As filed with the Securities and Exchange Commission on February 20, 2024
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common Shares - par value | PHG | New York Stock Exchange | ||
| Euro (EUR) 0.20 per share |
None
(Title of class)Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.None
(Title of class)Indicate the number of outstanding shares of each of the issuer's classes of capital or common stock as of the close of the period covered by the annual report.
| Class | Outstanding at December 31, 2023 | |
| KONINKLIJKE PHILIPS NV | 906,403,156 shares | |
| Common Shares par value EUR 0.20 per share |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☒Yes ☐ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒ No
Note - Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer," "accelerated filer,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
Large Accelerated Filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the
effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C.
7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ | International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
This document contains information required for the Annual Report on Form 20-F for the year ended December 31, 2023 of Koninklijke Philips N.V. (the 2023 Form 20-F). Reference is made to the Form 20-F cross reference table herein. Only (i) the information in this document that is referenced in the Form 20-F cross reference table, (ii) this introduction and the cautionary statement “forward-looking statements” on the next two pages and (iii) the Exhibits shall be deemed to be filed with the Securities and Exchange Commission for any purpose. Any additional information in this document which is not referenced in the Form 20-F cross reference table, or the Exhibits themselves, shall not be deemed to be so incorporated by reference, shall not be part of the 2023 Form 20-F and is furnished to the Securities and Exchange Commission for information only.
References to PhilipsReferences to the Company or company, to Philips or the (Philips) Group or group, relate to Koninklijke Philips N.V. and its subsidiaries, as the context requires. Royal Philips refers to Koninklijke Philips N.V.
IFRS based informationThe audited consolidated financial statements as of December 31, 2023 and 2022, and for each of the years in the three-year period ended December 31, 2023, included in the 2023 Form 20-F have been prepared in accordance with International Financial Reporting Standards (IFRS) as endorsed by the European Union (EU). All standards and interpretations issued by the International Accounting Standards Board (IASB) and the IFRS Interpretations Committee effective 2023 have been endorsed by the EU; consequently, the accounting policies applied by Philips also comply with IFRS as issued by the IASB. These accounting policies have been applied by group entities.
Use of non-IFRS informationIn presenting and discussing the Philips financial position, operating results and cash flows, management uses certain financial measures that are not measures of financial performance or liquidity under IFRS (‘non-IFRS’). These non-IFRS measures should not be viewed in isolation as alternatives to the equivalent IFRS measure and should be used in conjunction with the most directly comparable IFRS measures. Non-IFRS measures do not have standardized meaning under IFRS and therefore may not be comparable to similar measures presented by other issuers. A reconciliation of these non-IFRS measures to the most directly comparable IFRS measures is contained in this document. Reference is made in Reconciliation of non-IFRS information.
Third-party market share dataStatements regarding market share, contained in this document, including those regarding Philips’ competitive position, are based on outside sources such as specialized research institutes, industry and dealer panels in combination with management estimates. Where full year information regarding 2023 is not yet available to Philips, market share statements may also be based on estimates and projections prepared by management and/or based on outside sources of information. Management's estimates of rankings are based on order intake or sales, depending on the business.
Documents on displayPhilips’ SEC filings are publicly available through the SEC’s website at www.sec.gov. The SEC website contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Philips’ internet address is www.philips.com/investor. The contents of any websites referred to herein shall not be considered a part of or incorporated by reference into this document.
For definitions and abbreviations reference is made in Definitions and abbreviations
Due to rounding, amounts may not add up precisely to the totals provided in this report.
Pursuant to provisions of the United States Private Securities Litigation Reform Act of 1995, Philips is providing the following cautionary statement.
This document, including the information referred to in the Form 20-F cross reference table, contains certain forward-looking statements with respect to the financial condition, results of operations and business of Philips and certain of the plans and objectives of Philips with respect to these items, in particular, among other statements, certain statements in Item 4 “Information on the Company” with regard to management objectives, market trends, market standing, product volumes, business risks, the statements in Item 5 “Operating and financial review and prospects” with regards to trends in results of operations, margins overall, market trends, risk management, exchange rates, the statements in Item 8 “Financial Information” relating to legal proceedings and goodwill and statements in Item 11 “Quantitative and qualitative disclosure about market risks” relating to risk caused by derivative positions, interest rate fluctuations and other financial exposure are forward-looking in nature. Forward-looking statements can be identified generally as those containing words such as “anticipates”, “assumes”, “believes”, “estimates”, “expects”, “should”, “will”, “will likely result”, “forecast”, “outlook”, “projects”, “may” or similar expressions. By their nature, these statements involve risk and uncertainty because they relate to future events and circumstances and there are many factors that could cause actual results and developments to differ materially from those expressed or implied by these statements.
These factors include but are not limited to: Philips’ ability to gain leadership in health informatics in response to developments in the health technology industry; Philips’ ability to keep pace with the changing health technology environment; macroeconomic and geopolitical changes; integration of acquisitions and their delivery on business plans and value creation expectations; securing and maintaining Philips’ intellectual property rights, and unauthorized use of third-party intellectual property rights; ability to meet expectations with respect to ESG-related matters; failure of products and services to meet quality or security standards, adversely affecting patient safety and customer operations; breach of cybersecurity; challenges in simplifying our organization and our ways of working; the resilience of our supply chain; attracting and retaining personnel; challenges in driving operational excellence and speed in bringing innovations to market; compliance with regulations and standards including quality, product safety and (cyber) security; compliance with business conduct rules and regulations including privacy and upcoming ESG disclosure and due diligence requirements; treasury and financing risks; tax risks; reliability of internal controls, financial reporting and management process; global inflation.
As a result, Philips’ actual future results may differ materially from the plans, goals and expectations set forth in such forward-looking statements. For a discussion of factors that could cause future results to differ from such forward-looking statements, reference is made to the information in Risk factors.
Only (i) the information in this document that is referenced in the Form 20-F cross reference table, (ii) the Introduction and the cautionary statements concerning forward-looking statements of this report on pages 6-7, and (iii) the Exhibits shall be deemed to be filed with the Securities and Exchange Commission for any purpose. The content of Philips’ websites and other websites referenced herein should not be considered to be a part of or incorporated into the 2023 Form 20-F. Any additional information which is not referenced in the Form 20-F cross reference table or the Exhibits themselves shall not be deemed to be so incorporated by reference, shall not be part of the 2023 Form 20-F and is furnished to the Securities and Exchange Commission for information only.
The table below sets out the location in this document of the information required by SEC Form 20-F. The exact location is included in the column ‘Location in this document’. The page number refers to the starting page of the section for reference only (and is not intended to refer to the starting page of the specific subsection, if applicable).
The challenges of a world in turmoil amplify the sense of urgency I feel to make sure Philips delivers on its purpose and becomes an even stronger force for good, so people everywhere can look after their health and well-being and access the care they need, with us focusing on where we can help, from the hospital to the home.
In January 2023, we announced our multi-year plan to create value with sustainable impact. Throughout the year, Philips teams around the world worked relentlessly, in a volatile environment, to deliver on the first phase of that plan, laying a strong foundation for sustained future success.
Our products and services reached 1.9 billion people in 2023, including 221 million in underserved communities – taking us closer to our goal of improving 2.5 billion lives per year by 2030, including 400 million in underserved communities.
Our improved operational performance was driven by significant progress on the three pillars of our plan to create value for all our stakeholders: 1) focused organic growth; 2) scalable people- and patient-centric innovation; and 3) focus on execution to enhance patient safety and quality, strengthen our supply chain reliability, and establish a simplified, agile operating model.
We achieved our raised 2023 outlook with strong sales growth, improved profitability, and strong cash flow, despite the uncertainties brought about by an increasingly turbulent geopolitical environment. While the order book remains strong in absolute terms, order intake was down, for which the necessary improvement actions are under way. There is still a lot of work to be done, but 2023 represents a good start, and it reinforces our confidence in delivering on our three-year plan.
Patient safety and qualityResolving the consequences of the Respironics recall for our patients and customers is a key focus area, and I apologize for the distress caused. Globally, over 99% of the sleep therapy device registrations that are complete and actionable have been remediated, while the remediation of the ventilator devices remains ongoing.
We are fully committed to complying with the terms of the consent decree agreed with the US Department of Justice (DOJ), representing the US Food and Drug Administration (FDA), which primarily focuses on Philips Respironics in the US. The proposed consent decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business. Further details will become available once the consent decree has been finalized and submitted to the relevant US court for approval.
As well as implementing all measures agreed with the FDA and DOJ in connection with the Respironics recall, we will continue to rebuild relations with the FDA and other national regulators. In October, Philips Respironics received preliminary court approval for the class action settlement that would resolve all or nearly all private economic loss claims in the US related to the recall. The settlement does not include or constitute any admission of liability, wrongdoing, or fault by any of the Philips parties. The previously disclosed litigation, including the personal injury and medical monitoring claims, and investigation by the DOJ related to the Respironics recall are ongoing.
Driving progress on prioritiesWith patient safety and quality the number one priority, oversight now resides at Executive Committee level, and we have a new organization in place, with stronger processes and more effective early warning systems in the businesses. We are pro-actively addressing quality improvements and first-time-right design. One of the most inspiring events of the year took place in October – a company-wide Timeout for Patient Safety and Quality. All 70,000 employees came together in their teams to discuss how we are moving forward on patient safety and quality, and how to take it further.
In our drive to create more reliable and resilient supply chains, we have significantly reduced our high-risk components and our inventories, and the actions we have taken continued to have a positive impact on our sales and service levels. We continue to strengthen further through regionalization of the supply base and manufacturing capability to better respond to local requirements with a shorter value chain.
We also started the shift to our new, simplified operating model – with end-to-end businesses supported by a leaner enterprise layer, strong regions and a reinvigorated impact culture – and completed the realignment of workforce roles and reporting lines. This included the difficult but necessary reduction of approximately 8,000 roles to date, out of the planned reduction of 10,000 roles by 2025.
Reflecting our changing culture of people- and patient-centricity, accountability and impact, our Executive Committee was strengthened with the arrival of four new members in 2023, each bringing valuable experience and skills to the work of our leadership team. We also welcome the decision by Exor to take a 15% minority stake in our company – a sign of confidence in our plan, our people, and our future. And we marked the 100th anniversary of Philips in China, a remarkable achievement.
The opportunity to deliver better care for more peopleToday, millions of people around the world have little or no access to basic healthcare, and climate change is impacting both environmental and human health. Healthcare is simply not working as it needs to. There are not enough doctors and nurses to address the growing demand for care. In parallel, rising costs are stretching financial budgets to the limit.
That’s why we are advocating for systemic change, driven by all ecosystem players, that addresses technology, clinical practice, financing and regulation as a whole. Change that delivers better, more productive healthcare that works for everyone. Without this change, communities all around the world will increasingly face challenges to get the care they need.
Focusing our efforts on where we can make a difference, we want to help more healthcare providers help more patients, in a sustainable way, and empower more people to take care of their health and well-being – by applying our combined capabilities in innovation, design and sustainability.
There is a lot of work to be done, but we see the potential for a future where health systems run smoothly, efficiently and sustainably, with doctors and nurses seeing the patient at the right time and at the right point of care. Where they can be confident that the right choice is also the easy choice, with simpler workflows enabling them to give patients the best care and best experience.
With real-time and predictive insights supporting collaboration across the patient journey. And AI being used in a responsible manner to optimize workflows and improve efficiency, so that clinical staff get the time and space to focus on what matters and what they do best: caring for their patients.
We see a future where it is also easier for people everywhere to look after their health and well-being. For example, with solutions helping more parents and babies in the first 1,000 days and supporting the connection between good oral care and good overall health.
Recognizing that human health and environmental health go hand in hand, we are collaborating with our customers and suppliers to decarbonize healthcare and so create a more sustainable and resilient industry.
Looking aheadWhile realistic about the challenging economic environment, geopolitical risks, and uncertainties around ongoing litigation, I am confident we will continue to deliver on our multi-year plan to create value with sustainable impact – helping consumers lead healthy lives and healthcare providers deliver efficient, high-quality care to patients in a sustainable way. Based on our ongoing actions to enhance execution – driving patient safety and quality, increasing supply chain reliability, and further simplifying how we work – we expect further performance improvement in 2024.
Against this background, and reflecting the importance we attach to dividend stability, we propose to maintain the dividend at EUR 0.85 per share, to be distributed fully in shares.
On behalf of the Executive Committee, I would like to thank our consumers, our customers and their patients, our suppliers and ecosystem partners for their support, and the Supervisory Board for their support and guidance. I also want to express my deep gratitude to our employees for their dedication to improving people’s lives and our company’s performance, and to our shareholders and other stakeholders for their continued support.
As I look ahead, I am realistic about the challenges we face, optimistic about building on the momentum we have created, and excited about delivering on our purpose – for the benefit of patients, customers and consumers the world over.
Roy Jakobs
Chief Executive Officer
Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties. The Board of Management is entrusted with the management of the company. The other members of the Executive Committee have been appointed to support the Board of Management in the fulfilment of its managerial duties. Please also refer to Board of Management and Executive Committee within the chapter Corporate governance.
Members of the Board of ManagementRoy Jakobs joined Philips in 2010 and has held various global leadership positions across the company, starting as Chief Marketing & Strategy Officer for Philips Lighting. In 2012, he became Market Leader for Philips Middle East & Turkey, leading the Healthcare, Consumer, and Lighting businesses out of Dubai. Subsequently, he became Business Leader of Domestic Appliances, based in Shanghai, in 2015. In 2018, Roy joined the Executive Committee as Chief Business Leader of the Personal Health businesses and in early 2020 he started as Chief Business Leader of Connected Care. Prior to his career at Philips, he held various management positions at Royal Dutch Shell and Reed Elsevier.
Born 1961, Indian
Executive Vice President
Member of the Board of Management (since December 2015)
Chief Financial Officer
Abhijit Bhattacharya first joined Philips in 1987 and has held multiple senior leadership positions across various businesses and functions in Europe, Asia Pacific and the US. Between 2010 – 2014, he was the Head of Investor Relations of Philips, and subsequently, CFO of Philips Healthcare, Philips’ largest sector at the time. Prior to 2010, Abhijit was Head of Operations & Quality at ST-Ericsson, the joint venture of ST Microelectronics and Ericsson, and he was CFO of NXP’s largest business group.
Born 1973, Dutch
Executive Vice President
Member of the Board of Management (since November 2017)
Chief ESG & Legal Officer
Marnix van Ginneken joined Philips in 2007 and became Head of Group Legal in 2010. In 2014, Marnix became Chief Legal Officer of Royal Philips and Member of the Executive Committee. In 2017 he was appointed to the Board of Management. He is responsible for driving ESG efforts across the company, including Sustainability. He is also responsible for Legal, Intellectual Property & Standards and Government & Public affairs. Since January 1, 2024 he is Chairman of the Board of the Philips Foundation. In 2011, he was appointed Professor of International Corporate Governance at the Erasmus School of Law in Rotterdam. Before joining Philips, Marnix worked for Akzo Nobel and as an attorney in a private practice.
This page reflects the composition of the Executive Committee as per December 31, 2023. For a current overview of the Executive Committee members, see also https://www.philips.com/a-w/about/executive-committee.html 6.1Our strategic focus Today, most healthcare systems are struggling to keep up with the ever-rising need for, and cost of, healthcare, while systemic staff shortages and financial resource constraints increase the pressure.
The overview below is based on the International Integrated Reporting Council framework and includes resource inputs, value outcomes and societal impact across various financial and Environmental, Social and Governance (ESG) dimensions.
We identify the Environmental, Social and Governance topics which we believe have the greatest impact on our business and the greatest level of concern to stakeholders along our value chain, for instance patient safety and quality. We do this through a multi-stakeholder process. Please refer to Working with stakeholders and advocacy for more information. Assessing these topics enables us to prioritize and focus upon the most material topics and effectively address these in our policies, programs, targets and actions. We do this with reference to the GRI standard and identify and assess impacts on an ongoing basis, for example through discussions with our customers, suppliers, investors, employees, peer companies, social partners, regulators, NGOs, and academics. We also conduct a benchmark exercise, carry out trend analysis and run media searches to provide input for our materiality analysis. GRI has not yet published a sector standard for the healthcare industry. Philips’ impact on society at large is covered through our Lives Improved metric and the Environmental Profit & Loss account, as well as a number of other KPIs addressed in Environmental, Social and Governance. The result of our impact materiality assessment you will find below.
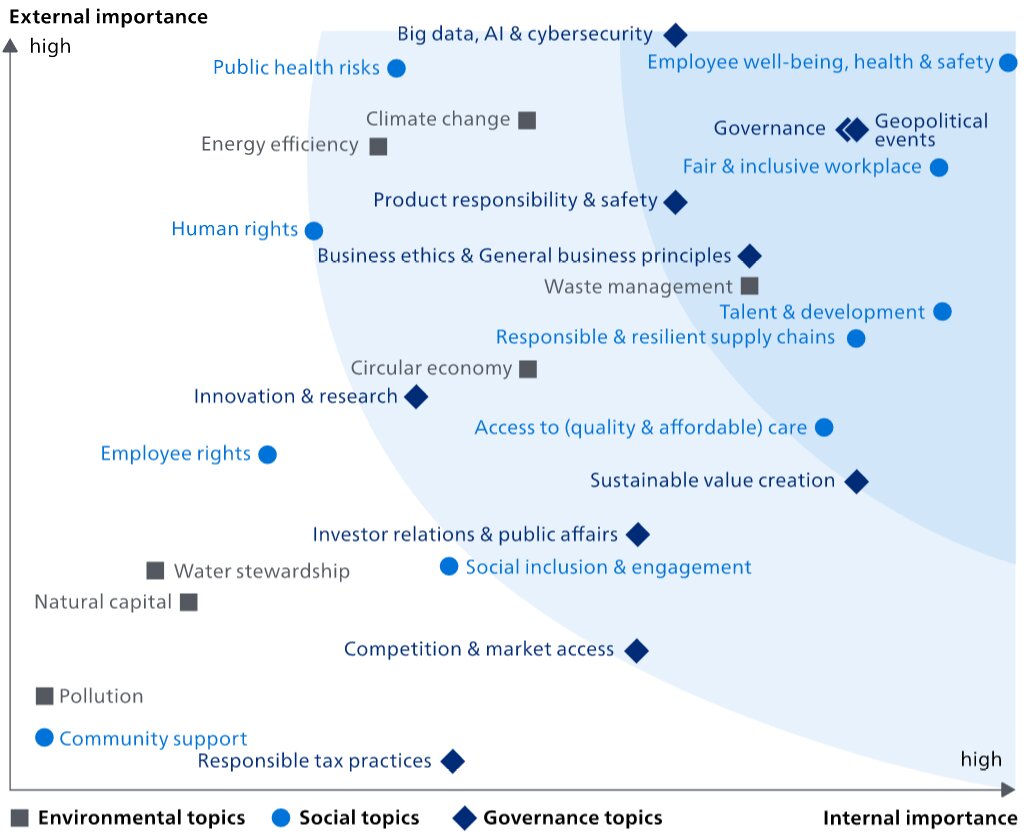
Similar to 2022, we used an evidence-based approach to materiality assessment, powered by a third-party AI-based application. The application allows automated sifting and analysis of millions of data points from publicly available sources, including corporate reports, mandatory regulations and voluntary initiatives, as well as news. With this data-driven approach to materiality assessment we have incorporated a wider range of data and stakeholders than was ever possible before and managed to get an evidence-based perspective on regulatory, strategic and reputational risks and opportunities. Topics were prioritized through a survey sent to a large and diverse set of internal and external stakeholders, combined with input from the application.
Changes in 2023On both external and internal importance axis, the most significant increases compared to 2022 were Waste management and Social inclusion & engagement. Innovation & research went down on both axes. On the internal importance axis, there was a significant decrease on Pollution.
Double materialityAfter completing the regular impact materiality assessment, we completed a preliminary 'double materiality' assessment, in preparation for the upcoming requirements of the EU Corporate Sustainability Reporting Directive (CSRD). The double materiality assessment addresses both financial materiality (the impact of society on Philips) as well as impact materiality (the impact of Philips on society): we only included the high and medium material topics from impact materiality and/or financial materiality. The data sources used for the financial materiality include corporate reports, mandatory regulations with sanctions, voluntary initiatives by e.g. central banks, and Sustainability Accounting Standards Board (SASB) accounting metrics. For impact materiality, we included sustainability data from corporate reports or sustainability reports, coverage in the news and voluntary initiatives and regulation. We calibrated the financial and impact materiality with a team of internal experts from Enterprise Risk Management, Group Control, Internal Audit, Insurance and Risk Management and Sustainability and aligned with our Enterprise Risk Management assessment. After this calibration the financial impact of Product responsibility & safety, Geopolitical events, and Big data, AI & Cybersecurity were increased. The results of the double materiality analysis are depicted below.
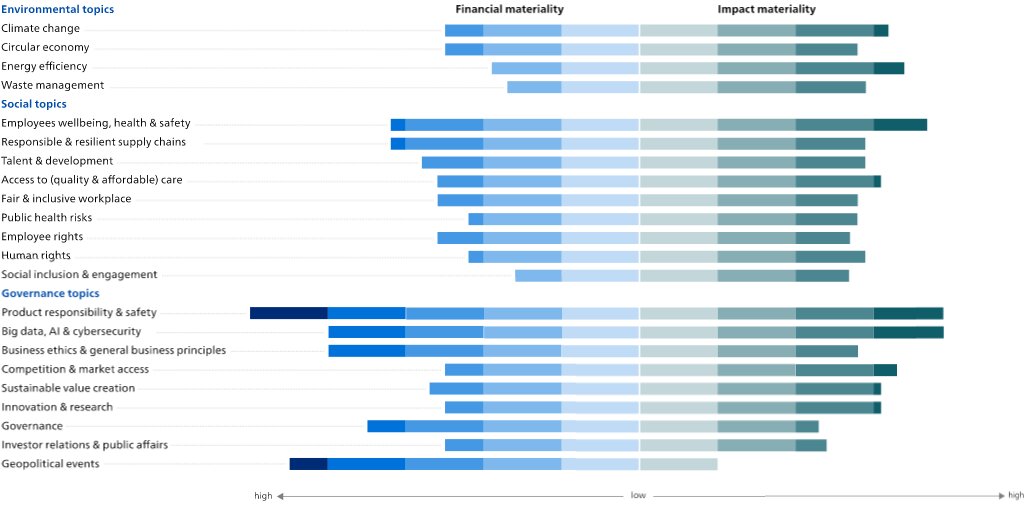
From the financial materiality assessment, the topics that ranked highest were: (1) Product responsibility & safety, (2) Geopolitical events, and (3) Big data, AI & Cybersecurity, as well as Business ethics & General business principles.
From the impact materiality assessment, the topics that ranked the highest were: (1) from the Environmental topics, Climate change, and Energy efficiency; (2) from the Social topics, Employee well-being, health & safety, and Access to (quality & affordable) care; and (3) from the Governance topics, Big data, AI & Cybersecurity, and Competition & market access. These topics are all covered in more detail in the Annual Report 2023 and monitored regularly.
The outcome of the double materiality assessment did not result in any significant changes in the material topics identified from impact materiality.
The results of our materiality assessment have been reviewed and approved by the Philips Board of Management and will be used to prepare for the upcoming EU legislation.
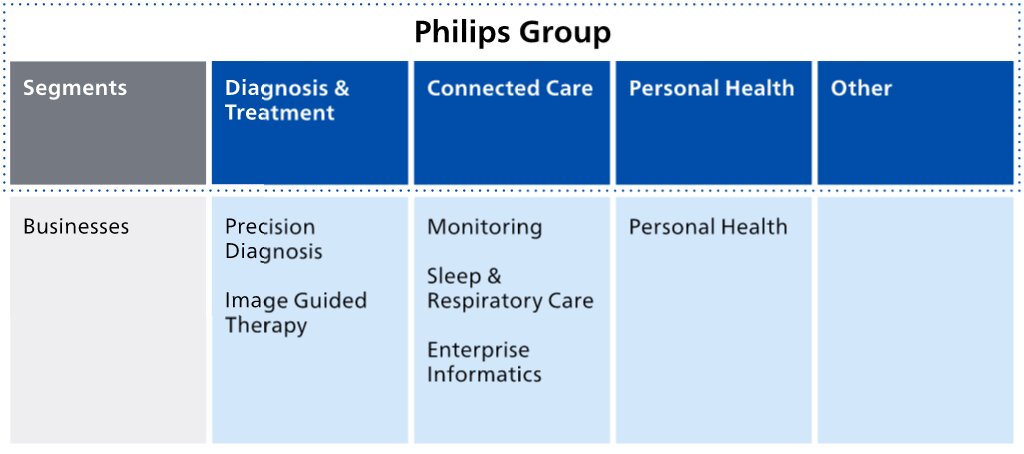
Koninklijke Philips N.V. (Royal Philips) is the parent company of the Philips Group. As announced on January 30, 2023, Philips has changed its operating model to end-to-end businesses with single accountability in order to make the company more agile in its drive to create value with sustainable impact. The segments Diagnosis & Treatment, Connected Care and Personal Health are each responsible for the management of their business activity worldwide, and are made up of the six businesses shown below. Additionally, Royal Philips identifies the segment Other.
Philips Group
Total sales by reportable segment
| 2023 | |
|---|---|
| Diagnosis & Treatment | 49% |
| Connected Care | 28% |
| Personal Health | 20% |
| Other | 3% |
Our Diagnosis & Treatment businesses create value through their portfolio of innovative AI-enabled solutions that support precision diagnosis and minimally invasive treatment in therapeutic areas such as cardiology, peripheral vascular, neurology, surgery, and oncology. With these solutions, we enable our customers to realize better health outcomes, improved patient and staff experience, and lower cost of care.
Serving diagnostic imaging markets globally, our goal is to improve customer performance in the radiology/imaging workflow. We see significant opportunity to enable precision diagnosis while at the same time supporting adjacent needs for care orchestration across care pathways and increasing departmental productivity. We do this through smart diagnostic systems, connected workflow solutions, and integrated AI-supported diagnostics and pathway informatics. These drive enterprise-wide operational efficiency and help clinicians to provide an early and definitive diagnosis, enabling them to select tailored care pathways with predictable outcomes for every patient, both inside and outside the hospital.
We also provide integrated solutions combining imaging systems, diagnostic monitoring data and therapeutic devices, which optimize interventional procedures to deliver more effective treatment, better outcomes and higher productivity. Building upon our leading-edge Azurion system, we continue to innovate, optimizing clinical and operational lab performance through advances in workflow and integration for routine procedures, and expanding the role of image-guided interventions to treat new groups of patients such as those with complex diseases including various cardiovascular conditions, stroke and lung cancer. We are also innovating the way we engage with our customers, using new business models across different care settings, including out-of-hospital settings such as office-based labs and ambulatory surgical centers, which offer clear clinical, financial and operational benefits.
In 2023, the Diagnosis & Treatment segment consisted of the following businesses:
Diagnosis & Treatment
Total sales by business
| 2023 | |
|---|---|
| Precision Diagnosis 1) | 61% |
| Image Guided Therapy | 39% |
Revenue is predominantly earned through the sale of products, leasing, customer services fees, recurring per-procedure fees for disposable devices, and software license fees. For certain offerings, per-study fees or outcome-based fees are earned over the contract term.
Sales channels are a mix of a direct sales force, especially in the larger markets, third-party distributors and an online sales portal. This varies by product, market and price segment. Our sales organizations have an intimate knowledge of technologies and clinical applications, as well as the solutions necessary to solve problems for our customers.
Sales at Philips’ Diagnosis & Treatment businesses are generally higher in the second half of the year, largely due to the timing of customer spending patterns.
At year-end 2023, Diagnosis & Treatment had 28,397 employees worldwide.
At RSNA23, Philips announced a raft of new innovations, including next-generation EPIQ Elite 10.0 and Affiniti ultrasound systems that increase diagnostic confidence and workflow efficiency, BlueSeal MRI Mobile, the world’s first and only mobile MRI system with helium-free operations, and new AI-enabled cloud solutions that enhance radiology efficiency and clinical confidence.
Philips launched its new MR 7700 3.0T system, which features an enhanced gradient system designed to deliver outstanding imaging results and speed to support confident diagnosis for every patient.
Five top hospitals in Shanghai, with a total of more than 10,000 beds, installed Philips’ advanced Spectral CT 7500 imaging system, helping physicians deliver first-time-right diagnosis through fast, low-dose X-ray scans.
Philips further expanded its ultrasound portfolio with the launch of the Ultrasound Compact 5500 CV, which delivers cart-based premium image quality in ultrasound exams for cardiology and vascular patients at the bedside.
Philips IntraSight Mobile received approval from the Chinese regulatory authority, paving the way for its commercial introduction in the Chinese market. IntraSight Mobile combines imaging and physiology applications on a mobile system for peripheral and coronary artery disease therapy.
Philips expanded its image-guided therapy portfolio with the launch of Philips Zenition 10, which provides a cost-effective imaging solution to guide high-volume routine surgery, as well as complex orthopedic and trauma procedures. Philips also launched the Zenition 30 Image Guided Therapy Mobile C-arm. Its workflow-enhancing features and excellent image quality enable surgeons to deliver enhanced care to more patients, helping alleviate the staff shortages faced by many hospitals.
Through the WE-TRUST study, Philips is driving innovation in stroke treatment. The trial examines the impact of the direct-to-angio treatment pathway on clinical outcomes, facilitated by a helical scan in the angio suite developed by Philips to reduce time to treatment. With 16 leading stroke centers and hospitals around the world involved in the trial and 100 patients enrolled, the WE-TRUST study has already achieved the scale and momentum needed to deliver a reliable evidence base on the potential benefits of the Direct to Angio Suite pathway for patients suspected of having a large vessel occlusion (LVO) ischemic stroke – a treatment pathway that focuses squarely on addressing the fact that the faster a patient is treated, the more likely they are to recover. Another significant milestone was the publication of health economics analysis results in the Journal of NeuroInterventional Surgery, demonstrating that this new stroke care pathway can save over USD 3,000 per patient.
With technology constantly advancing and becoming increasingly pervasive in healthcare, the Connected Care businesses aim to connect and elevate care for all. Philips connects patients and caregivers across care settings, delivering clinical, operational and therapeutic solutions that help our customers deliver better health outcomes, improve the patient and staff experience, and lower the cost of care across care settings. In 2023, the global economic situation continued to put additional pressure on customer budgets and worsened trends such as staff shortages, as well as increasing the need for solutions that enable more effective, sustainable and convenient care in hospital, clinics and the home – especially enabled by strong informatics and AI.
The Sleep & Respiratory Care business in particular continued to face multiple operational and regulatory challenges in 2023, but action has been taken to improve the ability of the Sleep & Respiratory Care organization to correctly assess potential patient safety or quality issues. Philips has reaffirmed its core activities, which put patient safety front and center in everything we do, and we believe that the implementation of a new simplified organization, which for Sleep & Respiratory Care began in 2022, will help to achieve this, as well as to improve productivity and increase agility. In the course of the year, Sleep & Respiratory Care gradually returned to the market for sleep therapy devices outside the US. For information about the Respironics recall and related remediation effort, please refer to Quality & Regulatory and patient safety.
With clinical depth and discovery, Philips Connected Care technologies help to cultivate a more accurate and complete view of the patient that drives better health and care. The combination of advanced technological solutions and a co-creation approach allows Philips to be an effective partner to its customers in their digital transformation, both across the enterprise and at the level of the individual clinician, nurse and patient. We help our customers to unlock actionable insights from pools of medical imaging data, patient monitoring data, and through the use of advanced AI, to improve outcomes and drive productivity.
Philips’ open, interoperable platforms aggregate and leverage information from clinical devices, patient and historical data to support care providers in patient engagement, diagnostics, and patient monitoring in the hospital, ambulatory and home settings.
In 2023, the Connected Care segment consisted of the following businesses:
Connected Care
Total sales by business
| 2023 | |
|---|---|
| Monitoring | 60% |
| Sleep & Respiratory Care | 17% |
| Enterprise Informatics | 23% |
In most of the Connected Care businesses, revenue is earned through the sale of products and solutions, as well as services and software licenses. Where bundled offerings result in solutions for our customers, or offerings are based on the number of people being monitored, we see more usage-based earnings models. In the area of patient care management (Ambulatory Monitoring & Diagnostics business unit and Sleep & Respiratory Care business), revenue is generated through clinical services, product sales and through rental models, whereby revenue is generated over time.
Sales channels include a mix of a direct sales force, partly paired with an online sales portal and distributors (varying by product, market and price segment). Sales are mostly driven by a direct sales force with an intimate knowledge of clinical settings and patient-specific diagnosis and treatment. Philips collaborates with customers and partners to co-create solutions, drive commercial innovation and adapt to new models such as monitoring-as-a-service and software-as-a-service.
Sales at Philips’ Connected Care businesses are generally higher in the second half of the year, largely due to customer spending patterns. However, the Philips Respironics voluntary recall notification in the Sleep & Respiratory Care business in June 2021 (as further discussed in Quality & Regulatory and patient safety) continued to have a negative impact on sales throughout 2023.
At year-end 2023, Connected Care had 17,549 employees worldwide.
Philips signed a 10-year, EUR 100 million Enterprise Monitoring as a Service agreement with one of the largest health systems in the US, covering 20 hospitals with over 3,000 beds. The agreement provides the health system with constant access to the latest technology, including software and services, while lowering initial investments.
Philips and NYU Langone Health announced an 8-year strategic partnership valued up to USD 115 million and aimed at enhancing patient care through further innovation. The partnership includes digital pathology, clinical informatics, and innovative AI-enabled diagnostics, with an Enterprise Monitoring as a Service model. With these new technologies, NYU Langone clinicians can collaborate in real time, sharing pathology, imaging studies or patient data to support diagnostic confidence and tailor individualized care plans.
Highlighting the strength of its comprehensive patient monitoring offering, Philips announced a multi-year partnership with Northwell Health to standardize and centralize patient monitoring across the hospital, allowing caregivers to see what is happening at each bedside.
Philips announced new interoperability capabilities that offer a comprehensive view of patient health for improved monitoring and care coordination. This is realized through the interoperability of Philips Capsule Medical Device Information Platform (MDIP) with Philips Patient Information Center iX (PIC iX) with streaming, vendor-neutral data that supports care delivery and collaboration.
Philips introduced the cloud-based Philips HealthSuite Imaging PACS on Amazon Web Services. This cloud-based enterprise imaging solution, which includes advanced AI-enabled applications, has been designed to enhance image access speed, reliability, and data orchestration for clinicians across the imaging workflow, while reducing costs for healthcare organizations.
Philips launched its ambulatory monitoring offering in Japan, combining Philips ePatch Holter monitors with ECG analysis through AI and advanced algorithms. This innovative approach aims to reduce clinician workload and improve the patient experience.
Our Personal Health business plays an important role in enabling healthy individual care routines with technology and solutions that support people’s long-term health and well-being.
We aim to drive profitable growth through a focus on innovation across three key areas:
The Personal Health segment consists of the Personal Health business, which comprises the following business units:
Personal Health
Total sales by business
| 2023 | |
|---|---|
| Personal Health 1) | 100% |
Through our Personal Health business, we offer a broad range of solutions in various consumer price segments to support people in proactively managing their health and well-being. Depending on the market, we offer an additional portfolio of locally relevant innovations and adjust our range to increase accessibility. A notable aspect of our commercial strategy is driving increased direct-to-consumer relationships and sales through our consumer communities and online store. About half of our Personal Health sales worldwide now take place online.
We are leveraging connectivity to offer new business models, partnering with other players in the health ecosystem, e.g. insurance companies and healthcare professionals, with the goal of extending opportunities for people to live healthily and prevent or manage disease. We are engaging consumers in their health journey in new and impactful ways through social media and digital innovation.
In Personal Health, improving lives also means caring for the world, with a key focus on environmental sustainability. For example, in 2023 we launched an initiative in Germany, Philips Refurb Editions, to give products a second life, with the same two-year guarantee as a new product. This is part of Personal Health’s commitment to driving a more circular economy, and we believe we need to keep finding innovative ways to support consumers with greater choices to live sustainably.
We also offer mobile solutions to support parents and parents-to-be for a more informed, more connected and healthier journey to parenthood. The Pregnancy+ app and Baby+ app offer parents supportive content at every stage of their first 1,000-day journey. Pregnancy+ also offers state-of-the-art, photo-realistic and interactive 3D fetal models to make the experience even more exciting, with new, personalized content for each day of the pregnancy. The Philips Pregnancy+ app was ranked among the best pregnancy apps of 2023 by Forbes*). It has more than 1.5 million daily active users and is available in 22 languages.
The revenue model is mainly based on product sale at the point in time the products are delivered to retailers and online platforms. We continue to increase revenue model diversity by expanding our new business models, including direct-to-consumer, subscriptions, try-and-buy offerings and services.
The Personal Health business experiences seasonality, with higher sales around key national and international events and holidays.
At year-end 2023, Personal Health employed 9,085 people worldwide.
Philips successfully launched the Sonicare DiamondClean 7900 Series electric toothbrush in China on major online shopping channels Alibaba and JD.com. Highlighting increasing customer demand, it claimed the number-one position in the high-end toothbrush category on Alibaba’s Tmall.
In partnership with JD.com, Philips launched the premium 7 Series Shaver in China, debuting as the #1 shaver on this major online shopping channel. Additionally, Philips’ DiamondClean 9000 premium electric toothbrush has become the best-selling high-end oral healthcare product on Alibaba.
To improve oral care habits among children, Philips introduced Sonicare for Kids 'Design a Pet Edition' with an entry price point designed to give more parents access to an electric toothbrush for their children.
Philips OneBlade packaging was named the 2023 Red Dot Communication Design Best of the Best in recognition of its paper-based model, illustrating how the use of less material, fewer parts, and less volume can go hand in hand with iconic presence and the best user experience.
Philips launched its 'Better than New' campaign in Germany, repositioning refurbished innovations and underscoring the company's commitment to circularity and sustainability. The campaign led to a significant year-on-year increase in sales revenue of refurbished Lumea and refurbished shaving products.
Philips announced Babybell Maternal & Child Supplies as the exclusive distributor for Philips Avent OneFeeding in China; the partnership aims to accelerate growth of the Philips Avent brand in the Chinese maternal health industry. The partnership combines the power of Philips’ latest innovations with Babybell's rich understanding of the local market and robust retail network to deliver on a faster innovation pace and expand market share.
In our external reporting on Other we report on the items Innovation & Strategy, IP Royalties, Central costs, and other small items. At year-end 2023, 14,626 people worldwide were working in these areas.
At Philips, we have set up our innovation teams to be as close to our customers and consumers as possible. The majority of our Research & Development (R&D) experts work in one of our business units, which allows them to directly hear customer and consumer needs and work closely with other stakeholders to turn innovations into actual products. Innovation at Philips is organized to encourage innovation anywhere along the value chain and not just at the product ideation stage.
The remaining R&D experts are part of our central Innovation & Strategy organization. The main job of these experts is to focus on industry-shifting ideas that advance a core product to fulfill the needs of a broad new customer segment. Innovation & Strategy focuses on enabling and accelerating innovation across our business units in different ways:
Strategy supports the business units in shaping their strategy to create a competitive advantage. The Enterprise Strategy team focuses on overall corporate strategy, and the Market Analysis & Forecasting team analyzes customer segments and market growth trends. Strategy also partners closely with the Mergers & Acquisitions and Finance teams to ensure our M&A activity is aligned with the business units and our enterprise strategic direction.
Research helps to define the future of healthcare by unlocking opportunities that have the potential to disrupt the healthcare industry. Breakthrough Innovation Teams (BRITE) and Exploratory Innovations Teams (XITE) are two ways Philips nurtures long-term ‘big bets’ in innovation and enables the growth of an overarching entrepreneurial mindset across all of Philips.
Experience Design plays an important role in making sure that the voice of the customer is heard and included in innovation. This means linking product development from inception with a patient and consumer view and ensuring that the highest product and experience performance requirements are embedded throughout all innovation projects. In 2023, the Philips brand won 160 awards for design excellence.
Innovation Engineering teams bring innovations to life by providing Philips business units with a central team of experienced, talented individuals with capabilities in software, hardware engineering, and AI. Innovation Engineering teams also enable business units to scale through shared platforms.
Innovation Excellence helps our business units to be the best they can in innovation by offering them an outside-in view and developing competencies, processes, data and tools they need to excel at innovation.
Innovation Effectiveness teams help to measure the return on innovation investments and guide enterprise-wide innovation initiatives.
Innovation & Strategy works from four main innovation sites – Eindhoven (Netherlands), Cambridge (USA), Bangalore (India) and Shanghai (China) – and smaller innovation and research sites in the Regions. Our global footprint enables us to understand, anticipate and react to local markets and needs.
IP RoyaltiesPhilips Intellectual Property & Standards (IP&S) proactively pursues the creation of new Intellectual Property (IP) in close co-operation with Philips’ operating businesses and Innovation & Strategy. IP&S is a leading industrial IP organization providing world-class IP solutions to Philips’ businesses to support their growth, competitiveness and profitability.
Royal Philips’ IP portfolio currently consists of approximately 53,000 patent rights, 31,500 trademarks, 135,000 design rights and 3,300 domain names. Philips filed 795 new patents in 2023, with a strong focus on the growth areas in health technology services and solutions.
Philips earns substantial annual income from license fees and royalties.
Philips believes its business as a whole is not materially dependent on any particular third-party patent or license, or any particular group of third-party patents and licenses.
Central CostsWe recharge the directly attributable part of the functional costs to the businesses. The remaining part is accounted for as 'central costs', and includes costs related to the Executive Committee and Group Functions such as Strategy, Legal and Audit fees.
Other small itemsOther small items refer to remaining items for intra-group services and legacy items relating to previously disposed businesses.
Geographically, our business is organized in three Regions: North America, Greater China and International Region (the latter made up of Europe and Growth groupings). Within our Regions, we further organize the business by Zones and Countries. Their primary accountability is to manage customer intimacy, relationships and understanding of their needs, (strategic) account management, service delivery, and indirect partner management. They are also accountable for government relations, local infrastructure needed to support Philips’ presence in a country (license to operate) and for statutory, fiscal & compliance duties, safety, sustainability and labor relations to secure compliant operations in the Region/Zone/Country.
For financial reporting purposes, we recognize four geographic areas: Western Europe, North America, Other mature geographies, and Growth geographies. Western Europe, North America and Other mature geographies are collectively recognized as Mature geographies in reporting on sales. This reflects the grouping of countries based on similar economic characteristics.
Leading health systems such as Northwell, TriHealth, and Atrium Health, have extended their long-term strategic partnerships (LSPs) with Philips to include enterprise informatics and precision diagnostics. In Canada, eHealth Saskatchewan elected to extend their enterprise informatics relationship with Philips, reinforcing the value of the partnership to clinicians and patients across the province.
The Enterprise Monitoring as a Service model (EMaaS) also drove innovation, giving health systems like NYU Langone Health, the University of California Irvine and Children’s Hospital of Orange County a predictable, scalable business model that enables them to standardize their patient monitoring platform across the enterprise. In addition to adopting EMaaS for their monitoring solutions, NYU Langone Health is also partnering with Philips to advance patient safety, quality and improve patient outcomes through digital innovation, including integrating patient data across the network, AI-enabled diagnostic imaging and digital pathology for precision diagnosis and treatment.
Philips Sonicare remains the leading electric rechargeable toothbrush in the United States and Canada, as well as the most-recommended rechargeable toothbrush brand in the United States. Additionally, Philips Norelco remains the leading electric male grooming brand in the US and Canada, reaching the next generation of young men with our One Blade multi-purpose shaver.
Philips continues to lead the way with innovation in its efforts to help address health disparities and maternal health access specifically – partnering with the state of Michigan to tailor the Philips Avent Pregnancy+ app, making it easier for moms within the state to find resources available to them, such as home-visiting nurses. During the first year, Pregnancy+ reached over 32,000 Michigan families, helping them get access to vital resources. The app was recognized by Forbes as the best pregnancy app of 2023. Forbes also recognized Philips for its Inclusion & Diversity efforts in North America, naming the company among the Best Employers for Diversity and Best Employers for Women for the second year in a row.
Greater ChinaIn 2023, we continued to deliver on our commitment to support China’s national health strategy, supplying hospitals with tailor-made solutions for their clinical and research needs, and empowering consumers to manage their health and well-being.
With the aim of better serving the Chinese market, we are committed to our ‘In China, For China’ strategy, which focuses on local innovation, manufacturing, services and partnership. We continue to drive ‘made in China’ fulfillment and create more locally relevant solutions by leveraging local ecosystems to serve both professional and consumer markets.
In the professional market, we have expanded our cooperation with local customers by providing cutting-edge imaging systems, informatics solutions and other products in support of delivering better care to patients in terms of precision diagnosis, interventional treatment, and smart hospital development. Key customers include many top hospitals: Huaxi Hospital in Sichuan Province, Renji Hospital, Xinhua Hospital, the Sixth People's Hospital, the 10th People's Hospital and Children’s Hospital in Shanghai, Jishuitan Hospital and Anzhen Hospital in Beijing, the First Affiliated Hospital of Dalian Medical University, the First Affiliated Hospital of Zhengzhou University, and Regional Imaging Center of Jiangxi Province, to name just a few.
In the consumer market, in line with our consistent ‘Professional, Young and Premium’ positioning, Philips’ brand strength increased in 2023, despite an overall weak consumer market. We leveraged new online and offline channels, including Healthy Living Lab, TikTok, O2O instant retail platforms like Meituan, JD to home, and Ele.me to engage with young consumers and grow business. Local innovation drove significant growth in Male Grooming and Oral Healthcare, which continue to solidify their leadership positions in China. Philips was recognized as a ‘gold brand’ (most favored consumer brand) in the Personal Health category for the fourth consecutive year by China Business Weekly.
2023 marked the 100th anniversary of Philips in China. This achievement stands as a testament to Philips’ commitment and dedication to improving people’s lives through meaningful innovation and fostering strong partnerships in China.
InternationalIn International Region we strive to execute on a shared global vision whilst meeting the unique local needs and circumstances of our customers. Our goal is to elevate customer relationships and move from being a trusted supplier of equipment, services and software to a transformational partner directly contributing to our customers’ long-term success. To support this vision we have made great progress on leveling up our go-to-market model, developing scalable solutions and software, expanding fit-for-future capabilities, reinvesting revenue to enable new business models, and establishing new partnerships.
In International Region, our Personal Health business plays an important role in enabling healthy individual care routines with technology and solutions that support people’s long-term health and well-being.
Philips entered into many new customer partnerships in 2023, including the following:
International – EuropePatients at Martini Hospital were the first in the Netherlands to use Philips' ePatch wearable sensor to diagnose cardiac arrhythmias. The hospital uses the sensor and Cardiologs software to detect atrial fibrillation after patients have had a stroke. The patch is designed to replace traditional Holter monitors, which are more cumbersome and can only be worn for a day. The new sensor is expected to improve detection of heart rhythm disorders and provide more personalized care, as well as reducing workload and lowering costs.
Philips and Gibraltar Health Authority announced a 16-year strategic partnership to transform patient imaging and cardiac care for local patients. The partnership will provide local coronary angiography and angioplasty services in a new interventional cardiac suite equipped with the latest diagnostic technology. The announcement represents a major reform in service delivery, improving the region’s access to life-saving interventions while reducing environmental impact, with patients no longer needing to travel abroad for treatment.
Developed by Dr David Tscholl and Dr Christoph Nöthiger, consulting anesthesiologists at University Hospital Zurich, the Visual Patient Avatar is a new approach to patient monitoring: patient data is translated into a simple visual design, reducing the time needed in the operating room to check and interpret vital signs. Together with Philips, this idea was further developed into a commercial solution that is now being implemented at University Hospital Bonn, the first hospital in Europe to use this type of display for faster decision support.
Philips is partnering with Assistance Publique-Hôpitaux de Paris, Hôpitaux Civils de Lyon and Incepto (a PACS AI application platform) to make Artificial Intelligence more accessible to radiologists. Philips has also joined forces with Hôpital Saint-Joseph (Paris) and Hôpital Marie-Lannelongue (Hauts-de-Seine) to improve personalized cancer care by integrating digital pathology into the imaging workflow. And Philips has opened its new healthcare innovation center, Health Innovation Paris (HIP).
Philips' innovative technologies feature across the Polish hospital network. In 2023, the first Incisive CT scanner in Central & Eastern Europe was installed to diagnose patients with cardiac disease at a private cardiology network. In addition, a state-of-the-art hybrid room was created at the University Hospital in Bydgoszcz. Longstanding cooperation with the American Heart of Poland has resulted in further contracts, including the installation of a monitoring network.
Philips and Norwegian Vestre Viken Health Trust deployed AI-enabled clinical care to help radiologists improve patient care. The large-scale deployment provides access to an AI-based bone fracture radiology application that will serve the needs of around half a million people across 22 Norwegian municipalities.
International – GrowthPhilips Japan officially launched the Turbo-Power laser atherectomy catheter, which debulks lesions in a single step and offers remote automatic rotation for precise directional control – a powerful tool for the treatment of peripheral vascular diseases. The Philips MR7700 3.0T imaging system with SmartSpeed AI was installed for the first time in Japan, at Hamamatsu University Hospital. The MR7700 achieves high image quality, while SmartSpeed utilizes the Compressed SENSE speed engine to reduce scan time.
Philips launched the Spectral CT 7500 imaging system with an event for the top 100 radiologists in India. This system performs low-dose scans without compromising speed, power or field of view. We also received an order for 28 Philips Incisive CT systems from a single state. This system combines operator and design efficiencies to improve patient and staff experience and support clinical decision-making. Philips Innovation Campus opened its new site in Bengaluru, home to over 5,000 engineers, scientists, business developers, and clinical experts.
In Australia, Philips signed a 7-year partnership agreement with the Queensland Government and Cairns and Hinterland Hospital and Health Service (CHHHS) to provide a turnkey solution including Vue PACS, Reporting & VNA Philips Software, and Infrastructure as a Service (IaaS) across a remote and large geography. The Philips solution will enable clinicians to access a complete imaging health record of their patients and provide a platform for the integration of all image data across the CHHHS enterprise, greatly enhancing the radiology workflow.
Demonstrating our commitment to high-quality, sustainable healthcare, Philips undertook multiple initiatives to expand helium-free MR operations in Brazil. Besides Brazil, this expansion reached Mexico, Panama, Puerto Rico, Colombia, Chile, Argentina and Ecuador, making a significant mark on the region's healthcare landscape. We are also working to localize the production of BlueSeal MRI magnets in Brazil. Other notable ventures included the Brazilian Company of Hospital Services (EBSERH) installing 14 Incisive CT imaging systems at Federal University Hospitals.
In Turkey, Philips has supplied high-grade medical equipment to the new Gaziantep City Hospital. The public city hospital complex, with 1,875 new beds, will serve Gaziantep and surrounding cities, adding much-needed healthcare capacity to the region, which was hit by a devastating earthquake in February 2023.
As part of a deal with Egypt’s Ministry of Health, Philips unveiled the first Mobile MRI Truck for the Middle East, Turkey & Africa region, to enhance healthcare delivery in remote, difficult-to-access and underserved locations. In just 3 months after implementation, more than 1,100 patients across Egypt had already benefited from this initiative.
In Kazakhstan, Philips provided advanced medical equipment to the National Coordination Center for Emergency HealthCare in Astana and the Hematology and Cardiology Center in Ust-Kamenogorsk. Both are multi-modality projects and have a high social importance, as the Emergency Center will be the flagship center for the National Stroke Program in Kazakhstan, and the Hematology and Cardiology Canter treats patients with serous blood diseases.
Philips is present in 75 countries globally and has its corporate headquarters in Amsterdam, Netherlands. Our real estate locations are spread around the globe, with key manufacturing and R&D sites in Europe, the Americas and Asia.
In 2023, we consolidated five different R&D locations into a new R&D Hub in Bangalore, India, which will host some 5,000 employees. We continued our right-sizing program through our Future of Work concepts to support hybrid working. The project to move Philips’ headquarters to a new location in Amsterdam in 2025 is progressing as planned.
We also continue to optimize our real estate portfolio in line with our Environmental ESG commitments towards 2025. Having met our goal of bringing our site-related CO₂ emissions under 35 kilotons per year in 2020, we further reduced our CO₂ emissions to 22 kilotons in 2023. In addition, we reached 78% renewable energy in 2023, already exceeding our target of 75% by 2025. Energy consumption decreased by 7.8% compared to 2022.
Over 75% of our locations are leased properties, and we manage vacancy closely to ensure the right level of space efficiency and flexibility to support our business dynamic. Our current facilities are adequate to meet the requirements of our present and foreseeable future operations. As expected, occupancy rates in our offices continued to stabilize in the first half of 2023. We continue to evaluate options to right-size our office footprint, to further adopt task-based working principles, and to cater for meaningful presence in inspiring layout and workplace solutions. The net book value of our land and buildings as of December 31, 2023, represented EUR 1,282 million; construction in progress represented EUR 32 million.
Philips Group
Key data
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Sales | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | (0.9)% | 3.9% | 1.9% |
| Comparable sales growth1) | (1.2)% | (2.8)% | 6.0% |
| Impairment of goodwill | (15) | (1,357) | (8) |
| Income from operations | 553 | (1,529) | (115) |
| as a % of sales | 3.2% | (8.6)% | (0.6)% |
| Financial expenses, net | (39) | (200) | (314) |
| Investments in associates, net of income taxes | (4) | (2) | (98) |
| Income tax (expense) benefit | 103 | 113 | 73 |
| Income from continuing operations | 612 | (1,618) | (454) |
| Discontinued operations, net of income taxes | 2,711 | 13 | (10) |
| Net income | 3,323 | (1,605) | (463) |
| Adjusted EBITA1) | 2,054 | 1,318 | 1,921 |
| as a % of sales | 12.0% | 7.4% | 10.6% |
| Income from continuing operations attributable to shareholders2) per common share (in EUR) - diluted | 0.64 | (1.76) | (0.50) |
| Adjusted income from continuing operations attributable to shareholders2) per common share (in EUR) - diluted1) | 1.58 | 0.92 | 1.25 |
The introduction of a simplified operating model, workforce reduction, an improved global supply chain, and the geopolitical environment contributed to the company’s business and results in 2023. Where relevant, the impact of these factors and the resulting uncertainties on the company’s results, balance sheet and cash flows have been considered and are reflected in amounts reported.
Macro-economic landscape 2023In 2023, global economic growth is estimated to have slowed compared to 2022, marked by easing price pressures and supply chain stress while major central banks were tightening their respective monetary policies. Global real GDP is estimated to have grown by 2.7% in 2023, compared with 3.1% in 2022. On the consumer side, households were dipping into their savings accumulated during the COVID period to maintain their spending levels and to cushion against the inflation surge since late 2021. However, consumer spending momentum is not expected to sustain due to the depletion of savings and expected labor market softening because of tighter financial conditions. The delayed effect of higher benchmark interest rates is expected to manifest further in 2024, leading to a further slowdown in global economic growth. Oxford Economics expects world real GDP growth of 2.3% in 2024.
In 2022, global economic activity slowed down compared to 2021, when the global economy had rebounded strongly from a COVID-induced recession. Several factors were at play. Firstly, the re-opening of the economy for most of the world in 2021 had disrupted global supply chains. Secondly, previous loose monetary policy, combined with supply chain issues, resulted in strong inflationary pressures commencing towards the end of 2021. Thirdly, to combat high inflation, central banks around the globe embarked on aggressive monetary policy tightening cycles.
Simplified operating modelOn January 30, 2023, Philips announced its plan to create value with sustainable impact, which is based on focused organic growth to deliver patient- and people-driven innovation at scale, with improved execution as a key value driver, prioritizing patient safety and quality, supply chain reliability and a simplified operating model. The simplified operating model aims to simplify the organization to increase agility and structurally lower the cost base by giving end-to-end accountability to the segments. Operating model productivity savings, procurement savings and other productivity programs contributed positively to the results of operations.
Workforce reductionIn addition to the reduction of its workforce by 4,000 roles announced in October 2022, in 2023 Philips announced plans to reduce its workforce by an additional 6,000 roles globally by 2025, in line with relevant local regulations and processes. These reductions are focused on Corporate and Functions optimization and non-core activities, and amounted to approximately 8,000 roles by year-end 2023. Workforce-related restructuring charges were EUR 196 million in 2023 and EUR 136 million in 2022.
Supply chain resilienceIn 2023, following significant actions to increase supply chain resilience and mitigate the impact of disruptions, our sales benefited from improved material availability and resolved shortages in components.
Limited availability and delays in the supply of certain components and products internationally – partly a consequence of the COVID pandemic and the Russia-Ukraine war – impacted our results in 2022. In addition, the supply chain constraints resulted in an increase in overall working capital, in particular inventories.
Geopolitical environmentHaving substantially reduced our operations in Russia in 2022, the remaining activities were focused on the delivery of medical systems, devices, and spare parts to healthcare providers. In 2023, increased sanctions and export controls led to a further reduction in sales activity. Philips’ operations in Russia and Ukraine on a combined basis represented less than 2% of group sales in both 2022 and 2023. The asset value of the activities in Russia and Ukraine, mainly working capital, was less than 1% of the consolidated total assets as of December 31, 2022 and 2023. The Russia-Ukraine war continues to put pressure on the global commodity landscape and supply chains, and contribute to higher levels of cost inflation.
The company’s global operations are exposed to geopolitical and macroeconomic changes (refer to Risk management and internal control). The current situation in the Middle East further increases economic and political uncertainty. Philips is present in Israel with several subsidiaries, mainly in Diagnosis & Treatment and Connected Care, that are primarily involved in manufacturing and research and development activities.
Climate-related mattersIn preparing the consolidated financial statements, management has considered the impact of climate change, specifically the financial impact of Philips meeting its internal and external climate-related aims, the potential impact of climate-related risks, and the costs incurred to pro-actively manage such risks. These considerations did not have a material impact on the financial reporting judgments, estimates or assumptions. The financial impacts considered include specific climate mitigation measures, such as the use of lower-carbon energy sources, the cost of developing more sustainable product offerings, and expenses incurred to mitigate against the impact of extreme weather conditions. To meet its long-term Science Based Targets and reduce its full value chain emissions in line with a 1.5°C global warming scenario, Philips has entered into a number of power purchase agreements.
The composition of sales growth in percentage terms in 2023, compared to 2022 and 2021, is presented in the following table.
Philips Group
Sales
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Diagnosis & Treatment | 7,825 | 8,290 | 8,818 |
| Nominal sales growth | 5.9% | 5.9% | 6.4% |
| Comparable sales growth1) | 8.3% | (0.8)% | 11.1% |
| Connected Care | 5,371 | 5,268 | 5,138 |
| Nominal sales growth | (14.9)% | (1.9)% | (2.5)% |
| Comparable sales growth1) | (19.0)% | (9.1)% | 1.1% |
| Personal Health | 3,429 | 3,626 | 3,602 |
| Nominal sales growth | 7.2% | 5.7% | (0.7)% |
| Comparable sales growth1) | 8.8% | 0.1% | 3.2% |
| Other | 530 | 643 | 612 |
| Philips Group | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | (0.9)% | 3.9% | 1.9% |
| Comparable sales growth1) | (1.2)% | (2.8)% | 6.0% |
Group sales in 2023 amounted to EUR 18,169 million, 1.9% higher than in 2022 on a nominal basis. Considering a 4.1% negative currency effect and consolidation impact, comparable sales growth*) was 6.0%. The negative currency effect was mainly due to depreciation of currencies against the euro, and affected all segments. In addition, provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, had a negative impact of 1%.
Comparable order intake decreased 5% in 2023, compared to a 3% decline in 2022. The order book (which covers around 40% of Group sales) remains strong, and we are taking the necessary actions to improve order intake by shortening lead times from order to delivery and building on the positive impact we are making with our innovations, for example in predictive data analytics and artificial intelligence across our portfolio, to help improve the quality and efficiency of care delivery. The order book remains strong and is expected to continue to support growth.
Group sales amounted to EUR 17,827 million in 2022, 3.9% higher than in 2021 on a nominal basis. Considering a 6.7% positive effect from currency and consolidation, comparable sales*) decreased by 2.8%. This was driven by a positive currency effect, mainly due to appreciation of currencies against the euro, and affected all segments.
The order book at year-end 2022 was 10% higher than at the end of 2021, ensuring a higher coverage for sales in 2023. The increase mainly relates to the Diagnosis & Treatment businesses driven by Diagnostic Imaging. Comparable order intake decreased 3%, compared to 4% growth in 2021.
Diagnosis & TreatmentIn 2023, sales amounted to EUR 8,818 million, 6.4% higher than in 2022 on a nominal basis. Considering a 4.7% negative currency effect and consolidation impact, comparable sales*) increased by 11.1%. This was due to double-digit growth in Ultrasound and Image-Guided Therapy and high-single-digit growth in Diagnostic Imaging, due to supply chain improvements.
In 2022, sales amounted to EUR 8,290 million, 5.9% higher than in 2021 on a nominal basis. Considering a 6.7% positive currency effect and consolidation impact, comparable sales*) decreased by 0.8%. This was due to mid-single-digit growth in Image-Guided Therapy and low-single-digit growth in Enterprise Diagnostic Informatics, which was more than offset by a decline in Ultrasound and in Diagnostic Imaging due to specific electronic component shortages.
Connected CareIn 2023, sales amounted to EUR 5,138 million, 2.5% lower than in 2022 on a nominal basis. Considering a 3.6% negative currency effect and consolidation impact, comparable sales*) increased by 1.1%. This growth was mainly driven by double-digit growth in Monitoring, partly offset by a decline in Sleep & Respiratory Care due to the consequences of the Respironics recall. In addition, sales were impacted by provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, which had a negative impact of 3.4%.
In 2022, sales amounted to EUR 5,268 million, 1.9% lower than in 2021 on a nominal basis. Considering a 7.2% positive currency effect and consolidation impact, comparable sales*) decreased by 9.1%. This was mainly due to the consequences of the Respironics recall and the impact of supply chain headwinds.
Personal HealthIn 2023, sales amounted to EUR 3,602 million, 0.7% lower than in 2022 on a nominal basis. Considering a 3.9% negative currency effect and consolidation impact, comparable sales*) increased by 3.2%. This was mainly driven by high-single-digit growth in Personal Care, partly offset by a decline in Oral Healthcare.
In 2022, sales amounted to EUR 3,626 million, 5.7% higher than in 2021 on a nominal basis. Considering a 5.6% positive currency effect and consolidation impact, comparable sales*) increased by 0.1%, consisting of a global increase of 2.5%, offset by a 2.4% decline in sales attributable to Russia due to the war with Ukraine. Oral Healthcare and Mother & Child Care recorded mid-single-digit growth, which was offset by a mid-single-digit decline in Personal Care.
OtherIn 2023, sales amounted to EUR 612 million, compared to EUR 643 million in 2022. The decrease was mainly due to the discontinuation of innovation consultancy activities provided to other companies until 2023.
In 2022, sales amounted to EUR 643 million, compared to EUR 530 million in 2021. The increase was mainly due to additional royalty income and supplies to the divested Domestic Appliances business.
Performance by geographic areaPhilips Group
Sales by geographic area
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Western Europe | 3,645 | 3,603 | 3,819 |
| North America | 6,781 | 7,588 | 7,562 |
| Other mature geographies | 1,694 | 1,643 | 1,626 |
| Mature geographies | 12,120 | 12,833 | 13,007 |
| Nominal sales growth | (2)% | 6% | 1% |
| Comparable sales growth1) | (3)% | (1)% | 4% |
| Growth geographies | 5,036 | 4,993 | 5,162 |
| Nominal sales growth | 1% | (1)% | 3% |
| Comparable sales growth1) | 3% | (7)% | 10% |
| Philips Group | 17,156 | 17,827 | 18,169 |
| Nominal sales growth | (0.9)% | 3.9% | 1.9% |
| Comparable sales growth1) | (1.2)% | (2.8)% | 6.0% |
Sales in Mature geographies in 2023 were 1% higher than in 2022 on a nominal basis and 4% higher on a comparable basis*). Sales in Western Europe were 6% higher year-on-year on a nominal basis and 7% higher on a comparable basis*), with double-digit growth in the Diagnosis & Treatment segment, mid-single-digit growth in the Personal Health segment, and low-single-digit decline in the Connected Care segment. Sales in North America were flat year-on-year on a nominal basis and 3% higher on a comparable basis*), as high-single-digit growth in the Diagnosis & Treatment segment was offset by a low-single-digit decline in the Personal Health segment. Sales in Other mature geographies decreased by 1% on a nominal basis and increased by 7% on a comparable basis*), with high-single-digit comparable sales growth*) in the Connected Care segment and mid-single-digit growth in the Diagnosis & Treatment and Personal Health segments.
Sales in mature geographies in 2022 were 6% higher than in 2021 on a nominal basis and 1% lower on a comparable basis*). Sales in Western Europe were 1% lower year-on-year on a nominal basis and 3% lower on a comparable basis*), with a double-digit decline in the Connected Care businesses, a low-single-digit decline in the Diagnosis & Treatment businesses, and flat growth in the Personal Health businesses. Sales in North America were 12% higher year-on-year on a nominal basis and were flat on a comparable basis*), as double-digit growth in the Personal Health businesses and low-single-digit growth in the Diagnosis & Treatment businesses were offset by a mid-single-digit decline in the Connected Care businesses, mainly due to the Sleep & Respiratory Care business. Sales in other mature geographies decreased by 3% on a nominal basis and 1% on a comparable basis*), with high-single-digit comparable sales growth*) in the Personal Health businesses offset by a high-single-digit decline in the Connected Care businesses.
Sales in Growth geographies in 2023 increased by 3% on a nominal basis and 10% on a comparable basis*), with double-digit growth in the Diagnosis & Treatment and Connected Care segments and low-single-digit growth in the Personal Health segment. The double-digit growth in comparable sales growth*) was driven by China, Middle East & Turkey and Latin America.
Sales in growth geographies in 2022 decreased by 1% on a nominal basis and 7% on a comparable basis*), with a double-digit decline in the Connected Care and Personal Health businesses and a low-single-digit decline in the Diagnosis & Treatment businesses. The high-single-digit decline in comparable sales growth*) was due to a double-digit decline in China and Russia & Central Asia, partly offset by double-digit growth in Middle East, Turkey & Africa.
Diagnosis & TreatmentDiagnosis & Treatment
Sales by geographic area
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Western Europe | 1,553 | 1,521 | 1,743 |
| North America | 2,664 | 3,019 | 3,172 |
| Other mature geographies | 805 | 782 | 766 |
| Mature geographies | 5,022 | 5,322 | 5,681 |
| Growth geographies | 2,803 | 2,968 | 3,137 |
| Sales | 7,825 | 8,290 | 8,818 |
| Nominal sales growth | 6% | 6% | 6% |
| Comparable sales growth1) | 8% | (1)% | 11% |
Sales in Growth geographies increased by 6% on a nominal basis in 2023, and on a comparable basis*) showed double-digit growth, which was mainly driven by China and Middle East & Turkey. Sales in Mature geographies increased by 7% on a nominal basis and showed double-digit growth on a comparable basis*), which was driven by double-digit growth in Western Europe and high-single-digit growth in North America.
Sales in growth geographies increased by 6% on a nominal basis in 2022, and on a comparable basis*) showed a low-single-digit decline, which was mainly due to China. Sales in mature geographies increased by 6% on a nominal basis and were flat year-on-year on a comparable basis*).
Connected CareConnected Care
Sales by geographic area
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Western Europe | 949 | 828 | 798 |
| North America | 3,019 | 3,227 | 3,132 |
| Other mature geographies | 649 | 587 | 573 |
| Mature geographies | 4,618 | 4,642 | 4,503 |
| Growth geographies | 753 | 626 | 635 |
| Sales | 5,371 | 5,268 | 5,138 |
| Nominal sales growth | (15)% | (2)% | (2)% |
| Comparable sales growth1) | (19)% | (9)% | 1% |
Sales in Growth geographies increased by 1% on a nominal basis in 2023, and on a comparable basis*) grew 7%, which was driven by Latin America and China. Sales in Mature geographies decreased by 3% on a nominal basis and were flat year-on-year on a comparable basis*), as growth in Other mature geographies was offset by Western Europe. In addition, provisions charged to sales of EUR 174 million, mainly in connection with the proposed Respironics consent decree, had a negative impact of 3.4%.
Sales in growth geographies decreased by 17% on a nominal basis in 2022, and on a comparable basis*) showed a double-digit decline, with a double-digit decline across most regions, mainly due to the consequences of the Respironics field action and the COVID situation in China. Sales in mature geographies increased by 1% on a nominal basis and showed a high-single-digit decline on a comparable basis*), with a double-digit decline in Western Europe and a mid-single-digit decline in North America.
Personal HealthPersonal Health
Sales by geographic area
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Western Europe | 894 | 902 | 961 |
| North America | 939 | 1,209 | 1,144 |
| Other mature geographies | 198 | 211 | 207 |
| Mature geographies | 2,032 | 2,322 | 2,312 |
| Growth geographies | 1,398 | 1,304 | 1,290 |
| Sales | 3,429 | 3,626 | 3,602 |
| Nominal sales growth | 7% | 6% | (1)% |
| Comparable sales growth1) | 9% | 0% | 3% |
Sales in Growth geographies decreased by 1% on a nominal basis in 2023, and on a comparable basis*) showed mid-single-digit growth, which was mainly driven by Middle-East & Turkey and China, partly offset by a decline in Russia & Central Asia. Sales in Mature geographies were flat on a nominal basis, and on a comparable basis*) showed low-single-digit growth, driven by Western Europe.
Sales in growth geographies decreased by 7% on a nominal basis in 2022, and on a comparable basis*) showed a double-digit decline, which was mainly attributable to China. Sales in mature geographies increased by 14% on a nominal basis, and on a comparable basis*) showed high-single-digit growth, driven by double-digit growth in North America.
Cost of salesPhilips Group
Cost of sales components
in millions of EUR unless otherwise stated
| 2021 | as a % of sales | 2022 | as a % of sales | 2023 | as a % of sales | |
|---|---|---|---|---|---|---|
| Costs of materials used | 4,142 | 24.1% | 4,320 | 24.2% | 4,626 | 25.5% |
| Salaries and wages | 2,245 | 13.1% | 2,462 | 13.8% | 2,381 | 13.1% |
| Depreciation and amortization | 479 | 2.8% | 535 | 3.0% | 461 | 2.5% |
| Other manufacturing costs | 3,123 | 18.2% | 3,316 | 18.6% | 3,252 | 17.9% |
| Cost of sales | 9,988 | 58.2% | 10,633 | 59.6% | 10,721 | 59.0% |
Cost of sales includes only expenses directly or indirectly attributable to the sale of products or services, such as cost of materials used, salaries and wages, depreciation and amortization of assets used in manufacturing, and other manufacturing costs (such as repair and maintenance costs related to production, expenses incurred for shipping and handling of internal movements of goods, and other expenses related to manufacturing).
Philips’ cost of sales increased by EUR 88 million to EUR 10,721 million in 2023, and decreased as a percentage of sales, compared to EUR 10,633 million in 2022, mainly due to an increase in Cost of materials used by EUR 306 million in 2023, mainly due to increased sales volume and cost inflation, partly offset by productivity measures and a favorable foreign currency impact. Other key factors influencing cost of sales were as follows:
Philips’ cost of sales increased by EUR 645 million to EUR 10,633 million in 2022, compared to EUR 9,988 million in 2021, mainly due to increased expenses of EUR 217 million in salaries and wages, driven by an unfavorable foreign currency impact and wage inflation, partly offset by productivity measures. Other key factors influencing cost of sales were as follows:
In 2023, Philips’ gross margin was EUR 7,448 million, or 41.0% of sales, compared to EUR 7,194 million, or 40.4% of sales, in 2022. Gross margin increased by EUR 254 million year-on-year, driven by increased sales and price & productivity measures, partly offset by cost inflation, an unfavorable foreign currency impact, and higher restructuring, acquisition-related and other charges.
In 2022, Philips’ gross margin was EUR 7,194 million, or 40.4% of sales, compared to EUR 7,168 million, or 41.8% of sales, in 2021. Gross margin was flat year-on-year due to cost inflation and a decrease in sales, which was offset by a favorable foreign currency impact, a decrease in restructuring, acquisition-related and other charges, and productivity and pricing measures.
Selling expensesSelling expenses amounted to EUR 4,524 million, or 24.9% of sales, in 2023, compared to EUR 4,621 million, or 25.9% of sales, in 2022. The year-on-year decrease in selling expenses of EUR 97 million was mainly driven by a favorable foreign currency impact, partly offset by higher restructuring, acquisition-related and other charges.
Selling expenses amounted to EUR 4,621 million, or 25.9% of sales, in 2022, compared to EUR 4,258 million, or 24.8% of sales, in 2021. The year-on-year increase in selling expenses of EUR 363 million was mainly due to an unfavorable foreign currency impact and an increase in restructuring, acquisition-related and other charges.
General and administrative expensesGeneral and administrative expenses amounted to EUR 608 million, or 3.3% of sales, in 2023, compared to EUR 671 million, or 3.8% of sales, in 2022. The year-on-year decrease of EUR 63 million was mainly driven by a favorable foreign currency impact and lower restructuring, acquisition-related and other charges.
General and administrative expenses amounted to EUR 671 million, or 3.8% of sales, in 2022, compared to EUR 599 million, or 3.5% of sales, in 2021. The year-on-year increase of EUR 72 million was mainly driven by higher restructuring, acquisition-related and other charges.
Research and development expensesResearch and development costs were EUR 1,890 million, or 10.4% of sales, in 2023, compared to EUR 2,091 million, or 11.7% of sales, in 2022. The year-on-year decrease of EUR 201 million was mainly driven by lower restructuring, acquisition-related and other charges and a favorable foreign currency impact. 2022 included R&D project impairment charges.
Research and development costs were EUR 2,091 million, or 11.7% of sales, in 2022, compared to EUR 1,806 million, or 10.5% of sales, in 2021. The year-on-year increase of EUR 285 million was mainly driven by higher restructuring, acquisition-related and other charges in relation to R&D project impairments and an unfavorable foreign currency impact.
Philips Group
Research and development expenses
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Diagnosis & Treatment | 762 | 894 | 827 |
| Connected Care | 670 | 822 | 663 |
| Personal Health | 190 | 200 | 197 |
| Other | 184 | 175 | 203 |
| Philips Group | 1,806 | 2,091 | 1,890 |
| As a % of sales | 10.5% | 11.7% | 10.4% |
In addition to the annual goodwill-impairment tests for Philips, trigger-based impairment tests were performed during the years 2023, 2022 and 2021. As a result of the tests, recorded goodwill impairments were EUR 1,357 million in 2022, and EUR 15 million in 2021. The goodwill impairment of EUR 1,331 million in 2022 was recorded in the Sleep & Respiratory Care business and was due to revisions to the expected future cash flows. In addition, in 2023 a EUR 8 million goodwill impairment was recognized for a business held for sale, whereas in 2022 a EUR 27 million goodwill impairment was recognized in the Precision Diagnosis Solutions business.
Goodwill impairment charges were EUR 8 million in 2023 and EUR 1,357 million in 2022. For further information refer to Goodwill.
Net income, Income from operations (EBIT) and Adjusted EBITA*)Net income amounted to a loss of EUR 463 million in 2023, an improvement of EUR 1.1 billion compared to 2022, which included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Higher earnings in 2023 were offset by a EUR 575 million litigation provision in connection with the Respironics recall. Net income in 2022 included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Net income is not allocated to segments, as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
The following overview shows Income from operations and Adjusted EBITA*) by segment.
Philips Group
Income from operations and Adjusted EBITA1)
in millions of EUR unless otherwise stated
| Income from operations | as a % of sales | Adjusted EBITA1) | as a % of sales | |
|---|---|---|---|---|
| 2023 | ||||
| Diagnosis & Treatment | 720 | 8.2% | 1,026 | 11.6% |
| Connected Care | (1,199) | (23.3)% | 369 | 7.2% |
| Personal Health | 552 | 15.3% | 597 | 16.6% |
| Other | (188) | (71) | ||
| Philips Group | (115) | (0.6)% | 1,921 | 10.6% |
| 2022 | ||||
| Diagnosis & Treatment | 538 | 6.5% | 788 | 9.5% |
| Connected Care | (2,347) | (44.6)% | 111 | 2.1% |
| Personal Health | 515 | 14.2% | 538 | 14.8% |
| Other | (235) | (119) | ||
| Philips Group | (1,529) | (8.6)% | 1,318 | 7.4% |
| 2021 | ||||
| Diagnosis & Treatment | 948 | 12.1% | 1,028 | 13.1% |
| Connected Care | (716) | (13.3)% | 553 | 10.3% |
| Personal Health | 576 | 16.8% | 590 | 17.2% |
| Other | (255) | (117) | ||
| Philips Group | 553 | 3.2% | 2,054 | 12.0% |
Income from operations amounted to a loss of EUR 115 million, or (0.6)% of sales, in 2023, compared to a loss of EUR 1,529 million, or (8.6)% of sales, in 2022, which included a charge of EUR 1.5 billion related to goodwill and R&D impairments. Higher earnings in 2023 were offset by a EUR 575 million Respironics litigation provision. Adjusted EBITA*) increased to EUR 1,921 million and the margin improved to 10.6%, compared to EUR 1,318 million and a margin of 7.4% in 2022, mainly driven by increased sales and pricing & productivity measures.
Amortization and goodwill impairment charges were EUR 298 million in 2023. This includes amortization charges of EUR 290 million and goodwill impairment charges of EUR 8 million. Amortization and goodwill impairment charges in 2022 were EUR 1,720 million. This included a charge of EUR 1,331 million related to an impairment of goodwill in the Sleep & Respiratory Care business, EUR 363 million amortization charges and a EUR 27 million goodwill impairment in the Precision Diagnosis Solutions business.
Restructuring, acquisition-related and other charges were EUR 1,739 million in 2023. This includes: a EUR 575 million Respironics litigation provision, EUR 363 million in connection with the proposed Respironics consent decree, and EUR 224 million Respironics field-action running remediation costs. In addition, it includes EUR 285 million restructuring charges, mainly related to workforce reduction, and charges in relation to quality remediation actions of EUR 175 million. 2022 charges were EUR 1,127 million and included: restructuring charges of EUR 185 million; EUR 148 million portfolio realignment impairments and charges; R&D project impairment charges of EUR 134 million; EUR 250 million for the Respironics field-action provision; EUR 210 million Respironics field-action running remediation costs; an approximately EUR 60 million provision for public investigations into tender irregularities; and EUR 59 million for provisions for quality actions in Connected Care.
Income from continuing operations attributable to shareholders per common share (in EUR) - diluted, was EUR (0.50) in 2023, compared to EUR (1.76) in 2022. Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted*) was EUR 1.25, compared to EUR 0.92 in 2022.
Net income amounted to a loss of EUR 1,605 million in 2022, a decrease of EUR 4.9 billion compared to 2021, mainly due to a charge of EUR 1.5 billion related to goodwill and R&D impairments in 2022 and a gain of EUR 2.5 billion on the sale of the Domestic Appliances business in 2021. Net income is not allocated to segments, as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Income from operations in 2022 amounted to a loss of EUR 1,529 million, or (8.6)% of sales, compared to EUR 553 million, or 3.2% of sales, in 2021, mainly impacted by a charge of EUR 1.5 billion related to goodwill and R&D impairments. Adjusted EBITA*) in 2022 was EUR 1,318 million and the margin amounted to 7.4%, compared to EUR 2,054 million and a margin of 12.0% in 2021, primarily due to the sales decline and cost inflation, partly offset by pricing and productivity measures.
Amortization and goodwill impairment charges in 2022 were EUR 1,720 million. This includes a charge of EUR 1,331 million related to an impairment of goodwill in the Sleep & Respiratory Care business, a EUR 27 million goodwill impairment in the Precision Diagnosis Solutions business, and amortization charges of EUR 22 million related to an impairment of a technology asset. In 2021, amortization and goodwill impairment charges were EUR 337 million and included a charge of EUR 13 million related to an impairment of goodwill and amortization charges of EUR 55 million related to an impairment of a technology asset.
Restructuring, acquisition-related and other charges in 2022 were EUR 1,127 million. This includes: restructuring charges of EUR 185 million; EUR 282 million portfolio realignment impairments and charges; EUR 250 million for the Respironics field-action provision; EUR 210 million Respironics field-action running remediation costs; a EUR 60 million provision for public investigations tender irregularities; and EUR 59 million for provisions for quality actions in Connected Care. 2021 charges were EUR 1,164 million and included: a field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification; provisions for quality actions of EUR 94 million and other matters of EUR 53 million in Connected Care; restructuring charges of EUR 80 million; acquisition-related charges of EUR 102 million partly offset by a EUR 87 million gain related to the re-measurement of contingent consideration liabilities; a loss of EUR 76 million related to a divestment; and separation costs of EUR 64 million related to the Domestic Appliances business. 2021 also included a release of a legal provision of EUR 38 million, a gain of EUR 33 million related to a minority participation, and a benefit from the re-measurement of environmental liabilities of EUR 22 million.
Income from continuing operations attributable to shareholders per common share (in EUR) - diluted, was EUR (1.84) in 2022, compared to EUR 0.67 in 2021. Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted*) was EUR 0.96 in 2022, compared to EUR 1.65 in 2021.
Diagnosis & TreatmentIncome from operations increased to EUR 720 million in 2023, compared to EUR 538 million in 2022. This was mainly driven by increased sales and pricing & productivity measures, partly offset by cost inflation. These factors also resulted in an increase in Adjusted EBITA*) to 11.6% of sales in 2023.
Amortization and goodwill impairment charges in 2023 were EUR 98 million and include EUR 89 million amortization charges and EUR 8 million goodwill impairment charges. 2022 charges were EUR 115 million and included EUR 22 million of charges related to an impairment of a technology asset in Image-Guided Therapy.
Restructuring, acquisition-related and other charges in 2023 were EUR 210 million and include EUR 81 million charges in relation to quality remediation actions and EUR 73 million restructuring charges, mainly related to workforce reduction. 2022 charges amounted to EUR 136 million and included R&D project impairment charges of EUR 73 million and a provision of approximately EUR 60 million for public investigations into tender irregularities.
Income from operations in 2022 decreased to EUR 404 million, compared to EUR 941 million in 2021. This was mainly due to cost inflation, partly offset by productivity measures. These factors also resulted in a decrease in Adjusted EBITA*) to 8.4% of sales in 2022.
Amortization and goodwill impairment charges in 2022 were EUR 115 million and included EUR 22 million of charges related to an impairment of a technology asset in Image-Guided Therapy. 2021 charges were EUR 144 million and included EUR 55 million of charges related to an impairment of a technology asset in Image-Guided Therapy.
Restructuring, acquisition-related and other charges in 2022 were EUR 201 million and include EUR 120 million portfolio realignment impairments and charges and a provision of EUR 60 million for public investigations tender irregularities. 2021 charges amounted to a gain of EUR 25 million and included: restructuring charges of EUR 44 million; acquisition-related charges of EUR 48 million offset by a EUR 85 million gain related to the re-measurement of contingent consideration liabilities; and the release of a legal provision of EUR 38 million.
Income from operations increased to EUR (1,199) million in 2023, compared to EUR (2,347) million in 2022, which included a charge of EUR 1.3 billion related to goodwill impairment. 2023 was mainly impacted by the consequences of the Respironics field action, in particular the EUR 575 million provision in connection with the Respironics litigation, partly offset by increased sales and productivity measures. Adjusted EBITA*) improved to 7.2% of sales and was also impacted by cost inflation.
Amortization and goodwill impairment charges in 2023 were EUR 178 million and include EUR 178 million amortization charges. 2022 charges were EUR 1,583 million and included EUR 1,331 million impairment of goodwill related to the Sleep & Respiratory Care business and a goodwill impairment of EUR 27 million in Precision Diagnosis Solutions.
Restructuring, acquisition-related and other charges in 2023 were EUR 1,390 million and include: charges of EUR 575 million Respironics litigation provision, EUR 363 million in connection with the proposed Respironics consent decree, and EUR 224 million Respironics field-action running remediation costs. In addition, it includes EUR 64 million restructuring charges, mainly related to workforce reduction, and charges in relation to quality remediation actions of EUR 94 million. 2022 charges were EUR 875 million and included: EUR 250 million for the Respironics field action provision; EUR 210 million Respironics running remediation costs; EUR 148 million portfolio realignment impairments and charges; and EUR 59 million provisions for quality actions in Connected Care.
Income from operations in 2022 decreased to EUR (2,246) million, compared to EUR (722) million in 2021. This was mainly due to the EUR 1.3 billion goodwill impairment, the sales decline, the consequences of the Respironics field action and cost inflation. Adjusted EBITA*) was 2.2% of sales in 2022 and was also impacted by the sales decline and cost inflation, partly offset by productivity measures.
Amortization and goodwill impairment charges in 2022 were EUR 1,530 million and include EUR 1,331 million impairment of goodwill related to the Sleep & Respiratory Care business and a goodwill impairment of EUR 27 million in Precision Diagnosis Solutions. 2021 charges were EUR 161 million and included a EUR 13 million impairment of goodwill related to the divested Personal Emergency Response Services (PERS) and Senior Living business.
Restructuring, acquisition-related and other charges in 2022 were EUR 811 million and include: EUR 250 million for the Respironics field action provision; EUR 210 million Respironics running remediation costs; EUR 160 million portfolio realignment impairments and charges; and EUR 59 million provisions for quality actions in Connected Care. 2021 charges were EUR 1,058 million and included: a field action provision of EUR 719 million in connection with the Philips Respironics voluntary recall notification; EUR 93 million of restructuring and acquisition-related charges; provisions for quality actions of EUR 94 million and other matters of EUR 53 million; and a gain of EUR 33 million related to a minority participation.
Personal HealthIncome from operations increased to EUR 552 million in 2023, compared to EUR 515 million in 2022. This was mainly driven by increased sales and pricing & productivity measures. These factors also resulted in an increase in Adjusted EBITA*) to 16.6% of sales.
Amortization charges in 2023 were EUR 14 million and include amortization charges related to intangible assets in Mother & Child Care. 2022 charges were EUR 15 million and included amortization charges related to intangible assets in Mother & Child Care.
Restructuring, acquisition-related and other charges in 2023 were EUR 31 million and include a EUR 23 million investment re-measurement loss and restructuring costs mainly related to workforce reduction of EUR 9 million. 2022 charges were not material.
Income from operations in 2022 decreased to EUR 515 million, compared to EUR 576 million in 2021. This was mainly driven by cost inflation and an adverse foreign currency impact, partly offset by pricing and productivity measures. These factors also resulted in a decrease in Adjusted EBITA*) to 14.8% of sales.
Amortization charges in 2022 were EUR 15 million and include amortization charges related to intangible assets in Mother & Child Care. 2021 charges were EUR 15 million and included amortization charges related to intangible assets in Mother & Child Care.
Restructuring, acquisition-related and other charges in 2022 and 2021 were not material.
In Other we report on the items Innovation & Strategy, IP Royalties, Central costs and Other.
Income from operations amounted to a loss of EUR 188 million in 2023, compared to a loss of EUR 235 million in 2022. Adjusted EBITA*) amounted to a loss of EUR 71 million, compared to a loss of EUR 119 million in 2022. The increase in Adjusted EBITA*) was mainly due to cost savings, partly offset by lower royalty income.
Restructuring, acquisition-related and other charges in 2023 were EUR 108 million and include EUR 139 million restructuring charges mainly related to workforce reduction and a gain of EUR 35 million due to a divestment. 2022 charges were EUR 108 million and included restructuring charges of EUR 61 million and a EUR 21 million impairment of intangible assets.
Income from operations in 2022 amounted to a loss of EUR 202 million, compared to a loss of EUR 242 million in 2021. Adjusted EBITA* in 2022 amounted to a loss of EUR 89 million, compared to a loss of EUR 105 million in 2021. Adjusted EBITA*) increased, mainly due to higher royalty income, partly offset by an adverse currency impact and investment in Quality & Regulatory.
Restructuring, acquisition-related and other charges in 2022 were EUR 107 million and include restructuring charges of EUR 61 million and a EUR 21 million impairment of intangible assets. 2021 charges were EUR 131 million and included a loss of EUR 76 million related to a divestment and EUR 64 million of separation costs related to the Domestic Appliances business, partly offset by a benefit from the re-measurement of environmental liabilities of EUR 22 million.
A breakdown of financial income and expenses is presented in the following table.
Philips Group
Financial income and expenses
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Interest expense, net | (141) | (210) | (230) |
| Net change in fair value of financial assets through profit or loss | 95 | 9 | (26) |
| Net foreign exchange gains (losses) | - | 9 | (23) |
| Other | 6 | (8) | (34) |
| Financial income and expenses | (39) | (200) | (314) |
Financial income and expenses resulted in a net expense of EUR 314 million in 2023, compared to a net expense of EUR 200 million in 2022. 2023 includes higher interest expense, fair value losses on minority investments and net foreign exchange losses compared to 2022. For further information, refer to Financial income and expenses.
Financial income and expenses resulted in a net expense of EUR 200 million, compared to a net expense of EUR 39 million in 2021. 2022 includes lower gains on the value of Philips' minority participations and higher interest expense, primarily due to financial charges related to early redemption of EUR and USD bonds and issuance of new EUR bonds in April 2022, compared to 2021. For further information, refer to Financial income and expenses.
Income taxesIncome tax expense increased by EUR 40 million year-on-year. The income tax benefit in 2023 is mainly driven by the negative income before tax, recognition of tax credits and tax incentives, partly offset by the tax effect on the economic loss class-action settlement provision relating to the Respironics recall. The income tax benefit in 2022 was mainly driven by the negative income before tax and tax incentives, partly offset by non-tax-deductible goodwill impairment.
Income tax expense decreased by EUR 10 million in 2022 compared to 2021, mainly due to lower income, partly offset by a non-deductible goodwill impairment in the Sleep & Respiratory Care business in 2022 and a one-off benefit relating to the recognition of tax assets due to a business transfer in 2021.
Investments in associatesResults related to investments in associates declined from a loss of EUR 2 million in 2022 to a loss of EUR 98 million in 2023. 2023 includes impairments of EUR 58 million and share of results of associates of EUR 40 million.
Results related to investments in associates improved from a loss of EUR 4 million in 2021 to a loss of EUR 2 million in 2022. In 2022, Philips recorded an impairment of EUR 66 million in relation to its interest in Candid Care Co. As part of the acquisition of Affera, Inc. by Medtronic plc in August 2022, the company sold its investment in Affera to Medtronic and recorded a gain of EUR 84 million on the sale.
Discontinued operationsPhilips Group
Discontinued operations, net of income taxes
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Domestic Appliances | 2,698 | 3 | (2) |
| Other | 13 | 10 | (7) |
| Net income of Discontinued operations | 2,711 | 13 | (10) |
In 2023 and 2022, Discontinued operations consisted primarily of the Domestic Appliances business and certain other divestments that were reported as discontinued operations. In 2021, the sale of the Domestic Appliance business resulted in an after-tax gain of EUR 2.5 billion.
For further information, refer to Discontinued operations and assets classified as held for sale.
Non-controlling interestsNet income attributable to non-controlling interests decreased from EUR 3 million in 2022 to EUR 2 million in 2023.
Net income attributable to non-controlling interests decreased from EUR 4 million in 2021 to EUR 3 million in 2022.
In 2023, Philips completed one acquisition involving a total net cash outflow of EUR 53 million (total equity price and settlement of debt). The acquisition is subject to final purchase price allocation procedures, which are expected to be finalized in the second quarter of 2024.
In 2022, Philips completed three acquisitions. The acquisition of Vesper Medical Inc., completed on January 11, 2022, was the most notable. Acquisitions in 2022 and prior years led to acquisition and post-merger integration charges of EUR 70 million in the Connected Care segment.
In 2021, Philips completed two acquisitions: BioTelemetry, which was completed on February 9, 2021, and Capsule Technologies, which was completed on March 4, 2021. Acquisitions in 2021 and prior years led to acquisition and post-merger integration charges of EUR 51 million in the Connected Care businesses.
DivestmentsIn 2023, Philips completed six divestments for a cash consideration of EUR 80 million, notably Philips Pharma Solutions in the US.
In 2022, Philips completed one divestment, which was not material.
n 2021, Philips completed three divestments. On September 1, 2021, Philips sold its Domestic Appliances business to a global investment firm, Hillhouse Investment, resulting in a EUR 2.5 billion gain after tax and transaction-related costs; reported in Discontinued Operations. In addition, Philips completed the divestment of the Personal Emergency Response Services (PERS) and Senior Living business on June 30, 2021, and September 17, 2021, respectively, as well as completing the divestment of a small business in segment Other. As part of the PERS divestment, Philips acquired shares in the buyer, Connect America Investment Holdings, LLC, with a value of EUR 40 million. The investment is classified as a financial asset measured at Fair Value through Other Comprehensive Income (FVTOCI) and is reported as part of Other non-current financial assets. The divestment resulted in a loss of EUR 76 million, which is included in Other business expenses in our Consolidated statements of income.
For details, please refer to Acquisitions and divestments.
The movements in cash and cash equivalents balance for the years ended December 31, 2021, 2022 and 2023 are presented and explained in the following table.
Philips Group
Condensed consolidated cash flows
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Beginning cash and cash equivalents balance | 3,226 | 2,303 | 1,172 |
| Net cash flows from operating activities | 1,629 | (173) | 2,136 |
| Net cash flows from investing activities | |||
| Net capital expenditures | (729) | (788) | (554) |
| Other cash flows from investing activities | (2,943) | (698) | (82) |
| Net cash flows from financing activities | |||
| Treasury shares transactions | (1,613) | (174) | (662) |
| Changes in debt | (251) | 1,092 | (181) |
| Dividend paid to shareholders of the company | (482) | (412) | (2) |
| Other cash flow items | 62 | 34 | (81) |
| Net cash flows from discontinued operations | 3,403 | (12) | 123 |
| Ending cash and cash equivalents balance | 2,303 | 1,172 | 1,869 |
Net cash flows from operating activities amounted to an inflow of EUR 2,136 million in 2023, compared to an outflow of EUR 173 million in 2022. This increase is mainly due to higher cash earnings and lower working capital, and includes a EUR 141 million payment related to the previously announced resolution of the economic loss class action in the US. Free cash flow*) amounted to a cash inflow of EUR 1,582 million in 2023, compared to an outflow of EUR 961 million in 2022.
Net cash flows from operating activities amounted to an outflow of EUR 173 million in 2022, compared to an inflow of EUR 1,629 million in 2021. This decrease is mainly due to lower cash earnings, increased working capital and cash costs related to the Philips Respironics field action. Free cash flow*) amounted to a cash outflow of EUR 961 million in 2022, compared to an inflow of EUR 900 million in 2021.
In 2021, net cash flows from operating activities amounted to EUR 1,629 million, compared to EUR 2,511 million in 2020. This decrease is mainly due to increased working capital and consumption of provisions, partly offset by lower income tax paid. Free cash flow*) amounted to EUR 900 million in 2021, compared to EUR 1,635 million in 2020.
Net cash flows from investing activitiesNet cash flows from investing activities consist of net capital expenditures and other cash flows from investing activities.
In 2023, other cash flows from investing activities amounted to a cash outflow of EUR 82 million, mainly due to a new business acquisition and minority investments, partly offset by divestment proceeds.
In 2022, other cash flows from investing activities amounted to a cash outflow of EUR 698 million, mainly due to the acquisitions of Vesper Medical and Cardiologs, amounting to EUR 414 million, and new minority investments.
In 2021, other cash flows from investing activities amounted to a cash outflow of EUR 2,943 million, mainly due to the acquisitions of BioTelemetry and Capsule Technologies amounting to EUR 2.8 billion.
Net cash flows from financing activitiesNet cash flows from financing activities consist of treasury shares transactions, changes in debt, dividend paid and other cash flow items.
In 2023, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 662 million net cash outflow. Changes in debt mainly includes new bonds issued of EUR 500 million and loan repayments amounting to EUR 500 million. The dividend was distributed fully in shares.
In 2022, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 174 million net cash outflow. Changes in debt mainly included new bonds issued of EUR 2 billion and a new term loan issued of EUR 500 million, partly offset by bond repayments of EUR 1.2 billion. Philips’ shareholders received a total dividend of EUR 741 million, including costs, of which the cash portion amounted to EUR 412 million.
In 2021, treasury shares transactions mainly included the share buyback activities, which resulted in EUR 1,613 million net cash outflow. Changes in debt mainly relates to short-term debt and lease repayments. Philips’ shareholders received a total dividend of EUR 773 million, including costs, of which the cash portion amounted to EUR 482 million.
Net cash flows from discontinued operationsIn 2023, net cash provided by discontinued operations was EUR 123 million, mainly related to a refund received of one-off advance tax payments of a previously disposed business.
In 2022, net cash used for discontinued operations was EUR 12 million, mainly related to previously disposed businesses.
In 2021, net cash provided by discontinued operations was EUR 3,403 million and consisted primarily of the net cash inflow of EUR 3,319 million from the sale of the Domestic Appliances business on September 1, 2021.
Condensed consolidated balance sheets as of December 31, 2021, 2022 and 2023 are presented in the following table:
Philips Group
Condensed consolidated balance sheets
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Intangible assets | 14,287 | 13,764 | 13,067 |
| Property, plant and equipment | 2,699 | 2,638 | 2,483 |
| Investments and financial assets | 1,121 | 1,334 | 1,050 |
| Deferred tax assets | 2,216 | 2,449 | 2,627 |
| Inventories | 3,450 | 4,049 | 3,491 |
| Receivables | 4,191 | 4,616 | 4,146 |
| Other assets | 693 | 665 | 672 |
| Payables | (3,784) | (3,635) | (3,886) |
| Provisions | (2,313) | (2,115) | (2,498) |
| Contract liabilities | (1,936) | (2,210) | (2,278) |
| Other liabilities | (1,473) | (1,244) | (993) |
| Net assets employed | 19,151 | 20,311 | 17,881 |
| Cash and cash equivalents | 2,303 | 1,172 | 1,869 |
| Debt | (6,980) | (8,201) | (7,689) |
| Net debt1) | (4,676) | (7,028) | (5,820) |
| Non-controlling interests | (36) | (34) | (33) |
| Shareholders' equity | (14,438) | (13,249) | (12,028) |
| Financing | (19,151) | (20,311) | (17,881) |
Total debt outstanding at the end of 2023 was EUR 7,689 million, compared with EUR 8,201 million at the end of 2022.
Philips Group
Balance sheet changes in debt
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| New lease liabilities | 164 | 104 | (233) |
| New borrowings long-term debt | 76 | 2,516 | (544) |
| Repayments long-term debt incl. leases | (302) | (1,472) | 754 |
| New borrowings (repayments) short-term debt | (25) | 47 | (29) |
| Forward contracts entered (matured) | (48) | (76) | 462 |
| Currency effects, consolidation changes and other | 180 | 101 | 102 |
| Changes in debt | 46 | 1,221 | 512 |
In 2023, total debt decreased by EUR 512 million compared to 2022. The decrease mainly comes from maturing forward contracts related to the share buyback program and long-term incentive and employee stock purchase plans, and repayments of long-term debt including leases, partly offset by new borrowings. In 2023, Philips issued EUR 500 million of fixed rate notes under the company’s EMTN program that mature in 2031 and used the proceeds for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in the fourth quarter of 2022. Changes in payment obligations from forward contracts are related to the maturity in 2023 of EUR 481 million of share buyback forwards (as announced in July 2021) and EUR 125 million of forwards relating to long-term incentive and employee stock purchase plans (as announced in March 2020 and May 2021), partially offset by EUR 138 million of forwards entered into relating to long-term incentive plans (as announced in June 2023).
In 2022, Philips announced a series of Liability Management transactions to optimize its debt maturity profile. The transactions included the issuance of three series of Notes under its EMTN program for a total of EUR 2 billion with maturities in 2027, 2029 and 2033. Part of the proceeds were used to tender certain of Philips’ outstanding US Dollar denominated bonds due 2025 and 2026 and Euro-denominated bonds due 2023, 2024 and 2025, as well as make-whole and fully redeem the Euro-denominated bonds due 2023 and 2024 that were not purchased as part of the Euro tender offer. Philips issued Commercial Paper of EUR 200 million in September 2022 and EUR 101 million in October 2022. These tranches were repaid throughout the fourth quarter of 2022. In addition, in October 2022 Philips entered into a EUR 1 billion credit facility that could be used for general corporate purposes. The credit facility was fully repaid in October 2023. Per year-end 2022, EUR 500 million was utilized and outstanding under the credit facility.
In 2021, total debt increased by EUR 46 million compared to 2020. The increase mainly comes from currency effects and consolidation changes, partly offset by net lease repayments and forward settlements. Repayments of long-term debt amounted to EUR 302 million. In February 2021, Philips entered into two bilateral loans amounting to a total of EUR 500 million that were repaid in September 2021. In addition, Philips issued commercial paper of EUR 300 million in May 2021 and EUR 150 million in July 2021 that was repaid in September 2021. Changes in payment obligations from forward contracts are mainly related to the forward contracts entered into of EUR 731 million relating to the EUR 1.5 billion share buyback program announced on July 26, 2021, and EUR 90 million relating to the long-term incentive and employee stock purchase plans announced on May 19, 2021. In addition, a total amount of EUR 745 million of forward contracts matured in 2021, which completed the settlement of the EUR 1.5 billion share buyback program announced on January 29, 2019, and a total amount of EUR 123 million of forward contracts matured in 2021 relating to the long-term incentive and employee stock purchase plans announced on October 22, 2018 and January 29, 2020. These payment obligations are recorded as financial liabilities under long-term debt. Other changes, mainly resulting from currency effects, led to an increase of EUR 175 million.
At the end of 2023, long-term debt as a proportion of the total debt stood at 91.5% with an average remaining term (including current portion) of 6.0 years, compared to 88.6% and 6.1 years respectively at the end of 2022.
At the end of 2022, long-term debt as a proportion of the total debt stood at 88.6% with an average remaining term (including current portion) of 6.1 years, compared to 92.7% and 6.0 years respectively at the end of 2021.
At the end of 2021, long-term debt as a proportion of the total debt stood at 92.7% with an average remaining term (including current portion) of 6.0 years, compared to 82.3% and 6.3 years respectively at the end of 2020.
For further information, please refer to Debt.
As of December 31, 2023, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility, the Philips Group had access to available liquidity of EUR 2,883 million, compared with gross debt (including short and long-term) of EUR 7,689 million.
As of December 31, 2022, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility and the EUR 500 million undrawn portion of the credit facility entered into in October 2022, the Philips Group had access to available liquidity of EUR 2,704 million, compared with gross debt (including short and long-term) of EUR 8,201 million.
As of December 31, 2021, including the cash position (cash and cash equivalents), as well as its EUR 1 billion committed revolving credit facility, the Philips Group had access to available liquidity of EUR 3,370 million, compared with debt (including short and long-term) of EUR 6,980 million.
Philips Group
Liquidity position
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Cash and cash equivalents | 2,303 | 1,172 | 1,869 |
| Listed equity investments at fair value1) | 67 | 32 | 14 |
| Committed revolving credit facility | 1,000 | 1,000 | 1,000 |
| Credit facility | 500 | ||
| Liquidity | 3,370 | 2,704 | 2,883 |
| Short-term debt | (506) | (931) | (654) |
| Long-term debt | (6,473) | (7,270) | (7,035) |
| Debt | (6,980) | (8,201) | (7,689) |
| Net available liquidity resources | (3,609) | (5,497) | (4,806) |
Philips has a EUR 1 billion committed revolving credit facility which was signed in April 2017 and refinanced in March 2022, which will expire in March 2027. In 2023, Philips extended the maturity of the facility to 2028 and has one 1-year extension option remaining. The facility can be used for general group purposes, such as a backstop of its Commercial Paper Program.
