Document
Praxis Precision Medicines Provides Corporate Update and Reports Second Quarter 2025 Financial Results
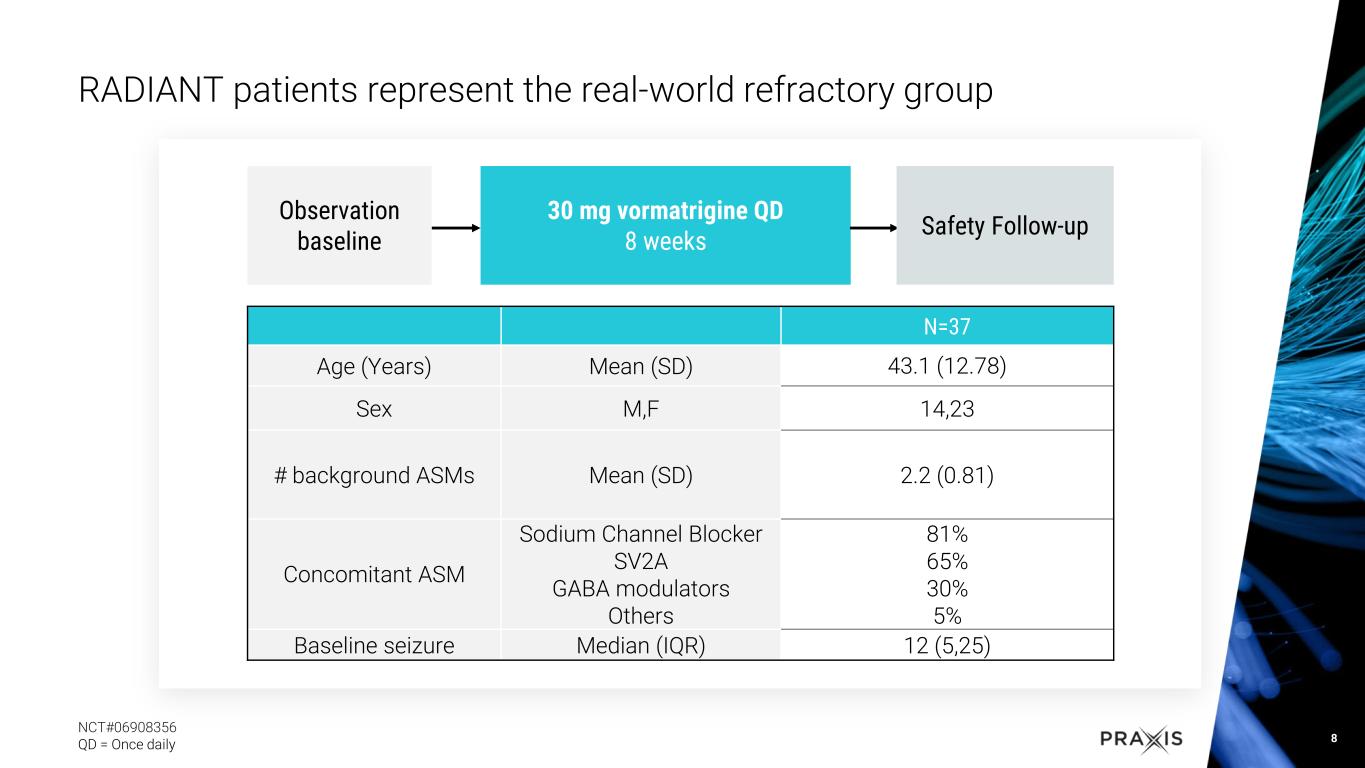
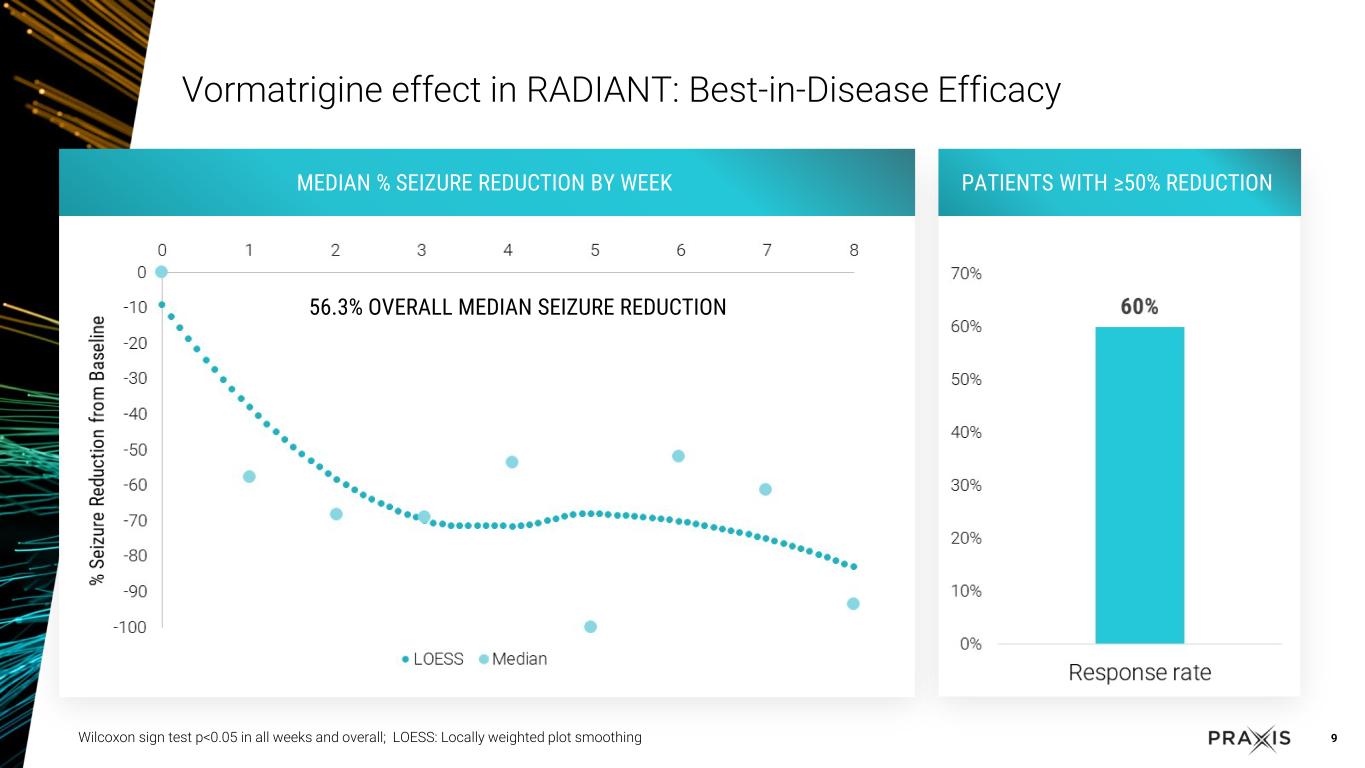
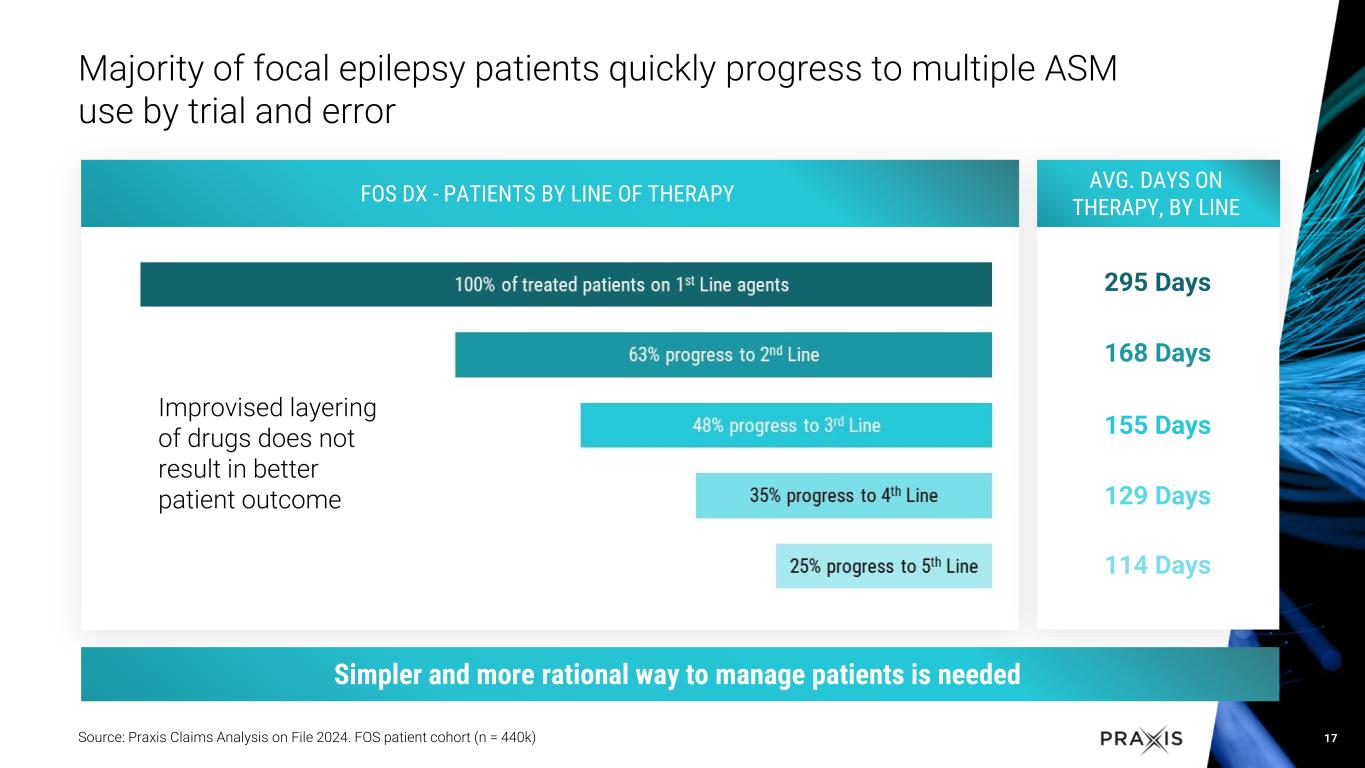
RADIANT study with vormatrigine in focal onset seizure (FOS) patients over eight weeks demonstrated 56.3% median reduction in seizure frequency from baseline, with 22% at 100% seizure reduction in last 28 days
Initiated two registrational studies for Developmental and Epileptic Encephalopathies (DEEs) programs: EMERALD for broad DEEs with relutrigine and EMBRAVE3 for SCN2A Gain-of-Function (GoF) with elsunersen
Relutrigine granted U.S. FDA Breakthrough Therapy Designation for the treatment of seizures associated with SCN2A and SCN8A DEEs, enabling expedited development
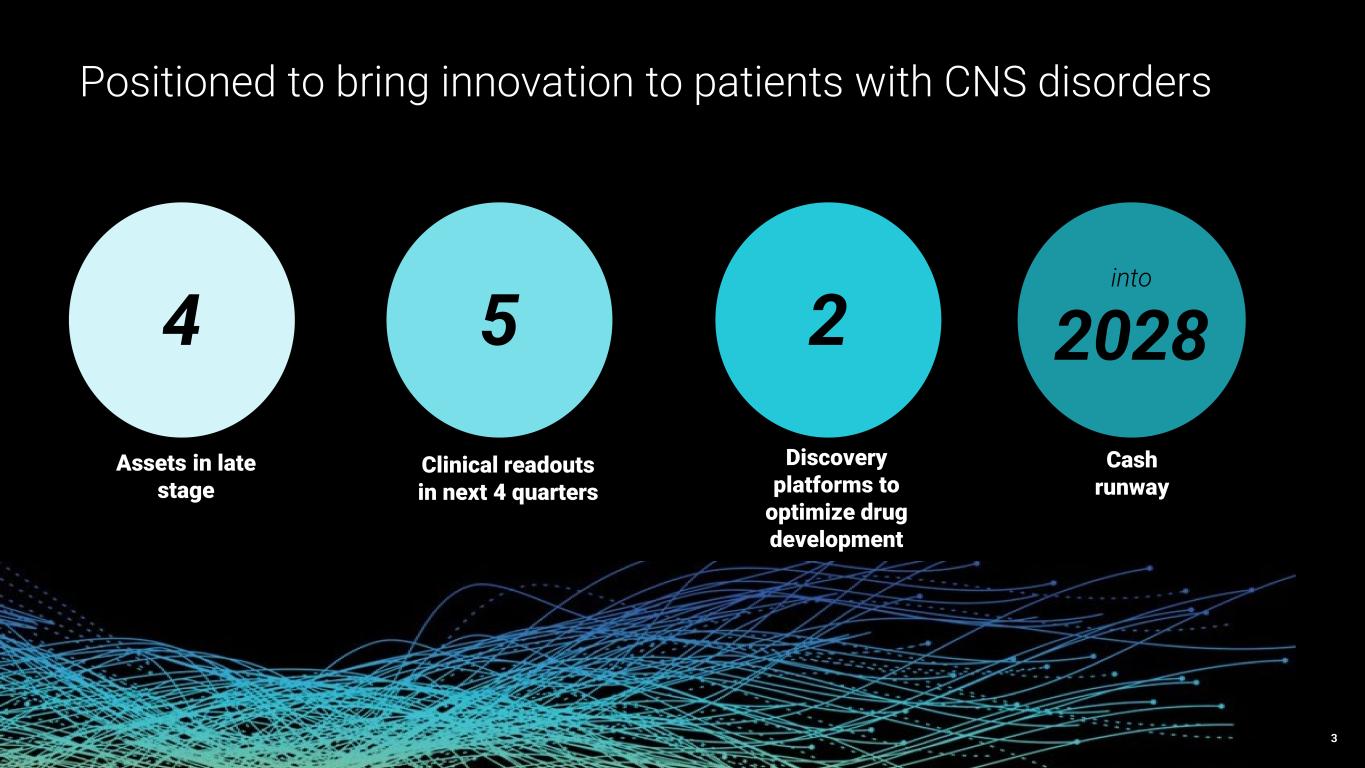
Cash and investments of approximately $447 million as of June 30, 2025 maintains runway into 2028
Praxis to host a conference call today, August 4, at 8:30am to discuss Phase 2 RADIANT study results and provide a corporate update
BOSTON, August 4, 2025 — Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies for central nervous system (CNS) disorders characterized by neuronal excitation-inhibition imbalance, today provided a corporate update and reported financial results for the second quarter 2025.
“In the second quarter, we continued to make remarkable progress across our portfolio and believe we are positioned to revolutionize treatment in both common and rare epilepsy. Earlier today we reported the positive results from the RADIANT study, where vormatrigine has shown an impressive 56.3% reduction in seizures in 8 weeks in a heavily pre-treated population. Enrollment is going well for the POWER1 pivotal study for vormatrigine, and we plan to shortly initiate POWER2 as well as the POWER3 study investigating vormatrigine as a standalone agent. Our relutrigine program continues to progress strongly, first with the breakthrough therapy designation for SCN2A and SCN8A DEEs, which we expect will allow us to expedite the regulatory process, and second with the initiation of the EMERALD registrational study in broad DEEs. Completing our progress across epilepsy, we also initiated the registrational EMBRAVE3 study of elsunersen in SCN2A patients. We remain well-capitalized as we look ahead to an exciting second half of 2025,” said Marcio Souza, president and chief executive officer of Praxis.
