
© L i s a t a T h e r a p e u t i c s , I n c . 2 0 2 5 . A l l r i g h t s r e s e r v e d . Targeted Therapy Delivered www.lisata.com Corporate Presentation | January 22, 2025 Nasdaq: LSTA Exhibit 99.2

2 Forward-looking statements advisory This presentation contains “forward-looking statements” that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, included in this communication regarding strategy, future operations, future financial position, future revenue, projected expenses, prospects, plans and objectives of management are forward-looking statements. In addition, when or if used in this communication, the words “may,” “could,” “should,” “anticipate,” “believe,” “estimate,” “expect,” “intend,” “plan,” “predict”, “target” and similar expressions and their variants, as they relate to Lisata or its management, may identify forward-looking statements. Examples of forward-looking statements include, but are not limited to, statements relating to Lisata’s continued listing on the Nasdaq Capital Market; expectations regarding the capitalization, resources and ownership structure of Lisata; the approach Lisata is taking to discover, develop and commercialize novel therapeutics; the adequacy of Lisata’s capital to support its future operations and its ability to successfully initiate and complete clinical trials; and the difficulty in predicting the time and cost of development of Lisata’s product candidates. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the safety and efficacy of Lisata’s product candidates, decisions of regulatory authorities and the timing thereof, the duration and impact of regulatory delays in Lisata’s clinical programs, Lisata’s ability to finance its operations, the likelihood and timing of the receipt of future milestone and licensing fees, the future success of Lisata’s scientific studies, Lisata’s ability to successfully develop and commercialize drug candidates, the timing for starting and completing clinical trials, rapid technological change in Lisata’s markets, the ability of Lisata to protect its intellectual property rights and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Lisata’s Annual Report on Form 10-K filed with the SEC on February 29, 2024, and in other documents filed by Lisata with the Securities and Exchange Commission. Except as required by applicable law, Lisata undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements, whether as a result of new information, future events or otherwise. Disclosure

Lisata at a Glance Company Overview 3

Lisata Therapeutics (Nasdaq: LSTA) Company Overview 4 OVERVIEW Clinical-stage therapeutics company rapidly developing a novel solid tumor targeting and penetration technology with tumor microenvironment (TME) modifying properties. MISSION To enhance the treatment benefits of existing and emerging therapies for solid tumors and similar diseases without additional side effects utilizing an approach that is patient-friendly and pharmacoeconomically attractive.

Company Overview 5 Lisata Therapeutics (Nasdaq: LSTA): Key attributes Projected cash runway into early 2026, funding all current development programs through data Seasoned management with successful international drug development experience and expertise Platform technology validated by existing partnerships with potential for many others Multiple product and business milestones projected over the next 12 - 18 months Proprietary field- leading technology with global IP protection extending beyond 2040

Seasoned leadership with proven history of drug approvals worldwide 6 Kristen K. Buck, MD Executive Vice President of R&D and Chief Medical Officer Dr. Buck is a board certified and licensed physician with >20 years of strategic global drug development, drug/device safety/epidemiology, FDA, and clinical practice experience. Gregory Berkin Chief Information Officer and Data Protection Officer James Nisco SVP of Finance and Treasury and Chief Accounting Officer Tariq Imam VP of BD and Operations and Corporate Counsel John Menditto VP of Investor Relations and Corporate Communications Bill Sietsema, PhD VP of Global Regulatory Affairs Ryan Quick VP of Chemistry, Manufacturing and Controls Detailed bios can be found at www.lisata.com David J. Mazzo, PhD President and Chief Executive Officer, Member of the Board of Directors With >40 years of experience, Dr. Mazzo is a global pharmaceutical executive noted for his strategic prowess and his vast experience developing and launching new products across all therapeutical areas. He recently was recognized as a 2024 PharmaVoice Top 100 Standout Leader. Company Overview

Therapeutic Focus and Rationale 7
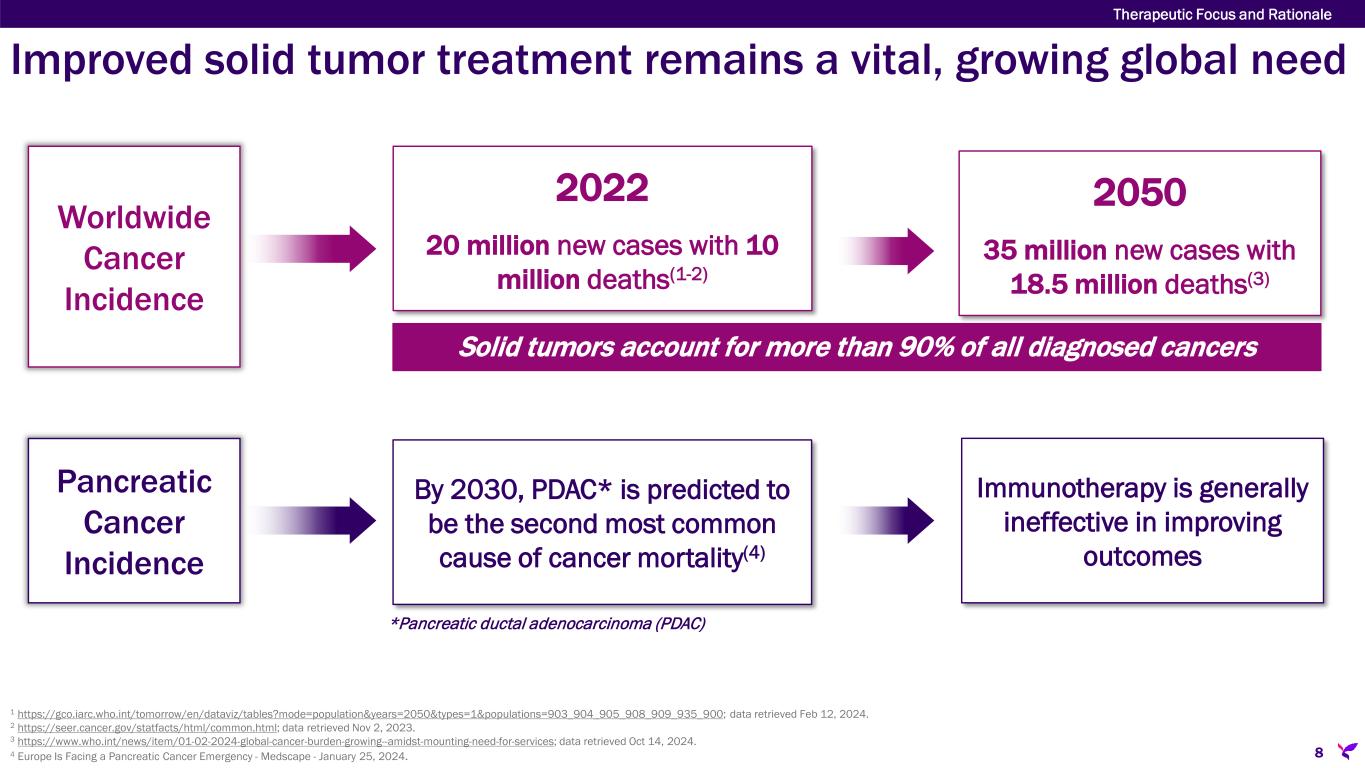
Improved solid tumor treatment remains a vital, growing global need Worldwide Cancer Incidence 2022 20 million new cases with 10 million deaths(1-2) 2050 35 million new cases with 18.5 million deaths(3) Solid tumors account for more than 90% of all diagnosed cancers Pancreatic Cancer Incidence By 2030, PDAC* is predicted to be the second most common cause of cancer mortality(4) Immunotherapy is generally ineffective in improving outcomes *Pancreatic ductal adenocarcinoma (PDAC) 1 https://gco.iarc.who.int/tomorrow/en/dataviz/tables?mode=population&years=2050&types=1&populations=903_904_905_908_909_935_900; data retrieved Feb 12, 2024. 2 https://seer.cancer.gov/statfacts/html/common.html; data retrieved Nov 2, 2023. 3 https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services; data retrieved Oct 14, 2024. 4 Europe Is Facing a Pancreatic Cancer Emergency - Medscape - January 25, 2024. 8 Therapeutic Focus and Rationale

Current solid tumor treatments & patient outcomes are suboptimal 9 Therapeutic Focus and Rationale Diagram source: Abizanda-Campo, S. et al, Microsyst Nanoeng 9, 154 (2023) Challenging tumor morphology and tumor microenvironment (TME) pose significant barriers to effective treatment and outcomes Tumor stroma acts as a physical barrier to anti-cancer agents An immunosuppressive TME contributes to tumor resistance and/or metastases Prolonged or escalated dosing of non-targeted anti-cancer therapies generally leads to intolerable off-target side effects

Certepetide is designed to optimize solid tumor treatment 1 Sugahara, et al. Mol Cancer Ther; 14(1) January 2015; Hamilton, et al., J MolMed. April 2015; and Miyamura, et al., bioRxiv. May 2023. 2 Yuan, D., Duda, D., et al. CCA Foundation Conf. 2024 Poster. Enhancing the efficacy of standard therapy in intrahepatic cholangiocarcinoma using LSTA1, a novel tumor targeting and penetration agent. Converts tumor stroma from a barrier to a conduit for anti-cancer drugs Selectively reduces TME immunosuppressive T cells and recruits cytotoxic T cells(1) Inhibits the metastatic cascade(2) Works with any modality of anti-cancer therapeutics Via co-administration or molecular tethering Approaching Phase 3-ready readiness in mPDAC** In mid-stage clinical development in multiple solid tumors Certepetide: a proprietary internalizing RGD* (iRGD) cyclic peptide with tumor specific targeting & penetration activity and tumor microenvironment modifying properties *internalizing RGD (iRGD): Arginylglycylaspartic acid **mPDAC: metastatic pancreatic ductal adenocarcinoma 10 Therapeutic Focus and Rationale

Partnerships Noteworthy existing relationships and potential for many more 11

Existing partnerships support certepetide’s promise and broad applicability Partnerships 12 R&D alliances contribute resources with minimal commercial interest in certepetide Australasian Gastro-Intestinal Trials Group - Clinical Trialists Consortium (Australia & New Zealand) WARPNINE - Foundation (Australia) Existing strategic commercial partnerships Qilu Pharmaceutical Qilu granted exclusive rights in China, Taiwan, Hong Kong and Macau Qilu assumes all development and commercialization responsibilities/costs in licensed territories Lisata collected $15 million in milestones to date Potential for additional $221 million in milestones plus royalties on sales to Lisata Kuva Labs Kuva granted exclusive worldwide rights to certepetide as a targeting agent/delivery vehicle in combination with Kuva’s NanoMark technology for diagnostic tumor imaging Includes a $1 million upfront license fee and potential for ~$20 million in milestones plus royalties on sales to Lisata Kuva assumes all development and commercialization responsibilities/costs Additional partnership opportunities exist for many combinations with certepetide By indication, modality of co-administered drug(s), and/or geography

13 Certepetide Strong Scientific Foundation and Rationale
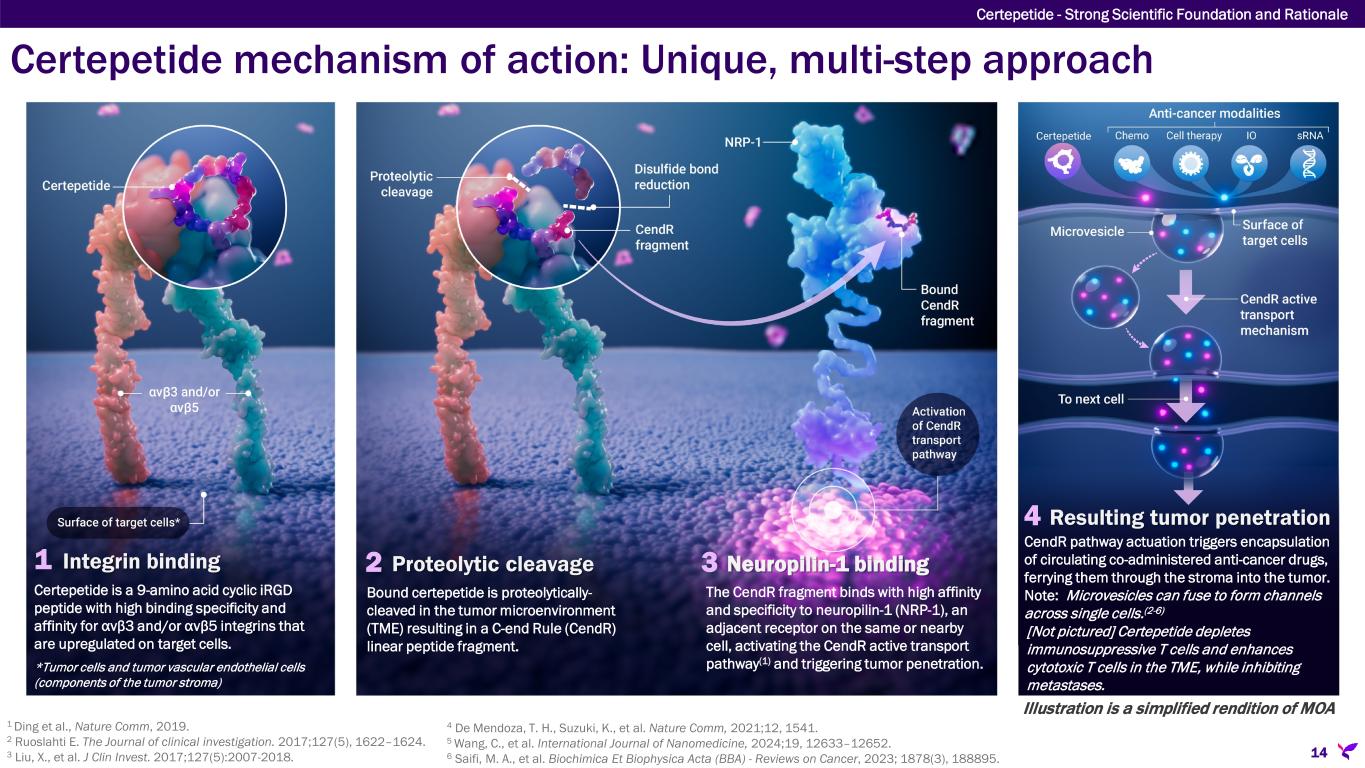
Certepetide mechanism of action: Unique, multi-step approach Certepetide - Strong Scientific Foundation and Rationale 14 1 Integrin binding 2 Proteolytic cleavage 3 Neuropilin-1 binding 4 Resulting tumor penetration Bound certepetide is proteolytically- cleaved in the tumor microenvironment (TME) resulting in a C-end Rule (CendR) linear peptide fragment. The CendR fragment binds with high affinity and specificity to neuropilin-1 (NRP-1), an adjacent receptor on the same or nearby cell, activating the CendR active transport pathway(1) and triggering tumor penetration. Certepetide is a 9-amino acid cyclic iRGD peptide with high binding specificity and affinity for αvβ3 and/or αvβ5 integrins that are upregulated on target cells. *Tumor cells and tumor vascular endothelial cells (components of the tumor stroma) CendR pathway actuation triggers encapsulation of circulating co-administered anti-cancer drugs, ferrying them through the stroma into the tumor. Note: Microvesicles can fuse to form channels across single cells.(2-6) [Not pictured] Certepetide depletes immunosuppressive T cells and enhances cytotoxic T cells in the TME, while inhibiting metastases. Illustration is a simplified rendition of MOA 1 Ding et al., Nature Comm, 2019. 2 Ruoslahti E. The Journal of clinical investigation. 2017;127(5), 1622–1624. 3 Liu, X., et al. J Clin Invest. 2017;127(5):2007-2018. 4 De Mendoza, T. H., Suzuki, K., et al. Nature Comm, 2021;12, 1541. 5 Wang, C., et al. International Journal of Nanomedicine, 2024;19, 12633–12652. 6 Saifi, M. A., et al. Biochimica Et Biophysica Acta (BBA) - Reviews on Cancer, 2023; 1878(3), 188895.

Certepetide/iRGD selectively promotes intratumoral penetration 1 Braun et al., Nature Mater. 2014. 2 Liu, Braun et al., Nature Comm. 2017. 3 Sugahara et al 2010. 15 Certepetide - Strong Scientific Foundation and Rationale Whole body imaging of mice with pancreatic ductal adenocarcinoma (arrow) dosed with Fluorescent Quantum Dots (FQDs) with and without certepetide(1),(2) Circulating FQDs result in whole body fluorescence Etching solution quenches fluorescence in circulation tumor FQDs + Etching solution tumor Certepetide + FQDs + Etching solution When co-administered with iRGD, nab- paclitaxel (Abraxane or ABX) is preferentially taken up by tumor tissue in mice(3) FQDs

Broad applicability & activity of certepetide/iRGD consistently demonstrated Sampling of >370 scientific publications showing improved survival 16 Certepetide - Strong Scientific Foundation and Rationale Breast cancer + Herceptin®Lung cancer + gemcitabine Zhang, et al., Plos One, 2015 Breast cancer + nanoparticle nab-paclitaxel GI cancer + adoptive cell therapy Ding, et al., Nature, 2019 PDAC + irinotecan nanoparticles Liu X et al., J Clin Invest, 2017 PDAC + gemcitabine Hurtado de Mendoza et al, Nature Comms, 2021 Sugahara, et al., Science, 2010Sugahara, et al., Science, 2010

Certepetide/iRGD consistently improves immunotherapy efficacy in multiple preclinical solid tumor models Certepetide - Strong Scientific Foundation and Rationale Solid Tumor Types Intrahepatic cholangiocarcinoma Pancreatic adenocarcinoma Prostate cancer Breast cancer Non-Small Cell Lung Cancer Gastric cancer Hepatocellular carcinoma Preclinical Observations Improved overall survival Reduced tumor size Reduced and/or inhibited metastases Sugahara, K., et al. bioRxiv 2023.05.24.542137; doi: https://doi.org/10.1101/2023.05.24.542137 Yuan, D., Duda, D., et al. 2024 CCA Foundation Conference. Poster: Enhancing the efficacy of standard therapy in intrahepatic cholangiocarcinoma using LSTA1, a novel tumor targeting and penetration agent. Sugahara, et al. 2015; Sugahara, et al. 2010 Yang, et al. 2019a; Yang, et al. 2019b Zhang et al 2016b Dong, et al. 2023 17

Certepetide improves immunotherapy impact in cholangiocarcinoma 18 Certepetide - Strong Scientific Foundation and Rationale Intrahepatic cholangiocarcinoma (ICC) has an immunosuppressive TME and a dense desmoplastic stroma with abnormal vasculature which together impede anti-cancer agent efficacy Lung metastases often lead to a significant decline in survival Human ICC SoC (gemcitabine/cisplatin/durvalumab) efficacy improved with certepetide in murine model Yuan, D., Duda, D., et al. CCA Foundation Conf. (2024) Poster. Enhancing the efficacy of standard therapy in intrahepatic cholangiocarcinoma using LSTA1, a novel tumor targeting and penetration agent Certepetide combined with chemo- and immunotherapy improves survival, reduces morbidity and inhibits metastasis in cholangiocarcinoma mouse model ICC mouse model *Certepetide was formerly known as LSTA1

iRGD enhances selective tumor penetration of trastuzumab Mouse model injected with human BT474 breast tumors Panel A shows greater staining for trastuzumab in breast cancer tissue with iRGD Panel B shows remarkable selectivity for tumor tissue with iRGD Panel C shows iRGD co-administered with trastuzumab leads to tumor shrinkage Sugahara, et al. 2010 Certepetide - Strong Scientific Foundation and Rationale 19 Trastuzumab + iRGDTrastuzumab A CB Tumor Liver Spleen HeartPancreas Lung Kidney Brain Certepetide is a proprietary iRGD; experiments denoting iRGD use a non-proprietary certepetide analog with differs in structure by a single acetyl group. Trastuzumab is a monoclonal Ab that inhibits HER2

Certepetide development strategy: A two-pillar approach 20 Certepetide - Strong Scientific Foundation and Rationale Pursue rapid global registration in mPDAC, initially combined with gemcitabine/nab-paclitaxel standard-of- care (SoC) Positive ASCEND Phase 2 initial results Phase 3 preparation underway Provisional approval application (TGA)* preparation underway Demonstrate certepetide effectiveness when combined with a variety of other SoC regimens (e.g., chemotherapy, immunotherapy, etc.) in a variety of solid tumors Multiple Phase 1 studies underway *TGA: Therapeutic Goods Administration (Australia)

21 Certepetide - Strong Scientific Foundation and Rationale Certepetide improved survival in mPDAC in two independent, multicenter Phase 1 trials 1 Von Hoff D, et al., New England Journal of Medicine, 2013. 2 Dean A, et al., The Lancet Gastroenterology & Hepatology, 2022 3 QILU Pharmaceutical 0.5 2.5 4.5 6.5 8.5 10.5 12.5 Median Overall Survival (Months) M on th s 13.2 months 8.5 months 11.1 months 55% improvement SoC + certepetide 31% improvement SoC CEND1-201 China Phase 1 Gemcitabine + Nab-paclitaxel + certepetide(3) Von Hoff 2013* Phase 3 Gemcitabine + Nab-paclitaxel(1) *Registration study establishing gemcitabine + nab-paclitaxel as SoC in mPDAC CEND1-001 Australia Phase 1 Gemcitabine + Nab-paclitaxel + certepetide(2)

Certepetide demonstrated internal consistency of response in two Phase 1 trials Certepetide - Strong Scientific Foundation and Rationale 1 Von Hoff D, et al., New England Journal of Medicine, 2013. 2 Dean A, et al., The Lancet Gastroenterology & Hepatology, 2022 3 QILU Pharmaceutical 22 Complete Response Partial Response Objective Response Rate Disease Control Rate (16 weeks) CA19-9 Drop >20% Median PFS 23% 59% 23% 3.4% 0.1% 55% 79% 96% 0% 42% 48% 61% 87% 91% SoC SoC SoC SoCSoC 5.5 mo 9.7 mo 5.8 mo 42% CEND1-201 China Phase 1 Gemcitabine + Nab-paclitaxel + certepetide(3) Von Hoff 2013* Phase 3 Gemcitabine + Nab-paclitaxel(1) *Registration study establishing gemcitabine + nab-paclitaxel as SoC in mPDAC CEND1-001 Australia Phase 1 Gemcitabine + Nab-paclitaxel + certepetide(2)

Remarkable evidence of certepetide activity in other solid tumors Certepetide potentiated a complete response in metastatic gastroesophageal adenocarcinoma (mGEAC) FDG-PET* scan June 2022 FDG-PET scan Sept. 2022 Reduction in FDG activity demonstrated(1) 53-year-old male with mGEAC with significant (> 5cm) nodal metastases (June 2022) SoC combination chemotherapy (FOLFIRINOX) and radiotherapy, with immunotherapy (pembrolizumab) later added, resulting in partial response Certepetide added to above regimen at cycle 7 and exploratory laparoscopy after cycle 18 (September 2022) showed no discernable disease 26+ months with sustained complete response 231 Buck, K.K, Dean, A., McSweeney, T. LSTA1 Potentiates Complete Response in Metastatic Gastroesophageal Adenocarcinoma. Oncol Cancer Case Rep. 2023, 9(6), 001-003 Certepetide - Strong Scientific Foundation and Rationale *Fluorodeoxyglucose (FDG)-positron emission tomography (PET)

ASCEND: Phase 2 study of certepetide in mPDAC Sponsor: Australasian Gastro-Intestinal Trials Group and NHMRC Clinical Trials Centre of University of Sydney (Australia) • Lisata-funded, data contractually sponsor- controlled • Restricts initial public announcement of results to scientific meetings or publications ‘Academic design’ overlooks global regulatory standards supporting eventual approval • Powered for 6-mos. PFS primary endpoint • Single cohort with one IV push of certepetide 3.2 mg/kg + SoC vs. SoC alone Investigator initiated trial inherited through acquisition of Cend Therapeutics Lisata amended protocol to ensure trial data will support global registration strategy Lisata clinical trial data rights defined/expanded ‘Product development design’ considers eventual regulatory review and approval Median overall survival (precedent registration endpoint) added for both cohorts Second cohort (Cohort B) added with two IV pushes of certepetide 3.2 mg/kg administered 4 hours apart to further evaluate pharmacodynamics of certepetide consistent with FDA Project Optimus 24 Certepetide - Strong Scientific Foundation and Rationale

ASCEND: Preliminary Cohort A progression-free survival data Cohort A ONE IV PUSH OF CERTEPETIDE + SoC Cohort A powered for 6-month PFS No statistically significant improvement shown with certepetide Data mature - 91% (86 out of 95 patients with Progressive Disease or Death) Cohort A: Progression-Free Survival (PFS) Data Treatment arm N Median PFS in months (95% CI) Certepetide 66 5.55 (5.29, 7.39) Placebo 21 5.52 (3.68, 7.39) median Moderately Censored Certepetide Placebo 25 Certepetide - Strong Scientific Foundation and Rationale
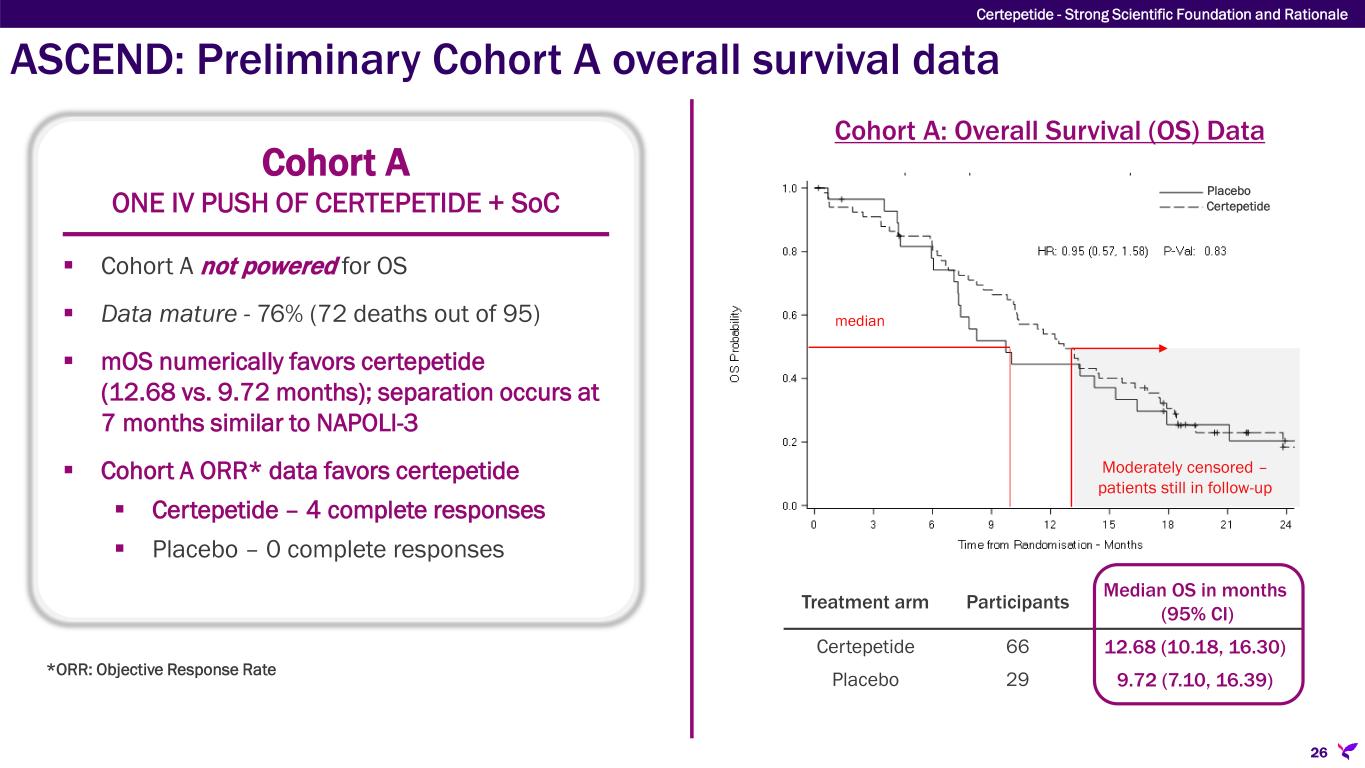
ASCEND: Preliminary Cohort A overall survival data 26 Certepetide - Strong Scientific Foundation and Rationale Cohort A ONE IV PUSH OF CERTEPETIDE + SoC Cohort A not powered for OS Data mature - 76% (72 deaths out of 95) mOS numerically favors certepetide (12.68 vs. 9.72 months); separation occurs at 7 months similar to NAPOLI-3 Cohort A ORR* data favors certepetide Certepetide – 4 complete responses Placebo – 0 complete responses Moderately censored – patients still in follow-up Cohort A: Overall Survival (OS) Data Treatment arm Participants Median OS in months (95% CI) Certepetide 66 12.68 (10.18, 16.30) Placebo 29 9.72 (7.10, 16.39) median Certepetide Placebo *ORR: Objective Response Rate

Certepetide improves clinical outcomes in mPDAC with benign safety profile Certepetide Clinical Data Summary to Date Two Phase 1 clinical trials (CEND1-001 in Australia and CEND1-201 in China) demonstrate that certepetide plus SoC chemotherapy improves overall survival in metastatic PDAC akin to the recently FDA-approved NALIRIFOX triplet therapy Certepetide is well tolerated with no dose-limiting toxicity Cumulative certepetide adverse events reflect companion therapy with which it is administered ASCEND Phase 2 trial Cohort A data demonstrate positive trend in overall survival, including 4 complete responses observed in certepetide treatment group compared to none in placebo group • Preliminary information suggests that Cohort B data will support a benefit of certepetide + SoC versus SoC alone; however, final, definitive data are expected by mid-2025 Phase 3 trial preparation underway 27 Certepetide - Strong Scientific Foundation and Rationale

28 Certepetide Clinical/Regulatory Development Portfolio

Certepetide – Clinical / Regulatory Development Portfolio Certepetide special regulatory designations and benefits 29 FDA Fast Track Designation • Pancreatic cancer (FDA) Eligible for Accelerated Approval, Priority Review and Rolling Review Provides for program-specific guidance from and frequent communication with FDA Orphan Drug Designations • Pancreatic cancer (FDA & EMA) • Malignant glioma (FDA) • Osteosarcoma (FDA) • Cholangiocarcinoma (FDA) Eligible for tax credits, marketing exclusivity, fee waivers and development grants Provides for specialized regulatory assistance from FDA's Office of Orphan Products Development FDA Rare Pediatric Disease Designation • Osteosarcoma (FDA) Eligible for Priority Review Voucher upon approval; redeemable for a priority review for any subsequent marketing application, or may be sold or transferred Vouchers have sold recently for $75-$100 million and, historically, for up to $350 million
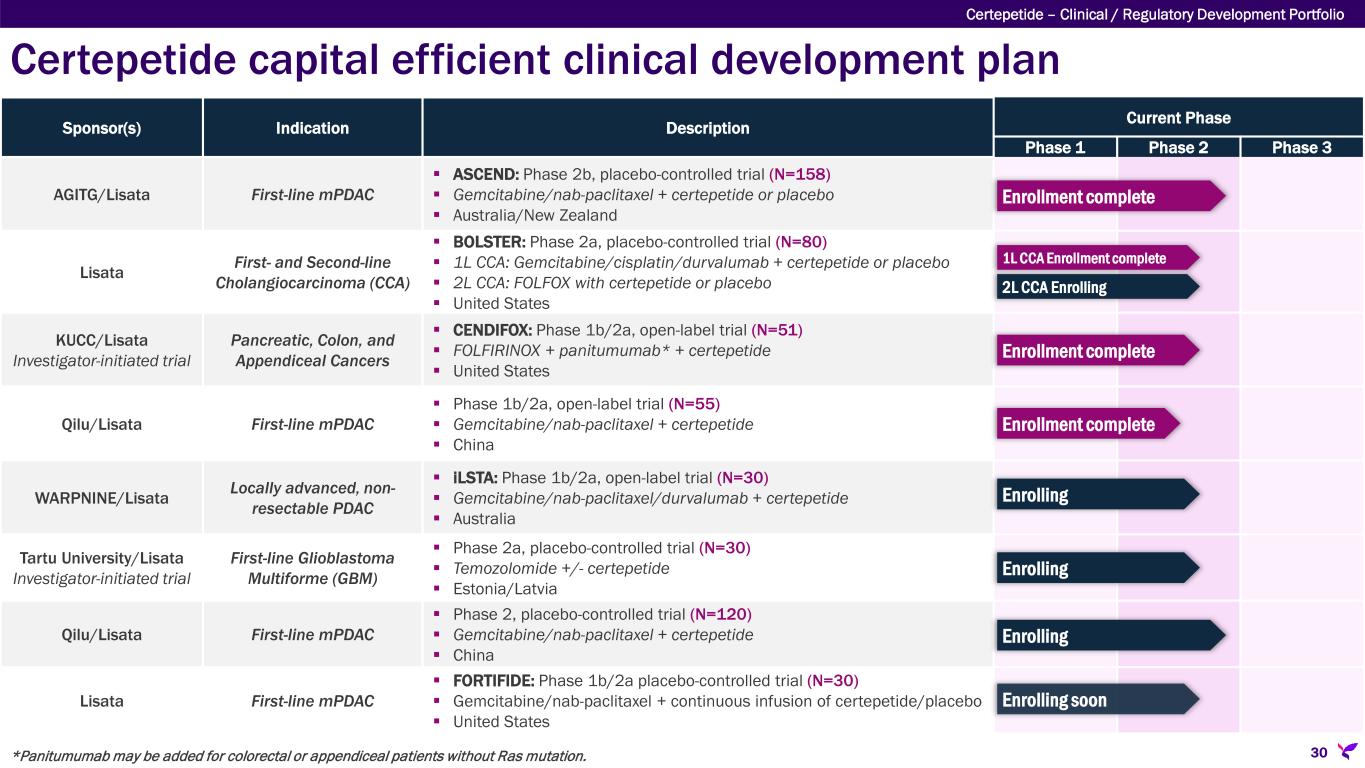
Certepetide capital efficient clinical development plan Certepetide – Clinical / Regulatory Development Portfolio 30 Sponsor(s) Indication Description Current Phase Phase 1 Phase 2 Phase 3 AGITG/Lisata First-line mPDAC ASCEND: Phase 2b, placebo-controlled trial (N=158) Gemcitabine/nab-paclitaxel + certepetide or placebo Australia/New Zealand Lisata First- and Second-line Cholangiocarcinoma (CCA) BOLSTER: Phase 2a, placebo-controlled trial (N=80) 1L CCA: Gemcitabine/cisplatin/durvalumab + certepetide or placebo 2L CCA: FOLFOX with certepetide or placebo United States KUCC/Lisata Investigator-initiated trial Pancreatic, Colon, and Appendiceal Cancers CENDIFOX: Phase 1b/2a, open-label trial (N=51) FOLFIRINOX + panitumumab* + certepetide United States Qilu/Lisata First-line mPDAC Phase 1b/2a, open-label trial (N=55) Gemcitabine/nab-paclitaxel + certepetide China WARPNINE/Lisata Locally advanced, non- resectable PDAC iLSTA: Phase 1b/2a, open-label trial (N=30) Gemcitabine/nab-paclitaxel/durvalumab + certepetide Australia Tartu University/Lisata Investigator-initiated trial First-line Glioblastoma Multiforme (GBM) Phase 2a, placebo-controlled trial (N=30) Temozolomide +/- certepetide Estonia/Latvia Qilu/Lisata First-line mPDAC Phase 2, placebo-controlled trial (N=120) Gemcitabine/nab-paclitaxel + certepetide China Lisata First-line mPDAC FORTIFIDE: Phase 1b/2a placebo-controlled trial (N=30) Gemcitabine/nab-paclitaxel + continuous infusion of certepetide/placebo United States Enrollment complete Enrollment complete Enrollment complete Enrolling *Panitumumab may be added for colorectal or appendiceal patients without Ras mutation. Enrolling Enrolling 1L CCA Enrollment complete 2L CCA Enrolling Enrolling soon

Certepetide preclinical activities and milestones Sponsor(s) Indication Objective and Description Upcoming Milestones University of Cincinnati/Lisata Endometriosis Assess the therapeutic effect of adding certepetide to bevacizumab (VEGF inhibitor) on the size and number of endometriotic lesions. Certepetide + bevacizumab Murine endometriosis model C57BL/6J United States Target date for data: 1Q2025 Valo Therapeutics/Lisata Melanoma Assess the therapeutic effects of PeptiCRAd (oncolytic virus), certepetide, and a checkpoint inhibitor (CPI) on systemic T cell responses, T cell infiltration into tumors, and impact on tumor growth control. Certepetide + PeptiCRAd + CPI Murine melanoma model B16-OVA Finland Target date for data: 2H2025 Certepetide – Clinical / Regulatory Development Portfolio 31
Clinical Development Milestones 32
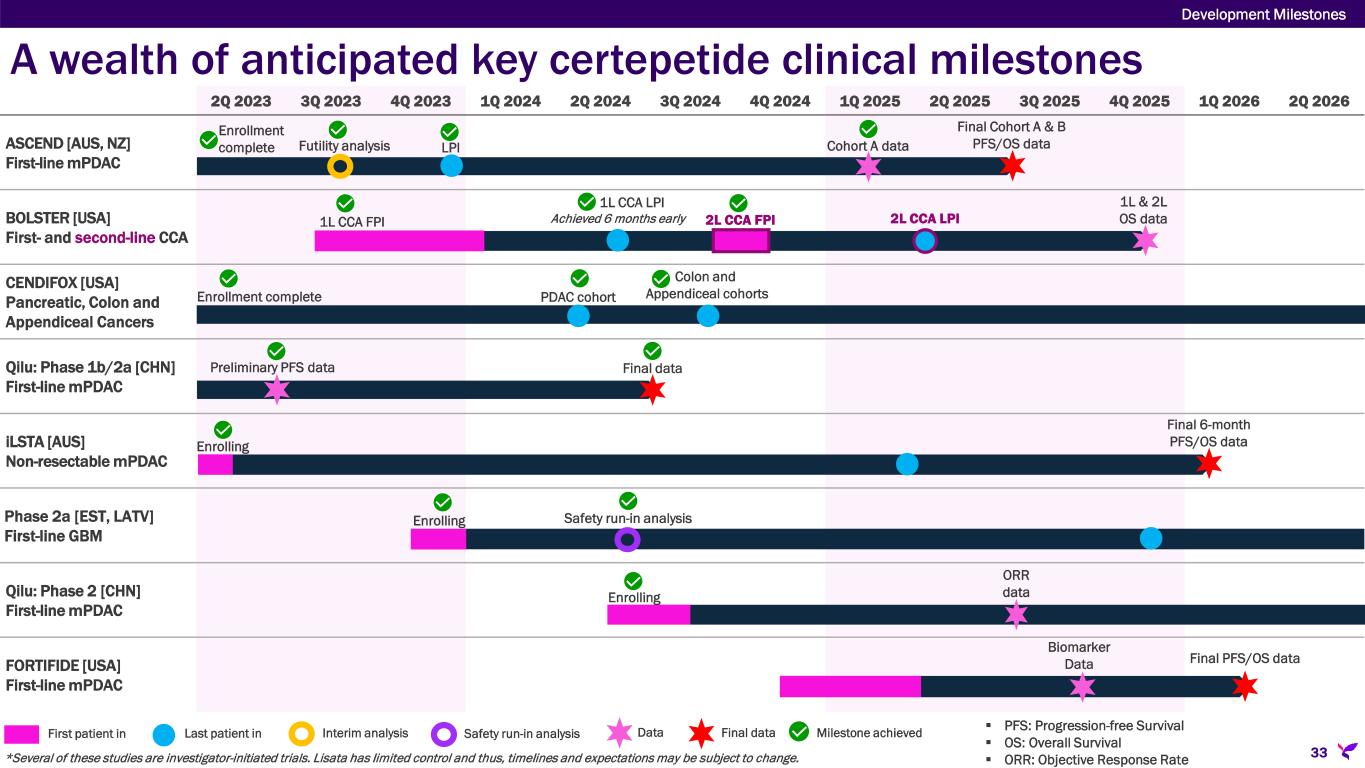
A wealth of anticipated key certepetide clinical milestones Development Milestones 33 2Q 2023 3Q 2023 4Q 2023 1Q 2024 2Q 2024 3Q 2024 4Q 2024 1Q 2025 2Q 2025 3Q 2025 4Q 2025 1Q 2026 2Q 2026 ASCEND [AUS, NZ] First-line mPDAC BOLSTER [USA] First- and second-line CCA CENDIFOX [USA] Pancreatic, Colon and Appendiceal Cancers Qilu: Phase 1b/2a [CHN] First-line mPDAC iLSTA [AUS] Non-resectable mPDAC Phase 2a [EST, LATV] First-line GBM Qilu: Phase 2 [CHN] First-line mPDAC FORTIFIDE [USA] First-line mPDAC PDAC cohort Colon and Appendiceal cohortsEnrollment complete Cohort A data Final Cohort A & B PFS/OS data Futility analysis Enrollment complete Preliminary PFS data Final data ORR dataEnrolling *Several of these studies are investigator-initiated trials. Lisata has limited control and thus, timelines and expectations may be subject to change. PFS: Progression-free Survival OS: Overall Survival ORR: Objective Response Rate First patient in Last patient in Interim analysis Data Final data Milestone achievedSafety run-in analysis Enrolling Safety run-in analysis Biomarker Data Final PFS/OS data 1L & 2L OS data1L CCA FPI 1L CCA LPI Achieved 6 months early 2L CCA LPI2L CCA FPI LPI Final 6-month PFS/OS data Enrolling

Financial Highlights 34

Capital projected to fund all clinical programs to data Financial Highlights 35 $35.9M Cash & Investments As of 9/30/2024 $0 Debt 1Q2026 Projected Cash Runway Into Common Shares Outstanding (9/30/2024): 8.3 million shares Options Outstanding (9/30/2024): Exercise Price: $0.02 - $4.22 = 1,217,400 shares Exercise Price: > $4.22 = 237,100 shares 1.5 million shares Warrants Outstanding (9/30/2024): Weighted Average Exercise Price: $40.52 1.5 million shares

* As of 9/30/2024; includes investments Key factors supporting investment in Lisata Therapeutics 36 Strong Investment Rationale PEOPLE Seasoned management with successful international drug development experience and expertise INTELLECTUAL PROPERTY Proprietary field- leading technology with global IP protection extending beyond 2040 MILESTONES Multiple product and business milestones projected over the next 12 - 18 months CAPITAL $35.9 million cash*- no debt; Current development funded through critical data milestones PARTNERING Platform technology validated by existing partnerships with potential for many others

© L i s a t a T h e r a p e u t i c s , I n c . 2 0 2 5 . A l l r i g h t s r e s e r v e d . Investor Relations Contact: John D. Menditto VP, IR & Corporate Communications Tel: (908) 842-0084 | Email: jmenditto@lisata.com Nasdaq: LSTA | www.lisata.com Targeted Therapy Delivered

Appendix 38

Appendix Certepetide capital efficient clinical development plan Development Partner(s) [Development Venue] Indication and Trial Product/Comparator Stage of Development Strategic Rationale Lisata/AGITG [Australia/New Zealand] First-line mPDAC; Gemcitabine/nab-paclitaxel with certepetide or placebo Phase 2b (ASCEND) Corroborate Phase 1b results in a placebo-controlled trial and evaluate 2 dose regimens of certepetide for dose optimization Lisata [United States] First- and Second-line Cholangiocarcinoma (CCA); 1L CCA: Gemcitabine/cisplatin/durvalumab + certepetide or placebo 2L CCA: FOLFOX + certepetide or placebo Phase 2a (BOLSTER) Assess certepetide safety and effectiveness in cholangiocarcinoma in a placebo-controlled trial (proof-of-concept) KUCC/Lisata* [United States] Pancreatic, Colon & Appendiceal Cancers; FOLFIRINOX + panitumumab** with certepetide Phase 1b/2a (CENDIFOX) Tumor immuno-profiling pre- & post- treatment and certepetide effectiveness assessment in combination with chemo and an EGFR inhibitor (open-label) Qilu [China] First-line mPDAC; Gemcitabine/nab-paclitaxel + certepetide Phase 1b/2a Assess safety, PK and therapeutic effect of certepetide in Chinese patients (open-label) WARPNINE/Lisata [Australia] Locally Advanced, Non-Resectable PDAC; Gemcitabine/nab-paclitaxel/durvalumab + certepetide Phase 1b/2a (iLSTA) Assess certepetide safety and effectiveness in combination with IO & Chemo in locally advanced PDAC; determine if inoperable tumors can become operable (open-label) Tartu University/Lisata* [Estonia/Latvia] First-line Glioblastoma Multiforme (GBM); Temozolomide +/- certepetide Phase 2a Assess certepetide safety and effectiveness in additional tumor type (GBM) in a placebo-controlled trial Qilu [China] First-line mPDAC; Gemcitabine/Nab-paclitaxel + certepetide Phase 2b Continue development of certepetide in China (placebo controlled) Lisata [United States] First-line mPDAC; Gemcitabine/nab-paclitaxel + continuous infusion of certepetide or placebo Phase 1b/2a (FORTIFIDE) Evaluate the safety, tolerability, and efficacy of a 4-hour continuous infusion of certepetide in combination with SoC in subjects with mPDAC who have progressed on FOLFIRINOX. Haystack MRD technology to measure ctDNA for early efficacy exploration. *Investigator-initiated trial **Panitumumab may be added for colorectal or appendiceal patients without Ras mutation 39

ASCEND: Phase 2b, blinded, randomized trial in mPDAC 40 Appendix Sponsor/Partner Australasian Gastro-Intestinal Trials Group (AGITG) in collaboration with the NHMRC Clinical Trials Centre at the University of Sydney Lisata funded (LSTA eligible for ~43% rebate on all qualified R&D expenses in AUS) Objective Corroborate Phase 1b results in a placebo-controlled study Determine if a second dose of certepetide further improves patient outcomes Design Phase 2b randomized, double-blind study in mPDAC testing gemcitabine + nab-paclitaxel SoC with one of two certepetide dose regimens or placebo Study Size N=158 (~30 sites in Australia and New Zealand) Endpoints Primary: Progression Free Survival Secondary: AEs, SAEs, Overall Survival, Objective Tumor Response Rate Timing Enrollment completed December 2023 Cohort A data released 1Q25 with Cohort B expected in 2Q25
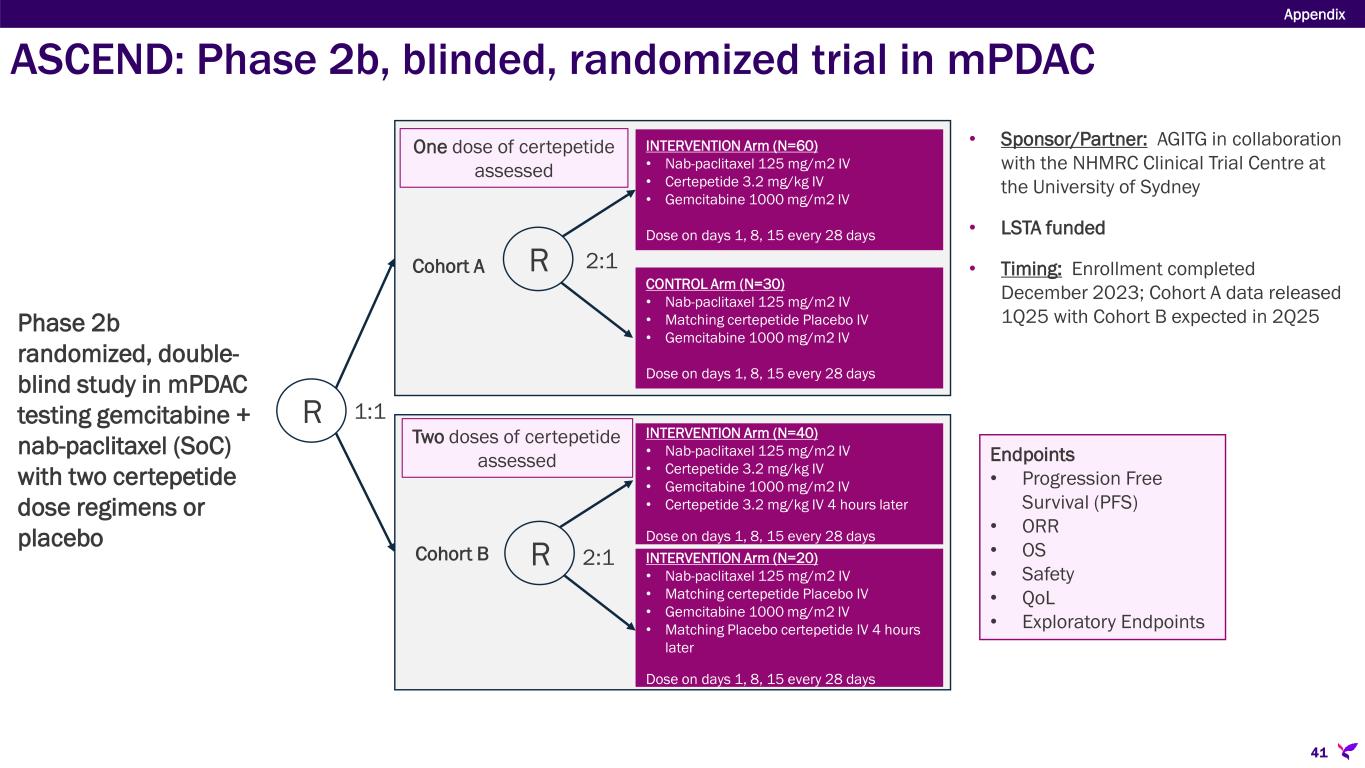
ASCEND: Phase 2b, blinded, randomized trial in mPDAC Appendix R INTERVENTION Arm (N=60) • Nab-paclitaxel 125 mg/m2 IV • Certepetide 3.2 mg/kg IV • Gemcitabine 1000 mg/m2 IV Dose on days 1, 8, 15 every 28 days CONTROL Arm (N=30) • Nab-paclitaxel 125 mg/m2 IV • Matching certepetide Placebo IV • Gemcitabine 1000 mg/m2 IV Dose on days 1, 8, 15 every 28 days INTERVENTION Arm (N=40) • Nab-paclitaxel 125 mg/m2 IV • Certepetide 3.2 mg/kg IV • Gemcitabine 1000 mg/m2 IV • Certepetide 3.2 mg/kg IV 4 hours later Dose on days 1, 8, 15 every 28 days INTERVENTION Arm (N=20) • Nab-paclitaxel 125 mg/m2 IV • Matching certepetide Placebo IV • Gemcitabine 1000 mg/m2 IV • Matching Placebo certepetide IV 4 hours later Dose on days 1, 8, 15 every 28 days R R Cohort A Cohort B 1:1 2:1 2:1 One dose of certepetide assessed Two doses of certepetide assessed Endpoints • Progression Free Survival (PFS) • ORR • OS • Safety • QoL • Exploratory Endpoints • Sponsor/Partner: AGITG in collaboration with the NHMRC Clinical Trial Centre at the University of Sydney • LSTA funded • Timing: Enrollment completed December 2023; Cohort A data released 1Q25 with Cohort B expected in 2Q25Phase 2b randomized, double- blind study in mPDAC testing gemcitabine + nab-paclitaxel (SoC) with two certepetide dose regimens or placebo 41

Sponsor/Partner Qilu Pharmaceutical (funds all development in China) Objective Evaluate safety, pharmacokinetics and preliminary efficacy of certepetide added to SoC in Chinese patients with mPDAC Design Phase 1b/2a open-label study in advanced mPDAC patients of Chinese ethnicity testing SoC chemotherapy (gemcitabine + Qilu-produced nab-paclitaxel) in combination with certepetide Study Size N=55 (~15 sites) Endpoints Primary: AEs, SAEs, Objective Response Rate, Duration of Response, Disease Control Rate, Overall Survival, and Progression Free Survival Secondary: Pharmacokinetic parameters Timing Final data anticipated 2H2025 Appendix 42 Phase 1b/2a open-label trial in mPDAC in China (CEND1-201)

7 Day Safety Evaluation Certepetide 1.6 mg/kg + nab-pac* + gem Days 1, 8, and 15 every 28 days Confirm Eligibility Informed Consent Extension Stage mPDAC 1.6 mg/kg certepetide Day 1 Phase 1b/2a study evaluating the safety, pharmacokinetics, and preliminary efficacy of certepetide for injection in Chinese patients with advanced metastatic pancreatic ductal adenocarcinoma mPDAC 3.2 mg/kg certepetide Day 1 7 Day Safety Evaluation Certepetide 3.2 mg/kg + nab-pac* + gem Days 1, 8, and 15 every 28 days Phase 1b N=3 N=3 Phase 2 Extension N=10-12 N=10-12 N=30-50 Disease Progression Response rates PFS OS Safety • Sponsor/Partner: Qilu Pharmaceutical (funds all development in China) • Timing: Final data anticipated 2H2024 *Qilu produced Phase 1b/2a open-label trial in mPDAC in China (CEND1-201) Appendix 43

Sponsor/Partner University of Kansas Medical Center (Investigator initiated trial in U.S.) KUCC funded; Lisata provides certepetide Objective Evaluate the safety and therapeutic effect of certepetide in combination with neoadjuvant FOLFIRINOX- based therapies and an EGFR inhibitor for the treatment of pancreatic, colon and appendiceal cancers and determine immuno-profiling in tumor pre- & post- treatment Design Phase 1b/2a open-label study in resectable pancreatic, colon with oligo metastases and appendiceal with peritoneal metastases cancers testing SoC chemotherapy (neoadjuvant FOLFIRINOX-based therapies) with certepetide ± panitumumab Study Size N=51 (21 PDAC, 15 colon and 15 appendiceal) Endpoints Primary: Drug Safety Secondary: Overall Survival, Disease-free Survival, Overall Response Rate, RO Resection Rate, Pathological Response Rate Timing Enrollment completed 4Q24 CENDIFOX: Phase 1b/2a open-label trial in PDAC and other cancers Appendix 44
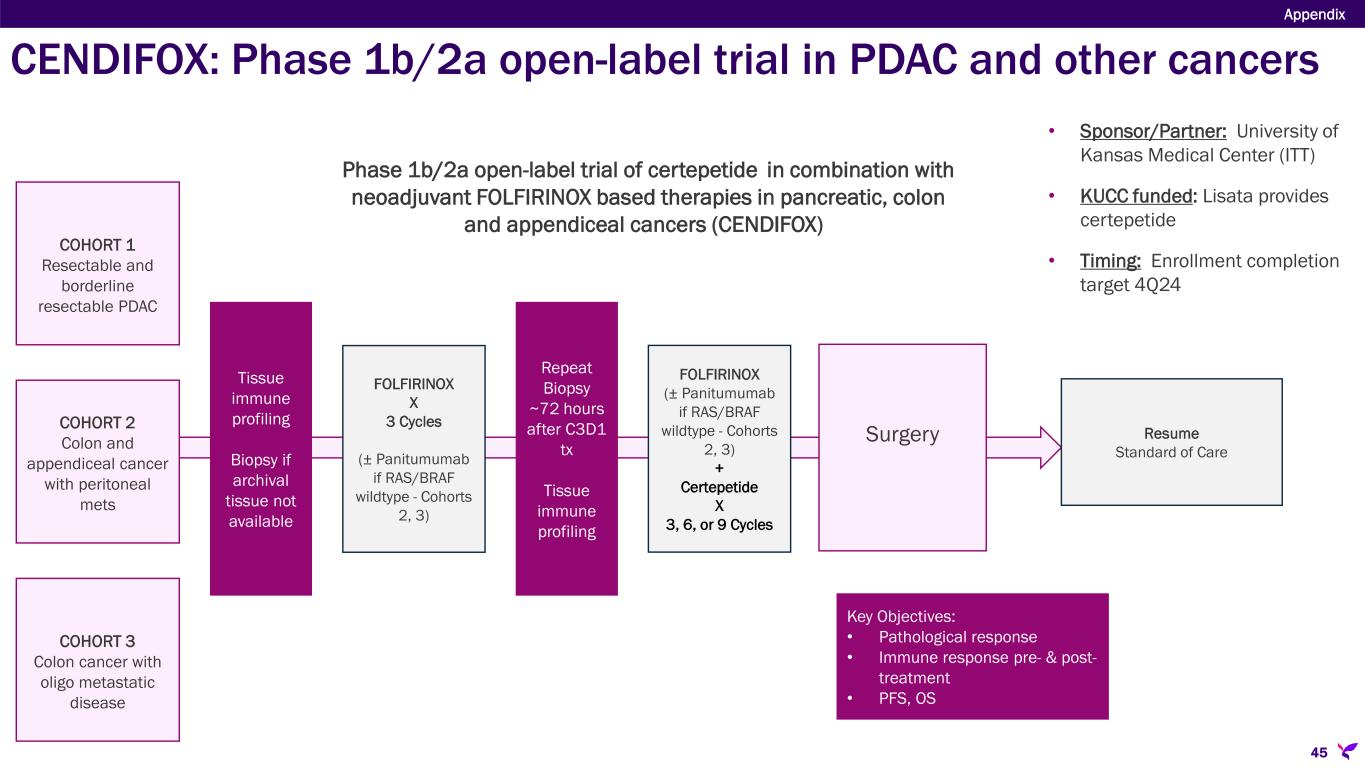
Surgery COHORT 1 Resectable and borderline resectable PDAC Key Objectives: • Pathological response • Immune response pre- & post- treatment • PFS, OS FOLFIRINOX X 3 Cycles (± Panitumumab if RAS/BRAF wildtype - Cohorts 2, 3) Tissue immune profiling Biopsy if archival tissue not available COHORT 2 Colon and appendiceal cancer with peritoneal mets COHORT 3 Colon cancer with oligo metastatic disease Repeat Biopsy ~72 hours after C3D1 tx Tissue immune profiling FOLFIRINOX (± Panitumumab if RAS/BRAF wildtype - Cohorts 2, 3) + Certepetide X 3, 6, or 9 Cycles Resume Standard of Care Phase 1b/2a open-label trial of certepetide in combination with neoadjuvant FOLFIRINOX based therapies in pancreatic, colon and appendiceal cancers (CENDIFOX) • Sponsor/Partner: University of Kansas Medical Center (ITT) • KUCC funded: Lisata provides certepetide • Timing: Enrollment completion target 4Q24 CENDIFOX: Phase 1b/2a open-label trial in PDAC and other cancers Appendix 45

Sponsor/Partner Lisata (U.S.) Objective Evaluate the preliminary efficacy, safety and tolerability of certepetide in combination with standards of care in subjects with first- and second-line cholangiocarcinoma Design Phase 2 randomized, double-blind, placebo-controlled, proof-of-concept trial in first- and second-line cholangiocarcinoma testing corresponding SoC with certepetide or placebo Study Size N=80 (N=40 per tumor type) 1:1 SoC + certepetide or SoC + placebo Endpoints Primary: OS Secondary: Safety, ORR, PFS Timing Enrollment completed for 1L CCA Enrollment commenced July 2024 for 2L CCA BOLSTER: Phase 2 blinded, randomized trial in Cholangiocarcinoma Appendix 46

Dosed on Days 1, 8 every 21 days X 8 cycles Disease Progression Response rates Safety Confirm Eligibility Informed Consent Survival Analysis Certepetide + cisplatin/gemcitabine/durvalumab 72-hour run-in without SoC 1st line Cholangiocarcinoma (CCA) Placebo + cisplatin/gemcitabine/durvalumab N=20 N=20 R Phase 2a, double-blind, placebo-controlled, multi-center, randomized study evaluating certepetide when added to standard of care (SoC) versus standard of care alone in subjects with first- and second-line cholangiocarcinoma • Sponsor: Lisata • Timing: • Enrollment completed for 1L CCA • Enrollment anticipated July 2024 for 2L CCA BOLSTER: Phase 2 blinded, randomized PoC trial in various cancers Appendix 47 Dosed every 14 days Disease Progression Response rates Safety Confirm Eligibility Informed Consent Survival Analysis Certepetide + FOLFOX 72-hour run-in without SoC 2nd line Cholangiocarcinoma (CCA) Placebo + FOLFOX N=20 N=20 R

Sponsor/Partner Qilu Pharmaceutical (funds all development in China) Objective Further evaluate safety and therapeutic efficacy of certepetide when added to SoC in Chinese patients with locally advanced unresectable mPDAC Design Phase 2b, double-blind, placebo-controlled, randomized study evaluating certepetide + SoC (Qilu-produced nab-paclitaxel and gemcitabine) vs. placebo + SoC Study Size N=120 (1:1 SoC + certepetide or SoC + placebo) Endpoints Objective response rate, progression free survival, duration of response, disease control rate, overall survival Safety Timing Trial initiated 2Q24 Appendix 48 Phase 2 double-blind, placebo-controlled trial in mPDAC in China

Days 1, 8, 15 and every 28 days Disease Progression Response rates PFS Safety Gemcitabine + Qilu produced nab-paclitaxel + certepetide 3.2 mg/kg Confirm Eligibility Informed Consent 1:1 Survival AnalysismPDAC R Gemcitabine + Qilu produced nab-paclitaxel + placebo N=60 N=60 Phase 2b, double-blind, placebo-controlled, randomized, multicenter study evaluating the safety and efficacy of certepetide when added to standard of care (nab-paclitaxel and gemcitabine) vs. standard of care alone and placebo in Chinese subjects with locally advanced unresectable mPDAC • Sponsor/Partner: Qilu Pharmaceutical (funds all development in China) • Timing: Trial initiated 2Q24 Phase 2 blinded, placebo-controlled trial in mPDAC in China Appendix 49

Sponsor/Partner WARPNINE, Inc. (registered charity in Australia) is funding trial Lisata providing study drug Objective Evaluate safety and therapeutic effect of LSTA1 in combination with IO & Chemo in locally advanced non-resectable pancreatic ductal adenocarcinoma (PDAC); determine if inoperable tumors can become operable Design Phase 1b/2a proof-of-concept safety and early efficacy study of LSTA1 in combination with durvalumab, gemcitabine and nab-paclitaxel, as first-line treatment in locally advanced non-resectable pancreatic adenocarcinoma Study Size N=30 Endpoints Safety and tolerability; 28-day DLTs Objective response rate, PFS, OS, duration of response, immune cell infiltration Timing Enrollment commenced April 2023 Appendix 50 iLSTA: Phase 1b/2a trial in locally advanced PDAC with chemo & IO

Cohort 2 Gemcitabine + nab-paclitaxel + LSTA1 + Placebo durvalumab N=5 Cohort 1 Gemcitabine + nab-paclitaxel + Placebo LSTA1 + Placebo durvalumab N=5 Cohort 3 Gemcitabine + nab-paclitaxel + LSTA1 + durvalumab N=up to 20 12 weeks 8 weeks tumor burden assessments until 24 months or recurrence 12 month Follow-up Primary Endpoint 24 months Follow-up completion EUS Biopsy Screening EUS & Biopsy At weeks 12-16 Tumor burden assessment at screening, cycle 2, and then 8-weekly thereafter. Patients are treated with 28-day cycles until surgery, definitive radiotherapy, unacceptable toxicity, progression or death Endpoints: safety, DLT, ORR, PFS, OS, DoR, immune profiling Ra nd om iz e Phase 1b/2a proof-of-concept safety and early efficacy study of LSTA1 in combination with durvalumab, gemcitabine and nab-paclitaxel, as first-line treatment in locally advanced non-resectable pancreatic ductal adenocarcinoma • Sponsor: WARPNINE, Inc. - funding trial • Timing: Enrollment commenced April 2023 Appendix 51 iLSTA: Phase 1b/2a trial in locally advanced PDAC with chemo & IO

Preliminary iLSTA Trial Results Poster at 2025 ASCO GI Appendix 52An abstract with a detailed summary of the poster presentation is also available on the ASCO GI website: https://meetings.asco.org/abstracts-presentations/241611

Sponsor/Partner Tartu University Hospital (Investigator initiated trial in Estonia) Lisata providing study drug and funding trial Objective Evaluate safety, tolerability, and therapeutic effect of certepetide in combination with standard-of-care (temozolomide) in patients with previously untreated Glioblastoma Multiforme Design Phase 2a proof-of-concept, double-blind, placebo-controlled, randomized study evaluating certepetide when added to standard of care (temozolomide) versus SoC and placebo in subjects with newly diagnosed Glioblastoma Multiforme (GBM) Study Size N=30 total (N=3 safety run-in, N=18 in main study schema) Endpoints Safety, tolerability ORR, PFS, OS, disease control rate Timing Enrollment commenced December 2023 Appendix 53 Phase 2a trial of certepetide with SoC in first-line GBM

Days 1, 2, 3, 4, 5 and every 28 days for 6 cycles Disease Progression Response rates Safety Temodar® + certepetideConfirm Eligibility Informed Consent 2:1 Survival Analysis 72-hour Run-in without SoC Newly Diagnosed GBM R Temodar® + certepetide matching placebo N=18 N=9 Phase 2a proof-of-concept double-blind, placebo-controlled, randomized, proof-of-concept study evaluating certepetide when added to standard of care (temozolomide) versus temozolomide and matching certepetide placebo in subjects with newly diagnosed GBM • Sponsor: Tartu University Hospital; Estonia • Funding: Lisata • Timing: Enrollment commenced December 2023 Appendix 54 Phase 2a trial of certepetide with SoC in first-line in GBM Main Study Schema Safety Lead-in Schema Confirm Eligibility Informed Consent Newly Diagnosed GBM 3-Day Run-in N=3 TMZ + certepetide Safety AnalysisCertepetide on Days 1, 2 which is repeated after 28 days for 2 cycles * TMZ + certepetide Days 1, 2, 3, 4, 5 and every 28 days for 4 remaining cycles • TMZ will be administered on Days 1, 2, 3, 4, and 5 of each cycle Disease Progression Response rates Safety Survival Analysis

Appendix 55 FORTIFIDE: Phase 1b/2a continuous infusion study of certepetide Sponsor/Partner Lisata (U.S. only) Objective Evaluate the safety, tolerability, pharmacodynamics, pharmacokinetics, and efficacy of certepetide when given as a 4-hour continuous infusion in combination with SoC in subjects with first-line mPDAC who have progressed on FOLFIRINOX. Haystack Oncology MRD technology to measure ctDNA for early efficacy exploration. Design Phase 1b/2a, double-blind, placebo-controlled, three-arm, randomized study evaluating the following treatment arms in subjects with first-line mPDAC who have progressed on FOLFIRINOX: an intravenous push of certepetide with continuous 4-hour infusion + SoC a single intravenous push of certepetide with continuous infusion of matching placebo + SoC an intravenous push of matching placebo with a continuous infusion of matching placebo + SoC Study Size N=30 Endpoints Safety and tolerability PFS, OS Timing First patient treated target 2Q25

FORTIFIDE: Phase 1b/2a continuous infusion study of certepetide 56 Subjects with 1L mPDAC Obtain Informed Consent Confirm Eligibility Gemcitabine, certepetide 3.2 mg/kg single IV push/ 4-hour continuous placebo infusion + nab-paclitaxel Days 1, 8, and 15 every 28 days Gemcitabine, certepetide placebo single IV push/ 4-hour continuous placebo infusion + nab-paclitaxel Days 1, 8, and 15 every 28 days N=10 N=10 Gemcitabine, certepetide 3.2 mg/kg single IV push/4-hour continuous certepetide infusion + nab-paclitaxel Days 1, 8, and 15 every 28 days N=10 R Safety Biomarkers PK Disease Progression Response rates PFS OS Primary Endpoint: • Safety Secondary Efficacy Endpoints: • Mean change from baseline in CA19-9 and CEA • Mean change from baseline in ctDNA (quantitative) • Pharmacokinetics of certepetide • ORR, PFS, OS Secondary Efficacy Endpoints to be assessed: • At baseline • C1 Day 1 (2 and 4 hours after dosing) • C1 Days 8, and 15 pre-dosing for Cycle 1 • Day 1 of each subsequent cycle until disease progression 72 hour Run-in without SoC 72 hour Run-in without SoC 72 hour Run-in without SoC























































