UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 20, 2023
MIND MEDICINE (MINDMED) INC.
(Exact name of Registrant as Specified in Its Charter)
British Columbia, Canada |
001-40360 |
98-1582438 |
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
|
|
|
One World Trade Center, Suite 8500 New York, New York |
|
10007 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
Registrant’s Telephone Number, Including Area Code: (212) 220-6633
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Shares |
MNMD |
The Nasdaq Stock Market LLC |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01 |
Regulation FD Disclosure. |
On June 20, 2023, Mind Medicine (MindMed) Inc. (the “Company”) hosted an Analyst Day in New York, NY focusing on the Company’s MM-120 program in generalized anxiety disorder (GAD). A copy of the presentation displayed during the Analyst Day, which the Company may use from time to time in communications or conferences, is filed herewith as Exhibit 99.1 and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
Exhibit No. |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
MIND MEDICINE (MINDMED) INC. |
|
|
|
|
|
Date: June 20, 2023 |
|
By: |
/s/ Robert Barrow |
|
|
Name: |
Robert Barrow |
|
|
Title: |
Chief Executive Officer |
Exhibit 99.1

MindMed Investor Presentation Investor Day 2023: MM-120 for Generalized Anxiety Disorder

Disclaimer This presentation (the “Presentation”) has been prepared by Mind Medicine (MindMed) Inc. (“MindMed” or the “Company”) solely for informational purposes. None of MindMed, its affiliates or any of their respective employees, directors, officers, contractors, advisors, members, successors, representatives or agents makes any representation or warranty as to the accuracy or completeness of any information contained in this Presentation and shall have no liability for any representations (expressed or implied) contained in, or for any omissions from, this Presentation. This Presentation does not constitute an offering of, or a solicitation of an offer to purchase, securities of MindMed and under no circumstances is it to be construed as a prospectus or advertisement or public offering of securities. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of MindMed. Any amounts are in USD unless otherwise noted. MindMed’s securities have not been approved or disapproved by the Securities and Exchange Commission (the "SEC") or by any state, provincial or other securities regulatory authority, nor has the SEC or any state, provincial or other securities regulatory authority passed on the accuracy or adequacy of this Presentation. Any representation to the contrary is a criminal offense. Cautionary Note Regarding Forward-Looking Statements This Presentation contains, and our officers and representatives may from time to time make, “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995 and other applicable securities laws. Forward-looking statements can often, but not always, be identified by words such as “plans”, “expects”, “is expected”, “budget”, “scheduled”, “estimates”, “forecasts”, “intends”, “anticipates”, will”, “projects”, or “believes” or variations (including negative variations) of such words and phrases, or statements that certain actions, events, results or conditions “may”, “could”, “would”, “might” or “will” be taken, occur or be achieved, and similar references to future periods. Except for statements of historical fact, examples of forward-looking statements include, among others, statements pertaining to: the development and commercialization of any medicine or treatment, or the efficacy of either of the foregoing, the success and timing of our development activities; the success and timing of our planned clinical trials; our ability to meet the milestones set forth herein; the likelihood of success of any clinical trials or of obtaining FDA or other regulatory approvals; the likelihood of obtaining patents or the efficacy of such patents once granted and the potential for the markets that MindMed is anticipating to access Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions as of the date of this Presentation. While MindMed considers these assumptions to be reasonable, the assumptions are inherently subject to significant business, social, economic, political, regulatory, competitive and other risks and uncertainties that are difficult to predict and many of which are outside of MindMed’s control, and actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to raise capital to complete its plans and fund its studies; the medical and commercial viability of the contemplated medicines and treatments being developed; MindMed’s history of negative cash flows; MindMed’s limited operating history; incurrence of future losses; lack of revenue; compliance with laws and regulations; difficulty associated with research and development; risks associated with clinical trials or studies; heightened regulatory scrutiny; early stage product development; clinical trial risks; regulatory approval processes; novelty of the psychedelic inspired medicines industry; as well as those risk factors discussed or referred to throughout the “Risk Factors” sections of MindMed’s most recently filed Annual Report on Form 10-K filed with the SEC and in other filings we make in the future with the SEC and the securities regulatory authorities in all provinces and territories of Canada, available under the Company’s profile on SEDAR at www.sedar.com. Any forward-looking statement made by MindMed in this Presentation is based only on information currently available to the Company and speaks only as of the date on which it is made. MindMed undertakes no obligation to publicly update any forwardlooking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Cautionary Note Regarding Regulatory Matters The United States federal government regulates drugs through the Controlled Substances Act. The Company works with a non-hallucinogenic synthetic derivative of the psychedelic substance ibogaine, known as zolunicant which is a synthetic organic molecule designed around a common coronaridine chemical backbone. Zolunicant is not a Schedule I substance in the United States and the Company does not foresee it becoming a Schedule I substance due to its non-hallucinogenic properties. While the Company is focused on programs using psychedelic or hallucinogenic compounds and non-hallucinogenic derivatives of these compounds, the Company does not have any direct or indirect involvement with the illegal selling, production or distribution of any substances in the jurisdictions in which it operates. The Company is a neuro-pharmaceutical drug development company and does not deal with psychedelic or hallucinogenic substances except within laboratory and clinical trial settings conducted within approved regulatory frameworks. The Company’s products will not be commercialized prior to applicable regulatory approval, which will only be granted if clinical evidence of safety and efficacy for the intended uses is successfully developed. Market and Industry Data This Presentation includes market and industry data that has been obtained from third party sources, including industry publications. MindMed believes that the industry data is accurate and that the estimates and assumptions are reasonable, but there is no assurance as to the accuracy or completeness of this data. Third party sources generally state that the information contained therein has been obtained from sources believed to be reliable, but there is no assurance as to the accuracy or completeness of included information. Although the data is believed to be reliable, MindMed has not independently verified any of the data from third party sources referred to in this Presentation or ascertained the underlying economic assumptions relied upon by such sources. References in this Presentation to research reports or to articles and publications should be not construed as depicting the complete findings of the entire referenced report or article. MindMed does not make any representation as to the accuracy of such information. Investor Day | June 2023 2

Today’s Agenda SPEAKER TOPIC Rob Barrow CEO, MindMed Maria Oquendo, MD David Feifel, MD, PhD Experts and Management Team Michael Kobernick, MD W. Chad Shear, JD Rob Barrow Experts and Management Team Rob Barrow Opening Remarks Unmet Need & Patient Journey in Generalized Anxiety Disorder (GAD) Practical Aspects of Monitored Therapies & Digital Medicine Questions and Answers (Q&A) – Sessions 2 and 3 Payer Considerations in New Medication Coverage The Intellectual Property (IP) Landscape Corporate Update Questions and Answers (Q&A) Concluding Remarks MindMed Investor Day | June 2023 3
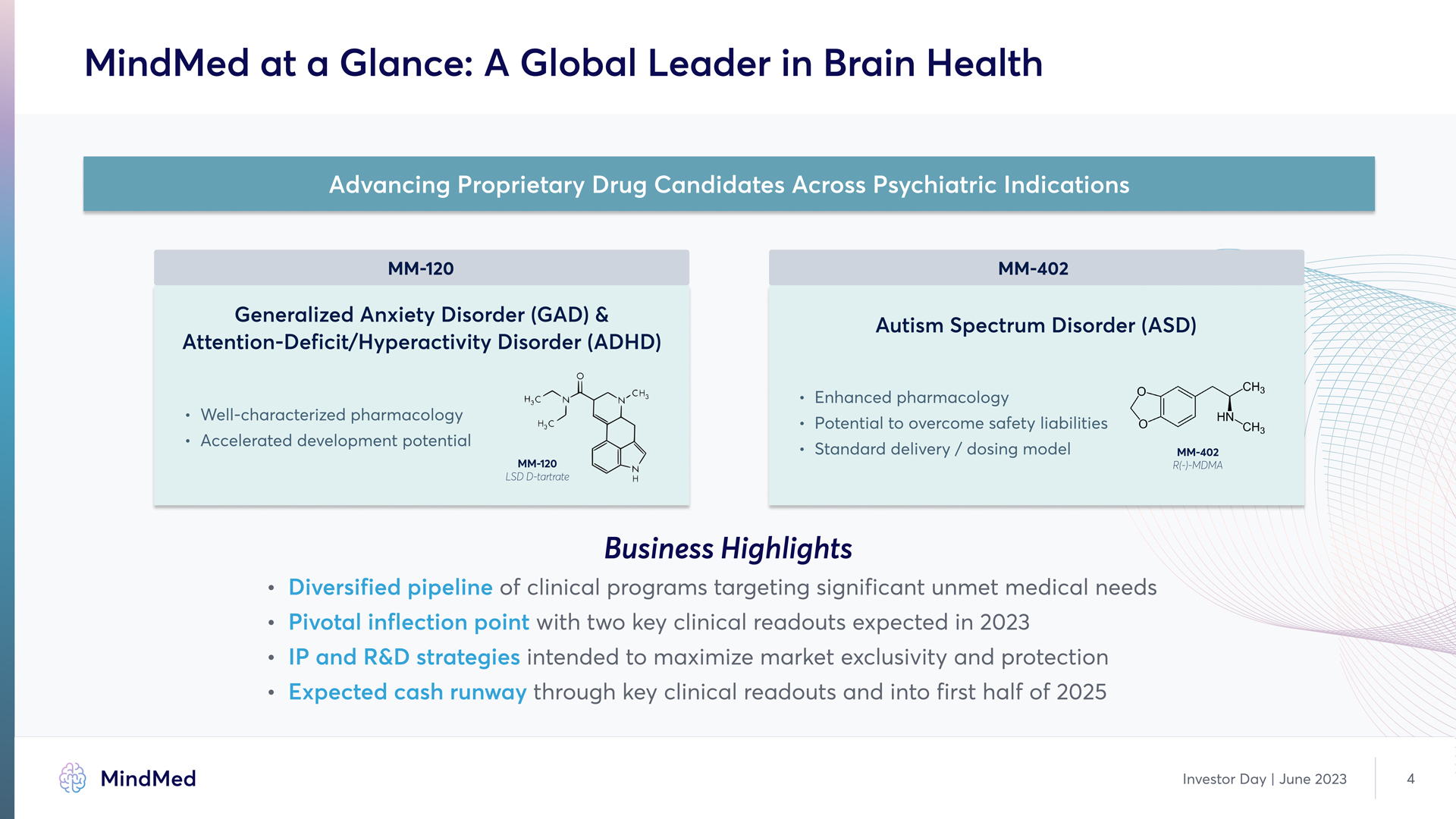
MindMed at a Glance: A Global Leader in Brain Health Advancing Proprietary Drug Candidates Across Psychiatric Indications MM-120 MM-402 Generalized Anxiety Disorder (GAD) & Attention-Deficit/Hyperactivity Disorder (ADHD) Well-characterized pharmacology Aelerated development potential Autism Spectrum Disorder (ASD) Enhanced pharmacology Potential to overcome safety liabilities Standard delivery / dosing model Business Highlights Diversified pipeline of of clinical programs targeting significant unmet medical needs Pivotal inflection point with two key clinical readouts expected in 2023 IP and R&D strategies intended to maximize market exclusivity and protection Expected cash runway through key clinical readouts and into first half of 2025 MindMed Investor Day | June 2023 4
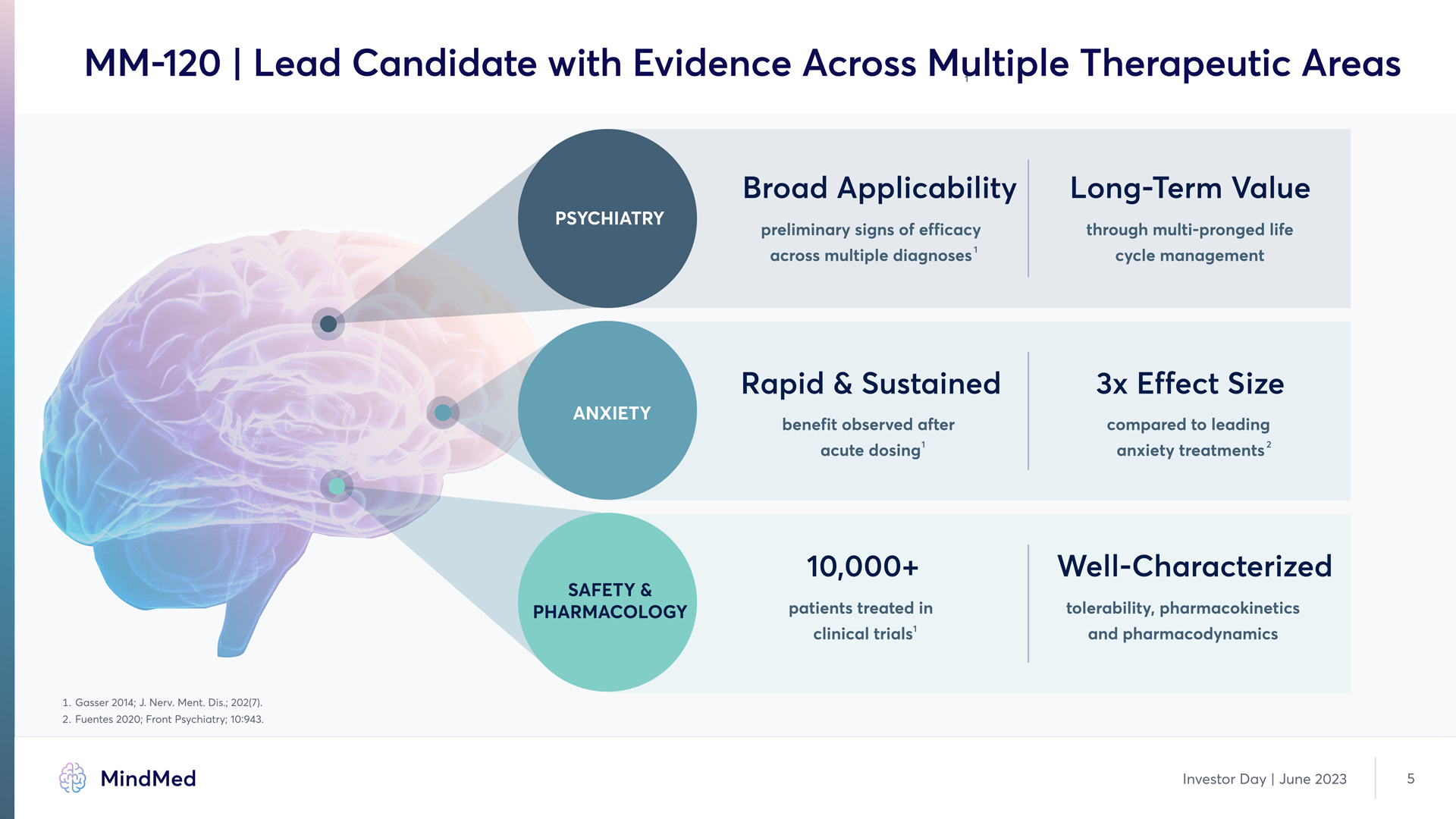
MM-120 | Lead Candidate with Evidence Across Multiple Therapeutic Areas Psychiatry ANXIETY SAFETY & PHARMACOLOGY 1 preliminary signs of efficacy across multiple diagnoses benefit observed after acute dosing Rapid & Sustained 10,000+ patients treated in clinical trials 1 Long-Term Value through multi-pronged life cycle management 3x Effect Size compared to leading anxiety treatments 2 Well-Characterized tolerability, pharmacokinetics and pharmacodynamics 1. Gasser 2014; J. Nerv. Ment. Dis.; 202(7). 2. Fuentes 2020; Front Psychiatry; 10:943. MindMed Investor Day | June 2023 5
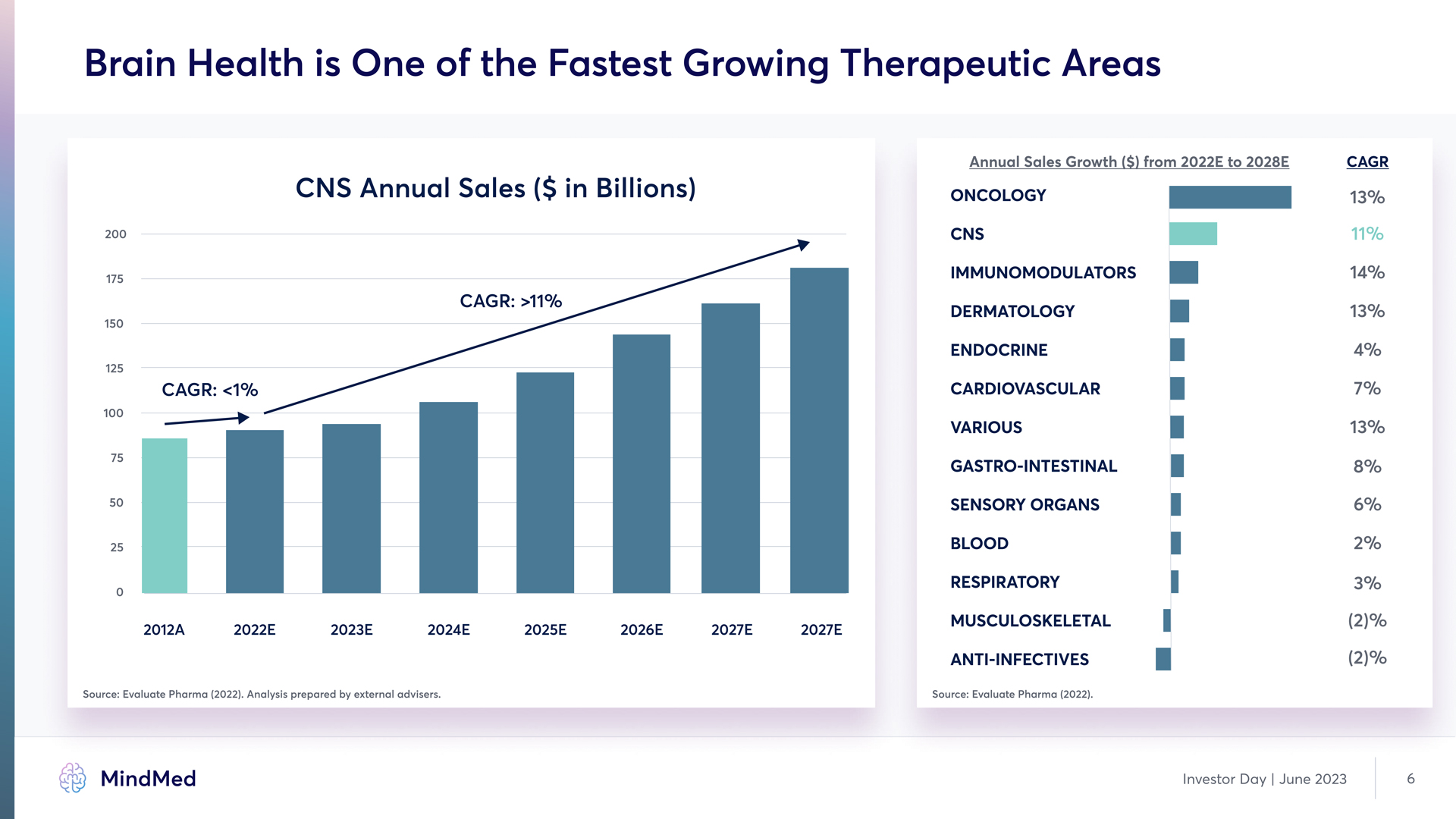
Brain Health is One of the Fastest Growing Therapeutic Areas CNS Annual Sales ($ in Billions) CAGR: >11% CAGR: <1% 200 175 150 125 100 75 50 25 0 2012A 2022E 2023E 2024E 2025E 2026E 2027E 2027E Annual Sales Growth ($) from 2022E to 2028E CAGR Oncology cns immunomodulators dermatology endocrine cardiovascular various gastro-intestinal sensory organs blood Respiratory Musculoskeletal anti-infectives 13% 11% 14% 13% 4% 7% 13% 8% 6% 2% 3% (2)% (2)% Source: Evaluate Pharma (2022). Analysis prepared by external advisers. Source: Evaluate Pharma (2022). MindMed Investor Day | June 2023 6
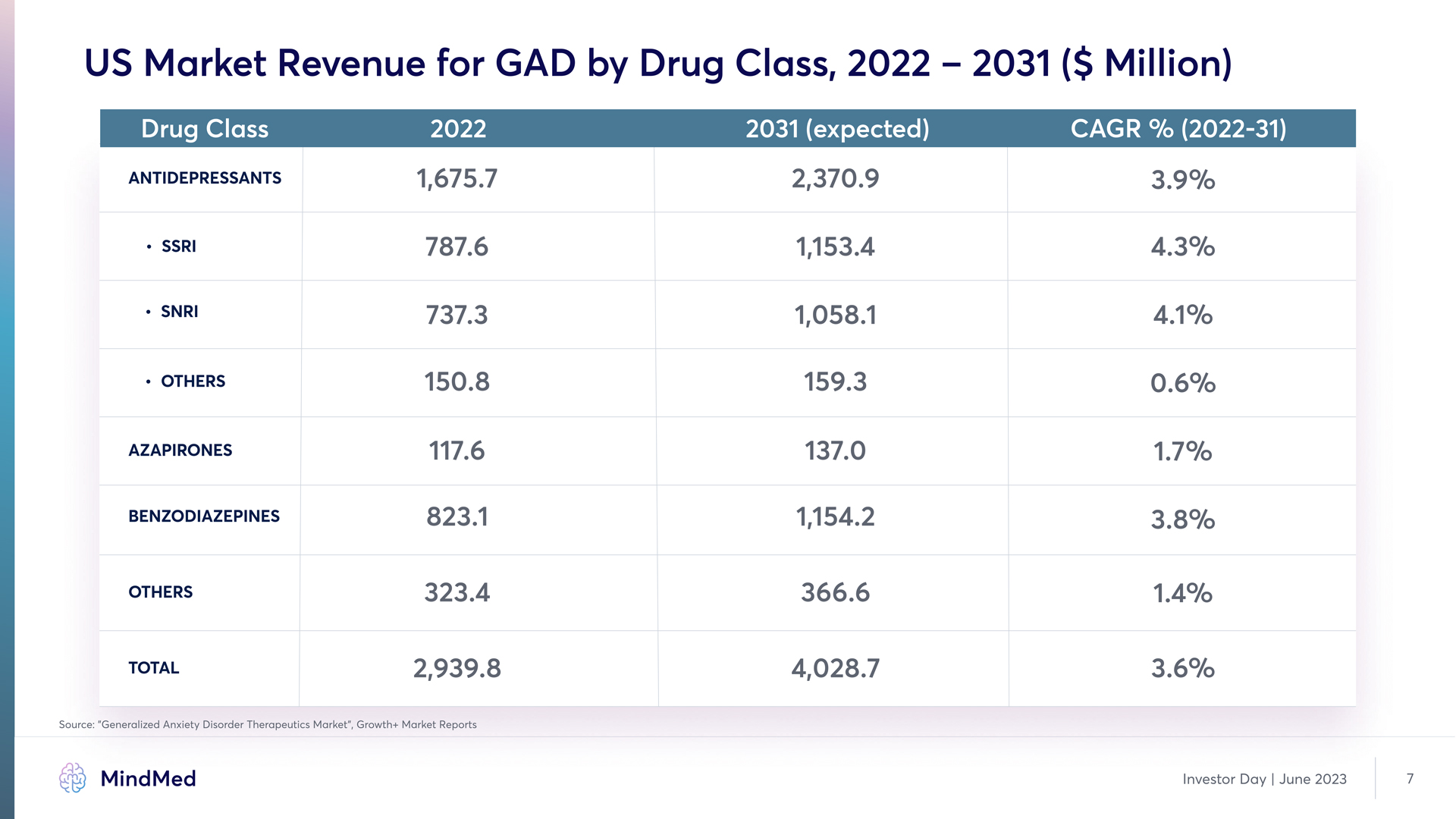
US Market Revenue for GAD by Drug Class, 2022 – 2031 ($ Million) Drug Class 2022 2031 (expected) cagr % (2022-31) Antidepressants SSRI SNRI Others Azapirones benzodiazepines Others TOTAL 1,675.7 787.6 737.3 150.8 117.6 823.1 323.4 2,939.8 2,370.9 1,153.4 1,058.1 159.3 137.0 1,154.2 366.6 4,028.7 3.9% 4.3% 4.1% 0.6% 1.7% 3.8% 1.4% 3.6% Source: ”Generalized Anxiety Disorder Therapeutics Market”, Growth+ Market Reports MindMed Investor Day | June 2023 7
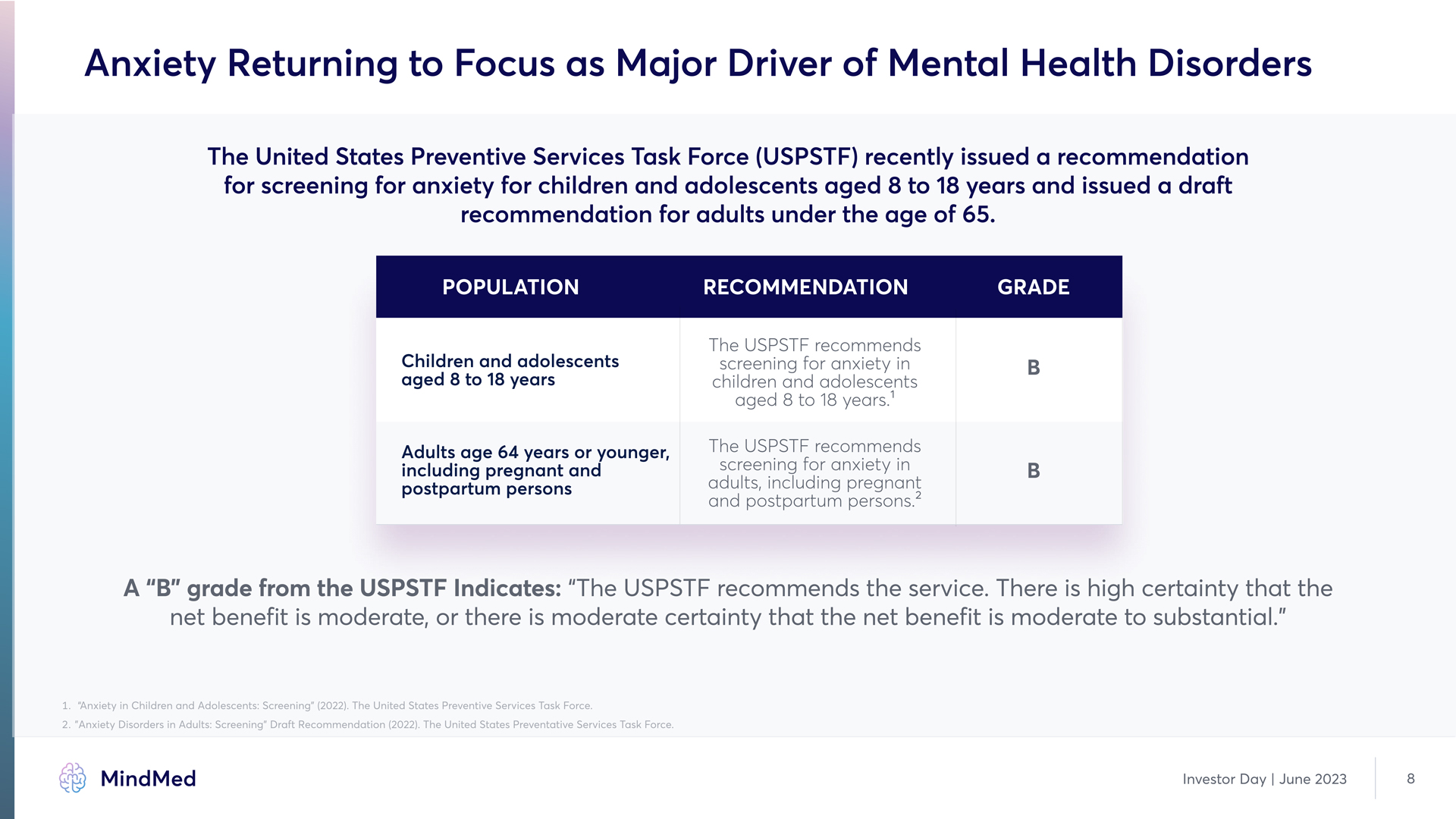
Anxiety Returning to Focus as Major Driver of Mental Health Disorders The United States Preventive Services Task Force (USPSTF) recently issued a recommendation for screening for anxiety for children and adolescents aged 8 to 18 years and issued a draft recommendation for adults under the age of 65. Population recommendation grade Children and adolescents aged 8 to 18 years B Adults age 64 years or younger, including pregnant and postpartum persons The USPSTF recommends screening for anxiety in children and adolescents aged 8 to 18 years.1 The USPSTF recommends screening for anxiety in adults, including pregnant and postpartum persons.2 B B A “B” grade from the USPSTF Indicates: “The USPSTF recommends the service3 There is high certainty that the net benefit is moderate, or there is moderate certainty that the net benefit is moderate to substantial.”1. “Anxiety in Children and Adolescents: Screening” (2022)3 The United States Preventive Services Task Force. 2. "Anxiety Disorders in Adults: Screening" Draft Recommendation (2022). The United States Preventative Services Task Force. MindMed Investor Day | June 2023 8
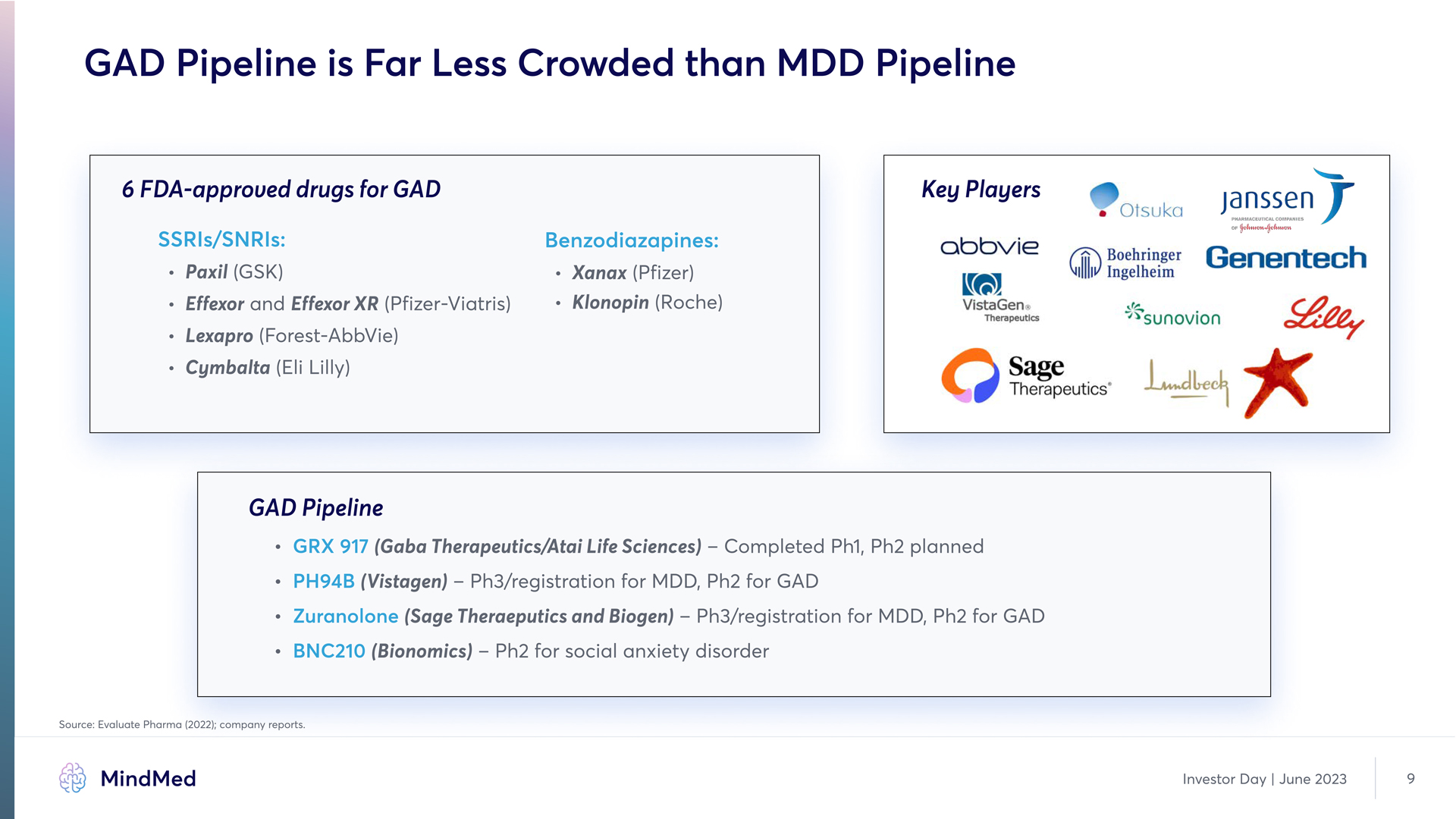
GAD Pipeline is Far Less Crowded than MDD Pipeline 6 FDA-approved drugs for GAD Key Players SSRIs/SORIs: Benzodiazapines: Paxil (GvKu) Effexor and Effexor XR (Pfizer-Viatris) Lexapro (Forest-AbbVie) Cymbalta (Eli Lilly) Xanax (Pfizer) Klonopin (Roche) Company Logo GAD Pipeline GRX 917 PH94B Zuranolone BOC210 (Gaba Therapeutics/Atai Life Sciences) – Completed Ph1, Ph2 planned (Vistagen) – Ph3/registration for MDD, Ph2 for GAD (Bionomics) – Ph2 for social anxiety disorder vource: Evaluate Pharma (2022); company reports. MindMed Investor Day | June 2023 9
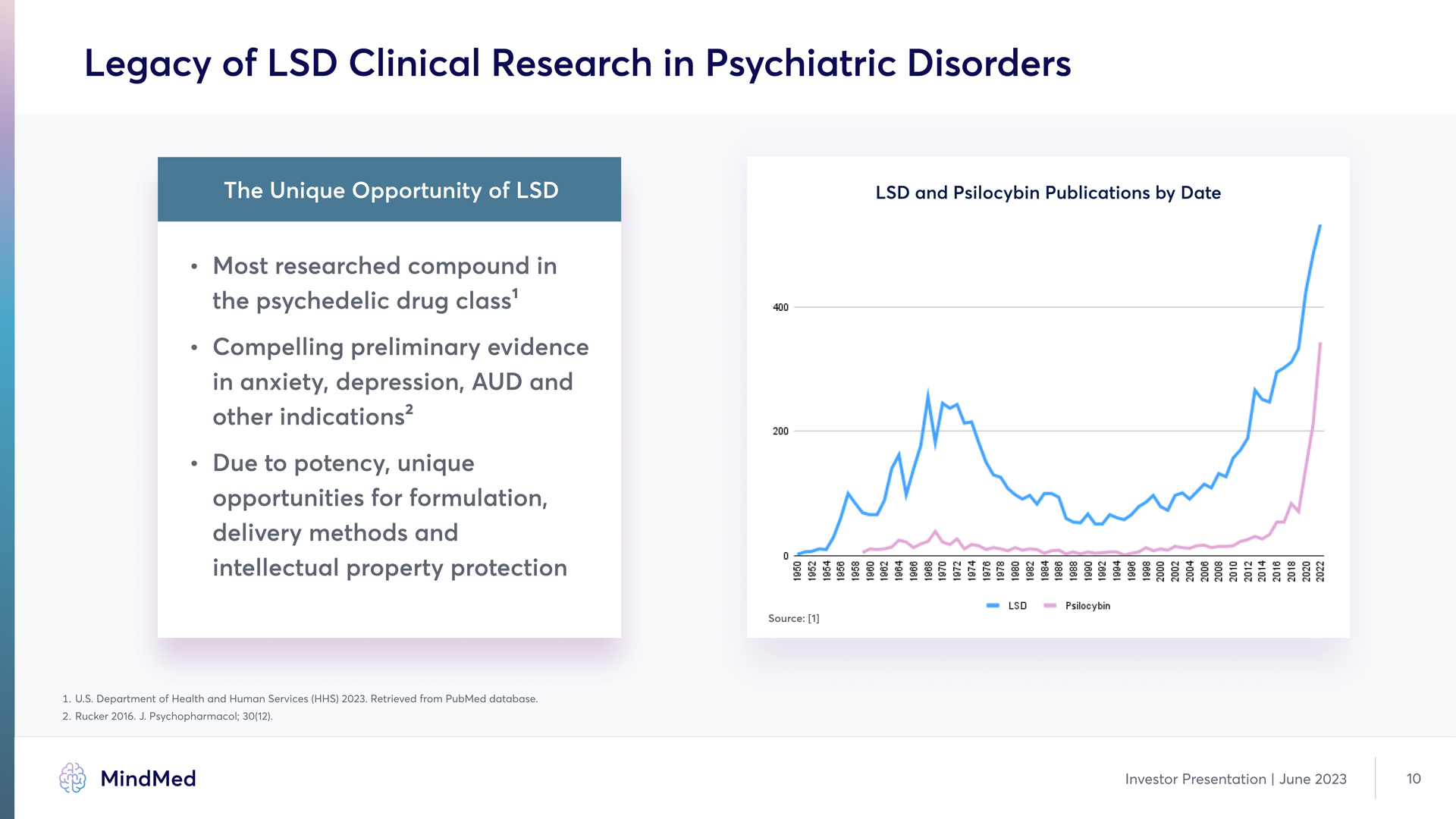
Legacy of LSD Clinical Research in Psychiatric Disorders The Unique Opportunity of LSD LSD and Psilocybin Publications by Date Most researched compound in the psychedelic drug class 1 Compelling preliminary evidence in anxiety, depression, AUD and other indicationsy 2 Due to potency, unique opportunities for formulation, delivery methods and intellectual property protection 400 200 0 1950 1952 1954 1956 1958 1960 1962 1964 1966 1968 1968 1972 1974 1976 1978 1980 1982 1984 1986 1988 1990 1992 1994 1996 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 2020 2022 LSD Psilocybin Source; [1Y] 1. UQSQ Department of Health and Human Services (HHS) 2023Q Retrieved from Pu4Med database. 2. Rucker 2016Q JQ Psychopharmacol; 30(12). MindMed Investor Presentation | June 2023 10
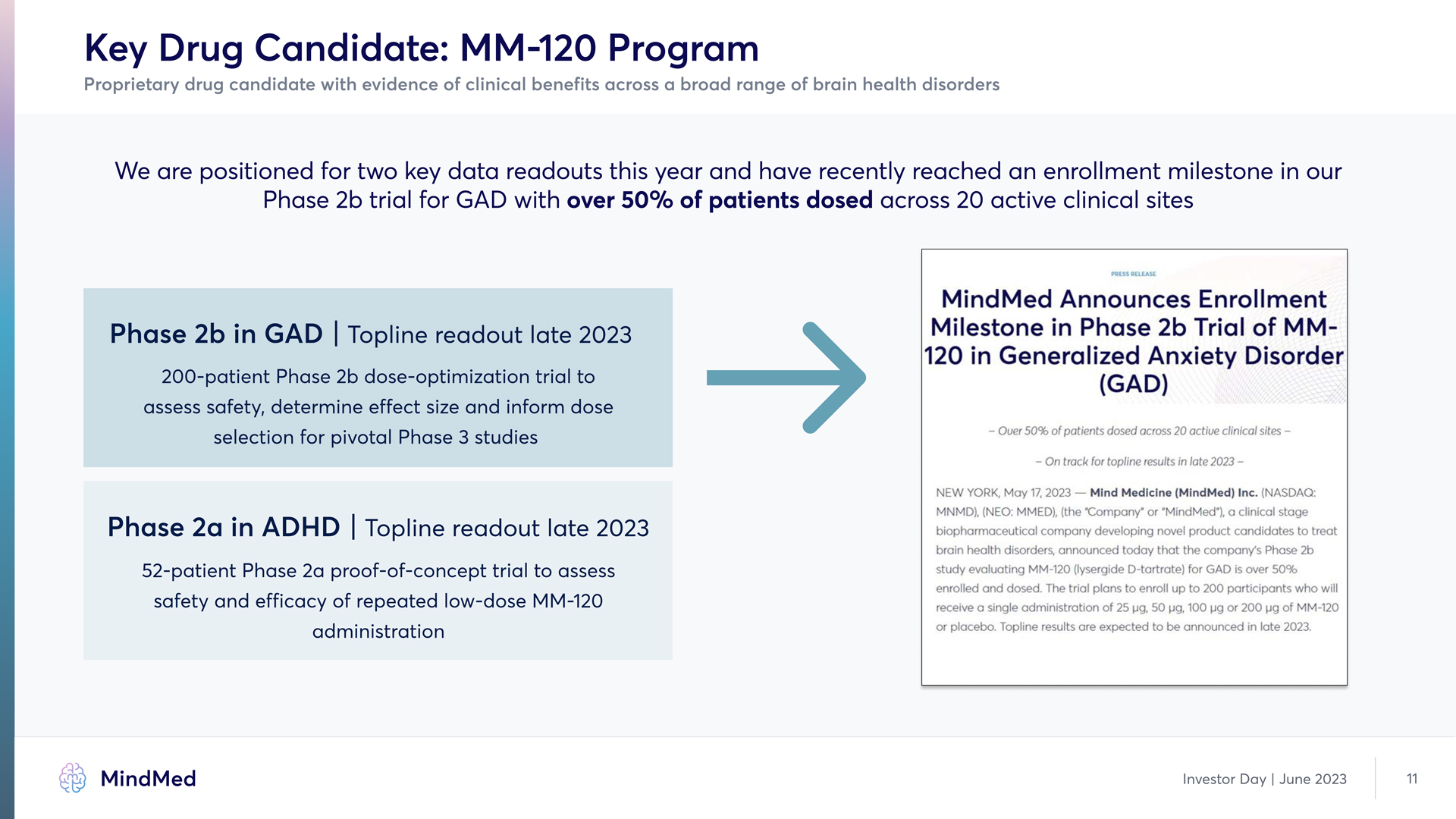
Key Drug Candidate: MM-120 Program Proprietary drug candidate with evidence of clinical benefits across a broad range of brain health disorders We are positioned for two key data readouts this year and have recently reached an enrollment milestone in our Phase 2b trial for GAD with over 50% of patients dosed across 20 active clinical sites Phase 2b in GAD | Topline readout late 2023 200-patient Phase 2b dose-optimization trial to assess safety, determine effect size and inform dose selection for pivotal Phase 3 studies Phase 2a in ADHD | Topline readout late 2023 52-patient Phase 2a proof-of-concept trial to assess safety and efficacy of repeated low-dose MM-120 administration PRESS RELEASE MindMed Announces Enrollment Milestone in Phase 2b Trial of MM- 120 in Generalized Anxiety Disorder (GAD) - Over 50% of patients dosed across 20 active clinical sites - - On track for topline results in late 2023 - NEW YORK, May 17, 2023-Mind Medicine (MindMed) Inc. (NASDAQ: MNMD), (NEO: MMED), (the "Company" or "MindMed"), a clinical stage biopharmaceutical company developing novel product candidates to treat brain health disorders, announced today that the company's Phase 2b study evaluating MM-120 (lysergide D-tartrate) for GAD is over 50% enrolled and dosed. The trial plans to enroll up to 200 participants who will receive a single administration of 25 µg, 50 µg, 100 µg or 200 µg of MM-120 or placebo. Topline results are expected to be announced in late 2023. MindMed Investor Day | June 2023 11
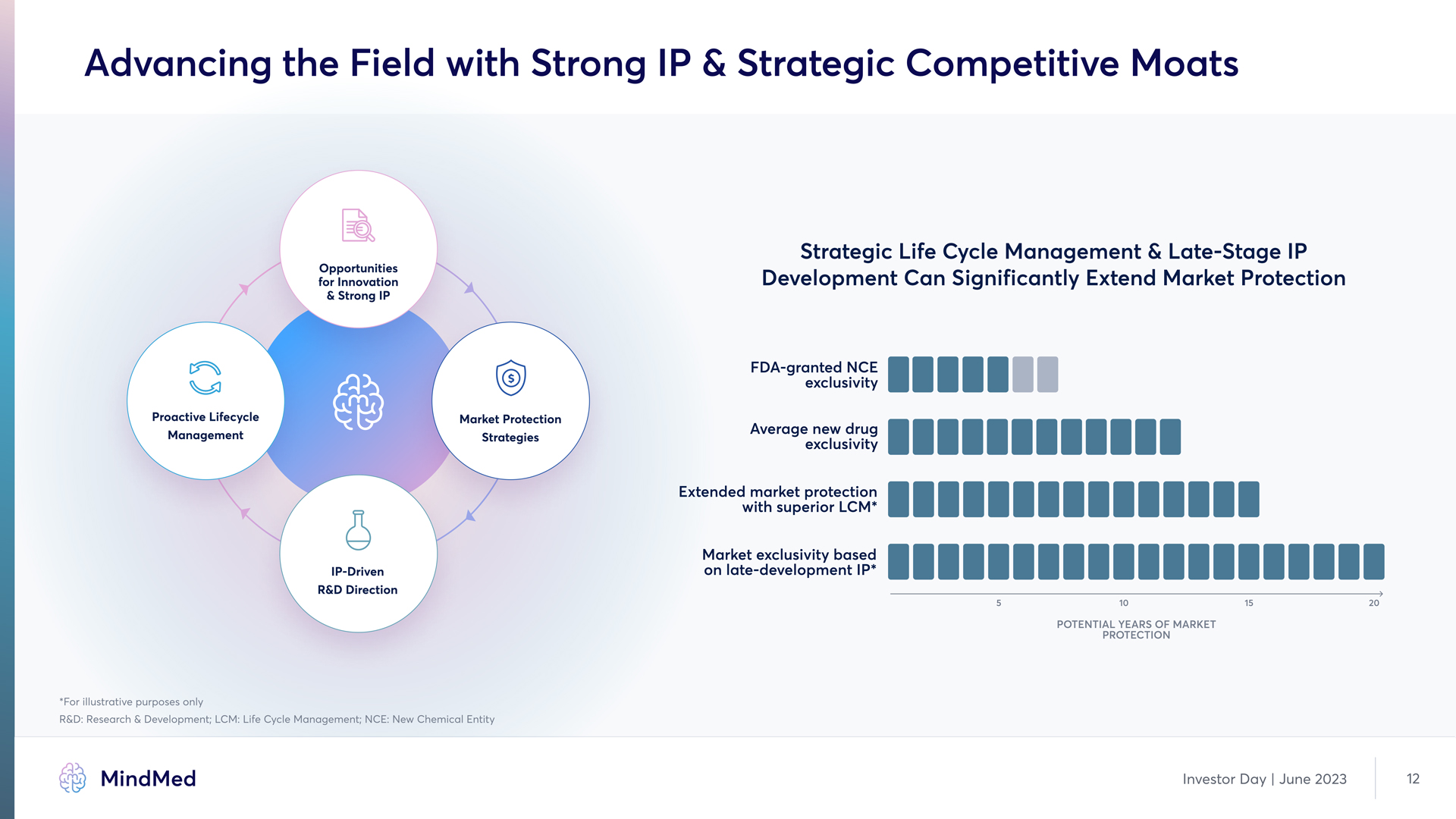
Advancing the Field with Strong IP & Strategic Competitive Moats Opportunities for Innovation & Strong IP Proactive Lifecycle Management Market Protection Strategies IP-Driven R&D Direction Strategic Life Cycle Management & Late-Stage IP Development Can Significantly Extend Market Protection FDA-granted NCE exclusivity Average new drug exclusivity Extended market protection with superior LCM* Market exclusivity based on late-development IP POTENTIAL YEARS OF MARKET 5 10 15 20 PROTECTION For illustrative purposes only R&D; Research & Development LCM Life Cycle Management NCE New Chemical E MindMed Investor Day | June 2023 12
Table of Contents 01 Opening Remarks 02 Unmet Need & Patient Journey in GAD 03 Practical Aspects of Monitored Therapies 04 Q&A for Sessions 2 and 3 Payer Considerations in New Medication Coverage 05 06 07 The IP Landscape Corporate Update 08 MindMed Q&A and Concluding Remarks Investor Day June 2023) 13

Unmet Need & Patient Journey in GAD - Maria Oquendo, MD Ruth Meltzer Professor and Chairman of Psychiatry at University of Pennsylvania Psychiatrist-in-Chief at the Hospital of the University of Pennsylvania President of the Board of Directors, American Foundation for Suicide Prevention Vice President, College of International Neuropsychopharmacology . Board of Trustees, Tufts University Over 450 peer-reviewed publications with over 18,000 citations Disclaimer Opinions expressed on the speaker's and in no way reflect the spoons of any organizations Member MindMed Scientific Advisory Board Professor of Psychiatry, Penn Medicine RAR Penn UNIVERSITY OF PENNSYLVANIA MindMed Investor Day | June 2023 14
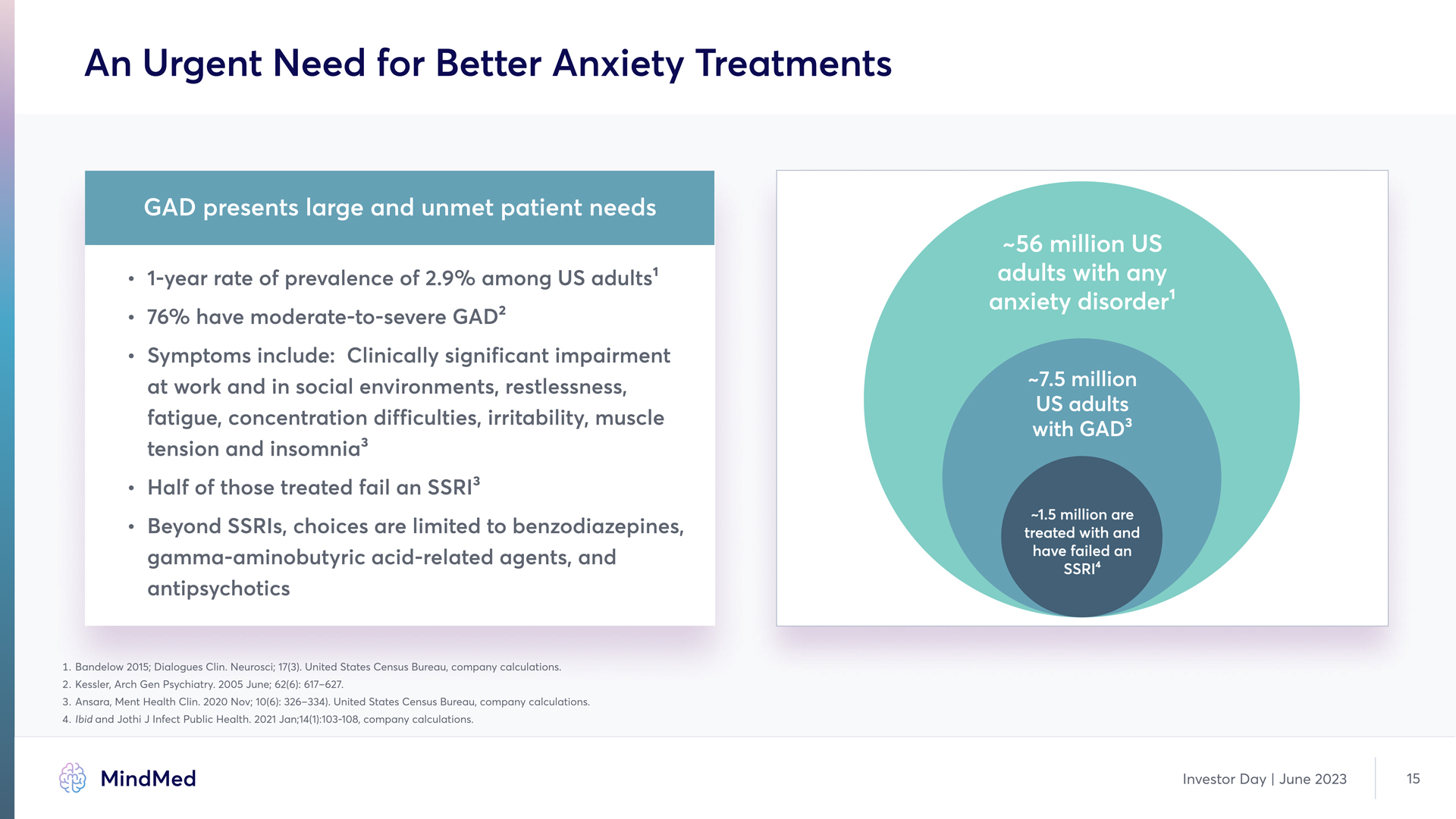
An Urgent Need for Better Anxiety Treatments GAD presents large and unmet patient needs 1-year rate of prevalence of 2.9% among US adults' 76% have moderate-to-severe GAD2 Symptoms include: Clinically significant impairment at work and in social environments, restlessness, fatigue, concentration difficulties, irritability, muscle tension and insomnia Half of those treated fail an SSRI' -56 million US adults with any anxiety disorder -7.5 million US adults with GAD Beyond SSRIs, choices are limited to benzodiazepines, gamma-aminobutyric acid-related agents, and antipsychotics -1.5 million are treated with and have failed an SSRI41. Bandelow 2015 Dialogues Clin Neurosci 131 United States Census Bureau company calculations. 2. Kessler, Arch Gen Psychiatry 2005 June 62(6): 617-627. 3. Ansara, Ment Health Clin. 2020 Nov: 10(6): 326-334) United States Census Bureau, company calculations. 4.
ibid and Jothi J Infect Public Health 2021 Jon,14(1):103-108, company calculations MindMed Investor Day | June 2023 15 Anxiety Correlated with Significant Impairment Evidence of greater impairment for those with higher anxiety severity 1 An anxiety disorder is also associated with less accomplishment at work, reduced labor force participation2 Associated with significantly higher rates of cardiac disorders, hypertension, gastrointestinal problems, genitourinary disorders and migraine3 1 Erickson SR, Guthrie S, VanEtten‐Lee M, et al. Severity of anxiety and work-related outcomes of patients with anxiety disorders. Depression and Anxiety. 2009;26(12)q1165-11o1 2 Waghorn G, Chant D, White P, Whiteford H. Disability, Employment and Work Performance Among People with ICD-10 anxiety disorders. Australian and New Zealand Journal of Psychiatry. 2005;39(1-2)q55-66. 3 Kariuki-Nyuthe C, Stein DJ, Kariuki-Nyuthe C, Stein DJ. Anxiety and Related Disorders and Physical Illness. In: Maj M, ed Key Issues in Mental Health 2014:81-8o. Investor Day | June 2023 16

Need for Additional Treatment Options is Clear. individual variations: GAD is not a once size fits all indication; it is a very diverse disorder Co-occurring conditions (e.g., depression or PTSD) may complicate treatment Treatment resistance: 50% fail first line (SSR.) treatment Side effects: Current choices come with the potential for long term side effects, which reduce compliance 1. Ansara, Ment Health Clin. 2020 Nov; 10(6): 326–334). United States Census Bureau, company calculations. Investor Day | June 2023 17
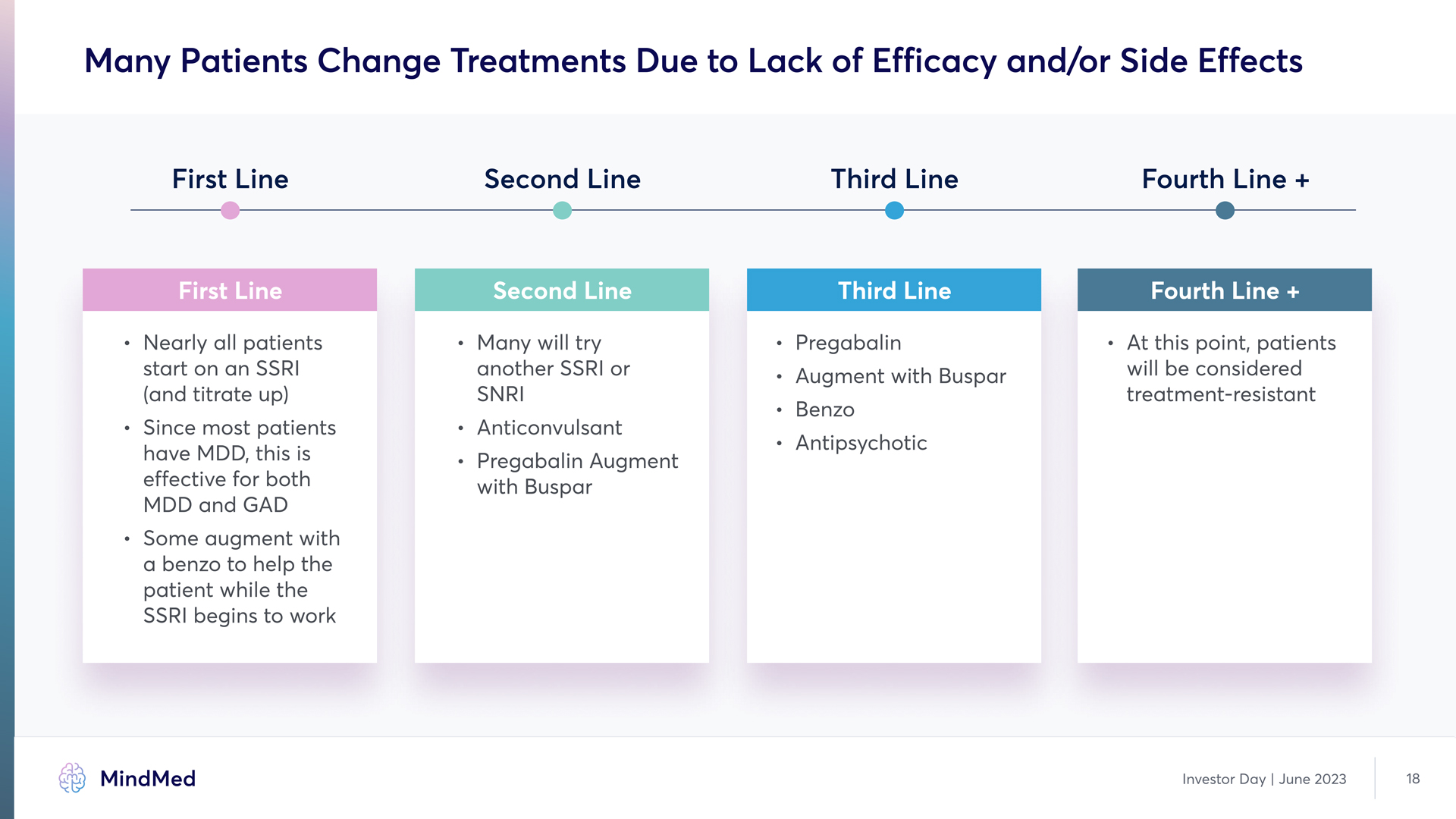
Many Patients Change Treatments Due to Lack of Efficacy and/or Side Effects First Line Second Line Third Line Fourth Line + First Line Nearly all patients start on an SSRI (and titrate up` l Since most patients have MDD, this is effective for both MDD and GAD l Some augment with a benzo to help the patient while the SSRI begins to work Second Line Many will try another SSRI or SNR Anticonvulsant Pregabalin Augment with Buspar Third Line Pregabalin augment with Buspar Benzo Antipsychotic Fourth Line + At this point, patients will be considered treatment-resistant Investor Day | June 2023 18
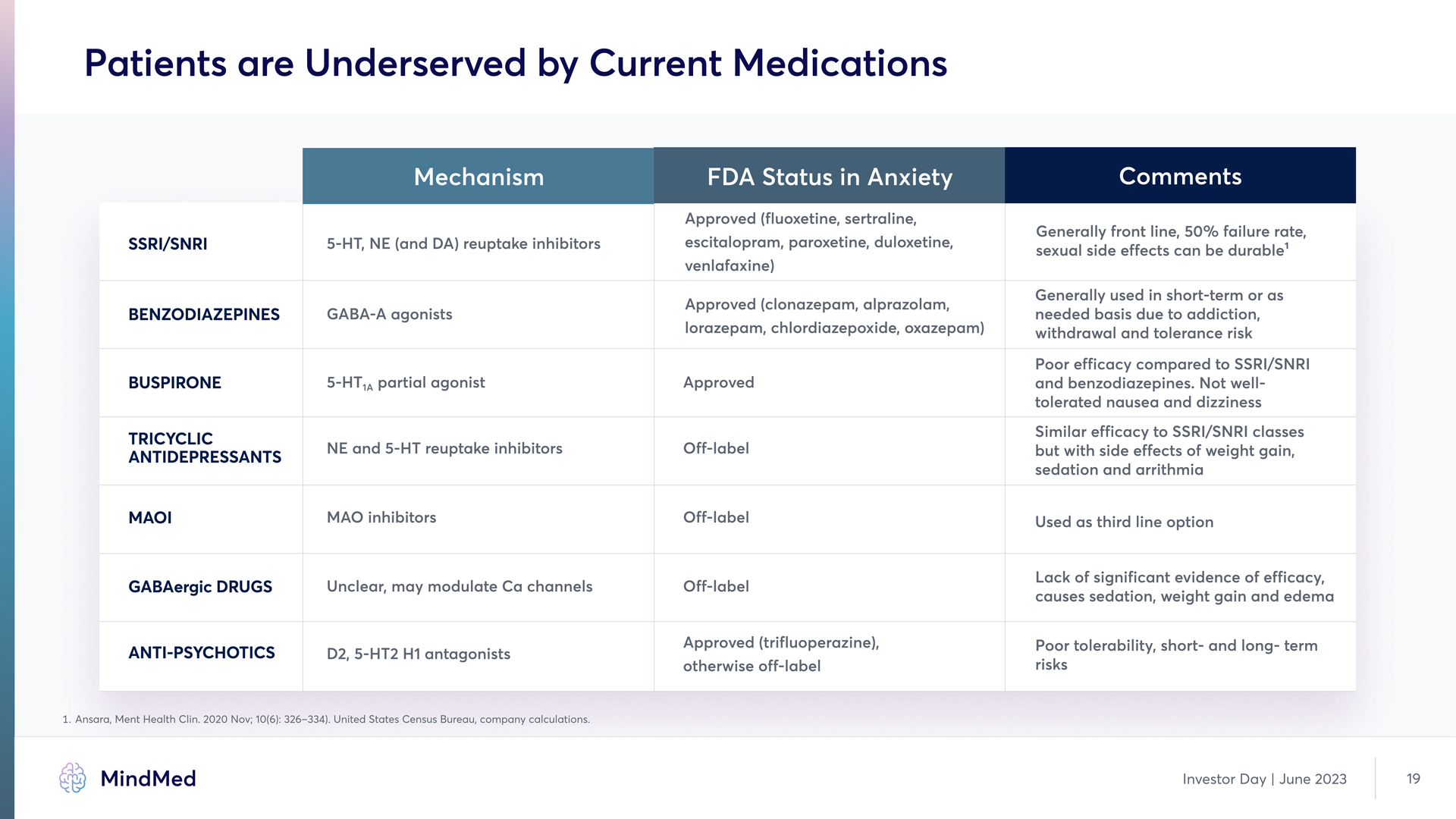
Patients are Underserved by Current Medications Mechanism FDA Status in Anxiety Comments SSRI/SNRI 5-HT, NE (and DA) reuptake inhibitors Approved (fluoxetine, sertraline, escitalopram, paroxetine, duloxetine, venlafaxine) Generally front line, 50% failure rate, sexual side effects can be durable1 Benzodiazepines GABA-A agonists Approved (clonazepam, alprazolam, lorazepam, chlordiazepoxide, oxazepam) Generally used in short-term or as needed basis due to addiction, withdrawal and tolerance risk Buspirone 5-HT1A partial agonist Approved Poor efficacy compared to SSRI/SNRI and benzodiazepines. Not well tolerated nausea and dizziness Tricyclic Antidepressants NE and 5-HT reuptake inhibitors Off-label Similar efficacy to SSRI/SNRI classes but with side effects of weight gain, sedation and arrithmia MAOI MAO inhibitors Off-label Used as third line option GABAergic drugs Unclear, may modulate Ca channels Off-label Lack of significant evidence of efficacy, causes sedation, weight gain and edema Anti-Psychotics D2, 5-HT2 H1 antagonists Approved (trifluoperazine), otherwise off-label Poor tolerability, short- and long- term risks 1 Ansara, Ment Health Clin. 2020 Nov; 10(6): 326–334). United States Census Bureau, company calculations. Investor Day | June 2023 19

Psychedelic Therapies Offer Promise Evidence of rapid and d3rable impacts in anxiety and depression Lesser concern for chronic safety issue Monitored and infreq3ent dosing promises greater compliance Investor Day | June 2023 20
Table of Contents 01 Opening Remarks 02 Unmet Need & Patient Journey in GAD 03 Practical Aspects of Monitored Therapies 04 Q&A for Sessions 2 and 3 05 Payer Considerations in New Medication Coverage 06 The IP Landscape 07 Corporate Update 08 Q&A and Concluding Remarks Investor Day | June 2023 21
Practical Aspects of Monitored Therapies – David Feifel, MD, PhD Founder and Medical Director, Kadima Neuropsychiatry institute l Professor Emeritus of Psychiatry, UC San Diego, where he was Director of the Neuropsychiatry and Behavioral Medicine Program and established world's first ketamine infusion program for depression l Member, American College of Neuropharmacology l Member, Psychedelic Task Force, National Network of Depression Centers l Author or co-author of over 140 peer-reviewed publications on topics related to treating mental illness, including novel treatments such as Transcranial Magnetic Stimulation (TMS) and ketamine therapy Founder, Kadima Neuropsychiatry institute Disclaimer: Opinions expressed are the speaker’s own and in no way reflect the opinions of Kadima or any other organization; Principal Investigator at a clinical site for MindMed’s MMED008 Phase 2b trial Investor Day | June 2023 22

The Kadima Neuropsychiatry Institute Founded to more effectively pursue cutting edge, non-invasive treatments for neuropsychiatric disorders Specializing in depression, anxiety, PTSD, OCD, eating disorders and related conditions Providing ~75 ketamine treatments daily Clinical site for MindMed, Beckley Psytech and Compass Pathways trials Investor Day | June 2023 23
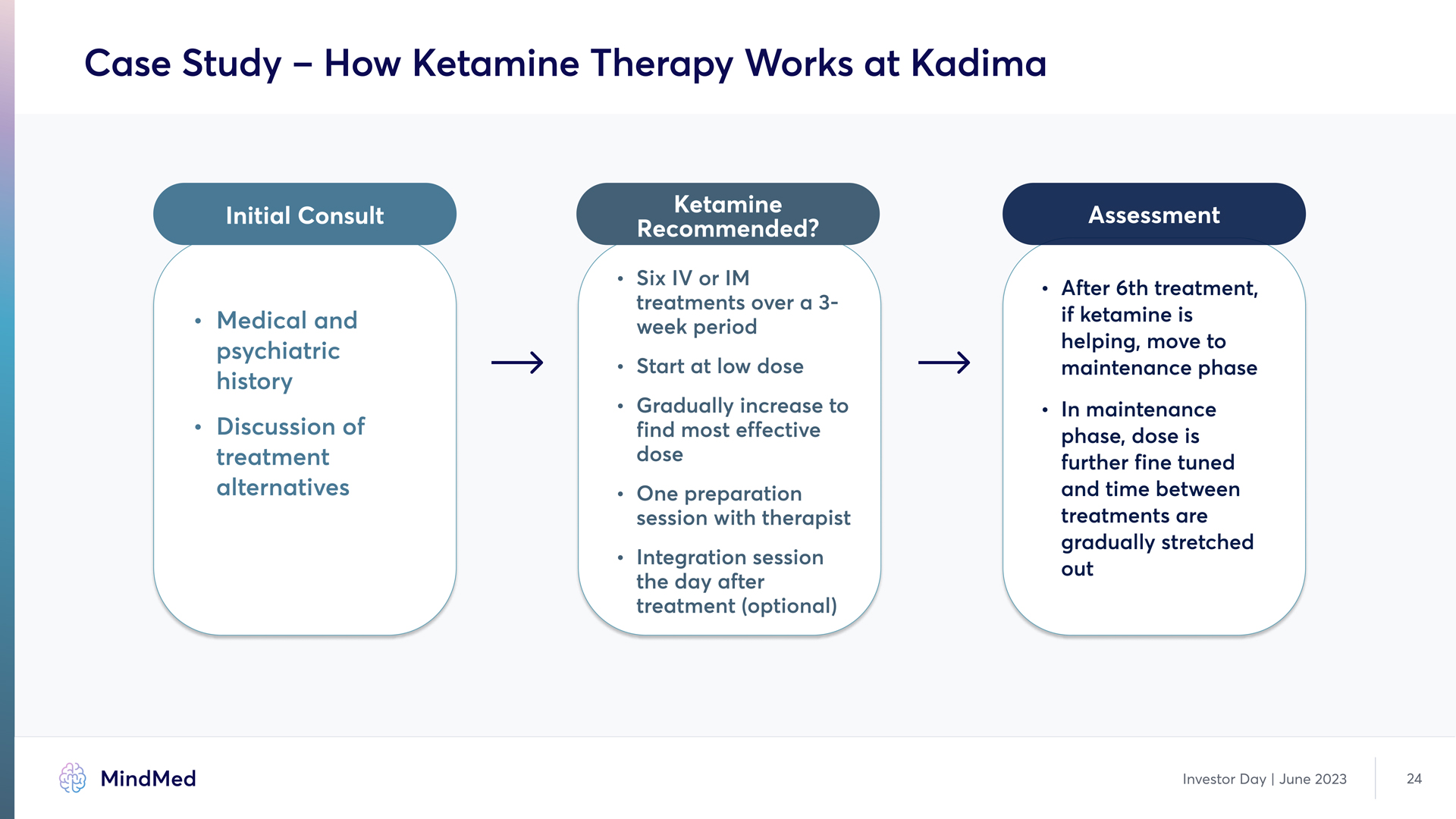
Case Study – How Ketamine Therapy Works at Kadima Initial Consult Medical and psychiatric history Discussion of treatment alternatives Ketamine Recommended? Six IV or IM treatments over a 3- week period` R Start at low dose R Gradually increase to find most effective dose R One preparation session with therapist R Integration session the day after treatment Optional Assessment After 6th treatment. if ketamine is helping. move to maintenance phase In maintenance phase. dose is further fine-tuned and time between treatments are gradually stretched out Investor Day | June 2023 24

Case Study – How Ketamine Therapy Works at Kadima Investor Day | June 2023 25
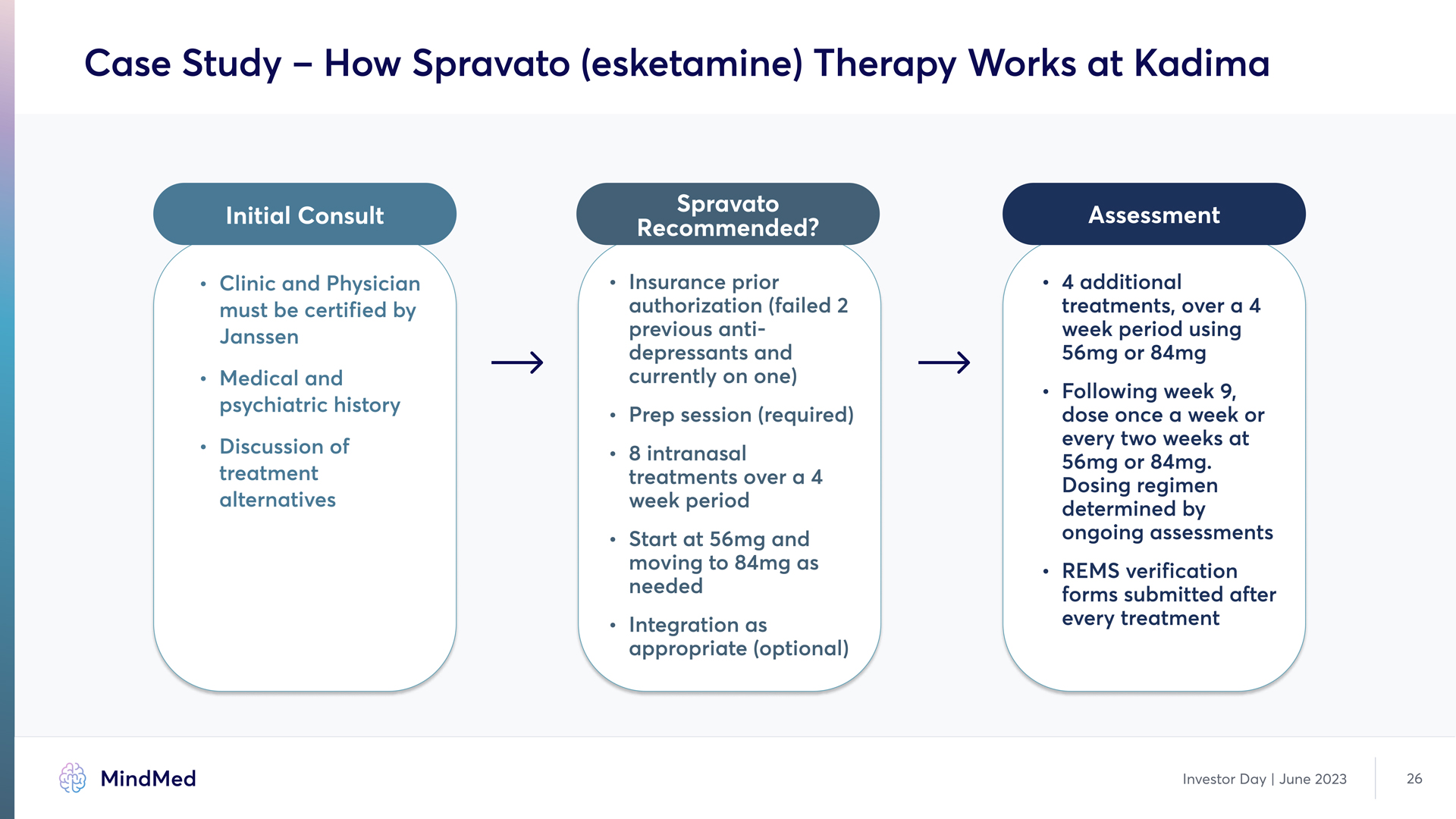
Case Study – How Spravato (esketamine) Therapy Works at Kadima Initial Consult Clinic and Physician must be certified by Janssen G Medical and psychiatric history Discussion of treatment alternatives Spravato Recommended? Insurance prior authorization (failed 2 previous antidepressants and currently on one~ G Prep session (required~ G 8 intranasal treatments over a 4 week period G Start at 56mg and moving to 84mg as needed G Integration as appropriate (optional) Assessment 4 additional treatments, over a 4 week period using 56mg or 84mœ G Following week 9, dose once a week or every two weeks at 56mg or 84mg. Dosing regimen determined by ongoing assessment£ G REMS verification forms submitted after every treatment Investor Day | June 2023 26
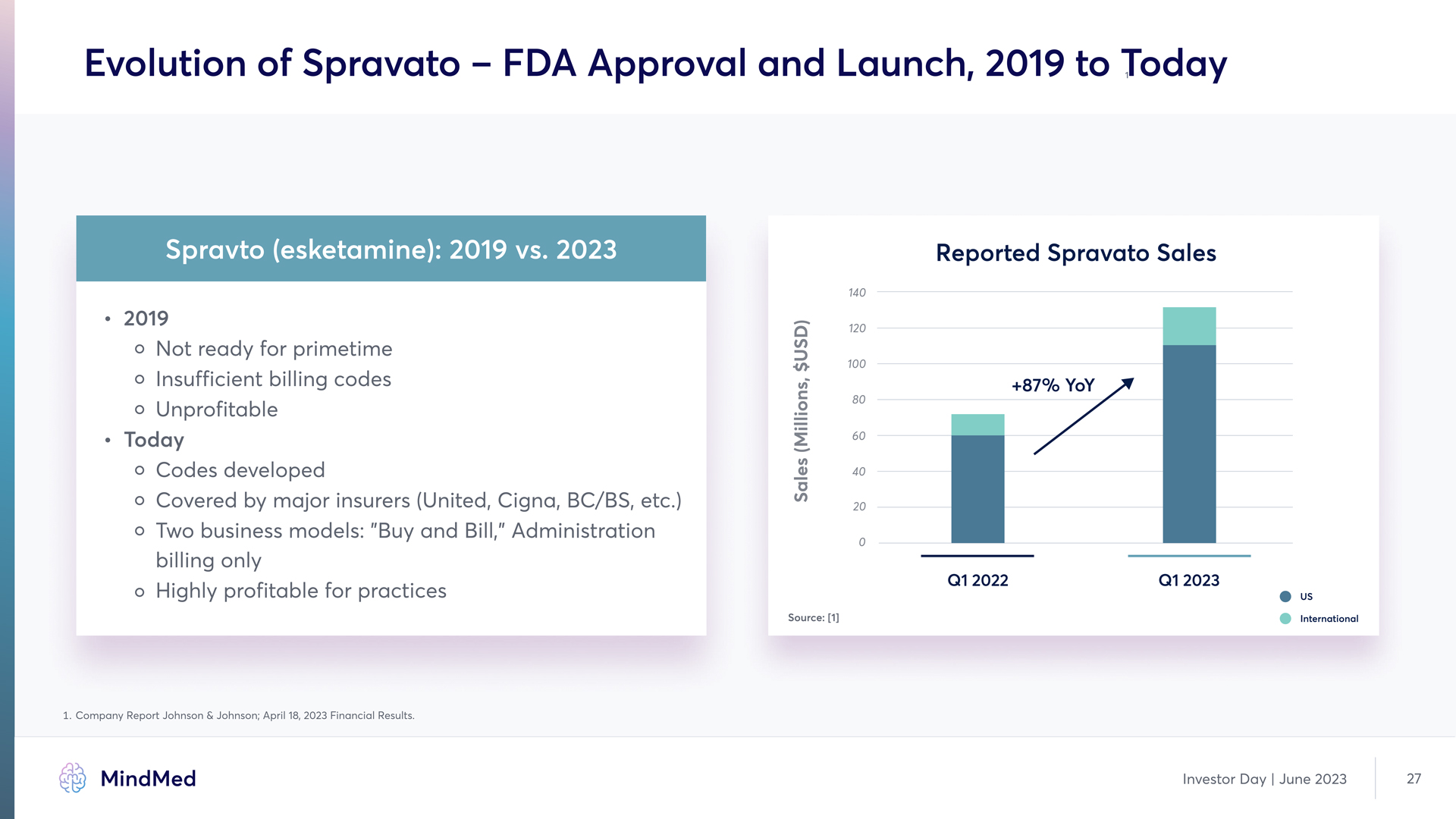
Evolution of Spravato – FDA Approval and Launch, 2019 to Today Spravto (esketamine): 2019 vs. 2023 2019 Not ready for primetime Insufficient billing codes Unprofitable Today Codes developed Covered by major insurers (Unite, Cigna, BC/BS, etc. Two business models: "Buy an Bill," Administration billing only Highly profitable for practices Reported Spravato Sales +87% YoY Graph 1. Company Report Johnson & Johnson; April 18, 2023 Financial Results. Investor Day | June 2023 27

The Experience with TMS Therapy Deep Transcranial Magnetic Stimulation (TMS) therapy is a cutting-edge, non-medication treatment that is FDA approved for depression, anxiety, OCD and smoking addiction. TMS uses a pulsating magnetic field designed to stimulate nerve cells Neurons) in the specific brain regions that are underactive in people with depression, anxiety, and other neuropsychiatric disorders. Involves 30-40 outpatient sessions of 18-36 minutes each over a course of 6 weeks (9-24 hours of total session time over that period) Widely accepted and reimbursed by insurers Investor Day | June 2023 28

Potential and Promise of Psychedelic Therapy Potential for rapid, long-lasting relief from a variety of symptoms we have years of experience and infrastructure for monitoring multiple sessions simultaneously through our work with ketamine and Spravato, as well as a participant in MindMed and Compass trial4 8 9hile the MM-120 monitoring session would potentially be longer than with ketamine and Spravato, total monitoring time is much shorter due to fewer session4 8 9ith profitability will come additional sites willing to administer and monitor the treatment Investor Day | June 2023 29

MindMed Q&A for Sessions 2 and 3 Investor Day | June 2023 30
Table of Contents 01 02 03 04 05 06 07 08 Opening Remarks Unmet Need & Patient Journey in GAD Practical Aspects of Monitored Therapies & Digital Medicine Q&A for Sessions 2 and 3 Payer Considerations in New Medication Coverage Corporate Update The IP Landscape Q&A and Concluding Remarks MindMed Investor Day | June 2023 31

Payer Considerations in New Medication Coverage – Michael Kobernick, MD Disclaimer: Opinions expressed are the speaker’s own and in no way reflect the opinions of Blue Cross Blue Shield of Michigan or any other organization. r Senior Medical Director, Blue Crosss/Blue Shield of Michigan Advises large employers on opportunities to improve quality of care and reduce cost Lecturer, Jefferson College of Population Health Adjunct Assistant Professor, Madonna University Former Chief Medical Officer, SmartHealth Roles including Vice President of Medical Affairs at St. John Providence Health System Senior Medical Director, BCBS of Michigan Blue Cross Blue Shield of michigan MindMed Investor Day | June 2023 32
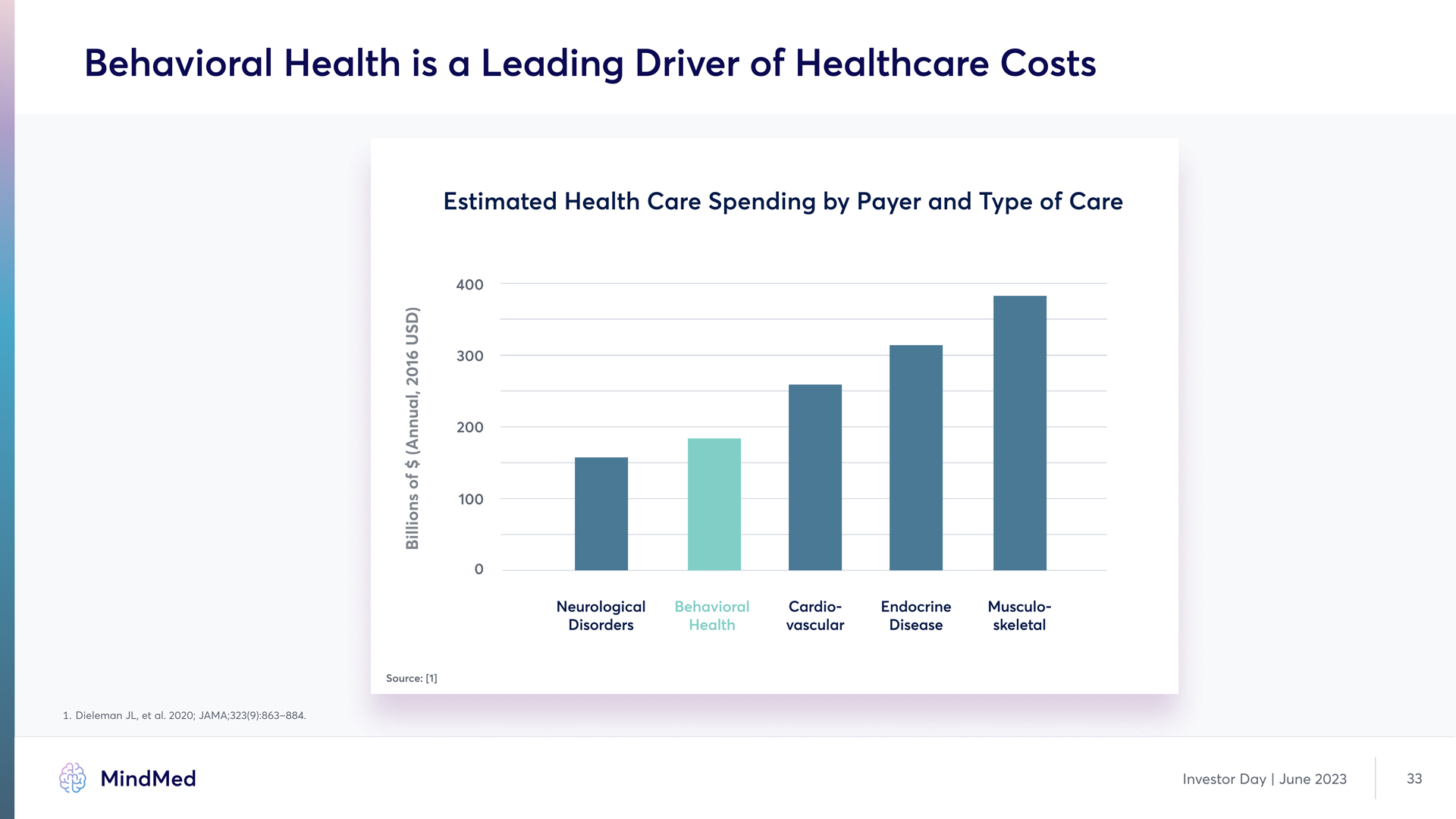
Behavioral Health is a Leading Driver of Healthcare Costs Estimated Health Care Spending by Payer and Type of Care 400 300 200 100 0 Neurological Disorders Behavioral Health Cardio-vascular Endocrine Disease Musculo- skeletal Billions of $ (Annual, 2016 USD) Source [1] HG Dieleman JL, et al. 2020; JAMA;323(9):863–884. MindMed Investor Day | June 2023 33
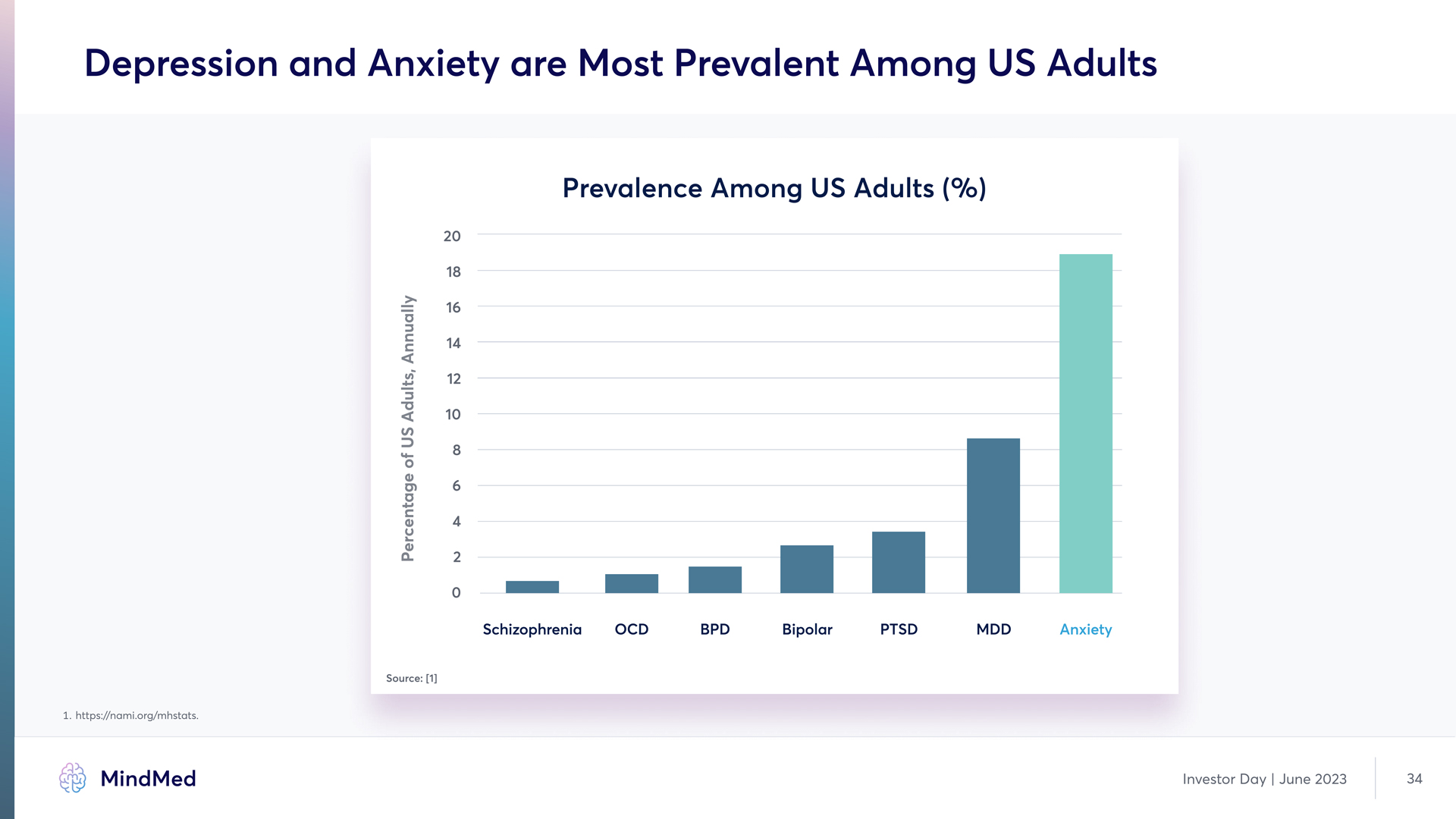
Depression and Anxiety are Most Prevalent Among US Adults Prevalence Among US Adults (%) 0 2 4 6 8 10 12 14 16 18 20 Schizophrenia OCD BPD Bipolar PTSD MDD Anxiety Percentage of US Adults, Annually Source: [1] 1. https://nami.org/mhstats. MindMed Investor Day | June 2023 34
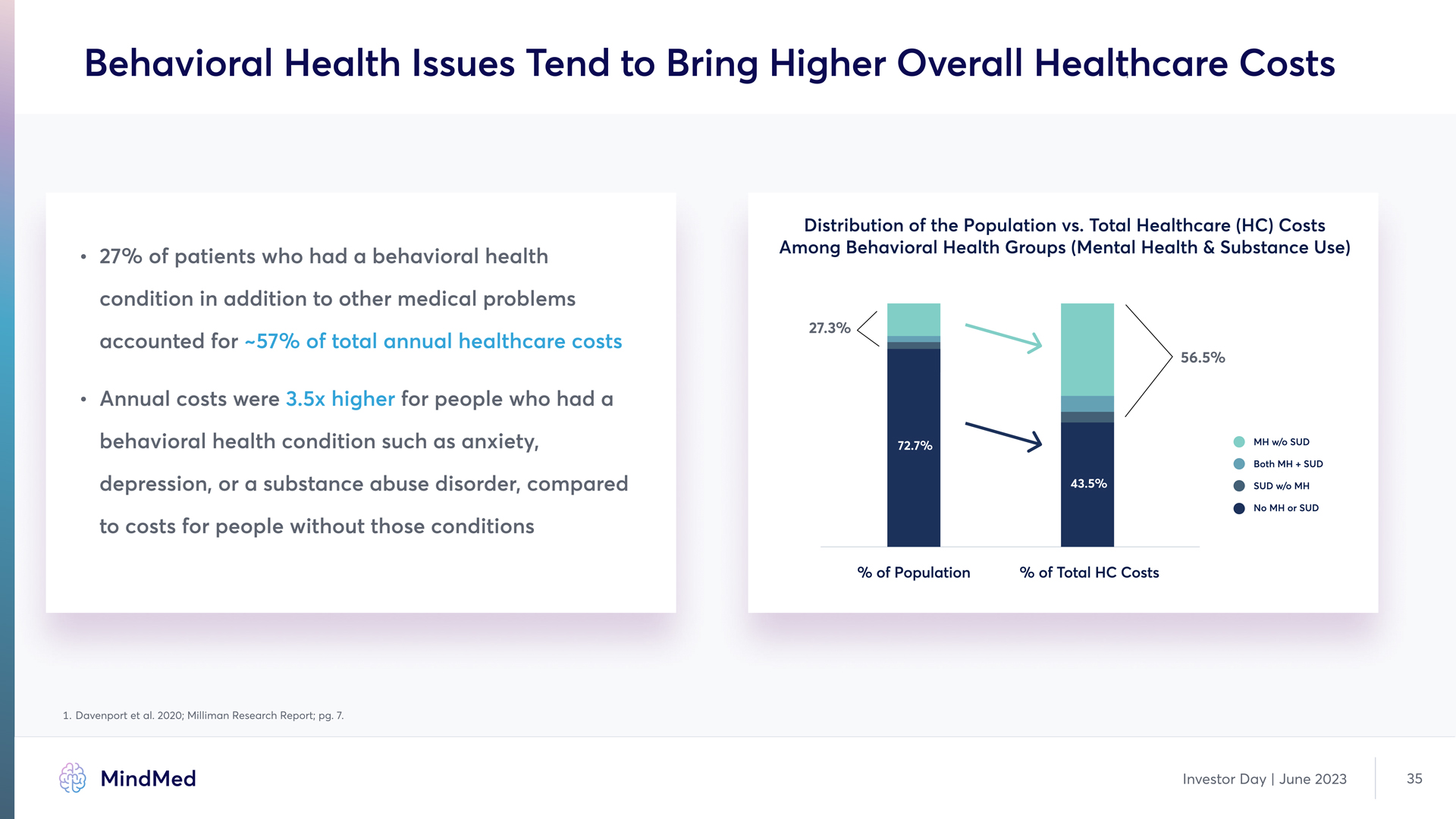
Behavioral Health Issues Tend to Bring Higher Overall Healthcare Costs a 27% of patients who had a behavioral health condition in addition to other medical problems accounted for a Annual costs were for people who had a behavioral health condition such as anxiety, depression, or a substance abuse disorder, compared to costs for people without those conditions ~57% of total annual healthcare costs 3.5x higher Distribution of the Population vs. Total Healthcare (HC) Costs Among Behavioral Health Groups (Mental Health & Substance Use) % of Population % of Total HC Costs MH w/o SUD Both MH + SUD SUD w/o MH No MH or SUD 27.3% 56.5% 1. Davenport et al. 2020; Milliman Research Report; pg. 7. MindMed Investor Day | June 2023 35

Behavioral Health is a Priority for Employers All employers want to address solutions for behavioral health Seen as an important social determinant of health All new approaches being embraced Virtual Care Models Co-Care Models – PCP + Psychiatrist Interest in Ketamine and Psychedelics MindMed Investor Day | June 2023 36

Factors For Medication Coverage Determination FDA Approval Clinical Efficacy Peer Reviewed Publications Informative but not Decisive Specialty Society Guidelines Subject Matter Expert Opinion MindMed Investor Day | June 2023 37

Monitoring and Administration Coverage Codes Currently Exist Observation Psychological Evaluation Evaluation and Management (EM) Precedents Spravato Sleep Studies MindMed Investor Day | June 2023 38
Table of Contents 01 02 03 04 05 06 07 08 Opening Remarks Unmet Need & Patient Journey in GAD Practical Aspects of Monitored Therapies Q&A for Sessions 2 and 3 Payer Considerations in New Medication Coverage The IP Landscape Corporate Update Q&A and Concluding Remarks MindMed Investor Day | June 2023 39
The IP Landscape – W. Chad Shear, JD Former principal in life sciences and pharmaceutical intellectual property (IP) at Fish & Richardson Led litigation on behalf of clients including Sunovion, Dainippon Sumitomo, Gilead Sciences, and Astellas Named an IP Trailblazer by The National Law Journal Former law clerk for the United States Court of Appeals for the Federal Circuit Partner, Intellectual Property at Cooley LLP Cooley Disclaimer: Opinions expressed are the speaker’s own and in no way reflect the opinions of Cooley LLP or any other organization; MindMed is a corporate client of Cooley LLP MindMed Investor Day | June 2023 40
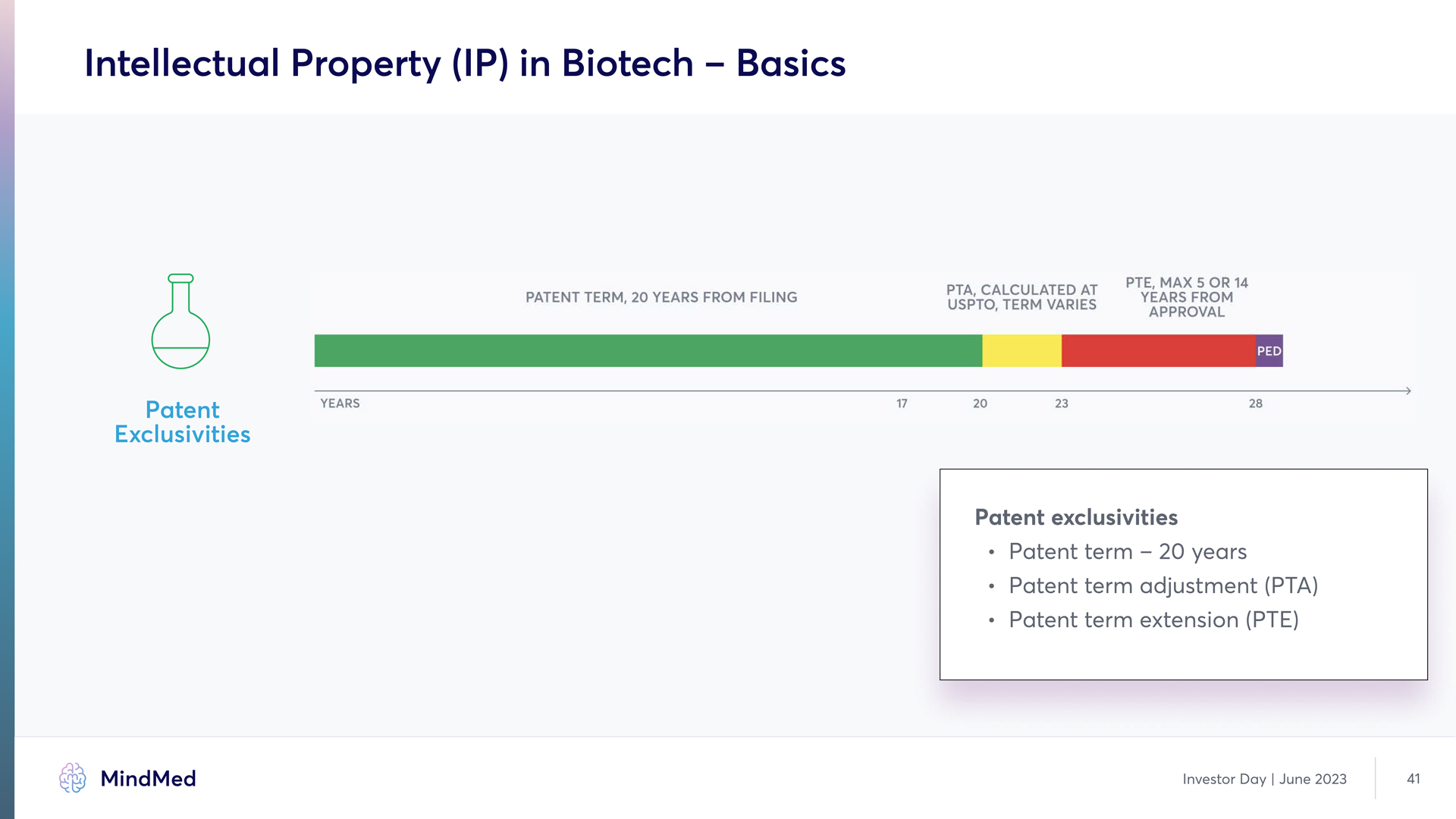
Intellectual Property (IP) in Biotech – Basics Patent Exclusivities PATENT TERN, 20 YEARS FROM FILING PTA, CALCULATED AT USPTO, TERM VERIES PTE, MAX 5 OR 14 YEAR FROM APPROVAL PED YEARS 17 20 23 28 Patent exclusivities Patent term – 20 years Patent term adjustment (PTA) Patent term extension (PTE) MindMed Analyst Day June 2023 41
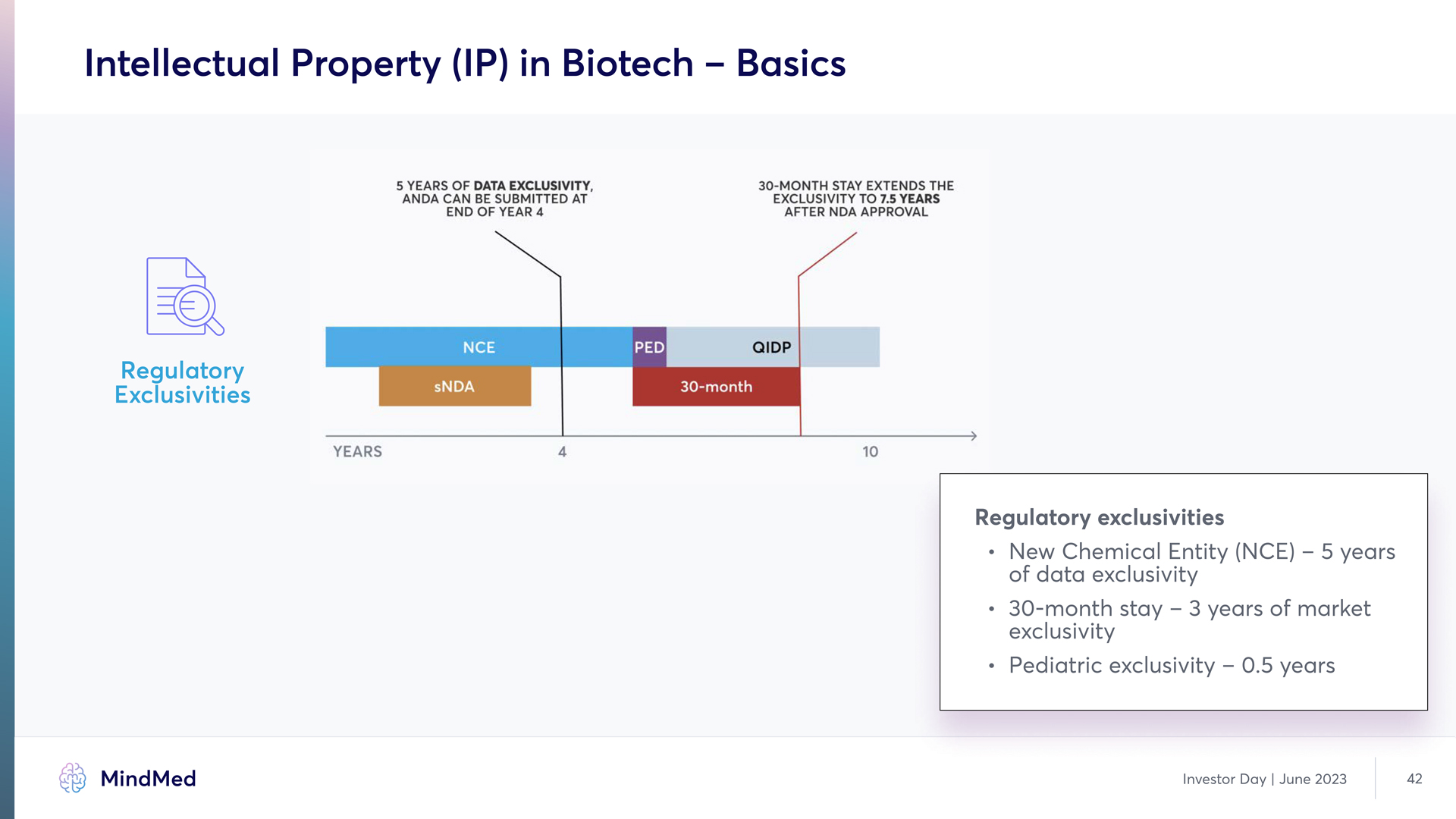
Intellectual Property (IP) in Biotech – Basics Regulatory Exclusivities 5 YEARS OF DATE EXCLUSIVITY ANDA CAN BE SUBMITTED AT END OF YEAR 4 30-MONTH STAY EXTENDS THE EXCLUSIVITY TO 7.5 YEARS AFTER NDA APPROVAL CE PED QIDP sNDA 30-MONTH YEARS 4 10 Regulatory exclusivities New Chemical Entity (NCE) – 5 years of data exclusivity 30-month stay – 3 years of market exclusivity Pediatric exclusivity – 0.5 years MindMed Analyst Day June 2023 42

What Can be Patented? New Compounds: These are new chemical compound or molecules that have never been described before Drug Formulations: Novel combinations, dosages, delivery systems, controlled-release formulations, or improved stability of the drug Manufacturing Processes: Novel synthesis routes, purification techniques, or formulation processes Medical Uses: Patents can be obtained for new therapeutic application techniques, or uses existing drugs Drug Delivery Systems: Novel drug system, Such as patches, implant, inhalers, or transdermal delivery methods, can be patented if they provide a unique and non-obvious solution REMS/Others: Specific technologies, or drug delivery systems that are part of the overall REMS program MindMed Investor Day June 2023 43

What are the Requirements for Patentability? Novelty: The invention should not have been previously known or used by others in any public form, such as in publications, patents, or public demonstrations Non-obviousness: The invention must involve an inventive step, meaning it must not be obvious to a person skilled in the relevant field Utility: The invention must have a specific and credible utility; it should be useful and serve a practical purpose MindMed Investor Day June 2023 44

Hatch-Waxman Act FDA Orange Book: Identifies dr7g prod7cts approved on the basis of safety and effectiveness by the Food and Drug Administration (FDA) under the Federal Food, Drug, and Cosmetic Act (the Act) and related patent and exclusivity information 30-month stay: Gives the brand product sponsor and patent holder a prescribed amount of time to assert patent rights in court before a generic competitor is approved and can market the drug MindMed Investor Day | June 2023 45
Table of Contents 01 Opening Remarks 02 Unmet Need & Patient Journey in GAD 03 Practical Aspects of Monitored Therapies 04 Q&A for Sessions 2 and 3 05 Payer Considerations in New Medication Coverage 06 The IP Landscape 07 Corporate Update 08 Q&A and Concluding Remarks MindMed Investor Day June 2023 46
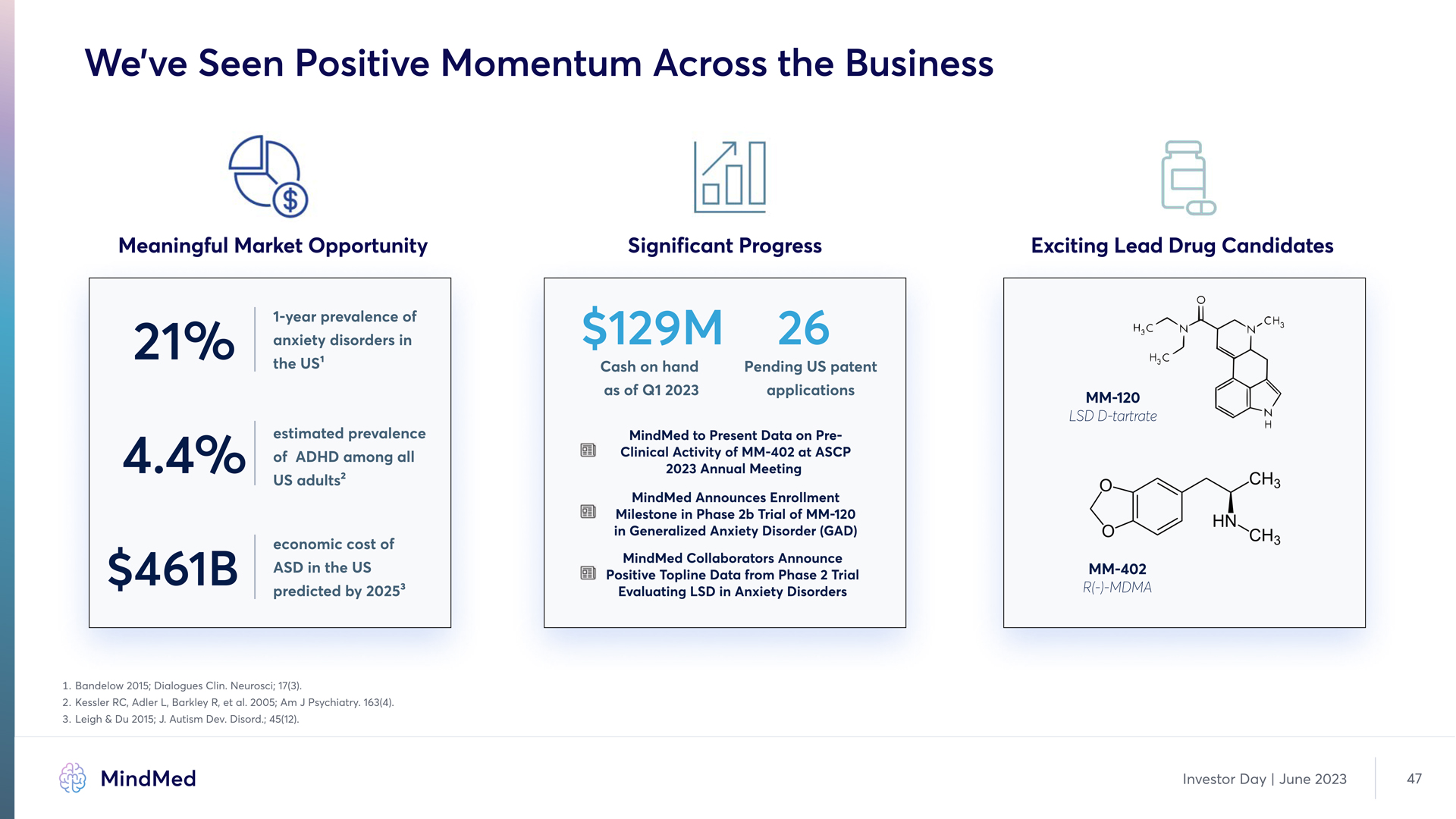
We’ve Seen Positive Momentum Across the Business Meaningful Market Opportunity 21% 1-year prevalence of anxiety disorders in 21% the US1 4.4% estimated prevalence of ADHD among all US adults2 $461B economic cost of ASD in the US predicted by 20253 Significant Progress $129M 26 Cash on hand as of Q1 2023 Pending US patent applications MindMed to Present Data on PreClinical Activity of MM-402 at ASCP 2023 Annual Meeting MindMed Announces Enrollment Milestone in Phase 2b Trial of MM-120 in Generalized Anxiety Disorder (GAD) MindMed Collaborators Announce Positive Topline Data from Phase 2 Trial Evaluating LSD in Anxiety Disorders Exciting Lead Drug Candidates MM-120 LSD D-tartrate MM-402 R(-)-MDMA 3. Bandelow 2015; Dialogues Clins Neurosci; 17(3) 2. Kessler RCd Adler Ld Barkley Rd et als 2005; Am J Psychiatrys 163(4) 3.Leigh & Du 2015; Js Autism Devs Disords; 45(12) MindMed Investor Day June 2023 47

Our Leadership Team Robert Barrow Chief Executive Officer and Board Director Miri Halperin Wernli, PhD Executive President Daniel Karlin, MD, MA Chief Medical Officer Schond Greenway, MBA Chief Financial Officer Mark Sullivan, JD Chief Legal Officer and Corporate Secretary Francois Lilienthal, MD, MBA Chief Commercial Officer Carrie Liao, CPA Chief Accounting Officer MindMed Usona Institute OLATEC MSD Roche ACTELION HealthMode Pfizer Tufts UNIVERSITY Avalo THERAPEUTICS Halozyme BARCLAYS Morgan Stanley Sesen bio M Modal Troutman Pepper MERCK Johnson & Johnson Bristol Myers Squibb TM ORIC Pharmaceuticals Mankind GenMarkDx MindMed Investor Day June 2023 48

Our R&D Leadership Team VP, Pharmaceuticals Development Bridget Walton, MS, RAC VP, Global Regulatory Affairs Robert Silva, PhD VP, Head of Development Carole Abel, MBA VP, Programs & Portfolio Office (PPO) AstraZeneca Pearl Liquidia sunovion NOVARTIS Johnson & Johnson Wyeth ZOGENIX IPSEN Innovation for patient care Sunovion Schering Plough ACTELION MindMed Investor Day June 2023 49
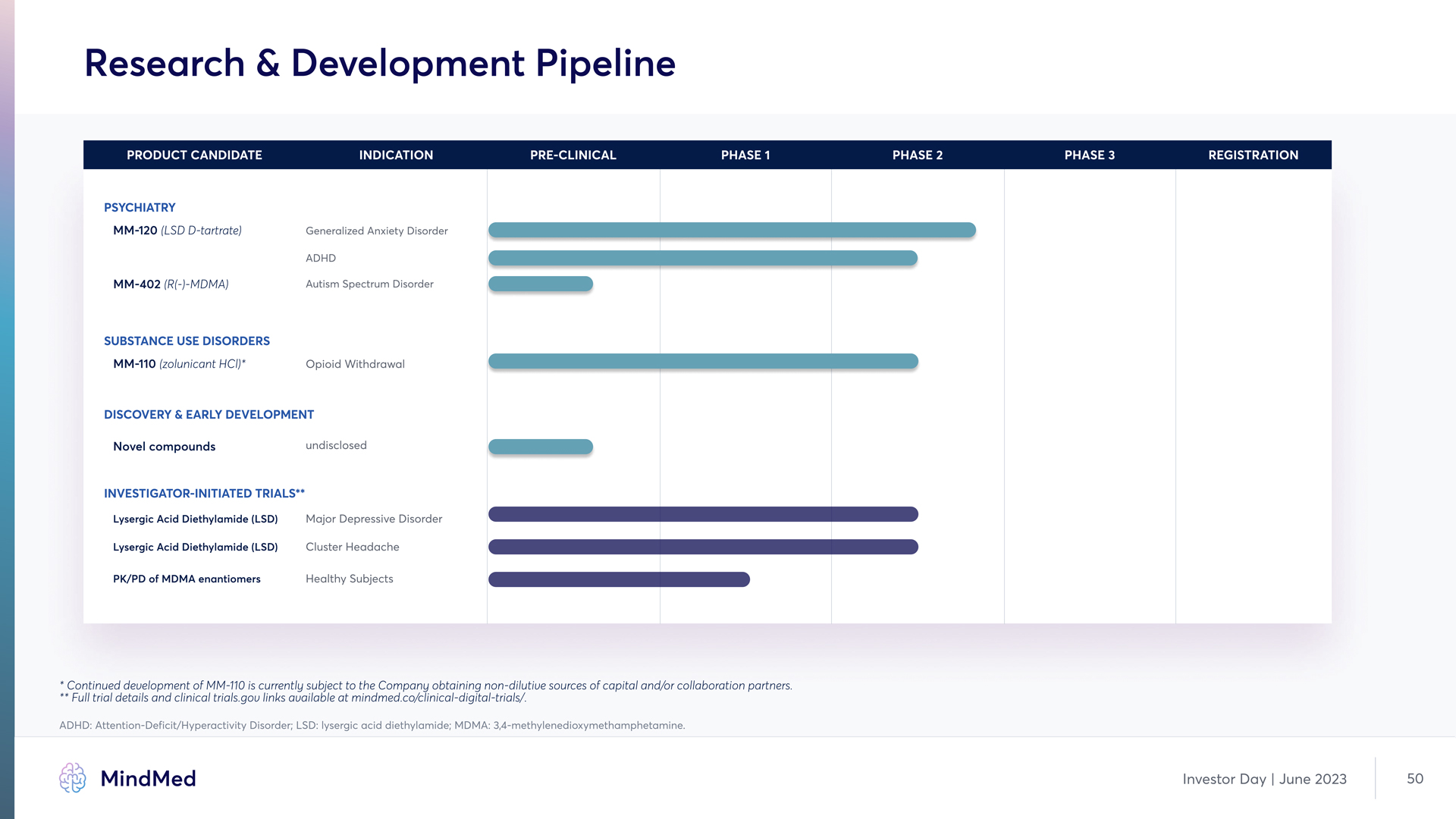
Research & Development Pipeline PRODUCT CANDIDATE INDICATION PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 REGISTRATION Psychiatry MM-120 (LSD D-tartrate) Generalized Anxiety Disorder ADHD MM-402 (R(-)-MDMA) Autism Spectrum Disorder SUBSTANCE USE DISORDERS MM-110 (zolunicant HCl)* Opioid Withdrawal DISCOVERY & Early Development Novel compounds undisclosed Investigator-initiated Trials** Lysergic Acid Diethylamide (LSD) Major Depressive Disorder Lysergic Acid Diethylamide (LSD) Cluster Headache PK/PD of MDMA enantiomers Healthy Subjects Continued development of MM-110 is currently subject to the Company obtaining non-dilutive sources of capital and/or collaboration partners. ** Full trial details and clinical trials.gov links available at mindmed.co/clinical-digital-trials/. ADHD: Attention-Deficit/Hyperactivity Disorder; LSD: lysergic acid diethylamide; MDMA: 3,4-methylenedioxymethamphetamine MindMed Investor Day June 2023 50
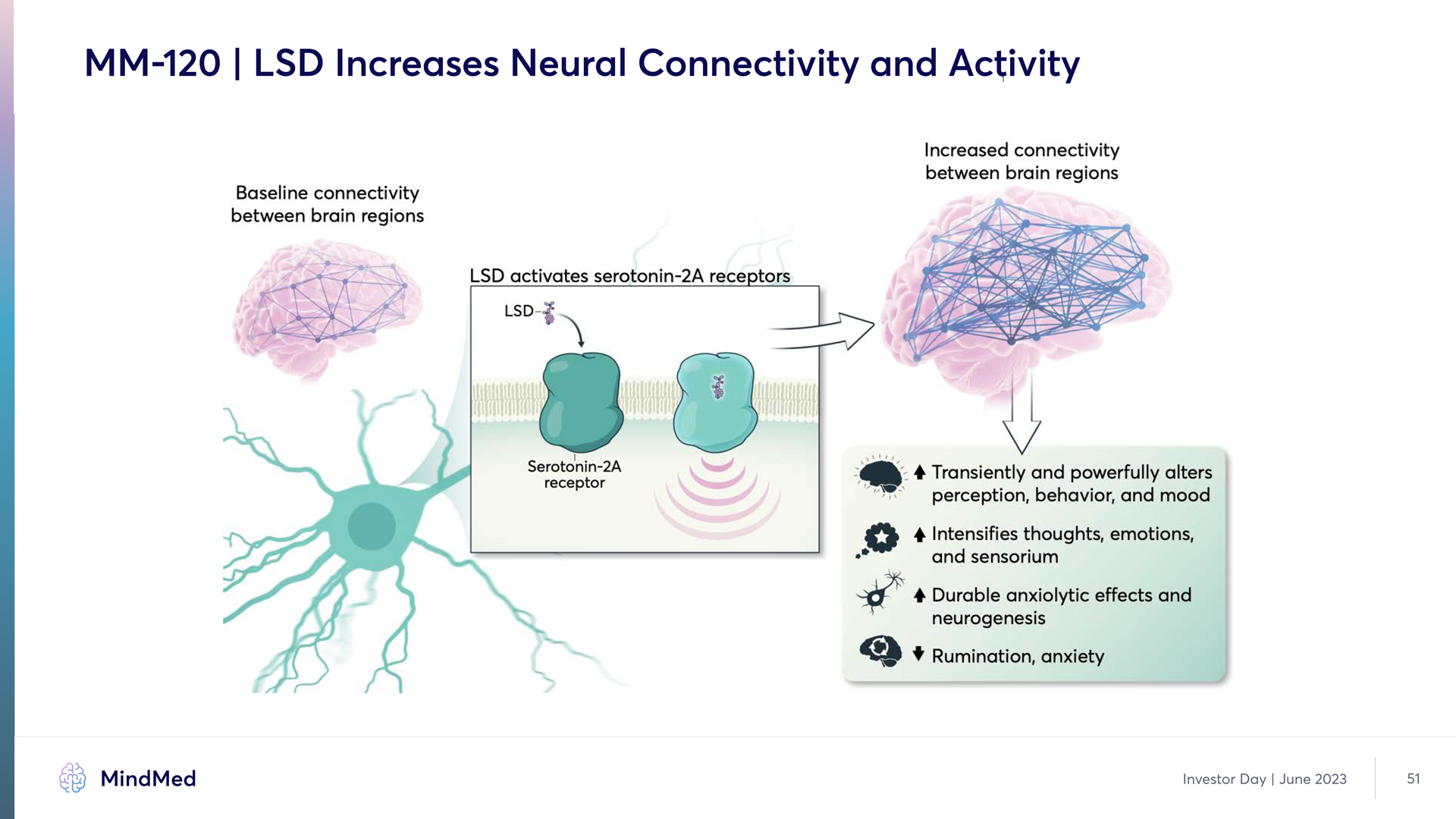
MM-120 | LSD Increases Neural Connectivity and Activity Baseline connectivity between brain regions LSD activates serotonin 2A receptors LSD Serotonin 2A receptor Increased connectivity between brain regions Transiently and powerfully alters perception, behavior, and mood Intensifies thoughts, emotions, and sensorium Durable anxiolytic effects and neurogenesis Rumination, anxiety MindMed Investor Day June 2023 51

MM-120 | Legacy of LSD Clinical Research in Psychiatric Disorders Studies Indication(s) Sample Size KEY FINDINGS 21 Studies PRIOR TO 1974 Anxiety, depression & neurotic illnesses 1 512 patients Up to 95% reduction in symptoms Gasser 2014 Anxiety in terminal illness 12 patients Effect size of 1.1 with durable reduction in anxiety at 1 year UHB’s LSD-Assist Anxiety 42 patients Rapid and durable reduction in symptoms post-treatment. Clinical response in 65% of LSD patients vs. 9% in placebo 1 Rucker 2016 J Psychopharmacol; 30(12) 2 Gasser 2014 J Nerv Ment Dis ; 202(7). 3 Holze, Gasser et al 2022 Biological Psychiatry MindMed Investor Day June 2023 52
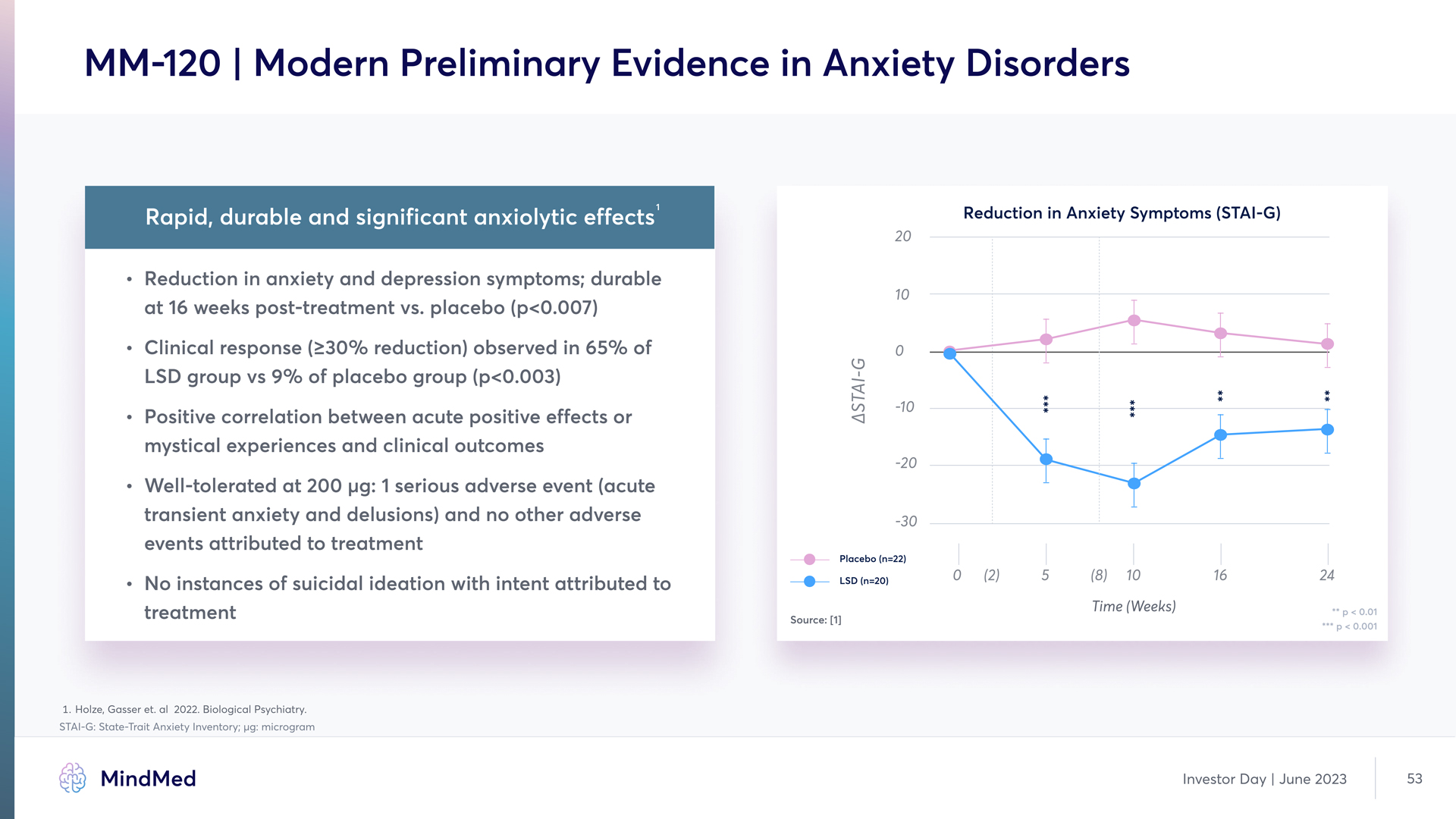
MM-120 | Modern Preliminary Evidence in Anxiety Disorders Rapid, durable and significant anxiolytic effects Reduction in anxiety and depression symptoms; durable at 16 weeks post-treatment vs. placebo (p<0.007) Clinical response (≥30% reduction) observed in 65% of LSD group vs 9% of placebo group £p<0.003) Positive correlation between acute positive effects or mystical experiences and clinical outcome Well-tolerated at 200 μg: 1 serious adverse event (acute transient anxiety and delusions) and no other adverse events attributed to treatment No instances of suicidal ideation with intent attributed to treatment Reduction in Anxiety Symptoms (STAI-G) (Chart) MindMed Investor Day June 2023 53
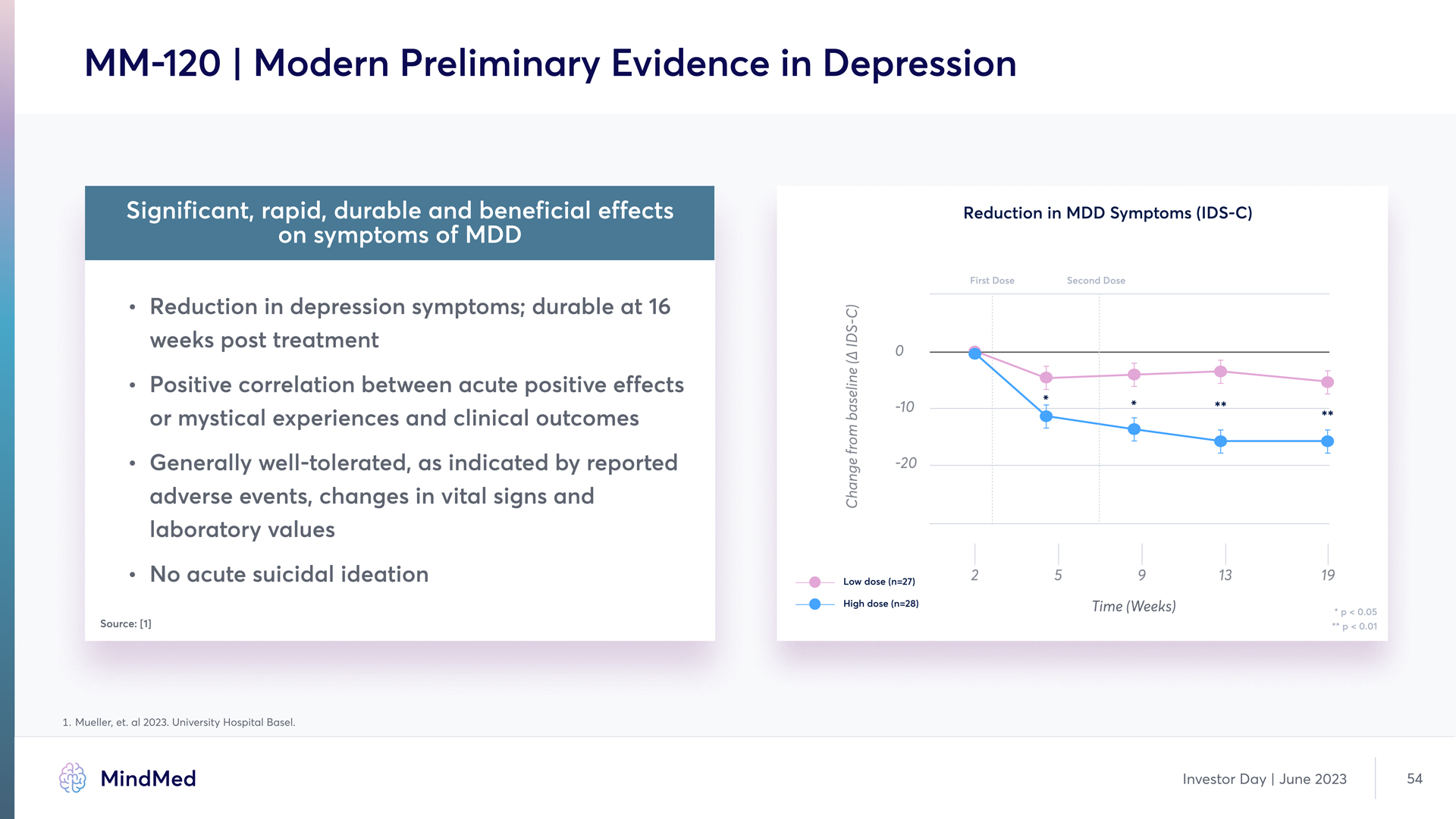
MM-120 | Modern Preliminary Evidence in Depression Significant, rapid, durable and beneficial effects on symptoms of MDD Reduction in depression symptoms; durable at 16 weeks post treatment Positive correlation between acute positive effect or mystical experiences and clinical outcome Generally well-tolerated, as indicated by reported adverse events, changes in vital signs and laboratory values No acute suicidal ideation 1 Mueller, et. al 2023. University Hospital Basel Reductioy iy MDD Symptomq (IDS-C) (Chart) MindMed Investor Day June 2023 54
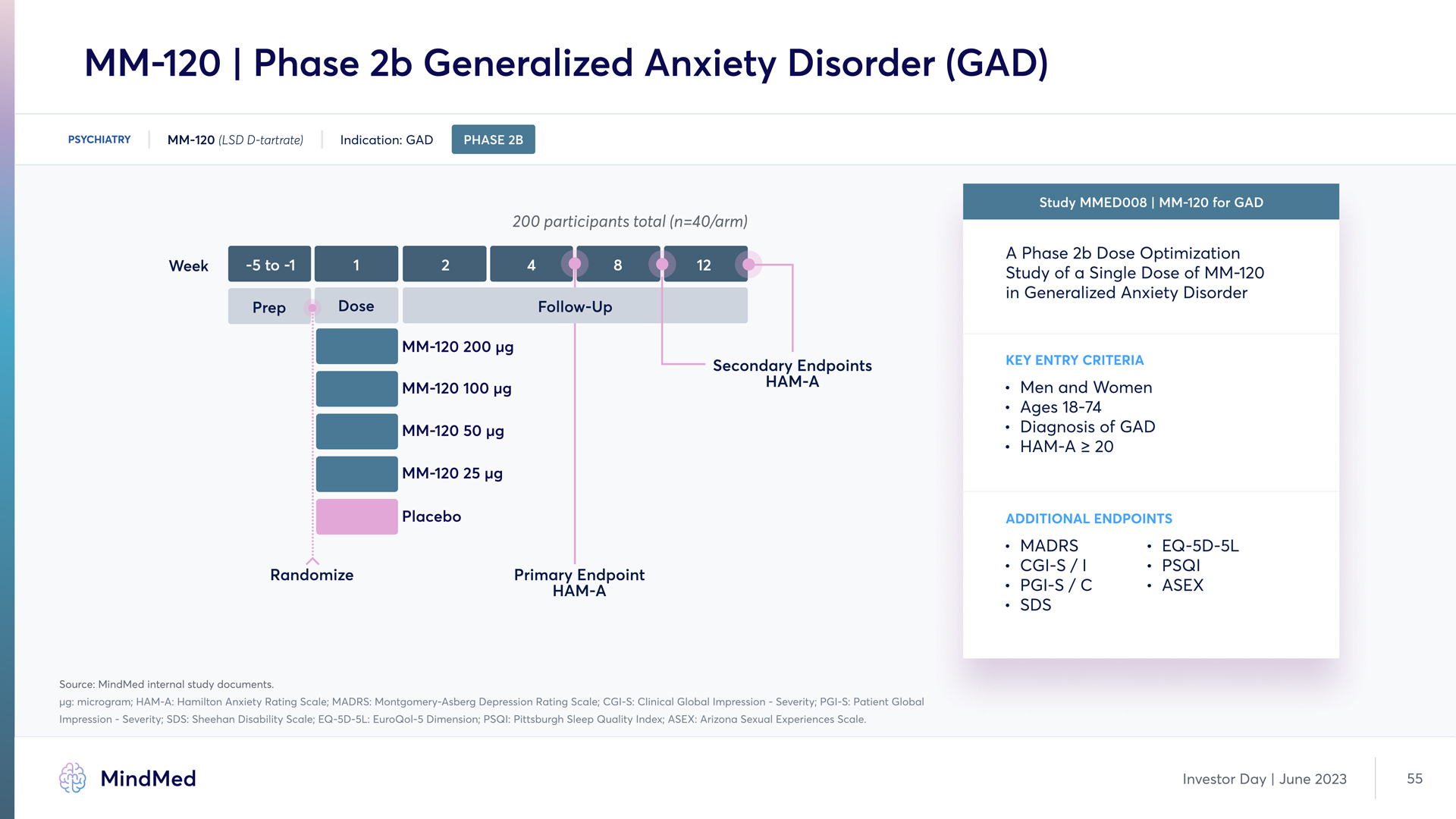
MM-120 | Phase 2b Generalized Anxiety Disorder (GAD) PSYCHIATRY MM-120 (LSD D-tartrate) Indication: GAD PHASE 2B 200 participants total (n=40/arm) Study MMED008 | MM-120 for GAD A Phase 2b Dose Optimization Study of a Single Dose of MM-120 in Generalized Anxiety Disorder Key Entry criteria Men and Women Ages 18-74 Diagnosis of GAD¶ HAM-A ≥ 20 Additional ENDPOINTS Source: MindMed internal study documents. µg: microgram; HAM-A: Hamilton Anxiety Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impression - Severity; PGI-S: Patient Global Impression - Severity; SDS: Sheehan Disability Scale; EQ 5D-5L: EuroQol-5 Dimension; PSQI: Pittsburgh Sleep Quality Index; ASEX: Arizona Sexual Experiences Scale MindMed Investor Day June 2023 55

MM-120 | Phase 2b Enrollment Update Over 50% Patients dosed across 20 active sites1 Next Update Additional update on enrollment and timing of data during Q2 2023 earnings (August) 1 Dosing update as of May 17, 2023 MindMed Investor Day June 2023 56

MM-120 | Potential Clinical Care Model Pre-Treatment During Treatment Post-Treatment Patient education, engagement, preparation Continuous monitoring by session monitors Follow-up psychosocial support Eligibility evaluation Non-directive psychosocial support Continuation of standard psychiatric care Care coordination with existing clinical team Accompanied discharge when release criteria met Remote monitoring for re-treatment needs MindMed Investor Day June 2023 57
MM-120 | Digital to Complement Delivery Through the Patient Journey Pre-Treatment During Treatment Post-Treatment Patient education, engagement, preparation In-session monitoring Real world monitoring of trends Deep digital diagnosis Clinician decision support Engagement in health maintenance Support for treatment selection Predictive models that link interventions and outcomes AI models to inform psychotherapies MindMed Investor Day June 2023 58
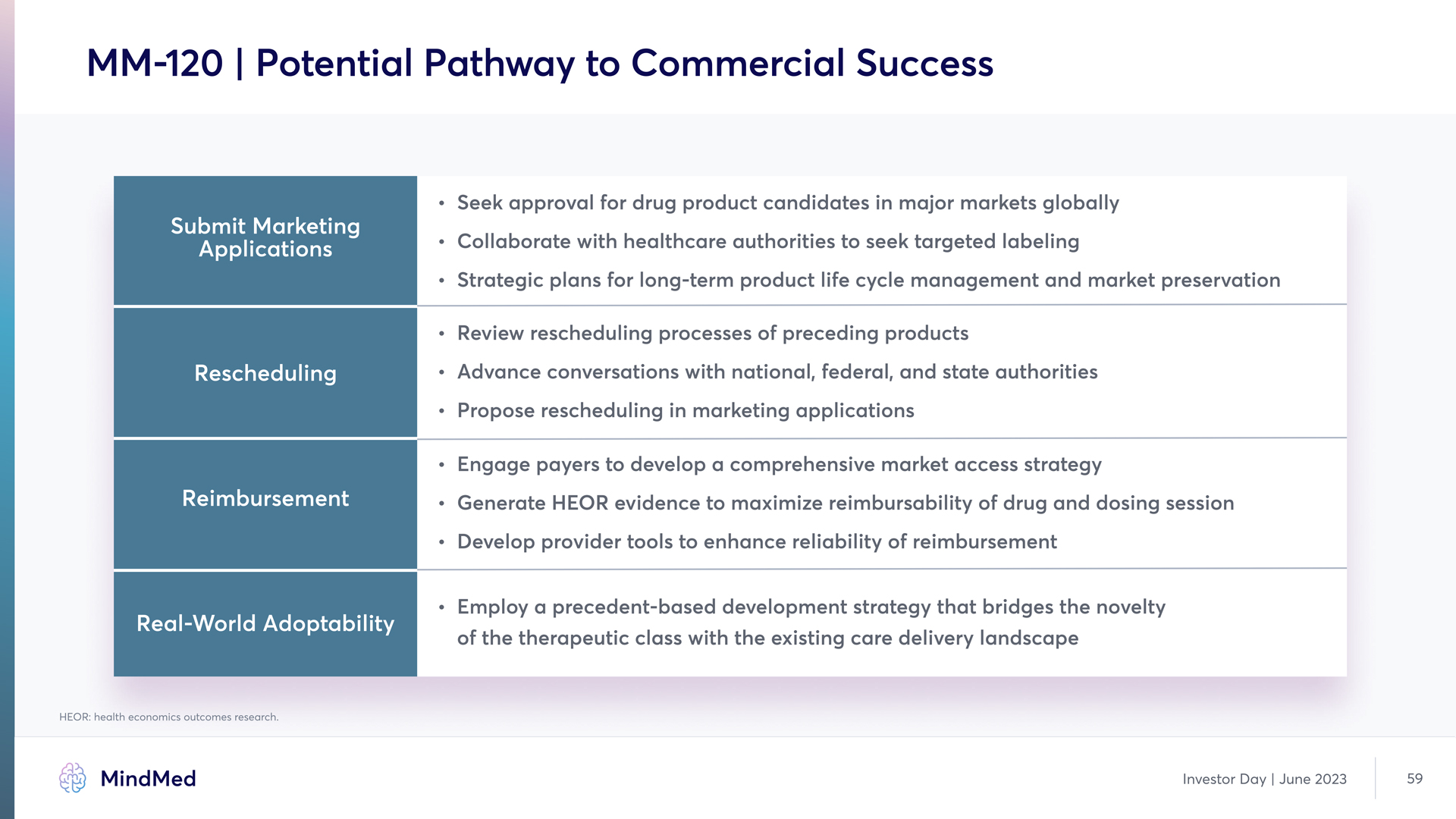
MM-120 | Potential Pathway to Commercial Success Soak approval for drug product candidates in major markets globally Collaborate with healthcare authorities t seek targeted labeling Strategic plans for long-term product life cycle management and market preservation Submit Marketing applications Review rescheduling processes of proceding products Rescheduling - Advance conversations with nations, fader and state authorities Propose rescheduling in marketing applications Engage payers to develop comprehensive market access strategy Generate HEOR evidence to maximize reimbursabillity of drug and dosing session Develop provider tools to enhance reliability of reimbursement of the therapeutic close with the exiting care delivery landscape. Real-World Adoptability Reimbursement Rescheduling HEOR: health economics outcomes research. MindMed Investor Day June 2023 59

Business Highlights A leader in developing psychedelic product candidates to treat brain health disorders Diversified pipeline of clinical programs targeting significant unmet medical needs IP and R&D strategies intended to maximize market exclusivity and protection 7 Z Leveraging decades of research on clinical and preclinical potential of product candi Expertise in drug and digital medicine development and commercialization Expected cash runway through key clinical readouts and into first half of 2025 MM-120 (LSD D-tartrate) for the treatment of GAD and ADHD Phase 2b dose-optimization study ongoing for the treatment of GAD; topline results expected in late 2023 Phase 2a study ongoing for the treatment of ADHD; topline results expected in late 2023 MM-402 or R(-)-MDMA for the treatment of core symptoms of ASD IND-enabling studies ongoing; initiation of a Phase 1 clinical trial is planned in 2023 = Phase 1 pharmacokinetic/pharmacodynamic (UHB) investigator-initiated trial of R-, S- and R/S-MDMA in healthy volunteers ongoing MindMed investor day june 2023 60

MindMed Q&A MindMed Investor Day June 2023 61