Philips' Commercial Paper Program amounts to USD 2.5 billion, under which commercial paper can be issued up to 364 days in tenor, both in the US and in Europe, in any major freely convertible currency. As of December 31, 2023, Philips had no commercial paper outstanding. Philips established a Euro Medium Term Note (EMTN) program which facilitates the issuance of notes for a total amount of up to EUR 10.0 billion. In 2023, Philips issued EUR 500 million fixed rate notes due 2031 under the program. The proceeds were used for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in the fourth quarter of 2022.
In terms of liquidity, the company has access to various sources. The company’s liquidity risk management procedures have not changed significantly during 2023. The access to existing lines of credit remains intact. These lines of credit, along with other financial risks to which Philips is exposed, are disclosed in Details of treasury and other financial risks. Further, with respect to potential claims related to the Respironics recall, please refer to Contingencies. The management continues to monitor the risks associated with such potential claims and its impact on liquidity position, if any.
Philips’ existing long-term debt is rated BBB+ (with stable outlook) by Fitch, Baa1 (with negative outlook) by Moody’s, and BBB+ (with negative outlook) by Standard & Poor’s. As part of our capital allocation policy, our net debt*) position is managed with the intention of retaining our strong investment grade credit rating. Ratings are subject to change at any time and there is no assurance that Philips will be able to achieve this goal. Philips' aim when managing the net debt*) position is dividend stability and a pay-out ratio of 40% to 50% of adjusted income from continuing operations attributable to shareholders*). Philips’ outstanding long-term debt and credit facilities do not contain financial covenants. Adverse changes in the company’s ratings will not trigger automatic withdrawal of committed credit facilities or any acceleration in the outstanding long-term debt (provided that the USD-denominated bonds issued by Philips in March 2008 and 2012 contain a ‘Change of Control Triggering Event’ and the EUR-denominated bonds contain a ‘Change of Control Put Event’). A description of Philips’ credit facilities can be found in Debt.
Philips Group
Credit rating summary
| long-term | short-term | outlook | |
| Fitch | BBB+ | Stable | |
| Moody's | Baa1 | P-2 | Negative |
| Standard & Poor's | BBB+ | A-2 | Negative |
Philips pools cash from subsidiaries to the extent legally and economically feasible. Cash not pooled remains available for local operational needs or general purposes. The company faces cross-border foreign exchange controls and/or other legal restrictions in a few countries, which could limit its ability to make these balances available on short notice for general use by the group.
Philips believes its current liquidity and direct access to capital markets is sufficient to meet its present financing needs.
The following table presents a summary of the Group's fixed contractual cash obligations and commitments as of December 31, 2023. These amounts are an estimate of future payments, which could change as a result of various factors such as a change in interest rates, foreign exchange, contractual provisions, as well as changes in our business strategy and needs. Therefore, the actual payments made in future periods may differ from those presented in the following table:
| payments due by period | |||||
|---|---|---|---|---|---|
| total | less than 1 year | 1-3 years | 3-5 years | after 5 years | |
| Long-term debt | 7,615 | 533 | 1,934 | 1,431 | 3,717 |
| Short-term debt | 122 | 122 | |||
| Interest on debt | 1,704 | 180 | 328 | 285 | 911 |
| Derivative liabilities | 39 | 38 | 1 | ||
| Purchase obligations3) | 668 | 355 | 286 | 27 | |
| Trade and other payables | 1,917 | 1,917 | |||
| Contractual cash obligations | 12,065 | 3,145 | 2,549 | 1,716 | 4,655 |
Included in debt are remaining forward contracts of EUR 167 million related to the EUR 1.5 billion share buyback program announced in July 2021 and EUR 224 million relating to the repurchase of shares to cover long-term incentive and employee stock purchase plans. In 2023, Philips entered into a total amount of EUR 138 million of forward contracts relating to the repurchase of up to 7.1 million shares to cover long-term incentive plans. In addition, in 2023 there were maturities of a total of EUR 481 million of forward contracts for 13.0 million shares related to the EUR 1.5 billion share buyback program announced in July 2021, as well as maturities of a total of EUR 125 million of forward contracts to repurchase shares to cover long-term incentive and employee stock purchase plans. Philips intends to cancel all of the shares acquired under the share buyback program and has canceled 15.1 million shares acquired in 2023, as the program was initiated for capital reduction purposes.
Philips offers voluntary supply chain finance programs with third parties, which provide participating suppliers with the opportunity to factor their trade receivables at the sole discretion of both the suppliers and the third parties. Philips continues to recognize these liabilities as trade payables and settles them accordingly on the invoice maturity date based on the terms and conditions of these arrangements. As of December 31, 2023, approximately EUR 114 million (2022: EUR 151 million) of the Philips accounts payable were transferred under these arrangements.
Other cash commitmentsThe company and its subsidiaries sponsor post-employment benefit plans in many countries in accordance with legal requirements, customs and the local situation in the countries involved. For a discussion of the plans and expected cash outflows, please refer to Post-employment benefits.
The company had various provisions by the end of 2023 which are expected to result in cash outflows in 2024. Refer to Provisions.
Philips has contracts with investment funds where it committed itself to make, under certain conditions, capital contributions to these funds of an aggregated remaining amount of EUR 153 million (2022: EUR 127 million). Capital contributions already made to these investment funds are recorded as non-current financial assets.
Please refer to Dividend for information on the proposed dividend distribution.
Please refer to Equity for information on other Long-term incentive and employee stock purchase plans.
GuaranteesPhilips’ policy is to provide guarantees and other letters of support only in writing. Philips does not provide other forms of support. The total fair value of guarantees recognized on the balance sheet amounts to EUR nil million for both 2023 and 2022. Remaining off-balance-sheet business-related guarantees on behalf of third parties and associates amount to EUR 2 million as of December 31, 2023 (December 31, 2022: EUR 2 million).
Philips’ dividend policy is aimed at dividend stability and a pay-out ratio of 40% to 50% of adjusted income from continuing operations attributable to shareholders*).
Proposed distributionA proposal will be submitted to the Annual General Meeting of Shareholders, to be held on May 7, 2024, to declare a distribution of EUR 0.85 per common share, in common shares, against retained earnings.
If the above dividend proposal is adopted, the shares will be traded ex-dividend as of May 9, 2024 at the New York Stock Exchange and Euronext Amsterdam. In compliance with the listing requirements of the New York Stock Exchange and Euronext Amsterdam, the dividend record date will be May 10, 2024.
The number of share dividend rights entitled to one new common share will be determined based on the volume-weighted average price of all traded common shares of Koninklijke Philips N.V. at Euronext Amsterdam on May 9, 10 and 13, 2024. The company will calculate the number of share dividend rights entitled to one new common share (the ratio), such that the gross dividend in shares will be approximately equal to EUR 0.85. The ratio and the number of shares to be issued will be announced on May 15, 2024. Distribution of the dividend (up to EUR 770 million) and delivery of new common shares, with settlement of any fractions in cash, will take place from May 16, 2024.
| ex-dividend date | record date | distribution from | |
| Euronext Amsterdam | May 9, 2024 | May 10, 2024 | May 16, 2024 |
| New York Stock Exchange | May 9, 2024 | May 10, 2024 | May 16, 2024 |
Further details will be given in the agenda with explanatory notes for the 2024 Annual General Meeting of Shareholders. The proposed distribution and all dates mentioned remain provisional until then.
Dividend in shares distributed out of retained earnings is subject to 15% dividend withholding tax, but only in respect of the par value of the shares (EUR 0.20 per share). Shareholders are advised to consult their tax advisor on the applicable situation with respect to taxes on the dividend received.
In May 2023, Philips settled a dividend of EUR 0.85 per common share, representing a total value of EUR 749 million (including costs). The dividend was distributed in the form of shares only, resulting in the issuance of 39,334,938 new common shares, leading to a 4.5% dilution. For more information refer to Shareholders’ equity.
Dividends and distributions per common shareThe following table sets forth in euros the gross dividends on the common shares in the fiscal years indicated (from prior-year profit distribution) and such amounts as converted into US dollars and paid to holders of shares of the New York Registry:
Philips Group
Gross dividends on the common shares
| 20191) | 20201) | 20212) | 20221) | 20232) | |
|---|---|---|---|---|---|
| in EUR | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| in USD | 0.96 | 0.95 | 1.03 | 0.90 | 0.93 |
Environmental, Social & Governance (ESG) are three key dimensions within which a company’s approach to doing business responsibly and sustainably, and its overall societal impact, are defined. They give expression to an increasingly widely held view – that companies that hold themselves accountable to their stakeholders and increase transparency will be more viable, and valuable, in the long term.
Philips is a purpose-driven company aiming to improve the health and well-being of 2.5 billion people annually by 2030. We believe that private-sector companies like ours have a vital role to play in collaborating with other partners across our supply chain, and with private and public organizations in society, to address the major challenges the world is facing.
Taking a multi-stakeholder approach, we draw inspiration from the societal impact we can have through our products and solutions, and through how we operate in the world. Our company is very conscious of our responsibility and our contribution to society and the environment. We are also witnessing growing interest in ESG on the part of our customers, who are increasingly turning to technology companies for support in addressing their sustainability objectives and are including ESG-related considerations in their procurement policies and criteria.
We aim to be a front-runner in the area of ESG and have been recognized as leading the way in, for example, sustainability, corporate governance practices and tax transparency.
Our reporting is aligned with the comprehensive and integrated Environmental, Social & Governance (ESG) commitments we have adopted for the period 2020-2025.
We have excluded the data from Domestic Appliances from the ESG information wherever possible. In a limited number of cases, for example for road logistics emissions, we have used proxies. If Domestic Appliances information was not available for past years, and could therefore not be excluded, we have indicated this in the respective section. The Employee Engagement Index (EEI) and General Business Principles (GBP) results have not been restated.
In September 2020, Philips reinforced its commitments as a purpose-driven company with the announcement of an enhanced and fully integrated approach to doing business responsibly and sustainably. Philips’ framework comprises a comprehensive set of commitments across all the Environmental, Social and Governance (ESG) dimensions that guide execution of the company’s strategy. It includes ambitious targets and detailed plans of action.
As a leading health technology company today, our purpose is to improve people’s health and well-being through meaningful innovation, positively impacting 2.5 billion lives per year by 2030. We aim to grow Philips responsibly and sustainably, and we therefore continuously set ourselves challenging environmental and social targets, and highest standards of governance. Acting responsibly towards the planet and society is part of our DNA. We believe that this is the best way for us to create superior, long-term value for Philips’ multiple stakeholders.
We act responsibly towards our planet in line with UN SDGs 12 and 13.
Our purpose is to improve people’s health and well-being through meaningful innovation, in line with UN SDG 3. We act responsibly towards society and partner with our stakeholders
We aim to deliver superior long-term value for our customers and shareholders, and we live up to the highest standards of ethics and governance in our culture and practices
We have an environmental impact through our global operations (including our supply chain), but even more so through our products and solutions. This is where we contribute to SDG 12 (Ensure sustainable consumption and production patterns) and SDG 13 (Take urgent action to combat climate change and its impacts).
Measuring our environmental impactPhilips has been performing Life-Cycle Assessments (LCAs) since 1990. LCAs provide insight into the lifetime environmental impact of our products. They are used to steer our EcoDesign efforts by reducing the environmental impact during the lifetime of our products and to grow our Green/EcoDesigned/EcoHero and Circular portfolio. As a next step, for the seventh year, we have measured our environmental impact on society at large via a so-called Environmental Profit & Loss (EP&L) account, which includes the hidden environmental costs associated with our activities and products. It provides insights into the main environmental hotspots and innovation areas to reduce the environmental impact of our products and solutions.
The EP&L account is based on LCA methodology, in which the environmental impacts are expressed in monetary terms using conversion factors developed by CE Delft. These conversion factors are subject to further refinement and are expected to change over time. We used expert opinions and estimates for some parts of the calculations. The figures reported are Philips’ best possible estimates. As we gain new insights and retrieve more and better data, we will enhance the methodology, use-cases and accuracy of results in the future. For more information and details we refer to our methodology document.
The definition of the use-case scenarios has a significant impact on the result, especially for consumer products, which have large sales volumes, long lifetimes and frequently high energy consumption.
The current EP&L account only includes the hidden environmental costs. It does not yet include the benefits to society that Philips generates by improving people’s health and well-being through our products and solutions. We have a well-established methodology to calculate the number of lives we positively touch with our products and solutions. We aim to look into valuing these societal benefits in monetary terms in the future.
The Philips products subject to the Respironics recall were evaluated as part of the 2023 EP&L calculation. In accordance with the EP&L methodology, products replaced during the recall by new products with lifetime guarantees were included in the 2023 EP&L calculation for all life cycle stages. Refurbished products and repair kits were not included.
Results 2023Compared to the adjusted 2022 EP&L impact of EUR 4.38 billion, Philips reduced its environmental impact in 2023 to EUR 4.21 billion. This is mainly due to differences in sales mix (including the impact of the Respironics recall).
The increase in the 2022 baseline was mainly driven was mainly driven by the update to the EcoInvent 3.9.1 database using ReCiPe 2016 (our Life Cycle Inventory database containing environmental impacts of products and services) from the EcoInvent 3.8 database using ReCiPe 2008, and the update to the 2023 CE Delft prices for EU27 from the 2017 CE Delft prices for Dutch territory only. Philips updates the EcoInvent database used on a yearly basis to utilize recent emission factors and in this case, to utilize the current ReCiPe 2016 methodology. Additionally, the CE Delft prices for EU27 were more representative of a global manufacturing company, like Philips.
To understand the changes to the 2022 EP&L and have a comparable baseline for the 2023 reporting, please refer to the following chart:
The majority of this increase can be attributed to the increase in the emission factors and/or prices for the following environmental impact categories on the lifecycle stages included in the 2022 EP&L:
Additionally, the environmental impact categories associated with biodiversity and ecosystem services were included, which amounted to approximately EUR 61 million. Therefore, the total increase attributed to methodology changes to the 2022 EP&L with the existing 2022 lifecycle stages is EUR 2.48 billion. Additionally, to compare the 2022 EP&L with the 2023 EP&L, the Raw Material Processing and Raw Material Waste lifecycle stages should be included (adding some EUR 302 million to the 2022 EP&L). With the inclusion of data quality improvements and corrections performed in 2023, the 2022 EP&L would be approximately EUR 4.38 billion.
The most significant environmental impact, 51% of the total, is related to the usage of our products, which is due to electricity consumption. Human toxicity, particulate matter formation, and climate change are other important impacts. The environmental costs include the environmental impact of the full lifetime of the products that we put on the market in 2023, e.g. 10 years in the case of a MRI or 5 years of usage in the case of a Sonicare toothbrush. Products identified as rentals are the only exception, with an energy consumption of one year. As we expand our EcoDesign activities, with a target to have all our products EcoDesigned by 2025, we expect to better report on its environmental impact in the years to come.
Of the total 2023 impact, just EUR 261 million (6%) is directly caused by Philips’ own operations, mainly driven by outbound logistics, followed by business travel. Compared to EUR 128 million in 2022, this is almost a two times increase, mainly due to updating the emission factors from EcoInvent 3.8 to EcoInvent 3.9.1 and the prices to the 2023 CE Delft prices for EU27, mitigating the downward trend in logistics emissions as presented in Sustainable Operations.
Our materials and components supply chain, including raw materials supply, raw materials processing, raw materials waste, and packaging currently has an environmental impact of some EUR 1.80 billion, which is 43% of our total environmental impact. The main contributors are the electronic components (including printed circuit boards), cables and metals used in our products. Through our Circular Economy and Supplier Sustainability programs we will continue to focus on reducing the environmental impact caused by the materials we source and apply in our products.
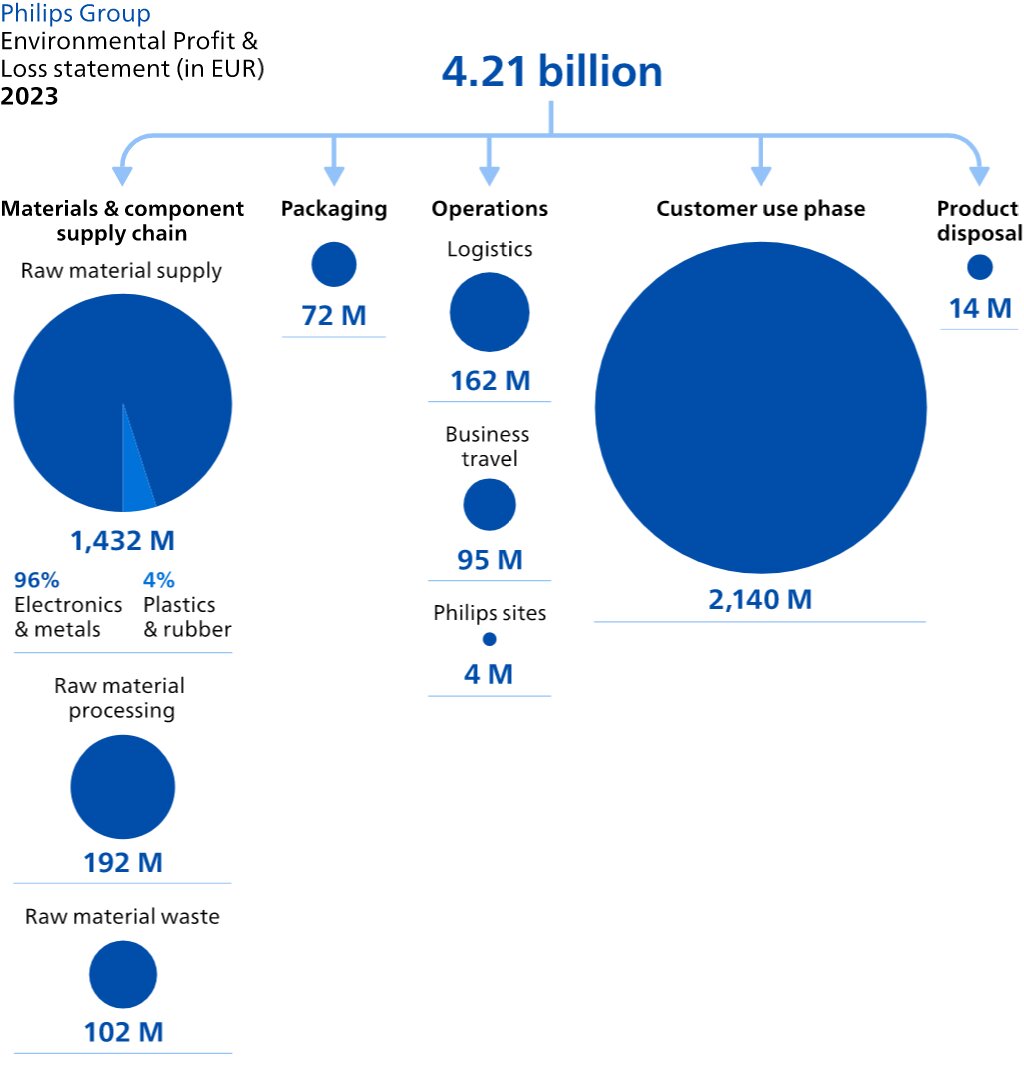
In 2018, we were the first health technology company to have its 2020-2040 targets (including purchased goods and services and the use of sold products) approved by the Science Based Targets initiative – showing our commitment to drive climate action across the value chain, from suppliers to customers, and ensuring that we contribute to the decarbonization required to stay in line with a 1.5 °C global warming scenario, as agreed in the Paris Agreement. Together with the insights gained through the EP&L we will optimize our climate impact by providing our businesses with actionable insights. For more information on our climate performance please refer to Climate Action.
For more information on our efforts to reduce emissions in the supply chain, please refer to Supplier sustainability.
For more information on our efforts to reduce emissions in the customer use-phase, please refer to Green/EcoDesigned Innovation and Green/EcoDesigned and EcoHero Revenues.
At Philips, we see climate change as a serious threat. Research from the Potsdam Institute for Climate Impact Research shows that over 4% of global CO2 emissions are caused by the healthcare sector. Therefore, we are taking action to rethink our business models and decouple economic growth from the impact we have on the environment. We believe large corporates should lead the transition to a low-carbon economy. This will not only benefit the environment, but will also positively impact social and economic aspects.
Operational carbon footprintDuring the COP21 United Nations Climate Conference in Paris in 2015, we committed to become carbon-neutral in our operations, pursue all efforts to reduce our operational emissions, source all our electricity from 100% renewable sources, and offset all unavoidable emissions by year-end 2020. We delivered on a comprehensive program that included energy-efficiency improvements, on-site renewables, and Power Purchase Agreements, as well as business travel improvements and transport mode shifts to low-carbon-emitting alternatives. As a result, we have significantly reduced our operational carbon footprint.
Since 2020, Philips has been carbon-neutral in its operations (scope 1, scope 2, and scope 3 - business travel and transportation & distribution). Although we prioritize carbon reduction, our comprehensive carbon offsetting program is still necessary to ensure carbon neutrality in our own operations.
Philips Group
Net operational carbon footprint
in kilotonnes CO2 -equivalent
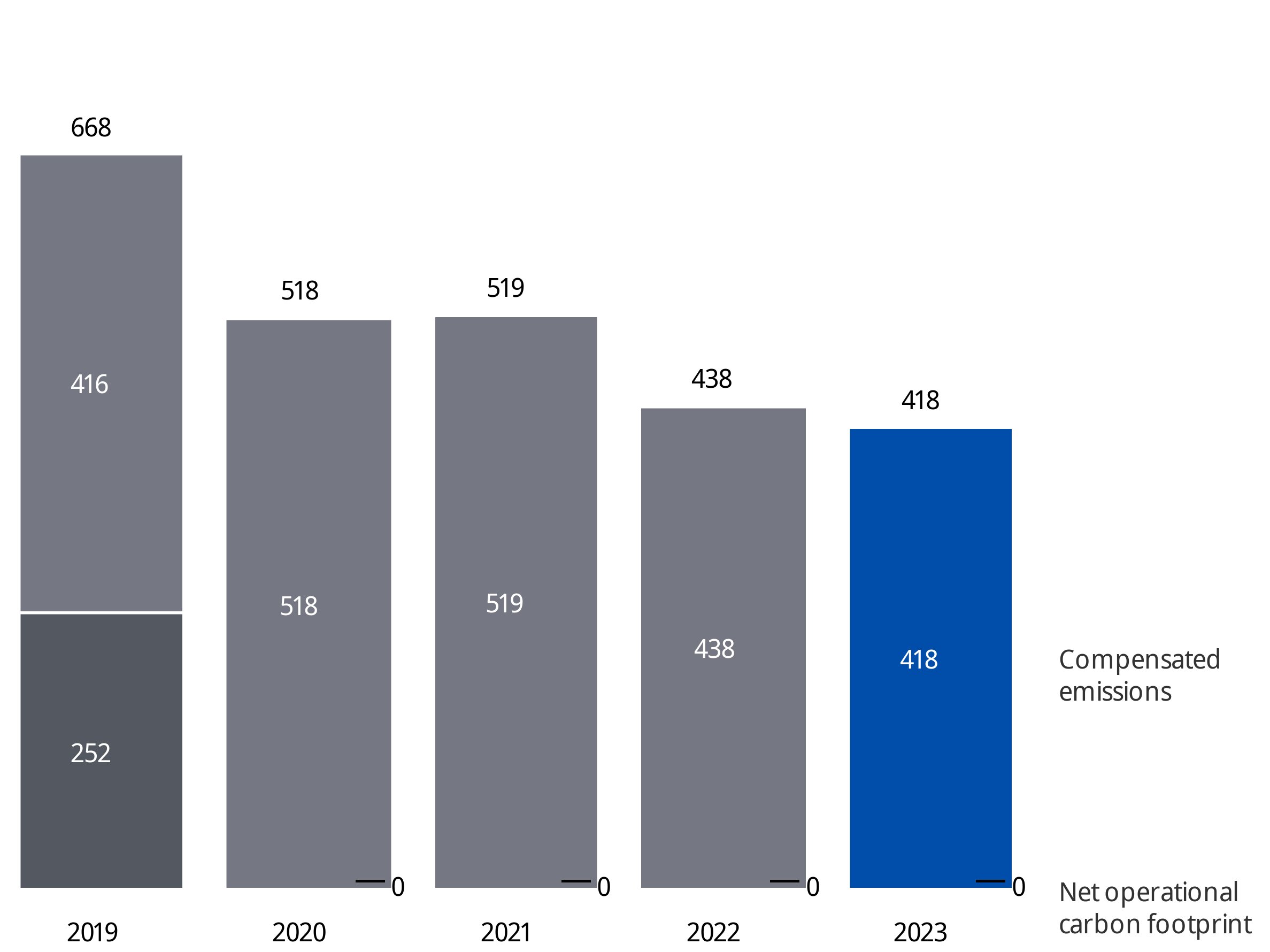
Having achieved our 2020 carbon neutrality target, we have raised the bar and set ambitious emission reduction targets to ensure we help limit the impact of global warming throughout our value chain – collaborating with suppliers and customers to amplify our impact. Philips is committed to addressing climate change by establishing ambitious long-term emission reduction targets, officially approved by the Science Based Targets initiative (SBTi). We have added the emissions from our (scope 3 categories) Purchased Goods & Services and Use of Sold Products retrospectively to define a baseline (2020). With these additions, we cover approximately 96% of our value chain emissions.
For all of our SBTi-approved and 1.5 °C-aligned targets, baselines and performance, please refer to the following table. These targets follow the cross-sector guidance of the SBTi. Philips was the first health technology company to have its targets approved by the SBTi.
Philips Group
Science Based Targets
reduction % compared to baseline
| Scope coverage | 2025 | 2030 | 2040 | ||
|---|---|---|---|---|---|
| Absolute Contraction Approach (ACA) emission reduction targets | Scope 1 & 2 (Baseline 2015) | 100% | -75% | -90% | |
| Scope 3 (Baseline 2020) | 96% | -42% |
In establishing our Greenhouse Gas (GHG) emissions baseline, the selection of the base year is guided by several considerations. More precisely, it is driven by historical data availability, the stability of operations during that period, and the desire to capture a representative snapshot of our emissions profile. In particular, we consider factors such as significant changes in business operations, facility expansions, or the implementation of emission reduction initiatives.
Despite the unprecedented challenges brought about by the COVID-19 pandemic, the year 2020 stands out as a significant year for Philips, marked by a level of relative stability in both customer base and emissions profile. In contrast, the year 2015 was selected as the baseline for scope 1 and 2 emissions due to it being the earliest feasible date for measurement and target-setting in alignment with the Paris Agreement. Should enhancements in data quality or methodological changes lead to an emission deviation exceeding 5% compared to our current baseline emissions, we are committed to restating the baseline in accordance with the Science Based Targets initiative.
How we will drive emission reduction across the value chainBy joining forces with customers and suppliers, we can reduce our shared carbon footprint and create a sustainable and more resilient healthcare industry. To deliver, we will focus on the following four objectives, in order of magnitude:
Designing energy-efficient products and collaborating with our customers to reduce emissions during the use-phaseMore and more, customers – both in healthcare and retail – are seeking solutions that are less impactful on the environment. To address that demand, we are continuously reducing the climate impact of our products by increasing the energy efficiency of our existing installed base and future product introductions. We see improving energy efficiency as a huge lever to deliver on our value chain emission reductions. More information can be found on our sustainability website.
Collaborating with our suppliers to reduce emissions in our supply chainThere is a pressing need for industry and business to manage and reduce CO2-e emissions across the entire value chain – including at supplier level. To this end, we have invited many of our largest suppliers – first-tier manufacturing and transportation-related suppliers – to report their climate performance and strategy as part of the Carbon Disclosure Project (CDP) Supply Chain program. Additionally, we engage with these suppliers to reduce their emissions as part of our Supplier Sustainability program. More information can be found on our sustainability website.
Minimizing our climate impact by adopting circular economy principlesFrom a climate perspective, applying circular business models can lead to a significant emission reduction. As the value of materials is retained, the need for virgin resources is significantly reduced, and consequently, the need for e.g. energy to produce those virgin materials, leading to reduced emissions. This is also part of our Circular Economy program. More information can be found on our sustainability website.
Transitioning to lower carbon-emitting energy at our sitesBy continuing to phase out fossil fuels at our sites and increase our global renewable energy share, we will be able to achieve our long-term emission targets (scope 1 and 2). This entails, for example, moving towards geothermal and renewable district heating and cooling solutions where available. More information can be found on our sustainability website.
RecognitionOur efforts are acknowledged by CDP (formerly known as the Carbon Disclosure Project), a global NGO that assesses the greenhouse gas (GHG) emission performance and management of reporting companies. In 2023, we were ranked on the CDP Climate Change ’A’ List for our continued climate performance and transparency for the 11th consecutive year. None of our peers can claim the same.
Actions related to the achievement of our targets are governed by our Environmental policy, which incorporates input from Philips' regulatory, design, sustainability, supply chain, and operations stakeholders, as well as the voice of our customers to minimize their environmental footprint.
Philips reports all its emissions in line with the Greenhouse Gas Protocol (GHGP).
A circular economy aims to decouple economic growth from the consumption of natural resources and ecosystems by optimizing their use, eliminating waste and pollution, and circulating products and materials for as long as possible, while giving natural systems the opportunity to regenerate themselves. The way we take, make and use materials has a significant impact on both climate and nature, as 45% of global GHG emissions come from the way products are made and used, and more than 90% of biodiversity loss stems from extraction and processing. Bringing this back to Philips’ impact on the planet, our use of materials accounts for over 40% of our environmental impact based on our EP&L methodology, which includes raw material supply, processing, waste and packaging. Therefore, in addition to the use of renewable resources and energy efficiency, the transition to a circular economy will be essential to meet our global climate goals.
Our Circular Economy programThe Circular Economy program at Philips ran for the 11th year in 2023, building on more than 30 years' experience of applying resource efficiency through our sustainability programs. Our ambition is to help our customers to ‘do more with less’ and drive the circular transformation across the value chain together with our partners. We apply Philips’ circularity principles ‘use less, use longer and use again’ across five strategic areas:
Philips Group
Progress towards Philips' 2025 circularity targets
| Metrics | Unit | 2020 Baseline | 2022 Results | 2023 Results | 2025 Targets | Key actions to deliver on 2025 targets |
|---|---|---|---|---|---|---|
| Resource inflows & outflows (products) | ||||||
| Circular revenues | % | 15 | 18.2 | 20.0 | 25 | Grow sales from products, services and solutions that use less virgin materials, optimize and extend product lifetime, recirculate materials |
| Resource inflows & outflows (waste) | ||||||
| Zero waste to landfill | % | 2.6 | 0.0 | 0.0 | less than 0.5 | Minimize waste to landfill |
| Circular materials management | % | 90 | 91 | 91 | 95 | Avoid waste by increasing the recirculation of discarded materials |
| Resource inflows (products) | ||||||
| Close the loop on medical equipment | # | Achieved for large medical equipment | Extend to small medical equipment | Adopt policy ensuring responsible end-of-use management |
Philips has committed to voluntary circularity targets to be delivered by 2025 as part of our externally communicated 2025 Sustainability Commitments. Key actions to deliver on these are stated in the above table. In 2023, Philips increased its circular revenues by 1.8% compared to the previous year, mainly driven by circular design of software and hardware. We implemented sharpened circular revenue requirements to further align with developments on circularity metrics and reporting disclosures. For example, external trends on metrics led to further sharpening of the definition of our software contributions. We also brought our circular revenue reporting more in line with our circular strategy on design and closing the loop.
In 2023, Philips achieved 91% circular materials management, comparable to 2022. We continued the emphasis on our Zero Waste to Landfill KPI, achieving 0.0% waste to landfill, compared to 0.0% in 2022.
We reclaimed more than 11,500 systems or pieces of equipment in 2023. The main driver was our take-back program for patient monitors.
Philips recognizes the importance of healthy ecosystems and biodiversity for our company, our employees, and society. Therefore, Philips has developed the Natural Capital program, which is an addition to existing sustainability programs. This program is dedicated to reducing Philips’ impacts on natural capital, focusing on our chemicals footprint, water consumption, and improving biodiversity and ecosystem services. By systematically quantifying and reducing the environmental impact of our operations, supply chain and the use-phase of our products, we actively aim to protect and restore biodiversity. Philips acknowledges its dependency and impact on natural capital and aims to iteratively improve its understanding to drive regenerative decisions.
Philips aims to restore and enhance biodiversity and ecosystem services (BES) at our industrial sites and to actively promote ecosystem restoration activities through partnerships with, among others, NGOs, local communities, and governments. The Natural Capital program is focused, taking our 23 manufacturing sites as a starting point; Philips has created a BES community and trained employees on all these sites in ecosystem services. As a result, the ecosystem services of Philips' global manufacturing sites have been mapped and quantified. Based on this data, Philips evaluated the total area and ecological value of each manufacturing site and established the first BES data baseline to measure BES improvements by 2024. Together with our partners, we are working to develop more advanced BES metrics suitable for industrial areas.
In 2022, our manufacturing sites delivered some 80 potential measures to enhance biodiversity on-site. Philips implemented 30% of the biodiversity improvement measures selected for the short term at a number of sites in 2023, e.g. planting native trees in India, creating flower gardens in China, and creating habitats for endangered bee species in Central America. Furthermore, we have published our first Taskforce on Nature-related Financial Disclosures (TNFD) report and aim to set ourselves Science Based Targets for Nature (SBTN) in the future.
Philips aims to expand BES improvements in 2024 and track BES performance at our manufacturing sites with a new ecosystem services mapping according to our Environmental Policy. Improving BES at our manufacturing sites, and thereby also improving the working environment, is a contributor to making Philips the ’best place to work’, one of the ESG commitments Philips announced in 2020. Furthermore, healthy ecosystems support our efforts to mitigate climate risks assessed in our TCFD report for our sites.
As can be derived from our Environmental Profit & Loss (EP&L) account, the environmental impact of Philips’ sites is limited, as they are not very energy-intensive, are 100%-powered with electricity from renewable sources, do not emit large quantities of high-impact substances, and are not water-intensive. At the same time, Philips is aware that the total environmental impact of the full value chain is substantial, especially upstream in the mining industry. Philips considers improving biodiversity on its own land as a first important step towards reducing biodiversity impact over the full value chain.
Having become carbon-neutral in our operations by year-end 2020, and with our drive to send zero waste to landfill, focus on circular materials management, and enhance BES, the environmental impact of our sites will be further optimized in the years to come.
At Philips, we recognize that human health and environmental health go hand in hand. In 2022, the United Nations declared the ability to live in a clean, healthy and sustainable environment a human right.
Climate change poses a threat to health and is expected to cause some 250,000 additional deaths per year globally, according to the World Health Organization. It creates a pressing need – together with global resource constraints, growing and aging populations, and the rise of chronic diseases – for resilient and sustainable healthcare models.
We see a growing demand from our customers, including hospitals, to reduce their environmental impact, reduce waste and decarbonize healthcare. Our Green/EcoDesigned Innovation – the Research & Development spend related to the development of new generations of Green/EcoDesigned products and solutions and Green technologies, addressing SDG 12 (Ensure sustainable consumption and production patterns) – is focused on addressing that impact.
Sustainable Innovation is the Research & Development spend related to the development of new generations of products and solutions that address the United Nations’ Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages) or 12.
In 2023, Philips invested EUR 142 million in Green/EcoDesigned Innovation, a decrease compared to 2022 due to more demanding EcoDesign criteria, a growing share of innovation spend in software, for which reporting processes still need to be further implemented, and reduced R&D investments at Philips. We expect Green/EcoDesigned Innovation spend to increase in the years to come, as one of our 2025 ESG commitments is to design all our new product introductions in line with our EcoDesign requirements by 2025. Over EUR 1.5 billion was invested in Sustainable Innovation in 2023.
Philips Group
Green/EcoDesigned Innovation per segment
in millions of EUR
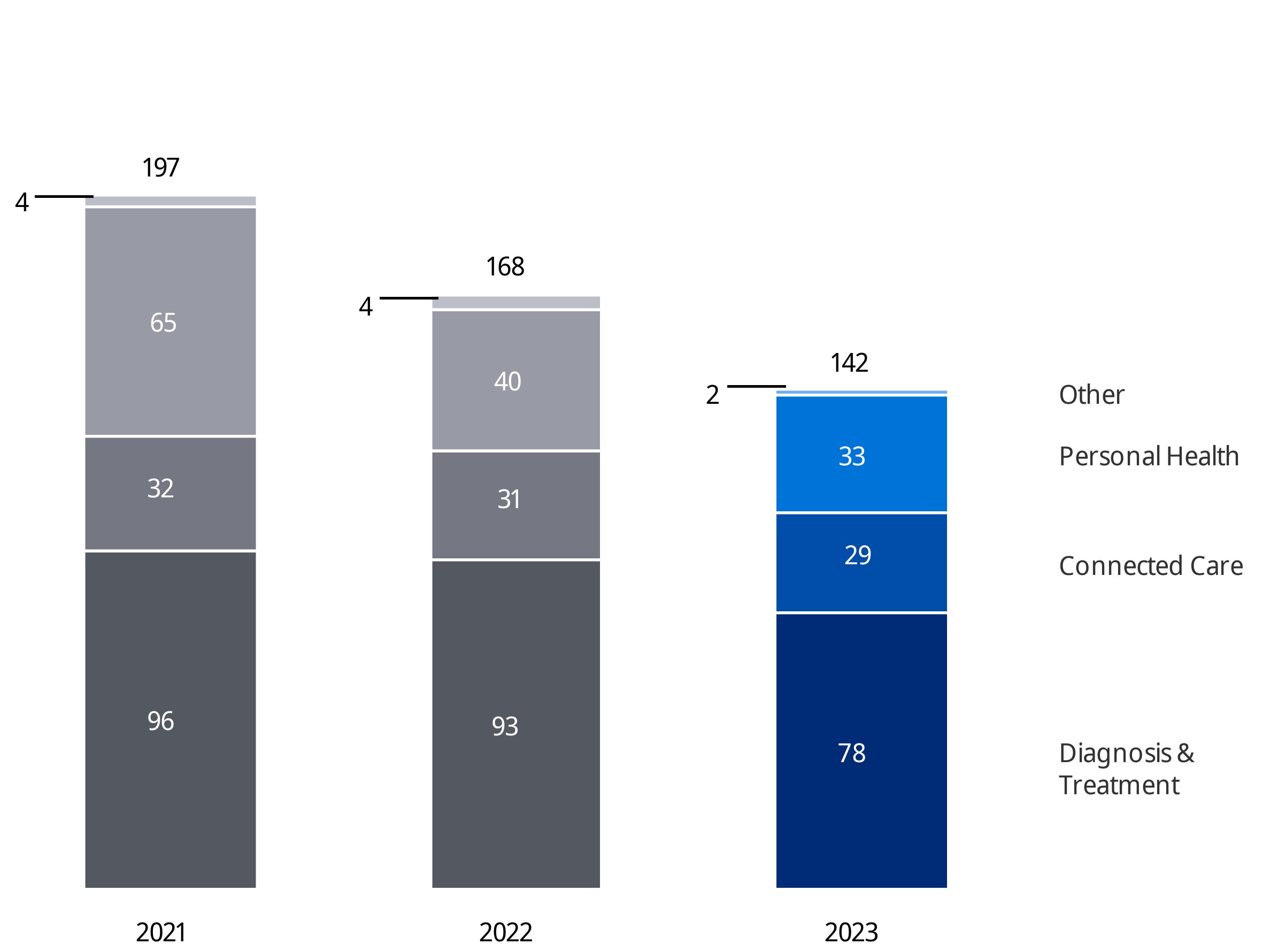
Philips develops innovative solutions that support precision diagnosis and effective, minimally invasive interventions and therapy, while respecting the limits of natural resources. Investments in Green Innovation in 2023 amounted to EUR 78 million, a reduction compared to EUR 93 million in 2022.
We aim to reduce environmental impact over the total lifecycle and our Green/EcoDesign innovations focus on four areas: Energy, Substances, Circularity, and Packaging. Energy efficiency is an area of focus, especially for our large imaging systems such as MRI. Through circular design, Philips also pays particular attention to enabling the reduction in use of virgin materials, for example, through designing for low weight and enabling the upgrading and re-use of our products. As a result, our customers can, for example, benefit from enhancements in workflow, dose management and imaging quality. In addition, we are reducing the substances of concern and improving our packaging. We continued to actively partner with multiple leading care providers to investigate innovative ways to reduce the environmental impact of healthcare, for example by maximizing energy-efficient use of medical equipment (e.g. by introducing EcoModes) and optimizing lifetime value. Philips closed the loop on large equipment by the end of 2020, by structurally embedding a responsible take-back policy into its customer trade-in offers. This means that for all equipment that a customer is willing to trade-in at end of use, Philips will take it back for refurbishment and parts recovery where feasible, or locally recycle it in a certified way to ensure it does not end up in landfill. Sustainable design and innovation help to further increase the value created and decrease the environmental impact Philips can deliver from returned systems.
Connected CarePhilips’ connected health solutions integrate, collect, combine and deliver quality data for actionable insights to help improve access to quality care, while respecting the limits of natural resources. It is our belief that well-designed e-health solutions can reduce the travel-related carbon footprint of healthcare, increase efficiency in hospitals, reduce waste, and improve access to care and outcomes. For example, our Philips Radiology Operations Command Center enables real-time collaboration and virtual imaging operations and can decrease staff travel time and costs. The value and adoption of e-health solutions also became apparent during the COVID-19 crisis. Green/EcoDesigned Innovation investments in 2023 amounted to EUR 29 million, in line with EUR 31 million in 2022. Over the coming years, Green/EcoDesigned Innovation projects will deliver, among other things, new EcoDesigned patient monitors with lower environmental footprints.
Personal HealthR&D investments at our Personal Health segment amounted to EUR 33 million in 2023, compared with EUR 40 million in 2022. Personal Health continued its work on improving the energy efficiency of its products, and the voluntary phase-out of polyvinyl chloride (PVC), brominated flame retardants (BFR), Bisphenol A (BPA) and phthalates from, among others, food contact and childcare products. New hairdryers have been launched that are more energy-efficient, with an improvement of more than 10% compared to the 2020 baseline. Personal Health also continues to increase circularity by, for example, using recycled materials in products and packaging. For packaging, we are increasingly shifting away from single use plastic materials. As part of our Fit for Future Packaging program, we have launched additional paper-based, mailbox-ready packaging solutions in our Grooming & Beauty portfolio, including OneBlades and hairdryers.
OtherThe segment Other invested EUR 2 million in Green/EcoDesigned Innovation, spread over projects focused on global challenges relating to water, air, energy, food, circular economy, and access to affordable healthcare.
Green/EcoDesigned Revenues are generated through products and solutions that offer a significant environmental improvement in one or more Green Focal Areas – Energy efficiency, Packaging, Substances, and Circularity – and thereby deliver a contribution to SDG 12 (Ensure sustainable consumption and production patterns). Green/EcoDesigned Revenues amounted to EUR 12.8 billion in 2023, or 70.5% of sales, comparable to 2022 (71.7% in 2022).
As the first EU Taxonomy delegated act, addressing Climate Change Adaptation and Climate Change Mitigation, only applies to sectors with the highest CO2 emissions, Philips’ activities are not within the scope of this delegated act and consequently none of Philips' revenues were eligible under this taxonomy during 2023.
Philips Group
Green/EcoDesigned Revenues per segment
in millions of EUR unless otherwise stated
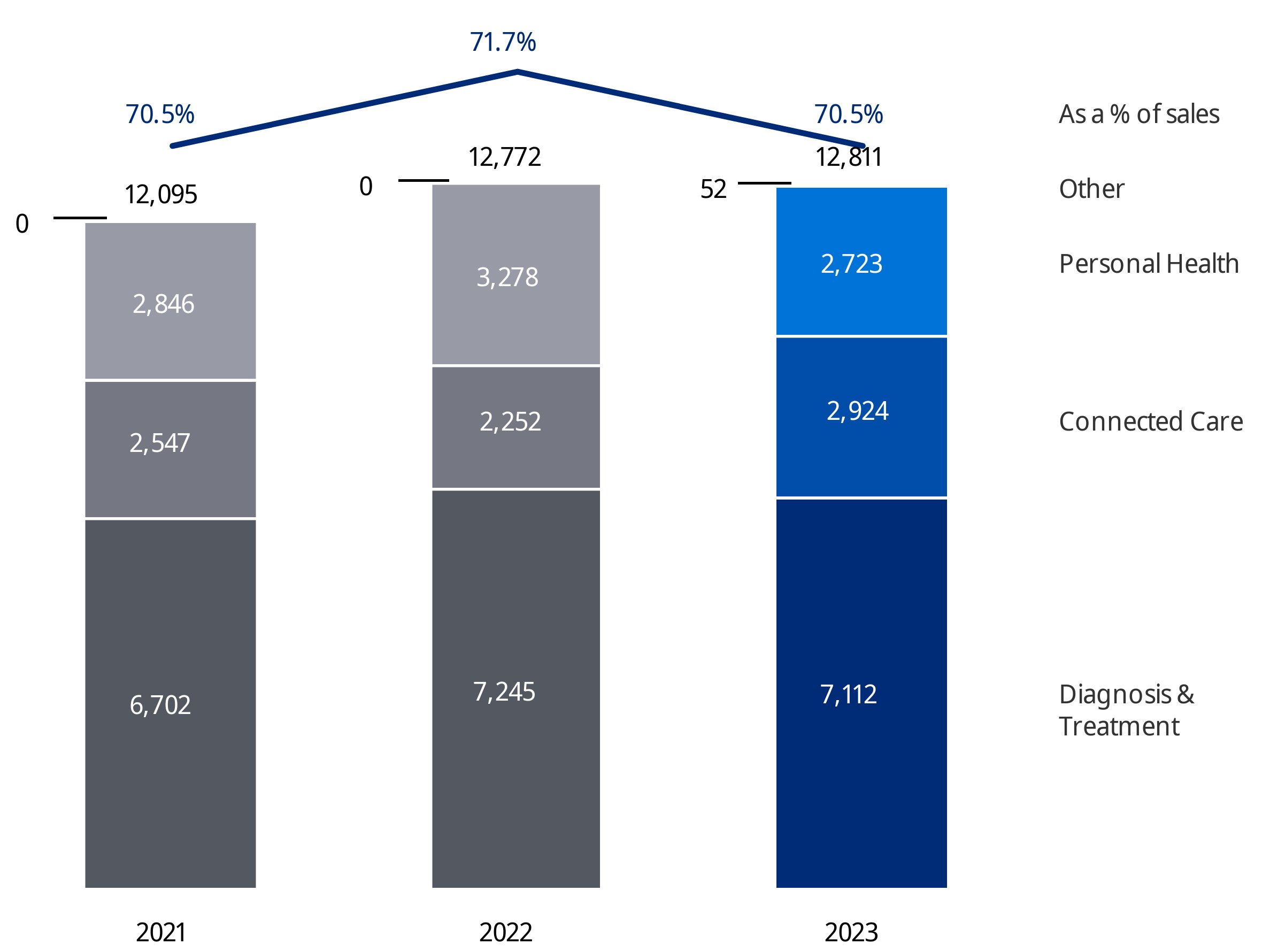
Through our EcoDesign process we aim to create products and solutions that have significantly less impact on the environment over their whole lifecycle. Overall, the most significant improvements have been in energy efficiency and lower weight (thus less resources), although increased attention was also given to substances of concern, packaging and circular design, in particular design for recyclability, in all segments in 2023.
By 2025, 25% of hardware revenues should come from EcoHeroes, which meet the EcoDesign requirements and outperform in at least one of the focal areas compared to their predecessor or relevant benchmarks, supported by a sustainability claim. In 2023, Philips achieved 15.9% in EcoHero Revenues, with most contributions from improvements in energy use.
Diagnosis & TreatmentIn 2023, a number of main platforms were launched. Specific attention was paid to preparing for future EcoDesigned product launches.
Connected CareNew Green/EcoDesigned products in Connected Care are expected in 2024 and beyond, with improvements in all EcoDesign focal areas.
Personal HealthIn our Personal Health business, the focus is on Green/EcoDesigned Products and Solutions that meet or exceed our minimum requirements in the areas of energy consumption, packaging, substances of concern, and application of recycled plastics. We continue to make progress in developing PVC/BFR-free products. More than 90% of our consumer product sales consist of PVC/BFR-free products, with the exception of power cords, for which there are not yet economically viable alternatives available. In our Oral Healthcare portfolio, we introduced the first brush heads containing 75% bio-based materials. In our Mother & Child Care portfolio, we launched the first baby monitor with recycled plastic. And we implemented recycled plastic in the interior parts of a significant proportion of the Male Grooming portfolio.
Philips’ Sustainable Operations programs focus on the main contributors to climate change, with the aim of reducing, re-using and/or recycling waste, reducing water consumption, and reducing emissions.
Annually, we undertake thorough assessments of both our operational sites and strategic suppliers to address potential water-related risks. We utilize publicly accessible tools such as the Aqueduct Water Risk Atlas by WRI and WWF Water Risk Filter to define and respond to these risks. This comprehensive process evaluates the susceptibility of our sites to various risks including water availability, wastewater quality, workplace water accessibility, and groundwater replenishment, encompassing physical, regulatory, and reputational concerns.
While Philips is not a water-intensive organization, this practice ensures the uninterrupted continuity of our operations and the provision of high-quality Water, Sanitation and Hygiene (WASH) services at all our sites and those of our strategic suppliers. Among our facilities, five locations have been identified as exposed to substantive financial and strategic risks related to water. These sites are situated either in areas highly vulnerable to coastal flooding or extremely high-water stress (>80%), with three in China, one in Indonesia, and one in the USA. In response, we have deployed engineers and experts to conduct further investigations, accurately identify risk exposure, anticipate potential losses, and implement proactive measures to mitigate property loss and business interruption.
We are proud to have received an 'A' score for disclosure transparency on water security in the CDP Europe 2023, demonstrating our ongoing commitment to water risk management and sustainability practices.
Total water withdrawal in 2023 was 661,076 m3, a 3% decrease compared to 2022 and a 7% reduction compared to 2019 (pre-COVID level).
Diagnosis & Treatment, which consumes 49% of total water usage, recorded a 5% increase, mainly caused by lower amounts of reused water in a site in North America. Personal Health recorded a 7% decrease. In 2022, one of our manufacturing sites in Asia experienced a water leakage which resulted in higher water intake in that year. This leakage was remedied. Connected Care showed a decrease of 11%, due to decreased production volumes at a site in Asia.
Philips Group
Water withdrawal
in thousands of m3
| 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| Diagnosis & Treatment | 295 | 286 | 337 | 310 | 324 |
| Connected Care | 150 | 116 | 119 | 111 | 99 |
| Personal Health | 265 | 221 | 247 | 257 | 238 |
| Philips Group | 710 | 623 | 703 | 678 | 661 |
In 2023, 99.6% of water was purchased and 0.4% was extracted from groundwater wells.
WasteIn 2023, our manufacturing sites generated 19,375 tonnes of waste, a decrease of 15% compared to 2022, mainly driven by the lower construction activities in different locations across the globe, lower paper/cardboard and plastic waste due to reduced production volumes at some sites and more efficient waste management. The reported re-used materials were 9% of the total waste.
Diagnosis & Treatment decreased waste by 12%, mainly driven by the decreased volume of one-time construction related re-used materials and lower construction activity, which was partially offset by the operational changes. Connected Care decreased waste by 21% due to the significant decrease in shipment packaging materials related to the recall, increased volume of re-used materials and operational changes. Personal Health reduced waste by 17% due to operational changes, lower production volumes and the start of a smart warehouse, that significantly reduced amounts of wood pallets and cardboard wastes by using reusable plastic trays.
Philips Environmental Policy addresses the waste hierarchy stating that Philips drives action by ensuring circular manufacturing and supply to increase circular practices at our sites and responsible waste management according to the waste hierarchy.
Philips Group
Total waste
in tonnes
| 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| Diagnosis & Treatment | 9,675 | 19,703 | 9,974 | 10,694 | 9,422 |
| Connected Care | 4,095 | 3,475 | 2,753 | 2,899 | 2,276 |
| Personal Health | 8,758 | 7,929 | 9,477 | 9,209 | 7,677 |
| Philips Group | 22,528 | 31,107 | 22,204 | 22,802 | 19,375 |
Until 2020, total waste consisted of waste that is delivered for landfill, incineration, waste to energy or recycling. We extended the scope with materials sent for re-use and other recovery as of 2021. Total waste does not include waste prevented.
Materials delivered for re-use, other recovery or recycling via an external contractor amounted to 17,446 tonnes, which equals 90% of the total waste. Materials delivered to incineration and landfill amounted to 1,929 tonnes, which equals 10% of the total waste, of which 74% comprised non-hazardous waste and 26% hazardous waste. We recorded 1,531 tonnes of waste prevented in our own activities in 2023, compared to 1,484 tonnes in 2022. Philips did not produce any radioactive waste in 2023.
Philips Group
Total waste by destination
in tonnes
| Total waste generated | Hazardous waste | Non-hazardous waste | |
|---|---|---|---|
| Re-use | 1,651 | 1 | 1,650 |
| Recycling | 15,762 | 1,536 | 14,226 |
| Other recovery | 33 | - | 33 |
| Waste diverted from disposal by recovery operation | 17,446 | 1,537 | 15,909 |
| Incineration (with energy recovery) | 1,480 | 199 | 1,281 |
| Incineration (without energy recovery) | 298 | 294 | 4 |
| Landfill | 1511) | 4 | 147 |
| Waste directed to disposal by disposal operation | 1,929 | 497 | 1,432 |
| Total waste generated | 19,375 | 2,034 | 17,341 |
The total waste destinations are fully categorized above. There is no waste generated that is destined for other disposal methods. Our sites addressed both the Circular Materials Management percentage as well as waste sent to landfill, as part of our ESG commitments; refer to Definitions and abbreviations for the definition of Circular Materials Management.
The Circular Materials Management percentage has replaced the recycling percentage in 2021. In 2023, it remained at 91%, the same level as in 2022.
Our Zero Waste to Landfill KPI excludes one-time-only waste and waste delivered to landfill due to regulatory requirements. According to this definition, in 2023 we reported 2.7 tonnes of waste sent to landfill, a small increase compared to 1 tonne in 2022. All our 23 industrial sites achieved Zero Waste to Landfill status at the end of 2023.
Philips Group
Total waste by composition
in tonnes
| Waste generated | Waste diverted from disposal | Waste directed to disposal | |
|---|---|---|---|
| Wood waste | 4,140 | 4,104 | 36 |
| Paper/cardboard waste | 3,527 | 3,522 | 5 |
| Metal waste | 3,338 | 3,291 | 48 |
| Plastic waste | 2,381 | 2,237 | 144 |
| Municipal (mixed) waste | 2,136 | 1,156 | 980 |
| Chemical waste | 2,020 | 1,532 | 487 |
| Electrical and electronic waste | 626 | 622 | 4 |
| Other | 1,208 | 983 | 225 |
2023 has been a year of change, focused on building the foundation to deliver greater impact in all we do by addressing challenges and shifting to our new operating model, whilst continuing to deliver for patients, customers and consumers. We laid the groundwork for our updated People strategy and the key pillars of growing our people, igniting our culture, simplifying how we work, and bringing oxygen to the organization through simple adaptive people processes and a focus on well-being and inclusion.
Aligned with these pillars, we continued driving the broader Talent agenda, with a strong focus on Executive Committee successor identification and development. This resulted in two internal executive appointments, our new Chief People Officer and Market Leader North America, and the appointment of two critical external executive female talents, Chief Business Leader Monitoring & Connected Care and Chief Medical Officer, further strengthening our MedTech expertise. We continued to support the development of our internal talents resulting in 33% internal mobility (vs. target 30%) and further improved our diversity ambitions with over 31% women in senior leadership. Overall, employee turnover is slightly up to 17.6% from 2022 (17.5%) and top talent has seen an increase to 12.3% from 10.2% in 2022.
In 2023, we began reinvigorating our culture using a phased approach to emphasize action over words, laying the groundwork for scaling this work in 2024. This included working on our culture through our most pressing challenges with the Philips Leadership Team and how to understand our disabling patterns as an organization so that we can begin to disrupt them. Deeper culture work was started in the businesses with specific needs, and preparations to ignite, embed and embody our culture at scale are under way. This culture work will run in parallel with our priority of scaling development for all 7,500 people leaders. Building on 2023 progress, we will enable leaders to model the skills and behaviors that align with our refreshed culture and position us to deliver results through our teams.
As a result of the shift in the operating model, we have made progress in simplifying how we work and are trending below the target headcount (69,656 actual vs. 72,295 target). Despite the amount of change in the organization, we saw an improvement in engagement scores between March and October 2023 by 5% to 73% indicating that the organization is starting to recover from the change, although the number is still below 2022 (78%) and benchmark levels.
In 2023, we gained momentum towards our strategy of becoming a more people-centric organization and we have put in place the foundations that will help us to deliver value with sustainable impact through our people.
Our cultureOur culture is crucial to meeting our goal of improving the lives of 2.5 billion people by 2030. The way we act and behave shapes our shared understanding of what is important and how we deliver. As a leader in health technology, we are on a journey to reinvigorate our culture so that we become even more people-focused, reduce complexity, and drive greater accountability to meet the needs of our patients, consumers, and customers more consistently.
We have launched a new global People strategy to accelerate this mission and significantly shape our organizational culture. This strategy rests on three pillars. Firstly, we simplify and enhance our core operations to empower employees to address pressing needs efficiently, all to prioritize patient safety and customer experience. Secondly, we focus on nurturing our workforce's skills and capabilities, adapting to the ever-evolving health technology industry. Thirdly, we instill a culture of inclusivity and belonging by integrating health, well-being, and diversity into our work practices, ensuring that every employee feels valued and connected to our shared mission. These pillars form the foundation of our talent ecosystem and reflect our commitment to fostering innovation and excellence on a global scale.
But our culture is more than words; it is also shaped by how our organization is structured. Our new operating model, implemented in 2023, defines how we work in a regulated environment to safely develop innovations that improve people's health and well-being responsibly and sustainably. We prioritize patient safety, quality, compliance, and integrity in everything we do. With this new structure, our culture-defining initiatives and programs are expected to have a greater impact as we focus on consistency and tangible action.
For example, in October we held a global Timeout for Patient Safety and Quality, where our entire company took time to reflect on how our daily work affects patient safety and quality, where we can commit to taking personal and collective responsibility, and how to create a culture centered on patients and people. Initiatives like this, along with targeted learning activities like our Clinical and Medical Learning Hub, which provides world-class clinical training and resources, contribute to making our culture ‘real’ and deliver a sense of pride for our people.
Considering the ever-changing economic, political, and health environment, we also remain flexible in our approach to how we work. We continue to adapt our hybrid model for more flexibility and collaboration, with a stronger focus on policies and programs that prioritize people and are tech-enabled for easier access and efficiency. This means we continue to:
This comprehensive and people-centric strategy – combined with our operating model and targeted interventions – is designed to transform Philips into an even more agile, high-performing organization with a thriving culture at the core.
Through 2023, our Strategic Priority recruitment team continued to focus on delivery of the roles most critical to the delivery of our strategy. Together, the Strategic Priority team delivered 1,427 hires within R&D, Q&R and Clinical roles at Corporate Grade 70 and above, and all Informatics roles. To further enhance our workforce of the future, Talent Acquisition delivered 20.2% of external candidates with MedTech experience.
Early Career TalentOur Philips-wide Graduate Development Program (GDP) continues to perform well and increased from 40 participants in 2021 to 240 in 2023. The GDP lasts two years and includes two job rotations, as well as offering the graduates a comprehensive learning and development track, and access to career centers to help guide future steps. Philips also gave meaningful work experience to 1,802 interns, offering 321 of them permanent employment after their internship.
Total Workforce StrategyWe continue our Total Workforce Strategy, which considers all sources of skills, capabilities, locations and changes in the labor market in order to deliver the Workforce of the Future. Our Right Shoring & Sourcing methodology is used to implement this strategy. This methodology steers improvements in workforce composition towards the ‘right shore’ (onshore, nearshore and offshore) and the ‘right source’ (employees, contingent workers and external services). The program delivered EUR 11.4 million in savings in 2023.
In 2023 we started with the implementation of our external workforce strategy. In addition, we have been looking at how we are attracting contingent workforce talent. Direct sourcing has been expanded to 32% in the Netherlands, to 14% in the United States, and has been rolled out to India. To strengthen the way we directly source contingent workforce talent, our own Philips employer value proposition is utilized for the different Functions to attract these contingent workers.
As a health technology leader, we attach great importance to the health and well-being of our workforce and to creating an environment of inclusion and belonging, where all employees feel psychologically safe. Our company’s success depends on our employees feeling valued, respected, and empowered to contribute fully. We are a diverse team made up of approximately 70,000 individuals across over 100 countries, all with different backgrounds, perspectives, and experiences. We fully value and leverage these differences to ensure that creativity and innovation can flourish. Philips’ commitment towards Inclusion & Diversity is reflected in our General Business Principles and the company-wide Inclusion & Diversity Policy and Fair Employment Policy.
RepresentationWe continue to put in place measures to enhance representation of diverse talent at all levels within the organization, and to ensure that representation at senior management levels reflects the diversity of our stakeholders, including consumers, our customers and their patients.
To this end, in 2022, Philips restated its commitment to having 35% of senior management positions held by women, by the end of 2025. Senior management positions (including senior directors and executives) amount to approximately 1,155 employees, 363 females (31.4%), 792 males (68.6%) at the end of 2023. As of year-end 2023, we had reached our initial goal (set in 2020) of a 30% representation of women in senior management.
Our Supervisory Board has adopted the Diversity Policy for the Supervisory Board, Board of Management and Executive Committee, which also includes the Supervisory Board’s aim that at least one-third of the members of each of the Board of Management and the Executive Committee are women and at least one-third are men. For more information on the Diversity Policy, please refer to Report of the Corporate Governance and Nomination & Selection Committee.
At year-end, the three members of the Board of Management remained male, with three out of 10 members of the Executive Committee being female. The Executive Committee numbers reflect a slight increase compared to 2022, where there were two women out of twelve in total, pending expected announcements of new leaders. The company generally seeks to fill vacancies by considering candidates that bring a diversity of (amongst others) gender. The selection of candidates is based on merit and there have been and may be pragmatic reasons – such as other relevant selection criteria and the availability of suitable candidates – that have impacted the achievement of our gender diversity goals.
Long-term Inclusion & Diversity ambitions are embedded in our People strategy. In our ongoing effort to increase transparency and accountability, we share data on the representation of women throughout our Businesses, Regions and Functions, including a monthly review with the Executive Committee. We closely monitor the inflow, advancement and outflow of talent, which makes it possible to customize goals and intervene where appropriate. We continue important initiatives that address unconscious bias, health and well-being, inclusion and development of underrepresented talent.
Philips Group
Gender diversity
in %
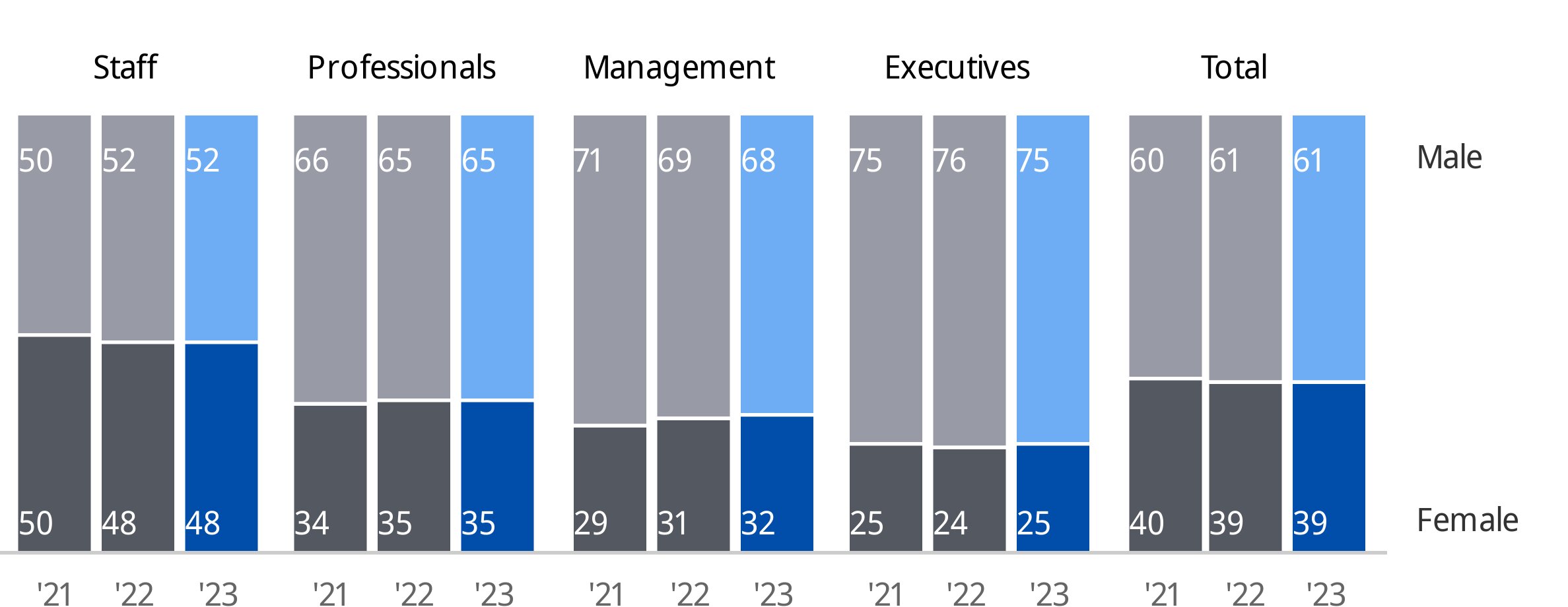
Philips Group
Generation diversity
in %
| 2023 | |
|---|---|
| Generation Z (1997 and onwards) | 11% |
| Generation Y / Millennials (1981 - 1996) | 48% |
| Generation X (1965 - 1980) | 33% |
| Baby Boomers (1946 - 1964) | 8% |
Philips Group
Employees by age group
| Employee age group | Under 30 | 30 to 50 | Above 50 | Total |
|---|---|---|---|---|
| % | 18 | 59 | 23 | 100 |
At Philips, we remain committed to a multi-generational workforce, as the presence of generational diversity is a key factor to foster creativity, productivity and innovation. The different life experiences of a multi-generational workforce support collaborative decision making and the inclusion of different beliefs and points of view.
Global Diversity CouncilOur Global Diversity Council is comprised of 10 senior leaders representing our Businesses, Regions and Functions. The Council provides governance and oversight on diversity efforts, promotes company-wide behavior change, and communicates on progress. Additionally, every Council member is an Executive Sponsor to one of our Employee Resource Groups.
Employee Resource GroupsEmployee Resource Groups (ERGs) provide an inclusive space for employees to support and care for one another, develop skills, experience meaningful cultural connections, expand their knowledge, all while strengthening relationships among the Philips community.
Philips currently has 11 ERGs globally, with over 10,000 employees participating: Able & Allies; Asian Employee Resource Group; Black Employee Resource Group; Future Leaders and Rising Employees; Latinx Employee Resource Group; Middle Eastern Employee Resource Group; Philips Empowering Parents; Philips Women Lead; Pride Network; Veterans and Family Coalition; and Neurodiversity Network.
Health & Well-beingIn 2023, we evolved our (mental) health and well-being framework to incorporate two additional pillars of well-being – career and environmental – enhancing our holistic approach and integrating global and local programs. We continued to address mental health by further rolling out the Employee Assistance Program (EAP).
We grew our Mental Health Champion program to 250 Champions across the globe, providing accredited training for peer-to-peer confidential support. We developed Compassionate leadership training for all our people managers, as well as encouraging dialogues around self-care, building trust and resilience. We also launched a Manager Mental Health toolkit, complemented by training sessions.
Along with International Women’s Day and PRIDE, Philips also recognized World Health Day and World Mental Health Day, with a variety of virtual mental well-being sessions and resilience practices that engaged employees across our Regions. In collaboration with Philips University, the Philips Energy Management well-being program was further extended across the organization.
Building capabilityIn 2023, we deployed bias training to all recruiters, continued the learning journey for employees with a focus on emotional wellness, and launched a learning series for all employees called Learn With Us, where we featured Diversity, Inclusion and Well-being best practices from across the globe.
External awardsMany stakeholders, including customers and potential partners and employees, view third-party assessments as objective indications of the strength of our commitment. Awards received in 2023 included Forbes Best Employers for Women, Forbes Best Employers for Diversity, and Forbes Best Employers in Texas.
External partnershipsAs we continue to focus on integrating inclusion, belonging and equity into the employee experience, we see value in partnering with diverse professional network organizations and job boards to sharpen our focus on the development and retention of our internal diverse talent and increasing representation of diverse talent across our organization. In 2023, we renewed a partnership with the National Black MBA Association and introduced partnerships with the National Sales Network, Circa and Mogul.
We continue to keep a close pulse on our employee sentiment through our bi-annual Employee Engagement Survey. Amidst the changes across the company, our Employee Engagement Survey (EES) saw an extremely high response rate of 73%, which means that almost 50,000 employees participated.
In 2023, average employee engagement scores decreased to 73% – lower than the Fortune 500 benchmark. The decrease in employee engagement scores, specifically in the first half of the year, did not come as a surprise, as we announced employee reductions and organizational changes in January and many of our employees were (pre-) informed of how they would be impacted. There was significant improvement (5 percentage points) in the EES scores in the second half of the year, as the employees settled within the new organization structure.
Philips Group
Employee Engagement Index
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Favorable | 79% | 77% | 73% |
| Neutral | 14% | 15% | 17% |
| Unfavorable | 7% | 8% | 10% |
In a changing environment, we listened actively to our employees to provide them with greater clarity on future direction and enable them to proactively deal with change to meet our customer and patient needs. In 2023, we introduced specific sessions around Patient Safety and Quality. We saw that our employees feel comfortable reporting a patient safety or quality concern, while some employees feel that we can learn more from reviewing quality and regulatory events, and that there is an opportunity to improve training and recognition on these topics. This is critical given the culture of people- and patient-centricity that we aspire to.
Using the Customer Experience Index, we look at how well employees think we focus on customer needs. These inputs are actively exchanged with the Customer Experience team.
Our employee engagement is primarily driven by a clear understanding of our customer needs and delivering on commitments that we make to each other. The results of the EES indicate that employees feel they can be themselves and have trusting relationships at work. Another significant factor driving engagement is our high scores on the Inclusion & Diversity index, which remains above the industry benchmark.
The total number of Philips Group employees was 69,656 at the end of 2023, compared to 77,233 at the end of 2022, a decrease of 7,577 FTEs.
On January 30, 2023, we launched a multi-year plan to create value with sustainable impact. Part of this plan is to improve performance and simplify our way of working to improve our agility and productivity. This includes the difficult, but necessary reduction of our workforce by around 6,000 roles globally by 2025. This is in addition to the 4,000 reductions announced in 2022. As we went through this change, we did it with the utmost care and respect for our people, with a strong focus on supporting those who were directly impacted by the reductions in finding a new role.
Subject to local country legislation, our support offers include:
Philips Group
Employees per segment
in FTEs at year-end
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Diagnosis & Treatment | 29,094 | 30,335 | 28,397 |
| Connected Care | 21,047 | 19,241 | 17,549 |
| Personal Health | 10,134 | 9,319 | 9,085 |
| Other | 17,913 | 18,337 | 14,626 |
| Philips Group | 78,189 | 77,233 | 69,656 |
Philips Group
Employment
in FTEs
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Balance as of January 1 | 75,001 | 78,189 | 77,233 |
| Consolidation changes: | |||
| Acquisitions | 2,594 | 87 | 27 |
| Divestments | (744) | (33) | (353) |
| Other changes | 1,338 | (1,010) | (7,251) |
| Balance as of December 31 | 78,189 | 77,233 | 69,656 |
Approximately 56% (2022: 58%) of the Philips workforce is located in Mature geographies and 44% (2022: 42%) in Growth geographies. In 2023, the number of employees in Mature geographies decreased by 5,392. The number of employees in Growth geographies decreased by 2,184.
Philips Group
Employees per geographic area
in FTEs at year-end
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Western Europe | 19,775 | 19,297 | 16,900 |
| North America | 21,807 | 20,618 | 18,094 |
| Other mature geographies | 4,683 | 4,576 | 4,105 |
| Mature geographies | 46,265 | 44,491 | 39,099 |
| Growth geographies | 31,923 | 32,742 | 30,558 |
| Philips Group | 78,189 | 77,233 | 69,656 |
In 2023, employee turnover amounted to 17.6%, of which 9.5% was voluntary, compared to 17.5% (11.1% voluntary) in 2022. External benchmarks show that our voluntary employee turnover remains in line with similar-sized companies, and that we are reasonably successful in retaining our employees.
Philips Group
Employee turnover 2023
in number of employees
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 2,935 | 2,261 | 237 | 19 | 5,452 |
| I choose not to self-identify | 1 | 1 | |||
| Male | 2,583 | 3,676 | 527 | 50 | 6,836 |
| Philips Group | 5,518 | 5,938 | 764 | 69 | 12,289 |
Philips Group
Employee turnover 2023
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 23.1% | 16.8% | 17.9% | 26.8% | 19.7% |
| I choose not to self-identify | 6.3% | 3.9% | |||
| Male | 18.7% | 14.5% | 18.3% | 23.4% | 16.1% |
| Philips Group | 20.8% | 15.2% | 18.1% | 24.3% | 17.6% |
Philips Group
Voluntary turnover 2023
| Staff | Professionals | Management | Executives | Total | |
|---|---|---|---|---|---|
| Female | 11.9% | 9.6% | 7.5% | 9.9% | 10.6% |
| I choose not to self-identify | 6.3% | 3.9% | |||
| Male | 11.1% | 7.8% | 7.1% | 6.1% | 8.8% |
| Philips Group | 11.5% | 8.4% | 7.2% | 7.0% | 9.5% |
The rate of employee turnover reported, is calculated in headcount as a monthly average across the reporting period.
As a company, Philips is committed to remunerating equal pay for equal work. We ensure that all employees are compensated fairly and without inequity based on gender, race, or any other characteristic protected by law.
A pay equity review is a crucial process that reflects our commitment to fostering fairness, equality, and transparency within our organization. Annually, Philips conducts a comprehensive analysis of its compensation structure to ensure that all employees are remunerated fairly for their skills, experience, and contributions, regardless of human characteristics that can lead to potential biases. The systematic measurement of gender pay gaps within Philips is an integral aspect of our operational practices. This commitment ensures that we not only provide equal opportunities, but also uphold fairness in compensation for the work undertaken by our diverse workforce. This dedication to transparency and proactive correction reflects our overarching commitment to fostering an environment of equality and inclusivity within the company.
In alignment with our global ethos, many countries in which Philips operates have already embraced the practice of regular pay equity reviews over several years. Notable examples include Australia, the United Kingdom, Sweden, India, and certain states within the United States. This demonstrates our unwavering dedication to upholding the principles of equity and inclusivity on a global scale. When an unexplained gap is brought to light, immediate actions are taken to rectify the situation. Furthermore, Philips is committed to understanding the underlying factors contributing to these unexplained gaps, and formulating proactive measures to prevent such occurrences in the future.
Specifically focusing on the United States, Philips embarked on a significant initiative in the form of a company-wide Pay Equity & Transparency Project starting in 2022 and culminating in 2023. This project builds upon the successful groundwork laid during pay equity reviews at the state level in the US. The objective has been to create a comprehensive and standardized approach to pay equity and pay transparency, ensuring that all employees across the US are remunerated fairly, in accordance with their skills and responsibilities. All US employees have the right to request pay transparency and understand their position against peers, which is over and above the current legislative requirements.
The Pay Equity Project is a testament to our commitment to not only meeting but exceeding legal and regulatory requirements. By proactively addressing pay equity concerns, we aim to create an inclusive work environment that fosters diversity and promotes equal opportunities for everyone within the organization. This initiative reinforces our belief that fair compensation is not only a legal obligation but a fundamental ethical responsibility that contributes to the overall success and sustainability of our company.
Through this proactive approach, we strive to set an industry standard for pay equity, demonstrating that a commitment to fairness and equality is not only beneficial for our employees but also the long-term success and reputation of Philips as a global leader in innovation and healthcare solutions.
Philips can only achieve its aim to improve the lives of 2.5 billion people per year by 2030 if we support and empower our people, so they can be their best and perform effectively. To this end, we conducted a living wage analysis on the lowest salaries in every country in which we currently operate.
The living wage is a concept defined by Anker and Anker (2017) as “Remuneration received by a worker in a particular place sufficient to afford a decent standard of living for the worker and her or his family. Elements of a decent standard of living include food, water, housing, education, healthcare, transport, clothing, and other essential needs, including provision for unexpected events”.
Based on the Living Wage analysis conducted in 2023, all Philips employees received wages and benefits that are consistent with at least the minimum Living Wage standard for an individual. Furthermore, approximately 97% of Philips employees received wages and benefits that are consistent with at least the minimum Living Wage standard for a family (based on reference data and guidance from Wage Indicator).
In 2023, the safety of our employees remained paramount. As the COVID-19 pandemic continued the endemic phase, the centralized controls put in place during the pandemic were relaxed in line with local governments’ advice. As Philips resumed normal operations, office occupancy started to rise, and business travel restarted. However, critical control measures were maintained. Philips has emerged from the pandemic with a good record of management and control that restricted the impact of the pandemic on employees and the wider business operations.
At Philips, we strive for an injury-free and illness-free work environment. Since 2016, the Total Recordable Cases (TRC) rate has been defined as a Key Performance Indicator (KPI). A TRC is a case where an injured employee is unable to work for one or more days, has medical treatment, or sustains an industrial illness. We set yearly TRC targets for the company, businesses and industrial sites.
We recorded 172 TRCs in 2023, the same as in 2022. While our workforce decreased in 2023, the TRC rate increased from 0.23 per hundred FTEs in 2022 to 0.24 in 2023.
In 2023 we recorded 90 Lost Workday Injury Cases (LWIC). These are occupational injury cases where an injured person is unable to work for one or more days after the injury. This represents a 9% increase compared with 81 in 2022. The LWIC rate increased to 0.12 per 100 FTEs in 2023, compared with 0.11 in 2022. The number of Lost Workdays caused by injuries decreased by 1,471 days (37%) to 2,549 days in 2023.
Philips strongly believes that companies have both the responsibility to respect human rights and the ability to protect them. Philips’ Human Rights Policy, General Business Principles, Supplier Sustainability Declaration and other relevant policies detail how Philips respects human rights, in line with the International Bill of Human Rights and the International Labor Organization’s Declaration on Fundamental Principles and Rights at Work.
In this regard, Philips follows the guidance given in the UN Guiding Principles on Business and Human Rights and the Organization for Economic Co-operation and Development (OECD) Guidelines for Multinational Enterprises. Philips has also been a signatory to the UN Global Compact since 2007. Philips’ Board of Management is responsible for strategy and oversight of all company activities across the three ESG dimensions, including human rights. The Board also monitors progress and takes corrective action where needed.
In addition, a cross-functional project team, comprised of a Human Rights Manager and professionals from several business functions, is in place to drive several human rights initiatives. The project team is overseen by the Human Rights Steering Committee, consisting of senior leaders from Operations, Legal, Human Resources and Sustainability.
In 2023, we continued to develop our due diligence strategy by conducting Human Rights Impact Assessments (HRIA). Philips conducted Human Rights Impact Assessments (HRIAs) at its sites in Pune (India) and Varginha (Brazil), living up to its commitment of conducting HRIAs at 100% of its at-risk sites by 2023. Philips intends to monitor the progress and findings from these sites and take them on a continuous improvement journey regarding human rights topics.
Although the Human Rights Impact Assessment of selected sites is primarily focused on Philips own operations, a derived deep-dive approach for certain suppliers has been rolled out since 2022. For 8 suppliers, a focused assessment on human rights was conducted, compared with the broader Supplier sustainability assessment approach which covers sustainability more holistically and is detailed in Supplier sustainability.
Our Human Rights Report contains detailed information regarding our progress, targets, and plans for continuous improvement.
Philips’ purpose to improve people’s health and well-being extends throughout our value chain. At Philips, we have a direct business relationship with approximately 4,900 product and component suppliers and 16,100 service providers. Our supply chain sustainability strategy is evaluated annually through a structured process, combined with multi-stakeholder dialogues. From this, we have developed multiple ESG programs aimed at driving sustainable improvement. These programs cover compliance with our policies, improvement of our suppliers’ sustainability performance, our approach towards responsible sourcing of minerals, and reducing the environmental impact of our supply base. Supplier engagement in these programs is driven by screening ESG opportunities and risks, evaluating materiality and impact along the lines of material, industry, and geographical characteristics.
Procurement and supplier information sessions are scheduled on an ongoing basis. During these sessions, our supplier ESG expectations are shared and clarified. Training courses are organized to support suppliers in meeting those expectations. In addition, suppliers are supported in improving their ESG performance via individual training. Where data is available, suppliers are informed on their performance compared to industry peers, and best practices are shared, and their adoption encouraged. During the sourcing process, supplier ESG indicators are evaluated. In addition to minimum requirements set out in our Code of Conduct, suppliers with a better ESG performance are considered favorably.
Supplier sustainability complianceTwo core policy documents form the basis of our supplier sustainability compliance approach: the Supplier Sustainability Declaration and the Regulated Substances List.
Supplier Sustainability Declaration (SSD)The SSD sets out the standards and behaviors Philips requires from its suppliers. The SSD is based on the Responsible Business Alliance (RBA) Code of Conduct, in combination with several additional Philips-specific expected behaviors. The Code is in alignment with the UN Guiding Principles on Business and Human Rights and key international human rights standards, including the ILO Declaration on Fundamental Principles and Rights at Work and the UN Universal Declaration of Human Rights. It covers topics such as Labor, Health & Safety, Environment, Ethics, and Management Systems. The RBA is the world’s largest industry coalition dedicated to responsible business conduct in global supply chains. As a Regular member of the RBA, Philips is required to commit publicly to the RBA Code of Conduct and actively pursue conformance to the Code and its standards, which must be regarded as a total supply chain initiative.
Regulated Substances List (RSL)The RSL specifies the chemical substances regulated by legislation. Suppliers are required to follow all the requirements stated in the RSL. Substances are marked as restricted or declarable.
All suppliers are required to commit to the SSD and RSL. Through integration of a Sustainability Agreement in our purchasing agreements, suppliers declare compliance to both the SSD and RSL. Upon request, they provide additional information and evidence.
Supplier Sustainability Performance (SSP) – 'Beyond Auditing'In 2016, Philips first piloted its 'Beyond Auditing' approach to engage suppliers on ESG matters, with a focus on:
This systematic approach is shown in the figure below and is a high-level representation of the SSP program.
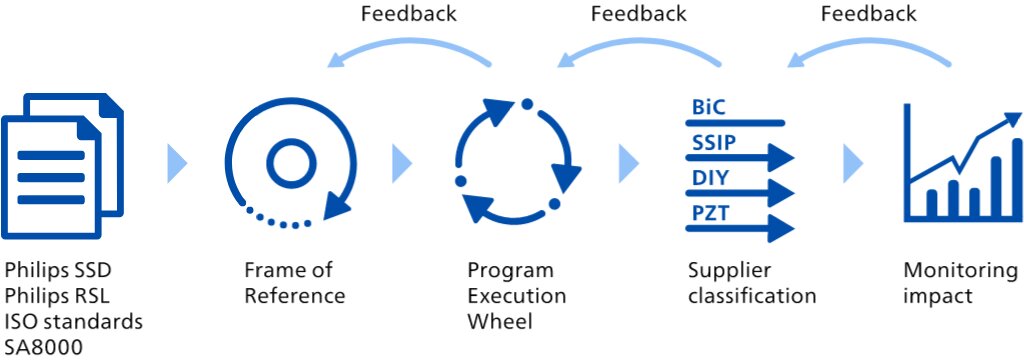
First, a set of references, international standards, and Philips requirements are used to develop the Frame of Reference, which covers management systems, environment, health & safety, business ethics, and human rights. For each, the maturity level of suppliers is identified in the Program Execution Wheel, which assesses suppliers against the Plan–Do–Check–Act (PDCA) cycle. Suppliers are then categorized through the Supplier Classification model, which differentiates on the basis of supplier maturity, resulting in supplier-specific proposals for improvement. The SSP process is monitored and adjusted through continuous feedback loops. The outcome of the SSP assessment is a supplier sustainability score ranging from 0 to 100. This score is based on supplier performance in environmental management, health & safety, business ethics, and human rights.
Supplier classificationSupplier selection for the program is based on significance. Significance of suppliers is determined through an assessment of the supplier’s associated ESG risks and opportunities, including material, industry, and geographical characteristics, as well as annual spend. In 2023, 152 of our suppliers were considered significant. After this initial assessment, the engagement strategy is tailored based on the suppliers’ current performance in terms of sustainability.
Philips Group
Significant suppliers - tier 1
| 2023 | |
|---|---|
| Number of suppliers | 152 |
| Spend as percentage of total | 20% |
There are four different engagement approaches: BiC (Best in Class), SSIP (Supplier Sustainability Improvement Plan), DIY (Do It Yourself) and PZT (Potential Zero Tolerance). The PZT status is a temporary status and requires immediate attention and action. Depending on the categorization, suppliers are engaged in different ways to improve their sustainability performance through agreed improvement plans.
If a (Potential) Zero Tolerance is identified, immediate action is taken. If the requested additional information and evidence lead to the conclusion that there is no structural Zero Tolerance, the supplier’s status will be changed and the supplier will go back to the original track in the program. If the conclusion gives rise to a structural Zero Tolerance, the supplier is required to:
Philips defines six Zero Tolerances:
For more details on the SSP process, refer to the SSP brochure.
Our 2023 resultsIn 2023, five zero tolerances were found across the following categories: health and safety, labor, and environmental impact. All five cases were successfully closed in 2023 after confirmation of completion of the corrective action plan. One zero tolerance, found in the last quarter of 2022, has also been closed during 2023.
Philips measures the impact of SSP engagements through the number of lives improved in the supply chain. This is derived from the improvements that suppliers make in their performance. To determine improvements, we calculate the pro rata change in performance from one year to the next.
Philips Group
Lives improved in the supply chain
in thousands of Lives
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Lives improved in the supply chain | 430 | 459 | 723 |
In 2023, the overall year-on-year improvement in performance was 37% for suppliers that received their first re-assessment in 2023. The number of employees impacted (first and second tier) suppliers participating in the SSP program was approximately 723,000. This figure includes suppliers assessed in the last three years, for which the supplier has communicated their number of employees via the self-assessment questionnaire, which was validated during the on-site assessment. For those workers, labor conditions improved, the risk of serious injury reduced, and the negative environmental impact of suppliers was brought down. This includes the workers at suppliers of the Domestic Appliances business, for which Philips continued the sustainability engagement. For a detailed break-down of percentage improvements realized by active suppliers in the past year, by comparing the assessment in 2023 to their previous assessment, refer to the following table.
Philips Group
SSP 2023 performance: pro-rata improvements
in %
| Topics | Policy | Procedures | Implementation | Management responsibility | Communication | Risk control | Target-setting & tracking | Corrective action approach | Supplier management |
|---|---|---|---|---|---|---|---|---|---|
| Environment | 2% | 7% | -2% | 10% | 2% | 23% | 15% | 10% | -8% |
| Health and Safety | 11% | 11% | 16% | 0% | 6% | 10% | 11% | 21% | 4% |
| Business Ethics | 11% | 20% | 9% | -4% | 26% | 21% | 33% | 26% | 1% |
| Human Capital | 13% | 13% | 19% | 11% | 7% | 4% | 10% | 12% | -2% |
Categories which showed the biggest improvement are:
In 2023, 158 suppliers were added to the SSP program (compared to 47 added in 2022). Of the population of suppliers that entered the program in the years before 2023 and have been assessed at least once in the past three years, 392 suppliers were still active in 2023 (compared to 249 in 2022). The combined group represents 77% of our significant suppliers who are in the program.
As part of our commitment to improve the lives of 1 million workers in the supply chain by 2025, we increased our engagement with second-tier suppliers. By teaming up with Tier 1 suppliers in conducting the assessment, Philips has supported in building ESG supplier management skills. In 2023, 110 second-tier suppliers entered the program, resulting in a total number of 138 second-tier suppliers engaged with in the last three years.
Additional progress made in 2023Philips is actively applying the latest insights in data science and machine learning methods to make the SSP program more efficient. Through the use of reference data collected over 1,600 assessments in the past years, Philips is working towards integrating maturity and improvement predictions in the program. This is expected to support us in determining the sustainability maturity of suppliers, while also increasing the effectiveness of our supplier improvement approach.
On an annual basis, Philips experts organize quality trainings in the sustainability area for suppliers in the scope of the SSP program.
Responsible sourcing of mineralsThe supply chains for minerals are long and complex. Philips does not source minerals directly from mines as there are typically 7+ tiers between end-user companies like Philips and the mines where the minerals are extracted. The extraction of minerals can take place in conflict-affected and high-risk regions, where mining is often informal and unregulated, and carried out at artisanal small-scale mines (ASM). These ASMs are vulnerable to exploitation by armed groups and local traders. Within this context, there is an increased risk of severe human rights violations (forced labor, child labor or widespread sexual violence), unsafe working conditions or environmental concerns.
Philips addresses the complexities of minerals supply chains through a continuous due diligence process, combined with active participation in multi-stakeholder initiatives to promote the responsible sourcing of minerals.
Each year, Philips investigates its supply chain to identify smelters of tin, tantalum, tungsten and gold in its supply chain and we have committed to not purchasing raw materials, sub-assemblies, or supplies found to contain conflict minerals.
Philips applies collective cross-industry leverage through active engagement via the Responsible Minerals Initiative (RMI). RMI identifies smelters that can demonstrate, through an independent third-party audit, that the minerals they procure are conflict-free. In 2023, Philips continued to actively direct its supply chain towards these smelters.
The Philips Conflict Minerals Due Diligence framework, measures and outcomes are described in the Conflict Minerals Report that we file annually to the US Securities and Exchange Commission (SEC). The conflict minerals report is also publicly available on Philips’ website. Philips fully supports and complies with the OECD Due Diligence Guidance for Responsible Supply Chains of Minerals from Conflict-Affected and High-Risk Areas (OECD Guidance).
Each year, we work with our suppliers on the quality of their due diligence reporting by setting minimum criteria for the Conflict Minerals Reporting Templates (CMRT). For the 2023 Conflict Minerals Report, Philips significantly strengthened the acceptance criteria for CMRTs as it intensified the required due diligence performed by suppliers towards the use of smelters of high concern. In addition, we strive to reduce the number of non-identified smelters. As a result, the percentage of CMRTs that satisfied minimum acceptance criteria has decreased by 13 percentage points.
Philips Group
Conflict Minerals Due Diligence results
| Key performance indicator | 2021 | 2022 | 2023 |
|---|---|---|---|
| Response rate of suppliers (%) | 99% | 95% | 95% |
| CMRTs that reached minimum acceptance criteria (%) | 84% | 78% | 65% |
| Non-listed smelters in our supply chain (#) | 0 | 0 | 0 |
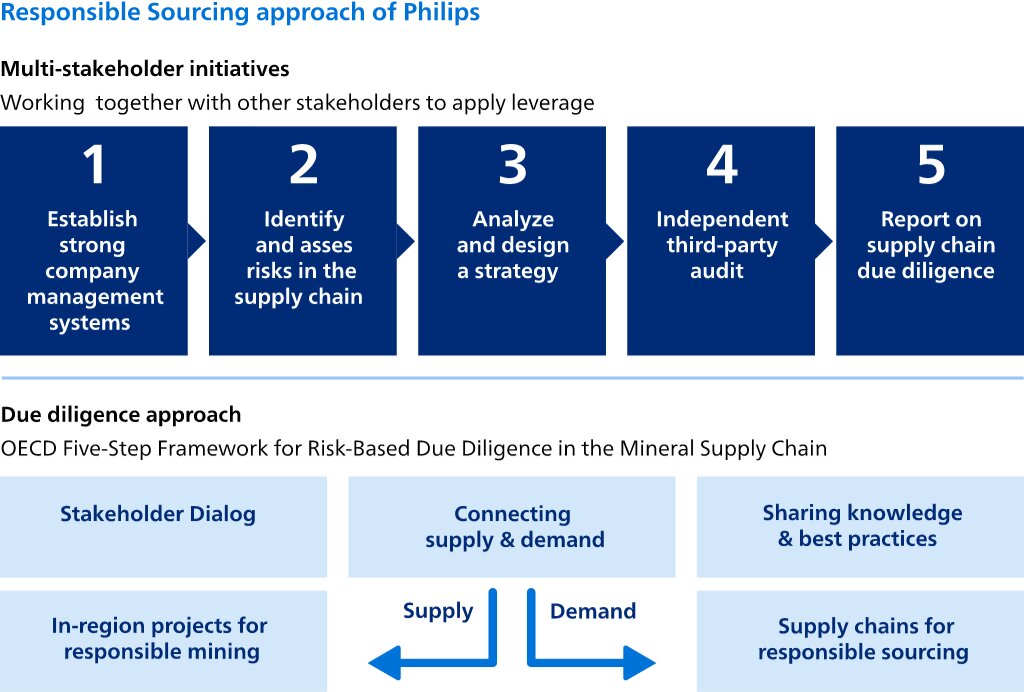
Philips has performed due diligence on cobalt since 2019. We use cobalt predominantly in lithium-ion batteries. As part of this initiative, we engaged suppliers that provide materials containing cobalt. In 2023, we again reached a 100% response rate (2022: 100%).
We believe that multi-stakeholder collaboration in the responsible sourcing of minerals is the most viable approach for addressing the complexities of minerals value chains.
European Partnership for Responsible Minerals (EPRM)Philips is a founding partner of EPRM and has been a strategic member since its inception in May 2016. EPRM is a multi-stakeholder partnership between governments, companies, and civil society actors working toward more sustainable minerals supply chains. The goal of EPRM is to create better social and economic conditions for mine workers and local mining communities by increasing the number of mines that adopt responsible mining practices in Conflict-Affected and High-Risk Areas (CAHRAs).
EPRM is an accompanying measure to the EU Conflict Minerals Regulation dedicated to making real change ‘on the ground’. Through EPRM, Philips supports activities to improve responsible mining practices in mining areas in CAHRAs and shares our knowledge and practice in conducting due diligence. Since 2018, Philips has actively participated in several working groups focused on strengthening the responsible production of minerals, as well as improving responsible sourcing practices.
Supplier decarbonization programSince 2003, Philips has looked at ways to improve the environmental performance of its suppliers. When it comes to climate change, we have adopted a multi-pronged approach: reducing the environmental impact of our products, committing to carbon neutrality in our own operations, and engaging with our supply chain to reduce their carbon footprint. Through initiatives such as the CDP supply chain program, Philips motivates its suppliers to disclose emissions, embed board responsibility on climate change, and actively work on reduction activities.
In October 2021, during COP26, Philips announced its target to have at least 50% of its suppliers (based on spend) committed to science-based targets for carbon reduction by 2025.
Philips Group
% of suppliers committed to science-based targets
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| % of suppliers committed to Science Based Targets | 28% | 41% | 46% |
We consider suppliers to have committed to science-based targets when this is communicated via the Science Based Targets initiative (SBTi), the suppliers' CDP disclosures, or public websites and announcements (on a 'Science Based Target', 'Net Zero Target', or equivalent). Multiple activities have been deployed to help us achieve this climate target. We consider spend to be relevant if it relates to product and component suppliers and relevant service providers, like logistics and information technology suppliers.
CDP engagement: Since 2011 we have been partnering with CDP Supply Chain, through which we invite suppliers to disclose their environmental performance and carbon intensity. In 2023, there was a response rate of 93% (2022: 85%). With 500 of our biggest suppliers included in the CDP engagement program in 2023, CDP confirmed Philips is in the top tier in terms of its supplier engagement coverage.
Of the group that responded, 60% engaged in emission-reduction initiatives (2022: 59%). In addition, 48% committed to carbon emission targets (2022: 47%). In the 2023 survey, our suppliers reported 14 million metric tonnes CO2 savings from improvement projects undertaken in 2023.
Philips Group
Supplier response rate to CDP questionnaire
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Supplier response rate to CDP questionnaire | 87% | 85% | 93% |
Data-driven insights: Through accurate data insights, Philips’ buyers are enabled to consider climate action in their supplier selection. In 2023, 46% of our purchases (in spend) were made at suppliers that have committed to science-based CO2 reduction targets.
Capability building: We support suppliers in advancing their company approach to climate action, offering guidance that is tailored to their climate action maturity. In 2023, we further grew the offering of tailored feedback and guidance for 80% of our suppliers to support their growth in capabilities and help improve their approach.
Opportunities for decarbonization: Through on-site assessments we identify energy efficiency opportunities that enable our suppliers to make cost-effective carbon reductions. Our team calculates for the supplier what the cost impact would be, and also the return. In 2023, 19 on-site assessments took place, which resulted in tailored plans for improvement.
To fulfill our company purpose, a responsible tax approach is required. We fully acknowledge our societal role when it comes to paying taxes in the geographies where value is created. We consider our tax payments as a contribution to the communities in which we operate, and part of our social value creation.
Our Approach to Tax sets the standard for our conduct, by which individual employees, the company and its subsidiaries must abide. We consider tax in the context of the broader society, inspired by our stakeholder dialogues, global initiatives of the Organization for Economic Cooperation and Development and United Nations, human rights, international tax laws and regulations, and relevant codes of conduct.
Under the ultimate responsibility of the Board of Management, the Chief Financial Officer annually reviews, evaluates, approves and where necessary adjusts Philips’ approach to tax. Part of our approach is to acknowledge the importance of transparency in respect of our tax contributions. Philips supports and participates in transparency initiatives such as the Dow Jones Sustainability Index (DJSI) and the Tax Transparency Benchmark of the Dutch Association of Investors for Sustainable Development (VBDO). The Tax Transparency Benchmark is a study conducted by the VBDO on tax transparency practices among Dutch and European listed companies. The 2023 benchmark assessed the tax transparency practices of 51 Dutch companies and 65 listed companies from Belgium, Denmark, France, Germany, Italy, Spain, and Sweden. Philips scored the maximum achievable 40 points. The jury noted our comprehensive Country Activity and Tax Report 2022, which explicitly linked to the GRI 207 Tax Standard and included information on environmental and social factors. Furthermore, the jury commended Philips for the clear description of the role taxes play within its value creation model. In addition, Philips scored a top score (100 out of 100) in the Tax Strategy section of the 2023 Dow Jones Sustainability Index.
Since 2020, we have been providing certain voluntary disclosures about taxes paid and collected in the countries in which we operate. The 2023 Country Activity and Tax Report is published on our website, in addition to, and simultaneously with the disclosures on tax included in this Annual Report.
Philips has a Tax Control Framework in place that forms part of its standard set of Internal Controls over Financial Reporting (ICFR). Philips' tax position is therefore reflected in its financial statements and covered by the Board of Management's report on ICRF and its conclusion regarding the effectiveness thereof, as referred to in the section Risk management and internal control.
Philips also endorses the ambitions expressed in the Tax Governance Code published by Dutch employers' organization VNO-NCW. We comply with the principles prescribed in the Code, available at www.vno-ncw.nl/taxgovernancecode, and we have touched upon the elements of this code in our Country Activity and Tax Report.
In 2023, Philips contributed to the communities where we operate through taxes paid (e.g. corporate income tax) and taxes collected (e.g. VAT). Philips' total tax contribution in 2023, amounting to EUR 3,051 million, is presented by tax type in the following table. Please refer to our 2023 Country Activity and Tax Report for more details.
Philips Group
Total contribution 2023 per tax type
in millions of EUR
| Corporate income tax paid | Customs duties | VAT1) | Payroll tax | Other taxes | Total | |
|---|---|---|---|---|---|---|
| Western Europe | (17) | 9 | 206 | 844 | 66 | 1,107 |
| North America | (31) | 38 | 123 | 721 | 8 | 859 |
| Other mature geographies | 35 | 3 | 77 | 116 | 1 | 232 |
| Growth geographies | 65 | 77 | 332 | 340 | 40 | 853 |
| Philips Group | 52 | 127 | 737 | 2,021 | 115 | 3,051 |
Stichting Philips Foundation, an independent foundation organized under Dutch law, is a registered charity established in 2014. In 2023, Royal Philips supported Philips Foundation with a contribution of EUR 6.7 million and provided the operating staff as well as the expert assistance of skilled volunteers in the execution of the Foundation’s programs.
Philips Foundation’s mission is to reduce healthcare inequality by providing access to quality healthcare for underserved communities through meaningful innovation. It does this through the provision and application of Philips’ healthcare expertise, innovation power, talent and resources and by financial support. Together with key partners around the globe (NGOs, academic partners, entrepreneurs), Philips Foundation seeks to identify challenges where a combination of healthcare technology expertise and partner experience can be used to create meaningful solutions that have a positive impact on people’s lives.
Philips Foundation works in projects (grant-based) and through impact investments (loans and equity). The instrument depends on the status and self-sustainability of the respective healthcare technology in serving the more disadvantaged communities.
Koninklijke Philips N.V. (Royal Philips), a company organized under Dutch law, is the parent company of the Philips group. Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties. The Board of Management is entrusted with the management of the company. The other members of the Executive Committee have been appointed to support the Board of Management in the fulfilment of its managerial duties. See the chapter Corporate governance of this Annual Report, where the company addresses the main elements of its corporate governance structure, reports on how it applies the principles and best practices of the Dutch Corporate Governance Code, and provides other information required under Dutch law.
Under the chairmanship of the President/Chief Executive Officer (CEO) and supported by the other members of the Executive Committee, the members of the Board of Management drive the company’s management agenda and share responsibility for the continuity of the Philips group, focusing on sustainable long-term value creation. In fulfilling their duties, the members of the Board of Management and Executive Committee are guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders.
The Supervisory Board supervises the policies, management and general affairs of Philips, and assists the Board of Management and the Executive Committee with advice on general policies related to the activities of the company, including setting and executing the strategy of the Philips Group.
Philips’ strategy, and the way it has been developed by the Board of Management under the supervision of the Supervisory Board, clearly integrates the company’s impact in the field of sustainability, including the effects on people and the environment. The Board of Management regularly convenes on ESG matters with other Executive Committee members (the Chief Operating Officer, the Chief Strategy & Innovation Officer, the Chief Human Resources Officer, the Chief Business Leader Precision Diagnosis and the Chief International Markets Market Leader) and certain functional executives. Together they define Philips’ ESG strategy, commitments, programs, targets and policies, they monitor and evaluate progress and take corrective action where needed. Progress on ESG is reported on a quarterly basis to the Audit Committee of the Supervisory Board, which assist the Supervisory Board in fulfilling its oversight responsibilities for the integrity and quality of the company’s sustainability statements,
ESG is also embedded in our core business processes, like innovation (EcoDesign), sourcing (Supplier Sustainability Program), manufacturing (Sustainable Operations), logistics (Green Logistics) and programs like the Circular Economy initiative.
Our operating model integrates key aspects of how we operate – from our strategy, structure & governance, policies, processes, systems & data, to our people & culture and performance management.
In 2023 we continued the process of simplifying the way we work to drive clear accountability and agility, and to unlock significant productivity and margin gains. This simplification – with end-to-end accountable businesses supported by a much leaner Group layer and strong Regions, together with a strengthened culture of patient- and people-centricity, innovation impact and clear accountability – is a primary enabler to create value with sustainable impact.