Recent Highlights and Anticipated Milestones
Cerebrum™ Small Molecule Platform
•Vormatrigine for FOS and Generalized Epilepsy: Vormatrigine is the most potent sodium-channel modulator ever designed to precisely target the hyperexcitable state of sodium-channels in adult common epilepsies
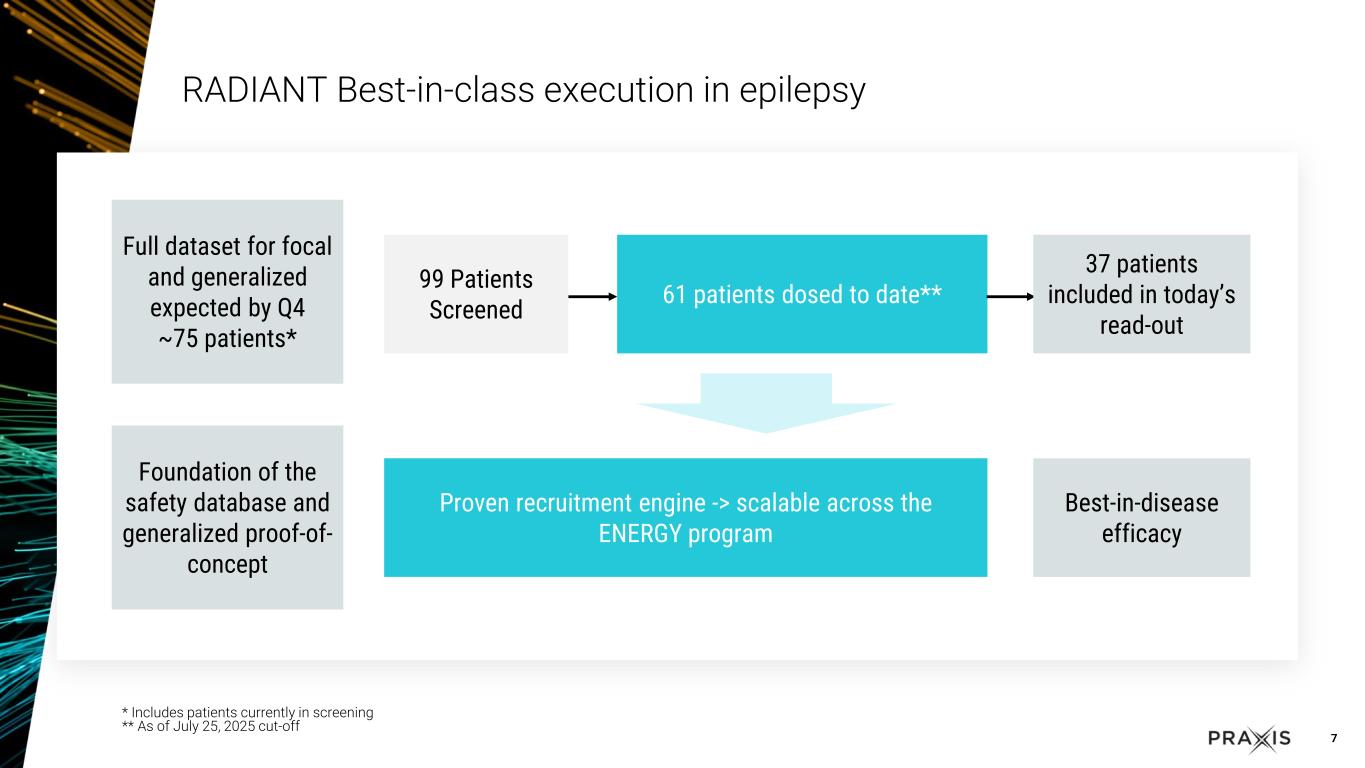
oEarlier today Praxis shared positive results of the RADIANT study evaluating patients with FOS
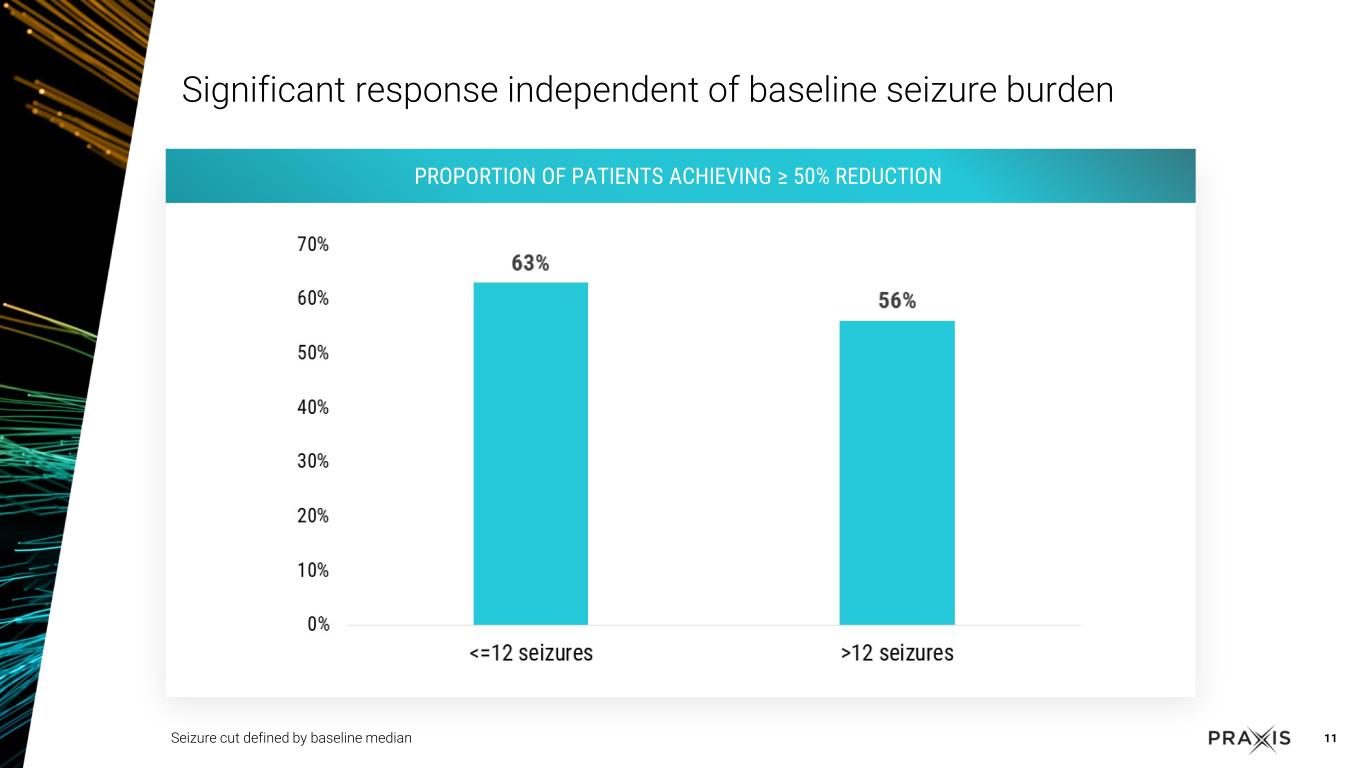
•Dosing with vormatrigine over 8 weeks led to 56.3% median reduction in seizure frequency
