0000721371--06-302024Q3falsehttp://fasb.org/us-gaap/2024#GainLossOnSalesOfAssetsAndAssetImpairmentCharges00007213712023-07-012024-03-3100007213712024-04-26xbrli:shares00007213712024-01-012024-03-31iso4217:USD00007213712023-01-012023-03-3100007213712022-07-012023-03-31iso4217:USDxbrli:shares00007213712024-03-3100007213712023-06-300000721371us-gaap:CommonStockMember2023-12-310000721371us-gaap:RetainedEarningsMember2023-12-310000721371us-gaap:TreasuryStockCommonMember2023-12-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310000721371us-gaap:NoncontrollingInterestMember2023-12-3100007213712023-12-310000721371us-gaap:NoncontrollingInterestMember2024-01-012024-03-310000721371us-gaap:CommonStockMember2024-01-012024-03-310000721371us-gaap:TreasuryStockCommonMember2024-01-012024-03-310000721371us-gaap:RetainedEarningsMember2024-01-012024-03-310000721371us-gaap:CommonStockMember2024-03-310000721371us-gaap:RetainedEarningsMember2024-03-310000721371us-gaap:TreasuryStockCommonMember2024-03-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-03-310000721371us-gaap:NoncontrollingInterestMember2024-03-310000721371us-gaap:CommonStockMember2022-12-310000721371us-gaap:RetainedEarningsMember2022-12-310000721371us-gaap:TreasuryStockCommonMember2022-12-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310000721371us-gaap:NoncontrollingInterestMember2022-12-3100007213712022-12-310000721371us-gaap:NoncontrollingInterestMember2023-01-012023-03-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-03-310000721371us-gaap:CommonStockMember2023-01-012023-03-310000721371us-gaap:TreasuryStockCommonMember2023-01-012023-03-310000721371us-gaap:RetainedEarningsMember2023-01-012023-03-310000721371us-gaap:CommonStockMember2023-03-310000721371us-gaap:RetainedEarningsMember2023-03-310000721371us-gaap:TreasuryStockCommonMember2023-03-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-03-310000721371us-gaap:NoncontrollingInterestMember2023-03-3100007213712023-03-310000721371us-gaap:CommonStockMember2023-06-300000721371us-gaap:RetainedEarningsMember2023-06-300000721371us-gaap:TreasuryStockCommonMember2023-06-300000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-06-300000721371us-gaap:NoncontrollingInterestMember2023-06-300000721371us-gaap:NoncontrollingInterestMember2023-07-012024-03-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-07-012024-03-310000721371us-gaap:CommonStockMember2023-07-012024-03-310000721371us-gaap:TreasuryStockCommonMember2023-07-012024-03-310000721371us-gaap:RetainedEarningsMember2023-07-012024-03-310000721371us-gaap:CommonStockMember2022-06-300000721371us-gaap:RetainedEarningsMember2022-06-300000721371us-gaap:TreasuryStockCommonMember2022-06-300000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-06-300000721371us-gaap:NoncontrollingInterestMember2022-06-3000007213712022-06-300000721371us-gaap:NoncontrollingInterestMember2022-07-012023-03-310000721371us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-07-012023-03-310000721371us-gaap:CommonStockMember2022-07-012023-03-310000721371us-gaap:TreasuryStockCommonMember2022-07-012023-03-310000721371us-gaap:RetainedEarningsMember2022-07-012023-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:CustomerConcentrationRiskMemberus-gaap:SalesRevenueNetMembercah:OptumRxMember2022-07-012023-06-30xbrli:pure0000721371cah:SpecialtyNetworksMember2024-01-012024-03-310000721371cah:SpecialtyNetworksMemberus-gaap:CustomerRelationshipsMember2024-03-180000721371cah:SpecialtyNetworksMembercah:TrademarksAndPatentsMember2024-03-180000721371cah:SpecialtyNetworksMemberus-gaap:DevelopedTechnologyRightsMember2024-03-180000721371cah:SpecialtyNetworksMember2024-03-180000721371cah:TransactionDataSystemInvestmentMember2024-03-310000721371cah:OutcomesDivestitureMember2023-07-012024-03-310000721371us-gaap:FairValueInputsLevel3Membercah:TransactionDataSystemInvestmentMemberus-gaap:FairValueMeasurementsNonrecurringMember2023-07-100000721371us-gaap:EmployeeSeveranceMember2023-06-300000721371us-gaap:FacilityClosingMember2023-06-300000721371us-gaap:EmployeeSeveranceMember2023-07-012024-03-310000721371us-gaap:FacilityClosingMember2023-07-012024-03-310000721371us-gaap:EmployeeSeveranceMember2024-03-310000721371us-gaap:FacilityClosingMember2024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMember2023-06-300000721371cah:GMPDMember2023-06-300000721371us-gaap:AllOtherSegmentsMember2023-06-300000721371cah:PharmaceuticalAndSpecialtySolutionsMember2023-07-012024-03-310000721371cah:GMPDMember2023-07-012024-03-310000721371us-gaap:AllOtherSegmentsMember2023-07-012024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMember2024-03-310000721371cah:GMPDMember2024-03-310000721371us-gaap:AllOtherSegmentsMember2024-03-310000721371cah:OptiFreightLogisticsMember2024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMember2022-07-012023-06-300000721371us-gaap:AllOtherSegmentsMember2022-07-012023-06-300000721371cah:GMPDMember2024-01-010000721371cah:OptiFreightLogisticsMemberus-gaap:AllOtherSegmentsMember2024-03-310000721371cah:GMPDMember2024-01-012024-03-310000721371cah:GMPDMember2024-03-31utr:Rate0000721371cah:GMPDMember2023-07-012023-09-300000721371cah:GMPDMember2022-10-012022-12-310000721371cah:GMPDMember2022-07-012022-09-300000721371cah:GMPDMember2023-03-310000721371cah:IPRDTrademarksandOtherMember2024-03-310000721371us-gaap:CustomerRelationshipsMember2024-03-310000721371cah:TrademarksAndPatentsMember2024-03-310000721371us-gaap:DevelopedTechnologyRightsMember2024-03-310000721371cah:IPRDTrademarksandOtherMember2023-06-300000721371us-gaap:CustomerRelationshipsMember2023-06-300000721371cah:TrademarksAndPatentsMember2023-06-300000721371us-gaap:DevelopedTechnologyRightsMember2023-06-300000721371cah:A3.079Notesdue2024Member2024-03-310000721371cah:A3.079Notesdue2024Member2023-06-300000721371cah:A3.5NotesDue2024Member2024-03-310000721371cah:A3.5NotesDue2024Member2023-06-300000721371cah:A3.75NotesDue2025Member2024-03-310000721371cah:A3.75NotesDue2025Member2023-06-300000721371cah:A3.41Notesdue2027Member2024-03-310000721371cah:A3.41Notesdue2027Member2023-06-300000721371cah:A5.125NotesDue2029Member2024-03-310000721371cah:A5.125NotesDue2029Member2023-06-300000721371cah:A5.45NotesDue2034Member2024-03-310000721371cah:A5.45NotesDue2034Member2023-06-300000721371cah:A4.6Notesdue2043Member2024-03-310000721371cah:A4.6Notesdue2043Member2023-06-300000721371cah:A4.5Notesdue2044Member2024-03-310000721371cah:A4.5Notesdue2044Member2023-06-300000721371cah:A4.9Notesdue2045Member2024-03-310000721371cah:A4.9Notesdue2045Member2023-06-300000721371cah:A4.368Notesdue2047Member2024-03-310000721371cah:A4.368Notesdue2047Member2023-06-300000721371cah:A7.0Debenturesduefiscal2027Member2024-03-310000721371cah:A7.0Debenturesduefiscal2027Member2023-06-300000721371cah:A5.125NotesDue2029Member2023-07-012024-03-310000721371cah:A5.45NotesDue2034Member2023-07-012024-03-310000721371cah:RemainingCashFromDebtProceedsMember2024-03-310000721371us-gaap:CommercialPaperMember2024-03-310000721371us-gaap:RevolvingCreditFacilityMember2024-03-310000721371cah:ShortTermCreditFacilitiesMembercah:CommittedReceivablesSalesFacilityProgramMember2024-03-310000721371cah:CommittedReceivablesSalesFacilityProgramMember2023-07-012024-03-310000721371cah:ShortTermCreditFacilitiesMember2024-03-310000721371cah:CVSHealthMember2014-07-312014-07-310000721371cah:NewYorkOpioidStewardshipActMember2018-04-300000721371cah:NewYorkOpioidStewardshipActMember2022-07-012023-06-300000721371cah:NewYorkOpioidStewardshipActMember2023-06-300000721371cah:TotalOpioidLitigationMember2024-03-310000721371cah:TotalOpioidLitigationMember2024-01-012024-03-310000721371cah:OpioidLawsuitsMember2024-03-31cah:statescah:numberOfUSTerritories0000721371stpr:ALcah:OpioidLawsuitsMember2023-11-300000721371stpr:ALcah:OpioidLawsuitsMember2023-07-012024-03-310000721371us-gaap:SubsequentEventMembercah:OpioidLawsuitsMember2021-07-012024-04-300000721371us-gaap:SubsequentEventMembercah:OpioidLawsuitsMember2024-05-012038-12-310000721371stpr:WVcah:OpioidLawsuitsMember2022-07-310000721371cah:NativeAmericanTribesMembercah:OpioidLawsuitsMember2022-10-310000721371cah:OpioidLawsuitsMember2024-01-012024-03-310000721371cah:PrivatePartiesMemberus-gaap:SubsequentEventMembercah:OpioidLawsuitsMember2024-04-26cah:lawsuit0000721371cah:PrivatePartiesMembercah:ClassActionLawsuitsMemberus-gaap:SubsequentEventMembercah:OpioidLawsuitsMember2024-04-260000721371cah:PrivatePartiesMembercah:ClassActionLawsuitsMembercah:OpioidLawsuitsMember2024-03-310000721371cah:PrivatePartiesMemberstpr:GAcah:OpioidLawsuitsMember2023-01-012023-03-31cah:plaintiff0000721371stpr:ALcah:PrivatePartiesMemberus-gaap:SubsequentEventMembercah:OpioidLawsuitsMember2024-07-012024-07-010000721371cah:OpioidLawsuitsMember2022-07-012023-06-300000721371us-gaap:SubsequentEventMembercah:ProductLiabilityLawsuitsMember2024-04-260000721371cah:AlamedaCountyMemberus-gaap:SubsequentEventMembercah:ProductLiabilityLawsuitsMember2024-04-262024-04-260000721371cah:ProductLiabilityLawsuitsMembercah:IVCApril2023AgreementMember2023-04-302023-04-300000721371cah:ProductLiabilityLawsuitsMembercah:IVCApril2023AgreementMember2023-04-300000721371cah:ProductLiabilityLawsuitsMembercah:IVCApril2023AgreementMember2023-05-012023-09-300000721371cah:ProductLiabilityLawsuitsMembercah:OtherAgreementsMemberus-gaap:SubsequentEventMember2021-07-012024-04-260000721371cah:ProductLiabilityLawsuitsMember2022-07-012023-06-300000721371cah:ProductLiabilityLawsuitsMembersrt:MinimumMember2024-03-310000721371srt:ScenarioForecastMembercah:GMPDMember2023-07-012024-06-300000721371cah:GMPDMemberus-gaap:SubsequentEventMember2024-04-012024-06-300000721371srt:MinimumMember2024-03-310000721371srt:MaximumMember2024-03-310000721371cah:CareFusionMember2024-03-310000721371cah:CareFusionMember2023-06-300000721371us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310000721371us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310000721371us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-03-310000721371us-gaap:FairValueMeasurementsRecurringMember2024-03-310000721371us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2023-06-300000721371us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2023-06-300000721371us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2023-06-300000721371us-gaap:FairValueMeasurementsRecurringMember2023-06-300000721371us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueHedgingMemberus-gaap:InterestRateSwapMember2024-03-310000721371us-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:FairValueHedgingMemberus-gaap:InterestRateSwapMember2023-03-310000721371us-gaap:CashFlowHedgingMember2024-01-012024-03-310000721371us-gaap:CashFlowHedgingMember2023-01-012023-03-310000721371us-gaap:CashFlowHedgingMember2023-07-012024-03-310000721371us-gaap:CashFlowHedgingMember2022-07-012023-03-310000721371us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2024-01-012024-03-310000721371us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2023-01-012023-03-310000721371us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2023-07-012024-03-310000721371us-gaap:CashFlowHedgingMemberus-gaap:ForeignExchangeContractMember2022-07-012023-03-310000721371srt:ScenarioForecastMemberus-gaap:CashFlowHedgingMemberus-gaap:SubsequentEventMemberus-gaap:ForeignExchangeContractMember2024-04-012025-03-310000721371cah:September2025Memberus-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMember2024-03-31iso4217:JPY0000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:June2027Member2024-03-310000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:January2023Member2024-03-310000721371cah:January2023Member2023-07-012024-03-310000721371cah:September2025Memberus-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMember2023-03-310000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:June2027Member2023-03-310000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:March2025Member2023-03-31iso4217:EUR0000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:March2026Member2023-03-310000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:March2022Member2023-03-310000721371us-gaap:CurrencySwapMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:NetInvestmentHedgingMembercah:September2018Member2023-03-310000721371cah:March2022Member2023-01-012023-03-310000721371cah:September2018Member2023-01-012023-03-310000721371us-gaap:ForeignExchangeContractMember2024-01-012024-03-310000721371us-gaap:ForeignExchangeContractMember2023-07-012024-03-310000721371us-gaap:ForeignExchangeContractMember2022-07-012023-03-310000721371us-gaap:ForeignExchangeContractMember2023-01-012023-03-310000721371us-gaap:NondesignatedMemberus-gaap:OtherNonoperatingIncomeExpenseMemberus-gaap:ForeignExchangeContractMember2022-07-012023-03-310000721371us-gaap:FairValueInputsLevel2Member2024-03-310000721371us-gaap:FairValueInputsLevel2Member2023-06-300000721371cah:A250MillionShareRepurchaseProgramMember2023-10-012023-12-310000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-10-012023-12-310000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-11-022023-11-020000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-12-132023-12-130000721371cah:A500MillionShareRepurchaseProgramMember2023-07-012023-09-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-07-012023-09-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-07-012023-12-310000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-10-312023-10-310000721371cah:A500MillionShareRepurchaseProgramMember2023-04-012023-06-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-04-012023-06-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-04-012023-09-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-08-162023-08-160000721371cah:A250MillionShareRepurchaseProgramMember2023-01-012023-03-310000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-01-012023-03-310000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-01-012023-02-280000721371cah:A250MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2023-02-282023-02-280000721371cah:A300MillionShareRepurchaseProgramMember2022-10-012022-12-310000721371us-gaap:TreasuryStockCommonMembercah:A300MillionShareRepurchaseProgramMember2022-10-012022-12-310000721371us-gaap:TreasuryStockCommonMembercah:A300MillionShareRepurchaseProgramMember2022-10-012023-03-310000721371us-gaap:TreasuryStockCommonMembercah:A300MillionShareRepurchaseProgramMember2023-01-132023-01-130000721371cah:A500MillionShareRepurchaseProgramMember2022-07-012022-09-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2022-07-012022-09-300000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2022-07-012022-12-230000721371cah:A500MillionShareRepurchaseProgramMemberus-gaap:TreasuryStockCommonMember2022-12-232022-12-230000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2023-06-300000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2023-06-300000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2023-06-300000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2023-07-012024-03-310000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2023-07-012024-03-310000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2023-07-012024-03-310000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2024-03-310000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2024-03-310000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2024-03-310000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2022-06-300000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2022-06-300000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2022-06-300000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2022-07-012023-03-310000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2022-07-012023-03-310000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2022-07-012023-03-310000721371us-gaap:AccumulatedForeignCurrencyAdjustmentIncludingPortionAttributableToNoncontrollingInterestMember2023-03-310000721371us-gaap:AccumulatedNetGainLossFromCashFlowHedgesIncludingPortionAttributableToNoncontrollingInterestMember2023-03-310000721371us-gaap:AociIncludingPortionAttributableToNoncontrollingInterestMember2023-03-31cah:segmentcah:Segments0000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2024-01-012024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2023-01-012023-03-310000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2024-01-012024-03-310000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2023-01-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:NuclearPrecisionHealthSolutionsMember2024-01-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:NuclearPrecisionHealthSolutionsMember2023-01-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:AtHomeSolutionsMember2024-01-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:AtHomeSolutionsMember2023-01-012023-03-310000721371cah:OptiFreightLogisticsMemberus-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2024-01-012024-03-310000721371cah:OptiFreightLogisticsMemberus-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2023-01-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2024-01-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2023-01-012023-03-310000721371us-gaap:OperatingSegmentsMember2024-01-012024-03-310000721371us-gaap:OperatingSegmentsMember2023-01-012023-03-310000721371us-gaap:CorporateNonSegmentMember2024-01-012024-03-310000721371us-gaap:CorporateNonSegmentMember2023-01-012023-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2023-07-012024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2022-07-012023-03-310000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2023-07-012024-03-310000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2022-07-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:NuclearPrecisionHealthSolutionsMember2023-07-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:NuclearPrecisionHealthSolutionsMember2022-07-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:AtHomeSolutionsMember2023-07-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMembercah:AtHomeSolutionsMember2022-07-012023-03-310000721371cah:OptiFreightLogisticsMemberus-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2023-07-012024-03-310000721371cah:OptiFreightLogisticsMemberus-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2022-07-012023-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2023-07-012024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2022-07-012023-03-310000721371us-gaap:OperatingSegmentsMember2023-07-012024-03-310000721371us-gaap:OperatingSegmentsMember2022-07-012023-03-310000721371us-gaap:CorporateNonSegmentMember2023-07-012024-03-310000721371us-gaap:CorporateNonSegmentMember2022-07-012023-03-310000721371country:US2024-01-012024-03-310000721371country:US2023-01-012023-03-310000721371us-gaap:NonUsMember2024-01-012024-03-310000721371us-gaap:NonUsMember2023-01-012023-03-310000721371country:US2023-07-012024-03-310000721371country:US2022-07-012023-03-310000721371us-gaap:NonUsMember2023-07-012024-03-310000721371us-gaap:NonUsMember2022-07-012023-03-310000721371cah:GMPDMember2022-07-012023-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2024-03-310000721371cah:PharmaceuticalAndSpecialtySolutionsMemberus-gaap:OperatingSegmentsMember2023-06-300000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2024-03-310000721371cah:GMPDMemberus-gaap:OperatingSegmentsMember2023-06-300000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2024-03-310000721371us-gaap:AllOtherSegmentsMemberus-gaap:OperatingSegmentsMember2023-06-300000721371us-gaap:CorporateNonSegmentMember2024-03-310000721371us-gaap:CorporateNonSegmentMember2023-06-300000721371us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-03-310000721371us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-03-310000721371us-gaap:PerformanceSharesMember2024-01-012024-03-310000721371us-gaap:PerformanceSharesMember2023-01-012023-03-310000721371us-gaap:RestrictedStockUnitsRSUMember2023-07-012024-03-310000721371us-gaap:RestrictedStockUnitsRSUMember2022-07-012023-03-310000721371us-gaap:PerformanceSharesMember2023-07-012024-03-310000721371us-gaap:PerformanceSharesMember2022-07-012023-03-310000721371us-gaap:RestrictedStockUnitsRSUMember2023-06-300000721371us-gaap:RestrictedStockUnitsRSUMember2024-03-310000721371cah:Fiscal2023Membersrt:MinimumMemberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371cah:Fiscal2022Membersrt:MinimumMemberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371srt:MaximumMembercah:Fiscal2023Memberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371cah:Fiscal2022Membersrt:MaximumMemberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371srt:MinimumMembercah:Fiscal2024Memberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371srt:MaximumMembercah:Fiscal2024Memberus-gaap:PerformanceSharesMember2023-07-012024-03-310000721371us-gaap:PerformanceSharesMember2023-06-300000721371us-gaap:PerformanceSharesMember2024-03-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
|
|
|
|
|
|
| ☑ |
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2024
or
|
|
|
|
|
|
| ☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________ to ________
Commission File Number: 1-11373
Cardinal Health, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ohio |
|
31-0958666 |
(State or other jurisdiction of
incorporation or organization) |
|
(IRS Employer
Identification No.) |
|
|
|
|
|
|
|
| 7000 Cardinal Place |
, |
Dublin |
, |
Ohio |
|
43017 |
| (Address of principal executive offices) |
|
(Zip Code) |
(614) 757-5000
(Registrant’s telephone number, including area code)
|
|
|
|
|
|
|
|
|
| Securities registered pursuant to Section 12(b) of the Act: |
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common shares (without par value) |
CAH |
New York Stock Exchange |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
☑ |
|
Accelerated filer |
☐ |
|
| Non-accelerated filer |
☐ |
|
Smaller reporting company |
☐ |
|
|
|
|
Emerging growth company |
☐ |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No þ
The number of the registrant’s common shares, without par value, outstanding as of April 26, 2024, was the following: 243,566,952.
|
|
|
|
Cardinal Health
Q3 Fiscal 2024 Form 10-Q
|
Table of Contents
About Cardinal Health
Cardinal Health, Inc., an Ohio corporation formed in 1979, is a global healthcare services and products company providing customized solutions for hospitals, healthcare systems, pharmacies, ambulatory surgery centers, clinical laboratories, physician offices and patients in the home. We provide pharmaceuticals and medical products and cost-effective solutions that enhance supply chain efficiency. We connect patients, providers, payers, pharmacists and manufacturers for integrated care coordination. Effective January 1, 2024, we began operating under an updated organizational structure and re-aligned our financial reporting structure under two reportable segments: Pharmaceutical and Specialty Solutions ("PSS") segment and Global Medical Products and Distribution ("GMPD") segment. All remaining operating segments that are not significant enough to require separate reportable segment disclosures are included in Other, which is comprised of Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics. As used in this report, “we,” “our,” “us,” and similar pronouns refer to Cardinal Health, Inc. and its majority-owned and consolidated subsidiaries, unless the context requires otherwise. Our fiscal year ends on June 30. References to fiscal 2024 and fiscal 2023 and to FY24 and FY23 are to the fiscal years ending or ended June 30, 2024 and June 30, 2023, respectively.
Forward-Looking Statements
This Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 (this "Form 10-Q") (including information incorporated by reference) includes "forward-looking statements" addressing expectations, prospects, estimates and other matters that are dependent upon future events or developments. Many forward-looking statements appear in Management’s Discussion and Analysis of Financial Condition and Results of Operations ("MD&A"), but there are others in this Form 10-Q, which may be identified by words such as “expect,” “anticipate,” “intend,” “plan,” “believe,” “will,” “should,” “could,” “would,” “project,” “continue,” “likely,” and similar expressions, and include statements reflecting future results or guidance, statements of outlook and expense accruals. These matters are subject to risks and uncertainties that could cause actual results to differ materially from those made, projected or implied. The most significant of these risks and uncertainties are described in this Form 10-Q, including Exhibit 99.1, and in "Risk Factors" in our Annual Report on Form 10-K for the fiscal year ended June 30, 2023 (our “2023 Form 10-K”), our Forms 10-Q for the quarters ending September 30, 2023 and December 31, 2023, and other SEC filings made since June 30, 2023. Forward-looking statements in this Form 10-Q speak only as of the date of this document. Except to the extent required by applicable law, we undertake no obligation to update or revise any forward-looking statement.
