
|
||||||||||
Operating income |
|
$ |
436.9 |
|
|
|
14.7 |
% |
|
$ |
432.7 |
|
|
|
17.1 |
% |
Non-GAAP adjustments: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Restructuring costs |
|
|
22.3 |
|
|
|
0.8 |
% |
|
|
4.8 |
|
|
|
0.2 |
% |
Acquisition-related costs |
|
|
19.3 |
|
|
|
0.7 |
% |
|
|
19.7 |
|
|
|
0.8 |
% |
Purchased intangible amortization |
|
|
47.1 |
|
|
|
1.6 |
% |
|
|
37.1 |
|
|
|
1.5 |
% |
Other costs |
|
|
20.7 |
|
|
|
0.6 |
% |
|
|
11.3 |
|
|
|
0.4 |
% |
Non-GAAP operating income |
|
$ |
546.3 |
|
|
|
18.4 |
% |
|
$ |
505.6 |
|
|
|
20.0 |
% |
Our non-GAAP operating margin was 18.4% and 20.0% for the years ended December 31, 2023, and 2022, respectively. The decrease in our non-GAAP operating margins in 2023 was primarily due to acquisitions and the impact of foreign exchange rate movements.
We can experience quarter-to-quarter fluctuations in our operating results as a result of various factors, some of which are outside our control, such as:
Several of these factors have in the past affected and may continue to affect the amount and timing of revenue recognized on sales of our products and receipt of related payments and will likely continue to do so in the future. Accordingly, our operating results in any particular quarter may not necessarily be an indication of any future quarter’s operating performance.
40
RESULTS OF OPERATIONS
A discussion regarding our results of operations for the fiscal year ended December 31, 2022 compared to 2021 can be found under Item 7 of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 1, 2023, which is available on the SEC’s website at www.sec.gov and our Investor Relations website at https://.ir.bruker.com under the “Financial Info” section.
Year Ended December 31, 2023, Compared to the Year Ended December 31, 2022
Consolidated Results
The following table presents our results for the years ended December 31, 2023, and 2022 (dollars in millions):
|
|
Year Ended December 31, |
|
|
|
|
|
|
|
|||||||
|
|
2023 |
|
|
2022 |
|
|
Dollar |
|
|
Percentage |
|
||||
Product revenue |
|
$ |
2,457.6 |
|
|
$ |
2,109.9 |
|
|
$ |
347.7 |
|
|
|
16.5 |
% |
Service revenue |
|
|
493.8 |
|
|
|
414.6 |
|
|
|
79.2 |
|
|
|
19.1 |
% |
Other revenue |
|
|
13.1 |
|
|
|
6.2 |
|
|
|
6.9 |
|
|
|
111.3 |
% |
Total revenue |
|
|
2,964.5 |
|
|
|
2,530.7 |
|
|
|
433.8 |
|
|
|
17.1 |
% |
Cost of product revenue |
|
|
1,165.2 |
|
|
|
984.0 |
|
|
|
181.2 |
|
|
|
18.4 |
% |
Cost of service revenue |
|
|
285.9 |
|
|
|
240.8 |
|
|
|
45.1 |
|
|
|
18.7 |
% |
Cost of other revenue |
|
|
0.1 |
|
|
|
0.2 |
|
|
|
(0.1 |
) |
|
|
(50.0 |
)% |
Total cost of revenue |
|
|
1,451.2 |
|
|
|
1,225.0 |
|
|
|
226.2 |
|
|
|
18.5 |
% |
Gross profit |
|
|
1,513.3 |
|
|
|
1,305.7 |
|
|
|
207.6 |
|
|
|
15.9 |
% |
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Selling, general and administrative |
|
|
729.4 |
|
|
|
607.4 |
|
|
|
122.0 |
|
|
|
20.1 |
% |
Research and development |
|
|
294.8 |
|
|
|
235.9 |
|
|
|
58.9 |
|
|
|
25.0 |
% |
Other charges, net |
|
|
52.2 |
|
|
|
29.7 |
|
|
|
22.5 |
|
|
|
75.8 |
% |
Total operating expenses |
|
|
1,076.4 |
|
|
|
873.0 |
|
|
|
203.4 |
|
|
|
23.3 |
% |
Operating income |
|
|
436.9 |
|
|
|
432.7 |
|
|
|
4.2 |
|
|
|
1.0 |
% |
Bargain purchase gain |
|
|
144.1 |
|
|
|
— |
|
|
|
144.1 |
|
|
|
|
|
Interest and other income (expense), net |
|
|
(36.8 |
) |
|
|
(18.8 |
) |
|
|
(18.0 |
) |
|
|
95.7 |
% |
Income before income taxes, equity in income of unconsolidated |
|
|
544.2 |
|
|
|
413.9 |
|
|
|
130.3 |
|
|
|
31.5 |
% |
Income tax provision |
|
|
117.7 |
|
|
|
116.4 |
|
|
|
1.3 |
|
|
|
1.1 |
% |
Equity in income of unconsolidated investees, net of tax |
|
|
2.0 |
|
|
|
1.0 |
|
|
|
1.0 |
|
|
|
100.0 |
% |
Consolidated net income |
|
|
428.5 |
|
|
|
298.5 |
|
|
|
130.0 |
|
|
|
43.6 |
% |
Net income attributable to noncontrolling interests in |
|
|
1.3 |
|
|
|
1.9 |
|
|
|
(0.6 |
) |
|
|
(31.6 |
)% |
Net income attributable to Bruker Corporation |
|
$ |
427.2 |
|
|
$ |
296.6 |
|
|
$ |
130.6 |
|
|
|
44.0 |
% |
Revenue
The following table presents revenue, change in revenue, and revenue growth by reportable segment for the years ended December 31, 2023, and 2022 (dollars in millions):
|
|
2023 |
|
|
2022 |
|
|
Dollar |
|
|
Percentage |
|
||||
BSI BioSpin |
|
$ |
798.5 |
|
|
$ |
696.7 |
|
|
$ |
101.8 |
|
|
|
14.6 |
% |
BSI CALID |
|
|
960.4 |
|
|
|
822.2 |
|
|
|
138.2 |
|
|
|
16.8 |
% |
BSI Nano |
|
|
941.9 |
|
|
|
787.0 |
|
|
|
154.9 |
|
|
|
19.7 |
% |
BEST |
|
|
280.7 |
|
|
|
237.1 |
|
|
|
43.6 |
|
|
|
18.4 |
% |
Eliminations (a) |
|
|
(17.0 |
) |
|
|
(12.3 |
) |
|
|
(4.7 |
) |
|
|
|
|
|
|
$ |
2,964.5 |
|
|
$ |
2,530.7 |
|
|
$ |
433.8 |
|
|
|
17.1 |
% |
41
Revenue increases were driven by strong demand for our differentiated instruments and solutions as well as pricing actions taken primarily to address inflation cost pressures. The BSI BioSpin Segment revenue increase was related primarily to the strong demand for our instruments across biopharma, academic government, industrial research and applied markets, our new integrated data solutions division and especially our Nuclear Magnetic Resonance (NMR) and related services. We had revenue from 4 gigahertz class NMR systems each in fiscal 2023 and fiscal 2022. The BSI CALID Segment revenue increase reflected strong demand for our differentiated instruments, primarily, in our life science mass spectrometry proteomics solutions, and our optics spectroscopy and microscopy instruments. Our growth in life science mass spectrometry was driven by the timsTOF platform and aftermarket business, as well as strong growth in applied mass spectrometry and our optics infrared, near infrared and Raman business. Microbiology and infectious disease revenue was up slightly as solid demand for MALDI Biotyper consumables was offset by a final drop of our modest COVID-19 molecular diagnostics revenue. The BSI NANO Segment revenue increase was driven by strong demand in its academic, government, industrial and semiconductor metrology markets. Revenue growth was primarily driven by our advanced X-ray solutions and nano surfaces, core tools and fluorescence microscopy. The BEST revenue increase was driven by strong superconductor demand from our magnetic resonance imaging original equipment manufacturer customers as well as by growth in big science, fusion research, and key new extreme ultraviolet EUV technologies for semiconductor lithography tools by large OEM customers, all in support of the strong artificial intelligence demand.
Geographically for the year ended December 31, 2023, our North America revenue grew 11.4% Asia Pacific increased by 22.9%, and European revenue increased by 16.9%, in each case, as compared to the same period in 2022.
Gross Profit
The increase in gross profit was a result of pricing and volume leverage, partially offset by net unfavorable impact of foreign exchange rate movements compared to 2022.
Selling, General and Administrative
Our selling, general and administrative expenses for the year ended December 31, 2023, increased to 24.6% of total revenue from 24.0% of total revenue for the comparable period in 2022. The year over year increase as a percentage of revenue was a result of inflation and increased planned spending in sales and marketing capabilities compared to 2022. Increased spending is related to additional headcount and personnel expenses as well as increased consulting and professional fees related to acquisitions, tax, audit and audit-related fees.
Research and Development
Our research and development expenses for the year ended December 31, 2023, increased to 9.9% of total revenue from 9.3% of total revenue for the comparable period in 2022. The slight increase as a percentage of revenue is a result of our increased investment in research and development capabilities, especially in our key Project Accelerate 2.0 initiatives. Investments are primarily related to additional headcount and personnel expenses as well as increased consulting and professional fees related to research and development activities.
Other Charges, Net
Other charges, net for the year ended December 31, 2023, consisted of $16.8 million of acquisition-related charges primarily completed in 2023, $18.8 million of restructuring costs related to closing facilities and implementing outsourcing and other restructuring initiatives mainly related to the PhenomeX acquisition as detailed in Note 21 Other Charges, net, $5.0 million of costs associated with our global IT transformation activities, $0.6 million related to professional fees and $5.7 million related to long-lived asset impairments. Acquisition-related charges relate primarily to the integration cost of newly acquired entities and the cost of post-combination employment services in the period acquired. The IT transformation initiative is a multi-year project aimed at updating and integrating our global enterprise resource planning and human resource information systems.
Other charges, net for the year ended December 31, 2022 consisted primarily of $19.3 million of acquisition-related charges related to transactions completed in 2021 and 2020, $3.9 million of restructuring costs related to closing facilities and implementing outsourcing and other restructuring initiatives, $3.0 million of costs associated with our global IT transformation activities, $2.4 million related to professional fees and $0.3 million related to long-lived asset impairments.
42
Operating Income
The following table presents operating income and operating margins on revenue by reportable segment for the years ended December 31, 2023 and 2022 (dollars in millions):
|
|
2023 |
|
|
2022 |
|
||||||||||
|
|
Operating |
|
|
Percentage of |
|
|
Operating |
|
|
Percentage of |
|
||||
BSI BioSpin |
|
$ |
195.9 |
|
|
|
24.5 |
% |
|
$ |
167.1 |
|
|
|
24.0 |
% |
BSI CALID |
|
$ |
209.3 |
|
|
|
21.8 |
% |
|
|
196.0 |
|
|
|
23.8 |
% |
BSI Nano |
|
$ |
109.1 |
|
|
|
11.6 |
% |
|
|
113.0 |
|
|
|
14.4 |
% |
BEST |
|
$ |
32.3 |
|
|
|
11.5 |
% |
|
|
31.3 |
|
|
|
13.2 |
% |
Corporate, eliminations and other (a) |
|
$ |
(109.7 |
) |
|
|
|
|
|
(74.7 |
) |
|
|
|
||
Total operating income |
|
$ |
436.9 |
|
|
|
14.7 |
% |
|
$ |
432.7 |
|
|
|
17.1 |
% |
The increase in operating income was due to higher gross profit resulting from, volume leverage, and price realization offset by planned sales and marketing investments, supply chain and logistics challenges, inflationary margin challenges in 2023, as compared to 2022, and investments in research and development capabilities.
Bargain purchase gain
In the fourth quarter of 2023, the Company recorded a gain of $144.1 million, or $0.99 per share, in connection with the PhenomeX acquisition, which closed on October 2, 2023. This bargain purchase gain reflects the excess of identifiable net assets acquired, including deferred tax assets related to acquired tax NOLs, over the purchase consideration paid.
Interest and Other Income (Expense), Net
The increase in interest and other income (expense), net in the year ended December 31, 2023, as compared to the same period in 2022 was primarily due to impairment charges recognized of $18.2 million on certain minority investments and higher foreign currency exchange losses of $13.3 million driven by weakening of the U.S. dollar against other currencies.
Income Tax Provision
The effective tax rates for years ended 2023 and 2022 were 21.6% and 28.1%, respectively. The decrease in our effective tax rate for the year ended December 31, 2023, compared to 2022, was primarily due to the non-taxable nature of the bargain purchase gain from the acquisition of PhenomeX.
Equity in Income of Unconsolidated Investees, net of tax
Equity in income of unconsolidated investees, net of tax represents the Company’s proportionate share of the earnings or losses as reported by equity-method investees.
Net Income Attributable to Noncontrolling Interests and Redeemable Noncontrolling Interests
The net income attributable to noncontrolling interests represented the minority shareholders’ proportionate share of the net income recorded by our majority-owned subsidiaries.
Net Income Attributable to Bruker Corporation
The increase in net income and earnings per diluted share was primarily driven by the increase in revenue, gross profit and operating profit as a result of strengthened demand and recovery in our business and end markets.
43
LIQUIDITY AND CAPITAL RESOURCES
We anticipate that our existing cash and credit facilities as of December 31, 2023, together with proceeds from notes offerings to be completed pursuant to note purchase agreements entered into in February 2024 and increased borrowing capacity under the amended and restated credit agreement entered into in January 2024, will be sufficient to support our operating and investing needs for at least the next twelve months. Our future cash requirements could be affected by acquisitions that we may complete, purchases of our common stock or the payment of dividends in the future. Historically, we have financed our growth and liquidity needs through cash flow generation from operations and debt financing. In the future, there are no assurances that we will continue to generate cash flow from operations or that additional financing alternatives will be available to us, if required, or if available, will be obtained on terms favorable to us.
Cash, cash equivalents and short-term investments at December 31, 2023, and 2022 totaled $488.3 million and $645.5 million, respectively, of which $398.4 million and $593.8 million, respectively, related to cash, cash equivalents and short-term investments is held outside of the United States in our foreign subsidiaries, most significantly in the Netherlands, Switzerland, China and Hong Kong.
The following table presents our cash flows from operating activities, investing activities and financing activities for the periods presented (in millions):
|
|
Year Ended December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net cash provided by operating activities |
|
$ |
350.1 |
|
|
$ |
274.4 |
|
Net cash used in investing activities |
|
|
(326.0 |
) |
|
|
(251.6 |
) |
Net cash provided by financing activities |
|
|
(193.4 |
) |
|
|
(415.3 |
) |
Effect of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
12.2 |
|
|
|
(30.5 |
) |
Net change in cash, cash equivalents and restricted cash |
|
$ |
(157.1 |
) |
|
$ |
(423.0 |
) |
Cash provided by operating activities during the year ended December 31, 2023, resulted from consolidated net income adjusted for non-cash items of $437.9 million, including the non-cash bargain purchase gain from the acquisition of PhenomeX, partially offset by a change in operating assets and liabilities, net of acquisitions and divestitures of $87.8 million. During the year ended December 31, 2022, net cash provided by operating activities resulted from consolidated net income adjusted for non-cash items of $418.5 million, partially offset by a change in operating assets and liabilities, net of acquisitions and divestitures of $144.1 million. The increase in net income adjusted for non-cash items was mainly due to higher revenue, gross profit, and operating income. The increase in operating cash flows was due to changes in operating assets and liabilities, and was impacted by improvements in receivable collections, timing of tax payments and accounts payable offset by an increase in inventory purchases and prepayments to meet increased order demand.
Cash used in investing activities during the year ended December 31, 2023, resulted primarily from the acquisitions and advances of $226.6 million, purchases of property, plant and equipment of $106.9 million, and minority investments of $24.8 million, partially offset by net proceeds from sales of property, plant and equipment of $11.1 million. Cash used in investing activities during the year ended December 31, 2022, was primarily from the acquisitions of $182.3 million, purchases of property, plant and equipment of $129.2 million, and minority investments of $60.2 million, partially offset by maturity of short-term investments of $100.0 million and $13.9 million of net proceeds from sales of property, plant and equipment.
We currently expect capital expenditures in 2024 to be approximately $125.0 million.
Net cash used in financing activities during the year ended December 31, 2023, was primarily attributable to cash payments made for shares of common stock under our repurchase program of $152.3 million, and $29.4 million for the payment of dividends. Net cash used in financing activities during the year ended December 31, 2022, was primarily attributable to cash payments made for shares of common stock under our repurchase program of $263.1 million, repayment of our 2012 Note Purchase Agreement of $105.0 million and $29.8 million for the payment of dividends.
Share Repurchase Program
In May 2021, the Company’s Board of Directors approved a share repurchase program (the “2021 Repurchase Program”) authorizing the purchase of up to $500.0 million of the Company’s common stock over a two-year period, in amounts, at prices, and at such times as management deems appropriate, subject to market conditions, legal requirements and other considerations. During the year ended December 31, 2023, the Company purchased a total of 315,318 shares at an aggregate cost of $22.2 million under the 2021 Repurchase Program. During the year ended December 31, 2022, the Company purchased a total of 4,215,094 shares at an aggregate cost of $264.7 million under the 2021 Repurchase Program. Authorization for the remaining $94.4 million on the 2021 Repurchase Program expired in May 2023.
44
In May 2023, the Company’s Board of Directors approved a share repurchase program (the “2023 Repurchase Program”) authorizing the purchase of up to $500.0 million of the Company’s common stock over a two-year period, in amounts, at prices, and at such times as management deems appropriate, subject to market conditions, legal requirements and other considerations. During the year ended December 31, 2023, the Company purchased a total of 2,097,119 shares at an aggregate cost of $130.1 million under the 2023 Repurchase Program. At December 31, 2023, $369.9 million remains available for future purchase under the 2023 Repurchase Program. We intend to fund any additional purchases from cash on hand, future cash flows from operations and available borrowings under the revolving credit facility.
In August 2022, the Inflation Reduction Act (“IRA”) was signed into law in the United States. The IRA introduced new tax provisions, including a 1.0% excise tax on stock repurchases. The Company expects additional guidance and regulations to be issued in future periods and will continue to assess its potential impact on its business as further information becomes available. The estimated excise tax on our stock repurchases was not material and was recorded in other current liabilities and additional paid in capital for the year ended December 31, 2023.
Shelf Registration
On June 2, 2023, the Company filed a registration statement on Form S-3ASR with the SEC (“2023 Shelf Registration”) for the issuance of common stock, preferred stock, debt securities, warrants, units, subscription rights and purchase contracts, which became effective immediately upon filing. At the time any of the securities covered by the 2023 Shelf Registration Statement are offered for sale, a prospectus supplement will be prepared and filed with the SEC containing specific information about the terms of any such offering.
Income Taxes
At December 31, 2023 and in accordance with the tax reform legislation signed by the President of the United States on December 22, 2017, or the 2017 Tax Act, we recorded state and foreign withholding taxes, as well as subsequent foreign currency translations on these withholding taxes as they are an obligation of the parent company, on the cash and liquid assets portion of the unremitted earnings and profits (E&P) of foreign subsidiaries expected to be repatriated from our foreign subsidiaries to the United States. We continue to be indefinitely reinvested in the amount of $1.4 billion of non-cash E&P that is subject to the 2017 Tax Act deemed repatriation. If this E&P is ultimately distributed to the United States in the form of dividends or otherwise, we would likely be subject to additional withholding tax. We will continue to evaluate our assertions on the cumulative historical outside basis differences in our foreign subsidiaries as of December 31, 2023. The amount of unrecognized deferred withholding taxes on the undistributed E&P was $95.6 million at December 31, 2023.
As of December 31, 2023, the Company has approximately $588.9 million of U.S. federal net operating loss carryforwards, of which $36.4 million begin to expire at various dates beginning in 2031 and the remainder $552.5 million will be carried forward indefinitely. The Tax Cuts and Jobs Act (TCJA) enacted on December 22, 2017, limits a taxpayer’s ability to utilize NOL deduction in a year to 80% taxable income for federal NOL arising in tax years beginning after 2017. The Company has approximately $676.6 million of state net operating loss carryforwards available to reduce state taxable income that are expected to expire at various times beginning in 2024. The Company also has approximately $97.9 million of German Trade Tax and Corporate Income Tax net operating losses that are carried forward indefinitely. Additionally, the Company has $28.7 million of other foreign net operating losses that are expected to expire at various times in the future. The Company has U.S. federal foreign tax credit carried forwards in the amount of $3.3 million. The Company also has U.S. federal and state research and development tax credit of $1.2 million and $10.3 million respectively. Utilization of these credits and net operating losses may be subject to annual limitations due to the ownership percentage change limitations provided by the Code Section 382 and similar state provisions. In the event of a deemed change in control under Code Section 382, an annual limitation on the utilization of net operating losses and credits may result in the expiration of all or a portion of the net operating loss and credit carryforwards. The Company is in the process of finalizing the impact of Section 382 related to the PhenomeX acquisition but believes the provisions will limit the availability of losses to offset future income. Additionally, the Company has $91.4 million of gross interest expense carryforward as provided by Code Section 163(j) that can be carried forward indefinitely.
Uncertain tax contingencies are positions taken or expected to be taken on an income tax return that may result in additional payments to tax authorities. If a tax authority agrees with the tax position taken or expected to be taken or the applicable statute of limitations expires, then additional payments will not be necessary.
45
Credit Facilities and Note Purchase Agreements
On February 8, 2024, the Company entered into a note purchase agreement pursuant to which the Company will issue and sell (i) CHF 50 million aggregate principal amount of 2.60% Series A Senior Notes due April 15, 2036 and (ii) CHF 50 million aggregate principal amount of its 2.62% Series B Senior Notes due April 15, 2039 in an offering exempt from the registration requirements of the Securities Act of 1933, as amended. The issuance and sale of the notes is subject to satisfaction of a variety of customary closing conditions and is expected to occur on or about April 15, 2024.
On February 1, 2024, the Company entered into a note purchase agreement, pursuant to which the Company will issue and sell (i) CHF 50 million aggregate principal amount of 2.56% Series A Senior Notes due April 15, 2034, (ii) CHF 146 million aggregate principal amount of its 2.62% Series B Senior Notes due April 15, 2036 and (iii) CHF 135 million aggregate principal amount of its 2.71% Series C Senior Notes due April 15, 2039 in an offering exempt from the registration requirements of the Securities Act of 1933, as amended. The issuance and sale of the notes is subject to satisfaction of a variety of customary closing conditions and is expected to occur on or about April 15, 2024.
On January 18, 2024, the Company, together with certain of its subsidiaries, as borrowers and guarantors, entered into an amended and restated credit agreement with Deutsche Bank Securities Inc., JPMorgan Chase Bank, N.A., TD Bank, N.A., and Wells Fargo Bank, National Association, as Co-Syndication Agents, BofA Securities, Inc., Deutsche Bank Securities Inc., JPMorgan Chase Bank, N.A., TD Bank, N.A. and Wells Fargo Securities, LLC, as Joint Bookrunners and Joint Lead Arrangers, Citizens Bank, N.A., Credit Suisse (Switzerland) Ltd., and U.S. Bank, N.A., as Co-Documentation Agents, ING Bank B.V. and PNC Bank, N.A., as Managing Agents, Bank of America, N.A., as Administrative Agent, Swing Line Lender and Issuing Bank, and the several banks or other financial institutions or entities from time to time party thereto as lenders. The amended and restated credit agreement amends and restates the credit agreement entered into by the Company and certain of its subsidiaries on December 11, 2019 with the other parties thereto.
The amended and restated credit agreement increases the aggregate principal amount from $600 million to $900 million and extends the maturity date to January 18, 2029, as may be further extended by the Company for the periods and on the terms set forth in the amended and restated credit agreement. In addition, the amended and restated credit agreement increases the uncommitted incremental facility whereby, under certain circumstances, the Company may, at its option, increase the amount of the revolving facility or incur term loans in an aggregate amount not to exceed $400 million.
In addition, we paid the remaining $100 million of our 4.46% Series 2012A Tranche D, private placement notes on January 18, 2024.
On January 12, 2024, the Company borrowed CHF 230 million under the Company's 2019, Revolving Credit Agreement.
As of December 31, 2023, we have several cross-currency and interest rate swap agreements with a notional value of $139.1 million of U.S. Dollar to Swiss Franc and a notional value of $239.1 million of U.S. Dollar to Euro to hedge the variability in the movement of foreign currency exchange rates on portions of our Euro and Swiss Franc denominated net asset investments. As a result of these agreements, we lowered our net interest expense by $18.3 million and $8.6 million during the year ended December 31, 2023, and 2022, respectively. We anticipate these swap agreements will lower net interest expense by approximately $12.7 million in 2024 and $8.8 million in 2025.
For a summary of the fair and carrying values of our outstanding debt as of December 31, 2023, and December 31, 2022, please read Note 12, Debt, to our consolidated financial statements included in our 2023 Form 10-K.
As of December 31, 2023, we had no off-balance sheet arrangements.
As of December 31, 2023, we were compliant with the financial covenants of these debt agreements.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, or U.S. GAAP, which requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period.
We consider our accounting estimates to be critical to the consolidated financial statements if (i) the estimate requires significant judgment or is complex in nature and (ii) if different estimates and assumptions were used, the results could have a material impact on our consolidated financial statements. We evaluate our estimates and the application of our policies on an ongoing basis.
46
We base our estimates and judgments on our historical experience, current market and economic conditions, industry trends, and other assumptions that we believe are reasonable. Actual results could differ from these estimates. Changes in estimates are recorded in the period in which they become known.
We believe the following critical accounting policies and estimates to be both those most important to the portrayal of our financial position and results of operations and those that require the most estimation and subjective judgment.
Revenue recognition
We recognize revenue in accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers (ASC 606). The key elements of ASC 606 are: 1) identifying a contract with the customer; 2) identifying the performance obligations in the contract; 3) determining the transaction price; 4) allocating the transaction price to the performance obligations in the contract; and 5) recognizing revenue when (or as) each performance obligation is satisfied.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. Some of our contracts have multiple performance obligations, most commonly due to providing additional goods or services along with a system, such as installation, accessories, parts and services. For contracts with multiple performance obligations, we allocate the contract’s transaction price to each performance obligation using the best estimate of the standalone selling price of each distinct good or service being provided to the customer. Our best evidence of standalone selling price is our normal selling pricing and discounting practices for the specific product or service when sold on a standalone basis. Alternatively, when not sold separately, we may determine standalone selling price using an expected cost plus a margin approach.
Our performance obligations are typically satisfied at a point in time, most commonly either on shipment, or customer acceptance. Certain performance obligations, such as maintenance contracts and extended warranty, are recognized over time based on the contractual obligation period. In addition, certain arrangements to provide more customized deliverables may be satisfied over time based on the extent of progress towards completion. For performance obligations recognized over time, revenue is measured by progress toward completion of the performance obligation that reflects the transfer of control. Typically, progress is measured using a cost-to-cost method based on cost incurred to date relative to total estimated costs upon completion as this best depicts the transfer of control to the customer. Application of the cost-to-cost method requires us to make reasonable estimates of the extent of progress toward completion and the total costs we expect to incur. Losses are recorded immediately when we estimate that contracts will ultimately result in a loss. Changes in the estimates could affect the timing of revenue recognition.
We include costs incurred in connection with shipping and handling of products within selling, general and administrative costs. Amounts billed to customers in connection with these costs are included in total revenues. When control of the goods transfers prior to the completion of our obligation to ship the products to our customers, we have elected the practical expedient to account for the shipping services as a fulfillment cost. We expense incremental costs of obtaining a contract as and when incurred if the expected amortization period is one year or less or the amount is immaterial. We exclude from the transaction price all taxes assessed by a governmental authority on revenue-producing transactions that are collected by us from a customer.
We recognize revenue from systems sales upon transfer of control in an amount that reflects the consideration we expect to receive. Transfer of control generally occurs upon shipment, or for certain systems, based upon customer acceptance for a system once installed at a customer facility. For systems that include customer-specific acceptance criteria, we are required to assess when we can demonstrate the acceptance criteria has been met, which generally is upon successful factory acceptance testing or customer acceptance and evidence of installation. For systems that require installation and where system revenue is recognized upon shipment, the standalone selling price of installation is deferred until customer acceptance. Revenue from accessories and parts is generally recognized based on shipment. Service revenue is recognized as the services are performed or ratably over the contractual obligation and includes maintenance contracts, extended warranties, training, application support and on-demand services.
When products are sold through an independent distributor or a strategic distribution partner, we recognize the system sale upon transfer of control which is typically on shipment. When we are responsible for installation, the standalone selling price of installation is deferred until customer acceptance. Our distributors do not have price protection rights or rights of return; however, our products are typically warranted to be free from defect for a period of one year.
We require an advance deposit based on the terms and conditions of contracts with customers for many of our contracts. Typically, revenue is recognized within one year of receiving an advance deposit. We do not have any material payment terms that extend beyond one year. There is minimal variable consideration included in the transaction price of our contracts.
Other revenues are primarily comprised of development arrangements recognized on a cost-plus-fixed-fee basis and licensing arrangements recognized either when the licenses are provided or ratably over the contract term depending on the nature of the arrangement.
47
Income taxes
The provision for income taxes includes federal, state, local and foreign taxes. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences of temporary differences between the financial statement carrying amounts and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which the temporary differences are expected to be recovered or settled. We evaluate the realizability of our deferred tax assets and establish a valuation allowance when it is more likely than not that all or a portion of deferred tax assets will not be realized.
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based upon the level of historical taxable income and income tax liability and projections for future taxable income over the periods in which the deferred tax assets are utilizable, we believe it is more likely than not that we will realize the net benefits of the deferred tax assets of our wholly owned subsidiaries, net of the recorded valuation allowance. In the event that actual results differ from our estimates, or we adjust our estimates in future periods, we may need to adjust or establish a valuation allowance, which could materially impact our consolidated financial position and results of operations.
We record liabilities related to uncertain tax positions in accordance with the guidance that clarifies the accounting for uncertainty in income taxes recognized in our financial statements. This guidance prescribes a minimum recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. We include accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, in income tax expense.
Goodwill, other intangible assets and other long-lived assets
Goodwill and indefinite-lived intangible assets are not amortized, but are evaluated for impairment on an annual basis, or on an interim basis when events or changes in circumstances indicate that the carrying value may not be recoverable. In assessing the recoverability of goodwill and indefinite-lived intangible assets, we must make assumptions regarding the estimated future cash flows, including forecasted revenue growth and the discount rate to determine the fair value of these assets. If these estimates or their related assumptions change in the future, we may be required to record impairment charges against these assets in the reporting period in which the impairment is determined.
We test goodwill for impairment at the reporting unit level, which is the operating segment or one level below an operating segment. We have the option of performing a qualitative assessment to determine whether further impairment testing is necessary before performing the quantitative assessment. If as a result of the qualitative assessment, it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount, a quantitative impairment test will be required. Otherwise, no further testing will be required. If a quantitative impairment test is performed, we compare the fair values of the applicable reporting units with their aggregate carrying values, including goodwill. We determine the fair value of reporting units using a weighting of both the market and the income methodologies. Estimating the fair value of the reporting units requires significant judgment by management. If the carrying amount of a reporting unit exceeds the fair value of the reporting unit, an impairment charge is recognized for the amount by which the carrying value amount exceeds the reporting unit’s fair value up to the total amount of goodwill allocated to the reporting unit.
In process research and development, or IPR&D, acquired as part of business combinations under the acquisition method represents ongoing development work associated with enhancements to existing products, as well as the development of next generation products. IPR&D is initially capitalized at fair value as an intangible asset with an indefinite life and assessed for impairment on an annual basis, or when indicators of impairment are identified. When the IPR&D project is complete, it is reclassified as a finite-lived intangible asset and is amortized over its estimated useful life. If an IPR&D project is abandoned before completion or is otherwise determined to be impaired, the value of the asset or the amount of the impairment is charged to the consolidated statements of income and comprehensive income in the period the project is abandoned or impaired.
Business Combinations
We account for business combinations under the acquisition method of accounting. Accordingly, at the date of each acquisition, we measure the fair value of all identifiable assets acquired (including intangible assets), liabilities assumed and any remaining noncontrolling interests and allocate the amounts paid to all items measured. The fair value of identifiable intangible assets acquired is based on valuations that use information and assumptions determined by management and which consider management’s best estimates of inputs and assumptions that a market participant would use. Any excess of fair value of acquired net assets, including identifiable intangible assets over the acquisition consideration, results in a bargain purchase gain.
48
RECENT ACCOUNTING PRONOUNCEMENTS
Information regarding recently issued accounting pronouncements may be found in Note 3 to our consolidated financial statements included in Item 8 of this Annual Report on Form 10-K.
ITEM 7A QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are potentially exposed to market risks associated with changes in foreign currency translation rates, interest rates and commodity prices. We selectively use financial instruments to reduce these risks. All transactions related to risk management techniques are authorized and executed pursuant to our policies and procedures. Analytical techniques used to manage and monitor foreign currency translation and interest rate risk include market valuations and sensitivity analysis.
We have estimated our market risk exposure using sensitivity analysis. To test the sensitivity of our market risk exposure, we have estimated the changes in fair value of market risk sensitive instruments assuming a hypothetical 10 percent adverse change in market prices or rates. The results of the sensitivity analyses are summarized below.
Foreign Currency Risk
We generate a substantial portion of our revenues in international markets, principally Germany and other countries in the European Union, Switzerland and Japan, which exposes our operations to the risk of exchange rate fluctuations. The impact of currency exchange rate movement can be positive or negative in any period. Our costs related to sales in foreign currencies are largely denominated in the same respective currencies, reducing our transaction risk exposure. However, for foreign currency denominated sales in certain regions, such as Japan, where we do not incur significant costs denominated in Japanese Yen, we are more exposed to the impact of foreign currency fluctuations.
For sales not denominated in U.S. Dollars, if there is an increase in the rate at which a foreign currency is exchanged for U.S. Dollars, it will require more of the foreign currency to equal a specified amount of U.S. Dollars than before the rate increase. In such cases, if we price our products in the foreign currency, we will receive less in U.S. Dollars than we would have received before the rate increase went into effect. If we price our products in U.S. Dollars and competitors price their products in local currency, an increase in the relative strength of the U.S. Dollar could result in our prices not being competitive in a market where business is transacted in the local currency. For example, if the U.S. Dollar strengthened against the Japanese Yen, our Japanese-based competitors would have a greater pricing advantage over us.
Our revenue by geography was as follows (dollars in millions):
|
|
2023 |
|
|
2022 |
|
||||||||||
|
|
Revenue |
|
|
Percentage of |
|
|
Revenue |
|
|
Percentage of |
|
||||
United States |
|
$ |
777.7 |
|
|
|
26.2 |
% |
|
$ |
696.1 |
|
|
|
27.5 |
% |
Europe |
|
|
981.2 |
|
|
|
33.1 |
% |
|
|
839.3 |
|
|
|
33.2 |
% |
Asia Pacific |
|
|
989.0 |
|
|
|
33.4 |
% |
|
|
804.9 |
|
|
|
31.8 |
% |
Rest of world |
|
|
216.6 |
|
|
|
7.3 |
% |
|
|
190.4 |
|
|
|
7.5 |
% |
Total revenue |
|
$ |
2,964.5 |
|
|
|
100.0 |
% |
|
$ |
2,530.7 |
|
|
|
100.0 |
% |
Changes in foreign currency exchange rates increased our revenue by approximately 0.4% in the year ended December 31, 2023, and decreased it by 2.2% in the year ended December 31, 2022.
Assets and liabilities of our foreign subsidiaries, where the functional currency is the local currency, are translated into U.S. Dollars using period-end exchange rates. Revenues and expenses of foreign subsidiaries are translated at the average exchange rates in effect during the year. Adjustments resulting from financial statement translations are included as a separate component of shareholders’ equity. We recorded a net gain of $15.1 million and a net loss of $22.0 million, in the years ended December 31, 2023, and December 31, 2022, respectively, from currency translation adjustments. A 10% depreciation in functional currencies, relative to the U.S. Dollar, at December 31, 2023, would have resulted in a reduction of shareholders’ equity of approximately $303.6 million. Gains and losses resulting from foreign currency transactions are reported in interest and other income (expense), net in the consolidated statements of income and comprehensive income. Our foreign currency transaction gain (loss), net were net losses of $13.3 million and $5.8 million for years ended December 31, 2023, and 2022, respectively.
49
The impact of currency exchange rate movement can be positive or negative in any period. We periodically enter into foreign currency contracts in order to minimize the volatility that fluctuations in currency translation have on our monetary transactions. Under these arrangements, we typically agree to purchase a fixed amount of a foreign currency in exchange for a fixed amount of U.S. Dollars or other currencies on specified dates with maturities of less than twelve months, with some agreements extending to longer periods. These transactions do not qualify for hedge accounting and, accordingly, the instrument is recorded at fair value with the corresponding gains and losses recorded in the consolidated statements of income and comprehensive income.
As of December 31, 2023, we have several cross-currency and interest rate swap agreements with a notional value of $139.1 million of U.S. Dollar to Swiss Franc and a notional value of $239.1 million of U.S. Dollar to Euro to hedge the variability in the movement of foreign currency exchange rates on portions of our Euro and Swiss Franc denominated net asset investments. Under the U.S. GAAP hedge accounting guidance, changes in fair value of the derivative that relates to changes in the foreign currency spot rate are recorded in the currency translation adjustment in comprehensive income (loss) and remain in accumulated comprehensive income (loss) in shareholders’ equity until the sale or substantial liquidation of the foreign operation. The difference between the interest rate received and paid under the interest rate cross-currency swap derivative agreement is recorded in interest income in the statement of income.
From time to time, we have entered into forward currency contracts designed to minimize the volatility that fluctuations in foreign currency have on our cash flows related to purchases and sales denominated in foreign currencies. Under these arrangements, we agree to purchase a fixed amount of a foreign currency in exchange for a fixed amount of U.S. Dollars or other currencies on specified dates typically with maturities of less than twelve months with some agreements extending to longer periods. These transactions are recorded at fair value with the corresponding gains and losses recorded in interest and other income (expense), net in the consolidated statements of income and comprehensive income. At December 31, 2023, and 2022, we had forward currency contracts with notional amounts aggregating $489.5 million and $187.2 million, respectively. We will continue to evaluate our currency risks, and in the future, may utilize foreign currency contracts more frequently.
Interest Rate Risk
We regularly invest excess cash in short-term investments that are subject to changes in interest rates. We believe that the market risk arising from holding these financial instruments is minimal because of our policy of investing in short-term financial instruments issued by highly rated financial institutions.
Our exposure related to adverse movements in interest rates is derived primarily from outstanding floating rate debt instruments that are indexed to short-term market rates. We currently have a higher level of fixed rate debt than variable rate debt, which limits the exposure to adverse movements in interest rates.
Commodity Price Risk
We are exposed to certain commodity risks associated with prices for various raw materials. The prices of copper and certain other raw materials, particularly niobium-tin, used to manufacture superconductors have increased significantly over the last decade. Copper and niobium-tin are the main components of low temperature superconductors and continued commodity price increases for copper and niobium, as well as other raw materials, may negatively affect our profitability. Periodically, we enter into commodity forward purchase contracts to minimize the volatility that fluctuations in the price of copper have on our sales of these products. At December 31, 2023, and 2022, we had fixed price commodity contracts with notional amounts aggregating $8.4 million and $8.9 million, respectively. The fair value of the fixed price commodity contracts at December 31, 2023, and 2022 was $0.3 million and $0.6 million, respectively. As commodity contracts settle, gains (losses) as a result of changes in fair values are adjusted to the contracts with the customers through revenues. We will continue to evaluate our commodity risks and may utilize commodity forward purchase contracts more frequently in the future.
Inflation Risk
We are subject to inflationary cost pressures across global operating supply networks. Certain components, parts, or materials are experiencing significant cost pressures that have impacted or may impact our cost of operations in future periods. Further, inflation has increased our selling, general and administrative expenses and may vary between countries in which we operate. We continue to evaluate these cost increases in relation to our new orders and may continue to see a negative impact on our financial results for a period of time.
50
ITEM 8 FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index of Consolidated Financial Statements
51
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Bruker Corporation
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of Bruker Corporation and its subsidiaries (the “Company”) as of December 31, 2023 and 2022, and the related consolidated statements of income, of comprehensive income, of redeemable noncontrolling interests and shareholders’ equity and of cash flows for each of the three years in the period ended December 31, 2023, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2023 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company’s internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
As described in Management’s Report on Internal Control over Financial Reporting, management has excluded Biognosys AG, Osthus Beteiligungs GmbH, MIRO Analytical AG, PhenomeX Inc. (Bruker Cellular Analysis), Acquifer Imaging GmbH, Deltabyte GmbH, Pinpoint Testing LLC, Fasmatech Science and Technology SA, Interherence GmbH, and Signals GmbH & Co. KG, from its assessment of internal control over financial reporting as of December 31, 2023 because they were acquired by the Company in purchase business combinations during 2023. We have also excluded Biognosys AG, Osthus Beteiligungs GmbH, MIRO Analytical AG, PhenomeX Inc. (Bruker Cellular Analysis), Acquifer Imaging GmbH, Deltabyte GmbH, Pinpoint Testing LLC, Fasmatech Science and Technology SA, Interherence GmbH, and Signals GmbH & Co. KG, from our audit of internal control over financial reporting. The total assets and total revenues of these acquired entities excluded from management’s assessment and our audit of internal control over financial reporting collectively represent approximately 9% and 1%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2023.
52
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue Recognition – System Sales
As described in Note 2 to the consolidated financial statements, the Company recognizes revenue from systems sales upon transfer of control in an amount that reflects the consideration it expects to receive. Transfer of control generally occurs upon shipment, or for certain systems, based upon customer acceptance for a system once delivered and installed at a customer facility. For systems that include customer-specific acceptance criteria, management is required to assess when it can demonstrate the acceptance criteria has been met, which generally is upon successful factory acceptance testing or customer acceptance and evidence of installation. The Company’s product revenue for the year ended December 31, 2023, was $2,457.6 million, of which a significant portion relates to system sales.
The principal consideration for our determination that performing procedures relating to revenue recognition for system sales is a critical audit matter is a high degree of auditor effort in performing procedures related to the Company’s system sales revenue recognition.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to system sales revenue recognition. These procedures also included, among others, evaluating the recognition of revenue for a sample of system sales transactions by obtaining and inspecting source documents, such as invoices, customer purchase orders, shipping or delivery documents, acceptance documents, and cash receipts, where applicable.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
February 29, 2024
We have served as the Company’s auditor since 2016.
53
BRUKER CORPORATION
CONSOLIDATED BALANCE SHEETS
(in millions, except share and per share data)
|
|
December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
ASSETS |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
488.3 |
|
|
$ |
645.5 |
|
Accounts receivable, net |
|
|
492.0 |
|
|
|
472.7 |
|
Inventories |
|
|
968.3 |
|
|
|
800.1 |
|
Assets held for sale |
|
|
— |
|
|
|
1.4 |
|
Other current assets |
|
|
215.6 |
|
|
|
193.5 |
|
Total current assets |
|
|
2,164.2 |
|
|
|
2,113.2 |
|
|
|
|
|
|
|
|
||
Property, plant and equipment, net |
|
|
599.7 |
|
|
|
487.0 |
|
Goodwill |
|
|
582.6 |
|
|
|
457.6 |
|
Intangible assets, net |
|
|
330.5 |
|
|
|
270.9 |
|
Operating lease assets |
|
|
91.7 |
|
|
|
51.2 |
|
Deferred tax assets |
|
|
297.2 |
|
|
|
76.8 |
|
Other long-term assets |
|
|
184.0 |
|
|
|
155.1 |
|
Total assets |
|
$ |
4,249.9 |
|
|
$ |
3,611.8 |
|
LIABILITIES, REDEEMABLE NONCONTROLLING INTERESTS AND |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Current portion of long-term debt |
|
$ |
121.2 |
|
|
$ |
18.7 |
|
Accounts payable |
|
|
202.7 |
|
|
|
178.4 |
|
Deferred revenue and customer advances |
|
|
400.0 |
|
|
|
370.2 |
|
Other current liabilities |
|
|
478.2 |
|
|
|
347.0 |
|
Total current Liabilities |
|
|
1,202.1 |
|
|
|
914.3 |
|
|
|
|
|
|
|
|
||
Long-term debt |
|
|
1,160.3 |
|
|
|
1,200.5 |
|
Long-term deferred revenue and customer advances |
|
|
91.5 |
|
|
|
101.6 |
|
Deferred tax liabilities |
|
|
67.7 |
|
|
|
62.3 |
|
Operating lease liabilities |
|
|
74.8 |
|
|
|
34.8 |
|
Accrued pension |
|
|
76.6 |
|
|
|
45.7 |
|
Other long-term liabilities |
|
|
163.6 |
|
|
|
120.8 |
|
Total Liabilities |
|
|
2,836.6 |
|
|
|
2,480.0 |
|
|
|
|
|
|
|
|
||
Commitments and contingencies (Note 18) |
|
|
|
|
|
|
||
Redeemable noncontrolling interests |
|
|
18.7 |
|
|
|
6.1 |
|
Shareholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $0.01 par value 5,000,000 shares authorized, none issued or |
|
|
— |
|
|
|
— |
|
Common stock, $0.01 par value 260,000,000 shares authorized, 175,943,705 |
|
|
1.7 |
|
|
|
1.7 |
|
Treasury stock at cost, 30,778,879 and 28,366,442 shares at December 31, 2023 |
|
|
(1,237.2 |
) |
|
|
(1,085.0 |
) |
Additional paid-in capital |
|
|
282.9 |
|
|
|
256.3 |
|
Retained earnings |
|
|
2,323.8 |
|
|
|
1,926.0 |
|
Accumulated other comprehensive income, net of tax |
|
|
6.0 |
|
|
|
14.8 |
|
Total shareholders’ equity attributable to Bruker Corporation |
|
|
1,377.2 |
|
|
|
1,113.8 |
|
Noncontrolling interests in consolidated subsidiaries |
|
|
17.4 |
|
|
|
11.9 |
|
Total shareholders’ equity |
|
|
1,394.6 |
|
|
|
1,125.7 |
|
Total liabilities, redeemable noncontrolling interests and shareholders’ equity |
|
$ |
4,249.9 |
|
|
$ |
3,611.8 |
|
The accompanying notes are an integral part of these consolidated financial statements.
54
BRUKER CORPORATION
CONSOLIDATED STATEMENTS OF INCOME
(in millions, except per share data)
|
|
Year Ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Product revenue |
|
$ |
2,457.6 |
|
|
$ |
2,109.9 |
|
|
$ |
2,017.3 |
|
Service revenue |
|
|
493.8 |
|
|
|
414.6 |
|
|
|
393.2 |
|
Other revenue |
|
|
13.1 |
|
|
|
6.2 |
|
|
|
7.4 |
|
Total revenue |
|
|
2,964.5 |
|
|
|
2,530.7 |
|
|
|
2,417.9 |
|
Cost of product revenue |
|
|
1,165.2 |
|
|
|
984.0 |
|
|
|
979.3 |
|
Cost of service revenue |
|
|
285.9 |
|
|
|
240.8 |
|
|
|
228.2 |
|
Cost of other revenue |
|
|
0.1 |
|
|
|
0.2 |
|
|
|
0.8 |
|
Total cost of revenue |
|
|
1,451.2 |
|
|
|
1,225.0 |
|
|
|
1,208.3 |
|
Gross profit |
|
|
1,513.3 |
|
|
|
1,305.7 |
|
|
|
1,209.6 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Selling, general and administrative |
|
|
729.4 |
|
|
|
607.4 |
|
|
|
561.2 |
|
Research and development |
|
|
294.8 |
|
|
|
235.9 |
|
|
|
220.8 |
|
Other charges, net |
|
|
52.2 |
|
|
|
29.7 |
|
|
|
14.3 |
|
Total operating expenses |
|
|
1,076.4 |
|
|
|
873.0 |
|
|
|
796.3 |
|
Operating income |
|
|
436.9 |
|
|
|
432.7 |
|
|
|
413.3 |
|
Bargain purchase gain |
|
|
144.1 |
|
|
|
— |
|
|
|
— |
|
Interest and other income (expense), net |
|
|
(36.8 |
) |
|
|
(18.8 |
) |
|
|
(19.7 |
) |
Income before income taxes, equity in income of unconsolidated investees, |
|
|
544.2 |
|
|
|
413.9 |
|
|
|
393.6 |
|
Income tax provision |
|
|
117.7 |
|
|
|
116.4 |
|
|
|
113.0 |
|
Equity in income of unconsolidated investees, net of tax |
|
|
2.0 |
|
|
|
1.0 |
|
|
|
— |
|
Consolidated net income |
|
|
428.5 |
|
|
|
298.5 |
|
|
|
280.6 |
|
Net income attributable to noncontrolling interests in |
|
|
1.3 |
|
|
|
1.9 |
|
|
|
3.5 |
|
Net income attributable to Bruker Corporation |
|
$ |
427.2 |
|
|
$ |
296.6 |
|
|
$ |
277.1 |
|
|
|
|
|
|
|
|
|
|
|
|||
Net income per common share attributable to Bruker |
|
|
|
|
|
|
|
|
|
|||
Basic |
|
$ |
2.92 |
|
|
$ |
2.00 |
|
|
$ |
1.83 |
|
Diluted |
|
$ |
2.90 |
|
|
$ |
1.99 |
|
|
$ |
1.81 |
|
Weighted average common shares outstanding: |
|
|
|
|
|
|
|
|
|
|||
Basic |
|
|
146.4 |
|
|
|
148.6 |
|
|
|
151.4 |
|
Diluted |
|
|
147.2 |
|
|
|
149.4 |
|
|
|
152.9 |
|
The accompanying notes are an integral part of these consolidated financial statements.
55
BRUKER CORPORATION
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in millions)
|
|
Year Ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Consolidated net income |
|
$ |
428.5 |
|
|
$ |
298.5 |
|
|
$ |
280.6 |
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|||
Foreign currency translation adjustments, net of tax benefit of ($3.2), ($1.4) |
|
|
93.3 |
|
|
|
(67.4 |
) |
|
|
(77.8 |
) |
Derivatives designated as hedging instruments net of tax expense (benefit) |
|
|
(77.1 |
) |
|
|
44.2 |
|
|
|
51.4 |
|
Pension and other postretirement benefit liability adjustments: |
|
|
|
|
|
|
|
|
|
|||
Pension and other postretirement benefit liability adjustments arising during the period |
|
|
(23.2 |
) |
|
|
43.8 |
|
|
|
11.6 |
|
Amortization of net loss and prior service benefit included in net periodic pension cost |
|
|
(0.7 |
) |
|
|
1.2 |
|
|
|
2.9 |
|
Total pension and other postretirement benefit liability adjustments, net of |
|
|
(23.9 |
) |
|
|
45.0 |
|
|
|
14.5 |
|
Total other comprehensive income (loss) |
|
|
(7.7 |
) |
|
|
21.8 |
|
|
|
(11.9 |
) |
Total Comprehensive income |
|
|
420.8 |
|
|
|
320.3 |
|
|
|
268.7 |
|
Less: Comprehensive income attributable to noncontrolling interests |
|
|
2.5 |
|
|
|
1.6 |
|
|
|
1.0 |
|
Less: Comprehensive loss attributable to redeemable noncontrolling |
|
|
(0.1 |
) |
|
|
(0.9 |
) |
|
|
(0.1 |
) |
Total Comprehensive income attributable to Bruker Corporation |
|
$ |
418.4 |
|
|
$ |
319.6 |
|
|
$ |
267.8 |
|
The accompanying notes are an integral part of these consolidated financial statements.
56
BRUKER CORPORATION
CONSOLIDATED STATEMENTS OF REDEEMABLE NONCONTROLLING INTERESTS AND SHAREHOLDERS’ EQUITY
(in millions, except share data)
|
|
Redeemable |
|
|
|
Number of Common |
|
|
Common |
|
|
Number of Treasury |
|
|
Treasury |
|
|
Additional |
|
|
Retained |
|
|
Accumulated |
|
|
Total |
|
|
Noncontrolling |
|
|
Total |
|
|||||||||||
Balance at December 31, 2020 |
|
$ |
— |
|
|
|
|
151,987,081 |
|
|
$ |
1.7 |
|
|
|
22,058,529 |
|
|
$ |
(667.0 |
) |
|
$ |
216.3 |
|
|
$ |
1,406.5 |
|
|
$ |
3.7 |
|
|
$ |
961.2 |
|
|
$ |
13.1 |
|
|
$ |
974.3 |
|
Stock options exercised |
|
|
— |
|
|
|
|
580,656 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
11.7 |
|
|
|
— |
|
|
|
— |
|
|
|
11.7 |
|
|
|
— |
|
|
|
11.7 |
|
Restricted stock units vested |
|
|
— |
|
|
|
|
278,769 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(4.7 |
) |
|
|
— |
|
|
|
— |
|
|
|
(4.7 |
) |
|
|
— |
|
|
|
(4.7 |
) |
Stock-based compensation |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
14.5 |
|
|
|
— |
|
|
|
— |
|
|
|
14.5 |
|
|
|
— |
|
|
|
14.5 |
|
Shares repurchased |
|
|
— |
|
|
|
|
(2,092,819 |
) |
|
|
— |
|
|
|
2,092,819 |
|
|
|
(153.3 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(153.3 |
) |
|
|
— |
|
|
|
(153.3 |
) |
Distributions to noncontrolling interests |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Cash dividends paid to common |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(24.1 |
) |
|
|
— |
|
|
|
(24.1 |
) |
|
|
— |
|
|
|
(24.1 |
) |
Formation of Acuity Spatial |
|
|
0.3 |
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
||||||
Consolidated net (loss) income |
|
|
(0.1 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
277.1 |
|
|
|
|
|
|
277.1 |
|
|
|
3.6 |
|
|
|
280.7 |
|
||||||
Other comprehensive loss |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(11.9 |
) |
|
|
(11.9 |
) |
|
|
(2.6 |
) |
|
|
(14.5 |
) |
Balance at December 31, 2021 |
|
$ |
0.2 |
|
|
|
|
150,753,687 |
|
|
$ |
1.7 |
|
|
|
24,151,348 |
|
|
$ |
(820.3 |
) |
|
$ |
237.8 |
|
|
$ |
1,659.5 |
|
|
$ |
(8.2 |
) |
|
$ |
1,070.5 |
|
|
$ |
14.1 |
|
|
$ |
1,084.6 |
|
Stock options exercised |
|
|
— |
|
|
|
|
251,006 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
5.8 |
|
|
|
— |
|
|
|
— |
|
|
|
5.8 |
|
|
|
— |
|
|
|
5.8 |
|
Restricted stock units vested |
|
|
— |
|
|
|
|
233,545 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3.0 |
) |
|
|
— |
|
|
|
— |
|
|
|
(3.0 |
) |
|
|
— |
|
|
|
(3.0 |
) |
Stock-based compensation |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
15.7 |
|
|
|
— |
|
|
|
— |
|
|
|
15.7 |
|
|
|
— |
|
|
|
15.7 |
|
Shares repurchased |
|
|
— |
|
|
|
|
(4,215,094 |
) |
|
|
— |
|
|
|
4,215,094 |
|
|
|
(264.7 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(264.7 |
) |
|
|
— |
|
|
|
(264.7 |
) |
Distributions to noncontrolling interests |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
(1.2 |
) |
|
|
(1.2 |
) |
||||||||
Cash dividends paid to common |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(29.8 |
) |
|
|
— |
|
|
|
(29.8 |
) |
|
|
— |
|
|
|
(29.8 |
) |
Consolidated net (loss) income |
|
|
(0.6 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
296.6 |
|
|
|
— |
|
|
|
296.6 |
|
|
|
2.5 |
|
|
|
299.1 |
|
PreOmics Acquisition - other shareholders |
|
|
6.8 |
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|||||
Acquisition of minority interest |
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(0.3 |
) |
|
|
(0.2 |
) |
|
|
(0.5 |
) |
|
|
(2.6 |
) |
|
|
(3.1 |
) |
||||
Other comprehensive (loss) income |
|
|
(0.3 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
23.2 |
|
|
|
23.2 |
|
|
|
(0.9 |
) |
|
|
22.3 |
|
Balance at December 31, 2022 |
|
$ |
6.1 |
|
|
|
|
147,023,144 |
|
|
$ |
1.7 |
|
|
|
28,366,442 |
|
|
$ |
(1,085.0 |
) |
|
$ |
256.3 |
|
|
$ |
1,926.0 |
|
|
$ |
14.8 |
|
|
$ |
1,113.8 |
|
|
$ |
11.9 |
|
|
$ |
1,125.7 |
|
Stock options exercised |
|
|
— |
|
|
|
|
285,030 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
9.7 |
|
|
|
— |
|
|
|
— |
|
|
|
9.7 |
|
|
|
— |
|
|
|
9.7 |
|
Restricted stock units vested |
|
|
— |
|
|
|
|
215,959 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3.4 |
) |
|
|
— |
|
|
|
— |
|
|
|
(3.4 |
) |
|
|
— |
|
|
|
(3.4 |
) |
Stock-based compensation |
|
|
0.1 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
17.6 |
|
|
|
— |
|
|
|
— |
|
|
|
17.6 |
|
|
|
— |
|
|
|
17.6 |
|
Shares repurchased |
|
|
— |
|
|
|
|
(2,412,437 |
) |
|
|
|
|
|
2,412,437.0 |
|
|
|
(152.2 |
) |
|
|
(1.2 |
) |
|
|
— |
|
|
|
— |
|
|
|
(153.4 |
) |
|
|
— |
|
|
|
(153.4 |
) |
|
Employee stock purchase plan |
|
|
— |
|
|
|
|
53,130 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
3.9 |
|
|
|
— |
|
|
|
— |
|
|
|
3.9 |
|
|
|
— |
|
|
|
3.9 |
|
Shares repurchased |
|
|
— |
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Cash dividends paid to common shareholders |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(29.4 |
) |
|
|
— |
|
|
|
(29.4 |
) |
|
|
— |
|
|
|
(29.4 |
) |
Proceeds from the sale of non- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5.0 |
|
|
|
5.0 |
|
|||||||||
Other shareholders of majority-owned acquisitions |
|
|
12.6 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
- |
|
|
Distributions to noncontrolling interest |
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(2.0 |
) |
|
|
(2.0 |
) |
Consolidated net (loss) income |
|
|
(0.7 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
427.2 |
|
|
|
— |
|
|
|
427.2 |
|
|
|
2.0 |
|
|
|
429.2 |
|
Other comprehensive income (loss) |
|
|
0.6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
(8.8 |
) |
|
|
(8.8 |
) |
|
|
0.5 |
|
|
|
(8.3 |
) |
|||||
Balance at December 31, 2023 |
|
$ |
18.7 |
|
|
|
|
145,164,826 |
|
|
$ |
1.7 |
|
|
|
30,778,879 |
|
|
$ |
(1,237.2 |
) |
|
$ |
282.9 |
|
|
$ |
2,323.8 |
|
|
$ |
6.0 |
|
|
$ |
1,377.2 |
|
|
$ |
17.4 |
|
|
$ |
1,394.6 |
|
The accompanying notes are an integral part of these consolidated financial statements.
57
BRUKER CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in millions)
|
|
Year Ended December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|||
Consolidated net income |
|
$ |
428.5 |
|
|
$ |
298.5 |
|
|
$ |
280.6 |
|
Adjustments to reconcile consolidated net income to cash flows from |
|
|
|
|
|
|
|
|
|
|||
Depreciation and amortization |
|
|
114.9 |
|
|
|
88.8 |
|
|
|
89.1 |
|
Stock-based compensation expense |
|
|
24.0 |
|
|
|
27.7 |
|
|
|
17.2 |
|
Deferred income taxes |
|
|
(24.4 |
) |
|
|
(14.8 |
) |
|
|
(5.8 |
) |
Bargain purchase gain on acquisition |
|
|
(144.1 |
) |
|
|
— |
|
|
|
— |
|
Impairment of minority investments and other long-lived assets |
|
|
22.2 |
|
|
|
1.6 |
|
|
|
— |
|
Gain on sale of minority investment |
|
|
(6.8 |
) |
|
|
— |
|
|
|
— |
|
Gain on sale of property, plant and equipment |
|
|
(8.5 |
) |
|
|
(10.1 |
) |
|
|
(1.1 |
) |
Other non-cash expenses, net |
|
|
32.1 |
|
|
|
26.8 |
|
|
|
28.0 |
|
Changes in operating assets and liabilities, net of acquisitions: |
|
|
|
|
|
|
|
|
|
|||
Accounts receivable |
|
|
(0.9 |
) |
|
|
(67.9 |
) |
|
|
(95.3 |
) |
Inventories |
|
|
(125.0 |
) |
|
|
(137.9 |
) |
|
|
(67.0 |
) |
Accounts payable and accrued expenses |
|
|
23.1 |
|
|
|
13.2 |
|
|
|
61.4 |
|
Income taxes payable |
|
|
43.7 |
|
|
|
(6.8 |
) |
|
|
(44.3 |
) |
Deferred revenue and customer advances |
|
|
(0.6 |
) |
|
|
52.7 |
|
|
|
38.8 |
|
Other changes in operating assets and liabilities |
|
|
(28.1 |
) |
|
|
2.6 |
|
|
|
(19.2 |
) |
Net cash provided by operating activities |
|
|
350.1 |
|
|
|
274.4 |
|
|
|
282.4 |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|||
Purchases of property, plant and equipment |
|
|
(106.9 |
) |
|
|
(129.2 |
) |
|
|
(92.0 |
) |
Purchases of short-term investments |
|
|
— |
|
|
|
— |
|
|
|
(148.0 |
) |
Maturity of short-term investments |
|
|
— |
|
|
|
100.0 |
|
|
|
98.2 |
|
Proceeds from sale of minority investment |
|
|
14.8 |
|
|
|
— |
|
|
|
— |
|
Cash paid for minority investments |
|
|
(24.8 |
) |
|
|
(60.2 |
) |
|
|
(0.5 |
) |
Cash paid for acquisitions, net of cash acquired |
|
|
(226.6 |
) |
|
|
(182.3 |
) |
|
|
(65.0 |
) |
Proceeds from sales of property, plant and equipment |
|
|
11.1 |
|
|
|
13.9 |
|
|
|
4.9 |
|
Net proceeds from cross-currency swap agreements |
|
|
6.4 |
|
|
|
6.2 |
|
|
|
10.0 |
|
Net cash used in investing activities |
|
|
(326.0 |
) |
|
|
(251.6 |
) |
|
|
(192.4 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|||
Proceeds from issuance (repayment) of long-term debt |
|
|
(6.5 |
) |
|
|
0.3 |
|
|
|
490.5 |
|
Repayment of 2012 Note Purchase Agreement |
|
|
— |
|
|
|
(105.0 |
) |
|
|
— |
|
Repayment of 2019 Term Loan Agreement |
|
|
(15.0 |
) |
|
|
(6.0 |
) |
|
|
(0.8 |
) |
Payment of deferred financing costs |
|
|
— |
|
|
|
— |
|
|
|
(0.1 |
) |
Proceeds from issuance of common stock, net |
|
|
9.5 |
|
|
|
2.8 |
|
|
|
7.0 |
|
Payment of contingent consideration |
|
|
(2.7 |
) |
|
|
(2.7 |
) |
|
|
(0.4 |
) |
Payment of dividends to common shareholders |
|
|
(29.4 |
) |
|
|
(29.8 |
) |
|
|
(24.2 |
) |
Repurchase of common stock |
|
|
(152.3 |
) |
|
|
(263.1 |
) |
|
|
(153.3 |
) |
Proceeds from (payment for) the sale (purchase) of noncontrolling interests |
|
|
3.0 |
|
|
|
(11.8 |
) |
|
|
— |
|
Net cash (used in) provided by financing activities |
|
|
(193.4 |
) |
|
|
(415.3 |
) |
|
|
318.7 |
|
Effect of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
12.2 |
|
|
|
(30.5 |
) |
|
|
(22.5 |
) |
Net change in cash, cash equivalents and restricted cash |
|
|
(157.1 |
) |
|
|
(423.0 |
) |
|
|
386.2 |
|
Cash, cash equivalents and restricted cash at beginning of year |
|
|
648.7 |
|
|
|
1,071.7 |
|
|
|
685.5 |
|
Cash, cash equivalents and restricted cash at end of year |
|
$ |
491.6 |
|
|
$ |
648.7 |
|
|
$ |
1,071.7 |
|
Supplemental cash flow information: |
|
|
|
|
|
|
|
|
|
|||
Cash paid for interest |
|
$ |
33.7 |
|
|
$ |
24.3 |
|
|
$ |
19.6 |
|
Cash paid for taxes |
|
$ |
96.6 |
|
|
$ |
147.4 |
|
|
$ |
145.5 |
|
Restricted cash period beginning balance |
|
$ |
3.2 |
|
|
$ |
3.5 |
|
|
$ |
3.7 |
|
Restricted cash period ending balance |
|
$ |
3.3 |
|
|
$ |
3.2 |
|
|
$ |
3.5 |
|
The accompanying notes are an integral part of these consolidated financial statements.