•Approximately 22% of patients reached 100% reduction in seizure frequency in the last 28 days
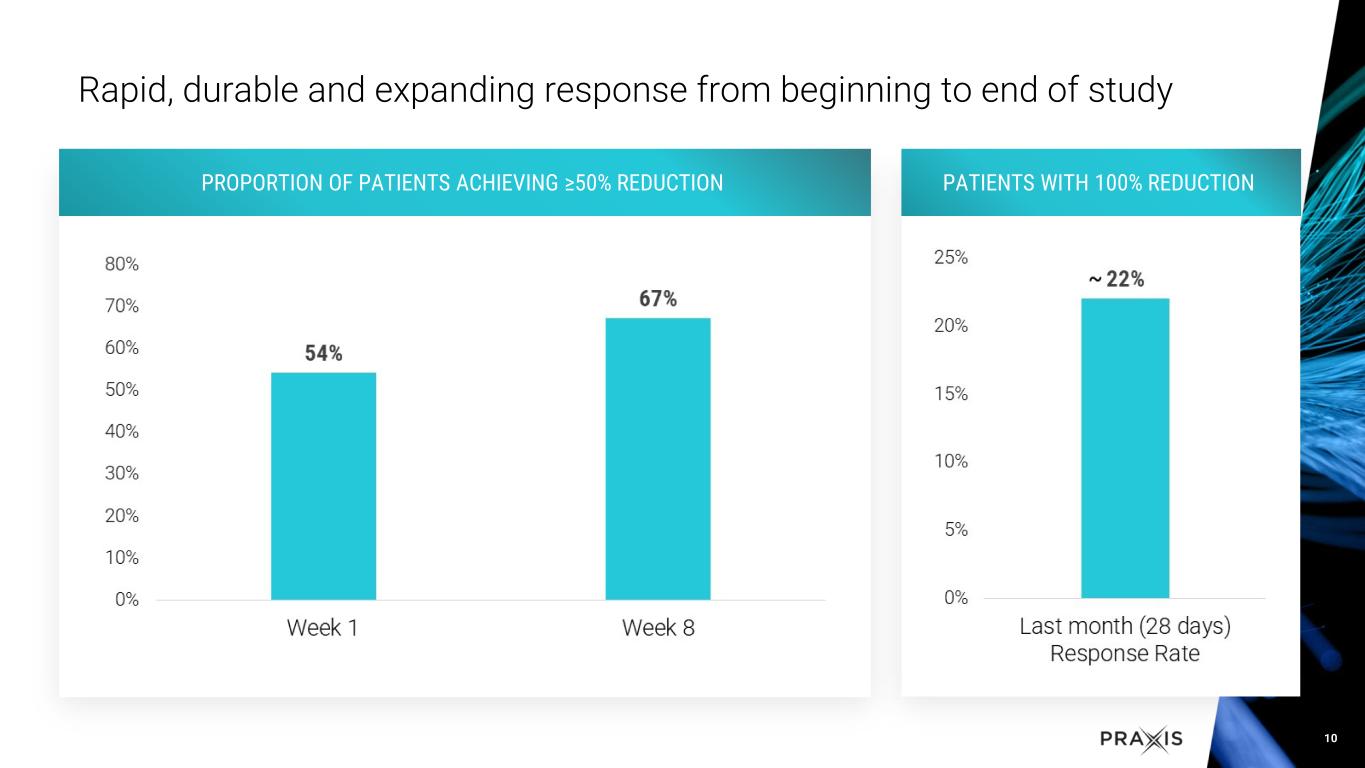
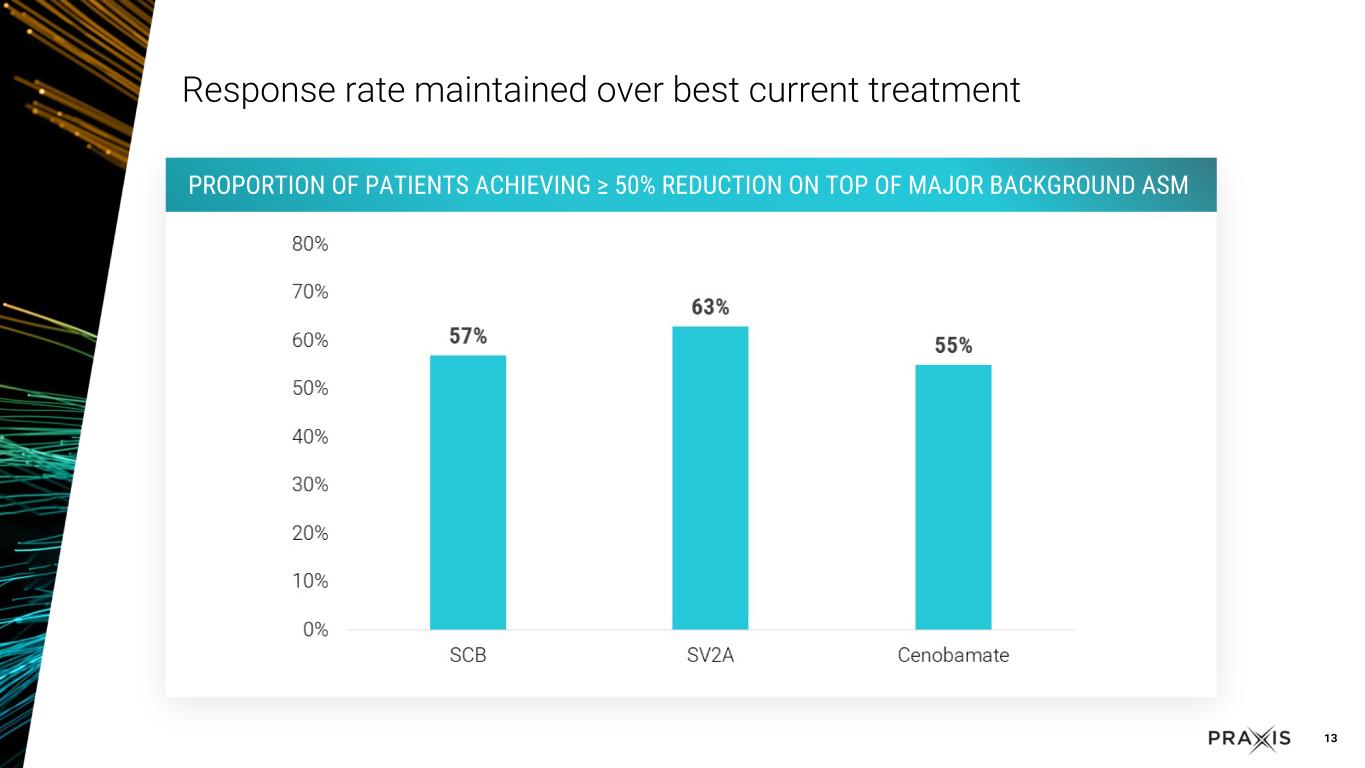
•Rapid and sustained response, with approximately 60% of patients achieving 50% response in the study
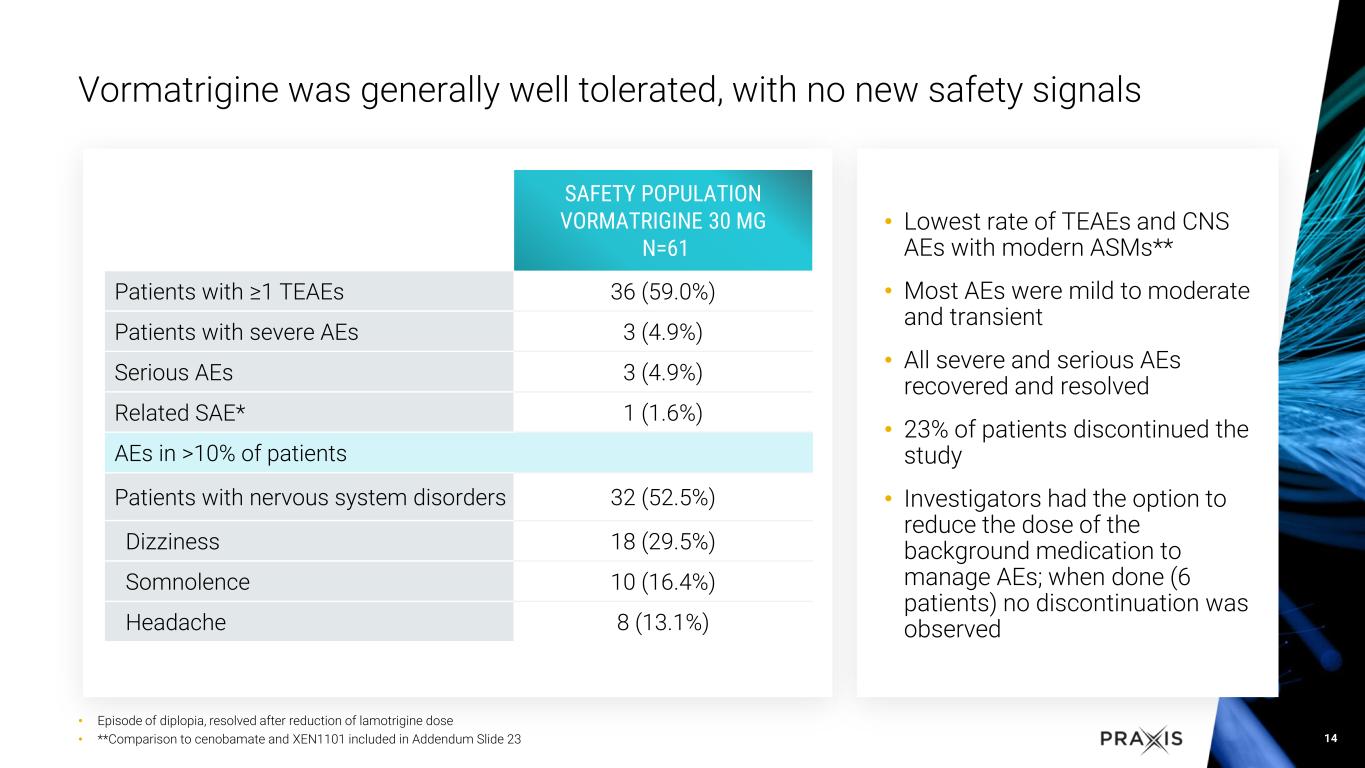
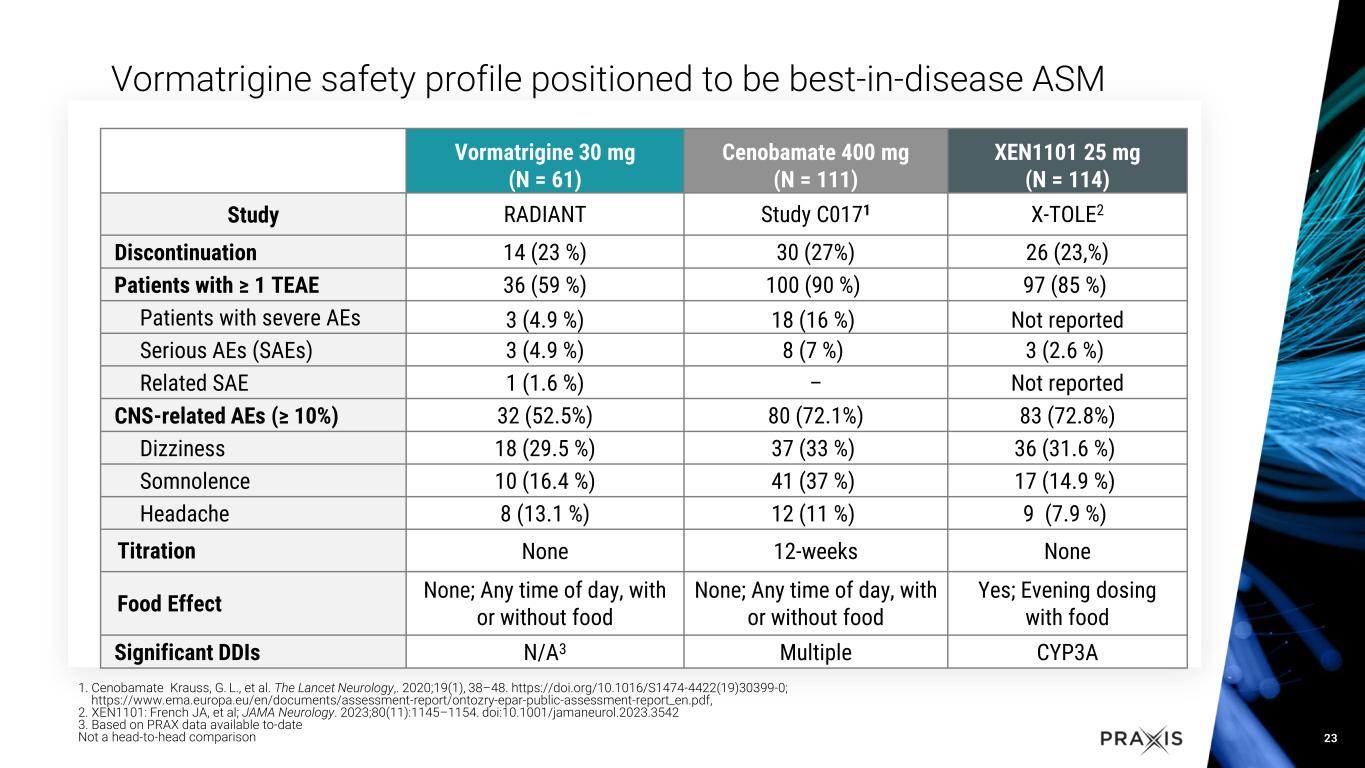
•Vormatrigine was generally well tolerated and continues to demonstrate a favorable safety profile
•Additional data are expected to be presented at the 36th International Epilepsy Congress on August 31, 2025, in Lisbon, Portugal. Praxis has also submitted a