UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): April 9, 2024
ADC Therapeutics SA
(Exact Name of Registrant as Specified in Its Charter)
|
Switzerland (State or Other Jurisdiction of Incorporation) |
001-39071 (Commission File Number) |
N/A (IRS Employer Identification Number) |
|
Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of Principal Executive Offices) (Zip Code) |
+41 21 653 02 00 (Registrant’s Telephone Number)
|
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered |
| Common Shares, par value CHF 0.08 per share | ADCT | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 C.F.R. §230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 C.F.R. §240.12b-2). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
On April 9, 2024, ADC Therapeutics SA (the “Company”) made available a presentation expected to be used in connection with the Company’s virtual research investor event on April 9, 2024 from 4:00 to 5:30 p.m. EDT. A copy of the presentation is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. The event will feature presentations from Ameet Mallik, Chief Executive Officer, and Patrick van Berkel, PhD, Chief Scientific Officer, highlighting recent updates and the Company’s novel exatecan-based antibody drug conjugate platform. A live question and answer session will follow the presentations.
The information contained in this Item 7.01 and Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit Number | Description |
| 99.1 | Presentation dated April 9, 2024 |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ADC Therapeutics SA | ||
| Date: April 9, 2024 | ||
| By: | /s/ Peter J. Graham | |
| Name: | Peter J. Graham | |
| Title: | Chief Legal Officer | |
Exhibit 99.1

April 9, 2024 2024 Research Investor Event 3 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates ▪ Forward Looking ▪ Closing Remarks ▪ Q&A

2 Our UK Labs


4 Forward - Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or ADC Therapeu tic s or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. Information provided in this presen tat ion and any accompanying oral presentation speak only as of the date hereof. This presentation contains forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward - looking statements by terminology such as “may”, “assumes”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estima te” , “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward - looking statements contain these identifying words. Forward - looking state ments are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the actual Zynlonta re venue for Q1 2024, the expected cash runway into Q4 2025, the timing and success of the Company’s early research programs in certain solid tumors with different targets, linkers and payloads; the effectiveness of the new commercial go - to - market strategy, competition from new technologies, the Company’s ability to grow ZYNLONTA® revenue in the United States; Swedish Orphan Biovitrum AB ( Sobi ®) ability to successfully commercialize ZYNLONTA® in the European Economic Area and market acceptance, adequate reimbursemen t coverage, and future revenue from the same; approval by the NMPA of the BLA for ZYNLONTA® in China submitted by Overland ADCT Bi oPharma and future revenue from the same, our strategic partners’, including Mitsubishi Tanabe Pharma Corporation, ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions, and the timing and a mou nt of future revenue and payments to us from such partnerships; the timing and results of the Company’s or its partners’ research and development projects or clinical trials including LOTIS 5 and 7, ADCT 601 and 602 as wel l as IITs in FL and MZL; the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Com pan y’s ability to enter into business development or research collaboration transactions; the Company’s indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imp ose d on the Company’s activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Co mpa ny’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities. Additional information concerning these and other factors that may cause actual results to differ mate ria lly from those anticipated in the forward - looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 10 - K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially di fferent from any future results, performance, achievements or prospects expressed in or implied by such forward - looking statements. The Company cautions investors not to place undue reliance on the forward - looking statements contained in this document. Forward - looking statements are based on our management’s beliefs and assumptions and on information currently available to our m anagement. No assurance can be given that such future results will be achieved. Such forward - looking statements contained in this presentation speak only as of the date of this presentation. The Company expressly disclaim any obligation or undertaking to update these forward - looking statements contained in this presentation to reflect any change in our expectations or any change in events, conditions, or circumstances on which su ch statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data d eri ved from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no repre sentation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is reliable, such research has not been verified by any independent so urc e.
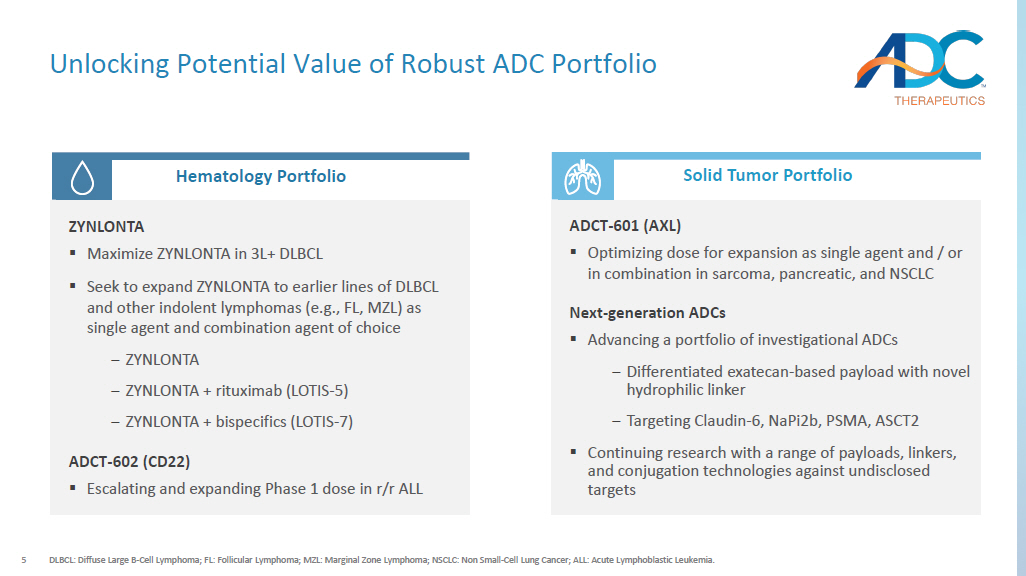
5 Unlocking Potential Value of Robust ADC Portfolio DLBCL: Diffuse Large B - Cell Lymphoma; FL: Follicular Lymphoma; MZL: Marginal Zone Lymphoma; NSCLC: Non Small - Cell Lung Cancer; ALL: Acute Lymphoblastic Leukemia. Short - Mid Term Mid - long Term Hematology Portfolio Solid Tumor Portfolio ZYNLONTA ▪ Maximize ZYNLONTA in 3L+ DLBCL ▪ Seek to expand ZYNLONTA to earlier lines of DLBCL and other indolent lymphomas (e.g., FL, MZL) as single agent and combination agent of choice ‒ ZYNLONTA ‒ ZYNLONTA + rituximab (LOTIS - 5) ‒ ZYNLONTA + bispecifics (LOTIS - 7) ADCT - 602 (CD22) ▪ Escalating and expanding Phase 1 dose in r/r ALL ADCT - 601 (AXL) ▪ Optimizing dose for expansion as single agent and / or in combination in sarcoma, pancreatic, and NSCLC Next - generation ADCs ▪ Advancing a portfolio of investigational ADCs ‒ Differentiated exatecan - based payload with novel hydrophilic linker ‒ Targeting Claudin - 6, NaPi2b, PSMA, ASCT2 ▪ Continuing research with a range of payloads, linkers, and conjugation technologies against undisclosed targets 6 ADC Therapeutics Business Update ▪ ZYNLONTA revenues in the first quarter of 2024 are expected to grow mid - single digit percentage sequentially vs. Q4 2023 ▪ Continued growth in the academic and community settings Commercial Performance Pipeline Progress ▪ LOTIS - 7 cleared dose escalation with no DLT, no ICANs, and no or low grade levels of CRS ▪ ADCT - 601 targeting AXL enrolling patients in Pancreatic; optimizing dose and schedule Corporate Update ▪ Multiple data catalysts in 2024 with expected cash runway into Q4 2025 ▪ Actively pursuing partnerships for solid tumor programs



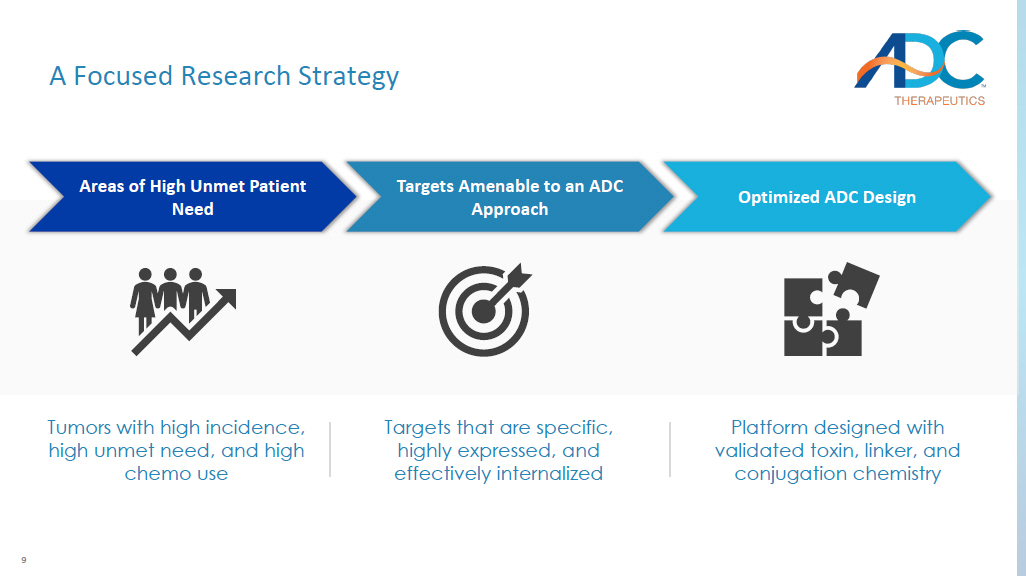
7 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates ▪ Forward Looking ▪ Closing Remarks ▪ Q&A 8 ADC Therapeutics: A Pioneer and Leader in the ADC Field Experienced Team ADC Pioneer Robust Pipeline Commercial - stage pioneer in the field of ADCs, with specialized end - to - end capabilities Seeking expansion of FDA - approved ZYNLONTA®, 2 novel compounds in clinical development, and multiple IN Ds filed since 2015 Array of payloads, linkers and conjugation chemistry enabling design of next - gen potent ADCs with enhanced therapeutic index Research Optionality An accomplished and multidisciplinary team with seasoned industry veterans 9 A Focused Research Strategy Areas of High Unmet Patient Need Targets Amenable to an ADC Approach Tumors with high incidence, high unmet need, and high chemo use Targets that are specific, highly expressed, and effectively internalized Optimized ADC Design Platform designed with validated toxin, linker, and conjugation chemistry
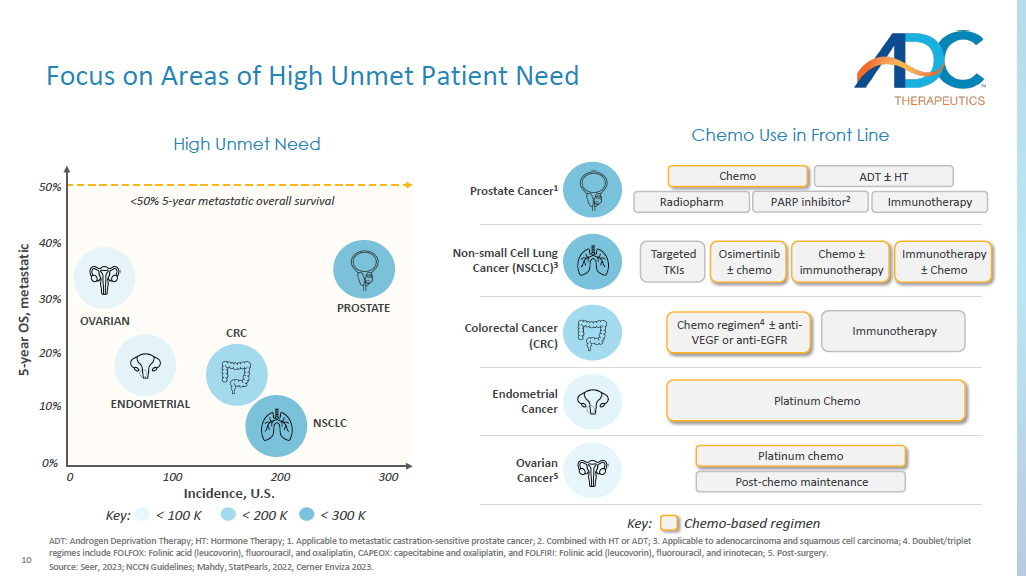
10 Focus on Areas of High Unmet Patient Need ADT: Androgen Deprivation Therapy; HT: Hormone Therapy; 1. Applicable to metastatic castration - sensitive prostate cancer; 2. C ombined with HT or ADT; 3. Applicable to adenocarcinoma and squamous cell carcinoma; 4. Doublet/triplet regimes include FOLFOX: Folinic acid (leucovorin), fluorouracil, and oxaliplatin, CAPEOX: capecitabine and oxaliplatin, and F OLF IRI: Folinic acid (leucovorin), fluorouracil, and irinotecan; 5 . Post - surgery. Source: Seer, 2023; NCCN Guidelines; Mahdy , StatPearls , 2022, Cerner Enviza 2023. Incidence, U.S. 5 - year OS, metastatic Chemo Use in Front Line Prostate Cancer 1 Chemo ADT ± HT Immunotherapy Radiopharm PARP inhibitor 2 Endometrial Cancer Platinum Chemo Ovarian Cancer 5 Platinum chemo Post - chemo maintenance Colorectal Cancer (CRC) Immunotherapy Chemo regimen 4 ± anti - VEGF or anti - EGFR High Unmet Need PROSTATE ENDOMETRIAL OVARIAN CRC NSCLC 0% 10% 20% 30% Non - small Cell Lung Cancer (NSCLC) 3 Targeted TKIs Osimertinib ± chemo Chemo ± immunotherapy Immunotherapy ± Chemo 0 100 200 300 Key: < 100 K < 200 K < 300 K Key: Chemo - based regimen 50% 40% <50% 5 - year metastatic overall survival 11 Identify Targets Amenable to an ADC Approach Internalization Properties Tissue Specificity Membrane Expression A membrane bound antigen that can be recognized by circulating ADCs Expressed exclusively or predominantly on tumor cells, but rare or low on normal tissues Internalized upon binding of the antigen - specific antibody so that the ADC can be internalized in tumor cells


