
November 8, 2022 nasdaq: axgn Corporate presentation

2 This presentation contains “forward-looking” statements as defined in the Private Securities Litigation Reform Act of 1995. These statements are based on management's current expectations or predictions of future conditions, events, or results based on various assumptions and management's estimates of trends and economic factors in the markets in which we are active, as well as our business plans. Words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “projects,” “forecasts,” “continue,” “may,” “should,” “will,” “goals,” and variations of such words and similar expressions are intended to identify such forward-looking statements. Actual results or event could differ materially from those described in any forward-looking statements as a result of various factors, including, without limitation, statements related to the impact of COVID-19 on our business, hospital staffing challenges and its impact on our business, statements regarding our growth, our financial guidance and performance, product development, product potential, regulatory process and approvals, APC renovation timing and expense, sales growth, product adoption, market awareness of our products, anticipated capital requirements, including the potential of future financings, data validation, expected clinical study enrollment, timing and outcomes, , our visibility at and sponsorship of conferences and our educational events, regulatory process and approvals and other factors, including legislative, regulatory, political, geopolitical, and economic developments, including global business disruption caused by Russia’s invasion of Ukraine and related sanctions, not within our control. The forward-looking statements are and will be subject to risks and uncertainties, which may cause actual results to differ materially from those expressed or implied in such forward-looking statements. Forward-looking statements contained in this presentation should be evaluated together with the many risks and uncertainties that affect our business and our market, particularly those risk factors described under Part I, Item 1A., “Risk Factors,” of our Annual Report on Form 10-K for the most recently ended fiscal year, as well as other risks and cautionary statements set forth in our filings with the U.S. Securities and Exchange Commission. Forward-looking statements are not a guarantee of future performance, and actual results may differ materially from those projected. The forward- looking statements are representative only as of the date they are made and, except as required by applicable law, we assume no responsibility to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances, or otherwise. Safe harbor statement revolutionizing the science of nerve repair™

3 The Axogen platform for nerve repair revolutionizing the science of nerve repair™
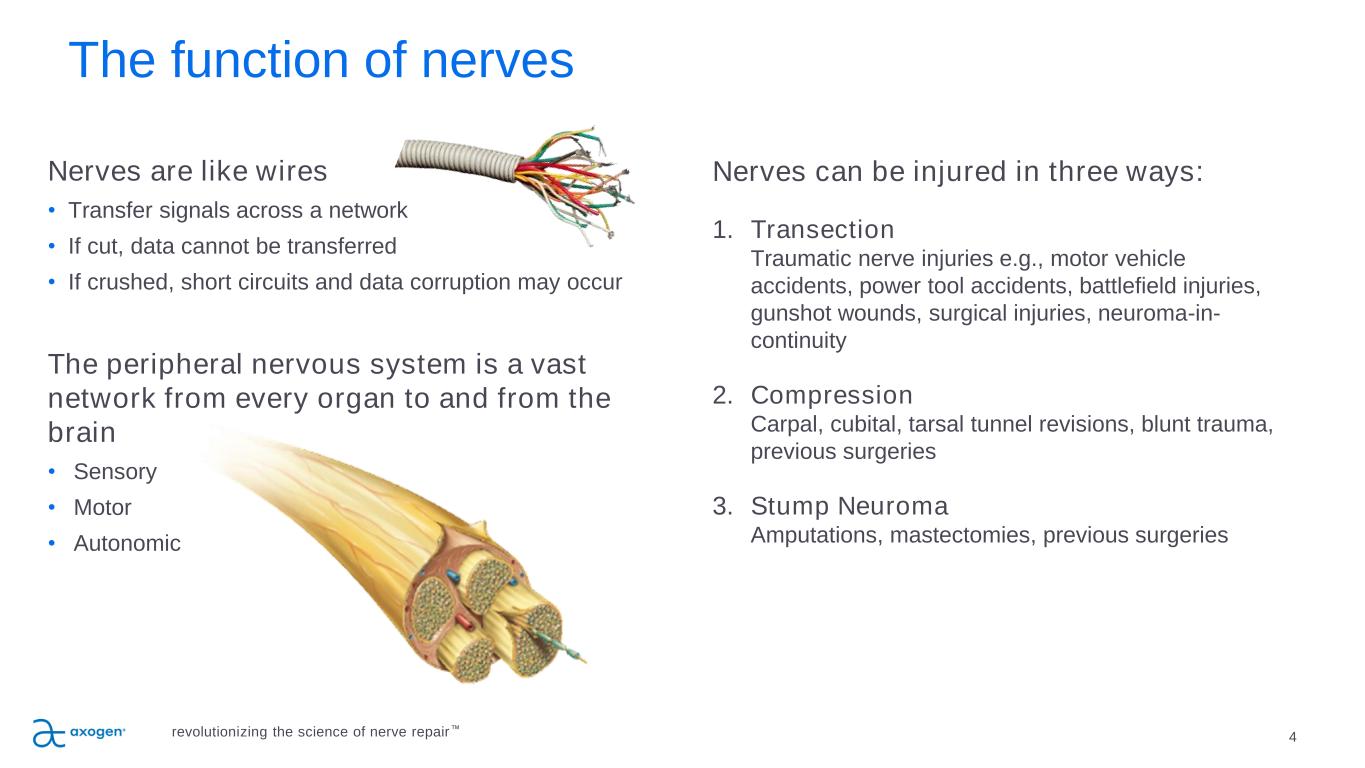
4 The function of nerves Nerves are like wires • Transfer signals across a network • If cut, data cannot be transferred • If crushed, short circuits and data corruption may occur The peripheral nervous system is a vast network from every organ to and from the brain • Sensory • Motor • Autonomic Nerves can be injured in three ways: 1. Transection Traumatic nerve injuries e.g., motor vehicle accidents, power tool accidents, battlefield injuries, gunshot wounds, surgical injuries, neuroma-in- continuity 2. Compression Carpal, cubital, tarsal tunnel revisions, blunt trauma, previous surgeries 3. Stump Neuroma Amputations, mastectomies, previous surgeries revolutionizing the science of nerve repair™

Connection Protection Termination 5 A comprehensive platform for addressing nerve injuries revolutionizing the science of nerve repair™
Axogen is the preeminent nerve repair company with a foundation for long-term sustainable growth 6 • Exclusively focused on peripheral nerve repair with a differentiated platform • 10+ years of demonstrated clinical outcome consistency • Featured in 207 peer-reviewed clinical publications • Over 50,000 Avance® Nerve Grafts implanted • Significant barriers to competitive entry • FDA granted Avance Regenerative Medicine Advanced Therapy (RMAT) designation • Commercial and surgeon education capabilities • Solid balance sheet provides resources to execute business plan • Experienced management team with strong track record of success revolutionizing the science of nerve repair™

Targeted nerve markets (U.S.) 7 Trauma $1.9B Breast $250M OMF $300M Carpal & Cubital Tunnel $270M U.S. potential procedural estimates >900,000** • Trauma: > 700,000 • Carpal Tunnel Revisions & Cubital Tunnel: 130,000 • Oral Maxillofacial (OMF): 56,000 • Breast Neurotization Procedures: 15,000 *$2.7B estimate does not include pain market **Referenced papers were used to derive specific assumptions in the procedure potential estimates. Papers used include both U.S. and OUS databases and studies. See Appendix for data sources. >$2.7 Billion* revolutionizing the science of nerve repair™
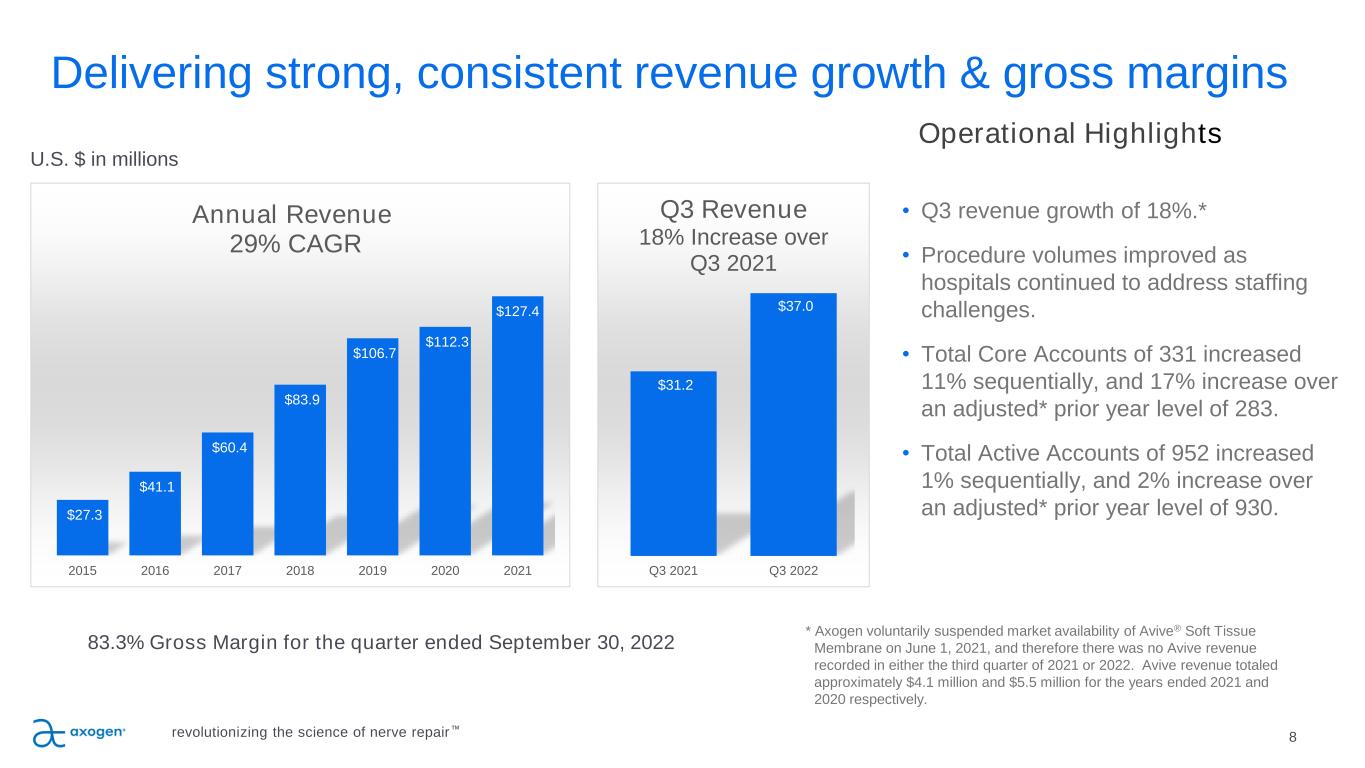
8 Delivering strong, consistent revenue growth & gross margins Operational Highlights • Q3 revenue growth of 18%.* • Procedure volumes improved as hospitals continued to address staffing challenges. • Total Core Accounts of 331 increased 11% sequentially, and 17% increase over an adjusted* prior year level of 283. • Total Active Accounts of 952 increased 1% sequentially, and 2% increase over an adjusted* prior year level of 930. 83.3% Gross Margin for the quarter ended September 30, 2022 U.S. $ in millions * Axogen voluntarily suspended market availability of Avive® Soft Tissue Membrane on June 1, 2021, and therefore there was no Avive revenue recorded in either the third quarter of 2021 or 2022. Avive revenue totaled approximately $4.1 million and $5.5 million for the years ended 2021 and 2020 respectively. $27.3 $41.1 $60.4 $83.9 $106.7 $112.3 $127.4 2015 2016 2017 2018 2019 2020 2021 Annual Revenue 29% CAGR revolutionizing the science of nerve repair™ $31.2 $37.0 Q3 2021 Q3 2022 Q3 Revenue 18% Increase over Q3 2021

Guidance 9 2022 Annual Financial Guidance – Updated 11/8/2022 • Full-year 2022 revenue is expected to be between $137.5 million and $140 million, compared to prior range of $135 million to $142 million. – Represents approximately 12% to 14% growth over 2021 revenue excluding the impact of $4.1 million of Avive revenue in 2021. • Full-year 2022 gross margin is expected to remain above 80%. revolutionizing the science of nerve repair™
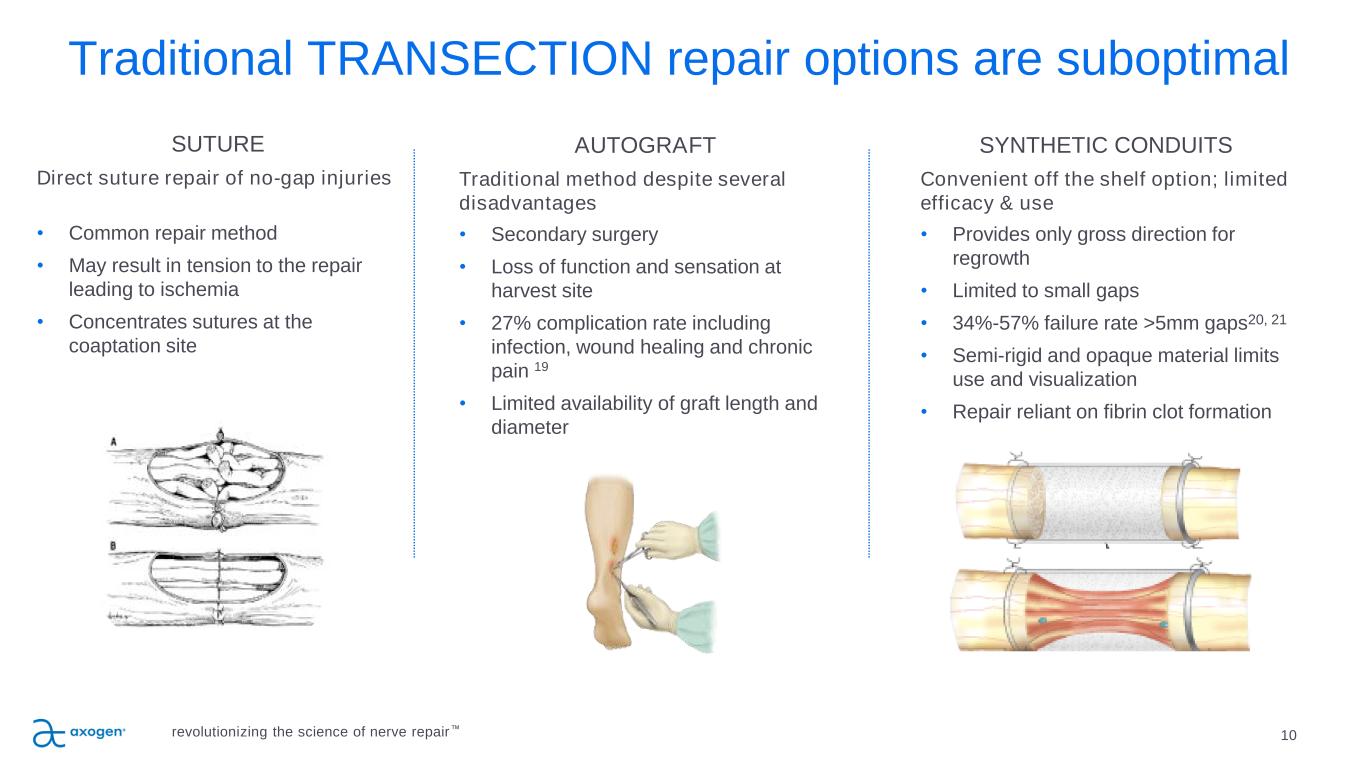
Traditional TRANSECTION repair options are suboptimal 10 SUTURE Direct suture repair of no-gap injuries • Common repair method • May result in tension to the repair leading to ischemia • Concentrates sutures at the coaptation site AUTOGRAFT Traditional method despite several disadvantages • Secondary surgery • Loss of function and sensation at harvest site • 27% complication rate including infection, wound healing and chronic pain 19 • Limited availability of graft length and diameter SYNTHETIC CONDUITS Convenient off the shelf option; limited efficacy & use • Provides only gross direction for regrowth • Limited to small gaps • 34%-57% failure rate >5mm gaps20, 21 • Semi-rigid and opaque material limits use and visualization • Repair reliant on fibrin clot formation revolutionizing the science of nerve repair™

11 25 µm Processed human nerve allograft for bridging nerve gaps Clinically studied off-the-shelf alternative • A biologically active nerve therapy with more than ten years of comprehensive clinical evidence • 82-84% meaningful recovery in sensory, mixed and motor nerve gaps in multi-center study22 • Eliminates need for an additional surgical site and risks of donor nerve harvest22 • May reduce OR time Structural support for regenerating axons • Cleansed and decellularized extracellular matrix (ECM) • Offers the benefits of human peripheral nerve micro-architecture and handling Revascularizes and remodels into patient’s own tissue similar to autologous nerve23 16 size options in a variety of lengths (up to 70mm) and diameters (up to 5mm) Only minimally processed porcine ECM for connector-assisted coaptation Alternative to direct suture repair • Reduces the risk of forced fascicular mismatch24, 25 Alleviates tension at critical zone of regeneration • Disperses tension across repair site26 • Moves suture inflammation away from coaptation face27, 28 Remodels into vascularized patient tissue28, 29, 30, 31, 32 14 size options in lengths of 10mm and 15mm, and diameters up to 7mm Axogen solutions for TRANSECTION repair revolutionizing the science of nerve repair™
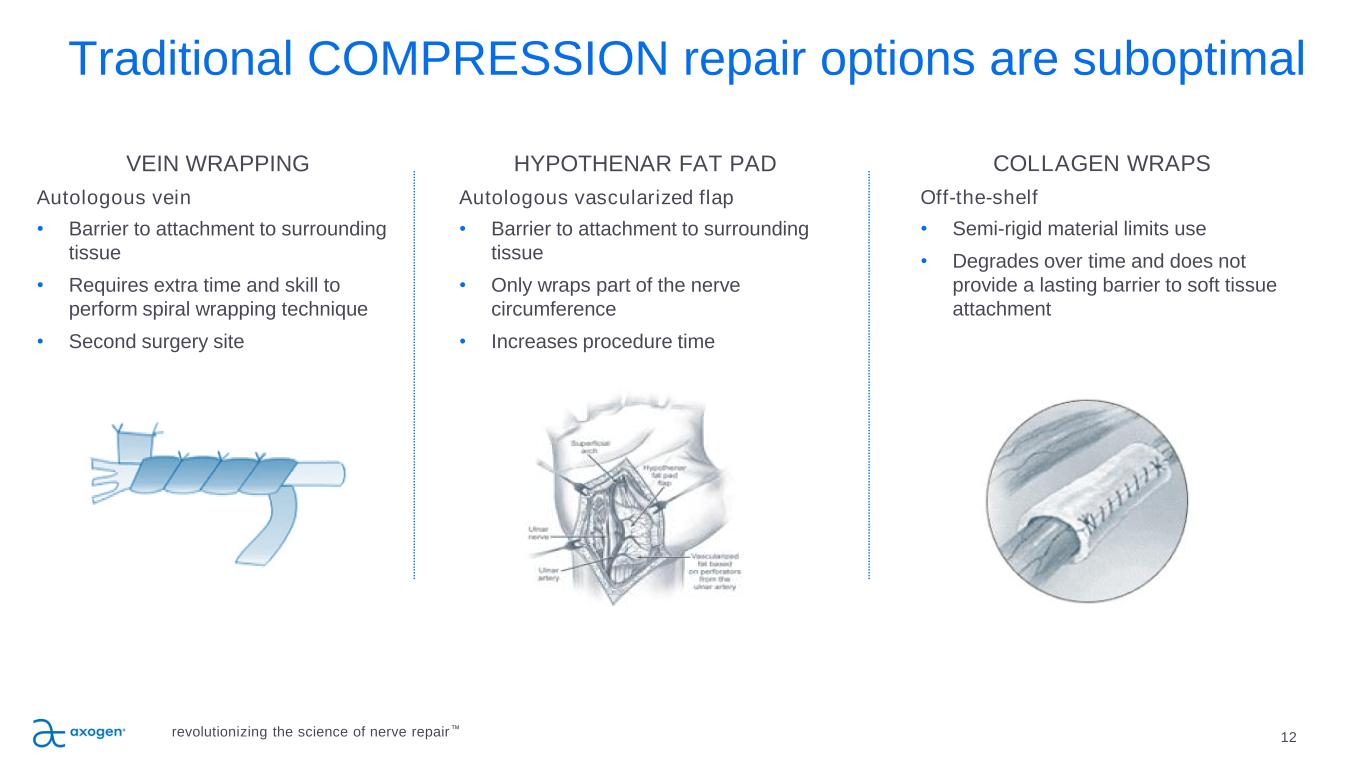
Traditional COMPRESSION repair options are suboptimal 12 VEIN WRAPPING Autologous vein • Barrier to attachment to surrounding tissue • Requires extra time and skill to perform spiral wrapping technique • Second surgery site HYPOTHENAR FAT PAD Autologous vascularized flap • Barrier to attachment to surrounding tissue • Only wraps part of the nerve circumference • Increases procedure time COLLAGEN WRAPS Off-the-shelf • Semi-rigid material limits use • Degrades over time and does not provide a lasting barrier to soft tissue attachment revolutionizing the science of nerve repair™