It is designed to help us to fulfill our purpose of improving the health and well-being of billions of people and ensure the highest standards of quality and integrity in everything we do.
The company’s risk management and internal control framework forms an integral part of the Philips business planning and performance review cycle. The purpose of our risk management is to identify and analyze the risks Philips faces in executing its strategy and activities, to set the risk appetite of the company, to take appropriate risk responses and to monitor its effectiveness. The objective of internal control is to maintain integrated management control of the company’s operations, reporting, and safeguarding compliance with applicable laws and regulations. As part of its internal control framework, Philips has implemented a standard set of Internal Controls over Financial Reporting (ICFR). Key elements of our framework include (but are not limited to) our General Business Principles, our corporate governance, authorization structures, our policy framework and internal reporting structures. Furthermore, it comprises various frameworks to help manage and control risk in line with our risk appetite. These frameworks include (but are not limited to) our Enterprise Risk Management framework (refer to Risk management), and other management systems and frameworks relevant to specific risk areas such as patient safety and quality, health & safety, environmental, tax, business continuity, information security and privacy. Please refer to Risk management for a more detailed description of Philips’ approach to risk management and more information on the risk factors that have been identified, and the risk responses that help to manage such risks in accordance with the relevant level of risk appetite.
Together with Philips’ established accounting procedures, our ICFR is designed to provide reasonable assurance that assets are safeguarded, that the books and records properly reflect transactions necessary to permit preparation of financial statements, that policies and procedures are carried out by qualified personnel, and that published financial statements are properly prepared and do not contain any material misstatements. With respect to financial reporting, a structured self-assessment and monitoring process is used company-wide to assess, document, review and monitor compliance with ICFR.
Each year, management’s accountability for ICFR is evidenced through the formal certification statement sign-off. Any deficiencies noted in the design and operating effectiveness of ICFR that were not completely remediated are evaluated at year-end by the Board of Management and the outcome reported to the Supervisory Board. The Board of Management’s report on ICFR, including its conclusions regarding the effectiveness thereof and its statement on compliance with section 404 of the US Sarbanes-Oxley Act, can be found in this report in the section Management’s annual report on internal control over financial reporting.
While pursuing our business objectives, we aim to be a responsible partner in society, acting with integrity towards our employees, customers, patients, business partners and shareholders, as well as the wider community in which we operate. To that end, our GBP – part of the Philips Operating Model – and their underlying policies incorporate and represent the fundamental principles by which all Philips businesses and employees around the globe must abide. They set the minimum standard for our business conduct as a health technology company, for our individual employees and for our subsidiaries, and Philips rigorously enforces compliance of its GBP throughout the company. Our GBP also serve as a reference for the business conduct, we expect from all our business partners.
The GBP include principles of doing business with integrity at work, integrity in the market and professional integrity outside work. They also set our integrity standard on inside information, aiming to prevent trading on or disclosure of non-public information, the publication of which would be likely to have a significant influence on the trading price of Philips securities or securities of companies that Philips is seeking to acquire. More specifically, Philips has adopted Rules of Conduct with respect to trading in Philips securities to promote compliance with applicable insider trading and other market abuse laws, rules and regulations, in particular the EU Market Abuse Regulation. The Rules of Conduct apply to all employees, the members of the Board of Management and the Supervisory Board of Royal Philips.
The GBP form an integral part of labor contracts in virtually every country in which Philips operates. Translations of the GBP text are available in 30 languages, allowing almost every employee to read the GBP in their native language. Detailed underlying policies, manuals, training, and tools are in place to give employees practical guidance on how to apply and uphold the GBP in their daily work environment. Details can be found at www.philips.com/gbp. Each year, employees reconfirm their commitment to the code of conduct after completing their GBP e-learning, and there is an additional annual sign-off for Executives. A similar sign-off is in place for Finance and Procurement staff for their respective codes of conduct.
The Executive Committee is responsible for the effective deployment of the GBP and for generally promoting a culture of compliance and ethics within the company. Furthermore, each quarter all our key Regions convene market compliance committees dealing with GBP-related matters in the local context. They are also responsible for the design and execution of localized compliance plans that are tailored to their market-specific risks and organizational set-up, and regularly review the relevant compliance metrics for their respective market through dashboards delivered by the legal compliance monitoring team. The GBP Program Office, together with a worldwide network of GBP Compliance Officers, supports the implementation of GBP initiatives.
As part of our continuous effort to raise GBP awareness and foster dialogue throughout the organization, each year a global GBP communications and training plan is deployed, including structured dialogues led by managers where quality, integrity and speaking up are discussed. This is part of a company-wide initiative aimed at reinforcing a culture of dialogue using ethical dilemma case studies that are relevant to our workforce. All functions at risk also receive annual training which includes content on, amongst others, antibribery and anticorruption and healthcare compliance via tailored case studies. Almost 60,000 (94%) of our assigned employees completed their yearly GBP e-learning. Specifically in 2023, we again focused on increasing awareness on integrity and on the importance of speaking up, through and following the deployment of our biennial Business Integrity Survey. Through this survey, more than 22,500 employees trusted us with their views and opinions on integrity within Philips. Ninety-four percent of employees expressed the belief that we act with integrity with Philips. To gain deeper insights into the results of the Business Integrity Survey, we execute deep-dive initiatives amongst our employees.
A key control to measure implementation of our GBP is the GBP monitoring and reporting program, which is part of our Internal Control framework. In addition, we continue to expand the capabilities of our legal compliance monitoring team, serving our business customers as well as our compliance networks with actionable data, thus further improving our compliance control framework.
The GBP are supported by established mechanisms with the aim of ensuring standardized reporting and enabling employees and third parties to escalate concerns 24/7. Concerns raised are registered consistently in a single database hosted outside of Philips servers to ensure confidentiality and security of identity and information. Encouraging people to speak up through the available channels if they have a concern will continue to be a cornerstone of our GBP communications and awareness campaigns. At least twice a year, the Executive Committee and Audit Committee of the Supervisory Board are informed on relevant GBP metrics, cases, trends and learnings.
In 2023, a total of 764 concerns were reported via Philips Speak Up (Ethics Line) and through our network of GBP Compliance Officers. This represents an increase of 8% from the total of 706 concerns in the previous reporting period (2022). This is a continuation of a year-on-year upward trend.
Through the Audit Committee of the Supervisory Board, the company also has procedures in place for the receipt, retention and treatment of complaints specifically relating to accounting, internal accounting controls, or auditing matters, enabling the confidential, anonymous submission of complaints.
Our remuneration policy is designed to encourage employees to deliver on our purpose and strategy and create stakeholder value, and to motivate and retain them. Our executive long-term incentive plan includes environmental and social commitments. A description of the composition of the remuneration of the individual members of the Board of Management and the Supervisory Board is included in the Report of the Remuneration Committee.
Enabling the delivery of patient-centric, safe, and high-quality care – the essence of patient safety and quality – is foundational to Philips' purpose to improve the health and well-being of people through meaningful innovation. This year we formed the Patient Safety and Quality organization, which brings together the Quality, Regulatory Affairs, and Clinical and Medical functions as one unified team. Positioned to support and enable the entire Philips organization and to foster the quality culture at Philips, the Patient Safety and Quality team is instrumental in ensuring we have the capabilities, processes, and tools required for operating in a highly regulated healthcare technology industry.
Throughout 2023, we continued to accelerate our work in crucial areas, with the goal of achieving and maintaining the highest level of patient safety and quality. Specific areas of focus included: preparation and manufacturing site readiness for audits and inspections; a review of quality records; new ways of undertaking product quality reviews; planning for IT and data enhancements; and simplification of our process framework and Quality Management Systems.
This year, we created the new role of Chief Patient Safety and Quality Officer, who is a member of the Philips Executive Committee and reports directly to the Chief Executive Officer. Additionally, we hired a new Chief Medical Officer to lead the team that is focused on clinical research, medical safety, and medical support for our global businesses.
Early in 2023, we organized CAPA management, Quality Management Systems, compliance training, internal quality audits, and other crucial areas for patient safety and quality into a Compliance and Quality shared service team. We set up a Transformations team, responsible for project management of Patient Safety and Quality programs. Regulatory Affairs formed new teams that are responsible for expanding our engagement with external stakeholders and regulators, and for the delivery of effective tools, processes, and services to facilitate timely and compliant market access. We appointed a new leader for Product Safety and Surveillance, Corrections and Removals, and strengthened our post-market processes and ways of working.
Across Philips, teams in all Businesses, Regions, and Functions continued to foster a quality culture and mindset, where all employees are encouraged to speak up and share ideas for improving the safety and efficacy of our products. In October, our employees took part in a Timeout for Patient Safety and Quality to solidify commitment and planning.
QualityWe strive to continuously raise our performance to deliver safe and high-quality products, services, and solutions, which are compliant with quality and safety standards and all applicable laws. In 2023, as part of our plan to create value with sustainable impact, we introduced a new operating model that enables us to simplify how we work and improve accountability and ownership. We are strengthening our engineering capabilities for new product development in areas such as quality systems engineering, reliability and software design.
We further reduced the number of Quality Management Systems in which we operate to increase focus, reduce complexity, and minimize risk. This year we closed nine QMS for a total of 66 Quality Management Systems by year-end 2023, with further significant reductions planned for 2024.
In 2023, our Accelerating Patient Safety & Quality program instituted an improved approach for management of skills and launched a training program on patient safety and quality topics. In collaboration with business units and Innovation & Strategy, we established programs to improve product design and reliability.
All Philips businesses are accountable for patient safety and quality. This year, we instituted a new Patient Safety and Quality performance review meeting with each business and at the Philips level in aggregate. We set Patient Safety and Quality key performance indicators for the company, and Quality performance metrics are part of the remuneration of all Philips Executives. Additionally, every employee has a patient safety and quality goal as part of people performance management.
Regulatory AffairsUnder Philips' new operating model, Regulatory Affairs sits on the leadership team of each Business and Region as a key partner. In 2023, we established internal governance and requirements for engagements with national government regulatory authorities (e.g. the US Food and Drug Administration (FDA), European Medicines Agency (EMA), China National Medical Products Administration, Notified Bodies, and National Competent Authorities in the European Union.
As a global business in a dynamic regulatory environment, we must ensure compliance with evolving regulations related to innovations in areas such as Artificial Intelligence, and healthcare informatics and software design. This year, we increased levels of investment in regulatory science and policy and in enterprise informatics. Teams are working on global harmonization of requirements, safe and innovative applications of AI, and secure transmission and storage of protected health information. Sought as strategic partners, the Regulatory Affairs team participated in international consensus standards groups alongside regulators and engaged with international regulators as invited experts and speakers at the International Medical Device Regulators Forum, Global Harmonization Working Party, and other meetings. We are working with the National Institutes of Health to establish ethical applications of Artificial Intelligence in medical devices.
In 2023, we also increased our collaborations with organizations supporting regulatory science like the Food, Drug, and Law Institute and the Regan-Udall Foundation for the FDA. In partnership with the Boston Globe and the Washington Post, we hosted an industry and customer panel on Transforming Healthcare with AI: Harnessing Technology to Promote Safety and Quality for Patients. Regulatory Affairs leaders moderated panel discussions among key international regulators and were featured speakers at events such as MedTech Europe and the AdvaMed annual conference.
Throughout 2023, we continued transitioning our portfolio to become European Union Medical Device Regulation (EU-MDR)-compliant. In March 2023, the grace period was extended until year-end 2027 or 2028, under strict conditions and depending on risk class. We continue to use this available grace period for placing a portion of our portfolio on the market under the European Union Medical Device Directive (EU-MDD).
Regulatory Affairs deployed a digital regulatory information management tool to all Regions in 2023, with continued deployments planned to Businesses in 2024. This tool will be a single source for regulatory data that will help increase speed to market.
Medical OfficeIn 2023, we formed a centralized medical office with expertise in clinical research, medical safety, healthcare economics, and a breadth of care specialties. This team is focused on supporting the global business, navigating the intricacies of addressing patients' and customers’ unmet needs across a variety of ecosystems, and looking across the entire product lifecycle to help teams develop solutions that are safe, effective, and relevant for patients, providers, and payers.
In 2023, we hosted our second Patient Safety Advisory Board, where external leaders from across the world, from a variety of professional backgrounds, participated in intensive workshops focused on themes identified as key to improving the safety and efficacy of our solutions. Experts from outside and within Philips exchanged ideas ranging from the role of innovation in human interface models, patient safety and clinical education programs and capability building at Philips, and other topics that are key to our overall way forward in innovation and safety. This year, Philips also introduced an extensive Clinical and Medical curriculum, available to all employees.
The team continued our advocacy and investment in topics such as radiation safety, medical device testing, and improved access to physician and staff training. At Harbor-UCLA Lundquist Institute, we host a facility for R&D pre-clinical testing, clinical and medical education proctoring, and fellows training. Our team continues to design, generate, and disseminate clinical and economic evidence to show the value of our solutions in improving patient outcomes.
Our Health Economics and Outcomes Research team continued to contribute economic evidence to support innovation and expand access to high-quality care, authoring 12 publications and 18 studies during 2023. The Ambulatory Monitoring & Diagnostics business unit increased in-network access to ambulatory patient monitoring for an additional 35 million patients in the United States through payer contracting initiatives. Our Image Guided Therapy-Devices team advocated with the Centers for Medicare and Medicaid (CMS) to improve Medicare payment to ambulatory surgery centers for cardiovascular and peripheral vascular treatments, ensuring patients have access to care in the setting that their providers determine is most appropriate.
Philips Respironics voluntary recallOn June 14, 2021, Philips’ subsidiary, Philips Respironics, initiated a voluntary recall notification in the United States, and field safety notice outside the US, for certain sleep and respiratory care products related to the polyester-based polyurethane (PE-PUR) sound abatement foam in these devices.
Since June 2021, together with five independent, certified testing laboratories and third-party experts, Philips Respironics has conducted extensive testing. Based on the results to date, Philips Respironics and the third-party experts concluded that use of the sleep therapy devices is not expected to result in any appreciable harm to health in patients. Further testing remains ongoing. Following ongoing communications, Philips Respironics agreed in October 2023 to the FDA’s recommendations to implement additional testing on the sleep and respiratory care devices to supplement current test data. Philips Respironics is in discussions with the FDA on the details of that further testing. Further testing remains ongoing. Since the start of the test and research program, Philips Respironics has endeavored to work cooperatively with the FDA on the program and to publish regular test updates as agreed with the FDA.
Following the FDA’s inspection of a Philips Respironics manufacturing facility in the US and the subsequent inspectional observations, the US Department of Justice (DOJ), acting on behalf of the FDA, began discussions with Philips in July 2022 regarding the terms of a proposed consent decree. On January 29, 2024, as part of the announcement of Philips’ fourth quarter and full year 2023 financial results, the company provided an update stating that Philips agrees on the terms of a consent decree with the DOJ and FDA. The consent decree primarily focuses on Philips Respironics’ business operations in the US. The consent decree is being finalized and will be submitted to the relevant US court for approval. The decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business.
In the US, Philips Respironics will continue to service sleep and respiratory care devices already with healthcare providers and patients, and supply accessories (including patient interfaces), consumables (including patient circuits), and replacement parts (including repair kits). Until the relevant requirements of the consent decree are met, Philips Respironics will not resume selling new CPAP or BiPAP sleep therapy devices or other respiratory care devices in the US. Outside the US, Philips Respironics will continue to provide new sleep and respiratory care devices, accessories, consumables, replacement parts and services, subject to certain requirements. Further details will become available once the proposed Respironics consent decree has been finalized and submitted to the relevant US court for approval. For more information, refer to Contingencies.
In 2023, we also maintained manufacturing capacity to produce the necessary devices and rework kits required for remediation of the Respironics recall. As of December 31, 2023, over 99% of the sleep therapy device registrations that are actionable had been remediated, while the remediation of the ventilator devices remains ongoing. We expect to continue such remediation activities in 2024.
Philips Respironics regularly communicates on product remediation and testing associated with the recall with global regulators and customers through a variety of channels. Philips Respironics continues to monitor complaints received following the recall/field safety notice via our Quality Management Systems, in accordance with the medical devices regulations and laws.
Consent decree – Emergency Care & ResuscitationIn October 2017, Philips North America LLC reached agreement on a consent decree with the DOJ, representing the FDA, related to compliance with current good manufacturing practice requirements arising from inspections conducted in 2015 and prior. The consent decree focuses primarily on Philips' Emergency Care and Resuscitation (ECR) business operations in Andover, Massachusetts, and Bothell, Washington.
Following a successful inspection in Bothell, Washington, in April 2020, the FDA determined Philips had met the conditions for resuming manufacturing and distribution of defibrillators in the US. The consent decree remains in effect for several years, during which the Emergency Care (formerly Emergency Care and Resuscitation) business unit will be subject to a series of annual assessments by an independent expert. Hospital Patient Monitoring (formerly Monitoring & Analytics), also named in the consent decree, is also under a heightened level of scrutiny over the same period.
We continue to make substantial progress in our compliance efforts. In October 2022, the FDA inspected Emergency Care in Bothell as a consent decree follow-up. Three observations (Form 483) were issued and subsequently remediated and reported to the FDA. In June 2023, Emergency Care in Bothell received the October 2022 Establishment Inspection Report marking closure. In late October 2023, the FDA conducted a follow-up inspection to the 2022 inspection that resulted in a 483 with two observations, which were rapidly addressed in formal responses and subsequently acknowledged by the FDA the first week of January 2024 as closed. Efforts in 2024 are focused on ensuring the sustained demonstrated state of substantial compliance to support seeking formal relief in the first half of 2025, which is the earliest time allowed in the consent decree.
We cannot predict the outcome of this matter, and the consent decree authorizes the FDA, in the event of any violations in the future, to order us to cease manufacturing and distributing Emergency Care or Hospital Patient Monitoring devices, recall products, pay liquidated damages, and take other actions. We cannot predict whether additional monetary investment will be incurred to resolve this matter or the matter’s ultimate impact on our business.
As a health technology company operating in a highly competitive industry, Philips’ brand reputation depends on the safety, quality and security of our products and services. Failure to meet cybersecurity standards may cause patient harm, negatively impact customer operations and their ability to provide healthcare or provide unauthorized access to patient records and medical devices. Philips furthermore relies on information technology to operate and manage its businesses, as well as store and process confidential data (relating to patients, employees, customers, intellectual property, suppliers and other partners). For a discussion of cybersecurity risks facing our business, see “Products and services may fail quality or security standards, which could adversely affect patient safety or customer operations" and “Philips could be exposed to a significant enterprise cybersecurity breach” in section Operational risks. As of the date of this Annual Report, there are no breaches of cybersecurity or other related risk threats that have, or are reasonably likely to, materially affect our business.
Security risk management is part of our broader risk management processes, and its aim is to protect the confidentiality, integrity, and availability of Philips’ products and services. To this end, the company has established a Group Security function and implemented security management processes, requirements and controls for the assessment, identification and management of material risks from, amongst others, cybersecurity threats. Our Head of Group Security, reporting to our Chief Financial Officer, leads the Group Security function in supporting the Board of Management in evaluating and setting the Group’s security strategy, issuing security policies and evaluating the progress and effectiveness of the deployment of the company’s security management framework. Our Chief Information Security Officer, reporting to our Head of Group Security, has nearly 26 years of technology and information security management experience in the industry, including prior roles with the Dutch Government and multinationals in the Consumer goods, Manufacturing, Chemical and Food processing industries, in various roles ranging from Chief Information Security Officer to IT security officer and Security Architect. Our Chief Information Security Officer is informed of and monitors the prevention, detection, mitigation and remediation of cybersecurity incidents through the Global Security Operations Center.
The company’s security management framework, including its cybersecurity policies and procedures, is maintained by the Group Security function and is designed to implement security requirements into all applicable business processes, information processing systems and infrastructure pertaining to our products and services and our supporting and enabling functions, during the entire lifecycle. The framework includes risk, vulnerability and penetration assessments, mandatory yearly security training for all employees, (including new hires Regular Phishing simulations for all employees three times a year), monitoring and response activities for vulnerabilities identified in products, services and infrastructure.
The Group Security function is also responsible for addressing security risks, including monitoring cybersecurity threats and responding to cybersecurity incidents. Philips’ Global Security Operations Center monitors the prevention, detection, mitigation and remediation of cybersecurity incidents on global enterprise systems, supported by certain external services and periodic/intermittent assessments. The severity and materiality of incidents are assessed through a dedicated security incident reporting process and, if necessary, incidents are escalated through central crisis management and (potentially) to the Philips Disclosure Committee, which assesses the need for public disclosure of (material) incidents. Incidents, where needed, are further escalated to Global Crisis Management.
Additionally, in order to address the security risks associated with our suppliers and the services they provide, security controls are embedded in our procurement and supplier management processes, covering due diligence when engaging with new suppliers, contracting, monitoring and managing existing supplier relationships, and terminating supplier relationships. These security controls check for existing security certificates and assurances reports for the services in scope, validate suppliers’ answers to security questionnaires in due diligence, and ensure that security schedules are part of the signed contracts.
The Board of Management is ultimately responsible for Philips’ cybersecurity management, which is overseen by the Supervisory Board (and specifically its Audit Committee). As part of this process, quarterly reports on cybersecurity risks and incidents are prepared by the IT Audit & Risk Committee (consisting of representatives from the Group Security and Group IT functions, Philips Internal Audit and the external auditor and submitted to the Board of Management and the Supervisory Board. This reporting includes the overall risk level, relevant changes in the risk environment, challenges in reaching and/or maintaining current risk levels and actual risk responses in the form of actions and owners.
Below we show how Philips performed in 2023 on the 21 Core metrics of the WEF ESG reporting framework, mapped to the three dimensions of our ESG commitments, as well as a number of additional Philips-specific metrics that we consider fundamental to the strategy and operation of our business.
Philips approaches risk management as a value-creating activity that is integral to innovation and entrepreneurship. As such, it is part of the Philips Operating Model. The key elements of our risk management and control system are described in this chapter. There can be no absolute assurance that our risk management will avoid or mitigate all risks that Philips faces. The material risks are described in the section Risk factors.
Risk management governanceThe Board of Management (BoM) is ultimately responsible for identifying, analyzing and managing the risks Philips faces in executing its strategy and activities, for setting the risk appetite of the company, and for the design, implementation and maintenance of our risk management and control system, including the monitoring of its effectiveness. As described below, the Executive Committee (ExCo), several experts, Enterprise functions and committees support the BoM in the discharge of its responsibilities.
The ExCo is primarily responsible for identifying and mitigating materials risks to Philips. The ExCo is supported by the Risk Management Support Team, consisting of experts on various categories of risk, through regular analysis of the enterprise risk profile and enhancement of the risk management framework. In addition, management across the company is responsible for identifying critical risks and implementing appropriate risk responses within their areas of responsibility.
Various Enterprise functions (e.g. Legal & ESG, Patient Safety & Quality, Finance and Group Security) support the ExCo and management with the process of risk identification, risk management, and monitoring of key risk areas. With the support of these functions certain designated frameworks and activities to structurally manage specific risk areas are maintained and deployed, such as:
For further details refer to the section Governance.
To ensure clarity and alignment on the status of, and to make recommendations on key risk areas these functions have recurring items on the BoM meeting agenda. The BoM discusses these topics with participation from relevant ExCo members and other senior executives and subject matter experts. Furthermore, dedicated reports on these key risk areas are shared and discussed with the Supervisory Board and external auditors in the relevant Audit & Risk Committees facilitated by Internal Audit.
The Internal Audit function assesses the quality of risk management and controls through the execution of a risk-based audit plan, as approved by the Audit Committee of the Supervisory Board. The BoM and leadership from Businesses, Regions/Zones and key Functions meet quarterly with Internal Audit in Audit and Risk Committees to discuss strengths and weaknesses of risk management and controls – as evaluated by internal and external auditors and by means of other (self) assessments – and take corrective action where necessary.
The Disclosure Committee oversees the company’s disclosure activities and assists the BoM in fulfilling its responsibilities in this respect. The Disclosure Committee seeks to ensure that the company implements and maintains internal procedures for the timely collection, evaluation, and disclosure of information potentially subject to public disclosure under the legal, regulatory and stock exchange requirements to which the company is subject.
The Supervisory Board oversees Philips’ risk management including the identification of material risks, in relation to the risk appetite of the company, the response measures put in place and the effectiveness thereof. The Audit Committee and the Quality & Regulatory Committee of the Supervisory Board assist the full Supervisory Board in fulfilling its risk management oversight responsibilities. The Audit Committee reviews the quality of risk management and controls, and the reported findings of internal and external audits. The Quality & Regulatory Committee’s role particularly relates to the quality and regulatory compliance of the company’s products (including software), services and systems throughout their lifecycle.
The chapter Corporate governance of this report addresses the main elements of the company’s corporate governance structure, reports on how it applies the principles and best practice provisions of the Dutch Corporate Governance Code and provides other information relevant to risk management governance.
Risk appetitePhilips seeks to manage risks consistently within its risk appetite. Risk appetite is set by the BoM, reviewed at least annually and published in the Philips risk management policy. It is effectuated through our Operating Model, of which various elements – such as our strategy, Philips General Business Principles (GBP) and behaviors, authority schedules, policies, process standards and performance management systems – include or reflect risk-taking guidance.
Philips’ risk appetite differs depending on the type of risk, ranging from an averse to a seeking approach. Philips operates within the dynamics of the health technology industry and aims to take the risks needed to ensure we continually revitalize our offerings and the way we work. At the same time, Philips is committed to act with integrity always and is averse to risks impacting our GBP, which include (but are not limited to) the Philips behavior ‘Patient safety, Quality, and Integrity always’. Our employees are expected to ensure compliance with our GBP, laws, and regulations, and to take action in the case of concerns or violations to our GBP. Please refer to the Philips General Business Principles (GBP) for more information. Philips’ risk appetite for the main risk categories is visualized below. Philips does not classify these risk categories in order of importance.
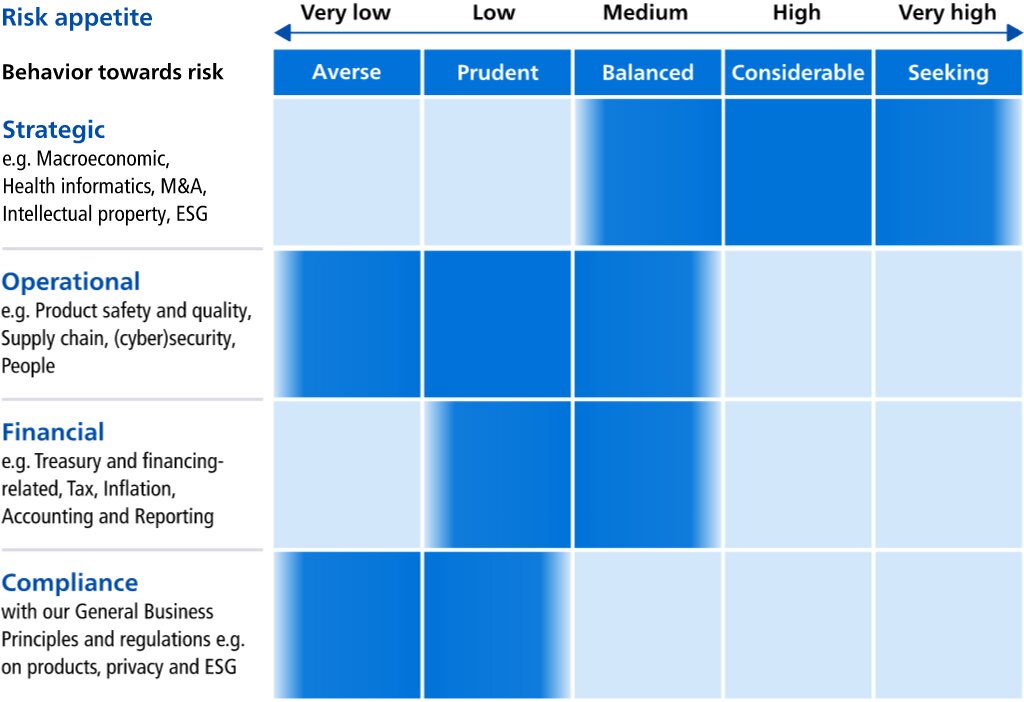
To provide a comprehensive view of Philips’ risks, structured risk assessments take place according to the Philips risk management process standard, applying a top-down and bottom-up approach. Our process standard is designed based on ‘Enterprise Risk Management: Integrating with Strategy and Performance’ (2017) from the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and on ISO 31000 - Risk Management.
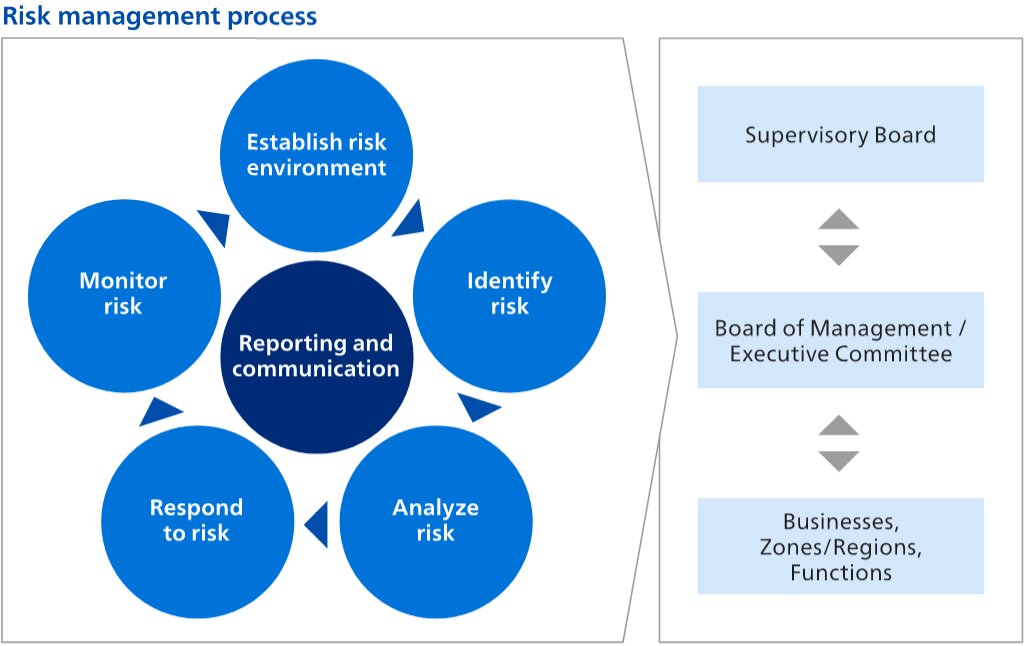
Key elements of the Philips risk management process are:
The measures taken during 2023 to further strengthen risk management include:
Philips believes the risks set out below are the material risks affecting Philips and its securities. These risk factors may not, however, include all the risks that ultimately may affect Philips. Some risks not yet known to Philips, or currently believed not to be material, may ultimately have a major impact on Philips’ business, revenue, income, assets, liquidity, capital resources, reputation and/or ability to achieve its business and ESG objectives. Please note that this section is not intended to describe risks that have materialized, as these are addressed in other sections and referenced where relevant. Philips defines risks in four main categories: Strategic, Operational, Compliance and Financial. Philips presents the risk factors within each category in order of our current view of their expected significance. Compared to the previous year we have further prioritized risk factors relating to patient safety and quality, supply chain, and the simplification of how we work. Although still relevant, we have de-emphasized risk factors related to pandemics. This does not mean that a lower-listed risk factor may not have a material and adverse impact on Philips’ business, revenue, income, assets, liquidity, capital resources, reputation, and/or ability to achieve its business and ESG objectives. Furthermore, other risk factors not listed below may ultimately prove to have more significant adverse consequences than the listed risk factors.
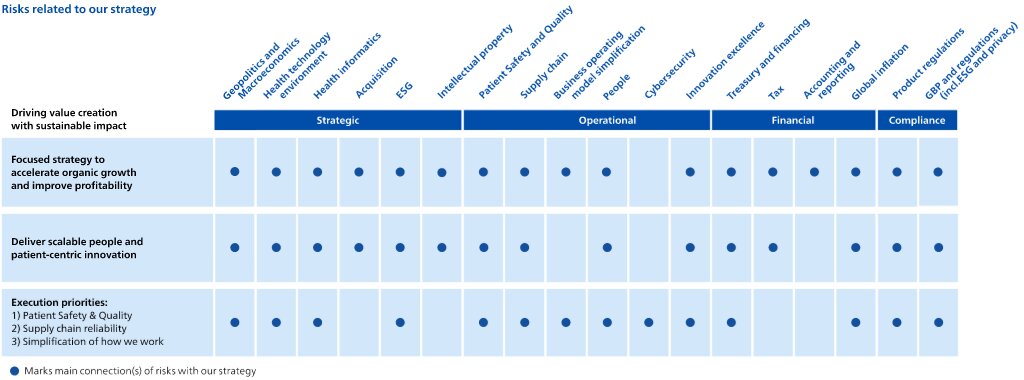
Philips’ business environment can be adversely impacted by macroeconomic and geopolitical conditions in global and individual markets. In 2023, Mature geographies accounted for 72% of Philips’ revenue, while Growth geographies accounted for the remaining 28%. Mature economies are currently the main source of Philips’ revenues, while growth economies are an increasing source of revenues. Philips produces, sources, and designs its products and services mainly from the United States (US), the European Union (EU) (primarily the Netherlands) and China, and the majority of Philips’ assets are located in these geographies. Changes in politics and monetary, trade and tax policies in the US, the EU and China may trigger reactions and countermeasures and may also have an adverse impact on other economies and international markets in which Philips is active. Philips continues to expect global market conditions to remain highly uncertain and volatile due to geopolitical and macroeconomic factors, whether or not they are related to or caused by the Russia-Ukraine war and/or the current situation in Israel and the larger Middle East region.
Philips observes a trend of geopolitical tensions and deglobalization which intensifies protectionism. Examples of protectionism measures are trade policies, tariffs, sanctions, local value creation and production requirements to obtain market access, custom duties, taxation, technology and data restrictions, cyberattacks, import or export controls, talent mobility restrictions, nationalization of assets, restrictions on repatriation of returns from foreign investments. In addition, there is general uncertainty on the development of local regulations and compliance thereto. Philips observes this trend in the major markets in which it operates and has a particular concern on the development of the US-China relationship and China’s drive to expand its global political footprint and become self-sufficient in critical technologies, including health-related ones.
If this trend continues, geopolitical relations deteriorate, and economies decouple, it is expected that existing global trade and investment restrictions will remain, and further regulatory and compliance challenges for doing business globally may emerge, resulting in continued pressure on market growth and investments.
Uncertainty and challenges regarding various global macroeconomic factors continue to persist. Examples of general factors are an overall weakening economic growth outlook, reduced government spending, declining customer and consumer confidence and spending, high inflation and interest rates, and the emergence of economic impacts related to the climate crisis. Although the ability to manage pandemics (for example, resurgences of COVID-19 or mutations thereof) has improved, pandemics may continue to affect Philips’ operations in the future. Examples of healthcare-specific potential factors include rising uncertainty over the future direction of public healthcare policy and the risk of declining public investment in healthcare ecosystems.
The Russia-Ukraine war has increased global economic and political uncertainty. Governments in the US, the UK, the EU, Canada, and Japan have each imposed export controls on certain products and sanctions on certain industry sectors and institutions in Russia, and additional controls and sanctions could be enacted in the future. Similarly, the conflict in Israel will further increase economic and political uncertainty and may affect the company’s results of operations, financial position and cash flows. Philips is present in Israel with several subsidiaries, mainly in Diagnosis & Treatment and Connected Care, that are primarily involved in manufacturing and research and development (R&D) activities. Upcoming elections in the US, the UK and the EU could also have an impact on the course of these conflicts. The ongoing conflicts may heighten the impact of other risks factors described herein, including but not limited to: volatility in prices for transportation, energy, commodities and other raw materials; disruptions in the global supply chain; decreased customer and consumer confidence and spending; increased cyberattacks; intensified protectionism; political and social instability; increased exposure to foreign currency fluctuations; rising inflation and interest rates; and constraints, volatility or disruptions in the credit and capital markets. It is possible that the conflict in Ukraine may escalate or expand and current or future sanctions and resulting geopolitical and macroeconomic disruptions could be significant. We cannot predict the impact the conflict may have on the global economy in the future.
Changes in geopolitical and macroeconomic conditions are difficult to predict, and the factors described above, or other factors, may lead to adverse impacts on global trade levels and flows, economic growth, and financial market and political stability, all of which could adversely affect the demand for, and supply of, Philips’ products and services. This may result in a material adverse impact on Philips’ business, financial condition, and operating results. These factors could also make it more difficult to budget and to make reliable financial forecasts or could have a negative impact on Philips’ access to funding.
Philips may be unable to keep pace with the changing health technology environmentWith Philips’ focus on health technology, our business model is transforming from transactional, product-focused business models to customer- and patient-centric, outcome-oriented business models, with multi-year customer partnerships enabled by a portfolio of innovative devices, solutions, platforms, insights and value-added services. If this transformation is made too slowly or is not successful, Philips may not meet the expectations of patients and other stakeholders in the health technology business environment. We may face a loss of customer relevance, fail to capture growth, and lose market share. In addition, because of our health technology focus, Philips may have a reduced ability to offset potential negative impacts (including, but not limited to, impacts on sales, operating results, liabilities, compliance, and financing) on its health technology business by other businesses through a more diversified portfolio. As a result of its focus on health technology, Philips is deepening customer engagement and entering into long-term solutions and services business arrangements and, as a result, is becoming more dependent on a number of key customers for long-term recurring revenues, thus increasing the risk that the loss of, or a significant reduction in, orders from one or more of our key customers could cause a significant decline in our revenues. As Philips looks to increase our use of indirect sales channels, Philips will increasingly rely on successfully leveraging new and existing partners to support end customers and patients. Any of these factors may have a material adverse impact on Philips’ brand value and reputation, business, financial condition, and operating results. More specific health technology risks and their potential impacts are included in the Operational, Financial and Compliance risk sections below as well as in the note Contingencies.
Philips may be unable to gain leadership in health informaticsNew digital technologies and ways of conducting business are fundamentally changing the health technology industry, and thus our competitive business environment. A key trend, started in radiology, is the application of artificial intelligence (AI) and machine learning (ML) to drive quality and efficiency in clinical and operational workflows. Customers need workflow-aware solutions that convert data from our imaging and monitoring systems into actionable insights. Another trend, accelerated by the pandemic, is the shift toward cloud-based Software as a Service (SaaS) business models and remotely upgradable and serviceable systems with suites of apps. These new types of offerings are enabled by hybrid cloud/on-premise digital platforms. Our informatics and systems businesses may fall behind established and new ‘born digital’ competitors if Philips fails to, in a timely way, develop the requisite capabilities, adjust its business models, and find ways to globally commercialize new products and services at scale. This could result in an inability to satisfy customer and patient needs, thereby missing out on revenue and margin growth opportunities, which may have a material adverse impact on Philips’ business, financial condition and operating results.
Acquisitions could fail to deliver on Philips’ business plans and value creation expectations, and we may not be able to successfully integrate acquired operationsAlthough Philips focusses on organic growth to deliver patient and people driven innovation at scale, selected acquisitions remain part of Philips’ growth strategy. We may not be able to integrate acquisitions successfully or efficiently with our existing operations, culture and systems, which may expose Philips to risks in areas such as sales and service, logistics, quality, regulatory compliance, legal claims, information technology, and finance. Integration challenges may adversely impact the realization of value creation expectations. Transactions may incur significant costs, result in unforeseen operating difficulties, divert management attention from other business priorities, and may ultimately be unsuccessful. Cost savings expected to be implemented, or other assumptions underlying the business case relating to a particular acquisition, may not be realized. If we are unable to accomplish any of our objectives in respect of any of our new acquisitions, we may not realize the anticipated benefits of such acquisitions and we may experience lower than anticipated profits, or even incur losses. Acquisitions may also lead to a substantial increase in long-lived assets, including goodwill, which may later be subject to write-down if an acquired business does not perform as expected, which may have a material adverse effect on Philips’ earnings.
Philips may be unable to meet internal or external aims or expectations with respect to ESG-related mattersEnvironmental, Social and Governance (ESG) factors may directly and indirectly impact the business environment in which Philips operates. Philips may, from time to time, disclose ESG-related initiatives or aims in connection with the conduct of its business and operations (for example, with respect to reducing greenhouse gas emissions in its supply chain). However, there is no guarantee that Philips will be able to implement such initiatives or meet such aims within anticipated timeframes, or at all. In addition, there is an increasing focus from Philips’ stakeholders – including customers, employees, regulators, and investors – on ESG matters, and those stakeholders may also have ESG-related expectations with respect to Philips’ business and operations. For example, customers may focus on ESG-related criteria in buying our products, and any inability by Philips to address concerns about ESG-related matters could negatively impact sentiment towards Philips and our products and brands. There are an increasing number of regulatory and legislative initiatives in the EU and other jurisdictions to address ESG issues, which will (once implemented) require Philips to significantly increase the scope of mandatory ESG disclosures, including the Corporate Sustainability Reporting Directive (CSRD), European Sustainability Reporting Standards (ESRS) and the SEC’s proposed climate disclosure rules. They will introduce or extend a duty of care, requiring Philips to identify and act on adverse environmental and human rights impacts across the organization and potentially the entire value chain, beyond or different from our current efforts. These regulatory and legislative initiatives, in turn, could also affect how customers or other stakeholders perceive our products or business operations. If our products or business operations do not meet the criteria for sustainability according to, for example, the EU Taxonomy Regulation (including the related delegated regulations) or any other similar regulations, this may negatively affect how customers or other stakeholders view Philips. Philips may fail to fulfill internal or external ESG-related initiatives, aims or expectations, or be perceived to do so, or we may fail to report performance or developments adequately or accurately with respect to such initiatives, aims or expectations. In addition, Philips could be criticized or held responsible for the scope of its initiatives or goals regarding ESG matters. Any of these factors may have an adverse impact on Philips’ reputation and brand value, or on Philips’ business, financial condition and operating results.
Philips may be unable to secure and maintain intellectual property rights for its products and services or may infringe others’ intellectual property rightsPhilips is dependent on its ability to obtain and maintain licenses and other intellectual property (IP) rights covering its products and services and its design and manufacturing processes. The IP portfolio is the result of an extensive IP generation process that could be influenced by a number of factors, including innovation and acquisitions. The value of the IP portfolio is dependent on the successful promotion and market acceptance of standards (co-)developed by Philips. This is particularly applicable to the segment ‘Other’, where licenses from Philips to third parties generate IP royalties and are important to Philips’ results of operations. The timing of licenses from Philips to third parties and associated revenues from IP royalties are uncertain and may vary significantly from period to period. Additionally, royalties are often based on sales by third parties, creating an exposure to macroeconomic effects and continuity of these third parties. A loss or impairment in connection with such licenses to third parties could have a material adverse impact on Philips’ financial condition and operating results. Philips is also exposed to the risk that a third party may claim to own IP rights to technology applied in Philips’ products and services. If any such claims of infringement of these IP rights are successful, Philips may be required to pay damages to such third parties or may incur other costs or losses.
The safety of patients and our brand reputation depends on the safety and quality of our products and services. Failure to meet product quality and security standards may cause patient harm, negatively impact customer operations and their ability to provide healthcare, provide unauthorized access to patient records and medical devices through cybersecurity incidents, and damage Philips' reputation and brand.
As a health technology innovator, our products and services must comply with rules and regulations that govern our operations, processes, and ways of working. Risks associated with non-compliance with quality, regulatory, and security assessments apply to pre-market activities (such as product design, production and supplier quality activities) and post-market activities. There are risks involving hardware, software and human error, spanning across the lifecycle, and involving third-party suppliers and components. Many of our products have multiple third-party software components, which may be exposed to security threats. We are subject to risk from known issues, and emerging potential issues. Potential consequences of these risks include damage to our brand reputation, competitive disadvantage, consent decrees (for example, the proposed Respironics consent decree described in note Subsequent events to the Consolidated financial statements), and losing our licenses to operate in specific markets, all of which may have a material adverse impact on Philips' business, financial condition, and operating results.
Philips may be unable to ensure a resilient supply chainMost of Philips’ operations are conducted internationally, which exposes Philips to supply chain challenges and uncertainties. Philips produces and procures products and parts in various countries globally. The production and shipping of products and parts, whether from Philips or from third parties, could be interrupted and may face increasing costs by various external factors, such as regional conflicts (e.g. the Middle East region), natural disasters, extreme weather events (the effects of which may be exacerbated by climate change) and geopolitics.
While macro trends around materials availability have improved in 2023, Philips’ medical systems stay in production for longer periods than the lifecycle of their semi-conductors and require continuous rejuvenation of their electronic components. Philips may fail to timely obtain or replace such components from existing supplies, and alternative sources of components could involve significant costs and regulatory challenges and may not be available to us on reasonable terms, adversely affecting our business and financial performance.
Our suppliers and our third-party service providers may also be exposed to labor shortages and potentially worsening macroeconomic and geopolitical trends. These factors may cause increased lead times and adversely impact our production capacity, which may negatively affect the delivery of products and services to customers, for example the postponement of equipment installations in hospitals. If Philips is not able to respond swiftly to those factors, this may result in an inability to deliver on customer needs, ultimately resulting in loss of revenue and margin.
Philips purchases raw materials, including rare-earth metals, copper, steel, aluminum, noble gases and oil-related products. While the macroeconomic trend of improved materials availability also positively impacts the raw materials and energy cost compared to 2022, there is no assurance that these raw materials will be available for purchase in the future or available at current costs.
Commodities have been subject to volatile markets, and such volatility is expected to continue and costs to increase. Costs may also increase as a result of stricter climate-change-related laws and regulations. Such legislation could require investments in technology to reduce energy use and greenhouse gas emissions, beyond what we expect in our existing plans, or could result in additional and increased carbon pricing. If Philips is not able to compensate for increased costs of energy, (sub-)components, (raw) materials, and transportation – either by reducing reliance thereon or passing on increased costs to customers – then price increases could have a material adverse impact on Philips’ business, financial condition, and operating results.
Philips may increase its dependency on a concentration of external suppliers, as a result of the continuing process of creating a leaner supply base and launching initiatives to replace internal capabilities with less costly outsourced products and services. These initiatives also need to be balanced with local-market value-creation requirements, including those relating to local manufacturing and data storage.
Although Philips works closely with its suppliers to avoid supply discontinuities, there can be no assurance that Philips will not encounter future supply issues, causing disruptions or unfavorable conditions. Furthermore, while the materials supply has improved in 2023, the challenges in our capability for the planning and synchronization of supply with demand continue, which combined with a drive for inventory reduction and cash flow improvements, can lead to further materials running out of stock, which could have a material adverse impact on Philips’ business, financial condition and operating results.
Philips may face challenges in simplifying the organization and the ways of workingAs announced in January 2023, a simplified, more agile operating model is a priority to improve the execution of our strategy. If we do not effectively simplify the organization and our ways of working, which changes include, but are not limited to, changes in governance, roles, processes, and IT landscape and architecture, this may result in limiting our ability to fully realize our business ambitions with respect to delivering sustainable impact, meeting critical patient and customer needs, delivering integral value proposition, growing the business, and/or maintaining business continuity. While Philips has implemented a new operating model to simplify the organization and improve its ways of working, Philips may need to undertake further changes and related restructuring in the future if the operating model ultimately proves to be wholly or partly unsuccessful.
To simplify ways of working and improve performance, Philips continuously seeks to create a more open, standardized, and cost-effective IT landscape. Approaches include outsourcing, offshoring, integration, and consolidation of IT systems. These changes may elevate third-party dependency risks regarding the delivery of IT services, the availability of IT systems, and the functionality offered by IT systems. Although Philips has sought to strengthen security measures and quality controls related to these systems, these measures may prove to be insufficient or unsuccessful, which may lead to a material adverse impact on Philips’ business, financial condition, and operating results.
Philips is dependent on its people for leadership and specialized skills and may be unable to attract and retain personnelThe attraction and retention of talented employees is critical to Philips’ success, and the loss of employees with specialized skills could result in business interruptions. There is strong competition for talent in key capability segments, and Philips needs to attract and retain critical talent. If employees perceive the workload following the recent operating model transformation and workforce reduction to be overly burdensome or prefer more flexibility than offered by our hybrid working policies, to mention two, employees may choose to terminate their employment with us. In this case, efficiencies in workflow may be impacted, or we may experience employee unrest, slowdowns, stoppages or other demands, such as overburden of the remaining employees. Philips is competing for the best talent and most sought-after skills, and there is no assurance of succeeding compared to other companies in attracting and retaining the highly qualified employees needed in the future. Wage inflation is increasing the competition for talent as well as the cost of labor. This may negatively impact our ability to realize our plan for creating value with sustainable impact, and if we are unable to offset the increased costs of labor through higher selling prices and increased productivity, then rising costs could also have a material adverse impact on Philips’ business, financial condition and operating results.
Philips could be exposed to a significant enterprise cybersecurity breachPhilips relies on information technology to operate and manage its businesses, as well as store and process confidential data (relating to patients, employees, customers, intellectual property, suppliers and other partners). Philips’ products, solutions and services increasingly contain sophisticated and complex information technology. The healthcare industry is subject to strict privacy, security and safety regulations with regard to a wide range of health information. At the same time, geopolitical conflicts and criminal activity continue to drive increases in the number, severity, and sophistication of cyberattacks globally. Considering the general increase in cybercrime, our customers and other stakeholders are becoming more demanding regarding the cybersecurity of our products and services. As a global health technology company, Philips is inherently and increasingly exposed to the risk of cyberattacks and potential impact of attacks on our suppliers. Information systems may be damaged, disrupted (including the provision of services to customers), or shut down due to cyberattacks. In addition, breaches in the security of our systems (or the systems of our customers, suppliers, or other partners) could result in the misappropriation, destruction, or unauthorized disclosure of confidential information (including intellectual property) or personal data belonging to us or our employees, customers, suppliers or other partners. These risks are particularly significant with respect to patient medical records. Cyberattacks may result in substantial costs and other negative consequences, which may include, but are not limited to, lost revenues, reputational damage, remediation and enhancement costs, penalties, and other liabilities to regulators, customers and other partners. Philips has not encountered any material breaches or other significant cybersecurity incidents in 2023. While Philips deals with the operational threat of cybercrime on a continuous basis and has so far been able to prevent significant damage or significant monetary cost in taking corrective action, there can be no assurance that future cyberattacks will not result in material or other consequences than as described above, which may result in a material adverse impact on Philips’ business, financial condition and operating results.
Philips may face challenges to drive excellence and speed in bringing innovations to marketTo gain sustainable competitive advantage and create value with sustainable impact, Philips aims to deliver scalable, people-centric, and patient-centric innovations. It is important that Philips innovates and delivers these innovations in close collaboration with its customers on a timely basis and at scale. The emergence of new low-cost competitors, particularly in Asia, the rise of artificial intelligence (AI) and data driven solutions, and the increasing importance of product and cyber security, further underlines the importance of improvements in the innovation process. Success in launching innovations depends on a number of factors, including development of value propositions, architecture and platform creation, product development, market acceptance, production, and delivery ramp-up. It is also dependent on addressing potential quality issues or other defects in the early stages of introduction, and on attracting and retaining skilled employees. Costs of developing new products and solutions may partially be reflected on Philips’ balance sheet and may be subject to write-down or impairment depending on the performance of such products or services. The significance and timing of such write-downs or impairments are uncertain, as is the ultimate commercial success of new product introductions. Accordingly, Philips cannot determine in advance the ultimate effect that innovations will have on its financial condition and operating results. If Philips fails to create and commercialize its innovations at scale, it may lose market share and competitiveness, which could have a material adverse effect on its financial condition and operating results.
Negative developments impacting the liquidity of global capital markets could affect Philips’ ability to raise or re-finance debt in the capital markets or could lead to significant increases in the cost of such borrowing in the future. If the markets expect a downgrade by the rating agencies, or if such a downgrade has actually taken place, this could increase the cost of borrowing, reduce our potential investor base and adversely affect our business.
Philips’ financing and liquidity position may also impact its ability to implement or complete any share-buyback program or distribute any dividends in accordance with its dividend policy or at all. Any announced share-buyback program or dividend policy may also be amended, suspended or terminated at any time, including at Philips’ discretion or as a result of applicable law, regulation or regulatory guidance, and any such amendment, suspension or termination could negatively affect the trading price of, increase trading price volatility of, or reduce the market liquidity of Philips’ shares or other securities. Additionally, any share-buyback program or distribution of dividend could diminish Philips’ cash or other reserves, which may impact its ability to finance future growth and to pursue potential future strategic opportunities. Any share-buyback program or dividend payment will depend on factors such as availability of financing, liquidity position, business outlook, cash flow requirements and financial performance, the state of the market and the general economic climate, and other factors, including tax and other regulatory considerations. Philips and its subsidiaries may also be subject to limitations on the distribution of shareholders’ equity under applicable law.
Philips operates in over 100 countries and its reported earnings and equity are therefore inevitably exposed to fluctuations in the exchange rates of foreign currencies against the euro. Philips’ sales and net investments in its foreign subsidiaries are sensitive in particular to movements in the US dollar, Japanese yen, Chinese renminbi, and a wide range of other currencies from developed and emerging economies. Philips’ sourcing and manufacturing spend is concentrated in the EU, the US and China. Income from operations is particularly sensitive to movements in currencies of countries where Philips has no or very small-scale manufacturing/local sourcing activities but significant sales of its products or services, such as Japan, Canada, Australia, the United Kingdom, and a range of emerging markets, such as South Korea, Indonesia, India and Brazil.
In view of the long lifecycle of health technology solution sales and long-term strategic partnerships, the financial risk of counterparties with outstanding payment obligations creates exposure risks for Philips, particularly in relation to accounts receivable from customers, liquid assets, and the fair value of derivatives and insurance contracts with financial counterparties. A default by counterparties in such transactions can have a material adverse effect on Philips’ financial condition and operating results.
Contingent liabilities may have a significant impact on the company’s consolidated financial position, results of operations and cash flows. For an overview of current cases please refer to the note Contingencies.
Philips is exposed to tax risks which could have a significant adverse financial impactPhilips is exposed to tax risks that could result in double taxation, penalties and interest payments. The source of the risks could originate from local tax laws and regulations as well as international and EU regulatory frameworks. These include transfer pricing risks on internal cross-border deliveries of goods and services, as well as tax risks relating to changes in the transfer pricing model. Examples of initiatives that may result in changing tax rules include, but are not limited to, the plans adopted by the Dutch parliament to abolish the tax exemption for dividend withholding tax on share buy backs with effect from 2025 and the OECD/G20 Inclusive Framework to address the allocation of income to user markets (Pillar One) and a 15% minimum corporate income tax rate (Pillar Two). The formal adoption of Council Directive (EU) 2022/2523 (the Pillar Two Directive) in December 2022 aims to achieve a coordinated implementation of Pillar Two in EU member states. The Dutch government adopted the Minimum Tax Rate Act 2024 (MTR Act) in December 2023 and the Pillar Two legislation will be applicable in local law with effect from 2024. As for Pillar One, it is too early to assess the potential impact. Philips is closely following the developments of this initiative.
As Philips maintains substance in the form of relevant assets and personnel in the countries in which it operates, and with the recently provided transitional safe harbor rules (based on Country-by-Country report) enacted by OECD, Philips currently expects to have limited exposure to taxation under Pillar Two. Pillar Two may still impose an additional tax burden on a jurisdiction-by-jurisdiction basis (which do not meet the transitional safe harbor rules) and increase Philips’ tax compliance burden significantly.
Furthermore, Philips is exposed to tax risks related to acquisitions and divestments, permanent establishments, tax loss, interest and tax credits carried forward, and potential changes in tax law that could result in higher tax expenses and payments. The risks may have a significant impact on local financial tax results, which could adversely affect Philips’ financial condition and operating results. The value of the deferred tax assets, such as tax losses carried forward, is subject to the availability of sufficient taxable income within the tax loss-carry-forward period. The ultimate realization of the company’s deferred tax assets is uncertain. Accordingly, there can be no absolute assurance that all deferred tax assets, such as (net) tax losses and credits carried forward, will be realized.
Flaws in internal controls could adversely affect our financial reporting and management processAccurate disclosures provide investors and other market professionals with significant information for a better understanding of Philips’ businesses. Failures in internal controls or other issues with respect to Philips’ public disclosures, including disclosures with respect to cybersecurity risks and incidents, could create market uncertainty regarding the reliability of the information (including financial data) presented. This could have a negative impact on the price of Philips securities. In addition, the reliability of revenue and expenditure data is key for steering the businesses and for managing top-line and bottom-line growth. The long lifecycle of health technology solution sales, from order acceptance to accepted installation and servicing, together with the complexity of the accounting rules recognizing revenue in the accounts, presents a challenge in terms of ensuring consistent and correct application of the accounting rules throughout Philips’ global business. Significant changes in the way of working, such as the changes made to our operating model, restructurings, and shifting processes to remote Global Business Services locations, may have an adverse impact on the environment under which controls are executed, monitored, reviewed, and tested. Any flaws in internal controls, or regulatory or investor actions in connection with flaws in internal controls, could have a material adverse effect on Philips’ business, financial condition, operation results, and reputation and brand.
Global inflation could materially adversely impact our business and results of operationsChanges in macroeconomic conditions, supply chain constraints, labor shortages, the conflict in Ukraine, and steps taken by governments and central banks, including in response to the COVID-19 pandemic as well as recent stimulus and spending programs, have led to higher inflation, which is likely, in turn, to lead to increased interest rates and adverse changes in the availability and cost of capital. These inflationary pressures could affect our manufacturing costs, operating expenses (including wages), and other expenses. We may not be able to compensate for increased costs by driving productivity to reduce costs and by passing these cost increases on through price measures in a timely manner, if at all, which could have an impact on our gross margins and profitability. Our business also operates in certain countries that have experienced hyperinflation, including Argentina and Turkey, and hyperinflationary conditions in any of the markets in which we operate may have a material adverse effect on our business, result of operations and financial condition. Inflation may also cause our customers to reduce or delay orders for our products, which could have a material adverse effect on our business, results of operations, and cash flows.
Our reputation and license to operate depends on our compliance with global regulations and standards. Operating in a highly regulated health-technology industry, our products and services, including parts and materials from suppliers, are subject to regulation by various government and regulatory agencies, e.g., FDA (US), EMA (Europe), NMPA (China), MHRA (UK), ASNM (France), BfArM (Germany), and IGZ (the Netherlands). In the EU, the Medical Device Regulation (EU MDR) became effective in May 2021 and imposes significant additional pre-market and post-market requirements. Examples of other product-related regulations are the EU’s Waste from Electrical and Electronic Equipment (WEEE), Restriction of Hazardous Substances (RoHS), Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and Energy-using Products (EuP) regulations. We are subject to various European, United States, and domestic, and foreign environmental laws and regulations, which are continuing to develop. Any failure to comply with such laws and regulations could jeopardize product quality, safety, and security or expose us to lawsuits, administrative penalties, and civil remedies, all of which may have a material adverse impact on Philips’ business, financial condition, and operating results.
Philips has observed an increase in safety and security requirements in a variety of new and upcoming legislation dealing with market access of consumer goods, medical devices, information and communication technology products, Cloud services, and specific areas such as data protection, cybersecurity, AI, and supply chain.
Both regulators and customers require us to demonstrate legal compliance and adequate security management using national and international standards and associated certifications. Non-compliance with conditions imposed by regulatory authorities, including in connection with the proposed Respironics consent decree relating to the Respironics recall or any similar regulatory undertakings, could result in product recalls, a temporary ban on products, stoppages at production facilities, remediation costs, fines, disgorgements of profits, and/or claims for damages. Product safety incidents or user concerns could jeopardize patient safety and/or trigger inspections by the FDA or other regulatory agencies, which, depending on the results of such inspections, could trigger the impacts described above, as well as other consequences. These issues could adversely impact Philips’ financial condition or operating result through lost revenue and cost of any required remedial actions, penalties or claims for damages. They could also negatively impact Philips’ reputation, brand, relationship with customers and market share. In particular, Philips is exposed to the ongoing impact of the Respironics voluntary recall/field action and related matters. Please refer to the section Quality & Regulatory and patient safety and the note Contingencies.
Philips is exposed to the risks of non-compliance with business conduct rules and regulations, including privacy and upcoming ESG disclosure and due diligence requirementsIn the execution of its strategy, Philips could be exposed to the risk of non-compliance with business conduct rules and regulations and our General Business Principles, including, but not limited to, patient safety, quality, anti-bribery, healthcare compliance, privacy and data protection, as well as upcoming ESG disclosure requirements and due diligence requirements. This risk is heightened in Growth geographies, as the legal and regulatory environment is less developed compared to Mature geographies. Examples of compliance risk areas include commission payments to third parties and remuneration payments to agents, distributors, consultants and similar entities, as well as the acceptance of gifts, which may be considered in some markets to be normal local business practice. The ongoing digitalization of Philips’ products and services, including its processing of personal data, increases the importance of compliance with privacy, data protection and similar laws. These risks could adversely affect Philips’ financial condition, reputation and brand and trigger the additional risk of exposure to governmental investigations, inquiries and legal proceedings and fines. In various jurisdictions, ESG disclosure requirements are currently being drafted. In Europe, the Corporate Sustainability Reporting Directive and European Sustainability Reporting Standards have been approved. The latter will significantly increase the scope of mandatory ESG disclosures. Philips needs to report over FY 2024 in line with the requirements of the CSRD and the ESRS. In addition, the proposed European Corporate Sustainability Due Diligence Directive and similar regulations and directives or other rules will (if implemented) require companies to identify and act on adverse environmental and human rights impacts across their organization – and potentially their entire value chain. Failure to meet these requirements could trigger the additional risk of exposure to inquiries from supervisory bodies and adversely affect Philips’ reputation and brand or could adversely impact Philips’ financial condition or operating result through lost revenue and cost of any required remedial actions, penalties or claims for damages.
For further details, please refer to the sub-section Legal proceedings within the note Contingencies.
In the two-tier corporate structure under Dutch law, the Supervisory Board is a separate body that is independent of the Board of Management and the company. The Supervisory Board supervises the policies, management and general affairs of Philips, and assists the Board of Management and the Executive Committee with advice. Please also refer to Supervisory Board within the chapter Corporate governance.
Former Group CEO of Singapore Telecommunications Limited and currently member of the Board of Directors of Prudential plc, Bharti Airtel Limited, Bharti Telecom Limited and Ayala Corporation. Member of the Council of Presidential Advisors of Singapore, Deputy Chairman of the Public Service Commission of Singapore.
Former CFO, President, Chairman and CEO of PepsiCo. Currently member of the Board of Directors and Chair of the Audit Committee of Amazon, Inc. Member of the International Board of Advisors of Temasek, member of the Board of Trustees of the Memorial Sloan Kettering Hospital, trustee of the national gallery of art.
Former Chief Operating Officer at VMware and President at SAP. Currently CEO and President of Cohesity and member of the Board of Directors of Snyk.
Currently CEO of PostNL, member of the Supervisory Board of ING Groep N.V., member of the Supervisory Board of Het Concertgebouw N.V. and member of the Advisory Board of Goldschmeding Foundation.
2023 was a challenging as well as an encouraging year for Philips, as the company started to deliver on its three-year plan to create value with sustainable impact. Improved operational performance was driven by a strong focus on execution to enhance patient safety and quality, strengthen supply chain reliability, and establish a simplified operating model. The company succeeded in achieving its raised 2023 outlook with strong sales growth, improved profitability, and strong cash flow. All despite the uncertainties brought about by an increasingly volatile geopolitical environment. That said, order intake and the Respironics recall, including litigation and the investigation by the US Department of Justice (DOJ), remain key areas of attention.
As we explain in our Report, we spent many sessions in 2023 engaging with the Board of Management and closely and actively reviewing key priority issues and actions to build further momentum and keep Philips on a value creation track for its stakeholders. The main topics discussed were patient safety and quality, supply chain strengthening, the workforce reduction, the new operating model and strategy for future business growth, as well as the composition of the Board of Management and Supervisory Board, the remuneration of the Board of Management, and the succession slate/bench across the organization.
Strengthening patient safety and quality across Philips is the highest priority, and resolving the consequences of the Respironics recall for our patients and customers is a key focus area. We feel encouraged by the progress made on the recall, as the company completes remediation of the sleep devices and strives to finalize remediation of the ventilator devices. Philips is fully committed to complying with the terms of the consent decree agreed with the DOJ, representing the US Food and Drug Administration (FDA), which primarily focuses on Philips Respironics in the US. The consent decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business. In 2023, the Supervisory Board spent time on further anchoring patient safety and quality in the organization, and it remains actively involved in the further outstanding steps which are expected to follow in respect of litigation and the investigation by the DOJ. For more information, please refer to Quality & Regulatory and patient safety.
We also discussed developments around the supply chain frequently and in depth – both the external situation and the further improvements planned internally to improve business and financial performance. The progress made in this area has been a strong driver of the company's increased growth.
The Supervisory Board endorses the simplification of Philips’ organizational structure, where the Businesses are leading, supported by the Regions and global Functions, with more focused KPIs. The workforce reduction of approximately 8,000 roles to date, out of the planned reduction of 10,000 roles by 2025, was an impactful and difficult, yet necessary, measure as the company drives a major step-up in productivity, including focusing its R&D activities on fewer, yet more impactful projects. The impact of this reduction became visible in the P&L in 2023. Philips will strive to implement the remaining reductions with due respect for every employee affected and in line with all local rules and regulations.
We welcome the decision by Exor, the Netherlands-based diversified holding company, to become a long-term investor in Philips, supporting the company’s strategy. Their purchase of a 15% shareholding underlines their confidence in Philips’ growth and value potential, and their trust in the leadership, and provides for Exor to nominate one member to our Supervisory Board. We are pleased with the availability of Mr. Benoît Ribadeau-Dumas as Exor nominee, and we will propose his appointment at the upcoming 2024 General Meeting of Shareholders.
We appreciate the support expressed by the shareholders for the current management and direction of Philips. The Supervisory Board has every confidence in CEO Roy Jakobs and his leadership team, as they focus on implementing the plan to create value with sustainable impact for our shareholders and all other stakeholders.
Together with my fellow members of the Supervisory Board, I look forward to providing continued oversight of Philips as the company continues its value creation trajectory and delivers on its purpose of improving people’s health and well-being through meaningful innovation.
Feike Sijbesma Chairman of the Supervisory Board In this context, the Supervisory Board and management also discussed the external environment in which the company operates, and the impact that the macro-economic outlook has on its performance.
Introduction Supervisory Board reportThe Supervisory Board supervises, advises and challenges the Board of Management in performing their management tasks as well as setting and executing the strategy of the Philips Group. The members of the Supervisory Board act in the interests of Royal Philips, its businesses and all its stakeholders. This report includes a more specific description of the Supervisory Board’s activities during the financial year 2023 and other relevant information on its functioning.
2023 focus areas and activities of the Supervisory BoardAgainst the background set forth in the Chairman’s letter above, the Supervisory Board was regularly updated by management on the company’s performance and outlook. The Supervisory Board engaged in discussions with management on improving performance and increasing productivity and agility, by addressing the enhancement of patient safety and accelerating the focus on quality, strengthening the supply chain reliability and establishing a simplified operating model at Philips. Progress, as well as the near-term and longer-term actions on those three priorities were reviewed and monitored by the Supervisory Board.
In 2023, the Supervisory Board devoted considerable time to the Respironics recall, as a recurring agenda item for each of its (regular) meetings. The Supervisory Board was kept apprised of the progress made with the repair and replacement program, and in particular discussed and tracked the comprehensive test and research program for the affected CPAP, BiPAP and mechanical ventilator devices. Putting the interest of patients first, the Supervisory Board challenged management to remain focused on keeping patients regularly updated on the status of the repair or replacement of their devices and to accelerate the repair and replacement program where possible, despite operational and supply challenges.
The Supervisory Board was also regularly updated on other aspects of the recall. This includes, amongst other things, the negotiations and preliminary approval of the economic loss class settlement, the ongoing engagements with the FDA and other competent authorities globally, and the discussions with the DOJ, acting on behalf of the FDA, regarding a proposed consent decree. It also includes the criminal and civil investigation opened by the DOJ’s Consumer Protection Branch and Civil Fraud Section, and the US Attorney’s Office for the Eastern District of Pennsylvania to which Philips Respironics is subject and the ongoing class-action lawsuits and individual personal injury claims in which Philips Respironics and other entities are defendants. The Supervisory Board specifically engaged with management on the potential impact of the consent decree as well as the litigation and investigations on the Philips Respironics business, both in North America and the rest of the world, and reviewed its plans to keep on serving patients with affected devices until market re-entry.
Recognizing the importance of patient safety and quality of products and solutions sold by the Philips Group generally, significant time was spent in 2023 on reviewing and tracking progress of the company-wide program Accelerating Patient Safety and Quality to improve and foster a culture, behaviors and a mindset that puts quality and patient safety first. In the context of this program, the Supervisory Board also discussed the process framework for product design and production controls in the company.
As presented on January 30, 2023, the Supervisory Board and the Board of Management together with the Executive Committee interacted on the company’s overall strategy to extend its leadership as a health technology company and its plan to create value with sustainable impact towards 2025 and beyond, based on focused organic growth and scalable innovation with improved execution as the key value driver. This plan is designed to restore Philips' value creation based upon sales growth and improvement of profitability and cash generation by strong focus on execution. It includes, amongst other things, the strategic plans and priorities of each of the Segments and Regions and at Enterprise level. The Supervisory Board engaged in multiple deep dive sessions on the strategy. These interactions led to more detailed strategy plans that were reviewed and signed-off by the Supervisory Board. Each strategic plan is supplemented with milestones and an execution plan to achieve the company’s ambitions. Specific attention was given to the role of China in the strategy moving forward given the dynamics of the China market.
Our oversight over the company’s overall strategy also included the restructuring and other actions designed to improve the operations and performance, to invest in quality, to simplify ways of working, to remove organizational complexity by putting businesses with single accountability in the lead enabled by strong Regions and lean Functions, and to reduce operational expenses in close alignment with the respective works councils and unions and with respect for the impacted employees and their colleagues.
The overview below indicates other key matters that were reviewed and/or discussed during one or more meetings in the course of 2023:
The Supervisory Board conducted four dedicated dialogues on Philips' positioning in the market and versus its competitors. Subsequently, the Supervisory Board ’deep dived’ into the overall Philips strategy and the strategy and performance of:
The Supervisory Board reviewed Philips’ annual and interim financial statements, including information related to ESG, prior to publication.
Supervisory Board meetings and attendanceIn 2023, the members of the Supervisory Board convened for seven regular meetings and four extraordinary meetings. Moreover, the Supervisory Board members collectively and individually interacted with members of the Board of Management, with members of the Executive Committee and with senior management outside the formal Supervisory Board meetings. The Chairman of the Supervisory Board and the CEO frequently had bilateral discussions about the company’s progress on a variety of matters.
The Supervisory Board meetings were well attended in 2023. All Supervisory Board members were present during the Supervisory Board meetings in 2023. The committees of the Supervisory Board also convened regularly (see the separate reports of the committees below) and the committees reported back on their activities to the full Supervisory Board. In addition, the Supervisory Board and Committees held private meetings. The members of the Supervisory Board concluded that they devoted sufficient time to engage (proactively if the circumstances so required) in their supervisory responsibilities.
In March 2023, a Supervisory Board member visited the European Congress of Radiology in Vienna, Austria. In June 2023, the Supervisory Board members visited Philips’ Image Guided Therapy-Devices site in Plymouth, Minnesota, USA. In the course of 2023, various Supervisory Board members visited Philips’ Diagnosis & Treatment manufacturing site in Best, the Netherlands.
Supervisory Board: composition, diversity and self-evaluationThe Supervisory Board is a separate corporate body that is independent of the Board of Management and the company. Its independent character is also reflected in the requirement that members of the Supervisory Board can be neither a member of the Board of Management nor an employee of the company. The Supervisory Board considers all its members (i.e. 100%) to be independent under the Dutch Corporate Governance Code. Furthermore, the members of its Audit Committee are independent under the rules of the US Securities and Exchange Commission, applicable to the Audit Committee.
The Supervisory Board currently consists of 10 members. Effective as per (the end of) the 2023 Annual General Meeting of Shareholders, David Pyott was re-appointed for a term of two years and Liz Doherty was re-appointed for a term of four years. The agenda for the upcoming 2024 Annual General Meeting of Shareholders will include proposals to re-appoint Feike Sijbesma and Peter Löscher as members of the Supervisory Board. The agenda will also include the proposal to appoint Benoît Ribadeau-Dumas as new member of the Supervisory Board, who will be nominated pursuant to the commitment of Exor N.V. to be a long-term minority investor in Philips and its right to propose one member to the Supervisory Board. We are very pleased with the availability of Mr. Benoît Ribadeau-Dumas and welcome him as new member of our Board (subject to his appointment at the 2024 AGM).
The Supervisory Board attaches great value to diversity in its composition and has adopted a Diversity Policy for the Supervisory Board, Board of Management and Executive Committee. For more information on the Diversity Policy, please refer to Report of the Corporate Governance and Nomination & Selection Committee. The Supervisory Board spent time in 2023 considering its composition, as well as the composition of the Executive Committee (including the Board of Management), taking into account the criteria set forth in the Diversity Policy.
The composition of the Supervisory Board furthermore follows its profile (which was updated in early 2023), as included in the Rules of Procedure of the Supervisory Board. The profile aims for an appropriate combination of knowledge and experience among the members of the Supervisory Board, encompassing general management, international business, environmental, social and governance (ESG) and sustainability, (consumer) health and medical technology, quality and regulatory, finance and accounting, human resources, manufacturing and supply chain, information technology and digital, marketing, and governmental and public affairs, all in relation to the global character of Philips’ businesses. The Supervisory Board also aims for having members with different nationalities and (cultural) backgrounds, working experiences or otherwise diverse qualities, as well as one or more members with an executive or similar position in business or society no more than five years ago. The composition of the Supervisory Board shall furthermore be in accordance with the Dutch Corporate Governance Code best practice provisions on independence, and each member of the Supervisory Board shall be capable of assessing the broad outline of the overall policy of the company. The size of the Supervisory Board may vary as it considers appropriate to support its profile.
Any (re-)appointments of members of the Supervisory Board must meet the gender quota, in accordance with Dutch law, requiring that at least one-third of the supervisory board members are women and at least one-third are men. (For calculation purposes, a total number of board members that cannot be divided by three, must be rounded up to the next number that can be divided by three.) Currently, the statutory quota is met, as out of ten Supervisory Board members, four members are female and six members are male.
In 2023, each member of the Supervisory Board completed a questionnaire to verify compliance with the applicable corporate governance rules and the Rules of Procedure of the Supervisory Board. The outcome of this survey was satisfactory.
An independent external party facilitated the 2023 self-evaluation process for the Supervisory Board and its committees. This included drafting and submitting relevant questionnaires, interviewing members of the Supervisory Board and aggregating and reporting on the results. The members of the Board of Management also provided their input. The questionnaires covered various topics such as composition, size, skills and experience, geographical coverage and diversity of the Supervisory Board, dynamics and focus of the meetings of the Supervisory Board, the effectiveness of the Supervisory Board’s oversight of various aspects such as strategy, business performance, risk management, succession planning and people, and engagement with management. All members of the Supervisory Board were invited to share recommendations to improve the Supervisory Board’s functioning and ways of working going forward. Furthermore, the performance of the Chairman, the other Supervisory Board members individually, and of the Supervisory Board’s committees was evaluated separately.
The reports on the results of the evaluation were discussed in a meeting of the Supervisory Board. The results of the self-evaluation indicated that the Supervisory Board is a well-functioning team of appropriate size that benefits from different expertise, diversity, and international geographical representation. The meetings and meeting dynamics were rated positively with good interactions, discussions and feedback and a strong relationship with management, and suggestions were made to further improve meeting efficiency. Board members also noted the importance of being challenged constructively and of the need to maintain a collective understanding of priorities and options for any relevant matters. The composition of the Supervisory Board and its succession plan is considered adequate. Already anticipating the expiry of the third term of appointment of Mr. Pyott in 2025, the Supervisory Board is tasked with identifying a candidate with equivalent MedTech expertise. In addition, attracting candidates with expertise in artificial intelligence will be part of the Supervisory Board’s succession planning. Supervisory Board members further noted the potential benefit from getting external views from various stakeholders, such as Exor, regulators, suppliers, employees, patients, and investors, to enhance its oversight and decision-making. Finally, the Supervisory Board members confirmed a number of current focus areas, such as Patient Safety and Quality, senior executive succession planning, strategy execution and value creation. Early 2024, the Chairman of the Supervisory Board had several meetings with individual members of the Supervisory Board to discuss ways to further enhance the functioning of the Supervisory Board and its individual members going forward. The Chairman also discussed the evaluation of his own functioning with the Vice-Chairman.
Supervisory Board composition
| Feike Sijbesma |
Paul Stoffels |
Chua Sock Koong |
Liz Doherty |
Marc Harrison |
Peter Löscher |
Indra Nooyi |
Sanjay Poonen |
David Pyott |
Herna Verhagen |
|
| Year of birth | 1959 | 1962 | 1957 | 1957 | 1964 | 1957 | 1955 | 1969 | 1953 | 1966 |
| Gender | Male | Male | Female | Female | Male | Male | Female | Male | Male | Female |
| Nationality | Dutch | Belgian | Singaporean | British/Irish | American | Austrian | American | American | British/American | Dutch |
| Initial appointment date | 2020 | 2018 | 2021 | 2019 | 2018 | 2020 | 2021 | 2022 | 2015 | 2022 |
| Date of (last) (re-)appointment | n/a | 2022 | n/a | 2023 | 2022 | n/a | n/a | n/a | 2023 | n/a |
| End of current term | 2024 | 2026 | 2025 | 2027 | 2026 | 2024 | 2025 | 2026 | 2025 | 2026 |
| Independent | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Committee memberships1) | RC & CGNSC | RC & CGNSC | AC | AC | QRC | AC & QRC | CGNSC | AC | RC & QRC | RC |
| Attendance at Supervisory Board meetings | (11/11) | (11/11) | (11/11) | (9/11) | (11/11) | (10/11) | (11/11) | (10/11) | (10/11) | (11/11) |
| Attendance at committee meetings | RC (8/8) CGNSC (6/6) |
RC (8/8) CGNSC (6/6) |
AC (6/6) | AC (6/6) | QRC (4/4) | AC (6/6) QRC (4/4) |
CGNSC (6/6) | AC (6/6) | RC (7/8) QRC (4/4) |
RC (8/8) |
| General management | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| International business | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| ESG & sustainability | yes | yes | yes | yes | ||||||
| (Consumer) health and medical technology | yes | yes | yes | yes | yes | yes | ||||
| Patient safety, quality & regulatory and product development | yes | yes | yes | yes | ||||||
| Finance and accounting | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Human Resources | yes | yes | yes | yes | yes | yes | yes | yes | yes | |
| Manufacturing and supply chain | yes | yes | yes | yes | yes | |||||
| Information technology and digital | yes | yes | yes | yes | yes | yes | yes | yes | yes | |
| Marketing | yes | yes | yes | yes | yes | yes | yes | |||
| Governmental and public affairs | yes | yes | yes | yes | yes | yes | yes | yes |
While retaining overall responsibility, the Supervisory Board has assigned certain of its tasks to the three long-standing committees, also referred to in the Dutch Corporate Governance Code: the Corporate Governance and Nomination & Selection Committee, the Remuneration Committee and the Audit Committee. In 2015, the Supervisory Board also established the Quality & Regulatory Committee. The separate reports of these committees are part of this Supervisory Board report and are published below.
The full Supervisory Board retains overall responsibility for the activities of its committees.
Financial statements 2023The financial statements of the company for 2023, as presented by the Board of Management, have been audited by Ernst & Young Accountants LLP, the independent external auditor appointed by the General Meeting of Shareholders. We have approved these financial statements, and all individual members of the Supervisory Board have signed these documents (as did the members of the Board of Management).
Finally, we would like to express our thanks to the members of the Board of Management, the Executive Committee and all other employees for their continued contribution throughout 2023.
The Supervisory Board
Feike Sijbesma
Paul Stoffels
Chua Sock Koong
Liz Doherty
Marc Harrison
Peter Löscher
Indra Nooyi
Sanjay Poonen
David Pyott
Herna Verhagen
The Corporate Governance and Nomination & Selection Committee is chaired by Feike Sijbesma. Its other members are Paul Stoffels and Indra Nooyi. The Committee is responsible for the review of the overall corporate governance, the selection criteria and appointment procedures for the Board of Management, the Executive Committee, certain other key management positions, as well as the Supervisory Board.
In 2023, the Corporate Governance and Nomination & Selection Committee held six meetings and all Committee members attended these meetings.
The Committee devoted time to the appointment or reappointment of candidates to fill current and future vacancies on the Supervisory Board. Following those consultations, it prepared decisions and advised the Supervisory Board, which resulted in the re-appointments of David Pyott and Liz Doherty as members of the Supervisory Board at the 2023 Annual General Meeting of Shareholders. This also resulted in the proposals to re-appoint Feike Sijbesma and Peter Löscher as members of the Supervisory Board, and to appoint Exor nominee Benoît Ribadeau-Dumas as new member of the Supervisory Board at the upcoming 2024 Annual General Meeting of Shareholders.
Under its responsibility for the selection criteria and appointment procedures for Philips’ senior management, the Committee reviewed the functioning of the Board of Management and its individual members, the Executive Committee succession plans and emergency candidates for key roles in the company. The review and evaluation consists of periodical performance review meetings with the individual members of the Board of Management and the Executive Committee, and evaluation of the results of these meetings by the Committee. The main findings and conclusions from these reviews were also shared with the Supervisory Board and the Remuneration Committee, and were taken into account in the performance evaluation of the Board of Management and Executive Committee members and the selection of succession candidates. Reference is made to 2023 Annual Incentive, setting out the performance review of the Board of Management members by the Remuneration Committee.
The Committee devoted time in 2023 to the selection and/or appointment of candidates to fill other current and future vacancies on the Board of Management and the Executive Committee. This resulted in the re-appointment, at the 2023 Annual General Meeting of Shareholders, of Abhijit Bhattacharya for a two-year’s term to his tenure as CFO that started in 2015, thereby ensuring continuity and enabling a smooth succession process in parallel. The Committee’s work furthermore resulted in appointments of four new members of the Executive Committee: Steve C. de Baca and Jeff DiLullo were appointed, effective February 6, 2023, as Chief Patient Safety & Quality Officer and Chief Market Leader of Philips North America, respectively. Furthermore, Julia Strandberg was appointed as the Chief Business Leader of the Connected Care segment, effective April 24, 2023, and Heidi Sichien was appointed as Chief People Officer, effective August 1, 2023. Philips expects to announce a new leader for its Precision Diagnosis business in 2024, which business is currently under the temporarily extended leadership of Bert van Meurs (Chief Business Leader for the Image Guided Therapy business).
With respect to corporate governance matters, the Committee discussed developments around the revised Dutch Corporate Governance Code, relevant legislation under consideration in the Netherlands and the regulatory regimes around disclosure requirements related to ESG. Finally, the Committee reviewed the Charter of the Corporate Governance and Nomination and Selection Committee and concluded it remains appropriate.
With respect to the productivity initiatives and other actions to improve the company’s performance (including the unfortunate but necessary reduction of roles), the Committee was updated by management on the impact on employees and the phased deployment approach and reviewed the simplification of the organization.
DiversityThe Diversity Policy for the Supervisory Board, Board of Management and Executive Committee was adopted in 2017 and revised in early 2023, and is published on the company website. The Committee periodically assesses the Diversity Policy and the size and composition of the Supervisory Board and makes recommendations, if relevant, relating to the profile for the Supervisory Board.
The criteria in the Diversity Policy aim to ensure that the Supervisory Board, the Board of Management and the Executive Committee have a sufficient diversity of views and the expertise needed for a good understanding of current affairs and longer-term risks and opportunities related to the company’s business. The nature and complexity of the company’s business is taken into account when assessing optimal diversity, as well as the social and environmental context in which the company operates.
Pursuant to the Diversity Policy, the selection of candidates for appointment to the Supervisory Board, Board of Management and Executive Committee is based on merit. With due regard to the criteria set forth in the Diversity Policy, the company shall seek to fill vacancies by considering candidates that represent a diversity of (among others) ages, gender, identities and educational and professional backgrounds. Please refer to the Supervisory Board report for more information on the diversity of the Supervisory Board.
The Diversity Policy includes the Supervisory Board’s aim that the Board of Management and the Executive Committee comprise members with different nationalities and (cultural) backgrounds, working experiences or otherwise diverse qualities. Effective 2022, Dutch law requires listed companies to set appropriate and ambitious gender diversity targets for the Board of Management and for a management level of a seniority to be determined by the company. To this end, the Diversity Policy includes the Supervisory Board’s aim that at least one-third of the members of each of the Board of Management and the Executive Committee are women and at least one-third are men. For more information, please refer to Diversity, Inclusion and Well-Being.
The Supervisory Board has determined the 2023 pay-outs and awards to the members of the Board of Management, upon the proposal of the Remuneration Committee, in accordance with the 2020 Remuneration Policy and the 2020 LTI Plan. In addition, the Supervisory Board has determined the 2023 vesting of the 2021 LTI grant, of which the performance period ended on December 31, 2023. This was done in accordance with the LTI Plan as approved during the 2020 Annual General Meeting of Shareholders.
The Remuneration Committee annually conducts a scenario analysis. This includes the calculation of remuneration under different scenarios, whereby different Philips performance assumptions and corporate actions are examined. The Supervisory Board concluded that the relationship between the strategic objectives and the chosen performance criteria for the 2023 Annual Incentive, as well as for the 2021 LTI, were adequate.
The annual base compensation of the members of the Board of Management has been reviewed as part of the regular remuneration review. No increase was applied to acknowledge the disappointing company performance in 2022 and to reflect the limited budget available for the annual compensation review for the wider population. As a result, the annual base compensation of Roy Jakobs, Abhijit Bhattacharya and Marnix van Ginneken remained unchanged in 2023.
The Annual Incentive performance has been assessed based on company financial results as well as individual results. Details are as follows:
Company financial results (80% weighting)In line with the Remuneration Policy, the company sets financial targets in advance of the year for all members of the Board of Management. For the year 2023, the financial targets set at Group level cover Comparable Sales Growth*), Adjusted EBITA*) and Free Cash Flow*). The realized performance for all three metrics was above target. Realized performance levels presented in the table below include the financial impact connected with the Respironics consent decree, and excluding this impact the Comparable Sales Growth*) and Adjusted EBITA*) would have been 7.0% and 10.5% respectively. Reviewing these performance levels, the Supervisory Board decided to apply two downward adjustments. First, to acknowledge the decrease in comparable order intake reported over 2023, the Supervisory Board lowered the payout on the Comparable Sales Growth metric from 200% to 175% of target . Second, to account for the upward effect on Adjusted EBITA resulting from excluding the financial impact connected with the proposed Respironics consent decree, the Supervisory Board also lowered the payout from 180% to 175% of target for this metric (corresponding to an Adjusted EBITA performance of 10.5% which excludes the financial impact referred to).
| Financial performance metric | Weighting as % of target Annual Incentive | Assessment of performance | Weighted pay-out as % of target Annual Incentive | ||||
|---|---|---|---|---|---|---|---|
| threshold performance | target performance | maximum performance | realized performance | resulting payout as % of target | |||
| Comparable Sales Growth1) | 30% | 0.0% | 1.5% | 3.5% | 6.0% | 175.0% | 52.5% |
| Adjusted EBITA margin1) | 30% | 7.5% | 9.0% | 11.0% | 10.6% | 175.0% | 52.5% |
| Free Cash Flow1) | 20% | 571 | 871 | 1,171 | 1,582 | 200.0% | 40.0% |
| Total | 80% | 145.0% | |||||
The individual performance criteria and assessment targets set at the beginning of the year have been disclosed in the following table. To determine the payout levels for the individual goals, the Supervisory Board typically applies a holistic assessment as to the performance against the set goals as well as the relative weighting of the goal categories. These relative weightings are not in all cases equal, but such that any goal category remains relevant and aligned with the strategic priorities for the year.
| Board of Management Member | Individual Performance criteria | Assessment of performance | Weighted pay-out as% of target Annual Incentive |
|---|---|---|---|
| Roy Jakobs | Strategy execution |
|
22.0% |
Quality & operational excellence |
|
||
People & organization |
|
||
Customer results |
|
||
ESG/Sustainability |
|
||
| Abhijit Bhattacharya | Strategy execution |
|
21.0% |
| Quality & operational excellence |
|
||
| People & organization |
|
||
| Customer results |
|
||
| ESG/Sustainability |
|
||
| Marnix van Ginneken | Strategy execution |
|
23.0% |
| Quality & operational excellence |
|
||
| People & organization |
|
||
| Customer results |
|
||
| ESG/Sustainability |
|
Overall, this leads to the following total Annual Incentive realization:
Annual Incentive realization 2023
in EUR unless otherwise stated
| Annual incentive opportunity | Realized annual incentive | |||||
|---|---|---|---|---|---|---|
| Target as a % of base compensation | Target Annual Incentive | Financial performance (weighted pay-out %) | Individual performance (weighted pay-out %) | Payout as % of target Annual Incentive1) | Realized annual incentive | |
| Roy Jakobs | 100% | 1,200,000 | 145.0% | 22.0% | 167.0% | 2,004,480 |
| Abhijit Bhattacharya | 80% | 648,000 | 145.0% | 21.0% | 166.0% | 1,075,939 |
| Marnix van Ginneken | 80% | 504,000 | 145.0% | 23.0% | 168.0% | 846,922 |
This section presents incentive performance metrics under the proposed 2024 Remuneration Policy for the Board of Management. In the event that the proposed 2024 Remuneration Policy would not be adopted by the 2024 AGM, the current 2020 Remuneration Policy would continue to apply.
In the proposed 2024 Remuneration Policy, the weighting of the non-financial element has increased to 30% (from 20%) and, correspondingly, the weighting of the financial element has decreased to 70% (from 80%). This change reflects the increased relative importance of factors relating to strategic priorities (such as patient safety and quality, supply chain reliability, and a simplified operating model), as well as our Environmental, Social and Governance (ESG) performance.
Financial element (70% weighting):For the year 2024, the following financial performance metrics are selected to ensure alignment with the key (strategic) priorities in the year:
At the start of each year, two to four performance categories are selected from the following list, whereby each selected category receives an equal weighting:
For each selected category, one or more performance objectives are determined at the start of the year for each of the members of the Board of Management.
For the year 2024, the following categories and objectives are selected to ensure alignment with the key (strategic) priorities in the year:
| Performance category | Performance objective | Applicable for | Weighting | Measurement description |
|---|---|---|---|---|
Patient Safety & Quality |
Drive Patient Safety & Quality as highest priority in the organization |
All members of Board of Management | 7.5% | This objective measures delivery on our company-wide program to strengthen our Patient Safety & Quality culture, capabilities and performance. Additionally, we measure the progress on the Respironics recall and delivery of the proposed consent decree commitments. |
Customer |
Improve customer experience | Roy Jakobs; Abhijit Bhattacharya |
7.5% | This objective is measured by the improvement of the customer NPS. |
| Improve supply chain reliability | Roy Jakobs | This objective is measured by the on-time delivery of orders as per customer expectations. | ||
Improve financial forecasting |
Abhijit Bhattacharya | Deliver Reliable Forecast as per plan. | ||
Manage legal issues |
Marnix van Ginneken | Develop and manage litigation strategy and potential liabilities. | ||
Strategy and Execution |
Drive focused strategy to win in the market |
Roy Jakobs | 7.5% | This objective measures delivery on our value creation plan and market share gain. |
| Abhijit Bhattacharya | This objective measures delivery on our value creation plan and delivery on cash program and productivity targets. | |||
| Marnix van Ginneken | This objective measures delivery on our value creation plan and delivery on legal & compliance commitments as per plan. | |||
Establish simplified, more agile operating model |
All members of Board of Management | This objective measures delivery on the operating model simplification and our headcount reduction plan. |
||
| ESG | Deliver on ESG Commitments |
All members of Board of Management | 7.5% |
This objective measures: - Performance on our ESG index (which includes various elements such as emission- and diversity targets) - Our employee engagement score - Talent and succession development of senior roles in the organization |
The 3-year performance period of the 2021 LTI grant, consisting of performance shares, ended on December 31, 2023. The realization of this grant is based on TSR achievement, adjusted EPS growth and sustainability objectives. The following performance achievement and vesting levels have been determined by the Supervisory Board in respect of the 2021 grant of performance shares:
Philips Group
Performance achievement and vesting levels
| achievement | weighting | vesting level | |
|---|---|---|---|
| TSR | 0.0% | 50.0% | 0.0% |
| EPS | 0.0% | 40.0% | 0.0% |
| Sustainability objectives | 175.0% | 10.0% | 17.5% |
| Total | 17.5% |
A ranking approach to TSR applies with Philips itself included in the TSR Performance Peer Group. TSR scores are calculated based on a local currency approach and by taking a 3-month averaging period prior to the start and end of the 3-year performance period. The performance incentive pay-out zone is outlined in the following table, which results in zero vesting for performance below the 40th percentile and 200% vesting for performance levels above the 75th percentile. The incentive zone range has been constructed such that the average pay-out over time is expected to be approximately 100%.
Philips Group
Performance-incentive zone for TSR
in %
| Position | 20-14 | 13 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 5-1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Vesting % | 0 | 60 | 80 | 90 | 100 | 120 | 140 | 160 | 180 | 200 |
The TSR achieved by Philips during the performance period was -51.10%, using a start date of October 2020 and end date of December 2023. This resulted in Philips being positioned at rank 20 in the TSR performance peer group shown in the following table, resulting in a TSR achievement of 0%.
Following Oracle’s acquisition of Cerner (completed June 2022), the Supervisory Board adopted the approach of recognizing Cerner’s performance through the delisting date. As a proxy for future performance, reinvestment in an index of the remaining 19 peer companies was assumed (effectively retaining a peer group of 20 companies).
TSR results LTI Plan 2021 grant: (51.10%)
| total return | rank number | |
|---|---|---|
| Canon | 119.99% | 1 |
| General Electric | 109.08% | 2 |
| Boston Scientific | 48.10% | 3 |
| Siemens Healthineers | 35.00% | 4 |
| Stryker | 28.06% | 5 |
| Getinge | 18.79% | 6 |
| Alcon | 16.73% | 7 |
| Johnson & Johnson | 12.57% | 8 |
| Cerner | 11.12% | 9 |
| Becton Dickinson | 10.29% | 10 |
| Terumo | 8.71% | 11 |
| Danaher | 7.33% | 12 |
| Hologic | (1.37)% | 13 |
| Reckitt Benckiser | (11.46)% | 14 |
| Elekta | (20.67)% | 15 |
| ResMed | (21.05)% | 16 |
| Medtronic | (24.84)% | 17 |
| Smith & Nephew | (27.58)% | 18 |
| Fresenius Medical | (45.30)% | 19 |
| Philips | (51.10)% | 20 |
The LTI Plan EPS payouts and targets set at the beginning of the performance period were as follows:
Philips Group
LTI Plan EPS payouts
| Below threshold | Threshold | Target | Maximum | Actual | |
|---|---|---|---|---|---|
| LTI plan EPS (euro) | <1.38 | 1.38 | 1.54 | 1.72 | 0.26 |
| Vesting % | 0% | 40% | 100% | 200% | 0% |
In respect of the 2021 LTI grant, the LTI plan EPS is calculated based on a reported net income attributable to shareholders divided by the number of common shares outstanding (after deduction of treasury shares) on the day prior to the beginning of the performance period (to eliminate the impact of any share buyback, stock dividend, etc.), resulting in an EPS of EUR (0.50). Furthermore, as per the 2020 LTI Plan, the LTI Plan EPS includes adjustments to account for events that were not planned when targets were set or were outside management’s control such as the profit and loss impact of acquisitions and divestments (balance is neutral), the profit and loss impact of unhedged foreign exchange variations versus plan (positive adjustment) and the profit and loss impact of legacy legal proceedings (positive impact). Overall, this resulted in an LTI Plan EPS of EUR 0.26 based on adjusted net income from continuing operations, leading to a realization of 0% of target.
Philips Group
LTI Plan EPS realization
in millions of EUR unless otherwise stated
Net income |
EPS (euro) |
|
|---|---|---|
Income from continuing operations attributable to shareholders |
(456) |
(0.50) |
Profit and loss impact of: |
||
- Acquisitions and divestitures 1) |
1 |
0.00 |
- Foreign exchange variations versus plan 2) |
60 |
0.07 |
- Legacy legal proceedings 3) |
628 |
0.69 |
Adjusted net income from continuing operations |
234 |
0.26 |
In order to further align the remuneration package for the Board of Management with our purpose and our ESG commitment, a sustainability criterion was introduced in the 2020 LTI Plan. Philips believes that ESG performance will improve the company’s performance as a whole and, therefore, that it should be explicitly linked to (long-term) remuneration. The criteria are based on three Sustainable Development Goals (SDGs) as defined by the United Nations that are included in Philips’ strategy on sustainability (no. 3, 12 and 13). These three SDGs are translated in five underlying objectives, which are measured against a specific target range.
At the beginning of the performance period, challenging target ranges are set for each of the five objectives. Based on a point-to-point method, performance achievement is measured at the end of the performance period (i.e., 3 years) versus the beginning of the performance period. The vesting level is determined based on the following scheme:
| No. of measures achieved on or above target | Vesting % |
|---|---|
| 1 | 0% |
| 2 | 0% |
| 3 | 50%-100% |
| 4 | 100%-150% |
| 5 | 150%-200% |
The realized performance is described in the following table. As five out of five objectives are achieved within or better than target range, the vesting % lies between 150% and 200% of target. Based on the overall performance of the five objectives, the Supervisory Board has assessed that a vesting level of 175% would reflect an appropriate positioning within the target range.
For more information on the realized performance on all five objectives please refer to our Environmental, Social and Governance.
| Sustainability category | Underlying objective | Target range | realized performance | |
|---|---|---|---|---|
| Ensure healthy lives and promote well-being for all at all ages (SDG3) Lives Improved |
Targeted # of Lives Improved in year 31) | 1,517 – 1,695 million | 1,880 million | Better than target range |
| Ensure sustainable consumption and production patterns (SDG12) Circularity |
Targeted circular revenue in year 32) | 15.0% – 20.1% | 20.0% | Within target range |
| Targeted waste to landfill in year 33) | 3.5% – 0.1% | 0.0% | Better than target range | |
| Targeted closing the loop in year 34) | 20.0% – 28.5% | 20.5% | Within target range | |
| Take urgent action to combat climate change and its impacts (SDG13) Carbon footprint |
Targeted CO2 equivalent (in Kilo Tonnes) in year 3 | 640 – 574 Ktonnes CO2 | 418 Ktonnes CO2 | Better than target range |
This section presents incentive performance metrics under the proposed 2024 Remuneration Policy for the Board of Management. In the event that the proposed 2024 Remuneration Policy will not be adopted by the 2024 AGM, the current 2020 Remuneration Policy will continue to apply.
The 2024 Long-Term Incentive grant remains to consist of 100% performance shares of which vesting is subject to performance over a period of 3 years. We have broadened the sustainability perspective to the full Environmental, Social and Governance ('ESG') spectrum, and subsequently increased the weighting of the ESG performance metric from 10% to 20%. By doing so, we aim to reflect the importance of ESG to our company and its increasing relevance to our stakeholders (as a strategic matter and in the context of our risk management), and to incentivize management’s focus on our policy objective to deliver superior, long-term value to our stakeholders, while acting responsibly towards our planet and society. As a result of this, the weighting of the relative TSR metric has been slightly reduced to 40% (from 50%) to keep a balanced weighting among the three LTI performance metrics. Lastly, the weighting of the adjusted EPS growth metric remains unchanged, resulting in the following performance metrics and weighting:
At the start of each performance year, we select four ESG objectives in line with our long-term strategic priorities. There is no exhaustive list of objectives that can be selected. To ensure that all objectives are material, auditable and measurable, we only select objectives which are reported in our Annual Report (in preparation for the Corporate Sustainability Reporting Directive) and therefore are subject to our external auditor’s reasonable assurance. Furthermore, we make sure that in any measurement year, the ESG objectives do not overlap with our non-financial performance objectives for the Annual Incentive.
The objectives selected for the 2024 LTI grant are shown in the following table, including the rationale for selecting these objectives and more details on the measurement approach.
2024-2026
ESG objective |
Weighting |
Rationale |
Measurement approach |
|---|---|---|---|
Targeted # of Lives Improved in year 3 1) |
5.0% |
Ensure healthy lives and promote well-being for all at all ages (SDG3) Lives Improved |
We have a Lives Improved calculation methodology, which follows a three-step approach. 1) We first determine the installed base of our health- and well-being solutions, 2) We determine the number of touchpoints per product per year, and 3) To avoid double-counting, we eliminate all direct- and indirect double-counts between products and solutions. |
Targeted circular revenue in year 3 2) |
5.0% |
Ensure sustainable consumption and production patterns (SDG12) Circularity |
Revenues generated through products and solutions that meet specific Circular Economy requirements (e.g. refurbished, reconditioned and remanufactured components). |
|
Targeted CO2 equivalent (in Kilotonnes) in year 3 |
5.0% |
Take urgent action to combat climate change and its impacts (SDG13) Carbon footprint |
Total greenhouse gas emissions caused by Philips, expressed in kilotonnes CO2-equivalent. Operational carbon footprint on a half-year basis, incl. industrial sites, non-industrial sites, business travel and logistics. |
Targeted Employee Engagement Score in year 3 |
5.0% |
Retain an engaged workforce Employee Engagement Score |
The Employee Engagement Score (EES) is the single measure of the overall level of employee engagement at Philips, measured on a bi-yearly basis. |
The following pension arrangement is in place for the members of the Board of Management working under a services agreement governed by Dutch law:
The following table gives an overview of the costs incurred by the company in 2023 and 2022 in relation to the remuneration of the Board of Management. Costs related to performance shares are based on accounting standards (IFRS), which prescribe that costs for each LTI grant are recognized over the full (multi-year) vesting period, proportionate to the relevant fiscal year. Therefore, the costs for any year reflect costs of multiple LTI grants, as opposed to the actual value for the holder of an LTI grant at the vesting date. Please refer to section 2021 Long-Term Incentive for more details on the actual vesting of the performance shares.
Philips Group
Remuneration Board of Management1)
in EUR
| Accounting costs in the year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| reported year | annual base compensation2) | base compensation | realized annual incentive | performance shares3) | pension allowances4) | pension scheme costs | other compensation5) | total cost | Fixed-variable remuneration6) | |
| R. Jakobs | 2023 | 1,200,000 | 1,200,000 | 2,004,480 | 968,922 | 267,798 | 31,891 | 109,256 | 4,582,347 | 35%-65% |
| 2022 | 1,200,000 | 256,438 | waived | 112,737 | 57,973 | 6,012 | 11,507 | 444,667 | 75%-25% | |
| A. Bhattacharya | 2023 | 810,000 | 810,000 | 1,075,939 | 793,429 | 197,133 | 31,891 | 94,516 | 3,002,907 | 38%-62% |
| 2022 | 810,000 | 806,250 | waived | 763,1407) | 237,250 | 28,133 | 61,308 | 1,896,081 | 60%-40% | |
| M.J. van Ginneken | 2023 | 630,000 | 630,000 | 846,922 | 614,840 | 125,298 | 31,891 | 53,446 | 2,302,397 | 37%-63% |
| 2022 | 630,000 | 626,250 | waived | 585,4907) | 141,622 | 28,133 | 35,343 | 1,416,837 | 59%-41% | |
| Total | 2023 | 2,640,000 | 3,927,341 | 2,377,191 | 590,228 | 95,673 | 257,218 | 9,887,650 | 36%-64% | |
| 2022 | 1,688,938 | waived | 1,461,367 | 436,845 | 62,278 | 108,158 | 3,757,585 | 61%-39% | ||
Internal pay ratios are a relevant input factor for determining the appropriateness of the implementation of the Remuneration Policy, as recognized in the Dutch Corporate Governance Code. For the 2023 financial year, the ratio between the annual total compensation for the CEO and the average annual total compensation for an employee was 46:1. The ratio decreased from 55:1 in 2022. Further details on the development of these amounts and ratios over time can be found in the following table. Please note that the amounts presented in the following table reflect total remuneration costs to the company which differ from the actual payout to the members of the Board of Management.