Non-GAAP Financial Measures
In the "Overview of Consolidated Results" section of MD&A, we use financial measures that are derived from our consolidated financial data but are not presented in our condensed consolidated financial statements prepared in accordance with U.S. generally accepted accounting principles ("GAAP"). These measures are considered "non-GAAP financial measures" under the United States Securities and Exchange Commission ("SEC") rules. The reasons we use these non-GAAP financial measures and the reconciliations to their most directly comparable GAAP financial measures are included in the “Explanation and Reconciliation of Non-GAAP Financial Measures” section following MD&A in this Form 10-Q.
|
|
|
|
|
|
|
|
|
|
|
|
1 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Management's Discussion and Analysis of Financial Condition and Results of Operations
The discussion and analysis presented below is concerned with material changes in financial condition and results of operations, including amounts and certainty of cash flows from operations and from outside sources, between the periods specified in our condensed consolidated balance sheets at March 31, 2024 and June 30, 2023, and in our condensed consolidated statements of earnings for the three and nine months ended March 31, 2024 and 2023. All comparisons presented are with respect to the prior-year period, unless stated otherwise. Our previously reported segment results have been recast to conform to our new reporting structure and reflect changes in the elimination of inter-segment revenue and allocated corporate technology and shared function expenses, which are driven by the reporting structure change. The discussion and analysis in this Form 10-Q should be read in conjunction with the MD&A included in our 2023 Form 10-K.
|
|
|
|
|
|
|
|
|
|
|
|
2 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Overview of Consolidated Results
Revenue
During the three and nine months ended March 31, 2024, revenue increased 9 percent and 10 percent to $54.9 billion and $167.1 billion, respectively, primarily due to branded and specialty pharmaceutical sales growth from existing customers.
GAAP and Non-GAAP Operating Earnings
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
GAAP operating earnings |
$ |
367 |
|
|
$ |
572 |
|
|
(36) |
% |
|
$ |
835 |
|
|
$ |
590 |
|
|
42 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
| State opioid assessment related to prior fiscal years |
— |
|
|
— |
|
|
|
|
— |
|
|
(6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholder cooperation agreement costs |
1 |
|
|
— |
|
|
|
|
1 |
|
|
8 |
|
|
|
| Restructuring and employee severance |
53 |
|
|
16 |
|
|
|
|
106 |
|
|
62 |
|
|
|
| Amortization and other acquisition-related costs |
80 |
|
|
74 |
|
|
|
|
207 |
|
|
216 |
|
|
|
| Impairments and (gain)/loss on disposal of assets, net |
84 |
|
|
20 |
|
|
|
|
622 |
|
|
883 |
|
|
|
| Litigation (recoveries)/charges, net |
81 |
|
|
(76) |
|
|
|
|
29 |
|
|
(256) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-GAAP operating earnings |
$ |
666 |
|
|
$ |
606 |
|
|
10 |
% |
|
$ |
1,799 |
|
|
$ |
1,497 |
|
|
20 |
% |
The sum of the components and certain computations may reflect rounding adjustments.
We had GAAP operating earnings of $367 million and $572 million during the three months ended March 31, 2024 and 2023, respectively, and $835 million and $590 million during the nine months ended March 31, 2024 and 2023, respectively. GAAP operating earnings during the three and nine months ended March 31, 2024 were favorably impacted by GMPD and Pharmaceutical and Specialty Solutions segment profit. GAAP operating earnings also reflects the pre-tax non-cash goodwill impairment charges related to the GMPD segment of $90 million during the three months ended March 31, 2024 and the $671 million and $863 million during the nine months ended March 31, 2024 and 2023, respectively. See "Critical Accounting Policies and Sensitive Accounting Estimates" section of this MD&A and
Note 5 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
GAAP operating earnings during the three months ended March 31, 2024 reflects $193 million of litigation charges recognized in connection with opioid-related matters, which were partially offset by a benefit of $105 million related to opioid-related prepayments. GAAP operating earnings during the three and nine months ended March 31, 2023 were favorably impacted by litigation recoveries and a reduction in litigation reserves. See "Results of Operations" section of this MD&A and
Note 7 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
Non-GAAP operating earnings during the three and nine months ended March 31, 2024 increased 10 percent and 20 percent, respectively, due to increases in GMPD and Pharmaceutical and Specialty Solutions segment profit.
|
|
|
|
|
|
|
|
|
|
|
|
3 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
GAAP and Non-GAAP Diluted EPS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| ($ per share) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
GAAP diluted EPS (1) |
$ |
1.05 |
|
|
$ |
1.34 |
|
|
(22) |
% |
|
$ |
2.49 |
|
|
$ |
1.23 |
|
|
N.M. |
|
|
|
|
|
|
|
|
|
|
|
|
| State opioid assessment related to prior fiscal years |
— |
|
|
— |
|
|
|
|
— |
|
|
0.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shareholder cooperation agreement costs |
— |
|
|
— |
|
|
|
|
— |
|
|
(0.02) |
|
|
|
| Restructuring and employee severance |
0.16 |
|
|
0.05 |
|
|
|
|
0.32 |
|
|
0.18 |
|
|
|
| Amortization and other acquisition-related costs |
0.24 |
|
|
0.21 |
|
|
|
|
0.62 |
|
|
0.61 |
|
|
|
Impairments and (gain)/loss on disposal of assets, net (2) |
0.44 |
|
|
0.35 |
|
|
|
|
2.14 |
|
|
2.82 |
|
|
|
| Litigation (recoveries)/charges, net |
0.19 |
|
|
(0.21) |
|
|
|
|
0.05 |
|
|
(0.60) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP diluted EPS (1) |
$ |
2.08 |
|
|
$ |
1.74 |
|
|
20 |
% |
|
$ |
5.62 |
|
|
$ |
4.24 |
|
|
33 |
% |
The sum of the components and certain computations may reflect rounding adjustments.
The reconciling items are presented within this table net of tax. See quantification of tax effect of each reconciling item in our GAAP to Non-GAAP Reconciliations in the "Explanation and Reconciliation of Non-GAAP Financial Measures."
(1)Diluted earnings per share attributable to Cardinal Health, Inc. ("diluted EPS").
(2)For the three and nine months ended March 31, 2024, impairments and (gain)/loss on disposal of assets, net includes pre-tax goodwill impairment charges of $90 million and $671 million, respectively, related to the GMPD segment. For fiscal 2024, the estimated net tax benefit related to the impairments is $56 million and is included in the annual effective tax rate. The incremental interim tax benefit recognized during the nine months ended March 31, 2024 is $36 million and will reverse in the fourth quarter of the fiscal year.
For the nine months ended March 31, 2023, impairments and (gain)/loss on disposal of assets, net included cumulative pre-tax goodwill impairment charges of $863 million related to the former Medical segment. For fiscal 2023, the net tax benefit related to the impairment was $68 million and was included in the annual effective tax rate. The incremental interim tax benefit recognized during the nine months ended March 31, 2023 was $66 million and reversed in the fourth quarter of fiscal 2023.
The changes in GAAP diluted EPS during the three and nine months ended March 31, 2024 were primarily due to the factors impacting GAAP operating earnings. GAAP diluted EPS was adversely impacted by goodwill impairment charges related to the GMPD segment, which had a $(0.29) per share after tax impact during the three months ended March 31, 2024, and a $(2.35) and $(2.76) per share after tax impact during the nine months ended March 31, 2024 and 2023, respectively. See "Critical Accounting Policies and Sensitive Accounting Estimates" section of this MD&A, and
Note 5 and
Note 8 of the "Notes to Condensed Consolidated Financial Statements" for additional details.
During the three and nine months ended March 31, 2024, non-GAAP diluted EPS increased 20 percent and 33 percent to $2.08 and $5.62 per share, respectively, due to higher non-GAAP operating earnings and a lower share count.
Cash and Equivalents
Our cash and equivalents balance was $3.7 billion at March 31, 2024 compared to $4.0 billion at June 30, 2023. During the nine months ended March 31, 2024, net cash provided by operating activities was $1.7 billion, which includes the impact of our annual payment of $378 million and prepayments of $239 million primarily related to the agreement to settle the vast majority of the opioid lawsuits filed by states and local governmental entities (the "National Opioid Settlement Agreement"). During the three months ended March 31, 2024, we issued additional long-term debt and received net proceeds of $1.14 billion, of which $589 million were classified as cash and equivalents in our condensed consolidated balance sheets as of March 31, 2024. The remaining proceeds were invested in short-term time deposits with initial effective maturities of more than three months and classified as prepaid expenses and other. In addition, during the nine months ended March 31, 2024, we deployed $1.2 billion for the Specialty Networks acquisition, $750 million for share repurchases, $377 million for cash dividends and $318 million for capital expenditures.
|
|
|
|
|
|
|
|
|
|
|
|
4 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Significant Developments in Fiscal 2024 and Trends
Operating and Segment Reporting Structure Changes
Effective January 1, 2024, we began operating under an updated organizational structure and re-aligned our financial reporting structure under two reportable segments: Pharmaceutical and Specialty Solutions segment and GMPD segment. All remaining operating segments that are not significant enough to require separate reportable segment disclosures are included in Other. The following indicates the changes from the second quarter of fiscal 2024 to the new reporting structure:
•Pharmaceutical and Specialty Solutions segment: This reportable segment is comprised of all businesses formerly within our Pharmaceutical segment except Nuclear and Precision Health Solutions.
•GMPD segment: This reportable segment is comprised of all businesses formerly within our Medical segment except at-Home Solutions and OptiFreight® Logistics.
•Other: This is comprised of the remaining operating segments, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics.
Our previously reported segment results have been recast to conform to our new reporting structure and reflect changes in the elimination of inter-segment revenue and allocated corporate technology and shared function expenses, which are driven by the reporting structure change.
Pharmaceutical and Specialty Solutions Segment
OptumRx Contracts
On April 22, 2024, we announced that our pharmaceutical distribution contracts with OptumRx, which expire at the end of June 2024, will not be renewed. Sales to OptumRx generated 16% of our consolidated revenue in fiscal 2023. Total sales to OptumRx generate a meaningfully lower operating margin than the overall Pharmaceutical and Specialty Solutions segment. We expect the nonrenewal of the OptumRx contracts to adversely impact our results of operations, including segment profit, financial condition and cash flows. In particular, we expect to generate lower than average operating cash flow in fiscal 2025 due to the unwinding of the negative net working capital associated with the contract.
Specialty Networks Acquisition
On March 18, 2024, we completed the acquisition of Specialty Networks for a purchase price of $1.2 billion in cash, subject to certain adjustments. Specialty Networks creates clinical and economic value for independent specialty providers and partners across multiple specialty group purchasing organizations ("GPOs"): UroGPO,Gastrologix and GastroGPO, and United Rheumatology. Specialty Networks’ PPS Analytics platform analyzes data from electronic medical records, practice management, imaging, and dispensing systems and transforms it into meaningful and actionable insights for providers and other stakeholders by using artificial intelligence and modern data analytics capabilities. The acquisition further expands our offerings in key therapeutic areas, accelerates our upstream data and research opportunities with biopharma manufacturers, and creates a platform for our expansion across therapeutic areas. We expect the Specialty Networks acquisition to positively impact Pharmaceutical and Specialty Solutions segment revenue and profit while increasing amortization and other acquisition-related costs during the remainder of fiscal 2024 and fiscal 2025.
COVID-19 Vaccine Distribution
Pharmaceutical and Specialty Solutions segment profit was favorably impacted during the nine months ended March 31, 2024 and on a year-over-year basis in part due to the company beginning to distribute the commercially available COVID-19 vaccines following U.S. Food and Drug Administration approval of updated vaccines in September 2023. The timing, magnitude and profit impact of vaccine distribution volume for the remainder of fiscal 2024 and beyond remains uncertain.
Generics Program
The performance of our Pharmaceutical and Specialty Solutions segment generics program positively impacted the year-over-year comparison of Pharmaceutical and Specialty Solutions segment profit during the three and nine months ended March 31, 2024. The Pharmaceutical and Specialty Solutions segment generics program includes, among other things, the impact of generic pharmaceutical product launches, customer volumes, pricing changes, the Red Oak Sourcing, LLC venture ("Red Oak Sourcing") with CVS Health Corporation ("CVS Health") and generic pharmaceutical contract manufacturing and sourcing costs.
|
|
|
|
|
|
|
|
|
|
|
|
5 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
The frequency, timing, magnitude and profit impact of generic pharmaceutical customer volumes, pricing changes, customer contract renewals, generic pharmaceutical manufacturer pricing changes and generic pharmaceutical contract manufacturing and sourcing costs all impact Pharmaceutical and Specialty Solutions segment profit and are subject to risks and uncertainties. These risks and uncertainties may impact Pharmaceutical and Specialty Solutions segment profit and consolidated operating earnings during the remainder of fiscal 2024.
Global Medical Products and Distribution Segment
Inflationary Impacts
Beginning in fiscal 2022, GMPD segment profit was negatively affected by incremental inflationary impacts, primarily related to transportation (including ocean and domestic freight), commodities and labor, and global supply chain constraints. Since that time, we have taken actions to partially mitigate these impacts, including implementing certain price increases and evolving our pricing and commercial contracting processes to provide us with greater pricing flexibility. In addition, decreases in some product-related costs have been recognized as the higher-cost inventory moved through our supply chain and was replaced by lower-cost inventory. These net inflationary impacts negatively affected the GMPD segment profit during fiscal 2023. The net inflationary impacts were less significant during the three and nine months ended March 31, 2024 and had a favorable impact on GMPD segment profit on a year-over-year basis.
We expect these net inflationary impacts to continue to affect GMPD segment profit during the remainder of fiscal 2024, but to a significantly lesser extent than in fiscal 2023 and prior periods due to our mitigation actions, together with continued decreases in certain product-related costs. However, these inflationary costs are difficult to predict and may be greater than we expect or continue longer than our current expectations. Our actions to increase prices and evolve our contracting strategies are subject to contingencies and uncertainties and it is possible that our results of operations will be adversely impacted to a greater extent than we currently anticipate or that we may not be able to mitigate the negative impact to the extent or on the timeline we anticipate.
Volumes
The GMPD segment profit was adversely impacted during fiscal 2023 in part due to lower volumes, which includes our Cardinal Health branded medical products. We have experienced Cardinal Health branded medical products sales growth during fiscal 2024 and expect further growth for the remainder of fiscal 2024 and beyond. The timing, magnitude and profit impact of this anticipated sales growth is subject to risks and uncertainties, which may impact GMPD segment profit.
Goodwill
The change in segment structure as discussed above resulted in changes to the composition of our reporting units. Accordingly, we are required to reallocate the goodwill in reporting units affected by the change using a relative fair value approach and assess goodwill for impairment both before and after the reallocation.
During the three months ended March 31, 2024, we allocated $90 million and $48 million of goodwill from the former Medical segment excluding our at-Home Solutions division (the "Medical Unit") to the GMPD reporting unit and the OptiFreight® Logistics reporting unit, respectively. We also assessed GMPD's goodwill for impairment and determined there was an impairment of GMPD’s remaining goodwill balance of $90 million. See "Critical Accounting Policies and Sensitive Accounting Estimates" section of this MD&A and
Note 5 of the "Notes to Condensed Consolidated Financial Statements" for additional detail.
Shareholder Cooperation Agreement
In September 2022, we entered into a Cooperation Agreement (the "Cooperation Agreement") with Elliott Associates, L.P. and Elliott International, L.P. (together, "Elliott") under which our Board of Directors (the "Board"), among other things, (1) appointed four new independent directors, including a representative from Elliott, and (2) formed an advisory Business Review Committee of the Board, which is tasked with undertaking a comprehensive review of our strategy, portfolio, capital-allocation framework and operations. In May 2023, we extended the term of the Cooperation Agreement until the later of July 15, 2024 or until Elliott's representative ceases to serve on, or resigns from, the Board. In connection with this extension, the Board has extended the term of the Business Review Committee until July 15, 2024.
The evaluation and implementation of any actions recommended by the Business Review Committee and the Board have impacted and may continue to impact our business, financial position and results of operations during the remainder of fiscal 2024 and beyond. We have incurred, and may incur additional legal, consulting and other expenses related to the Cooperation Agreement and the activities of the Business Review Committee.
|
|
|
|
|
|
|
|
|
|
|
|
6 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Results of Operations |
|
Results of Operations
Revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
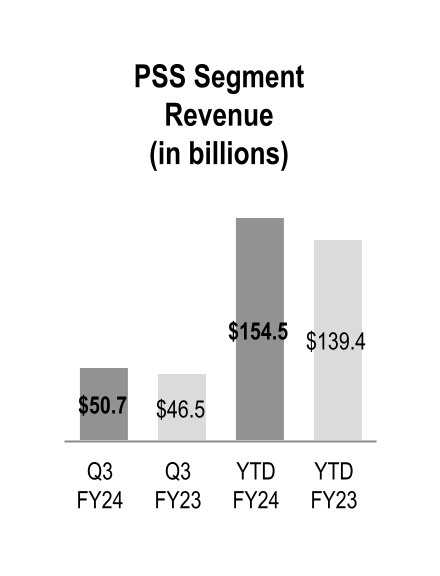
| Pharmaceutical and Specialty Solutions |
$ |
50,651 |
|
|
$ |
46,496 |
|
|
9 |
% |
|
$ |
154,524 |
|
|
$ |
139,441 |
|
|
11 |
% |
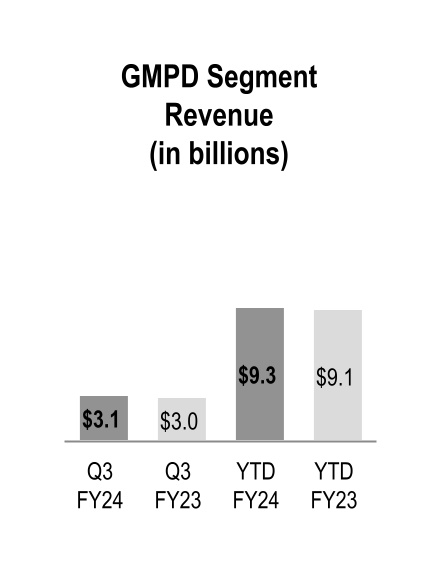
| Global Medical Products and Distribution |
3,113 |
|
|
2,989 |
|
|
4 |
% |
|
9,264 |
|
|
9,140 |
|
|
1 |
% |
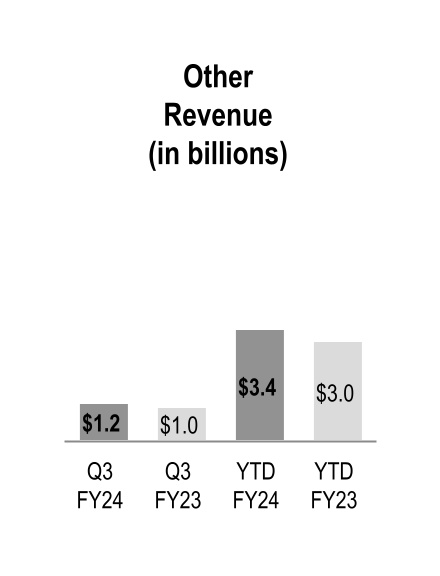
| Other |
1,167 |
|
|
1,025 |
|
|
14 |
% |
|
3,392 |
|
|
3,038 |
|
|
12 |
% |
| Total segment revenue |
54,931 |
|
|
50,510 |
|
|
9 |
% |
|
167,180 |
|
|
151,619 |
|
|
10 |
% |
| Corporate |
(20) |
|
|
(23) |
|
|
N.M. |
|
(61) |
|
|
(60) |
|
|
N.M. |
| Total revenue |
$ |
54,911 |
|
|
$ |
50,487 |
|
|
9 |
% |
|
$ |
167,119 |
|
|
$ |
151,559 |
|
|
10 |
% |
Pharmaceutical and Specialty Solutions
Pharmaceutical and Specialty Solutions segment revenue increased $4.2 billion and $15.1 billion during the three and nine months ended March 31, 2024, respectively, due to branded and specialty pharmaceutical sales growth from existing customers.
Global Medical Products and Distribution
GMPD segment revenue increased during the three and nine months ended March 31, 2024, driven by higher volumes from existing customers. Additionally, during the nine months ended March 31, 2024 GMPD segment revenue was adversely impacted by personal protective equipment ("PPE") volumes and pricing, partially offset by price increases to mitigate inflationary impacts.
Other
Other revenue increased during the three and nine months ended March 31, 2024 due to growth across the three operating segments: at-Home Solutions, Nuclear and Precision Health Solutions and OptiFreight® Logistics.
Cost of Products Sold
Cost of products sold for the three and nine months ended March 31, 2024 increased 9 percent and 10 percent to $53.0 billion and $161.6 billion, respectively, compared to the prior-year periods due to the factors affecting the changes in revenue and gross margin.
|
|
|
|
|
|
|
|
|
|
|
|
7 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Results of Operations |
|
Gross Margin
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
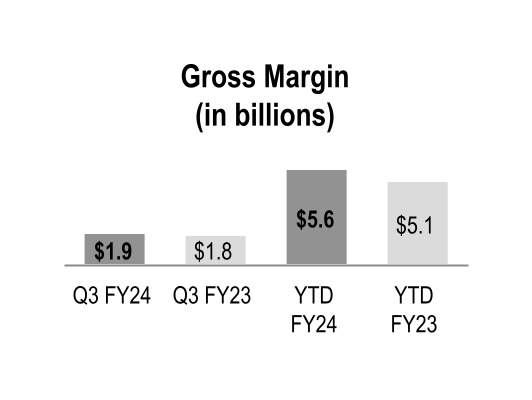
| Gross margin |
$ |
1,947 |
|
|
$ |
1,785 |
|
|
9 |
% |
|
$ |
5,561 |
|
|
$ |
5,062 |
|
|
10 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
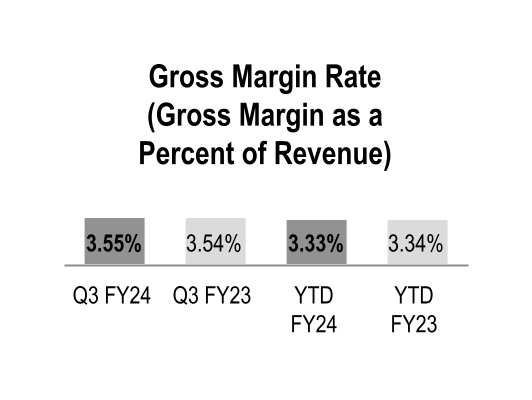
Gross margin increased during the three and nine months ended March 31, 2024 primarily due to the beneficial comparison of the prior-year net inflationary impacts in the GMPD segment and the performance of our generics program in the Pharmaceutical and Specialty Solutions segment. Gross margin also increased during the nine months ended March 31, 2024 due to increased contribution from branded pharmaceutical and specialty pharmaceutical products in the Pharmaceutical and Specialty Solutions segment, which includes the favorable impact of COVID-19 vaccine distribution.