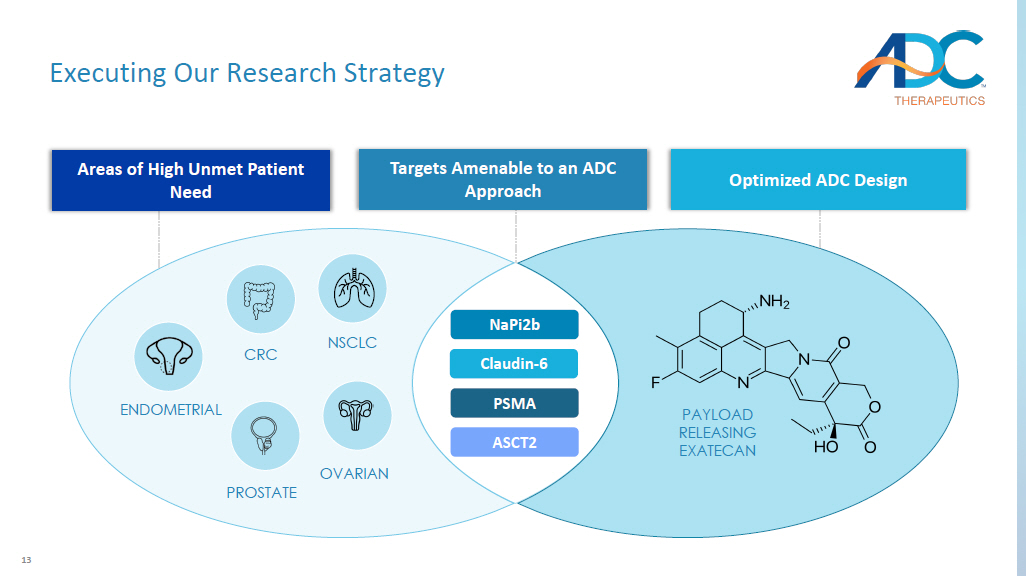
12 Optimize ADC Design 1. Based on preclinical data in cynomolgus monkey. Peptide Spacer Adaptor Linker Toxin(s) TOXIN Near term focus on exatecan toxin in lead candidates: x Clinically validated toxin with known toxicity profile x Superior therapeutic index supports strategy to dose ADC > 5 mg/kg in clinical setting 1 CONJUGATION Near term focus on hinge cysteine - conjugation in lead candidates: x Validated conjugation approach used in majority of approved ADCs x High homogeneous DAR can be achieved through IgG modification x Highly stable in plasma LINKER / PEPTIDE SPACER Near term focus on novel, high stable, hydrophilic linker: x Includes the clinically validated cleavable Val - Ala - PABC linker resulting in traceless release of exatecan x Offsets the hydrophobicity of exatecan using a novel peptide spacer 13 Executing Our Research Strategy NaPi2b Claudin - 6 PSMA ASCT2 NSCLC OVARIAN PAYLOAD RELEASING EXATECAN PROSTATE ENDOMETRIAL CRC Areas of High Unmet Patient Need Optimized ADC Design Targets Amenable to an ADC Approach
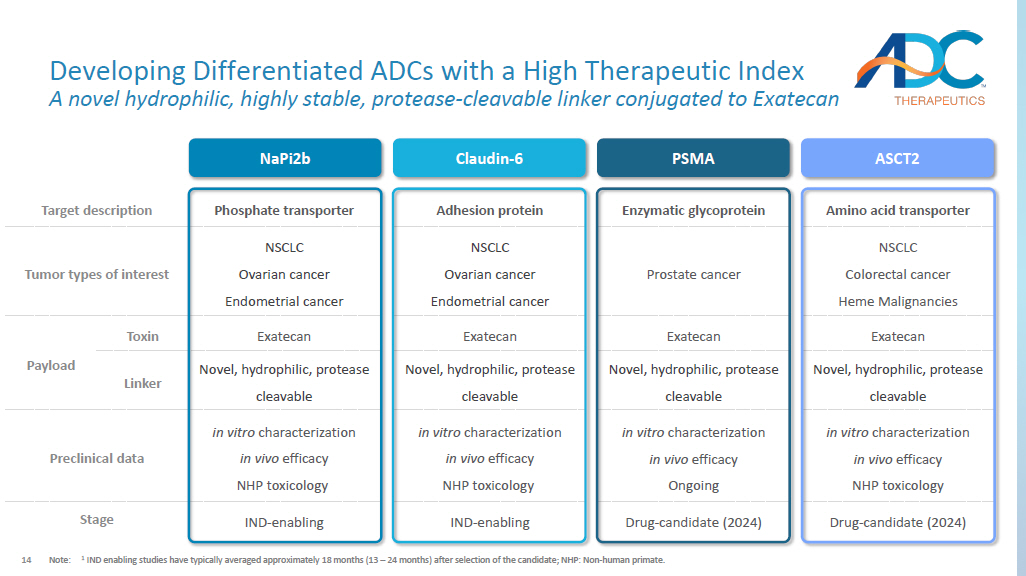
14 Amino acid transporter Enzymatic glycoprotein Adhesion protein Phosphate transporter Target description NSCLC Colorectal cancer Heme Malignancies Prostate cancer NSCLC Ovarian cancer Endometrial cancer NSCLC Ovarian cancer Endometrial cancer Tumor types of interest Exatecan Exatecan Exatecan Exatecan Toxin Payload Novel, hydrophilic, protease cleavable Novel, hydrophilic, protease cleavable Novel, hydrophilic, protease cleavable Novel, hydrophilic, protease cleavable Linker in vitro characterization in vivo efficacy NHP toxicology in vitro characterization in vivo efficacy Ongoing in vitro characterization in vivo efficacy NHP toxicology in vitro characterization in vivo efficacy NHP toxicology Preclinical data Drug - candidate (2024) Drug - candidate (2024) IND - enabling IND - enabling Stage Developing Differentiated ADCs with a High Therapeutic Index A novel hydrophilic, highly stable, protease - cleavable linker conjugated to Exatecan Note: 1 IND enabling studies have typically averaged approximately 18 months (13 – 24 months) after selection of the candidate; NHP: No n - human primate. NaPi2b Claudin - 6 PSMA ASCT2 15 Potential to be Highly Differentiated 1.
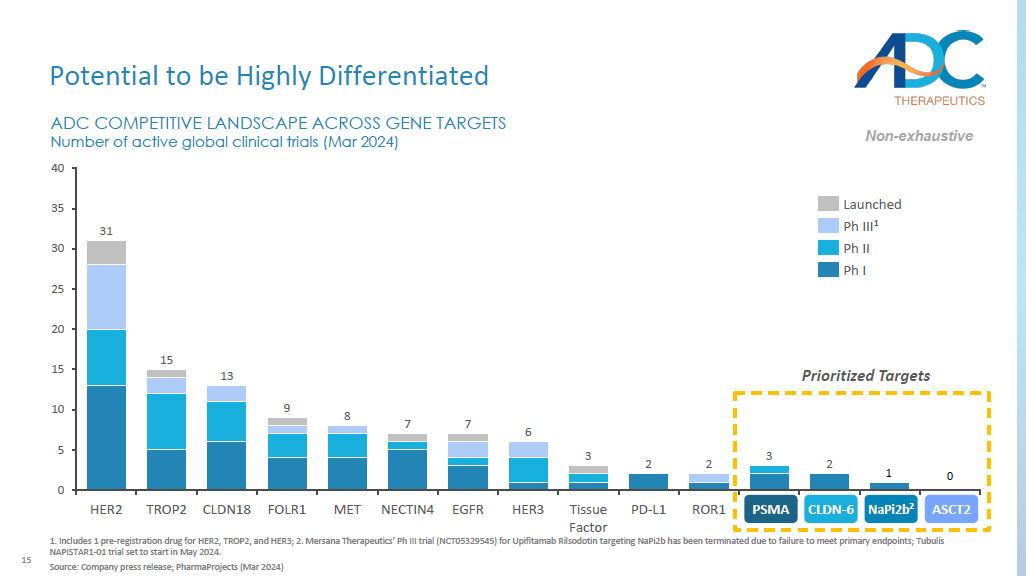
Includes 1 pre - registration drug for HER2, TROP2, and HER3; 2. Mersana Therapeutics’ Ph III trial (NCT05329545) for Upifitamab Rilsodotin targeting NaPi2b has been terminated due to failure to meet primary endpoints; Tubulis NAPISTAR1 - 01 trial set to start in May 2024. Source: Company press release; PharmaProjects (Mar 2024) Prioritized Targets 2 2 0 5 10 15 20 25 30 35 40 1 0 ADC COMPETITIVE LANDSCAPE ACROSS GENE TARGETS Number of active global clinical trials (Mar 2024) HER2 TROP2 CLDN18 FOLR1 MET NECTIN4 EGFR HER3 Tissue Factor PD - L1 ROR1 PSMA CLDN6 NaPi2b^ ASCT2 31 15 13 9 8 7 7 6 3 2 3 Launched Ph III 1 Ph II Ph I Non - exhaustive NaPi2b 2 ASCT2 CLDN - 6 PSMA 16 Research Focused in Large Market Opportunities 1.
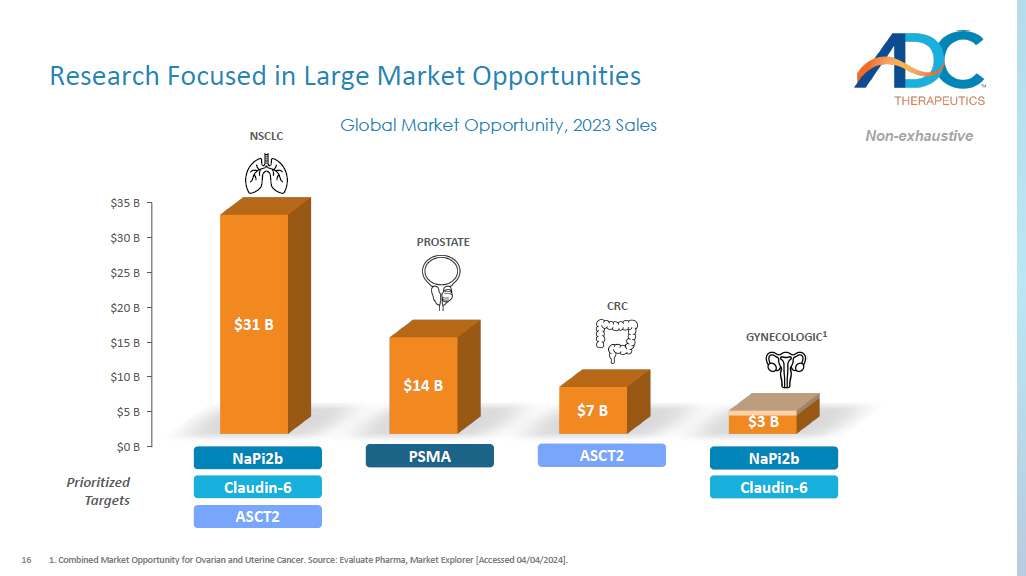
Combined Market Opportunity for Ovarian and Uterine Cancer. Source: Evaluate Pharma, Market Explorer [Accessed 04/04/2024] . $0 B $5 B $10 B $15 B $20 B $25 B $30 B $35 B $31 B $14 B $7 B Global Market Opportunity, 2023 Sales $3 B PROSTATE GYNECOLOGIC 1 CRC NSCLC PSMA NaPi2b Claudin - 6 ASCT2 ASCT2 NaPi2b Claudin - 6 Prioritized Targets Non - exhaustive 17 Proven Capabilities for Path Forward ADME: Absorb, Distribution, Metabolism, Excretion.1.

Prior INDs have taken between 13 and 24 months. ▪ Dose Schedule / optimization ▪ Safety ▪ Early signs of activity ▪ Proof of concept in selected indications ▪ Patient selection assay / biomarker validation when applicable ▪ Prevalence and expression data generation ▪ GMP manufacturing ▪ Toxicology / ADME ▪ Combination studies in vitro & in vivo with immune and/or targeted therapies ▪ In vivo benchmarking vs. competitors ▪ Assay Development INDs filed from 2015 – 2021 and entered the clinic 6 programs ongoing 2 programs completed early development 2 ~18 months 1 IND Readiness 12 – 24 months Early Development 18 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates ▪ Forward Looking ▪ Closing Remarks ▪ Q&A

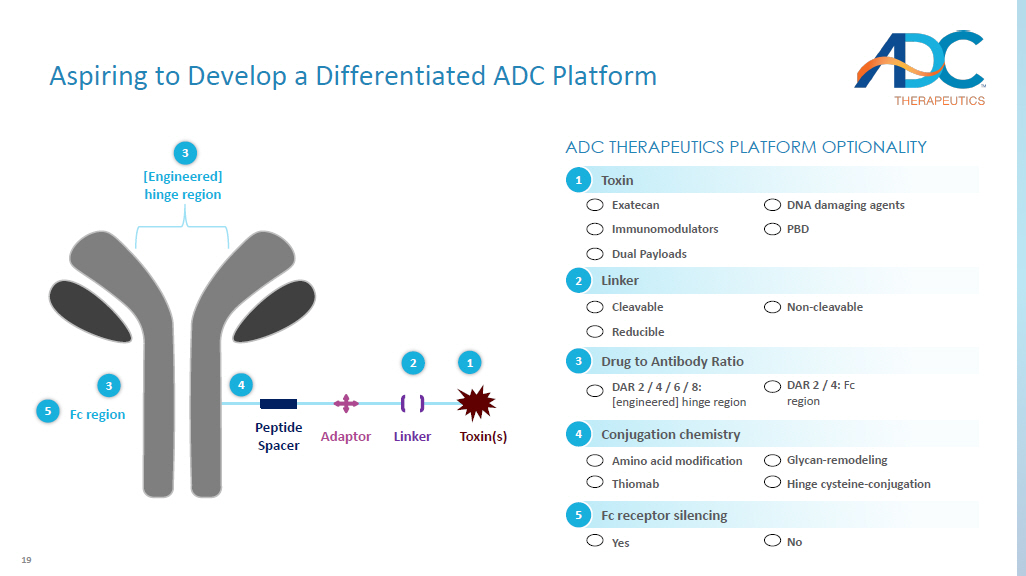
19 Peptide Spacer Adaptor Linker Fc region [Engineered ] hinge region Toxin(s) ADC THERAPEUTICS PLATFORM OPTIONALITY 3 3 5 4 2 1 DAR 2 / 4 / 6 / 8: [ engineered] hinge region Drug to Antibody Ratio 3 DAR 2 / 4: Fc region Fc receptor silencing Yes 5 No Cleavable Reducible Linker 2 Non - cleavable Amino acid modification Thiomab Conjugation chemistry 4 Glycan - remodeling Hinge cysteine - conjugation Toxin 1 Exatecan Immunomodulators Dual Payloads DNA damaging agents PBD Aspiring to Develop a Differentiated ADC Platform 20 Validation of Topoisomerase I Inhibitors in Oncology 1.

This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon veri fic ation and description of clinical benefit in a confirmatory trial. Source: Schoffski , Oncol Res Treat, 2024.

1990 2000 2010 2020 Topotecan Ovarian, SCLC, Cervical 1996 MM - 398 Pancreatic 1996 Irinotecan CRC 1996 HER2 - positive solid tumors 1 2019 Breast, Bladder 1 2020 Key: Chemo Antibody - drug Conjugate Regulatory Approval Timeline 21 A Novel Exatecan - based ADC Platform Val - Ala - PABC Exatecan = PL2202 ▪ Superior therapeutic index vs. DXd and other approved Topo1 inhibitors 1 — Preclinical data in cynomolgus monkey supports strategy to dose ADC > 5 mg/kg in patients — Enables combinability with standard of care ▪ No signs of interstitial lung disease (ILD) , a severe adverse event associated with DXd 1 ▪ Increased bystander effect and potency vs. DXd 1 ▪ Not a PgP substrate; enhanced intracellular presence ▪ Novel hydrophilic, highly stable, protease cleavable linker — Enables traceless release of exatecan — Offsets the hydrophobicity of exatecan Key Advantages of Our Exatecan Platform 1. Internal analysis.


22 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates — NaPi2b — Claudin - 6 — PSMA — ASCT2 23 NaPi2b: A Validated ADC Target Source: Wagner et al., Pflugers Arch (2014); Yin et al., Cancer Immun (2008); Banerjee et al., Cancer Treat Rev (2023); Gryshkova et al., Exp Oncol (2009) Hong et al., PLOS One (2013); Vlasenkova et al., Biomolecules (2021) ; Nurgalieva et al., Biochem Biophys (2021); Rubinfeld et al., Clin Cancer Res (2015); ADC Therapeutics Internal Studies. NaPi2b Overview: A membrane - localized phosphate transporter ▪ Plays a central role in cellular phosphate homeostasis regulation ▪ Induces tumorigenesis by disrupting phosphate homeostasis Role: x Clinically validated target Rationale: Low levels of expression in intestine, liver, testis, and lung on the apical site of bronchial epithelium NORMAL TISSUE PROFILE TUMOR EXPRESSION PROFILE NSCLC (N =30) ~80% positivity ~56% positivity Ovarian (N =70) Endometrial (N =45 ) ~81% positivity 24 1.
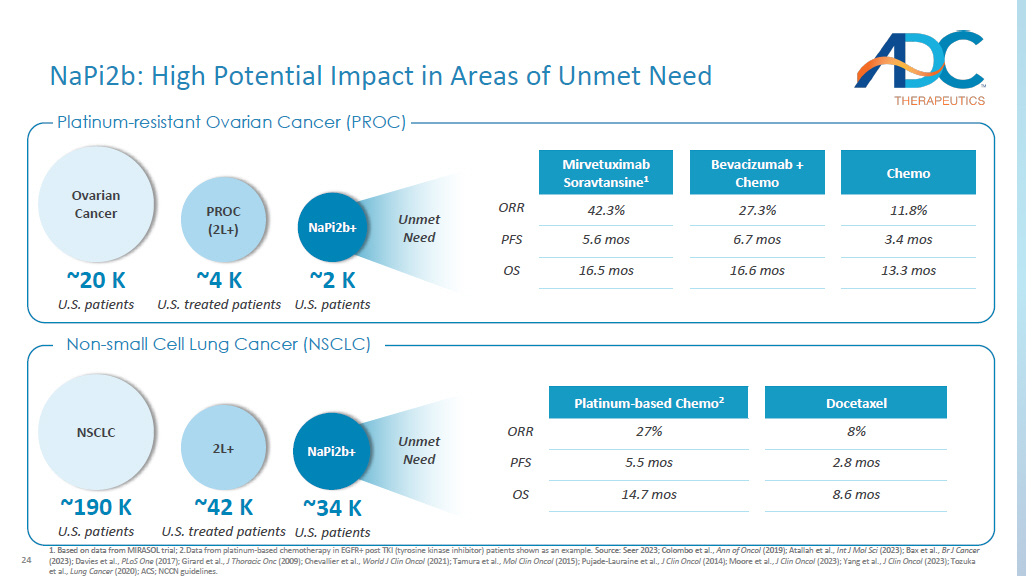
Based on data from MIRASOL trial; 2. Data from platinum - based chemotherapy in EGFR+ post TKI (tyrosine kinase inhibitor) patients shown as an example. Source: Seer 2023; Colombo et al., Ann of Oncol (2019); Atallah et al., Int J Mol Sci (2023); Bax et al., Br J Cancer (2023); Davies et al., PLoS One (2017); Girard et al., J Thoracic Onc (2009); Chevallier et al., World J Clin Oncol (2021); Tamura et al., Mol Clin Oncol (2015); Pujade - Lauraine et al., J Clin Oncol (2014); Moore et al., J Clin Oncol (2023); Yang et al., J Clin Oncol (2023); Tozuka et al., Lung Cancer (2020); ACS; NCCN guidelines. Platinum - resistant Ovarian Cancer (PROC) Non - small Cell Lung Cancer (NSCLC) Unmet Need NaPi2b: High Potential Impact in Areas of Unmet Need ~4 K U.S. treated patients PROC (2L+) ~2 K U.S. patients NaPi2b+ ~20 K U.S. patients Ovarian Cancer NSCLC ~190 K U.S. patients ~42 K U.S. treated patients 2L+ ~34 K U.S.

patients NaPi2b+ Unmet Need Docetaxel Platinum - based Chemo 2 8% 27% ORR 2.8 mos 5.5 mos PFS 8.6 mos 14.7 mos OS Chemo Bevacizumab + Chemo Mirvetuximab Soravtansine 1 11.8% 27.3% 42.3% ORR 3.4 mos 6.7 mos 5.6 mos PFS 13.3 mos 16.6 mos 16.5 mos OS 25 NaPi2b - PL2202 Active (preclinical) Status NaPi2b - specific humanized mAb Antibody x Efficiently internalized x Fc - silenced; mitigates target - independent toxicity Exatecan Payload x Clinically validated in tumors that are sensitive to Topo - 1 inhibitors (e.g., NSCLC) Proprietary hydrophilic, protease sensitive cleavable linker Linker Hinge cysteine x Site - specific conjugation eliminates heterogeneity Conjugation 6 DAR >10 TI 1 A Differentiated NaPi2b - ADC DAR: Drug Antibody Ratio ; TI: Therapeutic Index; 1. Calculated from Maximum Tolerated Dose (MTD) in cynomolgus monkey / Minimum Effective Dose (MED) in mouse. 2. Internal Ana lys is. Source: Company website; Conference presentations. Key Differences of Discontinued Assets ▪ Antibody with reduced internalization vs. our antibody ▪ Narrow therapeutic index 2 with risk of peripheral neuropathy and neutropenia given MMAE payload Lifastuzumab Vedotin (Lifa) ▪ Narrow therapeutic index 2 Upifitamab Rilsodotin ( UpRi ) ▪ Improved therapeutic index vs. UpRi but lower than NaPi2b - PL2202 2 XMT - 1592 26 Our Potential Advantage: Superior Cell Binding and Cytotoxicity Source: ADC Therapeutics Internal Studies.

IC 50 pM ADCs 107.3 NaPi2b - PL2202 2473 Lifastuzumab - PL2202 27168 B12 - VA - PL2202 x NaPi2b Ab has higher binding to OVCAR - 3 cells compared to lifastuzumab EC50 pM Antibodies 398.7 NaPi2b 1281 Lifastuzumab 12079 B12 Isotype IgG x NaPi2b - PL2202 exhibits higher in vitro cytotoxicity compared to lifastuzumab conjugated to PL2202 (Both DAR 6) In vitro Cell Binding In vitro Cytotoxicity 27 Our Potential Advantage: Stronger Bystander Activity Source: ADC Therapeutics Internal Studies.
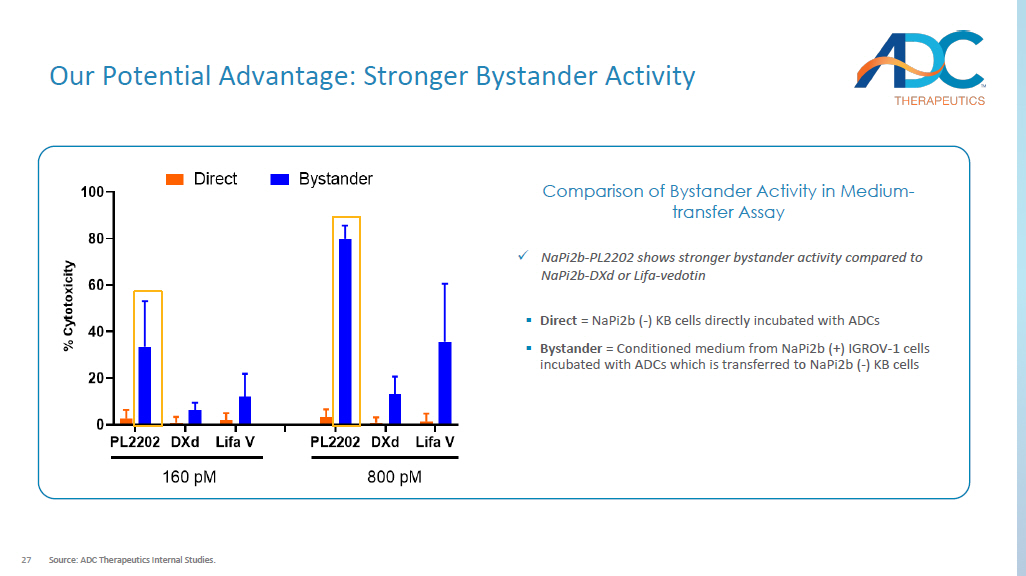
x NaPi2b - PL2202 shows stronger bystander activity compared to NaPi2b - DXd or Lifa - vedotin ▪ Direct = NaPi2b ( - ) KB cells directly incubated with ADCs ▪ Bystander = Conditioned medium from NaPi2b (+) IGROV - 1 cells incubated with ADCs which is transferred to NaPi2b ( - ) KB cells Comparison of Bystander Activity in Medium - transfer Assay 28 Strong in vivo Anti - tumor Activity with a Single Dose H441 Lung - papillary Adenocarcinoma Xenograft Model Source: ADC Therapeutics Internal Studies.

OVCAR - 3 Ovarian Xenograft Model TFS CR PR ADCs 0/10 0/10 9/10 NaPi2b - PL2202, 6.6mg/kg 0/10 0/10 0/10 B12 - VA - PL2202 10 mg/kg TFS CR PR ADCs 10/10 10/10 0/10 NaPi2b - PL2202 6 mg/kg 9/10 9/10 1/10 NaPi2b - PL2202 3 mg/kg 9/10 9/10 1/10 NaPi2b - PL2202 1 mg/kg 0/10 0/10 3/10 Lifastuzumab Vedo . 12 mg/kg 0/10 0/10 0/10 Lifastuzumab Vedo .
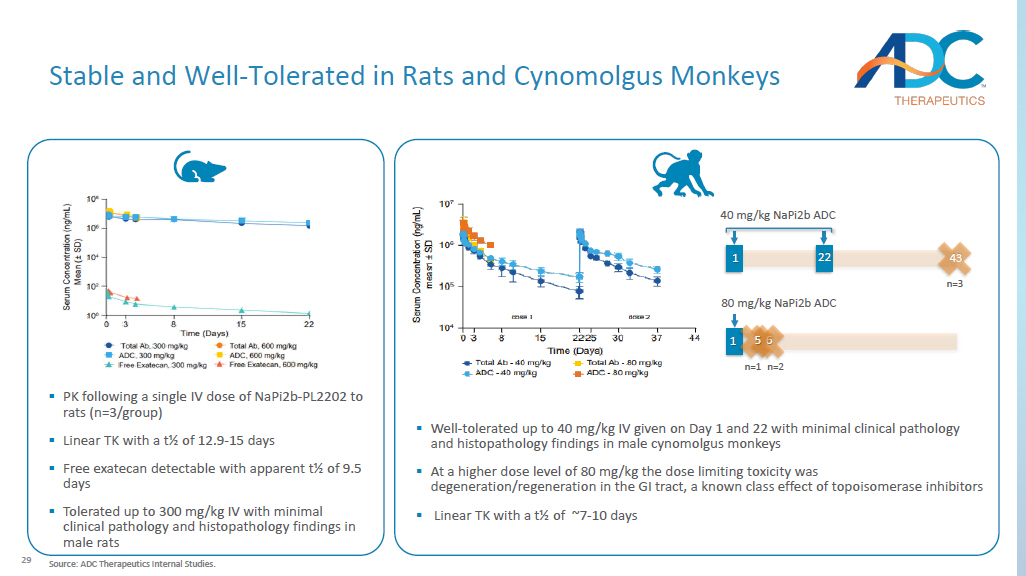
3 mg/kg 29 ▪ PK following a single IV dose of NaPi2b - PL2202 to rats (n=3/group) ▪ Linear TK with a t½ of 12.9 - 15 days ▪ Free exatecan detectable with apparent t½ of 9.5 days ▪ Tolerated up to 300 mg/kg IV with minimal clinical pathology and histopathology findings in male rats Stable and Well - Tolerated in Rats and Cynomolgus Monkeys ▪ Well - tolerated up to 40 mg/kg IV given on Day 1 and 22 with minimal clinical pathology and histopathology findings in male cynomolgus monkeys ▪ At a higher dose level of 80 mg/kg the dose limiting toxicity was degeneration/regeneration in the GI tract, a known class effect of topoisomerase inhibitors ▪ Linear TK with a t½ of ~7 - 10 days 40 mg/kg NaPi2b ADC 43 n=3 1 22 80 mg/kg NaPi2b ADC 6 n=2 1 5 n=1 Source: ADC Therapeutics Internal Studies.

30 Reasons to Believe TI: Therapeutic Index; IHC: Immunohistochemistry; ADME: Absorb, Distribution, Metabolism, Excretion. Source: ADC Therapeutics In ternal Studies. Validated Target With High Potential Impact x Membrane - bound antigen x Efficiently internalized x Expressed highly and specifically in NSCLC and Ovarian Cancer x Clinically validated with an ADC approach Path Forward ▪ Combination studies in vitro & in vivo with immune and/or targeted therapies ▪ In vivo benchmarking vs. competitors ▪ NaPi2b IHC assay development ▪ Progress towards IND readiness Differentiated Approach to Unlock Opportunity x Optimized ADC design with high TI x Strong antitumor activity with a single dose against Ovarian and Lung cancer xenograft models x Stable and well - tolerated in both rats and cynomolgus monkeys x Superior profile vs. Lifa x Higher Ab internalization x Higher tumor - specific cytotoxicity x Higher tumor regression at much lower dose x Stronger bystander killing vs. Lifa and DXd 31 Source: Kojima et al., Cancer (2020); Fierce Biotech; STAT news; Du et al., Mol Med Rep (2021); Cao et al., Onco Targets Ther (2018); ADC Therapeutics Internal Studies.
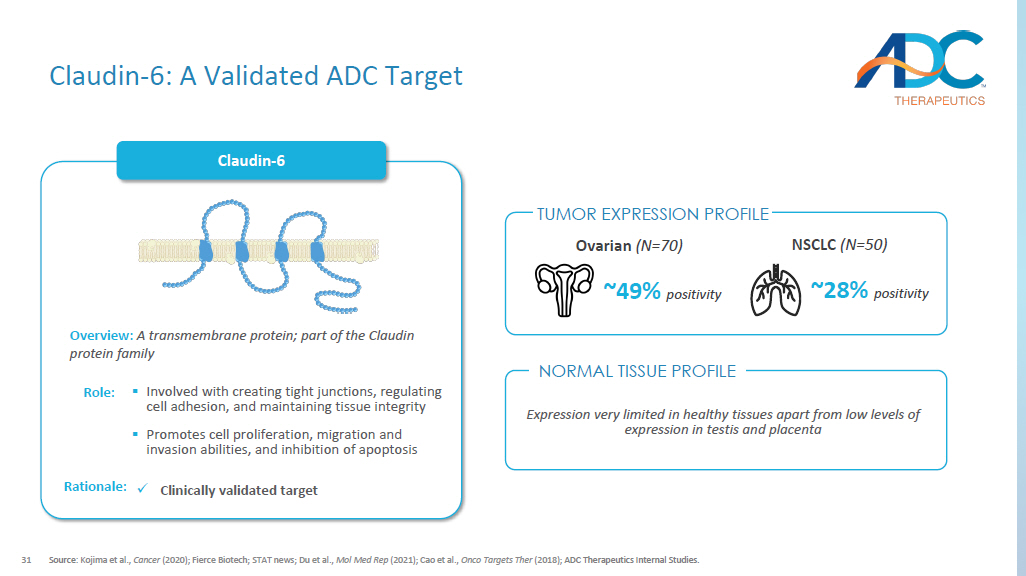
Claudin - 6 Overview: A transmembrane protein; part of the Claudin protein family ▪ Involved with creating tight junctions, regulating cell adhesion, and maintaining tissue integrity ▪ P romotes cell proliferation, migration and invasion abilities, and inhibition of apoptosis Role: x Clinically validated target Rationale: NSCLC (N =50) ~28% positivity ~49% positivity Ovarian (N =70) Expression very limited in healthy tissues apart from low levels of expression in testis and placenta Claudin - 6: A Validated ADC Target NORMAL TISSUE PROFILE TUMOR EXPRESSION PROFILE 32 1.
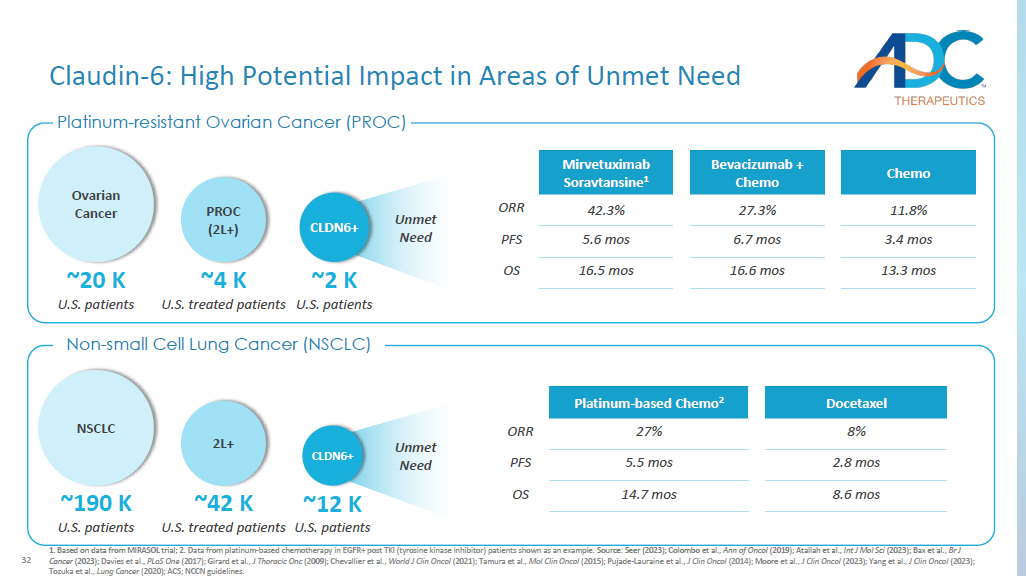
Based on data from MIRASOL trial; 2. Data from platinum - based chemotherapy in EGFR+ post TKI (tyrosine kinase inhibitor) patients shown as an example. Source: Seer (2023); Colombo et al., Ann of Oncol (2019); Atallah et al., Int J Mol Sci (2023); Bax et al., Br J Cancer (2023); Davies et al., PLoS One (2017); Girard et al., J Thoracic Onc (2009); Chevallier et al., World J Clin Oncol (2021); Tamura et al., Mol Clin Oncol (2015); Pujade - Lauraine et al., J Clin Oncol (2014); Moore et al., J Clin Oncol (2023); Yang et al., J Clin Oncol (2023); Tozuka et al., Lung Cancer (2020); ACS; NCCN guidelines. Docetaxel Platinum - based Chemo 2 8% 27% ORR 2.8 mos 5.5 mos PFS 8.6 mos 14.7 mos OS ~4 K U.S. treated patients PROC (2L+) ~20 K U.S. patients Ovarian Cancer ~2 K U.S. patients CLDN6+ Chemo Bevacizumab + Chemo Mirvetuximab Soravtansine 1 11.8% 27.3% 42.3% ORR 3.4 mos 6.7 mos 5.6 mos PFS 13.3 mos 16.6 mos 16.5 mos OS Unmet Need Claudin - 6: High Potential Impact in Areas of Unmet Need NSCLC ~190 K U.S. patients ~42 K U.S. treated patients 2L+ ~12 K U.S.

patients CLDN6+ Platinum - resistant Ovarian Cancer (PROC) Non - small Cell Lung Cancer (NSCLC) Unmet Need 33 GB01 - VA - PL2202 Asset name Active (preclinical) Status Humanized Claudin - 6 mAb Antibody x Efficiently internalized x Fc - silenced; mitigates target - independent toxicity Exatecan Payload x Clinically validated in tumors that are sensitive to Topo - 1 inhibitors (e.g., NSCLC) Proprietary hydrophilic, protease sensitive cleavable linker Linker Hinge cysteine x Site - specific conjugation eliminates heterogeneity Conjugation 6 DAR >10 TI 1 A Differentiated Claudin - 6 - ADC DAR: drug antibody ratio ; 1. Calculated from Maximum Tolerated Dose (MTD) in cynomolgus monkey / Minimum Effective Dose (MED) in mouse. 2. Internal Ana lys is. Source: Company website; Conference presentations. Key Differences of Competitor Asset ▪ Antibody with reduced internalization vs. GB01 ▪ MMAE payload is an MDR1 substrate ▪ Narrow therapeutic index 2 with risk of peripheral neuropathy and neutropenia given MMAE payload TORL - 1 - 23 34 Our Advantage: Superior Cell Binding and Cytotoxicity Source: ADC Therapeutics Internal Studies.

10 -2 10 0 10 2 10 4 10 6 0 20 40 60 80 100 120 Concentration (pM) % C e l l V i a b i l i t y AB3-7-VA-PL2202GB01-VA-PL2202 B12-VA-PL2202 IC50 pM ADCs 63.67 GB01 - VA - PL2202 359.9 AB3 - 7 - VA - PL2202 15496 B12 - VA - PL2202 EC50 pM Antibodies 412 GB01 1070 AB3 - 7 No binding B12 Isotype IgG In vitro Cell Binding In vitro Cytotoxicity 10 1 10 2 10 3 10 4 0 4×10 5 8×10 5 1.2×10 6 Concentration ng/mL M e d i a n F l u o r e s c e n c e I n t e n s i t y AB3-7GB01B12 x Claudin - 6 - specific Ab GB01 shows increased binding to NTERA - 2 cells compared to AB - 3 - 7 Ab x GB01 - VA - PL2202 exhibits increased in vitro cytotoxicity compared to AB - 3 - 7 - VA - PL2202 (both DAR 6)
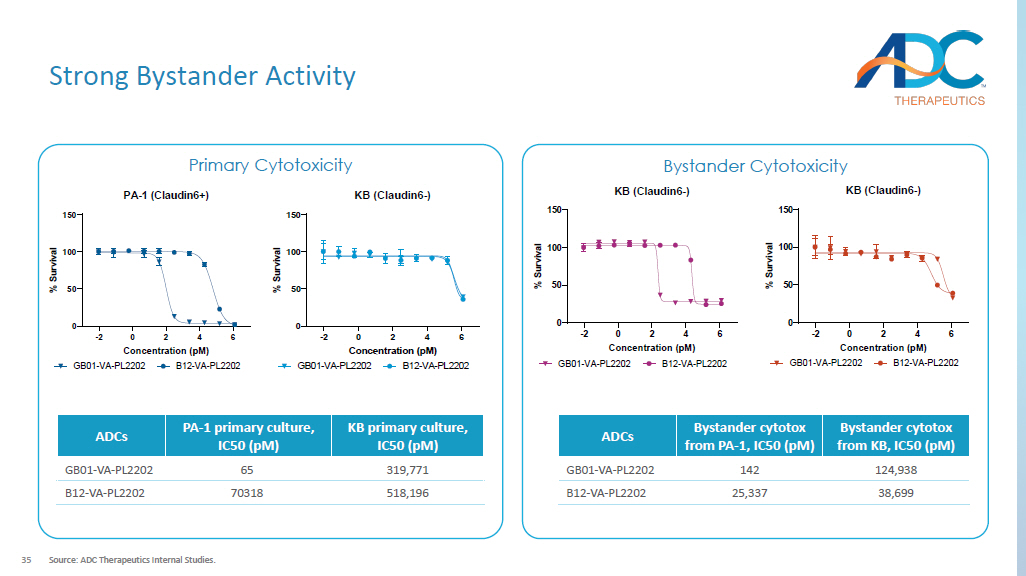
35 Strong Bystander Activity Source: ADC Therapeutics Internal Studies. Primary Cytotoxicity Bystander Cytotoxicity -2 0 2 4 6 0 50 100 150 PA-1 (Claudin6+) Concentration (pM) % S u r v i v a l GB01-VA-PL2202 B12-VA-PL2202 -2 0 2 4 6 0 50 100 150 KB (Claudin6-) Concentration (pM) % S u r v i v a l GB01-VA-PL2202 B12-VA-PL2202 KB primary culture, IC50 ( pM ) PA - 1 primary culture, IC50 ( pM ) ADCs 319,771 65 GB01 - VA - PL2202 518,196 70318 B12 - VA - PL2202 -2 0 2 4 6 0 50 100 150 KB (Claudin6-) Concentration (pM) % S u r v i v a l GB01-VA-PL2202 B12-VA-PL2202 Bystander cytotox from KB, IC50 ( pM ) Bystander cytotox from PA - 1, IC50 ( pM ) ADCs 124,938 142 GB01 - VA - PL2202 38,699 25,337 B12 - VA - PL2202 36 Strong in vivo Anti - tumor Activity with a Single Dose Source: ADC Therapeutics Internal Studies.
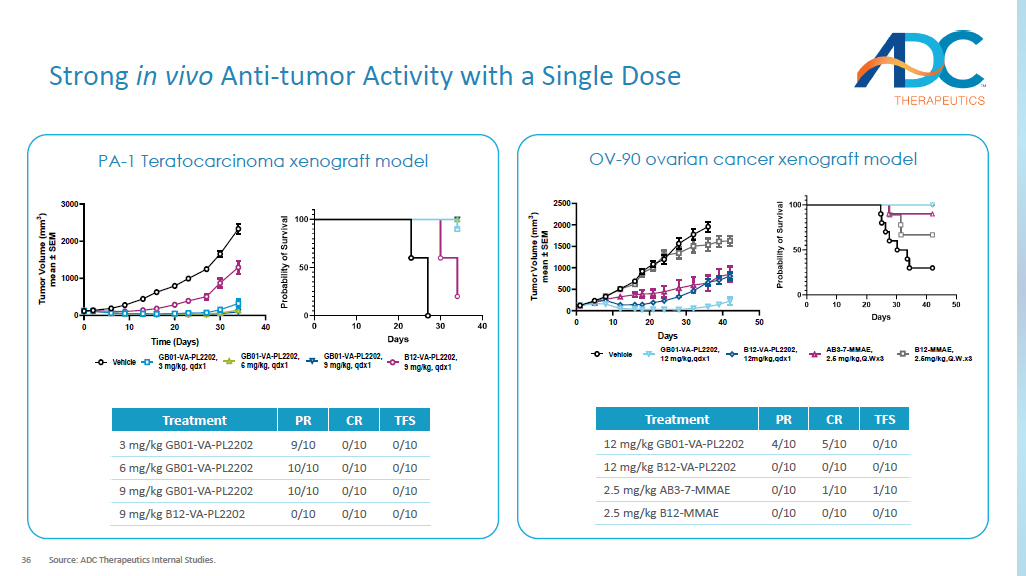
0 10 20 30 40 0 1000 2000 3000 Time (Days) T u m o r V o l u m e ( m m 3 ) m e a n ± S E M Vehicle GB01-VA-PL2202, 3 mg/kg, qdx1 GB01-VA-PL2202, 6 mg/kg, qdx1 GB01-VA-PL2202, 9 mg/kg, qdx1 B12-VA-PL2202, 9 mg/kg, qdx1 TFS CR PR Treatment 0/10 0/10 9/10 3 mg/kg GB01 - VA - PL2202 0/10 0/10 10/10 6 mg/kg GB01 - VA - PL2202 0/10 0/10 10/10 9 mg/kg GB01 - VA - PL2202 0/10 0/10 0/10 9 mg/kg B12 - VA - PL2202 PA - 1 Ovarian teratocarcinoma xenograft model OV - 90 ovarian cancer xenograft model 0 10 20 30 40 50 0 500 1000 1500 2000 2500 Days T u m o r V o l u m e ( m m 3 ) m e a n ± S E M Vehicle GB01-VA-PL2202, 12 mg/kg,qdx1 B12-VA-PL2202, 12mg/kg,qdx1 AB3-7-MMAE, 2.5 mg/kg,Q.Wx3 B12-MMAE, 2.5mg/kg,Q.W.x3 TFS CR PR Treatment 0/10 5/10 4/10 12 mg/kg GB01 - VA - PL2202 0/10 0/10 0/10 12 mg/kg B12 - VA - PL2202 1/10 1/10 0/10 2.5 mg/kg AB3 - 7 - MMAE 0/10 0/10 0/10 2.5 mg/kg B12 - MMAE 37 Stable and Well - tolerated in Cynomolgus Monkeys DLT: Dose limiting toxicity; GI: Gastrointestinal.

Source: ADC Therapeutics Internal Studies. ▪ Well - tolerated up to 40 mg/kg IV with minimal clinical pathology and no histopathology findings ▪ At 60 mg/kg, the DLT was degeneration/regeneration in the GI tract, consistent with a known class effect of topoisomerase inhibitors ▪ ADC exhibited linear PK with a t½ of ~8.5 – 10 days ▪ Free exatecan was detectable throughout the dosing period with an apparent t½ of 5.5 – 8 days 40 mg/kg Claudin - 6 ADC 43 n=3 1 22 20 mg/kg Claudin - 6 ADC 43 n=3 1 22 30 mg/kg Claudin - 6 ADC 7 n=3 60 mg/kg Claudin - 6 ADC 1 38 Reasons to Believe TI: Therapeutic Index; IHC: Immunohistochemistry; ADME: Absorb, Distribution, Metabolism, Excretion.
Source: ADC Therapeutics In ternal Studies. Path Forward Validated Target With High Potential Impact x Membrane - bound antigen x Efficiently internalized x Expressed highly and specifically in Ovarian cancer and NSCLC x Clinically validated with an ADC approach ▪ Combination studies in vitro & in vivo with immune and/or targeted therapies ▪ In vivo benchmarking vs. competitors ▪ Claudin - 6 IHC assay development ▪ Progress towards IND readiness Differentiated Approach to Unlock Opportunity x Optimized ADC design with high TI x High intracellular uptake x High tumor regression and responses x Strong antitumor activity with a single dose against Ovarian cancer xenograft model x Stable and well - tolerated in cynomolgus monkeys x Strong bystander killing activity 39 PSMA: A Validated ADC Target Source: Calais and Czernin , J Nucl Med (2021); O’Driscoll et al., Br J Pharmocol (2016); Kinoshita et al., World J Surg (2006); Sheehan et al ., Eur Urol Focus (2021); Caromile et al., Sci Signal (2017).
PSMA Overview: Prostate - specific membrane antigen (PSMA) is a type II membrane glycoprotein with enzymatic activity ▪ Facilitates neuronal glutamate synthesis in the brain; mediates folate absorption in the intestine ▪ Poorly defined physiological role in prostate cells ▪ May promote cancer progression by redirecting cell survival signaling to the pro - survival pathway mediated by PI3K - AKT signaling Role: x Clinically validated target Rationale: Limited expression on normal prostate tissue and healthy tissue in general ~90% expression in metastatic castration - resistant Prostate Cancer ( mCRPC ) NORMAL TISSUE PROFILE TUMOR EXPRESSION PROFILE 40 PSMA: High Potential Impact in Areas of Unmet Need 1.
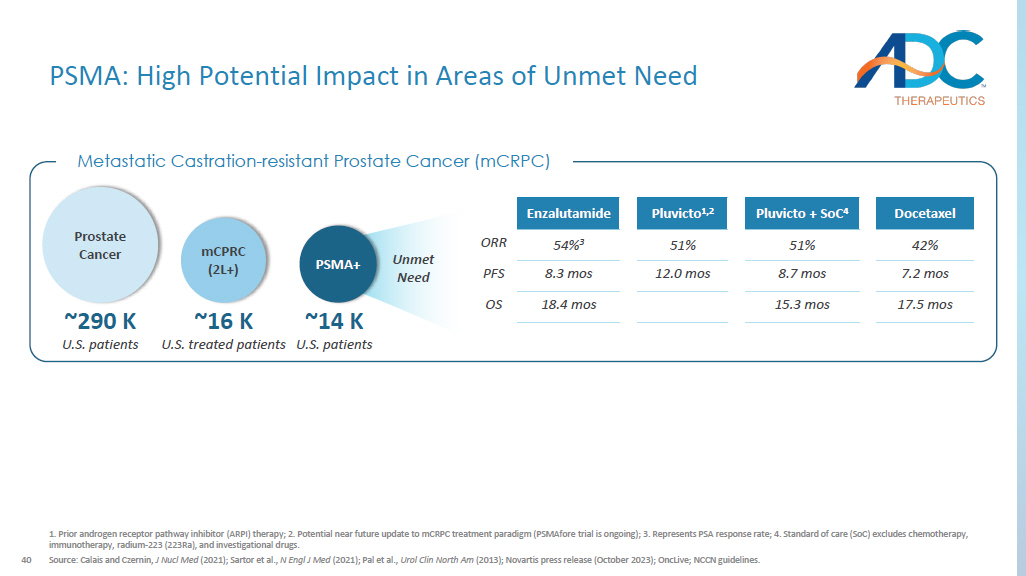
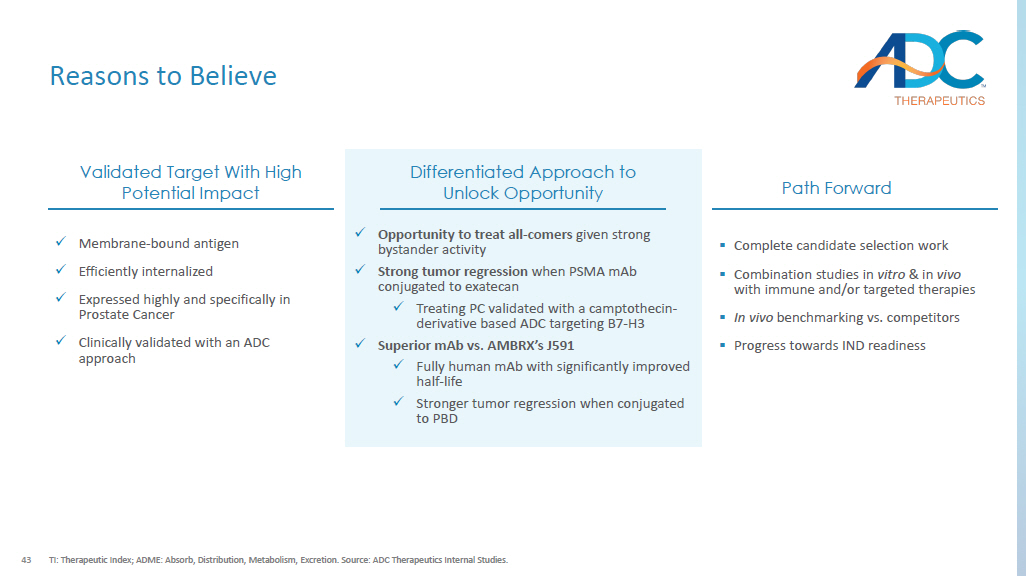
Prior androgen receptor pathway inhibitor (ARPI) therapy; 2. Potential near future update to mCRPC treatment paradigm ( PSMAfore trial is ongoing); 3. Represents PSA response rate; 4. Standard of care (SoC) excludes chemotherapy, immunotherapy, radium - 223 (223Ra), and investigational drugs. Source: Calais and Czernin , J Nucl Med (2021); Sartor et al., N Engl J Med (2021); Pal et al., Urol Clin North Am (2013); Novartis press release (October 2023); OncLive ; NCCN guidelines. Docetaxel Pluvicto + SoC 4 Pluvicto 1,2 Enzalutamide 42% 51% 51% 54% 3 ORR 7.2 mos 8.7 mos 12.0 mos 8.3 mos PFS 17.5 mos 15.3 mos 18.4 mos OS Metastatic Castration - resistant Prostate Cancer ( mCRPC ) ~16 K U.S. treated patients mCPRC (2L+) ~290 K U.S. patients Prostate Cancer ~14 K U.S. patients PSMA+ Unmet Need 41 DAR: drug - antibody - ratio; RNT: Radionuclide therapy 1.

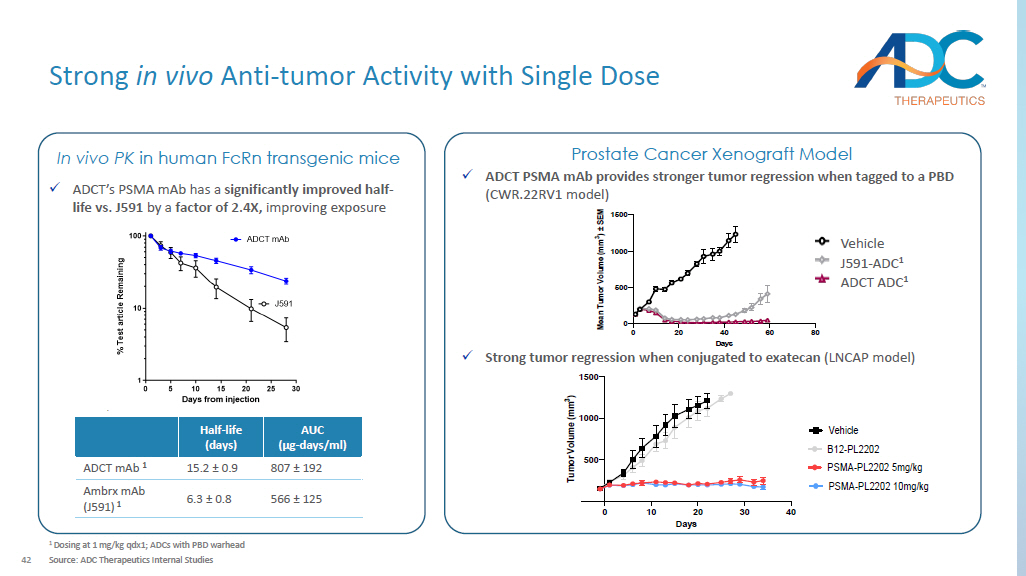
Contributing factors of resistance to PSMA - based RNT include heterogenei ty of tumor PSMA expression, failure to deliver a lethal dose of radiation to metastatic sites, tumor microenvironment, and tumor biological radioresistance . Source: Company websites; Gafita et al., Am Soc Clin Oncol Educ Book, 2022; Kratochwil et al., Semin Nucl Med, 2019; Fu, Nature, 2022, Conference presentations. PSMA - PL2202 Asset name Candidate selection Status Fully Human PSMA specific mAb Antibody x Improved half - life relative to widely used Chimeric J591 Exatecan Payload x Clinically validated in tumors that are sensitive to Topo - 1 inhibitors x Bystander killing effective in heterogenous tumors Proprietary hydrophilic, protease sensitive cleavable linker Linker Hinge cysteine Conjugation 4 DAR Key Differences of PSMA - targeted Clinical Assets ▪ No bystander killing with MMAF ; less effective in patients with heterogenous PSMA expression ▪ J591 chimeric antibody has non - specific binding to the liver ▪ Risk of neurotoxicity in post - taxane patients ARX517 ▪ ~30% of patients are reported to have inherent resistance to PSMA - based RNT 1 and acquired resistance is inevitable ▪ PSMA positive patients may be ineligible due to tumor heterogeneity ▪ Administration complexity; l imited combinability Lutetium (177Lu) vipivotide tetraxetan A Novel Approach to Treating PSMA All - comers 42 Strong in vivo Anti - tumor Activity with Single Dose 1 Dosing at 1 mg/kg qdx1; ADCs with PBD warhead Source: ADC Therapeutics Internal Studies AUC (µg - days/ml) Half - life (days) 807 ± 192 15.2 ± 0.9 ADCT mAb 1 566 ± 125 6.3 ± 0.8 Ambrx mAb (J591) 1 x ADCT’s PSMA mAb has a significantly improved half - life vs. J591 by a factor of 2.4X, improving exposure In vivo PK in human FcRn transgenic mice x ADCT PSMA mAb provides stronger tumor regression when tagged to a PBD (CWR.22RV1 model) Prostate Cancer Xenograft Model 0 20 40 60 80 0 500 1000 1500 Days M e a n T u m o r V o l u m e ( m m 3 ) ± S E M Vehicle J591-SG3249 0.6 mg/kg 2A10-SG3249 0.6 mg/kg ADCT ADC 1 J591 - ADC 1 Vehicle 0 10 20 30 40 500 1000 1500 Days T u m o r V o l u m e ( m m 3 ) B12-PL2202 PSMA-PL2202 10mg/kg Vehicle PSMA-PL2202 5mg/kg x Strong tumor regression when conjugated to exatecan (LNCAP model)
43 Reasons to Believe TI: Therapeutic Index; ADME: Absorb, Distribution, Metabolism, Excretion. Source: ADC Therapeutics Internal Studies. Validated Target With High Potential Impact x Membrane - bound antigen x Efficiently internalized x Expressed highly and specifically in Prostate Cancer x Clinically validated with an ADC approach Path Forward ▪ Complete candidate selection work ▪ Combination studies in vitro & in vivo with immune and/or targeted therapies ▪ In vivo benchmarking vs. competitors ▪ Progress towards IND readiness Differentiated Approach to Unlock Opportunity x Opportunity to treat all - comers given strong bystander activity x Strong tumor regression when PSMA mAb conjugated to exatecan x Treating PC validated with a camptothecin - derivative based ADC targeting B7 - H3 x Superior mAb vs. AMBRX’s J591 x Fully human mAb with significantly improved half - life x Stronger tumor regression when conjugated to PBD 44 ASCT2: A Promising Novel ADC Target Source: ADC Therapeutics Internal Studies.

ASCT2 ASCT2 is ubiquitously expressed in lung, skeletal, large intestine, kidney, testis, T - cells, brain and adipose tissue Overview: A Na+ - dependent aa exchanger (antiporter), localized at the plasma membrane and organised in homotrimers ▪ Responsible for regulating pools of amino acids in intra and extra cellular spaces with glutamine as the preferred substrate ▪ An essential transporter for glutamine uptake by tumors to promote growth and development Role: x Potential pharmacological target x Potential first - in - class opportunity Rationale: NSCLC (N=70) ~99% positivity ~100% positivity Colon (N=36) NORMAL TISSUE PROFILE TUMOR EXPRESSION PROFILE 45 ASCT2: High Potential Impact in Areas of Unmet Need 1.
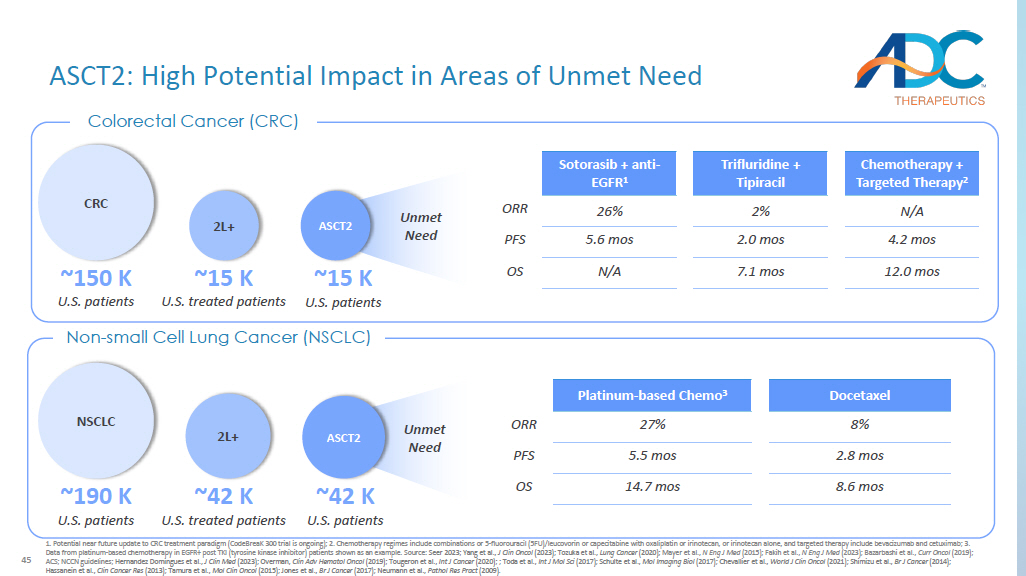
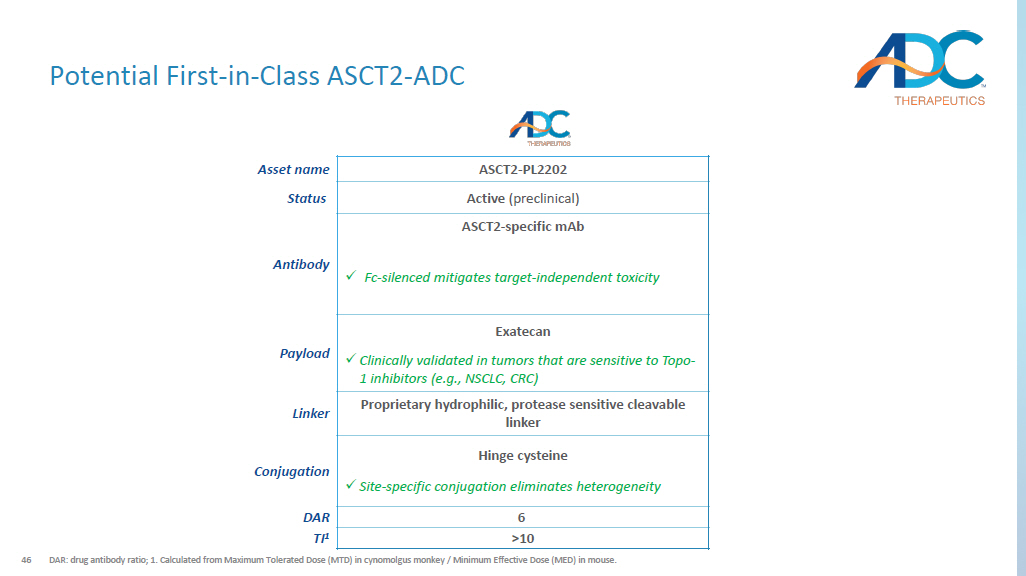
Potential near future update to CRC treatment paradigm ( CodeBreaK 300 trial is ongoing); 2. Chemotherapy regimes include combinations or 5 - fluorouracil (5FU)/leucovorin or capecitabine with oxa liplatin or irinotecan, or irinotecan alone, and targeted therapy include bevacizumab and cetuximab; 3. Data from platinum - based chemotherapy in EGFR+ post TKI (tyrosine kinase inhibitor) patients shown as an example. Source: Seer 2023 ; Yang et al., J Clin Oncol (2023); Tozuka et al., Lung Cancer (2020); Mayer et al., N Eng J Med (2015); Fakih et al., N Eng J Med (2023); Bazarbashi et al., Curr Oncol (2019); ACS; NCCN guidelines; Hernandez Domingues et al., J Clin Med (2023) ; Overman, Clin Adv Hematol Oncol (2019) ; Tougeron et al., Int J Cancer (2020) ; ; Toda et al., Int J Mol Sci (2017) ; Schulte et al., Mol Imaging Biol (2017); Chevallier et al., World J Clin Oncol (2021) ; Shimizu et al., Br J Cancer (2014) ; Hassanein et al., Clin Cancer Res (2013); Tamura et al., Mol Clin Oncol (2015); Jones et al., Br J Cancer (2017); Neumann et al., Pathol Res Pract (2009). Colorectal Cancer (CRC) Docetaxel Platinum - based Chemo 3 8% 27% ORR 2.8 mos 5.5 mos PFS 8.6 mos 14.7 mos OS NSCLC ~190 K U.S. patients ~42 K U.S. treated patients 2L+ ~42 K U.S. patients ASCT2 Non - small Cell Lung Cancer (NSCLC) Unmet Need CRC ~150 K U.S. patients ~15 K U.S. treated patients 2L+ ~15 K U.S. patients ASCT2 Unmet Need Chemotherapy + Targeted Therapy 2 Trifluridine + Tipiracil Sotorasib + anti - EGFR 1 N/A 2% 26% ORR 4.2 mos 2.0 mos 5.6 mos PFS 12.0 mos 7.1 mos N/A OS 46 Potential First - in - Class ASCT2 - ADC DAR: drug antibody ratio ; 1.
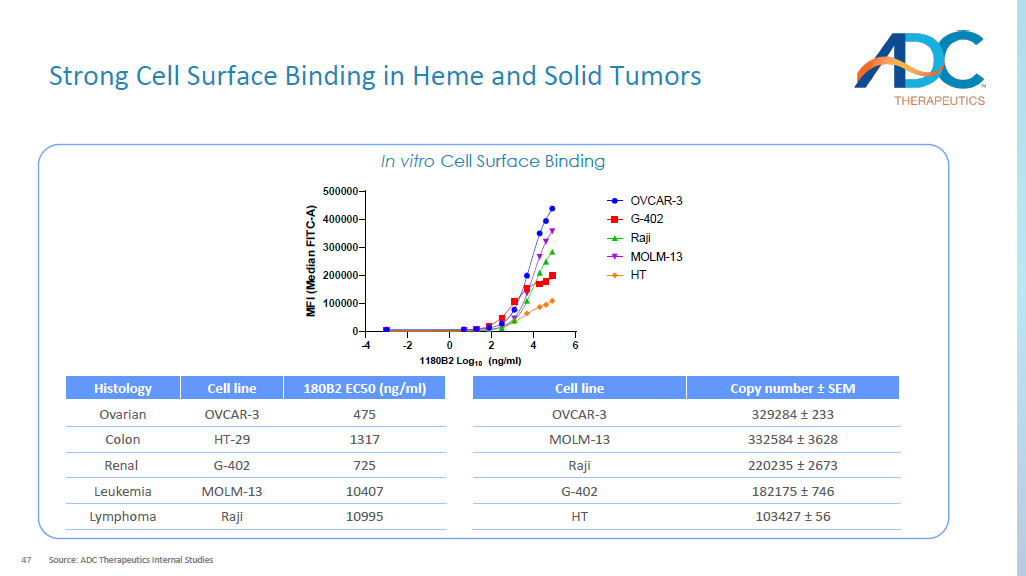
Calculated from Maximum Tolerated Dose (MTD) in cynomolgus monkey / Minimum Effective Dose (MED) in mouse. ASCT2 - PL2202 Asset name Active (preclinical) Status ASCT2 - specific mAb Antibody x Fc - silenced mitigates target - independent toxicity Exatecan Payload x Clinically validated in tumors that are sensitive to Topo - 1 inhibitors (e.g., NSCLC, CRC) Proprietary hydrophilic, protease sensitive cleavable linker Linker Hinge cysteine x Site - specific conjugation eliminates heterogeneity Conjugation 6 DAR >10 TI 1 47 Strong Cell Surface Binding in Heme and Solid Tumors Source: ADC Therapeutics Internal Studies In vitro Cell Surface Binding -4 -2 0 2 4 6 0 100000 200000 300000 400000 500000 1180B2 Log 10 (ng/ml) M F I ( M e d i a n F I T C - A ) OVCAR-3 G-402 Raji MOLM-13 HT Copy number ± SEM Cell line 329284 ± 233 OVCAR - 3 332584 ± 3628 MOLM - 13 220235 ± 2673 Raji 182175 ± 746 G - 402 103427 ± 56 HT 180B2 EC50 (ng/ml) Cell line Histology 475 OVCAR - 3 Ovarian 1317 HT - 29 Colon 725 G - 402 Renal 10407 MOLM - 13 Leukemia 10995 Raji Lymphoma
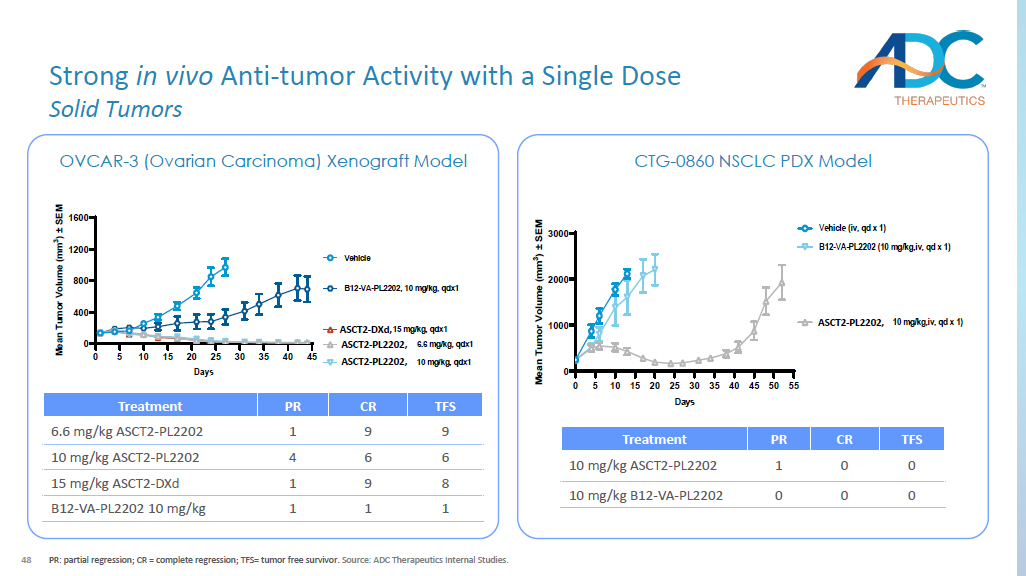
48 Strong in vivo Anti - tumor Activity with a Single Dose Solid Tumors PR: partial regression; CR = complete regression; TFS= tumor free survivor. Source: ADC Therapeutics Internal Studies. OVCAR - 3 (Ovarian Carcinoma) Xenograft Model 0 5 10 15 20 25 30 35 40 45 0 400 800 1200 1600 Days M e a n T u m o r V o l u m e ( m m 3 ) ± S E M Vehicle 1180B2-VA-PL2202, 6.6 mg/kg, qdx1 1180B2-VA-PL2202, 10 mg/kg, qdx1 B12-VA-PL2202, 10 mg/kg, qdx1 1180B2-DXd, 15 mg/kg, qdx1 TFS CR PR Treatment 9 9 1 6.6 mg/kg ASCT2 - PL2202 6 6 4 10 mg/kg ASCT2 - PL2202 8 9 1 15 mg/kg ASCT2 - DXd 1 1 1 B12 - VA - PL2202 10 mg/kg CTG - 0860 NSCLC PDX Model 0 5 10 15 20 25 30 35 40 45 50 55 0 1000 2000 3000 Days M e a n T u m o r V o l u m e ( m m 3 ) ± S E M Vehicle (iv, qd x 1) 1180B2-VA-PL2202 (10 mg/kg,iv, qd x 1) B12-VA-PL2202 (10 mg/kg,iv, qd x 1) TFS CR PR Treatment 0 0 1 10 mg/kg ASCT2 - PL2202 0 0 0 10 mg/kg B12 - VA - PL2202 ASCT2 - PL2202, ASCT2 - PL2202, ASCT2 - DXd, ASCT2 - PL2202, 49 Strong in vivo Anti - tumor Activity with a Single Dose Hematology PR: partial regression; CR = complete regression; TFS= tumor free survivor.

Source: ADC Therapeutics Internal Studies.
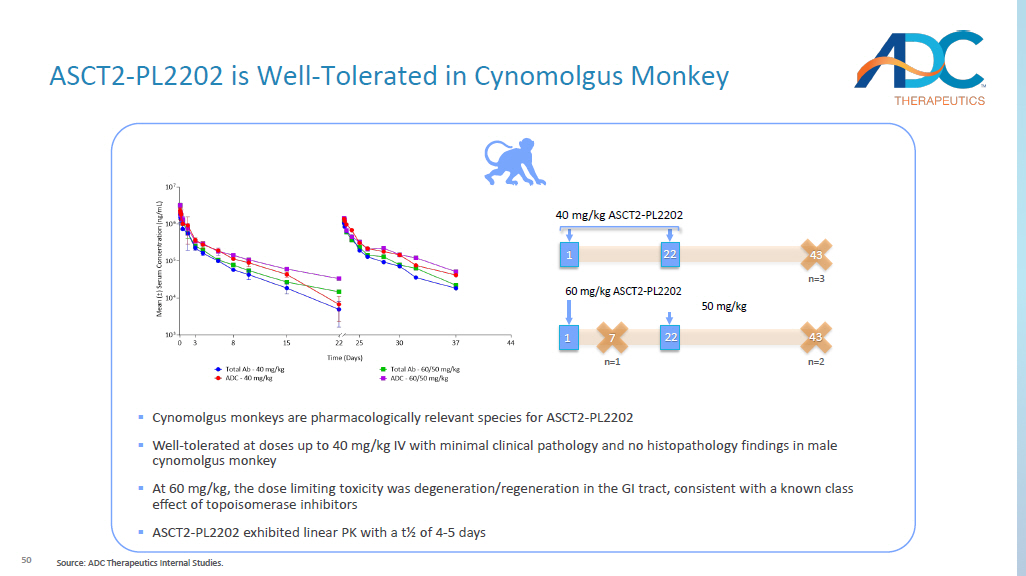
MOLM - 13 (AML) Xenograft Model Raji B (Burkitt’s lymphoma) Xenograft Model TFS CR PR Treatment 2 5 1 10 mg/kg ASCT2 - PL2202 0 0 0 10 mg/kg B12 - VA - PL2202 TFS CR PR Treatment 10 10 0 10 mg/kg ASCT2 - PL2202 0 0 0 10 mg/kg B12 - VA - PL2202 ASCT2 - PL2202, ASCT2 - PL2202, 50 60 mg/kg ASCT2 - PL2202 43 n=2 1 22 50 mg/kg 40 mg/kg ASCT2 - PL2202 43 n=3 1 22 7 n=1 ▪ Cynomolgus monkeys are pharmacologically relevant species for ASCT2 - PL2202 ▪ Well - tolerated at doses up to 40 mg/kg IV with minimal clinical pathology and no histopathology findings in male cynomolgus monkey ▪ At 60 mg/kg, the dose limiting toxicity was degeneration/regeneration in the GI tract, consistent with a known class effect of topoisomerase inhibitors ▪ ASCT2 - PL2202 exhibited linear PK with a t½ of 4 - 5 days ASCT2 - PL2202 is Well - Tolerated in Cynomolgus Monkey Source: ADC Therapeutics Internal Studies.
51 Reasons to Believe TI: Therapeutic Index; IHC: Immunohistochemistry; ADME: Absorb, Distribution, Metabolism, Excretion. Source: ADC Therapeutics In ternal Studies. Promising Target With High Potential Impact x Membrane - bound antigen x Efficiently internalized x Expressed highly in NSCLC, CRC, and hematologic malignancies Path Forward ▪ Complete candidate selection work ▪ Combination studies in vitro & in vivo with immune and/or targeted therapies ▪ ASCT2 IHC assay development ▪ Progress towards IND readiness Differentiated Approach to Unlock Opportunity x Optimized ADC design with high TI x Strong in vivo anti - tumor activity with a single dose in Ovarian, NSCLC, and hematologic xenograft and PDX models x Stable and well - tolerated in cynomolgus monkeys 52 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates ▪ Forward Looking ▪ Closing Remarks ▪ Q&A


53 A Diverse Spectrum of Payloads Camptothecin derivatives Class description Commercial / clinical payload examples Reported benefits DNA - damaging agents Immunomodulators PBD DNA crosslinker Activates immune system DNA cleavage and damage Disrupts DNA synthesis PBD TLR 7 agonist Duocarmycin Exatecan Tesirine TLR / STING agonists Calicheamicin, duocarmycin , doxorubicin Irinotecan, topotecan, deruxtecan ( DXd ) • High cytotoxic potency • Activity across a wide range of cancer cells • Rapid onset of DNA crosslinking • Short half - life • Maintains activity in drug - resistant cancer cells • Induces immune cell infiltration into the tumor • Avoids systemic immune - related toxicities • Promotes immunological memory of tumor cells • High cytotoxic potency • Ideal for tumors with low levels of target antigen • Targets non - dividing cancer cells with DNA repair inhibitors • Bypasses drug - resistance mechanisms • Higher efficacy and lower toxicity than other drugs Source: Hartley et al., Expert Opin Biol Ther (2021); Hartley et al., Sci Rep (2018); Li et al., Am J Cancer Res (2017); Janku et al., Cancer Immunol Res (2022); Ackerman et al., Nat Cancer (2021); Fu et al., Antib Ther (2018). ADCT toolbox payload 54 The Future of Our Payloads Source: ADC Therapeutics Internal Studies.

Exatecan Platform PA1 Ovarian teratocarcinoma xenograft TLR7 Platform Vehicle Target1, 2.5 mg/kg Target1 - TLR7, 2.5 mg/kg 50 HCC1954 Breast cancer xenograft Duocarmycin Platform PA1 Ovarian teratocarcinoma xenograft Target1 - Duocamycin, 1 mg/kg Target1 - Duocamycin, 3 mg/kg Vehicle, qdx1 55 The Forefront of Next - Generation ADC Development Branched Stable Linkers Dual Conjugation Sites More than one toxin per payload Toxin 1 Toxin 1 Alternative Toxins & Conjugation Sites Toxin 2 Toxin 2 Toxin 1 Toxin 1 Two drugs with orthogonal MOA MOA: Mode of Action Source: ADC Therapeutics Internal Studies.