Philips Group
Remuneration costs
in EUR
| 2019 | 2020 | 2021 | 2022 | 2023 | |
| Remuneration | |||||
| CEO Total Remuneration Costs (A)1) | 5,260,111 | 6,153,067 | 5,452,299 | 5,133,659 | 4,582,347 |
| CFO Total Remuneration Costs | 2,602,606 | 3,007,990 | 2,652,864 | 1,896,081 | 3,002,907 |
| CLO Total Remuneration Costs | 1,856,426 | 2,203,160 | 2,029,054 | 1,416,837 | 2,302,397 |
| Average Employee (FTE) Total Remuneration Costs (B)2) | 92,645 | 91,455 | 86,853 | 93,373 | 99,870 |
| Ratio A versus B3) | 57:1 | 67:1 | 63:1 | 55:1 | 46:1 |
| Company performance | |||||
| Annual TSR4) | 25.6% | 6.2% | (14.5)% | (60.0)% | 42.9% |
| Comparable Sales Growth%5) | 4.5% | 2.9% | (1.2)% | (2.8)% | 6.0% |
| Adjusted EBITA%5) | 13.2% | 13.2% | 12.0% | 7.4% | 10.6% |
| Free Cash Flow5) | 923 | 1,635 | 900 | (961) | 1,582 |
Please find below a brief summary of the Remuneration Policy for the Supervisory Board, as adopted at the Annual General Meeting of Shareholders 2020. The fee levels in this Remuneration Policy are the same as the Supervisory Board fee levels as determined by our shareholders at the 2018 Extraordinary General Meeting of Shareholders.
The overarching objective of the 2020 Remuneration Policy for the Supervisory Board is to enable its members to fulfill their duties, acting independently: supervising the policies, management and the general affairs of Philips, and supporting the Board of Management and the Executive Committee with advice. Also, the members of the Supervisory Board are guided by the company’s long-term interests, with due observance of the company’s purpose and strategy, taking into account the interests of shareholders and all other stakeholders.
To support the objectives mentioned above, the 2020 Remuneration Policy is aimed at attracting and retaining international Supervisory Board members of the highest caliber and with experience and expertise relevant to our health technology businesses.
In compliance with the Dutch Corporate Governance Code, the 2020 Remuneration Policy provides that the remuneration for the members of the Supervisory Board is not dependent on the results of the company and does not include any shares (or rights to shares). Nevertheless, members of the Supervisory Board are encouraged to hold shares in the company for the purpose of long-term investment to reflect their confidence in the future course of the company. The company does not grant personal loans to members of the Supervisory Board.
The Supervisory Board reviews fee levels in principle every three years, in order to monitor and take account of market developments and manage expectations of our key stakeholders. The levels are aimed at broadly median market levels (and around the 25th percentile market level for the Chairman) paid in the Quantum Peer Group (as used in the 2020 Remuneration Policy for the Board of Management).
The following table provides an overview of the current remuneration structure:
Philips Group
Remuneration Supervisory Board
in EUR
| Chair | Vice Chair | Member | |
|---|---|---|---|
| Supervisory Board | 155,000 | 115,000 | 100,000 |
| Audit Committee | 27,000 | n.a. | 18,000 |
| Remuneration Committee | 21,000 | n.a. | 14,000 |
| Corporate Governance and Nomination & Selection Committee | 21,000 | n.a. | 14,000 |
| Quality & Regulatory Committee | 21,000 | n.a. | 14,000 |
| Attendance fee per inter-European trip | 2,500 | 2,500 | 2,500 |
| Attendance fee per intercontinental trip | 5,000 | 5,000 | 5,000 |
| Entitlement to Philips product arrangement | 2,000 | 2,000 | 2,000 |
| Annual fixed net expense allowance | 11,345 | 2,269 | 2,269 |
| Other travel expenses | As reasonably incurred | ||
The members of the Supervisory Board benefit from coverage under the company’s Directors and Officers (D&O) liability insurance.
Remuneration of the Supervisory Board in 2023The individual members of the Supervisory Board received, by virtue of the positions they held, the following remuneration in 2023:
Philips Group
Remuneration of the Supervisory Board
in EUR
| membership | committees | other compensation1) | total | |
|---|---|---|---|---|
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 |
| P.A.M. Stoffels | 115,000 | 35,000 | 22,269 | 172,269 |
| D.E.I. Pyott | 100,000 | 35,000 | 19,769 | 154,769 |
| A.M. Harrison | 100,000 | 14,000 | 19,769 | 133,769 |
| M.E. Doherty | 100,000 | 27,000 | 27,269 | 154,269 |
| P. Löscher | 100,000 | 32,000 | 17,269 | 149,269 |
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 |
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 |
| H. Verhagen | 100,000 | 14,000 | 7,269 | 121,269 |
| S. Poonen | 100,000 | 18,000 | 19,769 | 137,769 |
| Total | 1,070,000 | 242,000 | 189,266 | 1,501,266 |
The Audit Committee is chaired by Liz Doherty. Its other members are Peter Löscher, Chua Sock Koong and Sanjay Poonen. Feike Sijbesma and Herna Verhagen also attend Audit Committee meetings. The Committee assists the Supervisory Board in fulfilling its supervisory responsibilities, including ensuring the integrity of the company’s financial statements, reviewing the company’s internal controls and overseeing the enterprise risk management process.
In 2023, the Audit Committee held five regular meetings and one extraordinary meeting, which all Audit Committee members attended.
The CEO, CFO, Chief ESG & Legal Officer, Head of Internal Audit, Chief Accounting Officer and external auditor (Ernst & Young Accountants LLP) were invited to and attended all regular meetings.
The Committee also met separately in private sessions with the CEO, CFO, Head of Internal Audit and external auditor after every regular quarterly meeting of the Committee. Prior to the Committee meetings, the Audit Committee chair met one-on-one with the Group Treasurer as well as with each of the management who regularly attend the Audit Committee meetings (as set out in the previous paragraph) and with the external auditor (Ernst & Young Accountants LLP).
The following overview highlights matters that were reviewed and/or discussed during Committee meetings in the course of, or in respect of, the financial year 2023:
In February 2024, the Committee reviewed, together with the other members of the Supervisory Board, the draft of the Annual Report 2023, as well as the key audit matters and the critical audit matters identified by the external auditor in relation to the 2023 financial statements included in the Annual Report 2023 and the Annual Report on Form 20-F, respectively. In February 2024, the Committee also reviewed the draft of the company’s 2023 Country Activity and Tax Report.
During each regular quarterly Audit Committee meeting, the Committee reviewed the quarterly report from the external auditor, in which the auditor set forth its findings and attention points during the relevant period. Apart from the Audit Committee meetings, the external auditor also attended all private sessions with the Audit Committee, where their observations were, if necessary, further discussed. The Annual Audit Letter was circulated to the full Supervisory Board, and planned actions to address the items raised were discussed with management in the subsequent Audit Committee meetings as well as in private sessions with management.
Finally, the Committee reviewed the Audit Committee Charter and concluded it remains appropriate.
The Quality & Regulatory Committee was established in view of the importance of patient safety and the quality of the company’s products, systems, services and solutions. The Committee provides broad oversight of compliance with the regulatory requirements that govern the development, manufacturing, marketing and servicing of the company’s products, systems, services and solutions. The Quality & Regulatory Committee assists the Supervisory Board in fulfilling its oversight responsibilities in these areas. It is chaired by David Pyott and its members are Marc Harrison and Peter Löscher.
In 2023, the Quality & Regulatory Committee held four meetings and all Committee members attended these meetings. The Quality & Regulatory Committee convened less frequently in 2023 (compared to 2022), as the quality related matters were a regular item on the agenda of the Supervisory Board meeting. The Chief Executive Officer, the Chief ESG & Legal Officer, the Chief Operations Officer and the Chief Quality & Regulatory Officer were present during these meetings.
The following overview indicates some of the matters that were discussed during meetings in the course of 2023:
Koninklijke Philips N.V. (Royal Philips), a company organized under Dutch law, is the parent company of the Philips group. Its shares have been listed on the Amsterdam stock exchange (Euronext Amsterdam) since 1912. Furthermore, its shares have been traded in the United States since 1962 and have been listed on the New York Stock Exchange since 1987.
Royal Philips has a two-tier board structure consisting of a Board of Management and a Supervisory Board, each of which is accountable to the General Meeting of Shareholders for the fulfillment of its respective duties.
The company is governed by Dutch corporate and securities laws, its Articles of Association, and the Rules of Procedure of the Board of Management and the Executive Committee and of the Supervisory Board, respectively. Its corporate governance framework is also based on the Dutch Corporate Governance Code (dated December 20, 2022) and US laws and regulations applicable to Foreign Private Issuers. Additionally, the Board of Management has implemented the Philips General Business Principles (GBP) and underlying policies, as well as separate codes of ethics that apply to employees working in specific areas of our business, i.e., the Financial Code of Ethics and the Procurement Code of Ethics. Many of the documents referred to are published on the company’s website and more information can be found in Our approach to risk management.
In this section of the Annual Report, the company addresses the main elements of its corporate governance structure, reports on how it applies the principles and best practices of the Dutch Corporate Governance Code, and provides the information required by the Dutch governmental Decree on Corporate Governance (Besluit inhoud bestuursverslag) and governmental Decree on Article 10 Takeover Directive (Besluit artikel 10 overnamerichtlijn). When deemed necessary in the interests of the company, the company may deviate from aspects of the company’s corporate governance structure, and any such deviations will be disclosed in the company’s corporate governance report.
In compliance with the Dutch Corporate Governance Code, other parts of the management report (within the meaning of article 2:391 of the Dutch Civil Code) included in the Annual Report address the strategy and culture of Philips aimed at sustainable long-term value creation. As described in more detail in Our strategic focus , Philips’ strategy of focused organic growth scalable patient- and people-centric innovation, and focus on reliable execution, is driven by our purpose: to improve people’s health and well-being through meaningful innovation. The Message from the CEO explains how this strategy was executed in 2023; refer also to Financial performance. Furthermore, reference is made to the Philips Operating Model, which among others includes standards for behaviors, quality and integrity within Philips.
Philips’ strategy, and the way it has been developed by the Board of Management under the supervision of the Supervisory Board, clearly integrates the company’s impact in the field of sustainability, including the effects on people and the environment. In How we create value with sustainable impact we report on resource inputs, value outcomes and societal impact across various financial and Environmental, Social and Governance (ESG) dimensions. We engage with our stakeholders and use a double materiality analysis to identify the ESG topics that we believe have the greatest impact: those having financial materiality (the impact of society on Philips) as well as those having impact materiality (the impact of Philips on society); refer to Working with stakeholders and advocacy and Double Materiality Assessment. The materiality analysis underpins the relevance of our fully integrated approach to doing business responsibly and sustainably, including a comprehensive set of key commitments across all the ESG dimensions that guide execution of our strategy; refer to Philips' ESG commitments. As one of these commitments, Philips considers its tax payments as a significant contribution to the communities in which it operates, and an integral part of its social value creation; refer to Total tax contribution.
The Board of Management is entrusted with the management of the company. Certain key officers have been appointed to support the Board of Management in the fulfilment of its managerial duties. The members of the Board of Management and these key officers together constitute the Executive Committee. In this Corporate governance report, wherever the Executive Committee is mentioned, this also includes the members of the Board of Management, unless the context requires otherwise. Please refer to Board of Management and Executive Committee for an overview of the current members.
Under the chairmanship of the President/Chief Executive Officer (CEO), and supported by the other members of the Executive Committee, the members of the Board of Management drive the company’s management agenda and share responsibility for the continuity of the Philips group, focusing on sustainable long-term value creation. Please refer to the Rules of Procedure of the Board of Management and the Executive Committee, which are published on the company’s website, for a description of further responsibilities and tasks, as well as procedures for meetings, resolutions, and minutes.
In fulfilling their duties, the members of the Board of Management and Executive Committee are guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders. The Board of Management and the Executive Committee have adopted a division of responsibilities based on the functional and business areas, each of which is monitored and reviewed by the individual members. The Board of Management is accountable for the actions and decisions of the Executive Committee and has ultimate responsibility for the company’s external reporting (including reporting to the shareholders of the company).
The Board of Management and the Executive Committee are supervised by the Supervisory Board. Members of the Board of Management and the Executive Committee will be present in the meetings of the Supervisory Board, if so invited. In addition, the CEO and other members of the Board of Management (and if needed, the other members of the Executive Committee) meet on a regular basis with the Chairman and other members of the Supervisory Board. The Board of Management and the Executive Committee are required to keep the Supervisory Board informed of all facts and developments concerning Philips that the Supervisory Board may need to be aware of in order to function as required and to properly carry out its duties.
Certain important decisions of the Board of Management require Supervisory Board approval, including decisions concerning: the operational and financial objectives of the company and the strategy designed to achieve these objectives; the issue, repurchase or cancellation of shares; and major acquisitions or divestments.
Appointment and compositionMembers of the Board of Management, including the CEO, are appointed by the General Meeting of Shareholders upon a binding recommendation drawn up by the Supervisory Board after consultation with the CEO. This binding recommendation may be overruled by a resolution of the General Meeting of Shareholders adopted by a simple majority of the votes cast and representing at least one-third of the issued share capital. If a simple majority of the votes cast is in favor of the resolution to overrule the binding recommendation, but such majority does not represent at least one-third of the issued share capital, a new meeting may be convened, at which the resolution may be passed by a simple majority of the votes cast, regardless of the portion of the issued share capital represented by such majority. In the event that a binding recommendation has been overruled, a new binding recommendation shall be submitted to the General Meeting of Shareholders. If such second binding recommendation has been overruled, the General Meeting of Shareholders shall be free to appoint a board member.
The CEO and the other members of the Board of Management are appointed for a (maximum) term of four years, it being understood that this term expires at the closing of the General Meeting of Shareholders to be held in the fourth calendar year after the year of their appointment or, if applicable, at a later retirement date or other contractual termination date in the fourth year, unless the General Meeting of Shareholders resolves otherwise. The same applies in the case of re-appointment, which is possible for consecutive terms of (a maximum of) four years. A (re-)appointment schedule for the Board of Management is published on the company’s website.
Pursuant to Dutch law, the members of the Board of Management are engaged by means of a services agreement (overeenkomst van opdracht). The term of the services agreement is aligned with the term for which the relevant member has been appointed by the General Meeting of Shareholders. In the event of termination of the services agreement by the company, severance payment is limited to a maximum of one year’s base salary. The services agreements provide no additional termination benefits.
Members of the Board of Management may be suspended by the Supervisory Board and by the General Meeting of Shareholders, and members of the Board of Management may be dismissed by the General Meeting of Shareholders (in each case in accordance with the Articles of Association). A shareholders’ resolution to suspend or dismiss a member of the Board of Management, other than a resolution proposed by the Board of Management or the Supervisory Board, may only be adopted by a simple majority of the votes cast, representing at least one third of the issued share capital. The other members of the Executive Committee are appointed, suspended and dismissed by the CEO, subject to approval by the Supervisory Board.
The Supervisory Board supervises the policies, management and general affairs of Philips, and assists the Board of Management and the Executive Committee with advice on general policies related to the activities of the company. In fulfilling their duties, the members of the Supervisory Board shall be guided by the interests of the company and its affiliated enterprise, taking into account the interests of its stakeholders.
In the two-tier corporate structure under Dutch law, the Supervisory Board is a separate body that is independent of the Board of Management and the company. Its independent character is also reflected in the requirement that members of the Supervisory Board can be neither a member of the Board of Management nor an employee of the company. Currently, the Supervisory Board considers all its members to be independent under the Dutch Corporate Governance Code. Furthermore, the members of its Audit Committee are independent under the rules of the US Securities and Exchange Commission, applicable to the Audit Committee.
The Supervisory Board must approve certain important decisions of the Board of Management, including decisions concerning the operational, business and financial objectives of the company and the strategy designed to achieve these objectives, the issue, repurchase or cancellation of shares and major acquisitions or divestments. The Supervisory Board and its individual members each have a responsibility to request from the Board of Management, the Executive Committee and the external auditor all information that the Supervisory Board needs in order to be able to carry out its duties properly as a supervisory body.
Please refer to the Rules of Procedure of the Supervisory Board, which are published on the company’s website, for a description of further responsibilities and tasks, as well as procedures for meetings, resolutions and minutes.
In its report (included in the company’s Annual Report), the Supervisory Board describes the composition and functioning of the Supervisory Board and its committees, their activities in the financial year, the number of committee meetings held and the main items discussed. Please refer to Supervisory Board report. Please also refer to Supervisory Board for an overview of the current members of the Supervisory Board.
Appointment and compositionMembers of the Supervisory Board are appointed by the General Meeting of Shareholders upon a binding recommendation drawn up by the Supervisory Board. This binding recommendation may be overruled by a resolution of the General Meeting of Shareholders adopted by a simple majority of the votes cast and representing at least one-third of the issued share capital. If a simple majority of the votes cast is in favor of the resolution to overrule the binding recommendation, but such majority does not represent at least one-third of the issued share capital, a new meeting may be convened. At this new meeting the resolution may be passed by a simple majority of the votes cast, regardless of the portion of the issued share capital represented by such majority. In the event that a binding recommendation has been overruled, a new binding recommendation shall be submitted to the General Meeting of Shareholders. If such second binding recommendation has been overruled, the General Meeting of Shareholders shall be free to appoint a board member.
The term of appointment of members of the Supervisory Board expires at the closing of the General Meeting of Shareholders to be held after a period of four years following their appointment. There is no age limit requiring the retirement of board members.
In line with the Dutch Corporate Governance Code, members of the Supervisory Board are eligible for re-appointment for a fixed term of four years once, and may subsequently be re-appointed for a period of two years, which appointment may be extended by at most two years. The report of the Supervisory Board must state the reasons for any re-appointment beyond an eight-year period.
A (re-)appointment schedule for the Supervisory Board is published on the company’s website.
Members of the Supervisory Board may be suspended or dismissed by the General Meeting of Shareholders in accordance with the Articles of Association. A resolution to suspend or dismiss a member of the Supervisory Board, other than a resolution proposed by the Supervisory Board, may only be adopted by a simple majority of the votes cast, representing at least one third of the issued share capital.
Candidates for appointment to the Supervisory Board are selected taking into account the company’s Diversity Policy, which is published on the company’s website. The Supervisory Board’s composition furthermore follows the profile included in the Rules of Procedure of the Supervisory Board, and the size of the board may vary as it considers appropriate to support its profile. Please refer to Supervisory Board report by the Supervisory Board. Typically, newly appointed members of the Supervisory Board follow an induction program and interact with Executive Committee members for deep-dives on matters such as strategy, finance and investor relations, quality, governance, legal, sustainability and digitization.
Effective 2022, Dutch law provides a mandatory gender quota, requiring that at least one-third of the Supervisory Board members are women and at least one-third men (for calculation purposes, a total number of board members that cannot be divided by three must be rounded up to the next number that can be divided by three). The quota is applicable to (i) the appointment of new Supervisory Board members, and (ii) the re-appointment of acting board members after eight years following their initial appointment. Except in certain exceptional circumstances, any appointment or re-appointment resulting in a Supervisory Board composition that does not meet (or no longer meets) the quota, will be invalid (null and void).
As announced on August 14, 2023, Philips and Exor N.V. entered into a relationship agreement on August 13, 2023, as a result of which Exor bought a 15% shareholding in the company. The relationship agreement with Exor includes Exor’s commitment to be a long-term minority investor and it's right to propose one member to the Supervisory Board. In this context, it is noted that, for as long as Exor has such nomination right pursuant to the relationship agreement, the independence exception of best practice provision 2.1.7(iii) of the Dutch Corporate Governance Code is deemed to apply to any Exor nominee that has been appointed upon such nomination in accordance with the Relationship Agreement. It is expected that the Supervisory Board will, upon Exor’s exercise of its right, submit a proposal for the appointment of the relevant nominee at the upcoming 2024 Annual General Meeting of Shareholders.
Supervisory Board committeesThe Supervisory Board, while retaining overall responsibility, has assigned certain tasks to four committees: the Corporate Governance and Nomination & Selection Committee, the Remuneration Committee, the Audit Committee, and the Quality & Regulatory Committee. Each committee reports to the full Supervisory Board. Please refer to the charters of the respective committees, which are published on the company’s website as part of the Rules of Procedure of the Supervisory Board, for a description of their responsibilities, composition, meetings and working procedures.
The Corporate Governance and Nomination & Selection Committee is responsible for preparing selection criteria and appointment procedures for members of the Supervisory Board, the Board of Management and the Executive Committee. The Committee makes proposals to the Supervisory Board for the (re)appointment of such members, and periodically assesses their functioning. The Committee also periodically assesses the Executive Committee succession planning and the Diversity Policy, and supervises the policy of the Executive Committee on the selection criteria and appointment procedures for Philips executives. At least once a year, the Committee reviews the corporate governance principles applicable to the company, and advises the Supervisory Board on any changes to these principles that it deems appropriate.
The Remuneration Committee is responsible for preparing decisions of the Supervisory Board on the remuneration of individual members of the Board of Management and the Executive Committee. The Committee prepares an annual remuneration report, which is published on the company’s website by the Supervisory Board ahead of the Annual General Meeting of Shareholders. In performing its duties and responsibilities, the Remuneration Committee is assisted by an external consultant and an in-house remuneration expert.
The Audit Committee assists the Supervisory Board in fulfilling its oversight responsibilities for: the integrity of the company’s financial statements; the financial and non-financial (ESG) reporting processes; the effectiveness (also in respect of the reporting process) of the risk management and internal controls framework; the internal and external audit process; the internal and external auditor’s qualifications, independence and performance; as well as the company’s process for monitoring compliance with laws and regulations and the GBP (including related manuals, training and tools). It reviews the company’s annual and interim financial statements, including non-financial information, prior to publication and advises the Supervisory Board on the adequacy and appropriateness of internal control policies and internal audit programs and their findings. The Committee furthermore supervises the Internal Audit function, maintains contact with and supervises the external auditor and prepares the nomination of the external auditor for appointment by the General Meeting of Shareholders.
The composition of the Audit Committee meets the relevant requirements under Dutch law and the applicable US rules. All of the members are considered to be independent and financially literate, and the Audit Committee as a whole has competence relevant to the sector in which the company is operating. In addition, Liz Doherty is designated as an Audit Committee financial expert, as defined under the regulations of the US Securities and Exchange Commission. The Supervisory Board considers the expertise and experience available in the Audit Committee, in conjunction with the possibility to take advice from internal and external experts and advisors, to be sufficient for the fulfillment of the tasks and responsibilities of the Audit Committee.
The Quality & Regulatory Committee has been established by the Supervisory Board in view of the central importance of the quality and (patient) safety of the company’s products, systems, services and software as well as the development, testing, manufacturing, marketing and servicing thereof, and the regulatory requirements relating thereto. The Quality & Regulatory Committee assists the Supervisory Board in fulfilling its oversight responsibilities in this area, while recognizing that the Audit Committee assists the Supervisory Board in its oversight of other areas of regulatory, compliance and legal matters.
The annual financial statements are prepared by the Board of Management and reviewed by the Supervisory Board upon the advice of its Audit Committee, taking into account the report of the external auditor. Upon approval by the Supervisory Board, the accounts are signed by all members of both the Board of Management and the Supervisory Board and are published together with the opinion of the external auditor. The Board of Management is responsible, under the supervision of the Supervisory Board, for the quality and completeness of such publicly disclosed financial reports. The annual financial statements are presented for discussion and adoption at the Annual General Meeting of Shareholders, to be convened subsequently.
The external auditor is appointed by the General Meeting of Shareholders in accordance with the Articles of Association. Philips’ current external auditor, Ernst & Young Accountants LLP, was appointed by the General Meeting of Shareholders held on May 7, 2015, for a term of four years starting January 1, 2016, was re-appointed at the Annual General Meeting of Shareholders held on May 9, 2019 for a term of three years starting January 1, 2020, was re-appointed at the Annual General Meeting of Shareholders held on May 10, 2022 for a term of one year starting January 1, 2023, and was re-appointed at the Annual General Meeting of Shareholders held on May 9, 2023 for a term of one year starting January 1, 2024.
PricewaterhouseCoopers Accountants N.V. was appointed at the Annual General Meeting of Shareholders held on May 9, 2023 as the company’s new external auditor for a term of four years starting January 1, 2025.
European and Dutch law requires the separation of audit and certain non-audit services. The external auditor may only provide audit and audit-related services and is prohibited from providing any other services. This is reflected in the Auditor Policy, which is published on the company’s website. The policy is also in line with (and in some ways stricter than) applicable US rules, under which the appointed external auditor must be independent from the company both in fact and appearance.
The Auditor Policy specifies certain audit services and audit-related services (also known as assurance services) that will or may be provided by the external auditor, and includes rules for the pre-approval by the Audit Committee of such services. Audit services must be pre-approved on the basis of the annual audit services engagement agreed with the external auditor. Proposed audit-related services may be pre-approved at the beginning of the year by the Audit Committee (annual pre-approval) or may be pre-approved during the year by the Audit Committee with respect to a particular engagement (specific pre-approval). The annual pre-approval is based on a detailed, itemized list of services to be provided, which is designed to ensure that there is no management discretion in determining whether a service has been approved, and to ensure that the Audit Committee is informed of each of the services it is pre-approving. Unless pre-approval with respect to a specific service has been given at the beginning of the year, each proposed service requires specific pre-approval during the year. Any annually pre-approved services where the fee for the engagement is expected to exceed pre-approved cost levels or budgeted amounts will also require specific pre-approval. The term of any annual pre-approval is 12 months from the date of the pre-approval unless the Audit Committee states otherwise. During 2023, there were no services provided to the company by the external auditor that were not pre-approved by the Audit Committee.
Stichting Preferente Aandelen Philips, a Foundation (stichting) organized under Dutch law, has been granted the right to acquire preference shares in the capital of Royal Philips, as stated in the company’s Articles of Association. In addition, the Foundation has the right to file a petition with the Enterprise Chamber of the Amsterdam Court of Appeal to commence an inquiry procedure within the meaning of article 2:344 of the Dutch Civil Code.
The object of the Foundation is to represent the interests of Royal Philips, the enterprises maintained by the company and its affiliated companies within the company’s group, in such a way that the interests of the company, these enterprises and all parties involved with them are safeguarded as effectively as possible, and that they are afforded maximum protection against influences which, in conflict with those interests, may undermine the autonomy and identity of Philips and those enterprises, and also to do anything related to the above ends or conducive to them. The Foundation's object includes the protection of Philips against (an attempt at) an unsolicited takeover or other attempt to exert (de facto) control of the company. The arrangement will allow Philips to determine its position in relation to the relevant third party (or parties) and its (their) plans, to seek alternatives and to defend the company’s interests and those of its stakeholders.
The mere notification that the Foundation exercises its right to acquire preference shares will result in such shares being effectively issued. The Foundation may exercise this right for as many preference shares as there are common shares in the company outstanding at that time. No preference shares have been issued as of December 31, 2023.
The members of the self-electing Board of the Foundation are Messrs J.P. de Kreij, J.V. Timmermans, J. van der Veer and P.N. Wakkie. No Philips Supervisory Board or Board of Management members or Philips officers are represented on the board of the Foundation.
Other protective measuresOther than the arrangements made with the Foundation referred to above, the company does not have any measures that exclusively or almost exclusively have the purpose of defending against unsolicited public offers for shares in the capital of the company. It should be noted that the Board of Management and the Supervisory Board remain under all circumstances authorized to exercise all powers vested in them to promote the interests of Philips.
The company has issued certain corporate bonds, the provisions of which contain a 'Change of Control Triggering Event' or a 'Change of Control Put Event’. Upon the occurrence of such events, the company might be required to offer to redeem or purchase any outstanding bonds at certain pre-determined prices. Please also refer to Debt. Furthermore, the Relationship Agreement entered into between the company and its long-term minority investor Exor N.V. (published on the company’s website) includes certain temporary lock-up obligations for Exor which fall away when any third party has ‘Acquired’ an ‘Interest’ of fifty percent (50%) or more in the company.
The company began as a limited partnership with the name Philips & Co in Eindhoven, the Netherlands, in 1891, and was converted into the company with limited liability N.V. Philips’ Gloeilampenfabrieken on September 11, 1912. The company’s name was changed to Philips Electronics N.V. on May 6, 1994, to Koninklijke Philips Electronics N.V. on April 1, 1998, and to Koninklijke Philips N.V. on May 15, 2013.
The majority of the shares in Royal Philips are held through the system maintained by the Dutch Central Securities Depository (Euroclear Nederland). In the past, Philips has also issued (physical) bearer share certificates ('Share Certificates'). A limited number of Share Certificates have not been surrendered yet, although the holders of Share Certificates are still entitled to a corresponding number of shares in Royal Philips. It is noted that, as a result of Dutch legislation that became effective in July 2019, the relevant shares were registered in the name of Royal Philips by operation of law per January 1, 2021. Owners of Share Certificates will continue to be entitled to a corresponding number of shares, but may not exercise the rights attached to such shares until they surrender their Share Certificates. Owners of Share Certificates may come forward to do so and to receive a corresponding number of shares until January 1, 2026, at the latest. As per January 2, 2026, entitlements attached to the Share Certificates not surrendered will expire by operation of law. For more information, please contact the Investor Relations department by email (investor.relations@philips.com) or telephone (+31-20-59 77222).
The statutory seat of the company is Eindhoven, the Netherlands, and the statutory list of all subsidiaries and affiliated companies, prepared in accordance with the relevant legal requirements (Dutch Civil Code, Book 2, articles 379 and 414), forms part of the notes to the financial statements and is deposited at the office of the Commercial Register in Eindhoven, the Netherlands (file no. 17001910). The executive offices of the company are located at the Philips Center, Amstelplein 2, 1096 BC Amsterdam, the Netherlands, telephone +31-20-59 77777.
Set forth below is a summary of certain provisions of the Articles of Association of the company, applicable Dutch law and related company policies. This summary does not constitute legal advice regarding those matters and should not be regarded as such.
Object and purposeThe objects of the company are to establish, participate in, administer and finance legal entities, companies and other legal forms for the purpose of the manufacture and trading of electrical, electronic, mechanical or chemical products, the development and exploitation of technical and other expertise, including software, or for the purpose of other activities, and to do everything pertaining thereto or connected therewith, including the provision of security in particular for commitments of business undertakings which belong to its group, all this in the widest sense, as may also be conducive to the proper continuity of the collectivity of business undertakings, in the Netherlands and abroad, which are carried on by the company and the companies in which it directly or indirectly participates. These objects can be found in Article 2 of the Articles of Association.
Share CapitalOn December 31, 2023, the issued share capital amounted to EUR 182,703,193.20 divided into 913,515,966 common shares and no preference shares.
Voting rightsAll issued and outstanding shares carry voting rights and each share confers the right to cast one vote in a shareholders’ meeting. Pursuant to Dutch law, no votes may be cast at a General Meeting of Shareholders in respect of shares which are held by the company. There are no special statutory rights attached to the shares of the company and no restrictions on the voting rights of the company’s shares exist. Major shareholders do not have different voting rights than other shareholders.
DividendsA dividend will first be declared on preference shares out of net income. The Board of Management has the power to determine what portion of the net income shall be retained by way of reserve, subject to the approval of the Supervisory Board. The remainder of the net income, after reservations made, shall be available for distribution to holders of common shares subject to shareholder approval after year-end.
Liquidation rightsIn the event of the dissolution and liquidation of the company, the assets remaining after payment of all debts and liquidation expenses are to be distributed in the following order of priority: to the holders of preference shares, the amount paid thereon; and the remainder to the holders of the common shares.
Preemptive rightsShareholders have a pro rata preferential right of subscription to any common share issuance unless the right is restricted or excluded. If designated by the General Meeting of Shareholders, the Board of Management has the power to restrict or exclude the preferential subscription rights. A designation of the Board of Management will be effective for a specified period of up to five years and may be renewed. Currently, the Board of Management has been granted the power to restrict or exclude the preferential right of subscription up to and including November 8, 2024. If the Board of Management has not been designated, the General Meeting of Shareholders has the power to restrict or exclude such rights, upon the proposal of the Board of Management, which proposal must be approved by the Supervisory Board. Resolutions by the General Meeting of Shareholders referred to in this paragraph require approval of at least two-thirds of the votes cast if less than half of the issued share capital is represented at the meeting.
The foregoing provisions also apply to the issuance of rights to subscribe for shares.
General Meeting of ShareholdersThe Annual General Meeting of Shareholders shall be held each year not later than the thirtieth day of June and, at the Board of Management’s option, in Eindhoven, Amsterdam, The Hague, Rotterdam, Utrecht or Haarlemmermeer (including Schiphol airport); the notice convening the meeting shall inform the shareholders accordingly. Without prejudice to applicable laws and regulations, the Board of Management may resolve to give notice to holders of its listed and traded via a stock exchange shares via the company’s website and/or by other electronic means representing a public announcement, which announcement remains directly and permanently accessible until the General Meeting of Shareholders. Holders of registered shares shall be notified by letter, unless the Board of Management resolves to give notice to holders of registered shares by electronic means of communication by sending a legible and reproducible message to the address indicated by the shareholder to the company for such purpose provided the relevant shareholder has agreed hereto.
In principle, all shareholders are entitled to attend a General Meeting of Shareholders, to address the meeting and to vote, except for shares held in treasury by the company. They may exercise the aforementioned rights at a meeting only for the common shares which on the record date are registered in their name. The record date is published in the above announcement and is, pursuant to Dutch law, set as the 28th day prior to the day of the relevant meeting. Holders of registered shares must advise the company in writing of their intention to attend the General Meeting of Shareholders. Holders of shares listed and traded via a stock exchange who either in person or by proxy wish to attend the General Meeting of Shareholders, should notify ABN AMRO Bank N.V., which is acting as agent for the company. They must submit a confirmation by a participating institution, in which administration they are registered as holders of the shares, that such shares are registered and will remain registered in its administration up to and including the record date, whereupon the holder will receive an admission ticket for the General Meeting of Shareholders. Holders of shares who wish to attend by proxy have to submit the proxy at the same time. A participating institution is a bank or broker which, according to the Dutch Securities Depository Act (Wet giraal effectenverkeer), is an intermediary (intermediair) of the Dutch Central Securities Depository (Euroclear Nederland).
In connection with the General Meeting of Shareholders, the company does not solicit proxies within the United States.
The Articles of Association of the company provide that there are no quorum requirements to hold a General Meeting of Shareholders. Subject to certain exceptions provided by Dutch law and/or the Articles of Association, resolutions of the General Meeting of Shareholders are passed by an absolute majority of votes cast and do not require a quorum.
Limitations on right to hold or vote Common SharesThere are no limitations imposed by Dutch law or by the Articles of Association on the right of non-resident owners to hold or vote the Common Shares.
Exchange controlsCash dividends paid in euros on Dutch registered shares and bearer shares may be officially transferred from the Netherlands and converted into any other currency without Dutch legal restrictions, except that for statistical purposes such payments and transactions must be reported to the Dutch Central Bank. Furthermore, no payments, including dividend payments, may be made to jurisdictions subject to sanctions adopted by the government of the Netherlands and implementing resolutions of the Security Council of the United Nations.
The Articles of Association of the company provide that cash distributions on New York Registry Shares shall be paid in US dollars, converted at the rate of exchange on the stock market of Euronext Amsterdam at the close of business on the day fixed and announced for that purpose by the Board of Management.
Significant differences in corporate governance practicesThe corporate governance rules established by the New York Stock Exchange (NYSE) allow Foreign Private Issuers, like Royal Philips, to follow home country practices on most corporate governance matters instead of those that apply to US domestic issuers, provided that they disclose any significant ways in which their corporate governance practices differ from those applying to listed US domestic issuers under the NYSE listing standards. The following paragraphs summarize what we believe to be the significant differences between certain Dutch practices on corporate governance matters and the corporate governance provisions applicable to US domestic issuers under the NYSE listing standards.
Dutch corporate governance codeThe company is a company organized under Dutch law, with its Common Shares listed on Euronext Amsterdam, and is subject to the Dutch Corporate Governance Code of December 20, 2022 (the Dutch Corporate Governance Code). Philips’ New York Registry Shares, representing Common Shares of the company, are listed on the NYSE.
Board structureThe NYSE listing standards prescribe regularly scheduled executive sessions of non-executive directors. The company has a two-tier corporate structure consisting of a Board of Management consisting of executive directors under the supervision of a Supervisory Board consisting exclusively of non-executive directors. Members of the Board of Management and other officers and employees cannot simultaneously act as member of the Supervisory Board. The Supervisory Board must approve specified decisions of the Board of Management.
Independence of members of our Supervisory BoardThe Dutch Corporate Governance Code sets forth certain best practices limiting the number of non-independent members of the Supervisory Board, and its committees. The Supervisory Board considers all its members to be independent under the Dutch Corporate Governance Code. The definitions of independence under the Dutch Corporate Governance Code, however, differ in their details from the definitions of independence under the NYSE listing standards. In some cases the Dutch requirements are stricter than the NYSE listing standards, and in other cases the NYSE listing standards are the stricter of the two. The members of the Audit Committee of the Supervisory Board are also independent under the NYSE listing standards.
Committees of our Supervisory BoardThe company has established four committees, consisting of members of the Supervisory Board only: the Audit Committee, the Remuneration Committee, the Corporate Governance and Nomination & Selection Committee and the Quality & Regulatory Committee. The roles, responsibilities and composition of these committees reflect the requirements of the Dutch Corporate Governance Code, the company’s Articles of Association and Dutch law, which differ from the NYSE listing standards in these respects. The role of each committee is to advise the Supervisory Board and to prepare the decision-making of the Supervisory Board. In principle, the entire Supervisory Board remains responsible for its decisions even if such decisions were prepared by one of the Supervisory Board’s committees.
The NYSE requires that, when an audit committee member of a listed US domestic issuer serves on four or more audit committees of public companies, the listed company should disclose (either on its website or in its Annual Report on Form 10-K) that the board of directors has determined that this simultaneous service would not impair the director’s service to the listed company. Dutch law does not require the company to make such a determination.
In accordance with the procedures laid down in the Philips Auditor Policy and as mandatorily required by Dutch law, the external auditor of the company is appointed by the General Meeting of Shareholders on the proposal of the Supervisory Board, after the latter has been advised by the Audit Committee and the Board of Management.
Equity compensation plansThe company complies with Dutch legal requirements regarding shareholder approval of equity compensation plans for the members of the Board of Management. Dutch law does not require shareholder approval of certain equity compensation plans for which the NYSE listing standards would require such approval. The company is subject to a Dutch requirement to seek shareholder approval for equity compensation plans for its members of the Board of Management.
Code of business conductThe listing standards of the NYSE prescribe certain parameters for listed company codes of business conduct and ethics. The company has implemented the Philips General Business Principles, which are applicable to all employees, and a Financial Code of Ethics, which is applicable to all employees performing an accounting or financial function. Waivers granted to Senior (Financial) Officers (as defined in our Financial Code of Ethics) must be disclosed. In 2023 the company did not grant any waivers of the Financial Code of Ethics.
Related party transactionsThe NYSE listing standards require certain transactions with related parties to be reviewed by a company’s audit committee or another independent body of the board of directors for potential conflicts of interest, and for the audit committee or other independent body to prohibit such a transaction if it determines it to be inconsistent with the interests of the company and its shareholders. However, foreign private issuers can rely on home country practice with respect to review and approval of related party transactions. Philips has internal procedures in place to confirm that related party transactions are entered into at arm’s length and, if and to the extent required under Dutch law, to enable the Supervisory Board to assess the terms of significant related party transactions.
New York Registry SharesCertain common shares of the company are registered in the register maintained by Deutsche Bank Trust Company Americas, as the New York transfer agent, registrar and dividend disbursing agent (the “New York Transfer Agent”), pursuant to a Transfer Agent Agreement, dated July 16, 2018, between the New York Transfer Agent and the company (such common shares, “New York Registry Shares”). As soon as practicable after receipt from the company, the New York Transfer Agent will provide holders of New York Registry Shares with a notice of any meeting or solicitation of consents or proxies with a notice prepared by the company stating (a) such information as is contained in such notice of meeting and any solicitation materials (or a summary thereof in English provided by the company), (b) that each registered holder at the close of business on the record date set by the company therefor will be entitled, subject to any applicable provisions of Dutch law and the Articles of Association, to exercise the voting rights pertaining to the New York Registry Shares, and (c) the manner in which such voting rights may be exercised. The New York Transfer Agent may, to the extent not prohibited by applicable law or by the requirements of the New York Stock Exchange, in lieu of distribution of the materials provided to it in connection with any meeting of, or solicitation of consents or proxies from, holders of common shares, distribute to the registered holders of New York Registry Shares a notice that provides such holders with, or otherwise publicizes to such holders, instructions on how to retrieve such materials or receive such materials upon request (i.e. by reference to a website containing the materials for retrieval or a contact for requesting copies of the materials).
Group financial statements contents
The Company’s Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of the design and operation of the company’s disclosure controls and procedures (as defined in Rules 13a15(e) and 15d15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by the Annual Report. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that these disclosure controls and procedures are effective as of December 31, 2023.
The Board of Management of Koninklijke Philips N.V. (Royal Philips) is responsible for establishing and maintaining an adequate system of internal control over financial reporting (as such term is defined in Rule 13a-15 (f) under the US Securities Exchange Act). Internal control over financial reporting is a process to provide reasonable assurance regarding the reliability of our financial reporting for external purposes in accordance with IFRS as issued by the IASB.
Internal control over financial reporting includes maintaining records that, in reasonable detail, accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of company assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of company assets that could have a material effect on our financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Also, projections of any evaluation of the effectiveness of internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Board of Management conducted an assessment of Royal Philips' internal control over financial reporting based on the “Internal Control Integrated Framework (2013)” established by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
Based on the Board of Management’s assessment of the effectiveness of Royal Philips' internal control over financial reporting as of December 31, 2023, it has concluded that, as of December 31, 2023, Royal Philips' internal control over Group financial reporting is considered effective.
The effectiveness of the Royal Philips’ internal control over financial reporting as of December 31, 2023, as included in this section Group financial statements, has been audited by Ernst & Young Accountants LLP, an independent registered public accounting firm, as stated in their report which follows hereafter.
There were no changes in our internal control over financial reporting during 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s report on internal control over financial reporting is set out on Management's annual report on internal control over financial reporting. The report set out on Independent auditor’s report on internal control over financial reporting, is provided in compliance with standards of the Public Company Accounting Oversight Board in the US and includes an opinion on the effectiveness of internal control over financial reporting as at December 31, 2022, based on COSO criteria.
Ernst & Young Accountants LLP (PCAOB ID: 1396) has also issued a report on the consolidated financial statements in accordance with the standards of the Public Company Accounting Oversight Board in the US, which is set out on Independent auditor’s report on the consolidated financial statements.
To: The Supervisory Board and Shareholders of Koninklijke Philips N.V.
Opinion on Internal Control over Financial ReportingWe have audited Koninklijke Philips N.V.’s internal control over financial reporting as of December 31, 2023, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, Koninklijke Philips N.V. (the Company) maintained, in all material respects, effective internal control over financial reporting as of December 31, 2023, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2023 and 2022, the related consolidated statements of income, comprehensive income, cash flows and changes in equity for each of the three years in the period ended December 31, 2023, and the related notes and our report dated February 20, 2024 expressed an unqualified opinion thereon.
The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying section ‘Management’s report on internal control’, of this Annual Report. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young Accountants LLP
Amsterdam, the Netherlands
February 20, 2024
Philips Group
Consolidated statements of income
in millions of EUR
For the year ended December 31
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Sales6 | 17,156 | 17,827 | 18,169 |
| Cost of sales | (9,988) | (10,633) | (10,721) |
| Gross margin | 7,168 | 7,194 | 7,448 |
| Selling expenses | (4,258) | (4,621) | (4,524) |
| General and administrative expenses | (599) | (671) | (608) |
| Research and development expenses | (1,806) | (2,091) | (1,890) |
| Impairment of goodwill11 | (15) | (1,357) | (8) |
| Other business income6 | 186 | 127 | 112 |
| Other business expenses6 | (123) | (109) | (645) |
| Income from operations6 | 553 | (1,529) | (115) |
| Financial income7 | 149 | 58 | 63 |
| Financial expenses7 | (188) | (258) | (376) |
| Investments in associates, net of income taxes | (4) | (2) | (98) |
| Income before taxes | 509 | (1,731) | (526) |
| Income tax (expense) benefit8 | 103 | 113 | 73 |
| Income from continuing operations | 612 | (1,618) | (454) |
| Discontinued operations, net of income taxes3 | 2,711 | 13 | (10) |
| Net income | 3,323 | (1,605) | (463) |
| Attribution of net income: | |||
| Net income attributable to shareholders of Koninklijke Philips N.V. | 3,319 | (1,608) | (466) |
| Net income attributable to non-controlling interests | 4 | 3 | 2 |
Philips Group
Earnings per common share attributable to shareholders of Koninklijke Philips N.V.
in EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Basic earnings per common share attributable to shareholders of Koninklijke Philips N.V. 1) | |||
| Income from continuing operations | 0.64 | (1.76) | (0.50) |
| Net income | 3.52 | (1.75) | (0.51) |
| Diluted earnings per common share attributable to shareholders of Koninklijke Philips N.V. 1) | |||
| Income from continuing operations | 0.64 | (1.76) | (0.50) |
| Net income | 3.50 | (1.75) | (0.51) |
Amounts may not add up due to rounding.
Philips Group
Consolidated statements of comprehensive income
in millions of EUR
For the year ended December 31
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net income for the period | 3,323 | (1,605) | (463) |
| Pensions and other-post employment plans:20 | |||
| Remeasurement, before tax | 134 | 101 | (26) |
| Income tax effect on remeasurements8 | (21) | (20) | 3 |
| Financial assets fair value through OCI: | |||
| Net current-period change, before tax | (39) | (32) | (20) |
| Income tax effect on net current-period change | 1 | 1 | 3 |
| Total of items that will not be reclassified to Income Statement | 74 | 49 | (40) |
| Currency translation differences: | |||
| Net current period change, before tax | 1,078 | 748 | (579) |
| Income tax effect on net current-period change8 | (5) | 2 | - |
| Reclassification adjustment for (gain) loss realized | 36 | - | (26) |
| Reclassification adjustment for (gain) loss realized, in discontinued operations | 69 | ||
| Cash flow hedges: | |||
| Net current-period change, before tax | (52) | (29) | 29 |
| Income tax effect on net current-period change8 | 18 | (10) | (2) |
| Reclassification adjustment for (gain) loss realized | (14) | 63 | (19) |
| Total of items that are or may be reclassified to Income Statement | 1,129 | 774 | (597) |
| Other comprehensive income for the period | 1,203 | 823 | (637) |
| Total comprehensive income for the period | 4,527 | (782) | (1,100) |
| Total comprehensive income (loss) attributable to: | |||
| Shareholders of Koninklijke Philips N.V. | 4,520 | (786) | (1,101) |
| Non-controlling interests | 7 | 4 | 1 |
Amounts may not add up due to rounding.
Philips Group
Consolidated balance sheets
in millions of EUR unless otherwise stated
As of December 31
| 2022 | 2023 | |
|---|---|---|
| Non-current assets | ||
| Property, plant and equipment 102 | 2,638 | 2,483 |
| Goodwill112 | 10,238 | 9,876 |
| Intangible assets excluding goodwill122 | 3,526 | 3,190 |
| Non-current receivables16 | 279 | 193 |
| Investments in associates5 | 537 | 381 |
| Other non-current financial assets13 | 660 | 619 |
| Non-current derivative financial assets28 | 4 | 3 |
| Deferred tax assets8 | 2,449 | 2,627 |
| Other non-current assets14 | 98 | 93 |
| Total non-current assets | 20,429 | 19,466 |
| Current assets | ||
| Inventories15 | 4,049 | 3,491 |
| Other current financial assets13 | 11 | 3 |
| Other current assets14 | 490 | 500 |
| Current derivative financial assets28 | 123 | 45 |
| Income tax receivable | 222 | 220 |
| Current receivables2516 | 4,115 | 3,733 |
| Assets classified as held for sale3 | 77 | 79 |
| Cash and cash equivalents29 | 1,172 | 1,869 |
| Total current assets | 10,259 | 9,940 |
| Total assets | 30,688 | 29,406 |
| Equity17 | ||
| Shareholders' equity | 13,249 | 12,028 |
| Common shares | 178 | 183 |
| Capital in excess of par value | 5,025 | 5,827 |
| Reserves | 1,488 | 879 |
| Other | 6,558 | 5,139 |
| Non-controlling interests17 | 34 | 33 |
| Group equity | 13,283 | 12,061 |
| Non-current liabilities | ||
| Long-term debt 18 | 7,270 | 7,035 |
| Non-current derivative financial liabilities28 | 4 | 3 |
| Long-term provisions2019 | 1,097 | 1,035 |
| Deferred tax liabilities8 | 91 | 71 |
| Non-current contract liabilities22 | 515 | 469 |
| Non-current tax liabilities 8 | 435 | 390 |
| Other non-current liabilities22 | 60 | 54 |
| Total non-current liabilities | 9,471 | 9,058 |
| Current liabilities | ||
| Short-term debt 18 | 931 | 654 |
| Current derivative financial liabilities28 | 207 | 40 |
| Income tax payable | 40 | 83 |
| Accounts payable25 | 1,968 | 1,917 |
| Accrued liabilities21 | 1,626 | 1,887 |
| Current contract liabilities22 | 1,696 | 1,809 |
| Short-term provisions2019 | 1,018 | 1,463 |
| Dividend payable | - | 11 |
| Liabilities directly associated with assets held for sale | - | 9 |
| Other current liabilities22 | 448 | 414 |
| Total current liabilities | 7,934 | 8,287 |
| Total liabilities and group equity | 30,688 | 29,406 |
Amounts may not add up due to rounding.
Philips Group
Consolidated statements of cash flows
in millions of EUR
For the year ended December 31
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Cash flows from operating activities | |||
| Net income (loss) | 3,323 | (1,605) | (463) |
| Results of discontinued operations, net of income tax | (2,711) | (13) | 10 |
| Adjustments to reconcile net income to net cash provided by (used for) operating activities: | |||
| Depreciation, amortization, and impairment of assets | 1,323 | 1,602 | 1,261 |
| Impairment of goodwill | 15 | 1,357 | 8 |
| Share-based compensation | 108 | 95 | 88 |
| Net loss (gain) on sale of assets | 55 | (115) | (71) |
| Interest income | (18) | (25) | (46) |
| Interest expense on debt, borrowings, and other liabilities | 152 | 226 | 255 |
| Investments in associates, net of income taxes | 4 | 112 | 107 |
| Income taxes | (103) | (113) | (71) |
| Decrease (increase) in working capital | (401) | (862) | 913 |
| Decrease (increase) in receivables and other current assets | (39) | (342) | 298 |
| Decrease (Increase) in inventories | (581) | (572) | 257 |
| Increase (decrease) in accounts payable, accrued and other current liabilities | 219 | 52 | 358 |
| Decrease (increase) in non-current receivables and other assets | (46) | 1 | (33) |
| Increase (decrease) in other liabilities | 33 | (84) | (38) |
| Increase (decrease) in provisions19 | 427 | (199) | 422 |
| Other items | (164) | (39) | 129 |
| Interest received | 17 | 15 | 53 |
| Interest paid | (151) | (205) | (250) |
| Dividends received from investments in associates | 14 | 12 | 13 |
| Income taxes paid | (249) | (333) | (152) |
| Net cash provided by (used for) operating activities | 1,629 | (173) | 2,136 |
| Cash flows from investing activities | |||
| Net capital expenditures | (729) | (788) | (554) |
| Purchase of intangible assets | (107) | (105) | (96) |
| Expenditures on development assets | (259) | (257) | (203) |
| Capital expenditures on property, plant and equipment | (397) | (444) | (345) |
| Proceeds from sales of property, plant and equipment | 33 | 18 | 90 |
| Net proceeds from (cash used for) derivatives and current financial assets23 | 48 | (72) | (46) |
| Purchase of other non-current financial assets23 | (124) | (116) | (92) |
| Proceeds from other non-current financial assets23 | 124 | 78 | 48 |
| Purchase of businesses, net of cash acquired45 | (3,098) | (712) | (73) |
| Net proceeds from sale of interests in businesses, net of cash disposed | 107 | 124 | 80 |
| Net cash provided by (used for) for investing activities | (3,672) | (1,487) | (636) |
| Cash flows from financing activities | |||
| Proceeds from issuance (payments on) short-term debt1823 | (25) | 47 | 29 |
| Principal payments on current portion of long-term debt1823 | (302) | (1,472) | (754) |
| Proceeds from issuance of long-term debt1823 | 76 | 2,516 | 544 |
| Re-issuance of treasury shares | 23 | 12 | |
| Purchase of treasury shares17 | (1,636) | (187) | (662) |
| Dividends paid to shareholders of Koninklijke Philips N.V. | (482) | (412) | (2) |
| Dividends paid to shareholders of non-controlling interests | (2) | (6) | (3) |
| Net cash provided by (used for) financing activities | (2,347) | 500 | (848) |
| Net cash provided by (used for) continuing operations | (4,390) | (1,160) | 652 |
| Net cash provided by (used for) discontinued operations3 | 3,403 | (12) | 123 |
| Net cash provided by (used for) continuing and discontinued operations | (986) | (1,172) | 776 |
| Effect of changes in exchange rates on cash and cash equivalents | 65 | 41 | (79) |
| Cash and cash equivalents at the beginning of the period | 3,226 | 2,303 | 1,172 |
| Cash and cash equivalents at the end of the period | 2,303 | 1,172 | 1,869 |
Amounts may not add up due to rounding.
Philips Group
Consolidated statements of changes in equity
in millions of EUR
For the year ended December 31
Common shares |
Capital in excess of par value |
Fair value through OCI |
Cash flow hedges |
Currency translation differences |
Retained earnings |
Treasury shares |
Total shareholders' equity |
Non-controlling interests |
Group equity |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reserves | Other | |||||||||||
| Balance as of January 1, 2021 | 182 | 4,400 | (305) | 23 | (58) | 7,828 | (199) | 11,870 | 31 | 11,901 | ||
| Total comprehensive income (loss) | (39) | (48) | 1,175 | 3,432 | 4,520 | 7 | 4,527 | |||||
| Dividend distributed | 1 | 290 | (773) | (482) | (2) | (484) | ||||||
| Minority Buy-out | - | - | ||||||||||
| Transfer of result on disposal of equity investments at FVTOCI to retained earnings | - | - | - | |||||||||
| Purchase of treasury shares | - | (758) | (757) | (757) | ||||||||
| Re-issuance of treasury shares | - | (150) | 18 | 143 | 11 | 11 | ||||||
| Forward contracts | 48 | (869) | (821) | (821) | ||||||||
| Share call options | 12 | (21) | (9) | (9) | ||||||||
| Cancellation of treasury shares | (7) | (1,221) | 1,228 | |||||||||
| Share-based compensation plans | 110 | 110 | 110 | |||||||||
| Income tax share-based compensation plans | (4) | (4) | (4) | |||||||||
| Balance as of December 31, 2021 | 177 | 4,646 | (344) | (25) | 1,117 | 9,344 | (476) | 14,438 | 36 | 14,475 | ||
| Total comprehensive income (loss) | (32) | 23 | 749 | (1,527) | (786) | 4 | (782) | |||||
| Dividend distributed | 3 | 326 | (741) | (412) | (6) | (418) | ||||||
| Minority Buy-out | - | - | ||||||||||
| Transfer of result on disposal of equity investments at FVTOCI to retained earnings | (1) | 1 | - | - | ||||||||
| Purchase of treasury shares | - | (24) | (24) | (24) | ||||||||
| Re-issuance of treasury shares | (43) | (28) | 77 | 7 | 7 | |||||||
| Forward contracts | 76 | (140) | (64) | (64) | ||||||||
| Share call options | 5 | (12) | (6) | (6) | ||||||||
| Cancellation of treasury shares | (2) | (298) | 299 | |||||||||
| Share-based compensation plans | 95 | 95 | 95 | |||||||||
| Income tax share-based compensation plans | 1 | 1 | 1 | |||||||||
| Balance as of December 31, 2022 | 178 | 5,025 | (376) | (2) | 1,866 | 6,832 | (275) | 13,249 | 34 | 13,283 | ||
| Total comprehensive income (loss) | (17) | 8 | (604) | (488) | (1,101) | 1 | (1,100) | |||||
| Dividend distributed | 8 | 741 | (816) | (68) | (3) | (70) | ||||||
| Transfer of result on disposal of equity investments at FVTOCI to retained earnings | 4 | (4) | - | - | ||||||||
| Purchase of treasury shares | - | - | - | |||||||||
| Re-issuance of treasury shares | (29) | (24) | 54 | - | - | |||||||
| Forward contracts | 465 | (608) | (143) | (143) | ||||||||
| Share call options | - | - | ||||||||||
| Cancellation of treasury shares | (3) | (563) | 566 | |||||||||
| Share-based compensation plans | 88 | 88 | 88 | |||||||||
| Income tax share-based compensation plans | 2 | 2 | 2 | |||||||||
| Balance as of December 31, 2023 | 183 | 5,827 | (390) | 6 | 1,263 | 5,402 | (262) | 12,028 | 33 | 12,061 | ||
Amounts may not add up due to rounding.
Koninklijke Philips N.V. (‘Royal Philips’), incorporated and domiciled in the Netherlands, is a public limited liability company organized under Dutch Law. Philips is headquartered in Amsterdam, the Netherlands and has its registered address at High Tech Campus 52, 5656 AG Eindhoven, the Netherlands. The consolidated financial statements of Royal Philips as of December 31, 2023 comprise Royal Philips and its subsidiaries (together referred to as the 'company’ or ‘Philips’ or the 'Group’). Philips is a leading health technology company primarily involved in diagnostic imaging, image-guided therapy, patient monitoring and health informatics, as well as in consumer health and home care.
Basis of preparationThe Consolidated financial statements are:
The preparation of financial statements requires management to make a number of estimates and judgments that affect the application of accounting policies and the reporting amounts of assets and liabilities, revenues and expenses, and the disclosure of contingent assets and liabilities. Amounts recognized are based on factors that are by default associated with uncertainty. Actual results may therefore differ from estimates. Estimates and underlying assumptions are reviewed on an ongoing basis. Revision to estimates are recognized prospectively. Where applicable, the estimates and judgments of specific financial statement items are described in the respective note to the consolidated financial statements.
The areas involving a higher degree of judgment and complexity in applying accounting principles and for which changes in the assumptions and estimates could result in significantly different results than those recorded in the consolidated financial statements are the following:
The company regularly updates its significant assumptions and estimates to support the reported amounts of assets, liabilities, income and expenses.
In preparing the consolidated financial statements management has considered the impact of climate change, specifically the financial impact of Philips meeting its internal and external climate related aims, the potential impact of climate related risks and the costs incurred to pro-actively manage such risks. These considerations did not have a material impact on the financial reporting judgments, estimates or assumptions. The specific financial impacts considered include, for example: specific climate mitigation measures, such as the use of lower carbon energy sources, the costs of developing more sustainable product offerings and expenses incurred to mitigate against the impact of extreme weather conditions. Philips uses 100% electricity from renewable sources, mainly through long-term Power Purchase Agreements thereby mitigating the impact of carbon taxes. The development of more sustainable products are covered through our EcoDesign program and already included in our R&D expenses. The physical risk related to climate change on our sites resulting from our TCFD-assessment is currently considered limited.
Material accounting policiesThe material accounting policies as generally applied throughout the financial statements are described below. Material accounting policies relating to specific financial statement items are included in the respective notes to the financial statements.
Basis of consolidationThe Consolidated financial statements comprise the financial statements of Koninklijke Philips N.V. and all subsidiaries that the company controls on a consolidated basis. Control exists when the company is exposed or has rights to variable returns from its involvement with the investee and the company has the ability to affect those returns through its power over the investee. Generally, there is a presumption that a majority of voting rights results in control. To support this presumption and in cases where Philips has less than a majority of the voting or similar rights of an investee, Philips considers all relevant facts and circumstances in assessing whether it has power over an investee, including the contractual arrangement(s) with the other vote holders of the investee, rights arising from other contractual arrangements and the company’s voting rights and potential voting rights. Subsidiaries are fully consolidated from the date that control commences until the date that control ceases. All intercompany balances and transactions have been eliminated in the Consolidated financial statements. Unrealized losses are eliminated in the same way as unrealized gains, but only to the extent that there is no evidence of impairment.
Foreign currency transactionsThe financial statements of all group entities are measured using the currency of the primary economic environment in which the entity operates (functional currency). The euro (EUR) is the functional currency of the company and the presentation currency of the consolidated financial statements. Foreign currency transactions are converted into the functional currency using the exchange rates prevailing at transaction date or the valuation date in cases where items are remeasured. Gains and losses resulting from the settlement of foreign currency transactions and those resulting from the conversion of foreign currency denominated monetary assets and liabilities at period-end exchange rates are recognized in the Consolidated statements of income, except for qualifying cash flow hedges, qualifying net investment hedges and equity investments measured at fair value through OCI which are recognized in other comprehensive income.
All foreign exchange differences are presented as part of Cost of sales, apart from tax items and financial income and expense, which are recognized in the same line item as they relate to in the Consolidated statements of income.
Non-monetary assets and liabilities denominated in foreign currencies that are measured at fair value are retranslated to the functional currency using the exchange rate at the date the fair value was determined. Non-monetary items in a foreign currency that are measured based on historical cost are translated using the exchange rate at the transaction date.
Foreign operationsThe assets and liabilities of foreign operations, including goodwill and fair value adjustments arising on acquisition, are translated to euros at the exchange rates prevailing at the reporting date. The income and expenses of foreign operations are translated to euros at the exchange rates prevailing at the dates of the transactions.
Foreign currency differences arising upon translation of foreign operations into euros are recognized in Other comprehensive income and presented as part of Currency translation differences in Equity. However, if the operation is not a wholly-owned subsidiary, the proportionate share of the translation difference is allocated to Non-controlling interests.
When a foreign operation is disposed of such that control, significant influence or joint control is lost, the cumulative amount in the Currency translation differences related to the foreign operation is reclassified to the Consolidated statements of income as part of the gain or loss on disposal. When the company disposes of only part of its interest in a subsidiary that includes a foreign operation while retaining control, the respective proportion of the cumulative amount is reattributed to Non-controlling interests. When the company disposes of only part of its investment in an associate or joint venture that includes a foreign operation while retaining significant influence or joint control, the relevant proportion of the cumulative amount is reclassified to the Consolidated statements of income.
New accounting policies effective in 2023No new IFRS accounting standards or amendments to existing standards, effective in 2023, had a significant impact on the consolidated financial statements. The company has not early adopted any standards or amendments to existing standards. Consistent with the IAS 12 amendment regarding Pillar Two taxation as issued by the IASB and adopted by the EU, Philips does not recognize and disclose deferred taxes arising from tax laws that implement Pillar Two model rules published by the Organisation for Economic Co-operation and Development. Furthermore, Philips will recognize and disclose the impact (if any) from Pillar Two income taxes on current tax effective from 2024.
New accounting policies effective after 2023The IASB has issued several IFRS accounting standards, or amendments to standards, with an effective date after 2023. The company does not anticipate that the application of these standards, or amendments to standards, will have a significant effect on the consolidated financial statements upon adoption.
Changes in presentation from the prior yearAccounting policies have been applied consistently for all periods presented in these consolidated financial statements. Certain prior-year amounts have been reclassified to conform to the current year presentation due to immaterial organizational changes.
Philips has realigned the composition of its reporting segments effective from April 1, 2023. The most notable change is the shift of the previous Enterprise Diagnostic Informatics business from the Diagnosis & Treatment segment to the Connected Care segment. This business, together with other informatics solutions in the Connected Care segment, now forms the Enterprise Informatics business. Accordingly, the comparative figures for the affected segments have been restated in the consolidated financial statements.
Per share calculations have been adjusted retrospectively for all periods presented to reflect the issuance of shares for the share dividend in respect of 2022.
Non-current assets (or disposal groups) are classified as held-for-sale if their carrying amounts are expected to be recovered through a sale transaction rather than through continuing use. Non-current assets (or disposal groups) classified as held-for-sale are measured at the lower of their carrying amount or the fair value less costs of disposal. Depreciation or amortization of an asset ceases when it is classified as held-for-sale. When non-current assets (or disposal groups) are classified as held-for-sale, comparative balances prior to such date are not represented in the Consolidated balance sheets.
Discontinued operationsA discontinued operation is a component of the company that has either been disposed of or is classified as held-for-sale and represents a separate major line of business or geographical area of operations or is a part of a single coordinated plan to dispose of a separate major line of business or geographical area of operations. Any gain or loss from disposal, together with the results of these operations until the date of disposal, are reported separately as discontinued operations in the Consolidated statements of income.
The financial information of discontinued operations is excluded from the respective captions in the Consolidated financial statements and related notes for all periods presented. Comparatives are re-presented for presentation of discontinued operations in the Consolidated statements of income and Consolidated statements of cash flows.
The determination of the fair value less costs of disposal involves the use of estimates and assumptions that tend to be uncertain. Circumstances to which these adjustments may relate include resolution of uncertainties that arise from the terms of the disposal transaction, such as the resolution of purchase price adjustments and indemnifications, resolution of uncertainties that arise from and are directly related to the operations of the component before its disposal, such as environmental and assurance-type product warranty obligations retained by the company, and the settlement of employee benefit plan obligations provided that the settlement is directly related to the disposal transaction.
In 2023 and 2022 discontinued operations consist of certain costs related to other divestments, which were previously reported as discontinued operations. In 2021 discontinued operations consist primarily of the Domestic Appliances business. The following table summarizes the results of discontinued operations, net of income taxes, reported in the consolidated statements of income.
Philips Group
Discontinued operations, net of income taxes
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Domestic Appliances | 2,698 | 3 | (2) |
| Other | 13 | 10 | (7) |
| Discontinued operations, net of income taxes | 2,711 | 13 | (10) |
On March 25, 2021, Philips signed an agreement to sell its Domestic Appliances business to global investment firm Hillhouse Investment. Since the first quarter of 2021, the Domestic Appliances business is presented as a discontinued operation, and comparative results have been restated to reflect the treatment of the Domestic Appliances business as a discontinued operation, because the sale of the Domestic Appliances business constitutes the discontinuance of a major line of business from the Personal Health segment.
The following table summarizes the results of Domestic Appliances included in the consolidated statements of income as a discontinued operation.
Philips Group
Results of Domestic Appliances
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Sales | 1,516 | 6 | - |
| Costs and expenses | (1,322) | (2) | (2) |
| Income from operations | 194 | 4 | (2) |
| Result on the sale of discontinued operations | 3,241 | 1 | (1) |
| Income before tax | 3,435 | 5 | (3) |
| Income tax benefit (expense)1) | 6 | (2) | 1 |
| Income tax related the sale of discontinued operations | (743) | ||
| Results from discontinued operations | 2,698 | 3 | (2) |
Costs of EUR 64 million incurred in relation to the separation of the Domestic Appliances business in 2021 have been accounted for in continuing operations, because these costs reflect expenses incurred by Royal Philips in the divestment process and are not considered representative of the core business results of the Domestic Appliances business.
On September 1, 2021, the company completed the sale of the Domestic Appliances business and recognized a transaction gain before tax of EUR 3,241 million. Philips received consideration of EUR 4,041 million, which is based on an enterprise value of EUR 3,850 million, increased by an amount of EUR 191 million for closing adjustments related to working capital and net indebtedness. The transaction gain before tax is the net effect of (i) the EUR 4,041 million consideration (ii) less the derecognition of net assets employed of EUR 715 million (iii) less transaction related costs of EUR 16 million, (iv) less the release of cumulative translation losses of EUR 69 million included in Other comprehensive income. The income tax charges related to the divestment process was EUR 743 million, resulting in an after-tax transaction gain of EUR 2,499 million. The income tax charge represents the consolidated tax expense resulting from asset transactions completed as part of the disentanglement of the business in anticipation of its sale, a significant portion of which relates to taxes payable in the Netherlands. In addition, Philips and the buyer entered into a 15-year brand license agreement with future annual payments that represents an estimated net present value of approximately EUR 0.7 billion, which will be received and recognized over time.
Discontinued operations: OtherCertain costs related to other divestments, which were previously reported as discontinued operations, resulted in a net loss of EUR (7) million in 2023, a net gain of EUR 10 million in 2022 and a net gain of EUR 13 million in 2021.
Discontinued operations cash flowsThe following table presents the net cash provided by (used for) discontinued operations reported in the Consolidated statements of cash flows.
Net cash provided by (used for) discontinued operations
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net cash provided by (used for) operating activities | 85 | (27) | 123 |
| Net cash provided by (used for) investing activities | 3,319 | 15 | |
| Net cash provided by (used for) discontinued operations | 3,403 | (12) | 123 |
In 2023, net cash provided by discontinued operations was EUR 123 million and consisted primarily of a refund received of one-off advance tax payments related to a previously divested business.
In 2022, net cash used for discontinued operations was EUR (12) million and consisted primarily of cash flows related to the tax claims from a previously divested business.
In 2021, net cash provided for discontinued operations was EUR 3,403 million and consisted primarily of the net cash inflow of EUR 3,319 million from the sale of the Domestic Appliances business on September 1, 2021.
Assets classified as held for saleAs of December 31, 2023, assets held for sale primarily consisted of assets and liabilities directly associated with a business held for sale.
As of December 31, 2022, assets held for sale consists of property, plant and equipment mainly related to the APAC Center Singapore building. The sale was finalized in January 2023.
The company accounts for business combinations using the acquisition method when control is transferred to the group. The consideration transferred in the acquisition is generally measured at fair value, as are the identifiable net assets acquired and the liabilities assumed. Transaction costs are expensed as incurred. Any contingent consideration is measured at fair value at the acquisition date and is initially presented in Long-term provisions. When the timing and amount of the consideration become more certain, it is reclassified to Accrued liabilities. If the contingent consideration that meets the definition of a financial instrument is classified as equity, it is not remeasured and settlement is accounted for within equity. Otherwise, subsequent changes in the fair value of the contingent consideration are recognized in the Consolidated statements of income.
Changes to the initial fair value of the acquired assets and liabilities, based on new information about the circumstances at the acquisition date, can be made up to twelve months after the acquisition date.
DivestmentsUpon loss of control, the company derecognizes the assets and liabilities of the subsidiary, any non-controlling interests and the other components of equity related to the subsidiary. Any surplus or deficit arising from the loss of control is recognized in the Consolidated statements of income. If the company retains any interest in the previous subsidiary, such interest is measured at fair value at the date the control is lost. Subsequently it is accounted for as either an equity-accounted investee (associate) or as a financial asset, depending on the level of influence retained. Further information on loss of control can be found in Discontinued operations and assets classified as held for sale.
Intangible assets acquired in a business acquisition and the financial liability related to non-controlling interest are measured at fair value at the date of the acquisition.
To determine the fair value of intangible assets at the acquisition date, estimates and assumptions are required. The valuation of the identifiable intangible assets involves estimates of expected sales, earnings and/or future cash flows and require use of key assumptions such as discount rate, royalty rate and growth rates.
Estimates are also applied when determining the fair value of legal cases and tax positions in the acquired entity. The fair value is based on estimates of the likelihood, the expected timing and the amount of the potential cash outflow. Provisions for legal cases and non-income tax positions are recognized at fair value even if it is not probable that an outflow will be required to settle the obligation. After initial recognition and until the liability is settled, cancelled or expired, the liability is measured at the higher of the amount that would be recognized in accordance with IAS 37 'Provisions, contingent liabilities and contingent assets' and the initial liability amount. For income tax positions, the company applies IAS 12 'Income Taxes', which requires recognition of provisions only when the likelihood of cash outflow is considered probable.
On May 5, 2023, Philips completed one acquisition within Ultrasound business unit to accelerate the growth of its Diagnosis & Treatment segment. The total equity purchase price and the settlement of debt, net of acquired cash, involved an amount of EUR 53 million and a contingent consideration of EUR 6 million at fair value, the latter recognized as a Long-term provision. Upon acquisition, the company recognized Goodwill of EUR 24 million, Other intangible assets of EUR 40 million and deferred tax asset and liability of EUR 5 million and EUR 2 million, respectively. The acquisition is subject to final purchase price allocation procedures, which is expected to be finalized in the second quarter of 2024. The primary provisional accounts subject to change are related to acquired intangible assets and goodwill.
Since the acquisition date through December 31, 2023, the contribution to sales to third parties and net income of the acquiree was not material. The sales and net income would not differ materially if the acquisition date had been January 1, 2023. Acquisition-related costs were recognized in General and administrative expenses and were not material.
DivestmentsDuring 2023 Philips completed six divestments for net cash consideration of EUR 80 million and a gain of EUR 50 million, which is included in Other business income of the Consolidated statements of income. The most notable was the sale of Philips Pharma Solutions in the US. The divestments were not material.
2022AcquisitionsIn 2022 Philips completed three acquisitions. The acquisitions involved aggregated net cash outflow of EUR 359 million. Including final purchase price adjustments processed in the course of 2023, the company recognized contingent consideration of EUR 96 million measured at fair value, aggregated Goodwill of EUR 307 million, Other intangible assets of EUR 179 million, Deferred tax assets of EUR 20 million and Deferred tax liabilities generated from the intangible assets of EUR 43 million.
Vesper Medical Inc. (Vesper) was the most notable acquisition and is discussed below. The remaining two acquisitions involved aggregated net cash outflow of EUR 139 million. Including final purchase price adjustments processed in the course of 2023, the company recognized contingent consideration of EUR 61 million measured at fair value, aggregated Goodwill of EUR 130 million, Other intangible assets of EUR 95 million and Deferred tax liabilities of EUR 23 million.
Since the respective acquisition dates through December 31, 2022, the contribution to sales to third parties and net income of the three acquired entities was not material. The sales and net income of the combined entities would not differ materially from these amounts if the acquisition date had been January 1, 2022. Acquisition-related costs were not material.
VesperOn January 11, 2022, Philips acquired all shares of Vesper for an amount of EUR 227 million in cash and EUR 34 million contingent consideration at fair value. Vesper, headquartered in Wayne, Pennsylvania, US, is a medical technology company that develops minimally-invasive peripheral vascular devices. The company is developing the Vesper DUO Venous Stent System®, commercialization of which is estimated to start after approval by the US Food and Drug Administration (FDA), expected in 2024. The Vesper DUO Venous Stent System® consists of venous stents intended to treat deep venous obstruction. It provides physicians with a modular portfolio to customize therapy, restore venous flow, and resolve the painful symptoms of deep venous disease for the broad range of patients suffering from chronic venous insufficiency. As of the acquisition date, Vesper forms part of the Image-Guided Therapy business portfolio of the Diagnosis & Treatment segment.
The condensed opening balance sheet of Vesper was as follows:
Opening balance sheet
in millions of EUR
| At acquisition date | |
|---|---|
| Vesper Medical Inc, | |
| Assets | |
| Intangible assets excluding goodwill | 84 |
| Deferred tax assets | 15 |
| Cash | 7 |
| Total Assets | 106 |
| Liabilities | |
| Accounts payable and other payables | (1) |
| Deferred tax liabilities | (20) |
| Total Liabilities | (21) |
| Total identifiable net assets at fair value | 85 |
| Goodwill arising on acquisition | 177 |
| Total purchase consideration | 262 |
| Of which: | |
| Purchase consideration transferred | 227 |
| Contingent consideration | 34 |
Goodwill recognized in the amount of EUR 177 million mainly represents revenue synergies expected from the combination of Philips’ peripheral vascular portfolio and Vesper's venous stenting solution to address the root cause of chronic deep venous disease (DVD). Strong clinical synergies between Vesper’s innovative stenting solution and Philips' existing peripheral vascular offering will help to better support clinicians to decide, guide, treat and confirm during the procedure, thereby enhancing patient care. Vesper goodwill is not tax-deductible.
The majority of the intangible assets balance relates to capitalized development costs, the fair value of which is determined using the multi-period excess earnings method, which is a valuation technique that estimates the fair value of an asset based on market participants’ expectations of the cash flows associated with that asset over its remaining useful life. The fair value of capitalized development costs is based on an estimate of positive future cash flows associated with incremental profits related to excess earnings, discounted at a rate of 12.0%. Capitalized development costs are tested for impairment on an annual basis until FDA approval is obtained and the asset is reclassified to an intangible asset that is depreciated over its economical useful life.
The contingent consideration arrangement requires Philips to pay the former owners of Vesper up to a maximum undiscounted amount of EUR 44 million contingent upon FDA approval of the Vesper DUO Venous Stent System. The fair value of the contingent consideration arrangement of EUR 34 million has been estimated by calculating the present value of the future expected cash flows. The estimate is based on a discount rate of 12% and assumed probability adjusted likelihood of FDA approval at a certain point in time.
During 2022 Philips completed two divestments that were not material.
Associates are all entities over which the company has significant influence, but not control or joint control. Significant influence is presumed with a shareholding of between 20% and 50% of the voting rights.
Investments in associates are accounted for using the equity method of accounting and are initially recognized at cost. The carrying amount of an investment in associate includes the carrying amount of goodwill identified on acquisition. An impairment loss on such investment is allocated to the investment as a whole.
The company’s share of the net income of these associates is included in Investments in associates, net of income taxes, in the Consolidated statements of income, after adjustments to align the accounting policies with those of the company. Dilution gains and losses arising from investments in associates are recognized in the Consolidated statements of income as part of Investments in associates, net of income taxes. Impairment losses and gains or losses on sale of investments are recorded in the Consolidated statements of income, more specifically on the line item ’Investments in associates, net of income taxes’.
When the company’s share of losses exceeds its interest in an associate, the carrying amount of that interest is reduced to zero and recognition of further losses is discontinued except to the extent that the company has an obligation or made payments on behalf of the associate.
The nature of the company’s interests in its consolidated entities and associates, and the effects of those interests on the company’s financial position and financial performance are discussed below.
Group companiesBelow is a list of material subsidiaries as of December 31, 2023 representing greater than 5% of either the consolidated group Sales, Income from operations or Income from continuing operations (before any intra-group eliminations) of Group legal entities. All of the entities are fully consolidated in the Group financial statements.
Philips Group
Interests in group companies
in alphabetical order by country
2023
| Legal entity name | Principal country of business |
| Philips (China) Investment Company, Ltd. | China |
| Philips Medizin Systeme Böblingen GmbH | Germany1) |
| Philips Japan, Ltd. | Japan |
| Philips Consumer Lifestyle B.V. | Netherlands |
| Philips Medical Systems (Cleveland), Inc. | United States |
| ATL International LLC | United States |
| Philips North America LLC | United States |
| Philips RS North America LLC | United States |
As of December 31, 2023, four consolidated subsidiaries are not wholly owned by Philips (December 31, 2022: four). In 2023, Sales to third parties and Net income for these subsidiaries in aggregate are EUR 492 million (December 31, 2022: EUR 472 million) and EUR 27 million (December 31, 2022: EUR 28 million), respectively.
Investments in associatesPhilips has investments in a number of associates. During 2023, Philips made two investments in associates for a total amount of EUR 3 million.
Due to the loss of significant influence in Candid Care during 2023, Philips reclassified the investment to Other non-current financial asset at FVTOCI (Level 3). On reclassification Philips recorded a loss of EUR 23 million in Other business expenses. For more information about Other non-current financial asset at FVTOCI, refer to Other financial assets and Fair value of financial assets and liabilities.
In 2023, Philips recorded its share in negative results of associates of EUR 51 million and impairment of EUR 58 million. The impairment losses mainly related to HALO Dx (EUR 33 million) and were recorded within the Investments in associates, net of income taxes line item.
Cumulative translation adjustments related to investments in associates were EUR (21) million as of December 31, 2023 (2022: EUR (22) million).
Involvement with unconsolidated structured entitiesPhilips founded three Philips Medical Capital (PMC) entities, in the US, France and Germany, in which Philips holds a minority interest. Philips Medical Capital, LLC in the US is the most significant entity. PMC entities provide healthcare equipment financing and leasing services to Philips customers for diagnostic imaging equipment, patient monitoring equipment, and clinical IT systems.
The company concluded that it does not control, and therefore should not consolidate the PMC entities. In the US, PMC operates as a subsidiary of De Lage Landen Financial Services, Inc. The same structure and treatment is applied to the PMC entities in the other countries, with other majority shareholders. Operating agreements are in place for all PMC entities, whereby acceptance of sales and financing transactions resides with the respective majority shareholder. After acceptance of a transaction by PMC, Philips transfers control and does not retain any obligations towards PMC or its customers, from the sales contracts.
As of December 31, 2023, Philips’ shareholding in Philips Medical Capital, LLC had a carrying value of EUR 27 million (December 31, 2022: EUR 29 million).
The company does not have any material exposures to losses from interests in unconsolidated structured entities other than the invested amounts.
The company recognizes revenue when it transfers control over a good or service to a customer, in an amount that reflects the consideration (i.e., transaction price) to which the company expects to be entitled to in exchange for the good or service. The consideration expected by the company may include fixed and/or variable amounts which can be impacted by sales returns, trade discounts and volume rebates. The company adjusts the consideration for the time value of money if the period between the transfer of the promised goods or services to the customer and payment by the customer exceeds six months.
Transfer of control varies depending on the individual terms of the contract of sale. For consumer-type products in the Personal Health segment, control is transferred when the product is shipped and delivered to the customer and title and risk have passed to the customer (depending on the delivery conditions) and acceptance of the product has been obtained.
Revenues from transactions relating to distinct goods or services are accounted for separately based on their relative stand-alone selling prices. The stand-alone selling price is the price that would be charged for the goods or service in a separate transaction under similar conditions to similar customers. The transaction price is determined (considering variable considerations) and allocated to performance obligations based on their relative stand-alone selling prices. These transactions mainly occur in the segments Diagnosis & Treatment and Connected Care and include arrangements that require subsequent installation and training activities to make distinct goods operable for the customer. As such, the related installation and training activities are part of equipment sales rather than separate performance obligations. Revenue is recognized when the performance obligation is satisfied, i.e., when the installation has been completed and the equipment is ready to be used by the customer in the way contractually agreed.
Variable consideration is included in the transaction price to the extent that it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur once associated uncertainties are resolved. Such assessment is performed on each reporting date to check whether it is constrained. For products for which a right of return exists during a defined period, revenue recognition is determined based on the historical pattern of actual returns, or in cases where such information is not available, revenue recognition is postponed until the return period has lapsed. Return policies are typically based on customary return arrangements in local markets. A provision is recognized for assurance-type product warranty at the time of revenue recognition and reflects the estimated costs of replacement and free-of-charge services that will be incurred by the company with respect to the products sold. For certain products, the customer has the option to purchase the warranty separately, which is considered a separate performance obligation on top of the assurance-type product warranty. For such warranties which provide distinct service, revenue recognition occurs on a straight-line basis over the extended warranty contract period. Occasionally, the company may offer a full or partial refund of consideration previously paid, for example as part of the resolution to warranty related matters. In such instances, a provision is recognized for the amounts expected to be refunded to customers, and remeasured at each reporting date to reflect changes in the estimated refunds, with a corresponding adjustment to revenue.
In the case of loss under a sales agreement, the loss is recognized immediately.
Sale of goodsRevenues are recognized at a point in time when control of the goods passes to the buyer, based on the allocation of the transaction price to the performance obligation.
Revenue from servicesRevenues are recognized over time as the company transfers control of the services to the customer which is demonstrated by the customer simultaneously receiving and consuming the benefits provided by the company. The amount of revenues is measured by reference to the progress made towards complete satisfaction of the performance obligation, which in general is evenly over time. Service revenue related to repair and maintenance activities for goods sold is recognized ratably over the service period or as services are rendered.
Income from royaltiesRoyalty income from brand license arrangements and from intellectual property rights, such as technology licenses or patents, is recognized on an accrual basis in accordance with the substance of the relevant agreement.
Shipping and handlingExpenses incurred for shipping and handling are mainly recorded as cost of sales. When shipping and handling are part of a project and billed to the customer, then the related expenses are recorded as cost of sales. Shipping and handling related to sales to third parties are partly recorded as selling expenses. When shipping and handling billed to customers are considered a distinct and separate performance obligation, the fees are recognized as revenue and costs included in cost of sales.
Other business income (expenses)Other business income (expenses) includes gains and losses on the sale of property, plant and equipment, gains and losses on the sale of businesses as well as other gains and losses not related to the company’s operating activities.
Government grantsGrants from governments are recognized at their fair value when there is a reasonable assurance that the grant will be received and the company will comply with the conditions. Grants related to costs are deferred in the consolidated balance sheet and recognized in the consolidated statement of income as a reduction of the related costs that they are intended to compensate. Grants related to assets are deducted from the cost of the asset and presented net in the consolidated balance sheets.
The company has sales promotions-related agreements with distributors and retailers designed to promote the sale of products. Among the programs are arrangements under which rebates and discounts can be earned by the distributors and retailers by attaining agreed upon sales levels, or for participating in specific marketing programs. Management estimates the sales-related accruals associated with these arrangements based on a combination of historical patterns and future expectations regarding which promotional targets are expected to be met by distributors and retailers. Accrued customer rebates are presented as other current liabilities, unless there is a right to offset against the respective accounts receivable.
A breakdown by nature of the income (loss) from operations is as follows:
Philips Group
Sales and costs by nature
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Sales | 17,156 | 17,827 | 18,169 |
| Costs of materials used | (4,142) | (4,320) | (4,626) |
| Employee benefit expenses | (6,246) | (6,952) | (6,903) |
| Depreciation and amortization1) | (1,323) | (1,602) | (1,261) |
| Impairment of goodwill | (15) | (1,357) | (8) |
| Shipping and handling | (645) | (756) | (668) |
| Advertising and promotion | (752) | (739) | (700) |
| Lease expenses | (19) | (39) | (51) |
| Other operational costs | (3,524) | (3,609) | (3,535) |
| Other business income (expenses) | 63 | 18 | (533) |
| Income from operations | 553 | (1,529) | (115) |
For information related to sales on a segment and geographical basis, refer to Information by segment and main country.
Philips Group
Sales composition
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Goods | 11,981 | 12,139 | 12,419 |
| Services | 4,374 | 4,878 | 4,926 |
| Royalties | 383 | 419 | 434 |
| Total sales from contracts with customers | 16,738 | 17,435 | 17,779 |
| Sales from other sources | 418 | 391 | 390 |
| Total sales | 17,156 | 17,827 | 18,169 |
Refer to Provisions.
Total sales from other sources mainly relates to operating leases EUR 234 million (2022: EUR 258 million 2021: EUR 293 million). Sales represent revenue from external customers.
As of December 31, 2023, the aggregate amount of the transaction price allocated to remaining performance obligations from a sale of goods and services was EUR 15,571 million. The company expects to recognize approximately 47% of the remaining performance obligations within 1 year. Revenue expected to be recognized beyond 1 year is mostly related to longer term customer service and software contracts.
Sales over time represent services and Other also includes royalties over time (2023: EUR 283 million 2022: EUR 292 million 2021: EUR 220 million).
Sales per geographic area are reported based on country of destination.
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2023 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Diagnosis & Treatment | 5,762 | 2,978 | 8,742 | 76 | 8,818 |
| Connected Care | 2,970 | 1,854 | 4,824 | 314 | 5,138 |
| Personal Health | 3,586 | 16 | 3,602 | 3,602 | |
| Other | 251 | 361 | 612 | - | 612 |
| Philips Group | 12,569 | 5,210 | 17,779 | 390 | 18,169 |
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2022 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Diagnosis & Treatment | 5,285 | 2,950 | 8,234 | 55 | 8,290 |
| Connected Care | 3,079 | 1,853 | 4,932 | 336 | 5,268 |
| Personal Health | 3,615 | 11 | 3,626 | 3,626 | |
| Other | 284 | 357 | 643 | - | 643 |
| Philips Group | 12,263 | 5,172 | 17,435 | 391 | 17,827 |
Philips Group
Disaggregation of Sales per segment
in millions of EUR
| 2021 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Diagnosis & Treatment | 5,120 | 2,656 | 7,776 | 50 | 7,825 |
| Connected Care | 3,399 | 1,604 | 5,002 | 369 | 5,371 |
| Personal Health | 3,423 | 6 | 3,429 | 3,429 | |
| Other | 200 | 330 | 530 | - | 530 |
| Philips Group | 12,142 | 4,596 | 16,738 | 418 | 17,156 |
Philips Group
Disaggregation of Sales per geographic area
in millions of EUR
| 2023 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Western Europe | 2,552 | 1,221 | 3,770 | 49 | 3,819 |
| North America | 4,859 | 2,608 | 7,470 | 92 | 7,562 |
| Other mature geographies | 980 | 398 | 1,378 | 248 | 1,626 |
| Mature geographies | 8,392 | 4,227 | 12,618 | 389 | 13,007 |
| Growth geographies | 4,177 | 984 | 5,161 | 1 | 5,162 |
| Sales | 12,569 | 5,210 | 17,779 | 390 | 18,169 |
Philips Group
Disaggregation of Sales per geographic area
in millions of EUR
| 2022 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Western Europe | 2,387 | 1,183 | 3,572 | 31 | 3,603 |
| North America | 4,889 | 2,612 | 7,502 | 86 | 7,588 |
| Other mature geographies | 972 | 399 | 1,369 | 274 | 1,643 |
| Mature geographies | 8,248 | 4,194 | 12,443 | 390 | 12,833 |
| Growth geographies | 4,015 | 978 | 4,992 | 1 | 4,993 |
| Sales | 12,263 | 5,172 | 17,435 | 391 | 17,827 |
Philips Group
Disaggregation of Sales per geographic area
in millions of EUR
| 2021 | |||||
|---|---|---|---|---|---|
| Sales at a point in time |
Sales over time |
Total sales from contracts with customers |
Sales from other sources |
Total sales |
|
| Western Europe | 2,537 | 1,087 | 3,624 | 21 | 3,645 |
| North America | 4,427 | 2,268 | 6,695 | 86 | 6,781 |
| Other mature geographies | 1,000 | 386 | 1,386 | 309 | 1,694 |
| Mature geographies | 7,964 | 3,741 | 11,705 | 415 | 12,120 |
| Growth geographies | 4,178 | 856 | 5,033 | 3 | 5,036 |
| Sales | 12,142 | 4,596 | 16,738 | 418 | 17,156 |
Cost of materials used represents the inventory recognized in cost of sales.
Employee benefit expensesPhilips Group
Employee benefit expenses
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Salaries and wages excluding share-based compensation | 5,014 | 5,594 | 5,635 |
| Share-based compensation | 115 | 104 | 97 |
| Post-employment benefit costs | 396 | 439 | 402 |
| Other social security and similar charges: | |||
| Required by law | 529 | 590 | 567 |
| Voluntary | 192 | 225 | 202 |
| Employee benefit expenses | 6,246 | 6,952 | 6,903 |
The employee benefit expenses relate to employees who are working on the payroll of Philips, both with permanent and temporary contracts.
For further information on post-employment benefit costs, refer to Post-employment benefits.
For details on the remuneration of the members of the Board of Management and the Supervisory Board, refer to Information on remuneration.
EmployeesThe average number (full-time equivalents, or FTEs) of employees by category is summarized as follows:
Philips Group
Employees by category
in FTEs
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Production | 38,618 | 39,742 | 35,281 |
| Research & development | 10,751 | 11,690 | 10,833 |
| Other | 22,543 | 23,019 | 23,001 |
| Employees | 71,912 | 74,451 | 69,115 |
| Third party workers | 4,533 | 4,086 | 3,149 |
| Philips Group | 76,445 | 78,538 | 72,264 |
Employees consist of those persons working on the payroll of Philips and whose costs are reflected in employee benefit expenses. Other consists of employees in commercial, general and administrative functions. Third party workers consist of personnel hired on a per-period basis, via external companies.
Philips Group
Employees by geographical location
in FTEs
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Netherlands | 11,142 | 11,180 | 9,794 |
| Other countries | 65,303 | 67,357 | 62,471 |
| Philips Group | 76,445 | 78,538 | 72,264 |
Depreciation of property, plant and equipment and amortization of intangible assets, including impairments, are as follows:
Philips Group
Depreciation and amortization1)
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Depreciation of property, plant and equipment | 630 | 711 | 689 |
| Amortization of software | 88 | 117 | 98 |
| Amortization of other intangible assets | 322 | 363 | 290 |
| Amortization of development costs | 284 | 411 | 184 |
| Depreciation and amortization | 1,323 | 1,602 | 1,261 |
Depreciation of property, plant and equipment is mainly included in cost of sales. Amortization of software is mainly included in general and administration expenses. Amortization of other intangible assets is included in selling expenses for brand names and customer relationships and is included in cost of sales for technology based and other intangible assets. Amortization of development costs is included in research and development expenses.
Impairment of goodwillIn 2023 a goodwill charge of EUR 8 million was recorded for the partial impairment of goodwill allocated to a business that was classified as held-for-sale as of December 31, 2023. During 2022, EUR 1,331 million of goodwill impairment charges were recorded in the Sleep & Respiratory Care business, due to revisions to the expected future cash flows. In addition, a EUR 27 million goodwill impairment was recognized in the Precision Diagnosis Solutions business. For further information refer to Goodwill.
Shipping and handlingShipping and handling costs are included in cost of sales and selling expenses in the Consolidated statements of income.
Advertising and promotionAdvertising and promotion costs are included in selling expenses in the Consolidated statements of income.
Lease expenseLease expense relates to short-term and low value leases.
Other operational costsOther operational costs contain items which are dissimilar in nature and individually insignificant in amount to disclose separately. These costs contain among others expenses for outsourcing services, mainly in Information Technology and Human Resources, third party workers, consultants, warranty, patents, costs for travelling and external legal service. Government grants of EUR 95 million were recognized as cost reduction in 2023 (2022: EUR 103 million 2021: EUR 104 million). The grants mainly relate to research and development activities and business development.
Audit and audit-related feesThe following table shows the fees attributable to the fiscal years 2021, 2022 and 2023 for services rendered by the external auditors.
Philips Group
Audit and audit-related fees
in millions of EUR
| 2021 | 2022 | 2023 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | EY NL1) | EY Network | Total | |
| Audit fees | 10.3 | 5.4 | 15.7 | 9.5 | 5.6 | 15.2 | 9.7 | 5.1 | 14.7 |
| consolidated financial statements | 10.3 | 2.7 | 13.0 | 9.5 | 3.1 | 12.6 | 9.7 | 2.6 | 12.3 |
| statutory financial statements | 2.7 | 2.7 | 2.5 | 2.5 | 2.5 | 2.5 | |||
| Audit-related fees2) | 0.6 | 0.3 | 0.9 | 0.8 | 0.2 | 1.0 | 0.9 | 0.2 | 1.0 |
| divestment | |||||||||
| sustainability assurance | 0.5 | 0.5 | 0.6 | 0.6 | 0.8 | 0.8 | |||
| other | 0.1 | 0.3 | 0.4 | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 |
| Tax fees | - | - | |||||||
| All other fees | |||||||||
| Fees | 10.9 | 5.7 | 16.6 | 10.3 | 5.8 | 16.2 | 10.6 | 5.2 | 15.8 |
Other business income (expenses) consists of the following:
Philips Group
Other business income (expenses)
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Result on disposal of businesses: | |||
| income | - | 4 | 50 |
| expenses | (75) | - | - |
| Result on disposal of fixed assets: | |||
| income | 24 | 3 | 12 |
| expenses | (5) | (1) | (1) |
| Result on other remaining businesses: | |||
| income | 161 | 121 | 49 |
| expenses | (43) | (109) | (643) |
| Other business income (expenses) | 63 | 18 | (533) |
| Total other business income | 186 | 127 | 112 |
| Total other business expenses | (123) | (109) | (645) |
The result on disposal of businesses mainly relates to divestment of non-strategic businesses. For more information refer to Acquisitions and divestments.
The result on disposal of fixed assets mainly relates to the sale of real estate assets.
The result on other remaining businesses mainly relates to the revaluation of contingent consideration and various legal matters. In 2023 Philips Respironics recorded a EUR 575 million provision in connection with the anticipated resolution of the economic loss class action. For more information on contingent consideration, refer to Provisions.
In 2023 a loss of EUR 23 million related to a minority participation was recognized in Other business expenses. For more information refer to Interests in entities.
Financial income and expenses are recognized on the accrual basis in the consolidated statements of income. Interest income and expense are measured using the effective interest method. Dividend income is recognized in the consolidated statements of income on the date that the company’s right to receive payment is established, which in the case of quoted securities is normally the ex-dividend date.
Philips Group
Financial income and expenses
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Interest income | 18 | 25 | 46 |
| Interest income from loans and receivables | 7 | 7 | 13 |
| Interest income from cash and cash equivalents | 11 | 18 | 33 |
| Dividend income from financial assets | 2 | 3 | 2 |
| Net gains from disposal of financial assets | - | - | - |
| Net change in fair value of financial assets through profit or loss | 95 | 9 | |
| Other financial income | 33 | 20 | 15 |
| Financial income | 149 | 58 | 63 |
| Interest expense | (159) | (235) | (277) |
| Interest expense on debt and borrowings | (126) | (200) | (229) |
| Finance charges under lease contract | (25) | (25) | (27) |
| Interest expense on pensions | (8) | (10) | (21) |
| Provision-related accretion expenses | (5) | (9) | (29) |
| Net foreign exchange gains (losses) | - | 9 | (23) |
| Net change in fair value of financial assets through profit or loss | (26) | ||
| Other financial expenses | (24) | (24) | (21) |
| Financial expenses | (188) | (258) | (376) |
| Financial income and expenses, net | (39) | (200) | (314) |
In 2023, financial income and expenses, net increased by EUR 114 million year-on-year, mainly due to fair value losses and net foreign exchange losses in 2023, compared to gains in 2022. The fair value losses mainly relate to power purchase agreements for renewable energy, limited-life funds (mainly Gilde Healthcare) and other investments recognized at fair value through profit and loss. Furthermore, provision-related accretion expenses and net interest expense were higher in 2023 compared to 2022. Net interest expense in 2023 was EUR 21 million higher than in 2022, mainly due the issuance of new debt in 2022 and 2023 and the impact of increasing interest rates.
In 2022, Financial income and expenses increased by EUR 161 million year-on-year, mainly due to higher interest expense and lower fair value gains. The lower fair value gains compared to 2021 are mainly from investments in limited-life funds (mainly Gilde Healthcare) and other investments recognized at fair value through profit or loss. Net interest expense in 2022 was EUR 69 million higher than in 2021, mainly due to the financial charges related to early redemption of EUR and USD bonds and the issuance of new EUR bonds in 2022. The decrease in 2022 compared to 2021 in other financial income is mainly due to higher interest income on tax in 2021.
Income taxes comprise of current, non-current and deferred tax. Income tax is recognized in the Consolidated statements of income except to the extent that it relates to items recognized directly within equity or in other comprehensive income. Current tax is the expected taxes payable on the taxable income for the year, using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax payable in respect of previous years.
In cases where it is concluded it is not probable that tax authorities will accept a tax treatment, the effect of the uncertainty is reflected in the recognition and measurement of tax assets and liabilities or, alternatively, a provision is made for the amount that is expected to be settled, where this can be reasonably estimated. This assessment relies on estimates and assumptions and may involve a series of judgments about future events. New information may become available that causes the company to change its judgment regarding the adequacy of existing tax assets and liabilities. Such changes to tax assets and liabilities will impact the income tax expense in the period during which such a determination is made.
Deferred tax assets and liabilities are recognized, using the consolidated balance sheet method, for the expected tax consequences of temporary differences between the carrying amounts of assets and liabilities and the amounts used for taxation purposes. Deferred tax is not recognized for the following temporary differences: (a) the initial recognition of goodwill; or (b) the initial recognition of an asset or liability in a transaction which: (i) is not a business combination, (ii) at the time of transaction, affects neither accounting profit nor taxable profit (tax loss), (iii) at the time of the transaction, does not give rise to equal amounts of taxable and deductible differences; or (c) differences relating to investments in subsidiaries, joint ventures and associates where the reversal of the respective temporary difference can be controlled by the company and it is probable that it will not reverse in the foreseeable future. Deferred taxes are measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the laws that have been enacted or substantively enacted by the reporting date. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity or on different taxable entities, but the company intends to settle current tax liabilities and assets on a net basis or their tax assets and liabilities will be realized simultaneously.
A deferred tax asset is recognized for unused tax losses, tax credits and deductible temporary differences to the extent that it is probable that there will be future taxable profits against which they can be utilized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in the countries where the deferred tax assets originated and during the periods when the deferred tax assets become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment.
Deferred tax liabilities for withholding taxes are recognized for subsidiaries in situations where the income is to be paid out as dividend in the foreseeable future and for undistributed earnings of unconsolidated companies to the extent that these withholding taxes are not expected to be refundable or deductible. Changes in tax rates and tax laws are reflected in the period when the change was enacted or substantively enacted by the reporting date.
Any subsequent adjustment to a tax asset or liability that originated in discontinued operations and for which no specific arrangements were made at the time of divestment, due to a change in the tax base or its measurement, is allocated to discontinued operations (i.e. backwards tracing). Examples are a tax rate change or change in retained assets or liabilities directly relating to the discontinued operation. Any subsequent change to the recognition of deferred tax assets is allocated to the component in which the taxable gain is or will be recognized. The above principles are applied to the extent the ‘discontinued operations’ are sufficiently separable from continuing operations.
Consistent with the IAS 12 amendment regarding Pillar Two taxation as issued by the IASB and adopted by the EU, Philips does not recognize and disclose deferred taxes arising from tax laws that implement Pillar Two model rules published by the Organisation for Economic Co-operation and Development. Furthermore, Philips will recognize and disclose the impact (if any) from Pillar Two income taxes on current tax effective from 2024.
Deferred tax assets are recognized to the extent that it is probable that there will be future taxable profits against which these can be utilized. Significant judgment is involved in determining whether such profits are probable. Management determines this on the basis of expected taxable profits arising from the reversal of recognized deferred tax liabilities, appropriate tax planning opportunities to support business goals and on the basis of forecasts.
Uncertain tax positionsUncertain tax positions are recognized as liabilities if and to the extent it is probable that additional tax will be due and the amount can be reliably measured. Significant judgment is involved in determining these positions.
The income tax benefit of continuing operations amounts to EUR 73 million (2022: EUR 113 million tax benefit, 2021: EUR 103 million tax benefit).
The components of income before taxes and income tax expense are as follows:
Philips Group
Income tax expense
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Income before taxes | 509 | (1,731) | (526) |
| Investments in associates, net of income taxes | (4) | (2) | (98) |
| Income before taxes and Investment in associates | 513 | (1,729) | (429) |
| Current tax (expense) benefit | (298) | (97) | (201) |
| Deferred tax (expense) benefit | 401 | 210 | 274 |
| Income tax (expense) benefit of continuing operations | 103 | 113 | 73 |
Income tax benefit of continuing operations excludes the tax benefit of the discontinued operations of EUR 9 million (2022: EUR 18 million benefit, 2021: EUR 737 million expense), mainly related to the release of provisions.