58
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business
Bruker Corporation, together with its consolidated subsidiaries (Bruker or the Company), develops, manufactures and distributes high-performance scientific instruments and analytical and diagnostic solutions that enable its customers to explore life and materials at microscopic, molecular and cellular levels. Many of the Company’s products are used to detect, measure and visualize structural characteristics of chemical, biological and industrial material samples. The Company’s products address the rapidly evolving needs of a diverse array of customers in life science research, pharmaceuticals, biotechnology, applied markets, cell biology, clinical research, microbiology, in-vitro diagnostics, nanotechnology and materials science research.
The Company has four reportable segments, Bruker Scientific Instruments (BSI) BioSpin, BSI CALID, BSI Nano and Bruker Energy & Supercon Technologies (BEST).
The BSI BioSpin Segment designs, manufactures and distributes enabling life science tools based on magnetic resonance technology. BSI BioSpin Segment’s revenues are generated by academic and government research customers, pharmaceutical and biotechnology companies and nonprofit laboratories, as well as chemical, food and beverage, clinical and other industrial companies.
The BSI CALID (Chemicals, Applied Markets, Life Science, In-Vitro Diagnostics, Detection) Segment designs, manufactures and distributes life science mass spectrometry and ion mobility spectrometry solutions, analytical and process analysis instruments and solutions based on infrared and Raman molecular spectroscopy technologies and radiological/nuclear detectors for Chemical, Biological, Radiological, Nuclear and Explosive (CBRNE) detection. Customers of the BSI CALID Segment include academic institutions and medical schools; pharmaceutical, biotechnology and diagnostics companies; contract research organizations; nonprofit and for-profit forensics laboratories; agriculture, food and beverage safety laboratories; environmental and clinical microbiology laboratories; hospitals and government departments and agencies.
The BSI Nano Segment designs, manufactures and distributes advanced X-ray instruments; atomic force microscopy instrumentation; advanced fluorescence optical microscopy instruments; analytical tools for electron microscopes and X-ray metrology; defect-detection equipment for semiconductor process control; handheld, portable and mobile X-ray fluorescence spectrometry instruments; spark optical emission spectroscopy systems; chipcytometry products and services for targeted spatial proteomics, multi-omic services, and products and services for spatial genomics research. Customers of the BSI Nano Segment include academic institutions, governmental customers, nanotechnology companies, semiconductor companies, raw material manufacturers, industrial companies, biotechnology and pharmaceutical companies and other businesses involved in materials research and life science research analysis.
The BEST reportable segment develops and manufactures superconducting and non-superconducting materials and devices for use in renewable energy, energy infrastructure, healthcare and “big science” research. The segment focuses on metallic low temperature superconductors for use in magnetic resonance imaging, nuclear magnetic resonance, fusion energy research and other applications.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and all majority and wholly owned subsidiaries. All intercompany accounts and transactions have been eliminated.
Noncontrolling Interests
Noncontrolling interests represents the minority shareholders’ proportionate share of the Company’s majority-owned subsidiaries. The portion of net income or net loss attributable to non-controlling interests is presented as net income attributable to noncontrolling interests in consolidated subsidiaries in the consolidated statements of income and comprehensive income, and the portion of other comprehensive income of these subsidiaries is presented in the consolidated statements of shareholders’ equity.
59
Redeemable Noncontrolling Interests
The Company has agreements with noncontrolling interest holders that provide the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, their remaining minority interest at a contractually defined redemption value. These rights can be accelerated in certain events. As the redemptions are contingently redeemable at the option of the noncontrolling interest shareholders, the Company classifies the carrying amount of the redeemable noncontrolling interest in the mezzanine section on the consolidated balance sheet, which is presented above the equity section and below liabilities. The redeemable noncontrolling interests are measured at the greater of the amount that would be paid if settlement occurred as of the balance sheet date based on the contractually defined redemption value and its carrying amount adjusted for net income (loss) attributable to the noncontrolling interest. Adjustments to the carrying value of the redeemable noncontrolling interest are recorded through retained earnings. During the years ended December 31, 2023, 2022 and 2021, carrying value adjustments were immaterial.
Business Combinations
The Company accounts for business combinations under the acquisition method of accounting. Accordingly, at the date of each acquisition, the Company measures the fair value of all identifiable assets acquired (including intangible assets), liabilities assumed and any remaining noncontrolling interests and allocates the amounts paid to all items measured. The fair value of identifiable intangible assets acquired is based on valuations that use information and assumptions determined by management and which consider management’s best estimates of inputs and assumptions that a market participant would use.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period.
Significant estimates and judgments made by management in preparing these financial statements include revenue recognition, write-downs for excess and obsolete inventory, estimated fair values used to record impairment charges related to intangible assets, goodwill, and other long-lived assets, amortization periods, expected future cash flows used to evaluate the recoverability of long-lived assets and to record intangible assets in business combinations, stock-based compensation expense, warranty allowances, restructuring and other related charges, contingent liabilities and the recoverability of the Company’s net deferred tax assets.
Changes in estimates are recorded in the period in which they become known. The Company bases its estimates on historical experience and various other assumptions that it believes to be reasonable under the circumstances. Actual results may differ from management’s estimates if these results differ from historical experience or other assumptions prove not to be substantially accurate, even if such assumptions were reasonable when made.
Subsequent Events Considerations
The Company considers events or transactions that occur after the balance sheet date but prior to the issuance of the financial statements to provide additional evidence for certain estimates or to identify matters that require additional disclosure. Subsequent events have been evaluated as required. The Company has evaluated all subsequent events and determined that, other than as reported herein, there are no material recognized or unrecognized subsequent events.
Cash and Cash Equivalents
Cash and cash equivalents primarily include cash on hand, money market funds and time deposits with original maturities of three months or less at the date of acquisition. Time deposits represent amounts on deposit in banks and temporarily invested in instruments with maturities of three months or less at the time of purchase. Certain of these investments represent deposits which are not insured by the Federal Deposit Insurance Corporation or any other government agency. Cash equivalents are carried at cost, which approximates fair value.
Short-term Investments
Short-term investments represent time and call deposits maturing within twelve months and with original maturities of greater than three months at the date of acquisition. Short-term investments are classified as available-for-sale and are reported at fair value.
60
Restricted Cash
Restricted cash is included as a component of cash, cash equivalents, and restricted cash on the Company’s consolidated statement of cash flows. The Company has certain subsidiaries that are required by local laws and regulations to maintain restricted cash balances to cover future employee benefit payments. Restricted cash balances are classified as non-current unless, under the terms of the applicable agreements, the funds will be released from restrictions within one year from the balance sheet date. The current and non-current portion of restricted cash is recorded within other current assets and other long-term assets, respectively, in the accompanying consolidated balance sheets.
Accounts Receivable, net
Accounts receivable have been reduced by an allowance for doubtful accounts. The allowance for doubtful accounts represents the Company’s best estimate of the amount of probable credit losses in our accounts receivable. The Company’s allowance is based on a number of factors, including an evaluation of customer credit worthiness, the age of the outstanding receivable, economic trends and historical experience. Provisions for doubtful accounts are recorded in selling, general and administrative expenses in the accompanying consolidated statements of income and comprehensive income.
Derivative Financial Instruments and Hedging Activities
All derivatives, whether designated in a hedging relationship or not, are recorded on the consolidated balance sheets at fair value. The accounting for changes in fair value of a derivative instrument depends on whether it has been designated and qualifies as part of a hedging relationship and further, on the type of hedging relationship. For those derivative instruments that are designated and qualify as hedging instruments, the Company must designate the hedging instrument, based on the exposure being hedged, as a fair value hedge, cash flow hedge, foreign currency hedge or a hedge of a net investment in a foreign operation. If a derivative is designated as a hedge, depending on the nature of the hedge, changes in the fair value of the derivative are either offset against the change in fair value of the hedged item through earnings or recognized in other comprehensive income until the hedged item is recognized in earnings. Derivatives that are not designated as hedges are recorded at fair value through earnings. The Company presents the cross-currency swap periodic settlements in investing activities and the interest rate swap periodic settlements in operating activities in the consolidated statements of cash flows. The Company records derivative assets and liabilities on a gross basis in the consolidated balance sheets.
Fair Value of Financial Instruments
The Company applies the following hierarchy to determine the fair value of financial instruments, which prioritizes the inputs used to measure fair value into three levels and bases the categorization within the hierarchy upon the lowest level of input that is available and significant to the fair value measurement. The levels in the hierarchy are defined as follows:
The valuation techniques that may be used by the Company to determine the fair value of Level 2 and Level 3 financial instruments are the market approach, the income approach and the cost approach. The market approach uses prices and other relevant information generated by market transactions involving identical or comparable assets or liabilities. The income approach uses valuation techniques to convert future amounts to a single present value based on current market expectations about those future amounts, including present value techniques, option-pricing models and the excess earnings method. The cost approach is based on the amount that would be required to replace the service capacity of an asset (replacement cost).
The Company’s financial instruments consist primarily of cash equivalents, restricted cash, derivative instruments consisting of forward foreign exchange contracts, cross-currency interest rate swap agreements, commodity contracts, derivatives embedded in certain purchase and sale contracts, derivatives embedded within noncontrolling interests, accounts receivable, accounts payable, contingent consideration and long-term debt. The carrying amounts of the Company’s cash equivalents, short-term investments and restricted cash, accounts receivable, borrowings under a revolving credit agreement and accounts payable approximate fair value because of their short-term nature. Derivative assets and liabilities are measured at fair value on a recurring basis.
61
The Company has evaluated the estimated fair value of financial instruments using available market information and management’s estimates. The use of different market assumptions and/or estimation methodologies could have a significant effect on the estimated fair value amounts.
Concentration of Credit Risk
Financial instruments that subject the Company to credit risk consist of cash, cash equivalents, derivative instruments, accounts receivables and restricted cash. The risk with respect to cash and cash equivalents is minimized by the Company’s policy of investing in short-term financial instruments issued by highly rated financial institutions. The risk with respect to derivative instruments is minimized by the Company’s policy of entering into arrangements with highly rated financial institutions. The risk with respect to accounts receivables is minimized by the creditworthiness and diversity of the Company’s customers. The Company performs periodic credit evaluations of its customers’ financial condition and generally requires an advanced deposit for a portion of the purchase price. Credit losses have been within management’s expectations and the allowance for doubtful accounts totaled $4.6 million and $5.3 million at December 31, 2023 and 2022, respectively. At December 31, 2023, and 2022, no single customer represented 10% or more of the Company’s accounts receivable. For the years ended December 31, 2023, 2022, and 2021, no single customer represented 10% or more of the Company’s total revenue.
Inventories
Components of inventory include raw materials, work-in-process, demonstration units and finished goods. Demonstration units include systems which are located in the Company’s demonstration laboratories or installed at the sites of potential customers and are considered available for sale. Finished goods include in-transit systems that have been shipped to the Company’s customers, but not yet installed and accepted by the customer. All inventories are stated at the lower of cost and net realizable value. Cost is determined principally by the first-in, first-out method for a majority of subsidiaries and by average-cost for certain other subsidiaries. The Company reduces the carrying value of its inventories for differences between cost and estimated net realizable value, taking into consideration usage in the preceding twelve months, expected demand, technological obsolescence and other information including the physical condition of demonstration inventories. The Company records a charge to cost of revenue for the amount required to reduce the carrying value of inventory to net realizable value. Costs associated with the procurement of inventories, such as inbound freight charges and purchasing and receiving costs, are capitalized as part of inventory and are also included in the cost of product revenue line item within the consolidated statements of income and comprehensive income.
Property, Plant and Equipment
Property, plant and equipment are stated at cost less accumulated depreciation and amortization. Major improvements that extend the useful lives are capitalized while expenditures for maintenance, repairs and minor improvements are charged to expense as incurred. When assets are retired or otherwise disposed of, the assets and related accumulated depreciation and amortization are eliminated from the accounts and any resulting gain or loss is reflected in the consolidated statements of income and comprehensive income. Depreciation and amortization are calculated on a straight-line basis over the estimated useful lives of the assets as follows:
|
|
Estimated Useful Life |
Buildings |
|
25 to 40 years |
Machinery and equipment |
|
3 to 10 years |
Computer and fixtures |
|
3 to 5 years |
Furniture and fixtures |
|
3 to 10 years |
Leasehold improvements |
|
Lesser of 15 years or the remaining lease term |
Goodwill and Intangible Assets
Goodwill and indefinite-lived intangible assets are not amortized, but are evaluated for impairment on an annual basis, or on an interim basis when events or changes in circumstances indicate that the carrying value may not be recoverable. In assessing the recoverability of goodwill and indefinite-lived intangible assets, the Company must make assumptions regarding the estimated future cash flows, including forecasted revenue growth, to determine the fair value of these assets. If these estimates or their related assumptions change in the future, the Company may be required to record impairment charges against these assets in the reporting period in which the impairment is determined.
The Company tests goodwill for impairment at the reporting unit level, which is the operating segment or one level below an operating segment. The Company has the option of performing a qualitative assessment to determine whether further impairment testing is necessary before performing the quantitative assessment. If as a result of the qualitative assessment, it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount, a quantitative impairment test will be required. Otherwise, no further testing will be required.
62
If a quantitative impairment test is performed, the Company compares the fair values of the applicable reporting units with their aggregate carrying values, including goodwill. The Company determines the fair value of reporting units using a weighting of both the market and the income methodologies. Estimating the fair value of the reporting units requires significant judgment by management. If the carrying amount of a reporting unit exceeds the fair value of the reporting unit, an impairment charge is recognized for the amount by which the carrying value amount exceeds the reporting unit’s fair value up to the total amount of goodwill allocated to the reporting unit.
In process research and development, or IPR&D, acquired as part of business combinations under the acquisition method represents ongoing development work associated with enhancements to existing products, as well as the development of next generation products. IPR&D is initially capitalized at fair value as an intangible asset with an indefinite life and assessed for impairment on an annual basis, or when indicators of impairment are identified. When the IPR&D project is complete, it is reclassified as a finite-lived intangible asset and is amortized over its estimated useful life. If an IPR&D project is abandoned before completion or is otherwise determined to be impaired, the value of the asset or the amount of the impairment is charged to the consolidated statements of income and comprehensive income in the period the project is abandoned or impaired.
Intangible assets with a finite useful life are amortized on a straight-line basis over their estimated useful lives as follows:
|
|
Estimated Useful Life |
Existing technology and related patents |
|
3 to 15 years |
Customer relationships |
|
5 to 15 years |
Trade names |
|
5 to 15 years |
Impairment of Long-Lived Assets
Impairment losses are recorded on long-lived assets used in operations when indicators of impairment are present and the quoted market price, if available or the estimated fair value of those assets are less than the assets’ carrying value and are not recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written down to their fair values. Impairment losses are charged to the consolidated statements of income and comprehensive income for the difference between the fair value and carrying value of the asset.
Warranty Costs and Deferred Revenue
The Company typically provides a one-year parts and labor warranty with the purchase of equipment. The anticipated cost for this warranty is accrued upon recognition of the sale and is included as a current liability on the accompanying consolidated balance sheets. The Company’s warranty reserve reflects estimated material and labor costs for potential product issues for which the Company expects to incur an obligation. The Company’s estimates of anticipated rates of warranty claims and costs are primarily based on historical information. The Company assesses the adequacy of the warranty reserve on a quarterly basis and adjusts the amount as necessary. If the historical data used to calculate the adequacy of the warranty reserve is not indicative of future requirements, additional or reduced warranty reserves may be required.
The Company also offers to its customers extended warranty and service agreements extending beyond the initial warranty for a fee. These fees are recorded as deferred revenue and recognized ratably into income over the life of the extended warranty contract or service agreement.
Income Taxes
The provision for income taxes includes federal, state, local and foreign taxes. Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the estimated future tax consequences of temporary differences between the financial statement carrying amounts and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the year in which the temporary differences are expected to be recovered or settled. The Company evaluates the realizability of its deferred tax assets and establishes a valuation allowance when it is more likely than not that all or a portion of deferred tax assets will not be realized.
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Based upon the level of historical taxable income and income tax liability and projections for future taxable income over the periods in which the deferred tax assets are utilizable, we believe it is more likely than not that we will realize the net benefits of the deferred tax assets of our wholly owned subsidiaries, net of the recorded valuation allowance. In the event that actual results differ from our estimates, or we adjust our estimates in future periods, we may need to adjust or establish a valuation allowance, which could materially impact our consolidated financial position and results of operations.
63
The Company records liabilities related to uncertain tax positions in accordance with the guidance that clarifies the accounting for uncertainty in income taxes recognized in a Company’s financial statements. This guidance prescribes a minimum recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. The Company includes accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, in income tax provision.
Customer Advances
The Company typically requires an advance deposit under the terms and conditions of contracts with customers. These deposits are recorded as a current or long-term liability until revenue is recognized on the specific contract in accordance with the Company’s revenue recognition policy.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 606, Revenue from Contracts with Customers. The key elements of ASC 606 are: 1) identifying a contract with the customer; 2) identifying the performance obligations in the contract; 3) determining the transaction price; 4) allocating the transaction price to the performance obligations in the contract; and 5) recognizing revenue when (or as) each performance obligation is satisfied.
A performance obligation is a promise in a contract to transfer a distinct good or service to the customer. A contract’s transaction price is allocated to each distinct performance obligation and recognized as revenue when, or as, the performance obligation is satisfied. Some of the Company’s contracts have multiple performance obligations, most commonly due to providing additional goods or services along with a system, such as installation, accessories, parts and services. For contracts with multiple performance obligations, the Company allocates the contract’s transaction price to each performance obligation using the best estimate of the standalone selling price of each distinct good or service being provided to the customer. The Company’s best evidence of standalone selling price is its normal selling pricing and discounting practices for the specific product or service when sold on a standalone basis. Alternatively, when not sold separately, the Company may determine standalone selling price using an expected cost plus a margin approach.
The Company’s performance obligations are typically satisfied at a point in time, most commonly either on shipment, or customer acceptance. Certain performance obligations, such as maintenance contracts and extended warranty, are recognized over time based on the contractual obligation period. In addition, certain arrangements to provide more customized deliverables may be satisfied over time based on the extent of progress towards completion. For performance obligations recognized over time, revenue is measured by progress toward completion of the performance obligation that reflects the transfer of control. Typically, progress is measured using a cost-to-cost method based on cost incurred to date relative to total estimated costs upon completion as this best depicts the transfer of control to the customer. Application of the cost-to-cost method requires the Company to make reasonable estimates of the extent of progress toward completion and the total costs the Company expects to incur. Losses are recorded immediately when the Company estimates that contracts will ultimately result in a loss. Changes in the estimates could affect the timing of revenue recognition.
The Company includes costs incurred in connection with shipping and handling of products within selling, general and administrative costs. Amounts billed to customers in connection with these costs are included in total revenues. When control of the goods transfers prior to the completion of the Company’s obligation to ship the products to its customers, the Company has elected the practical expedient to account for the shipping services as a fulfillment cost. The Company expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period is one year or less or the amount is immaterial. The Company excludes from the transaction price all taxes assessed by a governmental authority on revenue-producing transactions that are collected by the Company from a customer.
The Company recognizes revenue from systems sales upon transfer of control in an amount that reflects the consideration it expects to receive. Transfer of control generally occurs upon shipment, or for certain systems, based upon customer acceptance for a system once delivered and installed at a customer facility. For systems that include customer-specific acceptance criteria, the Company is required to assess when it can demonstrate the acceptance criteria has been met, which generally is upon successful factory acceptance testing or customer acceptance and evidence of installation. For systems that require installation and where system revenue is recognized upon shipment, the standalone selling price of installation is deferred until customer acceptance. Revenue from accessories and parts is generally recognized based on shipment. Service revenue is recognized as the services are performed or ratably over the contractual obligation and includes maintenance contracts, extended warranties, training, application support and on-demand services.
When products are sold through an independent distributor or a strategic distribution partner, the Company recognizes the system sale upon transfer of control which is typically on shipment. When the Company is responsible for installation, the standalone selling price of installation is deferred until customer acceptance.
64
The Company’s distributors do not have price protection rights or rights of return; however, the Company’s products are typically warranted to be free from defect for a period of one year.
The Company requires an advance deposit based on the terms and conditions of contracts with customers for many of its contracts. Typically, revenue is recognized within one year of receiving an advance deposit. The Company does not have any material payment terms that extend beyond one year. There is minimal variable consideration included in the transaction price of the Company’s contracts.
Other revenues are primarily comprised of development arrangements recognized on a cost-plus-fixed-fee basis and licensing arrangements recognized either when the licenses are provided or ratably over the contract term depending on the nature of the arrangement.
Contract Assets and Liabilities
Contract assets represent unbilled receivables when revenue recognized exceeds the amount billed to the customer, and the right to payment is not just subject to the passage of time. Contract assets typically result from system revenue recorded where a portion of the transaction price is not billable until a future event, such as customer acceptance, or from contracts recognized on a cost-to-cost or cost-plus-fixed-fee basis as revenue exceeds the amount billed to the customer. Amounts may not exceed their net realizable value. Contract assets are generally classified as current.
Contract liabilities consist of customer advances, deferred revenue and billings in excess of revenue from contracts recognized on a cost-to-cost or cost-plus-fixed-fee basis. Contract liabilities are classified as current or long-term based on the timing of when the Company expects to recognize revenue. Contract assets and liabilities are reported in a net position on a contract-by-contract basis at the end of each reporting period.
Deferred revenue previously included in other current liabilities on the consolidated balance sheets is currently presented together with customer advances. The change in the prior period was made to conform to the current period presentation.
Leases
The Company accounts for leases in accordance with ASC 842, Leases. At the inception of an arrangement, the Company determines whether the arrangement is or contains a lease based on the unique facts and circumstances present. Leases with a term greater than 12 months are recognized on the balance sheet as Right-of-use (“ROU”) assets with a corresponding lease liability. The Company has elected not to recognize on the consolidated balance sheets leases with an initial term of 12 months or less. Leases with an initial term of 12 months or less are directly expensed as incurred. Leases are classified as either operating or finance depending on the specific terms of the arrangement.
The Company’s leases mainly consist of facilities, office equipment, and vehicles. The majority of leases are classified as operating. The remaining lease term ranges from 2024 to 2039, with some leases including an option to extend the lease for varying periods of time or to terminate prior to the end of the lease term. Certain lease agreements contain provisions for future rent increases. Lease payments included in the measurement of the lease liability comprise fixed payments, future rent increases tied to an index or rate, and the exercise price of a Company option to purchase the underlying asset if the Company is reasonably certain to exercise the option. Future rent increases dependent on an index or rate are initially measured at the index or rate at the commencement date. The Company’s leases typically do not contain residual value guarantees.
At the commencement date, operating and finance lease liabilities, and their corresponding ROU assets, are recorded based on the present value of lease payments over the expected lease term. The lease term includes the non-cancelable period of the lease, plus any additional periods covered by either a Company option to extend (or not to terminate) the lease that the Company is reasonably certain to exercise, or an option to extend (or not to terminate) the lease controlled by the lessor. The interest rate implicit in lease contracts is typically not readily determinable, therefore an incremental borrowing rate is used to calculate the lease liability. The incremental borrowing rate is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment. Certain adjustments to the ROU asset may be required for items such as prepayments, lease incentives received, or initial direct costs paid.
Shipping and Handling Costs
The Company includes costs incurred in connection with shipping and handling of products within selling, general and administrative expenses in the accompanying consolidated statements of income and comprehensive income. Shipping and handling costs were $40.3 million, $39.4 million and $36.3 million in the years ended December 31, 2023, 2022 and 2021, respectively. Amounts billed to customers in connection with these costs are included in total revenues.
65
Research and Development
The Company commits substantial capital and resources to internal and collaborative research and development projects in order to provide innovative products and solutions to its customers. The Company conducts research primarily to enhance system performance and improve the reliability of existing products, and to develop revolutionary new products and solutions. Research and development costs are expensed as incurred and include salaries, wages and other personnel related costs, material costs and depreciation, consulting costs and facility costs.
Capitalized Software
Purchased software is capitalized at cost and is amortized over the estimated useful life, which is generally three years. Software developed for use in the Company’s products is expensed as incurred to research and development expense until technological feasibility is achieved. Subsequent to the achievement of technological feasibility, amounts are capitalizable; however, to date such amounts have not been material.
Advertising
The Company expenses advertising costs as incurred. Advertising expenses were $23.7 million, $19.3 million and $13.8 million during the years ended December 31, 2023, 2022 and 2021, respectively.
Stock-Based Compensation
The Company recognizes stock-based compensation expense in the consolidated statements of income and comprehensive income based on the fair value of the share-based award at the grant date. The Company’s primary types of share-based compensation are stock options, restricted stock awards and restricted stock units.
Compensation expense is amortized on a straight-line basis over the underlying vesting terms of the share-based award. Stock options to purchase the Company’s common stock are periodically awarded to executive officers and other employees of the Company subject to a vesting period of three to four years. The fair value of each option award is estimated on the date of grant using the Black-Scholes option-pricing model.
The determination of the fair value of stock-based payment awards using the Black-Scholes model is affected by our stock price and a number of assumptions, including expected volatility, expected life, risk-free interest rates and expected dividends. Risk-free interest rates are based on the yield on zero-coupon U.S. Treasury securities for a period that is commensurate with the expected life assumption. Expected life is determined through a calculation based on historical experience. Expected volatility is based the Company’s historical volatility results. Expected dividend yield is based on the estimated annualized dividend yield on our stock based on our history of paying dividends. The Company utilizes an estimated forfeiture rate derived from an analysis of historical data.
Assumptions regarding volatility, expected term, dividend yield and risk-free interest rates are required for the Black-Scholes model and are presented in the table below:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Risk-free interest rates |
|
|
4.28 |
% |
|
|
3.03 |
% |
|
|
0.62 |
% |
Expected life |
|
4.5 years |
|
|
4.4 years |
|
|
4.4 years |
|
|||
Volatility |
|
|
37 |
% |
|
|
36 |
% |
|
|
34 |
% |
Expected dividend yield |
|
|
0.30 |
% |
|
|
0.32 |
% |
|
|
0.20 |
% |
Weighted-average fair value per share |
|
$ |
23.02 |
|
|
$ |
19.68 |
|
|
$ |
21.69 |
|
Stock-based compensation for restricted stock awards and restricted stock units is expensed ratably over the vesting period based on the grant date fair value.
Earnings Per Share
Net income per common share attributable to Bruker Corporation shareholders is calculated by dividing net income attributable to Bruker Corporation, adjusted to reflect changes in the redemption value of the redeemable noncontrolling interest, by the weighted-average shares outstanding during the period. The diluted net income per share computation includes the effect of shares which would be issuable upon the exercise of outstanding stock options and the vesting of restricted stock, reduced by the number of shares which are assumed to be purchased by the Company under the treasury stock method. There was no redemption value adjustment of the redeemable noncontrolling interest for the years ended December 31, 2023, 2022 and 2021.
66
Post Retirement Benefit Plans
The Company recognizes the over-funded or under-funded status of defined benefit pension and other post retirement defined benefit plans as an asset or liability, respectively, in its consolidated balance sheets and recognizes changes in the funded status in the year in which the changes occur through other comprehensive income.
Other Comprehensive Income (Loss)
Other comprehensive income (loss) refers to revenues, expenses, gains and losses that are excluded from net income as these amounts are recorded directly as an adjustment to shareholders’ equity, net of tax. The Company’s other comprehensive income (loss) was composed of foreign currency translation adjustments, derivatives designated as hedging instruments and pension liability adjustments.
Foreign Currency Translation
Assets and liabilities of the Company’s foreign subsidiaries, where the functional currency is the local currency, are translated into U.S. Dollars using the current exchange rate as of the consolidated balance sheet date and shareholders’ equity is translated using historical rates. Revenues and expenses of foreign subsidiaries are translated at the average exchange rates in effect during the year. Adjustments resulting from financial statement translations are included as a separate component of shareholders’ equity. Gains and losses resulting from translation of foreign currency monetary transactions are reported in interest and other income (expense), net in the consolidated statements of income and comprehensive income for all periods presented. The Company has certain intercompany foreign currency transactions that are deemed to be of a long-term investment nature. Exchange adjustments related to those transactions are made directly to a separate component of shareholders’ equity.
Equity-method investments
The Company accounts for investments in common stock under the equity method if the Company has the ability to exercise significant influence, but not control, over an investee. Investments in equity-method investees are included within “Other long-term assets” in the consolidated balance sheets. The Company’s proportional share of the earnings or losses as reported by equity-method investees are classified as “Equity in income of unconsolidated investees, net of tax” in the consolidated statements of income and comprehensive income. The Company records investments, including incremental investments, of common shares in equity-method investees at cost. In the event the Company no longer has the ability to exercise significant influence over an equity-method investee, the Company would discontinue accounting for the investment under the equity method. The Company regularly evaluates these investments, which are not carried at fair value, for other-than-temporary impairment and records any impairment charge in earnings when the decline in value below the carrying amount of its equity method investment is determined to be other-than-temporary.
Minority investments
When the Company does not have control or the ability to exercise significant influence over an investee, it accounts for its minority investments in equity interests without a readily determinable fair value using the measurement alternative. The equity interest is initially recorded at cost, less impairment. The carrying amount is subsequently remeasured to its fair value when observable price changes occur or it is impaired. Any adjustments to the carrying amount are recorded in earnings.
The Company accounts for its minority investments in debt securities classified as available-for-sale at fair value. The Company regularly evaluates these investments for impairment. An available-for-sale debt security is impaired if its fair value is below its amortized cost basis. For available-for-sale debt securities with unrealized losses, management performs an analysis to assess whether the Company intends to sell or whether the Company would more likely than not be required to sell the security before the expected recovery of the amortized cost basis. Where the Company intends to sell a security, or may be required to do so, the security’s decline in fair value is deemed to be other-than-temporary and the full amount of the unrealized loss is reflected in earnings as an impairment loss. Regardless of the Company's intent to sell a security, additional analysis is performed on all securities with unrealized losses to evaluate losses associated with the creditworthiness of the security. Credit losses are identified where the Company does not expect to receive cash flows sufficient to recover the amortized cost basis of a security. If the Company does not intend to sell the security or is not more likely than not to sell the security and the decline in fair value is not due to credit-related factors, the loss is recorded in other comprehensive income.
67
Any impairment charges are included in “Interest and other income (expense), net” in the Consolidated Statements of Income and Comprehensive Income.
Risks and Uncertainties
The Company is subject to risks common to its industry including, but not limited to, global economic conditions, such as increasing inflation, uncertainties caused by recent banking industry volatility, rapid technological change, government and academic funding levels, geopolitical uncertainties, changes in commodity prices, spending patterns of its customers, protection of its intellectual property, availability of key raw materials and components and other supply chain challenges, compliance with existing and future regulation by government agencies and fluctuations in foreign currency exchange rates and interest rates.
The Company has experienced supply chain interruptions as a result of general global economic conditions, including inflation and the threat of a potential recession, a tighter labor market and other factors including natural events and disasters. Various factors, including increased demand for certain components and production delays, are contributing to shortages of certain components used in the Company’s products and increased difficulties in the Company’s ability to obtain a consistent supply of materials at stable pricing levels. Supply shortages and longer lead teams for components used in the Company's products, including limited source components, has resulted and may continue to cause disruptions to the Company’s production activities, which has had and may continue to have an adverse effect on the Company’s financial condition or result of operations. These factors have impacted and may continue to impact the timing of the Company’s revenue, and have also resulted, and may result in a delay of revenue, and an increase in manufacturing costs, all of which have adversely impacted and may continue to adversely impact the Company's operating results.
Additionally, world events, such as the conflict between Russia and Ukraine and related economic sanctions, the conflict in Israel, Palestine and surrounding areas, the possible expansion of such conflicts and potential geopolitical consequences, the ongoing tensions between the United States and China, tariff and trade policy changes, and increasing potential of conflict involving countries in Asia that are significant to the Company’s supply network operations, such as Taiwan and China, have resulted in increasing global instability and have created uncertainty for global commerce. As a result of the adverse economic impacts resulting from the conflict between Russia and Ukraine, such as increased prices for and a reduced supply of key metals used in our products, the Company has ceased its Russian operations. Sustained or worsening global economic conditions and increasing inflation and geopolitical tensions have increased the Company's cost of doing business, impacted the Company's supply chain operations, caused some of the Company's customers to reduce or delay spending and further intensified pricing pressures. Combined with increased inflation, potential energy shortages in Europe where we have significant operations, and overall higher energy and transportation costs, these factors have affected and may continue to affect the Company's financial condition and results of operations.
The preparation of the consolidated financial statements requires the Company to make estimates, judgments and assumptions that may affect the reported amounts of assets, liabilities, equity, revenues and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis the Company evaluates estimates, judgments and methodologies. Changes in estimates are recorded in the period in which they become known. The Company bases its estimates on historical experience and on various other assumptions that it believes are reasonable, the results of which form the basis for making judgments about the carrying values of assets, liabilities and equity and the amount of revenues and expenses. The full extent to which the global supply chain interruptions, higher energy costs and shortages, the global economy, including inflation and the threat of recession, and geopolitical instability will directly or indirectly impact future business, results of operations and financial condition, including sales, expenses, reserves and allowances, manufacturing, research and development costs and employee cost related amounts, will depend on future developments that are highly uncertain, including as a result of new developments concerning global supply chain and various global conflicts. The Company has made estimates of the impact of these disruptions within the financial statements and there may be changes to those estimates in future periods. Actual results may differ from management’s estimates if these results differ from historical experience.
Loss Contingencies
Loss contingency provisions are recorded if the potential loss from any claim, asserted or unasserted, or legal proceeding related to patents, products and other matters, is considered probable and the amount can be reasonably estimated, or a range of loss can be determined. These accruals represent management’s best estimate of probable loss. Disclosure is provided when a loss is considered probable but the loss is not reasonably estimable and when a material loss is reasonably possible but not probable.
68
3. Recent Accounting Pronouncements
In December 2023, the FASB issued ASU No. 2023-09 – Income Taxes (Topic 740): Improvements to Income Tax Disclosures (“ASU 2023-09”), which requires enhanced income tax disclosures, including disaggregation of information in the rate reconciliation table and disaggregation of information related to income taxes paid as presented on the Cash Flow Statement. This guidance is effective for annual reporting periods ending after December 15, 2024. The Company is evaluating the potential impact of this adoption on the consolidated financial statements and related disclosures.
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segments Disclosures (“ASU 2023-07”). The ASU expands public entities’ segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker (“CODM”). These significant segment expenses are included within each reported measure of segment profit or loss, an amount and description of its composition for other segment items, and interim disclosures of a reportable segment’s profit or loss and assets. All disclosure requirements under ASC 2023-07 are also required for public entities with a single reportable segment. This guidance is effective for annual reporting periods beginning after December 15, 2023, and interim periods after December 15, 2024. The Company is evaluating the potential impact of this adoption on the consolidated financial statements and related disclosures.
In March 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2023-01, Leases (Topic 842): Common Control Arrangements (“ASU 2023-01”), which provides additional guidance concerning related party leasing arrangements between entities under common control. Specifically, ASU 2023-01 provides a practical expedient for private companies in determining whether a related party arrangement qualifies as a lease and provides new guidance for all entities on the amortization period of leasehold improvements for common control arrangements. As the Company is a public company, the practical expedient for related party arrangements is not applicable to Bruker. This guidance is effective for annual and interim reporting periods beginning after December 15, 2023. Additionally, the Company evaluated the impact of adopting this new guidance regarding the amortization period of leasehold improvements for common control arrangements and concluded it is not expected to have a material impact on the consolidated financial statements and related disclosures.
In March 2020, the FASB issued ASU No. 2020-04, Reference Rate Reform (Topic 848): Facilitation of the Effects of Reference Rate Reform on Financial Reporting (“ASU 2020-04”), which provides temporary optional guidance to ease the potential burden in accounting for reference rate reform. The guidance provides optional expedients and exceptions for applying generally accepted accounting principles to transactions affected by reference rate reform if certain criteria are met. These transactions include: contract modifications, hedging relationships, and sale or transfer of debt securities classified as held-to-maturity. In January 2021, the FASB issued ASU 2021-01, Reference Rate Reform (Topic 848): Scope, to clarify that certain optional expedients and exceptions under the reference rate reform guidance for contract modifications and hedge accounting apply to derivatives that are affected by the discounting transition. Specifically, certain provisions in the reference rate reform guidance, if elected by an entity, apply to derivative instruments that use an interest rate for margining, discounting, or contract price alignment that is modified as a result of reference rate reform. This temporary guidance is effective for all entities as of March 12, 2020, through December 31, 2023. The Company elected to apply this guidance for all contract modifications or eligible hedging relationships during that time period subject to certain criteria.
4. Revenue
The following table presents the Company’s revenues by Segment for the years ended December 31 (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Revenue by Segment: |
|
|
|
|
|
|
|
|
|
|||
BSI BioSpin |
|
$ |
798.5 |
|
|
$ |
696.7 |
|
|
$ |
691.0 |
|
BSI CALID |
|
|
960.4 |
|
|
|
822.2 |
|
|
|
819.6 |
|
BSI Nano |
|
|
941.9 |
|
|
|
787.0 |
|
|
|
697.5 |
|
BEST |
|
|
280.7 |
|
|
|
237.1 |
|
|
|
223.8 |
|
Eliminations (a) |
|
|
(17.0 |
) |
|
|
(12.3 |
) |
|
|
(14.0 |
) |
Total revenue |
|
$ |
2,964.5 |
|
|
$ |
2,530.7 |
|
|
$ |
2,417.9 |
|
(a) Represents corporate costs and eliminations not allocated to the reportable segments.
69
Revenue for the Company recognized at a point in time versus over time is as follows for the years ended December 31 (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Revenue recognized at a point in time |
|
$ |
2,575.3 |
|
|
$ |
2,204.7 |
|
|
$ |
2,104.7 |
|
Revenue recognized over time |
|
|
389.2 |
|
|
|
326.0 |
|
|
|
313.2 |
|
Total revenue |
|
$ |
2,964.5 |
|
|
$ |
2,530.7 |
|
|
$ |
2,417.9 |
|
Remaining Performance Obligations
Remaining performance obligations represent the aggregate transaction price allocated to a promise to transfer a good or service that is fully or partially unsatisfied at the end of the period. As of December 31, 2023, remaining performance obligations were approximately $2,226.7 million.
Contract Balances
The timing of revenue recognition, billings and cash collections results in billed accounts receivable, notes receivables and unbilled receivables (contract assets) and deferred revenue, customer deposits and billings in excess of revenue recognized (contract liabilities) on the Company’s consolidated balance sheets.
Contract assets—Most of the Company’s long-term contracts are billed as work progresses in accordance with the contract terms and conditions, either at periodic intervals or upon achievement of certain milestones. Billing often occurs subsequent to revenue recognition, resulting in contract assets. Contract assets are classified as either other current assets or other long-term assets in the condensed consolidated balance sheets. The balance of contract assets as of December 31, 2023, and December 31, 2022, was $85.8 million and $61.3 million, respectively.
Contract liabilities—The Company often receives cash payments from customers in advance of the Company’s performance, resulting in contract liabilities. These contract liabilities are classified as either current or long-term in the consolidated balance sheet based on the timing of when revenue recognition is expected. The balance of contract liabilities as of December 31, 2023, and December 31, 2022, was $491.4 million and $471.7 million, respectively. The increase in the contract liability balance during the year ended December 31, 2023, is primarily a result of new performance obligations entered into during the period and delays in certain system installations due to supply chain and logistics challenges. Approximately $342.9 million of the contract liability balance on December 31, 2022, was recognized as revenue during the year ended December 31, 2023.
5. Other Current Assets
Other current assets consisted of the following (in millions):
|
|
December 31, |
|
|
December 31, |
|
||
Unbilled and notes receivable |
|
$ |
83.9 |
|
|
$ |
60.8 |
|
Income and other taxes receivable |
|
|
45.9 |
|
|
|
61.8 |
|
Prepaid expenses |
|
|
26.7 |
|
|
|
24.3 |
|
Deposits with vendors |
|
|
28.8 |
|
|
|
19.0 |
|
Interest rate cross-currency swap agreements |
|
|
12.0 |
|
|
|
14.8 |
|
Other assets |
|
|
18.3 |
|
|
|
12.8 |
|
Other current assets |
|
$ |
215.6 |
|
|
$ |
193.5 |
|
70
6. Acquisitions
Pro forma financial information has been presented for the PhenomeX acquisition and not for others because the impact, individually and collectively from the other acquisitions, on revenues and net income is not material. Amounts allocated to goodwill that are attributable to expected synergies are not expected to be deductible for tax purposes.
2023
On October 2, 2023, the Company acquired 100% of the outstanding stock of PhenomeX Inc. (“PhenomeX”), a publicly traded company, for a purchase price of $109.4 million, net of $11.8 million in cash acquired. Total cash consideration of $121.2 million consisted of $107.2 million for the acquisition of the outstanding stock including an $8.0 million payment to settle an employee award, and settlement of a $14.0 million note previously issued by the Company to PhenomeX during 2023. PhenomeX is a life science tools company with a focus on functional cell biology. Their products and services provide customers with, among other offerings, Optofluidic platforms such as the Beacon, Beacon Select and Beacon Quest as well as Proteomic Barcoding Platforms, such as the IsoLight System and the IsoSpark System. PhenomeX is domiciled in Emeryville, California and was integrated into the BSI Nano Segment. The Company renamed PhenomeX to Bruker Cellular Analytics (“BCA”) following acquisition.
The fair value of the identifiable intangible assets has been estimated by using the income approach through a discounted cash flow analysis of certain cash flow projections. The cash flow projections are based on forecasts used by the Company to price the acquisition, and the discount rates applied were benchmarked by referencing the implied rate of return of the Company’s pricing model and the weighted average cost of capital. The amortization period for the intangible assets acquired ranges from eight to twelve years for the technology and fifteen years for the customer relationships.
The components and fair value allocation of the consideration transferred in connection with the acquisition are as follows (in millions).
|
|
PhenomeX Inc. |
|
|
Segment |
|
BSI NANO |
|
|
Consideration Transferred: |
|
|
|
|
Cash paid |
|
$ |
121.2 |
|
Cash acquired |
|
|
(11.8 |
) |
Total consideration transferred |
|
$ |
109.4 |
|
Allocation of Consideration Transferred: |
|
|
|
|
Accounts receivable |
|
$ |
5.6 |
|
Inventories |
|
|
42.1 |
|
Other current assets |
|
|
7.6 |
|
Property, plant and equipment |
|
|
33.5 |
|
Deferred tax assets |
|
|
182.7 |
|
Other assets |
|
|
24.3 |
|
Intangible assets: |
|
|
|
|
Technology |
|
|
24.0 |
|
Customer relationships |
|
|
8.0 |
|
Liabilities assumed |
|
|
(74.3 |
) |
Total consideration allocated |
|
$ |
253.5 |
|
Bargain purchase gain |
|
$ |
144.1 |
|
The financial data presented above represents the provisional determination of the fair value of the identifiable assets acquired and liabilities assumed based on the information available to us as of the time of the issuance of these financial statements. The Company is in the process of reviewing and identifying acquisition accounting adjustments for several acquired tax positions including the final calculation of the acquired federal and state net operating loss carryforwards “NOLs”. Section 382(h) of the Tax Reform Act of 1986 imposed highly complex rules for the annual base limitation for NOL utilization, including the determination of built-in gains and losses. PhenomeX incurred significant losses prior to its acquisition by the Company and has undergone several ownership changes. Accordingly, the values recognized related to the deferred tax assets are based on provisional amounts and are subject to change until the Company finalizes its Section 382(h) study during the measurement period which is no later than one year from acquisition date. The final determination may result in asset and liability values that are different than the preliminary estimates and may result in an adjustment to the bargain purchase gain recorded in earnings.
71
ASC 805, Business Combinations, requires that any excess of fair value of acquired net assets, including identifiable intangible assets over the acquisition consideration, results in a gain from bargain purchase. The PhenomeX acquisition resulted in a gain on bargain purchase due to the estimated fair value of the identifiable net assets acquired exceeding the purchase consideration transferred by $144.1 million and is shown as a gain on bargain purchase on our consolidated statement of income. Prior to recognizing the gain, the Company reassessed the measurement and recognition of identifiable assets acquired, and liabilities assumed and concluded that the valuation procedures and resulting measures were appropriate. The acquisition resulted in a bargain purchase gain due to the following factors: (i) the sellers were motivated and were looking for new management to lead the strategic direction of the company, create economies of scale and return the company to profitable operations, (ii) recurring operating losses resulted in a valuation of PhenomeX that significantly affected its market capitalization that resulted in a sustained decrease in stock price and fair value and (iii) PhenomeX has accumulated significant NOL’s that can be utilized by the Company, subject to annual limitations, to reduce its US taxable income which could not be benefited by PhenomeX.
Results of acquired operations of BCA
The results of the acquired operations of BCA have been included in the consolidated financial statements of the Company since its acquisition date of October 2, 2023. For the period from October 2, 2023, through December 31, 2023, BCA had total revenues of $7.0 million and pre-tax net loss of $43.4 million. The tax effect on pre-tax net loss of BCA is included in the consolidated US tax return of Bruker Corporation.
In connection with the acquisition of PhenomeX, the Company incurred $10 million of acquisition-related expenses. These expenses primarily include legal and professional services. These acquisition-related expenses were recorded within Other Charges, Net in the consolidated statement of operations.
Shortly after acquiring PhenomeX, the Company initiated a restructuring plan. See Note 21, Other Charges, Net.
Supplemental Pro Forma Information (unaudited)
The supplemental pro forma financial information presented below is for illustrative purposes only, does not include the pro forma adjustments that would be required under Article 11 of Regulation S-X for pro forma financial information, is not necessarily indicative of the financial position or results of operations that would have been realized if the combination with PhenomeX had been completed on January 1, 2023, does not reflect synergies that might have been achieved, nor is it indicative of future operating results or financial position. The pro forma adjustments are based upon currently available information and certain assumptions that the Company believes are reasonable under the circumstances.
On March 21, 2023, pursuant to a merger agreement (“IsoPlexis Merger”), Berkeley Lights, Inc. (“BLI”) acquired and merged with IsoPlexis Corporation (“IsoPlexis”) with IsoPlexis surviving the merger as a wholly owned subsidiary of BLI. The newly merged company was renamed PhenomeX Inc. Historical financial statements of PhenomeX for periods prior to the IsoPlexis Merger are the historical financial statements of BLI. As a result, it would not be meaningful and would be impracticable to present comparative pro forma financial information as though the acquisition of PhenomeX had occurred as of the beginning of the comparable prior annual reporting period as if the business combination with PhenomeX had been completed on January 1, 2022.
|
|
Year Ended December 31, 2023 |
|
|||||||||
|
|
Before Adjustments |
|
|
Pro forma |
|
|
After Adjustments |
|
|||
Revenue |
|
$ |
3,005.9 |
|
|
$ |
— |
|
|
$ |
3,005.9 |
|
Net income (loss) |
|
$ |
285.0 |
|
|
$ |
(2.9 |
) |
|
$ |
282.1 |
|
|
|
|
|
|
|
|
|
|
|
|||
The supplemental pro forma financial information reflects pro forma adjustments which primarily include:
72
In the year ended December 31, 2023, the Company completed various other acquisitions that collectively complemented its existing product offerings of the Company’s existing businesses. The following table reflects the consideration transferred and the respective reportable segment for certain of the 2023 acquisitions (in millions):
|
|
Biognosys, AG |
|
|
Zontal Inc. |
|
|
Miro Analytical AG |
|
|||
Segment |
|
BSI CALID |
|
|
BSI BioSpin |
|
|
BSI CALID |
|
|||
Consideration Transferred: |
|
|
|
|
|
|
|
|
|
|||
Cash paid |
|
$ |
73.6 |
|
|
$ |
14.8 |
|
|
$ |
8.6 |
|
Cash acquired |
|
|
(9.5 |
) |
|
|
(0.3 |
) |
|
|
(0.5 |
) |
Holdback |
|
|
0.2 |
|
|
|
— |
|
|
|
1.0 |
|
Fair value of hybrid financial instruments - founders |
|
|
— |
|
|
|
18.5 |
|
|
|
9.3 |
|
Fair value of redeemable noncontrolling interest - other shareholders |
|
|
2.5 |
|
|
|
— |
|
|
|
0.5 |
|
Fair value of contingent consideration |
|
|
— |
|
|
|
0.5 |
|
|
|
— |
|
Total consideration transferred |
|
$ |
66.8 |
|
|
$ |
33.5 |
|
|
$ |
18.9 |
|
Allocation of Consideration Transferred: |
|
|
|
|
|
|
|
|
|
|||
Accounts receivable |
|
$ |
3.6 |
|
|
$ |
0.7 |
|
|
$ |
— |
|
Inventories |
|
|
0.4 |
|
|
|
— |
|
|
|
0.8 |
|
Other current assets |
|
|
0.9 |
|
|
|
0.3 |
|
|
|
— |
|
Property, plant and equipment |
|
|
8.0 |
|
|
|
— |
|
|
|
0.1 |
|
Other assets |
|
|
4.3 |
|
|
|
2.5 |
|
|
|
— |
|
Intangible assets: |
|
|
|
|
|
|
|
|
|
|||
Technology |
|
|
10.2 |
|
|
|
5.8 |
|
|
|
8.9 |
|
Customer relationships |
|
|
13.8 |
|
|
|
4.0 |
|
|
|
0.3 |
|
Backlog |
|
|
0.8 |
|
|
|
0.2 |
|
|
|
— |
|
Trade name |
|
|
2.7 |
|
|
|
1.1 |
|
|
|
0.2 |
|
Goodwill |
|
|
47.5 |
|
|
|
25.9 |
|
|
|
11.1 |
|
Liabilities assumed (a) |
|
|
(25.4 |
) |
|
|
(7.0 |
) |
|
|
(2.5 |
) |
Total consideration allocated |
|
$ |
66.8 |
|
|
$ |
33.5 |
|
|
$ |
18.9 |
|
(a) This amount includes an assumed liability for vested employee awards of $6.3 million on the acquisition date which was settled in the post-closing period ended March 31, 2023, for Biognosys, AG.
Biognosys, AG
On January 3, 2023, the Company acquired 97.15% of the outstanding stock of Biognosys, AG (“Biognosys”), a privately held company, for a cash consideration of CHF 75 million (approximately $80.1 million) less assumed liability for employee awards of CHF 5.9 million (approximately $6.3 million). Biognosys offers mass spectrometry based next-generation proteomics contract research services as well as proprietary proteomics software and laboratory consumables to support academic, pharma and biotech research and clinical development. Biognosys is domiciled in Zurich, Switzerland, and was integrated into the BSI CALID Segment.
Concurrent with the acquisition, the Company entered into an agreement with the noncontrolling interest holders that provides the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 2.85% of Biognosys for cash to the founders at a contractually defined redemption value exercisable beginning in 2028. The option price to acquire the remaining 2.85% equity interest will have a minimum redemption, or floor, value at each purchase or sell date, subject to post combination employment. The fair value at acquisition date of these put option rights has been bifurcated into two financial instruments to separately account for the amounts attributable to the put option rights to sell the non-controlling interests on exercise dates at (1) above the minimum redemption value and (2) the minimum redemption value or floor value that is subject to post combination employment (the hybrid instrument) services.
The rights (embedded derivative) to the option shares can be sold at a minimum redemption value provided certain post combination employment services are met or at fair value, if above the floor, on the purchase or sell date. Therefore, the portion assigned to the minimum redemption value of option value of the hybrid instrument, which is tied to continued employment of the noncontrolling interest holders, was classified as a long-term liability on the consolidated balance sheet. The hybrid instrument was initially measured at fair value on the acquisition date and shall be accreted over the post combination service period. The acquisition date fair value of the hybrid instrument which is an embedded derivative was not material.
73
The rights associated with the portion of the noncontrolling interest above the minimum redemption value are contingently redeemable at the option of the Company or the noncontrolling interest holders. As redemption of the rights is contingently redeemable at the option of the noncontrolling interest shareholders, the Company classifies the carrying amount of these rights in the mezzanine section on the consolidated balance sheet, which is presented above the equity section and below liabilities. The redeemable noncontrolling interest is initially measured at fair value on acquisition date and subsequently at the greater of the amount that would be paid if settlement occurred as of the balance sheet date based on fair value as defined in the purchase agreement and its carrying amount adjusted for net income (loss) attributable to the noncontrolling interest. Adjustments to the carrying value of the redeemable noncontrolling interest are recorded through retained earnings.
The amortization period for the intangible assets acquired is seven years for the technology and nine years for the customer relationships. The trade name was determined to have an indefinite life. The Company expects to amortize backlog through the end of 2025.
Zontal, Inc.
On May 4, 2023, the Company acquired 60% of the outstanding share capital of OSTHUS Beteiligungs GmbH and its wholly owned subsidiaries: OSTHUS Group GmbH, Zontal Inc., Zontal GmbH, and Zontal Data Information Technology (Dalian) co., Ltd, (hereinafter, the “Zontal Companies”) for cash consideration of EUR 13.4 million (approximately $14.8 million) with the potential for additional consideration of up to $14.4 million if certain revenue and EBITDA targets are met through 2025. Zontal, Inc, is the main operating company. The Zontal Companies offer various software applications and integrations including data management (Informatics and SDMS), which enables customers to harmonize, preserve and reuse their data to generate efficiencies and automate workflows. The Zontal Companies are domiciled in Aachen, Germany, and are integrated into the BSI BioSpin Segment.
Concurrent with the acquisition, the Company entered into an agreement with the noncontrolling interest holders that provides the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 40% of the Zontal Companies at a contractually defined redemption value exercisable beginning in 2027 and in 2031. The rights (embedded derivative) to the option shares can be exercised at a discounted redemption value upon certain events related to post combination employment services. As the options are tied to continued employment, the Company classified the hybrid instrument (noncontrolling interest with an embedded derivative) as a long-term liability on the consolidated balance sheet. The hybrid instrument associated with the options is initially measured at fair value on the acquisition date. Subsequent to the acquisition, the carrying value of the hybrid instrument is remeasured to fair value with changes recorded to stock-based compensation expense in proportion to the requisite service period vested.
The amortization period for the intangible assets acquired is eight years for technology, ten years for the trade name, and thirteen years for the customer relationships. The Company expects to amortize backlog through 2028.
MIRO Analytical AG
On October 2, 2023, the Company acquired 58.26% of the outstanding share capital of MIRO Analytical AG, (hereinafter, “MIRO”) for cash consideration of CHF 8.8 million (approximately $9.6 million). MIRO operates in the development, production, and distribution of equipment for gas analysis and related components. MIRO provides companies with tools to monitor and report on air pollution and greenhouse gases and therefore, empower them to act against air pollution and climate change. MIRO is domiciled in Switzerland and is integrated into the BSI CALID segment.
Concurrent with the acquisition, the Company entered into an agreement with the noncontrolling interest holders that provides the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 41.74% of MIRO at a contractually defined redemption value exercisable beginning in 2028 and in 2031. The redemption value and rights related to the noncontrolling interest vary for the founders and the other shareholder. The fair value of these rights has been bifurcated into two financial instruments to separately account for the amounts attributable to the founders and the amount attributable to other shareholders.
The rights (embedded derivative) associated with the founders shared may be exercised at a discounted redemption value upon certain events related to post combination employment services. As the options are tied to continued employment, the Company classified the hybrid instrument (noncontrolling interest with an embedded derivative) as a long-term liability on the consolidated balance sheet. The hybrid instrument associated with the founder’s options is initially measured at fair value on the acquisition date. Subsequent to the acquisition, the carrying value of the hybrid instrument is remeasured to fair value with changes recorded to stock-based compensation expense in proportion to the requisite service period vested.
74
The rights associated with the other noncontrolling interest holders are contingently redeemable at the option of the Company or the noncontrolling interest holders. As redemption of the rights is contingently redeemable at the option of the noncontrolling interest shareholders, the Company classifies the carrying amount of these rights in the mezzanine section on the consolidated balance sheet, which is presented above the equity section and below liabilities. The redeemable noncontrolling interest is initially measured at fair value and subsequently at the greater of the amount that would be paid if settlement occurred as of the balance sheet date based on the contractually defined redemption value and its carrying amount adjusted for net income (loss) attributable to the noncontrolling interest. Adjustments to the carrying value of the redeemable noncontrolling interest are recorded through retained earnings.
The amortization period for the intangible assets acquired is eleven years for technology. The Company has fully amortized the trade name and customer relationships as of December 31, 2023.
Other 2023 Acquisitions
In the year ended December 31, 2023, the Company completed various other acquisitions that complemented the Company's existing product offerings and are accounted for under the acquisition method. The following table reflects the consideration transferred and the respective reportable segment for these acquisitions (in millions):
Name of Acquisition |
|
Date Acquired |
|
Segment |
|
Total |
|
|
Cash |
|
||
Acquifer Imaging GmbH and Deltabyte GmbH |
|
January 4, 2023 |
|
BSI NANO |
|
$ |
7.6 |
|
|
$ |
7.6 |
|
Pinpoint Testing LLC |
|
March 28, 2023 |
|
BSI CALID |
|
|
8.6 |
|
|
|
3.6 |
|
Fasmatech Science SA |
|
March 3, 2023 |
|
BSI CALID |
|
|
10.3 |
|
|
|
8.4 |
|
Interherence GmbH |
|
July 3, 2023 |
|
BSI CALID |
|
|
17.3 |
|
|
|
3.9 |
|
Other majority owned acquisitions |
|
Various |
|
Various |
|
|
0.9 |
|
|
|
0.9 |
|
|
|
|
|
|
|
$ |
44.7 |
|
|
$ |
24.4 |
|
Minority and Equity-method investments
In the year ended December 31, 2023, the Company also made several minority investments. For these investments accounted for under the alternative measurement, these values also represent the carrying value at December 31, 2023. No impairments have been recognized on these investments. The following table reflects the consideration transferred and the respective reporting segment for the investments (in millions):
Name |
|
Acquisition / |
|
Financial |
|
Date Acquired |
|
Segment |
|
Total |
|
|
Cash |
|
||
Tome Biosciences |
|
Investment |
|
Other long-term assets |
|
July 26, 2023 |
|
Corporate |
|
$ |
10.0 |
|
|
$ |
10.0 |
|
Other Investments |
|
Investment |
|
Other long-term assets |
|
Various |
|
Various |
|
|
14.8 |
|
|
|
14.8 |
|
|
|
|
|
|
|
|
|
|
|
$ |
24.8 |
|
|
$ |
24.8 |
|
During the year ended December 31, 2023, the Company recorded a realized gain of $6.8 million from the sale of a minority investment. In the year ended December 31, 2022, the Company recorded an unrealized gain of $1.7 million from the remeasurement of a minority investment upon the occurrence of an observable transaction. The realized and unrealized gain is included in “Interest and other income (expense), net” in the Consolidated Statements of Income and Comprehensive Income.
For the year ended December 31, 2023, the Company recognized $18.2 million in impairment charges to write down the carrying value of certain minority investments. The Company did not recognize any impairment charges related to its minority investments for the year ended December 31, 2022. The impairment charges are included in “Interest and other income (expense), net” in the Consolidated Statements of Income and Comprehensive Income.
75
2024—Subsequent Event Acquisitions
On February 22, 2024, the Company acquired a minority equity interest in a privately held biotechnology company located in California for $10.0 million. The company utilizes precision proteomics to enable early detection of diseases.
On February 5, 2024, the Company acquired 100% of the outstanding stock of Nanophoton Corporation for a purchase price of $12.25 million, with potential for additional consideration of up to $5.25 million if certain revenue targets and non-revenue milestones are met through 2027.
On February 1, 2024, the Company acquired 100% of the outstanding stock of Spectral Instruments Imaging for a purchase price of $27.5 million, with potential for additional consideration of up to $10 million if certain revenue and EBITDA targets are met through 2025.
On January 2, 2024, the Company acquired 100% of the outstanding stock of Nion, LLC for a purchase price of $35 million, subject to a net working capital adjustment. The transaction also has the potential for additional contingent consideration of up to $23 million if certain revenue targets and non-revenue milestones are met.
On January 1, 2024, the Company acquired 100% of the outstanding stock of Tornado Spectral Systems for a purchase price of CAD 30 million ($23 million).
In December 2023, the Company entered into a binding put option agreement (the “Put Option Letter”) with Tecfin S.à r.l., a controlled affiliate of PAI Partners acting for itself and on behalf of the shareholders ( “the Sellers”) owning directly or indirectly 100% of the issued and outstanding securities of TecInvest S.à r.l, which does business as ELITechGroup. ELITechGroup’s subsidiaries are active in the molecular diagnostics, microbiology and biomedical testing equipment fields.
Following completion of required works council consultation processes in France and the Netherlands, on February 27, 2024, the Company entered into a Securities Purchase Agreement (the “Share Purchase Agreement”) with the Sellers to acquire all the outstanding securities of Tecfin S.a.r.l., Eliman S.A.S. and Eliman 2 S.A.S., which entities collectively, own a 100% interest in TecInvest S.a.r.l. Under the terms of the Share Purchase Agreement, the Company will acquire all issued and outstanding securities of ELITechGroup and their subsidiaries for a cash purchase price of EUR 870 million (approximately $962 million), subject to certain adjustments. The Share Purchase Agreement provides that closing of the acquisition is subject to the satisfaction or waiver of certain conditions, including certain regulatory approvals and notices. The Put Option Letter terminated upon execution of the Share Purchase Agreement.
76
2022
In the year ended December 31, 2022, the Company completed various acquisitions that collectively complemented its existing product offerings of to the Company’s existing businesses. The following table reflects the consideration transferred and the respective reportable segment for each of the 2022 acquisitions (in millions):
|
|
PreOmics GmbH |
|
|
Optimal Industrial Automation and Technologies |
|
|
Inscopix, Inc. |
|
|||
Segment |
|
BSI CALID |
|
|
BSI BioSpin |
|
|
BSI Nano |
|
|||
Consideration Transferred: |
|
|
|
|
|
|
|
|
|
|||
Cash paid |
|
$ |
52.1 |
|
|
$ |
40.3 |
|
|
$ |
101.5 |
|
Cash acquired |
|
|
(16.0 |
) |
|
|
(6.2 |
) |
|
|
(12.1 |
) |
Fair value of hybrid financial instruments - founders |
|
|
20.9 |
|
|
|
— |
|
|
|
— |
|
Fair value of redeemable noncontrolling interest - other shareholders |
|
|
6.8 |
|
|
|
— |
|
|
|
— |
|
Fair value of contingent consideration |
|
|
— |
|
|
|
0.4 |
|
|
|
— |
|
Working capital adjustment |
|
|
— |
|
|
|
— |
|
|
|
0.6 |
|
Total consideration transferred |
|
$ |
63.8 |
|
|
$ |
34.5 |
|
|
$ |
90.0 |
|
Allocation of Consideration Transferred: |
|
|
|
|
|
|
|
|
|
|||
Accounts receivable |
|
$ |
0.4 |
|
|
$ |
1.9 |
|
|
$ |
3.2 |
|
Inventories |
|
|
0.6 |
|
|
|
— |
|
|
|
2.5 |
|
Other current assets |
|
|
0.7 |
|
|
|
0.8 |
|
|
|
0.9 |
|
Property, plant and equipment |
|
|
1.3 |
|
|
|
0.1 |
|
|
|
0.5 |
|
Other assets |
|
|
0.4 |
|
|
|
0.8 |
|
|
|
4.7 |
|
Intangible assets: |
|
|
|
|
|
|
|
|
|
|||
Technology |
|
|
12.5 |
|
|
|
5.9 |
|
|
|
26.6 |
|
Customer relationships |
|
|
6.9 |
|
|
|
12.9 |
|
|
|
20.8 |
|
Backlog |
|
|
— |
|
|
|
1.1 |
|
|
|
— |
|
Trade name |
|
|
1.9 |
|
|
|
1.2 |
|
|
|
3.2 |
|
Goodwill |
|
|
47.0 |
|
|
|
18.6 |
|
|
|
52.2 |
|
Liabilities assumed |
|
|
(7.9 |
) |
|
|
(8.8 |
) |
|
|
(24.6 |
) |
Total consideration allocated |
|
$ |
63.8 |
|
|
$ |
34.5 |
|
|
$ |
90.0 |
|
PreOmics GmbH
On January 18, 2022, the Company acquired a 74.15% interest in PreOmics GmbH, (“PreOmics”), a privately held company, for a purchase price of EUR 46.1 million (approximately $52.1 million). PreOmics is a provider of sample preparation and automation solutions for proteomic analysis by mass spectrometry systems. PreOmics is located in Munich, Germany and was integrated into BSI CALID Segment.
Concurrent with the acquisition, the Company entered into an agreement with the noncontrolling interest holders that provides the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 25.85% of PreOmics for cash at a contractually defined redemption value for both the original founders and other shareholders of PreOmics, exercisable beginning in 2026. The fair value of these rights has been bifurcated into two financial instruments to separately account for the amounts attributable to the founders and the amount attributable to other shareholders.
The rights (embedded derivative) associated with the founders can be accelerated, at a discounted redemption value, upon certain events related to post combination employment services. As the options are tied to continued employment, the Company classified the hybrid instrument (noncontrolling interest with an embedded derivative) as a long-term liability on the consolidated balance sheet. The hybrid instrument associated with the founders is initially measured at fair value on the acquisition date. Subsequent to the acquisition, the carrying value of the hybrid instrument is remeasured to fair value with changes recorded to stock-based compensation expense in proportion to the requisite service period vested.
The rights associated with the other noncontrolling interest shareholders are contingently redeemable at the option. As redemption of the rights is contingently redeemable at the option of the noncontrolling interest shareholders, the Company classifies the carrying amount of the redeemable noncontrolling interest in the mezzanine section on the consolidated balance sheet, which is presented above the equity section and below liabilities. The redeemable noncontrolling interest is initially measured at fair value and subsequently at the greater of the amount that would be paid if settlement occurred as of the balance sheet date based on the contractually defined redemption value and its carrying amount adjusted for net income (loss) attributable to the noncontrolling interest. Adjustments to the carrying value of the redeemable noncontrolling interest are recorded through retained earnings.
77
The Company completed the fair value allocation during 2022. The amortization period for the intangible assets acquired is nine years for technology, and twelve years for the trade name and customer relationships.
Optimal Industrial Automation and Technologies
On April 1, 2022, the Company completed a share purchase agreement to acquire 100% of the outstanding stock of Optimal Industrial Technologies Limited (“OIT”) and Optimal Industrial Automation Limited (“OIA”), collectively, “Optimal”. The purchase price for the outstanding shares of Optimal was approximately GBP 30.7 million (approximately $40.3 million) with the potential for additional contingent consideration of up to GBP 3.4 million (approximately $4.5 million). OIA and OIT, provide industrial automation solutions and Process Analytical Technology (“PAT”) software and services to Life Sciences, Pharmaceutical, Chemical, Fast-Moving Consumer Goods, Power Generation and Food & Beverage customers around the globe. The Optimal Industrial Companies are closely related as OIA is a PAT software company, which develops and sells “turn-key industrial solution”, and OIT is a services company, which provides PAT expertise related to the software in addition to 24/7 integration support. Optimal is located in Bristol, England, and was integrated into the BSI BioSpin Segment. The Company accounted for the purchase of Optimal under the acquisition method.
The preliminary fair value allocation for Optimal included contingent consideration in the amount of GBP 0.3 million (approximately $0.4 million), which represented the estimated fair value of future payments to the former shareholders of Optimal based on achieving revenue targets in 2022. The Company completed the fair value allocation during the measurement period. The amortization period for the intangible assets acquired is ten years for the technology, between twelve years and fourteen years for the customer relationships, and thirteen years for the trade names. The Company amortized backlog through the second quarter of 2023.
Inscopix, Inc.
On November 7, 2022, the Company completed a share purchase agreement to acquire 100% of the outstanding stock of Inscopix, Inc. (“Inscopix”). The purchase price for the outstanding shares of Inscopix was $101.5 million. Inscopix is a private company founded in 2011 from Stanford University research that led to the invention of the miniscope. The innovation at the core of the Inscopix brain-mapping platform is the integration and miniaturization of the benchtop fluorescence microscope into a 2-gram device that can be mounted onto the head of a freely behaving animal to observe its brain activity. These miniscopes are empowering advances in neuroscience research and preclinical research for the development of transformational therapeutics. Inscopix is located in Mountain View, California and will be integrated into the BSI Nano Segment. The Company accounted for the purchase of Inscopix under the acquisition method.
The Company completed the fair value allocation during the measurement period. The amortization period for the intangible assets acquired is ten years for the technology and customer relationships, and twelve years for the trade name.
In addition to the PreOmics, Optimal and Inscopix acquisitions, in 2022 the Company completed various other acquisitions accounted for under the acquisition method that complemented the Company’s existing product offerings. The following table reflects the consideration transferred and the respective reportable segment for these acquisitions (in millions):
Name of Acquisition |
|
Date Acquired |
|
Segment |
|
Total |
|
|
Cash |
|
||
Prolab Instruments GmbH |
|
January 17, 2022 |
|
BSI CALID |
|
$ |
5.7 |
|
|
$ |
5.5 |
|
PepSep Holding ApS |
|
February 1, 2022 |
|
BSI CALID |
|
|
4.1 |
|
|
|
2.8 |
|
IonSense, Inc |
|
April 5, 2022 |
|
BSI CALID |
|
|
9.5 |
|
|
|
8.1 |
|
Neurescence, Inc. |
|
November 30, 2022 |
|
BSI Nano |
|
|
7.5 |
|
|
|
7.1 |
|
|
|
|
|
|
|
$ |
26.8 |
|
|
$ |
23.5 |
|
In addition to the acquisitions noted above, in 2022 the Company also made several minority investments. The following table reflects the consideration transferred and the respective reportable segment for the acquisitions (in millions):
Name |
|
Acquisition / |
|
Financial |
|
Date Acquired |
|
Segment |
|
Total |
|
|
Cash |
|
||
PrognomiQ, Inc |
|
Investment |
|
Other long-term assets |
|
February 16, 2022 |
|
BSI CALID |
|
$ |
12.0 |
|
|
$ |
12.0 |
|
Tofwerk, AG |
|
Investment |
|
Other long-term assets |
|
April 28, 2022 |
|
BSI CALID |
|
|
18.6 |
|
|
|
18.6 |
|
Kiyatec, Inc |
|
Investment |
|
Other long-term assets |
|
November 23, 2022 |
|
BSI CALID |
|
|
9.3 |
|
|
|
9.3 |
|
Other Investments |
|
Investment |
|
Other long-term assets |
|
Various |
|
BSI CALID |
|
|
20.3 |
|
|
|
20.3 |
|
|
|
|
|
|
|
|
|
|
|
$ |
60.2 |
|
|
$ |
60.2 |
|
78
7. Allowance for Doubtful Accounts
Accounts receivable on the consolidated balance sheets are presented net of allowance for doubtful accounts. The following is a summary of the components for allowance for doubtful accounts (in millions):
|
|
|
|
|
Balance at December 31, 2020 |
|
$ |
3.0 |
|
Additions |
|
|
2.2 |
|
Deductions |
|
|
(1.0 |
) |
Balance at December 31, 2021 |
|
|
4.2 |
|
Additions |
|
|
1.9 |
|
Deductions |
|
|
(0.8 |
) |
Balance at December 31, 2022 |
|
|
5.3 |
|
Additions |
|
|
1.3 |
|
Deductions |
|
|
(2.0 |
) |
Balance at December 31, 2023 |
|
$ |
4.6 |
|
8. Inventories
Inventories consisted of the following (in millions):
|
|
2023 |
|
|
2022 |
|
||
Raw materials |
|
$ |
371.2 |
|
|
$ |
300.9 |
|
Work-in-process |
|
|
314.9 |
|
|
|
278.7 |
|
Finished goods |
|
|
183.9 |
|
|
|
128.2 |
|
Demonstration units |
|
|
98.3 |
|
|
|
92.3 |
|
Total Inventories |
|
$ |
968.3 |
|
|
$ |
800.1 |
|
Finished goods include in-transit systems that have been shipped to the Company’s customers but not yet installed and accepted by the customer. At December 31, 2023, and 2022, finished goods inventory-in-transit was $48.6 million and $41.1 million, respectively.
9. Property, Plant and Equipment, Net
The following is a summary of property, plant and equipment, net by major asset class (in millions):
|
|
2023 |
|
|
2022 |
|
||
Land |
|
$ |
38.9 |
|
|
$ |
36.6 |
|
Building and leasehold improvements |
|
|
498.1 |
|
|
|
434.5 |
|
Machinery, equipment, software and furniture and fixtures |
|
|
586.9 |
|
|
|
462.6 |
|
|
|
|
1,123.9 |
|
|
|
933.7 |
|
Less accumulated depreciation and amortization |
|
|
(524.2 |
) |
|
|
(446.7 |
) |
Property, plant and equipment, net |
|
$ |
599.7 |
|
|
$ |
487.0 |
|
Depreciation expense, which includes the amortization of leasehold improvements, for the years ended December 31, 2023, 2022 and 2021 was $67.8 million, $51.6 million and $51.8 million, respectively.
79
10. Goodwill and Intangible Assets
Goodwill
The following table sets forth the changes in the carrying amount of goodwill by segment (in millions):
|
|
BSI BioSpin |
|
|
BSI CALID |
|
|
BSI Nano |
|
|
BEST |
|
|
Total |
|
|||||
Balance at December 31, 2020 |
|
$ |
30.8 |
|
|
$ |
61.3 |
|
|
$ |
228.0 |
|
|
$ |
0.3 |
|
|
$ |
320.4 |
|
Current period additions/adjustments |
|
|
12.8 |
|
|
|
0.6 |
|
|
|
16.6 |
|
|
|
— |
|
|
|
30.0 |
|
Foreign currency impact |
|
|
(1.2 |
) |
|
|
(4.0 |
) |
|
|
(5.7 |
) |
|
|
— |
|
|
|
(10.9 |
) |
Balance at December 31, 2021 |
|
|
42.4 |
|
|
|
57.9 |
|
|
|
238.9 |
|
|
|
0.3 |
|
|
|
339.5 |
|
Current period additions/adjustments |
|
|
18.6 |
|
|
|
55.3 |
|
|
|
58.0 |
|
|
|
— |
|
|
|
131.9 |
|
Foreign currency impact |
|
|
(3.2 |
) |
|
|
(5.8 |
) |
|
|
(4.8 |
) |
|
|
— |
|
|
|
(13.8 |
) |
Balance at December 31, 2022 |
|
|
57.8 |
|
|
|
107.4 |
|
|
|
292.1 |
|
|
|
0.3 |
|
|
|
457.6 |
|
Current period additions/adjustments |
|
|
25.8 |
|
|
|
83.8 |
|
|
|
(0.5 |
) |
|
|
— |
|
|
|
109.1 |
|
Foreign currency impact |
|
|
2.9 |
|
|
|
10.3 |
|
|
|
2.7 |
|
|
|
— |
|
|
|
15.9 |
|
Balance at December 31, 2023 |
|
$ |
86.5 |
|
|
$ |
201.5 |
|
|
$ |
294.3 |
|
|
$ |
0.3 |
|
|
$ |
582.6 |
|
The Company has historically evaluated its goodwill for impairment annually as of December 31 or more frequently if impairment indicators arose in accordance with Accounting Standards Codification (“ASC”) Topic 350, “Intangibles - Goodwill and Other.” In the fourth quarter of 2022, the Company changed the date of its annual assessment of goodwill to October 1st for all reporting units. The change in testing date for goodwill is a change in accounting principle, which management believes is preferable as the new date of the assessment better aligns with the Company’s budgeting process and will create a more efficient and timely process surrounding the impairment tests. The change in the assessment date does not delay, accelerate or avoid a potential impairment charge. The Company has determined that it is impracticable to objectively determine projected cash flows and related valuation estimates that would have been used as of each October 1st of prior reporting periods without the use of hindsight. As such, the Company prospectively applied the change in annual goodwill impairment testing date from October 1, 2022. No impairment was recognized during the years ended December 31, 2023, 2022, or 2021.
Intangible Assets
The following is a summary of intangible assets (in millions):
|
|
2023 |
|
|
2022 |
|
||||||||||||||||||
|
|
Gross |
|
|
|
|
|
Net |
|
|
Gross |
|
|
|
|
|
Net |
|
||||||
|
|
Carrying |
|
|
Accumulated |
|
|
Carrying |
|
|
Carrying |
|
|
Accumulated |
|
|
Carrying |
|
||||||
|
|
Amount |
|
|
Amortization |
|
|
Amount |
|
|
Amount |
|
|
Amortization |
|
|
Amount |
|
||||||
Existing technology and related patents |
|
$ |
428.3 |
|
|
$ |
(250.4 |
) |
|
$ |
177.9 |
|
|
$ |
354.7 |
|
|
$ |
(219.0 |
) |
|
$ |
135.7 |
|
Customer relationships |
|
|
227.4 |
|
|
|
(93.5 |
) |
|
|
133.9 |
|
|
|
192.3 |
|
|
|
(72.1 |
) |
|
|
120.2 |
|
Trade names |
|
|
28.4 |
|
|
|
(10.1 |
) |
|
|
18.3 |
|
|
|
22.3 |
|
|
|
(7.4 |
) |
|
|
14.9 |
|
Other |
|
|
2.2 |
|
|
|
(1.8 |
) |
|
|
0.4 |
|
|
|
1.0 |
|
|
|
(0.9 |
) |
|
|
0.1 |
|
Intangible assets |
|
$ |
686.3 |
|
|
$ |
(355.8 |
) |
|
$ |
330.5 |
|
|
$ |
570.3 |
|
|
$ |
(299.4 |
) |
|
$ |
270.9 |
|
For the years ended December 31, 2023, 2022 and 2021, the Company recorded amortization expense of approximately $47.1 million, $37.1 million and $37.4 million, respectively, in the consolidated statements of income and comprehensive income. At December 31, 2023, the net carrying amount of trade names includes $3.0 million related to indefinite-lived intangible assets which are not amortized but are evaluated for impairment.
The estimated future amortization expense related to amortizable intangible assets is as follows (in millions):
2024 |
|
$ |
46.9 |
|
2025 |
|
|
46.4 |
|
2026 |
|
|
43.7 |
|
2027 |
|
|
36.6 |
|
2028 |
|
|
35.9 |
|
2029 |
|
|
31.6 |
|
Thereafter |
|
|
86.4 |
|
Total |
|
$ |
327.5 |
|
80
On a quarterly basis, the Company reviews its intangible assets to determine if there have been any triggering events that could indicate an impairment. In connection with certain restructuring programs initiated in 2023, the Company performed impairment assessments of its long-lived assets comparing the carrying values to the sum of their undiscounted future cash flows. Based on the results of these analyses, the Company determined there were no impairments to its long-lived assets, including intangible assets and goodwill. No other triggering events were identified for the years ended December 31, 2023, and 2022. See Note 21, Other Charges, Net for discussion related to impairment of other long-lived assets in connection with the BCA restructuring plan.
11. Other Current Liabilities
The following is a summary of other current liabilities (in millions):
|
|
2023 |
|
|
2022 |
|
||
Accrued compensation |
|
|
166.5 |
|
|
|
136.1 |
|
Accrued warranty |
|
|
30.0 |
|
|
|
24.7 |
|
Contingent consideration |
|
|
7.2 |
|
|
|
8.1 |
|
Income taxes payable |
|
|
138.7 |
|
|
|
85.5 |
|
Other taxes payable |
|
|
18.7 |
|
|
|
18.5 |
|
Derivative liabilities |
|
|
0.9 |
|
|
|
0.9 |
|
Operating lease liabilities |
|
|
23.3 |
|
|
|
16.4 |
|
Legal and professional fees |
|
|
17.1 |
|
|
|
14.2 |
|
Hybrid instruments liability |
|
|
14.1 |
|
|
|
— |
|
Restructuring liabilities |
|
|
12.5 |
|
|
|
0.6 |
|
Other accrued expenses |
|
|
49.2 |
|
|
|
42.0 |
|
Other current liabilities |
|
$ |
478.2 |
|
|
$ |
347.0 |
|
The following table sets forth the changes in accrued warranty (in millions):
|
|
|
|
|
Balance at December 31, 2020 |
|
$ |
20.3 |
|
Accruals for warranties issued during the year |
|
|
23.4 |
|
Settlements of warranty claims |
|
|
(19.0 |
) |
Foreign currency impact |
|
|
(0.9 |
) |
Balance at December 31, 2021 |
|
|
23.8 |
|
Accruals for warranties issued during the year |
|
|
12.9 |
|
Settlements of warranty claims |
|
|
(10.9 |
) |
Foreign currency impact |
|
|
(1.1 |
) |
Balance at December 31, 2022 |
|
|
24.7 |
|
Accruals for warranties issued during the year |
|
|
16.8 |
|
Settlements of warranty claims |
|
|
(12.4 |
) |
Foreign currency impact |
|
|
0.9 |
|
Balance at December 31, 2023 |
|
$ |
30.0 |
|
81
12. Debt
The Company’s debt obligations consist of the following (in millions):
|
|
2023 |
|
|
2022 |
|
||
EUR notes (in U.S. Dollars) under the 2021 Note Purchase Agreement due 2031 |
|
$ |
165.8 |
|
|
$ |
160.6 |
|
CHF notes (in U.S. Dollars) under the 2021 Note Purchase Agreement due 2031 |
|
|
356.9 |
|
|
|
325.1 |
|
CHF notes (in U.S. Dollars) under the 2019 Note Purchase Agreement due 2029 |
|
|
353.3 |
|
|
|
321.9 |
|
U.S. Dollar notes under the 2019 Term Loan Agreement annual payments of |
|
|
278.3 |
|
|
|
293.3 |
|
U.S. Dollar notes under the 2012 Note Purchase Agreement due 2024 |
|
|
100.0 |
|
|
|
100.0 |
|
Unamortized debt issuance costs |
|
|
(1.3 |
) |
|
|
(1.7 |
) |
Other loans |
|
|
7.6 |
|
|
|
5.9 |
|
Total notes and loans outstanding |
|
|
1,260.6 |
|
|
|
1,205.1 |
|
Finance lease obligations |
|
|
20.9 |
|
|
|
14.1 |
|
Total debt |
|
|
1,281.5 |
|
|
|
1,219.2 |
|
Current portion of long-term debt and finance lease obligations |
|
|
(121.2 |
) |
|
|
(18.7 |
) |
Total long-term debt, less current portion |
|
$ |
1,160.3 |
|
|
$ |
1,200.5 |
|
2021 Note Purchase Agreement
On December 7, 2021, the Company entered into a note purchase agreement, referred to as the 2021 Note Purchase Agreement, with a group of institutional accredited investors. Pursuant to the 2021 Note Purchase Agreement, the Company issued and sold CHF 300 million aggregate principal amount of 0.88% series A senior notes and EUR 150 million aggregate principal amount of 1.03% series B senior notes due December 8, 2031, referred to as the 2021 Senior Notes. The obligations under the Note Purchase Agreement are unsecured and are fully and unconditionally guaranteed by certain of the Company’s subsidiaries.
Interest on the 2021 Senior Notes is payable semi-annually on June 7 and December 7 of each year, commencing June 7, 2022. The Company may prepay some or all of the 2021 Senior Notes at any time in an amount not less than 10% of the aggregate principal amount of the 2021 Senior Notes then outstanding at a price equal to the sum of (a) the principal amount to be prepaid, plus accrued and unpaid interest, (b) any applicable “make-whole” amount, and (c) certain other fees and expenses. In the event of a change in control (as defined in the 2021 Note Purchase Agreement) of the Company, the Company may be required to prepay the 2021 Senior Notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest and certain other fees and expenses.
The 2021 Note Purchase Agreement contains customary affirmative and negative covenants, including, among others, restrictions on the Company’s ability to incur liens, transfer or sell equity or assets, engage in certain mergers and consolidations, enter into transactions with affiliates, and engage or permit any subsidiary to engage in certain lines of business. The 2021 Note Purchase Agreement also includes customary representations and warranties and events of default.
Additionally, so long as any 2021 Senior Notes are outstanding, the Company may not permit (i) its leverage ratio (as determined pursuant to the 2021 Note Purchase Agreement) as of the end of any fiscal quarter to exceed 3.50 to 1.00 unless a material acquisition causes an adjusted leverage ratio to apply pursuant to the 2021 Note Purchase Agreement, (ii) its interest coverage ratio (as determined pursuant to the 2021 Note Purchase Agreement) as of the end of any fiscal quarter for any period of four consecutive fiscal quarters to be less than 2.50 to 1.00, or (iii) priority Debt at any time to exceed 15% of consolidated total assets (as determined pursuant to the 2021 Note Purchase Agreement).
2019 Transactions
On December 11, 2019, the Company entered into (1) a revolving credit agreement to establish a new revolving credit facility in the aggregate principal amount of $600 million; (2) a term loan agreement to establish a new term loan facility in the aggregate principal amount of $300 million; and (3) a note purchase agreement to issue and sell CHF 297 million aggregate principal amount of 1.01% senior notes due December 11, 2029.
Each of the revolving credit agreements, term loan agreement and note purchase agreements are described below.
82
2019 Revolving Credit Agreement
On December 11, 2019, the Company entered into a new credit agreement, referred to as the 2019 Revolving Credit Agreement. The 2019 Revolving Credit Agreement provides for a five-year revolving credit facility in the U.S. Dollar equivalent amount of $600 million, comprised of sub-facilities for revolving loans, swing-line loans, letters of credit and foreign borrowings. (See 2024 Amended and Restated Credit Agreement below.) The 2019 Revolving Credit Agreement also provides for an uncommitted incremental facility whereby, under certain circumstances, the Company may, at its option, increase the amount of the revolving facility or incur term loans in an aggregate amount not to exceed $250 million. Loans under the 2019 Revolving Credit Agreement will be repayable in full at maturity and may also be prepaid at the Company’s option in whole or in part without premium or penalty. Amounts borrowed under the 2019 Revolving Credit Agreement may be repaid and reborrowed from time to time prior to the maturity date. The obligations under the 2019 Revolving Credit Agreement are unsecured and are fully and unconditionally guaranteed by the Company and certain of its subsidiaries.
Borrowings under the 2019 Revolving Credit Agreement bear interest at a rate equal to, at the Company’s option, (a) the London Interbank Offered Rate (LIBOR) applicable to the relevant currency, plus a margin ranging from 1.000% to 1.500%, based on the Company’s leverage ratio, or (b) the highest of (i) the federal funds effective rate plus 1⁄2 of 1%, (ii) the prime rate announced by Bank of America, N.A., and (iii) LIBOR, as adjusted, plus 1%, plus, in each case, a margin rate ranging from 0.100% to 0.500%, based on the Company’s leverage ratio. The Company has also agreed to pay a quarterly facility fee based on the aggregate unused amount available under the 2019 Revolving Credit Agreement ranging from 0.100% to 0.200%, based on the Company’s leverage ratio.
The 2019 Revolving Credit Agreement includes affirmative, negative and financial covenants and events of default customary for financings of this type. The negative covenants include, among others, restrictions on liens, indebtedness of the Company and its subsidiaries, asset and equity sales, dividends, and transactions with affiliates. The financial covenants include maximum leverage ratio and minimum interest coverage ratios of the Company, specifically, the Company’s leverage ratio cannot exceed 3.5 and the interest coverage ratio cannot be less than 2.5. The events of default include, among others, payment defaults, defaults in the performance of affirmative and negative covenants, the inaccuracy of representations and warranties, bankruptcy and insolvency related events, certain ERISA events, material judgments, and the occurrence of a change of control.
The following is a summary of the maximum commitments and the net amounts available to the Company under the 2019 Revolving Credit Agreement and other lines of credit with various financial institutions located primarily in Germany and Switzerland that are unsecured and typically due upon demand with interest payable monthly (dollars in millions):
|
|
Weighted |
|
|
Total Amount |
|
|
Outstanding |
|
|
Outstanding |
|
|
Total |
|
|||||
2019 Credit Agreement (a) |
|
|
0.15 |
% |
|
$ |
600.0 |
|
|
$ |
— |
|
|
$ |
0.4 |
|
|
$ |
599.6 |
|
Bank guarantees and working capital line |
|
varies |
|
|
|
153.6 |
|
|
|
— |
|
|
|
153.6 |
|
|
|
— |
|
|
Total revolving lines of credit |
|
|
|
|
$ |
753.6 |
|
|
$ |
— |
|
|
$ |
154.0 |
|
|
$ |
599.6 |
|
|
(a) On January 12, 2024, the Company borrowed CHF 230 million under the Company’s 2019 Revolving Credit Agreement.
2019 Term Loan Agreement
On December 11, 2019, the Company, together with certain of its subsidiaries, as borrowers, entered into a term loan agreement, referred to as Term Loan Agreement with a bank consortium. The Term Loan Agreement provides for a $300 million seven-year term loan facility subject to terms and conditions substantially consistent with those provisions contained in the 2019 Revolving Credit Agreement. Loans under the Term Loan Agreement will be repayable in full at maturity, subject to scheduled amortization beginning in 2022, and may also be prepaid at the Company’s option in whole or in part without premium or penalty. The obligations under the Term Loan Agreement are unsecured and are fully and unconditionally guaranteed by certain of the Company’s subsidiaries.
Amounts outstanding under the Term Loan Agreement bear interest at a rate equal to, at the Company’s option, (a) the U.S. Dollar London Interbank Offered Rate (USD LIBOR), plus a margin ranging from 1.000% to 1.500%, based on the Company’s leverage ratio, or (b) the highest of (i) the federal funds effective rate plus 1⁄2 of 1%, (ii) the prime rate announced by Bank of America, N.A., and (iii) USD LIBOR, as adjusted, plus 1%, plus a margin ranging from 0.100% to 0.500%, based on the Company’s leverage ratio.
The other terms of the Term Loan Agreement are substantially similar to the terms of the 2019 Revolving Credit Agreement, including representations and warranties, affirmative, negative and financial covenants, and events of default.
83
2019 Note Purchase Agreement
On December 11, 2019, the Company entered into a note purchase agreement, referred to as the 2019 Note Purchase Agreement, with a group of institutional accredited investors. Pursuant to the 2019 Note Purchase Agreement, the Company issued and sold CHF 297 million aggregate principal amount of 1.01% senior notes due December 11, 2029, referred to as the 2019 Senior Notes. The obligations under the Note Purchase Agreement are unsecured and are fully and unconditionally guaranteed by certain of the Company’s subsidiaries.
Interest on the 2019 Senior Notes is payable semi-annually on June 11 and December 11 of each year, commencing June 11, 2020. The 2019 Senior Notes are unsecured obligations of the Company and are fully and unconditionally guaranteed by certain of the Company’s subsidiaries. The Company may prepay some or all of the 2019 Senior Notes at any time in an amount not less than 10% of the aggregate principal amount of the 2019 Senior Notes then outstanding at a price equal to the sum of (a) the principal amount to be prepaid, plus accrued and unpaid interest, (b) any applicable “make-whole” amount, and (c) certain other fees and expenses. In the event of a change in control (as defined in the 2019 Note Purchase Agreement) of the Company, the Company may be required to prepay the 2019 Senior Notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest and certain other fees and expenses.
The 2019 Note Purchase Agreement contains customary affirmative and negative covenants, including, among others, restrictions on the Company’s ability to incur liens, transfer or sell equity or assets, engage in certain mergers and consolidations, enter into transactions with affiliates, and engage or permit any subsidiary to engage in certain lines of business. The 2019 Note Purchase Agreement also includes customary representations and warranties and events of default.
Additionally, so long as any 2019 Senior Notes are outstanding, the Company may not permit (i) its leverage ratio (as determined pursuant to the 2019 Note Purchase Agreement) as of the end of any fiscal quarter to exceed 3.50 to 1.00 unless a material acquisition causes an adjusted leverage ratio to apply pursuant to the 2019 Note Purchase Agreement, (ii) its interest coverage ratio (as determined pursuant to the 2019 Note Purchase Agreement) as of the end of any fiscal quarter for any period of four consecutive fiscal quarters to be less than 2.50 to 1.00, or (iii) priority Debt at any time to exceed 15% of consolidated total assets (as determined pursuant to the 2019 Note Purchase Agreement).
2012 Note Purchase Agreement
In January 2012, the Company entered into a note purchase agreement, referred to as the 2012 Note Purchase Agreement, with a group of accredited institutional investors. Pursuant to the 2012 Note Purchase Agreement, the Company issued and sold $240.0 million of senior notes, referred to as the 2012 Senior Notes, which consist of the following:
Principal amounts of Tranches A, B and C together with any accrued interest thereon were paid on their respective due dates in accordance with the 2012 Note Purchase Agreement.
Under the terms of the 2012 Note Purchase Agreement, interest is payable semi-annually on January 18 and July 18 of each year. The 2012 Senior Notes are unsecured obligations of the Company and are fully and unconditionally guaranteed by certain of the Company’s direct and indirect subsidiaries. The 2012 Senior Notes rank pari passu in right of repayment with the Company’s other senior unsecured indebtedness. The Company may prepay some or all of the 2012 Senior Notes at any time in an amount not less than 10% of the original aggregate principal amount of the 2012 Senior Notes to be prepaid, at a price equal to the sum of (a) 100% of the principal amount thereof, plus accrued and unpaid interest, and (b) the applicable make-whole amount, upon not less than 30 and no more than 60 days’ written notice to the holders of the 2012 Senior Notes. In the event of a change in control of the Company, as defined in the Note Purchase Agreement, the Company may be required to prepay the Notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest.
The 2012 Note Purchase Agreement contains affirmative covenants, including, without limitation, maintenance of corporate existence, compliance with laws, maintenance of insurance and properties, payment of taxes, addition of subsidiary guarantors and furnishing notices and other information. The 2012 Note Purchase Agreement also contains certain restrictive covenants that restrict the Company’s ability to, among other things, incur liens, transfer or sell assets, engage in certain mergers and consolidations and enter into transactions with affiliates. The 2012 Note Purchase Agreement also includes customary representations and warranties and events of default. In the case of an event of default arising from specified events of bankruptcy or insolvency, all outstanding 2012 Senior Notes will become due and payable immediately without further action or notice. In the case of payment events of defaults, any holder of 2012 Senior Notes affected thereby may declare all 2012 Senior Notes held by it due and payable immediately.
84
In the case of any other event of default, a majority of the holders of the 2012 Senior Notes may declare all the 2012 Senior Notes to be due and payable immediately. Pursuant to the 2012 Note Purchase Agreement, so long as any 2012 Senior Notes are outstanding, the Company will not permit (i) its leverage ratio, as determined pursuant to the 2012 Note Purchase Agreement, as of the end of any fiscal quarter to exceed 3.50 to 1.00, (ii) its interest coverage ratio as determined pursuant to the 2012 Note Purchase Agreement as of the end of any fiscal quarter for any period of four consecutive fiscal quarters to be less than 2.50 to 1 or (iii) priority debt at any time to exceed 25% of consolidated net worth, as determined pursuant to the 2012 Note Purchase Agreement.
The existing $105 million 4.31% Series 2012A Senior Notes, Tranche C, was paid in January 2022, and the existing $100 million 4.46% Series 2012A Senior Notes, Tranche D, was paid on January 18, 2024.
Annual maturities of notes and loans outstanding are as follows (in millions):
2024 |
|
$ |
116.4 |
|
2025 |
|
|
16.9 |
|
2026 |
|
|
249.5 |
|
2027 |
|
|
1.6 |
|
2028 |
|
|
0.9 |
|
Thereafter |
|
|
876.6 |
|
Total |
|
$ |
1,261.9 |
|
As of December 31, 2023, the Company has several cross-currency and interest rate swap agreements with a notional value of $139.1 million of U.S. to Swiss Franc and a notional value of $239.1 million of U.S. to Euro to hedge the variability in the movement of foreign currency exchange rates on portions of our Euro and Swiss Franc denominated net asset investments. These agreements qualify for hedge accounting and accordingly the changes in fair value of the derivative are recorded in other comprehensive income and remain in accumulated comprehensive income (loss) attributable to Bruker Corporation in shareholders’ equity until the sale or substantial liquidation of the foreign operation. The difference between the interest rate received and paid under the interest rate and cross-currency swap agreements is recorded in interest and other income (expense) in the consolidated statements of income and comprehensive income. As a result of entering into these agreements, the Company lowered net interest expense by $18.3 million and $8.6 million during 2023 and 2022, respectively. The gains related to hedges of net asset investments in international operations that were recorded within the cumulative translation adjustment section of other comprehensive income were $76.8 million, $58.0 million and $36.4 million for the years ended December 31, 2023, 2022, and 2021 respectively. The Company presents the cross-currency swap periodic settlements in investing activities and the interest rate swap periodic settlements in operating activities in the statement of cash flow.
In June 2022, the Company entered into the First Amendment to the 2019 Credit Agreement to modify certain contract definitions within the agreement. Primarily, the current London Interbank Offered Rate (“LIBOR”) rates were changed to new alternative base rates for the respective currencies. As part of the change any related items, such as fall back rates and day conventions were also changed. No other material terms were modified with this agreement. In September 2022, the Company entered into the Second Amendment to the 2019 Term Loan Agreement and the Second Amendment to the 2019 Credit Agreement (collectively, the "Amendments"), to modify certain aspects of the 2019 Term Loan Agreement and 2019 Credit Agreement, respectively. The Amendments modify the reference rate thereunder from LIBOR to Secured Overnight Financing Rate (“SOFR”). There were no other changes to the 2019 Term Loan Agreement or 2019 Credit Agreement as a result of the Amendments. During 2022, the Company adopted the practical expedient for Reference Rate Reform related to its debt arrangements and as such, these amendments are treated as a continuation of the existing debt agreement and no gain or loss on the modification was recorded. The Company did not record any gains or losses on the conversion of the reference rate for Borrowings under the Term Loan Agreement from LIBOR to SOFR.
Interest expense for the years ended December 31, 2023, 2022 and 2021 was $16.4 million, $16.1 million and $14.3 million, respectively.
As of December 31, 2023, the Company was in compliance with the covenants of all debt agreements.
85
2024 Notes Purchase Agreement
On February 1, 2024, and February 8, 2024, the Company entered into two note purchase agreements, referred to as the 2024 Note Purchase Agreements, with a group of accredited institutional investors. Pursuant to the 2024 Note Purchase Agreements, the Company will issue and sell CHF 431.0 million (approximately $513 million) of senior notes, referred to as the 2024 Senior Notes, which consist of the following:
Under the terms of the 2024 Note Purchase Agreements, interest on the Notes is payable semi-annually on April 15 and October 15 of each year, commencing April 15 or October 15, 2024. The Notes are unsecured obligations of the Company and are fully and unconditionally guaranteed by certain of the Company’s subsidiaries. The Company may prepay some or all of the Notes at any time in an amount not less than 10% of the aggregate principal amount of the Notes then outstanding at a price equal to the sum of (a) the principal amount to be prepaid, plus accrued and unpaid interest, (b) any applicable “make-whole” amount, and (c) certain other fees and expenses. In the event of a change in control (as defined in the Note Purchase Agreement) of the Company, the Company may be required to prepay the Notes at a price equal to 100% of the principal amount thereof, plus accrued and unpaid interest and certain other fees and expenses.
The Note Purchase Agreements contain customary affirmative and negative covenants, including, among others, a most favored lender covenant, restrictions on the Company’s ability to incur liens, transfer or sell assets, engage in certain mergers and consolidations, enter into transactions with affiliates, and engage or permit any subsidiary to engage in certain lines of business. The Note Purchase Agreement also includes customary representations and warranties and events of default.
Additionally, commencing with the first full fiscal quarter ending following the closing of the offering, so long as any Notes are outstanding, the Company may not permit (i) its leverage ratio (as determined pursuant to the Note Purchase Agreement) as of the end of any fiscal quarter to exceed 3.50 to 1.00 unless a material acquisition causes an adjusted leverage ratio to apply pursuant to the Note Purchase Agreement, (ii) its interest coverage ratio (as determined pursuant to the Note Purchase Agreement) as of the end of any fiscal quarter for any period of four consecutive fiscal quarters to be less than 2.50 to 1.00, or (iii) Priority Debt at any time to exceed 15% of consolidated total assets (as determined pursuant to the Note Purchase Agreement). Commencing with the first full fiscal quarter ending following the closing of the offering, if the Leverage Ratio exceeds 3.50 to 1.00 an incremental leverage fee shall be due on the Notes in an aggregate amount equal to 0.50% of the aggregate outstanding principal amount of the Notes per annum, which shall be due and payable on the Notes.
The issuance and sale of the Notes is subject to satisfaction of a variety of customary closing conditions and is expected to occur on or about April 15, 2024.
2024 Amended and Restated Credit Agreement
On January 18, 2024, the Company, together with certain of its subsidiaries, as borrowers and guarantors, entered into an Amended and Restated Credit Agreement (the “Amended and Restated Credit Agreement”) with Deutsche Bank Securities Inc., JPMorgan Chase Bank, N.A., TD Bank, N.A., and Wells Fargo Bank, National Association, as Co-Syndication Agents, BofA Securities, Inc., Deutsche Bank Securities Inc., JPMorgan Chase Bank, N.A., TD Bank, N.A. and Wells Fargo Securities, LLC, as Joint Bookrunners and Joint Lead Arrangers, Citizens Bank, N.A., Credit Suisse (Switzerland) Ltd., and U.S. Bank, N.A., as Co-Documentation Agents, ING Bank B.V. and PNC Bank, N.A., as Managing Agents, Bank of America, N.A., as Administrative Agent, Swing Line Lender and Issuing Bank, and the several banks or other financial institutions or entities from time to time party thereto as lenders. The Amended and Restated Credit Agreement amends and restates the 2019 Revolving Credit Agreement entered into by the Company and certain of its subsidiaries on December 11, 2019 with the other parties thereto.
The Amended and Restated Credit Agreement increases the aggregate principal amount from $600 million to $900 million and extends the maturity date to January 18, 2029, as may be further extended by the Company for the periods and on the terms set forth in the Amended and Restated Credit Agreement. In addition, the Amended and Restated Credit Agreement increases the uncommitted incremental facility whereby, under certain circumstances, the Company may, at its option, increase the amount of the revolving facility or incur term loans in an aggregate amount not to exceed $400 million.
86
Amounts outstanding under the Amended and Restated Credit Agreement bear interest at a rate equal to, at the Company’s option, (a) the Secured Overnight Financing Rate (“SOFR”) applicable to the relevant currency, plus a margin ranging from 1.000% to 1.500%, based on the Company’s leverage ratio, or (b) the highest of (i) the federal funds effective rate plus 0.5%, (ii) the prime rate announced by Bank of America, N.A., and (iii) SOFR, as adjusted, plus 1.00%, plus a margin rate ranging from 0.000% to 0.500%, based on the Company’s leverage ratio. The Company has also agreed to pay a quarterly facility fee based on the aggregate amount available under the 2019 Revolving Credit Agreement ranging from 0.100% to 0.200%, based on the Company’s leverage ratio.
The Amended and Restated Credit Agreement includes affirmative, negative and financial covenants and events of default customary for financings of this type. The negative covenants include, among others, restrictions on liens, indebtedness of the Company and its subsidiaries, asset sales, dividends, and transactions with affiliates. The financial covenants include maximum leverage ratio and minimum interest coverage ratios of the Company. The events of default include, among others, payment defaults, defaults in the performance of affirmative and negative covenants, the inaccuracy of representations and warranties, bankruptcy and insolvency related events, certain ERISA events, material judgments, and the occurrence of a change of control.
13. Fair Value of Financial Instruments
The Company measures the following financial assets and liabilities at fair value on a recurring basis. The following tables set forth the Company’s financial instruments and presents them within the fair value hierarchy using the lowest level of input that is significant to the fair value measurement (in millions):
December 31, 2023 |
|
Total |
|
|
Quoted Prices |
|
|
Significant |
|
|
Significant |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Time deposits and money market funds |
|
$ |
226.9 |
|
|
$ |
— |
|
|
$ |
226.9 |
|
|
$ |
— |
|
Interest rate and cross-currency swap agreements |
|
|
20.3 |
|
|
|
— |
|
|
|
20.3 |
|
|
|
— |
|
Forward currency contracts |
|
|
1.3 |
|
|
|
— |
|
|
|
1.3 |
|
|
|
— |
|
Embedded derivatives in purchase and delivery contracts |
|
|
1.2 |
|
|
|
— |
|
|
|
1.2 |
|
|
|
— |
|
Fixed price commodity contracts |
|
|
0.3 |
|
|
|
— |
|
|
|
0.3 |
|
|
|
— |
|
Debt securities available for sale |
|
|
1.2 |
|
|
|
— |
|
|
|
— |
|
|
|
1.2 |
|
Total assets recorded at fair value |
|
$ |
251.2 |
|
|
$ |
— |
|
|
$ |
250.0 |
|
|
$ |
1.2 |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Contingent consideration |
|
$ |
12.3 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
12.3 |
|
Hybrid instruments liabilities |
|
|
70.5 |
|
|
|
— |
|
|
|
— |
|
|
|
70.5 |
|
Liability awards |
|
|
0.7 |
|
|
|
— |
|
|
|
— |
|
|
|
0.7 |
|
Interest rate and cross-currency swap agreements |
|
|
26.8 |
|
|
|
— |
|
|
|
26.8 |
|
|
|
— |
|
Forward currency contracts |
|
|
0.6 |
|
|
|
— |
|
|
|
0.6 |
|
|
|
— |
|
Total liabilities recorded at fair value |
|
$ |
110.9 |
|
|
$ |
— |
|
|
$ |
27.4 |
|
|
$ |
83.5 |
|
87
December 31, 2022 |
|
Total |
|
|
Quoted Prices |
|
|
Significant |
|
|
Significant |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Time deposits and money market funds |
|
$ |
198.7 |
|
|
$ |
34.0 |
|
|
$ |
164.7 |
|
|
$ |
— |
|
Interest rate and cross-currency swap agreements |
|
|
37.8 |
|
|
|
— |
|
|
|
37.8 |
|
|
|
— |
|
Forward currency contracts |
|
|
0.6 |
|
|
|
— |
|
|
|
0.6 |
|
|
|
— |
|
Embedded derivatives in purchase and delivery contracts |
|
|
0.1 |
|
|
|
— |
|
|
|
0.1 |
|
|
|
— |
|
Fixed price commodity contracts |
|
|
0.6 |
|
|
|
— |
|
|
|
0.6 |
|
|
|
— |
|
Debt securities available for sale |
|
|
10.5 |
|
|
|
— |
|
|
|
— |
|
|
|
10.5 |
|
Total assets recorded at fair value |
|
$ |
248.3 |
|
|
$ |
34.0 |
|
|
$ |
203.8 |
|
|
$ |
10.5 |
|
Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Contingent consideration |
|
$ |
9.6 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
9.6 |
|
Hybrid instruments liabilities |
|
|
34.2 |
|
|
|
— |
|
|
|
— |
|
|
|
34.2 |
|
Liability awards |
|
|
1.1 |
|
|
|
— |
|
|
|
— |
|
|
|
1.1 |
|
Interest rate and cross-currency swap agreements |
|
|
12.2 |
|
|
|
— |
|
|
|
12.2 |
|
|
|
— |
|
Forward currency contracts |
|
|
0.3 |
|
|
|
— |
|
|
|
0.3 |
|
|
|
— |
|
Total liabilities recorded at fair value |
|
$ |
57.4 |
|
|
$ |
— |
|
|
$ |
12.5 |
|
|
$ |
44.9 |
|
Derivative financial instruments are classified within level 2 because there is not an active market for each derivative contract. However, the inputs used to calculate the value of the instruments are obtained from active markets.
The Company measures certain assets and liabilities at fair value with changes in fair value recognized in earnings. Fair value treatment may be elected either upon initial recognition of an eligible asset or liability or, for an existing asset or liability, if an event triggers a new basis of accounting. The Company did not elect to remeasure any of its existing financial assets or liabilities and did not elect the fair value option for any financial assets or liabilities which originated during the year ended December 31, 2023 or 2022.
The fair value of the Company's long-term fixed interest rate debt was $883.3 million and $767.8 million as of December 31, 2023, and December 31, 2022, respectively. The fair value was based on market and observable sources with similar maturity dates and classified as Level 2 within the fair value hierarchy. The remaining long-term debt has variable interest rates and the carrying value approximates fair value accordingly.
Debt securities consist of investments in redeemable preferred stock. Debt securities are classified as either current or long-term investments based on their contractual maturities unless the Company intends to sell an investment within the next twelve months, in which case it is classified as current on the consolidated balance sheets. Debt securities are classified as available for sale and are carried at fair value.
Contingent consideration recorded within other current and other long-term liabilities represents the estimated fair value of future payments to the former shareholders as part of certain acquisitions. The contingent consideration is primarily based on the applicable acquired company achieving annual revenue and gross margin targets in certain years as specified in the relevant purchase and sale agreement. The Company initially values the contingent consideration on the acquisition date by using a Monte Carlo simulation or an income approach method. The Monte Carlo method models future revenue and costs of goods sold projections and discounts the average results to present value. The income approach method involves calculating the earnout payment based on the forecasted cash flows, adjusting the future earnout payment for the risk of reaching the projected financials, and then discounting the future payments to present value by the counterparty risk. The counterparty risk considers the risk of the buyer having the cash to make the earnout payments and is commensurate with a cost of debt over an appropriate term. Changes in fair value subsequent to acquisition are recognized in “Acquisition-related expenses, net” included in Other Charges, net, in the Consolidated Statements of Income and Comprehensive Income.
88
The following table sets forth the changes in contingent consideration liabilities (in millions):
Balance at December 31, 2021 |
|
$ |
6.6 |
|
Current period additions |
|
|
3.6 |
|
Current period adjustments |
|
|
5.6 |
|
Current period settlements |
|
|
(5.9 |
) |
Foreign currency effect |
|
|
(0.3 |
) |
Balance at December 31, 2022 |
|
|
9.6 |
|
Current period additions |
|
|
2.8 |
|
Current period adjustments |
|
|
7.6 |
|
Current period settlements |
|
|
(8.1 |
) |
Foreign currency effect |
|
|
0.4 |
|
Balance at December 31, 2023 |
|
$ |
12.3 |
|
As part of the 2018 Mestrelab Research, S.L. (“Mestrelab”), 2022 PreOmics, 2023 Biognosys, Zontal and MIRO acquisitions and certain other majority owned acquisitions, the Company entered into agreements with the noncontrolling interest holders that provide the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining ownerships for cash at contractually defined redemption values. These rights (embedded derivatives) can be accelerated, at discounted redemption values, upon certain events related to post combination employment services. As the options are tied to continued employment, the Company classified the hybrid instruments (noncontrolling interests with an embedded derivatives) as liabilities on the consolidated balance sheet. Subsequent to the acquisition dates, the carrying value of each hybrid instrument is remeasured to fair value with changes recorded to stock-based compensation expense in proportion to the respective requisite service period vested. The Company classified the hybrid instruments as Level 3 in the fair value hierarchy.
The following table sets forth the changes in hybrid instruments liability (in millions):
Balance at December 31, 2021 |
|
$ |
15.6 |
|
Current period additions |
|
|
20.9 |
|
Current period adjustments |
|
|
11.6 |
|
Current period settlements |
|
|
(11.6 |
) |
Foreign currency effect |
|
|
(2.3 |
) |
Balance at December 31, 2022 |
|
|
34.2 |
|
Current period additions |
|
|
36.1 |
|
Current period adjustments |
|
|
(2.1 |
) |
Foreign currency effect |
|
|
2.3 |
|
Balance at December 31, 2023 |
|
$ |
70.5 |
|
14. Derivative Instruments and Hedging Activities
Interest Rate Risk
The Company’s exposure to interest rate risk relates primarily to outstanding variable rate debt and adverse movements in the related market rates. Typically, the most significant component of the Company’s interest rate risk relates to amounts outstanding under the 2019 Revolving Credit Agreement, the 2019 Term Loan and the 2021 Senior Notes.
Commodity Price Risk Management
The Company has arrangements with certain customers under which it has a firm commitment to deliver copper-based superconductors at a fixed price. In order to minimize the volatility that fluctuations in the price of copper have on the Company’s sales of these commodities, the Company enters into commodity hedge contracts. As commodity contracts settle, gains (losses) as a result of changes in fair values are adjusted to the contracts with the customers through revenues.
Foreign Exchange Rate Risk Management
The Company generates a substantial portion of its revenues and expenses in international markets, principally Germany and other countries in the European Union and Switzerland, which subjects its operations to the exposure of exchange rate fluctuations. The impact of currency exchange rate movement can be positive or negative in any period. The Company periodically enters into foreign currency contracts in order to minimize the volatility that fluctuations in currency translation have on its monetary transactions.
89
Under these arrangements, the Company typically agrees to purchase a fixed amount of a foreign currency in exchange for a fixed amount of U.S. Dollars or other currencies on specified dates with maturities of less than twelve months, with some agreements extending to longer periods. These transactions do not qualify for hedge accounting and, accordingly, the instrument is recorded at fair value with the corresponding gains and losses recorded in the consolidated statements of income and comprehensive income.
In addition, the Company periodically enters into purchase and sales contracts denominated in currencies other than the functional currency of the Company's subsidiaries in the transaction. The Company accounts for these transactions separately valuing the “embedded derivative” component of these contracts. The contracts, denominated in currencies other than the functional currency of the transacting parties, amounted to $25.7 million and $15.3 million for the purchase of products as of December 31, 2023, and 2022, respectively. There were no contracts denominated in currencies other than the functional currency of the transacting parties for the delivery of products as of December 31, 2023, and 2022, respectively. The Company records the changes in the fair value of these embedded derivatives in interest and other income (expense), net in the consolidated statements of income and comprehensive income.
The Company had the following notional amounts outstanding under foreign exchange contracts, cross-currency interest rate swap agreements and long-term debt designated as net investment hedges and the respective fair value of the instruments recorded in the consolidated balance sheets as follows (in millions):
|
|
December 31, 2023 |
|
|
December 31, 2022 |
|
||||||||||
|
|
Notional |
|
|
Fair Value |
|
|
Notional |
|
|
Fair Value |
|
||||
Derivatives designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest rate cross-currency swap agreements |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other current assets |
|
|
|
|
$ |
12.0 |
|
|
|
|
|
$ |
14.8 |
|
||
Other assets |
|
|
|
|
|
8.3 |
|
|
|
|
|
|
23.0 |
|
||
Other long-term liabilities |
|
|
|
|
|
(26.8 |
) |
|
|
|
|
|
(12.2 |
) |
||
|
|
$ |
378.3 |
|
|
$ |
(6.5 |
) |
|
$ |
393.3 |
|
|
$ |
25.6 |
|
Long-term debt |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Long-term debt |
|
|
876.0 |
|
|
|
(85.3 |
) |
|
|
807.6 |
|
|
|
(17.0 |
) |
Total derivatives designated as hedging instruments |
|
$ |
1,254.3 |
|
|
$ |
(91.8 |
) |
|
$ |
1,200.9 |
|
|
$ |
8.6 |
|
Derivatives not designated as hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Forward currency contracts |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other current assets |
|
$ |
177.8 |
|
|
$ |
1.3 |
|
|
$ |
89.8 |
|
|
$ |
0.6 |
|
Other current liabilities |
|
|
311.7 |
|
|
|
(0.6 |
) |
|
|
97.4 |
|
|
|
(0.3 |
) |
Embedded derivatives in purchase and delivery contracts |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other current assets |
|
|
25.6 |
|
|
|
1.2 |
|
|
|
15.3 |
|
|
|
0.1 |
|
Other current liabilities |
|
|
0.1 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Fixed price commodity contracts |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other current assets |
|
|
8.4 |
|
|
|
0.3 |
|
|
|
8.9 |
|
|
|
0.6 |
|
Total derivatives not designated as hedging instruments |
|
$ |
523.6 |
|
|
$ |
2.2 |
|
|
$ |
211.4 |
|
|
$ |
1.0 |
|
Total derivatives |
|
$ |
1,777.9 |
|
|
$ |
(89.6 |
) |
|
$ |
1,412.3 |
|
|
$ |
9.6 |
|
90
The following is a summary of the gain (loss) included in the consolidated statements of income and comprehensive income related to the derivative instruments described above (in millions):
|
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
Financial Statement Classification |
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Derivatives not designated as |
|
|
|
|
|
|
|
|
|
|
|
|||
Forward currency contracts |
|
Interest and other income (expense), net |
|
$ |
12.4 |
|
|
$ |
(3.2 |
) |
|
$ |
(5.5 |
) |
Embedded derivatives in purchase and |
|
Interest and other income (expense), net |
|
|
1.1 |
|
|
|
(0.1 |
) |
|
|
0.1 |
|
|
|
|
|
|
13.5 |
|
|
|
(3.3 |
) |
|
|
(5.4 |
) |
Derivatives designated as cash flow |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate cross-currency swap agreements |
|
Interest and other income (expense), net |
|
|
10.4 |
|
|
|
0.1 |
|
|
|
(4.7 |
) |
Derivatives designated as net |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate cross-currency swap agreements |
|
Interest and other income (expense), net |
|
|
7.9 |
|
|
|
8.5 |
|
|
|
10.2 |
|
Total |
|
|
|
$ |
31.8 |
|
|
$ |
5.3 |
|
|
$ |
0.1 |
|
|
|
|
|
Year Ended December 31, |
|
|||||||||
|
|
Financial Statement Classification |
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Derivatives designated as cash flow hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate cross-currency swap agreements |
|
Accumulated other comprehensive income, net of tax |
|
$ |
(5.5 |
) |
|
$ |
21.2 |
|
|
$ |
15.0 |
|
Derivatives designated as net investment hedging instruments |
|
|
|
|
|
|
|
|
|
|
|
|||
Interest rate cross-currency swap agreements |
|
Accumulated other comprehensive income, net of tax |
|
|
(19.5 |
) |
|
|
9.1 |
|
|
|
25.6 |
|
Long-term debt |
|
Accumulated other comprehensive income, net of tax |
|
|
(52.1 |
) |
|
|
13.9 |
|
|
|
10.8 |
|
|
|
|
|
|
(71.6 |
) |
|
|
23.0 |
|
|
|
36.4 |
|
Total |
|
|
|
$ |
(77.1 |
) |
|
$ |
44.2 |
|
|
$ |
51.4 |
|
91
15. Income Taxes
The domestic and foreign components of income before income taxes for the years ended December 31 (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Domestic |
|
$ |
0.7 |
|
|
$ |
(13.3 |
) |
|
$ |
(15.4 |
) |
Foreign |
|
|
543.5 |
|
|
|
427.2 |
|
|
|
409.0 |
|
Total income before provision for income taxes |
|
$ |
544.2 |
|
|
$ |
413.9 |
|
|
$ |
393.6 |
|
The components of the income tax provision for the years ended December 31 (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Current income tax expense (benefit): |
|
|
|
|
|
|
|
|
|
|||
Federal |
|
$ |
2.3 |
|
|
$ |
(10.4 |
) |
|
$ |
1.0 |
|
State |
|
|
2.4 |
|
|
|
2.5 |
|
|
|
1.2 |
|
Foreign |
|
|
138.7 |
|
|
|
135.3 |
|
|
|
118.0 |
|
Total current income tax expense |
|
|
143.4 |
|
|
|
127.4 |
|
|
|
120.2 |
|
Deferred income tax expense (benefit): |
|
|
|
|
|
|
|
|
|
|||
Federal |
|
|
(18.1 |
) |
|
|
(3.1 |
) |
|
|
(7.8 |
) |
State |
|
|
(4.8 |
) |
|
|
(1.7 |
) |
|
|
(2.5 |
) |
Foreign |
|
|
(2.8 |
) |
|
|
(6.2 |
) |
|
|
3.1 |
|
Total deferred income tax benefit |
|
|
(25.7 |
) |
|
|
(11.0 |
) |
|
|
(7.2 |
) |
Income tax provision |
|
$ |
117.7 |
|
|
$ |
116.4 |
|
|
$ |
113.0 |
|
The income tax provision differs from the tax provision computed at the U.S. federal statutory rate due to the following significant components:
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Statutory tax rate |
|
|
21.0 |
% |
|
|
21.0 |
% |
|
|
21.0 |
% |
Foreign tax rate differential |
|
|
3.5 |
% |
|
|
5.2 |
% |
|
|
5.2 |
% |
Permanent differences |
|
|
2.8 |
% |
|
|
1.1 |
% |
|
|
1.2 |
% |
U.S. tax on foreign earnings |
|
|
1.0 |
% |
|
|
(0.1 |
)% |
|
|
1.6 |
% |
Stock compensation |
|
|
(0.3 |
)% |
|
|
(0.6 |
)% |
|
|
(2.5 |
)% |
Tax contingencies |
|
|
— |
|
|
|
3.3 |
% |
|
|
2.6 |
% |
Change in tax rates |
|
|
0.1 |
% |
|
|
0.1 |
% |
|
|
(0.1 |
)% |
Repatriation of foreign earnings |
|
|
0.3 |
% |
|
|
0.2 |
% |
|
|
(0.4 |
)% |
State income taxes, net of federal benefits |
|
|
(0.6 |
)% |
|
|
0.2 |
% |
|
|
(0.4 |
)% |
Research and development credits |
|
|
(2.0 |
)% |
|
|
(2.1 |
)% |
|
|
(0.9 |
)% |
Tax impact on bargain purchase gain |
|
|
(5.6 |
)% |
|
|
|
|
|
|
||
Other |
|
|
0.1 |
% |
|
|
(0.1 |
)% |
|
|
1.2 |
% |
Change in valuation allowance for unbenefited losses |
|
|
1.3 |
% |
|
|
(0.1 |
)% |
|
|
0.2 |
% |
Effective tax rate |
|
|
21.6 |
% |
|
|
28.1 |
% |
|
|
28.7 |
% |
92
The tax effect of temporary items that give rise to significant portions of the deferred tax assets and liabilities are as follows (in millions):
|
|
2023 |
|
|
2022 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Accrued expenses |
|
$ |
5.8 |
|
|
$ |
4.4 |
|
Compensation |
|
|
20.5 |
|
|
|
15.3 |
|
Section 174 capitalization |
|
|
50.5 |
|
|
|
15.8 |
|
Disallowed interest carryforwards |
|
|
20.7 |
|
|
|
9.1 |
|
Net operating loss carryforwards |
|
|
180.5 |
|
|
|
21.9 |
|
Foreign tax and other tax credit carryforwards |
|
|
16.7 |
|
|
|
20.3 |
|
Unrealized currency gain/loss |
|
|
18.6 |
|
|
|
13.1 |
|
Inventory |
|
|
5.5 |
|
|
|
1.9 |
|
Hedge unrealized FX gain/loss |
|
|
22.5 |
|
|
|
— |
|
Lease liabilities |
|
|
22.8 |
|
|
|
10.5 |
|
Other |
|
|
7.5 |
|
|
|
4.8 |
|
Gross deferred tax assets |
|
|
371.6 |
|
|
|
117.1 |
|
Less valuation allowance |
|
|
(13.8 |
) |
|
|
(6.6 |
) |
Total deferred tax assets |
|
|
357.8 |
|
|
|
110.5 |
|
Deferred tax liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
|
6.6 |
|
|
|
7.7 |
|
Hedge unrealized FX gain/loss |
|
|
— |
|
|
|
1.3 |
|
Deferred revenue |
|
|
2.6 |
|
|
|
0.9 |
|
Fixed assets |
|
|
17.2 |
|
|
|
10.1 |
|
Foreign patent reserves |
|
|
1.8 |
|
|
|
2.9 |
|
Intangibles |
|
|
64.1 |
|
|
|
53.8 |
|
Accrued expenses |
|
|
3.1 |
|
|
|
1.3 |
|
Accrued withholding tax |
|
|
10.2 |
|
|
|
7.9 |
|
Right-of-use asset |
|
|
21.5 |
|
|
|
10.5 |
|
Other |
|
|
1.1 |
|
|
|
— |
|
Total deferred tax liabilities |
|
|
128.2 |
|
|
|
96.4 |
|
Net deferred tax assets |
|
$ |
229.6 |
|
|
$ |
14.1 |
|
The Company uses the liability method to account for income taxes. Under this method, deferred income taxes are recognized for the future tax consequences of differences between the tax and financial accounting bases of assets and liabilities at each reporting period. Deferred income taxes are based on enacted tax laws and statutory tax rates applicable to the period in which these differences are expected to affect taxable income. A valuation allowance is established when necessary to reduce deferred tax assets to the expected realizable amounts.
The Company can only recognize a deferred tax asset to the extent this it is “more likely than not” that these assets will be realized. Judgments around realizability depend on the availability and weight of both positive and negative evidence. Changes in the valuation allowance for deferred tax assets were as follows (in millions):
Balance at December 31, 2020 |
|
$ |
6.6 |
|
Increases recorded as an expense to income tax provision |
|
|
0.5 |
|
Balance at December 31, 2021 |
|
|
7.1 |
|
Decreases recorded for valuation allowance release |
|
|
(0.5 |
) |
Balance at December 31, 2022 |
|
|
6.6 |
|
Increases recorded as an expense to income tax provision |
|
|
7.2 |
|
Balance at December 31, 2023 |
|
$ |
13.8 |
|
93
As of December 31, 2023, the Company has approximately $588.9 million of U.S. federal net operating loss carryforwards, of which $36.4 million begin to expire at various dates beginning in 2031 and the remainder $552.5 million will be carried forward indefinitely. The Tax Cuts and Jobs Act (TCJA) enacted on December 22, 2017, limits a taxpayer’s ability to utilize NOL deduction in a year to 80% taxable income for federal NOL arising in tax years beginning after 2017. The Company has approximately $676.6 million of state net operating loss carryforwards available to reduce state taxable income that are expected to expire at various times beginning in 2024. The Company also has approximately $97.9 million of German Trade Tax and Corporate Income Tax net operating losses that are carried forward indefinitely. Additionally, the Company has $28.7 million of other foreign net operating losses that are expected to expire at various times in the future. The Company has U.S. federal foreign tax credit carried forwards in the amount of $3.3 million. The Company also has U.S. federal and state research and development tax credit of $1.2 million and $10.3 million respectively. Utilization of these credits and net operating losses may be subject to annual limitations due to the ownership percentage change limitations provided by the Code Section 382 and similar state provisions. In the event of a deemed change in control under Code Section 382, an annual limitation on the utilization of net operating losses and credits may result in the expiration of all or a portion of the net operating loss and credit carryforwards. The Company is in the process of finalizing the impact of Section 382 related to the PhenomeX acquisition but believes the provisions will limit the availability of losses to offset future income. Additionally, the Company has $91.4 million of gross interest expense carryforward as provided by Code Section 163(j) that can be carried forward indefinitely.
At December 31, 2023 the Company recorded state income and foreign withholding taxes on the cash and liquid assets portion of the unremitted earnings and profits (E&P) of foreign subsidiaries expected to be repatriated from its foreign subsidiaries to the United States, except for amounts from certain subsidiaries, which the Company has asserted to be indefinitely reinvested. Specifically, the Company asserts that a total of $3.4 billion of unremitted foreign earnings is indefinitely reinvested. This figure is comprised of $2.0 billion in unremitted earnings as well as $1.4 billion of non-cash E&P in all jurisdictions not indefinitely reinvested. If this E&P is ultimately distributed to the United States in the form of dividends the Company would likely be subject to additional withholding tax. The Company estimates the amount of unrecognized deferred withholding taxes on the undistributed E&P to be approximately $95.6 million at December 31, 2023.
The Company had gross unrecognized tax benefits, excluding interest, of approximately $58.5 million as of December 31, 2023, that if recognized, would reduce the Company’s effective tax rate. In the next twelve months it is reasonably possible that the Company will reduce its unrecognized tax benefits by an immaterial amount due to the expiration of statutes of limitations. A tabular reconciliation of the Company’s gross unrecognized tax benefits is as follows (in millions):
Gross unrecognized tax benefits at December 31, 2020 |
|
$ |
22.7 |
|
Gross increases—tax positions in prior periods |
|
|
17.8 |
|
Gross increases—current period tax positions |
|
|
10.9 |
|
Gross unrecognized tax benefits at December 31, 2021 |
|
|
51.4 |
|
Gross decreases—tax positions in prior periods |
|
|
(8.2 |
) |
Gross increases—current period tax positions |
|
|
11.7 |
|
Gross unrecognized tax benefits at December 31, 2022 |
|
|
54.9 |
|
Gross decreases—tax positions in prior periods |
|
|
(3.8 |
) |
Gross increases—current period tax positions |
|
|
7.4 |
|
Gross unrecognized tax benefits at December 31, 2023 |
|
$ |
58.5 |
|
The Company’s policy is to include accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, in income tax expense. At December 31, 2023 and 2022, the Company had approximately $5.8 million and $4.2 million, respectively, of accrued interest and penalties related to uncertain tax positions included in other long-term liabilities in the consolidated balance sheets. The Company recorded expense of $1.3 million and $1.1 million for penalties and interest related to unrecognized tax benefits in the provision for income taxes during the years ended December 31, 2023 and 2022, respectively.
The Company files tax returns in the United States, which includes federal, state and local jurisdictions, and many foreign jurisdictions with varying statutes of limitations. The Company considers Germany, the United States and Switzerland to be its significant tax jurisdictions. The majority of the Company’s earnings are derived in Germany and Switzerland. Accounting for the various federal and local taxing authorities, the statutory rates for 2023 were approximately 30.0% and 20.0% for Germany and Switzerland, respectively. The mix of earnings in those two jurisdictions resulted in an increase of 4.2% from the U.S. statutory rate of 21% in 2023.
The Company has been subject to tax examinations for the years 2013-2017 with Germany taxing authorities and for the years 2013-2023 in various taxing authorities.
94
In 2020, the Company was granted an income tax holiday for the manufacturing facility in Malaysia. The tax holiday allows for tax-free operations through February 28, 2024, and with the option to apply for a 5 year extension if certain conditions are met. The company has applied for an extension of the tax holiday for another 5 years, which is currently under review by the local authorities. The effect of the tax holiday in Malaysia increased the Company's net income by $7.6 million, $2.5 million and $0.0 million and increased the Company's net income per diluted share by $0.05, $0.01 and $0.00 for the years ended December 31, 2023, 2022 and 2021, respectively.
In connection with the Tax Cuts and Jobs Act (“TCJA”) of 2017, the Company recorded a toll charge liability of $35.4 million and the liability decreased to $20.5 million due to its amended 2017 tax return filed in 2023. Of that amount, approximately $19.4 million has already been paid as of December 31, 2023.
In August 2022, the President of the United States signed into law the Inflation Reduction Act of 2022 (the “IRA”), a tax and spending package that introduced several tax provisions, including a 15% corporate alternative minimum tax (“CAMT”) on certain large corporations and a 1% excise tax on certain corporate stock repurchases. The IRA provisions, which became effective for the Company beginning on January 1, 2023, did not have a material impact on the Company during the year ended December 31, 2023. While the full impact of these provisions in the future depends on several factors, including interpretive regulatory guidance, which has not yet been released, we do not believe this legislation will have a material impact on our consolidated financial statements. We will continue to monitor the potential impact of this new legislation as new information and guidance becomes available.
The Organization for Economic Co-operation and Development (OECD) introduced its Pillar Two Framework Model Rules (“Pillar 2”), provides guidance for a global minimum tax. Certain aspects of Pillar 2 took effect on January 1, 2024, while other aspects go into effect on January 1, 2025. The Company is evaluating the potential impact of Pillar 2 on its business, as the countries in which it operates are enacting legislation implementing Pillar 2. While many aspects of the application of Pillar 2 remain to be clarified, the Company does not expect Pillar 2 to materially impact its tax liability.
95
16. Leases
Operating lease cost is recognized over the lease term on a straight-line basis, while finance lease cost is amortized over the expected term on a straight-line basis. Variable lease cost not dependent on an index or rate is recognized when incurred and typically consists of amounts owed by the Company to a lessor that are not fixed, such as reimbursement for common area maintenance and utilities cost.
The components of lease expense were as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Amortization of right-of-use assets |
|
$ |
4.1 |
|
|
$ |
3.3 |
|
|
$ |
1.5 |
|
Interest on lease liabilities |
|
|
1.0 |
|
|
|
0.6 |
|
|
|
0.1 |
|
Total finance lease cost |
|
|
5.1 |
|
|
|
3.9 |
|
|
|
1.6 |
|
Operating lease cost |
|
|
31.4 |
|
|
|
20.4 |
|
|
|
24.0 |
|
Short term lease cost |
|
|
4.4 |
|
|
|
5.6 |
|
|
|
4.2 |
|
Variable lease cost |
|
|
6.2 |
|
|
|
5.0 |
|
|
|
3.0 |
|
Impairment expense (a) |
|
|
3.2 |
|
|
|
— |
|
|
|
— |
|
Sublease income |
|
|
(2.0 |
) |
|
|
(1.7 |
) |
|
|
(2.0 |
) |
Total lease cost |
|
$ |
48.3 |
|
|
$ |
33.2 |
|
|
$ |
30.8 |
|
(a) Impairment charge recognized in 2023 is associated with the restructuring of PhenomeX. See Note, 21 Other Charges, Net
Supplemental balance sheet information related to leases was as follows (dollars in millions):
|
|
December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Operating leases |
|
|
|
|
|
|
||
Operating lease assets, net |
|
$ |
91.7 |
|
|
$ |
51.2 |
|
Other current liabilities |
|
|
23.3 |
|
|
|
16.4 |
|
Operating lease liability—long term |
|
|
74.8 |
|
|
|
34.8 |
|
|
|
December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Weighted average remaining lease term |
|
5.54 years |
|
|
4.6 years |
|
|
5.1 years |
|
|||
Weighted average discount rate |
|
|
5.3 |
% |
|
|
1.8 |
% |
|
|
1.6 |
% |
|
|
December 31, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Finance leases |
|
|
|
|
|
|
||
Property, plant and equipment, net |
|
$ |
21.9 |
|
|
$ |
14.2 |
|
Current portion of long-term debt |
|
|
5.1 |
|
|
|
2.4 |
|
Long-term debt |
|
|
15.8 |
|
|
|
11.7 |
|
|
|
December 31, |
|
|||||||||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Weighted average remaining lease term |
|
7.4 years |
|
|
10.2 years |
|
|
2.9 years |
|
|||
Weighted average discount rate |
|
|
4.6 |
% |
|
|
3.0 |
% |
|
|
1.6 |
% |
Supplemental cash flow information related to leases was as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Cash paid for amounts included in the measurement of lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Operating cash flows from finance leases |
|
$ |
0.9 |
|
|
$ |
0.6 |
|
|
$ |
0.1 |
|
Operating cash flows from operating leases |
|
|
24.0 |
|
|
|
20.1 |
|
|
|
21.2 |
|
Financing cash flows from finance leases |
|
|
5.0 |
|
|
|
3.3 |
|
|
|
1.6 |
|
Right-of-use assets obtained in exchange for lease liabilities |
|
|
|
|
|
|
|
|
|
|||
Operating leases |
|
$ |
73.5 |
|
|
$ |
22.8 |
|
|
$ |
21.2 |
|
Finance leases |
|
|
11.3 |
|
|
|
13.6 |
|
|
|
2.7 |
|
96
Future lease payments under operating leases and finance leases as follows (in millions):
|
|
Operating Leases |
|
|
Finance Leases |
|
||
Twelve months ending December 31: |
|
|
|
|
|
|
||
2024 |
|
$ |
27.0 |
|
|
$ |
5.4 |
|
2025 |
|
|
23.8 |
|
|
|
4.2 |
|
2026 |
|
|
17.8 |
|
|
|
2.9 |
|
2027 |
|
|
13.3 |
|
|
|
1.5 |
|
2028 |
|
|
11.2 |
|
|
|
1.0 |
|
Thereafter |
|
|
18.5 |
|
|
|
7.0 |
|
Total undiscounted lease payments |
|
|
111.6 |
|
|
|
22.0 |
|
Less: imputed interest |
|
|
(13.5 |
) |
|
|
(1.1 |
) |
Total lease liabilities |
|
$ |
98.1 |
|
|
$ |
20.9 |
|
17. Post Retirement Benefit Plans
Defined Contribution Plans
The Company sponsors various defined contribution plans that cover certain domestic and international employees. The Company may make contributions to these plans at its discretion. The Company contributed $13.7 million, $11.0 million and $9.4 million to such plans in the years ended December 31, 2023, 2022 and 2021, respectively.
Defined Benefit Plans
Substantially all of the Company’s employees in Switzerland, France, Japan and Thailand, as well as certain employees in Germany, are covered by Company-sponsored defined benefit pension plans. Retirement benefits are generally earned based on years of service and compensation during active employment. Eligibility is generally determined in accordance with local statutory requirements; however, the level of benefits and terms of vesting varies among plans.
The Company records pension service cost within cost of sales, selling, general and administrative, and research and development expenses while non-service related pension costs are recorded within interest and other income (expense), net in the consolidated statements of income and comprehensive income. The components of net periodic benefit costs included in the accompanying consolidated statements of income were as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Components of net periodic benefit costs: |
|
|
|
|
|
|
|
|
|
|||
Service cost |
|
$ |
5.5 |
|
|
$ |
6.5 |
|
|
$ |
8.1 |
|
Interest cost |
|
|
5.2 |
|
|
|
1.0 |
|
|
|
0.5 |
|
Expected return on plan assets |
|
|
(4.4 |
) |
|
|
(1.7 |
) |
|
|
(1.8 |
) |
Settlement (gain) loss recognized |
|
|
— |
|
|
|
(0.3 |
) |
|
|
0.1 |
|
Amortization of prior service (credit) cost |
|
|
(0.8 |
) |
|
|
(0.2 |
) |
|
|
0.9 |
|
Amortization of actuarial losses |
|
|
0.1 |
|
|
|
2.2 |
|
|
|
2.6 |
|
Net periodic benefit costs |
|
$ |
5.6 |
|
|
$ |
7.5 |
|
|
$ |
10.4 |
|
97
The Company measures its benefit obligation and the fair value of plan assets as of December 31st each year. The changes in benefit obligations and plan assets under the defined benefit pension plans, projected benefit obligation and funded status of the plans were as follows (in millions):
|
|
2023 |
|
|
2022 |
|
||
Change in benefit obligation: |
|
|
|
|
|
|
||
Benefit obligation at beginning of year |
|
$ |
197.8 |
|
|
$ |
253.9 |
|
Service cost |
|
|
5.5 |
|
|
|
6.5 |
|
Interest cost |
|
|
5.2 |
|
|
|
1.0 |
|
Plan participant contributions |
|
|
6.4 |
|
|
|
5.1 |
|
Plan amendments |
|
|
(2.4 |
) |
|
|
(2.9 |
) |
Plan settlements |
|
|
— |
|
|
|
(6.8 |
) |
Benefits paid |
|
|
(6.8 |
) |
|
|
(2.1 |
) |
Actuarial gain |
|
|
27.5 |
|
|
|
(48.8 |
) |
Premiums paid |
|
|
(2.3 |
) |
|
|
(1.7 |
) |
Plan combinations / acquisitions |
|
|
6.1 |
|
|
|
0.8 |
|
Impact of foreign currency exchange rates |
|
|
19.1 |
|
|
|
(7.2 |
) |
Benefit obligation at end of year |
|
|
256.1 |
|
|
|
197.8 |
|
|
|
|
|
|
|
|
||
Change in plan assets: |
|
|
|
|
|
|
||
Fair value of plan assets at beginning of year |
|
|
150.3 |
|
|
|
147.8 |
|
Return on plan assets |
|
|
1.5 |
|
|
|
2.5 |
|
Plan participant and employer contributions |
|
|
14.4 |
|
|
|
11.9 |
|
Benefits paid |
|
|
(6.8 |
) |
|
|
(2.1 |
) |
Plan settlements |
|
|
— |
|
|
|
(6.8 |
) |
Premiums paid |
|
|
(2.3 |
) |
|
|
(1.7 |
) |
Plan combinations / acquisitions |
|
|
4.8 |
|
|
|
0.4 |
|
Impact of foreign currency exchange rates |
|
|
15.5 |
|
|
|
(1.7 |
) |
Fair value of plan assets at end of year |
|
|
177.4 |
|
|
|
150.3 |
|
Net under-funded status |
|
$ |
(78.7 |
) |
|
$ |
(47.5 |
) |
Plan amendments relate to further reductions in the mandatory conversion rates for the pension plan in Switzerland that will be effective from 2025 onwards. The accumulated benefit obligation for the defined benefit pension plans is $226.4 million and $168.6 million at December 31, 2023 and 2022, respectively. All defined benefit pension plans have an accumulated benefit obligation and projected benefit obligation in excess of plan assets at December 31, 2023, and 2022.
The following amounts were recognized in the accompanying consolidated balance sheets for the Company’s defined benefit plans (in millions):
|
|
2023 |
|
|
2022 |
|
||
Current liabilities |
|
$ |
(2.1 |
) |
|
$ |
(1.8 |
) |
Non-current liabilities |
|
|
(76.6 |
) |
|
|
(45.7 |
) |
Net benefit obligation |
|
$ |
(78.7 |
) |
|
$ |
(47.5 |
) |
The following pre-tax amounts were recognized in accumulated other comprehensive income for the Company’s defined benefit plans (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Reconciliation of amounts recognized in the consolidated balance sheets: |
|
|
|
|
|
|
|
|
|
|||
Prior service cost |
|
$ |
14.6 |
|
|
$ |
12.0 |
|
|
$ |
9.4 |
|
Net actuarial gain (loss) |
|
|
(27.4 |
) |
|
|
4.2 |
|
|
|
(50.1 |
) |
Accumulated other comprehensive gain (loss) |
|
|
(12.8 |
) |
|
|
16.2 |
|
|
|
(40.7 |
) |
|
|
|
|
|
|
|
|
|
|
|||
Accumulated contributions in excess of net periodic benefit cost |
|
|
(65.9 |
) |
|
|
(63.7 |
) |
|
|
(65.4 |
) |
|
|
|
|
|
|
|
|
|
|
|||
Net amount recognized |
|
$ |
(78.7 |
) |
|
$ |
(47.5 |
) |
|
$ |
(106.1 |
) |
The amount in accumulated other comprehensive income at December 31, 2023, expected to be recognized as amortization of net loss within net periodic benefit cost in 2024 is $(0.5) million.
98
For the defined benefit pension plans, the Company uses a corridor approach to amortize actuarial gains and losses. Under this approach, net actuarial gains or losses in excess of ten percent of the larger of the projected benefit obligation or the fair value of plan assets are amortized over the average remaining service of active participants who are expected to receive benefits under the plans.
The following assumptions were used for defined benefit pension plans reflects the different economic environments within the various countries. The assumptions used to determine the net periodic benefit costs and the projected benefit obligations are as follows:
2023 |
|
Japan |
|
|
France |
|
|
Switzerland |
|
|
Germany |
|
|
Thailand |
|
|||||
Annual discount rate—defined benefit obligation |
|
|
1.1 |
% |
|
|
3.2 |
% |
|
|
1.4 |
% |
|
|
3.6 |
% |
|
|
3.5 |
% |
Annual discount rate—defined benefit cost |
|
|
0.9 |
% |
|
|
3.8 |
% |
|
|
2.4 |
% |
|
|
3.9 |
% |
|
|
3.7 |
% |
Expected return on plan assets |
|
|
— |
% |
|
|
3.0 |
% |
|
|
2.7 |
% |
|
|
— |
% |
|
|
— |
% |
Expected rate of compensation increase |
|
|
3.0 |
% |
|
|
3.0 |
% |
|
|
2.2 |
% |
|
|
2.6 |
% |
|
|
5.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
2022 |
|
Japan |
|
|
France |
|
|
Switzerland |
|
|
Germany |
|
|
Thailand |
|
|||||
Annual discount rate—defined benefit obligation |
|
|
0.9 |
% |
|
|
3.8 |
% |
|
|
2.4 |
% |
|
|
3.9 |
% |
|
|
3.7 |
% |
Annual discount rate—defined benefit cost |
|
|
0.4 |
% |
|
|
1.0 |
% |
|
|
0.4 |
% |
|
|
0.8 |
% |
|
|
2.5 |
% |
Expected return on plan assets |
|
|
— |
% |
|
|
3.0 |
% |
|
|
1.2 |
% |
|
|
— |
% |
|
|
— |
% |
Expected rate of compensation increase |
|
|
3.0 |
% |
|
|
3.0 |
% |
|
|
2.3 |
% |
|
|
2.6 |
% |
|
|
5.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
2021 |
|
Japan |
|
|
France |
|
|
Switzerland |
|
|
Germany |
|
|
Thailand |
|
|||||
Annual discount rate—defined benefit obligation |
|
|
0.4 |
% |
|
|
1.0 |
% |
|
|
0.4 |
% |
|
|
0.8 |
% |
|
|
— |
% |
Annual discount rate—defined benefit cost |
|
|
0.4 |
% |
|
|
0.6 |
% |
|
|
0.1 |
% |
|
|
0.5 |
% |
|
|
— |
% |
Expected return on plan assets |
|
|
— |
% |
|
|
3.0 |
% |
|
|
1.2 |
% |
|
|
— |
% |
|
|
— |
% |
Expected rate of compensation increase |
|
|
3.0 |
% |
|
|
2.0 |
% |
|
|
1.0 |
% |
|
|
2.6 |
% |
|
|
— |
% |
To determine the expected long-term rate of return on pension plan assets, the Company considers current asset allocations, as well as historical and expected returns on various asset categories of plan assets. For the defined benefit pension plans, the Company applies the expected rate of return to a market-related value of assets, which stabilizes variability in assets to which the expected return is applied.
Asset Allocations by Asset Category
The fair value of the Company’s pension plan assets by asset category and by level in the fair value hierarchy, is as follows (in millions):
December 31, 2023 |
|
Total |
|
|
Quoted Prices in |
|
|
Significant Other |
|
|
Significant |
|
||||
Plan Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Group BPCE Life (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
Swiss Life Collective BVG Foundation (b) |
|
|
177.4 |
|
|
|
— |
|
|
|
— |
|
|
|
177.4 |
|
Total plan assets |
|
$ |
177.4 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
177.4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
December 31, 2022 |
|
Total |
|
|
Quoted Prices in |
|
|
Significant Other |
|
|
Significant |
|
||||
Plan Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Group BPCE Life (a) |
|
$ |
0.1 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
0.1 |
|
Swiss Life Collective BVG Foundation (b) |
|
|
150.2 |
|
|
|
— |
|
|
|
— |
|
|
|
150.2 |
|
Total plan assets |
|
$ |
150.3 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
150.3 |
|
99
Contributions and Estimated Future Benefit Payments
For all of our plans except Switzerland, we do not have plan assets to payout benefit payments. Contributions are expected to be consistent with estimated benefit payments for the next fiscal year. The estimated future benefit payments are based on the same assumptions used to measure the Company’s benefit obligation at December 31, 2023. The following benefit payments reflect future employee service as appropriate (in millions):
2024 |
|
|
9.5 |
|
2025 |
|
|
9.8 |
|
2026 |
|
|
10.0 |
|
2027 |
|
|
11.2 |
|
2028 |
|
|
12.1 |
|
2029-2033 |
|
|
63.0 |
|
18. Commitments and Contingencies
Litigation and Related Contingencies
Lawsuits, claims and proceedings of a nature considered normal to its businesses may be pending from time to time against the Company. Third parties might allege that the Company or its collaborators are infringing their patent rights or that the Company is otherwise violating their intellectual property rights. The Company believes the outcome of pending proceedings, individually and in the aggregate, will not have a material impact on the Company’s business or its consolidated financial statements.
In September 2019, Luxendo GmbH (“Luxendo”), a subsidiary of Bruker Corporation, was sued in Germany by Carl Zeiss Microscopy GmbH, a subsidiary of Carl Zeiss AG (“Zeiss”), for infringement of a registered German utility model. After the utility model was canceled by the German Patent and Trademark Office in 2021, Zeiss withdrew its infringement action at the end of 2022 and the proceedings were terminated. However, a parallel European patent application, a family member of the utility model, is still pending in the European Patent Office (“EPO”). The Company is closely monitoring progress in the granting procedure and does not believe that a grant is imminent. The Company is presently unable to predict the EPO’s final decision on the patent application.
At December 31, 2023 and 2022, no material accruals have been recorded for potential contingencies.
Governmental Investigations
The Company is subject to regulation by national, state and local government agencies in the United States and other countries in which it operates. From time to time, the Company is the subject of governmental investigations often involving regulatory, marketing and other business practices. These governmental investigations may result in the commencement of civil and criminal proceedings, fines, penalties and administrative remedies which could have a material adverse effect on the Company’s financial position, results of operations and/or liquidity.
At December 31, 2023, and 2022, no material accruals have been recorded for potential contingencies related to these matters. The Company does not expect additional losses to be incurred in excess of the amounts accrued.
100
Unconditional Purchase Commitments
The Company has entered into unconditional purchase commitments, in the ordinary course of business, that include agreements to purchase goods, services or fixed assets and to pay royalties that are enforceable and legally binding and that specify all significant terms including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Purchase commitments exclude agreements that are cancelable at any time without penalty. The majority of these commitments are expected to be settled during 2024.
Unconditional purchase commitments that are fixed and determinable are as follows (in millions):
2024 |
|
$ |
375.1 |
|
2025 |
|
|
25.7 |
|
2026 |
|
|
0.7 |
|
2027 |
|
|
— |
|
2028 |
|
|
— |
|
Thereafter |
|
|
9.4 |
|
Total |
|
$ |
410.9 |
|
License Agreements
The Company has entered into license agreements allowing it to utilize certain patents. If these patents are used in connection with a commercial product sale, the Company pays royalties on the related product revenues. Licensing fees for the years ended December 31, 2023, 2022, and 2021, were $7.5 million, $6.4 million and $5.8 million, respectively, and are recorded in cost of product revenue in the consolidated statements of income and comprehensive income.
Letters of Credit and Guarantees
At December 31, 2023, and 2022, the Company had bank guarantees of $153.6 million and $130.7 million, respectively, related primarily to customer advances. These arrangements guarantee the refund of advance payments received from customers in the event that the merchandise is not delivered, or warranty obligations are not fulfilled in compliance with the terms of the contract. These guarantees affect the availability of the Company’s lines of credit.
Indemnifications
The Company enters into standard indemnification arrangements in the Company’s ordinary course of business. Pursuant to these arrangements, the Company indemnifies, holds harmless, and agrees to reimburse the indemnified parties for losses suffered or incurred by the indemnified party. These parties are generally the Company’s directors, officers, business partners or customers, in connection with any patent, or any copyright or other intellectual property infringement claim by any third party with respect to its products. The term of these indemnification agreements is generally perpetual any time after the execution of the agreement. The maximum potential amount of future payments the Company could be required to make under these agreements is unlimited. The Company believes the estimated fair value of these agreements is minimal based on historical experiences.
19. Earnings Per Share
The following table sets forth the computation of basic and diluted weighted average shares outstanding and net income per common share attributable to Bruker shareholders (in millions, except per share amounts):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Net income attributable to Bruker Corporation |
|
$ |
427.2 |
|
|
$ |
296.6 |
|
|
$ |
277.1 |
|
Weighted average common shares outstanding: |
|
|
|
|
|
|
|
|
|
|||
Weighted average common shares outstanding - basic |
|
|
146.4 |
|
|
|
148.6 |
|
|
|
151.4 |
|
Effect of dilutive securities: |
|
|
|
|
|
|
|
|
|
|||
Stock options, restricted stock awards, restricted |
|
|
0.8 |
|
|
|
0.8 |
|
|
|
1.5 |
|
Weighted average common shares outstanding - diluted |
|
|
147.2 |
|
|
|
149.4 |
|
|
|
152.9 |
|
Net income per common share attributable to Bruker |
|
|
|
|
|
|
|
|
|
|||
Basic |
|
$ |
2.92 |
|
|
$ |
2.00 |
|
|
$ |
1.83 |
|
Diluted |
|
$ |
2.90 |
|
|
$ |
1.99 |
|
|
$ |
1.81 |
|
101
Diluted earnings per share is calculated using the treasury stock method on a weighted average basis.
The following common share equivalents have been excluded from the computation of diluted weighted-average shares outstanding, as their effect would have been anti-dilutive (in millions of shares):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Stock options and ESPP purchase rights |
|
|
0.2 |
|
|
|
0.1 |
|
|
|
— |
|
Unvested restricted stock units |
|
|
— |
|
|
|
— |
|
|
|
0.1 |
|
20. Shareholders’ Equity
Share Repurchase Program
In May 2021, the Company’s Board of Directors approved a share repurchase program (the “2021 Repurchase Program”) authorizing the purchase of up to $500.0 million of the Company’s common stock over a two-year period, in amounts, at prices, and at such times as management deems appropriate, subject to market conditions, legal requirements and other considerations. During the year ended December 31, 2023, the Company purchased a total of 315,318 shares at an aggregate cost of $22.2 million under the 2021 Repurchase Program. During the year ended December 31, 2022, the Company purchased a total of 4,215,094 shares at an aggregate cost of $264.7 million under the 2021 Repurchase Program. Authorization for the remaining $94.4 million on the 2021 Repurchase Program expired in May 2023.
In May 2023, the Company’s Board of Directors approved a share repurchase program (the “2023 Repurchase Program”) authorizing the purchase of up to $500.0 million of the Company’s common stock over a two-year period, in amounts, at prices, and at such times as management deems appropriate, subject to market conditions, legal requirements and other considerations. During the year ended December 31, 2023, the Company purchased a total of 2,097,119 shares at an aggregate cost of $130.1 million under the 2023 Repurchase Program. At December 31, 2023, $369.9 million remains available for future purchase under the 2023 Repurchase Program. We intend to fund any additional purchases from cash on hand, future cash flows from operations and available borrowings under the revolving credit facility.
In August 2022, the Inflation Reduction Act (“IRA”) was signed into law in the United States. The IRA introduced new tax provisions, including a 1.0% excise tax on stock repurchases. The Company expects additional guidance and regulations to be issued in future periods and will continue to assess its potential impact on its business as further information becomes available. The estimated excise tax on our stock repurchases was not material and was recorded in other current liabilities and additional paid in capital for the year ended December 31, 2023.
Shelf Registration
On June 2, 2023, the Company filed a registration statement on Form S-3ASR with the SEC (“2023 Shelf Registration”) for the issuance of common stock, preferred stock, debt securities, warrants, units, subscription rights and purchase contracts, which became effective immediately upon filing. At the time any of the securities covered by the 2023 Shelf Registration Statement are offered for sale, a prospectus supplement will be prepared and filed with the SEC containing specific information about the terms of any such offering.
Cash Dividends on Common Stock
Dividends are declared by the Company’s Board of Directors (“Board”) in accordance with the Company’s dividend policy. Under the policy, the Company targeted a $0.20 per share cash dividend per annum to the Company’s shareholders payable in equal quarterly installments.
Subsequent dividend declarations and the establishment of record and payment dates for such future dividend payments, if any, are subject to the Board's continuing determination that the dividend policy is in the best interests of the Company’s shareholders. The dividend policy may be suspended or canceled at the discretion of the Board at any time. In February 2024, the Company announced that our Board had declared a quarterly dividend of $0.05 per share that will be payable in March 2024.
102
Stock Compensation Plans
On March 9, 2010, the Company’s Board of Directors unanimously approved and adopted the Bruker Corporation 2010 Incentive Compensation Plan (the “2010 Plan”), and on May 14, 2010, the 2010 Plan was approved by the Company’s shareholders. The 2010 Plan provided for the issuance of up to 8,000,000 shares of the Company’s common stock. The 2010 Plan allowed a committee of the Board of Directors determined to be the Compensation Committee, to grant incentive stock options, non-qualified stock options and restricted stock awards. The Compensation Committee had the authority to determine which employees would receive the awards, the amount of the awards and other terms and conditions of any awards. Awards granted under the 2010 Plan typically were made subject to a vesting period of three to five years. As of December 31, 2023, 5,545,090 options and 570,011 restricted stock awards have been granted under the 2010 Plan. At December 31, 2023, 304,098 options were outstanding under the 2010 Plan.
In May 2016, the Bruker Corporation 2016 Incentive Compensation Plan (the “2016 Plan”) was approved by the Company’s shareholders. With the approval of the 2016 Plan, no further grants will be made under the 2010 Plan. The 2016 Plan provides for the issuance of up to 9,500,000 shares of the Company’s common stock and permits the grant of awards of non-qualified stock options, incentive stock options, stock appreciation rights, restricted stock, unrestricted stock, restricted stock units, performance shares and performance units, as well as cash-based awards. The 2016 Plan is administered by the Compensation Committee. The Compensation Committee has the authority to determine which employees will receive awards, the amount of any awards, and other terms and conditions of such awards. Stock option awards granted under the 2016 Plan typically vest over a period of one to four years. As of December 31, 2023, 1,636,907 options and 2,745,861 restricted stock units have been granted under the 2016 Plan. At December 31, 2023, 663,462 options and 702,047 restricted stock units were outstanding under the 2016 Plan.
In June 2022, the Company's shareholders approved the 2022 Employee Stock Purchase Plan, under which eligible employees may contribute up to 10% of their earnings toward the semi-annual purchase of the Company's stock. The plan makes available 2,500,000 shares. Each plan enrollment period covers six months beginning June 1 and December 1 of each year. The purchase price per share of the Company's stock is equal to 90% of the lower of (1) the fair market value per share of the Company's stock on the first day of the applicable purchase period or (2) the fair market value per share of the Company's stock on the applicable purchase date, unless otherwise specified by the Plan Administrator before the start of any purchase period.
Members of the Company’s Board of Directors receive an annual award of restricted stock units which vest over a one-year service period. Stock options to purchase the Company’s common stock are periodically awarded to executive officers and other employees of the Company subject to a vesting period of three to four years. Restricted shares of the Company’s common stock were periodically awarded to executive officers, directors and certain key employees of the Company, subject to service restrictions, which vested ratably over periods of one to four years. The restricted shares of common stock may not be sold or transferred during the restriction period. Restricted stock units of the Company’s common stock are periodically awarded to executive officers, directors and certain employees of the Company which vest ratably over service periods of one to four years.
103
Stock-based Compensation
The following presents the impact of stock-based compensation expense on our consolidated statements of income (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Stock options |
|
$ |
1.6 |
|
|
$ |
1.5 |
|
|
$ |
1.3 |
|
Restricted stock units |
|
|
16.0 |
|
|
|
14.1 |
|
|
|
13.2 |
|
Employee Stock Purchase Plan |
|
|
0.8 |
|
|
|
0.1 |
|
|
|
— |
|
Total stock-based compensation |
|
$ |
18.4 |
|
|
$ |
15.7 |
|
|
$ |
14.5 |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Cost of product revenue |
|
$ |
1.4 |
|
|
$ |
1.1 |
|
|
$ |
2.2 |
|
Selling, general and administrative |
|
|
15.1 |
|
|
|
13.3 |
|
|
|
10.1 |
|
Research and development |
|
|
1.9 |
|
|
|
1.3 |
|
|
|
2.2 |
|
Total stock-based compensation |
|
$ |
18.4 |
|
|
$ |
15.7 |
|
|
$ |
14.5 |
|
In addition to the awards above, the Company recorded stock-based compensation within other charges, net of $5.6 million, $12.0 million and $2.7 million at December 31, 2023, 2022, and 2021, respectively, related to the Mestrelab, PreOmics, Biognosys, Zontal, and other majority owned acquisitions.
At December 31, 2023, the Company expects to recognize pre-tax stock-based compensation expense of $3.5 million associated with outstanding stock option awards granted under the Company’s stock plans over the weighted average remaining service period of 2.5 years. The Company also expects to recognize additional pre-tax stock-based compensation expense of $37.5 million associated with outstanding restricted stock units granted under the Company’s 2016 Incentive Compensation Plan over the weighted average remaining service period of 2.7 years.
Stock Option Awards
Stock option activity for the year ended December 31, 2023, is as follows:
|
|
Number of |
|
|
Weighted- |
|
|
Weighted - |
|
|
Aggregate |
|
||||
Outstanding at December 31, 2022 |
|
|
1,173,625 |
|
|
$ |
34.81 |
|
|
|
3.7 |
|
|
$ |
40.8 |
|
Granted |
|
|
77,719 |
|
|
|
69.37 |
|
|
|
|
|
|
|
||
Exercised |
|
|
(285,030 |
) |
|
|
34.05 |
|
|
|
|
|
|
|
||
Forfeited/Expired |
|
|
1,246 |
|
|
|
— |
|
|
|
|
|
|
|
||
Outstanding at December 31, 2023 |
|
|
967,560 |
|
|
$ |
37.78 |
|
|
|
3.7 |
|
|
$ |
35.5 |
|
Exercisable at December 31, 2023 |
|
|
763,544 |
|
|
$ |
29.78 |
|
|
|
3.0 |
|
|
$ |
33.8 |
|
Exercisable and expected to vest at |
|
|
965,990 |
|
|
$ |
37.77 |
|
|
|
3.7 |
|
|
$ |
35.4 |
|
The total intrinsic value of options exercised was $9.4 million, 10.7 million and $35.7 million for the years ended December 31, 2023, 2022 and 2021, respectively.
104
Restricted Stock Units
Restricted stock unit activity is presented below:
|
|
Shares |
|
|
Weighted- |
|
||
Outstanding at December 31, 2022 |
|
|
635,868 |
|
|
$ |
60.23 |
|
Granted |
|
|
354,265 |
|
|
|
66.20 |
|
Vested |
|
|
(266,395 |
) |
|
|
56.14 |
|
Forfeited |
|
|
(21,691 |
) |
|
|
63.54 |
|
Outstanding at December 31, 2023 |
|
|
702,047 |
|
|
$ |
64.68 |
|
The total fair value of restricted stock vested for the years ended December 31, 2023, 2022 and 2021was $17.8 million, $17.7 million and $27.1 million, respectively.
21. Other Charges, Net
The components of other charges, net, were as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Acquisition-related expenses, net |
|
$ |
16.8 |
|
|
$ |
19.3 |
|
|
$ |
6.1 |
|
Professional fees incurred in connection with investigation |
|
|
0.6 |
|
|
|
2.4 |
|
|
|
1.1 |
|
Information technology transformation costs |
|
|
5.0 |
|
|
|
3.0 |
|
|
|
2.8 |
|
Restructuring |
|
|
18.8 |
|
|
|
3.9 |
|
|
|
4.8 |
|
Long-lived asset impairments |
|
|
5.7 |
|
|
|
0.3 |
|
|
|
(0.5 |
) |
Other |
|
|
5.3 |
|
|
|
0.8 |
|
|
|
— |
|
Other charges, net |
|
$ |
52.2 |
|
|
$ |
29.7 |
|
|
$ |
14.3 |
|
Restructuring Initiatives
Restructuring charges for the years ended December 31, 2023, 2022 and 2021 included charges for various other programs which were recorded in the accompanying consolidated statements of income and comprehensive income as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Cost of revenues |
|
$ |
3.5 |
|
|
$ |
0.9 |
|
|
$ |
3.4 |
|
Other charges, net |
|
|
18.8 |
|
|
|
3.9 |
|
|
|
4.8 |
|
|
|
$ |
22.3 |
|
|
$ |
4.8 |
|
|
$ |
8.2 |
|
105
The following table sets forth the changes in the restructuring reserves (in millions):
|
|
Total |
|
|
Severance |
|
|
Exit Costs |
|
|
Provisions for |
|
||||
Balance at December 31, 2020 |
|
$ |
9.8 |
|
|
$ |
7.6 |
|
|
$ |
0.8 |
|
|
$ |
1.4 |
|
Restructuring charges |
|
|
8.2 |
|
|
|
5.3 |
|
|
|
1.5 |
|
|
|
1.4 |
|
Cash payments |
|
|
(10.3 |
) |
|
|
(9.3 |
) |
|
|
(1.0 |
) |
|
|
— |
|
Non-cash adjustments |
|
|
(1.1 |
) |
|
|
— |
|
|
|
(1.0 |
) |
|
|
(0.1 |
) |
Foreign currency impact |
|
|
(0.2 |
) |
|
|
(0.1 |
) |
|
|
— |
|
|
|
(0.1 |
) |
Balance at December 31, 2021 |
|
$ |
6.4 |
|
|
$ |
3.5 |
|
|
$ |
0.3 |
|
|
$ |
2.6 |
|
Restructuring charges |
|
|
4.8 |
|
|
|
2.4 |
|
|
|
2.3 |
|
|
|
0.1 |
|
Cash payments |
|
|
(7.8 |
) |
|
|
(5.4 |
) |
|
|
(2.4 |
) |
|
|
— |
|
Non-cash adjustments |
|
|
(1.4 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1.4 |
) |
Foreign currency impact |
|
|
(0.2 |
) |
|
|
(0.1 |
) |
|
|
— |
|
|
|
(0.1 |
) |
Balance at December 31, 2022 |
|
$ |
1.8 |
|
|
$ |
0.4 |
|
|
$ |
0.2 |
|
|
$ |
1.2 |
|
Restructuring charges |
|
|
22.3 |
|
|
|
20.5 |
|
|
|
0.8 |
|
|
|
1.0 |
|
Cash payments |
|
|
(13.1 |
) |
|
|
(12.3 |
) |
|
|
(0.8 |
) |
|
|
— |
|
Non-cash adjustments |
|
|
(1.6 |
) |
|
|
— |
|
|
|
— |
|
|
|
(1.6 |
) |
Acquired |
|
|
3.6 |
|
|
|
0.9 |
|
|
|
2.7 |
|
|
|
— |
|
Foreign currency impact |
|
|
0.1 |
|
|
|
0.1 |
|
|
|
— |
|
|
|
— |
|
Balance at December 31, 2023 |
|
$ |
13.1 |
|
|
$ |
9.6 |
|
|
$ |
2.9 |
|
|
$ |
0.6 |
|
In October 2023, the Company announced a restructuring plan associated with BCA (formerly PhenomeX), a component of the Nano reportable segment, to optimize costs and to facilitate integration efforts. The restructuring plan includes a reduction in headcount, consolidation of leased facilities, and a planned change in future product offerings. The restructuring plan is expected to be completed during 2024.
In connection with the BCA restructuring plan, the Company accrued severance and termination charges of $14.9 million. As of December 31, 2023, $9.8 million of these charges have been paid. The unpaid severance charges noted above will be paid in 2024 and 2025. As it relates to the consolidation of leased facilities, the Company recorded an impairment charge against operating lease right of use assets of $3.2 million, as detailed in Note 16, Leases, and expensed a negligible amount of associated leasehold improvements. As of December 31, 2023, no modification of operating leases connected to the restructuring plan had occurred. Finally, in connection with the planned change of future product offerings, the Company recorded an impairment charge against various equipment of $2.3 million.
The Company anticipates additional severance charges in 2024, which are not expected to be material. The Company is also likely to terminate additional leases as operations are consolidated and arrangements for early terminations with the landlord are reached. The Company does not expect termination payments to be significantly different from amounts recorded as outstanding lease liabilities as of December 31, 2023.
Certain other restructuring programs relating to in reductions in force recorded by the BSI NANO, BSI Biospin, BSI CALID and Corporate segments in 2023 were not material and substantially complete at the end of the year.
22. Interest and Other Income (Expense), Net
The components of interest and other income (expense), net, were as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Interest income |
|
$ |
7.5 |
|
|
$ |
2.8 |
|
|
$ |
0.9 |
|
Interest expense |
|
|
(16.4 |
) |
|
|
(16.1 |
) |
|
|
(14.3 |
) |
Impairment of minority investments |
|
|
(18.2 |
) |
|
|
— |
|
|
|
— |
|
Exchange losses on foreign currency transactions |
|
|
(13.3 |
) |
|
|
(5.8 |
) |
|
|
(4.1 |
) |
Pension components |
|
|
(0.1 |
) |
|
|
(1.0 |
) |
|
|
(2.2 |
) |
Other income (expense) |
|
|
3.7 |
|
|
|
1.3 |
|
|
|
— |
|
Interest and other income (expense), net |
|
$ |
(36.8 |
) |
|
$ |
(18.8 |
) |
|
$ |
(19.7 |
) |
106
23. Business Segment Information
The Company has four reportable segments, BSI BioSpin, BSI CALID, BSI Nano and BEST, as discussed in Note 1 to the consolidated financial statements. The Company's CEO is the chief operating decision maker.
Selected reportable segment information is presented below (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Revenue: |
|
|
|
|
|
|
|
|
|
|||
BSI BioSpin |
|
$ |
798.5 |
|
|
$ |
696.7 |
|
|
$ |
691.0 |
|
BSI CALID |
|
|
960.4 |
|
|
|
822.2 |
|
|
|
819.6 |
|
BSI Nano |
|
|
941.9 |
|
|
|
787.0 |
|
|
|
697.5 |
|
BEST |
|
|
280.7 |
|
|
|
237.1 |
|
|
|
223.8 |
|
Eliminations (a) |
|
|
(17.0 |
) |
|
|
(12.3 |
) |
|
|
(14.0 |
) |
Total revenue |
|
$ |
2,964.5 |
|
|
$ |
2,530.7 |
|
|
$ |
2,417.9 |
|
Operating Income: |
|
|
|
|
|
|
|
|
|
|||
BSI BioSpin |
|
$ |
195.9 |
|
|
$ |
167.1 |
|
|
$ |
158.2 |
|
BSI CALID |
|
|
209.3 |
|
|
|
196.0 |
|
|
|
227.2 |
|
BSI Nano |
|
|
109.1 |
|
|
|
113.0 |
|
|
|
73.4 |
|
BEST |
|
|
32.3 |
|
|
|
31.3 |
|
|
|
22.2 |
|
Corporate, eliminations and other (b) |
|
|
(109.7 |
) |
|
|
(74.7 |
) |
|
|
(67.7 |
) |
Total operating income |
|
$ |
436.9 |
|
|
$ |
432.7 |
|
|
$ |
413.3 |
|
Total assets by segment are as follows (in millions):
|
|
2023 |
|
|
2022 |
|
||
Assets: |
|
|
|
|
|
|
||
BSI BioSpin, BSI CALID, BSI Nano & Corporate |
|
$ |
4,110.6 |
|
|
$ |
3,508.4 |
|
BEST |
|
|
186.0 |
|
|
|
111.1 |
|
Eliminations and other (a) |
|
|
(46.7 |
) |
|
|
(7.7 |
) |
Total assets |
|
$ |
4,249.9 |
|
|
$ |
3,611.8 |
|
The Company is unable, without unreasonable effort or expense to disclose the amount of total assets by the BSI BioSpin, BSI CALID and BSI Nano Segments as well as the Corporate function and further, the Company’s chief operating decision maker does not receive any asset information by operating segment.
Total capital expenditures and depreciation and amortization by segment are presented below (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Capital Expenditures: |
|
|
|
|
|
|
|
|
|
|||
BSI BioSpin |
|
$ |
23.9 |
|
|
$ |
14.5 |
|
|
$ |
36.6 |
|
BSI CALID |
|
|
31.4 |
|
|
|
33.9 |
|
|
|
24.7 |
|
BSI Nano |
|
|
13.9 |
|
|
|
20.2 |
|
|
|
16.8 |
|
Corporate |
|
|
10.2 |
|
|
|
9.1 |
|
|
|
4.7 |
|
BEST |
|
|
27.5 |
|
|
|
51.5 |
|
|
|
9.2 |
|
Total capital expenditures |
|
$ |
106.9 |
|
|
$ |
129.2 |
|
|
$ |
92.0 |
|
Depreciation and Amortization: |
|
|
|
|
|
|
|
|
|
|||
BSI BioSpin |
|
$ |
25.7 |
|
|
$ |
22.6 |
|
|
$ |
21.3 |
|
BSI CALID |
|
|
29.3 |
|
|
|
20.7 |
|
|
|
19.8 |
|
BSI Nano |
|
|
48.0 |
|
|
|
35.6 |
|
|
|
37.4 |
|
Corporate |
|
|
4.7 |
|
|
|
3.9 |
|
|
|
4.3 |
|
BEST |
|
|
7.2 |
|
|
|
6.0 |
|
|
|
6.3 |
|
Total depreciation and amortization |
|
$ |
114.9 |
|
|
$ |
88.8 |
|
|
$ |
89.1 |
|
107
Revenue and long-lived assets (including property, plant and equipment, net and operating lease right of use assets) by geographical area are as follows (in millions):
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Revenue: |
|
|
|
|
|
|
|
|
|
|||
United States |
|
$ |
777.7 |
|
|
$ |
696.1 |
|
|
$ |
601.0 |
|
Germany |
|
|
281.5 |
|
|
|
247.4 |
|
|
|
262.6 |
|
Rest of Europe |
|
|
699.7 |
|
|
|
591.9 |
|
|
|
658.1 |
|
China |
|
|
528.1 |
|
|
|
396.5 |
|
|
|
357.6 |
|
Rest of Asia Pacific |
|
|
460.9 |
|
|
|
408.4 |
|
|
|
371.5 |
|
Other |
|
|
216.6 |
|
|
|
190.4 |
|
|
|
167.1 |
|
Total revenue |
|
$ |
2,964.5 |
|
|
$ |
2,530.7 |
|
|
$ |
2,417.9 |
|
|
|
2023 |
|
|
2022 |
|
|
2021 |
|
|||
Long-lived assets: |
|
|
|
|
|
|
|
|
|
|||
Germany |
|
$ |
350.2 |
|
|
$ |
309.6 |
|
|
$ |
244.0 |
|
Switzerland |
|
|
127.0 |
|
|
|
98.5 |
|
|
|
96.3 |
|
United States |
|
|
124.9 |
|
|
|
58.8 |
|
|
|
51.3 |
|
Rest of Europe |
|
|
57.4 |
|
|
|
42.5 |
|
|
|
45.2 |
|
Asia Pacific |
|
|
22.1 |
|
|
|
22.1 |
|
|
|
22.6 |
|
Other |
|
|
9.9 |
|
|
|
6.7 |
|
|
|
6.6 |
|
Total long-lived assets |
|
$ |
691.5 |
|
|
$ |
538.2 |
|
|
$ |
466.0 |
|
108
ITEM 9 CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We have established disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) that are designed to provide reasonable assurance that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and to ensure that such information is accumulated and communicated to management, including our Chief Executive Officer (principal executive officer) and Chief Financial Officer (principal financial officer), to allow timely decisions regarding required disclosures. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2023. Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures were effective at a reasonable assurance level as of December 31, 2023.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2023, based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework (2013). Based on this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2023.
We excluded Biognosys AG, Osthus Beteiligungs GmbH, MIRO Analytical AG, PhenomeX Inc. (Bruker Cellular Analysis), Acquifer Imaging GmbH, Deltabyte GmbH, Pinpoint Testing LLC, Fasmatech Science and Technology SA, Interherence GmbH, and Signals GmbH & Co. KG, from our assessment of internal control over financial reporting as of December 31, 2023, because they were acquired by the Company in business combinations during 2023. The total assets and total revenues of the acquired entities collectively represent approximately 9% and 1%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2023.
The effectiveness of our internal control over financial reporting as of December 31, 2023, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report, which appears in this Annual Report on Form 10-K.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2023, that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B OTHER INFORMATION
During the quarter ended December 31, 2023, none of the Company's directors or officers informed the Company of the adoption or termination of a “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement”, as those terms are defined in Regulation S-K, Item 408.
As previously disclosed, Bruker Invest AG, a Swiss stock corporation and a subsidiary of the Company (“Buyer”), entered into a binding put option agreement dated December 23, 2023 (the “Put Option Letter”), with Tecfin S.à r.l., a controlled affiliate of PAI Partners (the “Beneficiary”) acting for itself and on behalf of the shareholders (collectively, the “Sellers”) owning directly or indirectly 100% of the issued and outstanding securities of TecInvest S.à r.l, which does business as ELITechGroup (“Target”). Target’s subsidiaries (collectively, “EliTech”) are active in the molecular diagnostics, microbiology and biomedical testing equipment fields. Under the terms of the Put Option Letter, Buyer irrevocably and unconditionally agreed to sign the form of (i) Share Purchase Agreement attached to the Put Option Letter as Annex I (the “SPA”) and (ii) Warranty Agreement attached to the Put Option Letter as Annex II containing customary representations and warranties of the Sellers for transactions of this type (the “Warranty Agreement”), both pertaining to the acquisition of all issued and outstanding securities of Target on the terms and conditions set forth therein (the “Acquisition”), subject to the delivery by the Beneficiary sending by the Beneficiary, of a notice of Sellers’ decision to sell all of Target’s securities to Buyer in accordance with the terms and conditions of the SPA (the “Put Option”).
109
Concomitantly with the execution of the Put Option Letter, (i) the Company delivered to the Sellers a customary equity commitment letter covering all amounts payable by Buyer under the SPA (and which the Beneficiary relied upon in entering into the Put Option Letter) and (ii) Buyer secured an insurance policy to cover (as sole recourse) any and all claims for breach of the representations and warranties of the Sellers contained in the Warranty Agreement.
On February 27, 2024, following the exercise of the Put Option by the Sellers, the Buyer and the Sellers entered into the SPA, pursuant to which the Buyer will acquire (directly or indirectly) the Target and its subsidiaries for a cash purchase price derived from an Enterprise Value of EUR 870 million, subject to certain adjustments. The purchase price will be paid by Buyer with cash on hand. The SPA provides that closing of the Acquisition is subject to the satisfaction or waiver of certain conditions, notably certain regulatory approvals and notices and the carve out referred to above. In addition, in connection with the execution of the SPA, the Buyer and the Sellers entered into the Warranty Agreement.
The description of the Put Option Letter and the SPA and the transactions contemplated thereby, including the Acquisition, set forth above are not complete and are subject to, and qualified by, the full text of the Put Option Letter, the SPA and the Warranty Agreement, each of which is filed as an exhibit to this Annual Report on Form 10-K.
ITEM 9C DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS ITEM 10 DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Not applicable.
110
PART III
The full text of our code of conduct, which applies to our Principal Executive Officer, Principal Financial Officer, Principal Accounting Officer and Board of Directors is published on our Investor Relations website at www.bruker.com. We intend to disclose future amendments to certain provisions of our Code, or waivers of such provisions granted to executive officers and directors, on the website within four business days following the date of such amendment or waiver.
The information required by this item of Form 10-K is incorporated by reference to our definitive proxy statement (which will be filed with the SEC pursuant to Regulation 14A under the Exchange Act) relating to our 2024 Annual Meeting of Shareholders.
ITEM 11 EXECUTIVE COMPENSATION
The information required by this item of Form 10-K is incorporated by reference to our definitive proxy statement (which will be filed with the SEC pursuant to Regulation 14A under the Exchange Act) relating to our 2024 Annual Meeting of Shareholders.
ITEM 12 SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED SHAREHOLDER MATTERS
The information required by this item of Form 10-K is incorporated by reference to our definitive proxy statement (which will be filed with the SEC pursuant to Regulation 14A under the Exchange Act) relating to our 2024 Annual Meeting of Shareholders.
ITEM 13 CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item of Form 10-K is incorporated by reference to our definitive proxy statement (which will be filed with the SEC pursuant to Regulation 14A under the Exchange Act) relating to our 2024 Annual Meeting of Shareholders.
ITEM 14 PRINCIPAL ACCOUNTANT FEES AND SERVICES
Our independent registered public accounting firm is PricewaterhouseCoopers LLP, New York, NY, PCAOB ID 238.
The information required by this item of Form 10-K is incorporated by reference to our definitive proxy statement (which will be filed with the SEC pursuant to Regulation 14A under the Exchange Act) relating to our 2024 Annual Meeting of Shareholders.
111
PART IV
ITEM 15 EXHIBITS, FINANCIAL STATEMENTS AND SCHEDULES
The financial statements required by this item are filed as part of this report under Item 8—Financial Statements and Supplementary Data.
The financial statements required by this item are filed as part of this report under Item 8—Financial Statements and Supplementary Data.
EXHIBIT INDEX
112
113
10.29 |
|
|
|
10.30 |
|
|
|
10.31 |
|
|
|
21.1 ** |
|
|
|
|
|
|
|
23.1 ** |
Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm |
|
|
|
|
|
|
24.1 ** |
|
|
|
|
|
|
|
31.1 ** |
|
|
|
|
|
|
|
31.2 ** |
|
|
|
|
|
|
|
32.1 ** |
|
|
|
97.1 ** |
|
|
|
|
g |
|
|
101.INS ** |
Inline XBRL Instance Document |
|
|
|
|
|
|
101.SCH ** |
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
|
101.CAL ** |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
|
101.DEF ** |
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
101.LAB ** |
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
|
101.PRE** |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
|
104** |
The cover page from the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 has been formatted in Inline XBRL (included in Exhibit 101) |
|
|
114
* Certain portions have been omitted pursuant to an order granting confidential treatment and have been filed separately with the Securities and Exchange Commission.
† Designates management contract or compensatory plan or arrangement.
** Filed or furnished herewith.
No other instruments defining the rights of holders of long-term debt of the registrant or its subsidiaries have been filed as Exhibits because no such instruments met the threshold materiality requirements under Regulation S-K. The registrant agrees, however, to furnish a copy of any such instruments to the Commission upon request.
ITEM 16 FORM 10-K SUMMARY Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Not Applicable.
115
SIGNATURES
|
|
BRUKER CORPORATION |
|
|
|
|
|
Date: February 29, 2024 |
|
By: |
/s/ FRANK H. LAUKIEN, PH.D.
|
|
|
|
Name: Frank H. Laukien, Ph.D. |
|
|
|
Title: President, Chief Executive Officer and Chairman |
We, the undersigned officers and directors of Bruker Corporation, hereby severally constitute and appoint Frank H. Laukien, Ph.D. to sign for us and in our names in the capacities indicated below, the report on Form 10-K filed herewith and any and all amendments to such report, and to file the same, with all exhibits thereto and other documents in connection therewith, in each case, with the Securities and Exchange Commission, and generally to do all such things in our names and on our behalf in our capacities consistent with the provisions of the Securities Exchange Act of 1934, as amended, and all requirements of the Securities and Exchange Commission.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
116
Name |
Title |
Date |
|
|
|
/S/ FRANK H. LAUKIEN, PH.D. |
President, Chief Executive Officer and Chairman (Principal Executive Officer) |
February 29, 2024 |
Frank H. Laukien, Ph.D. |
|
|
|
|
|
/S/ GERALD N. HERMAN |
Executive Vice President and Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) |
February 29, 2024 |
Gerald N. Herman |
|
|
|
|
|
/S/ BONNIE H. ANDERSON |
Director |
February 29, 2024 |
Bonnie H. Anderson |
|
|
|
|
|
/S/ CYNTHIA M. FRIEND, PH.D. |
Director |
February 29, 2024 |
Cynthia Friend, Ph.D. |
|
|
|
|
|
/S/ WILLIAM A. LINTON |
Director |
February 29, 2024 |
William A. Linton |
|
|
|
|
|
/S/ Philip Ma |
Director |
February 29, 2024 |
Philip Ma |
|
|
|
|
|
/S/ JOHN ORNELL |
Director |
February 29, 2024 |
John Ornell |
|
|
|
|
|
/S/ RICHARD A. PACKER |
Director |
February 29, 2024 |
Richard A. Packer |
|
|
|
|
|
/S/ ADELENE Q. PERKINS |
Director |
February 29, 2024 |
Adelene Q. Perkins |
|
|
|
|
|
/S/ HERMANN REQUARDT, PH.D. |
Director |
February 29, 2024 |
Hermann Requardt, Ph.D. |
|
|
|
|
|
/S/ ROBERT ROSENTHAL, PH.D. |
Director |
February 29, 2024 |
Robert Rosenthal, Ph.D. |
|
|
|
|
|
117
EXHIBIT 10.29
From: |
Bruker Invest AG, a stock company, organized under the laws of Switzerland, having its registered office at Industriestrasse 26, Fallenden, CH-8117, Switzerland and registered with the Commercial register of canton Zurich under the number CHE-100.756.943 (the “Promisor”); |
To: |
Tecfin S.à r.l., a private limited liability company (société à responsabilité limitée) organized under the laws of Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L – 2449 Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B216066 (the “Beneficiary”) |
Strictly Private and Confidential
December 23, 2023
Re: Put Option
Dear Sirs:
Reference is made to our recent discussions relating to the contemplated acquisition of, directly or indirectly, all the issued interests in the share capital of TecInvest S.à. r.l, a private limited liability company (société à responsabilité limitée) incorporated under the laws of the Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L-2449 Luxembourg, Grand Duchy of Luxembourg and registered with the Trade and Companies Register of Luxembourg (Registre de Commerce et des Sociétés de Luxembourg) under number B 194689 which currently holds a majority stake in the EliTech group; collectively with all related transactions as contemplated under the Share Purchase Agreement and notably the Pre-Closing Reorganizations (as such term is defined in the Share Purchase Agreement) described in Annex 6.1 of the Share Purchase Agreement to be implemented by the Beneficiary at or prior to Closing, the “Transaction”.
The Promisor acknowledges that before the Beneficiary and the other Sellers are in a position to take any decision to enter into any binding agreement with respect to the contemplated Transaction, the French Works Council (as defined below) must be informed of and consulted with respect to the contemplated Transaction, and (ii) the Dutch Works Council (as defined below) must be informed of and requested for its advice in connection with one step of the Carve-Out Reorganization (as such term is defined in the Share Purchase Agreement), being the acquisition of 100% of the shares in Elitech Group B.V. by TecFin S.à.r.l. from ELITechGroup Benelux B.V.
This letter agreement (the “Put Option Agreement”) sets forth the Promisor’s irrevocable commitment to acquire all the Transferred Securities on the terms and subject to the conditions set forth in (i) a Share Purchase Agreement (including any and all schedules hereto) in the final and agreed form attached hereto as Annex I (the “Share Purchase Agreement”) and (ii) a Warranty Agreement (including any and all schedules hereto) in the final and agreed form attached hereto as Annex II (the “Warranty Agreement”, together with the Share Purchase Agreement, the “Transaction Agreements”).
Concomitantly with the execution of the Put Option Agreement by all its Parties, the Promisor shall secure an insurance policy for the benefit of it and/or the Promisor’s group to cover losses arising in respect of any breach of and claim in relation to the Business Representations set forth in the Warranty Agreement (the “W&I Insurance”).
Capitalized terms used in this Put Option Agreement and not otherwise defined shall have the meanings ascribed thereto in the form of Share Purchase Agreement attached hereto.
- 2 -
(such earlier time being the "Put Option Expiry Time").
The Promisor acknowledges that before the Beneficiary and the other Sellers are in a position to take any decision to enter into any binding agreement with respect to the Transaction:
For the purposes of this Put Option Agreement, the Consultation Process shall be deemed completed on the "Consultation Process Completion Date" which shall mean the date on which both (i) the French Works Council has been informed and consulted in connection with the contemplated Transaction pursuant to Article L. 2312-8 of the French Labor Code, as such date is described in Section 2.1(a), and (ii) the Dutch Works Council has been informed and requested for its advice in connection with the Contemplated Decision pursuant to Article 25 of the Dutch Works Councils Act (Wet op de Ondernemingsraden), as such date is described in Section 2.1(b).
- 3 -
- 4 -
- 5 -
- 6 -
- 7 -
- 8 -
The Beneficiary will provide the Promisor with a draft of the Execution Bring Down Certificate three (3) Business Days prior to the Closing Date to afford the Promisor the opportunity to review and discuss with the Sellers and the Company the draft Execution Bring Down Certificate.
- 9 -
- 10 -
- 11 -
Please acknowledge your receipt of this Put Option Agreement, and your acceptance of the benefit of the Put Option as an option to require the Promisor to purchase the Transferred Securities in accordance with the terms hereof (which may be exercised by you at your discretion and option), by countersigning this Put Option Agreement and the enclosed copy hereof in the space set forth below and returning one copy to us.
Yours faithfully,
BRUKER INVEST AG
By: /s/ JUERGEN SREGA_
Name: Mr. Juergen Srega
Title: Duly authorized
- 12 -
Acknowledged and agreed as of the date first set forth above:
TECFIN S.A.R.L.
By /s/ STEFANO DRAGO__
Name: Mr. Stefano Drago
Title: Class B Manager (Gérant de catégorie B)
By: /s/ BENJAMIN MATHU___
Name: Mr. Benjamin Mathu
Title: Class A Manager (Gérant de catégorie A)
- 13 -
Annex I
SHARE PURCHASE AGREEMENT
Annex II
WARRANTY AGREEMENT
EXHIBIT 10.30
SECURITIES PURCHASE AGREEMENT
BY AND BETWEEN
BRUKER INVEST AG,
on the one hand,
AND
THE SELLERS
IDENTIFIED HEREIN,
on the other hand,
WITH RESPECT TO
TECINVEST S.À R.L.
ELIMAN S.A.S.
ELIMAN 2 S.A.S.
Dated: February 27, 2024
- ii -
12761558_2
TABLE OF CONTENTS
Article Page
ARTICLE 1 INTERPRETATION |
3 |
|
1.1 |
Certain Definitions. |
3 |
1.2 |
Additional Definitions. |
11 |
1.3 |
Principles of Interpretation. |
13 |
1.4 |
Nature of the Sellers' Obligations. |
15 |
1.5 |
Shareholding Reorganizations and Scope of the Transaction. |
15 |
ARTICLE 2 PURCHASE AND SALE |
18 |
|
2.1 |
Agreement to Purchase and to Sell. |
18 |
2.2 |
Aggregate Consideration. |
18 |
2.3 |
Closing Purchase Price. |
18 |
2.4 |
Preliminary Closing Purchase Price. |
19 |
2.5 |
Balancing Amount. |
19 |
2.6 |
Payments made by the Purchaser to the Sellers. |
20 |
2.7 |
Repayment of Existing Indebtedness. |
20 |
2.8 |
Allocation of the Preliminary Closing Purchase Price and the Closing Purchase Price amongst the Sellers. |
21 |
2.9 |
Pre-Closing Certificate. |
21 |
2.10 |
Closing. |
22 |
2.11 |
Breach of Closing Obligations. |
25 |
ARTICLE 3 CONDITIONS PRECEDENT TO CLOSING |
26 |
|
3.1 |
Conditions Precedent. |
26 |
3.2 |
Responsibility for Satisfaction of the Regulatory Conditions. |
26 |
ARTICLE 4 REPRESENTATIONS AND WARRANTIES OF THE SELLERS |
29 |
|
4.1 |
Ownership of Securities; Sellers' Organization and Due Authorization. |
29 |
4.2 |
The Company. |
30 |
4.3 |
Eliman Entities. |
31 |
4.4 |
Group Structure. |
32 |
4.5 |
Incorporation – Existence of the Group Companies. |
33 |
4.6 |
Transaction Bonus Payments, No Transaction Costs |
33 |
ARTICLE 5 REPRESENTATIONS AND WARRANTIES OF THE PURCHASER |
33 |
|
5.1 |
Organization and Due Authorization. |
33 |
5.2 |
Governmental Authorizations. |
34 |
5.3 |
Financing. |
34 |
5.4 |
Acknowledgement. |
34 |
i
()
ARTICLE 6 PRE-CLOSING COVENANTS |
36 |
|
6.1 |
Pre-Closing Reorganizations. |
36 |
6.2 |
Ordinary Course of Business. |
37 |
6.3 |
Specific Undertakings |
39 |
6.4 |
Access and Information. |
39 |
6.5 |
Convening of Corporate Meetings. |
40 |
6.6 |
Termination of the SHAs. |
41 |
6.7 |
Interest rate period fixing for Existing Indebtedness. |
41 |
6.8 |
Public Announcements. |
41 |
6.9 |
"Know Your Client" (KYC) Requirements. |
42 |
6.10 |
Closing Bring Down Certificate |
42 |
6.11 |
Screening Tool |
43 |
6.12 |
Export Policies. |
43 |
6.13 |
Pre-Closing CC Transfers. |
44 |
6.14 |
Kreienbaum Dispute |
44 |
ARTICLE 7 POST-CLOSING COVENANTS |
45 |
|
7.1 |
Non-solicitation |
45 |
7.2 |
General solicitations |
45 |
7.3 |
Reversed CC Carve-Out |
45 |
ARTICLE 8 REPAYMENT AND INDEMNIFICATION OBLIGATIONS |
46 |
|
8.1 |
Repayment Obligations of the Sellers. |
46 |
8.2 |
Indemnification Obligations of the Purchaser. |
47 |
8.3 |
Limitations on Liability. |
47 |
8.4 |
W&I Insurance |
48 |
ARTICLE 9 TERMINATION |
49 |
|
9.1 |
Termination. |
49 |
9.2 |
Effect of Termination. |
49 |
ARTICLE 10 MISCELLANEOUS |
49 |
|
10.1 |
Sellers' Agent. |
49 |
10.2 |
Further Actions. |
50 |
10.3 |
Brokers and Finders. |
51 |
10.4 |
Records. |
51 |
10.5 |
Confidentiality. |
51 |
10.6 |
Costs and Expenses. |
53 |
10.7 |
Payments. |
53 |
10.8 |
No Hardship. |
54 |
10.9 |
Specific Performance. |
54 |
10.10 |
Express Waivers. |
54 |
10.11 |
Notices. |
54 |
10.12 |
Professional Advice. |
55 |
10.13 |
Entire Agreement. |
55 |
10.14 |
No Third Party Rights; Assignment. |
56 |
10.15 |
Severability. |
56 |
10.16 |
Waivers and Amendments. |
57 |
ii
()
10.17 |
Transfer Taxes. |
57 |
10.18 |
Purchaser's Tax election rights, Tax Refunds. |
57 |
10.19 |
Governing Law and Submission to Jurisdiction. |
57 |
10.20 |
Electronic Signature. |
58 |
Annex Page
Annex (A) Organisation Chart |
2 |
Annex (A)(bis) Security Holdings Table |
2 |
Annex (B) Company Securities |
2 |
Annex (C) Eliman 1 Securities |
2 |
Annex (D) Eliman 2 Securities |
3 |
Annex 1.1 Defined Terms and Interpretations |
3 |
Annex 1.1(bis) Data Room Documentation |
5 |
Annex 1.1(ter) Existing Financing |
6 |
Annex 1.1(quater) Existing Encumbrances |
6 |
Annex 1.1(quinquies) Required Antitrust Clearances |
9 |
Annex (b)(ii)(B) Resigning Persons |
23 |
Annex 3.2(b)(iv) Resigning Persons |
27 |
Annex 4.4(a) Group Structure Chart |
32 |
Annex 4.6(a) Transaction Bonus Payments |
33 |
Annex 6.1.1 Pre-Closing Reorganizations |
36 |
Annex 6.1.1 Pre-Closing Reorganizations |
36 |
Annex 6.2.1 Ordinary Course of Business |
37 |
Annex 6.2.1(vii) Seller's Agreements |
38 |
Annex 6.10(a) Closing Bring Down of Disclosures |
42 |
Annex 6.14(a) CC Trademark Transfer |
44 |
iii
()
SECURITIES PURCHASE AGREEMENT
THIS SECURITIES PURCHASE AGREEMENT, dated February 27, 2024 (as the same may be amended, supplemented or otherwise modified from time to time in accordance with its terms, this "Agreement"), is by and between:
1. BRUKER INVEST AG, a stock company, organized under the laws of Switzerland, having its registered office at Industriestrasse 26, Fallenden, CH-8117, Switzerland and registered with the Commercial Register of canton Zurich under the number CHE-100.756.943 (the "Purchaser"); on the one hand;
and each of:
2. TECFIN S.À R.L., a private limited liability company (société à responsabilité limitée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L – 2449 Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B 216066 ("TecFin");
3. MR. CHRISTOPH GAUER, a national of Germany residing Hagener Strasse 35, 82418 Murnau, Germany ("Christoph Gauer");
4. ELIMAN, a simplified joint stock company (société par actions simplifiée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 1A, Heienhaff, L-1736 Senningerberg, and registered with the Trade and Companies Register of Luxembourg under number B 216548 ("Eliman 1"); and
5. ELIMAN 2, a simplified joint stock company (société par actions simplifiée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 1A, Heienhaff, L-1736 Senningerberg, and registered with the Trade and Companies Register of Luxembourg under number B 216502 ("Eliman 2" and, together with Eliman 1, the "Eliman Entities").
TecFin, Christoph Gauer, Eliman 1 and Eliman 2 are hereinafter referred to collectively as the "Original Sellers" and, individually an "Original Seller", and together with the Purchaser, are hereinafter referred to collectively as the "Original Parties" and, individually an "Original Party".
RECITALS:
WHEREAS:
(A) TecInvest S.à r.l, a private limited liability company (société à responsabilité limitée) incorporated under the laws of the Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L-2449 Luxembourg, Grand Duchy of Luxembourg and registered with the Trade and Companies Register of Luxembourg (Registre de Commerce et des Sociétés de Luxembourg) under number B 194689 (the "Company"; and collectively with all its Subsidiaries other than ELITech Group B.V., and Elitech Clinical Systems SAS, the "Group Companies", the "Group" and ELITech Group B.V. and Elitech Clinical Systems SAS the "Carve-Out Companies") serves as the top holding company for the Elitech group of companies (including the Carve-Out Companies) whose organisation chart set out in Annex (A) TC "Annex (A) Organisation Chart" \f \l 2 together with true and accurate information on the allocation of its share capital as at the date of this Agreement and as at the Closing Date, in the form of the Security Holdings Table as set out in Annex (A)(bis) TC "Annex (A)(bis) Security Holdings Table" \f \l 2 ;
(B) On the date of this Agreement, the Company has issued the following securities which constitute all the securities issued by the Company (the "Company Securities"):
(1) 302,604,825 ordinary shares having a nominal value of €0.01 per share (the "Ordinary Shares");
(2) 21,786,383 series A preference shares having a nominal value of €0.01 per share and having the rights and preferences set forth in the Organizational Documents of the Company (the "Series A Preference Shares"); and
(3) 768,160,321 series A preferred equity certificates (the "Series A PECs");
which are held by the Original Sellers in the amounts indicated in Annex (B) TC "Annex (B) Company Securities" \f \l 2 .
(C) On the date of this Agreement, Eliman 1 has issued the following securities which constitute all the securities issued by Eliman 1 (the "Eliman 1 Securities"):
(1) 1,570,896 ordinary shares having a nominal value of €1 per share (the "Eliman 1 Ordinary Shares"); and
(2) 30,000 preference shares having a nominal value of €1 per share and having the rights and preferences set forth in the Organizational Documents of Eliman 1 (the "Eliman 1 Preferred Shares");
which are held by the Eliman 1 Shareholders in the amounts indicated in Annex (C) TC "Annex (C) Eliman 1 Securities" \f \l 2 .
(D) On the date of this Agreement, Eliman 2 has issued the following securities which constitute all the securities issued by Eliman 2 (the "Eliman 2 Securities"):
(1) 3,445,313 ordinary shares having a nominal value of €1 per share (the "Eliman 2 Ordinary Shares"); and
(2) 30,000 preference shares having a nominal value of €1 per share and having the rights and preferences set forth in the Organizational Documents of Eliman 2 (the "Eliman 2 Preferred Shares");
which are held by the Eliman 2 Shareholders in the amounts indicated in Annex (D) TC "Annex (D) Eliman 2 Securities" \f \l 2 .
(E) Prior to the signing of this Agreement on the date hereof (the "Signing Date"), the Purchaser and its advisers have had access to certain information concerning the Group Companies, and were able to participate in management and expert sessions concerning the Group Companies.
(F) The Sellers confirm that (i) the French social and economic committee of the Group Companies has been informed of and consulted with respect to (x) the carve-out of the Carve-out Companies and (y) the proposed sale of the relevant Group Company prior to the Signing Date and (ii) the Dutch works council at the level of Elitech Group B.V. has been informed of and requested for its advice in connection with one step of the Carve-Out Reorganizations, being the acquisition of 100% of the shares in Elitech Group B.V. by TecFin S.à.r.l. from ELITechGroup Benelux B.V.
(G) The Purchaser desires to purchase from the Sellers (as this term is defined below), and the Sellers desire to sell to the Purchaser, the Transferred Securities (as this term is defined below) in accordance with the provisions of this Agreement (the "Transaction"), being specified that concomitantly, the Parties have entered into the Warranty Agreement which forms an indivisible whole with this Agreement.
NOW, THEREFORE, the Parties hereto do hereby agree as follows:
ARTICLE 1 INTERPRETATION
1.1 Certain Definitions.
In addition to such terms as are defined elsewhere in this Agreement, wherever used in this Agreement (including the Recitals):
"Accounting Principles" shall mean the accounting principles and methodologies described in Parts 1 and 2 of Annex 1.1 TC "Annex 1.1 Defined Terms and Interpretations" \f \l 2 ;
"Adjusted Closing Statement" shall mean the closing statement for the purposes of this Agreement, in the form set out in the Part 2 of Annex 2.3, as agreed or determined in accordance with Annex 2.3;
"Affiliate" when used with reference to a specified Person, means any Person that directly or indirectly through one or more intermediaries controls, is controlled by or is under common control with the specified Person.
For such purposes, the term "control" (including the verb "to control" and the terms "controlling", "controlled by" and "under common control with") means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by Contract or otherwise; in such regard:
(a) a limited partnership shall be deemed to be controlled by its general partner(s);
(b) a "fonds professionnel de capital investissement" or a "fonds commun de placement" shall be deemed to be controlled by its management company (société de gestion);
(c) no portfolio company in which an investment fund holds, directly or indirectly, an equity investment shall be deemed to be an Affiliate of such investment fund; and
(d) after Closing, the Acquired Companies and the Group Companies shall be deemed to be Affiliates of the Purchaser;
"Allocable Portion" when used with respect to a Seller, shall mean the fraction having for numerator, the Portion of such Seller, and for denominator, as the case may be, the Preliminary Closing Purchase Price, and the Closing Purchase Price, except that:
(a) in relation to any claim made by the Purchaser that such Seller has breached any of its representations or warranties set forth in Section 4.1, such fraction shall be deemed to be equal to 100%;
(b) in relation to the Sellers' Agent Expenses, such fraction as determined by agreement between the Sellers' Agent and the CEO.
"Antitrust Clearance" shall mean the fact that, in accordance with the Antitrust Regulations, any Governmental Authority having jurisdiction with respect to the concentration resulting from the transactions contemplated by this Agreement:
(a) authorizes such concentration, or
(b) does not prevent such concentration within the applicable waiting period which is deemed to be an authorization from such Governmental Authority under the applicable Antitrust Regulations, or
(c) decides that such concentration falls outside the scope of the Antitrust Regulations;
for the avoidance of doubt, it is acknowledged that an Antitrust Clearance shall be deemed obtained even if subject to the implementation of certain commitments submitted by or required from the Purchaser or its Affiliates;
"Antitrust Regulations" shall mean any applicable Laws governing competition or antitrust pursuant to which the relevant Governmental Authority will be required to consider or review the concentration resulting from the acquisition by the Purchaser of the Transferred Securities in terms of its effect on the structure of the competition on the relevant markets; "Australian CC Assets" shall refer to the assets listed in Annex 6.15 owned by ELITechGroup Australia Pty Ltd as of the Put Option Date;
"Balancing Amount" shall mean an amount (whether positive or negative) equal to the Closing Purchase Price minus the Preliminary Closing Purchase Price;
"Business" shall mean the business of the Group Companies in the molecular diagnostics, the microbiology and the biomedical testing equipment fields, in particular the development, sale and marketing of PCR tests, assays, kits and other molecular diagnostics consumables, the sale of white-label testing machines for application in molecular diagnostics and microscopic slide stainers and osmometers as carried out as of the Put Option Date, the signing date of this Agreement and the Closing Date;
"Business Day" shall mean any day (other than a Saturday, a Sunday or a legal holiday in France, the Grand Duchy of Luxembourg, Germany, Switzerland and New York City) on which commercial banks in Paris, France; Luxembourg, Grand Duchy of Luxembourg; Frankfurt am Main, Germany; Zurich, Switzerland and New York City, New York/USA are open for business;
"Carve-Out Vendor Loan" shall have the meaning ascribed to such term in the Tax Step Plan;
"CC US Distribution Activity Value" shall mean a maximum amount of EUR 500,000; it being specified that the final amount shall be agreed between the Parties at the latest ten (10) Business Days prior to the Closing Date;
"CEO" shall mean the chief executive officer of the Company (i.e., Christoph Gauer);
"Contract" shall mean any written or oral contract, agreement, obligation, promise, commitment or other undertaking;
"COVID-19" means the SARS-CoV-2 or COVID-19 pandemic, and any evolutions thereof;
"Data Room" shall mean the electronic data room named "Elitech 2023" established by the Sellers via Intralinks and accessible for the Purchaser under https://services.intralinks.com/web/#hub/exchanges;
"Data Room Documentation" shall mean (i) the documents previously made available for the Purchaser's and its advisors' review in the Data Room, a list of which is attached as Annex 1.1(bis) TC "Annex 1.1(bis) Data Room Documentation" \f \l 2 and (ii) the written answers provided by the management of the Group Companies, the Sellers or their respective advisers to the queries made by the Purchaser and its advisers in connection with the Acquired Companies (through written Q&A or written minutes of (a) Q&A sessions, (b) experts sessions or (c) sites visits, in each case provided in the Data Room); and which, in both cases, have been copied onto a series of storage devices of which (x) one copy has been provided to the Purchaser and (y) one copy has been retained by the Sellers' Agent on the Put Option Date;
"Disclosed Information" shall mean, collectively:
(a) the management presentations provided in the Data Room Documentation and identified as:
(i) Project Channel - MP (Full) - 2023.03.13
(ii) CHANNEL Discussion Materials June 2023_07.06.2023
(iii) Project Channel - EBS Presentation - 17.06.2023 vSent
(iv) CHANNEL Turin Discussion Materials June 11 2023 vSent
(v) EG-Bruker visit R&D QARA overview 110723
(vi) CHANNEL Signes EM Discussion Materials July 18 2023 vFINAL
(vii) CHANNEL Signes EF Discussion Material July 18 2023 230717 vFINAL-3
(viii) CHANNEL Spankeren Discussion Materials July 19 2023 vFINAL
(ix) Project Channel – CC Carve-out and exit strategy as attached as Annex 6.1(a) to the SPA
(b) the Reports; and
(c) the Data Room Documentation;
in each case to the extent that the relevant fact, matter, circumstance or information regarding the Group Companies and their businesses contained therein were disclosed with sufficient detail to enable a reasonably diligent purchaser and its advisors to identify in a clear manner the nature and scope of the liability, claim, fact, matter, event or circumstance not being part of the Disclosed Information;
"Eliman 1 Shareholders" shall mean the shareholders of Eliman 1 listed in Annex (C);
"Eliman 2 Shareholders" shall mean the shareholders of Eliman 2 listed in Annex (C);
"Encumbrance" shall mean any pledge of real or personal property (nantissement or gage), mortgage (hypothèque), lien (privilège), right of retention (droit de rétention), fiducie-sûreté, charge (charge), ownership right (démembrement), seizure (saisie), escrow (séquestre) or other security (sûreté), option or other similar third party right (excluding: (x) any restrictions or limitations on transfer of any securities set forth in the Organizational Documents of the issuer of such securities, as applicable, and (y) any pledge, lien, right, charge or other security created or granted by the Purchaser or any of its Affiliates);
"Entity" shall mean any company (société), partnership (limited or general), joint venture, trust, association, economic interest group (groupement d'intérêt économique) or other organization, enterprise or entity, whether or not having a separate legal personality (personalité morale);
"Existing Financing" shall mean the financing agreements entered into by the Group Companies as borrower and evidenced by notes, bonds, debentures, debts, securities, or other similar Contracts, that will have to be repaid on the Closing, which are listed comprehensively in Annex 1.1(ter) TC "Annex 1.1(ter) Existing Financing" \f \l 2 ; "Existing Indebtedness" shall mean all outstanding and unpaid amounts owing as of the Closing Date (in principal, interest, penalties, commissions, fees and any other sums) due or becoming due by the Group Companies pursuant to, or in connection with, the Existing Financing (including, without limitation, all breakage costs due in connection with the prepayment of the Existing Financing) as determined in accordance with the terms of the Existing Financing;
"Existing Encumbrances" shall mean the Encumbrances pursuant to the Existing Financing listed in Annex 1.1(quater) TC "Annex 1.1(quater) Existing Encumbrances" \f \l 2 ;
"Foreign Investment Clearance" shall mean the fact that, in accordance with Foreign Investment Regulations, any Governmental Authority having jurisdiction with respect to a foreign investment resulting from the transactions contemplated by this Agreement (i) authorizes such foreign investment, (ii) does not prevent such foreign investment within the applicable waiting period which is deemed to be an official waiver from such Governmental Authority under applicable Foreign Investment Regulations, or (iii) decides that such foreign investment falls outside the scope of the relevant Foreign Investment Regulations; provided that, for the avoidance of doubt, it is acknowledged that a Foreign Investment Clearance shall be deemed obtained even if subject to implementation of certain commitments from the Purchaser or its Affiliates;
"Foreign Investment Regulations" shall mean any applicable Laws governing foreign investments pursuant to which the relevant Governmental Authority will be required to consider or review the foreign investment resulting from the transactions contemplated by this Agreement;
"Fundamental Representations" shall mean the representations and warranties of the Sellers set out in Article 4;
"Governmental Authority" shall mean any international, European, national, state, regional, departmental, municipal or local body with executive, legislative, judicial, regulatory, or administrative authority including any ministry, department, agency, commission, office, organization or other subdivision thereof and any Person having received delegated authority from any of the above, as well as any judicial court or tribunal of competent jurisdiction;
"Governmental Authorization" shall mean any approval, consent, permit, ruling, waiver, exemption or other authorization (including the lapse, without objection, of a prescribed time under a statute or regulation that states that a transaction may be implemented if a prescribed time lapses following the giving of notice without an objection being made) issued, granted, given or otherwise made available by or under the authority of any Governmental Authority (including, for the avoidance of doubt, the Governmental Authorities competent for the obtaining of the Required Regulatory Clearances but excluding, for the avoidance of doubt, any Foreign Investment Clearance and any Antitrust Clearance not explicitly listed in Annex 1.1Quater or 1.1Quinquies) or pursuant to any other Law;
"Individual Net Purchase Price" when used with respect to a Seller, shall mean an amount equal to (i) the Portion of such Seller, (ii) minus its Allocable Portion of the Sellers' Agent Expenses (including any VAT applicable to such Seller), which may be deducted from its Portion in accordance with Section 2.10(b); "Insolvency Proceeding" shall mean (i) in the case of an Entity, any bankruptcy (faillite), insolvency, winding-up, judicial or voluntary liquidation, moratorium, controlled management (gestion contrôlée), suspension of payment (sursis de paiement), voluntary arrangement with creditors (concordat préventif de la faillite), fraudulent conveyance, general settlement with creditors, reorganisation or similar order or Proceeding affecting the rights of creditors generally, as provided for under the respective applicable jurisdiction and (ii) in the case of a natural person, any personal bankruptcy or similar Proceeding applicable to individuals;
"Judgment" shall mean any award, decision, injunction, judgment, order or ruling entered, issued, made or rendered by any court, administrative agency or other Governmental Authority or by any arbitrator;
"Law" shall mean any treaty, convention, directive, law, ordinance, decree (décret), regulation (règlement), instruction, order (arrêté), rule (circulaire), principle of common law or code of any Governmental Authority (including any judicial or administrative interpretation thereof) in force;
"Long Stop Date" means 15 September, 2024 or any other date agreed upon in writing by the Purchaser and the Sellers' Agent;
"Loss" shall mean any damages (dommages) or losses (pertes) (including any reasonable attorney fees, procedural expenses and expenses) (but excluding any damages or losses which are contingent (as long as they are contingent), potential, reasonably unforeseeable consequential or punitive, any loss of future revenues, income or profits (manque à gagner), any loss of opportunity (perte d'une chance) and any diminution of value); it being specified that no price/earnings or similar multiplier or valuation factor (whether or not implicit in the Closing Purchase Price) shall be used for purposes of computing the amount of any Loss suffered by the Purchaser or any of the Acquired Companies;
"ordinary course of business" (or similar expressions) means the ordinary course of the operations of the Group Companies consistent with past practices and conduct (being specified that any specific change in the ordinary course of the operations of the Group Companies triggered directly by a Pandemic Measure shall be considered as ordinary course of business as long as such Pandemic Measure is applicable, but no longer afterwards);
"Organizational Documents" shall mean when used with respect to (x) any company (société) or other incorporated Entity, the memorandum and articles of association (statuts), charter or similar constitutive document of such company (société) or other incorporated Entity, as the same may be amended, supplemented or otherwise modified from time to time, and (y) any partnership or other unincorporated Entity, its certificate of formation, partnership agreement, governing agreement (contrat constitutif) or similar constitutive document, as the same may be amended, supplemented or otherwise modified from time to time;
"Pandemic Measures" means any mandatory quarantine, "shelter in place", "stay at home", workforce reduction, social distancing, shut down, closure, sequester or any other mandatory Law, order, directive, guidelines or recommendations by any Governmental Authority taken as a direct result of the COVID-19 situation; "Phase 2 Review" shall mean an in-depth investigation by a Governmental Authority of (x) the concentration resulting from the transactions contemplated by this Agreement under any applicable Antitrust Regulations or (y) the transactions contemplated by this Agreement under any applicable Foreign Investment Regulations;
"Person" shall mean a natural person, Entity, or Governmental Authority;
"PIK Debt Release Letter" means a pay-off and release letter in customary form setting out the mechanics for the repayment of all amounts outstanding under the PIK Debt and the release of the Encumbrances pursuant to the PIK Debt;
"PIK Debt" means the PIK facility agreement dated 2 August 2021 entered into by the Seller as borrower, which will be repaid on Closing and any other finance documents entered into in connection therewith;
"Pivot Account" shall mean the single bank account opened by the Sellers' Agent in the name of the Sellers, the details of which shall be notified by the Sellers' Agent to the Purchaser in the Pre-Closing Certificate;
"Portion", when used with respect to a Seller, shall mean the portion of the Preliminary Closing Purchase Price, or the Closing Purchase Price allocated to the Transferred Securities owned by such Seller in accordance with Section 2.3;
"Proceeding" shall mean any litigation, arbitration, dispute or other legal proceeding commenced, brought, conducted or heard by or before any Governmental Authority or arbitrator;
"Put Option Date" shall mean December 23, 2023;
"Receivable 1" shall have the meaning ascribed to such term in the Tax Step Plan and the Delegation Agreement 1;
"Receivable 2" shall have the meaning ascribed to such term in the Tax Step Plan and the Delegation Agreement 2;
"Representations" shall mean the Fundamental Representations together with the Business Representations;
"Reports" means; collectively:
(a) the tax fact book relating to the Transaction dated July 4, 2023 prepared by Hogan Lovells LLC; and
(b) the financial vendor due diligence report dated May 3, 2023 and its addendum dated June 5, 2023 prepared by Deloitte SAS relating to relating to the Transaction;
"Required Antitrust Clearances" shall mean the Antitrust Clearances listed in Annex 1.1(quinquies) TC "Annex 1.1(quinquies) Required Antitrust Clearances" \f \l 2 ; "Required Foreign Investment Clearances" shall mean the Foreign Investment Clearances listed in Annex 1.1(quinquies);
"Required Regulatory Clearances" shall mean, collectively, the Required Antitrust Clearances and the Required Foreign Investment Clearances;
"Restricted Payee" shall mean (i) in relation to any Seller which is an Entity, the Seller and its Affiliates, and (ii) in relation to any Seller which is a natural Person, the Seller and his or her Affiliates and his or her spouse, civil partner, ascendant or descendant; for the avoidance of doubt, "Restricted Payees" shall not include the Acquired Companies;
"Security Holdings Tables" shall mean the security holdings tables set forth in Annex (A)(bis), Annex (B), Annex (C) and Annex (D) (as such Annexes shall be updated by the Sellers' Agent to reflect any transfers of Company Securities, Eliman 1 Securities or Eliman 2 Securities, in accordance with the provisions thereof and notably the Shareholding Reorganizations);
"Sellers' Agent Expenses" shall mean the fees and expenses (including VAT or other similar charges, to the extent applicable) incurred by the Sellers' Agent on behalf of the Sellers in connection with the Transaction, as set out in the Pre-Closing Certificate;
"SHAs" shall mean the following securityholders' agreements:
(a) the securityholders agreement relating to the Company dated 25 July 2017 (as amended) entered into by and between, inter alia, the Original Sellers (the "Company SHA"); and
(b) the Manco securityholders agreement relating to the Eliman Entities dated November 10, 2017 (as amended from time to time) entered into by and between, inter alia, TecFin and the Eliman Shareholders (the "Manco SHA");
"Subsidiary" shall mean (i) any Entity of which more than 50% of the issued share capital and voting rights exercisable at a shareholders' meeting are at the time owned, directly or indirectly through one or more intermediaries, or both, by the Company, and, only with respect to the Representations, (ii) any Entity of which more than 50% of the issued share capital and voting rights exercisable at a shareholders' meeting are at the time owned, directly or indirectly through one or more intermediaries, or both, by the Company (excluding the Carve-Out Companies as of completion of the Carve-out Reorganizations);
"Tax" shall mean: (i) all forms of taxation, duties, imposts, levies, value added tax and contributions, including all social security charges, social and insurance contributions and employment and payroll taxes, and any associated interest, penalty, surcharge or fine; (ii) any liability for any amount of the types described in clause (i) as a result of being a member or a former member of a Tax consolidated group or similar arrangement or agreement and (iii) any liability for the payment of any amount of the types described in (i) or (ii) above as a result of being a transferee or successor to any person, or as a secondary liability, or due on behalf of third parties, or as a result of any obligation to indemnify any other person;
"Tax Benefit", with respect to any Loss, shall mean the aggregate of:
(a) the amount equal to any cash-effective Tax saving or benefit (other than VAT) arising directly out of such Loss (such as Tax reduction, Tax credit or Tax deduction or any creation or increase of carried back or forward Tax losses) for any Acquired Company or for any tax consolidated group or fiscal unity to which any Acquired Company belongs; and
(b) the amount of VAT which is actually recovered or can be offset by any Acquired Company as a direct result of such Loss;
"Ukraine Situation" shall mean any act of war (declared or not) or terrorism, military actions, armed hostilities, happening in Ukraine since February 24, 2022;
"US CC Activity" shall mean the distribution of clinical chemistry products (the "CC Products") and the manufacturing of buffers, washing solutions and accessories in relation with the CC Products in the United States of America conducted, as of the Put Option Date, by ELITechGroup Inc.;
"Vendor Loan 1" shall have the meaning ascribed to such term in the Tax Step Plan;
"Vendor Loan 2" shall have the meaning ascribed to such term in the Tax Step Plan;
"Vendor Loan 1 Amount" shall mean the amount of the Vendor Loan 1 which is equal to the amount of the Carve-Out Vendor Loan, it being understood that the Vendor Loan 1 Amount does not qualify as Cash, an offset to Indebtedness, nor Net Working Capital;
"Vendor Loan 2 Amount" shall mean the amount of the Vendor Loan 2 which is equal to the amount of the CC US Distribution Activity Value, it being understood that the Vendor Loan 2 Amount does not qualify as Cash, an offset to Indebtedness, nor Net Working Capital;
"W&I Insurer" shall mean Euclid Transactional;
"Warranty Agreement" shall mean the warranty agreement entered into by the Sellers and the Purchaser on the date hereof in connection with the Transaction;
"Working Hours" shall mean 9.00 a.m. to 6.00 p.m. on a Business Day.
1.2 Additional Definitions.
Each of the following additional terms has the meaning assigned thereto in the Section or Annex indicated below opposite such term:
Term Section
"Acquired Companies" 1.5.4
"Agreement" Parties' presentation
"Antitrust Condition" 3.1(a)
"Australian CC Asset Transfer" 6.13(a)(i)
"Australian CC Transfer" 6.13(a)(i)
"Base Closing Purchase Price" 2.3(a)(i)
"Business Representations" 8.4(a)
"Business Representations Cap" 8.4(b)
"Carve-Out Companies" Recitals (A)
"Carve-Out Reorganizations" Annex 6.1
"CC Trademark Transfers" 6.13(a)(ii)
"CC Transfers" 6.13(a)(ii)
"Christoph Gauer" Parties' presentation
"Closing" 2.10(a)
"Closing Bring Down Certificate" 6.10(a)
"Closing Bring Down of Disclosures" 6.10(a)
"Closing Date" 2.10(a)
"Closing Payments" 2.10(b)
"Closing Purchase Price" 2.3
"Company" Recitals (A)
"Company Securities" Recitals (B)
"Confidentiality Agreement" 6.4(a)
"Debt Release Letters" 2.7(c)
"Eliman 1" Parties' presentation
"Eliman 1 Ordinary Shares" Recitals (C)
"Eliman 1 Preferred Shares" Recitals (C)
"Eliman 1 Securities" Recitals (C)
"Eliman 2" Parties' presentation
"Eliman 2 Ordinary Shares" Recitals (D)
"Eliman 2 Preferred Shares" Recitals (D)
"Eliman 2 Securities" Recitals (D)
"Eliman Entities" Parties' presentation
"Enterprise Value" 2.3(a)(i)
"Extended Long Stop Date" 3.2(e)
"Foreign Investment Condition" 3.1(a)(ii)
"Group" Recitals (A)
"Group Companies" Recitals (A)
"Group Shares" 4.4(a)
"Group Structure Chart" 4.4(a)
"Hitachi" 3.1(a)(iii)
"Know Your Client" 6.9
"Ordinary Shares" Recitals (B)
"Original Parties" Parties' presentation
"Original Party" Parties' presentation
"Original Seller" Parties' presentation
"Original Sellers" Parties' presentation
"Parties" 1.5.2
"Party" 1.5.2
"Pending Dispute" 6.14(a)
"PIK Amount" 2.6
"Pre-Closing Certificate" 2.9
"Preliminary Closing Purchase Price" 2.4
"projections" 5.4(d)
"PSS" 6.3(b)
"Purchaser" Parties' presentation
"Regulatory Conditions" 3.1(a)(ii)
"Resigning Persons" 2.10(b)(i)(B)
"Restricted Persons" 7.1(b)
"Reversed CC Carve-Out" 7.3
"Sellers" 1.5.2
"Sellers' Agent" 10.1(a)
"Sellers' Representatives" 5.4(c)
"Series A PECs" Recitals (B)
"Series A Preference Shares" Recitals (B)
"Signing Date" Recitals (E)
"Share Registers" 2.10(b)(i)(A)
"TecFin" Parties' presentation
"Transaction" Recitals (G)
"Transferred Securities" 1.5.3
"US CC Activity Transfer" 6.13(a)(i)
"W&I Insurance" 8.4(a)
"W&I Insurer" 8.4(a)
1.3 Principles of Interpretation.
In this Agreement:
(a) All references herein to Articles, Sections, Schedules and Annexes shall be deemed references to articles and sections of, and schedules and annexes to this Agreement unless the context shall otherwise require. The descriptive headings to Articles, Sections, Schedules and Annexes are inserted for convenience only, and shall have no legal effect.
(b) The Annexes and Schedules to this Agreement shall be deemed to be a part of this Agreement, and references to "this Agreement" shall be deemed to include the same.
(c) The timeframes specified in this Agreement shall be calculated in accordance with Articles 640 to 642 of the French Civil Procedure Code, on the understanding that the references in Article 642 to ‘a public holiday or non-business day' and to the "first business day" ("un jour férié ou chômé" and "premier jour ouvrable") must be construed with reference to the definition of the expression ‘Business Day' set out herein.
(d) The following rules of interpretation shall apply unless the context shall require otherwise:
(i) Definitions used in this Agreement shall apply equally to both the singular and plural forms of the terms defined as well as to any gender. Pronouns of the masculine gender shall be deemed to include the feminine and neuter, and vice versa, as the context may require.
(ii) Whenever used in this Agreement:
(A) the words "include", "includes" and "including" shall be deemed to be followed by the phrase "without limitation";
(B) the words "hereof", "herein" and similar words shall be construed as references to this Agreement as a whole and not just to the particular Section or subsection in which the reference appears; and
(C) except when used with the word "either", the word "or" may have a disjunctive and not alternative meaning (i.e., where two items or qualities are separated by the word "or", the existence of one item or quality shall not be deemed to be exclusive of the existence of the other and, as the context may require, the word "or" may be deemed to include the word "and").
(iii) A reference to a specific time of day shall be to local time in Paris, France.
(iv) A reference to any Party to this Agreement or any other agreement or document includes such Party's successors and permitted assigns.
(v) A reference to any agreement or document is to that agreement or document as amended, novated, supplemented, varied or replaced from time to time, except to the extent prohibited by this Agreement.
(vi) A reference to any legislation or to any provision of any legislation includes any modification or re-enactment of such legislation, any legislative provision substituted for such legislation, and all regulations and statutory instruments issued under such legislation.
(vii) Each Party hereby expressly and irrevocably waives the provisions of articles 1190 and 1602 of the French Civil Code (Code Civil).
(viii) Unless specifically provided for in this Agreement, there shall be no requirement for a prior notice (mise en demeure préalable) for one Party to claim any right or implement any remedy, including termination of this Agreement, provided for hereunder and the Parties expressly waive the requirement for such a prior notice.
(ix) An obligation to use commercially reasonable, reasonable or best efforts or endeavors (or any similar obligation) shall be construed as an "obligation de moyens".
(x) When a French term is translated into English, such translation is for information purposes only and the French term shall prevail.
(xi) Any reference to any accounting term not specifically defined shall have the meaning of such accounting term under IFRS.
(xii) Unless the context otherwise requires, any reference to a statutory provision shall include such provision as it exists and is construed as of the Put Option Date.
1.4 Nature of the Sellers' Obligations.
The representations, warranties, covenants, agreements and other undertakings of the Sellers set forth in this Agreement, including the obligations of the Sellers set forth in Article 8 (Repayment and Indemnification Obligations), are all given or made by the Sellers severally but not jointly (conjointement mais non solidairement) for all purposes of this Agreement.
1.5 Shareholding Reorganizations and Scope of the Transaction.
1.5.1 Scope.
On the Closing Date:
(a) if the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, TecFin shall sell and deliver to the Purchaser and the Purchaser shall purchase from TecFin all of the Company Securities (including those previously held by Christoph Gauer other than Company Securities held by Eliman 1 and Eliman 2), all Eliman 1 Securities and all Eliman 2 Securities, pursuant to the terms of this Agreement,
(b) if the CG Shareholding Reorganization is completed but the Eliman Shareholding Reorganizations are not completed, (x) TecFin shall sell and deliver to the Purchaser and the Purchaser shall purchase from TecFin all of the Company Securities (including those previously held by Christoph Gauer) other than the Company Securities held by Eliman 1 and Eliman 2, (y) Eliman 1 shall sell and deliver to the Purchaser and the Purchaser shall purchase from Eliman 1 all of the Company Securities held by Eliman 1, and (z) Eliman 2 shall sell and deliver to the Purchaser and the Purchaser shall purchase from Eliman 2 all of the Company Securities held by Eliman 2, pursuant to the terms of this Agreement,
(c) if the CG Shareholding Reorganization is not completed but the Eliman Shareholding Reorganizations are completed, (x) TecFin shall sell and deliver to the Purchaser and the Purchaser shall purchase from TecFin all of the Company Securities held by TecFin (other than the Company Securities held by Eliman 1 and Eliman 2), all Eliman 1 Securities and all Eliman 2 Securities, and (y) Christoph Gauer shall sell and deliver to the Purchaser and the Purchaser shall purchase from Christoph Gauer all of the Company Securities held by Christoph Gauer, pursuant to the terms of this Agreement,
(d) if none of the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, (w) TecFin shall sell and deliver to the Purchaser and the Purchaser shall purchase from TecFin all of the Company Securities held by TecFin, (x) Christoph Gauer shall sell and deliver to the Purchaser and the Purchaser shall purchase from Christoph Gauer all of the Company Securities held by Christoph Gauer, (y) Eliman 1 shall sell and deliver to the Purchaser and the Purchaser shall purchase from Eliman 1 all of the Company Securities held by Eliman 1, and (z) Eliman 2 shall sell and deliver to the Purchaser and the Purchaser shall purchase from Eliman 2 all of the Company Securities held by Eliman 2, pursuant to the terms of this Agreement.
1.5.2 Sellers and Parties.
(a) As of the Signing Date, the term "Sellers" shall mean TecFin, Christoph Gauer, Eliman 1 and Eliman 2, for all purposes of this Agreement.
(b) As of the Closing Date:
(i) if the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, (x) the term "Sellers" shall mean TecFin, and (y) the Purchaser and TecFin shall refer collectively to the "Parties" and individually to a "Party", for all purposes of this Agreement,
(ii) if the CG Shareholding Reorganization is completed but the Eliman Shareholding Reorganizations are not completed, (x) the term "Sellers" shall mean TecFin, Eliman 1 and Eliman 2, and (y) the Purchaser, TecFin, Eliman 1 and Eliman 2 shall refer collectively to the "Parties" and individually to a "Party", for all purposes of this Agreement,
(iii) if the CG Shareholding Reorganization is not completed but the Eliman Shareholding Reorganizations are completed, (x) the term "Sellers" shall mean TecFin and Christoph Gauer, and (y) the Purchaser, TecFin and Christoph Gauer shall refer collectively to the "Parties" and individually to a "Party", for all purposes of this Agreement,
(iv) if none of the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, (x) the Original Sellers shall be the "Sellers", and (y) the Original Parties shall be the "Parties", for all purposes of this Agreement.
1.5.3 Transferred Securities.
(a) As of the Signing Date, the term "Transferred Securities" shall mean the Company Securities held by TecFin, Christoph Gauer, Eliman 1 and Eliman 2 for all purposes of the Agreement.
(b) As of the Closing Date:
(i) if the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, the term "Transferred Securities" shall mean the Company Securities, the Eliman 1 Securities and the Eliman 2 Securities held by TecFin for all purposes of the Agreement,
(ii) if the CG Shareholding Reorganization is completed but the Eliman Shareholding Reorganizations are not completed, the term "Transferred Securities" shall mean the Company Securities held by TecFin, Eliman 1 and Eliman 2 for all purposes of the Agreement,
(iii) if the CG Shareholding Reorganization is not completed but the Eliman Shareholding Reorganizations are completed, the term "Transferred Securities" shall mean the Company Securities, the Eliman 1 Securities and the Eliman 2 Securities held by TecFin and the Company Securities held by Christoph Gauer for all purposes of the Agreement,
(iv) if none of the CG Shareholding Reorganization and the Eliman Shareholding Reorganizations are completed, the term "Transferred Securities" shall mean the Company Securities held by TecFin, Christoph Gauer, Eliman 1 and Eliman 2 for all purposes of the Agreement.
1.5.4 Acquired Companies.
(a) As of the Signing Date, the term "Acquired Companies" shall mean the Group Companies for all purposes of this Agreement.
(b) As of the Closing Date:
(i) if the Eliman Shareholding Reorganizations are completed, the term "Acquired Companies" shall mean the Group Companies, Eliman 1 and Eliman 2 for all purposes of this Agreement; and
(ii) if the Eliman Shareholding Reorganizations are not completed, the term "Acquired Companies" shall mean the Group Companies, for all purposes of this Agreement.
ARTICLE 2 PURCHASE AND SALE
2.1 Agreement to Purchase and to Sell.
Upon the terms and subject to the conditions set forth in this Agreement (including the Regulatory Conditions), at the Closing, the Purchaser shall purchase from each Seller, and each Seller shall sell and deliver to the Purchaser, full and valid title to all but not less than all the Transferred Securities it holds set forth opposite each Seller's name in the Security Holdings Tables, free and clear of all Encumbrances, together with all rights and benefits attaching thereto at the Closing, provided that the Purchaser shall not be required to purchase less than all of the Transferred Securities, thus allowing the Purchaser to have full and valid title, directly or indirectly, to 100% of the Company Securities immediately following the Closing.
2.2 Aggregate Consideration.
The aggregate consideration to be paid for all the Transferred Securities shall be equal to the Closing Purchase Price (as defined below), calculated and paid in accordance with Sections 2.3 to 2.10.
2.3 Closing Purchase Price.
(a) The aggregate consideration to be paid for all the Transferred Securities (the "Closing Purchase Price") shall be an amount equal to:
(i) 870,000,000 euros (the "Enterprise Value") minus an amount of 6,000,000 euros to cover certain risks to result in an amount of 864,000,000 (eight hundred sixty-four million) euros (the "Base Closing Purchase Price");
(ii) plus, the Cash;
(iii) minus, the Indebtedness;
(iv) plus, the amount, if positive, equal to the Net Working Capital minus the Average Net Working Capital, or
(v) minus, the amount, if positive, equal to the Average Net Working Capital minus the Net Working Capital,
(vi) plus, the Vendor Loan 1 Amount,
(vii) plus, the Vendor Loan 2 Amount,
all items from (i) to (v) as determined in accordance with Annex 2.3 (Adjusted Closing Statement) and provided that (i) the Vendor Loan 1 Amount is subject to the Delegation Agreement 1 having been concluded, and (ii) the Vendor Loan 2 Amount is subject to the Delegation Agreement 2 having been concluded.
(b) The Closing Purchase Price shall be final and binding on the Parties and shall not be subject to any adjustment whatsoever, except as provided under Article 8 (Repayment and Indemnification);
(in which case the term "Closing Purchase Price" shall refer to the Closing Purchase Price so adjusted).
2.4 Preliminary Closing Purchase Price.
(a) The preliminary closing purchase price (the "Preliminary Closing Purchase Price") to be paid for all the Transferred Securities on the Closing Date shall be determined as follows:
(i) the Base Closing Purchase Price,
(ii) plus, estimated Cash as at the Closing Date (as defined in Annex 1.1),
(iii) minus, the estimated Indebtedness as at the Closing Date (as defined in Annex 1.1),
(iv) plus, the amount, if positive, equal to the estimated Net Working Capital as at the Closing Date minus the Average Net Working Capital (both as defined in Annex 1.1); or
(v) minus, the amount, if positive, equal to the Average Net Working Capital minus the estimated Net Working Capital as at the Closing Date,
(vi) plus, the Vendor Loan 1 Amount,
(vii) plus, the Vendor Loan 2 Amount,
provided that (i) the Vendor Loan 1 Amount is subject to the Delegation Agreement 1 having been concluded and (ii) the Vendor Loan 2 Amount is subject to the Delegation Agreement 2 having been concluded.
(b) Any payment to be made under Section 2.4 shall be made in cash (with the exception of (i) the Vendor Loan 1 Amount referred to in Section 2.4(a)(vi) that shall exclusively be settled at Closing by way of a set-off against the Receivable 1, and (ii) the Vendor Loan 2 Amount referred to in Section 2.4(a)(vii) that will exclusively be settled by way of a set-off in accordance with the Tax Step Plan) with respect to the portion of the Closing Purchase Price allocable to the Transferred Securities in compliance with the provisions of Section 2.6.
2.5 Balancing Amount.
(a) After final agreement on, or final determination of, the Adjusted Closing Statement in accordance with Annex 2.3:
(i) if the Balancing Amount is positive, the Purchaser shall pay to the Sellers an amount equal to the Balancing Amount in accordance with Section 2.5(b);
(ii) if the Closing Purchase Price is equal to the Preliminary Closing Purchase Price, no payment shall be made by either the Sellers or the Purchaser; or
(iii) if the Balancing Amount is negative, the Sellers shall pay to the Purchaser an amount equal to the absolute value of the Balancing Amount in accordance with Section 2.5(b)
(b) Any payment to be made under Section 2.5 shall be made in compliance with the provisions of Section 2.6.
2.6 Payments made by the Purchaser to the Sellers.
(a) Any payment to be made by the Purchaser under Sections 2.4 and 2.5 shall be made by electronic transfer of immediately available funds to the Pivot Account, except (i) the Vendor Loan 1 Amount that shall be settled at Closing by way of set-off against the Receivable, (ii) the Vendor Loan 2 Amount that will be settled by way of set-off in accordance with the Tax Step Plan, and (iii) the portion of the Preliminary Closing Purchase Price corresponding to the amount to be repaid under the PIK Debt, as indicated in the PIK Debt Release Letter (the "PIK Amount") that shall be paid on a specific bank account opened in the name of the Sellers or the PIK Agent (as provided under the PIK Debt Release Letter) and pledged to the lenders under the PIK Debt, which coordinates shall be set forth in the PIK Debt Release Letter or the Pre-Closing Certificate, as the case may be, on the understanding that the coordinates of such Pivot Account shall be set forth:
(i) in the Pre-Closing Certificate with respect to the Preliminary Closing Purchase Price; or
(ii) ten (10) Business Days in advance of the relevant due date in all other instances.
(b) The Sellers hereby represent that they shall:
(i) distribute, as applicable, the Preliminary Closing Purchase Price or the Closing Purchase Price amongst themselves; and,
(ii) subject to receipt (as evidenced in the manner indicated in Section 2.10) of the relevant payment by the Sellers, as apportioned on the Pivot Account, release the Purchaser from all liability in respect of each relevant payment and undertake not to exercise any remedy in this respect.
2.7 Repayment of Existing Indebtedness.
(a) The Purchaser acknowledges that the Existing Indebtedness will become repayable on the Closing Date in accordance with its terms as a consequence of the sale of the Transferred Securities.
(b) In addition to the payment of the Purchase Price, on the Closing Date, the Purchaser shall:
(i) repay and discharge (or procure that its Affiliates repay) the full amount of the Existing Indebtedness on behalf of the relevant Group Companies, with value date (date de valeur) on the Closing Date; or
(ii) make available to the Group Companies, in immediately available cleared funds, amounts sufficient to enable them to repay in full the Existing Indebtedness with value date (date de valeur) on the Closing Date and procure that the Group Companies repay such Existing Indebtedness, in immediately available cleared funds, with value date (date de valeur) on the Closing Date in accordance with the terms of the Existing Financing; in each case in accordance with the Debt Release Letters delivered to the Purchaser pursuant to this Agreement.
(c) The Purchaser shall reasonably cooperate with the Sellers' Agent, and the Sellers' Agent shall, as of the Signing Date, use all commercially reasonable efforts to obtain (or cause the Group Companies to obtain) the letters from the agent under the Existing Indebtedness which must (i) specify the amounts to be repaid by the Group Companies in connection with the repayment as at the Closing Date of all Existing Indebtedness (in principal, accrued and compound interest, prepayment indemnities charges), (ii) the bank account (including full IBAN details) on which such repayment shall be made, and (iii) irrevocably release, on the Closing Date, any and all Existing Encumbrances subject only to the full repayment of the sums payable under Section 2.7(b)(i) above (together the "Debt Release Letters"). The Sellers shall provide the Purchaser with final drafts of the Debt Release Letters no later than two (2) Business Days prior to the Closing.
2.8 Allocation of the Preliminary Closing Purchase Price and the Closing Purchase Price amongst the Sellers.
(a) The Preliminary Closing Purchase Price and the Closing Purchase Price shall be allocated, by the Sellers' Agent, amongst the Sellers and class of Transferred Securities based upon the number and class of Transferred Securities sold by each Seller to the Purchaser as determined in the Pre-Closing Certificate in accordance with the provisions set forth in this Agreement.
(b) The allocation of the Preliminary Closing Purchase Price and the Closing Purchase Price and, for the avoidance of doubt, the allocation of the Sellers' Agent Expenses, are the sole and exclusive responsibility of the Sellers and the Purchaser shall incur no liability whatsoever in respect thereof.
2.9 Pre-Closing Certificate.
Not less than ten (10) Business Days prior to the Closing Date, the Sellers' Agent shall deliver a certificate (the "Pre-Closing Certificate") to the Purchaser setting forth, by reference to a stated Closing Date:
(a) the Preliminary Closing Purchase Price determined as provided in Section 2.4 and indicating:
(i) the Sellers' good faith estimate of the Cash, such estimate to be made with the due care of a prudent businessman as at the Closing Date;
(ii) the Sellers' good faith estimate of the Indebtedness, such estimate to be made with the due care of a prudent businessman as at the Closing Date;
(iii) the Sellers' good faith estimate of the Net Working Capital, such estimate to be made with the due care of a prudent businessman as at the Closing Date; (iv) the difference between the Average Net Working Capital and the Sellers' good faith estimate of the estimated Net Working Capital;
(b) for each Seller, its Individual Net Purchase Price;
(c) each Seller's Allocable Portion;
(d) the details of the Pivot Account to which the Purchaser must transfer the Preliminary Closing Purchase Price;
(e) a copy of the Security Holdings Tables, as amended to reflect any transfers of Transferred Securities (upon completion of the Shareholding Reorganizations, if any) up to the date of the Pre-Closing Certificate;
(f) the amount of the Carve-Out Vendor Loan and the corresponding amount of the Vendor Loan 1 Amount and the Receivable 1 as at the Closing Date;
(g) the final amount of the CC US Distribution Activity Value agreed between the Parties and the corresponding amount of the Vendor Loan 2 Amount as at the Closing Date; and
(h) draft copies of the Debt Release Letters and PIK Debt Release Letter (stating the final payment amounts).
The Purchaser shall be entitled to waive delivering of the Pre-Closing Certificate in whole or in part by written notice to the Sellers’ Agent.
2.10 Closing.
(a) Provided that (x) the conditions to the respective obligations of the Parties set forth in Section 3.1 are satisfied, (y) this Agreement has not been previously terminated pursuant to Section 9.1, and (z) Seller's Agent having delivered the Pre-Closing Certificate (providing for all matters, statements and evidence set out in Section 2.9) to the Purchaser, the consummation of the purchase and sale of the Transferred Securities (the "Closing") shall be held at the offices of TecFin, Luxembourg, Grand Duchy of Luxembourg at 9 a.m. on the date which is the last Business Day of the calendar month in which the satisfaction of the last condition to Closing set forth in Section 3.1 occurred, or, if such condition is satisfied less than ten (10) Business Days prior to such date, on the last Business Day of the calendar month following the calendar month in which the last condition to Closing set forth in Section 3.1 occurred, or at such other location, time of day or date as the Purchaser and the Sellers' Agent may agree in writing. The date on which the Closing shall take place is referred to herein as the "Closing Date".
(b) At Closing, the following payments (the "Closing Payments") shall be made upon receipt of the executed Debt Release Letters and PIK Debt Release Letter:
(i) Sellers' Agent Expenses. The Sellers hereby expressly authorize the Sellers' Agent to withhold and deduct, from that part of the Preliminary Closing Purchase Price to be received by each of them, their Allocable Portion of the Sellers' Agent Expenses, and give an irrevocable instruction to the Sellers' Agent to pay on their behalf the Sellers' Agent Expenses directly to the Sellers' advisors.
(ii) Payment of the Preliminary Closing Purchase Price and Sellers' Agent Expenses. At Closing:
(A) the Purchaser shall pay the Preliminary Closing Purchase Price in accordance with Section 2.4;
(B) the Sellers' Agent shall pay the Sellers' Agent Expenses to the Sellers' advisors using the cash on the Pivot Account by wire transfers of immediately available cleared funds (free of any bank or other charges) to such accounts of the Seller's advisors, in accordance with Section 2.10(b)(i) above.
(iii) Repayment of the Existing Indebtedness. At Closing, the Purchaser shall, in accordance with the Debt Release Letters:
(A) repay in full the Existing Indebtedness on behalf of the relevant Acquired Company in immediately available cleared funds, with value date (date de valeur) on the Closing Date or make available to the relevant Acquired Company, in immediately available cleared funds, amounts sufficient to enable it to repay in full the Existing Indebtedness with value date (date de valeur) on the Closing Date; or
(B) procure that the relevant Acquired Company repays the Existing Indebtedness, in immediately available cleared funds, with value date (date de valeur) on the Closing Date in accordance with the terms of the Existing Financing.
(c) At Closing:
(i) the Sellers shall:
(A) register the Transferred Securities in the name of the Purchaser in the relevant register of each of the Company and of the Eliman Entities, as the case may be, to the extent that the Eliman Shareholding Reorganizations have been completed (the "Share Registers") on the Closing Date and deliver to the Purchaser a copy of such updated Share Registers certified by an authorized representative of the Company and such Eliman Entities (as the case may be);
(B) deliver to the Purchaser resignation letters duly signed by each of the persons listed on Annex 2.10(b)(i)(B) TC "Annex (b)(ii)(B) Resigning Persons" \f \l 2 (the "Resigning Persons"), in the form of Annex 2.10(b)(i)(B) Bis, evidencing his resignation from the positions indicated on Annex 2.10(b)(i)(B), effective on the Closing Date;
(C) deliver to the Purchaser the Closing Bring Down Certificate in the form set forth in Annex 6.10(a);
(D) deliver to the Purchaser FIRPTA compliance forms duly signed by the respective Group Companies;
(E) deliver to the Purchaser evidence reasonably satisfactory to the Purchaser of Mr Christoph Gauer having resigned as officer and/or director or any other office or employment with any of the Carve-Out Companies;
(F) if applicable, deliver to the Purchaser evidence of the completion of the Shareholding Reorganizations in accordance with Section 6.1;
(G) deliver to the Purchaser the Carve-Out Documentation;
(H) deliver to the Purchaser executed versions of the Debt Release Letters and PIK Debt Release Letter;
(ii) upon completion of the registration referred to in Section 2.10(c)(i)(A) and upon receipt of the resignation letters referred to in Section 2.10(c)(B), the Purchaser shall hold a shareholders' meeting or pass shareholders' resolutions of the relevant Acquired Companies incorporated in Luxembourg approving the following matters:
(A) acknowledgement of the resignations of the relevant Resigning Persons;
(B) grant of provisional, irrevocable and unconditional discharge to the relevant Resigning Persons for the execution of their mandates and the performance of their duties until the Closing Date, except, however, for cases of fraud or willful misconduct;
(C) change the registered office of the relevant Acquired Companies;
(D) with respect to Acquired Companies, undertaking to proceed to any registration with the Trade and Companies Register of Luxembourg and to any publication on the Recueil électronique des Sociétés et Associations as required by law; and;
(iii) the Purchaser shall deliver to the Sellers, the Carve-Out Documentation;
(iv) the Purchaser shall cause all shareholders of the Acquired Companies from which Resigning Persons resign at Closing to vote at the annual shareholders' meetings of the relevant Acquired Companies approving the annual accounts closing on December 31, 2023 (or at any such earlier date if the Purchaser decides to change the dates of the financial year of the relevant Acquired Companies) in favor of all resolutions irrevocably and unconditionally releasing the Resigning Persons from any liability arising from the performance of their duties until the Closing Date, except, however, for cases of fraud or willful misconduct.
All matters at Closing will be considered to take place simultaneously, and no delivery of any document at Closing will be deemed complete until all transactions and deliveries of documents required by this Agreement to be made at Closing are completed, and title to the Transferred Securities shall not be transferred and the Purchaser shall have no property rights or interest in the Transferred Securities and the transfer of the Transferred Securities shall not be recorded in the Share Registers unless and until the Closing actually takes place and the payments referenced in Section 2.10(b) have been effected, such payments being irrefutably deemed effected accordingly if the Purchaser provides evidence that the transferring bank(s) have irrevocably executed the transfer of the payments referenced in Section 2.10(b), e.g., by providing a copy of the respective SWIFT message (e.g., MT103) showing that the payments in Section 2.10(b) have been made in full and without any deductions (other than any costs charged by the receiving bank).
2.11 Breach of Closing Obligations.
(a) If either the Purchaser or any of the Sellers fails to comply with any of its material obligations under Section 2.10, the non-defaulting Party shall be entitled (in addition to or without prejudice to all other rights or remedies available to it, including the right to claim Losses or to seek the specific performance of this Agreement (exécution forcée)), by written notice to the defaulting Party (without the need for any prior notice (une mise en demeure)):
(i) to terminate this Agreement, without any liability on the part of the non-defaulting Party;
(ii) to effect the Closing so far as practicable taking into account the defaults which have occurred; or
(iii) to set a new date for Closing (not being more than ten (10) Business Days following the initially agreed date for Closing), in which case the provisions of Section 2.10 shall apply to the Closing as so deferred but provided that such deferral may only occur once.
(b) If the defaulting Party fails to comply with any of its material obligations under Section 2.10 on the second Closing Date as reset by the non-defaulting Parties in accordance with Section 2.11(a)(iii), the non-defaulting Party shall be entitled (in addition to or without prejudice to all other rights or remedies available to it, including the right to claim Losses or to seek the specific performance of this Agreement (exécution forcée)) to terminate this Agreement, without any liability on the part of the non-defaulting Party, by written notice to the defaulting Party sent on the date set for Closing (without the need for any prior notice (une mise en demeure)).
ARTICLE 3 CONDITIONS PRECEDENT TO CLOSING
3.1 Conditions Precedent.
(a) The obligation of each of the Parties to consummate the Transaction is subject to the satisfaction of the following conditions:
(i) that the Required Antitrust Clearances shall have been duly obtained and shall be in full force and effect (the "Antitrust Condition"); and
(ii) that the Required Foreign Investment Clearances shall have been duly obtained and shall be in full force and effect (the "Foreign Investment Condition"; and together with the Antitrust Condition, the "Regulatory Conditions");
(iii) that ELITechGroup S.p.A. shall have notified Hitachi High-Tech Corporation, a Japanese corporation, having its principal place of business at 1-17-1, Toranomon, Minato-ku, Tokyo, 105-6409, Japan ("Hitachi") in writing of the changes to its ownership as provided under the Joint Development Agreement dated 18 March 2021 and that Hitachi shall not have notified in writing ELITechGroup S.p.A. of its intention to terminate the Joint Development Agreement dated 18 March 2021; and
(iv) that the Carve-Out Reorganizations (excluding, for the avoidance of doubt, the CC Transfers) shall have been completed in accordance with Section 6.1 prior to the Closing.
(b) The satisfaction of the Regulatory Conditions shall not have any retroactive effect.
(c) None of the Parties may rely on the failure of any condition set forth in Article 3 (Conditions Precedent to Closing) to be satisfied if such failure was caused by the breach by such Party of any of the covenants or other undertakings set forth in this Agreement to be performed or observed by such Party prior to Closing.
3.2 Responsibility for Satisfaction of the Regulatory Conditions.
(a) The Parties acknowledge the importance that the Required Regulatory Clearances be obtained as soon as possible and that they are not aware of any reason that may prevent the satisfaction of the Regulatory Conditions on or prior to the Long Stop Date.
(b) The Purchaser shall (and shall cause its Affiliates to):
(i) provide the Sellers with a draft form of each notification, submission or filing required to be made in order to obtain the Required Regulatory Clearances (provided that privileged or commercially sensitive information relating to the Purchaser or its Affiliates may be first removed) and a reasonable opportunity to discuss its content with the Purchaser prior to its notification, submission or filing with the relevant Governmental Authorities and shall consider and take into account all reasonable comments or requests made by the Sellers' Agent in this respect;
(ii) submit or make at its (or their) own expense, as soon as reasonably practicable and in any event no later than on January 31, 2024 with respect to the Required Antitrust Clearances and February 16, 2024 with respect to the Required Foreign Investment Clearances:
(A) with respect to Required Regulatory Clearance for which a pre-notification process is required or customary, a first draft of such pre-notification that the Purchaser and the Sellers' Agent deem is reasonably complete with the competent Governmental Authority with respect to the transactions contemplated by this Agreement (and shall submit a formal and complete filing within two (2) Business Days after having obtained confirmation that the pre-notification is complete and can be submitted formally); and
(B) with respect to Required Regulatory Clearance for which no pre-notification process is required or customary, full and accurate filings that the Purchaser and the Sellers' Agent deem is reasonably complete with the other relevant Governmental Authorities with respect to the transactions contemplated by this Agreement;
(which notifications and filings the Purchaser shall confirm to the Sellers' Agent in writing promptly after their making) in order to obtain the Required Regulatory Clearances within the shortest possible delay;
(iii) supply as soon as reasonably practicable any additional information and documentary material in possession of the Purchaser that may be requested by the relevant Governmental Authorities in connection therewith;
(iv) use commercially reasonable efforts to obtain each of the Required Regulatory Clearances without undue delay on or before the Long-Stop Date, it being acknowledged and agreed that the Purchaser is (i) with respect to the Required Antitrust Clearances required to offer commitments and to accept all conditions or obligations or take any other action imposed, required or requested by any Governmental Authority competent for any Required Antitrust Clearance in order to grant the Required Antitrust Clearance and (ii) with respect to the Required Foreign Investment Clearances is under no obligation to offer commitments or accept conditions or obligations or take any other action other than the commitments set forth in Annex 3.2(b)(iv) TC "Annex 3.2(b)(iv) Resigning Persons" \f \l 2 in order to grant the Required Foreign Investment Clearance;
(v) not take any actions (including entering into any transaction, investment, agreement or other arrangement) that could reasonably be expected to make it more difficult to obtain the Required Regulatory Clearances or to result in any material delay in obtaining the Required Regulatory Clearances; (vi) keep the Sellers' Agent regularly and reasonably informed of the processing of the notifications, submissions and filings referred to above, including (v) notifying the Sellers' Agent promptly of any material communications (written or oral) from the relevant Governmental Authorities, (w) providing the Sellers' Agent with a draft form of any material written communications or with a summary of any material oral communications proposed to be made to the relevant Governmental Authorities (including notifications, briefs, arguments and proposals), including the proposed final form of or summary of any such communication, prior to filing or submitting such material communications with or to such Governmental Authorities, and taking into account any reasonable comments or requests made by the Sellers' Agent with respect thereto; (x) providing the Sellers' Agent promptly with the final form of any material written communications made to the relevant Governmental Authorities (including notifications, briefs, arguments and proposals); (y) organizing regular review with the Sellers' Agent (and its advisors) of the progress of any notifications, submissions and filings (including where necessary, seeking to identify appropriate commitments or remedies); and (z) informing the Sellers' Agent promptly if it becomes aware of anything that might result in any of the Required Regulatory Clearances being delayed or denied (including the opening of any in-depth review such as a Phase 2 Review), provided that in the case of all such documents, privileged or commercially sensitive information relating to the Purchaser or its Affiliates shall be redacted and un-redacted copies shall only be provided to external counsel of the Sellers' Agent on a counsel-to-counsel basis;
(vii) where practicable provide the Sellers' Agent and its advisors with a reasonable opportunity to participate in any meeting or reasonably relevant telephone conversations with the relevant Governmental Authorities; and
(viii) give notice to the Sellers' Agent of the receipt of any Required Regulatory Clearance within one (1) Business Day of its becoming aware of the same (including a copy of each Required Regulatory Clearance provided that any privileged or commercially sensitive information relating to the Purchaser or its Affiliates shall be redacted and un-redacted copies shall only be provided to external counsel of the Sellers' Agent on a counsel-to-counsel basis).
(c) The Sellers shall assist and cooperate, and shall do all things necessary or appropriate to ensure that the Acquired Companies assist and cooperate, with the Purchaser in preparing and filing all documents required to be submitted by the Purchaser or its Affiliates pursuant to this Section 3.2 and provide promptly any necessary information in the possession of or relating to the Sellers and/or the Acquired Companies which may be required by the Governmental Authorities, for the purpose of such filings or is necessary in order to respond to questions raised by such Governmental Authorities; provided that any privileged or commercially sensitive document or information relating to the Sellers or their Affiliates shall be redacted and un-redacted copies shall only be provided to external counsel of the Purchaser on a counsel-to-counsel basis. In addition, in relation to any jurisdictions in which a notification, submission or filing is required to be made by the Sellers or by an Acquired Company in order to obtain a Required Regulatory Clearance, the Sellers shall comply, and shall cause the relevant Acquired Companies to comply with, the procedures set forth in Sections 3.2(b)(iii), (v) through (viii) mutatis mutandis.
(d) All filing fees and similar charges incurred by the Parties in making any notifications, submissions or filings pursuant to this Section 3.2 shall be borne by the Purchaser, with the exception of the legal fees of the respective external counsel, which will be borne by the party having instructed the respective external counsel.
(e) In the event of the opening of an in-depth review such as a Phase 2 Review, each of the Sellers' Agent and the Purchaser shall be entitled (but shall not be obligated), by written notice to the Purchaser or the Sellers' Agent, as applicable, to postpone the Long Stop Date to a future date compatible with such in-depth review and which is no later than one (1) month after the Long Stop Date (the "Extended Long Stop Date"), in which event the provisions of this Agreement shall apply mutatis mutandis as if the Extended Long Stop Date were the Long Stop Date.
ARTICLE 4 REPRESENTATIONS AND WARRANTIES OF THE SELLERS
Each of the Sellers hereby represents and warrants to the Purchaser that (provided that (i) the representations and warranties set forth in Section 4.1 are made by each Seller only as to itself and (ii) the representations and warranties set forth in Section 4.3 are made only if the Eliman Shareholding Reorganizations are completed, by TecFin) as of the Put Option Date, as of the Signing Date and as of the Closing Date (except for such representations which are expressly made as of a specific date and are therefore made on such date only):
4.1 Ownership of Securities; Sellers' Organization and Due Authorization.
(a) Each Seller is the sole owner of the Transferred Securities listed opposite its name in the Security Holdings Tables. Such Transferred Securities will be free and clear of all Encumbrances and each Seller will be able to freely sell to the Purchaser, without any restrictions of any kind, full and valid title to all its Transferred Securities, thus allowing the Purchaser to own, on the Closing Date, directly and indirectly, all of the securities issued by the Company.
(b) The rights of each Seller on its Transferred Securities are not subject to any dispute whatsoever and, to the knowledge of such Seller, no such dispute is foreseeable.
(c) Each Seller, if an Entity, is duly incorporated and validly existing under the laws of its jurisdiction of incorporation or formation. Each Seller has the capacity and right to enter into this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby, and if an individual and married, no authorization or consent of such Seller's spouse is required for the sale of such Seller's Transferred Securities hereunder (it being noted that the Purchaser has received the spousal consent of Christoph Gauer's wife).
(d) The entering into of this Agreement and the performance of the Sellers' obligations hereunder have been duly authorized by all necessary corporate, partnership or similar action and proceedings on the part of the Seller, if such Seller is an Entity. This Agreement has been duly signed by each Seller and constitutes the valid and binding obligation of each relevant Seller, enforceable against the Sellers in accordance with its terms.
(e) Neither the entering into of this Agreement, nor the performance by the Sellers of their obligations hereunder, nor the consummation of the transactions provided for hereby does or will:
(i) conflict with or violate any provision of the Organizational Documents of any of the Sellers, which are Entities;
(ii) violate, conflict with or result in the breach or termination of, or constitute a default or event of default (or an event which with notice, lapse of time, or both, would constitute a default or event of default), under the terms of, any Contract or Governmental Authorization to which any of the Sellers is a party or by which any of the Sellers is bound; or
(iii) subject to obtaining the Regulatory Conditions, constitute a violation by any of the Sellers of any Laws or Judgments;
(f) Each Seller that is not an individual is a duly incorporated and properly registered company that is not insolvent and that is not the subject of any Insolvency Proceedings and no fact is liable to result in any such proceedings.
4.2 The Company.
(a) The Company is a private limited liability company (société à responsabilité limitée) duly incorporated and validly existing under the laws of the Grand Duchy of Luxembourg.
(b) No resolution has been adopted and no meeting has been convened with a view to winding up or liquidating the Company. No fact, application or, to the Sellers' Knowledge, circumstance, is liable to result in the Company being struck off the Trade and Companies Register or wound up, and no other fact, application or, to the Sellers' Knowledge, circumstance, is liable to affect the existence of the Company.
(c) The Company Securities are validly issued and fully paid-up and represent 100% of the capital and voting rights of the Company. Except for the Company Securities, the Company has not issued, nor approved the issuance of, any shares, warrants or securities of any nature whatsoever giving access immediately or in the future to the share capital of the Company; and there are no options or other agreements or undertakings pursuant to which the Company is or may become obliged to issue any shares, warrants or other securities of any nature whatsoever.
(d) The Company is not insolvent and is not the subject of any Insolvency Proceedings.
4.3 Eliman Entities.
4.3.1 Eliman 1.
(a) Eliman 1 is a simplified joint stock company (société par actions simplifiée) duly incorporated and validly existing under the laws of the Grand Duchy of Luxembourg.
(b) No resolution has been adopted and no meeting has been convened with a view to winding up or liquidating Eliman 1. No fact, application or, to the Sellers' Knowledge, circumstance, is liable to result in Eliman 1 being struck off the Trade and Companies Register or wound up, and no other fact, application or, to the Sellers' Knowledge, circumstance, is liable to affect the existence of Eliman 1.
(c) The Eliman 1 Securities are validly issued and fully paid-up and represent 100% of the capital and voting rights of Eliman 1. On the Closing Date, except for the Eliman 1 Securities, Eliman 1 has not issued, nor approved the issuance of, any shares, warrants or securities of any nature whatsoever giving access immediately or in the future to the share capital of Eliman 1; and there are no options or other agreements or undertakings pursuant to which Eliman 1 is or may become obliged to issue any shares, warrants or other securities of any nature whatsoever.
(d) Eliman 1 is not insolvent and is not the subject of any Insolvency Proceedings.
(e) Eliman 1 is the sole owner with full and valid title of the Company Securities listed opposite its name in the Security Holdings Tables. Such Company Securities will be free and clear of all Encumbrances at the time of Closing and are not subject to any dispute whatsoever and no such dispute is, to the Sellers' Knowledge, foreseeable.
(f) Eliman 1 has not engaged in any business activities other than the acquisition and holding of securities issued by the Company, does not have any employees, does not hold any material assets other than the Company Securities listed opposite its name in the Security Holdings Tables, and does (whether actual, potential, or threatened) not have any outstanding indebtedness for money borrowed or any other liabilities other than reflected in its net cash position or ordinary course operational and administrative costs and expenses which in any event are not expected to exceed EUR 30,000 on the Closing Date.
(g) The Company Securities listed opposite to Eliman 1's name in Annex B are owned with full and valid title by Eliman 1, and (ii) such Company Securities are fully paid and validly issued.
4.3.2 Eliman 2.
(a) Eliman 2 is a simplified joint stock company (société par actions simplifiée) duly incorporated and validly existing under the laws of the Grand Duchy of Luxembourg.
(b) No resolution has been adopted and no meeting has been convened with a view to winding up or liquidating Eliman 2. No fact, application or, to the Sellers' Knowledge, circumstance, is liable to result in Eliman 2 being struck off the Trade and Companies Register or wound up, and no other fact, application or, to the Sellers' Knowledge, circumstance, is liable to affect the existence of Eliman 2.
(c) The Eliman 2 Securities are validly issued and fully paid-up and represent 100% of the capital and voting rights of Eliman 2. On the Closing Date, except for the Eliman 2 Securities, Eliman 2 has not issued, nor approved the issuance of, any shares, warrants or securities of any nature whatsoever giving access immediately or in the future to the share capital of Eliman 2; and there are no options or other agreements or undertakings pursuant to which Eliman 2 is or may become obliged to issue any shares, warrants or other securities of any nature whatsoever.
(d) Eliman 2 is not insolvent and is not the subject of any Insolvency Proceedings.
(e) Eliman 2 is the sole owner with full and valid title of the Company Securities listed opposite its name in the Security Holdings Tables. Such Company Securities will be free and clear of all Encumbrances at the time of Closing and are not subject to any dispute whatsoever and, no such dispute is, to the Sellers' Knowledge, foreseeable.
(f) Eliman 2 has not engaged in any business activities other than the acquisition and holding of securities issued by the Company, does not have any employees, does not hold any material assets other than the Company Securities listed opposite its name in the Security Holdings Tables, and does (whether actual, potential, or threatened) not have any outstanding indebtedness for money borrowed or any other liabilities other than reflected in its net cash position or ordinary course operational and administrative costs and expenses which in any event are not expected to exceed EUR 30,000 on the Closing Date.
(g) The Company Securities listed opposite to Eliman 2's name in Annex (B) are owned with full and valid title by Eliman 2, and (ii) such Company Securities are fully paid and validly issued.
4.4 Group Structure.
(a) The group structure chart for the Acquired Companies attached as Annex 4.4(a) TC "Annex 4.4(a) Group Structure Chart" \f \l 2 ("Group Structure Chart") and the copies of the Organizational Documents of the Acquired Companies disclosed in the Data Room Documentation are complete and accurate. The respective Acquired Companies are the sole legal and beneficial owners, with full and valid title to all, of the shares and interests in the Acquired Companies as shown on the Group Structure Chart (the "Group Shares"), which are free and clear of any Encumbrances other than the Existing Encumbrances. Other than the Group Shares there is no (i) outstanding subscription, option, virtual option right, call, convertible note, warrant or right (whether nor not currently exercisable) to acquire any securities in any Acquired Company, (ii) outstanding security, instrument or obligation that is or may become convertible into or exchangeable for any securities of an Acquired Company, (iii) contract under which an Acquired Company is or may become obligated to sell or otherwise issue any securities of an Acquired Company, (iv) promise to grant, offer or otherwise provide any of the foregoing to any of the employees or directors or officers of an Acquired Company.
The respective Acquired Company is the sole legal and beneficial owner of the respective Group Shares and has unrestricted title to these Group Shares.
(b) None of the Acquired Companies owns any security or other interest in the share capital or partnership of any Entity other than other Acquired Companies and Elitech SR (Serbia) and FKGO GmbH (Germany).
4.5 Incorporation – Existence of the Group Companies.
(a) Each Group Company validly exists in accordance with the Laws of its jurisdiction of incorporation (or similar) and its articles of association.
(b) No resolution has been adopted and no meeting has been convened with a view to winding up or liquidating any of the Group Companies. To Seller's Knowledge, no fact, circumstance or application is liable to result in any of the Group Companies being struck off the Trade and Companies Register or wound up.
(c) To Seller's Knowledge, none of the Group Companies is the subject of any Insolvency Proceedings, it being acknowledged that FKGO GmbH (Germany) has been liquidated on February 9, 2024 under applicable law.
4.6 Transaction Bonus Payments, No Transaction Costs
(a) Except as set forth in Schedule 4.6(a) TC "Annex 4.6(a) Transaction Bonus Payments" \f \l 2 , none of the Group Companies has paid or promised to pay or is otherwise obliged to pay to their officers, directors or employees or to officers, directors or employees of any of their direct or indirect shareholders any bonus payments or other special incentives in connection with or as a direct result of the consummation of this Agreement or the transactions contemplated herein.
(b) None of the Group Companies has following 31 December 2022 paid, or is obligated to pay any brokerage, finder's fee, commission, advisory fees or expenses to any third party (including the Sellers or their Affiliates) in connection with or in respect of (i) the preparation, negotiation or execution of this Agreement, or (ii) the preparation and consummation of the Transaction or Project Elisir (i. e. the sale process for the Group Companies in 2020 and 2021).
ARTICLE 5 REPRESENTATIONS AND WARRANTIES OF THE PURCHASER
The Purchaser hereby represents, warrants and acknowledges to the Sellers that as of the Put Option Date, as of the Signing Date and as of the Closing Date (except for such representations which are expressly made as of a specific date and are therefore made on such date only):
5.1 Organization and Due Authorization.
(a) The Purchaser is a stock company duly organized under the laws of Switzerland. The Purchaser has the capacity and right to enter into this Agreement, to perform its obligations hereunder and to consummate the transactions contemplated hereby.
(b) The entering into of this Agreement and the performance of the Purchaser's obligations hereunder have been authorized by all necessary corporate action and proceedings on the part of the Purchaser. This Agreement has been duly signed by the Purchaser and constitutes a legal, valid and binding obligation of the Purchaser, enforceable against it in accordance with its terms.
(c) Neither the entering into of this Agreement, nor the performance by the Purchaser of its obligations hereunder, nor the consummation of the transactions provided for hereby does or will:
(i) conflict with or violate any provision of the Organizational Documents of the Purchaser;
(ii) violate, conflict with or result in the breach or termination of, or constitute a default or event of default (or an event which with notice, lapse of time, or both, would constitute a default or event of default), under the terms of, any Contracts or Governmental Authorizations to which the Purchaser or any of its Affiliates is a party or by which the Purchaser or any of its Affiliates is bound; or
(iii) subject to obtaining the Regulatory Conditions, constitute a violation by the Purchaser or any of its Affiliates of any Laws or Judgments.
(d) The Purchaser is not subject to any Insolvency Proceedings and no fact is liable to result in any such proceedings.
5.2 Governmental Authorizations.
No Governmental Authorization or other third party consent under any contract is required to be made or obtained by the Purchaser or any of its Affiliates prior to the Closing in connection with (a) the entering into of this Agreement by the Purchaser, (b) the performance by the Purchaser of its obligations hereunder, or (c) the consummation of any of the transactions contemplated by this Agreement.
5.3 Financing.
The Purchaser's obligations hereunder are not subject to any conditions regarding its or any other Person's ability to obtain or provide financing for the consummation of the transactions contemplated by this Agreement. On the Closing Date, the Purchaser will have obtained all the financing commitments in order to have sufficient funds necessary and immediately available funds in sufficient amounts to pay the Preliminary Closing Purchase Price and the Balancing Amount and to make such other payments required to be made by the Purchaser pursuant to this Agreement (regardless of whether or not it can obtain financing under its financing commitments). The Sellers hereby acknowledge that they have received an equity commitment letter from Bruker Corporation on the Put Option Date.
5.4 Acknowledgement.
(a) The Purchaser acknowledges that, prior to the execution of this Agreement and the Warranty Agreement, it and its advisors have been given access to the Disclosed Information and to meetings and Q&A sessions with the Sellers, the management of the Group Companies and their advisors. The Purchaser acknowledges that it has carried out an independent and satisfactory due diligence of the Group Companies, has reviewed the Disclosed Information to its reasonable satisfaction and has assessed the contents of the Disclosed Information, in particular by discussing the same with senior management of the Group Companies and asking any question which the analysis of the Disclosed Information might entail to such senior management and the advisers of the Sellers and the Group Companies. The Purchaser hereby acknowledges that it has taken the Disclosed Information and any such discussions and answers to its questions into account in the terms of its offer to acquire the Transferred Securities. In entering into this Agreement and the Warranty Agreement, the Purchaser has relied upon its own review and analysis of the Disclosed Information, upon the representations and warranties of the Sellers expressly set forth in this Agreement and the Warranty Agreement and upon its own independent investigations.
(b) The Purchaser acknowledges (i) that it has assessed the impact that the spread of COVID-19 and any Pandemic Measure has had, is having and is likely to have, on the operations and financial condition of the Group Companies and have been fully taken into account in the terms of this Agreement and (ii) that in no event would COVID-19, any Pandemic Measure or the Ukraine Situation in its current state constitute an event of force majeure on this Agreement and the Warranty Agreement. Consequently, the Purchaser hereby irrevocably waives any right that it has, or may have in the future, to terminate this Agreement as a result thereof.
(c) The Purchaser further acknowledges that the representations and warranties of the Sellers set forth in this Agreement and the Warranty Agreement supersede any and all earlier representations, warranties or statements made by any directors, officers, employees, agents, representatives or advisors of the Sellers (collectively, the "Sellers' Representatives") regarding the Transferred Securities, any of the Acquired Companies or any other matter referenced in this Agreement and the Warranty Agreement, and that neither any of the Sellers nor any of the Sellers' Representatives shall have any liability in respect of any such earlier representations, warranties or statements (and in respect of which the Purchaser undertakes not to, and to procure that none of its Affiliates shall, bring any claim or action against any of the Sellers or any of the Seller's Representatives).
Except as expressly set forth in this Agreement and the Warranty Agreement, neither any of the Sellers nor any of the Sellers' Representatives makes any representation or warranty, either express or implied, of any kind whatsoever with respect to the Transferred Securities, any of the Acquired Companies or any of the transactions contemplated hereby (including as to the accuracy or completeness of any information provided to the Purchaser or its representatives). Without limiting the generality of the foregoing, the Purchaser acknowledges that neither any of the Sellers nor any of the Sellers' Representatives makes any representation or warranty with respect to the future relations of any of the Acquired Companies with any customers or suppliers, or with regard to the future financial or business prospects of any of the Acquired Companies. In furtherance of the foregoing, to the fullest extent permitted by applicable Law, the Purchaser hereby irrevocably waives the benefit of any warranties generally available to purchasers under applicable Law except on the basis of willful misconduct (dol).
(d) The Purchaser further acknowledges that, except as expressly set forth in this Agreement and the Warranty Agreement, neither any of the Sellers nor any of the Sellers' Representatives makes any representation or warranty with respect to (i) the Disclosed Information or (ii) any financial projections, business plans, budgets, estimates, predictions or forecasts (collectively "projections") relating to any of the Acquired Companies (and in respect of which the Purchaser undertakes not to, and to procure that none of its Affiliates shall, bring any claim or action against any of the Sellers or any of the Sellers' Representatives). The Purchaser acknowledges that there are numerous assumptions reflected in such projections and significant uncertainties including uncertainties arising from the COVID-19, any Pandemic Measure and the Ukraine Situation (and has had in this respect the opportunity to discuss the same with the management of the Acquired Companies) inherent in attempting to make such projections, that the Purchaser is fully familiar with such types of assumptions and uncertainties, that the Purchaser is taking full responsibility for making its own evaluation of the adequacy and accuracy of all projections or forward-looking statements furnished to it, and that the Purchaser shall not have any claim against any of the Sellers or any of the Sellers' Representatives with respect thereto.
ARTICLE 6 PRE-CLOSING COVENANTS
6.1 Pre-Closing Reorganizations.
6.1.1 The Sellers shall procure that:
(i) the actions described in Annex 6.1.1 TC "Annex 6.1.1 Pre-Closing Reorganizations" \f \l 2 in connection with the Pre-Closing Reorganizations are taken by the Sellers or their relevant Affiliates; (ii) the Sellers and the relevant parties shall execute all required documents so that, on or prior to the Closing, the Carve-Out Companies shall be validly held by TecFin as provided under Annex 6.1.1 TC "Annex 6.1.1 Pre-Closing Reorganizations" \f \l 2 ; and
(iii) the Pre-Closing Reorganizations are consummated on or prior to the Closing, and all Tax returns required to be filed by the Sellers or any of their Affiliates with any Governmental Authority in respect of the Pre-Closing Reorganizations are duly and timely filed,
it being specified that the Parties agree to adjust the legal steps or actions necessary to complete the Pre-Closing Reorganizations (as described in the Tax Step Plan) should any modification are reasonably requested by the lenders under the Existing Financing.
6.1.2 The Purchaser shall, and shall cause its Affiliates to, cooperate and provide to the Sellers all documents and information that the Sellers may reasonably request in connection with the implementation of the Pre-Closing Reorganizations.
6.1.3 Any costs related to the preparation and implementation of (i) the Shareholding Reorganizations shall be borne by the Sellers, and (ii) the Carve-Out Reorganizations shall be borne by the Sellers.
6.2 Ordinary Course of Business.
6.2.1 During the period from the date of this Agreement to the Closing, except as may be (v) specifically contemplated by this Agreement or necessary or advisable to implement the transactions contemplated herein (including the Pre-Closing Reorganizations), (w) disclosed in Annex 6.2.1 TC "Annex 6.2.1 Ordinary Course of Business" \f \l 2 , (x) required by a contractual obligation existing on the Put Option Date, provided it was adequately disclosed in the Data Room (y) immediately required by applicable mandatory Law or immediately requested by a mandatory Governmental Authority, or (z) consented to in writing by the Purchaser (which consent shall not be unreasonably withheld, delayed or conditioned, having due consideration for the interests, as applicable, of the Acquired Companies), each of the Sellers shall, within the limits of its powers as a shareholder, director, officer or employee of the relevant Acquired Company, use its reasonable endeavors to ensure that each of the Acquired Companies maintains the value of its material assets and the relationships of the Business taken as a whole in all material respects and carries on its business only in the ordinary course of business consistent with past practices, and does not take any of the following actions:
(i) amending its Organizational Documents in any material respect which would be detrimental to the Purchaser or the consummation of the transactions contemplated herein, other than to comply with applicable Laws;
(ii) acquiring or selling, by merger, spin-off, consolidation, purchase or sale of shares or assets or otherwise, any Entity or business other than the disposal of the Carve-out Companies pursuant to the terms set forth in this Agreement or granting any Encumbrance on any such Entity or business, except for in each case, intercompany transactions or build-up acquisitions disclosed in the Disclosed Information;
(iii) altering its issued share capital or declaring, setting aside, making or paying any dividend or other distribution in respect of its share capital (in cash or otherwise), or purchasing or redeeming any shares in its share capital, except for in each case, to the benefit of another Acquired Company;
(iv) issuing or selling any shares in its share capital or any options, warrants or other rights to purchase any such shares or any securities convertible into or exchangeable for such shares, except for in each case, to the benefit of another Acquired Company;
(v) provided that there are fully covered under the Debt Release Letters and without any pre-payment obligation to any of the Group Companies, incurring (other than under the Debt Release Letters, as the case may be), other than in the ordinary course of business consistent with past practice, any material indebtedness for borrowed money (including through the issuance of debt securities), except for (x) indebtedness to another Acquired Company, (y) any drawing under the Existing Financing and (z) increase of the bank guarantee program in place with the Group;
(vi) change the payment procedures on accounts payable and accounts receivable (in particular any acceleration on accounts receivable and any deferred payment of accounts payable) and increase or decrease the volume of inventory, in each case on a Group Company level and throughout the Group Companies, unless consistent with past practice and in compliance with the duty of care of a prudent business man;
(vii) enter into, terminate or materially modify any of the agreements set out in Annex 6.2.1(vii) TC "Annex 6.2.1(vii) Seller's Agreements" \f \l 2 ;
(viii) offer or make any bonus or similar payments to any third party triggered by the consummation or any other event provided for under this Agreement;
(ix) hire any director, officer or, other than in the ordinary course of business and in line with the business plan and budget, employee;
(x) terminate any director, officer or employee of a Group Company with a fixed remuneration equal to or higher than EUR 150,000;
(xi) dispose of or limit any intellectual property rights owned by any of the Group Companies that are material to the Business or terminate, amend or otherwise change any right to use any third party intellectual property rights used by any of the Group Companies that are material to the Business, other than in the ordinary course of business; (xii) making any change in its accounting procedures or practices unless mandated by Law or the accounting principles generally accepted in its jurisdiction of incorporation or residence;
(xiii) enter into any related party transactions (other than any bona fide business transactions entered into in the ordinary course of business and on market terms with any portfolio company of TecFin's ultimate shareholder or any fund(s) managed or advised by PAI Partner S.à r.l.);
(xiv) terminate (whether unilaterally, by consent or by settlement), amend or change the service and/or employment agreements between the respective Group Company and any of Christoph Gauer, Mark Pegeot, Roberto Meda and/or Francesco Gorreta; or
(xv) committing to take any of the actions set forth in the foregoing subsections (i) through (xiii).
For the purposes of granting any consents which may be requested by the Sellers' Agent or an Acquired Company pursuant to this Section 6.2.1, the Purchaser hereby designates Wolfgang Pusch with immediate effect and represents and warrants to, and agrees with, the Sellers' Agent and each of the Sellers that Wolfgang Pusch shall have full capacity and right to give any such consents on behalf of the Purchaser until the Closing Date. Within five (5) Business Days of receipt of any request for consent by the Sellers' Agent or an Acquired Company, the Purchaser shall have the right to notify the Sellers' Agent or the relevant Acquired Company that it objects to the proposed action (which notice of objection shall indicate its reasons for so objecting). If the Purchaser shall not have notified the Sellers' Agent or the relevant Acquired Company, as the case may be, of its objection to a proposed action within such period of five (5) Business Days, the Purchaser shall be deemed to have consented to such proposed action. As an exception to Section 10.11, any notice to be made for the purpose of obtaining or giving any consent of the Purchaser under this Section 6.2.1 shall be given by e-mail only (without confirmation).
6.2.2 Nothing herein shall operate to restrict or prevent:
(i) any commercially reasonable action undertaken by any Acquired Company in an emergency or disaster situation with the intention of minimizing any adverse effect thereof; or
(ii) any commercially reasonable action taken as a response to any Pandemic Measure, and which (i) is reasonably necessary (a) to protect the health, safety and welfare of the directors, officers or employees of the Acquired Companies and other individuals having business dealing with the Acquired Companies, or (b) to respond to third party supply or service disruptions caused by COVID-19 or (ii) is consistent with actions taken or contemplated by other businesses or enterprises of similar size of the Group Companies operating in the same or substantially similar industry and a similar geographic presence;
in each case after consultation with the Purchaser if legally and practically possible.
6.2.3 For the avoidance of doubt, nothing contained in this Agreement is intended to give the Purchaser, directly or indirectly, the right to control or direct the Acquired Companies prior to the Closing and the Sellers shall not be under any obligation to provide any commercially sensitive information the disclosure of which would likely constitute a breach of applicable Law.
6.3 Specific Undertakings
As soon as reasonably practicable following the Signing Date, the Sellers shall use commercially reasonable efforts to procure that the Group Companies do the following:
(a) remediation of non-conformity addressed in the regulatory audit dated September 21, 2023 for ELITechGroup SpA;
(b) arrange for a visit with Hitachi and Precision System Science Co., Ltd., a Japanese corporation, having its principal place of business at 88 Kamihongou, Matsudo-shi, Chiba, 271-0064, Japan, ("PSS") in Japan for Christoph Gauer, Jürgen Srega and Wolfgang Pusch to introduce the Purchaser and Bruker group with the key competent representatives of Hitachi and PSS in charge for the commercial relationship with the Group Companies, as soon as possible, ideally in January or February 2024;
(c) deliver to the Purchaser the confirmation that the cybersecurity insurance policy covering all the Acquired Companies has been renewed for 2024.
6.4 Access and Information.
(a) During the period from the date of this Agreement to the Closing, upon the reasonable written request of the Purchaser and subject to compliance by the Purchaser with the terms of the confidentiality agreement entered into between TecInvest and Bruker Daltonik GmbH on October 6, 2020 (as amended from time to time) (the "Confidentiality Agreement") and the applicable Laws, the Sellers shall use their commercially reasonable efforts to ensure that the Acquired Companies (i) permit the Purchaser and its representatives reasonable access during Working Hours to the books and records and senior management of the Acquired Companies, and (ii) provide to the Purchaser any document or information in the possession of the Acquired Companies, in both cases, as may be reasonably requested by the Purchaser for the purpose of preparing the Closing and facilitating the debt financing of the transactions contemplated by this Agreement; and (iii) inform on regular basis on the progress of remediation of the past cyber incident.
(b) Notwithstanding anything in this Agreement to the contrary, it is agreed that:
(i) such access or assistance shall not interfere with the normal business and operations of the Acquired Companies and any such information being subject to the Confidentiality Agreement;
(ii) such access or assistance may be limited as a result of a Pandemic Measure; (iii) notwithstanding the foregoing, the Sellers' Agent shall not be required to provide access to any information which it reasonably believes that it may not provide to the Purchaser by reason of confidentiality undertakings with a third party or by reason of antitrust or cartel Laws;
(iv) the Purchaser shall, and shall cause its Affiliates to, refrain from, directly or indirectly, contacting any customer, supplier, agent, representative, creditor, co-contracting party (including franchisee, licensee or sublicense) or advisor of the Acquired Companies or any other Person which is in commercial relationship with the Acquired Companies, except for Persons with whom the Purchaser or its relevant Affiliate (i) sustain usual commercial and professional relationships in the ordinary course of business, or (ii) seek to develop in the future, and in each case, without reference to the Transaction or the Acquired Companies and for the sole purpose of such professional and commercial relationships;
(v) all fees, costs, expenses and other liabilities and obligations which may be incurred in connection with the debt financing of the transactions contemplated by this Agreement shall be for the sole account of the Purchaser; and
(vi) for the avoidance of doubt, nothing in this Section 6.4 shall be construed to be a condition precedent to the financing of the Transaction or the Closing or otherwise, directly or indirectly, serve as a basis for any delay in Closing under this Agreement.
6.5 Convening of Corporate Meetings.
If requested by the Purchaser by means of a notice sent to the Sellers' Agent at least fifteen (15) Business Days prior to the Closing Date, the Sellers shall cause the relevant Acquired Company (as requested by the Purchaser) to convene its shareholders or its directors to a meeting, pursuant to the Organizational Documents of such Group Companies, to resolve on the Closing Date (but after Closing) on the appointment of officers, directors or board members.
6.6 Termination of the SHAs.
(a) During the period from and including the date hereof until and including the Closing Date, each of the Sellers which is a party to any SHAs undertakes not to exercise any of its rights under the SHAs to the extent that they may prevent the completion of the transactions contemplated by this Agreement.
(b) Each of the Sellers which is a party to the Company SHA acknowledges and accepts that the Company SHA shall automatically terminate at Closing, without a requirement to complete any formality, provided that all the transactions contemplated to take place at Closing have been completed, in which case each of the Sellers which is a party to the Company SHA acknowledges that all of its rights under the Company SHA have been fully satisfied and that it has no claim and waives its rights in this respect against the other parties to the Company SHA (including, for the avoidance of doubt, the need to follow the procedures and complete the formalities contemplated by the Company SHA).
(c) Each of the Sellers which is a party to the Manco SHA acknowledges and accepts that the Manco SHA shall automatically terminate at Closing, without a requirement to complete any formality, provided that all the transactions contemplated to take place at Closing have been completed and notably the Eliman Shareholding Reorganizations, in which case each of the Sellers which is a party to the Manco SHA acknowledges that all of its rights under the Macnco SHA have been fully satisfied and that it has no claim and waives its rights in this respect against the other parties to the Manco SHA (including, for the avoidance of doubt, the need to follow the procedures and complete the formalities contemplated by the Manco SHA).
(d) Should this Agreement terminate or the transactions contemplated herein fail to be completed for any reason whatsoever, then each of the SHAs shall remain in full force and effect and the Sellers which are parties to SHAs shall be automatically released from the undertaking set forth in Section 6.6(a).
6.7 Interest rate period fixing for Existing Indebtedness.
The Sellers will, upon the reasonable request of the Purchaser following the Put Option Date, procure that the respective Acquired Companies select Interest Periods (as defined in the agreements governing the Existing Indebtedness) of one (1) month only until the Closing unless such an election could reasonably be materially adverse to the Acquired Companies and provided that the Acquired Companies shall not be under any obligation to break an Interest Period in order to facilitate a request to select a one (1) month Interest Period.
6.8 Public Announcements.
During the period from the date of this Agreement to the Closing Date, neither the Purchaser nor any of the Sellers shall, or shall permit any Affiliate which it controls, or any of its representatives or advisors, to issue or cause the publication of any press release or other public announcement or disclosure with respect to this Agreement or the transactions contemplated hereby (in case of any disclosure other than a press release or other public announcement except for (i) PAI Partners to any participants or investors or potential investors in any fund advised or managed by PAI Partners and (ii) the Purchaser to Bruker Corporation and any entity of Bruker Corporation's CALID Group, in each case of (i) and (ii) subject to market standard confidentiality arrangements) without the prior written consent of the other Parties, which consent shall not be unreasonably withheld, except that (i) each Party shall be permitted to make such public announcements as may be required by applicable Law and any announcements following thereon which are limited to the contents of then publicly available information and (ii) any announcements to the Purchaser's group in the ordinary course of business and without disclosing any non-public commercial details of the Transaction. In the event any such press release, public announcement or other disclosure is required by Law to be made by the Party proposing to issue the same, such Party shall notify the other Parties prior to the issuance or making of any such press release, public announcement or other disclosure and shall use its reasonable endeavors to consult in good faith with the other Parties and to take into account the reasonable requirements of such Parties as to the timing, contents and manner of making any such press release, public announcement or other disclosure.
6.9 "Know Your Client" (KYC) Requirements.
During the period from the date of this Agreement to the Closing:
(a) the Purchaser shall promptly deliver any documents reasonably requested by the Sellers' Agent in order to allow any of the Sellers to fulfill its obligations in relation to applicable Laws in force with regards to anti-money laundering and terrorism financing; and
(b) each Seller shall promptly deliver any documents reasonably requested by the Purchaser in order to allow it to fulfill its obligations in relation to applicable Laws in force with regards to anti-money laundering and terrorism financing.
6.10 Closing Bring Down Certificate
(a) No earlier than two (2) Business Days prior to the Closing Date and no later than one (1) Business Day prior to the Closing Date, the Sellers shall review the Business Representations and shall inquire each other and the persons listed in Annex (B) to the Warranty Agreement in order to disclose the exceptions to the Business Representations that reflect any facts, events or matters that have occurred between the Signing Date (included) and the Closing Date (excluded) and which render any of the Business Representations not being correct or complete as of the Closing Date ("Closing Bring Down of Disclosures"). The Sellers shall disclose the results of the Closing Bring Down of Disclosures by executing and delivering to the Purchaser a "closing bring down disclosure certificate", a draft of which is attached as Annex 6.10(a) TC "Annex 6.10(a) Closing Bring Down of Disclosures" \f \l 2 either (i) confirming that the Business Representations are still correct and complete as of the Closing Date or (ii) specifying in reasonable detail the facts, events and circumstances which render the Business Representations not being correct and complete as of the Closing Date (the "Closing Bring Down Certificate"). For the avoidance of doubt, (i) any Closing Bring Down of Disclosures made in the Closing Bring Down Certificate shall qualify any representations made as at the Closing Date and (ii) the Closing Bring Down Certificate shall not limit Seller(s)' liability for any breach of a representation given by the Sellers on the Put Option Date and/or on the Signing Date, as the case may be and (iii) the Closing Bring Down Certificate and the information and disclosures made in the Closing Bring Down Certificate do not preclude, hinder or limit the Purchaser in any way from making any claim (i) for specific performance, (ii) arising out of a breach of the representations and warranties given by the Sellers under Article 4 and/or (iii) under any other provision of the SPA.
(b) The Sellers' Agent will provide the Purchaser with a draft of the Closing Bring Down Certificate four (4) Business Days prior to the Closing Date to afford the Purchaser the opportunity to review and discuss with the Sellers and the Company the draft Closing Bring Down Certificate.
6.11 Screening Tool
As soon as reasonably practicable following the receipt of Required Antitrust Clearances, the Seller shall use commercially reasonable efforts to procure that the Group Companies (i) initiate the implementation of a commercially available reputable screening tool that is reasonably acceptable to the Purchaser with the goal to have such screening tool available and operative for all Group Companies as soon as practicable after the Closing Date to allow the Group Companies to screen on a daily basis automatically all names in the Group Companies' respective enterprise resource planning (ERP) systems, including, without limitation, all customers, distributors, resellers, suppliers, sales agents, known end users, freight forwarders, carriers, and other intermediaries and (ii) have the persons responsible for monitoring such screening tool undergo correlative training as reasonably required. The implementation may be pursued in a sequenced approach with ELITechGroup SpA, ELITechGroup Inc. and ELITechGroup MDx LLC becoming the pilot for such implementation. Such screening tool shall screen against all applicable sanctions lists, including, without limitation, the Specially Designated Nationals and Blocked Persons List, the Entity List, the Denied Persons List, the Unverified List, and the EU Consolidated Sanctions List.
6.12 Export Policies.
As soon as reasonably practicable following the receipt of Required Antitrust Clearances, the Seller shall use commercially reasonable efforts to procure that the Group Companies:
(a) cease to conduct any and all business in Afghanistan, Belarus, Burma/Myanmar, Crimea and all other embargoed regions of the Ukraine, Cuba, Democratic Republic of Congo, Iran, Iraq, North Korea, Russia, Syria, Venezuela, and Zimbabwe unless it complies with (i) all applicable U.S. export control and sanctions laws, and (ii) with all applicable EU export control and sanctions laws; and
(b) initiate the implementation of certain export control measures, including, as appropriate, (i) robust written U.S. and EU export control and sanctions compliance policies and procedures, including an order processing checklist for each business tailored to the products that it exports, (ii) the classification of the products of the Group Companies under the applicable U.S. and EU export control laws (unless such products have already been formally classified by the respective government agency), (iii) the preparation of de minimis analyses for non-U.S. equipment, (iv) the assignment of clearly defined export control compliance roles, and (v) provide U.S. and EU export control and sanctions awareness training to senior management and more specific compliance training to the managing directors and staff involved with international sales and exports in sales, order processing, and logistics/shipping.
6.13 Pre-Closing CC Transfers.
(a) The Parties acknowledge and agree that the Sellers intend to, prior to the Closing:
(i) transfer to the Carve-Out Companies (or any Affiliates thereof, such Affiliates not being either the Company or any Group Company) the US CC Activity (the "US CC Activity Transfer") and the Australian CC Assets (the "Australian CC Assets Transfer"); and (ii) have the Carve-Out Companies transfer to either the Company or any Group Company as designated by the Purchaser, the trademarks identified in green in Annex 6.13(a) TC "Annex 6.13(a) CC Trademark Transfer" \f \l 2 (the "CC Trademark Transfer", together with the US CC Activity Transfer and the Australian CC Assets Transfer, the "CC Transfers").
(b) The Sellers shall use their best efforts to complete the CC Transfers prior to the Closing.
(c) It is agreed between the Parties that the pre-Closing CC Transfers shall in no event delay the Closing Date or prevent the Transaction from taking place on the Closing Date in any manner whatsoever. In the event that the CC Transfers cannot be completed prior to the Closing, the Parties agree that the provisions of Section 7.3 (Reversed CC Carve-Out) shall apply.
6.14 Kreienbaum Dispute
(a) The Sellers shall have the right to control the defense of the pending dispute between ELITechGroup Inc. and Kreienbaum Neoscience GmbH before the U.S. District Court for the District of Utah (the "Pending Dispute") with full authority to conduct such defense and to settle the same both prior to or after the Closing. After the Closing, the Purchaser shall, and shall instruct the Group Companies to, fully cooperate in such defense.
(b) Any Loss arising out of the settlement or as a consequence of any final and non-appealable court decision in respect of the Pending Dispute shall, to the extent not yet paid by ELITechGroup Inc. on the Closing Date, be accounted as Indebtedness (as defined in Annex 1.1) or give rise to indemnification from the Sellers (prorate the number of Transferred Securities they hold) toward ELITechGroup Inc. to the extent not already paid or accounted as Indebtedness in the Closing Accounts, any such indemnification to be paid within fifteen (15) Business Days as from the date it falls due for ELITechGroup Inc.
ARTICLE 7 POST-CLOSING COVENANTS
7.1 Non-solicitation
As consideration for the purchase of the Transaction Securities for which this undertaking is a fundamental element for the Purchaser, each of Christoph Gauer and TecFin, acting individually and not jointly and severally, undertakes and covenants vis-à-vis the Purchaser and each of the Group Companies (as a third party beneficiary with the right to enforce rights under this Article 7 directly against TecFin) that for the period of two (2) years following the Closing Date, neither directly nor indirectly to:
(a) solicit or canvass away, directly or indirectly, any for a business activity that (i) competes with all or part of the Business or (ii), if solicited or canvassed away, would materially impair the or part of the Business, an entity which is or would be at the time of such solicitation a client, a supplier or more generally any entity which has an active business relation with the Group; (b) solicit or induce, directly or indirectly, any person who is at the Put Option Date employed by any Group Company as an officer, director, manager, department head or in any other managerial or leading position, limited to TecFin except the Group CFO (Marc Pégeot), (each a "Restricted Person") to interfere with the Business or discontinue his or her employment with any Group Company; or
(c) hire, retain or attempt to hire or retain any Restricted Person, or any person employed by a Group Company within the 12 months period prior to the Put Option Date.
7.2 General solicitations
Notwithstanding the foregoing, general solicitations of employment published in a newspaper, over the internet, or in another publication of general circulation and not specifically directed towards any Restricted Person shall not be deemed to constitute a solicitation or inducement for purposes of Section 7.1.
7.3 Reversed CC Carve-Out
(a) In the event that the CC Transfers cannot be completed prior to the Closing in accordance with Section 6.13 (Pre-Closing CC Transfers), the Purchaser shall cause ELITechGroup Inc. and the Sellers shall cause the Carve-Out Companies to take all corporate and other actions required to authorize, implement and complete the CC Transfers to the benefit of the Carve-Out Companies (or any substituted Affiliates, as instructed by the Sellers' Agent) (the "Reversed CC Carve-Out") as provided in the relevant Carve-Out Documentation and as described in step no. 7 of the Tax Step Plan.
(b) As from the Closing Date and until the date on which the Reversed CC Carve-Out is completed, the Purchaser undertakes:
(i) to cause ELITechGroup Inc. to perform its obligations under the Carve-Out Documentation;
(ii) to cause ELITechGroup Australia Pty Ltd to instruct its employees dedicated to the clinical chemistry distribution activities to continue to perform their activity, in particular by continuing to give them the required access to the laboratory and machines located in the premises of ELITechGroup Australia Pty Ltd;
(iii) to keep the Sellers' Agent regularly informed of the progress of the implementation of the Reversed CC Carve-Out including (y) organizing regular review with the Sellers' Agent (and its advisors) of the progress of the implementation of Reversed CC Carve-Out; and (z) informing the Sellers' Agent promptly if it becomes aware of anything that might result in the Reversed CC Carve-Out being delayed; and
(iv) to refrain from taking, directly or indirectly, any action that may impede or materially delay the completion of the Reversed CC Carve-Out.
(c) The Sellers' Agent shall use commercially reasonable efforts to, and cause the Carve-Out Companies to, cooperate and provide to the Purchaser all documents and information that the Purchaser may reasonably request in connection with the implementation of the Reversed CC Carve-Out, subject to availability, confidentiality and applicable antitrust law.
(d) It is expressly acknowledged and agreed by the Parties that the Reversed CC Carve-Out shall not have any consequence on the Closing Purchase Price as the price of the Reversed CC Carve-Out corresponds to the Vendor Loan 2 Amount. Any costs related to the preparation and implementation of the Reversed CC Carve-Out shall be borne by the Sellers.
(e) The undertakings set forth in this Section 7.3 shall survive until December 31, 2026.
ARTICLE 8 REPAYMENT AND INDEMNIFICATION OBLIGATIONS
8.1 Repayment Obligations of the Sellers.
(a) Subject to the provisions of Section 8.3, each of the Sellers shall pay to the Purchaser, as a partial repayment of the Closing Purchase Price, the amount of any and all Losses suffered by the Purchaser and/or – at Purchaser's election - any Group Company as a direct result of any breach of any representation or warranty of such Seller set forth in Article 4 of this Agreement. From and after the Closing, the right to a partial repayment of the Closing Purchase Price provided for in this Section 8.1 shall be the exclusive remedy of the Purchaser against the Sellers for any breach of the representation or warranty of such Seller set forth in Article 4 of this Agreement.
(b) It is specified that any reference to fraud or willful misconduct (dol) in this Article 8 shall be appreciated individually for each Party and that in no event can a Party be held liable for fraud or willful misconduct (dol) committed by another Party.
(c) The Parties hereby agree that any payment made by any Seller under this Section 8.1 shall be deemed to constitute a reduction of the Closing Purchase Price with respect to the portion of the Closing Purchase Price attributable to the Transferred Securities sold and agree to treat any such payment as such for all Tax, accounting and financial reporting purposes.
8.2 Indemnification Obligations of the Purchaser.
The Purchaser shall indemnify each of the Sellers from and against any and all Losses actually suffered by such Seller as a result of any breach of any representation or warranty of the Purchaser set forth in Sections 5.1 through 5.2 of this Agreement. From and after the Closing, the right to indemnification provided for in this Section 8.2 shall be the exclusive remedy of each of the Sellers for any breach of any representation or warranty of the Purchaser set forth in Sections 5.1 through 5.2 of this Agreement, it being understood that claims of the Sellers for specific performance and/or any other claims of the Sellers based on fraud or willful misconduct of the Purchaser shall remain unaffected.
8.3 Limitations on Liability.
(a) Except for any claims under the Warranty Agreement (where Section 8.3(a)(i) shall not apply), no Seller shall have any liability under Section 8.1 unless and to the extent:
(i) a claim has been notified by the Purchaser to such Seller (or, as the case may be, the Sellers' Agent) in writing on or before the earlier of (a) the expiry of the applicable statute of limitation and (b) December 22, 2026; and
(ii) if the relevant claim has not been agreed by the concerned Seller(s), Proceedings have been brought against the concerned Seller(s) within six (6) months of it being notified in accordance with Paragraph (i) above.
(b) In the event of a breach or alleged breach of this Agreement by any of the Sellers, the Purchaser shall not be entitled to rescind this Agreement or to treat this Agreement as terminated but shall only be entitled to claim for a partial repayment of the Closing Purchase Price in accordance with this Agreement in respect of such matter.
(c) In calculating the amount which may be due and payable by the Sellers as a result of any claim brought by the Purchaser pursuant to this Agreement, there shall be deducted the amount of any corresponding cash-effective Tax Benefits arising directly of an indemnified Loss.
(d) If the Purchaser is entitled to a claim against more than one Seller, the amount claimed by the Purchaser against any individual Seller under this Agreement shall not exceed the relative share of such Seller expressed as a percentage of the claimed amount calculated on the basis of such Seller's Allocable Portion in the Purchase Price in relation to the sum of all concerned Sellers' Allocable Portion in the Purchase Price among themselves.
(e) The aggregate liability of the Sellers (collectively) under this Agreement shall not exceed an amount equal to the Closing Purchase Price and the aggregate liability of each Seller under this Agreement shall not exceed an amount equal to the Seller's Allocable Portion of the Closing Purchase Price payable to such Seller.
(f) The Sellers shall not be liable to the Purchaser for any facts or matters disclosed to the Purchaser in this Agreement, in the Warranty Agreement or the Disclosed Information, provided, however, that the foregoing shall not apply to the warranties set out in Sections 4.1, 4.2, 4.3, 4.5, and 4.6.
(g) The Sellers shall not be liable to indemnify the Purchaser for Losses in respect of a breach of the representations and warranties given by the Sellers under Article 4 if and to the extent the Purchaser has been compensated by the W&I Insurance or any other insurance mechanism.
(h) For the avoidance of doubt, any limitations or exclusions of the Sellers' liability set forth in this Section 8.3 shall not apply in the case of fraud or wilful misconduct (dol).
8.4 W&I Insurance
(a) The Parties acknowledge that the Purchaser has taken out a warranty and indemnity insurance under an insurance policy that has been executed on or about the Put Option Date (the "W&I Insurance") and the relevant insurance provider(s) providing the W&I Insurance, "W&I Insurer") in respect of the representations and warranties set forth in the Warranty Agreement (the "Business Representations") and as the case may be, in this Agreement.
(b) The Purchaser acknowledges and agrees that:
(i) the maximum total aggregate liability of the Sellers in connection with a breach of any Business Representation shall in no event exceed one euro (EUR 1) ("Business Representation Cap");
(ii) the Purchaser will direct all claims for breach of a Business Representation to the extent they exceed the Business Representation Cap solely against the W&I Insurer under the W&I Insurance; and
(iii) the validity and collectability risks in respect of the W&I Insurance, if any, shall rest with the Purchaser; and
(iv) the W&I Insurance policy contains a provision according to which the W&I Insurer will not be entitled to make and will not make any recourse claim against the Sellers in respect of any breach of a Business Representation, except in cases of fraud or dol. The Purchaser shall not amend the wording of the W&I Policy on subrogation without the prior written consent of the Sellers.
(c) Following expiry of the fourth (4th) month as of the Put Option Date and up to the end of the fifth (5th) month following the Put Option Date, upon request of the Purchaser, the Sellers shall allow the Purchaser and its advisors to update its due diligence and provide the Purchaser and its advisors with such information on the Group Companies relevant for and strictly limited to the Business Representations that would have to be disclosed in the Closing Bring Down Disclosure if such bring down had to be made during the fifth (5th) month following the Put Option Date.
ARTICLE 9 TERMINATION
9.1 Termination.
Without prejudice to any other provision of this Agreement, this Agreement may be terminated, and the transactions contemplated hereby may be abandoned, at any time prior to the Closing:
(a) by the written agreement of the Purchaser and the Sellers' Agent;
(b) by the Sellers' Agent, if the Purchaser shall have failed to file (or caused to be filed) on a timely basis the notifications and other filings required to be filed by the Purchaser or its Affiliates pursuant to Section 3.2 provided (i) such breach has not been remedied within ten (10) Business Days following a formal notice sent to the Purchaser by the Sellers' Agent and (ii) the Sellers' Agent has materially complied with its undertakings under Section 3.2;
(c) by either the Purchaser or the Sellers' Agent if any Regulatory Condition is expressly denied by a final non-appealable decision of a competent Governmental Authority prior to the Long Stop Date (or the Extended Long Stop Date, as the case may be), unless such eventuality shall be due to the breach by the Party seeking to terminate this Agreement of any of the covenants, agreements or other undertakings set forth in this Agreement to be performed or observed by such Party prior thereto;
(d) by either the Purchaser or the Sellers' Agent, if the Closing shall not have occurred on or prior to the Long Stop Date (or the Extended Long Stop Date, as the case may be), unless such eventuality shall be due to the breach by the Party seeking to terminate this Agreement of any of the covenants, agreements or other undertakings set forth in this Agreement to be performed or observed by such Party prior thereto;
(e) by the Sellers' Agent pursuant to Section 2.11; or
(f) by the Purchaser pursuant to Section 2.11.
9.2 Effect of Termination.
Upon any termination of this Agreement pursuant to Section 9.1, all further obligations of the Parties hereunder, other than pursuant to Sections 10.6 (Costs and Expenses) and 10.19 (Governing Law and Jurisdiction) and as provided in the Confidentiality Agreement, shall terminate, except that nothing herein shall relieve any Party from liability for any antecedent breach of this Agreement.
ARTICLE 10 MISCELLANEOUS
10.1 Sellers' Agent.
(a) Until completion of the Shareholding Reorganizations or, if the Shareholding Reorganizations are not completed on the Closing Date, each of the Sellers hereby appoints irrevocably and exclusively TecFin, and expressly authorizes it to act on behalf and in the name of all or part of the Sellers, as its agent (mandataire) (the "Sellers' Agent") to, in its name and on its behalf:
(i) amend the terms of this Agreement;
(ii) receive notices under this Agreement (other than any notice given by the Purchaser of any breach or violation, or alleged breach or violation, by one or more Sellers of any term of this Agreement);
(iii) deliver any notices, certifications, consents, approvals or waivers required or appropriate under this Agreement;
(iv) receive or make any payment in connection with the transactions contemplated under this Agreement;
(v) make any filing required or appropriate under this Agreement (as determined in the reasonable judgment of the Sellers' Agent);
(vi) handle, dispute, compromise, settle or otherwise deal with any and all claims against by or against or disputes with the Purchaser under this Agreement; and
(vii) more generally, exercise the rights of the Sellers on their behalf under this Agreement (including the right to terminate this Agreement under Article 9.1).
(b) Any act or decision taken by the Sellers' Agent in accordance with this Agreement shall bind each of the Sellers, provided that such decision applies to each similarly situated Seller on the same basis.
(c) References to the "Sellers' Agent" appearing herein shall be deemed to be qualified by the phrase "(on behalf of each of the Sellers)", provided that the Sellers' Agent's so acting as the agent for each of the Sellers shall in no case cause the Sellers' Agent to be deemed to be liable for any obligations of a Seller hereunder or to establish any joint and several liability among the Sellers.
(d) The Sellers' Agent shall not bear any liability whatsoever, to either any of the Sellers or to the Purchaser, in its capacity as agent of the Sellers under this Agreement, except in case of willful misconduct (faute intentionnelle) or gross negligence (négligence grave), provided, however, that in the relationship with the Purchaser any actions and omissions of the Sellers' Agent shall be directly and fully attributable to the Sellers for which the Sellers' Agent acted or should have acted.
(e) It is specified that if the Shareholding Reorganizations are completed, any reference to the Sellers' Agent in this Agreement shall be deemed to be reference to TecFin.
10.2 Further Actions.
Subject to the terms and conditions herein provided, each of the Parties shall use its reasonable endeavors to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable under all applicable Laws to consummate and make effective the transactions contemplated by this Agreement.
10.3 Brokers and Finders.
(a) The Sellers shall indemnify and shall defend and hold the Purchaser and the Group Companies harmless against and in respect of all claims, losses, liabilities and expenses which may be asserted against the Purchaser (or any Affiliate of the Purchaser) by any broker or other Person who claims to be entitled to an investment banker's, financial advisor's, broker's, finder's or similar fee or commission in respect of the entering into of this Agreement, or the consummation of the transactions contemplated hereby, by reason of its acting at the request of the Seller, Sellers' Agent or any of Seller's Affiliates (including the Group Companies).
(b) The Purchaser shall indemnify and shall defend and hold the Sellers harmless against and in respect of all claims, losses, liabilities, fees, costs and expenses which may be asserted against the Sellers by any broker or other Person who claims to be entitled to an investment banker's, financial advisor's, broker's, finder's or similar fee or commission in respect of the entering into of this Agreement or the consummation of the transactions contemplated hereby, by reason of his acting at the request of the Purchaser or any of its Affiliates (excluding the Group Companies).
10.4 Records.
During the period from the Closing Date through the sixth (6th) anniversary of the Closing Date, the Purchaser shall not, and shall not permit any Acquired Company to, destroy or otherwise dispose of any books and records existing as of the Closing Date except with the prior written consent of the Sellers' Agent, which consent shall not be unreasonably withheld. The Purchaser shall, and shall cause each of the Acquired Companies to, upon reasonable request of the Sellers' Agent, make available to the Sellers and their respective representatives and agents all such books and records, and permit the Sellers and their respective representatives and agents to examine, make extracts from and, at their expense, copy such books and records at any time during Working Hours for any reasonable and proper business purpose or to allow the Sellers to reasonably comply with legal requirements they are subject to (including towards a Governmental Authority (including a Tax Governmental Authority)).
10.5 Confidentiality.
10.5.1 Confidentiality of this Agreement.
(a) Subject to Section 10.5.1(b), during the period from the date of this Agreement to the fifth (5th) anniversary of the Closing Date, each of the Parties shall, and shall cause its respective Affiliates to, treat the existence and the content of this Agreement as confidential and refrain from disclosing this Agreement, in whole or part, to any Person without the consent of the other Party.
(b) Section 10.5.1(a) does not apply to any disclosure:
(i) by a Party to any of its Affiliates or its or its Affiliates' directors, officers, employees, agents, representatives, advisors or auditors whose function requires him to receive such announcement or disclosure and, in the event of a disclosure to an agent, representative, advisor or auditor, provided that such disclosure is subject to customary confidentiality obligations;
(ii) (x) to the extent that it is required to be disclosed by applicable Law or rule of a listing authority or a stock exchange or requested by a Governmental Authority (including a Tax Governmental Authority) with relevant powers to which the disclosing Party or its relevant Affiliate is subject or submits, (y) as requested by a regulator with power to compel disclosure, or (z) as disclosed to a Governmental Authority (including a Tax Governmental Authority) in connection with any stamp or other documentary or transaction duties or other transfer Taxes, provided that the disclosing Party shall, to the extent permitted by Law, notify the Sellers' Agent (if the disclosing Party is the Purchaser or any of its Affiliates) or the Purchaser (if the disclosing Party is a Seller or the Affiliate of a Seller) prior to the issuance or making of any such announcement or disclosure and shall use its reasonable endeavors to consult in good faith with the other Party and to take into account the reasonable requirements of such other Party as to the timing, contents and manner of making any such announcement or disclosure;
(iii) by the Purchaser to any of its sources of debt financing;
(iv) by the Purchaser to investors or potential investors in the Purchaser but only to the extent that the disclosure is limited to an information relating to the direct and indirect shareholding held in the Group Companies and the financial terms relating to the equity investment in the Purchaser made in the context of the Transaction;
(v) by the Sellers to (y) PAI Partners or any of its Affiliates or (z) any participants or investors or potential investors in any fund advised or managed by PAI Partners or any of its Affiliates;
(vi) by a Group Company to its lenders under the Existing Financing for the purposes of facilitating the transactions contemplated herein (including the Carve-Out Reorganizations); or
(vii) by any of the Parties to enforce its rights under this Agreement.
10.5.2 Confidential Information of the Sellers.
(a) Subject to Section 10.5.2(b), after the Closing Date and for a period of five (5) years thereafter, the Purchaser shall, and shall cause its Affiliates to, treat as confidential and shall refrain from disclosing any information of a confidential or proprietary nature, in any form or medium, that relates exclusively to the Sellers or any of their Affiliates which they may possess.
(b) Section 10.5.2(a) does not apply to any disclosure (x) to the extent that it is required to be disclosed by applicable Law or rule of a listing authority or a stock exchange or requested by a Governmental Authority (including a Tax Governmental Authority) with relevant powers to which the Purchaser or its relevant Affiliate is subject or submits, (y) as
requested by a regulator with power to compel disclosure, or (z) as disclosed to a Governmental Authority (including a Tax Governmental Authority) in connection with any stamp or other documentary or transaction duties or other transfer Taxes, provided that the Purchaser or its relevant Affiliate shall, to the extent permitted by Law, notify the Sellers' Agent prior to the issuance or making of any such announcement or disclosure and shall use its reasonable endeavors to consult in good faith with the Sellers' Agent and to take into account its reasonable requirements of as to the timing, contents and manner of making any such announcement or disclosure.
(c) Any Affiliate of the Sellers, acting on its own or in cooperation with another Affiliate, may invoke and enforce Section 10.5.2(a).
10.6 Costs and Expenses.
Whether or not the transactions contemplated by this Agreement are consummated, except as may otherwise be expressly provided herein, each of the Sellers, on the one hand, and the Purchaser, on the other hand, shall bear their own expenses incurred in connection with the negotiation, preparation and signing of this Agreement and the consummation of the transactions contemplated herein.
10.7 Payments.
(a) Unless otherwise specified in this Agreement, all payments to be made (or caused to be made) under this Agreement shall be made in full without any set-off, restriction or condition, by wire transfers of immediately available cleared funds in such amounts, free of any bank or other charges (other than bank or other charges imposed by the recipient's bank) and without any deduction or withholding (unless such deduction or withholding is required by Law).
(b) If any deductions or withholdings are required by Law to be made from any payment to be made under this Agreement, the payer shall be obliged to pay to the recipient such additional amounts as will ensure that the recipient receives, in total, an amount which (after such deduction or withholding has been made) is no more and no less than it would have been entitled to receive in the absence of any such requirement to make a deduction or withholding.
(c) Any payment to be made under this Agreement which is not paid on its due date shall bear interest calculated from the due date until the effective date of payment at a rate per annum of five (5) per cent per annum above EURIBOR (with annual capitalization).
(d) For the purpose of converting amounts specified in one currency into another currency where required under this Agreement, the rate of exchange to be used in converting amounts specified in one currency into another currency shall be the foreign exchange rate as published on the website of the European Central Bank on the date of the relevant transaction or, if such rate is not available on such date, the date prior to such date for which such exchange is available; it being specified that for the purposes of any other amount labelled in a currency other than Euros set forth in the Pre-Closing Certificate, such reference date shall be the date on which the Pre-Closing Certificate is issued.
10.8 No Hardship.
(a) Each Party hereby acknowledges that the Agreement falls within the scope of article L. 211-40-1 of the French Monetary and Financial Code.
(b) Therefore, each Party hereby acknowledges that the provisions of article 1195 of the French Civil Code shall not apply to it with respect to its obligations under this Agreement and that it shall not be entitled to make any claim under article 1195 of the French Civil Code.
10.9 Specific Performance.
Pursuant to article 1221 of the French Civil Code, each Party acknowledges and agrees that money damages would not be an adequate remedy for any breach of this Agreement and that in addition to all other remedies which the non-breaching Parties may have, the non-breaching Parties shall be entitled to specific performance and injunctive or other equitable relief as a remedy for any such breach.
10.10 Express Waivers.
The Purchaser expressly and irrevocably waives (i) any right it may have under article 1226 of the French civil code to terminate this Agreement, (ii) any right it may have under articles 1186 and 1187 of the French civil code to claim that this Agreement has lapsed as a result of any other contract contributing to the completion of the transactions contemplated hereunder having terminated, lapsed or being ineffective for any reason whatsoever, (iii) to the fullest extent permitted by applicable Law, the benefits of articles 1130 et seq., 1190, 1602, 1626, 1641 and 1643 of the French civil code, and (iv) its right to benefit from the provisions of article 1223 of the French civil code and to accept a partial performance of the Agreement in exchange for a proportional discount of the price (unless otherwise provided).
10.11 Notices.
(a) All notices, demands or other communications given or made under or in connection with the matters contemplated by this Agreement shall only be effective if made in writing in English and:
(i) sent by registered mail or overnight courier service of recognized international standing delivering an acknowledgement of receipt (such as Fedex or DHL);
(ii) sent by email (which shall contain a scanned copy of the signed notice, demand or other communication) (with a confirmation including a copy of such email to be sent by no later than the next Business Day by registered mail or overnight courier service of recognized international standing delivering an acknowledgement of receipt (such as Fedex or DHL)); or
(iii) delivered by hand delivery against an acknowledgement of receipt dated and signed by the recipient;
to the relevant Party at its address and contact details set forth on Annex 10.11.
(b) Any notice, demand or other communication made in accordance with Section 10.11(a) shall be deemed to have been duly given or made as follows:
(i) if sent by registered mail or overnight courier service of recognized international standing, on the date of the first presentation of the courier;
(ii) if sent by email, on the date indicated on such email; and
(iii) if delivered by hand, on the date indicated on the corresponding acknowledgement of receipt signed by the recipient;
provided that if, in accordance with the above provisions, any such notice, demand or other communication is given or made pursuant to paragraphs (i) and (ii) above on a day which is not a Business Day or after 6.00 p.m. on a Business Day, such notice, demand or other communication shall be deemed to be given or made at the start of the next Business Day.
(c) A Party may notify the other Parties of a change to its name, relevant addressee, address, electronic address or fax number for the purposes of this Section 10.11 in accordance with provisions of this Section 10.11 provided that such notification shall only be effective:
(i) on the date specified in the notification as the date on which the change is to take place; or
(ii) if no date is specified or the date specified is less than one (1) Business Day after the date on which notice is given, the date which is one (1) Business Day after notice of any such change has been given.
10.12 Professional Advice.
Each of the Parties acknowledges and confirms that it was advised by its own lawyers and other professional advisors and, in such connection, has been able to independently assess the scope of its rights and obligations under this Agreement and has had the opportunity to negotiate the terms of this Agreement to its or his satisfaction. Consequently, no lawyer or other advisor (including, for the avoidance of doubt, the person or firm organizing the electronic execution of this Agreement) shall be deemed to be the sole drafter (rédacteur unique) on behalf of all the Parties and each of the Parties acknowledges and agrees that this Agreement shall not be deemed a contract of adhesion (contrat d'adhésion).
10.13 Entire Agreement.
This Agreement (together with the Confidentiality Agreement, the Put Option Agreement, the Carve-out Documentation and the Warranty Agreement as well as any ancillary agreements) represents the entire agreement and understanding of the Parties with reference to the transactions set forth herein and no representations or warranties have been made in connection with this Agreement other than those expressly set forth herein and in the Warranty Agreement.
This Agreement supersedes all prior negotiations, discussions, correspondence, communications, understandings and agreements between the Parties relating to the subject matter of this Agreement and all prior drafts of this Agreement, all of which are merged into this Agreement. No prior drafts of this Agreement may be used to show the intent of the Parties in connection with this Agreement or shall otherwise be admissible into evidence in any Proceeding or other legal action involving this Agreement. Any matter disclosed in any Annex to the Agreement or in the Disclosed Information shall be deemed disclosed for all purposes of this Agreement (notwithstanding the absence of any specific cross-references in the Annex in question) save for any liability of the Sellers under the representations and warranties pursuant to Article 4 (except Section 4.4). The Purchaser acknowledges that certain items to which the Annexes refer have been included to provide additional information to the Purchaser, and that such inclusion shall not be deemed to be an acknowledgment by the Sellers that such items are material.
10.14 No Third Party Rights; Assignment.
(a) This Agreement shall inure to the benefit of, and be binding upon, the Parties and their respective successors and assigns; provided, however, that none of the Parties shall assign any of its rights or delegate any of its obligations created under this Agreement without the prior written consent of the other Parties. Except as expressly provided herein, nothing set forth in this Agreement shall be construed to give any Person other than the Parties any right, remedy or claim under or with respect to this Agreement or any provision of this Agreement.
(b) Notwithstanding Section 10.14(a):
(i) the Purchaser is entitled to grant security over, or assign by way of security, all or any of its rights under this Agreement for the purposes of or in connection with the financing raised by the Purchaser in relation to the Transaction, provided that: (A) no assignee(s) shall be entitled to receive under this Agreement any greater amount than that to which the Purchaser would have been entitled; and (B) the Sellers shall not incur any liability or increasing liability, or be subject to any obligation or increased obligation, as a result thereof; and
(ii) the Purchaser shall be entitled to assign, prior to the Closing Date, all its rights and obligations under this Agreement to a wholly-owned Affiliate provided that: (A) the assignee benefits from the same Financing Commitments, (B), the initial Purchaser remains jointly and severally (solidairement) liable with the obligations of the assignee under this Agreement and (C) the initial Purchaser notifies such assignment to the Sellers' Agent at the latest ten (10) Business Days prior to the Closing Date.
10.15 Severability.
This Agreement shall be deemed severable, and the invalidity or unenforceability of any term or provision hereof shall not affect the validity or enforceability of this Agreement or of any other term or provision hereof.
Furthermore, in lieu of any such invalid or unenforceable term or provision, the Parties intend that there shall be added as a part of this Agreement a provision as similar in terms to such invalid or unenforceable provision as may be possible and be valid and enforceable.
10.16 Waivers and Amendments.
No modification of or amendment to this Agreement shall be valid unless in a writing signed by the Parties referring specifically to this Agreement and stating the Parties' intention to modify or amend the same. Any waiver of any term or condition of this Agreement must be in a writing signed by the Party sought to be charged with such waiver referring specifically to the term or condition to be waived, and no such waiver shall be deemed to constitute the waiver of any other breach of the same or of any other term or condition of this Agreement.
10.17 Transfer Taxes.
Any transfer or stamp taxes (including any droits d'enregistrement) or similar levies that may become payable as a result of the signing of this Agreement or the transfer of the Transferred Securities pursuant hereto shall be borne by the Purchaser and shall be paid on a timely basis in compliance with all statutory requirements. The Purchaser shall provide the Sellers' Agent with evidence of the payment of any such taxes or levies promptly upon the written request of the Sellers' Agent. If any Party is requested by a Governmental Authority to pay any such Taxes (and/or any related interest and penalties, as the case may be) in accordance with applicable Law for which the other Party is responsible, the non-responsible Party shall pay such Taxes (and/or any related interest and penalties, as the case may be) and the responsible Party shall promptly reimburse the non-responsible Party for such payment (including any related interest and penalties, as the case may be).
10.18 Purchaser's Tax election rights, Tax Refunds.
10.18.1 The Purchaser shall be entitled to make a Section 338(g) tax election at its discretion for US income tax purposes.
10.18.2 Any tax refunds, including tax refunds for periods prior to the Closing Date, shall remain with the respective Group Company without any compensation by the Purchaser to the Sellers.
10.19 Governing Law and Submission to Jurisdiction.
10.19.1 The Agreement shall be governed by French law without regard to its conflict of laws provision.
10.19.2 All disputes arising out of or in connection with this Agreement or its validity shall be finally settled under the Rules of Arbitration of the International Chamber of Commerce which are applicable on the date of this Agreement, without recourse to the ordinary courts of law.
10.19.3 The place of arbitration is Paris, France. The language of the arbitration is the English language. Notwithstanding, the Parties may submit evidence in the French language. The arbitral tribunal shall consist of three arbitrators.
The third arbitrator who will act as president of the arbitral tribunal shall be appointed by the two arbitrators appointed respectively be each Party, after consultation with the Parties.
10.19.4 Nothing in this Agreement restricts or prohibits any Party from applying to any court of competent jurisdiction for any interim measure of protection in respect of the subject matter of the dispute.
10.20 Electronic Signature.
The Parties:
(a) acknowledge and agree that the Agreement is entered into in writing in electronic form, in accordance with the terms of article 1366 of the French Civil Code, and signed electronically by means of a reliable identification process implemented by DocuSign®, guaranteeing the link between each signature and this Agreement in accordance with the provisions of article 1367 of the French Civil Code;
(b) acknowledge and agree that the Agreement has the same enforceability as a hard-copy written document pursuant to the provisions of article 1366 of the French Civil Code and the Agreement shall be validly invoked to evidence such enforceability;
(c) acknowledge and agree that this electronic signature has the same legal enforceability as their handwritten signature and give certainty (date certaine) to the date of/attributed to the signing of this Agreement by the DocuSign services (www.docusign.com);
(d) acknowledge and agree that (i) the requirement of having one original copy of the Agreement per Party shall be deemed to be fulfilled if the Agreement electronically signed is established and stored pursuant to articles 1366 and 1367 of the French Civil Code and (ii) that this process allows each Party to be provided with a copy of the Agreement on a material format or to have access to a copy of the Agreement, pursuant to the provisions of article 1375 of the French Civil Code;
(e) acknowledge and agree to designate Luxembourg (Grand-Duchy of Luxembourg) as the place of signature of the Agreement;
(f) acknowledge and agree that the Agreement shall take effect on the date hereof.
[remainder of page left intentionally blank]
BRUKER INVEST AG
By: /s/ JUERGEN SREGA
Name: Mr. Juergen Srega
Title: Duly authorized
TECFIN S.A R.L.
By: /s/ BENJAMIN MATHU
Name: Mr. Benjamin Mathu
Title: Class A Manager (Gérant de catégorie A)
By: /s/ FRANCOIS NADIN
Name: Mr. François Nadin
Title: Class A Manager (Gérant de catégorie A)
MR. CHRISTOPH GAUER
By: ___________________________
ELIMAN SAS
By: /s/ CHRISTOPH GAUER
Name: Mr. Christoph Gauer
Title: Président
ELIMAN 2 SAS
By: /s/ CHRISTOPH GAUER
Name: Mr. Christoph Gauer
Title: Président
EXHIBIT 10.31
WARRANTY AGREEMENT
BY AND BETWEEN
BRUKER INVEST AG
TECFIN S.À R.L.
MR. CHRISTOPH GAUER
ELIMAN
AND
ELIMAN 2
WITH RESPECT TO
TECINVEST S.À R.L.
TECBID S.À R.L.
ELITECH MEPCO SAS
ELITECH GROUP SAS
AND
TECBID US, INC.
AND ALL THEIR DIRECT AND INDIRECT SUBSIDIARIES
Dated: February 27, 2024
TABLE OF CONTENTS
Page
ARTICLE I INTERPRETATION |
3 |
|
1.1 |
Certain Definitions. |
3 |
1.2 |
Additional Definitions. (to be updated) |
10 |
1.3 |
Principles of Interpretation. |
11 |
ARTICLE II BRING DOWN DISCLOSURES |
12 |
|
2.1 |
Closing Bring Down Certificate. |
12 |
2.2 |
W&I Insurance & Subrogation |
12 |
ARTICLE III REPRESENTATIONS AND WARRANTIES |
13 |
|
3.1 |
Financial Statements. |
13 |
3.2 |
Changes in the Business of the Acquired Companies. |
14 |
3.3 |
Sufficiency of Assets |
14 |
3.4 |
Incorporation – Existence of the Group Companies. |
14 |
3.5 |
Real Property. |
14 |
3.6 |
Tax Matters. |
15 |
3.7 |
Personal Property. |
17 |
3.8 |
Intellectual Property |
17 |
3.9 |
Information Technology. |
19 |
3.10 |
Compliance with Law. |
20 |
3.11 |
Permits. |
20 |
3.12 |
Environmental Matters. |
21 |
3.13 |
Proceedings. |
21 |
3.14 |
Material Contracts. |
21 |
3.15 |
Insurance. |
23 |
3.16 |
Employment Matters. |
23 |
3.17 |
Subsidies. |
27 |
3.18 |
Relationships with the Sellers’ Group. |
27 |
3.19 |
Regulatory Matters |
27 |
3.20 |
Group Companies |
29 |
ARTICLE IV REPAYMENT OBLIGATION |
29 |
|
4.1 |
Repayment Obligation; Limitation on Quantum. |
29 |
4.2 |
Time Limits for Claims. |
29 |
4.3 |
Payment. |
30 |
4.4 |
Exclusions. |
30 |
ARTICLE V MISCELLANEOUS |
31 |
|
5.1 |
Sellers' Agent. |
31 |
5.2 |
Termination. |
31 |
5.3 |
Confidentiality. |
32 |
5.4 |
Costs and Expenses. |
32 |
5.5 |
Professional Advice. |
32 |
5.6 |
No Hardship. |
33 |
5.7 |
Specific Performance. |
33 |
5.8 |
Notices. |
33 |
5.9 |
Entire Agreement. |
34 |
- i -
5.10 |
No Third-Party Rights; Assignment. |
34 |
5.11 |
Severability. |
35 |
5.12 |
Waivers and Amendments. |
35 |
5.13 |
Governing Law and Submission to Jurisdiction. |
35 |
5.14 |
Signature of this Agreement. |
35 |
List of Annexes:
Annex A – Data Room Documentation
Annex B – Sellers' Knowledge
Annex C – Closing Bring Down Certificates
Annex D – Notices
List of Schedules:
Schedule 3.1(b) – Exceptions with respect to Financial Statements
Schedule 3.1(c) – Management Accounts
Schedule 3.1(f) – Bank Accounts
Schedule 3.2 – Changes in the Business of the Acquired Companies
Schedule 3.4(a) – Disclosures with regard to Incorporation - Existence of the Group Companies
Schedule 3.5(a) – Owned Real Estate
Schedule 3.5(b) – Lease Agreements
Schedule 3.5(d) – Disclosures with respect to the maintenance of the Owned Real Estate and the lease real property
Schedule 3.6 – Disclosures with respect to Tax Matters
Schedule 3.8(a) – Registered Intellectual Property Rights
Schedule 3.8(b) – Disclosures with respect to Owned Group Intellectual Property Rights
Schedule 3.8(g) – Disclosures with respect to infringements of intellectual Property Rights
Schedule 3.8(i) – Disclosures with respect to Software
Schedule 3.9(a) – Disclosures with respect to Information Technology
Schedule 3.9(c) – Disclosures with respect to malfunction of Information Technology infrastructures
Schedule 3.9(d) – Disclosures with respect to ownership or licenses re. Information Technology
Schedule 3.10(b) – Disclosures with respect to audit re. compliance with Law
Schedule 3.10(c) – Disclosures with respect to compliance with Law re. data protection
Schedule 3.11(a) – Disclosures with respect to permits, licenses, certifications, authorizations, registrations, qualifications and consents
Schedule 3.11(c) – Disclosures with respect to audit and inspections Schedule 3.14(a) – Material Contracts
Schedule 3.13(a) – Proceedings
- ii -
Schedule 3.15 – Insurance policies
Schedule 3.16(a) – Employees
Schedule 3.16(b) – Disclosures with respect to Contracts, plans or other arrangements (golden parachute and change of control provision) regarding Key Employees
Schedule 3.16(c) – Disclosures with respect to bonuses, wages, salaries, remuneration or advantage regarding Key Employees
Schedule 3.16(d) – Disclosures with respect to bonus and compensation agreements
Schedule 3.16(e) – Disclosures with respect to compensation obligations
Schedule 3.16(f) – Pensions and retirement agreements
Schedule 3.16(g) – Employee benefits
Schedule 3.16(h) – Collective agreements
Schedule 3.16(i) – Independent contractors and freelancers
Schedule 3.16(j) – Employment, labor and social security disputes
Schedule 3.16(k) – Disclosure with respect to the transfer of employees
Schedule 3.16(l) – Disclosures with respect to claims of formers employees
Schedule 3.16(m) – Disclosures with respect to employment-related illnesses
Schedule 3.16(n) – Disclosures with respect to Pension Commitments
Schedule 3.18 – Disclosures with respect to Relationships with the Sellers’ Group
Schedule 3.19(b) – Disclosure with respect to proper and valid CE or other certification
Schedule 3.19(c) – Disclosure with respect to the technical documentation and the quality management system
Schedule 3.19(d) – Inspections and audits
Schedule 3.19(e) – Disclosure with respect to fulfilment of IVDR and MDR
Schedule 3.19(f) – Disclosure with respect to clinical evaluations THIS WARRANTY AGREEMENT, dated February 27, 2024 (as the same may be amended, supplemented or otherwise modified from time to time in accordance with its terms, this "Agreement"), is by and between:
- iii -
WARRANTY AGREEMENT
1. BRUKER INVEST AG, a stock company, organized under the laws of Switzerland, having its registered office at Industriestrasse 26, Fallenden, CH-8117, Schwitzerland and registered with the Commercial register of canton Zurich under the number CHE-100.756.943 (the "Purchaser");
and each of:
2. TECFIN S.À R.L., a private limited liability company (société à responsabilité limitée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L – 2449 Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B216066 ("TecFin");
3. MR. CHRISTOPH GAUER, a national of Germany residing at Hagener Strasse 35, 82418 Murnau, Germany ("Christoph Gauer");
4. ELIMAN, a simplified joint stock company (société par actions simplifiée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 1A, Heienhaff, L-1736 Senningerberg, and registered with the Trade and Companies Register of Luxembourg under number B 216548 ("Eliman 1"); and
5. ELIMAN 2, a simplified joint stock company (société par actions simplifiée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 1A, Heienhaff, L-1736 Senningerberg, and registered with the Trade and Companies Register of Luxembourg under number B 216502 ("Eliman 2" and, together with Eliman 1, the "Eliman Entities").
TecFin, Christoph Gauer and the Eliman Entities are referred to collectively as the "Original Sellers" and individually as a "Original Seller".
The Sellers and the Purchaser are referred to collectively as the "Parties" and individually as a "Party".
For the purposes of this Agreement, the term "Sellers" shall have the meaning set out for such term in the SPA.
RECITALS:
WHEREAS:
(A) TecInvest S.à r.l. is a company (société à responsabilité limitée) incorporated under the laws of the Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L-2449 Luxembourg, Grand Duchy of Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B 194689 ("TecInvest").
(B) TecBid S.à r.l. is a company (société à responsabilité limitée) incorporated under the laws of the Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L-2449 Luxembourg, Grand Duchy of Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B 212299 ("TecBid").
(C) Elitech Mepco is a company (société par actions simplifiée) incorporated under the laws of France, having its registered office is 13-15, rue Jean Jaurès, 92800 Puteaux (France) and registered with the Registry of Commerce and Companies of Nanterre under number 528 320 450 ("Mepco").
(D) Elitech Group SAS is a company (société par actions simplifiée) incorporated under the laws of France, having its registered office is 13-15, rue Jean Jaurès, 92800 Puteaux (France) and registered with the Registry of Commerce and Companies of Nanterre under number 527 913 479 ("EG SAS").
(E) TecBid US, Inc. is a corporation incorporated under the law of the State of Utah and having its registered office at 370 West 1700 South, Logan, Utah 84321, USA ("TecBid US"; and together with TecInvest, TecBid, Mepco and EG SAS, collectively, the "Channel Holding Companies").
(F) The Sellers and the Purchaser have entered into a share purchase agreement, dated even-date herewith (as such agreement may be amended, supplemented or otherwise modified from time to time in accordance with its terms, the "SPA"), providing for the sale by the Sellers to the Purchaser of all the shares issued by TecInvest (the "Transferred Shares") on the terms, and subject to the conditions, set forth in the SPA.
(G) In connection with the sale of the Transferred Shares pursuant to the SPA, the Sellers have agreed to give the representations and warranties (together the “Business Representations”) set out in this Agreement.
- 2 -
NOW, THEREFORE, the Parties hereto do hereby agree as follows:
ARTICLE I INTERPRETATION
1.1 Certain Definitions.
In addition to such terms as are defined elsewhere in this Agreement (including the Recitals), in this Agreement:
"2022 Financial Statements" shall mean the (i) individual financial statements (to the extent they are audited – the audited individual financial statements) of each of the Acquired Companies and (ii) the audited consolidated financial statements of the Group, as of and for the year ended on December 31, 2022;
"Accounting Principles" shall mean, in respect of each relevant Acquired Company and each relevant Group Company, (i) IFRS for the consolidated accounts, and (ii) local generally accepted accounting principles and methods applied by such Acquired Company or Group Company consistently with past practices in compliance with local Laws and customs, whether for the drawing of its annual accounts or consolidated accounts (as the case may be);
"Acquired Companies" shall mean, collectively, the Channel Holding Companies and their respective direct and indirect Subsidiaries, except for the Carve-Out Companies;
"Affiliate" when used with reference to a specified Person, shall mean any Person that directly or indirectly through one or more intermediaries controls, is controlled by or is under common control with the specified Person. For such purposes, the term "control" (including the verb "to control" and the terms "controlling", "controlled by" and "under common control with") shall mean the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by Contract or otherwise; in such regard:
(a) a limited partnership shall be deemed to be controlled by its general partner(s);
(b) a "fonds professionnel de capital investissement" or a "fonds commun de placement" shall be deemed controlled by its management company (société de gestion);
(c) portfolio company in which an investment fund holds, directly or indirectly, an equity investment shall be deemed to be an Affiliate of such investment fund; and
(d) after the Closing Date, the Acquired Companies shall be deemed to be Affiliates of the Purchaser;
"Anti-Corruption Laws" shall mean (i) the U.S. Foreign Corrupt Practices Act of 1977 (as amended) and/or (ii) the United Kingdom Bribery Act and/or (iii) the German Criminal Act and/or (iv) anti-bribery legislation promulgated by the European Union and implemented by its member states and/or (v) legislation adopted in furtherance of the OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transaction and/or (vi) all other anti-bribery and anti-corruption Laws applicable to the Company or any of its Subsidiaries or their respective operations from time to time, to the extent applicable;
"Anti-Money Laundering Regulations" shall mean all anti-money laundering-related laws, regulations, reporting requirements and codes of practice applicable to the Company and its Subsidiaries, including without limitation (i) the EU Anti-Money Laundering Directives and any laws, decrees, administrative orders, circulars, or instructions implementing or interpreting the same and/or (ii) the applicable financial recordkeeping and reporting requirements of the U.S. Currency and Foreign Transaction Reporting Act of 1970, as amended and/or all other anti-money laundering-related Laws applicable to the Company and any of its Subsidiaries;
- 3 -
"Business" shall mean the research and development, manufacturing, purchase, sale, distribution of any In-Vitro Diagnostics or Life Science products;
"Business Day" shall mean any day, other than a Saturday or a Sunday, on which commercial banking institutions in Paris, France and Luxembourg, Grand Duchy of Luxembourg and Frankfurt am Main, Germany and New York City, New York/USA are open for ordinary banking business;
“Carve-out Companies” shall have the meaning ascribed to such term in the SPA;
"Closing Date" shall mean the date of completion of the sale of the Transferred Shares in accordance with the terms of the SPA;
"Code" shall mean the U.S. Internal Revenue Code of 1986, as amended;
"Confidentiality Agreement" shall have the meaning ascribed to such term in the SPA;
"Contract" shall mean any written contract, agreement, obligation, promise, commitment or other undertaking;
"COVID-19" shall mean SARS-CoV-2 or COVID-19 pandemic, and any evolutions thereof;
"Damages" shall mean any damages (dommages), liabilities, costs, loss of profit, expenses, fines, penalties or losses (pertes) (including but not limited to the costs of Proceedings, fees and expenses of lawyers, experts and consultants as well as late payment interests) provided that the determination of any Damages shall exclude any damages or losses which are contingent (for the time they are contingent), potential, unforeseen, consequential or potential, punitive and any loss of opportunity (perte d'une chance);
"Data Room" shall mean the virtual data room hosted by Intralinks containing the Data Room Documentation to which the Purchaser and its advisers have had access between November 13, 2023 and December 22, 2023;
"Data Room Documentation" shall mean (i) the documents previously made available for the Purchaser's and its advisors' review in the Data Room, a list of which is attached as Annex A and (ii) the written answers provided by the management of the Acquired Companies, the Sellers or their respective advisers to the queries made by the Purchaser and its advisers in connection with the Acquired Companies (whether through written Q&A or during site visits, Q&A sessions or calls and expert sessions or calls – it being specified that the Q&A and summaries of the information provided during the site visits, Q&A sessions or calls and expert sessions or calls shall only be relevant if and to the extent they have been included in the Data Room); and which, in both cases, have been copied onto a series of storage devices of which (x) one copy has been provided to the Purchaser and (y) one copy has been retained by the Sellers' Agent on the Put Option Date;
"Disclosed Information shall mean, collectively:
(a) the management presentations provided in the Data Room Documentation and identified as:
(i) Project Channel - MP (Full) - 2023.03.13
- 4 -
(ii) CHANNEL Discussion Materials June 2023_07.06.2023
(iii) Project Channel - EBS Presentation - 17.06.2023 vSent
(iv) CHANNEL Turin Discussion Materials June 11 2023 vSent
(v) EG-Bruker visit R&D QARA overview 110723
(vi) CHANNEL Signes EM Discussion Materials July 18 2023 vFINAL
(vii) CHANNEL Signes EF Discussion Material July 18 2023 230717
vFINAL-3
(viii) CHANNEL Spankeren Discussion Materials July 19 2023 vFINAL
(ix) Project Channel – CC Carve-out and exit strategy as attached as Annex 6.1(a) to the SPA
(b) the Reports; and
(c) the Data Room Documentation;
"Encumbrance" shall mean any pledge of real or personal property (nantissement or gage), mortgage (hypothèque), lien (privilège), right of retention (droit de rétention), fiducie-sûreté, charge (charge), ownership right (démembrement), seizure (saisie), escrow (séquestre) or other security (sûreté), option or other similar third party right in favour of any Person or any agreement, to create any of them (including inter alia a sale agreement, non-compete obligation, non-transferability obligation, pre-emptive right, preferential agreement, tag-along right, drag-along right, escrow arrangement, right of retention, retention of title clause, claim or demand) or any other third-party right or obligation of any kind that has a similar purpose or effect (excluding: (x) any restrictions or limitations on transfer of any securities set forth in the Organizational Documents of the issuer of such securities, as applicable, (y) any pledge, lien, right, charge or other security created or granted by the Purchaser or any of its Affiliates, and (z) for the avoidance of doubt, licenses of Intellectual Property granted in the Ordinary Course of Business);
"Entity" shall mean any company (société), partnership (limited or general), joint venture, trust, association, economic interest group (groupement d'intérêt économique) or other organization, enterprise or entity, whether or not having a separate legal personality (personnalité morale);
“Environment” shall mean soil, sediment, land surface or subsurface strata, surface waters (including navigable waters, ocean waters, streams, ponds, drainage basins, and wetlands), groundwaters, drinking water, ambient air (including indoor and outdoor air), plant, insect and animal life, and any other environmental medium, other geologic media, or natural resource;
“Environmental Condition” shall mean any environmental contamination, pollution, release, threatened release, or the presence of Hazardous Materials in, into, on, onto, under, migrating to, or migrating from the Environment, violation, actual or alleged, of Environmental Law, or any allegation of liability arising under any Environmental Law;
"Environmental Law" shall mean any federal, state, and local Law, and other provisions having the force or effect of Law, or other legal requirement, relating to pollution or the protection of the Environment (including all those related to public health and safety, noise and the safety of property and persons, including employees, and the production, generation, reporting, handling, storage, transportation, treatment, release, threatened release, or cleanup of any Hazardous Material, and the cleanup, removal, recovery, assessment, or remediation of any damage, release, emission, discharge, pollution, or contamination of or into the Environment), or any aspect of management, handling or use of any Hazardous Material or other materials, wastes, chemicals or substances regulated by any Governmental Authority, including but not limited to: the Comprehensive Environmental Response, Compensation and Liability Act, 42 U.S.C.
- 5 -
§§ 9601 et seq.; the Solid Waste Disposal Act, also known as the Resource Conservation and Recovery Act, 42 U.S.C. § 6901, et seq.; the Emergency Planning and Community Right-to-Know Act, 42 U.S.C. § 11001, et seq.; the Hazardous Materials Transportation Act, 49 U.S.C. §§ 5101 et seq.; the Clean Air Act, 42 U.S.C. §§ 7401 et seq.; the Federal Water Pollution Control Act, as amended by the Clean Water Act, 33 U.S.C. §§ 1251 et seq.; the National Environmental Policy Act of 1975, 42 USC Section 4321, et seq., the Occupational Safety and Health Act, as amended, 29 U.S.C. §§ 651, et seq.; the Toxic Substances Control Act, 15 U.S.C. §§ 2602 et seq.; the Rivers and Harbors Act of 1899, 33 U.S.C. § 401, et seq.; the Endangered Species Act of 1973, as amended, 16 USC Section 1531, et seq., the Oil Pollution Act of 1990, 33 U.S.C. § 2701, et seq.; the Safe Drinking Water Act of 1974, as amended, 42 USC Section 300(f), et seq., each as amended; any state or local law similar to the foregoing; all regulations issued pursuant to the foregoing; and all laws pertaining to all Environmental Permits issued to the Acquired Companies pursuant to the foregoing and all common law decisions applicable to the Acquired Companies related to the Environment; and all provisions of the French Civil Code and the rules concerning personal liability, liability for Third Parties, liability for property, nuisance between neighbours and all asbestos-related matters;
"ERISA" shall mean the U.S. Employee retirement Income Security Act of 1974, as amended.
"Execution Date" shall mean the signing date of this Agreement;
"Ex-Im Laws" shall mean all U.S. and applicable non-U.S. Laws relating to export, reexport, transfer, and import controls, including the Export Administration Regulations, the International Traffic in Arms Regulations, the customs and import Laws administered by U.S. Customs and Border Protection, and the EU’s Dual Use Regulation;
"Fairly Disclosed" shall mean with respect to a fact, information, circumstance or matter where such fact, information, circumstance or matter is disclosed with sufficient details to enable a reasonably diligent purchaser and its advisors to identify the nature and scope of the matter disclosed and make a reasonably informed assessment thereof based on a prima facie review (it being specified that when an estimate of the potential financial magnitude thereof has been disclosed, such estimate shall be deemed to have been disclosed in good faith, to the Sellers’ Knowledge, and, with respect to documents in the Data Room, where such documents are in (or are specifically referred to in) an appropriate folder of the Data Room, provided that the Parties agree that any reference in the Disclosed Information to information or documents that are not disclosed themselves shall not be construed as Fairly Disclosed;
"Governmental Authority" shall mean any domestic, foreign or supranational court or other judicial authority or governmental, administrative or regulatory body, department, agency, commission or authority;
"Governmental Authorization" shall mean any approval, consent, permit, ruling, waiver, exemption or other authorization (including the lapse, without objection, of a prescribed time under a statute or regulation that states that a transaction may be implemented if a prescribed time lapses following the giving of notice without an objection being made) issued, granted, given or otherwise made available by or under the authority of any Governmental Authority or pursuant to any Law;
- 6 -
"Group" shall mean the group composed of the Acquired Companies; “Hazardous Materials” shall mean collectively, any material, substance or waste that: (a) is classified or regulated or subject to standards of control or liability by any Governmental Authority under any applicable Environmental Law; (b) is included within statutory and/or regulatory definitions, designations or listings of “hazardous substance,” “contaminant,” “hazardous waste,” “extremely hazardous substance,” “solid waste,” “pollutant,” “regulated substance,” “hazardous materials,” “toxic substances,” “medical waste,” “mold”, or “air contaminant” or terms of similar significance under any Environmental Law; (c) any substance now listed in the United States Department of Transportation table or amendments thereto (49 C.F.R. 172.101) or by the United States Environmental Protection Agency (or any successor agency or department) as hazardous substances in 40 C.F.R. Part 302 and any amendments thereto; (d) any material, waste or substance which is any of the following: urea, asbestos or asbestos-containing materials, radon gas, pesticides, hydrocarbon, petroleum substance (including but not limited to crude oil or any fraction thereof, natural gas, natural gas liquids, gasoline, and synthetic gas), flammable, explosive, carcinogenic, infectious, mutagenic, toxic, lead paint, natural or synthetic gas, solid, liquid, or gaseous wastes, polychlorinated biphenyls (PCBs), formaldehyde foam insulation, per- and polyfluoroalkyl substances (PFAS), discharges of sewage or effluent, any material containing hydrated silicate (including amosite, actinolite, tremolite, chrysolite, crocidolite, and anthophyllite, whether friable or non-friable), and all toxic, hazardous, and radioactive wastes, chemicals, materials, pollutants, substances, and contaminants that are regulated or controlled by any Environmental Law, whether or not defined, classified, or identified as hazardous or toxic under those Laws, including any hazardous substance as defined in CERCLA, or source, byproduct or special nuclear material as defined in the Atomic Energy Act; (e) any industrial process and pollution control waste whether or not hazardous within the meaning of the Resource Conservation and Recovery Act, 42 U.S.C. 6901 et seq.; (f) any substance the presence of which requires investigation or remediation under any Environmental Law; (g) any substance the presence of which on any site or facility causes or threatens to cause a nuisance upon such site or facility or to any other premises or properties or poses or threatens to pose a hazard to the health or safety of persons on or about such site or facility or to any other premises or properties; (h) any substance, the presence of which on any site or facility could constitute a trespass by any person or entity; and (i) any substance which would otherwise constitute a Hazardous Material generated at the Acquired Companies’ facilities;
"Intellectual Property Right" shall mean any rights in the following: registered or unregistered patent, trademark, copyright, service mark, domain name, trade name, invention, logo, design right, know-how, trade secrets, Soleau envelope, company name, confidential information, drawing, software (including any specific software and software package as well as the related documentation concerning IT systems, application software and the revised objects and source code therefor), database right, domain name, licence or similar registered or unregistered intellectual and/or industrial property right (droit de propriété intellectuelle ou industrielle);
"Judgment" shall mean any award, decision, injunction, judgment, order or ruling entered, issued, made or rendered by any court, administrative agency or other Governmental Authority or by any arbitrator;
"Law" shall mean any treaty, convention, executive order, directive, law, ordinance, decree (décret), regulation (règlement), instruction, order (arrêté), rule (circulaire), principle of common law or code of any Governmental Authority (including any judicial or administrative interpretation thereof) in force;
"Lease Agreement" shall mean any lease agreement in respect of real properties to which any of the Acquired Companies is a party as of the Put Option Date and which provide for an annual net rent of more than seventy-five thousand euros (€75,000); "Ordinary Course of Business" shall mean the ordinary course of the operations of the Acquired Companies consistent with the diligence of a prudent businessman and with past practices and conduct (including any conduct, practice or action taken as Pandemic Measures, with respect to, or as a result of, COVID-19);
- 7 -
"Organizational Documents" shall mean when used with respect to (x) any company (société) or other incorporated Entity, the memorandum and articles of association (statuts), charter or similar constitutive document of such company (société) or other incorporated Entity, as filed with the relevant commercial registry, company registrar or other Governmental Authority, as the same may be amended, supplemented or otherwise modified from time to time, and (y) any partnership or other unincorporated Entity, its certificate of formation, partnership agreement, governing agreement (contrat constitutif) and/or similar constitutive document, as the same may be amended, supplemented or otherwise modified from time to time;
"Pandemic Measures" shall mean any quarantine, "shelter in place," "stay at home", workforce reduction, social distancing, shut down, closure, sequester or any other Law, order, directive, guidelines or recommendations by any Governmental Authority in connection with, or in respect of, COVID-19 or any other pandemic or epidemic;
"Person" shall mean a natural person, Entity, or Governmental Authority;
"Proceeding" shall mean any litigation, arbitration, dispute or other legal proceeding commenced, brought, conducted or heard by or before any Governmental Authority or arbitrator;
"Purchase Price" shall mean the aggregate consideration to be paid for the Transferred Shares pursuant to the SPA (as adjusted in accordance with the terms thereof);
"Put Option Date" shall mean December 23, 2023;
"Reports" shall mean, collectively:
(a) the tax fact book prepared by Hogan Lovells LLP; and
(b) the financial due diligence report and its addendum relating to the Transaction prepared Deloitte.
"Sanctioned Country" shall mean any country or region or government thereof that is, or has been in the last five years, the subject or target of a comprehensive embargo or sanctions under any applicable Trade Control Laws (including, at the time of signing, Belarus, Crimea, Iran, North Korea, Russia, Sudan, Syria, and the Donetsk and Luhansk regions of Ukraine);
"Sanctioned Person" shall mean any Person that is the subject or target of sanctions or restrictions under Sanctions Laws or Ex-Im Laws, including: (i) any Person listed on any U.S. or applicable non-U.S. sanctions- or export-related restricted party list, including the List of Specially Designated Nationals and Blocked Persons maintained by the U.S. Department of the Treasury, Office of Foreign Assets Controls (“OFAC”); (ii) any Person that is, in the aggregate, 50 percent or greater owned, directly or indirectly, or otherwise controlled by a Person or Persons described in clause (i); or (iii) any Person located, organized, or resident in a Sanctioned Country;
"Sanctions Laws" shall mean all U.S. and applicable non-U.S. Laws relating to economic or trade sanctions, including the sanctions Laws administered or enforced by the United States (including by OFAC or the U.S. Department of State), the European Union or its member states, the United Kingdom, or the United Nations. "Sanctions Regulations" shall mean any economic or trade sanctions or restrictive measures adopted, administered, imposed or enforced by the Office of Foreign Assets Control (OFAC) of the U.S. Department of the Treasury, the U.S. Department of State, the United Nations Security Council and/or the European Union and/or the Republic of France and/or Her Majesty's Treasury or any other relevant sanctions authority;
- 8 -
"Sellers’ Group" shall mean the group of companies composed of the Sellers and their Affiliates other than the Acquired Companies;
"Sellers' Knowledge" shall mean the actual knowledge and grossly negligent lack of knowledge and deemed knowledge of such persons based on files, books and records of the Acquired Companies (incl. e-mail) of the natural persons identified in Annex B-1 as of the Signing Date and as of the Closing Date, respectively, after the Sellers and these persons have made due enquiry on the Business Representations with the persons listed in Annex B-2. The Sellers shall not be obliged to carry out any other investigation.
"Sellers’ Representative" shall have the meaning ascribed to it in the SPA;
"Subsidiary" when used with reference to a specified Person, shall mean any Entity which is controlled (as this term is defined in the definition of Affiliates) by such Person, excluding Elitech SR (Serbia);
"Tax" shall mean: (i) all forms of taxation, duties, imposts, levies, customs duties, escheat or surrender of property deemed abandoned, remittance of taxes which should be or have been collected from employees or customers, repayment of investment grants and other forms of subsidies (including state aid), value added tax and contributions, including all social security charges, social and insurance contributions and employment and payroll taxes, and any associated interest, penalty, surcharge or fine under the Law of any jurisdiction; (ii) any liability for any amount of the types described in clause (i) as a result of being a member or a former member of a Tax consolidated group or similar arrangement or agreement and (iii) any liability for the payment of any amount of the types described in (i) or (ii) above as a result of being a transferee or successor to any person, or as a secondary liability, or due on behalf of third parties, or as a result of any obligation to indemnify any other person;
"Tax Authority" shall mean a Governmental Authority having authority to assess, determine, collect, audit or impose any Tax, the Tax base or elements thereof;
"Tax Returns" shall mean all returns, declarations, notices, self-assessment or similar documents, elections and forms including all schedules or attachments thereto which are filed with any Tax Authority with respect to Taxes;
“Third Party” shall mean any Person other than the Parties;
"Trade Control Laws" shall mean all applicable U.S. and non-U.S. Laws relating to export, reexport, transfer controls, customs, import, economic or trade sanctions, and anti-boycott, including but not limited to, the export administration regulations, the foreign trade regulations, and all applicable U.S. sanctions programs administered by the Office of Foreign Assets Control.
"Transaction" shall mean the sale and purchase of the Transferred Shares in accordance with the SPA;
"U.S. Employee Plan" shall mean any Pension Commitment or Employee Benefit maintained for the benefit of employees or directors (or their eligible dependents) of, or other providers of personal services to, a US Entity;
"US Entities" shall mean, collectively, TecBid US, ELITechGroup Inc., a Utah corporation and ELITechGroup MDx, LLC, a Washington limited liability company.
- 9 -
Each sometimes referred to separately as a "US Entity"; "VAT" shall mean (i) any tax imposed in compliance with the Council Directive of 28 November 2006 on the common system of value added tax (EC Directive 2006/112), as amended; and (ii) any other tax of a similar nature (including any sales or turnover Tax), whether imposed in a member state of the European Union in substitution for, or levied in addition to, such tax referred to in (i) above, or imposed elsewhere;
"W&I Insurer" shall mean Euclid Transactional UK Limited;
"W&I Policy" shall mean the insurance policy subscribed on or about the Put Option Date by the Purchaser with the W&I Insurer in respect of the representations and warranties made by the Sellers to the Purchaser hereunder and under the SPA; and
"Working Hours" shall mean 9.00 a.m. to 6.00 p.m. on a Business Day.
1.2 Additional Definitions. (to be updated)
Each of the following additional terms has the meaning specified therefor in the Section indicated below opposite such term:
Term Section
Agreement Recitals
Business Representations Recitals
EG SAS Recitals
Employee Benefits 3.16(g)
Channel Holding Companies Recitals
Closing Bring Down Certificate 2.1
Closing Bring Down Disclosures 2.1
Group Intellectual Property Rights 3.8(a)
In-bound License Agreements 3.8(d)
IVDR 3.19(b)
Information Technology 3.9(a)
Key Employees 3.16
Know-How 3.8(h)
Management Accounts 3.1(c)
Material Contracts 3.14
MDR 3.19(b)
Mepco Recitals
Owned Real Estate 3.5(a)
Owned Group Intellectual Property Rights 3.8(a)
Party Recitals
Pension Commitments 3.16(f)
Permits 3.11(a)
- 10 -
Purchaser Recitals
Registered Intellectual Property Rights 3.8(a)
Sellers Recitals
Sellers’ Agent 5.11.1(a)(a)
Software 3.8(i)
Subsidies 3.17(a)
TecBid Recitals
TecBid US Recitals
TecInvest Recitals
Transferred Shares Recitals
W&I Cap 4.1(a)
1.3 Principles of Interpretation.
In this Agreement:
(a) All references herein to Articles, Sections, Schedules and Annexes shall be deemed references to articles and sections of, and schedules and annexes to this Agreement unless the context shall otherwise require. The descriptive headings to Articles, Sections, Schedules and Annexes are inserted for convenience only, and shall have no legal effect.
(b) The Annexes and Schedules to this Agreement shall be deemed to be a part of this Agreement, and references to "this Agreement" shall be deemed to include the same.
(c) Days shall be to calendar days unless Business Days are specified (provided that articles 640 to 642 of the French Code of Civil Procedure shall be applied to calculate any period of time under the Agreement, provided that the references in article 642 to “un jour férié ou chômé” and “premier jour ouvrable” shall be interpreted by reference to the definition of “Business Day” appearing herein).
(d) The following rules of interpretation shall apply unless the context shall require otherwise:
(i) Definitions used in this Agreement shall apply equally to both the singular and plural forms of the terms defined as well as to any gender. Pronouns of the masculine gender shall be deemed to include the feminine and neuter, and vice versa, as the context may require.
(ii) Whenever used in this Agreement:
(A) the words "include", "includes" and "including" shall be deemed to be followed by the phrase "without limitation";
(B) the words "hereof", "herein" and similar words shall be construed as references to this Agreement as a whole and not just to the particular Section or subsection in which the reference appears; and
(C) except when used with the word "either", the word "or" may have a disjunctive and not alternative meaning (i.e., where two items or qualities are separated by the word "or", the existence of one item or quality shall not be deemed to be exclusive of the existence of the other and, as the context may require, the word "or" may be deemed to include the word "and").
- 11 -
(iii) A reference to a specific time of day shall be to local time in France.
(iv) A reference to any Party to this Agreement or any other agreement or document includes such Party's successors and permitted assigns.
(v) A reference to any agreement or document is to that agreement or document as amended, novated, supplemented, varied or replaced from time to time, except to the extent prohibited by this Agreement.
(vi) A reference to any legislation or to any provision of any legislation includes any modification or re-enactment of such legislation, any legislative provision substituted for such legislation, and all regulations and statutory instruments issued under such legislation.
(vii) Unless specifically provided for in this Agreement, there shall be no requirement for a prior notice (mise en demeure préalable) for one Party to claim any right or implement any remedy, including termination of this Agreement, provided for hereunder and the Parties expressly waive the requirement for such a prior notice.
(viii) An obligation to use commercially reasonable, reasonable or best efforts or endeavors (or any similar obligation) shall be construed as an "obligation de moyens".
(ix) When a French term is translated into English, such translation is for information purposes only and the French term shall prevail.
(e) Any representation or warranty qualified by the expression "to the Sellers’ knowledge" or any similar expression shall be deemed to refer exclusively to the actual (as opposed to constructive or imputed) knowledge of the persons whose names and position are set out in Annex B-1 after due enquiry with the persons listed in Annex B-2.
ARTICLE II BRING DOWN DISCLOSURES
2.1 Closing Bring Down Certificate.
(a) No earlier than two (2) Business Days prior to the Closing Date and no later than one (1) Business Day prior to the Closing Date, the Sellers shall review the Business Representations and shall inquire each other and the persons listed in Annex B-2 in order to disclose the exceptions to the Business Representations that reflect any facts, events or matters that have occurred between the Signing Date (included) and the Closing Date (excluded) and which render any of the Business Representations not being correct or complete as of the Closing Date ("Closing Bring Down Disclosures"). The Sellers shall disclose the results of the Closing Bring Down of Disclosures by executing and delivering to the Purchaser a “closing bring down disclosure certificate”, a draft of which is attached as Annex C either (i) confirming that the Business Representations are still correct and complete as of the Closing Date or (ii) specifying in reasonable detail the facts, events and circumstances which render the Business Representations not being correct and complete as of the Closing Date (the "Closing Bring Down Certificate"). For the avoidance of doubt, (i) any Closing Bring Down of Disclosures made in the Closing Bring Down Certificate shall qualify any representations made as at the Closing Date and (ii) the Closing Bring Down Certificate shall not limit Seller(s)' liability for any breach of a representation given by the Sellers on the Put Option Date and/or on the Signing Date, as the case may be.
(b) The Sellers’ Agent will provide the Purchaser with a draft of the Closing Bring Down Certificate four (4) Business Days prior to the Closing Date to afford the Purchaser the opportunity to review and discuss with the Sellers and the Company the draft Closing Bring Down Certificate.
2.2 W&I Insurance & Subrogation
- 12 -
The Purchaser acknowledges and agrees that the Business Representations are granted subject to the condition precedent that the W&I Policy contains a wording according to which the W&I Insurer will not be entitled to make, and will not make, any recourse claim against the Sellers in respect of any breach of a Business Representation, except in cases of fraud or willful misconduct. The Purchaser shall not amend the wording of the W&I Policy on subrogation without the prior written consent of the Sellers.
ARTICLE III REPRESENTATIONS AND WARRANTIES
The Sellers individually and not jointly (individuellement et non solidairement) hereby represent and warrant to the Purchaser that as of the Put Option Date, the date of this Agreement and as at the Closing Date (except for such representations and warranties which are expressly made as of a specific date and are therefore made as of such date only):
3.1 Financial Statements.
(a) The 2022 Financial Statements were prepared, in all material respects, in accordance with the Accounting Principles (save for the 2022 Financial Statements of EG GmbH that are not drawn-up as at the date hereof).
(b) Except as provided in Schedule 3.1(b), based on the facts known or to be known or taken into account by the managing directors of the Acquired Companies according to their duties under the Accounting Principles and the Laws as of the date of drawing-up the respective 2022 Financial Statements, to the Sellers’ Knowledge, (i) the 2022 Financial Statements are true and complete in all material respects, (ii) have been prepared in compliance with the Accounting Principles on a consistent basis with formal and material balance sheet continuity, in particular, all accounting principles have been retained and all election rights, have been exercised without change compared to the financial statements of the respective previous period in all material respects, and (iii) the 2022 Financial Statements provide, in all material respects, a true and fair view of the respective Acquired Company's/Acquired Companies' asset, liabilities, cash flows, financial and profit situation in accordance with the applicable Accounting Principles as at the respective balance sheet date and for the period ending on the respective balance sheet date.
(c) The consolidated management accounts of the Group Companies for the time period between 1 January 2023 and 31 October 2023 ("Management Accounts") which are attached as Schedule 3.1(c) (i) have been prepared by the managing directors of the Group Companies with the reasonable care and in accordance with the relevant Accounting Principles, and (ii) to the Sellers' Knowledge, do not materially misstate the Group Companies' assets, liabilities, cash flows, financial and profit situation in accordance with IFRS.
(d) All trade accounts receivables of an Acquired Company which are reflected in its books arise from sales or services in the Ordinary Course of Business. Since 1 January 2023 there has been no unusual acceleration of cash receipts or delays in paying any accounts payables of an Acquired Company. To the Sellers' Knowledge, there are no facts or circumstances which are likely to make impossible or materially delay the collection of such trade accounts receivables to an extent beyond normal historical bad debt losses of an Acquired Company.
(e) To the Sellers' Knowledge, the books and records of the Acquired Companies are in all material respects accurate, up-to-date and have been maintained in accordance with all applicable legal and accounting requirements To the Sellers' Knowledge, the Acquired Companies have unrestricted access at all times to all books and records of the Acquired Companies (including, without limitation, records and files stored on computer disks, tapes or other storage media).
- 13 -
(f) Schedule 3.1(f) contains a complete and correct list of all bank accounts of the Acquired Companies and authorized signatories for each account. There have been no payments into or out of any of these accounts since 1 November 2023, except for in the Ordinary Course of Business and as proper and consistent with past practice.
3.2 Changes in the Business of the Acquired Companies.
Except as provided in Schedule 3.2, since 1 January 2023, each of the Acquired Companies (i) has conducted its Business in all material respects in the Ordinary Course of Business in substantially the same manner as theretofore conducted, and (ii) has not taken any of the actions described in Section 6.2.1 (i) through (xv) of the SPA that would have required the consent of the Purchaser under the terms of the SPA if such action were to be taken between the date hereof and the Closing Date.
3.3 Sufficiency of Assets
To the Sellers’ Knowledge, the material assets owned, leased or otherwise used by the Acquired Companies constitute all material assets, properties or rights which are required for the conduct of the Business as currently conducted.
3.4 Incorporation – Existence of the Group Companies.
(a) Each Group Company was duly incorporated (or similar) and registered, and validly exists in accordance with the Laws of its jurisdiction of incorporation (or similar) and its articles of association. Except as disclosed in Schedule 3.4(a), each US Entity is duly qualified and in good standing as a foreign limited liability company authorized to do business in each jurisdiction in which the character of the properties owned or held under lease by it, or the nature of the business transacted by it, makes such qualification necessary.
(b) No resolution has been adopted and no meeting has been convened with a view to winding up or liquidating any of the Group Companies.
None of the Group Companies is insolvent or is the subject of any Insolvency Proceedings. The books, registers, minutes, shareholder accounts and security transfer registers have always been kept as materially required, by the Group Companies.
3.5 Real Property.
(a) Schedule 3.5(a) includes for each Acquired Company a correct and complete list of all real estate owned or co-owned by such Acquired Company, or subject to an inheritable building right, in-rem lease or similar right in favor of such Acquired Company (the "Owned Real Estate"). There is no further real estate owned by the Acquired Companies or to which they hold any of the afore-mentioned rights. There are no Encumbrances in or over the Owned Real Estate and there is no agreement or commitment to give or create any of them.
(b) Schedule 3.5(b) sets forth a complete list of all the Lease Agreements. To the Sellers' Knowledge and except as disclosed on Schedule 3.5(b):
(i) assuming valid title of the lessor of such real property, each Lease Agreement is in full force and effect, no Acquired Company has breached or is in breach of any material obligations of any Lease Agreement, and each of the Acquired Companies has the right to occupy and use all real property shown on such Schedule as leased by it; (ii) no party to any of the Lease Agreements has given any of the Acquired Companies written notice of: (i) any material increase after the Put Option Date in rent or charges, other than an increase in accordance with the terms of such lease or applicable Laws; (ii) any non-renewal of occupancy after the Put Option Date; (iii) any material variation or termination after the Put Option Date of any such lease; or (iv) any claim with respect to any breach or default under any such lease; and
- 14 -
(iii) no consent to the consummation of the Transaction is required from the lessor of any such Lease Agreements and, to the Sellers' Knowledge, there are no rights of any lessor to terminate a Lease Agreement or amend the terms and conditions.
(c) To the Sellers’ Knowledge, each Acquired Company has maintained the real property leased under a Lease Agreement with an annual rent exceeding EUR 100,000 in all material respects in accordance with the requirements of the applicable Lease Agreement and such real property and the Owned Real Estate in a manner sufficient to support the Business of the Acquired Companies as currently conducted. To the Sellers' Knowledge, (i) the real property leased under any Lease Agreement and the Owned Real Estate are in compliance with the zoning and permitting Laws and (ii) are not subject to any restrictions that limit or interfere with the intended use of the leased real property or the Owned Real Estate by the respective Acquired Company to carry on its Business.
(d) Except as provided in Schedule 3.5(d), to the Sellers' Knowledge, the Owned Real Estate and the leased real property, and the buildings and other fixtures thereon, have been properly maintained in all material respect, are in satisfactory order and repair (normal wear and tear excepted), are fit for the intended use and are in a condition adequate to conduct the business of the respective Acquired Company as currently conducted.
3.6 Tax Matters.
Except as disclosed on Schedule 3.6:
(a) each of the Acquired Companies has properly and timely filed or caused to be filed with the appropriate Governmental Authorities all Tax Returns that were required to be filed, and all such Tax Returns and, to the Sellers' Knowledge, all statements and information in such Tax Returns were true, complete and correct; in particular, all Tax credits shown in such Tax returns are valid and their amount is accurate so that such Tax credits could not be challenged by a Tax Authority;
(b) The Acquired Companies have always timely and completely paid or withheld and remitted when due all Taxes to the competent Tax Authorities or, if and to the extent not yet due, adequately accounted for such Taxes as a Tax liability or a Tax provision, as the case may be, in (x) the 2022 Financial Statements in accordance with the Accounting Principles (to the extent the Tax pertains to any period up to December, 31, 2022) or (y) the Final Closing Statement (to the extent the Tax pertains to the period after December, 31, 2022); to the Sellers' Knowledge, there are no facts which could give rise to any additional liability of any of the Acquired Companies for any Tax claims over and above that has already been paid or provided for and no further payments or penalties shall become due;
(c) (x) to the Sellers' Knowledge, there are no pending audits, examinations, investigations or other proceedings relating to the assessment or collection of Taxes for which any of the Acquired Companies may be liable, and (y) no claim for assessment or collection of Taxes relating to any of the Acquired Companies that is or, to the Sellers' Knowledge, may become payable by any of them has been notified in writing to the Sellers or any of the Acquired Companies by any Tax Authority;
(d) All material books, records, documents and accounts which each Acquired Company is required by Tax Law to present to the competent Tax Authorities upon their request have been properly prepared and are in the possession of the Acquired Companies; (e) To the Sellers' Knowledge, the Acquired Companies are registered for VAT purposes and, to the Sellers' Knowledge, (i) cannot be subjected to a recapture of VAT deducted on or before the Completion Date and (ii) have complied in all respects with all other applicable VAT legislation;
- 15 -
(f) The Acquired Companies have deducted or withheld all Tax which they have been obliged by applicable law to deduct or withhold from amounts paid by them to any employee, independent contractor, creditor, stockholder or any other person and have properly accounted to the relevant Tax Authority for all amounts of Tax so deducted or withheld and has otherwise complied with their legal obligations in respect of such deductions or withholdings;
(g) The Acquired Companies have not entered into any arrangements (being an arrangement which is not based on relevant legislation or any published practice) with, or obtained any (binding) ruling from, any Tax Authority nor have the Acquired Companies applied for or are negotiating such arrangements or rulings;
(h) No transaction, in respect of which any consent or clearance was required from any Tax Authority, has been entered into or carried out by the Acquired Companies, without such consent or clearance having first been properly obtained and all information supplied to any Tax Authority or other appropriate authority in connection with any such consent or clearance fully and accurately disclosed all facts and circumstances material to the giving of such consent or clearance; Any condition, commitment or formality required, according to the applicable Tax laws and any individual agreement granted by any Tax Authority, to benefit from an advantage with respect to Taxes have been duly met or made. The Acquired Companies have complied with all material conditions set out in any agreement applicable to them, granted by any Tax Authorities;
(i) The Acquired Companies have not had any dispute with any Tax Authority in relation to Taxes nor have the Acquired Companies been subject to any inspection, audit, or non-routine investigation by any Tax Authority for the last five (5) years prior to the date hereof. The Acquired Companies are not involved in any dispute with any Tax Authority;
(j) Each of the Acquired Companies has always been solely Tax-resident in its jurisdiction of incorporation or registered seat and has never been treated as resident in any other jurisdiction for any Tax purpose (including any double taxation arrangement); to the Sellers' Knowledge, each of the Acquired Companies has never had permanent establishments, VAT fixed establishments, or any other comparable taxable presence outside of its respective jurisdiction of incorporation;
(k) No bonus payments or similar payments to employees or the like have been made and/or promised by the Sellers, their Affiliates or the Acquired Companies that would result in any Tax withholding obligations of the Acquired Companies after the Closing Date;
(l) All rights to Tax refund claims recorded in the 2022 Financial Statements are legally valid and correct in terms of the amount;
(m) The Acquired Companies have not applied for or received or been granted any Tax relief measures in relation to, or in the context of, the COVID 19 pandemic;
(n) None of the Subsidiaries qualify as a French controlled foreign corporation per section 209 B and section 238 A of the French Tax Code;
(o) Within the two (2) years prior to the date hereof, no Acquired Company has been a “distributing corporation” or a “controlled corporation” in a distribution intended to qualify under Section 355(a) of the US Internal Revenue Code of 1986, as amended; (p) There are no Tax liens on any assets of any Acquired Company (other than permitted Encumbrances);
- 16 -
(q) No Acquired Company has been a party to any “listed transaction” within the meaning of US Treasury Regulation Section 1.6011-4(b);
(r) To the Sellers' Knowledge, no deferrals for Taxes have been requested by, or granted to, the Acquired Companies by a Tax Authority; and
(s) No Acquired Company which was not created in or organized under the Laws of, the United States or its political subdivisions is or, to the Sellers’ Knowledge, has been in the last five (5) years a “surrogate foreign corporation” within the meaning of Section 7874(a)(2)(B) of the US Internal Revenue Code of 1986, as amended, or is treated as a U.S. corporation under Section 7874(b) of the Code.
3.7 Personal Property.
(a) Each of the Acquired Companies has good title to (free and clear of all Encumbrances), or hold by valid and existing lease or license, all the tangible personal property assets which are material for the conduct of the Business. Each of the Acquired Companies owns all tangible assets purported to be owned by it, in particular all such assets referred to in the Management Accounts other than as transferred or otherwise disposed, worn-up or broke since 31 October 2023 in the Ordinary Course of Business.
(b) All such assets are (i) in reasonably good maintenance within the standards applicable to the industry of the Business, operating condition and repair, normal wear and tear excepted, other than machinery and equipment under repair or out of service in the Ordinary Course of Business and (ii) are sufficient for the conduct of the business activities of the Acquired Companies in a materially same way as currently conducted.
(c) To the Sellers’ Knowledge, the Acquired Companies own, lease, license or otherwise legally hold or have legal right to use all tangible assets which are presently used and necessary for the conduct of the business of the Acquired Companies as currently conducted.
3.8 Intellectual Property
(a) Schedule 3.8(a) contains a complete and correct list of all patents, registered trademarks, domain names and other Intellectual Property Rights registered with a Governmental Authority owned by the Acquired Companies as of the date hereof (collectively, "Registered Intellectual Property Rights"). To the Seller’s Knowledge, the Acquired Companies own or hold leases, licenses or otherwise legally hold or have a legal right to use all Intellectual Property Rights used by the Acquired Companies to operate its business activities as currently conducted by each of the Acquired Companies ("Group Intellectual Property Rights").
(b) Except as disclosed in Schedule 3.8(b), the Acquired Companies are the unrestricted and exclusive owners of the Registered Intellectual Property Rights, and all material non-registered Intellectual Property Rights owned by an Acquired Company ("Owned Group Intellectual Property Rights"). Except as disclosed in Schedule 3.8(b), the Owned Group Intellectual Property Rights are free from any ownership rights or Encumbrances or, to the Sellers' Knowledge, other rights of third parties (including, without limitation, current and former employees or directors of the Acquired Companies and current and former students, interns, freelancers and independent contractors) and, to the Sellers' Knowledge, no such third party has contested any of the Owned Group Intellectual Property Rights vis-à-vis any of the Acquired Companies. To the Sellers' Knowledge, there are no open payment obligations of any of the Acquired Companies towards any inventor of any invention which has been transferred to the relevant Acquired Company.
- 17 -
(c) All payments due for the maintenance of the Registered Intellectual Property Rights which are material for the business activities as currently conducted by the Acquired Companies have been made, all necessary applications for their renewal that are due have been filed within the relevant deadlines, and all other actions necessary for the maintenance of such Intellectual Property Rights as of the date hereof have been taken. To the Sellers' Knowledge, no material action has been done or omitted to be done by any Acquired Company by which the Owned Group Intellectual Property Rights has become invalid or unenforceable. To the Sellers' Knowledge, the relevant Acquired Company truly owning the registered Owned Intellectual Property Rights is listed as the owner of record in the relevant intellectual property office register, or correction of the relevant intellectual property office register with respect to the owner of record has been requested by the relevant Acquired Company for such Owned Intellectual Property Rights.
(d) As of the date hereof, the Group Intellectual Property Rights which are not owned by an Acquired Company have been leased or licensed by the respective Acquired Company ("In-bound License Agreements"), and, to the Seller’s Knowledge, such In-bound License Agreements are valid and enforceable and none of the In-bound License Agreements is terminated. To the Sellers' Knowledge, (i) no Acquired Company has breached any material obligation of any of the In-bound License Agreements and (ii) except as Fairly Disclosed in the Data Room, there are no factual circumstances (including the acquisition of the Acquired Companies according to the SPA) giving any of the contractual parties of any of the In-bound License Agreements a right to terminate.
(e) The Owned Group Intellectual Property Rights and, to the Sellers' Knowledge, the other Group Intellectual Property Rights, are not subject to any judicial or official proceedings challenging the validity, enforceability, effectiveness, use or ownership of such Intellectual Property Rights, or have been subject to such proceedings during the last three (3) years, which might affect the Acquired Companies' business activities as currently conducted in any material respect, nor are, to the Sellers' Knowledge, the Group Intellectual Property Rights being infringed by third parties.
(f) To the Sellers' Knowledge, each Acquired Company has timely paid all royalties, commissions, and similar amounts to the Persons, for the ownership or use of any material Group Intellectual Property, who are entitled to receive them.
(g) Except as disclosed in Schedule 3.8(g), to the Sellers' Knowledge, during the last three (3) years, neither the Acquired Companies nor the Acquired Companies’ use of any Group Intellectual Property violates or makes unlawful use of, or has infringed, misappropriated or otherwise violated or made unlawful use of, any Intellectual Property Rights of any third party. During the last the three (3) years, no Acquired Company has received any written notice alleging that an Acquired Company, or its use of any Group Intellectual Property Right, or any product or service of the Acquired Companies has infringed, misappropriated or otherwise violated or made unlawful use of any Intellectual Property Right of any third party.
(h) For all information, records, methods, techniques, processes, discoveries, unpatented inventions, innovations, unpatentable processes, specifications, formulae, algorithms, designs, plans, documentation, drawings, data and other technical information and software codes which are secret and not in the public domain and identified or identifiable in a tangible or electronic form and which are owned by an Acquired Company and material for the business activities as currently conducted by the Acquired Companies ("Know-How"), the Acquired Companies have taken reasonable steps as necessary to protect the confidentiality of such Know-How, including, without limitation, reasonable confidentiality and security measures.
- 18 -
To the Sellers' Knowledge, no material Know-How of the Acquired Companies has been disclosed up to the Closing Date to any unauthorized third party (i.e. other than in the ordinary course of business, e.g. as part of R&D co-operations and in each case subject to customary confidentiality provisions).
(i) Except as disclosed in Schedule 3.8(i), all documents, source codes and other information required for a software programmer of sufficient skill and expertise to adapt, modify or improve any material bespoke individual software developed wholly or partly by employees of an Acquired Company or commissioned by an Acquired Company ("Software") are in the possession of the Acquired Companies, and the relevant Acquired Company has the unrestricted right to adapt, modify or improve such Software for any purpose or use. The Software is free and clear of any Encumbrances. To the Sellers' Knowledge, except for licenses granted in the Ordinary Course of Business, the Acquired Companies have no duty or obligation (whether present, contingent or otherwise) to deliver, distribute, license or make available the Software to any third party, especially any escrow agent, and/or the public, other than in the Ordinary Course of Business.
(j) To the Seller’s Knowledge, the Software is not linked to or integrated with any open source software in a manner that would: (i) in any way restrict the commercialization of products using such Software (e.g. viral copyleft), or (ii) to the Sellers' Knowledge, have the effect that the source code or confidential documentation for the Software must be disclosed or otherwise be made available to third parties, or (iii) requires an Acquired Company to deliver, distribute, license or make available any Group Intellectual Property Right in connection with the Software, other than in the Ordinary Course of Business. To the Sellers' Knowledge, the relevant Acquired Company has complied with all material restrictions set out in the relevant open-source software licenses in connection with the Software.
3.9 Information Technology.
(a) Except as disclosed in Schedule 3.9(a), the information technology infrastructure (including the computer and telecommunication systems, software architecture and software programs, the functionalities, the tools used as well as the related hardware) used by the Acquired Companies in the operation of the Business (the "Information Technology") is, subject to the migration plans disclosed in the Data Room and other planned improvements Fairly Disclosed in the Data Room, fit for the requirements of the Business as conducted by the Acquired Companies, and in reasonable operating condition, taking into account normal wear and tear.
(b) The Acquired Companies comply, to the Sellers' Knowledge, in all material respects with all applicable IT security regulations (e.g. laws, directives, ordinances, standards, official requirements, etc.) and/or all applicable statutory confidentiality obligations.
(c) Except as disclosed in Schedule 3.9(c), in the last five (5) years, and at present, there has not been any material operational and/or functional malfunction of the Information Technology infrastructure, i.e. malfunctions of one or more material IT systems with an uninterrupted duration of at least twelve (12) hours, or loss of data attributable to a malfunction of the Information Technology infrastructure or software that has had an adverse effect on the operation of the Business.
(d) The Acquired Companies have full ownership of, or otherwise the right to use pursuant to license or other agreements, its Information Technology infrastructure. Except as disclosed in Schedule 3.9(d), (i) to the Sellers’ Knowledge, the Acquired Companies comply, in all material respects, with the licenses and lease conditions for the use of the software, databases and hardware in the Information Technology systems, (ii) all license and lease fees for licensed and leased Information Technology that are material for the conduct of the business of the Acquired Companies as currently conducted are paid, and (iii) no material license agreement has been terminated in writing nor do, to the Sellers' Knowledge, conditions for termination exist or arise in connection with the acquisition of the Acquired Companies in connection with the Transaction.
- 19 -
3.10 Compliance with Law.
(a) To the Sellers' Knowledge:
(i) the operations of each of the Acquired Companies are conducted in all material respects in accordance with all applicable Laws and Judgments (excluding export control and sanctions Laws); and
(ii) the Acquired Companies have in the last three (3) years and are at present in compliance in all material respects with applicable competition or public procurement Laws (excluding export control and sanctions Laws).
(b) Except as disclosed in Schedule 3.10(b), none of the Acquired Companies has received in the last three (3) years (i) written notice of any material violation by any of the Acquired Companies of any Law or of any material default with respect to any Judgment applicable to any of the Acquired Companies or (ii) received from any Governmental Authority or any other Person any written notice, inquiry, or allegation or conducted any internal investigation or audit concerning any actual or potential violation or wrongdoing, in each case related to, or in connection with, Trade Control Laws or Anti-Corruption Laws.
(c) Except as disclosed in Schedule 3.10(c), during the last three (3) years, each Acquired Company has complied with applicable legal requirements pertaining to the protection of personal data in all material respects. The collecting, processing, transfer, store and use of personal data by the Acquired Companies comply in all material respects with applicable data protection Laws. To the Sellers’ Knowledge, the Acquired Companies have fulfilled, in all material respects, all necessary legal requirements to comply with applicable data protection Laws.
(d) Neither any Acquired Company, nor any Sellers, nor, to the Sellers' Knowledge, any agents or employees of any Acquired Company when acting for or on behalf of an Acquired Company:
(i) has used any funds for improper or unlawful contributions, payments, briberies, gifts or entertainment, or made any improper or unlawful expenditures relating to political activity to domestic or foreign government officials or any other party, or
(ii) has accepted or received any improper or unlawful contributions, reimbursements, payments, gifts or expenditures, or
(iii) has otherwise engaged in any activity, practice of conduct which would constitute an offence under any Anti-Corruption Laws or applicable Anti-Money Laundering Regulations.
3.11 Permits.
(a) Except as disclosed in Schedule 3.11(a), each Acquired Company has all governmental permits, licenses, certifications, authorizations (public and private), registrations, qualifications and consents (public and private) which are required for the Acquired Companies in any jurisdiction currently relevant for the Acquired Companies to operate its Business as presently conducted ("Permits"). Each Permit is valid, binding and in full force and effect. No Permit has been revoked, withdrawn or challenged in writing by any third party. No Proceedings regarding a revocation or withdrawal of any Permit have been initiated or have been threatened in writing.
- 20 -
(b) To the Sellers’ Knowledge, the Acquired Companies are in, all material respects, in compliance, and during the last three (3) years have been in compliance, with all Permits that are material to the conduct of the businesses of the Acquired Companies.
(c) Except as disclosed in Schedule 3.11(c), other than in the Ordinary Course of Business, to the Sellers' Knowledge, there is currently no investigation or audit by any Governmental Authority pending or threatened in writing. No Governmental Authority has notified any Acquired Company in writing of an intention to conduct such an investigation or audit.
3.12 Environmental Matters.
(a) To the Sellers' Knowledge, there are no Environmental Conditions on any Owned Real Estate or leased real property or emanating from any Owned Real Estate or leased real property, which could reasonably be anticipated to result in any liability for or require action by one of the Acquired Companies and/or one of their legal predecessors.
(b) To the Sellers' Knowledge, there has been no release by the Acquired Companies at any Owned Real Estate or leased real property, or formerly used, owned or operated real property, that requires notice, investigation, mitigation, cleanup or remediation by the Acquired Companies pursuant to any applicable Environmental Law.
(c) To the Sellers’ Knowledge, the operations of the Acquired Companies are conducted and for the period of the last three (3) years have been conducted in all material respects in accordance with all applicable Environmental Laws.
(d) To the Sellers' Knowledge, there are no contractual or regulatory obligations of any Acquired Company in connection with any Environmental Conditions or Environmental Laws other than in the Ordinary Course of Business.
(e) To the Sellers' Knowledge, all Hazardous Materials have been used, stored and disposed of at properly licensed and applicable disposal locations by the Acquired Companies in compliance with all Environmental Laws, and there have been no releases of Hazardous Materials into the Environment and no Environmental Conditions caused by Hazardous Materials have occurred.
(f) To the Sellers' Knowledge, none of the Acquired Companies have received notice in writing in the past five (5) years from any Governmental Authority or Person of any violation by any of the Acquired Companies of any Environmental Law.
(g) To the Sellers' Knowledge, all Governmental Authorizations required to be obtained by any of the Acquired Companies under the Environmental Laws in order to conduct the Business have been obtained and are maintained in good standing.
3.13 Proceedings.
(a) Except as disclosed on Schedule 3.13(a), there is no Proceeding (i) pending or, (ii) threatened in writing against any of the Acquired Companies involving a claim for (α) a stated amount in excess of two hundred fifty thousand euros (€250,000), (β) alleging that one of the Acquired Companies has committed a criminal (pénal) act, or (γ) to which one of the Key Employees is a party.
(b) No enforceable (exécutoire) or final Judgment has been rendered against any of the Acquired Companies which has not been satisfied. There is no final Judgment that can reasonably be expected to have a material adverse effect on the Business of the Acquired Companies as conducted on the Put Option Date.
- 21 -
3.14 Material Contracts.
(a) Schedule 3.14(a) sets forth a list of all the following Contracts to which any of the Acquired Companies is a party as of the Put Option Date (the "Material Contracts"):
(i) the supply agreements (or description of contractual relationships) with the ten (10) most significant suppliers of the Business (taken as a whole) (determined by aggregate purchase invoices for 2022);
(ii) the customer agreements (or description of contractual relationships) with the ten (10) most significant customers of the Business (taken as a whole) (determined by aggregate sale invoices for 2022);
(iii) excluding any distribution agreements pursuant to which any Group Company is the distributor or pursuant to which any Group Company provides distribution rights, Contracts that materially limit the ability of the Acquired Company which is a party thereto to compete with any third party in any line of business which is material to such Acquired Company (other than any grant of exclusivity to a third party pursuant to the Contracts mentioned in (i) and (ii) above);
(iv) Contracts regarding the supply of instruments (including all amendments and side-agreements), in respect of the instruments InGenius, BeGenius and ELIverse;
(v) material research and development Contracts at the Group Companies level;
(vi) excluding any distribution agreements pursuant to which any Group Company is the distributor or pursuant to which any Group Company provides distribution rights, Contracts on the granting of exclusive rights by an Acquired Company to any of the Owned Group Intellectual Property Rights;
(vii) excluding any distribution agreements pursuant to which any Group Company is the distributor or pursuant to which any Group Company provides distribution rights, Contracts with third parties as licensor granting a license to an Acquired Company to use any material Intellectual Property Rights that are required for the Acquired Companies to conduct the Business as currently conducted, excluding licenses to generally available off-the-shelf software from third parties licensed under clickthrough, shrinkwrap or other standard terms and conditions;
(viii) guarantees, indemnities, suretyships, letters of comfort or similar securities issued by any of the Acquired Companies for the obligations of any other Person (other than another Acquired Company) in an amount exceeding EUR 100,000; and
(ix) any agency agreements and any agreements with independent distributors that each provide for a commission or remuneration of EUR 300,000 per year or Intellectual Property consultancy agreements;
(x) any agreements regarding swaps, options, forward sales or purchases, futures and other financial derivatives and combinations thereof;
(xi) partnership, shareholder or joint venture or joint development agreements.
(b) Each Material Contract is in full force and effect, and is a valid and binding agreement of the Acquired Company which is a party thereto.
(c) None of the Acquired Companies is in default of a material obligation under any Material Contract.
- 22 -
(d) None of the Material Contracts to which any of the Acquired Companies is a party may be terminated or amended commercially detrimentally for the Acquired Companies by the other party thereto as a result of the consummation of the Transaction.
3.15 Insurance.
(a) Schedule 3.15 sets forth a list of all material insurance policies maintained by the Acquired Companies (or by the Seller or any of its Affiliates to the extent relating to the Business) as of the Put Option Date. The policies of the Company listed in Schedule 3.15 and marked in [color] will not cease upon Closing but will continue to exist in full force and effect.
(b) All such policies (i) have been issued by a commercially reputable insurer, (ii) are in full force and effect and (iii) are, to the Sellers' Knowledge, enforceable. None of the Acquired Companies is in material default under such policies that would result in the termination of any such policy or the modification of the terms thereof in an adverse manner for the Acquired Companies. All claims thereunder have been filed in a due and timely fashion in the manner required by the policy or binder.
(c) To the Sellers' Knowledge, each Acquired Company was in the past, and still is, adequately insured against all risks which a prudent businessman usually insures itself against.
(d) All premiums due and payable under all such policies have been paid, the Acquired Companies will not be liable for retroactive premiums or similar payments, and the Acquired Companies are, to the Sellers' Knowledge, otherwise in material compliance with the terms of such policies. The Acquired Companies have not received any notice of cancellation, termination or material premium increase with respect to any such policy which was not replaced on substantially similar terms prior to the date of such cancellation.
3.16 Employment Matters.
(a) Schedule 3.16(a) sets forth a correct and complete list (on an anonymized basis) of the officers and employees of the Acquired Companies setting out for each officer and employee the following data (to the extent applicable): employer, date of birth or age, social security number, start of employment, notice periods of termination, fixed term, annual fixed gross salary or base wages, commissions and bonuses, as the case may be. The officers and employees listed on Schedule 3.16(a) and marked in [color] are referred to as the "Key Employees".
(b) Except as disclosed on Schedule 3.16(b), there are no Contracts, plans or other arrangements by which any of the Acquired Companies are bound, which contain any "change of control" or golden parachute provisions with respect to any of the Key Employees.
(c) Except as disclosed on Schedule 3.16(c), since January 1st, 2023, none of the Acquired Companies has paid or agreed to pay any bonuses or made or agreed to make any material increase in the rate of wages, salaries or other remuneration or advantage whatsoever of any of the Key Employees it employs, other than (i) as dictated by applicable Law or the applicable collective bargaining agreement or (ii) within the Ordinary Course of Business and in compliance with past practice.
(d) Except as disclosed on Schedule 3.16(d) (or where required by the applicable labor Laws), none of the Acquired Companies is bound by, sponsors or maintains:
(i) any severance agreements or other Contracts providing for compensation of the employees of the Acquired Companies in case of the signing or completion of the Transaction; or
(ii) any (v) bonus or profit-sharing plans or arrangements, (w) company savings plans or arrangements, (x) stock purchase or stock option plans or arrangements, (y) pension or retirement plans or arrangements, or (z) other employee funds or similar employee benefit plans or arrangements providing benefits of economic value to the employees of the Acquired Companies,
- 23 -
which are, in any material respect, more favorable to its employees than any mandatory plan, arrangement or fund provided by applicable labor Law or applicable collective bargaining agreements.
(e) Except as disclosed in Schedule 3.16(e), each of the Acquired Companies has in all material respects duly and timely fulfilled all payment and compensation obligations to its employees, consultants and directors, including the obligations pertaining to payment of salary, social security contributions, insurance premiums and tax withholdings, and has in all material respects duly calculated and accrued in accordance with applicable Laws in the books all entitlements not yet payable (including without limitation the deferred accrued indemnity, monthly payments, paid leaves and leaves).
(f) Schedule 3.16(f) sets forth a true and complete list for each Acquired Company of all agreements and other commitments, whether of an individual or collective nature, regarding pensions or retirement under which such Acquired Company has any obligations (the agreements or other commitments listed or to be listed in Schedule 3.16(f) the "Pension Commitments") other than required under applicable Law. All material obligations under or in connection with the Pension Commitments, including obligations arising by operation of law that have become due, have been fulfilled by the Acquired Companies.
(g) All agreements and other commitments, whether of (i) an individual nature if the benefits are exceeding an amount of fifty thousand euros (€50,000) or (ii) collective nature, in each case regarding employee benefits such as health, life or disability insurance, anniversary, holiday or jubilee payments, bonuses, profit participation, company car entitlements or other variable remuneration elements and stock options, stock appreciation rights or similar rights, other than Pension Commitments ("Employee Benefits") have been Fairly Disclosed in the Data Room and in Schedule 3.16(g).
(h) Schedule 3.16(h) contains a complete, true and correct list of all collective bargaining agreements and all agreements with unions, workers' councils and similar organizations, including without limitation reconciliation of interest agreements and social plans, general commitments, standard terms of employment, shop agreements or in any other legal form, to which any Acquired Company is bound.
(i) If and to the extent not Fairly Disclosed in the Data Room, Schedule 3.16(i) provides, a complete and correct list of all independent contractors and freelancers engaged by an Acquired Company. To the Sellers' Knowledge, no current or former independent contractor of any Acquired Company is or has been (or has the right to be) a misclassified employee.
(j) Schedule 3.16(j) contains a complete, true and correct list of all pending or, to the Sellers’ Knowledge, threatened in writing employment, labor and social security disputes to which each Acquired Company is a party involving a claim for a stated amount in excess of fifty thousand euros (€50,000).
(k) Except as disclosed in Schedule 3.16(k), none of the employees of the Carve-out Companies can ask for the transfer of its employment contract to or claim an employment relationship with an Acquired Company. To the Sellers' Knowledge, there is no reasonable basis for reinstatement of former employees, especially protected employees.
(l) Except as disclosed on Schedule 3.16(l), none of the former employees of the Acquired Companies is entitled to any remuneration, benefits, premiums and damages and the like save as reflected in the Management Accounts and there is no pending or, to the Sellers' Knowledge, threatened disputes between the Acquired Companies and any former employee.
- 24 -
(m) To the Sellers' Knowledge, there have been no employment-related illnesses suffered by any former employee which may lead to a claim in excess of fifty thousand euros (€ 50,000) against the Acquired Companies. To the Sellers' Knowledge, no work-related accident occurred in any of the Acquired Companies during the last three years and no declaration of occupational disease has been made leading to claims in excess of fifty thousand euros (€50,000) excluding amounts which are identifiably contained in the Management Accounts. Except as disclosed in Schedule 3.16(m), to the Sellers' Knowledge, no employees of the Acquired Companies have been exposed to hazardous substances by the Acquired Companies and no action has been taken and/or announced and/or threatened against the Acquired Companies, in particular in France on the ground of inexcusable fault ("faute inexcusable") of the Group Companies, in matter of exposure to asbestos. The Acquired Companies received no summon or observation from the Labour Inspection or the Regional social security body (for instance in France the so-called "CRAM") in this respect or failure to comply with Labour legislation.
(n) Except as disclosed in Schedule 3.16(n), none of the Acquired Companies has implemented a voluntary defined pensions or defined contributions pension plan or whatsoever to the benefit of employees, other than the Pension Commitments.
(o) Each US Entity has previously delivered or made available to the Purchaser, to the extent applicable for each U.S. Employee Plan and each U.S. based employee, true, correct and complete copies of (i) plan document or a written description of the material terms of any unwritten employee benefit plan or compensation arrangement, (ii) all determination or opinion letters from the IRS with respect to any plan intended to be qualified under Section 401 of the Code, (iii) all summary plan descriptions and summaries of material modifications, (iv) all trust agreements, insurance contracts, and other documents relating to the funding or payment of benefits under any plan, and (v) copies of all applicable nondiscrimination tests for the last three (3) years.
(p) Each U.S. Employee Plan has in the last five (5) years been, and is at present, in compliance in all material respects with the terms of such U.S. Employee Plan and the requirements prescribed by all applicable Law, including, but not limited to, ERISA, the Code the Health Insurance Portability and Accountability Act of 1996, as amended, and the Patient Protection and Affordable Care Act, as amended. There has been no nonexempt "prohibited transaction," as such term is defined in Section 4975 of the Code or Section 406 of ERISA, or breach of fiduciary duty with respect to any U.S. Employee Pan in connection with which, directly or indirectly, any Employee Plan (or any related funding medium) or US Entity (or any of its directors or employees) could, to the Sellers' Knowledge, be subject to either a material penalty or tax.
(q) The Elitech Group, Inc. Profit Sharing Plan is the only U.S. Employee Plan that is intended to be tax-qualified under Section 401(a) of the Code, it has received a favorable determination or opinion letter from the Internal Revenue Service as to its qualification and has not been operated in a manner which, to the Sellers' Knowledge, would reasonably be expected to cause it to be disqualified in operation, nor does it, to the Sellers' Knowledge, have any investment funds for which upon a plan termination, to the Sellers' Knowledge, there would be a negative market value adjustment or other penalty that would reduce the value of participants’ accounts
(r) Each U.S. Employee Plan that is a welfare benefit plan (as defined in Section 3(1) of ERISA) is fully insured, other than any flexible spending or health savings accounts, and is not subject to any retroactive premium adjustments. No U.S. Employee Plan provides health or life insurance benefits following retirement or other termination of employment, except to the extent required by the Consolidated Omnibus Budget Reconciliation Act of 1985 as amended or applicable state insurance law and at the sole expense of the participant or the participant’s covered dependents.
- 25 -
(s) No US Entity has at any time contributed to or been obligated to contribute, or has any current or, to the Sellers' Knowledge, potential liability to, any “multiple employer welfare arrangement” within the meaning of Section 3(40) of and subject to ERISA, any “multiple employer plan” sponsored by more than one employer within the meaning of Sections 4063 or 4064 of and subject to ERISA, a "multiemployer plan" as such term is defined in Section 4001(a)(3) of and subject to ERISA, or a pension plan subject to Title IV of ERISA or Section 412 of the Code.
(t) No U.S. Employee Plan is a “nonqualified deferred compensation plan” subject to Section 409A of the Code. None of the Acquired Companies has any obligation to gross-up, indemnify or otherwise reimburse any current or former employee, director, consultant or other service provider for any U.S. tax incurred by such service provider, including under Section 409A or 4999 of the Code or otherwise.
(u) To the Seller’s Knowledge, no officer, director or Key Employee of the Acquired Companies is subject to any Contracts or any order, writ or judgment that prohibits, limits or purports to limit such Person from engaging in or continuing any conduct, activity, duty or practice relating to the Business. To the Sellers' Knowledge, no former or current employee is a party to, or is otherwise bound by, any Contract that in any way adversely affected or affects the ability of the Seller or the Target Group to conduct the Business as currently conducted.
(v) There are no pending or, to the Sellers' Knowledge, threatened in writing, audits, investigations, information requests, claims, suits, demands or charges against any Seller or any of the Acquired Companies’ employees regarding any Laws relating to employment practices, terms and conditions of employment, leaves of absence, equal employment opportunity, non-harassment, non-discrimination, immigration (including but not limited to immigration related hiring practices and benefits), wages, hours, benefits, collective bargaining, the payment of social security and similar Taxes and occupational health and safety, which might lead to a financial burden of the Acquired Companies of more than fifty thousand euros (€50,000).
(w) To the Sellers' Knowledge, no Seller has implemented any plant closing or layoff of employees of the Acquired Companies that could implicate the United States’ WARN Act or any similar Law, nor shall the consummation of the contemplated transaction implicate the WARN Act or any similar Law.
(x) No Seller has received any notices or other correspondence from any United States government agency including but not limited to U.S. Citizenship and Immigration Services, U.S. Department of Homeland Security, Homeland Security Investigations and Immigration and Customs Enforcement alleging non- compliance with the requirements of the Immigration Reform and Control Act of 1986 including but not limited to Notices of Inspection, Notices of Suspect Documents, Notices of Intent to Fine, Social Security “no match” letters.
(y) None of the employees of the US Entities are currently sponsored for non-immigrant or immigrant visa status by employer including the specific nonimmigrant visa status and the status of any immigrant visa (“green card”) process.
(z) Each employee of the Acquired Companies whose regular work location is in the United States is authorized under applicable Law to work in the United States and, in connection therewith, the Acquired Companies are in compliance with all applicable immigration Laws, rules and regulations.
- 26 -
(aa) Since 1 January 2023, to the Sellers’ Knowledge, no formal allegations of sexual harassment have been made to or against any member of the Sellers' Group or employee of the Acquired Companies. To the Seller’s Knowledge, there are no facts occurring in the last year that would reasonably be expected to give rise to a claim of sexual harassment, other unlawful harassment or unlawful discrimination or retaliation against or involving any employee, director or independent contractor of any of the Acquired Companies.
3.17 Subsidies.
(a) The indirect or direct subsidies, allowances, grants, aids or other financial assistance ("Subsidies") made available or contributed to any Acquired Company since 1 January 2018 are comprehensively Fairly Disclosed in the Data Room. To the Sellers' Knowledge, no Acquired Company is in breach of any material obligation under the terms and conditions underlying any of the Subsidies.
(b) To the Sellers' Knowledge, none of the Subsidies (i) can be terminated and/or (ii) needs to be repaid by an Acquired Company as a consequence of the execution of this Agreement or the SPA or the consummation of the Transaction.
3.18 Relationships with the Sellers’ Group.
Subject as Fairly Disclosed in the Data Room and except as disclosed on Schedule 3.18:
(a) no member of the Sellers’ Group and no member of the Carve-out Companies has acquired any property, asset or right from any of the Acquired Companies which, is required for the Business as presently conducted;
(b) no member of the Sellers' Group and the Carve-out Companies is in possession of, or owns, any such asset or right; in particular, no member of the Sellers’ Group holds or retains any right with respect to (x) any tangible assets, (y) any Intellectual Property Rights or (z) any IT systems which is required for the Business as presently conducted;
(c) no member of the Sellers’ Group and the Carve-out Companies benefits from any guarantee, indemnity or similar right granted by any Acquired Company as surety for any of its own obligations; and
(d) no Acquired Company has entered into any agreement with any member of Sellers' Group (except as portfolio companies of funds advised by PAI Partners) or the Carve-out Companies or any of the Sellers' Group's or the Carve-out Companies' direct or indirect shareholders, relatives of any Seller or such shareholders, or with business entities (other than the Acquired Companies) in which any Seller or any of such shareholders has a direct or indirect participation.
3.19 Regulatory Matters
(a) To the Sellers’ Knowledge, (i) the Acquired Companies possess the certifications, clearances, approvals, authorizations, or licenses that are material to the conduct of the Business for the products it manufactures, sells and/or distributes and (ii) such certifications, clearances, approvals, authorizations, or licenses are in full force and effect. To the Sellers’ Knowledge, the Acquired Companies are in compliance in all material respects with the terms and conditions of the certifications, clearances, approvals, authorizations, or licenses and have received no written notices that any Acquired Company is in violation of any of the terms or conditions of any certifications, clearances, approvals, authorizations, or licenses or alleging the failure to maintain any certifications, approvals, authorizations, or licenses.
- 27 -
(b) Except as disclosed in Schedule 3.19(b), in accordance with the conduct of the business as presently conducted by the Acquired Companies, to the extent required, all essential products manufactured or sold by the Acquired Companies have proper and valid CE or other certification and have the legally required licenses according to the In-vitro Diagnostic Medical Device Regulation of the European Union (Directive 2017/746 - "IVDR") or, if applicable the Medical Device Regulation of the European Union (Directive 2017/746 – "MDR") and respective laws, rules and regulations under US laws., as it is currently in force.
(c) Except as disclosed in Schedule 3.19(c), to the Sellers' Knowledge, to the extent required by Law, the technical documentation and the quality management system are available in all essential parts for all products manufactured, sold or distributed by the Acquired Companies and essentially meet the requirements of the IVDR and MDR (insofar as these are legally mandatory) and ISO 13485:2016 and, to the Sellers' Knowledge, have not been disclosed to third parties (with the exception of the responsible notified body in accordance with the IVDR and employees and managing directors of the Acquired Companies) in the last three (3) years.
(d) Apart from the inspections/audits listed in Schedule 3.19(d) and the regularly recurring inspections/audits, no inspections, audits or other procedures are pending or have been carried out in the last three (3) years by the Federal Office for Drugs and Medical Devices or any other equivalent US, European, French, Italian, German or other national supervisory authority, notified body or public certification body.
(e) Except as disclosed in Schedule 3.19(e), to the Sellers’ Knowledge, each Acquired Company fulfils the requirements in all material respects (including obligations of follow-up product monitoring, product recall and labelling) arising from the IVDR and MDR for its business activities.
(f) Except as disclosed in Schedule 3.19(f), the clinical evaluations performed or initiated or supported by the Acquired Companies are in compliance with applicable Law in all material respects.
(g) The Acquired Companies are, and for the past three (3) years have been, in all material respects, in compliance with all applicable Laws administered or issued by the U.S. Food and Drug Administration (FDA) or any similar Governmental Authority, including the Federal Food, Drug, and Cosmetic Act (21 U.S.C. ch. 9 § 301 et seq.) and all other Laws regarding developing, testing, manufacturing, marketing, distributing, or promoting the products of the Acquired Companies, or complaint handling or adverse event reporting. The Acquired Companies also are, and for the past three (3) years have been, in all material respects, in compliance with the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b), the federal civil False Claims Act (31 U.S.C. § 3729 et seq.), the federal Civil Monetary Penalties Law (42 U.S.C. § 1320a-7a), the federal Exclusion Laws (42 U.S.C. § 1320a-7), the federal Program Fraud Civil Remedies Act (31 U.S.C. § 3801 et seq.), the federal Health Care Fraud Law (18 U.S.C. § 1347), the false statement and other health care fraud criminal provisions of HIPAA (18 U.S.C. § 1035), the Patient Protection and Affordable Care Act (P.L. 111-148), the Physician Payment Sunshine Act (42 U.S.C. § 1320a-7h), and applicable US state or US local Laws as amended from time to time.
(h) Neither the Acquired Companies, nor any officer, director, or, to the Seller’s Knowledge, employee or agent thereof has made any voluntary self-disclosure to any Governmental Authority of any non-compliance with any Law. The Acquired Companies are not and have not been subject to any corrective action plan, corporate integrity agreement, deferred prosecution agreement, consent decree, settlement agreement, or similar undertaking with or imposed by any Governmental Authority arising from any alleged violation of any Law. Neither the Acquired Companies, nor to the Seller’s Knowledge any officer, director, employee, or agent thereof has received from a Governmental Authority, in the last five years, any notice of any investigation or inquiry, including but not limited to a warning letter, subpoena, or civil investigative demand, relating to the conduct of any of the Acquired Companies’ business.
- 28 -
(i) Neither the Acquired Companies, nor any officer, director, nor, to the Sellers' Knowledge, any employee or agent thereof has been excluded, suspended, or debarred from participation in any Federal Health Care Program, FDA program, or the System for Award Management, or has been subject to any Action.
3.20 Group Companies
(a) Each Group Company is duly incorporated (or similar) and registered.
(b) To the Sellers' Knowledge, there is no fact or circumstance or application which is likely to affect the existence of any of the Group Companies or its capacity to operate its business.
(c) None of the Group Companies is the subject of any Insolvency Proceedings and no fact or circumstance which is likely to result in any such Insolvency Proceedings.
(d) FKGO GmbH (Germany) has been liquidated and deregistered from the Chamber of Commerce of Berlin on February 9, 2024. Except for the current ordinary costs and expenses and usual liquidation costs, there are no material liabilities in connection with FKGO GmbH (Germany) for which any Acquired Company can be held liable.
(e) None of the Group Companies is a member or shareholder of, or hold any Securities in, any Person or group whose members or shareholders have joint (solidaire) or unlimited liability.
(f) The Company’s rights to the securities of the Subsidiaries are not the subject of any dispute.
ARTICLE IV REPAYMENT OBLIGATION
4.1 Repayment Obligation; Limitation on Quantum.
(a) Subject to the occurrence of the Closing and subject to the provisions of this Article IV, the Sellers individually and not jointly (individuellement et non solidairement) shall be obligated to put the Purchaser or, at the Purchaser's election, the Acquired Companies into the same position they would have been in if the representation had been correct or, at the election of the Purchaser, to pay to Purchaser, as a partial repayment of the Purchase Price, the amount of all Damages suffered by the Purchaser and/or – at Purchaser’s election – any Acquired Company as a result of any inaccuracy or breach of any representation or warranty of the Sellers set forth in Article III, it being expressly agreed that the aggregate liability of the Sellers in respect of all claims, for any reason whatsoever, under this Agreement shall not exceed one euro (€1.00) ("W&I Cap").
(b) Without prejudice to any rights of the Purchaser under the W&I Policy, and subject to fraud or wilfull misconduct (dol), from and after the Closing Date, the right to repayment provided for in this Section 4.1 shall be the exclusive remedy of the Purchaser for any inaccuracy or breach of any representation or warranty of the Seller set forth in Article III.
(c) All payments made by the Seller to the Purchaser pursuant to this Article IV shall be treated by the Parties hereto for all purposes, including tax, accounting and financial reporting purposes, as a partial repayment of the Purchase Price to the fullest extent permitted by applicable Law.
- 29 -
4.2 Time Limits for Claims.
The liability of the Seller under Section 4.1 shall terminate on the date which is (i) twenty-four (24) months after the Closing Date in respect of any claim (other than any claim in respect of any inaccuracy or breach of a representation and warranty contained in Section 3.6 (Tax Matters) or Section 3.16 (Employment Matters)) and (ii) three (3) months after the expiry of the relevant statutory limitation period in respect of any claim in respect of any inaccuracy or breach of a representation and warranty contained in Section 3.6 (Tax Matters) or Section 3.16 (Employment Matters), in each case unless prior to such date the Purchaser has notified the Sellers’ Representative of a claim thereunder. For the avoidance of doubt, the Purchaser shall not be permitted to make any claim against the Sellers for any inaccuracy or breach of any representation or warranty of the Seller set forth in Article III unless the Closing (as such term is defined in the SPA) shall have occurred.
4.3 Payment.
No amount shall become due and payable by the Sellers to the Purchaser (x) in respect of any claim arising by reason of contingent liability, unless and to the extent that such contingent liability ceases to be contingent and has become an actual liability, and (y) in respect of any claim made by a third party unless and to the extent that the Purchaser or the relevant Acquired Company is under the obligation to actually pay the relevant Damages to the third party.
4.4 Exclusions.
(a) The Sellers shall not have any liability under this Article IV for any Damages resulting from or arising out of:
(i) any event, fact, matter or circumstance which was Fairly Disclosed in this Agreement (including the Schedules) or the Disclosed Information;
(ii) any matter which was specifically agreed in writing by the Purchaser;
(iii) any voluntary action of the Purchaser or, after the Closing Date, an Acquired Company which has caused the Damages at hand (including (x) any reorganization, any winding-up or cessation of any business or trade carried on by any of the Acquired Companies initiated after the Closing Date, by the Purchaser or its Affiliates or any of the Acquired Companies, or (y) any non-mandatory change in the Accounting Principles or Tax policies (including the transfer pricing policies and methods of submitting Tax Returns));
(iv) the passing of, or any change in, any Law or imposition of any new Tax not actually in force at the date of this Agreement (even if retroactive in effect);
(v) any amount is shown as a Tax liability or Tax provision in the Final Closing Statement and has reduced the Purchase Price;
(vi) any amount has been paid or otherwise settled until the Closing Date;
(vii) any amount that has been recovered by the Acquired Companies from a third party (excluding any employees of the Acquired Companies or the W&I Insurance) (including any cash Tax benefit actually received by the Purchaser or the Acquired Companies after the Closing Date), it being specified that an increase of Tax losses shall not be deemed to be a Tax benefit.
(b) For the avoidance of doubt, any limitations or exclusions of the Sellers’ liability set forth in this Article IV shall not apply in the case of fraud or wilful misconduct (dol).
- 30 -
ARTICLE V MISCELLANEOUS
5.1 Sellers' Agent.
(a) Each of the Sellers hereby appoints irrevocably and exclusively TecFin, and expressly authorizes it to act on behalf and in the name of all or part of the Sellers, as its agent (mandataire) (the "Sellers' Agent") to, in its name and on its behalf:
(i) amend the terms of this Agreement;
(ii) receive notices under this Agreement (other than any notice given by the Purchaser of any breach or violation, or alleged breach or violation, by one or more Sellers of any term of this Agreement);
(iii) deliver any notices, certifications, consents, approvals or waivers required or appropriate under this Agreement;
(iv) receive or make any payment in connection with the transactions contemplated under this Agreement;
(v) make any filing required or appropriate under this Agreement (as determined in the reasonable judgment of the Sellers’ Agent);
(vi) handle, dispute, compromise, settle or otherwise deal with any and all claims against by or against or disputes with the Purchaser under this Agreement; and
(vii) more generally, exercise the rights of the Sellers on their behalf under this Agreement (including the right to terminate this Agreement under Section 5.2).
(viii) Any act or decision taken by the Sellers' Agent in accordance with this Agreement shall bind each of the Sellers, provided that such decision applies to each similarly situated Seller on the same basis.
(ix) References to the "Sellers' Agent" appearing herein shall be deemed to be qualified by the phrase "(on behalf of each of the Sellers)", provided that the Sellers' Agent's so acting as the agent for each of the Sellers shall in no case cause the Sellers' Agent to be deemed to be liable for any obligations of a Seller hereunder or to establish any joint and several liability among the Sellers.
(x) The Sellers' Agent shall not bear any liability whatsoever, to either any of the Sellers or to the Purchaser, in its capacity as agent of the Sellers under this Agreement, except in case of willful misconduct (faute intentionnelle) or gross negligence (négligence grave).
5.2 Termination.
(a) This Agreement may be terminated, at any time prior to the Closing, by the written agreement of the Purchaser and the Seller.
(b) This Agreement shall also automatically terminate upon termination of the SPA in accordance with its terms.
(c) Upon any termination of this Agreement pursuant to paragraphs (a) or (b) of this Section 5.1, all further obligations of the Parties hereunder, other than pursuant to Section 5.3 (Confidentiality), 5.4 (Costs and Expenses), 5.10 (No Third-Party Rights; Assignment), 4.11 (Waiver and Amendments) and 5.13 (Governing Law and Submission to Jurisdiction), shall terminate.
- 31 -
5.3 Confidentiality.
(a) Subject to Section 5.3(b), during the period from the date of this Agreement to the fifth (5th) anniversary of the Closing Date, each of the Parties shall, and shall cause its respective Affiliates to, treat the existence and the content of this Agreement as confidential and refrain from disclosing this Agreement, in whole or part, to any Person without the consent of the other Party.
(b) Section 5.3(a) does not apply to any disclosure:
(i) by a Party to any of its Affiliates or its or its Affiliates' directors, officers, employees, agents, representatives, advisors or auditors whose function requires him to receive such announcement or disclosure and, in the event of a disclosure to an agent, representative, advisor or auditor, provided that such disclosure is subject to customary confidentiality obligations;
(ii) (x) to the extent that it is required to be disclosed by applicable Law or rule of a listing authority or a stock exchange or requested by a Governmental Authority (including a Tax Governmental Authority) with relevant powers to which the disclosing Party or its relevant Affiliate is subject or submits, (y) as requested by a regulator with power to compel disclosure, or (z) as disclosed to a Governmental Authority (including a Tax Governmental Authority) in connection with any stamp or other documentary or transaction duties or other transfer Taxes, provided that the disclosing Party shall, to the extent permitted by Law, notify the Sellers' Agent (if the disclosing Party is the Purchaser or any of its Affiliates) or the Purchaser (if the disclosing Party is a Seller or the Affiliate of a Seller) prior to the issuance or making of any such announcement or disclosure and shall use its reasonable endeavors to consult in good faith with the other Party and to take into account the reasonable requirements of such other Party as to the timing, contents and manner of making any such announcement or disclosure;
(iii) by the Sellers to (x) PAI Partners or any of its Affiliates or (y) any participants or investors or potential investors in any fund advised or managed by PAI Partners or any of its Affiliates;
(iv) by the Purchaser to its Affiliates or direct or indirect shareholders W&I brokers and W&I insurances, financing parties, as well as to their directors, officers, employees and agents and advisors at the relevant time as well as to third parties, in each case if they have specifically agreed in writing to non-disclosure in accordance with the terms and conditions hereof, or are bound by comparable statutory confidentiality obligations and who have a need to know such confidential information to the extent necessary for the performance of this Agreement; or
(v) by any of the Parties to enforce its rights under this Agreement.
5.4 Costs and Expenses.
(a) Except as may otherwise be expressly provided herein, each of the Sellers, on the one hand, and the Purchaser, on the other hand, shall bear their own expenses incurred in connection with the negotiation, preparation and signing of this Agreement.
(b) The Parties acknowledge that the costs of the W&I Insurance will be borne for a maximum amount of EUR 250,000 by the Sellers and the balance will be borne by the Purchaser.
5.5 Professional Advice.
Each of the Parties acknowledges and confirms that it was advised by its own lawyers and other professional advisors and, in such connection, has been able to independently assess the scope of its rights and obligations under this Agreement and has had the opportunity to negotiate the terms of this Agreement to its or his satisfaction.
- 32 -
Consequently, no lawyer or other advisor (including, for the avoidance of doubt, the person or firm organizing the electronic execution of this Agreement) shall be deemed to be the sole drafter (rédacteur unique) on behalf of all the Parties and each of the Parties acknowledges and agrees that this Agreement shall not be deemed a contract of adhesion (contrat d'adhésion).
5.6 No Hardship.
Each Party hereby acknowledges that the Agreement falls within the scope of article L. 211-40-1 of the French Monetary and Financial Code.
Therefore, each Party hereby acknowledges that the provisions of article 1195 of the French Civil Code shall not apply to it with respect to its obligations under this Agreement and that it shall not be entitled to make any claim under article 1195 of the French Civil Code.
5.7 Specific Performance.
Pursuant to article 1221 of the French Civil Code, each Party acknowledges and agrees that money damages would not be an adequate remedy for any breach of this Agreement and that in addition to all other remedies which the non-breaching Parties may have, the non-breaching Parties shall be entitled to specific performance and injunctive or other equitable relief as a remedy for any such breach.
5.8 Notices.
(a) All notices, demands or other communications given or made under or in connection with the matters contemplated by this Agreement shall only be effective if made in writing in English and:
(i) sent by registered mail or overnight courier service of recognized international standing delivering an acknowledgement of receipt (such as Fedex or DHL);
(ii) sent by email (which shall contain a scanned copy of the signed notice, demand or other communication) (with a confirmation including a copy of such email to be sent by no later than the next Business Day by registered mail or overnight courier service of recognized international standing delivering an acknowledgement of receipt (such as Fedex or DHL)); or
(iii) delivered by hand delivery against an acknowledgement of receipt dated and signed by the recipient;
to the relevant Party at its address and contact details set forth on Annex D.
(b) Any notice, demand or other communication made in accordance with Section 5.8(a) shall be deemed to have been duly given or made as follows:
(i) if sent by registered mail or overnight courier service of recognized international standing, on the date of the first presentation of the courier;
(ii) if sent by email, on the date indicated on such email; and
(iii) if delivered by hand, on the date indicated on the corresponding acknowledgement of receipt signed by the recipient;
provided that if, in accordance with the above provisions, any such notice, demand or other communication is given or made pursuant to paragraphs (i) and (ii) above on a day which is not a Business Day or after 6.00 p.m. on a Business Day, such notice, demand or other communication shall be deemed to be given or made at the start of the next Business Day.
- 33 -
(c) A Party may notify the other Parties of a change to its name, relevant addressee, address, electronic address or fax number for the purposes of this Section 5.8 in accordance with provisions of this Section 5.8 provided that such notification shall only be effective:
(i) on the date specified in the notification as the date on which the change is to take place; or
(ii) if no date is specified or the date specified is less than one (1) Business Day after the date on which notice is given, the date which is one (1) Business Day after notice of any such change has been given.
5.9 Entire Agreement.
This Agreement (together with the SPA, the Confidentiality Agreement, the Carve-out Documentation) represents the entire agreement and understanding of the Parties with reference to the transactions set forth herein and no representations or warranties have been made in connection with this Agreement other than those expressly set forth herein and in the SPA. This Agreement supersedes all prior negotiations, discussions, correspondence, communications, understandings and agreements between the Parties relating to the subject matter of this Agreement and all prior drafts of this Agreement, all of which are merged into this Agreement, it being understood that all terms agreed in the SPA, the Put Option Agreement and the Carve-Out Documentation shall remain unaffected. No prior drafts of this Agreement may be used to show the intent of the Parties in connection with this Agreement or shall otherwise be admissible into evidence in any Proceeding or other legal action involving this Agreement. The Purchaser acknowledges that certain items to which the Annexes refer have been included to provide additional information to the Purchaser, and that such inclusion shall not be deemed to be an acknowledgment by the Sellers that such items are material.
5.10 No Third-Party Rights; Assignment.
(a) This Agreement shall inure to the benefit of, and be binding upon, the Parties and their respective successors and assigns; provided, however, that none of the Parties shall assign any of its rights or delegate any of its obligations created under this Agreement without the prior written consent of the other Parties. Except as expressly provided herein, nothing set forth in this Agreement shall be construed to give any Person other than the Parties any right, remedy or claim under or with respect to this Agreement or any provision of this Agreement.
(b) Notwithstanding Section 5.10(a):
(i) the Purchaser is entitled to grant security over, or assign by way of security, all or any of its rights under this Agreement for the purposes of or in connection with the financing raised by the Purchaser in relation to the Transaction, provided that: (A) no assignee(s) shall be entitled to receive under this Agreement any greater amount than that to which the Purchaser would have been entitled; and (B) the Sellers shall not incur any liability or increasing liability, or be subject to any obligation or increased obligation, as a result thereof; and
(ii) the Purchaser shall be entitled to assign, prior to the Closing Date, all its rights and obligations under this Agreement to a wholly owned Affiliate provided that: (A) the initial Purchaser remains jointly liable with the obligations of the assignee under this Agreement and (B) the initial Purchaser notifies such assignment to the Sellers' Agent at the latest ten (10) Business Days prior to the Closing Date.
- 34 -
(iii) if a legal entity Seller is involved in a merger, demerger or any other form of restructuring, all of the rights and obligations of the Seller concerned under this Agreement shall be transferred to the Person or Persons resulting from the said merger, demerger or restructuring;
(iv) the provisions of this Agreement shall remain valid even if any Acquired Company ceases to exist, notably as a result of a merger or transfer of all of its assets and liabilities. Consequently, the Sellers may not rely on the fact that one of the Acquired Companies no longer exists in order to avoid fulfilling their undertakings under this Agreement. The Agreement shall remain in effect and unchanged (in its terms and scope) in favour of the Purchaser, notwithstanding the operation that has taken place.
5.11 Severability.
This Agreement shall be deemed severable, and the invalidity or unenforceability of any term or provision hereof shall not affect the validity or enforceability of this Agreement or of any other term or provision hereof. Furthermore, in lieu of any such invalid or unenforceable term or provision, the Parties intend that there shall be added as a part of this Agreement a provision as similar in terms to such invalid or unenforceable provision as may be possible and be valid and enforceable.
5.12 Waivers and Amendments.
No modification of or amendment to this Agreement shall be valid unless in a writing signed by the Parties referring specifically to this Agreement and stating the Parties' intention to modify or amend the same. Any waiver of any term or condition of this Agreement must be in a writing signed by the Party sought to be charged with such waiver referring specifically to the term or condition to be waived, and no such waiver shall be deemed to constitute the waiver of any other breach of the same or of any other term or condition of this Agreement.
5.13 Governing Law and Submission to Jurisdiction.
The Agreement shall be governed by French law and any dispute relating to it shall be subject to the exclusive jurisdiction of the relevant courts of the district of the Paris Court of Appeal (Cour d'appel de Paris), more precisely the Paris Commercial Court (Tribunal de commerce de Paris), the Parties having agreed that any such dispute could be brought before the international chamber of the Paris Commercial Court pursuant to the provisions of the protocol dated 7 February 2018 relating to proceedings before that chamber.
5.14 Signature of this Agreement.
The Parties:
(a) acknowledge and agree that the Agreement is entered into in writing in electronic form, in accordance with the terms of article 1366 of the French Civil Code, and signed electronically by means of a reliable identification process implemented by DocuSign®, guaranteeing the link between each signature and this Agreement in accordance with the provisions of article 1367 of the French Civil Code;
(b) acknowledge and agree that the Agreement has the same enforceability as a hard-copy written document pursuant to the provisions of article 1366 of the French Civil Code and the Agreement shall be validly invoked to evidence such enforceability;
(c) acknowledge and agree that this electronic signature has the same legal enforceability as their handwritten signature and give certainty (date certaine) to the date of/attributed to the signing of this Agreement by the DocuSign services (www.docusign.com);
- 35 -
(d) acknowledge and agree that (i) the requirement of having one original copy of the Agreement per Party shall be deemed to be fulfilled if the Agreement electronically signed is established and stored pursuant to articles 1366 and 1367 of the French Civil Code and (ii) that this process allows each Party to be provided with a copy of the Agreement on a material format or to have access to a copy of the Agreement, pursuant to the provisions of article 1375 of the French Civil Code;
(e) acknowledge and agree to designate Luxembourg (Grand-Duchy of Luxembourg) as the place of signature of the Agreement;
(f) acknowledge and agree that the Agreement shall take effect on the date hereof.
- 36 -
BRUKER INVEST AG
By: /s/ JUERGEN SREGA__
Name: Mr. Juergen Srega
Title: Duly authorized
TECFIN S.A R.L.
By: /s/ BENJAMIN MATHU__
Name: Mr. Benjamin Mathu
Title: Class A Manager (Gérant de catégorie A)
By: /s/ FRANCOIS NADIN__
Name: Mr. François Nadin
Title: Class A Manager (Gérant de catégorie A)
- 37 -
MR. CHRISTOPH GAUER
By: ___________________________
ELIMAN SAS
By: /s/ CHRISTOPH GAUER__
Name: Mr. Christoph Gauer
Title: Président
ELIMAN 2 SAS
By: /s/ CHRISTOPH GAUER__
Name: Mr. Christoph Gauer
Title: Président
- 38 -
Annex A
Data Room Documentation
- 39 -
Annex B
Sellers' Knowledge
Annex B-1 Knowledge Persons
Christoph Gauer – CEO
Marc Pégeot - CFO
Annex B-2 Persons consulted by the Knowledge Persons
Bryce McEuen
Roberto Meda
Francesco Gorreta
Stefan Lamouroux
Scott Johnston
- 40 -
Annex C
Closing Bring Down Certificates
From: |
TECFIN S.À R.L., a private limited liability company (société à responsabilité limitée) incorporated under the laws of Grand Duchy of Luxembourg, having its registered office at 53, boulevard Royal, L – 2449 Luxembourg and registered with the Trade and Companies Register of Luxembourg under number B216066, acting as Seller’s Agent under the SPA (as this term is defined below); |
To: |
BRUKER INVEST AG, a stock corporation (Aktiengesellschaft) organized under the laws of Switzerland having its registered office at Industriestrasse 26, 8117 Fällanden, Switzerland, and registered with the commercial register of canton Zurich (Handelsregisteramt des Kantons Zürich) under the number CHE-100.756.943, acting as Purchaser under the SPA. |
Strictly Private and Confidential
[date]
Re: Closing Bring Down Certificate within the meaning of Section [6.10(a)] of the SPA and Section 2.1(a) of the Warranty Agreement
Dear Sirs:
In the name and on behalf of the Sellers (as this term is defined in the SPA), reference is made to:
(i) The Securities Purchase Agreement entered into on [●] 2024 (the “Signing Date”) between the Sellers (hereinafter referred to as “we” or “us”) and the Purchaser (hereinafter referred to as “you” or “your”) (the “SPA”) and the Warranty Agreement entered into on [●] 2024 between the Sellers and the Purchaser (the “Warranty Agreement”).
(ii) Section [6.10(a)] of the SPA and Section 2.1(a) of the Warranty Agreement, pursuant to which, no earlier than two (2) Business Days prior to the Closing Date and no later than one (1) Business Day prior to the Closing Date, the Sellers shall have reviewed the Business Representations and the Sellers’ Agent shall deliver to the Purchaser the Closing Bring Down Certificate.
- 41 -
Capitalized terms used herein shall have the same meaning ascribed to them in the SPA, unless otherwise herein.
NOW, THEREFORE,
1. CONFIRMATION
1.1. [We hereby confirm to you that, after enquiry with the Sellers and the persons listed in Annex B-2 to the Warranty Agreement, no facts, events or matters have occurred between the Signing Date (included) and the Closing Date (excluded) which would render any of the Business Representations not correct or complete as of the Closing Date.]
1.2. [In accordance with Section [6.10(a)] of the SPA and Section 2.1 of the Warranty Agreement, you will find below facts, events or matters which have occurred between the Signing Date (included) and the Closing Date (excluded) which may render any of the Business Representations not correct or complete as of the Closing Date:
(i) [to be completed];
(ii) [to be completed].
For the avoidance of doubt, (i) any above of the disclosures made in this Closing Bring Down Certificate shall qualify any representations made as at the Closing Date and (ii) the Closing Bring Down Certificate shall not limit Seller(s)' liability for any breach of a representation given by the Sellers on the Put Option Date and/or on the Signing Date, as the case may be.]
2. FINAL PROVISIONS
Section [10.5] (Confidentiality) and [10.18] (Governing Law and Submission to Jurisdiction) of the SPA shall be incorporated herein in full.
- 42 -
Yours faithfully,
TECFIN S.A.R.L.
By ___________________
Name: [●]
Title: [●]
By: ___________________
Name: [●]
Title: [●]
- 43 -
Annex D
Notices
If to the Purchaser, to: Bruker Invest AG
Industriestrasse 26
8117 Fällanden, Switzerland
Attn: Jürgen Srega / Wolfgang Pusch / Carlos Mares
E-mail : Juergen.Srega@bruker.com
E-mail: Wolfgang.Pusch@bruker.com
E-mail: Carlos.Mares@bruker.com
with a copy to:
CMS Hasche Sigle Partnerschaft von Rechtsanwälten und Steuerberatern mbB
Neue Mainzer Straße 2-4
60311 Frankfurt am Main, Germany
Attn: Dr. Hendrik Hirsch
E-mail: hendrik.hirsch@cms-hs.com
If to the Sellers: TecFin S.à r.l
53, boulevard Royal
L-2449 Luxembourg
Attn: Stefano Drago / Arnaud Van Tichelen
E-mail: stefano.drago@paipartners.com
E-mail: arnaud.vantichelen@paipartners.com
with a copy to:
Willkie Farr & Gallagher LLP
21, boulevard Malesherbes
75008 Paris, France
Attn: Eduardo Fernandez
E-mail: efernandez@willkie.com
- 44 -
EXHIBIT 21.1
SUBSIDIARIES OF BRUKER CORPORATION
Name of Subsidiary |
Jurisdiction of Incorporation |
Acquifer Imaging GmbH (46) |
Germany |
Acuity Spatial Genomics, Inc. (33) |
Delaware, U.S.A. |
Agapetus GmbH (23) |
Austria |
Alicona Imaging GmbH (24) |
Austria |
Anasys Instruments Corporation (8) |
California, U.S.A. |
Bruker Norway AS (21) |
Norway |
Biognosys, AG (43) |
Switzerland |
Biognosys, Inc. (44) |
Delaware, U.S.A. |
Bruker (Beijing) Scientific Technology Co., Ltd. (11) |
China |
Bruker (Malaysia) SDN. BHD. (10) |
Malaysia |
Bruker Arabia Limited (23) |
Saudi Arabia |
Bruker Austria GmbH (4) |
Austria |
Bruker AXS GmbH (3) |
Germany |
Bruker AXS Holdings, Inc. |
Delaware, U.S.A. |
Bruker AXS LLC (8) |
Delaware, U.S.A. |
Bruker Belgium S.A./N.V. (26) |
Belgium |
Bruker BioSpin Corporation |
Massachusetts, U.S.A. |
Bruker BioSpin Holding GmbH (15) |
Germany |
Bruker BioSpin GmbH & Co. KG (31) |
Germany |
Bruker BioSpin Verwaltungs GmbH |
Germany |
Bruker Business Support Center sp. Z.o.o. (32) |
Poland |
Bruker Daltonics GmbH & Co., KG (17) |
Germany |
Bruker Daltonics Ltd. (18) |
United Kingdom |
Bruker Daltonik GmbH (16) |
Germany |
Bruker Detection Corporation (18) |
Massachusetts, U.S.A. |
Bruker do Brasil Ltda. (4) |
Brazil |
Bruker EAS GmbH (1) |
Germany |
Bruker Energy & Supercon Technologies, Inc. |
Delaware, U.S.A. |
Bruker Espanola S.A. (10) |
Spain |
Bruker Finance BV (18) |
Netherlands |
Bruker France S.A.S. (10) |
France |
Bruker India Scientific PVT, Ltd. (19) |
India |
Bruker Invest AG (9) |
Switzerland |
Bruker Italia S.r.l. (10) |
Italy |
Bruker Japan K.K. (10) |
Japan |
Bruker JV UK Ltd. (41) |
United Kingdom |
Bruker Korea Co. Ltd. (10) |
Korea |
Bruker Ltd. (10) |
Russia |
Bruker Ltd. (10) |
Canada |
Bruker Mexicana S.A. de C.V. (5) |
Mexico |
Bruker Microbiology Technology (Beijing) Co., Ltd (36) |
China |
Bruker Nano GmbH (4) |
Germany |
Bruker Nano, Inc. (27) |
Arizona, U.S.A. |
Bruker Nederland B.V. (10) |
Netherlands |
Bruker Nordic AB (18) |
Sweden |
Bruker Optics GmbH & Co. KG (35) |
Germany |
Bruker Optics Verwaltungs GmbH (35) |
Germany |
Bruker Optik Holding GmbH (18) |
Germany |
Bruker OST LLC (1) |
Delaware, U.S.A. |
Bruker Polska Sp. Z.o.o. (4) |
Poland |
Bruker Portugal Unipessoal LDA (10) |
Portugal |
Bruker PTY Ltd. (10) |
Australia |
Bruker s.r.o. (34) |
Czech Republic |
Bruker Scientific Instruments Hong Kong Co., Ltd. (10) |
Hong Kong |
Bruker Scientific Israel Ltd. (10) |
Israel |
Bruker Scientific LLC |
Delaware, U.S.A. |
Name of Subsidiary |
Jurisdiction of Incorporation |
Bruker Singapore Pte. Ltd. (10) |
Singapore |
Bruker South Africa (Pty) Ltd. (4) |
South Africa |
Bruker Switzerland AG (10) |
Switzerland |
Bruker Taiwan Co. Ltd. (18) |
Taiwan |
Bruker Technologies Ltd. (12) |
Israel |
Bruker Turkey Teknolojik Sistemler Ticaret Ltd. Sirketi (10) |
Turkey |
Bruker UK Ltd. (10) |
United Kingdom |
Bruker Verwaltungs GmbH (17) |
Germany |
Bruker-Physik GmbH (14) |
Germany |
Canopy Biosciences LLC (8) |
Delaware, U.S.A. |
Core Diagnostics Inc. (29) |
California, U.S.A. |
Deltabyte GmbH (46) |
Germany |
Gauss Fusion GmbH (40) |
Germany |
Gauss Fusion Inc. (45) |
Delaware, U.S.A. |
Fasmatech Science and Technology S.A. (10) |
Greece |
Hain LifeScience E.A. Ltd. (21) |
Kenya |
Hain LifeScience GmbH (34) |
Germany |
Hydrostatic Extrusions Ltd. (1) |
United Kingdom |
InCoaTec GmbH (4) |
Germany |
Inscopix Neuroscience, Inc. (25) |
Canada |
Inscopix, Inc. (8) |
California, U.S.A. |
Interherence GmbH (52) |
Germany |
InVivo Biotech Svx GmbH (34) |
Germany |
Lifescience Solutions Africa (Pty) Ltd. (21) |
South Africa |
Luxendo GmbH (10) |
Germany |
Merlin Diagnostika GmbH (34) |
Germany |
Mestrelab Research S.L. (22) |
Spain |
Molecubes, Inc. (13) |
Delaware, U.S.A. |
Molecubes, NV (10) |
Netherlands |
Neurescence, Inc. (6) |
Canada |
Optimal Industrial Automation Limited (10) |
United Kingdom |
Optimal Industrial Technologies Limited (10) |
United Kingdom |
Osthus Beteiligungs GmbH (47) |
Germany |
Osthus Group GmbH (48) |
Germany |
PepSep ApS (34) |
Denmark |
Pinpoint Testing, LLC (18) |
US (Where in USA) |
PMOD Technologies LLC (23) |
Switzerland |
Precision Diagnostics, Inc. (28) |
Delaware, U.S.A. |
PreOmics GmbH (37) |
Germany |
PreOmics Inc. (38) |
Delaware, U.S.A. |
Prolab Instruments GmbH (10) |
Switzerland |
Research Instruments GmbH (2) |
Germany |
SAS Biocentric (21) |
France |
SmartTip BV (30) |
Netherlands |
Vutara LLC (8) |
Delaware, U.S.A. |
XGLab S.r.l. (20) |
Italy |
Zellkraft Werk GmbH (28) |
Germany |
Zontal Inc. (49) |
Florida, U.S.A |
Zontal GmbH (50) |
Germany |
Zontal Data Information Technology (Dalian) Co., Ltd. (51) |
China |
Bruker Cellular Analysis, Inc. |
Delaware, U.S.A. |
BLI Europe International, LTD (53) |
United Kingdom |
BLI International LLC (53) |
Delaware, U.S.A. |
Berkeley Lights Life Science (Shanghai) Co. Ltd. (54) |
China |
Berkeley Lights Life Science (Singapore) Pte. Ltd. (54) |
Singapore |
IsoPlexis Corporation (53) |
Delaware, U.S.A. |
IsoPlexis (Shanghai) Trading Co., Ltd. (55) |
China |
Bruker Cellular Analysis Japan Co., Ltd. (54) |
Japan |
MIRO Analytical AG (56) |
China |
Signals GmbH & Co. KG (7) |
Germany |
EXHIBIT 23.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (No. 333-272381) and Form S-8 (Nos. 333-265396, 333-211686, 333-167333, 333-150430, 333-137090, 333-107294 and 333-47836) of Bruker Corporation of our report dated February 29, 2024 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
|
/s/ PricewaterhouseCoopers LLP
|
Boston, Massachusetts |
February 29, 2024 |
EXHIBIT 31.1
CERTIFICATION
I, Frank H. Laukien, certify that:
Date: February 29, 2024 |
|
By: |
/s/ FRANK H. LAUKIEN, PH.D.
|
|
|
|
Frank H. Laukien, Ph.D. |
|
|
|
President, Chief Executive Officer and Chairman |
|
|
|
(Principal Executive Officer) |
EXHIBIT 31.2
CERTIFICATION
I, Gerald N. Herman, certify that:
Date: February 29, 2024 |
|
By: |
/s/ GERALD N. HERMAN
|
|
|
|
Gerald N. Herman |
|
|
|
Executive Vice President and Chief Financial Officer |
|
|
|
(Principal Financial Officer and Principal Accounting Officer) |
EXHIBIT 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the Annual Report of Bruker Corporation (the “Company”) on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission on the date hereof (the “Report”), each of the undersigned, Frank H. Laukien, President, Chief Executive Officer and Chairman of the Board of Directors of the Company, and Gerald N. Herman, Executive Vice President and Chief Financial Officer of the Company, certifies, pursuant to 18 U.S.C. section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, that to the best of his knowledge:
Date: February 29, 2024 |
|
By: |
/s/ FRANK H. LAUKIEN, PH.D.
|
|
|
|
Frank H. Laukien, Ph.D. |
|
|
|
President, Chief Executive Officer and Chairman |
|
|
|
(Principal Executive Officer) |
|
|
|
|
Date: February 29, 2024 |
|
By: |
/s/ GERALD N. HERMAN
|
|
|
|
Gerald N. Herman |
|
|
|
Executive Vice President and Chief Financial Officer |
|
|
|
(Principal Financial Officer and Principal Accounting Officer) |
EXHIBIT 97.1
BRUKER CORPORATION
COMPENSATION RECOUPMENT POLICY
Bruker Corporation (“Bruker” or the “Company”) has adopted this Compensation Recoupment Policy (“Policy”) to implement the mandatory recoupment or “clawback” of compensation in the event of a Restatement in compliance with the applicable rules of the Nasdaq Stock Market (“Nasdaq”).
Capitalized terms used, but not immediately defined, in this Policy have the meaning set forth in section 13.
This Policy shall be administered in the sole discretion of the Committee, which shall have the discretion to interpret and make all determinations with respect to, and consistent with, applicable law and the provisions herein. Without limiting the foregoing, this Policy shall be interpreted in a manner that is consistent with the requirements of the Applicable Rules, and compliance with this Policy shall not be waived by the Committee, the Board, or the Company in any respect. Any interpretation and determination made by the Committee with respect to this Policy shall be final and binding on all affected individuals.
This Policy is effective as of October1, 2023 (“Effective Date”). This Policy applies to Incentive- Based Compensation that is Received by any Executive Officer on or after the Effective Date as described in section 7 below.
The Board may amend this Policy from time to time in its discretion, subject to any limitations under applicable law or listing standards, including the Applicable Rules.
Any right of recoupment under this Policy is in addition to, and not in lieu of, any other remedies or rights that may be available to Bruker pursuant to (a) the Company’s 2016 Incentive Compensation Plan or any successor plan thereto, the Company’s 2022 Short-Term Incentive Compensation Program or any successor plan thereto, or any other incentive plan of the Company or any of its subsidiaries, (b) the terms of any recoupment policy or provision in any employment agreement, compensation agreement or arrangement, or other agreement, or (c) any other legal remedies available to Bruker under applicable law.
In addition to recovery of compensation as provided for in this Policy, Bruker may take any and all other actions it deems necessary, appropriate, and in the Company’s best interest in connection with the Committee determining that this Policy should apply, including termination of the employment of, or initiating legal action against, an Executive Officer, and nothing in this Policy limits the Company’s rights to take any such appropriate actions.
In the event Bruker is required to prepare a Restatement, it shall reasonably promptly recover from an Executive Officer the amount of any erroneously awarded Incentive-Based Compensation that is Received by such Executive Officer during the Recovery Period. The amount of erroneously Received Incentive-Based Compensation will be the excess of the Incentive-Based Compensation Received by the Executive Officer (whether in cash or shares) based on the erroneous data in the original financial statements over the Incentive-Based Compensation (whether in cash or in shares) that would have been Received by the Executive Officer had such Incentive-Based Compensation been based on the restated results, without respect to any tax liabilities incurred or paid by the Executive Officer.
Without limiting the foregoing, for Incentive-Based Compensation based on Bruker’s stock price or total shareholder return, where the amount of erroneously awarded compensation is not subject to mathematical recalculation directly from the information in the Restatement, (a) the amount shall be based on Bruker’s reasonable estimate of the effect of the Restatement on the stock price or total shareholder return upon which the Incentive-Based Compensation was Received and (b) Bruker shall maintain documentation of the determination of that reasonable estimate and provide such estimate to the Regulators.
This Policy covers all persons who are Executive Officers at any time during the Recovery Period for which Incentive-Based Compensation is Received. Incentive-Based Compensation shall not be recovered under this Policy to the extent Received by any person before the date the person served as an Executive Officer. Subsequent changes in an Executive Officer’s employment status, including retirement or termination of employment, do not affect Bruker’s right to recover Incentive-Based Compensation pursuant to this Policy.
This Policy shall apply to Incentive-Based Compensation that is Received by any Executive Officer on or after the Effective Date and that results from attainment of a Financial Reporting Measure based on or derived from financial information for any fiscal period ending on or after the Effective Date.
The Committee shall determine, in its sole discretion, the method of recovering any Incentive- Based Compensation subject to this Policy, including those methods set forth in section 10.
No recovery shall be required if any of the following conditions are met and the Committee determines that, on such basis, recovery would be impracticable:
Bruker shall make all required disclosures and filings with the Regulators with respect to this Policy in accordance with the requirements of the Applicable Rules, and any other requirements applicable to the Company, including the disclosures required in connection with US Securities and Exchange Commission (“SEC”) filings.
Subject to section 8, in the event that the Committee determines that this Policy should apply, to the extent permitted by applicable law, Bruker shall, as determined by the Committee in its sole discretion, take any such actions as it deems necessary or appropriate to recover Incentive-Based Compensation. The actions may include, without limitation (and as applicable):
In addition, the Committee may authorize legal action for breach of fiduciary duty or other violation of law and take such other actions to enforce the obligations of the Executive Officer to the Company as the Committee deems appropriate.
Before Bruker takes action to seek recovery of compensation pursuant to this Policy against an Executive Officer, it shall take commercially reasonable steps to provide such individual with advance notice of such clawback; provided that such notice shall not in any way delay the reasonably prompt recovery of any erroneously awarded Incentive-Based Compensation.
Bruker shall not indemnify any current or former Executive Officer against the loss of erroneously awarded compensation, and shall not pay or reimburse any such person for premiums incurred or paid for any insurance policy to fund such person’s potential recovery obligations.
The following capitalized terms used in this Policy have the following meanings:
***
1 An executive officer of any Bruker subsidiary is an “Executive Officer” for purposes of this Policy if such executive officer performs significant policy-making functions described in the preceding sentence for the Company.
2 “Financial Reporting Measures” include, but are not limited to, the following examples of accounting-based measures and measures derived from: (i) revenues; (ii) net income;(iii) operating income;(iv) profitability of one or more reportable segments; (v) financial ratios (e.g., accounts receivable turnover and inventory turnover rates);
(vi) earnings before interest, taxes, depreciation, and amortization; (vii) funds from operations and adjusted funds from operations; (viii) liquidity measures(e.g., working capital and operating cash flow); (ix) return measures(e.g., return on invested capital and return on assets); (x) earnings measures (e.g., earnings per share); (xi) any of such financial reporting measures relative to a peer group; and (xii) tax basis income.
3 “Incentive-Based Compensation,” includes, but is not limited to, (i) non-equity incentive plan awards that are earned based wholly or in part on satisfying a Financial Reporting Measure performance goal; (ii) bonuses paid from a “bonus pool,” the size of which is determined based wholly or in part on satisfying a Financial Reporting Measure performance goal; (iii) other cash awards based on satisfaction of a Financial Reporting Measure performance goal;
(iv) performance stock units which vest based on satisfaction of a Financial Reporting Measure; and (v) proceeds received upon the sale of shares acquired through an incentive plan that were granted or vested based wholly or in part on satisfying a Financial Reporting Measure performance goal.