late-breaker abstract to present the full study results at the American Epilepsy Society Annual Meeting in December 2025 in Atlanta, Georgia
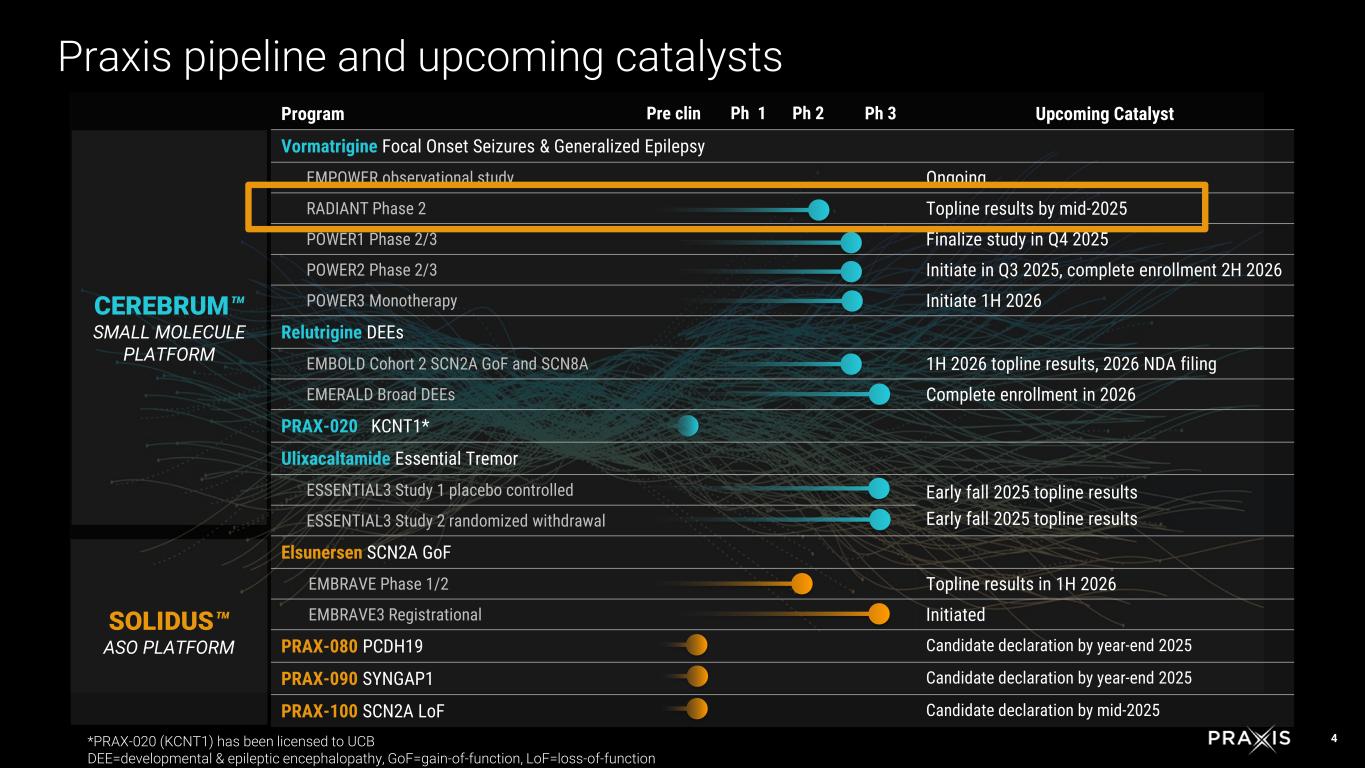
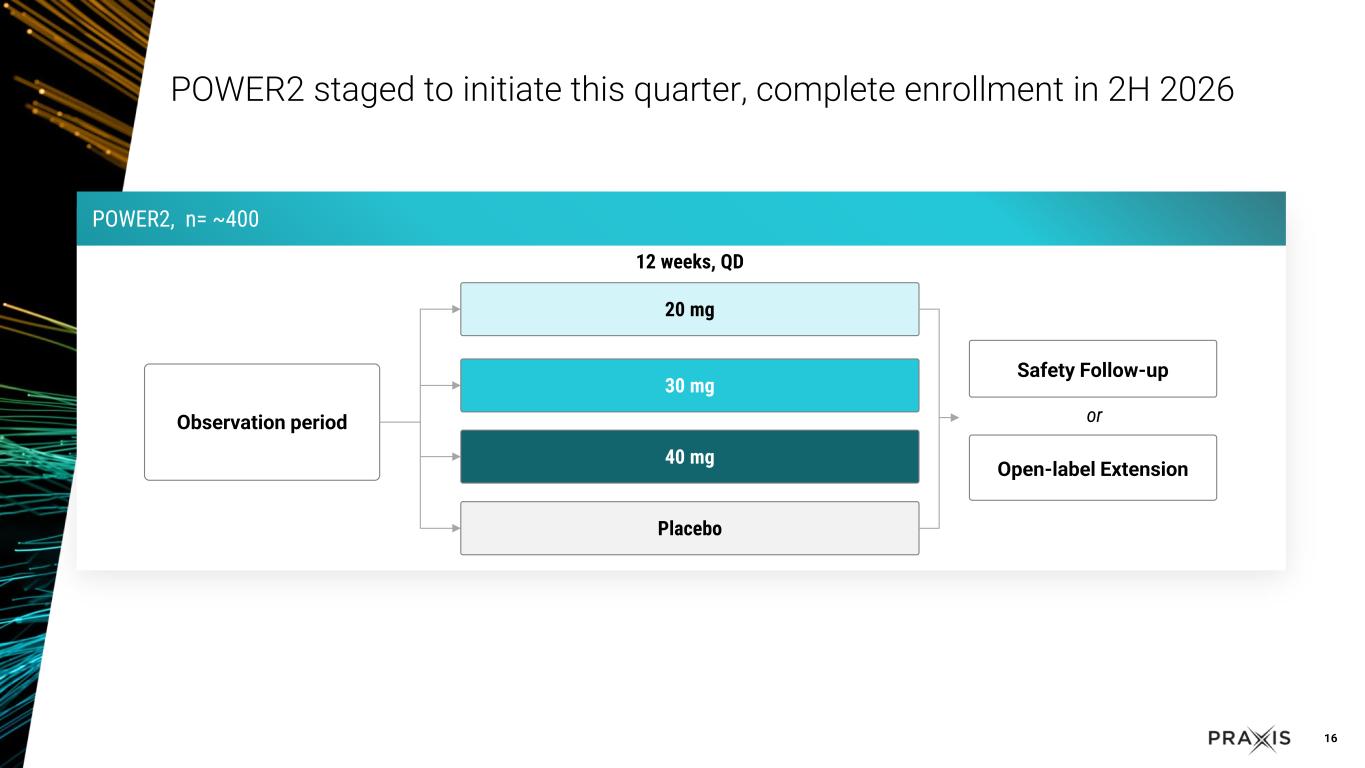
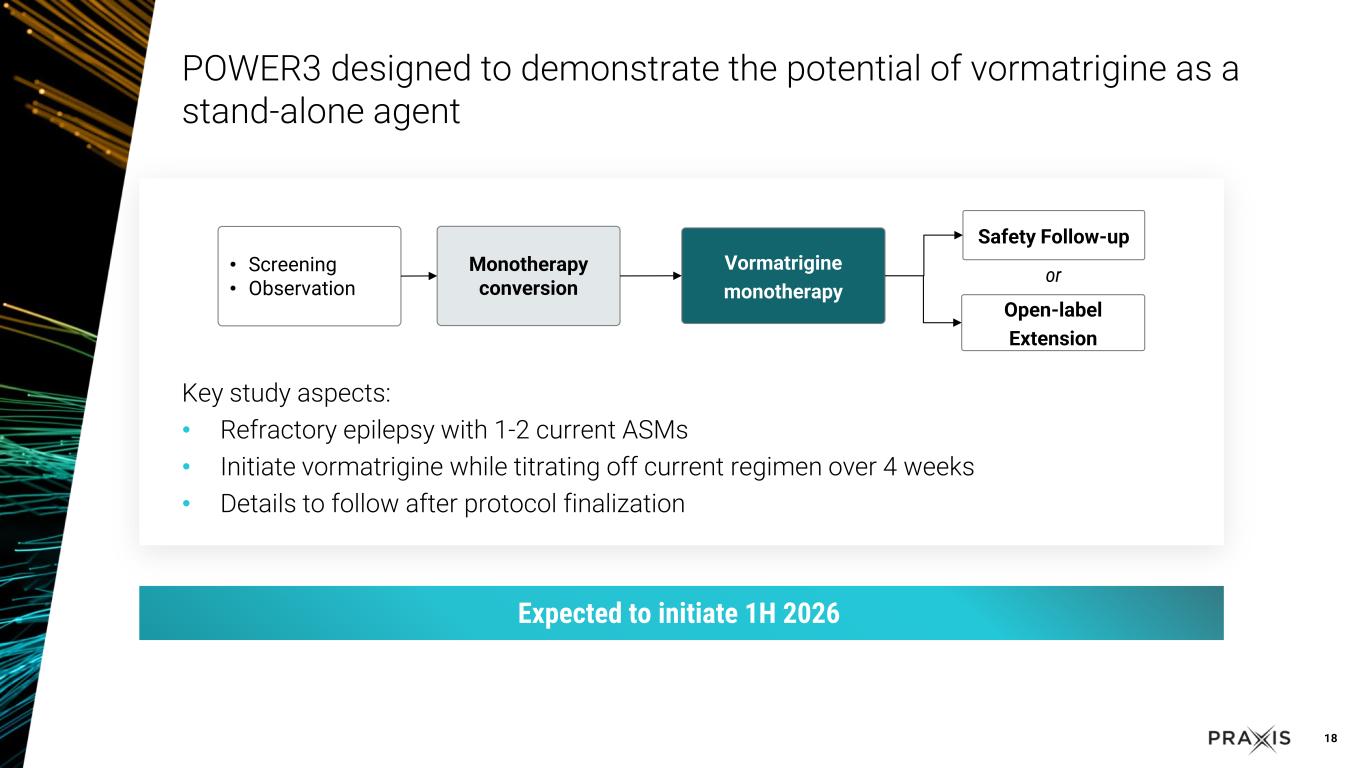
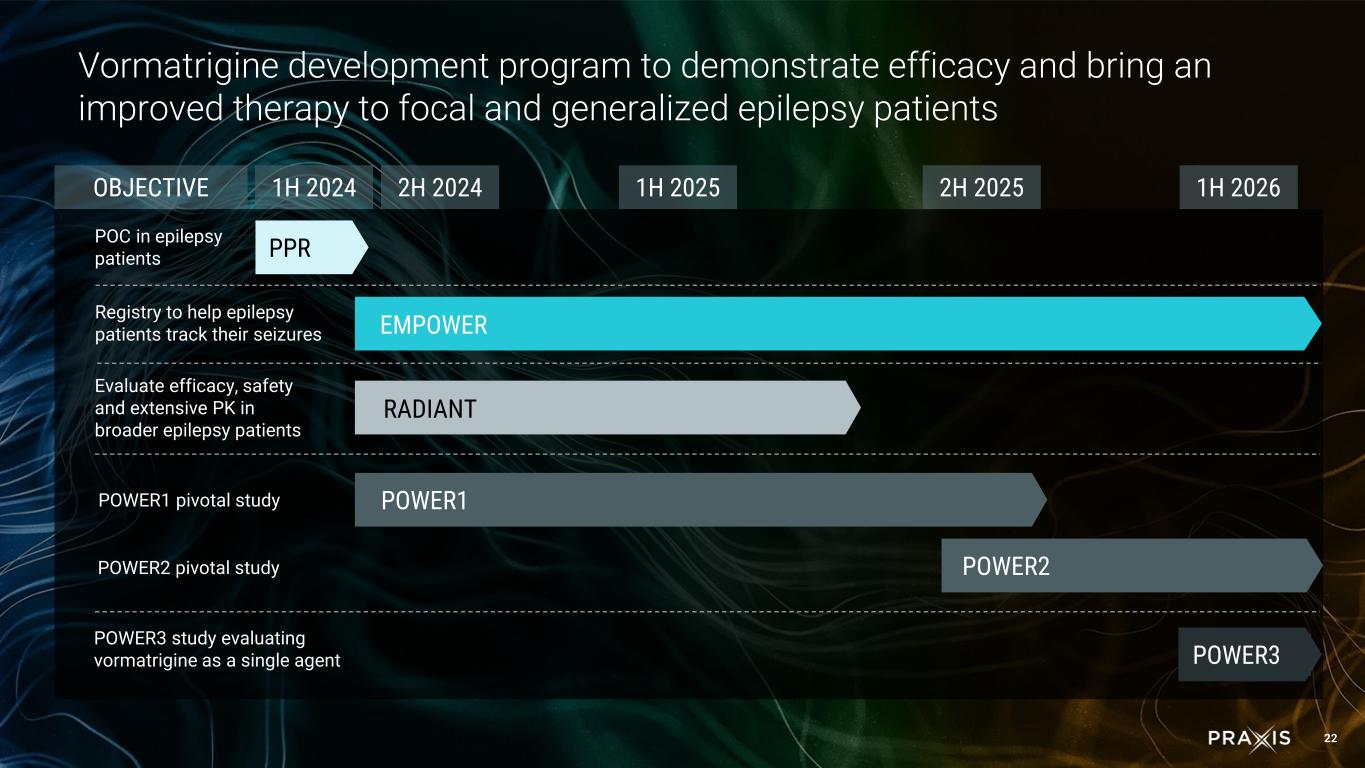
oThe POWER1 Phase 2/3 registrational study for FOS is enrolling well, and is on track to complete in the fourth quarter of 2025 oThe POWER2 Phase 2/3 registrational study for FOS trial design is completed based on data from RADIANT, and is expected to initiate in the third quarter of 2025 and complete enrollment in the second half of 2026
oThe POWER3 study to evaluate vormatrigine as a standalone agent is expected to initiate in the first half of 2026
oThe overall ENERGY program recruitment initiative, including the EMPOWER study, has attracted approximately 20,000 patients with epilepsy and continues to support patient identification for the epilepsy studies
•Relutrigine for DEEs: Relutrigine is Praxis’ second sodium channel modulator designed to precisely target the hyperexcitable state of sodium-channels, with therapeutic potential across developmental epilepsies.
oIn July 2025, relutrigine received FDA Breakthrough Therapy Designation (BTD) for the treatment of seizures associated with SCN2A and SCN8A DEEs, potentially expediting its development.
oIn May 2025, Praxis provided an update on the EMBOLD cohort 1 study group in SCN2A and SCN8A DEEs, showing patients in the open-label extension up to 11 months had a ~90% reduction in seizures from their baseline, and the mean period between seizures increased from three days at baseline to 67 days at month 11.
oThe EMBOLD registrational cohort 2 in SCN2A and SCN8A DEEs continues to enroll well, with topline results expected no later than the first half of 2026, followed by a potential NDA submission.
oThe EMERALD registrational study in broad DEEs has been initiated and is planned to include up to 160 patients, with enrollment expected to complete in 2026.
•Ulixacaltamide for Essential Tremor (ET): Essential Tremor is the largest movement disorder affecting approximately seven million people in the U.S. During the recruitment phase of the trial, started in November 2023, over 200,000 patients demonstrated interest in participating in the study.
oPraxis has completed enrollment of both Phase 3 studies in the Essential 3 program, with topline results expected in early fall of 2025.
oAfter reviewing the results of both studies, Praxis will determine if there is sufficient evidence to support an NDA submission.
Solidus™ Antisense Oligonucleotide (ASO) Platform
•Elsunersen for early-seizure-onset SCN2A DEE: SCN2A GoF-DEE is a rare, genetic epilepsy characterized by early-onset seizures and severe impact on development. Elsunersen is currently being evaluated in two registrational studies:
oThe EMBRAVE Part A Phase 1/2 study is continuing to enroll up to 16 patients. Patients are randomized 3:1 drug to sham for a six-month period on a once-monthly dose, with the potential to escalate from 1 mg to 8 mg. Topline results are expected in the first half of 2026.
oThe EMBRAVE3 registrational study for SCN2A GoF-DEE has been initiated. Cohort 1, aiming to enroll up to 40 patients ages 2 to 18 years, has started enrolling patients. In cohort 1, patients will be randomized 1:1 to receive 1 mg of elsunersen or sham once monthly for a six-month period. The primary endpoint assesses median percent change in seizure frequency from baseline. Cohorts 2 and 3 will evaluate patients from ages 1 to 2 and 0 to 1 years, respectively.
•Praxis remains on track to nominate a development candidate for each of its early stage ASO therapeutic initiatives in 2025:
oPRAX-080: Focused on targeting PCDH19 mosaic expression disorder, nominated by end of year.
oPRAX-090: Designed to address SYNGAP1 loss-of-function (LoF) mutations, a leading cause of severe intellectual disability and epilepsy in DEEs, nominated by end of year.
oPRAX-100: Targeting SCN2A LoF mutations, the predominant genetic link to de novo autism spectrum disorders, nominated mid-year.
Second Quarter 2025 Financial Results:
As of June 30, 2025, Praxis had $446.6 million in cash, cash equivalents and marketable securities, compared to $469.5 million in cash, cash equivalents and marketable securities as of December 31, 2024. The decrease of $22.9 million is primarily attributable to cash used in operating activities partially offset by net proceeds from at-the-market offerings of common stock. The Company’s cash, cash equivalents and marketable securities as of June 30, 2025 are expected to fund operations into 2028.
Praxis did not recognize any collaboration revenue during the three months ended June 30, 2025, compared to $0.4 million during the three months ended June 30, 2024. The decrease of $0.4 million is related to its Option and License Agreement with UCB. In December 2024, UCB exercised its option to in-license global development and commercialization rights for a KCNT1 small molecule development candidate, and as such, Praxis has no further research service obligations under the terms of the Option and License Agreement.
Research and development expenses were $63.0 million for the three months ended June 30, 2025, compared to $27.3 million for the three months ended June 30, 2024. The increase in research and development expenses of $35.7 million was primarily attributable to an increase of $32.0 million in Praxis’ Cerebrum™ platform, an increase of $3.0 million in personnel-related costs, an increase of $0.4 million in indirect expenses and an increase of $0.3 million in Praxis’ Solidus™ platform.
General and administrative expenses were $13.1 million for the three months ended June 30, 2025, compared to $10.6 million for the three months ended June 30, 2024. The increase in general and administrative expenses of approximately $2.5 million was primarily attributable to an increase of $1.3 million in indirect expenses, and an increase of $0.8 million in personnel-related costs.
Praxis reported a net loss of $71.1 million for the three months ended June 30, 2025, including $7.8 million of stock-based compensation expense, compared to $32.7 million for the three months ended June 30, 2024, including $5.9 million of stock-based compensation.
As of June 30, 2025, Praxis had 21.0 million shares of common stock outstanding.
Conference Call
Praxis will discuss the study results, as well as its second quarter 2025 financial results and business highlights on a conference call taking place today, August 4 at 8:30 am ET. Individuals may register for the conference call by clicking the registration link. Once registered, participants will receive dial-in details and a unique PIN which will allow them to access the call. An audio webcast will be accessible through the Events & Presentation page under the Investor Relations section of the Company’s website.
About Vormatrigine (PRAX-628)
Vormatrigine is a next-generation, functionally selective small molecule targeting the hyperexcitable state of sodium-channels in the brain that is currently being developed as a once daily, oral treatment for adult focal onset seizures and generalized epilepsy. Preclinical data demonstrates vormatrigine is differentiated from standard of care, with the potential to be best-in-class for focal epilepsy. In vitro, vormatrigine has demonstrated superior selectivity for disease-state NaV channel hyperexcitability. In vivo studies of vormatrigine have demonstrated unprecedented potency in the maximal electroshock seizure (MES) model, a highly predictive translational model for efficacy in focal epilepsy. Data from the first cohort of patients in the RADIANT study demonstrated a robust seizure reduction and generally safe and well tolerated profile. To learn more about the POWER1 study, please visit POWER1 study.