The components of income tax expense of continuing operations are as follows:
Philips Group
Current income tax expense
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Current year tax (expense) benefit | (291) | (111) | (211) |
| Prior year tax (expense) benefit | (7) | 14 | 10 |
| Current tax (expense) benefit | (298) | (97) | (201) |
Philips Group
Deferred income tax expense
In millions of EUR
| 2021 | 2022 | 2023 | ||
|---|---|---|---|---|
| Recognition of previously unrecognized tax loss and credit carryforwards | 138 | 2 | 72 | |
| Unrecognized tax loss and credit carryforwards | (10) | (13) | (41) | |
| Changes to recognition of temporary differences | (1) | (4) | (112) | |
| Prior year tax (expense) benefit | 20 | (1) | (2) | |
| Tax rate changes | 10 | (18) | 4 | |
| Origination and reversal of temporary differences, tax losses and tax credits | 245 | 244 | 353 | |
| Deferred tax (expense) benefit | 401 | 210 | 274 |
Philips’ operations are subject to income taxes in various foreign jurisdictions. The statutory income tax rate varies per country, which results in a difference between the weighted average statutory income tax rate and the Netherlands’ statutory income tax rate of 25.8% (2022: 25.8% 2021: 25.0%).
A reconciliation of the weighted average statutory income tax rate to the effective income tax rate of continuing operations is as follows:
Philips Group
Effective income tax rate
in %
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Weighted average statutory income tax rate in % | 22.7 | 23.6 | 22.0 |
| Recognition of previously unrecognized tax loss and credit carryforwards | (26.9) | 0.1 | 16.8 |
| Unrecognized tax loss and credit carryforwards | 1.9 | (0.7) | (9.6) |
| Changes to recognition of temporary differences | 0.3 | (0.2) | (26.2) |
| Non-taxable income and tax incentives | (40.6) | 5.8 | 22.8 |
| Non-deductible expenses | 19.3 | (22.9) | (10.7) |
| Withholding and other taxes | 7.2 | (1.4) | (5.1) |
| Tax rate changes | (1.9) | (1.0) | 0.9 |
| Prior year tax | (2.4) | 0.7 | 1.9 |
| Tax expense (benefit) due to change in uncertain tax treatments | 4.4 | 2.8 | 2.3 |
| Others, net | (4.0) | (0.2) | 1.9 |
| Effective income tax rate | (20.0) | 6.5 | 17.0 |
The effective income tax rate is lower than the weighted average statutory income tax rate in 2023 mainly due to the recognition of previously unrecognized tax loss and credit carryforwards, which is mainly related to a one-off recognition of tax credits and non-taxable income and tax incentives which includes recurring favorable tax incentives related to R&D investments, the innovation box regime in the Netherlands and export activities. This is partly offset by the changes to recognition of temporary differences, which mostly represents deferred tax assets not fully recognized in United States.
Due to the loss position in 2023, items such as non-deductible expense lead to a decrease of the effective income tax rate and items such as tax incentives lead to an increase in the effective income tax rate.
Global minimum tax (Pillar Two)In December 2021, the OECD released model rules to introduce a global minimum corporate income tax rate of 15% applicable to multinational enterprise groups with global revenue over EUR 750 million (“Pillar Two”). The formal adoption of Directive (EU) 2022/2523 in December 2022 aims to achieve a coordinated implementation of Pillar Two in the EU Member States. The Dutch implementation of Pillar Two, the so-called Minimum Tax Rate Act 2024 (the “MTR Act”), was enacted in December 2023 and will apply to Philips from the financial year ending December 31, 2024 and onwards. Under this legislation, Philips may be required to pay top-up taxes on profits if the related Pillar Two jurisdictional effective tax rate is less than 15%.
Philips will be affected by the “MTR Act” as well as the implementation of Pillar Two per local law in other jurisdictions and has performed an assessment of the Group’s potential exposure to the Pillar Two legislation.
This assessment indicates potential exposure from the constituent entities in Hong Kong and the United Arab Emirates, where the Pillar Two effective tax rate is below 15%. This would potentially have resulted in top-up taxes had Pillar Two legislation been effective in 2023. The assessment of the potential exposure to top-up taxes is based on the profits and tax expenses determined as part of the preparation of Philips’ consolidated financial statement, most recent tax fillings and country-by-country reporting. The Pillar Two effective tax rate is lower in these jurisdictions due to exempted income and domestic tax rates either below or close to 15%.
The group effective tax rate, had Pillar Two legislation been effective from 2023, would have been 16.4% which is 0.6% lower than the reported effective tax rate of 17% under IFRS. The decrease in the effective tax rate can be attributed to the reduction in the income tax benefit of continuing operations resulting from the inclusion of potential top-up tax exposure (expense).
Deferred tax assets and liabilitiesDeferred tax assets are recognized for temporary differences, unused tax losses, and unused tax credits to the extent that realization of the related tax benefits is probable. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income in the countries where the deferred tax assets originated and during the periods when the deferred tax assets become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment.
Net deferred tax assets relate to the following underlying assets and liabilities and tax loss carryforwards (including tax credit carryforwards) and their movements during the years 2023 and 2022 respectively are presented in the following tables.
The net deferred tax assets of EUR 2,556 million (2022: EUR 2,358 million) consist of deferred tax assets of EUR 2,627 million (2022: EUR 2,449 million) and deferred tax liabilities of EUR 71 million (2022: EUR 91 million). Of the total deferred tax assets of EUR 2,627 million as of December 31, 2023 (2022: EUR 2,449 million), EUR 1,676 million (2022: EUR 1,453 million) is recognized in respect of entities in various countries where there have been tax losses in the current or preceding period, primarily the United States (US). The increase is mainly related to the US where there has been a tax loss in 2023, among others due to the Philips Respironics’ business operations. Philips assessed the recoverability of the tax losses and recognized the related deferred tax asset only to the extent future tax profits are considered probable. For the recoverability assessment, the income projections were determined using similar methodology as used for goodwill impairment testing (for more information please refer to note Goodwill). The company evaluated multiple risk-adjusted scenarios which support the assumption that it is probable that the results of future operations will generate sufficient taxable income to utilize the tax losses as well the deductible temporary differences. The projections include forward-looking assumptions whereby the most recent available information was used to determine the expected period of recovery of the deferred tax assets. Relevant developments potentially impacting the period and probability of recovery will be monitored closely.
As of December 31, 2023 the temporary differences associated with investments, including potential income tax consequences on dividends, for which no deferred tax liabilities are recognized, aggregate to EUR 444 million (2022: EUR 355 million).
Philips Group
Deferred tax assets and liabilities
in millions of EUR
| Balance as of January 1, 2023 | recognized in income statement | other1) | Balance as of December 31, 2023 | Assets | Liabilities | |
|---|---|---|---|---|---|---|
| Intangible assets | 630 | 61 | (12) | 679 | 826 | (147) |
| Property, plant and equipment | (2) | 18 | (103) | (88) | 44 | (132) |
| Inventories | 464 | (26) | (78) | 360 | 363 | (2) |
| Other assets | 44 | 20 | 120 | 184 | 233 | (48) |
| Pensions and other employee benefits | 153 | 69 | (29) | 193 | 204 | (11) |
| Other liabilities | 483 | (56) | 69 | 496 | 521 | (25) |
| Deferred tax assets on tax loss carryforwards | 586 | 188 | (44) | 730 | 730 | |
| Set-off deferred tax positions | (294) | 294 | ||||
| Net deferred tax assets | 2,358 | 274 | (77) | 2,556 | 2,627 | (71) |
Philips Group
Deferred tax assets and liabilities
in millions of EUR
| Balance as of January 1, 2022 | recognized in income statement | other1) | Balance as of December 31, 2022 | Assets | Liabilities | |
|---|---|---|---|---|---|---|
| Intangible assets | 587 | 63 | (20) | 630 | 783 | (152) |
| Property, plant and equipment | 29 | (33) | 2 | (2) | 49 | (52) |
| Inventories | 372 | 75 | 17 | 464 | 473 | (8) |
| Other assets | 68 | (16) | (8) | 44 | 98 | (55) |
| Pensions and other employee benefits | 180 | 6 | (32) | 153 | 175 | (22) |
| Other liabilities | 499 | (34) | 17 | 483 | 560 | (77) |
| Deferred tax assets on tax loss carryforwards | 398 | 149 | 38 | 586 | 586 | |
| Set-off deferred tax positions | (275) | 275 | ||||
| Net deferred tax assets | 2,134 | 210 | 14 | 2,358 | 2,449 | (91) |
The company has available tax loss and credit carryforwards, which expire as follows:
Philips Group
Expiry years of net operating loss and credit carryforwards
in millions of EUR
| Total balance as of December 31, 2022 |
Unrecognized balance as of December 31, 2022 |
Total balance as of December 31, 2023 |
Unrecognized balance as of December 31, 2023 |
|
|---|---|---|---|---|
| Within 1 year | 4 | 3 | 17 | 15 |
| 1 to 2 years | 10 | 5 | 20 | 16 |
| 2 to 3 years | 9 | 3 | 7 | 2 |
| 3 to 4 years | 13 | 4 | 9 | 5 |
| 4 to 5 years | 38 | 3 | 38 | 16 |
| Later | 812 | 93 | 808 | 81 |
| Unlimited | 2,301 | 920 | 2,997 | 1,231 |
| Total | 3,187 | 1,032 | 3,896 | 1,366 |
The increase in the unrecognized balance as of December 31, 2023 is mainly explained by the US.
As of December 31, 2023, the amount of deductible temporary differences for which no deferred tax asset has been recognized in the balance sheet was EUR 125 million (2022: EUR 45 million).
Tax risksPhilips is exposed to tax risks and uncertainty over tax treatments. For particular tax treatments that are not expected to be accepted by tax authorities, Philips either recognizes a liability or reflects the uncertainty in the recognition and measurement of its current and deferred tax assets and tax attributes. For the measurement of the uncertainty, Philips uses the most likely amount or the expected value of the tax treatment. The expected liabilities resulting from the uncertain tax treatments are included in non-current tax liabilities (2023: EUR 390 million, 2022: EUR 435 million, decrease due to release of liabilities, in combination with higher tax losses or similar tax carryforwards that can be used if uncertain tax treatments were settled for the presumed amount at balance sheet date). The positions include, among others, the following:
Transfer pricing risksPhilips has issued transfer pricing directives, which are in accordance with international guidelines such as those of the Organization of Economic Co-operation and Development. In order to reduce the transfer pricing uncertainties, monitoring procedures are carried out by Group Tax to safeguard the correct implementation of the transfer pricing directives. However, tax disputes can arise due to inconsistent transfer pricing regimes and different views on "at arm's length" pricing.
Tax risks on general and specific service agreements and licensing agreementsDue to the centralization of certain activities (such as research and development, IT and group functions), costs are also centralized. As a consequence, these costs and/or revenues must be allocated to the beneficiaries, i.e. the various Philips entities. For that purpose, service contracts such as intra-group service agreements and licensing agreements are signed with a large number of group entities. Tax authorities review these intra-group service and licensing agreements, and may reject the implemented intra-group charges. Furthermore, buy in/out situations in the case of (de)mergers could affect the cost allocation resulting from the intragroup service agreements between countries. The same applies to the specific service agreements.
Tax risks due to disentanglements and acquisitionsWhen a subsidiary of Philips is disentangled, or a new company is acquired, tax risks may arise. Philips creates merger and acquisition (M&A) teams for these disentanglements or acquisitions. In addition to representatives from the involved business, these teams consist of specialists from various group functions and are formed, among other things, to identify tax risks and to reduce potential tax claims.
Tax risks due to permanent establishmentsA permanent establishment may arise when a Philips entity has activities in another country, tax claims could arise in both countries on the same income.
The cost of property, plant and equipment comprise all directly attributable costs (including the cost of material and direct labor).
Depreciation is generally calculated using the straight-line method over the useful life of the asset. Land and assets under construction are not depreciated. When assets under construction are ready for their intended use, they are transferred to the relevant asset category and depreciation starts. All other property, plant and equipment items are depreciated over their estimated useful lives to their estimated residual values.
The estimated useful lives of property, plant and equipment are as follows:
Philips Group
Useful lives of property, plant and equipment
| Buildings | from 5 to 50 years |
| Machinery and installations | from 3 to 20 years |
| Other equipment | from 1 to 10 years |
Property, plant and equipment are reviewed for impairment whenever events or changes in circumstances indicate that the book value of the assets concerned may not be recoverable. An impairment loss is recognized for the amount by which the asset's book value exceeds their recoverable amount. Impairments are reversed if and to the extent that the impairment no longer exists. The recoverable amount is defined as the higher of the asset’s fair value less costs of disposal and its value in use.
Gains and losses on the sale of property, plant and equipment are included in other business income. Costs related to repair and maintenance activities are expensed in the period in which they are incurred unless they extend the asset's original lifetime or capacity.
Right-of-use assetsThe company leases various items of real estate, vehicles and other equipment. The company determines whether an arrangement constitutes or contains a lease based on the substance of the arrangement at the lease inception. The arrangement constitutes or contains a lease if fulfillment is dependent on the use of a specific asset and the arrangement conveys a right to use the asset, even if that asset is not explicitly specified in the arrangement.
Company as a lesseeThe company recognizes right-of-use assets and lease liabilities for leases with a term of more than twelve months if the underlying asset is not of low value. Payments for short-term and low-value leases are expensed over the lease term. Extension options are included in the lease term if their exercise is reasonably certain. Right-of-use assets are measured at cost less accumulated depreciation and impairment losses, adjusted for any remeasurements. Right-of-use assets are depreciated using the straight-line method over the shorter of the lease term and the useful life of the underlying assets.
Company as a lessorWhen the company acts as a lessor, it determines at lease inception whether a lease is a finance lease or an operating lease. Leases in which the company does not transfer substantially all the risks and rewards incidental to ownership of an asset are classified as operating leases. The company recognizes lease payments received under operating leases as income on a straight-line basis over the lease term in the Consolidated statement of income.
Judgments are required, not only to determine whether there is an indication that an asset may be impaired, but also whether indications exist that impairment losses previously recognized may no longer exist or may have decreased (impairment reversal). After indications of impairment have been identified, estimates and assumptions are used in the determination of the recoverable amount of a fixed asset. These involve estimates of expected future cash flows (based on future growth rates and remaining useful life) and residual value assumptions, as well as discount rates to calculate the present value of the future cash flows.
Owned assetsEstimates are required to determine the (remaining) useful lives of fixed assets. Useful lives are determined based on an asset's age, the frequency of its use, repair and maintenance policy, technology changes in production and expected restructuring. The company estimates the expected residual value per asset item. The residual value is the higher of the asset's expected sales price (based on recent market transactions of similar sold items) and its material scrap value.
Right-of-use assetsJudgment is required to determine the lease term. The assessment of whether the company is reasonably certain to exercise extension options impacts the lease term, which could affect the amount of lease liabilities and right-of-use assets recognized.
Property, plant and equipment are fixed assets that are owned or right-of-use assets under a lease agreement. Owned and right-of-use assets are held for use in Philips' operating activities.
Philips Group
Property, plant and equipment
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Owned assets | 1,718 | 1,565 |
| Right-of-use assets | 919 | 919 |
| Total | 2,638 | 2,483 |
Philips Group
Property, plant and equipment - owned assets
in millions of EUR
| Land and buildings |
Machinery and installations | Other equipment |
Assets under construction | Total | |
|---|---|---|---|---|---|
| Balance as of January 1, 2023 |
|||||
| Cost | 1,135 | 1,779 | 1,454 | 309 | 4,676 |
| Accumulated depreciation | (621) | (1,291) | (1,046) | (2,958) | |
| Book value |
514 | 488 | 408 | 309 | 1,718 |
| Additions | 1 | 115 | 77 | 239 | 433 |
| Assets available for use | 20 | 90 | 144 | (262) | (8) |
| Depreciation | (56) | (196) | (167) | (420) | |
| Impairments | (5) | (23) | (17) | - | (45) |
| Transfer (to) from AHFS | (1) | (1) | (45) | (46) | |
| Reclassifications | 15 | 2 | (17) | (5) | (6) |
| Translation differences and other | (14) | (22) | (19) | (7) | (62) |
| Total change |
(39) | (35) | (45) | (35) | (154) |
| Balance as of December 31, 2023 |
|||||
| Cost | 1,114 | 1,731 | 1,404 | 274 | 4,521 |
| Accumulated depreciation | (638) | (1,278) | (1,041) | (2,957) | |
| Book value | 476 | 453 | 363 | 274 | 1,565 |
Philips Group
Property, plant and equipment - right-of-use assets
in millions of EUR
| Land and buildings |
Other equipment |
Total | |
|---|---|---|---|
| Balance as of January 1, 2023 | |||
| Cost | 1,365 | 206 | 1,571 |
| Accumulated depreciation | (543) | (108) | (651) |
| Book value |
822 | 98 | 919 |
| Additions | 175 | 62 | 236 |
| Assets available for use | 2 | 6 | 8 |
| Depreciation | (150) | (51) | (201) |
| Impairments | (23) | - | (23) |
| Transfer (to) from AHFS | (2) | (2) | |
| Reclassifications | - | 4 | 4 |
| Translation differences and other | (18) | (5) | (23) |
| Total change |
(16) | 15 | (1) |
| Balance as of December 31, 2023 | |||
| Cost | 1,425 | 216 | 1,641 |
| Accumulated depreciation | (619) | (104) | (722) |
| Book value | 806 | 112 | 919 |
Philips Group
Property, plant and equipment - owned assets
in millions of EUR
| Land and buildings |
Machinery and installations | Other equipment |
Assets under construction | Total | |
|---|---|---|---|---|---|
| Balance as of January 1, 2022 |
|||||
| Cost | 1,097 | 1,585 | 1,382 | 208 | 4,273 |
| Accumulated depreciation | (591) | (1,074) | (967) | (2,632) | |
| Book value |
506 | 511 | 415 | 208 | 1,641 |
| Additions | 1 | 102 | 77 | 314 | 494 |
| Assets available for use | 34 | 69 | 111 | (220) | (6) |
| Depreciation | (56) | (215) | (176) | - | (447) |
| Impairments | (3) | (20) | (18) | (1) | (42) |
| Transfer (to) from AHFS | (3) | - | (3) | ||
| Reclassifications | 18 | 14 | (5) | 2 | 29 |
| Translation differences and other | 16 | 26 | 2 | 5 | 50 |
| Total change |
8 | (23) | (8) | 100 | 78 |
| Balance as of December 31, 2022 |
|||||
| Cost | 1,135 | 1,779 | 1,454 | 309 | 4,676 |
| Accumulated depreciation | (621) | (1,291) | (1,046) | (2,958) | |
| Book value | 514 | 488 | 408 | 309 | 1,718 |
Philips Group
Property, plant and equipment - right-of-use assets
in millions of EUR
| Land and buildings |
Machinery and installations |
Other equipment |
Total | |
|---|---|---|---|---|
| Balance as of January 1, 2022 | ||||
| Cost | 1,332 | 176 | 216 | 1,724 |
| Accumulated depreciation | (418) | (139) | (109) | (666) |
| Book value |
914 | 37 | 107 | 1,058 |
| Additions | 52 | - | 54 | 106 |
| Assets available for use | 5 | 1 | 6 | |
| Depreciation | (155) | (2) | (58) | (214) |
| Impairments | (8) | - | - | (9) |
| Transfer (to) from AHFS | 3 | 3 | ||
| Reclassifications | (19) | (13) | - | (32) |
| Translation differences and other | 31 | (23) | (6) | 1 |
| Total change |
(92) | (37) | (9) | (139) |
| Balance as of December 31, 2022 | ||||
| Cost | 1,365 | - | 206 | 1,571 |
| Accumulated depreciation | (543) | (108) | (651) | |
| Book value | 822 | - | 98 | 919 |
Below are the references with respect to year-end disclosures as lessee:
Other qualitative and quantitative disclosures regarding the nature of lessee’s leasing activities and future lease obligations, refer to Debt.
Below are the references with respect to year-end disclosures as lessor:
Acquired finite-lived intangible assets are amortized using the straight-line method over their estimated useful life. The useful lives are evaluated annually. Intangible assets are initially capitalized at cost, with the exception of intangible assets acquired as part of a business combination, which are capitalized at their acquisition date fair value.
The company expenses all research costs as incurred. Expenditure on development activities, whereby research findings are applied to a plan or design for the production of new or substantially improved products and processes, is capitalized as an intangible asset if the product or process is technically and commercially feasible, the company has sufficient resources and the intention to complete development and can measure the attributable expenditure reliably.
The capitalized development expenditure comprises of all directly attributable costs (including the cost of materials and direct labor). Other development expenditures and expenditures on research activities are recognized in the Consolidated statements of income. Capitalized development expenditure is stated at cost less accumulated amortization and impairment losses. Amortization of capitalized development expenditure is charged to the Consolidated statements of income on a straight-line basis over the estimated useful lives of the intangible assets.
The expected useful lives of the intangible assets excluding goodwill are as follows:
Philips Group
Expected useful lives of intangible assets excluding goodwill
in years
| Brand names | 2-20 |
| Customer relationships | 2-25 |
| Technology | 3-20 |
| Other | 1-10 |
| Software | 1-10 |
| Product development | 3-10 |
The weighted average expected remaining life of brand names, customer relationships, technology and other intangible assets is 9.3 years as of December 31, 2023 (2022: 9.4 years).
Impairment of intangible assets not yet ready for useIntangible assets not yet ready for use are not amortized but are tested for impairment annually and whenever impairment indicators require. In the case of intangible assets not yet ready for use, either internal or external sources of information are considered to assess if there are indicators that an asset or a CGU may be impaired.
Impairment of non-financial assets other than goodwill, intangible assets not yet ready for use, inventories and deferred tax assetsNon-financial assets other than goodwill, intangible assets not yet ready for use, inventories and deferred tax assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is assessed by a comparison of the carrying amount of an asset with the greater of its value in use and fair value less cost of disposal. Value in use is measured as the present value of future cash flows expected to be generated by the asset. Fair value less cost of disposal is measured as the amount obtained from a sale of an asset in an arm’s length transaction, less costs of disposal. If the carrying amount of an asset is deemed not recoverable, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the recoverable amount. The review for impairment is carried out at the level where cash flows occur that are independent of other cash flows.
Impairment losses recognized in prior periods for Intangible assets other than goodwill are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if and to the extent that there has been a change in the estimates used to determine the recoverable amount. The loss is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of depreciation or amortization, if no impairment loss had been recognized. Reversals of impairment are recognized in the Consolidated statements of income.
The cash flow projections used in the value in use calculations for intangible assets excluding goodwill contain various judgments and estimations. For intangible assets excluding goodwill, estimates are required to determine the (remaining) useful lives.
Philips Group
Intangible assets excluding goodwill
in millions of EUR
| brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | |
|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2023 | ||||||||
| Cost | 647 | 2,735 | 2,947 | 2,605 | 648 | 869 | 152 | 10,602 |
| Amortization / impairments | (507) | (1,665) | (1,845) | (2,212) | (146) | (589) | (113) | (7,077) |
| Book value | 140 | 1,070 | 1,102 | 393 | 502 | 280 | 39 | 3,526 |
| Additions | 33 | - | 214 | 70 | - | 317 | ||
| Assets available for use | 157 | (157) | ||||||
| Acquisitions | 40 | - | - | 40 | ||||
| Amortization | (20) | (137) | (131) | (169) | (97) | (1) | (556) | |
| Impairments | - | - | - | (7) | (7) | (1) | - | (16) |
| Transfers to assets classified as held for sale | (1) | (20) | (8) | (2) | (32) | |||
| Translation differences and other | (1) | (37) | (30) | (38) | 1 | 18 | - | (87) |
| Total change | (22) | (195) | (89) | (57) | 42 | (13) | (1) | (335) |
| Balance as of December 31, 2023 | ||||||||
| Cost | 629 | 2,593 | 2,908 | 2,432 | 635 | 929 | 139 | 10,265 |
| Amortization / impairments | (511) | (1,718) | (1,895) | (2,096) | (91) | (662) | (101) | (7,075) |
| Book Value | 118 | 875 | 1,013 | 336 | 544 | 267 | 38 | 3,190 |
Philips Group
Intangible assets excluding goodwill
in millions of EUR
| brand names | customer relationships | technology | product development | product development construction in progress | software | other | total | |
|---|---|---|---|---|---|---|---|---|
| Balance as of January 1, 2022 | ||||||||
| Cost | 644 | 2,590 | 2,605 | 2,701 | 505 | 754 | 146 | 9,944 |
| Amortization / impairments | (481) | (1,447) | (1,605) | (2,102) | (91) | (467) | (101) | (6,294) |
| Book value | 162 | 1,143 | 1,000 | 599 | 414 | 287 | 44 | 3,650 |
| Additions | (3) | - | 51 | - | 257 | 109 | 1 | 416 |
| Assets available for use | 118 | (118) | - | - | - | |||
| Acquisitions | 1 | 3 | 177 | - | - | - | 180 | |
| Amortization | (24) | (141) | (140) | (206) | (1) | (100) | (3) | (614) |
| Impairments | - | (6) | (46) | (123) | (81) | (17) | (2) | (276) |
| Translation differences and other | 4 | 71 | 59 | 5 | 31 | 1 | (2) | 169 |
| Total change | (22) | (74) | 102 | (206) | 88 | (7) | (6) | (125) |
| Balance as of December 31, 2022 | ||||||||
| Cost | 647 | 2,735 | 2,947 | 2,605 | 648 | 869 | 152 | 10,602 |
| Amortization / impairments | (507) | (1,665) | (1,845) | (2,212) | (146) | (589) | (113) | (7,077) |
| Book Value | 140 | 1,070 | 1,102 | 393 | 502 | 280 | 39 | 3,526 |
Acquisitions in 2023 involved intangible assets of EUR 40 million in aggregate (2022: EUR 180 million). For more information, refer to Acquisitions and divestments.
Impairments in 2023 were EUR 16 million (2022: EUR 276 million) and mainly relate to product development construction in progress and product development.
The company uses scenarios in the business forecasting process and the most reasonable and supportable assumptions which represent management’s best estimate are used as the basis for the value-in-use calculations.
The amortization and impairment of intangible assets is further specified in Income from operations.
The most notable intangible assets as of December 31, 2023 relate to the BioTelemetry customer relationships and technology with a carrying value of EUR 327 million and EUR 123 million and a remaining amortization period of 13 years and 9 years, respectively and Spectranetics customer relationships and technology with a carrying value of EUR 261 million and EUR 175 million and a remaining amortization period of 14 years and 9 years, respectively. The most notable intangible assets as of December 31, 2022 relate to the BioTelemetry customer relationships and technology with value of EUR 385 million and EUR 150 million and a remaining amortization period of 14 years and 10 years, respectively and Spectranetics customer relationships and technology with a carrying value of EUR 291 million and EUR 203 million and a remaining amortization period of 15 years and 10 years, respectively.
The classification of financial assets at initial recognition depends on the financial asset’s contractual cash flow characteristics and the company’s business model for managing them.
The company initially measures a financial asset at its fair value plus, in the case of a financial asset not at fair value through profit or loss, transaction costs.
For the purposes of subsequent measurement, financial assets are classified into four categories:
The company recognizes a loss allowance for expected credit losses for trade receivables, contract assets, lease receivables, debt investments carried at amortized cost and fair value through other comprehensive income (FVTOCI).
At each balance sheet date, the company assesses whether there is objective evidence that a financial asset or a group of financial assets is impaired and recognizes a loss allowance for expected credit losses for financial assets measured at either amortized costs or at fair value through other comprehensive income. If, at the reporting date, the credit risk on a financial instrument has not increased significantly since initial recognition, the company measures the loss allowance for the financial instrument at an amount equal to 12 months of expected credit losses. If, at the reporting date, the credit risk on a financial instrument has increased significantly since initial recognition, the company measures the loss allowance for the financial instrument at an amount equal to the lifetime-expected credit losses. For all trade receivables, contract assets and lease receivables the company measures the loss allowance at an amount equal to lifetime-expected credit losses.
The determination of fair value is subject to estimates for investments that are not publicly traded. Refer to Fair value of financial assets and liabilities
Financial assets classified at amortized cost and at fair value through OCI are subject to impairment assessment. The calculation of expected credit losses requires the company to apply significant judgment and make estimates and assumptions that involve significant uncertainty at the time they are made. Changes to these estimates and assumptions can result in significant changes to the timing and amount of expected credit losses to be recognized.
In 2023, Other current financial assets decreased from EUR 11 million to EUR 3 million (2022: increased from EUR 2 million to EUR 11 million).
Other non-current financial assetsThe company’s investments in Other non-current financial assets mainly consist of investments in common shares of companies in various industries and investments in limited life funds. The changes during 2023 and 2022 were as follows:
Philips Group
Other non-current financial assets
in millions of EUR
| Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | |
|---|---|---|---|---|
| Balance as of January 1, 2023 | 322 | 284 | 54 | 660 |
| Changes: | ||||
| Acquisitions/additions | 71 | 14 | 20 | 105 |
| Sales/redemptions/reductions | (33) | (14) | (11) | (58) |
| Impairments | ||||
| Value adjustment through OCI | - | (17) | (17) | |
| Value adjustment through P&L | (39) | - | (39) | |
| Translation differences and other | (29) | (14) | (1) | (44) |
| Reclassifications | (8) | 5 | 15 | 12 |
| Balance as of December 31, 2023 | 284 | 258 | 77 | 619 |
Philips Group
Other non-current financial assets
in millions of EUR
| Non-current financial assets at FVTP&L | Non-current financial assets at FVTOCI | Non-current financial assets at Amortized cost | Total | |
|---|---|---|---|---|
| Balance as of January 1, 2022 | 283 | 300 | 47 | 630 |
| Changes: | ||||
| Acquisitions/additions | 114 | 18 | 18 | 150 |
| Sales/redemptions/reductions | (75) | (3) | (8) | (86) |
| Impairments | (3) | (1) | (5) | |
| Value adjustment through OCI | - | (35) | (35) | |
| Value adjustment through P&L | 5 | - | 5 | |
| Translation differences and other | (2) | 5 | (1) | 2 |
| Reclassifications | 1 | (2) | (1) | (2) |
| Balance as of December 31, 2022 | 322 | 284 | 54 | 660 |
As of December 31, 2023, equity investments of EUR 231 million (2022: EUR 259 million) are accounted under the FVTOCI category based on the company's election at initial recognition mainly because such investments are neither held for trading purposes nor primarily for their increase in value and the elected presentation is considered to reflect the nature and purpose of the investment.
The company recognizes contract assets for revenue earned from installation services because the receipt of consideration is conditional on successful completion of the installation. Upon completion of the installation and acceptance by the customer, the amount recognized as contract assets is reclassified to trade receivables.
Other assets are measured at amortized cost minus any impairment losses.
Other non-current assets as of December 31, 2023 were EUR 93 million (2022: EUR 98 million). These are mainly related to prepaid expenses.
Other current assetsOther current assets as of December 31, 2023 of EUR 500 million (2022: EUR 490 million) included contract assets of EUR 297 million (2022: EUR 292 million), accrued income of EUR 6 million (2022: EUR 24 million) and prepaid expenses of EUR 197 million (2022: EUR 174 million) mainly related to Diagnosis & Treatment businesses and Connected Care businesses.
Inventories are stated at the lower of cost or net realizable value. The cost of inventories comprises all costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. The costs of conversion of inventories include direct labor and fixed and variable production overheads, considering the stage of completion and the normal capacity of production facilities. Costs of idle facility and abnormal waste are expensed. The cost of inventories is determined using the first-in, first-out (FIFO) method. The write-down of inventories to net realizable value is included in cost of sales.
Inventory is reduced for the estimated losses due to obsolescence. This reduction is determined for groups of products based on sales in the recent past and/or expected future demand.
Inventories are summarized as follows:
Philips Group
Inventories
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Raw materials and supplies | 1,541 | 1,309 |
| Work in process | 648 | 552 |
| Finished goods | 1,860 | 1,629 |
| Inventories | 4,049 | 3,491 |
In 2023, overall global inventories have operationally decreased in all categories due to the deployment of strategic management of aging and unhealthy inventory accompanied by a more optimized tracking in both production and commercial inventories.
The write-down of inventories to net realizable value was EUR 339 million in 2023 (2022: EUR 215 million), including EUR 82 million related to the proposed Respironics consent decree (refer to Provisions).
Receivables are held by the company to collect the related cash flows. These receivables are measured at fair value and subsequently measured at amortized cost minus any impairment losses.
Receivables are derecognized when the company has transferred substantially all risks and rewards, which includes transactions in which the company enters into factoring transactions, or if the company does not retain control over the receivables.
Receivables are subject to impairment assessment, which involves estimating expected credit losses. Refer to Other financial assets for accounting policies on impairment of financial assets.
Non-current receivables are associated mainly with customer financing in the Diagnosis & Treatment businesses amounting to EUR 102 million (2022: EUR 69 million), for Signify indemnification amounting to EUR 4 million (2022: EUR 26 million), an income tax receivable amounting to EUR 12 million (which includes an interest receivable of EUR 3 million) for which Philips expects to get a refund (2022: EUR 126 million) and insurance receivables in the US amounting to EUR 33 million (2022: EUR 30 million).
Current receivablesCurrent receivables of EUR 3,733 million (2022: EUR 4,115 million) as of December 31, 2023 included trade accounts receivable (net of allowance) of EUR 3,546 million (2022: EUR 3,832 million), accounts receivable other of EUR 170 million (2022: EUR 228 million) and accounts receivable from investments in associates of EUR 18 million (2022: EUR 55 million).
The trade accounts receivable, net, per segment are as follows:
Philips Group
Trade accounts receivable, net
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Diagnosis & Treatment | 1,795 | 1,688 |
| Connected Care | 1,332 | 1,105 |
| Personal Health | 479 | 576 |
| Other | 226 | 177 |
| Trade accounts receivable, net | 3,832 | 3,546 |
The aging analysis of trade accounts receivable, net, representing current and overdue but not fully impaired receivables, is as follows:
Philips Group
Aging analysis
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Current | 3,280 | 3,132 |
| Overdue 1-30 days | 169 | 117 |
| Overdue 31-180 days | 282 | 234 |
| Overdue more than 180 days | 101 | 63 |
| Trade accounts receivable, net | 3,832 | 3,546 |
The changes in the allowance for doubtful accounts receivable are as follows:
Philips Group
Allowance for accounts receivable
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Balance as of January 1 | 190 | 226 |
| Additions charged to expense | 66 | 27 |
| Deductions from allowance1) | (51) | (26) |
| Transfer to assets held for sale | (1) | |
| Other movements | 21 | (10) |
| Balance as of December 31 | 226 | 216 |
The allowance for doubtful accounts receivable has been primarily established for receivables that are past due. The allowance presented also includes the allowance for Non-current customer finance receivables of EUR 8 million (2022: EUR 7 million). Other movements in the current period are mainly related to foreign currency valuations.
Included in the above balances as of December 31, 2023 are allowances for individually impaired receivables of EUR 210 million (2022: EUR 222 million).
Common shares are classified as equity. Incremental costs directly attributable to the issuance of shares are recognized as a deduction from equity. Where the company repurchases the company’s equity share capital (treasury shares), the consideration paid, including any directly attributable incremental transaction costs (net of income taxes), is deducted from shareholders’ equity until such treasury shares are cancelled or reissued.
Where such treasury shares are subsequently reissued, any consideration received, net of any directly attributable incremental transaction costs and the related income tax effects, is included in shareholders’ equity.
Call options on own shares are treated as equity instruments.
Dividends are recognized as a liability in the period in which they are declared and approved by shareholders. The income tax consequences of dividends are recognized when a liability to pay the dividend is recognized.
As of December 31, 2023, authorized common shares consist of 2 billion shares (December 31, 2022: 2 billion; December 31, 2021: 2 billion) and the issued and fully paid share capital consists of 913,515,966 common shares, each share having a par value of EUR 0.20 (December 31, 2022: 889,315,082; December 31, 2021: 883,898,969).
Preference sharesAs a means to protect the company against (an attempt at) an unsolicited takeover or other attempt to exert (de facto) control of the company, the ‘Stichting Preferente Aandelen Philips’ has been granted the right to acquire preference shares in the company. As of December 31, 2023, no such right has been exercised and no preference shares have been issued. Authorized preference shares consist of 2 billion shares as of December 31, 2023 (December 31, 2022: 2 billion; December 31, 2021: 2 billion).
Options, restricted and performance sharesUnder its share-based compensation plans, the company granted stock options on its common shares and other conditional rights to receive common shares in the future such as restricted shares and performance shares (refer to Share-based compensation).
Treasury sharesIn connection with the company’s share repurchase programs, shares which have been repurchased and are held in Treasury for the purpose of (i) delivery under share-based compensation plans upon exercise of options, or vesting of restricted or performance shares, and (ii) capital reduction, are accounted for as a reduction of shareholders’ equity. Treasury shares are recorded at cost, representing the market price on the acquisition date. When treasury shares are delivered by the company under its share-based compensation plans, such shares are removed from treasury shares on a first-in, first-out (FIFO) basis.
When treasury shares are delivered by the company upon exercise of options, the difference between the cost and the cash received is recorded in retained earnings. When treasury shares are delivered by the company upon vesting of restricted shares or performance shares (granted under the company’s share-based compensation plans), the difference between the market price of the shares and the cost is recorded in retained earnings, and the market price is recorded in capital in excess of par value.
The following table shows the movements in the outstanding number of shares over the last three years:
Philips Group
Outstanding number of shares
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Balance as of January 1 | 905,128,293 | 870,182,445 | 881,480,527 |
| Dividend distributed | 6,345,968 | 14,174,568 | 39,334,938 |
| Purchase of treasury shares | (45,486,392) | (5,080,693) | (15,964,445) |
| Delivery of treasury shares | 4,194,577 | 2,204,207 | 1,552,136 |
| Balance as of December 31 | 870,182,445 | 881,480,527 | 906,403,156 |
The following table reflects transactions that took place in relation to former and current share-based compensation plans:
Philips Group
Transactions related to share-based compensation plans
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Shares acquired | 3,996,576 | 2,142,445 | 3,000,000 |
| Average market price | EUR 36.15 | EUR 31.76 | EUR 41.59 |
| Amount paid | EUR 144 million | EUR 68 million | EUR 125 million |
| Shares delivered | 4,194,577 | 2,204,207 | 1,552,136 |
| Average price (FIFO) | EUR 34.14 | EUR 35.16 | EUR 34.59 |
| Cost of delivered shares | EUR 143 million | EUR 77 million | EUR 54 million |
| Total shares in treasury at year-end | 5,726,708 | 5,664,946 | 7,112,810 |
| Total cost | EUR 201 million | EUR 191 million | EUR 262 million |
The following transactions took place for capital reduction purposes:
Philips Group
Transactions related to capital reduction
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Shares acquired | 41,489,816 | 2,938,248 | 12,964,445 |
| Average market price | EUR 36.22 | EUR 36.61 | EUR 37.25 |
| Amount paid | EUR 1,503 million | EUR 108 million | EUR 483 million |
| Cancellation of treasury shares (shares) | 33,500,000 | 8,758,455 | 15,134,054 |
| Cancellation of treasury shares (EUR) | EUR 1,216 million | EUR 299 million | EUR 566 million |
| Total shares in treasury at year-end | 7,989,816 | 2,169,609 | |
| Total cost | EUR 287 million | EUR 83 million |
Share purchase transactions related to employee option and share plans, as well as transactions related to the reduction of share capital, involved a cash outflow of EUR 662 million in 2023. In 2023, the settlement of forward contracts resulted in a withholding tax liability for an amount of EUR 66 million relating to the dividend distribution. As of December 31, 2023, the remaining liability to be settled amounted to EUR 11 million.
Share repurchase methods for share-based remuneration plans and capital reduction purposesPhilips uses different methods to repurchase shares in its own capital: (i) share buyback repurchases in the open market via an intermediary; (ii) repurchase of shares via forward contracts for future delivery of shares; and (iii) the unwinding of call options on own shares. During 2023, Philips used method (ii) to repurchase shares for capital reduction purposes and share-based compensation plans.
Forward contracts to repurchase sharesFor share-based compensation plansOn June 14, 2023, Royal Philips announced that it will repurchase up to 7.1 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchases plans. Under this program, Philips entered into one forward contract for an amount of EUR 138 million to acquire 7.1 million shares with settlement dates varying between November 2024 and November 2025 and a weighted average forward price of EUR 19.43.
On June 13, 2022, Royal Philips announced that it will repurchase up to 3.2 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchases plans. Under this program, Philips entered into one forward contract for an amount of EUR 63 million to acquire 3.2 million shares with settlement dates in November 2024 and December 2024 and a weighted average forward price of EUR 19.75.
On May 19, 2021, Royal Philips announced that it will repurchase up to 2 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchase plans. Under this program, Philips entered into one forward contract for an amount of EUR 90 million to acquire 2 million shares with settlement dates in October 2023 and November 2023 and a weighted average forward price of EUR 44.85. As of December 31, 2023, all shares under this program were acquired (settled in the fourth quarter of 2023). This resulted in a EUR 90 million increase in retained earnings against treasury shares.
On January 29, 2020, Philips announced that it will repurchase up to 6 million shares to cover certain of its obligations arising from its long-term incentive and employee stock purchase plans. Under this program, Philips entered into three forward contracts to acquire in total 5 million for an amount of EUR 174 million to acquire with settlement dates varying between October 2021 and November 2022 and a weighted average forward price of EUR 34.85. On October 26, 2022, the original settlement date of two tranches entered into under this program (in total 1.75 million shares) has been extended from November 23, 2022 to November 2023, and November 2024, respectively. As of December 31, 2023, a total of 4.3 million shares (December 31, 2022: 3.3 million shares) under this program were acquired (settled in the fourth quarter of 2021, 2022, and 2023). This resulted in a EUR 35 million (2022: EUR 57 million) increase in retained earnings against treasury shares.
As of December 31, 2023, the remaining forward contracts to cover obligations under share-based compensation plans related to 11.1 million shares (December 31, 2022: 7.0 million shares) and amounted to EUR 224 million (December 31, 2022: EUR 211 million).
For capital reductionOn July 26, 2021, Philips announced a share buyback program for share cancellation purposes for an amount of up to EUR 1.5 billion. Consequently, in the third quarter of 2021 Philips entered into three forward contracts for an amount of EUR 731 million to acquire 20 million shares with settlement dates in 2022, 2023 and 2024 and a weighted average forward price of EUR 37.36. Philips executed the remainder of the program through open market purchases by an intermediary in the fourth quarter of 2021 (acquiring 21 million shares) and January 2022 (acquiring 0.8 million shares). This resulted in a EUR 781 million increase in retained earnings against treasury shares. As of December 31, 2023, a total of 15.1 million (December 31, 2022: 2.2 million) shares under this program were acquired (in the fourth quarter of 2022 and third and fourth quarters of 2023). This resulted in EUR 483 million (including dividend adjustment) increase in retained earnings against treasury shares (2022: EUR 83 million).
As of December 31, 2023, the remaining forward contracts entered into for capital reduction purposes relate to 4.4 million shares (December 31, 2022: 17.4 million shares) and amounted to EUR 167 million (December 31, 2022: EUR 648 million).
Share call optionsIn 2016, Philips purchased EUR-denominated and USD-denominated call options on its own shares to hedge options granted to employees up to 2013.
On December 31, 2022, there were 55,750 EUR-denominated call options outstanding. As of December 31, 2023, all outstanding EUR-denominated call options have expired.
Shares cancellationIn December 2023, Philips completed the cancellation of 15.1 million of its common shares (with a cost price of EUR 566 million). The cancelled shares were acquired as part of Philips’ EUR 1.5 billion share repurchase program announced on July 26, 2021.
Dividend distribution2023In May 2023, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 749 million (including costs). The dividend was distributed in the form of shares only, resulting in the issuance of 39,334,938 new common shares. Per share calculations have been adjusted retrospectively for all periods presented to reflect the issuance of shares for the share dividend in respect of 2022. Further reference is made to Earnings per share.
A proposal will be submitted to the 2024 Annual General Meeting of Shareholders to pay a dividend of EUR 0.85 per common share, in common shares only, against retained earnings for 2023.
2022In May 2022, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 741 million (including costs). Shareholders could elect for a cash dividend or a share dividend. Approximately 45% of the shareholders elected for a share dividend, resulting in the issuance of 14,174,568 new common shares. The settlement of the cash dividend involved an amount of EUR 411 million (including costs).
2021In June 2021, Philips distributed a dividend of EUR 0.85 per common share, representing a total value of EUR 773 million (including costs). Shareholders could elect for a cash dividend or a share dividend. Approximately 38% of the shareholders elected for a share dividend, resulting in the issuance of 6,345,968 new common shares. The settlement of the cash dividend involved an amount of EUR 482 million (including costs).
Limitations in the distribution of shareholders’ equityAs of December 31, 2023, pursuant to Dutch law, certain limitations exist relating to the distribution of shareholders’ equity of EUR 2,435 million. Such limitations relate to common shares of EUR 183 million, as well as to legal reserves required by Dutch law included under retained earnings of EUR 990 million and unrealized currency translation differences of EUR 1,263 million. The unrealized gain related to cash flow hedges of EUR 6 million and unrealized loss related to fair value through OCI financial assets of EUR 390 million qualify as revaluation reserves and reduce the distributable amount due to the fact that these reserves are negative.
The legal reserves required by Dutch law of EUR 990 million included under retained earnings relates to any legal or economic restrictions on the ability of affiliated companies to transfer funds to the parent company in the form of dividends.
As of December 31, 2022, these limitations in distributable amounts were EUR 3,054 million and related to common shares of EUR 178 million, as well as to legal reserves required by Dutch law included under retained earnings of EUR 1,010 million and unrealized currency translation differences of EUR 1,866 million. The unrealized losses related to fair value through OCI financial assets of EUR 376 million and unrealized loss related to cash flow hedges of EUR 2 million qualify as a revaluation reserve and reduce the distributable amount due to the fact that this reserve is negative.
Non-controlling interestsNon-controlling interests relate to minority stakes held by third parties in consolidated group companies.
Capital managementPhilips manages capital based upon the IFRS measures, net cash provided by operating activities and net cash used for investing activities as well as the non-IFRS measure net debt. The definition of this non-IFRS measure and a reconciliation to the IFRS measure is included below.
Net debt is defined as the sum of long and short-term debt minus cash and cash equivalents. Group equity is defined as the sum of shareholders’ equity and non-controlling interests. This measure is used by Philips Treasury management and investment analysts to evaluate financial strength and funding requirements. The Philips net debt position is managed with the intention of retaining the current strong investment grade credit rating. Furthermore, Philips’ aim when managing the net debt position is dividend stability and a pay-out ratio of 40% to 50% of Adjusted income from continuing operations attributable to shareholders (reconciliation to the most directly comparable IFRS measure, Net income, is provided at the end of this note).
Philips Group
Composition of net debt and group equity
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Long-term debt | 6,473 | 7,270 | 7,035 |
| Short-term debt | 506 | 931 | 654 |
| Total debt | 6,980 | 8,201 | 7,689 |
| Cash and cash equivalents | 2,303 | 1,172 | 1,869 |
| Net debt | 4,676 | 7,028 | 5,820 |
| Shareholders' equity | 14,438 | 13,249 | 12,028 |
| Non-controlling interests | 36 | 34 | 33 |
| Group equity | 14,475 | 13,283 | 12,061 |
| Net debt and group equity ratio | 24:76 | 35:65 | 33:67 |
Adjusted income from continuing operations attributable to shareholders is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted income from continuing operations attributable to shareholders to the most directly comparable IFRS measure, Net income for 2023 is included in the following table.
Philips Group
Adjusted income from continuing operations attributable to shareholders
1) in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net income | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (2,711) | (13) | 10 |
| Income from continuing operations | 612 | (1,618) | (454) |
| Income from continuing operations attributable to non-controlling interests | (4) | (3) | (2) |
| Income from continuing operations attributable to shareholders1) | 608 | (1,622) | (456) |
| Adjustments for: | |||
| Amortization and impairment of acquired intangible assets | 322 | 363 | 290 |
| Impairment of goodwill | 15 | 1,357 | 8 |
| Restructuring costs and acquisition-related charges | 95 | 202 | 381 |
| Other items: | 1,069 | 925 | 1,358 |
| Respironics litigation provision | 575 | ||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 |
| Respironics field-action running remediation cost | 94 | 210 | 224 |
| Quality remediation actions | 94 | 59 | 175 |
| R&D project impairments | 134 | ||
| Portfolio realignment charges | 109 | ||
| Impairment of assets in S&RC | 39 | ||
| Provision for public investigations tender irregularities | 60 | ||
| Provision for a legal matter | 31 | ||
| Investment re-measurement loss | 23 | ||
| Loss (gain) on divestment of business | 76 | (35) | |
| Remaining items | 87 | 63 | 2 |
| Net finance income/expenses | (84) | (4) | 18 |
| Tax impact of adjusted items and tax only adjusting items | (527) | (376) | (450) |
| Adjusted Income from continuing operations attributable to shareholders1) | 1,497 | 845 | 1,148 |
Debt is initially measured at fair value net of directly attributable transaction costs. Subsequently, debt is measured at amortized cost using the effective interest rate method. Amortized cost is calculated by taking into account any discount or premium on acquisition and fees or costs that are an integral part of the effective interest rate. Debt is derecognized when the obligation under the liability is discharged, cancelled or has expired.
Lease liabilitiesLease liabilities are measured at the present value of the lease payments due over the lease term, generally discounted using the incremental borrowing rate. Lease liabilities are subsequently measured at amortized cost using the effective interest method. Lease liabilities are remeasured in case of modifications or reassessments of the lease.
Philips has a USD 2.5 billion Commercial Paper Program and a EUR 1 billion committed standby revolving credit facility that can be used for general group purposes, such as a backstop of its Commercial Paper Program. As of December 31, 2023, Philips did not have any loans outstanding under either facility. These facilities do not have a material adverse change clause, have no financial covenants and no credit-rating-related acceleration possibilities. Philips established a Euro Medium-Term Note (EMTN) program, a framework that facilitates the issuance of notes for a total amount up to EUR 10 billion. As of December 31, 2023, Philips has EUR 3.3 billion outstanding under this program of which EUR 500 million fixed rate notes were issued in August 2023 with a maturity date in 2031.
The provisions applicable to all USD-denominated corporate bonds issued by the company in March 2008 and March 2012 (due 2038 and 2042) contain a ‘Change of Control Triggering Event’. If the company would experience such an event with respect to a series of corporate bonds the company might be required to offer to purchase the bonds that are still outstanding at a purchase price equal to 101% of their principal amount, plus accrued and unpaid interest, if any. Furthermore, the conditions applicable to the EUR-denominated corporate bonds issued since 2018 contain a similar provision (‘Change of Control Put Event’). Upon the occurrence of such an event, the company might be required to redeem or purchase any of such bonds at their principal amount together with interest accrued. Philips’ outstanding long-term debt do not contain financial covenants.
In 2023, Philips issued EUR 500 million of fixed rate notes under the company’s EMTN program that mature in 2031 and used the proceeds for general corporate purposes, including the repayment of EUR 500 million that was outstanding under the credit facility entered into in the fourth quarter of 2022. In 2023, Philips entered into a total amount of EUR 138 million forward contracts relating to the company’s long-term incentive plans. These forwards mature in the fourth quarters of 2024 (EUR 61m) and 2025 (EUR 77m). In addition, a total of EUR 125 million forward contracts relating to the long-term incentive and employee stock purchase plans and EUR 481 million of forwards related to the share buyback program announced in 2021 matured throughout 2023.
In 2022, Philips announced a series of Liability Management transactions to optimize its debt maturity profile. The transactions included the issuance of three series of Notes under its EMTN program for a total of EUR 2 billion with maturities in 2027, 2029 and 2033. Part of the proceeds were used to tender certain of Philips’ outstanding US Dollar denominated bonds due 2025 and 2026 and Euro-denominated bonds due 2023, 2024 and 2025, as well as make-whole and fully redeem the Euro-denominated bonds due 2023 and 2024 that were not purchased as part of the Euro tender offer. Philips issued Commercial Paper of EUR 200 million in September 2022 and EUR 101 million in October 2022. These tranches were repaid throughout the fourth quarter of 2022. In addition, in October 2022 Philips entered into a EUR 1 billion credit facility that could be used for general corporate purposes. The credit facility matured in October 2023 and had a 12-month extension option at Philips discretion. Per year-end 2022, EUR 500 million was utilized and outstanding under the credit facility. In 2022, Philips entered into a total amount of EUR 63 million forward contracts relating to the company’s long-term incentive and employee stock purchase plans. A total of EUR 57 million forward contracts relating to the long-term incentive and employee stock purchase plans and EUR 83 million of forwards related to the share buyback program announced in 2021 matured throughout 2022.
Long-term debtThe following tables present information about the long-term debt outstanding, its maturity and average interest rates in 2023 and 2022.
Philips Group
Long-term debt
in millions of EUR unless otherwise stated
| 2023 | |||||||
|---|---|---|---|---|---|---|---|
| amount outstanding | Current portion | Non-current portion | Between 1 and 5 years |
amount due after 5 years | average remaining term (in years) | average rate of interest | |
| USD bonds | 1,325 | 1,325 | 240 | 1,085 | 13.3 | 6.3% | |
| EUR bonds | 4,569 | 4,569 | 2,335 | 2,234 | 5.1 | 2.0% | |
| Forward contracts | 396 | 321 | 76 | 76 | 0.8 | 1.4% | |
| Lease liabilities | 1,074 | 211 | 864 | 505 | 358 | 3.9 | 3.1% |
| Bank borrowings | 203 | 1 | 201 | 201 | 1.2 | 4.2% | |
| Other long-term debt | - | - | - | - | - | 7.4 | 1.2% |
| Long-term debt | 7,568 | 532 | 7,035 | 3,357 | 3,678 | 6.0 | 2.9% |
Philips Group
Long-term debt
in millions of EUR unless otherwise stated
| 2022 | |||||||
|---|---|---|---|---|---|---|---|
| amount outstanding | Current portion | Non-current portion | Between 1 and 5 years |
amount due after 5 years | average remaining term (in years) | average rate of interest | |
| USD bonds | 1,378 | 1,378 | 250 | 1,128 | 14.3 | 6.3% | |
| EUR bonds | 4,061 | 4,061 | 1,836 | 2,225 | 5.7 | 1.7% | |
| Forward contracts | 858 | 606 | 252 | 252 | 1.0 | ||
| Lease liabilities | 1,082 | 230 | 852 | 504 | 348 | 3.9 | 2.4% |
| Bank borrowings | 705 | 2 | 702 | 702 | 1.9 | 1.7% | |
| Other long-term debt | 28 | 4 | 24 | 17 | 6 | 8.9 | 2.9% |
| Long-term debt | 8,111 | 842 | 7,270 | 3,562 | 3,706 | 6.1 | 2.4% |
The following table presents the amount outstanding and effective rate of bonds.
Philips Group
Unsecured Bonds
in millions of EUR unless otherwise stated
| effective rate | 2022 | 2023 | |
|---|---|---|---|
| Unsecured EUR Bonds | |||
| Due 30/03/2025; 1 3/8% | 1.509% | 346 | 346 |
| Due 22/05/2026; 1/2% | 0.608% | 750 | 750 |
| Due 05/05/2027; 1 7/8% | 2.049% | 750 | 750 |
| Due 02/05/2028; 1 3/8% | 1.523% | 500 | 500 |
| Due 05/11/2029; 2 1/8% | 2.441% | 650 | 650 |
| Due 30/03/2030; 2% | 2.128% | 500 | 500 |
| Due 08/09/2031; 4 2/8% | 4.330% | 500 | |
| Due 05/05/2033; 2 5/8% | 2.710% | 600 | 600 |
| Unsecured USD Bonds | |||
| Due 15/05/2025; 7 3/4% | 7.429% | 51 | 49 |
| Due 15/05/2025; 7 1/8% | 6.794% | 78 | 75 |
| Due 01/06/2026; 7 1/5% | 6.885% | 119 | 114 |
| Due 03/11/2038; 6 7/8% | 7.210% | 683 | 657 |
| Due 15/03/2042; 5% | 5.273% | 470 | 452 |
| Adjustments1) | (57) | (47) | |
| Unsecured Bonds | 5,439 | 5,894 |
The following table presents a reconciliation between the total of future minimum lease payments and their present value.
Philips Group
Lease liabilities
in millions of EUR
| 2022 | 2023 | |||||
|---|---|---|---|---|---|---|
| future minimum lease payments | interest | present value of minimum lease payments | future minimum lease payments | interest | present value of minimum lease payments | |
| Less than one year | 251 | 21 | 230 | 239 | 28 | 211 |
| Between one and five years | 554 | 49 | 505 | 572 | 67 | 505 |
| More than five years | 376 | 28 | 348 | 388 | 30 | 358 |
| Lease liabilities | 1,180 | 98 | 1,082 | 1,200 | 125 | 1,074 |
Philips Group
Short-term debt
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Short-term bank borrowings | 89 | 122 |
| Current portion of long-term debt | 842 | 532 |
| Short-term debt | 931 | 654 |
During 2023, the weighted average interest rate on the bank borrowings was 8.6% (2022: 5.7%). This increase was mainly driven by higher interest rate environments across various countries globally.
A provision is a liability of uncertain timing or amount. Provisions are recognized if, as a result of a past event, the company has a present legal or constructive obligation, it is probable that an outflow of economic benefits will be required to settle the obligation and the amount can be estimated reliably. Provisions are measured at the present value of the expenditures expected to be required to settle the obligation using a pre-tax discount rate that reflects current market assessments of the time value of money. The increase in the provision due to passage of time (accretion) is recognized as interest expense.
Restructuring-related provisionsProvisions for severance and termination benefits are recognized for those costs only when the company has a detailed formal plan for the restructuring and has raised a valid expectation with those affected that it will carry out the restructuring by starting to implement that plan or announcing its main features to those affected by it. Before a provision is established, the company recognizes any impairment loss on the assets associated with the restructuring.
By their nature, the recognition of provisions requires estimates and assumptions regarding the timing and the amount of outflow of resources. The main estimates include:
Philips Group
Provisions
in millions of EUR
| Post-employment benefits |
Respironics field-action |
Product warranty |
Environmental | Restructuring- related |
Legal | Contingent consideration |
Other | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 366 | 287 | 20 | 134 | 74 | 23 | 112 | 1,018 | |
| Non-current | 546 | 23 | 57 | 83 | 6 | 14 | 89 | 279 | 1,097 |
| Balance as of December 31, 2022 | 546 | 390 | 344 | 104 | 140 | 89 | 113 | 390 | 2,115 |
| Additions | 112 | 240 | 313 | 18 | 263 | 644 | 24 | 223 | 1,836 |
| Utilizations | (91) | (285) | (268) | (14) | (219) | (235) | (20) | (134) | (1,266) |
| Releases | (10) | (20) | (2) | (67) | (10) | (7) | (45) | (159) | |
| Accretion | 5 | 23 | 1 | (3) | 25 | ||||
| Acquisitions | 6 | 6 | |||||||
| Changes in discount rate | (6) | (6) | |||||||
| Translation differences and other | - | (10) | (12) | (3) | (2) | (23) | (2) | (1) | (53) |
| Total change | 12 | (55) | 13 | (2) | (24) | 399 | 2 | 39 | 383 |
| Current | 331 | 293 | 22 | 102 | 477 | 57 | 181 | 1,463 | |
| Non-current | 558 | 3 | 64 | 80 | 14 | 10 | 58 | 248 | 1,035 |
| Balance as of December 31, 2023 | 558 | 334 | 357 | 102 | 116 | 487 | 115 | 429 | 2,498 |
Philips Group
Provisions
in millions of EUR
| Post-employment benefits |
Respironics field-action |
Product warranty |
Environmental | Restructuring -related |
Legal | Contingent consideration |
Other | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Current | 525 | 207 | 26 | 58 | 39 | 52 | 92 | 998 | |
| Non-current | 659 | 52 | 32 | 99 | 8 | 53 | 156 | 257 | 1,315 |
| Balance as of December 31, 2021 | 659 | 577 | 238 | 124 | 66 | 91 | 208 | 349 | 2,313 |
| Additions | 61 | 250 | 320 | 15 | 154 | 89 | 160 | 1,049 | |
| Utilizations | (185) | (486) | (224) | (17) | (61) | (100) | (105) | (95) | (1,274) |
| Releases | (1) | (2) | (18) | (3) | (35) | (59) | |||
| Accretion | 4 | - | (3) | 2 | |||||
| Acquisitions | 4 | 96 | 99 | ||||||
| Changes in discount rate | (27) | (27) | |||||||
| Fair value changes | (86) | (86) | |||||||
| Translation differences and other | 12 | 49 | 9 | 7 | (1) | 7 | 14 | 97 | |
| Total change | (113) | (187) | 105 | (21) | 74 | (3) | (95) | 41 | (198) |
| Current | 366 | 287 | 20 | 134 | 74 | 23 | 112 | 1,018 | |
| Non-current | 546 | 23 | 57 | 83 | 6 | 14 | 89 | 279 | 1,097 |
| Balance as of December 31, 2022 | 546 | 390 | 344 | 104 | 140 | 89 | 113 | 390 | 2,115 |
On June 14, 2021, Philips’ subsidiary, Philips Respironics initiated a voluntary recall notification in the United States and field safety notice outside the US for certain sleep and respiratory care products related to the polyester-based polyurethane (PE-PUR) sound abatement foam in these devices. The remediation is progressing globally. As of December 31, 2023, the production required for the delivery of replacement devices to patients has been substantially completed and the total number of units expected to be remediated remained stable during the year at 5.6 million devices (specific CPAP, BiPAP and mechanical ventilator devices), excluding certain end-of-life-devices which are expected to be retired.
Philips has recognized a provision based on Philips’ best estimate of the costs to repair, replace or refund devices, subject to the Respironics field action. The provision is related to the cost to repair, replace or refund affected devices and includes, amongst others, the costs for the remaining production, the cost of intensified communication with physicians and patients, material costs, labor cost and logistics, as well as costs relating to the (partial) refunds provided to customers under the field action. The provision does not include any product liability costs or other claims.
The additions for the year primarily reflect the impact of the revised remediation approach in relation to the mechanical ventilator devices subject to the recall, following the agreed terms of the proposed consent decree (see below). The revised approach, which includes a revised repair program and assumes (partial) refunds to customers (refer to Income from operations), resulted in an increase in the costs associated with the remediation of these devices. Utilizations for the year reflect the costs incurred in executing the remediation during the year.
The completion of the field action continues to be subject to uncertainty, which requires management to make estimates and assumptions about items such as quantities and the portion to be replaced, repaired and refunded. An increase in the assumption for the refund portion by 10 percentage points, could have the effect of increasing the provision by an estimated EUR 19 million. Actual outcomes in future periods may differ from these estimates and affect the company’s results of operations, financial position and cash flows.
Further to the above, running remediation costs of EUR 224 million (2022: EUR 210 million) related to the remediation, such as testing, external advisory and regulatory response and additional right-of-return and warranty provisions, have been incurred.
Following the US Food and Drug Administration (FDA) inspection of certain of Philips Respironics' facilities in the US in 2021 and the subsequent inspectional observations, the US Department of Justice, acting on behalf of the FDA, in July 2022 started discussions with Philips regarding the terms of a consent decree to resolve the identified issues, which Philips has now agreed. As a consequence of addressing the consent decree, the company recorded charges of EUR 363 million in December 2023, mainly consisting of EUR 240 million addition to the Respironics field action provision, EUR 82 million inventory write-down (refer to Inventories), EUR 31 million onerous contract provision and EUR 6 million fixed asset impairment.
In addition to the above, Philips and its affiliates are defendants in a number of consumer class action lawsuits from users of the affected devices and a number of individual personal injury and other compensation claims. For legal matters including claims refer to the legal provisions section of this note as well as Contingencies.
Product warranty provisionsThe field action provision in connection with the Philips Respironics voluntary recall notification is shown separately above.
Additions in 2023 include quality remediation actions of EUR 81 million in the Diagnosis & Treatment segment.
The company expects the provisions to be utilized mainly within the next year.
Environmental provisionsThe environmental provisions include accrued costs recorded with respect to environmental remediation in various countries. In the US, subsidiaries of the company have been named as potentially responsible parties in state and federal proceedings for the clean-up of certain sites.
The additions and the releases of the provisions originate from additional insights in relation to factors like the estimated cost of remediation, changes in regulatory requirements and efficiencies in completion of various site work phases.
Approximately EUR 63 million of the long-term provision is expected to be utilized after one to five years, with the remainder after five years. For more details on the environmental remediation refer to Contingencies.
Restructuring-related provisionsPhilips Group
Restructuring-related provisions
in millions of EUR
| December 31, 2022 | December 31, 2023 | |
|---|---|---|
| Diagnosis & Treatment | 42 | 36 |
| Connected Care | 42 | 18 |
| Personal Health | 10 | 7 |
| Other | 47 | 56 |
| Philips Group | 140 | 116 |
Further to the workforce reduction in 2022, measures were announced on January 30, 2023 that primarily focus on the reduction of 6,000 positions by 2025. In 2023, the restructuring costs, net of releases, for these measures were EUR 140 million.
In addition, restructuring projects were executed during the year, of which the most significant impacted Connected Care and Other and mainly took place in the US and Netherlands. The restructuring mainly comprised product portfolio rationalization and the reorganization of global support functions. The company expects the provisions to be utilized mainly within the next year.
In 2022, Philips initiated general productivity actions aimed at simplifying the organization to streamline the way of working and reduce operating expenses. This includes an immediate reduction of around 4,000 positions globally across the organization, with severance and termination-related costs of EUR 80 million recorded in 2022.
Legal provisionsThe company and certain of its group companies and former group companies are involved as a party in legal proceedings, including regulatory and other governmental proceedings.
Additions mainly relate to a EUR 575 million in connection with the anticipated resolution of the economic loss class action in the US. The final cost of the settlement may vary based on, among other things, how many patients and other settlement class members participate in the settlement.
Utilizations mainly relate to the settlement the company reached with the US Securities and Exchange Commission (SEC) to resolve the SEC inquiry regarding alleged tender irregularities in the medical device industry in China, for which the company had recorded a provision of approximately EUR 60 million in 2022. The settlement reached was in line with the amount provided for and EUR 58 million was subsequently paid in 2023. In addition the company funded an amount of USD 155 million (EUR 141 million) into the Qualified Settlement Fund in relation to the economic loss class action settlement announced on September 7, 2023.
For details of other legal matters, including regulatory and other governmental proceedings, refer to Contingencies.
The company expects the provisions to be utilized mainly within the next three years.
Contingent consideration provisionsIn 2023, the addition of EUR 24 million is largely offset by utilizations of EUR 20 million. The acquisition of a business within Ultrasound resulted in a EUR 6 million increase.
Approximately EUR 28 million of the long-term provision is expected to be utilized within the next three years, with the remainder after four years.
Other provisionsThe main elements of other provisions are:
Philips Group
Other provisions
in millions of EUR unless otherwise stated
| 2022 | 2023 | |
|---|---|---|
| Employee jubilee funds | 83 |
77 |
| Self-insurance | 57 |
63 |
| Non-income taxes / social security | 46 |
51 |
| Rights of return | 36 |
39 |
| Decommissioning costs | 33 |
34 |
| Onerous contracts | 38 |
76 |
| Remaining | 97 | 89 |
| Balance as of December 31 | 390 | 429 |
Onerous contracts reflect non-cancellable commitments on supplies for which no future demand or alternative usage has been identified, including EUR 31 million in connection with the proposed Respironics consent decree as of December 31, 2023.
Remaining provisions relate to a variety of positions, for example provision for disability of employees and provision for royalty obligations.
Releases in 2022 and 2023 are due to the reassessment of the positions in other provisions throughout the year.
The company expects the other provisions to be utilized mainly within the next five years.
A defined contribution plan is a post-employment benefit plan for which the company pays fixed contributions into a separate entity and will have no legal or constructive obligation to pay further amounts. Obligations for contributions to defined contribution pension plans are recognized as an employee benefit expense in the Consolidated statements of income in the periods during which services are rendered by employees.
Defined benefit plansA defined benefit plan is a post-employment benefit plan that is not a defined contribution plan. Defined benefit plans define an amount of pension benefit that an employee will receive after retirement. That pension benefit typically depends on several factors such as years of service, age and salary.
The net pension asset or liability recognized in the Consolidated balance sheets in respect of defined benefit plans is the fair value of plan assets less the present value of the projected defined benefit obligation at the balance sheet date. The defined benefit obligation is calculated annually by qualified actuaries using the projected unit credit method. Recognized assets are limited to the present value of any reductions in future contributions or any future refunds. The net pension liability is presented as a long-term provision; no distinction is made for the short-term portion.
For the company’s major plans, a full discount rate curve of high-quality corporate bonds is used to determine the defined benefit obligation, where available. The curves are based on the Mercer Yield Curve methodology, which uses data of corporate bonds rated AA or equivalent. For the other plans the Mercer Yield Curve/Mercer Methodology has also been used taking into account the cash flows as much as possible in case there is a deep market in corporate bonds. For plans in countries without a deep corporate bond market, the discount rate is based on government bonds and the plan’s maturity.
Pension costs in respect of defined benefit plans primarily represent the increase of the actuarial present value of the obligation for post-employment benefits based on employee service during the year and the interest on the net recognized asset or liability in respect of employee service in previous years.
Remeasurements of the net defined benefit asset or liability comprise actuarial gains and losses, the return on plan assets (excluding interest) and the effect of the asset ceiling (excluding interest). The company recognizes all remeasurements in Other comprehensive income.
Past service costs arising from the introduction of a change to the benefit payable under a plan or a significant reduction of the number of employees covered by a plan (curtailment) are recognized in full in the Consolidated statements of income.
Short-term employee benefit obligations are measured on an undiscounted basis and are expensed as the related service is provided. The company recognizes a liability and an expense for bonuses and incentives based on a formula that takes into consideration the profit attributable to the company’s shareholders after certain adjustments.
The company’s net obligation in respect of other long-term employee benefits is the amount of future benefit that employees have earned in return for their service in the current and prior periods, such as jubilee entitlements. That benefit is discounted to determine its present value. Remeasurements are recognized in the Consolidated statements of income in the period in which they arise.
Further information on other employee benefits can be found in Provisions in the Other provisions section.
To make the actuarial calculations for the valuation of defined benefit obligations, assumptions are needed for interest rates, healthcare cost increases, future pension increases, life expectancy and employee turnover rates. The actuarial calculations are made by external actuaries based on inputs from observable market data, such as corporate bond returns and yield curves to determine the discount rates to apply, mortality tables to determine life expectancy and inflation rates to determine future salary and pension growth assumptions.
Employee post-employment benefit plans have been established in many countries in accordance with the legal requirements, customs and the local practice in the countries involved. The larger part of post-employment benefits are company pension plans, of which some are funded and some are unfunded. All funded post-employment benefit plans are considered to be related parties.
Most employees that take part in a company pension plan are covered by defined contribution (DC) pension plans. The main DC plans are in the Netherlands and the United States. The company also sponsors a number of defined benefit (DB) pension plans. The benefits provided by these plans are based on employees’ years of service and compensation levels.
The company also sponsors a limited number of DB retiree medical plans. The benefits provided by these plans typically cover a part of the healthcare costs after retirement. None of these plans are individually significant to the company and are therefore not further separately disclosed.
The larger funded DB and DC plans are governed by independent Trustees who have a legal obligation to protect the interests of all plan members and operate under the local regulatory framework.
The DB plans in Germany and the US make up most of the defined benefit obligation (DBO) and the net position. The company also has DB plans in the rest of the world; however these are individually not significant to the company and do not have a significantly different risk profile that would warrant separate disclosure.
The adjacent table provides a break-down of the present value of the funded and unfunded DBO, the fair value of plan assets and the net position in Germany, the US and in Other Countries. The table also provides the value of reimbursement rights.
Philips Group
Post-employment benefits
in millions of EUR
| Germany | United States | Other Countries | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Present value of funded DBO | (489) | (511) | (440) | (404) | (179) | (182) | (1,108) | (1,097) |
| Present value of unfunded DBO | (249) | (253) | (128) | (118) | (136) | (137) | (513) | (508) |
| Total present value of DBO | (738) | (764) | (568) | (522) | (315) | (319) | (1,621) | (1,605) |
| Fair value of plan assets | 477 | 481 | 474 | 442 | 171 | 166 | 1,122 | 1,089 |
| Net position | (261) | (283) | (94) | (80) | (144) | (153) | (499) | (516) |
| Value of reimbursement rights | 6 | 8 | 6 | 8 | ||||
The classification of the net position is as follows:
Philips Group
Classification net position
in millions of EUR
| Germany | United States | Other Countries | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Total asset for plans in a surplus | 9 | - | 34 | 39 | 4 | 2 | 46 | 41 |
| Total liability for plans in a deficit | (270) | (283) | (128) | (118) | (148) | (156) | (546) | (558) |
| Provisions for post-employment benefit plans under AHFS | ||||||||
| Net position | (261) | (283) | (94) | (80) | (144) | (153) | (499) | (516) |
The company has several DB plans in Germany, which are partially unfunded, meaning that after retirement the company is responsible for the benefit payments to retirees.
Due to the relatively high level of social security in Germany, the company’s pension plans mainly provide benefits for the higher earners. The plans are open for future pension accrual. Indexation is mandatory due to legal requirements. Some of the German plans have a DC design, but are accounted for as DB plans due to a legal minimum return requirement.
Company pension commitments in Germany are partly protected against employer bankruptcy via the “Pensions-Sicherungs-Verein” which charges a fee to all German companies providing pension promises.
Philips is one of the sponsors of Philips Pensionskasse VVaG in Germany, which is a multi-employer plan. The plan is classified and accounted for as a DC plan.
United StatesThe US DB pension plans are closed plans without future pension accrual. For the funding of any deficit in the US plan the Group adheres to the minimum funding requirements of the US Pension Protection Act.
The assets of the US funded pension plans are in Trusts governed by fiduciaries. The non-qualified pension plans that cover accrual above the maximum salary of the funded qualified plan are unfunded.
The company’s qualified pension commitments in the US are covered via the Pension Benefit Guaranty Corporation which charges a fee to US companies providing DB pension plans. The fee is also dependent on the amount of unfunded vested liabilities.
Risks related to DB plansDB plans expose the company to various demographic and economic risks such as longevity risk, investment risks, currency and interest rate risk and in some cases inflation risk. The latter plays a role in the assumed wage increase but more importantly in some countries where indexation of pensions is mandatory.
The company has an active de-risking strategy in which it constantly looks for opportunities to reduce the risks associated with its DB plans. Liability-driven investment strategies, lump sum cash-out options, buy-ins, buy-outs and a change to DC are examples of the strategy.
Investment policy in the largest pension plansPension fund trustees are responsible for and have full discretion over the investment strategy of the plan assets. The plan assets of the Philips pension plans are invested in well diversified portfolios. The interest rate sensitivity of the fixed income portfolio is closely aligned to that of the plan’s pension liabilities for most of the plans. Any contributions from the sponsoring company are used to further increase the fixed income part of the assets. As part of the investment strategy, any improvement in the funded ratio over time is used to further decrease the interest rate mismatch between the plan assets and the pension liabilities.
Summary of pre-tax costs for post-employment benefits and reconciliationsThe adjacent table contains the total of current and past service costs, administration costs and settlement results as included in Income from operations and the interest cost as included in Financial expenses.
Philips Group
Pre-tax costs for post-employment benefits
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Defined benefit plans | 36 | 50 | 47 |
| - included in income from operations | 28 | 39 | 25 |
| - included in financial expense | 8 | 10 | 21 |
| - included in Discontinued operations | 1 | ||
| Defined contribution plans | 375 | 400 | 376 |
| - included in income from operations | 368 | 400 | 376 |
| - included in Discontinued operations | 7 | ||
| Post-employment benefits costs | 411 | 449 | 423 |
The adjacent tables contain the reconciliations for the DBO and plan assets.
Philips Group
Defined benefit obligations
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Balance as of January 1 | 1,970 | 1,621 |
| Service cost | 32 | 32 |
| Interest cost | 36 | 71 |
| Employee contributions | 4 | 3 |
| Actuarial (gains) / losses | ||
| - demographic assumptions | 2 | |
| - financial assumptions | (366) | 48 |
| - experience adjustment | 12 | 2 |
| (Negative) past service cost | 16 | (9) |
| Settlements | - | 2 |
| Benefits paid from plan | (95) | (104) |
| Benefits paid directly by employer | (41) | (39) |
| Translation differences and other | 52 | (22) |
| Balance as of December 31 | 1,621 | 1,605 |
Philips Group
Plan assets
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Balance as of January 1 | 1,380 | 1,122 |
| Interest income on plan assets | 26 | 49 |
| Admin expenses paid | (1) | (1) |
| Return on plan assets excluding interest income | (254) | 23 |
| Employee contributions | 4 | 3 |
| Employer contributions | 17 | 14 |
| Settlements | ||
| Benefits paid from plan | (95) | (104) |
| Translation differences and other | 45 | (17) |
| Balance as of December 31 | 1,122 | 1,089 |
The past service cost in 2023 and 2022 mainly relate to the retiree medical plans in Brazil.
Plan assets allocationThe asset allocation in the company’s DB plans as of December 31, was as follows:
Philips Group
Plan assets allocation
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Assets quoted in active markets | ||
| - Debt securities | 560 | 513 |
| - Equity securities | ||
| - Other | 203 | 182 |
| Assets not quoted in active markets | ||
| - Debt securities | ||
| - Equity securities | 101 | 31 |
| - Other | 258 | 363 |
| Total assets | 1,122 | 1,089 |
The plan assets in 2023 contain 36% (2022: 32%) unquoted plan assets. Plan assets in 2023 do not include property occupied by or financial instruments issued by the company.
AssumptionsThe mortality tables used for the company’s largest DB plans are:
Germany: Heubeck-Richttafeln 2018 Generational, assuming 93% of mortality rates for male retirees between age 60 and 85
US: PRI-2012 Generational with MP2021 improvement scale + white collar adjustment
The weighted averages of the assumptions used to calculate the DBO as of December 31, were as follows:
Philips Group
Assumptions used for defined benefit obligations
in %
| Germany | United States | Other Countries | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | |
| Discount rate | 4.1% | 3.7% | 5.2% | 5.0% | 4.9% | 4.9% | 4.7% | 4.3% |
| Inflation rate | 2.0% | 2.0% | 2.3% | 2.3% | 2.6% | 2.5% | 2.2% | 2.2% |
| Salary increase | 2.8% | 2.8% | 0.0% | 0.0% | 3.3% | 4.3% | 2.9% | 3.0% |
The following table illustrates the approximate impact on the DBO from movements in key assumptions. The DBO was recalculated using a change in the assumptions of 1% which overall is considered a reasonably possible change. The impact on the DBO because of changes in discount rate is normally accompanied by offsetting movements in plan assets, especially when using matching strategies.
The average duration in years of the DBO of the DB plans is 10 (Germany: 11, United States: 8, and Other countries: 10) as of December 31, 2023 (2022: 8).
Philips Group
Sensitivity of key assumptions
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Increase | ||
| Discount rate (1% movement) | (122) | (123) |
| Pension increase (1% movement) | 57 | 60 |
| Salary increase (1% movement) | 12 | 12 |
| Longevity1) | 32 | 32 |
| Decrease | ||
| Discount rate (1% movement) | 145 | 147 |
| Pension increase (1% movement) | (49) | (52) |
| Salary increase (1% movement) | (11) | (11) |
Cash outflows in relation to post-employment benefits are estimated to amount to EUR 434 million in 2024, consisting of:
The service and administration cost for 2024 is expected to amount to EUR 30 million for DB plans. The net interest cost for 2024 for the DB plans is expected to amount to EUR 21 million. The cost for DC pension plans in 2024 is equal to the expected DC cash flow.
Accrued liabilities are initially measured at fair value and subsequently at amortized cost and are derecognized when the obligation under the liability is discharged, cancelled or has expired.
Accrued liabilities are summarized as follows:
Philips Group
Accrued liabilities
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Personnel-related costs: | ||
| - Salaries and wages | 490 | 791 |
| - Accrued holiday entitlements | 97 | 96 |
| - Other personnel-related costs | 101 | 93 |
| Fixed-asset-related costs: | ||
| - Gas, water, electricity, rent and other | 46 | 43 |
| Communication and IT costs | 64 | 61 |
| Distribution costs | 110 | 99 |
| Sales-related costs: | ||
| - Commission payable | 8 | 12 |
| - Advertising and marketing-related costs | 127 | 133 |
| - Other sales-related costs | 20 | 20 |
| Material-related costs | 132 | 138 |
| Interest-related accruals | 71 | 76 |
| Other accrued liabilities | 361 | 324 |
| Accrued liabilities | 1,626 | 1,887 |
Other liabilities are initially measured at fair value and subsequently at amortized cost and are derecognized when the obligation under the liability is discharged, cancelled or has expired.
The company recognizes contract liabilities if a payment is received or a payment is due (whichever is earlier) from a customer before the company transfers the related goods or services. Contract liabilities are recognized as revenue when the company performs under the contract (i.e., transfers control of the related goods or services to the customer).
Non-current liabilities were EUR 54 million as of December 31, 2023 (December 31, 2022: EUR 60 million).
Non-current liabilities are associated mainly with indemnification and non-current accruals.
Other current liabilitiesOther current liabilities are summarized as follows:
Philips Group
Other current liabilities
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Accrued customer rebates | 213 | 186 |
| Other taxes including social security premiums | 115 | 129 |
| Other liabilities | 120 | 98 |
| Other current liabilities | 448 | 414 |
Non-current contract liabilities were EUR 469 million as of December 31, 2023 (December 31, 2022: EUR 515 million) and current contract liabilities were EUR 1,809 million as of December 31, 2023 (December 31, 2022: EUR 1,696 million).
The current contract liabilities increased by EUR 113 million, which is mainly driven by an increase in deferred balances for customer service contracts.
The current contract liabilities as of December 31, 2022 resulted in revenue recognized of EUR 1,696 million in 2023.
A contingent liability is a liability of uncertain timing and amount. Contingencies are not recognized in the balance sheet because they are dependent on the occurrence or non-occurrence of one or more uncertain future events not wholly within the control of the company or because the risk of loss is estimated to be possible but not probable or because the amount cannot be measured reliably. Pursuant to IAS 37, Provisions, Contingent Liabilities and Contingent Assets, certain information is not disclosed for legal proceedings for which the company concludes that disclosure can be expected to seriously prejudice the outcome of the matter.
Contingent assetsContingent assets are disclosed if the inflow of economic benefits is probable, but not virtually certain. If the inflow of economic benefits becomes virtually certain, the asset would be considered no longer contingent and its recognition appropriate. Contingent assets are assessed continually and require management to apply judgment, especially to estimate the likelihood of the inflow of economic benefits.
Financial guaranteesPhilips’ policy is to provide guarantees and other letters of support only in writing. Philips does not stand by other forms of support. The company recognizes a liability at the fair value of the obligation at the inception of a financial guarantee contract. The guarantee is subsequently measured at the higher of the best estimate of the obligation or the amount initially recognized less, when appropriate, cumulative amortization.
Significant judgment is required to determine the likelihood of a potential outflow of resources. In addition, judgment is involved in determining whether the amount of an obligation can be measured with sufficient reliability. Contingencies involve inherent uncertainties including, but not limited to, court rulings, negotiations between affected parties, governmental actions, tax and environmental remediation.
The total fair value of guarantees recognized on the balance sheet amounts to EUR nil million for both 2023 and 2022. Remaining off-balance-sheet business related guarantees on behalf of third parties and associates to EUR 2 million in 2023 (December 31, 2022: EUR 2 million).
Environmental remediationThe company and its subsidiaries are subject to environmental laws and regulations. Under these laws, the company and/or its subsidiaries may be required to remediate the effects of certain manufacturing activities on the environment.
Legal proceedingsThe company and certain of its group companies and former group companies are involved as a party in legal proceedings, regulatory and other governmental proceedings, including discussions on potential remedial actions, relating to such matters as competition issues, commercial transactions, product liability, participations, and environmental pollution.
While it is not feasible to predict or determine the outcome of all pending or threatened legal proceedings, regulatory and governmental proceedings, the company is of the opinion that the cases described below may have, or have had in the recent past, a significant impact on the company’s consolidated financial position, results of operations and cash flows.
Public InvestigationsIn May 2023, the company reached a settlement with the US Securities and Exchange Commission (SEC) to resolve the SEC inquiry regarding alleged tender irregularities in the medical device industry in China, for which the company had recorded a provision of approximately EUR 60 million in 2022. The settlement reached was in line with the amount provided for. In addition, the previously disclosed SEC inquiry regarding alleged similar conduct in Brazil and Bulgaria has been discontinued. The US Department of Justice (DOJ) has closed its parallel inquiry into these matters.
In February 2023, the company received a statement of objections from the French Competition Authority (FCA) initiating a formal investigation to verify whether the company and certain other manufacturers of small domestic appliances breached antitrust rules in France in the period 2009-2014 through the alleged exchange of commercially sensitive information. The company filed its response to the statement of objections denying such allegations in May 2023 and is continuing to defend itself. The FCA is expected to organize a hearing and issue its decision in 2024. It is the company’s assessment that it is possible but not probable that this matter could lead to an outflow of economic resources. Given the uncertain outcome of the investigation and subsequent proceedings, the company is not able to reliably estimate the financial impact, if any, and no provision has been recognized as of December 31, 2023.
Respironics field actionOn June 14, 2021, Philips’ subsidiary Philips RS North America LLC (Philips Respironics) issued a voluntary recall notification in the United States and field safety notice outside the United States for specific Philips Respironics CPAP, Bi-Level PAP, and mechanical ventilator devices (the “Recalled Devices”).
Consent decreeOn August 26, 2021, the US Food and Drug Administration (FDA) commenced an inspection of the Philips Respironics manufacturing facility in Murrysville, Pennsylvania and provided Philips Respironics with its preliminary inspectional observations on November 9, 2021. Philips Respironics responded to the FDA's inspectional observations in December 2021, which described the actions already taken by the company, as well as additional planned actions. Philips Respironics is also providing periodic updates to the FDA on its progress for the planned actions. In July 2022, Philips started discussions with the DOJ, acting on behalf of the FDA on a consent decree that would, among other things, address compliance requirements for future sales, the resolution of the inspectional findings and the completion of the recall. On January 29, 2024, Philips announced that it agrees on the terms of a consent decree with the DOJ, representing the FDA. For further details please see Subsequent events.
DOJ investigationOn April 8, 2022, Philips Respironics and certain of Philips' subsidiaries in the US received a subpoena from the DOJ to provide information related to events leading to the Respironics recall. The relevant subsidiaries are cooperating with the investigation. The criminal and civil investigation is being conducted by the DOJ's Consumer Protection Branch and Civil Fraud Section, and the US Attorney’s Office for the Eastern District of Pennsylvania. Given the early stages of the investigation, the company is not able to reliably estimate the financial impact, if any.
Product liability claimsFollowing the voluntary recall notification, a number of civil complaints have been filed in several jurisdictions against Philips Respironics and certain of its affiliates (including the company) generally alleging economic loss, personal injury and/or the potential for personal injury allegedly caused by the Recalled Devices.
In the United States, consumer and commercial class action lawsuits have been filed alleging economic loss and medical monitoring claims. Individual personal injury lawsuits have also been filed. On October 8, 2021, a Multi-District Litigation (MDL) in the US District Court for the Western District of Pennsylvania was formed, and most of these class action and personal injury lawsuits have been consolidated in the MDL for pre-trial proceedings. As of December 31, 2023, plaintiffs have filed a consolidated economic loss class action complaint on behalf of device users, hospitals, and insurers and other third-party payers, a consolidated medical monitoring class action complaint on behalf of device users, and over 600 individual personal injury complaints. The company anticipates that the number of individual personal injury complaints will continue to increase in 2024.
On September 7, 2023, Philips Respironics reached agreement on a class action settlement in relation to the economic loss class action complaint, for which the company recorded a EUR 575 million provision in the first quarter of 2023. Under the agreement, which was preliminarily approved by the US District Court for the Western District of Pennsylvania on October 10, 2023, the Philips defendants will provide predefined cash awards to all eligible participants in the US depending on the type of device, extended warranties on all remediated devices provided as part of Respironics’ recall program, and an additional cash award if they return the Recalled Device to Philips Respironics. The settlement also provides for compensation for individuals who acquired replacement devices in the market after the recall and prior to the announcement of the settlement. The settlement also provides for compensation to private insurers and other third-party payers. The final cost of the settlement may vary based on, among other things, how many patients and other settlement class members participate in the settlement. The final approval hearing is scheduled for April 11, 2024.
In September 2022, the MDL court established a voluntary, court-approved census registry, and associated tolling, for potential claimants who have not filed claims, but may file claims in the future, relating to the Recalled Devices. The census registry replaced the private tolling agreement that had been in effect before the establishment of the census registry. At the time of termination, approximately 60,000 individuals had entered into the private tolling agreement. In the event these individuals wish to pursue or preserve their claims, they will need to file a lawsuit or register on the census registry. By December 31, 2023, approximately 57,000 individuals had joined the census registry. The company anticipates that the number of individuals on the census registry will increase in 2024. To better assess the claimed injuries and their relation, if any, to use of the Recalled Devices, Philips Respironics is working to require census registrants to supplement the information they are required to submit in the census registry established by the MDL court.
In Australia, a consumer class action lawsuit alleging personal injury was filed against the company’s subsidiary Philips Electronics Australia Ltd on October 4, 2021. In the course of 2022, the plaintiff in the case sought leave of the court to discontinue the class action citing that there is insufficient evidence to warrant the continuation of the class action and that since the issue of proceedings, Philips Respironics has been repairing, replacing, or (partially) refunding the devices which are the subject of the recall, meaning that any compensation relating to financial loss would be relatively confined. During the process for withdrawal of the case, a new lead plaintiff came forward in the second half of 2023 and is now continuing the class action.
Philips Respironics and certain of its affiliates (including the company) are also defendants in consumer class action lawsuits in Canada and Israel and collective actions in Chile, France and the Netherlands alleging economic loss and/or personal injury. In Canada, where various class actions had been filed, the court issued a decision on a carriage motion in April 2023, deciding that a class action filed in British Columbia may continue as a nationwide class action while defendants are seeking for all other class actions to be stayed.
While the company believes it is probable that these lawsuits will in the aggregate lead to an outflow of economic resources for Philips Respironics or other Philips entities, given the significant uncertainty regarding the nature of the relevant events and potential obligations, the company is not currently able to reliably estimate the amount of the obligation associated with these various lawsuits. The final outcome of the lawsuits and the cost to resolve them cannot currently be determined due to a number of variables, including uncertainty regarding the ultimate number of claimants and their allegations. Moreover, Philips Respironics has not yet completed its test and research program, including the additional testing requested by the FDA, for the Recalled Devices.
For the United States specifically, the lack of clarity around the nature of the specific injury each census registrant is claiming and its relation, if any, to use of the Recalled Devices contribute to the uncertainty. In addition, the MDL court has not yet decided several significant motions, and plaintiffs have not yet filed their motion for class certification in the medical monitoring action. Further, while document discovery has progressed, expert discovery has not yet begun, and the Court has not yet been asked to decide the question of whether any of the claimed injuries could have been caused by use of the Recalled Devices. An adverse outcome with respect to any or all of these lawsuits and/or any future claims could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
The company has product liability insurance in place that it expects to partially cover product liability-related cash outflows. Based on ongoing discussions with certain insurance carriers that took place during 2023, management of the company concluded that the likelihood of cash inflows changed to probable, but (consistent with prior periods) not virtually certain. Given the uncertainties associated with the cash outflows of the above claims and the applicable conditions of insurance coverage, no reliable estimate can be made or disclosed in relation to the expected insurance recovery.
Securities claimsOn August 16, 2021, a securities class action complaint was filed against the company, its former CEO and its CFO in the US District Court for the Eastern District of New York alleging violations of the Securities Exchange Act of 1934 causing damage to investors. On January 3, 2022, the lead plaintiff in the case filed its amended complaint seeking to represent individuals that purchased Philips shares between February 23, 2016, through November 12, 2021. Following the filing and briefing of the company’s motion to dismiss in the first half of 2022, plaintiff filed a second amended complaint on November 30, 2022, naming an additional defendant and expanding the alleged damage period to include certain share price declines that were allegedly based on disclosures made in 2022. The second amended complaint now focuses on share price declines that allegedly occurred as a result of various disclosures starting on April 26, 2021 through October 2022. The company's motion to dismiss the second amended complaint was filed in the first quarter of 2023. As of December 31, 2023, that motion is still pending with the Court.
In the Netherlands, in addition to the September 2022 letter from shareholders representative organization European Investors-VEB, holding the company and its directors liable for an alleged failure to make timely disclosures in relation to the Respironics recall, the company received letters from two other parties with similar allegations. As of December 31, 2023, no formal claims have been filed in this respect.
It is the company’s assessment that it is possible but not probable that these cases could lead to a certain outflow of economic resources. The company is not able to reliably estimate the financial impact, if any. An adverse outcome of these cases could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
Other claimsOn October 12, 2021, SoClean, a company offering ozone-based cleaning products for sleep devices, filed a lawsuit against the company and certain of its affiliates alleging that the defendants’ statements about the potential adverse effect ozone cleaning may have on the Recalled Devices has significantly damaged its business. Philips believes that the claim is without merit and will vigorously defend itself. In November 2023, the Court ruled on one of the motions to dismiss filed by defendants and partially dismissed some of SoClean’s claims. On January 4, 2024, Philips and its affiliates filed their answer and counterclaims against SoClean and one of its affiliates.
In addition, some of Philips Respironics’ business partners such as distributors and durable medical equipment providers have filed or threatened to file claims alleging economic losses suffered as a consequence of the voluntary recall. Philips Respironics is engaging with certain of its business partners on the level of compensation they allege to be entitled to under Philips Respironics’ replacement program of the Recalled Devices.
It is the company’s assessment that it is possible but not probable that these cases could lead to a certain outflow of economic resources. The company is not able to reliably estimate the financial impact, if any. In the event of an adverse outcome, these matters could have a material impact on the company’s consolidated financial position, results of operations and cash flows.
To date, other than for the economic loss class action settlement, no provisions have been recorded for the litigation and investigations associated with the Respironics field action.
OtherIn the second half of 2023, Electro Medical Systems S.A., a manufacturer of among others medical devices for dental prophylaxis, filed a lawsuit against the company alleging that the company materially breached its duties under a cooperation agreement entered into between the parties in 2016, claiming damages in excess of EUR 300 million, alleging loss of profit and lost increase in brand value. Philips disagrees with the allegations and will vigorously defend itself.
MiscellaneousFor details on other contractual obligations, please refer to liquidity risk in Details of treasury and other financial risks.
In 2023, the total remuneration costs relating to the members of the Executive Committee (consisting of 16 members throughout the year, including the members of the Board of Management) amounted to EUR 32.8 million (2022: EUR 25.6 million; 2021: EUR 33.4 million) consisting of the elements in the following table.
Philips Group
Remuneration costs of the Executive Committee1)
in EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Base salary/Base compensation | 9,598,588 | 9,528,279 | 8,729,458 |
| Annual incentive2) | 5,250,408 | 208,370 | 11,405,130 |
| Performance shares3) | 12,610,073 | 11,242,581 | 7,272,815 |
| Stock options | 13,358 | ||
| Restricted share rights3) | 1,380,644 | 1,191,529 | 1,907,511 |
| Pension allowances4) | 2,107,953 | 1,949,204 | 1,346,937 |
| Pension scheme costs | 306,694 | 288,179 | 260,554 |
| Other compensation5) | 2,104,044 | 1,216,163 | 1,900,224 |
| Total | 33,358,405 | 25,624,305 | 32,835,987 |
As of December 31, 2023, the members of the Executive Committee (including the members of the Board of Management) held 0 stock options (2022: 0; 2021: 184,900).
Remuneration of the Board of ManagementIn 2023, the total remuneration costs relating to the members of the Board of Management amounted to EUR 9.9 million (2022: EUR 8.4 million; 2021: EUR 10.3 million), see the following table.
Philips Group
Remuneration costs of individual members of the Board of Management
in EUR
| base compensation/salary | annual incentive1) | performance shares2) | restricted share rights2) | pension allowances3) | pension scheme costs | other compensation | total costs | |
|---|---|---|---|---|---|---|---|---|
| 2023 | ||||||||
| R. Jakobs | 1,200,000 | 2,004,480 | 968,922 | 267,798 | 31,891 | 109,256 | 4,582,347 | |
| A. Bhattacharya | 810,000 | 1,075,939 | 793,429 | 197,133 | 31,891 | 94,516 | 3,002,907 | |
| M.J. van Ginneken | 630,000 | 846,922 | 614,840 | 125,298 | 31,891 | 53,446 | 2,302,397 | |
| 2,640,000 | 3,927,341 | 2,377,191 | 590,228 | 95,673 | 257,218 | 9,887,650 | ||
| 2022 | ||||||||
| R. Jakobs4) | 256,438 | 112,737 | 57,973 | 6,012 | 11,507 | 444,667 | ||
| F.A. van Houten4) | 1,041,849 | 208,370 | 2,930,068 | 444,051 | 22,121 | 42,533 | 4,688,992 | |
| A. Bhattacharya | 806,250 | 763,140 | 237,250 | 28,133 | 61,308 | 1,896,081 | ||
| M.J. van Ginneken | 626,250 | 585,490 | 141,622 | 28,133 | 35,343 | 1,416,837 | ||
| 2,730,788 | 208,370 | 4,391,434 | 880,896 | 84,398 | 150,691 | 8,446,577 | ||
| 2021 | ||||||||
| F.A. van Houten | 1,325,000 | 850,915 | 2,626,295 | 565,403 | 27,462 | 57,224 | 5,452,299 | |
| A. Bhattacharya | 790,000 | 360,103 | 1,172,533 | 233,857 | 27,462 | 68,908 | 2,652,864 | |
| M.J. van Ginneken | 605,000 | 317,192 | 886,035 | 150,755 | 27,462 | 42,610 | 2,029,054 | |
| 2,720,000 | 1,528,211 | 4,684,863 | 950,014 | 82,387 | 168,742 | 10,134,217 |
The accumulated annual pension entitlements and the pension costs of individual members of the Board of Management are as follows:
Philips Group
Accumulated annual pension entitlements and pension-related costs
in EUR unless otherwise stated
| age at December 31, 2023 | accumulated annual pension as of December 31, 2023 | total pension related costs | |
|---|---|---|---|
| R. Jakobs | 49 | 56,383 | 299,689 |
| A. Bhattacharya | 62 | 40,324 | 229,024 |
| M.J. van Ginneken | 50 | 53,769 | 157,189 |
| Pension costs | 685,901 |
When pension rights are granted to members of the Board of Management, necessary payments (if insured) and all necessary provisions are made in accordance with the applicable accounting principles. In 2023, no (additional) pension benefits were granted to former members of the Board of Management.
Remuneration of the Supervisory BoardThe remuneration of the members of the Supervisory Board amounted to EUR 1.5 million (2022: EUR 1.5 million; 2021: EUR 1.3 million). Former members received no remuneration.
The members of the Supervisory Board do not receive any share-based remuneration. Therefore, as of December 31, 2023 the members of the Supervisory Board held no stock options, performance shares or restricted shares.
The individual members of the Supervisory Board received, by virtue of the positions they held, the following remuneration:
Philips Group
Remuneration of the Supervisory Board
in EUR
| membership | committees | other compensation1) | total | |
|---|---|---|---|---|
| 2023 | ||||
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 |
| P.A.M. Stoffels | 115,000 | 35,000 | 22,269 | 172,269 |
| D.E.I. Pyott | 100,000 | 35,000 | 19,769 | 154,769 |
| A.M. Harrison | 100,000 | 14,000 | 19,769 | 133,769 |
| M.E. Doherty | 100,000 | 27,000 | 27,269 | 154,269 |
| P. Löscher | 100,000 | 32,000 | 17,269 | 149,269 |
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 |
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 |
| H. Verhagen | 100,000 | 14,000 | 7,269 | 121,269 |
| S. Poonen | 100,000 | 18,000 | 19,769 | 137,769 |
| 1,070,000 | 242,000 | 189,266 | 1,501,266 | |
| 2022 | ||||
| F. Sijbesma | 155,000 | 35,000 | 16,345 | 206,345 |
| P.A.M. Stoffels | 115,000 | 35,000 | 27,269 | 177,269 |
| N. Dhawan | 35,616 | 6,411 | 5,808 | 47,836 |
| D.E.I. Pyott | 100,000 | 35,000 | 17,269 | 152,269 |
| A.M. Harrison | 100,000 | 14,000 | 12,269 | 126,269 |
| M.E. Doherty | 100,000 | 27,000 | 24,769 | 151,769 |
| P. Löscher | 100,000 | 32,000 | 24,769 | 156,769 |
| I. Nooyi | 100,000 | 14,000 | 17,269 | 131,269 |
| S.K. Chua | 100,000 | 18,000 | 22,269 | 140,269 |
| H. Verhagen | 100,000 | 14,000 | 7,269.0 | 121,269 |
| S. Poonen | 100,000 | 18,000 | 17,269 | 135,269 |
| 1,105,616 | 248,411 | 192,574 | 1,546,602 | |
| 2021 | ||||
| J. van der Veer | 53,507 | 12,082 | 3,916 | 69,505 |
| C.A. Poon | 39,699 | 16,915 | 783 | 57,397 |
| N. Dhawan | 100,000 | 18,000 | 2,269 | 120,269 |
| O. Gadiesh | 34,521 | 4,833 | 783 | 40,137 |
| D.E.I. Pyott | 100,000 | 36,370 | 2,269 | 138,639 |
| P.A.M. Stoffels | 109,863 | 27,808 | 4,769 | 142,440 |
| A.M. Harrison | 100,000 | 14,000 | 2,269 | 116,269 |
| M.E. Doherty | 100,000 | 27,000 | 4,769 | 131,769 |
| P. Löscher | 100,000 | 32,000 | 4,769 | 136,769 |
| F. Sijbesma | 141,301 | 27,808 | 8,237 | 177,346 |
| I. Nooyi | 100,000 | 14,000 | 2,269 | 116,269 |
| S.K. Chua | 65,753 | 11,836 | 1,492 | 79,081 |
| 1,044,644 | 242,652 | 38,595 | 1,325,891 |
Members of the Supervisory Board and of the Board of Management are prohibited from writing call and put options or similar derivatives of Philips securities.
| December 31, 2022 | December 31, 2023 | |
|---|---|---|
| R. Jakobs | 109,422 | 126,809 |
| A. Bhattacharya | 169,517 | 177,088 |
| M.J. van Ginneken | 123,914 | 129,447 |
| P. Stoffels | 17,000 | 17,759 |
| S. Poonen | 3,000 | 3,133 |
| I. Nooyi | 3,100 | 3,238 |
| D. Pyott | 19,000 | 19,848 |
| S.K. Chua | 2,000 | 2,089 |
| F. Sijbesma | 12,500 | 25,000 |
| M. Harrison | 1,500 | 1,567 |
| P. Löscher | 20,732 | 21,658 |
For financial reporting purposes, financial instruments are categorized into Level 1, 2 or 3, based on the degree to which the inputs to the fair value measurements are observable and the significance of the inputs to the fair value measurement in its entirety, which are as follows:
Transfers between levels of the fair value hierarchy are recognized at the end of the reporting period during which the change has occurred.