13 Axogen solution for COMPRESSION repair Minimally processed porcine extracellular matrix for wrapping and protecting injured peripheral nerve Protects repair site from surrounding tissue • Processing results in an implant that works with the body’s natural healing process33 • Minimizes soft tissue attachments34 Allows nerve gliding • Minimizes risk of entrapment34 • Creates a barrier between repair and surrounding tissue bed34 • ECM revascularizes and remodels into patient’s own tissue29,35 revolutionizing the science of nerve repair™
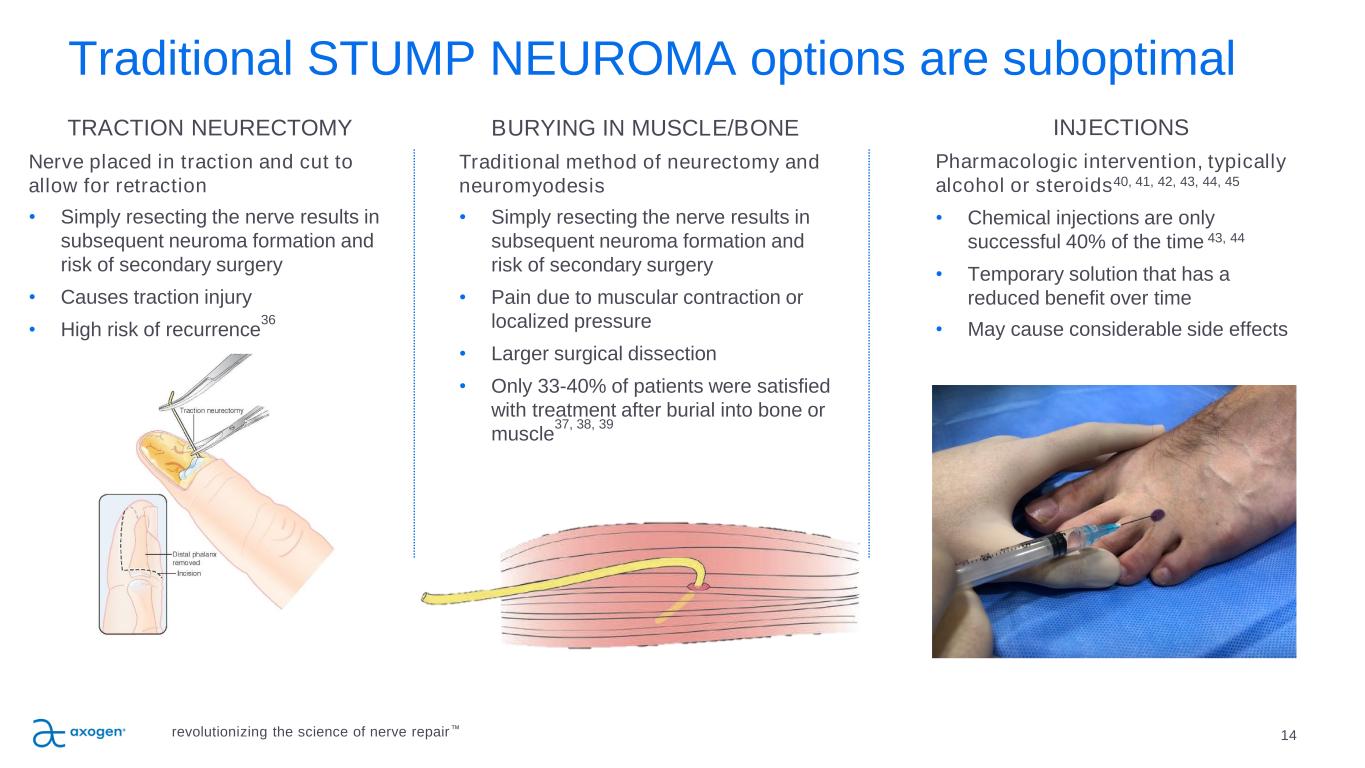
Traditional STUMP NEUROMA options are suboptimal 14 TRACTION NEURECTOMY Nerve placed in traction and cut to allow for retraction • Simply resecting the nerve results in subsequent neuroma formation and risk of secondary surgery • Causes traction injury • High risk of recurrence 36 BURYING IN MUSCLE/BONE Traditional method of neurectomy and neuromyodesis • Simply resecting the nerve results in subsequent neuroma formation and risk of secondary surgery • Pain due to muscular contraction or localized pressure • Larger surgical dissection • Only 33-40% of patients were satisfied with treatment after burial into bone or muscle 37, 38, 39 INJECTIONS Pharmacologic intervention, typically alcohol or steroids40, 41, 42, 43, 44, 45 • Chemical injections are only successful 40% of the time 43, 44 • Temporary solution that has a reduced benefit over time • May cause considerable side effects revolutionizing the science of nerve repair™
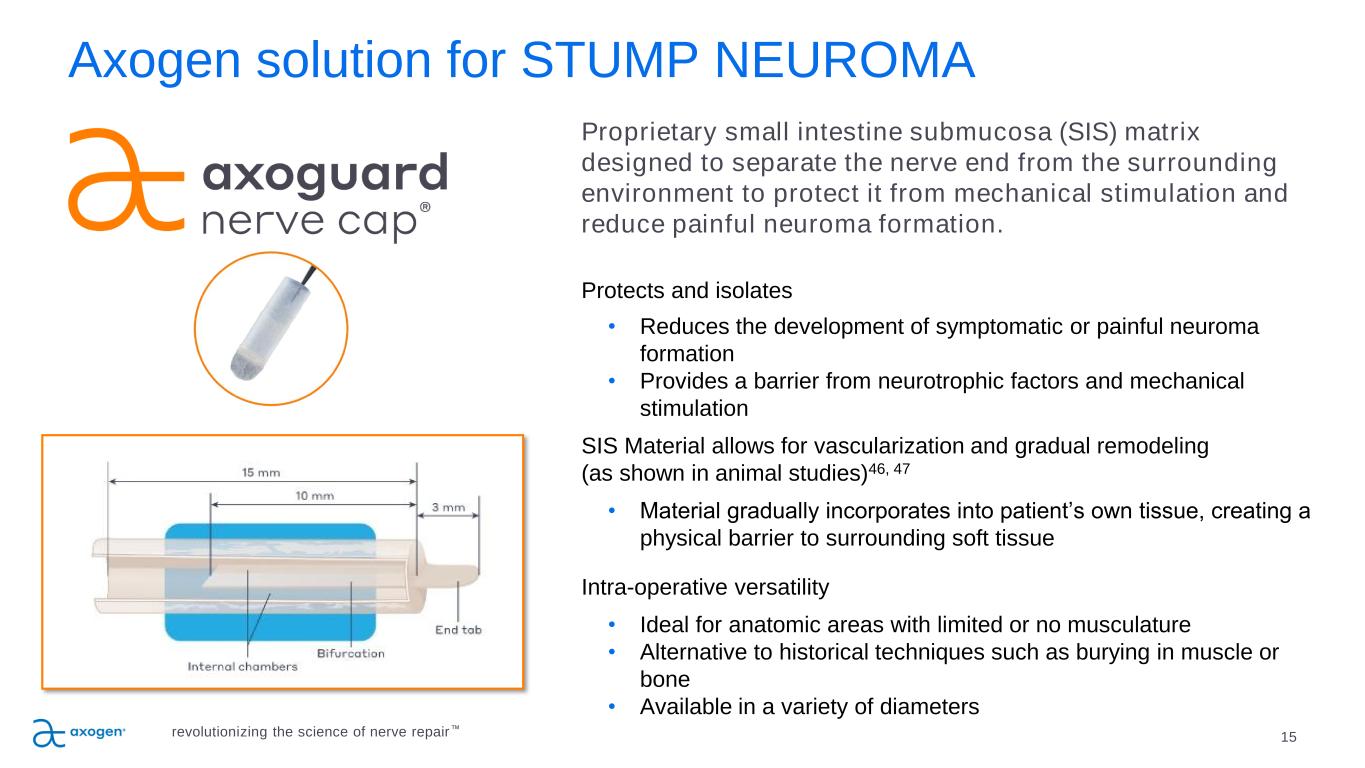
15 Axogen solution for STUMP NEUROMA Proprietary small intestine submucosa (SIS) matrix designed to separate the nerve end from the surrounding environment to protect it from mechanical stimulation and reduce painful neuroma formation. Protects and isolates • Reduces the development of symptomatic or painful neuroma formation • Provides a barrier from neurotrophic factors and mechanical stimulation SIS Material allows for vascularization and gradual remodeling (as shown in animal studies)46, 47 • Material gradually incorporates into patient’s own tissue, creating a physical barrier to surrounding soft tissue Intra-operative versatility • Ideal for anatomic areas with limited or no musculature • Alternative to historical techniques such as burying in muscle or bone • Available in a variety of diameters revolutionizing the science of nerve repair™
16 Avance IP and regulatory barriers to competitive entry Avance nerve graft is processed and distributed in accordance with US FDA requirements for Human Cellular and Tissue-based Products (HCT/P) Avance nerve graft Issued U.S. Patents Axogen has Enforcement Discretion from FDA allowing continued sales under controls applicable to HCT/Ps with agreed transition plan to regulation as a Biological Product under a Biologic License Application (BLA) if approved. A new (non-biosimilar) competitive processed nerve allograft, we believe, would need to complete clinical testing and obtain BLA approval prior to clinical release. Avance expected to be the reference product for the category of processed nerve allograft Avance nerve graft IP protection to 2023 and beyond New (non-biosimilar) competitive BLA product estimated 8 years Protection from potential biosimilars –12 years data exclusivity from BLA approval revolutionizing the science of nerve repair™ 6,972,168 9,690,975 7,402,319 9,996,729 7,732,200 10,311,281 7,851,447 10,441,681 8,758,794 10,783,349 8,986,733 10,813,643 9,402,868 11,147,558 9,572,911 11,156,595 9,597,429

RECONSM: A Multicenter, Prospective, Randomized, Subject & Evaluator Blinded Comparative Study of Nerve Cuffs & Avance Nerve Graft Evaluating Recovery Outcomes for the Repair of Nerve Discontinuities revolutionizing the science of nerve repair™ 17 Safety & efficacy non- inferiority comparison of Avance vs conduit Evaluated upper extremity digital nerve repair for nerve gaps 5-25mm 220 subjects from up to 25 U.S. centers stratified into gap lengths with two-thirds in the 5-14mm group and one- third in the 15-25mm group 17
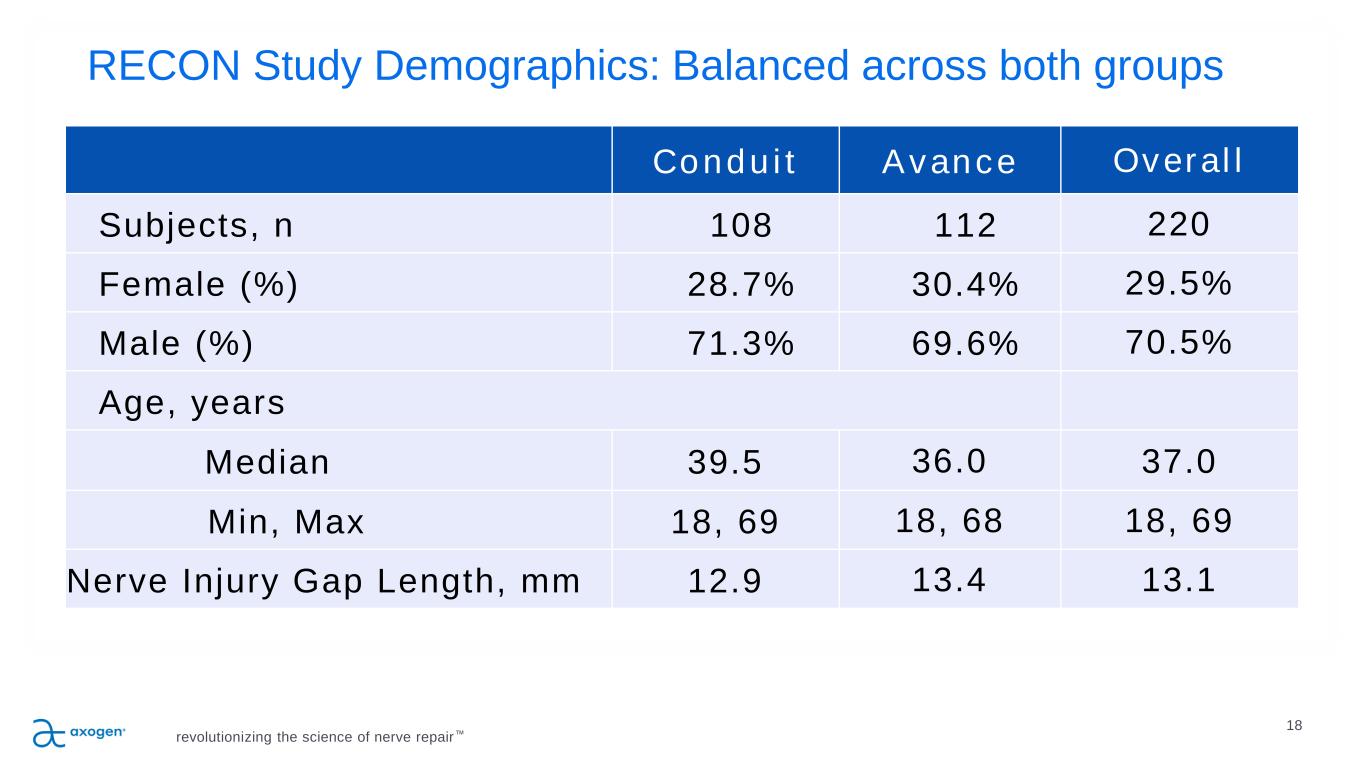
RECON Study Demographics: Balanced across both groups revolutionizing the science of nerve repair™ 18 Conduit Avance Overall Subjects, n 108 112 220 Female (%) 28.7% 30.4% 29.5% Male (%) 71.3% 69.6% 70.5% Age, years Median 39.5 36.0 37.0 Min, Max 18, 69 18, 68 18, 69 Nerve Injury Gap Length, mm 12.9 13.4 13.1 18
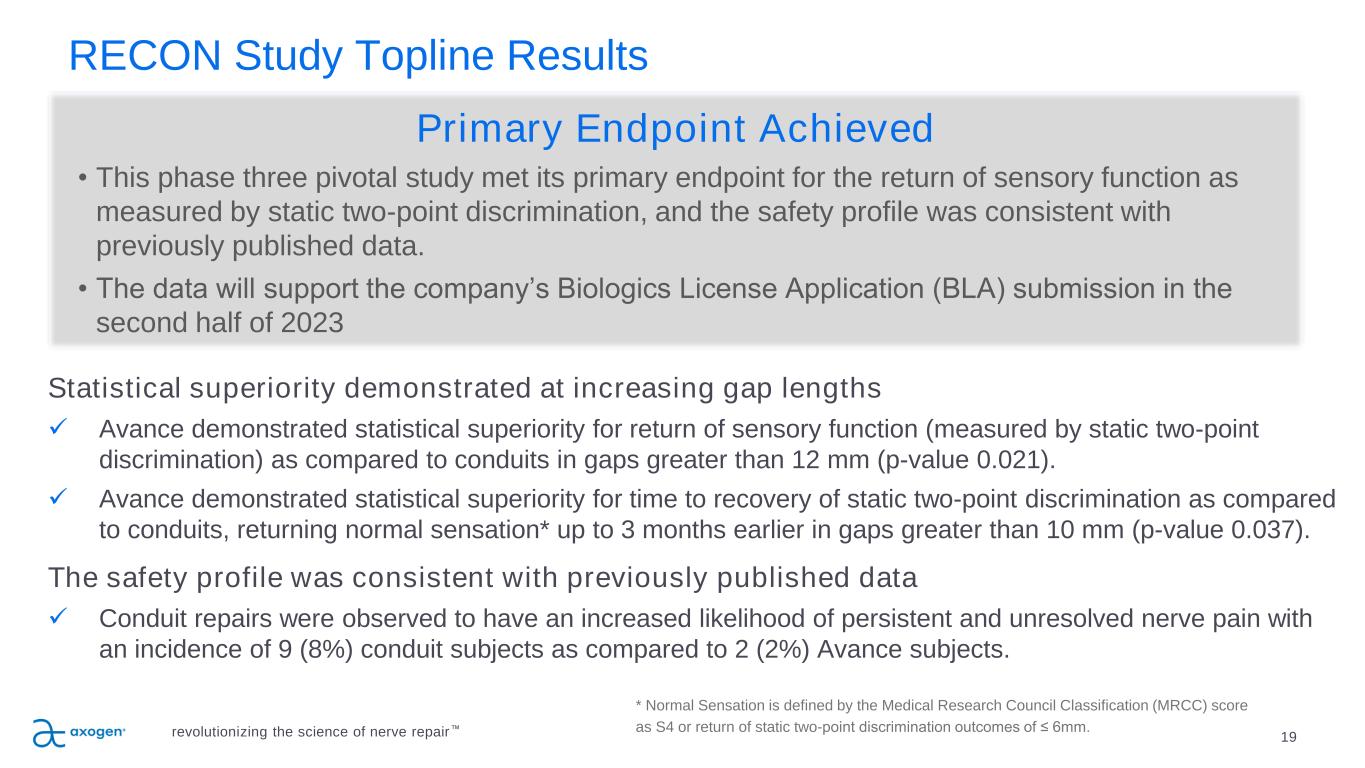
RECON Study Topline Results 19revolutionizing the science of nerve repair™ Statistical superiority demonstrated at increasing gap lengths ✓ Avance demonstrated statistical superiority for return of sensory function (measured by static two-point discrimination) as compared to conduits in gaps greater than 12 mm (p-value 0.021). ✓ Avance demonstrated statistical superiority for time to recovery of static two-point discrimination as compared to conduits, returning normal sensation* up to 3 months earlier in gaps greater than 10 mm (p-value 0.037). The safety profile was consistent with previously published data ✓ Conduit repairs were observed to have an increased likelihood of persistent and unresolved nerve pain with an incidence of 9 (8%) conduit subjects as compared to 2 (2%) Avance subjects. * Normal Sensation is defined by the Medical Research Council Classification (MRCC) score as S4 or return of static two-point discrimination outcomes of ≤ 6mm. Primary Endpoint Achieved • This phase three pivotal study met its primary endpoint for the return of sensory function as measured by static two-point discrimination, and the safety profile was consistent with previously published data. • The data will support the company’s Biologics License Application (BLA) submission in the second half of 2023
20 Market development strategy B u il d M a rk e t A w a re n e s s E d u c a te S u rg e o n s , D e v e lo p A d v o c a te s G ro w B o d y o f C li n ic a l E v id e n c e E x e c u te S a le s P la n E x p a n d P ro d u c t P ip e li n e + A p p li c a ti o n s revolutionizing the science of nerve repair™

21 Focus on building awareness among clinicians and patients • Increasing omnichannel engagement with clinicians and patients • Continuing clinical conference participation both virtually and in-person as appropriate • Ongoing patient ambassador program • Garnering positive media attention • Growing social media presenceB u il d M a rk e t A w a re n e s s revolutionizing the science of nerve repair™ resensation.com rethink-pain.com

22 P IL L A R 2 Emphasis on education • In-person and virtual national education programs • Providing customized multimodal learning programs to specific surgeon cohorts for advanced learning • Ongoing interactive webinar series covering the principles of nerve repair • Train more than three-quarters of all hand and micro-surgery fellows annuallyE d u c a te S u rg e o n s , D e v e lo p A d v o c a te s revolutionizing the science of nerve repair™
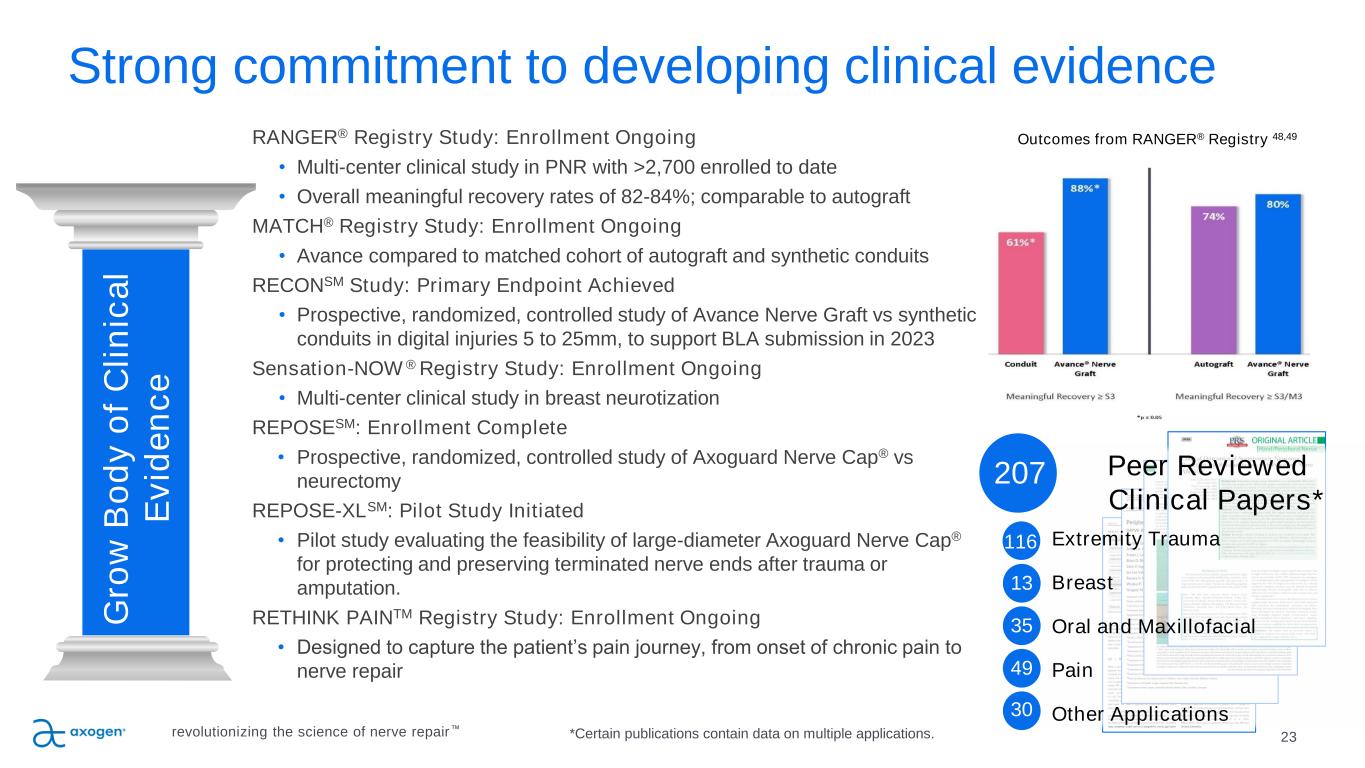
Outcomes from RANGER® Registry 48,49 23 P IL L A R 3 Strong commitment to developing clinical evidence RANGER® Registry Study: Enrollment Ongoing • Multi-center clinical study in PNR with >2,700 enrolled to date • Overall meaningful recovery rates of 82-84%; comparable to autograft MATCH® Registry Study: Enrollment Ongoing • Avance compared to matched cohort of autograft and synthetic conduits RECONSM Study: Primary Endpoint Achieved • Prospective, randomized, controlled study of Avance Nerve Graft vs synthetic conduits in digital injuries 5 to 25mm, to support BLA submission in 2023 Sensation-NOW ® Registry Study: Enrollment Ongoing • Multi-center clinical study in breast neurotization REPOSESM: Enrollment Complete • Prospective, randomized, controlled study of Axoguard Nerve Cap® vs neurectomy REPOSE-XLSM: Pilot Study Initiated • Pilot study evaluating the feasibility of large-diameter Axoguard Nerve Cap® for protecting and preserving terminated nerve ends after trauma or amputation. RETHINK PAINTM Registry Study: Enrollment Ongoing • Designed to capture the patient’s pain journey, from onset of chronic pain to nerve repair G ro w B o d y o f C li n ic a l E v id e n c e Peer Reviewed Clinical Papers* Extremity Trauma Breast Oral and Maxillofacial Pain Other Applications revolutionizing the science of nerve repair™ 116 13 35 49 30 207 *Certain publications contain data on multiple applications.
24 P IL L A R 4 Focused sales execution, increasing market penetration P IL L A R 3 E x e c u te S a le s P la n Sales execution focused on driving results • Continue driving penetration in Active and Core Accounts • 5,100 potential U.S. accounts perform nerve repair • 952 Active Accounts as of September 30, 2022 o Active Accounts represent approximately 85% of total revenue o Top 10% of Active Accounts represent approximately 35% of total revenue • 331 Core Accounts as of September 30, 2022 o Core Accounts represent approximately 60% of total revenue Expanded sales reach • U.S. direct sales team o 111 direct sales professionals at end of Q3 2022 • Supplemented by independent agencies • Revenue from direct sales channel represented approximately 90% of total revenue revolutionizing the science of nerve repair™
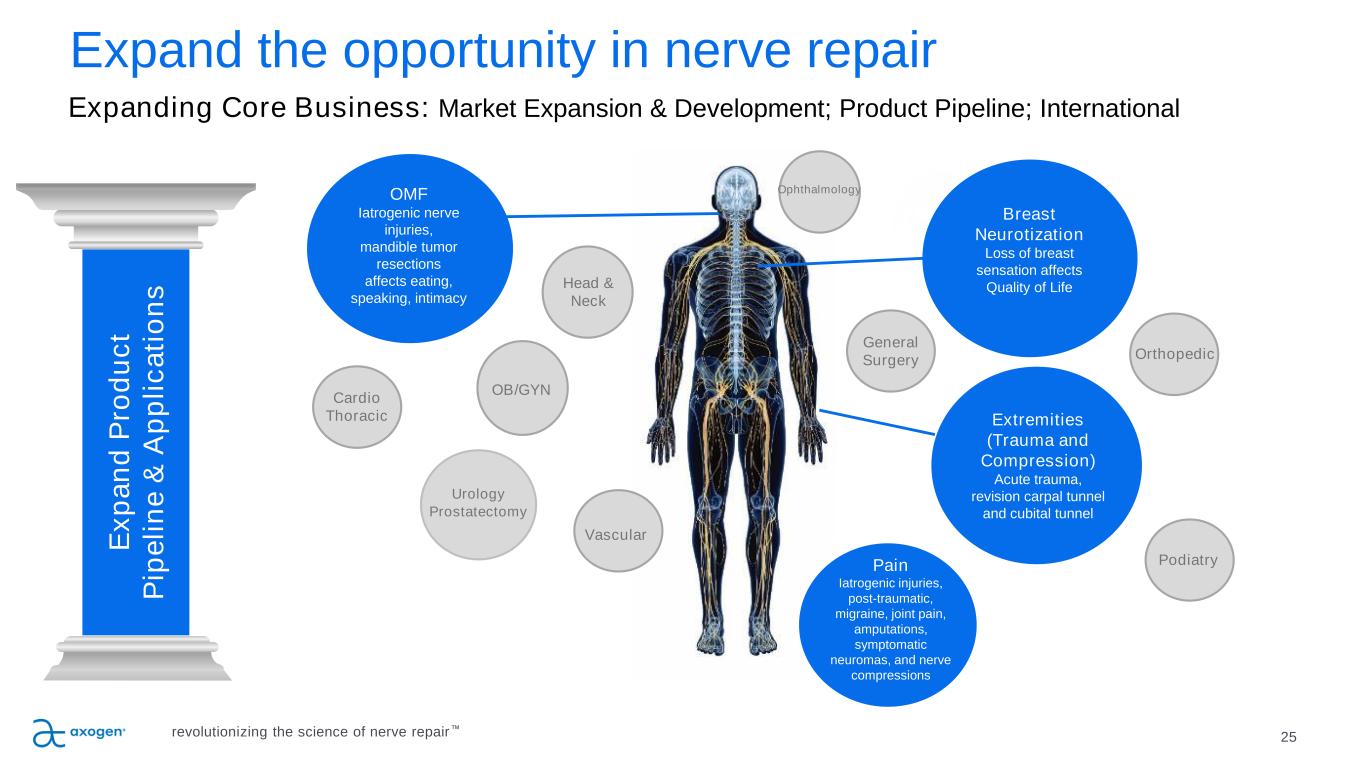
25 P IL L A R 5 Expand the opportunity in nerve repair P IL L A R 4 P IL L A R 3 E x p a n d P ro d u c t P ip e li n e & A p p li c a ti o n s Breast Neurotization Loss of breast sensation affects Quality of Life Urology Prostatectomy OMF Iatrogenic nerve injuries, mandible tumor resections affects eating, speaking, intimacy Extremities (Trauma and Compression) Acute trauma, revision carpal tunnel and cubital tunnel Head & Neck OB/GYN General Surgery Cardio Thoracic Orthopedic Podiatry Vascular Pain Iatrogenic injuries, post-traumatic, migraine, joint pain, amputations, symptomatic neuromas, and nerve compressions Ophthalmology Expanding Core Business: Market Expansion & Development; Product Pipeline; International revolutionizing the science of nerve repair™
26 Balance Sheet Highlights September 30, 2022 Cash, Cash Equivalents, and Investments $59.4 million Total Long-term Debt $50.0 million* Capital Structure (shares) September 30, 2022 Common Stock 42,272,223 Common Stock Options, RSUs, PSUs 6,998,426 Common Stock and Common Stock Equivalents 49,270,649 Balance sheet and capital structure * Total long-term debt includes debt proceeds under the terms of the agreement with Oberland Capital, inclusive of unamortized debt discount and deferred financing fees. revolutionizing the science of nerve repair™
Committed to our patients, the communities we serve, and our pursuit of advancing the science of nerve repair in ethical and sustainable ways Diversity, Equity, and Inclusion - Being the Company where exceptional people want to work Cybersecurity – Data Privacy, Training, and Policies Compliance - Quality Management System, Regulatory, and Good Manufacturing Practices Governance - Framework for Ethics Codes and Accountability Environment – Responsible, Sustainable Operations People Sustainability Business

Executive team 28 Erick DeVinney VP, Peripheral Nerve Science and Clinical Innovation Angiotech, PRA Intl Mike Donovan VP, Operations Zimmer Ivica Ducic, M.D., Ph.D. Medical Director Washington Nerve Institute Angelo Scopelianos, Ph.D. Chief Research & Development Officer J&J Peter J. Mariani Executive Vice President and Chief Financial Officer Guidant, Lensar, Hansen Isabelle Billet Chief Strategy & Business Development Officer J&J, C.R. Bard, Cardinal Karen Zaderej Chairman, CEO, & President J&J (Ethicon) Maria Martinez Chief Human Resources Officer HSNi, Bausch + Lomb revolutionizing the science of nerve repair™ Brad Ottinger General Counsel, Chief Compliance Officer MicroPort Orthopedics Doris Quackenbush Vice President of Sales Convatec

Axogen is the preeminent nerve repair company with a foundation for long-term sustainable growth 29 • Exclusively focused on peripheral nerve repair with a differentiated platform • 10+ years of demonstrated clinical outcome consistency • Featured in 207 peer-reviewed clinical publications • Over 50,000 Avance® Nerve Grafts implanted • Significant barriers to competitive entry • FDA granted Avance Regenerative Medicine Advanced Therapy (RMAT) designation • Commercial and surgeon education capabilities • Solid balance sheet provides resources to execute business plan • Experienced management team with strong track record of success revolutionizing the science of nerve repair™

Appendix • Key Clinical Data • Historical Core and Active Accounts • CMS outpatient and ASC reimbursement rates • Total Addressable Market • Axogen product portfolio and indications for use 30revolutionizing the science of nerve repair™

Avance Nerve Graft repairs found to be significantly better than conduit repairs 31 • Peer-reviewed publication from the MATCH cohort of the RANGER Registry • Includes outcomes from 110 subjects with 162 nerve injuries; 113 were repaired with Avance Nerve Graft and 49 were repaired with manufactured conduit • Findings show overall meaningful recovery rate was 88% for Avance Nerve Graft and 61% for conduit (p=0.001) for gaps up to 25mm • Average static two-point discrimination improved to 9.7mm for Avance Nerve Graft as compared to 12.2mm for conduit (p=0.018) • Note: lower measurement is reflective of improved discrimination and a better outcome • As gap length increased, Avance Nerve Graft outcome rates remained consistent while conduit rates declined significantly “Leversedge et al., A Multicenter Matched Cohort Study of Processed Nerve Allograft and Conduit in Digital Nerve Reconstruction” – Journal of Hand Surgery, September 202048 0% 20% 40% 60% 80% 100% ⱡMeaningful Recovery Rate by Gap Length Conduit Allograft <15mm 15-25mm ⱡMeaningful Recovery = ≥S3 on the MRCC Scale *p=0.008, **p=0.001 67% 92% 45% 85% * ** revolutionizing the science of nerve repair™