Gross margin rates were relatively flat during the three and nine months ended March 31, 2024 with the impact of the overall product mix mostly offset by the beneficial comparison to the prior-year net inflationary impacts in the GMPD segment. The changes in overall product mix were primarily driven by increased pharmaceutical distribution branded sales, which have a dilutive impact on our overall gross margin rate.
Distribution, Selling, General and Administrative ("SG&A") Expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
| SG&A expenses |
$ |
1,282 |
|
|
$ |
1,179 |
|
|
9 |
% |
|
$ |
3,762 |
|
|
$ |
3,567 |
|
|
5 |
% |
During the three and nine months ended March 31, 2024, SG&A expenses increased primarily due to investment projects, higher costs to support sales growth, and compensation-related costs.
|
|
|
|
|
|
|
|
|
|
|
|
8 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Results of Operations |
|
Segment Profit
We evaluate segment performance based on segment profit, among other measures. See
Note 13 of the "Notes to Condensed Consolidated Financial Statements" for additional information on segment profit.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
| Pharmaceutical and Specialty Solutions |
$ |
580 |
|
|
$ |
560 |
|
|
4 |
% |
|
$ |
1,541 |
|
|
$ |
1,394 |
|
|
11 |
% |
| Global Medical Products and Distribution |
20 |
|
|
(46) |
|
|
N.M. |
|
18 |
|
|
(175) |
|
|
N.M. |
| Other |
111 |
|
|
106 |
|
|
5 |
% |
|
319 |
|
|
305 |
|
|
5 |
% |
| Total segment profit |
711 |
|
|
620 |
|
|
15 |
% |
|
1,878 |
|
|
1,524 |
|
|
23 |
% |
| Corporate |
(344) |
|
|
(48) |
|
|
N.M. |
|
(1,043) |
|
|
(934) |
|
|
N.M. |
Total consolidated operating earnings |
$ |
367 |
|
|
$ |
572 |
|
|
(36) |
% |
|
$ |
835 |
|
|
$ |
590 |
|
|
(31) |
% |
Pharmaceutical and Specialty Solutions
Pharmaceutical and Specialty Solutions segment profit increased during the three and nine months ended March 31, 2024 primarily due to the performance of our generics program. During the nine months ended March 31, 2024, Pharmaceutical and Specialty Solutions segment profit also increased due to the increased contribution from branded pharmaceutical and specialty pharmaceutical products, which includes the favorable impact of COVID-19 vaccine distribution, partially offset by higher costs to support sales growth.
Global Medical Products and Distribution
Global Medical Product and Distribution segment profit increased during the three and nine months ended March 31, 2024 primarily due to beneficial comparison to the prior-year inflationary impacts, net of the effects of mitigation actions.
Other
Other segment profit increased during the three and nine months ended March 31, 2024 primarily due to the performance of OptiFreight® Logistics.
Corporate
The changes in Corporate during the three and nine months ended March 31, 2024 were due to the factors discussed in the "Other Components of Consolidated Operating Earnings" section that follows.
|
|
|
|
|
|
|
|
|
|
|
|
9 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Results of Operations |
|
Other Components of Consolidated Operating Earnings
In addition to revenue, gross margin and SG&A expenses discussed previously, consolidated operating earnings were impacted by the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
2024 |
|
2023 |
| Restructuring and employee severance |
$ |
53 |
|
|
$ |
16 |
|
|
$ |
106 |
|
|
$ |
62 |
|
| Amortization and other acquisition-related costs |
80 |
|
|
74 |
|
|
207 |
|
|
216 |
|
| Impairments and (gain)/loss on disposal of assets, net |
84 |
|
|
20 |
|
|
622 |
|
|
883 |
|
| Litigation (recoveries)/charges, net |
81 |
|
|
(76) |
|
|
29 |
|
|
(256) |
|
Restructuring and Employee Severance
Restructuring and employee severance costs during the three and nine months ended March 31, 2024 and 2023 include costs related to the implementation of certain enterprise-wide cost-savings measures, which include certain initiatives to rationalize our manufacturing operations. The increase in restructuring costs during the three and nine months ended March 31, 2024 was primarily due to these initiatives and certain projects resulting from the review of our strategy portfolio, capital-allocation framework and operations. During the three and nine months ended March 31, 2023, restructuring and employee severance costs also included costs related to the divestiture of the Cordis business.
Amortization and Other Acquisition-Related Costs
Amortization of acquisition-related intangible assets was $64 million and $69 million for the three months ended March 31, 2024 and 2023, respectively, and $191 million and $211 million for the nine months ended March 31, 2024 and 2023, respectively.
Impairments and (Gain)/Loss on Disposal of Assets, Net
We recognized a $90 million pre-tax non-cash goodwill impairment charge related to the GMPD segment during the three months ended March 31, 2024 and charges of $671 million and $863 million during the nine months ended March 31, 2024 and 2023, respectively, as discussed further in the "Critical Accounting Policies and Sensitive Accounting Estimates" section of this MD&A and
Note 5 of the "Notes to Condensed Consolidated Financial Statements."
Litigation (Recoveries)/Charges, Net
During the three months ended March 31, 2024, we recognized expense of $193 million in connection with opioid-related matters, which was offset by a benefit of $105 million related to prepayments at a prenegotiated discount of certain future payment amounts totaling $344 million. During the nine months ended March 31, 2024, we also recognized a $22 million charge related to an agreement in principle with the Alabama Attorney General. See
Note 7 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
We recognized income for net recoveries in class action antitrust lawsuit in which we were a class member or plaintiff of $6 million and $77 million during the three and nine months ended March 31, 2024, respectively, and $66 million during the nine months ended March 31, 2023.
During the three and nine months ended March 31, 2023, we recognized income of $71 million and $95 million, respectively, primarily related to a reduction of the reserve for the estimated settlement and defense costs for the Cordis OptEase and TrapEase inferior vena cava ("IVC") product liability due to the execution of certain settlement agreements.
During the nine months ended March 31, 2023, we recognized income of $93 million due to net proceeds from the settlement of a shareholder derivative litigation matter.
Earnings Before Income Taxes
In addition to the items discussed above, earnings before income taxes were impacted by the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
Change |
|
2024 |
|
2023 |
|
Change |
| Other (income)/expense, net |
$ |
(7) |
|
|
$ |
— |
|
|
N.M. |
|
$ |
(25) |
|
|
$ |
(5) |
|
|
N.M. |
| Interest expense, net |
33 |
|
|
28 |
|
|
18 |
% |
|
55 |
|
|
78 |
|
|
(29) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Results of Operations |
|
Interest Expense, Net
During the nine months ended March 31, 2024, interest expense decreased by 29 percent primarily due to increased interest income from cash and equivalents.
Provision for Income Taxes
The effective tax rate was 24.2 percent and 36.3 percent during the three months ended March 31, 2024 and 2023, respectively, and 23.2 percent and 36.7 percent during the nine months ended March 31, 2024 and 2023, respectively. These tax rates reflect the impact of the tax effects of goodwill impairment charges as well as certain other discrete items. See
Note 8 of the "Notes to Condensed Consolidated Financial Statements" for additional information, during the three and nine months ended March 31, 2024 and 2023.
Tax Effects of Goodwill Impairment Charges
During the nine months ended March 31, 2024, we recognized cumulative pre-tax goodwill impairment charges of $671 million related to the GMPD segment. The net tax benefit related to these charges is $56 million for fiscal 2024.
Unless an item is considered discrete because it is unusual or infrequent, the tax impact of the item is included in our estimated annual effective tax rate. When items are recognized through our estimated annual effective tax rate, we apply our estimated annual effective tax rate to the earnings before income taxes for the year-to-date period to compute our impact from income taxes for the current quarter and year-to-date period. The tax impacts of discrete items are recognized in their entirety in the period in which they occur.
The tax effect of the goodwill impairment charges recorded during the nine months ended March 31, 2024 was included in our estimated annual effective tax rate because it was not considered unusual or infrequent, given that we recorded goodwill impairments in prior fiscal years. The impact of the non-deductible goodwill increased the estimated annual effective tax rate for fiscal 2024. Applying the higher tax rate to the pre-tax income for the nine months ended March 31, 2024 resulted in recognizing an incremental interim tax benefit of approximately $36 million, which impacted the provision for income taxes in the condensed consolidated statements of earnings during the nine months ended March 31, 2024 and prepaid expenses and other assets in the condensed consolidated balance sheet at March 31, 2024. The incremental interim tax benefit will reverse in the fourth quarter of fiscal 2024.
|
|
|
|
|
|
|
|
|
|
|
|
11 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Liquidity and Capital Resources |
|
Liquidity and Capital Resources
We currently believe that, based on available capital resources and projected operating cash flow, we have adequate capital resources to fund our operations and expected future cash needs as described below. If we decide to engage in one or more acquisitions we may need to access capital markets for additional financing, depending on the size and timing of such transactions.
Cash and Equivalents
Our cash and equivalents balance was $3.7 billion at March 31, 2024 compared to $4.0 billion at June 30, 2023.
During the nine months ended March 31, 2024, net cash provided by operating activities was $1.7 billion, which includes the impact of our annual payment of $378 million and prepayments of $239 million primarily related to the National Opioid Settlement Agreement. During the three months ended March 31, 2024, we issued additional long-term debt and received net proceeds of $1.14 billion, of which $589 million were classified as cash and equivalents in our condensed consolidated balance sheets as of March 31, 2024. The remaining proceeds were invested in short-term time deposits with initial effective maturities of more than three months and classified as prepaid expenses and other. In addition, we deployed cash of $750 million for share repurchases, $377 million for cash dividends and $318 million for capital expenditures.
On March 18, 2024, we completed the acquisition of Specialty Networks for a purchase price of $1.2 billion in cash, subject to certain adjustments. See
Note 2 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
At March 31, 2024, our cash and equivalents were held in cash depository accounts with major banks or invested in high quality, short-term liquid investments.
Changes in working capital, which impact operating cash flow, can vary significantly depending on factors such as the timing of customer payments, inventory purchases, payments to vendors and tax payments in the regular course of business, as well as fluctuating working capital needs driven by customer and product mix.
The cash and equivalents balance at March 31, 2024 includes $650 million of cash held by subsidiaries outside of the United States.
Other Financing Arrangements and Financial Instruments
Credit Facilities and Commercial Paper
In addition to cash and equivalents and operating cash flow, other sources of liquidity at March 31, 2024 include a $2.0 billion commercial paper program, backed by a $2.0 billion revolving credit facility. We also have a $1.0 billion committed receivables sales facility. At March 31, 2024, we had no amounts outstanding under our commercial paper program, revolving credit facility, or our committed receivables sales facility.
In February 2023, we extended our $2.0 billion revolving credit facility through February 25, 2028. In September 2022, we renewed our committed receivables sales facility program through Cardinal Health Funding, LLC ("CHF") through September 30, 2025. In September 2023, Cardinal Health 23 Funding, LLC was added as a seller under our committed receivables sales facility.
Our revolving credit and committed receivables sales facilities require us to maintain a consolidated net leverage ratio of no more than 3.75-to-1. As of March 31, 2024, we were in compliance with this financial covenant.
Long-Term Debt and Other Short-Term Borrowings
We had total long-term obligations, including the current portion and other short-term borrowings, of $5.9 billion and $4.7 billion at March 31, 2024 and June 30, 2023, respectively.
In February 2024, we issued additional debt with the aggregate principal amount of $1.15 billion to fund the repayment of all of the aggregate principal amount outstanding of our 3.5% Notes due 2024 and 3.079% Notes due 2024, at their respective maturities, and for general corporate purposes. The notes issued are $650 million aggregate principal amount of 5.125% Notes that mature on February 15, 2029 and $500 million aggregate principal amount of 5.45% Notes that mature on February 15, 2034. The proceeds of the notes issued, net of discounts, premiums, and debt issuance costs were $1.14 billion. A portion of the proceeds were invested in short-term time deposits of $550 million with initial effective maturities of more than three months and classified as prepaid expenses and other in our condensed consolidated balance sheets as of March 31, 2024. The remaining proceeds of $589 million were invested in short-term time deposits classified as cash and equivalents in our condensed consolidated balance sheets as of March 31, 2024.
|
|
|
|
|
|
|
|
|
|
|
|
12 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| MD&A |
Liquidity and Capital Resources |
|
Capital Deployment
Opioid Litigation Settlement Agreement
We had $5.3 billion accrued at March 31, 2024 related to certain opioid litigation, as further described within
Note 7 of the "Notes to Condensed Consolidated Financial Statements." We expect the majority of the remaining payment amounts to occur through 2038. During the nine months ended March 31, 2024, we made our third annual payment of $378 million under the National Opioid Settlement Agreement. The amounts of future annual payments may differ from the payments that we have already made.
In January 2024, we made additional payments of approximately $239 million to prepay at a prenegotiated discount of certain future payment amounts totaling approximately $344 million owed under each of the National Opioid Settlement Agreement, West Virginia Subdivisions Settlement Agreement and settlement agreements with Native American tribes and Cherokee Nation. The majority of the prepayment relates to the seventh annual payment due under the National Opioid Settlement Agreement. As a result of these prepayments, we recognized income of $105 million in litigation charges/(recoveries), net in our condensed consolidated statements of earnings during the nine months ended March 31, 2024.
Capital Expenditures
Capital expenditures during the nine months ended March 31, 2024 and 2023 were $318 million and $264 million, respectively.
Dividends
On each of May 11, 2023, August 9, 2023, November 14, 2023 and February 6, 2024, our Board of Directors approved a quarterly dividend of $0.5006 per share, or $2.00 per share on an annualized basis, which were paid on July 15, 2023, October 15, 2023, January 15, 2024 and April 15, 2024 to shareholders of record on July 3, 2023, October 3, 2023, January 2, 2024 and April 1, 2024, respectively.
Share Repurchases
During the nine months ended March 31, 2024, we deployed $750 million of our common shares, in the aggregate, under accelerated share repurchase ("ASR") programs. We funded the ASR programs with available cash. See
Note 11 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
As of March 31, 2024, we have $3.5 billion remaining under our existing share repurchase authorization.
Specialty Networks Acquisition
On March 18, 2024, we completed the acquisition of Specialty Networks for a purchase price of $1.2 billion in cash, subject to certain adjustments. See
Note 2 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
|
|
|
|
|
|
|
|
|
|
|
|
13 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Other Items
The MD&A in our 2023 Form 10-K addresses our contractual obligations and cash requirements, as of and for the fiscal year ended June 30, 2023. Other than the acquisition of Specialty Networks and our debt issuance, there have been no subsequent material changes outside of the ordinary course of business to those items. See
Note 2 and
Note 6 of the "Notes to Condensed Consolidated Financial Statements" for additional information.
Critical Accounting Policies and Sensitive Accounting Estimates
The discussion and analysis presented below is a supplemental disclosure to the critical accounting policies and sensitive accounting estimates specified in our consolidated balance sheet at June 30, 2023. This discussion and analysis should be read in conjunction with the Critical Accounting Policies and Sensitive Accounting Estimates included in our 2023 Form 10-K and our Form 10-Q for the quarters ended September 30, 2023 and December 31, 2023.
Critical accounting policies are those accounting policies that (i) can have a significant impact on our financial condition and results of operations and (ii) require the use of complex and subjective estimates based upon past experience and management’s judgment. Other people applying reasonable judgment to the same facts and circumstances could develop different estimates. Because estimates are inherently uncertain, actual results may differ, including due to the risks discussed in "Risk Factors" and other risks discussed in our 2023 Form 10-K and our other filings with the SEC since June 30, 2023.
Goodwill
Purchased goodwill is tested for impairment annually or when indicators of impairment exist. Goodwill impairment testing involves a comparison of the estimated fair value of reporting units to the respective carrying amount, which may be performed utilizing either a qualitative or quantitative assessment. Qualitative factors are first assessed to determine if it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If it is determined that it is more likely than not that the fair value does not exceed the carrying amount, then a quantitative test is performed. The quantitative goodwill impairment test involves a comparison of the estimated fair value of the reporting unit to the respective carrying amount. A reporting unit is defined as an operating segment or one level below an operating segment (also known as a component).
Goodwill impairment testing involves judgment, including the identification of reporting units, qualitative evaluation of events and circumstances to determine if it is more likely than not that an impairment exists, and, if necessary, the estimation of the fair value of the applicable reporting unit. Our qualitative evaluation considers the weight of evidence and significance of all identified events and circumstances and most relevant drivers of fair value, both positive and negative, in determining whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount.
As discussed in the Overview section of this MD&A, effective January 1, 2024, we implemented a new enterprise operating and segment reporting structure. The updated structure is comprised of two reportable segments: Pharmaceutical and Specialty Solutions segment and Global Medical Products and Distribution segment. All remaining operating segments that are not significant enough to
require separate reportable segment disclosures are included in Other.
This change in segment structure resulted in changes to the composition of our former Medical operating segment excluding at-Home Solutions reporting unit ("Medical Unit"). Effective January 1, 2024, our reporting units are: Pharmaceutical and Specialty Solutions, GMPD, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics. GMPD and OptiFreight® Logistics comprised our former Medical Unit.
Accordingly, we allocated $90 million and $48 million of goodwill from the former Medical Unit to GMPD and OptiFreight® Logistics, respectively, based on the estimated relative fair values of the reporting units. We also assessed goodwill for impairment for these reporting units before and after the reallocation and determined there was no impairment for the Medical Unit and OptiFreight® Logistics during the three months ended March 31, 2024 as their fair values substantially exceeded their carrying values. However, the quantitative test resulted in an impairment of GMPD’s remaining goodwill balance of $90 million.
Our previously reported goodwill balances have been recast to conform to the new structure. Prior-period goodwill impairment charges related to the former Medical Unit were primarily driven by the performance and long-term financial plan assumptions of GMPD and have been fully allocated to GMPD under the new structure.
Global Medical Products and Distribution Goodwill
Our determinations of the estimated fair value of GMPD was based on a combination of the income-based approach (using a discount rate of 11 percent and a terminal growth rate of 2 percent) and market-based approaches at January 1, 2024. Additionally, we
|
|
|
|
|
|
|
|
|
|
|
|
14 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
assigned a weighting of 80 percent to the discounted cash flow method, 10 percent to the guideline public company method, and 10 percent to the guideline transaction method.
During the three months ended September 30, 2023, we elected to bypass the qualitative assessment and perform quantitative goodwill impairment testing for the former Medical Unit. The carrying amount exceeded the fair value, which resulted in a pre-tax impairment charge of $581 million for the three months ended September 30, 2023. We did not identify any indicators of impairment during the three months ended December 31, 2023.
During the three months ended December 31, 2022 and September 30, 2022, we performed quantitative goodwill impairment testing for the former Medical Unit and recorded impairment charges of $709 million and $154 million, respectively. We also performed interim quantitative goodwill impairment testing at March 31, 2023 and concluded that there was no impairment as of March 31, 2023 as the estimated fair value of the former Medical Unit exceeded its carrying value by approximately 4 percent.
|
|
|
|
|
|
|
|
|
|
|
|
15 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Explanation and Reconciliation of Non-GAAP Financial Measures |
|
|
Explanation and Reconciliation of Non-GAAP Financial Measures
The "Overview of Consolidated Results" section within MD&A in this Form 10-Q contains financial measures that are not calculated in accordance with GAAP.
In addition to analyzing our business based on financial information prepared in accordance with GAAP, we use these non-GAAP financial measures internally to evaluate our performance, engage in financial and operational planning, and determine incentive compensation because we believe that these measures provide additional perspective on and, in some circumstances are more closely correlated to, the performance of our underlying, ongoing business. We provide these non-GAAP financial measures to investors as supplemental metrics to assist readers in assessing the effects of items and events on our financial and operating results on a year-over-year basis and in comparing our performance to that of our competitors. However, the non-GAAP financial measures that we use may be calculated differently from, and therefore may not be comparable to, similarly titled measures used by other companies. The non-GAAP financial measures disclosed by us should not be considered a substitute for, or superior to, financial measures calculated in accordance with GAAP, and the financial results calculated in accordance with GAAP and reconciliations to those financial statements set forth below should be carefully evaluated.
Exclusions from Non-GAAP Financial Measures
Management believes it is useful to exclude the following items from the non-GAAP measures presented in this report for its own and for investors’ assessment of the business for the reasons identified below:
•LIFO charges and credits are excluded because the factors that drive last-in first-out ("LIFO") inventory charges or credits, such as pharmaceutical manufacturer price appreciation or deflation and year-end inventory levels (which can be meaningfully influenced by customer buying behavior immediately preceding our fiscal year-end), are largely out of our control and cannot be accurately predicted. The exclusion of LIFO charges and credits from non-GAAP metrics facilitates comparison of our current financial results to our historical financial results and to our peer group companies’ financial results. We did not recognize any LIFO charges or credits during the periods presented.
•State opioid assessments related to prior fiscal years is the portion of state assessments for prescription opioid medications that were sold or distributed in periods prior to the period in which the expense is incurred. This portion is excluded from non-GAAP financial measures because it is retrospectively applied to sales in prior fiscal years and inclusion would obscure analysis of the current fiscal year results of our underlying, ongoing business. Additionally, while states' laws may require us to make payments on an ongoing basis, the portion of the assessment related to sales in prior periods are contemplated to be one-time, nonrecurring items. Income from state opioid assessments related to prior fiscal years represents reversals of accruals due to changes in estimates or when the underlying assessments were invalidated by a Court or reimbursed by manufacturers.
•Shareholder cooperation agreement costs includes costs such as legal, consulting and other expenses incurred in relation to the agreement (the "Cooperation Agreement") entered into among Elliott Associates, L.P., Elliott International, L.P. (together, "Elliott") and Cardinal Health, including costs incurred to negotiate and finalize the Cooperation Agreement and costs incurred by the Business Review Committee of the Board of Directors, which was formed under this Cooperation Agreement. We have excluded these costs from our non-GAAP metrics because they do not occur in or reflect the ordinary course of our ongoing business operations and may obscure analysis of trends and financial performance.
•Restructuring and employee severance costs are excluded because they are not part of the ongoing operations of our underlying business and include, but are not limited to, costs related to divestitures, closing and consolidating facilities, changing the way we manufacture or distribute our products, moving manufacturing of a product to another location, changes in production or business process outsourcing or insourcing, employee severance and realigning operations.
•Amortization and other acquisition-related costs, which include transaction costs, integration costs, and changes in the fair value of contingent consideration obligations, are excluded because they are not part of the ongoing operations of our underlying business and to facilitate comparison of our current financial results to our historical financial results and to our peer group companies' financial results. Additionally, costs for amortization of acquisition-related intangible assets are non-cash amounts, which are variable in amount and frequency and are significantly impacted by the timing and size of acquisitions, so their exclusion facilitates comparison of historical, current and forecasted financial results. We also exclude other acquisition-related costs, which are directly related to an acquisition but do not meet the criteria to be recognized on the acquired entity’s initial balance sheet as part of the purchase price allocation. These costs are also significantly impacted by the timing, complexity and size of acquisitions.
|
|
|
|
|
|
|
|
|
|
|
|
16 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Explanation and Reconciliation of Non-GAAP Financial Measures |
|
|
•Impairments and gain or loss on disposal of assets, net are excluded because they do not occur in or reflect the ordinary course of our ongoing business operations and are inherently unpredictable in timing and amount, and in the case of impairments, are non-cash amounts, so their exclusion facilitates comparison of historical, current and forecasted financial results.
•Litigation recoveries or charges, net are excluded because they often relate to events that may have occurred in prior or multiple periods, do not occur in or reflect the ordinary course of our business and are inherently unpredictable in timing and amount.
•Loss on early extinguishment of debt is excluded because it does not typically occur in the normal course of business and may obscure analysis of trends and financial performance. Additionally, the amount and frequency of this type of charge is not consistent and is significantly impacted by the timing and size of debt extinguishment transactions.
The tax effect for each of the items listed above is determined using the tax rate and other tax attributes applicable to the item and the jurisdiction(s) in which the item is recorded. The gross, tax and net impact of each item are presented with our GAAP to non-GAAP reconciliations.
Definitions
Growth rate calculation: growth rates in this report are determined by dividing the difference between current-period results and prior-period results by prior-period results.
Non-GAAP operating earnings: operating earnings excluding (1) LIFO charges/(credits), (2) state opioid assessment related to prior fiscal years, (3) shareholder cooperation agreement costs, (4) restructuring and employee severance, (5) amortization and other acquisition-related costs, (6) impairments and (gain)/loss on disposal of assets, net and (7) litigation (recoveries)/charges, net.
Non-GAAP earnings before income taxes: earnings before income taxes excluding (1) LIFO charges/(credits), (2) state opioid assessment related to prior fiscal years, (3) shareholder cooperation agreement costs, (4) restructuring and employee severance, (5) amortization and other acquisition-related costs, (6) impairments and (gain)/loss on disposal of assets, net, (7) litigation (recoveries)/charges, net and (8) loss on early extinguishment of debt.
Non-GAAP net earnings attributable to Cardinal Health, Inc.: net earnings attributable to Cardinal Health, Inc. excluding (1) LIFO charges/(credits), (2) state opioid assessment related to prior fiscal years, (3) shareholder cooperation agreement costs, (4) restructuring and employee severance, (5) amortization and other acquisition-related costs, (6) impairments and (gain)/loss on disposal of assets, net, (7) litigation (recoveries)/charges, net and (8) loss on early extinguishment of debt, each net of tax.
Non-GAAP effective tax rate: provision for income taxes adjusted for the tax impacts of (1) LIFO charges/(credits), (2) state opioid assessment related to prior fiscal years, (3) shareholder cooperation agreement costs, (4) restructuring and employee severance, (5) amortization and other acquisition-related costs, (6) impairments and (gain)/loss on disposal of assets, net, (7) litigation (recoveries)/charges, net and (8) loss on early extinguishment of debt divided by (earnings before income taxes adjusted for the eight items above).
Non-GAAP diluted earnings per share attributable to Cardinal Health, Inc.: non-GAAP net earnings attributable to Cardinal Health, Inc. divided by diluted weighted-average shares outstanding.
|
|
|
|
|
|
|
|
|
|
|
|
17 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Explanation and Reconciliation of Non-GAAP Financial Measures |
|
|
GAAP to Non-GAAP Reconciliation
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions, except per common share amounts) |
|
|
|
|
Operating Earnings |
Operating Earnings Growth Rate |
Earnings Before Income Taxes |
Provision for Income Taxes |
Net Earnings1 |
Net Earnings1 Growth Rate |
Diluted EPS1 |
Diluted EPS1 Growth Rate |
|
|
|
|
|
Three Months Ended March 31, 2024 |
| GAAP |
|
|
|
|
$ |
367 |
|
(36) |
% |
$ |
341 |
|
$ |
82 |
|
$ |
258 |
|
(25) |
% |
$ |
1.05 |
|
(22) |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholder cooperation agreement costs |
|
|
|
|
1 |
|
|
1 |
|
— |
|
1 |
|
|
— |
|
|
| Restructuring and employee severance |
|
|
|
|
53 |
|
|
53 |
|
14 |
|
39 |
|
|
0.16 |
|
|
| Amortization and other acquisition-related costs |
|
|
|
|
80 |
|
|
80 |
|
21 |
|
59 |
|
|
0.24 |
|
|
Impairments and (gain)/loss on disposal of assets, net 2 |
|
|
|
|
84 |
|
|
84 |
|
(21) |
|
105 |
|
|
0.44 |
|
|
| Litigation (recoveries)/charges, net |
|
|
|
|
81 |
|
|
81 |
|
34 |
|
47 |
|
|
0.19 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-GAAP |
|
|
|
|
$ |
666 |
|
10 |
% |
$ |
640 |
|
$ |
130 |
|
$ |
509 |
|
14 |
% |
$ |
2.08 |
|
20 |
% |
|
|
|
|
|
Three Months Ended March 31, 2023 |
| GAAP |
|
|
|
|
$ |
572 |
|
N.M. |
$ |
544 |
|
$ |
197 |
|
$ |
345 |
|
N.M. |
$ |
1.34 |
|
N.M. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Restructuring and employee severance |
|
|
|
|
16 |
|
|
16 |
|
4 |
|
12 |
|
|
0.05 |
|
|
| Amortization and other acquisition-related costs |
|
|
|
|
74 |
|
|
74 |
|
19 |
|
55 |
|
|
0.21 |
|
|
Impairments and (gain)/loss on disposal of assets, net |
|
|
|
|
20 |
|
|
20 |
|
(69) |
|
89 |
|
|
0.35 |
|
|
| Litigation (recoveries)/charges, net |
|
|
|
|
(76) |
|
|
(76) |
|
(22) |
|
(54) |
|
|
(0.21) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-GAAP |
|
|
|
|
$ |
606 |
|
11 |
% |
$ |
578 |
|
$ |
129 |
|
$ |
447 |
|
11 |
% |
$ |
1.74 |
|
20 |
% |
|
|
|
|
|
Nine Months Ended March 31, 2024 |
GAAP |
|
|
|
|
$ |
835 |
|
42 |
% |
$ |
805 |
|
$ |
186 |
|
$ |
616 |
|
90 |
% |
$ |
2.49 |
|
N.M. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholder cooperation agreement costs |
|
|
|
|
1 |
|
|
1 |
|
— |
|
1 |
|
|
— |
|
|
| Restructuring and employee severance |
|
|
|
|
106 |
|
|
106 |
|
28 |
|
78 |
|
|
0.32 |
|
|
| Amortization and other acquisition-related costs |
|
|
|
|
207 |
|
|
207 |
|
54 |
|
153 |
|
|
0.62 |
|
|
Impairments and (gain)/loss on disposal of assets, net 2 |
|
|
|
|
622 |
|
|
622 |
|
92 |
|
530 |
|
|
2.14 |
|
|
| Litigation (recoveries)/charges, net |
|
|
|
|
29 |
|
|
29 |
|
17 |
|
12 |
|
|
0.05 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-GAAP |
|
|
|
|
$ |
1,799 |
|
20 |
% |
$ |
1,769 |
|
$ |
377 |
|
$ |
1,389 |
|
24 |
% |
$ |
5.62 |
|
33 |
% |
|
|
|
|
|
Nine Months Ended March 31, 2023 |
| GAAP |
|
|
|
|
$ |
590 |
|
N.M. |
$ |
517 |
|
$ |
189 |
|
$ |
325 |
|
N.M. |
$ |
1.23 |
|
N.M. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| State opioid assessment related to prior fiscal years |
|
|
|
|
(6) |
|
|
(6) |
|
(2) |
|
(4) |
|
|
0.02 |
|
|
| Shareholder cooperation agreement costs |
|
|
|
|
8 |
|
|
8 |
|
2 |
|
6 |
|
|
(0.02) |
|
|
| Restructuring and employee severance |
|
|
|
|
62 |
|
|
62 |
|
14 |
|
48 |
|
|
0.18 |
|
|
| Amortization and other acquisition-related costs |
|
|
|
|
216 |
|
|
216 |
|
56 |
|
160 |
|
|
0.61 |
|
|
Impairments and (gain)/loss on disposal of assets, net 2 |
|
|
|
|
883 |
|
|
883 |
|
138 |
|
745 |
|
|
2.82 |
|
|
| Litigation (recoveries)/charges, net |
|
|
|
|
(256) |
|
|
(256) |
|
(98) |
|
(158) |
|
|
(0.60) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-GAAP |
|
|
|
|
$ |
1,497 |
|
(3) |
% |
$ |
1,424 |
|
$ |
299 |
|
$ |
1,122 |
|
(1) |
% |
$ |
4.24 |
|
6 |
% |
1 Attributable to Cardinal Health, Inc.
2 For the three and nine months ended March 31, 2024, impairments and (gain)/loss on disposal of assets, net includes pre-tax goodwill impairment charges of $90 million and $671 million, respectively, related to the GMPD segment. For fiscal 2024, the estimated net tax benefit related to the impairments is $56 million and is included in the annual effective tax rate. The incremental interim tax benefit recognized during the nine months ended March 31, 2024 is $36 million and will reverse in the fourth quarter of the fiscal year.
For the nine months ended March 31, 2023, impairments and (gain)/loss on disposal of assets, net included cumulative pre-tax goodwill impairment charges of $863 million related to the former Medical segment. For fiscal 2023, the net tax benefit related to the impairment was $68 million and was included in the annual effective tax rate. The incremental interim tax benefit recognized during the nine months ended March 31, 2023 was $66 million and reversed in the fourth quarter of fiscal 2023.
The sum of the components and certain computations may reflect rounding adjustments.
We apply varying tax rates depending on the item's nature and tax jurisdiction where it is incurred.
|
|
|
|
|
|
|
|
|
|
|
|
18 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Quantitative and Qualitative Disclosures About Market Risk
There have been no material changes in the quantitative and qualitative market risk disclosures included in our 2023 Form 10-K since the end of fiscal 2023 through March 31, 2024.
Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We evaluated, with the participation of our principal executive officer and principal financial officer, the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Securities Exchange Act of 1934 (the "Exchange Act")) as of March 31, 2024. Based on this evaluation, our principal executive officer and principal financial officer have concluded that as of March 31, 2024, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed in our reports under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC rules and forms and that such information is accumulated and communicated to management as appropriate to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting during the quarter ended March 31, 2024 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
|
|
|
|
|
|
|
|
|
|
|
19 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Legal Proceedings
The legal proceedings described in
Note 7 of the "Notes to Condensed Consolidated Financial Statements" are incorporated in this "Legal Proceedings" section by reference.
Risk Factors
You should carefully consider the information in this Form 10-Q including the Risk Factors below and the risk factors discussed in "Risk Factors" and other risks discussed in our 2023 Form 10-K, our Forms 10-Q for the quarters ended December 31, 2023 and September 30, 2023, and our other filings with the SEC since June 30, 2023. These risks could materially and adversely affect our results of operations, financial condition, liquidity, and cash flows. Our business also could be affected by risks that we are not presently aware of or that we currently consider immaterial to our operations.
Changes to the U.S. healthcare environment may not be favorable to us.
Over a number of years, the U.S. healthcare industry has undergone significant changes designed to increase access to medical care, improve safety and patient outcomes, contain costs and increase efficiencies. These changes include a general decline in Medicare and Medicaid reimbursement levels, efforts by healthcare insurance companies to limit or reduce payments to pharmacies and providers, the basis for payments beginning to transition from a fee-for-service model to value-based payments and risk-sharing models and the industry shifting away from traditional healthcare venues like hospitals and into clinics, physician offices and patients’ homes.
We expect the U.S. healthcare industry to continue to change significantly in the future. Possible changes include changes in
legislation or regulations governing prescription pharmaceutical pricing, healthcare services, U.S.-based medical product manufacturing, mandated benefits, efforts to promote increased transparency in the pharmaceutical supply chain, drug shortages, further reduction of or limitations on governmental funding at the state or federal level or efforts by healthcare insurance companies to further limit payments for products and services. For example, the Federal Trade Commission has issued public requests for information related to pharmaceutical wholesalers' and group purchasing organizations' impacts on generic drug shortages and the impact of pharmacy benefits managers on drug affordability and access. These possible changes, and the uncertainty surrounding these possible changes, may directly or indirectly adversely affect us.
Unregistered Sales of Equity Securities and Use of Proceeds
Issuer Purchases of Equity Securities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Period |
Total Number
of Shares
Purchased (1) |
|
Average Price Paid per Share |
|
Total Number of Shares
Purchased
as Part of Publicly Announced Programs (2)
|
|
Approximate
Dollar Value of
Shares That May
Yet be Purchased
Under the Program (2)
(in millions)
|
| January 2024 |
106 |
|
|
$ |
105.98 |
|
|
— |
|
|
$ |
3,493 |
|
| February 2024 |
157 |
|
|
107.33 |
|
|
— |
|
|
3,493 |
|
| March 2024 |
51 |
|
|
110.14 |
|
|
— |
|
|
3,493 |
|
| Total |
314 |
|
|
$ |
107.33 |
|
|
— |
|
|
$ |
3,493 |
|
(1)Reflects 106, 157 and 51 common shares purchased in January, February, and March 2024, respectively, through a rabbi trust as investments of participants in our Deferred Compensation Plan.
(2)On June 7, 2023, our Board of Directors approved a new $3.5 billion share repurchase program, which will expire on December 31, 2027. As of March 31, 2024, we had $3.5 billion authorized for share repurchases remaining under this program.
Other Information
Rule 10b5-1 Plan Adoptions and Modifications
During the three months ended March 31, 2024, no director or officer adopted, modified or terminated a "Rule 10b5-1 trading arrangement" or "non-Rule10b5-1 trading arrangement" as each term is defined in Section 408(a) of Regulation S-K under the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
20 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Condensed Consolidated Statements of Earnings
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions, except per common share amounts) |
2024 |
|
2023 |
|
2024 |
|
2023 |
| Revenue |
$ |
54,911 |
|
|
$ |
50,487 |
|
|
$ |
167,119 |
|
|
$ |
151,559 |
|
| Cost of products sold |
52,964 |
|
|
48,702 |
|
|
161,558 |
|
|
146,497 |
|
| Gross margin |
1,947 |
|
|
1,785 |
|
|
5,561 |
|
|
5,062 |
|
|
|
|
|
|
|
|
|
| Operating expenses: |
|
|
|
|
|
|
|
| Distribution, selling, general and administrative expenses |
1,282 |
|
|
1,179 |
|
|
3,762 |
|
|
3,567 |
|
| Restructuring and employee severance |
53 |
|
|
16 |
|
|
106 |
|
|
62 |
|
| Amortization and other acquisition-related costs |
80 |
|
|
74 |
|
|
207 |
|
|
216 |
|
| Impairments and (gain)/loss on disposal of assets, net |
84 |
|
|
20 |
|
|
622 |
|
|
883 |
|
| Litigation (recoveries)/charges, net |
81 |
|
|
(76) |
|
|
29 |
|
|
(256) |
|
| Operating earnings |
367 |
|
|
572 |
|
|
835 |
|
|
590 |
|
|
|
|
|
|
|
|
|
| Other (income)/expense, net |
(7) |
|
|
— |
|
|
(25) |
|
|
(5) |
|
| Interest expense, net |
33 |
|
|
28 |
|
|
55 |
|
|
78 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Earnings before income taxes |
341 |
|
|
544 |
|
|
805 |
|
|
517 |
|
|
|
|
|
|
|
|
|
| Provision for income taxes |
82 |
|
|
197 |
|
|
186 |
|
|
189 |
|
| Net earnings |
259 |
|
|
347 |
|
|
619 |
|
|
328 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Less: Net earnings attributable to noncontrolling interests |
(1) |
|
|
(2) |
|
|
(3) |
|
|
(3) |
|
| Net earnings attributable to Cardinal Health, Inc. |
$ |
258 |
|
|
$ |
345 |
|
|
$ |
616 |
|
|
$ |
325 |
|
|
|
|
|
|
|
|
|
Earnings per common share attributable to Cardinal Health, Inc.: |
|
|
|
|
|
|
|
| Basic |
$ |
1.06 |
|
|
$ |
1.35 |
|
|
$ |
2.51 |
|
|
$ |
1.24 |
|
| Diluted |
1.05 |
|
|
1.34 |
|
|
2.49 |
|
|
1.23 |
|
|
|
|
|
|
|
|
|
| Weighted-average number of common shares outstanding: |
|
|
|
|
|
|
|
| Basic |
243 |
|
256 |
|
245 |
|
263 |
| Diluted |
245 |
|
258 |
|
247 |
|
264 |
|
|
|
|
|
|
|
|
| Cash dividends declared per common share |
$ |
0.5006 |
|
|
$ |
0.4957 |
|
|
$ |
1.5018 |
|
|
$ |
1.4871 |
|
See notes to condensed consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
21 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Condensed Consolidated Statements of Comprehensive Income
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
|
2024 |
|
2023 |
| Net earnings |
$ |
259 |
|
|
$ |
347 |
|
|
$ |
619 |
|
|
$ |
328 |
|
|
|
|
|
|
|
|
|
| Other comprehensive income/(loss): |
|
|
|
|
|
|
|
| Foreign currency translation adjustments and other |
1 |
|
|
4 |
|
|
(4) |
|
|
(34) |
|
|
|
|
|
|
|
|
|
| Net unrealized gain/(loss) on derivative instruments, net of tax |
(6) |
|
|
— |
|
|
(5) |
|
|
6 |
|
| Total other comprehensive income/(loss), net of tax |
(5) |
|
|
4 |
|
|
(9) |
|
|
(28) |
|
|
|
|
|
|
|
|
|
| Total comprehensive income |
254 |
|
|
351 |
|
|
610 |
|
|
300 |
|
|
|
|
|
|
|
|
|
| Less: comprehensive income attributable to noncontrolling interests |
(1) |
|
|
(2) |
|
|
(3) |
|
|
(3) |
|
|
|
|
|
|
|
|
|
| Total comprehensive income attributable to Cardinal Health, Inc. |
$ |
253 |
|
|
$ |
349 |
|
|
$ |
607 |
|
|
$ |
297 |
|
See notes to condensed consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
22 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Condensed Consolidated Balance Sheets
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
March 31, 2024 |
|
June 30, 2023 |
|
(Unaudited) |
|
|
| Assets |
|
|
|
| Current assets: |
|
|
|
| Cash and equivalents |
$ |
3,718 |
|
|
$ |
4,043 |
|
| Trade receivables, net |
11,566 |
|
|
11,344 |
|
| Inventories, net |
17,277 |
|
|
15,940 |
|
| Prepaid expenses and other |
3,161 |
|
|
2,362 |
|
| Assets held for sale |
12 |
|
|
144 |
|
| Total current assets |
35,734 |
|
|
33,833 |
|
|
|
|
|
| Property and equipment, net |
2,470 |
|
|
2,462 |
|
| Goodwill and other intangibles, net |
6,507 |
|
|
6,081 |
|
|
|
|
|
| Other assets |
1,169 |
|
|
1,041 |
|
| Total assets |
$ |
45,880 |
|
|
$ |
43,417 |
|
|
|
|
|
| Liabilities and Shareholders’ Deficit |
|
|
|
| Current liabilities: |
|
|
|
| Accounts payable |
$ |
32,089 |
|
|
$ |
29,813 |
|
| Current portion of long-term obligations and other short-term borrowings |
1,187 |
|
|
792 |
|
| Other accrued liabilities |
3,030 |
|
|
3,059 |
|
| Liabilities related to assets held for sale |
— |
|
|
42 |
|
| Total current liabilities |
36,306 |
|
|
33,706 |
|
|
|
|
|
| Long-term obligations, less current portion |
4,667 |
|
|
3,909 |
|
| Deferred income taxes and other liabilities |
8,169 |
|
|
8,653 |
|
|
|
|
|
| Shareholders’ deficit: |
|
|
|
| Preferred shares, without par value: |
|
|
|
Authorized—500 thousand shares, Issued—none |
— |
|
|
— |
|
| Common shares, without par value: |
|
|
|
Authorized—755 million shares, Issued—327 million shares at March 31, 2024 and June 30, 2023 |
2,887 |
|
|
2,747 |
|
| Accumulated deficit |
(289) |
|
|
(534) |
|
Common shares in treasury, at cost: 83 million shares and 76 million shares at March 31, 2024 and June 30, 2023, respectively |
(5,703) |
|
|
(4,914) |
|
| Accumulated other comprehensive loss |
(160) |
|
|
(151) |
|
| Total Cardinal Health, Inc. shareholders' deficit |
(3,265) |
|
|
(2,852) |
|
| Noncontrolling interests |
3 |
|
|
1 |
|
| Total shareholders’ deficit |
(3,262) |
|
|
(2,851) |
|
| Total liabilities and shareholders’ deficit |
$ |
45,880 |
|
|
$ |
43,417 |
|
See notes to condensed consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
23 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Condensed Consolidated Statements of Shareholders' Deficit
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Shares |
|
Accumulated Deficit |
|
Treasury Shares |
|
Accumulated Other
Comprehensive
Loss |
|
Noncontrolling Interests |
|
Total
Shareholders’ Deficit
|
|
| (in millions) |
Shares Issued |
|
Amount |
|
|
Shares |
|
Amount |
|
|
|
|
|
Three Months Ended March 31, 2024 |
|
| Balance at December 31, 2023 |
327 |
|
|
$ |
2,855 |
|
|
$ |
(425) |
|
|
(83) |
|
|
$ |
(5,724) |
|
|
$ |
(155) |
|
|
$ |
2 |
|
|
$ |
(3,447) |
|
|
Net earnings |
|
|
|
|
258 |
|
|
|
|
|
|
|
|
1 |
|
|
259 |
|
|
Other comprehensive loss, net of tax |
|
|
|
|
|
|
|
|
|
|
(5) |
|
|
|
|
(5) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Employee stock plans activity, net of shares withheld for employee taxes |
— |
|
|
32 |
|
|
|
|
— |
|
|
21 |
|
|
|
|
|
|
53 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Dividends declared |
|
|
|
|
(123) |
|
|
|
|
|
|
|
|
|
|
(123) |
|
|
| Other |
|
|
|
|
1 |
|
|
|
|
|
|
|
|
|
|
1 |
|
|
| Balance at March 31, 2024 |
327 |
|
|
$ |
2,887 |
|
|
$ |
(289) |
|
|
(83) |
|
|
$ |
(5,703) |
|
|
$ |
(160) |
|
|
$ |
3 |
|
|
$ |
(3,262) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, 2023 |
| Balance at December 31, 2022 |
327 |
|
|
$ |
2,747 |
|
|
$ |
(560) |
|
|
(68) |
|
|
$ |
(4,254) |
|
|
$ |
(146) |
|
|
$ |
1 |
|
|
$ |
(2,212) |
|
| Net earnings |
|
|
|
|
345 |
|
|
|
|
|
|
|
|
2 |
|
|
347 |
|
| Other comprehensive income, net of tax |
|
|
|
|
|
|
|
|
|
|
4 |
|
|
|
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Employee stock plans activity, net of shares withheld for employee taxes |
— |
|
|
21 |
|
|
|
|
— |
|
|
3 |
|
|
|
|
|
|
24 |
|
| Share repurchase program activity |
|
|
50 |
|
|
|
|
(4) |
|
|
(303) |
|
|
|
|
|
|
(253) |
|
| Dividends declared |
|
|
|
|
(128) |
|
|
|
|
|
|
|
|
|
|
(128) |
|
| Other |
|
|
|
|
1 |
|
|
|
|
|
|
|
|
(1) |
|
|
— |
|
| Balance at March 31, 2023 |
327 |
|
|
$ |
2,818 |
|
|
$ |
(342) |
|
|
(72) |
|
|
$ |
(4,554) |
|
|
$ |
(142) |
|
|
$ |
2 |
|
|
$ |
(2,218) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, 2024 |
| Balance at June 30, 2023 |
327 |
|
|
$ |
2,747 |
|
|
$ |
(534) |
|
|
(76) |
|
|
$ |
(4,914) |
|
|
$ |
(151) |
|
|
$ |
1 |
|
|
$ |
(2,851) |
|
Net earnings |
|
|
|
|
616 |
|
|
|
|
|
|
|
|
3 |
|
|
619 |
|
| Other comprehensive loss, net of tax |
|
|
|
|
|
|
|
|
|
|
(9) |
|
|
|
|
(9) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Employee stock plans activity, net of shares withheld for employee taxes |
— |
|
|
40 |
|
|
|
|
1 |
|
|
70 |
|
|
|
|
|
|
110 |
|
| Share repurchase program activity |
|
|
100 |
|
|
|
|
(9) |
|
|
(859) |
|
|
|
|
|
|
(759) |
|
| Dividends declared |
|
|
|
|
(372) |
|
|
|
|
|
|
|
|
|
|
(372) |
|
| Other |
|
|
|
|
1 |
|
|
1 |
|
|
|
|
|
|
(1) |
|
|
— |
|
| Balance at March 31, 2024 |
327 |
|
|
$ |
2,887 |
|
|
$ |
(289) |
|
|
(83) |
|
|
$ |
(5,703) |
|
|
$ |
(160) |
|
|
$ |
3 |
|
|
$ |
(3,262) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, 2023 |
| Balance at June 30, 2022 |
327 |
|
|
$ |
2,813 |
|
|
$ |
(280) |
|
|
(54) |
|
|
$ |
(3,128) |
|
|
$ |
(114) |
|
|
$ |
3 |
|
|
$ |
(706) |
|
| Net earnings |
|
|
|
|
325 |
|
|
|
|
|
|
|
|
3 |
|
|
328 |
|
| Other comprehensive loss, net of tax |
|
|
|
|
|
|
|
|
|
|
(28) |
|
|
|
|
(28) |
|
Purchase of noncontrolling interests |
|
|
|
|
|
|
|
|
|
|
|
|
(2) |
|
|
(2) |
|
| Employee stock plans activity, net of shares withheld for employee taxes |
— |
|
|
5 |
|
|
|
|
2 |
|
|
77 |
|
|
|
|
|
|
82 |
|
| Share repurchase program activity |
|
|
|
|
|
|
(20) |
|
|
(1,503) |
|
|
|
|
|
|
(1,503) |
|
| Dividends declared |
|
|
|
|
(388) |
|
|
|
|
|
|
|
|
|
|
(388) |
|
| Other |
|
|
|
|
1 |
|
|
|
|
|
|
|
|
(2) |
|
|
(1) |
|
| Balance at March 31, 2023 |
327 |
|
|
$ |
2,818 |
|
|
$ |
(342) |
|
|
(72) |
|
|
$ |
(4,554) |
|
|
$ |
(142) |
|
|
$ |
2 |
|
|
$ |
(2,218) |
|
See notes to condensed consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
24 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Condensed Consolidated Statements of Cash Flows
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Cash flows from operating activities: |
|
|
|
Net earnings |
$ |
619 |
|
|
$ |
328 |
|
|
|
|
|
Adjustments to reconcile net earnings to net cash provided by operating activities: |
|
|
|
| Depreciation and amortization |
524 |
|
|
516 |
|
| Impairments and (gain)/loss on disposal of assets, net |
622 |
|
|
883 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Share-based compensation |
88 |
|
|
69 |
|
|
|
|
|
| Provision for bad debts |
59 |
|
|
79 |
|
| Change in operating assets and liabilities, net of effects from acquisitions and divestitures: |
|
|
|
| Increase in trade receivables |
(262) |
|
|
(510) |
|
| Increase in inventories |
(1,371) |
|
|
(1,012) |
|
| Increase in accounts payable |
2,276 |
|
|
2,473 |
|
| Other accrued liabilities and operating items, net |
(870) |
|
|
(845) |
|
| Net cash provided by operating activities |
1,685 |
|
|
1,981 |
|
|
|
|
|
| Cash flows from investing activities: |
|
|
|
| Acquisition of subsidiaries, net of cash acquired |
(1,192) |
|
|
(10) |
|
| Proceeds from divestitures, net of cash sold |
9 |
|
|
— |
|
| Additions to property and equipment |
(318) |
|
|
(264) |
|
| Proceeds from disposal of property and equipment |
10 |
|
|
2 |
|
| Purchases of investments |
(3) |
|
|
(6) |
|
| Proceeds from investments |
1 |
|
|
1 |
|
| Proceeds from net investment hedge terminations |
28 |
|
|
29 |
|
Purchases of short-term time deposits |
(550) |
|
|
— |
|
| Net cash used in investing activities |
(2,015) |
|
|
(248) |
|
|
|
|
|
| Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
| Proceeds from long-term obligations, net of issuance costs |
1,139 |
|
|
— |
|
| Reduction of long-term obligations |
(23) |
|
|
(571) |
|
Net tax proceeds from share-based compensation |
23 |
|
|
11 |
|
|
|
|
|
| Dividends on common shares |
(377) |
|
|
(399) |
|
| Purchase of treasury shares |
(750) |
|
|
(1,500) |
|
| Net cash provided by/(used in) financing activities |
12 |
|
|
(2,459) |
|
|
|
|
|
| Effect of exchange rate changes on cash and equivalents |
(7) |
|
|
(1) |
|
|
|
|
|
|
|
|
|
| Net decrease in cash and equivalents |
(325) |
|
|
(727) |
|
| Cash and equivalents at beginning of period |
4,043 |
|
|
4,717 |
|
| Cash and equivalents at end of period |
$ |
3,718 |
|
|
$ |
3,990 |
|
See notes to condensed consolidated financial statements.
|
|
|
|
|
|
|
|
|
|
|
|
25 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
Notes to Condensed Consolidated Financial Statements
1. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation
Our condensed consolidated financial statements include the accounts of all majority-owned or consolidated subsidiaries, and all significant intercompany transactions and amounts have been eliminated. The results of businesses acquired or disposed of are included in the condensed consolidated financial statements from the date of the acquisition or up to the date of disposal, respectively.
References to "we," "our," and similar pronouns in this Quarterly Report on Form 10-Q for the quarter ended March 31, 2024 (this "Form 10-Q") are to Cardinal Health, Inc. and its majority-owned or consolidated subsidiaries unless the context requires otherwise.
Our fiscal year ends on June 30. References to fiscal 2024 and 2023 in these condensed consolidated financial statements are to the fiscal years ending or ended June 30, 2024 and June 30, 2023, respectively.
Our condensed consolidated financial statements have been prepared in accordance with the U.S. Securities and Exchange Commission ("SEC") instructions to Quarterly Reports on Form 10-Q and include the information and disclosures required by accounting principles generally accepted in the United States ("GAAP") for interim financial reporting. The preparation of financial statements in conformity with GAAP requires us to make estimates, judgments and assumptions that affect amounts reported in the condensed consolidated financial statements and accompanying notes. Actual amounts may differ from these estimated amounts.
In our opinion, all adjustments necessary for a fair presentation of the condensed consolidated financial statements have been included. Except as disclosed elsewhere in this Form 10-Q, all such adjustments are of a normal and recurring nature. In addition, financial results presented for this fiscal 2024 interim period are not necessarily indicative of the results that may be expected for the full fiscal year ending June 30, 2024. These condensed consolidated financial statements are unaudited and, accordingly, should be read in conjunction with the audited consolidated financial statements and related notes contained in our Annual Report on Form 10-K for the fiscal year ended June 30, 2023 (our "2023 Form 10-K").
Major Customers
On April 22, 2024, we announced that our pharmaceutical distribution contracts with OptumRx, which expire at the end of June 2024, will not be renewed. Sales to OptumRx generated 16% of our consolidated revenue in fiscal 2023.
Recently Issued Financial Accounting Standards
and Disclosure Rules Not Yet Adopted
We assess the adoption impacts of recently issued accounting standards by the Financial Accounting Standards Board ("FASB") on our condensed consolidated financial statements as well as material updates to previous assessments, if any, from our fiscal 2023 Form 10-K.
Segment Reporting
In November 2023, the FASB issued Accounting Standards Update ("ASU") 2023-07 Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which enhances reportable segment disclosure requirements, primarily through disclosures of significant segment expenses. This guidance will be effective for us in our fiscal 2025 Form 10-K and the guidance must be applied retrospectively to all prior periods presented. We are currently evaluating the impact of adoption of this guidance on our disclosures.
Income Tax Disclosure
In December 2023, the FASB issued ASU 2023-09 Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which enhances income tax disclosures primarily related to the rate reconciliation and income taxes paid information. This guidance also includes certain other amendments to improve the effectiveness of income tax disclosures. This guidance will be effective for us in our fiscal 2026 Form 10-K and should be applied on a prospective basis, with retrospective application permitted. We are currently evaluating the impact of adoption of this guidance on our disclosures.
Climate-Related Disclosures
In March 2024, the SEC issued final rules on climate-related disclosures that will require annual disclosure of material climate-related risks and material direct greenhouse gas emissions from operations owned or controlled (Scope 1) and material indirect greenhouse gas emissions from purchased energy consumed in owned or controlled operations (Scope 2). Additionally, the rules require disclosure in the notes to the financial statements of the effects of severe weather events and other natural conditions, subject to certain financial thresholds, as well as amounts related to carbon offsets and renewable energy credits or certificates. These rules also require disclosure of climate risk oversight practices of the Board of Directors and management, and the disclosure of governance, risk management and strategy related to material climate-related risks. In April 2024, the SEC voluntarily stayed the new rules pending the completion of judicial review. The disclosure requirements, if ultimately upheld as adopted, will begin phasing in for reports and registration statements including financial information with respect to annual periods beginning in our fiscal 2026. We are currently evaluating the impact of adoption of these final rules on our disclosures.
|
|
|
|
|
|
|
|
|
|
|
|
26 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
Recently Adopted Financial Accounting Standards
There were no new material accounting standards adopted during the nine months ended March 31, 2024.
Updated Segment Reporting Structure
Effective January 1, 2024, we operated under an updated organizational structure and re-aligned our reporting structure under two reportable segments: Pharmaceutical and Specialty Solutions segment and Global Medical Products and Distribution ("GMPD") segment. The remaining operating segments, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics, are not significant enough to require separate reportable disclosures and are included in Other. The Pharmaceutical and Specialty Solutions reportable segment consists of all businesses formerly within our Pharmaceutical segment, excluding Nuclear and Precision Health Solutions. The Global Medical Products and Distribution reportable segment consists of all businesses formerly within our Medical segment, excluding at-Home Solutions and OptiFreight® Logistics. Our previously reported segment results have been recast to conform to this re-aligned reporting structure and reflect changes in the elimination of inter-segment revenue and allocated corporate technology and shared function expenses, which are driven by the reporting structure change. See
Note 13 for segment results under the new reporting structure.
2. Acquisitions
On March 18, 2024, we completed the acquisition of Specialty Networks for a purchase price of $1.2 billion in cash, subject to certain adjustments. Specialty Networks creates clinical and economic value for providers and partners across multiple specialty group purchasing organizations ("GPOs"): UroGPO, Gastrologix and GastroGPO, and United Rheumatology.
Specialty Networks’ PPS Analytics platform analyzes data from electronic medical records, practice management, imaging, and dispensing systems and transforms it into meaningful and actionable insights for providers and other stakeholders by using artificial intelligence and modern data analytics capabilities. The acquisition further expands our offering in key therapeutic areas, accelerates our upstream data and research opportunities with biopharma manufacturers, and creates a platform for our expansion across therapeutic areas.
The pro forma results of operations and the results of operations for Specialty Networks have not been separately disclosed because the effects were not significant compared to the condensed consolidated financial statements.
Transaction costs associated with the Specialty Networks acquisition were $15 million during the three and nine months ended March 31, 2024, and are included in amortization and other acquisition-related costs in the condensed consolidated statements of earnings.
Fair Value of Assets Acquired and Liabilities Assumed
The allocation of the purchase price for the acquisition of Specialty Networks is not yet finalized and is subject to adjustment as we complete the valuation analysis of the acquisition. The purchase price is also subject to adjustment based on working capital requirements as set forth in the acquisition agreement.
The valuation of identifiable intangible assets utilizes significant unobservable inputs and thus represents a Level 3 nonrecurring fair value measurement. The estimated fair value of the identifiable intangible assets was determined using an income-based approach, which includes market participant expectations of the cash flows that an asset could generate over its remaining useful life, discounted back to present value using an appropriate rate of return. The discount rate used to arrive at the present value of the identifiable intangible assets was 10 percent, and reflects the internal rate of return and uncertainty in the cash flow projections.
The following table summarizes the estimated fair values of the assets acquired and liabilities assumed as of the acquisition date for Specialty Networks:
|
|
|
|
|
|
| (in millions) |
Specialty Networks |
Identifiable intangible assets: |
|
Customer relationships (1) |
$ |
404 |
|
Trade names (2) |
15 |
|
Developed technology (3) |
20 |
|
|
|
Total identifiable intangible assets acquired |
439 |
|
|
|
Identifiable net assets/(liabilities): |
|
Cash and equivalents |
23 |
|
Trade receivables, net |
19 |
|
|
|
Prepaid expenses and other |
2 |
|
|
|
|
|
|
|
Other accrued liabilities |
(15) |
|
Deferred income taxes and other liabilities |
(109) |
|
Total identifiable net assets/(liabilities) acquired |
359 |
|
Goodwill |
856 |
|
Total net assets acquired |
$ |
1,215 |
|
(1) The weighted-average useful life of customer relationships is 14 years.
(2) The weighted-average useful life of trade names is 8 years.
(3) The weighted-average useful life of developed technology is 9 years.
|
|
|
|
|
|
|
|
|
|
|
|
27 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
3. Divestitures
On June 5, 2023 we signed a definitive agreement to contribute the OutcomesTM business to Transaction Data Systems ("TDS"), a portfolio company of BlackRock Long Term Private Capital and GTCR, in exchange for a 16 percent equity interest in the combined entity. The transaction closed on July 10, 2023 and we recognized a pre-tax gain of $53 million during the nine months ended March 31, 2024, which was included in impairments and (gain)/loss on disposal of assets, net in our condensed consolidated statements of earnings. This gain includes our initial recognition of an equity method investment in the combined entity for $147 million.
We determined that the divestiture of the OutcomesTM business does not meet the criteria to be classified as discontinued operations. The OutcomesTM business operated within our former Pharmaceutical segment.
4. Restructuring and Employee Severance
The following tables summarize restructuring and employee severance:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Employee-related |
$ |
36 |
|
|
$ |
3 |
|
| Facility exit and other |
17 |
|
|
13 |
|
| Total restructuring and employee severance |
$ |
53 |
|
|
$ |
16 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Employee-related |
$ |
51 |
|
|
$ |
32 |
|
| Facility exit and other |
55 |
|
|
30 |
|
| Total restructuring and employee severance |
$ |
106 |
|
|
$ |
62 |
|
Employee-related costs primarily consist of termination benefits provided to employees who have been involuntarily terminated, duplicate payroll costs and retention bonuses incurred during transition periods. Facility exit and other costs primarily consist of project consulting fees, accelerated depreciation, professional, project management and other service fees to support divestitures, costs associated with vacant facilities, and certain other divestiture-related costs.
Restructuring and employee severance costs during the three and nine months ended March 31, 2024 and 2023 include costs related to the implementation of certain enterprise-wide cost-savings measures, which include certain initiatives to rationalize our manufacturing operations. The increase in restructuring costs during the three and nine months ended March 31, 2024 was primarily due to these initiatives and certain projects resulting from the review of our strategy portfolio, capital-allocation framework and operations. During the three and nine months ended March 31, 2023, restructuring and employee severance costs also included costs related to the divestiture of the Cordis business.
The following table summarizes activity related to liabilities associated with restructuring and employee severance:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Employee-
Related Costs |
|
Facility Exit
and Other Costs |
|
Total |
| Balance at June 30, 2023 |
$ |
44 |
|
|
$ |
2 |
|
|
$ |
46 |
|
| Additions |
38 |
|
|
13 |
|
|
51 |
|
| Payments and other adjustments |
(21) |
|
|
(6) |
|
|
(27) |
|
| Balance at March 31, 2024 |
$ |
61 |
|
|
$ |
9 |
|
|
$ |
70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
28 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
5. Goodwill and Other Intangible Assets
Goodwill
The following table summarizes the changes in the carrying amount of goodwill by segment and in total:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Pharmaceutical and Specialty Solutions |
|
Global Medical Products and Distribution (1) |
|
Other (2) (3) |
|
Total |
Balance at June 30, 2023 (4) |
$ |
2,762 |
|
|
$ |
677 |
|
|
$ |
1,170 |
|
|
$ |
4,609 |
|
| Goodwill acquired, net of purchase price adjustments |
856 |
|
|
(3) |
|
|
— |
|
|
853 |
|
| Foreign currency translation adjustments and other |
— |
|
|
(3) |
|
|
— |
|
|
(3) |
|
| Goodwill impairment |
— |
|
|
(671) |
|
|
— |
|
|
(671) |
|
| Balance at March 31, 2024 |
$ |
3,618 |
|
|
$ |
— |
|
|
$ |
1,170 |
|
|
$ |
4,788 |
|
(1) Prior-period goodwill impairment charges related to the former Medical segment were allocated to the GMPD segment. At March 31, 2024 and June 30, 2023, the GMPD segment accumulated goodwill impairment loss was $5.4 billion and $4.7 billion, respectively.
(2) Comprised of the remaining operating segments, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics.
(3) Reflects $48 million allocated to OptiFreight® Logistics.
(4) Reflects a $110 million reclassification between Pharmaceutical and Specialty Solutions and Other, which does not impact our previously reported condensed consolidated financial statements or results of our impairment tests.
The increase in the Pharmaceutical and Specialty Solutions segment goodwill is due to the Specialty Networks acquisition. Goodwill recognized in connection with this acquisition primarily represents the expected benefits from anticipated growth from new technology capabilities, synergies of integrating this business and the existing workforce of the acquired entity.
As a result of the segment change discussed in
Note 1, we allocated $90 million and $48 million of goodwill from the former Medical Unit to GMPD and OptiFreight® Logistics, respectively, based on the estimated relative fair values of the reporting units. We also assessed goodwill for impairment for these reporting units before and after the reallocation and determined there was no impairment for the Medical Unit and OptiFreight® Logistics during the three months ended March 31, 2024 as their fair values substantially exceeded their carrying values. However, the quantitative test resulted in an impairment of GMPD’s remaining goodwill balance of $90 million.
Our determination of the estimated fair value of the GMPD reporting unit is based on a combination of the income-based approach (using a discount rate of 11 percent and a terminal growth rate of 2 percent), and market-based approaches. Additionally, we assigned a weighting of 80 percent to the discounted cash flow method, 10 percent to the guideline public
company method, and 10 percent to the guideline transaction method.
During the three months ended December 31, 2023, we did not identify any indicators of impairment within our reporting units. During the three months ended September 30, 2023, we elected to bypass the qualitative assessment and perform quantitative goodwill impairment testing for the former Medical Unit due to an increase in the risk-free interest rate used in the discount rate. The carrying amount exceeded the fair value, which resulted in a pre-tax impairment charge of $581 million for the former Medical Unit, which was recognized during the three months ended September 30, 2023 and is included in impairments and (gain)/loss on disposal of assets, net in our condensed consolidated statements of earnings. This impairment charge was driven by an increase of 1 percent in the discount rate primarily due to an increase in the risk-free interest rate.
During the three months ended December 31, 2022 and September 30, 2022, we performed quantitative goodwill impairment testing for the former Medical Unit. This quantitative testing resulted in the carrying amount of the former Medical Unit exceeding the fair value, resulting in pre-tax goodwill impairment charges of $709 million and $154 million recorded during the three months ended December 31, 2022 and September 30, 2022, respectively. We also performed interim quantitative goodwill impairment testing at March 31, 2023 and concluded that there was no impairment as of March 31, 2023 as the estimated fair value of the former Medical Unit exceeded its carrying value by approximately 4 percent. The impairment charge recognized in the second quarter of fiscal 2023 was driven by certain reductions in our long-term financial plan assumptions, and the impairment charge recognized in the first quarter of fiscal 2023 was driven by an increase in the discount rate primarily due to an increase in the risk-free interest rate.
|
|
|
|
|
|
|
|
|
|
|
|
29 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
Other Intangible Assets
The following tables summarize other intangible assets by class at:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
| (in millions) |
Gross
Intangible |
|
Accumulated
Amortization |
|
Net
Intangible |
|
Weighted- Average Remaining Amortization Period (Years) |
| Indefinite-life intangibles: |
|
|
|
|
|
|
|
| Trademarks and patents |
$ |
11 |
|
|
$ |
— |
|
|
$ |
11 |
|
|
N/A |
| Total indefinite-life intangibles |
11 |
|
|
— |
|
|
11 |
|
|
N/A |
|
|
|
|
|
|
|
|
| Definite-life intangibles: |
|
|
|
|
|
|
|
| Customer relationships |
3,564 |
|
|
2,387 |
|
|
1,177 |
|
|
10 |
| Trademarks, trade names and patents |
561 |
|
|
401 |
|
|
160 |
|
|
7 |
| Developed technology and other |
1,043 |
|
|
672 |
|
|
371 |
|
|
7 |
| Total definite-life intangibles |
5,168 |
|
|
3,460 |
|
|
1,708 |
|
|
9 |
| Total other intangible assets |
$ |
5,179 |
|
|
$ |
3,460 |
|
|
$ |
1,719 |
|
|
N/A |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
| (in millions) |
Gross
Intangible |
|
Accumulated
Amortization |
|
Net
Intangible |
| Indefinite-life intangibles: |
|
|
|
|
|
| Trademarks and patents |
$ |
11 |
|
|
$ |
— |
|
|
$ |
11 |
|
| Total indefinite-life intangibles |
11 |
|
|
— |
|
|
11 |
|
|
|
|
|
|
|
| Definite-life intangibles: |
|
|
|
|
|
| Customer relationships |
3,174 |
|
|
2,274 |
|
|
900 |
|
| Trademarks, trade names and patents |
546 |
|
|
380 |
|
|
166 |
|
| Developed technology and other |
1,021 |
|
|
626 |
|
|
395 |
|
| Total definite-life intangibles |
4,741 |
|
|
3,280 |
|
|
1,461 |
|
| Total other intangible assets |
$ |
4,752 |
|
|
$ |
3,280 |
|
|
$ |
1,472 |
|
The increase in definite-life intangibles is due to the Specialty Networks acquisition. Total amortization of intangible assets was $64 million and $69 million for the three months ended March 31, 2024 and 2023, respectively, and $191 million and $211 million for the nine months ended March 31, 2024 and 2023, respectively. Estimated annual amortization of intangible assets for the remainder of fiscal 2024 through 2028 is as follows: $69 million, $256 million, $241 million, $213 million and $186 million.
6. Long-Term Obligations and Other Short-Term Borrowings
The following table summarizes long-term obligations and other short-term borrowings at:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) (1) |
March 31, 2024 |
|
June 30, 2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 3.079% Notes due 2024 |
$ |
753 |
|
|
$ |
764 |
|
| 3.5% Notes due 2024 |
402 |
|
|
404 |
|
| 3.75% Notes due 2025 |
508 |
|
|
513 |
|
| 3.41% Notes due 2027 |
1,192 |
|
|
1,184 |
|
| 5.125% Notes due 2029 |
644 |
|
|
— |
|
| 5.45% Notes due 2034 |
494 |
|
|
— |
|
| 4.6% Notes due 2043 |
311 |
|
|
306 |
|
| 4.5% Notes due 2044 |
330 |
|
|
331 |
|
| 4.9% Notes due 2045 |
428 |
|
|
428 |
|
| 4.368% Notes due 2047 |
564 |
|
|
561 |
|
| 7.0% Debentures due 2026 |
124 |
|
|
124 |
|
| Other Obligations |
104 |
|
|
86 |
|
| Total |
5,854 |
|
|
4,701 |
|
| Less: current portion of long-term obligations and other short-term borrowings |
1,187 |
|
|
792 |
|
| Long-term obligations, less current portion |
$ |
4,667 |
|
|
$ |
3,909 |
|
(1) Maturities are presented on a calendar year basis.
Maturities of existing long-term obligations and other short-term borrowings for the remainder of fiscal 2024 through 2028 and thereafter are as follows: $762 million, $436 million, $535 million, $1.3 billion, $11 million and $2.8 billion.
Long-Term Debt
We had total long-term obligations, including the current portion and other short-term borrowings, of $5.9 billion and $4.7 billion at March 31, 2024 and June 30, 2023, respectively. All the notes represent unsecured obligations of Cardinal Health, Inc. and rank equally in right of payment with all of our existing and future unsecured and unsubordinated indebtedness. Interest is paid pursuant to the terms of the obligations. These notes are effectively subordinated to the liabilities of our subsidiaries, including trade payables of $32.1 billion and $29.8 billion at March 31, 2024 and June 30, 2023, respectively.
In February 2024, we issued additional debt with the aggregate principal amount of $1.15 billion to fund the repayment of all of the aggregate principal amount outstanding of our 3.5% Notes due 2024 and 3.079% Notes due 2024, at their respective maturities, and for general corporate purposes. The notes issued are $650 million aggregate principal amount of 5.125% Notes that mature on February 15, 2029 and $500 million aggregate principal amount of 5.45% Notes that mature on February 15, 2034. The proceeds of the notes issued, net of discounts, premiums, and debt issuance costs, were $1.14 billion. A portion of the proceeds were invested in short-term time deposits of $550 million with initial effective maturities of more than three months and classified as prepaid
|
|
|
|
|
|
|
|
|
|
|
|
30 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
expenses and other in our condensed consolidated balance sheets as of March 31, 2024. The remaining proceeds of $589 million were invested in short-term time deposits classified as cash and equivalents in our condensed consolidated balance sheets as of March 31, 2024.
If we undergo a change of control, as defined in the notes, and if the notes receive specified ratings below investment grade by each of Standard & Poor's Ratings Services, Moody’s Investors Services and Fitch Ratings, any holder of the notes, excluding the debentures, can require with respect to the notes owned by such holder, or we can offer, to repurchase the notes at 101% of the principal amount plus accrued and unpaid interest.
Other Financing Arrangements
In addition to cash and equivalents and operating cash flow, other sources of liquidity include a $2.0 billion commercial paper program backed by a $2.0 billion revolving credit facility. We also have a $1.0 billion committed receivables sales facility. During the nine months ended March 31, 2024, under our commercial paper program and our committed receivables program, we had maximum combined total daily amounts outstanding of $1.3 billion and an average combined daily amount outstanding of $25 million. At March 31, 2024, we had no amounts outstanding under our commercial paper program, revolving credit facility or our committed receivables sales facility.
In February 2023, we extended our $2.0 billion revolving credit facility through February 25, 2028. In September 2022, we renewed our committed receivables sales facility program through Cardinal Health Funding, LLC (“CHF”) through September 30, 2025. In September 2023, Cardinal Health 23 Funding, LLC ("CH-23 Funding") was added as a seller under our committed receivables sales facility. Each of CHF and CH-23 Funding was organized for the sole purpose of buying receivables and selling undivided interests in those receivables to third-party purchasers. Although consolidated with Cardinal Health, Inc. in accordance with GAAP, each of CHF and CH-23 Funding is a separate legal entity from Cardinal Health, Inc. and from our respective subsidiary that sells receivables to CHF or CH-23 Funding, as applicable. Each of CHF and CH-23 Funding is designed to be a special purpose, bankruptcy-remote entity whose assets are available solely to satisfy the claims of its respective creditors.
Our revolving credit and committed receivables sales facilities require us to maintain a consolidated net leverage ratio of no more than 3.75-to-1. As of March 31, 2024, we were in compliance with this financial covenant.
7. Commitments, Contingent Liabilities and Litigation
Commitments
Generic Sourcing Venture with CVS Health
In July 2014, we established Red Oak Sourcing, LLC ("Red Oak Sourcing"), a U.S.-based generic pharmaceutical sourcing venture with CVS Health for an initial term of 10 years. Red Oak Sourcing negotiates generic pharmaceutical supply contracts on behalf of its participants. In August 2021, we amended our agreement to extend the term through June 2029. We are required to make quarterly payments to CVS Health for the term of the arrangement.
Contingencies
New York Opioid Stewardship Act
In April 2018, the State of New York passed a budget which included the Opioid Stewardship Act (the "OSA"). The OSA created an aggregate $100 million annual assessment on all manufacturers and distributors licensed to sell or distribute opioids in New York. Under the OSA, each licensed manufacturer and distributor would be required to pay a portion of the assessment based on its share of the total morphine milligram equivalents sold or distributed in New York during the applicable calendar year, beginning in 2017. Subsequently, New York passed a new statute that modified the assessment going forward and limited the OSA to two years (2017 and 2018).
We accrue contingencies if it is probable that a liability has been incurred and the amount can be estimated. During the fiscal year 2023, we recorded $6 million of income to reduce the previously estimated accrual to the invoiced amount for the calendar year 2018 assessment. At June 30, 2023, we had an outstanding liability of $3 million, which was paid in full during the first quarter of fiscal year 2024.
Legal Proceedings
We become involved from time to time in disputes, litigation and regulatory matters.
From time to time, we determine that products we distribute, source, manufacture or market do not meet our specifications, regulatory requirements, or published standards. When we or a regulatory agency identify a potential quality or regulatory issue, we investigate and take appropriate corrective action. Such actions have led to product recalls, costs to repair or replace affected products, temporary interruptions in product sales, restrictions on importation, product liability claims and lawsuits and can lead to action by regulators. Even absent an identified regulatory or quality issue or product recall, we can become subject to product liability claims and lawsuits.
From time to time, we become aware through employees, internal audits or other parties of possible compliance matters, such as complaints or concerns relating to accounting, internal accounting controls, financial reporting, auditing, or other ethical matters or relating to compliance with laws such as healthcare fraud and
|
|
|
|
|
|
|
|
|
|
|
|
31 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
abuse, anti-corruption or anti-bribery laws. When we become aware of such possible compliance matters, we investigate internally and take appropriate corrective action. In addition, from time to time, we receive subpoenas or requests for information from various federal or state agencies relating to our business or to the business of a customer, supplier or other industry participants. Internal investigations, subpoenas or requests for information could directly or indirectly lead to the assertion of claims or the commencement of legal proceedings against us or result in sanctions.
We have been named from time to time in qui tam actions initiated by private third parties. In such actions, the private parties purport to act on behalf of federal or state governments, allege that false claims have been submitted for payment by the government and may receive an award if their claims are successful. After a private party has filed a qui tam action, the government must investigate the private party's claim and determine whether to intervene in and take control over the litigation. These actions may remain under seal while the government makes this determination. If the government declines to intervene, the private party may nonetheless continue to pursue the litigation on his or her own purporting to act on behalf of the government.
We accrue for contingencies related to disputes, litigation and regulatory matters if it is probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Because these matters are inherently unpredictable and unfavorable developments or resolutions can occur, assessing contingencies is highly subjective and requires judgments about future events. We regularly review contingencies to determine whether our accruals and related disclosures are adequate. The amount of ultimate loss may differ from these estimates.
We recognize income from the favorable outcome of litigation when we receive the associated cash or assets.
We recognize estimated loss contingencies for certain litigation and regulatory matters and income from favorable resolution of litigation in litigation (recoveries)/charges, net in our condensed consolidated statements of earnings; however, losses and recoveries of lost profits from disputes that occur in the ordinary course of business are included within segment profit.
Opioid Lawsuits and Investigations
Cardinal Health, other Pharmaceutical wholesalers and other participants in the pharmaceutical supply chain have been named as a defendant in lawsuits related to the distribution of opioid pain medications. These lawsuits seek equitable relief and monetary damages based on a variety of legal theories, including various common law claims, such as public nuisance, negligence, unjust enrichment, personal injury, as well as violations of controlled substance laws, the Racketeer Influenced and Corrupt Organization Act and various other statutes. Plaintiffs in these lawsuits include governmental entities as well as private parties, such as unions and other health and welfare funds, hospital
system and other healthcare providers, businesses and individuals.
Additionally, we have received federal grand jury subpoenas issued in connection with investigations being conducted by the U.S. Attorney's Office for the Eastern District of New York and the Fraud Section of the U.S. Department of Justice ("DOJ"). We have also received civil requests for information, subpoenas and other requests from other DOJ offices. These investigations concern operation of our anti-diversion program, our anti-diversion policies and procedures and distribution of certain controlled substances. We are cooperating with these investigations. We are unable to predict the outcomes of any of these investigations.
In total, as of March 31, 2024, we have $5.3 billion accrued for these matters, of which $438 million is included in other accrued liabilities and the remainder is included in deferred income taxes and other liabilities in our condensed consolidated balance sheets. During the three months ended March 31, 2024, we recognized expense of $193 million in connection with opioid-related matters, which was partially offset by a benefit of $105 million related to prepayments at a prenegotiated discount of certain future payments totaling $344 million.
Because loss contingencies are inherently unpredictable and unfavorable developments or resolutions can occur, the assessment is highly subjective and requires judgements about future events. We regularly review these opioid litigation matters to determine whether our accrual is adequate. The amount of ultimate loss may differ materially from this accrual, whether as a result of settlement discussions, a judicial decision or verdict or otherwise, but we are not able to estimate a range of reasonably possible additional losses for these matters. We continue to strongly dispute the allegations made in these lawsuits and none of the settlements described below is an admission of liability or wrong-doing. Please see below for additional description of these matters.
States & Political Subdivisions
In February 2022, we along with two other national distributors (collectively, the "Distributors") independently approved a settlement agreement (the "National Opioid Settlement Agreement") to settle the vast majority of opioid lawsuits and claims brought by states and political subdivisions. This National Opioid Settlement Agreement became effective on April 2, 2022. In addition to the Distributors, parties to the National Opioid Settlement Agreement include 48 states, the District of Columbia and 5 U.S. territories. Over 99 percent of political subdivisions in settling states (by population as calculated under the National Opioid Settlement Agreement) that had brought opioid-related suits against us have chosen to join the National Opioid Settlement Agreement or have had their claims addressed by state legislation (together with settling states and territories, the "Settling Governmental Entities").
In February 2024, we finalized an agreement with the Alabama Attorney General, under which we agreed to pay approximately
|
|
|
|
|
|
|
|
|
|
|
|
32 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
$123 million to the State of Alabama over a period of ten years to resolve opioid-related claims brought by the State and its political subdivisions. This agreement is subject to certain contingencies, including subdivision participation. During the nine months ended March 31, 2024, we recognized a $22 million charge in litigation (recoveries)/charge, net in the condensed consolidated statements of earnings related to this agreement.
Through April 2024, we have paid the Settling Governmental Entities approximately $1.5 billion, which includes the January 2024 prepayment of certain future payment amounts described below. We expect to pay Settling Governmental Entities additional amounts up to $4.8 billion through 2038. The National Opioid Settlement Agreement also includes injunctive relief terms related to Distributors' controlled substance anti-diversion programs. A monitor is overseeing compliance with these provisions until 2027. In addition, the Distributors are engaging a third-party vendor to act as a clearinghouse for data aggregation and reporting, which Distributors will fund for 10 years. As a result of the National Opioid Settlement Agreement, the vast majority of lawsuits brought against us by State and other political subdivisions have been dismissed. We continue to engage in resolution discussions with certain nonparticipating political subdivisions. A trial in the case brought by the city of Baltimore, which is the largest remaining nonparticipating subdivision by population, is scheduled to begin in September 2024. We intend to defend ourselves vigorously against all remaining lawsuits.
Other Settlements
West Virginia subdivisions and Native American tribes were not a part of the National Opioid Settlement Agreement, and we had separate settlement negotiations with these groups. In July 2022, a judgment in favor of the Distributors was entered in bench trial before a federal judge in West Virginia in a case brought by Cabell County and City of Huntington. Plaintiffs have appealed this decision to the Fourth Circuit Court of Appeals. In July 2022, the Distributors reached an agreement to settle the opioid-related claims of the majority of the remaining West Virginia subdivisions. Under this agreement, we have agreed to pay eligible West Virginia subdivisions up to approximately $124 million over an eleven-year period. This agreement became effective in October 2022 when all participating subdivisions dismissed their cases.
In October 2022, we executed a final settlement agreement with the Native American tribes, pursuant to which we will pay up to approximately $136 million over five years. In connection with this settlement, the court entered dismissals for the Native American tribes' cases.
Prepayment of Future Payment Years
In January 2024, we made payments of approximately $239 million to prepay at a prenegotiated discount of certain future payment amounts totaling approximately $344 million owed under each of the National Opioid Settlement Agreement, West Virginia Subdivisions Settlement Agreement and settlement agreements with Native American tribes and Cherokee Nation. The majority of
the prepayment relates to the seventh annual payment as due under the National Opioid Settlement Agreement. As a result of these prepayments, we recognized income of approximately $105 million in litigation charges/(recoveries), net in our condensed consolidated statements of earnings during the three months ended March 31, 2024.
Private Plaintiffs
The National Opioid Settlement Agreement does not address claims by private parties, which includes unions and other health and welfare funds, hospital systems and other healthcare providers, businesses and individuals alleging personal injury. Lawsuits brought by private plaintiffs that were pending as of April 26, 2024 were approximately 380. Of these, 103 are purported class actions. The causes of action asserted by these plaintiffs are similar to those asserted by public plaintiffs. We are engaged in resolution discussions with certain private plaintiffs and have reached agreements in principle with counsel representing classes of third-party payors and acute care hospitals and have accrued $213 million in connection with those matters, which represents our anticipated share of those possible settlements. The agreements in principle remain subject to contingencies. We are vigorously defending ourselves in all these matters.
A trial in a case involving 21 plaintiffs began in state court in Georgia in January 2023 and concluded in March 2023 with a verdict for the company and other defendants on all claims. In July 2023, the judge denied the plaintiffs' motion for a new trial. Plaintiffs have filed a notice of appeal and defendants have filed a notice of cross-appeal. A trial involving eight hospital plaintiffs is scheduled to begin in Alabama in July 2024.
Insurance Litigation
We are involved in ongoing legal proceedings with insurers related to their obligations to reimburse us for defense and indemnity costs in connection with the lawsuits described above. During fiscal year 2023, we received approximately $10 million in insurance recoveries related to these matters.
Department of Justice Civil Investigative Demand
In November 2023, we received a Civil Investigative Demand ("CID") from the Department of Justice focused on potential violations of the Anti-Kickback Statute and False Claims Act in connection with a 2022 transaction in which we purchased a minority ownership interest in a rheumatology managed services organization and a group purchasing organization. We are cooperating with this investigation.
Cordis IVC Filter Matters
We have been named as a defendant in approximately 400 product liability lawsuits coordinated in Alameda County Superior Court in California involving claims by approximately 4,500 plaintiffs that allege personal injuries associated with the use of inferior vena cava ("IVC") filter products. These lawsuits sought a variety of remedies, including unspecified monetary damages. The divestiture of the Cordis business did not include product liability
|
|
|
|
|
|
|
|
|
|
|
|
33 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
related to the IVC filters in the U.S. and Canada, which we retained.
In April 2023, we executed a settlement agreement that, if certain conditions are satisfied, will resolve 4,375 claims for $275 million. This settlement agreement is subject to certain conditions, including certain opt-in thresholds. Between May and September 2023, we made settlement payments totaling $275 million into a qualified settlement fund, which will be disbursed to the plaintiffs if required conditions are satisfied. Since July 2021, we have also entered into other agreements to settle approximately 2,800 product liability claims. While these settlements will resolve the vast majority of IVC filter product liability claims, they will not resolve all of them and we intend to continue to vigorously defend ourselves in the remaining lawsuits.
We recognized income of $103 million during fiscal year 2023, primarily related to a reduction of the reserve for the estimated settlement and defense costs for these matters due to the execution of the settlements noted above. At March 31, 2024, we had a total of $296 million accrued for losses and legal defense costs, related to the IVC filter product liability lawsuits in our condensed consolidated balance sheets.
Other Civil Litigation
Generic Pharmaceutical Pricing Antitrust Litigation
In December 2019, pharmaceutical distributors including us were added as defendants in a civil class action lawsuit filed by indirect purchasers of generic drugs, such as hospitals and retail pharmacies. The indirect purchaser case is part of a multidistrict litigation consisting of multiple individual class action matters consolidated in the Eastern District of Pennsylvania. The indirect purchaser plaintiffs allege that pharmaceutical distributors encouraged manufacturers to increase prices, provided anti-competitive pricing information to manufacturers and improperly engaged in customer allocation. In May 2020, the court granted our motion to dismiss. In July 2022, the indirect purchasers filed an amended complaint and in August 2022, we filed a motion to dismiss the amended complaint. We are vigorously defending ourselves in this matter.
Antitrust Litigation Proceeds
We recognized income for net recoveries in class action antitrust lawsuits in which we were a class member or plaintiff of $6 million and $77 million during the three and nine months ended March 31, 2024, respectively, and $66 million during the nine months ended March 31, 2023.
8. Income Taxes
Fluctuations in our provision for income taxes as a percentage of pre-tax earnings (“effective tax rate”) are due to changes in international and U.S. state effective tax rates resulting from our business mix and discrete items.
Effective Tax Rate
During the three and nine months ended March 31, 2024, the effective tax rate was 24.2 percent and 23.2 percent, respectively, and reflects any impact of the tax effects of the goodwill impairment charges recognized during the three and nine months ended March 31, 2024.
During the three and nine months ended March 31, 2023, the effective tax rate was 36.3 percent and 36.7 percent, respectively, and reflects the impact of the tax effect of the goodwill impairment charges recognized during the nine months ended March 31, 2023.
Tax Effects of Goodwill Impairment Charges
During the nine months ended March 31, 2024, we recognized pre-tax goodwill impairment charges of $671 million. The net tax benefit related to these charges is $56 million for fiscal 2024.
Unless an item is considered discrete because it is unusual or infrequent, the tax impact of the item is included in our estimated annual effective tax rate. When items are recognized through our estimated annual effective tax rate, we apply our estimated annual effective tax rate to the earnings before income taxes for the year-to-date period to compute our impact from income taxes for the current quarter and year-to-date period. The tax impacts of discrete items are recognized in their entirety in the period in which they occur.
The tax effect of the goodwill impairment charges during the nine months ended March 31, 2024 was included in our estimated annual effective tax rate because it was not considered unusual or infrequent, given that we recorded goodwill impairments in prior fiscal years. The impact of the non-deductible goodwill increased the estimated annual effective tax rate for fiscal 2024. Applying the higher tax rate to the pre-tax income for the nine months ended March 31, 2024 resulted in recognizing an incremental interim tax benefit of approximately $36 million, which impacted the provision for income taxes in the condensed consolidated statements of earnings during the nine months ended March 31, 2024 and prepaid expenses and other assets in the condensed consolidated balance sheet at March 31, 2024. The incremental interim tax benefit will reverse in the fourth quarter of fiscal 2024.
Unrecognized Tax Benefits
We had $937.0 million and $1.0 billion of unrecognized tax benefits, at March 31, 2024 and June 30, 2023, respectively. The March 31, 2024 and June 30, 2023 balances include $850 million and $873 million of unrecognized tax benefits, respectively, that if recognized, would have an impact on the effective tax rate.
At March 31, 2024 and June 30, 2023, we had $57 million and $65 million, respectively, accrued for the payment of interest and
|
|
|
|
|
|
|
|
|
|
|
|
34 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
penalties related to unrecognized tax benefits, which we recognize in the benefit from income taxes in the condensed consolidated statements of earnings. These balances are gross amounts before any tax benefits and are included in deferred income taxes and other liabilities in the condensed consolidated balance sheets.
It is reasonably possible that there could be a change in the amount of unrecognized tax benefits within the next 12 months due to activities of the U.S. Internal Revenue Service ("IRS") or other taxing authorities, possible settlement of IRS and other audit issues, reassessment of existing unrecognized tax benefits or the expiration of statutes of limitations. We estimate that the range of the possible change in unrecognized tax benefits within the next 12 months is between zero and a net decrease of up to $20 million, exclusive of penalties and interest.
Other Tax Matters
We file income tax returns in the U.S. federal jurisdiction, various U.S. state jurisdictions, and various foreign jurisdictions. With few exceptions, we are subject to audit by taxing authorities for fiscal years 2015 through the current fiscal year.
We are a party to a tax matters agreement with CareFusion Corporation ("CareFusion"), a subsidiary of Becton, Dickinson and Company. Under the tax matters agreement, CareFusion is obligated to indemnify us for certain tax exposures and transaction taxes prior to our fiscal 2010 spin-off of CareFusion. In December 2023, the estimated tax exposure was updated to reflect adjustments based on settlement discussions with the IRS. Additionally, Cardinal received a partial payment from CareFusion to be applied towards the anticipated liability. The indemnification receivable was $20 million and $82 million at March 31, 2024 and June 30, 2023, respectively, and is included in other assets in the condensed consolidated balance sheets.
9. Fair Value Measurements
The following tables present the fair values for assets and (liabilities) measured on a recurring basis at:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
March 31, 2024 |
| (in millions) |
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
| Assets: |
|
|
|
|
|
|
|
| Cash equivalents |
$ |
2,013 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
2,013 |
|
|
|
|
|
|
|
|
|
| Other investments (1) |
105 |
|
|
— |
|
|
— |
|
|
105 |
|
|
|
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
|
|
|
| Forward contracts (2) |
— |
|
|
(70) |
|
|
— |
|
|
(70) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2023 |
| (in millions) |
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
| Assets: |
|
|
|
|
|
|
|
| Cash equivalents |
$ |
1,253 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
1,253 |
|
|
|
|
|
|
|
|
|
| Other investments (1) |
101 |
|
|
— |
|
|
— |
|
|
101 |
|
|
|
|
|
|
|
|
|
| Liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Forward contracts (2) |
— |
|
|
(73) |
|
|
— |
|
|
(73) |
|
(1)The other investments balance includes investments in mutual funds, which offset fluctuations in deferred compensation liabilities. These mutual funds invest in the equity securities of companies with both large and small market capitalization and high quality fixed income debt securities. The fair value of these investments is determined using quoted market prices.
(2)The fair value of interest rate swaps, foreign currency contracts, and net investment hedges is determined based on the present value of expected future cash flows considering the risks involved, including non-performance risk, and using discount rates appropriate for the respective maturities. Observable Level 2 inputs are used to determine the present value of expected future cash flows. The fair value of these derivative contracts, which are subject to master netting arrangements under certain circumstances, is presented on a gross basis in prepaid expenses and other, other assets, other accrued liabilities, and deferred income taxes and other liabilities within the condensed consolidated balance sheets.
Assets Measured on a Nonrecurring Basis
As discussed further in
Note 3, on July 10, 2023, we closed the transaction to contribute the Outcomes™ business to TDS, a portfolio company of BlackRock Long Term Private Capital and GTCR, in exchange for a 16 percent equity interest in the combined entity. We accounted for this investment initially at its fair value using Level 3 unobservable inputs under the discounted cash flow method. Accordingly, we recognized a $147 million equity method investment at closing during the first quarter of fiscal 2024.
10. Financial Instruments
We utilize derivative financial instruments to manage exposure to certain risks related to our ongoing operations. The primary risks managed through the use of derivative instruments include interest rate risk, currency exchange risk and commodity price risk. We do not use derivative instruments for trading or speculative purposes. While the majority of our derivative instruments are designated as hedging instruments, we also enter into derivative instruments that are designed to hedge a risk but are not designated as hedging instruments. These derivative instruments are adjusted to current
|
|
|
|
|
|
|
|
|
|
|
|
35 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
fair value through earnings at the end of each period. We are exposed to counterparty credit risk on all of our derivative instruments. Accordingly, we have established and maintain strict counterparty credit guidelines and only enter into derivative instruments with major financial institutions that are rated investment grade or better. We do not have significant exposure to any one counterparty and we believe the risk of loss is remote. Additionally, we do not require collateral under these agreements.
Interest Rate Risk Management
We are exposed to the impact of interest rate changes. Our objective is to manage the impact of interest rate changes on cash flows and the market value of our borrowings. We utilize a mix of debt maturities on our fixed-rate debt to manage changes in interest rates. In addition, we enter into interest rate swaps to further manage our exposure to interest rate variations related to our borrowings and to lower our overall borrowing costs.
Currency Exchange Risk Management
We conduct business in several major international currencies and are subject to risks associated with changing foreign exchange rates. Our objective is to reduce earnings and cash flow volatility associated with foreign exchange rate changes to allow management to focus its attention on business operations. Accordingly, we enter into various contracts that change in value as foreign exchange rates change to protect the value of existing foreign currency assets and liabilities, commitments and anticipated foreign currency revenue and expenses.
Commodity Price Risk Management
We are exposed to changes in the price of certain commodities. Our objective is to reduce earnings and cash flow volatility associated with forecasted purchases of these commodities to allow management to focus its attention on business operations. Accordingly, we enter into derivative contracts when possible to manage the price risk associated with certain forecasted purchases.
Fair Value Hedges
We enter into pay-floating interest rate swaps to hedge the changes in the fair value of fixed-rate debt resulting from fluctuations in interest rates. These contracts are designated and qualify as fair value hedges. Accordingly, the gain or loss recorded on the pay-floating interest rate swaps is directly offset by the change in fair value of the underlying debt. Both the derivative instrument and the underlying debt are adjusted to market value at the end of each period with any resulting gain or loss recorded in interest expense, net in the condensed consolidated statements of earnings. For the three and nine months ended March 31, 2024 and 2023, there were no gains or losses recorded to interest expense as changes in the market value of our derivative instruments offset changes in the market value of the underlying debt.
During the nine months ended March 31, 2024 and 2023, we entered into pay-floating interest rate swaps with total notional amounts of $500 million and $200 million, respectively. These
swaps have been designated as fair value hedges of our fixed rate debt and are included in deferred income taxes and other liabilities in our condensed consolidated balance sheets.
Cash Flow Hedges
We enter into derivative instruments to hedge our exposure to changes in cash flows attributable to interest rate, foreign currency and commodity price fluctuations associated with certain forecasted transactions. These derivative instruments are designated and qualify as cash flow hedges. Accordingly, the gain or loss on the derivative instrument is reported as a component of accumulated other comprehensive loss and reclassified into earnings in the same line item associated with the forecasted transaction and in the same period during which the hedged transaction affects earnings.
Pre-tax gains recognized in other comprehensive income/(loss) were $1 million and $2 million for the three months ended March 31, 2024 and 2023, respectively, and $2 million and $4 million for the nine months ended March 31, 2024 and 2023, respectively. Gains recognized in accumulated other comprehensive loss and reclassified into earnings were $2 million and $4 million for the three months ended March 31, 2024 and 2023, respectively, and $4 million and $8 million for the nine months ended March 31, 2024 and 2023, respectively. Gains currently included within accumulated other comprehensive loss associated with our cash flow hedges to be reclassified into net earnings within the next 12 months are $3 million.
Net Investment Hedges
We hedge the foreign currency risk associated with certain net investment positions in foreign subsidiaries. To accomplish this, we enter into cross-currency swaps that are designated as hedges of net investments.
In September 2023, we entered into ¥18 billion ($120 million) cross-currency swaps maturing in September 2025 and ¥18 billion ($120 million) cross-currency swaps maturing in June 2027.
In September 2023, we terminated the ¥38 billion ($300 million) cross-currency swaps entered into in January 2023 and received net settlement in cash of $28 million, recorded in proceeds from net investment hedge terminations in our condensed consolidated statements of cash flows.
In January 2023, we entered into ¥19 billion ($150 million) cross-currency swaps maturing in September 2025 and ¥19 billion ($150 million) cross-currency swaps maturing in June 2027. In March 2023, we entered into €100 million ($107 million) cross-currency swaps maturing in March 2025 and €100 million ($107 million) cross-currency swaps maturing in March 2026.
In January and March 2023, we terminated the ¥48 billion ($400 million) cross-currency swaps entered into in March 2022 and the €200 million ($233 million) cross-currency swap entered into in September 2018, respectively, and received net settlements of $10 million and $19 million in cash, respectively, recorded in proceeds from net investment hedge terminations in our condensed consolidated statements of cash flows.
|
|
|
|
|
|
|
|
|
|
|
|
36 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
Cross-currency swaps designated as net investment hedges are marked to market using the current spot exchange rate as of the end of the period, with gains and losses included in the foreign currency translation component of accumulated other comprehensive loss until the sale or substantial liquidation of the underlying net investments. To the extent the cross-currency swaps designated as net investment hedges are not highly effective, changes in carrying value attributable to the change in spot rates are recorded in earnings.
Pre-tax gains and losses from net investment hedges recorded in the foreign currency translation component of accumulated other comprehensive income/(loss) were a $19 million gain and an immaterial loss during the three months ended March 31, 2024 and 2023, respectively, and a $14 million gain and a $21 million loss during the nine months ended March 31, 2024 and 2023, respectively. Gains recognized in interest expense, net in the condensed consolidated statements of earnings for the portion of the net investment hedges excluded from the assessment of hedge effectiveness were $3 million and $4 million during the three months ended March 31, 2024 and 2023, respectively, and $10 million and $12 million during the nine months ended March 31, 2024 and 2023, respectively.
Economic (Non-Designated) Hedges
We enter into foreign currency contracts to manage our foreign exchange exposure related to sales transactions, intercompany financing transactions and other balance sheet items subject to revaluation that do not meet the requirements for hedge accounting treatment. Accordingly, these derivative instruments are adjusted to current market value at the end of each period through earnings. The gain or loss recorded on these instruments is substantially offset by the remeasurement adjustment on the foreign currency denominated asset or liability. The settlement of the derivative instrument and the remeasurement adjustment on the foreign currency denominated asset or liability are both recorded in other (income)/expense, net. We recorded an immaterial gain and an immaterial loss during the three months ended March 31, 2024 and 2023, respectively, and an immaterial loss and a $3 million loss during the nine months ended March 31, 2024 and 2023, respectively. The principal currencies managed through foreign currency contracts are Chinese renminbi, Canadian dollar, Indian rupee, Euro and British pound.
Fair Value of Financial Instruments
The carrying amounts of cash and equivalents, trade receivables, accounts payable and other accrued liabilities at March 31, 2024 and June 30, 2023 approximate fair value due to their short-term maturities.
The following table summarizes the estimated fair value of our long-term obligations and other short-term borrowings compared to the respective carrying amounts at:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
March 31, 2024 |
|
June 30, 2023 |
| Estimated fair value |
$ |
5,650 |
|
|
$ |
4,417 |
|
| Carrying amount |
5,854 |
|
|
4,701 |
|
The fair value of our long-term obligations and other short-term borrowings is estimated based on either the quoted market prices for the same or similar issues or other inputs derived from available market information, which represents a Level 2 measurement.
11. Shareholders' Deficit
We repurchased $750 million and $1.5 billion of our common shares, in the aggregate, through share repurchase programs during the nine months ended March 31, 2024 and 2023, respectively. We funded the repurchases with available cash. The common shares repurchased are held in treasury to be used for general corporate purposes.
During the three months ended December 31, 2023, we entered into an accelerated share repurchase ("ASR") program to repurchase common shares for an aggregate purchase price of $250 million. We received an initial delivery of 2.0 million common shares using a reference price of $101.66. The program concluded on December 13, 2023 at a volume weighted average price per common share of $103.67 resulting in a final delivery of 0.4 million common shares.
During the three months ended September 30, 2023, we entered into an ASR program to repurchase common shares for an aggregate purchase price of $500 million. We received an initial delivery of 4.4 million common shares using a reference price of $90.57. The program concluded on October 31, 2023 at a volume weighted average price per common share of $88.22 resulting in a final delivery of 1.3 million common shares.
During the three months ended June 30, 2023, we entered into an ASR program to repurchase common shares for an aggregate purchase of $500 million. We received an initial delivery of 4.6 million common shares using a reference price of $87.18. The program concluded on August 16, 2023 at a volume weighted average price per common share of $91.15 resulting in a final delivery of 0.9 million common shares.
During the three months ended March 31, 2023, we entered into an ASR program to repurchase common shares for an aggregate purchase price of $250 million. We received an initial delivery of 2.6 million common shares using a reference price of $77.03. The program concluded on February 28, 2023 at a volume weighted average price per common share of $77.27 resulting in a final delivery of 0.6 million common shares.
During the three months ended December 31, 2022, we entered into an ASR program to repurchase common shares for an aggregate purchase price of $250 million. We received an initial
|
|
|
|
|
|
|
|
|
|
|
|
37 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
delivery of 2.6 million common shares using a reference price of $76.58. The program concluded on January 13, 2023 at a volume weighted average price per common share of $77.50 resulting in a final delivery of 0.6 million common shares.
During the three months ended September 30, 2022, we entered into an ASR program to repurchase common shares for an aggregate purchase price of $1.0 billion. We received an initial delivery of 12.0 million common shares using a reference price of $66.74. The program concluded on December 23, 2022 at a volume weighted average price per common share of $73.36 resulting in a final delivery of 1.6 million common shares.
Accumulated Other Comprehensive Loss
The following tables summarize the changes in the balance of accumulated other comprehensive loss by component and in total:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Foreign
Currency
Translation
Adjustments |
|
Unrealized
Gain/(Loss) on
Derivatives,
net of tax |
|
Accumulated Other
Comprehensive
Loss |
| Balance at June 30, 2023 |
$ |
(137) |
|
|
$ |
(14) |
|
|
$ |
(151) |
|
| Other comprehensive income/(loss), before reclassifications |
(4) |
|
|
1 |
|
|
(3) |
|
| Amounts reclassified to earnings |
— |
|
|
(6) |
|
|
(6) |
|
Total other comprehensive loss attributable to Cardinal Health, Inc., net of tax expense of $4 million |
(4) |
|
|
(5) |
|
|
(9) |
|
| Balance at March 31, 2024 |
$ |
(141) |
|
|
$ |
(19) |
|
|
$ |
(160) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
Foreign
Currency
Translation
Adjustments |
|
Unrealized
Gain/(Loss) on
Derivatives,
net of tax |
|
Accumulated Other
Comprehensive
Loss |
| Balance at June 30, 2022 |
$ |
(102) |
|
|
$ |
(12) |
|
|
$ |
(114) |
|
| Other comprehensive income/(loss), before reclassifications |
(34) |
|
|
15 |
|
|
(19) |
|
| Amounts reclassified to earnings |
— |
|
|
(9) |
|
|
(9) |
|
Total other comprehensive income/(loss) attributable to Cardinal Health, Inc., net of tax benefit of $5 million |
(34) |
|
|
6 |
|
|
(28) |
|
| Balance at March 31, 2023 |
$ |
(136) |
|
|
$ |
(6) |
|
|
$ |
(142) |
|
12. Earnings Per Share Attributable to Cardinal Health, Inc.
The following tables reconcile the number of common shares used to compute basic and diluted earnings per share attributable to Cardinal Health, Inc.:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Weighted-average common shares–basic |
243 |
|
|
256 |
|
| Effect of dilutive securities: |
|
|
|
| Employee stock options, restricted share units and performance share units |
2 |
|
|
2 |
|
| Weighted-average common shares–diluted |
245 |
|
|
258 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Weighted-average common shares–basic |
245 |
|
|
263 |
|
| Effect of dilutive securities: |
|
|
|
| Employee stock options, restricted share units and performance share units |
2 |
|
|
1 |
|
| Weighted-average common shares–diluted |
247 |
|
|
264 |
|
The potentially dilutive employee stock options, restricted share units and performance share units that were antidilutive were immaterial and 1 million for the three and nine months ended March 31, 2024, respectively, and 2 million for both the three and nine months ended March 31, 2023.
13. Segment Information
Effective January 1, 2024, we operated under an updated organizational structure and re-aligned our reporting structure under two reportable segments: Pharmaceutical and Specialty Solutions segment and GMPD segment. All remaining operating segments that are not significant enough to require separate reportable segment disclosures are included in Other, which is comprised of Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics. The factors for determining the reportable segments include the manner in which management evaluates performance for purposes of allocating resources and assessing performance combined with the nature of the individual business activities. Our previously reported segment results have been recast to conform to this re-aligned reporting structure and reflect changes in the elimination of inter-segment revenue and allocated corporate technology and shared function expenses, which are driven by the reporting structure change.
Our Pharmaceutical and Specialty Solutions segment distributes branded and generic pharmaceutical, specialty pharmaceutical and over-the-counter healthcare and consumer products in the United States. This segment also provides services to pharmaceutical manufacturers and healthcare providers for
|
|
|
|
|
|
|
|
|
|
|
|
38 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
specialty pharmaceutical products; provides pharmacy management services to hospitals and operates a limited number of pharmacies, including pharmacies in community health centers; and repackages generic pharmaceuticals and over the counter healthcare products.
Our GMPD segment manufactures, sources and distributes Cardinal Health branded medical, surgical and laboratory products, which are sold in the United States, Canada, Europe, Asia and other markets. In addition to distributing Cardinal Health branded products, this segment also distributes a broad range of medical, surgical and laboratory products known as national brand products to hospitals, ambulatory surgery centers, clinical laboratories and other healthcare providers in the United States and Canada.
The remaining three operating segments included in Other are Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics. These operating segments respectively operate nuclear pharmacies and radiopharmaceutical manufacturing facilities, distribute medical products to patients' homes in the United States and provide supply chain services and solutions to our customers.
Revenue
The following tables present revenue for the two reportable segments and the remaining operating segments, included in Other, and Corporate:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Pharmaceutical and Specialty Solutions |
$ |
50,651 |
|
|
$ |
46,496 |
|
| Global Medical Products and Distribution |
3,113 |
|
|
2,989 |
|
| Nuclear and Precision Health Solutions |
352 |
|
|
313 |
|
at-Home Solutions |
743 |
|
|
651 |
|
OptiFreight® Logistics |
72 |
|
|
61 |
|
Other |
1,167 |
|
|
1,025 |
|
| Total segment revenue |
54,931 |
|
|
50,510 |
|
Corporate (1) |
(20) |
|
|
(23) |
|
| Total revenue |
$ |
54,911 |
|
|
$ |
50,487 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Pharmaceutical and Specialty Solutions |
$ |
154,524 |
|
|
$ |
139,441 |
|
| Global Medical Products and Distribution |
9,264 |
|
|
9,140 |
|
| Nuclear and Precision Health Solutions |
1,006 |
|
|
875 |
|
at-Home Solutions |
2,188 |
|
|
1,986 |
|
OptiFreight® Logistics |
198 |
|
|
177 |
|
Other |
3,392 |
|
|
3,038 |
|
| Total segment revenue |
167,180 |
|
|
151,619 |
|
Corporate (1) |
(61) |
|
|
(60) |
|
| Total revenue |
$ |
167,119 |
|
|
$ |
151,559 |
|
(1)Corporate revenue consists of the elimination of inter-segment revenue and other revenue not allocated to the segments.
The following tables present revenue by geographic area:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| United States |
$ |
53,715 |
|
|
$ |
49,343 |
|
| International |
1,216 |
|
|
1,167 |
|
| Total segment revenue |
54,931 |
|
|
50,510 |
|
| Corporate (1) |
(20) |
|
|
(23) |
|
| Total revenue |
$ |
54,911 |
|
|
$ |
50,487 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| United States |
$ |
163,548 |
|
|
$ |
148,186 |
|
| International |
3,632 |
|
|
3,433 |
|
| Total segment revenue |
167,180 |
|
|
151,619 |
|
| Corporate (1) |
(61) |
|
|
(60) |
|
| Total revenue |
$ |
167,119 |
|
|
$ |
151,559 |
|
(1)Corporate revenue consists of the elimination of inter-segment revenue and other revenue not allocated to the segments.
Segment Profit
We evaluate segment performance based on segment profit, among other measures. Segment profit is segment revenue, less segment cost of products sold, less segment distribution, selling, general and administrative ("SG&A") expenses. Segment SG&A expenses include share-based compensation expense as well as allocated corporate technology and shared function expenses, including corporate management, corporate finance, financial and customer care shared services, human resources, information technology and legal and compliance, including certain litigation defense costs. Corporate expenses are allocated to the operating segments based on headcount, level of benefit provided and other ratable allocation methodologies. The results attributable to noncontrolling interests are recorded within segment profit.
|
|
|
|
|
|
|
|
|
|
|
|
39 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
We do not allocate the following items to our segments:
•last-in first-out, or ("LIFO"), inventory charges/(credits);
•state opioid assessment related to prior fiscal years;
•shareholder cooperation agreement costs;
•restructuring and employee severance;
•amortization and other acquisition-related costs;
•impairments and (gain)/loss on disposal of assets, net; in connection with goodwill impairment testing for the GMPD segment as discussed further in
Note 5, we recognized pre-tax goodwill impairment charges of $90 million during the three months ended March 31, 2024 and $671 million and $863 million during the nine months ended March 31, 2024 and 2023, respectively;
•litigation (recoveries)/charges, net;
•other (income)/expense, net;
•interest expense, net;
•loss on early extinguishment of debt; or
•provision for/(benefit from) income taxes
In addition, certain investment spending, certain portions of enterprise-wide incentive compensation and other spending are not allocated to the segments. Investment spending generally includes the first-year spend for certain projects that require incremental investments in the form of additional operating expenses. Because approval for these projects is dependent on executive management, we retain these expenses at Corporate. Investment spending within Corporate was $17 million and $9 million for the three months ended March 31, 2024 and 2023, respectively, and $37 million and $20 million for the nine months ended March 31, 2024 and 2023, respectively.
The following tables present segment profit for the two reportable segments and the remaining operating segments, included in Other, and Corporate:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
Pharmaceutical and Specialty Solutions |
$ |
580 |
|
|
$ |
560 |
|
Global Medical Products and Distribution |
20 |
|
|
(46) |
|
| Other (1) |
111 |
|
|
106 |
|
| Total segment profit |
711 |
|
|
620 |
|
| Corporate |
(344) |
|
|
(48) |
|
Total operating earnings |
$ |
367 |
|
|
$ |
572 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
Pharmaceutical and Specialty Solutions |
$ |
1,541 |
|
|
$ |
1,394 |
|
Global Medical Products and Distribution |
18 |
|
|
(175) |
|
| Other (1) |
319 |
|
|
305 |
|
| Total segment profit |
1,878 |
|
|
1,524 |
|
| Corporate |
(1,043) |
|
|
(934) |
|
Total operating earnings |
$ |
835 |
|
|
$ |
590 |
|
(1)Comprised of the remaining operating segments, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics.
Segment Assets
The following table presents total assets for the two reportable segments and the remaining operating segments, included in Other, and Corporate:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions) |
March 31,
2024 |
|
June 30,
2023 |
Pharmaceutical and Specialty Solutions |
$ |
30,496 |
|
|
$ |
27,615 |
|
Global Medical Products and Distribution |
7,357 |
|
|
7,961 |
|
| Other (1) |
2,692 |
|
|
2,621 |
|
| Corporate |
5,335 |
|
|
5,220 |
|
| Total assets |
$ |
45,880 |
|
|
$ |
43,417 |
|
(1)Comprised of the remaining operating segments, Nuclear and Precision Health Solutions, at-Home Solutions and OptiFreight® Logistics.
14. Share-Based Compensation
We maintain stock incentive plans (collectively, the “Plans”) for the benefit of certain of our officers, directors and employees.
The following tables provide total share-based compensation expense by type of award:
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Restricted share unit expense |
$ |
20 |
|
|
$ |
17 |
|
|
|
|
|
| Performance share unit expense |
11 |
|
|
4 |
|
Total share-based compensation |
$ |
31 |
|
|
$ |
21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nine Months Ended March 31, |
| (in millions) |
2024 |
|
2023 |
| Restricted share unit expense |
$ |
58 |
|
|
$ |
49 |
|
|
|
|
|
| Performance share unit expense |
30 |
|
|
20 |
|
Total share-based compensation |
$ |
88 |
|
|
$ |
69 |
|
The total tax benefit related to share-based compensation was $4 million and $3 million for the three months ended March 31, 2024 and 2023, respectively, and $12 million and $9 million for the nine months ended March 31, 2024 and 2023, respectively.
Restricted Share Units
Restricted share units granted under the Plans generally vest in equal annual installments over three years. Restricted share units
|
|
|
|
|
|
|
|
|
|
|
|
40 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Notes to Financial Statements |
|
|
accrue cash dividend equivalents that are payable upon vesting of the awards.
The following table summarizes all transactions related to restricted share units under the Plans:
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions, except per share amounts) |
Restricted Share Units |
|
Weighted-Average
Grant Date Fair
Value per Share |
| Nonvested at June 30, 2023 |
2.2 |
|
|
$ |
57.37 |
|
| Granted |
0.9 |
|
|
90.78 |
|
| Vested |
(1.1) |
|
|
60.83 |
|
| Canceled and forfeited |
(0.1) |
|
|
71.21 |
|
| Nonvested at March 31, 2024 |
1.9 |
|
|
$ |
69.96 |
|
At March 31, 2024, the total pre-tax compensation cost, net of estimated forfeitures, related to nonvested restricted share units not yet recognized was $86 million, which is expected to be recognized over a weighted-average period of two years.
Performance Share Units
Performance share units vest over a three-year performance period based on achievement of specific performance goals. Based on the extent to which the targets are achieved, vested shares may range from zero to 234 percent of the target award amount for both the fiscal 2022 and 2023 grants and zero to 240 percent of the target award for the fiscal 2024 grant. Performance share units accrue cash dividend equivalents that are payable upon vesting of the awards.
The following table summarizes all transactions related to performance share units under the Plans (based on target award amounts):
|
|
|
|
|
|
|
|
|
|
|
|
| (in millions, except per share amounts) |
Performance
Share Units |
|
Weighted-Average
Grant Date Fair
Value per Share |
| Nonvested at June 30, 2023 |
1.2 |
|
|
$ |
82.17 |
|
| Granted |
0.5 |
|
|
94.66 |
|
| Vested |
(0.4) |
|
|
62.26 |
|
| Canceled and forfeited |
— |
|
|
— |
|
| Nonvested at March 31, 2024 |
1.3 |
|
|
$ |
96.80 |
|
At March 31, 2024, the total pre-tax compensation cost, net of estimated forfeitures, related to nonvested performance share units not yet recognized was $53 million, which is expected to be recognized over a weighted-average period of two years if performance goals are achieved.
|
|
|
|
|
|
|
|
|
|
|
|
41 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Exhibits
|
|
|
|
|
|
Exhibit
Number |
Exhibit Description |
| 3.1 |
|
| 3.2 |
|
10.1.1 |
|
10.1.2 |
|
10.1.3 |
|
10.1.4 |
|
10.2 |
|
| 31.1 |
|
| 31.2 |
|
| 32.1 |
|
| 99.1 |
|
|
|
| 101.SCH |
Inline XBRL Taxonomy Extension Schema Document |
| 101.CAL |
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
| 101.DEF |
Inline XBRL Taxonomy Definition Linkbase Document |
| 101.LAB |
Inline XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE |
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
| 104 |
Cover Page Interactive Data File - formatted in Inline XBRL (included as Exhibit 101) |
|
Cardinal Health Website
Cardinal Health uses its website as a channel of distribution for material company information. Important information, including news releases, financial information, earnings and analyst presentations, and information about upcoming presentations and events is routinely posted and accessible at ir.cardinalhealth.com. In addition, the website allows investors and other interested persons to sign up automatically to receive e-mail alerts when we post news releases, SEC filings and certain other information on its website.
|
|
|
|
|
|
|
|
|
|
|
|
42 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
|
|
|
|
|
|
|
|
|
| Form 10-Q Cross Reference Index |
|
|
Form 10-Q Cross Reference Index
|
|
|
|
|
|
|
|
|
| Item Number |
|
Page |
|
|
|
|
Part I. Financial Information |
|
| Item 1 |
|
|
| Item 2 |
|
|
| Item 3 |
|
|
| Item 4 |
|
|
|
|
|
|
Part II. Other Information |
|
| Item 1 |
|
|
| Item 1A |
|
|
| Item 2 |
|
|
| Item 3 |
Defaults Upon Senior Securities |
N/A |
| Item 4 |
Mine Safety Disclosures |
N/A |
| Item 5 |
|
|
| Item 6 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
43 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Cardinal Health, Inc. |
|
|
|
| Date: |
May 2, 2024 |
/s/ JASON M. HOLLAR |
|
|
Jason M. Hollar |
|
|
Chief Executive Officer |
|
|
|
|
|
/s/ AARON E. ALT |
|
|
Aaron E. Alt |
|
|
Chief Financial Officer |
|
|
|
|
|
|
|
|
|
|
|
|
44 |
Cardinal Health | Q3 Fiscal 2024 Form 10-Q |
|
EX-10.1 1
2
a10-qex1011xltiamendment.htm
EX-10.1 1
Document
FIRST AMENDMENT TO THE
CARDINAL HEALTH, INC. 2021 LONG-TERM INCENTIVE PLAN
This First Amendment to the Cardinal Health, Inc. 2021 Long-Term Incentive Plan (the “Plan”) was adopted on January 29, 2024 by the Human Resources and Compensation Committee of the Board of Directors of Cardinal Health, Inc. (the “Company”).
The Plan is hereby amended, effective as of January 29, 2024, as follows:
1.Section 20 of the Plan is hereby deleted in its entirety and in replacement thereof shall be the following:
“20. Clawback Policy.
To the extent applicable, Participants and Awards will be subject to the terms of the Cardinal Health, Inc. Clawback Policy adopted by the Company, as required by New York Stock Exchange listing standards and as may be in effect from time to time. In its discretion, moreover, the Administrator may require repayment to the Company of all or any portion of any Award if the amount of the Award was calculated based upon the achievement of financial results that were subsequently the subject of a restatement of the Company’s financial statements, the Participant engaged in misconduct that caused or contributed to the need for the restatement of the financial statements, and the amount payable to the Participant would have been lower than the amount actually paid to the Participant had the financial results been properly reported. This Section 20 will not be the Company’s exclusive remedy with respect to such matters.
EX-10.2
6
a10-qex102amendmentno1tomip.htm
EX-10.2
Document
FIRST AMENDMENT TO THE
CARDINAL HEALTH, INC. MANAGEMENT INCENTIVE PLAN
This First Amendment to the Cardinal Health, Inc. Management Incentive Plan (the “Plan”) was adopted on January 29, 2024 by the Human Resources and Compensation Committee of the Board of Directors of Cardinal Health, Inc. (the “Company”).
The Plan is hereby amended, effective as of January 29, 2024, as follows:
1.Section 5(b)(ii) of the Plan is hereby deleted in its entirety and in replacement thereof shall be the following:
“(ii) Mandatory Deferral. The Administrator additionally or alternatively may establish one or more programs under the Plan to require the deferral of payment of any Award for any specified period after the end of the applicable Performance Period, during which time the amount payable under the deferred Award may be reduced if and to the extent that the Administrator determines that the Participant whose Award is subject to reduction under Section 7 of the Plan. In such case, the Administrator will establish the conditions and mechanisms for payments of, and accrual of interest or other earnings, if any, on amounts so deferred, and such other terms, conditions, rules, and procedures that the Administrator deems advisable for the administration of any such deferral program.
2.The title of Section 7 of the Plan is changed to “Clawback Policy; Other Special Repayment and Forfeiture Events.”
3.Section 7(a) of the Plan is hereby deleted in its entirety and in replacement thereof shall be the following:
“(a) Clawback Policy. In addition to the other provisions of this Section 7, to the extent applicable, Participants and Awards will be subject to the terms of the Cardinal Health, Inc. Clawback Policy adopted by the Company, as required by New York Stock Exchange listing standards and as may be in effect from time to time. In its discretion, moreover, the Administrator may require repayment to the Company of all or any portion of any Award if the amount of the Award was calculated based upon the achievement of financial results that were subsequently the subject of a restatement of the Company’s financial statements, the Participant engaged in misconduct that caused or contributed to the need for the restatement of the financial statements, and the amount payable to the Participant would have been lower than the amount actually paid to the Participant had the financial results been properly reported. This Section 7(a) will not be the Company’s exclusive remedy with respect to such matters.
EX-31.1
7
a24q3_10qx33124xexhibit311.htm
EX-31.1
Document
I, Jason M. Hollar, certify that:
1.I have reviewed this Form 10-Q of Cardinal Health, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: May 2, 2024
|
|
|
|
|
|
| /s/ JASON M. HOLLAR |
|
| Jason M. Hollar |
|
| Chief Executive Officer |
|
EX-31.2
8
a24q3_10qx33124xexhibit312.htm
EX-31.2
Document
I, Aaron E. Alt, certify that:
1.I have reviewed this Form 10-Q of Cardinal Health, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
(a)Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
(b)Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
(c)Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
(d)Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
(a)All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
(b)Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: May 2, 2024
|
|
|
|
|
|
| /s/ AARON E. ALT |
|
| Aaron E. Alt |
|
| Chief Financial Officer |
|
EX-32.1
9
a24q3_10qx33124xexhibit321.htm
EX-32.1
Document
Certification of the Chief Executive Officer and the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
Jason M. Hollar, Chief Executive Officer of Cardinal Health, Inc. (the “Company”) and Aaron E. Alt, Chief Financial Officer of the Company, certify, pursuant to 18 U.S.C. Section 1350, that:
(1)the Periodic Report on Form 10-Q for the quarter ended March 31, 2024 containing the financial statements of the Company (the “Periodic Report”), which this statement accompanies, fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)); and
(2)the information contained in the Periodic Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Dated: May 2, 2024
|
|
|
|
|
|
|
/s/ JASON M. HOLLAR |
|
Jason M. Hollar |
|
Chief Executive Officer |
|
|
|
|
|
|
|
/s/ AARON E. ALT |
|
Aaron E. Alt |
|
Chief Financial Officer |
EX-99.1
10
a24q3_10qx33124xexhibit991.htm
EX-99.1
Document
Statement Regarding Forward-Looking Information
As used in this exhibit, “we,” “our,” “us” and similar pronouns refer to Cardinal Health, Inc. and its subsidiaries, unless the context requires otherwise. Our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the fiscal year ended June 30, 2023 (the “2023 Form 10-K”), and our quarterly reports on Form 10-Q, including this one, and our current reports on Form 8-K (along with any exhibits and amendments to such reports), as well as our news releases or any other written or oral statements made by or on behalf of us, including materials posted on our website, may include, directly or by incorporation by reference, forward-looking statements that reflect our current view (as of the date the forward-looking statement is first made) about future events, prospects, projections or financial performance. The matters discussed in these forward-looking statements are subject to certain risks and uncertainties that could cause actual results to differ materially from those projected, anticipated or implied in or by such statements. These risks and uncertainties include:
•competitive pressures in the markets in which we operate, including pricing pressures;
•uncertainties relating to the pricing of and demand for generic pharmaceuticals;
•significantly increased costs for commodities and other materials used in the Global Medical Products and Distribution segment manufacturing, including various components, compounds, raw materials or energy such as oil-based resins, pulp, cotton, latex and other commodities and the possibility that we may not successfully offset or mitigate these increases;
•uncertainties relating to the timing, frequency and profitability of generic pharmaceutical launches or other components of our pharmaceutical generics program;
•changes in the timing or frequency of the introduction of branded pharmaceuticals;
•uncertainties related to the timing, magnitude and profit impact of the distribution of commercially available COVID-19 vaccines;
•material reductions in purchases, pricing changes, non-renewal, early termination, or delinquencies or defaults under contracts with key customers;
•Risks associated with the nonrenewal of a large Pharmaceutical and Specialty Solutions segment customer at the end of fiscal year 2024, including the adverse impact of unwinding the negative net working capital associated with this customer and the risk that we may not be successful in mitigating the negative impact to segment profit;
•costs or claims resulting from quality issues, or other potential or alleged errors or defects in our manufacturing or sourcing of medical devices or other products or in our compounding, repackaging, information systems or pharmacy management services that may injure persons or damage property or operations, including costs from recalls, remediation efforts, and related product liability claims and lawsuits, including class action lawsuits;
•any compromise of our information systems or of those of a third-party service provider, including unauthorized access to or use or disclosure of company or customer information, disruption of access and ancillary risks associated with our ability to effectively manage any issues arising from any such compromise or disruption;
•continuing risks associated with the resolution and defense of the lawsuits and investigations in which we have been or will be named relating to the distribution of prescription opioid pain medication, including the investigations by the U.S. Department of Justice which concerns our anti-diversion program, our anti-diversion policies and procedures and our distribution of certain controlled substances;
•risks associated with the national opioid settlement agreement, including the risk that the maintenance of the required changes to distributors' controlled substance anti-diversion programs may result in unforeseen costs or operational challenges and the risk that if we fail to or are alleged to have failed to comply with the terms of the settlement agreement, we could incur monetary or other penalties or result in additional lawsuits being filed against us;
•uncertainties related to Cardinal Health Brand products, including our ability to manage cost and infrastructure, retain margin, increase volume and improve performance;
•risks arising from acquisitions, including possible liabilities relating to the operations or activities of such businesses prior to their acquisition, and uncertainties relating to our ability to achieve the anticipated results from acquisitions;
•risks associated with the tax benefit from our self-insurance loss claims, including, certain state courts' interpretation of laws and insurance policies in ways that may impact our self-insurance loss, which could negatively impact our financial position;
•disruption, damage or lack of access to, or failure of, our or our third-party service providers' information systems, our critical facilities, including our national logistics center, or our distribution networks;
•risks associated with our Corporate Integrity Agreement with the Office of Inspector General of the Department of Health and Human Services, including the risk that failure to comply with the requirements set forth therein could result in monetary or other penalties;
•our high sales concentration with certain key customers, including CVS Health Corporation and OptumRx;
•our ability to maintain the benefits of our generic pharmaceutical sourcing venture with CVS Health Corporation;
•actions of regulatory bodies and other governmental authorities, including the U.S. Drug Enforcement Administration, certain agencies within the U.S. Department of Health and Human Services (including the U.S. Food and Drug Administration, Centers for Medicare and Medicaid Services, the Office of Inspector General and the Office for Civil Rights), the U.S. Nuclear Regulatory Commission, the U.S. Federal Trade Commission, the U.S. Customs and Border Protection, various state boards of pharmacy, state controlled substance authorities, state health departments, state insurance departments, state Medicaid departments or comparable regulatory bodies or governmental authorities or foreign equivalents that, in each case, could delay, limit or suspend product development, manufacturing, distribution, importation or sales or result in warning letters, recalls, seizures, injunctions or monetary sanctions;
•shortages in commodities, components, compounds, raw materials or energy used by our businesses, including supply disruptions of radioisotopes;
•the loss of, or default by, one or more key suppliers for which alternative suppliers may not be readily available;
•uncertainties with respect to certain business process initiatives, including IT infrastructure activities and outsourcing relationships, including the ability to achieve the expected benefits from such initiatives, the risk that we could incur unexpected charges, and the risk that we may fail to retain key personnel;
•difficulties or delays in the development, production, manufacturing, sourcing and marketing of new or existing products and services, including difficulties or delays associated with obtaining or maintaining requisite regulatory consents, whether our own or third parties', or approvals associated with those activities;
•manufacturing disruptions, whether due to regulatory action, including regulatory action to reduce ethylene oxide ("EtO") emissions, production quality deviations, safety issues or raw material shortages or defects, or because a key product is manufactured at a single manufacturing facility with limited alternate facilities;
•risks associated with industry reliance on EtO to sterilize certain medical products that we manufacture or distribute, including the possibility that regulatory actions to reduce EtO emissions could become more widespread, which may result in increased costs or supply shortages; and risks that the lawsuits against us alleging personal injury resulting from EtO exposure could become more widespread;
•the possibility that we could be subject to adverse changes in the tax laws or challenges to our tax positions, including the possibility that the corporate tax rate in the U.S. could be increased;
•risks arising from possible violations of healthcare fraud and abuse laws;
•risks arising from possible violations of the U.S. Foreign Corrupt Practices Act and other similar anti-corruption laws in other jurisdictions and U.S. and foreign export control, trade embargo and customs laws;
•risks arising from our collecting, handling and maintaining patient-identifiable health information and other sensitive personal and financial information, which are subject to federal, state and foreign laws that regulate the use and disclosure of such information;
•risks arising from certain of our businesses being Medicare-certified suppliers or participating in other federal and state healthcare programs, such as state Medicaid programs and the federal 340B drug pricing program, which businesses are subject to accreditation and quality standards and other rules and regulations, including applicable reporting, billing, payment and record-keeping requirements;
•risks arising from pharmaceutical manufacturers' restriction of sales under the 340B drug pricing program to contract pharmacies, which may adversely impact our customers;
•risks arising from certain of our businesses manufacturing pharmaceutical and medical products or repackaging pharmaceuticals that are purchased or reimbursed through, or are otherwise governed by, federal or state healthcare programs, which businesses are subject to federal and state laws that establish eligibility for reimbursement by such programs and other applicable standards and regulations;
•changes in laws or changes in the interpretation or application of laws or regulations, as well as possible failures to comply with applicable laws or regulations, including as a result of possible misinterpretations or misapplications;
•unfavorable changes to the terms or with our ability to meet contractual obligations of key customer or supplier relationships, or changes in customer mix;
•risks arising from changes in U.S. or foreign tax laws and unfavorable challenges to our tax positions and payments to settle these challenges, which may adversely affect our effective tax rate or tax payments;
•uncertainties due to possible government healthcare reform, including proposals related to Medicare drug rebate arrangements, possible repeal or replacement of major parts of the Patient Protection and Affordable Care Act, proposals related to prescription drug pricing transparency and the possible adoption of Medicare-For-All;
•reductions or limitations on governmental funding at the state or federal level or efforts by healthcare insurance companies to limit payments for products and services;
•changes in manufacturers' pricing, selling, inventory, distribution or supply policies or practices;
•changes in legislation or regulations governing prescription drug pricing, healthcare services or mandated benefits;
•uncertainties arising as a result of the Supreme Court decision on Dobbs vs. Jackson, including uncertainties associated with states' proposed and adopted laws which may impact our ability to distribute or store certain pharmaceutical products and the risk that we could incur unforeseen costs to comply with these new laws in various jurisdictions;
•changes in hospital buying groups or hospital buying practices;
•changes in distribution or sourcing models for pharmaceutical and medical and surgical products, including an increase in direct and limited distribution;
•changes to the prescription drug reimbursement formula and related reporting requirements for generic pharmaceuticals under Medicaid;
•continuing consolidation in the healthcare industry, which could give the resulting enterprises greater bargaining power and may increase pressure on prices for our products and services or result in the loss of customers;
•risks to our business and information and controls systems in the event that business process improvements, infrastructure modernization or initiatives to use third-party service providers for key systems and processes are not effectively implemented;
•the risk that we may not effectively implement and maintain data governance structures across businesses to allow us to access and interpret our data, which could put us at a competitive disadvantage relative to our peers;
•the results, costs, effects or timing of any commercial disputes, government contract compliance matters, patent infringement claims, qui tam actions, government investigations, shareholder lawsuits or other legal proceedings;
•the possibility that our business performance or internal control over financial reporting may be adversely impacted if we are not successful at attracting, retaining and developing talent;
•losses relating to product liability lawsuits and claims regarding products for which we cannot obtain product liability insurance or for which such insurance may not be adequate to cover our losses, including the product liability lawsuits we are currently defending relating to alleged personal injuries associated with the use of Cordis inferior vena cava filter products;
•risks associated with the importation of products or source materials used in products that we manufacture or distribute, including risks associated with our country-of-origin determinations and the possibility that we could experience additional supply disruptions as a result of the Uyghur Forced Labor Prevention Act or other similar regulations;
•our ability to maintain adequate intellectual property protections;
•our ability to manage and complete divestitures or other strategic business combination transactions, including our ability to find buyers or other strategic exit opportunities and risks associated with the possibility that we could experience greater dis-synergies than anticipated or otherwise fail to achieve our strategic objectives;
•bankruptcy, insolvency or other credit failure of a customer or supplier that owes us a substantial amount;
•risks associated with global operations, including the effect of local economic environments, inflation, recession, currency volatility and global competition, in addition to risks associated with compliance with U.S. and international laws relating to global operations;
•uncertainties with respect to U.S. or international trade policies, tariffs, excise or border taxes and their impact on our ability to source products or materials that we need to conduct our business;
•risks associated with our use of and reliance on the global capital and credit markets, including our ability to access credit and our cost of credit, which may adversely affect our ability to efficiently fund our operations or undertake certain expenditures;
•our ability to introduce and market new products and our ability to keep pace with advances in technology;
•significant charges to earnings if goodwill or intangible assets become impaired;
•uncertainties relating to general political, business, industry, regulatory and market conditions; and
•other factors described in the “Risk Factors” section of the 2023 Form 10-K.
The words “expect,” “anticipate,” “intend,” “plan,” “believe,” “will,” “should,” “could,” “would,” “project,” “continue,” “likely,” and similar expressions generally identify “forward-looking statements,” which speak only as of the date the statements were made, and also include statements reflecting future results or guidance, statements of outlook and expense accruals. We undertake no obligation to update or revise any forward-looking statements, except to the extent required by applicable law.ted, anticipated or implied in or by such statements. These risks and uncertainties include: