UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended June 30, 2023
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to___________
Commission File Number: 001-36247
Meta Materials Inc.
(Exact Name of Registrant as Specified in its Charter)
Nevada |
74-3237581 |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
|
|
|
60 Highfield Park Drive Dartmouth, Nova Scotia, Canada |
B3A 4R9 |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (902) 482-5729
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Trading Symbol(s)
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 per share |
MMAT |
Nasdaq Capital Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
Non-accelerated filer |
☒ |
Smaller reporting company |
☒ |
|
|
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of August 7, 2023, the registrant had 477,261,145 shares of common stock, $0.001 par value per share, outstanding.
TABLE OF CONTENTS
2
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of the federal securities laws, which statements involve substantial risks and uncertainties. Forward-looking statements generally relate to future events or our future financial or operating performance. In some cases, you can identify forward-looking statements because they contain words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these words or other similar terms or expressions that concern our expectations, strategy, plans or intentions. Forward-looking statements contained in this Quarterly Report on Form 10-Q include statements about:
We caution you that the foregoing list may not contain all of the forward-looking statements made in this Quarterly Report on Form 10-Q.
You should not rely upon forward-looking statements as predictions of future events. We have based these forward-looking statements contained in this Quarterly Report on Form 10-Q largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition, results of operations, business strategy, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in Part II, Item 1A, "Risk Factors" in this Quarterly Report on Form 10-Q. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this Quarterly Report on Form 10-Q may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
3
The forward-looking statements made in this Quarterly Report on Form 10-Q relate only to events as of the date on which the statements are made. We undertake no obligation to update any forward-looking statements made in this Quarterly Report on Form 10-Q to reflect events or circumstances after the date of this Quarterly Report on Form 10-Q or to reflect new information or the occurrence of unanticipated events, except as required by law. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Quarterly Report on Form 10-Q, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
4
SUMMARY OF RISK FACTORS
Below is a summary of the principal factors that could materially harm our business, operating results and/or financial condition, impair our future prospects or cause the price of our common stock to decline. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Quarterly Report on Form 10-Q and our other filings with the Securities and Exchange Commission, or the SEC, before making an investment decision regarding our common stock.
5
6
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements
META MATERIALS INC.
CONDENSED CONSOLIDATED INTERIM BALANCE SHEETS (UNAUDITED)
|
|
As of |
|
|
As of |
|
||
|
|
June 30, 2023 |
|
|
December 31, 2022 |
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
13,955,519 |
|
|
$ |
10,090,858 |
|
Restricted cash |
|
|
514,350 |
|
|
|
1,720,613 |
|
Accounts and other receivables |
|
|
1,251,004 |
|
|
|
902,718 |
|
Notes receivable, net of allowance for credit loss |
|
|
333,300 |
|
|
|
2,211,900 |
|
Inventory |
|
|
477,197 |
|
|
|
468,027 |
|
Prepaid expenses and other current assets |
|
|
3,294,151 |
|
|
|
7,202,099 |
|
Due from related parties |
|
|
8,722 |
|
|
|
8,461 |
|
Total current assets |
|
|
19,834,243 |
|
|
|
22,604,676 |
|
Intangible assets, net |
|
|
53,906,464 |
|
|
|
56,313,317 |
|
Property, plant and equipment, net |
|
|
47,680,219 |
|
|
|
42,674,699 |
|
Operating lease right-of-use ("ROU") assets |
|
|
7,437,981 |
|
|
|
5,576,824 |
|
Goodwill |
|
|
— |
|
|
|
281,748,466 |
|
Total assets |
|
$ |
128,858,907 |
|
|
$ |
408,917,982 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
||
Trade and other payables |
|
$ |
11,664,885 |
|
|
$ |
16,694,211 |
|
Restructuring costs accrual |
|
|
942,770 |
|
|
|
— |
|
Current portion of long-term debt |
|
|
547,336 |
|
|
|
483,226 |
|
Current portion of deferred revenues |
|
|
743,255 |
|
|
|
730,501 |
|
Current portion of deferred government assistance |
|
|
825,132 |
|
|
|
799,490 |
|
Funding obligation |
|
|
981,873 |
|
|
|
— |
|
Current portion of operating lease liabilities |
|
|
1,059,105 |
|
|
|
967,126 |
|
Total current liabilities |
|
|
16,764,356 |
|
|
|
19,674,554 |
|
Deferred revenues |
|
|
467,405 |
|
|
|
479,808 |
|
Deferred government assistance |
|
|
394,945 |
|
|
|
319,017 |
|
Deferred tax liability |
|
|
2,491,922 |
|
|
|
3,253,985 |
|
Long-term operating lease liabilities |
|
|
5,309,123 |
|
|
|
3,375,031 |
|
Funding obligation |
|
|
— |
|
|
|
180,705 |
|
Long-term debt |
|
|
3,181,271 |
|
|
|
3,070,729 |
|
Total liabilities |
|
$ |
28,609,022 |
|
|
$ |
30,353,829 |
|
Stockholders’ equity |
|
|
|
|
|
|
||
Common stock - $0.001 par value; 1,000,000,000 shares authorized, 471,881,955 shares issued and outstanding at June 30, 2023, and $0.001 par value; 1,000,000,000 shares authorized, 362,247,867 shares issued and outstanding at December 31, 2022 |
|
|
450,059 |
|
|
|
340,425 |
|
Additional paid-in capital |
|
|
626,124,206 |
|
|
|
590,962,866 |
|
Accumulated other comprehensive loss |
|
|
(4,480,988 |
) |
|
|
(5,242,810 |
) |
Accumulated deficit |
|
|
(521,843,392 |
) |
|
|
(207,496,328 |
) |
Total stockholders’ equity |
|
|
100,249,885 |
|
|
|
378,564,153 |
|
Total liabilities and stockholders’ equity |
|
$ |
128,858,907 |
|
|
$ |
408,917,982 |
|
Commitments and contingencies (Note 21)
Subsequent events (Note 22)
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
7
META MATERIALS INC.
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (UNAUDITED)
|
|
Three months ended |
|
Six months ended |
||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
Revenue: |
|
|
|
|
|
|
|
|
Product sales |
|
$22,907 |
|
$334,113 |
|
$81,606 |
|
$502,240 |
Development revenue |
|
2,007,617 |
|
2,989,614 |
|
3,361,177 |
|
5,796,182 |
Total revenue |
|
2,030,524 |
|
3,323,727 |
|
3,442,783 |
|
6,298,422 |
Cost of sales (exclusive of items shown separately below) |
|
758,618 |
|
701,283 |
|
1,345,646 |
|
1,407,533 |
Depreciation and amortization expense included in cost of sales |
|
17,296 |
|
18,850 |
|
27,343 |
|
40,659 |
Stock-based compensation expense included in cost of sales |
|
(2,936) |
|
157,818 |
|
140,969 |
|
208,471 |
Gross profit |
|
1,257,546 |
|
2,445,776 |
|
1,928,825 |
|
4,641,759 |
Operating expenses: |
|
|
|
|
|
|
|
|
Selling & marketing |
|
780,233 |
|
951,750 |
|
3,087,586 |
|
1,967,850 |
General & administrative |
|
5,913,267 |
|
11,300,296 |
|
12,682,706 |
|
21,202,836 |
Research & development |
|
5,059,614 |
|
4,118,781 |
|
10,120,370 |
|
7,208,206 |
Depreciation & amortization expense |
|
3,358,527 |
|
1,801,319 |
|
6,593,022 |
|
3,453,504 |
Stock-based compensation expense |
|
(2,807,086) |
|
3,915,612 |
|
(948,647) |
|
7,860,400 |
Restructuring expense |
|
1,469,149 |
|
— |
|
1,469,149 |
|
— |
Goodwill impairment |
|
282,173,053 |
|
— |
|
282,173,053 |
|
— |
Total operating expenses |
|
295,946,757 |
|
22,087,758 |
|
315,177,239 |
|
41,692,796 |
Loss from operations |
|
(294,689,211) |
|
(19,641,982) |
|
(313,248,414) |
|
(37,051,037) |
Interest expense, net |
|
(4,522) |
|
(142,055) |
|
(117,520) |
|
(306,489) |
Gain (loss) on foreign exchange, net |
|
1,162,273 |
|
(971,713) |
|
1,447,184 |
|
(823,322) |
Other expenses, net |
|
(740,005) |
|
(336,993) |
|
(1,318,125) |
|
(1,346,436) |
Total other income (expense), net |
|
417,746 |
|
(1,450,761) |
|
11,539 |
|
(2,476,247) |
Loss before income taxes |
|
(294,271,465) |
|
(21,092,743) |
|
(313,236,875) |
|
(39,527,284) |
Income tax recovery |
|
618,079 |
|
109,985 |
|
914,811 |
|
109,985 |
Net loss |
|
$(293,653,386) |
|
$(20,982,758) |
|
$(312,322,064) |
|
$(39,417,299) |
Other comprehensive income (loss), net of tax |
|
|
|
|
|
|
|
|
Foreign currency translation gain (loss) |
|
439,035 |
|
(3,056,676) |
|
761,822 |
|
(2,151,294) |
Total other comprehensive income (loss) |
|
439,035 |
|
(3,056,676) |
|
761,822 |
|
(2,151,294) |
Comprehensive loss |
|
$(293,214,351) |
|
$(24,039,434) |
|
$(311,560,242) |
|
$(41,568,593) |
Basic and diluted loss per share |
|
$(0.65) |
|
$(0.07) |
|
$(0.76) |
|
$(0.13) |
Weighted average number of shares outstanding - basic and diluted |
|
452,837,999 |
|
301,488,660 |
|
411,090,600 |
|
293,481,943 |
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
8
META MATERIALS INC.
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (UNAUDITED)
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
Additional |
|
|
Other |
|
|
|
|
|
Total |
|
||||||
|
|
Common Stock |
|
|
Paid-in |
|
|
Comprehensive |
|
|
Accumulated |
|
|
Stockholders’ |
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Income (Loss) |
|
|
Deficit |
|
|
Equity |
|
||||||
Balance, April 1, 2023 |
|
|
383,744,889 |
|
|
$ |
361,922 |
|
|
$ |
603,693,239 |
|
|
$ |
(4,920,023 |
) |
|
$ |
(226,165,006 |
) |
|
$ |
372,970,132 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(293,653,386 |
) |
|
|
(293,653,386 |
) |
Other comprehensive income |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
439,035 |
|
|
|
— |
|
|
|
439,035 |
|
Issuance of common stock and warrants |
|
|
87,791,213 |
|
|
|
87,791 |
|
|
|
25,759,947 |
|
|
|
— |
|
|
|
— |
|
|
|
25,847,738 |
|
Stock issuance costs |
|
|
— |
|
|
|
— |
|
|
|
(2,883,446 |
) |
|
|
— |
|
|
|
— |
|
|
|
(2,883,446 |
) |
Exercise of stock options and warrants |
|
|
1,017 |
|
|
|
1 |
|
|
|
284 |
|
|
|
— |
|
|
|
— |
|
|
|
285 |
|
Settlement of restricted stock units |
|
|
344,836 |
|
|
|
345 |
|
|
|
(345 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
(2,470,473 |
) |
|
|
— |
|
|
|
— |
|
|
|
(2,470,473 |
) |
Deemed dividend for down round provision in warrants |
|
|
— |
|
|
|
— |
|
|
|
2,025,000 |
|
|
|
— |
|
|
|
(2,025,000 |
) |
|
|
— |
|
Balance, June 30, 2023 |
|
|
471,881,955 |
|
|
|
450,059 |
|
|
|
626,124,206 |
|
|
|
(4,480,988 |
) |
|
|
(521,843,392 |
) |
|
|
100,249,885 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance, April 1, 2022 |
|
|
286,927,265 |
|
|
$ |
265,106 |
|
|
$ |
467,692,775 |
|
|
$ |
608,446 |
|
|
$ |
(146,828,645 |
) |
|
$ |
321,737,682 |
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(20,982,758 |
) |
|
|
(20,982,758 |
) |
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(3,056,676 |
) |
|
|
— |
|
|
|
(3,056,676 |
) |
Issuance of common stock and warrants |
|
|
37,037,039 |
|
|
|
37,037 |
|
|
|
49,962,963 |
|
|
|
— |
|
|
|
— |
|
|
|
50,000,000 |
|
Stock issuance costs |
|
|
— |
|
|
|
— |
|
|
|
(3,680,666 |
) |
|
|
— |
|
|
|
— |
|
|
|
(3,680,666 |
) |
Exercise of stock options |
|
|
402,028 |
|
|
|
402 |
|
|
|
107,750 |
|
|
|
— |
|
|
|
— |
|
|
|
108,152 |
|
Issuance of stock in connection with acquisitions |
|
|
36,443,684 |
|
|
|
36,442 |
|
|
|
67,086,069 |
|
|
|
— |
|
|
|
— |
|
|
|
67,122,511 |
|
Stock-based compensation |
|
|
— |
|
|
|
— |
|
|
|
3,876,887 |
|
|
|
— |
|
|
|
— |
|
|
|
3,876,887 |
|
Balance, June 30, 2022 |
|
|
360,810,016 |
|
|
|
338,987 |
|
|
|
585,045,778 |
|
|
|
(2,448,230 |
) |
|
|
(167,811,403 |
) |
|
|
415,125,132 |
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
9
META MATERIALS INC.
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (UNAUDITED)
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|
|
|
Additional |
|
Other |
|
|
|
Total |
|
|
Common Stock |
|
Paid-in |
|
Comprehensive |
|
Accumulated |
|
Stockholders’ |
||
|
|
Shares |
|
Amount |
|
Capital |
|
(Loss) Income |
|
Deficit |
|
Equity |
Balance, January 1, 2023 |
|
362,247,867 |
|
$340,425 |
|
$590,962,866 |
|
$(5,242,810) |
|
$(207,496,328) |
|
$378,564,153 |
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(312,322,064) |
|
(312,322,064) |
Other comprehensive income |
|
— |
|
— |
|
— |
|
761,822 |
|
— |
|
761,822 |
Issuance of common stock and warrants |
|
105,365,182 |
|
105,365 |
|
36,219,443 |
|
— |
|
— |
|
36,324,808 |
Stock issuance costs |
|
— |
|
— |
|
(3,322,231) |
|
— |
|
— |
|
(3,322,231) |
Exercise of stock options and warrants |
|
2,635,261 |
|
2,635 |
|
708,896 |
|
— |
|
— |
|
711,531 |
Settlement of restricted stock units |
|
1,633,645 |
|
1,634 |
|
(1,634) |
|
— |
|
— |
|
— |
Stock-based compensation |
|
— |
|
— |
|
(468,134) |
|
— |
|
— |
|
(468,134) |
Deemed dividend for down round provision in warrants |
|
— |
|
— |
|
2,025,000 |
|
— |
|
(2,025,000) |
|
— |
Balance, June 30, 2023 |
|
471,881,955 |
|
$450,059 |
|
$626,124,206 |
|
$(4,480,988) |
|
$(521,843,392) |
|
$100,249,885 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, January 1, 2022 |
|
284,573,316 |
|
$262,751 |
|
$463,136,404 |
|
$(296,936) |
|
$(128,394,104) |
|
$334,708,115 |
Net loss |
|
— |
|
— |
|
— |
|
— |
|
(39,417,299) |
|
(39,417,299) |
Other comprehensive loss |
|
— |
|
— |
|
— |
|
(2,151,294) |
|
— |
|
(2,151,294) |
Issuance of common stock and warrants |
|
37,037,039 |
|
37,037 |
|
49,962,963 |
|
— |
|
— |
|
50,000,000 |
Stock issuance costs |
|
— |
|
— |
|
(3,680,666) |
|
— |
|
— |
|
(3,680,666) |
Exercise of stock options and warrants |
|
2,755,977 |
|
2,757 |
|
472,137 |
|
— |
|
— |
|
474,894 |
Issuance of stock in connection with acquisitions |
|
36,443,684 |
|
36,442 |
|
67,086,069 |
|
— |
|
— |
|
67,122,511 |
Stock-based compensation |
|
— |
|
— |
|
8,068,871 |
|
— |
|
— |
|
8,068,871 |
Balance, June 30, 2022 |
|
360,810,016 |
|
$338,987 |
|
$585,045,778 |
|
$(2,448,230) |
|
$(167,811,403) |
|
$415,125,132 |
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
10
META MATERIALS INC.
CONDENSED CONSOLIDATED INTERIM STATEMENTS OF CASH FLOWS (UNAUDITED)
|
|
Six months ended |
|
|||||
|
|
June 30, 2023 |
|
|
June 30, 2022 |
|
||
|
|
$ |
|
|
$ |
|
||
Cash flows from operating activities: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(312,322,064 |
) |
|
$ |
(39,417,299 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
||
Non-cash finance (income) expense |
|
|
740,777 |
|
|
|
(12,920 |
) |
Non-cash interest expense |
|
|
147,545 |
|
|
|
273,554 |
|
Non-cash interest income |
|
|
(822,898 |
) |
|
|
— |
|
Non-cash lease expense |
|
|
831,505 |
|
|
|
488,299 |
|
Non-cash goodwill impairment |
|
|
282,173,053 |
|
|
|
— |
|
Deferred income tax |
|
|
(914,811 |
) |
|
|
(109,985 |
) |
Depreciation and amortization expense |
|
|
6,620,365 |
|
|
|
3,494,163 |
|
Impairment of assets |
|
|
18,843 |
|
|
|
— |
|
Credit loss expense |
|
|
1,701,498 |
|
|
|
— |
|
Unrealized foreign currency exchange (gain) loss |
|
|
(1,674,111 |
) |
|
|
913,101 |
|
Change in deferred revenue |
|
|
(23,006 |
) |
|
|
(165,466 |
) |
Non-cash government assistance |
|
|
— |
|
|
|
(3,047 |
) |
Gain on sale of property, plant and equipment |
|
|
— |
|
|
|
(783 |
) |
Stock-based compensation expense |
|
|
(807,678 |
) |
|
|
7,623,207 |
|
Non-cash consulting expense |
|
|
— |
|
|
|
445,664 |
|
Changes in operating assets and liabilities |
|
|
(1,554,391 |
) |
|
|
(2,531,889 |
) |
Net cash used in operating activities |
|
|
(25,885,373 |
) |
|
|
(29,003,401 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
||
Purchases of property, plant and equipment |
|
|
(6,494,362 |
) |
|
|
(8,971,435 |
) |
Proceeds from sale of property, plant and equipment |
|
|
— |
|
|
|
39,140 |
|
Proceeds from short-term investments |
|
|
— |
|
|
|
1,620,281 |
|
Proceeds from below-market capital government loan |
|
|
256,240 |
|
|
|
— |
|
Proceeds from collection of notes receivable |
|
|
1,000,000 |
|
|
|
— |
|
Acquisition of business, net of cash acquired |
|
|
— |
|
|
|
(3,486,906 |
) |
Net cash used in investing activities |
|
|
(5,238,122 |
) |
|
|
(10,798,920 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
||
Proceeds from the issuance of common stock and warrants |
|
|
36,324,808 |
|
|
|
50,000,000 |
|
Stock issuance costs paid on the issuance of common stock and warrants |
|
|
(3,322,231 |
) |
|
|
(3,680,666 |
) |
Repayments of long-term debt |
|
|
(195,780 |
) |
|
|
(182,295 |
) |
Proceeds from stock option and warrants exercises |
|
|
711,531 |
|
|
|
474,894 |
|
Net cash provided by financing activities |
|
|
33,518,328 |
|
|
|
46,611,933 |
|
Net increase in cash, cash equivalents and restricted cash |
|
|
2,394,833 |
|
|
|
6,809,612 |
|
Cash, cash equivalents and restricted cash at beginning of the period |
|
|
11,811,471 |
|
|
|
47,434,472 |
|
Effects of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
263,565 |
|
|
|
(203,738 |
) |
Cash, cash equivalents and restricted cash at end of the period |
|
$ |
14,469,869 |
|
|
$ |
54,040,346 |
|
Supplemental cash flow information |
|
|
|
|
|
|
||
Accrued purchases of property, equipment and patents |
|
$ |
1,550,901 |
|
|
$ |
1,604,903 |
|
Right-of-use assets obtained in exchange for lease liabilities |
|
$ |
2,225,962 |
|
|
$ |
142,378 |
|
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
11
META MATERIALS INC.
NOTES TO CONDENSED CONSOLIDATED INTERIM FINANCIAL STATEMENTS (Unaudited)
1. Corporate information
Meta Materials Inc. (also referred to herein as the “Company”, “META”, “we”, “us”, or “our”) is a global leader in advanced materials and nanotechnology. We are developing materials that we believe can improve the performance and efficiency of many current products as well as allow new products to be developed that cannot otherwise be developed without such materials. We believe META is positioned for growth, by pioneering a new category of intelligent surfaces, which will allow us to become the metamaterials industry leader. We enable our potential customers across a range of industries - consumer electronics, 5G communications, healthcare, aerospace, automotive, and clean energy - to deliver improved products to their customers. For example, our nano-optic metamaterial technology is being used to develop anti-counterfeiting security features for a Central Bank customer and currencies and authentication for Global brands. We currently have over 500 active patent documents, of which over 300 patents have been issued.
Our principal executive office is located at 60 Highfield Park Drive, Dartmouth, Nova Scotia, Canada.
2. Significant accounting policies
Basis of Presentation
These unaudited condensed consolidated interim financial statements and related notes are presented in accordance with accounting principles generally accepted in the United States of America, or US GAAP. Our fiscal year-end is December 31. The condensed consolidated interim financial statements include the accounts of Meta Materials Inc. and its wholly-owned subsidiaries. All significant intercompany balances and transactions have been eliminated on consolidation.
These unaudited condensed consolidated interim financial statements do not include all of the information and notes required by US GAAP for annual financial statements. Accordingly, these unaudited condensed consolidated interim financial statements should be read in conjunction with our audited consolidated financial statements and notes for the years ended December 31, 2022 and 2021, filed with the Securities and Exchange Commission (the “SEC”) on Forms 10-K and 10-K/A, respectively.
Reclassification of Prior Year Presentation
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations. An adjustment has been made to the condensed consolidated interim statement of operations and comprehensive loss.
Recently Adopted Accounting Pronouncements
ASU 2021-08:
In October 2021, the Financial Accounting Standards Board, or FASB, issued ASU 2021-08, Business Combinations (Topic 805): Accounting for Contract Assets and Contract Liabilities from Contracts with Customers, which clarifies that an acquirer of a business should recognize and measure contract assets and contract liabilities in a business combination in accordance with ASC Topic 606, Revenue from Contracts with Customers. We adopted the guidance on January 1, 2023 and its adoption did not have a material effect on our condensed consolidated interim financial statements and related disclosures.
Recently Issued Accounting Pronouncements
We currently have no material recent accounting pronouncements yet to be adopted.
3. Going concern
At each reporting period, we evaluate whether there are conditions or events that raise substantial doubt about our ability to continue as a going concern within one year after the date that the financial statements are issued. Our evaluation entails analyzing prospective operating budgets and forecasts for expectations of our cash needs and comparing those needs to the current cash and cash equivalent balances. We are required to make certain additional disclosures if we conclude substantial doubt exists and it is not alleviated by our plans or when our plans alleviate substantial doubt about our ability to continue as a going concern.
In accordance with Accounting Standards Codification, or ASC, 205-40, Going Concern, we evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that these consolidated financial statements are issued. This evaluation initially does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented as of the date the financial statements are issued.
12
When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about our ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the condensed consolidated interim financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that these condensed consolidated interim financial statements are issued. In performing its analysis, management excluded certain elements of its operating plan that cannot be considered probable.
We have incurred net losses of $312.3 million (including $282.2 million of goodwill impairment) and $79.1 million for the six months ended June 30, 2023 and the twelve months ended December 31, 2022, respectively, and have an accumulated deficit of $521.8 million as of June 30, 2023. Our expectation to generate operating losses and negative operating cash flows in the future and the need for additional funding to support our planned operations, raise substantial doubt regarding our ability to continue as a going concern for a period of one year after the date that these condensed consolidated interim financial statements are issued. Management's plans to alleviate the conditions that raise substantial doubt include reduced spending, and the pursuit of additional capital. On June 6, 2023, our board of directors approved a revised operating plan (the "Realignment and Consolidation Plan") for the remainder of 2023 pursuant to which we have begun, but not yet completed, the process of realigning our corporate structure aimed at reducing our operating expenses and focusing on key applications which represent the greatest near-term revenue potential. As part of this plan, we are exploring alternatives for certain of our technologies including Holography Technology and Wireless Sensing and Radio Wave Imaging Technology. These alternatives may include a divestiture, entering into a joint venture and/or curtailing our investment in these technologies. See Note 20 for details. Management has concluded the likelihood that its plan to successfully obtain sufficient funding from one or more of these sources, or adequately reduce expenditures, while highly possible, is less than probable because these plans are not entirely within our control and/or have not been approved by our board of directors as of the date of these condensed consolidated interim financial statements. If we are unsuccessful in obtaining financing, we will be required to assess alternative forms of action. Accordingly, we have concluded that substantial doubt exists about our ability to continue as a going concern for a period of at least twelve months from the date of issuance of these condensed consolidated interim financial statements.
The accompanying condensed consolidated interim financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The condensed consolidated interim financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above. These adjustments may be material.
4. Acquisitions
Plasma App Ltd Acquisition
On April 1, 2022, we completed the purchase of 100% of the issued and outstanding shares of PAL, or the PAL acquisition. PAL is the developer of PLASMAfusion®, a proprietary manufacturing platform technology, which enables high speed coating of any solid material on any type of substrate. PAL’s team is located at the Rutherford Appleton Laboratories in Oxford, UK.
The acquisition was accounted for as a business combination in accordance with ASC 805.
The following table presents the final allocation of consideration paid for the PAL acquisition and fair value of the assets and liabilities acquired:
|
Amount |
Fair value of common stock issued |
$15,290,320 |
Fair value of deferred consideration |
$1,698,926 |
Total Consideration |
$16,989,246 |
Net assets of PAL: |
|
Cash and cash equivalents |
$13,822 |
Other assets |
36,104 |
Intangibles |
12,600,000 |
Deferred tax liability |
(3,150,000) |
Goodwill |
7,489,320 |
|
$16,989,246 |
Acquired intangible assets totaling $12.6 million relate to a developed technology intangible asset. The significant estimates and assumptions used by us in the determination of the fair value of the acquired developed technology intangible asset includes the revenue growth rate and the discount rate.
13
The goodwill resulting from the transaction is attributable to assembled workforce, synergies, technical know-how and expertise. The fair value of acquired assets and liabilities was measured as at the acquisition date based on a valuation report provided by a third-party valuation expert.
Losses from the PAL acquisition since the acquisition date included in the condensed consolidated interim statements of operations and comprehensive loss for the three and six months ended June 30, 2023 were $0.6 million and $1.2 million, respectively, excluding impairment of goodwill recognized as part of the PAL acquisition. During the three months ended June 30, 2023, we wrote off the entire amount of goodwill. See Note 10, Intangible assets and goodwill.
We have finalized the purchase price allocation to the individual assets acquired and liabilities assumed using the acquisition method. There were no further changes to the purchase price allocation, as disclosed in the audited consolidated financial statements and notes for the year ended December 31, 2022.
Optodot Acquisition
On June 22, 2022, we completed a purchase agreement with Optodot Corporation, or Optodot, a developer of advanced materials technologies, to acquire certain assets related to patents and intellectual property for the battery and other industries, or the Optodot acquisition. The consideration transferred included the following:
The acquisition was accounted for as a business combination in accordance with ASC 805. The transaction was structured as a tax-free re-organization pursuant to Internal Revenue Code Section 368(a)(1)(c). Accordingly, the tax basis of net assets acquired retain their carryover tax basis and holding period.
The following table presents the final allocation of consideration paid for the Optodot acquisition and fair value of the assets and liabilities acquired:
|
|
Amount |
|
|
Fair value of unrestricted common stock issued or to be issued |
|
$ |
41,791,115 |
|
Fair value of restricted common stock issued |
|
|
8,342,152 |
|
Cash consideration |
|
|
3,500,000 |
|
Total consideration |
|
$ |
53,633,267 |
|
|
|
|
|
|
Net assets of Optodot: |
|
|
|
|
Intangibles |
|
|
23,300,000 |
|
Deferred tax liability |
|
|
(4,893,000 |
) |
Goodwill |
|
|
35,226,267 |
|
|
|
$ |
53,633,267 |
|
Acquired intangible assets totaling $23.3 million relate to a developed technology intangible asset. The significant estimates and assumptions used by us in the determination of the fair value of the acquired developed technology intangible asset includes the revenue growth rate and the discount rate. The goodwill resulting from the transaction is attributable to assembled workforce, synergies, technical know-how and expertise. The fair value of acquired assets and liabilities has been measured as at the acquisition date based on a valuation report provided by a third-party valuation expert.
Losses/(profit) from the Optodot acquisition since the acquisition date included in the condensed consolidated interim statements of operations and comprehensive loss for the three and six months ended June 30, 2023 were ($0.2) million and $0.3 million, respectively, excluding impairment of goodwill recognized as part of the Optodot acquisition. During the three months ended June 30, 2023, we wrote off the entire amount of goodwill. See Note 10, Intangible assets and goodwill.
We have finalized the purchase price allocation to the individual assets acquired and liabilities assumed using the acquisition method. There were no further changes to the purchase price allocation, as disclosed in the audited consolidated financial statements and notes for the year ended December 31, 2022.
5. Related party transactions
As of June 30, 2023 and December 31, 2022, receivables due from a related party, Lamda Guard Technologies Ltd, or Lamda, were $8,722 and $8,461, respectively.
6. Notes receivable
Notes receivable consists of an amount due from Next Bridge Hydrocarbons Inc., or Next Bridge, which was previously a wholly-owned subsidiary of META until the completion of the spin-off transaction on December 14, 2022.
14
One note is partially secured by a combination of shares of META’s common stock and an interest in the Orogrande Project Property. The notes receivable has been recognized at their fair value, as of December 14, 2022, subsequent to the deconsolidation of Next Bridge from our consolidated financial results.
Amounts owing from Next Bridge include:
On March 31, 2023, in exchange for a prepayment of $1.0 million of the currently outstanding principal of the aforementioned loans, we agreed with Next Bridge on the following: (a) extended the maturity date of the 2022 Note from March 31, 2023 to October 3, 2023, and (b) increased the total amount of commitments and loans made by us to Next Bridge under the Loan Agreement by $2.6 million to reflect the aforementioned costs incurred in effecting the deconsolidation.
In addition, on March 31, 2023, we amended the 2021 Note issued and payable by Next Bridge to us, to extend the maturity date from March 31, 2023 to October 3, 2023. The existing liens securing the 2021 Note were also reaffirmed by the grantors of such liens.
We assessed the fair value of the notes receivable on the deconsolidation date in accordance with ASC 820, Fair Value Measurement, and recorded $2.2 million of fair value of the Next Bridge notes receivable as of December 31, 2022. In accordance with ASC 326, Financial Instruments – Credit losses, we elected a practical expedient to account for the Next Bridge notes receivable as collateral-dependent assets, whereby estimated credit losses are based on the fair value of the collateral. For the three months ended June 30, 2023, we recorded interest income of $0.4 million and credit losses of $0.7 million. For the six months ended June 30, 2023, we recorded interest income of $0.8 million and credit losses of $1.7 million. As of June 30, 2023, the carrying value, net of the credit losses, of notes receivable was $0.3 million.