Study finds Avance Nerve Graft (allograft) clinical outcomes recovery rates comparable to nerve autograft 32 “Safa et al., A Propensity Matched Cohort Study on Outcomes from Processed Nerve Allograft and Nerve Autograft in Upper Extremity Nerve Repairs”49 Presented at American Society for Surgery of the Hand (ASSH), Oct 2020 • Study of 156 nerve repairs found meaningful recovery rates for Avance Nerve Graft were comparable to autograft for both sensory and motor function Defined as MRCC Score ≥ S3/M3 Historical data on Nerve Autograft50,51,52,53,54,55, Mixed Nerve: 57-80%; Digital Nerve: 60-88% 68% 80% 70% 78% 72% 73% 0% 20% 40% 60% 80% 100% Sensory Mixed Sensory Mixed Motor ⱡMeaningful Recovery Outcomes by Nerve Function Autograft Allograft revolutionizing the science of nerve repair™
Studies find Avance Nerve Graft performed comparably to nerve autograft for both clinical outcomes and facility procedure costs 33 “Styron et al., Nerve Repair Hospital Index Procedure Costs – Allograft vs. Autograft Repair Type” Presented at the American Society for Surgery of the Hand (ASSH), October 202056 • Data from the 2018 Medicare Standard Analytic File57 • 340 claims reviewed for autograft and allograft, included inpatient and outpatient procedures • Found hospital facility procedure cost for Avance Nerve Graft was comparable to that of traditional nerve autograft • Did not evaluate the potential additional costs associated with managing the autograft donor site and subsequent morbidities “Styron et al., Comparative Effectiveness Evaluating Allograft, Autograft and Conduit Nerve Repairs: A Systematic Review” Presented at the American Association for Hand Surgery (AAHS), January 202158 • Systematic review of recovery outcomes from over 35 clinical studies and 1,500 nerve repairs with autograft, allograft and conduit repairs • Evaluated short and long gaps for both sensory and motor outcomes • Autograft and allograft outcome rates were found to be statistically better than conduit repairs* • Autograft and allograft outcome rates were found to be similar, regardless of gap length or nerve function • Cost comparison conducted with Medicare data on Hospital Index Procedure Costs for autograft and allograft were found to be similar *Conduits only had available data for short gap sensory nerve group revolutionizing the science of nerve repair™
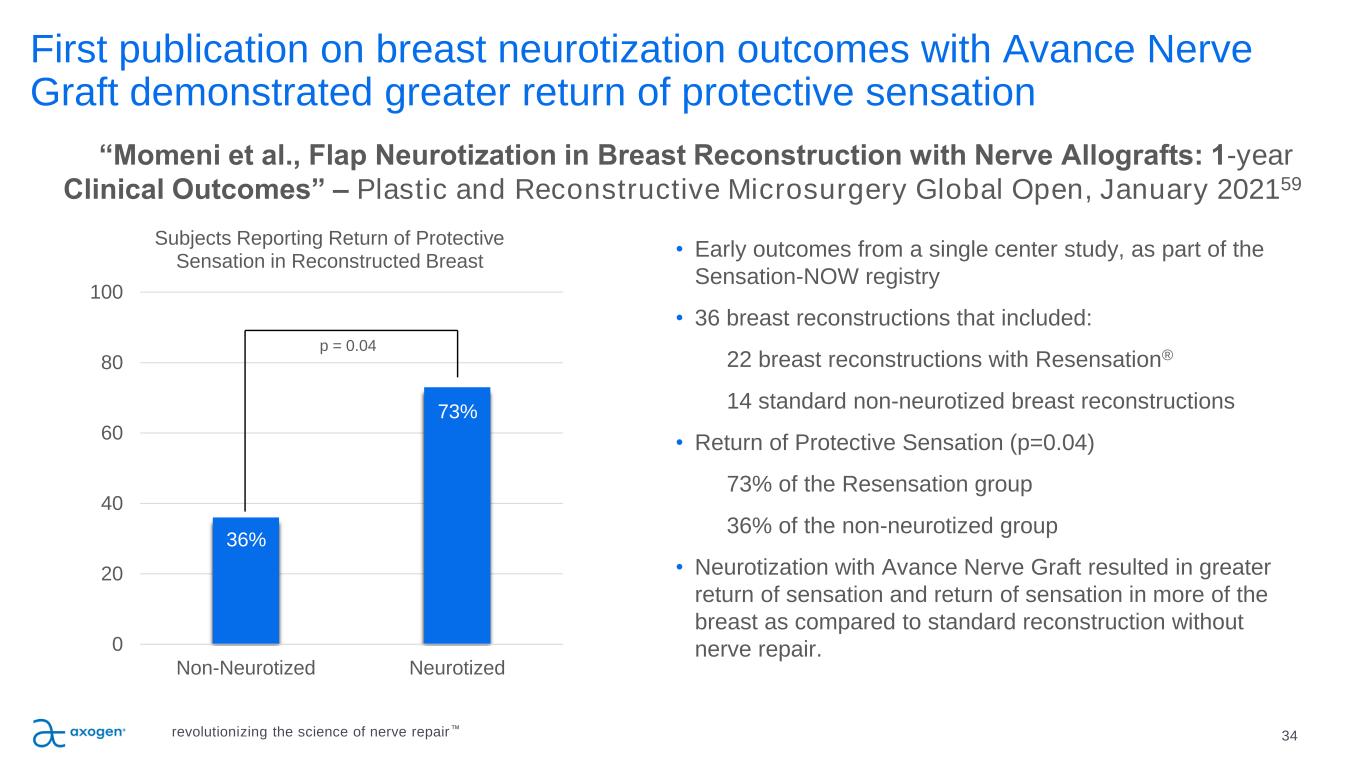
First publication on breast neurotization outcomes with Avance Nerve Graft demonstrated greater return of protective sensation 34 • Early outcomes from a single center study, as part of the Sensation-NOW registry • 36 breast reconstructions that included: 22 breast reconstructions with Resensation® 14 standard non-neurotized breast reconstructions • Return of Protective Sensation (p=0.04) 73% of the Resensation group 36% of the non-neurotized group • Neurotization with Avance Nerve Graft resulted in greater return of sensation and return of sensation in more of the breast as compared to standard reconstruction without nerve repair. “Momeni et al., Flap Neurotization in Breast Reconstruction with Nerve Allografts: 1-year Clinical Outcomes” – Plastic and Reconstructive Microsurgery Global Open, January 202159 36% 73% 0 20 40 60 80 100 Non-Neurotized Neurotized Subjects Reporting Return of Protective Sensation in Reconstructed Breast p = 0.04 revolutionizing the science of nerve repair™
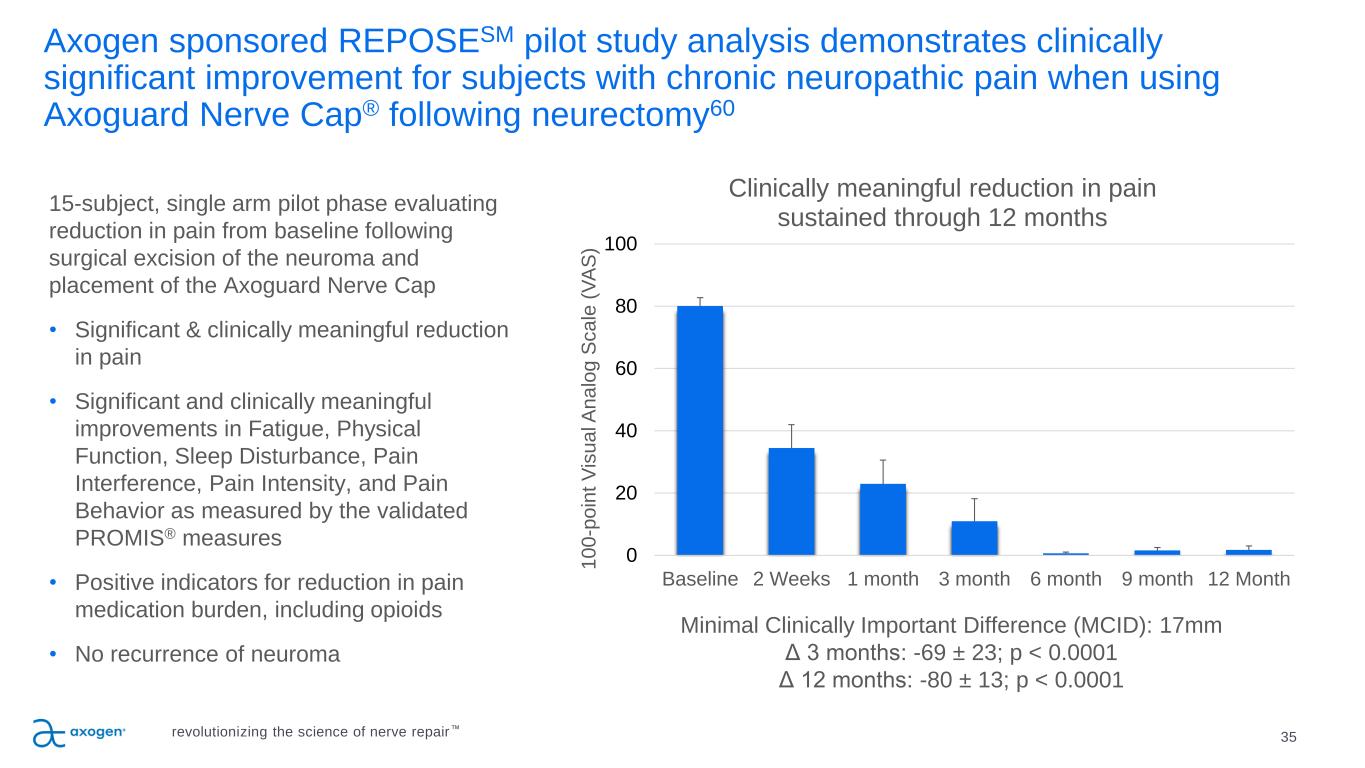
Axogen sponsored REPOSESM pilot study analysis demonstrates clinically significant improvement for subjects with chronic neuropathic pain when using Axoguard Nerve Cap® following neurectomy60 35 15-subject, single arm pilot phase evaluating reduction in pain from baseline following surgical excision of the neuroma and placement of the Axoguard Nerve Cap • Significant & clinically meaningful reduction in pain • Significant and clinically meaningful improvements in Fatigue, Physical Function, Sleep Disturbance, Pain Interference, Pain Intensity, and Pain Behavior as measured by the validated PROMIS® measures • Positive indicators for reduction in pain medication burden, including opioids • No recurrence of neuroma 0 20 40 60 80 100 Baseline 2 Weeks 1 month 3 month 6 month 9 month 12 Month 1 0 0 -p o in t V is u a l A n a lo g S c a le ( V A S ) Clinically meaningful reduction in pain sustained through 12 months Minimal Clinically Important Difference (MCID): 17mm Δ 3 months: -69 ± 23; p < 0.0001 Δ 12 months: -80 ± 13; p < 0.0001 revolutionizing the science of nerve repair™
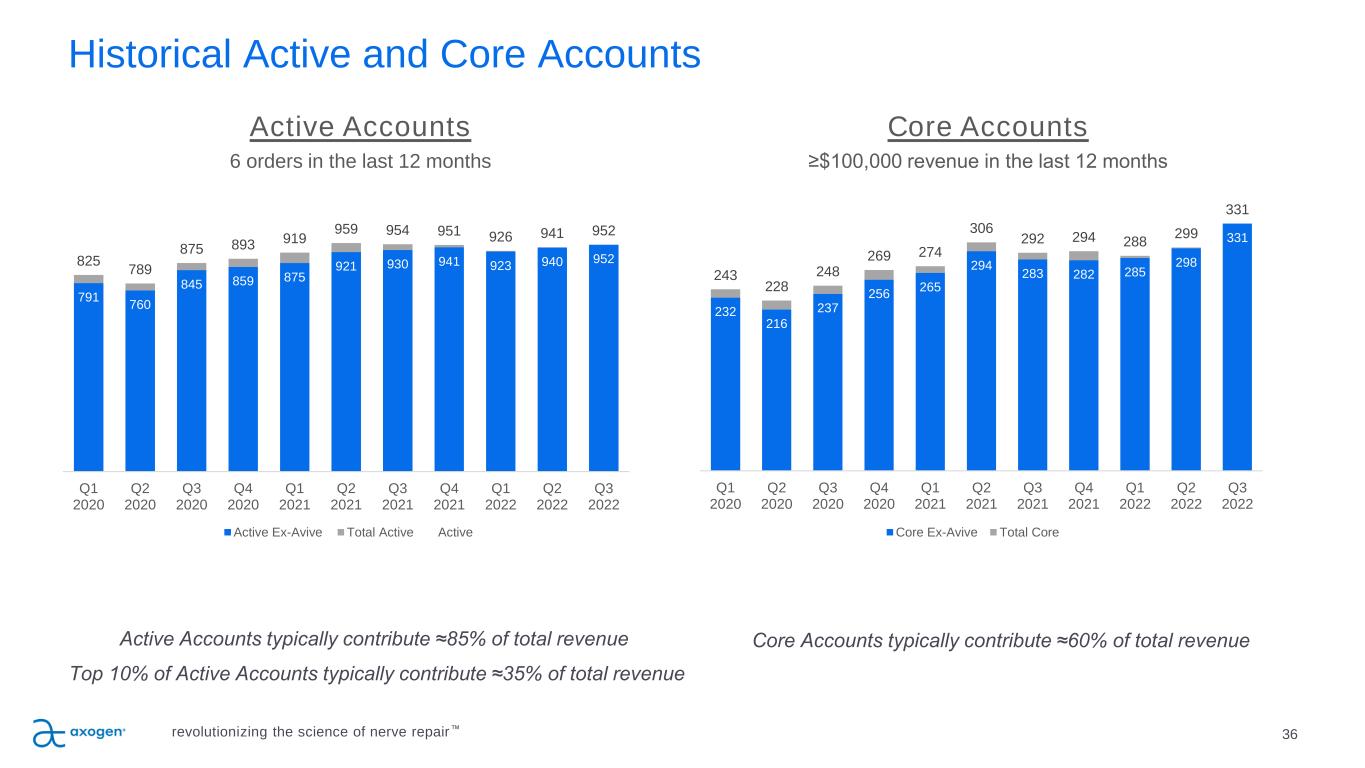
Historical Active and Core Accounts Active Accounts typically contribute ≈85% of total revenue Top 10% of Active Accounts typically contribute ≈35% of total revenue Active Accounts 6 orders in the last 12 months Core Accounts typically contribute ≈60% of total revenue Core Accounts ≥$100,000 revenue in the last 12 months 36revolutionizing the science of nerve repair™ 232 216 237 256 265 294 283 282 285 298 331 243 228 248 269 274 306 292 294 288 299 331 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Core Ex-Avive Total Core 791 760 845 859 875 921 930 941 923 940 952825 789 875 893 919 959 954 951 926 941 952 Q1 2020 Q2 2020 Q3 2020 Q4 2020 Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Active Ex-Avive Total Active Active

CPT Code Descriptor C-APC Hospital Outpatient (HOPD) Ambulatory Surgery Center (ASC) 2022 2023 % Change 2022 2023 % Change 64912 Nerve allograft repair2 5432 $ 5,824 $6,179 6.10% $3,868 $4,125 6.64% 64910 Conduit or vein allograft repair2 5432 $ 5,824 $6,179 6.10% $3,881 $3,905 0.62% 64886 Autograft repair (head and neck >4cm)2 5432 $ 5,824 $6,179 6.10% $4,245 $4,375 3.06% 64890 Autograft repair (hand and foot≤4cm) 6 5432 $ 5,824 $6,179 6.10% $3,249 $2,602 -19.91% 64891 Autograft repair (hand and foot>4cm) 2 5432 $ 5,824 $6,179 6.10% $3,249 $3,383 4.12% 64892 Autograft repair (arm and leg ≤4cm)2 5432 $5,824 $6,179 6.10% $3,718 $3,383 -9.01% 64893 Autograft repair (arm and leg >4cm)3 5432 $5,824 $6,179 6.10% $2,496 $3,383 35.54% 64897 Autograft repair (arm and leg ≤4cm multiple strands)3 5432 $5,824 $6,179 6.10% $2,496 $3,660 46.63% 64885 and 64895-96,98 Autograft repair (all other nerve type) 5 5432 $5,824 $6,179 6.10% $2,496 $2,632 5.45% 64834-36, 40, 56, 57, 62-64 Direct Repair (other hand/foot, arm/leg, repair/transpose, facial, low back,)5 5432 $5,824 $6,179 6.10% $2,496 $2,632 5.45% 64865 Direct Repair of facial nerve7 5432 $5,824 $6,179 6.10% $2,496 $3,383 35.54% 64831, 61 Direct Repair (digital, brachial plexus/arm) 4 5431 $1,793 $ 1,798 0.28% $825 $854 3.52% 64858 Direct Repair (sciatic)2 5431 $1,793 $ 1,798 0.28% $1,465 $1,481 1.09% 2023 CMS Final outpatient reimbursement rates - hospital and ASC 37 Although CMS rates1 only apply to Medicare cases, which represents a small percentage of traumatic injuries, private payors are often influenced by the analysis and decisions made by CMS 1. National average payment rates. Commercial payments are traditionally 1.5-2x higher than Medicare. 2. Nerve allograft repair CPT 64912, conduit repair CPT 64910, autograft repairs hand/foot >4cm CPT 64891, arm/leg≤4cm CPT code 64892, and head/neck >4cm CPT 64886 remain in C-APC 5432 and direct repair sciatic CPT 64858 remains in C-APC 5431 and all continue to meet ASC device intensive criteria 3. Autograft repair arm and leg >4cm CPT 64893, Autograft repair arm/leg ≤4cm multiple strands CPT 64897, remains in C-APC 5432 and meets ASC device intensive criteria in 2023 4. Direct repair digital and brachial plexus/arm CPT codes 64831 and 64861 remain in C-APC 5431 and do not meet ASC device intensive criteria. 5. Autograft repair all other nerve type CPT 64885 and 64895-96,98 and Direct repair other hand/foot CPT 64834-36, leg CPT 64840, repair/transpose CPT 64856, arm/leg CPT 64857, low back CPT 64862-64 remain in C-APC 5432 and do not meet ASC device intensive criteria 6. Autograft repair hand/foot ≤4cm CPT 64890 remains in C-APC 5432 no longer meets ASC device intensive criteria in 2023 7. Direct repair of facial nerve CPT 64865 meets ASC device intensive criteria in 2023 Note: Hospital inpatient rates for nerve repair align to DRGs 040, 041, 042 and range from $11.5k to $23.7k in the 2023 IPPS Proposed Rule
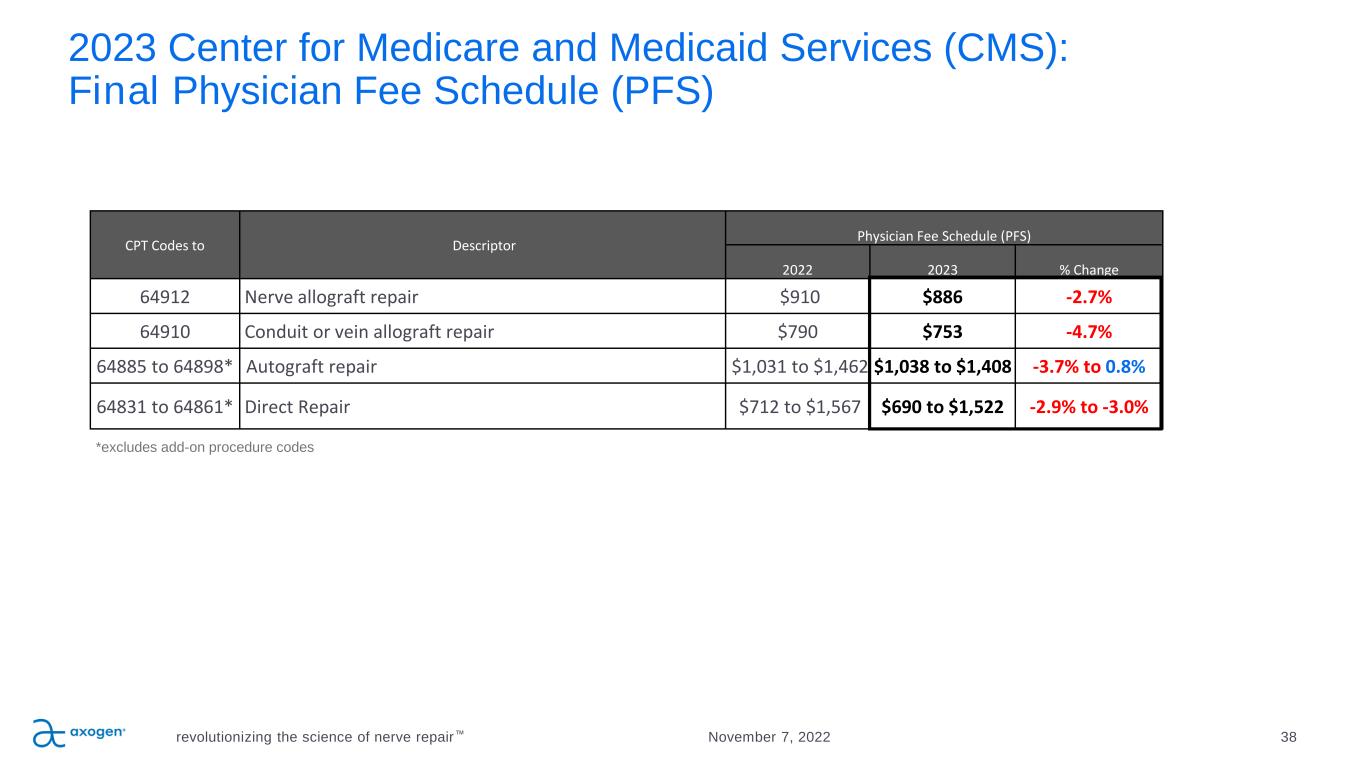
2023 Center for Medicare and Medicaid Services (CMS): Final Physician Fee Schedule (PFS) revolutionizing the science of nerve repair™ 38November 7, 2022 CPT Codes to Descriptor Physician Fee Schedule (PFS) 2022 2023 % Change 64912 Nerve allograft repair $910 $886 -2.7% 64910 Conduit or vein allograft repair $790 $753 -4.7% 64885 to 64898* Autograft repair $1,031 to $1,462 $1,038 to $1,408 -3.7% to 0.8% 64831 to 64861* Direct Repair $712 to $1,567 $690 to $1,522 -2.9% to -3.0% *excludes add-on procedure codes

Projected Incidence(a) Weighted Average Procedure Value Estimated Total Addressable Market Trauma Transection injuries >5mm (b) Other trauma injuries (c) 700,000 100% 203,000 29% 497,000 71% $2,715 $5,515 $1,570 $1,900M 100% $1,120M 59% $780M 41% Carpal and Cubital Tunnel 130,000 $2,100 $270M Oral and Maxillo-Facial (OMF) 56,000 $5,400 $300M Breast Reconstruction Neurotization 24,500 f laps (15,000 pat ients) $10,200 $250M Totals >900,000 (potent ia l ) >$2.7B a) Estimated Annual incidence of PNI surgery are figures rounded to the nearest thousandth except for Breast Reconstruction Neurotization (rounded to nearest hundredth). b) Transection injuries > 5mm assumes a factor of 1.22 nerve repairs per procedures, and utilization of the Axogen portfolio of products, based upon data observed in the RANGER® registry c) Other trauma injuries include transections < 5mm and crush injuries utilizing the Axoguard product line based upon literature and data observed in the RANGER® registry 39 Estimated $2.7B value of market opportunity in existing applications revolutionizing the science of nerve repair™
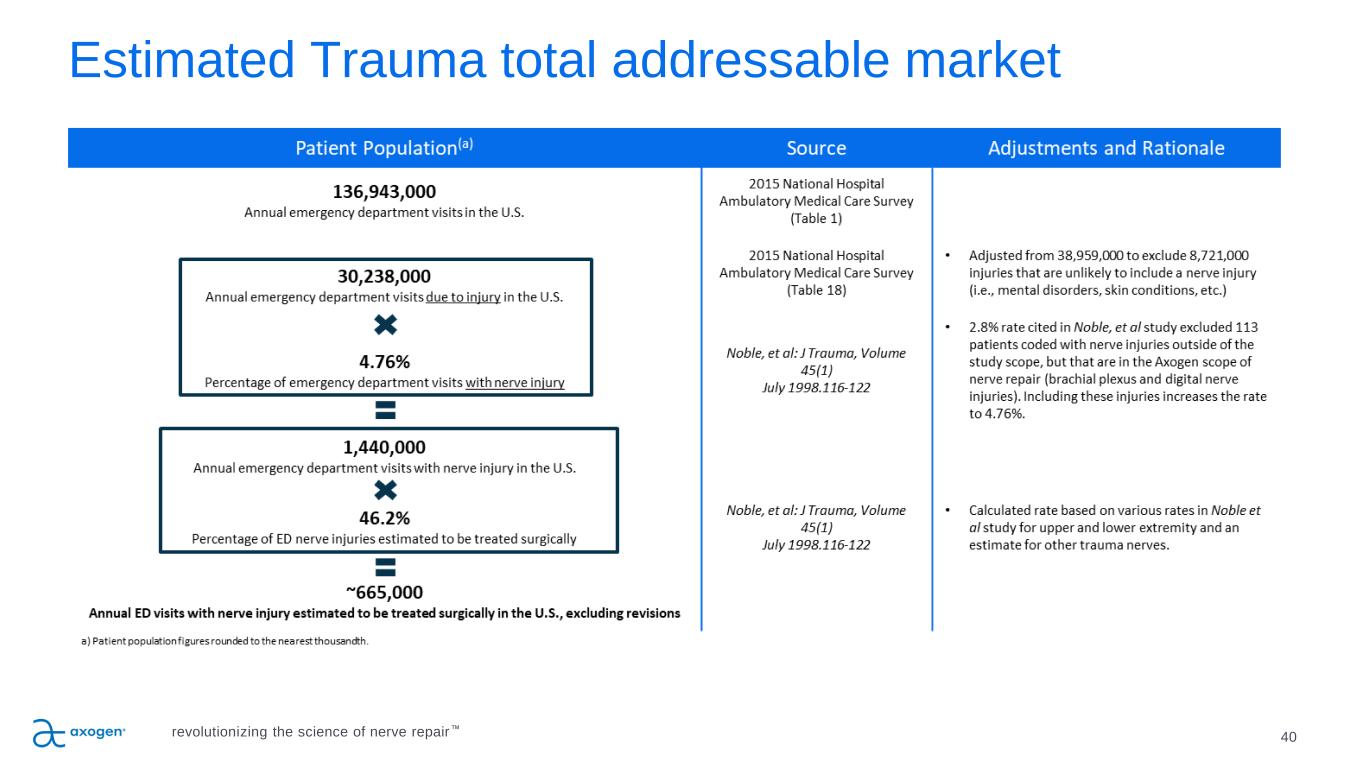
40 Estimated Trauma total addressable market revolutionizing the science of nerve repair™
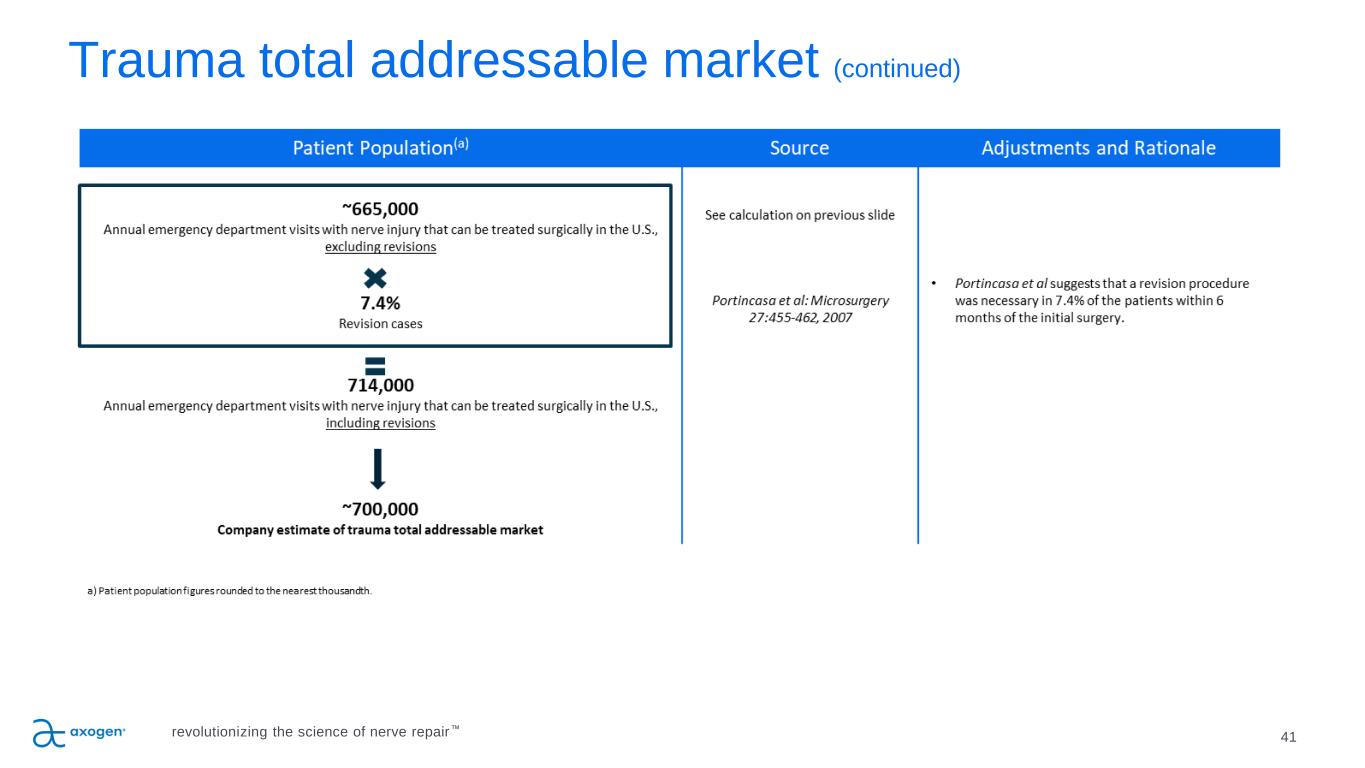
Trauma total addressable market (continued) 41revolutionizing the science of nerve repair™

Axogen comprehensive portfolio of products Avance® Nerve Graft • Regulatory Classification: Avance Nerve Graft is processed and distributed in accordance with U.S. Food and Drug Administration (FDA) requirements for Human Cellular and Tissue-based Products (HCT/P) under 21 CFR Part 1271 regulations, U.S. State regulations and the guidelines of the American Association of Tissue Banks (AATB). Additionally, international regulations are followed as appropriate. • Indication for Use: Avance Nerve Graft is processed nerve allograft (human) intended for the surgical repair of peripheral nerve discontinuities to support regeneration across the defect. • Contraindications: Avance Nerve Graft is contraindicated for use in any patient in whom soft tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing or evidence of a current infection. Axoguard Nerve Connector ® • Regulatory Classifications: Class II Medical Devices - 510(k) cleared, CE Marked • Indications for Use (EU and UK): The Axoguard Nerve Connector is indicated for the repair of peripheral nerve discontinuities with gaps up to 5 mm. The Axoguard Nerve Connector is supplied sterile and is intended for single use. • Indications for Use (ROW): Axoguard Nerve Connector is intended for the repair of peripheral nerve discontinuities where gap closure can be achieved by flexion of the extremity. The Axoguard Nerve Connector is supplied sterile and is intended for single use. • Contraindications: This device is derived from a porcine source and should not be used for patients with known sensitivity to porcine material. Axoguard Nerve Protector ® • Regulatory Classifications: Class II Medical Devices - 510(k) cleared, CE Marked • Indication for Use: Axoguard Nerve Protector is indicated for the repair of peripheral nerve injuries in which there is no gap. The Axoguard Nerve Connector is supplied sterile and is intended for single use. • Contraindications: This device is derived from a porcine source and should not be used for patients with known sensitivity to porcine material. Axoguard Nerve Cap® • Regulatory Classification: Class II Medical Device – 510(k) cleared • Indications for Use: Axoguard Nerve Cap is indicated to protect a peripheral nerve end and to separate the nerve from the surrounding environment to reduce the development of symptomatic or painful neuroma. • Contraindications: Axoguard Nerve Cap is derived from a porcine source and should not be used for patients with known sensitivity to porcine derived materials. Axoguard Nerve Cap is contraindicated for use in any patient for whom soft tissue implants are contraindicated; this includes any pathology that would limit the blood supply and compromise healing, or evidence of a current infection. Axoguard Nerve Cap should not be implanted directly under the skin. Note: This device is not intended for use in vascular applications. 42revolutionizing the science of nerve repair™

nasdaq: axgn 43revolutionizing the science of nerve repair™

44 Footnotes Trauma Market Data: 1. National Hospital Ambulatory Medical Care Survey: 2015 Emergency Department Summary Tables – Table 18. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf 2. Noble, et al.. Analysis of Upper and Lower Extremity Peripheral Nerve Injuries in a Population of Patients with Multiple Injuries. J Trauma. 1998; 45(1): 116-122. 3. Uzun, et al., Traumatic peripheral nerve injuries: demographic and electrophysiologic findings of 802 patients from a developing country. J Clin Neuromusc Dis. 2006; 7(3): 97–103. 4. Portincasa, et al. Microsurgical treatment of injury to peripheral nerves in upper and lower limbs: a critical review of the last 8 years. Microsurgery. 2007; 27(5): 455–462. Carpal Tunnel Revisions & Cubital Tunnel Market Data 5. Medicare National HCPS Aggregate Summary Table CY2016. https://data.cms.gov/Medicare-Physician-Supplier/Medicare-National-HCPCS-Aggregate-Summary-Table-CY/jtra-d83c/data 6. Sotereanos, et al. Vein wrapping for the treatment of recurrent carpal tunnel syndrome. Tech Hand Up Extrem Surg.1997; 1(1):35-40. 7. Seradge, et al. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998; 23(3): 483-491. 8. Papatheodorou, et al. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J Hand Surg Am. 2015; 40(5): 987-992 OMF Market Data 9. Lin, et al. Systematic Review and Meta-Analysis on Incidence of Altered Sensation of Mandibular Implant Surgery - PLoS One. 2016; 11(4): e0154082. 10. Hussaini. Procedure frequency in the jaws related to implant location. Dent Oral Craniofac Res. 2016; 2(2): 230-233. 11. Nguyen, et al. Risk factors for permanent injury of inferior alveolar and lingual nerves during third molar surgery. J Oral Maxillofac Surg. 2014; 72(12): 2394-2401. 12. Cheung, et al. Incidence of neurosensory deficits and recovery after lower third molar surgery: a prospective clinical study of 4338 cases. Int J Oral Maxillofac Surg. 2010; 39(4): 320–326. 13. Dental Implants Market (Product - Endosteal Implants, Subperiosteal Implants, Transosteal Implants, Intramucosal Implants; Material - Titanium Implants, Zirconium Implants; End User - Hospitals, Dental Clinics, and Academic & Research Institutes) - Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2017 – 2025. https://www.transparencymarketresearch.com/dental-implants-market.html 14. Cha, et al. Frequency of bone graft in implant surgery. Maxillofac Plast and Reconstr Surg. 2016; 38(1): 19. 15. Miloro, M (ed). Trigeminal Nerve Injuries. Springer; 2013. 16. Pogrel et al. Permanent nerve involvement resulting: From inferior alveolar nerve blocks. J Am Dent Assoc. 2000; 131(7): 901-907. 17. Agbaje, et al. Systematic review of the incidence of inferior alveolar nerve injury in bilateral sagittal split osteotomy and the assessment of neurosensory disturbances. Int. J Oral Maxillofac. Surg. 2015; 44(4): 447-451. Breast Neurotization Market Data, and Other Clinical References 18. ASPS 2017– Plastic Surgery Statistics Report. www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf 19. Rappaport, et al. Clinical utilization and complications of sural nerve biopsy. Am J Surg. 1993; 166(3): 252-256. 20. Weber, et al. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000; 106(5): 1036-1045. 21. Wangensteen, et al. Collagen tube conduits in peripheral nerve repair: A retrospective analysis. Hand. 2010; 5(3): 273-277. 22. Data on file at Axogen 23. Karabekmez, et al. Early clinical outcomes with the use of decellularized nerve allograft for repair of sensory defects within the hand. Hand. 2009; 4(3): 245-249. 24. Boeckstyns, et al. Collagen conduit versus microsurgical neurorrhaphy: 2-year follow-up of a prospective, blinded clinical and electrophysiological multicenter randomized, controlled trial. J hand Surg Am. 2013; 38(12): 2405-2411. 25. Brushart, et al. Selective reinnervation of distal motor stumps by peripheral motor axons. Exp Neurol. 1987; 97(2): 289-300. 26. Schmidhammer, et al. Alleviated tension at the repair site enhances functional regeneration: The effect of full range of motion mobilization on the regeneration of peripheral nerves--histologic, electrophysiologic, and functional results in a rat model. J Trauma. 2004; 56(3): 571-584 27. Tang, et al. The optimal number and location of sutures in conduit-assisted primary digital nerve repair. J Hand Surg Eur Vol. 2018; 43(6): 621-625. 28. Data on file at Axogen 29. Badylak, et al. Small intestinal submucosa: A substrate for in vitro cell growth. J Biomater Sci Polym Ed. 1998; 9(8): 863-878. 30. Hodde, et al. Effects of sterilization on an extracellular matrix scaffold: Part II. Bioactivity and matrix interaction. J Mater Sci Mater Med. 2007; 18(4): 545-550. 31. Nihsen, et al. Bioactivity of small intestinal submucosa and oxidized regenerated cellulose/collagen. Adv Skin Wound Care. 2008; 21(10): 479-486. 32. Zhukauskas et al., Comparative Study of Porcine Small Intestine Submucosa and Cross-Linked Bovine Type I Collagen as a Nerve Conduit. JHS GO 3(5), 282-288 Sep 2021 33. Hodde, et al. Vascular endothelial growth factor in porcine-derived extracellular matrix. Endothelium. 2001; 8(1): 11-24. 34. Data on file at Axogen 35. Kokkalis, et al. Assessment of processed porcine extracellular matrix as a protective barrier in a rabbit nerve wrap model. J Recon MicroSurg. 2011; 27(1): 19-28. 36. Pet MA, Ko JH, Friedly JL, Smith DG. Traction Neurectomy for Treatment of Painful Residual Limb Neuroma in Lower Extremity Amputees J Orthop Trauma. 29 (9), e321-5 Sep 2015. 37. Laborde K, et al. Results of surgical treatment of painful neuromas of the hand. The Journal of Hand Surgery. March 1981;7(2):190-193. revolutionizing the science of nerve repair™

45 Footnotes 38. Galeano M, et al. A free vein graft cap influences neuroma formation after nerve transection. Microsurgery. 2009;29(7):568-572. 39. Stokvis A. Surgical management of painful neuromas. Rotterdam, The Netherlands: Optima Grafische Communicatie; 2010. 40. Lin E, et al. Local administration of norephinephrine in the stump evokes dose-dependent pain in amputees. Clin J Pain. 2006;22(5):482-486. 41. O’Reilly MA, et al. Neuromas as the cause of pain in the residual limbs of amputees. An ultrasound study. Clin Radiology. May 1-6, 2016. 42. Rajput K, et al. Painful neuromas. The Clinical Journal of Pain. 2012;28(7):639-645 43. Gruber H, et al. Practical experience with sonographically guided phenol instillation of stump neuroma: predictors of effects, success, and outcome. Am J Roentgenol. 2008;190(5):1263-1269. 44. Fallat L. Cryosurgery or sclerosing injections: which is better for neuromas. Podiatry Today. 2004;17(6):58-66. 45. Bradley MD. Plantar neuroma: analysis of results following surgical excision in 145 patients. South Med J. 1976;69:853-845. 46. Kehoe S, et al. FDA-approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury. 2012;43:553-572. 47. Record RD, Hillegonds D, Simmons C, Tullius R, Rickey FA, Elmore D, Badylak SF. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials. 2001 Oct;22(19):2653-9. 48. Leversedge FJ, Zoldos J, Nydick J, Kao DS, Thayer W, MacKay B, McKee D, Hoyen H, Safa B, Buncke GM. A Multicenter Matched Cohort Study of Processed Nerve Allograft and Conduit in Digital Nerve Reconstruction. J Hand Surg Am. 2020 Dec;45(12):1148-1156. 49. Safa B, Power D, Liu A, Thayer WP, et al. A Propensity Matched Cohort Study on Outcomes from Processed Nerve Allograft and Nerve Autograft in Upper Extremity Nerve Repairs. In: The 75th Annual Meeting of the ASSH. Virtual Annual Meeting, October 1-2, 2020. 50. Safa B, Jain S, Desai MJ, Greenberg JA, Niacaris TR, Nydick JA, Leversedge FJ, Megee DM, Zoldos J, Rinker BD, McKee DM, MacKay BJ, Ingari JV, Nesti LJ, Cho M, Valerio IL, Kao DS, El-Sheikh Y, Weber RV, Shores JT, Styron JF, Thayer WP, Przylecki WH, Hoyen HA, Buncke GM. Peripheral nerve repair throughout the body with processed nerve allografts: Results from a large multicenter study. Microsurgery. 2020 Jul;40(5):527-537. 51. Sallam AA, El-Deeb MS, Imam MA. Nerve Transfer Versus Nerve Graft for Reconstruction of High Ulnar Nerve Injuries. J Hand Surg Am. 2017 Apr;42(4):265-273 52. Roganovic Z, Pavlicevic G. Difference in recovery potential of peripheral nerves after graft repairs. Neurosurgery. 2006 Sep;59(3):621-33; discussion 621-33. 53. Frykman G, Gramyk K. Results of nerve grafting. In: Gelberman R, ed. Operative nerve repair and reconstruction. Philadelphia: JB Lippincott, 1991:553–567 54. Vastamäki M, Kallio PK, Solonen KA. The results of secondary microsurgical repair of ulnar nerve injury. J Hand Surg Br. 1993 Jun;18(3):323-6. 55. Kallio PK, Vastamäki M, Solonen KA. The results of secondary microsurgical repair of radial nerve in 33 patients. J Hand Surg Br. 1993 Jun;18(3):320-2. 56. Styron JF, Thompson AK, Park LI, Watson GJ. Nerve Repair Hospital Index Procedure Costs – Allograft vs. Autograft Repair Type. In: The 75th Annual Meeting of the ASSH. Virtual Annual Meeting, October 1-2, 2020. 57. U.S. Centers for Medicare and Medicaid Services, Medicare Claims standard analytic file. 2018. 58. Styron JF, Lans-Valera J. Comparative Effectiveness Evaluating Allograft, Autograft and Conduit Nerve Repairs: A Systematic Review. American Association for Hand Surgery. Virtual Annual Meeting, January 2021 59. Momeni A, Meyer S, Shefren K, Januszyk M. Flap Neurotization in Breast Reconstruction with Nerve Allografts: 1-year Clinical Outcomes. Plast Reconstr Surg Glob Open. 2021 Jan 12;9(1):e3328 60. to Pereira R, Dauphinee D, Frania S, Garrett A, Martin C, Van Gils C, Thomajan C. Clinical evaluation of an innovative nerve termination cap for treatment and prevention of stump neuroma pain: Results from a prospective pilot clinical study. Fastrac. 2022; 2(2): 100179. © 2022 Axogen Corporation. The stylized “a” logo and Avance Nerve Graft are trademarks of Axogen Corporation. Axoguard is a registered trademark of Axogen Corporation. Axoguard Nerve Connector and Axoguard Nerve Protector are manufactured in the United States by Cook Biotech Incorporated, West Lafayette, Indiana, and are distributed exclusively by Axogen Corporation. LB-0588 revolutionizing the science of nerve repair™













