Offsetting and master netting agreementsFinancial assets and liabilities are offset and the net amount is reported in the balance sheet when, and only when, the company has currently a legally enforceable right to set-off the amounts and the group intends either to settle them on a net basis or to realize the asset and settle the liability simultaneously.
Determining the fair value of financial instruments requires the use of estimates according to the method applied for each type of financial asset of liability. The estimated fair value of financial instruments has been determined by the company using available market information and appropriate valuation methods. The estimates presented are not necessarily indicative of the amounts that will ultimately be realized by the company upon maturity or disposal. The use of different market assumptions and/or estimation methods may have a material effect on the estimated fair value amounts.
Specific valuation techniques used to value financial instruments include:
Level 1
Instruments included in level 1 are comprised primarily of listed equity investments classified as financial assets carried at fair value through profit or loss or carried at fair value through other comprehensive income. The fair value of financial instruments traded in active markets is based on quoted market prices at the balance sheet date. A market is regarded as active if quoted prices are readily and regularly available from an exchange, dealer, broker, industry group, pricing service, or regulatory agency, and those prices represent actual and regularly occurring market transactions on an arm’s length basis.
Level 2
The fair value of financial instruments that are not traded in an active market (for example, over-the-counter derivatives or convertible bond instruments) is determined by using valuation techniques. These valuation techniques maximize the use of observable market data where it is available and rely as little as possible on entity-specific estimates. If all significant inputs required to fair value an instrument are based on observable market data, the instrument is included in level 2. The fair value of derivatives is calculated as the present value of the estimated future cash flows based on observable interest yield curves, basis spread and foreign exchange rates. The valuation of convertible bond instruments uses observable market quoted data for the options and present value calculations using observable yield curves for the fair value of the bonds.
The fair value of debt is estimated on the basis of the quoted market prices for certain issuances, or on the basis of discounted cash flow analysis using market rates plus Philips’ spread for the particular tenors of the borrowing arrangement. Accrued interest is not included within the carrying amount or estimated fair value of debt.
Level 3
If one or more of the significant inputs are not based on observable market data, such as third-party pricing information without adjustments, the instrument is included in level 3.
The fair value of contingent consideration is dependent on the terms of the respective acquisition agreement that may require Philips to pay additional consideration to former shareholders if specified future events occur or conditions are met, such as the achievement of certain regulatory milestones or the achievement of certain commercial milestones. The fair value of the contingent consideration provision is generally determined using a probability-weighted and a risk-adjusted approach to estimate the achievement of future regulatory and commercial milestones, respectively. The discount rates used in the risk adjusted approach reflect the inherent risk related to achieving the commercial milestones. Both regulatory and commercial milestones are discounted for the time value of money at risk-free rates. The fair value measurement is based on management’s estimates and assumptions and hence classified as Level 3 in the fair value hierarchy.
The following tables show the carrying amounts and fair values of financial assets and financial liabilities, including their levels in the fair value hierarchy. Fair value information for financial assets and financial liabilities not carried at fair value is not included if the carrying amount is a reasonable approximation of fair value.
Philips Group
Fair value of financial assets and liabilities
in millions of EUR
| carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | |
|---|---|---|---|---|---|
| December 31, 2023 | |||||
| Financial assets | |||||
| Carried at fair value: | |||||
| Debt instruments | 226 | 226 | 226 | ||
| Equity instruments | 2 | 2 | 2 | ||
| Other financial assets | 56 | 56 | 34 | 22 | |
| Financial assets carried at FVTP&L | 284 | 284 | 34 | 250 | |
| Debt instruments | 27 | 27 | 26 | ||
| Equity instruments | 231 | 231 | 14 | 217 | |
| Current financial assets | 3 | 3 | 3 | ||
| Receivables - current | 32 | 32 | 32 | ||
| Financial assets carried at FVTOCI | 293 | 293 | 14 | 26 | 253 |
| Derivative financial instruments | 48 | 48 | 48 | ||
| Financial assets carried at fair value | 624 | 624 | 14 | 108 | 503 |
| Carried at (amortized) cost: | |||||
| Cash and cash equivalents | 1,869 | ||||
| Loans and receivables: | |||||
| Current loans receivables | - | ||||
| Other non-current loans and receivables | 77 | ||||
| Receivables - current | 3,701 | ||||
| Receivables - non-current | 193 | ||||
| Financial assets carried at (amortized) cost | 5,840 | ||||
| Total financial assets | 6,465 | ||||
| Financial liabilities | |||||
| Carried at fair value: | |||||
| Contingent consideration | (115) | (115) | (115) | ||
| Financial liabilities carried at FVTP&L | (115) | (115) | (115) | ||
| Derivative financial instruments | (43) | (43) | (43) | ||
| Financial liabilities carried at fair value | (158) | (158) | (43) | (115) | |
| Carried at (amortized) cost: | |||||
| Accounts payable | (1,917) | ||||
| Interest accrual | (76) | ||||
| Debt (Corporate bonds and leases) | (6,969) | (6,798) | (5,724) | (1,074) | |
| Debt (excluding corporate bonds and leases) | (721) | ||||
| Financial liabilities carried at (amortized) cost | (9,682) | ||||
| Total financial liabilities | (9,840) |
Philips Group
Fair value of financial assets and liabilities
in millions of EUR
| carrying amount | estimated fair value1) | Level 1 | Level 2 | Level 3 | |
|---|---|---|---|---|---|
| December 31, 2022 | |||||
| Financial assets | |||||
| Carried at fair value: | |||||
| Debt instruments | 232 | 232 | 232 | ||
| Equity instruments | 4 | 4 | 1 | 2 | |
| Other financial assets | 86 | 86 | 35 | 51 | |
| Financial assets carried at FVTP&L | 322 | 322 | 1 | 35 | 285 |
| Debt instruments | 25 | 25 | 25 | ||
| Equity instruments | 259 | 259 | 30 | 229 | |
| Current financial assets | 9 | 9 | 9 | ||
| Receivables - current | 26 | 26 | 26 | ||
| Financial assets carried at FVTOCI | 319 | 319 | 30 | 25 | 264 |
| Derivative financial instruments | 127 | 127 | 127 | ||
| Financial assets carried at fair value | 768 | 768 | 32 | 187 | 549 |
| Carried at (amortized) cost: | |||||
| Cash and cash equivalents | 1,172 | ||||
| Loans and receivables: | |||||
| Current loans receivables | 2 | ||||
| Other non-current loans and receivables | 54 | ||||
| Receivables - current | 4,088 | ||||
| Receivables - non-current | 279 | ||||
| Financial assets carried at (amortized) cost | 5,596 | ||||
| Total financial assets | 6,364 | ||||
| Financial liabilities | |||||
| Carried at fair value: | |||||
| Contingent consideration | (113) | (113) | (113) | ||
| Financial liabilities carried at FVTP&L | (113) | (113) | (113) | ||
| Derivative financial instruments | (211) | (211) | (211) | ||
| Financial liabilities carried at fair value | (324) | (324) | (211) | (113) | |
| Carried at (amortized) cost: | |||||
| Accounts payable | (1,968) | ||||
| Interest accrual | (71) | ||||
| Debt (Corporate bonds and leases) | (6,520) | (6,083) | (5,001) | (1,082) | |
| Debt (excluding corporate bonds and leases) | (1,680) | ||||
| Financial liabilities carried at (amortized) cost | (10,240) | ||||
| Total financial liabilities | (10,564) |
The following table shows the reconciliation from the beginning balance to the end balance for Level 3 fair value measurements.
Philips Group
Reconciliation of Level 3 fair value measurements
in millions of EUR
| Financial assets | Financial liabilities | |
|---|---|---|
| Balance as of January 1, 2023 | 549 | 113 |
| Acquisitions | 6 | |
| Purchase | 85 | |
| Sales | (56) | |
| Utilizations | (20) | |
| Recognized in profit and loss: | ||
| other business income | 16 | |
| financial income and expenses1) | (43) | 1 |
| Recognized in other comprehensive income2) | (40) | (2) |
| Receivables held to collect and sell | 6 | |
| Reclassification | 1 | |
| Balance as of December 31, 2023 | 503 | 115 |
Philips Group
Reconciliation of Level 3 fair value measurements
in millions of EUR
| Financial assets | Financial liabilities | |
|---|---|---|
| Balance as of January 1, 2022 | 523 | 208 |
| Acquisitions | 96 | |
| Purchase | 131 | |
| Sales | (76) | |
| Utilizations | (105) | |
| Recognized in profit and loss: | ||
| other business income | (85) | |
| financial income and expenses | 7 | (8) |
| Recognized in other comprehensive income1) | 8 | |
| Receivables held to collect and sell | (41) | |
| Reclassification from associates | 5 | |
| Balance as of December 31, 2022 | 549 | 113 |
Transactions in derivatives are subject to master netting and set-off agreements. In the case of certain termination events, under the terms of the master agreement, Philips can terminate the outstanding transactions and aggregate their positive and negative values to arrive at a single net termination sum (or close-out amount). This contractual right is subject to the following:
Philips Group
Financial assets subject to offsetting, enforceable master netting arrangements or similar agreements
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Derivatives | ||
| Gross amounts of recognized financial assets | 127 | 48 |
| Gross amounts of recognized financial liabilities offset in the balance sheet | ||
| Net amounts of financial assets presented in the balance sheet | 127 | 48 |
| Related amounts not offset in the balance sheet | ||
| Financial instruments | (54) | (34) |
| Net amount | 73 | 13 |
Philips Group
Financial liabilities subject to offsetting, enforceable master netting arrangements or similar agreements
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Derivatives | ||
| Gross amounts of recognized financial liabilities | (211) | (43) |
| Gross amounts of recognized financial assets offset in the balance sheet | ||
| Net amounts of financial liabilities presented in the balance sheet | (211) | (43) |
| Related amounts not offset in the balance sheet | ||
| Financial instruments | 54 | 34 |
| Net amount | (157) | (9) |
The company uses derivative financial instruments principally to manage its foreign currency risks and, to a more limited extent, interest rate and commodity price risks. All derivative financial instruments are accounted for at the trade date and classified as current or non-current assets or liabilities based on the maturity date or the early termination date. The company measures all derivative financial instruments at fair value that is derived from the market prices of the instruments, calculated on the basis of the present value of the estimated future cash flows based on observable interest yield curves, basis spread, credit spreads and foreign exchange rates, or derived from option pricing models, as appropriate. Gains or losses arising from changes in fair value of derivatives are recognized in the Consolidated statements of income, except for derivatives that are highly effective and qualify for cash flow or net investment hedge accounting.
Changes in the fair value of foreign exchange forward contracts attributable to forward points and changes in the time value of the option contracts are deferred in the cash flow hedges reserve within equity. The deferred amounts are recognized in the Consolidated statements of income against the related hedged transaction when it occurs.
Changes in the fair value of a derivative that is highly effective and that is designated and qualifies as a cash flow hedge are recorded in OCI until the Consolidated statements of income are affected by the variability in cash flows of the designated hedged item. To the extent that the hedge is ineffective, changes in the fair value are recognized in the Consolidated statements of income.
The company formally assesses, both at the hedge’s inception and on an ongoing basis, whether the derivatives that are used in hedging transactions are highly effective in offsetting changes in fair values or cash flows of hedged items. When it is established that a derivative is not highly effective as a hedge or that it has ceased to be a highly effective hedge, the company discontinues hedge accounting prospectively. When hedge accounting is discontinued because it is expected that a forecasted transaction will not occur, the company continues to carry the derivative on the Consolidated balance sheets at its fair value, and gains and losses that were accumulated in OCI are recognized immediately in the same line item as they relate to in the Consolidated statements of income.
Foreign currency differences arising upon retranslation of financial instruments designated as a hedge of a net investment in a foreign operation are recognized directly in the currency translation differences reserve through OCI, to the extent that the hedge is effective. To the extent that the hedge is ineffective, such differences are recognized in the Consolidated statements of income.
Financial assets are subject to impairment assessment, which involves estimating expected credit losses. Refer to Other financial assets for accounting policies on impairment of financial assets.
Philips is exposed to several types of financial risks which are further analyzed below. Philips does not purchase or hold derivative financial instruments for speculative purposes. Information regarding financial instruments is included in Fair value of financial assets and liabilities.
Liquidity riskLiquidity risk is the risk that an entity will encounter difficulty in meeting obligations associated with financial liabilities.
Liquidity risk for the group is monitored through the Treasury liquidity committee, which tracks the development of the actual cash flow position for the group and uses input from a number of sources in order to forecast the overall liquidity position on both short and longer term basis. Philips invests surplus cash in short-term deposits with appropriate maturities to ensure sufficient liquidity is available to meet liabilities when due and in money market funds.
The rating of the company’s debt by major rating agencies may improve or deteriorate. As a result, Philips’ future borrowing capacity may be influenced and its financing costs may fluctuate. Philips has various sources to mitigate the liquidity risk for the group. As of December 31, 2023, Philips had EUR 1,869 million in cash and cash equivalents (2022: EUR 1,172 million), within which short-term deposits of EUR 1,399 million (2022: EUR 482 million). Cash and cash equivalents include all cash balances, money market funds and short-term highly liquid investments with an original maturity of three months or less that are readily convertible into known amounts of cash. Philips pools cash from subsidiaries to the extent legally and economically feasible; cash not pooled remains available for the company’s operational or investment needs.
Philips faces cross-border foreign exchange controls and/or other legal restrictions in a few countries that could limit its ability to make these balances available on short notice for general use by the group.
Philips has a USD 2.5 billion Commercial Paper Program and a EUR 1 billion committed standby revolving credit facility that can be used for general group purposes, such as a backstop for its Commercial Paper Program. As of December 31, 2023, Philips did not have any loans outstanding under either facility. These facilities do not have a material adverse change clause, have no financial covenants and no credit-rating-related acceleration possibilities. As per March 9, 2020, Philips established a Euro Medium-Term Note (EMTN) program, a framework that facilitates the issuance of notes for a total amount up to EUR 10 billion. As of December 31, 2023, Philips has EUR 3.3 billion outstanding under this program of which EUR 500 million fixed rates notes were issued in August 2023 with maturity date in 2031. For a description of Philips’ credit facilities, refer to Debt.
In addition to cash and cash equivalents, as of December 31, 2023, Philips also held EUR 14 million of listed (level 1) equity investments at fair value (classified as other non-current financial assets).
The following table presents a summary of the Group’s fixed contractual cash obligations and commitments as of December 31, 2023. These amounts are an estimate of future payments which could change as a result of various factors such as a change in interest rates, foreign exchange, contractual provisions, as well as changes in business strategy and needs. Therefore, the actual payments made in future periods may vary from those presented in the following table:
| payments due by period | |||||
|---|---|---|---|---|---|
| total | less than 1 year | 1-3 years | 3-5 years | after 5 years | |
| Long-term debt | 7,615 | 533 | 1,934 | 1,431 | 3,717 |
| Short-term debt | 122 | 122 | |||
| Interest on debt | 1,704 | 180 | 328 | 285 | 911 |
| Derivative liabilities | 39 | 38 | 1 | ||
| Purchase obligations3) | 668 | 355 | 286 | 27 | |
| Trade and other payables | 1,917 | 1,917 | |||
| Contractual cash obligations | 12,065 | 3,145 | 2,549 | 1,716 | 4,655 |
Philips has contracts with investment funds where it committed itself to make, under certain conditions, capital contributions to these funds of an aggregated remaining amount of EUR 153 million (2022: EUR 127 million). As of December 31, 2023 capital contributions already made to these investment funds are recorded as non-current financial assets.
Philips offers voluntary supply chain finance programs with third parties which provide participating suppliers the opportunity to factor their trade receivables at the sole discretion of both the suppliers and the third parties. Philips continues to recognize these liabilities as trade payables and settles them accordingly on the invoice maturity date based on the terms and conditions of those arrangements. As of December 31, 2023 approximately EUR 114 million (2022: EUR 151 million) of the Philips account payable were transferred under these arrangements.
With respect to the Respironics field action, please refer to Contingencies. The management continues to monitor the risks associated with such potential claims and its impact on liquidity position, if any.
Leasing activitiesThe company leases various items of real estate, vehicles and other equipment where it acts as a lessee. The company has multiple extension and termination options in a number of lease contracts. These are used to maximize operational flexibility in terms of managing the assets used in the company's operations. The options considered reasonably certain are part of lease liabilities. In addition, the company is committed to leases not yet commenced to EUR 128 million. The company's lease contracts do not contain financial covenants.
The company enters into sale-and-leaseback transactions primarily for its Sleep & Respiratory Care businesses. These transactions are accounted for at market value. The payments for these leases are considered in determining lease liabilities. Principal repayments are part of cash flows used for financing activities and interest payments are part of cash flows used for operating activities. The cash inflows arising from the sales transactions are part of cash flows provided by financing activities. Lease payments under sale-and-leaseback arrangements for 2023 were EUR 55 million (2022: EUR 72 million). The remaining minimum payment under sale-and-leaseback arrangements included in lease obligations above are as follows:
Philips Group
Remaining minimum payments under sale-and-leaseback arrangements
in millions of EUR
| 2024 | 43 |
| 2025 | 30 |
| 2026 | 21 |
| 2027 | 12 |
| 2028 | 5 |
| Thereafter | 26 |
Philips has leasing activities where it acts as lessor. In such arrangements, Philips provides the customer with a right to use of medical equipment in exchange for a series of payments. Residual values of assets under lease form an insignificant part of the carrying amount of those assets. Residual values are influenced by asset market prices and are therefore subject to management estimation. Residual values are at least reassessed on an annual basis, or more often when necessary. Reassessments are based on a combination of realization of assets sold, expert knowledge and judgment of local markets. For lease receivables, the value of unguaranteed residual values as of December 31, 2023 was EUR 0.0 million (2022: EUR 0.6 million). In order to reduce residual value risk exposures there may be residual value guarantees or purchase options embedded in the customer contract. Credit risk for lease receivables is reviewed regularly and mitigated, for example, by retaining a security interest in the leased asset.
Currency riskCurrency risk is the risk that reported financial performance or the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rates. Philips operates in many countries and currencies and therefore currency fluctuations may impact Philips’ financial results. Philips is exposed to currency risk in the following areas:
It is Philips’ policy to reduce the potential year-on-year volatility caused by foreign-currency movements on its net earnings by hedging the anticipated net exposure of foreign currencies resulting from foreign-currency sales and purchases. In general, net anticipated exposures for the Group are hedged during a period of 15 months in layers of 20% up to a maximum hedge of 80%. Philips’ policy requires significant committed foreign currency exposures to be fully hedged, generally using forwards. However, not every foreign currency can or shall be hedged as there may be regulatory barriers or prohibitive hedging cost preventing Philips from effectively and/or efficiently hedging its currency exposures. As a result, hedging activities cannot and will not eliminate all currency risks for anticipated and committed transaction exposures.
The following table outlines the estimated nominal value in millions of EUR for committed and anticipated transaction exposure and related hedges for Philips’ most significant currency exposures consolidated as of December 31, 2023:
Philips Group
Estimated transaction exposure and related hedges
in millions of EUR
| Sales/Receivables | Purchases/Payable | |||
|---|---|---|---|---|
| exposure | hedges | exposure | hedges | |
| Balance as of December 31, 2023 | ||||
| Exposure currency | ||||
| USD | 1,793 | (1,449) | (888) | 805 |
| JPY | 547 | (319) | (14) | 14 |
| GBP | 312 | (196) | (12) | 12 |
| CNY | 439 | (304) | (98) | 95 |
| CAD | 249 | (161) | (1) | 1 |
| PLN | 91 | (103) | ||
| AUD | 226 | (137) | ||
| CHF | 103 | (63) | (1) | 1 |
| CZK | 63 | (70) | ||
| SEK | 33 | (18) | (3) | 3 |
| EUR | 233 | (232) | (114) | 113 |
| Others | 198 | (134) | (216) | 130 |
| Total 2023 | 4,287 | (3,185) | (1,346) | 1,173 |
| Total 2022 | 3,779 | (2,920) | (1,468) | 1,326 |
Philips uses foreign exchange spot and forward contracts, as well as zero cost collars in hedging the exposure. The derivatives related to transactions are, for hedge accounting purposes, split into hedges of on-balance-sheet accounts receivable/ payable and forecasted sales and purchases. Changes in the value of on-balance-sheet foreign-currency accounts receivable/payable, as well as the changes in the fair value of the hedges related to these exposures, are reported in the income statement under costs of sales. Hedges related to forecasted transactions, where hedge accounting is applied, are accounted for as cash flow hedges. The results from such hedges are deferred in other comprehensive income within equity to the extent that the hedge is effective. As of December 31, 2023, a gain of EUR 6 million was deferred in equity as a result of these hedges (2022: EUR 2 million loss). The result deferred in equity will be released to earnings mostly during 2024 at the time when the related hedged transactions affect the income statement. During 2023, nil (2022: EUR 1 million net gain) was recorded in the consolidated statement of income as a result of ineffectiveness on certain anticipated cash flow hedges. Ineffectiveness arises when anticipated exposures are no longer expected to be highly probable. During 2023, a gain of EUR 19 million included in the cash flow hedges reserve in equity pertaining to changes in fair value of foreign exchange forward and option contracts was released to income statement.
The total net fair value of hedges related to transaction exposure as of December 31, 2023, was an unrealized gain of EUR 10 million. The estimated impact of a 10% increase of value of the EUR is estimated to be EUR 116 million. The following table contains an overview of the instantaneous 10% increase in the value of EUR against major currencies.
Philips Group
Estimated impact of 10% increase of value of the EUR on the fair value of hedges
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| USD | 68 | 64 |
| JPY | 15 | 15 |
| GBP | 16 | 16 |
| CHF | 4 | 5 |
| PLN | 2 | 1 |
| RUB | - |
The EUR 116 million increase includes a gain of EUR 40 million that would impact the income statement, which would largely offset the opposite revaluation effect on the underlying accounts receivable and payable, and the remaining gain of EUR 77 million would be recognized in equity to the extent that the cash flow hedges were effective.
Foreign exchange exposure also arises as a result of inter-company loans and deposits. Where the company enters into such arrangements, the financing is generally provided in the functional currency of the subsidiary entity. The currency of the company’s external funding and liquid assets is matched with the required financing of subsidiaries, either directly through external foreign currency loans and deposits, or synthetically by using foreign exchange derivatives, including cross currency interest rate swaps and foreign exchange forward contracts. In certain cases where group companies may also have external foreign currency debt or liquid assets, these exposures are also hedged through the use of foreign exchange derivatives. Changes in the fair value of hedges related to this exposure are recognized within financial income and expenses in the statements of income. When such loans would be considered part of the net investment in the subsidiary, net investment hedging would be applied.
Translation exposure of foreign-currency equity invested in consolidated entities is generally not hedged. If a hedge is entered into, it is accounted for as a net investment hedge. Net current-period change, before tax, of the currency translation reserve of negative EUR 579 million mainly relates to the development of the USD versus the EUR. As of December 31, 2023, a weakening of USD by 10% versus the EUR would result in a decrease in the currency translation reserve in equity of approximately EUR 1,146 million, while a strengthening of USD by 10% versus the EUR would result in an increase in the currency translation reserve in equity of approximately EUR 1,400 million. Refer to the country risk paragraph for countries with significant foreign currency denominated equity invested.
As of December 31, 2023, external bond funding for a nominal value of USD 1,474 million (liability at book value: EUR 1,325 million) was designated as a net investment hedge of financing investments in foreign operations for an equal amount. During 2023 a total loss of EUR 2 million was recognized in the income statement as ineffectiveness on net investment hedges, arising from counterparty and own credit risk.
An instantaneous 10% increase in the value of the EUR against all currencies would lead to an decrease of EUR 52 million in the value of the derivatives, including a EUR 11 million increase related to the USD.
As of December 31, 2022, cross-currency interest rate swaps for a nominal value of USD 500 million (liability at fair value: EUR 147 million) and external bond funding for a nominal value of USD 1,490 million (liability at book value: EUR 1,378 million) were designated as a net investment hedge of financing investments in foreign operations for an equal amount. During 2022 a total gain of EUR 1.1 million was recognized in the income statement as ineffectiveness on net investment hedges, arising from counterparty and own credit risk.
The total net fair value of financing derivatives as of December 31, 2022, was a liability of EUR 147 million. An instantaneous 10% increase in the value of the EUR against all currencies would lead to an increase of EUR 192 million in the value of the derivatives, including a EUR 191 million increase related to the USD.
Philips does not currently hedge the foreign exchange exposure arising from equity interests in non-functional-currency investments in associates and other non-current financial assets.
Interest rate riskInterest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. As of December 31, 2023, Philips had outstanding debt of EUR 7,689 million (2022: EUR 8,201 million), which constitutes an inherent interest rate risk with potential negative impact on financial results. As of December 31, 2023, Philips held EUR 1,869 million in cash and cash equivalents (2022: EUR 1,172 million), and had total long-term debt of EUR 7,035 million (2022: EUR 7,270 million) and total short-term debt of EUR 654 million (2022: EUR 931 million). As of December 31, 2023, Philips had a ratio of fixed-rate long-term debt to total outstanding debt of approximately 89% compared to 80% one year earlier. Philips debt has a long maturity profile with an average tenor of long-term debt of 6.0 years with maturities up to 2042.
The following table provides the impact of a 1% increase/decrease of interest rates on the fair value of the debt and the annualized net interest expenses.
Philips Group
Net debt1) and interest rate sensitivity
in millions of EUR
| 2022 | 2023 | |
|---|---|---|
| Impact 1% interest increase on the fair value of the fixed-rate long-term debt2)3) | (274) | (283) |
| Impact 1% interest decrease on the fair value of the fixed-rate long-term debt2)3) | 274 | 284 |
| Impact 1% interest increase on the annualized net interest expense4) | 4 | 15 |
Equity price risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in equity prices.
Philips is a shareholder in some publicly listed companies and as a result is exposed to potential financial loss through movements in their share prices. The aggregate equity price exposure in such financial assets amounted to approximately EUR 14 million as of December 31, 2023 (2022: EUR 32 million). Philips does not hold derivatives in the above-mentioned listed companies. Philips also has shareholdings in several privately-owned companies amounting to EUR 219 million, mainly consisting of minority stakes in companies in various industries. As a result, Philips is exposed to potential value adjustments.
Commodity price riskCommodity price risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in commodity prices.
Philips is a purchaser of certain base metals, precious metals and energy. Philips may hedge certain commodity price risks using derivative instruments to minimize significant, unanticipated earnings fluctuations caused by commodity price volatility. As of December 31, 2023 and 2022, respectively, Philips did not have any significant outstanding financial commodity derivatives.
Credit riskCredit risk represents the loss that would be recognized at the reporting date, if counterparties failed completely to perform their payment obligations as contracted. Credit risk is present within Philips trade receivables and contract assets. To have better insights into the credit exposures, Philips performs ongoing evaluations of the financial and non-financial condition of its customers and adjusts credit limits when appropriate. In instances where the creditworthiness of a customer is determined not to be sufficient to grant the credit limit required, there are a number of mitigation tools that can be utilized to close the gap, including reducing payment terms, cash on delivery, pre-payments and pledges on assets.
Philips invests available cash and cash equivalents with various financial institutions and is exposed to credit risk with these counterparties. Philips is also exposed to credit risks in the event of non-performance by financial institutions with respect to financial derivative instruments. Philips actively manages concentration risk and on a daily basis measures the potential loss under certain stress scenarios, should a financial institution default. These worst-case scenario losses are monitored and limited by the company.
The company does not enter into any financial derivative instruments to protect against default by financial institutions. However, where possible the company requires all financial institutions with which it deals in derivative transactions to complete legally enforceable netting agreements under an International Swap Dealers Association master agreement or otherwise prior to trading, and whenever possible, to have a strong credit rating. Philips also regularly monitors the development of the credit risk of its financial counterparties. Wherever possible, cash is invested and financial transactions are concluded with financial institutions with strong credit ratings or with governments or government-backed institutions.
The following table shows the number of financial institutions with credit rating A- and above with which Philips has cash at hand and short-term deposits above EUR 10 million as of December 31, 2023.
Philips Group
Credit risk with number of counterparties
for deposits above EUR 10 million
| 10-100 million | 100-500 million | 500 million and above | |
|---|---|---|---|
| AAA rated bank counterparties | 4 | ||
| AA- rated bank counterparties | 1 | ||
| A+ rated bank counterparties | 2 | 3 | |
| A rated bank counterparties | 2 | 1 | |
| A- rated bank counterparties | 1 | ||
| 6 | 8 |
For an overview of the overall maximum credit exposure related to debt instruments, derivatives and loans and receivables, refer to Fair value of financial assets and liabilities.
Country riskCountry risk is the risk that political, legal, or economic developments in a single country could adversely impact performance. The country risk per country is defined as the sum of the equity of all subsidiaries and associated companies in country cross-border transactions, such as intercompany loans, accounts receivable from third parties and intercompany accounts receivable. The country risk is monitored on a regular basis.
As of December 31, 2023, the company had country risk exposure of EUR 13.3 billion in the United States, EUR 1.4 billion in the Netherlands, EUR 1.3 billion in China (including Hong Kong). Other countries higher than EUR 500 million are Germany EUR 786 million, United Kingdom EUR 731 million, and Japan EUR 614 million. Other countries with significant exposure are Singapore EUR 202 million and Israel EUR 214 million. The degree of risk of a country is taken into account when new investments are considered. The company does not, however, use financial derivative instruments to hedge country risk.
The impact of hyperinflation is also routinely assessed and was not material for the periods presented.
Other insurable risksPhilips is insured for a broad range of losses by global insurance policies in the areas of property damage/business interruption, general and product liability, transport, directors’ and officers’ liability, employment practice liability, crime and cybersecurity. The counterparty risk related to the insurance companies participating in the above-mentioned global insurance policies is actively managed. As a rule, Philips only selects insurance companies with a financial strength of at least A-. Throughout the year the counterparty risk is monitored on a regular basis.
To lower exposures and to avoid potential losses, Philips has a global Risk Engineering program in place. The main focus of this program is on property damage and business interruption risks including company interdependencies. Regular on-site assessments take place at Philips locations and business-critical suppliers by risk engineers of the insurer in order to provide an accurate assessment of the potential loss and its impact. The results of these assessments are shared across the company’s stakeholders. On-site assessments are carried out against the predefined Risk Engineering standards, which are agreed between Philips and the insurers. Recommendations are made in a Risk Improvement report and are monitored centrally. This is the basis for decision-making by the local management of the business as to which recommendations will be implemented.
For all policies, deductibles are in place, which vary from EUR 0 million to EUR 10 million per occurrence and this variance is designed to differentiate between the existing risk categories within Philips. Above a first layer of working deductibles, Philips operates its own re-insurance captive, which during 2023 retained EUR 25 million per claim and EUR 50 million in the annual aggregate for general, product, professional liability, and marine cargo claims and EUR 15 million aggregate for cyber.
New contracts were signed effective December 31, 2023, for the coming year, whereby the re-insurance captive retentions remained the same.
On January 29, 2024, Philips announced that it has agreed on the terms of a consent decree with the US Department of Justice (DOJ), representing the US Food and Drug Administration (FDA). The proposed consent decree primarily focuses on Philips Respironics’ business operations in the US. The proposed consent decree is being finalized and will be submitted to the relevant US court for approval. The decree will provide Philips Respironics with a roadmap of defined actions, milestones, and deliverables to demonstrate compliance with regulatory requirements and to restore the business.
As a consequence of addressing this proposed consent decree, which is a multi-year plan, Philips recorded charges of EUR 363 million in December 2023 that relate to remediation activities, inventory write-downs and onerous contract provisions. Refer to Provisions.
In this Annual Report Philips presents certain financial measures when discussing Philips’ performance that are not measures of financial performance or liquidity under IFRS (‘non-IFRS’). These non-IFRS measures (also known as non-GAAP or alternative performance measures) are presented because management considers them important supplemental measures of Philips’ performance and believes that they are widely used in the industry in which Philips operates as a means of evaluating a company’s operating performance and liquidity. Philips believes that an understanding of its sales performance, profitability, financial strength and funding requirements is enhanced by reporting the following non-IFRS measures:
Non-IFRS measures do not have standardized meanings under IFRS and not all companies calculate non-IFRS measures in the same manner or on a consistent basis. As a result, these measures may not be comparable to measures used by other companies that have the same or similar names. Accordingly, undue reliance should not be placed on the non-IFRS measures contained in this Annual Report and they should not be considered as substitutes for sales, net income, net cash provided by operating activities or other financial measures computed in accordance with IFRS.
This chapter contains the definitions of the non-IFRS measures used in this Annual Report as well as reconciliations from the most directly comparable IFRS measures. The non-IFRS measures discussed in this Annual Report are cross referenced to this chapter. These non-IFRS measures should not be viewed in isolation or as alternatives to equivalent IFRS measures and should be used in conjunction with the most directly comparable IFRS measures.
The non-IFRS financial measures presented are not measures of financial performance or liquidity under IFRS, but measures used by management to monitor the underlying performance of Philips’ business and operations and, accordingly, they have not been audited or reviewed by Philips’ external auditors.
Additionally, Philips provides forward-looking targets for comparable sales growth, adjusted EBITA margin improvement, free cash flow and organic ROIC, which are non-IFRS financial measures. Philips has not provided a quantitative reconciliation of these targets to the most directly comparable IFRS measures because certain information needed to reconcile these non-IFRS financial measures to the most comparable IFRS financial measures are dependent on specific items or impacts which are not yet determined, are subject to uncertainty and variability in timing and amount due to their nature, are outside of Philips’ control, or cannot be predicted, including items and impacts such as currency exchange rates, acquisitions and disposals, legal and tax gains and losses and pension settlements, charges and costs such as impairments, restructuring and acquisition-related charges, amortization of intangible assets and net capital expenditures. Accordingly, reconciliations of these non-IFRS forward looking financial measures to the most directly comparable IFRS financial measures are not available without unreasonable effort. Such unavailable reconciling items could significantly impact the results of operations and financial condition.
Comparable sales growthComparable sales growth represents the period-on-period growth in sales excluding the effects of currency movements and changes in consolidation. As indicated in General information to the Consolidated financial statements, foreign currency sales and costs are translated into Philips’ presentation currency, the euro, at the exchange rates prevailing at the respective transaction dates. As a result of significant foreign currency sales and currency movements during the periods presented, the effects of translating foreign currency sales amounts into euros could have a material impact on the comparability of sales between periods. Therefore, these impacts are excluded when presenting comparable sales in euros by translating the foreign currency sales of the previous period and the current period into euros at the same average exchange rates. In addition, the years presented were affected by a number of acquisitions and divestments, as a result of which various activities were consolidated or deconsolidated. The effect of consolidation changes has also been excluded in arriving at the comparable sales. For the purpose of calculating comparable sales, when a previously consolidated entity is sold or control is lost, relevant sales of that entity for the corresponding prior year period are excluded. Similarly, when an entity is acquired and consolidated, relevant sales of that entity for the current year period are excluded.
Comparable sales growth is presented for the Philips Group, operating segments and geographic area. Philips’ believes that the presentation of comparable sales growth is meaningful for investors to evaluate the performance of Philips’ business activities over time. Comparable sales growth may be subject to limitations as an analytical tool for investors, because comparable sales growth figures are not adjusted for other effects, such as increases or decreases in prices or quantity/volume. In addition, interaction effects between currency movements and changes in consolidation are not taken into account.
Philips Group
Sales growth composition by segment
in %
| nominal growth | consolidation changes | currency effects | comparable growth | |
|---|---|---|---|---|
| 2023 versus 2022 | ||||
| Diagnosis & Treatment | 6.4 | 0.2 | 4.5 | 11.1 |
| Connected Care | (2.5) | 0.3 | 3.3 | 1.1 |
| Personal Health | (0.7) | 0.0 | 3.9 | 3.2 |
| Philips Group | 1.9 | 0.2 | 3.9 | 6.0 |
|
2022 versus 2021 |
||||
| Diagnosis & Treatment | 5.9 | 0.0 | (6.7) | (0.8) |
| Connected Care | (1.9) | 0.0 | (7.2) | (9.1) |
| Personal Health | 5.7 | 0.0 | (5.7) | 0.1 |
| Philips Group | 3.9 | (0.3) | (6.4) | (2.8) |
|
2021 versus 2020 |
||||
| Diagnosis & Treatment | 5.9 | (0.1) | 2.5 | 8.3 |
| Connected Care | (14.9) | (6.3) | 2.2 | (19.0) |
| Personal Health | 7.2 | 0.0 | 1.6 | 8.8 |
| Philips Group | (0.9) | (2.5) | 2.3 | (1.2) |
Philips Group
Sales growth composition by geographic area
in %
| nominal growth | consolidation changes | currency effects | comparable growth | |
|---|---|---|---|---|
| 2023 versus 2022 | ||||
| Western Europe | 6.0 | 0.3 | 0.3 | 6.6 |
| North America | (0.3) | 0.2 | 2.7 | 2.5 |
| Other mature geographies | (1.0) | 0.1 | 8.2 | 7.3 |
| Mature geographies | 1.4 | 0.2 | 2.7 | 4.2 |
| Growth geographies | 3.4 | 0.2 | 6.9 | 10.5 |
| Philips Group | 1.9 | 0.2 | 3.9 | 6.0 |
|
2022 versus 2021 |
||||
| Western Europe | (1.2) | (1.3) | (0.4) | (2.8) |
| North America | 11.9 | 0.2 | (12.4) | (0.3) |
| Other mature geographies | (3.0) | 0.0 | 2.5 | (0.5) |
| Mature geographies | 5.9 | (0.3) | (6.7) | (1.1) |
| Growth geographies | (0.8) | (0.1) | (5.9) | (6.9) |
| Philips Group | 3.9 | (0.3) | (6.4) | (2.8) |
|
2021 versus 2020 |
||||
| Western Europe | (1.5) | (1.3) | (0.4) | (3.2) |
| North America | (1.5) | (5.5) | 3.6 | (3.4) |
| Other mature geographies | (3.2) | (0.1) | 3.6 | 0.3 |
| Mature geographies | (1.8) | (3.5) | 2.4 | (2.8) |
| Growth geographies | 1.2 | (0.3) | 2.1 | 3.0 |
| Philips Group | (0.9) | (2.5) | 2.3 | (1.2) |
The term Adjusted EBITA is used to evaluate the performance of Philips and its segments. EBITA represents Income from operations excluding amortization and impairment of acquired intangible assets and impairment of goodwill. Adjusted EBITA represents EBITA excluding gains or losses from restructuring costs, acquisition-related charges and other items.
Restructuring costs are defined as the estimated costs of initiated reorganizations, the most significant of which have been approved by the Executive Committee, and which generally involve the realignment of certain parts of the industrial and commercial organization.
Acquisition-related charges are defined as costs that are directly triggered by the acquisition of a company, such as transaction costs, purchase accounting related costs and integration-related expenses.
Other items are defined as any individual item with an income statement impact (loss or gain) that is deemed by management to be both significant and incidental to normal business activity. This includes the following: litigation costs and settlements in favor of (or against) the company, gains (or losses) on sale of businesses or assets, remediation costs, impairment of assets, portfolio realignment charges, environmental charges and other items which are individually above an amount of EUR 20 million in a quarter, or an individual item which is above EUR 40 million across multiple quarters. Refer to Net income, Income from operations (EBIT) and Adjusted EBITA within the Results of operations section of Financial performance.
Philips considers the use of Adjusted EBITA appropriate as Philips uses it as a measure of segment performance and as one of its strategic drivers to increase profitability through re-allocation of its resources towards opportunities offering more consistent and higher returns. This is done with the aim of making the underlying performance of the businesses more transparent.
EBITA excludes amortization and impairment of acquired intangible assets and impairment of goodwill, which primarily relates to brand names, customer relationships and technology, as Philips believes that such amounts are inconsistent in amount and frequency, are significantly impacted by the timing and/or size of acquisitions and do not factor into its decisions on allocation of its resources across segments. Although we exclude amortization and impairment of acquired intangible assets from the Adjusted EBITA measure, Philips believes that it is important for investors to understand that these acquired intangible assets contribute to revenue generation.
Philips believes Adjusted EBITA is useful to evaluate financial performance on a comparable basis over time by factoring out restructuring costs, acquisition-related charges and other incidental items which are not directly related to the operational performance of Philips Group or its segments.
Adjusted EBITA may be subject to limitations as an analytical tool for investors, as it excludes restructuring costs, acquisition-related charges and other incidental items and therefore does not reflect the expense associated with such items, which may be significant and have a significant effect on Philips’ net income.
Adjusted EBITA margin refers to Adjusted EBITA divided by sales expressed as a percentage.
Adjusted EBITA is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted EBITA to the most directly comparable IFRS measure, Net income, for the years indicated is presented in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Adjusted EBITDAAdjusted EBITDA is defined as Income from operations excluding amortization and impairment of intangible assets, impairment of goodwill, depreciation and impairment of property, plant and equipment, restructuring costs, acquisition-related charges and other items.
Philips understands that Adjusted EBITDA is broadly used by analysts, rating agencies and investors in their evaluation of different companies because it excludes certain items that can vary widely across different industries or among companies within the same industry. Philips considers Adjusted EBITDA useful when comparing its performance to other companies in the HealthTech industry. However, Adjusted EBITDA may be subject to limitations as an analytical tool because of the range of items excluded and their significance in a given reporting period. Furthermore, comparisons with other companies may be complicated due to the absence of a standardized meaning and calculation framework. Philips management compensates for the limitations of using Adjusted EBITDA by using this measure to supplement IFRS results to provide a more complete understanding of the factors and trends affecting the business rather than IFRS results alone. In addition to the limitations noted above, Adjusted EBITDA excludes items that may be recurring in nature and should not be disregarded in the evaluation of performance. However, we believe it is useful to exclude such items to provide a supplemental analysis of current results and trends compared to other periods. This is because certain excluded items can vary significantly depending on specific underlying transactions or events. Also, the variability of such items may not relate specifically to ongoing operating results or trends and certain excluded items, while potentially recurring in future periods and may not be indicative of future results. Net income, for the years indicated is included in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2023 | |||||
| Net Income | (463) | ||||
| Discontinued operations, net of income taxes | 10 | ||||
| Income taxes | (73) | ||||
| Investments in associates, net of income taxes | 98 | ||||
| Financial expenses | 376 | ||||
| Financial income | (63) | ||||
| Income from operations | (115) | 720 | (1,199) | 552 | (188) |
| Amortization and impairment of acquired intangible assets | 290 | 89 | 178 | 14 | 9 |
| Impairment of goodwill | 8 | 8 | - | ||
| EBITA | 183 | 816 | (1,020) | 567 | (179) |
| Restructuring and acquisition-related charges | 381 | 118 | 115 | 9 | 140 |
| Other items: | 1,358 | 92 | 1,275 | 22 | (32) |
| Respironics litigation provision | 575 | 575 | |||
| Respironics field-action connected to the proposed consent decree | 363 | 363 | |||
| Respironics field-action running remediation costs | 224 | 224 | |||
| Quality remediation actions | 175 | 81 | 94 | ||
| Provision for a legal matter | 31 | 31 | |||
| Investment re-measurement loss | 23 | 23 | |||
| Gain on divestment of business | (35) | (35) | |||
| Remaining items | 2 | 11 | (12) | (1) | 3 |
| Adjusted EBITA | 1,921 | 1,026 | 369 | 597 | (71) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 971 | 217 | 267 | 101 | 385 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (47) | (4) | (14) | (30) | |
| Adjusted EBITDA | 2,845 | 1,239 | 623 | 698 | 284 |
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2022 | |||||
| Net Income | (1,605) | ||||
| Discontinued operations, net of income taxes | (13) | ||||
| Income taxes | (113) | ||||
| Investments in associates, net of income taxes | 2 | ||||
| Financial expenses | 258 | ||||
| Financial income | (58) | ||||
| Income from operations | (1,529) | 538 | (2,347) | 515 | (235) |
| Amortization and impairment of acquired intangible assets | 363 | 115 | 226 | 15 | 8 |
| Impairment of goodwill | 1,357 | 1,357 | |||
| EBITA | 192 | 652 | (764) | 531 | (227) |
| Restructuring and acquisition-related charges | 202 | 3 | 125 | 11 | 62 |
| Other items: | 925 | 133 | 750 | (4) | 46 |
| Respironics field-action connected to the proposed consent decree | 250 | 250 | |||
| Respironics field-action running remediation costs | 210 | 210 | |||
| R&D project impairments | 134 | 73 | 59 | 3 | |
| Portfolio realignment charges | 109 | 109 | |||
| Impairments of assets in S&RC | 39 | 39 | |||
| Provision for public investigations tender irregularities | 60 | 60 | |||
| Quality remediation actions | 59 | 59 | |||
| Remaining items | 63 | - | 24 | (6) | 46 |
| Adjusted EBITA | 1,318 | 788 | 111 | 538 | (119) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 1,239 | 302 | 420 | 117 | 400 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (252) | (83) | (136) | (3) | (30) |
| Adjusted EBITDA | 2,305 | 1,008 | 394 | 652 | 250 |
Philips Group
Reconciliation of Net income to Adjusted EBITA and Adjusted EBITDA
in millions of EUR
| Philips Group | Diagnosis & Treatment | Connected Care | Personal Health | Other | |
|---|---|---|---|---|---|
| 2021 | |||||
| Net Income | 3,323 | ||||
| Discontinued operations, net of income taxes | (2,711) | ||||
| Income taxes | (103) | ||||
| Investments in associates, net of income taxes | 4 | ||||
| Financial expenses | 188 | ||||
| Financial income | (149) | ||||
| Income from operations | 553 | 948 | (716) | 576 | (255) |
| Amortization and impairment of acquired intangible assets | 322 | 142 | 158 | 15 | 6 |
| Impairment of goodwill | 15 | 2 | 13 | ||
| EBITA | 890 | 1,092 | (545) | 591 | (248) |
| Restructuring and acquisition-related charges | 95 | (30) | 130 | (1) | (5) |
| Other items: | 1,069 | (35) | 968 | - | 136 |
| Respironics field-action connected to the proposed consent decree | 719 | 719 | |||
| Respironics field-action running remediation costs | 94 | 94 | |||
| Quality remediation actions | 94 | 94 | |||
| Loss on divestment of business | 76 | 76 | |||
| Remaining items | 87 | (35) | 61 | - | 61 |
| Adjusted EBITA | 2,054 | 1,028 | 553 | 590 | (117) |
| Depreciation, amortization and impairment of fixed assets and other intangible assets | 1,001 | 221 | 314 | 116 | 351 |
| Adding back impairment of fixed assets included in Restructuring and acquisition-related charges and Other items | (70) | (4) | (68) | - | 2 |
| Adjusted EBITDA | 2,985 | 1,245 | 799 | 706 | 235 |
The term Adjusted income from continuing operations attributable to shareholders represents income from continuing operations less continuing operations non-controlling interests, amortization and impairment of acquired intangible assets, impairment of goodwill, excluding gains or losses from restructuring costs and acquisition-related charges, other items, adjustments to net finance expenses, adjustments to investments in associates and adjustments to tax expense. Shareholders refers to shareholders of Koninklijke Philips N.V.
Restructuring costs, acquisition-related charges and other items are all defined in the EBITA and Adjusted EBITA section above.
Net finance expenses are defined as either the financial income or expense component of an individual item already identified to be excluded as part of the Adjusted income from continuing operations, fair value movements of equity investments in limited life funds recognized at fair value through profit or loss or a financial income or expense component with an income statement impact (gain or loss) that is deemed by management to be both significant and incidental to normal business activity.
The adjustments to tax expense include the tax impact of the adjustments to income from continuing operations as well as tax only adjusting items, and uses the Weighted Average Statutory Tax Rate plus any recurring tax costs or benefits.
Philips considers the use of Adjusted income from continuing operations attributable to shareholders appropriate as Philips uses it as the basis for the Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted, a non-IFRS measure.
Adjusted income from continuing operations attributable to shareholders may be subject to limitations as an analytical tool for investors, as it excludes certain items and therefore does not reflect the expense associated with such items, which may be significant and have a significant effect on Philips’ net income. Net income, for the years indicated is included in the following table. Net income is not allocated to segments as certain income and expense line items are monitored on a centralized basis, resulting in them being shown on a Philips Group level only.
Adjusted income from continuing operations attributable to shareholders is not a recognized measure of financial performance under IFRS. The reconciliation of Adjusted income from continuing operations attributable to shareholders to the most directly comparable IFRS measure, Net income, for the years indicated is included in the following table.
Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted (Adjusted EPS)Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted is calculated by dividing the Adjusted income from continuing operations attributable to shareholders by the diluted weighted average number of shares (after deduction of treasury shares) outstanding during the period, as defined in General information to the Consolidated financial statements, earnings per share section.
Philips considers the use of Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted appropriate as it is a measure that is useful when comparing its performance to other companies in the HealthTech industry. However, it may be subject to limitations as an analytical tool for investors, as it uses Adjusted income from continuing operations attributable to shareholders which has certain items excluded.
Adjusted income from continuing operations attributable to shareholders per common share (in EUR) - diluted is not a recognized measure of financial performance under IFRS. The most directly comparable IFRS measure, income from continuing operations attributable to shareholders per common share (in EUR) - diluted for the years indicated, is included in the following table.
Philips Group
Adjusted income from continuing operations attributable to shareholders1)
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net income | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (2,711) | (13) | 10 |
| Income from continuing operations | 612 | (1,618) | (454) |
| Income from continuing operations attributable to non-controlling interests | (4) | (3) | (2) |
| Income from continuing operations attributable to shareholders1) | 608 | (1,622) | (456) |
| Adjustments for: | |||
| Amortization and impairment of acquired intangible assets | 322 | 363 | 290 |
| Impairment of goodwill | 15 | 1,357 | 8 |
| Restructuring costs and acquisition-related charges | 95 | 202 | 381 |
| Other items: | 1,069 | 925 | 1,358 |
| Respironics litigation provision | 575 | ||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 |
| Respironics field-action running remediation costs | 94 | 210 | 224 |
| Quality remediation actions | 94 | 59 | 175 |
| R&D project impairments | 134 | ||
| Portfolio realignment charges | 109 | ||
| Impairment of assets in S&RC | 39 | ||
| Provision for public investigations tender irregularities | 60 | ||
| Provision for a legal matter | 31 | ||
| Investment re-measurement loss | 23 | ||
| Loss (gain) on divestment of business | 76 | (35) | |
| Remaining items | 87 | 63 | 2 |
| Net finance income/expenses | (84) | (4) | 18 |
| Tax impact of adjusted items and tax only adjusting items | (527) | (376) | (450) |
| Adjusted Income from continuing operations attributable to shareholders1) | 1,497 | 845 | 1,148 |
| Earnings per common share: | |||
| Income from continuing operations attributable to shareholders1) per common share (in EUR) - diluted | 0.64 | (1.76) | (0.50) |
| Adjusted income from continuing operations attributable to shareholders1) per common share (in EUR) - diluted | 1.58 | 0.92 | 1.25 |
Free cash flow is defined as net cash flows from operating activities minus net capital expenditures. Net capital expenditures are comprised of the purchase of intangible assets, expenditures on development assets, capital expenditures on property, plant and equipment and proceeds from sales of property, plant and equipment.
Philips discloses free cash flow as a supplemental non-IFRS financial measure, as Philips believes it is a meaningful measure to evaluate the performance of its business activities over time. Philips understands that free cash flow is broadly used by analysts, rating agencies and investors in assessing its performance. Philips also believes that the presentation of free cash flow provides useful information to investors regarding the cash generated by the Philips operations after deducting cash outflows for purchases of intangible assets, capitalization of product development, expenditures on development assets, capital expenditures on property, plant and equipment and proceeds from disposal of property, plant and equipment. Therefore, the measure gives an indication of the long-term cash generating ability of the business. In addition, because free cash flow is not impacted by purchases or sales of businesses and investments, it is generally less volatile than the total of net cash provided by (used for) operating activities and net cash provided by (used for) investing activities.
Free cash flow may be subject to limitations as an analytical tool for investors, as free cash flow is not a measure of cash generated by operations available exclusively for discretionary expenditures and Philips requires funds in addition to those required for capital expenditures for a wide variety of non-discretionary expenditures, such as payments on outstanding debt, dividend payments or other investing and financing activities. In addition, free cash flow does not reflect cash payments that may be required in future for costs already incurred, such as restructuring costs.
Philips Group
Composition of free cash flow
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net cash flows provided by operating activities | 1,629 | (173) | 2,136 |
| Net capital expenditures: | (729) | (788) | (554) |
| Purchase of intangible assets | (107) | (105) | (96) |
| Expenditures on development assets | (259) | (257) | (203) |
| Capital expenditures on property, plant and equipment | (397) | (444) | (345) |
| Proceeds from disposals of property, plant and equipment | 33 | 18 | 90 |
| Free cash flow | 900 | (961) | 1,582 |
Net debt : group equity ratio is presented to express the financial strength of Philips. Net debt is defined as the sum of long- and short-term debt minus cash and cash equivalents. Group equity is defined as the sum of shareholders’ equity and non-controlling interests. This measure is used by Philips Treasury management and investment analysts to evaluate financial strength and funding requirements. This measure may be subject to limitations because cash and cash equivalents are used for various purposes, not only debt repayment. The net debt calculation deducts all cash and cash equivalents whereas these items are not necessarily available exclusively for debt repayment at any given time.
Philips Group
Composition of net debt to group equity
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Long-term debt | 6,473 | 7,270 | 7,035 |
| Short-term debt | 506 | 931 | 654 |
| Total debt | 6,980 | 8,201 | 7,689 |
| Cash and cash equivalents | 2,303 | 1,172 | 1,869 |
| Net debt | 4,676 | 7,028 | 5,820 |
| Shareholders' equity | 14,438 | 13,249 | 12,028 |
| Non-controlling interests | 36 | 34 | 33 |
| Group equity | 14,475 | 13,283 | 12,061 |
| Net debt : group equity ratio | 24:76 | 35:65 | 33:67 |
Organic Return on Invested Capital (ROIC) is defined as organic return which includes income from operations for the year excluding the impact of: Income or Loss from operations of businesses acquired in the five year period prior to the measurement date; certain tax gains and losses determined by management to be material in nature and require separate disclosure and; certain other items; and tax effects of the other adjustments (calculated at group effective tax rate) divided by average of the Net operating capital at the end of each of the five quarters ending on the relevant measurement date excluding the average net operating capital at the end of each of the five quarters ending on the relevant measurement date of the businesses acquired in the five year period prior to the measurement date, expressed as a percentage.
Net operating capital is defined as tangible fixed assets, intangible fixed assets, including goodwill, inventories and receivable balances, minus payable balances and provisions, all as further defined below. Net operating capital is adjusted to exclude assets and liabilities of businesses acquired in the five year period prior to the relevant measurement date, and adjustments determined by management to be necessary for comparability.
Other items are defined as material in nature and require separate disclosure and have the same nature as the items excluded from Adjusted EBITA. In the years 2020-2022 these other items included legal provisions, pension settlements, results of divestments, remediation costs, impairment of assets and portfolio realignment charges. Refer to Net income, Income from operations (EBIT) and Adjusted EBITA within the Results of operations section of Financial performance. Organic ROIC is calculated after taxes.
The term Organic Return on Invested Capital (ROIC) is used by management to evaluate Philips’ efficiency at allocating the capital under its control to profitable investments and how well the company uses capital to generate returns. Philips believes that Organic ROIC provides useful information to investors because it excludes the impact of recently acquired businesses, giving a more accurate representation of how the Philips Business System is leveraged to drive operational excellence and removes irregularity caused by various operating models of recently acquired businesses. Philips also believes that excluding certain items determined by management to be material in nature and requiring separate disclosure enhances comparability across several periods. Organic ROIC may be subject to limitations as an analytical tool for investors, as it excludes Income or Loss from operations of acquired businesses and tax gains and losses and certain other items, which may have a significant effect on ROIC. Organic ROIC is not a recognized measure of financial performance under IFRS.
The most comparable IFRS measure to Organic ROIC is Return on total assets, calculated as Income from operations for the year divided by total assets as of the end of the year. Return on total assets as of the balance sheet date for the years ended December 31, 2020, 2021 and 2022 is included in the following table.
Philips Group
Return on total assets
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Income from operations | 553 | (1,529) | (115) |
| Total assets | 30,961 | 30,688 | 29,406 |
| Return on total assets (%) | 1.8% | (5.0)% | (0.4)% |
The reconciliation of Average Net operating capital and the reconciliation of Net income to Organic ROIC for the years ended December 31, 2020, 2021 and 2022 are included in the following tables.
Philips Group
Reconciliation of Average Net operating capital1)
in millions of EUR
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Tangible fixed assets | 2,716 | 2,715 | 2,553 |
| Intangible assets (including goodwill) | 13,454 | 14,684 | 13,475 |
| Inventories | 3,248 | 3,999 | 3,984 |
| Receivable balances2) | 4,648 | 5,043 | 4,981 |
| Payable balances3) | (6,627) | (7,129) | (6,810) |
| Provisions4) | (2,178) | (2,313) | (2,420) |
| Group Average Net operating capital | 15,261 | 16,999 | 15,763 |
| Net operating capital of businesses acquired | (5,511) | (5,739) | (4,081) |
| Average Net operating capital | 9,750 | 11,260 | 11,681 |
Philips Group
Reconciliation of Net Income to Organic ROIC
in millions of EUR unless otherwise stated
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Net Income | 3,323 | (1,605) | (463) |
| Discontinued operations, net of income taxes | (2,711) | (13) | 10 |
| Income taxes | (103) | (113) | (73) |
| Investments in associates, net of income taxes | 4 | 2 | 98 |
| Financial expenses | 188 | 258 | 350 |
| Financial income | (149) | (58) | (36) |
| Income from operations | 553 | (1,529) | (115) |
| Loss from operations of businesses acquired | 124 | 178 | 253 |
| Tax gains and losses | (197) | (169) | (140) |
| Goodwill impairment | 15 | 1,357 | 8 |
| Other items: | 872 | 802 | 1,181 |
| Respironics litigation provision | 575 | ||
| Respironics field-action connected to the proposed consent decree | 719 | 250 | 363 |
| Respironics field-action running remediation costs | 94 | 210 | 224 |
| R&D project impairments | 134 | ||
| Portfolio realignment charges | 109 | ||
| Impairment of assets in S&RC | 39 | ||
| Provision for specified legal matters | (17) | 60 | 31 |
| Investment re-measurement loss | 23 | ||
| Loss (gain) on divestment of business | 76 | (35) | |
| Income taxes | 103 | 113 | 73 |
| Tax effects of other adjustments | (33) | (45) | (56) |
| Organic return | 1,437 | 707 | 1,204 |
| Average Net operating capital | 9,750 | 11,260 | 11,681 |
| Organic ROIC (%) | 14.7% | 6.3% | 10.3% |
In addition to monitoring the IFRS and non-IFRS financial measures discussed under Financial performance, Philips’ management also uses the following other key performance indicators to monitor the performance of the business and to manage the business. Comparative results have been restated to reflect the treatment of the Domestic Appliances business as a discontinued operation (for more information, please refer to Discontinued operations and assets classified as held for sale).
Philips Group
Other Key Performance Indicators
| 2021 | 2022 | 2023 | |
|---|---|---|---|
| Lives improved, in billions | 1.67 | 1.81 | 1.88 |
| Operational carbon footprint, in kilotonnes CO2-equivalent | 519 | 438 | 418 |
| Circular revenue | 16.0% | 18.1% | 20.0% |
| Waste to landfill | 0.1% | 0.0% | 0.0% |
| Closing the Loop | 34.0% | 35.3% | 20.5% |
| Comparable order intake | 4% | (3)% | (5)% |
Lives Improved
The purpose of Philips is to improve people’s health and well-being through meaningful innovation and we aim to improve the lives of 2 billion people a year by 2025, including 300 million in underserved communities, rising to 2.5 billion and 400 million respectively by 2030. We use Lives Improved as a measurement of our societal impact. In the course of 2021 we changed the definition of ‘lives improved’ (effective January 2021) to align more closely with our purpose. The new definition includes only products or solutions that contribute to people’s health and well-being, and no longer includes the contribution from our Green Products and Solutions that support a healthy ecosystem. Additionally, as we discontinued our Domestic Appliances business, we have removed the impact of this business from the Lives Improved results. The combined impact of these changes resulted in an overall drop of 223 million lives improved in 2021. We calculate Lives Improved as the number of individual interactions for each product sold (based on market intelligence and statistical data) and multiply by the number of those products delivered in a year (eliminating double counting for multiple different product touches per individual). See Improving people’s lives for more information on Lives Improved.
Operational Carbon Footprint
We aim to minimize our environmental impact and we use the Operational Carbon Footprint as one of the measurements of our impact. We define Operational Carbon Footprint as the total greenhouse gas emissions caused by an organization, event, product or person; expressed in kilotonnes CO2-equivalent. We calculate our Operational Carbon Footprint on a monthly basis and include industrial sites (manufacturing and assembly sites), non-industrial sites (offices, warehouses, IT centers and R&D facilities), business travel (lease and rental cars and airplane travel) and logistics (air, sea and road transport) See Sustainable Operations for more information on our Operational Carbon Footprint.
Circular Revenues
Circular Revenues are revenues from Philips products, services and solutions that contribute to circular practices. Propositions that qualify for circular revenues must comply with the requirements for at least one of the circular revenue categories. These include, among others, products with low weight or containing a minimum threshold of recycled or bio-based plastics, as-a-service models, software running in the cloud, telehealth, upgrades, lifetime extensions, and refurbished equipment or components.
Waste to Landfill
At Philips, as a responsible company, we strive to reduce our environmental impact. We define Waste to Landfill as total waste that is delivered for landfill and exclude one-time-only waste and waste delivered to landfill due to regulatory requirements. We calculate Waste to Landfill in kilotonnes per year. See Sustainable Operations for more information on Waste to Landfill.
Closing the Loop
Closing the loop means we are embedding a policy to responsibly take back all professional medical equipment sold directly to customers as part of a trade-in offer or as a service at customer request. As part of the policy, we will ensure that equipment coming back to us is, where feasible, made available for refurbishment and/or parts recovery, or locally recycled in a certified way to ensure it does not end up in landfill. We monitor the impact of our policies by measuring the amount of equipment that we collect from our customers. We report on this as ‘reclaimed equipment’.
Philips believes that the five other key performance indicators described above (Lives Improved, Operational Carbon Footprint, Circular Revenues, Waste to Landfill and Closing the Loop) provide important information to investors and are important to understanding the long-term performance and prospects of the business. In addition, these other key performance indicators are also used for management compensation purposes. Members of the Board of Management are eligible for grants of performance shares under the Long-Term Incentive (LTI) Plan, and the vesting of the performance shares is subject to performance over a period of 3 years and based on certain criteria, including a 10% weighting for Sustainability Objectives, which Philips defines as the five other key performance indicators described above: Lives Improved, Carbon Footprint, Circular Revenues, Waste to Landfill and Closing the Loop. Philips believes that including these other key performance indicators in our remuneration policy encourages management to act responsibly and sustainably, supporting the company’s overall performance and enhancing the long-term value of the company. See Remuneration of the Board of Management in 2023 for more information on the Philips’ Long-Term Incentive (LTI) Plan.
Philips currently intends to propose a 2024 Remuneration Policy for the Board of Management which would, among other things, provide for the vesting of performance shares subject to performance over a period of 3 years and based on certain criteria, including a 20% weighting for Sustainability Objectives, which would be defined under that plan as: Lives Improved, Carbon Footprint, Circular Revenues and Employee Engagement Score. The 2024 Remuneration Policy is subject to the approval of Philips shareholders at the 2024 AGM. See Remuneration of the Board of Management in 2023, starting on page 115 for more information on the Philips’ Long-Term Incentive (LTI) Plans under the 2020 Remuneration Policy and the currently proposed 2024 Remuneration Policy.
Comparable order intake
Comparable order intake represents the period-on-period growth, expressed as a percentage, in order intake excluding the effects of currency movements and changes in consolidation. Comparable order intake is reported for equipment and software in the Diagnoses & Treatment and Connected Care segments, and is defined as the total contractually committed value of equipment and software to be delivered within a specified timeframe, and is an approximation of expected future revenue growth in the respective businesses. Comparable order intake does not derive from the financial statements and a quantitative reconciliation is thus not provided. In 2023, comparable order book was tracked for businesses that represented approximately 40% of 2023 sales.
Philips has simplified its order intake policy by aligning horizons for all modalities to 18 months to revenue. Order intake for software contracts corresponds to the same 18 months to revenue horizon, meaning that only the next 18 months conversion to revenue under the contract is recognized. Philips believes this policy eliminates major variances in order intake growth and better reflects expected revenue in the short term from order intake booked in the reporting period.
Philips uses comparable order intake as an indicator of business activity and performance. Comparable order intake is not an alternative to revenue and may be subject to limitations as an analytical tool due to differences in amount and timing between booking orders and revenue recognition. Due to divergence in practice, other companies may calculate this or a similar measure (such as order backlog) differently and therefore comparisons between companies may be complicated.
The statements below are only a general summary of certain material Dutch tax consequences for holders of common shares that are non-residents of the Netherlands based on Dutch tax laws, presently in force, and the Tax Convention of December 18, 1992, as amended by the protocol that entered into force on December 28, 2004, between the United States of America and the Kingdom of the Netherlands (the US Tax Treaty) and are not to be read as extending by implication to matters not specifically referred to herein. As to individual tax consequences, investors in common shares should consult their own professional tax advisor.
With respect to a holder of common shares that is an individual who receives income or derives capital gains from common shares and this income received or capital gains derived are attributable to past, present or future employment activities of such holder, the income of which is taxable in the Netherlands, the Dutch tax position is not discussed in this summary.
Dividend withholding taxIn general, a distribution to shareholders by a company resident in the Netherlands (such as the company) is subject to a withholding tax imposed by the Netherlands at a rate of 15%. Share dividends paid out of the company’s paid-in share premium recognized for Dutch tax purposes are not subject to the abovementioned withholding tax. Share dividends paid out of the company’s retained earnings are subject to dividend withholding tax on the nominal value of the shares issued.
Relief at source is available to certain qualifying corporate holders of common shares if such common shares are attributable to a business carried out in the Netherlands, provided that such holder demonstrates that it is the beneficial owner of the dividend. Relief at source is available for dividend distributions to certain qualifying corporate holders of common shares resident in EU/EEA member states, and to certain qualifying corporate holders of common shares resident in non-EU/EEA states with which the Netherlands has concluded a tax treaty that includes a dividend article, provided that such holder demonstrates that it is the beneficial owner of the dividend unless such holder holds the common shares of the company with the primary aim or one of the primary aims to avoid the levy of Dutch dividend withholding tax from another person and the shareholding is put in place without valid commercial reasons that reflect economic reality.
Upon request and under certain conditions, certain qualifying non-resident individual and corporate holders of common shares resident in EU/EEA member states or in a qualifying non-EU/EEA state may be eligible for a refund of Dutch dividend withholding tax to the extent that the withholding tax levied is higher than the personal and corporate income tax which would have been due if they were resident in the Netherlands. However, this refund is not applicable when, based on the US Tax Treaty, the Dutch dividend withholding tax can be fully credited in the United States by the US holder.
Pursuant to the provisions of the US Tax Treaty, a reduced rate may be applicable in respect of dividends paid by the company to a beneficial owner holding directly 10% or more of the voting power of the company, if such owner is a company resident in the United States (as defined in the US Tax Treaty) and entitled to the benefits of the US Tax Treaty.
Pursuant to Dutch anti-dividend stripping legislation, a holder of common shares who is the recipient of dividends will in any case not be considered the beneficial owner of the dividends if (i) as a consequence of a combination of transactions, a person other than the recipient benefits, in full or in part, directly or indirectly, from the dividends; (ii) whereby such other person retains, directly or indirectly, an interest similar to that in the common shares on which the dividends were paid; and (iii) that other person is entitled to a credit, reduction or refund of dividend withholding tax that is less than that of the recipient.
Dividends paid to qualifying exempt US pension trusts and qualifying exempt US organizations are, under certain conditions, exempt from Dutch withholding tax under the US Tax Treaty. Qualifying exempt US pension trusts normally remain subject to withholding at the rate of 15% and are required to file for a refund of the tax withheld. Only if certain conditions are fulfilled, such pension trusts may be eligible for relief at source upon payment of the dividend. However, for qualifying exempt US organizations no relief at source upon payment of the dividend is currently available; such exempt US organizations should apply for a refund of the 15% withholding tax withheld. Further, under certain circumstances, certain exempt organizations (e.g. pension funds) may be eligible for a refund of Dutch withholding tax upon their request pursuant to Dutch tax law. From 1 January 2024 onwards, , provided certain conditions are met, such (US) organizations may be eligible for relief at source upon request.
The company may, with respect to certain dividends received from qualifying non-Dutch subsidiaries, credit taxes withheld from those dividends against the Dutch withholding tax imposed on certain qualifying dividends that are redistributed by the company, up to a maximum of the lesser of:
The reduction is applied to the Dutch dividend withholding tax that the company must pay to the Dutch tax authorities and not to the Dutch dividend withholding tax that the Company must withhold.
From 1 January 2024 onwards, in addition to Dutch dividend withholding tax, Dutch conditional withholding tax may apply at a statutory rate of 25.8% on dividends and other (deemed) distributions to certain affiliated (gelieerde) entities of the company for the purpose of the Dutch Withholding Tax Act 2021 (Wet bronbelasting 2021).
The Dutch conditional withholding tax only applies on dividends and other (deemed) distributions to entities that are resident (gevestigd), or have a permanent establishment to which the dividend or distribution is attributable, in a jurisdiction that is listed in the Dutch Regulation on low-taxing states and non-cooperative jurisdictions for tax purposes (Regeling laagbelastende staten en niet-coöperatieve rechtsgebieden voor belastingdoeleinden), and in certain deemed abusive situations.
An entity is generally affiliated within the meaning of the Dutch Withholding Tax Act 2021 if there is a controlling relationship between such entity and the distributing company.
Income and capital gainsIncome and capital gains derived from the common shares by a non-resident individual or non-resident corporate shareholder are generally not subject to Dutch income or corporation tax, unless (i) such income and gains are attributable to a (deemed) permanent establishment or (deemed) permanent representative of the shareholder in the Netherlands; or (ii) the shareholder is entitled to a share in the profits of an enterprise or (in the case of a non-resident corporate shareholder only) a co-entitlement to the net worth of an enterprise that is effectively managed in the Netherlands (other than by way of securities) and to which enterprise the common shares are attributable; or (iii) such income and capital gains are derived from a direct, indirect or deemed substantial participation in the share capital of the company (such substantial participation not being a business asset), and, in the case of a non-resident corporate shareholder only, it is being held with the primary aim or one of the primary aims to avoid the levy of income tax from another person and is put in place without valid commercial reasons that reflect economic reality; or (iv) in the case of a non-resident corporate shareholder, such shareholder is a resident of Aruba, Curacao or Saint Martin with a permanent establishment or permanent representative in Bonaire, Eustatius or Saba to which the common shares are attributable and certain conditions are met; or (v) in the case of a non-resident individual, such individual derives income or capital gains from the common shares that are taxable as benefits from ‘miscellaneous activities’ in the Netherlands (resultaat uit overige werkzaamheden, as defined in the Dutch Income Tax Act 2001), which includes the performance of activities with respect to the common shares that exceed regular portfolio management.
In general, a holder of common shares has a substantial participation if he holds either directly or indirectly and either independently or jointly with his partner (as defined in the Dutch Income Tax Act 2001), the ownership of, or certain other rights over, at least 5% of the total issued share capital or total issued particular class of shares of the company or rights to acquire direct or indirect shares, whether or not already issued, that represent at any time 5% or more of the total issued capital (or the total issued particular class of shares) or the ownership of certain profit participating certificates that relate to 5% or more of the annual profit or to 5% or more of the liquidation proceeds. A shareholder will also have a substantial participation in the company if one or more of certain relatives of the shareholder hold a substantial participation in the company. A deemed substantial participation amongst others exists if (part of) a substantial participation has been disposed of, or is deemed to have been disposed of, on a nonrecognition basis.
Estate and gift taxesNo estate, inheritance or gift taxes are imposed by the Netherlands on the transfer or deemed transfer of common shares by way of gift by or on the death of a shareholder if, at the time of the death of the shareholder or the gift of the common shares (as the case may be), such shareholder is not a (deemed) resident of the Netherlands.
Inheritance or gift taxes (as the case may be) are due, however, if such shareholder:
This section describes the material United States federal income tax consequences to a US holder (as defined below) of owning common shares. It applies only if the common shares are held as capital assets for United States federal income tax purposes. This discussion addresses only United States federal income taxation and does not discuss all of the tax consequences that may be relevant to a US holder in light of its individual circumstances, including foreign, state or local tax consequences, estate and gift tax consequences, and tax consequences arising under the Medicare contribution tax on net investment income or the alternative minimum tax. This section does not apply to a member of a special class of holders subject to special rules, including:
This section is based on the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations, published rulings and court decisions, all as currently in effect, as well as on the US Tax Treaty. These authorities are subject to change, possibly on a retroactive basis.
If an entity or arrangement that is treated as a partnership for United States federal income tax purposes holds the common shares, the United States federal income tax treatment of a partner will generally depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding the common shares should consult its tax advisor with regard to the United States federal income tax treatment of an investment in the common shares.
A US holder is defined as a beneficial owner of common shares that is, for United States federal income tax purposes:
A US holder should consult its own tax advisor regarding the United States federal, state and local tax consequences of owning and disposing of common shares in its particular circumstances.
The tax treatment of common shares will depend in part on whether or not we are classified as a passive foreign investment company, or PFIC, for United States federal income tax purposes. Except as discussed below under “—PFIC Rules”, this discussion assumes that we are not classified as a PFIC for United States federal income tax purposes.
Taxation of DistributionsUnder the United States federal income tax laws, the gross amount of any distribution paid in stock or cash out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes), other than certain pro-rata distributions of our common shares, will be treated as a dividend that is subject to United States federal income taxation. For a non-corporate US holder, dividends paid that constitute qualified dividend income will be taxable at the preferential rates applicable to long-term capital gains, provided that the non-corporate US holder holds the common shares for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and provided it meets other holding period requirements. Dividends paid with respect to the common shares generally will be qualified dividend income provided that, in the year in which the dividend is received, the common shares are readily tradable on an established securities market in the United States. Our common shares are listed on the New York Stock Exchange and we therefore expect that dividends will be qualified dividend income. A US holder must include any Dutch tax withheld from the dividend payment in this gross amount even though it does not in fact receive it. The dividend is taxable to a US holder when it receives the dividend, actually or constructively. The dividend will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations. For dividend payments made in euro, the amount of the dividend distribution that a US holder must include in its income will be the US dollar value of the euro payments made, determined at the spot euro/US dollar rate on the date the dividend is distributed, regardless of whether the payment is in fact converted into US dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend is distributed to the date a US holder converts the payment into US dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. Distributions in excess of current and accumulated earnings and profits, as determined for United States federal income tax purposes, will be treated as a non-taxable return of capital to the extent of a US holder’s basis in the common shares and thereafter as capital gain. However, we do not expect to calculate earnings and profits in accordance with United States federal income tax principles. Accordingly, US holders should expect to generally treat distributions we make as dividends.
Subject to certain limitations (including, but not limited to, those described in this paragraph), the Dutch tax withheld in accordance with the US Tax Treaty and paid over to the Netherlands will be creditable or deductible against a US holder’s United States federal income tax liability. However, the Dutch withholding tax may not be creditable or deductible to the extent that we reduce (as described above under “Dutch taxation - Dividend withholding tax”) the amount of withholding tax paid over to the Netherlands by crediting taxes withheld from certain dividends received by us. In addition, special rules apply in determining the foreign tax credit limitation with respect to dividends that are subject to the preferential tax rates. To the extent reduction or refund of the tax withheld is available under Dutch law, or under the US Tax Treaty, the amount of tax withheld that could have been reduced or that is refundable will not be eligible for credit against United States federal income tax liability. Dividends will generally be income from sources outside the United States, and will generally be “passive” income for purposes of computing the foreign tax credit allowable to the holder. In addition, to the extent an amount of Dutch tax withheld is contingent on the availability of a credit against the amount of income tax owed to another country, that amount of Dutch tax withheld will not be eligible for a credit against the US holder’s United States federal income tax liability.
Taxation of Capital GainsA US holder that sells or otherwise disposes of its common shares will recognize capital gain or loss for United States federal income tax purposes equal to the difference between the US dollar value of the amount that it realizes and its tax basis, determined in US dollars, in its common shares. Capital gain of a non-corporate US holder is generally taxed at preferential rates where the property is held more than one year. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
Passive Foreign Investment Company RulesWe believe that the common shares should currently not be treated as stock of a PFIC for United States federal income tax purposes, and we do not expect to become a PFIC in the foreseeable future. However, this conclusion is a factual determination that is made annually and thus may be subject to change. It is therefore possible that we could become a PFIC in a future taxable year. If we are treated as a PFIC, gain realized on the sale or other disposition of the common shares would in general not be treated as capital gain. Instead, unless a US holder elects to be taxed annually on a mark-to-market basis with respect to the common shares, a US holder would generally be treated as if it had realized such gain and certain “excess distributions” ratably over the holding period for the common shares and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, in addition to which an interest charge in respect of the tax attributable to each such year would apply. Any dividends received by a US holder will not be eligible for the special tax rates applicable to qualified dividend income if we are treated as a PFIC with respect to such US holder either in the taxable year of the distribution or the preceding taxable year, but instead will be taxable at rates applicable to ordinary income and subject to the excess distribution regime described above.
Financial calendar
| Annual General Meeting of Shareholders | |
| Record date 2024 AGM | April 9, 2024 |
| 2024 AGM | May 7, 2024 |
| Quarterly reports1) | |
| First quarter results 2024 | April 29, 2024 |
| Second quarter results 2024 | July 29, 2024 |
| Third quarter results 2024 | October 28, 2024 |
| Fourth quarter results 2024 | February 3, 2025 |
The Agenda and the explanatory notes to the Agenda for the Annual General Meeting of Shareholders on May 7, 2024, will be published on the company’s website.
Those persons who, on that date, hold shares in the company, and are registered as such in one of the registers designated by the Board of Management for the Annual General Meeting of Shareholders, will be entitled to participate in, and vote at, the meeting.
In the context of the Respironics recall, actionable registrations are those that contain the necessary information needed to complete the remediation and are not awaiting further information, including from patient registrants.
Artificial Intelligence (AI)Philips embraces the following formal definition of AI (source: European Commission High-Level Expert Group definition AI): Artificial intelligence (AI) systems are software (and possibly also hardware) systems designed by humans that, given a complex goal, act in the physical or digital dimension by perceiving their environment through data acquisition, interpreting the collected structured or unstructured data, reasoning on the knowledge, or processing the information, derived from this data and deciding the best action(s) to take to achieve the given goal.
AI systems can either use symbolic rules or learn a numeric model, and they can also adapt their behavior by analyzing how the environment is affected by their previous actions.
As a scientific discipline, AI includes several approaches and techniques, such as machine learning (of which deep learning and reinforcement learning are specific examples), machine reasoning (which includes planning, scheduling, knowledge representation and reasoning, search, and optimization), and robotics (which includes control, perception, sensors and actuators, as well as the integration of all other techniques into cyber-physical systems).
See also Philips AI Principles.
Brominated flame retardants (BFR)Brominated flame retardants are a group of chemicals that have an inhibitory effect on the ignition of combustible organic materials. Of the commercialized chemical flame retardants, the brominated variety are most widely used.
Business/Business unitIn the Philips Operating Model, our three operating segments are made up of six businesses, which are in turn comprised of 18 business units. See also the entry under Segment.
CO2-equivalentCO2-equivalent or carbon dioxide equivalent is a quantity that describes, for a given mixture and amount of greenhouse gas, the amount of CO2 that would have the same global warming potential (GWP), when measured over a specified timescale (generally 100 years).
Circular economyA circular economy aims to decouple economic growth from the consumption of natural resources by optimizing their use, eliminating waste and pollution, and circulating products and materials for as long as possible, while giving natural systems the opportunity to regenerate themselves.
Circular Materials ManagementCircular Materials Management is a KPI for promoting an increase in the proportion of waste treated using waste management hierarchy levels that are circular: prevention, re-use, and recycling. Circular Materials Management % is the proportion of materials managed circularly in comparison to the total used materials baseline. The total used materials baseline is the total of both circular and linear waste, excluding linear disposal of waste that is required by law. Circular Materials Management includes recycling, re-use, prevention and other recovery (e.g. repurposing). It excludes all linear disposal, which is classified as waste to energy, incineration and landfill.
Circular RevenuesCircular Revenues are revenues from Philips products, services and solutions that contribute to circular practices. Propositions that qualify for circular revenues must comply with the requirements for at least one of the circular revenue categories. These include, among others, products with low weight or containing a minimum threshold of recycled or bio-based plastics, as-a-service models, software running in the cloud, telehealth, upgrades, lifetime extensions, and refurbished equipment.
Closing the Loop / reclaimed equipmentClosing the loop means we are embedding a policy to responsibly take back all professional medical equipment sold directly to customers as part of a trade-in offer or as a service at customer request. As part of the policy, we will ensure that equipment coming back to us is, where feasible, made available for refurbishment and/or parts recovery, or locally recycled in a certified way to ensure it does not end up in landfill. We monitor the impact of our policies by measuring the amount of equipment that we collect from our customers. We report on this as ‘reclaimed equipment’.
Dividend yieldThe dividend yield is the annual dividend payment divided by Philips’ market capitalization. All references to dividend yield are as of December 31 of the previous year.
EcoHeroesPhilips’ ‘EcoHeroes’ concept aims to drive innovation beyond our EcoDesign requirements, delivering solutions that are demonstrably setting the pace in terms of environmental impact. An EcoHero product meets all EcoDesign requirements applicable to new product introductions and outperforms in at least one of the focal areas of EcoDesign (Energy, Packaging, Substances and Circularity).
Employee Engagement Index (EEI)The Employee Engagement Index (EEI) is the single measure of the overall level of employee engagement at Philips. It is a combination of perceptions and attitudes related to employee satisfaction, commitment and advocacy.
Energy-using Products (EuP)An energy-using product is a product that uses, generates, transfers or measures energy (electricity, gas, fossil fuel). Examples include boilers, computers, televisions, transformers, industrial fans and industrial furnaces.
FunctionsIn the Philips Operating Model, Philips' businesses are supported by lean Functions. The Functions deliver cost-effective services, ensure legal & regulatory requirements are deployed, and propose Enterprise policies, standards, guidance and infrastructure, as well as providing functional capabilities and expertise (e.g. via Centers of Excellence).
Full-time equivalent employee (FTE)Full-time equivalent is a way to measure a worker’s involvement in a project. An FTE of 1.0 means that the person is equivalent to a full-time worker, while an FTE of 0.5 signals that the worker works half-time.
Global Reporting Initiative (GRI)The Global Reporting Initiative (GRI) is a network-based organization that pioneered the world’s most widely used sustainability reporting framework. GRI is committed to the framework’s continuous improvement and application worldwide. GRI’s core goals include the mainstreaming of disclosure on environmental, social and governance performance.
Green/EcoDesigned InnovationGreen/EcoDesigned Innovation comprises all R&D activities directly contributing to the intended development of Green/EcoDesigned Products.
Green/EcoDesigned ProductsA Green/EcoDesigned Product must comply with all applicable legal requirements, Philips policies, and all stated EcoDesigned Product requirements in our four focal areas: Energy, Substances, Circularity and Packaging. The aim is to improve the energy efficiency of our products, use less resources and more recycled content, avoid the use of hazardous substances, design for circularity, and make our packaging easier to recycle and re-use.
Green/EcoDesigned RevenuesGreen/EcoDesigned Revenues are generated through products that meet the Green/EcoDesigned Products definition.
Growth geographiesGrowth geographies consists of the grouping 'Growth', which comprises the developing geographies Asia Pacific (excluding Japan, South Korea, Australia and New Zealand), Latin America, Central & Eastern Europe, Middle East & Turkey (excluding Israel) and Africa.
Hazardous substancesHazardous substances are generally defined as substances posing imminent and substantial danger to public health and welfare or the environment.
Income from operations (EBIT)Income from operations as reported on the IFRS consolidated statement of income. The term EBIT (earnings before interest and tax) has the same meaning as Income from operations.
Income from continuing operationsIncome from continuing operations as reported on the IFRS consolidated statement of income, which is net income from continuing operations, or net income excluding discontinued operations.
LeanThe basic insight of Lean thinking is that if every person is trained to identify wasted time and effort in their own job and to better work together to improve processes by eliminating such waste, the resulting enterprise will deliver more value at less expense.
Lives improved by PhilipsTo calculate how many lives we are improving, market intelligence and statistical data on the number of people touched by the products contributing to the social or ecological dimension over the lifetime of a product are multiplied by the number of those products delivered in a year. After elimination of double counts – multiple different product touches per individual are only counted once – the number of lives improved by our innovative solutions is calculated.
Long-term strategic partnershipMulti-year contractual agreement that represents a partnership to enable long-term collaboration.
Mature geographiesMature geographies are the highly developed markets constituting three geographic areas: Western Europe, North America, and Other mature (including Japan, South Korea, Israel, Australia and New Zealand).
Net Promoter ScoreNet Promoter Score®, or NPS®, measures customer experience and predicts business growth. NPS is calculated by taking the answer to a key question on a 0-10 scale: How likely is it that you would recommend [brand] to a friend or colleague?
Respondents are grouped as follows:
Subtracting the percentage of Detractors from the percentage of Promoters yields the Net Promoter Score, which can range from a low of -100 (if every customer is a Detractor) to a high of 100 (if every customer is a Promoter).
Operational carbon footprintA carbon footprint is the total set of greenhouse gas emissions caused by an organization, event, product or person; usually expressed in kilotonnes CO2-equivalent. Philips' operational carbon footprint is calculated on a monthly basis and includes industrial sites (manufacturing and assembly sites), non-industrial sites (offices, warehouses, IT centers and R&D facilities), business travel (lease and rental cars and airplane travel) and logistics (air, sea and road transport).
Philips Lighting/SignifyReferences to 'Signify' in this Annual Report relate to Philips' former Lighting segment (prior to deconsolidation as from the end of November 2017 and when reported as discontinued operations), Philips Lighting N.V. (before or after such deconsolidation) or Signify N.V. (after its renaming in May 2018), as the context requires.
Polyvinyl chloride (PVC)Polyvinyl chloride, better known as PVC or vinyl, is an inexpensive plastic so versatile it has become completely pervasive in modern society.
Quadruple AimAt Philips, we make value-based care principles actionable by addressing the Quadruple Aim – better health outcomes, improved patient experience, improved staff experience, and lower cost of care.
REACHRegistration, Evaluation, Authorization and Restriction of Chemicals (REACH;Regulation (EC) No 1907/2006) is a European Union regulation that addresses the production and use (e.g. in products) of chemical substances, and their potential impact on both human health and the environment. This regulation is covered in the Philips Regulated Substances List.
Regulated Substance ListPhilips Regulated Substances List (RSL) combines legal, industry, and voluntary Philips requirements regarding chemical substances used in Philips products and their packaging, either on a homogenous material level or present in the product as such. The RSL contains restricted and declarable substances.
Respironics recallThe voluntary recall notification in the United States and field safety notice outside the United States for certain sleep and respiratory care products initiated by Philips Respironics in 2021.
Responsible Business Alliance (RBA)The Responsible Business Alliance (formerly known as The Electronic Industry Citizenship Coalition (EICC)) was established in 2004 to promote a common code of conduct for the electronics and information and communications technology (ICT) industry. EICC now includes more than 100 global companies and their suppliers.
Restriction on Hazardous Substances (RoHS)The RoHS Directive prohibits all new electrical and electronic equipment placed on the market in the European Economic Area from containing lead, mercury, cadmium, hexavalent chromium, poly-brominated biphenyls (PBB) or polybrominated diphenyl ethers (PBDE)and four phthalates (DEHP, DBP, BBP and DiHP), except in certain specific applications, in concentrations greater than the values decided by the European Commission. These values have been established as 0.01% by weight per homogeneous material for cadmium and 0.1% for the other nine substances. This regulation is covered in the Philips Regulated Substances List.
SegmentThe Philips Operating Model identifies three operating segments – Diagnosis & Treatment, Connected Care and Personal Care – comprised of six businesses and 18 business units, as well as segment Other. Other includes Innovation & Strategy, IP Royalties, Central Costs, and other small items. See also the entry under Business/Business unit.
SolutionA combination of Philips (and 3rd-party) systems, devices, software, consumables and services, configured and delivered in a way to solve customer (segment)-specific needs and challenges.
Sustainable Development GoalsThe Sustainable Development Goals (SDGs) are a collection of 17 global goals set by the United Nations. The broad goals are interrelated though each has its own targets. The SDGs cover a broad range of social and economic development issues. These include poverty, hunger, health, education, climate change, water, sanitation, energy, environment and social justice.
Sustainable InnovationSustainable Innovation is the Research & Development spend related to the development of new generations of products and solutions that address the United Nations Sustainable Development Goals 3 (Ensure healthy lives and promote well-being for all at all ages) or 12 (Ensure sustainable consumption and production patterns). This includes all Diagnosis & Treatment and Connected Care innovation spend. In addition, innovation spend that contributes to Green Products and healthy living at Personal Health is included. Finally, innovation spend at Other that addresses the SDGs 3 and 12 is included.
VOCVolatile organic compounds (VOCs) are organic chemicals that have a high vapor pressure at ordinary room temperature. Their high vapor pressure results from a low boiling point, which causes large numbers of molecules to evaporate or sublimate from the liquid or solid form of the compound and enter the surrounding air, a trait known as volatility.
Voluntary turnoverVoluntary turnover covers all employees who resigned of their own volition.
Waste Electrical and Electronic Equipment (WEEE)The Waste Electrical and Electronic Equipment Directive (WEEE Directive) is the European Community directive on waste electrical and electronic equipment setting collection, recycling and recovery targets for all types of electrical goods. The directive imposes the responsibility for the disposal of waste electrical and electronic equipment on the manufacturers of such equipment.
Weighted Average Statutory Tax Rate (WASTR)The reconciliation of the effective tax rate is based on the applicable statutory tax rate, which is a weighted average of all applicable jurisdictions. This weighted average statutory tax rate (WASTR) is the aggregation of the result before tax multiplied by the applicable statutory tax rate without adjustment for losses, divided by the group result before tax.
| Exhibit 1 | English translation of the Articles of Association of the company (incorporated by reference to Exhibit 1 to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 27, 2019) |
| Exhibit 2 (a) |
Description of securities registered under Section 12 of the Exchange Act (Incorporated by reference to Exhibit 2 to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 25, 2020) |
| Exhibit 2 (b) |
Amended and Restated Trust Deed Related to a €10,000,000,000 Euro Medium Term Note Programme between the company and Citicorp Trustee Company Limited (as Trustee), dated March 8, 2022 Philips agrees to furnish copies of any or all other instruments under which the long-term debt securities of Philips or its subsidiaries are authorized to the Securities and Exchange Commission upon request. |
| Exhibit 4 | Material Contracts. |
| Exhibit 4 (a) | Services contract between the company and R.W.O. Jakobs (Incorporated by reference to Exhibit 4 (a) to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 21, 2023) |
| Exhibit 4 (b) | Services contract between the company and A. Bhattacharya |
| Exhibit 4 (c) | Services contract between the company and M.J. van Ginneken |
| Exhibit 4 (d) | Global Philips Performance Share Plan applicable to the Board of Management of Koninklijke Philips N.V. |
| Exhibit 4 (e) | Services contract between the company and F.A. van Houten (Incorporated by reference to Exhibit 4 (a) to the Annual Report on Form 20-F (File No. 001-05146-01) filed with the Securities and Exchange Commission on February 25, 2020) |
| Exhibit 4 (f) | Relationship Agreement between the company and Exor N.V. |
| Exhibit 8 | List of Subsidiaries. |
| Exhibit 12 (a) | Certification of R.W.O. Jakobs filed pursuant to 17 CFR 240. 13a-14(a). |
| Exhibit 12 (b) | Certification of A. Bhattacharya filed pursuant to 17 CFR 240. 13a-14(a). |
| Exhibit 13 (a) | Certification of R.W.O. Jakobs furnished pursuant to 17 CFR 240. 13a-14(b). |
| Exhibit 13 (b) | Certification of A. Bhattacharya furnished pursuant to 17 CFR 240. 13a-14(b). |
| Exhibit 15 (a) | EY Consent of independent registered public accounting firm. |
| Exhibit 97 | Clawback policy |
| 101.INS | XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. |
| 101.SCH | XBRL Taxonomy Extension Schema Document. |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document. |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
|
KONINKLIJKE PHILIPS N.V. (Registrant) | |
|
/s/ R.W.O. Jakobs (Chief Executive Officer, Chairman of the Board of Management and the Executive Committee) |
/s/ A. Bhattacharya (Chief Financial Officer, Member of the Board of Management and the Executive Committee) |
| Date: February 20, 2024 | |
I, R.W.O. Jakobs, certify that:
1. I have reviewed this Annual Report on Form 20-F of Koninklijke Philips N.V., a company incorporated under the laws of The Netherlands;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)), and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the period covered by the Annual Report that has materially affected or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5 The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 20, 2024
| /s/ R.W.O. Jakobs | |
| Name: R.W.O. Jakobs | |
| Title: Chief Executive Officer, | |
| Chairman of the Board of Management and the Executive Committee |
I, A. Bhattacharya, certify that:
1. I have reviewed this Annual Report on Form 20-F of Koninklijke Philips N.V., a company incorporated under the laws of The Netherlands;
2. Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3. Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4. The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)), and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the period covered by the Annual Report that has materially affected or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5. The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
Date: February 20, 2024
| /s/ A. Bhattacharya | |
| Name: A. Bhattacharya | |
| Title: Chief Financial Officer, | |
| Member of the Board of Management and the Executive Committee |
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), the undersigned officer of Koninklijke Philips N.V., a company incorporated under the laws of The Netherlands (the “Company”), hereby certifies, to such officer’s knowledge, that:
The Annual Report on Form 20-F for the year ended December 31, 2023 (the “Report”) of the Company fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934 and information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: February 20, 2024
| /s/ R.W.O. Jakobs | |
| Name: R.W.O. Jakobs | |
| Title: Chief Executive Officer, | |
| Chairman of the Board of Management and the Executive Committee |
The foregoing certification is being furnished solely pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code) and is not being filed as part of the Report or as a separate disclosure document.
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), the undersigned officer of Koninklijke Philips N.V., a company incorporated under the laws of The Netherlands (the “Company”), hereby certifies, to such officer’s knowledge, that:
The Annual Report on Form 20-F for the year ended December 31, 2023 (the “Report”) of the Company fully complies with the requirements of section 13(a) or 15(d) of the Securities Exchange Act of 1934 and information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
Date: February 20, 2024
| /s/ A. Bhattacharya | |
| Name: A. Bhattacharya | |
| Title: Chief Financial Officer, | |
| Member of the Board of Management and the Executive Committee |
The foregoing certification is being furnished solely pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code) and is not being filed as part of the Report or as a separate disclosure document.
We consent to the incorporation by reference in the Registration Statements (Form S-8 Nos. 333-140784, 333-151797, 333-157477, 333-165017, 333-172329, 333-179692, 333-186849) of Koninklijke Philips N.V. of our reports dated February 20, 2024, with respect to the consolidated financial statements of Koninklijke Philips N.V. and the effectiveness of internal control over financial reporting of Koninklijke Philips N.V. included in this Annual Report (Form 20-F) of Koninklijke Philips N.V. for the year ended December 31, 2023.
/s/ Ernst & Young Accountants LLP
Amsterdam, the Netherlands
February 20, 2024
KONINKLIJKE PHILIPS N.V.
AS ISSUER
AND
CITICORP TRUSTEE COMPANY LIMITED
AS TRUSTEE
AMENDED AND RESTATED
TRUST DEED
RELATING TO A
€10,000,000,000
EURO MEDIUM TERM NOTE PROGRAMME
CONTENTS
1. Definitions and Interpretation
2. Amount and Issue of the Notes
3. Covenant to Repay
4. The Notes
5. Covenant to Comply with the Trust Deed
6. Covenants by the Issuer
7. Waivers, Modifications, Substitution and Accession
8. Enforcement
9. Application of Moneys
10. Terms of Appointment
11. Costs and Expenses
12. Appointment and Retirement
13. Notices
14. Law and Jurisdiction
15. Severability
16. Contracts (Rights of Third Parties) Act 1999
17. Counterparts
Schedule 1 Terms and Conditions
Schedule 2 Forms of the Notes
Part A Form of Temporary Global Note
Part B Form of Permanent Global Note
Part C Form of Definitive Bearer Note
Part D Form of Coupon
Part E Form of Talon
Schedule 3 Provisions for Meetings of Noteholders
Schedule 4 Form of Authorised Officers' Certificate
THIS AMENDED AND RESTATED TRUST DEED is made on 8 March 2022
BETWEEN:
(1) KONINKLIJKE PHILIPS N.V. (the "Issuer"); and
(2) CITICORP TRUSTEE COMPANY LIMITED (the "Trustee", which expression includes, where the context admits, all persons for the time being the trustee or trustees of this Trust Deed).
WHEREAS
(A) The Issuer has established a programme (the "Programme") pursuant to which the Issuer may issue notes (the "Notes") from time to time as set out herein. Notes up to a maximum principal amount from time to time outstanding of €10,000,000,000 (subject to increase as provided in the Dealer Agreement (as defined below)) (the "Authorised Amount") may be issued pursuant to the Programme.
(B) In connection with the Programme, the Issuer and the Trustee entered into a trust deed dated 9 March 2020 (the "Original Trust Deed").
(C) The parties to this Trust Deed have agreed to make certain modifications to the Original Trust Deed.
(D) The Trustee has agreed to act as trustee under this Trust Deed on the following terms and conditions.
NOW THIS TRUST DEED WITNESSES AND IT IS HEREBY DECLARED as follows:
1. DEFINITIONS AND INTERPRETATION
1.1 Definitions
In this Trust Deed the following expressions have the following meanings:
"Agency Agreement" means, in relation to the Notes of any Series, the agreement appointing the initial Paying Agents in relation to such Series and any other agreement for the time being in force appointing Successor paying agents in relation to such Series, together with any agreement for the time being in force amending or modifying with the prior written approval of the Trustee any of the aforesaid agreements in relation to such Series;
"Agents" means, in relation to the Notes of any Series, the Principal Paying Agent, the other Paying Agents, the Calculation Agent or any of them;
"Appointee" means any delegate, agent, nominee or custodian appointed pursuant to the provisions of this Trust Deed;
"Auditors" means the independent auditors for the time being of the Issuer or, in the event of their being unable or unwilling promptly to carry out any action requested of them pursuant to the provisions of this Trust Deed, such other firm of accountants whose identity is approved in writing by the Trustee for the purposes of this Trust Deed;
"Authorised Signatory" means any Director of the Issuer or any other person or persons notified to the Trustee by any such Director as being an Authorised Signatory pursuant to Clause 6.19 (Authorised Signatories);
"Authority" means any competent regulatory, prosecuting, tax or governmental authority in any jurisdiction, domestic or foreign;
"Basic Terms Modification" means any proposal to:
(a) change the date of maturity of the Notes or any date for payment of interest thereon, reduce or cancel the amount of principal or interest payable or, where applicable, modify the method of calculating the amount payable (including interest) provided, however that, for the avoidance of doubt, any Benchmark Amendment or Benchmark Replacement Conforming Change and the selection of a Successor Rate, an Alternative Reference Rate or an Adjustment Spread (in each case in accordance with the provisions of Condition 5 (Interest) shall be excluded;
(b) alter the currency in which payments under the Notes and Coupons are to be made;
(c) effect the exchange, conversion or substitution of the Notes for, or the conversion of the Notes into, shares, bonds or other obligations or securities of the Issuer or any other person or body corporate formed or to be formed (other than as permitted under Clause 7.3 (Substitution) of this Trust Deed);
(d) alter the quorum or majority required to pass an Extraordinary Resolution; and
(e) alter the definition of a Basic Terms Modification or the proviso to paragraph 5 of Schedule 3.
"CGN Global Note" means a CGN Permanent Global Note or a CGN Temporary Global Note.
"CGN Permanent Global Note" means a Permanent Global Note representing Notes for which the relevant Final Terms specify that the New Global Note form is not applicable;
"CGN Temporary Global Note" means a Temporary Global Note representing Notes for which the relevant Final Terms specify that the New Global Note form is not applicable;
"Clearstream, Luxembourg" means Clearstream Banking S.A.;
"Common Safekeeper" means an ICSD in its capacity as common safekeeper or a person nominated by the ICSDs to perform the role of common safekeeper;
"Conditions" means the terms and conditions to be endorsed on, or incorporated by reference in, the Notes of any Series, in the form set out in Schedule 1 or in such other form, having regard to the terms of the Notes of the relevant Series, as may be agreed between the Issuer, the Principal Paying Agent, the Trustee and the relevant Dealer(s) as completed by the Final Terms applicable to such Series, as any of the same may from time to time be modified in accordance with this Trust Deed and any reference in this Trust Deed to a particular numbered Condition shall be construed in relation to the Notes of such Series accordingly;
"Contractual Currency" means, in relation to any payment obligation of any Note, the currency in which that payment obligation is expressed and, in relation to Clause 11.1 (Remuneration), pounds sterling or such other currency as may be agreed between the Issuer and the Trustee from time to time;
"Couponholder" means the holder of a Coupon;
"Coupons" means any bearer interest coupons in or substantially in the form set out in Part D of Schedule 2 appertaining to the Notes of any Series and for the time being outstanding or, as the context may require, a specific number thereof and includes any replacement Coupons issued pursuant to Condition 12 (Replacement of Notes and Coupons) and, where the context so permits, the Talons appertaining to the Notes of such Series;
"Director" means any director of the Issuer from time to time;
"Dealer Agreement" means the agreement between the Issuer and the Dealers named therein concerning the purchase of Notes to be issued pursuant to the Programme as amended from time to time or any restatement thereof for the time being in force;
"Dealers" means any person appointed as a Dealer by the Dealer Agreement and any other person which the Issuer may appoint as a Dealer and notice of whose appointment has been given to the Principal Paying Agent and the Trustee by the Issuer in accordance with the provisions of the Dealer Agreement but excluding any entity whose appointment has been terminated in accordance with the terms of the Dealer Agreement and notice of whose termination has been given to the Principal Paying Agent and the Trustee by the Issuer in accordance with the provisions of the Dealer Agreement and references to the "relevant Dealer(s)" mean, in relation to any Note, the Dealer(s) with whom the Issuer has agreed the issue and purchase of such Note;
"Electronic Consent" has the meaning set out in Schedule 3;
"euro", "€" and "EUR" means the single currency introduced at the start of the third stage of European economic and monetary union and as defined in Article 2 of Council Regulation (EC) No 974/98 of 3 May 1998 on the introduction of the euro, as amended;
"Euroclear" means Euroclear Bank SA/NV;
"Event of Default" means any one of the circumstances described in Condition 10 (Events of Default), but in the case of any of the events described in paragraphs (b) to (g) (other than (d)) only if such event is, pursuant to the provisions of Condition 10 (Events of Default) certified by the Trustee to be materially prejudicial to the interests of the Noteholders.;
"Extraordinary Resolution" has the meaning set out in Schedule 3;
"Final Terms" has the meaning ascribed to it in the Dealer Agreement;
"Fixed Rate Note" means a Note on which interest is calculated at a fixed rate payable in arrear on a fixed date or dates in each year and on redemption or on such other dates as may be agreed between the Issuer and the relevant Dealer(s) (as indicated in the relevant Final Terms);
"Floating Rate Note" means a Note on which interest is calculated at a floating rate payable on an Interest Payment Date (as defined in the Conditions) or Interest Payment Dates as may be agreed between the Issuer and the relevant Dealer(s) and on redemption (as indicated in the relevant Final Terms);
"Global Note" means a CGN Global Note or an NGN Global Note;
"ICSDs" means Clearstream, Luxembourg and Euroclear;
"Issue Date" means, in relation to any Note, the date of issue of such Note pursuant to the Dealer Agreement or any other relevant agreement between the Issuer and the relevant Dealer(s);
"Interest Commencement Date" means, in relation to any interest-bearing Note, the date specified in the relevant Final Terms from which such Note bears interest or, if no such date is specified therein, the Issue Date;
"Liabilities" means any loss, damage, cost, charge, claim, demand, expense, judgment, action, proceeding or other liability whatsoever (including, without limitation, in respect of taxes, duties, levies, imposts and other charges) including legal fees and expenses properly incurred on a full indemnity basis;
"London Business Day" means a day on which commercial banks are open for business in London.