Subsequent to June 30, 2023, we entered into a Loan Sale Agreement with Gregory McCabe ("Purchaser"), under which we sold and assigned to Purchaser all of our rights, title, interests, and obligations in and to the aforementioned loans owed by Next Bridge (“Assigned Loan Interests”) in exchange for cash consideration of $6.0 million and agreeing to enter into a Stock Purchase Agreement ("SPA") for an additional $6.0 million of shares of our common stock. Assigned Loan Interests represented $24.0 million of the outstanding balance of the Next Bridge notes receivable as of the closing date of August 7, 2023 ("Closing Date"). Pursuant to the SPA, the Purchaser will be required to purchase an aggregate of $6.0 million of shares of our common stock (beginning on September 1, 2023, or as soon as possible after such date if necessary under applicable law, in monthly amounts of $250,000 for the first six months and then in monthly amounts of $500,000 for the next nine months thereafter), at a per share purchase price equal to 120% of the 5-day volume weighted average price (“VWAP”) of our common stock on the trading day preceding the date of each such purchase. See Note 22, Subsequent events, for further information.
15
7. Inventory
Inventory consists of photosensitive materials, lenses, laser protection film and finished eyewear, and is comprised of the following:
|
|
As of |
|
|||||
|
|
June 30, |
|
|
December 31, |
|
||
Raw materials |
|
$ |
499,387 |
|
|
$ |
490,077 |
|
Supplies |
|
|
11,373 |
|
|
|
11,345 |
|
Work in process |
|
|
51,006 |
|
|
|
51,589 |
|
Finished goods |
|
|
45,390 |
|
|
|
42,058 |
|
Inventory provision |
|
|
(129,959 |
) |
|
|
(127,042 |
) |
Total inventory |
|
$ |
477,197 |
|
|
$ |
468,027 |
|
8. Prepaid expenses and other current assets
Prepaid expenses and other current assets consist of the following:
|
|
As of |
|
|||||
|
|
June 30, 2023 |
|
|
December 31, 2022 |
|
||
Prepaid expenses |
|
$ |
1,351,938 |
|
|
$ |
2,835,660 |
|
Other current assets |
|
|
544,425 |
|
|
|
365,583 |
|
Taxes receivable |
|
|
1,397,788 |
|
|
|
4,000,856 |
|
Total prepaid expenses and other current assets |
|
$ |
3,294,151 |
|
|
$ |
7,202,099 |
|
9. Property, plant and equipment, net
Property, plant and equipment consist of the following:
|
|
Useful life |
|
As of |
|
|||||
|
|
(years) |
|
June 30, |
|
|
December 31, |
|
||
Land |
|
N/A |
|
$ |
449,396 |
|
|
$ |
439,309 |
|
Building |
|
25 |
|
|
5,179,343 |
|
|
|
5,063,091 |
|
Computer equipment |
|
3-5 |
|
|
1,400,669 |
|
|
|
775,736 |
|
Computer software |
|
1 |
|
|
660,904 |
|
|
|
606,729 |
|
Manufacturing equipment |
|
2-5 |
|
|
23,358,248 |
|
|
|
22,701,761 |
|
Office furniture |
|
5-7 |
|
|
912,411 |
|
|
|
660,549 |
|
Leasehold improvements |
|
5-10 |
|
|
21,493,571 |
|
|
|
2,172,134 |
|
Enterprise Resource Planning software |
|
5 |
|
|
202,185 |
|
|
|
197,648 |
|
Assets under construction |
|
N/A |
|
|
7,588,023 |
|
|
|
20,337,338 |
|
|
|
|
|
|
61,244,750 |
|
|
|
52,954,295 |
|
Accumulated depreciation and impairment |
|
|
|
|
(13,564,531 |
) |
|
|
(10,279,596 |
) |
|
|
|
|
$ |
47,680,219 |
|
|
$ |
42,674,699 |
|
Depreciation expense was $1.6 million and $0.8 million for the three months ended June 30, 2023 and June 30, 2022, respectively, and $3.1 million and $1.6 million for the six months ended June 30, 2023 and 2022, respectively.
Property, plant and equipment is pledged as security under a General Security Agreement, or a GSA, signed in favor of the Royal Bank of Canada, or RBC, on July 14, 2014, which is related to our corporate bank account and credit card and includes all property, plant and equipment and intangible assets.
16
10. Intangible assets and goodwill
Intangible Assets
Intangible assets consist of the following:
|
|
Useful life |
|
As of |
|
|||||
|
|
(years) |
|
June 30, |
|
|
December 31, |
|
||
Patents |
|
5-10 |
|
$ |
42,986,894 |
|
|
$ |
42,111,143 |
|
Trademarks |
|
|
|
|
127,680 |
|
|
|
124,845 |
|
Developed technology |
|
20 |
|
|
14,297,583 |
|
|
|
13,976,668 |
|
Customer contract |
|
5 |
|
|
9,818,731 |
|
|
|
9,598,348 |
|
|
|
|
|
|
67,230,888 |
|
|
|
65,811,004 |
|
Accumulated amortization and impairment |
|
|
|
|
(13,324,424 |
) |
|
|
(9,497,687 |
) |
|
|
|
|
$ |
53,906,464 |
|
|
$ |
56,313,317 |
|
Amortization expense was $1.8 million and $1.0 million for the three months ended June 30, 2023 and 2022, respectively, and $3.5 million and $1.9 million for the six months ended June 30, 2023 and 2022, respectively.
Goodwill
Goodwill at December 31, 2022 |
|
$ |
281,748,466 |
|
Effect of foreign exchange on goodwill |
|
|
424,587 |
|
Impairment |
|
|
(282,173,053 |
) |
Goodwill at June 30, 2023 |
|
$ |
— |
|
Goodwill is tested for impairment annually as of December 31 or more frequently on a reporting unit basis when events or changes in circumstances indicate that impairment may have occurred. As defined in the authoritative guidance, a reporting unit is an operating segment, or one level below an operating segment. Historically, we conducted our business in a single operating segment and reporting unit.
As a result of the continued and sustained decline in the price of our common shares in the three months ended June 30, 2023, we determined there to be an indicator of impairment for our one reporting unit to which goodwill is assigned. As a result, we performed a quantitative interim goodwill impairment assessment as of June 30, 2023. We concluded that the carrying value of the reporting unit was higher than its estimated fair value, and a goodwill impairment loss totaling $282.2 million was recognized in the three months ended June 30, 2022, representing the entirety of the goodwill.
The estimated fair value of the reporting unit was determined using the market valuation method, which is consistent with the methodology we used in the annual impairment test conducted at December 31, 2022. The most significant assumption used in applying this method was our share price.
17
11. Long-term debt
|
|
As of |
|
|||||
|
|
June 30, |
|
|
December 31, |
|
||
Atlantic Canada Opportunities Agency, or ACOA, Business Development Program, or BDP, 2012 interest-free loan1 with a maximum contribution of CA$500,000, repayable in monthly repayments commencing October 1, 2015, of CA$5,952 until June 1, 2023. Loan repayments were temporarily paused effective April 1, 2020, until January 1, 2021, as a result of the COVID-19 outbreak. As of June 30, 2023, the amount of principal drawn down on the loan, net of repayments is CA$Nil (2022 - CA$35,714). |
|
$ |
— |
|
|
$ |
25,880 |
|
ACOA Atlantic Innovation Fund, or AIF, 2015 interest-free loan1,2 with a maximum contribution of CA$ 3,000,000. Annual repayments, commencing June 1, 2021, are calculated as a percentage of gross revenue for the preceding fiscal year, at Nil when gross revenues are less than CA$1,000,000, 5% when gross revenues are less than CA$10,000,000 and greater than CA$1,000,000, and CA$500,000 plus 1% of gross revenues when gross revenues are greater than CA$10,000,000. As of June 30, 2023, the amount or principal drawn down on the loan, net of repayments, is CA$2,661,293 (2022 - CA$2,661,293). |
|
|
1,442,185 |
|
|
|
1,449,493 |
|
ACOA BDP 2018 interest-free loan1,3 with a maximum contribution of CA$3,000,000, repayable in monthly repayments commencing June 1, 2021, of CA$31,250 until May 1, 2029. As of June 30, 2023, the amount of principal drawn down on the loan, net of repayments, is CA$2,218,750 (2022 - CA$2,406,250). |
|
|
1,106,506 |
|
|
|
1,136,556 |
|
ACOA PBS 2019 interest-free loan1 with a maximum contribution of CA$100,000, repayable in monthly repayments commencing June 1, 2021, of CA$1,400 until May 1, 2027. As of June 30, 2023, the amount of principal drawn down on the loan, net of repayments, is CA$65,278 (2022 - CA$73,611). |
|
|
32,853 |
|
|
|
34,750 |
|
ACOA Regional Relief and Recovery Fund, or RRRF, 2020 interest-free loan with a maximum contribution of CA$390,000, repayable on monthly repayments commencing April 1, 2023, of CA$11,000 until April 1, 2026. As of June 30, 2023, the principal amount drawn down on the loan is CA$357,000 (2022 - CA$390,000). |
|
|
171,732 |
|
|
|
159,642 |
|
Economic Development Agency of Canada for the Regions of Quebec, or EDC, 2022 interest-free loan4 with a maximum contribution of CA$2,000,000 (CA$1,000,000 for building renovations and CA$1,000,000 for acquisition of equipment for Nanotech). Repayable in 60 monthly installments of CA$ 30,000, with the first repayment due in January 2026. As of June 30, 2023, the principal amount drawn down on the loan is CA$1,800,000 (2022 - CA$1,454,167). |
|
|
975,331 |
|
|
|
747,634 |
|
|
|
|
3,728,607 |
|
|
|
3,553,955 |
|
Less: current portion |
|
|
547,336 |
|
|
|
483,226 |
|
|
|
$ |
3,181,271 |
|
|
$ |
3,070,729 |
|
1 We were required to maintain a minimum balance of positive equity throughout the term of the loan. However, on November 14, 2019, ACOA waived this requirement for the period ending June 30, 2019 and for each period thereafter until the loan is fully repaid.
2 The carrying amount of the ACOA AIF loan is reviewed each reporting period and adjusted as required to reflect management’s best estimate of future cash flows, discounted at the original effective interest rate.
3 A portion of the ACOA BDP 2018 loan was used to finance the acquisition and construction of manufacturing equipment resulting in $425,872 that was recorded as deferred government assistance, which is being amortized over the useful life of the associated equipment.
4 The EDC 2022 loan was used to finance building renovations and equipment purchase resulting in $409,401 of deferred government assistance as of June 30, 2023, which is being amortized over the useful life of the associated building and equipment.
12. Capital stock
Common Stock
Authorized: 1,000,000,000 common shares, $0.001 par value.
During the six months ended June 30, 2023, 2,635,161 stock options were exercised to purchase an equal number of common shares. In addition, 1,633,645 restricted stock units have vested and settled into an equal number of common shares.
18
At-the-Market Equity Offering Program:
On February 10, 2023, we entered into a sales agreement, or the ATM Agreement, which was amended on June 20, 2023, with an investment bank to conduct an "at-the-market" equity offering program, or the ATM, pursuant to which we may issue and sell, shares of our common stock, par value $0.001 per share, up to an aggregate of $100.0 million of shares of common stock, or the ATM Shares, from time to time.
Under the ATM Agreement, we set the parameters for the sale of ATM Shares, including the number of ATM Shares to be issued, the time period during which sales are requested to be made, limitations on the number of ATM Shares that may be sold in any one trading day and any minimum price below which sales may not be made. Sales of the ATM Shares, if any, under the ATM Agreement may be made in transactions that are deemed to be “at-the-market offerings” as defined in Rule 415 under the Securities Act of 1933, as amended, or the Securities Act.
During the three months ended June 30, 2023, we sold a total of 4,457,879 shares of our common stock under the ATM at a weighted average price of $0.19 per share, generating proceeds of $0.8 million, net of offering expenses.
During the six months ended June 30, 2023, we sold a total of 22,031,848 shares of our common stock under the ATM at a weighted average price of $0.51 per share, generating gross proceeds of $11.3 million and net proceeds of $11.0 million after offering expenses. As of June 30, 2023, $88.7 million of common stock remained eligible for sale under the ATM Agreement.
Registered Direct Offering:
April 2023 Offerings
On April 14, 2023, we entered into an underwriting agreement, or the Underwriting Agreement, with Ladenburg Thalmann & Co. Inc. and A.G.P/Global Alliance Partners, or the underwriters, relating to our public offering of (i) 83,333,334 shares of our common stock, par value $0.001 and (ii) warrants to purchase up to an aggregate of 83,333,334 shares of our common stock. The shares of common stock and warrants were sold together as a fixed combination, consisting of one share of common stock and a warrant to purchase one share of common stock, but are immediately separable and were issued separately in the offering. Each warrant is immediately exercisable to purchase one share of common stock at a price of $0.375 per share, or Exercise Price, subject to certain adjustments in the case of a Share Combination Event or a Dilutive Issuance as described below, and expires five years from the date of issuance. The combined price to the public in the offering for each share of common stock and accompanying warrant was $0.30. After deducting underwriting discounts and commissions and the offering expenses payable by us, the net proceeds were $22.1 million.
The gross proceeds of $25.0 million were allocated between common stock and accompanying warrants based on their relative fair values. The fair value of common stock was calculated based on the closing share price on the Issuance Date of $0.22. The fair value of the warrants was estimated using the Black-Scholes option pricing model. Accordingly, we have allocated $15.7 million as the fair value of common stock and $9.3 million as the fair value of warrants.
The warrants contain a down round provision that requires the exercise price to be adjusted if the Company sells shares of common stock below the current exercise price. If at any time and from time to time on or after April 18, 2023, the issue date of warrants, or Issue Date, and prior to the second anniversary of the Issue Date there occurs any Share Combination Event (as defined below) and the Event Market Price (as defined below) is less than the Exercise Price, then on the sixth trading day immediately following such Share Combination Event, the Exercise Price on such sixth trading day shall be reduced (but in no event increased) to the Event Market Price; provided, however, that in no event shall the Event Market Price be less than $0.076 (appropriately adjusted for any Share Combination Event occurring after the Issue Date), or the Floor Price.
A Share Combination Event occurs when we (i) pay a stock dividend or otherwise make a distribution or distributions on shares of our common stock or any other equity or equity equivalent securities payable in shares of common stock (which, for avoidance of doubt, shall not include any shares of common stock issued by us upon exercise of this warrant), (ii) subdivide outstanding shares of common stock into a larger number of shares, (iii) combine (including by way of reverse stock split) outstanding shares of common stock into a smaller number of shares, or (iv) issue by reclassification of shares of our common stock any shares of our capital stock. “Event Market Price” means, with respect to any Share Combination Event Date, the quotient determined by dividing (x) the sum of the volume weighted average price of the shares of common stock for each of the five trading days following such Share Combination Event divided by (y) five. All such determinations shall be appropriately adjusted for any stock dividend, stock split, stock combination, recapitalization or other similar transaction during such period.
In addition, if at any time prior to April 18, 2024, we grant, issue or sell, or are deemed to have granted issued or sold, any shares of our Common Stock, subject to certain exceptions, for a consideration per share, or the New Issuance Price less than a price equal to the exercise price of the warrants then in effect, the foregoing a Dilutive Issuance, then immediately after such Dilutive Issuance, the exercise price then in effect will be reduced to an amount equal to the greater of the New Issuance Price and the Floor Price.
19
We have evaluated the warrants as either equity-classified or liability-classified instruments based on an assessment of the warrants’ specific terms and applicable authoritative guidance in ASC 480, Distinguishing Liabilities from Equity, and ASC 815, Derivatives and Hedging. We have concluded that the warrants are considered indexed to our common shares and as such they have been classified as equity.
During the three months ended June 30, 2023, we sold shares of common stock for $0.174 under the ATM Arrangement; therefore, the exercise price of these warrants was adjusted from $0.375 to $0.174. The change in fair value between the value of the warrants using the new exercise price versus the old exercise price was $2.0 million. The amount was recorded as a deemed dividend in the condensed consolidated interim statement of changes in stockholders' equity during the three months ended June 30, 2023.
June 2022 Offerings
On June 24, 2022, we entered into a securities purchase agreement (the "SPA"), as amended and restated on June 27, 2022, with certain institutional investors for the purchase and sale in a registered direct offering of 37,037,039 shares of our common stock at a purchase price of $1.35 per share and warrants to purchase 37,037,039 shares at an exercise price of $1.75 per share. This resulted in gross proceeds from the offering of $50.0 million and net proceeds of $46.3 million.
The gross proceeds were allocated between common stock and accompanying warrants based on their relative fair values. The fair value of common stock was calculated based on the closing share price on June 27, 2022 of $1.15. The fair value of the warrants was estimated using the Black-Scholes option pricing model. Accordingly, we have allocated $27.9 million as the fair value of common stock and $18.5 million as the fair value of warrants.
The warrants are exercisable six months after the date of issuance, expire five and a half years from the date of issuance and have an exercise price of $1.75 per share of common stock. We have evaluated the warrants as either equity-classified or liability-classified instruments based on an assessment of the warrants’ specific terms and applicable authoritative guidance in ASC 480, Distinguishing Liabilities from Equity, and ASC 815, Derivatives and Hedging. We have concluded that the warrants are considered indexed to our common shares and as such they have been classified as equity.
The following table summarizes the changes in warrants:
|
|
Six months ended |
|
|||||
|
|
June 30, 2023 |
|
|||||
|
|
Number of |
|
|
|
|
||
|
|
warrants (#) |
|
|
Amount |
|
||
Outstanding, December 31, 2022 |
|
|
39,920,919 |
|
|
$ |
25,319,193 |
|
Issued |
|
|
83,333,334 |
|
|
|
9,317,080 |
|
Exercised |
|
|
(100 |
) |
|
|
(14 |
) |
Deemed dividend for down round provision in warrants |
|
|
— |
|
|
|
2,025,000 |
|
Outstanding, June 30, 2023 |
|
|
123,254,153 |
|
|
$ |
36,661,259 |
|
The fair value of warrants that were issued and estimated using the Black-Scholes option pricing model have the following inputs and assumptions:
|
|
Six months ended |
|
|
June 30, 2023 |
Weighted average grant date fair value ($) |
|
0.22 |
Weighted average expected volatility |
|
84.70% |
Weighted average risk-free interest rate |
|
3.65% |
Weighted average expected life of the warrants |
|
5.0 years |
13. Stock-based payments
On December 3, 2021, our shareholders approved the 2021 Equity Incentive Plan to utilize the 3,500,000 shares reserved and unissued under the Torchlight 2015 Stock Option and Grant Plan and the 6,445,745 shares reserved and unissued under the MMI 2018 Stock Option and Grant plan to set the number of shares reserved for issuance under the 2021 Equity Incentive Plan at 34,945,745 shares.
20
The 2021 Equity Incentive Plan allows the grants of non-statutory stock options, restricted stock, restricted stock units, or RSUs, stock appreciation rights, performance units and performance shares to employees, directors, and consultants.
DSU Plan
On March 28, 2013, we implemented a Deferred Stock Unit, or DSU, Plan for our directors, employees and officers. Directors, employees and officers are granted DSUs with immediate vesting as a form of compensation. Each unit is convertible at the option of the holder into one common share. Eligible individuals are entitled to receive all DSUs (including dividends and other adjustments) no later than December 1st of the first calendar year commencing after the time of termination of their services.
The following table summarizes the change in outstanding DSUs:
|
|
Number of |
|
|
Weighted |
|
||
|
|
# |
|
|
$ |
|
||
Outstanding, December 31, 2022 |
|
|
3,910,186 |
|
|
$ |
0.32 |
|
Granted |
|
|
525,210 |
|
|
$ |
0.22 |
|
Outstanding, June 30, 2023 |
|
|
4,435,396 |
|
|
$ |
0.31 |
|
X |
|
|
|
|
|
|
||
Vested, June 30, 2023 |
|
|
3,910,186 |
|
|
$ |
0.32 |
|
We recognized $15,045 of stock-based compensation expense related to DSUs during the three and six months ended June 30, 2023.
RSU Plan
Each unit is convertible at the option of the holder into one common share of our shares upon meeting the vesting conditions.
Total stock-based compensation expense related to RSUs included in the condensed consolidated interim statements of operations was as follows:
|
|
Three months ended |
|
|
Six months ended |
|
||||||||||
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Cost of sales |
|
$ |
(180,281 |
) |
|
$ |
157,818 |
|
|
$ |
(36,376 |
) |
|
$ |
208,471 |
|
Selling & marketing |
|
|
(246,314 |
) |
|
|
114,707 |
|
|
|
(125,770 |
) |
|
|
130,200 |
|
General & administrative |
|
|
(497,918 |
) |
|
|
396,997 |
|
|
|
(94,711 |
) |
|
|
517,162 |
|
Research & development |
|
|
(654,206 |
) |
|
|
1,096,021 |
|
|
|
(235,185 |
) |
|
|
1,193,232 |
|
|
(1) |
$ |
(1,578,719 |
) |
|
$ |
1,765,543 |
|
|
$ |
(492,042 |
) |
|
$ |
2,049,065 |
|
(1) Estimated forfeiture rate has been changed to 45% from 0% during the second quarter ended June 30, 2023 due to the Realignment and Consolidation Plan.
21
The following table summarizes the change in outstanding RSUs:
|
|
Number of |
|
|
Weighted |
|
||
Outstanding, December 31, 2022 |
|
|
6,506,922 |
|
|
$ |
1.71 |
|
Granted |
|
|
525,210 |
|
|
$ |
0.22 |
|
Vested and settled |
|
|
(1,633,645 |
) |
|
$ |
2.49 |
|
Awards forfeited |
|
|
(710,930 |
) |
|
$ |
1.38 |
|
Outstanding, June 30, 2023 |
|
|
4,687,557 |
|
|
$ |
1.34 |
|
|
|
|
|
|
|
|
||
Vested, but not yet settled, June 30, 2023 |
|
|
(85,169 |
) |
|
$ |
1.27 |
|
Employee Stock Option Plan
Each stock option is convertible at the option of the holder into one common share upon payment of the exercise price.
Total stock-based compensation expense related to stock options included in the condensed consolidated interim statements of operations and comprehensive loss was as follows:
|
|
Three months ended |
|
Six months ended |
||||
|
|
June 30, |
|
June 30, |
||||
|
|
2023 |
|
2022 |
|
2023 |
|
2022 |
Selling & marketing |
|
$(98,237) |
|
$60,622 |
|
$1,005 |
|
$65,015 |
General & administrative |
|
(865,612) |
|
985,932 |
|
(366,570) |
|
4,269,400 |
Research & development |
|
(282,498) |
|
815,669 |
|
34,881 |
|
1,239,727 |
|
(1) |
$(1,246,347) |
|
$1,862,223 |
|
$(330,684) |
|
$5,574,142 |
(1) Estimated forfeiture rate has been changed to 46% from 0% during the second quarter ended June 30, 2023 due to the Realignment and Consolidation Plan.
The following table summarizes the change in our outstanding stock options:
|
|
Number of |
|
|
Average |
|
|
Average |
|
|
Aggregate intrinsic |
|
||||
Outstanding, December 31, 2022 |
|
|
33,595,044 |
|
|
$ |
0.80 |
|
|
|
9.31 |
|
|
$ |
17,611,251 |
|
Granted |
|
|
1,519,336 |
|
|
$ |
0.23 |
|
|
|
|
|
|
— |
|
|
Forfeited |
|
|
(1,250,675 |
) |
|
$ |
0.84 |
|
|
|
|
|
|
— |
|
|
Exercised |
|
|
(2,635,161 |
) |
|
$ |
0.27 |
|
|
|
|
|
|
— |
|
|
Outstanding, June 30, 2023 |
|
|
31,228,544 |
|
|
$ |
0.82 |
|
|
|
6.61 |
|
|
$ |
109,875 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercisable, June 30, 2023 |
|
|
25,774,708 |
|
|
$ |
0.75 |
|
|
$ |
3.70 |
|
|
$ |
100,875 |
|
Below is a summary of the outstanding options as of June 30, 2023 and December 31, 2022:
|
|
June 30, |
|
|
December 31, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
||||||||||
Exercise price |
|
Number outstanding |
|
|
Number exercisable |
|
|
Number outstanding |
|
|
Number exercisable |
|
||||
$0.12 - $0.27 |
|
|
16,346,601 |
|
|
|
15,469,417 |
|
|
|
18,128,657 |
|
|
|
15,549,318 |
|
$0.89 - $1.00 |
|
|
2,984,668 |
|
|
|
1,822,394 |
|
|
|
2,984,668 |
|
|
|
1,822,394 |
|
$1.17 - $1.26 |
|
|
2,498,420 |
|
|
|
1,406,241 |
|
|
|
2,782,704 |
|
|
|
932,082 |
|
$1.31 - $1.58 |
|
|
6,429,744 |
|
|
|
4,107,545 |
|
|
|
6,729,904 |
|
|
|
3,054,672 |
|
$1.97 - 2.00 |
|
|
2,969,111 |
|
|
|
2,969,111 |
|
|
|
2,969,111 |
|
|
|
2,969,111 |
|
|
|
|
31,228,544 |
|
|
|
25,774,708 |
|
|
|
33,595,044 |
|
|
|
24,327,577 |
|
22
The fair value of options granted during six months ended June 30, 2023 are estimated at the grant date using the following weighted-average assumptions:
|
|
Six months ended |
|
|
June 30, 2023 |
Weighted average grant date fair value ($) |
|
0.19 |
Weighted average expected volatility |
|
84.56% |
Weighted average risk-free interest rate |
|
3.57% |
Weighted average expected life of the options |
|
1.48 |
14. Income taxes
We estimate our annual effective income tax rate in recording our quarterly provision for income taxes in the various jurisdictions in which we operate. Statutory tax rate changes and other significant or unusual items are recognized as discrete items in the quarter in which they occur.
Our effective tax rate for the three and six months ended June 30, 2023 differs from the statutory rates due to a valuation allowance as well as different domestic and foreign statutory tax rates.
Deferred tax recovery for the three months ended June 30, 2023 and 2022 was $0.6 million and $0.1 million, respectively. Deferred tax recovery for the six months ended June 30, 2023 and 2022 was $0.9 million and $0.1 million, respectively.
We have not yet been able to establish profitability or other sufficient significant positive evidence, to conclude that our deferred tax assets are more likely than not going to be realized. Therefore, we continue to maintain a valuation allowance against our deferred tax assets.
15. Net loss per share
The following table sets forth the calculation of basic and diluted net loss per share during the periods presented:
|
|
Three months ended |
|
|
Six months ended |
|
||||||||||
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net loss |
|
$ |
(293,653,386 |
) |
|
$ |
(20,982,758 |
) |
|
$ |
(312,322,064 |
) |
|
$ |
(39,417,299 |
) |
Deemed dividend for down round provision in warrants |
(1) |
$ |
(2,025,000 |
) |
|
$ |
- |
|
|
$ |
(2,025,000 |
) |
|
$ |
- |
|
|
|
$ |
(295,678,386 |
) |
|
$ |
(20,982,758 |
) |
|
$ |
(314,347,064 |
) |
|
$ |
(39,417,299 |
) |
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Weighted-average shares, basic |
|
|
452,837,999 |
|
|
|
301,488,660 |
|
|
|
411,090,600 |
|
|
|
293,481,943 |
|
Weighted-average shares, diluted |
|
|
452,837,999 |
|
|
|
301,488,660 |
|
|
|
411,090,600 |
|
|
|
293,481,943 |
|
Net loss per share |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Basic |
|
$ |
(0.65 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.76 |
) |
|
$ |
(0.13 |
) |
Diluted |
|
$ |
(0.65 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.76 |
) |
|
$ |
(0.13 |
) |
(1) Refer to April 2023 offerings in the Note 12.
The following potentially dilutive shares were not included in the calculation of diluted shares above as the effect would have been anti-dilutive:
|
|
Three months ended |
|
|
Six months ended |
|
||||||||||
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Options |
|
|
31,228,544 |
|
|
|
31,409,460 |
|
|
|
31,228,544 |
|
|
|
31,409,460 |
|
Warrants |
|
|
123,254,153 |
|
|
|
39,920,919 |
|
|
|
123,254,153 |
|
|
|
39,920,919 |
|
DSUs |
|
|
4,435,396 |
|
|
|
3,647,026 |
|
|
|
4,435,396 |
|
|
|
3,647,026 |
|
RSUs |
|
|
4,687,557 |
|
|
|
6,648,184 |
|
|
|
4,687,557 |
|
|
|
6,648,184 |
|
|
|
|
163,605,650 |
|
|
|
81,625,589 |
|
|
|
163,605,650 |
|
|
|
81,625,589 |
|
23
16. Additional cash flow information
The net changes in non-cash working capital balances related to operations consist of the following:
|
|
Six months ended |
|
|||||
|
|
June 30, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Grants receivable |
|
$ |
— |
|
|
$ |
125,558 |
|
Inventory |
|
|
1,533 |
|
|
|
(34,752 |
) |
Accounts and other receivables |
|
|
(332,424 |
) |
|
|
(67,162 |
) |
Prepaid expenses and other current assets |
|
|
3,995,939 |
|
|
|
(685,141 |
) |
Trade payables and accruals |
|
|
(5,522,384 |
) |
|
|
(1,603,016 |
) |
Restructuring costs accrual |
|
|
942,770 |
|
|
|
— |
|
Due to related party |
|
|
(261 |
) |
|
|
(108,102 |
) |
Operating lease right-of-use asset |
|
|
— |
|
|
|
(231 |
) |
Operating lease liabilities |
|
|
(639,564 |
) |
|
|
(159,043 |
) |
|
|
$ |
(1,554,391 |
) |
|
$ |
(2,531,889 |
) |
17. Fair value measurements
We use a fair value hierarchy, based on the relative objectivity of inputs used to measure fair value, with Level 1 representing inputs with the highest level of objectivity and Level 3 representing the lowest level of objectivity.
The fair values of cash and cash equivalents, restricted cash, short-term investments, grants and accounts receivable, due from related parties and trade and other payables approximate their carrying values due to the short-term nature of these instruments. The current portion of long-term debt has been included in the below table.
Our initial measurement of our notes receivable at fair value from Next Bridge is classified at Level 3 in the fair value hierarchy. See Note 6 for further details.
The funding obligation (a CA$1.3 million interest free loan obtained from an international engineering and support service provider under the Industrial and Regional Benefits program) was originally recorded at its fair value and had been adjusted based on the estimated timing of the repayment of the loan. During the three months ended June 30, 2023, we determined that the funding obligation’s carrying value equals its fair value because it is now repayable upon demand. The funding obligation has also been reclassified to current liabilities within the condensed consolidated interim balance sheet.
The fair values of the funding obligation and long-term debt are classified at Level 3 in the fair value hierarchy, as each instrument is estimated based on unobservable inputs including discounted cash flows using the market rate, which is subject to similar risks and maturities with comparable financial instruments as at the reporting date.
Carrying values and fair values of financial instruments that are not carried at fair value are as follows:
|
|
June 30, |
|
|
December 31, |
|
||||||||||
Financial liability |
|
Carrying value |
|
|
Fair value |
|
|
Carrying value |
|
|
Fair value |
|
||||
Funding obligation |
|
$ |
981,873 |
|
|
$ |
981,873 |
|
|
$ |
180,705 |
|
|
$ |
85,411 |
|
Long-term debt |
|
$ |
3,728,607 |
|
|
$ |
4,769,788 |
|
|
$ |
3,553,955 |
|
|
$ |
2,663,460 |
|
24
18. Revenue
We have one operating segment based on how management internally evaluates separate financial information, business activities and management responsibility.
Revenue is disaggregated as follows:
|
|
Three months ended |
|
|
Six months ended |
|
||||||||||
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Product sales |
|
$ |
22,907 |
|
|
$ |
334,113 |
|
|
$ |
81,606 |
|
|
$ |
502,240 |
|
Contract revenue (1) |
|
|
1,540,811 |
|
|
|
2,989,614 |
|
|
|
2,894,371 |
|
|
|
5,696,182 |
|
Other development revenue |
|
|
466,806 |
|
|
|
— |
|
|
|
466,806 |
|
|
|
100,000 |
|
Development revenue |
|
|
2,007,617 |
|
|
|
2,989,614 |
|
|
|
3,361,177 |
|
|
|
5,796,182 |
|
|
|
$ |
2,030,524 |
|
|
$ |
3,323,727 |
|
|
$ |
3,442,783 |
|
|
$ |
6,298,422 |
|
(1) A portion of contract revenue represents previously recorded deferred revenue that was recognized as revenue after satisfaction of performance obligations either through passage of time or after completion of specific performance milestones.
Customer Concentration
A significant amount of our revenue is derived from contracts with major customers. For the three and six months ended June 30, 2023, revenue from two customers, each representing more than 10% of the total revenue, accounted for $1.8 million, or 88.5%, and $3.1 million, or 88.8%, of total revenues, respectively.
During the six months ended June 30, 2023, we entered into a joint development agreement (the "JDA") with a global battery maker under which we provide research and development services in exchange for total consideration of $1.5 million. The contractual term of the JDA is 12-months ("Performance Period"). Because our research and development work, which is a single performance obligation, will be provided evenly throughout the Performance Period, we recognize revenue from the JDA based on a straight-line basis, as a time-based method, in accordance with ASC 606, Revenue Recognition. During the three and six months ended June 30, 2023, we recognized $0.4 million from the JDA.
We currently derive a significant portion of our revenue from contract services with a G10 central bank. In 2021, we acquired a development contract for up to $41.5 million over a period of up to five years. In 2022, we were awarded a $4.3 million purchase order under this contract. These contract services incorporate both nano-optic and optical thin film technologies and are focused on developing authentication features for future banknotes.
For the three and six months ended June 30, 2022, revenue from one customer accounted for $3.2 million, or 97%, and $5.9 million, or 94%, respectively, of total revenues.
19. Leases
During the six months ended June 30, 2023 and 2022, we commenced the following leases:
Columbia Office Lease
In September 2022, we entered into an operating lease agreement for the space of approximately 11,642 square feet for an office in a building located in Columbia, Maryland, USA for 11 years from the commencement date of June 23, 2023, with an option to renew the lease for an additional 5 years (the "Columbia office lease"). There are step-up lease payments for each 12-month period from lease commencement, with the first 12 months fully abated. The agreement also provides the one-time option to terminate the lease effective as of the last date of the 73rd month of the lease term. We did not include the effects of exercising those options in the lease term to calculate lease liability because we concluded it is not reasonably certain that we will exercise the options. As of June 30, 2023, we recorded $2.2 million of lease liability and ROU assets associated with the Columbia office lease.
Burnaby Lease Expansion
On February 25, 2022, we entered into an agreement to amend our Burnaby lease, or the expansion, to expand the premises by an additional 1,994 square feet, commencing on June 1, 2022, for a period of two years and eleven months. The agreement provides the tenant with early access to the premises at least three months prior to the commencement date to conduct leasehold improvements.
25
We obtained access to the premises on March 25, 2022 and consequently recognized a right-of-use asset and liability for the expansion as of March 31, 2022, of $146,822.
Billerica Office Lease
On October 1, 2022, we entered into an operating lease agreement for the space of approximately 12,655 square feet on the 2nd Floor of a commercial building located in Billerica, Massachusetts, USA, in two separate sections: 8,097 Rentable Square Footage (the "Phase 1 lease") and 4,558 Rentable Square Footage (the "Phase 2 lease"). The lease term is five years and six months for the Phase 1 lease commencing from July 1, 2023, the delivery date of the Phase 1 lease, on which the space became ready for use (the "lease commencement date"), with an option to renew the lease for an additional five years. The lease term for the Phase 2 lease depends on the delivery of the Phase 2 lease, commencing on the delivery date of Phase 2 lease, on which the space will be made readily available, and ending in December 2028, with an option to renew the lease for an additional five years. Since the lease commencement date, the Phase 1 lease requires us monthly lease payments of approximately $0.1 million over the entire lease term, subject to 3% annual upward adjustment, and additional monthly lease payments of approximately $0.1 million over five years for lease improvements made by a landlord. The Phase 2 lease is anticipated to commence in fiscal 2025. Both leases were not recognized as an operating lease as of June 30, 2023, as we had not yet occupied the premises as of June 30, 2023. We expect to recognize $1.1 million of lease liability and ROU asset for the Phase 1 lease, as of the lease commencement date. See Note 21, Commitments and contingencies, for further information.
Total operating lease expense included in the condensed consolidated interim statements of operations and comprehensive loss is as follows:
|
|
Three months ended |
|
|
Six months ended |
|
||||||||||
|
|
June 30, |
|
|
June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Operating lease expense |
|
$ |
469,369 |
|
|
$ |
443,263 |
|
|
$ |
869,819 |
|
|
$ |
885,720 |
|
Short term lease expense |
|
|
148,523 |
|
|
|
77,603 |
|
|
|
228,086 |
|
|
|
159,240 |
|
Variable and other lease expense |
|
|
135,630 |
|
|
|
55,525 |
|
|
|
225,759 |
|
|
|
114,342 |
|
Total |
|
$ |
753,522 |
|
|
$ |
576,391 |
|
|
$ |
1,323,664 |
|
|
$ |
1,159,302 |
|
We completed our evaluation of the provisions of ASC 842, Leases, and elected the practical expedient to not capitalize any leases with initial terms of less than twelve months on our balance sheet and include them as short-term lease expense in the condensed consolidated interim statements of operations and comprehensive loss.
Future minimum lease payments under non-cancelable operating lease obligations were as follows as of June 30, 2023:
Remainder of 2023 |
|
$ |
633,875 |
|
2024 |
|
|
1,468,819 |
|
2025 |
|
|
1,493,735 |
|
2026 |
|
|
1,351,197 |
|
2027 |
|
|
932,629 |
|
2028 |
|
|
940,836 |
|
Thereafter |
|
|
3,819,652 |
|
Total minimum lease payments |
|
|
10,640,743 |
|
Less: interest |
|
|
(4,272,515 |
) |
Present value of net minimum lease payments |
|
|
6,368,228 |
|
Less: current portion of lease liabilities |
|
|
(1,059,105 |
) |
Total long-term lease liabilities |
|
$ |
5,309,123 |
|
26
The table above does not include the future minimum lease payments for the Billerica office lease that haven't been commenced as of June 30, 2023. The total minimum lease payments for the Phase 1 lease of Billerica office lease are $1.3 million in the next 5.5 years. See Note 21 for further information.
20. Realignment and consolidation plan
On June 6, 2023, our board of directors approved the Realignment and Consolidation Plan for the remainder of 2023 pursuant to which we have begun, but not yet completed, the process of realigning our corporate structure aimed at reducing our operating expenses and focusing on key applications which represent the greatest near-term revenue potential. This strategic initiative is designed to adapt to changing market conditions, enhance efficiency, and reduce our cash burn rate.
We accrue costs in connection with ongoing restructuring actions. These accruals include estimates primarily related to employee headcount, local statutory benefits, and other employee termination costs. We calculate severance obligations based on standard customary practices. Accordingly, as of June 30, 2023, we recorded $0.9 million provisions for severance when probable and estimable since we committed to the Realignment and Consolidation Plan. These accruals are reviewed on a quarterly basis and changes to restructuring actions are appropriately recognized when identified.
Total estimated realignment and consolidation charges are expected to be $2.6 million, which includes employee termination costs and severance benefits. Of which $1.5 million of employee termination costs and severance benefits had been incurred as of June 30, 2023. The Realignment and Consolidation Plan is expected to be completed by the end of 2023. Cash payments in the three months ended June 30, 2023 were $0.5 million. The costs related to restructuring activities have been recorded in the restructuring expense on the condensed consolidated interim statement of operations and comprehensive loss.
21. Commitments and contingencies
Legal Matters
SEC Subpoena:
In September 2021, we received a subpoena from the Securities and Exchange Commission, Division of Enforcement, in a matter captioned In the Matter of Torchlight Energy Resources, Inc., or Torchlight. The subpoena requested that we produce certain documents and information related to, among other things, the merger involving Torchlight and Metamaterial Inc.
On July 20, 2023, the enforcement staff of the SEC provided us, our former Chief Executive Officer, John Brda, and our Chief Executive Officer, George Palikaras, with “Wells Notices.” See Note 22, Subsequent events, for details. We can offer no assurances as to the outcome of this investigation or its potential effect, if any, on us or our results of operation.
Securities Class Action:
On January 3, 2022, a putative securities class action lawsuit was filed in the U.S. District Court for the Eastern District of New York captioned Maltagliati v. Meta Materials Inc., et al., No. 1:21-cv-07203, against us, our Chief Executive Officer, our Chief Financial Officer, Torchlight’s former Chairman of the Board of Directors, and Torchlight’s former Chief Executive Officer. On January 26, 2022, a similar putative securities class action lawsuit was filed in the U.S. District Court for the Eastern District of New York captioned McMillan v. Meta Materials Inc., et al., No. 1:22-cv-00463. The McMillan complaint names the same defendants and asserts the same claims on behalf of the same purported class as the Maltagliati complaint. The complaints, purportedly brought on behalf of all purchasers of our publicly traded securities from September 21, 2020 through and including December 14, 2021, assert claims under Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, or the Exchange Act, arising primarily from a short-seller report and statements related to our business combination with Torchlight. The complaints seek unspecified compensatory damages and reasonable costs and expenses, including attorneys’ fees. On July 15, 2022, the Court consolidated these actions under the caption In re Meta Materials Inc. Securities Litigation, No. 1:21-cv-07203, appointed lead plaintiffs and approved the lead plaintiffs’ selection of lead counsel. Lead plaintiffs filed a consolidated complaint on August 29, 2022. We moved to dismiss that complaint on October 13, 2022. The motion was fully briefed on January 12, 2023. The Court held a hearing on the motion to dismiss on February 27, 2023 and took the motion under submission.
Shareholder Derivative Action:
On January 14, 2022, a shareholder derivative action was filed in the U.S. District Court for the Eastern District of New York captioned Hines v. Palikaras, et al., No. 1:22-cv-00248. The complaint names as defendants certain of our current officers and directors, certain former Torchlight officers and directors, and us (as nominal defendant). The complaint, purportedly brought on our behalf, asserts claims under Section 14(a) of the Exchange Act, contribution claims under Sections 10(b) and 21D of the Exchange Act, and various state law claims such as breach of fiduciary duties and unjust enrichment.
27
The complaint seeks, among other things, unspecified compensatory damages in our favor, certain corporate governance related actions, and an award of costs and expenses to the derivative plaintiff, including attorneys’ fees. On March 9, 2022, the Court entered a stipulated order staying this action until there is a ruling on a motion to dismiss in the securities class action.
Westpark Capital Group:
On July 25, 2022, WestPark Capital Group, LLC filed a complaint in Los Angeles County Superior Court against us for breach of contract, alleging that it is owed a $450,000 commission as a placement agent with respect to our June 2022 direct offering. On August 31, 2022, we filed an answer to the complaint. We dispute that WestPark Capital Group placed the investor in the direct offering and is owed a commission.
Contractual Commitments and Purchase Obligations
28
As of June 30, 2023, we had ongoing commitments for maintenance contracts and asset purchases as follows:
|
Long-term debt |
|
Lease signed |
|
Commitment in relation to Realignment and Consolidation Plan |
|
Other contractual commitment |
|
Total |
|
|||||
Remainder of 2023 |
$ |
576,915 |
|
$ |
117,255 |
|
$ |
942,770 |
|
$ |
1,063,700 |
|
$ |
2,700,640 |
|
2024 |
|
629,177 |
|
|
236,534 |
|
|
— |
|
|
382,593 |
|
|
1,248,305 |
|
2025 |
|
1,087,962 |
|
|
240,583 |
|
|
— |
|
|
— |
|
|
1,328,544 |
|
2026 |
|
1,324,910 |
|
|
244,631 |
|
|
— |
|
|
— |
|
|
1,569,541 |
|
2027 |
|
560,126 |
|
|
248,680 |
|
|
— |
|
|
— |
|
|
808,806 |
|
2028 |
|
555,136 |
|
|
206,322 |
|
|
— |
|
|
— |
|
|
761,458 |
|
Thereafter |
|
661,820 |
|
|
— |
|
|
— |
|
|
— |
|
|
661,820 |
|
|
$ |
5,396,046 |
|
$ |
1,294,005 |
|
$ |
942,770 |
|
$ |
1,446,293 |
|
$ |
9,079,114 |
|
|
|
|
|
|
|
|
|
|
|
|
|||||
22. Subsequent events
Sales Under the ATM Equity Offering Program
Subsequent to June 30, 2023, we sold a total of 3,756,991 shares of our common stock under the ATM at a weighted average price of $0.19 per share, generating gross proceeds of $0.7 million.
SEC Investigation
On July 20, 2023, the enforcement staff of the SEC provided us, our former Chief Executive Officer, John Brda, and our Chief Executive Officer, George Palikaras, with Wells Notices relating to the Investigation. The Wells Notices each state that the SEC staff has made a preliminary determination to recommend that the SEC file a civil enforcement action against the recipients alleging violations of certain provisions of the U.S. federal securities laws. Specifically, the Wells Notice received by us states that the proposed action would allege violations of Section 17(a) of the Securities Act; Sections 10(b), 13(a), 13(b)(2)(A), 13(b)(2)(B) and 14(a) of the Exchange Act of 1934 and Rules 10b-5 and 14a-9 thereunder; and Regulation FD.
A Wells Notice is neither a formal charge of wrongdoing nor a final determination that the recipient has violated any law. It allows the recipients the opportunity to address the issues raised by the enforcement staff before a decision is made by the SEC on whether to authorize any enforcement action. If the SEC were to authorize an action against us and/or any of the individuals, it could seek an injunction against future violations of provisions of the federal securities laws, the imposition of civil monetary penalties, and other equitable relief within the SEC’s authority. The SEC could also seek an order barring the individuals from serving as an officer or director of a public company. In addition, the SEC could seek disgorgement of an amount that may exceed our ability to pay.
The results of the Investigation and the Wells Notice process and any corresponding enforcement action against us or any of the individuals discussed above, and the costs, timing, and other potential consequences of responding and complying therewith, including any indemnification obligations of us, are unknown at this time.
Our Board of Directors is reviewing the Wells Notices but has not yet determined its next course of action regarding the Wells Notices. No financial impact of a loss contingency has been recognized in the condensed consolidated interim financial statements, as this matter does not meet the required criteria of being both probable and reasonably estimated at this time.
Sale of Next Bridge Notes Receivable
On August 7, 2023, we entered into a Loan Sale Agreement with Gregory McCabe, or Purchaser, under which we sold and assigned to Purchaser all of our rights, title, interests, and obligations in and to those certain loans due from Next Bridge described in Note 6, Notes receivable, in exchange for cash consideration of $6.0 million and agreeing to enter into an SPA for an additional $6.0 million of shares of our common stock. Assigned Loan Interests represented $24.0 million of the outstanding balance of the Next Bridge notes receivable as of the Closing Date.
Immediately after the Closing Date and by no later than August 28, 2023, we will negotiate with Purchaser and execute the SPA, escrow agreement and any other required transaction documents pursuant to which: (A) upon a monthly put by us, Purchaser will agree to purchase an aggregate of $6.0 million of shares of our common stock (beginning on September 1, 2023, or as soon as possible after such date if necessary under applicable law, in monthly amounts of $250,000 for the first six months and then in monthly amounts of $500,000 for the next nine months thereafter), at a per share purchase price equal to 120% of the 5-day VWAP of our common stock on the trading day preceding the date of each such purchase, (B) an escrow agent would hold all of the shares of our common stock that Purchaser has purchased after the Closing Date with any portion of this $6.0 million until the last payment is made, and (C) in the event of a default under such stock purchase agreement, Purchaser will forfeit all of the shares of our common stock described in clause (B) of this paragraph and held by such escrow agent and return such shares to us.
29
In addition, until Purchaser has purchased $6.0 million of shares of our common stock, Purchaser shall have a call option to purchase the then-remaining balance of the $6.0 million of shares of our common stock at a per share purchase price equal to 120% of the 5-day VWAP on the trading day preceding the date of Purchaser’s exercise of such call option (subject to compliance with applicable law and subject to compliance with all Nasdaq rules, including the “20% rule”).
30
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations should be read in conjunction with our condensed consolidated interim financial statements and related notes thereto included in Part I, Item I of this Quarterly Report on Form 10-Q. For additional information regarding our financial condition and results of operations, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Part II, Item 7 in the Amendment No. 1 to our Annual Report on Form 10-K/A for the year ended December 31, 2022, filed with the SEC on March 24, 2023, or the Form 10-K/A, as well as our consolidated financial statements and related notes thereto included in Part II, Item 8 of the Form 10-K/A. As discussed in the section titled “Forward-Looking Statements,” the following discussion contains forward-looking statements that involve risks and uncertainties. Factors that could cause or contribute to such differences include those identified below and those discussed in the "Part II, Item 1A (Risk Factors)" and other parts of this Quarterly Report on Form 10-Q and in the Form 10-K/A. Our historical results are not necessarily indicative of the results that may be expected for any period in the future.
OVERVIEW
Meta Materials Inc. (also referred to herein as the “Company”, “META®”, “META” “we”, “us”, or “our”) is a leader in advanced materials and nanotechnology. We are developing materials that we believe can improve the performance and efficiency of many current products as well as allow new products to be developed that cannot otherwise be developed without such materials. We believe META is positioned for growth, by pioneering a new category of intelligent surfaces, which will allow us to become the metamaterials industry leader. We enable our potential customers across a range of industries - consumer electronics, 5G communications, healthcare, aerospace, automotive, and clean energy - to deliver improved products to their customers. Our principal executive office is located at 60 Highfield Park Drive, Dartmouth, Nova Scotia, Canada.
During the second quarter ended June 30, 2023, we have begun to implement a plan to aimed at reducing our operating expenses and focusing on key applications which represent the greatest near-term revenue potential. We are focusing on NCORE™ and NPORE® which provides a solution for safer, lighter, and more sustainable batteries, expansion of our production capacity in our banknote and brand security lines, preparation for commercial launch of KolourOptik®Stripe technology and development of our multiple-gigapixel, wide area motion imagery system. For more information regarding our business, see Part I, Item I (Business), of Form 10-K/A filed on March 24, 2023.
Over the second quarter ended June 30, 2023, our net revenues increased compared to the first quarter ended March 31, 2023. Our net development revenue in the second quarter ended June 30, 2023 has increased compared to the first quarter ended March 31, 2023 mainly due to $0.4 million revenue from a new joint development agreement related to battery materials and a $0.2 million increase from contract services with a G10 central bank.
Over the past two quarters, our operating expenses have decreased. Our selling and marketing expenses decreased due to a decrease in salaries and benefits and accrued bonuses. Our general and administrative expenses decreased mainly due to a decrease in salaries and benefits, accrued bonuses and consulting fees. During the quarter ended June 30, 2023, our research and development expenses increased as compared to the quarter ended March 31, 2023, primarily resulting from an increase in research and development materials and subcontracting and consulting services, partially offset by a decrease in salaries and benefits and accrued bonuses.
Recent Developments
SEC Investigation
On July 20, 2023, the enforcement staff of the SEC provided us, our former Chief Executive Officer, John Brda, and our Chief Executive Officer, George Palikaras, with Wells Notices relating to the Investigation. The Wells Notices each state that the SEC staff has made a preliminary determination to recommend that the SEC file a civil enforcement action against the recipients alleging violations of certain provisions of the U.S. federal securities laws. Specifically, the Wells Notice received by us states that the proposed action would allege violations of Section 17(a) of the Securities Act; Sections 10(b), 13(a), 13(b)(2)(A), 13(b)(2)(B) and 14(a) of the Exchange Act of 1934 and Rules 10b-5 and 14a-9 thereunder; and Regulation FD.
A Wells Notice is neither a formal charge of wrongdoing nor a final determination that the recipient has violated any law. It allows the recipients the opportunity to address the issues raised by the enforcement staff before a decision is made by the SEC on whether to authorize any enforcement action. If the SEC were to authorize an action against us and/or any of the individuals, it could seek an injunction against future violations of provisions of the federal securities laws, the imposition of civil monetary penalties, and other equitable relief within the SEC’s authority. The SEC could also seek an order barring the individuals from serving as an officer or director of a public company. In addition, the SEC could seek disgorgement of an amount that may exceed our ability to pay.
31
The results of the Investigation and the Wells Notice process and any corresponding enforcement action against us or any of the individuals discussed above, and the costs, timing, and other potential consequences of responding and complying therewith, including any indemnification obligations of us, are unknown at this time.
Our Board of Directors is reviewing the Wells Notices but has not yet determined its next course of action regarding the Wells Notices. No financial impact of a loss contingency has been recognized in the condensed consolidated interim financial statements, as this matter does not meet the required criteria of being both probable and reasonably estimated at this time.
Realignment and Consolidation Plan
On June 6, 2023, our board of directors approved the Realignment and Consolidation Plan for the remainder of 2023 pursuant to which we have begun, but not yet completed, the process of realigning our corporate structure aimed at reducing our operating expenses and focusing on key applications which represent the greatest near-term revenue potential. This strategic initiative is designed to adapt to changing market conditions, enhance efficiency, and reduce our cash burn rate. As part of this plan, As part of this plan, we are exploring alternatives for certain of our technologies including Holography Technology and Wireless Sensing and Radio Wave Imaging Technology. These alternatives may include a divestiture, entering into a joint venture and/or curtailing our investment in these technologies.
The primary objectives of the Realignment and Consolidation Plan are as follows:
In order to accomplish the above objectives, we have begun to undertake the following key measures as part of the Realignment and Consolidation Plan:
Total estimated realignment and consolidation charges are expected to be $2.6 million, including employee termination costs and severance benefits, of which, $1.5 million of employee termination costs and severance benefits had been incurred as of June 30, 2023. Our average monthly cash burn rate before we started to implement the Realignment and Consolidation Plan was $5.7 million, which is expected to be reduced to between $2.6 million and $3.8 million, representing a reduction of approximately 54% to 33% respectively, by the end of the year ending December 31, 2023. We are currently engaged in a thorough and comprehensive assessment process to identify the best possible utilization of the assets related to Holography Technology and Wireless Sensing and Radio Wave Imaging Technology. As a result of the assessment, we may recognize additional impairment loss of other long-term assets. The implementation of the Realignment and Consolidation Plan will continue through the remainder of the fiscal year ending December 31, 2023.
Underwritten Public Offering
On April 14, 2023, we entered into the Underwriting Agreement, relating to our public offering of (i) 83,333,334 shares of our common stock, par value $0.001 and (ii) warrants to purchase up to an aggregate of 83,333,334 shares of our common stock. The shares of common stock and warrants were sold together as a fixed combination, consisting of one share of common stock and a warrant to purchase one share of common stock, but are immediately separable and will be issued separately in the offering. Each warrant is exercisable to purchase one share of common stock at a price of $0.375 per share, which was subsequently reduced to $0.174 based on the down round provisions in the case of a Share Combination Event or a Dilutive Issuance as described in Note 12, Capital stock, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q, and expires five years from the date of issuance.
32
The combined price to the public in the offering for each share of common stock and accompanying warrant was $0.30. After deducting underwriting discounts and commissions and estimated offering expenses payable by us, the net proceeds were $22.1 million. See Note 12, Capital stock, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q for further information.
Appointment of Chief Financial Officer and Chief Operating Officer; Appointment of Two New Directors
On April 19, 2023, Maurice Guitton notified our Board of his intention to resign and retire effective immediately. Mr. Guitton has indicated that his departure from the Board was not the result of any disagreement with management or the Board but was in order to pursue his retirement more fully.
Also on April 19, 2023, after taking into account Mr. Guitton’s retirement, the Board increased its size from seven to eight members and appointed Vyomesh Joshi and Eugenia Corrales to the Board. Ms. Corrales was also appointed to serve on the audit committee of the Board and Mr. Joshi was appointed to serve on the governance and nominating committee of the Board.
Mr. Joshi and Ms. Corrales each have participated in our Outside Director Compensation Plan, including an initial grant of Restricted Stock Units valued at $100,000. Each of Mr. Joshi or Ms. Corrales have entered into our standard form of indemnification agreement for directors and executive officers.
On April 20, 2023, Kenneth Rice was terminated as our Chief Financial Officer and Chief Operating Officer and decided to retire and on April 21, 2023, Jonathan Waldern was terminated as our Chief Technology Officer. In connection with their departures, each of Mr. Rice and Mr. Waldern received the severance payment and benefits provided under their respective employment agreements with us for a termination without cause, subject to his execution of a release and discharge agreement and compliance with post-termination restrictive covenants.
Also on April 20, 2023, Uzi Sasson was appointed as our Chief Financial Officer and Chief Operating Officer. Mr. Sasson assumed the duties of the principal financial officer and principal accounting officer as of his date of hire.
On April 20, 2023, we entered into an executive employment agreement with Mr. Sasson, or the Sasson Employment Agreement, pursuant to which we agreed to employ Mr. Sasson as our Chief Financial Officer and Chief Operating Officer, effective as of the Effective Date, for an indefinite term in consideration of an initial annual base salary of $375,000. Mr. Sasson is eligible to receive an annual bonus with a target of 65% of his base salary as determined by the Board in its sole discretion, based on achievement of Company performance and Mr. Sasson’s individual performance goals. This annual bonus will be prorated for 2023. In addition, in connection with the execution of the Employment Agreement with us, Mr. Sasson has received a stock option to purchase 300,000 shares of our common stock which will vest 25% annually over four years, subject to Mr. Sasson’s continued service with us through each vesting date. Mr. Sasson has received a long-term incentive equity grant with an aggregate grant date value of $500,000 (an “LTIP Grant”), computed using the Black Scholes valuation model, subject to Mr. Sasson’s continued employment through the grant date. The LTIP Grant will consist of 50% in stock options and 50% in restricted stock units and is expected to vest over a four-year period, with 25% of such options and restricted stock units vesting annually over the next four years after the date of grant. In the event that Mr. Sasson’s employment with us is terminated, he will be eligible for severance benefits in accordance with our established policies, if any, then in effect.
In accordance with our customary practice, we have also entered into our standard form of indemnification agreement with Mr. Sasson.
Appointment of Chief Legal Officer
On July 12, 2023, we entered into an executive employment agreement with Mr. D. Daniel (Dan) Eaton, as our Chief Legal Officer.
Mr. Eaton joined us as our Chief Legal Officer with a strong background in the technology industry. With previous General Counsel roles at Katena Computing Technologies, Inc., Netronome Systems, Inc., and Zilog Inc., Mr. Eaton brings a wealth of expertise in legal affairs and corporate governance. Beyond his legal expertise, Mr. Eaton has a successful track record in managing several 8-figure acquisitions, including significant transactions with Zilog, Inc. and IXYS Corporation, showcasing his strategic vision and ability to drive business growth through M&A activities. Mr. Eaton is licensed to practice law in the State of California and the United States Patent and Trademark Office and is well-versed in intellectual property rights and protection strategies. He also holds 12 issued patents on a variety of technical innovations. Mr. Eaton's education encompasses both legal and technical knowledge, having received a Juris Doctor from Santa Clara University and a Bachelor of Science degree from New Mexico State University.
33
Next Bridge Notes Receivable
As of June 30, 2023, we recorded credit losses of $1.7 million based on the Company's election to estimate credit loss based on the fair value of the collateral for the Next Bridge notes receivable in accordance with ASC 326.
Subsequent to June 30, 2023, we entered into a Loan Sale Agreement with Gregory McCabe, Purchaser, under which we sold and assigned to Purchaser all of our rights, title, interests, and obligations in and to those certain loans due from Next Bridge described in Note 6, Notes receivable, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q, in exchange for cash consideration of $6.0 million and agreeing to enter into an SPA for an additional $6.0 million of shares of our common stock. Pursuant to the SPA, the Purchaser will be required to purchase an aggregate of $6.0 million of shares of our common stock (beginning on September 1, 2023, or as soon as possible after such date if necessary under applicable law, in monthly amounts of $250,000 for the first six months and then in monthly amounts of $500,000 for the next nine months thereafter), at a per share purchase price equal to 120% of the 5-day VWAP of our common stock on the trading day preceding the date of each such purchase.
See Note 22, Subsequent events, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q for further information.
Known Trends and Uncertainties:
Impact from the Realignment and Consolidation Plan
With the implementation of the Realignment and Consolidation Plan, we anticipate achieving substantial cost savings and a lower cash burn rate that will positively support our sustainable growth. However, we expect to record a total of $2.6 million of one-time realignment and consolidation charges during fiscal 2023, which will reduce those benefits from the implementation of the Realignment and Consolidation Plan.
Expanding Operations and Facilities of Core Business
We are expanding capacity and facilities to support a growing range of market opportunities for our core business. This includes new development facilities in Maryland for Electro Optics and IR and in Massachusetts for the Battery Materials team. These activities require capital investments and increased overhead for leased facilities. Further, R&D expense related to our core business is expected to increase as a result of expanding operations of our core business. The timing of new customer programs and revenues associated with these expansions is uncertain and we may require additional financing to support the related cash consumption.
Inflation
A prolonged period of inflation in Europe, particularly for energy costs, could cause shortages or material cost increases for certain key raw materials, for which we depend on European suppliers. Specifically, shortages or cost increases in certain polymers used in our Holography products could result in higher costs for these products that may not be able to be passed on to our customers.
Inflation in North America, for labor costs and transportation costs in particular, could elevate our costs of hiring new team members and cause increases in our labor costs for existing team members. In addition, rising transportation costs are likely to increase our costs for shipping our products and the costs associated with our material purchases.
Vehicle Electrification
The transition from internal combustion engine, or ICE, vehicles to electric vehicles, or EVs, may be accelerated by recent disruptions in global oil supply, reduced investment in new domestic oil exploration, and increased government support for domestic EV production, battery and battery materials supply chain, and EV charging infrastructure. This may increase the opportunity for META to scale up battery materials production, acquire new battery component customers, and obtain government funding for capital projects. It could also accelerate demand for certain of our NANOWEB® products targeting EVs.
Expanding Focus and Emphasis on Information Technology
With the rapid growth of our global business, our data protection and cyber security needs have become a significant element of our business. Failure on our part to invest in the tools, equipment and personnel required to adequately manage these elements could result in regulatory issues, claims by customers and potential financial liabilities. Further, customer prospects identifying such failures could decide to delay or abandon orders from us.
34
NANOWEB® Capacity
Our NANOWEB® products have not yet reached the required manufacturing scale to enable us to address the volume demands of a number of our target vertical markets. We must either design, develop and procure additional internal capacity to produce NANOWEB® in higher volume and larger formats or identify outsourced suppliers capable of producing our designs. Internal capacity expansion may require higher capital expenditures and faces the risk of supply chain delays. Outsourced production may increase variable costs and put pressure on gross margins as we scale volumes. We are in negotiations for a strategic partnership to achieve 60cm capacity with a leading consumer electronics manufacturer.
Foreign Currency Fluctuation Risk
As we continue to expand our business globally, we presently have currency exposure arising from both sales and purchases denominated in foreign currencies. Fluctuations in the value of currencies, such as Canadian Dollar, British Pound and Euro against the US Dollar could adversely impact our revenue and operating and labor costs. In addition, items included in the financial statements of each of META and its subsidiaries are measured using the currency of the primary economic environment in which the entity operates (the “functional currency”) while our reporting currency is in US Dollar. As such, our financial results and positions are exposed to changes in exchange rates between the US Dollar and Canadian Dollar, the British Pound, and Euro. For the six months ended June 30, 2023, approximately 86.2% of our consolidated revenue and 15.8% of operating expenses were recorded in our entities whose functional currency is other than US Dollar and approximately 87.3% of Cash and Cash equivalents, 87.4% of Property and Equipment, and 49.4% of Accounts Payable and Accruals were recorded in such entities whose functional currency is other than US Dollar as of June 30, 2023.
Basis of Presentation
The following discussion highlights our results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described and provides information that management believes is relevant for an assessment and understanding of the condensed consolidated interim balance sheet and statements of operation and comprehensive loss presented herein. The following discussion and analysis are based on our condensed consolidated interim financial statements contained in this Quarterly Report, which we have prepared in accordance with U.S. GAAP. You should read the discussion and analysis together with such condensed consolidated interim financial statements and the related notes thereto.
Critical Accounting Estimates
Our condensed consolidated interim financial statements and the related notes thereto are prepared in accordance with U.S. GAAP. The preparation of condensed consolidated interim financial statements also requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs and expenses and related disclosures. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances. Actual results could differ significantly from our estimates. To the extent that there are differences between our estimates and actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected.
There have been no material changes to our critical accounting estimates as described in our Annual Report on Form 10-K/A for the year ended December 31, 2022.
Recent Accounting Pronouncements
For a description of recent accounting pronouncements, including the expected dates of adoption and estimated effects, if any, on our condensed consolidated interim financial statements, please see Note 2, Significant accounting policies, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q.
RESULT OF OPERATIONS
Revenue and Gross Profit:
Our revenue is generated from product sales as well as development revenue. We recognize revenue when we satisfy performance obligations under the terms of our contracts, and control of our products is transferred to our customers in an amount that reflects the consideration we expect to receive from our customers in exchange for those products or services.
35
Product Sales
Product sales include products, components, and samples sold to our customers. Revenue from the sale of prototypes and finished products is recognized at the point in time when control of the asset is transferred to the customer, generally on delivery of goods. We consider whether there are other obligations in the contract that are separate performance obligations to which a portion of the transaction price needs to be allocated. In determining the transaction price for the sale of prototypes, we consider the effects of variable consideration, the existence of significant financial components, non-cash consideration and consideration payable to the customer (if any).
Development Revenue
Development Revenue consists of revenues from contract services and research services, including non-recurring engineering services. Revenue from development activities is recognized over time, using an input method to measure progress towards complete satisfaction of the research activities and associated performance obligations identified within each contract have been satisfied.
Cost of Sales
Cost of Sales consists of direct material used in production, depreciation expenses of machinery and equipment used in production, salaries and benefits relating to the production staff, and other overheads allocated to production.
|
|
Three months ended June 30, |
|
Six months ended June 30, |
||||||||||||
|
|
2023 |
|
2022 |
|
Change |
|
2023 |
|
2022 |
|
Change |
||||
Product sales |
|
$22,907 |
|
$334,113 |
|
$(311,206) |
|
-93% |
|
$81,606 |
|
$502,240 |
|
$(420,634) |
|
-84% |
Development revenue |
|
2,007,617 |
|
2,989,614 |
|
(981,997) |
|
-33% |
|
3,361,177 |
|
5,796,182 |
|
(2,435,005) |
|
-42% |
Total revenue |
|
2,030,524 |
|
3,323,727 |
|
(1,293,203) |
|
-39% |
|
3,442,783 |
|
6,298,422 |
|
(2,855,639) |
|
-45% |
Cost of sales (exclusive of items shown separately below) |
|
758,618 |
|
701,283 |
|
57,335 |
|
8% |
|
1,345,646 |
|
1,407,533 |
|
(61,887) |
|
-4% |
Depreciation and amortization included in cost of sales |
|
17,296 |
|
18,850 |
|
(1,554) |
|
-8% |
|
27,343 |
|
40,659 |
|
(13,316) |
|
-33% |
Stock-based compensation included in cost of sales |
|
(2,936) |
|
157,818 |
|
(160,754) |
|
-102% |
|
140,969 |
|
208,471 |
|
(67,502) |
|
-32% |
Gross profit |
|
$1,257,546 |
|
$2,445,776 |
|
$(1,188,230) |
|
-49% |
|
$1,928,825 |
|
$4,641,759 |
|
$(2,712,934) |
|
-58% |
Gross profit percentage |
|
62% |
|
74% |
|
92% |
|
|
|
56% |
|
74% |
|
95% |
|
|
Comparison of the Three and Six Months Ended June 30, 2023 to the Three and Six Months Ended June 30, 2022:
Product Sales
The decrease in product sales for the three and six months ended June 30, 2023 of $0.3 million and $0.4 million, respectively, as compared to the same periods of 2022, is primarily due to a reduction in sales orders.
Development Revenue
The decrease in development revenue for the three and six months ended June 30, 2023 of $1.0 million and $2.4 million is mainly due to the decrease in our lithography revenue - banknotes applications due to larger contracts in 2022, partially offset by $0.4 million of revenue recognized from the joint development agreement with a global battery maker during the three months ended June 30, 2023. We derive a significant portion of our revenue from contract services with a confidential G10 central bank. In 2021, we acquired Nanotech which had a development contract for up to $41.5 million over a period of up to five years. These contract services incorporate both nano-optic and optical thin film technologies and are focused on developing authentication features for future banknotes.
Cost of Sales
The increase in cost of sales for the three months ended June 30, 2023 as compared to the three months ended June 30, 2022 was due to increased production cost, which is driven by more labor intensive tasks required for the current contracts compared to the contracts in the prior year, partially offset by lower material cost. The decrease in cost of sales for the six months ended June 30, 2023 as compared to the same period of 2022 was mainly due to a decrease in material consumption, partially offset by increased production cost.
36
Operating Expenses
|
|
Three months ended June 30, |
|
|
Six months ended June 30, |
|
||||||||||||||||||||||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
2023 |
|
|
2022 |
|
|
Change |
|
||||||||||||||
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Selling & marketing |
|
$ |
780,233 |
|
|
$ |
951,750 |
|
|
$ |
(171,517 |
) |
|
|
-18 |
% |
|
$ |
3,087,586 |
|
|
$ |
1,967,850 |
|
|
$ |
1,119,736 |
|
|
|
57 |
% |
General & administrative |
|
|
5,913,267 |
|
|
|
11,300,296 |
|
|
|
(5,387,029 |
) |
|
|
-48 |
% |
|
|
12,682,706 |
|
|
|
21,202,836 |
|
|
|
(8,520,130 |
) |
|
|
-40 |
% |
Research & development |
|
|
5,059,614 |
|
|
|
4,118,781 |
|
|
|
940,833 |
|
|
|
23 |
% |
|
|
10,120,370 |
|
|
|
7,208,206 |
|
|
|
2,912,164 |
|
|
|
40 |
% |
Depreciation & amortization expense |
|
|
3,358,527 |
|
|
|
1,801,319 |
|
|
|
1,557,208 |
|
|
|
86 |
% |
|
|
6,593,022 |
|
|
|
3,453,504 |
|
|
|
3,139,518 |
|
|
|
91 |
% |
Stock-based compensation expense |
|
|
(2,807,086 |
) |
|
|
3,915,612 |
|
|
|
(6,722,698 |
) |
|
|
-172 |
% |
|
|
(948,647 |
) |
|
|
7,860,400 |
|
|
|
(8,809,047 |
) |
|
|
-112 |
% |
Restructuring expense |
|
|
1,469,149 |
|
|
|
— |
|
|
|
1,469,149 |
|
|
|
100 |
% |
|
|
1,469,149 |
|
|
|
— |
|
|
|
1,469,149 |
|
|
|
100 |
% |
Goodwill impairment |
|
|
282,173,053 |
|
|
|
— |
|
|
|
282,173,053 |
|
|
|
100 |
% |
|
|
282,173,053 |
|
|
|
— |
|
|
|
282,173,053 |
|
|
|
100 |
% |
Total operating expenses |
|
$ |
295,946,757 |
|
|
$ |
22,087,758 |
|
|
$ |
273,858,999 |
|
|
|
1240 |
% |
|
$ |
315,177,239 |
|
|
$ |
41,692,796 |
|
|
$ |
273,484,443 |
|
|
|
656 |
% |
Selling & marketing
The decrease in selling & marketing expenses of $0.2 million for the three months ended June 30, 2023, as compared to the same period of 2022, is mainly due to a $0.6 million decrease in bonus accrual, partially offset by a $0.2 million increase in salaries and benefits and a $0.2 million increase in consulting fees.
The increase in selling & marketing expenses of $1.1 million for the six months ended June 30, 2023, as compared to the same period of 2022, is mainly due to a $0.9 million increase in salaries and benefits and a $0.7 million increase in consulting fees, partially offset by a $0.4 million reduction in bonus accrual.
The increased salary and benefit expenses in the three and six months ended June 30, 2023 as compared to the same periods of 2022 resulted from increases in headcount in the latter part of 2022 to acquire talent and grow the sales and marketing team.
General & administrative
The decrease in general & administrative expenses of $5.4 million for the three months ended June 30, 2023, as compared to the same period of 2022, is primarily due to a $4.4 million decrease in professional fees as a result of the reduced legal cost associated with the SEC investigation, the related lawsuits and acquisition costs and a $1.6 million decrease in bonus accrual and $0.4 million decrease in travel and other expenses, partially offset by a $0.5 million increase in insurance costs due to expanded insured assets, $0.3 million increase in rent and utilities, $0.1 million increase in stock exchange fee and $0.1 million increase in director fees.
The decrease in general & administrative expenses of $8.5 million for the six months ended June 30, 2023, as compared to the same period of 2022, primarily resulted from a $8.5 million decrease in professional fees mainly due to the reduced legal cost associated with the SEC investigation, the related lawsuits and acquisition costs and a $1.3 million decrease in bonus accrual, partially offset by a $0.5 million increase in insurance cost due to expanded insured assets, a $0.4 million increase in salaries and benefits as a result of increased headcount, a $0.1 million increase in stock exchange fee, a $0.1 million increase in IT & software related costs and a $0.1 million increase in director fees.
Research & development
The increase in research & development expenses of $0.9 million for the three months ended June 30, 2023, as compared to the same period of 2022, is primarily due to a $0.9 million increase in R&D materials, a $0.4 million increase in intellectual property related cost, a $0.4 million increase in salaries and benefits due to increased headcount through all locations as a result of 1) expansion in facilities and laboratories, and 2) the acquisitions of PAL and Optodot in Q2 2022, $0.2 million increase in outsourcing costs and a $0.1 million increase in travel and other expenses, partially offset by a $1.0 million reduction in bonus accrual.
The increase in research & development expenses of $2.9 million for the six months ended June 30, 2023, as compared to the same period of 2022, is primarily due to a $1.6 million increase in salaries and benefits due to increased headcount through all locations as a result of 1) expansion in facilities and laboratories, and 2) the acquisitions of PAL and Optodot in Q2 2022, $0.9 million increase in R&D materials, a $0.7 million increase in intellectual property related cost, a $0.2 million increase in outsourcing costs and a $0.2 million increase in rent and utilities, partially offset by a $0.7 million reduction in bonus accrual.
37
Depreciation & amortization expense in Operating expenses
For the three and six months ended June 30, 2023 as compared to the same periods of 2022, depreciation and amortization expense increased by $1.6 million and $3.1 million, respectively. The increase was mainly due to intangible assets acquired in Q2 2022 as part of the PAL and Optodot acquisitions as well as an increase in depreciation expense due to acquired equipment in different facilities.
Stock-based compensation expense in Operating expenses
For the three and six months ended June 30, 2023 as compared to the same periods of 2022, stock-based compensation expenses decreased by $6.7 million and $8.8 million, respectively. The decreases were mainly due to less equity-based awards granted during the three and six months ended June 30, 2023 compared to the same periods ended June 30, 2022 and more forfeitures of equity-based awards due to terminations in the three months ended June 30, 2023 compared to the same period ended June 30, 2022.
Restructuring expense
For the three and six months ended June 30, 2023, we recorded $1.5 million of restructuring expenses in relation to the 2023 Realignment and Consolidation Plan. There were no such expenses during the same periods ended June 30, 2022.
Goodwill impairment
For the three and six months ended June 30, 2023, we recorded $282.2 million of goodwill impairment as a result of a sustained decline in our market capitalization. Based on a quantitative interim goodwill impairment test, we concluded that the carrying value of the reporting unit was higher than its estimated fair value, which was determined using the market valuation method. This resulted in the impairment of the entire carrying value of goodwill as of June 30, 2023. There was no goodwill impairment recognized during the same periods ended June 30, 2022.
Other Expenses, net
|
|
Three months ended June 30, |
|
|
Six months ended June 30, |
|
||||||||||||||||||||||||||
|
|
2023 |
|
|
2022 |
|
|
Change |
|
|
2023 |
|
|
2022 |
|
|
Change |
|
||||||||||||||
Other expense: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
Interest expense, net |
|
$ |
(4,522 |
) |
|
$ |
(142,055 |
) |
|
$ |
137,533 |
|
|
|
-97 |
% |
|
$ |
(117,520 |
) |
|
$ |
(306,489 |
) |
|
$ |
188,969 |
|
|
|
-62 |
% |
Gain (loss) on foreign exchange, net |
|
|
1,162,273 |
|
|
|
(971,713 |
) |
|
|
2,133,986 |
|
|
|
-220 |
% |
|
|
1,447,184 |
|
|
|
(823,322 |
) |
|
|
2,270,506 |
|
|
|
-276 |
% |
Other expenses, net |
|
|
(740,005 |
) |
|
|
(336,993 |
) |
|
|
(403,012 |
) |
|
|
120 |
% |
|
|
(1,318,125 |
) |
|
|
(1,346,436 |
) |
|
|
28,311 |
|
|
|
-2 |
% |
Total other income (expense), net |
|
$ |
417,746 |
|
|
$ |
(1,450,761 |
) |
|
$ |
1,868,507 |
|
|
|
-129 |
% |
|
$ |
11,539 |
|
|
$ |
(2,476,247 |
) |
|
$ |
2,487,786 |
|
|
|
-100 |
% |
Interest expense, net
The change in net interest expense, net, of $0.1 million for the three months ended June 30, 2023, as compared to the same period of 2022, was primarily due to a decrease in interest accretion recognized during the quarter ended June 30, 2023.
The change in net interest expense, net, of $0.2 million for the six months ended June 30, 2023, as compared to the same period of 2022, was primarily due to a $0.2 million decrease in non-cash interest expense and bank charges during the quarter ended June 30, 2023.
Gain (loss) on foreign exchange, net
For the three and six months ended June 30, 2023 as compared to the same periods of 2022, the increases in net gain on foreign exchange were primarily driven by revaluations of intercompany balances in different currencies, mainly as a result of the devaluation of the Canadian dollar against the US dollar during 2023.
Other expenses, net
The other expenses, net, for the three months ended June 30, 2023, increased by $0.4 million as compared to the same period of 2022, primarily due to a $0.8 million adjustment made to funding obligation and a $0.3 million of credit loss provision expense, net of interest income, related to the notes receivable from Next Bridge in accordance with ASC 326, partially offset by a $0.4 million of Oil and Gas ("O&G") assets maintenance costs recorded in the three months ended June 30, 2022, and a $0.2 million of other income from the Scientific Research and Experimental Development ("SR&ED") tax incentive program recorded in the three months ended June 30, 2023.
38
The other expenses, net, for the six months ended June 30, 2023, slightly decreased as a result of the following changes: a $1.5 million decrease due to the O&G assets maintenance costs recorded in the six months ended June 30, 2022 and a $0.2 million of other income from the SR&ED tax incentive program recorded in the three months ended June 30, 2023, which are partially offset by a $0.9 million of credit loss provision expense, net of interest income, related to the notes receivable from Next Bridge in accordance with ASC 326 and a $0.8 million adjustment made to funding obligation.
Income Tax Recovery
|
|
Three months ended June 30, |
|
Six months ended June 30, |
||||||||||||
|
|
2023 |
|
2022 |
|
Change |
|
2023 |
|
2022 |
|
Change |
||||
Income tax recovery |
|
$618,079 |
|
$109,985 |
|
$508,094 |
|
462% |
|
$914,811 |
|
$109,985 |
|
$804,826 |
|
732% |
The increase in our income tax recovery for the three months ended June 30, 2023, as compared to the same period of 2022, was driven by an increase in accumulated losses that reduced our net deferred tax liability.
The increase in our income tax recovery for the six months ended June 30, 2023, as compared to the same period of 2022, was driven by an increase in accumulated losses that reduced our net deferred tax liability, partially offset by deferred tax expenses at a foreign entity.
We record deferred income tax liabilities for some of our foreign operations in Canada and the United Kingdom.
We have not yet been able to establish profitability or other sufficient significant positive evidence, to conclude that our deferred tax assets are more likely than not to be realized. Therefore, we continue to maintain a valuation allowance against our deferred tax assets.
LIQUIDITY AND CAPITAL RESOURCES
Liquidity risk is the risk that we will not meet our financial obligations as they become due after use of currently available cash. We have a planning and budgeting process to monitor operating cash requirements, including amounts projected for capital expenditures, which are adjusted as input variables change. These variables include, but are not limited to, our ability to generate revenue from current and prospective customers, general and administrative requirements and the availability of equity or debt capital and government funding. As these variables change, we may be required to issue equity or obtain debt financing.
On June 30, 2023, we had cash and cash equivalents of $14.5 million including $0.5 million in restricted cash compared to $11.8 million in cash and cash equivalents at December 31, 2022, including $1.7 million in restricted cash.
During the six months ended June 30, 2023, our principal sources of liquidity included $33.0 million of net proceeds obtained through the issuance of common stock under the At-the-Market Equity Offering Program and the equity offerings in April 2023, $1.0 million collection of notes receivable and $0.7 million proceeds from stock options exercise. See Note 12, Capital stock, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q for further information.
Our primary uses of liquidity for the six months ended June 30, 2023 included $13.4 million in salaries, $6.5 million in capital expenditures, $5.5 million in professional fees and stock exchange fees, $2.6 million in R&D materials and patents fees, $2.0 million in travel, advertisement and other costs, $1.6 million changes in working capital, $2.0 million in rent and utilities and $1.8 million in insurance.
During the second quarter ended June 30, 2023, our board of directors approved the Realignment and Consolidation Plan for the remainder of 2023 pursuant to which we have begun, but not yet completed, the process of realigning our corporate structure aimed at reducing our operating expenses and focusing on key applications which represent the greatest near-term revenue potential. Realignment and consolidation charges, not factoring in non-cash losses arising from the impairment of goodwill and long-term assets, are expected to be $2.6 million. We expect the Realignment and Consolidation Plan will reduce our operating expenses and drive operational efficiencies, excluding the non-cash losses from impairment of goodwill and long-term assets, during the year ending December 31, 2023 compared to the year ended December 31, 2022. Our average monthly cash burn rate before we started to implement the Realignment and Consolidation Plan was $5.7 million, which is expected to be reduced to between $2.6 million and $3.8 million, representing a reduction of approximately 54% to 33% respectively, by the end of the year ending December 31, 2023. We may incur additional expenses not currently contemplated due to events associated with the revised operating plan. The charges that we expect to incur in connection with the Realignment and Consolidation Plan are estimates subject to a number of assumptions, and actual results may differ materially.
39
We expect we will require additional funding to continue as a going concern and are dependent on raising capital to expand the commercialization of our products, fund our operations and further our research and development activities and ultimately attain profitable operations. Future capital requirements may vary materially from period to period and will depend on many factors, including the timing and extent of spending on research and development efforts and the ongoing investments to support the growth of our business.
Going Concern
In accordance with ASC 205-40, Going Concern, we evaluated whether there are conditions and events, considered in the aggregate, that raise substantial doubt about our ability to continue as a going concern within one year after the date that the condensed consolidated interim financial statements included in this Form 10-Q are issued. This evaluation initially does not take into consideration the potential mitigating effect of management’s plans that have not been fully implemented as of the date the condensed consolidated interim financial statements are issued. When substantial doubt exists under this methodology, management evaluates whether the mitigating effect of its plans sufficiently alleviates substantial doubt about our ability to continue as a going concern. The mitigating effect of management’s plans, however, is only considered if both (1) it is probable that the plans will be effectively implemented within one year after the date that the condensed consolidated interim financial statements are issued, and (2) it is probable that the plans, when implemented, will mitigate the relevant conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the condensed consolidated interim financial statements are issued. In performing its analysis, management excluded certain elements of its operating plan that cannot be considered probable. Under ASC 205-40, the receipt of potential funding from future partnerships, equity or debt issuance, potential achievement of milestones from customer agreements and reductions in workforce cannot be considered probable at this time because these plans are not entirely within our control and/or have not been approved by our Board of Directors as of the date of issuance of the condensed consolidated interim financial statements.
Our expectation to generate operating losses and negative operating cash flows in the future and the need for additional funding to support our planned operations, raise substantial doubt regarding our ability to continue as a going concern. Management's plans to alleviate the conditions that raise substantial doubt include reduced spending, and the pursuit of additional capital. Management has concluded the likelihood that its plan to successfully obtain sufficient funding from one or more of these sources, or adequately reduce expenditures, while highly possible, is less than probable. Accordingly, we have concluded that substantial doubt exists about our ability to continue as a going concern for a period of at least twelve months from the date of issuance of the condensed consolidated interim financial statements. See Note 3, Going concern, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q for further information.
The accompanying condensed consolidated interim financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the ordinary course of business. The condensed consolidated interim financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of the uncertainties described above.
The following table summarizes our cash flows for the periods presented:
|
|
Six months ended June 30, |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Net cash used in operating activities |
|
$ |
(25,885,373 |
) |
|
$ |
(29,003,401 |
) |
Net cash used in investing activities |
|
|
(5,238,122 |
) |
|
|
(10,798,920 |
) |
Net cash provided by financing activities |
|
|
33,518,328 |
|
|
|
46,611,933 |
|
Net increase in cash, cash equivalents and restricted cash |
|
$ |
2,394,833 |
|
|
$ |
6,809,612 |
|
Net cash used in operating activities:
During the six months ended June 30, 2023, net cash used in operating activities of $25.9 million was primarily driven by a net loss of $312.3 million for the period, and non-cash adjustments of $288.0 million mainly due to goodwill impairment of $282.2 million, depreciation and amortization of $6.6 million, a credit loss expense of $1.7 million, non-cash lease expense of $0.8 million, non-cash adjustment to funding obligation of $0.7 million, partially offset by a $1.7 million of net unrealized foreign currency exchange gain, non-cash interest income of $0.8 million, a $0.9 million decrease due to deferred income tax, and stock-based compensation of $0.8 million. In addition, there was $1.6 million cash used by working capital primarily due to a $5.5 million decrease in trade payables, a $0.6 million decrease in operating lease liabilities and a $0.3 million increase in accounts and other receivables, partially offset by a $4.0 million decrease in prepaid expenses and other current assets and a $0.9 million increase in restructuring costs accrual.
40
During the six months ended June 30, 2022, net cash used in operating activities of $29.0 million was primarily due to $39.4 million of net loss reported for the period, and non-cash adjustments of $13.0 million mainly due to stock-based compensation of $7.6 million, depreciation and amortization of $3.5 million, a $0.9 million of net unrealized foreign currency exchange loss, a $0.5 million of non-cash lease expense, a $0.4 million of non-cash consulting expense, partially offset by a decrease in deferred revenue of $0.2 million. In addition, there was $2.5 million cash used by working capital primarily due to a $1.6 million decrease in trade and other payables and a $0.7 million increase in prepaid expenses and other current assets.
Net cash used in investing activities:
During the six months ended June 30, 2023, net cash used in investing activities of $5.2 million was primarily driven by $6.5 million of capital expenditure associated with the construction of the Highfield Park Facility in Nova Scotia, Canada as well as the Thurso facility expansion in Quebec, partially offset by $1.0 million proceeds from collection of notes receivable and $0.3 million proceeds from a government loan.
During the six months ended June 30, 2022, net cash used by investing activities of $10.8 million was primarily due to $9.0 million of capital expenditure associated with the construction of the Highfield Park Facility in Canada as well as the equipment purchases for our facility in California, United States and $3.5 million cash paid for the Optodot acquisition, partially offset by $1.6 million proceeds from short-term investments.
Net cash provided by financing activities:
During the six months ended June 30, 2023, net cash provided by financing activities of $33.5 million was primarily driven by the $33.0 million net proceeds obtained through the issuance of common stock under the At-the-Market Equity Offering Program and the equity offering in April 2023, and $0.7 million proceeds from stock options and warrants exercise, partially offset by $0.2 million repayment of long-term debt.
During the six months ended June 30, 2022, net cash provided by financing activities of $46.6 million was primarily driven by the cash obtained through the proceeds from the issuance of common stock and warrants through Securities Purchase Agreements with institutional investors for the purchase and sale in a registered direct offering.
Commitments and Contractual Obligations
For a description of our commitments and contractual obligations, please see Note 21, Commitments and contingencies, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q.
Off-Balance Sheet Arrangements
Off-balance sheet firm commitments relating to outstanding letters of credit amounted to approximately $0.5 million as of June 30, 2023 which is secured by a performance security guarantee cover issued by Export Development of Canada. Further, this guarantee/standby letter of credit expires on October 5, 2023. Please see Note 21, Commitments and contingencies, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on this Form 10-Q. We do not maintain any other off-balance sheet arrangements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
This item is not required for a smaller reporting company.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic reports filed with the SEC is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our Chief Executive Officer, Chief Financial Officer and Chief Operating Officer as appropriate, to allow for timely decisions regarding required disclosure.
41
Our management, with the participation of our Chief Executive Officer, Chief Financial Officer and Chief Operating Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of June 30, 2023.
Although management believes there has been significant improvement in the design and implementation of our internal controls over financial reporting during the first six months of 2023, we still consider there to be a material weakness in our internal control over financial reporting that has not yet been remediated. Accordingly, our management, with the participation of our Chief Executive Officer, Chief Financial Officer and Chief Operating Officer, concluded that our disclosure controls and procedures were not effective as of June 30, 2023.
Nevertheless, giving full consideration to the material weakness and the progress made in addressing them since December 31, 2022, we have concluded that the condensed consolidated interim financial statements included in this Quarterly Report on Form 10-Q present fairly, in all material respects, our financial position, the results of our operations and our cash flows for each of the periods presented in conformity with U.S. GAAP.
Remediation of Previously Reported Material Weakness
Management has implemented a number of measures to address the material weakness disclosed in the Form 10-K/A for the year ended December 31, 2022. We are currently in the process of testing our key controls with the assistance of a third-party firm, with the intention to continue to strengthen our internal controls over financial reporting to ensure that management can routinely prepare our financial statements under GAAP and remain in compliance with the SEC reporting requirements.
To remediate the weakness described above, we have performed the following:
To remediate the weakness described above, we are in process of implementing the following:
Changes in Internal Controls
Except for the remediation activities described above, there were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the quarter ended June 30, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Internal Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, does not expect that disclosure controls or internal controls, when effective, will prevent all error and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. In addition, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
42
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple errors or mistakes.
Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management’s override of the control. The design of any systems of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving our stated goals under all potential future conditions. Over time, control may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of these inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. Individual persons may perform multiple tasks which normally would be allocated to separate persons and therefore extra diligence must be exercised during the period these tasks are combined.
43
PART II—OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, we are subject to threats of litigation or actual litigation in the ordinary course of business, some of which may be material. Other than as disclosed in Note 21, Commitments and contingencies, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q, we are not currently a party to any pending legal proceedings that, if determined adversely to us, would, in our opinion, have a material effect on our financial position, results of operations, or cash flows or that would not be covered by our existing liability insurance. The results of any litigation cannot be predicted with certainty, and regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Item 1A. Risk Factors
The following factors could materially affect our business, financial condition or results of operations and should be carefully considered in evaluating us and our business, in addition to other information presented elsewhere in this report. Before you invest in our securities, you should be aware that our business faces numerous financial and market risks, including those described below, as well as general economic and business risks. The following discussion provides information concerning the material risks and uncertainties that we have identified and believe may adversely affect our business, our financial condition and our results of operations. Before you decide whether to invest in our securities, you should carefully consider these risk factors together with all of the other information included in this Quarterly Report on Form 10-Q, and in our other public filings, which could materially affect our business, financial condition or future results. Our risk factors are not guarantees that no such conditions exist as of the date of this report and should not be interpreted as an affirmative statement that such risks or conditions have not materialized, in whole or in part.
Risks Related to the Realignment and Consolidation Plan
There is no assurance that we will be able to successfully complete the Realignment and Consolidation Plan, which may affect our ability to mitigate going concern risk.
Our ability to continue as a going concern is dependent upon our ability to consummate the Realignment and Consolidation Plan and to generate sufficient liquidity from the plan to meet our obligations and operating needs. As these plans and actions are complex, unforeseen factors could result in expected savings and benefits being delayed or not realized to the full extent planned (if at all), and our operations and business may be disrupted, which likely would adversely affect our financial condition, operating results and cash flow. These factors, together with our recurring losses from operations and accumulated deficit, create substantial doubt about our ability to continue as a going concern. See Note 20 of the notes to consolidated financial statements contained herein for more information on the Realignment and Consolidation Plan and the risks related thereto.
The Realignment and Consolidation Plan may require a substantial portion of the time and attention of our management, which may have an adverse effect on our business and results of operations, and we may face increased levels of employee attrition.
Our management has spent, and continues to be required to spend, a significant amount of time and effort focusing on the Realignment and Consolidation Plan. This diversion of attention may have an adverse effect on the conduct of our business, and, as a result, on our financial condition and results of operations, particularly if the Realignment and Consolidation Plan is protracted. During the pendency of the Realignment and Consolidation Plan, our employees may face considerable distraction and uncertainty and we may experience increased levels of employee attrition. A loss of key personnel or material erosion of employee morale could have a materially adverse effect on our ability to meet customer expectations, thereby adversely affecting our business and results of operations. The failure to retain or attract members of our management team and other key personnel could impair our ability to execute our strategy and implement operational initiatives, thereby having a material adverse effect on our financial condition and results of operations. Likewise, we could experience losses of customers who may be concerned about our ongoing long-term viability.
Risks Related to our Business
We expect to continue to incur losses from operations and negative cash flows, which raise substantial doubt about our ability to continue as a going concern.
We anticipate incurring additional losses until such time, if ever, we can achieve profitability. Substantial additional financing will be needed to fund our development, marketing and sales activities and generally to commercialize our technology. These factors raise substantial doubt about our ability to continue as a going concern.
We will seek to obtain additional capital through the issuance of debt or equity financing or other arrangements to fund operations; however, there can be no assurance we will be able to raise needed capital under acceptable terms, if at all. The sale of additional equity may dilute existing shareholders and newly issued shares may contain senior rights and preferences compared to currently outstanding shares of common stock.
44
Issued debt securities may contain covenants and limit our ability to pay dividends or make other distributions to shareholders. If we are unable to obtain such additional financing, future operations would need to be scaled back or discontinued. Due to the uncertainty in our ability to raise capital, we believe that there is substantial doubt as to our ability to continue as a going concern.
We have a limited operating history, which can make it difficult for investors to evaluate our operations and prospects and may increase the risks associated with investing in us.
We have incurred recurring consolidated net losses since our inception and expect our operating costs to continue to increase in future periods as we expend substantial financial and other resources on, among other things, business and headcount expansion in operations, sales and marketing, research and development, and administration as a public company. These expenditures may not result in additional revenues or the growth of our business. If we fail to grow revenues or to achieve profitability while our operating costs increase, our business, financial condition, results of operations and growth prospects will be materially, adversely affected.
We are expected to be subject to many of the risks common to early-stage enterprises for the foreseeable future, including challenges related to laws, regulations, licensing, integrating and retaining qualified employees; making effective use of limited resources; achieving market acceptance of existing and future products; competing against companies with greater financial and technical resources; acquiring and retaining customers; and developing new solutions; and challenges relating to identified material weaknesses in internal control.
We have a history of net losses, and we expect to continue to incur losses for the foreseeable future. If we ever achieve profitability, we may not be able to sustain it.
We have incurred losses from operations since our inception and expect to continue to incur losses from operations for the foreseeable future. We have incurred net losses of $312.3 million, including $282.2 million of goodwill impairment, and $79.1 million for the six months ended June 30, 2023 and the twelve months ended December 31, 2022, respectively. As a result of these losses, as of June 30, 2023, we had an accumulated deficit of $521.8 million. We expect to continue to incur significant sales and marketing, research and development, regulatory and other expenses as we grow our business. In addition, we expect our general and administrative expenses to increase due to the additional costs associated with being a public company. The net losses that we incur may fluctuate significantly from period to period. We will need to generate significant additional revenue in order to achieve and sustain profitability. Even if we achieve profitability, we cannot be sure that we will remain profitable for any substantial period of time.
We will need additional financing to execute our business plan and fund operations, which additional financing may not be available on reasonable terms or at all.
We will need to raise additional capital to expand the commercialization of our products, fund our operations and further our research and development activities. We will pursue sources of additional capital through various financing transactions or arrangements, including the sale/leaseback of certain properties, joint venturing of projects, debt financing, equity financing, or other means. We may not be successful in identifying suitable financing transactions in the time period required or at all, and we may not obtain the capital we require by other means.
Our ability to obtain financing, if and when necessary, may be impaired by such factors as the capital markets and our limited operating history.
Any additional capital raised through the sale of equity may dilute the ownership percentage of our stockholders. Raising any such capital could also result in a decrease in the fair market value of our equity securities because our assets would be owned by a larger pool of outstanding equity. The terms of securities we issue in future capital transactions may be more favorable to our new investors, and may include preferences, superior voting rights and the issuance of other derivative securities, and issuance of incentive awards under equity employee incentive plans, which may have a further dilutive effect.
In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we may issue, which may adversely impact our financial condition.
We may need to raise funds through debt financing in the future, which may require a significant amount of cash, and we may not have sufficient cash flow from our business to pay our indebtedness.
We may choose to raise additional funds in debt financing if it is available to us on terms we believe reasonable to increase our working capital, strengthen our financial position or to make acquisitions. Our ability to refinance any future indebtedness will depend on the capital markets and our financial condition at such time. We may not be able to engage in any of these activities or engage in these activities on desirable terms, which could result in a default on its debt obligations. In addition, any of our future debt agreements may contain restrictive covenants that may prohibit us from adopting any of these alternatives. Our failure to comply with these covenants could result in an event of default which, if not cured or waived, could result in the acceleration of our debt.
45
Our ability to make scheduled payments of a principal of future debt, to pay interest on or to refinance our indebtedness, including depends on our future performance, which is subject to economic, financial, competitive, and other factors beyond our control. Our business may not generate cash flow from operations in the future sufficient to service our debt and make necessary capital expenditures. If we are unable to generate such cash flow, we may be required to adopt one or more alternatives, such as selling assets, restructuring debt, or obtaining additional debt financing or equity capital on terms that may be onerous or highly dilutive.
In addition, future indebtedness, combined with our other financial obligations and contractual commitments, could have other important consequences. For example, it could:
Any of these factors could harm our business, results of operations, and financial condition. In addition, if we incur additional indebtedness, the risks related to our business and its ability to service or repay its indebtedness would increase.
When we hired the employees involved with our Vlepsis business, we agreed to share 50% of the profits, if any, from our Vlepsis business with the Vlepsis employees, thus our investment in the Vlepsis business will take longer to recoup. This may materially impact our profits from the Vlepsis business, and impact the rate of return on our investment in the Vlepsis business.
When we hired the Vlepsis team in March 2022, we adopted the Vlepsis Business Profit Sharing Plan whereby at the end of each year, generally, we are to calculate the profits from the Vlepsis business and then share 50% of such profits, if any, with certain of the Vlepsis employees. We have not yet had any profits from this business, but if we ever do, then this obligation to share such profits will impact our return on investment in Vlepsis and require more time to recoup our expenses and show a return. This may materially impact our ability to show an overall profit for the Company or delay our overall profitability of the Company, and your investment in the Company may be impacted.
Our operating results fluctuate significantly because of a number of factors, many of which are beyond our control.
Given the nature of the markets in which we participate, as well as macroeconomic uncertainties, we cannot reliably predict future revenues and profitability and unexpected changes may cause us to adjust our operations. Large portions of our costs are fixed, due in part to our significant sales, research and development and manufacturing costs. Thus, small declines in revenues could seriously negatively affect our operating results in any given quarter. Our operating results may fluctuate significantly from quarter-to-quarter and year-to-year. Some of the factors that may affect our quarterly and annual results are:
46
As a result of these factors, many of which are difficult to control or predict, we may experience materially adverse fluctuations in our future operating results on a quarterly or annual basis. Changes in demand for our products and in our customers’ needs could have a variety of negative effects on our competitive position and our financial results, and, in certain cases, may reduce our revenues, increase our costs, lower our gross margin percentage or require us to recognize impairments of our assets.
If we are unable to maintain effective disclosure controls and procedures, our business, financial position and results of operations could be adversely affected.
We are subject to the periodic reporting requirements of the Exchange Act. We designed our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Our management has concluded that a material weakness in our internal control over financial reporting exists at December 31, 2022. Management has further concluded that this material weakness resulted in our disclosure controls and procedures not being effective as of December 31, 2022. Please see Item 4 of Part I, Controls and Procedures, for more information about the material weakness that we identified.
We currently rely on revenue from development services and most of our products are in the development stage. Any delay in the development or introduction of our new products could adversely affect our business, financial condition, results of operations, and cash flows.
Most of our revenues are currently derived from development activities. While we strive to develop innovative products available for commercial use, we have not yet achieved mass commercial production or generated substantial revenues from product sales. The success of our future products remains uncertain, and there is no guarantee that we will be able to bring any products to market that are accepted by our customers. As a result, our financial performance and ability to sustain operations may be materially impacted.
We may be unable to develop new products, applications, and end markets for our products.
Our future success will depend in part on our ability to generate sales of our products as well as generating development revenue. Current and potential customers may have substantial investment in, and know-how related to our technologies. Customers may be reluctant to change from incumbent suppliers or cease using their own solutions, or our products may miss the design and procurement cycles of our customers. Many target markets have historically been slow to adopt new technologies. These markets often require long testing and qualification periods or lengthy government approval processes before admitting new suppliers or adopting new technologies. Introduction of new products and product enhancements will require that we effectively transfer production processes from research and development to manufacturing and coordinate efforts with those suppliers to achieve increased production volume rapidly. If we are unable to implement this strategy to develop new applications and end markets for products or develop new products, the business, financial condition, results of operations and growth prospects could be materially adversely affected. In addition, any newly developed or enhanced products may not achieve market acceptance or may be rendered obsolete or less competitive by the introduction of new products by other companies.
For example, we are currently developing VLEPSIS®, a ground-breaking, multiple-gigapixel, turnkey wide area motion imagery system, which tracks and monitors hundreds of objects/locations simultaneously, in stunning detail and resolution. It is set to launch in fiscal 2024. If we are unable to deliver products that meet our customers’ expectations, our business, financial condition, results of operations, and cash flows will be adversely affected.
Our research and marketing development activities and investments may not result in profitable, commercially viable or successfully produced and marketed products.
Although we, ourselves and through our investments, are committed to researching and developing new markets and products and improving existing products, there can be no assurances that such research and market development activities will prove profitable or that the resulting markets and/or products, if any, will be commercially viable or successfully produced and marketed. A failure in the demand for products to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations and financial condition of the companies in which we have or will invest in, and consequently, on us.
47
Because our products typically have lengthy sales cycles, we may experience substantial delays between incurring expenses related to research and development and the generation of revenues.
The time from initiation of design to volume production of new meta material products often takes 18 months or longer. We first work with customers to achieve a design win, which may take 12 months or longer. Our customers then complete the design, testing and evaluation process and begin to ramp up production, a period that may last an additional nine months or longer. As a result, a significant period of time may elapse between our research and development efforts and our realization of revenues, if any, from volume purchasing of our products by our customers.
Disruption in supply from our single source supplier of our holographic raw materials may cause a material adverse effect on our Holography-related products.
We purchase our holographic raw materials from a tier 1 German manufacturer, which is a single source supplier. Disruption in supply from this supplier for any number of factors may cause a material adverse effect on our Holography-related products, which would negatively impact our financial condition and results of operations.
Fluctuations in the mix of products sold may adversely affect our financial results.
Changes in the mix and types of products sold may have a substantial impact on our revenues and gross profit margins. In addition, more recently introduced products tend to have higher associated costs because of initial overall development costs and higher start-up costs. Fluctuations in the mix and types of our products may also affect the extent to which we are able to recover our fixed costs and investments that are associated with a particular product or wafer foundry, and, as a result, can negatively impact our financial results.
Variations in the amount of time it takes for us to sell our systems may cause fluctuations in our operating results, which could cause our stock price to decline.
Variations in the length of our sales cycles could cause our revenues and cash flows, and consequently, our business, financial condition, operating results and cash flows, to fluctuate widely from period to period. This variation could cause our stock price to decline. Our customers generally take a long time to evaluate our inspection and/or film metrology systems and many people are involved in the evaluation process. We expend significant resources educating and providing information to our prospective customers regarding the uses and benefits of our systems in the semiconductor fabrication process. The length of time it takes for us to make a sale depends upon many factors including, but not limited to:
Material weaknesses or significant deficiencies in our internal controls could materially and adversely affect our business, results of operations and financial condition.
Our management is responsible for establishing and maintaining effective internal control over financial reporting, as such term is defined in Securities Exchange Act Rule 13a‑15(f). Our internal control over financial reporting is a process designed by and under the supervision of our management, including our Chief Executive Officer and Chief Financial Officer, and effected by our management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with U.S. generally accepted accounting principles. As previously disclosed, our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2022 and, based on this evaluation, concluded that internal control over financial reporting was not effective as of December 31, 2022, due to material weaknesses in internal control over financial reporting. As of June 30, 2023, such material weaknesses had not yet been fully remediated.
Remediation efforts place a significant burden on our management and add increased pressure on our financial reporting resources and processes (see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Part I, Item 2 of this Quarterly Report on Form 10-Q for more information regarding such remediation efforts). If we are unable to successfully remediate these material weaknesses in a timely manner, or if any additional material weaknesses in our internal control over disclosure or financial reporting are identified, the accuracy of our financial reporting and our ability to timely file with the SEC may be adversely impacted. In addition, if our remedial efforts are insufficient, or if additional material weaknesses or significant deficiencies in our internal controls occur in the future, we could be required to restate our financial statements, which could materially and adversely affect our business, results of operations and financial condition, restrict our ability to access the capital markets, require us to expend significant resources to correct the material weaknesses or deficiencies, subject us to regulatory investigations and penalties, harm our reputation, and cause a decline in investor confidence or otherwise cause a decline in our stock price.
48
Impairment of our goodwill, other intangible assets and other long-term assets could materially and adversely affect our business, operating results, and financial condition.
During the three months ended June 30, 2023, we determined that a triggering event occurred as a result of a sustained decline in our market capitalization; therefore, we performed an interim impairment test for our reporting unit. As a result of the interim impairment testing, we recognized goodwill impairment charges of $282.2 million in the three months ended June 30, 2023.
Adverse events or changes in circumstances that may affect the estimated undiscounted future operating cash flows expected to be derived from our goodwill, intangible assets and long-term assets; therefore, we may be required to recognize additional impairment charges. Any impairment charges could have a material adverse effect on our operating results and net asset value in the quarter in which we recognize the impairment charge. We cannot provide assurances that we will not in the future be required to recognize impairment charges. Please see Item 7 of Part II, Management’s Discussion and Analysis of Financial Condition and Results of Operation – Goodwill, of the Form 10-K/A and Note 10, Intangible assets and goodwill, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q for more information.
We depend on our OEM customers and system integrators to incorporate our products into their systems.
Our revenues depend, in part, on our ability to maintain existing and secure new OEM customers. Our revenues also depend, in part, on the ability of our current and potential OEM customers and system integrators to incorporate our products into their systems, and to sell such systems successfully. Limited marketing resources, reluctance to invest in research and development and other factors affecting these OEM customers and third-party system integrators could have a substantial impact upon demand for our products, and in turn upon our revenues and financial results. If OEM customers or integrators are not able to adapt existing tools or develop new systems to take advantage of the features and benefits of our products or if they perceive us to be an actual or potential competitor, then the opportunities to expand our revenues and increase our margins may be severely limited or delayed. In addition, some of our OEM customers are developing their own competitive products. If they are successful, this may reduce our revenues from these customers.
Our revenues may be concentrated in a few customers, and if we lose any of these customers, or these customers do not pay us, our revenues could be materially adversely affected.
We rely on a few customers for a significant portion of our revenues. For the six months ended June 30, 2023, revenue from two customers, each of which individually represented more than 10% of the total revenue, accounted for $3.1 million or 88.8% of total revenue.
We currently derive a significant portion of our revenue from contract services with a G10 central bank. Although we are developing a new security feature under a framework contract with this customer. There can be no assurance that this project will be successful, or that it will result in long-term production revenue for this security feature.
The markets in which we participate are intensely competitive.
Many of our target markets are intensely competitive. Our ability to compete successfully in our target markets depends on the following factors:
In addition, our competitors or customers may offer new products based on new technologies, industry standards, end-user or customer requirements, including products that have the potential to replace our products or provide lower cost or higher performance alternatives to our products.
49
The introduction of new products by our competitors or customers could render our existing and future products obsolete or unmarketable.
Our agreements with various national governments and suppliers to such governments subject us to unique risks.
We must comply with, and are affected by, laws and regulations relating to the award, administration, and performance of various national government contracts. Awards received from such governments may be canceled or lose funding. Such government contracting parties may require us to increase or decrease production of certain products sold to such governments due to changes in strategy, priorities or other reasons, which could impact production of other products or sales to other customers to meet the requirements of such governments. In addition, such governments routinely retain rights to intellectual property developed in connection with government contracts. Such governments could exercise these rights in certain circumstances in the future, which could have the effect of decreasing the benefit we are able to realize commercially from such intellectual property.
National government agencies routinely audit and investigate government contractors and can decrease or withhold certain payments when it deems systems subject to its review to be inadequate. Additionally, any costs found to be misclassified may be subject to repayment. If an audit or investigation uncovers improper or illegal activities, we may be subject to civil or criminal penalties and administrative sanctions, including reductions of the value of contracts, contract modifications or terminations, forfeiture of profits, suspension of payments, penalties, fines and suspension, or prohibition from doing business with such governments. In addition, we could suffer serious reputational harm if allegations of impropriety were made against it. Any such imposition of penalties, or the loss of such government contracts, could materially adversely affect our business, financial condition, results of operations and growth prospects.
We are subject to the Foreign Corrupt Practices Act and similar anti-bribery and anti-corruption laws, as well as governmental export and import controls, all of which could subject us to liability or impair our ability to compete in international markets.
Our business activities may be subject to the U.S. Foreign Corrupt Practices Act (the FCPA), and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we operate. These laws generally prohibit companies and their employees and third-party business partners, representatives and agents from engaging in corruption and bribery, including offering, promising, giving or authorizing the provision of anything of value, either directly or indirectly, to a government official or commercial party in order to influence official action, direct business to any person, gain any improper advantage, or obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore involves significant interaction with government officials, including potentially officials of non-U.S. governments.
In addition to our own employees, we may in the future leverage third parties to conduct our business abroad, such as obtaining government licenses and approvals. We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies, state-owned or affiliated entities and we may be held liable for the corrupt or other illegal activities of our employees, our third-party business partners, representatives and agents, even if we do not explicitly authorize such activities. There is no certainty that our employees or the employees of our third-party business partners, representatives and agents will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in whistleblower complaints, adverse media coverage, investigations, loss of export privileges, debarment from U.S. government contracts, substantial diversion of management’s attention, significant legal fees and fines, severe criminal or civil sanctions against us, our officers, or our employees, disgorgement and other sanctions and remedial measures, and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, financial condition and stock price.
The U.S. and various foreign governments have imposed controls, export license requirements and restrictions on the import or export of certain products, technologies, and software. We must export our products in compliance with U.S. export controls and we may not always be successful in obtaining necessary export licenses. Our failure to obtain required import or export approval for our products or limitations on our ability to export or sell our products imposed by these laws may harm our international and domestic revenues. Noncompliance with these laws could have negative consequences, including government investigations, penalties and reputational harm.
Changes in our products or changes in export, import and economic sanctions laws and regulations may delay our introduction of new products in international markets, prevent our customers from deploying our products internationally or, in some cases, prevent the export or import of our products to or from certain countries altogether. In addition to the tariffs imposed by the U.S. Government on certain items imported from China, it is possible that additional sanctions or restrictions may be imposed by the United States on items imported into the United States from China. Similarly, in addition to the tariffs imposed by China on certain items imported from the United States, it is possible that additional sanctions or restrictions may be imposed by China on items imported into China from the United States. Any such measures could further adversely affect our ability to sell our products to existing or potential customers and harm our ability to compete internationally and grow our business. In addition, generally, tariffs may materially increase the cost of our raw materials and finished goods, may negatively impact our margins as we may not be able to pass on the additional cost through increasing the prices of our products, and may cause the contraction of certain industries, including the Industrial market.
50
Any change in export or import regulations or legislation, shift or change in enforcement, or change in the countries, persons or technologies targeted by these regulations, could result in decreased use of our products by, or in our decreased ability to export or sell our products to, existing or potential customers with international operations. In such an event, our business, financial condition, results of operations and growth prospects could be materially adversely affected.
We may experience delays in providing sufficient product for future testing of our products due to ongoing supply chain limitations.
Due to current supply chain disruptions, our contract manufacturing organizations may experience an inability to manufacture and produce sufficient quantities of our products as we progress through our regulatory testing and/or approval. Should this happen, we may not be able to provide sufficient quantities of our products which could delay our ability to bring products to market. Such a delay would cause us to use more capital than currently planned which may have a material adverse effect on our projected timing of product launches and financials.
Changes in laws, regulations or guidelines relating to our business plan and activities could adversely affect our business.
Our current and proposed operations are subject to a variety of laws, regulations and guidelines relating to production, the conduct of operations, transportation, storage, health and safety, medical device regulation and the protection of the environment. These laws and regulations are broad in scope and subject to evolving interpretations, which could require us to incur substantial costs associated with compliance or alter certain aspects of our business plan. In addition, violations of these laws, or allegations of such violations, could disrupt certain aspects of our business plan and result in a material adverse effect on certain aspects of our planned operations.
As an example, we launched a new product metaAIR® in March 2019 to provide laser glare protection to pilots in the airline industry. Currently, metaAIR® is not subject to any Federal Aviation Administration regulations. However, metaAIR® has yet to receive FDA approval/clearance and could become subject to evolving regulation by governmental authorities as the metaAIR® market evolves further.
If we are unable to make acquisitions, or successfully integrate them into our business, our results of operations and financial condition could be adversely affected.
We have completed a number of acquisitions during our operating history. We have spent and may continue to spend significant resources identifying and pursuing future acquisition opportunities. Acquisitions involve numerous risks including:
51
We cannot assure that we will be able to successfully acquire other businesses or product lines or integrate them into our operations without substantial expense, delay in implementation or other operational or financial problems. Failure to achieve the anticipated benefits of any prior and future acquisitions or to successfully integrate the operations of the acquired companies could have a material and adverse effect on our business, financial condition, and results of operations. Any future acquisitions could also result in potentially dilutive issuance of equity securities, acquisition or divestiture-related write-offs or the assumption of debt and contingent liabilities.
As a result of an acquisition, our financial results may differ from the investment community’s expectations in a given quarter. Further, if one or more of the foregoing risks materialize or market conditions or other factors lead us to change our strategic direction, we may not realize the expected value from such transactions. If we do not realize the expected benefits or synergies of such transactions, our consolidated financial position, results of operations, cash flows or stock price could be negatively impacted.
The regulatory approval process for our medical products in the United States and other countries around the world is time-consuming and complicated, and we may not obtain the approval required to market a product within the timeline required, or at all. Additionally, we may lose regulatory approval and/or our products may become subject to new and unanticipated foreign regulations.
Our wireless sensing technologies to enhance MRI and glucoWISE® non-invasive glucose® monitoring are under research and development. We have performed many pre-clinical experiments and we are preparing to perform clinical experiments as needed to continue the development of the related products. These products have not yet entered the clinical phase, and we have not engaged with any regulatory authorities regarding any medical uses subject to regulatory approval processes. We can provide no assurance that any clinical trials we commence will be successful, or that we will be successful in obtaining any regulatory approvals for any medical products we may develop in the future.
Development of medical devices and related operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
Any medical devices that we may develop in the future, and related operations, are subject to extensive regulation in the United States and elsewhere, including by the FDA and by the FDA’s foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development, manufacturing, and release; laboratory, preclinical, and clinical testing; labeling, packaging, content, and language of instructions for use and storage; product safety and efficacy claims; establishment, registration, and device listing; marketing, sales, and distribution; pre-market clearances, approvals, and certifications; service operations; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; post-market studies; and product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA and foreign counterparts enforce these regulatory requirements through, among other means, periodic unannounced inspections and periodic reviews of public marketing and promotion materials. We do not know whether we will be found compliant in connection with any future FDA or foreign counterparts’ inspections or reviews. Failure to comply with applicable regulations could jeopardize our ability to sell our medical devices and result in enforcement actions such as: warning letters; untitled letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances, approvals, or certifications; withdrawals or suspensions of current approvals or certifications, resulting in prohibitions on sales of our medical devices; and in the most serious cases, criminal penalties.
Legislative or regulatory reforms in the United States or other countries may make it more difficult and costly for us to obtain regulatory clearances, approvals, or certifications for our products or to manufacture, market, or distribute our products after clearance, approval, or certification is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations, or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. The FDA’s and other regulatory authorities’ policies may change, and additional government regulations may be promulgated that could prevent, limit, or delay regulatory clearance or approval of our product candidates.
52
We cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could harm our business, financial condition, and results of operations.
In the United States, there have been, and continue to be, a number of legislative initiatives to contain healthcare costs. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (ACA) was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. We expect additional state and federal healthcare policies and reform measures to be adopted in the future. Any of these could make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market, or distribute our products after clearance or approval is obtained. Any such reforms could have a material adverse effect on our industry generally and on our customers. In addition, any healthcare reforms that expand the government’s role in the U.S. healthcare industry may result in decreased sale of our products and lower reimbursement by payors for procedures using our products, any of which could affect demand for our products and/or result in additional pricing pressure, which in turn could impact our ability to successfully commercialize our products and could have an adverse material effect on our business, financial condition, and results of operations. Changes and reforms in the EU and other countries where we may decide to commercialize could have similar effects.
If coverage and reimbursement from third-party payors for procedures using our medical products, if authorized by a regulatory authority, significantly decline, physicians, hospitals, and other healthcare providers may be reluctant to use our products and our sales may decline.
In the United States, healthcare providers who purchase medical products generally rely on third-party payors, including Medicare, Medicaid, and private health insurance plans, to pay for all or a portion of the cost of the medical products that we may commercialize upon regulatory approval or clearance. Any decline in the amount payors are willing to reimburse our medical products, if cleared or approved for commercial use and distribution, may make it difficult for customers to adopt our products and could create additional pricing pressure for us. We may be unable to sell our products on a profitable basis if third-party payors deny coverage or reduce their current levels of reimbursement.
To contain costs of new technologies, governmental healthcare programs and third-party payors are increasingly scrutinizing new and existing treatments by requiring extensive evidence of favorable clinical outcomes. Physicians, hospitals, and other healthcare providers may not purchase our products if they do not receive satisfactory reimbursement from these third-party payors for the cost of using our products. If third-party payors issue non-coverage policies or if our customers are not reimbursed at adequate levels, this could adversely affect sales of our products. Outside of the United States, reimbursement systems vary significantly by country. The marketability of our products may suffer if government and commercial third-party payors fail to provide adequate coverage and reimbursement. Even if favorable coverage and reimbursement status is attained, less favorable coverage policies and reimbursement rates may be implemented in the future.
If we or our contractors fail to comply with healthcare and other governmental regulations, we could face substantial fines and penalties and our business, results of operations and financial condition could be adversely affected.
We are subject to certain federal, state, and foreign fraud and abuse laws, health information privacy and security laws, and transparency laws regarding payments and other transfers of value made to physicians and other healthcare professionals that could subject us to substantial penalties. Additionally, any challenge to, or investigation into, our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business. Our arrangements with physicians, hospitals and medical centers could expose us to broadly applicable fraud and abuse laws and other laws and regulations that may restrict the financial arrangements and relationships through which we may market, sell, and distribute our medical products after we receive the applicable marketing authorization. Our employees, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements. Federal and state healthcare laws and regulations that may affect our ability to conduct business, include, without limitation:
53
The scope and enforcement of each of the laws applicable to our business and products are uncertain and subject to rapid change in the current environment of healthcare reform. The U.S. Department of Justice has increased its scrutiny of interactions between manufacturers and healthcare providers, which has led to a number of investigations, prosecutions, convictions, and settlements in the healthcare industry. Responding to a government investigation is time and resource intensive and may cause harm to our business and reputation even if we are able to successfully defend against it. Any action brought against us for violations of these laws or regulations, even successfully defended, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. We may be subject to private “qui tam” actions brought by individual whistleblowers on behalf of the federal or state governments.
If our operations are found to be in violation of any of the federal, state and foreign laws described above or any other current or future fraud and abuse or other healthcare laws and regulations that apply to us, we may be subject to penalties, including significant criminal, civil, and administrative penalties, damages, fines, imprisonment for individuals, exclusion from participation in government programs, such as Medicare and Medicaid, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
54
If we fail to obtain and maintain necessary regulatory clearances, approvals, or certifications for our products, or if clearances, approvals or certifications for future products and indications are delayed or not issued, our commercial operations would be harmed.
Our medical products are subject to extensive regulation by the FDA in the United States and by regulatory agencies in other countries outside of the United States. Government regulations specific to medical devices are wide ranging and govern, among other things:
Before a new medical device, or a new intended use for an existing product, can be marketed in the United States, a company must first submit and receive 510(k) clearance pursuant to Section 510(k) of the Food, Drug and Cosmetic Act (FDCA), approval of a PMA by the FDA, or grant of a de novo classification request from the FDA, unless an exemption applies.
In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, in order to clear the proposed device for marketing. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence. In the PMA approval process, the FDA must determine that a proposed device is safe and effective for its intended use based on extensive data, including technical, pre-clinical, clinical trial, manufacturing, and labeling data. The PMA process is typically required for devices for which the 510(k) process cannot be used and that are deemed to pose the greatest risk, such as life sustaining, life supporting, or implantable devices. In the de novo classification process, a manufacturer whose novel device under the FDCA would otherwise be automatically classified as Class III and require the submission and approval of a PMA prior to marketing is able to request down-classification of the device to Class I or Class II on the basis that the device presents a low or moderate risk. If the FDA grants the de novo classification request, the applicant will receive authorization to market the device. This device type may be used subsequently as a predicate device for future 510(k) submissions. Modifications to products that are approved through a PMA application generally need prior FDA approval of a PMA supplement. Similarly, some modifications made to products cleared through a 510(k) submission may require a new 510(k) clearance, or such modification may put the device into Class III and require PMA approval or the grant of a de novo classification request.
The PMA approval, 510(k) clearance, and de novo classification processes can be expensive, lengthy, and uncertain. Any delay or failure to obtain necessary regulatory approvals, clearances or certifications would have a material adverse effect on our business, financial condition, and results of operations.
The FDA and foreign bodies can delay, limit, or deny clearance, approval, or certification of a device for many reasons, including:
55
Upon commercialization of any medical devices for which we receive FDA clearance or approval, we are required to investigate all product complaints we receive, and timely file reports with the FDA, including MDRs that require that we report to regulatory authorities if our products may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur. If these reports are not submitted in a timely manner, regulators may impose sanctions and we may be subject to product liability or regulatory enforcement actions, including warning letters, untitled letters, fines, civil penalties, recalls, seizures, operating restrictions, denial of requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products, withdrawal of current 510(k) clearances or premarket approvals, and narrowing of approved or cleared product labeling, all of which could harm our business. In addition, the FDA may provide notice of and conduct additional inspections, such as “for cause” inspections, of our business, sites, and facilities as part of its review process. Similar requirements may apply in foreign countries.
If we initiate a correction or removal action for our products to reduce a significant risk to health posed by our products, we would be required to submit a publicly available correction and removal report to the FDA and, in many cases, similar reports to other regulatory agencies. This report could be classified by the FDA as a device recall which could lead to increased scrutiny from the FDA, other international regulatory agencies, and our customers regarding the quality and safety of our products. Furthermore, the submission of these reports could be used by competitors against us and cause physicians to delay or cancel orders, which could harm our reputation.
The FDA and the Federal Trade Commission (FTC) also regulate the advertising, promotion, and labeling of our products to ensure that the claims we make are consistent with our regulatory authorizations, that there is adequate and reasonable scientific data to substantiate the claims, and that our promotional labeling and advertising is neither false nor misleading in any respect. If the FDA or FTC determines that any of our advertising or promotional claims are misleading, not substantiated, or not permissible, we may be subject to enforcement actions, including adverse publicity and/or warning letters, and we may be required to revise our promotional claims and make other corrections or restitutions.
The FDA, state authorities, and foreign counterparts have broad investigation and enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state agencies, or foreign counterparts, which may include any of the following sanctions:
If any of these events were to occur, our business and financial condition could be harmed. In addition, the FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of our products. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval or certification that we may have obtained and we may not achieve or sustain profitability, which would adversely affect our business, financial condition, and results of operations.
Semiconductors included as components in consumer products have shorter product life cycles than other types of products sold by our customers.
We believe that components are subject to shorter product life cycles, because of technological change, consumer preferences, trendiness and other factors, than other types of products sold by our customers. Shorter product life cycles result in more frequent design competitions for the inclusion of semiconductors in next generation consumer products, which may not result in design wins for us. Shorter product life cycles may lead to more frequent circumstances where sales of existing products are reduced or ended.
We may not be able to increase production capacity to meet the present and future demand for our products.
The semiconductor industry has been characterized by periodic limitations on production capacity. These limitations may result in longer lead times for product delivery than desired by many of our customers. If we are unable to increase our production capacity to meet future demand, some of our customers may seek other sources of supply, our future growth may be limited or our results of operations may be adversely affected.
56
Our gross margin is dependent on a number of factors, including our level of capacity utilization.
Semiconductor manufacturing requires significant capital investment, leading to high fixed costs, including depreciation expense. We are limited in our ability to reduce fixed costs quickly in response to any shortfall in revenues. If we are unable to utilize our manufacturing, assembly and testing facilities at a high level, the fixed costs associated with these facilities will not be fully absorbed, resulting in lower gross margins. Increased competition and other factors may lead to price erosion, lower revenues and lower gross margins for us in the future.
Our success depends on our ability to manufacture our products efficiently.
We manufacture our products in facilities that are owned and operated by us, as well as in external wafer foundries and subcontract assembly facilities. For various reasons, such as contamination in the manufacturing environment, defects in the masks used in the facilities and manufacturing equipment failures, we could experience a decrease in manufacturing yields. Additionally, if we increase our manufacturing output, the additional demands placed on existing equipment and personnel or the addition of new equipment or personnel may lead to a decrease in manufacturing yields. As a result, we may not be able to cost-effectively expand our production capacity in a timely manner.
Increasing raw material prices could impact our profitability.
From time to time, we have experienced price increases for many raw materials. If we are unable to pass price increases for raw materials onto our customers, our gross margins and profitability could be adversely affected.
Our revenues are dependent upon our products being designed into our customers’ products.
Some of our products are incorporated into customers’ products or systems at the design stage. The value of any design win largely depends upon the customer’s decision to manufacture the designed product in production quantities, the commercial success of the customer’s product and the extent to which the design of the customer’s products also accommodates incorporation of components manufactured by our competitors. In addition, our customers could subsequently redesign their products or systems so that they no longer require our products. The development of the next generation of products by our customers generally results in new design competitions for semiconductors, which may not result in design wins for us, potentially leading to reduced revenues and profitability. We may not achieve design wins or our design wins may not result in future revenues.
We rely on our distributors and sales representatives to sell some of our products.
Our distributors and sales representatives could reduce or discontinue sales of our products. They may not devote the resources necessary to sell our products in the volumes and within the time frames that we expect. In addition, we depend upon the continued viability and financial resources of these distributors and sales representatives, some of which are small organizations with limited working capital. These distributors and sales representatives, in turn, depend substantially on general economic conditions and conditions within the semiconductor industry. We believe that our success will continue to depend upon these distributors and sales representatives. Foreign distributors are typically granted longer payment terms, resulting in higher accounts receivable balances for a given level of sales than domestic distributors. Our risk of loss from the financial insolvency of distributors is, therefore, disproportionally weighted to foreign distributors. If any significant distributor or sales representative experiences financial difficulties, or otherwise becomes unable or unwilling to promote and sell our products, our business could be harmed.
Costs related to product defects and errata may harm our results of operations and business.
Costs associated with unexpected product defects and errata (deviations from published specifications) due to, for example, unanticipated problems in our manufacturing processes, include the costs of:
These costs could be substantial and may, therefore, increase our expenses and lower our gross margin. In addition, our reputation with our customers or users of our products could be damaged as a result of such product defects and errata, and the demand for our products could be reduced. These factors could harm our financial results and the prospects for our business.
57
We order materials and commence production in advance of anticipated customer demand. Therefore, revenue shortfalls may also result in inventory write-downs.
We typically plan our production and inventory levels based on our own expectations for customer demand. Actual customer demand, however, can be highly unpredictable and can fluctuate significantly. In response to anticipated long lead times to obtain inventory and materials, we order materials and production in advance of customer demand. This advance ordering and production may result in excess inventory levels or unanticipated inventory write-downs if expected orders fail to materialize.
Our international operations expose us to material risks.
For the six months ended June 30, 2023, almost all of our consolidated revenue was generated were in countries outside of the United States. We expect net revenues from foreign markets to continue to represent a significant portion of total net revenues. We maintain significant business operations in Canada. Some of the risks inherent in doing business internationally are:
Our financial performance is dependent on economic stability and credit availability in international markets. Actions by governments to address deficits or sovereign or bank debt issues, particularly in Europe, could adversely affect gross domestic product or currency exchange rates in countries where we operate, which in turn could adversely affect our financial results. If our customers or suppliers are unable to obtain the credit necessary to fund their operations, we could experience increased bad debts, reduced product orders and interruptions in supplier deliveries leading to delays or stoppages in our production. Alternatively, governmental actions in China or other emerging markets to address economic problems, such as inflation, asset or other “bubbles” or the transfer of capital out of the country, could also adversely affect gross domestic product or the growth thereof and result in reduced product orders or increased bad debt expense for us. In addition, the laws and courts of certain foreign countries may not protect our products or intellectual property rights to the same extent as do U.S. laws and courts. Therefore, the risk of piracy of our technology and products may be greater when we manufacture or sell our products in certain foreign countries.
Business interruptions may damage our facilities or those of our suppliers.
Our operations and those of our suppliers are vulnerable to interruption by fire, earthquake, flood and other natural disasters, as well as power loss, telecommunications failure and other events beyond our control. We do not have a detailed disaster recovery plan and our backup power sources have only a limited amount of time for the support of critical systems. Some of our facilities are located near major earthquake fault lines and have experienced earthquakes in the past. If a natural disaster occurs, our ability to conduct our operations could be seriously impaired, which could harm our business, financial condition and results of operations and cash flows. We cannot be sure that the insurance we maintain against general business interruptions will be adequate to cover all our losses.
58
We are exposed to risks that our employees, consultants, or other commercial partners and business associates may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, consultants, and other commercial partners and business associates may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless, or negligent conduct or other unauthorized activities that violate the regulations of the FDA and other regulators (both domestic and foreign), including those laws requiring the reporting of true, complete, and accurate information to such regulators, manufacturing standards, healthcare fraud and abuse laws, and regulations in the United States and internationally or laws that require the true, complete, and accurate reporting of financial information or data. In particular, sales, marketing, and business arrangements in the healthcare industry, including the sale of medical devices, are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. It is not always possible to identify and deter misconduct by our employees, consultants, and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal, and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid, and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of operations, any of which could adversely affect our business, financial condition and results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees and reputational harm, and divert the attention of management in defending ourselves against any of these claims or investigations.
Compliance with environmental laws and regulations could be expensive, and failure to comply with these laws and regulations could subject us to significant liability.
Our research and development and manufacturing operations involve the use of some hazardous substances and are subject to a variety of federal, state, local, and foreign environmental laws and regulations relating to the storage, use, discharge, disposal, remediation of, and human exposure to, hazardous substances and the sale, labeling, collection, recycling, treatment, and disposal of products containing hazardous substances. Liability under environmental laws and regulations can be joint and several and without regard to fault or negligence. Compliance with environmental laws and regulations may be expensive and noncompliance could result in substantial liabilities, fines and penalties, personal injury and third-party property damage claims and substantial investigation and remediation costs. Environmental laws and regulations could become more stringent over time, imposing greater compliance costs, and increasing risks and penalties associated with violations. We cannot assure you that violations of these laws and regulations will not occur in the future or have not occurred in the past as a result of human error, accidents, equipment failure or other causes. The expense associated with environmental regulation and remediation could harm our financial condition and operating results.
The risk of loss of our intellectual property, trade secrets or other sensitive business or customer confidential information or disruption of operations due to cyberattacks or data breaches could negatively impact our financial results.
Cyberattacks or data breaches could compromise confidential, business-critical information, cause disruptions in our operations, expose us to potential litigation, or harm our reputation. We have important assets, including intellectual property, trade secrets, and other sensitive, business-critical and/or confidential information which may be vulnerable to such incidents. While we are in the process of implementing a cybersecurity program that is continually reviewed, maintained, and upgraded, no assurance can be made that we are invulnerable to cyberattacks and data breaches which, if significant, could negatively impact our business and financial results.
Cybersecurity breaches and information technology failures could harm our business by increasing our costs and negatively impacting our business operations.
We rely extensively on information technology systems, including internet sites, computer software, data hosting facilities and other hardware and platforms, some of which are hosted by third parties, to assist in conducting our business. Our information technology systems, as well as those of third parties we use in our business operations, may be vulnerable to a variety of evolving cybersecurity risks, such as those involving unauthorized access or control, malicious software, data privacy breaches by employees or others with authorized access, cyber or phishing-attacks, ransomware and other security issues. Moreover, cybersecurity threat actors, whether internal or external, are becoming more sophisticated and coordinated in their attempts to access companies’ information technology systems and data, including the information technology systems of cloud providers and other third parties with whom we conduct our business.
The costs to us to eliminate or alleviate cyber or other security problems, bugs, viruses, worms, malicious software programs and security vulnerabilities could be significant, and our efforts to address these problems may not be successful and could result in interruptions, delays, cessation of service and loss of existing or potential customers that may impede our sales, manufacturing, distribution or other critical functions.
59
We manage and store proprietary information and sensitive or confidential data relating to our business and the businesses of third parties. Breaches of our security measures or the accidental loss, inadvertent disclosure or unapproved dissemination of proprietary information or sensitive or confidential data about us or our partners or customers, including the potential loss or disclosure of such information or data as a result of fraud, trickery or other forms of deception, could expose us, our partners and customers to a risk of loss or misuse of this information; result in regulatory investigations, fines, litigation and potential liability for us; damage our brand and reputation; or otherwise harm our business. In addition, the cost and operational consequences of implementing further data protection measures could be significant. Delayed sales, lower margins or lost customers resulting from these disruptions could adversely affect our financial results, stock price and reputation.
Changes in laws or regulations relating to privacy, information security and data protection, or any actual or perceived failure by us to comply with such laws and regulations or any other obligations, could adversely affect our business.
Personal privacy, information security and data protection are significant issues worldwide. The regulatory framework governing the collection, use, and other processing of personal data and other information is rapidly evolving. The United States federal and various state and foreign governments have adopted or proposed requirements regarding the collection, distribution, use, security and storage of personally identifiable information and other data relating to individuals, and federal and state consumer protection laws are being applied to enforce regulations related to the online collection, use and dissemination of data.
The costs of compliance with and other burdens imposed by laws, regulations, standards and other actual or asserted obligations relating to privacy, data protection and information security may be substantial, and they may require us to modify our data processing practices and policies. Any actual or alleged noncompliance with any of these laws, regulations, standards, and other actual or asserted obligations may lead to claims and proceedings by governmental actors and private parties, and significant fines, penalties or liabilities.
We are subject to taxation-related risks in multiple jurisdictions, and the adoption and interpretation of new tax legislation, tax regulations, tax rulings, or exposure to additional tax liabilities could materially affect our business, financial condition and results of operations.
We are a U.S. parented multinational group subject to income and other taxes in Canada, the United States, the United Kingdom, and other jurisdictions in which we do business. As a result, our provision for (benefit from) income taxes is derived from a combination of applicable tax rates in the various jurisdictions in which we operate. Significant judgment is required in determining our global provision for (benefit from) income taxes, value added and other similar taxes, deferred tax assets or liabilities and in evaluating our tax positions on a worldwide basis. It is possible that our tax positions may be challenged by tax authorities, which may have a significant impact on our global provision for (benefit from) income taxes. If such a challenge were to be resolved in a manner adverse to us, it could have a material adverse effect on our business, financial condition and results of operations.
Recent or future changes to United States, Canadian, United Kingdom and other non-U.S. tax laws could impact the tax treatment of our earnings. For example, the Inflation Reduction Act of 2022, enacted on August 16, 2022, imposes a one-percent non-deductible excise tax on repurchases of stock that are made by U.S. publicly traded corporations on or after January 1, 2023. In addition, as of January 1, 2022, the Tax Cuts and Jobs Act of 2017 requires research and experimental expenditures attributable to research conducted within the United States to be capitalized and amortized ratably over a five-year period. Any such expenditures attributable to research conducted outside the United States must be capitalized and amortized over a 15-year period. We generally conduct our international operations through wholly owned subsidiaries and report our taxable income in various jurisdictions worldwide based upon our business operations in those jurisdictions. The intercompany relationships between our legal entities are subject to complex transfer pricing regulations administered by taxing authorities in various jurisdictions. Although we believe we are compliant with applicable transfer pricing and other tax laws in the United States, Canada, the United Kingdom and other relevant countries, due to changes in such laws and rules, we may have to modify our international structure in the future, which will incur costs and may adversely affect our business, financial condition and results of operations.
If U.S., Canadian, United Kingdom or other non-U.S. tax laws change further, if our current or future structures and arrangements are challenged by a taxing authority, or if we are unable to appropriately adapt the manner in which we operate our business, we may have to undertake further costly modifications to our international structure, which may cause our tax liabilities to increase and adversely affect our business, financial condition and results of operations.
Our ability to use our deferred tax assets to offset future taxable income is subject to certain limitations, which may have a material impact on our business, financial condition or results of operations.
As of June 30, 2023, a valuation allowance has been recorded against our deferred tax assets that are more likely than not to be realized in the U.S. federal and state tax jurisdictions. We assess the available positive and negative evidence to estimate if sufficient future taxable income will be generated to utilize the existing deferred tax assets. Certain of our deferred tax assets may expire unutilized or underutilized, which could prevent us from offsetting future taxable income. We continue to assess the realizability of our deferred tax assets in the future. Future adjustments in our valuation allowance may be required, which may have a material impact on our quarterly and annual operating results.
60
Risks Related to Intellectual Property
If we fail to protect and enforce our intellectual property rights and our confidential information, our business could be adversely affected.
We rely on a combination of nondisclosure agreements and other contractual provisions and patent, trade secret and copyright laws to protect our technologies, products, product development and manufacturing activities from unauthorized use by third parties. Our patents do not cover all of our technologies, systems, products and product components and our competitors or others may design around our patented technologies. We cannot guarantee that these mechanisms will adequately protect our technology and intellectual property, nor can we guarantee that a court will enforce our intellectual property rights.
In addition, the laws and enforcement regimes of certain countries do not protect our technology and intellectual property to the same extent as do the laws and enforcement regimes of the U.S. In certain jurisdictions, we may be unable to protect our technology and intellectual property adequately against unauthorized use, which could adversely affect our business.
Our intellectual property revenues are uncertain and unpredictable in timing and amount.
We are unable to discern a pattern in or otherwise predict the amount of any payments for the sale or licensing of intellectual property that we may receive. Consequently, we are unable to plan on the timing of intellectual property revenues and our results of operations may be adversely affected by a reduction in future estimated intellectual property revenues.
We may become involved in material legal proceedings in the future to enforce or protect our intellectual property rights, which could harm our business.
From time to time, we may identify products that we believe infringes on our patents and may have to initiate litigation to enforce our patent rights against those products. Litigation stemming from such disputes could harm our ability to gain new customers, who may postpone licensing decisions pending the outcome of the litigation or who may, as a result of such litigation, choose not to adopt our technologies. Such litigation may also harm our business relationships with existing customers, who may, because of such litigation, cease making royalty or other payments to us or challenge the validity and enforceability of our patents or the scope of our related agreements.
In addition, the costs associated with legal proceedings are typically high, relatively unpredictable and not completely within our control. These costs may be materially higher than expected, which could adversely impair our working capital, affect our operating results and lead to volatility in the price of our common stock. Whether or not determined in our favor or ultimately settled, litigation would divert managerial, technical, legal and financial resources from our business operations. Furthermore, an adverse decision in any of these legal actions could result in a loss of our proprietary rights, subject us to significant liabilities, require us to seek licenses from others, limit the value of our technology or otherwise negatively impact the price of our common stock, business and financial position, results of operations and cash flows.
Even if we prevail in a legal action, significant contingencies may exist to the settlement and final resolution, including the scope of the liability of each party, our ability to enforce judgments against the parties, the ability and willingness of the parties to make any payments owed or agreed upon, and the dismissal of the legal action by the relevant court, none of which are completely within our control. Parties that may have financial obligations to us could be insolvent or decide to alter their business activities or corporate structure, which could affect our ability to collect royalties from such parties.
Our technologies may infringe on the intellectual property rights of others, which could lead to costly disputes or disruptions.
Various business segments in which we operate are characterized by frequent allegations of intellectual property infringement. Any allegation of infringement could be time consuming and expensive to defend or resolve, result in substantial diversion of management resources, cause suspension of operations or force us to enter into royalty, license, or other agreements rather than dispute the merits of such allegation. Furthermore, third parties making such claims may be able to obtain injunctive or other equitable relief that could block our ability to further develop or commercialize some or all of our technologies, and the ability of our customers to develop or commercialize their products incorporating our technologies, in the U.S. and abroad. If patent holders or other holders of intellectual property initiate legal proceedings, we may be forced into protracted and costly litigation. We may not be successful in defending such litigation and may not be able to procure any required royalty or license agreements on acceptable terms or at all.
61
Risk Related to Industry Adoption of our Products
We cannot provide assurance that markets will accept our various products at the expected market penetration rates, which may adversely affect our business operations and financial position.
We launched our first product, a laser glare protection eyewear named metaAIR®, in March 2019, with a primary focus on the aviation market. We have co-developed this product with Airbus through a strategic partnership. Airbus further extended its support by introducing us to Satair, an Airbus-owned company, which became the global distribution partner for metaAIR® to the aviation market. Since 2016, Airbus and Satair have invested a total of $2,000,000 for the product development and exclusive distribution rights to metaAIR®.
Despite our close collaboration with the Airbus Group and future plans for marketing and sales expansion, there can be no assurance that the aviation market will accept the metaAIR® product at the expected market penetration rates and a slower than forecasted market acceptance may have a material adverse effect on Holography laser glare protection related products and our financial position.
Slower than forecasted market acceptance of Lithography related products, partially in the automotive market, may have a material adverse effect on our financial position.
Our NANOWEB® applications have not yet reached the required manufacturing scale to enable us to address the volume demands of a number of our target vertical markets. We currently have only our first pilot scale, 300mm wide, roll-to-roll line, and we will need to add additional capacity and wider substrates to support our target applications. Broader sales and production are expected to be launched in two to three years’ time after successful completion of automotive and other vertical market product qualification and product introductions. We believe that the automotive market is a strategic high growth opportunity however, despite our close collaboration with automotive partners, there can be no assurance that the automotive market will accept the NANOWEB® product at the expected market penetration rates and a slower than forecasted market acceptance may have a material adverse effect on Lithography de-icing/de-fogging, transparent antenna and other related products and our financial position.
If products incorporating our technologies are used in defective products, we may be subject to product liability or other claims.
If our technology is used in defective or malfunctioning products, we could be sued for damages, especially if the defect or malfunction causes physical harm to people. While we will endeavor to carry product liability insurance, contractually limit our liability and obtain indemnities from our customers, there can be no assurance that we will be able to obtain insurance at satisfactory rates or in adequate amounts or that any insurance and customer indemnities will be adequate to defend against or satisfy any claims made against us. The costs associated with legal proceedings are typically high, relatively unpredictable and not completely within our control. Even if we consider any such claim to be without merit, significant contingencies may exist, similar to those summarized in the above risk factor concerning intellectual property litigation, which could lead us to settle the claim rather than incur the cost of defense and the possibility of an adverse judgment. Product liability claims in the future, regardless of their ultimate outcome, could have a material adverse effect on our business, financial condition and reputation, and on our ability to attract and retain customers.
We participate in markets that are subject to rapid technological change and require significant research and development expenses to develop and maintain products that can achieve market acceptance.
The markets for our products are characterized by:
We operate in a rapidly evolving industry subject to significant technological change and new product introductions and enhancements. Our future performance depends in part on the successful development, introduction and market acceptance of new and enhanced products that address these changes and current and potential customer requirements. To the extent customers defer or cancel orders for existing products due to a slowdown in demand or in the expectation of a new product release, or if there is any delay in development or introduction of our new products or enhancements of our products, our business and financial conditions, results of operations, and growth prospects would be materially adversely affected.
62
We also may not be able to develop the underlying core technologies necessary to create new products and enhancements, or to license these technologies from third parties.
Risks Related to Facilities and Human Resources
We have ongoing environmental costs, which could have a material adverse effect on our financial position or results of operations.
Certain of our operations and assets are subject to extensive environmental, health and safety regulations, including laws and regulations related to waste disposal and remediation of contaminated sites. The nature of our operations and products, including the raw materials we handle, exposes us to the risk of liabilities, obligations or claims under these laws and regulations due to the production, storage, use, transportation and sale of materials that can adversely impact the environment or cause personal injury, including, in the case of chemicals, unintentional releases into the environment. Environmental laws may have a significant effect on the costs of use, transportation and storage of raw materials and finished products, as well as the costs of storage, transportation and disposal of wastes.
The ultimate costs and timing of environmental liabilities are difficult to predict. Liabilities under environmental laws relating to contaminated sites can be imposed retroactively and on a joint and several basis. One liable party could be held responsible for all costs at a site, regardless of fault, percentage of contribution to the site or the legality of the original disposal. We could incur significant costs, including clean-up costs, natural resource damages, civil or criminal fines and sanctions and third-party lawsuits claiming, for example, personal injury and/or property damage, as a result of past or future violations of, or liabilities under, environmental or other laws.
In addition, future events, such as changes to or more rigorous enforcement of environmental laws, could require us to make additional expenditures, modify or curtail our operations and/or install additional pollution control equipment. It is possible that regulatory agencies may enact new or more stringent clean-up standards for chemicals of concern, including chlorinated organic products that we manufacture. This could lead to expenditures for environmental remediation in the future that are additional to existing estimates.
We may incur claims relating to our use, manufacture, handling, storage or disposal of hazardous materials.
Our research and development and manufacturing processes require the transportation, storage and use of hazardous materials, including chemicals, and may result in the generation of hazardous waste. National and local laws and regulations in many of the jurisdictions in which we operate impose substantial potential liability for the improper use, manufacture, handling, storage, transportation and disposal of hazardous materials as well as for land contamination, and, in some cases, this liability may continue over long periods of time. Despite our compliance efforts, we cannot eliminate the risk of industrial accidents that may lead to discharges or releases of hazardous materials and any resultant injury, property damage or environmental contamination from these materials. For example, real properties that we owned or used in the past or that we own or use now or in the future may contain detected or undetected contamination resulting from our operations at those sites or the activities of prior owners or occupants. We may suffer from expenses, claims or liability which may fall outside of or exceed our insurance coverage.
Furthermore, changes to current environmental laws and regulations may impose further compliance requirements on us that may impair our research, development and production efforts as well as our other business activities. New and evolving regulatory requirements include producer responsibility frameworks and regulations related to addressing climate change or other emerging environmental areas. Increased environment, health and safety laws, regulations and enforcement could result in substantial costs and liabilities to us and could subject our use, manufacture, handling, storage, transportation, and disposal of hazardous materials to additional constraints. Consequently, compliance with these laws could result in capital expenditures as well as other costs and liabilities, thereby adversely affecting business, financial position and results of operations.
Our failure to comply with applicable laws and regulations material to our operations, such as export control, environmental and climate related laws and regulations, or the inability to timely obtain requisite approvals necessary for the conduct of our business, such as fab land and construction approvals, could harm our business and operational results or subject us to potential significant legal liability.
Because we engage in manufacturing activities in multiple jurisdictions and conduct business with our customers located worldwide, such activities are subject to a myriad of governmental regulations. Our failure to comply with any such laws or regulations, as amended from time to time, and our failure to comply with any information and document sharing requests from the relevant authorities in a timely manner could result in:
63
Complying with applicable laws and regulations, such as environmental and climate related laws and regulations, could also require us, among other things, to do the following: (a) purchase, use or install remedial equipment; (b) implement remedial programs such as climate change mitigation programs; (c) modify our product designs and manufacturing processes, or incur other significant expenses such as obtaining renewable energy sources, renewable energy certificates or carbon credits, substitute raw materials or chemicals that may cost more or be less available for our operations.
Our inability to timely obtain approvals necessary for the conduct of our business could impair our operational and financial results. For example, if we are unable to timely obtain environmental related approvals needed to undertake the development and construction of a new fab or expansion project, then such inability may delay, limit, or increase the cost of our expansion plans that could also in turn adversely affect our business and operational results. In light of increased public interest in environmental issues, our operations and expansion plans may be adversely affected or delayed responding to public concern and social environmental pressures even if we comply with all applicable laws and regulations.
Delays in setting up facilities or receiving required permits could have an adverse effect on our financial position.
We are in the process of moving into a larger facility suitable to host the scale-up of production relating to Holography and Lithography. Lithography requires specific local government approvals to allow use of certain chemicals and their disposal. Any delay in setting up the facility and receiving permits may impact launch and/or development of related products and may have a material adverse effect on related products and consequently on our financial position.
We are highly dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to successfully manage and grow the business and to develop new products depends, in large part, on our ability to recruit and retain qualified employees, particularly highly skilled technical, sales, service, management, and key staff personnel. Competition for qualified resources is intense and other companies may have greater resources available to provide substantial inducements and to offer more competitive compensation packages. If we are not successful in attracting and retaining highly qualified personnel, it could have a material adverse effect on our business, financial condition, and results of operations.
Our future success depends on the continued service of management and key personnel and our ability to identify, hire and retain additional personnel.
Our success depends upon our ability to attract and retain highly skilled technical, managerial, marketing and finance personnel, and, to a significant extent, upon the efforts and abilities of our CEO and CFO and other members of senior management. The resignation or departure of our key personnel, including the CEO and CFO, could adversely affect our business, financial condition, and operations. These individuals play crucial roles in our strategic decision-making, day-to-day operations and financial management. Their departure may lead to uncertainties, loss of expertise, and potential disruptions in our business continuity. Additionally, attracting and retaining qualified replacements for such key positions may be challenging, and any delays or difficulties in finding suitable successors could further impact our operations. If we lose the services of or fail to recruit key management personnel, our business could be harmed.
Our results of operations could be adversely affected by labor shortages, turnover, labor cost increases and inflation.
A number of factors may adversely affect the labor force available to us in one or more of our geographies, including high employment levels, increasing market wages and other compensation costs, federal unemployment subsidies, and other government regulations, which include laws and regulations related to workers’ health and safety, wage and hour practices and immigration. These factors can also impact the cost of labor. Increased turnover rates within our employee base can lead to decreased efficiency and increased costs, such as increased overtime to meet demand and increased wage rates to attract and retain employees.
64
An overall labor shortage or lack of skilled labor, increased turnover or labor inflation could have a material adverse effect on results of operations.
Certain directors and officers may be subject to conflicts of interest.
Certain of our directors and officers may be involved in other business ventures through their direct and indirect participation in corporations, partnerships, joint ventures, etc. that may become potential competitors of the technologies, products and services we intend to provide. Situations may arise in connection with potential acquisitions or opportunities where the other interests of these directors' and officers' conflict with or diverge from our interests. In accordance with applicable corporate law, directors who have a material interest in or who are a party to a material contract or a proposed material contract with us are required, subject to certain exceptions, to disclose that interest and generally abstain from voting on any resolution to approve the contract. In addition, the directors' and officers' are required to act honestly and in good faith with a view to our best interests. However, in conflict-of-interest situations, our directors and officers may owe the same duty to another company and will need to balance their competing interests with their duties to us. Circumstances (including with respect to future corporate opportunities) may arise that may be resolved in a manner that is unfavorable to us.
Risks Related to Legal Matters
We are, and may in the future become, subject to various legal proceedings and claims that arise in or outside the ordinary course of business and which could adversely affect our business.
We are, and may in the future become, subject to various legal proceedings and claims that arise in or outside the ordinary course of business. We cannot predict the outcome of these proceedings or provide an estimate of potential damage, if any. We believe that the claims in the securities class actions are without merit and intend to defend against them vigorously. Regardless, failure by us to obtain a favorable resolution of the claims set forth in the complaints could require us to pay damage awards or otherwise enter into settlement arrangements for which our insurance coverage may be insufficient. Any such damage awards or settlement arrangements in current or future litigation could have a material adverse effect on our business, operating results or financial condition. Even if plaintiffs’ claims are not successful, defending against class action litigation is expensive and could divert management’s attention and resources, all of which could have a material adverse effect on our financial condition and operations, operating results and financial condition and negatively affect the price of our common stock. In addition, such lawsuits may make it more difficult for us to finance our operations in the future. See Note 21, Commitments and contingencies, to the condensed consolidated interim financial statements of this Form 10-Q. in the notes to the condensed consolidated interim financial statements that are included elsewhere in this Quarterly Report on Form 10-Q for more information regarding our legal proceedings.
We and a former CEO and our current CEO (the “CEOs”) have received “Wells Notices” from the SEC staff recommending that the SEC bring enforcement actions against us and the CEOs which could have a material adverse effect on our business, financial condition and results of operations, prospects, and/or our stock price.
In September 2021, we received a subpoena from the SEC, Division of Enforcement, in a matter captioned In the Matter of Torchlight Energy Resources, Inc. The subpoena requested that we produce certain documents and information related to, among other things, the merger involving Torchlight Energy Resources, Inc. and Metamaterial Inc. (the “Investigation”). On July 20, 2023, the enforcement staff of the SEC provided us, our former Chief Executive Officer, John Brda, and our current Chief Executive Officer, George Palikaras, with “Wells Notices” relating to the Investigation. The Wells Notices each state that the SEC staff has made a preliminary determination to recommend that the SEC file a civil enforcement action against the recipients alleging violations of certain provisions of the U.S. federal securities laws. Specifically, the Wells Notice received by the Company states that the proposed action would allege violations of Section 17(a) of the Securities Act; Sections 10(b), 13(a), 13(b)(2)(A), 13(b)(2)(B) and 14(a) of the Exchange Act of 1934 and Rules 10b-5 and 14a-9 thereunder; and Regulation FD.
Although a Wells Notice is neither a formal charge of wrongdoing nor a final determination that the recipient has violated any law, it is a formal notice that the SEC intends to bring an enforcement action against the recipient. If the SEC were to authorize an action against us and/or any of the individuals named above, it could seek an injunction against future violations of provisions of the federal securities laws, the imposition of civil monetary penalties, and other equitable relief within the SEC’s authority. The SEC could also seek an order barring the individuals from serving as an officer or director of a public company. In addition, the SEC could seek disgorgement of an amount from us that may exceed our ability to pay.
No financial impact of a loss contingency has been recognized in the condensed consolidated interim financial statements, as this matter does not meet the required criteria of being both probable and reasonably estimated at this time. At this time, we cannot predict with certainty the outcome of the Investigation, the Wells Notice process or any corresponding enforcement action against us or any of the individuals discussed above. The Investigation and the Wells Notice process may be expensive and disruptive, and we are subject to indemnifying each of the CEOs for their costs associated with the Wells Notices.
65
Our insurance, to the extent maintained, may not cover all claims that may be asserted against us or the CEOs, and we are unable to predict how long the Investigation will continue. An unfavorable outcome may have an adverse impact on our business, financial condition and results of operations, prospects, or our stock price. Any proceeding could also negatively impact our reputation among our stakeholders.
We are exposed to various risks related to the regulatory environment.
We are subject to various risks related to: (1) new, different, inconsistent or even conflicting laws, rules and regulations that may be enacted by legislative bodies and/or regulatory agencies in the countries in which we operate; (2) disagreements or disputes between national or regional regulatory agencies; and (3) the interpretation and application of laws, rules and regulations. If we are found by a court or regulatory agency not to be in compliance with applicable laws, rules or regulations, our business, financial condition and results of operations could be materially and adversely affected.
The scope, determination, and impact of claims, lawsuits, government and regulatory investigations, enforcement actions, disputes, and proceedings to which we are subject cannot be predicted with certainty, and may result in:
We may be affected by environmental laws and regulations.
We are subject to a variety of laws, rules and regulations related to the use, storage, handling, discharge and disposal of certain chemicals and gases used in our manufacturing process. Any of those regulations could require us to acquire expensive equipment or to incur substantial other expenses to comply with them. If we incur substantial additional expenses, product costs could significantly increase. Failure to comply with present or future environmental laws, rules and regulations could result in fines, suspension of production or cessation of operations.
Our insurance coverage strategy may not be adequate to cover all liabilities wo which we may be subject.
We will require insurance coverage for numerous risks related to our business, including our current and future litigations. Although our management believes that the events and amounts of liability covered by our insurance policies will be reasonable, taking into account the risks relevant to our business, and the fact that agreements with users contain limitations of liability, there can be no assurance that such coverage will be available or sufficient to cover claims to which we may become subject. If insurance coverage is unavailable or insufficient to cover any such claims, our financial resources, results of operations and prospects could be adversely affected. For example, a possible monetary penalty as a result of the aforementioned SEC investigation may exceed the insured limit which will adversely affect our financial condition and cash flows.
Risks Related to our Common Stock
Future sales and issuances of a substantial number of shares of our common stock or rights to purchase common stock by our stockholders in the public market could result in additional dilution of the percentage ownership of our stockholders and cause our stock price to fall.
66
If our stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market, the trading price of our common stock could decline.
We have and may continue to issue equity, convertible securities or other securities to investors in public and private offerings. In addition, we currently have effective resale shelf registration statements which enable the selling stockholders thereunder to sell shares in the public market pursuant thereto.
We also have outstanding as of June 30, 2023, 123,254,153 warrants to purchase 123,254,153 shares of our common stock at a weighted average exercise price of $1.56 per share. These warrants include 83,333,334 warrants issued in the April 2023 registered direct offering with an exercise price of $0.375 per share which is subsequently reduced to $0.174 per share based on the down round provision in the case of a Share Combination Event or a Dilutive Issuance as described in more detail in Note 12, Capital stock, in the Notes to the condensed consolidated interim financial statements of this Quarterly Report on Form 10-Q. The down round provision of the warrants issued in April 2023 allows the warrants holder to obtain our common stock with a lower price, which may cause significant dilution to existing shareholders, and otherwise have a material adverse effect on us. All of the warrants issued and outstanding as of June 30, 2023 are eligible for exercise.
Further, additional capital will be needed in the future to continue our planned operations, including commercialization efforts, expanded research and development activities and costs associated with operating a public company. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner, we determine from time to time. If we sell common stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our common stock. Additional issuances and sales of our common stock, including shares of our common stock available for issuance to our employees, directors and consultants, or a perception that such shares will be sold in the public market, could result in additional dilution and the trading price of our common stock could decline.
You may experience dilution as a result of future equity offerings or other equity issuances.
We will have to raise additional capital in the future. To raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may be lower than the price you paid per share. In addition, investors purchasing shares or other securities in the future could have rights superior to those of other investors. Any such issuance could result in substantial dilution to investors.
Our failure to satisfy certain Nasdaq listing requirements may result in our common stock being delisted from the Nasdaq Capital Market, which could eliminate the trading market for our common stock.
On March 20, 2023, we received written notice (“The Bid Price Letter”) from The Nasdaq Stock Market LLC, or Nasdaq, indicating that we are not in compliance with the $1.00 minimum bid price requirement for continued listing on The Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Rule”). In accordance with Nasdaq Listing Rule 5810(c)(3)(A), we have a period of 180 calendar days, or until September 18, 2023, to regain compliance with the Bid Price Rule. To regain compliance, the closing bid price of our common stock must meet or exceed $1.00 per share for a minimum of ten consecutive business days during this 180-day period. The Bid Price Letter was a notice of deficiency, not delisting, and does not currently affect the listing or trading of shares of our common stock on The Nasdaq Capital Market, which continues to trade under the symbol “MMAT.” We intend to continue actively monitoring the closing bid price of shares of our common stock and may, if appropriate, consider implementing available options to regain compliance with the Bid Price Rule.
If we do not regain compliance within the allotted compliance periods, including any extensions that may be granted by Nasdaq, Nasdaq will provide notice that our common stock will be subject to delisting. We would then be entitled to appeal that determination to a Nasdaq hearings panel. If the stock is delisted, we may trade on the over-the-counter market, or even in the pink sheets, which would significantly decrease the liquidity of an investment in our common stock. In addition, the stock may be deemed to be penny stock. If our common stock is considered penny stock, we would be subject to rules that impose additional sales practices on broker-dealers who sell our securities. For example, broker-dealers would have to make a special suitability determination for the purchaser and have received the purchaser’s written consent to the transaction prior to sale. Also, a disclosure schedule must be prepared prior to any transaction involving a penny stock and disclosure is required about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Monthly statements are also required to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock. Because of these additional obligations, some brokers may be unwilling to effect transactions in penny stocks. This could have an adverse effect on the liquidity of our common stock and the ability of investors to sell our common stock.
Subject to various spending levels approved by our board of directors, our management will have broad discretion in the use of the net proceeds from our capital raises and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from our capital raises, and our stockholders will not have the opportunity as part of their investment decision to assess whether the net proceeds from our capital raises are being used appropriately.
67
Because of the number and variability of factors that will determine our use of the net proceeds from our capital raises, their ultimate use may vary substantially from their currently intended use. You may not agree with our decisions, and our use of the proceeds from our capital raises may not yield any return to stockholders. Our failure to apply the net proceeds of our capital raises effectively could compromise our ability to pursue our growth strategy and we might not be able to yield a significant return, if any, on our investment of those net proceeds. Stockholders will not have the opportunity to influence our decisions on how to use our net proceeds from our capital raises. Pending their use, we may invest the net proceeds from our capital raises in interest and non-interest-bearing cash accounts, short-term, investment-grade, interest-bearing instruments and U.S. government securities. These temporary investments are not likely to yield a significant return.
An active, liquid and orderly trading market may not be sustained for our common stock, and, as a result, it may be difficult for you to sell your shares of our common stock.
The trading market for our common stock on the Nasdaq Capital Market may not be sustained. If the market for our common stock is not sustained, it may be difficult for you to sell your shares of common stock at an attractive price or at all. We cannot predict the prices at which our common stock will trade. It is possible that in one or more future periods our results of operations may not meet the expectations of public market analysts and investors, and, as a result of these and other factors, the price of our common stock may fall.
If equities or industry analysts do not publish research or reports about our company, or if they issue adverse or misleading opinions regarding us or our stock, our stock price and trading volume could decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us or our business. If no or few analysts commence coverage of us or if such coverage is not maintained, the market price for our stock may be adversely affected. Our stock price also may decline if any analyst who covers us issues an adverse or erroneous opinion regarding us, our business model, our intellectual property or our stock performance, or if our operating results fail to meet analysts’ expectations. If one or more analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline and possibly adversely affect our ability to engage in future financing.
The market price of our common stock has been and may continue to be volatile, and the value of your investment could decline significantly.
The trading price of our common stock has been and is likely to continue to be volatile. The trading price of our common stock since June 28, 2021 (the date of completion of the Arrangement) up to June 30, 2023, has ranged from a high of $7.96 to a low of $0.18. Factors that have caused, and could continue to cause, fluctuations in the trading price of our common stock include, but are not limited to, the following:
68
The stock market in general, and market prices for the securities of similar companies in particular, have from time to time experienced volatility that often has been unrelated to the operating performance of the underlying companies. These broad market and industry fluctuations may adversely affect the market price of our common stock, regardless of our operating performance, which might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate. In several recent situations when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation. If any of our stockholders were to bring a lawsuit against us, the defense and disposition of the lawsuit could be costly and divert the time and attention of our management and materially adversely affect our results of operations. We currently have ongoing lawsuits. See Part 1, Item 3, "Legal Proceedings" in this Annual Report on Form 10-K for more information regarding these lawsuits and the SEC’s investigation.
We may issue preferred stock whose terms could adversely affect the voting power or value of our common stock.
Our board of directors is authorized, without further stockholder action, and subject to Nasdaq rules, to issue preferred stock in one or more series and to designate the dividend rate, voting rights and other rights, preferences and restrictions of each such series. The terms of one or more classes or series of preferred stock could adversely impact the voting power or value of our common stock. Also, we might grant holders of preferred stock the right to elect some number of our directors in all events or on the happening of specified events or the right to veto specified transactions. Similarly, the repurchase or redemption rights or liquidation preferences we might assign to holders of preferred stock could affect the residual value of our common stock.
Anti-takeover provisions in our articles of incorporation and bylaws, as amended, as well as provisions in Nevada law, might discourage, delay, or prevent a change of control of us or changes in our management and, therefore, depress the trading price of our securities.
Our articles of incorporation and bylaws, as amended, as well as provisions in Nevada law, contain provisions that could have the effect of rendering more difficult or discouraging an acquisition deemed undesirable by our board. Our corporate governance documents include provisions:
These provisions, alone or together, could delay hostile takeovers and changes in control or changes in our management.
As a Nevada corporation, we are also subject to provisions of Nevada corporate law, including Section 78.411, et seq. of the Nevada Revised Statutes, which, among other things, prohibits a publicly-held Nevada corporation from engaging in a business combination with an interested stockholder, generally a person which together with its affiliates owns, or within the last two years has owned, 10% of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner, and, unless otherwise provided in our articles of incorporation or by-laws, restricts the ability of an acquiring person to obtain a controlling interest of 20% or more of our voting shares. Our articles of incorporation and by-laws, as amended, do not contain any provision which would currently keep the change of control restrictions of Section 78.378 from applying to it.
69
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of us, thereby reducing the likelihood that our stockholders could receive a premium for their common stock in an acquisition.
We are a smaller reporting company. We cannot be certain whether the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors or otherwise limit our ability to raise additional funds.
As of June 30, 2023, we are a “smaller reporting company” under applicable U.S. securities regulations. A smaller reporting company is a company that, as of the last business day of its most recently completed second fiscal quarter, has (i) an aggregate market value of the company’s voting stock held by non-affiliates, or public float, of less than $250 million or (ii) less than $100 million in revenue and less than $700 million in public float. In addition, a smaller reporting company is able to provide simplified executive compensation disclosures in our filings and has certain other reduced disclosure obligations in our SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Reduced disclosure in our SEC filings due to our status as a smaller reporting company may make it harder for investors to analyze our results of operations and financial prospects.
We have not paid cash dividends in the past and have no immediate plans to pay dividends.
Our current plan is to reinvest earnings, if any, to cover operating costs and otherwise remain competitive. We do not plan to pay any cash dividends with respect to our securities in the foreseeable future. We cannot assure you that we would, at any time, generate sufficient surplus cash that would be available for distribution to the holders of our common stock as a dividend. Therefore, you should not expect to receive cash dividends on our common stock.
Risks Related to the Spin-Off
Our spin-off of our oil and gas operations could be challenged under various state and federal fraudulent transfer laws.
In December 2022, we distributed all of our 165,472,241 outstanding shares of common stock of Next Bridge to holders of our Series A Non-Voting Preferred Stock on a pro rata basis, or the Spin-Off. It is possible that an unpaid creditor of Next Bridge or an entity vested with the power of such creditor (such as a trustee or debtor-in-possession in a bankruptcy) could claim under various state and federal fraudulent conveyance laws that the Spin-off left Next Bridge insolvent or with unreasonably small capital or that we intended or believed Next Bridge would incur debts beyond its ability to pay such debts as they mature. If a court were to agree with such a plaintiff, then such a court could void the Spin-off as a fraudulent transfer and seek recovery of Next Bridge’s liabilities from us. No assurance can be given as to what standard a court would apply to determine insolvency or that a court would determine that Next Bridge was insolvent at the time of or after giving effect to the Spin-off. Were a court to decide that the Spin-off was a fraudulent transfer and that we are responsible for any part of Next Bridge’s liabilities, our financial condition and results of operations could be materially and adversely affected.
Public attention to and inquiries regarding the “naked” short selling of our previously outstanding Series A Preferred Stock may negatively affect the trading value of our common stock.
Published reports assert that traders engaged in widespread violations of Regulation SHO by effecting “naked” short selling of our Series A Preferred Stock prior to the Spin-Off. The circumstances around the trading of the Series A Preferred Stock have generated significant public interest. We believe that the Financial Institutions Regulatory Authority and other entities may be reviewing the occurrence of naked short selling in general and possibly naked short selling in our Series A Preferred Stock in particular. The resulting public attention may make investors less willing to buy our common stock and could negatively affect the trading price of our common stock.
General Risk Factors
We are exposed to fluctuations in currency exchange rates.
Our majority of revenues are denominated in U.S. dollars which are recognized in the entity located outside of the United States, and therefore are exposed to significant currency exchange fluctuations. Recent events in the global financial markets have been coupled with increased volatility in the currency markets. Fluctuations in the exchange rate between the U.S. dollar, the Canadian dollar and the British Pound may have a material adverse effect on our business, financial condition, and operating results. We may, in the future, establish a program to hedge a portion of our foreign currency exposure with the objective of minimizing the impact of adverse foreign currency exchange movements.
70
With appropriate risk management and oversight this may be able to offset future risk, however a hedging strategy will result in additional operating costs.
Uncertain global macroeconomic conditions could adversely affect our results of operations and financial condition.
Uncertain global macroeconomic conditions that affect the economy and the economic outlook of the United States, Canada, Europe, UK and other parts of the world could adversely affect our customers and vendors, which could adversely affect our results of operations and financial condition. These uncertainties, including, among other things, sovereign and foreign bank debt levels, the inability of national or international political institutions to effectively resolve economic or budgetary crisis or issues, trade disputes or changes in trading rules and tariffs between nations, consumer confidence, unemployment levels, interest rates, availability of capital, fuel and energy costs, tax rates, and the threat or outbreak of terrorism or public unrest, could adversely impact our customers and vendors, which could adversely affect us. Recessionary conditions and depressed levels of consumer and commercial spending may cause customers to reduce, modify, delay or cancel plans to purchase our products and may cause vendors to reduce their output or change their terms of sales. We generally sell products to customers with credit payment terms. If customers’ cash flow or operating or financial performance deteriorates, or if they are unable to make scheduled payments or obtain credit, they may not be able to pay, or may delay payment to us. Likewise, for similar reasons vendors may restrict credit or impose different payment terms. Any inability of current or potential customers to pay us for our products or any demands by vendors for different payment terms may adversely affect our results of operations and financial condition.
Operating as a public company requires us to incur substantial costs and requires substantial management attention. In addition, certain members of our management team have limited experience managing a public company.
As a public company, we incur substantial legal, accounting and other expenses that we did not incur as a private company. For example, we are subject to the reporting requirements of the Exchange Act, the applicable requirements of the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the rules and regulations of the SEC and the listing standards of the Nasdaq Stock Market. For example, the Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business, financial condition and results of operations. We are also required to maintain effective disclosure controls and procedures and internal control over financial reporting. Compliance with these rules and regulations has increased and will continue to increase our legal and financial compliance costs and increase demand on our systems. In addition, as a public company, we may be subject to stockholder activism, which can lead to additional substantial costs, distract management and impact the manner in which we operate our business in ways we cannot currently anticipate. As a result of disclosure of information in filings required of a public company, our business and financial condition will become more visible, which may result in threatened or actual litigation, including by competitors.
Additionally, certain members of our management team have limited experience managing a publicly traded company, interacting with public company investors and complying with the increasingly complex laws pertaining to public companies. Our management team may not successfully or efficiently manage our transition to being a public company subject to significant regulatory oversight and reporting obligations under the federal securities laws and the continuous scrutiny of securities analysts and investors. These obligations and constituents will require significant attention from our senior management and could divert their attention away from the day-to-day management of our business, which could adversely affect our business, financial condition and results of operations.
Our results of operations could vary as a result of the methods, estimates, and judgments that we use in applying our accounting policies.
The methods, estimates, and judgments that we use in applying our accounting policies have a significant impact on our results of operations (see “Critical Accounting Policies and Estimates” in Part II, Item 7 of the Form 10-K/A). Such methods, estimates and judgments are, by their nature, subject to substantial risks, uncertainties and assumptions, and factors may arise over time that lead us to change our methods, estimates and judgments. Changes in those methods, estimates and judgments could significantly affect our results of operations.
Increased scrutiny of our environmental, social and governance responsibilities and practices may result in additional costs, liability risks, and may adversely impact our reputation, our ability to attract and retain a skilled workforce and willingness of customers and suppliers to do business with us.
Investor advocacy groups, institutional investors, proxy advisory services, stockholders, government, regulators, employees, customers and other stakeholders are increasingly focused on environmental, social and governance (“ESG”) practices of companies. Additionally, public interest and legislative pressure related to public companies’ ESG practices continues to grow. If our ESG practices fail to meet regulatory requirements or investor or other stakeholders' evolving expectations and standards for responsible business practices in numerous areas, including climate change and greenhouse gas emissions, environmental stewardship, support for communities where we operate, human and civil rights, director and employee diversity, human capital management, employee health and safety practices, product quality and safety, data security, supply chain management, regulatory compliance corporate governance and transparency and employing ESG strategies within business operations, our brand, reputation and employee retention may be negatively impacted and customers and suppliers may be unwilling to do business with us.
71
As we work to align our ESG practices with industry standards, we will be dealing with uncertainties and risks resulting from the forward-looking nature of many ESG issues, In addition, we will continue to expand our disclosures in these areas and doing so may result in additional costs and require additional resources to monitor, report, and comply with our various ESG practices. If we fail to adopt ESG standards or practices as quickly as stakeholders desire, report on our ESG efforts or practices accurately, or satisfy the expectations of stakeholders, our reputation, business, financial performance and growth may be adversely impacted.
72
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
None.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
On August 7, 2023, we entered into the Loan Sale Agreement with Gregory McCabe.
The Loan Sale Agreement provides for the nonrecourse transfer and assignment to Mr. McCabe of all of the Company’s rights and interests, and the assumption by Mr. McCabe of all of the Company’s obligations, under (i) the loan and extension of credit (the “Secured Loan”) made under and pursuant to, and evidenced by, that certain 8% Secured Promissory Note made by Next Bridge, in favor of us, with an original issue date of October 1, 2021 (as amended and in effect on August 7, 2023, the “Secured Note”), with an aggregate balance of principal and accrued interest of $17,109,444.52, (ii) certain loans under the Loan Agreement, dated as of September 2, 2022 (as amended from time to time, the “Loan Agreement”) among NBH, Torchlight, Torchlight Hazel LLC, a Texas limited liability company, or Hazel, Hudspeth Oil Corporation, a Texas corporation, or Hudspeth Oil, Wolfbone and Hudspeth Operating, LLC, a Texas limited liability company, or Hudspeth Operating, and together with NBH, Torchlight Energy, Hazel, Hudspeth Oil and Wolfbone, the Borrowers, with an aggregate balance of principal and accrued interest of $6,847,521.43, and (iii) the Loan Documents (as defined in the Loan Sale Agreement, and collectively with the Secured Note and the Loan Agreement, or the Assigned Loan Interests). Under the terms of the Loan Sale Agreement, Mr. McCabe has acquired the Assigned Loan Interests without warranty or recourse and has assumed all risks of non-collection.
The Assigned Loan Interests were assigned to Mr. McCabe in consideration of the payment to us by Mr. McCabe of $6,000,000. In addition, the parties agreed to negotiate and execute a stock purchase agreement, escrow agreement and related transaction documents no later than August 28, 2023, pursuant to which (i) upon a monthly put by us, Mr. McCabe will agree to purchase an aggregate of $6,000,000 of shares of our common stock (beginning on September 1, 2023, or as soon as possible after such date, in monthly amounts of $250,000 for the first six months and in monthly amounts of $500,000 for the next nine months thereafter), at a price per share equal to the 5-day VWAP on the trading day preceding the date of each such purchase, (ii) an escrow agent would hold all of the shares of common stock of our common stock purchased by Mr. McCabe with any portion of the $6,000,000 until the last payment is made, and (iii) in the event of a default under such agreement, Mr. McCabe will forfeit to us all of the shares of our common stock held by the escrow agent. In addition, Mr. McCabe will have a call option to purchase the then-remaining balance of the $6,000,000 of shares of our common stock at a purchase price per share equal to 120% of the 5-day VWAP on the trading day preceding the date of Mr. McCabe’s exercise of the call option.
From August 7, 2023 until Mr. McCabe has fulfilled the obligations set forth in the paragraph above, without our written consent, Mr. McCabe may not (i) sell, assign or otherwise transfer or (ii) create, incur, assume or permit to exist any lien or security interest on, the shares of our common stock which Mr. McCabe owned as of August 7, 2023 and will place such shares in escrow. If upon any put by us described in the paragraph above, Mr. McCabe is unable to provide funds to close on the purchase of such shares of our common stock within two trading days, then Mr. McCabe will be required to release from escrow and return to us that number of shares for which Mr. McCabe failed to provide such funds, with the number of shares of our common stock based on the per share purchase price that would have been applied for such month.
The foregoing description of the Loan Sale Agreement is not complete and is qualified in its entirety by reference to the full text of the Loan Sale Agreement which is filed as Exhibit 10.11 to this Current Report on Form 10-Q and incorporated herein by reference.
73
Item 6. Exhibits
Furnish the exhibits required by Item 601 of Regulation S-K (§ 229.601 of this chapter).
|
|
|
|
Incorporated by Reference |
||||
|
Exhibit
|
|
Exhibit Description
|
|
Form |
|
Exhibit |
|
Filing Date |
3.1.0 |
|
|
10-K |
|
3.1 |
|
18-Mar-19 |
|
3.1.1 |
|
Certificate of Amendment to Articles of Incorporation dated December 10, 2014 |
|
10-Q |
|
3.2 |
|
15-May-15 |
3.1.2 |
|
Certificate of Amendment to Articles of Incorporation dated September 15, 2015 |
|
10-Q |
|
3.3 |
|
12-Nov-15 |
3.1.3 |
|
Certificate of Amendment to Articles of Incorporation dated August 18, 2017. |
|
10-Q |
|
3.4 |
|
9-Nov-18 |
3.1.4 |
|
Amendment to the Articles of Incorporation of Torchlight Energy Resources, Inc., dated June 14, 2021 |
|
8-K |
|
3.1 |
|
16-Jun-21 |
3.1.5 |
|
Certificate of Amendment related to the Reverse Stock Split and Name Change, filed June 25, 2021 |
|
8-K |
|
3.1 |
|
29-Jun-21 |
3.2.0 |
|
|
8-K |
|
3.3 |
|
16-Jun-21 |
|
3.5.0 |
|
|
8-K |
|
3.1 |
|
26-Oct-16 |
|
4.1 |
|
|
8-K |
|
4.1 |
|
14-Apr-23 |
|
10.1 |
|
First Amendment to Meta Materials Inc. - Next Bridge Loan Agreement, dated as of December 21, 2022 |
|
8-K |
|
2.1 |
|
4-Apr-23 |
10.2 |
|
Second Amendment to Meta Materials Inc. - Next Bridge Loan Agreement, dated as of March 31, 2023 |
|
8-K |
|
2.2 |
|
4-Apr-23 |
10.3 |
|
|
8-K |
|
2.3 |
|
4-Apr-23 |
|
10.4 |
|
|
8-K |
|
2.4 |
|
4-Apr-23 |
|
10.5 |
|
|
8-K |
|
10.1 |
|
18-Apr-23 |
|
10.6 |
|
Employment Agreement with Uzi Sasson, dated as of April 20, 2023 |
|
|
|
|
|
Filed Herewith |
10.7 |
|
Employment Agreement with D. Daniel Eaton, dated as of June 27, 2023 |
|
|
|
|
|
Filed Herewith |
10.8 |
|
|
|
|
|
|
Filed Herewith |
|
10.9 |
|
Separation Agreement with Jonathan Waldern, dated as of June 14, 2023 |
|
|
|
|
|
Filed Herewith |
10.10 |
|
End of Employment Letter with Jonathan Waldern, dated as of April 25, 2023 |
|
|
|
|
|
Filed Herewith |
10.11 |
|
|
|
|
|
|
Filed Herewith |
|
31.1 |
|
|
|
|
|
|
Filed Herewith |
|
31.2 |
|
|
|
|
|
|
Filed Herewith |
|
32.1+ |
|
|
|
|
|
|
Furnished Herewith |
|
32.2+ |
|
|
|
|
|
|
Furnished Herewith |
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
|
|
|
|
|
Filed Herewith |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
Filed Herewith |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
Filed Herewith |
74
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
Filed Herewith |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
Filed Herewith |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
Filed Herewith |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
|
|
Filed Herewith |
+ |
The certifications attached as Exhibit 32.1 that accompany this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of the registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Quarterly Report on Form 10-Q, irrespective of any general incorporation language contained in such filing |
75
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
Meta Materials Inc. |
|
|
|
|
|
Dated: August 9, 2023 |
|
By: |
/s/ George Palikaras
|
|
|
|
George Palikaras |
|
|
|
President and Chief Executive Officer |
|
|
|
(Principal Executive Officer) |
|
|
|
|
Dated: August 9, 2023 |
|
By: |
/s/ Uzi Sasson
|
|
|
|
Uzi Sasson |
|
|
|
Chief Financial Officer and Chief Operating Officer |
|
|
|
(Principal Financial and Accounting Officer) |
76
Exhibit 10.6
METAMATERIAL TECHNOLOGIES USA INC.
EXECUTIVE EMPLOYMENT AGREEMENT
This Employment Agreement (this “Agreement”) is entered into as of April 20, 2023 (the “Effective Date”), by and between Metamaterial Technologies USA Inc. (the “Company”) and Uzi Sasson (“Executive”) (collectively referred to as the “Parties” or individually referred to as a “Party”). The terms of this Agreement and Employee’s employment with the Company will be effective as of the Effective Date.
RECITALS
WHEREAS, the Company is a wholly owned subsidiary of Meta Materials Inc. (“Parent”) (together with any sister corporations or affiliates of the Company, the “Company Group”);
WHEREAS, the Company wishes to retain the services of Executive performed for the Company Group and to enter into an agreement embodying the terms of such employment; and
WHEREAS, the Executive desires to accept such employment on the terms and subject to the conditions set forth in this Agreement.
AGREEMENT
NOW THEREFORE, in consideration of the foregoing recital and the respective undertakings of the Company and Executive set forth below, the Company and Executive agree as follows:
If to the Company:Metamaterial Technologies USA Inc.Attn: George Palikaras, President & CEO1 Research Drive, Dartmouth, Nova Scotia, Canada B2Y 4M9 If to Executive:at the last residential address known by the Company.
[intentionally blank; signature page follows]
IN WITNESS WHEREOF, each of the parties has executed this Agreement, in the case of the Company by their duly authorized officers, as of the day and year set forth below.
COMPANY:
METAMATERIAL TECHNOLOGIES USA INC.
By:/s/ George Palikaras Date:
George Palikaras
President and Chief Executive Officer
EXECUTIVE:
/s/ Uzi Sasson Date:
Uzi Sasson
Chief Financial Officer and Chief Operating Officer
[SIGNATURE PAGE TO EXECUTIVE EMPLOYMENT AGREEMENT]
Metamaterial Technologies USA, Inc
a subsidiary of META® Materials Inc., a Nevada Corporation,
headquartered in Nova Scotia, Canada
META Materials Inc.
5880 W. Las Positas Blvd, Suite 37
Pleasanton, California 94588
United States of America
Exhibit 10.7
June 27, 2023
David D. Eaton
[ ].com
Subject:Employment Offer – Chief Legal Officer
Dear Mr. Eaton,
On behalf of Metamaterial Technologies USA, Inc. (“MTI-US”), a wholly-owned subsidiary of Meta Materials Inc. (“META”), we are pleased to offer you a permanent, full-time position as a Chief Legal Officer. Should you decide to join us, you will become part of a fast-paced and dedicated team that works together to provide our clients with the highest possible level of service and product engineering.
As a member of our team, we would ask for your commitment to deliver outstanding quality and results that exceed client expectations. In addition, we expect your personal accountability in all the products, actions, advice and results that you provide as a representative of MTI-US. In return, we are committed to providing you with every opportunity to learn, grow and stretch to the highest level of your ability and potential.
We are confident that you will find this new opportunity both challenging and rewarding. The following points outline the terms that we are proposing.
1. Position title: Chief Legal Officer
2. Job description: You will perform the duties and have the responsibilities and authority customarily performed and held by that of a Chief Legal Officer, more particularly as set out in Appendix A attached hereto.
3. Start date: MTI-US requests that you begin work in this new position as soon as possible and, in any event, no later than July 12th, 2023 (or as soon as possible).
4. Hours of work: Core working hours will be defined by your reporting manager and you will perform your duties within those times. Normal hours of service is forty (40) hours per week.
5. Reporting relationship: You will be reporting to George Palikaras,CEO, working out of our MTI-US’s facilities in Pleasanton, California or remotely.
6. Salary: $285,000 per annum (the “Base Salary”).
60 HIGHFIELD PARK DRIVE I SUITE 102 I DARTMOUTH I NOVA SCOTIA I B3A 4R9 I CANADA I NASDAQ: MMAT I METAMATERIAL.COM
7. Vacation: You will be eligible for 20 days paid vacation, pro-rated for partial calendar year if appropriate.
8. Group benefits: Our standard PPO Medical Insurance (health, dental and vision) is also provided, in accordance with MTI-US’s current benefit plans in place from time to time, subject to the terms and conditions and coverage limitations of such plans. We also offer a wellness allowance of $250 per year to be used towards gym membership, sports equipment, or nutritional consultation. We reserve the right to modify, supplement and/or change the benefits plan from time to time.
9. Initial Option: At the first meeting of the Board following the Effective Date, the Company will recommend that Employee be granted a stock option to purchase 320,000 shares of Parent common stock (the “Initial Option”), subject to Employee’s continued employment through the grant date. The Initial Option shall have an exercise price equal to the fair market value of a share of Company’s common stock on the date of grant. The Company will recommend that the shares subject to the Initial Option be scheduled to vest 25% annually over four years, subject to Employee’s continued service with the Company through each vesting date. The Initial Option will be subject to the terms, definitions and provisions of the Company’s 2021 Equity Incentive Plan and a stock option agreement by and between Executive and the Parent, both of which documents are incorporated herein by reference.
10. Equity participation: Upon approval by META’s Board of Directors (the “Board”), you will be granted 100,000 RSUs in accordance with META's Employee Equity Incentive Compensation Plan. RSUs granted will vest in accordance with the plan.
11. Annual bonus: You are eligible to receive a performance-based bonus of up to 50% of the Base Salary, payable at the sole discretion of MTI-US. In order for any bonus to be awarded, the corporate objectives of META, as set by its Board in an agreed-upon scorecard, must be achieved. Once those objectives are achieved, you are eligible to earn this annual bonus on the achievement of specific annual personal objectives, in accordance with META’s policies (the "Bonus''). You understand and agree that the payment of a Bonus in any year does not give rise to any entitlement to a Bonus in another year and that, at all times, the determination of whether corporate or personal objectives have been achieved, whether a Bonus is payable and the amount of any such Bonus are within the absolute discretion of MTI-US. Participation in the bonus program is subject to the terms of such bonus plan(s), as may be amended from time to time by META.
You must be employed by MTI-US at the end of the fiscal year for which the Bonus is to be established in order to claim entitlement to such Bonus. For greater certainty, in the event that your employment terminates, (whether by resignation, dismissal, with or without cause, or for whatever other reason) in the course of the year for which a Bonus would otherwise be payable, you shall have no right to any portion of the Bonus, whether pro-rated or otherwise.
12.At-will employment: Please note that your employment with the Company constitutes “at will” employment. As a result, you are free to resign at any time, for any reason, or for no reason. Similarly, the Company is free to terminate its employment relationship with you at any time, with or without cause.
13.Termination: If the employment agreement ends due to Termination without Cause or Resignation for Good Reason, subject to the executive signing and not revoking a separate agreement and release, the Company will continue payment of the Executive’s Base Salary for a period equal to six (6) months following the termination date. In the event of a Change of Control the Company will continue payment of the Executive’s Base Salary for a period equal to twelve (12) months following the effective change date.
2
14. Pre-employment conditions: Should you decide to accept this offer, we shall require the following from you on or before your Start Date:
(a) Execution of our Confidentiality and Non-Solicitation Agreements;
(b) Documentary evidence of your identity and eligibility for employment in the USA;
This offer is open for acceptance until 3:00 pm Monday, 3rd July 2023, following which this offer is rescinded.
On your first day of employment, you will be provided with additional information about the company objectives and policies, benefit programs, and general employment conditions.
We look forward to the opportunity to work with you in an atmosphere that is successful and mutually challenging and rewarding.
Sincerely,
/s/ George Palikaras June 27, 2023
George Palikaras Date
President & CEO
Metamaterial Technologies USA, Inc.
With the signature below, I accept this offer for employment.
/s/ David D. (Dan) Eaton June 27, 2023
David D. Eaton Date
Encl:
Appendix A – Job Description
Appendix B - Confidentiality, Proprietary Property, and Invention Assignment
Exhibit A - List Of Prior Inventions And Original Works Of Authorship
Exhibit B - California Labor Code Section 2870
Exhibit C – Termination Certification
Exhibit D - Conflict Of Interest Guidelines
3
4
Exhibit 10.8
SEPARATION AGREEMENT AND RELEASE
Appendix C - Non-Solicitation Agreement This Separation Agreement and Release (“Agreement”) is made by and between Kenneth Rice (“Executive”) and Metamaterial Technologies USA Inc. (the “Company”) (collectively referred to as the “Parties” or individually referred to as a “Party”).
RECITALS
WHEREAS, Executive was employed by the Company, a subsidiary of Metamaterial Technologies Canada Inc., with an ultimate parent of Meta Materials, Inc. (the “Parent”) (together, with all other affiliates of the Company or the Parent, the “Company Group”);
WHEREAS, Executive signed an Executive Employment Contract with Metamaterial Inc. dated December 14, 2020 (the “Employment Agreement”);
WHEREAS, Executive signed an At-Will Employment, Confidential Information and Invention Assignment Agreement with Metamaterial Inc. dated December 11, 2020 (the “Confidentiality Agreement”);
WHEREAS, the Parent and Executive have entered into Stock Option Agreements granted as of the dates indicated in Exhibit A hereto, pursuant to which Executive was granted the option to purchase shares of the Company’s common stock (each such grant, an “Option Award”) and have entered into Restricted Stock Unit Award Agreements granted as of the dates indicated in Exhibit A hereto, granting Executive the right to receive an award of restricted stock units (each such award, an “RSU Award”), each subject to the terms and conditions of the Company’s Amended and Restated Executive Stock Option Plan (the “Stock Option Plan”) or the Company’s 2021 Equity Incentive Plan (the “2021 Plan” and, together with the Option Plan, the “Plans” and the Plans collectively with the Option Awards and RSU Awards, the “Stock Agreements”).
WHEREAS, the Company terminated Executive’s employment with the Company effective April 20, 2023 (the “Termination Date”); and
WHEREAS, the Parties wish to resolve any and all disputes, claims, complaints, grievances, charges, actions, petitions, and demands that the Executive may have against the Company and any of the Releasees as defined below, including, but not limited to, any and all claims arising out of or in any way related to Executive’s employment with or separation from the Company.
NOW, THEREFORE, in consideration of the mutual promises made herein, the Company and Executive hereby agree as follows:
COVENANTS
a. any and all claims relating to or arising from Executive’s employment relationship with the Company and the termination of that relationship or Executive’s relationship(s) with any member of the Company Group;
b. any and all claims relating to, or arising from, Executive’s right to purchase, or actual purchase of shares of stock of the Parent, including, without limitation, any claims for fraud, misrepresentation, breach of fiduciary duty, breach of duty under applicable state corporate law, and securities fraud under any state or federal law;
c. any and all claims for wrongful discharge of employment, termination in violation of public policy, discrimination, harassment, retaliation, breach of contract (both express and implied), breach of covenant of good faith and fair dealing (both express and implied), promissory estoppel, negligent or intentional infliction of emotional distress, fraud, negligent or intentional misrepresentation, negligent or intentional interference with contract or prospective economic advantage, unfair business practices, defamation, libel, slander, negligence, personal injury, assault, battery, invasion of privacy, false imprisonment, conversion, and disability benefits;
d. any and all claims for violation of any federal, state, or municipal statute, including, but not limited to, Title VII of the Civil Rights Act of 1964, the Civil Rights Act of 1991, the Rehabilitation Act of 1973, the Americans with Disabilities Act of 1990, the Equal Pay Act, the Fair Labor Standards Act, the Fair Credit Reporting Act, the Age Discrimination in Employment Act of 1967, the Older Workers Benefit Protection Act, the Employee Retirement Income Security Act of 1974, the Worker Adjustment and Retraining Notification Act, the Family and Medical Leave Act, the Immigration Reform and Control Act, the Sarbanes-Oxley Act of 2002, the Families First Coronavirus Response Act, any state and local employment laws regarding COVID-19, the Massachusetts Wage Act, M.G.L. c. 149, Sections 148-lS0C (the “Wage Act”), the California Family Rights Act, the California Labor Code, any California Industrial Welfare Commission Wage Order, and the California Fair Employment and Housing Act;
e. any and all claims for violation of the federal or any state constitution;
f. any and all claims arising out of any other laws and regulations relating to employment or employment discrimination;
g. any claim for any loss, cost, damage, or expense arising out of any dispute over the nonwithholding or other tax treatment of any proceeds received by Executive from the Company or any other member of the Company Group; and
h. any and all claims for attorneys’ fees and costs.
Executive agrees that the release set forth in this Section shall be and remain in effect in all respects as a complete general release as to the matters released. This release does not extend to any obligations incurred under this Agreement. This release does not release claims that cannot be released as a matter of law. Any and all disputed wage claims that are released herein shall be subject to binding arbitration in accordance with this Agreement, except as required by applicable law. This release does not extend to any right Executive may have to unemployment compensation benefits.
A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
Executive, being aware of said code section, agrees to expressly waive any rights Executive may have thereunder, as well as under any other statute or common law principles of similar effect.
“(a) Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time without using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either: (1) Relate at the time of conception or reduction to practice of the invention to the employer’s business, or actual or demonstrably anticipated research or development of the employer; or (2) Result from any work performed by the employee for the employer. (b) To the extent a provision in an employment agreement purports to require an employee to assign an invention otherwise excluded from being required to be assigned under subdivision (a), the provision is against the public policy of this state and is unenforceable.”
[THE REMAINDER OF THIS PAGE IS INTENTIONALLY LEFT BLANK; SIGNATURE PAGE FOLLOWS]
(a) Executive has read this Agreement;
(b) Executive has a right to consult with an attorney regarding this Agreement, and has been represented in the preparation, negotiation, and execution of this Agreement by an attorney of Executive’s own choice or has elected not to retain an attorney;
(c) Executive understands the terms and consequences of this Agreement and of the releases it contains;
(d) Executive is fully aware of the legal and binding effect of this Agreement; and
(e) Executive has not relied upon any representations or statements made by the Company that are not specifically set forth in this Agreement.
IN WITNESS WHEREOF, the Parties have executed this Agreement on the respective dates set forth below.
KENNETH RICE, an individual
Dated: /s/ Kenneth Rice May 3, 2023
Kenneth Rice
META MATERIALS INC.
Dated: /s/ George Palikaras By May 3, 2023
George Palikaras
President and Chief Executive Officer
Exhibit A
Option and RSU Awards
Options
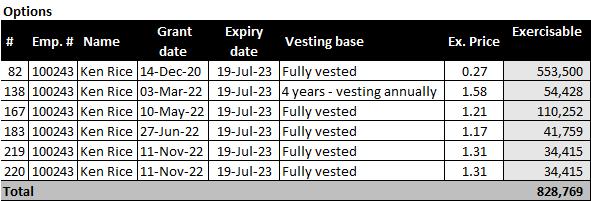
RSUs
Nil
Exhibit B
This Exhibit B lists the job titles and ages of all U.S.-based employees of Metamaterial technologies Canada Inc., Meta Materials, Inc., and Metamaterial Technologies USA, Inc. that were employed as of April 19, 2023, which is the decisional unit for this employment termination program. All listed individuals are covered by and eligible for this employment termination program by virtue of their membership in the decisional unit. An asterisk (*) denotes all employees whose employment was terminated in connection with the reduction in force and therefore, as of April 21, 2023, had been selected for this employment termination program. Two asterisks (**) denote all employees who (as of April 21, 2023) were slated to be terminated as part of this employment termination program after April 20, 2023.
Job Title |
Age |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]* |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]* |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
Exhibit 10.9
SEPARATION AGREEMENT AND RELEASE
This Separation Agreement and Release (“Agreement”) is made by and between Jonathan Waldern (“Executive”) and Metamaterial Technologies USA Inc. (the “Company”) (collectively referred to as the “Parties” or individually referred to as a “Party”).
RECITALS
WHEREAS, Executive was employed by the Company, a subsidiary of Metamaterial Inc., with an ultimate parent of Meta Materials, Inc. (the “Parent”) (together, with all other affiliates of the Company or the Parent, the “Company Group”);
WHEREAS, Executive signed an Executive Employment Contract with Metamaterial Inc. dated December 16, 2020 (the “Employment Agreement”);
WHEREAS, Executive signed an At-Will Employment, Confidential Information and Invention Assignment Agreement with Metamaterial Inc. dated December 16, 2020 (the “Confidentiality Agreement”);
WHEREAS, the Parent and Executive have entered into Stock Option Agreements granted as of the dates indicated in Exhibit A hereto, pursuant to which Executive was granted the option to purchase shares of the Company’s common stock (each such grant, an “Option Award”) and have entered into Restricted Stock Unit Award Agreements granted as of the dates indicated in Exhibit A hereto, granting Executive the right to receive an award of restricted stock units (each such award, an “RSU Award”), each subject to the terms and conditions of the Company’s Amended and Restated Executive Stock Option Plan (the “Stock Option Plan”) or the Company’s 2021 Equity Incentive Plan (the “2021 Plan” and, together with the Option Plan, the “Plans” and the Plans collectively with the Option Awards and RSU Awards, the “Stock Agreements”).
WHEREAS, the Company terminated Executive’s employment with the Company effective April 21, 2023 (the “Termination Date”); and
WHEREAS, the Parties wish to resolve any and all disputes, claims, complaints, grievances, charges, actions, petitions, and demands that the Executive may have against the Company and any of the Releasees as defined below, including, but not limited to, any and all claims arising out of or in any way related to Executive’s employment with or separation from the Company.
NOW, THEREFORE, in consideration of the mutual promises made herein, the Company and Executive hereby agree as follows:
COVENANTS
a. any and all claims relating to or arising from Executive’s employment relationship with the Company and the termination of that relationship or Executive’s relationship(s) with any member of the Company Group;
b. any and all claims relating to, or arising from, Executive’s right to purchase, or actual purchase of shares of stock of the Parent, including, without limitation, any claims for fraud, misrepresentation, breach of fiduciary duty, breach of duty under applicable state corporate law, and securities fraud under any state or federal law;
c. any and all claims for wrongful discharge of employment, termination in violation of public policy, discrimination, harassment, retaliation, breach of contract (both express and implied), breach of covenant of good faith and fair dealing (both express and implied), promissory estoppel, negligent or intentional infliction of emotional distress, fraud, negligent or intentional misrepresentation, negligent or intentional interference with contract or prospective economic advantage, unfair business practices, defamation, libel, slander, negligence, personal injury, assault, battery, invasion of privacy, false imprisonment, conversion, and disability benefits;
d. any and all claims for violation of any federal, state, or municipal statute, including, but not limited to, Title VII of the Civil Rights Act of 1964, the Civil Rights Act of 1991, the Rehabilitation Act of 1973, the Americans with Disabilities Act of 1990, the Equal Pay Act, the Fair Labor Standards Act, the Fair Credit Reporting Act, the Age Discrimination in Employment Act of 1967, the Older Workers Benefit Protection Act, the Employee Retirement Income Security Act of 1974, the Worker Adjustment and Retraining Notification Act, the Family and Medical Leave Act, the Immigration Reform and Control Act, the California Family Rights Act, the California Labor Code, the California Workers’ Compensation Act, and the California Fair Employment and Housing Act;
e. any and all claims for violation of the federal or any state constitution;
f. any and all claims arising out of any other laws and regulations relating to employment or employment discrimination;
g. any claim for any loss, cost, damage, or expense arising out of any dispute over the nonwithholding or other tax treatment of any proceeds received by Executive from the Company or any other member of the Company Group; and
h. any and all claims for attorneys’ fees and costs.
Executive agrees that the release set forth in this Section shall be and remain in effect in all respects as a complete general release as to the matters released. This release does not extend to any obligations incurred under this Agreement. This release does not release claims that cannot be released as a matter of law. Any and all disputed wage claims that are released herein shall be subject to binding arbitration in accordance with this Agreement, except as required by applicable law. This release does not extend to any right Executive may have to unemployment compensation benefits.
A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
Executive, being aware of said code section, agrees to expressly waive any rights Executive may have thereunder, as well as under any other statute or common law principles of similar effect.
“(a) Any provision in an employment agreement which provides that an employee shall assign, or offer to assign, any of his or her rights in an invention to his or her employer shall not apply to an invention that the employee developed entirely on his or her own time without using the employer’s equipment, supplies, facilities, or trade secret information except for those inventions that either: (1) Relate at the time of conception or reduction to practice of the invention to the employer’s business, or actual or demonstrably anticipated research or development of the employer; or (2) Result from any work performed by the employee for the employer. (b) To the extent a provision in an employment agreement purports to require an employee to assign an invention otherwise excluded from being required to be assigned under subdivision (a), the provision is against the public policy of this state and is unenforceable.”
[THE REMAINDER OF THIS PAGE IS INTENTIONALLY LEFT BLANK; SIGNATURE PAGE FOLLOWS]
(a) Executive has read this Agreement;
(b) Executive has a right to consult with an attorney regarding this Agreement, and has been represented in the preparation, negotiation, and execution of this Agreement by an attorney of Executive’s own choice or has elected not to retain an attorney;
(c) Executive understands the terms and consequences of this Agreement and of the releases it contains;
(d) Executive is fully aware of the legal and binding effect of this Agreement; and
(e) Executive has not relied upon any representations or statements made by the Company that are not specifically set forth in this Agreement.
IN WITNESS WHEREOF, the Parties have executed this Agreement on the respective dates set forth below.
JONATHAN WALDERN, an individual
Dated: /s/ Jonathan Waldern June 14, 2023
Jonathan Waldern
META MATERIALS INC.
Dated: /s/ George Palikaras By May 4, 2023
George Palikaras
President and Chief Executive Officer
Exhibit A
Option and RSU Awards
Options
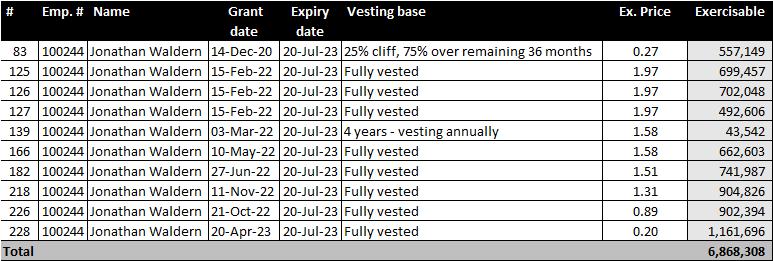
RSU
Nil
Exhibit B
This Exhibit B lists the job titles and ages of all U.S.-based employees of Metamaterial Inc., Meta Materials, Inc., and Metamaterial Technologies USA, Inc. that were employed as of April 19, 2023, which is the decisional unit for this employment termination program. All listed individuals are covered by and eligible for this employment termination program by virtue of their membership in the decisional unit. An asterisk (*) denotes all employees whose employment was terminated in connection with the reduction in force and therefore, as of April 21, 2023, had been selected for this employment termination program. Two asterisks (**) denote all employees who (as of April 21, 2023) were slated to be terminated as part of this employment termination program after April 20, 2023.
Job Title |
Age |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]* |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]* |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ]** |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
[ ] |
Metamaterial Technologies USA Inc.
a subsidiary of META® Materials Inc., a Nevada Corporation,
headquartered in Nova Scotia, Canada
META Materials Inc.5880 W. Las Positas Blvd., Suite 37
Pleasanton, California 94588
United States
Exhibit 10.10
April 25, 2023
Jonathan Waldern
Address:[ ]
Subject: Termination of Employment
Dear Jonathan,
With respect to your end of employment communicated verbally by George Palikaras on April 21, 2023, please find details below:
On Monday April 24, 2023, you were paid the following, minus any required withholdings:
We would also like to take this opportunity to remind you that, as with all employees, you continue to be bound by the company’s Confidentiality and Proprietary Property Agreement, Non-Solicitation Agreement and data protection policies.
Please acknowledge the above by signing this letter where indicated below and returning it, no later than May 9th, 2023.
We would like to thank you for your efforts to date and will do our best to support you in this transition.
Sincerely,
Nadine Geddes
Director of Operations
Meta Material Inc.
----------------------------------------------------------------------------------------------------------------------------
Employee acknowledgement
/s/ Jonathan Waldern May 4, 2023
Jonathan Waldern Date
60 HIGHFIELD PARK DRIVE I SUITE 102 I DARTMOUTH I NOVA SCOTIA I B3A 4R9 I CANADA I NASDAQ: MMAT I METAMATERIAL.COM
Execution Version
Exhibit 10.11
LOAN SALE AGREEMENT
This LOAN SALE AGREEMENT ("Agreement") is entered into as of this 7th of August 2023 ("Effective Date") by and between META MATERIALS INC., a Nevada corporation ("Seller"), and Gregory McCabe, individually ("Purchaser" and together with Seller, the "Parties").
RECITALS
WHEREAS, Seller desires to sell and assign to Purchaser all of Seller's rights, title, interests, and obligations in and to those certain Loans described on Schedule 1 hereto and made a part of this Agreement (each loan referred to individually as a "Loan" and collectively as the "Loans") together with the documents evidencing, securing or guarantying such Loans as set out on Schedule 2 hereto and made a part of this Agreement (collectively, the "Loan Documents"), on the terms and conditions set forth herein; and
WHEREAS, Purchaser desires to purchase and assume from Seller all Seller's rights, interests, and obligations in and to the Loans and the Loan Documents subject to the terms and conditions set forth herein.
NOW, THEREFORE, in consideration of the Purchase Price (as hereinafter defined) and the respective promises and agreements of the parties set forth herein, and intending to be legally bound, the Parties hereby agree as follows:
2
3
4
5
6
7
If to Seller: |
|
Meta Materials Inc. 1 Research Drive |
With a copy to (which shall not constitute notice): |
|
Wilson Sonsini Goodrich & Rosati, P.C. One Boston Place Boston, MA 02108 Attn: Tom Hornish Email: [ ]@wsgr.com |
If to Purchaser: |
|
Name: Gregory McCabe Midland, TX 79701 |
All Notices must be delivered by email (with electronic delivery receipt). Except as otherwise provided in this Agreement, a Notice is effective upon receipt by the receiving party provided the party giving the Notice has complied with the requirements of this Section.
8
9
List of Attachments:
Schedule 1 - Schedule of Loans
Schedule 2 - Schedule of Loan Documents
Schedule 3 - Seller's Wiring Instructions
Exhibit A - Form of Assignment and Assumption of Loans
Exhibit B – Form of Allonge to Note
Exhibit C - Form Notice of Loan Sale to Borrowers
Exhibit D – Form of Assignment of Deed of Trust, Mortgage, Security Agreement, Fixture Filing, Financing Statement and Assignment of Production [Signature Page to Loan Sale Agreement]
[SIGNATURE PAGE FOLLOWS]
10
Execution Version
IN WITNESS WHEREOF, the Parties hereto have executed this Agreement as of the date set forth above.
|
SELLER: META MATERIALS INC.
By /s/ George Palikaras Name: George Palikaras Title: CEO |
PURCHASER: GREGORY MCCABE
By /s/ Gregory McCabe Name: Gregory McCabe
|
|
|
|
|
|
Execution Version
SCHEDULE 1
Schedule of Loans
Loans, shall mean, collectively, all of which are described without representation or warranty of any kind by Seller:
1. The loan and extension of credit (the "Secured Loan") made under and pursuant to, and evidenced by, that certain 8% Secured Promissory Note made by Next Bridge Hydrocarbons, Inc. (f/k/a Oilco Holdings, Inc.), a Nevada corporation, in favor of Seller, with an original issue date of October 1, 2021 (as amended and in effect on or before the Closing Date, the "Secured Note"); and
2. The "Loans" as such term is defined in the Loan Agreement (defined in Schedule 2 hereto).
SCHEDULE 2
Schedule of Loan Documents
The documents described on this Schedule 2 shall collectively constitute the Loan Documents as that term is defined in this Agreement. All descriptions of the Loan Documents on this Schedule 2 are made without representation or warranty of any kind by Seller.
1. 8% Secured Promissory Note made by Next Bridge Hydrocarbons, Inc. (f/k/a Oilco Holdings, Inc.), a Nevada corporation, in favor of Seller, with an original issue date of October 1, 2021 (as amended from time to time, the "Secured Note")
2. First Amendment Agreement, dated as of September 2, 2022, among NBH, Gregory McCabe ("Pledgor"), Wolfbone Investments, LLC, a Texas limited liability company ("Wolfbone"), and Seller
3. Second Amendment to 8% Secured Promissory Note, dated as of March 31, 2023, among NBH, Pledgor, Wolfbone, and Seller
4. Stock Pledge Agreement dated as of September 30, 2021 (as amended from time to time, the "Stock Pledge Agreement"), made by Pledgor in favor of Seller
5. Reaffirmation and Ratification Agreement, dated as of May 15, 2023, between Pledgor and Seller
6. Deed of Trust, Mortgage, Security Agreement, Fixture Filing, Financing Statement and Assignment of Production, dated September 30, 2021 recorded under Document Number 00000153654 in the Official Records of Hudspeth County, Texas (as amended from time to time, the "Deed of Trust"), made by Wolfbone to Travis Vargo, as trustee, for the benefit of Seller
7. Extension of Lien, dated as of March 31, 2023, by Wolfbone, with respect to the Deed of Trust
8. Limited Waiver, Consent and Amendment Agreement, dated as of April 17, 2023, among Seller, NBH, Torchlight Energy, Inc., a Nevada corporation ("Torchlight Energy"), Torchlight Hazel, LLC, a Texas limited liability company ("Hazel"), Hudspeth Oil Corporation, a Texas corporation ("Hudspeth Oil"), Wolfbone, and Hudspeth Operating, LLC, a Texas limited liability company ("Hudspeth Operating", and together with NBH, Torchlight Energy, Hazel, Hudspeth Oil and Wolfbone, the "Borrowers")
9. Loan Agreement dated as of September 2, 2022 (as amended from time to time, the "Loan Agreement") among the Borrowers and Seller
10. First Amendment to Loan Agreement dated as of December 21, 2022 among the Borrowers and Seller
11. Second Amendment to Loan Agreement dated as of March 31, 2023 among the Borrowers and Seller
12. Note dated March 31, 2023 made by the Borrowers (other than Wolfbone) in favor of Lender
13. Note dated May 15, 2023 made by the Borrowers in favor of Lender (the "Amended and Restated Unsecured Note")
SCHEDULE 3
Seller's Wiring Instructions
All amounts due Seller shall be wired in immediately available US funds to:

Seller reserves the right to change or amend the wire instructions in this Schedule 3 at any time prior to Closing.
SCHEDULE 4
Shares of common stock of Seller beneficially owned by Purchaser
EXHIBITA
Form of Assignment and Assumption of Loans
12,000,000 shares of Common Stock This Assignment and Assumption of Loans ("Assignment and Assumption") is entered into as of August 7, 2023 ("Effective Date") by and between META MATERIALS INC., a Nevada corporation ("Assignor") and GREGORY MCCABE, individually ("Assignee" and together with Assignor, the "Parties").
RECITALS
Assignor and Assignee have entered into that certain Loan Sale Agreement effective as of the Effective Date ("Loan Sale Agreement"). Capitalized terms used but not defined herein are used as defined under the Loan Sale Agreement.
NOW, THEREFORE, in consideration of the Purchase Price, and for other good and valuable consideration as therein provided, Assignor agrees to sell and assign and Assignee agrees to purchase and assume the Assigned Loan Interests, pursuant to the terms and provisions set forth herein and in the Loan Sale Agreement.
1. Assignment and Assumption. Assignor hereby sells and assigns to Assignee and Assignee hereby purchases and assumes from Assignor all of Assignor's right, title, interests, and obligations in and to the Assigned Loan Interests, including, without limitation, the rights to receive principal, interest and fees and the duty to perform all obligations with respect the Loans and Loan Documents.
2. As-Is; No Recourse. The assignment and assumption evidenced hereby is made "AS-IS," and "WITH ALL FAULTS," WITHOUT REPRESENTATION BY ASSIGNOR, EXPRESS OR IMPLIED, OF ANY TYPE, CHARACTER, OR NATURE WHATSOEVER, EXCEPT AS EXPRESSLY SET FORTH IN SECTION 5 OF THE LOAN SALE AGREEMENT. Assignee further acknowledges and agrees that the assignment and assumption of the Assigned Loan Interests hereunder are made WITHOUT RECOURSE of any nature to Assignor.
3. Assignee's Waivers and Disclaims. Without limiting the generality of any of the foregoing, Assignee hereby waives and disclaims any express or implied representation or warranty by Assignor with respect to the Assigned Loan Interests transferred hereunder, including, without limitation: (a) the merchantability or fitness for a particular purpose of any Loan transferred hereunder, (b) the financial condition or corporate status of any obligor under the Loan Documents, (c) the condition of any guarantee or collateral securing any Loan or Loan Documents, (d) the collectability of any Loan, (e) the enforceability of any Loan Document or (f) the priority or perfection of any lien purportedly created under the Loan Documents.
4. Assumption and Release. From and after the date hereof, Assignee shall assume the Assigned Loan Interests under the Loans and Loan Documents and hereby releases Assignor of all further duties, obligations, and liabilities thereunder and under the Loan Sale Agreement.
[SIGNATURE PAGE FOLLOWS]
IN WITNESS WHEREOF, the Parties hereto have executed this Agreement as of the date set forth above.
|
ASSIGNOR:
META MATERIALS INC., a Nevada corporation
By_________________ Name: Title: |
ASSIGNEE:
GREGORY MCCABE
By_________________ Name: Gregory McCabe
|
EXHIBITB
Form of Allonge to Promissory Note
ALLONGE
This Allonge is attached to and made a part of that certain [NOTE TITLE] (the "Note") dated [insert date of Note], made by Next Bridge Hydrocarbons Inc. (f/k/a Oilco Holdings, Inc.), a Nevada corporation, in the original principal amount of [NUMBER IN WORDS] [NUMBER]/100 Dollars ($[NUMBER]), to the order of Meta Materials Inc., a Nevada corporation.
For value received, the undersigned hereby indorses the Note as follows:
Pay to the order of Gregory McCabe, without recourse, warranty or representation of any nature whatsoever.
Dated: August 7, 2023
|
META MATERIALS INC., a Nevada corporation
By_________________ Name: Title: |
EXHIBIT C
Form of Notice of Loan Sale to Borrowers
August 7, 2023
Next Bridge Hydrocarbons, Inc.
Torchlight Energy, Inc.
Torchlight Hazel, LLC
Hudspeth Oil Corporation
Wolfbone Investments, LLC
Hudspeth Operating, LLC
(collectively, the "Borrowers")
Dear Borrowers:
On August 7, 2023, the below loans (the "Loans") were sold by Meta Materials Inc. to Gregory McCabe ("Successor Lender") and, as of that date, the Loans were assigned to and assumed by Successor Lender.
Loans:
1. The loan and extension of credit (the "Secured Loan") made under and pursuant to, and evidenced by, that certain 8% Secured Promissory Note made by Next Bridge Hydrocarbons, Inc. (f/k/a Oilco Holdings, Inc.), a Nevada corporation, in favor of Meta Materials Inc., with an original issue date of October 1, 2021 (as amended from time to time, the "Secured Note"); and
2. The "Loans" as such term is defined in the Loan Agreement dated as of September 2, 2022 (as amended from time to time, the "Loan Agreement") among the Borrowers and Meta Materials Inc.
This letter is to notify you to send all future payments due under the Loans to the Successor Lender in the manner provided under the Secured Note and Loan Agreement, as applicable, at the following address: 500 W. Texas Ave. #890, Midland, TX 79701.
Until you are directed otherwise by Successor Lender, all notices and other communications to the Successor Lender with respect to the Loans should be delivered in accordance with the terms of the Secured Note and Loan Agreement, as applicable, to: 500 W. Texas Ave. #890, Midland, TX 79701.
|
Very truly yours, META MATERIALS INC., a Nevada corporation
|
|
By: ___________________________ Name: Title: |
EXHIBIT D
Assignment of Deed of Trust, Mortgage, Security Agreement, Fixture Filing, Financing Statement and Assignment of Production
|
PREPARED BY, RECORDING REQUESTED BY, AND WHEN RECORDED MAIL TO:
File #
|
|
SPACE ABOVE THIS LINE RESERVED FOR RECORDER'S USE
ASSIGNMENT OF
DEED OF TRUST, MORTGAGE, SECURITY AGREEMENT, FIXTURE FILING, FINANCING STATEMENT AND ASSIGNMENT OF PRODUCTION (Texas)
and Related Security Instruments
(hereinafter the "Assignment")
In consideration of the sum of Ten Dollars ($10.00) and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged META MATERIALS INC., a Nevada corporation, in its capacity as noteholder and secured party, having an address at 60 Highfield Park Drive, Dartmouth, Nova Scotia, B3A 4R9 ("Assignor"), does hereby grant, bargain, sell, convey, assign, transfer and set over unto GREGORY MCCABE, individually, having an address at 500 W. Texas Ave. #890, Midland, TX 79701 ("Assignee"), without recourse, all of the right, title and interest of Assignor in and to:
1. That certain Deed of Trust, Security Agreement, Assignment of Rents and Leases, Financing Statement (Texas) made by Next Bridge Hydrocarbons, Inc., as Grantor, in favor of Meta Materials Inc., dated September 30, 2021 and recorded as document number 00000153654 Real Property Records of Hudspeth County, Texas (the "Deed of Trust");
2. The bond(s), note(s) and/or obligation(s) secured by the Deed of Trust, the moneys due and to grow due thereon, with interest as specified therein, and all rights accrued or to accrue under the Deed of Trust; and
3. Any and all other related security instruments which secure the indebtedness and/or obligations secured by the Deed of Trust.
This Assignment is made without representation, recourse or warranty by Assignor.
IN WITNESS WHEREOF, the Assignor by its duly elected officers and pursuant to proper authority of its board of directors has duly executed, sealed, acknowledged and delivered this Assignment.
Dated as of August 7, 2023
ASSIGNOR:
Meta Materials Inc.,
in its capacity as Administrative Agent
By: __________________________
Name: ____________________
Title: ____________________
CORPORATE ACKNOWLEDGEMENT
(ASSIGNOR)
STATE OF
SS
COUNTY OF
On this ____ day of [__], before me, the undersigned, personally appeared, _________________________________________________, personally known and acknowledged himself/herself to me (or proved to me on the basis of satisfactory evidence) to be the _________________________________ of [__] (hereinafter, the "Corporation"), and that as such officer, being duly authorized to do so pursuant to its bylaws or a resolution of its board of directors, executed, subscribed and acknowledged the foregoing instrument for the purposes therein contained, by signing the name of the Corporation by himself/herself in their authorized capacity as such officer as his/her free and voluntary act and deed and the free and voluntary act and deed of said Corporation.
IN WITNESS WHEREOF, I hereunto set my hand and official seal.
___________________________________
Notary Public
[SEAL]
Exhibit 31.1
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, George Palikaras, certify that:
Date: August 9, 2023 |
|
|
By: |
|
/s/ George Palikaras |
|
|
|
|
|
George Palikaras |
|
|
|
|
|
President and Chief Executive Officer |
|
|
|
|
|
(Principal Executive Officer) |
Exhibit 31.2
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Uzi Sasson, certify that:
Date: August 9, 2023 |
|
|
By: |
|
/s/ Uzi Sasson |
|
|
|
|
|
Uzi Sasson |
|
|
|
|
|
Chief Financial Officer and Chief Operating Officer |
|
|
|
|
|
(Principal Financial and Accounting Officer) |
Exhibit 32.1
CERTIFICATION OF PRINCIPAL EXECUTIVE OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, I, George Palikaras, the Chief Executive Officer of Meta Materials Inc. (the “Company”), hereby certify, that, to my knowledge:
Date: August 9, 2023 |
|
|
By: |
|
/s/ George Palikaras |
|
|
|
|
|
George Palikaras |
|
|
|
|
|
President and Chief Executive Officer |
|
|
|
|
|
(Principal Executive Officer) |
Exhibit 32.2
CERTIFICATION OF PRINCIPAL FINANCIAL OFFICER
PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, I, Uzi Sasson, the Chief Financial Officer of Meta Materials Inc. (the “Company”), hereby certify, that, to my knowledge:
Date: August 9, 2023 |
|
|
By: |
|
/s/ Uzi Sasson |
|
|
|
|
|
Uzi Sasson |
|
|
|
|
|
Chief Financial Officer and Chief Operating Officer |
|
|
|
|
|
(Principal Financial and Accounting Officer) |