About Relutrigine (PRAX-562)
Relutrigine is a first-in-class small molecule in development for the treatment of developmental and epileptic encephalopathies (DEEs) as a preferential inhibitor of persistent sodium current, shown to be a key driver of seizure symptoms in severe DEEs. Relutrigine’s mechanism of precision sodium channel (NaV) modulation is consistent with superior selectivity for disease-state NaV channel hyperexcitability. In vivo studies of relutrigine have demonstrated dose-dependent inhibition of seizures up to complete control of seizure activity in SCN2A, SCN8A and other DEE mouse models. Relutrigine has been generally well-tolerated in three Phase 1 studies and has demonstrated biomarker changes indicative of NaV channel modulation. Data from cohort 1 of the Phase 2 EMBOLD study demonstrated a well-tolerated, robust, short- and long-term improvement in motor seizures in a heavily pre-treated population, alongside maintained seizure freedom in some patients with SCN2A- and SCN8A-DEE.
Relutrigine has received Orphan Drug Designation (ODD) and Rare Pediatric Disease Designation from the FDA for the treatment of SCN2A-DEE, SCN8A-DEE and Dravet syndrome; as well as Breakthrough Therapy Designation (BTD), and ODD from the European Medicines Agency for the treatment of SCN2A-DEE and SCN8A-DEE. To learn more about the EMERALD and EMBOLD studies, please visit ResilienceStudies.com.
About Ulixacaltamide
Ulixacaltamide is a differentiated and highly selective small molecule inhibitor of T-type calcium channels designed to block abnormal neuronal burst firing in the Cerebello-Thalamo-Cortical (CTC) circuit correlated with tremor activity. Ulixacaltamide, the most advanced program within Praxis’ Cerebrum™ small molecule platform, is currently in late-stage development for the treatment of essential tremor.
About Elsunersen (PRAX-222)
Elsunersen is an antisense oligonucleotide (ASO) designed to selectively decrease SCN2A gene expression, directly targeting the underlying cause of early-seizure-onset SCN2A-DEE to treat seizures and other symptoms in patients with gain-of-function SCN2A mutations. In vitro studies of elsunersen have demonstrated reduction in both SCN2A gene expression and protein levels. In vivo, elsunersen has demonstrated significant, dose-dependent reduction in seizures, improvement in behavioral and locomotor activity and increased survival in SCN2A mouse models, with potential to be the first disease-modifying treatment for SCN2A-DEE. Elsunersen has received ODD and RPDD from the FDA, and ODD and PRIME designations from the European Medicines Agency for the treatment of SCN2A-DEE. The elsunersen program is ongoing under a collaboration with Ionis Pharmaceuticals, Inc., and RogCon, Inc. To learn more about the EMBRAVE study, please visit https://www.embravestudy.com/.
About Praxis
Praxis Precision Medicines is a clinical-stage biopharmaceutical company translating insights from genetic epilepsies into the development of therapies for CNS disorders characterized by neuronal excitation-inhibition imbalance. Praxis is applying genetic insights to the discovery and development of therapies for rare and more prevalent neurological disorders through our proprietary small molecule platform, Cerebrum™, and antisense oligonucleotide (ASO) platform, Solidus™, using our understanding of shared biological targets and circuits in the brain. Praxis has established a diversified, multimodal CNS portfolio including multiple programs across epilepsy and movement disorders, with four clinical-stage product candidates. For more information, please visit www.praxismedicines.com and follow us on Facebook, LinkedIn and Twitter/X.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws, including express or implied statements regarding Praxis’ future expectations, plans and prospects, including, without limitation, statements regarding the anticipated timing of our clinical trials, the development of our product candidates and plans to initiate new clinical programs, the anticipated timing of regulatory submissions and interactions and our projected cash runway, as well as other statements containing the words “anticipate,” “believe,” “continue,” “could,” “endeavor,” “estimate,” “expect,” “anticipate,” “intend,” “may,” “might,” “plan,” “potential,” “predict,” “project,” “seek,” “should,” “target,” “will” or “would” and similar expressions that constitute forward-looking statements under the Private Securities Litigation Reform Act of 1995.
The express or implied forward-looking statements included in this press release are only predictions and are subject to a number of risks, uncertainties and assumptions, including, without limitation: uncertainties inherent in clinical trials; preliminary analyses from ongoing studies differing materially from final data from preclinical studies and completed clinical trials; the expected timing of clinical trials, data readouts and the results thereof, and submissions for regulatory approval or review by governmental authorities; regulatory approvals to conduct trials; and other risks concerning Praxis’ programs and operations as described in its Annual Report on Form 10-K for the year ended December 31, 2024 and as updated in the Quarterly Report on Form 10-Q for the period ended June 30, 2025, as well as other filings made with the Securities and Exchange Commission. Although Praxis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on information and factors currently known by Praxis. As a result, you are cautioned not to rely on these forward-looking statements.
Any forward-looking statement made in this press release speaks only as of the date on which it is made. Praxis undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise.
Investor Contact:
Praxis Precision Medicines
investors@praxismedicines.com
857-702-9452
Media Contact:
Dan Ferry
Life Science Advisors
Daniel@lifesciadvisors.com
617-430-7576
PRAXIS PRECISION MEDICINES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30, 2025 |
|
December 31, 2024 |
| Assets |
|
|
|
| Cash and cash equivalents |
$ |
157,415 |
|
|
$ |
215,372 |
|
| Marketable securities |
289,229 |
|
|
254,156 |
|
| Prepaid expenses and other current assets |
5,304 |
|
|
11,805 |
|
| Property and equipment, net |
258 |
|
|
230 |
|
| Operating lease right-of-use assets |
626 |
|
|
1,131 |
|
| Other non-current assets |
— |
|
|
416 |
|
| Total assets |
$ |
452,832 |
|
|
$ |
483,110 |
|
| Liabilities and stockholders’ equity |
|
|
|
| Accounts payable |
$ |
28,832 |
|
|
$ |
12,528 |
|
| Accrued expenses |
19,021 |
|
|
23,763 |
|
| Operating lease liabilities |
755 |
|
|
1,369 |
|
| Common stock |
14 |
|
|
14 |
|
| Additional paid-in capital |
1,380,978 |
|
|
1,281,522 |
|
| Accumulated other comprehensive gain |
395 |
|
|
654 |
|
| Accumulated deficit |
(977,163) |
|
|
(836,740) |
|
| Total liabilities and stockholders' equity |
$ |
452,832 |
|
|
$ |
483,110 |
|
PRAXIS PRECISION MEDICINES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share amounts)
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Three Months Ended
June 30,
|
|
Six Months Ended
June 30,
|
|
2025 |
|
2024 |
|
2025 |
|
2024 |
| Collaboration revenue |
$ |
— |
|
|
$ |
357 |
|
|
$ |
— |
|
|
$ |
788 |
|
| Operating expenses: |
|
|
|
|
|
|
|
| Research and development |
63,006 |
|
|
27,260 |
|
|
123,812 |
|
|
54,244 |
|
| General and administrative |
13,061 |
|
|
10,585 |
|
|
26,983 |
|
|
25,918 |
|
| Total operating expenses |
76,067 |
|
|
37,845 |
|
|
150,795 |
|
|
80,162 |
|
| Loss from operations |
(76,067) |
|
|
(37,488) |
|
|
(150,795) |
|
|
(79,374) |
|
| Other income: |
|
|
|
|
|
|
|
| Other income, net |
4,940 |
|
|
4,811 |
|
|
10,372 |
|
|
7,144 |
|
| Total other income |
4,940 |
|
|
4,811 |
|
|
10,372 |
|
|
7,144 |
|
| Net loss |
$ |
(71,127) |
|
|
$ |
(32,677) |
|
|
$ |
(140,423) |
|
|
$ |
(72,230) |
|
| Net loss per share attributable to common stockholders, basic and diluted |
$ |
(3.31) |
|
|
$ |
(1.74) |
|
|
$ |
(6.60) |
|
|
$ |
(4.41) |
|
| Weighted average common shares outstanding, basic and diluted |
21,474,827 |
|
18,824,479 |
|
21,266,490 |
|
16,364,421 |