"Material Subsidiary" means, at any time, a Subsidiary of the Issuer whose total assets represent at least 5 per cent. of the consolidated total assets of the Group or whose total net sales represent at least 7.5 per cent. of the consolidated net sales of the Group and in relation to which the Issuer has, directly or indirectly, the power to direct its management and policies whether through the ownership of voting capital, by contract or otherwise. For this purpose:
(a) in the case of each Subsidiary, the calculation shall be made by comparing the total assets or, as the case may be, total net sales of that Subsidiary individually (and not on a consolidated basis) to those of the Group;
(b) assets or sales which arise from transactions between members of the Group and which would be eliminated in the consolidated financial statements of the Group shall be excluded;
(c) the total assets or total net sales of a Subsidiary shall be calculated by reference to:
(i) the accounts of that Subsidiary used for the purpose of the latest audited consolidated financial statements of the Group; or
(ii) if the company became a Subsidiary after the end of the financial period to which the latest audited consolidated financial statements of the Group relate, its then latest audited accounts;
(b) the consolidated total assets or consolidated net sales of the Group shall be calculated by reference to the latest audited consolidated financial statements of the Group, adjusted as appropriate to reflect the total assets or total net sales of any company which has become or ceased to be a Subsidiary after the end of the financial period to which those accounts relate; and
(c) where a Material Subsidiary transfers all or substantially all of its assets to the Issuer or another Subsidiary, the transferor (if it is not the Holding Company of the transferee) shall cease to be a Material Subsidiary and (if the transferee is a Subsidiary but not a Material Subsidiary) the transferee shall become a Material Subsidiary.
For the purposes of this definition of Material Subsidiary, the term "Group" means the Issuer and its Subsidiaries, provided, however, that any reference to the financial statements of the Group shall be to the financial statements of the Issuer and its present and future subsidiaries, direct and indirect, within the meaning of Article 2:24 a of the Dutch Civil Code (Burgerlijk Wetboek); the term "Holding Company" of any other person means a company in respect of which that other person is a Subsidiary.
"NGN Global Note" means an NGN Permanent Global Note or an NGN Temporary Global Note.
"NGN Permanent Global Note" means a Permanent Global Note representing Notes for which the relevant Final Terms specify that the New Global Note form is applicable;
"NGN Temporary Global Note" means a Temporary Global Note representing Notes for which the relevant Final Terms specify that the New Global Note form is applicable;
"Noteholders" means the several persons who are for the time being bearers of Notes save that, in respect of the Notes of any Series, for so long as such Notes or any part thereof are represented by a Global Note deposited with a common depositary (in the case of a CGN Global Note) or common safekeeper (in the case of a NGN Global Note) for Euroclear and Clearstream, Luxembourg or, in respect of Notes in definitive form held in an account with Euroclear or Clearstream, Luxembourg, each person who is for the time being shown in the records of Euroclear or Clearstream, Luxembourg (other than Clearstream, Luxembourg, if Clearstream, Luxembourg shall be an accountholder of Euroclear and Euroclear, if Euroclear shall be an accountholder of Clearstream, Luxembourg) as the holder of a particular principal amount of the Notes of such Series (in which regard any certificate or other document issued by Euroclear or Clearstream, Luxembourg as to the principal amount of such Notes standing to the account of any person shall be conclusive and binding for all purposes save in the case of manifest error) shall be treated by the Issuer, the Paying Agents and the Trustee as the holder of such principal amount of such Notes for all purposes other than with respect to the payment of principal or interest on such principal amount of such Notes, for which purpose the bearer of the relevant Global Note shall be treated by the Issuer, any Paying Agent and the Trustee as the holder of such principal amount of such Notes in accordance with and subject to the terms of the relevant Global Note and the expressions "holder" and "holder of Notes" and related expressions shall be construed accordingly;
"Notes" means the bearer notes of each Series constituted in relation to or by this Trust Deed which shall be in or substantially in the form set out in Schedule 2 and, for the time being outstanding or, as the case may be, a specific number thereof and includes any replacement Notes of such Series issued pursuant to Condition 12 (Replacement of Notes and Coupons) and (except for the purposes of Clause 4.1 (Global Notes) and 4.3 (Signature)) each Global Note in respect of such Series for so long as it has not been exchanged in accordance with the terms thereof;
"outstanding" means, in relation to the Notes of any Series, all the Notes of such Series other than:
(a) those which have been redeemed in accordance with this Trust Deed;
(b) those in respect of which the date for redemption in accordance with the Conditions has occurred and for which the redemption moneys (including all interest accrued thereon to the date for such redemption) have been duly paid to the Trustee or the Principal Paying Agent in the manner provided for in the Agency Agreement (and, where appropriate, notice to that effect has been given to the Noteholders in accordance with Condition 13 (Notices)) and remain available for payment in accordance with the Conditions;
(c) those which have been purchased and surrendered for cancellation as provided in Condition 7 (Redemption and Purchase);
(d) those which have become void under Condition 9 (Prescription);
(e) those mutilated or defaced Notes which have been surrendered or cancelled and in respect of which replacement Notes have been issued pursuant to Condition 12 (Replacement of Notes and Coupons);
(f) (for the purpose only of ascertaining the aggregate principal amount of Notes outstanding and without prejudice to the status for any other purpose of the relevant Notes) those Notes which are alleged to have been lost, stolen or destroyed and in respect of which replacements have been issued pursuant to Condition 12 (Replacement of Notes and Coupons);
provided that for each of the following purposes, namely:
(i) the right to attend and vote at any meeting of the holders of Notes of any Series and any direction or request by the holders of the Notes of any Series;
(ii) the determination of how many and which Notes of any Series are for the time being outstanding for the purposes of Clauses 8.1 (Legal Proceedings) and 7.1 (Waiver), Conditions 10 (Events of Default) and 14 (Meetings of Noteholders, Modification, Waiver, Authorisation and Determination, Substitution) and Schedule 3; and
(iii) any discretion, power or authority, whether contained in this Trust Deed or provided by law, which the Trustee is required to exercise in or by reference to the interests of the holders of the Notes of any Series or any of them;
those Notes (if any) of the relevant Series which are for the time being held by any person (including but not limited to the Issuer) for the benefit of the Issuer shall (unless and until ceasing to be so held) be deemed not to remain outstanding;
"Paying Agents" means, in relation to the Notes of any Series, the several institutions (including, where the context permits, the Principal Paying Agent) at their respective specified offices initially appointed pursuant to the relative Agency Agreement and/or, if applicable, any Successor paying agents in relation to such Series at their respective specified offices;
"Permanent Global Note" means, in relation to any Series, a Global Note to be issued pursuant to Clause 4.1 (Global Notes) in the form or substantially in the form set out in Part B of Schedule 2, with such modifications (if any) as may be agreed between the Issuer, the Paying Agent, the Trustee and the relevant Dealer(s);
"Potential Event of Default" means an event or circumstance which could, with the giving of notice, lapse of time, the issuing of a certificate and/or fulfilment of any other requirement provided for in Condition 10 (Events of Default), become an Event of Default;
"Principal Paying Agent" means, in relation to the Notes of any Series, the institution at its Specified Office initially appointed as issuing and principal paying agent in relation to such Series pursuant to the relative Agency Agreement or, if applicable, any Successor principal paying agent in relation to such Series at its Specified Office;
"repay" includes "redeem" and vice versa and "repaid", "repayable", "repayment", "redeemed", "redeemable" and "redemption" shall be construed accordingly;
"Series" means a Tranche of Notes together with any further Tranche or Tranches of Notes expressed to be consolidated and form a single series with the Notes of the original Tranche and the terms of which are identical (save for the Issue Date and/or the Interest Commencement Date but including as to whether or not the Notes are listed);
"specified office" means, in relation to any Agent in respect of any Series, either the office identified with its name in the Conditions of such Series or any other office notified to any relevant parties pursuant to the Agency Agreement;
"Subsidiary" has the meaning set out in Condition 1.1 (Interpretation – Definitions);
"Successor" means in relation to the Paying Agents, such other or further person as may from time to time be appointed pursuant to the Agency Agreement as a Paying Agent, notice of whose appointment has been given to the Noteholders pursuant to Clause 6.13 (Notification of change in Principal Paying Agent) in accordance with Condition 13 (Notices).
"Talonholder" means the holder of a Talon;
"Talons" means any bearer talons appertaining to the Notes of any Series or, as the context may require, a specific number thereof and includes any replacement Talons issued pursuant to Condition 12 (Replacement of Notes and Coupons);
"Temporary Global Note" means, in relation to any Series, a Global Note to be issued pursuant to Clause 4.1 (Global Notes) in the form or substantially in the form set out in Part A of Schedule 2, with such modifications (if any) as may be agreed between the Issuer, the Paying Agent, the Trustee and the relevant Dealer(s);
"the Stock Exchange" means, in relation to the Notes of any relevant series, the stock exchange or exchanges (if any) on which such Notes are for the time being quoted or listed;
"this Trust Deed" means this Trust Deed and the Schedules (as from time to time modified in accordance with the provisions contained herein) and (unless the context requires otherwise) includes any deed or other document executed in accordance with the provisions hereof (as from time to time modified as aforesaid) and expressed to be supplemental hereto;
"Tranche" means all Notes of the same Series with the same Issue Date and Interest Commencement Date;
"Trustee Acts" means both the Trustee Act 1925 and the Trustee Act 2000 of England and Wales;
"VAT" means any value added tax, goods and services tax, sales or use tax or similar tax, including, without limitation, such tax as may be chargeable under or pursuant to Council Directive 2006/112/EC.
"Written Resolution" has the meaning set out in Schedule 3; and
"Zero Coupon Note" means a Note that is in bearer form and that constitutes a claim for a fixed sum against the Issuer and on which interest does not become due during their tenor or other Notes which qualify as savings certificates as defined in the Dutch Savings Certificates Act (Wet inzake spaarbewijzen).
1.2 Principles of interpretation
In this Trust Deed:
1.2.1 Statutory modification: a provision of any statute shall be deemed also to refer to any statutory modification or re-enactment thereof or any statutory instrument, order or regulation made thereunder or under such modification or re-enactment;
1.2.2 Additional amounts: all references in this Trust Deed to principal and/or interest in respect of the Notes or to any moneys payable by the Issuer under this Trust Deed shall be deemed to include, in the case of amounts of principal payable, a reference to any specific redemption price (as described in the Conditions) and, in any case, a reference to any additional amounts which may be payable under Condition 8 (Taxation) or, if applicable, under any undertaking or covenant given pursuant to Clause 3.1 (Covenant to repay and to pay interest);
1.2.3 Relevant Currency: "relevant currency" shall be construed as a reference to the currency in which payments in respect of the Notes and/or Coupons of the relevant Series are to be made as indicated in the relevant Final Terms;
1.2.4 Enforcement of rights: an action, remedy or method of judicial proceedings for the enforcement of rights of creditors shall include, in respect of any jurisdiction other than England, references to such action, remedy or method of judicial proceedings for the enforcement of rights of creditors available or appropriate in such jurisdictions as shall most nearly approximate thereto;
1.2.5 Clauses and Schedules: a Schedule or a Clause, sub-clause, paragraph or sub-paragraph is, unless otherwise stated, to a Schedule hereto or a clause, sub-clause, paragraph or sub-paragraph hereof respectively;
1.2.6 Clearing systems: Euroclear and/or Clearstream, Luxembourg shall, wherever the context so admits, be deemed to include references to any additional or alternative clearing system approved by the Issuer and the Trustee;
1.2.7 Trust corporation: a trust corporation denotes a corporation entitled by rules made under the Public Trustee Act 1906 to act as a custodian trustee or entitled pursuant to any other legislation applicable to a trustee in any jurisdiction other than England to act as trustee and carry on trust business under the laws of the country of its incorporation;
1.2.8 Coupons: in the case of any Notes which are Zero Coupon Notes references to Coupons and Couponholders in this Trust Deed are not applicable to such Notes;
1.2.9 Interpretation: words denoting individuals shall include companies, corporations and partnerships, words importing the singular number shall include the plural and, in each case, vice versa;
1.2.10 Records: any reference to the records of an ICSD shall be to the records that each of the ICSDs holds for its customers which reflect the amount of such customers' interests in the Notes (but excluding any interest in any Notes of one ICSD shown in the records of another ICSD).
1.3 The Conditions
In this Trust Deed, unless the context requires or the same are otherwise defined, words and expressions defined in the Conditions and not otherwise defined herein shall have the same meaning in this Trust Deed.
1.4 Headings
The headings and sub-headings are for ease of reference only and shall not affect the construction of this Trust Deed.
1.5 The Schedules
The Schedules are part of this Trust Deed and shall have effect accordingly.
1.6 Amendment and Restatement
Save in relation to all Series of Notes issued during the period up to and including the day last preceding the date of this Trust Deed and any Notes issued on or after the date of this Trust Deed so as to be consolidated and form a single Series with the Notes of any Series issued up to and including such last preceding day, with effect on and from the date of this Trust Deed.
(a) the Original Trust Deed is amended and restated on the terms of this Trust Deed; and
(b) the provisions of the Original Trust Deed insofar as the same still have effect and shall cease to have effect and in lieu thereof the provisions of this Trust Deed shall have effect.
2. AMOUNT AND ISSUE OF THE NOTES
2.1 Amount of the Notes
The Notes will be issued in Series in an aggregate principal amount from time to time outstanding not exceeding the Authorised Amount and for the purpose of determining such aggregate principal amount clause 14 (Increase in Authorised Amount) of the Dealer Agreement shall apply.
2.2 Prior to each Issue Date
By not later than 3.00 p.m. (London time) on the London Business Day (which for this purpose shall be a day on which commercial banks are open for business in London) preceding each proposed Issue Date, the Issuer shall:
(a) deliver or cause to be delivered to the Trustee a copy of the relevant Final Terms; and
(b) notify the Trustee in writing without delay of the Issue Date and the principal amount of the Notes of the relevant Tranche.
2.3 Constitution of Notes
Upon the issue of the Temporary Global Note, initially representing the Notes of any Tranche, such Notes shall become constituted by this Trust Deed without further formality.
2.4 Further legal opinions
Before the first issue of Notes occurring after each anniversary of this Trust Deed, on each occasion as the Trustee so requests (on the basis that the Trustee considers it reasonably necessary in view of a change (or proposed change) in applicable law affecting the Issuer or in English law affecting the Issuer, this Trust Deed or the Agency Agreement, the Issuer will procure at its cost that further legal opinions in such form and with such content as the Trustee may require from the legal advisers specified in the Dealer Agreement or in the relevant jurisdiction approved by the Trustee are delivered to the Trustee.
3. CONVENANT TO REPAY
3.1 Covenant to repay and to pay interest
The Issuer covenants with the Trustee that it shall, as and when the Notes of any Series or any of them become due to be redeemed or any principal on the Notes of any Series or any of them becomes due to be repaid in accordance with the Conditions, unconditionally pay or procure to be paid to or to the order of the Trustee in immediately available freely transferable funds in the relevant currency the principal amount of the Notes of such Series or any of them becoming due for payment on that date and shall (subject to the provisions of the Conditions and except in the case of Zero Coupon Notes), until all such payments (both before and after judgment or other order of any court of competent jurisdiction) are duly made, unconditionally pay or procure to be paid to or to the order of the Trustee as aforesaid on the dates provided for in the Conditions interest on the principal amount (or such other amount as may be specified in the Final Terms) of the Notes or any of them of such Series outstanding from time to time as set out in the Conditions (subject to Clause 3.3 (Interest on Floating Rate Notes following Event of Default)), provided that:
3.1.1 every payment of principal or interest in respect of such Notes or any of them made to or to the order of the Principal Paying Agent in the manner provided in the Agency Agreement shall satisfy, to the extent of such payment, the relevant covenant by the Issuer contained in this Clause 3 except to the extent that there is default in the subsequent payment thereof to the relevant Noteholders or Couponholders (as the case may be) in accordance with the Conditions;
3.1.2 if any payment of principal or interest in respect of such Notes or any of them is made after the due date, payment shall be deemed not to have been made until either the full amount is paid to the relevant Noteholders or Couponholders (as the case may be) or, if earlier, the seventh day after notice has been given to the relevant Noteholders in accordance with the Conditions that the full amount has been received by the Principal Paying Agent or the Trustee.
3.1.3 in any case where payment of the whole or any part of the principal amount due in respect of any Note is improperly withheld or refused upon due presentation of the relevant Note, interest shall accrue on the whole or such part of such principal amount (except in the case of Zero Coupon Notes, to which the provision of Condition 7 (Redemption and Purchase) shall apply) from the date of such withholding or refusal until the date either on which such principal amount due is paid to the relevant Noteholders or, if earlier, the seventh day after notice has been given to the relevant Noteholders in accordance with the Conditions that the full amount payable in respect of the said principal amount is available for collection by the relevant Noteholders provided that on further due presentation of the relevant Note such payment is in fact made.
The Trustee will hold the benefit of this covenant and the covenant in Clause 5 (Covenant to comply with the Trust Deed) on trust for the Noteholders in accordance with this Trust Deed.
3.2 Following an Event of Default
At any time after any Event of Default or Potential Event of Default shall have occurred, the Trustee may:
3.2.1 by notice in writing to the Issuer, the Principal Paying Agent and the other Agents require the Principal Paying Agent and the other Agents or any of them:
(a) to act thereafter, until otherwise instructed by the Trustee, as Agents of the Trustee under the provisions of this Trust Deed on the terms provided in the Agency Agreement (with consequential amendments as necessary and save that the Trustee's liability under any provisions thereof for the indemnification, remuneration and payment of out-of-pocket expenses of the Agents shall be limited to amounts for the time being held by the Trustee on the trusts of this Trust Deed in relation to the Notes on the terms of this Trust Deed and available to the Trustee for such purpose) and thereafter to hold all Notes and Coupons and all sums, documents and records held by them in respect of Notes and Coupons on behalf of the Trustee; and/or
(b) to deliver up all Notes and Coupons and all sums, documents and records held by them in respect of Notes and Coupons to the Trustee or as the Trustee shall direct in such notice provided that such notice shall be deemed not to apply to any document or records which the relevant Agent is obliged not to release by any law or regulation; and
3.2.2 by notice in writing to the Issuer require the Issuer to make all subsequent payments in respect of Notes and Coupons to or to the order of the Trustee and, with effect from the issue of any such notice until such notice is withdrawn, proviso 3.1.1 to Clause 3.1 (Covenant to repay and to pay interest) and (so far as it concerns payments by the Issuer) Clause 9.4 (Payment to Noteholders and Couponholders) shall cease to have effect.
3.3 Interest on Floating Rate Notes following Event of Default
If Floating Rate Notes become immediately due and repayable under Condition 10 (Events of Default) the rate and/or amount of interest payable in respect of them will be calculated by the Principal Paying Agent at the same intervals as if such Notes had not become due and repayable, the first of which will commence on the expiry of the Interest Period (as defined in the Conditions) during which the Notes become so due and repayable in accordance with Condition 5 (Interest) (with consequential amendments as necessary) except that the rates of interest need not be published.
3.4 Currency of payments
All payments in respect of, under and in connection with this Trust Deed and the Notes to the relevant Noteholders and Couponholders shall be made in the relevant currency as required by the Conditions.
3.5 Separate Series
The Notes of each Series shall form a separate Series of Notes and accordingly, unless for any purpose the Trustee in its absolute discretion shall otherwise determine, all the provisions of this Trust Deed shall apply mutatis mutandis separately and independently to the Notes of each Series and in such Clauses and Schedule the expressions "Notes", "Noteholders", "Coupons", "Couponholders", "Talons" and "Talonholders" shall be construed accordingly.
4. THE NOTES
4. 1 Global Notes
4.1.1 The Notes of each Tranche will initially be together represented by a Temporary Global Note. Each Temporary Global Note shall (save as may be specified in the relevant Final Terms) be exchangeable, in accordance with its terms, for interests in a Permanent Global Note or Notes in definitive form.
4.1.2 Each Permanent Global Note shall be exchangeable, in accordance with its terms, for Notes in definitive form.
4.1.3 All Global Notes shall be prepared, completed and delivered to a common depositary (in the case of a CGN Global Note) for Clearstream, Luxembourg and Euroclear or, as the case may be, a Common Safekeeper (in the case of an NGN Global Note), in accordance with the Dealer Agreement or to another appropriate depositary in accordance with any other agreement between the Issuer and the relevant Dealer(s) and, in each case, in accordance with the Agency Agreement. The relevant Final Terms shall be annexed to each Global Note.
4. 2 Notes in definitive form
Notes in definitive form will be security printed in accordance with applicable legal and stock exchange requirements substantially in the form set out in Part C of Schedule 2. Any Coupons and Talons will also be security printed in accordance with the same requirements and will be attached to the Notes in definitive form at the time of issue. Notes in definitive form will be endorsed with the Conditions.
4. 3 Signature
The Global Notes and the Notes in definitive form will be signed manually or in facsimile by an Authorised Signatory of the Issuer and will be authenticated manually by or on behalf of the Principal Paying Agent and, if applicable, will be effectuated manually by or on behalf of the Common Safekeeper. The Issuer may use the facsimile signature of a person who at the date such signature was originally produced was an Authorised Signatory even if at the time of issue of any Global Note or Note in definitive form such person no longer holds that office. Global Notes and Notes in definitive form so executed and duly authenticated and, if applicable, duly effectuated will be binding and valid obligations of the Issuer.
4.4 Entitlement to treat holder as owner
Each of the Issuer, the Trustee and any Agent may deem and treat the holder of any Note or Coupon or of a particular principal amount of the Notes as the absolute owner of such Note or principal amount, as the case may be, free of any equity, set- off or counterclaim on the part of the Issuer against the original or any intermediate holder of such Note or Coupon or principal amount (whether or not such Note or Coupon or principal amount shall be overdue and notwithstanding any notation of ownership or other writing thereon or any notice of previous loss or theft of such Note or Coupon) for all purposes and, except as ordered by a court of competent jurisdiction or as required by applicable law, the Issuer, the Trustee and the Paying Agent shall not be affected by any notice to the contrary. All payments made to any such holder shall be valid and, to the extent of the sums so paid, effective to satisfy and discharge the liability for the moneys payable in respect of such Note or principal amount, as the case may be.
5 COVENANT TO COMPLY WITH THE TRUST DEED
5.1 Covenant to comply with the Trust Deed
The Issuer covenants with the Trustee to comply with those provisions of this Trust Deed and the Conditions which are expressed to be binding on it and to perform and observe the same. The Notes and the Coupons are subject to the provisions contained in this Trust Deed, all of which shall be binding upon the Issuer, the Noteholders, the Couponholders and all persons claiming through or under them respectively.
5.2 Trustee may enforce Conditions
The Trustee shall itself be entitled to enforce the obligations of the Issuer under the Notes and the Conditions as if the same were set out and contained in this Trust Deed which shall be read and construed as one document with the Notes.
5.3 Covenant held upon Trust
The Trustee shall hold the benefit of the covenant of this Clause 5 upon trust for the Noteholders and the Couponholders according to their respective interests.
6. COVENANTS BY THE ISSUER
The Issuer covenants with the Trustee that, so long as any of the Notes remain outstanding, it will:
6.1 Information
So far as permitted by applicable law, give or procure to be given to the Trustee such opinions, certificates, information and evidence as it shall reasonably require and in such form as it shall reasonably require (including without limitation the procurement by the Issuer of all such certificates called for by the Trustee pursuant to Clause 10.1(c) for the purpose of the discharge or exercise of the duties, trusts, powers, authorities and discretions vested in it under this Trust Deed or by operation of law.
6.2 Books of account
At all times keep proper books of account as may be necessary to comply with all applicable laws as so to enable the financial statements of the Issuer to be prepared and, at any time after the occurrence of an Event of Default or a Potential Event of Default or if the Trustee has grounds to believe that an Event of Default or a Potential Event of Default has occurred, allow so far as permitted by applicable law the Trustee and any person appointed by the Trustee to whom the Issuer shall have no reasonable objection free access to such books of account at all reasonable times during normal business hours.
6.3 Financial and other information
Send to the Trustee two copies in English of its annual audited accounts and every document issued or sent to holders of debt securities (including the Noteholders), in each case as soon as practicable after the issue or publication thereof.
6.4 List of Material Subsidiaries
Give to the Trustee (i) on the date hereof and (ii) at the same time as sending to it the certificate referred to in Clause 6.7 (Certificate of compliance), a certificate signed by two Authorised Signatories of the Issuer addressed to the Trustee (with a form and content satisfactory to the Trustee) listing those Subsidiaries of the Issuer which as at the date of such certificate are Material Subsidiaries.
6.5 Changes in list of Material Subsidiaries
Give to the Trustee, as soon as reasonably practicable after the completion of the acquisition or disposal of any company which thereby becomes or ceases to be a Material Subsidiary or after any transfer is made to any Subsidiary which thereby becomes a Material Subsidiary, a certificate signed by two Authorised Signatories of the Issuer to such effect.
6.6 Events of Default
Forthwith give notice in writing to the Trustee upon becoming aware of any Event of Default or any Potential Event of Default without waiting for the Trustee to take any further action.
6.7 Certificate of compliance
Give to the Trustee (i) within 14 days after demand by the Trustee therefor and (ii) (without the necessity for any such demand) within 14 days after the publication of its audited accounts in respect of each financial year commencing with the financial year ending 31 December 2022 and in any event not later than 180 days after the end of each such financial year a certificate in or substantially in the form set out in Schedule 4 signed by two Authorised Signatories of the Issuer to the effect that as at the date which is not earlier than 7 days prior to the date of such certificate (the "certification date") there did not exist and had not existed since the certification date of the previous certificate (or in the case of the first such certificate the date hereof) any Event of Default or any Potential Event of Default (or if such exists or existed specifying the same) and that during the period from and including the certification date of the last such certificate (or in the case of the first such certificate the date hereof) to and including the certification date of such certificate the Issuer has complied with all its obligations contained in this Trust Deed or (if such is not the case) specifying the respects in which it has not complied.
6.8 Further assurance
So far as permitted by applicable law, at all times execute and do all such further documents, acts and things as may be necessary at any time or times in the opinion of the Trustee to give effect to this Trust Deed.
6.9 Maintenance of Principal Paying Agent
At all times maintain a Principal Paying Agent in accordance with the Conditions.
6.10 Notification of non-payment
Use all reasonable endeavours to procure the Principal Paying Agent to notify the Trustee forthwith in the event that the Principal Paying Agent does not, on or before the due date for any payment in respect of the Notes or any of them or any of the Coupons, receive unconditionally pursuant to the Agency Agreement payment of the full amount in the requisite currency of the moneys payable on such due date on all such Notes or Coupons as the case may be.
6.11 Notification of late payment
In the event of the unconditional payment to the Principal Paying Agent or the Trustee of any sum due in respect of the Notes or any of them or any of the Coupons being made after the due date for payment thereof forthwith give or procure to be given notice to the Noteholders in accordance with Condition 13 (Notices) that such payment has been made.
6.12 Listing
If the Notes are admitted to listing, trading and/or quotation, use all reasonable endeavours to maintain the listing of the Notes on the Stock Exchange or, if it is unable to do so having used all reasonable endeavours or if the maintenance of such listing is agreed by the Trustee to be unduly onerous or impractical use all reasonable endeavours to obtain and maintain an admission to listing, trading and/or quotation of the Notes on such other listing authority, stock exchange(s), securities market(s) and/or quotation system(s) (if any) as the Issuer may (with the prior written approval of the Trustee) decide and shall also upon obtaining an admission to listing, trading and/or quotation of the Notes on such other listing authority, stock exchange(s), securities market(s) and/or quotation system(s) (if any) enter into a trust deed supplemental to this Trust Deed to effect such consequential amendments to this Trust Deed as the Trustee may require or as shall be requisite to comply with the requirements of any such listing authority, stock exchange(s), securities market(s) and/or quotation systems (if any) and promptly give notice of the identity of such listing authority, stock exchange(s), securities market(s) and/or quotation systems (if any) to the Noteholders.
6.13 Notification of change in Principal Paying Agent
Give notice to the Noteholders in accordance with Condition 13 (Notices) of any appointment, resignation or removal of the Principal Paying Agent (other than the appointment of the initial Principal Paying Agent) after having obtained the prior written approval of the Trustee thereto or any change of the Principal Paying Agent's specified office and (except as provided by the Agency Agreement or the Conditions) at least 30 days prior to such event taking effect; provided always that so long as any of the Notes or Coupons remains liable to prescription in the case of the termination of the appointment of the Principal Paying Agent no such termination shall take effect until a new Principal Paying Agent has been appointed on terms previously approved in writing by the Trustee.
6.14 Notices to Noteholders
Send or procure to be sent to the Trustee, not less than 7 days prior to which any such notice is to be given, the form of every notice to be given to the Noteholders in accordance with Condition 13 (Notices) and obtain the prior written approval of the Trustee to, and promptly give to the Trustee two copies of, the final form of every notice to be given to the Noteholders in accordance with Condition 13 (Notices) (such approval, unless so expressed, not to constitute approval for the purposes of Section 21 of the Financial Services and Markets Act 2000 of the United Kingdom (as amended, the "FSMA") of a communication within the meaning of Section 21 of the FSMA).
6.15 Compliance with Agency Agreement
Comply with and perform all its obligations under the Agency Agreement and use all reasonable endeavours to procure that the Principal Paying Agent complies with and performs all its respective obligations thereunder and any notice given by the Trustee pursuant to Clause 3.2.1 and not make any amendment or modification to the Agency Agreement without the prior written approval of the Trustee.
6.16 Notes held by the Issuer and its Subsidiaries
In order to enable the Trustee to ascertain the principal amount of Notes of each series for the time being outstanding for any of the purposes referred to in the proviso to the definition of outstanding in Clause 1.1 (Definitions), deliver to the Trustee forthwith upon being so requested in writing by the Trustee a certificate in writing signed by two Authorised Signatories of the Issuer setting out the total number and aggregate principal amount of Notes of each series which:
(a) up to and including the date of such certificate have been purchased by the Issuer or any of its Subsidiaries and cancelled; and
(b) are at the date of such certificate held by, for the benefit of, or on behalf of, the Issuer or any of its Subsidiaries.
6.17 Clearing systems
Use all reasonable endeavours to procure that Euroclear and/or Clearstream, Luxembourg (as the case may be) issue(s) any certificate or other document requested by the Trustee under Clause 10.1(t) as soon as practicable after such request.
6.18 Redemption of Notes
Give notice to the Trustee of the proposed redemption of the Notes not less than the number of days specified in the Conditions prior to the redemption date in respect of such Note or Coupon and duly proceed to redeem such Notes or Coupons accordingly.
6.19 Authorised Signatories
Upon the execution hereof and thereafter as soon as reasonably practicable upon any change of the same, deliver to the Trustee (with a copy to the Principal Paying Agent) a list of the Authorised Signatories of the Issuer, together with certified specimen signatures of the same.
6.20 Notification of amendment to Dealer Agreement
Notify the Trustee of any amendment to the Dealer Agreement.
6.21 Benchmark Amendment Certificate
No later than notifying the Trustee, pursuant to Condition 5 (Interest), the Issuer shall deliver to the Trustee and the Agents a certificate (on which the Trustee shall be entitled to rely on without further enquiry or liability) signed by two Authorised Signatories of the Issuer certifying (i) that each change which the Issuer requests the Trustee to make pursuant to Condition 5.2(m) (Benchmark Discontinuation (Independent Adviser)) is a Benchmark Amendment (as defined in the Conditions) and that the effect of the drafting of such change is solely to implement a Benchmark Amendment (as defined in the Conditions) and/or (ii) that each change which the Issuer requires the Trustee to make pursuant to Condition 5.2(f)(Interest – Floating Rate Notes referencing SOFR) is a Benchmark Replacement Conforming Change (as defined in the Conditions) and that the effect of the drafting of such change is solely to implement a Benchmark Replacement Conforming Change (as defined in the Conditions).
7 WAIVERS, MODIFICATIONS, SUBSTITUTION AND ACCESSION
7.1 Waiver
The Trustee may, without any consent or sanction of the Noteholders or Couponholders and without prejudice to its rights in respect of any subsequent breach, Event of Default or Potential Event of Default, from time to time and at any time, but only if and in so far as in its opinion the interests of the Noteholders shall not be materially prejudiced thereby, authorise or waive, any breach or proposed breach by the Issuer of any of the covenants or provisions contained in the Conditions, this Trust Deed or the Agency Agreement or determine that any Event of Default or Potential Event of Default shall not be treated as such for the purposes of this Trust Deed; any such authorisation, waiver or determination shall be binding on the Noteholders and the Couponholders and, if, but only if, the Trustee shall so require, the Issuer shall cause such authorisation, waiver or determination to be notified to the Noteholders as soon as practicable thereafter in accordance with the Conditions, provided that the Trustee shall not exercise any powers conferred upon it by this Clause 7 in contravention of any express direction by an Extraordinary Resolution or of a request in writing made by the holders of not less than 25 per cent. in aggregate principal amount of the Notes then outstanding (but so that no such direction or request shall affect any authorisation, waiver or determination previously given or made).
7.2 Modifications
7.2.1 The Trustee may from time to time and at any time without any consent or sanction of the Noteholders or Couponholders concur with the Issuer in making any modification (a) to the Conditions, the Notes, this Trust Deed or the Agency Agreement (other than any Basic Terms Modification) provided that the Trustee is of the opinion that such modification will not be materially prejudicial to the interests of the Noteholders or (b) to the Conditions, the Notes, this Trust Deed or the Agency Agreement if in the opinion of the Trustee such modification is of a formal, minor or technical nature or made to correct a manifest error or an error which is, in the opinion of the Trustee, proven or which modification is required to comply with a mandatory provision of law. Any such modification shall be binding on the Noteholders and the Couponholders and, unless the Trustee otherwise agrees, the Issuer shall cause such modification to be notified to the Noteholders as soon as practicable thereafter in accordance with the Conditions.
7.2.2 In addition, the Trustee shall agree to vary or amend the Conditions, this Trust Deed and/or the Agency Agreement to give effect to certain amendments without any requirement for the consent or approval of the Noteholders, on the basis set out in Condition 5.2(m) (Benchmark Discontinuation (Independent Adviser)) and Condition 5.2(f) (Interest – Floating Rate Notes referencing SOFR) but it shall not be obliged to concur with the Issuer in respect of any Benchmark Amendments and/or Benchmark Replacement Conforming Changes (each as defined in the Conditions) which, in its sole opinion, would have the effect of (i) imposing more onerous obligations upon it or exposing it to any additional duties, responsibilities or liabilities or reducing or amending the protective provisions afforded to the Trustee in this Trust Deed, the Agency Agreement and/or the Conditions or (ii) exposing the Trustee and/or the Agents (as applicable) to any additional liabilities against which it has not been indemnified and/or secured and/or prefunded to its satisfaction.
7.3 Substitution
7.3.1 Subject to Clause 7.3.2 below, the Trustee may without the consent of the Noteholders or Couponholders at any time agree with the Issuer to the substitution in place of the Issuer (or of any previous substitute under this Clause 7) as the principal debtor under this Trust Deed in relation to the Notes and Coupons of any Series and under such Notes and Coupons of (a) any Subsidiary of the Issuer or (b) any company which directly or indirectly owns 100 per cent. of the shares or other equity interests (as the case may be) carrying the right to vote in the Issuer in place of the Issuer as issuer and principal debtor under this Trust Deed and the Notes (each substituted entity hereinafter called the "Substituted Obligor") if a trust deed is executed or some other written form of undertaking is given by the Substituted Obligor to the Trustee, in form and manner satisfactory to the Trustee, agreeing to be bound by the terms of this Trust Deed, the Notes and the Coupons with any consequential amendments which the Trustee may deem appropriate as fully as if the Substituted Obligor had been named in this Trust Deed and on the Notes and the Coupons as the principal debtor in place of the Issuer (or of any previous substitute under this Clause 7) in the case of a substitution of the Issuer (or any such previous substitute).
7.3.2 The following further conditions shall apply to Clause 7.3.1 above:
(a) the Issuer and the New Company shall comply with such other requirements as the Trustee may direct in order that the substitution is fully effective in the interests of the Noteholders and the Couponholders;
(b) a legal opinion addressed to the Trustee has been provided confirming that (i) the Substituted Obligor has obtained all governmental and regulatory approvals and consents necessary for its assumption of liability as principal debtor in respect of the Notes and the Coupons in place of the Issuer (or such previous substitute as aforesaid) and (ii) such approvals and consents are at the time of substitution in full force and effect;
(c) (without prejudice to the generality of the preceding sub-clauses of this sub-Clause 7.3.2) where the Substituted Obligor is incorporated, domiciled or resident in or is otherwise subject generally to the taxing jurisdiction of any territory or any political sub-division thereof or any authority of or in such territory having power to tax (the "Substituted Territory") other than or in addition to the territory, the taxing jurisdiction of which (or to any such authority of or in which) the Issuer is subject generally (the "Issuer's Territory"), the Substituted Obligor will (unless the Trustee otherwise agrees) give to the Trustee an undertaking in form and manner satisfactory to the Trustee in terms corresponding to the terms of Condition 8 (Taxation) with the substitution for the reference in that Condition to the Issuer's Territory of references to the Substituted Territory and in such event the Trust Deed and Notes and Coupons will be interpreted accordingly; and
(d) any two Authorised Signatories of the Substituted Obligor certify that immediately prior to the assumption of its obligations as Substituted Obligor under this Trust Deed the Substituted Obligor is solvent after taking account of all prospective and contingent liabilities resulting from its becoming the Substituted Obligor, the Trustee need not have regard to the financial condition, profits or prospects of the Substituted Obligor or compare the same with those of the Issuer (or of any previous substitute under this Clause 7.3).
7.3.3 Release: Any agreement by the Trustee pursuant to sub-clause 7.3.1 shall, if so expressed, operate to release the Issuer (or any such previous substitute as aforesaid) the subject of such release from any or all of its obligations as principal debtor under the Notes and this Trust Deed. Not later than fourteen days after the execution of any such documents as aforesaid and after compliance with the said requirements of the Trustee, the Substituted Obligor shall cause notice thereof to be given to the Noteholders.
7.3.4 Completion of substitution: Upon the execution of such documents and compliance with the requirements in this Clause 7.3.4, the Substituted Obligor shall be deemed to be named in this Trust Deed and the Notes and as the principal debtor in place of the Issuer (or of any previous substitute under this Clause 7.3) and this Trust Deed, the Notes and the Coupons shall thereupon be deemed to be amended in such manner as shall be necessary to give effect to the substitution and without prejudice to the generality of the foregoing any references in this Trust Deed, in the Notes and Coupons to the Issuer shall be deemed to be references to the Substituted Obligor.
8. ENFORCEMENT
8.1 Legal proceedings
The Trustee may at any time, at its discretion and without further notice, institute such proceedings and/or take such action against the Issuer as it may think fit to enforce the provisions of this Trust Deed or the Conditions but it shall not be bound to take any such proceedings or action or to take any other action under or pursuant to this Trust Deed or the Notes unless it shall have been so directed by an Extraordinary Resolution or so requested in writing by the holders of at least one-quarter in principal amount of the outstanding Notes and it shall have been indemnified and/or secured and/or prefunded to its satisfaction against all Liabilities to which it may thereby become liable and or which it may incur by so doing provided that the Trustee shall not be held liable for the consequence of taking any such action and may take such action without having regard to the effect of such action on individual Noteholders or Couponholders. Only the Trustee may enforce the provisions of the Notes or this Trust Deed and no Noteholder or Couponholder shall be entitled to proceed directly against the Issuer unless the Trustee, having become bound so to proceed, fails to do so within a reasonable time and such failure is continuing.
8.2 Evidence of default
If the Trustee (or any Noteholder or Couponholder where entitled under this Trust Deed so to do) makes any claim, institutes any legal proceeding or lodges any proof in a winding up or insolvency of the Issuer under this Trust Deed or under the Notes, proof therein that:
8.2.1 as regards any specified Note within a given Series, the Issuer has made default in paying any principal due in respect of such Note shall (unless the contrary be proved) be sufficient evidence that the Issuer has made the like default as regards all other Notes in respect of which a corresponding payment is then due;
8.2.2 as regards any specified Coupon the Issuer has made default in paying any interest due in respect of such Coupon shall (unless the contrary be proved) be sufficient evidence that the Issuer has made the like default as regards all other Coupons in respect of which a corresponding payment is then due; and
8.2.3 as regards any Talon, the Issuer has made default in exchanging such Talon for further Coupons and a further Talon as provided by its terms shall (unless the contrary be proved) be sufficient evidence that the Issuer has made the like default as regards all other Talons which are then available for exchange,
and for the purposes of Clauses 8.2.1 and 8.2.2 a payment shall be a "corresponding" payment notwithstanding that it is due in respect of a Note of a different denomination from that in respect of the above specified Note.
9. APPLICATION OF MONEYS
9.1 Application of moneys
All moneys received by the Trustee in respect of the Notes of any Series or amounts payable under this Trust Deed will despite any appropriation of all or part of them by the Issuer (including any moneys which represent principal or interest in respect of Notes or Coupons which have become void under the Conditions) be held by the Trustee on trust to apply them (subject to Clause 9.2 (Investment of moneys)):
(a) firstly, in payment or satisfaction of those costs, charges, expenses and liabilities incurred by the Trustee in the preparation and execution of the trusts of this Trust Deed (including remuneration of the Trustee);
(b) secondly, in or towards payment pari passu and rateably of all interest remaining unpaid in respect of the Notes of the relevant Series and all principal moneys due on or in respect of the Notes of that Series provided that where the Notes of more than one Series have become so due and payable, such monies shall be applied as between the amounts outstanding in respect of the different Series
(c) pari passu and rateably (except where, in the opinion of the Trustee, such monies are paid in respect of a specific Series or several specific Series, in which event such monies shall be applied solely to the amounts outstanding in respect of that Series or those Series respectively); and
(d) thirdly, the balance (if any) in payment to the Issuer
9.2 Investment of moneys
The Trustee may at its absolute discretion and pending payment invest moneys at any time available for the payment of principal and interest on the Notes of any series in some or one of the investments hereinafter authorised for such periods as it may consider expedient with power from time to time at the like absolute discretion to vary such investments and to accumulate such investments and the resulting interest and other income derived therefrom. The accumulated investments shall be applied under this Clause 9. All interest and other income deriving from such investments shall be applied first in payment or satisfaction of all amounts then due and unpaid under this Trust Deed, including without limitation Clause 9.1 (Application of Moneys) to the Trustee and/or any Appointee and otherwise held for the benefit of and paid to the holders of the Notes of such series or the holders of the related Coupons, as the case may be.
9.3 Authorised Investments
Any moneys which under this Trust Deed may be invested by the Trustee may be invested in the name or under the control of the Trustee in any of the investments for the time being authorised by English law for the investment by trustees of trust moneys or in any other investments, whether similar to those aforesaid or not, which may be selected by the Trustee or by placing the same on deposit in the name or under the control of the Trustee with such bank or other financial institution as the Trustee may think fit and in such currency as the Trustee in its absolute discretion may determine and The Trustee may at any time vary or transfer any of such investments for or into other such investments or convert any moneys so deposited into any other currency and shall not be responsible for any Liability occasioned by reason of any such investments or such deposit whether by depreciation in value, fluctuation in exchange rates or otherwise.
9.4 Payment to Noteholders and Couponholders
The Trustee shall give notice to the Noteholders in accordance with Condition 13 (Notices) of the date fixed for any payment under Clause 9.1 (Application of Moneys). Any payment to be made in respect of the Notes or Coupons of any Series by the Issuer or the Trustee may be made in the manner provided in the Conditions, the Agency Agreement and this Trust Deed and any payment so made shall be a good discharge to the extent of such payment by the Issuer or the Trustee (as the case may be). Any payment in full of interest made in respect of a Coupon in the manner aforesaid shall extinguish any claim of a Noteholder which may arise directly or indirectly in respect of such interest.
9.5 Production of Notes and Coupons
Upon any payment under Clause 9.4 (Payment to Noteholders and Couponholders) of principal or interest, the Note or Coupon in respect of which such payment is made shall, if the Trustee so requires, be produced to the Trustee or the Paying Agent by or through whom such payment is made and the Trustee shall in respect of a Note or Coupon, (a) in the case of part payment, enface or cause such Paying Agent to enface a memorandum of the amount and date of payment thereon (or, in the case of part payment of an NGN Global Note cause the Principal Paying Agent to procure that the ICSDs make appropriate entries in their records to reflect such payment) or (b) in the case of payment in full, cause such Note or Coupon to be surrendered or shall cancel or procure the same to be cancelled and shall certify or procure the certification of such cancellation.
9.6 Noteholders to be treated as holding all Coupons
Wherever in this Trust Deed the Trustee is required or entitled to exercise a power, trust, authority or discretion under this Trust Deed, the Trustee shall, notwithstanding that it may have express notice to the contrary, assume that each Noteholder is the holder of all Coupons and Talons appertaining to each Note of which they are the holder.
10. TERMS OF APPOINTMENT
By way of supplement to the Trustee Acts, it is expressly declared as follows:
10.1 SUPPLEMENT TO TRUSTEE ACTS
Section 1 of the Trustee Act 2000 of England and Wales shall not apply to the duties of the Trustee in relation to the trusts constituted by this Trust Deed. Where there are any inconsistencies between the Trustee Acts and the provisions of this Trust Deed, the provisions of this Trust Deed shall, to the extent allowed by law, prevail and, in the case of any such inconsistency with the Trustee Act 2000 of England and Wales, the provisions of this Trust Deed shall constitute a restriction or exclusion for the purposes of that Act. The Trustee shall have all the powers conferred upon trustees by the Trustee Acts and by way of supplement thereto it is expressly declared as follows:
(a) The Trustee may in relation to this Trust Deed act on the advice, opinion or certificate of or any information (whether addressed to the Trustee or not) obtained from any lawyer, valuer, accountant, surveyor, banker, broker, auctioneer or other expert whether obtained by the Issuer, the Trustee or otherwise and which advice may be provided on such terms (including as to limitations on liability) as the Trustee may consider in its sole discretion to be consistent with prevailing market practice with respect to advice or opinions of that nature and shall not be responsible for any Liability occasioned by so acting.
(b) Any such advice, opinion, certificate or information may be sent or obtained by letter, facsimile transmission or email and the Trustee shall not be liable for acting on any advice, opinion, certificate or information purporting to be conveyed by any such letter, facsimile transmission or email although the same shall contain some error or shall not be authentic.
(c) The Trustee may call for and shall be at liberty to accept as sufficient evidence of any fact or matter or the expediency of any transaction or thing a certificate signed by any two Directors or Authorised Signatories of the Issuer and the Trustee shall not be bound in any such case to call for further evidence or be responsible for any Liability that may be occasioned by it acting on such certificate.
(d) The Trustee shall not be responsible for the receipt or application of the proceeds of the issue of any of the Notes by the Issuer, the exchange of any Global Note for another Global Note or definitive Notes, the delivery of any Global Note or definitive Notes to the person(s) entitled to it or them.
(e) The Trustee shall not be bound to give notice to any person of the execution of any documents comprised or referred to in this Trust Deed or to take any steps to ascertain whether any Event of Default or any Potential Event of Default has happened and, until it shall have actual knowledge or express notice pursuant to this Trust Deed to the contrary, the Trustee shall be entitled to assume that no Event of Default or Potential Event of Default has happened and that the Issuer is observing and performing all its obligations under this Trust Deed.
(f) Save as expressly otherwise provided in this Trust Deed, the Trustee shall have absolute and uncontrolled discretion as to the exercise or non-exercise of its trusts, powers, authorities and discretions under this Trust Deed (the exercise or non-exercise of which as between the Trustee and the Noteholders and Couponholders shall be conclusive and binding on the Noteholders and Couponholders) and shall not be responsible for any Liability which may result from their exercise or non-exercise and in particular the Trustee shall not be bound to act at the request or direction of the Noteholders or otherwise under any provision of this Trust Deed or to take at such request or direction or otherwise any other action under any provision of this Trust Deed, without prejudice to the generality of Clause 9.1 (Application of Moneys), unless it shall first be indemnified and/or secured and/or prefunded to its satisfaction against all Liabilities to which it may render itself liable or which it may incur by so doing.
(g) The Trustee shall not be liable to any person by reason of having acted upon any Extraordinary Resolution in writing or any Extraordinary Resolution or other resolution purporting to have been passed at any meeting of holders of Notes of all or any series in respect whereof minutes have been made and signed or any direction or request of holders of Notes of all or any series even though subsequent to its acting it may be found that there was some defect in the constitution of the meeting or the passing of the resolution or (in the case of an Extraordinary Resolution in writing) that not all Noteholders had signed the Extraordinary Resolution or (in the case of a direction or request) it was not signed by the requisite number of Noteholders or that for any reason the resolution, direction or request was not valid or binding upon such Noteholders and the relative Couponholders.
(h) The Trustee shall not be liable to any person by reason of having accepted as valid or not having rejected any Note or Coupon purporting to be such and subsequently found to be forged or not authentic.
(i) Any consent or approval given by the Trustee for the purposes of this Trust Deed may be given on such terms and subject to such conditions (if any) as the Trustee thinks fit and notwithstanding anything to the contrary in this Trust Deed may be given retrospectively. The Trustee may give any consent or approval, exercise any power, authority or discretion or take any similar action if it is satisfied that the interests of the Noteholders will not be materially prejudiced thereby whether or not such consent, approval, power, authority, discretion or action is specifically referred to in this Trust Deed as being so determinable, but without prejudice to any provision of this Trust Deed to the contrary. For the avoidance of doubt, the Trustee shall not have any duty to the Noteholders in relation to such matters other than that which is contained in the preceding sentence where exercised by the Trustee.
(j) The Trustee shall not (unless and to the extent ordered so to do by a court of competent jurisdiction) be required to disclose to any Noteholder or Couponholder any information (including, without limitation, information of a confidential, financial or price sensitive nature) made available to the Trustee by the Issuer or any other person in connection with this Trust Deed and no Noteholder or Couponholder shall be entitled to take any action to obtain from the Trustee any such information.
(k) Where it is necessary or desirable for any purpose in connection with this Trust Deed to convert any sum from one currency to another it shall (unless otherwise provided by this Trust Deed or required by law) be converted at such rate or rates, in accordance with such method and as at such date for the determination of such rate of exchange, as may be agreed by the Trustee in consultation with the Issuer and any rate, method and date so agreed shall be binding on the Issuer, the Noteholders and the Couponholders.
(l) The Trustee may certify that any of the conditions, events and acts set out in subparagraphs (b) to (g) (other than (d)) of Condition 10 (Events of Default) (each of which conditions, events and acts shall, unless in any case the Trustee in its absolute discretion shall otherwise determine, for all the purposes of this Trust Deed be deemed to include the circumstances resulting therein and the consequences resulting therefrom), is in its opinion materially prejudicial to the interests of the Noteholders and any such certificate shall be conclusive and binding upon the Issuer, the Noteholders and the Couponholders.
(m) The Trustee as between itself and the Noteholders and Couponholders may determine all questions and doubts arising in relation to any of the provisions of this Trust Deed. Every such determination, whether or not relating in whole or in part to the acts or proceedings of the Trustee, shall be conclusive and shall bind the Trustee and the Noteholders and Couponholders.
(n) In connection with the exercise by it of any of its trusts, powers, authorities and discretions under this Trust Deed (including, without limitation, any modification, waiver, authorisation or determination), the Trustee shall have regard to the general interests of the Noteholders as a class and shall not have regard to any interests arising from circumstances particular to individual Noteholders or Couponholders (whatever their number) and, in particular but without limitation, shall not have regard to the consequences of any such exercise for individual Noteholders or Couponholders (whatever their number) resulting from their being for any purpose domiciled or resident in, or otherwise connected with, or subject to the jurisdiction of, any particular territory or any political sub-division thereof and the Trustee shall not be entitled to require, nor shall any Noteholder or Couponholder be entitled to claim, from the Issuer, the Trustee or any other person any indemnification or payment in respect of any tax consequence of any such exercise upon individual Noteholders or Couponholders except to the extent already provided for in Condition 8 (Taxation) and/or any undertaking given in addition thereto or in substitution therefor under this Trust Deed.
(o) Any trustee of this Trust Deed being a lawyer, accountant, broker or other person engaged in any profession or business shall be entitled to charge and be paid all usual professional and other charges for business transacted and acts done by such person or such person's firm in connection with the trusts of this Trust Deed and also their properly incurred charges in addition to disbursements for all other work and business done and all time spent by such person or such person's firm in connection with matters arising in connection with this Trust Deed.
(p) The Trustee may in the execution and exercise of all or any of the trusts, powers, authorities and discretions vested in it by this Trust Deed whenever it thinks fit delegate by power of attorney or otherwise to any person or persons or fluctuating body of persons (whether being a joint trustee of this Trust Deed or not) all or any of its trusts, powers, authorities and discretions under this Trust Deed. Such delegation may be made upon such terms (including power to sub-delegate) and subject to such conditions and regulations as the Trustee may in the interests of the Noteholders think fit. The Trustee shall not be under any obligation to supervise the proceedings or acts of any such delegate or sub-delegate or be in any way responsible for any Liability incurred by reason of any misconduct or default on the part of any such delegate or sub-delegate provided that the Trustee shall have exercised reasonable care in selecting such person.
(q) The Trustee may in the conduct of the trusts of this Trust Deed instead of acting personally employ and pay an agent (whether being a lawyer or other professional person) to transact or conduct, or concur in transacting or conducting, any business and to do, or concur in doing, all acts required to be done by the Trustee in connection with this Trust Deed (including the receipt and payment of money). The Trustee shall not be in any way responsible for any Liability incurred by reason of any misconduct or default on the part of any such agent or be bound to supervise the proceedings or acts of any such agent provided that the Trustee shall have exercised reasonable care in selecting such person.
(r) The Trustee may appoint and pay any person to act as a custodian or nominee on any terms in relation to such assets of the trusts constituted by this Trust Deed as the Trustee may determine, including for the purpose of depositing with a custodian this Trust Deed or any document relating to the trusts constituted by this Trust Deed and the Trustee shall not be responsible for or required to insure against any Liability incurred in connection with such deposit or by reason of the misconduct, omission or default on the part of any person appointed by it hereunder or be bound to supervise the proceedings or acts of such person and may pay all sums required to be paid on account of or in respect of any such deposit; the Trustee is not obliged to appoint a custodian if the Trustee invests in securities payable to bearer provided that the Trustee shall have exercised reasonable care in selecting such person.
(s) The Trustee shall not be responsible for the execution, delivery, legality, effectiveness, adequacy, genuineness, validity, performance, enforceability or admissibility in evidence of this Trust Deed or any other document relating or expressed to be supplemental thereto and shall not be liable for any failure to obtain any licence, consent or other authority for the execution, delivery, legality, effectiveness, adequacy, genuineness, validity, performance, enforceability or admissibility in evidence of this Trust Deed or any other document relating or expressed to be supplemental thereto.
(t) The Trustee may call for any certificate or other document issued by Euroclear, Clearstream, Luxembourg or any other relevant clearing system in relation to any matter. Any such certificate or other document shall, in the absence of manifest error, be conclusive and binding for all purposes. Any such certificate or other document may comprise any form of statement or print out of electronic records provided by the relevant clearing system (including Euroclear's EUCLID or Clearstream, Luxembourg's Creation Online system) in accordance with its usual procedures and in which the account holding a particular principal or nominal amount of the Notes is clearly identified together with the amount of such holding. The Trustee shall not be liable to any person by reason of having accepted as valid or not having rejected any certificate or other document to such effect purporting to be issued by Euroclear or Clearstream, Luxembourg or any other applicable clearing system and subsequently found to be forged or not authentic.
(u) The Trustee shall not be responsible to any person for failing to request, require or receive any legal opinion relating to the Notes or for checking or commenting upon the content of any such legal opinion and shall not be responsible for any Liability incurred thereby.
(v) Notwithstanding anything else herein contained, the Trustee may refrain without liability from doing anything which in its reasonable opinion would or may be illegal or contrary to any law of any state or jurisdiction (including but not limited to the laws of the United States of America or any jurisdiction forming part of it and England & Wales) or any directive or regulation of any agency of any such state or jurisdiction and may without Liability do anything which is, in its reasonable opinion, necessary to comply with any such law, directive or regulation.
(w) No provision of this Trust Deed shall require the Trustee to do anything which may cause it to expend or risk its own funds or otherwise incur any Liability in the performance of any of its duties or in the exercise of any of its rights, powers or discretions, if it shall have reasonable grounds for believing that repayment of such funds or adequate indemnity against such risk or Liability is not assured to it.
(x) In the absence of knowledge or express notice to the contrary, the Trustee shall be entitled to assume without enquiry (other than requesting a certificate pursuant to Clause 6.16 (Notes held by the Issuer and its Subsidiaries) that no Notes are held by, for the benefit of, or on behalf of, the Issuer or any of its Subsidiaries).
(y) The Trustee shall not be responsible for, or for investigating any matter which is the subject of, any recital, statement, representation, warranty or covenant of any person contained in this Trust Deed, or any other agreement or document relating to the transactions contemplated in this Trust Deed or under such other agreement or document.
(z) The Trustee shall be under no obligation to monitor or supervise the functions of any other person under the Notes or Coupons or any other agreement or document relating to the transactions herein or therein contemplated and shall be entitled, in the absence of actual knowledge of a breach of obligation, to assume that each such person is properly performing and complying with its obligations.
(aa) When determining whether an indemnity or any security or pre-funding is satisfactory to it, the Trustee shall be entitled, acting reasonably, to evaluate its risk in any given circumstance by considering the worst-case scenario and, for this purpose, it may take into account, without limitation, the potential costs of defending or commencing proceedings in England or elsewhere.
(bb) The Trustee shall not incur any Liability to the Issuer, Noteholders or any other person in connection with any approval given by it pursuant to Clause 6.14 to any notice to be given to Noteholders by the Issuer; the Trustee shall not be deemed to have represented, warranted, verified or confirmed that the contents of any such notice are true, accurate or complete in any respects or that it may be lawfully issued or received in any jurisdiction.
(cc) The Trustee shall not be responsible for monitoring whether any notices to Noteholders are given in compliance with the requirements of the Stock Exchange or with any other legal or regulatory requirements.
(dd) A certificate of the Auditors that in their opinion a Subsidiary is or is not or was or was at any particular period a Material Subsidiary shall, in the absence of manifest error, be conclusive and binding on the Issuer, the Trustee, the Noteholders and the Couponholders.
(ee) Notwithstanding anything contained in this Trust Deed, to the extent required by any applicable law, if the Trustee is or will be required to make any deduction or withholding from any distribution or payment made by it hereunder or if the Trustee is or will be otherwise charged to, or is or may become liable to, tax (other than, for the avoidance of doubt, taxes imposed in respect of net income by a taxing jurisdiction wherein the Trustee is incorporated or resident for tax purposes or carries on or is deemed to carry on business) as a consequence of performing its duties hereunder whether as principal, agent or otherwise, and whether by reason of any assessment, prospective assessment or other imposition of liability to taxation of whatsoever nature and whensoever made upon the Trustee, and whether in connection with or arising from any sums received or distributed by it or to which it may be entitled under this Trust Deed (other than in connection with its remuneration as provided for herein) or any investments or deposits from time to time representing the same, including any income or gains arising therefrom or any action of the Trustee in connection with the trusts of this Trust Deed (other than the remuneration herein specified) or otherwise, then the Trustee shall be entitled to make such deduction or withholding or, as the case may be, to retain out of sums received by it an amount sufficient to discharge any liability to tax which relates to sums so received or distributed or to discharge any such other liability of the Trustee to tax from the funds held by the Trustee upon the trusts of this Trust Deed.
10.2 TRUSTEE'S LIABILITY
10.2.1 Subject to section 750 of the Companies Act 2006 (if applicable), nothing in this Trust Deed shall in any case in which the Trustee has failed to show the degree of care and diligence required of it as trustee having regard to the provisions of this Trust Deed conferring on it any trusts, powers, authorities or discretions exempt the Trustee from or indemnify it against any Liability for its own gross negligence, wilful default or fraud which it may be guilty in relation to its duties under this Trust Deed.
10.2.2 Notwithstanding any provision of this Trust Deed to the contrary, the Trustee shall not in any event be liable for:
(a) loss of profit, loss of business, loss of goodwill, loss of opportunity, whether direct or indirect; and
(b) special, indirect, punitive or consequential loss or damage of any kind whatsoever,
whether or not foreseeable, even if the Trustee has been advised of the likelihood of such loss or damage, unless the claim for loss or damage is made in respect of fraud on the part of the Trustee.
10.3 Disapplication
Section 1 of the Trustee Act 2000 shall not apply to the duties of the Trustee in relation to the trusts constituted by this Trust Deed. Where there are any inconsistencies between the Trustee Acts and the provisions of this Trust Deed, the provisions of this Trust Deed shall, to the extent allowed by law, prevail and, in the case of any such inconsistency with the Trustee Act 2000, the provisions of this Trust Deed shall constitute a restriction or exclusion for the purposes of that Act.
10.4 Trustee liable for negligence
10.4.1 Subject to section 750 of the Companies Act 2006 (if applicable), nothing in this Trust Deed shall in any case in which the Trustee has failed to show the degree of care and diligence required of it as trustee having regard to the provisions of this Trust Deed conferring on it any trusts, powers, authorities or discretions exempt the Trustee from or indemnify it against any Liability for its own gross negligence, wilful default or fraud of which it may be guilty in relation to its duties under this Trust Deed.
10.4.2 Notwithstanding any provision of this Trust Deed to the contrary, the Trustee shall not in any event be liable for:
(a) loss of profit, loss of business, loss of goodwill, loss of opportunity, whether direct or indirect; and
(b) special, indirect, punitive or consequential loss or damage of any kind whatsoever,
whether or not foreseeable, even if the Trustee has been advised of the likelihood of such loss or damage, unless the claim for loss or damage is made in respect of fraud on the part of the Trustee.
11. COSTS AND EXPENSES
11.1 Remuneration
11.1.1 Normal remuneration: The Issuer shall pay to the Trustee remuneration for its services as trustee as from the date of this Trust Deed, such remuneration to be at such rate and on such dates as may from time to time be agreed in writing between the Issuer and the Trustee. Such remuneration shall accrue from day to day and be payable (in priority to payments to the Noteholders and Couponholders) up to and including the date when, all the Notes having become due for redemption, the redemption moneys and interest thereon to the date of redemption have been paid to the Principal Paying Agent or, as the case may be, the Trustee provided that if upon due presentation of any Note or Coupon, payment of the moneys due in respect thereof is improperly withheld or refused, remuneration will commence again to accrue.
11.1.2 Extra remuneration: In the event of the occurrence of an Event of Default or a Potential Event of Default, if the Trustee considers it expedient or necessary or is requested by the Issuer to undertake duties which the Trustee and the Issuer agree to be of an exceptional nature or otherwise outside the scope of the normal duties of the Trustee under this Trust Deed the Issuer shall pay to the Trustee such additional remuneration as shall be agreed between them (and which may be calculated by reference to the Trustee's normal hourly rates in force from time to time).
11.1.3 Failure to agree: In the event of the Trustee and the Issuer failing to agree:
(a) (in a case to which Clause 11.1.1 (Normal remuneration) above applies) upon the amount of the remuneration; or
(b) (in a case to which Clause 11.1.2 (Extra remuneration) above applies) upon whether such duties shall be of an exceptional nature or otherwise outside the scope of the normal duties of the Trustee under this Trust Deed, or upon such additional remuneration,
such matters shall be determined by a merchant or investment bank (acting as an expert and not as an arbitrator) selected by the Trustee and approved by the Issuer or, failing such approval, nominated (on the application of the Trustee) by the President for the time being of The Law Society of England and Wales (the expenses involved in such nomination and the fees of such merchant or investment bank being payable by the Issuer) and the determination of any such merchant or investment bank shall be final and binding upon the Trustee, the Issuer the Noteholders and the Couponholders.
11.1.4 Indemnity: Without prejudice to the right of indemnity by law given to trustees, but subject to Clauses 11.1.9 (Value Added Tax) and 11.1.10 (Income taxes), the Issuer shall indemnify the Trustee and every Appointee and keep such person indemnified against all Liabilities to which such person may be or become subject or which may be incurred by such person in the preparation and execution or purported execution of any of their trusts, powers, authorities and discretions under this Trust Deed or its or their functions under any such appointment or in respect of any other matter or thing done or omitted in any way relating to this Trust Deed or any such appointment (including all Liabilities incurred in disputing or defending any of the foregoing).
11.1.5 Expenses: The Issuer shall also pay or discharge all costs, charges and expenses (including stamp duties, levies, imposts, issue, registration, documentary and other similar taxes or duties to the extent provided for in Clause 11.2 (Stamp duties), and VAT in accordance with Clause 11.1.9 (Value Added Tax) but excluding all other taxes) incurred by the Trustee in relation to the preparation and execution of, the exercise of its powers and the performance of its duties under, and in any other manner relating to, this Trust Deed, including but not limited to travelling expenses and any stamp, issue, registration, documentary and other similar taxes or duties paid or payable by the Trustee in connection with any action properly taken by or on behalf of the Trustee for enforcing this Trust Deed.
11.1.6 Third parties: Where any amount which would otherwise be payable by the Issuer under this Clause 11.1 has instead been paid by any person or persons other than the Issuer (each, an "Indemnifying Party"), the Issuer shall pay to the Trustee an equal amount for the purpose of enabling the Trustee to reimburse the Indemnifying Parties.
11.1.7 Payments of amounts due: All amounts payable pursuant to Clause 11.1.6 (Third parties) above and/or this Clause 11.1.7 shall be payable by the Issuer on the date specified in a demand by the Trustee and in the case of payments actually made by the Trustee prior to such demand shall carry interest at the rate of three per cent. per annum above the base rate (on the date on which payment was made by the Trustee) of National Westminster Bank Plc from the date specified in such demand, and in all other cases shall (if not paid within 30 days after the date of such demand or, if such demand specifies that payment is to be made on an earlier date, on such earlier date) carry interest at such rate from such 30th day of such other date specified in such demand. All remuneration payable to the Trustee shall carry interest at such rate from the due date therefor.
11.1.8 Payments: The Issuer hereby further undertakes to the Trustee that all monies payable by the Issuer to the Trustee under this Clause 11.1 shall be made without set-off, counterclaim, deduction or withholding unless required by law. In the event of deduction or withholding being required by law, the Issuer will pay such additional amounts as will result in the receipt by the Trustee of the amounts which would otherwise have been payable by the Issuer to the Trustee under this Clause 11.1 in the absence of any such deduction or withholding.
11.1.9 Value Added Tax: All amounts payable by the Issuer under this Trust Deed are exclusive of VAT. If the Trustee or the representative member (as that term is used in the Value Added Tax Act 1994) of a group to which it belongs for VAT purposes is liable to account for VAT in respect of any service made to the Issuer in accordance with this Trust Deed, the Issuer shall pay to the Trustee (in addition to and at the same time as paying any other remuneration for such supply, and upon receipt of a valid VAT invoice) an amount equal to the amount of such VAT. Where the Issuer is required to reimburse or indemnify the Trustee for any cost or expense, the Issuer shall reimburse or indemnify (as the case may be) the Trustee for the full amount of such cost or expense, including such part thereof as represents VAT, save to the extent that the Trustee or the representative member of a group to which it belongs for VAT purposes is entitled to credit or repayment in respect of such VAT.
11.1.10 Income taxes: For the avoidance of doubt, the Trustee shall be responsible for its own corporate income tax and nothing in this Trust Deed shall require the Issuer to pay taxes imposed in respect of net income by a taxing jurisdiction wherein the Trustee is incorporated or resident or carries on or is deemed to carry on business for tax purposes.
11.1.11 Discharge: Unless otherwise specifically stated in any discharge of this Trust Deed the provisions of this Clause 11.1 shall continue in full force and effect notwithstanding such discharge.
11.2 Stamp duties
The Issuer will pay any stamp, issue, registration, documentary and other similar fees, duties and taxes, including interest and penalties, payable (a) in the United Kingdom or the Netherlands on or in connection with (i) the execution and delivery of this Trust Deed and (ii) the constitution and original issue of the Notes and the Coupons and (b) in any jurisdiction on or in connection with any action properly taken by or on behalf of the Trustee or (where permitted under this Trust Deed so to do) any Noteholder or Couponholder to enforce this Trust Deed.
11.3 Currency indemnity
The Issuer shall indemnify the Trustee, every Appointee, the Noteholders and the Couponholders and, subject to Clauses 11.1.9 (Value Added Tax) and 11.1.10 (Income taxes), keep them indemnified against:
11.3.1 any Liability incurred by any of them arising from the non-payment by the Issuer of any amount due to the Trustee or the Noteholders or Couponholders under this Trust Deed by reason of any variation in the rates of exchange between those used for the purposes of calculating the amount due under a judgment or order in respect thereof and those prevailing at the date of actual payment by the Issuer; and
11.3.2 any deficiency arising or resulting from any variation in rates of exchange between (i) the date as of which the local currency equivalent of the amounts due or contingently due under this Trust Deed (other than this Clause 11.3) is calculated for the purposes of any bankruptcy, insolvency or liquidation of the Issuer and (ii) the final date for ascertaining the amount of claims in such bankruptcy, insolvency or liquidation. The amount of such deficiency shall be deemed not to be reduced by any variation in rates of exchange occurring between the said final date and the date of any distribution of assets in connection with any such bankruptcy, insolvency or liquidation.
11.4 Indemnities separate
The indemnities in this Clause 11 constitute separate and independent obligations from the other obligations in this Trust Deed, will give rise to separate and independent causes of action, will apply irrespective of any indulgence granted by the Trustee and/or any Noteholder or Couponholder and will continue in full force and effect despite any judgment, order, claim or proof for a liquidated amount in respect of any sum due under this Trust Deed or the Notes or the Coupons or any other judgment or order. Any such Liability as referred to in this Clause 11 shall be deemed to constitute a Liability suffered by the Trustee, the Noteholders and the Couponholders and no proof or evidence of any actual Liability shall be required by the Issuer or its liquidator or liquidators.
12. APPOINTMENT AND RETIREMENT
12.1 Appointment of Trustees
The power of appointing new trustees of this Trust Deed shall be vested in the Issuer but no person shall be appointed who shall not previously have been approved by an Extraordinary Resolution of the Noteholders. A trust corporation may be appointed sole trustee hereof but subject thereto there shall be at least two trustees hereof one at least of which shall be a trust corporation. Any appointment of a new trustee hereof shall as soon as practicable thereafter be notified by the Issuer to the Agents and the Noteholders. The Noteholders shall together have the power, exercisable by Extraordinary Resolution, to remove any trustee or trustees for the time being hereof. The removal of any trustee shall not become effective unless there remains a trustee hereof (being a trust corporation) in office after such removal or until a trust corporation is appointed as successor.
12.2 Co-trustees
12.2.1 Notwithstanding the provisions of Clause 12.1 (Appointment of Trustees), the Trustee may, upon giving prior notice to the Issuer but without the consent of the Issuer or the Noteholders or the Couponholders, appoint any person established or resident in any jurisdiction (whether a trust corporation or not) to act either as a separate trustee or as a co-trustee jointly with the Trustee:
(a) if the Trustee considers such appointment to be in the interests of the Noteholders or the Couponholders; or
(b) for the purposes of conforming to any legal requirements, restrictions or conditions in any jurisdiction in which any particular act or acts are to be performed; or
(c) for the purposes of obtaining a judgment in any jurisdiction or the enforcement in any jurisdiction either of a judgment already obtained or of this Trust Deed.
12.2.2 The Issuer hereby irrevocably appoints the Trustee to be its attorney in its name and on its behalf to execute any such instrument of appointment.
12.2.3 Such a separate trustee or co-trustee shall (subject always to the provisions of this Trust Deed) have such trusts, powers, authorities and discretions (not exceeding those conferred on the Trustee by this Trust Deed) and such duties and obligations as shall be conferred on such person or imposed by the instrument of appointment.
12.2.4 The Trustee shall have power in like manner to remove any such person.
12.2.5 Such proper remuneration as the Trustee may pay to any such person, together with any attributable costs, charges and expenses incurred by it in performing its function as such separate trustee or co-trustee, shall for the purposes of this Trust Deed be treated as costs, charges and expenses incurred by the Trustee.
12.3 Retirement of Trustees
A trustee of this Trust Deed may retire at any time on giving not less than 90 days' prior written notice to the Issuer without giving any reason and without being responsible for any costs, charges and expenses properly incurred by reason of such retirement. The Noteholders may by Extraordinary Resolution remove any trustee or trustees for the time being of this Trust Deed in relation to Notes. The Issuer undertakes that in the event of the only trustee of this Trust Deed which is a Trust Corporation (for the avoidance of doubt, disregarding for this purpose any separate or co-trustee appointed under Clause 12.2 (Co-trustees) giving notice under this Clause 12.3 or being removed by Extraordinary Resolution it will use all reasonable endeavours to procure that a new trustee of this Trust Deed being a Trust Corporation is appointed as soon as reasonably practicable thereafter. The retirement or removal of any such trustee shall not become effective until a successor trustee being a Trust Corporation is appointed. If, in such circumstances, no appointment of such a new trustee has become effective within 90 days of the date of such notice or Extraordinary Resolution, the Trustee shall be entitled to appoint a Trust Corporation as trustee of this Trust Deed, but no such appointment shall take effect unless previously approved by an Extraordinary Resolution.
12.4 Competence of a majority of Trustees
Whenever there shall be more than two trustees hereof the majority of such trustees shall (provided such majority includes a trust corporation) be competent to execute and exercise all the trusts, powers, authorities and discretions vested by this Trust Deed in the Trustee generally.
12.5 Powers additional
The powers conferred by this Trust Deed upon the Trustee shall be in addition to any powers which may from time to time be vested in it by general law or as the holder of any of the Notes or the Coupons.
12.6 Merger
Any corporation into which the Trustee may be merged or converted or with which it may be consolidated, or any corporation resulting from any merger, conversion or consolidation to which the Trustee shall be a party, or any corporation succeeding to all or substantially all the corporate trust business of the Trustee, shall be the successor of the Trustee hereunder, provided such corporation shall be otherwise qualified and eligible under this Clause 12.6, without the execution or filing of any paper or any further act on the part of any of the parties hereto.
13. NOTICES
13.1 Addresses for notices
All notices and other communications hereunder shall be made in writing and in English (by letter or email) and shall be sent as follows:
13.1.1 Issuer: if to the Issuer, to it at:
Koninklijke Philips N.V.
Philips Center
Amstelplein 2
1096 BC Amsterdam
The Netherlands
Attention: Group Treasury
Email: treasury.middleoffice@philips.com
13.1.2 Trustee: if to the Trustee, to it at:
Citicorp Trustee Company Limited
Citigroup Centre
Canada Square
London E14 5LB
England
Email: emea.at.debt@citi.com
Attention: Agency & Trust
13.2 Effectiveness
Every notice or other communication sent in accordance with Clause 13.1 (Addresses for notices) shall be effective as follows:
13.2.1 Letter: if sent by letter, it shall be deemed to have been delivered 7 days after the time of despatch; and
13.2.2 Email: if sent by email, it shall be deemed to have been delivered at the time of delivery to the recipient's email address,
provided that any such notice or other communication which would otherwise take effect after 4.00 p.m. on any particular day shall not take effect until 10.00 a.m. on the immediately succeeding Business Day in the place of the addressee, and further provided that in the case of a notice or demand given by email a delivery receipt is received by the sending party confirming the email has been delivered to the recipient's correct email address.
13.3 No Notice to Couponholders
Neither the Trustee nor the Issuer shall be required to give any notice to the Couponholders for any purpose under this Trust Deed and the Couponholders shall be deemed for all purposes to have notice of the contents of any notice given to the Noteholders in accordance with Condition 13 (Notices).
14. LAW AND JURISDICTION
14.1 Governing Law
This Trust Deed and the Notes and any non-contractual obligations arising out of or in connection with them are governed by English law.
14.2 English Courts
The courts of England have exclusive jurisdiction to settle any disputes (a "Dispute"), arising from or connected with this Trust Deed or the Notes (including a dispute regarding the existence, validity or termination of, and all non-contractual obligations arising out of or in connection with, this Trust Deed or the Notes) or the consequences of their nullity.
14.3 Appropriate forum
The parties agree that the courts of England are the most appropriate and convenient courts to settle Disputes and, accordingly that they will not argue to the contrary.
14.4 Rights of the Issuer and the Trustee to take proceedings outside England
Notwithstanding Clauses 14.2 (English Courts) and 14.3 (Appropriate forum), to the extent allowed by law, the Issuer and the Trustee may, in respect of any Dispute or Disputes, take (i) proceedings in any other court with jurisdiction and (ii) concurrent proceedings in any number of jurisdictions.
14.5 Process agent
The Issuer agrees that the documents which start any Proceedings and any other documents required to be served in relation to those Proceedings may be served on it by being delivered to Philips Electronics UK Limited (Attention: Company Secretary), Philips Centre, Guildford Business Park, Guildford, Surrey GU2 8XG. If such person is not or ceases to be effectively appointed to accept service of process on behalf of the Issuer, the Issuer shall, on the written demand of the Trustee, appoint a further person in England to accept service of process on their behalf and, failing such appointment within 15 days, the Trustee shall be entitled to appoint such a person by written notice addressed to the Issuer. Nothing in this paragraph shall affect the right of the Trustee or (when they are entitled to do so) any of the Noteholders to serve process in any other manner permitted by law.
14.6 Power of Attorney
If the Issuer is represented by an attorney or attorneys in connection with the signing and/or execution and/or delivery of this Trust Deed or any agreement or document referred to herein or made pursuant hereto and the relevant power or powers of attorney is or are expressed to be governed by the laws of the Netherlands, it is hereby expressly acknowledged and accepted by the other parties hereto that such laws shall govern the existence and extent of such attorney's or attorney's authority and the effects of the exercise thereof.
15. SEVERABILITY
In case any provision in or obligation under this Trust Deed shall be invalid, illegal or unenforceable in any jurisdiction, the validity, legality and enforceability of the remaining provisions or obligations, or of such provision or obligation in any other jurisdiction, shall not in any way be affected or impaired thereby.
16. CONTRACTS (RIGHTS OF THIRD PARTIES) ACT 1999
No person shall have any right to enforce any provision of this Trust Deed under the Contracts (Rights of Third Parties) Act 1999.
17. COUNTERPARTS
This Trust Deed may be executed in any number of counterparts, each of which shall be deemed an original.
IN WITNESS WHEREOF this Trust Deed has been executed as a deed by the parties hereto and is intended to be and is hereby delivered on the date first before written.
SCHEDULE 1
TERMS AND CONDITIONS
[to be inserted]
SCHEDULE 2
FORMS OF THE NOTES
PART A
FORM OF TEMPORARY GLOBAL NOTE
Series Number: [ ]
Serial Number: [ ]
[Tranche Number: [ ]]
[ANY UNITED STATES PERSON WHO HOLDS THIS OBLIGATION WILL BE SUBJECT TO LIMITATIONS UNDER THE UNITED STATES INCOME TAX LAWS, INCLUDING LIMITATIONS PROVIDED IN SECTIONS 165(j) AND 1287(a) OF THE UNITED STATES INTERNAL REVENUE CODE OF 1986, AS AMENDED.][1]
KONINKLIJKE PHILIPS N.V.
EURO MEDIUM TERM NOTE PROGRAMME
TEMPORARY GLOBAL NOTE
representing up to
[Aggregate principal amount of tranche]
[Title of Notes ]
€10,000,000,000
Euro Medium Term Note Programme
This global Note is a Temporary Global Note without interest coupons issued in respect of an issue of [aggregate principal amount of Tranche] in aggregate principal amount of [title of Notes] (the "Notes") by Koninklijke Philips N.V. a public limited liability company (naamloze vennootschap) incorporated under the law of the Netherlands registered at the Dutch Chamber of Commerce with number 17001910 (the "Issuer").
This Temporary Global Note is issued subject to and in accordance with the Conditions and trust deed (as amended and/or supplemented and/or restated from time to time, the "Trust Deed") dated 9 March 2020 and as amended and restated on 8 March 2022 between, amongst others, the Issuer and Citicorp Trustee Company Limited as Trustee (the "Trustee", which expression includes all persons for the time being appointed Trustee or Trustees under the Trust Deed) and is subject to an amended and restated Agency Agreement (as amended and/or supplemented and/or restated from time to time, the "Agency Agreement") dated 8 March 2022 between the Issuer, amongst others, Citibank, N.A., London Branch, the Trustee and certain other financial institutions names therein. References herein to the "Conditions" shall be to the Terms and Conditions of the Notes as set out in Schedule 1 to the Trust Deed as completed by the final terms applicable to the Notes (the "Final Terms") but, in the event of any conflict between the provisions of the Conditions and the information in the Final Terms, the Final Terms will prevail. Words and expressions defined in the Conditions shall bear the same meanings when used in this Temporary Global Note.
[1] Legend to appear on every Temporary Global Note representing Notes with a maturity of more than one year.
1. PROMISE TO PAY
1.1 Pay to bearer
The Issuer, for value received, promises to pay to the bearer of this Temporary Global Note, in respect of each Note represented by this Temporary Global Note, the Redemption Amount on the Maturity Date or on such earlier date or dates as the same may become payable in accordance with the Conditions (or to pay such other amounts of principal on such dates as may be specified in the Final Terms), and to pay interest on each such Note on the dates and in the manner specified in the Conditions, together with any additional amounts payable in accordance with the Conditions, all subject to and in accordance with the Conditions; provided, however, that such interest shall be payable only:
(a) Before the Exchange Date: in the case of interest falling due before the Exchange Date (as defined below), to the extent that a certificate or certificates issued by Euroclear Bank SA/NV ("Euroclear") and/or Clearstream Banking S.A. ("Clearstream, Luxembourg", together with Euroclear, the international central securities depositaries or "ICSDs") and/or any other relevant clearing system dated not earlier than the date on which such interest falls due and in substantially the form set out in Schedule 3 (Form of Euroclear/Clearstream, Luxembourg Certification) hereto is/are delivered to the Specified Office of the Principal Paying Agent; or
(b) Failure to exchange: in the case of interest falling due at any time, to the extent that the Issuer has failed to procure the exchange for a permanent global note of that portion of this Temporary Global Note in respect of which such interest has accrued.
1.2 NGN Principal Amount
If the Final Terms specify that the New Global Note form is applicable, this Temporary Global Note shall be a "New Global Note" or "NGN" and the principal amount of Notes represented by this Temporary Global Note shall be the aggregate amount from time to time entered in the records of both ICSDs. The records of the ICSDs (which expression in this Temporary Global Note means the records that each ICSD holds for its customers which reflect the amount of such customers' interests in the Notes (but excluding any interest in any Notes of one ICSD shown in the records of another ICSD)) shall be conclusive evidence of the principal amount of Notes represented by this Temporary Global Note and, for these purposes, a statement issued by an ICSD (which statement shall be made available to the bearer upon request) stating the principal amount of Notes represented by this Temporary Global Note at any time shall be conclusive evidence of the records of the ICSD at that time.
1.3 CGN Principal Amount
If the Final Terms specify that the New Global Note form is not applicable, this Temporary Global Note shall be a "Classic Global Note" or "CGN" and the principal amount of Notes represented by this Temporary Global Note shall be the amount stated in the Final Terms or, if lower, the principal amount most recently entered by or on behalf of the Issuer in the relevant column in Schedule 1 (Payments, Exchange and Cancellation of Notes).
2. NEGOTIABILITY
This Temporary Global Note is negotiable and, accordingly, title to this Temporary Global Note shall pass by delivery.
3. EXCHANGE
3.1 Permanent Global Note
If the Final Terms specify the form of Notes as being "Temporary Global Note exchangeable for a Permanent Global Note", then on or after the day following the expiry of 40 days after the date of issue of this Temporary Global Note (the "Exchange Date"), the Issuer shall procure (in the case of first exchange) the delivery of a Permanent Global Note (which expression has the meaning given in the Trust Deed) in accordance with the Agency Agreement to the bearer of this Temporary Global Note or (in the case of any subsequent exchange) an increase in the principal amount of the Permanent Global Note in accordance with its terms against:
(a) Presentation and surrender: presentation and (in the case of final exchange) presentation and surrender of this Temporary Global Note to or to the order of the Principal Paying Agent; and
(b) Certification: receipt by the Principal Paying Agent of a certificate or certificates issued by Euroclear and/or Clearstream, Luxembourg and/or any other relevant clearing system dated not earlier than the Exchange Date and in substantially the form set out in Schedule 3 (Form of Euroclear/Clearstream, Luxembourg Certification) hereto.
The principal amount of Notes represented by the Permanent Global Note shall be equal to the aggregate of the principal amounts specified in the certificates issued by Euroclear and/or Clearstream, Luxembourg and/or any other relevant clearing system and received by the Principal Paying Agent; provided, however, that in no circumstances shall the principal amount of Notes represented by the Permanent Global Note exceed the initial principal amount of Notes represented by this Temporary Global Note.
3.2 Definitive Notes; Not D Rules
If the Final Terms specify the form of Notes as being "Temporary Global Note exchangeable for Definitive Notes" and also specify that the C Rules are applicable or that neither the C Rules nor the D Rules are applicable, then on or after the day following the expiry of 40 days after the date of issue of this Temporary Global Note (the "Exchange Date"), the Issuer shall procure the delivery of Definitive Notes (which expression has the meaning given in the Agency Agreement) in accordance with the Agency Agreement with Coupons and Talons (if so specified in the Final Terms) attached and in an aggregate principal amount equal to the principal amount of Notes represented by this Temporary Global Note to the bearer of this Temporary Global Note against presentation and surrender of this Temporary Global Note to or to the order of the Principal Paying Agent.
3.3 Definitive Notes; D Rules
If the Final Terms specify the form of Notes as being "Temporary Global Note exchangeable for Definitive Notes" and also specifies that the D Rules are applicable, then on or after the day following the expiry of 40 days after the date of issue of this Global Note (the "Exchange Date"), the Issuer shall procure the delivery of Definitive Notes (which expression has the meaning given in the Agency Agreement) in accordance with the Agency Agreement with Coupons and Talons (if so specified in the Final Terms) attached against:
(a) Presentation and surrender: presentation and (in the case of final exchange) surrender of this Temporary Global Note to or to the order of the Principal Paying Agent; and
(b) Certification: receipt by the Principal Paying Agent of a certificate or certificates issued by Euroclear and/or Clearstream, Luxembourg and/or any other relevant clearing system dated not earlier than the Exchange Date and in substantially the form set out in Schedule 3 (Form of Euroclear/Clearstream, Luxembourg Certification) hereto.
The Definitive Notes so delivered from time to time shall be in an aggregate principal amount equal to the aggregate of the principal amounts specified in the certificates issued by Euroclear and/or Clearstream, Luxembourg and/or any other relevant clearing system and received by the Principal Paying Agent; provided, however, that in no circumstances shall the aggregate principal amount of Definitive Notes so delivered exceed the initial principal amount of Notes represented by this Temporary Global Note.
4. DELIVERY OF PERMANENT GLOBAL OR DEFINITIVE NOTES
4.1 Permanent Global Note
Whenever any interest in this Temporary Global Note is to be exchanged for an interest in a Permanent Global Note, the Issuer shall procure (in the case of first exchange) the prompt delivery (free of charge to the bearer) of such Permanent Global Note, duly authenticated, to the bearer of this Temporary Global Note or (in the case of any subsequent exchange) an increase in the principal amount of Notes represented by such Permanent Global Note in accordance with its terms, in each case in an aggregate principal amount equal to the aggregate of the principal amounts specified in the certificates issued by Euroclear and/or Clearstream, Luxembourg and/or any other relevant clearing system and received by the Principal Paying Agent against presentation and (in the case of final exchange) surrender of this Temporary Global Note to or to the order of the Principal Paying Agent within 7 days of the bearer requesting such exchange.
4.2 Definitive Notes
Whenever this Temporary Global Note is to be exchanged for Definitive Notes, the Issuer shall procure the prompt delivery (free of charge to the bearer) of such Definitive Notes, duly authenticated and with Coupons and Talons attached (if so specified in the Final Terms), in an aggregate principal amount equal to the principal amount of Notes represented by this Temporary Global Note to the bearer of this Temporary Global Note against the surrender of this Temporary Global Note to or to the order of the Principal Paying Agent within 30 days of the bearer requesting such exchange.
5. WRITING DOWN
On each occasion on which:
(a) Permanent Global Note: the Permanent Global Note is delivered or the principal amount of Notes represented thereby is increased in accordance with its terms in exchange for a further portion of this Temporary Global Note; or
(b) Definitive Notes: Definitive Notes are delivered in exchange for this Temporary Global Note; or
(c) Cancellation: Notes represented by this Temporary Global Note are to be cancelled in accordance with Condition 7.8 (Redemption and Purchase - Cancellations),
the Issuer shall procure that:
(i) if the Final Terms specify that the New Global Note form is not applicable, (i) the principal amount of Notes represented by the Permanent Global Note, the principal amount of such increase or (as the case may be) the aggregate principal amount of such Notes and (ii) the remaining principal amount of Notes represented by this Temporary Global Note (which shall be the previous principal amount of Notes represented by this Temporary Global Note less the aggregate of the amounts referred to in (i)) are entered in Schedule 1 (Payments, Exchange and Cancellation of Notes) hereto, whereupon the principal amount of Notes represented by this Temporary Global Note shall for all purposes be as most recently so entered; and
(ii) if the Final Terms specify that the New Global Note form is applicable, details of the exchange or cancellation shall be entered pro rata in the records of the ICSDs.
6. PAYMENTS
6.1 Recording of Payments
Upon any payment being made in respect of the Notes represented by this Temporary Global Note, the Issuer shall procure that:
(a) CGN: if the Final Terms specify that the New Global Note form is not applicable, details of such payment shall be entered in Schedule 1 (Payments, Exchange and Cancellation of Notes) hereto and, in the case of any payment of principal, the principal amount of the Notes represented by this Temporary Global Note shall be reduced by the principal amount so paid; and
(b) NGN: if the Final Terms specify that the New Global Note form is applicable, details of such payment shall be entered pro rata in the records of the ICSDs and, in the case of any payment of principal, the principal amount of the Notes entered in the records of ICSDs and represented by this Temporary Global Note shall be reduced by the principal amount so paid.
6.2 Discharge of Issuer's obligations
Payments due in respect of Notes for the time being represented by this Temporary Global Note shall be made to the bearer of this Temporary Global Note and each payment so made will discharge the Issuer's obligations in respect thereof. Any failure to make the entries referred to above shall not affect such discharge.
7. CONDITIONS APPLY
Until this Temporary Global Note has been exchanged as provided herein or cancelled in accordance with the Agency Agreement, the bearer of this Temporary Global Note shall be subject to the Conditions and, subject as otherwise provided herein, shall be entitled to the same rights and benefits under the Conditions as if the bearer were the holder of Definitive Notes and any related Coupons and Talons in the smallest Specified Denomination and in an aggregate principal amount equal to the principal amount of the Notes represented by this Temporary Global Note.
8. AUTHENTICATION
This Temporary Global Note shall not be valid or enforceable for any purpose unless and until it has been authenticated for and on behalf of Citibank, N.A., London Branch as principal paying agent.
9. EFFECTUATION
If the Final Terms specify that the New Global Note form is applicable, this Temporary Global Note shall not be valid or enforceable for any purpose unless and until it has been effectuated for and on behalf of the entity appointed as common safekeeper by the ICSDs.
10. CONTRACTS (RIGHTS OF THIRD PARTIES) ACT 1999
No rights are conferred on any person under the Contracts (Rights of Third Parties) Act 1999 to enforce any term of this Temporary Global Note, but this does not affect any right or remedy of a third party which exists or is available apart from that Act.
11. NOTICES
Any notice or demand to the Issuer or the Trustee to be given, made or served for any purposes under this Temporary Global Note shall be given, made or served by sending the same by pre-paid post (first class if inland, first class airmail if overseas), email (if applicable), facsimile transmission (if applicable) or by delivering it by hand as follows:
to the Issuer: Koninklijke Philips N.V.
Philips Center
Amstelplein 2
1096 BC Amsterdam
The Netherlands
Attention: Group Treasury
Email: treasury.middleoffice@philips.com
to the Trustee: Citicorp Trustee Company Limited
Citigroup Centre
Canada Square
London E14 5LB
England
Email: emea.at.debt@citi.com
Attention: Agency & Trust
or to such other address or email address (if applicable) as shall have been notified (in accordance with this Clause 11) to the other party hereto and any notice or demand sent by post as aforesaid shall be deemed to have been given, made or served two days in the case of inland post or seven days in the case of overseas post after despatch, any notice or demand sent by email as aforesaid shall be deemed to have been given, made or served at the time of despatch and any notice or demand given by facsimile, when a transmission report showing the successful transmission of the facsimile is received by the sender provided that in the case of a notice or demand given by email a delivery receipt is received by the sending party confirming the email has been delivered to the recipient's correct email address.
12. GOVERNING LAW
This Temporary Global Note and any non-contractual obligations arising out of or in connection with it are governed by English law.
AS WITNESS the [manual/facsimile] signature of a duly authorised person on behalf of the Issuer.
KONINKLIJKE PHILIPS N.V.
By: ………………………………..
[manual or facsimile signature]
(duly authorised)
Name: ……………………………
Title: Authorised Signatory
ISSUED on the Issue Date
AUTHENTICATED for and on behalf of
CITIBANK, N.A., LONDON BRANCH
as principal paying agent without recourse, warranty or liability
By: ………………………………..
[manual signature]
(duly authorised)
EFFECTUATED for and on behalf of
as common safekeeper without
recourse, warranty or liability
By: ………………………………..
[manual signature]
(duly authorised)
Schedule 1
Payments, Exchange and Cancellation of Notes
[2]
|
Date of payment, delivery or cancellation |
Amount of interest then paid |
Principal amount of Permanent Global Note then delivered or by which Permanent Global Note then increased or aggregate principal amount of Definitive Notes then delivered |
Aggregate principal amount of Notes then cancelled |
Remaining principal amount of this Temporary Global Note |
Authorised Signature |
[2] Schedule 1 should only be completed where the Final Terms specify that the New Global Note form is not applicable.
Schedule 2
Form of Accountholder's Certification
KONINKLIJKE PHILIPS N.V.
EURO MEDIUM TERM NOTE PROGRAMME
This is to certify that as of the date hereof, and except as set forth below, the above-captioned Securities held by you for our account (a) are owned by persons that are not citizens or residents of the United States, United States partnerships, United States corporations or any estate or trust the income of which is subject to United States Federal income taxation regardless of its source ("United States persons"), (b) are owned by United States person(s) that (i) are foreign branches of a United States financial institution (as defined in U.S. Treasury Regulations Section 1.165-12(c)(1)(iv)) ("financial institutions") purchasing for their own account or for resale, or (ii) acquired the Securities through foreign branches of United States financial institutions and who hold the Securities through such United States financial institutions on the date hereof (and in either case (i) or (ii), each such United States financial institution hereby agrees, on its own behalf or through its agent, that you may advise the issuer or the issuer's agent that it will comply with the requirements of Section 165(j)(3)(A), (B) or (C) of the Internal Revenue Code of 1986, as amended, and the regulations thereunder), or (c) are owned by United States or foreign financial institution(s) for purposes of resale during the restricted period (as defined in U.S. Treasury Regulations Section 1.163-5(c)(2)(i)(D)(7)), and in addition if the owner of the Securities is a United States or foreign financial institution described in clause (c) (whether or not also described in clause (a) or (b)) this is to further certify that such financial institution has not acquired the Securities for purposes of resale directly or indirectly to a United States person or to a person within the United States or its possessions.
As used herein, "United States" means the United States of America (including the States and the District of Columbia); and its "possessions" include Puerto Rico, the U.S. Virgin Islands, Guam, American Samoa, Wake Island and the Northern Mariana Islands.
We undertake to advise you promptly by tested telex on or prior to the date on which you intend to submit your certification relating to the Securities held by you for our account in accordance with your operating procedures if any applicable statement herein is not correct on such date, and in the absence of any such notification it may be assumed that this certification applies as of such date.
[This certification excepts and does not relate to [currency] [amount] of such interest in the above Securities in respect of which we are not able to certify and as to which we understand exchange and delivery of definitive Securities (or, if relevant, exercise of any rights or collection of any interest) cannot be made until we do so certify.]
We understand that this certification is required in connection with certain tax laws and, if applicable, certain securities laws of the United States. In connection therewith, if administrative or legal proceedings are commenced or threatened in connection with which this certification is or would be relevant, we irrevocably authorise you to produce this certification to any interested party in such proceedings.
Dated: [ ]
[name of account holder]
as, or as agent for,
the beneficial owner(s) of the Securities
to which this certificate relates.
By:................................................................
Authorised signatory
Schedule 3
Form of Euroclear/Clearstream, Luxembourg Certification
KONINKLIJKE PHILIPS N.V.
EURO MEDIUM TERM NOTE PROGRAMME
This is to certify that, based solely on certifications we have received in writing, by tested telex or by electronic transmission from member organisations appearing in our records as persons being entitled to a portion of the principal amount set forth below (our "Member Organisations") substantially to the effect set forth in the temporary global note issued in respect of the securities, as of the date hereof, [currency] [amount] principal amount of the above-captioned Securities (a) is owned by persons that are not citizens or residents of the United States, United States partnerships, United States corporations or any estate or trust the income of which is subject to United States Federal income taxation regardless of its source ("United States persons"), (b) is owned by United States persons that (i) are foreign branches of United States financial institutions (as defined in U.S. Treasury Regulations Section 1.165-12(c)(1)(iv)) ("financial institutions") purchasing for their own account or for resale, or (ii) acquired the Securities through foreign branches of United States financial institutions and who hold the Securities through such United States financial institutions on the date hereof (and in either case (i) or (ii), each such United States financial institution has agreed, on its own behalf or through its agent, that we may advise the Issuer or the Issuer's agent that it will comply with the requirements of Section 165(j)(3)(A), (B) or (C) of the Internal Revenue Code of 1986, as amended, and the regulations thereunder), or (c) is owned by United States or foreign financial institutions for purposes of resale during the restricted period (as defined in U.S. Treasury Regulations Section 1.163-5(c)(2)(i)(D)(7)), and to the further effect that United States or foreign financial institutions described in clause (c) (whether or not also described in clause (a) or (b)) have certified that they have not acquired the Securities for purposes of resale directly or indirectly to a United States person or to a person within the United States or its possessions.
We further certify (1) that we are not making available herewith for exchange (or, if relevant, exercise of any rights or collection of any interest) any portion of the temporary global security excepted in such certifications and (2) that as of the date hereof we have not received any notification from any of our Member Organisations to the effect that the statements made by such Member Organisations with respect to any portion of the part submitted herewith for exchange (or, if relevant, exercise of any rights or collection of any interest) are no longer true and cannot be relied upon as of the date hereof.
We understand that this certification is required in connection with certain tax laws and, if applicable, certain securities laws of the United States. In connection therewith, if administrative or legal proceedings are commenced or threatened in connection with which this certification is or would be relevant, we irrevocably authorise you to produce this certification to any interested party in such proceedings.
Dated: [ ]
Euroclear Bank SA/NV
or
Clearstream Banking S.A.
By:................................................................
Authorised signatory
FORM OF PERMANENT GLOBAL NOTE
[ANY UNITED STATES PERSON WHO HOLDS THIS OBLIGATION WILL BE SUBJECT TO LIMITATIONS UNDER THE UNITED STATES INCOME TAX LAWS, INCLUDING THE LIMITATIONS PROVIDED IN SECTIONS 165(j) AND 1287(a) OF THE INTERNAL REVENUE CODE.][3]
[3] Legend to appear on every Permanent Global Note representing Notes with a maturity of more than one year.
KONINKLIJKE PHILIPS N.V.
EURO MEDIUM TERM NOTE PROGRAMME
PERMANENT GLOBAL NOTE
representing up to
[Aggregate principal amount of Tranche]
[Title of Notes]
€10,000,000,000
Euro Medium Term Note Programme
This global Note is a Permanent Global Note without interest coupons issued in respect of an issue of [aggregate principal amount of Tranche] in aggregate principal amount of [title of Notes] (the "Notes") by Koninklijke Philips N.V. a public limited liability company (naamloze vennootschap) incorporated under the law of the Netherlands registered at the Dutch Chamber of Commerce with number 17001910 (the "Issuer").
This Permanent Global Note is issued subject to and in accordance with the Conditions and a trust deed (as amended and/or supplemented and/or restated from time to time, the "Trust Deed") dated 9 March 2020 and as amended and restated on 8 March 2022 between, amongst others, the Issuer and Citicorp Trustee Company Limited as trustee (the "Trustee", which expression includes all persons for the time being appointed Trustee or Trustees under the Trust Deed) and is subject to an amended and restated Agency Agreement (as amended and/or supplemented and/or restated from time to time, the "Agency Agreement") dated 8 March 2022 between the Issuer, amongst others, Citibank, N.A., London Branch, the Trustee and certain other financial institutions names therein. References herein to the "Conditions" shall be to the Terms and Conditions of the Notes as set out in Schedule 1 to the Trust Deed as completed by the final terms applicable to the Notes (the "Final Terms") attached hereto but, in the event of any conflict between the provisions of the Conditions and the information in the Final Terms, the Final Terms will prevail. Words and expressions defined in the Conditions shall bear the same meanings when used in this Permanent Global Note.
1. PROMISE TO PAY
1.1 Pay to bearer
The Issuer, for value received, promises to pay to the bearer of this Global Note, in respect of each Note represented by this Global Note, the Redemption Amount on the Maturity Date or on such earlier date or dates as the same may become payable in accordance with the Conditions (or to pay such other amounts of principal on such dates as may be specified in the Final Terms), and to pay interest on each such Note on the dates and in the manner specified in the Conditions, together with any additional amounts payable in accordance with the Conditions, all subject to and in accordance with the Conditions.
1.2 NGN Principal Amount
If the Final Terms specify that the New Global Note form is applicable, this Global Note shall be a "New Global Note" or "NGN" and the principal amount of Notes represented by this Global Note shall be the aggregate amount from time to time entered in the records of both Euroclear Bank SA/NV ("Euroclear") and/or Clearstream Banking S.A. ("Clearstream, Luxembourg", together with Euroclear, the international central securities depositaries or "ICSDs"). The records of the ICSDs (which expression in this Global Note means the records that each ICSD holds for its customers which reflect the amount of such customers' interests in the Notes (but excluding any interest in any Notes of one ICSD shown in the records of another ICSD)) shall be conclusive evidence of the principal amount of Notes represented by this Global Note and, for these purposes, a statement issued by an ICSD (which statement shall be made available to the bearer upon request) stating the principal amount of Notes represented by this Global Note at any time shall be conclusive evidence of the records of the ICSD at that time.