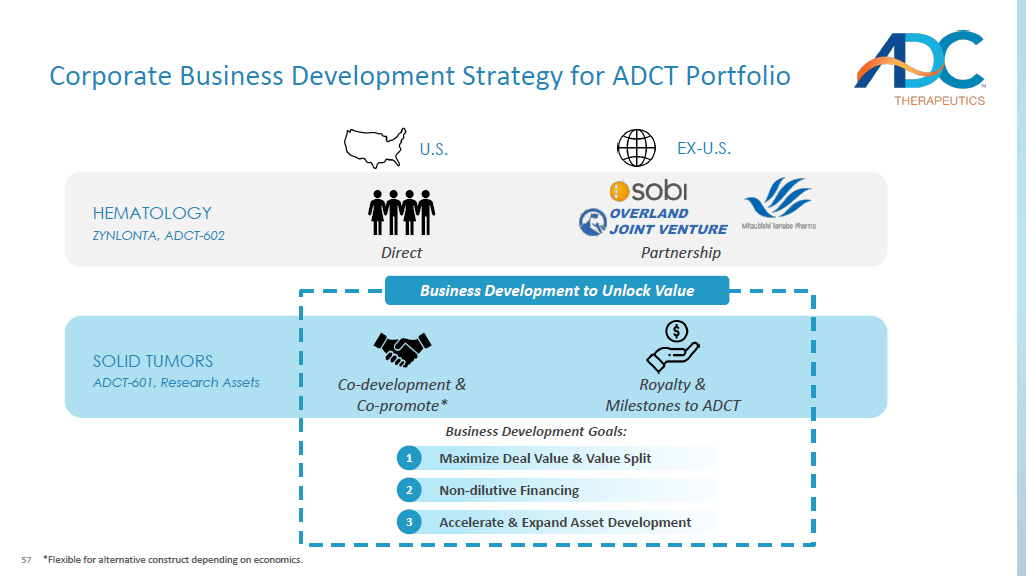
56 Agenda ▪ Business Update ▪ Our Research Strategy ▪ Our Platform ▪ Lead Candidates ▪ Forward Looking ▪ Closing Remarks ▪ Q&A 57 HEMATOLOGY ZYNLONTA, ADCT - 602 SOLID TUMORS ADCT - 601, Research Assets Business Development to Unlock Value Business Development Goals: U.S. EX - U.S. Co - development & Co - promote* Royalty & Milestones to ADCT Accelerate & Expand Asset Development 3 Non - dilutive Financing 2 Maximize Deal Value & Value Split 1 Direct Partnership *Flexible for alternative construct depending on economics.

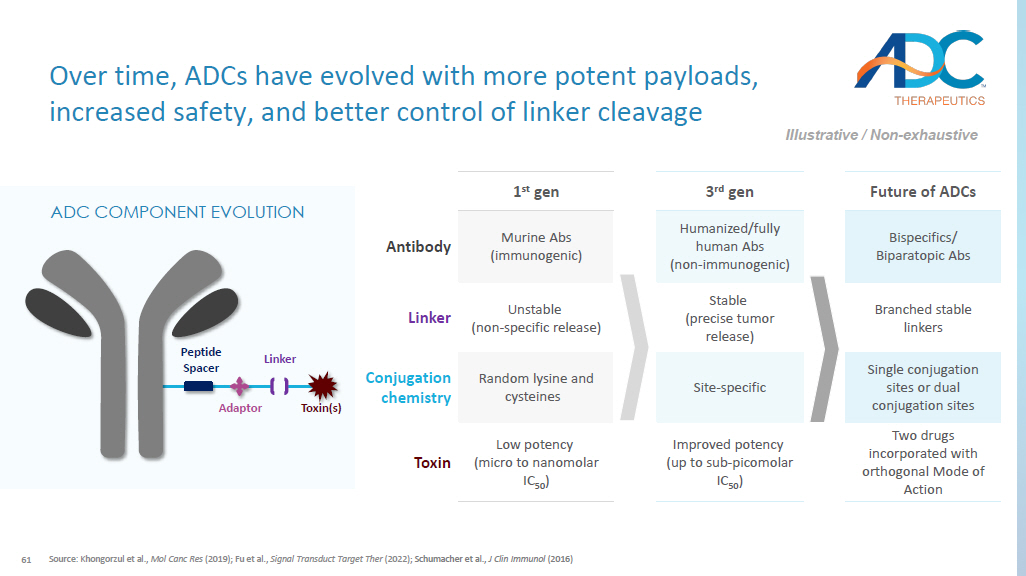
OVERLAND JOINT VENTURE Corporate Business Development Strategy for ADCT Portfolio 58 Closing Summary ▪ Pioneer in the ADC space with an approved product and multiple clinical assets ▪ At the forefront of next - generation ADC development with a proven track record of selecting the optimal targeting moiety, linker, and payload to establish a therapeutic window ▪ Lead preclinical candidates are built on a novel, proprietary, exatecan - based platform ▪ Differentiated exatecan platform design that has enabled reproducible in vivo efficacy and a high therapeutic index in all of our preclinical candidates ▪ High potential patient impact in tumors with unmet medical need ▪ A unique opportunity to accelerate and expand the development of multiple transformational ADC therapies through internal development and innovative collaborations 61 Future of ADCs 3 rd gen 1 st gen Bispecifics / Biparatopic Abs Humanized/fully human Abs (non - immunogenic) Murine Abs (immunogenic) Antibody Branched stable linkers Stable (precise tumor release) Unstable (non - specific release) Linker Single conjugation sites or dual conjugation sites Site - specific Random lysine and cysteines Conjugation chemistry Two drugs incorporated with orthogonal Mode of Action Improved potency (up to sub - picomolar IC 50 ) Low potency (micro to nanomolar IC 50 ) Toxin Source: Khongorzul et al., Mol Canc Res (2019); Fu et al., Signal Transduct Target Ther (2022); Schumacher et al., J Clin Immunol (2016) Over time, ADCs have evolved with more potent payloads, increased safety, and better control of linker cleavage Illustrative / Non - exhaustive ADC COMPONENT EVOLUTION Peptide Spacer Adaptor Linker Toxin(s)

Q&A

Appendix

62 Each of the Exatecan programs has a composition of matter filing for the antibody drug conjugate per se with long expiry dates In - licensed composition of matter patent protection (exclusive) for Claudin - 6 antibody US, EU, CN, JP – base expiry 05/2041 with possible extensions up to 05/2046 2041 2042 2045 Proprietary hydrophilic linker technology Base Expiry 10/2044 KEY PATENT PORTFOLIO 2046 2043 2044 2047 2048 2049 Composition of matter (Exatecan - based ADCs) – NaPi2b, Claudin - 6, PSMA, ASCT2 US, EU, CN and JP – base expiry 10/2044 or 12/2044 with possible extensions up to 10/2049 or 12/2049 63 Regulatory Exclusivities REGULATORY DATA PROTECTION ▪ 12 years from approval with a possible pediatric extension of 6 months ▪ 10 years from approval ▪ 8 - year re - examination period with a possible 2 - year pediatric extension

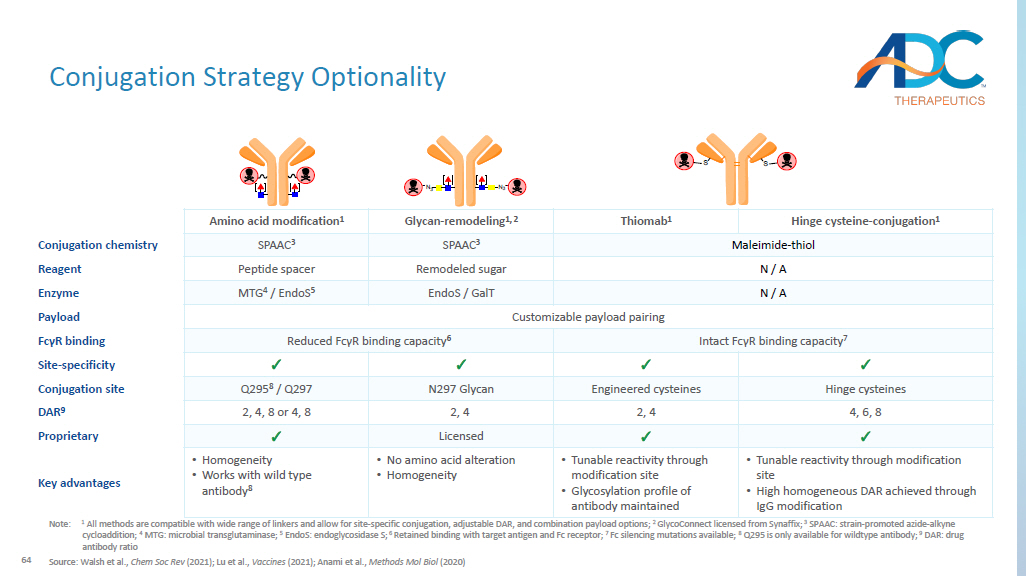
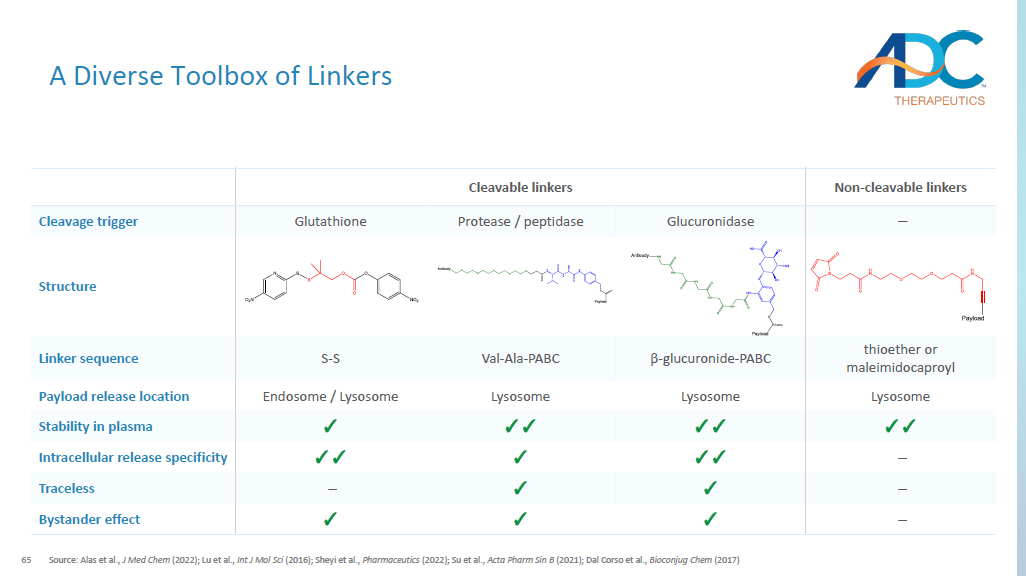
64 CORPORATE Conjugation Strategy Optionality Note: 1 All methods are compatible with wide range of linkers and allow for site - specific conjugation, adjustable DAR, and combination payload options; 2 GlycoConnect licensed from Synaffix ; 3 SPAAC: strain - promoted azide - alkyne cycloaddition; 4 MTG: microbial transglutaminase; 5 EndoS : endoglycosidase S; 6 Retained binding with target antigen and Fc receptor; 7 Fc silencing mutations available; 8 Q295 is only available for wildtype antibody; 9 DAR: drug antibody ratio Source: Walsh et al., Chem Soc Rev (2021); Lu et al., Vaccines (2021); Anami et al., Methods Mol Biol (2020) Hinge cysteine - conjugation 1 Thiomab 1 Glycan - remodeling 1, 2 Amino acid modification 1 Maleimide - thiol SPAAC 3 SPAAC 3 Conjugation chemistry N / A Remodeled sugar Peptide spacer Reagent N / A EndoS / GalT MTG 4 / EndoS 5 Enzyme Customizable payload pairing Payload Intact Fc γ R binding capacity 7 Reduced Fc γ R binding capacity 6 Fc γ R binding ✓ ✓ ✓ ✓ Site - specificity Hinge cysteines Engineered cysteines N297 Glycan Q295 8 / Q297 Conjugation site 4, 6, 8 2, 4 2, 4 2, 4, 8 or 4, 8 DAR 9 ✓ ✓ Licensed ✓ Proprietary • Tunable reactivity through modification site • High homogeneous DAR achieved through IgG modification • Tunable reactivity through modification site • Glycosylation profile of antibody maintained • No amino acid alteration • Homogeneity • Homogeneity • Works with wild type antibody 8 Key advantages MTGase PNGase F Endo-S N 3 N 3 amine-PEG(3u)-azide MTGase amine-PEG(3u)-azide N 3 N 3 DBCO- DBCO-65 A Diverse Toolbox of Linkers Source: Alas et al., J Med Chem (2022); Lu et al., Int J Mol Sci (2016); Sheyi et al., Pharmaceutics (2022); Su et al., Acta Pharm Sin B (2021); Dal Corso et al., Bioconjug Chem (2017) Non - cleavable linkers Cleavable linkers – Glucuronidase Protease / peptidase Glutathione Cleavage trigger Structure thioether or maleimidocaproyl β - glucuronide - PABC Val - Ala - PABC S - S Linker sequence Lysosome Lysosome Lysosome Endosome / Lysosome Payload release location ✓✓ ✓✓ ✓✓ ✓ Stability in plasma – ✓✓ ✓ ✓✓ Intracellular release specificity – ✓ ✓ – Traceless – ✓ ✓ ✓ Bystander effect