1.3 CGN Principal Amount
If the Final Terms specify that the New Global Note form is not applicable, this Global Note shall be a "Classic Global Note" or "CGN" and the principal amount of Notes represented by this Global Note shall be the amount stated in the Final Terms or, if lower, the principal amount most recently entered by or on behalf of the Issuer in the relevant column in Schedule 1 (Payments, Exchange and Cancellation of Notes).
2. NEGOTIABILITY
This Global Note is negotiable and, accordingly, title to this Global Note shall pass by delivery.
3. EXCHANGE
Upon the occurrence of an Exchange Event (as further described below), this Global Note will become exchangeable, in whole but not in part only and at the request of the bearer of this Global Note, for Definitive Notes (which expression has the meaning given in the Trust Deed) in accordance with the Agency Agreement.
An Exchange Event will occur:
(a) upon the happening of any of the events defined as an "Event of Default" in Condition 10 (Events of Default); or
(b) if the Issuer has been notified that both Euroclear Bank SA/NV ("Euroclear") and Clearstream Banking, S.A. ("Clearstream, Luxembourg" and, together with Euroclear, the "relevant Clearing Systems") have announced an intention permanently to cease business or have in fact done so and no successor clearing system satisfactory to the Trustee is available; or
(c) if the Issuer has or will become subject to adverse tax consequences which would not be suffered were the Notes in definitive form and a certificate to such effect signed by an Authorised Signatory (as defined in the Trust Deed) of the Issuer is given to the Trustee.
4. DELIVERY OF DEFINITIVE NOTES
Whenever this Global Note is to be exchanged for Definitive Notes, the Issuer shall procure the prompt delivery (free of charge to the bearer) of such Definitive Notes, duly authenticated and with Coupons and Talons attached (if so specified in the Final Terms), in an aggregate principal amount equal to the principal amount of Notes represented by this Global Note to the bearer of this Global Note against the surrender of this Global Note to or to the order of the Principal Paying Agent within 30 days of the bearer requesting such exchange.
5. WRITING DOWN
On each occasion on which:
(a) Payment of principal: a payment of principal is made in respect of this Global Note;
(b) Definitive Notes: Definitive Notes are delivered; or
(c) Cancellation: Notes represented by this Global Note are to be cancelled in accordance with Condition 8 (Redemption and Purchase - Cancellations),
the Issuer shall procure that:
(i) if the Final Terms specify that the New Global Note form is not applicable, (i) the amount of such payment and the aggregate principal amount of such Notes; and (ii) the remaining principal amount of Notes represented by this Global Note (which shall be the previous principal amount hereof less the aggregate of the amounts referred to in (i) above) are entered in Schedule 1 (Payments, Exchanges against Temporary Global Note, Delivery of Definitive Notes and Cancellation of Notes) hereto, whereupon the principal amount of Notes represented by this Global Note shall for all purposes be as most recently so entered; and
(ii) if the Final Terms specify that the New Global Note form is applicable, details of the exchange or cancellation shall be entered pro rata in the records of the ICSDs.
6. WRITING UP
6.1 Initial Exchange
If this Global Note was originally issued in exchange for part only of a temporary global note representing the Notes, then all references in this Global Note to the principal amount of Notes represented by this Global Note shall be construed as references to the principal amount of Notes represented by the part of the temporary global note in exchange for which this Global Note was originally issued which the Issuer shall procure:
(a) CGN: if the Final Terms specify that the New Global Note form is not applicable, is entered in Schedule 1 (Payments, Exchanges against Temporary Global Note, Delivery of Definitive Notes and Cancellation of Notes) hereto, whereupon the principal amount of Notes represented by this Global Note shall for all purposes be as most recently so entered; and
(b) NGN: if the Final Terms specify that the New Global Note form is applicable, is entered by the ICSDs in their records.
6.2 Subsequent Exchange
If at any subsequent time any further portion of such temporary global note is exchanged for an interest in this Global Note, the principal amount of Notes represented by this Global Note shall be increased by the amount of such further portion, and the Issuer shall procure that the principal amount of Notes represented by this Global Note (which shall be the previous principal amount of Notes represented by this Global Note plus the amount of such further portion) is:
(a) CGN: if the Final Terms specify that the New Global Note form is not applicable, entered in Schedule 1 (Payments, Exchanges against Temporary Global Note, Delivery of Definitive Notes and Cancellation of Notes) hereto, whereupon the principal amount of this Global Note shall for all purposes be as most recently so entered; and
(b) NGN: if the Final Terms specify that the New Global Note form is applicable, entered by the ICSDs in their records.
7. PAYMENTS
7.1 Recording of Payments
Upon any payment being made in respect of the Notes represented by this Global Note, the Issuer shall procure that:
(a) CGN: if the Final Terms specify that the New Global Note form is not applicable, details of such payment shall be entered in Schedule 1 (Payments, Exchange and Cancellation of Notes) hereto and, in the case of any payment of principal, the principal amount of the Notes represented by this Global Note shall be reduced by the principal amount so paid; and
(b) NGN: if the Final Terms specify that the New Global Note form is applicable, details of such payment shall be entered pro rata in the records of the ICSDs and, in the case of any payment of principal, the principal amount of the Notes entered in the records of ICSDs and represented by this Global Note shall be reduced by the principal amount so paid.
7.2 Discharge of Issuer's obligations
Payments due in respect of Notes for the time being represented by this Global Note shall be made to the bearer of this Global Note and each payment so made will discharge the Issuer's obligations in respect thereof. Any failure to make the entries referred to above shall not affect such discharge.
8. CONDITIONS APPLY
Until this Global Note has been exchanged as provided herein or cancelled in accordance with the Agency Agreement, the bearer of this Global Note shall be subject to the Conditions and, subject as otherwise provided herein, shall be entitled to the same rights and benefits under the Conditions as if the bearer were the Holder of Definitive Notes and any related Coupons and Talons in the smallest Specified Denomination and in an aggregate principal amount equal to the principal amount of Notes represented by this Global Note.
9. AUTHENTICATION
This Global Note shall not be valid or enforceable for any purpose unless and until it has been authenticated for and on behalf of Citibank, N.A., London Branch as principal paying agent.
10. EFFECTUATION
If the Final Terms specify that the New Global Note form is applicable, this Permanent Global Note shall not be valid or enforceable for any purpose unless and until it has been effectuated for and on behalf of the entity appointed as common safekeeper by the ICSDs.
11. CONTRACTS (RIGHTS OF THIRD PARTIES) ACT 1999
No rights are conferred on any person under the Contracts (Rights of Third Parties) Act 1999 to enforce any term of this Global Note, but this does not affect any right or remedy of a third party which exists or is available apart from that Act.
12. NOTICES
Any notice or demand to the Issuer or the Trustee to be given, made or served for any purposes under this Global Note shall be given, made or served by sending the same by pre-paid post (first class if inland, first class airmail if overseas), email (if applicable), facsimile transmission (if applicable) or by delivering it by hand as follows:
to the Issuer: Koninklijke Philips N.V.
Philips Center
Amstelplein 2
1096 BC Amsterdam
The Netherlands
Attention: Group Treasury
Email: treasury.middleoffice@philips.com
to the Trustee: Citicorp Trustee Company Limited
Citigroup Centre
Canada Square
London E14 5LB
England
Email: emea.at.debt@citi.com
Attention: Agency & Trust
or to such other address or email address (if applicable) as shall have been notified (in accordance with this Clause 12) to the other party hereto and any notice or demand sent by post as aforesaid shall be deemed to have been given, made or served two days in the case of inland post or seven days in the case of overseas post after despatch, any notice or demand sent by email as aforesaid shall be deemed to have been given, made or served at the time of despatch and any notice or demand given by facsimile, when a transmission report showing the successful transmission of the facsimile is received by the sender provided that in the case of a notice or demand given by email a delivery receipt is received by the sending party confirming the email has been delivered to the recipient's correct email address.
13. GOVERNING LAW
This Global Note and any non-contractual obligations arising out of or in connection with it are governed by English law.
AS WITNESS the [manual/facsimile] signature of a duly authorised person on behalf of the Issuer.
KONINKLIJKE PHILIPS N.V.
By: ………………………………..
[manual or facsimile signature]
(duly authorised)
Name: ……………………………
Title: Authorised Signatory
ISSUED on the Issue Date
AUTHENTICATED for and on behalf of
CITIBANK, N.A., LONDON BRANCH as principal paying agent without recourse, warranty or liability
By:................................................................
[manual signature]
(duly authorised)
EFFECTUATED for and on behalf of
as common safekeeper without
recourse, warranty or liability
By:................................................................
[manual signature]
(duly authorised)
SCHEDULE 1
PAYMENTS, EXCHANGES AGAINST TEMPORARY GLOBAL NOTE, DELIVERY OF DEFINITIVE NOTES AND CANCELLATION OF NOTES
[4]
|
Date of payment, exchange delivery or cancellation |
Amount of interest then paid |
Amount of principal then paid |
Principal amount of Temporary Global Note then exchanged |
Aggregate principal amount of Definitive Notes then delivered |
Aggregate principal amount of Notes then cancelled |
New principal amount of this Global Note |
Authorised Signature |
[4] Schedule 1 should only be completed where the Final Terms specify that the New Global Note form is not applicable.
PART C
FORM OF DEFINITIVE BEARER NOTE
[On the face of the Note:]
Series Number: [ ]
Serial Number: [ ]
[Tranche Number: [ ]]
[Denomination]
[ANY UNITED STATES PERSON WHO HOLDS THIS OBLIGATION WILL BE SUBJECT TO LIMITATIONS UNDER THE UNITED STATES INCOME TAX LAWS, INCLUDING THE LIMITATIONS PROVIDED IN SECTIONS 165(j) AND 1287(a) OF THE UNITED STATES INTERNAL REVENUE CODE OF 1986, AS AMENDED.][5]
[Pursuant to the Dutch Savings Certificates Act (Wet inzake spaarbewijzen), each transfer and acceptance of this Note within, from or into The Netherlands (other than between individuals who do not act in the conduct of a profession or trade):
(a) must be made through the mediation of either the Issuer or a member of Euronext Amsterdam N.V.; and
(b) if it involves its physical delivery, must be recorded in a transaction note which includes the name and address of each party, the nature of the transaction and the number and serial numbers of the Notes transferred.][6]
[Pursuant to the Dutch Savings Certificates Act (Wet inzake spaarbewijzen), each transfer and acceptance of this Note within, from or into The Netherlands (other than between individuals who do not act in the conduct of a profession or trade):
(c) must be made through the mediation of either the Issuer or a member of Euronext Amsterdam N.V.; and
(d) unless it is made between a professional borrower and a professional lender, if it involves its physical delivery, must be recorded in a transaction note which includes the name and address of each party, the nature of the transaction and the number and serial numbers of the Notes transferred.][7]
[5] Legend to appear on every Note with a maturity of more than one year.
[6] This legend should be placed on Notes on which interest does not become due during their tenor or other Notes which qualify as savings certificates as defined in the Dutch Savings Certificates Act and which are (a) not admitted to trading on Eurolist by Euronext Amsterdam N.V.'s stock market, (b) issued within The Netherlands, or issued outside The Netherlands but distributed within The Netherlands in the course of initial distribution or immediately thereafter and (c) do not qualify as commercial paper or certificates of deposit.
[7] This legend should be placed on Notes on which interest does not become due during their tenor or other Notes which qualify as savings certificates as defined in the Dutch Savings Certificates Act and which are (a) not admitted to trading on Eurolist by Euronext Amsterdam N.V.'s stock market, (b) issued within The Netherlands, or issued outside The Netherlands but distributed within The Netherlands in the course of initial distribution or immediately thereafter and (c) qualify as commercial paper or certificates of deposit.
KONINKLIJKE PHILIPS N.V.
EURO MEDIUM TERM NOTE PROGRAMME
[Aggregate principal amount of Tranche]
[Title of Notes]
KONINKLIJKE PHILIPS N.V. (the "Issuer"), subject to and in accordance with the Conditions [endorsed hereon/set out in Schedule 1 to the Trust Deed (as defined below) which shall be incorporated by reference herein and have effect as if set out herein] (the "Conditions") as completed by the relevant information appearing in the final terms (the "Final Terms") and a trust deed (as modified and/or supplemented and/or restated from time to time, the "Trust Deed") dated 9 March 2020 and as amended and restated on 8 March 2022 and made between, amongst others, the Issuer and Citicorp Trustee Company Limited as trustee for the holders of the Notes (the "Trustee", which expression includes all persons for the time being appointed Trustee or Trustees under the Trust Deed) and an amended and restated Agency Agreement (as amended and/or supplemented and/or restated from time to time, the "Agency Agreement") dated 8 March 2022 between, amongst others, the Issuer, Citibank, N.A., London Branch, the Trustee and certain other financial institutions named therein, for value received promises to pay to the bearer hereof on the Maturity Date, or on such earlier date as this Note may become due and repayable in accordance with the Conditions and the Trust Deed, the amount payable on redemption of this Note, and to pay interest (if any) on the principal amount of this Note calculated and payable as provided in the Conditions and the Trust Deed together with any other sums payable under the Conditions and the Trust Deed.
In the event of any conflict between the provisions of the Conditions and such information set out in the Final Terms, such information set out in the Final Terms will prevail.
[This Note shall not/Neither this Note nor any of the interest coupons [or talons] appertaining hereto shall] be valid for any purpose until this Note has been authenticated by or on behalf of Citibank, N.A., London Branch as Principal Paying Agent.
This Note and any non-contractual obligations arising out of or in connection with it are governed by English law.
IN WITNESS WHEREOF the Issuer has caused this Note to be signed in facsimile on its behalf.
KONINKLIJKE PHILIPS N.V.
By: ………………………………..
(duly authorised)
Name: ……………………………
Title: Authorised Signatory
ISSUED in London as of [ ] 20 [•]
AUTHENTICATED for and on behalf of
CITIBANK, N.A., LONDON BRANCH,
as Principal Paying Agent without recourse,
warranty or liability
By:................................................................
(duly authorised)
[On the reverse of the Notes:]
TERMS AND CONDITIONS
[Conditions to be as set out in Schedule 1 to the Trust Deed or such other form as may be agreed between the Issuer, the Principal Paying Agent, the Trustee and the relevant Dealer(s), but shall not be endorsed if not required by the relevant Stock Exchange]
FINAL TERMS
[Here to be set out the relevant information completing the Conditions which appears in the Final Terms relating to the Notes]
[At the foot of the Terms and Conditions:]
PRINCIPAL PAYING AGENT
Citibank, N.A., London Branch
Citigroup Centre
Canada Square
Canary Wharf
London E14 5LB
England
PART D
FORM OF COUPON
[On the face of the Coupon:]
KONINKLIJKE PHILIPS N.V. a public limited liability company (naamloze vennootschap) incorporated under the law of The Netherlands registered at the Dutch Chamber of Commerce with number 17001910
Euro Medium Term Note Programme
[Amount and title of Notes]
Series No: [ ]
Serial Number of Note: [ ]
[Tranche No: [ ]]
Coupon for [set out the amount due] due on [date]
Such amount is payable, subject to the terms and conditions (the "Conditions") endorsed on the Note to which this Coupon relates (which are binding on the holder of this Coupon whether or not it is for the time being attached to such Note), against presentation and surrender of this Coupon at the specified office for the time being of any of the agents shown on the reverse of this Coupon (or any successor or additional agents appointed from time to time in accordance with the Conditions).
IN WITNESS WHEREOF the Issuer has caused this Coupon to be signed in facsimile on its behalf.
KONINKLIJKE PHILIPS N.V.
By: ………………………………..
(duly authorised)
Name: ……………………………
Title: Authorised Signatory
KONINKLIJKE PHILIPS N.V.
Euro Medium Term Note Programme
[Amount and title of Notes]
Coupon for the amount of interest due on the Interest Payment Date falling in [month and year].
Such amount is payable, subject to the terms and conditions (the "Conditions") endorsed on the Note to which this Coupon relates (which are binding on the holder of this Coupon whether or not it is for the time being attached to such Note), against presentation and surrender of this Coupon at the specified office for the time being of any of the agents shown on the reverse of this Coupon (or any successor or additional agents appointed from time to time in accordance with the Conditions).
The Note to which this Coupon relates may, in certain circumstances specified in the Conditions, fall due for redemption before the maturity date of this Coupon. In such event, this Coupon shall become void and no payment will be made in respect hereof.
IN WITNESS WHEREOF the Issuer has caused this Coupon to be signed in facsimile on its behalf.
KONINKLIJKE PHILIPS N.V.
By: ………………………………..
(duly authorised)
Name: ……………………………
Title: Authorised Signatory
[ANY UNITED STATES PERSON WHO HOLDS THIS OBLIGATION WILL BE SUBJECT TO LIMITATIONS UNDER THE UNITED STATES INCOME TAX LAWS, INCLUDING THE LIMITATIONS PROVIDED IN SECTIONS 165(j) AND 1287(a) OF THE INTERNAL REVENUE CODE.][8]
[On the reverse of the Coupon:]
PRINCIPAL PAYING AGENT:
Citibank, N.A., London Branch, Citigroup Centre, Canada Square, Canary Wharf, London E14 5LB, England
[8] Legend to appear on every Coupon relating to a Note with a maturity of more than one year.
PART E
FORM OF TALON
[On the face of the Talon]
KONINKLIJKE PHILIPS N.V. a public limited company (naamloze vennootschap) incorporated under the law of The Netherlands registered at the Dutch Chamber of Commerce with number 17001910
Euro Medium Term Note Programme
[Amount and title of Notes]
Series No: [ ]
Serial Number of Note: [ ]
[Tranche No: [ ]]
On or after the maturity date of the final Coupon which is (or was at the time of issue) part of the Coupon Sheet to which this Talon is (or was at the time of issue) attached, this Talon may be exchanged at the specified office for the time being of the principal paying agent shown on the reverse of this Talon (or any successor principal paying agent appointed from time to time in accordance with the terms and conditions (the "Conditions") of the Notes to which this Talon relates) for a further Coupon Sheet (including a further Talon but excluding any Coupons in respect of which claims have already become void pursuant to the Conditions).
The Note to which this Talon relates may, in certain circumstances specified in the Conditions, fall due for redemption before the maturity date of such final Coupon. In such event, this Talon shall become void and no Coupon will be delivered in respect hereof.
[ANY UNITED STATES PERSON WHO HOLDS THIS OBLIGATION WILL BE SUBJECT TO LIMITATIONS UNDER THE UNITED STATES INCOME TAX LAWS, INCLUDING THE LIMITATIONS PROVIDED IN SECTIONS 165(j) AND 1287(a) OF THE INTERNAL REVENUE CODE.][9]
[On the reverse of the Talon:]
PRINCIPAL PAYING AGENT:
Citibank, N.A., London Branch, Citigroup Centre, Canada Square, Canary Wharf, London E14 5LB, England
[9] Legend to appear on every Talon relating to a Note with a maturity of more than one year.
SCHEDULE 3
PROVISIONS FOR MEETINGS OF NOTEHOLDERS
1.
(a) As used in this Schedule the following expressions shall have the following meanings unless the context otherwise requires:
(i) "voting certificate" shall mean an English language certificate issued by the Principal Paying Agent and dated in which it is stated:
(A) that on the date thereof Notes (whether in definitive form or represented by a Global Note and not being Notes in respect of which a block voting instruction has been issued and is outstanding in respect of the meeting specified in such voting certificate or any adjourned such meeting) were deposited with the Principal Paying Agent or (to the satisfaction of the Principal Paying Agent) were held to its order or under its control or blocked in an account with Euroclear, Clearstream, Luxembourg or any other relevant clearing system and that no such Notes will cease to be so deposited, held or blocked until the first to occur of:
(1) the conclusion of the meeting specified in such certificate or, if applicable, of any adjourned such meeting; and
(2) the surrender of the certificate to the Principal Paying Agent who issued the same; and
(B) that the bearer thereof is entitled to attend and vote at such meeting and any adjourned such meeting in respect of the Notes represented by such certificate;
(ii) "block voting instruction" shall mean an English language document issued by the Principal Paying Agent and dated in which:
(A) it is certified that Notes (whether in definitive form or represented by a Global Note and not being Notes in respect of which a voting certificate has been issued and is outstanding in respect of the meeting specified in such block voting instruction and any adjourned such meeting) have been deposited with the Principal Paying Agent or (to the satisfaction of the Principal Paying Agent) were held to its order or under its control or blocked in an account with Euroclear, Clearstream, Luxembourg or any other relevant clearing system and that no such Notes will cease to be so deposited, held or blocked until the first to occur of:
(1) the conclusion of the meeting specified in such document or, if applicable, of any adjourned such meeting; and
(2) the surrender to the Principal Paying Agent not less than 48 hours before the time for which such meeting or any adjourned such meeting is convened of the receipt issued by the Principal Paying Agent in respect of each such deposited or blocked Note which is to be released or (as the case may require) the Note or Notes ceasing with the agreement of the Principal Paying Agent to be held to its order or under its control and the giving of notice by the Principal Paying Agent to the Issuer in accordance with paragraph A hereof of the necessary amendment to the block voting instruction;
(B) it is certified that each holder of such Notes has instructed the Principal Paying Agent that the vote(s) attributable to the Note or Notes so deposited, held or blocked should be cast in a particular way in relation to the resolution or resolutions to be put to such meeting or any adjourned such meeting and that all such instructions are during the period commencing 48 hours prior to the time for which such meeting or any adjourned such meeting is convened and ending at the conclusion or adjournment thereof neither revocable nor capable of amendment;
(C) the aggregate principal amount of the Notes so deposited, held or blocked are listed distinguishing with regard to each such resolution between those in respect of which instructions have been given as aforesaid that the votes attributable thereto should be cast in favour of the resolution and those in respect of which instructions have been so given that the votes attributable thereto should be cast against the resolution; and
(D) one or more persons named in such document (each hereinafter called a "proxy") is or are authorised and instructed by the Principal Paying Agent to cast the votes attributable to the Notes so listed in accordance with the instructions referred to in (B) above as set out in such document;
(iii) "24 hours" shall mean a period of 24 hours including all or part of a day upon which banks are open for business in both the place where the relevant meeting is to be held and in each of the places where the Principal Paying Agent have their specified offices (disregarding for this purpose the day upon which such meeting is to be held) and such period shall be extended by one period or, to the extent necessary, more periods of 24 hours until there is included as aforesaid all or part of a day upon which banks are open for business in all of the places as aforesaid; and
(iv) "48 hours" means two consecutive periods of 24 hours.
(b) A holder of a Note (whether in definitive form or represented by a Global Note) may obtain a voting certificate in respect of such Note from the Principal Paying Agent or require the Principal Paying Agent to issue a block voting instruction in respect of such Note by depositing such Note with the Principal Paying Agent or (to the satisfaction of the Principal Paying Agent) by such Note being held to its order or under its control or blocked in an account with Euroclear, Clearstream, Luxembourg or any other relevant clearing system, in each case not less than 48 hours before the time fixed for the relevant meeting and on the terms set out in subparagraph (a)(i)(A) or (a)(ii)(A) above (as the case may be), and (in the case of a block voting instruction) instructing the Principal Paying Agent to the effect set out in subparagraph (a)(ii)(C) above. The holder of any voting certificate or the proxies named in any block voting instruction shall for all purposes in connection with the relevant meeting or adjourned meeting of Noteholders be deemed to be the holder of the Notes to which such voting certificate or block voting instruction relates.
2. The Issuer or the Trustee may at any time and the Trustee shall (subject to it being indemnified and/or secured and/or prefunded to its satisfaction) upon a requisition in writing signed by the holders of not less than one-quarter in aggregate principal amount of the Notes for the time being outstanding convene a meeting of the Noteholders. Every such meeting shall be held at such time and place as the Trustee may appoint or approve in writing.
3. At least 21 days' notice (exclusive of the day on which the notice is given and the day on which the meeting is to be held) specifying the place, day and hour of meeting shall be given to the Noteholders, prior to any meeting of the Noteholders, in the manner provided by Condition 13.1 (Notices – Notices to the Noteholders). Such notice, which shall be in the English language, shall state generally the nature of the business to be transacted at the meeting thereby convened but (except for an Extraordinary Resolution) it shall not be necessary to specify in such notice the terms of any resolution to be proposed. Such notice shall state that Notes may, not less than 48 hours before the time fixed for the meeting, be deposited the Principal Paying Agent or (to its satisfaction) held to their order or under their control for the purpose of obtaining voting certificates or appointing proxies. A copy of the notice shall be sent to the Trustee (unless the meeting is convened by the Trustee) and to the Issuer (unless the meeting is convened by the Issuer).
4. A person (who may, but need not be, a Noteholder) nominated in writing by the Trustee shall be entitled to take the chair at the relevant meeting or adjourned meeting but if no such nomination is made or if at any meeting or adjourned meeting the person nominated shall not be present within 15 minutes after the time appointed for holding the meeting or adjourned meeting the Noteholders present shall choose one of their number to be Chairperson, failing which the Issuer may appoint a Chairperson. The Chairperson of an adjourned meeting need not be the same person as was Chairperson of the meeting from which the adjournment took place.
5. The quorum at any such meeting for passing an Extraordinary Resolution shall (subject as provided below) be one or more persons present holding Notes in definitive form or voting certificates or being proxies and holding or representing in the aggregate more than 50 per cent. in aggregate principal amount of the Notes for the time being outstanding provided that at any meeting the business of which includes a Basic Terms Modification (each of which shall, subject only to Clause 7.2.2 of the Trust Deed, only be capable of being effected after having been approved by Extraordinary Resolution) the quorum for passing the requisite Extraordinary Resolution shall be one or more persons present holding Notes in definitive form or voting certificates or being proxies and holding or representing in the aggregate not less than two-thirds of the aggregate principal amount of the Notes for the time being outstanding.
6. If within 15 minutes (or such longer period not exceeding 30 minutes as the Chairperson may decide) after the time appointed for any such meeting a quorum is not present for the transaction of any particular business, then, subject and without prejudice to the transaction of the business (if any) for which a quorum is present, the meeting shall if convened upon the requisition of Noteholders be dissolved. In any other case it shall stand adjourned to the same day in the next week (or if such day is a public holiday the next succeeding business day) at the same time and place (except in the case of a meeting at which an Extraordinary Resolution is to be proposed in which case it shall stand adjourned for such period, being not less than 13 clear days nor more than 42 clear days, and to such place as may be appointed by the Chairperson either at or subsequent to such meeting and approved by the Trustee). If within 15 minutes (or such longer period not exceeding 30 minutes as the Chairperson may decide) after the time appointed for any such adjourned meeting a quorum is not present for the transaction of any particular business, then, subject and without prejudice to the transaction of the business (if any) for which a quorum is present, the Chairperson may either (with the approval of the Trustee) dissolve such meeting or adjourn the same for such period, being not less than 13 clear days (but without any maximum number of clear days), and to such place as may be appointed by the Chairperson either at or subsequent to such adjourned meeting and approved by the Trustee, and the provisions of this sentence shall apply to all further adjourned such meetings. At any such adjourned meeting one or more persons present holding Notes in definitive form or voting certificates or being proxies (whatever the aggregate principal amount of the Notes so held or represented by them) shall (subject as provided below) form a quorum and shall (subject as provided below) have power to pass any Extraordinary Resolution or other resolution and to decide upon all matters which could properly have been dealt with at the meeting from which the adjournment took place had the requisite quorum been present provided that at any such adjourned meeting the quorum for the transaction of business comprising any of the matters specified in the proviso to paragraph 5 above shall be one or more persons present holding Notes in definitive form or voting certificates or being proxies and holding or representing in the aggregate not less than one-third of the aggregate principal amount of the Notes for the time being outstanding.
7. Notice of any adjourned meeting at which an Extraordinary Resolution is to be submitted shall be given in the same manner as notice of an original meeting but as if 10 were substituted for 21 in paragraph 3 above and such notice shall state the relevant quorum requirements that apply to the adjourned meeting. Subject as aforesaid it shall not be necessary to give any notice of an adjourned meeting.
8. Every question submitted to a meeting shall be decided in the first instance by a show of hands and in case of equality of votes the Chairperson shall both on a show of hands and on a poll have a casting vote in addition to the vote or votes (if any) to which such person may be entitled as a Noteholder or as a holder of a voting certificate or as a proxy.
9. At any meeting unless a poll is (before or on the declaration of the result of the show of hands) demanded by the Chairperson, the Issuer, the Trustee or any person present holding a Note in definitive form or a voting certificate or being a proxy (whatever the aggregate principal amount of the Notes so held or represented by such person) a declaration by the Chairperson that a resolution has been carried or carried by a particular majority or lost or not carried by a particular majority shall be conclusive evidence of the fact without proof of the number or proportion of the votes recorded in favour of or against such resolution.
10. Subject to paragraph 12 below, if at any such meeting a poll is so demanded it shall be taken in such manner and subject as hereinafter provided either at once or after an adjournment as the Chairperson directs and the result of such poll shall be deemed to be the resolution of the meeting at which the poll was demanded as at the date of the taking of the poll. The demand for a poll shall not prevent the continuance of the meeting for the transaction of any business other than the motion on which the poll has been demanded.
11. The Chairperson may with the consent of (and shall if directed by) any such meeting adjourn the same from time to time and from place to place but no business shall be transacted at any adjourned meeting except business which might lawfully (but for lack of required quorum) have been transacted at the meeting from which the adjournment took place.
12. Any poll demanded at any such meeting on the election of a Chairperson or on any question of adjournment shall be taken at the meeting without adjournment.
13. The Trustee and its lawyers and any director, officer or employee of a corporation being a trustee of this Trust Deed and any director or officer of the Issuer and its lawyers and any other person authorised so to do by the Trustee may attend and speak at any meeting. Save as aforesaid, but without prejudice to the proviso to the definition of "outstanding" in Clause 1 (Definitions and Interpretation) of the Trust Deed, no person shall be entitled to attend and speak nor shall any person be entitled to vote at any meeting of the Noteholders or join with others in requesting the convening of such a meeting or to exercise the rights conferred on the Noteholders by Conditions 10 (Events of Default) and 11.1 (Enforcement – Enforcement by the Trustee) unless they either produce the Note or Notes in definitive form of which they are the holder or a voting certificate or is a proxy. No person shall be entitled to vote at any meeting in respect of Notes which are deemed to be not outstanding by virtue of the proviso to the definition of "outstanding" in Clause 1 (Definitions and Interpretation) of the Trust Deed. Nothing herein shall prevent any of the proxies named in any block voting instruction or form of proxy from being a director, officer or representative of or otherwise connected with the Issuer.
14. Subject as provided in paragraph 13 hereof at any meeting:
(a) on a show of hands every person who is present in person and produces a Note in definitive form or voting certificate or is a proxy shall have one vote; and
(b) on a poll every person who is so present shall have one vote in respect of each €1,000 in aggregate principal amount of the outstanding Notes so produced in definitive form or represented by the voting certificate so produced or in respect of which they are a proxy or in respect of which (being in definitive form) they are the holder.
Without prejudice to the obligations of the proxies named in any block voting instruction any person entitled to more than one vote need not use all their votes or cast all the votes to which they are entitled in the same way.
15. The proxies named in any block voting instruction need not be Noteholders.
16. Each block voting instruction together (if so requested by the Trustee) with proof satisfactory to the Trustee of its due execution on behalf of the Principal Paying Agent shall be deposited by the Principal Paying Agent at such place as the Trustee shall approve not less than 24 hours before the time appointed for holding the meeting or adjourned meeting at which the proxies named in the block voting instruction propose to vote and in default the block voting instruction shall not be treated as valid unless the Chairperson of the meeting decides otherwise before such meeting or adjourned meeting proceeds to business. A copy of each block voting instruction shall be deposited with the Trustee before the commencement of the meeting or adjourned meeting but the Trustee not thereby be obliged to investigate or be concerned with the validity of or the authority of the proxies named in any such block voting instruction.
17. Any vote given in accordance with the terms of a block voting instruction shall be valid notwithstanding the previous revocation or amendment of the block voting instruction or of any of the Noteholders' instructions pursuant to which it was executed provided that no intimation in writing of such revocation or amendment shall have been received from the Principal Paying Agent by the Issuer at its registered office (or such other place as may have been required or approved by the Trustee for the purpose) by the time being 24 hours and 48 hours respectively before the time appointed for holding the meeting or adjourned meeting at which the block voting instruction is to be used.
18. A meeting of the Noteholders shall in addition to the powers hereinbefore given have the following powers exercisable only by Extraordinary Resolution (subject to the provisions relating to quorum contained in paragraphs 5 and 6 above) namely:
(a) Approve any Basic Terms Modification.
(b) Power to sanction any compromise or arrangement proposed to be made between the Issuer, the Trustee, any Appointee and the Noteholders and Couponholders or any of them.
(c) Power to sanction any abrogation, modification, compromise or arrangement in respect of the rights of the Trustee, any Appointee, the Noteholders, the Couponholders or the Issuer against any other or others of them or against any of their property whether such rights shall arise under this Trust Deed or otherwise.
(d) Power to assent to any modification of the provisions of this Trust Deed which shall be proposed by the Issuer or the Trustee.
(e) Power to give any authority or sanction which under the provisions of this Trust Deed is required to be given by Extraordinary Resolution.
(f) Power to appoint any persons (whether Noteholders or not) as a committee or committees to represent the interests of the Noteholders and to confer upon such committee or committees any powers or discretions which the Noteholders could themselves exercise by Extraordinary Resolution.
(g) Power to approve of a person to be appointed a trustee and power to remove any trustee or trustees for the time being of this Trust Deed.
(h) Power to discharge or exonerate the Trustee and/or any Appointee from all Liability in respect of any act or omission for which the Trustee and/or such Appointee may have become or may become responsible under this Trust Deed.
(i) Power to authorise the Trustee and/or any Appointee (subject to it being indemnified and/or secured and/or prefunded to its satisfaction) to concur in and execute and do all such deeds, instruments, acts and things as may be necessary to carry out and give effect to any Extraordinary Resolution.
(j) Power to sanction any scheme or proposal for the exchange or sale of the Notes for or the conversion of the Notes into or the cancellation of the Notes in consideration of shares, stock, notes, bonds, debentures, debenture stock and/or other obligations and/or securities of the Issuer or any other company formed or to be formed, or for or into or in consideration of cash, or partly for or into or in consideration of such shares, stock, notes, bonds, debentures, debenture stock and/or other obligations and/or securities as aforesaid and partly for or into or in consideration of cash.
(k) (Other than as permitted under Clause 7.3 (Substitution) of the Trust Deed), power to approve the substitution of any entity for the Issuer (or any previous substitute) as principal debtor under this Trust Deed.
19. Any Extraordinary Resolution (i) passed at a meeting of the holders duly convened and held in accordance with this Trust Deed, (ii) passed as an Extraordinary Resolution in writing in accordance with this Trust Deed or (iii) passed by way of electronic consents communicated through the electronic communications systems of the relevant clearing system(s) to the Trustee in accordance with their operating rules and procedures shall be binding upon all the holders whether or not present or whether or not represented at any meeting and whether or not voting on such Extraordinary Resolution and upon all Couponholders and each of them shall be bound to give effect thereto accordingly and the passing of any such Extraordinary Resolution shall be conclusive evidence that the circumstances justify the passing thereof. Notice of the result of the voting on any Extraordinary Resolution duly considered by the holders shall be published in accordance with Condition 13 (Notices) by the Issuer within 14 days of such result being known, provided that the non- publication of such notice shall not invalidate such result.
20. The expression "Extraordinary Resolution" when used in this Trust Deed means (i) a resolution passed at a meeting duly convened and held in accordance with this Trust Deed by a majority consisting of not less than two thirds of the votes cast on such resolution, (ii) a resolution in writing signed by or on behalf of the holders of not less than two-thirds in principal amount of the Notes for the time being outstanding or (iii) a resolution passed by way of electronic consents communicated through the electronic communications systems of the relevant clearing system(s) to the Trustee in accordance with their operating rules and procedures by or on behalf of the holders of not less than two-thirds in principal amount of the Notes outstanding.
21. Minutes of all resolutions and proceedings at every meeting of the Noteholders shall be made and entered in books to be from time to time provided for that purpose by the Issuer and any such Minutes as aforesaid if purporting to be signed by the Chairperson of the meeting at which such resolutions were passed or proceedings transacted shall be conclusive evidence of the matters therein contained and until the contrary is proved every such meeting in respect of the proceedings of which Minutes have been made shall be deemed to have been duly held and convened and all resolutions passed or proceedings transacted thereat to have been duly passed or transacted.
22. Subject to all provisions of this Trust Deed the Trustee may:
(a) without the consent of the Issuer, the Noteholders or the Couponholders prescribe such other or further regulations ("Further Regulations") regarding the requisitioning and/or the holding of meetings of Noteholders and attendance and voting thereat as the Trustee may in its sole discretion think fit; or
(b) concur with the Issuer in making Further Regulations if it is of the opinion that to do so is not materially prejudicial to the Noteholders.
SCHEDULE 4
FORM OF AUTHORISED OFFICERS' CERTIFICATE
[on Issuer letterhead]
[date]
To: Citicorp Trustee Company Limited (as Trustee)
KONINKLIJKE PHILIPS N.V.
€10,000,000,000
Euro Medium Term Note Programme
This certificate is delivered to you in accordance with Clause 6.7 of the trust deed dated 8 March 2022 (the "Trust Deed") and made between the Issuer and Citicorp Trustee Company Limited (the "Trustee"). All words and expressions defined in the Trust Deed shall (save as otherwise provided herein or unless the context otherwise requires) have the same meanings herein.
We hereby certify that to the best of our knowledge, information and belief, and having made all reasonable enquiries:
(a) as at the date herewith, no Event of Default or Potential Event of Default existed [other than [●]] and no Event of Default, Potential Event of Default had existed or happened at any time since [insert date of last certificate delivered]/[the certification date (as defined in the Trust Deed) of the last certificate delivered under Clause 6.7] [other than [●]]; and
(b) from and including [insert date of last certificate delivered]/[the certification date of the last certificate delivered under Clause 6.7] to and including [date], the Issuer has complied in all respects with its obligations under this Trust Deed [other than [●]] .
For and on behalf of
KONINKLIJKE PHILIPS N.V.
By:……………..
Authorised Signatory
By:……………..
Authorised Signatory
SIGNATORIES TO THIS TRUST DEED
The Issuer
EXECUTED as a deed by )
KONINKLIJKE PHILIPS N.V. )
)
acting by: )
Title: Authorised Signatory )
)
in the presence of: )
Witness's Signature:
Witness's Name:
Witness's Address:
The Trustee
EXECUTED as a deed by )
CITICORP TRUSTEE )
COMPANY LIMITED )
acting by )
The following contract is the services contract of Mr A. Bhattacharya, containing terms and conditions for the provision of services and other arrangements that apply with effect from May 9, 2023 (“the Commencement Date”) as member of the Board of Management of Royal Philips (“Koninklijke Philips N.V.”, hereinafter also referred to as “the Company”).
1. Commencement of Engagementa. Subject to the terms and conditions of this contract for the provision of services (the “Contract“) the Company hereby engages you as independent contractor starting on the Commencement Date to fulfill the role of member of the Board of Management of the Company as Chief Financial Officer and, in conjunction with such role, of member of the Executive Committee of the Company. As a member of the Executive Committee you will perform your duties and responsibilities attached to that function within the corporate governance framework of the Company. In your capacity as member of the Board of Management of the Company you will have and observe all rights and obligations pursuant to the articles of association of the Company, the Rules of Procedure of the Board of Management and Executive Committee, and statutory provisions. By signing this Contract, you declare that you have received a copy of the Company’s articles of association and abovementioned Rules of Procedure and that you are familiar with their content.
b. The terms and conditions set forth in this Contract and its annexes replace the terms and conditions as laid down in any (previous) employment or services agreements and/ or other written or verbal understandings you may have (had) with the Company and/ or other companies belonging to the Philips Group. By entering into this Contract all prior contracts of employment and/or prior contracts for the provision of services (if any) with the Company and/or other companies belonging to the Philips Group are explicitly terminated.
c. This Contract is a contract for the provision of services, as defined in articles 7:400 and further of the Dutch Civil Code (“DCC”). You acknowledge and agree that, pursuant to article 2:132 section 3 DCC, your relationship with the Company and/or this Contract cannot be regarded an employment agreement as defined in article 7:610 DCC and further.
d. In this Contract the Company and you are together referred to as the “Parties” and each of you as a “Party”.
2. Duration of the Engagementa. The Contract shall be entered into for a fixed period of time. The Contract shall start on the Commencement Date and shall terminate by operation of law, without any prior notice being required, on the last day of the quarter in which the Annual General Meeting of Shareholders of the Company in the second calendar year following the Commencement Date takes place (the “Contract End Date”).
b. Both Parties shall have the right to terminate this Contract before the Contract End Date or (if renewed) before any later Contract expiration date against the end of a calendar month by giving written notice of termination. In this respect, the Parties agree to adhere to a notice period of six (6) months. If notice of termination is given by a Party for urgent cause (‘dringende reden’), no notice period applies for the Party giving notice. For the definition of urgent cause (‘dringende reden’), reference is made to article 7:678 DCC and further.
c. If you are dismissed by the General Meeting of Shareholders of the Company, not for urgent cause as defined below, or if you resign, as member of the Board of Management of the Company (and, in direct relation thereto, as member of the Executive Committee of the Company) this Contract is terminated by operation of law without any prior notice of termination being required, which termination shall take effect (i) as per the date six (6) months after the end of the calendar month in which the General Meeting of Shareholders has adopted the resolution pursuant to which you are dismissed as member of the Board of Management of the Company, or, as the case may be, (ii) as per the date six (6) months after the end of the calendar month in which you have submitted your written resignation as member of the Board of Management of the Company. In deviation from the previous sentence, this Contract shall terminate with immediate effect as from the date per which (i) the General Meeting of Shareholders has dismissed you as member of the Board of Management of the Company, or, as the case may be, (ii) you have resigned as member of the Board of Management of the Company, in the event such dismissal or resignation (as the case may be) is given/made for urgent cause (‘dringende reden’). For the definition of urgent cause (‘dringende reden’), reference is made to article 7:678 DCC and further.
d. In deviation from clause 2 (b), the Company cannot terminate this Contract during the first two (2) years of your sickness or incapacity for work (although it can already give notice of termination), except when notice of termination is given by the Company (i) for urgent cause (‘dringende reden’) or (ii) prior to the first day of your sickness/incapacity for work. In deviation from clause 2 (c), in the event of your dismissal as member of the Board of Management of the Company by the General Meeting of Shareholders during your sickness or incapacity for work other than for urgent cause (‘dringende reden’) and after the first day of your sickness/incapacity for work, this Contract shall terminate at the later of (i) the date which is six (6) months after the end of the calendar month in which the General Meeting of Shareholders has adopted the resolution pursuant to which you are dismissed as member of the Board of Management of the Company, or (ii) the date of your recovery from sickness/incapacity for work, but no later than at the date on which the incapacity for work has lasted for two (2) years. For the definition of urgent cause (‘dringende reden’), reference is made to article 7:678 DCC and further. The Parties acknowledge and agree that this clause does not prevent the competent body from dismissing you as member of the Board of Management of the Company.
e. If the Contract is terminated at the initiative of the Company (whereby your dismissal by the General Meeting of Shareholders as member of the Board of Management of the Company shall also be deemed a termination “at the initiative of the Company” for the purposes of this clause) or by mutual agreement (at the initiative of the Company) before the Contract End Date, or before any other expiration date if the Contract has been renewed, other than for urgent cause (‘dringende reden’), you shall be entitled to a one off compensation in the amount of one time your Annual Base Compensation as defined in clause 3 hereof. For the definition of urgent cause (‘dringende reden’), reference is made to article 7:678 DCC and further. You shall not be entitled to such payment if the Contract is terminated immediately following a period of your long lasting sickness or disability which has lasted two years or longer (periods of incapacity for work that follow one another at intervals of less than four weeks shall be deemed one consecutive period of incapacity for work for the purposes of this clause).
f. In case of termination of the Contract, you will resign, with effect from a date to be determined by the Company but ultimately per the effective date of such termination, as member of the Board of Management and, in direct relation thereto, as member of the Executive Committee of the Company.
g. The compensation as referred to in clause e) above, shall be deemed to include any amounts that may be payable to you in connection with the enforcement of the non- competition clause as set forth in the General Terms of Employment that are – mutatis mutandis – applicable to you.
3. CompensationYour annual compensation as of the Commencement Date amounts to EUR 810,000 gross, which amount includes holiday allowances, to be paid in twelve equal monthly installments after deduction of the statutory tax and social security premiums to be withheld by the Company. Annual review and subsequent upwards adjustment, if any, of your annual compensation, will be determined at the discretion of the Supervisory Board of the Company and on the advice of the Remuneration Committee of the Supervisory Board. Only compensation increases determined and approved by the Supervisory Board will replace the compensation amount mentioned above. You will be informed in writing by means of a compensation statement. The annual compensation as may be amended on the basis of this clause from time to time shall be referred to as the Annual Base Compensation.
4. Annual IncentiveIn addition to the Annual Base Compensation, you shall be eligible each year for an annual incentive, subject to certain targets being met. This incentive shall be determined annually by the Supervisory Board. You shall be notified in writing of these annual incentive targets.
The on-target (= 100% score) annual incentive amount to be realized by you is currently set by the Supervisory Board at 80% of your Annual Base Compensation.
The Supervisory Board shall determine in its sole reasonable discretion to what extent the annual incentive targets have been met.
5. Long Term Incentive PlanThe Supervisory Board, where relevant within the framework approved by the Company’s General Meeting of Shareholders, can decide by discretion to grant Performance Shares under the Global Philips Performance Share Plan and/or other equity related incentives to the members of the Board of Management on a year-to-year basis. As a member of the Board of Management you are in principle eligible to participate in such plan.
The Long Term Incentive grant value equals 150% of your Annual Base Compensation.
To improve Philips’ Corporate Governance and to further align the interests of senior Philips Executives with the interests of our shareholders, you are required to hold a certain level of Philips shares equal to 300% of your actual Annual Base Compensation. The Supervisory Board may decide to adapt the Philips Share Ownership Guidelines on an annual basis.
The minimum number of Philips shares required to be held can be accumulated by:
For further details you are referred to the Philips Share Ownership Guidelines Executive Committee in the enclosed Information Package.
6. Claw backThe Supervisory Board may in its sole discretion but acting in good faith, resolve to recoup some or all of the incentive compensation -including any benefits derived therefrom- in all appropriate cases (taking into account all relevant factors, including whether the assertion of a recoupment claim may in its opinion prejudice the interests of the Company and its group companies in any related proceeding or investigation), granted to you as an Annual Incentive, as Performance Shares grants, as shares acquired by you under such grants, as other equity related incentive or otherwise (hereinafter referred to as ‘Incentive Compensation’), if:
a. The Incentive Compensation has been paid, granted, vested and/or delivered on the basis of incorrect financial or other data; or
b. In assessing the extent to which the relevant performance conditions and/or targets in relation to the payment, grant, vesting and/or delivery of the Incentive Compensation was satisfied, such assessment was based on an error, inaccurate or misleading information or assumptions and that such error, information or assumptions would have resulted or did in fact result either directly or indirectly in that payment, grant, vesting and/or delivery (or being capable thereof) to a greater degree than would have been the case had that error not been made; or
c. There are circumstances which would allow the Company to terminate this Contract for urgent cause (‘dringende reden’) (whereby for the definition of urgent cause (‘dringende reden’) reference is made to article 7:678 DCC and further), where such circumstances arose in, or related to, a period relevant to the date of payment, grant, vesting and/or delivery; or
d. You were involved in, or directly or indirectly responsible for a serious violation of the Philips General Business Principles or applicable law; or
e. The Company or the business in which you work/worked, or for which you were responsible, suffered a material failure of risk management, or
f. Something which occurred in the period relevant to the payment, grant, vesting and/ or delivery has a sufficiently significant impact on the reputation of the Company or its group members to justify the operation of a recoupment claim.
By accepting a payment, grant, vesting and/or delivery of the Incentive Compensation, you agree to fully co-operate with the Company in order to give effect to this clause.
Furthermore by accepting any payment, grant, vesting and/or delivery of the Incentive Compensation you provide an irrevocable power of attorney to the Company to transfer any shares held by you in the account administered by the Company’s global plan administrator and to perform any other acts necessary or desirable to give effect to this clause. This power of attorney is governed by Dutch law exclusively.
7. Pension RightsAs from the Commencement Date, you shall be included in the Pension Regulations of “Stichting Philips Pensioenfonds” applicable to executives, in respect of your pensionable salary up to the current statutory limit of EUR 128,810 which may change from time to time (“Statutory Pensionable Salary”) if and as soon as you meet the requirements set out in those pension regulations. In respect of your pensionable salary exceeding the Statutory Pensionable Salary, you shall be entitled to the pension allowance applicable to members of the Executive Committee, in accordance with the rules and conditions governing this pension allowance. The level of the pension allowance is and remains at the discretion of the Company. Currently the pension allowance for the part of your Annual Base Compensation exceeding the Statutory Pensionable Salary is set at 25% of your Annual Base Compensation exceeding the Statutory Pensionable Salary.
8. Car/Mobility AllowanceYou are entitled to a monthly Car/Mobility Allowance amounting to EUR 2,630. The Car/ Mobility allowance can be used for a leasing an electric Vehicle or can be paid out in monthly (gross) installments.
9. Business Entertainment Expenses AllowanceWith respect to your position within the Company, you may be eligible for a fixed allowance for business entertainment expenses. Currently the tax-free allowance in your case is EUR 6,000 per annum (i.e. EUR 500 per month). This sum is meant to enable you amongst others to cover the expenses you incur in entertaining guests on behalf of the Company.
Parties agree that changes in fiscal legislation could make it necessary or desirable for the Company to change the above arrangement.
10. Senior Executive Ambassador ProgramYou are invited to participate in the Senior Executive Ambassador Program to use Philips products that will be made available to you at your home.
11. Insurancesa. Accident Insurance
You will be covered by a 24-hours accident insurance policy. The maximum sum insured is three times your gross Annual Base Compensation. We refer you to the chapter benefits in the Information Package.
b. Directors and Officers Liability Insurance
You will be an Insured Person under the Directors and Officers liability insurance taken out by the Company. Subject to its terms and conditions, the Directors and Officers liability insurance policy protects your personal assets against liabilities and reimburse defense costs that arise based on your acts or omissions in your capacity as member of the Board of Management and Executive Committee. A copy of the Directors and Officers liability insurance policy (or a summary thereof) will be made available upon your request.
12. Incapacity for workThe present Company policy for Executive Committee members with regard to incapacity for work or sickness is that for a maximum period of three years from the start of disablement, but at the very latest up to the end of the Contract, the balance between your Annual Base Compensation at the start of the total disability and the aggregate amount of any statutory allowance distributed to you on account of the total disablement together with possible allowances distributed for the same reason by the Philips Pension Fund will - subject to your compliance with the Company’s directives - be paid by the Company.
The Company shall not be bound by the aforesaid obligation if you have a claim against third parties in respect of your disablement. Upon surrender to the Company of such claim - in so far as it relates to loss of Annual Base Compensation - an amount equal to the aforesaid balance shall - but for no longer than the period stated in the foregoing clause - be paid by the Company in advance.
This policy is subject to change at the discretion of the Company. No compensation will be paid in case the new policy is less favorable than the present policy.
13. HolidaysThe holiday entitlement for members of the Board of Management is 25 working days per calendar year.
14. General Terms of engagementBy signing the Contract, you declare to have received, to have read and to agree with the General Terms of Employment of the Company, which apply mutatis mutandis to your engagement and are attached to this Contract as Annex 1. These General Terms of Employment amongst others contain a non-competition clause. You hereby acknowledge and agree that you are fully bound by the restrictions set out in the aforementioned non-competition clause for the duration of such non-competition clause as set out in the clause itself.
15. Philips rules about corporate governance and corporate citizenshipUnderpinning Philips’ commitment to responsible corporate citizenship, integrity and transparency, the following terms and principles have been set.
These terms and principles apply equally to corporate actions and to the behavior of members of the Executive Committee in conducting Philips’ business. By signing this Contract, you declare that you are bound by, and that you shall adhere to and act according to, the terms and principles mentioned above. The Company may alter the terms and principles unilaterally at its discretion. For more information on the terms and principles, we refer you to the Information Package. Any changes will be available on the Philips Global Intranet website.
The Compliance Officer with respect to Inside Information will contact you, as you are designated as “Qualified Insider”.
16. Privacy and data protectionYou acknowledge that Philips may process your personal data for legitimate business purposes, such as human resources and personnel management, business process execution and internal management, internal communications, health safety and security, compliance with legal obligations, exercise or defense of legal claims. The processing of such personal data is further described in the relevant privacy notice(s) which is attached to this agreement or otherwise made available to you. By signing this agreement, you acknowledge to have read and agreed with the processing of your personal data, as described in the relevant privacy notice(s) attached to this agreement or otherwise made available to you.
During your employment with Philips, you agree to comply with all Philips privacy and security related policies, procedures, rules and regulations (including the Philips Privacy Rules), as announced by Philips from time to time or made available to you. At all times, you must maintain the confidentiality of the personal data that you have access to and cannot share, disclose or otherwise transfer personal data to any unauthorized third parties.
17. Applicable Law and jurisdictiona. This Contract is governed by the laws of the Netherlands.
b. All disputes arising from this Contract, including disputes concerning the existence and validity thereof, shall be resolved in accordance with the Arbitration Rules of the Netherlands Arbitration Institute.
RELATIONSHIP AGREEMENT
Between Koninklijke Philips N.V. and EXOR N.V.
Dated 13 August 2023
Contents
Clause
1 Definitions and construction
2 Terms of Relationship and support
3 Philips Supervisory Board composition
3.1 Appointment and dismissal
3.2 Nomination of the Exor Nominee
3.3 Conflict of interest
3.4 Expiry of nomination right
3.5 Resignation Exor Nominee
3.6 Dismissal Exor Nominee
4 Standstill
5 Non-compete
6 Lock-Up and Sell Down
6.1 General
6.2 Lock-up period
6.3 Post Lock-Up Sell Down
6.4 Intragroup disposal of shares
7 Public Communications
7.1 Non-disparagement
7.2 General communications
7.3 Communication on Permitted Disposals
8 Information Requirements
8.1 Duty to disclose
8.2 No selective disclosure
8.3 Price sensitive information relating to the other party
8.4 Periodical Information Meetings
8.5 Interest disclosure
8.6 Exor information rights
8.7 Confidentiality
9 General Restrictions
9.1 General
9.2 Constitutional documents
10 Duration and termination
10.1 Duration and termination
11 Validity
11.1 Signing
11.2 Invalidity
12 Entire Agreement
13 Amendments and waivers
13.1 Amendments and waivers
13.2 No deemed waivers
13.3 Further assurances
14 Third party rights
15 Rescission, errors and suspension
15.1 No rescission; errors
15.2 No suspension
16 No assignment
17 Notices
17.1 Communications in writing
17.2 Addresses
18 Governing law and dispute resolution
18.1 Governing law
18.2 Dispute resolution
Schedules
Schedule 2 Press release
Schedule 3 Deed of Adherence
Relationship agreementTHIS AGREEMENT IS DATED 13 AUGUST 2023 AND MADE BETWEEN:
Schedule 1 Definitions and interpretation (1) Koninklijke Philips N.V., a public limited liability company, incorporated under the laws of the Netherlands, with seat in Eindhoven, the Netherlands, and address at High Tech Campus 52, Eindhoven, the Netherlands and registered with the Dutch Trade Register under number 17001910 ("Philips"); and
(2) EXOR N.V., a public limited liability company, incorporated under the laws of the Netherlands, with seat in Amsterdam, the Netherlands, and address at Gustav Mahlerplein 25 A, Amsterdam, The Netherlands and registered with the Dutch Trade Register under number 64236277 ("Exor", together with Philips, the "Parties" and each a "Party").
BACKGROUND:
(A) Exor has bought fifteen percent (15%) of the issued and outstanding Ordinary Shares and the voting rights in respect thereof ("15% Threshold Stake"), with the possibility to further increase the amount of Ordinary Shares and the voting rights in respect thereof legally and/or beneficially held (“Interest”), but shall in any event cause its and its Affiliates combined Interest not to exceed twenty percent (20%) of the issued and outstanding Ordinary Shares and the voting rights in respect thereof ("20% Threshold Stake").
(B) The Parties acknowledge and agree that the relationship is intended to be mutually beneficial and long term.
(C) Philips considers this investment from Exor to be beneficial to the overall long-term strategy, including but not limited to its plan to create long-term value with sustainable impact, as announced in January 2023.
(D) In turn, Exor intends to support Philips’ strategy (including, but not limited to, its plan to create long-term value with sustainable impact, as announced in January 2023) and exercise its voting rights and other shareholder rights and powers to contribute to the long-term multi-stakeholder value creation of Philips and its enterprise.
(E) The Parties wish to enter into this relationship agreement (this "Agreement") to agree on certain arrangements relating to the governance of Philips and to manage the relationship between Philips and Exor as a shareholder of Philips, all in accordance with the laws and regulations applicable to Philips and Exor as companies listed on Euronext Amsterdam, a regulated market of Euronext Amsterdam N.V., and to Philips as a company listed on the New York Stock Exchange.
(F) The Parties agree that this Agreement will be announced and published on the Signing Date and for as long as this Agreement is in effect.
THE PARTIES AGREE AS FOLLOWS:
1 Definitions and construction
The definitions and provisions of Schedule 1 (Definitions and interpretation) shall apply throughout this Agreement.
2 Terms of Relationship and support
2.1.1 As soon as possible after the Signing Date and in any event prior to the opening of Euronext Amsterdam on the first trading day after the Signing Date, the Parties shall announce their relationship by way of the joint press release in the form attached as Schedule 2 (Press release).
3 Philips Supervisory Board composition
3.1 Appointment and dismissal
3.1.1 The members of the supervisory board of Philips (the "Philips Supervisory Board" and the "Philips Supervisory Board Members") shall be appointed, suspended and dismissed in accordance with the procedures set out in (i) the Articles of Association, (ii) the Rules of Procedure Supervisory Board, (iii) this Agreement, and (iv) applicable laws and regulations.
3.2 Nomination of the Exor Nominee
3.2.1 Notwithstanding Clause 3.1 (Appointment and dismissal), Exor shall have the right to nominate one (1) individual to serve as Philips Supervisory Board Member (the "Exor Nominee") for appointment by the general meeting of shareholders of Philips ("General Meeting of Shareholders"), unless such nomination right has expired in accordance with Clause 3.4 (Expiry of nomination right).
3.2.2 For the purpose of Clause 3.2.1, Exor shall only nominate the Exor Nominee after consultation with the CGNS Committee and shall only nominate an individual who (i) has knowledge and experience encompassing one or more of the aspects included in the profile included in the Rules of Procedure Supervisory Board, (ii) does not hold a board position in an entity that undertakes activities that materially compete with the Philips business, (iii) is not subject to any material criminal, administrative or similar investigation by any authority or proceedings, and (iv) is eligible for appointment to the Philips Supervisory Board under Dutch law.
3.2.3 Philips shall amend the Philips Supervisory Board profile to reflect Exor’s nomination right as set out herein and to reflect that the Exor Nominee qualifies for the independence exception of principle 2.1.7(iii) of the Dutch Corporate Governance Code. Philips confirms that at the date of this Agreement, the composition of the Supervisory Board shall not restrict the nomination or appointment of the Exor Nominee in light of the diversity quorum of article 2:142b DCC.
3.2.4 Philips shall use its reasonable efforts to cause the Philips Supervisory Board to nominate the Exor Nominee in accordance with the Articles of Association for appointment as member to the Philips Supervisory Board in the next General Meeting of Shareholders after receiving Exor's proposal.
3.2.5 Subject to compliance with applicable rules and regulations in relation to such nominations, if the Exor Nominee must be replaced, or its position is vacant for any reason, Exor may nominate a new Exor Nominee. Philips shall use reasonable efforts to cause the Philips Supervisory Board to nominate the Exor Nominee for appointment in accordance with Clause 3.2.4 and to determine that the relevant designated individual shall temporarily be allowed to attend Supervisory Board meetings until appointment by the General Meeting of Shareholders at the next General Meeting of Shareholders held after Exor has nominated a qualifying individual in writing to the Philips Supervisory Board.
3.2.6 If the General Meeting of Shareholders fails to appoint the Exor Nominee, Exor shall have the right to nominate one (1) other Exor Nominee in accordance with Clause 3.2.1, whereby clauses 3.2.2 through 3.2.5 shall apply mutatis mutandis.
3.2.7 If the General Meeting of Shareholders fails to appoint the Exor Nominee during the second General Meeting of Shareholders, the Parties shall discuss and identify a further nomination and the relevant process for appointment, provided that Exor shall be entitled to terminate this Agreement in accordance with Clause 10.1.1(a), provided that the run-off period in respect of Clauses 4.1.1 and 6 (except for Clauses 6.3.2(a) and 6.3.2(b)) will not apply in case no Exor Nominee has ever been appointed to Philips Supervisory Board in accordance with this Agreement.
3.3 Conflict of interest
3.3.1 Exor acknowledges and shall procure that the Exor Nominee shall, in fulfilling its role as member of the Philips Supervisory Board, solely be guided by the best interest of Philips and its business, taking into account the interests of all the Philips shareholders and stakeholders of the Philips Group, as also set out in the Dutch Corporate Governance Code.
3.3.2 Exor shall procure that the Exor Nominee shall abstain from participating in the deliberation and decision-making on any matter presented to the Philips Supervisory Board in which it has a conflict of interest, as set out in the Rules of Procedure Supervisory Board, including any transaction, arrangement or agreement between any member of the Philips Group and Exor or any of its Affiliates, or any of the legal entities referred to in article 1.10(2) of the Rules of Procedure Supervisory Board.
3.3.3 No conflict of interest shall be deemed to arise:
(a) by the mere fact that Exor owns Ordinary Shares and the Exor Nominee is also involved in Exor or any of its Affiliates as official, director, shareholder or otherwise;
(b) by the mere fact that the Exor Nominee disagrees with another member of the Philips Supervisory Board;
(c) on matters that affect Philips shareholders generally or require a vote or discussion in the General Meeting of Shareholders; or
(d) on the positioning of the Philips Supervisory Board in relation to potential takeover offers or activist campaigns (unless Exor is in breach of the Standstill (as defined below)).
3.3.4 In case the Exor Nominee votes against a proposal by Philips to the General Meeting of Shareholders (i) to change the composition of the Philips Board of Management (the ''Philips BoM'') or the Philips Supervisory Board, or (ii) to significantly change the identity or nature of Philips or the enterprise affiliated with it, and Exor has informed Philips, subject to Clause 3.5 that Exor and/or its Affiliates intend to exercise their shareholder rights to vote against the proposal, then Philips can require the Exor Nominee to abstain from the further deliberations and decision-making on the matter until the vote of the General Meeting of Shareholders on the matter is completed.
3.4 Expiry of nomination right
3.4.1 Subject to Clauses 3.4.3 and 3.5, Exor shall no longer have the right to nominate the Exor Nominee pursuant to Clause 3.2.1, if Exor and its Affiliates combined Interest no longer equals or exceeds the 15% Threshold Stake and Exor and its Affiliates combined Interest has remained below the 15% Threshold Stake for six (6) consecutive months, as a result of (i) a Disposal of Ordinary Shares or (ii) a choice to receive cash dividend by Exor or its Affiliates.
3.4.2 The expiration of the right referred to in Clause 3.4.1 is definitive and Exor cannot remedy such expiration, unless agreed otherwise between Exor and Philips.
3.4.3 If Exor and its Affiliates combined Interest threatens to fall below the 15% Threshold Stake due to an issuance of new shares or rights to shares for a cash consideration or a consideration in kind (including in connection with M&A transactions), then Philips shall offer Exor to participate in such issuance pro rata up to a maximum number of Ordinary Shares to maintain the 15% Threshold Stake. In case Exor does not participate in such issuance in full and its and its Affiliates' combined Interest is diluted by more than one-and-a-half percent (1.5%) of the issued and outstanding Ordinary Shares as a result of such issuance, Exor shall retain its right to nominate the Exor Nominee pursuant to Clause 3.2.1, provided that:
(a) its and its Affiliates' combined Interest equals or exceeds at least ten percent (10%) of the issued and outstanding Ordinary Shares and the voting rights in respect thereof (the "10% Temporary Threshold Stake") following the issuance of Ordinary Shares in which Exor did not participate in full; and
(b) Exor and its Affiliates shall use reasonable best efforts, including, but not limited to, electing any stock dividend or reinvesting any cash dividends in any stock of Philips, in order to obtain the 15% Threshold Stake within three (3) years after the aforementioned issuance of new shares or rights to shares, it being understood that if Exor and its Affiliates combined Interest falls below the 10% Temporary Threshold Stake at any time and has remained below the 10% Temporary Threshold Stake for six (6) consecutive months after the issuance in which Exor did not fully participate, Exor shall no longer have the right to nominate the Exor Nominee pursuant to Clause 3.2.1.
3.4.4 Exor shall inform the Philips Supervisory Board in writing within five (5) Business Days if Exor and its Affiliates combined Interest no longer reaches the 15% Threshold Stake. Exor shall provide Philips with sufficient information to confirm the exact date on which Exor and its Affiliates combined Interest failed to reach the 15% Threshold Stake.
3.4.5 If Exor and its Affiliates combined Interest again reaches the 15% Threshold Stake before the expiration referred to in Clause 3.4.1, it will inform Philips in writing within five (5) Business Days and shall provide Philips with sufficient information to confirm the exact date on which Exor and its Affiliates combined Interest again reached the 15% Threshold Stake.
3.5 Resignation Exor Nominee
3.5.1 In case Exor and/or its Affiliates intends to undertake any of the actions as set out below in Clauses 3.5.2(a), 3.5.2(b), 3.5.2(c) or 3.5.2(d), the Parties shall take the following actions in the following order with the aim of reaching an amicable solution prior to Exor and/or its Affiliates taking any such action:
(a) first, Exor shall inform the chairman of the Philips Supervisory Board;
(b) secondly, the Parties shall each designate one senior executive to, in good faith, discuss and to seek mutual understanding on how to deal with the relevant matter;
(c) thirdly, if no mutual understanding can be reached by the relevant senior executives, the chairman of the Philips Supervisory Board and the CEO of Exor shall discuss the relevant matter.
3.5.2 In case the Parties have not reached an amicable solution pursuant to Clause 3.5.1 and Exor and/or its Affiliates have pursued any of the actions set out in Clauses 3.5.2(a), 3.5.2(b), 3.5.2(c) or 3.5.2(d) below, Clauses 3.5.3 through 3.5.5 will apply:
(a) Exor and/or its Affiliates vote (i) in favour of a resolution that is not proposed or supported by the Philips Supervisory Board and that is not adopted by the General Meeting of Shareholders, or (ii) against a proposal that is proposed or supported by the Philips Supervisory Board and adopted by the General Meeting of Shareholders;
(b) Exor and/or its Affiliates exercise any of their shareholder rights and powers attached to any Ordinary Shares held by Exor or its respective Affiliates, as the case may be, to request any items to be included on the agenda of the General Meeting of Shareholders within the meaning of article 2:114a DCC, without approval of the Philips Supervisory Board and that is not adopted by the General Meeting of Shareholders;
(c) Exor and/or its Affiliates initiate legal proceedings against Philips including but not limited to proceedings at the Dutch Enterprise Chamber within the meaning of article 2:345 DCC; or
(d) Exor and/or its Affiliates breach Clauses 4, 5, 6 or 7.1
3.5.3 For any action taken pursuant to Clauses 3.5.2(a) or 3.5.2(b) Philips and Exor shall reasonably discuss in good faith over at least a period of two (2) weeks after the General Meeting of Shareholders at which the relevant proposal is voted on, whether the Exor Nominee should resign from the Philips Supervisory Board, and after such discussions have taken place, the Supervisory Board shall decide whether the Exor Nominee should resign.
3.5.4 In case of Clauses 3.5.2(a) or 3.5.2(b), where the General Meeting of Shareholders follows the position or proposal (as the case may be) of Exor, Philips and Exor shall reasonably discuss in good faith whether the Exor Nominee should resign from the Philips Supervisory Board.
3.5.5 For any action taken pursuant to Clauses 3.5.2(c) or 3.5.2(d), unless such action or breach is reasonably capable of being remedied and has not been remedied by Exor and/or its Affiliates within forty-five (45) days after receipt of a written notice of such breach from Philips, Exor shall procure that the Exor Nominee shall resign immediately upon first request by the Philips Supervisory Board, unless the Philips Supervisory Board (excluding the Exor Nominee) decides that the resignation may take place later.
3.5.6 Notwithstanding Clauses 3.5.1 through 3.5.5, Exor shall procure that the Exor Nominee shall resign immediately upon first request by the Philips Supervisory Board, unless the Philips Supervisory Board (excluding the Exor Nominee) decides that the resignation may take place later in case:
(a) Exor no longer has the right to nominate the Exor Nominee pursuant to Clause 4 (Expiry of nomination right); or
(b) the Exor Nominee engages in gross negligence, wilful misconduct, fraud or maladministration (onbehoorlijk bestuur).
3.5.7 In case of a resignation of the Exor Nominee pursuant to Clauses 3.5.2 or 3.5.6(a), Exor shall no longer have the right to nominate an Exor Nominee in accordance with Clause 3.2, provided that a resignation pursuant to maladministration (onbehoorlijk bestuur) or gross negligence as set out in Clause 3.5.6(b) shall require the prior consent of Exor (not to be unreasonably withheld) and that a resignation pursuant to Clause 3.5.6(b) shall not affect the right of Exor to nominate a replacement on the terms of this Agreement for appointment at the next General Meeting of Shareholders.
3.6 Dismissal Exor Nominee
The Philips Supervisory Board shall not be permitted to propose a suspension or dismissal of the Exor Nominee to the General Meeting of Shareholders, unless:
(a) in case of gross negligence, wilful misconduct, fraud, maladministration (onbehoorlijk bestuur); or
(b) the Exor Nominee, whose resignation was to be procured by Exor under Clause 5 (Resignation Nominee), did not resign immediately after such obligation arose,
provided that a dismissal pursuant to maladministration (onbehoorlijk bestuur) or gross negligence as set out in Clause 3.6(a) shall require the prior consent of Exor (not to be unreasonably withheld) and that a dismissal pursuant to Clause 3.6(a) shall not affect the right of Exor to nominate a replacement on the terms of this Agreement for appointment at the same or next General Meeting of Shareholders, as indicated by Exor.
4 Standstill
4.1 .1Exor shall not, and shall procure that its Affiliates and its other Representatives acting on its or any of its Affiliates’ behalf shall not, without the prior written consent of Philips, directly or indirectly:
(a) Acquire more than the 20% Threshold Stake;
(b) make or announce, or cause, assist, advise or coordinate with another Person to make or announce, a public offer for any Ordinary Shares that is not recommended by the Philips BoM;
(c) offer, sell or tender their Ordinary Shares, in whole or in part, whether or not in the open market to any party, or parties acting together, that have made, have announced to make or are reasonably expected to make or partake in a public offer on the Ordinary Shares in accordance with Article 5:70 or 5:74 of the Dutch Financial Supervision Act (Wet op het financieel toezicht) that is not recommended by the Philips BoM, unless such party, or such parties, have Acquired an Interest of more than fifty percent (50%) in Philips or more (not caused or assisted by any action of Exor or any of its Affiliates);
(d) propose to enter into, directly or indirectly, any merger or business combination involving Philips or any of its Affiliates or to purchase, directly or indirectly, a material portion of the assets of Philips or any of its Affiliates;
(e) take any short position in any securities issued by Philips, except (i) as permitted under Clause 2.1(a) through 6.2.1(c) or (ii) in connection with a Permitted Disposal;
(f) act in concert with any hedge fund publicly engaged in any activist campaign against Philips at that point in time aimed at seeking control or influence over Philips' Supervisory Board, the Philips' BoM or policies; or
(g) advise, assist or encourage any Person in connection with any of the foregoing.
(such obligations together, the "Standstill").
4.1.2 If Philips intends to restructure its capital, in any way, as a result of which Exor would come to hold such a percentage of the Ordinary Shares that it would become obligated to make a Mandatory Offer, Philips shall inform Exor in writing at least thirty (30) Business Days before initiating such restructuring. In such case, Parties shall reasonably discuss in good faith to take such measures (including to participate in such restructuring, or to repurchase Exor’s Ordinary Shares if appropriate) as are required to avoid that Exor will have to make such Mandatory Offer.
4.1.3 In case of any share buy-back program initiated by Philips, Parties shall reasonably discuss in good faith for Exor or its Affiliates to participate in such share buy-back program to the extent required to avoid that Exor and its Affiliates combined Interest will exceed the 20% Threshold Stake.
4.1.4 If, for whatever other reason Exor and its Affiliates combined Interest exceeds the 20% Threshold Stake, Philips may request that Exor and/or its Affiliates will Dispose of the excess Ordinary Shares in an orderly market manner within forty (40) Business Days, unless a longer period is agreed upon with Philips, failing which Philips will have the right (but not the obligation) to repurchase the excess Ordinary Shares at the prevailing market price.
5 Non-compete
For as long as Exor has a nomination right pursuant to Clause 2 (Nomination of the Exor Nominee), Exor shall not, and shall procure that its Affiliates and its other Representatives acting on its or any of its Affiliates' behalf shall not, without the prior written consent of Philips, directly or indirectly, either alone or together with another Person, Acquire any shares or other (options to) securities of a Philips Competitor, exceeding two percent (2%) of such Competitor's total issued share capital, provided that this restriction shall not apply to investments by or on behalf of the on arm’s length, independently managed, asset management company Lingotto.
6 Lock-Up and Sell Down
6.1 General
Notwithstanding any other provision in this Agreement:
(a) Exor’s obligations in this Clause 6 will fall away with immediate effect when any third party has Acquired an Interest of fifty percent (50%) or more in Philips (not caused, assisted or supported by any action of Exor or any of its Affiliates in breach of this Agreement); and
(b) the Lock-Up Period (as defined below) will fall away with immediate effect when Philips' long term issuer credit rating is downgraded to below investment grade.
6.2 Lock-up period
6.2.1 Subject to Clause 4 (Standstill), Clause 5 (Non-compete) and Clause 6 (Lock-Up and Sell Down), during the period commencing on the Signing Date and ending three (3) years thereafter (both days inclusive) (the “Lock-Up Period”), Exor shall not, and shall procure that its Affiliates shall not, undertake any action that causes Exor and its Affiliates combined Interest to fall below the 15% Threshold Stake or, for the avoidance of doubt, exceed the 20% Threshold Stake (the “Lock-Up”), provided that nothing in this Clause 6 shall prevent:
(a) the entry into, exercise of rights and performance of obligations under, or any transfer pursuant to, an agreement documenting, or relating to, the Permitted Derivatives Transaction or Permitted Securities Lending Transaction;
(b) the grant of a pledge, charge or any other security interest over any Ordinary Shares or the assignment of any rights in relation to any Ordinary Shares (including the creation and exercise of a right of use on one or more occasions at any time) (a “Security Interest”) to or for the benefit of any Permitted Derivative Transaction Counterparty and/or any agent or trustee acting for any such counterparty; or
(c) the sale, transfer or appropriation of any Ordinary Shares or exercise of any other enforcement rights pursuant to and following any enforcement of a Security Interest over, or in relation to, any Ordinary Shares granted by Exor to or for the benefit of any Permitted Derivative Transaction Counterparty.
6.3 Post Lock-Up Sell Down
6.3.1 Subject to Clause 4 (Standstill), and Clause 5 (Non-compete), and Clause 6 (Lock-Up and Sell Down) (for the avoidance of doubt, including Clause 6.2 (Lock-up period)), Exor and its Affiliates may Dispose all or part of their Ordinary Shares, whether or not in the open market (a "Permitted Disposal"). Notwithstanding the preceding sentence, Exor shall use reasonable best efforts to conduct (and shall procure that its Affiliates will use reasonable best efforts to conduct) any Permitted Disposal in an orderly market manner.
6.3.2 Any Permitted Disposal by Exor or any of its Affiliates (other than in regular stock market transactions in which Exor, nor its Affiliates, brokers or intermediaries acting on its behalf reasonably know the identity of the counterparty or have control over the settlement of the respective transaction) to the following parties requires prior written approval from Philips:
(a) Philips Competitors;
(b) activist parties:
(i) publicly acting at that point in time as an activist vis-à-vis Philips; or
(ii) other activist parties with a track-record of shareholder activism, as regularly determined by Philips and Exor jointly, each acting in good faith;
(c) investors that as a result of the Permitted Disposal would come to legally or beneficially hold five percent (5%) or more of the issued and outstanding Ordinary Shares; and
(d) investors that as a result of the Permitted Disposal would become required to make a Mandatory Offer.
6.3.3 In case of an intended Permitted Disposal by Exor and its Affiliates of more than three percent (3%) over a one-month period of the issued and outstanding Ordinary Shares in Philips, Exor will, to the extent allowed by applicable laws and regulations, consult with Philips as soon as reasonably possible about the (intended) Permitted Disposal and, if applicable, the intended bookrunner(s) to be appointed.
6.3.4 Philips shall reasonably cooperate with Exor in good faith in connection with any Permitted Disposal by Exor and its Affiliates of more than three percent (3%) of the issued and outstanding Ordinary Shares in Philips, including, but not limited to (i) providing access to information required for a due diligence which is appropriate for a company of the size and nature of Philips and which is customary and market practice for similar transactions, (ii) providing cooperation and assistance in connection with the preparation of a prospectus or a similar offering document required under applicable law to consummate such Permitted Disposal, (iii) providing cooperation and assistance with requests from the underwriters or advisers involved in the Permitted Disposal, including for management involvement in a Marketed Offering that is being carried out in order to consummate such Permitted Disposal or being a party to an underwriting agreement in connection with a Marketed Offering on terms that are customary and market practice for similar transactions, including indemnification provisions, it being understood that nothing in this Clause 6.3.4, implies an obligation on the part of Philips to apply for a (secondary) listing of the Ordinary Shares and (b) Philips will be under no obligation to share inside information (as defined by the MAR) relating to Philips in respect of the foregoing except to the extent allowed under the MAR. Furthermore, Philips may delay its compliance with its obligations under this Clause 6.3.4, if Philips determines in good faith that such compliance would violate applicable law, stock exchange requirements or Philips' insider trading policy.
6.3.5 The Parties will maintain an ongoing dialogue with Philips regarding investors who potentially could be interested in acquiring the Ordinary Shares held by Exor and/or its Affiliates, provided that Exor will be under no obligation to share inside information (as defined by the MAR) with Philips in this respect.
6.3.6 The Parties acknowledge and agree that the obligation of Philips to reasonably cooperate in good faith with due diligence under Clause 6.3.4 includes but is not limited to (i) management interviews, (ii) a review of the minutes of meetings of the Philips BoM and the Philips Supervisory Board and (iii) a limited documentary review relating to major litigation, acquisitions and disposals.
6.3.7 Each Party will bear its own fees and expenses in connection with a Permitted Proposal, provided that any fees and expenses incurred by Philips in connection with the preparation of such Permitted Disposal as a direct result of a request by Exor to co-operate with such Permitted Disposal will be borne by Exor, it being understood that if the Permitted Disposal also includes the issue or sale of Ordinary Shares by Philips, Exor and Philips will each bear its pro rata share of such fees and external expenses based on the number of Ordinary Shares actually issued or sold by them in such Permitted Disposal.
6.3.8 In case of an Accelerated Bookbuilding Offering or a Marketed Offering, Exor will give Philips the opportunity to provide suggestions on the execution thereof including the allocation of placement of Ordinary Shares, provided that the final allocations will be decided between Exor and its banks.
6.4 Intragroup disposal of shares
The Parties acknowledge that Exor may at any time Dispose its (directly or indirectly held) Ordinary Shares to a Permitted Exor Transferee, provided that (i) the Permitted Exor Transferee first becomes a party to this Agreement by signing a Deed of Adherence and (ii) Exor remains jointly and severally liable with such Permitted Exor Transferee for all obligations under this Agreement. If the Permitted Exor Transferee is at any time no longer an Affiliate of Exor, the Ordinary Shares shall be transferred back to Exor or such other Permitted Exor Transferee of Exor in compliance with this Clause 4.
7 Public Communications
7.1 Non-disparagement
Each Party shall refrain from making, and shall cause its Affiliates and its and their respective Representatives not to make or cause to be made, any false or bad faith statement or announcement (including through any press, media, analysts or other Persons) that derogates or is reasonably likely to damage the reputation of the other Party and any of its Affiliates, or any of its or their respective current or former Representatives.
7.2 General communications
Any communication about Philips by Exor and/or its Affiliates, including any filings with the SEC (e.g., Schedule 13D and/or 13G) upon and after obtaining the 15% Threshold Stake, shall be made only after consultation with Philips. Such consultation shall not be required for any communication:
(a) which is in line with communication arrangements pre-agreed between the Parties, if any;
(b) which is in the ordinary course of business of investor communication; or
(c) confirming facts or information that are already in the public domain other than as a result of a breach of this Agreement.
7.3 Communication on Permitted Disposals
7.3.1 In view of the necessity of a clear and coordinated communication regarding any Permitted Disposal, public communications by either Party with respect to a Permitted Disposal will be made only in accordance with applicable law and after consultation with the other Party regarding the contents of such communication, to the extent reasonably practicable and subject to Clause 8 (Information Requirements). Such consultation shall not be required for any communication:
(a) which is in line with communication arrangements pre-agreed between the Parties, if any;
(b) which is in the ordinary course of business of investor communication and not disclosing specific information on an actual Permitted Disposal; or
(c) confirming facts or information that are already in the public domain other than as a result of a breach of this Agreement.
7.3.2 Each Party shall ensure that any communication by it relating to a Permitted Disposal will not result in violations of securities laws or inconsistencies with any prospectus or similar offering document regarding such Permitted Disposal.
8 Information Requirements
8.1 Duty to disclose
Nothing in this Agreement shall prohibit or restrict either Party from disclosing (in accordance with article 17 MAR or such other laws, or applicable rules or regulations, including the rules and regulations of any relevant stock exchange or other regulatory body (including the AFM and the SEC) to which either Party is or becomes subject) any inside information, as defined in the MAR, if and when such disclosure is in the reasonable opinion of such Party required and cannot or can no longer be delayed under applicable law or by any rules or regulations (including the rules and regulations of any relevant stock exchange or other regulatory body such as the AFM and the SEC).
8.2 No selective disclosure
Nothing in this Agreement will require a providing Party to disclose inside information, as defined in the MAR, to the receiving Party to the extent that such disclosure without general publication would violate applicable law. The Parties confirm their view, which view is based on the current interpretation of the relevant courts of applicable laws pertaining to inside information and the disclosure thereof, that to the extent that the information a Party discloses to another Party pursuant to this Agreement qualifies as inside information, this disclosure is made in the normal course of the exercise of that Party's duties, within the meaning of article 10(1) MAR.
8.3 Price sensitive information relating to the other party
8.3.1 The Parties acknowledge that each Party is subject to certain duties under the MAR and that Philips is also subject to certain duties under US federal securities laws and the rules of the New York Stock Exchange, and that such laws and rules may impose duties and restrictions as to the timely publication and/or use of inside information or other material information.
8.3.2 Each Party acknowledges that any disclosure of price sensitive information (voorwetenschap), as defined in the MAR, relating to such Party and/or its shares could also qualify as price sensitive information in relation to the other Party and/or its shares.
8.4 Periodical Information Meetings
Subject to applicable rules and regulations, including Philips' investor relations policy, Philips shall undertake that Exor shall have the opportunity to meet with Philips' investor relations team, the Philips BoM and chairman of the Philips Supervisory Board on a regular basis.
8.5 Interest disclosure
Philips may at any time reasonably request Exor to inform Philips how many Ordinary Shares Exor and its Affiliates legally or beneficially hold. Upon such request, Exor shall inform Philips as requested in writing within fifteen (15) Business Days.
8.6 Exor information rights
8.6.1 To the extent permitted under applicable law and regulation (including the MAR), Philips shall supply Exor with all such information reasonably required by Exor:
(a) to complete any tax return or other filing which may be required by law or regulation;
(b) for any audit or regulatory reason; or
(c) to meet its financial reporting requirements.
8.7 Confidentiality
8.7.1 Subject to Clause 8.7.2, each Party shall keep confidential all non-public information provided to it by the other Party or otherwise obtained by it under or in connection with this Agreement regarding the business and financial affairs of the other Party or any of its Affiliates (“Confidential Information”).
8.7.2 Each Party shall be entitled to disclose Confidential Information:
(a) to any of its officers, employees, auditors, bankers or professional advisers, whose position makes it necessary to know that information in order to assist that Party, as applicable; provided that the recipient thereof agrees to be bound by the same duty of confidentiality as applies to the disclosing Party and that such Party shall be responsible for any breach of confidentiality by such recipient;
(b) in respect of Exor or the Exor Nominee, to any of its respective direct or indirect Affiliates and its respective officers, employees, auditors, bankers or professional advisers, in any event only if and when it is necessary that such party or person receives that information to assist Exor in relation to its shareholding in Philips provided that the recipient thereof agrees to be bound by the same duty of confidentiality as applies to the disclosing Party and that the disclosing Party shall be responsible for any breach of confidentiality by such recipient;
(c) if such information has ceased to be Confidential Information as a result of having become public without breach of this Agreement or any other duty of confidentiality relating to that information of which the relevant Party was aware;
(d) as may be required by law, rules or regulations or by any relevant securities exchange or governmental authority, regulatory body or antitrust authority to which that Party is subject (wherever situated), including information required to be disclosed in any shareholder circular, or for tax, financial reporting, audit or accounting purposes, whether or not the requirement for disclosure of such information has the force of law;
(e) as may be required for the purpose of any arbitral or judicial proceedings arising out of this Agreement or the related agreements; or
(f) with the written consent of the other Party.
9 General Restrictions
9.1 General
9.1.1 Exor shall, and shall procure that its Affiliates shall, not take any action that would have the effect of preventing the Philips Group from:
(a) complying with their obligations under applicable laws and regulations; or
(b) managing their affairs in accordance with the principles of good governance set out in the Dutch Corporate Governance Code (save as disclosed by Philips).
9.1.2 Exor shall, and shall procure that its Affiliates shall, not exercise any of its voting rights or other shareholder rights and powers attached to any Ordinary Shares held by Exor or its Affiliates, as the case may be, in a way that would be inconsistent with, or breach any of the provisions of this Agreement, applicable laws and regulations (including related to insider trading) or the Dutch Corporate Governance Code (including applicable deviations).
9.1.3 Other than the obligations set out in Clause 9.1.1 and 9.1.2, no limitations shall apply to Exor and its Affiliates in exercising their voting rights or other shareholder rights and powers attached to any Ordinary Shares held by Exor or its respective Affiliates, as the case may be.
9.2 Constitutional documents
Philips and Exor shall procure that the Philips BoM and the Philips Supervisory Board shall not propose, implement or approve any amendment to (i) the Articles of Association and (ii) the Rules of Procedure Supervisory Board, if such amendment would be contradictory to the arrangements set forth in this Agreement.
10 Duration and termination
10.1 Duration and termination
10.1.1 This Agreement shall enter into force on the date hereof and terminate automatically upon the occurrence of the earlier of:
(a) Exor no longer having the right to nominate the Exor Nominee pursuant to Clause 4 (Expiry of nomination right), the Exor Nominee ceasing to be a member of the Philips Supervisory Board, without Exor having nominated a replacement within eight (8) weeks (if applicable), or no Exor Nominee has been appointed pursuant to and subject to Clause 3.2.7, provided that in each of these events Clause 4.1.1 will continue to apply for a period of eighteen (18) months, the Lock-Up (as set out in Clause 6.2) will continue to apply until the earlier of (i) the expiry of the Lock-Up Period and (ii) a period of six (6) months and the other provisions of Clause 6 shall continue to apply for a period of twelve (12) months;
(b) a Party becoming subject to bankruptcy or suspension of payments;
(c) the Ordinary Shares ceasing to be admitted to listing on the regulated market of Euronext Amsterdam; or
(d) the dissolution or liquidation of a Party, provided that if a Party ceases to exist as a result of a merger, demerger, conversion, or other similar corporate transaction, such Party's legal successor shall be deemed to have become a party to this Agreement in such Party's place and this Agreement shall not terminate,
provided that this Clause 10, Clause 2 and Clauses 12 up to and including 18 shall survive termination of this Agreement.
10.1.2 Except as provided in Clause 10.1.1, this Agreement may only be terminated by mutual agreement of the Parties in writing.
11 Validity
11.1 Signing
11.1.1 This Agreement does not have any legal effect until each Party has validly signed this Agreement.
11.1.2 If this Agreement is signed in counterparts, these counterparts will count as one agreement.
11.2 Invalidity
11.2.1 In this Clause 11.2 "enforceable" includes legal, valid and binding (and derivative terms are to be construed accordingly).
11.2.2 If any provision in this Agreement is held to be or becomes unenforceable (in each case either in its entirety or in part) under any law of any jurisdiction:
(a) that provision will to the extent of its unenforceability be deemed not to form part of this Agreement but, subject to the restrictions of article 3:41 of the Dutch Civil Code, the enforceability of the remainder of this Agreement will not be affected; and
(b) the Parties shall use reasonable efforts to agree a replacement provision that is enforceable to achieve so far as possible the intended effect of the unenforceable provision.
12 Entire Agreement
This Agreement contains the entire agreement of the Parties in relation to its subject matter. All previous agreements and arrangements made by the Parties in relation to that subject matter are hereby terminated.
13 Amendments and waivers
13.1 Amendments and waivers
This Agreement may not be amended, supplemented or waived except by a written agreement between the Parties.
13.2 No deemed waivers
No failure to exercise, nor any delay in exercising, by a Party, any right or remedy under this Agreement will operate as a waiver. No single or partial exercise of any right or remedy will prevent any further or other exercise or the exercise of any other right or remedy.
13.3 Further assurances
The Parties shall at their own costs and expenses from time to time execute and procure to be executed such documents and perform and procure to be performed such acts as may be reasonable required by each of them to give the Parties the full benefit of this Agreement.
14 Third party rights
Except where this Agreement expressly provides otherwise:
(a) it contains no stipulations for the benefit of a third party (derdenbedingen) which may be invoked by a third party against a Party; and
(b) where this Agreement contains a stipulation for the benefit of a third party, this Agreement (including the relevant third party's rights under this Agreement) may be terminated, amended, supplemented or waived (in each case either in its entirety or in part) without that third party's consent.
15 Rescission, errors and suspension
15.1 No rescission; errors
15.1.1 No Party may fully or partly rescind (ontbinden) this Agreement.
15.1.2 If a Party has made an error (heeft gedwaald) in relation to this Agreement, it shall bear the risk of that error.
15.2 No suspension
No Party may suspend (opschorten) performance of its obligations under or in connection with this Agreement on whatever grounds.
16 No assignment
No Party may fully or partly assign or encumber rights and obligations under this Agreement without the other Party's prior written consent. Without this consent, no assignment or encumbrance is effected.
17 Notices
17.1 Communications in writing
Any communication to be made under or in connection with this Agreement must be made in writing and sent by regular mail or e-mail.
17.2 Addresses
The address and e-mail addresses (and the department of the officer, if any, for whose attention the communication is made) of each Party for any communication to be made under or in connection with this Agreement are any substitute address or department or officer as the Party may notify to the other Party by not less than five (5) days' notice.
18 Governing law and dispute resolution
18.1 Governing law
This Agreement shall be governed by and construed in accordance with the laws of the Netherlands.
18.2 Dispute resolution
18.2.1 Any dispute arising out of, or in connection with, this Agreement or other agreements and arrangements connected to or resulting from this Agreement, whether contractual or non-contractual, shall be submitted to the CEOs of Exor and Philips from time to time to be settled and resolved by them within twenty (20) Business Days of the matter being referred to them, following and upon the written request of either of the Parties.
18.2.2 If the dispute cannot be resolved by the CEOs of Exor and Philips within twenty (20) Business Days of the matter being referred to them in accordance with Clause 18.2.1, the Parties shall refer the dispute to proceedings under the rules of arbitration of the Netherlands Arbitration Institute (Nederlands Arbitrage Instituut) ("NAI", unless it concerns an urgent matter as referred to in Section 254 of the Dutch Code of Civil Procedure, in which case the dispute will be finally resolved in accordance with the Dutch Code of Civil Procedure. The place of arbitration will be in Amsterdam, the Netherlands. The language of arbitration will be in English.
18.2.3 The arbitral tribunal will consist of three (3) arbitrators to be nominated and/or appointed as follows:
(a) The claimant Party shall nominate one arbitrator in its request for arbitration, and the respondent Party shall nominate one arbitrator in its answer. If a Party fails to nominate an arbitrator, the relevant arbitrator will be appointed by the NAI;
(b) The third arbitrator will act as chairman of the arbitral tribunal. The third arbitrator will be nominated jointly by the two arbitrators referred to in paragraph (a) above within 30 days of the date of the last of their confirmations and/or appointments. If these two arbitrators fail to nominate jointly the third arbitrator, that arbitrator will be appointed by the NAI.
18.2.4 The arbitral tribunal shall decide and make its arbitral award or awards in accordance with the applicable rules of law. The arbitral tribunal shall not assume the powers of an amiable compositeur or decide ex aequo et bono.
18.2.5 An arbitration pursuant to this Clause 18.2 shall not be consolidated with any other arbitration, whether on the basis of article 1046 of the Dutch Code of Civil Procedure (Wetboek van Burgerlijke Rechtsvordering) or otherwise, except for another arbitration pursuant to this Clause 18.2.
18.2.6 The Parties shall not be entitled to any form of discovery or disclosure, and the arbitral tribunal shall have no power to order discovery or disclosure of (a) documentary evidence, (b) oral testimony, or (c) any other materials.
18.2.7 Severability
If any provision of this Agreement is held by any court or other competent authority to be void or unenforceable in whole or in part, the other provisions of this Agreement and the remainder of the effective provisions will continue to be valid. The Parties will then use all reasonable endeavours to replace the invalid or unenforceable provision(s) with a valid and enforceable substitute provision(s) the effect of which is as close as possible to the intended effect of the invalid or unenforceable provision(s).
THIS AGREEMENT HAS BEEN SIGNED ON THE DATE STATED AT THE BEGINNING OF THIS AGREEMENT BY:
Koninklijke Philips N.V.
By: W.R.O. Jakobs
Title:CEO
By: F.Sijbesma
Title: Chairman of the Supervisory Board
EXOR N.V.
By: G.de Boer
Title: CFO
Schedule 1 Definitions and Interpretation
1 Definitions
"10% Temporary Threshold Stake" has the meaning given to it in Clause 3.4.3;
"15% Threshold Stake" has the meaning given to it in Recital (A);
"20% Threshold Stake" has the meaning given to it in Recital (A);
"Accelerated Bookbuilding Offering" means an offering of Ordinary Shares for which the risk has not been transferred to a third party (such as in a Bought Deal);
''Acquire'' means the act or process of obtaining, directly or indirectly, (economic) ownership, possession, or control including lending or holding for another Person;
"Affiliates" means, in respect of a Party, a Person which is Controlling or Controlled by such Party, or Controlled by a Person who also Controls such Party, or which otherwise qualifies as a "subsidiary" or "group company" of that Party as referred to in articles 2:24a and 2:24b Dutch Civil Code (Burgerlijk Wetboek); for the avoidance of doubt, for purposes of this Agreement, Philips and its Affiliates shall not be considered to be Affiliates of Exor and vice versa;
"AFM" means Stichting Autoriteit Financiële Markten;
"Agreement" has the meaning given to it in Recital (A);
"Articles of Association" means the articles of association of Philips, as amended from time to time;
"Bought Deal" means a sale and transfer of Ordinary Shares in which an investment bank or other third party is taking a risk position other than taking a settlement risk;
"Business Day" mean a day (other than a Saturday or a Sunday) on which banks are open for general business in the Netherlands;
''CGNS Committee” means the Corporate Governance and Nomination & Selection Committee of the Philips Supervisory Board;
"Clause" means a clause of this Agreement;
''Confidential Information” has the meaning given in Clause 8.7.1;
"Control" means the possession, directly or indirectly, solely or jointly, whether through ownership of voting interests, by contract or otherwise, of (a) more than 50% of the voting power at general meetings of a Person, (b) the power to appoint and dismiss a majority of the managing directors or supervisory directors of a Person or (c) the power to otherwise direct or cause the direction of the management and policies of a Person, and "Controlling" and "Controlled" shall be construed accordingly;
"DCC" means the Dutch Civil Code;
"Deed of Adherence" as attached as Schedule 3 (Deed of Adherence);
"Disposal" or "Dispose" means (i) to directly or indirectly, sell, transfer, assign or otherwise dispose of any legal or beneficial interest in any Ordinary Share and the voting rights in respect thereof, (ii) to directly or indirectly grant any Encumbrance over any Ordinary Share or (iii) any arrangement, structuring device or other transaction having a similar economic or legal effect to the transactions referred to under (i) and (ii), it being understood that a Disposal of Ordinary Shares to a legal successor under universal title shall not be considered a Disposal, and where “Encumbrance” means any security interest, claim, lien, charge, pledge, or other restriction that creates or may create a security interest over the Ordinary Shares;
"Dutch Corporate Governance Code" means the Dutch Corporate Governance Code of 20 December 2022;
"Exor" has the meaning given in the preamble of this Agreement;
"Exor Nominee" has the meaning given in Clause 3.2.1;
"General Meeting of Shareholders" has the meaning given to it in Clause 3.2.1;
“Interest” has the meaning given to it in Recital (A);
“Lock-Up” has the meaning given to it in Clause 6.2.1;
“Lock-Up Period” has the meaning given to it in Clause 6.2.1;
"Mandatory Offer" means a mandatory public offer for Philips in accordance with Articles 5:70 and 72(1) of the Financial Supervision Act (Wet op het financieel toezicht);
"MAR" means Regulation (EU) No 596/2014 of the European Parliament and of the Council of 16 April 2014 on market abuse (market abuse regulation);
"Marketed Offering" means an offering of Philips securities which entails Philips' involvement in the form of a management road show and/or the preparation of a prospectus, registration statement or similar offering document;
"NAI" has the meaning given to it in Clause 18.2.1;
"Ordinary Shares" means the ordinary shares in the share capital of Philips;
"Parties" or "Party" has the meaning given to it in the preamble of this Agreement;
"Permitted Derivative Transaction" means the financial instrument entered into by Exor on or around the date of this Agreement to facilitate the purchase of Ordinary Shares by Exor to increase its shareholding to the initial 15% Threshold Stake, which may provide price protection in relation to such purchase and which has a final maturity date falling not more than 18 months following the date of this Agreement;
"Permitted Derivative Transaction Counterparty" means any party to a Permitted Derivative Transaction;
"Permitted Disposal" has the meaning given in Clause 6.3.1;
"Permitted Exor Transferee" means Exor Nederland N.V., Exor S.A., Ancom USA Inc, Exor SN LLC, and Exor Investments Limited, as well as any other Person that Exor notifies to Philips and is Controlled by Exor;
"Permitted Securities Lending Transaction" means any stock borrowing or lending, or repurchase or collateral arrangement or right of use relating to Ordinary Shares created pursuant to or in accordance with the terms of a Permitted Derivative Transaction or Security Interest to facilitate the transfer of Ordinary Shares to the Permitted Derivative Transaction Counterparty for the purposes of facilitating the hedging activities of the Permitted Derivative Transaction Counterparty to prevent the termination of the Permitted Derivative Transaction and under which Exor has a right to receive equivalent Ordinary Shares at or prior to maturity of the relevant Permitted Derivative Transaction;
"Person" means any individual, company, legal entity, partnership or unincorporated association, whether or not having separate legal personality;
"Philips BoM" has the meaning given to it in Clause 3.3.4;
"Philips Competitor" means any party identified between the Parties at the date hereof, provided that the list of Philips Competitors can be updated in good faith between the Parties every two years following the date of this Agreement and that Clause 5 shall not apply to investments held by Exor or its Affiliates in a party at the date that such party is qualified as a Philips Competitor;
"Philips Group" means Philips and its Affiliates;
"Philips Shareholder" means any Person holding any Ordinary Shares;
"Philips Supervisory Board Member" has the meaning given to it in Clause 3.2.1;
"Philips Supervisory Board" has the meaning given to it in Clause 3.2.1;
"Philips" has the meaning given in the preamble of this Agreement;
"Representatives" means, in respect of a Party, its Affiliates as well as the directors, officers, employees, agents and professional advisers (including lawyers, accountants, consultants and financial advisers) of such Party or any of its Affiliates;
''Rules of Procedure Philips Supervisory Board'' means the rules of procedure of the Philips Supervisory Board, as amended from time to time;
"Schedule" means a schedule to this Agreement;
"SEC" means the United States' Securities and Exchange Commission;
''Security Interest" has the meaning given to it in Clause 6.2.1(b);
"Signing Date" means the date on which the Agreement is duly signed by all Parties;
"Standstill" has the meaning given to it in Clause 4.1.1;
2 Headings and references to Clauses and Schedules
2.1Headings have been inserted for convenience of reference only and do not affect the interpretation of any of the provisions of this Agreement.
2.2A reference in this Agreement to:
(a) a Clause is to the relevant clause of this Agreement; and
(b) a Schedule is to the relevant schedule to this Agreement.
3 Legal terms
In respect of any jurisdiction other than the Netherlands, a reference to any Dutch legal term shall be construed as a reference to the term or concept which most nearly corresponds to it in that jurisdiction.
4 Other references
4.1 Whenever used in this Agreement, the words "include", "includes" and "including" shall be deemed to be followed by the phrase "without limitation".
4.2 Whenever used in this Agreement, the words "as of" shall be deemed to include the day or moment in time specified thereafter.
4.3 Any reference in this Agreement to any gender shall include all genders, and words importing the singular shall include the plural and vice versa.
Schedule 2 Press release
Schedule 3 Deed of Adherence
THIS DEED is made on [●]
BETWEEN:
WHEREAS:
(A) The Company and the Original Shareholder at the date of this deed of adherence are parties to a relationship agreement dated [●] (the "Agreement";)
(B) The Original Shareholder intends to transfer the Agreement to the New Shareholder
(C) This Deed is made by the New Shareholder in compliance with Clause [●] of the Agreement.
IT IS AGREED as follows:
1. The New Shareholder confirms that it has been supplied with a copy of the Agreement.
2. The New Shareholder undertakes to the each of the parties to the Agreement to be bound by the Agreement in all respects as if the New Shareholder was a Party to the Agreement and named in it as Exor and to observe and perform all the provisions and obligations of the Agreement applicable to or binding on Exor under the Agreement insofar as they fall to be observed or performed on or after the date of this Deed.
3. This Deed is made for the benefit of (a) the Parties to the Agreement and (b) every other person who after the date of the Agreement (and whether before or after the execution of this deed) assumes any rights or obligations under the Agreement or adheres to it.
4. The contact details of the New Shareholder are as follows:
Attn:
E-mail:
Address:
With copy to:
5. This Deed shall be governed by and construed in accordance with the laws of the Netherlands. Any dispute arising out of, or in connection with, this Deed shall be resolved in accordance with the provisions of the Agreement.
IN WITNESS WHEREOF this Deed has been executed and has been delivered on the date which appears on the first page of this Deed.
[New Shareholder]
By:
Its:
The Company
By:
Its: