0001836564FALSE2024FYP3YP1YP2Yxbrli:sharesiso4217:EURxbrli:pureiso4217:EURxbrli:sharesvaln:vaccinevaln:employeeiso4217:USDvaln:performance_obligationvaln:customervaln:grantiso4217:GBPvaln:planvaln:trancheutr:Yvaln:member00018365642024-01-012024-12-310001836564dei:BusinessContactMember2024-01-012024-12-310001836564valn:AmericanDepositorySharesMember2024-01-012024-12-310001836564ifrs-full:OrdinarySharesMember2024-01-012024-12-3100018365642024-12-310001836564valn:PricewaterhouseCoopersMember2024-01-012024-12-310001836564valn:PricewaterhouseCoopersMember2023-01-012023-12-310001836564valn:PricewaterhouseCoopersMember2022-01-012022-12-310001836564valn:DeloitteAssociesMember2024-01-012024-12-310001836564valn:DeloitteAssociesMember2023-01-012023-12-310001836564valn:DeloitteAssociesMember2022-01-012022-12-310001836564valn:StatutoryAuditorMembervaln:PricewaterhouseCoopersMember2024-01-012024-12-310001836564valn:StatutoryAuditorMembervaln:PricewaterhouseCoopersMember2023-01-012023-12-310001836564valn:StatutoryAuditorMembervaln:PricewaterhouseCoopersMember2022-01-012022-12-310001836564valn:StatutoryAuditorMembervaln:DeloitteAssociesMember2024-01-012024-12-310001836564valn:StatutoryAuditorMembervaln:DeloitteAssociesMember2023-01-012023-12-310001836564valn:StatutoryAuditorMembervaln:DeloitteAssociesMember2022-01-012022-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:PricewaterhouseCoopersMember2024-01-012024-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:PricewaterhouseCoopersMember2023-01-012023-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:PricewaterhouseCoopersMember2022-01-012022-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:DeloitteAssociesMember2024-01-012024-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:DeloitteAssociesMember2023-01-012023-12-310001836564valn:StatutoryAuditorsNetworkMembervaln:DeloitteAssociesMember2022-01-012022-12-3100018365642023-01-012023-12-3100018365642022-01-012022-12-3100018365642023-12-3100018365642022-12-3100018365642021-12-310001836564ifrs-full:IssuedCapitalMember2023-12-310001836564ifrs-full:SharePremiumMember2023-12-310001836564ifrs-full:OtherReservesMember2023-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2023-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2023-12-310001836564ifrs-full:OtherReservesMember2024-01-012024-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2024-01-012024-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2024-01-012024-12-310001836564ifrs-full:IssuedCapitalMember2024-01-012024-12-310001836564ifrs-full:SharePremiumMember2024-01-012024-12-310001836564ifrs-full:IssuedCapitalMember2024-12-310001836564ifrs-full:SharePremiumMember2024-12-310001836564ifrs-full:OtherReservesMember2024-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2024-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2024-12-310001836564ifrs-full:IssuedCapitalMember2022-12-310001836564ifrs-full:SharePremiumMember2022-12-310001836564ifrs-full:OtherReservesMember2022-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2022-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2022-12-310001836564ifrs-full:OtherReservesMember2023-01-012023-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2023-01-012023-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2023-01-012023-12-310001836564ifrs-full:IssuedCapitalMember2023-01-012023-12-310001836564ifrs-full:SharePremiumMember2023-01-012023-12-310001836564ifrs-full:IssuedCapitalMember2021-12-310001836564ifrs-full:SharePremiumMember2021-12-310001836564ifrs-full:OtherReservesMember2021-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2021-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2021-12-310001836564ifrs-full:OtherReservesMember2022-01-012022-12-310001836564ifrs-full:RetainedEarningsProfitLossForReportingPeriodMember2022-01-012022-12-310001836564ifrs-full:RetainedEarningsExcludingProfitLossForReportingPeriodMember2022-01-012022-12-310001836564ifrs-full:IssuedCapitalMember2022-01-012022-12-310001836564ifrs-full:SharePremiumMember2022-01-012022-12-3100018365642024-02-022024-02-020001836564valn:DebtFinancingAgreementDue2027Member2024-03-182024-03-180001836564ifrs-full:GrossCarryingAmountMembervaln:AmendedDebtFinancingAgreementDueThirdQuarter2028Member2024-12-310001836564valn:StrategicPartnershipAgreementMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2024-07-222024-07-220001836564valn:StrategicPartnershipAgreementMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2019-07-252019-07-250001836564valn:StrategicPartnershipAgreementMembervaln:LimmaTechBiologicsAGMembervaln:ShigellaVaccineMember2024-08-012024-08-010001836564valn:StrategicPartnershipAgreementMembervaln:LimmaTechBiologicsAGMembervaln:ShigellaVaccineMembervaln:IntellectualProperty1Member2024-08-012024-08-010001836564valn:StrategicPartnershipAgreementMembervaln:LimmaTechBiologicsAGMembervaln:ShigellaVaccineMembersrt:ScenarioForecastMember2025-01-012025-12-310001836564valn:PrivatePlacement1Member2024-09-132024-09-130001836564valn:PrivatePlacement1Memberifrs-full:OrdinarySharesMember2024-09-1300018365642024-09-130001836564valn:PrivatePlacement1Membervaln:PriorToPrivatePlacementMember2024-09-130001836564valn:PrivatePlacement1Membervaln:SubsequentToPrivatePlacementMember2024-09-130001836564valn:VaccinesHoldingsSwedenABMember2024-12-312024-12-310001836564valn:VaccinesHoldingsSwedenABMember2023-12-312023-12-310001836564valn:ValnevaAustriaGmbHMember2024-12-312024-12-310001836564valn:ValnevaAustriaGmbHMember2023-12-312023-12-310001836564valn:ValnevaCanadaIncMember2024-12-312024-12-310001836564valn:ValnevaCanadaIncMember2023-12-312023-12-310001836564valn:ValnevaFranceSASMember2024-12-312024-12-310001836564valn:ValnevaFranceSASMember2023-12-312023-12-310001836564valn:ValnevaScotlandLtdMember2024-12-312024-12-310001836564valn:ValnevaScotlandLtdMember2023-12-312023-12-310001836564valn:ValnevaSwedenABMember2024-12-312024-12-310001836564valn:ValnevaSwedenABMember2023-12-312023-12-310001836564valn:ValnevaUKLtdMember2024-12-312024-12-310001836564valn:ValnevaUKLtdMember2023-12-312023-12-310001836564valn:ValnevaUSAIncMember2024-12-312024-12-310001836564valn:ValnevaUSAIncMember2023-12-312023-12-310001836564valn:VBC3ErrichtungsGmbHMember2024-12-312024-12-310001836564valn:VBC3ErrichtungsGmbHMember2023-12-312023-12-310001836564valn:VBC3ErrichtungsGmbHMember2023-10-010001836564valn:VBC3ErrichtungsGmbHMembervaln:ValnevaSEMember2023-10-010001836564valn:VBC3ErrichtungsGmbHMembervaln:ValnevaAustriaGmbHMember2023-10-010001836564valn:VBC3ErrichtungsGmbHMember2023-10-310001836564ifrs-full:NotLaterThanOneYearMemberifrs-full:LiquidityRiskMember2024-12-310001836564ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMemberifrs-full:LiquidityRiskMember2024-12-310001836564ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMemberifrs-full:LiquidityRiskMember2024-12-310001836564ifrs-full:LaterThanFiveYearsMemberifrs-full:LiquidityRiskMember2024-12-310001836564ifrs-full:LiquidityRiskMember2024-12-310001836564ifrs-full:NotLaterThanOneYearMemberifrs-full:LiquidityRiskMember2023-12-310001836564ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMemberifrs-full:LiquidityRiskMember2023-12-310001836564ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMemberifrs-full:LiquidityRiskMember2023-12-310001836564ifrs-full:LaterThanFiveYearsMemberifrs-full:LiquidityRiskMember2023-12-310001836564ifrs-full:LiquidityRiskMember2023-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2022-01-012022-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2024-01-012024-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2023-01-012023-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2024-12-312024-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Memberifrs-full:BottomOfRangeMember2024-12-312024-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Memberifrs-full:TopOfRangeMember2024-12-312024-12-310001836564valn:IXIAROMember2024-01-012024-12-310001836564valn:IXIAROMember2023-01-012023-12-310001836564valn:IXIAROMember2022-01-012022-12-310001836564valn:DUKORALMember2024-01-012024-12-310001836564valn:DUKORALMember2023-01-012023-12-310001836564valn:DUKORALMember2022-01-012022-12-310001836564valn:ThirdPartyProductsMember2024-01-012024-12-310001836564valn:ThirdPartyProductsMember2023-01-012023-12-310001836564valn:ThirdPartyProductsMember2022-01-012022-12-310001836564valn:IXCHIQChikungunyaVLA1553Member2024-01-012024-12-310001836564valn:IXCHIQChikungunyaVLA1553Member2023-01-012023-12-310001836564valn:IXCHIQChikungunyaVLA1553Member2022-01-012022-12-310001836564valn:CovidVLA2001ProductMember2024-01-012024-12-310001836564valn:CovidVLA2001ProductMember2023-01-012023-12-310001836564valn:CovidVLA2001ProductMember2022-01-012022-12-310001836564valn:RoyaltiesReceivedMember2024-01-012024-12-310001836564valn:RoyaltiesReceivedMember2023-01-012023-12-310001836564valn:RoyaltiesReceivedMember2022-01-012022-12-310001836564valn:RevenuesFromShippingAndHandlingMember2024-01-012024-12-310001836564valn:RevenuesFromShippingAndHandlingMember2023-01-012023-12-310001836564valn:RevenuesFromShippingAndHandlingMember2022-01-012022-12-310001836564valn:MilestonePaymentLicencesMember2024-01-012024-12-310001836564valn:MilestonePaymentLicencesMember2023-01-012023-12-310001836564valn:MilestonePaymentLicencesMember2022-01-012022-12-310001836564valn:OtherServicesMember2024-01-012024-12-310001836564valn:OtherServicesMember2023-01-012023-12-310001836564valn:OtherServicesMember2022-01-012022-12-310001836564valn:ThereofCOVID19Member2024-01-012024-12-310001836564valn:ThereofCOVID19Member2023-01-012023-12-310001836564valn:ThereofCOVID19Member2022-01-012022-12-310001836564valn:VaccineSupplyAgreementMembervaln:UKAuthorityMembervaln:CovidVLA2001ContractMember2022-01-012022-12-310001836564valn:AmendedAdvancePurchaseAgreementAPAMembervaln:EuropeanCommissionMembervaln:CovidVLA2001ContractMember2022-01-012022-12-310001836564ifrs-full:GoodsSoldDirectlyToConsumersMember2024-01-012024-12-310001836564ifrs-full:GoodsSoldDirectlyToConsumersMember2023-01-012023-12-310001836564ifrs-full:GoodsSoldDirectlyToConsumersMember2022-01-012022-12-310001836564ifrs-full:GoodsSoldThroughIntermediariesMember2024-01-012024-12-310001836564ifrs-full:GoodsSoldThroughIntermediariesMember2023-01-012023-12-310001836564ifrs-full:GoodsSoldThroughIntermediariesMember2022-01-012022-12-310001836564country:US2024-01-012024-12-310001836564country:US2023-01-012023-12-310001836564country:US2022-01-012022-12-310001836564country:CA2024-01-012024-12-310001836564country:CA2023-01-012023-12-310001836564country:CA2022-01-012022-12-310001836564country:GB2024-01-012024-12-310001836564country:GB2023-01-012023-12-310001836564country:GB2022-01-012022-12-310001836564country:DE2024-01-012024-12-310001836564country:DE2023-01-012023-12-310001836564country:DE2022-01-012022-12-310001836564country:AT2024-01-012024-12-310001836564country:AT2023-01-012023-12-310001836564country:AT2022-01-012022-12-310001836564valn:NordicsMember2024-01-012024-12-310001836564valn:NordicsMember2023-01-012023-12-310001836564valn:NordicsMember2022-01-012022-12-310001836564country:FR2024-01-012024-12-310001836564country:FR2023-01-012023-12-310001836564country:FR2022-01-012022-12-310001836564valn:OtherEuropeMember2024-01-012024-12-310001836564valn:OtherEuropeMember2023-01-012023-12-310001836564valn:OtherEuropeMember2022-01-012022-12-310001836564valn:RestOfWorldMember2024-01-012024-12-310001836564valn:RestOfWorldMember2023-01-012023-12-310001836564valn:RestOfWorldMember2022-01-012022-12-310001836564country:USvaln:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2022-01-012022-12-310001836564country:GBvaln:VaccineSupplyAgreementMembervaln:UKAuthorityMembervaln:CovidVLA2001ContractMember2022-01-012022-12-310001836564valn:ConcentrationRiskByCustomerMembervaln:OneCustomerMember2024-01-012024-12-310001836564valn:LargestCustomerMember2024-01-012024-12-310001836564valn:LargestCustomerMember2023-01-012023-12-310001836564valn:LargestCustomerMember2022-01-012022-12-310001836564valn:ScotlandMember2024-01-012024-12-310001836564valn:ScottishEnterpriseMember2023-01-012023-12-310001836564valn:ScottishEnterpriseMember2024-01-012024-12-310001836564valn:PartneringSecondAgreementWithCEPIMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2024-01-012024-12-310001836564valn:PartneringAgreementWithCEPIMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2023-01-012023-12-310001836564ifrs-full:AssetsAndLiabilitiesClassifiedAsHeldForSaleMembervaln:CTMUnitSwedenMember2023-01-012023-12-310001836564ifrs-full:LegalProceedingsProvisionMember2022-01-012022-12-310001836564valn:ScottishEnterpriseMember2022-02-012022-02-280001836564ifrs-full:TopOfRangeMembervaln:ScottishEnterpriseMember2022-02-012022-02-280001836564valn:CovidVLA2001ProductMembervaln:ScottishEnterpriseMember2022-02-012022-02-280001836564valn:CovidVLA2001ProductMembervaln:ScottishEnterpriseMember2023-05-012023-05-310001836564valn:ScottishEnterpriseMember2023-05-012023-05-310001836564valn:PartneringAgreementWithCEPIMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2024-01-012024-12-310001836564valn:ValnevaSEMember2024-12-310001836564valn:ValnevaSEMember2023-12-310001836564valn:ValnevaAustriaGmbHMember2024-12-310001836564valn:ValnevaAustriaGmbHMember2023-12-310001836564valn:ValnevaScotlandLtdMember2024-12-310001836564valn:ValnevaScotlandLtdMember2023-12-310001836564valn:ValnevaSwedenABMember2024-12-310001836564valn:ValnevaSwedenABMember2023-12-310001836564valn:VaccinesHoldingsSwedenABMember2024-12-310001836564valn:VaccinesHoldingsSwedenABMember2023-12-310001836564ifrs-full:UnusedTaxLossesMember2024-12-310001836564ifrs-full:UnusedTaxLossesMember2023-12-310001836564valn:FixedAssetsRelatedTemporaryDifferencesMember2024-12-310001836564valn:FixedAssetsRelatedTemporaryDifferencesMember2023-12-310001836564valn:InventoryRelatedTemporaryDifferencesMember2024-12-310001836564valn:InventoryRelatedTemporaryDifferencesMember2023-12-310001836564valn:BorrowingsAndAccruedInterestRelatedTemporaryDifferenceMember2024-12-310001836564valn:BorrowingsAndAccruedInterestRelatedTemporaryDifferenceMember2023-12-310001836564valn:ProvisionRelatedTemporaryDifferencesMember2024-12-310001836564valn:ProvisionRelatedTemporaryDifferencesMember2023-12-310001836564ifrs-full:OtherTemporaryDifferencesMember2024-12-310001836564ifrs-full:OtherTemporaryDifferencesMember2023-12-310001836564valn:UnrecognisedDeferredTaxAssetsNettingMember2024-12-310001836564valn:UnrecognisedDeferredTaxAssetsNettingMember2023-12-310001836564valn:DeferredTaxAssetsTotalMember2024-12-310001836564valn:DeferredTaxAssetsTotalMember2023-12-310001836564valn:IntangibleAssetsRelatedTemporaryDifferencesMember2024-12-310001836564valn:IntangibleAssetsRelatedTemporaryDifferencesMember2023-12-310001836564valn:DeferredTaxLiabilitiesTotalMember2024-12-310001836564valn:DeferredTaxLiabilitiesTotalMember2023-12-310001836564ifrs-full:ShareOptionsMember2024-01-012024-12-310001836564ifrs-full:ShareOptionsMember2023-01-012023-12-310001836564ifrs-full:ShareOptionsMember2022-01-012022-12-310001836564ifrs-full:ComputerSoftwareMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:ComputerSoftwareMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564valn:AcquiredRDTechnologyAndProjectsMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564valn:GeneralCapitalisedDevelopmentExpenditureMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564valn:GeneralCapitalisedDevelopmentExpenditureMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564valn:AcquiredRDTechnologyAndProjectsMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564valn:IXIAROMember2024-01-012024-12-310001836564ifrs-full:ComputerSoftwareMember2023-12-310001836564valn:AcquiredRDTechnologyAndProjectsMember2023-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMember2023-12-310001836564ifrs-full:IntangibleAssetsUnderDevelopmentMember2023-12-310001836564ifrs-full:ComputerSoftwareMember2024-01-012024-12-310001836564valn:AcquiredRDTechnologyAndProjectsMember2024-01-012024-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMember2024-01-012024-12-310001836564ifrs-full:IntangibleAssetsUnderDevelopmentMember2024-01-012024-12-310001836564ifrs-full:ComputerSoftwareMember2024-12-310001836564valn:AcquiredRDTechnologyAndProjectsMember2024-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMember2024-12-310001836564ifrs-full:IntangibleAssetsUnderDevelopmentMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2024-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:AcquiredRDTechnologyAndProjectsMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:CapitalisedDevelopmentExpenditureMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:IntangibleAssetsUnderDevelopmentMember2024-12-310001836564ifrs-full:GrossCarryingAmountMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ComputerSoftwareMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembervaln:AcquiredRDTechnologyAndProjectsMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:CapitalisedDevelopmentExpenditureMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:IntangibleAssetsUnderDevelopmentMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2024-12-310001836564ifrs-full:ComputerSoftwareMember2022-12-310001836564valn:AcquiredRDTechnologyAndProjectsMember2022-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMember2022-12-310001836564ifrs-full:IntangibleAssetsUnderDevelopmentMember2022-12-310001836564ifrs-full:ComputerSoftwareMember2023-01-012023-12-310001836564valn:AcquiredRDTechnologyAndProjectsMember2023-01-012023-12-310001836564ifrs-full:CapitalisedDevelopmentExpenditureMember2023-01-012023-12-310001836564ifrs-full:IntangibleAssetsUnderDevelopmentMember2023-01-012023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerSoftwareMember2023-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:AcquiredRDTechnologyAndProjectsMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:CapitalisedDevelopmentExpenditureMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:IntangibleAssetsUnderDevelopmentMember2023-12-310001836564ifrs-full:GrossCarryingAmountMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ComputerSoftwareMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembervaln:AcquiredRDTechnologyAndProjectsMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:CapitalisedDevelopmentExpenditureMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:IntangibleAssetsUnderDevelopmentMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMember2023-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:IXIAROMember2024-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:IXIAROMember2023-12-310001836564valn:IXIAROMember2024-12-310001836564valn:IXIAROMember2023-12-310001836564ifrs-full:TopOfRangeMember2024-01-012024-12-310001836564ifrs-full:BottomOfRangeMembercountry:SE2024-01-012024-12-310001836564ifrs-full:TopOfRangeMembercountry:SE2024-01-012024-12-310001836564ifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2023-12-310001836564ifrs-full:MachineryMember2023-12-310001836564ifrs-full:FixturesAndFittingsMember2023-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2024-01-012024-12-310001836564ifrs-full:MachineryMember2024-01-012024-12-310001836564ifrs-full:FixturesAndFittingsMember2024-01-012024-12-310001836564ifrs-full:RightofuseAssetsMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2024-12-310001836564ifrs-full:MachineryMember2024-12-310001836564ifrs-full:FixturesAndFittingsMember2024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2022-12-310001836564ifrs-full:MachineryMember2022-12-310001836564ifrs-full:FixturesAndFittingsMember2022-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2023-01-012023-12-310001836564ifrs-full:MachineryMember2023-01-012023-12-310001836564ifrs-full:FixturesAndFittingsMember2023-01-012023-12-310001836564ifrs-full:RightofuseAssetsMember2023-01-012023-12-310001836564country:SE2024-12-310001836564country:SE2023-12-310001836564valn:CovidVLA2001ContractMembervaln:LandBuildingsAndLeaseholdImprovementsMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564ifrs-full:MachineryMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:MachineryMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564ifrs-full:FixturesAndFittingsMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:FixturesAndFittingsMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564ifrs-full:ComputerEquipmentMemberifrs-full:BottomOfRangeMember2024-01-012024-12-310001836564ifrs-full:ComputerEquipmentMemberifrs-full:TopOfRangeMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2023-12-310001836564ifrs-full:MachineryMember2023-12-310001836564ifrs-full:ComputerEquipmentMember2023-12-310001836564ifrs-full:FixturesAndFittingsMember2023-12-310001836564ifrs-full:ConstructionInProgressMember2023-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2024-01-012024-12-310001836564ifrs-full:MachineryMember2024-01-012024-12-310001836564ifrs-full:ComputerEquipmentMember2024-01-012024-12-310001836564ifrs-full:FixturesAndFittingsMember2024-01-012024-12-310001836564ifrs-full:ConstructionInProgressMember2024-01-012024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2024-12-310001836564ifrs-full:MachineryMember2024-12-310001836564ifrs-full:ComputerEquipmentMember2024-12-310001836564ifrs-full:FixturesAndFittingsMember2024-12-310001836564ifrs-full:ConstructionInProgressMember2024-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:LandBuildingsAndLeaseholdImprovementsMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2024-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembervaln:LandBuildingsAndLeaseholdImprovementsMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ComputerEquipmentMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:FixturesAndFittingsMember2024-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2024-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2022-12-310001836564ifrs-full:MachineryMember2022-12-310001836564ifrs-full:ComputerEquipmentMember2022-12-310001836564ifrs-full:FixturesAndFittingsMember2022-12-310001836564ifrs-full:ConstructionInProgressMember2022-12-310001836564valn:LandBuildingsAndLeaseholdImprovementsMember2023-01-012023-12-310001836564ifrs-full:MachineryMember2023-01-012023-12-310001836564ifrs-full:ComputerEquipmentMember2023-01-012023-12-310001836564ifrs-full:FixturesAndFittingsMember2023-01-012023-12-310001836564ifrs-full:ConstructionInProgressMember2023-01-012023-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:LandBuildingsAndLeaseholdImprovementsMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:MachineryMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ComputerEquipmentMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:FixturesAndFittingsMember2023-12-310001836564ifrs-full:GrossCarryingAmountMemberifrs-full:ConstructionInProgressMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMembervaln:LandBuildingsAndLeaseholdImprovementsMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:MachineryMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ComputerEquipmentMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:FixturesAndFittingsMember2023-12-310001836564ifrs-full:AccumulatedDepreciationAmortisationAndImpairmentMemberifrs-full:ConstructionInProgressMember2023-12-310001836564ifrs-full:CostOfSalesMember2024-01-012024-12-310001836564ifrs-full:CostOfSalesMember2023-01-012023-12-310001836564valn:ResearchAndDevelopmentExpensesMember2024-01-012024-12-310001836564valn:ResearchAndDevelopmentExpensesMember2023-01-012023-12-310001836564valn:MarketingAndDistributionExpensesMember2024-01-012024-12-310001836564valn:MarketingAndDistributionExpensesMember2023-01-012023-12-310001836564valn:GeneralAndAdministrativeExpensesMember2024-01-012024-12-310001836564valn:GeneralAndAdministrativeExpensesMember2023-01-012023-12-310001836564country:GB2024-12-310001836564country:GB2023-12-310001836564country:AT2024-12-310001836564country:AT2023-12-310001836564valn:NordicsMember2024-12-310001836564valn:NordicsMember2023-12-310001836564country:FR2024-12-310001836564country:FR2023-12-310001836564country:US2024-12-310001836564country:US2023-12-310001836564country:CA2024-12-310001836564country:CA2023-12-310001836564valn:IXIAROMember2024-12-310001836564valn:DUKORALMember2024-12-310001836564valn:IXCHIQChikungunyaVLA1553Member2024-12-310001836564valn:ShigellaVaccineMember2024-12-310001836564valn:ServicesRelatedToClinicalTrialMaterialMember2024-12-310001836564valn:IXIAROMember2023-12-310001836564valn:DUKORALMember2023-12-310001836564valn:IXCHIQChikungunyaVLA1553Member2023-12-310001836564valn:ShigellaVaccineMember2023-12-310001836564valn:ServicesRelatedToClinicalTrialMaterialMember2023-12-310001836564valn:IXIAROMember2024-01-012024-12-310001836564valn:DUKORALMember2024-01-012024-12-310001836564valn:ShigellaVaccineMember2024-01-012024-12-310001836564valn:ServicesRelatedToClinicalTrialMaterialMember2024-01-012024-12-310001836564valn:DUKORALMember2023-01-012023-12-310001836564valn:ShigellaVaccineMember2023-01-012023-12-310001836564valn:ServicesRelatedToClinicalTrialMaterialMember2023-01-012023-12-310001836564ifrs-full:CurrencyRiskMember2024-01-012024-12-310001836564currency:USDifrs-full:CurrencyRiskMember2024-01-012024-12-310001836564currency:USDifrs-full:CurrencyRiskMember2023-01-012023-12-310001836564currency:GBPifrs-full:CurrencyRiskMember2024-01-012024-12-310001836564currency:GBPifrs-full:CurrencyRiskMember2023-01-012023-12-310001836564currency:SEKifrs-full:CurrencyRiskMember2024-01-012024-12-310001836564currency:SEKifrs-full:CurrencyRiskMember2023-01-012023-12-310001836564currency:CADifrs-full:CurrencyRiskMember2024-01-012024-12-310001836564currency:CADifrs-full:CurrencyRiskMember2023-01-012023-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsAAACountryMember2024-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsAAACountryMember2023-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsAACountryMember2024-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsAACountryMember2023-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsACountryMember2024-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsACountryMember2023-12-310001836564valn:AAMember2024-12-310001836564valn:AAMember2023-12-310001836564valn:AMember2024-12-310001836564valn:AMember2023-12-310001836564valn:CounterpartiesWithoutExternalCreditRatingOrRatingBelowAMember2024-12-310001836564valn:CounterpartiesWithoutExternalCreditRatingOrRatingBelowAMember2023-12-310001836564valn:CovidVLA2001ProductMember2024-12-310001836564valn:CovidVLA2001ProductMember2023-12-310001836564valn:IXIARODUKORALAndIXCHIQMember2024-12-310001836564valn:IXIARODUKORALAndIXCHIQMember2023-12-310001836564valn:IXIAROAndIXCHIQAndDUKORALMember2024-12-310001836564valn:IXIAROAndIXCHIQAndDUKORALMember2023-12-310001836564valn:ReceivablesFromGovernmentalInstitutionsBCountryMember2023-12-310001836564valn:Shigella4VS4VMembervaln:LimmaTechBiologicsAGMember2024-01-012024-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2024-01-012024-12-310001836564valn:BliNKBiomedicalSASMember2023-09-072023-09-070001836564valn:BliNKBiomedicalSASMemberifrs-full:NoncurrentAssetsHeldForSaleMember2023-09-082023-09-0800018365642024-09-132024-09-130001836564valn:OtherRegulatedReservesMember2023-12-310001836564ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-12-310001836564ifrs-full:TreasurySharesMember2023-12-310001836564ifrs-full:ReserveOfSharebasedPaymentsMember2023-12-310001836564valn:OtherRevenueReserveMember2023-12-310001836564valn:OtherRegulatedReservesMember2024-01-012024-12-310001836564ifrs-full:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310001836564ifrs-full:TreasurySharesMember2024-01-012024-12-310001836564ifrs-full:ReserveOfSharebasedPaymentsMember2024-01-012024-12-310001836564valn:OtherRevenueReserveMember2024-01-012024-12-310001836564valn:OtherRegulatedReservesMember2024-12-310001836564ifrs-full:AccumulatedOtherComprehensiveIncomeMember2024-12-310001836564ifrs-full:TreasurySharesMember2024-12-310001836564ifrs-full:ReserveOfSharebasedPaymentsMember2024-12-310001836564valn:OtherRevenueReserveMember2024-12-310001836564valn:OtherRegulatedReservesMember2022-12-310001836564ifrs-full:AccumulatedOtherComprehensiveIncomeMember2022-12-310001836564ifrs-full:TreasurySharesMember2022-12-310001836564ifrs-full:ReserveOfSharebasedPaymentsMember2022-12-310001836564valn:OtherRevenueReserveMember2022-12-310001836564valn:OtherRegulatedReservesMember2023-01-012023-12-310001836564ifrs-full:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310001836564ifrs-full:TreasurySharesMember2023-01-012023-12-310001836564ifrs-full:ReserveOfSharebasedPaymentsMember2023-01-012023-12-310001836564valn:OtherRevenueReserveMember2023-01-012023-12-310001836564valn:StockOptionPlansMember2024-01-012024-12-310001836564valn:StockOptionPlansMember2023-01-012023-12-310001836564valn:StockOptionPlansMember2022-01-012022-12-310001836564valn:FreeOrdinarySharesProgramMember2024-01-012024-12-310001836564valn:FreeOrdinarySharesProgramMember2023-01-012023-12-310001836564valn:FreeOrdinarySharesProgramMember2022-01-012022-12-310001836564valn:PhantomSharesMember2024-01-012024-12-310001836564valn:PhantomSharesMember2023-01-012023-12-310001836564valn:PhantomSharesMember2022-01-012022-12-310001836564valn:StockOptionPlansMember2024-12-310001836564valn:ESOPPlans2013To2017Membervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlans2013To2017Membervaln:TrancheOneMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlans2013To2017Membervaln:TrancheTwoMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlan2019OnwardsMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlan2019OnwardsMembervaln:TrancheOneMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlan2019OnwardsMembervaln:TrancheTwoMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:ESOPPlan2019OnwardsMembervaln:TrancheThreeMembervaln:StockOptionPlansMember2024-01-012024-12-310001836564valn:StockOptionPlansMember2023-12-310001836564valn:StockOptionPlansMember2022-12-310001836564valn:ESOP2013Membervaln:StockOptionPlansMember2023-01-012023-12-310001836564valn:AwardsMaturing2025Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2025Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2026Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2026Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2027Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2027Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2029Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2029Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2032Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2032Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2033Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2033Membervaln:StockOptionPlansMember2023-12-310001836564valn:AwardsMaturing2034Membervaln:StockOptionPlansMember2024-12-310001836564valn:AwardsMaturing2034Membervaln:StockOptionPlansMember2023-12-310001836564valn:StockOptionPlansMember2024-10-222024-10-220001836564ifrs-full:BottomOfRangeMembervaln:StockOptionPlansMember2024-10-222024-10-220001836564ifrs-full:TopOfRangeMembervaln:StockOptionPlansMember2024-10-222024-10-220001836564valn:ManagementBoardMembervaln:FreeOrdinarySharesProgramMember2024-01-012024-12-310001836564valn:ManagementBoardMembervaln:FreeOrdinarySharesProgramMember2023-01-012023-12-310001836564valn:SeniorLeadershipGroupMembervaln:FreeOrdinarySharesProgramMember2024-01-012024-12-310001836564valn:SeniorLeadershipGroupMembervaln:FreeOrdinarySharesProgramMember2023-01-012023-12-310001836564valn:FreeOrdinarySharesProgramMember2023-12-310001836564valn:FreeOrdinarySharesProgramMember2022-12-310001836564valn:FreeOrdinarySharesProgramMember2024-12-310001836564valn:PhantomSharesMember2023-12-310001836564valn:PhantomSharesMember2022-12-310001836564valn:PhantomSharesModificationOfProgramMembervaln:PhantomSharesMember2023-01-012023-12-310001836564valn:PhantomSharesMember2024-12-310001836564valn:AwardsMaturing2027Membervaln:PhantomSharesMember2024-12-310001836564valn:AwardsMaturing2027Membervaln:PhantomSharesMember2023-12-310001836564valn:AwardsMaturing2029Membervaln:PhantomSharesMember2024-12-310001836564valn:AwardsMaturing2029Membervaln:PhantomSharesMember2023-12-310001836564valn:AwardsMaturing2030Membervaln:PhantomSharesMember2024-12-310001836564valn:AwardsMaturing2030Membervaln:PhantomSharesMember2023-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2024-12-310001836564valn:ResearchTaxCreditMember2024-12-310001836564valn:ResearchTaxCreditMember2023-12-310001836564valn:CEPIMember2024-12-310001836564valn:CEPIMember2023-12-310001836564ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2024-12-310001836564ifrs-full:LaterThanOneYearAndNotLaterThanThreeYearsMember2023-12-310001836564ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMember2024-12-310001836564ifrs-full:LaterThanThreeYearsAndNotLaterThanFiveYearsMember2023-12-310001836564ifrs-full:LaterThanFiveYearsMember2024-12-310001836564ifrs-full:LaterThanFiveYearsMember2023-12-310001836564currency:EUR2024-12-310001836564currency:EUR2023-12-310001836564currency:USD2024-12-310001836564currency:USD2023-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2020-02-012024-12-310001836564ifrs-full:GrossCarryingAmountMembervaln:AmendedDebtFinancingAgreementDueFirstQuarter2027Member2024-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2023-08-160001836564valn:TrancheOneMembervaln:AmendedDebtFinancingAgreementDue2028Member2024-12-310001836564valn:TrancheTwoMembervaln:AmendedDebtFinancingAgreementDue2028Member2024-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2023-01-012023-12-310001836564srt:MaximumMember2024-01-012024-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2023-12-310001836564valn:AmendedDebtFinancingAgreementDue2028Member2022-12-310001836564currency:SEK2024-12-310001836564currency:SEK2023-12-310001836564valn:OtherCurrenciesMember2024-12-310001836564valn:OtherCurrenciesMember2023-12-310001836564valn:MasterCollaborationAndLicenseAgreementMembervaln:SerumInstituteOfIndiaMembervaln:IXCHIQChikungunyaVLA1553Member2024-01-012024-12-310001836564valn:AdvancePurchaseAgreementAPAMembervaln:KingdomOfBahrainMembervaln:CovidVLA2001ProductMember2023-01-012023-12-3100018365642023-07-012023-07-010001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2024-12-310001836564valn:SupplyAgreementMembervaln:GSKMember2024-12-310001836564valn:AmendedCollaborationAndLicenseAgreementMembervaln:PfizerIncMembervaln:LymeVLA15Member2023-12-310001836564valn:SupplyAgreementMembervaln:GSKMember2023-12-310001836564ifrs-full:BottomOfRangeMember2024-12-310001836564ifrs-full:TopOfRangeMember2024-12-310001836564ifrs-full:BottomOfRangeMember2023-12-310001836564ifrs-full:TopOfRangeMember2023-12-310001836564ifrs-full:PresentValueOfDefinedBenefitObligationMember2023-12-310001836564ifrs-full:PresentValueOfDefinedBenefitObligationMember2022-12-310001836564ifrs-full:PresentValueOfDefinedBenefitObligationMember2024-01-012024-12-310001836564ifrs-full:PresentValueOfDefinedBenefitObligationMember2023-01-012023-12-310001836564ifrs-full:PresentValueOfDefinedBenefitObligationMember2024-12-310001836564ifrs-full:LegalProceedingsProvisionMember2024-12-310001836564ifrs-full:LegalProceedingsProvisionMember2023-12-310001836564valn:PartneringSecondAgreementWithCEPIMembervaln:CoalitionForEpidemicPreparednessInnovationsCEPIMembervaln:IXCHIQChikungunyaVLA1553Member2024-12-310001836564valn:UKAuthorityMember2022-01-012022-12-310001836564valn:VitalMeatSASMember2022-01-012022-12-310001836564valn:ResearchTaxCreditMembervaln:BPIFranceMember2022-11-300001836564valn:ResearchTaxCreditMembervaln:BPIFranceMember2024-12-310001836564valn:ResearchTaxCreditMembervaln:BPIFranceMemberifrs-full:BottomOfRangeMember2022-11-300001836564valn:ResearchTaxCredit2021Membervaln:BPIFranceMember2024-12-310001836564valn:ResearchTaxCredit2022Membervaln:BPIFranceMember2024-12-310001836564valn:ResearchTaxCredit2023Membervaln:BPIFranceMember2024-12-310001836564valn:ExecutiveCommitteeFormerManagementBoardMember2024-01-012024-12-310001836564valn:ExecutiveCommitteeFormerManagementBoardMember2023-01-012023-12-310001836564valn:ExecutiveCommitteeFormerManagementBoardMember2022-01-012022-12-310001836564valn:ExecutiveCommitteeFormerManagementBoardMember2024-12-310001836564valn:BoardOfDirectorsMember2024-01-012024-12-310001836564valn:BoardOfDirectorsMember2023-01-012023-12-310001836564valn:BoardOfDirectorsMember2022-01-012022-12-310001836564valn:UnitedStatesU.S.DepartmentOfDefenseDoDMembervaln:MajorPartnershipAgreementMembervaln:IXIAROMember2025-01-302025-01-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
☐ REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2024
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 13(d) OF THE SECURITIES EXCHANGE ACT OF 1934
OR
☐ SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Date of event requiring this shell company report ________________
Commission File Number 001-40377
Valneva SE
(Exact name of Registrant as specified in its charter and translation of Registrant’s name into English)
France
(Jurisdiction of incorporation or organization)
6 rue Alain Bombard
44800 Saint-Herblain, France
(Address of principal executive offices)
Thomas Lingelbach
Chief Executive Officer, Valneva SE
6 rue Alain Bombard
44800 Saint-Herblain, France
Tel: +33 2 28 07 37 10
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading Symbol(s) |
|
Name of each exchange on which registered |
| American Depositary Shares, each representing two ordinary shares, €0.15 nominal value per share |
|
VALN |
|
The Nasdaq Global Select Market |
| Ordinary shares, €0.15 nominal value per share |
|
* |
|
The Nasdaq Global Select Market* |
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act. None.
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None.
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report. Ordinary Shares: 162,521,524 outstanding as of December 31, 2024
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒ No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ☐ Yes ☒ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ☒ Yes ☐ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). ☒ Yes ☐ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer, “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer ☒ Accelerated filer ☐ Non-accelerated filer ☐ Emerging growth company ☐
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that require a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to § 240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
U.S. GAAP ☐ International Financial Reporting Standards as issued by the International Accounting Standards Board ☒ Other ☐
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes ☒ No
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
| Item |
|
Page |
|
|
4 |
|
|
4 |
|
|
6 |
| PART I |
|
|
| Item 1 |
|
7 |
| Item 2 |
|
7 |
| Item 3 |
|
7 |
| Item 4 |
|
60 |
| Item 4A |
|
101 |
| Item 5 |
|
102 |
| Item 6 |
|
129 |
| Item 7 |
|
150 |
| Item 8 |
|
154 |
| Item 9 |
|
155 |
| Item 10 |
|
156 |
| Item 11 |
|
168 |
| Item 12 |
|
169 |
| PART II |
|
|
| Item 13 |
|
172 |
| Item 14 |
|
172 |
| Item 15 |
|
174 |
| Item 16 |
|
178 |
| Item 16A |
|
178 |
| Item 16B |
|
178 |
| Item 16C |
|
178 |
| Item 16D |
|
179 |
| Item 16E |
|
179 |
| Item 16F |
|
179 |
| Item 16G |
|
179 |
| Item 16H |
|
180 |
| Item 16I |
|
180 |
| Item 16J |
|
180 |
| Item 16K |
|
180 |
| PART III |
|
|
| Item 17 |
|
181 |
| Item 18 |
|
181 |
| Item 19 |
|
182 |
INTRODUCTION
Unless otherwise indicated in this Annual Report (this “Annual Report”), “Valneva,” “the company,” “our company,” “we,” “us” and “our” refer to Valneva SE and its consolidated subsidiaries.
“Valneva,” the Valneva logo, “IXIARO,” “JESPECT,” “DUKORAL”, “IXCHIQ” and other trademarks or service marks of Valneva SE of any of our business partners appearing in this Annual Report are the property of Valneva, its subsidiaries or its business partners, as applicable. Solely for convenience, the trademarks, service marks and trade names referred to in this Annual Report are listed without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their right thereto. All other trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners. We do not intend to use or display other companies’ trademarks and trade names to imply any relationship with, or endorsement or sponsorship of us by, any other companies.
Our audited consolidated financial statements have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. Our consolidated financial statements are presented in euros, and unless otherwise specified, all monetary amounts are in euros. All references in this Annual Report to “$,” “US$,” “U.S.$,” “U.S. dollars,” “dollars” and “USD” mean U.S. dollars and all references to “€” and “euros” mean euros, unless otherwise noted. Throughout this Annual Report, references to ADSs mean American Depositary Shares or ordinary shares represented by such ADSs, as the case may be.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than present and historical facts and conditions contained in this Annual Report, including statements regarding our future results of operations and financial position, business strategy, plans and our objectives for future operations, are forward-looking statements. When used in this Annual Report, the words “anticipate,” “believe,” “can,” “could,” “estimate,” “expect,” “intend,” “is designed to,” “may,” “might,” “plan,” “potential,” “predict,” “objective,” “should,” or the negative of these and similar expressions identify forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
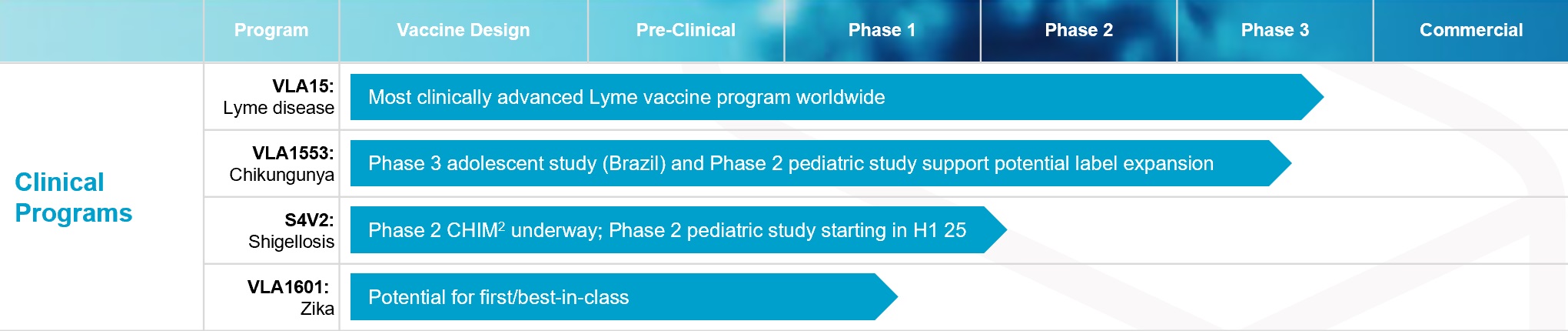
•timing and expected outcomes of clinical trials and pre-clinical studies, particularly with respect to the Phase 3 clinical trial of our Lyme disease vaccine candidate VLA15 as well as the ongoing Phase 2 and 3 clinical trials and the planned Phase 4 clinical trials of our approved chikungunya vaccine IXCHIQ and the ongoing Phase 2 trials of the shigellosis vaccine candidate S4V2;
•the likelihood, timing, and expected outcomes of regulatory filings and approvals, including, in the short-term, potential approval of the chikungunya vaccine candidate VLA1553 in Brazil and other markets and the potential filings and approvals of VLA15 and in the mid-term, potential filings and approvals of S4V2;
•our ability to successfully market IXCHIQ in the United States, Europe, Canada, the UK, and other markets where it may be approved;
•our expectations with respect to Pfizer’s regulatory and commercialization plans for our Lyme disease candidate;
•our expectations and forecasts for sales of our approved products, particularly IXCHIQ, and estimates of market opportunity for our approved products and vaccine candidates;
•our ability to expand, develop, and advance our pipeline of product candidates;
•our ability to supply a sufficient quantity of our products and product candidates and to safely and effectively scale up our manufacturing capabilities, including at our Almeida manufacturing facility in Scotland;
•the effectiveness and profitability of our collaborations and partnerships, our ability to maintain our current collaborations and partnerships and our ability to enter into new collaborations and partnerships;
•the potential safety and effectiveness of our vaccine candidates in development;
•the effects of any pandemics on our sales and operations, including our expectations and assumptions regarding the resumption of travel and the future demand for travel vaccines;
•our expectations related to future milestone and royalty payments and other revenue under our collaborations and partnerships;
•our ability to meet our obligations under our various collaboration, partnership, and distribution arrangements;
•the effects of increased competition as well as innovations by new and existing competitors in our industry;
•our ability to obtain, maintain, protect and enforce our intellectual property rights and proprietary technologies and to operate our business without infringing the intellectual property rights and proprietary technology of third parties;
•regulatory and political developments in the United States, Europe, and other countries, including in particular regulatory developments relating to environmental, social, and governance issues and in response to anti-vaccine sentiments in the United States and elsewhere;
•statements regarding future revenue, cash situation, hiring plans, expenses, capital expenditures, capital requirements, stock performance, and financing opportunities; and
•other risks and uncertainties, including those listed in the section of this Annual Report titled “Item 3.D—Risk Factors.”
You should refer to the section of this Annual Report titled “Item 3.D—Risk Factors” for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. The Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act do not protect any forward-looking statements that we make in connection with this Annual Report.
In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements.
You should read this Annual Report and the documents that we reference in this Annual Report and have filed as exhibits to this Annual Report completely and with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
Unless otherwise indicated, information contained in this Annual Report concerning our industry and the markets in which we operate, including our general expectations and market position, market opportunity and market size estimates, is based on information from independent industry analysts, third-party sources and management estimates. Management estimates are derived from publicly available information released by independent industry analysts and third-party sources, as well as data from our internal research, and are based on assumptions made by us based on such data and our knowledge of such industry and market, which we believe to be reasonable. In addition, while we believe the market opportunity information included in this Annual Report is generally reliable and is based on reasonable assumptions, such data involve risks and uncertainties and are subject to change based on various factors, including those discussed under the section of this Annual Report titled “Item 3.D—Risk Factors.”
SUMMARY RISK FACTORS
Our business is subject to a number of risks and uncertainties, including those risks discussed at-length in the section below titled “Risk Factors.” These risks include, among others, the following:
•Our business is capital intensive, and given our level of planned investments over the medium term, we may not achieve or maintain profitability.
•Our future success is substantially dependent on the successful clinical development, regulatory approval, and commercialization of our product candidates in a timely manner. This risk is heightened in the short-term given that our Lyme disease vaccine candidate is undergoing Phase 3 clinical trials. If we are not able to obtain the regulatory approvals we target, whether as a result of clinical trials results or other factors, we will not be able to commercialize our product candidates according to our plans or at all, and our ability to generate product revenue will be adversely affected. Delays in clinical development may also lead to delays in our expected regulatory and commercial timelines, which could materially impact our business plans and our financial projections.
•We may require ongoing funding to finance our operations. If we are unable to raise capital when needed, we could be forced to delay, reduce, or terminate certain of our planned investments, including in-development programs or other parts of our operations.
•Our products are aimed at diseases that largely threaten travelers. If international travel is substantially disrupted, as it was at the height of the COVID-19 pandemic or may be in future due to a similar event or adverse economic conditions, this will significantly adversely affect the sale of these vaccines. Additionally, future outbreaks of disease, in regions where we or third parties on which we rely have significant manufacturing facilities, concentrations of clinical trial sites, or other business operations, could materially affect our operations globally and at our clinical trial sites, as well as the business or operations of our manufacturers, CROs, or other third parties with whom we conduct business.
•Our future growth depends on continuing to build our pipeline of product candidates. If we are unable to progress existing clinical-stage and pre-clinical stage product candidates or to initiate new clinical or pre-clinical programs, this could have a material impact on our business plans and financial projections.
•We depend upon our existing collaboration partner, Pfizer, and other third parties to advance our business and provide other key services. If we are unable to maintain such existing agreements or enter into additional arrangements as needed, or if such third parties do not provide such services as anticipated, our business could be adversely affected.
•We operate in a highly regulated industry and may fail to comply with applicable regulatory obligations, including after product approval is obtained. Additionally, we may not be able to adapt in a timely manner or at all to changes in the regulatory landscape applicable to our business, including regulations related to environmental, social and governance matters, and changes in the functioning of regulatory agencies could have a significant impact on our ability to seek approval for and market our products.
•If we are unable to obtain and maintain patent protection for our product candidates and technology, or if the scope of the patent protection obtained is not sufficiently broad or robust, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our product candidates and technology may be adversely affected.
•We rely primarily on our manufacturing facilities and rely in part on third parties’ manufacturing facilities as the source of manufacturing for our products and for certain of our product candidates.
•The terms of our financing arrangements place restrictions on our operating and financial flexibility.
•We may face competition, and our competitors may have significantly greater resources and experience, which may negatively impact our commercial opportunities.
•We are dependent on single source suppliers for some of the components and materials used in our products.
•We may encounter difficulties in managing our growth, which could disrupt our operations.
•If we are unable to maintain effective internal controls over financial reporting, the accuracy and timeliness of our financial reporting may be adversely affected, which could hurt our business, lessen investor confidence, and depress the market price of our securities.
•Our information systems and data, and those of third-parties connected to us, are vulnerable to cyber attacks and security breaches which could have a material impact on our operations, reputation, and/or financial results.
•The rights of shareholders in companies subject to French corporate law differ in material respects from the rights of shareholders of corporations incorporated in the United States. As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than a U.S. company.
PART I
Item 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
Item 3. Key Information
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Our business faces significant risks. You should carefully consider all of the information set forth in this Annual Report and in our other filings with the United States Securities and Exchange Commission, or the SEC, including the following risk factors which we face and which are faced by our industry. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. This report also contains forward-looking statements that involve risks and uncertainties. Our results could materially differ from those anticipated in these forward-looking statements, as a result of certain factors including the risks described below and elsewhere in this Annual Report and our other SEC filings. See “Special Note Regarding Forward-Looking Statements” above.
Risks Related to Our Financial Position and Capital Needs
Our business is capital intensive, and given our level of planned investments over the medium term, we may not achieve or maintain profitability.
We have previously incurred significant net losses, and we might not succeed in becoming profitable over the next several years. Our net loss was €12.2 million, €101.4 million and €143.3 million for the years ended December 31, 2024, 2023 and 2022, respectively. As of December 31, 2024 we had an accumulated net loss of €563.9 million. Our planned investments with respect to our approved products and product candidates and to seek and develop additional product candidates are in excess of the revenues that we expect to generate in the short term, and we expect to continue to incur substantial operating losses for the next several years. The net losses we incur may fluctuate significantly from quarter to quarter and year to year.
To achieve profitability, we must generate significant sales of our commercial products and obtain regulatory approval for and successfully commercialize our product candidates, including the Lyme disease candidate for which we would obtain milestones and royalties upon successful commercialization. We anticipate that if the Phase 3 trial of our Lyme disease vaccine candidate is successful, Pfizer will apply for approval in the United States and European Union in 2026. Our future revenues from these products depend on obtaining regulatory approval in additional markets, generating market acceptance, obtaining reimbursement from third-party payors, and increasing market share. In addition, we also generate revenue from sales of third-party products, licensing and service agreements, and grants.
If we are required by regulatory authorities to perform studies in addition to those expected (for example, the Phase 4 clinical trials of IXCHIQ which were mandated by the regulatory agencies in connection with approval of IXCHIQ) or if there are any delays in our clinical trials, particularly the Phase 3 clinical trial for our Lyme disease vaccine candidate, or any delays in the development of any of our product candidates, our anticipated expenses could change. Even if we do receive regulatory approval for a product, we may not be able to generate revenue from it, according to the timing or quantities expected or at all. For example, although we received several regulatory approvals for VLA2001, our vaccine against the SARS-CoV-2 virus causing COVID-19, we were not able to generate meaningful sales in our target markets and ultimately discontinued the product.
Because of the numerous risks and uncertainties associated with biopharmaceutical product development and commercialization, we cannot accurately predict when or if we will be able to achieve or maintain profitability. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable could decrease the value of our company and could impair our ability to raise capital, maintain our research and development efforts, expand our business, or continue our operations.
We market our products primarily to travelers to regions where the targeted diseases are endemic. If international travel is substantially disrupted, this will significantly adversely affect the sale of these vaccines.
We market IXCHIQ, DUKORAL, and IXIARO primarily to travelers to particular regions. During the COVID-19 pandemic, travel significantly decreased worldwide, and sales of DUKORAL and IXIARO decreased significantly in 2020 and 2021, adversely affecting our financial results. In addition, our failure to correctly forecast the recovery of the travel vaccine market in 2022 and produce sufficient quantities of DUKORAL and IXIARO resulted in a loss of potential sales. While international travel has resumed significantly, if another disruption or adverse economic conditions cause a substantial decrease in international travel, our revenues will be significantly adversely affected, and we may not be able to finance our operations and continue the development of one or more of our vaccine candidates without additional financing.
We may require additional funding to finance our operations and achieve our strategic ambitions. If we are unable to raise capital when needed, we could be forced to delay, reduce, or terminate certain of our planned investments, including development programs or other parts of our operations.
Investment in product development in the healthcare industry, including of biopharmaceutical products, is highly speculative because it entails substantial expenditures and significant risk that any potential product candidate will fail to demonstrate adequate effect or an acceptable safety profile, gain regulatory approval, and become commercially successful. We may need to raise additional capital to complete the development and commercialization of our product candidates and fund certain of our existing manufacturing and other commitments. We expect to finance our cash needs through public or private equity or debt financings, third-party (including government) funding, and marketing and distribution arrangements, as well as other collaborations, strategic alliances, and licensing arrangements, or any combination of these approaches. However, our operating plans may change due to factors we cannot predict, and we may need to seek additional funds sooner than planned.
Global financial markets may be negatively impacted by macroeconomic factors including as a result of inflation, changes in interest rates, changes in trade policies and geopolitical conflicts in Europe and the Middle East. If these disruptions persist or deepen, or if other global events have a significant impact on the global financial markets, we could experience an inability to access additional capital or an increase in our costs of borrowing, which could in the future negatively affect our capacity for certain corporate development transactions or our ability to make other important, opportunistic investments. Adequate additional financing may not be available to us when we require it in sufficient amounts or on acceptable terms, or at all. Additionally, investors are using sustainability and environmental, social and governance, or ESG, criteria to evaluate possible investments, and we cannot guarantee that we will be able to implement effective sustainable practices that will make us attractive for such investors, in a timely fashion or at all. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce, or altogether terminate certain of our research and development programs or future commercialization efforts. We may need to seek funds through arrangements with collaborative partners or otherwise at an earlier stage of product development than otherwise would be desirable, and we may be required to monetize rights to some of our technologies or product candidates at an earlier stage of development or otherwise agree to terms unfavorable to us.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. Under French law, our share capital may be increased only with shareholders’ approval at an extraordinary general shareholders’ meeting on the basis of a report from the Board of Directors. In addition, the French Commercial Code imposes certain limitations on our ability to price certain offerings of our share capital without preferential subscription rights (droit préférentiel de souscription), which limitation may prevent us from successfully completing any such offering.
Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares or the ADSs to decline. The sale of additional equity or convertible securities would dilute our shareholders.
The terms of our financing arrangements place restrictions on our operating and financial flexibility.
In February 2020, we entered into a debt financing agreement, or the Financing Agreement, with Deerfield and OrbiMed. The loans bear interest at 9.95%. As of December 31, 2024, we had $200 million (€192.5 million) drawn down in four tranches under the Financing Agreement, including an additional $100 million (€93.4 million) made available to us in an amendment signed in August 2023. This additional loan will mature in the third quarter of 2028, and repayments begin in the first quarter of 2027. The original loan of $100 million will mature in the first quarter of 2027, and repayments begin in the first quarter of 2026.
The Financing Agreement contains covenants for minimum revenue and liquidity which were in effect during 2024, and are currently set to €115 million and €35 million, respectively. As a result of deferred recognition of revenues and the effects of COVID-19 on product sales, we were previously at risk of not meeting the minimum revenue covenant and have amended these covenants several times since 2020. Compliance with these covenants could require raising additional funds or changing our clinical development plans, either of which could limit our flexibility to operate our business in a way that might be advantageous to us and our shareholders. If our consolidated net revenues (excluding grants) or our liquidity were to fall below the amounts required, this would constitute an event of default that could trigger various consequences.
For example, the interest rate on the loans could increase by up to 10 additional interest points if the duration of the default is longer than 15 days, or we could be required to immediately repay the full principal amount of the loans, including all fees and interest associated with repayment. If we were unable to pay the full amount due in case of these and certain other events of default, our lenders could exercise their rights to take possession and dispose of the collateral, which includes substantially all of our intellectual property, securing the Financing Agreement for their benefit. Our business, financial condition, and results of operations could be substantially harmed if this occurs. Further details about possible events of default are described in the Financing Agreement, which is filed as an exhibit to this Annual Report as Exhibit 4.27.
Additionally, we announced in February 2022 that Valneva Scotland had received two grants worth up to £20 million (approximately €23.9 million) from Scottish Enterprise, Scotland’s national economic development agency, to support research and development relating to the manufacturing processes of our COVID-19 vaccine and our other vaccine candidates. Following the termination of our COVID-19 vaccine program, in May 2023 we amended the grant relating to this program to reduce the available funding by £0.7 million and to adjust how the funds will be used. The funds under these grants will be received over three years, beginning in March 2022. Valneva SE has provided a parent guarantee in connection with these grants, and if we fail to comply with the terms of the grants, Scottish Enterprise may stop payments under the grants and require repayment of the funds provided to date.
Risks Related to the Development and Commercialization of Our Product Candidates
Our future success is substantially dependent on the successful clinical development, regulatory approval, and commercialization of our product candidates in a timely manner.
Only a small percentage of products in development in our industry successfully complete regulatory authorities’ approval processes and are commercialized. The regulatory approval or marketing authorization process for our product candidates takes many years and depends on numerous factors outside of our control, including the unpredictability of future clinical trial results, and the discretion of regulatory authorities. Even if we believe that the pre-clinical or clinical data for our product candidates are promising, such data may not be sufficient to support approval by the regulatory authorities. Approval by one regulatory authority does not guarantee approval by another regulatory authority, with the same scope or at all, on the basis of the same data. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. Generally, failure to develop a vaccine that we can successfully commercialize could result in the total loss of our investment in its development and consequently could have a significant impact on shareholder value.
Even if we eventually complete clinical testing and receive approval of our product candidates, regulatory authorities may grant approval or other marketing authorization contingent on the performance of costly additional clinical trials, including post-marketing clinical trials. For example, the continued approval of IXCHIQ is conditioned upon our completion of two Phase 4 clinical trials. The timely completion of these trials will require successful coordination with regulatory agencies, who will need to approve the proposed plans, and with local partners. Additionally, execution of the Phase 4 clinical trials will require approval of IXCHIQ in Brazil. Regulatory authorities may also provide approval for a product candidate for a more limited indication or patient population than we originally request and may not approve or authorize the labeling that we believe is necessary or desirable for the successful commercialization of a product candidate, or may withdraw, vary, or suspend their approval of the product or impose restrictions on its distribution in the form of a risk evaluation and mitigation strategy, or REMS, or foreign equivalent. Any delay in obtaining, or inability to obtain, applicable regulatory approval would delay, inhibit, or prevent commercialization of that product candidate and would adversely impact our business and prospects.
Successful commercialization of our products following approval also depends on recommendations for their use from local bodies, such as the U.S. Centers for Disease Control’s Advisory Committee for Immunization Practices, or ACIP. In 2024, ACIP issued recommendations relating to vaccination against chikungunya, and the scope of the recommendation is narrower than the label for IXCHIQ in the United States. There is a high reliance on shared clinical decision-making for IXCHIQ vaccination, and decisions to vaccinate depend on high awareness of risk factors on both the traveler and healthcare practitioner sides. The ACIP recommendation could decrease the demand relative to a broader recommendation for vaccination and could affect IXCHIQ’s sales in the United States. Furthermore, the ACIP recommendations have yet to be published in the Morbidity and Mortality Weekly Report, or MMWR. As a result, our access to the retail channel for IXCHIQ has been limited, and sales in the United States in 2024 were lower than initially expected. Similar differences or delays in recommendations in other markets may also impact sales of IXCHIQ or other products that may be approved in the future.
In addition, regulations and policies may be added or revised in the EU, the U.S., or other jurisdictions, which may prevent or delay approval of our future product candidates under development on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain regulatory approvals, increase the costs of compliance, or restrict our ability to maintain any marketing authorizations we may have obtained. Furthermore, the U.S. Supreme Court’s June 2024 decision in Loper v. Bright Enterprises v. Raimondo overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statutes. The Loper decision could result in additional legal challenges to regulations and decisions issued by federal agencies, including the FDA, on which we rely. Any such legal challenges, if successful, could have a material impact on our business. Additionally, the Loper decision may result in increased regulatory uncertainty, inconsistent judicial interpretations, and other impacts to the agency rulemaking process, any of which could adversely impact our business and operations. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative or executive action, either in the United States or abroad.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may be subject to enforcement action and we may not achieve or sustain profitability.
Successful commercialization of our products depends on numerous factors, some of which may be outside of our control.
Our business is substantially dependent on our ability to commercialize our products in a timely manner in the markets in which they are approved. To successfully commercialize our products, we need to accomplish a number of tasks, including the following:
•Developing the commercial organization to support commercialization of the product or entering into partnerships for commercialization in certain geographies;
•Establishing a commercially viable pricing structure;
•Obtaining approval for coverage and adequate reimbursement from third-party and government payors, including government health administration authorities, including through the dissemination of recommendations from regulatory bodies such as ACIP; and
•Generating knowledge of and demand for our products, including through government or other large-scale contracts.
If we are unable to accomplish any of the above, either ourselves or by partnering with third parties, it could affect our ability to generate sales. For example, our COVID-19 vaccine received four marketing approvals but ultimately was not a commercial success due to lack of interest from potential government purchasers. Our partnership with Bavarian Nordic for the distribution of IXIARO and DUKORAL in Germany, one of our key markets, will terminate at the end of 2025, and our sales of these products could be impacted if we are unable to arrange for an alternative commercial approach in a timely manner. In addition, in prior periods, we have experienced shortages of IXIARO and DUKORAL relative to demand, resulting in losses of potential sales.
In addition, we supply the U.S. Department of Defense with IXIARO, our vaccine against Japanese encephalitis. Contracts with the U.S. government are subject to extensive regulations that are subject to change. The U.S government may also modify or terminate its contracts with us, without prior notice and at its convenience. Funding may be reduced or withheld as part of its annual U.S. Congressional appropriations process due to fiscal constraints, changing priorities, or other reasons. As a result, we could face increased compliance costs, withheld payments and/or reduced future business, which could have an impact on our operating results.
If we are unable to successfully commercialize our product candidates, including through contracting with third parties, we may not generate significant product revenue, which would limit the return on our investment and may prevent us from becoming profitable.
If the market opportunities for our products and product candidates are smaller than we believe they are or any approval we obtain is based on a narrower definition of the patient population, our business may suffer.
We currently focus our efforts on commercialization of our approved products for prevention of chikungunya, Japanese encephalitis, and cholera. Our estimated market opportunity, pricing estimates, and available coverage and reimbursement may differ significantly from the actual market addressable by our products and product candidates.
Further, new studies may change the estimated incidence or prevalence of the diseases we are targeting, and the number of people impacted may turn out to be lower than expected. In addition, the disease for which we are developing a product vaccine may cease to be a public health concern. Our efforts to educate physicians, patients, third-party payors, and others in the medical community on the benefits of our product candidates require significant resources and may never be successful. Such efforts may require more resources than are typically required due to the complex and distinctive nature of our product candidates.
Likewise, the potentially addressable patient population for each of our products or product candidates may be limited or may not be receptive to receiving our vaccines or vaccine candidates, and new patients may become increasingly difficult to identify or access. This may be due in part to reputational challenges that the vaccine industry is facing related to the growing momentum of the anti-vaccine movement in some regions, including in the United States, or to a distrust of certain types of vaccines, of vaccines against certain diseases or of the adjuvants contained in our vaccines. For example, there has been some negative public perception of Lyme disease vaccines as a result of the Lyme disease vaccine LYMErix, which was marketed by Smith Kline Beecham Biologicals and discontinued due to lack of market access and safety concerns, although its benefit/risk profile was confirmed by an FDA advisory committee even post-approval. If the market opportunities for our products or product candidates are smaller than we estimate, this could have an adverse effect on our business, financial condition, results of operations, and prospects. Similarly, if the estimates and forecasts of investment analysts regarding the market for one of our product candidates differ significantly from the actual addressable market, there could be an impact on Valneva’s valuation and on the trading price of our ordinary shares and ADSs.
Success in pre-clinical studies or earlier clinical trials may not be indicative of results in future clinical trials, and any ongoing, planned, or future clinical trials might not produce results sufficient for the necessary regulatory approvals.
Success in pre-clinical testing and earlier clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Pre-clinical and proof-of-concept studies and Phase 1 clinical trials for vaccines are primarily designed to evaluate safety and immunogenicity. Our ongoing clinical trials might not produce data consistent with those of prior trials. There can be significant variability in safety or efficacy results between different trials of the same product candidate due to numerous factors, including changes in trial protocols, differences in composition of the trial participants, adherence to the dosing regimen and other trial protocols, and the rate of dropout among clinical trial participants. Our product candidates may fail to show the desired characteristics in clinical development sufficient to obtain or maintain regulatory approval, despite positive results in pre-clinical studies, successful advancement through earlier clinical trials, or initial data that we may publish, which may materially change as clinical trials progress. A number of companies in the biotechnology and product development industry have suffered significant setbacks in advanced clinical trials, even after experiencing promising results in early animal and human testing.
A trial design that is considered appropriate for regulatory approval includes an adequate sample size with appropriate statistical power, as well as proper control of bias, to allow a meaningful interpretation of the results. The required sample size also depends on the type of vaccine candidate, type of clinical trial design, and regulatory pathway agreed with regulatory agencies. In a late-phase clinical trial with multiple end points, we may not achieve a statistically significant result, even with a large sample size.
In addition, the design of a clinical trial can determine whether its results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced. As an organization, we may be unable to design and execute a clinical trial to support regulatory approval, including conditional approval or emergency use authorization for any given current or future product candidate. Data obtained from pre-clinical and clinical activities are subject to varying interpretations, which may delay, limit, or prevent regulatory approval. In addition, we may experience regulatory delays or rejections as a result of many factors, including changes in regulatory policy or results of audits of clinical trial partners by regulatory authorities during the period of our product candidate development.
Clinical product development is uncertain and time consuming, and we may incur additional costs or encounter substantial delays or difficulties in our clinical trials.
Clinical testing is expensive, is difficult to design and implement, can take many years to complete, and is uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. If we or our partners experience delays in the commencement or completion of our clinical trials, or if we terminate a clinical trial prior to completion, the commercial prospects of our product candidates could be negatively impacted, and our ability to generate revenue from our product candidates may be delayed. Any delays of clinical trials would lead to delays in the regulatory approval process, increase development costs, and could lead to a negative perception of Valneva or the product candidate.
We may experience numerous unforeseen events prior to, during, or as a result of, clinical trials that could delay or prevent our ability to receive marketing approval or commercialize our product candidates, including the following:
•We may be unable to generate sufficient pre-clinical, toxicology, or other in vivo or in vitro data to support the initiation of clinical trials.
•We may struggle to reach a consensus with regulatory authorities on the design or implementation of our clinical trials, or any modification thereto.
•Regulators or institutional review boards and ethics committees may prevent us or our investigators from commencing a clinical trial or conducting a clinical trial at a prospective trial site.
•We may experience delays in reaching agreement on acceptable terms with prospective clinical research organizations, or contract research organizations, or CROs, and clinical trial sites.
•We or our manufacturing partners may experience delays or fail to comply with current Good Clinical Practice, or GCP, good manufacturing practices, or cGMP, or other applicable regulations.
•The number of subjects required for clinical trials of our product candidates may be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate, participants may drop out of these clinical trials at a higher rate than we anticipate or fail to return for follow-up, or we may fail to recruit suitable subjects to participate in a trial.
•We may have difficulty collaborating with investigators.
•We or our CROs, partners, other third parties may fail to adhere to clinical trial requirements.
•Clinical trials for our product candidates may have inconclusive results.
•Regulatory authorities may impose a clinical hold on our trials, as a result of a serious adverse event or concerns with a class of product candidates, after an inspection of our clinical trial operations, trial sites or manufacturing facilities, after review of an investigational new drug application, or IND, or IND amendment, after an application for the authorization of a clinical trial or related amendment, or equivalent application or amendment, or after the finding that the investigational protocol or plan is clearly deficient to meet its stated objectives.
•Serious adverse events associated with the product candidate may occur that regulatory authorities view as outweighing the potential benefits of the product candidate.
•We may need to amend or submit new clinical protocols due to changes in regulatory requirements or guidance.
•We may need to run new or additional trials due to changes in the standard of care on which a clinical development plan was based.
•We may decide or be required by regulators to conduct additional clinical trials or abandon product development programs.
•We may face disruptions caused by man-made or natural disasters, public health pandemics or epidemics, global instability, or other business interruptions.
For example, we have experienced a delay in receiving all the required regulatory approvals to initiate the Phase 2 pediatric study for S4V2, the vaccine candidate for shigellosis that we have in-licensed from LimmaTech Technologies. Likewise, following Pfizer’s decision in February 2023 to discontinue approximately half of the participants then enrolled in the Phase 3 trial of our Lyme disease vaccine candidate as a result of violations of GCP at certain trial sites run by a third party, the target for submission of a BLA shifted from 2025 to 2026.
In addition, if we make manufacturing or formulation changes to our product candidates, we may need to conduct additional testing to bridge our modified product candidate to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates, if approved, or allow our competitors to bring competing products to market before we do, which could impair our ability to successfully commercialize our product candidates.
Our product development costs will also increase if we experience delays in testing or obtaining marketing approvals. The risk of increased development costs is more pronounced for our Lyme disease vaccine candidate given that it is currently in Phase 3 clinical trials.
We depend on strategic collaborations with partners, which may require us to relinquish rights to and control over the development and commercialization of our product candidates or to make payments upon achievement of milestone events.
We have in the past and may in the future enter into agreements or engage in strategic collaborations in order to advance our business strategy. For example, in April 2020 we entered into a research collaboration and license agreement with Pfizer in connection with VLA15, our Lyme disease vaccine candidate. Pursuant to this agreement, Pfizer is leading late-stage development of the vaccine candidate, including conducting the ongoing Phase 3 clinical trial, and, if the Phase 3 data are positive, will have sole control over the timing and nature of its commercialization, which will have a significant impact on Valneva’s financial condition after 2025. In addition, in July 2024, we entered into a development, collaboration, license and commercialization agreement with LimmaTech Biologics in connection with S4V2, their shigellosis vaccine candidate, pursuant to which we have assumed development of the product candidate and will be responsible for commercializing the product. Under this agreement, we will be required to make milestone payments to LimmaTech Biologics at various stages of product development, regardless of our ability to ultimately successfully commercialize the product.
In addition, we may in the future explore strategic collaborations, which may never materialize or may require that we relinquish rights to and control over the development and commercialization of our product candidates. We cannot predict what form such strategic collaborations or licenses might take in the future. If we do seek additional strategic collaborations, we are likely to face significant competition in seeking appropriate strategic collaborators, and strategic collaborations and licenses can be complicated and time-consuming to negotiate and document. We may not be able to negotiate strategic collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional strategic collaborations or licenses because of the numerous risks and uncertainties associated with establishing them. Any delays in entering into new strategic collaborations or licenses that we have deemed important for the development and commercialization of any of our product candidates could delay or limit those processes in certain geographies for certain indications, which would harm our business prospects, financial condition, and results of operations.
Our current and future collaborations and licenses could subject us to a number of risks, including the following:
•Collaborators and partners have significant discretion in determining the efforts and amount and timing of resources that they devote to the development or commercialization of our product candidates, and their priorities may differ from ours.
•Business combinations or significant changes in a strategic collaborator’s business strategy may adversely affect a strategic collaborator’s willingness or ability to devote resources and complete its obligations under any arrangement.
•A collaborator or partner may not pursue development and commercialization of our products or product candidates or may elect not to continue or renew development or commercialization of our products or product candidates based on clinical trial results or delays, changes in their strategic focus, availability of funding, or other external factors, such as a business combination that diverts resources or creates competing priorities.
•Disputes may arise between us and our strategic collaborators that result in the delay or termination of the research, development, or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management’s attention and consumes resources.
•Strategic collaborators may select indications or design clinical trials in a way that may be less successful than if we were doing so.
•Strategic collaborators may not pursue further development and commercialization of products resulting from the strategic collaboration arrangement or may elect to discontinue research and development programs.
•Strategic collaborators may delay or encounter unanticipated problems with clinical trials, provide insufficient funding, terminate a clinical trial or abandon a product candidate, repeat or conduct new clinical trials, or require a new version of a product candidate for clinical testing.
•Strategic collaborators may not commit adequate resources to the marketing and distribution of our product candidates, limiting our potential revenue from these products.
•We may be required to undertake the expenditure of substantial operational, financial, and management resources, including expenditure beyond the amount originally agreed.
•We may not have the right to control the preparation, filing, prosecution, and maintenance of patents and patent applications covering the technology that we license, and we cannot always be certain that these patents and patent applications will be prepared, filed, prosecuted, and maintained in a manner consistent with the best interests of our business.
•We may be required to issue equity securities that would dilute our shareholders’ percentage ownership of our company.
•We may acquire and develop product candidates that fail to receive market approval or gain commercial approval.
•We may be required to assume substantial actual or contingent liabilities.
•Strategic collaborators may experience financial difficulties.
•Strategic collaborators may not properly maintain, enforce, or defend our intellectual property rights or may use our proprietary information in a manner that could jeopardize or invalidate our proprietary information or expose us to potential litigation.
•Strategic collaborators could decide to move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors.
•Strategic collaborators could terminate the arrangement or allow it to expire, which would delay the development and may increase the cost of developing our product candidates.
Furthermore, license agreements we enter into in the future may not provide exclusive rights to use intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our technology and products. As a result, we may not be able to prevent competitors from developing and commercializing competitive products in territories included in all of our licenses.
Enrollment and retention of subjects in clinical trials is an expensive and time-consuming process and could be delayed, made more difficult, or rendered impossible by multiple factors outside our control.
Identifying and qualifying subjects in a timely manner to participate in our clinical trials is critical to our success. We may encounter difficulties in enrolling subjects in our clinical trials, and such difficulties may delay or prevent development, approval, and commercialization of our product candidates. Even once enrolled, we may be unable to retain a sufficient number of subjects to complete any of our trials. Subject enrollment and retention in clinical trials depends on many factors, including the nature of the trial protocol, the existing body of safety and efficacy data, the number and nature of competing vaccines already in the market, ongoing clinical trials of competing vaccine candidates for the same indication, the proximity of subjects to clinical sites, and the eligibility criteria for the trial. In addition, enrollment and retention of subjects in clinical trials could be disrupted by man-made or natural disasters, public health events such as pandemics, or other business interruptions. In addition, public perception of a specific clinical trial or of vaccine safety issues may adversely influence willingness of subjects to participate in clinical trials. Additionally, granted emergency use authorizations, or EUAs, may saturate the marketplace prior to our advancement or commercialization, as allowed, for any of the vaccine areas in which we are developing products.
Any negative results we or other study sponsors may report in clinical trials of our product candidates may make it difficult or impossible to recruit and retain subjects in other clinical trials of that same product candidate. Delays or failures in planned subject enrollment or retention may result in increased costs, program delays or both, which could have a harmful effect on our ability to develop our product candidates or could render further development impossible. In addition, we may rely on CROs and clinical trial sites to ensure proper and timely conduct of our current and future clinical trials and, while we enter into agreements governing their services, we will be limited in our ability to ensure their actual performance, including adherence to GCP, and any issues with their performance could have substantial negative effects on our clinical development programs.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences following any potential marketing approval.
During the conduct of clinical trials, subjects report changes in their health, including illnesses, injuries, and discomforts, to their physician. Often, it is not possible to determine whether or not the product candidate being studied caused these conditions. If subjects in our clinical trials experience any side effects, and if regulatory authorities determine that such side effects are being caused by our vaccine candidates, they may require additional testing to confirm these determinations.
In addition, it is possible that as we test our product candidates in larger, longer, and more extensive clinical trials, or as use of these product candidates becomes more widespread if they receive regulatory approval, illnesses, injuries, discomforts, and other adverse events that were not observed in earlier trials, as well as conditions that did not occur or went undetected in previous trials, will be reported by subjects. Many times, side effects are only detectable after investigational products are tested in large-scale pivotal trials or, in some cases, after they are made available to patients on a commercial scale after approval. For example, in February 2025, the CDC provided public notice that it was investigating five hospitalizations for cardiac (heart) or neurologic (nervous system) events following vaccination with IXCHIQ among individuals who were 65 years and older. The CDC intends to discuss the findings from its investigation with ACIP. If additional clinical experience indicates that any of our product candidates have side effects or cause serious or life-threatening side effects, the development of the product candidate may fail or be delayed, or, if the product candidate has received regulatory approval, such approval may be revoked or limited or require labeling updates.
In addition, we are currently conducting a technology transfer of the drug product manufacturing process with strategic partners in Brazil and India. We intend to provide our partners with the drug substance for our chikungunya vaccine, while our partners will complete manufacturing and be responsible for seeking and maintaining regulatory approval of the vaccine. While we are not responsible for the clinical trials conducted by our partners, we have input on the clinical design and must ensure that all relevant pharmacovigilance and safety monitoring is conducted according to ICH guidelines. If the results of these trials are not favorable, or if the vaccine candidate causes serious side-effects not seen in our own clinical trials, this may have reputational consequences for our vaccine, may require us to expend our resources to establish that such side-effects were not caused by our drug substance, may cause regulators in countries where our vaccine has been approved to raise additional questions or require further post-approval trials, which would have a material impact on our business.
The development of additional product candidates is risky and uncertain, and we might not be able to successfully develop additional vaccines for other diseases.
A core element of our business strategy is to expand our product pipeline. In August 2024 we acquired the exclusive worldwide license for LimmaTech Biologic’s S4V2 Shigella vaccine candidate, which is currently in Phase 2 development. We also continue to evaluate the possibilities for the other clinical and preclinical candidates in our pipeline as well as the possibilities for acquiring candidates from third parties or partnering with third parties to co-develop candidates. Efforts to identify, acquire or in-license, and then develop product candidates require substantial technical, financial, and human resources, whether or not any product candidates are ultimately identified, and there are a limited number of third-party programs to evaluate for this purpose. Our efforts may initially show promise in identifying potential product candidates yet fail to yield product candidates for clinical development, approved products, or commercial revenue for many reasons, including the following:
•Our methodology may not be successful in identifying potential product candidates.
•Competitors may develop alternatives that render any product candidates we develop obsolete.
•A product candidate may be shown to have harmful side effects or other characteristics that indicate it is unlikely to be effective or otherwise fail to meet applicable regulatory criteria, or limit its commercial potential.
•A disease we may target may cease to be a public health concern.
•A product candidate may not be capable of being produced in commercial quantities at an acceptable cost, or at all.
•A product candidate may not be accepted as safe and effective by physicians, patients, the medical community, or third-party payors.
We have limited financial, manufacturing, and management resources and, as a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater market potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing, or other royalty arrangements in circumstances under which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. In addition, we may not be successful in replicating our approach to development for other disease indications. If we are unsuccessful in identifying and developing additional product candidates or are unable to do so, our business and shareholder value may be harmed.
Our current products are, and any future product candidates for which we obtain regulatory approval will be, subject to ongoing regulatory oversight.
Our currently approved products, and any future products we commercialize, if any, are subject to ongoing regulatory requirements for manufacturing, labeling, packaging, storage, advertising, promotion, sampling, record keeping, applicable product tracking and tracing requirements, and submission of safety and other post-market information. Any regulatory approvals that we receive for our product candidates may also be subject to a REMS or foreign equivalents or contain requirements for potentially costly post-marketing testing, including Phase 4 trials (such as those required for IXCHIQ), and surveillance to monitor the quality, safety, and efficacy of the product. Such regulatory requirements may differ from country to country depending on where we receive regulatory approval. Regulators may also subsequently limit or revise the indicated uses for which the product was originally marketed, which could significantly impact our sales. For example, the agency supervising pharmaceutical products in Canada, which is our principal market for DUKORAL, contacted us in July 2021 to request further information in support of DUKORAL’s indications and labeling. While this matter has been resolved, if DUKORAL’s indications or labeling were to change significantly in Canada or elsewhere in the future, this could have a significant negative impact on our sales which in turn could result in the product no longer being economically viable.
In addition, biopharmaceutical manufacturers and their facilities are subject to ongoing review and periodic inspections by the competent authorities of individual EEA countries, FDA, or other comparable regulatory authorities for compliance with applicable regulatory requirements, including with cGMP requirements and with commitments made in the application for regulatory approval. If we, or a regulatory authority, discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, or if a regulatory authority disagrees with the promotion, marketing, or labeling of that product, a regulatory authority may impose restrictions relative to that product, the manufacturing facility, or us. These restrictions could include requesting a recall or requiring withdrawal of the product from the market, suspension of manufacturing, or suspension, variation or withdrawal of the related approval.
If we fail or if a third party fails to comply with applicable regulatory requirements for our products or any of our product candidates that receive regulatory approval in the future, a regulatory authority may:
•issue an untitled letter or warning letter asserting that we are in violation of the law;
•seek an injunction or impose administrative, civil, or criminal penalties or monetary fines;
•suspend, vary, or withdraw regulatory approval;
•suspend or vary any ongoing clinical trials;
•refuse to approve a pending BLA or comparable foreign application for regulatory approval or any supplements thereto submitted by us or our partners;
•restrict the labeling, distribution, marketing, or manufacturing of the product or clinical trial material;
•seize or detain the product or otherwise require the withdrawal of the product from the market or product recalls;
•require conduct of additional post-marketing studies or clinical trials;
•refuse to permit the import or export of product candidates; or
•refuse to allow us to enter into supply contracts, including government contracts.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and harm our business, financial condition, results of operations, and prospects.
Regulatory authorities’ policies may change, and additional government regulations may be enacted that could prevent, limit, or delay regulatory approval of our product candidates. In addition, we cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative or executive action in any geography where we market a product.
It is difficult to predict how these executive actions, including any executive orders, will be implemented and the extent to which they will affect the regulatory authorities’ ability to exercise their authority. If these executive actions impose constraints on the regulatory authorities’ ability to engage in oversight and implementation activities in the normal course, our business, financial condition, results of operations, and prospects may be negatively impacted.
We may be liable if regulatory enforcement agencies determine we engaged in the off-label promotion of our products, pre-approval promotion of our product candidates, or dissemination of false or misleading labeling, advertising, or promotional materials.
Our promotional activities, materials, and training methods are strictly regulated, with prohibitions on marketing claims that promote the off-label use of our products or that omit material facts or make false or misleading statements about the safety or efficacy of our products. Pre-approval promotion of product candidates is also prohibited. However, in the United States and certain other countries, the FDA or the equivalent regulatory authority does not restrict or regulate a physician’s choice of treatment within the practice of medicine. Therefore, physicians may use our products off-label if deemed appropriate in their independent medical judgment.
A regulatory authority could disagree with the manner in which we advertise and promote our products or communicate about our product candidates. It could conclude that a claim is misleading if it determines that there are inadequate non-clinical and/or clinical data supporting the claim, or if a claim fails to reveal material facts about the safety or efficacy of our products, or claim that we have engaged in pre-approval promotion of a product candidate.
If a regulatory authority in the United States or certain other countries determines that our promotional activities or advertising materials promote an off-label use or make false or misleading claims, or that our communications about product candidates constitute pre-approval promotion, it could request that we modify our promotional materials, training content, or other communications or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fines, and criminal penalties. In the case of a claim of pre-approval promotion, these consequences could result in a delay in the review of any dossiers we have submitted for regulatory review and approval. Equivalent limitations and penalties are provided in the EU, both at the EU level and at the national level in individual EU Member States.
In the United States, violations of the Federal Food, Drug, and Cosmetic Act, or FDCA, may also lead to investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws, which may lead to costly penalties and may adversely impact our business. Recent court decisions in the United States have impacted FDA’s enforcement activity regarding off-label promotion in light of First Amendment considerations such that companies may share truthful and not misleading information that is otherwise consistent with a product’s FDA approved labeling; however, there are still significant risks in this area, in part due to the potential for False Claims Act exposure.
In addition, the off-label use of our products may increase the risk of product liability claims. Product liability claims are expensive to defend and could result in substantial damage awards against us and harm our reputation.
If we are unable to maintain and expand our sales and marketing capabilities on our own or with others, we may not be successful in increasing sales of our current products and commercializing future products, if approved.
To increase sales of our current products and third-party products pursuant to distribution agreements, as well as successfully commercialize any product candidate that may result from our development programs, we will need to maintain and continue to build out our sales and marketing capabilities, either on our own or with others. The continued development of our sales and marketing team will be expensive and time-consuming and could delay any product launch. We compete with many companies that currently have extensive, experienced, and well-funded marketing and sales operations to recruit, hire, train, and retain marketing and sales personnel, and will have to compete with those companies to recruit, hire, train, and retain any of our own marketing and sales personnel. If we are unable to sustain and expand our sales and marketing team, we may be unable to compete successfully against these more established companies. Alternatively, if we choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems, we will be required to negotiate and enter into arrangements with such third parties relating to the proposed collaboration. If we are unable to enter into such arrangements when needed, on acceptable terms, or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations.
Our future growth depends, in part, on our ability to penetrate multiple markets, in which we would be subject to additional regulatory burdens and other risks and uncertainties.
Our future profitability will depend, in part, on our ability to continue to commercialize our products and, if approved, our product candidates in markets in Europe, the United States, and other countries where we maintain commercialization rights. As we continue to commercialize our products and begin to commercialize our product candidates, if approved, in multiple markets, we are subject to additional risks and uncertainties, including:
•foreign currency exchange rate fluctuations and currency controls;
•economic weakness, including inflation and rising interest rates, or political instability in particular economies and markets;
•potentially adverse and/or unexpected tax consequences, including penalties due to the failure of tax planning or due to the challenge by tax authorities on the basis of transfer pricing and liabilities imposed from inconsistent enforcement;
•the burden of complying with complex and changing regulatory, tax, accounting. and legal requirements, many of which vary between countries;
•different medical practices and customs in multiple countries affecting acceptance of drugs in the marketplace;
•differing payor reimbursement regimes, governmental payors. or patient self-pay systems and price controls;
•tariffs, trade barriers, import or export licensing requirements. or other restrictive actions;
•compliance with tax, employment, immigration. and labor laws for employees living or traveling abroad;
•workforce uncertainty in countries where labor unrest is common;
•reduction or loss of protection of intellectual property rights in some foreign countries, and related prevalence of generic alternatives to therapeutics; and
•becoming subject to the different, complex. and changing laws, regulations, and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties, and regulations.
These and other risks associated with international operations may adversely affect our ability to attain or maintain profitable operations. Future sales of our products or our product candidates, if they are approved, will be dependent on purchasing decisions of and recommendations from government health administration authorities. As a result of adverse conditions affecting the global economy and credit and financial markets, including disruptions due to political instability, armed conflict, wars, or otherwise, these organizations may defer purchases or may be unable to satisfy their purchasing or reimbursement obligations, which may affect milestone payments or royalties for our products or any of our product candidates that are approved for commercialization in the future.
Our failure to obtain marketing approval in jurisdictions other than the United States and the European Union would prevent our product candidates from being marketed in these other jurisdictions, and any approval we are granted for our product candidates in the United States and the European Union would not assure approval of product candidates in other jurisdictions.
In order to market and sell our product candidates in jurisdictions other than the United States and the European Union, we must obtain separate marketing approvals in such jurisdictions and comply with numerous and varying regulatory requirements. The approval process varies among countries and can involve additional testing aside from that which is required to obtain such approval in the United States and the European Union. The time required to obtain approval may differ from that required to obtain approval from the FDA or regulatory authorities in the European Union. The regulatory approval process outside the United States and the European Union generally includes all of the risks associated with obtaining FDA approval or approvals from regulatory authorities in the European Union. In addition, some countries outside the United States and the European Union require approval of the sales price of a product before it can be marketed. In many countries, separate procedures must be followed to obtain reimbursement, and a product may not be approved for sale in the country until it is also approved for reimbursement. We may not obtain marketing, pricing, or reimbursement approvals outside the United States and the European Union on a timely basis, if at all. Approval by the FDA or regulatory authorities in the European Union does not ensure approval, with the same scope or at all, by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States and the European Union does not ensure similar approval by regulatory authorities in other countries or jurisdictions or by the FDA or regulatory authorities in the European Union. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. Marketing approvals in countries outside the United States and the European Union do not ensure pricing approvals in those countries or in any other countries where such approvals are required, and marketing approvals and pricing approvals do not ensure that reimbursement will be obtained.
Product liability lawsuits against us could divert our resources, cause us to incur substantial liabilities, damage our reputation, and limit commercialization of any product candidate that we may develop as well as continued commercialization of our current products.
We face an inherent risk of product liability exposure related to the sale and use of our products and the testing of our product candidates in clinical trials. Side effects of, or manufacturing defects in, products that we develop could result in injury or even death. For example, our liability could be sought after by subjects participating in the clinical trials in the context of the development of the vaccine candidates tested and unexpected side effects resulting from the administration of these products. Once a product is approved for sale and commercialized, the likelihood of product liability lawsuits increases. Criminal or civil proceedings might be filed against us by subjects, regulatory authorities, biopharmaceutical companies, and any other third party using or marketing our products. These actions could include claims resulting from acts by our partners, licensees, and subcontractors over which we have little or no control. These lawsuits may divert our management from pursuing our business strategy, result in withdrawal of clinical trial participants, result in decreased demand for our products, and may be costly and time-consuming to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities, may be forced to limit or forgo further development or commercialization of the affected products, and may suffer damage to our reputation.
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our products or our product candidates.
To date, we have obtained product liability insurance with a coverage amount of €40 million per claim, up to 1.5 times per year. Our product liability insurance will need to be adjusted in connection with the commercial sales of our products and our product candidates or our strategic partnerships, and may be unavailable in meaningful amounts or at a reasonable cost. Our insurance coverage may not be sufficient to cover any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial. A successful product liability claim, or series of claims, brought against us could cause our share price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
In addition, product liability claims relating to our own or similar products may result in increases in insurance premiums or deductibles that may make insurance coverage more costly or prohibitively expensive. Additionally, insurance providers may refuse to provide coverage for a category of related products if one such product is removed from the market for safety reasons. We cannot guarantee that we will be able to maintain product liability insurance coverage for all of our products. If we are the subject of a successful product liability claim that exceeds the limits of any insurance coverage we obtain, we would incur substantial charges that would adversely affect our earnings and require the commitment of capital resources that might otherwise be available for the development and commercial launch of our product programs. Should any of these risks materialize, this could have a material adverse effect on our business, prospects, financial condition, and results of operations.
Risks Related to Competition
We operate in a highly competitive industry, and our competitors may have significantly greater resources and experience, which may negatively impact our commercial opportunities.
The biotechnology and pharmaceutical industries are subject to intense competition and rapid and significant technological change. We have many potential competitors, including major pharmaceutical companies, specialized biotechnology firms, academic institutions, government agencies, and private and public research institutions. Many of our competitors have significantly greater financial and technical resources, as well as experience and expertise across a wide range of capabilities, which are required in our industry.
Principal competitive factors in our industry include the following:
•the quality and breadth of an organization’s technology,
•management of the organization and the execution of the organization’s strategy,
•the skill and experience of an organization’s employees and its ability to recruit and retain skilled and experienced employees,
•an organization’s intellectual property portfolio,
•the capabilities of an organization throughout the product pipeline, from target identification and validation to discovery and development to manufacturing and marketing, and
•the availability of substantial capital resources to fund discovery, development, and commercialization activities.
Large and more established companies compete in the general vaccine market. In particular, these companies may have greater experience and expertise in the following areas:
•securing government contracts and grants to support their research and development efforts,
•conducting testing and clinical trials,
•obtaining regulatory approvals to market products,
•manufacturing such products on a broad scale, and
•marketing approved products.
Smaller or early-stage companies and research institutions also may prove to be significant competitors, particularly through collaborative arrangements with large and established pharmaceutical companies. As these companies and research institutions develop their technologies, they may develop proprietary positions, which may prevent or limit our product development and commercialization efforts. If any of our competitors succeed in obtaining approval from regulatory authorities for their products sooner than we do or for products that are more effective or less costly than ours, or if the scope of approval for a competing product is broader than an approval granted for our product, our commercial opportunity could be significantly reduced. Mergers and acquisitions, including of specific assets, in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors and in changes to the competitive landscape in regions where we market and distribute our products.
Some of our competitors have developed or are developing vaccines for the same diseases that we are targeting, and we may not be able to compete effectively or may lose market share to these products.
We are aware of companies with competing products or product candidates for Japanese encephalitis, cholera (one of which is currently available in the U.S. and a limited number of European markets) and chikungunya (including one that was recently approved in the U.S. and Europe). If and as these vaccines become available in the markets in which we compete, sales of our vaccines may be adversely affected.
We may not be successful in gaining significant market share for any approved product candidate and may not continue to be successful maintaining or gaining market share for our currently marketed products if competing products enter the market. Our technologies and vaccines also may be rendered obsolete or non-competitive because of products introduced by our competitors to the marketplace more rapidly and at a lower cost.
Risks Related to Our Reliance on Third Parties
We are dependent on single-source suppliers for some of the components and materials used in our products.
In certain cases, we rely on single suppliers for all of our requirements for some of our materials or components. In most cases we do not have long term contracts with these suppliers, and even in the cases where we do the contracts include significant qualifications that would make it extremely difficult for us to force the supplier to provide us with their services, materials, or components should they choose not to do so. We are therefore subject to the risk that these third-party suppliers will not be able or willing to continue to provide us with materials and components that meet our specifications, quality standards, and delivery schedules. Factors that could impact our suppliers’ willingness and ability to continue to provide us with the required materials and components include disruption at or affecting our suppliers’ facilities, such as work stoppages or natural disasters, adverse weather or other conditions that affect their supply, the financial condition of our suppliers, and deterioration in our relationships with these suppliers. In addition, we cannot be sure that we will be able to obtain these materials and components on satisfactory terms. Any increase in material and component costs could reduce our sales and harm our gross margins. In addition, any loss of a material supplier may permanently cause a change in one or more of our products that may not be accepted by our customers or that may cause us to eliminate that product altogether.
For example, we rely on a single-source supplier for fetal bovine serum, a critical and scarce raw material which is only available from our supplier and is used in the manufacturing of IXIARO. We also rely on a single-source supplier for the adjuvant contained in certain vaccine candidates. A loss of the supplier or any shortages of these or other materials for which we rely on a single supplier could adversely affect our ability to manufacture our products and significantly raise our cost of production.
We have not qualified secondary sources for all materials or components that we source through a single supplier, and we cannot assure investors that the qualification of a secondary supplier would prevent future supply issues. Disruption in the supply of materials or components would impair our ability to sell our products and meet customer demand and also could delay the launch of new products, any of which could harm our business and results of operations. If we were to have to change suppliers, the new supplier may not be able to provide us materials or components in a timely manner and in adequate quantities that are consistent with our quality standards and on satisfactory pricing terms. In addition, alternative sources of supply may not be available for materials that are scarce or components for which there are a limited number of suppliers.
If we experience shortages in the supply of our marketed products, our results could be materially impacted.
The marketing and distribution of our products and the late-stage development of our product candidates may depend on our ability to establish and maintain collaborations with biopharmaceutical companies.
We rely on collaboration, research, and license agreements with other biopharmaceutical companies to assist us in the marketing and distribution of our products and the development of product candidates and the financing of their development. For example, in prior periods, our distribution agreements with Bavarian Nordic have been important to our business, both for the sale of our own products IXIARO and DUKORAL and for the revenue we have earned from our distribution of two of Bavarian Nordic’s vaccines. As a result of Bavarian Nordic’s acquisition of another cholera vaccine, we amended our agreements with effect in May 2023. The agreements relating to our distribution of Bavarian Nordic’s rabies vaccine in Canada and the United Kingdom terminated on December 31, 2024, and the remaining distribution agreements between Valneva and Bavarian Nordic will terminate on December 31, 2025. These remaining distribution agreements provide for Bavarian Nordic’s distribution of our products in Germany and Switzerland and for our distribution of Bavarian Nordic’s RABIPUR and ENCEPUR vaccines in France, Austria, and the Benelux region. We are now making plans to ensure continued distribution of our products in Germany and Switzerland, but the termination of these distribution agreements and the transition to alternate arrangements could have an impact on our sales in these countries.
As we continue to commercialize our products and identify new product candidates, we will determine the appropriate strategy for development and marketing, which may result in the need to establish additional collaborations with other biopharmaceutical companies. We may also enter into agreements with institutions and universities to participate in our other research programs and to share intellectual property rights.
We may fail to maintain or find collaboration partners and to sign new agreements for our other product candidates and programs. The competition for partners is intense, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may not be able to maintain a collaboration if, for example, development or approval of a product candidate is delayed, actual or expected sales of an approved product candidate do not meet expectations, or the collaborator terminates the collaboration, including because of changes in the collaborator’s business. Any collaboration, or other strategic transaction, may also require us to incur non-recurring or other charges, increase our near- and long-term expenditures, and pose significant integration or implementation challenges or disrupt our management or business. However, the failure to explore or enter into a collaboration or other strategic cooperation might also cause us to forego beneficial opportunities to develop and commercialize our product candidates.
We rely on third parties to supply key materials used in our research and development, to manufacture our products and product candidates, to provide services to us, and to assist with clinical trials.
We make considerable use of third-party suppliers for the key materials used in our business, such as the fetal bovine serum used in IXIARO and the adjuvant used in certain vaccine candidates. We also rely upon several, and in the future may rely on additional, third-party contract manufacturing organizations, or CMOs, for the manufacture and supply of components and substances for all of the product candidates we are developing. For example, we have outsourced an important step in the manufacturing of IXCHIQ to a third party, and another third party performs the filling process for IXIARO and the filling of IXCHIQ diluent.
In the biopharmaceutical industry, supplier changes require lengthy validation and regulatory approval processes. A loss of any CMO or component supplier and delay in establishing a replacement could delay our clinical development and regulatory approval process or interrupt supply.
The failure of third-party suppliers to comply with regulatory standards could result in the imposition of sanctions on us. These sanctions could include fines, injunctions, civil penalties, refusal by regulatory organizations to grant approval to conduct clinical trials or marketing authorization for our products, delays, suspension, variations or withdrawal of approvals, license revocation, seizure or recalls of our products, operating restrictions, and legal proceedings. Furthermore, the presence of non-conformities, as may be detected in regulatory toxicology studies, could result in delays in the development of one or more of our product candidates or in the supply of a commercial product and would require further tests to be financed. Although we are involved in establishing the protocols for the production of these materials, we do not control all the stages of production and cannot guarantee that the third parties will fulfil their contractual and regulatory obligations or that we will be informed in a timely manner of any non-conformities or other failure to comply with obligations. In particular, a partner’s failure to comply with protocols or regulatory constraints, or repeated delays by a partner, could compromise the development or manufacturing of our products. Such events could also inflate our product development or manufacturing costs.
We also use third parties to provide certain services such as scientific, medical, or strategic consultancy services. These service providers are generally selected for their specific expertise, as is the case with the academic partners with whom we collaborate. We face intense competition to build and maintain such a network under acceptable terms. Such external collaborators may terminate their involvement at any time, and we can exert only limited control over their activities. We may not be able to obtain the intellectual property rights to the product candidates or technologies developed under collaboration, research, and license agreements under acceptable terms or at all. Moreover, our scientific collaborators may assert intellectual property rights or other rights beyond the terms of their engagement.
Finally, we use third parties to assist with conducting clinical trials. All clinical trials are subject to strict regulations and quality standards. Should any of these risks materialize, as in the case of the Phase 3 trial of VLA15 involving GCP violations by a third party engaged by Pfizer to conduct certain clinical trial sites, this could have a material adverse effect on our business.
Risks Related to Our Business Operations, Employee Matters and Managing Growth
We are highly dependent on our key personnel, and if we are not able to retain these members of our management team or recruit and retain additional management, clinical, and scientific personnel, our business will be harmed.
We are highly dependent on our management, scientific, and medical personnel, particularly our Chief Executive Officer Thomas Lingelbach, who we heavily rely on for a variety of matters. Our key personnel may currently terminate their employment with us at any time. The loss of the services of any of these persons could impede the achievement of our research, development, and commercialization objectives. Additionally, we do not currently maintain “key person” life insurance on the lives of our executives or other employees.
Recruiting and retaining other senior executives, qualified scientific and clinical personnel, and commercialization, manufacturing and sales and marketing personnel will be critical to our success. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval of, and commercialize our product candidates. Competition to hire from this limited pool is intense, and we may be unable to hire, train, retain, or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies, as well as universities and research institutions, for similar personnel. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high-quality personnel, our ability to pursue our growth strategy will be limited.
We also rely, and for the foreseeable future will continue to rely, in part, on certain independent organizations, advisors, and consultants to provide certain services. Such assistance might not continue to be available to us on a timely or cost-effective basis when needed, and we might not find qualified replacements. In addition, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed, or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business.
Our future performance will also depend, in part, on our ability to successfully integrate newly hired staff, particularly at the senior level. Failure to do so could result in inefficiencies in the development and commercialization of our product candidates and other aspects of our business, which could negatively impact our results of operations.
We may encounter difficulties in managing our growth, which could disrupt our operations.
Our strategy involves continuing to grow our business organically. However, we may also grow through selective acquisitions of complementary products and technologies, or of companies with such assets, although no such plan is currently contemplated. As our development progresses, we expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of research, drug development, regulatory affairs, and sales, marketing and distribution for our approved products.
To manage our anticipated future growth, we must continue to implement and improve our managerial, operational, and financial systems, expand our facilities, and recruit and train additional qualified personnel. Due to our limited financial resources and the extent of our anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel.
Our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing organic or inorganic growth. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure and give rise to operational errors, loss of business opportunities, loss of employees, and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of existing and additional product candidates. If our management is unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced, and we may not be able to implement our business strategy.
If we were to acquire assets or companies, the success of such an acquisition would depend on our capacity to carry out such acquisitions and to integrate such assets or companies into our existing operations. The implementation of such a strategy could impose significant constraints, including:
•human resources: recruiting, integrating, training, managing, motivating, and retaining a growing number of employees;
•financial and management system resources: identification and management of appropriate financing and management of our financial reporting systems; and
•infrastructure: expansion or transfer of our laboratories or the development of our information technology system.
In addition, an acquisition could result in shareholder litigation, which could be costly and time consuming and divert management’s attention and resources. For example, following the merger between Vivalis SA and Intercell AG in 2013, certain former Intercell shareholders initiated legal proceedings to request a revision of either the cash compensation paid to departing shareholders or the exchange ratio between Intercell and Valneva shares used for the non-departing shareholders who received Valneva shares in the merger. On February 8, 2021, the judicial committee in charge of these proceedings appointed an expert and requested that he give an opinion on the exchange ratio applied to this latter group. On October 6, 2021, we received the expert’s opinion. With respect to the exchange ratio, the expert confirmed the prior calculation used but also recommended the calculation of safety margins. Additionally, the expert addressed the cash compensation paid to departing shareholders and recommended an increase in such compensation. If this increase is approved by the court, it would result in a liability lower than our current litigation reserves, which pertain to this plaintiff group specifically. The expert provided a supplemental opinion in April 2022, and the judicial committee in charge of the proceedings provided an initial opinion to the Vienna commercial court in April 2023 and a supplemental opinion in June 2024 that reiterated its initial opinion. The court has not made a decision yet. The results of this litigation or any other legal proceedings are inherently uncertain, and adverse judgments or settlements in some of these legal disputes may result in adverse and potentially substantial monetary damages, penalties, or injunctive relief against us.
We have engaged and may in the future engage in strategic transactions, such as acquisitions or investments in other companies or technologies, which could divert our management’s attention and in some cases result in dilution to our shareholders and otherwise disrupt our operations and adversely affect our operating results.
We have engaged and may in the future engage in strategic transactions that may divert the attention of management and incur various expenses in identifying, investigating, and pursuing suitable transactions, whether or not they are consummated. For example, we may seek to acquire or invest in additional businesses and/or technologies that we believe complement or expand our current products or product candidates, enhance our technical capabilities, or otherwise offer growth opportunities in the United States and internationally. In 2015 we acquired Crucell Sweden AB and all assets, licenses, and privileges related to DUKORAL. We may also consider divestment of specific assets to support different strategic objectives.
Realizing the benefits of acquisitions depends upon the successful integration of the acquired technology into our existing and future product candidates. Furthermore, we may not be able to integrate the acquired personnel, operations, and technologies successfully, or effectively manage the combined business following the acquisition. We also may not realize the anticipated benefits from any acquired business. The risks we face in connection with acquisitions and investments, whether or not consummated, include the following:
•unanticipated costs or liabilities associated with the acquisition;
•diversion of management’s attention from other business concerns;
•adverse effects on our existing strategic collaborations as a result of the acquisition;
•assimilation of operations, intellectual property, and products of an acquired company;
•the potential loss of key employees;
•difficulty integrating the accounting systems, operations, and personnel of the acquired business;
•the assumption of additional indebtedness or contingent or unknown liabilities, or adverse tax consequences or unfavorable accounting treatment;
•claims and disputes by shareholders and third parties, including intellectual property claims and disputes;
•risks and uncertainties associated with the other party to such a transaction, including the prospects of that party and their existing products or product candidates and regulatory approvals;
•increased operating expenses and cash requirements; and
•use of substantial portions of our available cash to consummate the acquisition.
A significant portion of the purchase price of companies we acquire may be allocated to acquired goodwill and other intangible assets, which must be assessed for impairment at least annually. If our acquisitions do not yield expected returns, we may in the future be required to take charges to our operating results based on this impairment assessment process, which could adversely affect our business, financial condition, results of operations, and prospects.
Acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could adversely affect our operating results. In addition, if an acquired business fails to meet our expectations, our business, financial condition, results of operations, and prospects may suffer. We cannot assure you that we will be successful in integrating the businesses or technologies we may acquire. The failure to successfully integrate these businesses could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our CMOs, CROs, and other contractors and consultants, could be subject to cybersecurity attacks, earthquakes, power shortages, information technology or telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, armed conflict, wars, public health pandemics or epidemics, and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses.
Our business has been and could in the future be materially adversely affected by the effects of pandemics or epidemics, including COVID-19 and future outbreaks of the disease. COVID-19 adversely affected economic activity across virtually all sectors and industries on a local, national, and global scale. We are unable to accurately predict the impact that any future developments of COVID-19 or a similar event would have on our business or those of our manufacturers, CROs, and related third parties due to numerous uncertainties, including the duration of the outbreak, the result of vaccination efforts, resurgence of the virus including any new variants, actions that may be taken by governmental authorities, impacts on international travel, the impact on the business of our service providers and partners, and the impact on the global financial markets, which could limit our access to capital and affect our liquidity. These and similar, and perhaps more severe, disruptions in our operations could materially impact our business, operating results and financial condition.
Our ability to develop and commercialize our product candidates could be disrupted if our operations or those of our suppliers are affected by man-made or natural disasters or other business interruptions.
We may be negatively impacted by volatility in the political and economic environment across the markets in which we operate, including as a result of military conflicts, elections and changes in leadership, economic downturns, increases in interest rates, tariffs, sustained inflation, and changes in regulatory practice or policy that apply to our company or industry. Any of these could result in higher operating costs and may negatively impact our business and financial performance.
Trade, monetary and fiscal policies, and political and economic conditions may substantially change, and credit markets may experience periods of constriction and variability. These conditions may impact our business. Furthermore, rising inflation may negatively impact our business, increase costs, and reduce profitability. While we would take actions, wherever possible, to mitigate the impact of the effects of inflation, in the case of sustained inflation across several of the markets in which we operate, it could become increasingly difficult to effectively mitigate the increases to our costs. If we are unable to take actions to effectively mitigate the effect of the resulting higher costs, our profitability and financial position could be negatively impacted.
Due to the conflict between Russia and Ukraine, the United States, the European Union, and other jurisdictions have imposed various sanctions against Russia and Belarus. The military conflict and the retaliatory measures that have been taken, or could be taken in the future, by the U.S., the European Union, and other jurisdictions against Russia and Belarus have created global security concerns that could result in a regional conflict and otherwise have a lasting impact on regional and global economies, any or all of which could adversely affect our business. Other conflicts, such as the ongoing conflict between Israel and Hamas, could cause similar disruption and adversely impact our business. Any or all of these actions, as well as actions such as cyber-attacks by state-sponsored or non-state actors, could disrupt our operations and supply chain and adversely affect our ability to conduct and analyze ongoing and future clinical trials of our product candidates, among other possible consequences. Additionally, concerns about security and any increase in the cost of travel resulting from the rising cost of fuel could impact the travel industry. Any of these results could materially harm our business.
The U.S. Federal Reserve and European Central Bank have raised interest rates multiple times in response to concerns about inflation, among other things, and they may raise them again. Higher interest rates, coupled with reduced government spending and volatility in financial markets may increase economic uncertainty.
Similarly, the ongoing military conflicts between Russia and Ukraine and in the Middle East have created volatility in the global capital markets and are expected to have further global economic consequences, including ongoing disruptions of the global supply chain and energy markets. Any such volatility and disruptions may adversely affect our business or the third parties on whom we rely. If the equity and credit markets deteriorate, including as a result of political unrest or war, it may make any necessary debt or equity financing more difficult to obtain in a timely manner or on favorable terms, including relative to cost or dilution. Increased inflation rates can adversely affect us by increasing our costs, including labor and employee benefit costs. In addition, higher inflation and macro turmoil and uncertainty could also adversely affect our customers, which could reduce demand for our products.
We could also be affected by new and increased tariffs between the United States and other countries, and any retaliatory tariffs by those other countries. If our activities, or those of our current or future service providers, manufacturers, suppliers and other partners, fall within the scope of any of these or other tariffs, our costs may increase significantly.
Our available cash and cash equivalents are held in accounts managed by third party financial institutions in the United States and in Europe and consist of cash in our operating accounts. At any point in time, the funds in our operating accounts at U.S. financial institutions may exceed the Federal Deposit Insurance Corporation insurance limits. While we monitor the cash balances in our operating accounts and adjust the cash balances as appropriate, these cash balances could be impacted if the underlying financial institutions fail as a result of adverse conditions in the financial markets or otherwise.
Additionally, disruptions at governmental agencies such as the FDA, CDC, and SEC, or similar agencies outside the United States, resulting from funding or staffing cuts, changes in leadership or priorities, or other actions of the executive or legislative branches of the U.S. or other governments may have a significant and negative impact on our business. Similarly, legislative changes may be instituted, or enforcement priorities may shift, creating uncertainty about the regulatory environment in which we operate. Such disruptions may affect an agency’s ability to perform routine functions, thereby extending the time necessary for review of new product candidates or previously approved products. The FDA may operate with fewer resources and otherwise adapt its practices moving forward in ways that could hinder review of product candidates or result in changes to prior product approvals, notably of vaccines. Efforts to reduce regulations and expenditures across the U.S. government, presently directed by executive orders or memoranda from the Office of Management and Budget, may lead to proposed or actual policy changes that create additional uncertainty for our business and could affect the FDA’s relationship with the pharmaceutical industry, transparency in decision making and ultimately the cost and availability of vaccines and other prescription drugs, which could have a material adverse effect on our business. If funding for the FDA or other regulatory authorities is reduced, priorities change, a prolonged government shutdown occurs, or current or future global health concerns prevent the FDA or other regulatory authorities from conducting regulator inspections, reviews or other activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business. Similar disruptions may occur in other markets where we operate, including less familiar markets where we have established strategic collaborations with local partners.
Further, in our operations as a public company, future government disruptions or shutdowns could impact our ability to access the public markets and obtain necessary capital in order to properly capitalize and continue our operations.
Our IT systems and data, and those of our collaborators, consultants, service providers, and other contractors, are vulnerable to cyberattacks and security breaches, which could significantly disrupt our core operations, product development programs, and overall business and adversely affect our business strategy, financial condition, results of operations, and prospects.
Our computer and information technology systems, networks, infrastructure, hardware, software, and cloud-based computing services, collectively referred to as IT Systems, and those of our current and future collaborators, service providers, and other contractors or consultants are vulnerable to malware (such as ransomware), malicious code (such as computer viruses and worms), data corruption, cyber-based attacks (including attacks enhanced or facilitated by AI), malfeasance by insiders, human error, natural disasters, public health pandemics or epidemics, terrorism, war, and telecommunication and electrical failures, all of which threaten the confidentiality, integrity, and availability of our IT Systems, key business processes, and intellectual property, proprietary business information, personal information, and other important data we process or maintain, collectively referred to as our Confidential Information.
We and certain of our third-party providers have in the past experienced cyberattacks and other security incidents, and we expect that to continue in varying degrees in the future. We expect cyberattacks to accelerate on a global basis in both frequency and magnitude as threat actors are increasingly sophisticated in using techniques and tools – including artificial intelligence – that can circumvent controls, evade detection, and remove forensic evidence. As a result, we may be unable to detect, investigate, remediate, or recover from future attacks or incidents or to avoid a material adverse impact on our IT Systems, Confidential Information, or business. Cybersecurity threats are increasingly difficult to detect and come from a variety of sources, including traditional computer “hackers,” threat actors, “hacktivists,” organized criminal threat actors, insiders and other personnel (such as through theft or misuse), sophisticated nation-states, and nation-state-supported actors. Remote and hybrid working arrangements at our company (and at many third-party providers) also increase cybersecurity risks due to the challenges associated with managing remote computing assets and the security vulnerabilities that are present in many non-corporate and home networks. In addition, we cannot comprehensively identify all misconfigurations, “bugs”, or vulnerabilities in proprietary or third-party systems or software used by our business or guarantee that patches or compensating controls will be applied before vulnerabilities can be exploited by a threat actor. Moreover, any use or integration of generative or other artificial intelligence in our, or any third parties’, operations, products, or services will pose new and/or unknown cybersecurity risks and challenges.
There can also be no assurance that our cybersecurity risk management program and processes, including our policies, controls, or procedures, will be fully implemented, complied with, or effective in protecting our IT Systems and Confidential Information. Any significant system failure, accident, attack, or security breach could have a material adverse effect on our business, financial condition, and results of operations. The costs to us to mitigate network security problems, bugs, viruses, worms, malicious software programs, and security vulnerabilities or to respond to or recover from a cyberattack or security incident could be significant and could result in unexpected interruptions, delays, cessation of service, and other harm to our business and our competitive position, as well as regulatory investigations, litigation (including class action suits), reputational impacts, and the loss of partners, collaborators, and customers. If such an event were to occur and cause interruptions in our operations, it could also result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other proprietary information or other similar disruptions. For example, the loss or corruption of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, including but not limited to information related to our product candidates, we could incur liability, our competitive and reputational position could be harmed, and the further development and commercialization of our product candidates could be delayed.
In addition, our IT Systems and those of our current and any future collaborators, service providers, and other contractors or consultants are potentially vulnerable to data security breaches, whether by employees, contractors, consultants, malware, phishing attacks, or other cyberattacks, that may expose Confidential Information to unauthorized persons. For example, we have experienced phishing attacks in the past, and we expect to be a target of phishing attacks and other cyberattacks in the future. In addition, our IT Systems include cloud-based applications that are hosted by third-party service providers with security and information technology systems subject to similar risks. Consequently, successful cyberattacks that disrupt or result in unauthorized access to third-party IT Systems can materially impact our operations and financial results. If a data security breach affects our systems, corrupts our data, or results in the unauthorized disclosure or release of personally identifiable information, for example, our reputation could be materially damaged. In addition, such a breach may require notification to governmental agencies, supervisory bodies, credit reporting agencies, the media, or individuals pursuant to various data protection, privacy, and security laws, regulations, and guidelines, as applicable, such as the EU and UK GDPR (as defined below). Accordingly, a data security breach or privacy violation that leads to unauthorized access to, disclosure, or modification of personal information (including protected health information), that prevents access to personal information, or that materially compromises the privacy, security, or confidentiality of the personal information, could result in fines, increased costs, or loss of revenue, and we could incur liability, our competitive position could be harmed, and the further development and commercialization of our product candidates could be delayed.
Furthermore, laws and regulations around the globe, such as the EU and UK GDPR, can expose us to enforcement actions and investigations by regulatory authorities and potentially result in regulatory penalties and significant legal liability, if our information technology security efforts fail and if we fail to disclose any material cybersecurity incident in an adequate and timely manner. We cannot be sure that our insurance coverage will be adequate or sufficient to protect us from or to mitigate liabilities arising out of our privacy and security practices, that such coverage will continue to be available on commercially reasonable terms or at all, or that such coverage will pay future claims.
In addition, third parties may gather, collect, or infer sensitive information about us from public sources, data brokers, or other means that reveals competitively sensitive details about our organization and could be used to undermine our competitive advantage or market position. Additionally, our sensitive information could be leaked, disclosed, or revealed as a result of or in connection with our employees’ or vendor’s use of generative AI technologies.
Our employees, principal investigators, consultants, and commercial partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants, and commercial partners. Misconduct by these parties could include intentional failures, reckless and/or negligent conduct, or unauthorized activities that violate any of the following:
•the laws and regulations of the EEA countries, FDA, and other regulatory authorities, including those laws requiring the reporting of true, complete, and accurate information to competent regulatory authorities,
•manufacturing standards,
•federal and state data privacy, security, fraud and abuse, and other healthcare laws and regulations in the EEA, the United States, and elsewhere and
•laws that require the true, complete, and accurate reporting of financial information or data.
In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct also could involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, creating fraudulent data in our pre-clinical studies or clinical trials, or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation.
It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in significant civil, criminal, and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participating in government-funded healthcare programs, such as Medicare and Medicaid or comparable foreign programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, contractual damages, reputational harm, and the curtailment or restructuring of our operations.
Our business may be exposed to foreign exchange risks.
We operate internationally and are exposed to foreign exchange risks arising from various currencies, primarily with respect to the Euro (EUR), the British Pound (GBP), the Canadian Dollar (CAD), the Swedish Krona (SEK), and the U.S. Dollar (USD). As we grow and as a result of our strategic collaborations, in particular in Brazil and India, our exposure to foreign exchange risks will increase. Foreign exchange risks arise from future commercial transactions, recognized assets and liabilities, and net investments in foreign operations. Because a substantial part of sales of IXIARO and IXCHIQ are, or are expected to be, generated in the United States, with a significant part of production costs in GBP, and in Canada for DUKORAL, with production costs in SEK, we are exposed to foreign exchange risks, principally with respect to the USD, GBP, SEK, and CAD. However, our results of operations continue to be impacted by exchange rate fluctuations. For example, an increase in the value of the euro against the U.S. dollar could be expected to have a negative impact on our revenue and earnings growth as U.S. dollar revenue and earnings, if any, would be translated into euro at a reduced value. While we entered into currency option contracts in 2020 to limit the risk of foreign exchange losses, we cannot predict the impact of foreign currency fluctuations, and foreign currency fluctuations in the future may adversely affect our financial condition, results of operations and cash flows. Our ADSs are quoted in U.S. dollars on Nasdaq, while our ordinary shares trade in euro on Euronext Paris. Our financial statements are prepared in euro. Therefore, fluctuations in the exchange rate between the euro and the U.S. dollar will also affect, among other matters, the value of our ordinary shares and ADSs. We could also sign contracts denominated in other currencies, which would increase our exposure to currency risk. In accordance with our business decisions, our exposure to this type of risk could change depending on:
•the currencies in which we receive our revenues;
•the currencies chosen when agreements are signed, such as licensing agreements, or co-marketing or co-development agreements;
•the location of clinical trials on product candidates; and
•our policy for insurance coverage.
In addition, in light of the ongoing military conflict between Russia and Ukraine and the resulting tensions between the European Union, the United Kingdom, the United States and other countries with Russia, any resulting material change to the valuation of European and U.S. currencies could adversely impact our operating results.
Risks Related to the Manufacture of Our Products and Product Candidates
We may be unable to successfully manufacture our products or product candidates in sufficient quality and quantity, which would impact commercialization of our products and delay development of our product candidates.
We perform most of the manufacturing of our products and our product candidates in-house. Delays in manufacturing or inability to manufacture sufficient doses of a product or product candidate could adversely affect our business, financial condition, prospects, and results of operations. If we, or any third-party manufacturing partners, are unable to manufacture sufficient quantities of any vaccine, we may not be able to meet demand or fulfill our obligations under any agreements, or we may be forced to forego additional partnerships or supply agreements which would be advantageous for our business. We may encounter unexpected challenges relating to manufacturing efficiency, quality control, or stability profile that could impact the quantity of products or product candidates manufactured, the consistency of quantity across batches, or the length of time that manufactured material can be used. These problems could impact our supply of the market and require us to manufacture more than previously expected, leading to delays and added costs. Additionally, any supply shortages due to an inability to manufacture sufficient doses could result in fines.
We experienced supply shortages for both IXIARO and DUKORAL in 2022, 2023, and 2024 due primarily to the faster than expected recovery of the travel market from the COVID-19 pandemic and delays in internal processes.
Our manufacturing facility in Livingston, Scotland is the sole source of commercial quantities of drug substance of our Japanese encephalitis vaccine IXIARO and our chikungunya vaccine IXCHIQ. Our manufacturing facility in Solna, Sweden, is the sole source of commercial quantities of DUKORAL. Our Vienna facility is involved in the product release process for products manufactured in Livingston. Any event, such as a fire or pandemic, that would prevent any aspect of the production process in these facilities would prevent us from manufacturing the relevant product and supplying our customers or clinical trial centers, which could lead to significant delays and shortages.
We may be required to increase our manufacturing capacity to meet demand for approved products, and we may be unable to do this in a timely or cost-effective manner, or at all. We do not have experience manufacturing on the scale that would be required for a large-scale commercialization of vaccine candidates that may receive approval in the future.
The process of developing additional manufacturing capacity is complex and affected by multiple external factors, many of which are beyond our control.
We, our contract manufacturers, any future collaborators, and their contract manufacturers could be subject to periodic unannounced inspections by the FDA or other comparable regulatory authorities to monitor and ensure compliance with cGMP or other applicable regulations. Despite our efforts to audit and verify regulatory compliance, we or one or more of our third-party manufacturing vendors may be found on regulatory inspection by the authorities to be noncompliant with cGMP or other applicable regulations. This may result in shutdown of the relevant facility or invalidation of drug product lots or processes, as well as delays in clinical development programs which could ultimately negatively impact our regulatory and commercialization timelines and expectations. In some cases, a product recall may be warranted or required, which would materially affect our ability to supply and market our drug products.
We have outsourced to third parties an important step in the manufacturing of IXCHIQ, the filling process for IXIARO, and the filling of IXCHIQ diluent. Outsourcing of manufacturing could result in delays, concerns about manufacturing consistency, or other manufacturing failures. Per the standard industry practice, we rather than the third-party provider would bear the risk of such problems. In addition, we have to compete with other companies for capacity, which might limit our ability to scale up our production as and when we need to. If we are unable to scale up our production as per our plans for this or any other reason, this could affect our ability to produce our vaccines or vaccine candidates at a reasonable price.
Any of these factors impacting manufacturing quantity or quality could delay clinical trials, regulatory submissions, and/or commercialization of our products, interfere with current sales, entail higher costs, and result in our inability to effectively sell our products.
We rely upon third parties to manufacture and supply components of certain substances necessary to manufacture our products and product candidates.
We currently rely upon several, and in the future may rely on additional, third-party CMOs for the manufacture and supply of components and substances for all of the product candidates we are developing. Additionally, certain component materials are currently available from a single supplier, or a small number of suppliers. We cannot be sure that these suppliers will remain in business, or that they will not be purchased by one of our competitors or another company that is not interested in continuing to manufacture these materials for us. We cannot assure you that, if required, we will be able to identify alternate sources with the desired scale and capability and establish relationships with such sources. Additionally, in the biopharmaceutical industry, supplier changes require lengthy validation and regulatory approval processes. A loss of any CMO or component supplier and delay in establishing a replacement could delay our clinical development and regulatory approval process and interrupt supply.
Manufacturing facilities and clinical trial sites are subject to significant government regulations and approvals. If we or any third parties fail to comply with these regulations or maintain these approvals, our business could be materially harmed.
Our manufacturing facilities and those of our third-party manufacturing partners are subject to ongoing regulation and periodic inspection by national authorities, including the competent authorities of EEA countries, the FDA, and other regulatory bodies to ensure compliance with cGMP and other applicable regulations when producing batches of our products and product candidates for clinical trials. CROs and other third-party research organizations must also comply with Good Laboratory Practice, or GLP, when carrying out regulatory toxicology studies. Any failure to follow and document our or their adherence to such cGMP and GLP regulations or other regulatory requirements may lead to significant delays in the availability of products for commercial sale or clinical trials, may result in the termination of or a hold on a clinical trial, may delay or prevent filing or approval of marketing applications for our products, or may cause us to not meet our obligations under our commercial agreements.
Failure to comply with applicable regulations at our manufacturing sites or at clinical trial sites could also result in national authorities, the competent authorities of EEA countries, the FDA, or other applicable regulatory authorities taking various actions, including:
•levying fines and other civil penalties;
•imposing consent decrees or injunctions;
•requiring us to suspend or put on hold one or more of our clinical trials;
•requiring an additional audit or validation of clinical trial data;
•suspending, varying, or withdrawing regulatory approvals;
•delaying or refusing to approve pending applications or supplements to approved applications;
•requiring us to suspend manufacturing activities or product sales, imports, or exports;
•requiring us to communicate with physicians and other customers about concerns related to actual or potential safety, efficacy, and other issues involving our products;
•mandating product recalls or seizing products;
•imposing operating restrictions; and
•seeking criminal prosecutions.
Any of the foregoing actions could be detrimental to our reputation, business, financial condition, or operating results. Furthermore, we or our key suppliers and partners may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all, or in delays to our clinical trials. In addition, before any additional products would be considered for marketing authorization in the EEA, the United States, or other jurisdictions, our suppliers will have to pass an inspection by the applicable regulatory authorities. We are dependent on our suppliers’ cooperation and ability to pass such inspections, and the inspections and any necessary remediation may be costly. Failure to pass such inspections by us or any of our suppliers would adversely affect our ability to commercialize our products or product candidates in the EEA, the United States, or other jurisdictions. Moreover, many of the third parties with whom we contract may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting product development activities that could harm our competitive position. Should any of these risks materialize, this could have a material adverse effect on our business, prospects, financial condition, and results of operations.
Our production costs may be higher than we currently estimate.
Our products and our product candidates are manufactured according to manufacturing best practices applicable to drugs for clinical trials and to specifications approved by the applicable regulatory authorities. If any of our products were found to be non-compliant, we would be required to manufacture the product again, which would entail additional costs and may prevent delivery of the product on time.
Other risks inherent in the production process may have the same effect, such as:
•contamination of the controlled atmosphere area;
•unusable premises and equipment;
•new regulatory requirements requiring a partial and/or extended stop to the production unit to meet the requirements;
•unavailable qualified personnel;
•power failure of extended duration; and
•logistical error.
Additionally, if we externalize any aspect of manufacturing that we have historically performed internally, this could result in an increase in production costs. Should any of these risks materialize, this could have a material adverse effect our business, prospects, financial condition, and results of operations.
We use hazardous chemicals and biological materials in our business and any claims relating to improper handling, storage or disposal of these materials could be time-consuming and costly.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including chemicals and biological materials. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We also handle genetically recombined material, genetically modified species, and pathological biological samples. Consequently, in France, Sweden, and Scotland where we have production facilities and in the jurisdictions where we conduct clinical trials, we are subject to environment and safety laws and regulations governing the use, storage, handling, discharge, and disposal of hazardous materials, including chemical and biological products. We impose preventive and protective measures for the protection of our workforce and waste control management in accordance with applicable laws, including part four of the French Labor Code, relating to occupational health and safety.
If we fail to comply with applicable regulations, particularly those applicable to all BSL classifications, we could be subject to criminal prosecutions, fines, damages, and the suspension of all or part of our operations. Compliance with environmental, health, and safety regulations involves additional costs, and we may have to incur significant costs to comply with future laws and regulations in relevant jurisdictions. Compliance with environmental laws and regulations could require us to purchase equipment, modify facilities, and undertake considerable expenses. We do not have insurance that specifically covers liability relating to hazardous materials and could be liable for any inadvertent contamination, injury, or damage, which could negatively affect our business and engage the civil and/or criminal liability of the Company and/or its representatives.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our product candidates and technology, or if the scope of the patent protection obtained is not sufficiently broad or robust, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our product candidates and technology may be adversely affected.
Our success depends, in large part, on our ability to obtain and maintain patent protection in the United States and other countries with respect to our product candidates and our technology. We and our licensors have sought, and intend to seek, to protect our proprietary position by filing patent applications in Europe, the United States and other jurisdictions related to our product candidates and our technology that are important to our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has, in recent years, been the subject of much litigation. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or product candidates or which effectively prevent others from commercializing competitive technologies and product candidates. Because patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file a patent application relating to any particular aspect of a product candidate. Foreign patents may be subject also to opposition or comparable proceedings in the corresponding foreign patent office.
The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection.
We or our licensors have not pursued or maintained, and may not pursue or maintain in the future, patent protection for our product candidates in every country or territory in which we may sell our products, if approved. In addition, the laws of some countries do not protect intellectual property rights to the same extent as European laws and federal and state laws in the United States. Consequently, we may not be able to prevent third parties from infringing our patents in all countries outside the EEA or the United States, or from selling or importing products that infringe our patents in and into the EEA or the United States or other jurisdictions.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if the patent applications we license or own do issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity, or enforceability, and our patents may be challenged in the courts or patent offices in EEA countries, the United States, and other jurisdictions. Such challenges may result in loss of exclusivity or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products or could limit the duration of the patent protection of our technology and product candidates. For example, one of our patents that relates to VLA84 has been limited in scope in opposition proceedings in Europe. In another case in 2023, we decided to withdraw a patent covering IXIARO following an opposition proceeding in Europe. More recently, we have also received a further opposition by a third party against a European patent that is directed at IXIARO, and VLA1601. The proceeding started in June 2023, and we will defend our position with one or more submissions. Although we do not expect these developments to have a significant impact on further commercialization of IXIARO, we may face similar proceedings in the future that could have a significant effect on our ability to commercialize our products. We have also recently received an opposition by a third party against a European patent that is directed to our Zika product candidate, VLA1601. The proceeding began in February 2023, and we will defend our position with one or more submissions. A further opposition by a third party against a European patent that is directed at IXIARO, VLA1601 and VL15 was made on January 2025 with the EPO.
Given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. In addition, if the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, it could dissuade companies from collaborating with us to develop, and threaten our ability to commercialize, our product candidates.
Furthermore, our owned and in-licensed patents may be subject to a reservation of rights by one or more third parties. As a result, such third parties, including governments and non-for-profit organizations, may have certain rights, including “march-in” rights, to such patent rights and technology. When new technologies are developed with such partners, they generally obtain certain rights in any resulting patents, including a nonexclusive license authorizing the party to use the invention for noncommercial purposes. These rights may permit the funding partner to disclose our confidential information to third parties and to exercise “march-in” rights to use or allow third parties to use our licensed technology. The funding partner can exercise its “march-in” rights if it determines that action is necessary because we fail to achieve practical application of the government-funded technology, because action is necessary to alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. or other country industry. In addition, our rights in such inventions may be subject to certain requirements to manufacture products embodying such inventions in the United States or other countries. Any exercise by the funding partners of such rights could harm our competitive position, business, financial condition, results of operations, and prospects.
Obtaining and maintaining our patent rights depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent application process. In addition, periodic maintenance fees, renewal fees, annuity fees and various other government fees on patents and/or patent applications will have to be paid to the USPTO and various government patent agencies outside the United States over the lifetime of our owned and licensed patents and/or applications and any patent rights we may own or license in the future. We rely on our service providers or our licensors to pay these fees. We employ reputable law firms and other professionals to help us comply, and we are also dependent on our licensors to take the necessary action to comply with these requirements with respect to our licensed intellectual property. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, nonpayment of fees, and failure to properly legalize and submit formal documents. If we or our licensors fail to maintain the patents and patent applications covering our product candidates or technologies, we may not be able to use such patents and patent applications or stop a competitor from marketing products that are the same as or similar to our product candidates, which would have an adverse effect on our business. In many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules. There are situations, however, in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, potential competitors might be able to enter the market and this circumstance could harm our business.
In addition, if we fail to apply for applicable patent term extensions or adjustments, we will have a more limited time during which we can enforce our granted patent rights. In addition, if we are responsible for patent prosecution and maintenance of patent rights in-licensed to us, any of the foregoing could expose us to liability to the applicable patent owner.
Patent terms may be inadequate to protect our competitive position on our products and product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after its first effective filing date. Although various extensions may be available, the life of a patent and the protection it affords is limited. In addition, although upon issuance in the United States a patent’s life can be extended based on certain delays caused by the USPTO, this increase can be reduced or eliminated based on certain delays caused by the patent applicant during patent prosecution. If we do not have sufficient patent life to protect our products, our business and results of operations could be adversely affected.
Given the amount of time required for the development, testing, and regulatory review of our product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we have or will obtain patent rights. The Hatch-Waxman Act in the United States, and similar legislation in the European Union, permit a patent term extension of up to five years beyond the normal expiration of the patent, provided that the patent is not enforceable in the U.S. for more than 14 years from the date of drug approval, which is limited to the approved indication (or any additional indications approved during the period of extension). Furthermore, in the United States, only one patent per approved product can be extended and only those claims covering the approved product, a method for using it, or a method for manufacturing it may be extended. In the EEA, supplementary protection certificates, or SPCs, provide protection for the active ingredient of a patented and authorized medicinal product, which may extend for up to five years beyond the normal patent expiry date (providing together with the patent up to 15 years exclusivity from the first EU marketing authorization). In some cases an additional six months of SPC protection may be obtained by performing pediatric trials of the product. The protection afforded by an SPC extends only to the active ingredient of the authorized medicinal product, within the scope of the granted base patent. However, the applicable authorities may not agree with our assessment of whether such extensions are available and may refuse to grant extensions to our patents or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and pre-clinical data and may be able to launch their product earlier than might otherwise be the case.
Third parties may initiate legal proceedings alleging that we are infringing, misappropriating, or otherwise violating their intellectual property rights, the outcome of which would be uncertain and could have a negative impact on the success of our business.
Our commercial success depends, in part, upon our ability and the ability of others with whom we may collaborate to develop, manufacture, market, and sell our current and any future product candidates and use our proprietary technologies without infringing, misappropriating, or otherwise violating the proprietary rights and intellectual property of third parties. The biotechnology and pharmaceutical industries are characterized by extensive and complex litigation regarding patents and other intellectual property rights. Numerous U.S.- and foreign-issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk may increase that our product candidates may give rise to claims of infringement of the patent rights of others. We may in the future become party to, or be threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our current and any future product candidates and technology, including interference proceedings, post grant review and inter partes review before the USPTO. Foreign patents may be subject also to opposition or comparable proceedings in the corresponding foreign patent office. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit. There is a risk that third parties may choose to engage in litigation with us to enforce or to otherwise assert their patent rights against us. Even if we believe such claims are without merit, a court of competent jurisdiction could hold that these third-party patents are valid, enforceable and infringed, which could have a negative impact on our ability to commercialize our current and any future product candidates.
In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this is a high burden and requires us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. Moreover, given the vast number of patents in our field of technology, we cannot be certain that we do not infringe existing patents or that we will not infringe patents that may be granted in the future. While we have in the past and may in the future decide to initiate proceedings to challenge the validity of these or other patents in the future, we may be unsuccessful, and courts or patent offices in Europe, the United States, and other jurisdictions could uphold the validity of any such patent. Even if we are successful in obtaining a first-instance judgement from a court or patent office that such patents are invalid, such judgements may be subject to appeal procedures which suspend revocation of the patent until a final appeal judgment is reached. This may result in many years of uncertainty and could ultimately lead to reversal of the original judgment and the patent being upheld. Furthermore, because patent applications can take many years to issue and are typically confidential for 18 months or more after filing, and because pending patent claims can be revised before issuance, there may be applications now pending which may later result in issued patents that may be infringed by the manufacture, use, or sale of our product candidates. Regardless of when filed, we may fail to identify relevant third-party patents or patent applications, or we may incorrectly conclude that a third-party patent is invalid or not infringed by our product candidates or activities. If a patent holder believes that our product candidate or technology platform infringes its patent, the patent holder may sue us even if we have received patent protection for our technology. Moreover, we may face patent infringement claims from nonpracticing entities that have no relevant product revenue and against whom our own patent portfolio may thus have no deterrent effect. If a patent infringement suit were threatened or brought against us, we could be forced to stop or delay research, development, manufacturing, or sales of the product or product candidate that is the subject of the actual or threatened suit.
If we are found to infringe a third party’s valid and enforceable intellectual property rights, we could be required to obtain a license from such third party to continue developing, manufacturing, and marketing our product candidate(s) and technology. Under any such license, we would most likely be required to pay various types of fees, milestones, royalties, or other amounts. Moreover, we may not be able to obtain any required license on commercially reasonable terms or at all, and if such an instance arises, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business. Parties making claims against us may also seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates.
The licensing or acquisition of third-party intellectual property rights is a competitive area, and more established companies may also pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources, and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or any return on our investment at all. If we are unable to successfully obtain rights to required third-party intellectual property rights or maintain the existing intellectual property rights we have, we may have to abandon development of the relevant program or product candidate, which could have an adverse effect on our business, financial condition, results of operations, and prospects. Furthermore, even if we were able to obtain a license, it could be nonexclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. We could be forced, including by court order, to cease developing, manufacturing, and commercializing the infringing technology or product candidate. We may also have to redesign our products, which may not be commercially or technically feasible or may require substantial time and expense. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right. We may be required to indemnify collaborators or contractors against such claims. A finding of infringement could prevent us from manufacturing and commercializing our current or any future product candidates or force us to cease some or all of our business operations, which could harm our business. Even if we are successful in defending against such claims, litigation can be expensive and time-consuming and would divert management’s attention from our core business. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our ordinary shares and ADSs.
Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business, financial condition, results of operations, and prospects.
We may be subject to claims asserting that our employees, consultants, or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property.
Certain of our employees, consultants, or advisors are currently, or were previously, employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants, and advisors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these individuals or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s current or former employer. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel.
Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
In addition, we may in the future be subject to claims by our former employees or consultants asserting an ownership right in our patents or patent applications as a result of the work they performed on our behalf. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Although it is our policy to require our employees and contractors who may be involved in the conception or development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who, in fact, conceives or develops intellectual property that we regard as our own, and we cannot be certain that our agreements with such parties will be upheld in the face of a potential challenge or that they will not be breached, for which we may not have an adequate remedy. The assignment of intellectual property rights may not be self-executing or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property.
In some countries, the national law may stipulate that certain inventions made by an employee belong to the employer or employee and may restrict the ability of employment or other contracts to define which inventions belong ab initio to the employer. Thus in some countries employees could claim ownership of inventions by operation of national law and assignments may not be enforceable. Inventors may also assert additional rights relating to their inventive contribution, without necessarily claiming ownership. For instance, in some countries inventors are entitled to adequate remuneration or other benefit from an invention, even if the invention belongs by law to their employer. In some cases employee-inventors may also be entitled to pursue patent applications that the employer decides to abandon. Inventors claiming such rights may require us to pay additional compensation or might bring claims against us using the patent applications they acquire.
We may be involved in lawsuits to protect or enforce our patents, the patents of our licensors, or our other intellectual property rights, which could be expensive, time-consuming, and unsuccessful.
Competitors may infringe, misappropriate, or otherwise violate our patents, the patents of our licensors, or our other intellectual property rights, or may allege that we have infringed on their intellectual property rights. To counter infringement or unauthorized use or defend against such claims, we may be required to file legal claims, which can be expensive and time-consuming and are likely to divert significant resources from our core business, including distracting our technical and management personnel from their normal responsibilities. For example, Takeda initiated an inter partes review proceeding before the U.S. Patent and Trademark Office on our Zika U.S. PATENT NO. 11,219,681. This proceeding ended as the Patent Trial and Appeal Board decided to deny Institution for this proceeding following our withdrawal of some of the claims. The remaining claims continue to cover VLA1601.
In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our owned or licensed patents at risk of being invalidated or interpreted narrowly and could put our owned or licensed patent applications at risk of not issuing. The initiation of a claim against a third party might also cause the third party to bring counterclaims against us, such as claims asserting that our patent rights are invalid or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, non-enablement, or lack of statutory subject matter. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant material information from the USPTO or similar foreign authorities or made a materially misleading statement during prosecution. Third parties may also raise similar validity claims before the USPTO in post-grant proceedings such as ex parte reexaminations, inter partes review, post-grant review, or oppositions or similar proceedings outside the United States, in parallel with litigation or even outside the context of litigation. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We cannot be certain that there is or will be no invalidating prior art, of which we and the patent examiner were unaware during prosecution. For the patents and patent applications that we have licensed, we may have limited or no right to participate in the defense of any licensed patents against challenge by a third party. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of any future patent protection on our current or future product candidates. Such a loss of patent protection could harm our business.
We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Our business could be harmed if in litigation the prevailing party does not offer us a license, or if the license offered as a result is not on commercially reasonable terms. Any litigation or other proceedings to enforce our intellectual property rights may fail and, even if successful, may result in substantial costs and distract our management and other employees.
We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon, misappropriating, or successfully challenging our intellectual property rights. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have an adverse effect on our ability to compete in the marketplace.
Developments in patent law could have a negative impact on our business.
Changes in either the patent laws or interpretation of the patent laws could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. For example, from time to time, the U.S. Congress, the USPTO, or similar foreign authorities may change the standards of patentability, and any such changes could have a negative impact on our business. In addition, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in September 2011, includes a number of significant changes to U.S. patent law. These changes include a transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged, and changes to the way patent applications are disputed during the examination process, such as allowing third-party submission of prior art to the USPTO during patent prosecution. These changes may favor larger and more established companies that have greater resources to devote to patent application filing and prosecution. Under a first-to-file system, assuming that other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor made the invention earlier. The USPTO has developed new regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the America Invents Act, and, in particular, the first-to-file provisions, became effective in March 2013. Substantive changes to patent law associated with the America Invents Act, or any subsequent U.S. legislation regarding patents, may affect our ability to obtain patents, and if obtained, to enforce or defend them. Accordingly, it is not clear what, if any, impact the America Invents Act will have on the cost of prosecuting our U.S. patent applications, our ability to obtain U.S. patents based on our discoveries, and our ability to enforce or defend any patents that may issue from our patent applications, all of which could have a material adverse effect on our business, prospects, financial condition, and results of operations.
In addition, changes to or different interpretations of patent laws in the United States and other countries may permit others to use our or our partners’ discoveries or to develop and commercialize our technology and product candidates without providing any compensation to us, or may limit the number of patents or claims we can obtain. The patent positions of companies in the biotechnology and pharmaceutical market are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of U.S. patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In Europe, the Enlarged Board of Appeal of the EPO has recently indicated that it is prepared to apply a “dynamic” interpretation of certain patent law provisions in view of political developments and thus could reverse previously pro-patentee positions relating to biotechnological and pharmaceutical inventions. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, the USPTO, and the EPO, as well as similar bodies in other countries, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future, which could have a material adverse effect on our business, prospects, financial condition, and results of operations.
We may not be able to protect our intellectual property rights throughout the world, which could negatively impact our business.
Filing, prosecuting, and defending patents covering our current and any future product candidates and technology platforms in all countries throughout the world would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we or our licensors have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we may obtain patent protection but where patent enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents, and any future patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, including certain developing countries such as Brazil and India, where we have entered into strategic collaborations, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our intellectual property and proprietary rights generally. Proceedings to enforce our intellectual property and proprietary rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly, could put our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. For example, such a license may be issued in circumstances where demand for a product cannot be met by the patent holder in cases of a public health emergency, such as the COVID-19 pandemic. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent and trademark protection for our product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. Because we rely on third parties to help us discover, develop, and manufacture our current and any future product candidates, or if we collaborate with third parties for the development, manufacturing, or commercialization of our current or any future product candidates, we must, at times, share trade secrets with them. We may also conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements.
We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements, or other similar agreements with our advisors, employees, third-party contractors, and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of these parties to use or disclose our confidential information, including our trade secrets. We also enter into invention or patent assignment agreements with our employees, advisors, and consultants. Despite our efforts to protect our trade secrets, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Moreover, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our confidential information or proprietary technology and processes. Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary technologies will be effective. If any of the collaborators, scientific advisors, employees, contractors, and consultants who are parties to these agreements breaches or violates the terms of any of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets as a result. Moreover, if confidential information that is licensed or disclosed to us by our partners, collaborators, or others is inadvertently disclosed or subject to a breach or violation, we may be exposed to liability to the owner of that confidential information. Enforcing a claim that a third party illegally or unlawfully obtained and is using our trade secrets, like patent litigation, is expensive and time-consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets.
In addition, our competitors may independently develop knowledge, methods, and know-how equivalent to our trade secrets. Competitors could purchase our products and replicate some or all of the competitive advantages we derive from our development efforts for technologies on which we do not have patent protection. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure could have an adverse effect on our business, financial condition, results of operations, and prospects.
We also face the risk of potential unauthorized disclosure or misappropriation of our intellectual property by our collaborators, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. Our collaborators also may use our proprietary information and intellectual property in such a way as to invite litigation or other intellectual property-related proceedings that could jeopardize our proprietary information or invalidate our intellectual property.
We also seek to preserve the integrity and confidentiality of our data and other confidential information by maintaining physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and detecting the disclosure or misappropriation of confidential information and enforcing a claim that a party illegally disclosed or misappropriated confidential information is difficult, expensive, and time-consuming, and the outcome is unpredictable. Further, we may not be able to obtain adequate remedies for any breach. In addition, our confidential information may otherwise become known or be independently discovered by competitors, in which case we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us.
Any trademarks we have and that we may obtain may be infringed or successfully challenged, resulting in harm to our business.
We rely on trademarks as one means to distinguish any of our product candidates that are approved for marketing from the products of our competitors. Third parties may oppose our trademark applications or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks, and we may not have adequate resources to enforce our trademarks. We entered into a co-existence agreement with respect to the VALNEVA trademark. The agreement places restrictions on how we can use this mark and how we can seek trademark protection for this mark.
In addition, any proprietary name we propose to use with our current or any other product candidate in the United States must be approved by the FDA, regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable proprietary product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business. The following examples are illustrative:
•others may be able to make compounds or formulations that are similar to our product candidates but that are not covered by the claims of any patents, should they issue, that we own or license;
•others may be able to develop technologies that are similar to our technology platforms but that are not covered by the claims of any patents, should they issue, that we own or license;
•we or our licensors might not have been the first to file patent applications covering certain of our inventions;
•others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights;
•it is possible that our pending patent applications will not lead to issued patents;
•issued patents that we own or license may not provide us with any competitive advantages or may be held invalid or unenforceable as a result of legal challenges;
•our competitors might conduct research and development activities in the United States and other countries that are covered by a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights, and then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
•we may not develop additional proprietary technologies that are patentable; and
•the patents of others may have an adverse effect on our business.
If we breach our license agreements or any of the other agreements under which we acquired, or will acquire, the intellectual property rights to our product candidates, we could lose the ability to continue the development and commercialization of the related product candidates.
We have in-licensing agreements relating to certain of our products and product candidates, including with LimmaTech Biologics for S4V2 (Shigella).
If we fail to meet our obligations under these agreements, our licensors may have the right to terminate our licenses. If any of our license agreements are terminated, and we lose our intellectual property rights under such agreements, this may result in a complete termination of our product development and any commercialization efforts for the product candidates which we are developing under such agreements. While we would expect to exercise all rights and remedies available to us, including seeking to cure any breach by us, and otherwise seek to preserve our rights under such agreements, we may not be able to do so in a timely manner, at an acceptable cost or at all.
Disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement, including those related to:
•the scope of rights granted under the license agreement and other issues relating to interpretation of the relevant agreement;
•whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the license granted to us;
•our right to sublicense patent and other rights to third parties under collaborative development relationships;
•our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations; and
•the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors, on the one hand, and us and our sublicensees, on the other hand.
Risks Related to Regulatory Compliance
We may fail to obtain regulatory approval for our products on a timely basis or comply with our continuing regulatory obligations after approval is obtained.
Delays in obtaining regulatory approval can be extremely costly in terms of lost sales opportunities, loss of any potential marketing advantage of being early to market, and increased clinical trial costs. The speed with which we begin and complete our pre-clinical studies, clinical trials, and applications for marketing approval will depend on several factors, including the following:
•regulatory authority review and approval of proposed clinical trial protocols;
•approval of clinical trials protocols and informed consent forms by institutional review boards responsible for overseeing the ethical conduct of the trial, or positive ethics committee opinions, as part of the single decision on the authorization of a clinical trial issued by EU Member States including input from the national competent authorities and ethics committee;
•the rate of participant enrollment and retention, which is a function of many factors, including among others the size of the participant population, the proximity of participants to clinical sites, the eligibility criteria for the clinical trial, and the nature of the protocol;
•unfavorable test results or side effects experienced by clinical trial participants;
•analysis of data obtained from pre-clinical and clinical activities, which are susceptible to varying interpretations and which interpretations could delay, limit, or prevent regulatory approval or delay, limit, prevent, or result in the suspension, variation, or termination of clinical studies;
•the availability of skilled and experienced staff to conduct and monitor clinical trials and to prepare the appropriate regulatory applications;
•compliance with GCP and other applicable regulations by CROs and personnel conducting a clinical trial; and
•changes in the policies of regulatory authorities for drug or vaccine approval during the period of product development.
We may not be permitted to continue or commence additional clinical trials. Regulatory authorities may require us or our collaborators to delay, restrict, or discontinue clinical trials on various grounds, including a finding that the participants are being exposed to an unacceptable health risk or as a result of non-compliance with applicable regulations such as GCP.
In addition, we or our collaborators may be unable to submit applications to regulatory authorities within the timeframe we currently expect. Once submitted, applications must be approved by various regulatory authorities before we or our collaborators can commercialize the product described in the application.
Accelerated regulatory review and approval procedures do not guarantee faster development, review, or approval or that approval will ultimately be granted.
Regulatory authorities such as the EMA and FDA offer various options for accelerated review and approval of product candidates, such as the EMA’s PRIME designation for priority medicines and the FDA’s Fast Track designation and accelerated approval pathway. We seek to take advantage of these opportunities in order to facilitate the development, review, and approval processes for our product candidates.
IXCHIQ, or VLA1553 in jurisdictions where it is subject to ongoing regulatory review, received PRIME designation from the EMA. The PRIME scheme is intended to encourage drug development in areas of unmet medical need and provides accelerated assessment of products that may offer a major therapeutic advantage over existing treatments or benefit patients without treatment options, reviewed under the centralized procedure.
VLA15, our candidate against Lyme disease, and S4V2, the vaccine candidate against shigellosis that we are developing with LimmaTech Biologics, each received Fast Track designation from the FDA. Fast Track designation may be available to help expedite the development or approval process for a drug that is intended for the treatment of a serious or life-threatening condition and that demonstrates the potential to address an unmet medical need for this condition.
PRIME or Fast Track designation does not change the standards for product approval or ensure that the product will receive marketing approval at all or within any particular timeframe. In addition, the FDA may withdraw Fast Track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast Track designation alone does not guarantee qualification for the FDA’s priority review procedures. We may seek PRIME and/or Fast Track designation for other vaccine candidates in the future. If we do seek such designations for our other vaccine candidates, we may not receive such designations, and even if we receive designations, we may not experience a faster development process, review, or approval compared to conventional EMA or FDA procedures.
Although VLA15 and S4V2 have received Fast Track designation, this designation might not result in a faster or more successful development or review process or in ultimate approval of these product candidates by the FDA.
Finally, we received approval for IXCHIQ under the FDA’s accelerated approval pathway and may seek such approval for other vaccine candidates in the future. A product may be eligible for accelerated approval if it treats a serious or life-threatening condition, generally provides a meaningful advantage over available therapies, and demonstrates an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit. As a condition of approval, the FDA may require that a sponsor of a product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials, such as the Phase 4 clinical trials that are required for IXCHIQ. These confirmatory trials must be completed with due diligence. Even if we do receive accelerated approval for a future product candidate, we may not experience a faster development or regulatory review or approval process, and receiving accelerated approval does not provide assurance of ultimate full FDA approval. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of any future products on this pathway.
If approved, our investigational products regulated as biologics may face competition from biosimilars approved through an abbreviated regulatory pathway.
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated approval pathway for biologic products that are biosimilar to or interchangeable with an FDA-licensed reference biologic product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed.
During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a BLA for the competing product containing the sponsor’s own pre-clinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity, and potency of the other company’s product.
We believe that any of our product candidates approved as a biologic product under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our investigational medicines to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once licensed, will be substituted for any one of our reference products in a way that is similar to traditional generic substitution for non-biologic products is not yet clear and will depend on a number of marketplace and regulatory factors that are still developing. If competitors are able to obtain marketing approval for biosimilars referencing our products, our products may become subject to competition from such biosimilars, with the attendant competitive pressure and consequences.
The European Union provides opportunities for data and market exclusivity related to marketing authorizations. Upon receiving a marketing authorization, innovative medicinal products are generally entitled to receive eight years of data exclusivity and 10 years of market exclusivity. Data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar marketing authorization application can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the European Union until 10 years have elapsed from the initial marketing authorization of the reference product in the European Union. The overall ten-year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. However, there is no guarantee that a product will be considered by the European Union’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity.
In the EU, there is a special regime for biosimilars, or biological medicinal products that are similar to a reference medicinal product but that do not meet the definition of a generic medicinal product. For such products, the results of appropriate preclinical or clinical trials must be provided in support of an application for marketing authorization. Guidelines from the EMA detail the type of quantity of supplementary data to be provided for different types of biological product.
We also believe that our product candidates in the EEA should benefit from this data and market exclusivity. As with the United States, however, if competitors obtain marketing authorization for their biosimilar products, our products may become subject to competition from these biosimilars, with the attendant competitive pressure and consequences.
Even if we successfully commercialize any of our vaccine candidates, either alone or in collaboration, we face uncertainty with respect to pricing, third-party reimbursement, and healthcare reform, all of which could adversely affect any commercial success of our vaccine candidates.
Market acceptance and sales of any vaccine candidates that we commercialize, if approved, will depend in part on the extent to which reimbursement for these products and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations, and other private health insurers. Therefore, our ability to collect revenue from the commercial sale of our vaccines may depend on our ability, and that of any current or potential future collaboration partners or customers, to obtain recommendations for us or adequate levels of approval, coverage, and reimbursement for such products from third-party payors such as:
•government health administration authorities, such as ACIP in the United States;
•private health insurers;
•managed care organizations;
•pharmacy benefit management companies; and
•other healthcare related organizations.
Third-party payors decide which therapies and vaccines they will pay for and establish reimbursement levels. Travel vaccines are rarely reimbursed in Europe and, while no uniform policy for coverage and reimbursement exists in the United States, third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, decisions regarding the extent of coverage and amount of reimbursement to be provided for any product candidates that we develop will be made on a payor-by-payor basis. Therefore, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage, and adequate reimbursement, for the product. Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Each payor determines whether or not it will provide coverage for a product, what amount it will pay the manufacturer for the product and on what tier of its formulary it will be placed. The position on a payor’s list of covered drugs, biological, and vaccine products, or formulary, generally determines the co-payment that a patient will need to make to obtain the product and can strongly influence the adoption of such product by patients and physicians. Even if favorable coverage and reimbursement status is attained for one or more product candidates for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Even patients for whom our products are recommended by the local health authority may be less likely to use our products if the level of coverage and reimbursement provided, if any, is not adequate relative to the level of potential risk of travelling while unvaccinated, as determined by the patient and their physician. In addition, because our product candidates are physician-administered, separate reimbursement for the product itself may or may not be available. Instead, the administering physician may only be reimbursed for providing the treatment or procedure in which our product is used.
Third-party payors are increasingly challenging the prices charged for medical products and may deny coverage or offer inadequate levels of reimbursement if they determine that a prescribed product has not received appropriate clearances from the applicable regulatory authorities, is not used in accordance with cost-effective treatment methods as determined by the third-party payor, or is experimental, unnecessary or inappropriate. Prices could also be driven down by managed care organizations that control or significantly influence utilization of healthcare products. Outside the United States, pricing of competitive products by third parties is the biggest driver of the prices of our products. In the United States, we may be significantly adversely affected if the federal pricing rules change to require a greater discount than the current minimum of 24% compared to non-federal average manufacturer price for products listed on the federal supply schedule.
Third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular product. We cannot be sure that coverage and reimbursement will be available for any vaccine that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Inadequate coverage and reimbursement may impact the demand for, or the price of, any product for which we obtain marketing approval. If coverage and adequate reimbursement are not available, or are available only at limited levels, we may not be able to successfully commercialize any vaccine candidates that we develop.
In both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals and initiatives to change the health care system in ways that could affect our ability to sell vaccines and could adversely affect the prices that we receive for our vaccine candidates, if approved. Some of these proposed and implemented reforms could result in reduced pharmaceutical pricing or reimbursement rates for medical products, which could adversely affect our business strategy, operations, and financial results.
For example, in the United States, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, contains several cost containment measures that could adversely affect our future revenue, including, for example, increased drug rebates under Medicaid for brand name prescription drugs, extension of Medicaid rebates to Medicaid managed care organizations, and extension of so-called 340B discounted pricing on pharmaceuticals sold to certain healthcare providers. Additional provisions of various laws, including the ACA, that may negatively affect our future revenue and prospects for profitability include the assessment of an annual fee based on our proportionate share of sales of brand name prescription drugs to certain government programs, including Medicare and Medicaid, as well as mandatory discounts on drugs (including vaccines) sold to certain Medicare Part D beneficiaries in the coverage gap (the so-called “donut hole”).
Other aspects of healthcare reform, such as expanded government enforcement authority and heightened standards that could increase compliance-related costs, could also affect our business in the United States or elsewhere. In addition, we face uncertainties because there are ongoing federal legislative and administrative efforts to repeal, substantially modify, or invalidate some or all of the provisions of the ACA in the United States. We cannot predict the ultimate content, timing, or effect of any healthcare reform legislation or the impact of potential legislation on us. If we are unable to obtain and maintain sufficient third-party coverage and adequate reimbursement, the commercial success of our vaccine products may be greatly hindered, and our financial condition and results of operations may be materially and adversely affected.
Legislators, policymakers, and healthcare insurance funds in the EU may continue to propose and implement cost-containing measures to keep healthcare costs down, particularly due to the financial strain that the COVID-19 pandemic placed on national healthcare systems of the EU Member States. These measures could include limitations on the prices we would be able to charge for product candidates that we may successfully develop and for which we may obtain regulatory approval or the level of reimbursement available for these products from governmental authorities or third-party payors. Further, an increasing number of EU and other foreign countries use prices for medicinal products established in other countries as “reference prices” to help determine the price of the product in their own territory. Consequently, a downward trend in prices of medicinal products in some countries could contribute to similar downward trends elsewhere.
Our relationships with customers, healthcare providers, and third-party payors are subject, directly or indirectly, to healthcare fraud and abuse laws, false claims laws, health information privacy and security laws, and other healthcare laws and regulations. If we are unable to comply, or have not fully complied, with such laws, we could face substantial penalties.
Healthcare providers and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers, and third-party payors subject us to various fraud and abuse laws and other healthcare laws.
These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell, and distribute our product candidates, if approved. Restrictions under applicable U.S. federal, state, and foreign healthcare laws and regulations include, but are not limited to, the following:
•the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving, or providing any remuneration (including any kickback, bribe, or certain rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order, or recommendation of, any good, facility, item, or service, for which payment may be made, in whole or in part, under any U.S. federal healthcare program, such as Medicare and Medicaid. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers, on the one hand, and prescribers, purchasers, and formulary managers, on the other. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•the U.S. federal civil and criminal false claims, including the civil False Claims Act, which can be enforced through civil whistleblower or qui tam actions, and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the U.S. federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease, or conceal an obligation to pay money to the U.S. federal government. Pharmaceutical manufacturers can cause false claims to be presented to the U.S. federal government by engaging in impermissible marketing practices, such as the off-label promotion of a product for an indication for which it has not received FDA approval. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
•the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the healthcare fraud statute implemented under HIPAA or specific intent to violate it in order to have committed a violation;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, also imposes certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy and security of individually identifiable health information of covered entities subject to the rule, such as health plans, healthcare clearinghouses, and certain healthcare providers as well as their business associates, independent contractors of a covered entity that perform certain services involving the use or disclosure of individually identifiable health information on their behalf, and their subcontractors that use, disclose, or otherwise process individually identifiable health information;
•the Federal Food Drug and Cosmetic Act, or FDCA, which prohibits, among other things, the adulteration or misbranding of drugs, biologics, and medical devices;
•the U.S. Physician Payments Sunshine Act and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics, and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program, with specific exceptions, to report annually to the government information related to certain payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists, and chiropractors), other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members; and
•similar healthcare laws and regulations in other jurisdictions, such as state anti-kickback and false claims laws, state laws that require biopharmaceutical companies to comply with the biopharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, state laws that require the reporting of information relating to drug and biologic pricing; state and local laws that require the registration of pharmaceutical sales representatives and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect as HIPAA, thus complicating compliance efforts. Outside the United States, interactions between pharmaceutical companies and healthcare professionals are also governed by strict laws, such as national anti-bribery laws of European countries, national sunshine rules, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct. These laws may include the French “Bertrand Law”, French Ordinance n° 2017-49 of January 19, 2017 and Decree No. 2020-730 of June 15, 2020 relating to benefits offered by persons manufacturing or marketing health products or services, and the UK’s Bribery Act 2010, which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state marketing, and/or transparency laws applicable to manufacturers or any company providing services related to their products that may be broader in scope than the federal requirements. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines, or imprisonment.
Ensuring that our internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations is and will continue to be costly. It is possible that governmental authorities will conclude that our business practices, including our relationships with physicians and other healthcare providers, may not comply with current or future statutes, regulations, or case law involving applicable fraud and abuse or other healthcare laws and regulations.
If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal, and administrative penalties, as well as damages, fines, disgorgement, imprisonment, exclusion from participating in U.S. government-funded healthcare programs, such as Medicare and Medicaid, or comparable foreign programs, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, contractual damages, reputational harm, and the curtailment or restructuring of our operations.
Even if resolved in our favor, litigation or other legal proceedings relating to healthcare laws and regulations may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our ordinary shares and ADSs. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development, manufacturing, sales, marketing, or distribution activities. Uncertainties resulting from the initiation and continuation of litigation or other proceedings relating to applicable healthcare laws and regulations could have an adverse effect on our ability to compete in the marketplace. Further, if the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to significant civil, criminal, or administrative sanctions, including exclusions from U.S. government-funded healthcare programs.
Healthcare legislative reform measures may have a negative impact on our business, financial condition, results of operations, and prospects.
In the United States, the European Union and some foreign jurisdictions, there have been, and we expect there will continue to be, several legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare. For example, in March 2010, the ACA was passed, which substantially changed the way healthcare is financed by both governmental and private payors in the United States. Among the provisions of the ACA, those of greatest importance to the pharmaceutical and biotechnology industries include increasing the minimum level of Medicaid rebates payable by manufacturers of brand name drugs; requiring collection of rebates for drugs paid by Medicaid managed care organizations; requiring manufacturers to participate in a coverage gap discount program, under which they must agree to offer point-of-sale discounts (increased to 70 percent, effective as of January 1, 2019) off negotiated prices of applicable brand drugs to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D; imposing a non-deductible annual fee on pharmaceutical manufacturers or importers who sell certain “branded prescription drugs” to specified federal government programs; implementing a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; expanding the types of entities eligible for the 340B drug discount program; expanding eligibility criteria for Medicaid programs; creating a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and establishing a Center for Medicare Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
There have been amendments to and executive, judicial, and congressional challenges to certain aspects of the ACA. For example, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is unclear how any healthcare reform measures of the second Trump administration will impact the ACA and our business.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of 2% per fiscal year pursuant to the Budget Control Act of 2011, which began in 2013 and through subsequent legislation will remain in effect through 2032, unless additional Congressional action is taken. Additionally, on March 11, 2021, the American Rescue Plan Act of 2021 was signed into law, which eliminates the statutory Medicaid drug rebate cap, currently set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, effective January 1, 2024.
Further, in the United States there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries, Presidential executive orders, and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug and biological product pricing, reduce the cost of prescription drugs and biological products under government payor programs, and review the relationship between pricing and manufacturer patient programs. At the federal level, the IRA, among other things, (i) directs HHS to negotiate the price of certain high-expenditure, single-source biologics that have been on the market for at least 11 years covered under Medicare, and subjects drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” for such drugs and biologics under the law, or the Medicare Drug Price Negotiation Program and (ii) imposes rebates with respect to certain drugs and biologics covered under Medicare Part B or Medicare Part D to penalize price increases that outpace inflation. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. These provisions take effect progressively and began in 2023. On August 15, 2024, HHS announced the agreed-upon reimbursement price of the first ten drugs that were subject to price negotiations, although the Medicare Drug Price Negotiation Program is currently subject to legal challenges.
On January 17, 2025, HHS selected fifteen additional drugs products covered under Part D for price negotiation in 2025. Each year thereafter more Part B and Part D products will become subject to the Medicare Drug Price Negotiation Program. Further, on December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework.
At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine which drugs, biological products, and suppliers will be included in their healthcare programs. Furthermore, there has been increased interest by third-party payors and governmental authorities in reference pricing systems and publication of discounts and list prices.
We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our current or any future product candidates or additional pricing pressures. We cannot predict the likelihood, nature, or extent of government regulation that may arise from future legislation or administrative action in the United States or any other jurisdiction, particularly given recent U.S. Congressional and Presidential elections. The current Trump administration is pursuing policies to reduce regulations and expenditures across government including at HHS, the FDA, CMS and related agencies. These actions, presently directed by executive orders or memoranda from the Office of Management and Budget, may propose policy changes that create additional uncertainty for our business. These actions may include, for example, directives to reduce agency workforce, rescinding a Biden administration executive order tasking the Center for Medicare and Medicaid Innovation, or CCMI, to consider new payment and healthcare models to limit drug spending and eliminating the Biden administration’s executive order that directed HHS to establishing an AI task force and developing a strategic plan. The newly elected Presidential administration also may be more skeptical of the safety and efficacy of vaccine products, which could lead to increased regulatory scrutiny and more restrictive coverage policies regarding our products and product candidates. Additionally, in its June 2024 decisions in Loper Bright Enterprises v. Raimondo, or Loper Bright, the U.S. Supreme Court overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statues. The Loper Bright decisions could result in additional legal challenges to current regulations and guidance issued by federal agencies applicable to our operations, including those issued by the FDA. Congress may introduce and ultimately pass health care related legislation that could impact the drug approval process and make changes to the Medicare Drug Price Negotiation Program created under the IRA. If we or any third parties we may engage are slow or unable to adapt to changes in existing or new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our current or any future product candidates we may develop may lose any regulatory approval that may have been obtained, and we may not achieve or sustain profitability. Further, any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products.
In some countries, the proposed pricing for a biopharmaceutical product must be approved before it may be lawfully marketed. In addition, in certain foreign markets, the pricing of biopharmaceutical products is subject to government control, and reimbursement may in some cases be unavailable. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. An EU Member State may approve a specific price for the medicinal product, refuse to reimburse a product at the price set by the manufacturer, or adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market.
Moreover, in the EEA some countries require the completion of additional studies that compare the cost-effectiveness of a particular medicinal product candidate to currently available therapies. This Health Technology Assessment, or HTA process, which is currently governed by the national laws of the individual EU Member States, is the procedure according to which the assessment of the public health impact, therapeutic impact, and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States. On January 31, 2018, the European Commission adopted a proposal for a regulation on health technologies assessment. The proposed regulation is intended to boost cooperation among EU Member States in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas. In December 2021, the HTA Regulation was adopted, and it entered into force on January 11, 2022. It will apply from 2025.
There can be no assurance that any country that has price controls or reimbursement limitations for biopharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, biopharmaceutical products launched in the European Union do not follow price structures of the United States and generally tend to have significantly lower prices.
In addition, the policies of the FDA, the competent authorities of the EU Member States, the EMA, the European Commission, and other comparable regulatory authorities with respect to clinical trials may change and additional government regulations may be enacted.
For instance, the regulatory landscape related to clinical trials in the EU recently evolved. The EU Clinical Trials Regulation, or CTR, which was adopted in April 2014 and repeals the EU Clinical Trials Directive, became applicable on January 31, 2022. The CTR allows sponsors to make a single submission to both the competent authority and an ethics committee in each EU Member State, leading to a single decision for each EU Member State. The assessment procedure for the authorization of clinical trials has been harmonized as well, including a joint assessment by all EU Member States concerned, and a separate assessment by each EU Member State with respect to specific requirements related to its own territory, including ethics rules. Each EU Member State’s decision is communicated to the sponsor via the centralized EU portal. Once the clinical trial is approved, clinical study development may proceed. The CTR foresaw a three-year transition period that ended on January 31, 2025. Since this date, all new or ongoing trials are subject to the provisions of the CTR. Compliance with the CTR requirements by us and our third-party service providers, such as CROs, may impact our developments plans.
It is currently unclear to what extent the UK will seek to align its regulations with the EU in the future. The UK regulatory framework in relation to clinical trials is derived from existing EU legislation (as implemented into UK law, through secondary legislation).
On January 17, 2022, the UK Medicines and Healthcare products Regulatory Agency, or MHRA, launched an eight-week consultation on reframing the UK legislation for clinical trials. The UK Government published its response to the consultation on March 21, 2023 confirming that it would bring forward changes to the legislation and such changes were laid before parliament on December 12, 2024. These resulting legislative amendments will, if implemented in their current form, bring the UK into closer alignment with the CTR. Failure of the UK to closely align its regulations with the EU may have an effect on the cost of conducting clinical trials in the UK as opposed to other countries and/or make it harder to seek a marketing authorization for the Company's product candidates on the basis of clinical trials conducted in the United Kingdom.
In addition, on April 26, 2023, the European Commission adopted a proposal for a new Directive and Regulation to revise the existing pharmaceutical legislation and on April 10, 2024, the Parliament adopted its related position. The proposed revisions remain to be agreed and adopted by the European Council. Moreover, on December 1, 2024, a new European Commission took office. The proposal could, therefore, still be subject to revisions. If adopted in the form proposed, the European Commission proposals to revise the existing EU laws governing authorization of medicinal products may result in a number of changes to the regulatory framework governing medical products, including a decrease in data and market exclusivity opportunities for our product candidates in the EU and make them open to generic or biosimilar competition earlier than is currently the case with a related reduction in reimbursement status.
If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies governing clinical trials, our development plans may be impacted.
We are subject to various risks in connection with sustainability (environmental, social, and governance), including evolving and potentially conflicting regulatory regimes related to sustainability, which may impact our reputation, business, financial condition, and results of operations.
As a publicly listed company in France and the United States, we are subject to regulations related to sustainability in both jurisdictions, including most notably the European Union’s Corporate Sustainability Reporting Directive (CSRD), under which we were required to report extensive information about our environmental, social, and governance practices and associated impacts, risks, and opportunities. This information is included in our annual report for 2024 filed with the French Financial Markets Authority (Authorité des marchés financiers, or AMF). On February 26, 2025, the European Commission issued a proposal for significant changes to the CSRD and related regulations which, if implemented, would substantially reduce Valneva’s future sustainability-related reporting obligations from a European perspective. We cannot guarantee that these proposed changes will be implemented, in whole or in part, and the timing of such implementation will determine numerous aspects of Valneva’s sustainability-related activities, including the amount of resources allocated to support compliance with reporting on the scale required for the year ended December 31, 2024. Compliance with the CSRD and with the related EU Taxonomy Regulation requires significant time, attention, and resources. Similarly, we would be subject to the SEC’s climate disclosure rules which were published in March 2024 and are currently suspended. We cannot predict if, when, or in what form such rules may apply to Valneva in the future. Additionally, pushback against sustainability-related regulations and practices has gained momentum in the United States at the federal level and in certain states. Most notably, executive orders signed in January 2025 by President Trump addressing diversity, equity, and inclusion (DEI) could result in additional compliance obligations, scrutiny of Valneva’s practices, and potential harm to Valneva’s business, given that the U.S. Department of Defense is one of our key customers. Monitoring and adjusting to ongoing regulatory developments related to sustainability may impose unexpected costs or result in other interruptions to our operations that could have a material adverse effect on our business, financial condition, and results of operations.
Furthermore, expectations of stakeholders regarding sustainability also continue to evolve and may conflict with one another. Relevant stakeholders include investors, governments, customers, employees, third party business partners, and the communities in which we operate. While some stakeholders wish for companies to make commitments to sustainability, for example related to climate change, others may oppose such activities. In this context, we may not be able to meet stakeholder expectations, by failing to meet commitments, failing to make commitments or commitments of sufficient ambition, or by making commitments that may be perceived as misaligned with our strategy or corporate interests. Failing to meet stakeholder expectations related to sustainability could result in litigation or regulatory actions and impact our reputation, share price, recruitment and retention, financial condition, and results of operations. Our actions related to sustainability could also have a long-term and potentially negative impact on our operations and achievement of our strategic goals.
Additionally, our sustainability-related information published in compliance with the CSRD is subject to a different, more expansive standard of materiality than is understood in the context of U.S. federal securities laws and regulations. The double materiality standard requires the reporting entity to consider both financial materiality (i.e., sustainability matters which generate risks or opportunities that affect, or could reasonably be expected to affect, the Company’s financial position, financial performance, cash flows, access to finance, or cost of capital over the short, medium, or long term) and impact materiality (i.e., the Company’s material actual or potential, positive or negative impacts on people or the environment over the short-, medium-, and long-term). Impacts, risks, and opportunities are material if they satisfy one or both of these materiality tests. Such information should therefore not necessarily be interpreted as material in the U.S. context, even if the words “material” or “materiality” are used.
We are subject to anti-corruption laws, as well as export control laws, customs laws, sanctions laws, and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures, and legal expenses, which could adversely affect our business, results of operations, and financial condition.
We are subject to other laws and regulations governing our international operations, including regulations administered by the authorities in the United States, European Union, and United Kingdom, including applicable export control regulations, economic sanctions on countries and persons, and customs requirements and currency exchange regulations, collectively referred to as the trade control laws. Specifically, as a result of the Russian invasion of Ukraine in February 2022, the United States, the European Union, the United Kingdom, and other jurisdictions adopted a series of financial and trade sanctions in relation to Russia and Belarus and Russian and Belarussian listed citizens and entities.
Exports of our products and product candidates must be made in compliance with trade control laws. In some cases, certain licensing, authorization, or reporting requirements may need to be performed. In addition, these laws may restrict or prohibit altogether the supply of certain of our products, product candidates, or services to certain governments, persons, entities, countries, and territories. Changes in our products and product candidates or changes in applicable trade control laws may create delays in the introduction or provision of our products and product candidates in certain jurisdictions, prevent others from using our products and product candidates or, in some cases, prevent the export or import of our products and product candidates to certain countries, governments, or persons altogether. Any limitation on our ability to export or provide our products, product candidates, and services could adversely affect our business, financial condition, and results of operations.
We are also subject to anti-corruption laws in the countries where we operate, including the United States. The Foreign Corrupt Practices Act, or FCPA, prohibits companies and their employees, third-party intermediaries, and other associated persons from paying, offering, authorizing payment, or providing anything of value, directly or indirectly, to any foreign official, political party, or candidate for the purpose of influencing any act or decision of a foreign entity in order to obtain or retain business. The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls. An executive order issued in February 2025 has created considerable uncertainty regarding whether and how the FCPA will continue to be enforced.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the biopharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials.
French anti-corruption laws also prohibit acts of bribery and influence peddling:
•Article 433-1-1° of the French Criminal Code (bribery of domestic public officials);
•Article 433-1-2° of the French Criminal Code (influence peddling involving domestic public officials);
•Article 434-9 of the French Criminal Code (bribery of domestic judicial staff);
•Article 434-9-1 of the French Criminal Code (influence peddling involving domestic judicial staff);
•Articles 435-1 and 435-3 of the French Criminal Code (bribery of foreign or international public officials);
•Articles 435-7 and 435-9 of the French Criminal Code (bribery of foreign or international judicial staff);
•Articles 435-2, 435-4, 435-8 and 435-10 of the French Criminal Code (active and passive influence peddling involving foreign or international public officials and foreign or international judicial staff);
•Articles 445-1 and 445-2 of the French Criminal Code (bribery of private individuals); and
•French Law n°2016-1691 of December 9th, 2016 on Transparency, the Fight Against Corruption and the Modernization of the Economy (Sapin 2 Law), which provides for numerous new obligations for large companies such as the obligation to draw up and adopt a code of conduct defining and illustrating the different types of behavior to be proscribed as being likely to characterize acts of corruption or influence peddling, to set up an internal warning system designed to enable the collections of reports from employees relating to the existence of conduct or situations contrary to the company’s code of conduct, to set up accounting control procedures, whether internal or external, designed to ensure that the books, registers and accounts are not used to conceal acts of corruption or influence peddling, to set up a disciplinary system for sanctioning company employees in the event
of a breach of the company’s code of conduct or a system for monitoring and evaluating the measures implemented.
We are also subject to the UK Bribery Act 2010, which makes it a criminal offense to:
•Offer, promise, or give a financial or other advantage to a person to induce them to perform improperly or reward a person for improper performance (directly or via a third party). A bribe can be of any form, size, or value that would provide the intended recipient with some form of benefit or advantage. Bribes can include money, discounts, vouchers, loans, gifts, hospitality, accommodation, use of assets, preferential treatment, business advantage, and employment opportunities, among others;
•Request, agree to receive or accept a financial or other advantage with the intention of or as reward for improper performance (directly or via a third party);
•Attempt bribery of a foreign public official in order to obtain or retain business or an advantage in the conduct of business, either directly or via a third party;
•As a commercial organization, fail to prevent bribery, as a result of not having adequate procedures in place to prevent a person directly or associated with a company to commit any of the other offenses.
For the purposes of the UK Bribery Act 2010, “foreign public official” means an individual who:
•is an official or agent of a public international organization; or
•exercises a public function:
◦for or on behalf of a country or territory outside the Island (or any subdivision of such a country or territory); or
◦for any public agency or public enterprise of that country or territory (or subdivision).
We might not be effective in ensuring compliance by our employees, representatives, contractors, business partners, and agents with all applicable anti-corruption laws, including the FCPA, the French anti-corruption laws, or other applicable legal requirements, including trade control laws. If we are not in compliance with the FCPA, the French anti-corruption laws, and other anti-corruption laws or trade control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations, and liquidity. Likewise, any investigation of any potential violations of the FCPA, the French anti-corruption laws, other anti-corruption laws or trade control laws by U.S. or other authorities could also have an adverse impact on our reputation, our business, results of operations, and financial condition.
We (and our service providers) receive, process, store, and use personal information and other data, which subjects us to stringent and evolving U.S. and foreign laws, regulations, rules, contractual obligations, policies, and other obligations related to data privacy and security. Our (and our service providers’) actual or perceived failure to comply with such obligations could harm our reputation, subject us to significant fines and liability, and otherwise adversely affect our business.
We, and our service providers, receive, process, store, and use personal information and other data about our clinical trial participants, employees, partners, and others. We, and our service providers, must comply with numerous foreign and domestic laws, regulations, guidance, industry standards, external and internal privacy and security policies, contractual requirements, and other obligations regarding privacy and the storing, sharing, use, processing, disclosure, security, and protection of personal information and other data, such as information that we collect about patients and healthcare providers in connection with clinical trials in Europe, the United States, and elsewhere. We strive to comply with all applicable requirements and obligations; however, new laws, policies, codes of conduct, and legal obligations may arise, continue to evolve, be interpreted and applied in a manner that is inconsistent from one jurisdiction to another, and conflict with one another. Any failure or perceived failure by us or third parties working on our behalf to comply with applicable laws and regulations, any privacy and data security obligations pursuant to contract or pursuant to our stated privacy or security policies, or obligations to third parties may result in governmental enforcement actions (including fines, penalties, judgments, settlements, imprisonment of company officials and public censure), civil claims (to which we have been subject), litigation, damage to our reputation, and loss of goodwill, any of which could have a material adverse effect on our business, operations, and financial performance. With substantial uncertainty over the interpretation and application of these laws, regulations, and other obligations, we may face challenges in addressing their requirements and making necessary changes to our policies and practices, and may incur significant costs and expenses in our efforts to do so.
In the United States, federal, state, and local governments have enacted numerous data privacy and security laws, including data breach notification laws, personal data privacy laws, consumer protection laws (e.g., Section 5 of the Federal Trade Commission Act), and other similar laws (e.g., state surveillance and wiretapping laws such as California Invasion of Privacy Act). For example, the federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes specific requirements relating to the privacy, security, and transmission of individually identifiable protected health information. In addition, in the past few years, numerous U.S. states—including California, Virginia, Colorado, Connecticut, and Utah—have enacted comprehensive privacy laws that impose certain obligations on covered businesses, including providing specific disclosures in privacy notices and affording residents with certain rights concerning their personal data. As applicable, such rights may include the right to access, correct, or delete certain personal data, and to opt-out of certain data processing activities, such as targeted advertising, profiling, and automated decision-making.
The exercise of these rights may impact our business and ability to provide our products and services. Certain states also impose stricter requirements for processing certain personal data, including sensitive information, such as conducting data privacy impact assessments. These state laws allow for statutory fines for noncompliance. For example, the California Consumer Privacy Act of 2018, as amended by the California Privacy Rights Act of 2020, together referred to as the CCPA, applies to personal data of consumers, business representatives, and employees who are California residents, and requires businesses to provide specific disclosures in privacy notices and honor requests of such individuals to exercise certain privacy rights, such as those noted below. The CCPA provides for fines and allows private litigants affected by certain data breaches to recover significant statutory damages. Although the CCPA exempts some data processed in the context of clinical trials, the CCPA increases compliance costs and potential liability with respect to other personal data we maintain about California residents. Similar laws are being considered in several other states, as well as at the federal and local levels, and we expect more states to pass similar laws in the future. While these states, like the CCPA, also exempt some data processed in the context of clinical trials, these developments further complicate compliance efforts and increase legal risk and compliance costs for us, the third parties upon whom we rely, and our customers.
Our employees and personnel use generative artificial intelligence, or AI, technologies to perform their work, and the disclosure and use of personal data in generative AI technologies is subject to various privacy laws and other privacy obligations. Governments have passed and are likely to pass additional laws regulating generative AI. Our use of this technology could result in additional compliance costs, regulatory investigations and actions, and lawsuits. If we are unable to use generative AI, it could make our business less efficient and result in competitive disadvantages.
The global data protection landscape is rapidly evolving, and we expect that there will continue to be new and proposed laws, regulations, and industry standards concerning privacy, data protection, and information security, and we cannot yet determine the impact that such future laws, regulations, and standards may have on our business. For example, in Canada, the Personal Information Protection and Electronic Documents Act and various related provincial laws, as well as Canada’s Anti-Spam Legislation, apply to our operations. The EU General Data Protection Regulation and United Kingdom’s implementation of the General Data Protection Regulation, known respectively as the EU and UK GDPR, as well as EEA Member States’ and the United Kingdom’s implementing national legislation, apply to the collection and processing of personal data, including health-related information, by companies located in the EEA or the United Kingdom. In certain circumstances, the EU and UK GDPR also apply to companies located outside of the EEA or United Kingdom who are processing personal data of individuals located in the EEA or United Kingdom. The EU and UK GDPR have increased compliance burdens on us, such as requiring the following:
•processing personal data only for specified, explicit, and legitimate purposes for which personal data were collected;
•establishing a legal basis for processing personal data and creating obligations for controllers and processors to appoint data protection officers in certain circumstances;
•increasing transparency obligations to data subjects for controllers (including presentation of certain information in a concise, intelligible, and easily accessible form about how their personal data is used and their rights vis-à-vis that data and its use);
•introducing the obligation to carry out so-called data protection impact assessments in certain circumstances;
•establishing limitations on collection and retention of personal data through “data minimization” and “storage limitation” principles;
•establishing obligations to implement “privacy by design”;
•introducing obligations to honor increased rights for data subjects (such as rights for individuals to be “forgotten,” rights to data portability, and rights to object, etc., in certain circumstances);
•formalizing a heightened and codified standard of data subject consent;
•establishing obligations to implement certain technical and organizational safeguards to protect the security and confidentiality of personal data;
•introducing obligations to agree to certain specific contractual terms and to take certain measures when engaging third party processors and joint controllers;
•introducing the obligation to provide notice of certain personal data breaches to the relevant supervisory authority or authorities and affected individuals; and
•mandating the appointment representatives in the United Kingdom and/or EEA in certain circumstances.
The processing of sensitive personal data, such as health information, is subject to compliance with specific exceptions under the EU and UK GDPR which may impose heightened compliance burdens and is a topic of active interest among foreign regulators. The EU and UK GDPR increase our obligations with respect to clinical trials conducted in Europe (including the EEA, United Kingdom and Switzerland) by expressly expanding the definition of personal data to include “pseudonymized” or key-coded data and requiring changes to informed consent practices and more detailed notices for clinical trial subjects and investigators.
The EU and UK GDPR also provide for more robust regulatory enforcement and greater penalties for noncompliance than previous data protection laws, including fines of up to 20 million euros under the EU GDPR, 17.5 million pound sterling under the UK GDPR, or in each case, 4% of global annual revenue for the preceding financial year, whichever is higher.
In addition to administrative fines, a wide variety of other potential enforcement powers are available to competent supervisory authorities in respect of potential and suspected violations of the EU and UK GDPR, including extensive audit and inspection rights, and powers to order temporary or permanent bans on all or some processing of personal data carried out by non-compliant actors. The EU and UK GDPR also confer a private right of action on data subjects and consumer associations to lodge complaints with supervisory authorities, seek judicial remedies, and obtain compensation for damages resulting from violations of the EU and UK GDPR.
In the ordinary course of business, we may transfer personal data from Europe and other jurisdictions to the United States or other countries. Europe and other jurisdictions have enacted laws requiring data to be localized or limiting the transfer of personal data to other countries. In particular, the EEA and the United Kingdom have significantly restricted the transfer of personal data to the United States and other countries whose privacy laws are generally believed to be inadequate. Other jurisdictions may adopt similarly stringent interpretations of their data localization and cross-border data transfer laws. Although there are currently various mechanism that may be used to transfer personal data from the EEA and United Kingdom to the United States in compliance with law, such as the EEA standard contractual clauses, the UK’s International Data Transfer Agreement / Addendum, and the EU-U.S. Data Privacy Framework and the UK extension thereto (which allows for transfers to relevant U.S.-based organizations who self-certify compliance and participate in the Framework), these mechanisms are subject to legal challenges, and there is no assurance that we can satisfy or rely on these measures to lawfully transfer personal data to the United States.
If there is no lawful manner for us to transfer personal data from the EEA or UK, including, for example, obtaining individuals’ explicit consent to transfer their personal data from the EEA or UK to the United States or other countries, we will face increased exposure to regulatory actions, substantial fines, and injunctions against processing personal data from the EEA or United Kingdom. The inability to transfer personal data from the EEA, United Kingdom, or Switzerland may also restrict our clinical trials activities in such jurisdictions, limit our ability to collaborate with contract research organizations as well as other service providers, contractors and other companies subject to European data protection laws, and require us to increase our data processing capabilities in the EEA, United Kingdom, or Switzerland, likely at significant expense. Additionally, other countries outside of Europe have enacted or are considering enacting similar cross-border data transfer restrictions and laws requiring local data residency, which could increase the cost and complexity of delivering our services and operating our business. The type of challenges we face in Europe will likely also arise in other jurisdictions that adopt laws similar in construction to the EU and UK GDPR or regulatory frameworks of equivalent complexity.
The EU GDPR provides that EEA countries may make their own further laws and regulations to introduce specific requirements related to the processing of “special categories of personal data,” including personal data related to health, biometric data used for unique identification purposes, and genetic information, as well as personal data related to criminal offences or convictions. In the United Kingdom, the United Kingdom Data Protection Act 2018 complements the UK GDPR in this regard. This fact may lead to greater divergence on the law that applies to the processing of such data types across the EEA and/or United Kingdom, compliance with which, as and where applicable, may increase our costs and could increase our overall compliance risk. Such country-specific regulations could also limit our ability to collect, use, and share data in the context of our EEA and/or United Kingdom establishments (regardless of where any processing in question occurs), and/or could cause our compliance costs to increase, ultimately having an adverse impact on our business, and harming our business and financial condition.
For example, in France, the conduct of clinical trials is subject to compliance with specific provisions. The French Law No.78-17 of 6 January 1978 on Information Technology, Data Files and Civil Liberties, as amended, establishes a strict framework applicable to the processing of personal data in the health sector. This framework requires, among others, the filing of compliance undertakings with “reference methodologies” (such as the MR-001) adopted by the French Data Protection Authority, or CNIL, or, if not complying, obtaining an authorization from the CNIL. Failure to comply with the stringent provisions of the reference methodologies or failure to obtain the CNIL’s authorization could expose us to adverse consequences, including the interruption of our clinical trials in France, increased exposure to regulatory actions, or the need to relocate part of or all of our data processing activities to other jurisdictions at significant expense.
It is possible that the EU and UK GDPR or other laws and regulations relating to privacy and data protection may be interpreted and applied in a manner that is inconsistent from jurisdiction to jurisdiction or inconsistent with our current policies and practices, and compliance with such laws and regulations could require us to change our business practices and compliance procedures in a manner adverse to our business. We cannot guarantee that we are in compliance with all such applicable data protection laws and regulations, and we cannot be sure how these regulations will be interpreted, enforced, or applied to our operations. Furthermore, other jurisdictions outside the EEA are similarly introducing or enhancing privacy and data security laws, rules, and regulations, which could increase our compliance costs and the risks associated with noncompliance. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices, and our efforts to comply with the evolving data protection rules may be unsuccessful. We cannot guarantee that we, our third-party collaborators, or our vendors are in compliance with all applicable data protection and privacy laws and regulations as they are enforced now or as they evolve. Further, for example, our privacy policies may be insufficient to protect any personal information we collect, or may not comply with applicable laws. Our non-compliance could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level may be costly and require ongoing modifications to our policies, procedures, and systems. In addition, if we are unable to properly protect the privacy and security of protected health information, we could be found to have breached our contracts.
In addition to data privacy and security laws, we may be subject to contractual obligations based on industry standards adopted by industry groups, such as best practices governing the conduct of clinical trials, and we are, or may become, subject to such obligations in the future. We are also subject to contractual obligations related to data privacy and security. Our efforts to comply with such obligations may not be successful. For example, certain privacy laws, such as the EU and UK GDPR and CCPA, may require us to impose specific contractual restrictions on certain service providers that have access to personal data, such as clinical trial patient data or personal data of clinical trial site personnel. We publish privacy policies, marketing materials, and other statements regarding data privacy and security on our website. If these policies, materials or statements are found to be deficient, lacking in transparency, deceptive, or unfair, or to misrepresent our practices, we may be subject to investigation, enforcement actions by regulators (such as the Federal Trade Commission), or other adverse consequences.
Our actual or perceived failure to adequately comply with applicable laws and regulations relating to privacy and data protection, or to protect personal data and other data we process or maintain, could result in regulatory enforcement actions against us, including fines, penalties, orders that require a change in our practices, additional reporting requirements and/or oversight, imprisonment of company officials and public censure, claims for damages by affected individuals, other lawsuits, or reputational damage.
We benefit from tax credits in Austria, France, and Scotland that could be reduced or eliminated.
As a company with research and development activity, we benefit from certain tax advantages, including the Austrian Research and Development tax credit, the French Research Tax Credit (Crédit Impôt Recherche) and the Scottish Research and Development tax credit, which are tax credits aimed at stimulating research and development. Our Austrian Research and Development tax credits were €3.6 million, €5.7 million and €13.9 million for the years ended December 31, 2024, 2023, and 2022, respectively. Our French Research Tax Credits were €1.3 million, €1.1 million, and €1.5 million for the years ended December 31, 2024, 2023, and 2022, respectively. Our Scottish Research and Development tax credit amounted to €5.2 million in the year ended December 31, 2024. The Austrian Research and Development tax credit is calculated based on claimed amount of eligible research and development in Austria, while the French Research Tax credit is calculated based on our claimed amount of eligible research and development expenditures in France. The main differences between the Austrian and French research tax credits are the applicable percentage of and the basis for the tax credit. The tax credits are a source of financing to us that could be reduced or eliminated by the Austrian and French tax authorities or by changes in Austrian and French tax law or regulations.
The Austrian Research and Development tax credit is reimbursed to us. While the Austrian Research and Development tax credit is reviewed as a part of the issuance of a certificate by the local auditor and the research and development projects need an approval from the Austrian Research Promotion Agency (FFG), the Austrian tax authority may audit each research and development claim. The Austrian tax authorities may challenge our eligibility for, or our calculation of, certain tax reductions in respect of our research and development activities (and therefore the amount of Research and Development Tax Credit claimed). Furthermore, the Austrian Parliament may decide to eliminate, or to reduce the scope or the rate of, the Research Tax Credit benefit, either of which it could decide to do at any time.
The French Research Tax Credit can be offset against French corporate income tax due with respect to the year during which the eligible research and development expenditures have been made. The portion of tax credit in excess which is not being offset, if any, represents a receivable against the French Treasury which can in principle be offset against the French corporate income tax due by the company with respect to the three following years. The remaining portion of tax credit not being offset upon expiry of such a period may then be refunded to the company. The French Research Tax credit is reimbursed within the expiry of a period of three years.
The French tax authorities, with the assistance of the Higher Education and Research Ministry, may audit each research and development program in respect of which a Research Tax Credit benefit has been claimed and assess whether such program qualifies in their view for the Research Tax Credit benefit. The French tax authorities may challenge our eligibility for, or our calculation of, certain tax reductions or deductions in respect of our research and development activities (and therefore the amount of Research Tax Credit claimed). Furthermore, the French Parliament may decide to eliminate, or to reduce the scope or the rate of, the Research Tax Credit benefit, either of which it could decide to do at any time.
The United-Kingdom Research and Development (R&D) tax relief supports companies that work on innovative projects in science and technology. To qualify for R&D relief, a project must seek an advance in a field of science or technology. Only companies chargeable to UK Corporation Tax can qualify for this relief. The British tax authority may audit each research and development claim. The British tax authorities may challenge our eligibility for, our calculation of, certain tax reductions in respect of our research and development activities (and therefore the amount of Research and Development Tax Credit claimed).
If we fail to receive future Research Tax Credit amounts or if our calculations are challenged, even if we comply with the current requirements in terms of documentation and eligibility of its expenditure, our business, prospects, financial condition, and results of operations could be adversely affected.
We may be unable to carry forward existing tax losses.
We have accumulated tax loss carry forwards of €843.6 million, €879.1 million, and €821.6 million for the years ended December 31, 2024, 2023, and 2022, respectively in several locations. Applicable French law provides that, for fiscal years ending after December 31, 2012, the use of these tax losses is limited to €1.0 million, plus 50% of the portion of net earnings exceeding this amount.
The unused balance of the tax losses in application of such rule can be carried forward to future fiscal years, under the same conditions and without time restriction. If we decide to change our corporate structure, this could have an effect on our ability to use these tax loss carry forwards. In addition, future changes to applicable tax law and regulation could eliminate or alter these or other provisions in a manner unfavorable to us.
Comprehensive tax reform legislation could adversely affect our business and financial condition.
Corporate tax reform, anti-base-erosion rules and tax transparency continue to be high priorities in many jurisdictions. As a result, policies regarding corporate income and other taxes in numerous jurisdictions are under heightened scrutiny and tax reform legislation has been, and will likely continue to be, proposed or enacted in a number of jurisdictions in which we operate.
In August 2022, the Inflation Reduction Act was signed into law in the United States incorporating some of the Biden Administration’s proposals for corporate tax reform. Other recently enacted legislation in the United States includes the Tax Act, the Families First Coronavirus Response Act, and the CARES Act. The U.S. Department of Treasury has broad authority to issue regulations and interpretative guidance that may have a significant impact on our results of operations in the period issued, including our effective tax rate.
In addition, many countries are implementing legislation and other guidance to align their international tax rules with those of the Organization for Economic Co-operation and Development, or OECD, whose base erosion and profit shifting recommendations and action plan aim to standardize and modernize global corporate tax policy, including changes to cross-border tax, transfer pricing documentation rules, and nexus-based tax incentive practices. The OECD is also continuing discussions surrounding fundamental changes in allocation of profits among tax jurisdictions in which companies do business, as well as the implementation of a global minimum tax (namely the “Pillar One” and “Pillar Two” proposals). As a result of this heightened scrutiny, prior decisions by tax authorities regarding treatments and positions of corporate income taxes could be subject to enforcement activities and legislative investigation and inquiry, which could also result in changes in tax policies or prior tax rulings. Any such changes may also result in the taxes we previously paid being subject to change.
Risks Related to Ownership of Our Ordinary Shares and the ADSs
We do not currently intend to pay dividends on our securities and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of the ordinary shares and ADSs. In addition, French law may limit the amount of dividends we are able to distribute.
We have never declared or paid any cash dividends on our ordinary shares and do not currently intend to do so for the foreseeable future. We currently intend to invest our future earnings, if any, to fund our growth.
Therefore, the holders of our ordinary shares and ADSs are not likely to receive any dividends for the foreseeable future and the success of an investment in our ordinary shares and ADSs will depend upon any future appreciation in value. Consequently, investors may need to sell all or part of their holdings of the ordinary shares or ADSs after price appreciation, which may never occur, as the only way to realize any future gains on their investment. There is no guarantee that the ordinary shares or ADSs will appreciate in value or even maintain the price at which our shareholders have purchased them.
Further, under French law, the determination of whether we have been sufficiently profitable to pay dividends is made on the basis of our statutory financial statements prepared and presented in accordance with accounting standards applicable in France. Moreover, pursuant to French law, we must allocate 5% of our unconsolidated net profit for each year to our legal reserve fund before dividends, should we propose to declare any, may be paid for that year, until the amount in the legal reserve is equal to 10% of the aggregate nominal value of our issued and outstanding share capital. In addition, payment of dividends may subject us to additional taxes under French law. Therefore, we may be more restricted in our ability to declare dividends than companies that are not incorporated in France.
In addition, exchange rate fluctuations may affect the amount of euro that we are able to distribute, and the amount in U.S. dollars that our shareholders receive upon the payment of cash dividends or other distributions we declare and pay in euro, if any. These factors could harm the value of the ADSs, and, in turn, the U.S. dollar proceeds that holders receive from the sale of the ADSs.
Future sales of ordinary shares or ADSs by existing shareholders could depress the market price of the ordinary shares or ADSs.
Future sales of a substantial number of our ADSs or ordinary shares, or the perception that such sales will occur, could cause a decline in the market price of our ADSs and/or ordinary shares. Sales in the United States of our ADSs and ordinary shares held by our directors, officers, and affiliated shareholders or ADS holders are subject to restrictions. If these shareholders or ADS holders sell substantial amounts of ordinary shares or ADSs in the public market, or the market perceives that such sales may occur, the market price of our ADSs or ordinary shares and our future ability to raise capital through an issue of equity securities on favorable terms could be adversely affected.
The dual listing of our ordinary shares and the ADSs may adversely affect the liquidity and value of the ADSs.
Our ADSs are listed on the Nasdaq Global Select Market and our ordinary shares are listed on Euronext Paris. Trading of the ADSs or ordinary shares in these markets takes place in different currencies (U.S. dollars on Nasdaq and euro on Euronext Paris), and at different times (resulting from different time zones, different trading days and different public holidays in the United States and France).
The trading prices of our ordinary shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on Euronext Paris could cause a decrease in the trading price of the ADSs on Nasdaq. Investors could seek to sell or buy our ordinary shares to take advantage of any price differences between the markets through a practice referred to as arbitrage. Any arbitrage activity could create unexpected volatility in both our share prices on one exchange, and the ordinary shares available for trading on the other exchange. In addition, holders of ADSs will not be immediately able to surrender their ADSs and withdraw the underlying ordinary shares for trading on the other market without effecting necessary procedures with the depositary. This could result in time delays and additional cost for holders of ADSs. We cannot predict the effect of this continued dual listing on the value of our ordinary shares and the ADSs. However, the continued dual listing of our ordinary shares and ADSs may reduce the liquidity of these securities in one or both markets and may adversely affect the development of an active trading market for the ADSs in the United States.
The rights of shareholders in companies subject to French corporate law differ in material respects from the rights of shareholders of corporations incorporated in the United States.
We are a European public company with limited liability (Societas Europaea or SE), with our registered office in France. Our corporate affairs are governed by our bylaws and by the laws governing companies incorporated in France. The rights of shareholders and the responsibilities of members of our Board of Directors are in many ways different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. For example, in the performance of its duties, our Board of Directors is required by French law to consider the interests of our company, its shareholders, its employees and other stakeholders, rather than solely our shareholders and/or creditors. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a shareholder or holder of ADSs. Further, in accordance with French law, as long as a double voting right is attached to each ordinary share which is held in registered form in the name of the same shareholder for at least two years, ordinary shares deposited with the depositary will not be entitled to double voting rights. Therefore, holders of ADSs who wish to obtain double voting rights will need to surrender their ADSs, withdraw the deposited shares, and take the necessary steps to hold such ordinary shares in registered form in the holder’s name for at least two years. See “Item 16G—Corporate Governance.”
U.S. investors may have difficulty enforcing civil liabilities against our company and members of the Executive Committee and the Board of Directors.
Most of the members of our Executive Committee and Board of Directors and the experts named in this Annual Report are non-residents of the United States, and all or a substantial portion of our assets and the assets of such persons are located outside the United States. As a result, it may not be possible to serve process on such persons or us in the United States or to enforce judgments obtained in U.S. courts against them or us based on civil liability provisions of the securities laws of the United States. Additionally, it may be difficult to assert U.S. securities law claims in actions originally instituted outside of the United States. Foreign courts may refuse to hear a U.S. securities law claim because foreign courts may not be the most appropriate forums in which to bring such a claim. Even if a foreign court agrees to hear a claim, it may determine that the law of the jurisdiction in which the foreign court resides, and not U.S. law, is applicable to the claim. Further, if U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process, and certain matters of procedure would still be governed by the law of the jurisdiction in which the foreign court resides. In particular, there is some doubt as to whether French courts would recognize and enforce certain civil liabilities under U.S. securities laws in original actions or judgments of U.S. courts based upon these civil liability provisions. In addition, awards of punitive damages in actions brought in the United States or elsewhere may be unenforceable in France. An award for monetary damages under the U.S. securities laws would be considered punitive if it does not seek to compensate the claimant for loss or damage suffered but is intended to punish the defendant. French law provides that a shareholder, or a group of shareholders, may initiate a legal action to seek indemnification from the directors of a corporation in the corporation’s interest if it fails to bring such legal action itself. If so, any damages awarded by the court are paid to the corporation and any legal fees relating to such action may be borne by the relevant shareholder or the group of shareholders. The enforceability of any judgment in France will depend on the particular facts of the case as well as the laws and treaties in effect at the time. The United States and France do not currently have a treaty providing for recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters.
Our bylaws and French corporate law contain provisions that may delay or discourage a takeover attempt.
Provisions contained in our bylaws and French corporate law could make it more difficult for a third party to acquire us, even if doing so might be beneficial to our shareholders. In addition, provisions of our bylaws impose various procedural and other requirements, which could make it more difficult for shareholders to effect certain corporate actions. These provisions include the following:
•under French law, the owner of 90% of the share capital and voting rights of a public company listed on a regulated market in a Member State of the European Union or in a state party to the EEA Agreement, including from the main French stock exchange, has the right to force out minority shareholders following a tender offer made to all shareholders;
•under French law, a non-resident of France as well as any French entity controlled by non-residents of France may have to file a declaration for statistical purposes with the Bank of France (Banque de France) within 20 working days following the date of certain direct foreign investments in us, including any purchase of our ADSs. In particular, such filings are required in connection with investments exceeding €15,000,000 that lead to the acquisition of at least 10% of our share capital or voting rights or cross such 10% threshold;
•under French law, certain investments in a French company relating to certain strategic industries (such as research and development in biotechnologies and activities relating to public health) and activities by individuals or entities not French, not resident in France or controlled by entities not French or not resident in France, are subject to prior authorization of the Ministry of Economy;
•a merger (i.e., in a French law context, a share for share exchange following which our company would be dissolved into the acquiring entity and our shareholders would become shareholders of the acquiring entity) of our company into a company incorporated in the European Union would require the approval of our Board of Directors as well as a two-thirds majority of the votes held by the shareholders present, represented by proxy or voting by mail at the relevant meeting;
•a merger of our company into a company incorporated outside of the European Union would require 100% of our shareholders to approve it;
•under French law, a cash merger is treated as a share purchase and would require the consent of each participating shareholder;
•our shareholders have granted our Board of Directors broad authorizations to increase our share capital or to issue additional ordinary shares or other securities (for example, warrants) to our shareholders, the public or qualified investors, including as a possible defense following the launching of a tender offer for our ordinary shares;
•our shareholders have preferential subscription rights on a pro rata basis on the issuance by us of any additional securities for cash or a set-off of cash debts, which rights may only be waived by the extraordinary general meeting (by a two-thirds majority vote) of our shareholders or on an individual basis by each shareholder;
•our Board of Directors appoints the members of the Executive Committee, notably the Chief Executive Officer (Directeur Général) and Associate Managing Officers (Directeurs Généraux Délégués);
•our Board of Directors has the right to appoint members of the Board to fill a vacancy created by the resignation or death of a member of the Board for the remaining duration of such member’s term of office, and subject to the approval by the shareholders of such appointment at the next shareholders’ meeting, which prevents shareholders from having the sole right to fill vacancies on our Board;
•our Board of Directors can be convened by the Chair, Vice-Chair, or Lead Independent Member or, if there has been no Board meeting for more than two months, by Directors representing one-third of the Board;
•our Board of Directors meetings can take place in person, by way of videoconference or teleconference or by written consultation and for decisions of the Board of Directors to be valid, at least half of the Directors must be present or represented;
•approval of at least a majority of the votes held by shareholders present, represented by a proxy, or voting by mail at the relevant ordinary shareholders’ general meeting is required to remove members of the Board of Directors with or without cause;
•the crossing of certain ownership thresholds has to be disclosed and can impose certain obligations;
•advance notice is required for nominations to the Board of Directors or for proposing matters to be acted upon at a shareholders’ meeting, except that a vote to remove and replace a member of the Board can be proposed at any shareholders’ meeting without notice;
•transfers of shares shall comply with applicable insider trading rules and regulations, and in particular with the Market Abuse Regulation 596/2014 of April 16, 2014; and
•pursuant to French law, our bylaws, including the sections relating to the number of members of the Board of Directors and Associate Managing Officers, and election and removal of members of the Board of Directors, the Chief Executive Officer, and Associate Managing Officers from office may only be modified by a resolution adopted by two-thirds of the votes of our shareholders present, represented by a proxy or voting by mail at the meeting.
If we are unable to maintain effective internal controls over financial reporting, the accuracy and timeliness of our financial reporting may be adversely affected, which could hurt our business, lessen investor confidence, and depress the market price of our securities.
We must maintain effective internal control over financial reporting in order to accurately and timely report our results of operations and financial condition. In addition, as a public company listed in the United States, the Sarbanes-Oxley Act requires, among other things, that we assess the effectiveness of our internal control over financial reporting at the end of each fiscal year.
We previously identified material weaknesses in our internal control over financial reporting in connection with the preparation of the consolidated financial statements for the years ended December 31, 2021 and 2022. We took steps to address these material weaknesses and implemented remediation plans, and management concluded that the previously identified material weakness had been remediated as of December 31, 2023. However, the process to document and evaluate our internal control over financial reporting is both costly and challenging, and we need to continue to dedicate significant internal resources to this process. The controls that we have judged to be effective for the year ended December 31, 2024 may not continue to be effective and we might not be able to prevent any future material weaknesses in our internal control over financial reporting.
If we fail to staff our accounting and finance function adequately or maintain internal control over financial reporting adequate to meet the demands that will be placed upon us as a public company listed in the United States, our business and reputation may be harmed, and the price of our ordinary shares and ADSs may decline. In addition, undetected material weaknesses in our internal control over financial reporting could lead to restatements of financial statements and require us to incur the expense of remediation. Any of these developments could result in investor perceptions of us being adversely affected, which could cause a decline in the market price of our securities.
Existing and potential investors in our ordinary shares or ADSs may have to request the prior authorization from the French Ministry of Economy prior to acquiring a significant ownership position in our ordinary shares or ADSs.
Under French law, investments of more than 10% by certain individuals or entities in a French-listed company deemed to be a strategic industry may be subject to prior authorization of the French Ministry of Economy pursuant to Articles L. 151-1 et seq. and R. 151-1 et seq. of the French Monetary and Financial code.
If an investment requiring the prior authorization of the French Minister of Economy is completed without such authorization having been granted, the French Minister of Economy might direct the relevant investor to nonetheless (i) submit a request for authorization, (ii) have the previous situation restored at its own expense or (iii) amend the investment. The relevant investor might also be found criminally liable and might be sanctioned with a fine which cannot exceed the greater of: (i) twice the amount of the relevant investment, (ii) 10% of the annual turnover before tax of the target company and (iii) €5 million (for an entity) or €1 million (for an individual). Criminal penalties may also be imposed upon complaint by the French Minister of Economy in accordance with the French Customs Code.
Failure to comply with such measures could result in significant consequences on the applicable investor. Such measures could also delay or discourage a takeover attempt, and we cannot predict whether these measures will result in a lower or more volatile market price of our ADSs.
Purchasers of ADSs are not directly holding our ordinary shares.
A holder of ADSs is not treated as one of our shareholders and does not have direct shareholder rights, unless he or she withdraws the ordinary shares underlying his or her ADSs. French law governs our shareholder rights. The depositary, through the custodian or the custodian’s nominee, is the holder of the ordinary shares underlying ADSs. Purchasers of ADSs have ADS holder rights. The deposit agreement among us, the depositary, and ADS holders sets out ADS holder rights, as well as the rights and obligations of us and the depositary. ADS holders are encouraged to read the deposit agreement, which is filed as an exhibit to this Annual Report.
Your right as a holder of ADSs to participate in any future preferential subscription rights offering or to elect to receive dividends in shares may be limited, which may cause dilution to your holdings.
According to French law, if we issue additional securities for cash, current shareholders will have preferential subscription rights for these securities on a pro rata basis unless they waive those rights at an extraordinary meeting of our shareholders (by a two-thirds majority vote) or individually by each shareholder. However, our ADS holders in the United States will not be entitled to exercise or sell such rights unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. In addition, the deposit agreement provides that the depositary will not make rights available to you unless the distribution to ADS holders of both the rights and any related securities are either registered under the Securities Act or exempted from registration under the Securities Act. Further, if we offer holders of our ordinary shares the option to receive dividends in either cash or shares, under the deposit agreement the depositary may require satisfactory assurances from us that extending the offer to holders of ADSs does not require registration of any securities under the Securities Act before making the option available to holders of ADSs. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, ADS holders may be unable to participate in our rights offerings or to elect to receive dividends in shares and may experience dilution in their holdings. In addition, if the depositary is unable to sell rights that are not exercised or not distributed or if the sale is not lawful or reasonably practicable, it will allow the rights to lapse, in which case you will receive no value for these rights.
You may not be able to exercise your right to vote the ordinary shares underlying your ADSs.
Holders of ADSs may exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the provisions of the deposit agreement. The deposit agreement provides that, upon receipt of notice of any meeting of holders of our ordinary shares, the depositary will fix a record date for the determination of ADS holders who shall be entitled to give instructions for the exercise of voting rights. Upon timely receipt of notice from us, if we so request, the depositary shall distribute to the holders as of the record date (i) the notice of the meeting or solicitation of consent or proxy sent by us and (ii) a statement as to the manner in which instructions may be given by the holders.
You may instruct the depositary of your ADSs to vote the ordinary shares underlying your ADSs. Otherwise, you will not be able to exercise your right to vote, unless you withdraw the ordinary shares underlying the ADSs you hold. However, you may not know about the meeting far enough in advance to withdraw those ordinary shares. If we ask for your instructions, the depositary, upon timely notice from us, will notify you of the upcoming vote and arrange to deliver our voting materials to you. We cannot guarantee you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your ordinary shares or to withdraw your ordinary shares so that you can vote them yourself.
If the depositary does not receive timely voting instructions from you, it may give a proxy to a person designated by us to vote the ordinary shares underlying your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote, and there may be nothing you can do if the ordinary shares underlying your ADSs are not voted as you requested.
You may be subject to limitations on the transfer of your ADSs and the withdrawal of the underlying ordinary shares.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its books at any time or from time to time when it deems expedient in connection with the performance of its duties. The depositary may refuse to deliver, transfer, or register transfers of your ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government, or governmental body, or under any provision of the deposit agreement, or for any other reason subject to your right to cancel your ADSs and withdraw the underlying ordinary shares. Temporary delays in the cancellation of your ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting, or we are paying a dividend on our ordinary shares. In addition, you may not be able to cancel your ADSs and withdraw the underlying ordinary shares when you owe money for fees, taxes, and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities.
ADSs holders may not be entitled to a jury trial with respect to claims arising under the deposit agreement, which could result in less favorable outcomes to the plaintiffs in any such action.
The deposit agreement governing the ADSs representing our ordinary shares provides that, to the fullest extent permitted by law, ADS holders, including holders who acquire ADSs in the secondary market, waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our shares, the ADSs or the deposit agreement, including any claim under the U.S. federal securities laws.
If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable based on the facts and circumstances of that case in accordance with the applicable state and federal law. To our knowledge, the enforceability of a contractual pre-dispute jury trial waiver in connection with claims arising under the federal securities laws has not been finally adjudicated by the United States Supreme Court. However, we believe that a contractual pre-dispute jury trial waiver provision is generally enforceable, including under the laws of the State of New York, which govern the deposit agreement, by a federal or state court in the City of New York, which has non-exclusive jurisdiction over matters arising under the deposit agreement. In determining whether to enforce a contractual pre-dispute jury trial waiver provision, courts will generally consider whether a party knowingly, intelligently and voluntarily waived the right to a jury trial. We believe that this is the case with respect to the deposit agreement and the ADSs. It is advisable that you consult legal counsel regarding the jury waiver provision before entering into the deposit agreement.
If you or any other holders or beneficial owners of ADSs bring a claim against us or the depositary in connection with matters arising under the deposit agreement or the ADSs, including claims under federal securities laws, you or such other holder or beneficial owner may not be entitled to a jury trial with respect to such claims, which may have the effect of limiting and discouraging lawsuits against us and the depositary. If a lawsuit is brought against either or both of us and the depositary under the deposit agreement, it may be heard only by a judge or justice of the applicable trial court, which would be conducted according to different civil procedures and may result in different outcomes than a trial by jury would have, including results that could be less favorable to the plaintiffs in any such action. Nevertheless, if this jury trial waiver provision is not permitted by applicable law, an action could proceed under the terms of the deposit agreement with a jury trial. No condition, stipulation or provision of the deposit agreement or ADSs serves as a waiver by any holder or beneficial owner of ADSs or by us or the depositary of compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder.
As a foreign private issuer, we are exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than a U.S. company.
We are a foreign private issuer, as defined in the SEC’s rules and regulations and, consequently, we are not subject to all of the disclosure requirements applicable to public companies organized within the United States. For example, we are exempt from certain rules under the Exchange Act that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents, or authorizations applicable to a security registered under the Exchange Act, including the U.S. proxy rules under Section 14 of the Exchange Act. In addition, the members of our Board of Directors and Executive Committee are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Moreover, while we currently make annual and semi-annual filings with respect to our listing on Euronext Paris and expect to file financial reports on an annual and semi-annual basis, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies and are not required to file quarterly reports on Form 10-Q or current reports on Form 8-K under the Exchange Act. In addition, foreign private issuers are not required to file their Annual Report on Form 20-F until four months after the end of each fiscal year. Accordingly, there is less publicly available information concerning our company than there would be if we were not a foreign private issuer.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from Nasdaq corporate governance listing standards, and these practices may afford less protection to shareholders than they would enjoy if we complied fully with Nasdaq corporate governance listing standards.
As a foreign private issuer listed on Nasdaq, we are subject to Nasdaq’s corporate governance listing standards. However, Nasdaq rules permit foreign private issuers to follow the corporate governance practices of its home country. Some corporate governance practices in France may differ significantly from Nasdaq corporate governance listing standards. We intend to continue to rely on exemptions for foreign private issuers and follow French corporate governance practices in lieu of Nasdaq corporate governance standards, to the extent possible. For example, neither the corporate laws of France nor our bylaws require a majority of the members of our Board of Directors to be independent, and although the corporate governance code to which we currently refer (the Middlenext Code) recommends that at least two of the members of the Board of Directors be independent (as construed under such code), this code only applies on a “comply-or-explain” basis, and we may in the future either decide not to apply this recommendation or change the corporate code to which we refer. Furthermore, we could include non-independent members of the Board of Directors as members of our Nomination, Governance and Compensation committee, and the independent members of our Board of Directors would not necessarily hold regularly scheduled meetings at which only independent members of the Board are present. In addition, we follow French law with respect to shareholder approval requirements in lieu of the various shareholder approval requirements of Nasdaq. Currently, we intend to continue to follow home country practice to the maximum extent possible. Therefore, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
We may lose our foreign private issuer status in the future, which could result in significant additional cost and expense.
While we currently qualify as a foreign private issuer, the determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, our next determination will be made on June 30, 2025. In the future, we would lose our foreign private issuer status if we to fail to meet the requirements necessary to maintain our foreign private issuer status as of the relevant determination date. For example, if more than 50% of our securities are held by U.S. residents and more than 50% of the members of our Board of Directors or Executive Committee are residents or citizens of the United States, we could lose our foreign private issuer status. As of December 31, 2024, approximately 23% of our outstanding ordinary shares (including ordinary shares in the form of ADSs) were held by U.S. residents (assuming that all holders of ADSs as of such date are residents of the United States).
The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly more than costs we incur as a foreign private issuer. If we are not a foreign private issuer in the future, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive in certain respects than the forms available to a foreign private issuer. We would be required under current SEC rules to prepare our financial statements in accordance with U.S. generally accepted accounting principles, or U.S. GAAP, rather than IFRS, and modify certain of our policies to comply with corporate governance practices required of U.S. domestic issuers. Such conversion of our financial statements to U.S. GAAP would involve significant time and cost. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers such as the ones described above and exemptions from procedural requirements related to the solicitation of proxies.
If we are a passive foreign investment company, there could be adverse U.S. federal income tax consequences to U.S. holders.
Under the Code, a non-U.S. company will be considered a passive foreign investment company, or PFIC, for any taxable year in which (1) 75% or more of its gross income consists of passive income or (2) 50% or more of the weighted-average quarterly value of its assets consists of assets that produce, or are held for the production of, passive income. For purposes of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property, and certain rents and royalties. In addition, for purposes of the above calculations, a non-U.S. corporation that directly or indirectly owns at least 25% by value of the shares of another corporation or partnership is treated as if it held its proportionate share of the assets and received directly its proportionate share of the income of such other corporation or partnership. If we are a PFIC for any taxable year during which a U.S. holder (as defined in Item 10D, “Taxation”) holds our ordinary shares or ADSs, we will continue to be treated as a PFIC with respect to such U.S. holder in all succeeding years during which the U.S. holder owns the ordinary shares or ADSs, regardless of whether we continue to meet the PFIC test described above, unless the U.S. holder makes a specified election once we cease to be a PFIC. If we are classified as a PFIC for any taxable year during which a U.S. holder holds our ordinary shares or ADSs, the U.S. holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements.
We do not believe that we were characterized as a PFIC for the taxable year ending December 31, 2024. The determination of whether we are a PFIC is a fact-intensive determination made on an annual basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation. As a result, there can be no assurance regarding if we currently are treated as a PFIC, or may be treated as a PFIC in the future. In addition, for our current and future taxable years, the total value of our assets for PFIC testing purposes may be determined in part by reference to the market price of our ordinary shares or ADSs from time to time, which may fluctuate considerably. Under the income test, our status as a PFIC depends on the composition of our income which will depend on the transactions we enter into in the future and our corporate structure.
The composition of our income and assets is also affected by how we spend the cash we raise in any offering. Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the Internal Revenue Service, or IRS, will agree with our conclusion and that the IRS would not successfully challenge our position. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status for any prior, current or future taxable year.
For further discussion of the PFIC rules and the adverse U.S. federal income tax consequences in the event we are classified as a PFIC, see Item 10D of this Annual Report.
If a United States person is treated as owning at least 10% of our ordinary shares, such holder may be subject to adverse U.S. federal income tax consequences.
If a U.S. holder is treated as owning, directly, indirectly or constructively, at least 10% of the value or voting power of our ordinary shares or ADSs, such U.S. holder may be treated as a “United States shareholder” with respect to each “controlled foreign corporation” in our group, if any. Our group currently includes one U.S. subsidiary and, therefore, under current law our current non-U.S. subsidiaries and any future newly formed or acquired non-U.S. subsidiaries will be treated as controlled foreign corporations, regardless of whether we are treated as a controlled foreign corporation. A United States shareholder of a controlled foreign corporation may be required to annually report and include in its U.S. taxable income its pro rata share of “Subpart F income,” “global intangible low-taxed income”, and investments in U.S. property by controlled foreign corporations, regardless of whether we make any distributions. An individual that is a United States shareholder with respect to a controlled foreign corporation generally would not be allowed certain tax deductions or foreign tax credits that would be allowed to a United States shareholder that is a U.S. corporation. Failure to comply with controlled foreign corporation reporting obligations may subject a United States shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any United States shareholder information that may be necessary to comply with the reporting and tax paying obligations applicable under the controlled foreign corporation rules of the Code. U.S. holders should consult their tax advisors regarding the potential application of these rules to their investment in our ordinary shares or ADSs.
Tax authorities may disagree with our positions and conclusions regarding certain tax positions, or may apply existing rules in an unforeseen manner, resulting in unanticipated costs, taxes, or non-realization of expected benefits.
A tax authority may disagree with tax positions that we have taken, which could result in increased tax liabilities. For example, the Internal Revenue Service or another tax authority could challenge our allocation of income by tax jurisdiction and the amounts paid between our affiliated companies pursuant to our intercompany arrangements and transfer pricing policies, including amounts paid with respect to our intellectual property development. Similarly, a tax authority could assert that we are subject to tax in a jurisdiction where we believe we have not established a taxable connection, often referred to as a “permanent establishment” under international tax treaties, and such an assertion, if successful, could increase our expected tax liability in one or more jurisdictions.
A tax authority may take the position that material income tax liabilities, interest, and penalties are payable by us, for example where there has been a technical violation of contradictory laws and regulations that are relatively new and have not been subject to extensive review or interpretation, in which case we expect that we might contest such assessment. High-profile companies can be particularly vulnerable to aggressive application of unclear requirements. Many companies must negotiate their tax bills with tax inspectors who may demand higher taxes than applicable law appears to provide. Contesting such an assessment may be lengthy and costly and if we were unsuccessful in disputing the assessment, the implications could increase our anticipated effective tax rate, where applicable.
General Risk Factors
The trading price of our equity securities has been and may continue to be volatile, and purchasers of our ordinary shares or ADSs could incur substantial losses.
The price of our ordinary shares and ADSs has been, and likely will continue to be, significantly affected by events such as announcements regarding scientific and clinical results concerning product candidates currently being developed by us, our collaboration partners, or our main competitors, changes in market conditions related to our sector of activity, announcements of new contracts or amendments or terminations to existing contracts, technological innovations and collaborations by us or our main competitors, developments concerning intellectual property rights, the development, regulatory approval and commercialization of new products by us or our main competitors, and changes in our financial results.
Equity markets are subject to considerable price fluctuations, and often, these movements do not reflect the operational and financial performance of the listed companies concerned. In particular, biotechnology companies’ share prices have been highly volatile and may continue to be highly volatile in the future. As we operate in a single industry, we are especially vulnerable to these factors to the extent that they affect our industry. Fluctuations in the stock market as well as the macro-economic environment could significantly affect the price of our ordinary shares. As a result of this volatility, investors may not be able to sell their ordinary shares or ADSs at or above the price originally paid for the security. The market price for our ordinary shares and ADSs may be influenced by many factors, including:
•actual or anticipated fluctuations in our financial condition and operating results, including as a result of clinical trial results and other announcements related to our clinical pipeline;
•adverse results or delays in our or any of our competitors’ pre-clinical studies or clinical trials or regulatory timelines;
•adverse regulatory decisions, including failure to receive regulatory approval for any of our product candidates;
•the termination or amendment of a strategic alliance, partnership, or collaboration or the inability to establish additional strategic alliances, partnerships, or collaborations;
•actual or expected regulatory or legal developments in the United States, European Union and other jurisdictions;
•failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public;
•actual or anticipated changes in our growth rate relative to our competitors;
•competition from existing products or new products that may emerge;
•announcements by us or our competitors of significant acquisitions, divestitures, strategic partnerships, joint ventures, collaborations, or capital commitments;
•issuance of new or updated research or reports by securities analysts;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•ordinary share and ADS price and volume fluctuations attributable to inconsistent trading volume levels of our ordinary shares and ADSs;
•price and volume fluctuations in trading of our ordinary shares on Euronext Paris;
•additions or departures of key management or scientific personnel;
•disputes or other developments related to proprietary rights, including patents, litigation matters, and our ability to obtain patent and other intellectual property protection for our technologies;
•changes to coverage policies or reimbursement levels by commercial third-party payors and government payors and any announcements relating to coverage policies or reimbursement levels;
•announcement or expectation of additional debt or equity financing efforts;
•sales of our ordinary shares or ADSs by us, our insiders or our other shareholders; and
•general economic and market conditions, including macroeconomic factors such as geopolitical instability, rising interest rates and inflation.
These and other market and industry factors may cause the market price and demand for our ordinary shares and ADSs to fluctuate substantially, regardless of our actual operating performance, which may limit or prevent investors from readily selling their ordinary shares or ADSs and may otherwise negatively affect the liquidity of the trading market for the ordinary shares and ADSs. In addition, in the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could be costly and time consuming and divert management’s attention and resources. We are aware through publicly available information that U.S.-based law firms specializing in shareholder lawsuits have announced the commencement of “investigations” of our company following a decline in ADS trading price allegedly in connection with a notice posted on the U.S. CDC’s website regarding an investigation of five hospitalizations that occurred after vaccination with IXCHIQ. As of the date of this Annual Report, we have not received notice of any actual claims, but we cannot exclude the possibility of a lawsuit relating to these investigations or any others that may be announced.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, the price of the ordinary shares or ADSs and their trading volume could decline.
The trading market for the ADSs and ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. As a public company in France since 2013 and in the United States since May 2021, our equity securities are currently subject to coverage by a number of analysts. If fewer securities or industry analysts cover our company, the trading price for our ADSs and ordinary shares could be negatively impacted. If one or more of the analysts who covers us downgrades our equity securities or publishes incorrect or unfavorable research about our business, the price of our ordinary shares and ADSs would likely decline. Additionally, if one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our ordinary shares and ADSs could decrease, which could cause the price of our ordinary shares and ADSs or their trading volume to decline.
Item 4. Information on the Company
A. History and Development of the Company
Our legal name is “Valneva SE”. We are a public company listed on the Nasdaq Global Select Market and Euronext Paris that was formed in 2013 through the merger of Intercell, an Austrian vaccine biotech company listed on the Vienna Stock Exchange, and Vivalis, a French biotech company listed on Euronext Paris. We were incorporated on March 24, 1999 as a limited liability company and converted into a European Company (Societas Europaea, or SE) on May 28, 2013. Our registered office is located at 6 rue Alain Bombard, 44800 Saint-Herblain, France. We are registered at the Nantes Trade and Companies Registry under the number 422 497 560. Our telephone number at our principal executive offices is +33 2 28 07 37 10.
We have nine wholly owned subsidiaries—Valneva Austria GmbH, a limited liability company formed under the laws of Austria in 2013, Valneva Scotland Ltd., a private company limited by shares formed under the laws of Scotland in 2003, Valneva USA, Inc., a Delaware corporation formed in 1997, Vaccines Holdings Sweden AB, a private limited company formed under the laws of Sweden in 2014, Valneva Sweden AB, a private limited company formed under the laws of Sweden in 1992, Valneva Canada, Inc., a corporation formed under the laws of Canada in 2015, Valneva UK Ltd., a private company formed under the laws of England and Wales in 2015, Valneva France SAS, a société par actions simplifiée formed under the laws of France in 2019, and VBC 3 Errichtungs GmbH, a limited liability company formed under the laws of Austria that we acquired in 2023 in connection with the purchase of the office building we occupy in Vienna. Our agent for service of process in the United States is Valneva USA, Inc.
Our website address is www.valneva.com. The reference to our website is an inactive textual reference only and information contained in, or that can be assessed through, our website is not incorporated by reference into this Annual Report and does not constitute a part of this Annual Report.
The SEC maintains an internet site at http://www.sec.gov that contains reports and other information that we file electronically with the SEC.
Our full capital expenditures in the years ended December 31, 2024, 2023 and 2022 totaled €17.2 million, €18.3 million and €29.0 million respectively, primarily related to investments in our manufacturing facilities in Scotland and Sweden. We expect our capital expenditures in 2025 to be primarily financed from our existing cash and cash equivalents.
B. Business Overview
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best-, or only-in-class vaccine solutions. We have a strong track record, having advanced multiple vaccines from early Research & Development (R&D) to approvals, and currently market three proprietary travel vaccines, including the world’s first chikungunya vaccine, IXCHIQ, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine development pipeline. Our clinical portfolio is composed of highly differentiated vaccine candidates that are designed to provide preventative solutions for diseases with high unmet need. Our pipeline includes the only Lyme disease vaccine candidate (VLA15) in advanced clinical development, which we are developing in partnership with Pfizer, as well as the world’s most clinically advanced tetravalent Shigella vaccine candidate, under development in collaboration with LimmaTech Biologics AG (LimmaTech), and proprietary vaccine candidates against the Zika virus and other global public health threats.
VLA15 is a Phase 3 vaccine candidate targeting Borrelia, the bacterium that causes Lyme disease, under development in collaboration with Pfizer, and it is the only vaccine candidate against Lyme disease currently undergoing late-stage clinical trials. Pfizer and Valneva are currently executing the Phase 3 field efficacy study for VLA15 called VALOR (Vaccine Against Lyme for Outdoor Recreationists). Enrollment in the trial was completed in December 2023 and the primary vaccination series were completed in July 2024. Participants will be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025.
VLA15 targets the six most prevalent serotypes, or variations, of Borrelia in the United States, where approximately 476,000 people are diagnosed with Lyme disease each year and in Europe, where at least a further 200,000 cases occur annually.
VLA1553 is a vaccine candidate which was approved by the U.S. Food and Drug Administration (FDA), the European Commission (EC), Health Canada, and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) under the brand name IXCHIQ. It is indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 18 years of age and older. At the end of February 2024, the U.S. Advisory Committee on Immunization Practices (ACIP) provided recommendations on how to use IXCHIQ and these recommendations were then adopted by the U.S. Centers for Disease Control and Prevention (CDC). In 2024, Valneva submitted label extension applications to the FDA, the European Medicines Agency (EMA) and Health Canada to potentially extend the use of IXCHIQ, which is currently approved in adults, to adolescents 12 to 17 years of age. The applications to the FDA and Health Canada also included the two-year antibody persistence data to the product label, which is a key differentiator for IXCHIQ. In February 2025, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion recommending authorization of a label extension for IXCHIQ in adolescents 12 years of age and older. The EC will now review the CHMP recommendation, and a decision on the label extension application in the European Union (EU), Norway, Liechtenstein, and Iceland is expected within sixty days following the CHMP opinion. A marketing application is also under review in Brazil, which would represent the first approval in an endemic country. The vaccine is still undergoing several clinical trials with a view to expand access, support label extensions and enhance the product profile.
Shigella4V2 (S4V2) is a Phase 2 tetravalent bioconjugate vaccine candidate against shigellosis, a diarrheal infection caused by Shigella bacteria, under development in collaboration with LimmaTech. Shigellosis is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year, particularly among children in Low and Middle Income Countries (LMICs). No approved Shigella vaccine is currently available outside of Russia or China, and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO). In October 2024, the FDA granted Fast Track designation to S4V2, recognizing its potential to address a serious condition and fill an unmet medical need.
VLA1601 is a Phase 1 vaccine candidate targeting the Zika virus (ZIKV), a mosquito-borne viral disease whose transmission has been reported in 89 countries and territories and persists in several countries in the Americas and other endemic regions. There are no preventive vaccines or effective treatments available. As such, Zika remains a public health threat and is included in the FDA’s Tropical Disease Priority Review Voucher Program. VLA1601 is being developed on the original manufacturing platform of our licensed Japanese encephalitis vaccine IXIARO, which was further optimized to develop our inactivated, adjuvanted COVID-19 vaccine VLA2001, the first COVID-19 vaccine to receive a standard marketing authorization in Europe.
We have already successfully licensed and commercialized a portfolio of traveler vaccines, which is composed of IXIARO (also marketed as JESPECT in Australia and New Zealand), indicated for the prevention of Japanese encephalitis in travelers and military personnel, and DUKORAL, indicated for the prevention of cholera and, in Canada, Switzerland, New Zealand, and Thailand, prevention of diarrhea caused by Enterotoxigenic Escherichia coli, or ETEC, the leading cause of travelers’ diarrhea. During the course of 2024, we launched our chikungunya vaccine IXCHIQ in the United States, France and Canada and expect to launch it in additional countries in 2025. Additionally, we distribute vaccines for third parties in selected countries where we have a commercial infrastructure.
We have a highly developed, nimble and sophisticated manufacturing infrastructure with facilities across Europe to meet our clinical and commercial needs, including BioSafety Level 3 (BSL-3) manufacturing and R&D facilities. We have assembled a team of experts with deep scientific, clinical and business expertise in biotechnology and specifically in vaccine development, manufacturing and commercialization. Our senior leadership team has extensive experience and demonstrated ability to move vaccines through the clinic and into successful commercialization. Members of our team have previously worked at industry leaders such as Novartis, Chiron, GlaxoSmithKline and Daiichi Sankyo.
Our Pipeline and Proprietary Commercial Portfolio
Our pipeline consists of assets at all stages of research & development. Our goal is to develop vaccine candidates that are first-, best-, or only-in-class and address unmet needs in infectious diseases. Our aim is to develop these assets for future commercialization either in-house or through and with partners.
Our advanced clinical pipeline and commercialized products are summarized below:
1. Indications differ by country. ETEC stands for Enterotoxigenic Escherichia coli (E. Coli) bacterium. 2 Controlled human infection model
Our clinical pipeline includes:
•VLA15 – a vaccine candidate against Borrelia, the bacterium that causes Lyme disease. VLA15 is a multivalent recombinant protein vaccine that targets six serotypes of Borrelia representing the most common serotypes found in North America and Europe. VLA15 is the only Lyme disease program in advanced clinical development today and has received Fast Track designation from the FDA. We reported results for three Phase 2 clinical trials of VLA15 in both adult and pediatric populations, in which we observed high levels of antibodies against all six serotypes. These include the announcement in September 2023 of positive Phase 2 pediatric and adolescent immunogenicity and safety data following a booster vaccination with VLA15. These results from the VLA15-221 Phase 2 study showed a strong anamnestic antibody response for all serotypes in pediatric (5 to 11 years of age) and adolescent participants (12 to 17 years of age), as well as in adults (18 to 65 years of age), one month after administration of a booster dose (month 19). The safety and tolerability profile of VLA15 after a booster dose was consistent with previous studies. In August 2022, jointly with Pfizer, we initiated a Phase 3 clinical study, “Vaccine Against Lyme for Outdoor Recreationists (VALOR)”, to investigate the efficacy, safety, and immunogenicity of VLA15 in participants five years of age and older in highly endemic regions in the United States and Europe. In February 2023, Pfizer, as the study sponsor, decided to discontinue approximately half of the total enrolled participants in the trial following violations of Good Clinical Practice, or GCP, at certain clinical trial sites run by a third party clinical trial site operator. The clinical trial remains ongoing with other sites not operated by the third party and new sites in the U.S. and Canada. In December 2023, we and Pfizer announced that we completed recruitment for the study. 9,437 participants 5 years of age and older were enrolled in the trial to receive three doses of VLA15 or a saline placebo (1:1 ratio) within the first year, and one booster dose approximately one year after vaccination with the first three doses, as part of the primary series. In July 2024, we announced completion of the primary vaccination series. Participants will receive their remaining booster dose and then be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025. Subject to positive data, Pfizer aims to submit a Biologics Licence Application (BLA) to the FDA and a marketing authorization application (MAA) to the EMA in 2026. According to the terms of our collaboration, Pfizer leads late phase development of VLA15. If VLA15 is approved, Pfizer will have sole control over its commercialization, and we will be eligible to receive milestone and royalty payments. In June 2022, the terms of our collaboration with Pfizer were updated, and Pfizer invested €90.5 ($95) million in Valneva as part of an Equity Subscription Agreement.
•VLA1553 – a single-dose, live-attenuated vaccine candidate against CHIKV. VLA1553 was approved by the FDA under the brand name IXCHIQ in November 2023 and with this approval, became the world’s first licensed chikungunya vaccine available to address this unmet medical need. It is indicated in the United States for the prevention of disease caused by CHIKV in individuals 18 years of age and older who are at increased risk of exposure to CHIKV. Alongside the approval, we received a Priority Review Voucher which we sold for $103 million in February 2024. At the end of February 2024, ACIP provided recommendations on how to use IXCHIQ, and these recommendations were then adopted by the CDC. We now expect expanded access to the retail channel via publication of the ACIP recommendations in the Morbidity and Mortality Weekly Report (MMWR). IXCHIQ was also approved in Canada in June 2024, in Europe in July 2024 and in the UK in February 2025 for the prevention of disease caused by the chikungunya virus in individuals 18 years of age and older. A Marketing application is also under review in Brazil. Label extensions to potentially extend the use of IXCHIQ to adolescents aged 12 to 17 years are also under review in the U.S., Europe and Canada. In February 2025, the Committee for Medicinal Products for Human Use of the EMA adopted a positive opinion recommending authorization of a label extension for IXCHIQ to individuals 12 years of age and older. The recommendation is now being reviewed by the EC and a decision on the label extension is expected within sixty days. The Canadian and U.S. label extension applications also include two-year antibody persistence data, which is a key differentiator for IXCHIQ that was already included in the initial filing with the EMA. The pivotal Phase 3 data, showing a high seroresponse rate of 98.9% in participants 28 days after receiving a single administration, were published in The Lancet, the world’s leading peer-reviewed medical journal, in June 2023. We have continued to evaluate antibody persistence in a dedicated trial which will extend over ten years. Additionally, a clinical study in 754 adolescents 12 to 17 years of age, VLA1553-321, is ongoing in Brazil, for which we reported positive pivotal Phase 3 immunogenicity and safety data in November 2023. Funded by the Coalition for Epidemic Preparedness Innovations (CEPI) and conducted in collaboration with Instituto Butantan, this adolescent trial is intended to support the label extension in this age group following approvals in adults. The trial is also expected to support licensure of the vaccine in Brazil, which would be the first potential approval for use in endemic populations. We also initiated a pediatric trial in children 1 to 11 years of age, VLA1553-221, in January 2024 to support a Phase 3 pivotal pediatric study and potentially extend the label to this age group following initial regulatory approvals in adults and possibly in adolescents. In 2025, we will also start preparing the initiation of two Phase 4 post-marketing studies required as part of our approval under the FDA’s accelerated approval pathway. These Phase 4 studies, and several ongoing and planned studies, are supported through an expanded partnership and additional grant funding of $41.3 million from CEPI with co-funding from the European Union. As part of the CEPI partnership, we signed an exclusive license agreement for our chikungunya vaccine with the Serum Institute of India (SII), the world’s largest manufacturer of vaccines by number of doses, in December 2024 to support broader access to the vaccine in low and middle-income countries (LMICs).
•S4V2 – a tetravalent bioconjugate vaccine candidate against shigellosis, a diarrheal infection caused by Shigella bacteria, under development in collaboration with LimmaTech. In October 2024, we announced the launch of a Phase 2b controlled human infection model (CHIM) study to assess the safety and preliminary efficacy in approximately 120 healthy Shigella-naïve participants aged 18 to 50 years at three sites in the United States. In addition to the CHIM study, LimmaTech will conduct a Phase 2 pediatric study in LMICs expected to begin in the first half of 2025. Valneva will assume all further development, including CMC (chemistry, manufacturing and controls) and regulatory activities, and be responsible for its commercialization worldwide, if approved. Shigellosis is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year, particularly among children in LMICs. No approved Shigella vaccine is currently available outside of Russia or China and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO). In October 2024, the FDA granted Fast Track designation to S4V2, recognizing its potential to address a serious condition and fill an unmet medical need.
•VLA1601 – a highly purified inactivated, adjuvanted novel vaccine candidate against the mosquito-borne viral disease caused by the Zika virus, or ZIKV. Disease outbreaks have been reported in tropical Africa, Southeast Asia, the Pacific Islands, and, since 2015, in the Americas. Zika virus transmission persists in several countries in the Americas and in other endemic regions. To date, a total of 89 countries and territories have reported evidence of mosquito-transmitted Zika virus infection; however, surveillance remains limited globally. There are no preventive vaccines or effective treatments available. As such, Zika remains a public health threat and is included in the FDA’s Tropical Disease Priority Review Voucher Program. VLA1601 is being developed on the original manufacturing platform of our licensed Japanese encephalitis vaccine IXIARO, which was further optimized to develop our inactivated, adjuvanted COVID-19 vaccine VLA2001, the first COVID-19 vaccine to receive a standard marketing authorization in Europe. We reported positive Phase 1 results for the first generation of our Zika vaccine candidate in 2019, showing a favorable safety profile and immunogenicity in all tested doses and schedules, comparable to IXIARO and other clinical stage ZIKV vaccines. In March 2024, we initiated a Phase 1 clinical trial to investigate the safety and immunogenicity of our optimized vaccine candidate. The randomized, placebo-controlled, Phase 1 trial, VLA1601-102, is planned to enroll approximately 150 participants aged 18 to 49 years in the United States. A vaccine against the Zika virus would nicely complement Valneva’s portfolio of travel vaccines against mosquito-borne diseases, which already includes IXCHIQ and IXIARO.
In addition to our clinical-stage assets, our portfolio includes a series of pre-clinical assets against disease targets that reflect our strategy of providing prophylactic solutions to significant diseases that lack a preventative and effective therapeutic treatment option. These include VLA2112, a vaccine candidate targeting the Epstein-Barr virus, or EBV, which is one of the most common human viruses. EBV can cause infectious mononucleosis and is strongly associated with the development of several types of cancer and multiple sclerosis. We have also been working on a vaccine candidate targeting the enteric disease caused by Enterotoxigenic Escherichia coli (ETEC).
We commercialize our fully-owned travel vaccines IXIARO/JESPECT, DUKORAL, and IXCHIQ and previously supplied our inactivated COVID-19 vaccine VLA2001 under government contracts.
•IXIARO is an inactivated Vero cell culture-derived Japanese encephalitis vaccine that is the only Japanese encephalitis vaccine licensed and available in the United States, Canada, and Europe. IXIARO is indicated for active immunization against Japanese encephalitis, the most prevalent cause of viral encephalitis in Asia, for adults, adolescents, children, and infants aged two months and older. Sales of IXIARO were €94.1 million in 2024 compared to €73.5 million in 2023. The 28% increase in sales reflect a double-digit sales growth to both travelers and the U.S. military compared to 2023. In 2024, Valneva supplied additional doses of IXIARO to the U.S. Department of Defense (DoD) under the contract it signed in September 2023, which allowed the DoD to purchase additional doses during the following twelve months. In January 2025, Valneva announced the signing of a new $32.8 million contract with the DoD for the supply of IXIARO. Under this new one-year contract, the DoD will buy a minimum of $32.8 million worth of IXIARO vaccines and has the possibility to purchase additional doses during the following twelve months.
•DUKORAL is an oral vaccine for the prevention of diarrhea caused by Vibrio cholera and, in Canada and certain other countries, heat-labile toxin producing ETEC, the leading cause of travelers’ diarrhea. We acquired DUKORAL in 2015 and recorded €32.3 million of sales in the year ended December 31, 2024 compared to €29.8 million in the year ended December 31, 2023. DUKORAL sales benefited from the resumption of marketing investments in the second half of 2024 following successful regulatory inspection of our new manufacturing site in Sweden. DUKORAL is authorized for use in the European Union and Australia to protect against cholera and in Canada, Switzerland, New Zealand, and Thailand to protect against cholera and ETEC.
•IXCHIQ is a single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus in individuals 18 years of age and older who are at increased risk of exposure to CHIKV. In 2024, we received marketing approval for IXCHIQ in the United States, Europe and Canada. With these approvals, IXCHIQ became the world’s first licensed chikungunya vaccine available to address this unmet medical need and the third vaccine Valneva brought from early R&D to approval. Valneva started commercializing IXCHIQ in the United States in the first quarter of 2024 and in Canada and France at the end of 2024. Sales of IXCHIQ were €3.7 million in the year ended December 31, 2024. We expect to launch in additional countries in 2025, notably in Europe.
Sales from these products are currently complemented by sales from the distribution of third-party products in markets where Valneva operates its own marketing and sales infrastructure (United States, Canada, Nordic countries, United Kingdom, Austria, and France).
Our Strategy
Our strategy supports our vision to contribute to a world in which no one dies or suffers from a vaccine-preventable disease. Our strategy is based on an integrated business model that has allowed us to build a portfolio of differentiated clinical and pre-clinical assets as well as a growing commercial business. We are focused on utilizing our proven and validated product development capabilities to rapidly advance solutions addressing unmet needs in infectious diseases towards regulatory approval, with the goal of becoming first-, best-, or only-in-class. We have entered into strategic partnerships with other well-established pharmaceutical companies to leverage their clinical and commercial capabilities to optimize the potential value of select assets. As we advance our late-stage portfolio, we also remain focused on investing in our research and development pipeline in order to develop our earlier-stage assets as well as identify new targets and indications where we believe we can make a significant difference.
In order to execute upon this strategy as an independent, financially sustainable company, we are pursuing the following strategic goals:
•Advance VLA15 for the prevention of Lyme disease in collaboration with Pfizer. We are developing VLA15 as a vaccine against Borrelia, the bacterium that causes Lyme disease in North America and Europe. We reported results for three Phase 2 clinical trials of VLA15 in both adult and pediatric populations, in which VLA15 generated high levels of antibodies against all six Borrelia strains. Together with Pfizer, we announced the initiation of a Phase 3 clinical study, “Vaccine Against Lyme for Outdoor Recreationists (VALOR)”, in August 2022, completed recruitment of 9,437 participants for the study in December 2023 and the primary vaccination series in July 2024. Participants will receive their remaining booster dose and then be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025. Pfizer aims to submit a BLA to the FDA and an MAA to the EMA in 2026, subject to positive data. If VLA15 is approved, Pfizer will have sole responsibility for ts commercialization, and we will be eligible to receive milestone and royalty payments.
•Grow product sales focusing on proprietary products, and build a leading position in the travel vaccines market. We will focus on continuing to grow sales of our proprietary travel vaccines (IXIARO, DUKORAL and IXCHIQ) and launch IXCHIQ in the markets where the vaccine is and may be approved. In 2025, we expect to receive regulatory decisions on the marketing applications we filed in Brazil and the label extensions we filed in the U.S., Europe, and Canada.The third-party product business supported Valneva’s revenues as a complement to its existing travel vaccine portfolio, especially during the COVID-19 pandemic. However, third-party sales over the past years yielded much lower gross margin than our proprietary products, diluting our overall margins. We have therefore decided to focus resources on direct sales of our proprietary products.
•Progress our pipeline of clinical and pre-clinical programs to develop new vaccines addressing diseases with significant unmet need. To remain an industry leader in the development of prophylactic vaccines, we intend to continue to progress vaccine candidates against unmet medical needs and identify disease targets with the potential to be effectively prevented by vaccines with a view to potentially develop vaccine candidates against those targets. In 2025, we notably expect the first Phase 2 efficacy results of our Shigella vaccine candidate and the Phase 1 results of our Zika vaccine candidate.
•Opportunistically pursue strategic collaborations to maximize full potential of our clinical and commercial portfolios. We intend to continue to selectively evaluate collaborations to leverage the clinical and commercial expertise of large pharmaceutical companies.
•Focus on stringent cost management. In the mid-term, we will continue focusing on stringent cost management with a particular focus on marketing and distribution as well as general and administrative costs. We believe that we have sufficient resources to finance our operational business, excluding debt repayment, until potential milestone and commercial revenues from our program against Lyme disease enable the Company to operate in a sustained profitable way.
•Maintain an attractive workplace by strengthening our ESG (environmental, social, and governance) strategy. As a member of the United Nations Global Compact, we intend to ramp up ESG initiatives and continue to develop the three pillars of our responsible business commitments: Protecting Lives, Reaching People and Preserving the Planet.
Background to Vaccine Development
Infectious diseases have widely affected, and continue to widely affect, humankind. Prevention of infectious diseases through vaccination, known as prophylactic vaccination, is considered one of the most beneficial and cost-effective health care interventions. Prophylactic vaccines often represent the preferred solution to debilitating and widespread infectious diseases given their capacity to bring about significant health benefits to both individuals and communities, while remaining highly cost effective. This is a result of the fact that vaccines provide health benefits not only to individuals who have actually received the vaccine, but also to the broader community as the vaccinated population brings the immunological benefits of protection to non-vaccinated populations through the “herd immunity” effect that helps to reduce the spread of the disease.
Despite the large and growing need for vaccines, many urgent medical needs remain unaddressed, including infectious diseases, such as Lyme disease, Shigellosis and Zika, and hospital-acquired infections, such as infections with C. difficile. Developing vaccines for such diseases remains a high priority for the research and development world.
There are a number of approaches to engineering vaccine candidates. Most vaccines in use today utilize one of the following five technological approaches:
•Live attenuated vaccines. Live attenuated vaccines use a weakened, or attenuated, form of the virus or bacteria that causes a disease. Live attenuated vaccines typically provoke more durable immunological responses. However, they may not be safe for use in immunocompromised individuals, and on rare occasions can mutate to a virulent form and cause disease. Live attenuated vaccines protect against diseases such as measles/mumps/rubella, rotavirus, smallpox, chickenpox and yellow fever. Our chikungunya virus vaccine IXCHIQ is an example of a live attenuated vaccine.
•Inactivated vaccines. Inactivated vaccines use a version of the disease-causing virus or bacteria that has been destroyed with chemicals, heat or radiation. Inactivated vaccines have a long history of use and are among the safest types of vaccine, with possibilities for use in special target populations, such as patients with weakened immune systems. We believe that the extensive knowledge and experience with the existing viral inactivation procedures for vaccine manufacture will continue to serve as a foundation of vaccinology for novel inactivated vaccines. Today millions of people are, and will be, protected worldwide with inactivated viral vaccines. Inactivated vaccines protect against diseases such as hepatitis A, flu, polio, and rabies. Our products IXIARO and VLA2001 are both inactivated vaccines.
•Subunit, recombinant, polysaccharide, and conjugate vaccines. Subunit, recombinant, polysaccharide, and conjugate vaccines use specific pieces of the virus or bacteria, such as its protein, sugar, or casing, to generate an immune response. Rather than introducing an inactivated or attenuated microorganism to an immune system (which would constitute a “whole-agent” vaccine), a subunit vaccine uses a fragment of the microorganism to generate an immune response. Subunit vaccines can produce a long-lived immunity and are relatively safe since only parts of the virus are used and can be applicable to people with weakened immune systems. These vaccines protect against diseases such as Hib (Haemophilus influenza type b), hepatitis B, HPV (human papillomavirus), whooping cough (part of the DTaP combined vaccine), pneumococcal disease, meningococcal disease, and shingles. Our clinical development and manufacturing technology have allowed us to develop our VLA15 vaccine candidate, a multivalent, protein subunit vaccine for prevention of Lyme disease.
•Toxoid vaccines. Toxoid vaccines use a toxin made by the virus or bacteria that causes a disease. These vaccines are used to protect against diseases such as diphtheria and tetanus.
•Messenger RNA (mRNA) vaccines. mRNA vaccines are one of the newest areas in vaccine technology. As shown during the COVID-19 pandemic, they can be developed quickly using the pathogen’s genetic code. When an mRNA vaccine is delivered, the RNA material teaches our body how to make a specific type of protein that is unique to the virus, but does not make the person sick. The protein triggers an immune response, which includes the generation of antibodies that recognize the protein. That way, if a person is ever exposed to that virus in the future, the body would likely have the tools (antibodies) to fight against it.
Additionally, there are companies pursuing novel technologies such as DNA vaccines, which transfect a specific antigen DNA-coding sequence onto the cells of an immunized species, and dendritic cell vaccines, which combine dendritic cells with antigens in order to present the antigens to the body’s white blood cells, thus stimulating an immune reaction. Although some of these novel technologies have shown promise, they largely remain in the early stages of development and face significant challenges related to manufacturing and distribution.
Our deep expertise and capabilities across many of these approaches gives us the flexibility to follow our strategy of first targeting diseases that lack a preventative solution and then developing an efficacious and safe vaccine candidate based on our determination of the most effective approach.
In addition to the vaccine’s primary component, such as an inactivated virus, vaccines may contain adjuvants, which are used to improve the immune response to the vaccine, for example through producing more antibodies. Adjuvants used in human vaccines include alum (aluminium hydroxide) and others (e.g. CpG-1018, manufactured by Dynavax). Adjuvants have a proven safety record based on more than 60 years of use. Effective use of adjuvants requires expertise around vaccine formulation and development. We have utilized different adjuvants in a number of our vaccine candidates or licensed vaccines.
Vaccines are administered through various routes such as orally, subcutaneously, intramuscularly, intradermally and intranasally. These various methods of administration help to simplify the vaccination process, allowing more people to be vaccinated and promoting adherence to the recommendations, such as receiving a follow-up dosage.
The different approaches to vaccine development cannot be universally applied to infectious diseases and be effective; instead, each approach must be targeted against a disease according to a compelling biological rationale. As such, development of vaccines are intensive and complicated processes that require evaluation of multiple modalities, endpoints and clinically meaningful data points. The efficacy and safety of vaccines are measured using multiple methodologies and approaches, although research and regulatory bodies often focus on the following measures:
•Immunogenicity — the ability of a foreign substance, such as an antigen, to provoke an immune response
•Seroconversion rates (SCR) — the proportion of subjects in a trial for whom a specific antibody develops and becomes detectable in blood
•Seroconversion — an antibody response capable of preventing infection
•Titer — a laboratory test that measures the presence and amount of antibodies in the blood
•Viremia — the presence of a virus in the blood
Our Clinical Pipeline
VLA15— Our vaccine candidate targeting Lyme disease
We are developing VLA15 as an investigational vaccine against Borrelia, the bacterium that causes Lyme disease. VLA15 is a recombinant protein vaccine candidate that targets six serotypes of Borrelia representing the most common serotypes found in North America and Europe. We have reported initial results of three Phase 2 clinical trials of VLA15 in over 900 healthy adults, and results have demonstrated the presence of high titers of antibodies against all six serotypes. In August 2022, together with Pfizer, we initiated a Phase 3 clinical study, “Vaccine Against Lyme for Outdoor Recreationists (VALOR)”, to investigate the efficacy, safety, and immunogenicity of VLA15 in participants five years of age and older in highly endemic regions in the United States and Europe. In February 2023, we announced that Pfizer, as the study sponsor, decided to discontinue approximately half of the total recruited participants in the trial following violations of GCP at certain clinical trial sites run by a third-party clinical trial site operator. The discontinuation of these participants was not due to any safety concerns with the investigational vaccine and was not prompted by a participant-reported adverse event. The clinical trial remains ongoing with other sites not operated by the third party and new sites in the U.S. and Canada. In December 2023, we and Pfizer announced that we completed recruitment for the study. 9,437 participants five years of age and older were enrolled to receive three doses of VLA15 or a saline placebo (1:1 ratio) within the first year, and one booster dose approximately one year after completion of the first three doses, as part of the primary immunization. In July 2024, we announced completion of the three-doses primary vaccination series. Participants are now receiving their booster dose and will be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025. Pfizer aims to submit a BLA to the FDA and a MAA to the EMA in 2026, subject to positive data.
We announced our collaboration with Pfizer for late phase development and commercialization of VLA15, if approved, in April 2020 and received a $130 million upfront payment on signing. In June 2022, the terms of this agreement were updated and Pfizer invested €90.5 ($95) million in Valneva as part of an Equity Subscription Agreement. As per the updated terms, Pfizer will fund 60% of the remaining shared development costs. We will receive tiered royalties ranging from 14% to 22%, which will be complemented by up to $100 million in milestones payable to us based on cumulative sales. Other development and early commercialization milestones were unchanged, of which $143 million remain to date. We received a $25 million milestone payment from Pfizer following initiation of the Phase 3 study. See “Item 10.C—Material Contracts—Pfizer License Agreement” for more details.
Overview of Lyme disease
Lyme disease is a systemic infection caused by Borrelia bacteria transmitted to humans by infected Ixodes ticks. It is considered the most common vector-borne illness in the Northern Hemisphere. According to the U.S. Centers for Disease Control and Prevention, approximately 476,000 people in the United States are diagnosed with Lyme disease each year, and at least a further 200,000 cases occur in Europe. Research suggests that Lyme disease cases may rise 92% by 2100 in the United States due to climate change. Although most patients recover from Lyme disease, 10-20% have persistent symptoms, which for some are chronic and disabling. Studies indicate that Lyme disease costs up to approximately $1.3 billion each year in direct medical costs in the United States alone. The global market for a Lyme disease vaccine is estimated to exceed $1 billion.
The transmission of Lyme disease infection is well understood and documented. Borrelia bacteria colonize in the salivary glands of ticks. When a tick attaches for feeding, it injects its saliva into the human or animal host, bringing along with it antihistamines, cytokine blockers, and anticoagulants and, in the case of an infected tick, Borrelia bacteria as well.
Early symptoms of Lyme disease can often be overlooked or misinterpreted as they are often associated with other, often less severe, illnesses. These symptoms include fever, chills, headache, fatigue, muscle and joint aches, as well as swollen lymph nodes. In 70-80% of cases, a gradually expanding rash called Erythema migrans forms. As this rash enlarges, it appears as a target or bulls-eye, three to thirty days after infection. Left untreated, the disease can disseminate beyond this initial area into the circulation, the joints, the heart, the brain, and the rest of the central nervous system. If not treated, once the infection has progressed it can cause serious complications, including arthritis with severe joint paint, heart palpitations or irregular heartbeat, and inflammation of the brain and spinal cord.
When diagnosed sufficiently early, Lyme disease can be successfully treated with a two-week to four-week course of oral antibiotics. However, given that the disease is often misdiagnosed in its early stages, patients often miss this therapeutic window. Additionally, chronic symptoms can commonly persist beyond antibiotic treatment, a set of conditions referred to as Post-Treatment Lyme Disease Syndrome, or PTLDS. There are no proven treatments for PTLDS, which often resolves over time but unfortunately may take many months. There is therefore a strong emphasis on prophylactic approaches to preventing the disease through behavior modification – avoiding areas where ticks are prevalent, wearing clothing which minimizes tick exposure, using insect repellants and physically removing ticks that have attached. However, even with education and behavior modification, Lyme disease remains a serious and prevalent disease in the regions where it is endemic.
VLA15 Approach
VLA15 provides a potential prophylactic solution to Lyme disease by generating antibodies that target the OspA protein on the surface of Borrelia, killing the bacteria before it can be transmitted from the infected tick to the human host. Third-party studies have shown that antibodies against OspA, which are immunoglobulin G, or IgG, antibodies, in the blood of an animal bitten by an infected tick are transmitted to the tick during feeding and kill the Borrelia in the tick’s gut before it can migrate to the tick’s salivary glands and be transmitted to the animal. VLA15 is a recombinant protein subunit vaccine that is designed to achieve this protective effect using a truncated form of the OspA protein to generate IgG antibodies against the OspA protein through a process summarized in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
| Step 1 |
Step 2 |
Step 3 |
Step 4 |
| Vaccine, when injected, elicits high levels of anti-OspA antibodies |
Tick attaches to vaccinated human and begins feeding on blood (24- to 48-hour attachment needed to transmit B. burgdorferi) |
Anti-OspA antibodies from vaccine enter tick via consumed blood |
Antibodies kill B. burgdorferi in midgut, preventing transmission
to human host |
There are multiple serotypes or variants of Borrelia that lead to Lyme disease. The difference among the serotypes includes the fact that they have variant genetic sequences in the code for the OspA protein, meaning that each serotype requires a specific antigen targeting its OspA protein. In the United States, Lyme disease is predominantly associated with B. burgdorferi infection, or serotype 1 (ST1), while in Europe, there are multiple serotypes with B. afzelii, or serotype 2 (ST2), accounting for slightly more than half of infections. We have developed VLA15 as a single vaccine candidate that includes the OspA antigens from the six most frequently observed serotypes of Borrelia in North America and Europe.
To simplify production of the antigenic proteins, we linked the antigenic regions of two OspA proteins from different serotypes into a fusion construct. This allows us to produce the antigens against the six primary serotypes of Borrelia with just three protein constructs.
Phase 1 Clinical Trial and Results
We evaluated VLA15 in a partially randomized, multi-center dose escalation Phase 1 clinical trial conducted in Belgium and the United States in 179 healthy adults below 40 years of age. The first 24 subjects were included in an open-label trial in which they participated in a staggered dose escalation design. The remaining 155 subjects were enrolled in one of six blinded treatment groups, receiving VLA15 at a dose of either 12 µg, 48 µg, or 90 µg, with or without alum as an adjuvant, by intramuscular injection on Days 0, 28, and 56. The trial was designed to investigate the safety and tolerability as well as immunogenicity of VLA15. The primary endpoint was safety and tolerability of VLA15 up to three months after enrollment (Day 84).
The final Phase 1 data supported the tolerability profile observed at all time-points, as reported in the interim analysis. The Phase 1 trial met its study endpoints in terms of safety and immunogenicity. The majority of adverse events were mild or moderate, and there were no vaccine-related serious adverse events, allergic reactions, or reactions potentially related to Lyme borreliosis observed. The most common local adverse events were injection site pain (67.0%) and tenderness (84.4%). Solicited systemic adverse events were reported by 58.1% (48 µg with alum group, 90 µg with alum group) to 76.7% (90 µg without alum group) of subjects. The most common solicited systemic adverse events were headache (44.7%), excessive fatigue (25.1%), and myalgia (25.1%). Adverse event rates following subsequent doses in the primary series declined compared to the first dose, indicating no enhanced reactogenicity risk with subsequent vaccinations.
In addition, the final Phase 1 immunogenicity results indicated that the alum-adjuvanted formulations elicited higher immune responses at all time-points, confirming interim data findings as compared to respective non-adjuvanted groups of the same dose level. As expected, based on the interim Phase 1 data, antibody titers declined post Day 84 across all groups, trending towards baseline at approximately one year after initial vaccination.
For some vaccines, immunity begins to decline after a certain period of time, at which point a “booster” dose is needed to raise immunity levels. To evaluate the benefit of a booster dose, 64 subjects across the two higher dose groups (48 µg and 90 µg, both with and without alum) from the Phase 1 trial received a booster in the period 12 to 15 months after their initial dose in the primary immunization. Safety and immunogenicity of VLA15 was evaluated up to month 19, with an interim analysis four weeks after the booster. This booster dose resulted in a significant anamnestic response, yielding OspA antibody titers at levels from 2.7-fold for ST2 and ST3 to 5.8-fold for ST1 over the initial titers observed at Day 84. This potent immunogenic response was observed against all six OspA variants. Additional data about a booster dose follow in the Phase 2 discussion below.
Phase 2 Clinical Trials and Results
We have evaluated the safety and immunogenicity of VLA15 at different dosage levels and schedules in three Phase 2 clinical trials in Europe and the United States. Together, these trials enrolled 1443 healthy participants of 5 to 65 years of age.
VLA15-201 Clinical Trial and Results
Our first Phase 2 clinical trial, VLA15-201, was a randomized, observer-blind, placebo-controlled, multi-center Phase 2 clinical trial conducted in Belgium, Germany, and the United States, consisting of a “run-in phase” and a “main study phase.” In the run-in phase, a total of 120 participants aged 18-40 were randomized into one of four groups: a placebo group and three groups at different dosage levels of VLA15 with alum (90 µg, 135 µg, or 180 µg). The participants received intramuscular injections on Days 1, 29, and 57. Based on the elicited higher antibody responses across all serotypes observed from the run-in phase, we selected the two higher VLA15 dose levels to be evaluated in the main study phase. A total of 452 subjects aged 18-65 were randomized 2:2:1 to receive one of two VLA15 doses (135 µg or 180 µg) or placebo and received intramuscular injections on Days 1, 29, and 57. The primary endpoint for the trial was GMTs for IgG against each OspA serotype ST1 to ST6. Secondary endpoints examined SCR, geometric mean fold rise, or GMFR, and occurrence of adverse events.
In July 2020, we announced results from our Phase 2 clinical trial of VLA15-201 in which we observed VLA15 was immunogenic across all dose groups tested. Compared to results from the Phase 1 clinical trial, the higher doses used in our Phase 2 clinical trial elicited higher antibody responses across all serotypes than those observed after the primary series in the Phase 1 clinical trial. SCR in the highest dose ranged from 81.5% (ST1) to 95.8% (ST2) on Day 85. No statistically significant differences between 135 µg and 180 µg treatment groups were observed.
In the age group comparable to the age group investigated in the Phase 1 clinical trial (18-39 years), SCRs ranged from 85.6% to 97%. The immunological response in older adults (50-65 years), one of the main target groups for a Lyme vaccine, had SCRs ranging from 71.9% to 93%. Results indicated that prior exposure to Borrelia burgdorferi sensu lato (Bb sl), the bacteria that causes Lyme disease (baseline Bb sl sero-positivity) did not have an impact on immunogenicity or safety.
VLA15 was generally well tolerated across all dose and age groups tested. No serious adverse events related to VLA15 were observed in any treatment group. The most common solicited local adverse events were injection site pain (68.4%) and tenderness (76.6%), whereas the most common solicited systemic adverse events were headache (33.2%), fatigue (31.6%), and muscle pain (myalgia) (41.1%). The proportion of adverse events decreased with subsequent vaccinations and were transient.
Overall, the tolerability profile including rates of fever appeared to be comparable to what has been observed in third-party trials of other lipidated recombinant vaccines or lipid-containing formulations.
VLA15-202 Clinical Trial and Results
Our second Phase 2 clinical trial, VLA15-202, was a randomized, observer-blind, placebo-controlled multi-center Phase 2 clinical trial conducted in the United States with 246 healthy volunteers aged 18-65. The subjects were randomized 2:2:1 to receive either VLA15 with alum (either 135 µg or 180 µg) or placebo, administered through intramuscular injection at month zero, two, and six. The primary endpoint of the trial was GMTs for IgG against each OspA serotype, measured at month 7 to highlight the importance of further increases in OspA-specific IgG titers after the primary immunization series, which are likely necessary to achieve a successful vaccine candidate. Secondary endpoints evaluated SCRs, GMFRs, and the occurrence of adverse events.
On October 20, 2020, we reported interim results from VLA15-202. Compared to VLA15-201, immunogenicity was further enhanced using an immunization schedule of vaccinating at zero, two, and six months. SCRs, after completion of the primary vaccination series, showed similar responses and ranged from 93.8% (ST1) to 98.8% (ST2, ST4).
Antibody responses were comparable in the two dose groups tested as of Day 208. The immunological response in older adults, one of the main target groups for a Lyme vaccine, was consistent with our observations in VLA15-201. Furthermore, results did not indicate that prior exposure to Borrelia burgdorferi sensu lato (Bb sl), the bacteria that causes Lyme disease (baseline Bb sl sero-positivity) has an impact on immunogenicity or safety, also consistent with our observations in VLA15-201.
Unlike our previous trials, we also performed a Serum Bactericidal Assay, or SBA, assessing the functional immune response against Lyme disease after vaccination with VLA15. Assays, such as SBAs, are commonly used to enable a potential prediction of vaccine efficacy via the measurement of vaccine-induced functional immune responses. Over the course of our trial, the SBAs demonstrated functionality of antibodies against all OspA serotypes.
VLA15 was generally well tolerated across all doses and age groups tested in VLA15-202. The tolerability profile including fever rates was comparable to what has been observed in trials of other lipidated recombinant vaccines or lipid containing formulations. Overall, 232 of 246 participants (94.3%) reported any adverse event, solicited or unsolicited, up to Day 208. Rates of participants who experienced adverse events were similar in the VLA15 treatment groups: 96.9% (135 µg group) and 99.0% (180 µg group), compared with 80.4% in the placebo group. Most adverse events were mild or moderate in severity and no related serious adverse events were reported. A total of 6.1% of participants experienced severe related adverse events; 5.7% of participants experienced at least one severe solicited Grade 3 reactogenicity event, and as such, were considered to be related, including 6.2% in the 135 µg group, 7.1% in the 180 µg group, and 2.0% in the placebo group. One participant in the 135 µg group experienced a severe unsolicited adverse event of ventricular extrasystoles 13 days after the second vaccination, which was assessed as possibly related to the study vaccine by the investigator. The participant had a history of benign premature ventricular contractions, was treated with propranolol and recovered after 39 days. Six unrelated serious adverse events were reported: 3.1% in the 135 µg group (invasive ductal breast carcinoma, prostate cancer, and vertigo) and 2.0% in the 180 µg group (intervertebral disc protrusion, osteoarthritis). One case of Lyme disease (135 µg group) was reported as an adverse event of significant interest: erythematous rash, developed approximately two weeks after the first vaccination.
On September 28, 2021, we announced further positive results from VLA15-202. Continued evaluation at Month 18 showed that antibody titers declined thereafter across all dose groups, remaining above baseline and confirming the need for a booster strategy. Participants who received a complete primary vaccination series with the 180 µg dose of VLA15 were invited to continue the trial in a booster extension phase and were randomized 2:1 to receive an additional 180 µg dose of VLA15 or placebo at Month 18. VLA15’s acceptable safety profile was confirmed through one-month post-booster. No related serious adverse events were observed in any treatment group. Administration of the booster dose elicited a strong anamnestic response yielding a 2.9-fold (ST3) to 4.2-fold (ST1, ST4) increase (GMT) in anti-OspA IgG antibody titers compared with titers observed after primary immunization. All participants seroconverted to anti-OspA IgG after the booster dose, meaning SCRs were 100% for all OspA serotypes. SCR was defined as the rate of participants that changed from seronegative at baseline to seropositive. Additionally, participants who were seropositive at baseline needed to show at least a 4-fold increase in anti-OspA IgG compared to baseline titer. Functionality of elicited antibodies was demonstrated by SBA, leading to SCRs ranging from 86.8% (ST2) to 100.0% (ST3) after the booster. The trial is continuing to monitor persistence of antibody responses.
The results of the VLA15-201 and VLA15-202 trials were published in the peer-reviewed medical journal, The Lancet Infectious Diseases, in June 2024. The article, titled “Optimization of Dose Level and Vaccination Schedule for the VLA15 Lyme Borreliosis Vaccine Candidate Among Healthy Adults: Two Randomized, Phase 2 Studies” provides a detailed analysis of the clinical results for the two trials.
VLA15-221 Clinical Trial
On December 2, 2020, we announced the acceleration of the pediatric development of VLA15. The Phase 2 clinical trial VLA15-221, which commenced in March 2021, is the first clinical trial of VLA15 that includes a pediatric test population between 5 and 17 years old. We announced completion of recruitment for VLA15-221 in July 2021 and reported positive topline and booster data in February 2022 and September 2023, respectively. The dosing of the first subject in this trial triggered a milestone payment from Pfizer of $10 million.
VLA15-221 is a randomized, observer-blind, placebo-controlled Phase 2 clinical trial. A total of 625 participants, 5 to 65 years of age and in groups with age ranges of 5-11, 12-17 and 18-65, were randomized to receive VLA15 at Month 0-2-6 or Month 0-6 (approximately 200 volunteers each) or placebo at Month 0-2-6 (approximately 200 volunteers). The trial was conducted at sites in the US which are located in areas where Lyme disease is endemic and has enrolled volunteers with a cleared past infection with Borrelia burgdorferia as well as Borrelia burgdorferi-naïve volunteers. Participants received VLA15 at a dose of 180µg, which was selected based on data generated in the two previous Phase 2 clinical trials.
The main safety and immunogenicity readout was performed approximately one month after completion of the primary vaccination schedule (i.e. at Month 7), when peak antibody titers were anticipated. A subset of participants received a booster dose of VLA15 or placebo at Month 18 (Booster Phase) and will be followed for three additional years to monitor antibody persistence. The objective of the trial is to show safety and immunogenicity down to 5 years of age and to evaluate the optimal vaccination schedule for use in Phase 3 clinical development.
In the sub-analysis of participants 18-65 years old who received VLA15 in either the two-dose schedule (N=90) or the three-dose schedule (N=97), performed one month after the last vaccination, VLA15 was found to be immunogenic with both vaccination schedules tested. These data are consistent with the strong immunogenicity profile observed for this age group in previous Phase 2 studies. However, the induction of anti-OspA IgG (anti-outer surface protein A immunoglobulin G) antibody titers was higher in participants who received the three-dose primary series compared to those who received the two-dose primary series. Based on these results, we and Pfizer proceeded with a three-dose primary series vaccination schedule in the Phase 3 clinical trial discussed below. The analysis was also consistent with the acceptable safety and tolerability profile observed in previous studies of VLA15. No vaccine-related serious adverse events were observed.
In April 2022, together with Pfizer, we reported positive pediatric data for the VLA15-221 trial. In pediatric participants (5-17 years old) who received VLA15 in either the two-dose schedule (N=93) or three-dose schedule (N=97), VLA15 was found to be more immunogenic than in adults with both vaccination schedules tested. The safety and tolerability profile observed in the 5- to 17-year age group was similar to the previously reported profile in adult participants. No vaccine-related serious adverse events (SAEs) were observed. Like in adults, the immunogenicity and safety data supported a three-dose primary vaccination schedule in pediatric participants in the Phase 3 trial.
Additionally, in September 2023, we reported positive booster results for this trial. The results showed a strong anamnestic antibody response for all serotypes in pediatric, adolescent, and adult participants one month after administration of a booster dose (month 19). Depending on the primary schedule they received (month 0-2-6 or month 0-6), participants seroconverted after the booster dose, yielding seroconversion rates of 95.3% and 94.6% for all OspA serotypes in all age groups, respectively. Additionally, OspA antibody titers were significantly higher one month after the booster dose compared to one month after the primary schedule with 3.3- to 3.7-fold increases (GMT) in adults, 2.0- to 2.7- fold increases in adolescents and 2.3- to 2.5-fold increases in children for all serotypes. The safety and tolerability profile of VLA15 after a booster dose was consistent with previous studies as the vaccine candidate was well-tolerated in all age groups regardless of the primary vaccination schedule. No vaccine-related serious adverse events and no safety concerns were observed by an independent Data Safety Monitoring Board.
In September 2024, we and Pfizer reported further booster results following vaccination with a second booster dose given one year after receiving the first booster dose. The immune response and safety profile of VLA15 one month after receiving the second booster dose were similar to those reported after receiving the first booster dose, showing compatibility with the anticipated benefit of a booster vaccination prior to each Lyme season. These latest booster results again demonstrated a significant anamnestic antibody response across all six serotypes covered by the vaccine candidate in pediatric (5 to 11 years of age) and adolescent (12 to 17 years of age) participants, as well as in adults (18 to 65 years of age), measured one month after administration of this second booster dose (month 31). A high proportion of participants seroconverted after the second booster dose, yielding seroconversion rates* (SCRs) above 90% for all outer surface protein A (OspA) serotypes in all age groups, in-line with SCRs after the first booster. Geometric Mean Titers at one month post first and second booster (i.e. month 19 vs. month 31) were comparably high. The safety and tolerability profile of VLA15 after a second booster dose was comparable to the profile observed after the first booster. To date, no safety concerns have been observed by an independent Data Monitoring Committee (DMC) in any treatment or age group.
Phase 3 Trial
In August 2022, together with Pfizer, we announced the initiation of a Phase 3 clinical trial, Vaccine Against Lyme for Outdoor Recreationists (VALOR), to investigate the efficacy, safety, and immunogenicity of VLA15.
The randomized, placebo-controlled, Phase 3 VALOR trial has been enrolling participants five years of age and older and is being conducted in areas where Lyme disease is highly endemic, including Finland, Germany, the Netherlands, Poland, Sweden, Canada, and the United States.
As per the terms of our collaboration, we received a $25 million milestone payment from Pfizer following initiation of the Phase 3 study. In February 2023, Pfizer, as the study sponsor, decided to discontinue a significant percentage of enrolled U.S. study participants following violations of Good Clinical Practice at certain clinical trial sites run by a third-party clinical trial site operator. The discontinuation of these participants was not due to any safety concerns with the investigational vaccine and was not prompted by a participant-reported adverse event. The trial has continued with other sites not operated by the third party and new sites in the U.S. and Canada. In December 2023, we and Pfizer announced that we completed recruitment for the study. 9,437 participants five years of age and older were enrolled to receive, as part of the full primary series, three doses of VLA15 180 µg or a saline placebo (1:1 ratio) within the first year, and one booster dose of VLA15 or saline placebo approximately one year after vaccination with the first three doses. In July 2024, we announced completion of the three-doses primary vaccination series. Participants are continuing to receive their booster dose and will be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025.
Pfizer aims to submit a BLA to the FDA and an MAA to the EMA in 2026, subject to positive data.
VLA1553 / IXCHIQ—Our vaccine targeting the chikungunya virus
VLA1553 is a single-dose, live-attenuated vaccine candidate against CHIKV, which was approved under the brand name IXCHIQ in the United States, Canada, Europe, and the United Kingdom in November 2023, June 2024, July 2024, and February 2025, respectively. It is indicated for the prevention of disease caused by CHIKV in individuals 18 years of age and older.
The vaccine is still undergoing several clinical trials with a view to expand access, support label extensions, and enhance the product profile.
In 2024, we submitted label extensions to extend the use of IXCHIQ, which is currently approved in adults, to adolescents aged 12 to 17 years, in the U.S., Europe, and Canada. The Canadian and U.S. label extension applications also included two-year antibody persistence data, which is a key differentiator for IXCHIQ that was already included in the initial filing with the EMA. In February 2025, EMA’s CHMP adopted a positive opinion recommending authorization of IXCHIQ’s label extension to adolescents. The European Commission will now review the CHMP recommendation, and a decision on the adolescent label extension in the EU, Norway, Liechtenstein and Iceland is expected within sixty days following the CHMP recommendation. The label extension applications are supported by positive six-month adolescent Phase 3 data collected through a study in Brazil funded by the Coalition for Epidemic Preparedness Innovations (CEPI) and the European Union’s (EU) Horizon Program, This study is also expected to support marketing approval in Brazil.
In addition to our adolescent study, we have continued to monitor persistence in a dedicated antibody persistence trial, VLA1553-303. We reported positive twelve, twenty-four, and thirty-six month antibody persistence data in December 2022, 2023, and 2024, respectively, demonstrating a very high level of seroconversion, with 99%, 97%, and 96% of participants, respectively, showing protective CHIKV neutralizing antibodies, after receiving a single vaccination. We will continue to evaluate persistence in the VLA1553-303 trial for a period of ten years.
Additionally, we initiated a Phase 2 pediatric trial in children 1 to 11 years of age, VLA1553-221, in January 2024 to support a Phase 3 pivotal pediatric study and potentially extend the label in this age group following initial regulatory approvals in adults and possibly in adolescents. In January 2025, we announced positive results for VLA1553-221. The trial, which was evaluating the safety and immunogenicity of two different dose levels of IXCHIQ in 304 children, met its primary endpoint. The vaccine was well tolerated by children aged one to eleven years regardless of the dose (half dose or full dose) or previous chikungunya infection (CHIKV), and, to a similar extent, to an active control MenACYW vaccine (Nimenrix). Overall, the safety profile was consistent with the profile observed in Valneva’s pivotal Phase 3 trials in adults and adolescents. Valneva’s vaccine was highly immunogenic in both dose groups. A full dose of the vaccine exhibited a more robust immune response compared to a half dose by providing protective antibody titers already at Day 15 and Day 29 post-vaccination, confirming the excellent immunogenicity previously observed in adults and adolescents. An independent Data Safety Monitoring Board (DSMB) rigorously monitored safety data throughout the trial and confirmed the absence of any safety concerns. In 2025, we also expect to initiate the Phase 4 post-marketing studies required as part of our approval under the FDA’s accelerated approval pathway.
Using our positive pivotal Phase 3 trial in over 4,000 healthy adults, we sought licensure under the accelerated approval pathway which eliminates the need to execute a time-intensive and costly field trial where a group of patients receiving a placebo is compared to groups of patients receiving VLA1553. However, this approach requires that vaccine effectiveness, i.e., the proof that the vaccine can prevent cases of disease, is demonstrated post-licensure.
Overview of the chikungunya virus
Chikungunya is a mosquito-borne virus posing a serious public health problem in tropical and sub-tropical regions. Chikungunya virus often causes sudden large outbreaks with high attack rates, affecting one-third to three-quarters of the population in areas where the virus is circulating and can cause a significant economic impact. Between 2013 and 2023, more than 3.7 million cases were reported in the Americas. The true incidence of chikungunya is likely to be much higher due to the level of under-reporting, with available studies suggesting an under-reporting factor of five times due to difficulty in diagnosing the symptoms, which can be similar to those of dengue and Zika, and due to lack of access to good medical care in certain areas where outbreaks are prevalent. It is estimated that the global market for a chikungunya vaccine, including travel and endemic markets, will exceed $500 million annually by 2032.
Chikungunya infection is characterized by an acute onset of fever, rash, myalgia, and sometimes debilitating arthritic pain in multiple joints. Chikungunya causes symptomatic infection in 72-92% of infected humans around four to seven days after infection. Mortality of chikungunya is low (<1%) but the chronicity of its joint pain (arthralgia) and inflammatory symptoms represent a significant burden of disease with potential long-term debilitating impact. For example, following a significant outbreak in 2005, 94% of symptomatic travelers infected in La Reunion, an island in the Indian Ocean, complained of joint or bone pain six months after the epidemic peak, and this pain was constant in 41% of the cases. The effect of chronic symptoms on the quality of life was defined as totally disabling or important in almost half of the patients. Even at 32 months post-infection, 83% of people continued to report joint pain.
In addition to having significant impact on patients who become infected, chikungunya is highly transmissible, and prior outbreaks have led to significant spread of the virus. For example, in 2004, a chikungunya epidemic in Kenya triggered the spread of this virus to nearly all regions of the world with cases reported in Africa, Asia, Europe, the Americas, the Indian Ocean, the Pacific Ocean, and the Caribbean islands. Cases in Europe and the United States are typically tied to recent travel to endemic areas.
However, one of the vector mosquitos, the tiger mosquito, is established in southern regions of Europe and the United States, and travel-related cases have generated local outbreaks as reported from Italy and France.
Without vaccination, we believe the spread of chikungunya will continue to increase rapidly, driven by a number of key factors:
•The recent development that chikungunya can be spread by a second species of mosquitos, one that has a broader worldwide distribution, is tolerant to colder temperatures, and is highly abundant in large parts of the world;
•The current lack of herd immunity in large portions of the human population;
•The ease of chikungunya’s spread by travel, which can occur if an uninfected mosquito feeds on an infected person who has returned home from an endemic area; and
•An increase in the geographic distribution and size of the population at risk due to climate change.
The current standard of care to treat individuals who have become infected with chikungunya is the application of non-steroidal anti-inflammatory drugs to relieve symptoms. To date, apart from our vaccine IXCHIQ which has recently been launched in the U.S., Canada and certain countries in Europe, preventive measures in other territories rely on avoiding mosquito bites. Effective mosquito control has proven challenging, even in higher income countries.
VLA1553 / IXCHIQ Approach
IXCHIQ is a live-attenuated chikungunya vaccine based on the East, Central, and Southern African, or ECSA, strain which has spread across the Indian Ocean. It is cross-reactive with other strains, meaning that it is designed to protect against those as well, including the strain of Asian lineage which is rapidly spreading across the Americas as observed in pre-clinical studies. Additionally, given that we have engineered IXCHIQ as a live-attenuated vaccine, we believe it may confer life-long immunity.
IXCHIQ is engineered using a strain of chikungunya, where specific segments of the virus have been deleted, thereby weakening, or attenuating, the virus. This approach enables IXCHIQ to catalyze the patient’s immune system into generating the antibodies necessary to provide protection against the virus while the weakened strain does not cause the patient to develop significant symptoms. In our pre-clinical studies, growth of this strain on Vero cells resulted in a viral titer 35 times lower than observed with the original unattenuated strain, demonstrating the attenuation of our chikungunya strain. The deleted segment also remained absent following replication of the virus in the Vero cells, suggesting that the weakness of the virus is sustained.
Phase 1 Clinical Trial and Results
We conducted a single blind, randomized dose-escalation Phase 1 clinical trial of VLA1553 in 120 adults, at multiple centers in the United States, the results of which were published in the Lancet Infectious Diseases in 2020. Chikungunya virus neutralizing antibodies were observed in 100% of patients for 12 months at all three of the doses evaluated. A single vaccination was sufficient to induce sustaining high-titer neutralizing antibodies at 12 months post-vaccination.
We found that 100% of participants had seroconverted by day 14 at all three of the doses tested and this seroconversion persisted for one year across all dose groups. When re-evaluated with the assay that was used to define the seroresponse threshold for Phase 3, we confirmed that 100% of participants had seroresponded by day 14.
Individuals that received a single high dose of VLA1553 did not exhibit an increase in antibody titers following subsequent re-vaccination at month six. No viremia was detected in any participant after any re-vaccination, suggesting that a single dose provides sufficient protection.
The majority of adverse events across the dose groups were assessed as mild or moderate and were reported after the single vaccination. No adverse event of special interest, meaning adverse events resembling a chikungunya-like infection, and no vaccine-related serious adverse events were reported. Injection site reactogenicity was low, with less than 7% of individuals in the high-dose group reporting any local adverse event, all of which were mild in severity. Systemic adverse events were predominantly headache (32.5%), fever (26.7%) and fatigue (24.2%), followed by muscle pain (20.0%) and joint pain (13.3%), all of which were transient and are typical reactions after immunization and similar to those reported after vaccination with other vaccines in the general population. Severe fever (a temperature of 102.1°F or higher) was reported by seven participants. Adverse events decreased on re-vaccination at month six.
Phase 3 Clinical Trials
VLA1553-301 Clinical Trial
In September 2020, we initiated our pivotal Phase 3 clinical trial, VLA1553-301, in the United States. In this double-blind, multi-center, randomized Phase 3 clinical trial, 4,115 participants aged 18 years and above were randomized 3:1 into two groups to receive either VLA1553 0.5mL or placebo. Immunogenicity was determined with a µPRNT50 assay.
The primary endpoint was safety and immunogenicity 28 days after a single vaccination with VLA1553. The trial met its primary endpoint, inducing protective CHIKV neutralizing antibody titers in 98.9% of participants 28 days after receiving a single shot. The seroconversion rate result of 98.9%, and specifically the lower bound of the 95%CI of 96,7%, exceeded the 70% threshold (for non-acceptance) agreed with the FDA. The excellent immunogenicity profile was maintained over time, with 96.3% of participants showing protective CHIKV neutralizing antibody titers six months after receiving a single vaccination.
VLA1553 was highly immunogenic, with a GMT of approximately 3,362, confirming the immunogenicity profile seen in the Phase 1 clinical trial.
VLA1553 was generally well tolerated across all age groups among the 3,082 subjects evaluated for safety. An independent Data Safety Monitoring Board, or DSMB, continuously monitored the study and identified no safety concerns. The final data safety profile is consistent with results from the Phase 1 clinical trial. The majority of solicited adverse events were mild or moderate and resolved within three days. 2.0% of study participants reported severe solicited adverse events, most commonly fever. Approximately 50% of trial participants experienced solicited systemic adverse events, most commonly headache, fatigue, and myalgia. The local tolerability profile showed that approximately 15% of participants experienced solicited local adverse events.
Additionally, VLA1553 was highly immunogenic in elderly study participants (65 years of age or older), who achieved equally high seroconversion rates and neutralizing antibody titers over time as younger adults.
The final pivotal Phase 3 data were published in The Lancet in June 2023.
VLA1553-302 Clinical Trial
We also initiated a lot-to-lot consistency Phase 3 trial, VLA1553-302, in February 2021 to show manufacturing consistency of VLA1553, which is a requirement for licensure. We announced completion of recruitment for this trial in June 2021 and positive topline and final data from this trial in December 2021 and May 2022, respectively.
The VLA1553-302 trial met its primary endpoint, demonstrating that three consecutively manufactured vaccine lots elicited equivalent immune responses measured by neutralizing antibody titer GMT ratios on Day 29 after vaccination. The trial included 408 participants aged 18 to 45 and confirmed the excellent immunogenicity profile observed in the pivotal Phase 3 trial, VLA1553-301. All three lots were equally well tolerated and the safety profile was consistent with results in VLA1553-301. The trial therefore confirmed clinical equivalence as well as manufacturing consistency of the three lots.
The lot-to-lot data were part of our submission to the FDA which we completed in December 2022.
VLA1553-303 Clinical Trial
In April 2021, we initiated an antibody persistence trial that will follow annually up to 375 subjects in the immunogenicity subset of the VLA1553-301 trial for a period of ten years after a single immunization with IXCHIQ. In December 2022, 2023 and 2024, we reported 12-, 24- and 36-month data, respectively, for this trial. 12, 24, and 36 months after the single-dose vaccination, 99%, 97% and 96% of participants, respectively, retained neutralizing antibody titers above the seroresponse threshold of 150. The antibody persistence was similar in older adults aged ≥65 years, who retained neutralizing antibody titers comparable to younger adults throughout the follow-up. No safety concerns were identified for the duration of the follow-up study, confirming the safety profile observed in previous studies.
VLA1553-321 Clinical Trial
In January 2022, we announced the initiation of a Phase 3 trial of VLA1553 in 754 adolescents 12 to 17 years of age. Conducted in Brazil by Institution Butantan and funded by CEPI, the VLA1553-321 trial is intended to support the label extension in this age group following the initial regulatory approval in adults from the FDA. This trial is also expected to support licensure of the vaccine in Brazil, which would be the first potential approval for use in endemic populations.
In November 2023, we reported positive Phase 3 immunogenicity and safety data showing that a single-dose vaccination with VLA1553 induced a robust immune response in adolescents, thereby confirming the excellent immunogenicity previously observed in adults. VLA1553 induced levels of protective antibody titers in 98.8% of participants 28 days after a single vaccination significantly exceeding the FDA’s requirement for study success of the lower bound of the 95% CI for seroresponse rate >70%. Additionally, VLA1553 was generally well tolerated in adolescents, irrespective of previous CHIKV infection, and showed a similar safety profile as reported in adults. In May 2024, we reported six-month data which confirmed the positive immunogenicity and safety data reported previously. A single-dose vaccination with VLA1553 induced a high, sustained immune response with a seroresponse rate of 99.1% (232 out of 234 participants) at Day 180 in an immunogenicity subset of individuals who were CHIKV negative at baseline. Additionally, the six-month data confirmed that a single dose of the vaccine was generally safe and well tolerated in adolescents receiving VLA1553, irrespective of previous infection with the chikungunya virus. Throughout the trial, an independent DSMB consistently assessed safety data and found no safety issues. The majority of solicited adverse events observed following VLA1553 administration were mild or moderate and resolved within three days post vaccination. In January 2025, we reported positive twelve-month Phase 3 data which showed a sustained seroresponse rate in 98.3% of adolescents one-year after single vaccination. These results support and strengthen the pivotal data previously reported for adolescents (12 to 17 years old) which supported filing for potential label extension to this age group in the U.S. , Europe, and Canada . Data from this trial are also expected to support licensure of IXCHIQ in Brazil, which would be the first potential approval for use in endemic populations.
VLA1553-221 Clinical Trial
In January 2024, we initiated a Phase 2 pediatric trial in children one to eleven years of age to evaluate the safety and immunogenicity of two different dose levels of IXCHIQ. The multicenter, prospective, randomized, observer-blinded Phase 2 clinical trial enrolled 304 healthy children at three trial sites in the Dominican Republic and Honduras. Following a safety run-in phase, participants were randomized to receive either a full dose formulation of the vaccine (120 participants), a half dose formulation (120 participants), or a control vaccine (60 participants). In January 2025, we announced positive results for VLA1553-221.
The trial met its primary endpoint demonstrating that the vaccine was well tolerated by children aged one to eleven years regardless of the dose (half dose or full dose) or previous chikungunya infection (CHIKV), and, to a similar extent, to an active control MenACYW vaccine (Nimenrix). Overall, the safety profile was consistent with the profile observed in Valneva’s pivotal Phase 3 trials in adults and adolescents. Valneva’s vaccine was highly immunogenic in both dose groups. A full dose of the vaccine exhibited a more robust immune response compared to a half dose by providing protective antibody titers already at Day 15 and Day 29 post-vaccination, confirming the excellent immunogenicity previously observed in adults and adolescents. An independent DSMB rigorously monitored safety data throughout the trial and confirmed the absence of any safety concerns. The Phase 2 pediatric data are intended to support a Phase 3 pivotal study in children with the objective to extend the label to this age group following initial regulatory approvals in adults and possibly in adolescents.
S4V2— The most advanced tetravlent Shigellosis vaccine candidate
In August 2024, Valneva entered into a collaboration with LimmaTech Biologics AG for the development of S4V2, a tetravalent bioconjugate vaccine candidate against shigellosis, a diarrheal infection caused by Shigella bacteria.
Shigellosis is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year, particularly among children in LMICs. No approved Shigella vaccine is currently available outside of Russia or China, where two vaccines exist for limited use. The development of Shigella vaccines has been identified as a priority by the WHO. In October 2024, the U.S. FDA granted Fast Track designation to S4V2, recognizing its potential to address a serious condition and fill an unmet medical need.
At the time the collaboration was established, LimmaTech had already generated initial safety and immunogenicity data with its S4V2 candidate in adults and infants. In November 2024, Valneva and LimmaTech announced the launch of a parallel-group, randomized, double-blind, multicenter, placebo-controlled Phase 2b controlled human infection model (CHIM) study to assess the safety, immunogenicity, and preliminary efficacy in approximately 120 healthy Shigella-naïve participants aged 18 to 50 years at three sites in the United States.
In addition to the CHIM study, LimmaTech is preparing for a Phase 2 pediatric safety and immunogenicity study in low- and middle-income countries. This study is expected to begin in the first half of 2025. LimmaTech is the regulatory sponsor of the ongoing clinical studies. Upon completion of these studies, Valneva will become the IND holder and assume all further development, including CMC (chemistry, manufacturing and controls) and regulatory activities, and be responsible for its commercialization worldwide, if approved.
Our other clinical programs
VLA1601—Our Zika virus development program
Zika is a mosquito-borne viral disease caused by the Zika virus (ZIKV). It is the first and only flaviviral disease that was declared a public health emergency because of devastating birth defects following maternal infection. According to the World Health Organization, there is scientific consensus that Zika virus is a cause of microcephaly and Guillain-Barré syndrome.
VLA1601 is a highly purified inactivated, adjuvanted vaccine candidate against the Zika virus. It is being developed on the original manufacturing platform of Valneva’s licensed Japanese encephalitis vaccine IXIARO, which was further optimized to develop the Company’s inactivated, adjuvanted COVID-19 vaccine VLA2001, the first one to receive a standard marketing authorization in Europe. Valneva reported Phase 1 results from its first-generation Zika vaccine candidate in 2019. The inactivated vaccine candidate met the study’s primary endpoint showing a favorable safety profile in all doses and schedules tested comparable to IXIARO and other clinical stage ZIKV vaccines. VLA1601 was also immunogenic in all treatment groups and induced both dose- and schedule-dependent neutralizing antibodies against the Zika virus with the kinetics expected for an inactivated, alum-adjuvanted whole-virus vaccine. Seroconversion rates reached up to 85.7% on Day 35 for the highest dose level tested. Antibodies declined during six-month follow-up, as expected for this vaccine class, with seroconversion rates remaining up to 40%.
The incidence of Zika significantly declined after its peak in 2016 due to high population level immunity in affected countries. Back in November 2018, we therefore decided to put this program on hold and chose to prioritize our Lyme disease and chikungunya programs representing a greater health crisis. However, Zika virus transmission persists in several countries in the Americas and in other endemic regions. According to the WHO, a total of 89 countries and territories have reported evidence of mosquito transmitted Zika virus infection to date however, surveillance remains limited globally. There are no preventive vaccines or effective treatments available and, as such, Zika remains a public health threat and is included in the FDA’s Tropical Disease Priority Review Voucher Program. As a result, we have decided to re-initiate the program and expect to start the clinical evaluation of our second-generation vaccine in the coming weeks. A vaccine against the Zika virus would nicely complement Valneva’s portfolio of travel vaccines against mosquito-borne diseases, which already includes IXCHIQ and IXIARO.
VLA84—Our Clostridium difficile vaccine candidate that remains on hold
We have developed VLA84, a vaccine candidate targeting the prevention of primary symptomatic Clostridium difficile infection, or CDI, a leading cause of life-threatening, healthcare-associated infections worldwide. VLA84 is designed to produce an immune response to neutralize the effects of C. difficile toxins A and B, considered to be largely responsible for CDI. We completed Phase 2 development of VLA84.
The key objectives of the Phase 2 trial were met, the vaccine candidate generated strong immune responses against C. difficile toxins A and B, and the safety and tolerability profile was good. We could advance into Phase 3 if we find a suitable partner to reactivate this program.
Our Pre-clinical Portfolio
In addition to our clinical-stage assets, our portfolio includes several pre-clinical assets against disease targets that reflect our strategy of providing prophylactic solutions to significant diseases that lack a preventative and effective therapeutic treatment option.
Our pre-clinical work involves exploratory study of a given disease, including extensive review of existing literature and early data that will inform our view of whether and how we could develop a vaccine for that disease.
Our preclinical portfolio is summarized below:
Our two most advanced pre-clinical assets against EBV and Enterotoxigenic Escherichia coli (ETEC) are presented below. Additionally, we initiated pre-clinical work on vaccine candidates against different enteric diseases.
VLA2112 - Our vaccine candidate targeting Epstein-Barr Virus (EBV)
Epstein-Barr virus (EBV), also known as human herpesvirus 4, is a member of the herpes virus family. It is found all over the world and is one of the most common human viruses. Most people get infected with EBV by early adulthood. EBV spreads most commonly through bodily fluids, primarily saliva. EBV can cause infectious mononucleosis, also called mono, and is strongly associated with different cancers and multiple sclerosis.
Our EBV vaccine candidate, VLA2112, is based on adjuvanted, subunit viral glycoproteins to elicit high titers of EBV-neutralizing antibodies.
The selection of antigens that best neutralize infection of both epithelial cells and B cells was completed in 2023 and confirmatory preclinical research is ongoing.
Our vaccine candidate targeting Enterotoxigenic Escherichia Coli (ETEC)
We have started research work on a vaccine candidate against ETEC. Antigen selection is ongoing for the candidate, which we expect to be highly differentiated compared to other existing ETEC vaccine candidates.
ETEC is the most common cause of traveler’s diarrhea and a major cause of children diarrhea in LMICs. There is currently no specific treatment or vaccine available against ETEC.
Our Commercial Portfolio
Our commercial portfolio is composed of three vaccines, our travel vaccines IXIARO/JESPECT, DUKORAL, and IXCHIQ. We stopped manufacturing our inactivated COVID-19 vaccine, VLA2001, in August 2022 in light of reduced order volumes. Our travel vaccines serve a wide range of potential travelers where the diseases they prevent are endemic, from business and leisure travelers to government and military personnel traveling on behalf of their government. We also distribute certain third-party vaccines in countries where we operate our own marketing and sales infrastructure. Our commercial activities have generated meaningful revenues, much of which we have reinvested in our research and development capabilities in order to advance our clinical assets and drive future growth.
IXIARO—Our Japanese encephalitis vaccine
IXIARO, or JESPECT in Australia and New Zealand, is an inactivated Vero cell culture-derived Japanese encephalitis vaccine and is the only Japanese encephalitis vaccine currently approved for use in the United States, Canada, and Europe. IXIARO is indicated for active immunization against Japanese encephalitis in adults, adolescents, children, and infants aged two months and older, and is a required vaccine for U.S. military personnel who are deployed to areas of risk for Japanese encephalitis. The pediatric indication of IXIARO was granted Orphan Drug designation by the FDA.
Japanese encephalitis virus, or JEV, is spread by mosquitos and is the most important cause of viral encephalitis in Asia and the Western Pacific.
Japanese encephalitis background
Japanese encephalitis is a considerable public health problem for many Asian countries, with recent estimates pointing to 67,900 cases annually. Close to three billion people live in regions at risk for this mosquito-borne viral disease. JEV is transmitted to humans by mosquitos that have bitten an infected animal and less than 1% of infected individuals develop the disease. Those that do develop the disease face a 20-30% mortality rate and up to 50% of survivors have significant permanent neurological damage. Many individuals infected by JEV develop symptoms within five to 15 days, usually starting as a flu-like illness with fever, chills, tiredness, headache, nausea, and vomiting. Confusion and agitation also occur in the early stage of Japanese encephalitis. Later symptoms may include swelling around the brain and coma, which can result in death.
In 2023, over 32 million people traveled from Europe and North America to the countries where JEV is endemic. Vaccination remains the single most important control measure against Japanese encephalitis worldwide.
IXIARO Overview
IXIARO is an inactivated vaccine administered as two doses either seven or 28 days apart. In a randomized clinical trial, high titers of neutralizing antibodies were detected in 96.4% of adults 28 days after the last dose. The immune response to IXIARO was durable, with high levels of neutralizing antibodies in 84.9% of participants three years after initial immunization. A separate trial administration of a booster dose at 14 months after completion of the initial two doses resulted in 100% of participants having neutralizing antibodies.
IXIARO is approved for the prevention of disease caused by JEV in individuals two months of age and older. This intramuscular vaccine is administered in two parts, between seven and 28 days apart depending on the age of the recipient, and with the second dose completed at least a week prior to potential exposure to JEV. A booster shot may be given at least 11 months after completion of the primary immunization series if ongoing exposure or re-exposure to JEV is expected. In 2020, the FDA approved the extension of IXIARO’s shelf life from 24 months to 36 months.
Sales of IXIARO
IXIARO was first approved by the FDA and European Commission in 2009, and reached pre-pandemic sales of €94.1 million during the year ended December 31, 2019. In the year ended December 31, 2022, sales of €41.3 million were impacted by lower sales to the U.S. Department of Defense (DoD) due to lower travel vaccine use during the pandemic. This decrease was partly offset by the significant recovery of the private travel markets, with private sales reaching €28.8 million in the year ended December 31, 2022 compared to €7.1 million in the year ended December 31, 2021. In the year ended December 31, 2023, IXIARO sales increased 78% to €73.5 million primarily benefiting from the continued travel market recovery and price increases. At the end of September 2023, we also signed a new one-year contract with the DoD worth a minimum of $32 million for the supply of IXIARO. In 2024, IXIARO sales increased by 28% to €94.1 million showing double-digit sales growth to both travelers and the DoD compared to 2023. In 2024, Valneva supplied additional doses of IXIARO to the DoD under the contract signed in September 2023, which allowed the DoD to purchase additional doses during the next twelve months. In January 2025, we announced the signing of a new $32.8 million contract with the DoD. Similar to previous contracts, it includes the possibility to purchase additional doses during the following twelve months.
DUKORAL—Our vaccine against cholera and ETEC
DUKORAL is an oral vaccine containing four inactivated strains of the bacterium Vibrio cholerae serotype O1, and part of a toxin from one of these strains as active substances. DUKORAL is authorized for use in the European Union and Australia to protect against cholera and in Canada, Switzerland, New Zealand, and Thailand to protect against cholera and ETEC, the leading cause of travelers’ diarrhea. Originally licensed in Sweden by SBL Vaccines in 1991, and subsequently in the European Union in 2004 through a centralized procedure followed by other international markets, the vaccine was acquired by us in 2015 from Janssen Pharmaceuticals as part of our strategic vision to extend our proprietary travel vaccine portfolio.
Cholera disease background
Cholera is an acute diarrheal disease caused by ingestion of food or water contaminated with the bacterium V. cholerae. Cholera remains a global threat to public health and an indicator of inequity and lack of social development. Researchers have estimated that every year, there are roughly 1.3 to 4.0 million cases, and 21,000 to 143,000 deaths worldwide due to cholera. Cholera is an extremely virulent disease that can cause severe acute watery diarrhea. It takes between 12 hours and five days for a person to show symptoms after ingesting contaminated food or water. Cholera affects both children and adults and can kill within hours if untreated.
Most people infected with V. cholerae do not develop any symptoms, although the bacteria are present in their feces for up to 10 days after infection and are shed back into the environment, potentially infecting other people. Among people who develop symptoms, the majority have mild or moderate symptoms, while a minority develop acute watery diarrhea with severe dehydration. This can lead to death if left untreated.
ETEC disease background
ETEC is the leading cause of travelers’ diarrhea and a major cause of diarrheal disease in lower-income countries. There are approximately 5-18 million reported cases of ETEC per year worldwide. ETEC is transmitted by food or water contaminated with animal or human feces. Infection by ETEC can cause profuse watery diarrhea and abdominal cramping. Illness develops one to three days after exposure and usually lasts three to four days. Most patients recover without any specific treatment other than rehydration.
DUKORAL Overview
DUKORAL is intended for active immunization against cholera (and LT-ETEC diarrhea in certain jurisdictions) in adults and children from two years of age who will be visiting endemic/epidemic areas. The use of DUKORAL should be determined on the basis of official recommendations, taking into account the variability of epidemiology and the risk of contracting disease in different geographical areas and travelling conditions. DUKORAL is a drinkable vaccine that helps prevent diarrhea caused by heat-labile toxin-producing ETEC as well as cholera.
DUKORAL is administered orally after dissolving the product in a glass of water. Vaccination requires two doses given one to six weeks apart. In an efficacy trial done in Bangladesh in 89,596 adults and children aged two years and older, the efficacy of DUKORAL against cholera was 85% in the six months after the third dose and 57% in the second year after immunization. Protective efficacy declined over the three-year trial period. DUKORAL conferred 67% protection against episodes of diarrhea caused by ETEC during the initial three months of follow-up but demonstrated no protection thereafter.
Sales of DUKORAL
DUKORAL was granted marketing authorization throughout the European Union in 2004, having previously been licensed in Sweden and Norway in 1991 through national licensure processes. DUKORAL was approved in Canada in 2003. Sales of DUKORAL were €32.3 million, €29.8 million and €17.3 million in the years ended December 31, 2024, 2023, and 2022, respectively, of which Canada represented €17.5 million, €11.4 million, and €0.6 million, respectively, of global sales due to the strong overlap between Canadian travelers to regions of high ETEC prevalence and the vaccine’s approved indication. In 2024, DUKORAL sales grew 8% reaching €32.3 million supported by increased sales in Canada, the biggest travel market for DUKORAL, as well as improved product availability resulting in replenishment orders from indirect markets. Similar to other travel vaccines, sales in 2021 were significantly impacted by ongoing COVID-19 travel restrictions. In 2022, DUKORAL sales started to benefit from the recovery in the private travel markets, and this recovery intensified in 2023 and 2024.
IXCHIQ—Our Chikungunya Vaccine
IXCHIQ is the world’s first licensed chikungunya vaccine available to address this unmet medical need and the third vaccine we brought from early R&D to approval. We received marketing approval for IXCHIQ from the FDA in November 2023 under an accelerated pathway based on anti-CHIKV neutralizing antibody titers. Continued approval for this indication is contingent upon verification of clinical benefit in Phase 4 confirmatory studies. IXCHIQ is also approved in Canada, Europe, and the United Kingdom.
Chikungunya disease background
Chikungunya is a mosquito-borne viral disease caused by the chikungunya virus, a Togaviridae virus, transmitted by Aedes mosquitoes. Infection leads to symptomatic disease in up to 97% of humans after four to seven days following the mosquito bite. While mortality with CHIKV is low, morbidity is high. Clinical symptoms include acute onset of fever, debilitating joint and muscle pain, headache, nausea, rash and chronic arthralgia.
Chikungunya virus often causes sudden large outbreaks with high attack rates, affecting one-third to three-quarters of the population in areas where the virus is circulating. The high-risk areas of infection for travelers are places where chikungunya virus-carrying mosquitos are endemic, including the Americas, parts of Africa, and Southeast Asia, and the virus has spread to more than 110 countries. Between 2013 and 2023, more than 3.7 million cases were reported in the Americas and the economic impact is considered to be significant. The medical and economic burden is expected to grow as the CHIKV primary mosquito vectors continue to spread geographically. Before IXCHIQ, there were no preventive vaccines or effective treatments available and, as such, the World Health Organization (WHO) has highlighted chikungunya as a major public health threat.
IXCHIQ Overview
IXCHIQ is a single-dose, live-attenuated vaccine licensed in the U.S., Europe, Canada, and the UK. It is indicated for the prevention of disease caused by chikungunya virus in individuals 18 years of age and older. At the end of February 2024, the ACIP provided recommendations on how to use IXCHIQ and these recommendations were then adopted by the CDC. We now expect expanded access to the retail channel via publication of the ACIP recommendations in the MMWR. A marketing authorization application is also under review in Brazil, which would represent the first approval in an endemic country. We submitted label extension applications to potentially extend the use of IXCHIQ, which is currently approved in adults, to adolescents aged 12 to 17 years, in the U.S., Europe and Canada. The Canadian and U.S. label extension applications also include two-year antibody persistence data, which is a key differentiator for IXCHIQ that was already included in the initial filing with EMA. In February 2025, EMA’s CHMP adopted a positive opinion recommending authorization of a label extension for IXCHIQ to adolescents. The EC will now review the CHMP recommendation, and a decision on the label extension in the EU, Norway, Liechtenstein, and Iceland is expected within sixty days following the CHMP’s positive opinion.
Sales of IXCHIQ
Our commercial teams launched the vaccine in the U.S., Canada, and France in March, October and November 2024, respectively. Considering IXCHIQ is the first vaccine worldwide against this unmet need, we focused in 2024 on raising awareness on the disease, shaping the market and booking first sales. We recognized initial sales of €3.7 million in 2024.
Third-party Vaccines
We distribute certain third-party vaccines in countries where we operate our own marketing and sales infrastructure. In June 2020, we entered into a distribution agreement with Bavarian Nordic, pursuant to which we agreed to commercialize their marketed vaccines for rabies and tick-borne encephalitis, leveraging our commercial infrastructure in Canada, the United Kingdom, France and Austria during the COVID-19 outbreak. The agreements relating to our distribution of Bavarian Nordic’s rabies vaccine in Canada and the United Kingdom terminated on December 31, 2024, and the remaining distribution agreements between us and Bavarian Nordic will terminate on December 31, 2025. In September 2022, we also announced a partnership with VBI Vaccines for the marketing and distribution of the only 3-antigen Hepatitis B vaccine, PreHevbri, in select European markets. This contract ended in 2024 when VBI voluntarily withdrew PreHevbri from the markets following insolvency proceedings. In the year ended December 31, 2024, third-party sales decreased by €2.5 million to €33.2 million compared to €35.7 million in 2023 as a result of external supply constraints in the first half of the year. Valneva expects to gradually wind down third-party sales to less than 5% of overall product sales by 2026/2027, expected to result in overall gross margin improvement.
For additional information about our commercial agreements, see “Item 10.C—Material Contracts” of this Annual Report.
The following table summarizes our current third-party agreements:
Competition
We compete in an industry characterized by rapidly advancing technologies, significant competition and a complex intellectual property landscape. We face substantial competition from large pharmaceutical, specialty pharmaceutical, and biotechnology companies. Academic research institutions and governmental agencies can and will continue to compete with support from public and private research institutions. Many of our competitors, either alone or through their collaborations, have significantly greater financial resources and expertise in research and development, manufacturing, pre-clinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products than we do. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient enrollment in clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. As a result, our competitors may discover, develop, license, commercialize, and market products before or more successfully than we do. Below is a description of competition surrounding each of our disease targets and other technologies in development in the vaccines field.
IXIARO/JESPECT Competition
Our commercial vaccine against Japanese encephalitis, IXIARO (marketed as JESPECT in Australia and New Zealand), is the only approved and marketed vaccine for travelers to Japanese encephalitis endemic areas who originate in the U.S., Canada, and European countries.
Given the large population in the Japanese encephalitis endemic region, consisting of over 3 billion people, and the inclusion of the Japanese encephalitis vaccine in many national immunization programs, the competitive landscape in the endemic region is more crowded. Many of the first generation, locally manufactured mouse-brain derived vaccines have been phased out over the past 5-10 years, making way for the introduction of second-generation technologies. This includes companies such as Biken and Kaketsuken (Japan), both with inactivated vero-cell based vaccines, Chengdu (China and GAVI/UNICEF markets) with a live-attenuated vaccine, and Sanofi’s live-attenuated chimeric vaccine, IMOJEV (Australia/some Asian territories). None of these vaccines are currently approved for sale in the European Union, Canada or the United States. Therefore, there is currently no direct competitor to IXIARO in those markets, which represented over 96% of total IXIARO revenues in 2024.
The only country where our Japanese encephalitis vaccine currently faces direct competition is Australia, where it splits market share with the IMOJEV vaccine, originally manufactured by Sanofi and now owned by Substipharm, a French company. This acquisition may result in future competition for IXIARO in travel markets.
DUKORAL Competition
DUKORAL has historically been the only vaccine licensed and marketed to travelers within the European Union, Canada, and Australia against cholera and, in certain countries including Canada, Switzerland, and New Zealand, ETEC. Canada, the Nordic countries, and Australia accounted for approximately 80% of DUKORAL sales in 2024, with Canada alone representing over 58%. DUKORAL is also registered in several endemic countries, and is on the WHO’s list of prequalified vaccines, meaning it has been assessed as safe and effective.
While DUKORAL is relevant for both traveler and endemic segments, our commercial strategy focuses on the traveler market, which included approximately 453 million travelers to Asia, South America, and Africa in 2019.
Endemic market sales currently represent less than 3% of DUKORAL sales. This segment is supplied directly and through UNICEF procurement programs by an Indian vaccine, Shancol, and a Korean vaccine, Euvichol.
Product sales for DUKORAL are driven by typical factors associated with travelers’ vaccines, including the number of travelers in endemic regions, national recommendations, awareness about the illness, and the perception of risk by health practitioners and tourists.
An indication for ETEC diarrhea in Canada, in conjunction with educational and promotional efforts, has resulted in higher penetration rates of DUKORAL in this market.
Another cholera vaccine, Vaxchora, received FDA approval in the United States in 2016. The clinical trial attempting to demonstrate the vaccine’s protection against ETEC was not successful in the Phase 1 clinical trial. Vaxchora was approved by the European Commission in April 2020 for protection against cholera only.
IXCHIQ Competition
Following FDA approval in November 2023, IXCHIQ became the world’s first chikungunya vaccine to be approved for adults aged 18 and older. In addition to the U.S., IXCHIQ is currently approved in Canada, Europe, and the UK. Additionally, a marketing authorization application is currently under review in Brazil, which would represent the first approval in an endemic country. Label extensions applications were submitted in the U.S., Canada, and Europe and, in February 2025, EMA’s CHMP adopted a positive opinion on the application recommending authorization of a label extension to adolescents 12 years of age and older. Another chikungunya vaccine received marketing approval in the U.S. and Europe in February 2025 and a third vaccine entered Phase 2/3 clinical trial in August 2021.
We are aware of other companies developing early clinical stage vaccine candidates with neutralizing antibodies mechanism of action for chikungunya and companies developing vaccine candidates with similar mechanism of action although they are currently at pre-clinical stage of development.
Competition related to our product pipeline
Lyme disease
We are aware of companies developing mRNA vaccines such as Moderna, therapeutic antibiotic drug candidates such as Ixodes, or antibody-mediated treatment such as Takeda Pharmaceuticals, Inovio Pharmaceuticals, Tarsus, and Euroimmun. However, their programs are in pre-clinical and/or Phase 1/2 clinical stage. Other companies such as GlaxoSmithKline, Sanofi, and Baxter had clinical programs against Lyme disease. LYMErix, from GSK, achieved approval in the U.S. and was later taken out of the market due to lack of market access and potential safety concerns, although it was later proven to be safe by a FDA advisory committee. Sanofi and Baxter were not successful and stopped their programs before requesting a marketing authorization.
Shigellosis
No approved Shigella vaccine is currently available outside of Russia or China, where two vaccines exist for limited (primarily military) use, neither of which are tetravalent. Through our exclusive partnership with LimmaTech Biologics AG, we are currently developing the world’s most clinically advanced tetravalent Shigella vaccine candidate (Phase 2). Our candidate includes the four most prevalent Shigella species/serotypes which we believe is a strong advantage compared to competition. We are aware of a Phase 3 Shigella vaccine candidate developed by Beijing Zhifei Lvzhu but it is a bivalent one. Two other trivalent vaccine candidates developed by Institut Pasteur and GVGH are also in Phase 2. An additional five shigella vaccine candidates, including four oral ones, are in Phase 1.
Sales and Marketing
We have a specialist commercial capability comprising approximately 60 employees for the distribution of our travelers’ vaccines, IXCHIQ, IXIARO and DUKORAL, and third-party vaccines.
We have established our own commercial operations in certain travel vaccine markets including the United States, Canada, the United Kingdom, Sweden, France, Austria, Norway, Denmark, Finland, Belgium, and the Netherlands. We commercialize our own and third-party vaccine brands to both private and government customers, including the U.S. military. In other markets, we have entered into marketing and distribution agreements with companies that specialize in the promotion of travel brands and/or for which there is a strategic fit with their product portfolio.
Examples of such distribution partnerships include Germany (Bavarian Nordic), Eastern Europe (IMED), Israel (Kamada), and Australia and New Zealand (Seqirus/CSL).
Commercial Operations in Key Markets
We manage nearly all of our global product sales revenues through our own commercial operations. Local operations include expertise in Sales, Marketing, Medical Affairs, Governmental Affairs (U.S.), business support functions, and General Management.
Our commercial teams work continuously to improve service and performance, including embracing digital technology, which allows us to better connect with travelers, physicians, and other health care professionals. We put the customer at the heart of our activities and focus on their needs for improved awareness, a deeper understanding of the travel health landscape, and tailor-made services to achieve their objectives.
We have also continued to leverage our commercial organization to distribute third-party products and aim to attract additional products to further leverage our commercial infrastructure. We entered into a partnership with Seqirus in 2016 to commercialize two differentiated flu vaccines in Austria. We also entered into a marketing and distribution partnership with Kamada in 2018 to commercialize their Rabies immunoglobulin in Canada and with Bavarian Nordic in 2020 to commercialize their Rabipur and Encepur brands in Austria, the UK, France, Belgium, the Netherlands, and Canada. In September 2022, we announced a marketing and distribution agreement with VBI Vaccines Inc. to commercialize their Hepatitis B vaccine PreHevbri in the United Kingdom, Sweden, Norway, Denmark, Finland, Belgium, and the Netherlands which ended in 2024.
Manufacturing
Manufacturing of vaccines is considered one of the most complex pharmaceutical manufacturing operations. It can take between six to 36 months to produce, package, and deliver high quality vaccines to those who need them. The process includes testing each batch of vaccine at every step of its journey, and repeat quality control of batches by different authorities around the world.
Our manufacturing base provides a long-term and sustainable industrial network to supply clinical trial material and commercial products based on objectives for delivery schedule, costs, flexibility and quality.
We operate three manufacturing sites augmented by contract manufacturing partners. Our manufacturing network has been operating and producing licensed vaccines for more than ten years. We have a highly experienced management team and workforce operating our production network. We have the expertise and capability to produce most types of viral or bacterial vaccines.
Livingston (Edinburgh), Scotland, UK
We own two neighboring facilities in Livingston, Scotland. that operate under a Manufacturers License from MHRA. One of these facilities is fully operational, with a size of 3,547 square meters. The second facility was added in August 2020 and comprises approximately 6,500 square meters. The sites are qualified to meet required quality standards of several regulatory bodies including FDA, the European Commission, EMA, TGA and Health Canada. We employ currently around 155 staff on the site. The site is a multi-product, FDA-registered manufacturing site and viral vaccines center of excellence.
The Livingston site operates dedicated bulk production units for IXIARO and a BioSafety Level 3 multi-purpose unit used for IXCHIQ clinical supply and commercial manufacturing.
In addition, and as part of our COVID-19 vaccine program, the Livingston site was expanded to include two additional production units in a state-of-the-art manufacturing facility. In light of reduced order volume from EU Member States, we stopped manufacturing of our COVID-19 vaccine and took the decision to transfer the production of IXIARO and IXCHIQ to the new manufacturing facility. This transfer is ongoing.
Solna (Stockholm), Sweden
Our Solna facility can operate on a multi-product basis and comprises approximately 11,000 square meters. The site is qualified to meet required standards of several regulatory bodies including the competent Swedish authorities, Health Canada, and TGA. Our Solna site has a heritage and history from more than 100 years in vaccines operations. It is currently our center of excellence for fill-finish operations. As part of our COVID-19 activities, we expanded our fill-finish capacity by fitting out a nearby site for formulation, filling, and packaging and have now transferred our manufacturing activities to this new site. With around 130 employees, the site operates as a dedicated and integrated production unit for DUKORAL. In 2023, as part of a review of our global R&D strategy, we took the decision to divest our Clinical Trial Manufacturing (CTM) unit to NorthX Biologics. Our Solna site is operated on a long-term lease under a Manufacturers License from MPA.
Vienna, Austria
Our facility in Vienna includes a dedicated Quality unit for Quality control (in vitro and in vivo) and Quality Assurance. This unit covers both proprietary and third party products. As such, this facility is registered with the FDA and operated under respective licenses from the Austrian Agency for Health and Food Safety. In Vienna, where we have centralized our product development capabilities we also have a GMP technical development unit that establishes our new vaccines prior to the final industrialization stage.
The management of all contract manufacturing partners is managed by a dedicated external manufacturing unit based in Vienna.
Intellectual Property
Our commercial success depends in part on obtaining and maintaining patent, trade secret, and other intellectual property and proprietary protection of our technology, current and future products, and product candidates and methods used to develop and manufacture them. We cannot be sure that patents will be granted with respect to any of the pending patent applications or to any patent applications that we file in the future, nor can we be sure that any of our existing patents or any patents that may be granted to us in the future will be sufficient to protect our technology or will not be challenged, invalidated. or circumvented. Our success also depends on our ability to operate our business without infringing, misappropriating, or otherwise violating any patents and other intellectual property or proprietary rights of third parties.
We manage our intellectual property by:
•seeking protection for our products, technologies, and processes by actively using the patent, trademark, copyright, and trade secrets systems in Europe, the United States, Canada, Brazil, India, and other jurisdictions where we might have business interests;
•defending, and if needed, enforcing our property rights in selected jurisdictions; and
•identifying, reviewing and monitoring third party patent rights and challenging and invalidating such rights where applicable, in order to establish and ensure the unrestricted use and operation of our products, product candidates, and technologies, in those jurisdictions where we have business interests.
Patents and patent applications
We consider protecting technologies and products through patents and patent applications essential to the success of our businesses.
As of December 31, 2024, we had a portfolio of 448 issued patents, including 83 granted with effect in Germany, France, the United Kingdom, Spain, and Italy, 50 issued in the United States, and 158 pending patent applications, including 24 pending in Europe and four pending international, or PCT, patent applications.
In countries where we seek legal protection through patents, the duration of legal protection for a particular product, method, or use is generally 20 years from the filing date. This protection may be extended in some countries, particularly in the European Union, China, Japan, South Korea, Australia, Canada, and the United States. The protection, which may also vary by country, depends on the type of patent and its scope. In most industrialized countries, any new active substance, formulation, indication, or manufacturing process may be legally protected. We conduct ongoing checks to protect our inventions and to act against any infringement of our patents.
IXIARO
In regards to our Japanese encephalitis vaccine, IXIARO, as of December 31, 2024, we own a patent family that includes five issued U.S. patents (9,884,115; 9,895,437; 9,913,898; 10,668,146; and 11,110,170) with claims covering the aqueous composition of IXIARO and methods for preparing IXIARO, and one pending U.S. patent application. This patent family also includes two granted European patents with claims directed to compositions comprising IXIARO and/or methods for preparing IXIARO, and one pending European patent application. One of the granted European patents directed to a method for preparing an aqueous composition comprising aluminium, a reactive compound and a protein, was opposed at the EPO in June 2023. A second of the granted European patents directed to an aqueous composition comprising aluminium, a reactive compound and a protein, was opposed at the EPO on January 2025. Patent applications, if issued, and patents in this family are expected to expire in 2032, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a pending U.S. and a European patent application with claims covering the manufacturing processes of IXIARO and potentially other vaccines. Patent applications, if issued, are expected to expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Valneva also owns a pending U.S., Canadian, and European patent application with claims covering a particular use of the IXIARO formulation and potentially other vaccines. Patent application, if issued, are expected to expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
DUKORAL
In regards to our DUKORAL product, as of December 31, 2024, we own a pending U.S., Canadian, and European patent application with claims directed to stable pharmaceutical compositions covering a currently non-commercialized formulation of DUKORAL and methods of use thereof, and patent applications or applications related to these applications, if issued, are expected to expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Patents covering the composition of matter of DUKORAL are expired.
VLA15—Borrelia vaccine candidate
In regards to our Borrelia vaccine candidate VLA15 which is currently licensed to Pfizer, as of December 31, 2024, we own a patent family which includes six issued U.S. patents, two pending U.S. patent applications and two European patents that are validated, one in 38 of the European Patent Convention member states and the other in 12 of those member states, as well as 26 foreign patents and two patent applications with claims covering the composition of matter of VLA15. We further own a second patent family which includes three issued U.S. patents and one granted European patent as well as 16 foreign patents and six patent applications with claims covering the composition of matter of VLA15. Patent applications, if issued, and patents in these families are expected to expire in 2033 and 2035, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a patent family with claims directed to immunogenic polypeptides with C-terminus domains of OspA to induce a protective immune response that includes a U.S. and a European patent validated as a Unitary patent, UK patent, and Spanish patent and patent applications pending in the U.S., Canada, and Hong Kong. Patent applications, if issued, in this family are expected to expire in 2038, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
As of December 31, 2024, we also own two patent families with claims directed to compositions comprising OspA fusion proteins including uses thereof and to improved methods for producing a vaccine. Both families have been nationalized in Europe, the U.S., and Canada in 2022. Patent applications claiming priority to these patent applications, if issued, are expected to expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees. We further co-own with a third party a patent family which includes pending patent applications in Europe, the U.S., and 13 further foreign jurisdictions. Patent applications claiming priority to these patent applications, if issued, are expected to expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a European patent directed to an aqueous composition comprising aluminium, a reactive compound and a protein. This case has been opposed at the EPO on January 2025.
IXCHIQ
In regards to our IXCHIQ product, as of December 31, 2024, we own two patent families that include four granted U.S. patents and two granted European patents with claims covering methods of preparing and methods of purifying VLA1553 and two pending European and one U.S. patent applications. Patent applications, if issued, and patents in this family are expected to expire in 2036, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a patent family with claims directed to pharmaceutical compositions of VLA1553 that includes three U.S. patents, two New Zealand patents, Brazilian, Chinese, Japanese, Mexican and Taiwanese patents and over 20 pending patent applications in such jurisdictions as the U.S., Europe, Australia, Brazil, Canada, China, India, Japan, and Mexico. Patent applications, if issued, and patents in this family are expected to expire in 2038, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
As of December 31, 2024, we also own two patent families with claims covering formulations, secondary uses, and manufacturing processes of VLA1553. Each of these two families were nationalized in 17 jurisdictions and all are still pending except in South Africa. Patent applications, if issued, are expected to expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own three patent families with claims directed to the administration of IXCHIQ in immunocompromised subjects, and two directed to particular formulations and combination uses. As of December 31, 2024, these families are either in the priority year or recently filed as an international patent application but none of the three families are published yet. Patent applications if issued, are expected to expire in 2044 or 2045, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
VLA2401-Shigella vaccine candidate
In regards to our Shigella vaccine VLA2401, as of December 31, 2024, we have in-licensed twelve patent families of which most are related to a bioconjugation technology using mutated PglB oligosaccharyltransferases and its uses to produce vaccine candidates including vaccine candidate VLA2401. The latest of these patents, which relates to an immunogenic composition of VLA2401, if maintained, is expected to expire in 2041, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
VLA84—Clostridium difficile candidate
In regards to our C. difficile candidate VLA84, as of December 31, 2024, we own a patent family with five granted U.S. patents with claims covering the composition of matter of VLA84 and methods of use thereof, one pending U.S. patent application, and 12 granted foreign patents in such jurisdictions as Australia, China, and Japan. This patent family also includes a granted European patent validated in over 35 countries that has been opposed now has been maintained by the European Patent Office in amended form, which still covers VLA84. A second European patent has not been opposed and a third European patent application is pending. Patent applications, if issued, and patents in this family are expected to expire in 2031, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also filed an opposition against two European patents owned by a third party that has claims that might cover our C. difficile vaccine candidate. The European Patent Office revoked both of these in opposition proceedings and after the patentee withdrew both appeals, they were canceled without substantive decisions.
We filed also a further opposition against an European patent that has claims that might cover VLA84 in July of 2024. The European patent office has revoked the patent in the meantime.
VLA1601—Zika vaccine candidate
In regards to our Zika vaccine candidate VLA1601, as of December 31, 2024, we own a patent family that includes five issued U.S. patents with claims covering the aqueous composition of VLA1601 and methods for preparing IXIARO, and one pending U.S. patent application. This patent family also includes two granted European patents with claims directed to compositions comprising VLA1601 and/or methods for preparing VLA1601 and one pending European patent application. One of the granted European patents, directed to a method for preparing an aqueous composition comprising aluminium, a reactive compound and a protein, was opposed at the EPO in June 2023. A second of the granted European patents directed to an aqueous composition aluminium, a reactive compound and a protein was opposed at the EPO on January 2025. Patent applications, if issued, and patents in this family are expected to expire in 2032, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
Furthermore, we own a patent family with five granted U.S. patents with claims covering the formulation VLA1601, one pending U.S. patent applications, one granted European patent validated in thirty countries, one pending European patent application, seven further foreign patents, and ten further pending foreign patent applications. The granted European patent directed to a Zika virus vaccine was opposed at the EPO in November 2023. Patent applications, if issued, and patents in this family are expected to expire in 2036, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees. A third party has filed an Inter Partes Review Proceeding against one of the U.S. patents, for which the U.S. Patent Trial and Appeal Board has now issued a decision denying Institution after we withdrew some of the claims.
We also own two patent families that include three granted U.S. patents with claims covering methods of preparing and methods of purifying VLA1601, one pending U.S. patent application and two granted and two pending European patent applications. Patent applications, if issued, and patents in these families are expected to expire in 2036, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a pending U.S. and a European patent application with claims covering the manufacturing processes of VLA1601 and potentially other vaccines. Patent applications, if issued, are expected to expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also own a patent family with claims directed to large scale manufacturing processes of VLA1601. As of December 31, 2024, this family is still in the international patent application phase. Patent applications if issued, are expected to expire in 2044, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity, or other governmental fees.
We also won a patent family with claims directed to a second generation Zika vaccine including VLA1601. As of December 31, 2024, this family is still in the international patent application phase. Patent applications if issued, are expected to expire in 2044, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
Other protection mechanisms
Our core technologies and products and many of our projects for the development of products candidates depend upon the knowledge, experience, and skills of our scientific and technical personnel. In order to protect our trade secrets, proprietary know-how, and technologies, we generally require all employees, contractors, advisors, and collaborators to enter into confidentiality agreements. These agreements prohibit the disclosure of our confidential information. Agreements with employees and consultants also require disclosure and assignment to us of any ideas, developments, discoveries, and inventions. Furthermore, we have a number of identified trade secrets in its manufacturing processes which are particularly marked and handled by Valneva and its sublicensees.
The expiration of a patent for a product may result in significant competition, due to the emergence of biosimilar or similar products, and in a strong reduction of product sales which benefited from patent protection. However, the vaccine field is largely protected from direct substitutions, as regulatory and manufacturing complexity has for now blocked the pathway in developed markets for vaccine biosimilars. However, this is not the case regarding similar products relying on a full or abbreviated regulatory approval process and this situation may also change in the future, thus opening a pathway to biosimilars.
Nevertheless, in many cases, we may still continue to reap commercial benefits from our product manufacturing secrets, even when the patents for such product have expired.
Trademarks
The trademark rights we hold are national, international and European-wide in scope. The rights are generally granted for a period of ten years and are indefinitely renewable, although in some cases, their validity is contingent on the trademark’s continued use. We hold the title to the names of the products used and those associated therewith.
Our trademarks benefit primarily from protection for pharmaceutical products included in Class 5 and for services in Class 42 of the International Classification of Products and Services.
Our company name, key products, technologies, and product candidates, namely VALNEVA, IXIARO, JESPECT, DUKORAL, IXCHIQ and EB66, and the number of trademarks related to these products and our company held by us at December 31, 2024 are shown in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
| Trademarks |
Number of registrations or applications |
|
Valneva®, Valneva logos |
88 |
|
| IXIARO®, IXIARO logo |
137 |
|
| JESPECT® |
19 |
|
| DUKORAL® |
60 |
|
IXCHIQ® |
58 |
|
| EB66® |
11 |
|
CHIK-A-WHAT® |
3 |
|
We also hold registrations for our different entities names, as well as the slogan and logo which constitute our graphic charter. We defend our trademark rights by filling a notice of opposition against applications for identical or similar trademarks, and initiate, if such is the case, legal actions to have our rights recognized.
“VALNEVA” trademark
Valneva SE and the company KRKA, tovarna zdravil, d.d., Novo Mesto signed a co-existence agreement on January 20, 2014, with respect to KRKA’s earlier trademark DALNEVA covering goods of Class 5. We agreed on restricting the specification of goods for the trademark Valneva, by adding the limitation “none of the afore-mentioned goods for the treatment of cardiovascular diseases” to the European Union Trademark (EUTM) application No. 011441268, and to any future applications.
Moreover, we also filed a notice of opposition before the European Union Intellectual Property Office, or EUIPO, against the trademark application VALNECOR (application No. 13.519889) of the company Vetpharma Animal Health S.L., for Class 5, invoking articles 8(1)b and 8(4) of the Regulation (EC) No. 207/2009 on the Community trademark (EUTMR—as amended). On February 19, 2016, the Opposition Division of the EUIPO decided in our favor and upheld the opposition (No. B 2508755) for all the contested goods in Class 5.
A letter of undertakings effective as of July 25, 2016 has been signed by VALNÉVA, a French Simplified Joint Stock company, and Valneva SE, in order to:
•acknowledge our prior rights; and
•record VALNÉVA’s undertaking never to contest or challenge the company name and the trademarks Valneva—registered or filed—for any goods and services.
VALNÉVA further agreed not to use the name VALNÉVA for scientific R&D in the fields of medicine, antibodies and vaccines.
We and Boehringer Ingelheim International GmbH also signed a prior rights agreement on July 28, 2016. In this agreement, we undertake not to use the trademark Valneva as a product name or part of a product name for the identification of specific products, but only to identify the fabricant of the product (“house mark” or “manufacturers brand”). We also undertake to limit the registration of the mark “Valneva” in Class 5 to the “Pharmaceutical products for human and veterinary use, namely vaccines and antibodies and fragments thereof, blood serum, adjuvants for medical or veterinary use”, only if so specifically requested by Boehringer Ingelheim.
We filed a notice of opposition before EUIPO against the trademark application VALNOBI n°17579525 made in Class 5 in the name of Bayer AG. On February 4, 2019, the Opposition Division of the EUIPO decided in our favor and upheld the opposition (No. B 3 047 941) for all the contested goods in Class 5.
We filed notices of opposition against the EU trademark application VALENA no. 017895207 and the Austrian trademark application VALENA no. 295810. The Austrian trademark application was withdrawn and the EU trademark application was rejected to a large part of the contested goods and services, and in particular to all of the goods in class 5.
“IXIARO” trademark
On October 30, 2015, Valneva Austria GmbH acquired from GSK (GlaxoSmithKline Biologics SA, GlaxoSmithKline GmbH and CO.KG) the trademark “IXIARO” and the related trademarks and domain names, for all jurisdictions. No co-existence or prior rights agreements exist for the trademark IXIARO.
OxARO v IXIARO
We filed an Opposition in 2021 and signed a prior rights agreement with the result that SafeRx withdrew the application OxARO in the U.S. The Settlement Agreement was signed on January 26, 2022. According to the Settlement Agreement SafeRx undertakes to refrain from asserting rights deriving from U.S. Application Serial No. 90/233,007 or use of the trademark OXARO for pharmaceutical preparations and agrees to expressly abandon U.S. Application Serial No. 90/233,007. SafeRx agrees never to use OXARO by itself on a product distributed in the marketplace and will instead use “OxARO ER” and “OxARO IR”. SafeRx may use OXARO solely for fundraising for product development and FDA review, but once through FDA review, SafeRx agrees never to use the mark OXARO by itself, but instead will use the marks “OxARO ER” and “OxARO IR”.
“DUKORAL” trademark
Various prior rights agreements related to the trademark “DUKORAL” were executed in the years 1996 to 2002. A further prior rights and delimitation agreement between Crucell Sweden AB, now Valneva Sweden AB, and Berlin-Chemie AG was signed on June 29, 2012. For mutual settlement of the opposition filed by then Crucell Sweden AB, Berlin Chemie AG undertakes not to derive any rights from the registration and use of their German trademark DUCORA against the Community Trademark registration of DUKORAL, and to tolerate new applications and modifications of the prior DUKORAL trademark, provided that Crucell Sweden AB shall not apply for the trademark DUCORA. Berlin-Chemie AG restricted the goods and services of their German registration of DUCORA. Crucell then agreed to the registration or use of German trademark DUCORAL under the conditions specified and to withdraw the opposition. Since this agreement is effective worldwide, the party who possesses prior rights in any country agrees to consent to the registration or use of the other party’s respective mark under the same conditions as mentioned in this agreement.
Domain names
As at December 31, 2024, we hold 199 domain names (reserved or in the process of being reserved).
Government Regulation
Government authorities in the United States at the federal, state, and local level and in other countries and jurisdictions including the European Union, or EU, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, and export and import of biological products, such as our products, product candidates, and any future product candidates we develop. We, along with our third-party contractors, will be required to navigate the various pre-clinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies, seek approval or licensure of our product candidates, and distribute and market our products, if approved. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local, and foreign statutes and regulations requires the expenditure of substantial time and financial resources.
Regulatory Approval in the United States
In the United States, biological products are subject to regulation under the Federal Food, Drug, and Cosmetic Act, or FDCA, the Public Health Service Act, or PHSA, and other federal, state, local, and foreign statutes and regulations. The process required by the FDA before biologic product candidates may be marketed in the United States generally involves the following:
•completion of extensive pre-clinical laboratory and animal studies in accordance with applicable regulations, including studies conducted in accordance with the FDA’s Good Laboratory Practice, or GLP, requirements;
•submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin;
•approval by an institutional review board, or IRB, or independent ethics committee at each clinical trial site before each clinical trial may be commenced;
•performance of adequate and well-controlled human clinical trials in accordance with applicable IND regulations, Good Clinical Practice, or GCP, requirements and other clinical trial-related regulations to establish the safety, purity, and potency of the product candidate for each proposed indication;
•preparation and submission to the FDA of a biologics license application, or BLA, after completion of all clinical trials;
•payment of any user fees for FDA review of the BLA;
•a determination by the FDA within 60 days of its receipt of a BLA to accept the application for review;
•satisfactory completion of an FDA Advisory Committee review, if applicable;
•satisfactory completion of one or more FDA pre-approval inspections of the manufacturing facility or facilities where the biologic, or components thereof, will be produced to assess compliance with current Good Manufacturing Practice, or cGMP requirements to assure that the facilities, methods, and controls are adequate to preserve the biologic’s identity, strength, quality and purity;
•satisfactory completion of any potential FDA audits of the clinical trial sites that generated the data in support of the BLA to assure compliance with GCPs and integrity of the clinical data; and
•FDA review and approval of the BLA, to permit commercial marketing of the product for particular indications for use in the United States.
Pre-clinical Studies
Before testing any biological product candidates in humans, the product candidate must undergo rigorous pre-clinical testing. Pre-clinical studies include laboratory evaluation of product chemistry and formulation, as well as in vitro and animal studies to assess the potential for adverse events and in some cases to establish a rationale for therapeutic use. The conduct of pre-clinical studies is subject to federal regulations and requirements, including GLP regulations for safety/toxicology studies. An IND sponsor must submit the results of the pre-clinical tests, together with manufacturing information, analytical data, any available clinical data or literature, and plans for clinical studies, among other things, to the FDA as part of an IND. An IND is a request for authorization from the FDA to administer an investigational product to humans and must become effective before human clinical trials may begin. Some long-term pre-clinical testing may continue after the IND is submitted. An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to one or more proposed clinical trials and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. As a result, submission of an IND may not result in the FDA allowing clinical trials to commence.
Clinical Trials
The clinical stage of development involves the administration of the investigational product to healthy volunteers or patients under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with GCPs, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated in the trial. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. Furthermore, each clinical trial must be reviewed and approved by an IRB for each institution at which the clinical trial will be conducted to ensure that the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative and must monitor the clinical trial until completed.
There also are requirements governing the reporting of ongoing clinical trials and completed clinical trial results to public registries. Information about certain clinical trials, including clinical trial results, must be submitted within specific timeframes for publication on the www.clinicaltrials.gov website. Information related to the product candidate, patient population, phase of investigation, clinical trial sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Disclosure of the results of these clinical trials can be delayed in certain circumstances.
A sponsor who wishes to conduct a clinical trial outside of the United States may, but need not, obtain FDA authorization to conduct the clinical trial under an IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of a BLA. The FDA will accept a well-designed and well-conducted foreign clinical trial not conducted under an IND if the clinical trial was conducted in accordance with GCP requirements, and the FDA is able to validate the data through an onsite inspection if deemed necessary.
For purposes of BLA submission and approval, clinical trials are generally conducted in three sequential phases, known as Phase 1, Phase 2, and Phase 3, which may overlap or be combined:
•Phase 1 clinical trials generally involve a small number of healthy volunteers or disease-affected patients who are initially exposed to a single dose and then multiple doses of the product candidate. The primary purpose of these clinical trials is to assess the safety, dosage tolerance, absorption, metabolism, and distribution of the product candidate in humans, the side effects associated with increasing doses, and, if possible, early evidence of effectiveness.
•Phase 2 clinical trials generally involve studies conducted in a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials.
•Phase 3 clinical trials generally involve a large number of patients at multiple sites and are designed to provide statistically significant evidence of clinical efficacy of the product for its intended use, further evaluate its safety, and to establish the overall benefit/risk relationship of the product and provide an adequate basis for product approval. In most cases, the FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the biologic.
Phase 1, Phase 2, Phase 3, and other types of clinical trials may not be completed successfully within any specified period, if at all. The FDA, the IRB, or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including non-compliance with regulatory requirements or a finding that the patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the biologic has been associated with unexpected serious harm to patients. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether a trial may move forward at designated checkpoints based on access to certain data from the trial.
Concurrent with clinical trials, companies usually complete additional animal studies and also must develop additional information about the chemistry and physical characteristics of the biologic as well as finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product and, among other things, companies must develop methods for testing the identity, strength, quality, potency, and purity of the final product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biologic does not undergo unacceptable deterioration over its shelf life.
FDA Review Processes
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies, and clinical trials are submitted to the FDA as part of a BLA requesting approval to market the product for one or more indications. The BLA must include all relevant data available from pre-clinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. Data can come from company-sponsored clinical studies intended to test the safety and effectiveness of a use of the product, or from a number of alternative sources, including studies initiated by independent investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety, purity, and potency of the investigational product to the satisfaction of the FDA. FDA approval of a BLA must be obtained before a biologic may be marketed in the United States.
The FDA reviews a submitted BLA to determine if it is substantially complete before the FDA accepts it for filing and may request additional information from the sponsor. The FDA will make a decision on accepting a BLA for filing within 60 days of receipt, and may refuse to file any BLA that it deems incomplete or not properly reviewable at the time of submission. In this event, the BLA must be resubmitted with any additional information requested in order to be reviewed by FDA. Once the submission is accepted for filing, the FDA begins an in-depth review of the BLA. The FDA reviews a BLA to determine, among other things, whether a product is safe, pure, and potent and whether the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety, purity, and potency. Under the goals agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA targets 10 months from the filing date in which to complete its initial review of an original BLA and respond to the applicant, and six months from the filing date of an original BLA designated for priority review. The review process for both standard and priority review may be extended by the FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission. The FDA does not always meet its PDUFA goal dates for standard and priority BLAs, and the review process can be extended by FDA requests for additional information or clarification.
The cost of preparing and submitting a BLA is substantial. Under PDUFA, each BLA must be accompanied by a substantial user fee. The FDA adjusts the PDUFA user fees on an annual basis. Fee waivers or reductions are available in certain circumstances, including a waiver of the application fee for the first application filed by a small business. Additionally, no user fees are assessed on BLAs for products designated as orphan drugs, unless the product also includes a non-orphan indication. The applicant under an approved BLA is also subject to an annual program fee.
Before approving a BLA, the FDA will typically conduct a pre-approval inspection of the manufacturing facilities for the new product to determine whether such facilities comply with cGMP requirements. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
The FDA also may audit data from clinical trials to ensure compliance with GCP requirements and the integrity of the data supporting safety, purity, and potency of the product candidate. Additionally, the FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation, and a recommendation as to whether the application should be approved and under what conditions, if any. The FDA is not bound by recommendations of an advisory committee, but it generally considers such recommendations carefully when making decisions on approval.
After the FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product is produced, it will issue either an approval letter or a Complete Response Letter, or CRL. A CRL or deferred action on the application may also occur where FDA is unable to complete required pre-approval inspections due to travel restrictions. An approval letter authorizes commercial marketing of the biologic with specific prescribing information for specific indications. A CRL indicates that the review cycle of the application is complete and the application will not be approved in its present form. A CRL generally outlines the deficiencies in the BLA and may require additional clinical data, additional pivotal clinical trial(s), and/or other significant and time-consuming requirements related to clinical trials, pre-clinical studies, or manufacturing in order for FDA to reconsider the application. If a CRL is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, withdraw the application, or request an opportunity for a hearing.
The FDA has committed to reviewing such resubmissions in two or six months from receipt, depending on the type of information included. Even if data and information are submitted in response to the deficiencies identified in a CRL, the FDA may decide that the BLA does not satisfy the criteria for approval.
If regulatory approval of a product is granted, such approval will be granted for particular indications and may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may require a REMS to help ensure that the benefits of the biologic outweigh the potential risks to patients. A REMS is a safety strategy implemented to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use. A REMS can include medication guides, communication plans for healthcare professionals, and elements to assure a product’s safe use, or ETASU. An ETASU can include, but is not limited to, special training or certification for prescribing or dispensing the product, dispensing the product only under certain circumstances, special monitoring, and the use of patient-specific registries. The requirement for a REMS can materially affect the potential market and profitability of the product. FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States but for which there is no reasonable expectation that the cost of developing and making the product for this type of disease or condition will be recovered from sales of the product in the United States.
Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
Among the benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA application user fee. In addition, if a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same product for the same indication for seven years from the date of such approval, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity by means of greater effectiveness, greater safety, or providing a major contribution to patient care, or in instances of drug supply issues. Competitors, however, may receive approval of either a different product for the same indication or the same product for a different indication. In the latter case, because healthcare professionals are free to prescribe products for off-label uses, the competitor’s product could be used for the orphan indication despite another product’s orphan exclusivity.
A designated orphan drug many not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or, as noted above, if a second applicant demonstrates that its product is clinically superior to the approved product with orphan exclusivity or the manufacturer of the approved product is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. For example, Fast Track designation may be granted for products that are intended to treat a serious or life-threatening disease or condition for which there is no effective treatment and where pre-clinical or clinical data demonstrate the potential to address unmet medical needs for the disease condition. Fast Track designation applies to a combination of the product and the specific indication for which it is being studied. The sponsor of a biological product candidate can request the FDA to designate the candidate for a specific indication for Fast Track status concurrent with, or after, the submission of the IND for the candidate. The FDA must determine if the biologic candidate qualifies for Fast Track designation within 60 days of receipt of the sponsor’s request. The sponsor of a Fast Track product has opportunities for more frequent interactions with the applicable FDA review team during product development and, once a BLA is submitted, the product candidate may be eligible for priority review. A Fast Track product may also be eligible for rolling review, where the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the BLA, the FDA agrees to accept sections of the BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the BLA. Any product submitted to the FDA for marketing, including under a Fast Track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval.
Breakthrough therapy designation may be granted for products that are intended, alone or in combination with one or more other products, to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over currently approved therapies on one or more clinically significant endpoints.
Under the breakthrough therapy program, the sponsor of a new biologic candidate may request that the FDA designate the candidate for a specific indication as a breakthrough therapy concurrent with, or after, the submission of the IND for the biologic candidate. The FDA must determine if the biological product qualifies for breakthrough therapy designation within 60 days of receipt of the sponsor’s request. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process, providing timely advice to the product sponsor regarding development and approval, involving more senior staff in the review process, assigning a cross-disciplinary project lead for the review team and taking other steps to design the clinical studies in an efficient manner. The designation also includes all of the Fast Track program features, including eligibility for rolling review of BLA submissions if the relevant criteria are met.
Priority review may be granted for products that are intended to treat a serious or life-threatening condition and, if approved, would provide a significant improvement in safety and effectiveness compared to available therapies. The FDA will attempt to direct additional resources to the evaluation of an application designated for priority review in an effort to facilitate the review. For original BLAs, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date (as compared to ten months under standard review).
Accelerated approval may be granted for products that are intended to treat a serious or life-threatening condition and that generally provide a meaningful therapeutic advantage to patients over existing treatments. A product eligible for accelerated approval may be approved on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. The accelerated approval pathway is most often used in settings in which the course of a disease is long, and an extended period of time is required to measure the intended clinical benefit of a product, even if the effect on the surrogate or intermediate clinical endpoint occurs rapidly. The accelerated approval pathway is contingent on a sponsor’s agreement to conduct additional post-approval confirmatory studies to verify the product’s clinical benefit in relationship to the surrogate endpoint. These confirmatory trials must be completed with due diligence and, in some cases, the FDA may require that the trial be designed, initiated, and/or fully enrolled prior to approval. Failure to conduct required post-approval studies, or to confirm a clinical benefit during post-marketing studies, would allow the FDA to withdraw the product from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or the time period for FDA review or approval may not be shortened. Furthermore, Fast Track designation, breakthrough therapy designation, priority review, and accelerated approval do not change the standards for approval, but may expedite the development or approval process.
Additional Controls for Biologics
To help reduce the increased risk of the unintentional introduction of other microorganisms, the PHSA emphasizes the importance of manufacturing controls for products whose attributes cannot be precisely defined. The PHSA also provides authority to the FDA to immediately suspend licenses in situations where there exists a danger to public health, to prepare or procure products in the event of shortages and critical public health needs, and to authorize the creation and enforcement of regulations to prevent the introduction or spread of communicable diseases in the United States and between states.
After a BLA is approved, the product may also be subject to official lot release as a condition of approval. As part of the manufacturing process, the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official release by the FDA, the manufacturer submits samples of each lot of product to the FDA together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products, such as viral vaccines, before releasing the lots for distribution by the manufacturer. In addition, the FDA conducts laboratory research related to the regulatory standards on the safety, purity, potency, and effectiveness of biological products. As with drugs, after approval of biologics, manufacturers must address any safety issues that arise, are subject to recalls or a halt in manufacturing, and are subject to periodic inspection after approval.
Pediatric Information
Under the Pediatric Research Equity Act, or PREA, BLAs or supplements to BLAs must contain data to assess the safety and effectiveness of the biological product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the biological product is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA generally does not apply to any biological product for an indication for which orphan designation has been granted.
The Best Pharmaceuticals for Children Act, or BPCA, provides a six-month extension of any exclusivity—patent or non-patent—for a biologic if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new biologic in the pediatric population may produce health benefits in that population, FDA making a written request for pediatric studies, and the applicant agreeing to perform, completing, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as applications, with all of the benefits that designation confers.
Post-Approval Requirements
Any products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims, are subject to prior FDA review and approval. Once a BLA is approved, a product will be subject to certain additional post-approval requirements.
The FDA also may require post-marketing testing, known as Phase 4 testing, impose a REMS and/or post-market surveillance to monitor the effects of an approved product, or place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, biological product manufacture, packaging, and labeling procedures must continue to conform to cGMPs after approval. Biologics manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies. Manufacturers are subject to periodic unannounced inspections by the FDA, including those focused on manufacturing facilities to assess compliance with cGMPs. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs.
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information, imposition of post-market studies or clinical studies to assess new safety risks or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
•restrictions on the marketing or manufacturing of the product, suspension of the approval, complete withdrawal of the product from the market or product recalls;
•fines, warnings, or other enforcement-related letters or holds on post-approval clinical studies;
•refusal of the FDA to approve pending BLAs or supplements to approved BLAs, or suspension or revocation of product license approvals;
•product seizure or detention, or refusal to permit the import or export of products;
•consent decrees, corporate integrity agreements, debarment, or exclusion from federal healthcare programs;
•mandated modification of promotional materials and labeling and the issuance of corrective information;
•the issuance of safety alerts, Dear Healthcare Provider letters, press releases, and other communications containing warnings or other safety information about the product; or
•injunctions or the imposition of civil or criminal penalties.
The FDA closely regulates the marketing, labeling, advertising, and promotion of biologics. A company can make only those claims relating to safety and efficacy, purity, and potency that are consistent with the provisions of the FDA-approved label. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, issuance of warning or untitled letters, requirements to issue corrective advertising, and potential civil and criminal penalties. Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict the manufacturer’s communications on the subject of off-label use of their products, as well as actions taken on behalf of the manufacturer, such as sponsored scientific and educational activities conducted by a third party.
Biosimilars and Reference Product Exclusivity
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, created an abbreviated approval pathway for biological products shown to be biosimilar to, or interchangeable with, an FDA-licensed reference biological product. Biosimilarity, which requires that the biological product be highly similar to the reference product notwithstanding minor differences in clinically inactive components and that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical trial or trials. Interchangeability requires that a biological product be biosimilar to the reference product and that the product can be expected to produce the same clinical results as the reference product in any given patient and, for products administered multiple times to an individual, that the product and the reference product may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biological product without such alternation or switch.
Under the BPCIA an application for a biosimilar or interchangeable product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date of first licensure of the reference product. “First licensure” typically means the initial date the particular product at issue was licensed in the United States. Date of first licensure does not include the date of licensure of (and a new period of exclusivity is not available for) a biological product if the licensure is for a supplement for the biological product or for a subsequent application by the same sponsor or manufacturer of the biological product (or licensor, predecessor in interest, or other related entity) for a change (not including a modification to the structure of the biological product) that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device, or strength, or for a modification to the structure of the biological product that does not result in a change in safety, purity, or potency.
During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing that applicant’s own pre-clinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity, and potency of its product.
The BPCIA is complex and continues to be interpreted and implemented by the FDA. In addition, government proposals have sought to reduce the 12-year reference product exclusivity period.
Regulatory Approval in the EU
In order to market any product outside of the United States, a company also must comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety, and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales, and distribution of products. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable foreign regulatory authorities before it can initiate clinical trials or market product in those countries or jurisdictions. Specifically, the process governing approval of medicinal products in the EU generally follows the same lines as in the United States. It entails satisfactory completion of pharmaceutical development, nonclinical studies, and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. It also requires the submission to relevant competent authorities for clinical trials authorization and to the EMA or to competent authorities in EU Member States for a marketing authorization application, or MAA, and granting of a marketing authorization, or MA, by competent authorities in EU Member States or the European Commission before the product can be marketed and sold in the EU.
Clinical Trial Approval
In the EU, clinical trials are governed by the Clinical Trials Regulation (EU) No 536/2014, or CTR, which entered into application on January 31, 2022, repealing and replacing the former Clinical Trials Directive 2001/20, or CTD.
The CTR is intended to harmonize and streamline clinical trial authorizations, simplify adverse-event reporting procedures, improve the supervision of clinical trials, and increase transparency. Specifically, the Regulation, which is directly applicable in all EU Member States, introduces a streamlined application procedure through a single-entry point, the “EU portal”, the Clinical Trials Information System, or CTIS; a single set of documents to be prepared and submitted for the application; as well as simplified reporting procedures for clinical trial sponsors. A harmonized procedure for the assessment of applications for clinical trials has been introduced and is divided into two parts. Part I assessment is led by the competent authorities of a reference Member State selected by the trial sponsor and relates to clinical trial aspects that are considered to be scientifically harmonized across EU Member States. This assessment is then submitted to the competent authorities of all concerned Member States in which the trial is to be conducted for their review. Part II is assessed separately by the competent authorities and Ethics Committees in each concerned EU Member State concerned. Individual EU Member States retain the power to authorize the conduct of clinical trials on their territory.
The CTR foresaw a three-year transition period that ended on January 31, 2025. Since this date, all new or ongoing trials are subject to the provisions of the CTR.
In all cases, clinical trials must be conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki. Medicines used in clinical trials must be manufactured in accordance with the guidelines on cGMP and in a GMP licensed facility, which can be subject to GMP inspections.
Orphan Drug Designation and Exclusivity
Regulation (EC) No. 141/2000 as implemented by Regulation (EC) No. 847/2000 provides that a product can be designated as an orphan drug by the European Commission if its sponsor can establish: that the product is intended for the diagnosis, prevention, or treatment of (1) a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the EU when the application is made, or (2) a life-threatening, seriously debilitating, or serious and chronic condition in the EU and that without incentives it is unlikely that the marketing of the drug in the EU would generate sufficient return to justify the necessary investment. For either of these conditions, the applicant must demonstrate that there exists no satisfactory method of diagnosis, prevention, or treatment of the condition in question that has been authorized in the EU or, if such method exists, the drug has to be of significant benefit compared to products available for the condition.
In the EU, an application for designation as an orphan product can be made any time prior to the filing of the MAA. Orphan medicinal product designation entitles an applicant to incentives such as fee reductions or fee waivers, protocol assistance, and access to the centralized MA procedure. Upon grant of an MA, orphan medicinal products are entitled to a ten-year period of market exclusivity for the approved therapeutic indication, which means that the EMA cannot accept another MAA, or grant an MA, or accept an application to extend an MA for a similar product for the same indication for a period of ten years. The period of market exclusivity is extended by two years for orphan medicinal products that have also complied with an agreed Pediatric Investigation Plan, or PIP. No extension to any supplementary protection certificate can be granted on the basis of pediatric studies for orphan indications. Orphan medicinal product designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
The period of market exclusivity may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria on the basis of which it received orphan medicinal product destination, including where it can be demonstrated on the basis of available evidence that the original orphan medicinal product is sufficiently profitable not to justify maintenance of market exclusivity or where the prevalence of the condition has increased above the threshold. Additionally, an MA may be granted to a similar medicinal product with the same orphan indication during the ten-year period if: (i) the MA holder of the authorized product consents to a second original orphan medicinal product application, (ii) the manufacturer of the original orphan medicinal product is unable to supply sufficient quantities; or (iii) the second applicant can establish that its product, although similar, is safer, more effective, or otherwise clinically superior to the authorized orphan medicinal product. A company may voluntarily remove a product from the register of orphan products. A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication.
Pediatric Development
In the EU, Regulation (EC) No 1901/2006 provides that all MAAs for new medicinal products have to include the results of trials conducted in the pediatric population, in compliance with a pediatric investigation plan, or PIP, agreed with the EMA’s Pediatric Committee, or PDCO. The PIP sets out the timing and measures proposed to generate data to support a pediatric indication of the medicinal product for which MA is being sought. The PDCO can grant a deferral of the obligation to implement some or all of the measures provided in the PIP until there are sufficient data to demonstrate the efficacy and safety of the product in adults. Further, the obligation to provide pediatric clinical trial data can be waived by the PDCO when these data are not needed or appropriate because the product is likely to be ineffective or unsafe in children, the disease or condition for which the product is intended occurs only in adult populations, or when the product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. Once the MA is obtained in all EU Member States and study results are included in the product information, even when negative, the product is eligible for a six-month extension to the Supplementary Protection Certificate, or SPC, if any is in effect at the time of authorization or, in the case of orphan medicinal products, a two-year extension of orphan market exclusivity.
Marketing Authorization
To obtain a marketing authorization for a product in the EU, an applicant must submit a marketing authorization application, or MAA, either under a centralized procedure administered by the European Medicines Agency, or EMA, or one of the procedures administered by competent authorities in the EU Member States (decentralized procedure, national procedure, or mutual recognition procedure). An MA may be granted only to an applicant established in the EU.
The centralized procedure provides for the grant of a single MA by the European Commission that is valid for all EU Member States. Pursuant to Regulation (EC) No 726/2004, the centralized procedure is compulsory for specific products, including for (i) medicinal products derived from biotechnological processes, (ii) products designated as orphan medicinal products, (iii) advanced therapy medicinal products (ATMPs), and (iv) products with a new active substance indicated for the treatment of HIV/AIDS, cancer, neurodegenerative diseases, diabetes, auto-immune and other immune dysfunctions, and viral diseases. For products with a new active substance indicated for the treatment of other diseases and products that are highly innovative or for which a centralized process is in the interest of patients authorization through, the centralized procedure is optional on related approval.
Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use (CHMP) is responsible for conducting the initial assessment of a product. The CHMP is also responsible for several post-authorization and maintenance activities, such as the assessment of modifications or extensions to an existing MA.
Under the centralized procedure in the EU, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions of the CHMP. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product targeting an unmet medical need is expected to be of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts a request for accelerated assessment, the time limit of 210 days will be reduced to 150 days (not including clock stops). The CHMP can, however, revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment.
Unlike the centralized authorization procedure, the decentralized MA procedure requires a separate application to, and leads to separate approval by, the competent authorities of each EU Member State in which the product is to be marketed. This application is identical to the application that would be submitted to the EMA for authorization through the centralized procedure. The reference EU Member State prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. The resulting assessment report is submitted to the concerned EU Member States who, within 90 days of receipt, must decide whether to approve the assessment report and related materials. If a concerned EU Member State cannot approve the assessment report and related materials due to concerns relating to a potential serious risk to public health, disputed elements may be referred to the Heads of Medicines Agencies’ Coordination Group for Mutual Recognition and Decentralised Procedures – Human (CMDh) for review. The subsequent decision of the European Commission is binding on all EU Member States.
The mutual recognition procedure allows companies that have a medicinal product already authorized in one EU Member State to apply for this authorization to be recognized by the competent authorities in other EU Member States. Like the decentralized procedure, the mutual recognition procedure is based on the acceptance by the competent authorities of the EU Member States of the MA of a medicinal product by the competent authorities of other EU Member States. The holder of a national MA may submit an application to the competent authority of an EU Member State requesting that this authority recognize the MA delivered by the competent authority of another EU Member State.
An MA has an initial validity of five years in principle. The MA may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the EU Member State in which the original MA was granted. To support the application, the MA holder must provide the EMA or the competent authority with a consolidated version of the eCTD (Common Technical Document) providing up-to-date data concerning the quality, safety, and efficacy of the product, including all variations introduced since the MA was granted, at least nine months before the MA ceases to be valid. The European Commission or the competent authorities of the EU Member States may decide, on justified grounds relating to pharmacovigilance, to proceed with one further five year renewal period for the MA. Once subsequently definitively renewed, the MA shall be valid for an unlimited period. Any authorization which is not followed by the actual placing of the medicinal product on the EU market (for a centralized MA) or on the market of the authorizing EU Member State within three years after authorization ceases to be valid (the so-called sunset clause).
Innovative products that target an unmet medical need and are expected to be of major public health interest may be eligible for a number of expedited development and review programs, such as the Priority Medicines, or PRIME, scheme, which provides incentives similar to the breakthrough therapy designation in the U.S. PRIME is a voluntary scheme aimed at enhancing the EMA’s support for the development of medicinal products that target unmet medical needs. Eligible products must target conditions for which there is an unmet medical need (there is no satisfactory method of diagnosis, prevention, or treatment in the EU or, if there is, the new medicinal product will bring a major therapeutic advantage) and they must demonstrate the potential to address the unmet medical need by introducing new methods of therapy or improving existing ones. Benefits accrue to sponsors of product candidates with PRIME designation, including but not limited to, early and proactive regulatory dialogue with the EMA, frequent discussions on clinical trial designs and other development program elements, and potentially accelerated MAA assessment once a dossier has been submitted.
In the EU, a “conditional” MA may be granted in cases where all the required safety and efficacy data are not yet available. The European Commission may grant a conditional MA for a medicinal product if it is demonstrated that all of the following criteria are met: (i) the benefit-risk balance of the medicinal product is positive; (ii) it is likely that the applicant will be able to provide comprehensive data post-authorization; (iii) the medicinal product fulfils an unmet medical need; and (iv) the benefit of the immediate availability to patients of the medicinal product is greater than the risk inherent in the fact that additional data are still required. The conditional MA is subject to conditions to be fulfilled for generating the missing data or ensuring increased safety measures. It is valid for one year and must be renewed annually until all related conditions have been fulfilled. Once any pending studies are provided, the conditional MA can be converted into a traditional MA. However, if the conditions are not fulfilled within the timeframe set by the EMA and approved by the European Commission, the MA will cease to be renewed.
An MA may also be granted “under exceptional circumstances” where the applicant can show that it is unable to provide comprehensive data on efficacy and safety under normal conditions of use even after the product has been authorized and subject to specific procedures being introduced. These circumstances may arise in particular when the intended indications are very rare and, in the state of scientific knowledge at that time, it is not possible to provide comprehensive information, or when generating data may be contrary to generally accepted ethical principles. Like a conditional MA, an MA granted in exceptional circumstances is reserved to medicinal products intended to be authorized for treatment of rare diseases or unmet medical needs for which the applicant does not hold a complete data set that is required for the grant of a standard MA. However, unlike the conditional MA, an applicant for authorization in exceptional circumstances is not subsequently required to provide the missing data. Although the MA “under exceptional circumstances” is granted definitively, the risk-benefit balance of the medicinal product is reviewed annually, and the MA will be withdrawn if the risk-benefit ratio is no longer favorable.
In addition to an MA, various other requirements apply to the manufacturing and placing on the EU market of medicinal products. Manufacture of medicinal products in the EU requires a manufacturing authorization, and import of medicinal products into the EU requires a manufacturing authorization allowing for import. The manufacturing authorization holder must comply with various requirements set out in the applicable EU laws, regulations and guidance. These requirements include compliance with EU GMP standards when manufacturing medicinal products and APIs, including the manufacture of APIs outside of the EU with the intention to import the APIs into the EU. Similarly, the distribution of medicinal products within the EU is subject to compliance with the applicable EU laws, regulations, and guidelines, including the requirement to hold appropriate authorizations for distribution granted by the competent authorities of the EU Member States. MA holders and/or manufacturing and import authorization, or MIA holders and/or distribution authorization holders may be subject to civil, criminal or administrative sanctions, including suspension of manufacturing authorization, in case of non-compliance with the EU or EU Member States’ requirements applicable to the manufacturing of medicinal products.
Data and Market Exclusivity
The EU provides opportunities for data and market exclusivity related to MAs. Upon receiving an MA, innovative medicinal products are generally entitled to receive eight years of data exclusivity and 10 years of market exclusivity. Data exclusivity, if granted, prevents regulatory authorities in the EU from referencing the innovator’s data to assess a generic application or biosimilar application for eight years from the date of authorization of the innovative product, after which a generic or biosimilar MAA can be submitted, and the innovator’s data may be referenced. The market exclusivity period prevents a successful generic or biosimilar applicant from commercializing its product in the EU until ten years have elapsed from the initial MA of the reference product in the EU. The overall ten-year period may, occasionally, be extended for a further year to a maximum of 11 years if, during the first eight years of those ten years, the MA holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
However, there is no guarantee that a product will be considered by the EU’s regulatory authorities to be a new chemical/biological entity, and products may not qualify for data exclusivity.
Regulatory Requirements after Marketing Authorization
Where an MA is granted in relation to a medicinal product in the EU, the holder of the MA is required to comply with a range of regulatory requirements applicable to the manufacturing, marketing, promotion, and sale of medicinal products.
Similar to the United States, both MA holders and manufacturers of medicinal products are subject to comprehensive regulatory oversight by the EMA, the European Commission, and/or the competent regulatory authorities of the individual EU Member States. The holder of an MA must establish and maintain a pharmacovigilance system and appoint an individual qualified person for pharmacovigilance who is responsible for oversight of that system. Key obligations include expedited reporting of suspected serious adverse reactions and submission of periodic safety update reports, or PSURs.
All new MAAs must include a risk management plan, or RMP, describing the risk management system that the company will put in place and documenting measures to prevent or minimize the risks associated with the product. The regulatory authorities may also impose specific obligations as a condition of the MA. Such risk-minimization measures or post-authorization obligations may include additional safety monitoring, more frequent submission of PSURs, or the conduct of additional clinical trials or post-authorization safety studies.
Advertising Regulation
In the EU, the advertising and promotion of medicinal products are subject to both EU and EU Member States’ laws governing promotion of medicinal products, interactions with physicians and other healthcare professionals, misleading and comparative advertising and unfair commercial practices. Although general requirements for advertising and promotion of medicinal products are established under EU directives, the details are governed by regulations in individual EU Member States and can differ from one country to another. For example, applicable laws require that promotional materials and advertising in relation to medicinal products comply with the product’s Summary of Product Characteristics, or SmPC, as approved by the competent authorities in connection with an MA. The SmPC is the document that provides information to physicians concerning the safe and effective use of the product. Promotional activity that does not comply with the SmPC is considered off-label and is prohibited in the EU. Direct-to-consumer advertising of prescription medicinal products is also prohibited in the EU.
Regulatory Approval in the United Kingdom
On January 31, 2020, the United Kingdom, or UK, left the EU (commonly referred to as “Brexit”) and accordingly is no longer an EU Member State. As the UK is no longer an EU Member State, the UK’s participation in the European Medicines Regulatory Network has ceased and the UK Medicines and Healthcare products Regulatory Agency, or MHRA, has assumed the functions that were previously undertaken by the EU institutions for human medicines on the UK market. The UK regulatory framework in relation to clinical trials is derived from existing EU legislation existing as at the date that the UK left the EU (as implemented into UK law, through secondary legislation).
On January 17, 2022, the MHRA launched an eight-week consultation on reframing the UK legislation for clinical trials. The consultation closed on March 14, 2022 and aims to streamline clinical trials approvals, enable innovation, enhance clinical trials transparency, enable greater risk proportionality, and promote patient and public involvement in clinical trials. The UK Government published its response to the consultation on March 21, 2023, confirming that it would bring forward changes to the legislation and such changes were laid before parliament on December 12, 2024. These resulting legislative amendments will, if implemented in their current form, bring the UK into closer alignment with the CTR. In October 2023, the MHRA announced a new Notification Scheme for clinical trials which enables a more streamlined and risk-proportionate approach to initial clinical trial applications for Phase 4 and low-risk Phase 3 clinical trial applications.
Marketing authorizations in the UK are governed by the Human Medicines Regulations (SI 2012/1916), as amended. Since January 1, 2021, an applicant for the EU centralized procedure marketing authorization can no longer be established in the UK. As a result, since this date, companies established in the UK cannot use the EU centralized procedure and instead must follow one of the UK national authorization procedures or one of the remaining post-Brexit international cooperation procedures to obtain a marketing authorization to market products in the UK. All existing EU marketing authorizations for centrally authorized products were automatically converted or grandfathered into UK marketing authorization, effective in Great Britain only, free of charge on January 1, 2021, unless the marketing authorization holder opted-out of this possibility. Northern Ireland remained within the scope of EU authorizations in relation to centrally authorized medicinal products until January 1, 2025. On January 1, 2025, a new arrangement as part of the so-called “Windsor Framework” came into effect and reintegrated Northern Ireland under the regulatory authority of the MHRA with respect to medicinal products. Pursuant to the Windsor Framework holders of Great Britain marketing authorizations converted into UK marketing authorizations on January 1, 2025 unless the entities holding such marketing authorizations also held a Northern Ireland marketing authorization that they failed to cancel before December 31, 2024. The Windsor Framework removes EU licensing processes and EU labeling and serialization requirements in relation to Northern Ireland and introduces a UK-wide licensing process for medicines. Companies must follow one of the United Kingdom’s national authorization procedures or one of the remaining post-Brexit international cooperation procedures to obtain a marketing authorization to market products in the United Kingdom.
The MHRA has also introduced changes to national marketing authorization procedures. This includes introduction of procedures to prioritize access to new medicines that will benefit patients, including a 150-day assessment route, a rolling review procedure, and the International Recognition Procedures which entered into application on January 1, 2024. Since January 1, 2024, the MHRA may rely on the International Recognition Procedure, or IRP, when reviewing certain types of marketing authorization applications. This procedure is available for applicants for marketing authorization who have already received an authorization for the same product from a reference regulator. These include the FDA, the EMA, and national competent authorities of individual EEA countries. A positive opinion from the EMA and CHMP, or a positive end of procedure outcome from the mutual recognition or decentralized procedures, are considered to be authorizations for the purposes of the IRP.
There is no pre-marketing authorization orphan designation for medicinal products in the UK. Instead, the MHRA reviews applications for orphan designation in parallel to the corresponding marketing authorization application. The criteria are essentially the same as those in the EU, but have been tailored for the market. This includes the criterion that prevalence of the condition in the United Kingdom, rather than the EU, must not be more than five in 10,000. Upon the grant of a marketing authorization with orphan status, the medicinal product will benefit from up to 10 years of market exclusivity from similar products in the approved orphan indication. The start of this market exclusivity period will be set from the date of first approval of the product in the United Kingdom.
International Regulation
In addition to regulations in the United States and the EU, a variety of foreign regulations govern clinical trials, commercial sales, and distribution of product candidates. The approval process varies from country to country and the time to approval may be longer or shorter than that required for FDA, European Commission, or EU Member State competent authority approval.
Other Healthcare Laws and Regulations and Legislative Reform in the United States and the EU
U.S. Healthcare Laws and Regulations
Healthcare providers and third-party payors will play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our operations, including any arrangements with healthcare providers, third-party payors, and customers may expose us to broadly applicable fraud and abuse and other healthcare laws that may affect the business or financial arrangements and relationships through which we would market, sell, and distribute our products. Our current and future operations are subject to regulation by various federal, state, and local authorities in addition to the FDA, including but not limited to the Centers for Medicare & Medicaid Services, or CMS, the Department of Health and Human Services, or HHS, (including the Office of Inspector General, Office for Civil Rights and the Health Resources and Services Administration), the U.S. Department of Justice, or DOJ, and individual U.S. Attorney offices within the DOJ, and state and local governments. The healthcare laws that may affect our ability to operate include, but are not limited to:
•The federal Anti-Kickback Statute, which prohibits any person or entity from, among other things, knowingly and willfully soliciting, receiving, offering, or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order, or recommendation of an item or service reimbursable, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs. The term “remuneration” has been broadly interpreted to include anything of value. The federal Anti-Kickback Statute has also been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other hand. There are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, but the exceptions and safe harbors are drawn narrowly and require strict compliance in order to offer protection. Additionally, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•Federal civil and criminal false claims laws, such as the False Claims Act, which can be enforced by private citizens through civil qui tam actions, and civil monetary penalty laws prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, false, fictitious, or fraudulent claims for payment of federal funds, and knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to avoid, decrease, or conceal an obligation to pay money to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes “any request or demand” for money or property presented to the U.S. government. Drug manufacturers can be held liable under the False Claims Act even when they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent claims. For example, pharmaceutical companies have been prosecuted under the False Claims Act in connection with their alleged off-label promotion of drugs, purportedly concealing price concessions in the pricing information submitted to the government for government price reporting purposes, and allegedly providing free product to customers with the expectation that the customers would bill federal healthcare programs for the product. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act;
•The Health Insurance Portability and Accountability Act, or HIPAA, among other things, imposes criminal liability for executing or attempting to execute a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, or willfully obstructing a criminal investigation of a healthcare offense, and creates federal criminal laws that prohibit knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious, or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious, or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items, or services. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their implementing regulations, which impose privacy, security and breach reporting obligations with respect to individually identifiable health information upon entities subject to the law, such as health plans, healthcare clearinghouses, and certain healthcare providers, known as covered entities, and their respective business associates and their covered subcontractors that perform services for them that involve individually identifiable health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in U.S. federal courts to enforce HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions;
•Federal and state consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•The federal transparency requirements under the Physician Payments Sunshine Act, created under the ACA, which requires, among other things, certain manufacturers of drugs, devices, biologics, and medical supplies reimbursed under Medicare, Medicaid, or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to payments and other transfers of value provided to physicians, defined to include doctors, dentists, optometrists, podiatrists and chiropractors, and other healthcare professionals (such as physician assistants and nurse practitioners), and teaching hospitals and physician ownership and investment interests, including such ownership and investment interests held by a physician’s immediate family members;
•Federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs;
•Similar healthcare laws and regulations in other jurisdictions, such as anti-kickback and false claims laws, that may impose similar or more prohibitive restrictions, and may apply to items or services reimbursed by non-governmental third-party payors, including private insurers, and state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information; state and foreign laws that require pharmaceutical companies to implement compliance programs, comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government; state and local laws that require the registration of pharmaceutical sales representatives and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect as HIPAA, thus complicating compliance efforts; and
•State laws that require the reporting of marketing expenditures or drug pricing, including information pertaining to and justifying price increases; state laws that prohibit various marketing-related activities, such as the provision of certain kinds of gifts or meals; state laws that require the posting of information relating to clinical trials and their outcomes.
If our operations are found to be in violation of any of these laws or any other current or future healthcare laws that may apply to us, we may be subject to significant civil, criminal, and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, contractual damages, reputational harm, diminished profits and future earnings, additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, and the curtailment or restructuring of our operations, any of which could substantially disrupt our operations. Although effective compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, if any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to significant criminal, civil, or administrative sanctions, including exclusions from government funded healthcare programs.
U.S. Legislative Reform
We operate in a highly regulated industry, and new laws, regulations, and judicial decisions, or new interpretations of existing laws, regulations, and decisions, related to healthcare availability, the method of delivery and payment for healthcare products and services could negatively affect our business, financial condition, and prospects. There is significant interest in promoting healthcare reforms, and it is likely that federal and state legislatures within the United States and the governments of other countries will continue to consider changes to existing healthcare legislation.
For example, the United States and state governments continue to propose and pass legislation designed to reduce the cost of healthcare. In 2010, the U.S. Congress enacted the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA, which included changes to the coverage and reimbursement of drug products under government healthcare programs.
The ACA, among other things, addressed a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected, increased the minimum Medicaid rebates owe by manufacturers under the Medicaid Drug Rebate Program, extended the rebate program to individuals enrolled in Medicaid managed care organizations and established annual fees and taxes on manufacturers of certain prescription drugs.
There have been executive, judicial, and congressional challenges and amendments to certain aspects of the ACA. For example, on August 16, 2022, the Inflation Reduction Act of 2022, or IRA, was signed into law, which, among other things, extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. The IRA also eliminates the “donut hole” under the Medicare Part D program beginning in 2025 by significantly lowering the beneficiary maximum out-of-pocket cost and creating a new manufacturer discount program. It is unclear how any healthcare reform measures of the second Trump administration will impact the ACA and our business.
In addition, there have been and continue to be a number of initiatives at the United States federal and state levels that seek to reduce healthcare costs. In 2011, the U.S. Congress enacted the Budget Control Act, which included provisions intended to reduce the federal deficit. The Budget Control Act resulted in the imposition of 2% reductions in Medicare payments to providers beginning in 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2032, unless additional Congressional action is taken. Additionally, on March 11, 2021, the American Rescue Plan Act of 2021 was signed into law, which eliminates the statutory Medicaid drug rebate cap, previously set at 100% of a drug’s average manufacturer price, for single source and innovator multiple source drugs, beginning January 1, 2024. If government spending is further reduced, anticipated budgetary shortfalls may also impact the ability of relevant agencies, such as the FDA, to continue to function at current levels, which may impact the ability of relevant agencies to timely review and approve research and development, manufacturing, and marketing activities, which may delay our ability to develop, market, and sell any product candidates we may develop. Moreover, any significant spending reductions affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented, or any significant taxes or fees that may be imposed on us, as part of any broader deficit reduction effort or legislative replacement to the Budget Control Act, could have an adverse impact on our anticipated product revenues.
Furthermore, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several congressional inquiries, Presidential executive orders, and proposed legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products.
For example, the IRA, among other things, (i) directs HHS, to negotiate the price of certain high-expenditure, single-source biologics covered under Medicare that have been on the market for at least 11 years, or the Medicare Drug Price Negotiation Program, and subjects drug manufacturers to civil monetary penalties and a potential excise tax by offering a price that is not equal to or less than the negotiated “maximum fair price” for such drugs and biologics under the law, and (ii) imposes rebates with respect to certain drugs and biologics covered under Medicare Part B or Medicare Part D to penalize price increases that outpace inflation. The IRA permits HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. These provisions began to take effect progressively starting in fiscal year 2023. On August 15, 2024, HHS announced the agreed-upon reimbursement price of the first ten drugs that were subject to price negotiations, although the Medicare Drug Price Negotiation Program is currently subject to legal challenges. On January 17, 2025, HHS selected fifteen additional drugs products covered under Part D for price negotiation in 2025. Each year thereafter more Part B and Part D products will become subject to the Medicare Drug Price Negotiation Program. Further, on December 8, 2023, the National Institute of Standards and Technology published for comment a Draft Interagency Guidance Framework for Considering the Exercise of March-In Rights which for the first time includes the price of a product as one factor an agency can use when deciding to exercise march-in rights. While march-in rights have not previously been exercised, it is uncertain if that will continue under the new framework. Additionally, on July 9, 2021, an executive order was issued directing the FDA to, among other things, continue to clarify and improve the approval framework for biosimilars, including the standards for interchangeability of biological products, facilitate the development and approval of biosimilar and interchangeable products, clarify existing requirements and procedures related to the review and submission of BLAs, and identify and address any efforts to impede biosimilar competition.
Additional health reform measures may continue and affect our business in unknown ways, particularly given the recent change in administration. The current Trump administration is pursuing policies to reduce regulations and expenditures across government including at HHS, the FDA, CMS and related agencies. These actions, presently directed by executive orders or memoranda from the Office of Management and Budget, may propose policy changes that create additional uncertainty for our business. These actions may include, for example, directives to reduce agency workforce, rescinding a Biden administration executive order tasking the Center for Medicare and Medicaid Innovation, or CCMI, to consider new payment and healthcare models to limit drug spending and eliminating the Biden administration’s executive order that directed HHS to establish an AI task force developing a strategic plan. The newly elected Presidential administration also may be more skeptical of the safety and efficacy of vaccine products, which could lead to increased regulatory scrutiny and more restrictive coverage policies regarding our products and product candidates. Additionally, in its June 2024 decision in Loper Bright Enterprises v. Raimondo, or Loper Bright, the U.S. Supreme Court overturned the longstanding Chevron doctrine, under which courts were required to give deference to regulatory agencies’ reasonable interpretations of ambiguous federal statutes. The Loper Bright decision could result in additional legal challenges to current regulations and guidance issued by federal agencies applicable to our operations, including those issued by the FDA. Congress may introduce and ultimately pass health care related legislation that could impact the drug approval process and make changes to the Medicare Drug Price Negotiation Program created under the IRA.
Individual states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access, and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. In addition, regional healthcare authorities and individual hospitals are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription drug and other healthcare programs. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, particularly in light of the change in administration.
Coverage and Reimbursement
Market acceptance and sales of any vaccine candidates that we commercialize, if approved, will depend in part on the extent to which reimbursement for these product and related treatments will be available from third-party payors, including government health administration authorities, managed care organizations, and other private health insurers.
Third-party payors decide which therapies they will pay for and establish reimbursement levels. Travel vaccines are rarely reimbursed in Europe and, while no uniform policy for coverage and reimbursement exists in the United States, third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. One payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage, and adequate reimbursement, for the product. Additionally, a third-party payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Each payor determines whether or not it will provide coverage for a product, what amount it will pay the manufacturer for the product, and on what tier of its formulary it will be placed. The position on a payor’s list of covered drugs, biological, and vaccine products, or formulary, generally determines the co-payment that a patient will need to make to obtain the product and can strongly influence the adoption of such product by patients and physicians. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. In addition, because our product candidates are physician-administered, separate reimbursement for the product itself may or may not be available. Instead, the administering physician may only be reimbursed for providing the treatment or procedure in which our product is used. Further, coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained for one or more products for which we receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Third-party payors are increasingly challenging the prices charged for medical products and may deny coverage or offer inadequate levels of reimbursement if they determine that a prescribed product has not received appropriate clearances from the EMA, FDA, or other government regulators; is not used in accordance with cost-effective treatment methods as determined by the third-party payor; or is experimental, unnecessary, or inappropriate. Prices could also be driven down by managed care organizations that control or significantly influence utilization of healthcare products. Outside the United States, pricing of competitive products by third-parties is the biggest driver of the prices of our products.
In both the United States and some foreign jurisdictions, there have been a number of legislative and regulatory proposals and initiatives to change the health care system in ways that could affect our ability to sell vaccines and could adversely affect the prices that we receive for our vaccine candidates, if approved. Some of these proposed and implemented reforms could result in reduced pharmaceutical pricing or reimbursement rates for medical products. For example, in the United States, the ACA contains several cost containment measures that could adversely affect our future revenue, including, for example, increased drug rebates under Medicaid for brand name prescription drugs, extension of Medicaid rebates to Medicaid managed care organizations, and extension of so-called 340B discounted pricing on pharmaceuticals sold to certain healthcare providers. Additional provisions of various laws including the ACA, that may negatively affect our future revenue and prospects for profitability include the assessment of an annual fee based on our proportionate share of sales of brand name prescription drugs to certain government programs, including Medicare and Medicaid, as well as mandatory discounts on drugs (including vaccines) sold to certain Medicare Part D beneficiaries in the coverage gap (the so-called “donut hole”).
In the EU, pharmaceutical companies, products and distributors are also generally subject to extensive governmental price controls and other market regulations. In many EU Member States, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products, but monitor and control company profits.
In various EU Member States, continuous cost-cutting measures, such as lower maximum prices, lower or lack of reimbursement coverage and incentives to use cheaper products as an alternative apply. Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU Member States, including countries representing major markets. The HTA process, which is currently governed by the national laws of these countries, is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of use of a given medicinal product in the national healthcare systems of the individual country is conducted. The outcome of HTA regarding specific medicinal products will often influence the pricing and reimbursement status granted to these medicinal products by the competent authorities of individual EU Member States.
On December 15, 2021, the Health Technology Regulation, or HTA Regulation, was adopted. The HTA Regulation is intended to boost cooperation among EU member states in assessing health technologies, including new medicinal products, and providing the basis for cooperation at EU level for joint clinical assessments in these areas.
When it enters into application in 2025, the HTA Regulation will be intended to harmonize the clinical benefit assessment of HTA across the European Union. In light of the fact that the United Kingdom has left the EU, Regulation No 2021/2282 on HTA will not apply in the United Kingdom. However, the UK Medicines and Healthcare Products Regulation Agency is working with UK HTA bodies and other national organizations, such as the Scottish Medicines Consortium (“SMC”), the National Institute for Health and Care Excellence, and the All-Wales Medicines Strategy Group, to introduce new pathways supporting innovative approaches to the safe, timely and efficient development of medicinal products, including, effective as of March 31, 2025, relaunching the Innovative Licensing and Access Pathway with more predictable timelines and closer involvement of the National Health Service.
C. Organizational Structure
The chart below presents our significant subsidiaries as of December 31, 2024. Each subsidiary shown is 100% owned by the relevant parent company unless otherwise noted.
D. Property, Plants and Equipment
Our registered office is located at 6 rue Alain Bombard, 44800 Saint-Herblain, France. We also have key manufacturing facilities located in Scotland and Sweden. We believe that our existing facilities are adequate for our near-term needs, and we believe that suitable additional or alternative manufacturing and office space will be available as required in the future on commercially reasonable terms.
We own the following facilities:
•a 3,178 square meter building located at 6 rue Alain Bombard in Saint-Herblain, France, used as laboratories and offices;
•two neighboring facilities in Livingston, Scotland, used primarily for vaccine production, storage, and offices. One of these facilities is fully operational with a size of 3,547 square meters. The second facility was added in August 2020 and has approximately 6,500 square meters. This expansion of the Almeida facility is discussed further in“Item 5.B—Liquidity and Capital Resources” ; and
•a 10,725 square meter building located in Vienna, Austria, used as laboratories and offices. We acquired the building in October 2023 after the previous lease agreement expired on September 30, 2023.
We lease the following facilities:
•premises of approximately 766 total square meters across two office spaces located in the same building in Lyon, France, dedicated to sales and marketing activities. Valneva France SAS subleases around 152 square meters to Valneva SE for offices;
•a 10,739 square meter facility located in Solna, Sweden, including:
◦4,005 square meters used for industrial operation manufacturing, including production activities and housing laboratories and offices; ◦1,450 square meters used for the development and manufacture of Clinical Trial Material, in addition to laboratories and offices (now subleased to NorthX Biologics Matfors AB following the divestment in July 2023);
◦1,504 square meters supporting supply chain activities and customer service, including pick and pack activities, in addition to office space;
◦1,206 square meters of laboratories and offices supporting quality control; and
◦2,574 square meters of office space for commercial operations, quality assurance, administration, legal, information technology, and other support functions;
•a 4,000 square meter facility in Solna, Sweden, including:
◦630 square meters used for industrial operation manufacturing, including fill-finish activities and a GMP area;
◦3,370 square meters used for Clean Not Classified areas, media production, cool rooms, goods reception and offices for industrial operations and quality assurance;
•72 square meters of office space in Fleet, England, dedicated to sales and marketing activities;
•Approximately 1,800 square meters of warehouse space in Livingston, Scotland, located near Valneva’s owned sites;
•136 square meters of office space in Kirkland, Quebec, dedicated primarily to sales and marketing activities; and
•470 square meters of offices in Bethesda, Maryland, dedicated to sales and marketing activities.
Currently, about 183 square meters of our facility in Saint Herblain are subleased to Vital Meat SAS, a Groupe Grimaud affiliate, and a total of 2,128 square meters of the larger facility in Solna are subleased to NorthX Biologics Matfors AB.
Item 4A. Unresolved Staff Comments.
Not applicable.
Item 5. Operating and Financial Review and Prospects
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related Notes included elsewhere in this Annual Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Annual Report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in “Item 3.D—Risk Factors” of this Annual Report, our actual results could differ materially from the results described in or implied by these forward-looking statements.
Our audited consolidated financial statements as of and for the years ended December 31, 2024 and 2023 and the three years ended December 31, 2024 have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB.
For ease of presentation, numbers have been rounded and, where indicated, are presented in thousands of Euros. Calculations, however, are based on exact figures. Therefore, the sum of the numbers in a column of a table may not conform to the total figure displayed in the column.
Overview
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best-, or only-in-class vaccine solutions. We have a strong track record, having advanced multiple vaccines from early Research & Development (R&D) to approvals, and currently market three proprietary travel vaccines, including the world’s first chikungunya vaccine, IXCHIQ, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine development pipeline. Our clinical portfolio is composed of highly differentiated vaccine candidates that are designed to provide preventative solutions for diseases with high unmet need. Our pipeline includes the only Lyme disease vaccine candidate (VLA15) in advanced clinical development, which we are developing in partnership with Pfizer, as well as the world’s most clinically advanced tetravalent Shigella vaccine candidate, under development in collaboration with LimmaTech Biologics AG (LimmaTech), and proprietary vaccine candidates against the Zika virus and other global public health threats.
VLA15 is a Phase 3 vaccine candidate targeting Borrelia, the bacterium that causes Lyme disease, under development in collaboration with Pfizer, and it is the only vaccine candidate against Lyme disease currently undergoing late-stage clinical trials. Pfizer and Valneva are currently executing the Phase 3 field efficacy study for VLA15 called VALOR (Vaccine Against Lyme for Outdoor Recreationists). Enrollment in the trial was completed in December 2023 and the primary vaccination series were completed in July 2024. Participants will be monitored for the occurrence of Lyme disease cases until the end of the Lyme disease season in 2025. VLA15 targets the six most prevalent serotypes, or variations, of Borrelia in the United States, where approximately 476,000 people are diagnosed with Lyme disease each year and in Europe, where at least a further 200,000 cases occur annually.
VLA1553 is a vaccine candidate which was approved by the U.S. Food and Drug Administration (FDA), the European Commission (EC), Health Canada, and the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) under the brand name IXCHIQ. It is indicated for the prevention of disease caused by chikungunya virus (CHIKV) in individuals 18 years of age and older. At the end of February 2024, the U.S. Advisory Committee on Immunization Practices (ACIP) provided recommendations on how to use IXCHIQ and these recommendations were then adopted by the U.S. Centers for Disease Control and Prevention (CDC). In 2024, Valneva submitted label extension applications to the FDA, the European Medicines Agency (EMA) and Health Canada to potentially extend the use of IXCHIQ, which is currently approved in adults, to adolescents 12 to 17 years of age. The applications to the FDA and Health Canada also included the two-year antibody persistence data to the product label, which is a key differentiator for IXCHIQ. In February 2025, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion recommending authorization of a label extension for IXCHIQ in adolescents 12 years of age and older. The EC will now review the CHMP recommendation, and a decision on the label extension application in the European Union (EU), Norway, Liechtenstein, and Iceland is expected within sixty days following the CHMP opinion. A marketing application is also under review in Brazil, which would represent the first approval in an endemic country. The vaccine is still undergoing several clinical trials with a view to expand access, support label extensions and enhance the product profile.
Shigella4V2 (S4V2) is a Phase 2 tetravalent bioconjugate vaccine candidate against shigellosis, a diarrheal infection caused by Shigella bacteria, under development in collaboration with LimmaTech. Shigellosis is the second leading cause of fatal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths are attributed to Shigella each year, particularly among children in Low and Middle Income Countries (LMICs). No approved Shigella vaccine is currently available outside of Russia or China, and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO). In October 2024, the FDA granted Fast Track designation to S4V2, recognizing its potential to address a serious condition and fill an unmet medical need.
VLA1601 is a Phase 1 vaccine candidate targeting the Zika virus (ZIKV), a mosquito-borne viral disease whose transmission has been reported in 89 countries and territories and persists in several countries in the Americas and other endemic regions.
There are no preventive vaccines or effective treatments available. As such, Zika remains a public health threat and is included in the FDA’s Tropical Disease Priority Review Voucher Program. VLA1601 is being developed on the original manufacturing platform of our licensed Japanese encephalitis vaccine IXIARO, which was further optimized to develop our inactivated, adjuvanted COVID-19 vaccine VLA2001, the first COVID-19 vaccine to receive a standard marketing authorization in Europe.
We have already successfully licensed and commercialized a portfolio of traveler vaccines, which is composed of IXIARO (also marketed as JESPECT in Australia and New Zealand), indicated for the prevention of Japanese encephalitis in travelers and military personnel, and DUKORAL, indicated for the prevention of cholera and, in Canada, Switzerland, New Zealand, and Thailand, prevention of diarrhea caused by Enterotoxigenic Escherichia coli, or ETEC, the leading cause of travelers’ diarrhea. During the course of 2024, we launched our chikungunya vaccine IXCHIQ in the United States, France and Canada and expect to launch it in additional countries in 2025. Additionally, we distribute vaccines for third parties in selected countries where we have a commercial infrastructure.
We have a highly developed, nimble and sophisticated manufacturing infrastructure with facilities across Europe to meet our clinical and commercial needs, including BioSafety Level 3 (BSL-3) manufacturing and R&D facilities. We have assembled a team of experts with deep scientific, clinical and business expertise in biotechnology and specifically in vaccine development, manufacturing and commercialization. Our senior leadership team has extensive experience and demonstrated ability to move vaccines through the clinic and into successful commercialization. Members of our team have previously worked at industry leaders such as Novartis, Chiron, GlaxoSmithKline and Daiichi Sankyo.
Our operations have focused on organizing and staffing our company, business planning, raising capital, establishing and maintaining our intellectual property portfolio, establishing our commercial infrastructure, growing our commercial portfolio, establishing and advancing our manufacturing capabilities, and conducting pre-clinical studies and clinical trials. As of December 31, 2024, we had €168.3 million in cash and cash equivalents. This includes the gross proceeds of $103 million for the sale of our Priority Review Voucher (PRV) which we received in February 2024. Our operating income was €13.3 million for the year ended December 31, 2024, and operating losses were €82.1 million and €113.4 million for the years ended December 31, 2023 and 2022, respectively. Our net losses were €12.2 million, €101.4 million, and €143.3 million for the years ended December 31, 2024, 2023, and 2022, respectively. We expect to continue to incur significant operating expenses and net losses for the foreseeable future.
Factors Affecting Our Results
We believe that our financial performance has been and for the foreseeable future will continue to be primarily driven by the factors discussed below. While many of these factors present opportunities for our business, they also pose challenges that we must successfully address in order to sustain our growth and improve our results of operations. Our ability to successfully address the factors below is subject to various risks and uncertainties, including those described in “Item 3.D—Risk Factors”.
Revenues
We principally derive our revenues from the sale of our commercialized travel vaccines in their respective markets, as well as from sales of third-party products. In the years covered by this Annual Report, revenues from our products derived from the sale of IXIARO, DUKORAL, IXCHIQ, and VLA2001. We also derive revenues from royalties, partnerships related to our vaccine candidates, as well as from collaborations, services, and licensing agreements and by offering our technologies and services to third parties.
Product Sales of IXIARO, DUKORAL, IXCHIQ, VLA2001, and Third-party Products
Product sales of IXIARO and DUKORAL represented in aggregate 77.4%, 71.4%, and 51.1% of our revenues for the years ended December 31, 2024, 2023, and 2022, respectively. Our primary markets for these products are the United States and Germany for IXIARO and Canada for DUKORAL.
In 2024, we generated our first revenue from the sale of IXCHIQ following approval of the vaccine by the the U.S. Food and Drug Administration (FDA), EMA, and Health Canada. For the year ended December 31 2024 product sales of IXCHIQ represented 2.3% of our revenues.
Product sales of VLA2001 represented 3.9% of our revenues for the year ended December 31 2023, and 25.8% for the year ended December 31, 2022,
In addition, we generate revenues by leveraging our existing sales and marketing infrastructure to sell third-party products. Revenues from sales of third-party products represented 20.3%, 24.7%, and 23.1% of our revenues for the years ended December 31, 2024, 2023, and 2022, respectively.
Sales trends in travel vaccines are primarily driven by travel volume to endemic regions, national travel advisories, awareness about illness, and the perception of risk by health practitioners and tourists. Although the COVID-19 pandemic and the impact on travel caused a material reduction in our revenues during 2020 and 2021, the progressive recovery of the travel market allowed for increased sales since 2022.
While COVID-19 impacted sales of our travel vaccines to the general public, sales of IXIARO to the U.S. Department of Defense (DOD) Defence Logistics Agency (DLA), which purchases the Japanese encephalitis vaccine for military personnel being deployed to endemic regions, have remained significant over the periods presented herein. Sales of IXIARO to the DLA derived from one contract signed in September 2020 and another signed in September 2023. The terms of the 2020 agreement contemplated an initial base year followed by two option years, each with a range of minimum and maximum potential dose orders.
In September 2021, we announced that DLA had exercised the first year option of this agreement. Due to the ongoing impact of the COVID-19 pandemic on DLA’s operations, the option terms were amended such that the minimum number of doses for the first option year was 200,000 with an approximate value of $28.8 million. We also agreed to provide additional inventory to the DLA after September 2023 to mitigate the potential impact of unused stock that may expire. This replacement inventory was provided free of charge in 2024 and resulted in the release of the contract liability of $5.2 million (€4.7 million) recognized as at December 31, 2023 (December 31, 2022; $5.2 million; December 31, 2021: $5.4 million). In August 2022, we announced that DLA had decided not to exercise the second option year of the initial contract, as DLA considered its existing IXIARO supply sufficient to meet current needs. The agreement signed in September 2023 was for one year and has a minimum value of approximately $32.3 million for approximately 200,000 doses. In January 2025, we signed a new $32.8 million IXIARO supply contract with the DLA.
For the years ended December 31, 2024, 2023, and 2022, 30.9%, 25.5%, and 30.3%, respectively, of our total product sales of IXIARO were from sales to the DLA.
Other revenues
Revenues from Collaboration
We derive revenues from collaboration and partnership agreements. One source of collaboration revenues is through our research collaboration and license agreement, or the Collaboration and License Agreement, with Pfizer Inc. In April 2020, Valneva signed the Collaboration and License Agreement with Pfizer to co-develop and commercialize the Group’s Lyme disease vaccine candidate (VLA15). This is classified as an agreement with a customer as defined by IFRS 15 guidance on revenue contracts with customers, and accordingly, amounts received or payable by Valneva under the Collaboration and License Agreement are accounted for in the Group’s revenues.
In 2021 and 2022 several amendments to the transaction price were made via amendments to the Collaboration and License Agreement and resulted in a reduction to the constrained (i.e. highly probable) transaction price, reflecting an increase in expected payments to customer related to Valneva’s contribution to Pfizer’s future development costs.
In addition, Valneva considered the constraint to determine whether it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur. Valneva concluded that it is no longer highly probable that it will be entitled to the consideration as payments to customers might further increase in the future. Therefore, for the year ended December 31, 2022, the accumulated revenue recognized since the inception of the agreement with Pfizer amounting to €45.9 million was reversed as other revenues from contracts with customers. In the years ended December 31, 2024 and December 31, 2023, no revenues were recognized.
While license and equipment purchase orders were fulfilled in prior periods, the R&D activities and additional services were ongoing through 2024 and satisfy the performance obligation over time. During this period Valneva funded 40% of the remaining shared development costs.
Items not included in the transaction price as of December 31, 2024 are (i) $143 million from early commercialization milestones, (ii) royalties, ranging from 14% to 22%, and (iii) $100 million in sales-based milestones ,which will be recognized as and when they occur.
As at December 31, 2024, the refund liability amounted to €18.6 million (December 31, 2023: €33.1 million). The decrease was mainly due to a) payments made by Valneva in the period in connection with the terms of this agreement and partly compensated with b) services provided by Valneva for which revenues were deferred and added to the refund liability.
As at December 31, 2024, Valneva has completed its payment obligations of the Phase 3 costs to Pfizer. As of December 31, 2024 and 2023, €3.7 million in contract costs were included in other assets. In 2024 and 2023, no contract liabilities were disclosed while in 2022 €4.2 million were included.
Revenues from Technologies and Services
We also derive revenues from sales of our technologies and services. Revenues from our technologies consists of revenues from our EB66 cell line, which is derived from duck embryonic stem cells and provides an alternative to the use of chicken eggs for large scale manufacturing of human and veterinary vaccines, and our IC31 vaccine adjuvant, which is a synthetic adjuvant targeting antigens to improve immune response and has been licensed to several pharmaceutical companies. Services revenues consist of research and development services we provide to third parties, including process and assay development and production and testing of clinical trial material.
Key Cost Drivers
Research and Development
We generate a significant amount of research and development expenses due to the nature of our business. Research and development expenses were €74.1 million, €59.9 million, and €104.9 million for the years ended December 31, 2024, 2023, and 2022, respectively. Research and development expenses generally track development of our underlying product candidate portfolio. Investment in research and development is required to support advancing programs through increasingly expensive stages of clinical development.
We have seen an increase in research and development costs during 2024 compared to 2023, mainly due to incremental expenses incurred in relation to the development collaboration and licensing agreement signed for the Shigella4V vaccine candidate.
In addition we have seen increased expenses associated with transferring manufacturing of our commercial products to the new manufacturing site in Scotland. In 2023, the decrease compared to 2022 was due to the phasing of clinical trial expenses and accelerated wind-down of VLA2001-related activities in 2022.
Our research and development costs in 2024 mainly comprised expenses relating to the Phase 3 (in adolescents and children) and Phase 4 clinical trial for our chikungunya vaccine IXCHIQ, our Phase 2 study of the Shigella4V2 vaccine candidate, the Lyme program (in partnership with Pfizer), the development of our Zika vaccine candidate, and work on pre-clinical projects. We expect R&D expenses to increase in the medium/long term as we advance other candidates in our pipeline.
Marketing and Distribution
We have developed an established commercial infrastructure that is dedicated to promoting and selling our products and educating physicians and travelers about our products and the diseases they target. We are continually investing in our commercial infrastructure and have identified markets where we can increase our sales and marketing efforts and market penetration. We have also been able to leverage our commercial infrastructure for third-party product distribution.
Marketing and distribution expenses were €52.4 million for the year ended December 31, 2024, compared to €48.8 million in 2023 and €23.5 million in 2022. In 2024, advertising and promotional spend increased in line with a significant resumption of international travel and as a result of launch preparation spend following the FDA licensure of IXCHIQ in November 2023. We expect that marketing and distribution expenses will continue to increase, though at much lower rates, driven by incremental spend in creating awareness for IXCHIQ and by further building our commercial infrastructure.
Cost of Goods and Services
Historically, manufacturing costs have experienced limited cost increases. Manufacturing costs comprise site infrastructure, employees to operate the manufacturing, and the bill of materials. Incremental cost increase is driven by the variable cost in the bill of materials. We are manufacturing our chikungunya vaccine at our facilities in Livingston, Scotland. We need limited additional infrastructure and employees for this program, and we incur relatively low raw materials costs.
Cost of goods and services were €98.5 million in the year ended December 31, 2024 (2023: €100.9 million, 2022: €324.4 million), of which €0.9 million (2023: €5.5 million, 2022: €159.4 million) related to VLA2001.The slight decrease between 2024 and 2023 mainly resulted from a reduction in failed manufacturing batches of IXIARO. The considerably higher amounts in 2023 and 2022 stem from costs of goods of the VLA2001 doses sold, write-downs for materials which could not be used, failed batches, and batches at risk of failure as well as product which is not expected to be sold. €5.3 million of costs of goods and services in 2023 related to onerous agreements provision and settlement costs of which €0.6 million have been released in 2024.
General and Administrative Expenses
General and administrative expenses decreased in the year ended December 31, 2024 compared to the year ended in December 31, 2023. In 2023 Valneva put in place some new processes as we became a more complex organization, requiring additional corporate support and therefore incurred initialization expenses in connection with these processes. In 2024, we benefited furthermore from lower directors and officers’ insurance costs, lower fees for professional services and personnel costs included in this line item were lower, all of which led to a decrease in general and administrative expenses. In 2023, employee-related expenses were lower due to the favorable effect of our share price development on our employee share-based compensation programs.
Gain from sale of Priority Review Voucher, net
The Company sold the PRV received from the FDA for $103 million (€95 million) on February 2, 2024. The Company was awarded a tropical disease PRV in November 2023 following the FDA’s approval of IXCHIQ, Valneva’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus. The net gain from the sale of the PRV amounted to €90.8 million, after deducting expenses in the amount of €4.2 million, which included transaction fees as well as expenses in connection with contractual payment obligations related to the PRV sale.
Grants
We seek grants from governmental agencies and non-governmental organizations to partially offset our increasing research and development costs. Grant income also includes research and development tax credits. Grants, which are recorded in other income, increased to €20.2 million for the year ended December 31, 2024 from €18.1 million for the year ended December 31, 2023, mainly due to the recognition of a €3.7 million grant income received from Scottish Enterprise, Scotland’s national economic development agency, for developing non-COVID-19 vaccines (IXCHIQ and IXIARO). In 2024, we recognized research and development tax credits in Austria of €3.6 million. In 2023 and 2022, the amounts recognized were €5.7 million and €13.9 million, respectively, which were mainly related to the then COVID-19 and chikungunya vaccine candidates.
In July 2019, we entered into a funding agreement with CEPI pursuant to which we are eligible to receive up to $23.4 million (paid in a series of six-month tranches) for vaccine manufacturing and late-stage clinical development of a single-dose live attenuated vaccine against chikungunya (VLA1553) in return for equitable access to project results. In 2022, CEPI agreed to increase our funding to up to $24.6 million. We are obligated to pay CEPI up to $7.0 million in commercial and related milestones. See “Item 10.C—Material Contracts—CEPI Funding Agreement” for more details on the terms of this grant.
The partnership with CEPI was extended in 2024 when the Group signed the second funding agreement. Valneva will receive up to $41.3 million in the next five years to support broader access to the world’s first chikungunya vaccine, IXCHIQ, in Low- and Middle-Income countries (LMICs), as well as post-marketing trials and potential label extensions in children, adolescents, and pregnant women. The proceeds from CEPI are treated under IAS 20 and presented as grant income. In the year ended December 31, 2024, €6.5 million of grant income related to the second agreement with CEPI was recognized.
In February 2022 the Group received two grants worth up to £20.0 million (approximately €23.9 million) from Scottish Enterprise, Scotland’s national economic development agency, to support research and development relating to the manufacturing processes of the COVID-19 vaccine and other vaccine candidates. Following the termination of the COVID-19 vaccine program, in May 2023 the grant relating to this program was amended, reducing the available funding from £7.5 million to zero. In 2024, there have been no amendments to the grants. The funds under the remaining grant were received over three years, beginning in March 2022. In the year ended December 31, 2024, €3.7 million (£3.1 million) of grant funds from Scottish Enterprise were recognized. In the year ended December 31, 2023, €11.1 million (£9.6 million) of grant funds from Scottish Enterprise were recognized.
We plan to continue evaluating and pursuing grant opportunities.
International Operations and Foreign Currency Exchange Risks
We operate on a global basis with facilities, sales, and activities throughout the world, and our global operations subject our financial results to fluctuations in foreign currency exchange rates. Because we generate a substantial part of sales in the United States for IXIARO, with production costs in the British Pound, or GBP, and in Canada for DUKORAL, with production costs in Swedish Krona, or SEK, and we received proceeds in USD from the capital raises in May 2021, October 2021, June 2022, and October 2022, we are exposed to foreign exchange risks, principally with respect to the U.S. Dollar, or USD, GBP, SEK and the Canadian dollar, or CAD. Our results of operations continue to be impacted by exchange rate fluctuations.
Impact of COVID-19
The COVID-19 pandemic had a number of significant impacts on our business since March 2020. Notably, we initiated development of a COVID-19 vaccine, VLA2001, and sold VLA2001 to certain European countries and Bahrain. Other than recording sales related to shipment of VLA2001 doses to Bahrain and residual costs for VLA2001 clinical studies, the COVID-19 pandemic had no further impact on the income statement for the years ended December 31, 2024 or 2023.
All raw material and work in progress related to VLA2001 which could not be repurposed and used for other products were written down during prior years.
Financial Operations Overview
Revenue
Our product revenue is primarily derived from the sale of our commercialized products IXIARO and DUKORAL in their approved markets and sales of third-party products pursuant to distribution partnerships. In 2024, we generated our first revenue from the sale of IXCHIQ following approval of the vaccine by the FDA, EMA, and Health Canada. We distribute products both directly and through the use of third-party distributors. We primarily sell IXIARO in the United States (in the private market as well as to the U.S. Department of Defense for military personnel being deployed to endemic areas), Canada, Germany, the Nordics (being Denmark, Finland, Norway and Sweden together), France, and Benelux. We primarily sell DUKORAL in Canada and the Nordics. We derived product revenues from the sale of our COVID-19 vaccine to certain European countries in 2022 and to the Kingdom of Bahrain in 2022 and 2023.
Our other revenue (from collaboration, licensing, and services) consists of milestone payments, upfront licensing payments, and reimbursement of services. Certain of these payments are initially recorded on our statement of financial position and subsequently recognized as revenue in accordance with our accounting policy as described further under “Critical Accounting Estimates and Judgments” and Note 5.2 to our consolidated financial statements as of and for the years ended December 31, 2024 and 2023 included elsewhere in this Annual Report. We generate revenues from licensing and service agreements for our product candidates and proprietary technologies. We contract with third parties to provide a variety of services such as manufacturing services, leases arrangements, research licenses, commercial licenses, and research and development services. The terms of such licenses include license fees payable as initial fees, annual license maintenance fees, and fees to be paid upon achievement of milestones, as well as license option fees and fees for the performance of research services. In addition, our licensing arrangements generally provide for royalties payable on the licensee’s future sales of products developed within the scope of the license agreement.
In the years ended December 31, 2023 and 2022, our other revenues included certain amounts from the agreements relating to our COVID-19 vaccine: a) the UK Supply Agreement executed in September 2020 and b) the EC APA executed in October 2021 and c) the Bahrain APA executed in December 2021.
For more detailed information, see Note 5.5 to the financial statements included elsewhere in this Annual Report.
Operating Expenses
Cost of Goods and Services
Cost of goods and services consist primarily of personnel costs, costs for materials, royalties, and costs for third-party services, as well as building and energy costs, depreciation and amortization, impairment charges of tangible assets, and other direct and allocated costs incurred in connection with the production of our products. Costs of goods and services also include costs of product sales from inventory produced in the prior year, idle production costs, and costs related to expired and faulty products which have been written off. Cost of goods and services also include costs relating to our revenue-generating collaboration, services, and licensing agreements.
Research and Development Expenses
The nature of our business and the primary focus of our activities generate a significant amount of research and development expenses. Research and development expenses include the costs associated with research and development conducted by us or for us by outside contractors, research partners, or clinical study partners, and expenses associated with research and development carried out by us in connection with strategic collaboration and licensing agreements. Our research and development expenses are primarily incurred as a result of the following activities:
•discovery efforts leading to product candidates,
•development efforts for our clinical programs, and
•development of our manufacturing technology and infrastructure.
The costs of the above activities driving research and development expenses comprise the following categories:
•expenses related to our research and development personnel, including salaries, social security expense, share-based compensation expense, and other related expenses,
•expenses incurred under agreements with third parties, such as consultants, investigative sites, contract research organizations, or CROs, that conduct our pre-clinical studies and clinical trials, and in-licensing arrangements,
•costs of acquiring, developing, and manufacturing materials for pre-clinical studies and clinical trials, including both internal manufacturing and third-party contract manufacturing organizations, or CMOs,
•expenses incurred for the procurement of materials, laboratory supplies, and non-capital equipment used in the research and development process, and
•facilities, depreciation and amortization, and other direct and allocated expenses incurred as a result of research and development activities.
The substantial majority of our direct expenses incurred for the years ended December 31, 2024, 2023, and 2022, including for CROs, other contracted research and development activities, and raw materials, related to our chikungunya vaccine, our Shigella4V (S4V) vaccine candidate, COVID-19 vaccine (in 2022), and our Lyme disease vaccine candidate. We also incur indirect research and development expenses primarily related to facilities, energy, and office costs as well as the cost of research and development personnel.
Research and development expenses are generally recognized in the period in which they are incurred. However, research and development expenses of €7.0 million related to VLA2001 for which no future benefit is expected were provisioned as of December 31, 2022 and fully released, against the incoming invoices received, in the period ended December 31, 2023.
Research and development expenses incurred in connection with product candidates are capitalized and recorded as intangible assets when the following criteria are met:
•the technical feasibility of completing the asset has been achieved so that it will be available for use or sale;
•we have the intention to complete the asset and use or sell it;
•we have the ability to use or sell the asset;
•we believe the asset will generate probable future economic benefits and can demonstrate the existence of a market or the usefulness of the asset if it is to be used internally;
•we are confident of the availability of adequate technical, financial, and other resources to complete development of the asset and to use or sell it; and
•we have the ability to reliably measure the expenditure attributable to the intangible asset.
In the years ended December 31, 2024, 2023, and 2022, no research and development expenses were recorded as intangible assets. As of December 31, 2024 and 2023, we had previously capitalized research and development costs recorded as intangible assets in an aggregate amount of €1.1 million and €1.2 million, respectively.
Research and development activities are a key component of our business model. The successful development and commercialization of a product candidate involves significant costs, which may vary from year to year depending upon factors such as the progress of clinical trials and other research and development activities, the timing of regulatory approvals, the duration of the regulatory approvals process and the possibility of, and potential expenses related to, filing, prosecuting, defending, or enforcing any patent claims or other intellectual property or proprietary rights. The most expensive stages in the regulatory approval process in the United States and the European Union are late-stage clinical trials, which are the longest and largest trials conducted during the approval process. The significant cost factors in our clinical trials include manufacturing compounds for product candidates, organizing clinical trials, including participant enrollment, production and testing of product candidates involved in clinical trials, and laboratory testing and analysis of clinical parameters.
By contrast, pre-clinical research and development expenses primarily depend on the number of scientific staff employed. We expect that our research and development expenses will continue to increase in the foreseeable future as we initiate and progress clinical trials for our vaccine candidates.
Marketing and Distribution Expenses
Marketing and distribution expenses consist primarily of expenses relating to marketing and distribution personnel, including salaries, social security contributions, share-based compensation expense, and other employee-related expenses, advertising, media, and public relations expenses, warehousing and distribution costs, costs related to third-party services and other direct and allocated expenses incurred in connection with our own commercial sales infrastructure, business development, and other marketing and distribution activities. We have incurred incremental costs for preparation of market access and launch activities of IXCHIQ following licensure of the vaccine in the U.S. in November 2023.
General and Administrative Expenses
General and administrative expenses consist primarily of non-research and development personnel-related costs, including salaries, social security contributions, share-based compensation expense, and other employee-related expenses for general management, finance, legal, human resources, investor relations, internal audit, and other administrative and operational functions, fees for professional services, such as consulting, legal, and financial services, information technology, and facility-related costs. These costs relate to the operation of our business and are unrelated to our research and development function or any individual product candidate program.
We anticipate that our general and administrative expenses in the near term will remain comparable to the costs incurred in the year ended December 31, 2024. We also anticipate continued material expenses associated with being a public company in the United States, including costs related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with U.S. exchange listing and Securities and Exchange Commission, or SEC, requirements, director and officer insurance premiums, and investor relations costs. In particular, we will continue to incur additional accounting expenses to comply with the Sarbanes-Oxley Act of 2002 in the United States that require us to test the effectiveness of our internal controls over financial reporting. We also anticipate increased expenses associated with new sustainability reporting requirements applicable to us as a publicly listed company in both France and the United States.
Other Income (Expenses)
Our other income results principally from grants and research tax credits. We expect to continue to be eligible for these tax credits and subsidies for so long as we incur eligible expenses.
Grants
Grants from governmental agencies and non-governmental organizations are recognized where there is reasonable assurance that the grant will be received and that we will comply with all conditions.
In 2019 the Group signed a funding agreement with CEPI. Valneva received $24.6 million for vaccine manufacturing and late-stage clinical development of a single-dose, live attenuated vaccine against chikungunya. In line with CEPI’s commitment to equitable access, the funding underwrote a partnership effort to accelerate regulatory approval of Valneva’s chikungunya vaccine for use in regions where outbreaks occur and to support World Health Organization prequalification to facilitate broader access in lower- and middle-income countries. Valneva had to pay back part of the consideration upon achievement of certain milestones. The refundable consideration is accounted for as a loan and measured in accordance with IFRS 9 (see Note 5.24.1). The difference between the proceeds from CEPI and the carrying amount of the loan is treated under IAS 20 and presented as “Borrowings”. The amount from the CEPI grant which benefits Instituto Butantan is recognized as revenue (see Note 5.5). In the year ended December 31, 2024, nil grant income (2023: €0.2 million) and €0.4 million of other revenues (2023: €5.0 million) related to the first CEPI agreement were recognized.
The partnership with CEPI was extended in 2024 when the Group signed the second funding agreement. Valneva will receive up to $41.3 million in the next five years to support broader access to the world’s first chikungunya vaccine, IXCHIQ, in Low- and Middle-Income countries (LMICs), as well as post-marketing trials and potential label extensions in children, adolescents, and pregnant women. The proceeds from CEPI are treated under IAS 20 and presented as grant income. In the year ended December 31, 2024, €6.5 million of grant income related to the second agreement with CEPI was recognized.
In February 2022 the Group received two grants worth up to £20.0 million (approximately €23.9 million) from Scottish Enterprise, Scotland’s national economic development agency, to support research and development relating to the manufacturing processes of the COVID-19 vaccine and other vaccine candidates. Following the termination of the COVID-19 vaccine program, in May 2023 the grant relating to this program was amended, reducing the available funding from £7.5 million. to zero . In 2024, there have been no amendments to the grants. The funds under the remaining grant were received over three years, beginning in March 2022. In the year ended December 31, 2024, €3.7 million (£3.1 million) of grant funds from Scottish Enterprise were recognized. In the year ended December 31, 2023, €11.1 million (£9.6 million) of grant funds from Scottish Enterprise were recognized.
Research Tax Credits
We benefit from Austrian research tax credit, Scottish tax credit, and French tax credit (known as Crédit d’Impôt Recherche, or CIR). The qualifications for the tax credits are similar, as the Austrian, British, and French tax authorities encourage companies to conduct technical and scientific research. To be eligible, companies need to demonstrate that they have expenses that meet certain required criteria. The main differences between the three jurisdictions’ tax credits are the applicable percentage of and the basis for the tax credit.
We have concluded that research tax credits in the three countries meet the definition of a government grant, as defined in IAS 20, Accounting for Government Grants and Disclosure of Government Assistance, and, as a result, it has been classified as other income within operating income in our statement of operations.
In December 31, 2024 we received research and development tax credits mainly from Scotland (€5.2 million) and from Austria (€3.6 million) whereas in the previous period we received tax credits primarily from Austria (€5.7 million) and to a lesser extent from France.
Finance Income (Expenses)
Finance income relates primarily to interest income received from cash and cash equivalents deposits. Our cash and cash equivalents are deposited primarily into cash accounts and term deposit accounts with short maturities and therefore generate only a modest amount of interest income.
Finance expenses relate primarily to interest expense paid to banks and government agencies and on other loans as well as to interest expense on lease liabilities and refund liabilities.
We also incur foreign exchange gains and losses related to our international operations, primarily with respect to the U.S. Dollar, the British Pound, the Swedish Krona, and the Canadian Dollar, which amounts are recorded as finance income or expenses.
Income Tax
Income tax income or expense reflects our current income tax, as well as our deferred tax income (expense).
Adjusted EBITDA
To provide investors with additional information regarding our financial results, we have provided within this Annual Report Adjusted EBITDA, a non-IFRS financial measure, which is defined as earnings (loss) from the period before income taxes, finance income/expense, foreign currency gain/(loss) – net, result from investments in associates, amortization, depreciation, and impairment. Adjusted EBITDA is a common supplemental measure of performance used by investors and financial analysts. Management uses Adjusted EBITDA as a supplemental measure for assessing operating performance in conjunction with related GAAP amounts. It also uses Adjusted EBITDA in connection with matters such as strategic planning, annual budgeting, operating decision making, evaluating company performance, and comparing operating results with historical periods and with industry peer companies.
Management uses and presents IFRS results as well as the non-IFRS measure of Adjusted EBITDA to evaluate and communicate its performance. While non-IFRS measures should not be construed as alternatives to IFRS measures, management believes non-IFRS measures are useful to further understand our current performance, performance trends, and financial condition.
We have provided reconciliations in “Item 5A—Operating Results—Results of Operations” to operating loss, which is the most directly comparable IFRS measure, for the years ended December 31, 2024, 2023, and 2022. Our use of Adjusted EBITDA has limitations as an analytical tool, and you should not consider it in isolation or as a substitute for analysis of our results as reported under IFRS. For example:
•Although depreciation and amortization are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future, and Adjusted EBITDA does not reflect cash capital expenditure requirements for such replacements or for new capital expenditure requirements.
•Adjusted EBITDA does not reflect changes in, or cash requirements for, our working capital needs.
•Adjusted EBITDA does not reflect interest expense or income tax payments that may represent a reduction in cash available to us.
Item 5A. Operating Results
Results of Operations
Overview
Results of Operations—Consolidated
Our results of operations for the years ended December 31, 2024, 2023, and 2022 are summarized in the table below.
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Product sales |
163,253 |
|
144,624 |
|
114,797 |
|
| Other revenues |
6,325 |
|
9,088 |
|
246,506 |
|
| REVENUES |
169,579 |
|
153,713 |
|
361,303 |
|
| Cost of goods and services |
(98,538) |
|
(100,875) |
|
(324,441) |
|
| Research and development expenses |
(74,143) |
|
(59,894) |
|
(104,922) |
|
| Marketing and distribution expenses |
(52,356) |
|
(48,752) |
|
(23,509) |
|
| General and administrative expenses |
(42,750) |
|
(47,799) |
|
(34,073) |
|
| Gain from sale of Priority Review Voucher, net |
90,833 |
|
— |
|
— |
|
| Other income and expense, net |
20,706 |
|
21,520 |
|
12,199 |
|
| OPERATING PROFIT/(LOSS) |
13,330 |
|
(82,087) |
|
(113,443) |
|
| Finance income |
2,362 |
|
1,210 |
|
260 |
|
| Finance expenses |
(23,984) |
|
(23,325) |
|
(19,054) |
|
| Foreign exchange gain/(loss), net |
(3,193) |
|
5,574 |
|
(12,587) |
|
| Result from investments in associates |
— |
|
— |
|
9 |
|
| PROFIT/(LOSS) BEFORE INCOME TAX |
(11,486) |
|
(98,629) |
|
(144,815) |
|
| Income tax income/(expense) |
(761) |
|
(2,800) |
|
1,536 |
|
| PROFIT/(LOSS) FOR THE PERIOD |
(12,247) |
|
(101,429) |
|
(143,279) |
|
Results of Operations
Our Executive Committee, as our chief operating decision maker (“CDM”), considers our operating business in its entirety to allocate resources and assess performance. The CDM evaluates all vaccine candidates and vaccine products together as a single operating segment, “development and commercialization of prophylactic vaccines”. Therefore, the split used to allocate resources and assess performance is based on a functional view, thus correlating to the income statement format. We have changed our internal reporting process as at January 1, 2023 to present a single operating segment instead of the previously disclosed product-based segments.
Comparisons for the Years Ended December 31, 2024 and 2023
Revenue
Consolidated Revenue
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Product sales |
163,253 |
|
144,624 |
|
|
| Other revenues from contracts with customers |
5,622 |
|
8,075 |
|
|
| Other non-IFRS 15 revenue |
704 |
|
1,014 |
|
|
| REVENUES |
169,579 |
|
153,713 |
|
|
Revenue increased by €15.9 million, or 10%, to €169.6 million for the year ended December 31, 2024. The increase from 2023 to 2024 is mainly related to higher product sales.
Our total product sales reached €163.3 million for the year ended December 31, 2024, compared to €144.6 million in the same period of 2023. This 13% increase was driven by the continued recovery of travel vaccine sales, higher sales of IXIARO to the U.S. Department of Defense (DoD) as well as the first sales of IXCHIQ. Currency fluctuations of €0.5 million also favorably impacted product sales.
Product sales
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| IXIARO |
94,069 |
|
73,483 |
|
|
| DUKORAL |
32,303 |
|
29,775 |
|
|
| Third party products |
33,185 |
|
35,675 |
|
|
| IXCHIQ |
3,696 |
|
— |
|
|
| VLA2001 |
— |
|
5,691 |
|
|
| PRODUCT SALES |
163,253 |
|
144,624 |
|
|
IXIARO product sales were €94.1 million in the year ended December 31, 2024 compared to €73.5 million in 2023. The 28% increase in sales is primarily the result of the continued travel market recovery, higher sales to the U.S. Department of Defense (DoD), and price increases. The increase in IXIARO product sales included a favorable €0.4 million foreign currency impact.
DUKORAL sales were €32.3 million in the year ended December 31, 2024 compared to €29.8 million in 2023. This 8% increase is also a result of the significant recovery in the private travel markets and price increases. Foreign currency fluctuations had a negative impact of €0.2 million on DUKORAL sales.
The first IXCHIQ product sales were recorded in 2024, following the adoption of the U.S. Advisory Committee on Immunization Practices (ACIP)’s recommendations by the U.S. Centers for Disease Control and Prevention (CDC) in March 2024.
Third-party product sales were €33.2 million in the year ended December 31, 2024 compared to €35.7 million in 2023, a (7)% decrease, which was mainly driven by lower sales of Rabipur/RabAvert and Encepur under the distribution agreement with Bavarian Nordic.
There were no COVID-19 vaccine sales in 2024 compared to €5.7 million in 2023, following the discontinuation of the program.
Product Sales—By Geography
We also monitor product sales generated in the countries and regions where we operate. The following table presents product sales by geography and is based on the final location where our distribution partner sells the product or where the customer or partner is located.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| United States |
48,446 |
|
32,606 |
|
|
| Canada |
32,321 |
|
28,193 |
|
|
| Germany |
18,154 |
|
13,238 |
|
|
| Nordics |
12,865 |
|
12,426 |
|
|
| United Kingdom |
19,393 |
|
20,216 |
|
|
| Austria |
13,998 |
|
13,915 |
|
|
| France |
6,685 |
|
5,303 |
|
|
| Other Europe |
7,984 |
|
8,168 |
|
|
| Rest of World |
3,408 |
|
10,560 |
|
|
| PRODUCT SALES |
163,253 |
|
144,624 |
|
|
Total product sales in the United States increased by €15.8 million, or 49%, to €48.4 million in the year ended December 31, 2024, compared to €32.6 million in the year ended December 31, 2023. They increased primarily as a result of higher sales to the U.S. Department of Defense (DoD), higher demand in private markets, and first sales of IXCHIQ.
Product sales in Canada increased by €4.1 million, or 15%, from €28.2 million in the year ended 2023, to €32.3 million in 2024 as a result of the significant recovery in the private travel markets.
Sales in Germany increased by €4.9 million, or 37%, to €18.2 million in the year ended December 31, 2024 due to continued resumption of travel positively impacting sales of our travel vaccines.
Other revenues from contracts with customers
The following table presents our other revenues (from collaboration, licensing, and services) for the years ended December 31, 2024 and 2023.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2024 |
2023 |
|
| Royalties received |
2,410 |
|
2,129 |
|
|
| Revenues from shipping and handling |
1,368 |
|
21 |
|
|
| Milestone payment - licenses |
712 |
|
3,637 |
|
|
| Other services |
1,133 |
|
2,288 |
|
|
| thereof COVID-19 |
— |
|
1,973 |
|
|
| OTHER REVENUES FROM CONTRACTS WITH CUSTOMERS |
5,622 |
|
8,075 |
|
|
In the year ended December 31, 2024, total other revenues were €5.6 million, a decrease of €2.5 million compared to €8.1 million in the year ended December 31, 2023. Revenues from shipping and handling increased in 2024 to €1.4 million due to a revised customer agreement that includes an additional charge for freight costs. Revenues from milestone payments and licenses decreased by €2.9 million in 2024 compared to 2023, mainly resulting from lower revenue recognition related to the R&D collaboration activities for chikungunya with Instituto Butantan in Brazil.The amount in the year ended December 31, 2023 included recognition of COVID-related revenues stemming from the previous COVID-19 vaccine supply agreement with the Kingdom of Bahrain.
Operating Income and Expenses
Cost of Goods and Services
Cost of goods and services (COGS) decreased by €2.3 million, or 2.3%, to €98.5 million for the year ended December 31, 2024. The slight reduction in cost of goods and services was primarily due to lower batch write-offs for failed batches in manufacturing, reductions in IXIARO related royalty payments and lower cost of services related to the Pfizer VLA-15 collaboration with Pfizer.
The gross margin on commercial product sales amounted to 50.6% in the year ended December 31, 2024 compared to 46.0% in the year ended December 31, 2023. COGS of €36.7 million related to IXIARO product sales, yielding a product gross margin of 61.0%. COGS of €19.8 million related to DUKORAL product sales, yielding a product gross margin of 38.7%. Of the remaining COGS in 2024, €22.3 million related to the third-party products distribution business, €7.2 million to IXCHIQ and €8.5 million to cost of services. In 2023, overall COGS were €100.9 million, of which €90.7 million related to cost of goods and €10.2 million related to cost of services. In 2023, COGS of the COVID-19 vaccine program amounted to €5.3 million.
Research and Development Expenses
Research and development expenses increased by €14.2 million, or 23.8%, to €74.1 million for the year ended December 31, 2024 from €59.9 million in the year ended December 31, 2023. Research and development expenses were 28% of our total operating expenses for the year ended December 31, 2024. This increase was mainly resulting from Tech transfer activities performed in relation to IXIARO between the two manufacturing sites in Scotland. Reductions due to the discontinuation of the COVID-19 vaccine, VLA2001, partly offset the overall increase in research and development expenses.
For the year ended December 31, 2024, research and development expenses consisted primarily of i) €25.5 million of employee-related expenses, consisting of wages, salaries, social security and pension costs, and share-based compensation paid to employees in research and development functions, ii) €25.5 million of external research and development services, including costs for clinical studies and external manufacturing, and iii) €8.5 million of material consumption.
For the year ended December 31, 2023, research and development expenses consisted primarily of i) €20.2 million of employee-related expenses, consisting of wages, salaries, social security and pension costs, and share-based compensation paid to employees in research and development functions, ii) €30.1 million of external research and development services, including costs for clinical studies and external manufacturing, and iii) €3.5 million of material consumption.
We track our research and development expenses by product or development program. The following table sets forth our research and development expenses by product or development program for the periods indicated:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
| IXCHIQ |
31,237 |
|
31,953 |
|
| IXIARO |
14,358 |
|
1,175 |
|
| Zika vaccine candidate (VLA1601) |
11,307 |
|
12,828 |
|
| Shigella vaccine |
6,121 |
|
— |
|
| EBV |
4,065 |
|
2,565 |
|
| DUKORAL |
838 |
|
875 |
|
| Lyme borreliosis vaccine candidate (VLA15) |
239 |
|
277 |
|
| Human metapneumovirus vaccine candidate (VLA1554) |
170 |
|
739 |
|
| VLA2001 |
78 |
|
5,796 |
|
| Other research projects (*) |
5,729 |
|
3,685 |
|
| TOTAL RESEARCH AND DEVELOPMENT EXPENSES |
74,143 |
|
59,894 |
|
* In 2024 and 2023, other research projects included €2.5 million and €1.4 million of expenses related to IFRS 2 (share-based and cash-based compensation) programs, which have not been allocated to the projects.
IXCHIQ. Our research and development expenses related to our chikungunya vaccine IXCHIQ decreased by €0.7 million, or 2.2%, to €31.2 million in the year ended December 31, 2024 from €32.0 million in the year ended December 31, 2023. The majority of our research and development activities have shifted towards Phase 4 obligations after the approval of the vaccine by the FDA in November 2023 and adolescents and pediatrics studies.
VLA1601. Our research and development expenses related to our Zika vaccine candidate program decreased in the year ended December 31, 2024 by €1.5 million, or 11.9%, to €11.3 million from €12.8 million in the year ended December 31, 2023. In 2023, we re-initiated clinical development of the program, including manufacturing of clinical trial materials, which resulted higher costs than we incurred in 2024.
VLA2001. Our research and development expenses related to our COVID-19 vaccine program decreased by €5.7 million, or 98.6%, to €0.1 million in the year ended December 31, 2024 from €5.8 million in the year ended December 31, 2023. This decrease was a result of discontinuing the program.
IXIARO. Our research and development expenses related to IXIARO increased to €14.4 million for the year ended December 31, 2024 from €1.2 million for the year ended December 31, 2023. This increase resulted from technology transfer activities performed in Scotland during 2024.
VLA15. Our research and development expenses remained relatively unchanged. In 2024, Lyme disease clinical studies for Phase 3 of €7.2 million were included in COGS, since these studies were related to the Pfizer partnership accounted under IFRS15 .
Marketing and Distribution Expenses
Marketing and distribution expenses for the year ended December 31, 2024 amounted to €52.4 million compared to €48.8 million in 2023, which mainly related to higher staff costs to support product sales growth across the direct markets. The employee-related expenses were positively affected in 2023 by the release of the employer contribution provision and therefore an income to the social security contributions. Marketing and distribution expenses comprised 20% of our total operating expenses for the year ended December 31, 2024, compared to 19% of our total operating expenses for the year ended December 31, 2023.
For the year ended December 31, 2024, marketing and distribution expenses consisted primarily of €17.3 million of employee-related expenses, including salaries, social security contributions, share-based compensation income/expense, and other employee-related expenses, €16.8 million of advertising expenses, including media and public relations expenses, €5.8 million of warehousing and distribution costs, and €6.0 million of expenses related to third-party services.
For the year ended December 31, 2023, marketing and distribution expenses consisted primarily of €13.1 million of employee-related expenses, including salaries, social security contributions, share-based compensation expense and other employee-related expenses, €13.4 million of advertising expenses, including media and public relations expenses, €3.9 million of warehousing and distribution costs, and €11.2 million of costs related to third-party services.
General and Administrative Expenses
General and administrative expenses decreased by €5.0 million, or 11%, to €42.8 million for the year ended December 31, 2024 from €47.8 million for the year ended December 31, 2023. General and administrative expenses comprised 16% of our total operating expenses for the year ended December 31, 2024 compared to 19% of our total operating expenses for the year ended December 31, 2023.
For the year ended December 31, 2024, general and administrative expenses consisted primarily of €20.5 million of employee-related expenses (salaries, social security contributions, share-based compensation expense and other employee-related expenses paid to employees), as well as of €16.7 million in costs and fees for professional services, such as consulting, legal and financial services. For the year ended December 31, 2023, general and administrative expenses consisted primarily of €21.1 million of non-research and development employee-related expenses, consisting of salaries, social security contributions, share-based compensation expense, and other employee-related expenses paid to employees in general and administrative functions, as well as of €21.8 million in costs and fees for professional services, such as consulting, legal, and financial services. The employee-related expenses were positively affected in 2023 by the release of the employer contribution provision and therefore an income to the social security contributions.
Expenses by Nature
The table below summarizes our cost of goods and services, research and development expenses, marketing and distribution expenses, and general and administrative expenses by nature of cost:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
| Employee benefit expense other than share-based compensation (*) |
83,028 |
|
72,997 |
|
| Consulting and other purchased services |
65,783 |
|
80,988 |
|
| Raw materials and consumables used |
21,982 |
|
14,113 |
|
| Depreciation and amortization and impairment |
19,586 |
|
16,853 |
|
| Advertising costs |
16,781 |
|
13,361 |
|
| Building and energy costs |
13,908 |
|
13,088 |
|
| Cost of services and change in inventory |
13,681 |
|
11,417 |
|
| Share-based compensation expense |
8,710 |
|
6,276 |
|
| Supply, office and IT costs |
7,682 |
|
11,663 |
|
| Warehousing and distribution costs |
5,790 |
|
3,939 |
|
| License fees and royalties |
4,065 |
|
5,492 |
|
| Other expenses |
3,593 |
|
4,432 |
|
| Travel and transportation costs |
3,197 |
|
2,700 |
|
| OPERATING EXPENSES |
267,788 |
|
257,320 |
|
The increase in operating expenses of €10.5 million from €257.3 million in the year ended December 31, 2023 to €267.8 million in the year ended December 31, 2024 primarily resulted from higher employee benefit expenses with 2023 positively impacted by a release of the provision of employer contribution charges resulting from decreasing share price. The increase in raw materials and consumables is mainly driven by clinical trial materials and consumables used for IXCHIQ trials In addition, we incurred higher advertising expenses related to continuing launch activities for IXCHIQ across the United States, Canada, and some European markets and higher depreciation charges related to the completion of the Almeida manufacturing facility in Scotland.
Expenses for “consulting and other purchased services” reduced substantially in the year ended December 31, 2024, due to lower service fees, for clinical studies related to research and development of the Zika vaccine candidate and lower expenditures on the COVID-19 vaccine, VLA2001.
The increase of “advertising costs” in the year ended December 31, 2024 compared to the year ended December 31, 2023 was driven by the launch of IXCHIQ in different markets.
Gain from sale of Priority Review Voucher, net
The Company sold the PRV received from the FDA for $103 million (€95 million) on February 2, 2024.
The Company was awarded a tropical disease PRV in November 2023 following the FDA’s approval of IXCHIQ, Valneva’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus.
The net gain from the sale of the PRV amounted to €90.8 million, after deducting expenses in the amount of €4.2 million, which included transaction fees as well as expenses in connection with contractual payment obligations related to the PRV sale.
Other Income (Expenses), net
The table below summarizes the other operating income (expenses), net for the years ended December 31, 2024 and 2023:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
| Research and development tax credit |
10,028 |
|
6,797 |
|
| Grant income |
10,194 |
|
11,350 |
|
| Gain/(loss) on disposal of fixed assets and intangible assets, net |
(445) |
|
(21) |
|
| Gain/(loss) from revaluation of lease agreements |
711 |
|
45 |
|
| Taxes, duties, fees, charges, other than income tax |
(346) |
|
(475) |
|
| Miscellaneous income/(expenses), net |
564 |
|
3,824 |
|
| OTHER INCOME AND EXPENSES, NET |
20,706 |
|
21,520 |
|
Other operating income and expenses, net decreased by €0.8 million, or 4%, to €20.7 million for the year ended December 31, 2024 from €21.5 million for the year ended December 31, 2023.
In the year ended December 31, 2024, research and development tax credit increased to €10.0 million from €6.8 million in the year ended December 31, 2023. This was mainly a result of tax credits received in Scotland in relation to eligible development activities performed on the Scottish site.
In the year ended December 31, 2024, grant income decreased as activities related to the first chikungunya-related grant received by CEPI were completed and spending related to the second grant obtained in 2024 ramped up during the second half of 2024, resulting in a net decrease.
The decrease in the “miscellaneous income/(expenses), net” in the year ended December 31, 2024 is due mainly to the one-time income of €4.7 million recognized in the year ended December 31, 2023, which was related to a settlement with a supplier in connection with COVID-19 activities and the one-time expense of €1.4 million related to the divestment of the CTM unit in Solna.
Financial Income (Expense)
The table below summarizes our financial income (expense) for the years ended December 31, 2024 and 2023:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2024 |
2023 |
|
|
|
|
|
| Interest income from other parties |
2,362 |
|
1,210 |
|
|
| Fair value gains on derivative financial instruments |
— |
|
— |
|
|
| TOTAL FINANCE INCOME |
2,362 |
|
1,210 |
|
|
|
|
|
|
| Interest expense on loans |
(22,808) |
|
(13,681) |
|
|
| Interest expense on refund liabilities |
(360) |
|
(8,419) |
|
|
| Interest expenses on lease liabilities |
(813) |
|
(1,183) |
|
|
| Other interest expense |
(3) |
|
(42) |
|
|
| Fair value losses on derivative financial instruments |
— |
|
— |
|
|
| TOTAL FINANCE EXPENSES |
(23,984) |
|
(23,325) |
|
|
| FOREIGN EXCHANGE GAIN/(LOSSES), NET |
(3,193) |
|
5,574 |
|
|
| FINANCE INCOME/(EXPENSES), NET |
(24,816) |
|
(16,541) |
|
|
Finance expenses, net were €24.8 million for the year ended December 31, 2024 compared to net expenses of €16.5 million for the year ended December 31, 2023. This increase in finance income/expenses, net was mainly due to foreign exchange losses in the year ended December 31, 2024, primarily related to the appreciation of the USD exchange rate against the Euro affecting our corresponding balance sheet accounts (liabilities and borrowings denominated in USD), whereas in the year ended December 31, 2023, the movements of the USD against the Euro resulted in profits incurred during that period.
The increase in interest expense on loans is due to the increase in Valneva’s average loan principal in 2024 compared to 2023. In the year ended December 31, 2023 further tranches of the D&O Loan Agreement were drawn. The interest expense on refund liabilities for the year ended December 31, 2024 decreased to €0.4 million as significant payments were made to Pfizer during the second half of 2023 and payment obligations were fulfilled during the first half of 2024.The interest expense on refund liabilities for the year ended December 31, 2023 of €8.4 million was mainly caused by accumulated payment deferrals related to the Pfizer agreement.
Income Tax
We recorded €0.8 million of income tax expenses for the year ended December 31, 2024 compared to €2.8 million of income tax gains for the year ended December 31, 2023. This change in income tax was primarily driven by deferred tax income in 2023 due to high impairment charges.
Profit/(Loss) for the Period
Our loss for the period ended December 31, 2024 was €12.2 million, decreased from a loss of €101.4 million for the period ended December 31, 2023. The improvement was mainly a result of the sale of the priority review voucher (PRV) received following the licensure of IXCHIQ by the U.S. FDA. The loss in 2023 was primarily driven by one-off expenses of goods and services related to valuation of inventory and onerous agreement provisions for material in connection with our COVID-19 vaccine and the discontinuation of this program.
Adjusted EBITDA
Our Adjusted EBITDA profit was €32.9 million for the year ended December 31, 2024 compared to a loss of €65.2 million for the year ended December 31, 2023. The Adjusted EBITDA loss improved by €98.1 million, which was primarily driven by the lower net loss. A reconciliation of Adjusted EBITDA to net loss, the most directly comparable IFRS measure, is set forth below:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
| PROFIT/(LOSS) FOR THE PERIOD |
(12,247) |
|
(101,429) |
|
| Add: |
|
|
| Income tax (benefits)/expense |
761 |
|
2,800 |
|
| Total finance income |
(2,362) |
|
(1,210) |
|
| Total finance expense |
23,984 |
|
23,325 |
|
| Foreign currency (gain)/loss – net |
3,193 |
|
(5,574) |
|
|
|
|
| Amortization |
4,881 |
|
5,831 |
|
| Depreciation |
14,705 |
|
11,753 |
|
| Impairment |
— |
|
(731) |
|
| ADJUSTED EBITDA |
32,916 |
|
(65,234) |
|
Comparisons for the Years Ended December 31, 2023 and 2022
Revenue
Consolidated Revenue
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2023 |
2022 |
|
| Product sales |
144,624 |
|
114,797 |
|
|
| Other revenues from contracts with customers |
8,075 |
|
245,709 |
|
|
| Other non-IFRS 15 revenue |
1,014 |
|
797 |
|
|
| REVENUES |
153,713 |
|
361,303 |
|
|
Revenue decreased by €207.6 million, or 57%, to €153.7 million for the year ended December 31, 2023 compared to €361.3 million for the year ended December 31, 2022. The decrease was mainly related to one-off revenues recorded in the prior year linked to our COVID-19 program, which has been discontinued.
Our total product sales reached €144.6 million for the year ended December 31, 2023, compared to €114.8 million in the same period of 2022. This 26% increase was driven by the continued recovery of travel vaccine sales. Currency fluctuations of €2.8 million adversely impacted product sales.
Product sales
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2023 |
2022 |
|
| IXIARO |
73,483 |
|
41,349 |
|
|
| DUKORAL |
29,775 |
|
17,334 |
|
|
| Third party products |
35,675 |
|
26,545 |
|
|
|
|
|
|
| VLA2001 |
5,691 |
|
29,568 |
|
|
| PRODUCT SALES |
144,624 |
|
114,797 |
|
|
IXIARO product sales were €73.5 million in the year ended December 31, 2023 compared to €41.3 million in 2022. The 78% increase in sales is primarily the result of the continued travel market recovery, as well as price increases. The increase in IXIARO product sales included an adverse €1.5 million foreign currency impact.
DUKORAL sales were €29.8 million in the year ended December 31, 2023 compared to €17.3 million in the same period of 2022. This 72% increase is also a result of the significant recovery in the private travel markets and price increases. Foreign currency fluctuations reduced DUKORAL sales by €0.9 million.
Third party product sales were €35.7 million in the year ended December 31, 2023 compared to €26.5 million in the comparison period of 2022, a 34% increase which was mainly driven by sales of Rabipur/RabAvert and Encepur under the distribution agreement with Bavarian Nordic.
COVID-19 vaccine sales in 2023 amounted to €5.7 million compared to €29.6 million in 2022, which was a result of our decision to suspend the program.
Product Sales—By Geography
We also monitor product sales generated in the countries and regions where we operate. The following table presents product sales by geography and is based on the final location where our distribution partner sells the product or where the customer or partner is located.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2023 |
2022 |
|
| United States |
32,606 |
|
21,992 |
|
|
| Canada |
28,193 |
|
18,904 |
|
|
| Germany |
13,238 |
|
20,341 |
|
|
| Nordics |
12,426 |
|
8,560 |
|
|
| United Kingdom |
20,216 |
|
10,901 |
|
|
| Austria |
13,915 |
|
13,749 |
|
|
| France |
5,303 |
|
2,625 |
|
|
| Other Europe |
8,168 |
|
6,245 |
|
|
| Rest of World |
10,560 |
|
11,480 |
|
|
| PRODUCT SALES |
144,624 |
|
114,797 |
|
|
|
|
|
|
Total product sales in the United States increased by €10.6 million, or 48%, to €32.6 million in the year ended December 31, 2023, compared to €22.0 million in the year ended December 31, 2022. The increase was primarily a result of higher sales to the U.S. DLA and higher demand in private markets.
Product sales in Canada increased by €9.3 million, or 49%, from €18.9 million in the year ended 2022, to €28.2 million in 2023 as a result of the significant recovery in the private travel markets.
Sales in the United Kingdom increased by €9.3 million, or 85%, to €20.2 million in the year ended December 31, 2023 due to continued resumption of travel positively impacting sales of our travel vaccines and of product distributed for third parties.
Other revenues from contacts with customers
The following table presents our other revenues (from collaboration, licensing, and services), for the years ended December 31, 2023 and 2022.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2023 |
2022 |
|
| Royalties received |
2,129 |
|
843 |
|
|
| Revenues from shipping and handling |
21 |
|
21 |
|
|
| Milestone payment - licenses |
3,637 |
|
(38,805) |
|
|
| Other services |
2,288 |
|
283,650 |
|
|
| thereof COVID-19 |
1,973 |
|
280,010 |
|
|
| OTHER REVENUES FROM CONTRACTS WITH CUSTOMERS |
8,075 |
|
245,709 |
|
|
In the year ended December 31, 2023, total other revenues were €8.1 million, a decrease of €237.6 million compared to €245.7 million in the year ended December 31, 2022. The amount in the year ended December 31, 2022 included recognition of COVID-related revenues stemming from previous COVID-19 vaccine supply agreements with the UK Authority and the European Commission.
In the year ended December 31, 2022, €45,9 million of negative revenue were recorded for the Lyme disease vaccine candidate, primarily resulting from a reversal of revenue from amendments of the Collaboration and License Agreement with Pfizer.
Operating Income and Expenses
Cost of Goods and Services
Cost of goods and services (COGS) decreased by €223.6 million, or 68.9%, to €100.9 million for the year ended December 31, 2023. The significant reduction in cost of goods was primarily due to impairment and scrap of short-dated or expired product in 2022 related to the wind down of COVID-19 manufacturing.
The gross margin on commercial product sales amounted to 46.0% in the year ended December 31, 2023 compared to 45.5% in the year ended December 31, 2022. COGS of €35.1 million related to IXIARO product sales, yielding a product gross margin of 52.3%. The IXIARO gross margin was impacted by batch write-offs in the Scottish manufacturing site. COGS of €17.1 million related to DUKORAL product sales, yielding a product gross margin of 42.4%. Of the remaining COGS in 2023, €22.8 million related to the third-party products distribution business, €5.3 million to VLA2001 and €10.2 million to cost of services. In 2022, overall COGS were €324.4 million, of which €314.7 million related to cost of goods and €9.7 million related to cost of services. In 2022, COGS of the COVID-19 vaccine program amounted to €267.1 million and included effects from the significant reduction of sales volumes to the European Union Member States which resulted in impairment of fixed assets and inventories.
Research and Development Expenses
Research and development expenses decreased by €45.0 million, or 42.9%, to €59.9 million for the year ended December 31, 2023 from €104.9 million in the year ended December 31, 2022. Research and development expenses were 23% of our total operating expenses for the year ended December 31, 2023. This decrease was exclusively driven by the lower spend on our COVID-19 vaccine, VLA2001 due to discontinuation of the program while we intensified our work on IXCHIQ leading in sum to 25.0% increased expenses of €32.0 million for IXCHIQ as of December 31, 2023. Further, costs related to the Zika vaccine candidate increased as we have been working towards re-initiation of clinical development.
For the year ended December 31, 2023, research and development expenses consisted primarily of i) €20.2 million of employee-related expenses, consisting of wages, salaries, social security and pension costs, and share-based compensation paid to employees in research and development functions, ii) €30.1 million external research and development services, including costs for clinical studies and external manufacturing, and iii) €3.5 million of material consumption.
For the year ended December 31, 2022, research and development expenses consisted primarily of i) €12.5 million of employee-related expenses, consisting of wages, salaries, social security and pension costs, and share-based compensation paid to employees in research and development functions, ii) €59.1 million of external research and development services, including costs for clinical studies and external manufacturing, and iii) €7.8 million of material consumption. In 2022, employee-related expenses profited from a favorable effect of our share price development on the employee share-based compensation programs.
We track our research and development expenses by product or development program. The following table sets forth our research and development expenses by product or development program for the periods indicated:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2023 |
2022 |
|
| IXCHIQ |
31,953 |
|
25,558 |
|
|
| Zika vaccine candidate (VLA1601) |
12,828 |
|
2,143 |
|
|
| VLA2001 |
5,796 |
|
72,762 |
|
|
| IXIARO |
1,175 |
|
504 |
|
|
| DUKORAL |
875 |
|
563 |
|
|
| Human metapneumovirus vaccine candidate (VLA1554) |
739 |
|
1,562 |
|
|
| Lyme borreliosis vaccine candidate (VLA15) |
277 |
|
1,016 |
|
|
| EBV |
2,565 |
|
— |
|
|
| Other research projects (*) |
3,685 |
|
815 |
|
|
| TOTAL RESEARCH AND DEVELOPMENT EXPENSES |
59,894 |
|
104,922 |
|
|
* In 2023 and 2022, other research projects included €1.4 million of expenses and €1.3 million of income respectively, related to IFRS 2 (share-based and cash-based compensation) programs, which have not been allocated to the projects.
VLA1553. Our research and development expenses related to our chikungunya vaccine IXCHIQ increased by €6.4 million, or 25.0%, to €32.0 million in the year ended December 31, 2023 from €25.6 million in the year ended 2022. This increase related to the progress of the chikungunya vaccine towards BLA submission and ultimately U.S. licensure in November 2023.
VLA1601. Our research and development expenses related to our Zika vaccine candidate program increased in the year ended December 31, 2023 by €10.7 million, or 499%, to €12.8 million from €2.1 million in the year ended December 31, 2022. The increase was caused by our re-initiation of clinical development of the program in 2023.
VLA2001. Our research and development expenses related to our COVID-19 vaccine program decreased by €67.0 million, or 92%, to €5.8 million in the year ended December 31, 2023 from €72.8 million in the year ended December 31, 2022. This decrease was primarily driven by reduced clinical study costs due to the progress of the program as well as wind-down activities.
VLA15. Our research and development expenses related to our Lyme disease vaccine candidate program decreased by €0.7 million, or 73%, to €0.3 million in the year ended December 31, 2023 from €1.0 million in the year ended December 31, 2022. This decrease was primarily driven by the completion of our Phase 2 clinical studies. In 2023, Lyme disease clinical studies for Phase 2 and Phase 3 of €8.2 million were included in COGS, as these studies were related to the Pfizer partnership.
Our research and development expenses for our commercial products and the rest of our development pipeline increased by €5.6 million, or 163%, to €9.0 million in the year ended December 31, 2023 from €3.4 million in the year ended December 31, 2022. This increase was primarily related to increased expenses related to our pre-clinical stage programs as well as IFRS 2.
Marketing and Distribution Expenses
Marketing and distribution expenses for the year ended December 31, 2023 amounted to €48.8 million compared to €23.5 million in 2022, which mainly related to €20.7 million of expenses for the launch preparations for the chikungunya vaccine candidate, VLA1553, compared to €7.3 million in the year ended December 31, 2022. The employee-related expenses were positively affected in 2022 by the release of the employer contribution provision and therefore an income to the social security contributions. Marketing and distribution expenses comprised 19% of our total operating expenses for the year ended December 31, 2023, compared to 5% of our total operating expenses for the year ended December 31, 2022.
For the year ended December 31, 2023, marketing and distribution expenses consisted primarily of €13.1 million of employee-related expenses, including salaries, social security contributions, share-based compensation income/expense, and other employee-related expenses, €13.4 million of advertising expenses, including media and public relations expenses, €3.9 million of warehousing and distribution costs, and €11.2 million of expenses related to third-party services.
For the year ended December 31, 2022, marketing and distribution expenses consisted primarily of €3.4 million of employee-related expenses, including salaries, social security contributions, share-based compensation expense and other employee-related expenses, €7.3 million of advertising expenses, including media and public relations expenses, €1.9 million of warehousing and distribution costs, and €5.4 million of costs related to third-party services. The employee-related expenses were positively affected in 2022 by the release of the employer contribution provision and therefore an income to the social security contributions.
General and Administrative Expenses
General and administrative expenses increased by €13.7 million, or 40%, to €47.8 million for the year ended December 31, 2023 from €34.1 million for the year ended December 31, 2022. General and administrative expenses comprised 19% of our total operating expenses for the year ended December 31, 2023 compared to 7% of our total operating expenses for the year ended December 31, 2022. This increase was primarily driven by a positive effect in 2022 from the release of an employer contribution provision on share-based compensation plans due to the decrease of the share price.
For the year ended December 31, 2023, general and administrative expenses consisted primarily of €21.1 million of employee-related expenses (salaries, social security contributions, share-based compensation expense and other employee-related expenses paid to employees), as well as of €21.8 million in costs and fees for professional services, such as consulting, legal and financial services. For the year ended December 31, 2022, general and administrative expenses consisted primarily of €11.6 million of non-research and development employee-related expenses, consisting of salaries, social security contributions, share-based compensation expense, and other employee-related expenses paid to employees in general and administrative functions, as well as of €18.5 million in costs and fees for professional services, such as consulting, legal, and financial services. The employee-related expenses were positively affected in 2022 by the release of the employer contribution provision and therefore an income to the social security contributions.
Expenses by Nature
The table below summarizes our cost of goods and services, research and development expenses, marketing and distribution expenses, and general and administrative expenses by nature of cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2023 |
2022 |
| Consulting and other purchased services |
80,988 |
|
141,631 |
|
| Cost of services and change in inventory |
11,417 |
|
190,086 |
|
| Employee benefit expense other than share-based compensation (*) |
72,997 |
|
56,393 |
|
| Share-based compensation expense |
6,276 |
|
(5,215) |
|
| Raw materials and consumables used |
14,113 |
|
12,723 |
|
| Depreciation and amortization and impairment |
16,853 |
|
44,285 |
|
| Building and energy costs |
13,088 |
|
14,696 |
|
| Supply, office and IT costs |
11,663 |
|
11,739 |
|
| License fees and royalties |
5,492 |
|
6,830 |
|
| Advertising costs |
13,361 |
|
7,343 |
|
| Warehousing and distribution costs |
3,939 |
|
1,898 |
|
| Travel and transportation costs |
2,700 |
|
2,208 |
|
| Other expenses |
4,432 |
|
2,329 |
|
| OPERATING EXPENSES |
257,320 |
|
486,945 |
|
|
|
|
* As of December 31, 2022, the position “employee benefit expense other than share-based compensations” includes an amount of €23.2 million resulting from the release of the provision of employer contribution charges fees, which are payable at the exercise of the share-based payment programs.
The decrease in operating expenses of €229.6 million from €486.9 million in the year ended December 31, 2022 to €257.3 million in the year ended December 31, 2023 primarily resulted from one-off expenses recorded in the prior year related to the suspended COVID-19 program. These expenses included the write-down of COVID-19 vaccine inventory of €159.4 million (presented under “cost of services and change in inventory”) as well as impairment charges of fixed assets, leading to a total expense of €44.3 million for depreciation, amortization, and impairment charges.
Expenses for “consulting and other purchased services” reduced substantially in the year ended December 31, 2023, as the comparison period of 2022 included considerable expenses for COVID-19 related to research and development and external manufacturing costs.
Expenses for “cost of services and change in inventory” strongly decreased in 2023, as in the year ended 2022 effects from the significant changes to the ordered volumes and the expected future demand for VLA2001, in particular a write-down of inventory of €159.4 million, were recorded.
The expense position “depreciation and amortization and impairment” contains a reversal of a fixed asset impairment in the amount of €1.9 million related to production equipment in the year ended December 31, 2023, whereas 2022 included one-off charges of €14.8 million for the impairment of COVID-19 related fixed assets including idle manufacturing equipment, leasehold improvements and Right of Use assets.
“Employee benefit expenses other than share-based compensation” increased in the year ended December 31, 2023 compared to December 31, 2022 because of a €23.2 million release of the employer contribution provision and therefore an income to the social security contributions in 2022. In the same year “Share-based compensation expense” showed an income due to share-based payment program valuations resulting from the reduction in the share price.
Other Income (Expenses)
The table below summarizes the other operating income (expenses) for the years ended December 31, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2023 |
2022 |
| Research and development tax credit |
6,797 |
|
15,348 |
|
| Grant income |
11,350 |
|
191 |
|
| Gain/(loss) on disposal of fixed assets and intangible assets, net |
(21) |
|
(38) |
|
| Gain/(loss) from revaluation of lease agreements |
45 |
|
(32) |
|
| Taxes, duties, fees, charges, other than income tax |
(475) |
|
(217) |
|
| Miscellaneous income/(expenses), net |
3,824 |
|
(3,054) |
|
| OTHER INCOME AND EXPENSES, NET |
21,520 |
|
12,199 |
|
|
|
|
Other operating income and expenses, net increased by €9.3 million, or 76%, to €21.5 million for the year ended December 31, 2023 from €12.2 million for the year ended December 31, 2022.
In the year ended December 31, 2023, grant income increased due to the recognition of grant income received from Scottish Enterprise in the amount of €11.1 million. The research and development tax credit decrease was linked to the decrease of our research and development expenses. As in 2022, €13.9 million related to the research and development programs executed in Austria, mainly for the COVID-19 and chikungunya vaccine (candidates), were recognized.
In the “Miscellaneous income/(expenses), net”, in the year ended December 31, 2023, a gain from a final settlement with a supplier in connection with COVID-19 activities was recognised in the amount of €4.7 million, partly offset by a loss of €1.4 million from the divestment of the CTM Unit in Solna. A €0.3 million gain from the sale of our equity investment BliNK Biomedical SAS was recorded. In the year ended December 31, 2022, this position was negatively impacted by an increase in a litigation provision in the amount of €3.1 million.
Financial Income (Expense)
The table below summarizes our financial income (expense) for the years ended December 31, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
2023 |
2022 |
|
| FINANCE INCOME |
|
|
|
| Interest income from other parties |
1,210 |
|
260 |
|
|
| Fair value gains on derivative financial instruments |
— |
|
— |
|
|
| TOTAL FINANCE INCOME |
1,210 |
|
260 |
|
|
| FINANCE EXPENSES |
|
|
|
| Interest expense on loans |
(13,681) |
|
(8,238) |
|
|
| Interest expense on refund liabilities |
(8,419) |
|
(9,597) |
|
|
| Interest expenses on lease liabilities |
(1,183) |
|
(955) |
|
|
| Other interest expense |
(42) |
|
(264) |
|
|
|
|
|
|
| TOTAL FINANCE EXPENSES |
(23,325) |
|
(19,054) |
|
|
| FOREIGN EXCHANGE GAIN/(LOSSES), NET |
5,574 |
|
(12,587) |
|
|
| FINANCE INCOME/(EXPENSES), NET |
(16,541) |
|
(31,381) |
|
|
|
|
|
|
Finance expenses, net were €16.5 million for the year ended December 31, 2023 compared to net expenses of €31.4 million for the year ended December 31, 2022. This decrease in finance income/expenses, net was mainly due to foreign exchange gains in the year ended December 31, 2023, primarily related to the depreciation of the USD and GBP exchange rates against the Euro affecting our corresponding balance sheet accounts (liabilities and borrowings denominated in USD), whereas in the year ended December 31, 2022, the appreciation of the USD and the GBP against the Euro resulted in losses incurred during that period.
Income Tax
We recorded €2.8 million of income tax expenses for the year ended December 31, 2023 compared to €1.5 million of income tax gains for the year ended December 31, 2022. This change in income tax was primarily driven by deferred tax income in 2022 due to high impairment charges.
Profit/(Loss) for the Period
Our loss for the period ended December 31, 2023 was €101.4 million, decreased from a loss of €143.3 million for the period ended December 31, 2022. The higher loss in 2022 was primarily driven by one-off expenses of goods and services related to valuation of inventory and onerous agreement provisions for material in connection with our COVID-19 vaccine and its program suspension.
Adjusted EBITDA
Our Adjusted EBITDA loss was €65.2 million for the year ended December 31, 2023 compared to a loss of €69.2 million for the year ended December 31, 2022. The Adjusted EBITDA loss decreased by €3.9 million, which was primarily driven by a lower net loss largely offset by high impairment losses in 2022.A reconciliation of Adjusted EBITDA to net loss, the most directly comparable IFRS measure, is set forth below:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2023 |
2022 |
| PROFIT/(LOSS) FOR THE PERIOD |
(101,429) |
|
(143,279) |
|
| Add: |
|
|
| Income tax (benefits)/expense |
2,800 |
|
(1,536) |
|
| Total finance income |
(1,210) |
|
(260) |
|
| Total finance expense |
23,325 |
|
19,054 |
|
| Foreign currency (gain)/loss – net |
(5,574) |
|
12,587 |
|
| Result from investments in associates |
— |
|
(9) |
|
| Amortization |
5,831 |
|
7,024 |
|
| Depreciation |
11,753 |
|
14,012 |
|
| Impairment |
(731) |
|
23,249 |
|
| ADJUSTED EBITDA |
(65,234) |
|
(69,159) |
|
B. Liquidity and Capital Resources.
Overview
We have financed our operations primarily through a combination of equity offerings, secured debt, and revenues from product sales. As at December 31, 2024, we had €168.3 million in cash and cash equivalents. Based upon our current operating plan, we believe that our existing cash and cash equivalents as of December 31, 2024 will fund our current operating plans for at least the next 12 months following the publication of the full-year 2024 financial statements.
Sources and Uses of Cash
We have financed our operations through revenue from product sales, payments under historical collaborative research alliances, as well as research tax credits and subsidies granted by various public institutions. In addition, we have borrowed secured debt to finance our operations.
In June 2022, we signed an Equity Subscription Agreement with Pfizer. Pursuant to the Equity Subscription Agreement, Pfizer invested €90.5 million ($95 million) in Valneva, representing 8.1% of Valneva’s share capital at a price of €9.49 per share. The per share purchase price was determined based on the average closing price of the Company’s shares on Euronext Paris during the 10 trading days preceding the date of the Equity Subscription Agreement.
On August 12, 2022, we entered into an Open Market Sale Agreement, or the Sales Agreement, with Jefferies LLC, or Jefferies, pursuant to which we may issue and sell ADSs, each representing two ordinary shares, having an aggregate offering price of up to $75,000,000 (subject to French regulatory limits), from time to time, in one or more at-the-market offerings, for which Jefferies will act as sales agent and/or principal. The at-the-market facility has been registered under the Securities Act pursuant to our Registration Statement on Form F-3 (File No. 333-266839). As of December 31, 2024, no issuances or sales had been made pursuant to the Sales Agreement.
In October 2022, we announced the closing of our global offering to specified categories of investors of an aggregate 21,000,000 new ordinary shares, consisting of a public offering of 375,000 ADSs, each representing two ordinary shares, in the United States at an offering price of $9.51 per ADS, and a concurrent private placement of 20,250,000 ordinary shares in Europe (including France) and other countries outside of the United States at the corresponding offering price of €4.90 per ordinary share. Gross proceeds of this global offering were €102.9 million, whereas related expenses of €7.4 million incurred.
In September 2024, we announced the private placement of a total of 23,000,000 new ordinary shares (the “Offer Shares”), each with a nominal value of €0.15, have been issued at a price of €2.66 each, without shareholders’ preferential subscription rights, to a limited number of institutional investors within the United States and outside of the United States to non-U.S. investors. We raised gross proceeds of €61.2 million from this global offering, while incurring related expenses of €4.0 million, resulting in net proceeds of €57.1 million.
As of December 31, 2024, we had borrowings and lease liabilities of €216.3 million, of which €187.4 million were other loans and €28.9 million were lease liabilities.
In February 2020, we entered into a debt financing agreement, or the Financing Agreement, with Deerfield and OrbiMed. The intended use of proceeds was to repay existing borrowings from the European Investment Bank and allow us to continue to advance our Lyme and chikungunya development programs in the short term. We have amended the Financing Agreement several times, most recently in March 2024. For further information about these amendments, refer to “Item 3.D—Risk Factors” and the Notes to our consolidated financial statements. As of December 31, 2024, $200.0 million (€192.5 million) was outstanding under the Financing Agreement of which a total of $100.0 million was drawn in two installments, in August and December 2023.
The loan bears interest at 9.95%. Due to the quarterly interest calculation method, the aggregate annual interest actually paid is an amount equivalent to 10.09%. The interest-only period for the initial tranches amounting to $100.0 million extends until the first quarter of 2026, and this portion of the loan will mature in the first quarter of 2027. The interest-only period for the tranches drawn in 2023 amounting to $100.0 million extends until the first quarter of 2027, and this portion of the loan will mature in the fourth quarter of 2028. The loan is secured by substantially all of our assets, including our intellectual property, and is guaranteed by Valneva SE and certain of its subsidiaries. The Financing Agreement contains covenants, including a minimum liquidity in the amount of €35.0 million and minimum consolidated net revenue in the amount of €115.0 million on a consecutive twelve month basis. If our consolidated liquidity or net revenues were to fall below the covenant minimum values, we would be in default, which may trigger various consequences. For example, the interest rate on the loans could increase by up to 10 additional interest points if the duration of the default is longer than 15 days, or we could be required to immediately repay the full principal amount of the loans, including all fees and interest associated with repayment. We do not expect the limitations of these covenants to affect our ability to meet our cash obligations.
In February 2022, we announced that Valneva Scotland had received two grants worth up to £20.0 million (approximately €23.9 million) from Scottish Enterprise to support research and development relating to the manufacturing processes of our COVID-19 vaccine and our other vaccine candidates. Following the termination of our COVID-19 vaccine program, in May 2023 the grant relating to this program was amended, reducing the available funding from £7.5 million (€9.0 million) to nil. In 2024, there were no amendments to the grants. The funds under the remaining grant will be received over three years, beginning in March 2022. Valneva SE has provided a parent guarantee in connection with these grants, and if we fail to comply with the terms of the grants, Scottish Enterprise may stop payments under the grants and require repayment of the funds provided to date. As of the date of this Annual Report, we have received for the Years Ended December 31, 2024 and 2023 respectively €3.7 million (£3.1 million) and €11.1 million (£9.6 million) of grant funds from Scottish Enterprise.
In November 2023, we obtained licensure of VLA1553, our chikungunya vaccine candidate, in the United States and consequently were awarded a Priority Review Voucher (PRV) from the US Food and Drug Administration (FDA). The PRV was sold in February 2024 and generated proceeds of $103 million.
As we continue to develop and commercialize our products and product candidates in the coming years, we will likely continue relying on some or all of these sources of financing, as well as potential milestone payments and royalties that may result from licensing agreements for our products and product candidates.
Cash Flows
Comparisons for the Years Ended December 31, 2024 and 2023
The table below summarizes our cash flows for the years ended December 31, 2024 and 2023:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| (In € thousand) |
2024 |
2023 |
| Net cash generated from/(used in) operating activities |
(67,218) |
|
(202,744) |
|
| Net cash generated from/(used in) investing activities |
76,916 |
|
(20,585) |
|
| Net cash generated from/(used in) financing activities |
30,682 |
|
63,081 |
|
| NET CHANGE IN CASH AND CASH EQUIVALENTS |
40,380 |
|
(160,248) |
|
Operating Activities
Net cash used in operating activities for the year ended December 31, 2024 was €67.2 million compared to €202.7 million of net cash used in the year ended December 31, 2023. Net cash used in operating activities for the year ended December 31, 2024 was primarily derived from the loss for the period amounting to €12.2 million and from decreases in working capital in the amount of €11.4 million, which largely were related to payments to Pfizer in conjunction with Valneva’s contribution to the Phase 3 costs of the Lyme VLA15 R&D program, reducing the refund liability. These amounts have been partly offset by adjustments for non-cash transactions of €49.0 million mostly for depreciation and amortization of tangible and intangible assets of €19.6 million and interest expenses of €24.0 million.
Net cash used in operating activities for the year ended December 31, 2023 was €202.7 million, which derived from the loss for the period, amounting to €101.4 million, and from decreases in working capital in the amount of €145.6 million, which largely were related to payments to Pfizer in conjunction with Valneva’s contribution to the Phase 3 costs of the Lyme VLA15 R&D program, reducing the refund liability. These amounts have been partly offset by adjustments for non-cash transactions of €45.0 million mostly for depreciation and amortization of tangible and intangible assets of €17.6 million and interest expenses of €23.3 million.
Investing Activities
Net cash generated from investing activities for the year ended December 31, 2024 was €76.9 million mainly resulting from proceeds received for the sale of the priority review voucher partly offset by €13.9 million purchases for property, plant and equipment. This compared to cash used in investing activities of €20.6 million for the year ended December 31, 2023 and was comprised primarily of €14.2 million purchases for property, plant and equipment as well as of the acquisition of the VBC3 building in Vienna.
Financing Activities
Net cash generated from financing activities was €30.7 million for the year ended December 31, 2024 compared to €63.1 million for the year ended December 31, 2023. Net cash generated from financing activities during the year ended December 31, 2024 was primarily due to €57.1 million of net proceeds from a private placement of ordinary shares. Interest payments amounting to €20.0 million reduced the net cash generated from financing activities.
Net cash generated from financing activities during the year ended December 31, 2023 was primarily due to €81.1 million of net proceeds from borrowings, namely the additional tranches from the financing agreement with Deerfield and OrbiMed drawn in the second half of the year. Interest payments amounting to €12.6 million reduced the net cash generated from financing activities.
Comparisons for the Years Ended December 31, 2023 and 2022
The table below summarizes our cash flows for the years ended December 31, 2023 and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| (In € thousand) |
2023 |
2022 |
| Net cash generated from/(used in) operating activities |
(202,744) |
|
(245,343) |
|
| Net cash generated from/(used in) investing activities |
(20,585) |
|
(29,054) |
|
| Net cash generated from/(used in) financing activities |
63,081 |
|
215,116 |
|
| NET CHANGE IN CASH AND CASH EQUIVALENTS |
(160,248) |
|
(59,282) |
|
|
|
|
Operating Activities
Net cash used in operating activities for the year ended December 31, 2023 was €202.7 million compared to €245.3 million of net cash used in the year ended December 31, 2022. Net cash used in operating activities for the year ended December 31, 2023 was primarily derived from the loss for the period amounting to €101.4 million and from decreases in working capital in the amount of €145.6 million, which largely were related to payments to Pfizer in conjunction with Valneva’s contribution to the Phase 3 costs of the VLA15 R&D program, reducing the refund liability. These amounts have been partly offset by adjustments for non-cash transactions of €45.0 million mostly for depreciation and amortization of tangible and intangible assets of €17.6 million and interest expenses of €23.3 million.
Net cash used in operating activities for the year ended December 31, 2022 was primarily derived from the loss for the period amounting to €143.3 million and from changes in non-current assets and liabilities in the amount of minus €147.7 million (which mainly related to refund liabilities recorded in line with the Pfizer agreement amendments as they are no longer non-current). These amounts have been partly offset by depreciation and amortization of €21.0 million as well as impairment of tangible and intangible assets of €23.2 million, interest expenses of €19.1 million, share-based compensation of minus €8.7 million, and by other non-cash expenses amounting to minus €9.2 million. Changes in working capital amounted to €1.7 million.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2023 was €20.6 million, compared to €29.1 million for the year ended December 31, 2022 and was comprised primarily of €14.2 million of purchases for property, plant and equipment as well as of the acquisition of the VBC3 building in Vienna.
Net cash used in investing activities for the year ended December 31, 2022 was €29.1 million, compared to €93.1 million for the year ended December 31, 2021 and was comprised primarily of €29.2 million of purchases for property, plant and equipment.
Financing Activities
Net cash generated from financing activities was €63.1 million for the year ended December 31, 2023 compared to €215.1 million for the year ended December 31, 2022. Net cash generated from financing activities during the year ended December 31, 2023 was primarily due to €81.1 million of net proceeds from borrowings, namely the additional tranches from the financing agreement with Deerfield and OrbiMed drawn in the second half of the year. Interest payments amounting to €12.6 million reduced the net cash generated from financing activities.
Net cash generated from financing activities for the year ended December 31, 2022 consisted primarily of €189.8 million net proceeds from the Equity Subscription Agreement signed with Pfizer in June 2022 and closing of a global offering in October 2022 as well as €39.3 million of proceeds mainly from disbursements from the Deerfield and OrbiMed Financing Agreement during 2022, partially offset by €9.2 million of interest payments and lease payments amounting to €3.0 million.
Operating and Capital Expenditure Requirements
We have previously incurred significant operating losses, including in the years discussed in this annual report. As of December 31, 2024 and 2023, we had accumulated a net loss of €563.9 million and €551.7 million, respectively. Our net loss was €12.2 million the year ended December 31, 2024 and our net loss was €101.4 million, and €143.3 million for the years ended December 31, 2023 and 2022, respectively.
We expect to continue to incur significant expenses, and we may incur substantial operating losses over the next several years as we market our approved products, advance clinical development of our product candidates and continue our research and development efforts in the United States, Europe, and endemic markets. Our net losses may fluctuate significantly from quarter to quarter and year to year, depending on the timing of our clinical trials and our expenditures on other research and development activities.
We anticipate that our expenses will increase in connection with our ongoing activities, as we:
•invest in our vaccine candidate programs, including ZIKA and Shigella, and our other pre-clinical and research programs;
•invest into fulfilling Phase 4 post-marketing obligations and progress commercial launch activities related to IXCHIQ, our chikungunya vaccine; and
•invest in our working capital and general corporate purposes.
Our present and future funding requirements will depend on many factors, including, among other things:
•costs of continued commercial activities, including product sales, marketing, manufacturing and distribution, for our approved products;
•the scope, progress, timing, and successful completion of our clinical trials of our current or future product candidates, especially the Phase 3 clinical trial for VLA15 and the Phase 4 clinical trials of IXCHIQ;
•the number of potential new product candidates we identify and decide to develop;
•our ability to establish and maintain collaborations on favorable terms, if at all;
•the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims of infringement raised by third parties;
•the time and costs involved in obtaining regulatory approval for our product candidates and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these product candidates; and
•the amount of revenues, if any, we may derive either directly, or in the form of royalty payments from any current or future collaboration agreements.
For more information as to the risks associated with our future funding needs, see “Item 3.D—Risk Factors”.
We expect to finance these expenses and our operating activities through a combination of revenue from sales of our products and third-party products, grants, milestone and service payments from our collaboration with Pfizer regarding our Lyme disease vaccine candidate, and our existing liquidity. If we are unable to generate sufficient revenue from product sales and through our collaboration agreements in accordance with our expected timeframes, we will need to raise additional capital through the issuance of our shares, through other equity or debt financings or through collaborations with other companies. However, we may be unable to raise additional funds or enter into other funding arrangements when needed on favorable terms, or at all, which would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our development programs or commercialization efforts or grant others rights to develop or market drug candidates that we would otherwise prefer to develop and market ourselves. Our ability to successfully transition to profitability will be dependent upon achieving a level of revenues adequate to support our cost structure. We cannot assure you that we will ever be profitable or generate positive cash flow from operating activities.
Although it is difficult to predict future liquidity requirements, we believe that our existing cash and cash equivalents as of December 31, 2024 will be sufficient to fund our operations through at least 12 months after publication of this document.
Contractual Obligations
The following table discloses aggregate information about our material long-term contractual obligations as of December 31, 2024 and the periods in which payments are due. Future events could cause actual payments and timing of payments to differ from the contractual cash flows set forth below.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Less than 1 year |
Between 1 and 3 years |
Between 3 and 5 years |
Over 5 years |
Total |
| Borrowings |
20,852 |
|
132,489 |
|
33,349 |
|
683 |
|
187,373 |
|
| Lease liabilities |
2,508 |
|
5,203 |
|
5,083 |
|
16,147 |
|
28,941 |
|
| Refund liabilities |
19,650 |
|
6,491 |
|
— |
|
— |
|
26,141 |
|
| TOTAL |
43,010 |
|
144,183 |
|
38,432 |
|
16,830 |
|
242,455 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The amounts disclosed in the table above are the contractual undiscounted cash flows.
Borrowings
As of December 31, 2024, the carrying amount of bank borrowings and other loans was €187.4 million. Of this, €180.8 million related to the Financing Agreement with Deerfield and OrbiMed. Following amendments to the Financing Agreement, most recently in March 2024, the interest-only period on the initial $100 million tranche extends until the first quarter of 2026, and this portion of the loan and will mature in the first quarter of 2027. The interest-only period for the tranches drawn in 2023 amounting to $100.0 million extends until the first quarter of 2027, and this portion of the loan will mature in the fourth quarter of 2028. The interest rate is 9.95% (equivalent to 10.09% on an annual basis). Other borrowings related to financing of research and development expenses and CIR (research and development tax credit in France) of €3.5 million and the CEPI loan in the amount of €3.0 million, which relates to advanced payments received which are expected to be paid back in the future.
As of December 31, 2023, the carrying amount of bank borrowings and other loans was €176.8 million. Of this, €167.5 million related to the Financing Agreement with Deerfield and OrbiMed. Other borrowings related to financing of research and development expenses and CIR (research and development tax credit in France) of €3.6 million and the CEPI loan in the amount of €5.7 million, which relates to advanced payments received which are expected to be paid back in the future.
Lease Liabilities
As of December 31, 2024, the outstanding, discounted amount of lease liabilities was €28.9 million. Of this, €26.9 million related to the lease agreements for two premises in Sweden, which we expect will terminate in 2031 and 2037, respectively. Base rent will increase based on an inflation index. In October 2023, the lease agreement for premises in Vienna, Austria, expired and we acquired the premises via takeover of the legal entity VBC 3, which possessed the premises. For further information, see Note 5.13 of our financial statements. Regular installment payments are variable and based on EURIBOR. Other lease liabilities of €2.0 million related to a number of minor agreements with various conditions (interest rates) and terms (maturities).
As of December 31, 2023, the outstanding, discounted amount of lease liabilities was €32.0 million. Of this, €28.3 million related to the lease agreements for two premises in Sweden. Base rent will increase based on an inflation index. In October 2023, the lease agreement for premises in Vienna, Austria, expired whereby the lease liability at the end of September 2023 amounted to €22.5 million. Other lease liabilities of €3.6 million related to a number of minor agreements with various conditions (interest rates) and terms (maturities).
Refund Liabilities
As of December 31, 2024, the carrying amount of refund liabilities was €26.1 million. Of this, €18.6 million related to the collaboration with Pfizer, as we are funding 40% of Phase 3 clinical trial costs performed by Pfizer, and €6.5 million (all non-current) related to the expected payment to GSK related to the termination of the strategic alliance agreement in 2019, and €1.1 million (all current) related to refund liabilities to customers related to rebate and refund programs as well as right to return of commercialized products.
As of December 31, 2023, the carrying amount of refund liabilities was €39.9 million. Of this, €33.1 million related to the collaboration with Pfizer, as we are funding 40% of Phase 3 clinical trial costs performed by Pfizer, and €6.5 million (all non-current) related to the expected payment to GSK related to the termination of the strategic alliance agreement in 2019, and €0.3 million (all current) related to refund liabilities to customers related to rebate and refund programs as well as right to return of commercialized products.
C. Research and Development, Patents and Licenses
For a discussion of our research and development activities, see “Item 4.B—Business Overview” and “Item 5.A—Operating Results.”
D. Trend Information
For a discussion of trends, see “Item 4.B—Business Overview,” “Item 5.1—Operating Results” and “Item 5.B—Liquidity and Capital Resources.”
E. Critical Accounting Estimates
Our consolidated financial statements are prepared in accordance with IFRS as issued by the IASB. Some of the accounting methods and policies used in preparing our consolidated financial statements under IFRS are based on complex and subjective assessments by our management or on estimates based on past experience and assumptions deemed realistic and reasonable based on the circumstances concerned. Critical accounting policies and practices are tailored to specific events in the current year, and the accounting policies and practices that are considered critical might change from year to year. The actual value of our assets, liabilities, and shareholders’ equity and of our accumulated deficit could differ from the value derived from these estimates if conditions change and these changes had an impact on the assumptions adopted.
Our management applied judgement and estimates on the following critical accounting topic:
Revenue Recognition of Other Revenue
Management’s judgement is required to determine the identification of performance obligations (especially when determining whether the license is distinct, which is the case when the customer can benefit from the license without further involvement), the determination of the transaction price (including the judgement of payables to customers), and allocation of the transaction price to the performance obligations on relative standalone selling price. The standalone selling price is sometimes not available or are hard to value intangible assets, so various valuation techniques are used. In addition, management’s judgement is required whether revenue from collaborations and licensing is recognized over time or at a point in time.
In April 2020, we entered into a collaboration to co-develop and commercialize our Lyme disease vaccine candidate with Pfizer. This agreement included a $130.0 million (€116.9 million) upfront payment from Pfizer, which we received in June 2020 and booked in an amount of €116.9 million, and a $10.0 million milestone payment from Pfizer, which we received in April 2021 and booked in an amount of €8.4 million. While we are obligated to contribute 40% (before May 2022: 30%) of all ongoing and future development costs through completion of the development program, as of December 31, 2024, 2023 and 2022, €18.6 million, €33.1 million and €135.5 million, respectively, have been recognized as discounted refund liabilities to reflect the requirement to pay 40% (before May 2022: 30%) of Pfizer’s research and development costs. As of December 31, 2024 revenue recognition for €18.6 million was deferred and recorded as refund liability. We determined that entitlement to the consideration is not yet highly probable, due to the possibility of increased payments to customers while R&D activities are progressing ahead of BLA licensure submission (including Phase 3 study) to the FDA.
Item 6. Directors, Senior Management and Employees
A. Directors and Senior Management
Until December 20, 2023, we had a two-tier corporate governance system consisting of a Management Board (Directoire), which was responsible for managing the Company, and a Supervisory Board (Conseil de Surveillance), which oversaw the Management Board. On December 20, 2023, our shareholders approved the Supervisory Board’s recommendation to change to a one-tier governance system under which the Company is led by a Board of Directors. We refer to our senior management as the Executive Committee, which includes the members of our former Management Board.
The following table sets forth information concerning the members of our Executive Committee and Board of Directors as of the date of this annual report.
|
|
|
|
|
|
|
|
|
| Name |
Age |
Position |
Executive Committee |
|
|
| Thomas Lingelbach |
61 |
Director, President & Chief Executive Officer |
| Peter Bühler |
55 |
Chief Financial Officer |
| Franck Grimaud |
58 |
Chief Business Officer |
| Juan Carlos Jaramillo |
54 |
Chief Medical Officer |
| Dipal Patel |
51 |
Chief Commercial Officer |
| Frédéric Jacotot |
61 |
General Counsel and Corporate Secretary (until July 31, 2024) |
| Vincent Dequenne |
57 |
Chief Operating Officer |
| Petra Pesendorfer |
39 |
Chief People Officer |
Hanneke Schuitemaker |
61 |
Chief Scientific Officer |
Kendra Wergin |
39 |
General Counsel and Corporate Secretary (from August 1, 2024) |
| Non-Employee Directors |
|
|
| Anne-Marie Graffin |
63 |
Chair of the Board of Directors |
| James Sulat |
74 |
Vice Chair of the Board of Directors |
| James Connolly |
60 |
Member of the Board of Directors |
| Maïlys Ferrère |
62 |
Representative of Bpifrance Participations SA, member of the Board of Directors |
Danièle Guyot-Caparros |
66 |
Member of the Board of Directors |
| Kathrin Jansen |
67 |
Member of the Board of Directors |
Executive Committee
The members of our Executive Committee are appointed by the Board of Directors and are responsible for the day-to-day management of the Company. Certain members of the Executive Committee were also appointed as Associate Managing Officers (Directeurs Généraux Délégués), as described further in this Item 6.
Thomas Lingelbach has served as our President and Chief Executive Officer (CEO) since 2013. He served as Chairman of our Management Board until December 2023, and in connection with the change to a one-tier governance structure, he was appointed as a member of our Board of Directors alongside his role as President and CEO and as Directeur Général. He serves as the Chairman of the Executive Committee. Prior to becoming our founding CEO, Mr. Lingelbach served in a variety of increasingly senior roles, most recently as CEO of Intercell AG from 2011 until its merger with Vivalis SA in 2013. He is an established vaccine industry leader with breadth of experience. Prior to joining Intercell, he served as Vice President and Executive Committee Member, Global Industrial Operations-Vaccines of Chiron Corporation. Upon Chiron’s acquisition by Novartis Vaccines & Diagnostics GmbH & Co KG, he served as Managing Director of Chiron Behring GmbH & Co KG and General Manager until he joined Intercell. During his more than 30 years in vaccines, he held a variety of positions from product development to commercialization, with a strong emphasis on technical development and operations. In different capacities, he contributed to the successful development and licensure of more than ten vaccines. Mr. Lingelbach holds an M.S. in Engineering, specialised in bioprocess engineering, from Technische Hochschule Mittelhessen (THM) and complemented his education with a business administration program.
Peter Bühler has served as our Chief Financial Officer since January 2022. Mr. Bühler previously served as Chief Financial Officer of Quotient Limited, a position he held from February 2020 until December 2021. From May 2017 to March 2019, Mr. Bühler served as Group Chief Financial Officer at Zaluvida Corporate AG. From April 2013 to April 2017, Mr. Bühler served as Group Chief Financial Officer at Stallergenes Greer SA. Mr. Bühler is a Swiss Chartered Accountant, a member of the Swiss Institute of Certified Accountants and Tax Consultants and received an MBA from SBS Swiss Business School.
Franck Grimaud has served as our Chief Business Officer since 2013. Prior to joining us, he served as Chief Executive Officer of Vivalis SA from 1999 until its merger with Intercell AG in 2013. Mr. Grimaud has served as Chair of the Governing Board of Fonds Pays de la Loire Participations since September 2016 and as President of the Board of Directors of Atlanpole Biothérapies since February 2018, where he served as Treasurer from January 2015 to February 2018. Mr. Grimaud holds an M.B.A. from University of Ottawa and received his Licence AES from Université de Poitiers.
Juan Carlos Jaramillo, M.D., has served as our Chief Medical Officer since October 2020. Prior to joining us, Dr. Jaramillo served as Senior Vice President, Market Access & Medical Affairs and then as Senior Vice President, Head of Global Market Access & Pricing at Daiichi Sankyo, GmbH from April 2013 to September 2020. Prior to Daiichi Sankyo, Dr. Jaramillo served as Senior Vice President, Medical Affairs & Clinical Development at Grünenthal, Inc. and prior to that held a variety of positions at GlaxoSmithKline plc. Dr. Jaramillo received his M.D. and B.S. in Pre-Medicine from Universidad Central Del Este.
Dipal Patel has served as our Chief Commercial Officer since November 2022. Ms. Patel has over 23 years’ experience in the pharmaceutical industry. Prior to joining us, Ms. Patel served as Vice President, Vaccines Commercialization Lead at GlaxoSmithKline, a position she held from January 2020, and as Vice President, Commercial Head (Respiratory) Emerging Markets from August 2017 to January 2020. Prior to that Ms. Patel held multiple roles at GlaxoSmithKline covering commercial strategy, execution, market access and lifecycle management. Over her career, she has held roles of increasing responsibilities across multiple countries including the United States, Australia, Belgium, Singapore, Thailand, and the European and emerging markets regions. Ms. Patel graduated with a B.Sc. (Honors) from Macquarie University, Sydney in 1998 followed by an M.B.A. from Macquarie Graduate School of Management in 2006.
Frédéric Jacotot served as our General Counsel and Corporate Secretary from 2013 to July 2024. Prior to joining us, he served as counsel at Abbott Laboratories from 2010 to 2013. Mr. Jacotot received his Diplôme d’études approfondies in business law from Paris 1 Panthéon-Sorbonne University.
Vincent Dequenne has served as our Chief Operating Officer since June 2022 and as a member of our Executive Committee since January 2024. Prior to this position, he served as our Senior Vice President of Global Industrial Operations from July 2021 to May 2022. Prior to that, he served as Managing Director Biologics at Eurogentec from January 2020 to June 2021 and as CDMO Managing Director at Pierre Fabre from October 2017 to January 2020. Before that, he served in roles of increasing responsibility at GSK for more than 10 years and at Eli Lilly for more than 15 years. Mr. Dequenne holds a Master of Engineering, Electromechanical Engineering from L'institut Supérieur Industriel in Mons, Belgium.
Petra Pesendorfer has served as our Chief People Officer and as a member of our Executive Committee since January 2024. She has more than 18 years’ experience in strategic and operational Human Resources, leading large teams across different countries and regions in rapidly growing business environments. During her career, she has held a variety of positions with increasing international responsibilities. Prior to joining us, Ms. Pesendorfer served as Vice President & Global HR Business Unit & Functional Head (2019 - 2023), Regional HR Head for USA and Canada (2022-2023) and Global Head of Human Capital Development & Talent Acquisition (2016-2019) at ams OSRAM. Prior to that, Ms. Pesendorfer held multinational HR leadership roles at Rentokil Initial and Soravia Group from 2006 to 2016. Ms. Pesendorfer holds an International Master of Business Administration from FH Wien University of Applied Sciences, Vienna, and the University of Texas at Brownsville.
Hanneke Schuitemaker joined Valneva in June 2024 as our Chief Scientific Officer. She has more than two decades of experience in vaccine discovery and development. She worked at Janssen Vaccines and Prevention, which is part of the Johnson and Johnson group, including as Global Head of Viral Vaccine Discovery and Translational Medicine, with responsibility for the strategy and execution of vaccine programs on COVID-19, HIV, RSV, Ebola, and multiple other viral disease targets. Prior to that, she worked at Sanquin, the Dutch blood supply foundation, as Chair of the Department of Clinical Viro-Immunology, and at the Amsterdam University Medical Center, as Chair of the Department of Experimental Immunology. Dr. Schuitemaker received her Ph.D. in Medicine from the University of Amsterdam.
Kendra Wergin has served as our General Counsel and Corporate Secretary and as a member of our Executive Committee since August 2024. Ms. Wergin is admitted to the bars of New York, California, and the District of Columbia. She joined Valneva in 2020 as Senior Corporate Counsel and served as Vice President, Legal and Associate General Counsel from March 2023 until her appointment to the Executive Committee. Prior to joining Valneva, Ms. Wergin served as Corporate Counsel at Intuit (2020) and as an Associate in the London and Paris offices of Latham & Watkins LLP (2014-2019), where she practiced corporate law. Ms. Wergin brings additional skills from prior experience in public education, local government, and consulting. Ms. Wergin holds a Juris Doctor from the University of Virginia School of Law, a Master droit économique (Master in Economic Law) from l’Institut d’Etudes Politiques de Paris (Sciences Po), a Master of Arts in Teaching from American University, and a Bachelor of Arts from the College of William & Mary.
Board of Directors
The Board of Directors is composed of a minimum of three and a maximum of eighteen members. Directors are appointed for a renewable term of three years at the general meeting of shareholders. The general meeting of shareholders may revoke the appointments of directors at any time during the meeting by a simple majority vote. The appointees are selected by the shareholders and may be individuals or entities (represented by a designated individual).
The age limitation for directors is 80 years old, and no more than 20% of the directors may be over 75 years old. The limitations on holding such an appointment concurrently with an appointment in another company are those set forth in the applicable statutory and regulatory provisions.
Our Board of Directors is currently composed of the following non-employee directors, in addition to Thomas Lingelbach:
Anne-Marie Graffin joined our Supervisory Board in 2013 and was appointed to the new Board of Directors in December 2023. She was elected Chair of our Board of Directors in December 2023. She served as Chief Executive Officer of the Big Booster Acceleration Program, an international non-profit acceleration program for startups, from 2011 to May 2017. Prior to that, she served in a variety of positions, most recently as Executive Vice President and member of the Executive Committee at Sanofi Pasteur MSD, a European vaccine company, from 1998 to 2011. Ms. Graffin has served on the supervisory board of Nanobiotix S.A. (Nasdaq: NBTX) since January 2014, on the board of Sartorius Stedim Biotech SA since April 2015, and on the Board of Directors of Vetoquinol SA since 2022. Ms. Graffin received her MBA from ESSEC Business School Paris. We believe Ms. Graffin’s experience in the vaccine space and her experience advising biotech companies qualifies her to serve on our Board of Directors.
James Sulat joined our Supervisory Board in 2013 and was appointed to the new Board of Directors in December 2023. He is currently Vice Chairman of our Board of Directors. Previously, he served on the Supervisory Board of Intercell AG from 2005 until its merger with Vivalis SA in 2013. From 2009 to 2013, Mr. Sulat served as Chief Executive Officer and Chief Financial Officer of Maxygen, Inc., and as a member of Maxygen’s Board of Directors from 2003 to 2013. From 2005 to 2009, Mr. Sulat served in a variety of roles at Memory Pharmaceuticals Corp., including as President and Chief Executive Officer from 2005 to 2008 and as a member of Memory’s Board of Directors from 2005 to 2009. Previously, Mr. Sulat served as Chief Financial Officer for Chiron Corporation and Stanford Health Services. Mr. Sulat has served on the Board of GS Holdings, Inc. since October 2021. He previously served on the Board of Directors of Exicure, Inc. from 2021 until December 2022, on the Board of Directors of Arch Therapeutics, Inc. from 2015 until December 2021 and on the Board of Directors of AMAG Pharmaceuticals, Inc. from 2014 to November 2020. Mr. Sulat received an MBA and an M.S. in Health Services Administration from Stanford University and a B.S. in Administrative Sciences from Yale University. We believe Mr. Sulat’s experience in the pharmaceutical industry, expertise in corporate finance and public company board experience qualifies him to serve on our Board of Directors.
James Connolly joined our Supervisory Board in June 2022 and was appointed to the new Board of Directors in December 2023. Since 2013, Mr. Connolly has been providing broad based consulting and advisory services to a variety of vaccine, biopharmaceutical and investment organizations. From 2010 to 2013, Mr. Connolly was President and CEO of Aeras (now IAVI). Prior to this, he spent 24 years at Wyeth (now Pfizer) in a series of increasingly senior roles, including Executive Vice President and General Manager, Wyeth Vaccines and President, Wyeth Canada. Mr. Connolly currently serves on the Board of Directors of IAVI. He previously served on the Board of Directors of Vaxess Technologies (2013-2019), Aeras (2013-2018), PaxVax (2014-2018), Tivorsan Pharmaceuticals (2015-2020) and Ambulatus Robotics (2020-2021). Mr. Connolly earned a B.S. in Business Administration from Washington University in St Louis. We believe Mr. Connolly’s experience in the vaccines and pharmaceutical industries and his experience advising biotech companies qualifies him to serve on our Board of Directors.
Maïlys Ferrère joined our Supervisory Board in June 2022 as representative of Bpifrance Participations, member of the Supervisory Board, and now continues to represent Bpifrance Participations on our Board of Directors. Ms. Ferrère has served as a Director, Head of the Large Venture Investment Activity at Bpifrance, France’s public investment bank, since October 2013. Ms. Ferrère served on the board of directors of Sequans Communications S.A., a publicly traded French designer, developer and supplier of cellular semiconductor solutions, and serves on the Board of DBV Technologies, a publicly traded French company that develops a treatment against peanut allergy. Ms. Ferrère served on the board of directors of Innate Pharma SA., a French global oncology-focused biotech company, from 2017 to 2021. Ms. Ferrère served on the board of directors at Gensight Biologics S.A., a French publicly traded biotechnology company, from 2016 to 2019. She graduated from Institut d’Etudes Politiques Paris and began her career with the Internal Audit of Société Générale before working for multiple French banks in the equity capital markets origination department. We believe that Ms. Ferrère’s experience in the banking industry and her knowledge of capital markets qualify her to serve on our Board of Directors.
Danièle Guyot-Caparros joined our Board of Directors in 2024. She started her career in Audit and Corporate Finance with PWC specializing in the Chemical/Pharma Industry. In 1992, she joined Rhône-Poulenc-Rorer (later Aventis and Sanofi) where she held several senior finance positions (CFO Global R&D, CFO Europe, Group Planning). In 2008, she became Senior Advisor for Deloitte France to support the development of the Life Sciences and Health Care Industry practice. She has supported multiple engagements with a large diversity of clients (big and mid-size pharma companies, biotech, foundations etc.) focusing on transformation, governance issues and M&A. Ms. Guyot-Caparros is also an experienced non-executive director with a focus on Biotech/Medtech. From 2015 to 2017, she was board and audit committee member at Diaxonhit (now Eurobio Scientific) listed on Euronext Growth. She chaired the audit committee of Supersonic Imagine from July 2018 to September 2019 until the acquisition of the company by US group Hologic. From 2013 to June 2022, she chaired the audit committee of ONXEO (listed on Euronext, OMX Copenhagen and now Euronext Growth) and chaired the board from May 2019 to July 2021. In October 2022, she joined the board of DBV Technologies, a company listed on Euronext and Nasdaq, and is a member of the audit committee and of the compensation committee. Ms. Guyot-Caparros is a graduate from ICN (Institut Commercial de Nancy), with specialization in finance and accounting. She holds a chartered accountant degree and a non-executive director qualification awarded by IFA-Science-Po. We believe that Ms. Guyot-Caparros’s experience in finance and business operations qualify her to serve on our Board of Directors.
Kathrin Jansen joined our Supervisory Board in June 2023 and was appointed to our new Board of Directors in December 2023. Dr. Jansen has over 30 years of vaccine R&D experience focused on the development of vaccines addressing large unmet medical needs. From 2015 to 2022 she served as Senior Vice President and Head of Vaccine Research and Development at Pfizer Inc, and as a member of Pfizer’s Worldwide Research, Development and Medical leadership team. She led a fully integrated, global vaccines research and development organization, with responsibilities ranging from discovery to clinical development, registration, and postmarketing commitments of all of Pfizer’s vaccines, including partnered ones.
Most notably she led the development of several highly successful and licensed vaccines such as Pfizer/BioNtech’s SARS-CoV-2 (COMIRNATY), the first-ever licensed mRNA vaccine, Pfizer’s Streptococcus pneumoniae (Prevnar 20), Respiratory syncytial virus (Abrysvo), and Meningococcal B Group B (Trumenba) vaccines. From 2006 to 2015, Dr. Jansen served as Senior Vice President at Wyeth Pharmaceuticals and then Pfizer and was responsible for vaccine discovery, early development, and clinical testing operations. Prior to Wyeth, Dr. Jansen spent 12 years at Merck Research Laboratories supporting several vaccine efforts and leading the R&D activities of Gardasil, the world’s first cervical cancer vaccine. Dr. Jansen received her Ph.D. in microbiology, biochemistry & genetics from Phillips Universitaet, Marburg, Germany, in 1984 followed by postdoctoral training at Cornell University. Dr. Jansen was appointed an Adjunct Professor at the University of Pennsylvania School of Medicine in 2010 and has authored and co-authored over 200 publications. She is a member of the National Academy of Medicine, National Academy of Engineering, a Fellow of the Royal Society of Medicine and recipient of the Albert E Sabin Gold Medal. We believe that Dr. Jansen’s experience in the vaccines industry qualifies here to serve on our Board of Directors.
Diversity of the Board of Directors
Our Board of Directors consists of 4 women (including one permanent representative of a corporate board member) and 3 men. We therefore comply with the applicable provisions of the French Commercial Code, which requires that the difference between the number of directors of each gender should be no greater than two.
Family Relationships
There are no family relationships among any of the members of our Board of Directors and Executive Committee.
B. Compensation
Compensation of Members of the Board of Directors
We pay all our Board members, other than as described below, for their service on our Board of Directors. This compensation consists of base compensation (made up of basic fees and supplements depending on their role on the Board and Committees, except for the Chair) and additional compensation as described below (which is based on the length of their service on the Board). Fees are fixed but may be reduced if meeting attendance is under 75%. At our General Meetings of shareholders held on December 20, 2023 and June 26, 2024, shareholders approved the compensation scheme for base compensation as shown below, payable to our directors in respect of 2024:
|
|
|
|
|
|
| Member Role |
Maximum Allowable Compensation (per year) |
| Chair of the Board |
€90,000 |
| Other Board members |
€45,000 plus the supplements listed below, as the case may be |
| Vice-Chair supplement |
€15,000 |
| Lead Independent Member supplement |
€15,000 |
Committee Chair supplement (includes membership of the chaired Committee) |
€15,000 |
Committee membership supplement (per Committee) |
€7,500 |
The shareholders also approved the additional compensation component for each Board member, which is structured as follows (and may be prorated in case the Board member leaves the Board before the date triggering the payment of this compensation):
•€13,300 to be paid approximately one year after the Annual General Meeting of June 2022 (or after the date of appointment of the member concerned, if later);
•€26,600 to be paid approximately two years after the Annual General Meeting of June 2022 (or after the date of appointment of the member concerned, if later);
•€39,900 to be paid approximately three years after the Annual General Meeting of June 2022 (or after the date of appointment of the member concerned, if later) and again annually thereafter.
The following table sets forth the total compensation earned by members of the Board during the year ended December 31, 2024 for their service on our Board, in accordance with the principles described above:
|
|
|
|
|
|
| Member |
Compensation |
| Anne-Marie Graffin |
€116,600 |
| James Sulat |
€107,225 |
| James Connolly |
€86,600 |
| Kathrin Jansen |
€80,800 |
Danièle Guyot-Caparros |
€28,844.17 |
Ms. Maïlys Ferrère did not receive any compensation in connection with her representation of Bpifrance Participations on the Board of Directors during the year ended December 31, 2024, as Bpifrance Participations waived its right to receive such compensation. In addition, in accordance with the decision of the Board of Directors held on December 20, 2023, Mr. Thomas Lingelbach does not receive any compensation for his role on the Board of Directors.
Note: In 2024, we also paid €39,789 in total as prorated additional compensation to former members of the Supervisory Board who served until December 20, 2023 (namely Frédéric Grimaud, Sharon Tetlow and Johanna Pattenier).
Compensation of CEO and Associate Managing Officers — 2024
The Company’s general management is currently represented by our CEO (Directeur Général), Mr. Thomas Lingelbach, and the Associate Managing Officers (Directeurs Généraux Délégués) listed below, also appointed in order to assist Mr. Lingelbach in the performance of his duties:
•Franck Grimaud, Chief Business Officer;
•Peter Bühler, Chief Financial Officer;
•Juan Carlos Jaramillo, Chief Medical Officer; and
•Dipal Patel, Chief Commercial Officer.
Note: During the fiscal year 2024, the General Management comprised an additional member, Mr. Frédéric Jacotot, General Counsel & Corporate Secretary, whose functions terminated on July 31, 2024.
The current term of office of Mr. Lingelbach will end at the 2026 General Meeting called to approve the annual financial statements for the fiscal year ended December 31, 2025.
The current terms of office of Mr. Grimaud, Mr. Jaramillo, Mr. Bühler and Ms. Patel will end at the 2025 General Meeting called to approve the annual financial statements for the fiscal year ended December 2024.
The method and amount of compensation for the CEO and each Associate Managing Officer is determined by the Board of Directors, after recommendation by the Nomination, Governance and Compensation Committee.
The following tables set forth compensation earned by the CEO and Associate Managing Officers with respect to the year ended December 31, 2024.
Mr. Thomas Lingelbach – Chief Executive Officer and member of the Board of Directors since December 20, 2023 (previously Chief Executive Officer and Chair of the Management Board)
Mr. Lingelbach’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Mr. Lingelbach and Valneva Austria GmbH, which came into force at the end of our Combined General Meeting held on June 23, 2022 (as amended, notably on December 20, 2023), and (b) the decisions of our Board of Directors, as applicable.
|
|
|
|
|
|
|
|
|
| Type of Compensation |
Amount of compensation earned |
Description |
| Fixed compensation |
€573,195 |
As per Board of Directors decision dated December 20, 2023. |
| Annual variable compensation |
€378,308.70 |
75% of 2024 gross annual salary set by the Board of Directors on December 20, 2023.
Validation by the Board of Directors of 75% of the objectives set with respect to the year 2024, on February 14, 2025.
|
Exceptional compensation |
€0 |
|
Compensation in connection with Board membership |
€0 |
In accordance with the decision of the Board of Directors of December 20, 2023 |
| Fringe benefits : |
|
|
| – Car rental |
Lease fee: €15,840
Insurance: €4,813.64
Other car related expenses
(except fuel) : €5,801.76
|
Maximum €1,320 per month for the lease fee as per Mr. Lingelbach’s Management Agreement. |
| – Death and endowment insurance policy |
€18,000 |
Long-term life insurance policy as a retirement savings product. |
| – Reimbursement of home workplace journeys made by flights, and associated costs |
€3,145.82 |
The current Management Agreement executed between Mr. Lingelbach and our subsidiary, Valneva Austria GmbH, provides that Mr. Lingelbach be reimbursed for the costs of weekend flights between hometowns in Germany and Austria and sites of Valneva, these costs including the transfers from and to the airport. |
| Total compensation |
€999,104.92 |
|
Mr. Franck Grimaud – Chief Business Officer and Associate Managing Officer since December 20, 2023 (previously Chief Business Officer, Management Board member and Managing Director)
Mr. Grimaud’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Mr. Grimaud and Valneva SE, which came into force at the end of our Combined General Meeting held on December 20, 2023, and (b) the decisions of our Board of Directors, as applicable.
|
|
|
|
|
|
|
|
|
| Type of compensation |
Amount of compensation earned |
Description |
| Fixed compensation |
€291,747.50 |
As per Board of Directors decision dated December 20, 2023. |
| Annual variable compensation |
€135,662.59 |
50% of 2024 gross annual salary set by the Board of Directors on December 20, 2023.
Validation by the Board of Directors of 93% of the objectives set with respect to the year 2024, on February 14, 2025.
|
| Fringe benefits : |
|
|
| – Car rental |
Lease fee: €15,840
Insurance: €1,899.54
French taxes on the use of vehicles: €20
|
Maximum €1,320 per month for the lease fee as per Mr. Grimaud’s Management Agreement. |
| – Garantie Sociale des Chefs et Dirigeants d’Entreprises |
€9,021 |
An unemployment insurance contract for Company Directors and Managers (Convention Garantie Sociale des Chefs et Dirigeants d’Entreprise) has been granted to Mr. Grimaud. The purpose of this contract is to guarantee the payment of compensation in case of unemployment (up to 70% of the last professional net income filed with the tax authorities). This GSC was set up pursuant to an authorization of the Supervisory Board of October 26, 2000. |
| Total compensation |
€454,190.63 |
|
Mr. Peter Bühler – Chief Financial Officer and Associate Managing Officer since December 20, 2023 (previously Chief Financial Officer and Management Board member)
Mr. Bühler’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Mr. Bühler and Valneva Austria GmbH, , which came into force at the end of our Company's Combined General Meeting held on June 23, 2022 (as amended, notably on December 20, 2023), and (b) the decisions of our Board of Directors, as applicable.
|
|
|
|
|
|
|
|
|
| Type of compensation |
Amount of compensation earned |
Description |
| Fixed compensation |
€410,970 |
As per Board of Directors decision dated December 20, 2023. |
| Annual variable compensation |
€195,210.75 |
50% of 2023 gross annual salary set by the Board of Directors on December 20, 2023.
Validation by the Board of Directors of 95% of the objectives set with respect to the year 2024, on February 15, 2025.
|
| Fringe benefits : |
|
|
| – Car allowance |
€15,840.00
|
Maximum €1,320 per month as per Mr. Bühler’s Management Agreement. |
| – Death and endowment insurance policy |
€18,000 |
Long-term life insurance policy as a retirement savings product. |
| Total compensation |
€640,020.75 |
|
Mr. Frédéric Jacotot – General Counsel & Corporate Secretary, Associate Managing Officer from December 20, 2023 until July 31, 2024 (previously General Counsel & Corporate Secretary, Management Board member)
Mr. Jacotot’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Mr. Jacotot and Valneva SE, which came into force at the end of our Company's Combined General Meeting held on December 20, 2023, and (b) the decisions of our Board of Directors, and (c) the provisions of the Settlement Agreement executed with Valneva SE on April 30, 2024 (in the context of Mr. Jacotot’s leaving Valneva), as applicable.
|
|
|
|
|
|
|
|
|
| Type of compensation |
Amount of compensation earned |
Description |
| Fixed compensation |
€133,054.55 |
Amount prorated to take account of Mr. JACOTOT's departure date. The officer's fixed annual compensation was set at €228,093.50 by a decision of our Board of Directors on December 20, 2023. |
| Annual variable compensation |
€0 |
In accordance with the provision of the Settlement Agreement executed with Valneva SE. |
| Fringe benefits : |
|
|
| – Garantie Sociale des Chefs et Dirigeants d’Entreprises |
€10,223.20 |
Unemployment insurance contract for Company Directors and Managers (Convention Garantie Sociale des Chefs et Dirigeants d’Entreprise) has been granted to Mr. Jacotot with effect as from January 1, 2020. The purpose of this contract is to guarantee the payment of compensation in case of unemployment (up to 70% of the last professional net income filed with the tax authorities). |
| Total compensation |
€143,277.75 |
|
Dr. Juan Carlos Jaramillo – Chief Medical Officer and Associate Managing Officer since December 20, 2023 (previously Chief Medical Officer and Management Board member)
Dr. Jaramillo’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Dr. Jaramillo and Valneva Austria GmbH, , which came into force at the end of the Company's Combined General Meeting held on June 23, 2022 (as amended, notably on December 20, 2023), and (b) the decisions of our Board of Directors, as applicable.
|
|
|
|
|
|
|
|
|
| Type of compensation |
Amount of compensation earned |
Description |
| Fixed compensation |
€375,486.50 |
As per Board of Directors decision dated December 20, 2023. |
| Annual variable compensation |
€163,336.63 |
50% of 2024 gross annual salary set by the Board of Directors on December 20, 2023.
Validation by the Board of Directors of 87% of the objectives set with respect to the year 2024, on February 14, 2025.
|
Exceptional compensation |
€0 |
|
|
Fringe benefits:
– Car allowance
|
€15,840 |
€1,320 per month as per Dr. Jaramillo’s Management Agreement. |
| – Death and endowment insurance policy |
€18,000 |
€1,500 per month as per Dr. Jaramillo’s Management Agreement. |
| – Reimbursement of home workplace journeys made by flights, and associated costs |
€12,112.26 |
The current Management Agreement executed between Dr. Jaramillo and the subsidiary Valneva Austria GmbH provides that Dr. Jaramillo be reimbursed for the costs of weekend flights between hometown in Spain and site of Valneva Austria, these costs including the transfers from and to the airport. |
| Total compensation |
€584,775.39 |
|
Ms. Dipal Patel – Chief Commercial Officer and Associate Managing Officer since December 20, 2023 (previously Chief Commercial Officer and Management Board member)
Ms. Patel’s 2024 compensation is defined in accordance with (a) the provisions of the Management Agreement executed between Ms. Patel and Valneva UK Ltd, which came into force on November 18, 2022 (as amended, notably on December 20, 2023), and (b) the decisions of our Board of Directors, as applicable.
|
|
|
|
|
|
|
|
|
| Type of compensation |
Amount of compensation earned (*) |
Description |
| Fixed compensation |
£314,150, or €379,389.89 |
As per Board of Directors decision dated December 20, 2023. |
| Annual variable compensation |
£138,226, or €166,931.55 |
50% of 2024 gross annual salary set by the Board of Directors on December 20, 2023.
Validation by the Board of Directors of 88% of the objectives set with respect to the year 2024, on February 14, 2025.
|
|
Fringe benefits:
– Car allowance
|
£13,392 or €16,173.13 |
£1,116 per month per Ms. Patel’s Management Agreement. |
| – Contribution to UK pension plan |
£23,561.25, or €28,454.24 |
7.5% of the gross annual salary |
| Total compensation |
€590,948.81 |
|
(*) Exchange rate applied: €1 for £0.82804 (average rate for December 2024). This rate will be updated, in particular with regard to the annual variable compensation, at the time of the bonus payment expected in July 2025 (subject to prior approval by the Company's Annual Ordinary General Meeting to be held in June 2025).
Compensation of the CEO and Associate Managing Officers —2025
The Board of Directors, during its meeting held on March 19, 2025, confirmed the following base salaries for the current CEO and Associate Managing Officers with respect to the year ended December 31, 2025:
|
|
|
|
|
|
General Management |
2025 Base Salary |
| Thomas Lingelbach |
€581,793 |
| Franck Grimaud |
€291,748 |
| Peter Bühler |
€417,135 |
| Juan Carlos Jaramillo |
€413,035 |
| Dipal Patel |
£318,077 |
Adoption of Clawback Policy
In November 2023, in accordance with Rule 10D-1 promulgated under the Exchange Act and Nasdaq Listing Rule 5608, we adopted an incentive compensation recoupment policy which is filed herewith as Exhibit 97.1.
Limitations on Liability and Indemnification Matters
Under French law, provisions of bylaws that limit the liability of the members of the Board of Directors, the Chief Executive Officer and Deputy Chief Executive Officer(s) (together with the Chief Executive Officer, the “Executive Officers”) are prohibited. However, French law allows sociétés européennes to contract for and maintain liability insurance against civil liabilities incurred by members of the Board and Executive Officers involved in a third-party action, provided that they acted in good faith and within their capacities as members of such board or management of the company. Criminal liability cannot be indemnified under French law, whether directly by the company or through liability insurance.
We have liability insurance for our Board members, Executive Officers and other members of our Executive Committee and have obtained insurance coverage for liability under the Securities Act. We also have entered into agreements with our Board members and Executive Officers to provide contractual indemnification. With certain exceptions and subject to limitations on indemnification under French law, these agreements provide for indemnification for damages and expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding arising out of his or her actions in that capacity. We believe that this insurance and these agreements are necessary to attract qualified Board members and Executive Officers.
These agreements may discourage shareholders from bringing a lawsuit against our Board members and Executive Officers for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our Board members and Executive Officers, even though such an action, if successful, might otherwise benefit us and our shareholders. Furthermore, a shareholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our Board members and Executive Officers pursuant to these insurance agreements.
Equity Incentives
We believe our ability to grant equity incentives is a valuable and necessary compensation tool that allows us to attract and retain the best personnel for positions of substantial responsibility, provides additional incentives to employees and promotes the success of our business. In accordance with French corporate law, we have historically granted several different equity incentive instruments to our management and our employees, including stock options and free ordinary shares.
Our Board of Directors’ authority to grant these stock options and free ordinary shares and the aggregate amount authorized to be granted must be approved by two-thirds of the shareholders voting in the relevant extraordinary shareholders’ meeting. Once approved by our shareholders, our Board of Directors can continue to grant such awards for a specified period.
We currently have various long-term incentive plans for our management and employees that have been approved by our shareholders. In the event of certain changes in our share capital structure, such as a consolidation or share split or dividend, French law and applicable grant documentation provides for appropriate adjustments of the conversion ratio and/or the exercise price of the outstanding stock options.
Stock Options
The beneficiaries receive a number of options, depending notably on their job functions, that they can convert into ordinary shares during specific exercise periods that are announced by the management and subject to applicable vesting periods.
Under our plans that are currently in force (or were in force during 2024), each option converts into one ordinary share. Our stock option plans currently in force do not include a discount on the exercise price.
All stock options not exercised within ten years of the grant date lapse without compensation.
The following table sets forth the stock options outstanding as of December 31, 2024 (or which were in force during 2024):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plan name |
ESOP
2015
|
ESOP
2016
|
ESOP
2017
|
ESOP
2019
|
SLG SOP
2022
|
ESOP
2022
|
SLG SOP
2023
|
ESOP
2023
|
SLG SOP 2024 |
ESOP 2024 |
| General Meeting date |
June 26, 2014 |
June 30, 2016 |
June 30, 2016 |
June 28, 2018 |
June 23, 2022 |
June 23, 2022 |
June 21, 2023 |
June 21, 2023 |
December 20, 2023 |
December 20, 2023 |
| Grant date |
July 28, 2015 |
October 7, 2016 |
December 7, 2017 |
September 30, 2019 |
October 10, 2022 |
October 10, 2022 |
December 15, 2023 |
December 15, 2023 |
October 22, 2024 |
October 22, 2024 |
| Subscription price |
€3.92 |
€2.71 |
€2.85 |
€3.05 |
€6.47 |
€6.47 |
€5.25 |
€5.25 |
€2.62 |
€2.62 |
| Option/share conversion ratio |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
1: 1 |
| Stock options granted to employees and/or corporate officers by the Management Board at launch of plan |
712,000 |
584,250 |
1,269,500 |
2,670,010 |
1,159,751 |
2,154,500 |
1,284,519 |
2,156,750 |
2,619,966 (including 1,520,269 to corporate officers) |
2,337,750 |
| Vesting dates |
July 28,
2017 (for
50% of
the
options)
July 28,
2019 (for
the
remaining
50%) |
October 7,
2018 (for
50% of
the
options)
October 7,
2020 (for
the
remaining
50%) |
December 7,
2019 (for
50% of the
options)
December 7,
2021 (for
the
remaining
50%) |
September 30, 2020 (for 1/3
of the
options)
September 30, 2021 (for
another 1/3 of
the options)
September 30, 2022 (for the
remainder) |
October 10,
2023 (for 1/3
of the
options)
October 10,
2024 (for
another 1/3 of
the options)
October 10,
2025 (for the
remainder) |
October 10,
2023 (for 1/3
of the
options)
October 10,
2024 (for
another 1/3 of
the options)
October 10,
2025 (for the
remainder) |
December 15, 2024 (for 1/3
of the
options)
December 15, 2025 (for
another 1/3 of
the options)
December 15, 2026 (for the
remainder) |
December 15, 2024 (for 1/3
of the
options)
December 15, 2025 (for
another 1/3 of
the options)
December 15, 2026 (for the
remainder) |
October 22,
2025 (for 1/3
of the
options)
October 22,
2026 (for
another 1/3 of
the options)
October 22,
2027 (for the
remainder) |
October 22,
2025 (for 1/3
of the
options)
October 22,
2026 (for
another 1/3 of
the options)
October 22,
2027 (for the
remainder) |
Stock options exercised as of December 31, 2024 |
478,845 |
383,250 |
427,025 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Shares resulting from exercise of stock options |
478,845 |
383,250 |
427,025 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
Outstanding stock options as of December 31, 2024 |
43,655 |
14,500 |
551,475 |
1,596,166 |
937,224 |
1,402,750 |
1,128,809 |
1,877,000 |
2,619,966 |
2,300,500 |
| Of which outstanding stock options held by corporate officers |
0 |
0 |
0 |
0 |
686,315 |
0 |
842,347 |
0 |
1,520,269 |
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares potentially resulting from stock option exercise after December 31, 2024 |
43,655 |
14,500 |
551,475 |
1,596,166 |
937,224 |
1,402,750 |
1,128,809 |
1,877,000 |
2,619,966 |
2,300,500 |
Stock options having lapsed as of December 31, 2024 |
189,500 |
186,500 |
291,000 |
1,073,844 |
222,527 |
742,750 |
155,710 |
279,750 |
0 |
37,250 |
Free Ordinary Shares
Free ordinary shares (actions ordinaires gratuites) are employee equity incentive instruments pursuant to which the beneficiaries are granted, for free, the possibility to receive our ordinary shares under certain conditions.
In 2024, the Company’s Board of Directors granted 991,643 free ordinary shares for the benefit of Executive Committee and members of the Company’s senior management (2023: 445,320). The purpose of these free share plans is to provide a long-term incentive program for the Company’s senior management.
The following table shows the free ordinary shares outstanding as of December 31, 2024:
|
|
|
|
|
|
| Plan name |
2019-2023 Free Share Plan (Terminated on March 18, 2024) |
| General Meeting date |
June 27, 2019 |
| Management Board decision |
December 19, 2019 |
| Free ordinary shares granted by the Management Board |
2,191,947 allocated in three tranches, each amounting to one third of the total individual allocation. If one third is not a whole number, the number of free ordinary shares will be rounded down for the first two tranches and rounded up for the third tranche. |
| Duration of vesting period |
The vesting period was set at two (2) years as from December 19, 2019 for the first tranche, three (3) years as from December 19, 2019 for the second tranche, and four (4) years as from December 19, 2019 for the third tranche. The vesting of free ordinary shares thus became final, for each tranche, at the end of the aforementioned vesting period, subject to the fulfillment of the performance and employment conditions described below. |
| Date of availability |
Following free ordinary shares vesting, no compulsory holding period will be applicable to the beneficiaries that are non-executive employees.
However, in accordance with Section II (4th paragraph) of Article L.225-197-1 of the French Commercial Code, in their meeting held on November 21, 2019, the Supervisory Board decided that the beneficiaries that are corporate officers should keep not less than 20% of the vested free shares of each tranche until termination of their office as Management Board member or corporate officer.
|
Free ordinary shares fully vested as of December 31, 2024 |
1,551,039 |
Free ordinary shares being vested as of December 31, 2023 |
0 |
Free ordinary shares lapsed as of December 31, 2023 |
640,908 |
|
|
|
|
|
|
Performance and employment conditions set under the plan |
Concerning non-corporate officers employees, the vesting of each tranche was contingent upon the beneficiary’s performance in the Relevant Year having been rated not lower than “Meets Expectations” (regardless of any qualifying sign), as assessed by his/her supervisor under the Company’s employee performance appraisal rules.
Concerning corporate officers, the vesting of each tranche was contingent upon the level of achievement of the Management Board member’s collective and individual goals in the Relevant Year (as defined below), as assessed by the Supervisory Board (or the Board of Directors), starting above 60% (60% = no vesting) and increasing in a linear way, so that 80% goal achievement resulted in vesting of 50% of the relevant tranche and 100% goal achievement resulted in vesting of 100% of the relevant tranche.
Relevant Year means 2021 for the first tranche, 2022 for the second tranche and 2023 for the third tranche. When a vesting period expired before the performance has been assessed for the Relevant Year, the vesting of the relevant tranche will be postponed until all Participants had been assessed.
Additionally, the beneficiaries had to continuously remain a corporate officer or employee (full time or not less than 80%) of the Company or a direct or indirect subsidiary of the Company until vesting of the free ordinary shares granted to them, subject to the retirement exception below or any individual exemption.
|
| Provisions relating to retirement |
The beneficiaries who, prior to the vesting of all or part of the free ordinary shares granted to them, retired in accordance with the age requirements of their pension plan, retained a portion of their free ordinary shares, and this applied to each of the tranches that had not yet vested. When applicable, the number of shares thus retained has been calculated according to the period elapsed between the date of the initial allocation of free ordinary shares until the date of the executive’s retirement, in relation to the total duration of the tranche in question (two, three or four years) – provided, however, that the performance condition defined in the plan was declared satisfied during the performance appraisal immediately preceding the retirement of the beneficiary in question. In the case of beneficiaries who were corporate officers, the level of performance had also an impact on the amount of free ordinary shares they could keep. |
| Provisions relating to a change of control |
If (a) a Change of Control (as defined below) had occurred not earlier than December 19, 2021, and (b) the performance condition stated above had been met for the calendar year immediately preceding the year of Change of Control (or for the year of Change of Control if already assessed), all tranches would have vested immediately. In the case of beneficiaries who were corporate officers, their level of performance would also have affected the amount of shares that would have been subject to this accelerated vesting.
Change of Control means that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L 233-3 of the French Commercial Code.
|
|
|
|
|
|
|
| Plan name |
2022-2025 Free Share Plan |
| General Meeting date |
June 23, 2021 |
| Management Board decision |
October 10, 2022 |
| Free ordinary shares granted by the Management Board |
374,390 allocated in three tranches, each amounting to one third of the total individual allocation. If one third is not a whole number, the number of free ordinary shares will be rounded down for the first two tranches and rounded up for the third tranche. |
| Duration of vesting period |
The vesting period is set at two (2) years as from October 10, 2022 for the first and the second tranches, and three (3) years as from October 10, 2022, for the third tranche. The vesting of free ordinary shares thus becomes final, for each tranche, at the end of the aforementioned vesting period, subject to the fulfillment of the employment condition described below. |
| Date of availability |
Following free shares vesting, no compulsory holding period will be applicable to the beneficiaries that are non-executive employees.
However, in accordance with Section II (4th paragraph) of Article L.225-197-1 of the French Commercial Code, in their meeting held on June 22, 2022, the Supervisory Board decided that the beneficiaries that are corporate officers should keep not less than 20% of the vested free shares of each tranche until termination of their office as Management Board member or corporate officer.
|
Free ordinary shares fully vested as of December 31, 2024 |
217,102 |
Free ordinary shares being vested as of December 31, 2024 |
104,904 |
Free ordinary shares lapsed as of December 31, 2024 |
52,384 |
| Performance and employment conditions |
No performance condition. However, the beneficiaries of the plan must, on an ongoing basis, remain corporate officers or employees (full time or at least 80%) of the Company or of a direct or indirect subsidiary of the Company until the grant of the free ordinary shares allocated to them. |
| Provisions relating to retirement |
The beneficiaries who retire in accordance with the age requirements of their pension plan prior to full vesting will be entitled to a pro rata number of shares for each unvested tranche based on the period from the date of grant to retirement in relation to the total term of the tranche in question, provided, however, that for purposes of this calculation, the term of the first tranche shall be considered to be one year. |
| Provisions relating to a change of control |
In the event of a Change of Control, beneficiaries will immediately and definitively receive all of their free ordinary shares in the process of being acquired under all tranches of the plan.
“Change of Control” means that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L.233-3 of the French Commercial Code.
|
|
|
|
|
|
|
| Plan name |
2022 Special Free Share Plan Number 2 (Plan terminated on October 10, 2024) |
| General Meeting date |
June 23, 2021 |
| Management Board decision |
December 6, 2022 |
| Free ordinary shares granted by the Management Board |
December 6, 2022 |
| Duration of vesting period |
The vesting period for the shares was set at two (2) years from December 6, 2022. The vesting of free ordinary shares thus became final, for each tranche, at the end of the aforementioned vesting period, subject to the fulfillment of the employment condition described below. |
| Date of availability |
No holding period is applicable to ordinary shares vested under this plan. However, in accordance with Section II (4th paragraph) of Article L.225-197-1 of the French Commercial Code, in their meeting held on May 4 and October 12, 2022, the Supervisory Board decided that the beneficiary should keep not less than 10% of the vested free shares of each tranche until termination of his office as Management Board member or corporate officer. |
Free ordinary shares fully vested as of December 31, 2024 |
27,521 |
Free ordinary shares being vested as of December 31, 2024 |
0 |
Free ordinary shares lapsed as of December 31, 2024 |
0 |
| Performance and employment conditions |
The beneficiary of the plan had to retain, on an ongoing basis, the status of corporate officer or employee (full-time or at least 80%) of the Company or of a direct or indirect subsidiary of the Company until the vesting of the free ordinary shares allocated to him. There is no performance condition. |
| Provisions relating to a change of control |
If (a) a Change of Control had occurred before December 6, 2024, and (b) the above-mentioned employment conditions was satisfied until the Change of Control, and (c) Article L.225-197-1, III of the French Commercial Code did not apply, the plan would have been cancelled and the Company would have indemnified the beneficiaries for the loss of unvested free ordinary shares granted under the cancelled plan, subject however to the shareholders’ approval to the indemnity so allocated. The gross amount of this indemnity would have been calculated as though such free shares had been vested upon the Change of Control.
“Change of Control” means that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L.233-3 of the French Commercial Code.
|
|
|
|
|
|
|
| Plan name |
2023-2026 Free Share Plan |
| General Meeting date |
June 23, 2021 |
| Management Board decision |
December 15, 2023 |
| Free ordinary shares granted by the Management Board |
445,320 allocated in three tranches, each amounting to one third of the total ordinary shares granted by the Management Board. If one third is not a whole number, the number of free shares will be rounded down for the first two tranches and rounded up for the third tranche. |
| Duration of vesting period |
The vesting period is set at two (2) years as from December 15, 2023 for the first and the second tranches, and three (3) years as from December 15, 2023, for the third tranche. The vesting of free ordinary shares thus becomes final, for each tranche, at the end of the aforementioned vesting period, subject to the fulfillment of the employment condition described below. |
| Date of availability |
Following free shares vesting, no compulsory holding period will be applicable to the beneficiaries that are non-executive employees.
However, in accordance with section II (fourth paragraph) of Article L.225-197-1 of the French Commercial Code, in their meeting held on March 9, 2023 (confirmed on June 21, 2023), the Supervisory Board decided that the beneficiaries that are corporate officers should keep not less than 20% of the vested free shares of each tranche until termination of their office as Management Board member or corporate officer.
|
Free ordinary shares fully vested as of December 31, 2024 |
0 |
Free ordinary shares being vested as of December 31, 2024 |
388,472 (including 216,600 for executive corporate officers) |
Free ordinary shares lapsed as of December 31, 2024 |
56,848 |
| Performance and employment conditions |
No performance conditions.
However, the beneficiaries of the plan must, on an ongoing basis, remain corporate officers or employees (full time or at least 80%) of the Company or of a direct or indirect subsidiary of the Company until the grant of the free ordinary shares allocated to them, except for the retirement provisions described below.
|
| Provisions relating to retirement |
The beneficiaries who retire in accordance with the age requirements of their pension plan prior to full vesting will be entitled to a pro rata number of shares for each unvested tranche based on the period from the date of grant to retirement in relation to the total term of the tranche in question, provided, however, that for purposes of this calculation, the term of the first tranche shall be considered to be one year. |
| Provisions relating to a change of control |
If a Change of Control takes place before December 14, 2025, and Article L.225-197-1, III of the French Commercial Code does not apply, the plan will be canceled and the Company will indemnify the beneficiaries for the loss of unvested free ordinary shares granted under the canceled plan, subject however for the beneficiaries that are corporate officers to the shareholders’ approval to the indemnity so allocated. The gross amount of this indemnity will be calculated as though such free shares had been vested upon the Change of Control. The conditions and limitations set forth in the applicable plan rules will apply to this calculation mutatis mutandis.
“Change of Control” shall mean that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L.233-3 of the French Commercial Code.
|
|
|
|
|
|
|
| Plan name |
Free ordinary share plan 2024-2027 |
| General Meeting date |
December 20, 2023 |
Board of Directors decision |
October 22, 2024 |
Free ordinary shares granted by the Board of Directors |
991,643 allocated in three tranches, each amounting to one third of the total ordinary shares granted by the Board of Directors. If one third is not a whole number, the number of free shares will be rounded down for the first two tranches and rounded up for the third tranche. |
| Duration of vesting period |
The vesting period is set at two (2) years as from October 22, 2024 for the first and the second tranches, and three (3) years as from October 22, 2024, for the third tranche. The vesting of free ordinary shares thus becomes final, for each tranche, at the end of the aforementioned vesting period, subject to the fulfillment of the employment condition described below. |
| Date of availability |
In accordance with the decisions of the Board of Directors of June 25, 2024 and October 22, 2024, and pursuant to Section II (5th paragraph) of Article L. 225-197-1 of the French Commercial Code when applicable, the executive corporate officers (CEO - Directeur Général, and Associate Managing Officers - Directeurs Généraux Délégués) as well as each other members of the Executive Committee, should keep not less than 20% of the vested free shares of each tranche until termination of their Executive Committee membership and, when applicable, their corporate office. |
Free ordinary shares fully vested as of December 31, 2024 |
0 |
Free ordinary shares being vested as of December 31, 2024 |
991,643 (including 436,534 by executive corporate officers) |
Free ordinary shares lapsed as of December 31, 2024 |
0 |
| Performance and employment conditions |
No performance conditions.
However, the beneficiaries of the plan must, on an ongoing basis, remain corporate officers or employees (full time or at least 80%) of the Company or of a direct or indirect subsidiary of the Company until the grant of the free ordinary shares allocated to them, except for the retirement provisions described below.
|
| Provisions relating to retirement |
The beneficiaries who retire in accordance with the age requirements of their pension plan prior to full vesting will be entitled to a pro rata number of shares for each unvested tranche based on the period from the date of grant to retirement in relation to the total term of the tranche in question, provided, however, that for purposes of this calculation, the term of the first tranche shall be considered to be one year. |
| Provisions relating to a change of control |
If a Change of Control takes place before October 22, 2026, and Article L.225-197-1, III of the French Commercial Code does not apply, the plan will be canceled and the Company will indemnify the beneficiaries for the loss of unvested free ordinary shares granted under the canceled plan, subject however for the beneficiaries that are corporate officers to the shareholders’ approval to the indemnity so allocated. The gross amount of this indemnity will be calculated as though such free shares had been vested upon the Change of Control. The conditions and limitations set forth in the applicable plan rules will apply to this calculation mutatis mutandis.
“Change of Control” shall mean that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L.233-3 of the French Commercial Code.
|
Phantom Shares
In recent years, we established Phantom Stock Option Programs with terms and conditions similar to the then-existing Employee Stock Option Plans (or “ESOPs”) described above, for employees who are U.S. tax citizens.
The Phantom Stock Option Programs are based on our share price and entitle the participants to a potential cash bonus if there has been an increase in our share price compared to the strike price at the grant date. Each employee participating in the program has phantom stock options potentially giving right to a certain number of phantom shares, which will be settled in cash instead of equity.
The overall objectives of the Phantom Stock Option Programs were (i) to retain certain employees who are U.S. citizens, (ii) to create long-term incentive for the participants, because they were not eligible for the ESOPs, and consequently (iii) to align the interests of our employees who are U.S. citizens and our employees eligible for the ESOPs.
The strike price per phantom share for each program is calculated on the basis of the volume-weighted average closing price of our shares on Euronext Paris during a period of 20 trading days prior to the grant of options under the parallel ESOP. Current strike prices are set in a range from €2.71 to €3.92. The phantom shares will be settled in cash until 2030 by subtracting the entry price per share from the market price per share and multiplying the result by the total number of granted phantom shares, but only if our market price per share at that date exceeds the strike price. The market price per share will be based on the closing price of our shares on Euronext Paris on the date of receipt of the exercise notice.
In 2020, we established a Phantom Free Share Plan for the benefit of senior managers who could not receive free ordinary shares under the free ordinary share plan 2019-2023 because they were not members of the Management Committee. This plan includes vesting and performance conditions similar to those of the free ordinary share plan 2019-2023, but provides for a settlement in cash instead of equity.
As of December 31, 2024, the Phantom Stock Option Programs and Phantom Free Share plan consisted of an aggregate of 39,500 phantom shares.
The Phantom Stock Option Programs and Phantom Free Share plan do not have any dilutive effect on our shareholders, as the phantom shares do not constitute or qualify for our ordinary shares.
The liability for the phantom plans is measured (initially at the end of each reporting period until settled) at the fair value of the share options rights, by applying an option pricing model taking into account the terms and conditions on which the phantom rights were granted and the extent to which the employees have rendered services to date.
C. Board Practices
On December 20, 2023, our shareholders approved the change from a two-tier governance system, under which we were governed by a Supervisory Board and Management Board, to a one-tier governance system led by a Board of Directors, with our senior management comprising an Executive Committee. Responsibility for the management of the Company rests with our Executive Committee, notably through our Chief Executive Officer (Directeur Général) and four other members appointed as Associate Managing Officers (Directeurs Généraux Délégués) who together form the Company’s General Management.
We present the details of the Board of Directors below.
Composition
The Board of Directors is composed of a minimum of three and a maximum of eighteen members. Directors are appointed for a renewable term of three years at the general meeting of shareholders. The age limit for the members of the Supervisory Board is 80, and no more than 20% of the directors may be over 75 years old. The limitations on holding such an appointment concurrently with an appointment in another company are subject to the applicable legal and regulatory provisions. Six out of seven of our directors were appointed by our shareholders during our Combined General Meeting on December 20, 2023, and one was newly appointed by our shareholders during our Combined General Meeting on June 26, 2024. The terms of Mr. Connolly, Ms. Graffin, Dr. Jansen, and Mr. Lingelbach will expire at the end of the annual General Meeting of shareholders to be held in June 2026. The terms of Mr. Sulat and Bpifrance Participations, represented as of the date of this annual report by Ms. Maïlys Ferrère, will expire at the end of the annual General Meeting of shareholders to be held in June 2025. The terms of Ms. Guyot-Caparros will expire at the end of the annual General Meeting of shareholders to be held in June 2027. The Ordinary General Meeting of shareholders may revoke the appointments of directors at any time by a simple majority vote. The directors are appointed by the shareholders and may be individuals or companies (represented by a designated individual). With the exception of Mr. Lingelbach, our Chief Executive Officer and member of the Board of Directors who has a Management Agreement with our subsidiary Valneva Austria GmbH, none of our directors serve pursuant to a service contract providing benefits upon termination of service as a director.
Role of the Board in Risk Oversight
Our Board of Directors is primarily responsible for the oversight of our risk management activities and has delegated to the Audit, Compliance and Risk Committee (as defined below) the responsibility to assist the Board in this task. While our Board oversees our risk management, our management, through the Executive Committee, is responsible for day-to-day risk management processes. Our Board expects our management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the Board.
We believe this division of responsibilities is the most effective approach for addressing the risks we face.
Committees
The Board of Directors has established four committees, each of which operates pursuant to rules of procedure adopted by the Board. These committees are the Audit, Compliance and Risk Committee, the Nomination, Governance and Compensation Committee, the Science and Technology Committee, and the ESG Committee. The Science and Technology Committee was previously referred to as the “Scientific Committee” until it was renamed in the internal rules adopted by our Board of Directors on September 25, 2024. The respective responsibilities of the Audit, Compliance and Risk Committee and the ESG Committee have been reviewed during 2024 to take account of the latest legislation on sustainability reporting.
Subject to available exemptions, the composition and functioning of all of our Committees will comply with all applicable requirements of the French Commercial Code, the Exchange Act, the Nasdaq listing rules and SEC rules and regulations.
In accordance with French law, Committees of our Board only have an advisory role and can only make recommendations to our Board of Directors. As a result, decisions are made by our Board of Directors taking into account non-binding recommendations of the relevant Board Committee.
Audit, Compliance and Risk Committee
The Audit, Compliance and Risk Committee assists our Board in its oversight of our corporate accounting and financial reporting and oversees the selection of our auditors, their remuneration and independence and keeps the Board informed on control systems, key processes and procedures, security and risks. In accordance with the operating rules adopted by the board, the committee is composed of at least three members (or their permanent representatives) appointed by the Board.
The members of our Audit, Compliance and Risk Committee as of the date of this annual report are Danièle Guyot-Caparros (chair), James Sulat and James Connolly. Our Board has determined that Ms. Guyot-Caparros, Mr. Sulat and Mr. Connolly are independent within the meaning of the applicable listing rules and the independence requirements contemplated by Rule 10A-3 under the Exchange Act. Our Board has further determined that Ms. Guyot-Caparros and Mr. Sulat are “Audit Committee Financial Experts” as defined by the Nasdaq listing rules and that each of the members qualifies as financially sophisticated under the Nasdaq listing rules.
The principal responsibility of our Audit, Compliance and Risk Committee is to monitor the existence and efficacy of our financial audit and risk control procedures on an ongoing basis.
The Committee supports the Board in fulfilling the Board’s oversight responsibilities with respect to the following:
•our corporate accounting and financial reporting processes,
•our sustainability information reporting processes,
•our systems of internal control over financial and sustainability reporting, and
•audits of our financial statements.
Additionally, the committee monitors and discusses the quality and integrity of our financial statements and our reports, including sustainability information included therein, by evaluating and overseeing the qualifications, independence and performance of the firm or firms engaged as our independent external auditors, both for the purpose of preparing or issuing an audit report or performing financial audit services, and for the purpose of certifying our reporting of sustainability information.
Nomination, Governance and Compensation Committee
Our Nomination, Governance and Compensation Committee assists our Board with respect to the appointment and the compensation of the members of our Board and Executive Committee. In accordance with operating rules adopted by the Board, the Nomination, Governance and Compensation Committee is composed of at least three members (or their permanent representatives) appointed by the Board.
The members of our Nomination, Governance and Compensation Committee as of the date of this annual report are Anne-Marie Graffin, James Sulat and James Connolly, all of whom are independent. Ms. Graffin served as the chair of the Committee until December 18, 2024 and was replaced by Mr. Connolly on that date.
Our Board of Directors has assigned the following duties specifically to the Nomination, Governance and Compensation Committee:
•reviewing our compensation policy, in particular the description of our collective objectives (applicable company-wide) and individual objectives (for members of the Executive Committee),
•reviewing the compensation of the members of our Executive Committee,
•examining and making proposals with respect to the various components of corporate officers’ compensation, the policy concerning the distribution of equity such as warrants, stock options, grants and capital increases reserved for members of our savings plan the allocation of incentive bonuses and all the provisions relating to retirement benefits and any other kind of benefit,
•examining the amount of attendance fees among Board members,
•assisting the Board in the selection of directors, the members of the Executive Committee, and the members of Board committees, and
•making recommendations with respect to the independence of the members of the Board and committees.
Science and Technology Committee (previously known as “Scientific Committee” until September 25, 2024)
Our Science and Technology Committee (initially established on December 20, 2023 as “Scientific Committee”) assists our Board in oversight of the Company’s research and development programs, portfolio strategy and capital allocation, and scientific and technological expertise.
In accordance with operating rules adopted by the Board, the Science and Technology Committee is composed of at least two members (or their permanent representatives) appointed by the Board. The members of our Science and Technology Committee are Kathrin Jansen and Thomas Lingelbach. Dr. Jansen is the chair of the committee.
Environmental, Social and Governance (ESG) Committee
Our Environmental, Social, and Governance Committee, or the ESG Committee, assists our Board in fulfilling its responsibilities relating to ESG matters under applicable law and as the Board may otherwise determine, including review of the Company’s ESG strategy and capital allocations and public disclosures on ESG matters. In accordance with operating rules adopted by the Board, the ESG Committee is composed of at least three members (or their permanent representatives) appointed by the Board. The members of our ESG Committee as of the date of this annual report are Thomas Lingelbach, Kathrin Jansen and Bpifrance Participations, represented by Maïlys Ferrère. Mr. Lingelbach is the chair of the committee.
D. Employees
As of December 31, 2024, we had a headcount of 713 employees located in Austria, Canada, France, Sweden, the United Kingdom and the United States. The table below shows the number of employees employed by us and each of our subsidiaries:
|
|
|
|
|
|
|
|
| Location |
Number of Employees |
| Valneva Austria GmbH |
305 |
| Valneva Canada Inc. |
10 |
| Valneva France SAS |
14 |
| Valneva Scotland Ltd |
155 |
| Valneva SE |
58 |
| Valneva Sweden AB |
129 |
| Valneva UK Ltd |
12 |
| Valneva USA, Inc. |
30 |
| Total |
713 |
Of these employees, approximately 41% were primarily engaged in manufacturing, 25% in research and development, 23% in general and administrative functions, and 10% in commercial operations.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing, and integrating our existing and new employees, advisors, and consultants. The principal purposes of our equity incentive plans are to attract, retain, and reward personnel through the granting of equity-based compensation awards in order to increase shareholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Pursuant to local laws, including the laws of France and Austria, some of our employees are covered by collective bargaining agreements.
E. Share Ownership
For information regarding the share ownership of our directors and executive officers, see “Item 6.B—Compensation” and “Item 7.A—Major Shareholders.”
F. Disclosure of Action to Recover Erroneously Awarded Compensation
Not applicable.
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
The following table and accompanying footnotes sets forth, as of December 31, 2024, information regarding beneficial ownership of our ordinary shares by:
•each person, or group of affiliated persons, known by us to beneficially own more than 5% of our ordinary shares;
•each of the members of our Board of Directors (including the former Chair of the Management Board) and our Associate Managing Officers (former Management Board members), individually or as a group.
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including free ordinary shares that vest within 60 days of December 31, 2024 and options that are currently exercisable or exercisable within 60 days of December 31, 2024. Ordinary shares subject to free ordinary shares that vest within 60 days of December 31, 2024, and options currently exercisable or exercisable within 60 days of December 31, 2024 are deemed to be outstanding for computing the percentage ownership of the person holding these free ordinary shares or options and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all ordinary shares shown that they beneficially own, subject to community property laws where applicable. The information does not necessarily indicate beneficial ownership for any other purpose, including for purposes of Sections 13(d) and 13(g) of the Securities Act.
Unless otherwise indicated in the notes under the table, the address of each beneficial owner listed in the table below is c/o Valneva SE, 6 rue Alain Bombard, 44800 Saint-Herblain, France.
|
|
|
|
|
|
|
|
|
|
Number of
Ordinary
Shares
Owned |
Percentage of
Ordinary Shares
Beneficially Owned |
|
| 5% Shareholders: |
|
|
CDC (Bpifrance Participations, CDC Croissance and CNP Assurances) (1) |
14,093,426 |
8.67 |
| Including Bpifrance Participations SA (legal entity member of the Board of Directors) |
8,619,478 |
5.30 |
Pfizer Inc.(2) |
9,554,395 |
5.88 |
Members of the Board of Directors (3): |
|
|
| Mr. Thomas Lingelbach |
556,536 |
* |
| Ms. Anne-Marie Graffin |
25,250 |
* |
| Mr. James Sulat |
97,367 |
* |
| Mr. James Connolly |
— |
|
— |
|
Ms. Danièle Guyot-Caparros |
— |
|
— |
|
| Ms. Kathrin Jansen |
— |
|
— |
|
Bpifrance Participations (represented by Maïlys Ferrère) |
8,619,478 |
5.30 |
Associate Managing Officers (members of the Management Board until December 20, 2023) (3): |
|
|
| Mr. Franck Grimaud |
685,831 |
* |
| Mr. Peter Bühler |
137,373 |
* |
| Mr. Juan Carlos Jaramillo |
99,305 |
* |
| Ms. Dipal Patel |
45,932 |
* |
| All members of our Board of Directors and Associate Managing Officers as a group |
15,741,020 |
9.54 |
% |
* Represents beneficial ownership of less than 1%.
(1)As reported in a Schedule 13D filed with the SEC on September 17, 2024. As of September 17, 2024, (i) Bpifrance Participations held directly 8,639,886 Ordinary Shares and 17,259,364 Voting Rights, and (ii) CDC Croissance held, through CDC PME CROISSANCE, 5,473,240 Ordinary Shares and 5,473,240 Voting Rights. Neither Bpifrance nor EPIC held any Ordinary Shares directly. Bpifrance may be deemed to be the beneficial owner of 8,639,886 Ordinary Shares and 17,259,364 Voting Rights indirectly through its 99.99%ownership of Bpifrance Participations. EPIC may be deemed to be the beneficial owner of 8,639,886 Ordinary Shares and 17,259,364 Voting Rights indirectly through its joint ownership and control of Bpifrance. CDC may be deemed to be the beneficial owner of (x) 8,639,886 Ordinary Shares and 17,259,364 Voting Rights, indirectly through its joint ownership and control of Bpifrance, (y) 5,473,240 Ordinary Shares and 5,473,240 Voting Rights, indirectly through its ownership of CDC Croissance and (z) 708 Ordinary Shares and 708 Voting Rights, indirectly through its ownership of CNP Assurances. And as completed by a change in balance of quantities of securities for Bpifrance Participations after a transaction on November 8, 2024.
(2)As reported in a notification of statutory threshold crossing addressed to the Company on September 23, 2024. The principal address for Pfizer Inc. is 235 E. 42nd Street, New York, NY 10017.
(3)The business address of Messrs. Thomas Lingelbach, Juan Carlos Jaramillo and Peter Bühler is located at Valneva Austria GmbH, Campus Vienna Biocenter 3, 1030, Vienna (Austria). The business address of Ms. Dipal Patel is located at: Valneva UK Ltd, Centaur House, Ancells Business Park, Ancells Road, Fleet, Hampshire, U51 2UJ (United Kingdom).
Significant Changes in Percentage Ownership
The significant changes in the percentage ownership held by our principal shareholders since January 1, 2022 were primarily the result of (i) our issuance and sale of 8,145,176 ordinary shares (including in the form of ADSs) in our May 2021 U.S. public offering and European private placement, (ii) our issuance and sale of 5,175,000 ordinary shares (including in the form of ADSs) in our November 2021 U.S. public offering and European private placement, (iii) our issuance and sale of 9,549,761 ordinary shares to Pfizer in June 2022, (iv) our issuance and sale of 21,000,000 ordinary shares (including in the form of ADSs) in our October 2022 U.S. public offering and European private placement and (v) our issuance and sale of 23,000,000 ordinary shares in our September 2024 Private Placement.
Voting Rights
A double voting right is attached to each registered share which is held in the name of the same shareholder for at least two years, starting from the registration of the Company in the form of a European company.
Shareholders in the United States
To our knowledge, as of October 31, 2024, 37,345,270 shares, or 22.98% of our ordinary shares outstanding at that date, were held of record by 50 residents of the United States.
B. Related Party Transactions
Related Person Transaction Policy
We comply with French law regarding approval of transactions with related parties.
The latest version of our Related Person Transaction Policy was adopted by the Board of Directors in December 2023. This policy sets forth our procedures for the identification, review, consideration and approval or ratification of Related Person Transactions (as defined below). For purposes of our policy only, a related person transaction is a transaction, arrangement or similar contractual relationship, or any series of similar transactions, arrangements or relationships, in which we and any related person are, were or will be participants and (a) the amount involved in the transaction exceeds $120,000 or (b) certain transactions that fall within the scope of the relevant provisions of the French Commercial Code, with the exception of usual transactions concluded under normal conditions. A related person is any member of the Board of Directors, a Directeur Général Délégué (certain members of our Executive Committee), or beneficial owner of more than 5% of any class of our voting securities, including any of their immediate family members and any entity owned or controlled by such persons.
Under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not initially identified as a related person transaction prior to consummation, our management must present information regarding the related person transaction to the Board of Directors for review, consideration, assessment, and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests, direct and indirect, of the related persons, the benefits to us of the transaction, and whether the transaction is on terms that are comparable to the terms available to or from, as the case may be, an unrelated third party or to or from employees generally. Under the policy, we will collect information that we deem reasonably necessary from each member of our Board of Directors and Executive Committee and, to the extent feasible, significant shareholders to enable us to identify any existing or potential related person transactions and to effectuate the terms of the policy.
In addition, under our Code of Conduct, our employees and the members of our Board of Directors and Executive Committee have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest.
In considering related person transactions, the Board of Directors will take into account the relevant available facts and circumstances including, but not limited to:
•the risks, costs and benefits to us;
•the impact on the independence of a member of the Board of Directors in the event the related person is a member of the Board of Directors, an Immediate Family Member of a member of the Board or an entity with which a member of the Board of Directors or a Directeur Général Délégué is affiliated;
•the availability of other sources for comparable services or products; and
•the terms available to or from, as the case may be, unrelated third parties or to or from employees generally.
The policy requires that, in determining whether to approve, ratify, or reject a Related Person Transaction, the Board of Directors must consider, in light of known circumstances, whether the transaction is in, or is not inconsistent with, our best interests and those of our shareholders, as the Board of Directors determines in the good faith exercise of its discretion.
Related Person Transactions
Since January 1, 2024, we have engaged in the following Related Person Transactions:
Indemnification Agreements
We entered into an indemnification agreement in 2024 with Ms. Danièle Guyot-Caparros, who became a member of our Board of Directors on June 26, 2024, under which she qualified as a Related Person.
We first entered into indemnification agreements with each of member of our then-existing Management Board (now members of the Executive Committee) and Supervisory Board (now the Board of Directors) in connection with our global offerings in 2021 and 2022, and we have since entered into new agreements with each of our new directors and officers.
With certain exceptions and subject to limitations on indemnification under French law, indemnification agreements provide for indemnification for damages and expenses including, among other things, attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in any action or proceeding arising out of his or her actions in that capacity. We have liability insurance for the members of our Executive Committee and Board of Directors. We believe that this insurance and these agreements are necessary to attract qualified members of the Executive Committee and Board of Directors.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Settlement Agreement with Mr. Frédéric Jacotot, former Associate Managing Officer (Directeur Général Délégué) of our Company
We entered into a settlement agreement to document the terms agreed with our former General Counsel, Mr. Frédéric Jacotot, regarding his retirement from Valneva on July 31, 2024.
Pursuant to the terms of the Settlement Agreement and in accordance with his Management Agreement, Mr. Jacotot received a gross termination indemnity of €152,062, equal to his 2024 annual base salary less the salary paid during his notice period (four months). This was approved by our shareholders at the June 2024 Annual General Meeting.
The Settlement Agreement further provides that Mr. Jacotot will keep the benefit of all tranches (three tranches) of free ordinary shares granted to him under Valneva’s 2022-2025 Free Share Plan.
Service Agreement (Contrat de Prestation de Services d’Accompagnement) executed with Bpifrance Participations
Pursuant to the European Corporate Sustainability Reporting Directive, we have an obligation to provide certain data relating to the environmental impact of our operations and to put in place a strategy relating to such impact.
In this context, we entered into a service agreement (Contrat de Prestation de Services d’Accompagnement) with a provider specialized in climate strategy to support the calculation of Scope 3 emissions and assist with preparing a climate strategy.
Bpifrance Participations, also a party to this agreement, acts as a financing provider, covering part of the fees we owe to the service provider (financing provided for the total amount of €18,300).
Transactions With Groupe Grimaud and Affiliates
In September 2018, we entered into a collaboration and research license agreement, or CRLA, with Groupe Grimaud La Corbière SA (now Groupe Grimaud Corbière SAS), or Groupe Grimaud, which was subsequently assigned to Vital Meat SAS, or Vital Meat, a French company and affiliate of Groupe Grimaud, for the purpose of collaborating with Groupe Grimaud to explore the possibility of using our avian cell lines to produce nutritional meat-like substances. Under this agreement, we granted Groupe Grimaud non-exclusive research license to use our EBx platform (excluding EB66), provided Groupe Grimaud with certain assistance and provided office space and certain equipment to Groupe Grimaud in connection with such research.
Following expiration of the CRLA, in May 2022 we entered into a sale and licensing agreement, or EBx Agreement, with Vital Meat, pursuant to which we agreed to sell our “Cleanmeat” patent and the EBx platform (excluding EB66) to Vital Meat and granted Vital Meat an exclusive commercial license to use certain Valneva know-how and patents in the context of developing nutritional meat-like substances. The EBx Agreement provides for upfront and milestone payments to Valneva of €4.0 million, royalties equal to three percent of net sales of products developed using the EBx platform, and sublicensing revenues ranging from 25% to 75%. We received €450,000 pursuant to the EBx Agreement in 2024 (€0 in 2023).
In June 2023, we amended certain non-financial terms of the EBx Agreement, including to allow for sublicensing and subcontracting relating to development activities.
Vital Meat also continues to rent certain of our office space and equipment pursuant to a premises and equipment provision agreement dating to September 2018 which was last amended in December 2024. Groupe Grimaud and affiliates paid €145,754 under this agreement in 2024 (2023: €129.3 thousand).
Since August 26, 2024, Groupe Grimaud La Corbière no longer holds more than 5% of our outstanding share capital, and the Groupe and its affiliates have therefore ceased to be related persons under our policy.
C. Interests of Experts and Counsel
Not applicable.
Item 8. Financial Information
A. Consolidated Statements and Other Financial Information
Consolidated Statements
Our consolidated financial statements are included as part of this Annual Report, starting at page F-1.
Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. For a description of certain legal matters, see the Notes to our consolidated financial statements included elsewhere in this Annual Report.
Dividend Policy
We have never declared or paid any dividends on our ordinary shares. Under our credit facility, except with respect to certain permitted dividend distributions, we are generally not permitted to declare or make any dividend with respect to our share capital. We do not anticipate paying cash dividends on our equity securities in the foreseeable future and intend to retain all available funds and any future earnings for use in the operation and expansion of our business, given our state of development.
Subject to the requirements of French law and our bylaws, dividends may only be distributed from our distributable profits, plus any amounts held in our available reserves which are reserves other than legal and statutory and revaluation surplus. Dividend distributions, if any in the future, will be made in euro and converted into U.S. dollars with respect to the ADSs, as provided in the deposit agreement.
B. Significant Changes
Not applicable.
Item 9. The Offer and Listing
A. Offer and Listing Details
Our ADSs have been listed on the Nasdaq Global Select Market under the symbol “VALN” since May 6, 2021. Our ordinary shares have been trading on Euronext Paris under the symbol “VAL” since May, 2013. Prior to that date, there was no public trading market for our ADSs or our ordinary shares.
B. Plan of Distribution
Not applicable.
C. Markets
Our ADSs have been listed on Nasdaq under the symbol “VALN” since May 6, 2021. Our ordinary shares have been trading on Euronext Paris under the symbol “VAL” May, 2013.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
Item 10. Additional Information
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
The information set forth in Exhibit 2.3 “Description of Securities” is incorporated herein by reference.
C. Material Contracts
Agreements Relating to Product Sales
Department of Defense Contracts
In September 2020, the U.S. Department of Defense, Defense Logistics Agency, or DLA, awarded us a new contract for the supply of IXIARO, following previous contracts we have had with DLA since January 2019. The terms of the agreement contemplated an initial base year followed by two option years, each with a range of minimum and maximum potential dose orders. The base year had a minimum value of approximately $53 million for 370,000 doses, and the first option year, which DLA exercised, had a minimum value of approximately $28.8 million for 200,000 doses. The second option year, which DLA did not exercise, had a minimum value of approximately $36 million for 250,000 doses. We also agreed to provide additional inventory after September 2023 to mitigate the potential impact of unused stock that may expire. This replacement inventory was to be provided without cost to DLA resulting in a contract liability amounting to $5.2 million (€4.7 million) recognized as of December 31,, 2023. The replacement inventory were provided during 2024, therefore the contract liability is nil as of December 31, 2024
In September 2023, the DLA awarded us a new one-year contract for the supply of IXIARO. The contract has a minimum value of approximately $32.3 million for approximately 200,000 doses. In January 2025, the DLA awarded us with a further one-year contract for the supply of IXIARO, with a minimum value of approximately $32.8 million for approximately 200,000 doses.
Since 2009, we have also had a Federal supply schedule contract with the Department of Veterans Affairs listing IXIARO.
Bavarian Nordic Distribution Agreements
In November 2020, Valneva Austria GmbH, or Valneva Austria, entered into a distribution agreement, or the IXIARO Distribution Agreement, with Bavarian Nordic A/S, or BN, pursuant to which Valneva Austria granted BN an exclusive right to import, market, promote, distribute, and sell IXIARO in Germany. In parallel, Valneva Sweden AB, or Valneva Sweden, entered into a distribution agreement, or the DUKORAL Distribution Agreement, with BN pursuant to which Valneva Sweden granted BN an exclusive right to import, market, promote, distribute, and sell DUKORAL in Germany. The IXIARO Distribution Agreement and the DUKORAL Distribution Agreement together are referred to as the BN Distribution Agreements.
In connection with BN’s purchase of the Vaxchora cholera vaccine, the DUKORAL Distribution Agreement was amended with effect in May 2023 to convert BN’s exclusive right to distribute DUKORAL to a non-exclusive distribution right and to terminate the agreement on December 31, 2025. The IXIARO Distribution Agreement will also terminate on December 31, 2025.
The BN Distribution Agreements include sub-distribution rights. Each of Valneva Austria and Valneva Sweden has a co-exclusive right to deliver, distribute, market, sell, promote, and import IXIARO and DUKORAL, as applicable, in Germany solely with respect to certain non-profit organizations. Pursuant to the BN Distribution Agreements, BN is required to use reasonable commercial efforts to promote, sell and distribute IXIARO and DUKORAL in Germany and is required to purchase an agreed upon minimum quantity of IXIARO and DUKORAL doses during each year of the BN Distribution Agreements. The DUKORAL Distribution Agreement, as amended, maintains this requirement for a minimum quantity but provides that this condition will lapse automatically if Valneva appoints another distributor of DUKORAL in Germany.
VBI Distribution Agreement
In December 2022, Valneva Austria GmbH entered into an agreement, or the VBI Distribution Agreement, with VBI Vaccines B.V., or VBI, relating to Valneva’s distribution of VBI’s hepatitis B vaccine PreHevbri, or the Product. The VBI Distribution Agreement had an initial term until December 31, 2025, with the possibility of renewal for an additional two years.
In July and August 2024, VBI’s parent company, VBI Vaccines Inc., commenced bankruptcy proceedings in Canada under the Companies’ Creditors Arrangement Act and in the United States under Chapter 15 of the Bankruptcy Code, and delisted from the Nasdaq Stock Market. In connection with these proceedings, VBI voluntarily withdrew PreHevbri from the market and Marketing Authorization for the product in the European Union was withdrawn. In light of these events, we consider the agreement to be terminated, and Valneva has taken steps to destroy any remaining stock of the product.
Agreements Relating to Product Development and Manufacturing
Pfizer License Agreement
In April 2020, we entered into a research collaboration and license agreement, or the Pfizer License, with Pfizer. In June 2022, Valneva Austria and Pfizer amended the Pfizer License. In connection with the Pfizer License, as amended, we granted to Pfizer (a) an exclusive, worldwide, sublicensable license under certain patents, know-how, and materials and (b) a non-exclusive, worldwide, sublicensable license under all patents, know-how or other intellectual property rights controlled by us, in each case to use, have used, develop, have developed, manufacture, have manufactured, commercialize, have commercialized and otherwise exploit VLA15 and related products for all therapeutic, diagnostic and prophylactic human and veterinary use. Under the Pfizer License, we also obtained, during the development term, a non-exclusive, royalty-free, fully paid-up, worldwide license with the right to sublicense to subcontractors under certain patents and know-how controlled by Pfizer and patents and know-how developed under the Pfizer License to perform development activities relating to VLA15 and related products.
We are obligated to grant licenses or sublicenses that are consistent with the Pfizer License directly to affiliates of Pfizer upon Pfizer’s written request. Each party also granted the other a non-exclusive, irrevocable, perpetual, royalty-free, fully paid-up worldwide license for research purposes with the right to sublicense to affiliates under its know-how, materials, and confidential information disclosed under the agreement.
In connection with the Pfizer License, we may not develop or exploit a competing product, and we must use commercially reasonable efforts to perform assigned obligations under a development plan. As partial consideration for the license grant, Pfizer paid us a one-time upfront payment of $130 million on June 15, 2020. We and Pfizer will each contribute towards development costs, and Pfizer is obligated to pay us up to $178 million in development milestones and low double-digit tiered royalties starting at 14% on net sales of licensed products, subject to specified offsets and reductions. Of this $178 million, (i) $143 million is comprised of additional payments related to the first stages of commercialization of VLA15 in the United States and Europe as well as the approval of the vaccine, (ii) $10 million is comprised of payments linked to development milestones related to the initiation of the VLA15-221 clinical study and was received in 2021, and (iii) $25 million related to the initiation of the Phase 3 clinical trial and was received in 2022. Royalties are payable on a licensed product-by-licensed product and country-by-country basis beginning with the first commercial sale of such licensed product in such country and ending on the last to occur of the date on which the sale, offer for sale or importation of such licensed product in such country would infringe, but for the license granted here, a valid claim covering such licensed product in such country and fifteen years after the first commercial sale of such licensed product in such country. In addition, the royalties will be supplemented by milestone payments of up to $100 million, payable to Valneva based on cumulative sales.
The Pfizer License expires on a country-by-country and licensed product-by-licensed product basis upon the expiration of the last royalty term for any licensed product in such country. Pfizer may terminate the agreement (a) on a licensed product-by-licensed product and country-by-country basis or in its entirety for convenience or any uncured material breach by us, (b) in whole or relevant part for certain violations of global trade control laws prior to the first regulatory approval of a licensed product, or (c) for our breach of certain representations and warranties or other failure to comply with specified laws. We may terminate the agreement on a licensed product-by-licensed product and country-by-country basis for any uncured material breaches by Pfizer of any of its diligence obligations, or in its entirety for any uncured material breach of the agreement by Pfizer.
Following the signature of the amendment to the Pfizer License in June 2022, Valneva will finance 40% of the costs of Phase 3 costs, compared to 30% in the initial agreement. In addition, Pfizer is paying Valneva royalties ranging from 14% to 22%, compared to royalties starting at 19% in the initial agreement.
On June 22, 2022, Pfizer invested €90.5 million ($95 million), or 8.1% of Valneva’s share capital at a price of €9.49 per share, through a reserved capital increase designed to strengthen the strategic partnership between the two companies in Lyme disease. Valneva used the proceeds of this investment to finance a portion of its contribution to the Phase 3 Lyme program.
CEPI Funding Agreements
In July 2019, we entered into a funding agreement, or the CEPI Agreement, with CEPI. In connection with the CEPI Agreement, we were awarded up to $23.4 million in funding (paid in a series of six-month tranches) to further develop a chikungunya vaccine, or the product, and we are obligated to provide equitable access to project results on the terms and conditions of the CEPI Agreement. Under the CEPI Agreement, equitable access means the regular supply of chikungunya vaccines in all Non-Traveler’s Market Countries (as defined in the CEPI Agreement, covering mostly low and middle income countries) that have a demand for the vaccines at an affordable price (as defined in the CEPI Agreement) and, in the context of an outbreak or increased outbreak preparation need, means that vaccines are first available to populations in the affected territory when and where they are needed. In addition, we granted CEPI a limited non-exclusive, fully paid-up, sublicensable license, referred to as the Public Health License, under the project results and other intellectual property necessary to enable CEPI or a third party designated by CEPI to develop, manufacture, market, and/or supply the product worldwide solely to end users in an affected territory in preparation for or response to an outbreak.
Such Public Health License shall only be effective upon specified license triggers.
Under the agreement, we were obligated to pay CEPI up to $7.0 million in commercial and related milestones, of which $3.0 million was paid in 2024, and to supply CEPI with specified quantities of the chikungunya drug product or investigational product in case of an outbreak or increased outbreak preparation need. This includes maintaining at our cost a one-year rolling safety stock comprised of not less than 200,000 doses of chikungunya vaccine, referred to as the Safety Stock. In case the Safety Stock is used to address an outbreak or increased outbreak preparation need, and CEPI wishes to replenish such Safety Stock, CEPI shall pay us the related production costs.
Either party may terminate the CEPI Agreement upon an uncured material breach of the agreement or insolvency of the other party. CEPI may also terminate the agreement if we are unable to discharge our obligations, for safety, regulatory, or ethical issues, if we do not satisfy specified criteria for funding, if there are material changes to the development plan without CEPI’s prior written consent, or during the term any affiliate to whom we have assigned or transferred the agreement ceases to be our affiliate. We may also terminate the agreement (in whole or with respect to certain markets) for convenience at any time after 10 years following the grant of U.S. marketing approval for the product, at any time after three years following the grant of U.S. marketing approval for the product if we are unable to sell the product at a viable price, or if CEPI transfers or assigns the agreement other than to specified entities. Following the last to occur of (a) the granting of U.S. marketing approval for the product and (b) such approval in the first low income country, in the event we undergo a change of control or sell the entire chikungunya business, we may also terminate the agreement. In each of these terminations by Valneva, we have obligations to collaborate with CEPI for two years to find a third party supplier to whom our obligations under the CEPI Agreement will be assigned and to transfer the drug substance and drug product technology and related intellectual property (with the exception of trademarks) to such third party supplier. In lieu of such transfer, after two years following termination, the CEPI Agreement will be suspended, except for certain continuing obligations, until we and CEPI agree to continue the program appropriate to the circumstances.
In connection with our obligations under the CEPI Agreement, and following the execution of a binding term sheet in May 2020, in January 2021 we entered into definitive agreements with Instituto Butantan, a Brazilian public institute, and Fundação Butantan, a Brazilian non-profitable private foundation of the Instituto Butantan, which we refer to jointly as Butantan, engaged in the research, development, manufacture, and commercialization of vaccines in Brazil, pursuant to which we and Butantan intend to collaborate to transfer our drug product technology to Butantan, to enable Butantan to develop, manufacture, and commercialize our chikungunya vaccine in Latin America and in certain low and middle income countries and obtain WHO prequalification. In turn, Butantan will support certain clinical and Phase 4 observational studies that we will use to meet regulatory requirements with the FDA. Butantan will also have to comply with certain CEPI requirements, among others, equitable access to the product and outbreak related obligations, including maintaining a Safety Stock.
In July 2024, we entered into a further funding agreement, or the 2024 CEPI Agreement, with CEPI, under which we will receive an additional $41.3 million in funding over the next five years from CEPI, with support from the European Union’s Horizon Europe program. This funding will be paid in a series of six-month tranches in advance of agreed milestones, and is intended to support post-marketing trials for IXCHIQ and potential label extensions to enable the vaccine to be administered to children, adolescents and pregnant women, including retroactive funding for certain activities commenced prior to the entry into the agreement.
The 2024 CEPI Agreement sets up a framework that applies to our relationship with Butantan and the Serum Institute of India (SII), which is further discussed below. Under the CEPI framework, we are required to prioritize the public health systems in Non Traveler’s Market Countries, taking into consideration public sector demand, production capacity and contractual obligations existing prior to any public sector purchase agreements. CEPI also retains the first right to provide additional funding and support for the further development, manufacture and deployment of the IXCHIQ vaccine in Non-Traveler’s Market Countries.
The 2024 CEPI Agreement sets up a governance structure that enables CEPI to be further involved in meetings with our partners, Butantan and SII, as well as in meetings with regulatory authorities to the extent permitted by such authorities.
Serum Institute of India Master Collaboration and License Agreement
In December 2024, we entered into an exclusive license agreement with the Serum Institute of India (SII) to expand access to our chikungunya vaccine to low and medium income countries in Asia. The agreement falls within the framework of the 2024 CEPI Agreement. Under the agreement, the parties will conduct a technology transfer of the drug product manufacturing process to SII, which will establish its own drug product manufacturing process and along with the drug substance from us, will manufacture its own vaccine and be responsible for seeking and maintaining regulatory approval of the vaccine in India and certain other countries in Asia.
SII will also be required to comply with certain CEPI requirements, including equitable access to the product and outbreak-related obligations, including maintaining a Safety Stock.
LimmaTech Development, Collaboration, License and Commercialization Agreement
In July 2024, Valneva Austria GmbH entered into an exclusive agreement with LMBT Biologics AG, or LimmaTech, to develop, manufacture and commercialize globally Shigella4V, or S4V, a vaccine candidate for the prevention of shigellosis. Under the terms of the agreement, LimmaTech received an upfront payment of €10 million, LimmaTech is also eligible to receive milestone payments over the course of the product candidate’s development, and royalties on sales of the product, if approved.
The level of royalty payments varies between developed countries, where the vaccine will be marketed to travelers, and low and middle income countries, or LMICs.
LimmaTech is responsible for the development of the product until the first Phase 2 controlled human infection model, or CHIM study, and we are responsible for subsequent development of the product candidate, as well as filing for regulatory approvals and commercialization. LimmaTech will be responsible for development costs up to a capped amount, and we will assume all subsequent development costs.
LimmaTech will retain ownership of its own IP assets under the agreement. After the end of Phase 2 trials, we have the right to take over the prosecution and maintenance of any of LimmaTech’s patent rights that solely cover S4V.
The agreement expires on a country-by-country basis, upon the expiration of the last royalty term for any licensed product in such country, up to the end of 2046 at the latest. Either party may terminate the agreement if there is a material breach of the terms which is not cured within the specified time period. LimmaTech is also permitted to terminate the agreement if we challenge the patent rights licenced under the agreement. Finally, by the end of the Phase 2 trials, if we are not in ongoing negotiations, or fail to enter into a sub-licence agreement with a partner to commercialize the product through publicly funded or other not-for-profit channels in LMIC territories within six months of the end of the Phase 2 trials, LimmaTech has the right to terminate the agreement only with respect to such LMIC territories.
After the completion of the Phase 2 CHIM study, we have the right to terminate the agreement, either with respect to the LMIC territory or in its entirety. We also have the right to terminate the agreement if the expected results in the Phase 2 trials is not met.
IDT Commercial Manufacturing Services Agreement and VLA1553 Product Schedule
In November 2021, Valneva Austria GmbH entered into a non-exclusive commercial manufacturing services agreement, or the IDT Agreement, with IDT Biologika GmbH, or IDT, pursuant to which IDT would provide contract manufacturing services under separate product schedules. The IDT Agreement will expire in November 2026 unless previously terminated. Valneva may terminate the IDT Agreement for convenience. Either party may terminate the IDT Agreement or the separate product schedules, in whole or in part, in case of material breach, insolvency, or certain compliance failures.
Valneva and IDT entered into a product schedule pertaining to the manufacturing of our chikungunya vaccine, or the VLA1553 Product Schedule, in December 2022. Pursuant to the VLA1553 Product Schedule, IDT performs the fill and finish and lyophilization of the drug product of our chikungunya vaccine (VLA1553) using bulk drug substance batches received from Valneva. The VLA1553 Product Schedule will remain in place until December 31, 2029 and will automatically renew thereafter unless previously terminated.
Manufacturing Agreement with Vetter Pharma International
In April 2023, Valneva Austria GmbH entered into a non-exclusive commercial manufacturing services agreement, or the Vetter Agreement, with Vetter Pharma International GmbH, or Vetter, pursuant to which Vetter will provide syringes pre-filled with sterilized water in connection with the manufacturing of IXCHIQ. The maximum estimated value of the Vetter Agreement during the initial term is approximately €26.9 million. The Vetter Agreement will expire in April 2028 unless previously terminated and may be renewed for subsequent terms. Either party may terminate the Vetter Agreement in case of breach or insolvency on the part of the other party, and Vetter may terminate in case of a change of control of Valneva involving a Vetter competitor or in case the parties cannot agree on changes to manufacturing processes or prices that may be requested by Valneva.
D. Exchange Controls
Under current French foreign exchange control regulations there are no limitations on the amount of cash payments that we may remit to residents of foreign countries. Laws and regulations concerning foreign exchange controls do, however, require that all payments or transfers of funds made by a French resident to a non-resident such as dividend payments be handled by an accredited intermediary. All registered banks and substantially all credit institutions in France are accredited intermediaries.
E. Taxation
Material U.S. federal income tax considerations for U.S. Holders
The following is a description of the material U.S. federal income tax consequences to the U.S. Holders described below of owning and disposing of our ordinary shares or ADSs. It is not a comprehensive description of all tax considerations that may be relevant to a particular person’s decision to acquire securities. This discussion applies only to a U.S. Holder that holds our ordinary shares or ADSs as a capital asset for tax purposes (generally, property held for investment). In addition, it does not describe all of the tax considerations that may be relevant in light of a U.S. Holder’s particular circumstances, including state, local, and non-U.S. tax considerations, estate and gift tax considerations, the impact of special tax accounting rules under Section 451(b) of the Code or the alternative minimum tax provisions of the Code, the potential application of the Medicare contribution tax, and tax considerations applicable to U.S. Holders subject to special rules, such as:
•banks, insurance companies, and certain other financial institutions;
•U.S. expatriates and certain former citizens or long-term residents of the United States;
•dealers or traders in securities who use a mark-to-market method of tax accounting;
•persons holding ordinary shares or ADSs as part of a hedging transaction, “straddle,” wash sale, conversion transaction or integrated transaction, or persons entering into a constructive sale with respect to ordinary shares or ADSs;
•persons whose “functional currency” for U.S. federal income tax purposes is not the U.S. dollar;
•brokers, dealers, or traders in securities, commodities, or currencies;
•tax-exempt entities or government organizations;
•S corporations, partnerships, or other entities or arrangements classified as partnerships for U.S. federal income tax purposes (and investors therein);
•regulated investment companies or real estate investment trusts;
•persons who acquired our ordinary shares or ADSs pursuant to the exercise of any employee stock option or otherwise as compensation;
•persons holding shares or ADSs in connection with a trade or business outside the United States;
•persons that own or are deemed to own ten percent or more of our shares (by vote or value); and
•persons holding our ordinary shares or ADSs in connection with a trade or business, permanent establishment, or fixed base outside the United States.
If an entity that is classified as a partnership for U.S. federal income tax purposes holds ordinary shares or ADSs, the U.S. federal income tax treatment of a partner will generally depend on the status of the partner and the activities of the partnership. Partnerships holding ordinary shares or ADSs and partners in such partnerships are encouraged to consult their tax advisors as to the particular U.S. federal income tax consequences of holding and disposing of ordinary shares or ADSs.
The discussion is based on the Code, administrative pronouncements, judicial decisions, final, temporary and proposed Treasury Regulations, and the income tax treaty between France and the United States, or the Treaty, all as of the date hereof, changes to any of which may affect the tax consequences described herein — possibly with retroactive effect.
A “U.S. Holder” is a holder who, for U.S. federal income tax purposes, is a beneficial owner of ordinary shares or ADSs and is:
(1)an individual who is a citizen or resident of the United States;
(2)a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, any state therein or the District of Columbia;
(3)an estate the income of which is subject to U.S. federal income taxation regardless of its source; or
(4)a trust if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) the trust has a valid election to be treated as a U.S. person under applicable U.S. Treasury Regulations.
U.S. Holders are encouraged to consult their tax advisors concerning the U.S. federal, state, local, and non-U.S. tax consequences of owning and disposing of ordinary shares or ADSs in their particular circumstances.
The discussion below assumes that the representations contained in the deposit agreement are true and that the obligations in the deposit agreement and any related agreement will be complied with in accordance with their terms. Generally, a holder of an ADS should be treated for U.S. federal income tax purposes as holding the ordinary shares represented by the ADS. Accordingly, no gain or loss will be recognized upon an exchange of ADSs for ordinary shares.
Passive Foreign Investment Company rules
Under the Code, we will be a PFIC for any taxable year in which (1) 75% or more of our gross income consists of passive income or (2) 50% or more of the value of our assets (generally determined on the basis of a weighted quarterly average) consists of assets that produce, or are held for the production of, passive income. For purposes of these tests, passive income includes dividends, interest, gains from the sale or exchange of investment property, and certain rents and royalties. Cash and cash-equivalents are passive assets for these purposes. In addition, for purposes of the above calculations, a non-U.S. corporation that directly or indirectly owns at least 25% by value of the shares of another corporation or partnership is treated as holding and receiving directly its proportionate share of assets and income of such corporation or partnership. If we are a PFIC for any taxable year during which a U.S. Holder holds our shares, the U.S. Holder may be subject to adverse tax consequences regardless of whether we continue to qualify as a PFIC, including ineligibility for any preferred tax rates on capital gains or on actual or deemed dividends, interest charges on certain taxes treated as deferred, and additional reporting requirements.
We do not believe that we were characterized as a PFIC for the year ended December 31, 2023. However, the determination of whether we are a PFIC is a fact-intensive determination made on an annual basis applying principles and methodologies that in some circumstances are unclear and subject to varying interpretation.
As a result, there can be no assurance that we will not be treated as a PFIC for the current or any future taxable year. In addition, the total value of our assets for PFIC testing purposes (including goodwill) may be determined in part by reference to the market price of our ordinary shares or ADSs from time to time, which may fluctuate considerably. Accordingly, if our market capitalization declines while we hold a substantial amount of cash and cash-equivalents for any taxable year we may be a PFIC for that taxable year. Under the income test, our status as a PFIC depends on the composition of our income for the relevant taxable year which will depend on the transactions we enter into in the future and our corporate structure. The composition of our income and assets is also affected by how we spend the cash we raise in any offering. Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position. Accordingly, our U.S. counsel expresses no opinion with respect to our PFIC status for any prior, current, or future taxable year.
If we are classified as a PFIC in any year with respect to which a U.S. Holder owns the ordinary shares or ADSs, we will continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which the U.S. Holder owns the ordinary shares or ADSs, regardless of whether we continue to meet the tests described above unless we cease to be a PFIC and the U.S. Holder has made a “deemed sale” election under the PFIC rules. If such a deemed sale election is made, a U.S. Holder will be deemed to have sold the ordinary shares or ADSs the U.S. Holder holds at their fair market value and any gain from such deemed sale would be subject to the rules described below. After the deemed sale election, so long as we do not become a PFIC in a subsequent taxable year, the U.S. Holder’s ordinary shares or ADSs with respect to which such election was made will not be treated as shares in a PFIC and the U.S. Holder will not be subject to the rules described below with respect to any “excess distribution” the U.S. Holder receives from us or any gain from an actual sale or other disposition of the ordinary shares or ADSs. U.S. Holders should consult their tax advisors as to the possibility and consequences of making a deemed sale election if we are a PFIC and cease to be a PFIC and such election becomes available.
For each taxable year that we are treated as a PFIC with respect to U.S. Holders, U.S. Holders will be subject to special tax rules with respect to any “excess distribution” such U.S. Holder receives and any gain such U.S. Holder recognizes from a sale or other disposition (including a pledge) of ordinary shares or ADSs, unless our ordinary shares or ADSs constitute “marketable stock” and such U.S. Holder makes a mark-to-market election (as discussed below). Distributions a U.S. Holder receives in a taxable year that are greater than 125% of the average annual distributions a U.S. Holder received during the shorter of the three preceding taxable years or the U.S. Holder’s holding period for the ordinary shares or ADSs will be treated as an excess distribution. Under these special tax rules:
•the excess distribution or gain will be allocated ratably over a U.S. Holder’s holding period for the ordinary shares or ADSs;
•the amount allocated to the taxable year of the disposition or distribution (as applicable), and any taxable year prior to the first taxable year in which we became a PFIC, will be treated as ordinary income; and
•the amount allocated to each other year will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year.
The tax liability for amounts allocated to years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the ordinary shares or ADSs cannot be treated as capital, even if a U.S. Holder holds the ordinary shares or ADSs as capital assets.
If we are a PFIC, a U.S. Holder will generally be subject to similar rules with respect to distributions we receive from, and our dispositions of the stock of, any of our direct or indirect subsidiaries or any other entities in which we hold equity interests that also are PFICs, or lower-tier PFICs, as if such distributions were indirectly received by, and/or dispositions were indirectly carried out by, such U.S. Holder. U.S. Holders should consult their tax advisors regarding the application of the PFIC rules to lower-tier PFICs.
U.S. Holders can avoid the interest charge on excess distributions or gain relating to the ordinary shares or ADSs by making an effective QEF Election. However, a U.S. Holder can only make a QEF election with respect to ordinary shares or ADSs in a PFIC if such company agrees to furnish such U.S. Holder with certain tax information annually. We do not presently intend to provide the information required to allow a U.S. Holder to make a QEF election if we are a PFIC.
U.S. Holders can avoid the interest charge on excess distributions or gain relating to the ordinary shares or ADSs by making a mark-to-market election with respect to the ordinary shares or ADSs, provided that the ordinary shares or ADSs are “marketable stock.” Ordinary shares or ADSs will be marketable stock if they are “regularly traded” on certain U.S. stock exchanges or on a non-U.S. stock exchange that meets certain conditions. For these purposes, the ordinary shares or ADSs will be considered regularly traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Any trades that have as their principal purpose meeting this requirement will be disregarded. Our ADSs will be listed on the Nasdaq Global Select Market, which is a qualified exchange for these purposes. Consequently, if our ADSs remain listed on the Nasdaq Global Select Market and are regularly traded, and you are a holder of ADSs, we expect the mark-to-market election would be available to U.S. Holders if we are a PFIC. Each U.S. Holder should consult its tax advisor as to the whether a mark-to-market election is available or advisable with respect to the ordinary shares or ADSs.
A U.S. Holder that makes a mark-to-market election must include in ordinary income for each year an amount equal to the excess, if any, of the fair market value of the ordinary shares or ADSs at the close of the taxable year over the U.S.
Holder’s adjusted tax basis in the ordinary shares or ADSs. An electing U.S. Holder may also claim an ordinary loss deduction for the excess, if any, of the U.S. Holder’s adjusted basis in the ordinary shares or ADSs over the fair market value of the ordinary shares or ADSs at the close of the taxable year, but this deduction is allowable only to the extent of any net mark-to-market gains for prior years. Gains from an actual sale or other disposition of the ordinary shares or ADSs in any year in which we are a PFIC will be treated as ordinary income, and any losses incurred on a sale or other disposition of the shares will be treated as an ordinary loss to the extent of any net mark-to-market gains for prior years. Once made, the election cannot be revoked without the consent of the IRS unless the ordinary shares or ADSs cease to be marketable stock.
However, a mark-to-market election generally cannot be made for equity interests in any lower-tier PFICs that we own, unless shares of such lower-tier PFIC are themselves “marketable stock.” As a result, even if a U.S. Holder validly makes a mark-to-market election with respect to our ordinary shares or ADSs, the U.S. Holder may continue to be subject to the PFIC rules (described above) with respect to its indirect interest in any of our investments that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. U.S. Holders should consult their tax advisors as to the availability and desirability of a mark-to-market election, as well as the impact of such election on interests in any lower-tier PFICs.
Unless otherwise provided by the U.S. Treasury, each U.S. shareholder of a PFIC is required to file an Annual Report containing such information as the U.S. Treasury may require. A U.S. Holder’s failure to file the Annual Report may result in substantial penalties and extend the statute of limitations with respect to the U.S. Holder’s federal income tax return. U.S. Holders should consult their tax advisors regarding the requirements of filing such information returns under these rules.
WE STRONGLY URGE YOU TO CONSULT YOUR TAX ADVISOR REGARDING THE IMPACT OF OUR PFIC STATUS ON YOUR INVESTMENT IN THE ORDINARY SHARES OR ADSs AS WELL AS THE APPLICATION OF THE PFIC RULES TO YOUR INVESTMENT IN THE ORDINARY SHARES OR ADSs.
Taxation of distributions
Subject to the discussion above under “Passive Foreign Investment Company rules,” distributions paid on ordinary shares or ADSs, other than certain pro rata distributions of ordinary shares or ADSs, will generally be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because we may not calculate our earnings and profits under U.S. federal income tax principles, we expect that distributions generally will be reported to U.S. Holders as dividends. Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be taxable at preferential rates applicable to “qualified dividend income.” However, the qualified dividend income treatment will not apply if we are treated as a PFIC with respect to the U.S. Holder for our taxable year of the distribution or the preceding taxable year. The amount of a dividend will include any amounts withheld by us in respect of French income taxes. The amount of the dividend will be treated as foreign-source dividend income to U.S. Holders and will not be eligible for the dividends-received deduction generally available to U.S. corporations under the Code. Dividends will generally be included in a U.S. Holder’s income on the date of the U.S. Holder’s receipt of the dividend. The amount of any dividend income paid in foreign currency will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt. Such gain or loss would generally be treated as U.S.-source ordinary income or loss. The amount of any distribution of property other than cash (and other than certain pro rata distributions of ordinary shares or ADSs or rights to acquire ordinary shares or ADSs) will be the fair market value of such property on the date of distribution.
For foreign tax credit purposes, our dividends will generally be treated as passive category income. Subject to applicable limitations, some of which vary depending upon the U.S. Holder’s particular circumstances, any French income taxes withheld from dividends on ordinary shares or ADSs at a rate not exceeding the rate provided by the Treaty will be creditable against the U.S. Holder’s U.S. federal income tax liability. U.S. Treasury regulations may in some circumstances prohibit a U.S. Holder from claiming a foreign tax credit with respect to certain foreign taxes that are not creditable under applicable tax treaties. The rules governing foreign tax credits are complex and U.S. Holders should consult their tax advisors regarding the creditability of foreign taxes in their particular circumstances. The rules governing foreign tax credits are complex and U.S. Holders should consult their tax advisors regarding the creditability of foreign taxes in their particular circumstances. In lieu of claiming a foreign tax credit, U.S. Holders may, at their election, deduct foreign taxes, including any French income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year.
Sale or other taxable disposition of ordinary shares and ADSs
Subject to the discussion above under “Passive Foreign Investment Company rules,” gain or loss realized on the sale or other taxable disposition of ordinary shares or ADSs will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the ordinary shares or ADSs for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ordinary shares or ADSs disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes. The deductibility of capital losses is subject to limitations.
If the consideration received by a U.S. Holder is not paid in U.S. dollars, the amount realized will be the U.S. dollar value of the payment received determined by reference to the spot rate of exchange on the date of the sale or other disposition.
However, if the ordinary shares or ADSs are treated as traded on an “established securities market” and you are either a cash basis taxpayer or an accrual basis taxpayer that has made a special election (which must be applied consistently from year to year and cannot be changed without the consent of the IRS), you will determine the U.S. dollar value of the amount realized in a non-U.S. dollar currency by translating the amount received at the spot rate of exchange on the settlement date of the sale. If you are an accrual basis taxpayer that is not eligible to or does not elect to determine the amount realized using the spot rate on the settlement date, you will recognize foreign currency gain or loss to the extent of any difference between the U.S. dollar amount realized on the date of sale or disposition and the U.S. dollar value of the currency received at the spot rate on the settlement date.
Information reporting and backup withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries generally are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding.
Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle it to a refund, provided that the required information is timely furnished to the IRS.
Information with respect to foreign financial assets
Certain U.S. Holders who are individuals and certain closely-held entities may be required to report information relating to the ordinary shares or ADSs, subject to certain exceptions (including an exception for ordinary shares or ADSs held in accounts maintained by financial institutions, in which case the accounts themselves may have to be reported if maintained by non-U.S. financial institutions). U.S. Holders should consult their tax advisors regarding their reporting obligations with respect to their ownership and disposition of the ordinary shares or ADSs.
Material French Tax Considerations
The following describes the material French income tax consequences to U.S. holders of purchasing, owning, and disposing of our ADSs.
This discussion does not purport to be a complete analysis or listing of all potential tax effects of the acquisition, ownership, or disposition of our ADSs to any particular investor, and does not discuss tax considerations that arise from rules of general application or that are generally assumed to be known by investors. All of the following is subject to change. Such changes could apply retroactively and could affect the consequences described below.
The following discussion does not address the French tax consequences applicable to securities (including ADSs) held in trusts. If ADSs are held in trust, the grantor, trustee, and beneficiary are advised to consult their own tax advisor regarding the specific tax consequences of acquiring, owning and disposing of such securities.
The description of the French income tax and real estate wealth tax consequences set forth below is based on the double tax treaty entered into between the Government of the United States of America and the Government of the French Republic for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and Capital of August 31, 1994, or the Treaty, which came into force on December 30, 1995 (as amended by any subsequent protocols, including the protocol of January 13, 2009), and the tax guidelines issued by the French tax authorities in force as of the date of this Annual Report, or the Treaty.
If a partnership holds ADSs, the tax treatment of the partnership and a partner in such partnership generally will depend on the status of the partner and the activities of the partnership. Such partner or partnership is urged to consult its own tax advisor regarding the specific tax consequences of acquiring, owning and disposing of ADSs.
This discussion applies only to investors that hold ADSs as capital assets that are entitled to Treaty benefits under the “Limitation on Benefits” provision contained in the Treaty, and whose ownership of the ADSs is not effectively connected to a permanent establishment or a fixed base in France. Certain U.S. holders may be subject to special rules not discussed below, and are advised to consult their usual tax advisor regarding the specific tax consequences which may apply to their particular situation.
U.S. holders are advised to consult their own tax advisor regarding the tax consequences of the purchase, ownership, and disposition of ADSs in light of their particular circumstances, especially with regard to the “Limitations on Benefits” provision contained in the Treaty.
Tax on Sale or Other Disposals
As a matter of principle, under French tax law, a U.S. holder should not be subject to any French tax on any capital gain from the sale, exchange, repurchase, or redemption by us of ADSs, provided such U.S. holder is not a French resident for French tax purposes and has not held more than 25% of our dividend rights, known as “droits aux benefices sociaux,” at any time during the preceding five years, either directly or indirectly, and, as relates to individuals, alone or with relatives (as an exception, a U.S. holder resident, established or incorporated in certain non-cooperative States or territories as defined in Article 238-0 A of the French tax code (“Code général des impôts,” or the FTC), other than those mentioned in Article 238-0 A, 2 bis, 2° of the FTC, may be subject to a 75% withholding tax in France on any such capital gain, regardless of the fraction of the dividend rights it holds).
Under application of the Treaty, a U.S. holder who is a U.S. resident for purposes of the Treaty and is entitled to Treaty benefits will not be subject to French tax on such capital gain unless the ADSs form part of the business property of a permanent establishment or fixed base that the U.S. holder has in France. U.S. holders who own ADSs through U.S. partnerships that are not resident for Treaty purposes are advised to consult their own tax advisor regarding their French tax treatment and their eligibility for Treaty benefits in light of their own particular circumstances. A U.S. holder that is not a U.S. resident for Treaty purposes or is not entitled to Treaty benefits (and in both cases is not resident, established or incorporated in certain non-cooperative States or territories as defined in Article 238-0 A of the FTC) and has held more than 25% of our dividend rights, known as “droits aux bénéfices sociaux” at any time during the preceding five years, either directly or indirectly, and, as relates to individuals, alone or with relatives may be subject to a levy in France (i) at the rate of 12.8% for individuals, and (ii) a rate of 25% for legal persons. Pursuant to Article 244 bis B of the FTC, such legal persons, whatever their form, may obtain a refund of the portion of such withholding tax which exceeds the corporate income tax which they would have been liable to pay if their registered seat had been located in France, provided that (i) they do not effectively either participate in our management or our control and (ii) their registered office is located in a State or territory that has concluded a tax treaty with France that contains an administrative assistance clause on the exchange of information and the fight against tax fraud and tax evasion and that is not a non-cooperative State or territory within the meaning of Article 238-0 A of the FTC.
Financial Transactions Tax and Registration Duties
Pursuant to Article 235 ter ZD of the FTC, purchases of ADSs of a French company listed on a regulated market of the European Union or on a foreign regulated market formally acknowledged by the AMF are subject to a 0.3% French tax on financial transactions for purchases made before April 1, 2025 and 0.4% as from this date provided that the issuer’s market capitalization exceeds 1 billion euros as of December 1 of the year preceding the taxation year. A list of companies whose market capitalization exceeds 1 billion euros as of December 1 of the year preceding the taxation year, within the meaning of Article 235 ter ZD of the FTC, is published annually by the French tax authorities in their official guidelines.
As at December 1, 2024, our market capitalization did not exceed 1 billion euros, pursuant to BOI-ANNX-000467-23/12/2024.
As a result, the acquisition of ADSs is currently out of the scope of the tax on financial transactions, but this may change in the future. Purchases of our ADSs may, however, become subject to the tax on financial transactions as from January 1, 2026, provided that our market capitalization exceeds 1 billion euros as at December 1, 2025 and that the Nasdaq Global Market is acknowledged by the French AMF.
In the case where Article 235 ter ZD of the FTC is not applicable, the French tax code provides that transfers of shares—issued by a French company which are listed on a regulated or organized market within the meaning of Articles L421-1 and L424-1 of French monetary code (Code monétaire et financier) or, pursuant to French tax administrative doctrine (BOI-ENR-DMTOM-40-10 24/04/2024 # 30), listed on another similar regulated or organized market operating under similar conditions—are subject to uncapped registration duties at the rate of 0.1% if the transfer is evidenced by a written deed (acte) executed either in France or outside France.
However neither the French tax code, nor case law or official guidelines published by the French tax authorities indicate if the transfer of ADSs should be in the scope of the above-mentioned registration duties. As a result, transfer of ADSs should remain outside of the scope of such registration duties. U.S. Holders are urged to consult their own tax advisor about the possible application of the registration duty upon the transfer of ADSs.
Taxation of Dividends
Dividends paid by a French corporation to non-residents of France are generally subject to French withholding tax at a rate of currently (i) 25% for payment benefiting legal persons which are not French tax residents, and (ii) 12.8% for payment benefiting individuals who are not French tax residents. Dividends paid by a French corporation in non-cooperative States or territories, as defined in Article 238-0 A of the FTC other than those mentioned in Article 238-0 A, 2 bis, 2° of the FTC, will generally be subject to French withholding tax at a rate of 75% unless the company which pays the dividend proves that the distribution of such proceeds in that State or territory has neither the object nor the effect of permitting their location in such State or territory for the purpose of tax evasion).
However, eligible U.S. holders entitled to Treaty benefits under the “Limitation on Benefits” provision contained in the Treaty who are U.S. residents, as defined pursuant to the provisions of the Treaty, will not be subject to this 12.8% or 25%, or 75% withholding tax rate, but may be subject to the withholding tax at a reduced rate (as described below).
Under the Treaty, the rate of French withholding tax on dividends paid to an eligible U.S. holder who is a U.S. resident as defined pursuant to the provisions of the Treaty and whose ownership of the ADSs is not effectively connected with a permanent establishment or fixed base that such U.S. holder has in France, is generally reduced to 15%, or to 5% if such U.S. holder is a corporation and owns directly or indirectly at least 10% of the share capital of the issuer; such U.S. holder may claim a refund from the French tax authorities of the amount withheld in excess of the Treaty rates of 15% or 5%, if any.
For U.S. holders that are not individuals but are U.S. residents, as defined pursuant to the provisions of the Treaty, the requirements for eligibility for Treaty benefits, including the reduced 5% or 15% withholding tax rates contained in the “Limitation on Benefits” provision of the Treaty, are complex, and certain technical changes were made to these requirements by the protocol of January 13, 2009. U.S. holders are advised to consult their own tax advisor regarding their eligibility for Treaty benefits in light of their own particular circumstances.
Dividends paid to an eligible U.S. holder may immediately be subject to the reduced rates of 5% or 15% provided that:
•such holder establishes before the date of payment that it is a U.S. resident under the Treaty by completing and providing the depositary with a treaty form (Form 5000) in accordance with French guidelines (BOI-INT-DG-20-20-20-20-12/09/2012); or
•the depositary or other financial institution managing the securities account in the U.S. of such holder provides the French paying agent with a document listing certain information about the U.S. holder and its ADSs and a certificate whereby the financial institution managing the U.S. holder’s securities account in the United States takes full responsibility for the accuracy of the information provided in the document.
Otherwise, dividends paid to a U.S. holder, if such U.S. holder is a legal person, will be subject to French withholding tax at the rate of 25%, or 75% if paid in certain non-cooperative States or territories (as defined in Article 238-0 A of the FTC other than those mentioned in Article 238-0 A, 2 bis, 2° of the FTC), and then reduced at a later date to 5% or 15%, provided that such holder duly completes and provides the French tax authorities with the treaty forms Form 5000 and Form 5001 before December 31 of the second calendar year following the year during which the dividend is paid. Certain qualifying pension funds and certain other tax-exempt entities are subject to the same general filing requirements as other U.S. holders except that they may have to supply additional documentation evidencing their entitlement to these benefits.
Form 5000 and Form 5001, together with instructions, will be provided by the depositary to all U.S. holders registered with the depositary. The depositary will arrange for the filing with the French tax authorities of all such forms properly completed and executed by U.S. holders of ADSs and returned to the depositary in sufficient time so that they may be filed with the French tax authorities before the distribution in order to immediately obtain a reduced withholding tax rate. Otherwise, the depositary must withhold tax at the full rate of 25% or 75% as applicable. In that case, the U.S. holders may claim a refund from the French tax authorities of the excess withholding tax.
Estate and Gift Taxes
In general, a transfer of securities by gift or by reason of death of a U.S. holder that would otherwise be subject to French gift or inheritance tax, respectively, will not be subject to such French tax by reason of the double tax treaty entered into between the Government of the United States of America and the Government of the French Republic for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Estates, Inheritances and Gifts, dated November 24, 1978 which came into force on October 1, 1980 (as amended by any subsequent protocols, including the protocol of December 8, 2004), unless (i) the donor or the transferor is domiciled in France at the time of making the gift or at the time of his or her death, or (ii) the ADSs were used in, or held for use in, the conduct of a business through a permanent establishment or a fixed base in France.
Wealth Tax
Since January 1, 2018, the French wealth tax (impôt de solidarité sur la fortune) has been repealed and replaced by the French real estate wealth tax (impôt sur la fortune immobilière). Its scope is narrowed to real estate assets (and certain assets deemed to be real estate assets) or rights, held directly or indirectly through one or more legal entities and whose net taxable assets amount at least to €1,300,000.
Broadly, subject to provisions of double tax treaties and to certain exceptions, individuals who are not residents of France for tax purposes within the meaning of Article 4 B of the FTC, are subject to real estate wealth tax (impôt sur la fortune immobilière) in France pursuant to French official guidelines BOI-INT-CVB-USA-10 19/02/2020 # 25) in respect of the portion of the value of their shares of our company representing real estate assets (Article 965, 2° of the FTC). Some exceptions are provided by the FTC. For instance, any participations representing less than 10% of the share capital of an operational company and shares representing real estate for the professional use of the company considered shall not fall within the scope of the French real estate wealth tax (impôt sur la fortune immobilière).
Under the Treaty (the provisions of which should be applicable to this real estate wealth tax (impôt sur la fortune immobilière) in France), the French real estate wealth tax (impôt sur la fortune immobilière) will however generally not apply to securities held by an eligible U.S. holder who is a U.S. resident, as defined pursuant to the provisions of the Treaty, provided that such U.S. holder (i) does not own directly or indirectly more than 25% of the issuer’s financial rights and (ii) that the ADSs do not form part of the business property of a permanent establishment or fixed base in France.
U.S. holders are advised to consult their own tax advisor regarding the specific tax consequences which may apply to their particular situation with respect to such French real estate wealth tax (impôt sur la fortune immobilière).
THE DISCUSSION ABOVE IS A SUMMARY OF THE MATERIAL FRENCH AND U.S. FEDERAL INCOME TAX CONSEQUENCES OF AN INVESTMENT IN OUR ADSs AND IS BASED UPON LAWS AND RELEVANT INTERPRETATIONS THEREOF IN EFFECT AS OF THE DATE OF THIS ANNUAL REPORT, ALL OF WHICH ARE SUBJECT TO CHANGE, POSSIBLY WITH RETROACTIVE EFFECT. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN ADSs IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. Nevertheless, we file with the SEC an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with an opinion expressed by an independent registered public accounting firm.
We maintain a corporate website at www.valneva.com. We intend to post our Annual Report on Form 20-F on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference.
The Securities and Exchange Commission maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding registrants, such as us, that file electronically with the SEC.
With respect to references made in this Annual Report to any contract or other document of our company, such references are not necessarily complete and you should refer to the exhibits attached or incorporated by reference to this Annual Report for copies of the actual contract or document.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders.
Not applicable.
Item 11. Quantitative and Qualitative Disclosures about Market Risk
We operate internationally and are exposed to foreign exchange risks arising from various currencies, primarily with respect to the British Pound (GBP), the Canadian Dollar (CAD), the Swedish Krona (SEK) and the U.S. Dollar (USD). The foreign exchange risks from the exposure to other currencies, including the Danish Krone, the Swiss Franc and the Norwegian Krone, are relatively limited. Foreign exchange risks arise from future commercial transactions, recognized assets and liabilities, and net investments in foreign operations. Our objective is to limit the potential negative impact of the foreign exchange rate changes, for example by currency conversion of cash and cash equivalents denominated in foreign currency and by using foreign currency options. We have certain investments in foreign operations, the net assets of which are exposed to foreign currency translation risk.
With all other variables held constant, the impact from changes in exchange rates on the pre-tax result would be as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| USD/EUR +10% |
(16,973) |
|
(24,079) |
|
|
| USD/EUR -10% |
20,745 |
|
29,430 |
|
|
| GBP/EUR +10% |
8,525 |
|
4,760 |
|
|
| GBP/EUR -10% |
(10,420) |
|
(5,817) |
|
|
| SEK/EUR +10% |
(5,725) |
|
(8,846) |
|
|
| SEK/EUR -10% |
6,998 |
|
10,812 |
|
|
| CAD/EUR +10% |
3,178 |
|
2,368 |
|
|
| CAD/EUR -10% |
(3,884) |
|
(2,894) |
|
|
The effect in the $/EUR relationship is mostly due to borrowings denominated in US-Dollar while the cash and working capital is predominantly on a Euro basis. Due to higher borrowings in the year ended December 31, 2024, the Group’s sensitivity has slightly increased. The Group has not used any hedging instruments to reduce the impact of foreign exchange rate changes.
A. Interest Rate Risk
We are exposed to market risks in connection with hedging both of our liquid assets and of our medium and long-term indebtedness and borrowings subject to variable interest rates. Borrowings issued at variable rates expose us to cash flow interest rate risks, which are offset by cash and financial assets held at variable rates. During 2024, as well as 2023 and 2022, the Group’s investments at variable rates, as well as the borrowings at variable rates, were denominated in EUR, SEK, USD, CAD, and GBP. We analyze our interest rate exposure on a dynamic basis. Based on this analysis, we calculate the impact on profit and loss of a defined interest rate change. The same interest rate change is used for all currencies. The calculation only includes investments in financial instruments and cash in banks that represent major interest-bearing positions. As at December 31, 2024 and December 31, 2023, no material interest risk was identified. In case of increasing interest rates, the positive effect from cash in banks will be higher than the negative effect from variable interest-bearing liabilities. In case of decreasing interest rates, there will be no material negative impact.
B. Credit Risk
We are exposed to credit risk. We hold bank accounts, cash balances, and securities at sound financial institutions with high credit ratings. To monitor the credit quality of our counterparts, we rely on credit ratings as published by specialized rating agencies such as Standard & Poor’s, Moody’s, and Fitch. We have policies that limit the amount of credit exposure to any single financial institution. We are also exposed to credit risks from our trade debtors, as our income from product sales, collaborations, licensing, and services arises from a small number of transactions. We have policies in place to enter into such transactions only with highly reputable, financially sound counterparts. If customers are independently rated, these ratings are used. Otherwise, when there is no independent rating, a risk assessment of the credit quality of the customer is performed, taking into account its financial position, past payment experience and other relevant factors. Individual credit limits are set based on internal or external ratings in accordance with signature authority limits as set by the Management Board.
C. Interim Periods
Not applicable.
D. Safe Harbor
This Annual Report contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act and as defined in the Private Securities Litigation Reform Act of 1995. See “Special Note Regarding Forward-Looking Statements.”
Item 12. Description of Securities Other than Equity Securities
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Citibank, as depositary, registers and delivers our ADSs. Each ADS represents two ordinary shares deposited with Citibank Europe plc, located at 1 North Wall Quay, Dublin 1 Ireland or any successor, as custodian for the depositary. Each ADS will also represent any other securities, cash or other property that may be held by the depositary. The depositary’s corporate trust offices at which the ADSs will be administered are located at 388 Greenwich Street, New York, New York 10013.
A deposit agreement among us, the depositary and the ADS holders sets out the ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. A copy of the deposit agreement is incorporated by reference as an exhibit to this Annual Report.
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
|
|
|
|
|
|
| Service |
Fees |
| Issuance of ADSs (e.g., an issuance of ADS upon a deposit of ordinary shares, upon a change in the ADS(s)-to-ordinary share ratio, or for any other reason), excluding ADS issuances as a result of distributions of ordinary shares |
Up to U.S. 5¢ per ADS issued |
| Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property, upon a change in the ADS(s)-to ordinary share ratio, or for any other reason) |
Up to U.S. 5¢ per ADS cancelled |
| Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) |
Up to U.S. 5¢ per ADS held |
| Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions, or (ii) exercise of rights to purchase additional ADSs |
Up to U.S. 5¢ per ADS held |
| Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) |
Up to U.S. 5¢ per ADS held |
| ADS Services |
Up to U.S. 5¢ per ADS held on the applicable record date(s) established by the depositary |
| Registration of ADS transfers (e.g., upon a registration of the transfer of registered ownership of ADSs, upon a transfer of ADSs into DTC and vice versa, or for any other reason) |
Up to U.S. 5¢ per ADS (or fraction thereof) transferred |
| Conversion of ADSs of one series for ADSs of another series (e.g., upon conversion of Partial Entitlement ADSs for Full Entitlement ADSs, or upon conversion of Restricted ADSs (each as defined in the Deposit Agreement) into freely transferable ADSs, and vice versa). |
Up to U.S. 5¢ per ADS (or fraction thereof) converted |
ADS holders are also responsible for paying certain charges such as:
•taxes (including applicable interest and penalties) and other governmental charges;
•the registration fees as may from time to time be in effect for the registration of ordinary shares on the share register and applicable to transfers of ordinary shares to or from the name of the custodian, the depositary, or any nominees upon the making of deposits and withdrawals, respectively;
•certain cable, telex, and facsimile transmission and delivery expenses;
•the fees, expenses, spreads, taxes, and other charges of the depositary and/or service providers (which may be a division, branch or affiliate of the depositary) in the conversion of foreign currency;
•the reasonable and customary out-of-pocket expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs and ADRs; and
•the fees, charges, costs, and expenses incurred by the depositary, the custodian, or any nominee in connection with the ADR program.
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person for whom the ADSs are issued (in the case of ADS issuances) and to the person for whom ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and (ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs.
In the case of (i) registration of ADS transfers, the ADS transfer fee will be payable by the ADS holder whose ADSs are being transferred or by the person to whom the ADSs are transferred, and (ii) conversion of ADSs of one series for ADSs of another series, the ADS conversion fee will be payable by the Holder whose ADSs are converted or by the person to whom the converted ADSs are delivered.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary. You will receive prior notice of such changes. The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Taxes
ADS holders are responsible for the taxes and other governmental charges payable on the ADSs and the securities represented by the ADSs. We, the depositary, and the custodian may deduct from any distribution the taxes and governmental charges payable by holders and may sell any and all property on deposit to pay the taxes and governmental charges payable by holders. You are liable for any deficiency if the sale proceeds do not cover the taxes that are due.
The depositary may refuse to issue ADSs, to deliver, transfer, split, and combine ADRs or to release securities on deposit until all taxes and charges are paid by the applicable holder. The depositary and the custodian may take reasonable administrative actions to obtain tax refunds and reduced tax withholding for any distributions on your behalf. However, you may be required to provide to the depositary and to the custodian proof of taxpayer status and residence and such other information as the depositary and the custodian may require to fulfill legal obligations. You are required to indemnify us, the depositary, and the custodian for any claims with respect to taxes based on any tax benefit obtained for you.
PART II
Item 13. Defaults, Dividend Arrearages and Delinquencies.
Not applicable.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
A. Not applicable.
B. Not applicable.
C. Not applicable.
D. Not applicable.
E. Use of Proceeds
May 2021 Global Offering
In May 2021, we announced the closing of a global offering to specified categories of investors of an aggregate of 8,145,176 new ordinary shares, after full exercise of the overallotment option granted to the underwriters. The public offering consisted of 2,850,088 ADSs, each representing two ordinary shares, in the United States at an offering price of $26.41 per ADS and a concurrent private placement of 2,445,000 ordinary shares in Europe (including in France) and other countries outside of the United States at the corresponding offering price of €11.00 per ordinary share. Gross proceeds of this global offering, after full exercise of the underwriters’ option were €89.6 million. Net proceeds of this global offering were €82.8 million.
Goldman Sachs Bank Europe SE, Jefferies International Limited, Jefferies GmbH, and Jefferies LLC were the representatives of the underwriters in this offering.
The net proceeds from this offering have been used, and are expected to continue to be used, as described in the final prospectus for the global offering filed with the U.S. Securities and Exchange Commission on May 7, 2021. None of the net proceeds of the global offering were paid directly or indirectly to any director, officer, or general partner of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
November 2021 Global Offering
In November 2021, we announced the closing of a global offering to specified categories of investors of an aggregate of 5,175,000 new ordinary shares, after full exercise of the overallotment option granted to the underwriters. The public offering consisted of 354,060 ADSs, each representing two ordinary shares, in the United States at an offering price of $39.4160 per ADS and a concurrent private placement of 4,466,880 ordinary shares in Europe (including in France) and other countries outside of the United States at the corresponding offering price of €17.00 per ordinary share. Gross proceeds of this global offering, after full exercise of the underwriters’ option, were approximately €88.0 million, whereas related expenses of €6.7 million were incurred. Net proceeds of this global offering were €81.3 million.
Goldman Sachs Bank Europe SE, Jefferies International Limited, Jefferies GmbH, and Jefferies LLC were the representatives of the underwriters in this offering.
The net proceeds from this offering have been used, and are expected to continue to be used, as described in the final prospectus for the global offering filed with the U.S. Securities and Exchange Commission on November 1, 2021. None of the net proceeds of the global offering were paid directly or indirectly to any director, officer, or general partner of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
October 2022 Global Offering
In October 2022, we announced the closing of a global offering to specified categories of investors of an aggregate of 21,000,000 new ordinary shares, after full exercise of the over-allotment option granted to the underwriters. The public offering consisted of 375,000 ADSs, each representing two ordinary shares, in the United States at an offering price of $9.51 per ADS and a concurrent private placement of 20,250,000 ordinary shares in Europe (including in France) and other countries outside of the United States at the corresponding offering price of €4.90 per ordinary share. Gross proceeds of this global offering, after full exercise of the underwriters’ option, were approximately €102.9 million, whereas related expenses of €7.4 million were incurred. Net proceeds of this global offering were €96.0 million.
Goldman Sachs Bank Europe SE, Jefferies GmbH, and Jefferies LLC were the representatives of the underwriters in this offering.
The net proceeds from this offering have been used, and are expected to continue to be used, as described in the final prospectus for the global offering filed with the U.S. Securities and Exchange Commission on September 30, 2022. None of the net proceeds of the global offering were paid directly or indirectly to any director, officer, or general partner of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
Item 15. Controls and Procedures
A. Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer (principal executive officer) and our chief financial officer (principal financial officer), has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13(a)-15(e) and 15(d)-15(e) under the Securities Exchange Act of 1934, as amended, “the Exchange Act”), as of December 31, 2024. Based on that evaluation, our principal executive officer and principal financial officer have concluded that our disclosure controls and procedures were effective as of December 31, 2024.
B. Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined under the Exchange Act) and for the assessment of the effectiveness of our internal control over financial reporting. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with International Financial Reporting Standards.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements, including the possibility of human error, the circumvention or overriding of controls, or fraud. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. Effective internal controls can provide only reasonable assurance with respect to the preparation and fair presentation of financial statements.
Under the supervision and with the participation of our chief executive officer (principal executive officer) and chief financial officer (principal financial officer), management conducted an assessment of the effectiveness of our internal control over financial reporting based upon the framework in “Internal Control — Integrated Framework (2013)” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this evaluation, management has concluded that our internal control over financial reporting was effective as of December 31, 2024. The effectiveness of our internal control over financial reporting as of December 31, 2024, has been audited by PricewaterhouseCoopers Audit and Deloitte & Associés, independent registered public accounting firms, as stated in their report which appears under Item 15 C.
C. Attestation Report of the Registered Public Accounting Firm
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMS
To the Shareholders and the Board of Directors of Valneva SE
Opinion on Internal Control over Financial Reporting
We have audited the internal control over financial reporting of Valneva SE and its subsidiaries (together the “Company”) as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the COSO.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated statement of financial position of the Company as of December 31, 2024, and the related consolidated statement of profit or loss, statement of comprehensive income, statement of changes in equity and statement of cash flows for the year ended December 31, 2024 (collectively the “consolidated financial statements”). Our report dated March 24, 2025 expressed an unqualified opinion on those financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying ”Management’s Annual Report on Internal Control over Financial Reporting” appearing under Item 15B. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are public accounting firms registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers Audit /s/ Deloitte & Associés
Neuilly-sur-Seine and Paris-La-Défense, France
March 24, 2025
PricewaterhouseCoopers Audit and Deloitte & Associés have served as the Company’s auditors since 2013 and 2007, respectively.
D. Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) that occurred during the period covered by this Annual Report that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [Reserved]
A. Audit Committee Financial Expert
Our Board of Directors has determined that Mr. Sulat, Mr. Connolly, and Ms. Guyot are independent within the meaning of the applicable listing rules and the independence requirements contemplated by Rule 10A-3 under the Exchange Act. Our Board of Directors has further determined that Mr. Sulat is an “audit committee financial expert” as defined by the Nasdaq listing rules and that each of the members qualifies as financially sophisticated under the Nasdaq listing rules.
B. Code of Ethics
We have adopted a Code of Conduct & Ethics applicable to all of our employees and members of our Board of Directors and Executive Committee. Our Code of Conduct & Ethics is available on our website. We expect that any amendments to the Code of Conduct & Ethics, or any waivers of its requirements, will be disclosed on our website. Under Item 16B of Form 20-F, if a waiver or amendment of the Code of Conduct & Ethics applies to our principal executive officer, principal financial officer, principal accounting officer, or controller and relates to standards promoting any of the values described in Item 16B(b) of Form 20-F, we are required to disclose such waiver or amendment on our website in accordance with the requirements of Instruction 4 to such Item 16B. The reference to our website is an inactive textual reference only and information contained in, or that can be assessed through, our website is not incorporated by reference into this Annual Report and does not constitute a part of this Annual Report.
C. Principal Accountant Fees and Services
PricewaterhouseCoopers Audit and Deloitte & Associés served as our independent auditors for the year ended December 31, 2024 and for all other reporting periods presented. The table below shows fees charged by those firms and member firms of their networks to Valneva and consolidated subsidiaries in the years ended December 31, 2024, 2023 and 2022.
Principal Accountant Fees and Services:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
|
PricewaterhouseCoopers |
Deloitte & Associés |
|
| in € thousand |
2024 |
% |
2023 |
% |
2022 |
% |
2024 |
% |
2023 |
% |
2022 |
% |
|
| Audit fees |
1,710 |
89 |
% |
2,076 |
98 |
% |
1,891 |
99 |
% |
1,311 |
89 |
% |
1,902 |
99 |
% |
1,678 |
99 |
% |
|
| provided by the statutory auditor |
1,272 |
66 |
% |
1,539 |
73 |
% |
1,386 |
72 |
% |
1,185 |
80 |
% |
1,622 |
84 |
% |
1,376 |
81 |
% |
|
| provided by the statutory auditor's network |
439 |
23 |
% |
537 |
25 |
% |
505 |
26 |
% |
126 |
9 |
% |
280 |
15 |
% |
302 |
18 |
% |
|
| Audit-related Fees |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
13 |
1 |
% |
|
| provided by the statutory auditor |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
13 |
1 |
% |
|
| provided by the statutory auditor's network |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
|
| Tax Fees |
45 |
2 |
% |
40 |
2 |
% |
25 |
1 |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
|
| provided by the statutory auditor's network |
45 |
2 |
% |
40 |
2 |
% |
25 |
1 |
% |
0 |
– |
% |
0 |
– |
% |
0 |
– |
% |
|
| All Other Fees |
163 |
8 |
% |
0 |
– |
% |
0 |
– |
% |
163 |
11 |
% |
19 |
1 |
% |
0 |
– |
% |
|
| Total |
1,918 |
100 |
% |
2,116 |
100 |
% |
1,916 |
100 |
% |
1,474 |
100 |
% |
1,921 |
100 |
% |
1,691 |
100 |
% |
|
“Audit fees” are the aggregate fees billed for the audit of our annual financial statements. This category also includes services that PricewaterhouseCoopers and Deloitte & Associés provides, such as consents and assistance with and review of documents filed with the SEC.
“Audit-related Fees” are the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit and are not reported under Audit Fees.
“Tax fees” are the aggregate tax fees billed for services related to the production of certification in the context of the declaration of expenses to obtain grants and prepare special reports relating to certain operations on the Company’s capital.
“All other fees” are the aggregate fees billed for the limited assurance review of sustainability reporting and verification of disclosure requirements set out in article 8 of Regulation (EU) 2020/852.
|
|
|
|
|
|
|
|
|
| Auditor Name |
Auditor Location |
Auditor Firm ID |
| PricewaterhouseCoopers Audit |
Neuilly-sur-Seine, France |
1347 |
| Deloitte & Associés |
Paris, France |
1756 |
Audit and Non-Audit Services Pre-Approval Policy
French law requires that audit committees pre-approve any non-audit services to be performed by a company’s statutory auditors. Additionally, French law requires audit committees to ensure that such non-audit services will not affect the independence of the statutory auditors in performing their audit services, and the fees received for non-audit services cannot exceed 70% of the total fees for audit services.
Accordingly, our Audit and Governance Committee, or the Committee, has authority to propose the retention and compensation of the Company’s registered public accounting firms and oversees the independence and performance of such firms with respect to both audit-related and non-audit-related services. The Committee may approve the provision of services other than the certification of financial statements by the auditors following an analysis of the potential impact of providing such services on the auditors’ independence and the approval of any safeguards that may be required to mitigate such impact.
Prior to engagement of any prospective auditors, the Committee reviews a written disclosure by the prospective auditors of all relationships between the prospective auditors, or their affiliates, and the Company, or persons in financial oversight roles at the Company, that may reasonably be thought to bear on independence and discusses with the prospective auditors the potential effects of such relationships on the independence of the prospective auditors, consistent with Ethics and Independence Rule 3526, Communication with Audit Committees Concerning Independence (“Rule 3526”), of the Public Company Accounting Oversight Board (United States). Consistent with Rule 3526, at least annually, the Committee receives and reviews written disclosures from the auditors delineating all relationships between the auditors, or their affiliates, and the Company, or persons in financial oversight roles at the Company, that may reasonably be thought to bear on independence and a letter from the auditors affirming their independence, and considers and discusses with the auditors any potential effects of any such relationships on the independence of the auditors as well as any compensation or services that could affect the auditors’ objectivity and independence.
The Committee has considered the non-audit services provided by PricewaterhouseCoopers and Deloitte & Associés as described above and believes that they are compatible with maintaining PricewaterhouseCoopers and Deloitte & Associés’s independence as our independent registered public accounting firms.
D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
Not applicable.
F. Changes to Certifying Accountant
Not applicable.
G. Corporate Governance
As a French société européenne, we are subject to various corporate governance requirements under French law. We are a “foreign private issuer” under the U.S. federal securities laws and the Nasdaq listing rules. The foreign private issuer exemption will permit us to follow home country corporate governance practices instead of certain Nasdaq listing requirements. A foreign private issuer that elects to follow a home country practice instead of Nasdaq listing requirements must submit to Nasdaq a written statement from an independent counsel in such issuer’s home country certifying that the issuer’s practices are not prohibited by the home country’s laws.
We apply the Middlenext code, which recommends that a majority of the members of the Board of Directors be independent (as such term is defined under the code). Neither the corporate laws of France nor our bylaws requires that (i) our compensation committee include only independent members of the Board of Directors, (ii) each committee of the Board of Directors have a formal written charter, or (iii) our independent members of the Board of Directors hold regularly scheduled meetings at which only independent members of the Board of Directors are present. We intend to continue to follow French corporate governance practices in lieu of Nasdaq listing requirements for each of the foregoing.
These exemptions do not modify the independence requirements for the audit and governance committee, and we intend to comply with the requirements of the Sarbanes-Oxley Act and the Nasdaq listing rules, which require that our audit and governance committee be composed of at least three independent members. Rule 10A-3 under the Exchange Act provides that the audit committee must have direct responsibility for the nomination, compensation and choice of our auditors, as well as control over the performance of their duties, management of complaints made, and selection of consultants.
Under Rule 10A-3, if the laws of a foreign private issuer’s home country require that any such matter be approved by the board of directors or our shareholders, the audit committee’s responsibilities or powers with respect to such matter may instead be advisory. Under French law, the audit committee may only have an advisory role and appointment of our statutory auditors, in particular, must be decided by our shareholders at our annual meeting.
In addition, Nasdaq rules require that a listed company specify that the quorum for any meeting of the holders of share capital be at least 331/3% of the outstanding shares of the company’s ordinary voting shares. We intend to continue to follow our French home country practice rather than complying with this Nasdaq rule. Consistent with French law, when first convened, general meetings of shareholders may validly convene only if the shareholders present or represented hold at least (i) 20% of the voting shares in the case of an ordinary general meeting or of an extraordinary general meeting where shareholders are voting on a capital increase by capitalization of reserves, profits, or share premium (the ordinary general meeting shall make its decision on a majority of half of the votes cast by the shareholders present or represented), or (ii) 25% of the voting shares in the case of any other extraordinary general meeting (the general meeting shall make its decision on a majority of two thirds of the votes cast by the shareholders present or represented). If such quorum required by French law is not met, the meeting is adjourned. There is no quorum requirement under French law when an ordinary general meeting or an extraordinary general meeting is reconvened where shareholders are voting on a capital increase by capitalization of reserves, profits or share premium, but the reconvened meeting may consider only questions that were on the agenda of the adjourned meeting. When any other extraordinary general meeting is reconvened, the required quorum under French law is 20% of the shares entitled to vote. If a quorum is not met at a reconvened meeting requiring a quorum, then the meeting may be adjourned for a maximum of two months.
Furthermore, Nasdaq’s corporate governance rules require listed U.S. companies to seek shareholder approval for the implementation of certain equity compensation plans and issuances of securities, which we are not required to, and do not intend to, follow as a foreign private issuer.
H. Mine Safety Disclosure.
Not applicable.
I. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
J. Insider Trading Policy
We have adopted an insider trading policy governing the purchase, sale, and other dispositions of our securities by directors, senior management, and employees that is reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, and the listing standards of Euronext Paris and the Nasdaq Global Select Market. A copy of our insider trading policy is filed as Exhibit 19.1 to this Annual Report on Form 20-F.
K. Cybersecurity
Risk management and strategy
We have implemented and maintain various information security processes designed to identify, assess and manage material risks from cybersecurity threats to our critical systems and information (collectively, our “Information Systems and Data”).
Our Information Technology department, with support from members of our Legal and Compliance teams and our Head of Risk Management, helps identify and assess cybersecurity risks and prepare the Company to respond to these risks. We use various methods for monitoring and evaluating threats to our environment including, for example: using manual and automated tools to detect anomalies and attempted attacks, subscribing to reports and services that identify cybersecurity threats, evaluating our and our industry’s risk profile, analyzing reports of threats and actors, conducting scans of our environment, evaluating threats reported to us, conducting internal and external audits, conducting threat assessments for internal and external threats, and conducting vulnerability assessments, including penetration tests.
Depending on the environment and system, we implement and maintain various technical, organizational, and physical measures, processes, standards and policies designed to manage and mitigate material risks from cybersecurity threats to our Information Systems and Data. These include, in addition to others discussed in this Item 16K, system monitoring, an incident detection and response plan, a disaster recovery plan, encryption and segregation of certain data, network security controls, and measures for the physical security of our technology infrastructure. We provide an annual information security awareness training to our employees and ask them to review certain information security policies on an annual basis.
Our identification, assessment and management of material risks from cybersecurity threats are integrated into the Company’s overall risk management processes. For example, we include information on cybersecurity risk evaluations conducted by management in reports provided to our internal Risk Management Committee, elements of which are shared with the Audit, Risk, and Compliance Committee (“the Audit Committee”) of our Board of Directors. Additionally, our Executive Committee may discuss cybersecurity risks and mitigation activities as part of its general risk management oversight.
Our Chief Financial Officer (CFO) is the member of our Executive Committee with functional responsibility for cybersecurity and may elevate cybersecurity topics for the attention of the Executive Committee, Audit Committee, and Board of Directors.
We use third-party service providers to assist us from time to time to identify, assess, and manage material risks from cybersecurity threats, including for example cybersecurity consultants, threat intelligence service providers, cybersecurity software and service providers, penetration testing firms, dark web monitoring services, forensic investigators, and professional services firms, including legal counsels.
Support elements for a variety of functions across our business are performed by third parties, such as distributors, contract manufacturing organizations, contract research organizations, application providers, and supply chain resources. We consider cyber risks in evaluating third parties and services, and our vendor management processes are tailored to our assessment of a particular vendor’s risk profile and criticality to our operations. Those processes may include, for example, some combination of the following: performing a risk assessment or issuing a security questionnaire, reviewing written security programs, performing certain vulnerability scans, conducting security assessment calls with the vendor’s security personnel, performing audits on the vendor’s compliance with our security requirements, or imposing contractual obligations relating to information security. Depending on the nature of the services provided, the sensitivity of the Information Systems and Data at issue, and the identity of the provider, our vendor management process may involve different levels of assessment designed to help identify and manage cybersecurity risks associated with a provider.
We have not identified risks from any known cybersecurity threats, including as a result of any prior cybersecurity incidents, that have materially affected us, including our operations, business strategy, results of operations, or financial condition. For a description of the risks from cybersecurity threats that may be reasonably likely to materially affect the Company and how they may do so, see our risk factors under Item 3D. Risk Factors in this Annual Report, including those described in “Risks Related to our Business Operations, Employee Matters and Managing Growth”.
Governance
Our Board of Directors considers the Company’s cybersecurity risk as part of its general oversight function. The Audit Committee is responsible for overseeing the Executive Committee’s implementation and enforcement of our cybersecurity risk management processes.
Our cybersecurity risk assessment and management processes are implemented and maintained by a management team comprised of our Vice President of Information Technology and Analytics (“VP of IT/Analytics”) and our CFO, to whom our VP of IT/Analytics reports. This management team is responsible for hiring appropriate personnel, managing spending relating to cybersecurity, providing information on cybersecurity risks, preparing for cybersecurity incidents, reviewing security assessments, approving cybersecurity processes and resources, and managing our response to significant cybersecurity incidents. The management team stays informed about and monitors efforts to prevent, detection, mitigate and remediate cybersecurity incidents through various means, which may include briefings with operational cybersecurity team members, outside threat intelligence sources, and from tooling described above that is deployed in our IT environment.
Individuals responsible for cybersecurity at an operational level within the Company have a minimum of five years experience in the field of information technology. For example, our Head of Information Security and Audit is certified within the TÜV Austria as a Manager and Auditor according to ISO 27001 & ISO 27002. We also have a Cyber Incident Response Team that includes the Head of Information Security, Data Protection Officer, and Director of Information Technology Infrastructure. This group may be expanded as needed to include representatives from our Legal and Corporate Communications teams as well as our Executive Committee, which is responsible for communicating with the Audit Committee or full Board of Directors as needed.
The Audit Committee receives regular reports from the VP of IT/Analytics concerning the Company’s significant cybersecurity threats and risks and the processes the Company has implemented to address them, as well as cybersecurity incidents deemed significant by the management team. The Audit Committee also has access to various reports, summaries or presentations related to cybersecurity threats, risk and mitigation.
Item 17. Financial Statements
See the financial statements beginning on page F-1 of this Annual Report.
Item 18. Financial Statements
Not applicable.
Item 19. Exhibits
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exhibit
Number |
Description of Document |
Incorporated by Reference |
|
Schedule/
Form
|
File Number |
Exhibit |
Filing Date |
| 1.1* |
|
20-F |
|
|
|
| 2.1 |
|
F-1/A |
333-255155 |
4.1 |
April 29, 2021 |
| 2.2 |
|
F-1/A |
333-255155 |
4.2 |
April 29, 2021 |
| 2.3* |
|
20-F |
|
|
|
| 4.1† |
|
F-1 |
333-255155 |
10.1 |
April 9, 2021 |
| 4.2† |
|
6-K |
001-40377 |
10.5 |
August 15, 2022 |
| 4.3† |
|
6-K |
001-40377 |
10.6 |
August 15, 2022 |
| 4.4† |
|
6-K |
001-40377 |
10.7 |
August 15, 2022 |
| 4.5† |
|
20-F |
001-40377 |
4.3 |
March 30, 2023 |
| 4.6† |
|
20-F |
001-40377 |
10.3 |
March 24, 2022 |
| 4.7† |
|
20-F |
001-40377 |
10.7 |
March 24, 2022 |
| 4.8† |
|
20-F |
001-40377 |
4.9 |
March 30, 2023 |
| 4.9† |
|
F-1 |
333-255155 |
10.4 |
April 9, 2021 |
4.10*† |
|
20-F |
|
|
|
4.11*† |
|
20-F |
|
|
|
4.12*† |
|
20-F |
|
|
|
4.13† |
|
F-1 |
333-255155 |
10.8 |
April 9, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4.14† |
|
F-1 |
333-260507 |
10.9 |
October 26, 2021 |
4.15† |
|
20-F |
001-40377 |
4.1 |
March 22, 2024 |
4.16*† |
|
20-F |
|
|
|
4.17† |
|
F-1 |
333-255155 |
10.10 |
April 9, 2021 |
4.18† |
|
F-1 |
333-255155 |
10.11 |
April 9, 2021 |
4.19† |
|
20-F |
001-40377 |
4.15 |
March 22, 2024 |
4.20† |
|
20-F |
001-40377 |
4.3 |
March 30, 2023 |
4.21† |
|
20-F |
001-40377 |
4.2 |
March 22, 2024 |
4.22† |
|
F-1 |
333-255155 |
10.6 |
April 9, 2021 |
| 4.23† |
|
F-1 |
333-255155 |
10.7 |
April 9, 2021 |
4.24† |
|
20-F |
001-40377 |
4.20 |
March 22, 2024 |
4.25† |
|
20-F |
001-40377 |
4.21 |
March 22, 2024 |
4.26† |
|
20-F |
001-40377 |
4.22 |
March 22, 2024 |
4.27†# |
Credit Agreement, dated February 3, 2020, by and among Valneva Austria GmbH, Valneva SE, Wilmington Trust, National Association and the Lenders, as amended to date on June 24, 2020, July 31, 2020, January 15, 2021, November 30, 2021, January 3, 2022, April 25, 2022, September 22, 2022, August 16, 2023, October 30, 2023 and March 18, 2024. |
20-F |
001-40377 |
4.23 |
March 22, 2024 |
| 4.28 |
|
6-K |
001-40377 |
1.1 |
August 15, 2022 |
4.29† |
|
20-F |
001-40377 |
4.25 |
March 22, 2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.38 |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.31+ |
|
F-1 |
333-255155 |
10.1 |
April 9, 2021 |
4.32+ |
|
F-1 |
333-255155 |
10.1 |
April 9, 2021 |
4.33+ |
|
F-1 |
333-255155 |
10.1 |
April 9, 2021 |
4.34+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.35+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.36+ |
|
20-F |
001-40377 |
4.32 |
March 22, 2024 |
4.37+ |
|
20-F |
001-40377 |
4.33 |
March 22, 2024 |
4.38+* |
|
20-F |
|
|
|
4.39+ |
|
20-F |
001-40377 |
4.38 |
March 30, 2023 |
4.40+ |
|
20-F |
001-40377 |
4.35 |
March 22, 2024 |
4.41+* |
|
20-F |
|
|
|
4.42+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.43+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.44+ |
|
20-F |
001-40377 |
4.38 |
March 22, 2024 |
4.45+* |
|
20-F |
|
|
|
4.46+ |
|
20-F |
001-40377 |
4.4 |
March 22, 2024 |
4.47+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.48+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
4.49+ |
|
F-1 |
333-255155 |
10.2 |
April 9, 2021 |
| 8.1 |
|
20-F |
001-40377 |
8.1 |
March 22, 2024 |
| 12.1* |
|
|
|
|
|
| 12.2* |
|
|
|
|
|
| 13.1** |
|
|
|
|
|
| 13.2** |
|
|
|
|
|
15.1* |
|
|
|
|
|
| 15.2* |
|
|
|
|
|
19.1* |
|
|
|
|
|
| 97.1 |
|
20-F |
001-40377 |
97.1 |
March 22, 2024 |
| 101.INS* |
XBRL Instance Document |
|
|
|
|
| 101.SCH* |
XBRL Taxonomy Extension Schema Document |
|
|
|
|
| 101.CAL* |
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
| 101.DEF* |
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 101.LAB* |
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
| 101.PRE* |
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
* Filed herewith.
** Furnished herewith.
+ Indicates management contract or compensatory plan.
† Certain portions of this exhibit have been omitted because they are not material and would likely cause competitive harm to the registrant if disclosed.
# Certain exhibits and schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant hereby undertakes to furnish supplementally a copy of any omitted exhibit or schedule upon request by the Securities and Exchange Commission.
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing this Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
VALNEVA SE
By: /s/ Thomas Lingelbach
Thomas Lingelbach
Chief Executive Officer
Date: March 24, 2025
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
|
|
|
|
|
|
|
| Report of Independent Registered Public Accounting Firm |
|
| Consolidated Financial Statements as at December 31, 2022 |
F-1 |
| Consolidated Statements of Income (Loss) and Comprehensive Income (Loss) |
F-6 |
| Consolidated Balance Sheets |
F-8 |
| Consolidated Statements of Cash Flows |
F-9 |
| Consolidated Statements of Changes in Equity |
F-10 |
| Notes to the Consolidated Financial Statements |
F-11 |
|
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRMS
To the Shareholders and the Board of Directors of Valneva SE
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Valneva SE and its subsidiaries (together the "Company") as of December 31, 2024 and 2023, and the related consolidated statement of profit or loss, statement of comprehensive income, statement of changes in equity and statement of cash flows for each of the three years in the period ended December 31, 2024, including the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company's internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 24, 2025 expressed an unqualified opinion on the Company's internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's consolidated financial statements based on our audits. We are public accounting firms registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits of these consolidated financial statements in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Other Revenues and Refund Liabilities – Research Collaboration and License Agreement with Pfizer
As described in Notes 5.3.1 Critical Judgments in Applying the Group’s Accounting Policies, 5.5.2 Other Revenues, and 5.29 Refund Liabilities to the Consolidated Financial Statements, in April 2020, the Company signed the Collaboration and License Agreement (the “Agreement”) with Pfizer to co-develop and commercialize a Lyme disease vaccine candidate. The Agreement is within the scope of IFRS 15 “Revenue from Contracts with Customers” and includes an exclusive license as well as research and development (“R&D”) and support services. In 2021 and 2022 several amendments were made to this Collaboration and License Agreement.
The considerations for this Agreement include a variable part. Variable considerations derive from upfront and milestone payments received and to be received from Pfizer, contribution from Valneva to Pfizer in shared development costs, as well as specific facts and circumstances which could potentially increase future payments to Pfizer. At the end of each reporting period, Valneva updates the estimated transaction price and its assessment of whether an estimate of variable considerations is constrained.
Revenue is recognized when the variable consideration constraint is removed and if it is highly probable that a significant revenue reversal will not occur.
Upfront, milestones and other payments received from Pfizer, net of contributions paid by Valneva to Pfizer amounted to €18.6 million as of December 31, 2024, and are recognized as refund liabilities, as Valneva concluded it is not highly probable that the Company will be entitled to the variable considerations, due to the possibility of additional payments to Pfizer while R&D activities are still progressing. Therefore, no revenue relating to the Agreement was recognized in 2024.
The principal considerations for our determination that performing procedures relating to other revenues and refund liabilities – research collaboration and license agreement with Pfizer - is a critical audit matter are (i) the significant judgment by management when estimating the transaction price and assessing whether an estimate of variable considerations is constrained and (ii) a high degree of auditor subjectivity in performing procedures in evaluating management’s estimates .
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the revenue recognition process, including control over the estimate of the transaction price. These procedures also included, among others, (i) evaluating the reasonableness of management’s assessment of whether the variable consideration is constrained and it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur; (ii) testing, on a sample basis, the contributions of Valneva and Pfizer to the refund liability; (iii) confirming with Pfizer the current terms of the Agreement and relevant balance sheet positions; and (iv) evaluating the sufficiency of the disclosure in the consolidated financial statements.
/s/ PricewaterhouseCoopers Audit /s/ Deloitte & Associés
Neuilly-sur-Seine and Paris-La-Défense, France
March 24, 2025
PricewaterhouseCoopers Audit and Deloitte & Associés have served as the Company’s auditors since 2013 and 2007, respectively.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
2 |
TABLE OF CONTENTS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 1 CONSOLIDATED STATEMENT OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME |
|
| 1.1 Consolidated Statement of Profit or Loss |
|
| 1.2 Consolidated Statement of Comprehensive Income |
|
| 2 CONSOLIDATED STATEMENT OF FINANCIAL POSITION |
|
| 3 CONSOLIDATED STATEMENT OF CASH FLOWS |
|
| 4 CONSOLIDATED STATEMENT OF CHANGES IN EQUITY |
|
| 5 NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS |
|
| 5.1 General information |
|
| 5.2 Summary of significant accounting policies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5.3 Critical accounting judgements and key sources of estimation uncertainty |
|
|
|
|
|
|
|
| 5.4 Segment information |
|
|
|
|
|
|
|
| 5.5 Revenues |
|
|
|
|
|
|
|
|
|
| 5.6 Expenses by nature |
|
| 5.7 Employee benefit expense |
|
| 5.8 Other income/(expenses), net |
|
|
|
|
|
| 5.9 Finance income/(expenses), net |
|
| 5.10 Income tax income/(expense) |
|
|
|
|
|
| 5.11 Earnings (Losses) per share |
|
| 5.12 Intangible assets |
|
| 5.13 Leases (right of use assets) |
|
|
|
|
|
| 5.14 Property, plant and equipment |
|
|
|
|
|
|
|
| 5.15 Impairment testing |
|
| 5.16 Financial instruments |
|
|
|
|
|
|
|
|
|
| 5.17 Inventories |
|
| 5.18 Trade receivables |
|
| 5.19 Other assets |
|
| 5.20 Cash and cash equivalents |
|
| 5.21 Assets classified as held for sale |
|
| 5.22 Equity |
|
|
|
|
|
| 5.23 Share-based compensation |
|
|
|
|
|
|
|
|
|
|
|
| 5.24 Borrowings |
|
|
|
|
|
|
|
| 5.25 Trade payables and accruals |
|
| 5.26 Tax and employee-related liabilities |
|
| 5.27 Lease liabilities |
|
| 5.28 Contract liabilities |
|
| 5.29 Refund liabilities |
|
| 5.30 Provisions |
|
|
|
|
|
| 5.31 Other liabilities |
|
| 5.32 Cash flow information |
|
|
|
|
|
| 5.33 Commitments and contingencies |
|
|
|
|
|
| 5.34 Related-party transactions |
|
|
|
|
|
|
|
| 5.35 Events after the reporting period |
|
|
|
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
3 |
1 CONSOLIDATED STATEMENT OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME
1.1 Consolidated Statement of Profit or Loss
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
Note |
2024 |
2023 |
2022 |
| Product sales |
5.5 |
163,253 |
|
144,624 |
|
114,797 |
|
| Other revenues |
5.5 |
6,325 |
|
9,088 |
|
246,506 |
|
| REVENUES |
|
169,579 |
|
153,713 |
|
361,303 |
|
| Cost of goods and services |
5.6 |
(98,538) |
|
(100,875) |
|
(324,441) |
|
| Research and development expenses |
5.6 |
(74,143) |
|
(59,894) |
|
(104,922) |
|
| Marketing and distribution expenses |
5.6 |
(52,356) |
|
(48,752) |
|
(23,509) |
|
| General and administrative expenses |
5.6 |
(42,750) |
|
(47,799) |
|
(34,073) |
|
| Gain from sale of Priority Review Voucher, net |
5.8 |
90,833 |
|
— |
|
— |
|
| Other income and expenses, net |
5.8 |
20,706 |
|
21,520 |
|
12,199 |
|
| OPERATING PROFIT/(LOSS) |
|
13,330 |
|
(82,087) |
|
(113,443) |
|
| Finance income |
5.9 |
2,362 |
|
1,210 |
|
260 |
|
| Finance expenses |
5.9 |
(23,984) |
|
(23,325) |
|
(19,054) |
|
| Foreign exchange gain/(loss), net |
5.9 |
(3,193) |
|
5,574 |
|
(12,587) |
|
| Result from investments in associates |
|
— |
|
— |
|
9 |
|
| PROFIT/(LOSS) BEFORE INCOME TAX |
|
(11,486) |
|
(98,629) |
|
(144,815) |
|
| Income tax benefit/(expense) |
5.10 |
(761) |
|
(2,800) |
|
1,536 |
|
| PROFIT/(LOSS) FOR THE PERIOD |
|
(12,247) |
|
(101,429) |
|
(143,279) |
|
|
|
|
|
|
| EARNINGS/(LOSSES) PER SHARE |
|
|
|
|
for profit/(loss) for the period attributable to the equity holders of the Company (expressed in € per share) |
|
|
|
|
| Basic |
5.11 |
(0.08) |
|
(0.73) |
|
(1.24) |
|
| Diluted |
5.11 |
(0.08) |
|
(0.73) |
|
(1.24) |
|
The accompanying Notes form an integral part of these financial statements.
1.2 Consolidated Statement of Comprehensive Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
Note |
2024 |
2023 |
2022 |
| PROFIT/(LOSS) FOR THE PERIOD |
|
(12,247) |
|
(101,429) |
|
(143,279) |
|
| OTHER COMPREHENSIVE INCOME/(LOSS) |
|
|
|
| Items that may be reclassified to profit or loss |
|
|
|
| Currency translation differences |
5.22.2 |
(1,329) |
|
3,300 |
|
(73) |
|
| Items that will not be reclassified to profit or loss |
|
|
|
| Defined benefit plan actuarial gains/(losses) |
5.30.1 |
49 |
|
(130) |
|
178 |
|
| Other comprehensive income/(loss) for the period, net of tax |
|
(1,281) |
|
3,170 |
|
105 |
|
| TOTAL COMPREHENSIVE INCOME/(LOSS) FOR THE PERIOD |
(13,527) |
|
(98,258) |
|
(143,174) |
|
The accompanying Notes form an integral part of these financial statements.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
4 |
2 CONSOLIDATED STATEMENT OF FINANCIAL POSITION
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
| in € thousand |
Note |
2024 |
2023 |
|
| ASSETS |
|
|
|
|
| Non-current assets |
|
201,020 |
|
197,238 |
|
|
| Intangible assets |
5.12 |
25,258 |
|
25,567 |
|
|
| Right of use assets |
5.13 |
19,232 |
|
20,392 |
|
|
| Property, plant and equipment |
5.14 |
138,883 |
|
136,198 |
|
|
|
|
|
|
|
| Deferred tax assets |
5.10.2 |
9,605 |
|
6,592 |
|
|
| Other non-current assets |
5.19 |
8,041 |
|
8,490 |
|
|
| Current assets |
|
299,012 |
|
262,824 |
|
|
| Inventories |
5.17 |
53,663 |
|
44,466 |
|
|
| Trade receivables |
5.18 |
35,205 |
|
41,645 |
|
|
| Other current assets |
5.19 |
41,874 |
|
50,633 |
|
|
| Cash and cash equivalents |
5.20 |
168,271 |
|
126,080 |
|
|
|
|
|
|
|
| TOTAL ASSETS |
|
500,032 |
|
460,062 |
|
|
| EQUITY |
|
|
|
|
| Share capital |
5.22 |
24,378 |
|
20,837 |
|
|
| Share premium |
5.22 |
647,600 |
|
594,003 |
|
|
| Other reserves |
5.22.2 |
73,203 |
|
65,088 |
|
|
| Retained earnings/(Accumulated deficit) |
|
(551,682) |
|
(450,253) |
|
|
| Profit/(Loss) for the period |
|
(12,247) |
|
(101,429) |
|
|
| TOTAL EQUITY |
|
181,253 |
|
128,247 |
|
|
| LIABILITIES |
|
|
|
|
| Non-current liabilities |
|
204,199 |
|
172,952 |
|
|
| Borrowings |
5.24 |
166,521 |
|
132,768 |
|
|
| Lease liabilities |
5.27 |
26,432 |
|
29,090 |
|
|
|
|
|
|
|
| Refund liabilities |
5.29 |
6,491 |
|
6,303 |
|
|
| Provisions |
5.30 |
546 |
|
1,074 |
|
|
| Deferred tax liabilities |
5.10.2 |
4,162 |
|
3,638 |
|
|
| Other non-current liabilities |
5.31 |
46 |
|
79 |
|
|
| Current liabilities |
|
114,580 |
|
158,863 |
|
|
| Borrowings |
5.24 |
20,852 |
|
44,079 |
|
|
| Trade payables and accruals |
5.25 |
35,522 |
|
44,303 |
|
|
| Income tax liability |
|
1,742 |
|
632 |
|
|
| Tax and Employee-related liabilities |
5.26 |
19,458 |
|
16,209 |
|
|
| Lease liabilities |
5.27 |
2,508 |
|
2,879 |
|
|
| Contract liabilities |
5.28 |
3,010 |
|
5,697 |
|
|
| Refund liabilities |
5.29 |
19,650 |
|
33,637 |
|
|
| Provisions |
5.30 |
6,686 |
|
10,835 |
|
|
| Other current liabilities |
5.31 |
5,152 |
|
592 |
|
|
|
|
|
|
|
| TOTAL LIABILITIES |
|
318,779 |
|
331,815 |
|
|
| TOTAL EQUITY AND LIABILITIES |
|
500,032 |
|
460,062 |
|
|
The accompanying Notes form an integral part of these financial statements.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
5 |
3 CONSOLIDATED STATEMENT OF CASH FLOWS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
| in € thousand |
Note |
2024 |
2023 |
2022 |
|
|
|
|
|
|
|
| CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
| Profit/(Loss) for the period |
|
(12,247) |
|
(101,429) |
|
(143,279) |
|
|
| Gain from sale of Priority Review Voucher, net |
|
(90,833) |
|
— |
|
— |
|
|
| Adjustments for non-cash transactions |
5.32.1 |
48,979 |
|
44,984 |
|
44,070 |
|
|
| Changes in non-current operating assets and liabilities |
5.32.1 |
(180) |
|
514 |
|
(147,713) |
|
|
| Changes in working capital |
5.32.1 |
(11,394) |
|
(145,578) |
|
1,732 |
|
|
| Cash used in operations |
5.32.1 |
(65,674) |
|
(201,509) |
|
(245,189) |
|
|
| Income tax paid |
|
(1,545) |
|
(1,236) |
|
(154) |
|
|
| NET CASH GENERATED FROM/(USED IN) OPERATING ACTIVITIES |
|
(67,218) |
|
(202,744) |
|
(245,343) |
|
|
|
|
|
|
|
|
| CASH FLOWS FROM INVESTING ACTIVITIES |
|
|
|
|
|
| Acquisition of subsidiaries, net of cash acquired |
5.1.2 |
— |
|
(10,951) |
|
— |
|
|
| Purchases of property, plant and equipment |
|
(13,865) |
|
(14,231) |
|
(29,246) |
|
|
| Proceeds from sale of property, plant and equipment |
|
165 |
|
111 |
|
8 |
|
|
| Purchases of intangible assets |
|
(2,579) |
|
(81) |
|
(76) |
|
|
|
|
|
|
|
|
| Proceeds from assets classified as held for sale |
|
— |
|
3,358 |
|
— |
|
|
|
|
|
|
|
|
| Proceeds from sale of Priority Review Voucher |
|
90,833 |
|
— |
|
— |
|
|
| Interest received |
|
2,362 |
|
1,210 |
|
260 |
|
|
| NET CASH GENERATED FROM/(USED IN) INVESTING ACTIVITIES |
|
76,916 |
|
(20,585) |
|
(29,054) |
|
|
|
|
|
|
|
|
| CASH FLOWS FROM FINANCING ACTIVITIES |
|
|
|
|
|
| Proceeds/(payments) from issuance of common stock, net of costs of equity transactions |
5.22 |
57,139 |
|
(240) |
|
189,837 |
|
|
|
|
|
|
|
|
| Proceeds from borrowings, net of transaction costs |
5.24 |
(34) |
|
81,111 |
|
39,331 |
|
|
| Repayment of borrowings |
5.24 |
(3,734) |
|
(2,097) |
|
(1,793) |
|
|
| Payment of lease liabilities |
5.27 |
(2,719) |
|
(3,127) |
|
(3,048) |
|
|
Interest paid1 |
|
(19,969) |
|
(12,567) |
|
(9,211) |
|
|
| NET CASH GENERATED FROM FROM/(USED IN) FINANCING ACTIVITIES |
|
30,682 |
|
63,081 |
|
215,116 |
|
|
|
|
|
|
|
|
| NET CHANGE IN CASH AND CASH EQUIVALENTS |
|
40,380 |
|
(160,248) |
|
(59,282) |
|
|
Cash and cash equivalents at beginning of the year2 |
5.20 |
126,080 |
|
286,532 |
|
346,642 |
|
|
| Exchange gains/(losses) on cash |
|
1,811 |
|
(204) |
|
(828) |
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS AT END OF THE PERIOD |
|
168,271 |
|
126,080 |
|
286,532 |
|
|
(1) Cash flows relating to the interest on the lease liabilities amounted to €0.8 million as at December 31, 2024 (2023: €1.2 million and 2022: €0.8 million)
(2) Cash and cash equivalents as at December 31, 2022 amounted to €289.4 million as it included restricted cash of €2.9 million.
The accompanying Notes form an integral part of these financial statements.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
6 |
4 CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Note |
|
Share capital |
Share premium |
Other reserves |
Retained earnings/
(Accumulated deficit) |
Profit/(loss)
for the period |
Total
equity |
| BALANCE AS AT JANUARY 1, 2024 |
|
|
20,837 |
|
594,003 |
|
65,088 |
|
(450,253) |
|
(101,429) |
|
128,247 |
|
| Total comprehensive income/(loss) |
|
|
— |
|
— |
|
(1,281) |
|
— |
|
(12,247) |
|
(13,527) |
|
| Income appropriation |
|
|
— |
|
— |
|
— |
|
(101,429) |
|
101,429 |
|
— |
|
| Share-based compensation expense: |
|
|
|
|
|
|
|
|
| Value of services |
5.23 |
|
— |
|
— |
|
9,395 |
|
— |
|
— |
|
9,395 |
|
| Exercises |
5.23 |
|
91 |
|
(91) |
|
— |
|
— |
|
— |
|
— |
|
| Capital Increase |
5.22 |
|
3,450 |
|
57,730 |
|
— |
|
— |
|
— |
|
61,180 |
|
| Cost of equity transaction, net of tax |
|
|
— |
|
(4,041) |
|
— |
|
— |
|
— |
|
(4,041) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE AS AT DECEMBER 31, 2024 |
|
|
24,378 |
|
647,600 |
|
73,203 |
|
(551,682) |
|
(12,247) |
|
181,253 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Note |
|
Share capital |
Share premium |
Other reserves |
Retained earnings/
(Accumulated deficit) |
Profit/(loss)
for the period |
Total
equity |
| BALANCE AS AT JANUARY 1, 2023 |
|
|
20,755 |
|
594,043 |
|
55,252 |
|
(306,974) |
|
(143,279) |
|
219,797 |
|
| Total comprehensive income/(loss) |
|
|
— |
|
— |
|
3,170 |
|
— |
|
(101,429) |
|
(98,258) |
|
| Income appropriation |
|
|
— |
|
— |
|
— |
|
(143,279) |
|
143,279 |
|
— |
|
| Share-based compensation expense: |
|
|
|
|
|
|
|
|
| Value of services |
5.23 |
|
— |
|
— |
|
6,666 |
|
— |
|
— |
|
6,666 |
|
| Exercises |
5.23 |
|
82 |
|
(39) |
|
— |
|
— |
|
— |
|
42 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE AS AT DECEMBER 31, 2023 |
|
|
20,837 |
|
594,003 |
|
65,088 |
|
(450,253) |
|
(101,429) |
|
128,247 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Note |
|
Share capital |
Share premium |
Other reserves |
Retained earnings/
(Accumulated deficit) |
Profit/(loss)
for the period |
Total
equity |
| BALANCE AS AT JANUARY 1, 2022 |
|
|
15,786 |
|
409,258 |
|
52,512 |
|
(233,549) |
|
(73,425) |
|
170,581 |
|
| Total comprehensive income/(loss) |
|
|
— |
|
— |
|
105 |
|
— |
|
(143,279) |
|
(143,174) |
|
| Income appropriation |
|
|
— |
|
— |
|
— |
|
(73,425) |
|
73,425 |
|
— |
|
| Share-based compensation expense: |
|
|
|
|
|
|
|
|
| Value of services |
5.23 |
|
— |
|
— |
|
2,636 |
|
— |
|
— |
|
2,636 |
|
| Exercises |
5.23 |
|
387 |
|
3,371 |
|
— |
|
— |
|
— |
|
3,758 |
|
| Capital Increase |
5.22 |
|
4,582 |
|
188,945 |
|
— |
|
— |
|
— |
|
193,527 |
|
| Cost of equity transaction, net of tax |
5.22 |
|
— |
|
(7,531) |
|
— |
|
— |
|
— |
|
(7,531) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE AS AT DECEMBER 31, 2022 |
|
|
20,755 |
|
594,043 |
|
55,252 |
|
(306,974) |
|
(143,279) |
|
219,797 |
|
The accompanying Notes form an integral part of these financial statements.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
7 |
5 NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
5.1 General information
5.1.1 Corporate Information
Valneva SE (the Company) together with its subsidiaries (the Group or Valneva) is a company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical needs. The Company takes a highly specialized and targeted approach, applying deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions. The Group has a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently markets three proprietary travel vaccines as well as certain third-party vaccines leveraging the Group’s established commercial infrastructure. Revenues from the growing commercial business help fuel the continued advancement of the vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, as well as vaccine candidates against the Zika virus, Shigella4V and other global public health threats.
VLA2001, the only inactivated whole-virus COVID-19 vaccine approved in Europe, was first commercialized in late 2021. Valneva suspended manufacturing of the vaccine in August 2022 and inventories were fully written down as of December 31, 2022. In order to save additional costs linked to the vaccine including license fees, Valneva requested the withdrawal of VLA2001’s marketing authorization in Europe. The withdrawal was accepted by EMA and became effective on December 1, 2023.
As at December 31, 2024, the Group’s portfolio includes three commercial vaccines:
▪IXIARO (also marketed as JESPECT), indicated for the prevention of Japanese encephalitis;
▪DUKORAL, indicated for the prevention of cholera, and, in some countries, prevention of diarrhea caused by enterotoxigenic Escherichia coli; and
▪IXCHIQ, indicated for the prevention of disease caused by the chikungunya virus.
The Company is registered at 6 rue Alain Bombard, 44800 Saint-Herblain, France. Valneva has operations in Austria, Sweden, the United Kingdom, France, Canada and the United States and had an average of 695 employees.
Valneva SE is a public company listed on the Euronext Paris (symbol: VLA) and on the Nasdaq Global Select Market (symbol: VALN) since May 2021.
Significant events of the period and significant agreements
Sale of Priority Review Voucher for $103 million
The Company sold the Priority Review Voucher (PRV) it received from the U.S. Food and Drug Administration (FDA) for $103 million (€95 million) on February 2, 2024.
The Company was awarded a tropical disease PRV in November 2023 following the FDA’s approval of IXCHIQ, Valneva’s single-dose, live-attenuated vaccine against the chikungunya virus (CHIKV). With this approval, IXCHIQ became the world’s first licensed chikungunya vaccine available to address this unmet medical need.
Valneva invested proceeds from the sale of the PRV into its R&D projects, including the co-development of its Phase 3 vaccine candidate against Lyme disease, additional clinical trials for IXCHIQ, and the expansion of the Company’s clinical pipeline.
Amendment of the D&O Loan Agreement
On March 18, 2024, Valneva signed an amendment to its loan agreement (the D&O Loan Agreement) with U.S. Healthcare funds Deerfield Management Company and OrbiMed.
Reimbursements of the first tranche will now start in January 2026 instead of July 2024. Maturity of this first tranche will remain in the first quarter of 2027. The interest-only period has been extended by 18 months. The terms of the second $100 million tranche remain unchanged. (see Note 5.24).
Health Canada Approval of the World’s First Chikungunya Vaccine, IXCHIQ
On June 24, 2024 Valneva announced that Health Canada had approved IXCHIQ for the prevention of disease caused by the chikungunya virus in individuals 18 years of age and older. This decision was the second approval the Company received for IXCHIQ following approval from the FDA in November 2023.
Marketing Authorization in Europe for the World’s First Chikungunya Vaccine,IXCHIQ
On July 1, 2024 Valneva announced that the European Commission (EC) had granted marketing authorization in Europe for IXCHIQ for the prevention of disease caused by the chikungunya virus in individuals 18 years of age and older. The approval was unanimously endorsed by Member States following a stringent assessment by the European Medicines Agency (EMA). The EC decision was the third approval the Company received for IXCHIQ.
|
|
|
|
|
|
|
|
|
|
VALNEVA SE - CONSOLIDATED FINANCIAL STATEMENTS 2024 |
8 |
Expanded partnership with CEPI
On July 22, 2024, Valneva announced that it had expanded its existing partnership with the Coalition for Epidemic Preparedness Innovations (CEPI) to support broader access to the world’s first chikungunya vaccine, IXCHIQ, in Low- and Middle-Income countries (LMICs), as well as post-marketing trials and potential label extensions in children, adolescents, and pregnant women. CEPI will provide Valneva up to $41 million of additional funding over the next five years, with support from the European Union’s (EU) Horizon Europe program.
The project will help generate additional data to potentially support extended IXCHIQ labels in chikungunya-endemic countries and vulnerable populations at risk of being infected with this debilitating mosquito-borne disease.
The expanded partnership strengthens an earlier agreement signed in 2019, which awarded Valneva $24.6 million in CEPI-EU funding to develop, manufacture, and market its single-shot vaccine in certain LMICs affected by chikungunya. Under this initial agreement, Valneva partnered with Brazil’s Instituto Butantan in 2021 and conducted an adolescent clinical trial in Brazil to support licensure of the vaccine in this country.
For more information please refer to Note 5.8.
Strategic Partnership with LimmaTech for development of a Shigella vaccine
On August 1, 2024, Valneva announced that it had entered into a strategic partnership with LimmaTech Biologics AG (Schlieren, Switzerland), a clinical-stage biotech company developing vaccines for the prevention of life-threatening diseases.
This includes an exclusive licensing agreement for the development, manufacturing, and commercialization of Shigella4V (S4V), a tetravalent bioconjugate vaccine candidate against shigellosis.
Under the terms of the agreement with Valneva, LimmaTech received an upfront payment of €10.0 million in exchange for granting an exclusive license for the development, manufacturing, and commercialization of Shigella4V. This payment also covers LimmaTech’s involvement in conducting specific clinical trials. The upfront payment was allocated across these activities, with a portion attributed to the licensing of intellectual property in the amount of €2.5 million and the remainder allocated to the development services provided.
LimmaTech is eligible to receive additional regulatory, development, and sales-based milestone payments as well as low double-digit royalties on sales. Valneva expects to pay €4.3 million for first milestones in 2025 and the remaining milestones thereafter. In addition to these milestones Valneva will also reimburse LimmaTech for development costs based on the contracted Development Plan.
LimmaTech is responsible for conducting a Phase 2 Controlled Human Infection Model (CHIM), started in the second half of 2024 and a Phase 2 pediatric study in LMICs, expected to begin in 2025. Valneva will be responsible for all further development, including CMC (chemistry, manufacturing, and controls) additional Phase 2 clinical trials, Phase 3 clinical trials and regulatory activities, and Valneva will be responsible for its commercialization worldwide if approved.
Successful Private Placement
On September 13, 2024, the Company announced the successful pricing of its Private Placement for a final amount of €61.2 million. A total of 23,000,000 new ordinary shares (the “Offer Shares”), each with a nominal value of €0.15, were issued at a price of €2.66 each, without shareholders’ preferential subscription rights, to a limited number of institutional investors within the United States or to U.S. persons who have represented that they are qualified institutional buyers, and outside the United States, to qualified investors under the European Prospectus Regulation and certain categories of institutional investors in countries outside of the European Union.
The Offer Shares represented 16.5% of the Company’s share capital on a non-diluted basis prior to the completion of the Private Placement and 14.2% of the Company’s share capital on a non-diluted basis following the Private Placement.
For more information please refer to Note 5.22.
Valneva Successfully Expands Access to Asia for its chikungunya vaccine with Serum Institute of India
On December 19, 2024, Valneva and the Serum Institute of India (SII) announced an exclusive license agreement for Valneva’s single-shot chikungunya vaccine to enable supply of the vaccine in Asia. The collaboration to support broader access to the vaccine in low-and-middle-income countries (LMICs) in the region falls within the framework of the $41.3 million funding agreement Valneva signed with the Coalition for Epidemic Preparedness Innovations (CEPI) in July 2024 with co-funding from the European Union.
Under the agreement, the Companies are conducting a technology transfer of the current drug product manufacturing process. Valneva will supply its chikungunya vaccine drug substance to SII, which will complete manufacturing and be responsible for seeking and maintaining regulatory approval of the vaccine in India and other countries in Asia. Future commercialization will be based on a profit-sharing model along with single-digit million milestone payments towards technology transfer and regulatory approvals to Valneva.
5.1.2 Group information
The following list shows all subsidiaries held by the Company directly or indirectly:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Name |
Country of incorporation |
Consolidation Method |
Interest held as at |
| December 31, 2024 |
December 31, 2023 |
| Vaccines Holdings Sweden AB |
SE |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva Austria GmbH |
AT |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva Canada Inc. |
CA |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva France SAS |
FR |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva Scotland Ltd. |
UK |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva Sweden AB |
SE |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva UK Ltd. |
UK |
Full Consolidation |
100 |
% |
100 |
% |
| Valneva USA, Inc. |
US |
Full Consolidation |
100 |
% |
100 |
% |
| VBC 3 Errichtungs GmbH |
AT |
Full Consolidation |
100 |
% |
100 |
% |
The closing date for the consolidated financial statements is December 31 of each year.
The Company’s site in Saint-Herblain houses general and administrative functions as well as research and development facilities. Valneva SE has a site in Lyon which serves as a base for commercial activities.
Vaccines Holdings Sweden AB, located in Solna, Sweden, is the holding company of Valneva Sweden AB, also located in Solna, which manufactures DUKORAL and commercializes DUKORAL, IXIARO, and third-party products such as Moskito Guard and other vaccines in the Nordic countries.
Valneva Austria GmbH, located in Vienna, Austria, focuses on pre-clinical and clinical development activities of vaccines. The facilities accommodate departments for pre-clinical R&D, technical/clinical product development, quality and regulatory affairs, general and administrative as well as commercial functions. Valneva Austria GmbH commercializes IXIARO, DUKORAL, and third-party products such as FLUCELVAX TETRA, FLUAD, Rabipur and Encepur.
Valneva Canada Inc., located in Kirkland, Canada, focuses on commercializing IXIARO, DUKORAL, IXCHIQ, and third-party products such as KAMRAB and Rabipur/RabAvert.
Valneva France SAS, located in Lyon, France, focuses on commercializing IXIARO, DUKORAL, IXCHIQ, and third-party products such as Rabipur and Encepur.
Valneva Scotland Ltd., located in Livingston, Scotland (United Kingdom) is primarily involved in the production of IXIARO and IXCHIQ.
Valneva UK Ltd., located in Fleet, England (United Kingdom), focuses on commercializing DUKORAL, IXIARO, and third-party products such as Rabipur in the United Kingdom.
Valneva USA, Inc., located in Bethesda, Maryland (USA), focuses on the commercialization of IXIARO and IXCHIQ to the U.S. military and the U.S. private market.
VBC 3 Errichtungs GmbH (Vienna, Austria), owns the administration and research building used by Valneva Austria GmbH.
Acquisition of VBC 3 Errichtungs GmbH (VBC3)
On October 31, 2023, the Group acquired 100% of the equity of VBC 3 Errichtungs GmbH, located in Vienna, Austria, whereby Valneva SE purchased 6% and Valneva Austria GmbH 94% of the equity. VBC3 owns the building in which Valneva Austria GmbH carries out central administrative and R&D activities. Previously, the building was under a finance lease. The purchase was treated as an acquisition of a group of assets, and the cost of the purchase was allocated to the individual identifiable assets and liabilities on the basis of their relative fair values at the date of purchase.
The following table summarizes the recognized amounts of identifiable net assets based on their relative fair values at the acquisition date which was determined as October 1, 2023 as per contract details:
|
|
|
|
|
|
| in € thousand |
October 1, 2023 |
| Cash |
1,003 |
|
| Property, Plant & Equipment |
22,373 |
|
| Loans and borrowings |
(11,296) |
|
| Other liabilities |
(126) |
|
| TOTAL IDENTIFIABLE NET ASSETS |
11,955 |
|
The fair value of the consideration transferred, excluding the entity’s cash of €1.0 million, was €11.0 million and was settled in cash. Acquisition-related costs were of minor relevance and are not included as part of consideration transferred. They have been recognized as an expense in the consolidated statement of profit or loss, as part of other expenses.
5.2 Summary of significant accounting policies
The principal accounting policies applied in preparing these consolidated financial statements are outlined below. These policies have been consistently applied to all years presented.
5.2.1 Basis of preparation
These 2024 Consolidated Financial Statements have been prepared in accordance with the International financial reporting standards, which comprise IFRS (International Financial Reporting Standards), IAS (International Accounting Standard) and their interpretations, and IFRIC (International Financial Reporting Interpretations Committee), as issued by the International Accounting Standards Board (IASB).
The preparation of financial statements in conformity with IFRS as issued by the IASB requires the use of certain critical accounting estimates. It also requires the Group’s management to exercise its judgement in applying the Group’s accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in Note 5.3.
For ease of presentation, numbers have been rounded and, where indicated, are presented in thousands of Euros. Calculations, however, are based on exact figures. Therefore, the sum of the numbers in a column of a table may not conform to the total figure displayed in the column.
These consolidated financial statements were approved and authorized for issuance by the Board of Directors on March 19, 2025.
5.2.2 Impact of new, revised or amended Standards and Interpretations
Standards, amendments to existing standards and interpretations issued by IASB whose application has been mandatory since January 1, 2024
|
|
|
|
|
|
|
|
|
|
|
|
| New standards, Interpretations and amendments adopted by the Group |
Effective date |
Effects |
| IFRS 16 |
Amendments to IFRS 16 Leases: Lease Liability in a Sale and Leaseback |
January 1, 2024 |
none |
| IAS 1 |
Amendments to IAS 1: Classification of Liabilities as Current or Non-current and Non-current liabilities with covenants |
January 1, 2024 |
none |
| IAS 7 & IFRS 7 |
Amendments to IAS 7 Statement of Cash Flows and IFRS 7 Financial Instruments: Disclosures: Supplier Finance Arrangements |
January 1, 2024 |
none |
The amendments listed above did not have any material impact on the amounts recognized in prior periods and are not expected to significantly affect the current or future periods.
Standards, amendments to existing standards and interpretations whose application is not yet mandatory.
The Group did not elect to apply early the following new standards, amendments, and interpretations which were issued but not mandatory as at January 1, 2024.
|
|
|
|
|
|
|
|
|
|
|
|
| New standards, Interpretations and amendments |
Effective date |
Effects |
IAS 21 |
Amendments to IAS 21 The Effects of Changes in Foreign Exchange Rates: Lack of Exchangeability |
January 1, 2025 |
none |
| IFRS 18 |
New standard, IFRS 18 Presentation and Disclosures in Financial Statements |
January 1, 2027 |
under assessment |
| IFRS 19 |
New standard, IFRS 19 Subsidiaries without Public Accountability: Disclosures |
January 1, 2027 |
none |
| IFRS 7 & IFRS 9 |
Amendments IFRS 9 and IFRS 7 regarding the classification and measurement of financial instruments |
January 1, 2026 |
none |
| Annual improvements to IFRS – Volume 11 |
Amendments to IFRS 1 First-time Adoption of International Financial Reporting Standards: Hedge accounting by a first-time adopter |
January 1, 2026 |
none |
| Amendments to IFRS 7 Financial Instruments: Disclosures: Gain or loss on derecognition, Disclosure of deferred difference between fair value and transaction price, Introduction and credit risk disclosures |
January 1, 2026 |
none |
| Amendments to IFRS 9 Financial Instruments: Lessee derecognition of lease liabilities, Transaction price |
January 1, 2026 |
none |
| Amendments to IFRS 10 Consolidated Financial Statements: Determination of a ‘de facto agent’ |
January 1, 2026 |
none |
| Amendments to IAS 7 Statement of Cash Flows: Cost method |
January 1, 2026 |
none |
| IFRS 7 & IFRS 9 |
Amendments IFRS 9 and IFRS 7 regarding the application of the ‘own use’ exemption to Power Purchase Agreements (PPAs) |
January 1, 2026 |
none |
These standards and amendments are not expected to have a material impact on the entity in the current reporting periods and on foreseeable future transactions, except IFRS 18 which is currently under assessment.
5.2.3 Consolidation
Subsidiaries
Subsidiaries are entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are deconsolidated from the date that control ceases.
The Group uses the acquisition method of accounting to account for business combinations. The consideration transferred for the acquisition of a subsidiary is the fair value of assets transferred, the liabilities incurred, and the equity interests issued by the Company. The consideration transferred includes the fair value of any asset or liability resulting from a contingent consideration arrangement. Acquisition-related costs, other than those associated with the issue of debt or equity securities, are expensed as incurred. Identifiable assets acquired, liabilities, and contingent liabilities assumed in a business combination are measured initially at their fair values at the acquisition date. The excess of the consideration transferred over the fair value of the Company’s share of the identifiable net assets acquired is recorded as goodwill. If the fair value of the net assets of the acquired subsidiary exceeds the consideration, the difference is recognized directly in the income statement as a bargain purchase gain. Intercompany transactions, balances and unrealized gains on transactions between Group companies are eliminated.
5.2.4 Foreign currency translation
Functional and presentation currency
Items included in the financial statements of each of the Group’s entities are measured using the currency of the primary economic environment in which the entity operates (the functional currency). The consolidated financial statements are presented in Euros, which is Valneva SE’s functional and presentation currency.
Transactions and balances
Foreign currency transactions are converted into the functional currency using exchange rates applicable on the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of monetary assets and liabilities denominated in foreign currencies at year-end exchange rates are recognized in the income statement.
Subsidiaries
The results and financial position of all subsidiaries (none of which have the currency of a hyperinflationary economy) that have a functional currency different from the presentation currency are converted into the presentation currency as follows:
•assets and liabilities presented for each balance sheet are converted according to the exchange rate valid on the balance sheet date;
•income and expenses for each income statement are converted at monthly average exchange rates (unless this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are converted on the dates of the transactions); and
•all resulting exchange differences are recognized as other comprehensive income and are shown as other reserves.
When a foreign operation is partially disposed of or sold, exchange differences that had been recorded in equity are recognized in the income statement as part of the gain or loss on sale.
5.2.5 Financial risk management
The Group’s activities expose it to a variety of financial risks: market risk (including currency risk and interest rate risk), credit risk, and liquidity risk. The Group’s overall risk management program focuses on the unpredictability of financial markets and seeks to minimize potential adverse effects on the Group’s financial performance.
Financial risk management is carried out under the responsibility of the CFO. The Group’s risk management systems identify, evaluate, and manage financial risks. The Audit Committee of the Group’s Board of Directors receives regular reports on the Group’s risk management systems, including the management of financial risks.
Market risk
Foreign exchange risk
The Group operates internationally and is exposed to foreign exchange risks arising from various currencies, primarily with respect to the British Pound (GBP), the Canadian Dollar (CAD), the Swedish Krona (SEK), and the US Dollar (USD). The foreign exchange risks from the exposure to other currencies are relatively limited. Foreign exchange risks arise from future commercial transactions, recognized assets and liabilities, and net investments in foreign operations.
The objective of the Group is to limit the potential negative impact of the foreign exchange rate changes, for example by currency conversion of cash and cash equivalents denominated in foreign currency and by balancing foreign currency assets and liabilities to the extent possible. The Group has certain investments in foreign operations, the net assets of which are exposed to foreign currency translation risk.
Interest rate risk
The Group is exposed to market risks in connection with hedging both its liquid assets and its medium and long-term indebtedness and borrowings subject to variable interest rates.
Borrowings issued at variable rates expose the Group to cash flow interest rate risks, which are offset by cash and financial assets held at variable rates. During 2024, as well as 2023, both the Group’s financial assets as well as financial liabilities at variable rates were denominated in EUR, SEK, USD, CAD, and GBP.
The Group analyzes its interest rate exposure on a dynamic basis. Based on this analysis, the Group calculates the impact on profit and loss of a defined interest rate change. The same interest rate change is used for all currencies. The calculation only includes investments in financial instruments and cash in banks that represent major interest-bearing positions. As at December 31, 2024 and December 31, 2023, no material interest risk was identified. In case of increasing interest rates the positive effect from cash in banks will be higher than the negative effect from variable interest-bearing liabilities; in case of decreasing interest rates there will be no material negative impact.
Credit risk
The Group is exposed to credit risk which is the risk of financial loss if customers or counterparties to a financial instrument fail to meet their contractual obligations.
Valneva holds bank accounts, cash balances, and securities at sound financial institutions with high credit ratings. To monitor the credit quality of its counterparts, the Group relies on credit ratings as published by specialized rating agencies such as Standard & Poor’s, Moody’s, and Fitch. The Group has policies that limit the amount of credit exposure to any single financial institution. The Group is also exposed to credit risks from its trade debtors, as its income from product sales, collaborations, licensing, and services arises from a small number of transactions. The Group has policies in place to enter into such transactions only with highly reputable, financially sound counterparts. If customers are independently rated, these ratings are used. Otherwise, when there is no independent rating, a risk assessment of the credit quality of the customer is performed, taking into account its financial position, past payment experience and other relevant factors. Individual credit limits are set based on internal or external ratings in accordance with signature authority limits. The credit quality of financial assets is described in Note 5.16.4.
Liquidity risk
The Group is exposed to liquidity risk due to the maturity of its financial liabilities and the fluctuations of its operating cash flow, and the potential implementation of early repayment clauses in loan or grant agreements. Furthermore, fluctuations in the Group’s operating cash flow during accounting periods also generate liquidity risks. Prudent liquidity risk management therefore implies maintaining sufficient cash resources, cash equivalents and short-term deposits in order to satisfy ongoing operating requirements and the ability to close out market positions. Extraordinary conditions on the financial markets may, however, temporarily restrict the possibility to liquidate certain financial assets.
Although it is difficult to predict future liquidity requirements, the Group considers that the existing cash and cash equivalents as at December 31, 2024 will be sufficient to fund its operations for at least 12 months from the date of authorization for issuance of these consolidated financial statements. For the existing D&O Loan Agreement with covenants, amendments were agreed to reduce the minimum liquidity covenant and the minimum revenue covenant to prevent a breach of the covenants (see Note 5.24.1). No adjustments were made to these covenants in 2023 or 2024.
The table below analyzes the Group’s financial liabilities into relevant maturity groupings based on the remaining period from the balance sheet date to the contractual maturity date. The amounts disclosed in the table are the contractual undiscounted cash flows.
Balance as at December 31, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Less than 1 year |
Between 1 and 3 years |
Between 3 and 5 years |
Over 5 years |
Total |
| Borrowings |
20,852 |
|
132,489 |
|
33,349 |
|
683 |
|
187,373 |
|
| Lease liabilities |
2,508 |
|
5,203 |
|
5,083 |
|
16,147 |
|
28,941 |
|
| Refund liabilities |
19,650 |
|
6,491 |
|
— |
|
— |
|
26,141 |
|
| Trade payables and accruals |
35,522 |
|
— |
|
— |
|
— |
|
35,522 |
|
Tax and employee-related liabilities (1) |
13,107 |
|
— |
|
— |
|
— |
|
13,107 |
|
| Other liabilities |
79 |
|
— |
|
— |
|
— |
|
79 |
|
| TOTAL |
91,719 |
|
144,183 |
|
38,432 |
|
16,829 |
|
291,163 |
|
(1) Social security and other tax payables are excluded from the tax and employee-related liabilities balance, as this analysis is required for financial instruments only.
Balance as at December 31, 2023
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Less than 1 year |
Between 1 and 3 years |
Between 3 and 5 years |
Over 5 years |
Total |
| Borrowings |
44,079 |
|
62,378 |
|
70,390 |
|
— |
|
176,847 |
|
| Lease liabilities |
2,879 |
|
5,313 |
|
5,414 |
|
18,362 |
|
31,969 |
|
| Refund liabilities |
33,637 |
|
6,303 |
|
— |
|
— |
|
39,941 |
|
| Trade payables and accruals |
44,303 |
|
— |
|
— |
|
— |
|
44,303 |
|
Tax and employee-related liabilities (1) |
10,815 |
|
— |
|
— |
|
— |
|
10,815 |
|
| Other liabilities |
34 |
|
— |
|
— |
|
— |
|
34 |
|
| TOTAL |
135,747 |
|
73,995 |
|
75,804 |
|
18,362 |
|
303,908 |
|
(1) Social security and other tax payables are excluded from the tax and employee-related liabilities balance, as this analysis is required for financial instruments only.
The fair values as well as the book values of the Group’s borrowings are disclosed in Note 5.24. To manage liquidity risk, the Group holds a combination of cash, cash equivalents and short-term deposit balances.
5.2.6 Capital risk management
The Group’s objectives when managing capital are to safeguard the Group’s ability to continue as a going concern in order to provide benefits for shareholders and for other stakeholders and to maintain an optimal capital structure to reduce the cost of capital. The Group actively manages its funds to primarily ensure liquidity and principal preservation while seeking to maximize returns. The Group’s cash and short-term deposits are located at several different banks. In order to maintain or adjust the capital structure, the Group may issue new shares or sell assets to reduce debt.
In order to pursue its business strategy to grow into a major, self-sustained vaccine company through organic growth and opportunistic mergers & acquisitions, the Group may rely on additional equity and debt financing. Capital consists of “Equity” as shown in the consolidated balance sheet.
5.2.7 Fair value estimation
The carrying value less impairment provision of trade receivables and payables are assumed to approximate their fair values due to the relatively short maturity of the respective instruments.
5.3 Critical accounting judgements and key sources of estimation uncertainty
In applying the Group’s accounting policies, which are described in Note 5.2: Summary of significant accounting policies, management is required to make judgements (other than those involving estimations) that have a significant impact on the amounts recognized and to make estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period, or in the period of the revision and future periods if the revision affects both current and future periods.
5.3.1 Critical judgements in applying the Group’s accounting policies
The following is the critical judgement, apart from those involving estimations (which are presented separately below), that management has made in the process of applying the Group’s accounting policies and that has the most significant effect on the amounts recognized in financial statements:
•Note 5.5.2 Other revenues and Note 5.29 Refund liabilities: Revenue recognition of other revenues/refund liabilities: management’s judgement is required to determine the identification of performance obligations (especially when determining whether the license is distinct, which is the case when the customer can benefit from the license without further involvement), the determination of the transaction price (including the judgement of payables to customers), and allocation of the transaction price to the performance obligations on relative standalone selling price. The standalone selling price is sometimes not available or is based on hard-to-value intangible assets, so various valuation techniques are used.
In addition, management’s judgement is required regarding whether revenue from collaborations and licensing and service agreements is recognized over time or at a point in time. Revenue is only recognized when it is highly likely that it will not reverse in future, and this is a judgement required from management. In particular, Note 5.5.2 underlines the judgements made in applying accounting policies, which are most relevant with respect to the Research Collaboration and License Agreement with Pfizer.
5.3.2 Key sources of estimation uncertainty
The key assumptions concerning the future, and other key sources of estimation uncertainty in the reporting period that may have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below:
•Note 5.5 Revenues: Revenue recognition of product sales: estimate of expected returns and replacements, and supply of products free of charge;
•Note 5.5.2 Other revenues: Likelihood of refund liabilities and of revenue recognition in accordance with the actual costs compared to the budget;
•Note 5.10 Income tax income/(expense): Recognition of deferred tax assets: availability of future taxable profit against which deductible temporary differences and tax losses carried forward can be utilized and whether sufficient evidence is provided for entities;
•Note 5.12 Intangible assets: Amortization period of development expenditures and acquired technologies. The most significant criteria considered for the determination of the useful life of an intangible asset include the patent life and the estimated period during which Valneva can benefit from asset. These assumptions are considered to be a key source of estimation uncertainty as relatively small changes in the assumptions used may have a significant effect on the Group’s financial statements within the next year;
•Note 5.14 Property, plant and equipment: Depreciation period - assessment of useful life;
•Note 5.15 Impairment testing: Impairment test of intangible and tangible assets and right of use assets: key assumptions underlying recoverable amounts. Budgets comprise forecasts of revenue, staff costs and overheads based on current and anticipated market conditions that have been considered and approved by the Executive Committee. The revenue projections are inherently uncertain due to the short-term nature of the business and unstable market conditions. If the Group does not successfully develop vaccine candidates and receive regulatory approval, or if Valneva fails to successfully manufacture or commercialize approved vaccines, an impairment may be required. For the main estimates and sensitivities related to the impairment test regarding the CGU, see Note 5.15;
•Note 5.17 Inventories: Write-down analysis for inventories: For the assessment of write-down of raw material the current production plans have been taken into account. Raw material which will not be used before its expiry date is written down. For this assessment the status of the expiry dates as of the balance sheet date is used. For the assessment of write-downs of work in progress, finished goods, and purchased goods, the forecasted sales plans and a minimum shelf life at the time of the most current sales expectation are taken into account. In addition, inventories are assessed on the likelihood of the release of the relevant products.
•Note 5.23 Share-based compensation: Share-based payments and related expected employer contribution costs: assumption for fair value determination as well as the determination of accelerated vesting in the event of a change of control (as considered remotely);
•Note 5.29 Refund liabilities: Recognition and classification of the refund obligation related to the Pfizer Collaboration and License Agreement;
•Notes 5.30 Provisions and 5.33 Commitments and contingencies: Recognition and measurement of provisions and contingencies: key assumptions about the likelihood and magnitude of an outflow of resources. In estimating the provision for onerous contracts, management makes assumptions regarding the likelihood of termination costs for certain agreements;
•Note 5.18 Trade receivables and 5.16.5 Impairment of financial assets: A simplified approach based on historical loss rates is used to determine the loss allowances in order to recognize expected credit losses (ECL) for short-term financial assets such as trade receivables.
5.3.3 Measurements of fair values
A number of the Group’s accounting policies and disclosures require the measurement of fair values, for both financial and non-financial assets and liabilities.
When measuring the fair value of an asset or a liability, the Group uses observable market data as far as possible. Fair values are categorized into different levels in a fair value hierarchy based on the inputs used in the valuation techniques as follows:
•Level 1: quoted prices (unadjusted) in active markets for identical assets or liabilities.
•Level 2: inputs other than quoted prices included in Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices).
•Level 3: inputs for the asset or liability that are not based on observable market data (unobservable inputs).
If the inputs used to measure the fair value of an asset or a liability fall into different levels of the fair value hierarchy, then the fair value measurement is categorized in its entirety in the same level of the fair value hierarchy as the lowest level input that is significant to the entire measurement.
The Group recognizes transfers between levels of the fair value hierarchy at the end of the reporting period during which the change has occurred.
Further information about the assumptions made in measuring fair values is included in the following Notes:
•Note 5.16: Financial instruments and
•Note 5.23: Share-based compensation.
5.4 Segment information
The Executive Committee, as the Company’s chief operating decision maker (“CDM”), considers Valneva’s operating business in its entirety to allocate resources and assess performance. The Executive Committee evaluates all vaccine candidates and vaccine products together as a single operating segment, “development and commercialization of prophylactic vaccines”. Therefore, the split used to allocate resources and assess performance is based on a functional view, thus correlating to the income statement.
5.5 Revenues
Revenues include both revenues from contracts with customers and other revenues (mainly subleases) which are out of scope from IFRS 15:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Product sales |
163,253 |
|
144,624 |
|
114,797 |
|
| Other revenues from contracts with customers |
5,622 |
|
8,075 |
|
245,709 |
|
| Other non-IFRS 15 revenue |
704 |
|
1,014 |
|
797 |
|
| REVENUES |
169,579 |
|
153,713 |
|
361,303 |
|
5.5.1 Product sales
The Group mostly generates product sales revenues from the sale of its commercialized travel vaccines and from the sale of third-party products.
The Group’s product sales contracts generally include one type of performance obligation. Revenue is recognized at the point in time when the identified performance obligation is transferred to the customer, either when the customer obtains control over the goods at the time of shipment or when the product is received by the customer, which generally happens within a few days, depending on the terms of the agreement. Sales contracts with retailers, the U.S. Department of Defense (DOD), and IXCHIQ distributors in the U.S. and Canada are shown as “direct product sales”, whereas sales to the other distributors are reported as “indirect sales - sales through distributors”.
Some of the Group’s product sales agreements include retrospective rebates, charge-back clauses, discounts, and under certain conditions return rights, which give rise to variable consideration under IFRS 15. The constraints on variable consideration (expected rebates, discounts and considerations for product returns) are taken into account and recognized on an accrual basis and reported as refund liabilities or as contract liabilities (for replacement doses) in the consolidated balance sheet.
In most cases, Valneva sells its products through retailers. When more than one party is involved in providing or distributing goods or services, the standard requires an entity to determine whether it and its retailers are principals or agents in these transactions by evaluating the nature of its promises to the customer. An entity is a principal if it controls a promised good or service before transferring that good or service to the customer. An entity is an agent if its role is to arrange for another entity to provide the goods or services. Indicators that control has been transferred are as follows: a) the retailer is primarily responsible for fulfilling the promise to its customers, b) the retailer has inventory risk, and c) the retailer has discretion in establishing the price for the sale to its customers. One of Valneva’s retailers has extensive rights to return and consequently takes no inventory risk and does not have the power to establish the price for sales to its customers. Therefore, this retailer acts as agent rather than as principal. All of Valneva’s other retailers act as principal. While revenue from product sales to principals is recognized when the control is transferred to the principals, revenue from product sales to agents are recognized when the control is transferred to the final customer, which occurs when the goods are delivered to the final customer. Distribution costs and other amounts payable to customers are deducted from the revenue from principals, and costs paid to agents are recognized as “Marketing and distribution expenses”.
Valneva also sells products acquired from third parties. Valneva considers that it is acting as principal given that it controls such products before transferring them to the final customer. More specifically, Valneva assumes the inventory risk before the goods are transferred to customers and has discretion in establishing prices for such goods. Revenue is recognized when the product is delivered to the customers. Products purchased from third parties are recognized as “inventory” in the balance sheets and when sold as “cost of goods” in the statements of income.
5.5.2 Other revenues
The Group generates other revenues from its products, product candidates and proprietary technologies. The contracts in place often include several different promised goods or services such as research licenses, commercial licenses, and further R&D services.
The terms of such agreements include license fees received as initial fees, annual license maintenance fees, and fees to be paid upon achievement of milestones, as well as license option fees and fees for the performance of research services. In addition, the Group’s licensing arrangements generally provide for royalties payable on the licensee’s future sales of products developed within the scope of the license agreement. Furthermore, revenue recognized due to the termination of agreements is recognized in other revenues.
The Group’s existing license contracts provide distinct right to use licenses, and therefore revenue is recognized at the point in time at which the licensee is able to direct the use of and benefit from the license. The consideration for licensing contracts may consist of fixed and variable parts. In case of right-to-use licenses, the fixed part of the consideration is recognized at the point in time when the licensee is able to direct the use and benefit from the license. For any variable consideration, revenue is recognized at the point in time when the variable consideration constraint is removed.
Revenue for research and development services within the Group’s contracts currently in place is recognized over time. The progress is measured on an input basis (costs incurred related to total costs expected). This input method is considered an appropriate measure of the progress towards complete satisfaction of these performance obligations under IFRS 15.
Variable considerations are included in revenues only to the extent that it is highly probable that a significant reversal in the amount of the cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. At the end of each reporting period, the Group updates the estimated transaction price and its assessment of whether an estimate of variable consideration is constrained. Amounts allocated to a satisfied performance obligation are recognized as revenue, or as a reduction of revenue, in the period in which a change in estimate of variable consideration occurs. Revenues from license royalties are recognized when the underlying product sales occur.
Lyme - Pfizer Collaboration and License Agreement
In April 2020, Valneva signed the Collaboration and License Agreement with Pfizer to co-develop and commercialize the Group’s Lyme disease vaccine candidate (VLA15). This is classified as an agreement with a customer as defined by IFRS 15 guidance on revenue contracts with customers, and accordingly, amounts received by or payable to Valneva under the Collaboration and License Agreement are accounted for in the Group’s revenues.
In 2021 and 2022 several amendments were made to the Collaboration and License Agreement. This resulted in an increase in the expected payments to customer related to Valneva’s contribution to Pfizer’s future development costs.
As a result, Valneva considered the constraint to determine whether it is highly probable that a significant reversal in the amount of cumulative revenue recognized will not occur. Valneva concluded that it is no longer highly probable that it will be entitled to the consideration as payments to customers might further increase in the future. Therefore, for the year ended December 31, 2022, the accumulated revenue recognized since the inception of the agreement with Pfizer amounting to €45.9 million was reversed as other revenues from contracts with customers. In the years ended December 31, 2024 and December 31, 2023, no revenues were recognized as Valneva determined that entitlement to the consideration is not yet highly probable, due to the possibility of increased payments to customers while R&D activities are progressing ahead of BLA licensure submission (including Phase 3 study) to FDA.
While license and equipment purchase orders were fulfilled in prior periods, the R&D activities and additional services were ongoing through 2024 and satisfy the performance obligation over time. During this period Valneva funded 40% of the remaining shared development costs. As of December 31, 2024, Valneva has completed its expected payment obligations of the Phase 3 costs to Pfizer.
Items not included in the transaction price as of December 31, 2024 are (i) $143 million from early commercialization milestones, (ii) royalties, ranging from 14% to 22%, and (iii) $100 million in sales based milestones,which will be recognized as and when they occur.
5.5.3 Disaggregated revenue information
The Group’s revenues are disaggregated as follows:
Type of goods or service
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| IXIARO |
94,069 |
|
73,483 |
|
41,349 |
|
| DUKORAL |
32,303 |
|
29,775 |
|
17,334 |
|
| Third party products |
33,185 |
|
35,675 |
|
26,545 |
|
| IXCHIQ |
3,696 |
|
— |
|
— |
|
VLA20011 |
— |
|
5,691 |
|
29,568 |
|
| PRODUCT SALES |
163,253 |
|
144,624 |
|
114,797 |
|
| Royalties received |
2,410 |
|
2,129 |
|
843 |
|
| Revenues from shipping and handling |
1,368 |
|
21 |
|
21 |
|
| Milestone payment - licenses |
712 |
|
3,637 |
|
(38,805) |
|
| Other services |
1,133 |
|
2,288 |
|
283,650 |
|
| thereof COVID-19 |
— |
|
1,973 |
|
280,010 |
|
| OTHER REVENUES FROM CONTRACTS WITH CUSTOMERS |
5,622 |
|
8,075 |
|
245,709 |
|
| Other non-IFRS 15 revenue |
704 |
|
1,014 |
|
797 |
|
| REVENUES |
169,579 |
|
153,713 |
|
361,303 |
|
(1) Revenues from these products were derived from contractual arrangements and do not represent product sales.
In the year ended December 31, 2024 product sales for all active products increased by €18.6 million compared to the same period in 2023.
IXIARO sales showed a 28% increase in sales, which was the result of the U.S. Military returning to a more regular supply pattern and double-digit growth in the travel market..
DUKORAL sales in 2024 were 8% higher compared to 2023. This increase is a result of the significant recovery in the private travel market and price increases. Foreign currency fluctuations had an immaterial impact on DUKORAL sales.
The first IXCHIQ product sales were recorded in 2024, following the adoption of the U.S. Advisory Committee on Immunization Practices (ACIP)’s recommendations by the U.S. Centers for Disease Control and Prevention (CDC) in March 2024.
Third party product sales in 2024 were 7% lower compared to 2023, which was mainly driven by lower sales of Rabipur/RabAvert, following supply shortages primarily in the first quarter of the year.
Product sales for the COVID-19 vaccine VLA2001 product decreased by €5.7 million as the program was discontinued.
Revenues from shipping and handling increased in 2024 to €1.4 million due to a revised customer agreement including an additional charge for freight costs.
Revenues from milestone payments and licenses decreased by €2.9 million in 2024 compared to 2023, mainly resulting from lower revenue recognition related to the R&D collaboration activities for chikungunya with Instituto Butantan in Brazil.
Other revenues decreased from €2.3 million in 2023 to €1.1 million in 2024. This change is mainly due to the discontinuation of the VLA2001 program.
In the year ended December 31, 2022, other revenues from other services from contracts with customers were strongly influenced by one-off effects. An income of €169.2 million was related to the termination of the UK Supply Agreement and further €110.8 million to the termination of the EC APA . This was partially offset by €45.9 million of negative revenue for milestone payments and licenses due to an increase in the refund liability resulting from the amendment to the Collaboration and License Agreement with Pfizer.
Sales channels for product sales
Products are sold via the following sales channels:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Direct product sales |
137,889 |
|
119,305 |
|
75,968 |
|
| Indirect product sales |
25,365 |
|
25,320 |
|
38,828 |
|
| TOTAL PRODUCT SALES |
163,253 |
|
144,624 |
|
114,797 |
|
Geographical markets
In presenting information on the basis of geographical markets, revenue is based on the final location where Valneva’s distribution partner sells the product or where the customer/partner is located.
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| United States |
48,593 |
|
32,964 |
|
(23,803) |
|
| Canada |
32,321 |
|
28,193 |
|
18,904 |
|
| United Kingdom |
19,489 |
|
20,266 |
|
181,129 |
|
| Germany |
18,374 |
|
13,503 |
|
68,529 |
|
| Austria |
15,897 |
|
14,583 |
|
21,793 |
|
| Nordics |
13,937 |
|
12,695 |
|
12,043 |
|
| France |
7,220 |
|
5,866 |
|
46,608 |
|
| Other Europe |
9,056 |
|
9,335 |
|
18,740 |
|
| Rest of World |
4,691 |
|
16,308 |
|
17,360 |
|
| REVENUE TOTAL |
169,579 |
|
153,713 |
|
361,303 |
|
Nordics includes Finland, Denmark, Norway and Sweden.
In the year ended December 31, 2024, total revenues increased by €15.9 million in comparison to the year ended December 31, 2023. Revenues from the United States, Germany, and Canada positively contributed to this result. Sales to the U.S. military increased considerably, primarily as a result of the U.S. military returning to a more regular supply pattern in 2024, while no sales to the U.S. military were recorded in the first half of 2023.
Revenues in the year ended December 31, 2022 were strongly influenced by one-off effects. Revenues from the United States included net negative revenue of €45.9 million as a result of the amendment to the Collaboration and License Agreement with Pfizer. Further 2022 revenues from the United Kingdom included non-product revenues of €169.2 million from the UK Authority following the UK Settlement Agreement. During 2022, Valneva also received a release of non-refundable advance payments from several EU member states, affecting specifically revenues from Germany, France, Austria, the Nordics, and Other Europe.
Information about major customers
The concentration risk from the customer portfolio of the Group is limited. In 2024, there was one single customer with a contribution exceeding 10% of the annual revenue (with a share of 14%).
Product sales to the largest customer amounted to €23.0 million in 2024 (2023: €17.7 million, 2022: €16.0 million). Other revenues from the largest customer amounted to €1.2 million in 2024 (2023: €5.0 million, 2022: €169.2 million). In 2022, the UK Authority was the largest customer due to the UK Supply Agreement.
5.5.4 Assets and liabilities related to contracts with customers
See Note 5.18 for details on trade receivables, Note 5.19 for details on costs to obtain a contract, Note 5.28 for details of contract liabilities and Note 5.29 for details of refund liabilities.
5.6 Expenses by nature
The consolidated income statement line items cost of goods and services, research and development expenses, marketing and distribution expenses and general and administrative expenses include the following items by nature of cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
Note |
2024 |
2023 |
2022 |
| Consulting and other purchased services |
|
65,783 |
|
80,988 |
|
141,631 |
|
| Cost of services and change in inventory |
|
13,681 |
|
11,417 |
|
190,086 |
|
| Employee benefit expense other than share-based compensation (*) |
5.7 |
83,028 |
|
72,997 |
|
56,393 |
|
| Share-based compensation expense |
5.7 |
8,710 |
|
6,276 |
|
(5,215) |
|
| Raw materials and consumables used |
|
21,982 |
|
14,113 |
|
12,723 |
|
| Depreciation and amortization and impairment |
5.12/13/14 |
19,586 |
|
16,853 |
|
44,285 |
|
| Building and energy costs |
|
13,908 |
|
13,088 |
|
14,696 |
|
| Supply, office and IT costs |
|
7,682 |
|
11,663 |
|
11,739 |
|
| License fees and royalties |
|
4,065 |
|
5,492 |
|
6,830 |
|
| Advertising costs |
|
16,781 |
|
13,361 |
|
7,343 |
|
| Warehousing and distribution costs |
|
5,790 |
|
3,939 |
|
1,898 |
|
| Travel and transportation costs |
|
3,197 |
|
2,700 |
|
2,208 |
|
| Other expenses |
|
3,593 |
|
4,432 |
|
2,329 |
|
| OPERATING EXPENSES |
|
267,788 |
|
257,320 |
|
486,945 |
|
|
|
|
|
|
Operating expenses in the year ended December 31, 2024 amounted to €267.8 million, which was a slight increase compared to the €257.3 million in the year ended December 31, 2023.
The €229.6 million decrease in operating expenses from €486.9 million in the year ended December 31, 2022 to €257.3 million in the year ended December 31, 2023 primarily resulted from one-off expenses recorded in 2022 which were related to the suspended COVID-19 program. These expenses included the write-down of COVID-19 vaccine inventory of €159.4 million (presented under “cost of services and change in inventory”) as well as impairment charges related to fixed assets.
Expenses for “consulting and other purchased services” reduced substantially in the year ended December 31, 2024 to €65.8 million. During the comparison period of 2023, we incurred higher service fees for clinical studies related to research and development of the Zika vaccine candidate and higher expenditures on the COVID-19 vaccine, VLA2001. The comparison period of 2022 included significant expenses for VLA2001, related to research and development and external manufacturing costs.
Expenses for “cost of services and change in inventory” and “Raw materials and consumables used” increased in the year ended December 31, 2024 by €2.3 million and €7.9 million respectively, compared to 2023, mostly due to higher level of sales and more expenditure on research and development activities on commercialized products. These expenses strongly decreased in the year ended December 31, 2023 compared to the year ended December 31, 2022 as effects from the significant changes to the ordered volumes and the expected future demand for VLA2001, including in particular a write-down of inventory of €159.4 million.
“Employee benefit expenses other than share-based compensation” increased in the year ended December 31, 2024 compared to December 31, 2023 and December 31, 2022 due to inflation-related higher salaries and social security contributions. During 2024, the Group had an average of 695 employees (in 2023: 684 employees).
“Share-based compensation” expenses increased in the year ended December 31, 2024 compared to 2023 by €2.4 million due to a new plan introduced as at 22nd of October, 2024. In 2022 we recorded an income under “Share-based compensation expense” due to a change in the valuation of the share-based payment program resulting from the reduction in our share price.
The expense under “depreciation and amortization and impairment” increased in the year ended December 31, 2024 compared to 2023 by €2.7 million due to the capitalization of the Almeida facility in Livingston. In the year ended December 31, 2022 the Group recorded one-off charges of €14.8 million for the impairment of VLA2001-related fixed assets including idle manufacturing equipment, leasehold improvements and Right of Use assets.
The increase of “advertising costs” in the year ended December 31, 2024 compared to the year ended December 31, 2023 and 2022 was driven by higher staff costs to support product sales growth across the direct markets.
Principal Accountant Fees and Services
PricewaterhouseCoopers Audit and Deloitte & Associés served as independent auditors for the year ended December 31, 2024 and for all other reporting periods presented. The table below shows fees charged by those firms and member firms of their networks to Valneva and consolidated subsidiaries in the years ended December 31, 2024 and 2023.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
PricewaterhouseCoopers |
Deloitte & Associés |
| in € thousand |
2024 |
% |
2023 |
% |
2022 |
% |
2024 |
% |
2023 |
% |
2022 |
% |
| Audit fees |
1,710 |
|
89 |
% |
2,076 |
|
98 |
% |
1,891 |
|
99 |
% |
1,311 |
|
89 |
% |
1,902 |
|
99 |
% |
1,678 |
|
99 |
% |
| provided by the statutory auditor |
1,272 |
|
66 |
% |
1,539 |
|
73 |
% |
1,386 |
|
72 |
% |
1,185 |
|
80 |
% |
1,622 |
|
84 |
% |
1,376 |
|
81 |
% |
| provided by the statutory auditor's network |
439 |
|
23 |
% |
537 |
|
25 |
% |
505 |
|
26 |
% |
126 |
|
9 |
% |
280 |
|
15 |
% |
302 |
|
18 |
% |
| Audit-related Fees |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
13 |
|
1 |
% |
| provided by the statutory auditor |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
13 |
|
1 |
% |
| provided by the statutory auditor's network |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
| Tax Services |
45 |
|
2 |
% |
40 |
|
2 |
% |
25 |
|
1 |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
| provided by the statutory auditor's network |
45 |
|
2 |
% |
40 |
|
2 |
% |
25 |
|
1 |
% |
— |
|
— |
% |
— |
|
— |
% |
— |
|
— |
% |
| All Other Fees |
163 |
|
8 |
% |
— |
|
— |
% |
— |
|
— |
% |
163 |
|
11 |
% |
19 |
|
1 |
% |
— |
|
— |
% |
| Total |
1,918 |
|
100 |
% |
2,116 |
|
100 |
% |
1,916 |
|
100 |
% |
1,474 |
|
100 |
% |
1,921 |
|
100 |
% |
1,691 |
|
100 |
% |
Audit-related fees comprised mainly the aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit and are not reported under Audit Fees. All other fees are the aggregate fees billed for the limited assurance review of sustainability reporting and verification of disclosure requirements set out in article 8 of Regulation (EU) 2020/852.
5.7 Employee benefit expense
Employee benefit expenses include the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Salaries |
63,313 |
|
55,793 |
|
57,272 |
|
| Social security contributions |
16,300 |
|
14,359 |
|
(3,035) |
|
| Share-based compensation expense |
8,710 |
|
6,276 |
|
(5,215) |
|
| Training and education |
1,582 |
|
1,292 |
|
840 |
|
| Other employee benefits |
1,833 |
|
1,553 |
|
1,317 |
|
| TOTAL EMPLOYEE BENEFIT EXPENSE |
91,739 |
|
79,273 |
|
51,178 |
|
In the year ended December 31, 2024, the social security contributions included an income of €1.6 million (December 31, 2023: €1.6 million, December 31, 2022: €23.2 million) resulting from the release of the provision of employer contribution charges on share-based payment programs due to the reduction in the share price.
During 2024, the Group had an average of 695 employees (2023: 684 employees, 2022: 778 employees).
5.8 Other income/(expenses), net
Gain from sale of Priority Review Voucher, net Other income and expenses, net
The Company sold the PRV received from the FDA for $103 million (€95 million) on February 2, 2024.
The Company was awarded a tropical disease PRV in November 2023 following the FDA’s approval of IXCHIQ, Valneva’s single-dose, live-attenuated vaccine indicated for the prevention of disease caused by chikungunya virus.
The net gain from the sale of the PRV amounted to €90.8 million, after deducting expenses in the amount of €4.2 million, which included transaction fees as well as expenses in connection with contractual payment obligations related to the PRV sale.
Other income and expenses, net include the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Research and development tax credit |
10,028 |
|
6,797 |
|
15,348 |
|
| Grant income |
10,194 |
|
11,350 |
|
191 |
|
| Gain/(loss) on disposal of fixed assets and intangible assets, net |
(445) |
|
(21) |
|
(38) |
|
| Gain/(loss) from revaluation of lease agreements |
711 |
|
45 |
|
(32) |
|
| Taxes, duties, fees, charges, other than income tax |
(346) |
|
(475) |
|
(217) |
|
| Miscellaneous income/(expenses), net |
564 |
|
3,824 |
|
(3,054) |
|
| OTHER INCOME AND EXPENSES, NET |
20,706 |
|
21,520 |
|
12,199 |
|
Other operating income and expenses decreased by €0.8 million, or 4%, to €20.7 million for the year ended December 31, 2024 from €21.5 million for the year ended December 31, 2023 primarily due to lower grant income and net miscellaneous income.
Income from the research and development tax credit increased in 2024 due to the research and development tax credit in Scotland amounting to €5.2 million. However, this increase was offset by the reduction in the research and development tax credit in Austria by €2.1 million due to a lower eligible expense base compared to 2023.
In the year ended December 31, 2023, the Group recognized a grant income of €11.1 million from Scottish Enterprise, Scotland’s national economic development agency, for developing non-COVID-19 vaccines (the IXCHIQ and IXIARO) while in the year ended December 31, 2024 the amount recognized was €3.7 million.
In the year ended December 31, 2024, Valneva was awarded with additional funding of $41.3 million to be split over the next five years from CEPI (Coalition for Epidemic Preparedness Innovations). By December 31, 2024, Valneva had received and recognized €6.5 million (December 31, 2023 €0.2 million) as grant income related to this agreement.
The decrease in the “miscellaneous income/(expenses), net” for the year ended December 31, 2024 is due mainly to the one-time income of €4.7 million recognized for the year ended December 31, 2023, which was related to a settlement with a supplier in connection with COVID-19 activities and the one-time expense of €1.4 million related to the divestment of the CTM unit in Solna. In the year ended December 31, 2022, this position was negatively impacted by a litigation provision in the amount of €3.1 million.
5.8.1 Grants
Grants from governmental agencies and non-governmental organizations are recognized where there is reasonable assurance that the grant will be received and the Group will comply with all conditions.
Grants received as reimbursement of approved research and development expenses are recognized as other income when the related expenses have been incurred and there is reasonable assurance that funds will be received. Advance payments received under such grants are deferred and recognized when the relevant conditions are met. Advance payments received which need to be repaid are recognized as borrowings (see Note 5.24.1).
Government grants received to support the purchase of property, plant and equipment are included in non-current liabilities as deferred government grants and are credited to the income statement on a straight-line basis over the expected lives of the related assets.
In February 2022, the Group received two grants worth up to £20.0 million (approximately €23.9 million) from Scottish Enterprise, Scotland’s national economic development agency, to support research and development relating to the manufacturing processes of the COVID-19 vaccine and other vaccine candidates. Following the termination of the COVID-19 vaccine program, in May 2023 the grant relating to this program was amended, reducing the available funding from £7.5 million to nil. In 2024, there were no amendments to the grants. The funds under the remaining grant were received over three years, beginning in March 2022. In the year ended December 31, 2024, €3.7 million (£3.1 million) of grant funds from Scottish Enterprise were recognized. In the year ended December 31, 2023, €11.1 million (£9.6 million) of grant funds from Scottish Enterprise were recognized.
In 2019 the Group signed a funding agreement with CEPI. Valneva received $24.6 million for vaccine manufacturing and late-stage clinical development of a single-dose, live attenuated vaccine against chikungunya. In line with CEPI’s commitment to equitable access, the funding underwrote a partnership effort to accelerate regulatory approval of Valneva’s chikungunya vaccine for use in regions where outbreaks occur and to support World Health Organization prequalification to facilitate broader access in lower- and middle-income countries. Valneva had to pay back part of the consideration upon achievement of certain milestones. The refundable consideration is accounted for as a loan and measured in accordance with IFRS 9 (see Note 5.24.1). The difference between the proceeds from CEPI and the carrying amount of the loan is treated under IAS 20 and presented as “Borrowings”. The amount from the CEPI grant which benefits Instituto Butantan is recognized as revenue (see Note 5.5). In the year ended December 31, 2024, nil grant income (2023: €0.2 million) and €0.4 million of other revenues (2023: €5.0 million) related to the first CEPI agreement were recognized.
The partnership with CEPI was extended in 2024 when the Group signed the second funding agreement. CEPI provides up to $41.3 million additional funding in the next five years to support broader access to IXCHIQ, in Low- and Middle-Income countries (LMICs), as well as post-marketing trials and potential label extensions in children, adolescents, and pregnant women. The proceeds from CEPI are treated under IAS 20 and presented as Grant income. In the year ended December 31, 2024, €6.5 million of grant income related to the second agreement with CEPI was recognized.
5.8.2 Research and development tax credits
Research and development tax credits granted by tax authorities are accounted for as grants under IAS 20. As a consequence, the portion of the research tax credit covering operating expenses is recognized in the income statement in “Other income and expenses, net” and the portion covering capitalized development expenditures under “Intangible assets” is recorded as deduction from the assets relating to fixed assets.
In December 31, 2024, the position included research and development tax credits mainly from Scotland (€5.2 million) and from Austria (€3.6 million) whereas in the previous period Valneva received tax credits primarily from Austria (€5.7 million) and to a lesser extent from France.
5.9 Finance income/(expenses), net
Interest income is recognized on a time-proportion basis using the effective interest method.
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| FINANCE INCOME |
|
|
|
| Interest income from other parties |
2,362 |
|
1,210 |
|
260 |
|
|
|
|
|
| TOTAL FINANCE INCOME |
2,362 |
|
1,210 |
|
260 |
|
| FINANCE EXPENSES |
|
|
|
| Interest expense on loans |
(22,808) |
|
(13,681) |
|
(8,238) |
|
| Interest expense on refund liabilities |
(360) |
|
(8,419) |
|
(9,597) |
|
| Interest expenses on lease liabilities |
(813) |
|
(1,183) |
|
(955) |
|
| Other interest expense |
(3) |
|
(42) |
|
(264) |
|
|
|
|
|
| TOTAL FINANCE EXPENSES |
(23,984) |
|
(23,325) |
|
(19,054) |
|
| FOREIGN EXCHANGE GAIN/(LOSSES), NET |
(3,193) |
|
5,574 |
|
(12,587) |
|
| FINANCE INCOME/(EXPENSES), NET |
(24,816) |
|
(16,541) |
|
(31,381) |
|
The increase in interest expense on loans is due to the further draw-down in the second half of the year 2023, for further details see Note 5.24.
The interest expense on refund liabilities for the year ended December 31, 2023 of €8.4 million was mainly caused by accumulated payment deferrals related to the Pfizer agreement. The interest expense on refund liabilities for the year ended December 31, 2024 decreased to €0.4 million due to the significant payments made to Pfizer during the second half of 2023 and the fulfillment of all payment obligations during the first half of 2024. Please refer to Note 5.29 for more information on the refund liability balances.
The foreign exchange gain/(losses), net are primarily driven by non-cash revaluation results of non-Euro denominated balance sheet positions, especially caused by USD denominated liabilities (increase of the USD against the EUR of 7% in 2024).
5.10 Income tax income/(expense)
The tax expense for the period comprises current and deferred tax. Tax is recognized in the income statement, except to the extent that it relates to items recognized in other comprehensive income or directly in equity. In this case, the tax is also recognized in other comprehensive income or directly in equity, as the case may be. The current Income tax income/(expense) is calculated on the basis of the tax laws enacted or substantively enacted at the balance sheet date in the countries where the Group’s subsidiaries operate and generate taxable income. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation. It establishes provisions, where appropriate, based on amounts expected to be paid to the tax authorities.
Deferred income tax is provided in full, using the liability method, on temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the consolidated financial statements. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantially enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled.
Deferred income tax assets are recognized to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized.
Deferred income tax is provided on temporary differences arising on investments in subsidiaries and associates, except where the timing of the reversal of the temporary difference is controlled by the Group and it is probable that the temporary difference will not be reversed within the foreseeable future.
5.10.1 Current income tax
Income tax income/(expense) is comprised of current and deferred tax.
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
|
|
|
|
| CURRENT TAX |
|
|
|
| Current income tax charge |
(2,595) |
|
(931) |
|
(1,029) |
|
| Adjustments in respect of current income tax of previous year |
(119) |
|
(175) |
|
97 |
|
|
|
|
|
| DEFERRED TAX |
|
|
| Relating to origination and reversal of temporary differences |
1,953 |
|
(1,695) |
|
2,468 |
|
| INCOME TAX BENEFIT/(EXPENSE) |
(761) |
|
(2,800) |
|
1,536 |
|
The individual entities’ reconciliations, which are prepared on the basis of the tax rates applicable in each country while taking consolidation procedures into account, have been summarized in the reconciliation below. The estimated tax charge is reconciled to the effective tax charge disclosed.
The tax on the Group’s loss before tax differs from the theoretical amount that would arise using the weighted average tax rate applicable to profits of the consolidated companies as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| PROFIT/(LOSS) BEFORE TAX |
(11,486) |
(98,629) |
(144,815) |
| Tax calculated at domestic tax rates applicable to profits in the respective countries |
2,953 |
|
23,400 |
|
37,203 |
|
| Income not subject to tax (mainly R&D tax credit) |
3,630 |
|
190 |
|
7,435 |
|
| Expenses not deductible for tax purposes |
(3,391) |
|
(1,902) |
|
(26) |
|
| Deferred tax asset not recognized |
(3,896) |
|
(23,360) |
|
(45,955) |
|
| Utilization of previously unrecognized tax losses/ Recognition of tax asset previously not recognized |
172 |
|
(1,593) |
|
2,628 |
|
| Income tax credit/withholding tax/other adjustments |
168 |
|
553 |
|
101 |
|
| Effect of change in applicable tax rate |
(34) |
|
(160) |
|
586 |
|
| Exchange differences |
(244) |
|
(25) |
|
(526) |
|
| Income tax of prior years |
(119) |
|
98 |
|
90 |
|
| Minimum income tax |
— |
|
(2) |
|
(2) |
|
| INCOME TAX BENEFIT/(EXPENSE) |
(761) |
|
(2,800) |
|
1,536 |
|
| Effective income tax rate |
— |
|
— |
|
— |
|
In the year ended December 31, 2024, although the Group operated at a loss overall, there were profitable entities with revenues from the sale of commercialized travel vaccines and from the sale of third-party products.
5.10.2 Deferred tax
As at December 31, 2024, the deferred tax assets of €201.4 million (December 31, 2023: €204.5 million) were not recognized as there was not sufficient evidence that adequate taxable profit will be available against which the unused tax losses can be utilized in the foreseeable future. Deferred tax assets were only recognized for entities where sufficient evidence has been provided that adequate taxable profit will be available against which the unused tax losses can be utilized in the foreseeable future.
As at December 31, 2024, the Group had tax losses carried forward of €843.6 million (December 31, 2023: €879.1 million), of which €307.3 million related to Valneva SE (December 31, 2023: €290.0 million), €516.0 million related to Valneva Austria GmbH (December 31, 2023: €564.2 million), €10.7 million related to Valneva Scotland, Ltd. (December 31, 2023: €10.4 million), €9.2 million related to Valneva Sweden AB (December 31, 2023: €13.7 million) and €0.8 million related to Vaccines Holdings Sweden AB (December 31, 2023: €0.9 million).
Tax losses carried forward in France, Austria, United Kingdom and Sweden have no expiry date.
The gross movement on the deferred income tax account was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| BEGINNING OF THE YEAR |
2,954 |
|
4,943 |
|
2,292 |
|
| Exchange differences |
536 |
|
(294) |
|
171 |
|
| Income statement charge/(credit) |
1,953 |
|
(1,695) |
|
2,480 |
|
| END OF THE YEAR |
5,443 |
|
2,954 |
|
4,943 |
|
The deferred tax assets and liabilities are allocable to the various balance sheet items as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| DEFERRED TAX ASSET FROM |
|
|
|
| Tax losses carried forward |
200,153 |
|
207,858 |
|
|
| Fixed assets |
1,444 |
|
1,765 |
|
|
| Inventory |
7,325 |
|
4,388 |
|
|
| Borrowings and accrued interest |
9,909 |
|
4,722 |
|
|
| Provision |
1,564 |
|
1,501 |
|
|
| Other items |
318 |
|
217 |
|
|
| Non-recognition of deferred tax assets |
(201,356) |
|
(204,529) |
|
|
| TOTAL DEFERRED TAX ASSETS |
19,357 |
|
15,921 |
|
|
| DEFERRED TAX LIABILITY FROM |
|
|
|
| Fixed assets |
(6,963) |
|
(6,364) |
|
|
| Intangible assets |
(5,517) |
|
(5,157) |
|
|
| Other items |
(1,435) |
|
(1,446) |
|
|
| TOTAL DEFERRED TAX LIABILITY |
(13,915) |
|
(12,967) |
|
|
| DEFERRED TAX, NET |
5,443 |
|
2,954 |
|
|
The corporate income tax rate in Austria was set at 23% in 2024 (2023: 24%, 2022: 25%).
The corporate income tax rate in the United Kingdom was set at 25% in 2024 (2023: 19% until March 2023 and increased to 25% from April 2023 onward).
The corporate income tax rate in France reduced to 25% from 2022 onward.
The deferred tax assets and liabilities presented above as at December 31, 2024 and December 31, 2023 have been adjusted for these changes in tax rates.
5.11 Earnings (Losses) per share
Basic
Basic earnings (losses) per share are calculated by dividing the profit attributable to equity holders of the Company by the weighted average number of outstanding shares during the year, excluding shares purchased by the Company and held as treasury shares (see Notes 5.22 and 5.23).
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
|
2024 |
2023 |
2022 |
Net profit (loss) from continuing operations attributable to equity holders of the Company (in € thousand) |
(12,247) |
|
(101,429) |
|
(143,279) |
|
| Weighted average number of outstanding shares |
145,705,876 |
|
138,624,381 |
|
115,473,914 |
|
BASIC EARNINGS (LOSSES) FROM CONTINUING OPERATIONS PER SHARE (€ PER SHARE) |
(0.08) |
|
(0.73) |
|
(1.24) |
|
Diluted
Diluted earnings per share are calculated by adjusting the weighted average number of ordinary outstanding shares to assume conversion of all dilutive potential ordinary shares. The Company has share options as dilutive potential ordinary shares. For the share options, a calculation is done to determine the number of shares that could have been acquired at fair value (determined as the average annual market share price of the Company’s shares) based on the monetary value of the subscription rights attached to outstanding share options.
The number of shares calculated as above is compared with the number of shares that would have been issued assuming the exercise of the share options.
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
|
2024 |
2023 |
2022 |
Profit used to determine diluted earnings per share (in € thousand) |
(12,247) |
|
(101,429) |
|
(143,279) |
|
Weighted average number of outstanding shares for diluted earnings (losses) per share (1) |
145,705,876 |
|
138,624,381 |
|
115,473,914 |
|
DILUTED EARNINGS/(LOSSES) FROM CONTINUING OPERATIONS PER SHARE (€ PER SHARE) |
(0.08) |
|
(0.73) |
|
(1.24) |
|
(1) Potentially dilutive securities (2024: 1,627,520 share options; 2023: 2,861,904 share options, 2022: 1,504,892) have been excluded from the computation of diluted weighted-average shares outstanding, because such securities had an antidilutive impact due to the losses reported.
5.12 Intangible assets
Computer software
Acquired computer software licenses are capitalized on the basis of the costs incurred to acquire and implement the specific software. These costs are amortized on a straight-line basis over their estimated useful lives, which is generally between three to six years.
Costs associated with developing or maintaining computer software programs are recognized as expenses when they were incurred.
The costs of computer software subject to a software as a service agreement (SaaS) are recognized as expenses when they are incurred.
Acquired research and development technology and projects
Acquired research and development technology projects are capitalized. In case of a purchase with variable payments for an intangible asset, Valneva applies the cost accumulation method. Each component of the agreement such as up-front payments, development milestone payments, and sales milestone payment are considered and assessed separately to determine if they meet the capitalization criteria. Valneva chooses to apply the cost accumulation approach for the capitalization of variable or contingent payments and does not estimate and record a liability at the acquisition date.
Amortization of the intangible asset over its useful life starts when the product has been fully developed and is ready for use. These costs are amortized on a straight-line basis over their useful lives. This useful life is determined on a case-by-case basis according to the nature and characteristics of the items included under this heading. The main current acquired research and development technology project is amortized over periods of 24 years, which is based on the patent life and technological replacement of a newer vaccine generation.
Development costs
Research expenses are recognized as expenses when incurred. Development expenses incurred on clinical projects (related to the design and testing of new or significantly improved products) are recognized as intangible assets when the following criteria have been fulfilled:
•it is technically feasible to complete the intangible asset so that it will be available for use or sale;
•management intends to complete the intangible asset and to utilize or sell it;
•there is an ability to utilize or sell the intangible asset;
•it can be demonstrated how the intangible asset will generate probable future economic benefits;
•adequate technical, financial, and/or other resources to complete the development and to utilize or sell the intangible asset are available; and
•the expenditure attributable to the intangible asset during its development can be reliably measured.
Other development expenditures that do not meet these criteria are recognized as expenses when they are incurred. Development costs previously recognized as an expense are not recognized as an asset in a subsequent period. Capitalized development costs are recorded as intangible assets and amortized from the point at which the asset is ready for use on a straight-line basis over its useful life, generally 10 - 15 years. In 2024 and 2023, no development costs were capitalized.
Amortization
Amortization of intangible assets is calculated using the straight-line method to allocate their cost amounts to their residual values over their estimated useful lives, as follows:
•Software 3 - 6 years
•Acquired R&D technology and projects 1 - 24 years
•Development costs 1 - 15 years
The useful life is determined on a case-by-case basis according to the nature and characteristics of the items included under this heading. The main current acquired research and development technology project is amortized over periods of 24 years (with a remaining useful life period of 8 years) which is based on estimated period where Valneva benefits from the patent.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Software |
Acquired R&D technology and projects |
Development costs |
Intangible assets in the course of construction |
Total |
|
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2024 |
|
|
|
|
|
| Opening net book value |
255 |
|
24,073 |
|
1,239 |
|
— |
|
25,567 |
|
| Additions |
79 |
|
2,500 |
|
— |
|
— |
|
2,579 |
|
| Amortization charge |
(126) |
|
(2,687) |
|
(145) |
|
— |
|
(2,958) |
|
|
|
|
|
|
|
| Exchange rate differences |
(2) |
|
62 |
|
11 |
|
— |
|
71 |
|
| CLOSING NET BOOK VALUE |
205 |
|
23,948 |
|
1,104 |
|
— |
|
25,258 |
|
|
|
|
|
|
|
| AS AT DECEMBER 31, 2024 |
|
|
|
|
|
| Cost |
5,823 |
|
83,349 |
|
7,345 |
|
— |
|
96,516 |
|
| Accumulated amortization and impairment |
(5,618) |
|
(59,400) |
|
(6,240) |
|
— |
|
(71,258) |
|
| CLOSING NET BOOK VALUE |
205 |
|
23,948 |
|
1,104 |
|
— |
|
25,258 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Software |
Acquired R&D technology and projects |
Development costs |
Intangible assets in the course of construction |
Total |
|
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2023 |
|
|
|
|
|
| Opening net book value |
585 |
|
26,731 |
|
1,394 |
|
— |
|
28,711 |
|
| Additions |
85 |
|
— |
|
— |
|
— |
|
85 |
|
| Amortization charge |
(420) |
|
(2,683) |
|
(160) |
|
— |
|
(3,262) |
|
| Disposals |
— |
|
— |
|
— |
|
— |
|
— |
|
| Exchange rate differences |
4 |
|
24 |
|
4 |
|
— |
|
33 |
|
| CLOSING NET BOOK VALUE |
255 |
|
24,073 |
|
1,239 |
|
— |
|
25,567 |
|
|
|
|
|
|
|
| AS AT DECEMBER 31, 2023 |
|
|
|
|
|
| Cost |
6,368 |
|
80,562 |
|
7,314 |
|
— |
|
94,244 |
|
| Accumulated amortization and impairment |
(6,113) |
|
(56,489) |
|
(6,075) |
|
— |
|
(68,677) |
|
| CLOSING NET BOOK VALUE |
255 |
|
24,073 |
|
1,239 |
|
— |
|
25,567 |
|
As at December 31, 2024, acquired research and development increased by €2.5 million when the Group entered into a strategic partnership with LimmaTech Biologics to accelerate the development of the world’s most clinically advanced tetravalent Shigella vaccine candidate.
As at December 31, 2023, there were no acquired research and development technology project assets with a definite useful life that not yet amortized.
Significant intangible assets (included in acquired R&D technology and projects as well as in development costs) with definite useful life are comprised primarily of the already commercialized vaccine against Japanese encephalitis (IXIARO) with acquisition costs amounting to €79.1 million (December 31, 2023: €78.8 million) and a net book value of €22.3 million (December 31, 2023: €25.0 million).
For impairment tests, please see Note 5.15.
5.13 Leases (right of use assets)
The Group leases various premises, equipment, and vehicles. Rental contracts are typically made for fixed periods ranging from a few months to five years. The rental contracts for the premises in Sweden (10 and 15 years) include a significantly longer fixed period. Generally, the rental contracts do not include an option for early termination or prolongation of the rental period. The rental contracts for the premises in Sweden include options to terminate the agreements earlier. The notice periods in these contracts are between one and six years. At the commencement date, it was not reasonably certain that these early termination options were to be exercised, so they were not included in the valuation of the lease liabilities and right of use assets. Contracts may contain both lease and non-lease components. The Group allocates the consideration in the contract to the lease and non-lease components based on their relative stand-alone prices.
Lease terms are negotiated on an individual basis and contain a wide range of different terms and conditions. The lease agreements do not impose any covenants other than the security interests in the leased assets that are held by the lessor. Leased assets may not be used as security for borrowing purposes.
The lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date, discounted by using the rate implicit in the lease. If this rate cannot be readily determined, which is generally the case for leases in the Group, the Group uses its incremental borrowing rate. The incremental borrowing rate depends on the term, currency, and start date of the lease and is determined based on a series of inputs including: the risk-free rate based on government bond rates, a country-specific risk adjustment, a credit risk adjustment based on bond yields, and an entity-specific adjustment when the risk profile of the entity that enters into the lease is different than that of the Group and the lease does not benefit from a guarantee from the Group. Valneva uses incremental borrowing rates between 0.183% and 7.000%, depending on the currency and the remaining term until maturity. For the rental contracts for the premises in Sweden interest rates of 2.493% and 3.401% were determined.
The Group is exposed to potential future increases in variable lease payments based on an index or rate, which are not included in the lease liability until they take effect. When adjustments to lease payments based on an index or rate take effect, the lease liability is reassessed and adjusted against the right-of-use asset. This includes also the major contracts for the premises in Sweden, which contain variable payments based on inflation rates or on published interest rates.
Lease payments are allocated between principal and finance cost. The finance cost is charged to profit or loss over the lease period so as to produce a constant periodic rate of interest on the remaining balance of the liability for each period.
Right-of-use assets are generally depreciated over the shorter of the asset's useful life and the lease term on a straight-line basis. If the Group is reasonably certain to exercise a purchase option, the right-of-use asset is depreciated over the underlying asset’s useful life.
Payments associated with short-term leases of equipment and vehicles and all leases of low-value assets (below €10,000) are recognized on a straight-line basis as an expense in profit or loss. Short-term leases are leases with a lease term of 12 months or less and for which there is no option for the lessee to prolong the contract to more than 12 months or there is no reasonable certainty that such an option will be exercised. Low-value assets comprise mainly IT equipment and small items of office furniture.
The Group does not have residual value guarantees in the rental contracts.
5.13.1 Development of right-of-use assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Land, buildings and leasehold improvements |
Manufacturing and laboratory equipment |
Furniture, fittings and other |
Total assets |
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2024 |
|
|
|
|
| Opening net book value |
20,141 |
|
— |
|
251 |
|
20,392 |
|
| Additions |
38 |
|
— |
|
199 |
|
237 |
|
| Amortization |
(1,788) |
|
— |
|
(135) |
|
(1,923) |
|
| Impairment charge |
980 |
|
— |
|
— |
|
980 |
|
| Revaluation due to variable payments |
1,404 |
|
— |
|
— |
|
1,404 |
|
| Termination of contracts |
(1,246) |
|
— |
|
(12) |
|
(1,258) |
|
| Exchange rate differences |
(596) |
|
— |
|
(3) |
|
(599) |
|
| CLOSING NET BOOK VALUE |
18,932 |
|
— |
|
300 |
|
19,232 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Land, buildings and leasehold improvements |
Manufacturing and laboratory equipment |
Furniture, fittings and other |
Total assets |
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2023 |
|
|
|
|
| Opening net book value |
41,365 |
|
— |
|
238 |
|
41,603 |
|
| Additions |
3,593 |
|
— |
|
189 |
|
3,781 |
|
| Amortization |
(2,428) |
|
— |
|
(141) |
|
(2,569) |
|
|
|
|
|
|
|
|
|
|
|
| Termination of contracts |
(22,516) |
|
— |
|
(32) |
|
(22,548) |
|
| Exchange rate differences |
127 |
|
— |
|
(2) |
|
125 |
|
| CLOSING NET BOOK VALUE |
20,141 |
|
— |
|
251 |
|
20,392 |
|
In the year ended December 31, 2024, right of use assets decreased from €20.4 million to €19.2 million, mainly due to termination of contracts and amortizations.
The largest remaining active lease contract is for the building in Solna, Sweden, which has a book value of €15.2 million as at December 31, 2024 (December 31, 2023: €15.5 million).
Due to the termination of the contract for a leased building, an impairment charge of €1.0 million relating to VLA2001 was derecognised in 2024.
For details on lease liabilities, see Note 5.27. For details on the impairment charge, see Note 5.15.
5.13.2 Other amounts recognized in the consolidated income statement
Expense relating to short-term leases and leases of low-value assets as well as expenses relating to termination of lease contracts have not been material in 2024 and 2023. There have been no substantive revaluations recognized to the income statement in 2024 and 2023.
5.14 Property, plant and equipment
Property, plant and equipment mainly comprise a manufacturing facility and leasehold improvements in rented office and laboratory space. All Property, plant and equipment are stated at historical cost less depreciation and less impairment losses when necessary. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Subsequent costs are included in the asset’s carrying amount or are recognized as a separate asset, only when it is probable that future economic benefits associated with the item will flow to the Group and that the cost of the item can be measured reliably. All other repairs and maintenance are charged to the income statement during the financial period in which they incur.
Property, plant and equipment include machinery, for which validation is required to bring the asset to its working condition. The costs of such validation activities are capitalized together with the cost of the asset. Validation costs beyond the normal validation costs, which are usually required to bring an asset to its working condition, are expensed immediately. The usual validation costs are capitalized on the asset and depreciated over the remaining life of the asset or the shorter period until the next validation is usually required.
Depreciation of assets is calculated using the straight-line method to allocate their cost amounts to their residual values over their estimated useful lives, as follows:
▪Buildings, leasehold improvements 5 - 40 years
▪Machinery, laboratory equipment 1 - 15 years
▪Furniture, fittings and office equipment 4 - 10 years
▪Hardware 3 - 5 years
Leasehold improvements are depreciated over the shorter of their useful life or the lease term, unless the entity expects to use the assets beyond the lease term.
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at each balance sheet date.
An asset’s carrying amount is immediately written down to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
Gains and losses on disposals are determined by comparing proceeds with the carrying amount. These gains and losses are included in the income statement “other income and expenses, net” (see Note 5.8).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Land, buildings and leasehold improvements |
Manufacturing and laboratory equipment |
Computer hardware |
Furniture, fittings and other |
Assets in the course of construction |
Total |
|
|
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2024 |
|
|
|
|
|
|
| Opening net book value |
99,100 |
|
31,761 |
|
1,053 |
|
560 |
|
3,724 |
|
136,198 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Additions |
10,356 |
|
3,720 |
|
569 |
|
68 |
|
(292) |
|
14,421 |
|
| Depreciation charge |
(7,828) |
|
(6,248) |
|
(467) |
|
(161) |
|
— |
|
(14,705) |
|
| Impairment charge/reversal |
— |
|
322 |
|
— |
|
— |
|
— |
|
322 |
|
| Disposals |
(102) |
|
(800) |
|
(20) |
|
(11) |
|
— |
|
(932) |
|
| Exchange rate differences |
3,151 |
|
302 |
|
20 |
|
21 |
|
86 |
|
3,579 |
|
| CLOSING NET BOOK VALUE |
104,678 |
|
29,056 |
|
1,155 |
|
477 |
|
3,518 |
|
138,883 |
|
|
|
|
|
|
|
|
| AS AT DECEMBER 31, 2024 |
|
|
|
|
|
|
| Cost |
151,034 |
|
74,445 |
|
3,542 |
|
1,512 |
|
3,518 |
|
234,051 |
|
| Accumulated depreciation and impairment |
(46,357) |
|
(45,389) |
|
(2,386) |
|
(1,035) |
|
— |
|
(95,167) |
|
| CLOSING NET BOOK VALUE |
104,678 |
|
29,056 |
|
1,155 |
|
477 |
|
3,518 |
|
138,883 |
|
The additions in 2024 were primarily from the construction of the Almeida facility in Livingston. The depreciation charges for the fiscal year were conducted in accordance with standard practices, and present a slight increase due to the investments from previous years.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Land, buildings and leasehold improvements |
Manufacturing and laboratory equipment |
Computer hardware |
Furniture, fittings and other |
Assets in the course of construction |
Total |
|
|
|
|
|
|
|
| YEAR ENDED DECEMBER 31, 2023 |
|
|
|
|
|
|
| Opening net book value |
74,493 |
|
34,544 |
|
1,140 |
|
675 |
|
1,583 |
|
112,435 |
|
|
|
|
|
|
|
|
| Change in consolidation scope |
22,373 |
|
— |
|
— |
|
— |
|
— |
|
22,373 |
|
| Additions |
9,088 |
|
2,884 |
|
414 |
|
33 |
|
1,985 |
|
14,404 |
|
| Depreciation charge |
(6,008) |
|
(4,372) |
|
(442) |
|
(155) |
|
— |
|
(10,976) |
|
| Impairment charge/reversal |
— |
|
1,869 |
|
— |
|
— |
|
— |
|
1,869 |
|
| Disposals |
(1,837) |
|
(3,547) |
|
(61) |
|
(2) |
|
— |
|
(5,448) |
|
| Exchange rate differences |
991 |
|
383 |
|
3 |
|
9 |
|
155 |
|
1,541 |
|
| CLOSING NET BOOK VALUE |
99,100 |
|
31,761 |
|
1,053 |
|
560 |
|
3,724 |
|
136,198 |
|
|
|
|
|
|
|
|
| AS AT DECEMBER 31, 2023 |
|
|
|
|
|
|
| Cost |
125,580 |
|
73,686 |
|
3,438 |
|
1,895 |
|
3,724 |
|
208,323 |
|
| Accumulated depreciation and impairment |
(26,479) |
|
(41,926) |
|
(2,384) |
|
(1,335) |
|
— |
|
(72,125) |
|
| CLOSING NET BOOK VALUE |
99,100 |
|
31,761 |
|
1,053 |
|
560 |
|
3,724 |
|
136,198 |
|
The change in the scope of consolidation in 2023 came from the acquisition of VBC3. The additions were primarily related to the Almeida facility in Livingston. The reversal of impairment was due to a reversal of a fixed asset impairment in the amount of €1.9 million related to production equipment in Livingston.
From the total of €19.6 million (2023: €16.9 million) of depreciation, amortization and impairment expenses, €12.0 million (2023: €12.5 million) were charged to cost of goods and services, €6.3 million (2023: €3.0 million) were charged to research and development expenses, €0.7 million (2023: €0.8 million) were charged to marketing and distribution expenses and €0.4 million (2023: €0.5 million) were charged to general and administrative expenses.
Non-current operating assets by region
Non-current operating assets for this purpose consist of intangible assets, right of use assets and property, plant and equipment. The main non-current operating assets are allocated to sites where production and research and development activities take place. Sales activities by distribution sites do not require major non-current operating assets. Revenues by region (see Note 5.5) are structured according to the location of the final customer. In some countries there are customers, but no assets.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| United Kingdom |
93,309 |
|
87,646 |
|
|
| Austria |
48,533 |
|
49,460 |
|
|
| Nordics |
36,264 |
|
39,111 |
|
|
| France |
4,350 |
|
4,839 |
|
|
| United States |
781 |
|
934 |
|
|
| Canada |
138 |
|
166 |
|
|
| NON-CURRENT ASSETS |
183,373 |
|
182,156 |
|
|
5.15 Impairment testing
At the end of each reporting period Valneva assesses whether there is any indication that an asset may be impaired. Indicators for the necessity of an impairment test are, among others, actual or expected declines in sales or margins and significant changes in the economic environment with an adverse effect on Valneva’s business. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less selling costs and value in use.
For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units or CGUs). The cash-generating units correspond with the specific vaccine products and vaccine candidates. Non-financial assets, other than goodwill, that suffered impairment are reviewed for possible reversal of the impairment at each reporting date.
An assessment of triggering events performed for the year ended December 31, 2024 resulted in no triggering events identified for the IXIARO and DUKORAL CGU’s. Following the identification of a triggering event for the IXCHIQ CGU, an impairment test was performed. No material impairment reversal was booked in the year ended December 31, 2024.
A dedicated CGU was identified for Shigella resulting from the development collaboration license and commercialization agreement signed with LimmaTech Biologics AG in July 2024. For this newly identified CGU there were no indications of impairment.
IXIARO
No triggering event was identified for the year ended December 31, 2024. Further, the impairment test for the CGU of IXIARO did not result in any impairment for the year ended December 31, 2023.
DUKORAL
As at December 31, 2024, no triggering event was identified therefore no impairment testing was conducted that could have resulted in impairment charges. The impairment testing on DUKORAL for the year ended December 31, 2023 did not result in any impairment charges.
IXCHIQ
The impairment test for the IXCHIQ CGU as at December 31, 2024 did not result in any impairment requirement as the value in use for the CGU was considerably higher than the book value of its assets. Further details can be seen in the below sensitivity analysis.
Sensitivity to changes in assumptions
The net present value calculations are based upon assumptions regarding market size, expected sales volumes resulting in sales value expectations, expected royalty income or expected milestone payments. The calculations are based on projected sales of product but although on expected royalties and milestones payments to received. The net present value calculations are most sensitive to the following assumptions:
–discount rate
–reduction of expected revenues
The following table shows these parameters and their sensitivity to the overall result in case of described changes:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand (except ratios) |
IXIARO |
DUKORAL |
IXCHIQ |
Shigella |
CTM* |
| WEIGHTED AVERAGE COST OF CAPITAL (WACC) |
|
|
|
|
|
| 2024 |
— |
% |
— |
% |
8.79 |
% |
— |
% |
— |
% |
| 2023 |
9.08 |
% |
8.94 |
% |
9.04 |
% |
— |
% |
— |
% |
| BREAK-EVEN WACC |
| 2024 |
— |
% |
— |
% |
27.04 |
% |
— |
% |
— |
% |
| 2023 |
81.06 |
% |
8.04 |
% |
113.62 |
% |
— |
% |
— |
% |
IMPAIRMENT IF WACC INCREASES BY 1% (in € thousand) |
| 2024 |
— |
|
— |
|
NO |
— |
|
— |
|
| 2023 |
NO |
3,330 |
|
NO |
— |
|
— |
|
Impairment if sales reduce by 10% (in € thousand) |
|
|
|
|
|
| 2024 |
— |
|
— |
|
NO |
— |
|
— |
|
| 2023 |
NO |
6,508 |
|
NO |
— |
|
— |
|
* CTM CGU was sold in July 2023, see Note 5.21.
5.16 Financial instruments
Derivatives are initially recognized at fair value on the date a derivative contract is entered into and are subsequently re-measured at their fair value at each balance sheet date.
The valuation techniques utilized for measuring the fair values of assets and liabilities are based on observable and unobservable inputs. Observable inputs reflect readily obtainable data from independent sources, while unobservable inputs reflect management’s market assumptions.
The fair value of instruments that are quoted in active markets are determined using the quoted prices where they represent those at which regularly and recently occurring transactions take place. Furthermore, the Group uses valuation techniques to establish the fair value of instruments where prices, quoted in active markets, are not available.
5.16.1 Financial instruments by category
The Group has materially only short-term assets and all of the financial instruments are categorized as assets at amortized costs. Financial instruments can be found in the following positions within the assets:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| FINANCIAL INSTRUMENTS IN ASSETS |
|
|
|
| Trade receivables |
35,205 |
|
41,645 |
|
|
Other assets (1) |
1,256 |
|
1,109 |
|
|
| Cash and cash equivalents |
168,271 |
|
126,080 |
|
|
| TOTAL ASSETS |
204,731 |
|
168,834 |
|
|
(1) Prepayments and tax receivables and other non-financial assets are excluded from the other assets balance, as this analysis is required only for financial instruments.
The Group has only financial instruments which are categorized as liabilities at amortized costs. Financial instruments can be found in the following positions within the liabilities:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| FINANCIAL INSTRUMENTS IN LIABILITIES |
|
|
|
| Borrowings |
187,373 |
|
176,847 |
|
|
| Trade payables and accruals |
35,522 |
|
44,303 |
|
|
Tax and employee-related liabilities (1) |
13,107 |
|
10,815 |
|
|
| Lease liabilities |
28,941 |
|
31,969 |
|
|
| Refund liabilities |
26,141 |
|
39,941 |
|
|
Other liabilities (2) |
79 |
|
34 |
|
|
| TOTAL LIABILITIES |
291,163 |
|
303,908 |
|
|
(1) Social security and other tax payables are excluded from the tax and employee-related liabilities balance, as this analysis is required only for financial instruments.
(2) Deferred income is excluded from the other liabilities balance, as this analysis is required only for financial instruments.
5.16.2 Fair value measurements
As at December 31, 2024 and December 31, 2023, the Group did not have assets and liabilities measured through profit and loss. In both periods, the Group also did not subscribe to any foreign currency options nor foreign currency forwards. Due to the short-term nature of its financial instruments fair valuation has no effect on the financial position.
5.16.3 Foreign currency sensitivity analysis
The following table details the Group’s sensitivity of financial instruments to a 10% increase and decrease in currency units against the relevant foreign currencies. 10% is the sensitivity rate used when reporting foreign currency risk internally to key management personnel and represents management’s assessment of the reasonably possible change in foreign exchange rates. The sensitivity analysis includes only outstanding foreign currency denominated monetary items and adjusts their translation at the year-end for a 10% change in foreign currency rates. The sensitivity analysis includes external loans as well as loans to foreign operations within the Group where the denomination of the loan is in a currency other than the currency of the lender or the borrower. A positive number below indicates an increase in pre-tax profit or a reduction in pre-tax loss.
With all other variables held constant, the impact from changes in exchange rates on the pre-tax result would be as follows:
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
| USD/EUR +10% |
(16,973) |
|
(24,079) |
|
| USD/EUR -10% |
20,745 |
|
29,430 |
|
| GBP/EUR +10% |
8,525 |
|
4,760 |
|
| GBP/EUR -10% |
(10,420) |
|
(5,817) |
|
| SEK/EUR +10% |
(5,725) |
|
(8,846) |
|
| SEK/EUR -10% |
6,998 |
|
10,812 |
|
| CAD/EUR +10% |
3,178 |
|
2,368 |
|
| CAD/EUR -10% |
(3,884) |
|
(2,894) |
|
The effect in the USD/EUR relationship is mostly due to borrowings denominated in USD while the cash and working capital is predominantly on a EUR basis. The Group has not used any hedging instruments to reduce the impact of foreign exchange rate changes.
5.16.4 Credit quality of financial assets
The credit quality of financial assets that are not impaired can be assessed by reference to external credit ratings (if available) or to historical information about counterparty default rates as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| TRADE RECEIVABLES |
|
|
|
| Receivables from governmental institutions (AAA-country) |
— |
|
205 |
|
|
| Receivables from governmental institutions (AA-country) |
5,202 |
|
11,535 |
|
|
| Receivables from governmental institutions (A-country) |
— |
|
— |
|
|
| AA |
— |
|
— |
|
|
| A |
3,184 |
|
— |
|
|
| Counterparties without external credit rating or rating below A |
26,819 |
|
29,905 |
|
|
| TRADE RECEIVABLES |
35,205 |
|
41,645 |
|
|
|
|
|
|
| OTHER ASSETS |
|
|
|
| A |
— |
|
— |
|
|
| Assets from governmental institutions (AA-country) |
— |
|
— |
|
|
| Counterparties without external credit rating or rating below A |
1,256 |
|
1,109 |
|
|
| OTHER ASSETS |
1,256 |
|
1,109 |
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS |
|
|
|
| AA |
8,921 |
|
17,581 |
|
|
| A |
156,135 |
|
108,253 |
|
|
| Counterparties without external credit rating or rating below A |
3,214 |
|
245 |
|
|
| CASH AND CASH EQUIVALENTS |
168,271 |
|
126,080 |
|
|
The rating information refers to long-term credit ratings as published by Standard & Poor’s or another rating organization (equivalent to the Standard & Poor’s rating).
The maximum exposure to credit risk at the reporting date is the fair value of the financial assets.
5.16.5 Impairment of financial assets
Trade receivables
According to IFRS 9.5.5.15, the simplified approach (measuring the loss allowance at an amount equal to lifetime expected credit losses) has to be used for trade receivables, which do not contain a significant financing component. This is the case for the Group, as all trade receivables are short-term with a maturity lasting less than 12 months.
Loss allowances have to be established for each trade receivable based on the expected credit losses. Accordingly, at the end of each reporting period, trade receivables were adjusted through a loss allowance in accordance with the revised expected outcome.
According to IFRS 9.5.5.17, default probabilities should be determined on the basis of historical data but must be adjusted on the balance sheet date on the basis of up-to-date information and forward looking information.
The analysis of the historical data showed as at December 31, 2024 and December 31, 2023 that losses incurred were immaterial, taking further into account the limited number of customers as well as credit checks mentioned in Note 5.2.5. Therefore, loss allowance was considered immaterial as at December 31, 2024 and December 31, 2023.
Other assets and cash and cash equivalents
Historically, no losses have been incurred on other assets measured at amortized costs and on cash and cash equivalents. As at December 31, 2024 and December 31, 2023, the expected credit loss was calculated using the cumulative expected default rate based on the counterparties’ ratings and was immaterial.
5.17 Inventories
Inventories are stated at the lower of cost and net realizable value. The cost of finished goods and work in progress comprises raw materials, direct labor, other direct costs and related production overheads (based on normal operating capacity) at standard costs. The variances between the actual costs and the standard costs are calculated monthly and allocated to the inventory, so there is no difference between actual cost of manufacture and the value of inventory. Inventories exclude borrowing costs. Provisions for batches which fail to meet quality requirements and may not be sold (failed batches) are deducted from the value of inventories.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Raw materials |
28,086 |
|
35,379 |
|
|
| Work in progress |
36,832 |
|
38,094 |
|
|
| Finished goods |
19,493 |
|
12,968 |
|
|
| Purchased goods (third party products) |
4,762 |
|
3,626 |
|
|
| GROSS AMOUNT OF INVENTORIES BEFORE WRITE-DOWN |
89,173 |
|
90,067 |
|
|
| Less: write-down provision |
(35,510) |
|
(45,601) |
|
|
| INVENTORIES |
53,663 |
|
44,466 |
|
|
As of December 31, 2024 the decrease in gross amounts of inventories before write-down primarily related to a decrease in the inventory of raw materials and work in progress, which was partially offset by an increase in finished goods, namely, IXIARO, IXCHIQ and DUKORAL, and purchased goods from third parties.
The total write-down provision on inventory amounted to €35.5 million as of December 31,, 2024 (December 31, 2023: €45.6 million). The decrease in the write-down provision compared to the prior year was mainly attributable to the scrapping of the COVID-19 vaccine, VLA2001, in an amount of €14.0 million in 2024 (2023: €145.7 million) following the suspension of manufacturing of the product in 2022. All raw materials and work in progress related to VLA2001 that could not be repurposed and used for other products were written down during prior years.
Write-down provisions related to the inventory categories as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Raw materials |
21,728 |
|
28,158 |
|
|
| Work in progress |
12,600 |
|
15,177 |
|
|
| Finished goods |
591 |
|
1,524 |
|
|
| Purchased goods (third party products) |
591 |
|
743 |
|
|
| TOTAL WRITE-DOWN PROVISION |
35,510 |
|
45,601 |
|
|
As at December 31, 2024, €17.2 million of the inventory reserves were attributable to VLA2001 (December 31, 2023: €31.2 million), of which €17.2 million related to raw materials (December 31, 2023: €26.6 million).
As at December 31, 2024, the remaining write-down provision of €17.2 million in raw materials and work in progress related to Valneva's commercialized vaccines IXIARO, DUKORAL and IXCHIQ (December 31, 2023: €12.2 million).
As at December 31, 2024, the write-down provision for finished goods for Valneva's commercialized vaccines IXIARO IXCHIQ and DUKORAL based on sales expectations and limited shelf life of the products, amounted to €0.6 million (December 31, 2023: €1.5 million).
A slight decrease in the provision for third party products was visible as at December 31, 2024 (December 31, 2023: €0.7 million).
5.18 Trade receivables
Trade receivables are initially recognized at fair value. The carrying amount of trade receivables is reduced through an allowance for doubtful account. When a trade receivable is considered uncollectible, it is written off against this allowance account.
Subsequent recoveries of amounts previously written off are credited against the allowance account. Changes in the carrying amount of the allowance account are recognized in the profit or loss.
Trade receivables include the following:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Trade receivables |
35,257 |
|
41,714 |
|
|
| Less: loss allowance of receivables |
(52) |
|
(69) |
|
|
| TRADE RECEIVABLES, NET |
35,205 |
|
41,645 |
|
|
In 2024 and 2023, no material impairment losses were recognized. As at December 31, 2024, the amount of trade receivables past due (which is defined as being more than 30 days late) reached €2.0 million (December 31, 2023: €4.5 million). On December 31, 2023, an amount of €3.4 million came from a governmental authority with a credit rating of B+/B2, which was paid in the course of 2024 and led to the decrease in the outstanding amount.
Due to the short-term nature of the current receivables, their carrying amount is considered to be the same as their fair value.
As at December 31, 2024, trade receivables included €35.2 million (December 31, 2023: €41.6 million) of receivables from contracts with customers. The decrease is mainly due to payments received from Pfizer and a governmental authority.
5.19 Other assets
Other assets include the following:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| R&D tax credit receivables |
31,208 |
|
43,762 |
|
|
| Advance payments |
399 |
|
759 |
|
|
| Tax receivables |
3,686 |
|
3,921 |
|
|
| Prepaid expenses |
8,878 |
|
4,468 |
|
|
| Contract costs |
3,710 |
|
3,710 |
|
|
| Consumables and supplies on stock |
767 |
|
872 |
|
|
| Miscellaneous current assets |
11 |
|
522 |
|
|
| OTHER NON-FINANCIAL ASSETS |
48,659 |
|
58,014 |
|
|
| Deposits |
198 |
|
194 |
|
|
| Miscellaneous financial assets |
1,058 |
|
916 |
|
|
| OTHER FINANCIAL ASSETS |
1,256 |
|
1,109 |
|
|
| OTHER ASSETS |
49,915 |
|
59,123 |
|
|
| Less non-current portion |
8,041 |
|
8,490 |
|
|
| CURRENT PORTION |
41,874 |
|
50,633 |
|
|
Due to the short term nature of the financial instruments included in other assets, their carrying amount is considered to be the same as their fair value.
The “R&D tax credit receivables” mainly relate to the research and development tax credit in Austria, Scotland, and France. The decrease in 2024 is due to the received payment of Austrian R&D tax credit related to the year 2021.
The increase in prepaid expenses is due to the upfront payment to Limmatech Biologics AG concerning the Shigella4V program amounting to €3.8 million.
The miscellaneous financial assets mainly relate to the grant received from Scottish Enterprise. For more information see Note 5.8.1.
5.20 Cash and cash equivalents
Cash includes cash at bank, cash in hand, and deposits held at call with banks. Cash equivalents include short-term bank deposits and medium-term notes with a maximum maturity of three months that can be assigned or sold on very short notice and are subject to insignificant risk of changes in value in response to fluctuations in interest rates.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Cash in hand |
1 |
|
9 |
|
|
| Cash at bank |
168,269 |
|
126,071 |
|
|
|
|
|
|
| Clearing accounts |
— |
|
(1) |
|
|
|
|
|
|
|
|
|
|
| CASH AND CASH EQUIVALENTS |
168,271 |
|
126,080 |
|
|
As at December 31, 2024, and December 31, 2023, the Group had no restricted cash.
In 2024, the minimum liquidity requirement for the Group according to the D&O Loan Agreement (see Note 5.24.1) was €35 million.
5.21 Assets classified as held for sale
As at December 31, 2024, no assets were classified as held for sale and no transactions involving the reclassification of assets into this category occurred during the current financial year. This status reflects the fact that all assets continue to be used in the Group’s operations and that, at the date of preparation of the financial statements, no disposal is anticipated that would meet the criteria for reclassification as held for sale.
BliNK Biomedical SAS
Valneva previously held a 48.9% equity interest in BliNK Biomedical SAS, Marseille (BliNK), a private company not listed on a stock exchange. As a result of the management's intent to sell the equity interest, it was classified as an asset held for sale as of June 30, 2022.
On September 8, 2023, the Company sold its equity interest in BliNK. The proceeds of the sale amounted to €2.4 million. For the year ended December 31, 2023 the final sale resulted in a profit of €0.2 million. The transaction stipulates an earn-out component which entitles the Company to receive 0.006491% for each equity interest share of BliNK’s net revenue over a period of seven years. The Company has assessed the fair value of the earn-out component as at December 31, 2024 and 2023 to be immaterial.
Divestment of CTM Unit in Solna, Sweden
Valneva decided to divest its Clinical Trial Materials unit in Solna. The transfer of ownership of the unit took effect on July 1, 2023. With the payment of the proceeds, no remaining assets or liabilities held for sale were shown for the CTM unit as of December 31, 2024 and 2023.
5.22 Equity
5.22.1 Share capital and share premium
The ordinary shares and convertible preferred shares are classified as equity.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| Number of shares |
2024 |
2023 |
|
Ordinary shares issued (€0.15 par value per share) |
162,521,524 |
138,912,142 |
|
| Convertible preferred shares registered |
— |
— |
|
| TOTAL SHARES ISSUED |
162,521,524 |
138,912,142 |
|
| Less Treasury shares |
(124,322) |
(124,322) |
|
| OUTSTANDING SHARES |
162,397,202 |
|
138,787,820 |
|
|
Incremental costs directly attributable to the issue of new shares are shown in equity as a deduction, net of tax, if any, from the proceeds.
When the Company purchases its own equity share capital (treasury shares), the consideration paid, including any directly attributable incremental costs (net of income taxes, if any) is deducted from equity attributable to the Company’s equity holders until the shares are cancelled, reissued or otherwise disposed of. In cases where such shares are subsequently sold or reissued, any consideration received, net of any directly attributable incremental transaction costs and related income tax effects is included in equity attributable to the Company’s equity holders.
The profit or loss for the year is fully included in net result, while other comprehensive income solely affects retained earnings and other reserves.
The following table shows the development of the number of outstanding shares:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| Number of shares |
2024 |
2023 |
|
| OUTSTANDING AS AT JANUARY 1 |
138,787,820 |
138,243,160 |
|
| Share-based compensation exercises |
609,382 |
|
544,660 |
|
|
| Capital Increase |
23,000,000 |
|
— |
|
|
|
|
|
|
|
|
|
|
| OUTSTANDING AT YEAR END |
162,397,202 |
|
138,787,820 |
|
|
The Company has issued stock options to employees under various employee stock option plans (ESOPs) established in the last 10 years. For details, please refer to Note 5.23.
On September 13, 2024, the Company announced the successful pricing of its Private Placement for a total of 23,000,000 new ordinary shares having a nominal value of €0.15, being issued at a price of €2.66 each. Aggregate gross proceeds of the Global Offering, before deducting underwriting commissions and expenses payable by the Company, were €61.2 million. The €4.0 million transaction costs directly attributable to the issue of new shares are shown in equity as a deduction, net of tax resulting in a net proceeds of €57.1 million.
Conditional and authorized capital
As at December 31, 2024, the Company had 14,003,064 (December 31, 2023: 9,919,432) shares of conditional capital in connection with (see Note 5.23):
•the possible exercise of existing stock options; and
•the possible final grant of existing Free Ordinary Shares.
Pursuant to resolution No. 37 of the Combined General Meeting held on June 26, 2024, the maximum aggregate amount of capital increases that may be carried out, with immediate effect or in the future, under resolutions 29 to 37 of said Meeting, may not exceed €5.175 million, it being specified that to this maximum aggregate amount will be added the additional nominal amount of shares or securities to be issued in accordance with applicable legal or regulatory provisions and, if applicable, with contractual provisions providing for other forms of adjustment, in order to preserve the rights of the holders of securities or other rights giving immediate and/or future access to the capital of the Company.
5.22.2 Other reserves
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Other regulated reserves |
Other comprehensive income |
Treasury shares |
Capital from Share-based compensation |
Other revenue reserves |
Total |
| BALANCE AS AT JANUARY 1, 2024 |
52,820 |
|
(1,871) |
|
(645) |
|
24,301 |
|
(9,517) |
|
65,088 |
|
| Currency translation differences |
— |
|
(1,329) |
|
— |
|
— |
|
— |
|
(1,329) |
|
| Defined benefit plan actuarial losses |
— |
|
49 |
|
— |
|
— |
|
— |
|
49 |
|
| Share-based compensation expense |
— |
|
— |
|
— |
|
9,395 |
|
— |
|
9,395 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE AS AT DECEMBER 31, 2024 |
52,820 |
|
(3,151) |
|
(645) |
|
33,696 |
|
(9,517) |
|
73,203 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Other regulated reserves |
Other comprehensive income |
Treasury shares |
Capital from Share-based compensation |
Other revenue reserves |
Total |
| BALANCE AS AT JANUARY 1, 2023 |
52,820 |
|
(5,041) |
|
(645) |
|
17,636 |
|
(9,517) |
|
55,252 |
|
| Currency translation differences |
— |
|
3,300 |
|
— |
|
— |
|
— |
|
3,300 |
|
| Defined benefit plan actuarial gains |
— |
|
(130) |
|
— |
|
— |
|
— |
|
(130) |
|
| Share-based compensation expense |
— |
|
— |
|
— |
|
6,666 |
|
— |
|
6,666 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| BALANCE AS AT DECEMBER 31, 2023 |
52,820 |
|
(1,871) |
|
(645) |
|
24,301 |
|
(9,517) |
|
65,088 |
|
Other regulated reserves contain a non-distributable mandatory legal reserve from the merger with Intercell AG.
The Company did not obtain a dividend from its subsidiaries or pay a dividend to its shareholders in 2024 and 2023.
5.23 Share-based compensation
The Company operates various share-based compensation plans, both equity-settled and cash-settled plans. The consolidated statement of profit or loss includes the following expenses arising from share-based payments:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
|
| Stock option plans |
7,894 |
|
5,152 |
|
1,916 |
|
|
|
|
|
|
|
| Free ordinary shares program |
1,501 |
|
1,514 |
|
719 |
|
|
|
|
|
|
|
| Phantom shares |
(685) |
|
(390) |
|
(11,291) |
|
|
| SHARE-BASED COMPENSATION EXPENSE /(INCOME) |
8,710 |
|
6,276 |
|
(8,656) |
|
|
5.23.1 Stock option plans
The fair value of such share-based compensation is recognized as an expense for employee services received in exchange for the grant of the options. The total amount to be expensed over the vesting period is determined by reference to the fair value of the options granted, excluding the impact of any non-market vesting conditions. Non-market vesting conditions are included in assumptions about the number of options that are expected to become exercisable. Annually, the Group revises its estimates of the number of options that are expected to become exercisable. It recognizes the impact of the revision of original estimates, if any, in the income statement and makes a corresponding adjustment to equity.
The proceeds received net of any directly attributable transaction costs are credited to nominal capital (nominal value) and share premium (amount exceeding nominal value) when the options are exercised.
Beginning in 2013, the Company granted stock options to employees and management pursuant to eight successive plans.
Stock options granted from 2013 to 2017 are exercisable in two equal portions after being held for two and for four years (the vesting periods), while stock options granted from 2019 onwards are exercisable in three equal portions after being held for one year, two years and three years. Stock options granted in 2019 are subject to performance conditions.
All options expire no later than ten years after being granted. Stock options are not transferable or negotiable and unvested options lapse without compensation upon termination of employment with the Group (forfeiture). Stock options granted from 2013 onwards vest with the effectiveness of the takeover of more than 50% of the outstanding voting rights of the Group. As this change of control event was considered remote, it has not been considered in the determination of the vesting period.
Changes in the number of stock options outstanding and their related weighted average exercise prices are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2024 |
2023 |
|
Number of options |
Number of shares available |
Average exercise price (in € per share) |
Number of options |
Number of shares available |
Average exercise price (in € per share) |
| OUTSTANDING AS AT JANUARY 1 |
8,550,802 |
|
8,550,802 |
|
5.02 |
|
5,774,339 |
|
5,776,114 |
|
4.90 |
|
| Granted |
4,957,716 |
|
4,957,716 |
|
2.62 |
|
3,441,269 |
|
3,441,269 |
|
5.25 |
|
| Expired |
— |
|
— |
|
N/A |
(3,648) |
|
(4,015) |
|
2.92 |
|
| Forfeited |
(1,036,473) |
|
(1,036,473) |
|
4.47 |
|
(647,024) |
|
(647,024) |
|
5.25 |
|
| Exercised |
— |
|
— |
|
N/A |
(14,134) |
|
(15,542) |
|
2.92 |
|
| OUTSTANDING AT YEAR END |
12,472,045 |
12,472,045 |
3.44 |
|
8,550,802 |
8,550,802 |
5.02 |
|
| Exercisable at year end |
4,766,957 |
4,766,957 |
4.61 |
3,296,856 |
3,296,856 |
3.98 |
No employee stock options were exercised in 2024, whereas 14,134 employee stock options (of which 14,134 were granted from ESOP 2013) were exercised in 2023.
Stock options outstanding at the end of the period have the following expiry dates and exercise prices:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise price (in € per share) |
Number of options as at December 31, (presentation as number of convertible shares) |
|
| Expiry date |
2024 |
2023 |
|
|
|
|
|
|
| 2025 |
3.92 |
|
43,655 |
43,655 |
|
| 2026 |
2.71 |
|
14,500 |
14,500 |
|
| 2027 |
2.85 |
|
551,475 |
551,475 |
|
| 2029 |
3.05 |
|
1,596,166 |
1,770,676 |
|
| 2032 |
6.47 |
|
2,339,974 |
2,750,477 |
|
| 2033 |
5.25 |
|
3,005,809 |
3,420,019 |
|
| 2034 |
2.62 |
|
4,920,466 |
— |
|
| OUTSTANDING AT YEAR END |
|
12,472,045 |
|
8,550,802.00 |
|
|
During 2024, 4,957,716 stock options were granted (2023: 3,441,269). The weighted average grant date fair value of options granted during 2024 was €1.84 (2023: €3.22). The fair value of the granted options was determined using the Black Scholes valuation model.
The significant inputs into the models were:
|
|
|
|
|
|
|
As at Oct 2024 |
Expected volatility (%), based on historical volatility |
75.42 |
% |
Expected vesting period (term in years) |
5.50 – 6.50 |
Risk-free interest rate (%) |
2.03% – 2.43% |
5.23.2 Free ordinary shares
In 2024, the Company’s Board of Directors granted 991,643 free ordinary shares for the benefit of Executive Committee and members of the Company’s senior management (2023: 445,320). The purpose of these free share plans is to provide a long-term incentive program for the Company’s senior management.
The number of free ordinary shares granted was as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| Number of free ordinary shares granted |
2024 |
2023 |
|
| Executive Committee (formerly Management Board) |
572,793 |
263,842 |
|
| Senior Leadership Group |
418,850 |
181,478 |
|
| FREE ORDINARY SHARES GRANTED |
991,643 |
445,320 |
|
In accordance with the foregoing, changes in the outstanding free ordinary shares are as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| Number of free shares |
2024 |
2023 |
|
| OUTSTANDING AS AT JANUARY 1 |
1,368,630 |
|
1,487,667 |
|
|
| Granted |
991,643 |
|
445,320 |
|
|
| Forfeited |
(265,872) |
|
(14,725) |
|
|
| Exercised |
(609,382) |
|
(549,632) |
|
|
| OUTSTANDING AT YEAR END |
1,485,019 |
1,368,630 |
|
Subject to vesting conditions (service conditions), the free share granted to a participant will vest in and be definitely delivered to that participant in three tranches. Each tranche will amount to one third of the total individual allocation. If one third is not a whole number, the number of free shares will be rounded down for the first two tranches and rounded up for the third tranche.
The first and the second tranche for the free shares granted in 2024 will vest on October 22, 2026, and the third tranche will vest on October 22, 2027.
Following the vesting of the free shares, no compulsory holding period will apply to the vested shares.
The expenses arising from the free ordinary share plan is the number of shares granted expected to vest multiplied with the share price at the grant date.
The 2024, 2023 and 2022 plans further provide for accelerated vesting of the free shares in the event of a Change of Control (as defined in the applicable terms & conditions) occurring no earlier than two years after the grant date. For the 2022 plan that is October 10, 2024, for the 2023 plan it is December 15, 2025, and for the 2024 plan it is October 22, 2026. As management considered the chance of a Change of Control remote at the grant date, this was not included in the determination of the vesting period. In addition, the plan provides for the possibility to remain entitled to a prorated number of shares, for any unvested tranche, in case of retirement of a beneficiary before complete vesting.
Finally, the terms and conditions applicable to the free share plans state that if a Change of Control takes place before the specified date and section III of Article L. 225-197-1 of the French Commercial Code does not apply, the plan will be canceled and the Company will indemnify the participants for the loss of unvested free shares, subject however to all required shareholder approvals where corporate officers are concerned. The gross amount of this indemnity will be calculated as though such free shares had been vested upon the Change of Control. The conditions and limitations set forth in the applicable terms and conditions of the plan will apply to this calculation, mutatis mutandis.
In accordance with the decisions of the Board of Directors of June 25, 2024 and October 22, 2024, and by application of section II (5th paragraph) of Article L. 225-197-1 of the French Commercial Code when applicable, the beneficiaries that are corporate officers, i.e. the CEO (Directeur Général) and the Associate Managing Officers (Directeurs Généraux Délégués), and each of the other members of the Executive Committee should keep not less than 20% of the vested free shares of each tranche until termination of both their Executive Committee membership and (as applicable) their corporate office.
5.23.3 Phantom shares
In 2017, 2019 and 2020, phantom share plans were issued for employees who are U.S. citizens, with the same conditions as the stock option and free ordinary share programs (see above) but which will not be settled in equity, but in cash. Therefore, it is considered as a cash settled plan. The liability for the phantom shares is measured (initially and at the end of each reporting period until settled) at the fair value of the share options rights, by applying an option pricing model taking into account the terms and conditions on which the phantom rights were granted and the extent to which the employees have rendered services to date.
No new phantom shares were granted in 2024 or in 2023.
In accordance with the foregoing, changes in the outstanding phantom shares are as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| Number of phantom shares |
2024 |
2023 |
|
| OUTSTANDING AS AT JANUARY 1 |
410,500 |
|
670,500 |
|
|
| Granted |
— |
|
— |
|
|
| Forfeited |
(161,000) |
|
(50,000) |
|
|
| Exercised |
(210,000) |
|
(210,000) |
|
|
| OUTSTANDING AT YEAR END |
39,500 |
|
410,500 |
|
|
The carrying amount of the liability relating to the phantom shares as at December 31, 2024 was €1.4 thousand (December 31, 2023: €1.4 million). The fair values of the granted options were determined on the balance sheet dates using the Black Scholes valuation model.
Phantom shares outstanding at the end of the period have the following expiry dates and exercise prices:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercise price (in € per share) |
Number of phantom shares as at December 31, |
|
| Expiry date |
2024 |
2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2027 |
2.85 |
|
6,250 |
|
6,250 |
|
|
| 2029 |
3.05 |
|
33,250 |
|
194,250 |
|
|
| 2030 |
— |
|
— |
|
210,000 |
|
|
| OUTSTANDING AT YEAR END |
|
39,500 |
|
410,500 |
|
|
The significant inputs into the models were:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
|
2024 |
2023 |
|
| Expected volatility (in %) |
53.47 |
% |
51.26 |
% |
|
| Expected vesting period (term in years) |
— |
|
— |
|
|
| Risk-free interest rate (in %) |
2.31 |
% |
2.10 |
% |
|
5.24 Borrowings
Borrowings are initially recognized at fair value if determinable, net of transaction costs incurred. Borrowings are subsequently stated at amortized cost. Any difference between the proceeds (net of transaction costs) and the redemption value is recognized in the income statement over the period of the borrowings using the effective interest method.
Borrowings are classified as current liabilities unless the Group has an unconditional right to defer settlement of the liability for at least 12 months after the balance sheet date.
Borrowings of the Group at period-end include the following:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| NON-CURRENT |
|
|
|
| Debentures and other loans |
166,521 |
|
132,768 |
|
|
| CURRENT |
|
|
|
| Debentures and other loans |
20,852 |
|
44,079 |
|
|
| TOTAL BORROWINGS |
187,373 |
|
176,847 |
|
|
As at December 31, 2024, the carrying amount of bank borrowings and other loans was €187.4 million. Of this, €180.8 million related to the D&O Loan Agreement. Other borrowings related to financing of research and development expenses included the CIR (research and development tax credit in France) of €3.5 million (December 31, 2023: €3.6 million) and the CEPI grant in the amount of €3.0 million (December 31, 2023: €5.7 million), which relates to advance payments that are expected to be paid back in the future.
The maturity of the borrowings is as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Between 1 and 3 years |
132,489 |
|
62,378 |
|
|
| Between 3 and 5 years |
33,349 |
|
70,390 |
|
|
| Over 5 years |
683 |
|
— |
|
|
| NON-CURRENT BORROWINGS |
166,521 |
|
132,768 |
|
|
| Current borrowings |
20,852 |
|
44,079 |
|
|
| TOTAL BORROWINGS |
187,373 |
|
176,847 |
|
|
The carrying amounts of the Group’s borrowings are denominated in the following currencies:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Borrowings denominated in EUR |
3,540 |
|
3,581 |
|
|
| Borrowings denominated in USD |
183,833 |
|
173,266 |
|
|
| TOTAL BORROWINGS |
187,373 |
|
176,847 |
|
|
5.24.1 Other loans
As at December 31, 2024, a total of $200.0 million has been drawn under the D&O Loan Agreement. The book value of the loan amounts to $187.9 million (€180.8 million). Following amendments to the D&O Loan Agreement, in March 2024, the interest-only period on the initial $100.0 million (€93.4 million) tranche lasts until the first quarter of 2026, and this portion of the loan will mature in the first quarter of 2027. The interest-only period for the tranches drawn in 2023 amounting to $100.0 million lasts until the first quarter of 2027, and this portion of the loan will mature in the third quarter of 2028. The interest rate on all tranches remains unchanged at 9.95%, translating into an effective interest rate for the first draw of 14.17% and for the second draw of 13.47% as of December 31, 2024. Transaction costs amounting to $0.9 million ($11.2 million in December 31, 2023) have been deducted from the loan proceeds received in December 31, 2024. The net present value of the modified loan was less than 10% different from the previous net present value of the loan, therefore the modification is not treated as an extinguishment under IFRS 9.
The loan is secured by substantially all of Valneva’s assets, including its intellectual property, and is guaranteed by Valneva SE and certain of its subsidiaries. The minimum liquidity requirement is €35.0 million for 2024. The twelve-month rolling minimum revenue requirement of €115.0 million is effective for 2024. The Group does not expect these limitations to affect its ability to meet its cash obligations. As at December 31, 2024, the Group’s consolidated liquidity or net revenues did not fall below the covenant minimum values.
The D&O Loan Agreement is included in the balance sheet item “Borrowings” and developed as follows:
|
|
|
|
|
|
|
|
|
|
| in € thousand |
2024 |
2023 |
|
| BALANCE AS AT JANUARY 1 |
167,520 |
|
89,182 |
|
|
| Proceeds of issue |
— |
|
91,111 |
|
|
| Transaction costs |
(944) |
|
(11,198) |
|
|
| Accrued interest |
22,530 |
|
12,942 |
|
|
| Payment of interest |
(18,978) |
|
(11,022) |
|
|
| Exchange rate difference |
10,713 |
|
(3,494) |
|
|
| BALANCE AS AT CLOSING DATE |
180,841 |
|
167,520 |
|
|
| Less: non-current portion |
(161,420) |
|
(127,119) |
|
|
| CURRENT PORTION |
19,421 |
|
40,401 |
|
|
5.24.2 Borrowings and other loans secured
As at December 31, 2024, €184.4 million (December 31, 2023: €171.1 million) of the outstanding borrowings and other loans were guaranteed, secured, or pledged. These borrowings and other loans related to financing of research and development expenses, fixed assets and CIR (R&D tax credit in France) and have various conditions (interest rates) and terms (maturities).
5.24.3 Fair value of borrowings and other loans
The fair value of the borrowings and other loans are calculated by discounting the contractual cash flows with interest rates derived from relevant bond yields and swap rates and adjusted for any further potential risk and liquidity risks related to the nature of each loan. The relevant bond yields were determined by an internal analysis based on Moody’s RiskCalc corporate rating methodology. As at December 31, 2024, and December 31, 2023, the resulting calculations revealed no material difference between the carrying amount and the fair value.
5.25 Trade payables and accruals
Trade payables and accruals include the following:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Trade payables |
12,639 |
|
17,564 |
|
|
| Accrued expenses |
22,883 |
|
26,739 |
|
|
| TOTAL |
35,522 |
|
44,303 |
|
|
| Less non-current portion |
— |
|
— |
|
|
| CURRENT PORTION |
35,522 |
|
44,303 |
|
|
The carrying amounts of trade and other payables are considered to be the same as their fair values, due to their short-term nature. All trade payables and accruals are current.
5.26 Tax and employee-related liabilities
Liabilities for tax and employee-related liabilities are generally measured at amortized costs. Liabilities related to employees comprise mainly accruals for bonuses and unconsumed vacations. The line social security and other taxes consists of amounts owed to tax authorities and social security institutions.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Employee-related liabilities |
13,107 |
|
10,815 |
|
|
| Social security and other taxes |
6,350 |
|
5,394 |
|
|
| BALANCE AS AT DECEMBER 31 |
19,458 |
|
16,209 |
|
|
| Less non-current portion |
— |
|
— |
|
|
| CURRENT PORTION |
19,458 |
|
16,209 |
|
|
5.27 Lease liabilities
Lease liabilities are effectively secured as the rights to the leased assets revert to the lessor in the event of default.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| OPENING NET BOOK VALUE |
31,969 |
|
53,574 |
|
|
| Additions |
237 |
|
3,759 |
|
|
| Revaluation due to variable payments |
1,399 |
|
(2) |
|
|
| Termination of contracts |
(1,100) |
|
(22,539) |
|
|
| Lease payments |
(3,425) |
|
(4,286) |
|
|
| Interest expenses |
813 |
|
1,183 |
|
|
| Exchange rate differences |
(952) |
|
280 |
|
|
| CLOSING NET BOOK VALUE |
28,941 |
|
31,969 |
|
|
In 2024, lease liabilities decreased by €3.0 million, mainly due to a lease contract termination in Scotland, an early termination of some leased office spaces in France and redemption of lease liabilities in Sweden. In addition, variable lease payments connected to an index in Sweden were revalued to the current index value.
The maturity of non-current lease liabilities is as follows:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Between 1-3 years |
5,203 |
|
5,313 |
|
|
| Between 3-5 years |
5,083 |
|
5,414 |
|
|
| Over 5 years |
16,147 |
|
18,362 |
|
|
| NON-CURRENT LEASE LIABILITIES |
26,432 |
|
29,090 |
|
|
| Current lease liabilities |
2,508 |
|
2,879 |
|
|
| TOTAL LEASE LIABILITIES |
28,941 |
|
31,969 |
|
|
The carrying amounts of the Group’s lease liabilities are denominated in the following currencies:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| EUR |
1,078 |
|
1,479 |
|
|
| SEK |
26,870 |
|
28,308 |
|
|
| Other |
992 |
|
2,182 |
|
|
| TOTAL LEASE LIABILITIES |
28,941 |
|
31,969 |
|
|
5.28 Contract liabilities
A contract liability has to be recognized when the customer has already provided the consideration or part of the consideration before an entity has fulfilled its performance obligation (agreed goods or services which should be delivered or provided) resulting from the “contract”.
Development of contract liabilities is presented in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
|
| in € thousand |
2024 |
2023 |
|
|
| BALANCE AS AT JANUARY 1 |
5,697 |
|
9,411 |
|
|
|
| Revenue recognition |
(462) |
|
(4,394) |
|
|
|
| Redemption |
(4,777) |
|
— |
|
|
|
| Addition |
2,500 |
|
1,870 |
|
|
|
| Other releases |
— |
|
(1,032) |
|
|
|
| Exchange rate differences |
53 |
|
(159) |
|
|
|
| BALANCE AS AT CLOSING DATE |
3,010 |
|
5,697 |
|
|
|
| Less non-current portion |
— |
|
— |
|
|
|
| CURRENT PORTION |
3,010 |
|
5,697 |
|
|
|
As at December 31, 2024, the redemption in the amount of €4.8 million mainly relates to U.S. distributor agreements, for which the obligation to replace vaccine doses was fulfilled in 2024. An upfront payment of €2.5 million is shown under additions and refer to the master collaboration and license agreement with Serum Institute of India regarding IXCHIQ.
In the year ended December 31, 2023, we recognized revenue in the amount of €3.8 million from the Advanced Purchase Agreement (APA) for VLA2001 with the Kingdom of Bahrain. The other releases of €1.0 million were from the divestment of Valneva’s CTM Unit in Solna as of July 1, 2023.
5.29 Refund liabilities
A refund liability has to be recognized when the customer has already provided a consideration which is expected to be refunded partially or totally. It is measured at the amount the Company has an obligation to repay or amounts which did not meet the criteria for revenue recognition in the past, but there are no remaining goods and services to be provided in future.
Development of refund liabilities during the period is presented below:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| BALANCE AS AT JANUARY 1 |
39,941 |
|
143,085 |
|
|
| Additions |
4,013 |
|
465 |
|
|
| Payments |
(979) |
|
(352) |
|
|
| Other releases |
(18,922) |
|
(108,542) |
|
|
| Revenue recognition |
— |
|
(40) |
|
|
| Interest expense capitalized |
360 |
|
8,419 |
|
|
| Exchange rate difference |
1,728 |
|
(3,095) |
|
|
| BALANCE AS AT CLOSING DATE |
26,141 |
|
39,941 |
|
|
| Less non-current portion |
(6,491) |
|
(6,303) |
|
|
| CURRENT PORTION |
19,650 |
|
33,637 |
|
|
As at December 31, 2024, from the total refund liability of €26.1 million, €18.6 million (all-current) are connected to the Collaboration and License Agreement with Pfizer. In the first half of 2024 payments were made by Valneva in connection with the terms and schedule of the Agreement, which are disclosed under other releases. In addition, services provided by Valneva during 2024 resulted in additions to the refund liability.
Refund liabilities of €6.5 million (of which €6.5 million is non-current) relate to the expected payment to GlaxoSmithKline (GSK) due to the termination of the strategic alliance agreements (SAA) in 2019.
An amount of €1.1 million relates to refund liabilities mainly due to statistical return provision and rebates as at December 31, 2024 (December 31, 2023: €0.3 million).
As at December 31, 2023, €33.1 million originated from the collaboration with Pfizer and €6.5 million (of which €6.3 million was non-current) related to the expected payment to GSK from the termination of the SAA in 2019.
5.30 Provisions
5.30.1 Provisions for employee commitments
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Employer contribution costs on share-based compensation plans |
81 |
|
1,684 |
|
|
| Phantom shares |
1 |
|
1,421 |
|
|
| Retirement termination benefits |
515 |
|
459 |
|
|
| Leaving indemnities and restructurings |
964 |
|
670 |
|
|
| BALANCE AS AT CLOSING DATE |
1,561 |
|
4,234 |
|
|
| Less non-current portion |
546 |
|
490 |
|
|
| CURRENT PORTION |
1,015 |
|
3,744 |
|
|
Share-based provisions
Employer contribution costs on share-based compensation plans and phantom shares are calculated at the balance sheet date using the share price of Valneva as at December 31, 2024: €2.16 (December 31, 2023: €4.72). The decrease in these provisions as at December 31, 2024 is mainly due to the share price movement.
Retirement termination benefits
Some Group companies provide retirement termination benefits to their retirees.
For defined benefit plans, retirement costs are determined once a year:
•From December 31, 2021 onward, under the new calculation method proposed by the IFRS IC and according to the updated recommendation of the ANC n 2013-02 as at December 31, 2021: under this method, when the plan provides for the payment of an indemnity to the employee, if he or she is present at the date of retirement, the amount of which depends on seniority and is capped at a certain years of service, the commitment must be calculated solely on the basis of the years of service prior to the retirement date.
The final obligation is then discounted. These calculations mainly use the following assumptions:
•a discount rate;
•a salary increase rate;
•an employee turnover rate.
Actuarial gains and losses arising from experience adjustments and changes in actuarial assumptions are charged or credited to equity in other comprehensive income in the period in which they arise.
For basic schemes and defined contribution plans, the Group recognizes the contributions as expenses when payable, as it has no obligations over and above the amount of contributions paid.
Assumptions used
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
|
| in € thousand |
2024 |
2023 |
|
|
| Discount rate |
3.40 |
% |
3.20 |
% |
|
|
| Salary increase rate |
2.50 |
% |
2.50 |
% |
|
|
| Turnover rate |
0%-21.35% |
0%-21.35% |
|
|
| Social security rate |
43.00%-47.00% |
43.00%-47.00% |
|
|
Average remaining lifespan of employees (in years) |
20 |
22 |
|
|
Changes in defined benefit obligation
Present value of obligation development:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| BALANCE AS AT JANUARY 1 |
459 |
|
330 |
|
|
| Current service cost |
105 |
|
(1) |
|
|
| Actuarial losses/(gains) |
(49) |
|
130 |
|
|
| BALANCE AS AT CLOSING DATE |
515 |
|
459 |
|
|
5.30.2 Other provisions
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| Non-current |
— |
|
584 |
|
|
| Current |
5,671 |
|
7,091 |
|
|
| PROVISIONS |
5,671 |
|
7,675 |
|
|
The position mostly comprises €5.2 million from a provision for expected legal and settlement costs under a court proceeding related to the Intercell AG/Vivalis SA merger (December 31, 2023: €5.2 million). For further information on this court proceeding, please see Note 5.33.
5.31 Other liabilities
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Deferred income |
5,028 |
|
513 |
|
|
| Other financial liabilities |
79 |
|
34 |
|
|
| Miscellaneous liabilities |
91 |
|
125 |
|
|
| OTHER LIABILITIES |
5,198 |
|
671 |
|
|
| Less non-current portion |
(46) |
|
(79) |
|
|
| CURRENT PORTION |
5,152 |
|
592 |
|
|
As at December 31, 2024 other liabilities increased to €5.2 million mainly due to deferred income related to the second agreement signed with CEPI.
5.32 Cash flow information
5.32.1 Cash generated from operations
The following table shows the adjustments to reconcile net loss to net cash generated from operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
|
2024 |
2023 |
2022 |
| PROFIT/(LOSS) FOR THE PERIOD |
|
(12,247) |
|
(101,429) |
|
(143,279) |
|
|
|
|
|
|
| Gain from sale of Priority Review Voucher, net |
|
(90,833) |
|
— |
|
— |
|
| ADJUSTMENTS FOR NON-CASH TRANSACTIONS: |
|
|
|
|
| Depreciation and amortization |
|
19,586 |
|
17,584 |
|
21,036 |
|
| Write-off/impairment fixed assets/intangibles |
|
— |
|
(731) |
|
23,249 |
|
| Share-based compensation expense |
|
7,975 |
|
5,111 |
|
(8,656) |
|
| Income tax expense/(income) |
|
761 |
|
2,800 |
|
(1,536) |
|
| Dividends received from associated companies |
|
— |
|
— |
|
— |
|
| (Profit)/loss from disposal of property, plant, equipment and intangible assets |
|
(266) |
|
(12) |
|
38 |
|
| Share of (profit)/loss from associates |
|
— |
|
— |
|
(9) |
|
| (Profit)/loss from disposal held for sale |
|
— |
|
580 |
|
— |
|
| Provision for employer contribution costs on share-based compensation plans |
|
(1,594) |
|
(1,659) |
|
(22,933) |
|
| Other non-cash (income)/expense |
|
895 |
|
(804) |
|
14,088 |
|
| Interest income |
|
(2,362) |
|
(1,210) |
|
(260) |
|
| Interest expense |
|
23,984 |
|
23,325 |
|
19,054 |
|
| TOTAL ADJUSTMENTS FOR NON-CASH TRANSACTIONS |
|
48,979 |
|
44,984 |
|
44,070 |
|
| CHANGES IN NON-CURRENT OPERATING ASSETS AND LIABILITIES (EXCLUDING THE EFFECTS OF ACQUISITION AND CONSOLIDATION): |
|
|
|
|
| Other non-current assets |
|
449 |
|
(192) |
|
10,981 |
|
| Long term contract liabilities |
|
— |
|
— |
|
(5,241) |
|
| Long term refund liabilities |
|
— |
|
1,136 |
|
(154,833) |
|
| Other non-current liabilities and provisions |
|
(629) |
|
(430) |
|
1,379 |
|
| TOTAL CHANGES IN NON-CURRENT OPERATING ASSETS AND LIABILITIES |
|
(180) |
|
514 |
|
(147,713) |
|
| CHANGES IN WORKING CAPITAL (EXCLUDING THE EFFECTS OF ACQUISITION AND EXCHANGE RATE DIFFERENCES ON CONSOLIDATION): |
|
|
|
|
| Inventory |
|
(6,803) |
|
(9,165) |
|
84,224 |
|
| Trade and other receivables |
|
15,707 |
|
(2,855) |
|
12,401 |
|
| Contract liabilities |
|
(2,793) |
|
(3,471) |
|
(114,603) |
|
| Refund liabilities |
|
(14,183) |
|
(112,689) |
|
33,764 |
|
| Trade and other payables and provisions |
|
(3,321) |
|
(17,398) |
|
(14,053) |
|
| Total changes in working capital |
|
(11,394) |
|
(145,578) |
|
1,732 |
|
| CASH GENERATED/(USED) IN OPERATIONS |
|
(65,674) |
|
(201,509) |
|
(245,189) |
|
(1) In the year ended December 31, 2022, the position “employee benefit other than share-based compensation” includes an income of €23.2 million, which resulted from release of the employer contribution provision, which was accounted for as of December 31, 2021 for the payable at the exercise of the IFRS 2 programs.
(2) As at December 31, 2022, the terms of the royalty and the CAPEX obligation towards the UK Authority were redefined under the 2022 settlement agreement. Management assessed the likelihood for this future obligation as remote. This resulted in a reduction of refund liabilities and recognition of other revenues recognized of €169.2 million.
5.32.2 Reconciliation of liabilities arising from financing activities
Liabilities arising from financing activities are those for which cash flows were (or future cash flows will be) classified in the Group’s consolidated statement of cash flows as cash flows from financing activities. The below table illustrates the development of borrowings. For development of lease liabilities see Note 5.27.
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31 |
| in € thousand |
2024 |
2023 |
|
| BALANCE AS AT JANUARY 1 |
176,847 |
|
98,806 |
|
|
| Proceeds of issue |
910 |
|
92,309 |
|
|
| Transaction costs |
(944) |
|
(11,198) |
|
|
| Repayments |
(3,734) |
|
(2,097) |
|
|
| Revaluations |
(385) |
|
393 |
|
|
| Accrued interest |
22,862 |
|
13,365 |
|
|
| Payment of interest |
(19,156) |
|
(11,025) |
|
|
| Exchange rate difference |
10,974 |
|
(3,706) |
|
|
| BALANCE AS AT DECEMBER 31 |
187,373 |
|
176,847 |
|
|
5.33 Commitments and contingencies
As at December 31, 2024, there were €3.5 million of capital expenditure contracted, mainly related to manufacturing sites (December 31, 2023: €3.7 million). The contracts are all related to the finalization of the Almeida building in Scotland, the new manufacturing facility and production site for IXIARO and IXCHIQ and to manufacturing equipment in Sweden for DUKORAL production site.
As at December 31, 2024 there were €39.3 million related to R&D collaboration agreements These commitments include milestone payments depending on successful clinical development or meeting specific revenue targets. The amount disclosed represents the maximum that would be paid if all milestones achieved.
5.33.1 Other commitments, pledges and guarantees
The other commitments mainly relate to royalty payments and consist of:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
| Loans and grants |
— |
|
6 |
|
|
| Royalties |
6,025 |
|
6,798 |
|
|
| OTHER COMMITMENTS |
6,025 |
|
6,804 |
|
|
The pledges consist of:
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
|
|
|
|
|
| Pledges on bank accounts |
164,546 |
|
121,085 |
|
|
|
|
|
|
| GUARANTEES AND PLEDGES |
164,546 |
|
121,085 |
|
|
The stated pledges on cash at banks originate from the requirements of the D&O Loan Agreement which in addition is secured by substantially all of Valneva’s assets, including its intellectual property, and is guaranteed by the Company and certain of its subsidiaries. For more information about this loan agreement, please refer to Note 5.24.
5.33.2 Contingencies and litigations
Following the merger between the companies Vivalis SA and Intercell AG in 2013, certain former Intercell shareholders initiated legal proceedings before the Commercial Court of Vienna to request a revision of either the cash compensation paid to departing shareholders or the exchange ratio between Intercell and Valneva shares used in the merger.
In October 2021, Valneva received an opinion from a court-appointed expert regarding the exchange ratio. The expert confirmed the calculation used previously, but also recommended the calculation of safety margins. Additionally, the expert addressed the cash compensation paid to departing shareholders and recommended an increase in such compensation. The expert provided a supplemental opinion in April 2022, and the judicial committee in charge of the proceedings gave its opinion to the Commercial Court of Vienna in April 2023.
Nonetheless, the final outcome will depend on the court’s position on specific legal points and the Court has not made a final decision yet. The Company therefore assessed the probability of several scenarios and decided to hold a provision of €5.2 million to cover the reassessed risk and potential legal costs (December 31, 2023: €5.2 million).
5.34 Related-party transactions
In the year ended December 31, 2024, there have been some changes to related parties. As the business evolved, management reassessed the contract with Groupe Grimaud La Corbière SAS, Sevremoine (France) and its affiliate Vital Meat SAS. It was determined that they are no longer considered as related parties. Bpifrance, Maisons-Alfort (France) is still considered as related party with significant influence through a membership in the Company’s Board of Directors.
Additionally, there have been some changes in the key management personnel during the year. Since the transition to a one-tier governance model, in December 2023, the key management consists of the Board of Directors as well as the Executive Committee while until December 2023, it included the Management Board and the Supervisory Board.
5.34.1 Rendering of services
Transactions with related parties are carried out similar to those of the market:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Provision of services: |
|
|
|
| Operating activities |
391 |
|
260 |
|
1,200 |
|
| Financing activities |
194 |
|
76 |
|
8 |
|
| PROVISION OF SERVICES |
585 |
|
335 |
|
1,208 |
|
Services provided by Valneva to Groupe Grimaud La Corbière SAS, a shareholder of Valneva, were considered related party transactions until September 30, 2024 and consist of services within a collaboration and research license agreement and of the provision of premises and equipment and sale of patents and cells.
Operating activities include Valneva’s agreement with Vital Meat SAS, an affiliate of Group Grimaud La Corbière SAS, to which Valneva transferred certain assets (patent and cell lines) for a consideration of €1.0 million in the year ended December 31, 2022.
From June 2022 onward, Bpifrance qualifies as a related party since it is a shareholder of Valneva with significant influence through its membership of the Company's Board of Directors. Valneva has borrowed amounts amounting to 80% of French Tax Authorities receivables relating to Research Tax Credits for 2021, 2022 and 2023 from Bpifrance. The total amount borrowed from Bpifrance is €3.5 million. A commitment fee of 0.5% as well as interest at the EURIBOR one-month average rate of the previous month (the rate mentioned is a variable rate deducted at nil percent if it were to be negative) plus 1.7% p.a. is applicable to these borrowed amounts (see table above).
The borrowings related to the Research Tax Credits outstanding:
|
|
|
|
|
|
|
|
|
|
|
|
| in € thousand |
Amount |
Grant date |
|
|
|
|
|
| BPI payable relating to Research tax credit 2021 |
1,419 |
|
November 2022 |
|
| BPI payable relating to Research tax credit 2022 |
1,198 |
|
December 2023 |
|
| BPI payable relating to Research tax credit 2023 |
910 |
|
November 2024 |
|
|
|
|
|
5.34.2 Key management compensation
The aggregate compensation of the key management (including Executive Committee and Board of Directors) was as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, |
| in € thousand |
2024 |
2023 |
2022 |
| Salaries and other short-term employee benefits |
4,624 |
|
3,439 |
|
3,172 |
|
| Other long-term benefits |
83 |
|
52 |
|
45 |
|
| Share-based payments (expense of the year) |
3,128 |
|
2,145 |
|
722 |
|
| KEY MANAGEMENT COMPENSATION |
7,835 |
|
5,636 |
|
3,939 |
|
In the year ended December 31, 2024, the aggregate compensation of the members of the Company’s Executive Committee (former Management Board) amounted to €7.4 million (2023: €5.2 million, 2022: €3.6 million) and represents primarily salaries and share-based payments. The increase in ‘Salaries and other short-term employee benefits’ relates to additional two Executive Committee members. ‘Share-based payments’ increased due to the new free ordinary shares and stock options granted during 2024.
The presented key management compensation includes that of the Board of Directors in the amount of €0.5 million for the year ended December 31, 2024 (2023: €0.5 million; 2022: €0.4 million).
5.35 Events after the reporting period
IXIARO® new Supply Contract with the U.S. Government
Valneva SE signed a new $32.8 million contract with the United States (U.S.) Department of Defense (DoD) for the supply of its Japanese encephalitis (JE) vaccine, IXIARO. Under this new one year contract, the DoD will buy a minimum of $32.8 million worth of IXIARO vaccines and has the possibility to purchase additional doses during the coming twelve months
EX-1. 1
2
exhibit11articlesofassocia.htm
EX-1. 1
Document
|
|
|
VALNEVA SE
European company with a Board of Directors
with a share capital of 24,378,228.60 Euros
Registered office: 6 rue Alain Bombard, 44800 Saint-Herblain
Identification N° 422 497 560 RCS Nantes
(the Company)
-----
ARTICLES OF ASSOCIATION
As amended on December 6, 2024 |
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
TITLE I
FORM - COMPANY NAME - COMPANY OBJECT -
REGISTERED OFFICE - DURATION
Article 1. Form
The Company was incorporated in the form of a limited liability company with a Board of Directors under the terms of a private deed of 24 March 1999.
The shareholders of the Company modified the form of management and governance, adopting the formula of a Management Board and Supervisory Board, by decision of the Extraordinary General Meeting of 29 November 2002.
On May 28 2013, the Company was transformed into a European Company (Societas Europaea or SE) with a management board and supervisory board through a cross-border merger between Intercell AG, a company governed by Austrian law, with a share capital of 55,183,961 Euros, with registered office at Campus Vienna Biocenter 3, 1030 Vienna, Austria, formerly entered in the Trade and Companies Register of Vienna under number FN 166438m and Vivalis SA, a limited liability company governed by French law with a share capital of 3,224,379.30 Euros, with registered office at La Corbière - 49450 Roussay, and with the unique identification number 422 497 560 RCS Angers.
The shareholders of the Company modified the form of management and governance, adopting the formula of a Board of Directors, by decision of the Combined General Meeting of December 20, 2023.
The Company is governed by the European Community and national regulations in effect, as well as by these Articles of Association.
Article 2. Name
The company name is: Valneva.
In all of the instruments and documents deriving from the Company and intended for third parties, the name must be immediately preceded or followed by the words "European company" or the initials "SE" and a statement of the amount of the share capital.
Article 3. Object
The Company has as its object, within France and in every country:
o research and development within the field of biomedicine and pharmacology;
o the commercial exploitation of patents and know-how;
o trading in products of all kinds and the provision of services in the field of data processing and information technology;
o the production, monitoring and marketing of all products, services and research programs with applications to human and animal health, using the technologies of molecular and cellular biology and all of the associated techniques;
o the participation of the Company by all means, direct or indirect, in all operations which may be associated with its company object, through the creation of new companies, contributions, subscription or purchase of securities or company rights, mergers or otherwise, the creation, acquisition, leasing, lease management of all operating assets or facilities; o the acquisition, exploitation or sale of all procedures and patents regarding these activities, within France and abroad;
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
o and more generally, all industrial, commercial or financial, securities or property operations, which may be directly or indirectly associated with its business object or likely to favour its exploitation, realisation or development.
Article 4. Registered office
The registered office of the Company is located at 6 rue Alain Bombard, 44800 Saint-Herblain.
The registered office may be transferred to any location within France, upon simple decision by the Board of Directors and subject to ratification by the shareholders at their next Ordinary General Meeting or by a decision of the Extraordinary General Meeting in accordance with applicable statutory provisions. The transfer of the registered office to another member State of the European Community is subject to ratification of the Special Meeting of the shareholders in accordance with L. 229-2 of the French commercial code. In the case of a transfer decided in accordance with the law by the Board of Directors, the latter is authorized to modify the Articles of Association in consequence.
Article 5. Duration - Financial year
The duration of the Company shall be ninety-nine (99) years from its first registration in the Trade and Companies Register, except in cases of extension or early dissolution.
The financial year shall begin on January 1 and shall end on December 31.
TITLE II
SHARE CAPITAL – SHARES
Article 6. Share capital
The share capital is set at 24,378,228.60 Euros. It is divided into 162,521,524 fully subscribed and paid-up ordinary shares of 0.15 Euro par value each.
Article 7. Change in the share capital
The share capital shall be increased by any means and by all procedures provided by law. The Extraordinary General Meeting, on the report of the Board of Directors, has sole competence for deciding on the share capital increase and may delegate such competence as provided by law.
The shareholders shall have a preferential subscription right, in proportion to their shares, for subscribing to shares in the context of a share capital increase. Shareholders may waive their preferential subscription right in an individual capacity.
The right to the allocation of new shares to the shareholders, following the capitalisation of reserves, profits or issuance premiums, shall belong to the bare owner, subject to the rights of the usufructuary.
Article 8. Paying up of the shares
Shares subscribed in cash shall mandatorily be paid up for at least a quarter of their nominal value on subscription and, if necessary, for the entire issuance premium.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The paying in of the surplus shall take place, on one or several occasions, at the decision of the Board of Directors, within five years of the date on which the share capital increase has become final.
Calls for funds shall be brought to the attention of subscribers by registered letter with notice of receipt, sent at least fifteen (15) days before the date set for each payment. Payments shall be made either to the registered office or to any other place indicated for this purpose.
Any delay in the payment of amounts due on the unpaid amount of the shares shall entail, ipso jure and without any formality being necessary, the payment of interest at the legal rate, starting from the due date, without prejudice to the personal action that the Company may take against the defaulting shareholder and the enforcement measures provided by law.
Article 9. Reduction - amortisation of the share capital
The reduction of the share capital shall be authorised or decided by the Extraordinary General Meeting, which may delegate all of the powers to the Board of Directors for the execution of the same. In no case may it infringe the equal standing of shareholders.
The reduction of the share capital to an amount less than the legal minimum may only be decided under the condition precedent of a share capital increase intended to bring it to an amount at least equal to this minimum, unless the Company is transformed into a company of another form.
In the event of failure to comply with these provisions, any interested party may apply to a court for the dissolution of the Company.
At the same time, the court cannot pronounce the dissolution if the adjustment has taken place on the day on which it rules on the merits.
The share capital may be amortised in accordance with the law.
Article 10. Form of the shares
1. The fully paid up shares may take nominative or bearer form, at the choice of the shareholder, subject to the legal and regulatory provisions in effect.
The shares are recorded in the shareholders’ accounts under the conditions and pursuant to the procedures provided by law. The securities recorded in the account are transferred by transfer from account to account. Records in the accounts, payments and transfers are carried out in accordance with legal and regulatory requirements.
2. For the purposes of identifying the holders of bearer shares, the Company is entitled, according to legal and regulatory requirements, to ask at its own expense the central depository responsible for maintaining the securities issuance account (the Central Depositary), as per the case, for the name or company name, nationality, year of birth or year of incorporation and the addresses of the holders of securities conferring immediate or future voting rights at its meetings and the number of shares held by each of them, as well as, if applicable, the restrictions which may affect the securities.
With regard to the list provided to the Company by the Central Depositary, the Company has the right to request either from the Central Depository, or directly from the persons on this list and which the Company believes may be registered as an intermediary and on behalf of third party owners of securities, the information provided in the preceding paragraph regarding the owners of the securities.
These persons shall be required, if they have the capacity of intermediary, to disclose the identity of the owners of these securities. The information shall be provided directly to the authorised financial intermediary which holds the account, with the obligation of this latter party to notify it, as appropriate, to the Issuer or to the Central Depository.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The Company is also entitled, with regard to the securities in the nominative form, to ask, at any time, the intermediary registered on behalf of third party owners of the securities to disclose the identity of the owners of these securities.
For as long as the Company considers that certain holders of securities, in bearer or nominative form, whose identity has been disclosed to it are acting as holders on behalf of third party owners of the shares, it shall be entitled to ask these owners to reveal the identity of the owners of the securities, under the conditions provided above.
Following the requests for information cited above, the Company shall be entitled to request that any legal person owning shares of the Company representing more than 2% of its share capital or voting rights reveals the identity of persons holding directly or indirectly more than one third of the share capital of this legal person or of the voting rights which are exercised at the general meetings of the same person.
When the person forming the object of a request pursuant to the stipulations of this Article has not submitted the information so requested within the legal and regulatory deadlines or has transmitted incomplete or erroneous information regarding either its capacity or the owners of the securities, the shares or the securities giving immediate or future access to the share capital for which the person has been entered in the account shall be deprived of voting rights for all General Meetings to be held until the date of regularisation of identification, with the payment of dividends deferred until that date.
Article 11. Indivisibility of shares
Shares are indivisible with respect to the Company. The undivided joint owners of shares shall be represented at General Meetings by one of their number or by a joint representative of their choice. In the absence of agreement among them on the choice of a representative, the latter shall be designated by order of the President of the Commercial Court ruling in summary proceedings at the request of the first joint owner to take action.
The bare owner and the usufructuary have the right to participate in collective decisions. The voting right attached to the share belongs to the usufructuary for the Ordinary General Meetings and to the bare owner for the Extraordinary General Meetings. Shareholders may nevertheless agree among themselves on any other allocation for the exercise of the voting right at General Meetings. In this event, they shall bring their agreement to the attention of the Company by registered letter addressed to the registered office, with the Company obliged to observe this agreement for any General Meeting to be convened after the expiry of a one-month deadline after sending the registered letter, with the postmark serving as evidence of the date of dispatch.
The right of the shareholder to obtain notification of the company documents or to consult them may also be exercised by each of the joint owners of the undivided shares, by the usufructuary and the bare owner of shares.
Article 12. Transfer and Transmission of shares - Crossing of Threshold
The transfer of shares shall be made by transfer from account to account, pursuant to the law.
In the event of a share capital increase, the shares shall be negotiable as of its final conclusion.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Movements of securities for which due payments have not been made shall not be authorised.
In addition to the legal obligation to inform the Company of holdings of certain fractions of the share capital and to make any resulting declaration of intent, each natural or legal person, acting alone or in concert, who comes to hold or ceases to hold, directly or indirectly, a fraction equal to 2% of the share capital or voting rights, or any multiple of this percentage, shall be obliged to notify the Company of the same within four (4) stock exchange trading days, as soon as one of these thresholds is crossed, by registered letter with notice of receipt, addressed to the registered office of the Company, specifying the number of shares, corresponding voting rights and securities giving access to the share capital that it holds alone or in concert.
In order to determine the stipulated thresholds, account shall also be taken of the shares held indirectly and of shares regarded as owned shares, as defined by the provisions of Articles L. 233-7 et seq. of the French Commercial Code.
In each of the declarations cited above, the declaring party shall certify that the declaration made includes all shares held or possessed pursuant to the provisions of Articles L. 233-7 et seq. of the French Commercial Code. It shall also indicate the date or dates of acquisition.
This disclosure obligation applies in all cases of crossing thresholds stipulated above, including the thresholds prescribed by law.
Failure to observe the notification obligation cited above shall be sanctioned, at the demand (recorded in the minutes of the Meeting) of one or several shareholders who together hold a fraction of at least 2% of the share capital or voting rights of the Company, by suspension of voting rights attached to the shares which exceed the fraction that has not been regularly declared for each General Meeting of shareholders held until the date of regularisation of the notification.
Furthermore, in the event that the registered shareholder knowingly disregards the notification obligation for threshold crossing with regard to the Company, the Commercial Court within the jurisdiction of which the Company has its registered office may, at the request of the Company or of a shareholder, pronounce the complete or partial suspension of voting rights, for a total period not exceeding five years, against any shareholder who has not made the declarations cited above or who has not observed the content of the declaration of intent provided in Article L. 233-7 VII of the French Commercial Code within six (6) months of the publication of the said declaration.
Article 13. Rights and obligations attached to the shares
1. Each share gives the right to participate in collective decisions, as well as the right to be informed of the progress of the Company and to receive certain documents at times and under the conditions provided by law and these Articles of Association.
2. Shareholders shall only bear losses up to the limit of their contributions.
Subject to the provisions of the law and of these Articles of Association, no majority may impose an increase in their commitments. The rights and obligations attached to the share shall follow the security regardless of its holder.
3. The ownership of a share shall entail the ipso jure adhesion to the decisions of the General Meeting and to these Articles of Association.
The assignment shall include all dividends fallen due and falling due, as well as any portion of the reserve fund, unless otherwise notified to the Company.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The heirs, creditors, assignees or other representatives of a shareholder may not, under any pretext, require the sealing of the property and company documents, demand the division or the sale by auction of these assets or interfere in the administration of the Company. In order to exercise their rights, they shall refer to the company inventories and to the decisions of the General Meeting.
4. Whenever it is necessary to possess a certain number of shares in order to exercise any right, in the event of an exchange, consolidation or attribution of securities or for an increase or reduction in the share capital, a merger or any other transaction, shareholders holding a number of shares less than that required shall only be able to exercise these rights provided that they personally ensure that they obtain the required number of shares.
5. Each share confers a right of ownership of the Company’s assets, to profit-sharing and to the liquidation surplus, to a share proportional to the stake in the share capital which it represents, taking into account, where appropriate, amortised and unamortised, paid up and unpaid share capital, for the nominal amount of the shares and the rights of the different classes of shares.
6. Except in cases where the law provides otherwise and with the exception of the double voting right provided below, each shareholder shall have as many voting rights and express as many votes at Meetings as he has shares fully paid up for all of the due payments. For the same nominal value, each capital or participating share shall confer one vote.
7. A double voting right, considering the proportion of the share capital which they represent, shall be attributed to all fully paid up shares, which shall be documented by a registration in the nominative form for at least two years, starting from the registration of the Company in the form of a European company, in the name of the same shareholder. This right is also granted on issuance, in the event of a share capital increase through incorporation of reserves, profits or issue premiums, to the shares attributed as a bonus to a shareholder by virtue of former shares for which it has already benefited from this right.
TITLE III
ADMINISTRATION OF THE COMPANY
Article 14. Composition of the Board of Directors
The Board of Directors consists of at least three (3) members and at most eighteen (18) members, appointed by the Ordinary General Meeting of shareholders, subject to legal exemptions.
Subject to the stipulations of Articles 15 and 21 below, the members of the Board of Directors (including the Chair) who are natural persons must be aged less than eighty (80), it being specified, however, that the Board of Directors shall continuously comprise a minimum of 80% of members aged less than seventy-five (75).
A legal person may be appointed as member of the Board of Directors but must, under the conditions provided by the law, designate a natural person who shall be its permanent representative on the Board of Directors. The age limits set forth in respect of the members of the Board of Directors who are natural persons shall equally apply to such permanent representatives, subject to the stipulations of Article 15 below.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 15. Duration of duties – Renewal – Co-opting
The term of office of the members of the Board of Directors is set at three (3) years (with one year understood as the interval between two consecutive Ordinary General Meetings), subject to the following stipulations.
By way of exception, the General Meeting may, in order to implement a staggered renewal of Directors’ terms of office, appoint any Director for a term of less than three (3) years. The term of office of any such Director will expire at the close of the General Meeting called to approve the financial statements for the year ended and held in the year in which the Director’s term of office expires.
The term of office of any member of the Board of Directors shall be limited to the remaining period until the annual Ordinary General Meeting to be held in the year during which the member of the Board of Directors in question reaches the age limit applicable to him or her in accordance with the provisions of Article 14 of these Articles of Association.
A member of the Board of Directors put under guardianship shall be deemed to have resigned automatically. Such compulsory resignation shall not invalidate the discussions and decisions in which the member of the Board of Directors deemed to have resigned automatically took part.
The members of the Board of Directors shall be re-elected on one or several occasions, subject to the above stipulations concerning the age limit. They may be dismissed at any time by decision of the Ordinary General Meeting, under the conditions and pursuant to the procedures provided by law.
In the event of a vacancy, due to death or resignation, of one or several positions on the Board of Directors, the Board of Directors may make appointments in a provisional capacity between two General Meetings. These appointments shall be submitted for the ratification of the following Ordinary General Meeting. In the absence of ratification, the decisions taken and the acts previously carried out by the Board shall nevertheless remain valid.
When the number of members of the Board of Directors has fallen below the legal minimum, the Board of Directors shall call the Ordinary General Meeting within the shortest possible period, with a view to establishing a full board.
The member appointed as a replacement for another whose mandate has not expired, shall only remain in office during the remaining time of the mandate of his predecessor.
Furthermore, the Board of Directors may include elected members representing employees, pursuant to the provisions of Article L. 225-27-1 and, as appropriate, L. 225-23 and L. 22-10-5 of the French Commercial Code.
Article 16. Bureau and resolutions of the Board
1. The Board shall, among its members, appoint a Chair upon the terms set out in Article 20.
The Board may also appoint a Vice-Chair from among its members if it deems it appropriate. The Vice-Chair’s term of office shall be set by the Board and shall not exceed his/her term as Board member. The Vice-Chair, if he/she is independent, may be appointed as Lead Independent Member with the duties specified in the Board’s internal rules.
The Board may appoint a Secretary who may not be a shareholder. The Secretary, the Chair and the Vice-Chair (if any) make up the Board committee (“bureau du conseil")
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The Chair, the Lead Independent Member and the Vice-Chair (if any) shall be natural persons. They shall be appointed for the duration of their office as Directors and shall always be re-electable.
In the event of absence or impediment of the Chair, the session of the Board of Directors shall be chaired by the Vice-Chair (if any), or in the absence of the Vice-Chair, by a Director specifically appointed for this purpose by the Board members attending that meeting.
2. The Board of Directors shall meet as often as the interests of the Company require and at least once per quarter, at the request of the Chair, the Vice-Chair (if any), or the Lead Independent Member, made by any written means, including by email or even verbally.
However, Directors representing at least one third of the total number of Directors may request the Chair to call a Board meeting, if there has been no Board meeting for more than two (2) months, provided that they should specify the meeting agenda in such a request. The Directeur Général, if he is not the Chair, may also request the Chair to call a Board meeting, based on a specified agenda. Beyond these cases, and unless the meeting is called by the Vice-Chair, the agenda shall be set by the Chair and may be set only at the time of the meeting.
Board of Directors meetings may also be held (i) by videoconference or any other electronic means of telecommunication, or (ii) by written decision on the conditions and within the limits provided for by law.
In-person meetings shall take place at the registered office or at any other location indicated in the convening notice.
For decisions to be valid, at least half of the Directors must be present or represented. Decisions shall be taken by a majority of votes of present or represented members; in the event of a tie vote, the chair of the session shall have the deciding vote.
In addition, the Board of Directors shall set internal rules which may provide that Directors who take part in Board meetings by videoconference or telecommunication means enabling them to be identified and guaranteeing their effective participation, the nature and conditions of application of which are determined by the legislative and regulatory provisions in force, are deemed to be present for the purposes of calculating the quorum and majority. However, for as long as prohibited by law, the use of videoconferencing or other means of telecommunication will not be applicable for the approval of the annual financial statements and, where applicable, the consolidated financial statements, as well as for the approval of the management report (including, where applicable, the Group management report).
The members of the Board of Directors may be represented at each session by another Director, but a Director may represent only one other. These powers shall only be valid for a single session and must be granted in writing (including, for example, a simple letter or e-mail).
An attendance register shall be kept at the registered office, which shall be signed by the members of the Board of Directors who take part in the board meeting. The attendance register may be kept in electronic format, in accordance with applicable laws and regulations.
The production of an extract or copy of the minutes shall serve as sufficient evidence for the number of members in office and their attendance or representation.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The decisions of the Board shall be noted in the minutes drawn up in a special register or on numbered and initialled loose sheets, possibly in electronic format, pursuant to the conditions set by the current legislation.
These minutes shall be signed by the chair of the session and by another director who effectively attended the relevant meeting(s), possibly in electronic format, in accordance with applicable laws and regulations.
In the event of impediment of the chair of the session, the minutes shall be signed by at least two Directors, who effectively attended the relevant meeting(s).
Copies or excerpts of these minutes shall be validly certified by the Chair or Vice-Chair (if any), the Directeur Général, a Directeur Général Délégué (if any) or a Director temporarily acting as meeting Chair, or by a proxy authorised for this purpose, possibly in electronic format, in accordance with applicable laws and regulations.
The members of the Board of Directors, as well as any person taking part in the meetings of the Board of Directors, shall be bound by a confidentiality obligation with regard to the resolutions of the Board of Directors, as well as to the information of a confidential nature or presented as such by the Chair of the Board of Directors or the Directeur Général.
The Statutory Auditors shall be convened to all of the meetings of the Board of Directors which examine or draw up the annual or interim financial statements.
Article 17. Powers and attributions of the Board of Directors
The Board of Directors determines the direction of the Company’s business activities and oversees their implementation in accordance with its corporate interests, taking into account the social, environmental, cultural and sporting challenges of its activity. Subject to the powers expressly granted to shareholders’ meetings, and within the limits of the corporate purpose, the Board deals with all matters concerning the proper operation of the Company and settles all matters concerning the Company through its deliberations.
In dealings with third parties, the Company shall even be committed by the actions of the Board of Directors which do not relate to the Company object, unless it demonstrates that the third party was aware that this action exceeded this object or could not have been unaware of the same in view of the circumstances, mere publication of the Articles not being sufficient to constitute such proof.
The Company shall carry out the verifications and inspections which it considers appropriate at any time of the year and may order the forwarding of documents which it considers necessary for carrying out its mission.
Without prejudice to the foregoing and to the powers vested in it by law, the Board of Directors, acting by a majority of its members present or represented, and in accordance with the legal and regulatory provisions in force, authorizes the following agreements and transactions prior to their conclusion:
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
i. approval of the annual budget;
ii. approval of the business plan;
iii. approval of any significant change in the Company’s activities;
iv. approval of material changes in accounting policies;
v. any share capital reductions and share buy back programs;
vi. acquisition and disposal of business branches, equity interests or assets for an amount exceeding 7 million Euros as well as any lease management (location-gérance) of all or part of the fonds de commerce, except for the transactions previously approved as part of the annual budget or business plan;
vii. creation, sale, dissolution, or liquidation of a subsidiary or joint venture;
viii. acquisition, assignment, or licensing of product rights (including all intellectual property rights, but excluding commercial distribution rights) in excess of 7 million Euros;
ix. any capital expenditure for an amount exceeding 7 million Euros not previously approved as part of the annual budget;
x. any operation or contract involving an operating expense for an amount exceeding 7 million Euros not previously approved as part of the annual budget;
xi. any implementation, refinancing or amendment to the terms of any borrowings (including any bonds) for an amount exceeding 7 million Euros, and not previously approved as part of the annual budget;
xii. any merger, demerger, asset contribution, dissolution, liquidation, or other restructurings operation in which the assets or liabilities involved represent a value in excess of 7 million Euros;
xiii. any settlement or compromise relating to any litigation of an amount exceeding 7 million Euros;
xiv. any decision to initiate litigation against a third party in which the Company’s claim(s) would represent an amount exceeding 7 million Euros;
xv. any decision to delist all or part of the Company’s shares from one of the markets on which they are admitted to trading, or to admit them to trading on a new market; and
xvi. any agreement or undertaking to do any of the foregoing.
Any decision to transfer out of France the registered office and/or the research & development centre(s) operated by the Company in France shall be subject, as from the date hereof, to the prior authorisation of the Board of Directors resolving unanimously.
Each Director receives all the information necessary for the performance of their duties, and may obtain from the Chair or Directeur Général all the documents necessary for the performance of their duties.
Members of the Board of Directors must not divulge, even after they no longer hold office, any information in their possession concerning the Company, the disclosure of which could prejudice the Company’s interests, with the exception of cases where such disclosure is required or permitted by the legal or regulatory provisions in force or in the public interest.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The Board of Directors may grant all of the special mandates or specific missions to one or several of its members, or to third parties, whether shareholders or not, for one or several given objects.
The Board of Directors may also appoint, from among its members, one or several specialised committees, the composition and attributions of which it shall set and which shall carry out their activities at its liability, without the said attributions having the object of delegating to the committees the powers exclusively attributed to the Board of Directors by the law or these Articles of Association, or the effect of reducing limiting the powers of the Board of Directors.
Article 18. Remuneration of the Board of Directors
The members of the Board of Directors may receive by way of remuneration of their activity a fixed annual amount, the amount of which, determined by the Ordinary General Meeting of shareholders, shall be maintained until a decision to the contrary and shall be charged to the general expenses of the Company.
The Board shall share these benefits among its members in a manner which it considers appropriate.
The Board of Directors may also allocate exceptional remuneration to certain of its members for missions or mandates entrusted to them in the cases and under the conditions provided by law.
Article 19. Observers
The Board of Directors may appoint one or several observers who take part in meetings of the Board of Directors.
The observer or observers are called to attend the meetings of the Board of Directors in their observational capacity, without voting rights. The observer or observers must receive the same information as the members of the Board of Directors.
The observers may be consulted by members of the Board of Directors, as necessary, on all questions within their competences and for which they can deliver an opinion or an advice.
Observers may not be remunerated and, like Directors, are subject to the obligations set out in the Board of Directors’ internal rules, including, in particular, the confidentiality obligations set out in the internal rules and these Articles of Association.
Article 20. Chairmanship of the Board of Directors
The Board of Directors elects a Chair from among its members, who must be a natural person. The Board determines the term of office, which may not exceed the Director’s term of office, and may dismiss the director at any time. The Board sets any remuneration.
The Chair of the Board of Directors organises and directs the Board’s work, and reports to the General Meeting. The Chair ensures that the Company’s governing bodies operate smoothly, and in particular that the Directors can fulfil their duties.
Article 21. General Management
1. The general management of the Company is the responsibility either of the Chair of the Board of Directors, or of another natural person, who may or may not be a Director of the Company, appointed by the Board of Directors and titled Directeur Général. When the Chair of the Board of Directors assumes responsibility for the Company’s general management, the provisions applicable to the Directeur Général apply.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The Directeur Général represents the Company in dealings with third parties. They are vested with the broadest powers to act on the Company’s behalf in all circumstances. They exercise their powers within the limits of the corporate purpose and subject to those powers expressly assigned by law to shareholders’ meetings and the Board of Directors. The Company shall even be committed by the actions of the Directeur Général which do not relate to the Company’s provided object or attributions, unless the Company demonstrates that the third party was aware that this action exceeded these limits or could not have been unaware of the same in view of the circumstances, mere publication of the Articles not being sufficient to constitute such proof.
The Directeur Général may not be older than 70. The Directeur Général is deemed to have resigned automatically on turning 70. Nonetheless, their term would extend until the next Board meeting, where the new Directeur Général is appointed.
The term of office of the Directeur Général is set by decision of the Board of Directors without exceeding their term as Director if the Directeur Général is also a director.
The Board of Directors may dismiss the Directeur Général at any time.
2. On simple deliberation by a majority of the votes of the Directors present or represented, the Board of Directors chooses between the two methods of exercising general management referred to in the first paragraph of section 21.1.
Shareholders and third parties are informed of this choice in accordance with legal and regulatory requirements.
The Board’s choice remains in force until a contrary decision by the Board or, at the Board’s discretion, for the duration of the Directeur Général’s term of office.
3. On the recommendation of the Directeur Général, the Board of Directors may appoint one or more individuals to assist the Directeur Général as Directeur Général Délégué.
In agreement with the Directeur Général, the Board of Directors determines the scope and duration of the powers granted to the Directeurs Généraux Délégués. The Board of Directors sets their remuneration. Their term of office is set in the Board decision that appoints them and may not exceed their term as Director (if applicable), subject to the provisions of Article L. 225-55 of the French Commercial Code.
With respect to third parties, the Directeurs Généraux Délégués have the same powers as the Directeur Général; in particular, the Directeurs Généraux Délégués have the power to commence legal proceedings.
The number of Directeurs Généraux Délégués may not exceed five (5).
The Directeurs Généraux Délégués may be dismissed at any time by the Board of Directors, on recommendation of the Directeur Général.
A Directeur Général may not be older than 70. A Directeur Général Délégué is deemed to have resigned automatically if they turn 70 while in office.
If the Directeur Général ceases to hold office or is prevented from carrying out their duties, the Directeurs Généraux Délégués shall retain their functions and powers until a new Directeur Général is appointed, unless the Board of Directors decides otherwise.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 22. Agreements between the Company, its Directeur Général, one of its Directeurs Généraux Délégués, one of its Directors or a shareholder
All agreements entered into directly, or through an intermediary, between the Company and its Directeur Général, one of its Directeurs Généraux Délégués, one of its Director or one of its shareholders holding more than 10% of the voting rights or in the case of an entity shareholder, its controlling company within the meaning of Article L. 233-3 of the French Commercial Code, shall be subject to the prior authorisation of the Board of Directors.
The same applies to agreements in which one of the persons mentioned in the preceding paragraph has an indirect interest as well as agreements which take place between the Company and an entity, if the Directeur Général, one of the Directeurs Généraux Délégues or one of the Directors of the Company is the owner, general partner having unlimited liability, manager, Director, member of the supervisory board or, generally, an executive officer of such entity.
The prior authorisation of the Board of Directors is motivated by giving reasons indicating the interest of the agreement for the Company, in particular, by specifying the financial conditions attached to it.
The party directly or indirectly interested shall inform the Board of Directors as soon as he or she is aware of an agreement subject to authorisation. If this party serves on the Board of Directors, they shall not have the right to take part in the discussions and the vote on the requested authorisation.
The Chair of the Board of Directors shall inform the Statutory Auditors of all authorised agreements entered into and shall submit them for approval to the General Meeting. The Statutory Auditors submit a report on these agreements to the meeting of shareholders which must vote on this report. The party directly or indirectly interested in the agreement shall not have the right to take part in the vote and its shares shall not be taken into account for the calculation of the majority.
The agreements approved by the shareholders’ Meeting, together with those not approved, shall be effective with respect to third parties except when declared null and void in cases of fraud. However, and even in the absence of fraud, any prejudicial consequences for the Company of agreements that have not been approved may be borne by the interested party.
Regardless of the liability of the interested party, all agreements for which the prior authorisation by the Board of Directors is required, which are concluded without such prior authorisation by the Board of Directors may be declared null and void if the consequences thereof were prejudicial to the Company. An action to render the agreement null and void shall be time barred after three years as of the date of the agreement. However, if such agreement has been hidden, this period shall be calculated as of the date on which its existence was revealed. The nullity can be remedied by a vote by the General Meeting held on a special report by the Statutory Auditors’ stating the circumstances under which the authorisation procedure was not followed. In such case, the interested party may not take part in the vote and his or her shares shall not be taken into account for the calculations of quorum and majority.
The foregoing provisions do not apply to agreements concerning current operations and entered under normal conditions or agreements entered into between two companies, one of which holds, directly or indirectly, all of the share capital of the other, if applicable, less the minimum number of shares required to satisfy the requirements of article 1832 of the French Civil Code, or articles L. 225-1 and L. 22-10-2 of the French Commercial Code.
The Board of Directors must set up a procedure to periodically assess whether agreements relating to current operations and entered into on customary terms meet these criteria. The persons directly or indirectly interested in one of these agreements shall not take part in this assessment.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 23. Statutory auditors
One or several Statutory Auditors shall be appointed and shall carry out their monitoring mission pursuant to the law.
They shall have the permanent mission, to the exclusion of any interference in the management, of verifying the books and values of the Company and of monitoring the regularity and fairness of the Company accounts.
TITLE IV
SHAREHOLDERS’ MEETINGS
Article 24. Nature of the Meetings
The decisions of the shareholders shall be taken at a General Meeting.
The Ordinary General Meetings shall be those which are convened on to take all of the decisions which do not modify the Articles of Association.
The Extraordinary General Meetings shall be those convened on to decide or authorise direct or indirect modifications of the Articles of Association.
The Special Meetings shall bring together the holders of shares of a given category to rule on a modification of the rights of the shares of this category and all other decisions provided by law or by these Articles of Association.
The resolutions of the General Meetings shall oblige all of the shareholders, even if absent, dissenting or incapable.
Article 25. Calling and convening of the General Meetings
The General Meetings shall be convened either by the Board of Directors or failing this, by the Statutory Auditors or by a representative designated by the court, at the demand, either of any interested party or the Social and Economic Committee in the event of an emergency or by several shareholders representing at least 5% of the share capital.
During the liquidation period, the Meetings shall be convened by the liquidator(s).
The General Meetings shall be convened at the registered office or at any other location indicated in the notice of calling.
The Company shall be obliged, within the time limits set out in applicable laws, to publish a notice of meeting in the Bulletin des Annonces Légales Obligatoires (BALO) (Bulletin of Obligatory Legal Announcements) containing the mentions provided by the laws in effect.
The convening of the General Meetings shall be realised by the inclusion in a newspaper authorised to receive legal announcements in the Department of the registered office and in addition, in the Bulletin des Annonces Légales Obligatoires (BALO), within the time limits set out in applicable laws.
When a Meeting has been unable to deliberate in regular fashion, due to failure to reach the necessary quorum, the second Meeting and as per the case, the second extended Meeting, shall be convened, in the same forms as the first, within the time limits set out in applicable laws and the notice of calling shall recall the date of the first calling and reproduce its agenda.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 26. Agenda
1. The agenda of the Meetings shall be drawn up by the author of the calling.
2. One or several shareholders, representing at least the required proportion of the share capital and acting under the conditions and pursuant to the deadlines set by the law, shall be entitled to request the inclusion of draft resolutions in the agenda of the Meeting by registered letter with a request for notice of receipt.
3. If a Social and Economic Committee exists, it may request the entering of draft resolutions on the agenda of a Meeting.
These draft resolutions must be notified to the shareholders and be entered in the agenda and submitted to the vote of the Meeting.
4. The Meeting may not deliberate on an issue which is not entered on the agenda, which may not be modified at a second calling. It may nevertheless dismiss one or several members of the Board of Directors under any circumstances and replace them.
Article 27. Admissions to Meetings - Powers
All of the shareholders shall be entitled to take part in the Meetings on providing proof of their identity, though subject to compliance with the following provisions:
- for holders of registered shares, their registration in the registered share account maintained by the Company before the second business day preceding the Meeting date;
- for holders of ordinary bearer shares, issuance of a certificate of participation (attestation de participation) by an authorised intermediary confirming they are registered in a securities account before the second business day preceding the Meeting date.
Any shareholder may vote by post through a form, the details of which are set forth by a decree of the Conseil d’État, and a copy of which may further be obtained under the conditions indicated by the notice of calling of the Meeting.
A shareholder may also vote by proxy, in accordance with the provisions of Articles L. 225-106 and L. 22-10-39 of the French Commercial Code, and thus be represented either by another shareholder who provides evidence of a power of attorney, by his/her spouse or partner with whom he/she has concluded a civil solidarity pact, or by any other natural or legal person of his/her choice (and this under the conditions provided in Articles L. 22-10-40, R. 225-79 and R. 22-10-24 of the French Commercial Code).
In the event of existence of a Social and Economic Committee within the Company, two of its members designated by the counsel, of which one belongs to the category of technical staff and supervisors and the other to the category of employees and workers, or where appropriate, the persons mentioned in Articles L. 2312-74 and L. 2312-75 of the Labour Code, may attend the General Meetings. They shall be heard at their request for all of the resolutions which require the unanimity of shareholders.
Shareholders may, upon decision of the Board of Directors, take part in the General Meetings by videoconference or by any other means of telecommunication, including the Internet, which allow their identification in accordance with the conditions and procedures set forth by the applicable regulations in force. Where applicable, this decision shall be communicated in the convening notice of the General Meeting.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Upon decision of the Board of Directors, the shareholders may access and use the proxy form or voting form in electronic format, under the conditions and in accordance with the conditions and procedures set forth by the applicable regulations in force.
Article 28. Holding of the Meeting - Bureau - Minutes
An attendance sheet shall be signed by the attending shareholders and representatives, to which shall be attached the powers granted to each representative and, as appropriate, the postal voting forms. It shall be certified as accurate by the bureau of the Meeting.
The Meetings shall be chaired by the Chair of the Board of Directors or, in his absence, by the Vice-Chair (if any) or by a member of the Board especially appointed for this purpose. Failing this, the Meeting shall itself elect its chair. In the event of convening by a Statutory Auditor or court-appointed agent, the Meeting shall be chaired by the author of the convening notice.
The two present and accepting shareholders, representing the largest number of votes, both as themselves and as representatives, shall serve as scrutineers. The bureau so established shall designate a secretary, who may be selected from outside the members of the Meeting.
The deliberations of the meetings shall be recorded in minutes signed by the members of the bureau and drawn up in a special register, possibly in electronic format, in accordance with the applicable laws and regulations. Copies and extracts of these minutes shall be certified under the conditions set by applicable laws and regulations, possibly in electronic format.
Article 29. Quorum - Vote
1. The quorum shall be calculated on all of the shares comprising the share capital, except in the Special Meetings, where it shall be calculated on all of the shares for the category in question, all of which minus the shares deprived of the voting rights by virtue of the provisions of the law. In the event of a postal vote or a proxy form sent by mail or, as the case may be, by email, only those forms duly completed and received by the Company at least three (3) days before the date of the Meeting, i.e. no later than the fourth day before the date of the Meeting, shall be considered for the calculation of the quorum. However, in the event of a postal or proxy vote via Internet pursuant to Article R. 225-61 of the French Commercial Code, only those electronic forms received by the Company no later than 3 p.m., Paris time, on the day immediately preceding the Meeting, shall be taken into account for quorum purposes.
2. Subject to the double voting right cited in Article 13 of these Articles of Association, the voting rights attached to shares shall be proportional to the stake in the share capital which they represent.
3. The vote shall be expressed by a show of hands, by a roll-call or by a secret ballot, pursuant to what the bureau of the Meeting or the shareholders decide. The shareholders may also vote by post, or by proxy under the conditions of Article 27 of these Articles of association, including, upon decision of the Board of Directors, by videoconference or by any other means of telecommunication, including the Internet, which allow their identification in accordance with the conditions and procedures set forth by the applicable regulations in force.
4. For the purposes of calculating the quorum and majority, shareholders shall be considered to be present who take part in the Meeting via videoconference or telecommunications media, including the Internet, which permit their identification and guarantee their effective participation, the nature and conditions of application of which are determined by legislative and regulatory provisions in effect.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 30. Ordinary General Meeting
The Ordinary General Meeting shall take all of the decisions exceeding the powers of the Board of Directors, which do not have the object of modifying the Articles of Association.
The Ordinary General Meeting shall meet at least once a year, within six months of the end of the financial year, to rule on the financial statements for the financial year, subject to the extension of the deadline by a court decision.
It shall only deliberate validly, on a first convening, if the present and represented shareholders, or those voting by postal vote, hold at least the number of shares set out in applicable laws. No quorum shall be required for the second convening.
It shall rule with a majority of the votes validly cast by the present or represented shareholders or shareholders voting by post. Abstention and votes blank or void shall not be considered as votes cast.
For the purposes of calculating the quorum and majority, shareholders shall be considered to be present who take part in the General Meetings via videoconference or any telecommunications media as detailed in the Article 29, 4th paragraph, of these Articles of Association.
Article 31. Extraordinary General Meeting
The Extraordinary General Meeting may amend the Articles of Association in all of their provisions and notably decide on the conversion of the Company into a limited liability company. It may nevertheless increase the commitments of the shareholders, subject to the operations resulting from a consolidation of shares effected in regular fashion.
The Extraordinary General Meeting may only deliberate validly if the present or represented shareholders or shareholders voting by postal vote possess on the first convening or on the second convening the number of shares set out by applicable laws. In the absence of this latter quorum, the second Meeting may be extended until a date two months later than the one on which it had been convened.
The Extraordinary General Meeting shall rule with a majority of two thirds of the votes validly cast by the present or represented shareholders, or voting by postal vote, unless there is a legal exemption. Abstention and votes blank or void shall not be considered as votes cast.
In constituent Extraordinary General Meetings, i.e. those convened to deliberate on the approval of a contribution in kind or the granting of a particular benefit, the grantor or beneficiary shall not have a vote, either for itself or as a representative.
For the purposes of calculating the quorum and majority, shareholders shall be regarded as present who take part in the General Meetings via videoconference or any telecommunications media as detailed in the Article 29, 4th paragraph, of these Articles of Association.
Article 32. Special Meetings
If there are several categories of share, no modification may be made to the rights of the shares in one of these categories, without a requisite vote of an Extraordinary General Meeting, open to all of the shareholders and furthermore, without an equally requisite vote of a Special Meeting, open only to the owners of shares of the category in question.
The special Meetings may only deliberate validly if the present or represented shareholders hold on the first convening or on the second convening the number of shares of the relevant category set out by applicable laws.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Other meetings shall be convened and shall deliberate under the same conditions as the Extraordinary General Meetings, subject to the particular provisions applicable to Meetings of holders of shares with a priority dividend, but without voting rights.
For the purposes of calculating the quorum and majority, shareholders shall be regarded as present who take part in the Meeting via videoconference or telecommunications media as detailed in the Article 29, 4th paragraph, of these Articles of Association.
Article 33. Right of notification of the shareholders
Every shareholder has the right to receive, under the conditions and at times set by law, the documents required for it to be able to pronounce knowledgeably and draw up a ruling on the management and control of the Company.
The nature of these documents and the conditions of their dispatch or provision shall be determined by the law and regulations.
TITLE V
COMPANY ACCOUNTS -
ALLOCATION AND DISTRIBUTION OF PROFITS
Article 34. Inventory - Annual Financial Statements
The Company shall maintain regular accounts of its operations, pursuant to the law and commercial practice.
At the end of each financial year, the Board of Directors shall draw up an inventory of the various elements of the assets and liabilities. It shall also draw up the annual reports and as appropriate, the consolidated financial statements, pursuant to the provisions of the French Commercial Code.
It shall attach a statement of guarantee deposits, endorsements and guarantees given by the Company to the balance sheet, together with a statement of sureties granted by it.
It shall draw up a management report containing the indications set by law.
The management report shall include, as per the case, the report on the management of the group, when the Company must draw up and publish consolidated accounts under the conditions provided by law.
As appropriate, the Board of Directors shall draw up provisional accounting documents under the conditions provided by law.
All of these documents shall be made available to the Statutory Auditors under the appropriate legal and regulatory conditions.
Article 35. Allocation and distribution of profits
First of all, amounts to be provisioned in legal reserves shall be deducted from the net profit for each financial year minus previous losses, if any. In this way, 5% shall be deducted to establish the legal reserve fund; this deduction shall cease to be obligatory when the said fund has reached one tenth of the share capital; it shall resume if, for any reason, the legal reserve has fallen below this fraction.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
The distributable profits shall then consist of the net profit for the financial year minus previous losses and the amounts provisioned to reserves by way of application of the law and the Articles of Association plus retained earnings.
For this profit, the General Meeting shall then deduct the amounts which it considers appropriate to allocate to optional, ordinary or extraordinary reserves or as retained earnings.
The balance, if any, may be allocated among all of the shares in proportion to their paid-up and unamortised amount and their respective pecuniary rights.
At the same time, except in the case of a capital reduction, no distribution may be made to the shareholders when the shareholders’ equity is or becomes, following this distribution, less than the amount of the share capital plus the reserves for which distribution is prohibited, pursuant to the law or the Articles of Association.
The General Meeting may decide to distribute the amounts deducted from the optional reserves, either to provide or supplement a dividend, or by way of an exceptional distribution; in this event, the decisions shall expressly indicate the reserve items from which the deductions shall be made. At the same time, the dividends shall be distributed as a priority from the distributable profit for the financial year.
The losses, if any, shall be attributed, after the approval of the financial statements by the General Meeting, to a special account, for attribution to profits for future financial years, until they are extinguished.
Article 36. Payment of dividends
Ruling on the annual financial statements, the General Meeting has the right to grant an option to each shareholder for all or part of the distributed dividend or interim dividends, for payment of the dividend or interim dividends in cash or in shares.
The procedures for payment of dividends in cash shall be set by the General Meeting or failing this, by the Board of Directors.
However, the payment of dividends must take place within at most nine months of the end of the financial year, unless this deadline is extended by a judicial authorisation.
When financial statements drawn up during or at the end of the financial year and certified by a Statutory Auditor reveal that the Company has generated a profit, after the end of the preceding financial year, after establishing the necessary depreciation and provisions and deducting previous losses, if any, as well as amounts to be attributed to reserves by way of application of the law or Articles of Association and taking account of retained earnings, interim dividends may be distributed before approval of the annual financial statements. The amount of these interim dividend payments may not exceed the amount of the profit so defined.
The Company may only demand a repeat of the dividend from the shareholders if the distribution has been carried out in violation of the legal provisions and if the Company establishes that the beneficiaries were aware of the regular character of this distribution when it was made or could not have been unaware of the same in view of the circumstances. Actions for the return of undue payments shall be prescribed five years after the payment of these dividends. Dividends unclaimed within five years of their payment falling due shall be prescribed.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
TITLE VI
SHAREHOLDERS’ EQUITY - PURCHASE BY THE COMPANY
CONVERSION - EXTENSION - DISSOLUTION - LIQUIDATION
Article 37. Shareholders’ equity less than half of the share capital
If, as a result of losses recorded in the accounting records, the Company's shareholder equity falls below half of the share capital, the Board of Directors is required, within four months of approval of the financial statements showing the loss, to convene an Extraordinary General Meeting to decide whether to dissolve the Company early.
If the Company is not dissolved, it is required, no later than the end of the second fiscal year following the year in which the losses were recognized, to restore its shareholders' equity to a value at least equal to half of the share capital or, subject to Article L. 224-2 of the French Commercial Code, to reduce its share capital by the amount necessary to bring the value of its shareholders' equity to at least half of its amount.
In both cases, the resolution adopted by the General Meeting is published in accordance with regulatory requirements.
If, before the deadline referred to in the second paragraph of this article, shareholders' equity has not been reconstituted to a value at least equal to half of the share capital, even though the Company's share capital exceeds a threshold set by decree by the Conseil d’État in relation to the size of its balance sheet, the Company is required, no later than the end of the second fiscal year following this deadline, to reduce its share capital, subject to article L. 224-2 of the French Commercial Code, to a value less than or equal to this threshold.
When, in application of the fourth paragraph of this article, the Company has reduced its share capital without reconstituting its shareholders' equity, and subsequently carries out a capital increase, it must comply with the provisions of the same fourth paragraph before the end of the second fiscal year following the year in which the increase took place.
In the event of failure to convene a General Meeting, or if the Meeting is unable to deliberate validly on final notice, any interested party may apply to the courts for the Company to be wound up. The same applies if the provisions of the fourth paragraph have not been applied. In all cases, the court may grant the Company a maximum period of six months to regularize the situation. It may not order the dissolution of the Company if, on the day it rules on the merits of the case, the situation has been regularized.
The provisions of this article do not apply to companies in safeguard or receivership proceedings or benefiting from a safeguard or receivership plan.
Article 38. Conversion
Pursuant to Article L. 229-10 of the French Commercial Code, the Company may be transformed into a limited liability Company, if, at the time of conversion, it has been in existence for at least two years and if it has drawn up financial statements for the last two financial years and these have been approved by its shareholders.
The conversion decision shall be taken on the basis of a report by one or several conversion auditors designated by a decision of the court, which attests that the shareholders’ equity is at least equal to the share capital.
This document is a free translation. In case of discrepancy between the French and the English version, the French version shall prevail.
Article 39. Extension
At least one year before the expiry date of the Company, the Board of Directors must convene the Extraordinary General Meeting of shareholders for the purpose of deciding, under the conditions required for the amendment of the articles of Association, whether the Company must be extended.
The shareholders who oppose the said extension shall be obliged to assign their shares to the other shareholders within three (3) months, starting from the resolution of the General Meeting which has decided on the extension, at the express demand of these latter parties by registered letter with notice of receipt. The assignment price of the shares shall be determined by an expert under the conditions provided in Article 1843-4 of the Civil Code. In the event that the purchase requests exceed the number of shares to be assigned, the allocation shall be made pro rata to the number of shares already held by the acquirers and within the limits of the shares to be assigned.
Article 40. Dissolution - Liquidation
Except in the cases of judicial dissolution provided by the law, and unless the Company is extended in regular fashion, it shall be dissolved on expiry of a deadline set by the Articles of Association or following a decision of an Extraordinary General Meeting of the shareholders.
One or several liquidators shall then be appointed by this Extraordinary General Meeting under the conditions of a quorum and majority provided for the Ordinary General Meetings.
The liquidator shall represent the Company. The entire company assets shall be realized, and the liabilities discharged, by the liquidator, who shall be vested with the broadest powers. He shall then allocate the available balance between the shares, pro rata to their participation in the share capital.
The General Meeting of shareholders may authorise it to continue with current business transactions or to undertake new ones for the purposes of the liquidation.
In the event that all of the shares are acquired by a single shareholder, any dissolution decision, whether voluntary or judicial, shall entail the transmission of the Company’s assets, to the sole shareholder, under the conditions provided by law, without a liquidation being necessary.
TITLE VII
DISPUTES
Article 41. Disputes
Any disputes which may arise regarding the business of the company or the execution of the provisions of the Articles of Association, during the life of the Company or during its liquidation, whether between the shareholders, the management or controlling bodies of the Company or the Statutory Auditors, or between the shareholders themselves, shall be submitted to the competent courts with jurisdiction over the registered office.
* *
*
EX-2.3
3
exhibit23-descriptionofsec.htm
EX-2.3
Document
Exhibit 2.3
DESCRIPTION OF SECURITIES REGISTERED PURSUANT TO
SECTION 12 OF THE SECURITIES EXCHANGE ACT OF 1934
The following description of the ordinary shares, the American Depositary Shares and the articles of association, or bylaws, of Valneva SE (“Valneva,” the “Company,” “us” or “we”) is a summary and does not purport to be complete. This summary is subject to, and qualified in its entirety by reference to, the complete text of the Company’s bylaws, which are incorporated by reference as Exhibit 3.1 of the Company’s Annual Report on Form 20-F to which this description is also an exhibit. The Company encourages you to read the Company’s bylaws carefully.
As of December 31, 2024, Valneva had the following series of securities registered pursuant to Section 12(b) of the Securities Exchange Act of 1934, as amended, or the Exchange Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of Each Class |
Trading Symbol |
Name of Each Exchange on Which
Registered |
| Ordinary Shares, nominal value €0.15 per share* |
* |
The Nasdaq Global Select Market* |
| American Depositary Shares, each representing two ordinary shares, nominal value €0.15 per share |
VALN |
The Nasdaq Global Select Market |
* Not for trading, but only in connection with the registration of the American Depositary Shares.
Reconciliation of the Ordinary Shares Outstanding
The following table shows the reconciliation of the number of ordinary shares issued and outstanding as of December 31, 2023 and 2024:
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
Ordinary
Shares |
Ordinary Shares issued at December 31, 2023 |
|
138,912,142 |
Number of ordinary shares issued in connection with the definitive acquisition of free shares |
|
609,382 |
Number of ordinary shares issued in connection with private placement |
|
23,000,000 |
Ordinary Shares issued at December 31, 2024 |
|
162,521,524 |
History of Securities Issuances
From January 1, 2022 through December 31, 2024, the following events have changed the number of our issued and outstanding ordinary shares:
•On January 11, 2022, we issued 772,070 ordinary shares, in connection with the conversion of Free Convertible Preferred Shares on January 3 and 4, 2022, carried out by a total cash contribution of €111,558.30 as nominal value.
•On January 26, 2022, we issued 1,179,516 ordinary shares, in connection with the exercise of equity warrants on January 21, 2022, and stock options between January 4 and 11, 2022, carried out by a total cash contribution of €3,917,031.12 (including €176,927.40 as nominal value).
•On February 25, 2022, we issued 3,125 ordinary shares, in connection with the exercise of equity warrants on February 4, 2022, carried out by a total contribution of €8,043.75 (including €468.75 as nominal value).
•On March 25, 2022, we issued 636,648 ordinary shares, in connection with the end of the vesting period of free Ordinary Shares, carried out through incorporation of an issue premium of €95,497.20.
•On June 19, 2022, we issued 9,549,761 ordinary shares, in connection with a reserved capital increase whose total cash contributions amounted to €90,627,231.89 (including €1,432,464.15 as nominal value).
•On September 29, 2022, we issued 21,000,000 ordinary shares, in connection with a global offering whose total cash contributions amounted to €102,900,000 (including €3,150,000 as nominal value).
•On October 31, 2022, we issued 3,125 ordinary shares, in connection with the exercise of equity warrants on October 21, 2022, carried out by a total contribution of €8,043.75 (including €468.75 as nominal value).
•On December 1st, 2022, we issued 6,250 ordinary shares, in connection with the exercise of equity warrants on November 30, 2022, carried out by a total contribution of €16,087.50 (including €937.50 as nominal value).
•On December 23, 2022, we issued 6,250 ordinary shares, in connection with the exercise of equity warrants on December 8 & 10, 2022, carried out by a total contribution of €16,087.50 (including €937.50 as nominal value).
•On January 4, 2023, we cancelled 20,514 preferred shares convertible into ordinary shares, carried out by a total reduction of €3,077.10 as nominal value.
•On May 10, 2023, we issued 549,632 ordinary shares, in connection with the end of the vesting period of free Ordinary Shares, carried out through incorporation of an issue premium of €82,444.80.
•On August 7, 2023, we issued 15,542 ordinary shares, in connection with the exercise of stock-options between July 24 & 28, 2023, to employees or corporate officers, carried out by a total contribution of €45,367.10 (including €2,331.30 as nominal value).
•On March 18, 2024, we issued 364,759 ordinary shares, in connection with the end of the vesting period of free Ordinary Shares, carried out through incorporation of an issue premium of €54,713.85.
•On September 17, 2024, we issued 23,000,000 ordinary shares, in connection with a private placement whose total cash contributions amounted to €61,180,000 (including €3,450,000 as nominal value).
•On October 10, 2024, we issued 217,102 ordinary shares, in connection with the end of the vesting period of free Ordinary Shares, carried out through incorporation of an issue premium of €32,565.30.
•On December 6, 2024, we issued 27,521 ordinary shares, in connection with the end of the vesting period of free Ordinary Shares, carried out through incorporation of an issue premium of €4,128.15.
Shareholder Authorizations Regarding Share Capital
The following list shows the current authorizations granted at our shareholders’ meeting held on June 26, 2024, or the 2024 Shareholders’ Meeting, to the Board of Directors of the Company in respect of capital increases:
•authorization granted to the Board of Directors to cancel treasury shares;
•delegation of authority to the Board of Directors to increase the share capital by issuing ordinary shares or any securities giving access to the capital, while maintaining the preferential subscription right of the shareholders;
•delegation of authority to the Board of Directors to increase the capital by issuing ordinary shares or any securities giving access to the capital through a public offering (other than those referred to in Article L. 411-2, 1° of the French Monetary and Financial Code), canceling preferential subscription rights of the shareholders though including an option for a priority period;
•delegation of authority to the Board of Directors to increase the share capital by issuing shares and/or securities giving immediate and/or future access to the Company's share capital, with cancellation of preferential subscription rights of the shareholders, through a public offering referred to in Article L. 411-2, 1° of the French Monetary and Financial Code;
•delegation of authority to the Board of Directors in the event of an issue of the Company’s ordinary shares and/or securities giving immediate and/or future access to the Company’s share capital, with cancellation of preferential subscription rights of the shareholders, to set the issue price, up to a limit of 10% of the share capital per year;
•delegation of authority to the Board of Directors to increase the share capital by issuing shares and/or securities giving immediate and/or future access to the Company’s share capital, with cancellation of preferential subscription rights of the shareholders for the benefit of certain categories of persons meeting specified characteristics;
•delegation of authority to the Board of Directors to increase the number of shares to be issued in the case of a capital increase, with or without preferential subscription rights for existing shareholders, within the limit of 15% of the initial issue amount;
•delegation of authority to the Board of Directors in order to increase the share capital through the capitalization of reserves, earnings or premium;
•delegation of authority to the Board of Directors to increase the share capital by issuing shares and/or securities giving immediate and/or future access to the Company’s share capital, with cancellation of preferential subscription rights of the shareholders, in consideration for contributions in kind for equity securities or other securities giving access to the capital.
The following authorizations granted to our Board of Directors by the shareholders’ meeting held on held on December 20, 2023 are still outstanding:
•delegation of authority to the Board of Directors for the purpose of granting stock options, through one or more issues, for the benefit of employees and/or corporate officers of the Company and its affiliates, entailing waiver by shareholders of their preferential subscription right;
•authorization granted to the Board of Directors to allocate existing or new free shares for the benefit of employees and/or corporate officers of the Company and its affiliates.
ORDINARY SHARES
As of December 31, 2024, our issued share capital consisted of a total of 162,521,524 ordinary shares with a nominal value of €0.15 per share. Of these 162,521,524 issued ordinary shares, 124,322 are treasury shares.
The description below reflects the terms of our bylaws and summarizes the material rights of holders of our ordinary shares under French law. Please note that this is only a summary and is not intended to be exhaustive. For further information, please refer to the full text of our bylaws, which are incorporated by reference as Exhibit 3.1 of the Company’s Annual Report on Form 20-F to which this description is also an exhibit.
Business Purpose
Our business purpose, within France and in every country is the following:
•research and development within the field of biomedicine and pharmacy;
•commercial exploitation of patents and know-how;
•trading in products of all kinds, and the provision of services in the field of data processing and information technology;
•production, monitoring and marketing of all products, services and research programs with applications to human and animal health, using the technologies of molecular and cellular biology and all of the associated techniques;
•participation of the Company by all means, direct or indirect, in all operations which may be associated with its company object, though the creation of new companies, contributions, subscription or purchase of securities or company rights, mergers or otherwise, the creation, acquisition, leasing, lease management of all operating assets or facilities;
•the acquisition, exploitation or sale of all procedures and patents regarding these activities, within France and abroad;
•and more generally, all industrial, commercial or financial, securities or property operations, which may be directly or indirectly associated with its business object or likely to favor its exploitation, realization or development.
Governance Structure
On December 20, 2023, our shareholders approved the change from a two-tier governance system under which we were governed by a Supervisory Board and Management Board to a one-tier governance system led by a Board of Directors, with Executive Officers in charge of the general management. Our former Chair of the Management Board was appointed by our Board as the Company’s Chief Executive Officer (directeur général), and five former Management Board members were appointed as Deputy Chief Executive Officers (directeurs généraux délégués): our Chief Financial Officer, Chief Medical Officer, Chief Business Officer, and Chief Commercial Officer. These Deputy Chief Executive Officers, together with the other members of our Executive Committee (which includes our Chief Operating Officer, Chief People Officer, Chief Scientific Officer and General Counsel), assist our Chief Executive Officer with the operational management of the Company. We present the details of both the Supervisory Board and the Board of Directors below.
Board of Directors (formerly the Supervisory Board)
Unless otherwise indicated, the details presented below apply with respect to the former Supervisory Board and the present Board of Directors, both of which are referred to as the "Board" for convenience.
Members
The Board is made up of a minimum of three members and a maximum of eighteen. The members of the Board are appointed for a renewable term of three years at the General Meeting of shareholders, which may revoke their appointments at any time. The directors may be individuals or companies.
The maximum age for membership on the Board is 80 years old, and no more than 20% of the members of the Board of Directors may be over 75 years old.
Chair of the Board
The Board appoints a Chair from its members who are individuals and may appoint a Vice Chair. The Chair, or in his or her absence the Vice Chair, is in charge of convening the Board and directing its discussions.
In a report to the General Meeting of shareholders attached to the Management Report, the Chair of the Board reports on the conditions for preparing and organizing the work of the Board as well as the internal control procedures set up by us.
Meetings and Powers of the Board
The Board meets as often as is in our interests but least once per quarter. Meetings are called under the circumstances and according to the conditions set forth in the bylaws, by the Chair or, if any, the Vice Chair or Lead Independent Director.
Board meetings may also be held (i) by videoconference or any other electronic means of telecommunication or remote transmission, or (ii) by written decision on the conditions and within the limits provided for by law.
At least half of the members of the Board must be present to constitute a quorum and decisions are made by a majority of the members of the Board present or represented. In the case of a tie vote, the Chair of the session shall have the deciding vote.
Executive Officers are required to obtain the Board’s approval prior to the conclusion of certain agreements or transactions. Prior to the change from a two-tier to a one-tier governance system, the Supervisory Board exercised permanent control over the Management Board, pursuant to the powers explicitly conferred on it by French law.
Under French law, any agreement entered into, directly or through an intermediary, between us and one of the members of the Board of Directors, our Chief Executive Officer, our Deputy Chief Executive Officers, or a shareholder that holds over 10% of the voting rights, or, if such shareholder is a company, the controlling company thereof, must be subject to prior authorization from the Board of Directors. The interested member cannot vote on such decision. The same applies to agreements in which a person referred to above has an indirect interest. Such prior authorization also applies to agreements between us and another company if one of our Executive Officers or Board members is the owner, a partner with unlimited liability, manager, director, managing director, member of the supervisory board or of the board of directors, or, in a general manner is in a position of responsibility within the other company. These provisions are not applicable to agreements concerning ordinary operations entered into under normal conditions.
Compensation of the Board
The General Meeting of shareholders may allocate an annual fixed sum and our Board allocates this sum among its members as it sees fit. In addition, the Board may allocate exceptional compensation (rémunération exceptionnelle) for missions or mandates entrusted to its members; in this case, this remuneration is subject to the provisions regarding related-parties agreements.
Committees
The Board may decide to establish committees responsible for reviewing matters which the Board or its Chair wish to submit to them for examination and advice.
Observers
The Board may appoint one or more observers. The observer or observers are called to attend the meetings of the Board of Directors in their observational capacity, without voting rights. They hold the same information and communication rights as the Board’s members and they are bound to the same confidentiality obligations.
Rights and Obligations Attached to Ordinary Shares
Each of our ordinary shares gives the right to a share of the profits and assets in proportion to the amount of capital it represents. It also gives the right to vote and be represented in the General Meeting of shareholders under the conditions set forth by French law and the bylaws.
If we are liquidated, any assets remaining after payment of the debts, liquidation expenses and all of the remaining obligations will first be used to repay in full the par value of our ordinary shares. Any surplus will be distributed pro rata among shareholders in proportion to the number of ordinary shares respectively held by them, taking into account, where applicable, of the rights attached to ordinary shares of different classes.
Shareholders are liable for corporate liabilities only up to the par value of the ordinary shares they hold; they are not liable to further capital calls.
We have not issued any ordinary shares giving holders privileged rights compared to those attached to other ordinary shares.
Shareholders’ rights may be modified as allowed by French law. Only the extraordinary shareholders’ meeting is authorized to amend any and all provisions of our bylaws. It may not, however, increase shareholder commitments without the prior approval of each shareholder.
Voting Rights
The voting rights attached to the ordinary shares are in proportion to the amount of capital they represent and each share gives the right to one vote. However, ordinary shares fully paid up and evidenced as having been held in registered form in the name of the same shareholder for at least two years, carry a double voting right in respect to that granted to other ordinary shares, according to the portion of share capital they represent. The ownership of a share implies, ipso facto, the acceptance of our bylaws and any decision of our shareholders. However, ADSs are not eligible for double voting rights.
Under French law, treasury shares or ordinary shares held by entities controlled by us are not entitled to voting rights and do not count for quorum purposes.
There is no limitation on voting rights in our bylaws nor limit the right of non-residents of France or non-French persons to own or, where applicable, to vote our securities.
Under French law, the holders of warrants of the same class (i.e., warrants that were issued at the same time and with the same rights), including founders’ warrants, are entitled to vote as a separate class at a general meeting of that class of warrant holders under certain circumstances, principally in connection with any proposed modification of the terms and conditions of the class of warrants or any proposed issuance of preferred shares or any modification of the rights of any outstanding class or series of preferred shares.
Dividends
We may only distribute dividends out of our distributable profits, plus any amounts held in our reserves that the shareholders decide to make available for distribution, other than those reserves that are specifically required by law. The conditions for payment of dividends in cash shall be set at the shareholders’ meeting.
“Distributable Profits” consist of our statutory net profit in each fiscal year, calculated in accordance with accounting standards applicable in France, as increased or reduced by any profit or loss carried forward from prior years, less any contributions to the reserve accounts. Pursuant to French law, we must allocate at least 5% of our statutory net profit for each year to our legal reserve fund before dividends may be paid with respect to that year. Such allocation is compulsory until the amount in the legal reserve is equal to 10% of the aggregate par value of our issued and outstanding share capital.
Dividends are distributed to shareholders pro rata according to their respective holdings of ordinary shares. In the case of interim dividends, distributions are made to shareholders on the date set by our Board during the meeting in which the distribution of interim dividends is approved. The actual dividend payment date is decided by the shareholders at an ordinary general shareholders’ meeting or by our Board in the absence of such a decision by the shareholders. Shareholders that own ordinary shares on the actual payment date are entitled to the dividend.
Pursuant to French law, dividends distributed out of our distributable profits must be paid within a maximum of nine months after the close of the relevant fiscal year, unless extended by court order. Dividends not claimed within five years after the payment date shall be deemed to expire and revert to the French state.
Shareholders may be granted an option to receive dividends or interim dividends in cash or in ordinary shares, in accordance with legal conditions.
Change in Share Capital
Any change to the capital or the rights attached to the ordinary shares is subject to legal provisions, as our bylaws do not set forth any particular requirements.
Increase in Share Capital
Pursuant to French law, our share capital may be increased only with shareholders’ approval at an extraordinary general shareholders’ meeting following the recommendation of our Board of Directors. The shareholders may delegate to our Board either the authority (délégation de compétence) or the power (délégation de pouvoir) to carry out any increase in share capital.
Increases in our share capital may be effected by:
•issuing additional shares;
•increasing the nominal value of existing shares;
•creating a new class of equity securities (preference shares); and
•exercising the rights attached to securities giving access to the share capital.
Increases in share capital by issuing additional securities may be effected through one or a combination of the following issuances:
•in consideration for cash;
•in consideration for assets contributed in kind;
•through an exchange offer or merger;
•by conversion of previously issued debt instruments;
•by exercise of the rights attached to securities giving access to the share capital;
•by capitalization of profits, reserves or share premium; and
•subject to certain conditions, by way of offset against debt incurred by us.
Decisions to increase the share capital through the capitalization of reserves, profits and/or share premium require shareholders’ approval at an extraordinary general shareholders’ meeting, acting under the quorum and majority requirements applicable to ordinary shareholders’ meetings. Increases effected by an increase in the nominal value of shares require unanimous approval of the shareholders, unless effected by capitalization of reserves, profits or share premium. All other capital increases require shareholders’ approval at an extraordinary general shareholders’ meeting acting under the regular quorum and majority requirements for such meetings.
Reduction in Share Capital
Pursuant to French law, any reduction in our share capital requires shareholders’ approval at an extraordinary general shareholders’ meeting. The shareholders may delegate to our Board the power (délégation de pouvoir) to carry out the share capital reduction. The share capital may be reduced either by decreasing the nominal value of the outstanding shares or by reducing the number of outstanding shares. The number of outstanding shares may be reduced by the repurchase and cancellation of shares. Holders of each class of shares must be treated equally unless each affected shareholder agrees otherwise, depending on the contemplated operations.
Preferential Subscription Rights
According to French law, if we issue additional securities for cash, current shareholders will have preferential subscription rights to these securities on a pro rata basis. Preferential subscription rights entitle the individual or entity that holds them to subscribe pro rata based on the number of shares held by them to the issuance of any securities increasing, or that may result in an increase of, our share capital by means of a cash payment or a set-off of cash debts. Pursuant to French law, the preferential subscription rights are transferable during a period equivalent to the subscription period relating to a particular offering but starting two days prior to the opening of the subscription period and ending two days prior to the closing of the subscription period.
The preferential subscription rights with respect to any particular offering may be waived at an extraordinary general meeting by a two-thirds vote of our shareholders or individually by each shareholder.
Our Board of Directors and our independent auditors are required by French law to present reports to the shareholders’ meeting that specifically address any proposal to waive the preferential subscription rights.
Form, Holding and Transfer of Shares
Form of Shares
The ordinary shares are held under registered or bearer form, if the legislation so permits, according to the shareholder’s choice.
Further, in accordance with applicable laws, we may request at any time from the central depository responsible for holding our shares, the information referred to in Article L. 228-2 of the French Commercial Code. Thus, we are, in particular and at any time, entitled to request the name and year of birth or, in the case of a legal entity, the name and the year of incorporation, nationality and address of holders of securities conferring immediate or long-term voting rights at the Company’s shareholders meeting and the amount of securities owned by each of them and, where applicable, the restrictions that the securities could be affected by.
Holding of Shares
In accordance with French law concerning the “dematerialization” of securities, the ownership rights of shareholders are represented by book entries instead of share certificates. Shares issued are registered in individual accounts opened by us or any authorized intermediary, in the name of each shareholder and kept according to the terms and conditions laid down by the legal and regulatory provisions.
Ownership of ADSs by Non-French Residents
Neither the French Commercial Code nor our bylaws currently impose any restrictions on the right of non-French residents or non-French shareholders to own and vote shares. However, non-French residents must file a declaration for statistical purposes with the Bank of France (Banque de France) within 20 working days following the date of certain direct foreign investments in us, including any purchase of our ADSs. In particular, such filings are required in connection with investments exceeding €15,000,000 that lead to the acquisition of at least 10% of our share capital or voting rights or cross such 10% threshold. Violation of this filing requirement may be sanctioned by five years of imprisonment and a fine of up to twice the amount of the relevant investment. This amount may be increased fivefold if the violation is made by a legal entity.
Certain foreign investments in French companies are subject to prior authorization from the Minister of the Economy when all or a portion of the target’s business activity is related to a strategic sector, such as energy, transport, public health, telecommunications, etc. We operates certain activities covered by the regulation on foreign investments in France, particularly for public health. Due to the operation of activities, we fall within the scope of the laws and regulations governing foreign investments in France set forth by Articles L. 151-3 and R. 151-2 et. seq. of the French Monetary and Financial Code.
Under these provisions, the acquisition by a non-French citizen, a French citizen who does not reside in France, a non-French entity or a French entity controlled by such persons or entities, of control, within the meaning of Article L. 233-3 of the French Commercial Code, or of all or a portion of a branch of activity of the Company or one of its French subsidiaries conducted activities enumerated by the aforementioned provisions, is subject to the prior authorization of the French Minister of the Economy. Moreover, the acquisition by an investor that is not a citizen of a member State of the European Union, or of a State that is a party to the agreement on the European Economic Area (EEA), that results, directly or indirectly, in exceeding, alone or in concert, the threshold of 10% of the voting rights of the Company or of one of its French subsidiaries conducting these activities, is subject to this same procedure.
In the context of the prior authorization procedure, the Minister of the Economy is charged with verifying that the conditions of the planned transaction preserves the national interests; in this respect the Minister may attach one or more conditions to the authorization of such a transaction in order to ensure the continuity of the concerned activities, industrial capacities, research and development capacities or related expertise, or even, on the basis of a motivated decision, refuse such an authorization, particularly if national interests cannot be protected.
If an investment requiring the prior authorization of the French Minister of Economy is completed without such authorization having been granted, the French Minister of Economy might direct the relevant investor to nonetheless (i) submit a request for authorization, (ii) have the previous situation restored at its own expense or (iii) amend the investment. The relevant investor might also be found criminally liable and might be sanctioned with a fine which cannot exceed the greater of: (i) twice the amount of the relevant investment, (ii) 10% of the annual turnover before tax of the target company and (iii) €5 million (for an entity) or €1 million (for an individual). Criminal sanctions may also be imposed upon complaint by the French Minister of Economy in accordance with the French Customs Code.
Foreign Exchange Controls
Under current French foreign exchange control regulations there are no limitations on the amount of cash payments that we may remit to residents of foreign countries. Laws and regulations concerning foreign exchange controls do, however, require that all payments or transfers of funds made by a French resident to a non-resident such as dividend payments be handled by an accredited intermediary. All registered banks and substantially all credit institutions in France are accredited intermediaries.
Availability of Preferential Subscription Rights
Under French law, shareholders have preferential rights to subscribe for cash issues of new ordinary shares or other securities giving rights to acquire additional ordinary shares on a pro rata basis. Holders of our securities in the United States (which may be in the form of ordinary shares or ADSs) may not be able to exercise preferential subscription rights for their securities unless a registration statement under the Securities Act is effective with respect to such rights or an exemption from the registration requirements imposed by the Securities Act is available. We may, from time to time, issue new ordinary shares or other securities giving rights to acquire additional ordinary shares (such as warrants) at a time when no registration statement is in effect and no Securities Act exemption is available. If so, holders of our securities in the United States will be unable to exercise any preferential subscription rights and their interests will be diluted. We are under no obligation to file any registration statement in connection with any issuance of new ordinary shares or other securities. We intend to evaluate at the time of any rights offering the costs and potential liabilities associated with registering the rights, as well as the indirect benefits to us of enabling the exercise by holders of ADSs in the United States of the subscription rights, and any other factors we consider appropriate at the time, and then to make a decision as to whether to register the rights. We cannot assure you that we will file a registration statement.
For holders of our ordinary shares in the form of ADSs, the depositary may make these rights or other distributions available to ADS holders. If the depositary does not make the rights available to ADS holders and determines that it is impractical to sell the rights, it may allow these rights to lapse. In that case the holders will receive no value for them. The section herein titled “American Depositary Shares-Dividends and Other Distributions” explains in detail the depositary’s responsibility in connection with a rights offering. See also “Risk Factors-Your right as a holder of ADSs to participate in any future preferential subscription rights or to elect to receive dividends in shares may be limited, which may cause dilution to your holdings” in the Company’s Annual Report on Form 20-F to which this description is filed as an exhibit.
Assignment and Transfer of Shares
Shares are freely negotiable, subject to applicable legal and regulatory provisions. Market Abuse Regulation 596/2014 of April 16, 2014 and French law notably provide for standstill obligations and prohibition of insider trading.
Repurchase and Redemption of Ordinary Shares
Under French law, we may acquire our own ordinary shares. Such acquisition may be challenged on the ground of market abuse regulations. However, Market Abuse Regulation 596/2014 of April 16, 2014 and its delegated regulations, or MAR, provides for safe harbor exemptions when the acquisition is made (i) under a buy-back program to be authorized by the shareholders in accordance with the provisions of Article L. 22-10-62 of the French Commercial Code and with the General Regulations of the French Financial Markets Authority, or AMF and (ii) for the following purposes:
•to decrease our share capital, with the approval of the shareholders at an extraordinary general meeting; in this case, the ordinary shares repurchased must be cancelled within one month from the expiry of the purchase offer;
•to meet obligations arising from debt securities that are exchangeable into equity instruments;
•to provide ordinary shares for distribution to employees or managers under a profit-sharing, free ordinary share or share option plan; or
•we benefit from a simple exemption when the acquisition is made under a liquidity contract complying with the General Regulations of, and market practices accepted by, the AMF.
All other purposes, and especially share buy-backs made for external growth operations in pursuance of Article L. 22-10-62 of the French Commercial Code, while not forbidden, must be pursued in strict compliance with market manipulation and insider dealing rules.
Under MAR and in accordance with the General Regulations of the AMF, a corporation shall report to the competent authority of the trading value on which the shares have been admitted to trading or are traded, no later than by the end of the seventh daily market session following the date of the execution of the transaction, all the transactions relating to the buy-back program, in a detailed form and in an aggregated form.
No such repurchase of ordinary shares may result in us holding, directly or through a person acting on our behalf, more than 10% of our issued share capital (5% in case of repurchase of shares to be used in payment or in exchange in the context of a merger, division or transfer of assets). Ordinary shares repurchased by us continue to be deemed “issued” under French law but are not entitled to dividends or voting rights so long as we hold them directly or indirectly, and we may not exercise the preemptive rights attached to them.
Sinking Fund Provisions
Our bylaws do not provide for any sinking fund provisions.
General Meeting of Shareholders
General Meetings of shareholders are called by the Board. They can also be called by the auditor(s) or an officer appointed by a court upon request, by any interested party or by the Works Council in an emergency, by one or more shareholders holding at least five percent of the ordinary shares or by an association of our shareholders. Meetings are held at our registered offices or at any other location indicated in the convening notice.
The meeting announcement is published in the French Bulletin of Mandatory Legal Notices (Bulletin des Annonces Légales Obligatoires or BALO) at least 35 days prior to the date of a General Meeting of shareholders. In addition to the information concerning us, the notice indicates in particular the agenda of the General Meeting of shareholders and the draft resolutions that will be presented.
In the 21 days preceding the meeting, we will publish the information and documents relating to the meeting on our web site.
The General Meeting of shareholders must be announced at least 15 days beforehand, by a notice placed in a journal that publishes legal announcements in the department where the headquarters are located, and in the BALO. Holders of registered ordinary shares who have owned them for at least one month as of the date on which the latest notice is published receive individual notices. When a General Meeting of shareholders is unable to take action because the requisite quorum is not present, a second meeting is called at least ten days in advance using the same procedure as the first one.
The General Meeting of shareholders may only take action related to items on the agenda. However, it may dismiss and replace one or more members of the Board any time. One or more shareholders representing at least the percentage of share capital fixed by law, and acting according to the legally required conditions and deadlines, are allowed to request that items and/or draft resolutions be added to the agenda of the General Meeting of shareholders. The work council may also request the entering of draft resolutions on the agenda of a General Meeting.
Each shareholder has the right to attend the meetings and take part in deliberation (i) personally; (ii) by granting proxy to another shareholder, his or her spouse or partner in a civil union or any other natural or legal person of his or her choice; (iii) by sending a proxy to the company without indication of the beneficiary; (iv) by voting by correspondence; or (v) by videoconference or another means of telecommunication, including internet, in accordance with applicable laws and regulations that allow identification; by presenting proof of identity and ownership of ordinary shares, subject to:
•for holders of registered ordinary shares, an entry in the shareholder registry at least two business days before the General Meeting of shareholders; and
•for holders of bearer ordinary shares, filing, under the conditions provided by law, of a certificate of participation issued by an authorized intermediary two days before the date of the General Meeting of shareholders.
The final date for returning voting ballots by correspondence is set by the Board and disclosed in the notice of meeting published in the BALO. This date cannot be earlier than three days prior to the meeting as provided in the bylaws.
A shareholder who has voted by correspondence will no longer be able to participate directly in the meeting or to be represented. In the case of returning the proxy form and the voting by correspondence form, the proxy form is taken into account, subject to the votes cast in the voting by correspondence form.
A shareholder may be represented at meetings by any individual or legal entity by means of a proxy form which we send to such shareholder either at the shareholder’s request or at our initiative. A shareholder’s request for a proxy form must be received at the registered office at least five days before the date of the meeting. The proxy is only valid for a single meeting, for two meetings (an ordinary and an extraordinary meeting convened for the same day or within 15 days) or for successive meetings convened with the same agenda.
A shareholder may vote by correspondence by means of a voting form, which we send to such shareholder either at the shareholder’s request or at our initiative, or which we include in an appendix to a proxy voting form under the conditions provided for by current laws and requirements. A shareholder’s request for a voting form must be received at the registered office at least six days before the date of the meeting. The voting form is also available on our website at least 21 days before the date of the meeting. The voting by correspondence form addressed by a shareholder is only valid for a single meeting or for successive meetings convened with the same agenda.
The above legislation provides that shareholders (and all the persons who may attend the general meeting of shareholders) may participate in the meeting by means of a teleconference or audio-visual conference call if this conference allows for the identification of the participants, transmits at least the voice of the participants and allows the continuous and simultaneous retransmission of the debates.
Our Bylaws and French Corporate Law Contain Provisions that May Delay or Discourage a Takeover Attempt
Provisions contained in our bylaws and French corporate law could make it more difficult for a third-party to acquire us, even if doing so might be beneficial to our shareholders. In addition, provisions of our bylaws impose various procedural and other requirements, which could make it more difficult for shareholders to effect certain corporate actions. These provisions include the following:
•under French law, the owner of 90% of the share capital or voting rights of a public company listed on a regulated market in a Member State of the European Union or in a state party to the European Economic Area, or EEA, Agreement, including from the main French stock exchange, has the right to force out minority shareholders following a tender offer made to all shareholders;
•under French law, a non-resident of France as well as any French entity controlled by non-residents of France may have to file a declaration for statistical purposes with the Bank of France (Banque de France) within 20 working days following the date of certain direct foreign investments in us, including any purchase of our ADSs. In particular, such filings are required in connection with investments exceeding €15,000,000 that lead to the acquisition of at least 10% of our share capital or voting rights or cross such 10% threshold. See “Ownership of ADSs by Non-French Residents” herein;
•under French law, certain investments in a French company relating to certain strategic industries (such as research and development in biotechnologies and activities relating to public health) and activities by individuals or entities not French, not resident in France of controlled by entities not French or not resident in France are subject to prior authorization of the Ministry of Economy. See “Ownership of ADSs by Non-French Residents” herein;
•a merger (i.e., in a French law context, a share for share exchange following which our company would be dissolved into the acquiring entity and our shareholders would become shareholders of the acquiring entity) of our company into a company incorporated in the European Union would require the approval of our Board as well as a two-thirds majority of the votes held by the shareholders present, represented by proxy or voting by mail at the relevant meeting;
•a merger of our company into a company incorporated outside of the European Union would require 100% of our shareholders to approve it;
•under French law, a cash merger is treated as a share purchase and would require the consent of each participating shareholder;
•our shareholders may grant in the future our Board broad authorizations to increase our share capital or to issue additional ordinary shares or other securities (for example, warrants) to our shareholders, the public or qualified investors, including as a possible defense following the launching of a tender offer for our ordinary shares;
•our shareholders have preferential subscription rights on a pro rata basis on the issuance by us of any additional securities for cash or a set-off of cash debts, which rights may only be waived by the extraordinary general meeting (by a two-thirds majority vote) of our shareholders or on an individual basis by each shareholder;
•our Board has the right to appoint members of the Board to fill a vacancy created by the resignation or death of a member of the Board for the remaining duration of such member’s term of office, and subject to the approval by the shareholders of such appointment at the next shareholders’ meeting, which prevents shareholders from having the sole right to fill vacancies on our Board;
•our Board can be convened by the Chair, the Vice Chair, or the Lead Independent Member of the Board or by our Chief Executive Officer. One-third of the members of the Board may send a written request to the Chair to convene the Board if there has not been a meeting for more than two months;
•our Board meetings can only be regularly held if at least half of its members attend either physically or by way of videoconference or teleconference enabling the members’ identification and ensuring their effective participation in the Board’s decisions;
•approval of at least a majority of the votes held by shareholders present, represented by a proxy, or voting by mail at the relevant ordinary shareholders’ general meeting is required to remove members of the Board with or without cause;
•the crossing of certain ownership thresholds has to be disclosed and can impose certain obligations; see “Key Provisions of Our Bylaws and French Law Affecting Our Ordinary Shares” herein;
•specific items are required in the shareholders' meeting agenda for nominations to the Board or for proposing matters to be acted upon at said shareholders’ meeting, except that a vote to remove and replace a member of the Board can be proposed at any shareholders’ meeting without notice;
•transfers of shares shall comply with applicable insider trading rules and regulations, and in particular with the Market Abuse Regulation 596/2014 of April 16, 2014; and
•pursuant to French law, our bylaws, including the sections relating to the number of members of the Board, and election and removal of members of the Board from office may only be modified by a resolution adopted by two-thirds of the votes of our shareholders present, represented by a proxy or voting by mail at the meeting.
Shareholder Identification
Ordinary shares may be registered or bearer ordinary shares, at the option of the shareholder, subject to the applicable legal requirements.
To identify the holders of bearer ordinary shares, we are authorized to ask in accordance with current legal and regulatory requirements, the central depositary that maintains the records of the issue of these ordinary shares, in exchange for a fee, for the holders’ name or business name, year of birth or year of incorporation, address and nationality, e-mail address, number of securities held giving immediate or future access to the capital and any restrictions to which the securities are subject.
Modification of the Bylaws
Our bylaws may only be amended by approval at an extraordinary shareholders’ meeting. Our bylaws may not, however, be amended to increase shareholder commitments without the approval of each shareholder. Decisions are made by a two-thirds majority of the votes held by the shareholders present, represented by proxy, or voting by mail.
Crossing the Threshold Set in the Bylaws
Without prejudice to the legal or regulatory stipulations, any natural person or legal entity who goes above or below, directly or indirectly, acting alone or in concert (de concert), a percentage of the share capital or voting rights equal to or higher than 2% or a multiple of this percentage, must inform us of the total number of ordinary shares, voting rights and securities giving access to capital or voting rights that it, he or she owns immediately or eventually, within four trading days of the date on which such ownership threshold is crossed.
If shareholders fail to comply with these obligations, shares or voting rights exceeding the fraction that should have been declared are deprived of voting rights at General Meetings of Shareholders for any meeting that would be held until the expiry of a period of two years from the date of regularization of the notification in accordance with Article L. 233-14 of the Commercial Code, if the failure to declare has been determined and one or several shareholders holding at least 2% of the capital make a request thereof, as recorded in the minutes of the General Meeting.
These requirements are without prejudice to the threshold crossing declarations provided for under French law in Articles L. 233-7, L. 233-9 and L. 233-10 of the French Commercial Code, which impose a declaration to us and to the French Financial Markets Authority (AMF) upon crossing, either upward or downward, of the following thresholds in share capital or voting rights no later than the fourth trading day following the crossing: 5%, 10%, 15%, 20%, 25%, 30%, 33.33%, 50%, 66.66%, 90% and 95%.
Furthermore, any shareholder crossing upward, alone or acting in concert, these 10%, 15%, 20% or 25% thresholds shall file a declaration pursuant to which it shall set out its intention for the following 6 months, including notably whether it intends to continue acquiring shares of the company or to acquire control over the company and its intended strategy for the company.
In addition, and subject to certain exemptions, any shareholder crossing upward, alone or acting in concert, the 30% threshold shall file a mandatory public tender offer. Also, any shareholder holding directly or indirectly a number between 30% and 50% of the capital or voting rights and who, in less than 12 consecutive months, increases their holding of capital or voting rights by at least 1% of the company’s capital or voting rights, shall file a mandatory public tender offer.
Differences in Corporate Law
We are a société européenne à conseil d'administration, or S.E., incorporated under the laws of France. The laws applicable to French S.E. differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the French Commercial Code applicable to us and the Delaware General Corporation Law, the law under which many public companies in the United States are incorporated. This summary is not intended to be a complete discussion of the respective rights.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
France |
|
Delaware |
| Number of directors |
|
Under French law, a société européenne à conseil d’administration must have at least three and may have up to 18 directors. The number of directors is fixed by or in the manner provided in the bylaws. In addition, the composition of the board of directors endeavors to seek a balanced representation of women and men. Since January 1, 2017, the number of directors of each gender may not be less than 40% when the company is listed on a regulated market or when the company meets certain criteria of turnover and number of employees, if not listed on a regulated market. Any appointment made in violation of this limit that is not remedied as well as the deliberations taken by the director irregularly appointed will be null and void. The directors are appointed by the shareholders’ general meetings. |
|
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws, unless the certificate of incorporation fixes the number of directors. |
| Director qualifications |
|
Under French law, a corporation may prescribe qualifications for directors under its bylaws. In addition, under French law, directors of a corporation may be legal entities (with the exception of the chair of the board of directors), and such legal entities must designate an individual to represent them and to act on their behalf at meetings of the board of directors. |
|
Under Delaware law, a corporation may prescribe qualifications for directors under its certificate of incorporation or bylaws. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Removal of directors |
|
Under French law, directors may be removed from office, with or without cause, by the shareholders at any shareholders’ general meeting without notice or justification, by a simple majority vote of the shareholders present and voting at the meeting in person or by proxy. |
|
Under Delaware law, unless otherwise provided in the certificate of incorporation, directors may be removed from office, with or without cause, by a majority stockholder vote, though in the case of a corporation whose board is classified, stockholders may effect such removal only for cause. |
|
|
|
|
|
| Vacancies on the Board of Directors |
|
Under French law, vacancies on the board of directors resulting from death or resignation, provided that at least three directors remain in office, may be filled by a majority of the remaining directors pending ratification at the next shareholders’ general meeting. |
|
Under Delaware law, vacancies on a corporation’s board of directors, including those caused by newly created directorships, may be filled by a majority of the remaining directors (even though less than a quorum). |
|
|
|
|
|
| Annual General Meeting |
|
Under French law, the annual general meeting of shareholders shall be held at such place, on such date and at such time as decided each year by the board of directors and notified to the shareholders in the convening notice of the annual meeting, within six months following the end of the relevant fiscal year unless such period is extended by court order. |
|
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be provided by the certificate of incorporation or by the bylaws, or by the board of directors if neither the certificate of incorporation or the bylaws so provide. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| General Meeting |
|
Under French law, general meetings of the shareholders may be called by the board of directors or, failing that, by the statutory auditors, or by a court appointed agent (mandataire ad hoc) or liquidator in certain circumstances, or by the majority shareholder in capital or voting rights following a public tender offer or exchange offer or the transfer of a controlling block on the date decided by the board of directors or the relevant person. |
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
| Notice of General Meetings |
|
A meeting announcement is published in the French Bulletin of Mandatory Legal Notices (BALO) at least 35 days prior to a meeting and made available on the website of the company at least 21 days prior to the shareholders’ general meeting. Subject to special legal provisions, a meeting notice is sent out at least 15 days prior to the date of the shareholders’ general meeting, by means of a notice inserted both in a newspaper for legal notices (journal d’annonces légales) of the registered office department and, if relevant, in the BALO. Further, shareholders holding registered shares for at least a month at the time of the latest insertion of the notice shall be summoned individually, by regular letter (or by registered letter if they request it and include an advance of expenses) sent to their last known address. This notice to shareholders holding registered shares may also be transmitted by electronic means of telecommunication, in lieu of any such mailing, to any shareholder requesting it beforehand by registered letter with acknowledgment of receipt in accordance with legal and regulatory requirements, specifying their e-mail address. When the shareholders’ general meeting cannot deliberate due to lack of required quorum, the second meeting must be called at least ten calendar days in advance in the same manner as used for the first notice. The convening notice shall specify the name of the company, its legal form, share capital, registered office address, registration number with the French Registry of Trade and Companies (registre du commerce et des sociétés), the place, date, hour and agenda of the meeting and its nature (ordinary and/or extraordinary meeting). The convening notice must also indicate the conditions under which the shareholders may vote by correspondence and the places and conditions in which they can obtain voting forms by mail. |
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than 10 nor more than 60 days before the date of the meeting and shall specify the place, date, hour, means of remote communication, if any, by which stockholders and proxy holders may be deemed to be present in person and vote, the record date for voting if it is different from the record date determining notice and, in the case of a special meeting, purpose or purposes for which the meeting is called. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Proxy |
|
Each shareholder has the right to attend the shareholders’ general meetings and participate in the discussions (i) personally, or (ii) by granting proxy to his/her spouse, his/her partner with whom he/she has entered into a civil union or to another shareholder or to any individual or legal entity of his choice; or (iii) by sending a proxy to the company without indication of the beneficiary (in which case, such proxy shall be cast in favor of the resolutions supported by the board of directors and against all other resolutions), or (iv) by voting by correspondence, or (v) by videoconference or another means of telecommunication in accordance with applicable French laws that allow identification. The proxy is only valid for a single meeting or for successive meetings convened with the same agenda. It can also be granted for two shareholders’ general meetings, one ordinary, and the other extraordinary, held on the same day or within a period of 15 days. |
|
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director’s voting rights as a director. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholder action by written consent |
|
Under French law, shareholders’ action by written consent is not permitted in a société européenne. |
|
Under Delaware law, a corporation’s certificate of incorporation (1) may permit stockholders to act by written consent if such action is signed by all stockholders, (2) may permit stockholders to act by written consent signed by stockholders having the minimum number of votes that would be necessary to take such action at a meeting or (3) may prohibit actions by written consent. |
|
|
|
|
|
| Preemptive Rights |
|
Under French law, in case of issuance of additional shares or other securities for cash or set-off against cash debts, the existing shareholders have preferential subscription rights (droits préférentiels de souscription) to these securities on a pro rata basis of his/her share ownership unless such rights are waived by a two-thirds majority of the votes held by the shareholders present or represented at the extraordinary general meeting deciding or authorizing the capital increase, voting in person or represented by proxy or voting by mail. In case such preferential subscription rights have not been waived by the shareholders' extraordinary general meeting, each shareholder may individually either exercise, assign or not exercise its preferential subscription rights. Further, preferential subscription rights may only be exercised during the subscription period. In accordance with French law, the exercise period cannot be less than five trading days in duration. Preferential subscription rights are transferable during the subscription period, but starting two business days prior to the start of the subscription period and ending two business days prior to its closing. |
|
Under Delaware law, unless otherwise provided in a corporation’s certificate of incorporation, a stockholder does not, by operation of law, possess preemptive rights to subscribe to additional issuances of the corporation’s stock or to any security convertible into such stock. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Sources of Dividends |
|
Under French law, dividends may only be paid by a French société européenne out of distributable profits (bénéfices distribuables) plus any distributable reserves and “distributable premium” that the shareholders decide to make available for distribution, other than those reserves that are specifically required by law. “Distributable profits” (bénéfices distribuables) consist of the unconsolidated net profits of the relevant corporation for each fiscal year, as increased or reduced by any profit or loss carried forward from prior years.
“Distributable premium” refers to the contribution paid by the shareholders in addition to the par value of their ordinary shares for their subscription that the shareholders decide to make available for distribution.
Except in case of a share capital reduction, no distribution can be made to the shareholders when the net equity is, or would become, lower than the amount of the share capital plus the reserves which cannot be distributed in accordance with the law or the company's bylaws.
|
|
Under Delaware law, dividends may be paid by a Delaware corporation either out of (1) surplus as defined in and computed in accordance with Delaware law or (2) in case there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year, except when the capital is diminished by depreciation in the value of its property, or by losses, or otherwise, to an amount less than the aggregate amount of capital represented by issued and outstanding stock having a preference on the distribution of assets. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Repurchase of Ordinary Shares |
|
Under French law, a corporation may acquire its own ordinary shares. Such acquisition may be challenged on the ground of market abuse regulations. However, the Market Abuse Regulation 596/2014 of April 16, 2014 (MAR) provides for safe harbor exemptions when the acquisition is made for the following purposes:
•to decrease its share capital, provided that such decision is not driven by losses and that a purchase offer is made to all shareholders on a pro rata basis, with the approval of the shareholders at the extraordinary general meeting deciding the capital reduction, in which case, the shares repurchased must be cancelled within one month from the expiry of the purchase offer;
•with a view to distributing within one year of their repurchase the relevant shares to employees or managers under a profit-sharing, free share or share option plan; not to exceed 10% of the share capital, in which case the shares repurchased must be distributed within 12 months from their repurchase failing which they must be cancelled; or
•to meet obligations arising from debt securities, that are exchangeable into equity instruments.
A simple exemption is provided when the acquisition is made under a buy-back program to be authorized by the shareholders in accordance with the provisions of Article L. 22-10-62 of the French Commercial Code and in accordance with the General Regulation of the Financial Markets Authority (Règlement Général de l’AMF).
All other purposes, and especially share buy-backs for external growth operations pursuant to Article L. 22-10-62 of the French Commercial Code, while not forbidden, must be pursued in strict compliance of market manipulations and insider dealing rules. Under the MAR and in accordance with the General Regulation of the AMF, a corporation shall report to the competent authority of the trading venue on which the shares have been admitted to trading or are traded, no later than by the end of the seventh daily market session following the date of the execution of the transaction, all the transactions relating to the buy-back program, in a detailed form and in an aggregated form.
By exception, a company shall provide to the AMF, on a monthly basis, and to the public on a biannual basis, a summary report of the transactions made under a liquidity contract.
No such repurchase of ordinary shares may result in the company holding, directly or through a person acting on its behalf, more than 10% of its issued share capital (5% in case of repurchase of shares to be used in payment or in exchange in the context of a merger, division or transfer of assets).
|
|
Under Delaware law, a corporation may generally redeem or repurchase shares of its stock unless the capital of the corporation is impaired or such redemption or repurchase would impair the capital of the corporation. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Liability of directors and officers |
|
Under French law, a company's bylaws may not include any provisions limiting the liability of directors. Civil liabilities of the directors may be sought for (1) an infringement of laws and regulations applicable to a company, (2) breach of the bylaws and (3) management failure. |
|
Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation or its stockholders for damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for:
• any breach of the director’s duty of loyalty to the corporation or its stockholders;
• acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
• intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or
• any transaction from which the director derives an improper personal benefit.
|
|
|
|
|
|
| Voting Rights |
|
French law provides that, unless otherwise provided in the bylaws, each shareholder is entitled to one vote for each share of capital stock held by such shareholder. Double voting rights are automatically granted to the shares held in registered form (au nominatif) for more than two years, unless provided otherwise in the bylaws. |
|
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholder Vote on Certain Transactions |
|
Generally, under French law, completion of a merger, dissolution, sale, lease or exchange of all or substantially all of a corporation’s assets requires:
•the approval of the board of directors; and
•approval by a two-thirds majority of the votes held by the shareholders present, represented by proxy or voting by mail at the relevant shareholders’ meeting or, in the case of a merger that will result in an increase of the shareholders’ commitments or with a non-EU company, approval of all shareholders of the corporation (by exception, the extraordinary general meeting of the acquiring company may delegate to the board of directors authority to decide a merger-absorption or to determine the terms and conditions of the merger plan).
|
|
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires:
• the approval of the board of directors; and
• approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter.
|
|
|
|
|
|
| Dissent or Dissenters’ Appraisal Rights |
|
French law does not provide for any such right but provides that a merger is subject, depending on the circumstances of the merger, to shareholders’ approval by a two-thirds majority vote, or unanimous decisions of the shareholders, as stated above. |
|
Under Delaware law, a holder of shares of any class or series has the right, in specified circumstances, to dissent from a merger or consolidation by demanding payment in cash for the stockholder’s shares equal to the fair value of those shares, as determined by the Delaware Chancery Court in an action timely brought by the corporation or a dissenting stockholder. Delaware law grants these appraisal rights only in the case of mergers or consolidations and not in the case of a sale or transfer of assets or a purchase of assets for stock. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Further, no appraisal rights are available for shares of any class or series that is listed on a national securities exchange or held of record by more than 2,000 stockholders, unless the agreement of a merger or consolidation requires the holders to accept for their shares anything other than:
• shares of stock of the surviving corporation;
• shares of stock of another corporation that are either listed on a national securities exchange or held of record by more than 2,000 stockholders;
• cash in lieu of fractional shares of the stock described in the two preceding bullet points; or
• any combination of the above.
• In addition, appraisal rights are not available to holders of shares of the surviving corporation in specified mergers that do not require the vote of the stockholders of the surviving corporation.
|
|
|
|
|
|
| Standard of Conduct for directors |
|
French law does not contain specific provisions setting forth the standard of conduct of a director. However, directors have a duty of loyalty, a duty to act without self-interest, on a well-informed basis and they cannot make any decision against a corporation’s corporate interest (intérêt social) taking into consideration the social and environmental aspects of their activity, where applicable. |
|
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the stockholders. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Shareholder Suits |
|
French law provides that a shareholder, or a group of shareholders, may initiate a legal action to seek indemnification from the directors of a corporation in the corporation’s corporate interest if it fails to bring such legal action itself. If so, any damages awarded by the court are paid to the corporation and legal fees relating to such action may be borne by the relevant shareholder or the group of shareholders.
The plaintiff must remain a shareholder through the duration of the legal action.
There is no other case where shareholders may initiate a derivative action to enforce a right of a corporation.
A shareholder may alternatively or cumulatively bring individual legal action against the directors, provided he has suffered distinct damages from those suffered by the corporation. In this case, any damages awarded by the court are paid to the relevant shareholder.
|
|
Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must:
• state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiff’s shares thereafter devolved on the plaintiff by operation of law; and allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or
• state the reasons for not making the effort.
Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery.
|
|
|
|
|
|
| Amendment of Certificate of Incorporation |
|
Under French law, corporations are not required to file a certificate of incorporation with the French Registry of Trade and Companies (registre du commerce et des sociétés) and only have bylaws (statuts) as organizational documents. As indicated in the paragraph below, only the extraordinary shareholders’ meeting is authorized to adopt or amend the bylaws. |
|
Under Delaware law, generally a corporation may amend its certificate of incorporation if:
• its board of directors has adopted a resolution setting forth the amendment proposed and declared its advisability; and
• the amendment is adopted by the affirmative votes of a majority (or greater percentage as may be specified by the corporation) of the outstanding shares entitled to vote on the amendment and a majority (or greater percentage as may be specified by the corporation) of the outstanding shares of each class or series of stock, if any, entitled to vote on the amendment as a class or series.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amendment of Bylaws |
|
Under French law, only the extraordinary shareholders’ meeting is authorized to adopt or amend the bylaws. The extraordinary shareholders’ meeting may authorize the board of directors to amend the bylaws to comply with legal provisions, subject to the ratification of such amendments by the next extraordinary shareholders’ meeting. The board of directors is authorized to amend the bylaws as a result of a decision to relocate the company’s registered office in France, subject to ratification by the next ordinary shareholders’ meeting. |
|
Under Delaware law, the stockholders entitled to vote have the power to adopt, amend or repeal bylaws. A corporation may also confer, in its certificate of incorporation, that power upon the board of directors. |
AMERICAN DEPOSITARY SHARES
Citibank is the depositary for the ADSs representing our ordinary shares. Citibank’s depositary offices are located at 388 Greenwich Street, New York, New York 10013. ADSs represent ownership interests in securities that are on deposit with the depositary. ADSs may be represented by certificates that are commonly known as American Depositary Receipts, or ADRs. The depositary typically appoints a custodian to safekeep the securities on deposit. In this case, the custodian is Citibank Europe plc, located at 1 North Wall Quay, Dublin 1 Ireland.
We have appointed Citibank as depositary pursuant to a deposit agreement. The form of the deposit agreement is on file with the SEC under cover of a registration statement on Form F-6. You may obtain a copy of the deposit agreement from the SEC’s website (www.sec.gov). Please refer to registration number 333-255301 when retrieving such copy. The portions of this summary description that are italicized describe matters that may be relevant to the ownership of ADSs but that may not be contained in the deposit agreement.
We are providing you with a summary description of the material terms of the ADSs and of your material rights as an owner of ADSs. Please remember that summaries by their nature lack the precision of the information summarized and that the rights and obligations of an owner of ADSs will be determined by reference to the terms of the deposit agreement and not by this summary. We urge you to review the deposit agreement in its entirety.
Each ADS represents the right to receive, and to exercise the beneficial ownership interests in, two ordinary shares that are on deposit with the depositary and/or custodian. An ADS also represents the right to receive, and to exercise the beneficial interests in, any other property received by the depositary or the custodian on behalf of the owner of the ADS but that has not been distributed to the owners of ADSs because of legal restrictions or practical considerations. We and the depositary may agree to change the ADS-to-Share ratio by amending the deposit agreement. This amendment may give rise to, or change, the depositary fees payable by ADS owners. The custodian, the depositary and their respective nominees will hold all deposited property for the benefit of the holders and beneficial owners of ADSs. The deposited property does not constitute the proprietary assets of the depositary, the custodian or their nominees. Beneficial ownership in the deposited property will under the terms of the deposit agreement be vested in the beneficial owners of the ADSs. The depositary, the custodian and their respective nominees will be the record holders of the deposited property represented by the ADSs for the benefit of the holders and beneficial owners of the corresponding ADSs. A beneficial owner of ADSs may or may not be the holder of ADSs. Beneficial owners of ADSs will be able to receive, and to exercise beneficial ownership interests in, the deposited property only through the registered holders of the ADSs, the registered holders of the ADSs (on behalf of the applicable ADS owners) only through the depositary, and the depositary (on behalf of the owners of the corresponding ADSs) directly, or indirectly, through the custodian or their respective nominees, in each case upon the terms of the deposit agreement.
If you become an owner of ADSs, you will become a party to the deposit agreement and therefore will be bound to its terms and to the terms of any ADR that represents your ADSs. The deposit agreement and the ADR specify our rights and obligations as well as your rights and obligations as an owner of ADSs and those of the depositary. As an ADS holder you appoint the depositary to act on your behalf in certain circumstances. The deposit agreement and the ADRs are governed by New York law. However, our obligations to the holders of ordinary shares will continue to be governed by the laws of France, which may be different from the laws in the United States.
In addition, applicable laws and regulations may require you to satisfy reporting requirements and obtain regulatory approvals in certain circumstances. You are solely responsible for complying with such reporting requirements and obtaining such approvals. Neither the depositary, the custodian, us or any of their or our respective agents or affiliates shall be required to take any actions whatsoever on your behalf to satisfy such reporting requirements or obtain such regulatory approvals under applicable laws and regulations.
The manner in which you own the ADSs (e.g., in a brokerage account vs. as registered holder, or as holder of certificated vs. uncertificated ADSs) may affect your rights and obligations, and the manner in which, and extent to which, the depositary’s services are made available to you.
As an owner of ADSs, we will not treat you as one of our shareholders and you will not have direct shareholder rights. The depositary will hold on your behalf the shareholder rights attached to the ordinary shares underlying your ADSs. As an owner of ADSs, you will be able to exercise the shareholders rights for the ordinary shares represented by your ADSs through the depositary only to the extent contemplated in the deposit agreement. To exercise any shareholder rights not contemplated in the deposit agreement you will, as an ADS owner, need to arrange for the cancellation of your ADSs and become a direct shareholder.
As an owner of ADSs, you may hold your ADSs either by means of an ADR registered in your name, through a brokerage or safekeeping account, or through an account established by the depositary in your name reflecting the registration of uncertificated ADSs directly on the books of the depositary (commonly referred to as the “direct registration system” or “DRS”). The direct registration system reflects the uncertificated (book-entry) registration of ownership of ADSs by the depositary. Under the direct registration system, ownership of ADSs is evidenced by periodic statements issued by the depositary to the holders of the ADSs. The direct registration system includes automated transfers between the depositary and The Depository Trust Company, or DTC, the central book-entry clearing and settlement system for equity securities in the United States. If you decide to hold your ADSs through your brokerage or safekeeping account, you must rely on the procedures of your broker or bank to assert your rights as ADS owner. Banks and brokers typically hold securities such as the ADSs through clearing and settlement systems such as DTC. The procedures of such clearing and settlement systems may limit your ability to exercise your rights as an owner of ADSs. Please consult with your broker or bank if you have any questions concerning these limitations and procedures. All ADSs held through DTC will be registered in the name of a nominee of DTC, which nominee will be the only “holder” of such ADSs for purposes of the deposit agreement and any applicable ADR. This summary description assumes you have opted to own the ADSs directly by means of an ADS registered in your name and, as such, we will refer to you as the “holder.” When we refer to “you,” we assume the reader owns ADSs and will own ADSs at the relevant time.
The registration of the ordinary shares in the name of the depositary or the custodian shall, to the maximum extent permitted by applicable law, vest in the depositary or the custodian the record ownership in the applicable ordinary shares with the beneficial ownership rights and interests in such ordinary shares being at all times vested with the beneficial owners of the ADSs representing the ordinary shares. The depositary or the custodian shall at all times be entitled to exercise the beneficial ownership rights in all deposited property, in each case only on behalf of the holders and beneficial owners of the ADSs representing the deposited property.
Dividends and Distributions
As a holder of ADSs, you generally have the right to receive the distributions we make on the securities deposited with the custodian. Your receipt of these distributions may be limited, however, by practical considerations and legal limitations. Holders of ADSs will receive such distributions under the terms of the deposit agreement in proportion to the number of ADSs held as of the specified record date, after deduction of the applicable fees, taxes and expenses.
Distributions of Cash
Whenever we make a cash distribution for the securities on deposit with the custodian, we will deposit the funds with the custodian. Upon receipt of confirmation of the deposit of the requisite funds, the depositary will arrange for the funds received in a currency other than U.S. dollars to be converted into U.S. dollars and for the distribution of the U.S. dollars to the holders, subject to the laws and regulations of France.
The conversion into U.S. dollars will take place only if practicable and if the U.S. dollars are transferable to the United States. The depositary will apply the same method for distributing the proceeds of the sale of any property (such as undistributed rights) held by the custodian in respect of securities on deposit.
The distribution of cash will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. The depositary will hold any cash amounts it is unable to distribute in a non-interest bearing account for the benefit of the applicable holders and beneficial owners of ADSs until the distribution can be effected or the funds that the depositary holds must be escheated as unclaimed property in accordance with the laws of the relevant states of the United States.
Distributions of Shares
Whenever we make a free distribution of ordinary shares for the securities on deposit with the custodian, we will deposit the applicable number of ordinary shares with the custodian. Upon receipt of confirmation of such deposit, the depositary will either distribute to holders new ADSs representing the ordinary shares deposited or modify the ADS-to-ordinary shares ratio, in which case each ADS you hold will represent rights and interests in the additional ordinary shares so deposited. Only whole new ADSs will be distributed. Fractional entitlements will be sold and the proceeds of such sale will be distributed as in the case of a cash distribution.
The distribution of new ADSs or the modification of the ADS-to-ordinary shares ratio upon a distribution of ordinary shares will be made net of the fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes or governmental charges, the depositary may sell all or a portion of the new ordinary shares so distributed.
No such distribution of new ADSs will be made if it would violate a law (e.g., the U.S. securities laws) or if it is not operationally practicable. If the depositary does not distribute new ADSs as described above, it may sell the ordinary shares received upon the terms described in the deposit agreement and will distribute the proceeds of the sale as in the case of a distribution of cash.
Distributions of Rights
Whenever we intend to distribute rights to subscribe for additional ordinary shares, we will give prior notice to the depositary and we will assist the depositary in determining whether it is lawful and reasonably practicable to distribute rights to subscribe for additional ADSs to holders.
The depositary will establish procedures to distribute rights to subscribe for additional ADSs to holders and to enable such holders to exercise such rights if it is lawful and reasonably practicable to make the rights available to holders of ADSs, and if we provide all of the documentation contemplated in the deposit agreement (such as opinions to address the lawfulness of the transaction). You may have to pay fees, expenses, taxes and other governmental charges to subscribe for the new ADSs upon the exercise of your rights. The depositary is not obligated to establish procedures to facilitate the distribution and exercise by holders of rights to subscribe for new ordinary shares other than in the form of ADSs.
The depositary will not distribute the rights to you if:
•We do not timely request that the rights be distributed to you or we request that the rights not be distributed to you; or
•We fail to deliver satisfactory documents to the depositary; or
•It is not reasonably practicable to distribute the rights.
The depositary will sell the rights that are not exercised or not distributed if such sale is lawful and reasonably practicable. The proceeds of such sale will be distributed to holders as in the case of a cash distribution. If the depositary is unable to sell the rights, it will allow the rights to lapse.
Elective Distributions
Whenever we intend to distribute a dividend payable at the election of shareholders either in cash or in additional shares, we will give prior notice thereof to the depositary and will indicate whether we wish the elective distribution to be made available to you. In such case, we will assist the depositary in determining whether such distribution is lawful and reasonably practicable.
The depositary will make the election available to you only if it is reasonably practicable and if we have provided all of the documentation contemplated in the deposit agreement. In such case, the depositary will establish procedures to enable you to elect to receive either cash or additional ADSs, in each case as described in the deposit agreement.
If the election is not made available to you, you will receive either cash or additional ADSs, depending on what a shareholder in France would receive upon failing to make an election, as more fully described in the deposit agreement.
Other Distributions
Whenever we intend to distribute property other than cash, ordinary shares or rights to subscribe for additional ordinary shares, we will notify the depositary in advance and will indicate whether we wish such distribution to be made to you. If so, we will assist the depositary in determining whether such distribution to holders is lawful and reasonably practicable.
If it is reasonably practicable to distribute such property to you and if we provide to the depositary all of the documentation contemplated in the deposit agreement, the depositary will distribute the property to the holders in a manner it deems practicable.
The distribution will be made net of fees, expenses, taxes and governmental charges payable by holders under the terms of the deposit agreement. In order to pay such taxes and governmental charges, the depositary may sell all or a portion of the property received.
The depositary will not distribute the property to you and will sell the property if:
•We do not request that the property be distributed to you or if we request that the property not be distributed to you; or
•We do not deliver satisfactory documents to the depositary; or
•The depositary determines that all or a portion of the distribution to you is not reasonably practicable.
The proceeds of such a sale will be distributed to holders as in the case of a cash distribution.
Redemption
Whenever we decide to redeem any of the securities on deposit with the custodian, we will notify the depositary in advance. If it is practicable and if we provide all of the documentation contemplated in the deposit agreement, the depositary will provide notice of the redemption to the holders.
The custodian will be instructed to surrender the shares being redeemed against payment of the applicable redemption price. The depositary will convert into U.S. dollars upon the terms of the deposit agreement the redemption funds received in a currency other than U.S. dollars and will establish procedures to enable holders to receive the net proceeds from the redemption upon surrender of their ADSs to the depositary. You may have to pay fees, expenses, taxes and other governmental charges upon the redemption of your ADSs. If less than all ADSs are being redeemed, the ADSs to be retired will be selected by lot or on a pro rata basis, as the depositary may determine.
Changes Affecting Ordinary Shares
The ordinary shares held on deposit for your ADSs may change from time to time. For example, there may be a change in nominal or par value, split-up, cancellation, consolidation or any other reclassification of such ordinary shares or a recapitalization, reorganization, merger, consolidation or sale of assets of the Company.
If any such change were to occur, your ADSs would, to the extent permitted by law and the deposit agreement, represent the right to receive the property received or exchanged in respect of the ordinary shares held on deposit. The depositary may in such circumstances deliver new ADSs to you, amend the deposit agreement, the ADRs and the applicable Registration Statement(s) on Form F-6, call for the exchange of your existing ADSs for new ADSs and take any other actions that are appropriate to reflect as to the ADSs the change affecting the Shares. If the depositary may not lawfully distribute such property to you, the depositary may sell such property and distribute the net proceeds to you as in the case of a cash distribution.
Issuance of ADSs upon Deposit of Ordinary Shares
The depositary may create ADSs on your behalf if you or your broker deposit ordinary shares with the custodian. The depositary will deliver these ADSs to the person you indicate only after you pay any applicable issuance fees and any charges and taxes payable for the transfer of the ordinary shares to the custodian. Your ability to deposit ordinary shares and receive ADSs may be limited by U.S. and French legal considerations applicable at the time of deposit.
The issuance of ADSs may be delayed until the depositary or the custodian receives confirmation that all required approvals have been given and that the ordinary shares have been duly transferred to the custodian. The depositary will only issue ADSs in whole numbers.
When you make a deposit of ordinary shares, you will be responsible for transferring good and valid title to the depositary. As such, you will be deemed to represent and warrant that:
•The ordinary shares are duly authorized, validly issued, fully paid, non-assessable and legally obtained.
•All preemptive (and similar) rights, if any, with respect to such ordinary shares have been validly waived or exercised.
•You are duly authorized to deposit the ordinary shares.
•The ordinary shares presented for deposit are free and clear of any lien, encumbrance, security interest, charge, mortgage or adverse claim, and are not, and the ADSs issuable upon such deposit will not be, “restricted securities” (as defined in the deposit agreement).
•The ordinary shares presented for deposit have not been stripped of any rights or entitlements.
If any of the representations or warranties are incorrect in any way, we and the depositary may, at your cost and expense, take any and all actions necessary to correct the consequences of the misrepresentations.
Transfer, Combination, and Split Up of ADRs
As an ADR holder, you will be entitled to transfer, combine or split up your ADRs and the ADSs evidenced thereby. For transfers of ADRs, you will have to surrender the ADRs to be transferred to the depositary and also must:
•ensure that the surrendered ADR is properly endorsed or otherwise in proper form for transfer;
•provide such proof of identity and genuineness of signatures as the depositary deems appropriate;
•provide any transfer stamps required by the State of New York or the United States; and
•pay all applicable fees, charges, expenses, taxes and other government charges payable by ADR holders pursuant to the terms of the deposit agreement, upon the transfer of ADRs.
To have your ADRs either combined or split up, you must surrender the ADRs in question to the depositary with your request to have them combined or split up, and you must pay all applicable fees, charges and expenses payable by ADR holders, pursuant to the terms of the deposit agreement, upon a combination or split up of ADRs.
Withdrawal of Ordinary Shares Upon Cancellation of ADSs
As a holder, you will be entitled to present your ADSs to the depositary for cancellation and then receive the corresponding number of underlying ordinary shares at the custodian’s offices. Your ability to withdraw the ordinary shares held in respect of the ADSs may be limited by U.S. and French legal considerations applicable at the time of withdrawal. In order to withdraw the ordinary shares represented by your ADSs, you will be required to pay to the depositary the fees for cancellation of ADSs and any charges and taxes payable upon the transfer of the ordinary shares. You assume the risk for delivery of all funds and securities upon withdrawal. Once canceled, the ADSs will not have any rights under the deposit agreement.
If you hold ADSs registered in your name, the depositary may ask you to provide proof of identity and genuineness of any signature and such other documents as the depositary may deem appropriate before it will cancel your ADSs. The withdrawal of the ordinary shares represented by your ADSs may be delayed until the depositary receives satisfactory evidence of compliance with all applicable laws and regulations. Please keep in mind that the depositary will only accept ADSs for cancellation that represent a whole number of securities on deposit.
You will have the right to withdraw the securities represented by your ADSs at any time except for:
•Temporary delays that may arise because (i) the transfer books for the ordinary shares or ADSs are closed, or (ii) ordinary shares are immobilized on account of a shareholders’ meeting or a payment of dividends.
•Obligations to pay fees, taxes and similar charges.
•Restrictions imposed because of laws or regulations applicable to ADSs or the withdrawal of securities on deposit.
The deposit agreement may not be modified to impair your right to withdraw the securities represented by your ADSs except to comply with mandatory provisions of law.
Voting Rights
As a holder, you generally have the right under the deposit agreement to instruct the depositary to exercise the voting rights for the ordinary shares represented by your ADSs.
At our request, the depositary will distribute to you any notice of shareholders’ meeting received from us together with information explaining how to instruct the depositary to exercise the voting rights of the securities represented by ADSs. In lieu of distributing such materials, the depositary may distribute to holders of ADSs instructions on how to retrieve such materials upon request.
If the depositary timely receives voting instructions from a holder of ADSs, it will endeavor to vote the securities (in person or by proxy) represented by the holder’s ADSs in accordance with such voting instructions.
Securities for which no voting instructions have been received will not be voted (except as otherwise contemplated in the deposit agreement). Please note that the ability of the depositary to carry out voting instructions may be limited by practical and legal limitations and the terms of the securities on deposit. We cannot assure you that you will receive voting materials in time to enable you to return voting instructions to the depositary in a timely manner.
Fees and Charges
As an ADS holder, you will be required to pay the following fees under the terms of the deposit agreement:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Service |
|
Fees |
| Issuance of ADSs (e.g., an issuance of ADS upon a deposit of ordinary shares, upon a change in the ADS(s)-to-ordinary share ratio, or for any other reason), excluding ADS issuances as a result of distributions of ordinary shares |
|
Up to U.S. 5¢ per ADS issued |
|
|
|
| Cancellation of ADSs (e.g., a cancellation of ADSs for delivery of deposited property, upon a change in the ADS(s)-to ordinary share ratio, or for any other reason) |
|
Up to U.S. 5¢ per ADS cancelled |
|
|
|
| Distribution of cash dividends or other cash distributions (e.g., upon a sale of rights and other entitlements) |
|
Up to U.S. 5¢ per ADS held |
|
|
|
| Distribution of ADSs pursuant to (i) stock dividends or other free stock distributions, or (ii) exercise of rights to purchase additional ADSs |
|
Up to U.S. 5¢ per ADS held |
|
|
|
| Distribution of securities other than ADSs or rights to purchase additional ADSs (e.g., upon a spin-off) |
|
Up to U.S. 5¢ per ADS held |
|
|
|
| ADS Services |
|
Up to U.S. 5¢ per ADS held on the applicable record date(s) established by the depositary |
|
|
|
Registration of ADS transfers (e.g., upon a registration of the transfer of registered ownership of ADSs, upon a transfer of ADSs into DTC and vice versa, or for any other reason) |
|
Up to U.S. 5¢ per ADS (or fraction thereof) transferred |
|
|
|
Conversion of ADSs of one series for ADSs of another series (e.g., upon conversion of Partial Entitlement ADSs for Full Entitlement ADSs, or upon conversion of Restricted ADSs (each as defined in the Deposit Agreement) into freely transferable ADSs, and vice versa). |
|
Up to U.S. 5¢ per ADS (or fraction thereof) converted |
As an ADS holder, you will also be responsible to pay certain charges such as:
•taxes (including applicable interest and penalties) and other governmental charges;
•the registration fees as may from time to time be in effect for the registration of ordinary shares on the share register and applicable to transfers of ordinary shares to or from the name of the custodian, the depositary or any nominees upon the making of deposits and withdrawals, respectively;
•certain cable, telex and facsimile transmission and delivery expenses;
•the fees, expenses, spreads, taxes and other charges of the depositary and/or service providers (which may be a division, branch or affiliate of the depositary) in the conversion of foreign currency;
•the reasonable and customary out-of-pocket expenses incurred by the depositary in connection with compliance with exchange control regulations and other regulatory requirements applicable to ordinary shares, ADSs and ADRs; and
•the fees, charges, costs and expenses incurred by the depositary, the custodian, or any nominee in connection with the ADR program.
ADS fees and charges for (i) the issuance of ADSs, and (ii) the cancellation of ADSs are charged to the person for whom the ADSs are issued (in the case of ADS issuances) and to the person for whom ADSs are cancelled (in the case of ADS cancellations). In the case of ADSs issued by the depositary into DTC, the ADS issuance and cancellation fees and charges may be deducted from distributions made through DTC, and may be charged to the DTC participant(s) receiving the ADSs being issued or the DTC participant(s) holding the ADSs being cancelled, as the case may be, on behalf of the beneficial owner(s) and will be charged by the DTC participant(s) to the account of the applicable beneficial owner(s) in accordance with the procedures and practices of the DTC participants as in effect at the time. ADS fees and charges in respect of distributions and the ADS service fee are charged to the holders as of the applicable ADS record date. In the case of distributions of cash, the amount of the applicable ADS fees and charges is deducted from the funds being distributed. In the case of (i) distributions other than cash and (ii) the ADS service fee, holders as of the ADS record date will be invoiced for the amount of the ADS fees and charges and such ADS fees and charges may be deducted from distributions made to holders of ADSs. For ADSs held through DTC, the ADS fees and charges for distributions other than cash and the ADS service fee may be deducted from distributions made through DTC, and may be charged to the DTC participants in accordance with the procedures and practices prescribed by DTC and the DTC participants in turn charge the amount of such ADS fees and charges to the beneficial owners for whom they hold ADSs. In the case of (i) registration of ADS transfers, the ADS transfer fee will be payable by the ADS holder whose ADSs are being transferred or by the person to whom the ADSs are transferred, and (ii) conversion of ADSs of one series for ADSs of another series, the ADS conversion fee will be payable by the Holder whose ADSs are converted or by the person to whom the converted ADSs are delivered.
In the event of refusal to pay the depositary fees, the depositary may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder. Note that the fees and charges you may be required to pay may vary over time and may be changed by us and by the depositary. You will receive prior notice of such changes. The depositary may reimburse us for certain expenses incurred by us in respect of the ADR program, by making available a portion of the ADS fees charged in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Amendments and Termination
We may agree with the depositary to modify the deposit agreement at any time without your consent. We undertake to give holders of ADSs 30 days’ prior notice of any modifications that would materially prejudice any of their substantial rights under the deposit agreement. We will not consider to be materially prejudicial to your substantial rights any modifications or supplements that are reasonably necessary for the ADSs to be registered under the Securities Act or to be eligible for book-entry settlement, in each case without imposing or increasing the fees and charges you are required to pay. In addition, we may not be able to provide you with prior notice of any modifications or supplements that are required to accommodate compliance with applicable provisions of law.
You will be bound by the modifications to the deposit agreement if you continue to hold your ADSs after the modifications to the deposit agreement become effective. The deposit agreement cannot be amended to prevent you from withdrawing the ordinary shares represented by your ADSs (except as permitted by law).
We have the right to direct the depositary to terminate the deposit agreement. Similarly, the depositary may in certain circumstances on its own initiative terminate the deposit agreement. In either case, the depositary must give notice to the holders at least 30 days before termination. Until termination, your rights under the deposit agreement will be unaffected.
After termination, the depositary will continue to collect distributions received (but will not distribute any such property until you request the cancellation of your ADSs) and may sell the securities held on deposit. After the sale, the depositary will hold the proceeds from such sale and any other funds then held for the holders of ADSs in a non-interest bearing account.
At that point, the depositary will have no further obligations to holders other than to account for the funds then held for the holders of ADSs still outstanding (after deduction of applicable fees, taxes and expenses).
In connection with any termination of the deposit agreement, the depositary may make available to owners of ADSs a means to withdraw the ordinary shares represented by ADSs and to direct the depositary of such ordinary shares into an unsponsored American depositary share program established by the depositary. The ability to receive unsponsored American depositary shares upon termination of the deposit agreement would be subject to satisfaction of certain U.S. regulatory requirements applicable to the creation of unsponsored American depositary shares and the payment of applicable depositary fees.
Books of Depositary
The depositary will maintain ADS holder records at its depositary office. You may inspect such records at such office during regular business hours but solely for the purpose of communicating with other holders in the interest of business matters relating to the ADSs and the deposit agreement.
The depositary will maintain in New York facilities to record and process the issuance, cancellation, combination, split-up and transfer of ADSs. These facilities may be closed from time to time, to the extent not prohibited by law.
Transmission of Notices, Reports and Proxy Soliciting Material
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. Subject to the terms of the deposit agreement, the depositary will send you copies of those communications or otherwise make those communications available to you if we ask it to.
Limitations on Obligations and Liabilities
The deposit agreement limits our obligations and the depositary’s obligations to you. Please note the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary are obligated only to take the actions specifically stated in the deposit agreement without negligence or bad faith. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
The depositary disclaims any liability for any failure to carry out voting instructions, for any manner in which a vote is cast or for the effect of any vote, provided it acts in good faith and in accordance with the terms of the deposit agreement. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
The depositary disclaims any liability for any failure to accurately determine the lawfulness or practicality of any action, for the content of any document forwarded to you on our behalf or for the accuracy of any translation of such a document, for the investment risks associated with investing in ordinary shares, for the validity or worth of the ordinary shares, for any tax consequences that result from the ownership of ADSs or other deposited property, for the credit-worthiness of any third party, for allowing any rights to lapse under the terms of the deposit agreement, for the timeliness of any of our notices or for our failure to give notice or for any act or omission of or information provided by DTC or any DTC participant. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
The depositary shall not be liable for acts or omissions of any successor depositary in connection with any matter arising wholly after the resignation or removal of the depositary. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary will not be obligated to perform any act that is inconsistent with the terms of the deposit agreement. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary disclaim any liability if we or the depositary are prevented or forbidden from or subject to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required by the terms of the deposit agreement, by reason of any provision, present or future of any law or regulation including regulations of any stock exchange, or by reason of present or future provision of any provision of our Articles of Incorporation, or any provision of or governing the securities on deposit, or by reason of any act of God or war or other circumstances beyond our control. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary disclaim any liability by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our Articles of Incorporation or in any provisions of or governing the securities on deposit. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary further disclaim any liability for any action or inaction in reliance on the advice or information received from legal counsel, accountants, any person presenting Shares for deposit, any holder of ADSs or authorized representatives thereof, or any other person believed by either of us in good faith to be competent to give such advice or information. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary also disclaim liability for the inability by a holder or beneficial holder to benefit from any distribution, offering, right or other benefit that is made available to holders of ordinary shares but is not, under the terms of the deposit agreement, made available to you. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary may rely without any liability upon any written notice, request or other document believed to be genuine and to have been signed or presented by the proper parties. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary also disclaim liability for any consequential or punitive damages for any breach of the terms of the deposit agreement. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
We and the depositary disclaim liability arising out of losses, liabilities, taxes, charges or expenses resulting from the manner in which a holder or beneficial owner of ADSs holds ADSs, including resulting from holding ADSs through a brokerage account. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
• |
No disclaimer of any Securities Act liability is intended by any provision of the deposit agreement. |
Nothing in the deposit agreement gives rise to a partnership or joint venture, or establishes a fiduciary relationship, among us, the depositary and you as ADS holder.
Nothing in the deposit agreement precludes Citibank (or its affiliates) from engaging in transactions in which parties adverse to us or the ADS owners have interests, and nothing in the deposit agreement obligates Citibank to disclose those transactions, or any information obtained in the course of those transactions, to us or to the ADS owners, or to account for any payment received as part of those transactions.
As the above limitations relate to our obligations and the depositary’s obligations to you under the deposit agreement, we believe that, as a matter of construction of the clause, such limitations would likely to continue to apply to ADS holders who withdraw the ordinary shares from the ADS facility with respect to obligations or liabilities incurred under the deposit agreement before the cancellation of the ADSs and the withdrawal of the ordinary shares, and such limitations would most likely not apply to ADS holders who withdraw the ordinary shares from the ADS facility with respect to obligations or liabilities incurred after the cancellation of the ADSs and the withdrawal of the ordinary shares and not under the deposit agreement.
In any event, you will not be deemed, by agreeing to the terms of the deposit agreement, to have waived our or the depositary’s compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder. In fact, you cannot waive our or the depositary’s compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder.
Taxes
You will be responsible for the taxes and other governmental charges payable on the ADSs and the securities represented by the ADSs. We, the depositary and the custodian may deduct from any distribution the taxes and governmental charges payable by holders and may sell any and all property on deposit to pay the taxes and governmental charges payable by holders. You will be liable for any deficiency if the sale proceeds do not cover the taxes that are due.
The depositary may refuse to issue ADSs, to deliver, transfer, split and combine ADRs or to release securities on deposit until all taxes and charges are paid by the applicable holder. The depositary and the custodian may take reasonable administrative actions to obtain tax refunds and reduced tax withholding for any distributions on your behalf. However, you may be required to provide to the depositary and to the custodian proof of taxpayer status and residence and such other information as the depositary and the custodian may require to fulfill legal obligations. You are required to indemnify us, the depositary and the custodian for any claims with respect to taxes based on any tax benefit obtained for you.
Foreign Currency Conversion
The depositary will arrange for the conversion of all foreign currency received into U.S. dollars if such conversion is practical, and it will distribute the U.S. dollars in accordance with the terms of the deposit agreement. You may have to pay fees and expenses incurred in converting foreign currency, such as fees and expenses incurred in complying with currency exchange controls and other governmental requirements.
If the conversion of foreign currency is not practical or lawful, or if any required approvals are denied or not obtainable at a reasonable cost or within a reasonable period, the depositary may take the following actions in its discretion:
•Convert the foreign currency to the extent practical and lawful and distribute the U.S. dollars to the holders for whom the conversion and distribution is lawful and practical.
•Distribute the foreign currency to holders for whom the distribution is lawful and practical.
•Hold the foreign currency (without liability for interest) for the applicable holders.
Governing Law/Waiver of Jury Trial
The deposit agreement, the ADRs and the ADSs will be interpreted in accordance with the laws of the State of New York. The rights of holders of ordinary shares (including ordinary shares represented by ADSs) are governed by the laws of France.
AS A PARTY TO THE DEPOSIT AGREEMENT, YOU IRREVOCABLY WAIVE, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, YOUR RIGHT TO TRIAL BY JURY IN ANY LEGAL PROCEEDING ARISING OUT OF THE DEPOSIT AGREEMENT OR THE ADRs AGAINST US AND/OR THE DEPOSITARY.
The deposit agreement provides that, to the extent permitted by law, ADS holders waive the right to a jury trial of any claim they may have against us or the depositary arising out of or relating to our ordinary shares, the ADSs or the deposit agreement, including any claim under U.S. federal securities laws. If we or the depositary opposed a jury trial demand based on the waiver, the court would determine whether the waiver was enforceable in the facts and circumstances of that case in accordance with applicable case law. However, you will not be deemed, by agreeing to the terms of the deposit agreement, to have waived our or the depositary’s compliance with U.S. federal securities laws and the rules and regulations promulgated thereunder.
EX-4.10
4
exhibit410-cepifundingagre.htm
EX-4.10
Document
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) CUSTOMARILY AND ACTUALLY TREATED BY THE REGISTRANT AS PRIVATE OR CONFIDENTIAL.
EXECUTION VERSION
ANNEX A: TERMS AND CONDITIONS
C E PI
Funding Agreement
(CEPI Identification: Valneva 0002)
Agreement Summary
|
|
|
|
|
|
| AWARDEE INFORMATION |
| Name: |
Valneva Austria GmbH |
| Mailing Address: |
Campus Vienna Biocenter 3, 1030 Vienna, Austria |
| Project Lead: |
[***] |
|
Project Management
Contact:
|
[***] |
| Bank Account Details: |
Account Name: Valneva Austria GmbH
Name of Bank: [***]
IBAN:[***]
BIC/Swift Code: [***]
|
|
|
|
|
|
|
| CEPI INFORMATION |
| Mailing Address: |
Post Box 1030 Hoff, 0218 Oslo, Norway |
| Project Lead: |
[***] |
| Management Contact: |
[***] |
|
|
|
|
|
|
| AGREEMENT INFORMATION |
| Project Name |
Expanding the Profile of Live-Attenuated Chikungunya Vaccine |
| CEPI Program Name |
CEPI CfP3iii Chikungunya Vaccines |
| Effective Date |
Date of last signature below |
|
|
|
|
1
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
|
|
|
|
|
|
| Pre-Activities Start Date |
1 September 2023 |
|
This Agreement includes and incorporates by
reference:
|
The agreement (referred to as the “Agreement”) means this Agreement Summary together with the following:
-CEPI Policies and Procedures as of Effective Date (Schedule C)
-Integrated Product Development Plan (“IPDP”) (Annex C)
|
|
|
|
|
2
Sensitivity: Official Use
|
|
|
|
|
|
|
|
-IPDP Reporting Templates (Annex D)
-Project Budget including Pre-Award Cost (Annex E)
-Payment Request Form and Financial Report Templates (Annex F)
-Equitable Access Plan (Annex G)
|
THIS AGREEMENT is between Valneva Austria GmbH (“Awardee” or “You”) and the Coalition for Epidemic Prepared ness Innovations (“CEPI”) and is effective as of the Effective Date. Each party to this Agreement may be referred to individually as a “Party” and together as the “Parties.” This Agreement sets out the terms and conditions governing the performance of the Project, funding of the Project and how the results of the Project will be used to further CEPI’s mission. As a condition of this funding award, the Parties enter into this Agreement by having their authorized representatives sign below.
Signed for and on behalf of COALITION FOR EPIDEMIC PREPAREDNESS INNOVATIONS by:
Signature: [***]
[***]
Name:…………………………………………..
[***]
Title:…………………………………………….
19-07-2024
Date:…………………………………………….
Signed for and on behalf of Valneva Austria GmbH by:
[***]
Name:…………………………………………..
[***]
Title:…………………………………………….
19-07-2024
Date:…………………………………………….
Signature:………………………………………
[***]
Name:…………………………………………..
[***]
Title:…………………………………………….
19-07-2024
Date:…………………………………………….
|
|
|
|
3
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
ANNEX A: TERMS AND CONDITIONS
CfP3iii Award Terms and Conditions
1.These Terms and Conditions
1.1These “Terms and conditions” (or “T&Cs”) describe the contractual relationship between cepi and Awardee for a particular Project under CEPI’s CfP3iii Programme. They describe each Party’s rights and obligations, and provide instructions on the conduct of funded activities and the intended use of the results from funded activities. The Parties commit to participate in the Project with good intent and in good faith.
1.2A glossary of defined terms used in these T&Cs is set out i
n schedule a. a table setting out the effects of termination may be found in
Schedule B to the T&Cs.
1.3CEPI, Awardee and the Serum Institute of India Private Limited (“SII”) are currently discussing and aim to enter into a tripartite side letter (“Side Letter”) not later than within two (2) calendar months after the date of this Agreement whereby, provided SII and Awardee fulfil certain conditions included in the Side Letter, [***]. If there is any conflict between the finalized Side Letter and this Agreement, the Side Letter will prevail. For the avoidance of doubt, nothing in this Clause shall be construed as CEPI providing consent to SII becoming a Sub-Awardee until the Side Letter has been executed.
2.Project Organization and Management
2.1IPDP and Work Packages. the awardee’s project activities, which are intended to further develop a Chikungunya Vaccine are set out in the Integrated Product Development Plan (IPDP), which may be found in
Annex C. Awardee will use commercially reasonable endeavours to achieve the associated deliverables, milestones and timelines of each Work Package and achieve agreed upon Technical Review criteria by the agreed deadline (the “Technical Review Point”) set forth in Annex C. In accordance with Clause 4.6 below, additional Work Package(s) may be agreed in writing by the Parties after the Effective Date, which, upon execution by both Parties, shall be annexed to and become a part of this Agreement. Work Packages may also be modified or extended with the mutual written consent of both Parties in accordance with Clause
24.14.
2.2Technical Reviews. awardee will notify the JMAG when it is reasonably assured that a technical Review Point will be achieved in the near term, and promptly provide the JMAG with relevant information and agreed upon data. [***]
2.3Project Organization. the project will be organized and managed as described in the team charter in
Annex B. The Project management shall be Awardee’s sole responsibility provided that Awardee shall consult with CEPI concerning the management of the Project to the extent required by the Team Charter and/or this
|
|
|
|
4
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
Agreement and will consider CEPI’s comments in good faith. [***] Notwithstanding the foregoing, CEPI shall be entitled to be party to tripartite meetings between CEPI, Awardee and each LMIC Manufacturer [***]
2.4Joint Monitoring and Advisory Group. the team charter establishes a joint monitoring and Advisory Group (or “JMAG”) to facilitate communications and interactions between the Parties and any LMIC Manufacturers, as well as review Project activities in terms of timelines and budget. [***]
2.5the awardee will:
a.undertake the activities and comply with the obligations described in the team charter;
b.participate in the designated activities and meetings of the jmag;
c.keep accurate, complete and reliable records of activities performed and results arising as a result of the activities set out in the IPDP (“IPDP Records”);
d.maintain the IPDP records for [***] after the termination or expiry of the project, or for any longer period as required by law, the CEPI Clinical Trials Policy or Awardee’s own policies;
e.monitor progress of the project and make IPDP reports to the JMAG as described in the IPDP;
f.keep the JMAG (until the equitable access group is established in accordance with clause
16.3 in which case Awardee will inform the Equitable Access Group) informed of its adherence to the Equitable Access Plan and its progress in meeting its objectives;
g.propose amendments to the IPDP and project budget to the JMAG, as may be required; however, such amendments may require CEPI approval beyond the JMAG level; and
h.notify CEPI if the project lead designated in the ipdp becomes unavailable and designate areplacement reasonably satisfactory to CEPI within [***].
i.
3.Sub-Awardee Participation in the Project
3.1Sub-Awardees. Awardee’s activities under the project may be undertaken by affiliates, and contracted collaborators, including LMIC Manufacturers (collectively, “Sub-Awardees”) designated in the IPDP and Project Budget. Awardee will be responsible for the acts and omissions of its Sub-Awardees.
3.2Hungarian Act IX Restriction. none of the funds provided under this agreement (whether via a sub-contract or otherwise) shall be used in any way directly or indirectly to provide support, resources or assets to any public interest trusts established on the basis of the Hungarian Act IX of 2021 or any entity maintained by such a public interest trust.
3.3CEPI Approval of Additional Sub-Awardees. any proposed sub-awardee not expressly referred to in the IPDP or Project Budget must be approved by CEPI in writing before a sub-award has been made. Such approval not to be unreasonably withheld, conditioned or delayed by CEPI.
|
|
|
|
5
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
3.4Sub-Awardee Obligations. a sub-awardee must agree to comply with all of the relevant obligations applicable to Awardee, whether explicitly identified as such or as is reasonable from the nature of the obligation. Each sub-agreement with a Sub-Awardee must:
a.be consistent with the work package stream structure as well as the associated milestones and budgets;
b.require the same record keeping obligations and provide CEPI the same access (either directly or indirectly through Awardee) to IPDP Records and Financial Records (as are applicable to Awardee);
c.require compliance with the same laws, policies and procedures as are applicable under these T&Cs, including the CEPI Policies and Procedures;
d.be consistent with awardee’s obligation in this agreement, including without limitation in the sections related to Financial Management and Oversight (Clause 5); Dissemination and Publication of Project Data (Clause 13); Dissemination of Project Materials (Clause 14); Intellectual Property (Clause 15); Equitable Access (Clause 16) and the Equitable Access Plan (Annex G); Sharing of Commercial Benefits (Clause 17); Preparation for Outbreaks (Clause 18); the Public Health License (Clause 19); and Term and Termination (Clause 22); and
e.prohibit the sub-awardee from subcontracting its obligations without CEPI`s consent. such consent not to be unreasonably withheld, conditioned or delayed.
3.5the awardee will:
a.sign an agreement with each sub-awardee, prior to their conducting any activities under the Project or amend any relevant agreement signed with a Sub-Awardee prior to the Effective Date of this Agreement, to be consistent with Awardee’s relevant obligations to CEPI under this Agreement and the IPDP;
b.in addition to, and without in any way diminishing or otherwise altering, awardee’s obligations under this Agreement and under the IPDP with respect to use of Sub-Awardees in LMICs, cooperate with CEPI in good faith and to the extent reasonably possible to preferentially use Sub-Awardees operating in LMICs where Outbreaks are likely to occur in order to build infrastructure and develop experienced personnel in the relevant territory; and
c.promptly provide a copy of each sub-awardee agreement or amendment thereto to CEPI, provided that Awardee shall have the right to redact any confidential information contained therein that is not necessary for CEPI to determine compliance with Clause
3.4.
4.Project Funding and Work Package Streams
4.1Work Package Streams. the IPDP will be organized into discrete phases, corresponding with the Project Budget. The associated activities, budgets, deliverables and timelines for each phase are set out in Work Package streams in the IPDP (each a “Work Package Stream”).
4.2Project Payments. payments for the project will be made in us dollars ($) to awardee’s bank account identified on the Agreement Summary. CEPI will make payments in advance covering the planned activities for the subsequent six (6) month period beginning on the date specified in the Budget. The initial payment will additionally cover pre-award costs commencing on the Pre-Activities Start Date, as agreed between the Parties and included in the Budget.
|
|
|
|
6
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
4.3Subsequent Tranches. CEPI will pay the initial tranche of funding within [***] of signature of this Agreement. All subsequent 6-month tranches will be paid by CEPI within [***] after receipt of all of the following: (i) a payment request by Awardee; and (ii) the required IPDP Report (Annex D) and Financial Reports (Annex F), adjusted appropriately for any underspend from any previous payments.
4.4Payment when there is a Breach. CEPI is not obliged to pay any tranches of funding for any work Package for so long as Awardee is in breach of a material obligation under this Agreement.
4.5Delayed Payments. CEPI may delay or condition a payment if:
a.awardee has not achieved a milestone by the agreed time, unless such delay has been approved by the JMAG;
b.CEPI has been notified that awardee or any of its sub-awardees are no longer in compliance with the Warranties under Clause
20 at the time the tranche is requested; or
c.awardee has not completed the payment request form or submitted satisfactory IPDP reports and/or Financial Reports.
4.6Funding of Additional Work Packages.
a.CEPI shall have the first right subject to section 4.6 c., in its sole discretion, to provide further funding and other support for the further development, manufacture and deployment of the Product in Non-Traveler’s Market countries. Such activities would be negotiated in good faith and set out in an additional Work Package(s) with associated project budget for such additional Work Packages amending Annex C. Nothing in this Clause
4.6 confers any obligation on CEPI to fund additional Work Packages.
b.CEPI may decide not to proceed with any additional work package in accordance with clause 4.6 a. if it is not in the best interest of CEPI’s mission. If CEPI decides not to fund additional Work Packages, it will notify Awardee as soon as such a decision is made.
c.in the event that (a) awardee reasonably requires any third party funding for the development, manufacture or deployment of the Product in Non-Traveler’s Market countries; or (b) Awardee receives any offer or indication of interest from a third party, or identifies a call for proposal from a third party, to provide funding support for such development, manufacture or deployment, Awardee shall provide prompt written notice to CEPI, including a summary of the amount of funding required or offered and the terms (if any) offered by any potential third party funder (each a “Further Funding Notice”). CEPI shall provide written notice to Awardee that it does, or does not wish to provide such further funding within [***] of receipt by CEPI of a Further Funding Notice. After this period Awardee shall have the right to accept any third party funding support for the development, manufacture and deployment of the Product in Non Traveler’s Market countries provided CEPI (i) notifies Awardee that it does not wish to provide additional funding, or (ii) fails to provide Awardee with such notice within the [***] time period.
4.7Retained Payment. CEPI will retain [***] of the final payment tranche until awardee submits the final IPDP Report and Financial Report.
|
|
|
|
7
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
4.8Withholding tax: payments under this agreement are to be made without withholding for or on account of any tax unless required by law, in which case, any such tax withheld shall be treated as having been paid by the paying Party to the other Party for all purposes under this Agreement, and the paying Party shall duly account for such tax withheld to the relevant tax authority and provide reasonable evidence of this to the other Party. The paying Party will notify the other Party in writing as soon as reasonably practicable once it becomes aware it has an obligation to so withhold and the Parties will cooperate with respect to reasonable requests by that other Party to secure a reduction in the rate of applicable withholding tax or to permit that other Party to obtain a repayment of, or credit for, tax withheld.
4.9The Awardee will:
a.use award payments only in accordance with the IPDP, agreed work package streams and project Budget, and for the period between the Pre-Activities Start Date and 30 June 2024 as set forth in the relevant section of Annex E
b.provide a financial report to CEPI within [***] of the end of each six (6) month period during the Term of the Project, regarding its expenditures pursuant to the Project Budget, using the template provided in Annex F. In the first Financial Report Awardee will include details of its expenditures incurred in the period from the Pre-Activities Start Date until the date of the first Financial Report;
c.provide a separate final financial report for a work package within [***] after the completion of any Work Package; and
d.reimburse CEPI for any funding underspend.
5.Financial Management and Oversight
5.1Financial Practices. from the Pre-Activities Start Date, awardee’s financial management of the Project will be governed by controls, good management practices, procedures and standards at least as rigorous as its local Generally Accepted Accounting Principles (GAAP), or International Financial Reporting Standards (IFRS) if adopted by the Awardee, as confirmed in Awardee’s annual audited financial statement.
5.2Financial Oversight. subject to the confidentiality provisions contained in clause
24.4, CEPI, or its designee, will have on-site access to Awardee’s Financial Records annually, at such times as CEPI may request, provided CEPI has given not less than [***] notice, in order that CEPI may monitor Awardee’s expenditure of Project funds. CEPI or its designee will have such on-site access to Awardee’s Financial Records more than annually in the following circumstances:
i.where CEPI has reasonable grounds indicating that the Awardee is in material breach of this Agreement or has misapplied CEPI Funding; and
|
|
|
|
8
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
ii.where required in the context of an audit of CEPI by one or more of its funders.
5.3the awardee will:
a.from the Pre-Activities Start Date, keep accurate, complete and reliable records of revenues and expenditures for the period between the Pre-Activities Start Date and 30 June 2024 as set forth in the relevant section of Annex E;
b.from the effective date, keep accurate, complete and reliable records of revenues and expenditures under the Project Budget (“Financial Records”) against an individual project code;
c.retain all financial records and details of the pre-activities start date expenditure for [***] after termination or expiry of the Project or for any longer period as required by law or Awardee’s own policies and allow CEPI access to such records as set out in Clause
5.2 for such retention period;
d.provide [***] written notice to CEPI before destroying financial records;
e.provide up-to-date audited financial statements, as requested by CEPI, and relevant extracts from the auditors’ report for such financial statement as well as the management letter to the auditors;
f. if requested by CEPI, awardee will permit awardee’s external auditors or an independent audit firm appointed by CEPI to conduct a Project audit (on and off site). The audit will be conducted at CEPI’s reasonable cost and expense;
g.procure a project audit as identified above from sub-awardees at CEPI’s request and at CEPI’s reasonable cost and in accordance with relevant audit and assurance standards, including but not limited to, ISA800 or ISRS4400; and
h.provide information required by the European communities court of auditors and anti-fraud office.
6.Compliance with Applicable Laws and CEPI Policies and Procedures
6.1Compliance Requirements. Awardee will comply with relevant national and supranational laws and governmental regulations that apply to Awardee’s Project-related activities.
6.2CEPI’s Third Party Code. The third party Code is a statement of CEPI’s values and of the policies, practices and principles applicable to recipients of CEPI funding. Awardee:
a.acknowledges the statement of CEPI’s values in Section 1 of the Code;
b.will adhere to business practices, ethical principles and legal requirements that are at least substantially similar to those described in Sections 2 to 10 of the Code;
c.will comply with the requirements for reporting compliance concerns and misconduct to CEPI (Sections 4 and 11 of the Code);
d.will cooperate as may be reasonably requested by CEPI in the submission of information related to Project activities and expenditures in accordance with the International Aid Transparency Initiative (Section 12 of the Code); and
|
|
|
|
9
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
e.will, for any sub-contractor not listed in the Team Charter or IPDP, comply with the provisions of the Third Party Code related to Sub-Contractors (Section 14 of the Code).
6.3Amendment of CEPI Policies and Procedures. CEPI may notify awardee from time-to-time that the CEPI Policies and Procedures have been amended. Such amended CEPI Policies and Procedures will become effective with respect to Awardee and Sub-Awardees [***] after notification from CEPI, absent notification of objection by the Awardee. In case Awardee sends CEPI a notification of objection, the compliance officers from Awardee and CEPI shall decide on the matter. If the compliance officers are unable to make a decision within [***] from the date of receipt by CEPI of the notification of objection from Awardee, the Parties shall initiate the escalation process described in Clause
23.1.
6.4the awardee will:
a.comply with applicable laws and regulations;
b.subject to clause
24.6, comply with CEPI policies and procedures;
c.provide access to information to the EC court of auditors and anti-fraud office as required;
d.notify CEPI promptly to discuss any amended CEPI policies and procedures that raise concerns about Awardee’s ability to perform its obligations under this Agreement.
7.Clinical Studies
For the purposes of this section, where an LMIC Manufacturer will be undertaking clinical studies, and provided such clinical studies have been funded by CEPI under this Agreement, Awardee will obligate the relevant LMIC Manufacturer to comply with the obligations applicable to Awardee, mutatis mutandis.
7.1Clinical Studies. if any work package includes research involving human subjects, such activities must comply with applicable laws, the requirements of any relevant regulatory agency and with CEPI’s Clinical Trials Policy.
7.2Clinical Data. where applicable, the data arising in the conduct of a clinical trial will be collected in a way that ensures that each subject, prior to enrolment and in accordance with all applicable laws and regulations, including the EU’s General Data Protection Regulation (GDPR), provides informed consent to allow:
a.direct access to her or his medical records;
|
|
|
|
10
Sensitivity: Official Use
|
|
|
|
ANNEX A: TERMS AND CONDITIONS |
b.the processing of data relating to her or him and to the movement of that data to other countries, including countries outside of the European Economic Area;
c.the transfer of such data to awardee;
d.the transfer of anonymised data to CEPI;
e.the collection and use of clinical study data (duly anonymised and, at CEPI’s request, blinded) in accordance with and for the purposes indicated in Clause
13; f.the collection and use of biological samples and the use of data (duly anonymised and, at CEPI’s request, blinded) derived from such samples by CEPI or its designated Assessors in accordance with and for the purposes indicated in Clause
14; and
g.the use of such data for the purpose of obtaining approval from applicable regulatory agencies.
7.3Priority for Certain Clinical Studies. Awardee acknowledges that the pool of subjects available in areas of Outbreak to participate in a clinical study to test products such as the Product may be limited. Accordingly, if CEPI reasonably determines in consultation with experts (for example a sub-group or subcommittee of CEPI’s Scientific Advisory Committee that CEPI determines has appropriate expertise) that a product other than the Awardee’s Product has substantially greater potential, as determined in accordance with WHO guidance or relevant local regulatory guidance and should be used for a particular clinical study of subjects in areas of Outbreak, the Awardee agrees that it shall abide by such decision and will not proceed with any clinical study of the Product with subjects from areas of Outbreak unless agreed with CEPI. In the event that Awardee must discontinue a clinical study of the Product in areas of Outbreak according to CEPI´s determination pursuant to this Clause
7.3, then CEPI shall (i) cooperate with Awardee in an appropriate wind down of the study and (ii) to the extent not funded in advance by CEPI, reimburse Awardee for Awardee’s reasonably incurred non-cancellable expenses relating to such discontinued clinical study. For clarity, Awardee shall not pay back any sums already received from CEPI that have been actually spent by Awardee in connection with such discontinued clinical study. For the purposes of this Clause, CEPI agrees that nothing in this Clause
7.3 will prevent (i) Awardee from undertaking a Pivotal Study in any country or (ii) Awardee fulfilling its obligations under its risk management plan prepared by Awardee in connection with its biologics license application in any country, including but not limited to post registration efficacy trials or any other commitment with any relevant regulatory authority to conduct a clinical study that would support the development of the Product. For the purposes of this Agreement, “Pivotal Study” shall mean a clinical study designed to fulfil the requirement for the filing of an application for a marketing authorization for a Product and that is acceptable to the relevant regulatory authority as a basis for the grant of a marketing authorization.
7.4The Awardee will:
a.be the sponsor of any clinical study, or ensure that a sub-awardee acting as local sponsor, fulfils all sponsor obligations as detailed below;
11 11
|
|
|
ANNEX A: TERMS AND CONDITIONS |
b.be responsible for obtaining and maintaining all regulatory approvals (including ethical committee approvals) necessary or reasonably useful for the conduct of the clinical trial and appropriate clinical trial insurance cover;
c.publish details of any clinical study in a publicly accessible clinical study register, where patient privacy is upheld, as required under law and, as applicable, prior to the commencement of patient recruitment for such clinical study;
d.ensure that any informed consent form permits the use of project results described in these T&Cs and in the IPDP;
e.establish a data safety monitoring board (DSMB);
f. notify the JMAG in writing immediately following any safety issues or similar events;
g.verify that the clinical study data are complete and include all completed case report forms and all other clinical study documentation required to be in the possession of a clinical trial sponsor by applicable law; and
h.subject to the confidentiality provisions contained in clause
24.4, permit a CEPI representative or nominee (except for any matters that should remain blinded to CEPI in the interests of the integrity of the clinical study and except for closed sessions) to:
a.attend meetings of the DSMB for the clinical study as an observer (either in person or by electronic means); and
b. receive all papers that a member of the TSC (as defined in Clause
7.5a) or DSMB would be entitled to receive.
7.5In order to support the clinical studies funded by CEPI (whether in whole or in part), the awardee:
a.will establish a trial steering committee (TSC) or any other appropriate clinical oversight setting, as agreed between the Parties; and
b.shall use commercially reasonable endeavours to adhere to any further requirements under this Clause 7.5, as agreed between the Parties. Such requirements may be similar to and may include but not be limited to those requirements outlined under Clause 7.4 (f) and 7.4 (h) above.
8.Quality Requirements and Responsibilities
8.1Awardee shall ensure that all activities performed under this agreement shall be performed in accordance with all applicable safety, legal, ethical and regulatory authority requirements or standards, including the CEPI Third Party Code, any associated regulatory approval, clinical trials application and/or all applicable GxPs.
8.2During the term, awardee will:
8.2.1inform CEPI of any significant quality-related issues, events or changes that are reasonably likely to adversely affect the supply of Products to Non-Traveler’s Market Countries;
8.2.2within [***], notify CEPI of the outcome of GxP regulatory inspections and any material adverse quality findings and any critical quality events (including serious breaches, deviations, audit findings, breaches of data integrity etc.) that are reasonably likely to adversely affect the supply of Products to Non-Traveler’s Market Countries; and
|
|
|
ANNEX A: TERMS AND CONDITIONS |
8.2.3consider in good faith quality recommendations identified by CEPI relating to the Project as may arise throughout the course of the Term in respect of the supply of Products to Non-Traveler’s Market Countries.
8.3Quality-related disputes between the parties that are not resolved in the normal course of business shall be brought to the attention of the JMAG in writing. Both Parties shall use all reasonable endeavours to agree to a prompt resolution of the disagreement and agree to work jointly to develop a strategy for such solution. If the quality-related dispute relates to the LMIC Manufacturer, Awardee shall encourage the LMIC Manufacturer to work jointly with CEPI to develop a strategy for solution. The Parties shall record any such resolution in writing.
8.4Awardee shall, and shall use commercially reasonable endeavours to facilitate that the LMIC Manufacturers, permit CEPI, or its designee (subject to prior written consent by Awardee and/or the LMIC Manufacturer (as applicable) or such designee, not to be unreasonably conditioned, withheld or delayed), to conduct a detailed due diligence assessment of the relevant party’s quality systems on-site, provided such site visits shall be restricted to [***] visit per manufacturer each [***] during the Term. CEPI shall cause its designee to enter into a reasonably acceptable confidentiality agreement with the relevant party obliging such designee to retain all such information in confidence pursuant to such confidentiality agreement. In the event that such assessment identifies any major or critical deficiencies in the relevant party’s quality system, Awardee shall, or shall obligate the LMIC Manufacturer to, take all actions reasonably necessary to correct such deficiencies.
8.5Pharmacovigilance meetings with sub-awardees. Awardee will keep CEPI informed about any official meetings between Awardee and Sub-Awardees, or Sub-Awardees and any regulatory authority, concerning significant Pharmacovigilance matters related to the Product. Awardee will schedule regular meetings with each Sub-awardee’s Pharmacovigilance Team and with CEPI’s Pharmacovigilance Team to share a routine safety update on the Product and to discuss any significant safety related issues, as applicable.
9.Animal and Toxicology Studies
9.1Animal Studies. If any work package includes studies using animals, such activities must comply with applicable laws as well as CEPI’s Animals in Research Policy.
9.2The awardee will:
a.obtain and maintain all regulatory approvals (including ethical committee approvals) necessary or reasonably useful for the conduct of research involving animals;
b.share details of its animal and toxicology protocols with CEPI through the JMAG meetings and provide CEPI with a draft of each trial protocol for any animal or toxicological studies it intends to conduct prior to their initiation and will consult with and consider any reasonable suggestions made by CEPI; and
c.inform JMAG of any anticipated deviations from the original design of animal studies described in the IPDP and obtain JMAG approval before implementing those changes.
10.Standards and Assays
10.1Standards Development. If any work package relates to the development of biological reference materials, Awardee will provide relevant materials and data and shall grant rights to their use for International Standards development, to one of either the WHO or the Paul-Ehrlich-Institute (PEI) in Germany or, if agreed by the Parties, another independent standards development agency.
|
|
|
ANNEX A: TERMS AND CONDITIONS |
10.2Assay Development. A work package may include the development of new or improved assays (including immunogenicity and potency/release assays), as will be described in the IPDP.
10.3CEPI Service Providers. CEPI has entered into certain service agreements with CEPI service Providers that have agreed to provide preferential charging to CEPI Awardees. CEPI may make available various laboratory services or other support to Awardee provided by a CEPI Service Provider, for example by providing testing of clinical serum samples, evaluation of immunity of Product in animal models and various analytical services. Awardee may utilise any CEPI Service Provider for the provision of services as may be, and solely if and to the extent, specified in a Work Package and agreed in writing between the Parties. Awardee and the CEPI Service Provider may, at their own discretion, enter directly into an appropriate agreement between themselves setting out the terms on which the services will be provided. CEPI shall, through the JMAG or otherwise, discuss with Awardee protocols and data management related to any services provided by any CEPI Service Provider.
10.4The Awardee will:
a.as described in the IPDP, participate in collaborative interlaboratory studies for evaluation of a candidate reference material. Such studies ultimately will be included in reports to the WHO Expert Committee on Biological Standardization; and
b.provide written standard operating procedures (“SOPs”) for any assays developed and qualified with CEPI funding (in whole or in part) or with the use of samples or biological material facilitated by CEPI. Transfer capacity and technology relating to such assays to a designated, independent third party laboratory if required by CEPI for the assay to be validated for Phase 3 clinical trials. If and to the extent any SOPs incorporate Trade Secret Information or Confidential Information within Awardee Background IP, CEPI will maintain the confidentiality of such information in accordance with Clause
24.4 and Awardee and the designated third party laboratory shall first enter into a customary confidentiality agreement with Awardee governing the use and non-disclosure of such information, provided that Awardee and such third party laboratory shall not delay the execution of such agreement.
11.Regulatory Activities
For the purposes of this section, where an LMIC Manufacturer will be undertaking regulatory activities for a particular Product, Awardee will use commercially reasonable endeavours to ensure that the relevant LMIC Manufacturer adhere to the obligations applicable to Awardee, mutatis mutandis.
11.1Meetings with Regulatory Authorities. Awardee will keep CEPI informed about any product related official meetings with regulatory authorities (including WHO-PQ). In case Awardee is participating in the meeting with the relevant regulatory authority, the Awardee shall provide an opportunity to have a CEPI representative in the meeting as a silent observer, provided the regulatory authority and the LMIC Manufacturer so accept. At CEPI’s reasonable request, Awardee will request a meeting with regulatory authorities to address any significant unresolved issues.
11.2Regulatory Filings. Awardee will regularly inform CEPI regarding its, and the LMIC Manufacturer’s, regulatory strategy for the Product and will inform CEPI of any discussions with regulatory authorities that may have a significant impact on future development of the Product through the bilateral and multilateral meetings referred to in the Team Charter. Awardee will provide CEPI with copies of:
11.2.1key regulatory filings and submissions in respect of the Product, including M1 and M2 of the Common Technical Document (CTD), clinical study reports, proposed product specifications and product label information;
|
|
|
ANNEX A: TERMS AND CONDITIONS |
11.2.2official meeting minutes of meetings between the regulatory authority and Awardee/LMIC Manufacturer relating to the Product, and any written communications from the regulatory authority on significant matters related to the Product which may impact the Project.
11.3Cross Referencing. Awardee will co-operate with CEPI to allow CEPI to cross-reference specific parts of Awardee’s Project Results in order to support regulatory pandemic preparedness, to be further discussed and agreed upon between the Parties.
11.4Regulatory Approvals in LMICs. Awardee will ensure the LMIC manufacturer will use commercially reasonable endeavours to obtain regulatory approvals and licensure for the Product in Non-Traveler's Market countries where there is a demand for the Product. The Parties, through the JMAG, may discuss and agree on a list of such Non-Traveler's Market countries in which to seek such approvals and licensure and on a schedule for seeking such approvals and licensure, and Awardee will, or will obligate its Sub- Awardee(s) to, use commercially reasonable endeavours to meet such schedule in such countries.
11.5WHO Prequalification. Awardee will ensure that the LMIC manufacturer will use commercially reasonable endeavours to obtain WHO prequalification for the Product.
12.Project Results and their Ownership
12.1Project Results. The “Project results”, meaning the outcomes and results of the project, may comprise biological samples, data, intellectual property, materials, any Product and Investigational Product, publications, reference standards, technology and other results and shall include all Project IP, Project Data and Project Materials.
12.2Ownership of Project Results. Awardee will own the project results.
12.3The awardee will:
a.share with CEPI, subject to the confidentiality provisions contained in clause
24.4, all significant Project Results as soon as is practical;
b.record project results accurately, completely and reliably in awardee’s IPDP records; and
c.identify project results in the IPDP reports provided to the JMAG.
13.Dissemination and Publication of Project Data
13.1Reporting of Project Data. Subject to the confidentiality provisions contained in clause
24.4, Awardee shall provide CEPI with access to all data and information, including all pre-clinical and clinical study data, produced or arising as a result of the Project (“Project Data”), and will report Project Data regularly to the JMAG. Notwithstanding the foregoing, with respect to Project Data produced or arising as a result of any Awardee-Funded Study, Awardee shall provide summaries of such Project Data to the JMAG and, at CEPI’s request (including through CEPI’s members of the JMAG), Awardee shall provide additional information and details relating to such Project Data as reasonably requested by CEPI.
13.2Sharing of Project Data with the Research Community. Awardee will share with the research community Project Data relevant to topics of interest to the research community, such as disease-specific assays, animal models, correlates of protection or diagnostics and epidemic preparedness mechanisms, as described in the IPDP and agreed in the JMAG, subject to the Awardee’s right, prior to such Project Data entering the public domain, (i) to remove Trade Secret Information and Confidential Information within Awardee Background IP, if any, included in such Project Data and (ii) in case there is any patentable subject matter included in such Project Data, to delay such Project Data entering the public domain for a reasonable period of time, not to exceed [***].
|
|
|
ANNEX A: TERMS AND CONDITIONS |
13.3Publication of Project Results. CEPI encourages awardee’s timely publication of project data and other Project Results, including pre-clinical studies, in scientific publications and manuscripts, congress posters and congress presentations. No less than [***] prior to submission of any such proposed publication, Awardee shall submit such publication to CEPI for review. In the event that CEPI has any comments on the proposed publication, Awardee shall cooperate with CEPI in good faith to incorporate CEPI’s comments prior to publication. All such publications (other than publications that relate exclusively to any Awardee-Funded Study) shall include a statement that the work was “co-funded by the European Union and CEPI” (translated into local languages, where appropriate) and, provided the relevant publisher accepts, shall display the European flag (emblem):
Co-funded by the European Union
With respect to publications relating to clinical trials other than the Awardee-Funded Studies, Awardee shall credit where appropriate the country in which the clinical trials were performed and make the results of such clinical trials available to the relevant country’s Ministry of Health or equivalent. In the event CEPI wishes to publish any Project Results, CEPI shall submit such proposed publication to Awardee for review no less than [***] prior to submission for publication and if, within [***] after receipt of such proposed publication, (i) Awardee notifies CEPI of specific content in such proposed publication that constitutes Trade Secret Information or Confidential Information within Awardee Background IP, then CEPI shall remove such specific content from the proposed publication, or (ii) Awardee notifies CEPI that there is patentable subject matter contained in such proposed publication, CEPI shall delay submission of the proposed publication for a reasonable period of time requested by Awardee, not to exceed [***].
13.4Additional requirements for communication and dissemination. Any communication or dissemination activity (i.e. publications, manuscripts, congress posters and congress presentations) related to the Project must use factually accurate information. Moreover, it must indicate the following disclaimer (translated into local languages, where appropriate): “Co-Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or Horizon Europe. Neither the European Union nor the granting authority can be held responsible for them.”
13.5Clinical Study Data. CEPI’s clinical trials policy requires that clinical data and results (including negative results) must be disclosed publicly in as close to real time as possible. Accordingly, such data and results must be shared through an easily discoverable public route (website or system) that includes a metadata description, where patient privacy is upheld, and the system follows a request- for-information approach (where requests are fulfilled subject to an independent review and approval step). Clinical study data will be submitted for publication within [***] after each final study report or report submitted to CEPI unless Awardee has reasons for a delay of the publication of the clinical study data and said delay is agreed in writing with CEPI. The Clinical Trial ID or registry identifier code/number shall be included in all publications of clinical trials. Notwithstanding the foregoing, the terms of this Clause 13.5 shall not be mandatory with respect to clinical data and results arising from any Awardee-Funded Study.
|
|
|
ANNEX A: TERMS AND CONDITIONS |
13.6Outbreak-Related Publications. Additionally, project data will be shared in accordance with WHO’s 2016 Guidance for Managing Ethical Issues in Infectious Disease Outbreaks and WHO’s 2016 Guidance on Good Participatory Practices in Trials of Interventions Against Emerging Pathogens.
13.7Open Access. CEPI requires “Open access” for project data. this means that a copy of the final manuscript of all research publications, journal articles, scholarly monologues and book chapters published under this Clause
13 must be deposited into PubMed Central (or Europe PubMed Central) or otherwise made freely available upon acceptance for publication or immediately after the publisher’s official date of final publication. Moreover, all peer-reviewed published research that is funded, in whole or in part, by CEPI shall be published in accordance with the principles of “Plan S” - Accelerating the transition to full and immediate Open Access to scientific publications, a UK and European data sharing initiative for research funded by public grants.
13.8The awardee will:
a.notify the JMAG on an ongoing basis as project data is produced and disseminated in accordance with Clause
13.1;
b.disseminate project data consistent with the requirements set out above in this clause
13; and
c.cooperate in regard to data analysis, to the extent relevant under a given work package, by CEPI’s Assessors, subject to Clause
24.4, by:
i.providing data or other information generated under this Agreement to CEPI’s designated Assessor as CEPI may reasonably request, including data regarding the results of any of its pre-clinical or clinical trials (duly anonymized and, upon CEPI’s request, blinded);
ii.providing CEPI’s designated Assessor with other data (duly anonymised and, upon CEPI’s request, blinded) as CEPI may reasonably request in order to conduct comparative assessments; and
i.providing CEPI’s designated Assessor with clinical study data (duly de-identified and, at CEPI’s request, blinded) for the purposes of signal detection or meta-analyses of safety data (including across candidate vaccines).
14.Dissemination of Project Materials
14.1Dissemination and Sharing of Project Materials. Awardee will share with CEPI project materials produced under the Project. CEPI undertakes to keep the Project Materials confidential in accordance with the terms of Clause
24.4. For purposes of this Agreement, “Project Materials” means the drug product and the clinical trial materials described in Clause
14.4 (c) (ii). For clarity, “Project Materials” shall not include any intermediates or assays relating to the manufacturing process.
14.2Comparator Samples. The awardee will use commercially reasonable endeavours to make a limited amount of samples of marketed Product and/or Product in use under emergency use license available as comparator for other research in the Field, if reasonably justified by requestor under terms and conditions (including but not limited to the scope of research, protocol and reporting) agreed between the Parties, with the spirit to advance research in the Field.
14.3Comparative Evaluation of Samples. CEPI may engage one or more independent third party laboratories or collaborators (“Assessors”) (which may include but is not limited to the Task Force for Global Health and its Safety Platform for Emergency vACcines (SPEAC Project)) to perform additional testing on Project Materials as specified under Clause 14.4c, at CEPI’s expense, in order to provide CEPI with directly comparable evaluations of similar materials produced under CEPI’s portfolio of awarded projects. All such Assessors shall be bound by confidentiality obligations at least as stringent as those contained in Clause 24.4. CEPI shall inform Awardee through the JMAG about potential Assessors prior to their engagement by CEPI. CEPI may not engage Awardee Competitors as Assessors without Awardee’s consent, such consent not to be unreasonably withheld, delayed or conditioned. Awardee shall have the right to veto the engagement of an Awardee Competitor that CEPI seeks to appoint as an Assessor. CEPI may appoint any other third party as an Assessor provided that, if Awardee raises reasonable objections to the appointment of an Assessor (other than an Awardee Competitor), the matter shall be submitted to the JMAG for decision. If the JMAG is unable to decide, then the escalation process according to Clause 23.1 shall apply. CEPI may, in its sole discretion and at its own expense, also engage certain independent third party entities to transport the samples from Awardee to the Assessor, address import/export issues, or provide any documentation CEPI may determine is required for such samples. The results of the testing, analysis, meta-analysis or other assessments (“Results”) will be subject to the confidentiality obligations under this Agreement. CEPI will provide to the Awardee the Results as are relevant to Awardee’s activities under the Project. In no event will CEPI publish or otherwise disclose any Results without Awardee’s consent, such consent not to be unreasonably withheld, delayed or conditioned.
14.4The awardee will:
a.notify the JMAG on an ongoing basis as project materials are produced under the IPDP;
b.disseminate and share project materials consistent with the requirements set out above in this Clause
14; and
c.cooperate with CEPI’s assessor, to the extent relevant under a given work package, subject to Clause
24.4, by:
i.providing CEPI’s designated Assessor a reasonable number of doses of a candidate vaccine (Product) representative of the final Product, for animal immunogenicity studies;
ii.providing CEPI’s designated Assessors with an agreed number of biological samples from clinical studies under the Project funded by CEPI and provided such clinical studies collects biological specimen as samples, (excluding 1) the Awardee-Funded Study and 2) the completed Phase 3 clinical study VLA1553-301) for use in future research carried out by or on behalf of CEPI including agreed volumes of biological samples (for example, serum, and peripheral blood mononuclear cells (PBMCs)) from human participants vaccinated with the Project vaccines (excluding subjects vaccinated in Awardee’s Phase 1 clinical trial completed prior to the Effective Date or in the Awardee-Funded Study) at specified timepoints agreed with CEPI for immunology testing; and
iii.ensuring that any samples to be transferred or exported by or on behalf of Awardee from a clinical trial site or sample storage site are transferred and/or exported pursuant to the terms and conditions of a suitable to-be-agreed-upon material transfer agreement (containing, among other terms, confidentiality and use restrictions) to be entered into between Awardee and the Assessor in addition to any other applicable laws and regulations.
15.Intellectual Property
15.1Protection for Project IP. Awardee has the right, but not the obligation, to seek protection, at its own cost, for the discoveries, inventions, know-how, patents, trademarks and other forms of intellectual property that arise under the Project (“Project IP”).
15.2Third Party Patents. The parties will notify each other promptly regarding any third-party intellectual property they become aware of that raises concerns about Awardee’s ability to perform its obligations under this Agreement, including the Equitable Access Plan, or the potential use by CEPI of the Public Health License described in Clause
19. The Parties will cooperate in good faith to resolve any such matters.
15.3The awardee will:
a.notify the JMAG as Project IP is created, discovered or made; any applications for any rights to Project IP are submitted or are otherwise prosecuted; any application regarding the registration of any Project IP is granted, including the granting of any patent or trade mark, as part of its regular IPDP reports; and
b.ensure that it has enforceable policies or written agreements with all of its employees, agents and subcontractors which assign to the Awardee ownership of all Project IP.
16.Equitable Access
16.1Equitable Access. CEPI is committed to achieving equitable access to the outputs of all CEPI- supported programmes, including access to all applicable Project Results in accordance with this Agreement, pursuant to CEPI’s “Equitable Access” Policy. Equitable Access to Chikungunya vaccines means the regular supply of the Product(s) to public health systems in all Non-Traveler’s Market Countries that have a demand for the vaccines at an affordable price (as outlined in Clause 16.4) and, in the context of an Outbreak or Increased Outbreak Preparation Need means that appropriate vaccines are first available to populations in the Affected Territory when and where they are needed, including to end an Outbreak or curtail an epidemic, regardless of ability to pay. Consistent with CEPI’s Equitable Access Policy, CEPI is also committed to supporting Equitable Access so that the economics are sustainable to the manufacturer.
16.2Equitable Access Plan. The initial plan to support such Equitable Access commitment (the “Equitable Access Plan”) is set out in Annex G and sets out among other things how the Product will be suitable for Non Traveler’s Market use and made available to all populations in Affected Territories without undue delay and at an affordable but sustainable price (subject always to Claus
e 16.4). This Equitable Access Plan shall be reviewed by JMAG no less than every [***] and shall take into account production [***] and shall be updated throughout the Term to reflect such reviews or as otherwise agreed between the Parties. The Parties agree that a more detailed Equitable Access Plan will be agreed promptly after the Equitable Access Group is established. The Awardee will keep the JMAG and, if established, the Equitable Access Group, fully and regularly informed of its adherence to the Equitable Access Plan and its progress, or lack thereof, in meeting its objectives.
16.3Equitable Access Group. Within [***] of the effective date the parties will establish an Equitable Access Group (the “Equitable Access Group”) that will meet regularly to monitor the progress of, and advance the Awardee’s commitment to, Equitable Access. The LMIC Manufacturer will be a member of the Equitable Access Group and will keep the other members regularly informed of its commitment to Equitable Access.
Within [***] of setting up the Equitable Access Group, the Parties will update the Team Charter to describe the organisation and management of the Equitable Access Group.
16.4Pricing: The parties recognize that the price of the product to public health systems in all non-Traveler’s Market Countries is critical to achieving Equitable Access. To the extent that Awardee, a Trusted Collaborator, any Sub-Awardee or LMIC Manufacturer commercializes the Product in public health systems in the Non-Traveler’s Market Countries which utilizes or otherwise benefits from, whether directly or indirectly, any Project Result, [***].
16.5Information about Production, Supply, Pricing and Sales.
16.5.1Upon written request by CEPI, Awardee will provide, and will procure that its Sub-Awardees, LMIC Manufacturers and Trusted Collaborator provides, reasonable information about its production, supply, pricing and sales of Product, including its audited financial statements sufficient to enable CEPI to evaluate whether such activities are consistent with Awardee’s obligations under this Agreement.
16.5.2Awardee shall ensure that its Sub-Awardee Instituto Butantan provides CEPI with updates on Sub-Awardee’s pricing discussions with relevant pricing authorities within Brazil and the other Non-Traveler’s Market Countries awarded to Butantan, including public sector procurement agencies such as AVAT, GAVI, , PAHO, and various Ministries of Health, as applicable. Further, Sub-Awardee shall share with CEPI any official pricing data submitted to CMED and CONITEC in Brazil and to other relevant pricing authorities outside of Brazil.
16.6Supply Commitment.
16.6.1Awardee shall ensure its Sub-Awardees, to the greatest extent possible, prioritise the supply of Product to public health systems in Non-Traveler’s Market Countries taking into consideration public sector demand, production capacity and contractual obligations existing prior to any public sector purchase agreements entered into in accordance with 16.6.2.
16.6.2Awardee shall ensure its Sub-Awardees shall use all reasonable endeavours to bid on applicable public sector tenders for the supply of Product in Non-Traveler’s Market Countries in time and at the price specified in the relevant tender (and [***]). Awardee shall ensure its Sub-Awardees use all reasonable endeavours to deliver the Product in accordance with the tender, or any subsequent purchase agreements with any public sector purchaser and meet the lead-time specified in the tender and/or purchase agreement.
16.6.3Nothing in this Clause 16.6 shall preclude Awardee and its Sub-Awardees from supplying
Product to the private market in Non-Traveler’s Market Countries as long as the Awardee or Sub-Awardee does not discriminate against the public health system in the Non-Traveler’s Market Countries in favour of the private market in such countries. In the event there is insufficient Product for Sub-Awardees to be able to fulfil orders to public sector markets, Awardee shall ensure its Sub-Awardees prioritise supply to the public market in accordance with Clause
16.6.1.
16.6.4Except in the event that Awardee has transferred the manufacturing processes for drug substance to Awardee’s Sub-Awardee Instituto Butantan and any potential future Sub-Awardee, Awardee shall use reasonable commercial endeavours to manufacture and supply drug substance in the quantities needed to meet public sector demand in Non-Traveler’s Market Countries for the Term and for [***] following the grant of marketing approval for VLA1555 by ANVISA.
16.6.5Within [***] of the expected launch date of each of VLA1555 and VLA1556, Awardee shall ensure that its Sub-Awardees develop and provide Awardee and CEPI with a launch readiness supply plan for Non-Traveler’s Market Countries.
16.6.6Awardee undertakes to ensure that no LMIC Manufacturer has or will have the right to supply Product in Awardee’s Traveler’s Market.
17.Sharing of Commercial Benefits
17.1Sharing of Commercial Benefits. CEPI has committed to its own funders to obtain a share of Awardee’s Commercial Benefits as a contribution to support CEPI’s programme activities.
17.2The Awardee will: ensure any LMIC manufacturer - other than Instituto Butantan who shall remain bound by the safety stock requirements agreed under the prior funding agreement between the Parties, effective as of 1 April 2019, as amended - shall make the following contributions to CEPI:
i.Until the rolling safety stock has been established by each LMIC Manufacturer in accordance with Clause
17.2(ii), Awardee will ensure that the LMIC Manufacturer, at its own cost, maintain and make available to CEPI an Investigational Product reserve stockpile of [***] doses of drug product
ii.Awardee will ensure the LMIC Manufacturer produces, at its own cost, a one-year rolling safety stock comprised of not less than [***] doses of Finished Drug Product within [***] of receipt of marketing approval for the Product from a competent regulatory authority having jurisdiction over the relevant LMIC Manufacturer. The stock in paragraph
17.2(i) and this paragrap
h 17.2(ii) is referred to as (“Safety Stock”).
iii.Awardee will provide details of the Safety Stock to the global Technical Advisory Group to monitor Global Virtual Pooled Inventory (“TAG-GVPI”) once the TAG-GVPI is established.
iv.In case of an Outbreak or Increased Outbreak Preparation Need, CEPI may utilize such Safety Stock in the Affected Territory by giving notice in writing to Awardee and Awardee will ensure the LMIC Manufacturer dispatches all or some only of the Safety Stock, as instructed by CEPI and CEPI shall pay any reasonable costs incurred in connection with the utilization of the Safety Stock, including but not limited to transportation, distribution and storage in the Affected Territory. For clarity, Awardee shall ensure the LMIC Manufacturer makes no charge for the supply of the Safety Stock allocated to and used by CEPI in accordance with this paragrap
h 17.2(iv) and the storage costs of such Safety Stock, incurred prior to dispatch to the Affected Territory, shall be borne by the LMIC Manufacturer.
v.If the Safety Stock is used by CEPI in the case of an Outbreak or Increased Outbreak Preparation Need, CEPI or such third parties as CEPI may nominate shall be responsible for the costs of transportation of such Safety Stock from the LMIC Manufacturer’s facility. If, following the use of the Safety Stock as directed by CEPI, CEPI wishes to replenish the Safety Stock, Awardee shall, or shall obligate its LMIC Manufacturers to, produce such quantities of Product as are required to replenish the Safety Stock and CEPI shall pay Awardee for the costs of the production of such Product.
18.Preparation for Outbreaks
18.1Outbreak. CEPI will notify awardee in writing in the event of an outbreak or if there is an increased Outbreak Preparation Need, in each case identifying the Affected Territory (“Outbreak Notice”). Once an Outbreak Notice has been provided by CEPI, CEPI shall have the right to direct how the Safety Stock referred to in Clause 17.2. or any Product manufactured pursuant to Clause 18.2 may be used and to whom it may be provided in the Affected Territory. In consultation with relevant public health authorities in the Affected Territory, CEPI may request that Awardee discuss in good faith whether and how the Project Results could be utilized in response to the Outbreak Notice. Awardee is committed to use commercially reasonable endeavours to address Outbreaks and Increased Outbreak Preparation Need wherever they occur in the world. Following receipt of an Outbreak Notice, Awardee will use its commercially reasonable endeavours to increase the supply of Product available for use by CEPI or its nominees to an amount which equals at least [***] of the production forecast for the Products prepared by Awardee immediately prior to service of the Outbreak Notice and Awardee will use its commercially reasonable endeavours to ensure that such increased capacity is available for delivery to CEPI within [***] of the date of service of the Outbreak Notice. For clarity, Awardee will use commercially reasonable endeavours to keep such deadline of [***] (including discussing with Awardee’s contract manufacturers how they can meet the proposed deadlines), however, Awardee’s ability to meet deadlines will be subject to the lead times of Awardee`s contract manufacturers and the time required for the release testing of the Product. In the event that CEPI’s request for Product to meet the increased demand during an Outbreak or Increased Outbreak Preparation Need is in excess of the quantities that Awardee is able to supply to CEPI based on Awardee`s commercially reasonable endeavours, Awardee shall not be obliged to supply Product to CEPI under this Clause 18.1 to the extent that the supply of such quantities of Product to CEPI would result in Awardee being in breach of any binding contracts in existence on the date of service of the Outbreak Notice (which for the avoidance of doubt may include the supply of Products to customers for Awardee’s Traveler’s Market or in connection with any clinical trials). In such event, provided that Awardee has supplied Product in accordance with this Clause 18.1, Awardee shall not be considered to be in default, and Clauses 18 and 19 shall not apply.
18.2Additional Product Development. Pursuant to an outbreak notice, CEPI may request that awardee undertake additional Product development at CEPI’s expense or undertake other activities, including the pursuit of regulatory approvals and licensure to the extent not already obtained, with the aim of addressing the needs of the Affected Territory. An additional Work Package covering these activities will be negotiated expeditiously and in good faith by the Parties.
18.3Multidose presentation. Awardee shall assist and support each LMIC manufacturer in the potential development of a multi-dose presentation of the Product.
18.4Additional Investigational Product or Product Stockpiles. In addition to the safety stock referred to in Clause
17.2ii., CEPI may request that Awardee undertake, at CEPI’s expense, the manufacturing and maintenance of an additional stockpile of Investigational Product or Product for use in or for the Affected Territory. Such Product may be used for further clinical trials in Outbreak conditions to advance vaccine development, or pursuant to an emergency use authorization, in each case in emergency situations based on national or international guidance (such as WHO), or in such other manner within an Affected Territory as CEPI may reasonably determine. An additional Work Package covering this activity will be negotiated expeditiously and in good faith by the Parties.
18.5Trusted Collaborator. promptly after receipt of a written request from (or at any earlier time), Awardee will propose a third party, for example, a Sub-Awardee, as a preferred alternative to itself (“Trusted Collaborator”), that is capable of performing the work and would be prepared to undertake activities pursuant to Clause
18.2 or
18.4 in the event that Awardee declines CEPI’s request to do so, or if Awardee and CEPI do not reach agreement on a new Work Package. CEPI may also propose a Trusted Collaborator to Awardee. Neither Party may unreasonably decline to accept the designation of a proposed Trusted Collaborator.
18.6Technology Transfer. As described in the IPDP, awardee will be transferring technology to LMIC Manufacturers. Awardee will promptly and diligently provide all necessary guidance, information, materials and assistance reasonably required to transfer Awardee’s technology to each such LMIC Manufacturer as outlined in the IPDP. Pursuant to an Outbreak Notice, CEPI may request to accelerate the timelines for transfer of Awardee’s technology to one or both of such Sub-Awardees and/or CEPI may request an expansion of the transfer to another Trusted Collaborator (other than such LMIC Manufacturers) if that would achieve the transfer more quickly. If CEPI requests transfer of Awardee’s technology to another Trusted Collaborator, Awardee will promptly and diligently provide all necessary guidance, information, materials and assistance reasonably required by such Trusted Collaborator to accomplish the activities that may be requested by CEPI under Clause 18.2 or 18.4 (“Technology Transfer”) at CEPI’s cost. Awardee shall carry out the Technology Transfer to such other Trusted Collaborator pursuant to the terms and conditions of a to-be-agreed-upon confidentiality agreement in accordance with this Agreement to be entered into between Awardee and the Trusted Collaborator governing the Trusted Collaborator’s use and non-disclosure of information and materials provided in connection with the Technology Transfer, provided that Awardee and the Trusted Collaborator shall not delay the execution of such agreement.
18.7The Awardee will: use commercially reasonable endeavours to cooperate with CEPI in developing a response to an Outbreak or Increased Outbreak Preparation Need which may include opportunities for Awardee and its Sub-Awardees to receive additional Work Packages and funding from CEPI. CEPI is engaging with global stakeholders and in the future, it is likely that Outbreak response will be coordinated by a global entity. In such event, Awardee shall use all reasonable endeavours to collaborate with such entity and to comply with its requirements concerning the Outbreak.
18.8Outbreak in Awardee`s Traveler`s Market. Notwithstanding anything to the contrary herein, in the event any country in the Awardee’s Traveler’s Market is included in the Affected Territory, Clauses
18 and
19 shall not apply to such country in the Awardee`s Traveler`s Market on the condition that Awardee shall, at the request of public health agencies in such country in the Awardee’s Traveler’s Market, supply the Product to all such public health agencies that request the Product in a quantity and at a price as agreed with the relevant public health agencies. The price agreed with the relevant public health agency shall not exceed the lowest supply price of the Product for similar volumes of Product agreed by Awardee with any customer in the Affected Territory in the [***] preceding the receipt of the Outbreak Notice by Awardee. For purposes of this Clause
18.8, “similar volume” shall mean a volume within the range of [***]. For clarity, if Awardee fails to comply with the foregoing supply obligation with respect to any country in the Awardee’s Traveler’s Market that is included in the Affected Territory, the terms of Clauses
18 and
19 shall apply to such country in the Awardee’s Traveler’s Market that is included in the Affected Territory. However, if the reason why Awardee cannot comply with the supply obligation is that (i) the quantity of Product requested by the relevant public health agency is impossible to fulfil due to Awardee´s capacities or (ii) the price proposed by the public health agency would be unsustainable to Awardee, Clauses
18 and
19 shall not apply in such case. In any case, “sustainable price” shall never be below Awardee`s manufacturing costs.
26
19.Public Health License
19.1Grant of a Public Health License. Awardee hereby grants the public health license to CEPI (subject to Clause
18.8), on the condition that CEPI may only exercise the rights granted under the Public Health License in the following circumstances:
a.awardee’s activities supported by CEPI under the project have meaningfully advanced the Product; and
b.the awardee has not notified CEPI that it wishes to terminate the agreement pursuant to clause
22.2; and
c.one or more of the triggers set out in clause
19.2 has occurred.
19.2Public Health License Triggers. Consistent with clause
19.1, CEPI’s right to exercise the public Health License will be triggered when:
a.awardee or trusted collaborator declines to participate in activities requested by CEPI under Clause
18.1 or
18.2;
b.CEPI determines, in good faith and having taken expert advice (for example from a sub-group or subcommittee of CEPI’s Scientific Advisory Committee that CEPI determines has appropriate expertise), that Awardee or Trusted Collaborator will not be able to perform the activities under Clause
18.1 or
18.2 if requested by CEPI;
c.awardee is in material breach of this agreement or the equitable access plan;
d.[***] have passed since an outbreak notice in accordance with clause
18.1 and the Parties including Trusted Collaborator have not signed an agreement or new Work Package for the activities contemplated under Clause
18.1 or Clause, as applicable, despite CEPI’s request; or
19.3Agreement with Trusted Collaborator. In the event that the public health license becomes exercisable in accordance with Clause
19.1, CEPI may endeavour in good faith to reach agreement with a Trusted Collaborator to perform such activities as CEPI may deem necessary. If despite CEPI’s good faith efforts those negotiations do not result, or CEPI reasonably deems that such negotiations are unlikely to result, in an agreement on a timely basis, then CEPI may grant rights under its Public Health License to a third party unilaterally designated as a Trusted Collaborator by CEPI.
19.4The awardee will:
a.identify enabling rights to CEPI as of the signature date of this agreement and provide updates to the JMAG regarding the Enabling Rights during the course of the Project;
b.promptly provide to CEPI an updated list of enabling rights and project results in the event that the Public Health License becomes exercisable;
c.make no encumbrances regarding ownership or access to project results or enabling rights that would conflict or interfere with the Public Health License without the express written permission of CEPI, such permission not to be unreasonably withheld, conditioned or delayed;
d.upon exercise of the public health license by CEPI, promptly and diligently make available to CEPI all guidance, information, Project IP, Enabling Rights, materials and assistance reasonably required to accomplish the Project activities identified by CEPI.
20.Warranties
20.1Warranties. As of the date of signature of this agreement, Awardee warrants that the following statements (“Warranties”) are true and correct:
a.it has the full power and authority to enter into and assume its obligations under this agreement;
b.it is in material compliance with all statutes, regulations, directives and requirements of any governmental entity that relate to its activities and obligations;
c.to the best of its knowledge and belief, it does not infringe, misappropriate or violate the intellectual property, privacy or publicity rights of any third party that are relevant to the Project;
d.it has not granted rights to any third party in respect of project results (other than in accordance with the terms of this Agreement);
e.to the best of its knowledge and belief, no person has any right or claim to any payment or other compensation in respect of the use or exploitation of the Project Results, except as set out in pre-existing or contemplated licence agreements with third parties, copies of which have been provided to CEPI prior to the date of signature of this Agreement;
f.to the best of its knowledge and belief, none of awardee and its sub-awardees, nor any officer or employee of the foregoing has been debarred or is subject to debarment by a regulatory authority or funding agency anywhere;
g.it is not a restricted party; in breach of sanctions; or subject to or involved in any complaint, claim, proceeding, formal notice, investigation or other action by any regulatory or enforcement authority or third party concerning any Sanctions;
h.none of the funds provided under this agreement (whether via a sub-contract, sub-grant or otherwise) are used in any way directly or indirectly to provide support, resources, assets or any other benefit to, a Restricted Party in a manner that would violate Sanctions;
i.all financial statements and budgets submitted to CEPI as of the date of signature of this Agreement are true, complete and accurate;
j.to the best of its knowledge and belief, all encumbrances have been disclosed that could affect CEPI’s use of the Public Health License; and
k.the pre-award costs that CEPI has agreed to fund commencing on the pre-activities start date and included in the Budget have been performed in accordance with (i) the CEPI Policies and Procedures agreed upon in the prior funding agreement between the parties, effective as of 1 April 2019, as amended and (ii) the principles and requirements contained in the Third Party Code, as agreed between the Parties in the Declaration dated 30 September 2019.
20.2The Awardee will: undertake during the term of this agreement that all of the statements warrantied above will remain true and correct, and shall notify CEPI promptly in the event that this changes.
21.Indemnification and Insurance
21.1Awardee Indemnification for Third Party Claims. Awardee will indemnify and defend CEPI, its Affiliates, third party contractors and employees from and against any and all claims, damages, and liabilities asserted by third parties (including claims for negligence) which arise directly or indirectly from: (i) Awardee’s, or its Sub-Awardee’s activities under this Agreement, or (ii) the use of the Product, Project Results or Enabling Rights (including for the avoidance of doubt, the use of the Product in development activities and clinical studies), save to the extent such claim, damage or liability is caused by CEPI’s negligence or intentional misconduct or is required to be indemnified by CEPI pursuant to Clause
21.2.
21.2CEPI Indemnification for Third Party Claims. Solely in the event that CEPI has exercised the public Health Licence, CEPI will indemnify and defend Awardee, its Affiliates, Sub-Awardees, third party contractors and employees from and against any and all claims, damages, and liabilities asserted by third parties (including claims for negligence) which arise directly or indirectly from the use of the Product, Project Results or Enabling Rights by CEPI or a Trusted Collaborator designated by CEPI in the course of exercising the Public Health Licence, save to the extent such claim, damage or liability is caused by Awardee’s or its Sub-Awardee’s activities under this Agreement (including manufacture of drug substance or Product) or by Awardee’s negligence or intentional misconduct.
21.3Conduct of Responses to Third Party Claims. Each party shall use its reasonable endeavours to inform the other Party promptly of any circumstances that are likely to give rise to a third party claim which may be covered by Clause
21.1 or Clause
21.2 together with copies of all relevant papers and official documents. The indemnifying Party shall not take any material action in respect of any third party claim without the consent of the indemnified Party, including settlement of any such third party claim, provided such consent is not unreasonably conditioned, withheld or delayed. The indemnifying Party will keep the indemnified Party fully informed of the progress of all relevant third party claims which are covered by Clause
21.1 or Clause
21.2 and shall fully consult the indemnified Party on the nature of any defence to be advanced in advance.
21.4Exclusions. Neither party shall be liable to the other party for any loss of profits or economic loss; or indirect, incidental or consequential damages, whether in contract, warranty, negligence, tort, strict liability or otherwise, arising out of any breach of or failure to perform any of the provisions of this Agreement.
21.5Liability Cap. CEPI’s maximum liability in aggregate to awardee arising out of this agreement shall not exceed the aggregate of the total Work Package budget unless CEPI has exercised the Public Health Licence in which event CEPI’s maximum liability to Awardee arising out of this Agreement shall not [***]. Awardee’s maximum liability in aggregate to CEPI arising out of this Agreement shall not exceed the greater of:(a) the aggregate of the actual funding received from CEPI up to the date on which the claim is brought against Awardee or (b) Awardee’s total insurance coverage as set out in Clause
21.8.
21.6Exclusions from Liability Cap. Notwithstanding the foregoing, nothing in this agreement shall limit the liability of either Party in respect of:
a.personal injury or death arising out of that party’s negligence or intentional misconduct; or
b.fraud or fraudulent misrepresentation.
21.7Clinical Studies by CEPI under the Public Health License. In the event that the public health License becomes exercisable and CEPI intends to exercise such rights, CEPI will procure insurance protection consistent with the requirements for Awardee below.
21.8The awardee will:
a.satisfy the indemnification obligations arising under this clause
21;
b.obtain and continuously maintain, until [***] after completion of the project, insurance on a claims-made basis with an insurance company of a credit rating of A or better to cover reasonably foreseeable claims that may arise in connection with its activities under the Project;
c.if awardee is the sponsor of a clinical trial pursuant to this agreement, it will obtain and will ensure that any Sub-awardee that is the sponsor of a clinical trial will obtain, clinical trial insurance on a claims-made basis pursuant relevant local guidelines for the country in which the clinical study is conducted. Such insurance is to be effective from the commencement date of the clinical study until five (5) years after completion of the clinical study;
d.without limiting the foregoing, awardee shall maintain the following insurance coverage: general Third Party and Products Liability Insurance limited to [***].
e.if requested by CEPI, awardee will:
i.ensure that the insurer records CEPI’s interest on each such insurance policy;
ii.provide CEPI with a copy of each such certificate of insurance and annually on renewal;
iii.notify CEPI of any claims made under these policies relating to the subject matter of this Agreement during the Term and for at least the duration of any applicable statutory period of limitation afterwards; and
iv.comply with the terms of these insurance policies for the Term and for at least the duration of any applicable statutory period of limitation afterwards.
22.Term and Termination
22.1Term. this agreement shall commence on the effective date identified in the agreement summary and will continue in full force and effect until the activities set out in the IPDP and all agreed Work Packages have been completed, or as otherwise terminated pursuant to this Clause
22 (the “Term”).
22.2Termination for Default. If either party (the “Defaulting party”):
a.breaches a material obligation in this agreement and either fails to cure that breach within a cure period of [***] (or longer time agreed in writing) after notice from the other Party (the “Terminating Party”) or if that breach is not capable of cure; or
b.makes any arrangement with its creditors, resolves to or undergoes any insolvency proceeding anywhere in the world (except for the purpose of solvent amalgamation or reconstruction);
then the Terminating Party may terminate this Agreement by giving written notice of termination to the Defaulting Party effective immediately or at the end of any cure period if later.
22.3Additional CEPI Termination Rights. In addition to clause
22.2, CEPI shall be entitled to terminate this Agreement with immediate effect, unless otherwise indicated below, by providing written notice to Awardee in the following circumstances:
a.if following escalation to the senior officers pursuant to the process referred to in clause
23.1 (for clarity, excluding submission to arbitration), CEPI reasonably determines, in good faith, that Awardee is unable or will become unable to discharge its obligations under this Agreement, for example if key personnel or technology resources required for successful completion of the Project become unavailable to Awardee, and Awardee does not promptly and reasonably alleviate CEPI’s concerns;
b.there are safety, regulatory or ethical issues with continuing the project, as reasonably determined by CEPI;
c.if awardee has committed fraud or a financial irregularity. for the purposes of this clause “Financial Irregularity” includes any and all kinds of corruption, including bribery, nepotism and illegal gratuities; misappropriation of cash, inventory and all other kinds of assets; and making fraudulent financial and non-financial statements to CEPI;
d.awardee does not satisfy the criteria in clause 4.5 required for CEPI to pay funding tranches under the Project and fails to satisfy those criteria in full within a cure period of [***] (or longer time agreed in writing) after written notice from CEPI; or
e.any material changes or amendments are made to the IPDP (including awardee’s traveler’s Market Development Plan) without CEPI’s prior written consent.
22.4Effects of Termination. in all termination events:
a.CEPI will not be required to make any further payments to awardee under this agreement or any Work Package other than to reimburse Awardee for any non-cancellable expenses incurred in accordance with the Work Package in accordance with
Schedule B;
b.awardee will return any CEPI funds which are unspent at the date of termination within [***] of the date of termination;
c.each party shall return or destroy, as requested by the other party, the confidential information of the other Party except (i) CEPI may retain the Project Results subject to the obligations of confidentiality set out in Clause
24.4, (ii) each Party may keep one (1) copy of such Confidential Information for monitoring compliance and, (iii) solely in the event that the Public Health License has been exercised, CEPI may retain such other Confidential Information which embodies the Enabling Rights as may be required by CEPI to exercise and benefit from the Public Health License. Neither Party shall be required to delete copies of Confidential Information stored on automatic electronic backup systems;
d.if there is an on-going clinical study funded by CEPI (whether in whole or in part), unless Awardee decides in its sole discretion to continue such clinical study at Awardee`s cost or unless agreed otherwise by the Parties in writing, Awardee will ensure that no additional trial subjects are enrolled and the Parties will work together to plan and implement a wind-down of the study in an orderly fashion, with due regard for patient safety and the rights of any participating subjects; and
e.the parties will give effect to the relevant termination or expiration obligations described in
22.5Survival of Rights and Identified Clauses. Termination of this agreement shall be without prejudice to the rights and duties of either Party accrued prior to termination. The following sections will continue to be enforceable notwithstanding termination or expiration: Clauses
2.5c.,
2.5d.,
4.9d.,
5.3, 15, and
21 – 24, as well as any other provision, which by its nature, is intended to survive termination.
22.6 The parties will:
a.perform all acts necessary to comply with the relevant effects of termination described above; and
b.honour the rights and duties that survive termination.
23.Resolving Differences
23.1Escalation process. Any question, difference or dispute which may arise concerning the construction, meaning or effect of this Agreement, or concerning the rights or liabilities of the Parties hereunder, or any other matter arising out of or in connection with this Agreement shall first be submitted to the Chief Executive Officer of CEPI and to the Chief Executive Officer of the Awardee (the “Senior Officers”) for resolution (each of whom may call on others to advise them as they see fit). The Senior Officers shall discuss the matter arising in good faith and in a timely manner and endeavour to reach a mutually agreeable solution. If the Parties are unable to resolve such dispute through such negotiations within [***] of such dispute being escalated to the Senior Officers, then in respect of any dispute, controversy or claim the Parties irrevocably submit to arbitration in accordance with Clause
23.2.
23.2Arbitration. Any disputes to be resolved by binding arbitration pursuant to clause
23 (including any question regarding its existence, validity or termination or this Agreement), shall be referred to and finally resolved by arbitration under the Rules of the London Court of International Arbitration, which Rules are deemed to be incorporated by reference into this Clause. The number of arbitrators shall be one. The seat, or legal place, of arbitration shall be London, England. The language to be used in the arbitral proceedings shall be English. Notwithstanding the foregoing, any Party may seek specific performance, interim or final injunctive relief or any other relief of similar nature or effect in any court of competent jurisdiction.
23.3Public Health License. If CEPI invokes its rights under a public health license, then the parties will pursue an expedited resolution of any differences under Clause
23 within [***]. However, because of the exigent circumstances when there is an Outbreak, Awardee agrees that CEPI may proceed under a Public Health License, but Awardee retains its right to seek injunctive relief in addition to any other rights or remedies it may have under this Agreement, at law or in equity.
23.4The Parties will: cooperate in good faith to resolve differences and disputes pursuant to this clause
23.
24.General Provisions
24.1Defined Terms. The terms defined in these T&Cs shall have the meaning explicitly ascribed to them.
24.2Announcements. The parties will agree in writing upon the form of all press releases and public announcements concerning this Agreement except that:
a.either may disclose a description of the project subject to the confidentiality provisions of Clause 1.4 as well as the names of participating organizations and investigators;
b.CEPI may publish the summarized progress and outcomes of the project (provided that the confidentiality provisions of Clause
24.4 shall apply, except to the extent that such publication is made in accordance with the procedures of Clause
13.2 and
13.3), a summary of the terms and conditions of this Agreement, the name of Awardee and the Project Lead, and the amount of the CEPI funding; and
c.as required by law or any competent regulatory authority.
24.3Assignment. Neither party will, without the prior written consent of the other party assign, transfer, convey or declare a trust over this Agreement or make any other disposition (whether in whole or in part) of any of its rights and obligations to any third party, including by novation except that:
a.CEPI may transfer its rights and obligations under this agreement to an organisation of equivalent charitable mission, if CEPI determines (in good faith) that CEPI will not be in a position to fulfil its obligations or exercise its rights in the future. Except if the organization to which CEPI is transferring its rights and obligations is either The Wellcome Trust Limited or the Bill and Melinda Gates Foundation or their respective successors in title, Awardee shall have the right to terminate this Agreement without cause by giving [***] written notice to the assignee. Awardee may exercise its termination right under this Clause
24.3 a. within [***] of receipt of CEPI`s notification of the assignment.
b.awardee may transfer its rights and obligations under this agreement as part of a sale of the entire business required for the satisfaction of Awardee’s obligations under this Agreement either:
i. to an Affiliate of Awardee, provided that, if the assignee ceases to be an Affiliate of Awardee at any time the other provisions of this Clause
24.3 will apply, then CEPI will have the right to terminate this Agreement at any time unless and until the novation agreement referred to in Clause
24.3b. (ii) has been entered into; or
ii.to a third party provided that (a) the assignee has, in CEPI’s reasonable opinion, sufficient capital, expertise and commitment to carry on that business as a going concern and to meet Awardee’s obligations under this Agreement at least at the same level as Awardee prior to such transfer, and (b) the assignee, Awardee and CEPI enter into a novation agreement in a form reasonably acceptable to CEPI at the time of the assignment or other conveyance in the event of the transfer of all or a substantial part of Awardee’s activities related to the Project.
24.4Confidential Information. “Confidential information” means any and all non-public information disclosed on or after the Effective Date of this Agreement by one Party to the other Party whether orally or in writing or in any other form. Each Party undertakes that both during the term of this Agreement and for a period of [***] after its termination or expiry, it shall keep confidential and not disclose to any person other than its employees, agents, consultants, professional advisers, Sub-Awardees, permitted subcontractors and regulatory authorities, and, in the case of CEPI, its funders, other members of the CEPI Group and Assessors (all of the foregoing, other than regulatory authorities, “Representatives”), in each case who have a need to know any Confidential Information of the other Party disclosed to or obtained by it in connection with this Agreement. Each Party shall take commercially reasonable security precautions to protect against unauthorized access to or disclosure of such Confidential Information. Each Party shall ensure that all Representatives to which Confidential Information of the other Party is disclosed are: (i) informed of the confidentiality provisions of this Agreement; and (ii) bound by confidentiality and non-use obligations at least as stringent as these. Notwithstanding the foregoing, (A) the obligations of confidentiality under this Clause 24.4 (x) with respect to Trade Secret Information shall continue for as long as Awardee maintains such information as trade secret in accordance with applicable laws, rules or regulations, and (y) with respect to Confidential Information within Awardee Background IP shall continue for a period of [***] after disclosure of such Awardee Background IP, and (B) Trade Secret Information shall not be disclosed to third parties except in connection with a Technology Transfer pursuant to Clause 18.7 and, if applicable, CEPI’s exercise of the Public Health License pursuant to Clause 19 and, in each case, subject to the preceding clauses (i) and (ii); provided, that nothing herein shall restrict any rights of reference and access to Confidential Information within the Project Results for regulatory purposes, including for purposes of seeking, obtaining and maintaining regulatory approvals for the Product; and provided, further, that the reference to “third parties” in clause (B) (with respect to disclosure of Trade Secret Information) shall not mean or include CEPI’s employees, agents, consultants and professional advisers who receive the information for internal use by CEPI and who are informed of the confidentiality provisions of this Agreement and are bound by confidentiality and non-use obligations at least as stringent as these. Confidential information will not include:
a.information that is or was already known to the receiving party at the time of disclosure, as shown by written records, without any obligation to keep it confidential;
b.information that is independently developed by employees, agents, consultants and professional advisers of the receiving Party who have not had access to the Confidential Information of the disclosing Party as evidenced by contemporaneous written records;
c.information that at the time of being disclosed or obtained by the receiving party or at any time thereafter, is published or otherwise generally available to the public other than due to default by the receiving Party of its obligations hereunder;
d.information properly obtained by the receiving party from a source which, to the best knowledge of the receiving Party, is not known to be bound by a confidentiality agreement, fiduciary obligation or other legal or contractual restriction that may prohibit the disclosure of such Confidential Information; and
e.information to the limited extent that is required to be disclosed by a competent court or regulatory authority or otherwise by applicable law (including any requirements for disclosure under the Freedom of Information Act 2000); provided, that where it is free to do so, the receiving Party shall give notice of such disclosure to the disclosing Party as soon as reasonably practicable.
For clarity, Project Results shall be considered Awardee`s Confidential Information, but may be disclosed and utilized by the Parties to the extent as set out in this Agreement and, in particular, pursuant to Clauses
13, 19 and
24.2.
In the event CEPI exercises its Public Health License pursuant to Clause
19, CEPI and/or its designated Trusted Collaborator may use Awardee`s Confidential Information to the extent required to give effect to such license, but shall otherwise comply with the provisions of this Clause
24.4.
24.5Entire Agreement. This agreement, including its agreement summary and annexes, including CEPI Policies and Procedures, constitutes the entire agreement and understanding between the Parties relating to its subject matter and supersedes and replaces all prior arrangements, whether written or oral, between the Parties relating to the subject matter of this Agreement.
24.6Conflicts Between Components of this Agreement. If there is any conflict between the provisions of this Agreement, any Work Package or the CEPI Policies and Procedures, then the provisions of this Agreement will prevail, followed by the provisions of the Work Package and finally the terms of the CEPI Policies and Procedures. For clarity, the Funding Agreement between the Parties dated 1 April 2019 remains in full force and effect and shall not be superseded by this Agreement for any Work Packages performed thereunder.
24.7Force Majeure. Neither party shall be deemed to have defaulted under or to be in breach of this Agreement for failure or delay in fulfilling material obligations when such failure or delay is directly caused by an event outside of their reasonable control, including but not limited to acts of war, insurrections, acts of terrorism, acts of God or acts, omissions or delays in acting or failure to act by any of CEPI’s funders (collectively a “Force Majeure Event”). Each Party shall inform the other promptly and in writing of any Force Majeure Event and the Parties will discuss the situation, and acting in good faith, agree on the appropriate course of action under the circumstances. Notwithstanding the foregoing, in the case of an Outbreak or Increased Outbreak Preparation Need, the Parties will be expected to continue to carry out their obligations pursuant to applicable Work Packages with all due health and safety precautions.
24.8Further Assurances. Each party will perform such acts and execute such documents as reasonably may be required for securing to or vesting in the other Party the rights agreed to be granted to it pursuant to this Agreement.
24.9No Rights for Third Parties. A person who is not a party to this agreement has no right under the Contracts (Rights of Third Parties) Act 1999 or otherwise to enforce or to enjoy the benefit of any term of this Agreement.
24.10Notices. Any notice to be given pursuant to this agreement shall be in writing in the English language and shall be delivered by overnight courier, by registered, recorded delivery or certified mail (postage prepaid) to the address of the recipient Party provided in the Agreement Summary or such other address as a Party may from time to time designate by written notice. Any notice given pursuant to this clause shall be deemed to have been received on the day of receipt, provided receipt occurs on a Business Day of the recipient Party or otherwise on the next following Business Day of the recipient. The Parties agree that email and fax are not valid methods of giving notice under this Agreement.
24.11No Waiver. Neither party shall be deemed to have waived any of its rights or remedies under this Agreement unless the waiver is expressly made in writing and signed by a duly authorized representative of that Party.
24.12Awardee Efforts. Awardee will use all reasonable endeavours in achieving the milestones and objectives of the Project in the applicable timeframe.
24.13Relationship of the Parties. Neither party shall by reason of this agreement be empowered to act as agent for the other Party or to pledge the credit of the other Party. Neither Party will be held liable for or incur liability in respect of the acts or defaults of the other Party.
24.14Variation. No variation, amendment, modification or supplement to this agreement will be valid unless and until it is made in writing and signed by a duly authorised representative of each Party.
24.15Choice of Law. This agreement and any dispute arising out of this agreement or its formation will be governed by and construed in accordance with the laws of England and Wales without giving effect to any choice of law or conflict of law provisions or rules that would cause the application of the laws of any other jurisdiction.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
Schedule A: Glossary of Defined Terms
“Affected Territory” means any country, or any geographic area within a country, in which there is an Outbreak or for which there is an Increased Outbreak Preparation Need. For clarity, the Affected Territory includes any country in Awardee’s Traveler’s Market and any Non-Traveler’s Market Countries, in each case in which there is an Outbreak or for which there is an Increased Outbreak Preparation Need.
“Affiliate” means any business entity Controlled by, Controlling or under common Control with a Party. For the purposes of this definition, “Control” (with correlative meanings, “Controlled by” or “Controlling”) means direct or indirect beneficial ownership of more than fifty percent (50%) of the voting interest in an entity, or more than fifty percent (50%) interest in the income of the entity in question, or the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of an entity.
“Agreement Summary” means the cover page to this Agreement signed by the Parties. “Assessor” has the meaning set out in Clause
14.3.
“Awardee Background IP” means discoveries, inventions, know-how, patents and patent applications, trademarks and trademark applications, copyrights and copyrightable materials and other intellectual property rights that are owned or controlled by Awardee at the Effective Date or that Awardee develops, acquires or otherwise comes to own or control after the Effective Date outside the scope of the Project and without any CEPI funding.
“Awardee Competitor” means any commercial entity researching, developing or manufacturing a Chikungunya vaccine for use anywhere in the world.
“Awardee-Funded Study” means the VLA1553-304 (HIV+), VLA1553-305 (YF co-vacc), VLA1553-222 (dose finding <1y), VLA1553-323 (pediatric <1y), VLA1553-401 (PASS) and VLA-India (adults) studies of the Product referred to in the IPDP to be conducted, at Awardee’s sole expense.
“Awardee’s Traveler’s Market Development Plan” has the meaning set out in Clause
2.1.
“Awardee’s Traveler’s Market” means those countries listed below and any country that is defined by the Organization for Economic Co-operation and Development from time to time as a high income country; provided that if any such country becomes an LMIC, such country will no longer be included in the Awardee’s Traveler’s Market and will become a Non-Traveler’s Market Country.
1.USA, Canada; and
2.Austria, Belgium, Bulgaria, Croatia, Republic of Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden and the United Kingdom, Andorra, Iceland, Lichtenstein, Malta, Monaco, Norway, San Marino, Switzerland; and
3.Japan, South Korea, Taiwan, Singapore, Hong-Kong; and
4.Australia, New Zealand; and
5.Israel, Bahrain, Turkey, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates.
“Awardee`s Traveler`s Market Development Plan” has the meaning set out in Clause
2.1.
“Business Day” means a day on which banks are normally open for business and which is not a Saturday or Sunday, or a bank or public holiday in Norway and Austria.
“CEPI Group” means the nodes of CEPI established in Norway, England, India, the United States of America and any other node of CEPI which may be established from time to time.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
“CEPI Policies and Procedures” means the policies and procedures listed in
Schedule C of this Agreement, as updated (including by the addition of CEPI policies and procedures) or amended from time to time pursuant to Clause
6.3.
“CEPI Programme” means the third phase of CEPI’s award programme under its Third Call for Proposals to develop vaccines against Chikungunya.
“Chikungunya Vaccine” means a candidate vaccine that induces a specific immune response against at least one Chikungunya antigen in the prophylaxis of infection or therapeutic use against Chikungunya virus.
“Commercial Benefits” means any economically quantifiable benefits that arise from the commercial exploitation of the Project Results other than in preparation for or in response to an Outbreak or Increased Outbreak Preparation Need. Examples of Commercial Benefits include the commercial licensing of Project IP, receipt of government-granted incentives such as Priority Review Vouchers and revenue from the commercialization of combination, derivative or follow-on products (including antibody products, assays and vaccines) or application of production technology.
“Confidential Information” has the meaning set out in Clause
24.4.
“Data Safety and Monitoring Board” or “DSMB” means an independent group that reviews and evaluates clinical study data for participant safety and makes recommendations concerning the continuation, modification, or termination of a study.
“Effective Date” means the start date of this Agreement referred to on the first page of this Agreement.
“Enabling Rights” means any and all rights owned or controlled by the Awardee at the Effective Date, together with those which arise on or after the Effective Date, which in each case, relate to the development, manufacture, supply or marketing of the Product, including improvements to the Project Results and Product existing at the date that CEPI is first entitled to utilize the Public Health License pursuant to Clause
19, whether or not arising under the Project. Enabling Rights include applicable Awardee Background IP but do not include any rights that Awardee is contractually precluded from granting to CEPI.
“Equitable Access” means that vaccines and other products developed, in whole or in part, with CEPI’s financial support must be first available to populations when and where they are needed to end an outbreak or curtail an epidemic, regardless of ability to pay, while at a price that is sustainable to the manufacturer, as further detailed in CEPI’s “Equitable Access” Policy.
“EU” means the economic, scientific, and political organization of member states known as the European Union, as its membership may be altered from time to time, and any successor thereto. For clarity, the United Kingdom shall be considered part of the EU at all times for the purposes of this Agreement.
“Field” means Chikungunya virus in humans.
“Finished Drug Product” means the finished product formulation, containing drug substance, filled into unit packages together with a diluent, if applicable, and as finally labelled and packaged in a form ready for administration.
“Financial Records” has the meaning set out in Clause
5.3.
“Financial Report” means Awardee’s report to CEPI of its expenditures under the Project Budget on the Financial Report Template in Annex F and Awardee’s report of its activities under the IPDP.
“Financial Report Template” means the form of report in Annex F to be used by Awardee for its reports to the JMAG.
“Good Clinical Practice” or “GCP” means (i) all laws, rules, regulations, guidelines and generally accepted standards and requirements regarding the ethical conduct of clinical trials including designing, recording and reporting trials that involve the participation of human subjects as promulgated by any competent authority having jurisdiction in the country in which the organisation is registered or any country in which the clinical trial is to be conducted, (ii) Good Clinical Practice as set forth in ICH GCP Guidelines E6(R1), as amended (iii) all applicable standards and laws and regulations for current good clinical practices promulgated by the US Food and Drug Administration and the European Medicines Agency and (iv) any other good clinical practice regulations and guidance set out in the applicable Work Package.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
“Good Distribution Practice” or “GDP” means the WHO Good Storage and Distribution Practices for Medical Products Annex 7, WHO Technical Report Series 1025, 2020 and all applicable standards and laws and regulations for current good distribution practices for medicinal products (including vaccines) promulgated by any competent authority having jurisdiction in any country in which the Products are distributed.
“Good Laboratory Practice” or “GLP” means (i) all laws, rules, regulations, guidelines and generally accepted standards and requirements regarding quality control for laboratories to ensure the consistency and reliability of results promulgated by any competent authority having jurisdiction in the country in which the organisation is registered, or any country in which the laboratory testing is to be conducted, (ii) Good Laboratory Practice as set forth in OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring (iii) all applicable standards and laws and regulations for current good laboratory practices promulgated by the US Food and Drug Administration and the European Medicines Agency and (iv) any other good Laboratory practice regulations and guidance set out in the applicable Work Package.
“Good Manufacturing Practice” or “GMP” means (i) all applicable standards and laws and regulations for current good manufacturing practices for medicinal products (including vaccines) promulgated by any competent authority having jurisdiction in the country in which the organisation is registered, or any country in which the Product is distributed, (ii) Good Manufacturing Practice as set forth in the World Health Organization’s Technical Reports Series TRS 986 - Annex 2 Good Manufacturing Practices for pharmaceutical products: main principles and TRS 999 - Annex 2 WHO good manufacturing practices for biological products (TRS no 999), (iii) all applicable standards and laws and regulations for current good manufacturing practices for medicinal products (including vaccines) ingredients, testing, storage, handling, seed lots, cell banks and intermediates, bulk and finished products promulgated by the US Food and Drug Administration and the European Medicines Agency and (iv) any other good manufacturing practice regulations and guidance set out in the applicable Work Package.
“Good Practice” or “GxP” means all or any one (as the context requires) of GCP, GDP, GLP or GMP.
“High Income Countries” or “HICs” means those countries identified by the OECD as having high income economies, as of the Effective Date, as set out at
https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending- groups.
“Increased Outbreak Preparation Need” means when, having considered all reasonably accessible and relevant information including epidemiological data, travel and migration patterns and the likely availability of other products or product candidates, CEPI determines, in its sole discretion in consultation with experts (for example a sub-group or subcommittee of CEPI’s Scientific Advisory Committee that CEPI determines has appropriate expertise), that there is a heightened need for the Product to address potential Outbreaks.
“Integrated Product Development Plan” or “IPDP” means the document i
n Annex C that describes the research and development activities related to the Product and associated deliverables, milestones and timelines, as may be amended from time-to-time.
“International Standard” means a biological standard accepted by WHO for use as an International Reference Preparation.
“Investigational Product” means a Product that has not received a marketing authorization.
“IPDP Records” has the meaning set out in Clause
2.5.
“IPDP Reports” has the meaning set out in Clause
2.3.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
“IPDP Report Template” means the form of report in Annex D to be used by Awardee for its reports to the JMAG.
“Joint Monitoring and Advisory Group” or “JMAG” has the meaning set out in Clause
2.4.
“LMIC Manufacturer” means a manufacturer based in a Non-Traveler’s Market country engaged by Awardee to manufacture and/or supply the Product as outlined in the IPDP.
“Low and Middle Income Countries” or “LMICs” are those countries defined by the Organisation for Economic Co-operation and Development as being least developed or having low-income or lower-middle income economies from time to time, and as of the Effective Date set out at: https://www.oecd.org/dac/financing- sustainable-development/development-finance-standards/daclist.htm under the columns ‘Least Developed Countries’, ‘Low Income Countries’ and ‘Lower Middle-Income Countries and Territories’.
“Net Sales” means the gross amount invoiced or received by Awardee, its Affiliates, licensees or assignees in respect of sales of Product to third party purchasers in bona fide arm’s length transactions less deductions allowed by applicable licensing agreements or otherwise customary in the biopharmaceutical industry, booked on an accrual basis pursuant to Generally Accepted Accounting Principles (GAAP) or International Financial Reporting Standards (IFRS), as relevant.
“Non-Traveler’s Market Countries” means all countries of the world other than the countries in the Awardee’s Traveler’s Market.
“Outbreak” means a Public Health Emergency of International Concern declared by WHO, or a public health emergency on a national or regional scale declared by one or more public health agencies, in each case as a result of a material increase in the number of cases of people infected with CHIK including any regional out-break, an epidemic or a pandemic.
“Outbreak Notice” has the meaning set out in Clause
18.
“Pre-Activities Start Date” means the date referred to on the first page of this Agreement.
“Product” means a Chikungunya Vaccine under the Project (including, for the avoidance of doubt, VLA1553 VLA1555 and VLA1556 in Non-Traveler’s Market countries,) and includes any form or dosage of pharmaceutical composition, including combination products, or preparation for use in humans that is developed in whole or in part as part of the Project, including any Investigational Product.
“Project” means Awardee’s activities as described under the IPDP or as otherwise funded by CEPI pursuant to this Agreement.
“Project Budget” means the documents in Annex D that describes CEPI’s funding award, payment sched-ules, and any co-funding or in-kind contributions by Awardee.
“Project Data” has the meaning set out in Clause
13.1.
“Project IP” has the meaning set out in Clause
15.1.
“Project Lead” means the principal investigator named by Awardee in the IPDP or otherwise agreed by the Parties.
“Project Materials” has the meaning set out in Clause
14.1.
“Project Results” has the meaning set out in Clause
12.1.
“Public Health License” means a non-exclusive, fully paid-up, royalty free, irrevocable, sublicensable license under the Project Results and Enabling Rights that is necessary or reasonably useful to develop, manufacture, market and/or supply the Product worldwide, provided that all end users of the Product are located in the Affected Territory; in each case for the purpose of achieving Equitable Access during the Term and for twenty (20) years thereafter. For the purposes of this definition, the term ‘Product’ shall mean the Chikungunya Vaccine in any form or dosage of pharmaceutical composition or preparation for use in humans.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
“Restricted Party” means a person that is:
(a)listed on any Sanctions List or targeted by Sanctions (whether designated by name or by reason of being included in a class of persons); or
(b)located in or incorporated under the laws of any country or territory that is the target of country- or territory-wide Sanctions; or
(c)directly or indirectly, in the aggregate, 50% or more owned, or controlled by, or acting on behalf, at the direction, or for the benefit of, one or more persons referred to in (a) and/or (to the extent relevant under Sanctions) (b) above.
“Retained Amount” means the ten per cent (10%) of the final payment tranche retained by CEPI under Clause
4.7.
“Safety Issues” means any material concerns regarding safety or efficacy of any Product studied under the Project, including serious adverse events or serious adverse reaction, safety-related signals, product recalls or relevant recommendations from the Data Safety Monitoring Board to place a hold on or to end a clinical study.
“Safety Stock” has the meaning set out in Clause
17.2.
“Sanctions” means any applicable laws, regulations or orders concerning any trade, economic or financial sanctions or embargoes administered by the Sanctions Authorities and any other regime administering trade, economic or financial sanctions applicable to this Agreement;
“Sanctions Authority” means that Norwegian State, the United Nations, the European Union, the Member States of the European Union, the United Kingdom, the United States of America, Australia, Canada and any authority acting on behalf of any of them or their respective legislative, executive, enforcement and/or regulatory authorities or bodies acting in connection with Sanctions.
“Sanctions List” means:
(a)the lists of Sanctions designations and/or targets maintained by any Sanctions Authority; and/or
(b)any other Sanctions designation or target listed and/or adopted by a Sanctions Authority,
in all cases, as amended, supplemented or replaced from time to time.
“Sub-Awardee” has the meaning set out in Clause
3.1.
“Team Charter” means the description of how the Project will be organized and managed as described in
Annex B.
“Technical Review” means the mutually agreed milestones that trigger a programmatic review as further detailed in the IPDP.
“Technical Review Point” has the meaning set out in Clause
2.1.
“Technology Transfer” has the meaning set out in Clause
18.6.
“Term” has the meaning set out in Clause
22.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
“Terms and Conditions” or “T&Cs” shall have the meaning set out in Clause
1.1.
“Trade Secret Information” means Confidential Information that Awardee maintains as trade secret in compliance with applicable laws, rules or regulations and that is labeled as confidential or proprietary or, if not so labeled, is of a nature that a reasonable person with knowledge of the subject matter would recognize, based upon its content and/or the context of its disclosure, to be a trade secret.
“Trial Steering Committee” or “TSC” solely with regard to clinical studies funded or co-funded by CEPI means a group of experts that will provide advice on the clinical study design, clinical study protocol including any changes to the protocol and any feedback from regulatory and other national authorities; and monitor the progress of the clinical trial.
“Trusted Collaborator” has the meaning set out in Clause
18.
"VLA1553” means Awardee’s single-shot, live-attenuated vaccine, marketed under the name ‘IXCHIQ’ in Non-Traveler’s Market countries.
“VLA1555” means a single-shot, live-attenuated vaccine, to be developed, manufactured and commercialized by Awardee’s Sub-Awardee Butantan for specific Non-Traveler’s Market countries.
“VLA1556” means a single-shot, live-attenuated vaccine, potentially to be developed, manufactured and commercialized by a Sub-Awardee, an LMIC Manufacturer or a Trusted Collaborator in specific Non-Traveler’s Market countries, subject to contract with such Sub-Awardee and approval by CEPI.
“Warranties” has the meaning set out in Clause
20.
“WHO” means the World Health Organization.
“Work Package” means the complete Project (as a single Work Package consisting of the Work Package Streams set out in the IPDP) or any additional activities related to research, development, manufacture or supply of a Chikungunya Vaccine that CEPI may decide to proceed with or request to be performed hereunder.
“Work Package Stream” has the meaning set out in Clause
4.
ANNEX A: TERMS AND CONDITIONS – SCHEDULE B
Schedule B: Effects of Termination
|
|
|
OBLIGATIONS ON TERMINATION BY AWARDEE PURSUANT TO CLAUSE 22.2 (Termination for Default) |
CEPI shall reimburse Awardee for all reasonably incurred non-cancellable expenses relating to the Project which were authorised by CEPI and which arise after the termination date, solely to the extent they are not otherwise covered by CEPI funding.
|
|
|
OBLIGATIONS ON EXPIRATION OR TERMINATION PURSUANT TO CLAUSE 22.3(b) (Termination due to Safety, Regulatory or Ethical Issues) |
CEPI shall reimburse Awardee for all reasonably incurred non-cancellable expenses which were authorised by CEPI and which arise after the termination date, solely to the extent they are not otherwise covered by CEPI funding, and the Parties will work together to plan and implement a wind-down of the Work Package in an orderly fashion relating to the Project. |
OBLIGATIONS ON TERMINATION BY CEPI PURSUANT TO CLAUSES
22.2, 22.3a, 22.3b,
(Termination For Default; CEPI’s Reasonable Determination that Awardee is or will be Unable to Perform;; Financial
|
|
|
| Irregularity; or Failure to Satisfy Clause 4.5, respectively) |
Solely at CEPI’s discretion, CEPI may reimburse Awardee for some or all or Awardee’s reasonably incurred non-cancellable expenses relating to the Project which were authorised by CEPI and which arise after the termination date.
|
|
|
Subject to Clause 13.2, Awardee shall promptly make all Project Data publicly available in such manner as CEPI may direct, save to the extent that to do so would result in the public disclosure of Enabling Technology which would not otherwise be publicly disclosed. |
CEPI shall have the right to require Awardee, at CEPI’s discretion, to either: (i) perform Technology Transfer to a Trusted Collaborator (including any Trusted Collaborator appointed pursuant to Clause
19.3) on an expedited basis at the Awardee’s cost, or (ii) if Technology Transfer has already occurred at the date of termination and certain costs in relation to such Technology Transfer were borne by CEPI, reimburse CEPI for such costs.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
|
|
|
CEPI shall have the right to exercise the Public Health License, pursuant to Clause 19.2d). Awardee shall use all reasonable endeavours to promptly transfer to CEPI (or its nominee), at Awardee’s cost, any regulatory approvals and applications for regulatory approvals relating to the Product.
|
Awardee shall ship to CEPI (or its nominee) all Project Materials within [***] of CEPI requesting such Materials.
|
|
|
Awardee shall provide CEPI with a list of all sub-license, contract manufacturing agreements and other agreements and arrangement to which Awardee is a party which relate to the development and marketing of the Product (the “Contracts”), within [***] of the Termination Date. CEPI may request copies of any Contracts, which Awardee will promptly provide. |
CEPI shall have the right to require Awardee to: (i) assign the benefit (subject to the assumption of the burden) of one or more Contracts to CEPI or its nominee and, where consent of a third party is required, seek to obtain such consent; (ii) novate one or more Contracts to CEPI or its nominee; or (iii) terminate one or more Contracts in accordance with its terms at Awardee’s cost.
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
|
|
|
Where termination is due to any financial irregularity or fraudulent or illegal activity by Awardee, Awardee shall repay to CEPI the amount of funds related to such financial irregularity or fraudulent or illegal activity within [***] of the notice of termination. “Financial irregularity” refers to all kinds of: corruption, including bribery, nepotism and illegal gratuities; misappropriation of cash, inventory and all other kinds of assets; and financial and non-financial fraudulent statements. |
|
|
|
ANNEX A: TERMS AND CONDITIONS – SCHEDULE A |
Schedule C: CEPI Policies and Procedures as of Effective Date
Animals in Research Policy
Anti-Corruption Policy
Clinical Trials Policy
Cost Guidance
Equitable Access Policy
Information Security Policy
International Sanctions Policy
Managing Conflicts Of Interest Policy
Procurement Policy
Scientific Integrity Policy
Transparency & Confidentiality Policy
Travel Policy
Third Party Code
Annex B: Team Charter
|
|
|
|
43
Sensitivity: Official Use
|
Team Charter
For the CfP-3iii agreement package for project
|
|
|
“Expanding the Profile of Live-Attenuated chikungunya Vaccine” |
Awardee: Valneva
This “Team Charter” describes the operation and the governance of the CfP-3iii project titled “Expanding the Profile of Live-Attenuated chikungunya Vaccine”.
The aim of this Team Charter is to provide project management and operational guidance. It is designed to function as an operational document to explain the roles and responsibilities of Awardee (“Project Team”) and CEPI while also setting out how the Project will operate in practice. In the event of any conflict between this Team Charter and the Terms and Conditions of the Agreement, the Terms and Conditions shall prevail in all circumstances.
Unless specifically defined in this Team Charter, all defined terms shall have the same meaning as set out in the Terms and Conditions.
The objectives of the Project are described in the Integrated Product Development Plan (“IPDP”). For document locations, please refer to section 7 “Document Management and Archiving”, below.
1.Project Team Composition and Responsibility
It is the responsibility of the Project Team to perform the work described in the IPDP pursuant to certain Work Packages on the terms of the Agreement.
The Project Team will be comprised of:
|
|
|
|
|
|
|
|
|
|
Contact Name |
Contact Details |
| Awardee’s Project Lead |
[***] |
Email:
[***]
Tlf/mobile:
|
| CEPI Project Lead |
[***] |
Email: [***]
Tlf/mobile:
|
Additional details of the Project Team members and Project Team structure are set out in Schedule 1 and 2 to this Annex.
2.CEPI Team Composition and Responsibility
The CEPI team will be composed of a Project Lead, a Project Manager, a Project Finance Manager, and an Equitable Access lead. This team will be supplemented by relevant experts, internal or external to CEPI under appropriate confidentiality arrangements to be parts of the JMAG or sub-teams depending on the specific needs of the project, such as CMC, Regulatory, Clinical subject matter experts.
The responsibility of the CEPI team in the Project is:
1.To mobilize experts and consultants in order to support the Project Team to overcome obstacles during the Project. This function is offered on an ad-hoc basis;
2.To be part of the JMAG and sub committees with its responsibilities
3.To oversee that project milestones and deliverables are met according to the Agreement and its schedules; and
4.To ensure that CEPI’s investment is closely monitored and that funds are distributed in accordance with the Agreement.
5.To ensure that Project Continuity and Equitable Access plans are developed and implemented as required throughout the Project life cycle.
3.Project Governance Structure
The Project governance structure is set out in Schedule 2 to this Annex.
1.The Awardee’s Project Lead should encourage all Project Team members to contribute to the development strategy.
2.The style of communication is encouraged to be objective, open and direct.
3.Personal accountability in the Project Team should be pre-defined and clearly articulated along with a set of measurable actions and deadlines, together with definition of roles and responsibilities.
4.If there is a risk that deadlines or other Project requirements will not be met, the first approach is for precautions and counter measures to be taken by the Project Team.
4.Joint Monitoring and Advisory Group Composition (JMAG)
JMAG’s Remit
The JMAG shall be entitled to:
1.monitor the performance and technical content of each Work Package against the milestones and their dates, and critically assess the results on an on-going basis to identify and address any weaknesses or delays in any Work Package;
2.approve the achievement of milestones and Technical Reviews (but shall not have the right to approve final Project completion);
3.provide a forum for discussion as to whether the activities currently agreed to are sufficient to satisfy CEPI’s Mission;
4.Provide a forum for discussion of items and issues raised in the various sub-committee meetings described in Table 1, potentially for JMAG deliberations and decisions where within scope.
5.have the authority to approve extensions to Work Package timelines within [***] of the originally planned timeframe as set out in the relevant Work Package, provided that each such extension is at no cost to CEPI and does not impact the overall completion date of the Project;
6.have the authority to approve transfer of funds between cost categories within a Work Package Project Budget, to the extent that any such changes are cost neutral;
7.review and approve proposed changes and updates to the IPDP;
8.review and discuss pre-clinical/nonclinical and clinical trial protocols including any substantial changes;
9.review and discuss the regulatory strategy for the use of the Product and receive regular updates on regulatory filings and submissions;
10.review the contractual and operational status and capabilities of Trusted Collaborator(s) (if applicable);
11.review and discuss publications;
12.coordinate the sharing of any Project Results identified in the Work Package as intended for use by other CEPI awardees;
13.review and update the Equitable Access plans;
14.discuss plans, as appropriate, for the development and manufacturing and its scale-up and scale-out;
15.approve the progress reports on an agreed upon schedule;
16.review any reports and updates provided by any site visit groups;
17.provide a forum for coordinating the Parties’ responses to issues with respect to the Project Vaccine, to the extent relating to CEPI’s use in the Field, including unexpected disruptions to the supply of the Project Vaccine, recalls, safety issues or withdrawals of the Project Vaccine;
18.receive written notification of all Project Results; and
19.make such other decisions as may be delegated to the JMAG pursuant to the Agreement or by written agreement of the Parties.
Limitations on JMAG
The JMAG has no right to do any of the acts set out below. These acts can only be done by CEPI or jointly by the Parties as set out in the Agreement:
1.confirm willingness to fund any additional Work Packages (such decision is to be made solely by CEPI);
2.approve the Financial Reports;
3.approve completion of the Project;
4.amend or vary the provisions of the Terms and Conditions,
5.approve any Sub-Awardee(s) to the extent not identified in the IPDP
6.alter the fundamental scope or objectives or agreed completion date of the Project; and
7.approve an overall increase to a Project Budget or timeline.
JMAG Composition
The JMAG Members shall be comprised of the following persons:
1.Two “Voting Members”, who will be the Awardee Project Lead (who shall also be the chairperson of the JMAG) and the CEPI Project Lead. The Awardee Project Lead or nominee and the CEPI representative or nominee shall both have the right to vote on matters brought before the JMAG and falling within the JMAG remit. CEPI may, at its sole discretion, appoint or remove the CEPI representative or nominee by notice in writing to the Awardee; and
2.“Non-Voting Members” Each of the Parties may invite those persons whose special skills or knowledge might advance the Project, to attend and address the JMAG meetings as observers. Such Non-Voting Members shall not have a right to participate in the JMAG decision-making process. The Project Lead shall ensure that any such attendees sign confidentiality agreements in a form acceptable to all Parties. Each Party shall pay for the reasonable documented travel expenses and/or consulting fees of the Non-Voting Members it proposed and shall ensure that such travel is conducted in accordance with CEPI’s Travel Policy.
Quorum
The quorum for JMAG meetings shall be the two (2) Voting Members. Decisions of the JMAG shall be made by unanimity of the Voting Members. Where consensus cannot be reached, the matter shall be escalated in accordance with Clause 23.1 of the Agreement.
5.Project Team and JMAG Meetings, and committee meetings
Each new team, sub-team and JMAG will have a kick-off meeting to start its activities and each team, sub-team and JMAG will have a final close-out meeting to end the operation of the team/sub-team and capture the lessons learned.
The Awardee’s Project Lead or his/her designee, shall be responsible for organising all meetings, including preparing meeting agenda, papers and ensuring that minutes of meetings are produced promptly after each meeting and circulated to Members in a timely manner.
For this CfP-3iii grant, and the nature of proposed arrangement with 3 partners including Valneva, Instituto Butantan and Serum Institute of India, the organisation and management of all meetings described in the Table 1 will lie with the awardee, i.e. Valneva, unless stated otherwise. Decisions at submeetings will be brought to the JMAG for information, and for decision where applicable, as described in JMAG remit in Section 4 of this document.
The agreed governance structure in Table 1 follows the principles of allowing enough exchange between all Parties for areas where this is needed, while at the same time keeping committees and meetings limited and as small as possible, to allow efficient management of all collaborations considering the number of Parties and time zones involved. General operational guidelines for the Parties include that (1) presentations are to be shared at a minimum [*** in advance, (2) meeting minutes are to be drafted within [***] following the meeting, and (3) meeting minutes comments are to be received within [***] after distribution of the draft minutes.
Table 1. Overview of Committees Meetings with Member Parties, Frequency and Topics Discussed
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Meetings |
VLA |
CEPI |
IB |
SII |
Frequency |
Existing? |
Topics Discussed |
Comments related to limited member |
| CEPI Monthly meeting |
✓ |
✓ |
|
|
[***] |
Y (CfP-3i) |
[***] |
|
| CMC |
✓ |
✓ |
|
|
[***] |
Y (CfP-3i) |
[***] |
|
| RA |
✓ |
✓ |
|
|
[***] |
Y (CfP-3i) |
[***] |
|
| JMAG |
✓ |
✓ |
|
|
[***] |
Y (CfP-3i) |
[***] |
|
| JSC VLA-IB |
✓ |
|
✓ |
|
[***] |
Y (CfP-3i) |
[***] |
|
| JSC VLA-SII |
✓ |
|
|
✓ |
[***] |
New |
[***] |
|
Tech Transfer Committee →Tech Devel. Committee |
✓ |
(✓) |
✓ |
|
[***] |
Y (CfP-3i) → New scope & members |
[***] |
IB to lead TDC. Assuming IB's consent, 1 person defined by CEPI will join as an observer for relevant and non-confidential info. and until multidose development is complete. CEPI's comments to be followed-up with designated VLA member. |
| Tech Transfer Committee |
✓ |
(✓) |
|
✓ |
[***] |
New |
[***] |
One person defined by CEPI will join as an observer for relevant and non-confidential info.. CEPI's comments to be followed-up with VLA chair. |
| Development Committee |
✓ |
(✓) |
✓ |
|
[***] |
Y (CfP-3i) |
[***] |
One person defined by CEPI will join as an observer for relevant and non-confidential info.. CEPI's comments to be followed-up with VLA chair. |
| Development Committee |
✓ |
(✓) |
|
✓ |
[***] |
New |
[***] |
One person defined by CEPI will join as an observer for relevant and non-confidential info.. CEPI's comments to be followed-up with VLA member. |
LMIC Launch Readiness Committee |
✓ |
|
✓ |
|
[***] |
Y (CfP-3i) |
[***] |
|
LMIC Launch Readiness Committee |
✓ |
|
|
✓ |
[***] |
New |
[***] |
|
| Trial Steering Committee |
✓ |
✓ |
✓ |
✓ |
[***] |
Y (CfP3.i) → Additional party SII |
[***] |
|
| Quadripartite |
✓ |
✓ |
✓ |
✓ |
[***] |
New |
[***] |
|
Bipartite Tripartite Quadripartite Member
Limited Member
6.Communication with Stakeholders
Stakeholder communications regarding the Project between CEPI and the Awardee should be reviewed and approved by the JMAG. In order to maintain open communication with these stakeholders, copies of the final minutes will be distributed on request to CEPI and Awardee organization members, who are bound by confidentiality restrictions. The two Project Leads (Awardee and CEPI) will consult each other prior to any communication to third parties, presentations, press releases, conference presentation about the Project.
7.Document Management and Archiving
CEPI shall provide and maintain a secure computer platform (for example Microsoft TEAM site) to serve as a collaborative site repository for Awardee and CEPI to share Project documents during the conduct and until completion of the Project (“the Secure Portal”).
It is the responsibility of the Awardee Project Manager to ensure that relevant documents are posted on the Secure Portal in a timely manner, including but not limited to the following: agenda and meeting minutes of JMAGs and Project Team meetings, the IPDP, planning documents, Risk Register, Project Budget, Financial and CPP Reports and organizational charts. The Project Teams will agree to a list of team members that will have access to the Secure Portal.
In addition, CEPI utilizes an electronic Project Management system (Salesforce) for progress and financial reporting to which the Awardee PL, PM, and financial manager will have access.
Such approved access to both the secure Portal and Salesforce will be governed by the terms and conditions of Confidentiality as outlined in the Agreement.
The Awardee is responsible for ensuring that they hold their archive of any required documents related to the Project, in accordance with applicable ICH Guidelines (GLP, GMP, GCP, etc) and local legislation and / or regulation as applicable.
8.Planning
The Project Lead, or their nominated deputy, is responsible for maintaining the following plans for the Project (“Project Plans”), the frequency of updates of which will be agreed by the JMAG:
Project Plans:
1.IPDP: should be regarded as a “living document” and updated regularly (Microsoft Word document). These updates should be reviewed by JMAG.
a.Project Gantt chart: used for tracking and risk management (Microsoft Project file)
b.Risk Register
9.Budgets
Within each Work Package, the Awardee Project Lead is accountable for managing the Project budget, and CEPI is accountable for making payments in accordance with the Agreement.
10.Review of Team Charter
This Team Charter may be amended as required by a decision of the JMAG.
11.Appendices
Schedule 1: Awardee Project Team Members at signature
Schedule 2: Awardee Project Governance Structure
Schedule 3: CEPI Project Governance Structure
Schedule 4: Reporting Template
|
|
|
Schedule 1: Awardee Project Team Members at signature (subject to change throughout the project) |
|
|
|
|
|
|
|
|
|
| Name |
Role in Project, Function |
Contact details (email / mobile) |
[***] |
Chief Medical Officer |
[***] |
[***] |
VP Business & Corporate Development |
[***] |
[***] |
Business & Corporate Development Senior Manager |
[***] |
[***] |
Head of Project Management Chikungunya |
[***] |
[***] |
Senior Project Manager |
[***] |
[***] |
VP Clinical Development |
[***] |
[***] |
Director Clinical Strategy and Project Lead Chikungunya Vaccine Development |
[***] |
[***] |
Head of CTM External Manufacturing - Drug Product |
[***] |
[***] |
VP Technical Development & Operations |
[***] |
[***] |
VP Analytical Development |
[***] |
[***] |
VP Regulatory Affairs |
[***] |
[***] |
Director Clinical
Serology
|
[***] |
[***] |
Director Late-Stage
Clinical Development
|
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ANNEX B – TEAM CHARTER CfP-3iii CHIKVACCINE Page 8 of 10 |
Annex C Integrated Product Development Plan (IPDP)
Sensitivity: Official Use
Integrated Product Development Plan (iPDP)
Project Name: “Expanding the Profile of Live-Attenuated chikungunya
Vaccine”
For VLA1553, VLA1555, VLA1556, a Lyophilized, Single-Dose, Live-
Attenuated Chikungunya Virus Vaccine
VALNEVA Austria GmbH
(July 16th, 2024)
Integrated Product Development Plan (iPDP) 1
For VLA1553, VLA1555, VLA1556, a Lyophilized, Single-Dose, Live-Attenuated Chikungunya Virus Vaccine 1
1.Project description 2
1.1.Introduction and background 2
1.3.Expected work package outcome 6
2.Target Product Profile 11
5.Manufacturing strategy 24
1.Project description
1.1.Introduction and background
|
|
|
|
Valneva’s objective is to make the world’s first chikungunya vaccine broadly available in both developed and developing countries, to prevent the impact this disease has on communities and individuals. Our candidate vaccine, a single-shot, live-attenuated vaccine candidate (VLA1553) aiming to provide long-lasting immunity with a single dose, is as of recently approved by the US FDA, and licensure reviews are ongoing in Europe, Canada and Brazil. Based on its live attenuated technology, and corroborated by all clinical data generated to date, VLA1553 is able to induce a fast, strong and long-lasting immune response that will translate into long-lasting protection from chikungunya disease after a single dose.
VLA1553 / brand name IXCHIQ has completed all necessary Phase 3 clinical studies to obtain FDA approval under the accelerated approval pathway in adults. It is Valneva’s objective to improve and extend the future use of Valneva’s chikungunya vaccine. Planned studies aim at generating evidence for:
1.Data to facilitate and improve the implementation of the vaccine: Such as, antibody persistence long-term safety, safety and immunogenicity in special populations (e.g. pregnant women, immunocompromised; Phase 3 studies), local registration studies as well as data to strengthen the case of effectiveness of the vaccine against chikungunya virus in endemic areas (Phase 4 studies).
2.Paediatric population – a vulnerable and therefore important target population for immunization, since in case of an outbreak they may lack immunity from previous outbreaks and the majority of use of the chikungunya vaccine in the paediatric population is expected to occur in LMIC countries (Dose finding Phase 2 and Phase 3 studies).
Since 2019, with support from CEPI and EU Horizon 2020, Valneva has developed the product with a two-pronged strategy that prioritizes both establishing a vaccine to protect travellers from high-income to endemic countries and immunizing people living in LMIC to reduce the burden of the disease. To provide a sustainable commercial basis and stable market for this vaccine despite the epidemic nature of chikungunya, Valneva has developed the vaccine for high-income traveller’s markets like US and Europe. Simultaneously, to combat the huge burden from chikungunya afflicted on endemic regions, we have implemented a partnering strategy as foreseen under our 2019 CEPI grant and partnered with Instituto Butantan of Brazil for making the product available to Low- and Middle-Income Countries (LMICs).
The Butantan-Valneva partnership also enables significant development synergies, whereby data generated with Valneva’s chikungunya vaccine VLA1553 will be supporting LMIC partners to achieve licensure. For example, Valneva’s pivotal Phase 3 studies with VLA1553 in US adults, complemented by data generated in adolescents in Brazil with VLA1553 and enabled through
|
CEPI funding, have served as the basis for the ANVISA submission of IXCHIQ, and will continue to support future licensure submission of VLA1555 (which is locally produced after tech-transfer of VLA1553 lyophilisation and packaging to Instituto Butantan). Physical-chemical comparability between the products can be leveraged to ensure that data generated for either vaccine can be used from a regulatory perspective for the other vaccines. FDA has from a scientific perspective agreed to that concept, and also EMA has agreed to this concept. Therefore, any clinical data generated by Valneva for VLA1553 will support any regulatory submission in LMIC, and vice versa. In the case of the post marketing commitments under the accelerated approval, however, FDA has determined during its review process that from a legal perspective, these studies must be carried out with the same (regulatory-wise) product as the one registered under the accelerated approval. Therefore, the post marketing effectiveness studies will be carried out with VLA1553, which, to achieve that, has been submitted for licensure review in Brazil.
Approval of a vaccine without efficacy data is seen as the most important hurdle in LMIC countries. However, with the approval of the product by FDA and in the near future by Health Canada, EMA and ANVISA, it is expected to convince LMIC competent authorities to approve this product based on immunogenicity endpoints, and the support from CEPI is expected to support regulatory alignment across to the LMIC countries.
Valneva does not apply for the CEPI funding as a formal consortium. Instead, as with the last funding agreement, we are proposing to be the awardee and contractual counterpart to CEPI and extend obligations to our subcontractors. This setup has proven very efficient in the current collaboration with IB and will greatly reduce complexity in the future set-up.
In order for VLA and IB to focus their efforts and increase the likelihood of success, VLA and IB have agreed in principle for VLA to take back the India, Asian and Middle Eastern LMICs rights and supply to UNICEF from IB and to enter into a similar partnership as the VLA-IB one with Serum Institute of India (SII), including a DP TT and a clinical, regulatory and commercialization collaboration to make the product available in India, Asian and Middle Eastern LMICs. With the development of this additional CHIKV product, the following codes are used to distinguish between the different products:
•VLA1553: VLA’s chikungunya vaccine candidate (IXCHIQ)
•VLA1555: Drug Product manufactured by IB following tech transfer from VLA
•VLA1556: Drug Product manufactured by SII following tech transfer from VLA
In order to make Valneva’s, Butantan (IB)’s and Serum Institute of India (SII)’s chikungunya vaccines broadly accessible, all efforts will be made to continue submitting and registering the CHIKV products in HIC and LMIC countries. Health Canada and EMA applications were filed in May and October 2023, respectively, aiming to receive approval in the second half of 2024. For MHRA/UK approval, VLA will follow the reliance route from EMA. The strategy for Brazil submission recently changed; VLA1553 was submitted in December 2023, to comply with FDA’s legal requirements to perform the Phase 4 studies with an identical product. This will get the
|
|
|
|
vaccine to the Brazil market earlier. ANVISA agreed that VLA1555 will be submitted under a separate license, planned in 2024. VLA1555 multidose is to be developed with plans to be submitted to ANVISA in 2026. VLA1556 will be submitted to India and Asian Key Countries by Serum Institute of India.
Valneva remains committed to the further development of its chikungunya vaccine and will continue to drive significant efforts to augment CEPI’s effort to ensure broad implementation of a chikungunya vaccine in LMIC. Under the CHIKV CfP3.iii grant, Valneva will continue and expand its collaboration with Instituto Butantan, and Serum Institute of India.
|
1.2.Work package objectives
1.2.1WP1- Clinical & effectiveness studies
|
|
|
|
•Conduct of trial VLA1553-303 to investigate long-term antibody persistence (for up to 10 yrs) following vaccination with VLA1553
•Conduct of trial VLA1553-321 in Chikungunya endemic regions with Valneva DP to demonstrate safety and immunogenicity of VLA1553 in an adolescent population (12-17 yrs) and individuals previously exposed to ChikV.
•Conduct of trial VLA1553-221 in Chikungunya endemic regions with Valneva DP to identify the optimal dose level of VLA1553 in a paediatric population aged 1-11 years based on safety and immunogenicity.
•Conduct of trial VLA1553-322 in Chikungunya endemic regions with Valneva DP to establish safety and immunogenicity of VLA1553 in a paediatric population aged 1-11 years
•Conduct an effectiveness study (VLA1553-402) in Brazil to estimate vaccine efficacy of VLA1553, Valneva DP/Ixchiq, in the prevention of symptomatic laboratory-confirmed CHIKV cases after a single vaccination with VLA1553 in Brazil; the pilot vaccine program, a prerequisite for running this effectiveness study, will be accompanied by a serosurvey and prospective safety cohort study.
•Conduct a pregnancy surveillance study (VLA1553-403) as part of the pilot vaccination program in Brazil to monitor women who have inadvertently been vaccinated during pregnancy.
•Conduct an effectiveness study (VLA1553-404) using Valneva DP/Ixchiq, a pragmatic randomised controlled trial to assess vaccine effectiveness in preventing symptomatic virologically-confirmed Chikungunya virus (CHIKV) disease among adults; in addition, safety of the vaccine will be monitored (chikungunya-like adverse reactions)
|
1.2.2WP2- CMC & Technology Transfer activity
|
|
|
|
•Transfer of documentation and complete a risk assessment
•Lyo cycle successfully developed
•Transfer DP Lyo process to Indian Lyo manufacturer
•Successful PPQ campaign
•Analytical transfer of CHIK vaccines analytical method successfully completed
•Comparability report between VLA1553 and VLA1556 accepted by Indian Regulatory authorities
|
1.2.3WP3 – Regulatory engagement and licensure (Activity not funded by CEPI, included for
visibility/completeness and alignment with milestone & deliverable table as linked to Technical Reviews #1, #2 and #3 listed in following section)
•IXCHIQ (VLA 1553) to be approved in Brazil (dossier submitted in Dec 2023)
•Butantan will, in collaboration with Valneva, apply for the Brazilian licensure of the Butantan
Product (VLA1555) after completion of the Drug Product Technology Transfer and apply for and use reasonable endeavors to apply for WHO pre-qualification of the Butantan Product immediately following but no later than twelve (12) months after obtaining ANVISA Regulatory approval and use reasonable endeavors to obtain WHO pre-qualification of the Butantan Product
•SII will, in collaboration with Valneva, apply for the Indian licensure of the SII product (VLA1556)
1.2.4– 1.2.5
WPS4 and WPS5 are not included in this grant.
1.2.6WP6 – Project management
•Lead program management of the entire chikungunya program throughout the duration of the contract. This will include total project lifecycle management, comprising project planning, execution, monitoring, controlling, and closure
•Monitoring and maintaining the Scope of Work (SOW)
•Monitoring the program budget and timelines
•Coordinating project communications internally and with external project stakeholders
•Preparing of reports, and assist in satisfying the CEPI reporting requirements
1.3.Expected work package outcome (Milestones, Deliverables and Technical Reviews)
(WPS1: Clinical & effectiveness studies, WPS2: CMC & Tech Transfer activity and WPS3: Project Management)
|
|
|
|
|
|
|
|
|
|
|
|
| Work Package |
Milestones |
Deliverable/s |
Expected by |
| WP1 #1 |
[***] |
[***] |
[***] |
| WP1 #2 |
[***] |
[***] |
[***] |
| WP1 #3 |
[***] |
[***] |
[***] |
| WP1 #4 |
[***] |
[***] |
[***] |
| WP1 #5 |
[***] |
[***] |
[***] |
| WP1 #6 |
[***] |
[***] |
[***] |
| WP1 #7 |
[***] |
[***] |
[***] |
| WP1 #8 |
[***] |
[***] |
[***] |
| WP1 #9 |
[***] |
[***] |
[***] |
| WP1 #10 |
[***] |
[***] |
[***] |
| WP1 #11 |
[***] |
[***] |
[***] |
| WP1 #12 |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| WP1 #13 |
[***] |
[***] |
[***] |
| WP1 #14 |
[***] |
[***] |
[***] |
| WP1 #15 |
[***] |
[***] |
[***] |
| WP1 #16 |
[***] |
[***] |
[***] |
| WP1 #17 |
[***] |
[***] |
[***] |
| WP1 #18 |
[***] |
[***] |
[***] |
| WP1 #19 |
[***] |
[***] |
[***] |
| WP1 #20 |
[***] |
[***] |
[***] |
| WP1 #21 |
[***] |
[***] |
[***] |
| WP1 #22 |
[***] |
[***] |
[***] |
| WP1 #23 |
[***] |
[***] |
[***] |
| WP1 #24 |
[***] |
[***] |
[***] |
| WP1 #25 |
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
| WP1 #26 |
[***] |
[***] |
[***] |
| WP2 #1 |
[***] |
[***] |
[***] |
| WP2 #2 |
[***] |
[***] |
[***] |
| WP2 #3 |
[***] |
[***] |
[***] |
| WP2 #4 |
[***] |
[***] |
[***] |
| WP2 #5 |
[***] |
[***] |
[***] |
| WP2 #6 |
[***] |
[***] |
[***] |
| WP2 #7 |
[***] |
[***] |
[***] |
| WP2 #8 |
[***] |
[***] |
[***] |
| WP2 #9 |
[***] |
[***] |
[***] |
|
WPS2 #10
(Not funded by CEPI, included for completeness)
|
[***] |
[***] |
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
WPS3 activities not in scope of CHIKV CfP3.iii. Milestones and deliverables included for visibility/completeness |
| WPS3 #1 |
[***] |
[***] |
[***] |
| WPS3 #2 |
[***] |
[***] |
[***] |
| WPS3 #3 |
[***] |
[***] |
[***] |
Technical Review Criteria and Technical Review Points for CfP3.iii, with milestones and deliverables to be submitted for CEPI review – CEPI to be notified 2 months in advance of expected TR which is to take place within 3 months of the respective licensure |
|
Technical
Review #1
|
[***] |
Technical Review [***] |
Technical
Review Point:
[***]
|
|
Technical
Review #2
|
[***] |
Technical Review Criteria:
[***]
|
Technical
Review Point:
[***]
|
|
Technical
Review #3
|
[***] |
Technical Review Criteria: [***] |
Technical Review Point: [***] |
2.Target Product Profile
Vaccine Target Product Profile: VLA1553
|
|
|
|
|
|
Vaccine characteristic |
Expected |
| Product |
Live-attenuated chikungunya Vaccine (VLA1553,) |
| Indication for use |
Active immunization for the prevention of disease caused by chikungunya virus in individuals aged 1 year and above. Initial approval: 18 yrs (US/EU/CAN) and 12 years (ANVISA) |
| Target population |
Travelers (US EU CAN) / Outbreak use (US EU BRA) / Endemic areas (BRA) |
| Safety/Reactogenicity |
Generally safe and well tolerated, comparable to other licensed Vaccines:
Safety was already evaluated in 3,610 participants who received at least one dose of VLA1553 (3 clinical studies)
|
| Protective efficacy |
VLA1553 has shown to induce a fast, strong, and persistent immune response following a single-dose vaccination across all age groups evaluated in the clinical studies (individuals ≥18 years of age):
-Seroresponse rate of 99% 28 days after a single dose, in adults and adolescents.
-Persistence demonstrated with 97% seroresponse retained after 24 months
|
| Dose regimen |
Each approximately 0.5-mL dose contains not less than 3.0 log10 TCID50 (Tissue Culture Infectious Dose 50%) of live, attenuated chikungunya virus. |
Duration of protection |
Potentially lifelong protection, based on Live Attenuated Virus (LAV) Technology. Up to 10 years demonstrated in clinical trials. |
Route of administration |
Intramuscular administration |
Product stability and storage |
Shelf life: 24 months (at launch) |
| Presentation |
Lyophilized requiring reconstitution; storage at +2 to 8°C |
Cost of Goods / Cost per Dose |
Confidential |
Registration and prequalification |
Registered in a broad range of countries, including HIC / travellers markets and Brazil |
Vaccine Target Product Profile: VLA1555/1556
|
|
|
|
|
|
Vaccine characteristic |
Expected |
| Product |
Live-attenuated chikungunya Vaccine (VLA1555 and VLA1556) |
| Indication for use |
Active immunization for the prevention of disease caused by chikungunya virus in individuals aged 1 year and above. Initial approval: 12 years and above |
| Target population |
Outbreak use / Endemic areas |
| Safety/Reactogenicity |
Generally safe and well tolerated, relying on the clinical data generated with VLA1553, see above, in addition to physico-chemical comparability data. |
| Protective efficacy |
99% Seroresponse, relying on the clinical data generated with VLA1553, see above, in addition to physico-chemical comparability data. |
| Dose regimen |
Each approximately 0.5-mL dose contains not less than 3.0 log10 TCID50 (Tissue Culture Infectious Dose 50%) of live, attenuated chikungunya virus. |
Duration of protection |
Potentially lifelong protection, based on Live Attenuated Virus (LAV) Technology. Up to 10 years demonstrated in studies, relying on the clinical data generated with VLA1553, see above, in addition to physicochemical comparability data. |
Route of administration |
Intramuscular administration |
Product stability and storage |
Shelf life: 24 months (at launch) |
| Presentation |
Lyophilized requiring reconstitution; storage at +2 to 8°C |
Cost of Goods / Cost per Dose |
Confidential |
Registration and prequalification |
Registered in a broad range of LMICs with medical need, who pre-qualified, suitable presentation for use in NIP’s (e.g., multi-dose, diluent in vial) |
3.Non-clinical strategy
|
|
|
|
|
|
| WP |
Non-clinical strategy |
|
The safety, immunogenicity and protective potency of the CHIKV vaccine candidate VLA1553 have been assessed in numerous non-clinical studies.
The non-clinical development program has focused on the establishment of a small animal as well as non-human primate (NHP) model allowing for the evaluation of the CHIKV candidate vaccine with respect to safety and efficacy, i.e., immunogenicity and protection (Hallengärd et al. 2014). To this end, mouse models have been investigated and were shown to be permissive for infection with a wild-type (wt) CHIKV isolate, LR2006 OPY-1. Thus, infection of mice with wt CHIKV was shown to cause significant viremia, a major sign also in humans (Couderc and Lecuit 2009). In addition, NHPs serve as excellent animal models for understanding CHIKV pathogenesis as they are a natural amplification host for CHIKV and share significant genetic and physiological homology with humans. CHIKV infection in NHPs results in acute fever, rash, viremia and the production of CHIKV-specific neutralizing antibodies, type I interferon and pro- inflammatory cytokines. CHIKV establishes a persistent infection in NHPs, particularly in cynomolgus macaques (Broeckel, Haese et al. 2015).
Valneva’s preclinical data package generated with the CHIKV vaccine candidate demonstrates that a single dose of the CHIKV del5nsP3 vaccine/VLA1553:
•is highly immunogenic and induces a strong and long-lasting neutralizing
antibody response in a mouse and NHP model;
•protects NHPs from a high-dose wt CHIKV challenge;
•causes no clinical manifestations typically associated with wt CHIKV infections in
the NHP model;
•shows a delayed and strongly reduced viremia as compared to wt CHIKV infection
in a mouse and NHP model;
•shows strongly reduced cytokine production compared to wt CHIKV infection;
•shows a more sporadic, transient and lower dissemination in tissues of VLA1553
immunized NHPs compared to wt CHIKV infected NHPs;
•confirms the stability of the virus del5nsP3 attenuation in humans post
vaccination;
•is able to protect NHPs from CHIKV infection based on passive transfer of human
immune sera to NHPs followed by wt CHIKV challenge and;
|
• shows a negligible risk of VLA1553 virus transmission from vaccinated to non-vaccinated humans by mosquitoes.
An overview of further non-clinical studies can be found in Table 2 below.
|
|
|
|
|
|
|
|
|
Table 2. Non-clinical Studies with VLA1553 |
| Study |
Species |
Summary |
|
Repeat-Dose
Toxicity
|
Rabbits (New Zealand Whites) |
Upon two high dose vaccinations at a two-week interval, all findings were transient and resolved within the 30 days recovery period; No adverse findings. |
Persistence of infection and biodistribution |
NHPs
(Cynomolgus macaques)
|
•VLA1553 replication in blood was 3 logs lower
than replication of wt CHIKV;
•shedding of VLA1553 in saliva and vaginal fluids
was much lower than wt CHIKV;
•in cerebrospinal fluid, synovial fluid and urine,
no shedding of VLA1553 nor wt CHIKV was detected;
•VLA1553 dissemination in tissues, when
detected, was more sporadic, transient and lower than observed for wild-type CHIKV;
•VLA1553 was not detected in joints and muscles.
|
Mosquito Transmission Study |
Mosquitos (Aedes albopictus) |
Probability of mosquitoes transmitting VLA1553 virus from a human vaccinated with the vaccine appears to be very low (threshold titer of 3.875 log10 CCID50/mL). |
|
Passive transfer in NHPs using human serum from
VLA1553-101
|
NHPs (Cynomolgus macaques) |
After vaccination with VLA1553 a neutralizing antibody titer of ≥50 determined by µPRNT50 is proposed as a titer reasonably likely to predict protection. |
4 of 49
4.Clinical strategy
|
|
|
|
|
|
| WP |
Clinical strategy (Phase 3-4) |
| WP 1 |
Valneva’s objective is to improve and extend the future use of Valneva’s chikungunya vaccine. Planned studies aim at generating evidence to:
1.Facilitate and improve the implementation of the vaccine: Such as, antibody persistence, long-term safety, safety and immunogenicity in special populations (e.g. pregnant women, immune- compromised); very importantly, data to demonstrate effectiveness of the vaccine against chikungunya virus in endemic areas, as well as – if necessary - local registration studies (e.g. India)
•VLA1553-303: Antibody persistence study:
[***]
•VLA1553-304: Trial in moderately immunocompromised HIV patients: (Not in scope of
CHIKV CfP3.iii, included for visibility/completeness)
[***]
|
|
|
|
|
|
|
|
|
Three real-world evidence Phase 4 studies will include:
•VLA1553-401, Post-Authorization Safety Study [PASS]: (Not in scope of CHIKV CfP3.iii,
included for visibility/completeness)
[***]
•VLA1553-402, Effectiveness study: [***]
•VLA1553-403, Pregnancy study:
[***]
•VLA1553-404, Effectiveness study 2:
[***]
2. Extend the licensure to the paediatric population – a vulnerable and therefore important target population for immunization, since in case of an outbreak they may lack immunity from previous outbreaks. Paediatric use of a chikungunya vaccine is primarily expected to occur in LMIC.
•VLA1553-321, Adolescent study:
[***]
•VLA1553-221, Dose-finding study:
[***]
•VLA1553-322, Pediatric study:
[***]
VLA1553-222, Dose-finding study (<1y) / VLA1553-323 Pediatric study (<1y): (Not
requested for CEPI funding, included for visibility/completeness)
|
|
|
|
|
|
|
Within each study arm subjects will be stratified 3:1, in two age subsets: - 6 months to 12 months of age (n=150);
- <6 months (n=150).
|
|
Clinical sample analysis:
All testing of neutralizing antibody titer will be performed at Nexelis in the validated µPRNT in order to ensure comparability of results (this includes specimens from any studies performed by IB or SII). The WHO international standard (1502/19) for anti-chikungunya virus immunoglobulin G will be used to determine a correlation factor to allow conversion of results reported in µPRNT50 into International Units.
For the Phase 4 effectiveness studies, a serological method and a molecular diagnostic method using dried blood spots will be developed and validated with a local Brazilian lab that will serve as a central laboratory for those trials.
|
1.Regulatory strategy
|
|
|
|
|
|
| WP |
Regulatory strategy |
|
•FDA/US: VLA1553 was awarded FDA Fast Track designation and FDA Breakthrough
Therapy Designation. BLA submission was completed end of 2022 and reviewed under an accelerated review pathway with a Prescription Drug User Fee Act (PDUFA) action date at the end of November 2023. On Nov. 09th, the FDA approved VLA1553, IXCHIQ.
•EMA/EU: EMA Priority Medicines (PRIME) designation was obtained in October 2020.
MAA submission was performed in October 2023, with approval expected H2/2024. Based on the discussions with EMA, Valneva agrees to the OPEN procedure for the MAA.
•ANVISA and WHO have agreed to participate in the OPEN procedure from EMA
regarding review of the MAA dossier. VLA and IB would benefit from including ANVISA as it is a very public health focused agency and this way, they get more familiar with the vaccine. CHMP opinion is expected on May 30th, 2024, according to the timelines and assumptions on stop clocks. Based on the CHMP opinion in May, EC/EU approval will follow 2 months later.
•Health Canada/Canada: Regulatory application has been filed end of May 2023.
Dossier was validated on Aug. 25th 2023, which initiated the review process of 300 days. The Approval is expected end of June 2024.
•MHRA/UK: The submission in UK will follow a reliance process: the International
Recognition Process (IRP) with EMA as reference authority. VLA will submit the dossier after the positive CHMP opinion. Assuming no clock stops /questions, the approval is expected within 2024.
•Label Extension (VLA1553-321): Label extension variation for use in adolescent population
(above 12 yrs of age) will be submitted to NRAs that have approved VLA1553.
o US FDA: Q4/2024
o EU: Q4/2024
o ANVISA: Q1/2025 (can be earlier if approval of VLA1553 is earlier than expected)
o UK: Q1/2025
o CAN: Q4/2024
•LMICs:
o Brazil: Due to legal requirements FDA requested in October 2023 to perform the phase 4 study (-402) with exactly the same product as the one approved in US (VLA1553 VLA and IB agreed to register VLA1553 in Brazil. As a next step, VLA1555 is also planned to be licensed in Brazil. ANVISA agreed that VLA1555 will be a separate license. VLA1553 was submitted in Dec 2023 (under Open procedure). The VLA1553 dossier is currently under review at ANVISA.
|
8 of 49
o Valneva & IB are currently working out strategies for approval of VLA1553 & VLA1555.
o VLA1555 multidose is to be developed with plans to be submitted to ANVISA later.
o After ANVISA approval of VLA1553, engagement will be sought rapidly with WHO to discuss VLA1555.Based on the current assumption, VLA1555 will be submitted for WHO PQ by Q2 2026. Earlier submission is also possible provided WHO is willing to accept the submission.
o The new territories split between IB and SII are as follows (amendments and agreements not yet signed):
▪ IB Territory is to include all LatAm countries, China, Africa, plus PAHO and GAVI; and
▪ SII Territory is to include India, Asia LMICs, Middle East LMICs, plus UNICEF.
o Valneva and IB finalized the re-negotiations of IB’s territory, which was agreed to include all LatAm countries, including, for avoidance of doubt, LatAm HICs, China Africa, PAHO and GAVI.
o Meanwhile, Valneva and SII are negotiating a full deal like that of Valneva/IB wherein SII will be an exclusive licensee to VLA for the development, manufacturing (including a DP tech transfer), and commercialization of a Chikungunya vaccine in India, Asian and Middle Eastern LMICs, and UNICEF.
o The way GAVI, PAHO and UNICEF operate is currently being evaluated to decide on the best solution, especially in light of the new 2024 GAVI investment strategy.
o Latin America
▪ Private market: Butantan to perform NRA submissions to different countries directly or via partners (selection of distributors is ongoing). Submission and approval timelines TBD.
▪ Public market: Butantan to follow the regulatory processes in LatAm via WHO PQ + PAHO, which gains access to most countries. In case of WHO PQ absence, NRA submissions to different countries to be performed directly or via partners. Submission and approval timelines TBD.
o India/Asia
▪ Local route to licensure will entail safety and immunogenicity evaluation in a clinical study with VLA1556. VLA and SII are of the opinion that comparability between the products will be acceptable to DCGI at a biophysical and biochemical level without any clinical comparison to VLA1553 (no immunobridging required between the two products, but only data to bridge immunogenicity and safety data from foreign to Indian population). Furthermore, since this is based on the consideration that the DS will be identical for VLA1553 & VLA1556, therefore no immunobridging is expected to be required.
SII expects to have first interactions with DCGI after preliminary data from tech transfer. Prior to the registration in India, which will need local clinical data, our partner SII will obtain to achieve an approval of VLA1556 for export use; based on the clinical dossier of VLA1553 and the local PPQ and physico-chemical comparability. This way, the vaccine could reach other markets that do not require local clinical data potentially before an approval in India. This regulatory strategy has been applied for other vaccines from SII in the past.
▪ NRA submission and review timelines for the rest of Asia TBD will be worked out with SII.
▪ Public market: Following WHO PQ, the Indian partner will supply any Asian LMIC public market.
▪ Our future partner SII will also push for ‘Export Only’ license for VLA1556 whereby the product can be exported out of India without product approval in India.
o Africa: IB to stockpile and supply outbreaks via WHO PQ and supranationals (GAVI, UNICEF). African private and public markets to be further assessed.
o Supranationals (PAHO/GAVI/UNICEF): interactions have started and will continue in parallel to the WHO PQ process, so that once WHO PQ is available, the product is considered eligible for all supranational procurement.
2.Pharmacovigilance strategy
|
|
|
|
The Corporate Pharmacovigilance Team at Valneva is responsible for managing all pharmacovigilance tasks and activities worldwide.
The Valneva’s Corporate PV Team is located at Valneva Austria and is led by the Director Pharmacovigilance & QPPV. The Director Pharmacovigilance & QPPV reports directly to the Chief Medical Officer. The Corporate PV department is divided into two divisions – Global PV Strategy lead by the Director Pharmacovigilance & QPPV and in global PV Operations overseen by Head of Global PV Operations & Deputy QPPV.
As member of the Global PV Strategy team, the Director Pharmacovigilance & QPPV is involved in planning and set up of projects with other Valneva Departments and international partners. The global PV Operations team tasks include routine pharmacovigilance activities as well as the continuous development of the pharmacovigilance system.
With the help of international partners and specialized PV service providers, the Corporate Pharmacovigilance Team operates a state-of-the-art global pharmacovigilance system around the core PV activities, like collection, and management of safety data and the continues monitoring of the risks and benefits of Valneva’s vaccines. For more than ten years now, Valneva has outsourced case management and processing of ICSRs into Valneva’s global safety database to the PV service provider SCRATCH. The dedicated team at SCRATCH handles all case management activities as well as the medical assessment and submission of ICSRs to regulatory Authorities. Although the activities are outsourced, the Corporate PV Team has the full oversight about all cases handled by the SCRATCH team.
In addition to the global responsibilities, Valneva’s Corporate PV team is responsible for local PV activities in Germany, Austria, the United Kingdom and the United States. In those countries the Corporate PV team is conducting all local PV activities like literature screening, regulatory intelligent screening and any communication related to safety topics with authorities.
Since some countries require a dedicated local PV person (who is also able to speak the local language) Valneva has teamed up with the service provider Propharma Group to be able to fulfil this local requirement. Propharma Group handles the local PV person (LPPV) network, including local literature search, local regulatory intelligent screening and supports with any communication to local authorities if necessary. In countries where no local PV representative is necessary or Propharma Group cannot cover the territory, Valneva’s local partner covers basic PV tasks like safety information reporting, local literature screening and communication to local authorities on behalf of Valneva.
Although, Valneva’s Corporate PV team conducts and oversees all audit activities of the PV system, Valneva works with PV auditors provided by Propharma Group to conduct PV system audits of partners.
Each partner who is considered part of Valneva’s PV system has concluded a corresponding contract with the Corporate PV team for the tasks or services assumed.
Furthermore, Valneva’s Corporate PV Team ensures that all safety relevant data received from any global source, is entered into Valneva’s global safety database, and is processed in accordance with current legal requirements. All computerized systems used by the Corporate PV Team are validated and are regularly audited. Valneva utilizes an integrated Quality Management System (QMS) to facilitate, integrate and manage its quality processes and systems. The QMS facilitates the development, revision, review and approval of
|
controlled documents, subsequent training and any required archival of controlled documents. All procedural documents are reviewed, approved, and version controlled. Periodic review is performed at a minimum every 2 (two) years to ensure continued accuracy. All pharmacovigilance activities are covered in standard operating procedures (SOP) in the QMS and controlled by a dedicated QA team in terms of compliance with current quality standards. In addition to the core activities, the Corporate PV Team performs the qualification of new business partners, plans, and oversees the audits of the PV system of partners and service providers and conducts the yearly global pharmacovigilance training for all Valneva employees and external work force if applicable.
Furthermore, the performance of key PV activities (defined as KPIs) is monitored and reviewed by the Director Pharmacovigilance & QPPV on a quarterly basis.
The key aspiration of Valneva’s Corporate PV Team is to operate an appropriate safety system, to enable Valneva to optimize the monitoring of the benefit-risk of its vaccines, so that we can better serve and safeguard our patients.
Signal and Risk Management procedures
Continuous monitoring of the risk benefit profile includes the following processes:
•Each ICSR or other safety information is assessed and fully processed by qualified personnel at the service provider; medical assessment is documented in the electronic case file
•For serious ICSRs, the respective CIOMS I form is forwarded from the service provider to Valneva Corporate PV Department (QPPV or delegate) for review and approval of the medical assessment before submission to the relevant competent authorities is performed by service provider according to respective timelines.
•Signal detection is performed on a regular basis, i.e., at least quarterly, or ad hoc. This includes comparison to the previous quarter and cumulative information.
•PSURs are prepared and reviewed according to regulatory requirements.
•Information from other sources, including but not limited to product technical complaints, QA investigations, regulatory information (e.g., from regulator websites), or publications is continually reviewed, and appropriate measures initiated, as needed.
•The Director Pharmacovigilance & QPPV is actively involved in the compilation / update of RMPs and approves the final version of the RMP. The Director Pharmacovigilance & QPPV is responsible for the monitoring of RMP commitments, as well as for monitoring the effectiveness of risk minimization measures, if applicable.
Routine signal detection is performed by the PV Operations team on a regular basis. The PV Operations team generates a list of ICSRs received from worldwide sources of the previous quarter from the global safety database, for analysis and evaluation.
Data are analyzed as follows:
•by absolute figures
•by rates, i.e., ICSR rate per 100.000 doses of Valneva product sold.
|
|
|
|
•by batch number where available
•the rates of the current quarter are compared to the previous quarter as well as to cumulative data.
•by country / region
For further evaluation, serious unexpected Adverse Drug Reactions (ADRs) are covered as narratives, including the company medical assessment. If any peculiar / unusual ICSRs have been received - irrespective if serious or non-serious - these are also discussed in the SD report.
To complete the report a signal evaluation and a risk evaluation of the new data is performed. The analysis includes the interval data as well as cumulative information available.
A conclusion and summary section are covered at the beginning of the SD Report for fast orientation of the members of the Quality & Product Safety Management Board (Q&PSMB) as Valneva’s decision making body for quality and safety related matters, receive the SD reports for information.
In case a potential signal or risk is identified, the PV Operations team performs a preliminary signal evaluation. All information from routine signal detection and the preliminary signal evaluation are compiled and forwarded to the QPPV. The QPPV and the Chief Medical Officer, will assess the evidence for causality and perform an analysis. External experts or consultants may be involved in such evaluation. If this assessment verifies that there is sufficient evidence for the existence of a new potentially causal association, or a new aspect of a known association, the signal is considered validated, requiring further analysis.
Validated signals are escalated to the Q&PSMB as Valneva’s decision making body for quality and safety related matters. For signals that may impact Public Health, an ad hoc Q&PSMB meeting is scheduled immediately, but no later than within 24 hours.
For other signals, an ad hoc Q&PSMB meeting is scheduled no later than within 3 business days. During the Q&PSMB meeting, the validation of a signal is confirmed. Depending on the potential impact on Public Health, the type of actions and the corresponding timelines are agreed.
|
3.Quality management strategy
Quality management strategy
The Global Valneva Quality Management System (QMS) defines the quality governance structure including roles and responsibilities.
Preclinical manufacturing:
Since the VLA1553 vaccine was designed by reverse genetics all relevant ICH guidelines (especially ICHQ5A-Q5E, ICHQ6A-Q6B, ICHQ7; ICHQ8, ICHQ9, ICH Q10 and ICH Q11) were considered in pharmaceutical development, subsequent technology transfer, GMP manufacturing and later commercial manufacturing.
For product development strategies outlined in the ICHQ8 guideline were considered. A design space for the proposed manufacturing process as well as for the lyophilisation development was established and critical process parameters were tested in comprehensive design of experiments studies, before transfer to a CMO.
The preclinical material for example was produced under GMP conditions using the same production scale, production method, production site and panel of QC test methods like the later GMP material for clinical Phase 1. Scientific advices from several Competent Authorities including FDA were considered in the final process design and for control of materials. The Vero cell bank derives from the commercial IXIARO production process and is fully characterized and approved. The viral seed banks were fully characterized using the ICHQ5 guideline series. Process and formulation development was followed using the ICHQ11 guidelines. The process robustness and high product quality was confirmed meanwhile by running the process at different sites and scales under PD and GMP conditions. The CEPI/Wellcome NC3Rs was provided for guidance and considered by Valneva.
GMP manufacturing:
The well designed and robust manufacturing process is complemented by a control and risk management strategy, embedded into a GMP compliant pharmaceutical quality system according to ICH Q7, ICHQ9 and ICHQ10, respectively. Suitable analytical test methods, in-process controls and product specifications are established to control each stage of the product (please refer to Figure 8 & 9). All involved sites for GMP production have implemented a Pharmaceutical Quality System according to ICH Q7, ICH Q10, current EU and US GMP regulations. The GMP manufacturing and testing sites have appropriate GMP licenses, are regularly inspected by EU GMP Authorities and successfully passed FDA inspections. According to the EU GMP legislation each batch of VLA1553 Drug Substance and VLA1553 Drug Product for clinical or commercial supply have to be released by a registered Qualified Person having the appropriate product knowledge, quality oversight, experience and education. Qualified Persons are fulfilling these requirements at Valneva Austria GmbH.
Finally, a manufacturing strategy was chosen to develop a scalable, efficient, high yield process of excellent product quality and robustness.
Clinical conduct:
Valneva performs its clinical studies in compliance with ICH and its applicable regulation. Adherence of the involved parties is being assured applying different measures:
a)Vendors (e.g., CRO, Central lab) selected for Valneva’s project have to undergo a formal vendor selection and qualification process which routinely includes a system audit; if an already qualified vendor is contracted for study conduct, the vendor still has to undergo routine audits according to time intervals defined in Valneva’s standard operating procedure (SOP).
|
|
|
|
a)Study sites qualification for study participation is reviewed and evaluated: prior to study site selection, site qualification visits are performed to understand the site’s previous experience in performing (vaccine) clinical studies, their knowledge of GCP and applicable regulation, their resources (in terms of staff as well as equipment); if necessary, specific training is performed early on prior to study execution in order to assure compliance. During study conduct, on top of routine monitoring visits, Valneva selects sites for the performance of study site audits in line with Valneva’s respective SOP.
b)As per ICH-E6 requirement, risk-based quality management is applied. A risk management plan and risk log is developed for each study to allow timely identification of risks, to identify appropriate risk mitigation measures and to track risks, changes thereof as well as effectiveness of defined measures.
c)Analysis of clinical samples is performed in GCLP certified laboratories following Standard Operating Procedures. Assay validation is done according to ICH Q2 (R1) guidelines.
|
4.Management
Valneva operates according to the most stringent compliance rules, including all applicable GxP requirements, business compliance requirements, corporate social responsibility, and environmental sustainability rules, and is a participant of the United Nations Global Compact. Based on this fundament of expertise Valneva plans to drive the project via the below mentioned key employees supporting the project with existing staff. In addition, we will be expanding the team bringing on board a highly experienced individual to steer the efforts on the post-marketing studies, expected to on-board in January 2024.
A.Project governance
Refer to Team charter for governance arrangements and meeting cadence
Valneva´s project management will lead program management of the entire chikungunya program throughout the duration of the contract. This will include total project lifecycle management, comprising project planning, execution, monitoring, controlling, and closure. Project management will monitor and maintain the Scope of Work (SOW), monitor the program budget and timeline, coordinate project communications internally and with external project stakeholders, prepare reports, and assist in satisfying the CEPI reporting requirements.
Governance bodies with Butantan have been set-up in order to oversee the new Technical Development activities, the Butantan Development plan, the interactions with ANVISA and WHO-PQ as well as the clinical activities in Brazil. The governance bodies encompass:
-A Joint Steering Committee to oversee the development of the BUTANTAN Product based on the BUTANTAN Development Plan
-A Development Committee to (i) approve updates and changes to the BUTANTAN Development Plan; (ii) supervise the activities of the Development Team and the development of the VLA1555 Vaccine Candidate; (iii) resolve issues referred by members of the Development Team; (iv) make strategic decisions related to the BUTANTAN Development Plan; (v) review the progress of the BUTANTAN Development Plan and any related activities; (vi) approve budgets associated with the BUTANTAN Development Plan; (vii) otherwise oversee the BUTANTAN Development Plan, and (vii) monitor the LatAm Studies.
-The former Tech Transfer Committee evolved into the Technical Development committee to oversee the next development activities for VLA1555. This committee and the activities associated are led by Butantan with the support of VLA if needed.
A Joint Steering Committee, Development Committee and Tech Transfer Committee will also be set up with Serum Institute of India.
B.Valneva’s management team
[***]
This dedicated Valneva team is representing a vast range of capabilities and expertise in state-of-the- art vaccine development from bench to global licensure. It will be supported by all functions at Valneva, which are needed to successfully conduct the proposed Project, leveraging the company´s unique and proven development experience.
i.. Planning
1.Annex A: Work Breakdown Structure.
ii.Annex B: RASCI chart:
R- Responsible
A- Approve
S-Support
C-Consulted
I-Informed
iii.Annex C: High level Gantt chart with stage gate decisions for the work packages described above.
iv.Annex D: Detailed Gantt chart, with milestones.
v.Annex E: Risk Table.
Appendix A
Work Breakdown Structure for activities under current funding application
[***]
Appendix B
The outlined RASCI Chart reflects the Valneva project team environment responsible for execution of the project. Valneva internal approvals will be done by the outlined committees:
The Research & Development Operational Committee (RDOC) oversees and steers VLA´s global research & development programs within agreed strategies and budgets ensuring most appropriate, cross-functionally aligned scientific, medical and technical execution of R&D activities, including the company´s innovation agendas
The Operations Committee (OpsCo) oversees and steers VLA’s global industrial operations including manufacturing, supply, quality & regulatory compliance, EH&S and facilities (global), ensuring most appropriate manufacturing & site operations and product supply to demand & quality to internal and external customers.
The Management Board (MB) beside its responsibility for overall corporate and financial strategy and its reporting obligations to the Supervisory Board will focus on the legal representation of the company under the relevant jurisdictions. Further key aspects covered by the MB will be organizational structure, top management and governance development, corporate development, business compliance, investor relations and communications. Functional representatives will also be invited as needed for relevant topics.
[***]
EX-4.11
5
exhibit411-siiagreementred.htm
EX-4.11
Document
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) CUSTOMARILY AND ACTUALLY TREATED BY THE REGISTRANT AS PRIVATE OR CONFIDENTIAL.
MASTER COLLABORATION AND LICENSE AGREEMENT
concluded by and between:
Valneva Austria GmbH, CIN: FN 389960 x / HG Wien, with its registered office at A-1030 Vienna, Campus Vienna Biocenter 3, Austria (hereinafter referred to as “VALNEVA”)
and
Serum Institute of India Private Limited, a company incorporated in India under the Companies Act, 1956, having its registered office at 212/2, Off Soli Poonawalla Road, Hadaspar, Pune 411 028, Maharashtra, India (hereinafter referred to as “SIIPL”)
VALNEVA and SIIPL are hereinafter jointly referred to as the “Parties”, and individually as a “Party”.
WHEREAS:
(A)VALNEVA is a specialty vaccine company focused on the development, manufacturing and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, and is the exclusive owner or licensee of proprietary rights in the VALNEVA Product (as defined below);
(B)SIIPL is a leading pharmaceutical company engaged in the business of researching, developing, manufacturing, supplying, distributing and marketing of biological products and vaccines for human use and lifesaving drugs;
(C)VALNEVA holds, owns and/or otherwise Controls the Licensed Technology (as defined below) related to the VALNEVA Product;
(D)VALNEVA has entered into a funding agreement with CEPI (as defined below) aiming to accelerate Regulatory Approval of the VALNEVA Product, secure supply of the chikungunya vaccine in regions where there is an Outbreak or an Increased Outbreak Preparation Need and, support WHO prequalification to facilitate broader access in low- and middle-income countries. VALNEVA and SIIPL have agreed to enter into this Agreement to address the needs of a chikungunya vaccine in the SIIPL Territory (as defined below);
(E)SIIPL desires to obtain requisite licenses from VALNEVA to use Licensed Technology, either by itself or through its Sub-licensees (as defined below), to Develop, Manufacture, and Commercialize the SIIPL Product (all defined below) in the SIIPL Territory in the Field (as defined below) during the Term (as defined below) of this Agreement;
(F)The Parties have discussed specific CEPI requirements together with CEPI and have signed the CEPI Side Letter (as defined below);
(G)The Parties have agreed to enter into this Master and License Collaboration Agreement to set forth their mutual understandings and agreement on the framework of their collaboration and exploitation efforts as described below, while the specifics for the
Technology Transfer from VALNEVA to SIIPL, the supply by VALNEVA of VALNEVA Drug Substance (as defined below) for use in the Manufacture of the SIIPL Product will be set out in the “Project Agreements”, namely the Technology Transfer Agreement and the Drug Substance Supply Agreement and related quality agreement.
NOW THEREFORE, THE PARTIES HEREBY AGREE as follows:
1. Interpretation and Definitions
Unless specifically set forth to the contrary in this Master Collaboration and License Agreement, the following terms, when capitalized, whether used in the singular or plural, shall have the respective meanings set forth below, and derivative forms of these terms when capitalized shall be interpreted accordingly.
“Affected Territory” means any country, or any geographic area within a country, in which there is an Outbreak or for which there is an Increased Preparation Need.
“Affiliate” With respect to VALNEVA shall mean any Person that is controlled by, controls, or is under common control with VALNEVA as of or after the Effective Date. For such purpose, the term “control” means direct or indirect beneficial ownership of more than fifty percent (50%) of the voting interest in an entity, or more than fifty percent (50%) interest in the income of the entity in question, or the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of an entity.
“Agreement” shall mean this Master Collaboration and License Agreement including any Annexes attached hereto.
“Annual Recalculation Certificate” is defined in Section 11.4.
“Applicable Law(s)” shall mean any applicable national, supranational, or local statute, ordinance, decree or other law, regulation or by-law or any code, rule or direction, including but not limited to GLP, GCP, cGMP and cGDP, and Anti-Corruption Laws or any license, consent, permit, authorization or other approval of any Regulatory Authority, Governmental Entity or any other statutory Person which has appropriate jurisdiction in the countries where the Parties shall perform activities in furtherance of this Agreement and the Project Agreements.
“Anti-Corruption Laws” shall mean any and all anti-corruption and anti-bribery laws applicable to this Agreement and as may be applicable to each Party, including but not limited to the U.S. Foreign Corrupt Practices Act and the UK Bribery Act.
“Batch Record” means the written record documenting the manufacturing process and the history of a batch Manufactured.
“Business Collaborators” shall mean any licensor, licensee, seller, service provider or financing source that has or envisions having a business relationship with a Party or its
Affiliates.
“Business Day(s)” shall mean any day other than a Saturday, Sunday or statutory holiday in Austria or India.
“CEPI” means the Coalition for Epidemic Preparedness Innovations.
“CEPI Side Letter” shall mean the Letter Agreement signed on 18 December 2024 by CEPI, SIIPL and VALNEVA, attached hereto as Annex 6.
“Certified Auditor(s)” is defined in Section 10.9 e.
“Change of Control” means, with respect to a Party, that: (a) any Third Party acquires directly or indirectly the beneficial ownership of more than 50% voting security of such Party, or if the percentage ownership of such Third Party in the voting securities of such Party is increased through stock redemption, cancellation, or other recapitalization, and immediately after such acquisition or increase such Third Party is, directly or indirectly, the beneficial owner of voting securities representing more than 50% of the total voting power of all of the then outstanding voting securities of such Party; (b) any merger, consolidation, recapitalization, or reorganization of such Party is consummated that would result in shareholders or equity holders of such Party owning 50% or less of the outstanding voting securities of the surviving entity (or its parent entity) immediately following such transaction; (c) the shareholders or equity holders of such Party approve any plan of complete liquidation of such Party, or an agreement for the sale or disposition by such Party of all or substantially all of such Party’s assets, in each case, through one or more related transactions, other than to an Affiliate or pursuant to one or more related transactions that would result in shareholders or equity holders of such Party immediately prior to such transaction owning more than 50% of the outstanding voting securities of the surviving entity (or its parent entity) immediately following such transaction; or (d) the sale or transfer to any Third Party, in one or more related transactions, of all or substantially all of such Party’s consolidated assets taken as a whole.
“Claims” is defined in Section 16.4 a.
“Clinical Trial(s)” shall mean any clinical trial involving the administration of a product to a human subject for the purpose of evaluating the safety, efficacy, immunogenicity, performance, or other characteristic of such product.
“Commercialize” means any and all activities directed to the marketing, promotion, distribution, pricing, reimbursement, offering for sale, and sale of the SIIPL Product and interaction with a Governmental Entity in the applicable country or region within the SIIPL Territory for such SIIPL Product regarding the foregoing, but excluding activities directed to Manufacturing, Medical Affairs, or the Development thereof. “Commercialize”, “Commercializing”, and “Commercialized” and “have Commercialized” will be construed
accordingly.
“Commercial License” has the meaning set forth in Section 3.1.
"Commercially Reasonable Efforts" shall mean those efforts in accordance with the subject Party’s efforts and resources normally used by it for a product owned by it, or to which it has rights, which is of similar market potential at a similar stage in its product life, taking into account the competitiveness of the marketplace, the proprietary position of the product, the regulatory structure involved, the profitability of the applicable product, and other relevant factors including technical, legal, scientific or medical factors. Commercially Reasonable Efforts requires, with respect to an obligation, that the Party: (a) promptly assign responsibility for such obligation to specific employees who are held accountable for progress and monitor such progress on an on-going basis, (b) set and consistently seek to achieve specific and meaningful objectives for carrying out such obligation, and (c) consistently make and implement decisions and allocate resources designed to advance progress with respect to such objectives.
“Confidential Information” shall mean and include all data and proprietary information and materials of a Party or its Affiliates or their Business Collaborators, not in the public domain, including without limitation, data, information and materials relating to that Party’s or its Affiliates` or their Business Collaborators’ products and technology, business, affairs, research and development activities, results of pre-clinical and clinical trials, national and multinational regulatory proceedings and affairs, finances, plans, contractual relationships and operations. The terms and conditions of this Agreement shall be considered Confidential Information of both Parties.
"Conforming Product(s)" shall mean SIIPL Product(s) that have been Manufactured in conformance with, and are otherwise themselves in conformance with, Applicable Law (including but not limited to cGMP), Regulatory Approvals, and other terms and conditions of this Agreement and the Project Agreements.
“Control” means, with respect to any assets, including but not limited to materials, or intellectual property rights, the possession (whether by ownership or license) by a Party (or by any Affiliate of a Party) of the ability to grant to the other Party a license to such asset without violating the terms of any agreement, other arrangement or any patent rights or Know-How of any Third Party.
"Current Good Manufacturing Practices" or "cGMP" means manufacturing standards that are consistent with all applicable International Conference on Harmonization Guidelines.
“Develop” or “Developing” means all internal and external research, development, and regulatory activities related to the SIIPL Product, including (a) non-clinical testing, toxicology, testing and studies, non-clinical and preclinical activities, and Clinical Trials, and (b) preparation, submission, review, and development of data or information for the purpose of submission to a Regulatory Authority to obtain authorization to conduct Clinical Trials and to obtain, support, or maintain Regulatory Approval of the SIIPL Product in the SIIPL Territory, but excluding activities directed to Manufacturing, Medical Affairs, or
Commercialization. Development will include development and regulatory activities for additional forms, formulations, or indications for the SIIPL Product after receipt of Regulatory Approval of such product (including label expansion), including Clinical Trials initiated following receipt of Regulatory Approval or any Clinical Trial to be conducted after receipt of Regulatory Approval that was mandated by the applicable Regulatory Authority as a condition of such regulatory approval with respect to an approved formulation or indication (such as post-marketing studies, observational studies, paediatric studies, implementation and management of registries and analysis thereof, in each case, if required by any Regulatory Authority in the SIIPL Territory to support or maintain Regulatory Approval for the SIIPL Product in the SIIPL Territory). “Develop”, “Developing”, “Development”, and “Developed” and “have Developed” will be construed accordingly.
“Development Plan” has the meaning set forth in Section 6.2.
“Distributors” has the meaning set forth in Section 8.27.
“Documentation” is defined in the Technology Transfer Agreement.
“Drug Substance Aliquot” [***].
“Drug Substance Supply Price” means the price of Drug Substance Aliquot paid by SIIPL to VALNEVA under the Drug Substance Supply Agreement which cost agreed on the Effective Date are set forth in Annex 4.
“Drug Product Manufacturing Cost” means the cost incurred by SIIPL in its Manufacturing of the SIIPL Product which costs agreed on the Effective Date are set forth in Annex 4.
“Effective Date” shall mean the date of last signature of this Agreement.
“Equitable Access Plan” is defined in Section 8.25.
“Exploit” or “Exploitation” means to make, have made, use, offer to sell, sell, Develop, have Developed, Manufacture, have Manufactured, perform Medical Affairs,
Commercialize or have Commercialized and other activities related to the SIIPL Product. When used as a noun, exploitation means any and all activities involved in Exploiting.
“Field” shall mean the field of vaccine for the prevention or treatment of chikungunya in humans.
“First Commercial Sale” shall mean, with respect to the SIIPL Product in a country within the SIIPL Territory, the first sale of the SIIPL Product to a Third Party for distribution, use, or consumption in such country after receipt of Regulatory Approval for such SIIPL Product in such country.
“Force Majeure” is defined in Section 20.6.
"Good Clinical Practices" or "GCP" means clinical practices and standards that are consistent with all applicable International Conference on Harmonization Guidelines, including, without limitation, ICH: E6 Good Clinical Practice in its then current version.
"Good Distribution Practices" or "GDP" means, as relevant to the SIIPL Product, the then-current good distribution practices and similar rules, regulations and guidelines, as amended from time to time, applicable to the proper handling, transport, storage, importation, marketing, promotion, sale and distribution of pharmaceutical products in the SIIPL Territory.
"Good Laboratory Practices" or "GLP" means laboratory practices and standards that are consistent with all applicable International Conference on Harmonization Guidelines.
“Governmental Entity” shall mean any court, agency, authority, department, regulatory body or other instrumentality of any government or country or of any national, federal, state, provincial, regional, county, city or other political subdivision of any such government or any supranational organization of which any such country is a member, which has competent and binding authority to decide, mandate, regulate, enforce, or otherwise control the activities of the Parties contemplated by this Agreement and the Project Agreements.
“Intellectual Property Rights” or “IP” shall mean and refer to, throughout the world, (a) Patent Rights, trademarks, trade names, trade dress, service marks, domain names, (b) copyrights, moral rights, related rights (including without limitation so called "neighboring rights" and "sui generis" rights), database rights, trade secrets and know-how, rights in and to databases (including rights to prevent the extraction or reutilization of information from a database), design rights, industrial design rights, utility model rights, topography rights and all other rights associated with works of authorship (including computer programs), creations or performances, whether published or unpublished, and (c) all rights or forms of protection of a similar nature whether registered or applications for registration thereof, including but not limited to all other intellectual property, industrial property, and similar rights.
“Increased Outbreak Preparation Need” shall mean when, having considered all reasonably accessible and relevant information including epidemiological data, travel and migration patterns and the likely availability of other products or product candidates, CEPI determines, in its sole discretion in consultation with experts (for example a sub-group or subcommittee of CEPI’s Scientific Advisory Committee that CEPI determines has appropriate expertise), that there is a heightened need for the SIIPL Product to address potential Outbreaks.
“Joint Steering Committee” or “JSC” shall have the meaning assigned to it in Article 4.
“Key Countries” are defined in Annex 1.
“Know-How” means any proprietary information and documents containing records, discoveries, improvements, modifications, processes, techniques, methods, assays, chemical or biological materials, designs, protocols, formulas, data (including physical data, chemical data, toxicology data, animal data, raw data, clinical data, and analytical and quality control data), dosage regimens, control assays, product specifications, marketing, pricing and distribution costs, inventions, algorithms, technology, forecasts, profiles, strategies, plans, results in any form whatsoever, trade secrets (in each case, patentable, copyrightable, or otherwise).
“Latent Defect” means a defect in the VALNEVA Drug Substance that (i) was not reasonably discoverable using routine quality control procedures and (ii) was discovered after administration of the SIIPL Product on mass scale.
“Licensed Manufacturing Know-How” means all Know-How that (a) is Controlled by VALNEVA or any of its Affiliates as of the Effective Date, (b) is Developed by VALNEVA during the Term of this Agreement, and (c) directly relates to the fill & finish Manufacture of the VALNEVA Product but does not include the Know-How relating to VALNEVA Drug Substance and/or related processes. The detailed scope of the Licensed Manufacturing Know-How is defined in the Technology Transfer Agreement.
“Licensed Patents” means i) the patents listed in Annex 2 of this Agreement and ii) any other future patents that may be filed during the Term of this Agreement by VALNEVA both i) and ii) in as far they are related to the Licensed Manufacturing Know-How.
“Licensed Technology” means the Licensed Manufacturing Know-How, and the Licensed Patents.
“Manufacture” or “Manufacturing” means activities which include, without limitation, the manufacturing, formulation, processing, packaging, labelling, filling, finishing, assembly, shipping, storage, or freight of the SIIPL Product (or any components or process steps involving any product or any companion diagnostic), placebo, or comparator agent, as the case may be, including quality assurance and stability testing, characterization testing, quality control release testing of VALNEVA Drug Substance and SIIPL Product, quality assurance review and release of product, process development, qualification, and validation, scale-up, preclinical, clinical, and commercial manufacture
and analytic development, and product characterization, but excluding activities directed to Development, Medical Affairs, or Commercialization. “Manufacturing” and “Manufactured” and “have Manufactured” will be construed accordingly.
“Manufacturing Report” is defined in Section 10.5.
“Medical Affairs” shall mean the following activities of medical affairs personnel (including medical science liaisons) related to the SIIPL Product in the SIIPL Territory: (i) providing input and assistance with consultancy meetings, and delivering non-promotional scientific exchanges and conducting non-promotional activities such as presenting scientific information; (ii) providing grants to support continuing medical education or symposia for educational needs related to the SIIPL Product, including with respect to its therapeutic use; (iii) development, publication, presentation and dissemination of publications relating to the SIIPL Product; (iv) responding to medical inquiries and providing medical information services in response to inquiries received from healthcare professionals; and (v) arranging meetings with or giving presentations to (in-person or
otherwise) healthcare professionals and other relevant professionals.
“Milestone Payments” are defined in Annex 5.
“Net Profit” means [***].
“Net Sales” means [***].
No deduction will be made for any cost incurred by SIIPL in Developing, Manufacturing, or Commercializing the SIIPL Product except as permitted pursuant to sections (i) to (iv) above; provided that SIIPL Product transferred to Third Parties in reasonable quantities for Clinical Trials (if applicable), compassionate use or expanded access programs, indigent programs, in each case, will give rise to Net Sales only to the extent that SIIPL or its Sublicensee invoices or receives amounts therefor. If a single item falls into more than one of the categories set forth in sections (i)-(iv) above, then such item may not be deducted more than once. All deductions in sections (i) through (iv) above will be fairly and equitably allocated between the SIIPL Product and other products of SIIPL or its Sub-licensee such that the SIIPL Product does not bear a disproportionate portion of such deductions. Calculations of Net Sales will be consistently applied across all of SIIPL’s products and will be consistent between periods. Such amounts will be determined from the books and records of the selling party and will be calculated in accordance with GAAP or IFRS, as applicable.
“Outbreak” means a public health emergency on a national or regional scale declared by one or more public health agencies, in each case as a result of a material increase in the number of cases of people infected with chikungunya including any regional outbreak, an
epidemic or a pandemic.
“Party” shall have the meaning assigned to such term in the preamble hereof.
“Profit Share” is defined in Section 10.3.
"Patent Rights" means all rights, title and interests in and to (a) all national, regional, and international patents and patent applications filed in any country of the world including provisional patent applications and all supplementary protection certificates, (b) all patent applications filed either from such patents, patent applications, or provisional applications or from an application claiming priority from any of these, including any continuation, continuation-in part, divisional, provisional, converted provisional, or continued prosecution application, or any substitute applications, (c) any patent issued with respect to or in the future issued from any such patent applications, including utility models, petty patents and design patents and certificates of invention, and (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations, and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications.
“Payment” is defined in Section 11.2.
“Person” shall mean any individual, corporation, limited liability company, partnership, firm, joint venture, association, joint-stock company, publicly listed company, trust, unincorporated organization, governmental body or other entity.
“Project Agreements” means those agreements listed in Section 2.5, and other agreements as may be necessary for the Parties (or appropriate Affiliates) to implement the collaboration contemplated under this Agreement.
“Quarterly Certificates” is defined in Section 10.6.
"Raw Materials" shall mean any and all compounds, excipients, commodities, and other materials required for the Manufacture of the SIIPL Product.
“Regulatory Approval” means with respect to a certain pharmaceutical product, all approvals by the competent Regulatory Authority in a certain country necessary to manufacture and sell such pharmaceutical product commercially in such country.
“Regulatory Authority” shall mean any federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other Governmental Entity with authority over the marketing, pricing and/or sale of a pharmaceutical product in a country.
“Representatives” is defined in Section 21.2.
“Results” shall mean data generated by either Party during the Term of this Agreement and the Project Agreements.
“SIIPL Claims” is defined in Section 16.2.
“SIIPL Facility” is defined in the Technology Transfer Agreement.
“SIIPL Improvements” shall mean and include all Know-How, invented or Developed during the Term of this Agreement solely by SIIPL or its Sub-licensees relating to the
Manufacture, Development and Commercialization of the SIIPL Product under this Agreement.
“SIIPL Indemnities” is defined in Section 16.2.
“SIIPL Losses” is defined in Section 16.2.
“SIIPL Product” shall mean the finished drug product Developed by SIIPL or its Sublicensees based on the Licensed Technology.
“SIIPL Proprietary Rights” shall mean all proprietary rights, including any and all Intellectual Property Rights, in the SIIPL Improvements.
“SIIPL Territory” is defined in Annex 1 attached to this Agreement.
“Sales and Marketing Cost” means [***].
“Subcontractor” means a Third Party engaged by SIIPL to perform certain obligations or exercise certain rights on behalf of SIIPL under this Agreement without transferring or disclosing without VALNEVA’s prior written consent any trade secrets of VALNEVA more specifically mentioned under Annex 8 of the Technology Transfer Agreement, or having any subcontractor’s right created in Licensed Technology.
“Sub-licensee” means a Third Party to whom SIIPL has granted a sub-license pursuant to Article 3 of this Agreement.
“Successful Completion” as it relates to the technology transfer is defined in the Technology Transfer Agreement.
“Term” is defined in Section 17.1.
“Technology Transfer” shall mean the transfer of Licensed Manufacturing Know-How (as further defined in the Technology Transfer Agreement), relating to the formulation, fill, lyophilization and finish processes necessary for the proper Development and Manufacturing of the SIIPL Product in the SIIPL Territory.
"Technology Transfer Agreement" shall mean that certain Technology Transfer Agreement between the Parties, dated as of even date herewith, setting forth terms and conditions relating to the Technology Transfer of the Licensed Manufacturing Know-How from VALNEVA to SIIPL.
“Third Party” shall mean any Person other than VALNEVA and SIIPL and VALNEVA Affiliates.
“Upfront Payment” is defined in Annex 5.
“Valid Claim” shall mean (a) an unexpired claim (where the claim relates to any composition of matter or method of use) of an issued Patent Right, which claim has not been found to be unpatentable, invalid or unenforceable by a court or other Governmental Entity in the subject country, from which decision no appeal is taken or can be taken; or
(b) a claim (where the claim relates to any composition of matter or method of use) of a pending application, which application claims a first priority no more than seven (7) years prior to the date upon which pendency is determined; provided, however, when any and all such Patent Rights issued based on such pending application, any claim contained therein shall be deemed a Valid Claim as of that point in time. If, in any country, there should be two (2) or more such decisions conflicting with respect to the validity of the same claim, the decision of the higher or highest tribunal shall thereafter control; however, should the tribunals be of equal rank, then the decision or decisions upholding the claim shall prevail when the conflicting decisions are equal in number, and the majority of decisions shall prevail when the conflicting decisions are unequal in number.
“VALNEVA Claims” is defined in Section 16.1.
“VALNEVA Drug Substance” shall mean the active ingredient of the VALNEVA Product as further defined in the Drug Substance Supply Agreement.
“VALNEVA Indemnitees” is defined in Section 16.1.
“VALNEVA Know-How” means all Know-How that (a) is Controlled by VALNEVA or any of its Affiliates on the Effective Date or is developed by VALNEVA during the Term of this Agreement, and that (b) directly relates to the VALNEVA Product and/or the VALNEVA Drug Substance.
“VALNEVA Losses” is defined in Section 16.1.
“VALNEVA Product” shall mean VALNEVA’s chikungunya vaccine in finished form, also known as IXCHIQ® in the VALNEVA Territory.
“VALNEVA Territory” shall mean all countries of the world excluding the SIIPL Territory.
1.1References to sections and paragraphs are to sections and paragraphs to this Agreement (unless the context otherwise requires).
1.2References to this Agreement shall include the Annexes attached hereto.
1.3The headings in this Agreement are inserted for convenience only and shall not constitute a part of this Agreement, and they shall not affect its meaning, construction or effect.
2.Background.
2.1The objectives of this Agreement and the Project Agreements are (i) to speed up the development of a chikungunya vaccine in the SIIPL Territory, which are high-risk outbreak areas, (ii) to ensure that there is a regular supply of the SIIPL Product in countries that have a demand for the vaccine at an affordable price, and (iii) in the context of an Outbreak or an Increased Outbreak Preparation Need to ensure that the SIIPL Product is first available to populations in the Affected Territory when and where they are needed.
2.2The Parties’ activities under this Agreement and the Project Agreements are as follow:
a.VALNEVA will transfer its Licensed Manufacturing Know-How to SIIPL under the terms and conditions of the Technology Transfer Agreement within a period agreed therein. SIIPL will obtain Regulatory Approval of the single-dose SIIPL Product in the SIIPL Territory, including, but not limited to, India and other Key Countries, and WHO prequalification as further set forth in Section 6.5 below.
b.SIIPL will Develop, Manufacture, Commercialize and otherwise Exploit the SIIPL Product in the SIIPL Territory at SIIPL’s sole responsibility, financial risk, cost, and expense, according to the terms and conditions of this Agreement.
c.VALNEVA will supply VALNEVA Drug Substance to SIIPL for purposes of the Technology Transfer and thereafter for the commercial Manufacturing of the SIIPL Product.
d.In parallel with the Technology Transfer of the single-dose SIIPL Product in India, SIIPL may, in its sole discretion, decide to develop a multi-dose version of the SIIPL Product provided such development is not detriment to the technology transfer and registration of the single dose SIIPL Product. However, such development shall be SIIPL’s sole responsibility, conducted at SIIPL’s sole risk, cost, and expense.
2.3R&D Evaluation Agreement. Within [***] following the Effective Date of this Agreement, SIIPL shall provide VALNEVA with a draft pre-clinical development and evaluation agreement, including a work plan pertaining to the evaluation of the feasibility of a potential Dengue - Chikungunya combination vaccine at SIIPL’s own cost (“Evaluation Agreement”). VALNEVA shall promptly review such draft and the Parties shall negotiate in good faith and use Commercially Reasonable Efforts to finalize such an agreement on mutually acceptable terms within [***] following VALNEVA’s receipt of the draft Evaluation Agreement. A potential license to a Dengue – Chikungunya combination vaccine requires a separate collaboration and license agreement between VALNEVA and SIIPL.
2.4Drug Substance Technology Transfer. In case of a significant and consistent increased demand for the Product, the Parties agree that they shall negotiate in good faith and use Commercially Reasonable Efforts to finalize and enter into an agreement for the technology transfer of VALNEVA’s Drug Substance from Valneva to SIIPL on mutually agreed terms and conditions.
2.5Certain specific terms and conditions of the undertakings under Section 2.2 a)-d) above will be set out in separate agreements, including but not limited to, a Technology Transfer Agreement, a Drug Substance Supply Agreement and a Quality Agreement (hereinafter individually referred to as a “Project Agreement” and jointly referred to as “the Project Agreements”).
2.6All terms and conditions of this Agreement shall be incorporated by reference into the Project Agreements. The Parties agree that this Agreement and the Project Agreements will be read harmoniously. To the extent there is any inconsistency or conflict between the
terms of any Project Agreement and the terms of this Agreement, the terms of this Agreement shall prevail.
2.7The Parties will comply with Applicable Law and the agreed obligations under the CEPI Side Letter and will use Commercially Reasonable Efforts to perform their respective obligations under this Agreement and the Project Agreements.
3.License Grant to SIIPL.
3.1Commercial License. Subject to terms and conditions of this Agreement, VALNEVA hereby grants to SIIPL an exclusive, sub-licensable, and profit sharing license to use the Licensed Technology solely together with the VALNEVA Drug Substance supplied by VALNEVA to Develop, Manufacture Commercialize and otherwise Exploit, either through SIIPL directly or through its pre-approved Sub-licensees or Subcontractors, the SIIPL Product, in the SIIPL Territory in the Field during the Term of this Agreement (“Commercial License”). For clarification, the license granted as it relates to the use of VALNEVA’s regulatory dossier (“Dossier”) is restricted to the limited use necessary for regulatory purposes.
3.2Covenant Not to Sue. SIIPL will not sue VALNEVA and/or any of VALNEVA Affiliates or sublicensee(s) on any matter relating to any Improvement made and/or Controlled by VALNEVA and/or its Affiliates to its Licensed Technology and further SIIPL will not challenge the validity of the Licensed Patents.
3.3Sublicense and Subcontractors. The Parties agree that SIIPL may grant, subject to preapproval by VALNEVA, a sublicense (in whole or in part) under the licenses as set forth in this Section 3 to a Sub-licensee. A sublicense to SIIPL’s affiliates does not require any pre-approval, however SIIPL agrees to provide VALNEVA with not less than [***] prior written notice of such affiliate. For clarity, a sub-license to any Third Party requires VALNEVA’s prior written consent. SIIPL shall provide VALNEVA with a redacted copy of each agreement with such pre-approved Sub-licensee. SIIPL shall ensure that any sublicense granted in accordance with the terms of this Agreement shall be on similar terms and conditions as stipulated in this Agreement, however such terms and conditions may not be less stringent than those provided in this Agreement. SIIPL shall remain responsible for the performance of its Sub-licensees, hereunder. For the sake of clarity, it is hereby agreed between the Parties that, as of the Effective Date of this Agreement, SIIPL will be the sole legal entity provided with a license to the Licensed Technology.
3.4SIIPL and / or its Sub-licensees may engage Subcontractors (without transferring or disclosing VALNEVA’s trade secrets as mentioned below without VALNEVA’s prior consent pertaining to the Licensed Technology) to perform SIIPL’s and / or its Sublicensees’ obligations under this Agreement. The Subcontractors shall be in compliance with the Quality Agreement. As between VALNEVA and SIIPL, SIIPL shall remain responsible for its performance, and for the performance of its and its Sub-licensees’ Subcontractors, hereunder. For clarity, any disclosure or transfer of
VALNEVA’s trade secrets require VALNEVA’s prior written consent. Such trade secrets are listed in Annex 8.
3.5Nothing stated in this section shall apply to any Distributors engaged by SIIPL for the Commercialization and Exploitation of the Product in any country within the SIIPL Territory to the extent such Distributors are acting solely in such distribution capacity.
4.Joint Steering Committee (JSC)
4.1Purpose of the JSC. The Parties shall form a Joint Steering Committee (JSC) to oversee the progress of the Development of the SIIPL Product based on the SIIPL Development Plan, such SIIPL Development Plan setting forth inter alia timelines for the Technology Transfer of the Licensed Technology, for the conduct of Clinical Trials (if any), and submission(s) of marketing authorization(s) and WHO prequalification.
4.2Composition of the JSC. The JSC shall be composed of up to 4 (four) members of each Party, respectively, no later than [***] after the Effective Date. The chairperson of the JSC shall be designated annually on an alternating basis between the Parties. The initial chairperson shall be selected by VALNEVA. Each Party shall designate as its representatives’ individuals who have the requisite experience, knowledge and seniority to be able to make decisions on behalf of the Party designating such individual.
4.3Meetings of the JSC. The Joint Steering Committee shall meet at least quarterly and at such other times as the Parties may agree or may be necessary under this Agreement or the Project Agreements. The first meeting of the Joint Steering Committee shall be held as soon as reasonably practicable, but in no event later than [***] after the Effective Date. Meetings shall be held by teleconference or videoconference; provided, however, that there shall be at least [***] face-to-face meeting per calendar year, unless the Parties agree otherwise. Face-to-face meetings shall alternate between the principal business locations of the Parties or be held at such other location as may be mutually agreed upon by the Parties. Meetings may also be called by either Party, on ten (10) days written notice to the other.
4.4At least [***] prior to each meeting of the JSC, SIIPL shall submit to VALNEVA an updated report on the status of activities under the SIIPL Development Plan.
4.5All decisions of the JSC shall be made by unanimous consent of the members present in person or by telephone or teleconferences/videoconferences at any meeting, with SIIPL members cumulatively having one (1) vote and VALNEVA members cumulatively having one (1) vote. A quorum for a meeting shall require at least one (1) representative from SIIPL and at least one (1) representative from VALNEVA.
In the event that unanimity cannot be reached by the JSC with respect to a matter that is subject to its decision-making authority, then the matter shall be referred for further review and resolution to the CEO of VALNEVA or such other similar position designated by VALNEVA from time to time, and the CEO of SIIPL, or such other similar position designated by SIIPL from time to time. The designated persons at each
Party shall use reasonable efforts to resolve the matter within [***] after the matter is referred to them. In the event that the designated officers fail to resolve the matter, the Parties agree to submit the matter to be resolved to a suitably qualified independent individual acceptable to both Parties. In the event that the matter can still not be resolved within thirty (30) days of its referral to the independent person jointly approved by both Parties, the dispute resolution provisions according to Section 20.5 shall apply.
4.6Within [***] following the meeting of the Joint Steering Committee, the JSC chair will circulate among the JSC members the meeting minutes, including any decisions made by the JSC and any action items to be completed.
4.7The Joint Steering Committee shall not have the authority to amend, modify or waive compliance with any term or condition of, or take any action inconsistent with or in violation of this Agreement and the Project Agreements or any Applicable Law.
4.8The JSC shall be entitled to appoint subcommittees, specifically for the clinical development and the Technology Transfer.
4.9Costs. The Parties agree that the costs incurred by each Party in connection with its participation in the Joint Steering Committee shall be borne solely by such Party.
5.CEPI Side Letter Requirements
5.1 The Parties hereby agree that:
5.1.1VALNEVA has entered into a funding agreement with CEPI aiming to accelerate Regulatory Approval of the VALNEVA Product, secure supply of the chikungunya vaccine in regions where there is an Outbreak or Increased Outbreak Preparation Need and, support WHO prequalification to facilitate broader access in low- and middle-income countries.
5.1.2Both Parties mutually acknowledge that they have entered into an understanding with CEPI, and, as a result of this understanding, both Parties agree that the CEPI Side Letter shall be incorporated herein by reference hereto and is applicable to the licenses provided to SIIPL under this Agreement.
6.Development Plan, Regulatory Activities, Diligence, and potential Clinical Trials
6.1The Parties agree to form a Development Committee to oversee the Development Plan and the Development activities performed by SIIPL under this Agreement and the Project Agreements. The Parties acknowledge that development oversight is necessary solely for proper reporting by both VALNEVA and SIIPL to CEPI on the progress of the SIIPL Product to be accessible to the public health market in the SIIPL Territory, at an affordable price.
6.2Development Plan. Not later than [***] after the Effective Date, SIIPL shall provide VALNEVA with a detailed written plan for SIIPL’s Development of the SIIPL Product
throughout the SIIPL Territory including but not limited to Development and regulatory strategies and timelines for Development activities, deliverables, CMC strategy and path towards Regulatory Approval (the “Development Plan”), subject to and as further described in this Section 6.2. SIIPL will obtain and maintain all Regulatory Approvals required to Develop and Commercialize the SIIPL Product throughout the SIIPL Territory at SIIPL’s own cost. SIIPL will perform all Development of the SIIPL Product in accordance with the Development Plan. The Development Plan and any updates thereto will include a high-level summary of key Development activities for the SIIPL Product and approximate timelines for such activities, provided that such timelines are subject to change due to applicable timelines and requirements of Regulatory Authorities, including for obtaining and maintaining permissions and other Regulatory Approvals. The Development Plan and updates thereto will include all Development activities necessary to obtain and maintain all Regulatory Approvals to Commercialize the SIIPL Product in each country in the SIIPL Territory and any other activities otherwise recommended or required by the applicable Regulatory Authority in any country in the SIIPL Territory to obtain or maintain such Regulatory Approvals. SIIPL will update the Development Plan as necessary during the Term and will provide each such update to VALNEVA for review and comment. SIIPL will incorporate all reasonable comments received from VALNEVA regarding Development activities for the SIIPL Product that are relevant to obtaining or maintaining Regulatory Approvals to Commercialize the SIIPL Product in any country in the SIIPL Territory.
6.3Development Committee. The Parties shall appoint a development committee of equal number of members designated by VALNEVA and SIIPL, respectively, no later than [***] after the Effective Date ("Development Committee"). The members from each Party collectively will have one (1) vote. The function of the Development Committee shall be to: (i) review and comment upon updates and changes to the SIIPL Development Plan; (ii) advise on the Development of the SIIPL Product generally; (iii) review the progress of the SIIPL Development Plan and any related activities; (iv) appoint subcommittees (with equal participation by the Parties) and set forth the applicable procedures of operation for such subcommittees, and (v) otherwise oversee the SIIPL Development Plan. The Development Committee shall hold meetings at least quartery and at such other times as the Parties may agree or may be necessary. Meetings shall be held by teleconference or videoconference; provided, however, that there shall be at least [***] per calendar year, unless the Parties agree otherwise. Face-to-face meetings shall alternate between the principal business locations of the Parties or be held at such other location as may be mutually agreed upon by the Parties. Within [***] following the meeting of the Development Committee, SIIPL will provide VALNEVA with the draft meeting minutes for review, including any decisions made by the Development Committee and any action items to be completed. Each Party shall bear its own costs and expenses incurred in connection with such meetings.
6.4Regulatory Activities. Subject to and as further described in this Section 6.4, SIIPL will have sole control over and decision-making authority with respect to all regulatory activities for
the SIIPL Product in the SIIPL Territory, including obtaining and maintaining, in its name or the name of its designee, all Regulatory Approvals, licenses, and permits required to Commercialize the SIIPL Product in the SIIPL Territory, and any correspondence or meetings with Regulatory Authorities regarding any of the foregoing, provided that SIIPL shall give VALNEVA [***] prior written notice that SIIPL will be providing any such submissions for VALNEVA’s review, which review shall not unreasonably delay such filings, or as may be decided by the Parties mutually, in advance of SIIPL ’s filing or submission thereof, and SIIPL will incorporate any reasonable comments received from VALNEVA into such regulatory submissions (including with respect to the inclusion or exclusion of VALNEVA’s Confidential Information). SIIPL shall allow VALNEVA and CEPI to participate in meetings with the relevant Regulatory Authority as a silent observer, provided the Regulatory Authority so accepts. At VALNEVA’s and CEPI’s reasonable request, SIIPL shall request a meeting with Regulatory Authorities to address any significant unresolved issues. SIIPL will conduct such regulatory activities in accordance with the then-current Development Plan. SIIPL will be solely responsible for all costs and expenses incurred by it to obtain and maintain such Regulatory Approvals required to Commercialize the SIIPL Product in the SIIPL Territory. Upon SIIPL’s request and without incremental charge to SIIPL, VALNEVA will provide reasonable support to SIIPL (which support shall be detailed in the Development Plan) to obtain or maintain Regulatory Approval to Commercialize the SIIPL Product in any country in the SIIPL Territory, and in the activities in support thereof, to the extent VALNEVA has the resources to do so. VALNEVA will provide letters of authorization as necessary for SIIPL to exercise its right of reference to drug master files and VALNEVA may, at either Party’s request, participate in any meetings (in person or by teleconference) with any Regulatory Authority regarding any Regulatory Approval necessary to Commercialize the SIIPL Product in the SIIPL Territory, in each case, without incremental charge to SIIPL.
6.5Diligence Obligations. SIIPL will perform all activities set forth in the Development Plan in accordance with Applicable Law and use its best efforts to perform all such activities in accordance with the applicable timeframes set forth in the Development Plan, including but not limited to the obligation to apply for and use Commercially Reasonable Efforts to 1) obtain Regulatory Approval and/or licensure of the SIIPL Product within the SIIPL Territory, 2) obtain WHO prequalification, and 3) Commercialize the SIIPL Product in the SIIPL Territory. The Parties have agreed that SIIPL shall prioritize and use Commercially Reasonable Efforts to obtain Regulatory Approval and licensure of the SIIPL Product in the Key Countries. SIIPL shall use best efforts to implement the Launch Readiness Plan for Key Countries in accordance with agreed timelines. In case SIIPL fails to provide and implement such Launch Readiness Plan within the agreed timelines, Section 4.5 shall apply. SIIPL shall use Commercially Reasonable Efforts to apply for WHO prequalification no later than [***] after Regulatory Approval in India. SIIPL shall further use Commercially Reasonable Efforts to pursue UNICEF procurement of the SIIPL Product within the SIIPL Territory within [***] of obtaining WHO prequalification. SIIPL shall use best efforts to ensure the SIIPL Product is added onto the National Immunization Program of India and other Key
Countries within [***] of Regulatory Approval of the SIIPL Product in India and other Key Countries, as applicable.
6.6Development Costs. SIIPL shall bear its own costs and expenses incurred in connection with the performance of its obligations under the Development of the SIIPL Product.
6.7Clinical Trials. In the event SIIPL would like to conduct any Clinical Trials such Clinical Trial shall be agreed upon and regulated in a separate agreement between VALNEVA and SIIPL. The Parties agree that only in the event SIIPL decides to accept any funding directly from CEPI for the conduct of such Clinical Trials then the terms and conditions of such funding would be agreed upon in a separate agreement with CEPI as agreed in the CEPI Side Letter.
6.8Development and Regulatory Records. SIIPL will maintain and retain for a period of [***], complete, current and accurate records of all Development and regulatory activities conducted by it hereunder, and all data and other information resulting from such activities. Such records will fully and properly reflect all work done and Results achieved in the performance of the Development and regulatory activities in good scientific manner appropriate for regulatory and patent purposes. These records will be made available to VALNEVA upon request.
7.Intellectual Property, Infringement and Prosecution
7.1Ownership of IP. Both Parties shall keep all right, title and interest in and to their respective IP. Unless specifically agreed hereunder otherwise, nothing herein shall grant any Party a license to the other Party’s IP. For clarity, all Intellectual Property Rights in relation to Licensed Technology shall be VALNEVA’s IP and shall remain the exclusive property of VALNEVA, and all Intellectual Property Rights in SIIPL Improvements and excluding VALNEVA Licensed Technology are owned and Controlled by SIIPL as on the Effective Date of the Agreement and anytime thereafter shall be SIIPL’s IP and shall remain the exclusive property of SIIPL.
7.2SIIPL Product Trademark. SIIPL have control over and the decision-making with respect to, the creation, development, selection, and approval of all trademarks and trade dress under which the SIIPL Product is Commercialized in the SIIPL Territory. In addition, SIIPL will have sole control over and decision-making with respect to, filing, prosecuting, registering, maintaining, and protecting the trademarks and trade dress to be used to Commercialize the SIIPL Product in the SIIPL Territory at its own costs and expense.
7.3SIIPL Improvements, Results and IPR. SIIPL Improvements, Results and Intellectual Property Rights generated solely by SIIPL under this Agreement pertaining to the SIIPL Product shall be exclusively owned by SIIPL.
7.4Tangible Ownership of the SIIPL Product. SIIPL will have tangible ownership of the SIIPL Product(s) Manufactured by SIIPL or on behalf of SIIPL, as well as the right to Commercialize and Exploit the same under the license granted in Section 3.1.
7.5No Implied Licenses. Neither Party is granted any rights to any Patent Rights, Know-How,
or other Intellectual Property Rights owned or Controlled by the other Party, other than as explicitly identified herein. Nothing herein will affect the Parties’ respective ownership of any Patent Rights, Know-How, or other Intellectual Property Rights owned by such Party.
7.6Prosecution and Maintenance of Licensed Patents. VALNEVA shall, at its own judgment and expense and in its sole discretion, control the preparation, filing, prosecution and maintenance and conduct any other matters relating to the Licensed Patents.
7.7Third Party Infringement with respect to Licensed Technology. In the event that during the Term of this Agreement and thereafter SIIPL obtains actual knowledge that a Third Party is infringing in the SIIPL Territory any Licensed Patent licensed under this Agreement, it shall promptly notify VALNEVA thereof, setting forth the facts of which it has knowledge in reasonable detail. VALNEVA shall have the first right to effect termination of such infringement, including bringing suit or other proceedings against the infringer in its own name, and shall keep SIIPL informed of the progress of such proceedings. VALNEVA shall bear all its costs incurred in connection with any such lawsuit or other proceeding which it may institute, including the reasonable out of pocket expenses incurred by SIIPL in providing any assistance which VALNEVA may request, and VALNEVA shall be entitled to collect and retain for its own account any damages or profits as may be awarded as a result of such lawsuit or other proceeding.
In case VALNEVA elects, in its sole discretion, not to pursue the infringer, SIIPL may, in is discretion, bring suit or other proceedings against the infringer in its own name and at its own cost; however, in no event shall SIIPL, through any court action or proceeding, any settlement arrangement or any proceeding, filing or communication with any patent office, admit to the abandonment, invalidity of or unenforceability of, or otherwise impair VALNEVA’s rights in, any Licensed Patents, without VALNEVA’s prior written consent. Under no circumstances shall SIIPL agree to restrict or take any actions that would be to the detriment of any Licensed Technology in a potential settlement agreement with any infringer. Should SIIPL wish to cease the prosecution of an infringer of such Licenced Technology, SIIPL shall promptly notify VALNEVA thereof and VALNEVA shall, in its sole discretion, be entitled to continue to pursue or not pursue the infringer. For clarity, nothing in this Agreement shall be construed as obligating either Party to proceed against an infringer.
7.8Infringement Actions. Subject to Section 7.6-7.7, the Party exercising any enforcement
rights:
(i)shall have full control over the conduct of the action; and
(ii)shall keep the other Party reasonably informed of the progress of and developments in any proceedings against infringers.
7.9Defense of Licensed Technology. VALNEVA shall have, at its own judgment and expense
and in its sole discretion, sole control the defence of the Licensed Patents and/or Licensed Manufacturing Know How. However, VALNEVA agrees that SIIPL can also have the same defence on any SIIPL Results, Know-How and Intellectual Property Rights generated under this Agreement.
8.Manufacture and Commercialization.
8.1SIIPL and VALNEVA shall mutually discuss and agree on a Launch Readiness Plan including the information set forth in Annex 3 (“Launch Readiness Plan”). Such Launch Readiness Plan shall be agreed, with regard to India within [***] of the Effective Date of this Agreement and with regard to any other country within the SIIPL Territory, [***] prior to the First Commercial Sale.
8.2Right of Reference. Regulatory Cooperation. VALNEVA will provide SIIPL with applicable information, reports, documents, certificates, and any other materials regarding the Licensed Technology that are reasonably requested by SIIPL and necessary for SIIPL to Manufacture, have Manufactured the SIIPL Product, to Develop the SIIPL Product and to obtain and maintain Regulatory Approval for the SIIPL Product in the SIIPL Territory.
8.3Regulatory Inquiries. VALNEVA will promptly notify SIIPL in writing of any governmental or regulatory inquiries, inspections, or audits directly related to the Licensed Technology and any findings related to the same. SIIPL will promptly notify VALNEVA in writing of any governmental or regulatory inquiries, inspections, or audits directly related to the SIIPL Product and any findings related to the same.
8.4Commencement of Commercial License. The Parties agree that with the completion of the transfer of Documentation of the Licensed Manufacturing Know-How to SIIPL as set forth in the Technology Transfer Agreement SIIPL is able to use and benefit from the Commercial License under Section 3.1.
8.5Manufacture of Conforming Product. SIIPL shall at its sole cost and expense Manufacture Conforming Product in accordance with this Agreement, including the Quality Agreement, in sufficient quantities to meet market demand in the SIIPL Territory based on the Forecasted Quantities according to Section 8.20. SIIPL and/or its Sub-licensees shall be the exclusive manufacturer of the SIIPL Product intended for sale in the SIIPL Territory.
8.6VALNEVA Rights Outside the SIIPL Territory. Nothing herein or in any Project Agreement, shall in any way limit VALNEVA’s right and ability to Develop, Manufacture, Commercialize and Exploit, either by itself or through its Affiliates and contracted Third Parties any product, including without limitation the VALNEVA Product, anywhere in the world outside the SIIPL Territory.
8.7VALNEVA Drug Substance. During the Term of this Agreement VALNEVA will supply VALNEVA Drug Substance to SIIPL for the Manufacture of the SIIPL Product. The terms and conditions of such VALNEVA Drug Substance supply are set forth in the Drug
Substance Supply Agreement. For clarity, any Sub-licensee will have to enter into a separate supply agreement with VALNEVA for the supply of VALNEVA Drug Substance to such Sub-licensee.
8.8Raw Materials and Equipment. SIIPL shall at its sole cost and expense (i) procure all Raw Materials, machines, format pieces and equipment required for the Manufacture of Conforming Product hereunder.
8.9Quality Agreement. As between the Parties, all matters pertaining to quality control and quality assurance, stability testing and waste under this Agreement shall be governed by a separate Quality Agreement to be entered into between the Parties. Such Quality Agreement is incorporated herein by reference hereto and shall be concluded as soon as possible following the Effective Date.
8.10Fill & Finish. In accordance with Section 8.5 SIIPL shall at its sole cost and expense Manufacture (including the fill & finish) the SIIPL Product. Manufacture shall be done at a SIIPL Facility or a facility of the Sub-licensee that (i) complies with and is maintained in accordance with Applicable Law, applicable facility specifications and other terms and conditions of the Project Agreements.
8.11Packaging and labelling. SIIPL shall at its sole cost and expense be responsible for the packaging and labelling of the SIIPL Product and procure all required materials therefore in accordance with the Quality Agreement.
8.12Product Testing. All SIIPL Product Manufactured hereunder will be tested for conformance according to the Quality Agreement. SIIPL shall conduct all tests that are SIIPL's responsibility according to the Quality Agreement, at SIIPL’s sole cost and expense.
8.13Retention of Samples. In accordance with the Quality Agreement, SIIPL shall retain a sufficient quantity of samples of each batch of SIIPL Product Manufactured hereunder to allow at least full duplicate testing by or for VALNEVA and as otherwise required by Applicable Law, including stability samples for the period required by Applicable Law. Upon VALNEVA’s request, SIIPL shall make such samples available to VALNEVA for inspection and testing in accordance with the Quality Agreement. SIIPL shall properly store the retained samples in a suitable storage facility and otherwise in accordance with Applicable Law.
8.14Retention of Records. In accordance with the Quality Agreement, SIIPL shall maintain and retain books and records, excluding financial records, pertaining to the Manufacture and testing of SIIPL Product and as otherwise required by Applicable Law for the period required by Applicable Law including without limitation sufficient books and records to ensure SIIPL's and VALNEVA's ability to perform a complete lot history via lot tracing of the SIIPL Product and to otherwise ensure compliance with Applicable Law. Without limiting the generality of the foregoing, such books and records shall contain testing and quality assurance records, batch production records, deviation reports, Raw Materials
data, analytical assays and data, standard operating procedures and other process documents and records directly related to the manufacture of SIIPL Product and any other information that may be required to be retained under Applicable Law.
8.15Inspections and Audits. In accordance with the Quality Agreement, VALNEVA shall have the right to conduct inspections, audits and investigations of the SIIPL Facility, equipment, record keeping procedures and records related to the Manufacture of the SIIPL Product up to [***] per calendar year with at least [***] prior written notice. VALNEVA shall also have the right at its discretion to have a representative reasonably acceptable to SIIPL present in the SIIPL Facility to observe and monitor the Manufacture of the SIIPL Product. SIIPL shall provide prompt written notice to VALNEVA of any inspections or investigations by the applicable Regulatory Authority directed towards the SIIPL Product or the SIIPL Facility used in the Manufacture of SIIPL Product and shall also provide VALNEVA with an English translation of the communication received form the Regulatory Authority, such translation to be prepared by a qualified translator. Prior to submitting any written communication to the Regulatory Authorities, SIIPL shall submit to VALNEVA for review and approval, the draft communication and an English translation thereof prepared by a qualified translator. Notwithstanding anything to the contrary, should SIIPL receive a citation as a result of a regulatory inspection or should SIIPL be found by VALNEVA to be in violation of any Applicable Law or any terms or conditions of this Agreement, VALNEVA shall be entitled to perform for cause inspections, audits and investigations of SIIPL’s applicable facilities, equipment, record keeping procedures and records related to the Manufacture of SIIPL Product as necessary to verify that SIIPL is thereafter in full compliance with Applicable Law and all terms or conditions of this Agreement.
8.16Regulatory Licenses. SIIPL shall obtain and maintain at its sole cost and expense any and all licenses, permits and consents, including, without limitation, facility licenses and permits required by Applicable Law or by Regulatory Authorities, to perform its obligations and carry out its activities under this Agreement, including without limitation, the Manufacture of SIIPL Product for sale in the SIIPL Territory and other Exploitation activities in accordance with this Agreement.
8.17Safety and Environmental Procedures. SIIPL shall maintain and enforce at its sole cost and expense all health, environmental and safety procedures for the Manufacture, handling, storage and shipping of the SIIPL Product and Raw Materials and the generation, treatment, handling, storage and/or disposal of wastes relating thereto that comply with Applicable Law and Section 21.
8.18Personnel. SIIPL represents and warrants that it has and will continue to have the appropriate skills, personnel, equipment and other resources necessary to perform its obligations under this Agreement. SIIPL shall in no event engage any Third Party in the Manufacture of the SIIPL Product or permit any Third Party to gain access to Licensed Technology except to the extent expressly permitted by Article 3.
8.19Manufacturing Capacity. SIIPL shall at all times during the Term of this Agreement maintain the capability to Manufacture the SIIPL Product in sufficient quantities 1) to satisfy market demands in the SIIPL Territory based on the Forecasted Quantities according to Section 8.20, and 2) the Safety Stock agreed under the CEPI Side Letter.
8.20Market Demand Forecasts. No later than [***] prior to the anticipated First Commercial Sale of the SIIPL Product, and thereafter on a quarterly basis, SIIPL shall submit to VALNEVA a [***] rolling forecast of the estimated quantity of SIIPL Product to be Manufactured by SIIPL to satisfy market demands in the SIIPL Territory ("Forecasted Quantities”).
8.21Manufacturing Shortfall. Manufacturing Shortfall and Failure to Supply. In the event that
SIIPL anticipates, or in the event SIIPL’s actual Manufacturing capacity will, or does not meet Forecasted Quantities resulting in a shortfall (“Manufacturing Shortfall”), SIIPL shall immediately notify VALNEVA in writing of any such anticipated or actual Manufacturing Shortfall. If an anticipated or actual Manufacturing Shortfall is expected to last more than [***], the Parties shall promptly upon receipt of notice meet to discuss SIIPL's efforts to remedy the Manufacturing Shortfall and ways to maintain its Manufacturing capacity. Furthermore, upon VALNEVA’s request, SIIPL shall provide VALNEVA with all materials, documents and other information relevant to confirm SIIPL's efforts and their respective status.
8.22During a period whereby SIIPL is in a Manufacturing Shortfall situation, and upon notice of such Manufacturing Shortfall situation, the Parties shall discuss in good faith the potential supply of VALNEVA’s or any designated Third Party’s, chikungunya product into the SIIPL Territory for as long as the Manufacturing Shortfall situation remains so as to preserve the availability of a chikungunya product within the SIIPL Territory.
8.23In the event that (i) SIIPL is in Manufacturing Shortfall and does not cure such Manufacturing Shortfall within [***] from the date of VALNEVA’s receipt of notice thereof, such Manufacturing Shortfall shall be deemed a material breach for purposes of this Agreement.
8.24Marketing and Sales. SIIPL or, if applicable, its Sublicensee, shall at its sole cost and expense be responsible for all advertising, marketing and sales activities with respect to SIIPL Product in the SIIPL Territory and use Commercially Reasonable Efforts to market and sell SIIPL Product in the SIIPL Territory in which Regulatory Approval for the SIIPL Product has been obtained in accordance with terms and conditions of this Agreement and Applicable Law. SIIPL or, if applicable, its Sublicensee, shall (i) at all times conduct business in a manner that reflects favourably on the SIIPL Product; (ii) not disparage the good name, good will or reputation of VALNEVA; (iii) not engage in deceptive, misleading or unethical practices; (iv) not make any false or misleading representations or other statements with regard to VALNEVA or the SIIPL Product.
8.25Equitable Access Plan. SIIPL acknowledges and agree that the objectives with this Agreement are to (i) speed up the development of a chikungunya vaccine in the SIIPL
Territory, (ii) ensure that there is a regular supply of the SIIPL Product in countries that have a demand for the vaccine at an affordable price, and (iii) in the context of an Outbreak or an Increased Outbreak Preparation Need to ensure that the SIIPL Product is first available to populations in the Affected Territory when and where they are needed. Based on the aforementioned, the initial plan to support such objectives (the “Equitable Access Plan”) set out in the CEPI Side Letter outlining, among other things, how the SIIPL Product will be made available to all populations in the SIIPL Territory and Affected Territories without undue delay and at an affordable price.
8.26Supply Commitment.
8.26.1Diligent Commercial Efforts. SIIPL shall at all times during the Term of this Agreement diligently Commercialize and otherwise Exploit the SIIPL Product in the SIIPL Territory in sufficient quantities to satisfy market demands the same being subject to fulfilment of VALNEVA’s obligations under the Drug Substance Supply Agreement to adequately supply VALNEVA Drug Substance without material delay. In addition, SIIPL shall supply the SIIPL Product in accordance with the requirements set forth in the CEPI Side Letter.
8.27Distributors. SIIPL may market, advertise, distribute, and sell SIIPL Product either directly or through its resellers, distributors and other marketing partners or collaborators (“Distributors”), provided however that (i) such Distributors are subject to a written agreement with SIIPL and will not be contrary to any provision herein mentioned. SIIPL shall remain responsible and liable towards VALNEVA for any acts and omissions of its Distributors.
8.28Regulatory Inquiries. VALNEVA will promptly notify SIIPL in writing of any governmental or regulatory inquiries, inspections, or audits directly related to the Licensed Technology or any findings related to the same. SIIPL will promptly notify VALNEVA in writing of any governmental or regulatory inquiries, inspections or audits directly related to the SIIPL Product and any findings related to the same.
8.29SIIPL Product Recalls and Withdrawals. Each Party will promptly notify the other in writing in detail if (a) any batch of SIIPL Product is alleged or proven to be the subject of a recall, market withdrawal, or correction in the SIIPL Territory; (b) such Party reasonably determines that a recall is necessary; or (c) such Party becomes aware of any quality or risk issues related to SIIPL Product. SIIPL will be responsible for instituting a recall, market withdrawal, or correction of the SIIPL Product at its own cost and expense unless a recall is required due solely to any Latent Defects in the VALNEVA Drug Substance. Each Party will cooperate as reasonably requested by the Party responsible for recall.
8.30Pharmacovigilance (PV) Agreement. The Parties shall enter into a Pharmacovigilance Agreement as and when required and on mutually agreed terms between the Parties.
9.Trade Name and Trademarks.
9.1Product Trade Name and Product Trademarks. SIIPL shall market, distribute, sell or otherwise Commercialize the SIIPL Product in the SIIPL Territory under a different trade name and/or trademark(s) than the VALNEVA Product ("SIIPL Trade Name" and “SIIPL Trademark” respectively). SIIPL shall be the owner and exclusively hold all rights in the SIIPL Trade name and SIIPL Trademark.
9.2Trademark. Neither Party shall use any trademark of, or any trademark similar to that of, the other Party.
9.3Third Party Infringement. Each Party shall promptly notify the other Party in writing upon learning of any actual, alleged or threatened infringement of the other Party’s trade name and/or trademark(s), Each Party shall be responsible, at its sole cost and expense, to take actions and bring suit or other proceedings against such Third Party infringer, and shall keep other Party reasonably informed on the status of such actions and proceedings.
10.Financial Terms
10.1Upfront Payment. As consideration for the rights and licenses to be granted by VALNEVA
to SIIPL under this Agreement, and the Project Agreements, SIIPL shall make an Upfront Payment to VALNEVA as more particularly described in Annex 5.
10.2Milestone Payments. In addition to the Upfront Payment, SIIPL shall make Milestone Payments to VALNEVA as described in Annex 5.
10.3Profit Sharing. As further consideration for the rights and licenses to be granted by VALNEVA to SIIPL under this Agreement, SIIPL shall pay to VALNEVA the compensation set forth in Annex 4 on the Net Profit (“Profit Share”). The Profit Share shall be paid to VALNEVA by SIIPL on a country-by-country basis starting on the date of the First Commercial Sale of the SIIPL Product in the respective country within SIIPL Territory and continuing until the expiration or termination of this Agreement The Payments pertaining to Profit Share, shall be invoiced on a quarterly basis and be paid in accordance with the payment terms set forth in Section 10.4. Payments will be certified in accordance with Sections 10.9 e. and 11.3 and amounts based on the Sales Reports outlined in Section 10.6.
10.4Payment Terms. During the [***] after the Effective Date, SIIPL shall pay invoices within [***] after receipt of invoice with respect to the Profit Share (Annex 4). Payment terms thereafter shall be [***] after receipt of invoice. As regards the Upfront Payment the payment term is [***] after receipt of invoice and as regards the Milestone Payments, payment terms shall be [***] after receipt of invoice.
10.5Manufacturing Reports. Upon the finalization of a Manufacturing campaign, SIIPL shall furnish to VALNEVA the following documentation: 1) the Batch Record for the batch of VALNEVA Drug Substance used for Manufacturing, and 2) a Manufacturing report indicating amount of doses of SIIPL Product Manufactured, Drug Substance Aliquots used for the Manufacture, failed batches and losses together with the amount of
the then current stock of VALNEVA Drug Substance Aliquots and the amount of SIIPL Product Manufactured (“Manufacturing Report”). The Parties shall discuss and mutually agree on the format to be used for the Manufacturing Report within [***] after the Effective Date.
10.6Sales Reports. As agreed in this Agreement, SIIPL shall furnish to VALNEVA on a country-by-country basis: 1) a sales report including Net Sales, Net Profit, amount of doses of SIIPL Product sold, the Ex Manufacturing Price of SIIPL Product sold (in both local currency and in Euros (as per Section 11.1) and specifying Batch Record and percentage of SIIPL Product sold) pertaining to the corresponding SIIPL Product sold during the preceding calendar quarter, and 2) a certificate from its Certified Auditors for the calculation of its Payment obligations for every calendar quarter (the “Quarterly Certificates”) within [***] of the end of each such quarter.
10.7Records. During the Term of this Agreement and for a minimum period of [***] thereafter, SIIPL shall keep detailed, accurate and up to date records and books of account accessible electronically from a single location, showing the quantity, description and price of all SIIPL Product(s) supplied in each country of the SIIPL Territory and all sums paid to VALNEVA during the previous [***]. SIIPL shall ensure that such records and books of accounts are sufficient to ascertain the calculation of its Payment obligations with respect to SIIPL Product supplied by SIIPL in the SIIPL Territory.
10.8Costs. [***]
10.9Consistent with this Agreement and the CEPI Side Letter, SIIPL shall:
a.) comply with controls, good management practices, procedures and standards at least as rigorous as its local Generally Accepted Accounting Principles (GAAP), or International Financial Reporting Standards (IFRS) if adopted by SIIPL, as confirmed in SIIPL’s annual audited financial statement;
b.) keep accurate, complete and reliable records of revenues and expenditures under the project (hereinafter “Financial Records”) against an individual project code;
c.) retain all Financial Records for [***] after termination or expiration of this Agreement for whatever reason or for any longer period as required by Applicable Law or SIIPL’s own policies and allow VALNEVA access to such records as set out in Section 6.8 for such retention period;
d.) upon VALNEVA`s request, provide up-to-date audited financial statements, and relevant extracts from the auditors’ report for such financial statement as well as the management letter to the auditors;
e.) Financial audits. SIIPL shall furnish a certificate from its Certified Auditors for the calculation of SIIPL’s Payment. As used herein, “Certified Auditors” means an auditor firm duly licensed to practice as an auditor and whose lead individual responsible for audits has sufficient experience for auditing biotechnology, biopharma or pharmaceutical companies and who is responsible and liable under Applicable Law. Notwithstanding the foregoing, VALNEVA
shall have the right to, on an annual basis, request SIIPL to engage, at its own cost, the European accounting firm KNAV (contact details to be provided separately) to provide an additional certificate confirming the certificate from the Certified Auditor. For clarity, the financial terms agreed under this Section 10 do not apply to any potential combination vaccine development. If the Parties enter into a separate agreement on such combination vaccine development pursuant to Section 2.3 of this Agreement, the financials related to such development shall be agreed in such separate agreement.
11.Payment Terms.
11.1Currency and Currency Conversion. All payments by either Party to the other Party under this Agreement shall be made in [***]. Where calculation of the Profit Share or other amounts due under this Agreement requires the conversion to [***] of Net Sales generated or other amounts received in any other currency, conversion to Euros will be calculated based on the average of the mid-market exchange rates of the relevant country’s currency to [***] of the relevant quarter, as quoted by the Reserve Bank of India (https://www.rbi.org.in).
11.2Taxes. The amounts payable by SIIPL to VALNEVA pursuant to this Agreement (each, a
“Payment”) shall be paid free and clear of any and all taxes, except for any withholding taxes required by Applicable Law. Except as provided in this section, VALNEVA shall be solely responsible for paying any and all taxes on income (other than withholding taxes required by Applicable Law to be deducted from Payments and remitted by SIIPL), excluding applicable Indian GST levied on account of, or measured in whole or in part by reference to, any Payments it receives. SIIPL shall deduct or withhold from the Payments any taxes that it is required by Applicable Law to deduct or withhold. Notwithstanding the foregoing, if VALNEVA is entitled under any applicable tax treaty to a reduction rate of, or the elimination of, applicable withholding tax, it may deliver to SIIPL or the appropriate Governmental Entity (with the assistance of SIIPL to the extent that this is reasonably required and is requested in writing) the prescribed forms necessary to reduce the applicable rate of withholding or to relieve SIIPL of its obligation to withhold such tax and SIIPL shall apply the reduced rate of withholding or dispense with withholding, as the case may be; provided that SIIPL has received evidence of VALNEVA’s delivery of all applicable forms (and, if necessary, its receipt of appropriate governmental authorization) at least [***] prior to the time that the Payments are due. If, in accordance with the foregoing, SIIPL withholds any amount, it shall pay to VALNEVA the balance when due, make timely payment to the proper Governmental Entity of the withheld amount and promptly send to VALNEVA the relevant withholding tax certificates.
11.3Certifications. As agreed in this Agreement, SIIPL shall furnish to VALNEVA Quarterly Certificates from their Certified Auditors for the calculation of its Payment obligations under this Agreement. SIIPL shall pay to VALNEVA any underpayment reflected in such Quarterly Certificate within [***] of the end of the applicable calendar quarter and may credit any overpayment based on the results disclosed by such Quarterly Certificates against future Payment obligations of SIIPL due to VALNEVA.
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
11.4Reconciliation. The Parties agree to conduct an annual reconciliation of the Payments made in accordance with Section 10 and Annex 5. Within [***] after the end of each calendar year, SIIPL shall furnish to VALNEVA a certificate issued by its Certified Auditors certifying the total amount of its respective Payment obligations accrued in such preceding Calendar Year (the “Annual Recalculation Certificate”). Along with the delivery of an Annual Recalculation Certificate, SIIPL shall pay to VALNEVA any underpayment reflected in such Annual Recalculation Certificate and may credit.
11.5Any disputes with respect to any amount due under Section 11.4 may be referred by either Party for dispute resolution in accordance with Section 20.5 (Negotiation; Resolution).
11.6Payment Dispute. Either Party shall promptly notify the other Party about any dispute on any Payment due under this Agreement. The paying Party shall pay any uncontested amount in accordance with the terms agreed herein and shall not withhold any uncontested portion of the due Payment. The Parties shall consult in good faith to promptly resolve any disputed amount hereunder within [***] following the original due date. Any amounts subsequently resolved shall be due and payable within ten (10) Business Days of such resolution. Any disputes with respect to any amount due which cannot be resolved between the Parties may be referred by either Party for dispute resolution in accordance with Section 20.5 (Negotiation; Resolution) hereof.
11.7Interest. If any Payment under this Agreement is not made by the date on which the same becomes due and payable, the Parties shall automatically, without any further notification being given by the other Party, [***].
12.Non-compete.
12.1Subject to Section 12.3, SIIPL covenants not to whether directly or indirectly for the term of this Agreement, manufacture or have manufactured for commercial use, file applications for Regulatory Approval, commercialize or have commercialized or otherwise exploit, in the SIIPL Territory, any competing chikungunya product or any combination thereof.
12.2Notwithstanding anything to the contrary in Section 12.1, SIIPL shall have the right to perform research and development on a competing chikungunya product or any combination thereof, up to the point of commercial manufacturing of such product, provided such chikungunya product or combination product, is not a competing live attenuated chikungunya product or any combination thereof.
12.3In case of termination of this Agreement pursuant to Article 17, Section 12. 1 and 12.2 will not apply from the date of termination.
13.Confidential Information.
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
13.1All Confidential Information disclosed, revealed or otherwise made available by one Party ("Disclosing Party") to the other Party ("Receiving Party"), including prior to the Effective Date, in connection with, under, or as a result of, this Agreement and/or the Project Agreements is furnished to the Receiving Party solely to permit the Receiving Party to exercise its rights, and perform its obligations, under this Agreement and the Project Agreements. The Receiving Party shall not use any of the Disclosing Party’s Confidential Information for any other purpose, and shall not disclose, reveal or otherwise make any of the Disclosing Party’s Confidential Information available to any Third Party, without the prior written consent of the Disclosing Party. Notwithstanding the foregoing, SIIPL agrees that VALNEVA may, without SIIPL’s consent, disclose SIIPL’s Confidential Information to CEPI to the extent necessary to monitor SIIPL’s compliance with the CEPI Side Letter. VALNEVA will inform SIIPL at the JSC meetings what type of SIIPL Confidential Information VALNEVA has shared with CEPI.
13.2In addition to the Receiving Party's obligations under Section 13.1, the Receiving Party shall take all appropriate steps, and shall implement all appropriate safeguards, to prevent the unauthorized use or disclosure of any of the Disclosing Party's Confidential Information. Without limiting the generality of this Section 13.2, the Receiving Party shall disclose any of the Disclosing Party's Confidential Information only to those of its Affiliates, officers, directors, employees, licensees, sublicensees, potential sublicensees, consultants and potential or actual financial investors that have a need to know the Disclosing Party's Confidential Information, in order for the Receiving Party to exercise its rights and perform its obligations under this Agreement or the Project Agreements, and only if (i) such Affiliates, officers, directors, employees, licensees, sublicensees, potential sublicensees, consultants and potential or actual financial investors have executed appropriate non-disclosure agreements containing substantially similar terms regarding confidentiality as those set out in this Agreement and/or the Project Agreements or are otherwise bound by obligations of confidentiality effectively prohibiting the unauthorized use or disclosure of the Disclosing Party's Confidential Information, and (ii) documents containing Confidential Information have been redacted from all information that is not strictly necessary to be disclosed to such Person. The Receiving Party shall furnish the Disclosing Party with immediate written notice of any unauthorized use or disclosure of any of the Disclosing Party's Confidential Information and shall take all actions that the Disclosing Party reasonably requests in order to prevent any further unauthorized use or disclosure of the Disclosing Party's Confidential Information.
13.3The Receiving Party's obligations under Sections 13.1 and 13.2 shall not apply to the extent that the Receiving Party can prove by competent written evidence that any of the Disclosing Party's Confidential Information:
(a)is (at the time of disclosure) or becomes (after the time of disclosure) publicly known through no breach of this Agreement by the Receiving Party or its Affiliates, Sublicensees, or Subcontractors;
(b)was known to, or was otherwise in the possession of, the Receiving Party or its Affiliates prior to the time of disclosure by the Disclosing Party or its Affiliates, as evidenced by prior or contemporaneous written records;
(c)is disclosed to the Receiving Party or its Affiliates on a non-confidential basis by a Third Party who is entitled to disclose it without breaching any confidentiality obligation to the Disclosing Party or any of its Affiliates;
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
(d)is independently developed by or on behalf of the Receiving Party or its Affiliates, as evidenced by its prior or contemporaneous written records, without reference to the Confidential Information disclosed by the Disclosing Party or its Affiliates under this Agreement; or
(e)required (i) by Applicable Law, (ii) by the listing standards or agreements of any national or international securities exchange or other similar laws of a Governmental Entity, (iii) to respond to an inquiry of a Governmental Entity or Regulatory Authority, or (iv) as may be required in a judicial, administrative or arbitration proceeding. Such disclosure shall be only for the sole purpose of and solely to the extent required by such laws and requests. The Receiving Party shall, to the extent permitted by law, furnish the Disclosing Party with prior written notice of such disclosure requirement as reasonably practicable and permissible under Applicable Law, so as to permit the Disclosing Party, in its sole discretion, to take appropriate action, including seeking a protective order, in order to prevent the Disclosing Party's Confidential Information from passing into the public domain or becoming generally available to the public.
13.4Upon expiration or termination of this Agreement and the Project Agreements for any reason whatsoever, the Receiving Party shall cease all use of and return to the Disclosing Party, or destroy, as the Disclosing Party shall specify in writing, all copies of all documents and other materials that contain or embody any of the Disclosing Party's Confidential Information, except to the extent that the Receiving Party is required by Applicable Law to retain such documents and materials. Within thirty [***] after the date of expiration or termination of this Agreement, the Receiving Party shall furnish the Disclosing Party with a certificate, duly executed by an officer of the Receiving Party, confirming that the Receiving Party has complied with its obligations under this Section 13.4.
13.5All of the Receiving Party's obligations under Sections 13.1 and 13.2 hereof, with respect to the protection of the Disclosing Party's Confidential Information, shall for a period of [***] survive the expiration or termination of this Agreement and/or the Project Agreements for any reason whatsoever. Notwithstanding the foregoing, trade secrets shall be kept confidential as long as such information remains a trade secret under Applicable Law.
13.6Public Announcement of this Agreement. On or about the Effective Date, the Parties will issue a mutually agreed joint press release. Notwithstanding the foregoing, no public announcement concerning the existence of, terms, or subject matter of this Agreement or the Project Agreements shall be made, either directly or indirectly, by any Party, without first obtaining the prior written approval of the other Party and agreement upon the nature and text of such public announcement. Such agreement and approval shall not be unreasonably withheld, conditioned or delayed.
13.7Notwithstanding the foregoing, SIIPL agrees that VALNEVA may with prior written information share the existence of, the terms, or subject matter of this Agreement and the Project Agreements with. any of the following:
Page 30
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
a.) CEPI; and
b.) existing and other investors in connection with an offering or placement of securities for purposes of obtaining financing or investment; and actual and prospective lenders for the purpose of obtaining financing or investment; and
c.) potential acquirers or merger partners included in a due diligence process of all or a portion of VALNEVA`s business to which this Agreement and the Project Agreements relate.
In the event of b.) and c.), such disclosures shall be subject to a confidentiality agreement between VALNEVA and such Third Parties. As for CEPI, the Agreements and Project Agreements shall be shared by Valneva in redacted form, which form shall be approved by SIIPL. Upon such approval, Valneva may share the Agreement and the Project Agreements to CEPI.
13.8Non-Use of Names. No Party shall use, either directly or indirectly, the logo, name, trade names or trademarks of the other Party or its Affiliates, in any publicity, marketing or advertising material or other disclosures unless a copy or transcript of the proposed disclosure is submitted to and approved in advance in writing by the other Party in its sole discretion.
14.Insurance.
14.1SIIPL shall procure and shall maintain a general liability and product liability insurance policy with “A-rated” insurance carriers according to AM Best rating agency or equivalent (e.g. “AA” rating of S&P; “Aa” rating of Moody’s, valid for those countries where the SIIPL Products are Manufactured and Commercialized with a a limit of two times 20 million EUR per claim and as an annual aggregate for personal injury, property damage and financial loss, and naming VALNEVA its Affiliates, and their respective directors, officers, employees, and agents as an additional insured party.
14.2SIIPL shall provide, or cause to be provided, to VALNEVA written evidence of such insurance promptly upon request of VALNEVA. Notwithstanding anything to the contrary, the naming of VALNEVA as an additional insured party in SIIPL’s insurance policy shall in no way limit or otherwise affect SIIPL’s liability or obligations towards VALNEVA under this Agreement.
14.3VALNEVA shall procure and maintain a general liability and product liability insurance policy, with “A-”rated insurance carriers according to AM Best Rating Agency or equivalent (e.g. “AA” rating of S&P; “Aa” rating of Moody’s), with a limit of two times 20 million EUR per claim and as an annual aggregate for personal injury, property damage and financial loss, and naming SIIPL and its directors, officers, employees, and agents as an additional insured party.
14.4VALNEVA shall provide, or cause to be provided, to SIIPL written evidence of such insurance promptly upon request of SIIPL. Notwithstanding anything to the contrary, the naming of SIIPL, its directors, officers, employees, and agents as an additional insured
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
party in VALNEVA’s insurance policy shall in no way limit or otherwise affect VALNEVA`s liability or obligations towards SIIPL under this Agreement.
15.Warranties and Liabilities
15.1Representations, Warranties and Covenants of each Party. As of the Effective Date, each of VALNEVA and SIIPL hereby represents, warrants and covenants to the other Party as follows:
a.it is a corporation or entity duly organized and validly existing under the laws of the state or other jurisdiction of its incorporation or formation; and
b.the execution, delivery and performance of this Agreement by such Party does not conflict with any other agreement by which it is bound, and has been duly authorized by all requisite corporate action and does not require any shareholder action or approval; and
c.it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder; and
d.it shall at all times comply in all material respects with all Applicable Laws relating to its activities under this Agreement and the Project Agreements; and
e.there is no action or proceeding pending or, to the knowledge of such Party, threatened that could reasonably be expected to impair or delay the ability of such Party to perform its obligations under this Agreement; and
f.to the best of its knowledge and belief, neither Party, nor any officer or employee of either Party has been debarred or is subject to debarment by a Regulatory Authority or funding agency anywhere.
15.2VALNEVA Warranties. In addition to the representations, warranties and covenants made under Section 15.1, VALNEVA hereby represents, warrants and covenants as of the Effective Date that:
(a)VALNEVA owns, Controls all right, title and interests in and to the Licensed Technology as of the Effective Date of the Agreement, and in the event any or all of the Licensed Technology is jointly owned, Controlled with any Third Party, then VALNEVA represents and warrants to SIIPL that (i) such Third Party shall have no objections and shall raise no encumbrances to the transfer of the Licensed Technology to SIIPL in accordance with Section 2.2 and (ii) in relation to Licensed Technology, no part of the business understanding or arrangement between VALNEVA and any Third Party and/or no document executed between VALNEVA and any Third Party, shall adversely affect or be deemed to obstruct and interfere with any Development, Manufacturing, and Commercialization activity undertaken by SIIPL for the SIIPL Product, or any right, title or interest of SIIPL in the SIIPL Improvements;
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
(b)to VALNEVA’s knowledge, the Licensed Technology is not infringed, is freely licensable, and is aligned with all Applicable Laws, rules and regulations; and that the Licensed Technology is free of any Third-Party infringement or claims; and that there is no objection or restriction; under any Third Party agreements to enter into, or perform its obligations, or for SIIPL to exercise its rights under this Agreement;
(c)There is no restriction, implied or active on the Licensed Technology preventing VALNEVA from licensing the same, and that VALNEVA is aware of no Third-Party patent rights that would affect SIIPL’s right to import or use the Licensed Technology in any country in the SIIPL Territory;
(d)The rights, title and interest asserted by VALNEVA in and to the Licensed Technology has neither been challenged by any Third Party(ies) nor has VALNEVA received any claims nor faced any action, penalty, proceedings, prosecution, demands, infringement suit, passing off action, made or initiated or imposed by any Third Party(ies) in respect of the Licensed Technology, on grounds of infringement of any patent rights and / or passing off claims and/or of infringement of any other Intellectual Property Rights of such Third Party(ies), as would affect the rights of SIIPL and / or its Sub-licensees or impede SIIPL and / or its Sub-licensees from importing, receiving, transferring, handling, storing or utilizing the Licensed Technology;
(e)VALNEVA warrants that VALNEVA shall be responsible for prosecution and maintenance of Licensed Patents. VALNEVA further warrants that it shall file requisite patent applications, including but not limited to any current or future variants of concern, and / or any improvements of the Licensed Technology, as per SIIPL’s instructions, as may be necessary and expedient to secure Patent Rights for protecting and covering the Licensed Technology (including but not limited to any current or future variants of concern, and / or any improvements of the Licensed Technology) in SIIPL Territory, SIIPL agrees to reimburse the costs incurred by VALNEVA in securing Patent Rights in all such geographies;
(f)VALNEVA further warrants that it shall commercially reasonably maintain any and all Licensed Patents; and
(g)In the event VALNEVA is entering into any financial, funding or loan arrangement with a Third Party for any purpose, then, (i) the responsibility of such financial, funding or loan arrangements (including any and all financial obligations) shall be at the sole cost, risk and consequence of VALNEVA without any liability on SIIPL whatsoever, and (ii) VALNEVA agrees that SIIPL shall not be liable and shall not be a party or connected to any financial, funding or loan arrangement and shall not bear any direct or indirect responsibility with respect to the same.
15.3Warranties and Covenants of SIIPL.
[***]
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
15.4DISCLAIMER OF WARRANTIES. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT OR THE PROJECT AGREEMENTS, NEITHER PARTY MAKES ANY, AND HEREBY EXPRESSLY DISCLAIMS ANY AND ALL, REPRESENTATIONS, GUARANTEES, OR WARRANTIES, IMPLIED, STATUTORY OR OTHERWISE, IN CONNECTION WITH THIS AGREEMENT OR THE PROJECT AGREEMENTS OR THE SUBJECT MATTER HEREOF, INCLUDING, WITHOUT LIMITATION ANY IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, TITLE, AND NON-INFRINGEMENT, AND ANY AND ALL WARRANTIES THAT MAY ARISE OUT OF COURSE OF DEALING, COURSE OF PERFORMANCE, OR USAGE OF TRADE. NOTHING IN THIS AGREEMENT OR ANY PROJECT AGREEMENT SHALL CONSTITUTE OR BE CONSTRUED TO CONSTITUTE A REPRESENTATION OR WARRANTY THAT NO THIRD-PARTY RIGHTS ARE OR MAY BE REQUIRED TO CARRY OUT THE COLLABORATION AS CONTEMPLATED IN THIS AGREEMENT AND IN THE PROJECT AGREEMENTS, AND NOTHING HEREIN SHALL CONSTITUTE OR BE CONSTRUED TO CONSTITUTE A REPRESENTATION OR WARRANTY AS TO THE VALIDITY OF ANY PATENTS.
16.Indemnification and Limitation of Liability
16.1Indemnification by SIIPL. SIIPL will indemnify, defend, and hold harmless VALNEVA, its Affiliates, and their respective directors, officers, employees, and agents (collectively, the “VALNEVA Indemnitees”) from and against [***] suffered by VALNEVA Indemnitees in connection with any suits or claims brought by Third Parties (“VALNEVA Claims”) arising out of or resulting from any bona fide claim with respect to [***];. Further, SIIPL will indemnify, defend, and hold harmless VALNEVA Indemnitees of VALNEVA Losses suffered to the extent that resulting from or arising in connection with a VALNEVA Claim based on, resulting from, or arising in connection with [***].
provided, however, that SIIPL shall not be obligated to indemnify, defend or hold harmless VALNEVA Indemnitees from any VALNEVA Claims or for any VALNEVA Losses incurred by VALNEVA Indemnitees to the extent arising out of, or attributable to suits or claims brought by Third Parties in relation to Section 16.2 (i) – (iii).
16.2Indemnification by VALNEVA. VALNEVA shall indemnify, defend and hold harmless SIIPL and its respective directors, officers, employees, and agents (collectively "SIIPL Indemnitees") from and against [***] suffered by SIIPL Indemnitees in connection with any suits or claims brought by Third Parties (“SIIPL Claims”) arising out of or resulting from any bona fide claim with respect to [***] provided, however, that VALNEVA shall not be obligated to indemnify, defend or hold harmless SIIPL Indemnitees from any SIIPL Claim or for any SIIPL Losses incurred by SIIPL or a SIIPL Indemnitees to the extent arising out of or attributable to suits or claims brought by Third Parties in relation to Section 16.1 (i) –(ii).
16.3Liability Cap. The Indemnifying Party’s liability under this Agreement and the Project Agreements is limited to the amount specified in the Sections 14.1 and 14.3.
16.4Indemnification Procedures.
a.Each indemnified Party shall notify the indemnifying Party in writing (and in reasonable detail) of any suits or claims brought by Third Parties within ten (10) Business Days after receipt by such indemnified Party of notice of the VALNEVA Claim or SIIPL Claim, as the case may be, or otherwise becoming aware of the existence or threatened existence thereof (such VALNEVA Claim or SIIPL Claim being referred to as a "Claim"). Failure to give such notice shall not constitute a defence, in whole or in part, to any claim by an indemnified Party hereunder except to the extent the rights of the indemnifying Party are materially prejudiced by such failure to give notice. The indemnifying Party shall notify the indemnified Party in writing of its intentions as to defence of the Claim or potential Claim in writing within [***] after receipt of notice of the Claim. If the indemnifying Party assumes the defence of a Claim against an indemnified Party, an indemnifying Party shall have no obligation or liability under this Section 16.4 as to any Claim for which settlement or compromise of such Claim or an offer of settlement or compromise of such Claim is made by an indemnified Party without the prior written consent of the indemnifying Party, which consent shall not be unreasonably withheld, conditioned or delayed.
b.The indemnifying Party shall assume exclusive control of the defence and settlement (including all decisions relating to litigation, defence and appeal) of any such Claim which seeks solely monetary remedies (so long as it has confirmed its indemnification obligation responsibility to such indemnified Party under this Section 16.3 with respect to a given Claim); provided, however, that (i) the indemnifying Party may not settle such Claim in any manner that would require payment by the indemnified Party, or would materially adversely affect the rights granted to the indemnified Party hereunder, or would materially conflict with the terms of this Agreement or the Project Agreements, without first obtaining the indemnified Party’s prior written consent, which consent shall not be unreasonably withheld, conditioned or delayed; and (ii) the conduct of proceedings relating to Patent Rights shall be subject to specific provisions in Section 7.
c.The indemnified Party shall reasonably cooperate with the indemnifying Party in its defence of the Claim (including, without limitation, making documents and records available for review and copying and making Persons within its control available for pertinent testimony in accordance with the confidentiality provisions of Article 13, and neither Party shall be required to divulge privileged material to the other) at the indemnifying Party’s expense. If the indemnifying Party assumes defence of the Claim, an
indemnified Party may participate in, but not control, the defence of such Claim using attorneys of its choice and at its sole cost and expense, with such cost and expense not being covered by the indemnifying Party. If an indemnifying Party does not agree to assume the defence of the Claim asserted against the indemnified Party (or does not give notice that it is assuming such defence), or if the indemnifying Party assumes the defence of the Claim in accordance with Section 16.4 yet fails to defend or take other reasonable, timely action, in response to such Claim asserted against the indemnified Party, the indemnified Party shall have the right to defend or take other reasonable action to defend its interests in such proceedings, and shall have the right to litigate, settle or otherwise dispose of any such Claim; provided, however, that no Party shall have the right to settle a Claim in a manner that would adversely affect the rights granted to the other Party hereunder, or would materially conflict with this Agreement or the Project Agreements or would require a payment by the Party, or adversely affect the Party or its Affiliates, without the prior written consent of the Party entitled to control the defence of such Claim.
d.Limitation of liability. In no event shall either Party be liable to the other, or the VALNEVA Indemnitees or the SIIPL Indemnitees, as applicable, for [***]. The foregoing limitation shall not apply, however, to a Party's indemnification obligations pursuant to this Article 16 or liability arising from the breach of the Intellectual Property Rights provisions under Article 7, the non-compete under Article 12 and the confidentiality obligations under Article 13.
17.Term and Termination
17.1Term. This Agreement shall come into force on the Effective Date and, unless terminated earlier in accordance with the provisions under Article 17, this Agreement shall expire on a country-by-country basis on the later of: the expiry in such country of the last Valid Claim of the Licensed Patents licensed to SIIPL under this Agreement or [***] after the First Commercial Sale of the SIIPL Product in such country (collectively “Term”)
17.2This Agreement may be terminated by either Party for cause in case of:
(a)Material Breach. Immediately in case of material breach in accordance with Section 21.12 and otherwise upon written notice to the other Party if the other Party materially breaches this Agreement or any Project Agreement and such material breach is not discontinued or cured within [***] after the breaching Party’s receipt of an initial written notice by the non-breaching Party with reasonable detail as to the nature and scope of the applicable breach; or
(b)License Conversion. In the event VALNEVA provide SIIPL with a notice of termination due to a material breach of SIIPL of its obligations of this Agreement then VALNEVA may, due to the aforesaid breach, provide notice to SIIPL of its intent to convert the exclusive license granted hereunder to a non-exclusive license. If the Parties are in agreement, then the Parties agree to mutually discuss in good faith the terms, conditions and payment structure with respect to such non-exclusive license and the same will be duly recorded by the Parties in a separate agreement. VALNEVA shall have no obligation
to enter into such a non-exclusive license agreement, and may, in its discretion, decide to terminate this Agreement pursuant to Section 17.2(a); and
(c)Bankruptcy. By giving [***] prior written notice to the other Party if the other Party becomes insolvent or a bankruptcy action or any other insolvency proceeding is instituted against it and not dismissed within [***].
17.3Withdrawal of Regulatory Approval. In addition to Sections 17.2 and 17.4, SIIPL, will have the right to terminate for cause immediate upon written notice in case of a withdrawal of the EMA Market Authorization of the VALNEVA Product due to safety concerns.
17.4This Agreement may be terminated by SIIPL for any reason, with or without cause, upon not less than [***] prior written notice to VALNEVA.
18.Consequences of Termination
18.1Reversion of Rights. Subject to Section 18.2, upon termination of this Agreement for whatever reason, all of SIIPL`s rights and licenses under this Agreement, including but not limited to the Commercial License granted by VALNEVA to SIIPL under Section 3.1 of this Agreement, shall automatically terminate and all of SIIPL's rights to the VALNEVA Licensed Technology shall automatically revert to VALNEVA.
18.2Continued Supply of SIIPL Product. Upon the expiration or termination of this Agreement the following shall apply:
(i)the provisions under the CEPI Side Letter, including the Business Continuity Plan, shall apply with respect to continued supply of SIIPL Product in the SIIPL Territory for a limited period post expiration or termination as set forth in the CEPI Side Letter;
(ii)in case SIIPL terminates this Agreement and any Project Agreement in accordance with Sections 17.4, or if VALNEVA terminates in accordance with Section 17.2 SIIPL shall continue the Manufacture and Commercialization of the SIIPL Product for a period up to [***], or for such shorter period of time as VALNEVA may decide at its own discretion. SIIPL shall use Commercially Reasonable Efforts to assist VALNEVA with any transfer of the Licensed Technology to a new licensee, at SIIPL’s own cost.
During periods of continued supply in accordance with Section 18 (i) and (ii), the Profit Share set forth in Section 10.3 applies and remains in effect during this period of continued supply.
18.3No Compensation. Upon early termination of this Agreement by SIIPL in accordance with Section 18.2, 18.3 and 18.4, neither Party shall in any event be entitled to any compensation or damages or other payment whatsoever, whether in respect of goodwill, loss of profit or otherwise. Further the Parties agree that either Party shall only be entitled to receive any amounts and/or Payments due till the date of termination.
18.4Termination of Project Agreements. Termination of this Agreement by either Party for whatever reason shall automatically terminate any and all Project Agreements without further notice required. For the avoidance of doubt, termination of any Project Agreement according to Article 18 or any additional termination rights granted in the respective Project Agreement shall have no effect on the validity of this Agreement and any other Project Agreement not so terminated, which shall remain in full force and effect.
18.5License after Patent Expiration. Upon the expiration the later of a 1) Valid Claim of a Licensed Patent, or [***] after First Commercial Sale of the SIIPL Product under this Agreement on a country-by-country basis, VALNEVA agrees to grant, and hereby does grant to SIIPL a fully-paid, non-exclusive, royalty-free license under the Licensed Technology to Manufacture and Commercialize the SIIPL Products in any such country in the SIIPL Territory as constituted as of the date of the applicable expiration. For the avoidance of doubt, the expiration of this Agreement will not have an effect on the Drug Substance Supply Agreement, which will remain in force for as long as SIIPL Manufactures and Commercializes the SIIPL Product, subject to an agreement to be negotiated in good faith by and between the Parties, including terms of a mutually acceptable price of Drug Substance following the expiration of this Agreement.
18.6Survival. Sections 3.2 (Covenant not to Sue), 6.8 (Regulatory Records), Article 7 (Intellectual Property; Infringement and Prosecution), Articles 10 (Financial Terms) and 11 (Payment Terms), Article 13 (Confidential Information), Section 15.4 (Disclaimer of Warranties), Article 16 (Indemnification and Limitation of Liability) Article 18 (Consequences of Termination and Continued Supply), Article 20 (General Provisions and Governing Law and Dispute Resolution) shall survive the expiration or termination of this Agreement. For clarity, expiration or termination of this Agreement shall not relieve the Parties of any obligation accruing prior to such expiration or termination, including but not limited to any Payment or reporting obligations as agreed under this Agreement and the Project Agreements.
19.Conditions Precedent
19.1Management Approval. Both Parties hereby confirm that they have acquired any required approval of their management boards and/or supervisory boards for the execution of this Agreement and the Project Agreements.
20.General Provisions
20.1Assignment. Neither Party shall assign this Agreement or any Project Agreement without the prior written consent of the other Party, such consent not to be unreasonably withheld, conditioned or delayed. Either Party may transfer any and all of its rights and/or obligations hereunder to any of its affiliates and inform the other Party thereafter. to which this Agreement primarily relates, with prior information.
20.2Change of Control. This Agreement will be binding on and inure to the benefit of the Parties, their executors, administrators, successors, and permitted assigns. In the event of any Change of Control with respect to the other Party occurs, then such acquiring party
shall be bound by the terms and conditions of this Agreement. The Parties shall promptly inform each other of such Change of Control.
20.3No Third-Party Beneficiary. Except as expressly provided otherwise in this Agreement no Person other than each Party and any of its permitted assigns and assignee Affiliate(s) hereunder will be deemed an intended beneficiary hereunder or have any right to enforce any obligation under this Agreement.
20.4Notices. All notices, reports and other communications between the Parties under this Agreement and the Project Agreements shall be sent by registered mail, postage prepaid and return receipt requested, by international courier, or by email, with a confirmation copy sent by registered mail or international courier (and deemed to be delivered on the date of receipt of such confirmation copy), addressed as follows:
To VALNEVA:
[***]
Legal notices to be sent in copy to: [***]
To SIIPL:
[***]
Email: [***]
20.5Governing Law, Dispute Resolution and Jurisdiction. The Parties agree to settle their disputes amicably and in good faith. If the executives dealing with the subject matter of this Agreement cannot settle the dispute among themselves then the matter will be referred to the Managing Director / Chief Executive Officer of the respective Parties who shall discuss the dispute and try to arrive at an amicable solution. In the event the Parties are unable to resolve the disputes as mentioned above then this Agreement, and the obligations contained herein, shall be governed by and interpreted in accordance with the laws of the State of New York, USA and the Parties irrevocably submit to the exclusive jurisdiction of the courts of New York, USA, without giving effect to the conflicts of law provisions thereof.
20.6Force Majeure. Except as otherwise expressly set forth in this Agreement, neither Party will have breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement when such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party, including, but not limited to, an act of God, war, act of terror, civil commotion, labor strike or lock-out, epidemic, pandemic, quarantine, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe, acts, omissions, or delays in acting, by any Governmental Entity (“Force Majeure”). The Party affected by any event of Force Majeure shall promptly notify the other Party in writing, explaining the nature, details and expected duration of the Force Majeure event. Such
Party shall also notify the other Party from time to time as to when the affected Party reasonably expects to resume performance in whole or in part of its obligations under this Agreement, and to notify the other Party of the termination of the event of Force Majeure. The Party affected by an event of Force Majeure shall use its Commercially Reasonable Efforts to remedy, remove, or mitigate such force majeure event and the effects thereof. If a Party anticipates that an event of Force Majeure may occur, such Party shall promptly notify the other Party in writing of the nature, details and expected duration of the Force Majeure event. Upon termination of the event of Force Majeure, the performance of any suspended obligation or duty shall promptly recommence. In case the Force Majeure event continues for a period of [***] the unaffected Party may terminate this Agreement with immediate effect.
20.7Severability. If any provision of this Agreement or the Project Agreements is determined by any court or administrative tribunal of competent jurisdiction to be invalid or unenforceable, the Parties shall negotiate in good faith a replacement provision that is commercially equivalent, to the maximum extent permitted by Applicable Law, to such invalid or unenforceable provision. The invalidity or unenforceability of any provision of this Agreement or the Project Agreements shall not affect the validity or enforceability of the other provisions of this Agreement and the Project Agreements. Nor shall the invalidity or unenforceability of any provision of this Agreement or the Project Agreements in one country or jurisdiction affect the validity or enforceability of such provision in any other country or jurisdiction in which such provision would otherwise be valid or enforceable.
20.8Entire Agreement and Amendments. This Agreement, together with all Project Agreements, constitutes the entire agreement between the Parties, and supersedes all prior agreements, understandings and communications between the Parties, with respect to the subject matter hereof. No modification or amendment of this Agreement or the Project Agreements shall be binding upon the Parties unless in writing and executed by the duly authorized representatives of each of the Parties; this shall also apply to any change of this clause.
20.9Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein will be deemed to encompass references to either or both genders, and the use of the singular will be deemed to include the plural (and vice versa), (b) the words "include," "includes," and "including" will be deemed to be followed by the phrase "without limitation," (c) the word "will" will be construed to have the same meaning and effect as the word "shall," (d) any definition of or reference to any agreement, instrument, or other document herein will be construed as referring to such agreement, instrument, or other document as from time to time amended, supplemented, or otherwise modified(subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any person will be construed to include the person's successors and assigns, (f) the words "herein," "hereof," and "hereunder" and words of similar import, will each be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Articles, Sections, Schedules, or Exhibits will be construed to refer to Articles, Sections, Schedules, or
Exhibits of this Agreement, and references to this Agreement include all Schedules hereto, (h) the word "notice" means notice in writing (whether or not specifically stated) and will include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder "agree," "consent, "approve," or the like will require that such agreement, consent, or approval be specific and in writing, whether by written agreement, letter, approved minutes, or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or Section or other division thereof, will be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, (k) the term "or" will be interpreted in the inclusive sense commonly associated with the term "and/or," and (l) in the event of any conflict between the terms and conditions of this Agreement and any terms and conditions that may be set forth on any order, invoice, verbal agreement, in the Quality Agreement, in any Project Agreement and in the Pharmacovigilance Agreement, this Agreement shall prevail except for any quality-related matters in which case the Quality Agreement shall prevail.
20.10Data Protection. The Parties do not envisage that any personal data will be exchanged between the Parties in the performance of this Agreement. Each Party will comply with applicable data protection laws in connection with this Agreement and the Project Agreements and may enter into data processing agreements if necessary.
20.11Waivers. The failure by either Party to assert any of its rights hereunder or under the Project Agreements, including the right to terminate this Agreement and the Project Agreements due to a breach or default by the other Party, shall not be deemed to constitute a waiver by that Party of its right thereafter to enforce each and every provision of this Agreement and the Project Agreements in accordance with their terms.
20.12Counterparts. This Agreement and the Project Agreements may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
20.13Independent Contractors. The Parties are independent contractors and this Agreement and the Project Agreements shall not constitute or give rise to an agency, partnership or joint venture relationship among the Parties and each Party’s performance hereunder is that of a separate, independent entity.
20.14Language. This Agreement and the Project Agreements, and any amendments or modifications thereto, shall be executed in English. Any communications between the Parties under this Agreement and the Project Agreements, including but not limited to any notices provided, shall be in English only.
20.15Headings. The headings are placed herein merely as a matter of convenience and shall not affect the construction or interpretation of any of the provisions of this Agreement.
20.16Costs. Except as is otherwise expressly set forth herein or in the Project Agreements, each Party shall bear its own expenses in connection with the activities contemplated and performed hereunder and under the Project Agreements.
20.17Foreign Corrupt Practices. By signing this Agreement, each Party agrees to conduct the business contemplated herein and in the Project Agreements in a manner, which is consistent with both Applicable Law and good business ethics. Both SIIPL and VALNEVA warrant, that neither SIIPL nor VALNEVA, nor any person employed by or representing VALNEVA or SIIPL, has ever made, offered, provided or authorized, and each of VALNEVA and SIIPL covenants that neither it, nor any person employed by it or representing it, will make, offer, provide or authorize, directly or indirectly, any payment or transfer of anything of value to any official, representative or employee of any Governmental Entity or instrumentality, any political party or officer thereof, or any candidate for public office for the purpose of influencing a decision by any of them in their official capacity.
20.18Contract formation. This document is not an offer unless signed by a Party and is not a contract unless signed by both Parties.
21.Good Business Practices; Anti-Bribery; Human Rights.
21.1Each Party agrees to conduct the cooperation contemplated herein in a manner which is consistent with Applicable Law and good business ethics. Each Party shall comply with applicable Anti-Corruption Laws in the performance of its activities hereunder. Without limiting the foregoing, neither Party shall make any payments, or offer or transfer anything of value, to any government official or government employee, to any political party official or candidate for political office or to any other Third Party related to the cooperation in a manner that would violate Anti-Corruption Laws.
21.2Each Party shall, and shall cause persons employed or engaged by it who perform activities hereunder (“Representatives”) to, comply with Applicable Laws, any and all Anti-Corruption Laws in all respects.
21.3Notwithstanding anything to the contrary herein, each Party hereby agrees that it shall not, and shall cause its Representatives not to, take any actions (i) that are prohibited by Anti-Corruption Laws, and/or (ii) which would make the other Party liable for a violation of Anti-Corruption Laws and Human Rights.
21.4Each Party represents and warrants that it will:
i.not disparage the name, good will, or reputation of the other Party;
ii.not engage in deceptive, misleading, or unethical practices;
iii.not make any false or misleading representations or other statements with
regard to the other Party or Product;
iv.represent only such facts about Product as are in accordance with the Regulatory Approval, the summary of Product characteristics or its equivalent, and
v.in no event make any representations, warranties, guarantees or other statements in the other Party’s name or on the other Party’s behalf, except as approved in advance in writing by the other Party;
vi.directly or indirectly, make or authorize or promise an offer, payment or gift, of anything of value, to any government employee, any official (including but not limited to any governmental or regulatory official), any political party or official thereof, or any candidate for political office, or any other Third Party that may have any influence in relation to the activities contemplated hereunder, that would violate Anti-Corruption Laws;
vii.engage in any activity that would expose the other Party or its Affiliates, to a risk of penalties or of violations under laws or regulations of any relevant jurisdiction that prohibit improper payments, including but not limited to bribes, to officials of any government of any agency, instrumentality or political subdivision thereof, to political parties or political party officials or candidates for public office, or to any employee of any customer or supplier.
21.5During the Term of this Agreement, each Party shall have in place, maintain and follow a code of business conduct/reasonable procedures designed to prevent, detect and manage possible violations of Anti-Corruption Laws.
21.6Each Party represent and warrants as of the Effective Date, that:
(a)it has not been convicted of, pleaded guilty to or charged with any offence involving fraud, corruption or bribery, or breach of any Applicable Laws in any jurisdiction or country,
(b)it is not subject to or threatened by any actions, suits or proceedings for any alleged violation of any Applicable Laws.
21.7Each Party agrees to immediately inform the other Party of the occurrence of any possible violation by such Party and/or its Representatives of any Anti-Corruption Laws.
21.8Each Party hereby represents and warrants that all Representatives are appropriately trained on Anti-Corruption Laws on a regular basis and at least once per year.
21.9Each Party shall on an annual basis confirm at the other Party that:
(a)appropriate training and training materials on Anti-Corruption Laws have been provided to all Representatives; and
(b)to the best of such Party's knowledge, there have been no violations of Anti-Corruption Laws by such Party or its Representatives in the performance of their activities hereunder.
21.10Human Rights. Each Party further represents that, with respect to its respective obligations under this Agreement, it will:
(a)not use child labor in circumstances that could cause physical or emotional impairment to the child;
(b)not use forced labor (prison, indentured, bonded or otherwise);
(c)provide a safe and healthy workplace; safe housing (if applicable); and access to clean water, food, and emergency healthcare in the event of accidents in the workplace;
(d)not discriminate against employees on any grounds (including race, religion, disability or gender);
(e)not use corporal punishment or cruel or abusive disciplinary practices;
(f)pay at least the minimum wage, where applicable, and provide any legally mandated benefits;
(g)comply with laws on working hours and employment rights;
(h)respect employees’ right to join and form independent trade unions;
(i)encourage subcontractors under this Agreement to comply with these standards; and (j) maintain a complaints process to address any breach of these standards.
21.11SIIPL undertakes to comply with VALNEVA’s Business Partners Code of Conduct attached hereto as Annex 7 without any amendments, and in case Valneva make any amendments to the VALNEVA’s Business Partners Code of Conduct it will not be applicable to SIIPL, for the sake of clarity SIIPL will only comply to what has been attached under (Annex 7).
21.12The Parties agree that violation of Sections 21.1, 21.3 and 21.10 above and violation of the following sections (i) Anti-Bribery, Anti-Corruption, Business Expenses and Money Laundering, (ii) Anti-Trust, Competition and Fair Dealing, and (iii) Human Rights,
Discrimination, Harassment and Bullying under the VALNEVA’s Business Partners Code of Conduct annexed to this Agreement, shall be regarded as material breach of this Agreement allowing for immediate termination.
21.13VALNEVA has the right to terminate this Agreement in accordance with 21.12. However, prior to exercising such right, the Parties will discuss in good faith, including whether, as determined by VALNEVA, such breach can be remedied and if so, what the appropriate remedy period shall be. To the extent any provision of the Business Ethics Code conflicts with the terms of this Agreement, the terms of this Agreement shall prevail.
List of Annexes:
•Annex 1 – SIIPLTerritory
•Annex 2 – Licensed Patents
•Annex 3 – Launch Readiness Plan Requirements
•Annex 4 – Profit Share – Calculation and Reconciliation
•Annex 5 – Upfront Payment and Milestone Payments
•Annex 6- CEPI Side Letter
•Annex 7 – VALNEVA’s Business Partners Code of
Conduct
SIGNATURE PAGE FOLLOWS
IN WITNESS WHEREOF, intending to be legally bound, the Parties hereto have caused this Master Collaboration and License Agreement to be executed by their duly authorized representatives.
VALNEVA AUSTRIA GmbH Serum Institute of India Private Limited
Name: [***] Name: [***]
Title: [***] Title: [***]
Date: 12/18/2024 Date: 12/18/2024
Annex 1
SIIPL Territory
Key Countries:
[***]
Additional countries:
[***]
Key Countries and additional countries listed above together referred to as the SIIPL Territory.
In addition, within the SIIPL Territory, SIIPL shall have the exclusive right to supply and sell the SIIPL Product to:
(i)UNICEF, and
(ii)any Public and governmental agency.
The Parties acknowledge and agree that they shall engage in discussions, together with VALNEVA’s other licensees, regarding the territorial scope of exclusive, or non-exclusive supplies to UNICEF and GAVI respectively before end of 2025.
Annex 2
[***]
Annex 3
[***]
Annex 4
[***]
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
ANNEX- 5
Upfront Payment and milestone Payments
[***]
Annex 6
CEPI Side Letter
|
|
|
Docusign Envelope ID: B27B032E-53C5-4594-AC8F-38C3F2302C3B |
Annex 7
VALNEVA’s Business Partners Code of Conduct
(Under the Business Partners Code Of Conduct the term “Business Partner” shall be read and interpreted as “Collaborator”)
Annex 8
Valneva Trade Secrets
[***]
EX-4.12
6
exhibit412-limmatechvalnev.htm
EX-4.12
Document
[***] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS OMITTED BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) CUSTOMARILY AND ACTUALLY TREATED BY THE REGISTRANT AS PRIVATE OR CONFIDENTIAL.
DEVELOPMENT COLLABORATION, LICENSE AND COMMERCIALIZATION
AGREEMENT
by and between
VALNEVA AUSTRIA GMBH
and
LIMMATECH BIOLOGICS AG
TABLE OF CONTENTS
EXHIBITS VII
PREAMBLE 1
1.DEFINITIONS 2
2.LICENSE GRANTS AND TECHNOLOGY TRANSFER 16
2.1.Exclusive License from LimmaTech to Valneva 16
2.2.Exclusive Sublicense from LimmaTech to Valneva 17
2.3.1.Permitted Sublicenses 17
2.3.3.Other Sublicenses 18
2.4.Non-Exclusive License from Valneva to LimmaTech 18
2.5.Right of Reference 18
2.6.Exclusivity Obligation of LimmaTech. 18
2.7.Exclusivity Obligation of Valneva 19
2.8.No Implied Rights. 19
2.9.Safe Harbor. 19
2.10.Initial Data Transfer 19
2.11.Samples of Tangible Materials 19
2.12.Continuing Disclosure and Knowledge Transfer 20
2.13.Development Data Transfer 20
2.14.Technology Transfer 20
3.PAYMENTS AND COSTS 20
3.1.Up-Front Payment. 20
3.2.Development Costs 20
3.2.1.Accounting for and Refunding of Development Costs 21
3.2.2.Audits of Development Costs 21
3.2.3.Third Party Grants 22
3.3.Development Milestone Payments 22
3.4.Commercial Milestone Payments. 24
3.5.1.Royalties Rates 24
3.5.2.Royalties Rates Reductions 25
3.5.3.Fully Paid-Up, Royalty Free License 25
3.5.4.Adjustment for Additional Third Party License 25
3.5.5.No Adjustment for LimmaTech Third Party Agreements 25
3.6.1.Commercialization by the Global Health Partner(s) within the LMIC Territory 26
3.6.2.Commercialization by Valneva within the LMIC Territory private market 27
3.6.3.Participation or Royalty Rates Reductions 27
3.6.4.Fully Paid-Up, Royalty Free License 28
3.6.5.Adjustment for Additional Third Party License 28
3.7.Compensation for Costs Incurred by LimmaTech Beyond 31 December 2046. 28
3.8.1.No Cumulative Royalties 29
3.8.2.Blended Royalty 29
3.8.3.Royalty Report and Payments 29
3.8.5.Currency 31
3.8.6.Method of Payment 31
3.8.7.Record Keeping 31
3.8.8.LimmaTech Audits 31
3.8.9.Underpayments/Overpayments following LimmaTech Audits 32
3.8.10.Confidentiality 32
4.DEVELOPMENT PLAN 32
4.1.Scope of the Development Plan 32
4.2.1.General 32
4.2.2.Development Obligations; Subcontractors 32
4.2.3.Personnel Matters 33
4.2.4.Valneva Oversight of Development Activities 33
4.2.5.Manufacturing Technology Transfer 33
4.2.6.LimmaTech Disclosure and Knowledge Transfer Obligations 34
5.LICENSED PRODUCT DEVELOPMENT AND COMMERCIALIZATION 38
5.1.General 38
5.3.1.Development Diligence 39
5.3.2.Commercial Diligence 39
5.3.3.Exceptions to Diligence Obligations 39
5.3.4.Deemed Satisfaction of Valneva Diligence Obligations 40
5.3.5.Performance by the Parties’ Affiliates or Valneva’s Sublicensees 40
5.4.1.Regulatory Reporting 40
5.4.2.Regulatory Approvals 41
5.4.3.Cooperation 41
5.4.4.Right of Reference 41
5.4.5.Pharmacovigilance 41
5.5.1.General 42
5.5.2.Branding 42
5.6.Manufacturing 42
5.7.Progress Reporting Post JSC Dissolution 42
6.INTELLECTUAL PROPERTY 42
6.1.Platform Patent Rights. 42
6.1.1.Management and Enforcement of Platform Patent Rights 42
6.2.1.Ownership of LimmaTech Technology 44
6.2.4.Other Actions by Third Parties 47
6.2.5.Purple Book Listings 47
6.2.6.Paragraph IV Type Notices 48
6.2.7.Notice of Infringement 48
6.2.8.Third Party Infringement Suits 48
6.2.9.Misappropriation Actions Relating to LimmaTech Know-How 49
6.3.1.Ownership of Valneva Technology 49
6.3.2.License from Valneva to LimmaTech over Valneva Technology 49
6.3.3.Filing, Prosecution and Maintenance 49
6.3.4.Enforcement and defense 50
6.3.5.Misappropriation Actions 50
7.CONFIDENTIALITY, PUBLICATION AND DATA PROTECTION. 50
7.1.Obligation of Confidentiality. 50
7.2.Limitations 50
7.3.1.Disclosure to Party Representatives 51
7.3.2.Disclosure to Third Parties 51
7.3.3.Required Disclosures 52
7.4.Unauthorized Use or Disclosure. 53
7.5.1.Announcements 53
7.5.2.Publications 53
7.6.Obligations in Connection with Change of Control 53
7.7.Data Protection 54
8.REPRESENTATIONS, WARRANTIES AND COVENANTS 54
8.1.Mutual Representations and Warranties 55
8.2.Mutual Covenants 55
8.3.Representations and Warranties of LimmaTech 55
8.6.Accuracy of Valneva's Representations and Warranties 58
8.7.LimmaTech Covenants 59
8.8.Valneva Covenants 61
8.9.Representation by Legal Counsel 61
8.10.Disclaimer 61
9.TERM AND TERMINATION 61
9.1.Term 62
9.2.Termination for Cause by LimmaTech 62
9.3.2.Termination for Cause 63
9.4.2.Accrued Rights 67
9.4.3.Survival Period 67
9.5.1.Termination Right 67
9.5.2.Rights to Intellectual Property 68
9.5.3.No Limitation of Rights 68
10.LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE 68
10.1.No Consequential Damages 68
10.2.Indemnification by Valneva. 69
10.3.Indemnification by LimmaTech. 69
10.4.1.Notice 70
10.4.2.Control 70
10.4.3.Settlement 71
10.4.4.Insurance 71
11.MISCELLANEOUS. 72
11.1.Assignment 72
11.2.Change of Control 72
11.3.Further Actions. 72
11.4.Force Majeure. 72
11.5.Interpretation 72
11.6.Notices 73
11.7.Amendment 74
11.8.Waiver 74
11.9.Severability 74
11.10.Descriptive Headings. 74
11.11.Global Trade Control Laws 74
11.12.1.Anti Bribery and Anti-Corruption 75
11.12.2.Ethical Standards and Human Rights 75
11.12.3.Ethical Care of Animals 75
11.13.1.Escalation 76
11.13.2.Governing Law 76
11.13.3.Arbitration 76
11.13.4.Expert Determination 77
11.14.Entire Agreement 77
11.15.Independent Contractors. 78
11.16.Counterparts 78
11.17.No Third Party Rights or Obligations 78
11.18.Electronic Signatures 78
EXHIBITS
|
|
|
|
|
|
| Exhibit 1.21 |
Development Budget |
| Exhibit 1.26 |
Development Plan |
| Exhibit 1.49 |
Licensed Know-How |
| Exhibit 1.54 |
LimmaTech Materials |
| Exhibit 1.55 |
LimmaTech Patent Rights |
| Exhibit 1.58 |
LimmaTech Third Party Agreements |
| Exhibit 1.78 |
Platform Materials |
| Exhibit 1.79 |
Platform Patent Rights |
| Exhibit 2.3.2 Part A |
List of Global Health Partners [***] |
| Exhibit 2.3.2 Part B |
Terms and conditions reasonable and necessary to be included in the Global Health License |
| Exhibit 3.6.1 |
Payments to Third Party Licensors for the LMIC Territory |
| Exhibit 4.2.2 |
Current Subcontractors of LimmaTech |
| Exhibit 7.5.1 |
Draft Joint Press Release |
| Exhibit 8.8.1 |
Obligations under Third Party Licenses |
DEVELOPMENT COLLABORATION, LICENSE AND COMMERCIALIZATION
AGREEMENT
This Development Collaboration, License and Commercialization Agreement (“Agreement”) is entered into as of the Effective Date (as defined below) by and between:
Valneva Austria GmbH, a corporation organized and existing under the laws of Austria and having a principal place of business at Campus Vienna Biotech 3, AT-1030 Vienna, AUSTRIA (“Valneva”);
and
LimmaTech Biologics AG, a company organized and existing under the laws of Switzerland and having a principal place of business at Grabenstrasse 3, CH-8952 Schlieren, Switzerland (“LimmaTech”);
Individually referred to as a “Party” and collectively as the “Parties”.
PREAMBLE
WHEREAS, LimmaTech Controls (as defined below) certain Patent Rights (as defined below) and Know-How (as defined below) relating to the Development, Manufacture and Commercialization of Licensed Products (as defined below);
WHEREAS, Valneva has extensive experience and expertise in the development and commercialization of pharmaceutical and biopharmaceutical products;
WHEREAS, subject to the terms of this Agreement, LimmaTech wishes to grant to Valneva, and Valneva wishes to receive from LimmaTech, an exclusive license to the Licensed Technology (as defined below) to Develop, Manufacture, Commercialize and otherwise Exploit the Licensed Product in the Field of Use in the Territory (as defined below);
WHEREAS, Valneva and LimmaTech wish to engage in collaborative clinical Development pursuant to the Development Plan for Licensed Products alone (i.e., excluding any Combination; both as defined below) to be advanced through Clinical Trials (as defined below) and Commercialized by Valneva in the Territory outside the Global Health Market and by Global Health Partner(s) in the Global Health Market (as defined below); and
WHEREAS, Valneva is aware that LimmaTech Controls part of the Licensed Technology (i.e., the Platform Technology - as defined below) under an exclusive license from GlaxoSmithKline Biologicals SA (“GSK”), effective as of 22 November 2022, including sublicenses from several parties as outlined in said exclusive license (“GSK License”). Taking into account the GSK License in the drafting of this Agreement, the Parties have agreed that:
-Valneva shall assume certain obligations and covenants originally incumbent on LimmaTech under the GSK License, to the exclusion of any financial obligations, for which LimmaTech is and will remain solely liable to GSK; and
-certain modifications to the GSK License were a necessary precondition to the execution and
effective performance of this Agreement, as a result of which, prior to the execution of this Agreement, LimmaTech and GSK entered into an amendment to the GSK License on July 26, 2024.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants promises and covenants contained herein, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
1.DEFINITIONS.
As used in this Agreement, the following terms will have the meanings set forth below:
1.1.“Affiliate” means any entity directly or indirectly controlled by, controlling, or under common control with, a Party, but only for so long as such control will continue. In this context, “control” (including, with correlative meanings, “controlled by”, “controlling” and “under common control with”) means: (a) beneficial ownership by one entity, directly or indirectly, of more than 50% (or the maximum ownership interest permitted by Applicable Law) of the voting securities or other ownership or general partnership interest (whether directly or pursuant to any option, warrant or other similar arrangement) or other comparable equity interests of another entity, or (b) possession, direct or indirect, of the power to direct or cause direction of the management or policies of another entity (whether through ownership of securities or other ownership interests, by contract or otherwise); provided, however, that where an entity owns a majority of the voting power necessary to elect a majority of the board of directors or other governing board of another entity, but is restricted from electing such majority by contract or otherwise, such entity will not be considered to be in control of such other entity until such time as such restrictions are no longer in effect.
1.2.“Agreement” means this Development Collaboration, License and Commercialization Agreement,
including its Exhibits, as may be amended from time to time.
1.3.“Applicable Law” means all applicable laws, statutes, rules, regulations, industry standards, orders, judgments or ordinances and other pronouncements having the effect of law of any federal, national, multinational, regional, state, provincial, county, city or other political subdivision, government or Regulatory Authority.
1.4.“Binding Obligation” means, with respect to a Party (a) any agreement or arrangement that binds or affects such Party’s operations or property, including any assignment, license agreement, loan agreement, guaranty, or financing agreement, including the LimmaTech Third Party Agreements; (b) the provisions of such Party’s charter, bylaws or other organizational documents or (c) any order, writ, injunction, decree or judgment of any court or Governmental Authority entered against such Party or by which any of such Party’s operations or property are bound.
1.5.“Business Day” means a day on which banks in Brussels, Vienna and Zurich are open for business, but in any event excluding (i) calendar days beginning on December 24th and continuing through January 2nd of each Calendar Year during the Term and (ii) all Saturdays and Sundays.
1.6.“Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending
on March 31, June 30, September 30 and December 31.
1.7.“Calendar Year” means any twelve (12) month period beginning on January 1 and ending on the
next subsequent December 31.
1.8.“Change of Control” means, with respect to a Party (a) the acquisition of beneficial ownership, directly or indirectly, by any Person (other than such Party or an Affiliate of such Party, and other than by virtue of obtaining irrevocable or other proxies) of securities or other voting interest of such Party representing a majority or more of the combined voting power of such Party’s then outstanding securities or other voting interests, (b) any merger, reorganization, consolidation or business combination involving such Party with a Third Party that results in the holders of beneficial ownership (other than by virtue of obtaining irrevocable proxies) of the voting securities or other voting interests of such Party (or, if applicable, the ultimate parent of such Party) immediately prior to such merger, reorganization, consolidation or business combination ceasing to hold beneficial ownership of at least fifty percent (50%) of the combined voting power of the surviving entity immediately after such merger, reorganization, consolidation or business combination, (c) any sale, lease, exchange, contribution or other transfer (in one transaction or a series of related transactions) of all or substantially all of the assets of such Party to which this Agreement relates, other than a sale or disposition of such assets to an Affiliate of such Party or (d) the approval of any plan or proposal for the liquidation or dissolution of such Party (other than in circumstances where such Party is deemed a Debtor pursuant to Section
9.5).
1.9.“Clinical Trial” means a human clinical study conducted on sufficient numbers of human subjects that is designed to (a) establish that a pharmaceutical product is reasonably safe for continued testing, (b) investigate the safety and efficacy of the pharmaceutical product for its intended use, and to define warnings, precautions and adverse reactions that may be associated with the pharmaceutical product in the dosage range to be prescribed or (c) support Regulatory Approval of such pharmaceutical product or label expansion of such pharmaceutical product. Without limiting the foregoing, Clinical Trial includes any Phase 2 Clinical Trial, Phase 3 Clinical Trial or Phase 4 Trial conducted by or on behalf of one or both Parties in connection with this Agreement.
1.10.“CMO” (contract manufacturing organizations), also called “CDMOs” (contract development and manufacturing organizations), means a company that provides drug development and drug manufacturing services.
1.11.“Combination” means a Licensed Product combining one or more Active Ingredients which are not licensed under this Agreement with Active Ingredients licensed hereunder (as identified in Section
1.51., wherein “Active Ingredient” shall mean any component of the Licensed Product which is biologically active and that is responsible for the intended therapeutic.
1.12.“Commercialize” or “Commercializing” means to (a) market, promote, distribute, offer for sale, sell, have sold, import, have imported, export, have exported or otherwise commercialize a Licensed Product and (b) conduct discovery, pre-clinical, research or other Development activities with respect to a Licensed Product that has received Regulatory Approval. When used as a noun, “Commercialization” means any and all activities involved in Commercializing.
1.13.“Commercially Reasonable Efforts” means with respect to a Party, each and every effort, activity or conduct commonly used in the biopharmaceutical industry by a company of comparable size, under similar circumstances (comparable market potential, market size, profit margin, competitive landscape and risk profile) in a comparable development, manufacture and commercialization program of similar scope and at a similar stage, in order to ensure achieving the purposes of this Agreement but in any event, not less than the level of effort that such Party would use to conduct its own comparable business. Notwithstanding anything to the contrary contained herein, it is understood and agreed that, with respect to Valneva, “Commercially Reasonable Efforts” will not take into account any amounts paid or payable to LimmaTech under this Agreement.
1.14.Section
1.14 intentionally omitted.
1.15.“Confidential Information” means information disclosed, supplied or otherwise made available in any form (e.g. oral, electronically stored, captured in print, etc.) by or on behalf of one Party or any of its Affiliates, Sublicensees or Subcontractors (in such capacity, the “Disclosing Party”) to the other Party or any of its Affiliates, Sublicensees or Subcontractors (in such capacity, the “Receiving Party”) in connection with this Agreement on or after the Effective Date including, with respect to each Party, all Know-How, results, Intellectual Property or information including, but not limited to, proprietary information and materials (whether or not patentable) regarding or embodying such Party’s or its Representatives’ technology, products, business information or objectives, which is marked or otherwise identified as “confidential” or with a similar designation or, if it is not, which a reasonable Person would recognize as information that should be treated as confidential without being marked or otherwise identified as “confidential” or with a similar designation. For the avoidance of doubt, Confidential Information does not include any such information that is covered by any limitation or exclusions set forth in Section
7.2.
1.16.“Control” or “Controlled” means when used in reference to any Intellectual Property Right, other intangible property or Materials, the ability (whether by ownership, license or otherwise, other than pursuant to this Agreement) to, without violating the terms of any agreement with a Third Party, grant a license or sublicense or provide access or other right in, to or under such Intellectual Property Right, other intangible property or Materials.
1.17.“Copyright” means any copyright Controlled by Valneva, which copyright pertains to the promotional materials and literature utilized by Valneva in connection with the Commercialization of Licensed Products in the Territory.
1.18.“Cover” means, with respect to any given Licensed Products and Patent Rights, that a Valid Claim of such Patent Rights would, absent a license hereunder or thereunder or ownership thereof, be infringed by the sale, offer for sale, use, manufacture, having manufactured or importation of such Licensed Product. Cognates of the word “Cover” will have correlative meanings.
1.19.“CRO” (contract research organization) means a company that provides research and development
services for the pharmaceutical, biotechnology, and medical device industries.
1.20.“Develop” or “Developing” means to discover, research or otherwise develop a process, Licensed Product, including conducting non-clinical and clinical research and development activities prior to Regulatory Approval. When used as a noun, “Development” means any and all activities involved in Developing.
1.21.“Development Budget” means the budget plan annexed to this Agreement as Exhibit 1.21 that sets forth in reasonable detail the estimated Development Costs for each activity to be performed by LimmaTech and Valneva, respectively, and the estimated time periods within which such Development Costs have been or are to be incurred by the Party responsible for the corresponding activity.
1.22.“Development Costs” means, with respect to the Licensed Product, the costs incurred by a Party,
on a country-by-country basis prior to Regulatory Approval in each such country, during the Term and in connection with such Party’s performance under this Agreement, and, in each case, subject to the Development Plan and the Development Budget. Development Costs include FTEs of each Party at the respective Direct Cost FTE Rate, external costs incurred (including costs of Subcontractors), and costs for Materials purchased by either Party for the performance of its obligations under the Development Plan, any such costs to be calculated on an “as is” basis, without mark-up.
1.23.“Development Costs Cap” means the cap on the Development Costs incurred by LimmaTech prior to the End of Phase 2 to conduct activities assigned to LimmaTech under the Development Plan to carry on the LimmaTech Phase 2 Clinical Trials, such cap to [***] to be spent in accordance with the Development Budget.
1.24.“Development Data” means any non-clinical or clinical findings, results and other research data relating to the Licensed Products, in any format; formal reports of Clinical Trials; and chemistry manufacturing and controls (CMC) development data (including records of Manufactured batches), INDs and other regulatory filings and registration dossiers, to the extent required for the Development, Manufacture and Commercialization of the Licensed Products.
1.25.“Development Event” means each Development event listed in the table that appears in Section
1.26.“Development Plan” means the project plan annexed to this Agreement as Exhibit 1.26 that sets forth in reasonable detail the main clinical and other Development work that is expected (subject to technical, scientific, clinical, economic and other applicable factors) to be conducted in the Development and Manufacture of the Licensed Product in the Field of Use for the Travelers’ Territory and for the LMIC Territory after the Effective Date and until first Regulatory Approval in a Major Market Country. The Development Plan will also outline, inter alia, the activities to be performed with respect to the transfer of the Licensed Technology from LimmaTech to Valneva and the binding development milestones to be met by Valneva (“Binding Development Milestones”), including the corresponding deadlines. LimmaTech will be contributing to the Development and Manufacture up to the End of Phase 2, and for such activities the Development Plan will delineate the respective activities to be performed by LimmaTech, and the activities to be performed by Valneva and estimated timelines and milestones.
1.27.“Development Term” means the period of time beginning on the Effective Date and expiring on
completion of the Development Plan.
1.28.“Disclosing Party” has the meaning set forth in Section
1.15.
1.29.“Effective Date” means the date on which both Parties have executed this Agreement, as indicated
at the end of this Agreement.
1.30.“EMA” means the European Medicines Agency or any successor agency thereto.
1.31.“End of Phase 2” means the date of the final meeting with the FDA to discuss the final reports of
the Phase 2 Clinical Trials and prepare for Phase 3 Clinical Trials.
1.32.“Exploit” means to Develop, Manufacture, Commercialize, use or otherwise exploit. Cognates of
the word “Exploit” will have correlative meanings.
1.33.“FDA” means the United States of America (“US”) Food and Drug Administration or any
successor agency thereto.
1.34.“Field of Use” means the prevention or treatment of the disease caused by Shigella in humans.
1.35.“First Commercial Sale” means, with respect to any Licensed Product and with respect to any country of the Territory, the first sale of such Licensed Product by Valneva or an Affiliate or Sublicensee of Valneva to a Third Party in such country after such Licensed Product has been granted Regulatory Approval by the appropriate Regulatory Authority in such country.
1.36.“FTE” means, with respect to a person, the equivalent of the work of one (1) employee full time for one (1) year (consisting of at least 1,700 working hours per year (with no further reductions for vacations and holidays)). Overtime, and work on weekends, holidays and the like will not be counted with a multiplier toward the number of hours that are used to calculate the FTE contribution. The portion of a FTE billable by a Party for one (1) individual during a given accounting period shall be determined by dividing the number of hours worked by said individual on the work to be conducted under the Agreement during such accounting period by the number of FTE hours applicable for such accounting period based on 1,700 working hours per year.
1.37.“FTE Rate” means (i) for the period commencing on the Effective Date and until End of Phase 2, an annual collaboration rate for direct costs of [***] per FTE (“Direct Cost FTE Rate”); and (ii) for the period commencing on the day after the End of Phase 2, an annual fully loaded rate of [***] per FTE (“Fully Loaded FTE Rate”).
1.38.“GCP” means the Good Clinical Practices officially published by EMA, FDA and the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) that may be in effect from time to time and are applicable to the testing of the compounds.
1.39.“Global Health Market” means public sales (e.g., through Gavi, government, NGOs or other not
for profit organizations) in the LMIC Territory.
1.40.“Governmental Authority” means any court, agency, department, authority or other instrumentality of any national, state, county, city or other political subdivision.
1.41.Section
1.41 intentionally omitted.
1.42.“Human Material” means cells, cell cultures, blood, fluids, tissues, genetic material and genetic information (including data or results derived from DNA or RNA) of one or more Subjects provided or utilized by either Party to conduct the Development Plan pursuant to this Agreement.
1.43.“ICF” means an “Informed Consent Form” that was approved by a qualified Institutional Review
Board or Independent Ethics Committee in accordance with all Applicable Law and recognized international standards for the protection of human research subjects.
1.44.“IFRS” means International Financial Reporting Standards.
1.45.“IND” means an “Investigational New Drug” application submitted under the FD&C Act for the purpose of obtaining permission to conduct Clinical Trials in the US, and/or any analogous application or submission with any analogous agency or Regulatory Authority to conduct Clinical Trials outside of the US, such as, for example, a “CTA” or “Clinical Trial Application” to conduct Clinical Trials in the European Union (“EU”) under Applicable Law.
1.46.“Intellectual Property Rights” means any and all rights and privileges in inventions, discoveries, findings, advances, technical information, Confidential Information, other proprietary information, KnowHow, materials, data or technology (each whether or not patentable), Trademarks, images, works of authorship (including, as the case may be, computer applications, programs, software, files, compilations, databases, documentation and related items), including all Patent Rights, trademark rights, copyrights, database rights, design rights relating thereto, and derivative works thereof, and any other forms of proprietary or intellectual property rights however denominated throughout the world.
1.47.“Joint Steering Committee” or “JSC” means the steering committee described in Section
4.3.1.
1.48.“Know-How” means Trade Secrets, Development Data, technical information (including, without limitation, data and information relating to inventions, methods, systems, processes, discoveries, concepts, methodologies, models, research, development and testing procedures, tests and trials, manufacturing processes, techniques and specifications, quality control data, analyses, reports, methods of production and purification and formulations and submissions) and any document containing such Trade Secrets, Development Data and information, whether or not patentable.
1.49.“Licensed Know-How” means Platform Know-How and LimmaTech Know-How. The Licensed
Know-How as of the Effective Date is identified in Exhibit 1.49 hereto.
1.50.“Licensed Patent Rights” means Platform Patent Rights and LimmaTech Patent Rights.
1.51.“Licensed Product(s)” means any product which is Developed, Manufactured, Commercialized
or otherwise Exploited using the Licensed Know-How, and/or is otherwise Covered by a Licensed Patent Right, where such product is a vaccine whose Active Ingredients are O-antigens from Shigella sonnei, Shigella Flexneri serotypes 2a, 3a and 6, all conjugated to an exoprotein A (EPA) carrier protein, and produced using “bioconjugation” technology using an oligosaccharyltransferase selected from wild-type PglB, PglB containing mutations described in WO 16/107818 or Pg1L. Unless otherwise set forth herein, for the purposes of this Agreement, Licensed Product includes Combination(s).
1.52.“Licensed Technology” means Licensed Patent Rights and Licensed Know-How.
1.53.“LimmaTech Know-How” means (a) any Know-How that (i) is owned by LimmaTech or any of its Affiliates before or on the Effective Date and (ii) relates to any Licensed Products and is useful or necessary for the Development, Manufacture, Commercialization or other Exploitation of any Licensed Products; (b) any Know-How Developed solely by or on behalf of LimmaTech or its Representatives after the Effective Date and before the End of Phase 2 in the conduct of activities under this Agreement; and (c) any Know-How owned by LimmaTech before the End of Phase 2 that directly relates to O-antigens from Shigella sonnei, Shigella Flexneri serotypes 2a, 3a and 6, all conjugated to an exoprotein A (EPA) carrier protein, and produced using “bioconjugation” technology using an oligosaccharyltransferase selected from wild-type PglB, PglB containing mutations described in WO 16/107818 or Pg1L. Know-How of any Person that becomes an Affiliate of LimmaTech after the Effective Date as a result of a Change of Control of LimmaTech will not be included within LimmaTech Know-How; provided that, and only so long as, none of LimmaTech Technology used in the Development or Manufacture of a Licensed Product is used by such Person or its Affiliates, other than LimmaTech, in the Development, Manufacture or Commercialization of any Licensed Product. If such Person uses, in any manner LimmaTech Technology for the Development, Manufacture or Commercialization of a Licensed Product in the Field of Use, then any Intellectual Property Rights conceived, discovered, developed or otherwise made, by or on behalf of such Person in the course of such use will be included in LimmaTech Know-How.
1.54.“LimmaTech Materials” means any Materials owned by LimmaTech or any of its Affiliates on
the Effective Date, including but not limited to Clinical Trial Materials, as identified in Exhibit 1.54.
1.55.“LimmaTech Patent Rights” means any Patent Rights that may arise on (a) the inventions listed
in Exhibit 1.55 attached hereto; and (b) any other invention(s) discovered, created, conceived, developed or reduced to practice solely by LimmaTech or its Representatives during the performance of Phase 2 Clinical Trials. Patent Rights of any Person that becomes an Affiliate of LimmaTech after the Effective Date as a result of a Change of Control of LimmaTech will not be included within LimmaTech Patent Rights; provided that, and only so long as none of LimmaTech Technology used in the Development or Manufacture of a Licensed Product is used by such Person or its Affiliates, other than LimmaTech, in the Development or Manufacture of any Licensed Product. If such Person uses LimmaTech Technology in any manner for the Development or Manufacture of a Licensed Product in the Field of Use, then any Intellectual Property Rights conceived, discovered, developed or otherwise made, by or on behalf of such Person in the course of such use will be included in LimmaTech Patent Rights.
1.56.“LimmaTech Phase 2 Clinical Trials” means the “Phase 2 Human Challenge Study (S.sonnei)”
and the “Pediatric Phase 2 Study in endemic countries” to be conducted by LimmaTech as further outlined in the Development Plan in Exhibit 1.26.
1.57.“LimmaTech Technology” means LimmaTech Patent Rights and LimmaTech Know-How.
1.58.“LimmaTech Third Party Agreement” means any agreement between LimmaTech (or any of its
Affiliates) and any Third Party (such Third Party, a “Third Party Licensor”) that (a) relates to any of the Licensed Technology or (b) otherwise grants a license or otherwise transfers any right to practice under any Patent Rights or Know-How, in each case that relates to the Licensed Products or activities under this Agreement, as listed in Exhibit 1.58.
1.59.“LMIC GAVI” means LMIC eligible for new vaccine introduction as per “Gavi Alliance
Eligibility and Transition Policy” and subsequent revisions (Board Document Template (gavi.org); if a country is removed from such list, then the corresponding change shall become effective between the Parties at the end of the Calendar Year in which such removal takes place.
1.60.“LMIC non-GAVI” means all LMIC excluding LMIC GAVI.
1.61.“LMIC Territory” means all LMIC.
1.62.“Low- and Middle-Income Country(ies)” or “LMIC” means the countries identified by the World Bank based on gross national income per capita (in U.S. dollars, converted from local currency using the Atlas method), including low, lower middle and higher middle-income countries.
1.63.“Major EU Market Country(ies)” means any of France and Germany.
1.64.“Major Market Country(ies)” means any of the Major EU Market Countries, the United
Kingdom, Australia and the US.
1.65.“Manufacture” or “Manufacturing” means to make, produce, manufacture, process, fill, finish,
package, label, perform quality assurance testing, release, ship or store, and for the purposes of further Manufacturing, distribute, import or export, a Licensed Product or any component thereof. When used as a noun, “Manufacture” or “Manufacturing” means any and all activities involved in Manufacturing a Licensed Product or any component thereof.
1.66.“Marketing Approval Application” or “MAA” means an application submitted to a competent
Regulatory Authority necessary to Commercialize a drug in a country or jurisdiction of the Territory, including, in the US, the biologics license application (“BLA”) requesting permission to introduce, or deliver for introduction, a biological product into interstate commerce or, in EU, the marketing authorization application (“EU-MAA”).
1.67.“Materials” means any and all biopharmaceutical, biological, chemical or other physical materials (but not information and documents about such materials, which constitute Know-How), including without limitation production strains, nucleotide sequences and protein sequences, in each case related to the Licensed Product.
1.68.“Material Breach” means a breach that, having regard to the circumstances, defeats the main purpose of the Agreement, or relates to an essential element of the Agreement, and deprives the aggrieved non-breaching Party of a material benefit that it reasonably expected, so that it would be unreasonable to require such Party to remain bound by the Agreement.
1.69.“Net Sales” means the gross invoiced price for sales of Licensed Products sold by Valneva or its Affiliates or Sublicensees (in final form for end use, but exclusive of inter-company transfers), less the following deductions (to be documented in writing) from such invoiced amounts which are actually incurred or accrued:
(a)credits or allowances granted for damaged, outdated, returned, rejected or recalled Licensed Products, and retroactive price reductions;
(b)normal and customary trade, cash and quantity discounts;
(c)sales and excise taxes and duties (including value added taxes (VAT) and import duties) paid or allowed by a selling party and any other governmental charges imposed upon the sale of a Licensed Product;
(d)chargebacks and rebates, including those granted to managed health care organizations, wholesalers, buying groups, retailers or to governments, their agencies and purchasers and reimbursers; and
(e)freight, insurance, charges or other fees related to the handling and distribution of Licensed Products (to the extent not paid by the Third Party customer).
All of the foregoing elements of Net Sales calculations shall be determined on an accrual basis in accordance with applicable IFRS.
Sales between or among Valneva, its Affiliates and Sublicensees shall be excluded from the computation of Net Sales, but Net Sales shall include the first sales to Third Parties (other than Sublicensees) by Valneva, any such Affiliates or Sublicensees. The supply of Licensed Products as samples, for use in non-clinical or clinical studies, or for use in any tests or studies reasonably necessary to comply with any Applicable Law, regulation or request by a Regulatory or Governmental Authority or as is otherwise normal and customary in the industry shall not be included within the computation of Net Sales.
In the event a Combination is sold, then for each Calendar Quarter payment period and on a country-bycountry basis for the remainder of this paragraph, the gross amount invoiced for the Licensed Product combined therein shall be calculated by multiplying the gross amount invoiced for such Combination by the fraction A/(A+B), where “A” is the gross amount invoiced for the Licensed Product sold separately and “B” is the gross amount invoiced for the other Active Ingredient(s) sold separately. In the event that the other Active Ingredient is not sold separately, then the gross amount invoiced for that Licensed Product shall be calculated by multiplying the gross amount invoiced for the Combination by the fraction A/C, where “A” is the gross invoice amount for the Licensed Product, if sold separately, and “C” is the gross invoice amount for the Combination.
In the event that a particular Combination is not addressed by the foregoing, Net Sales for royalty determination shall be determined by the Parties in good faith.
Valneva may sell Licensed Products as part of a bundle with other Valneva products or offer package deals to customers that include Licensed Products, provided that for the calculation of Net Sales, the gross amount invoiced for Licensed Products so treated will be deemed to be the weighted average unit invoice price in the same Calendar Quarter for the Licensed Product sold separately and the rebate or discount with respect to the Licensed Product may not exceed the weighted average rebate or discount on all other products sold as part of the bundle or package.
1.70.Section
1.70 intentionally omitted.
1.71.Section
1.71 intentionally omitted.
1.72.“Patent Rights” means issued patents, pending patent applications, together with any patent resulting therefrom, including provisional applications, divisionals, continuations, continuations-in-part, continued prosecution applications, reissues, substitutions, revalidations, divisions and renewals, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms, including patent term adjustments, patent term extensions, supplementary protection certificates or the equivalent thereof, registrations and confirmations thereof which relate to an invention, other forms of government-issued rights substantially similar to any of the foregoing, and all foreign counterparts of any of the foregoing.
1.73.“Person” means any individual, firm, corporation, partnership, limited liability company, trust,
business trust, joint venture, Governmental Authority, association or other entity.
1.74.“Phase 2 Clinical Trial(s)” means a Clinical Trial, the principal purpose of which is to make a preliminary determination as to whether a pharmaceutical product is safe for its intended use and to obtain sufficient information about such product’s efficacy or immunogenicity, in a manner that is generally consistent with the Applicable Law, to permit the design of further Clinical Trials.
1.75.“Phase 3 Clinical Trial” means a pivotal Clinical Trial with a defined dose or a set of defined doses of a pharmaceutical product designed to ascertain efficacy or immunogenicity and safety of such product, in a manner that is generally consistent with the Applicable Law, for the purpose of enabling the preparation and submission of a BLA.
1.76.“Phase 4 Trial” means any study initiated in the Territory for a Licensed Product following the first Regulatory Approval for the sale of such Licensed Product for the indication being studied whether or not required by a Governmental Authority. Phase 4 Trials may include epidemiological studies, modeling and pharmacoeconomic studies, and post-marketing surveillance studies, as well as any clinical study or research study sponsored and conducted by a Person not employed by or on behalf of either Party.
1.77.“Platform Know-How” means any Know-How which LimmaTech has been granted a license or any right to use before or on the Effective Date under the LimmaTech Third Party Agreements, in each case that relates to one or more Licensed Products, excluding any Know-How related to Active Ingredients in a Combination which are not licensed under this Agreement, including the Development, Manufacture, Commercialization or other Exploitation of the Licensed Products (excluding Active Ingredients in a Combination which are not licensed under this Agreement).
1.78.“Platform Materials” means any Materials which LimmaTech has been granted a license or any right to use before or on the Effective Date under the LimmaTech Third Party Agreements, including but not limited to the Materials listed in Exhibit 1.78, in each case that relates to one or more Licensed Products, excluding any Material relating to Active Ingredients in a Combination which are not licensed under this Agreement, including the Development, Manufacture, Commercialization or other Exploitation of the Licensed Products (excluding Active Ingredients in a Combination which are not licensed under this Agreement).
1.79.“Platform Patent Rights” means any Patent Rights which LimmaTech has been granted a license
or any right to use before or on the Effective Date under the LimmaTech Third Party Agreements, and which it Controls on the Effective Date, as listed in Exhibit 1.79, in each case that relates to one or more Licensed Products (excluding any Patent Rights Covering Active Ingredients in a Combination which are not licensed under this Agreement), including the Development, Manufacture, Commercialization or other Exploitation of the Licensed Products (excluding Active Ingredients in any Combination which are not licensed under this Agreement).
1.80.“Platform Technology” means Platform Patent Rights and Platform Know-How.
1.81.“Price Approval” means, in any country where a Governmental Authority authorizes
reimbursement for, or approves or determines pricing for, pharmaceutical products, receipt (or, if required to make such authorization, approval or determination effective, publication) of such reimbursement authorization or pricing approval or determination (as the case may be).
1.82.“Receiving Party” has the meaning set forth in Section
1.15.
1.83.“Regulatory Approval” means all technical, medical and scientific licenses, registrations,
authorizations and approvals (including approvals of BLAs, supplements and amendments, pre- and postapprovals and labeling approvals) of any Regulatory Authority, necessary or useful for the use, Development, Manufacture, and Commercialization of a pharmaceutical or biopharmaceutical product in a regulatory jurisdiction, including commercially reasonable Price Approvals and commercially reasonable Third Party reimbursement approvals.
1.84.“Regulatory Authority” means, with respect to a country in the Territory, any national (e.g., the FDA, the Belgian Federal Agency for Medicines and Health Products (FAGG/AFMPS)) or supranational (e.g., the European Commission, the Council of the European Union, or the EMA) Governmental Authority involved in the granting of a Regulatory Approval or, to the extent required in such country, Price Approval, for pharmaceutical products in such country.
1.85.“Representatives” means (a) with respect to Valneva, Valneva, its Affiliates, its Sublicensees and each of their respective officers, directors, employees, consultants, contractors, Subcontractors and agents and (b) with respect to LimmaTech, LimmaTech, its Affiliates and each of their respective officers, directors, employees, consultants, contractors, Subcontractors and agents.
1.86.“Right of Reference” means the authority to rely upon, and otherwise use, an investigation, data, regulatory filings and registration dossiers for the purpose of obtaining a Regulatory Approval, including the ability to make available the underlying raw data for audit by the applicable Regulatory Authority, if necessary.
1.87.“Royalty Term” means, with respect to any particular Licensed Product in any particular country in the Territory (on a country-by-country basis), the period commencing on the First Commercial Sale of such Licensed Product in such country and ending on the last to occur of (a) the date on which the Manufacture, sale, offer for sale or importation of such Licensed Product in such country would no longer infringe, but for the license granted hereunder, a Valid Claim in such country; (b) the date on which Regulatory Exclusivity in such country with respect to such Licensed Product expires, “Regulatory Exclusivity” to mean any exclusive marketing rights or data exclusivity rights conferred by any Regulatory Authority with respect to a Licensed Product other than Patent Rights; or (c) [***] after First Commercial Sale of such Licensed Product in such country. Notwithstanding the foregoing, the Parties agree that, in no event, and for the entire Territory, the Royalty Term will end no later than (and including) [***].
1.88.“Subcontractor” means a CRO, CMO, CDMO or other service provider to which a Party or a
Party’s Affiliate may, subject to the conditions specified in Section
4.2.2, subcontract part of its responsibilities under the Development Plan and/or, in case of LimmaTech, in execution of the LimmaTech Phase 2 Clinical Trials.
1.89.“Subject” means the individual donor of the Human Material or of the original tissues from which
the Human Material was derived.
1.90.“Sublicensee” means any Person to whom Valneva may, subject to the conditions specified in
Section
2.3, grant, directly or indirectly, a sublicense of rights licensed by LimmaTech to Valneva under this Agreement.
1.91.“Territory” means worldwide.
1.92.“Third Party” means any Person other than Valneva, LimmaTech or their respective Affiliates.
1.93.“Trademark(s)” means any trademark, trade name, service mark, service name, brand, product name, corporate name, domain name, trade dress, logo, slogan or other indication of origin or ownership, including the goodwill and activities associated with each of the foregoing.
1.94.“Trade Secrets” means Confidential Information that qualifies as “trade secret” under Applicable Law including any Confidential Information that, during the Term, either Party expressly identifies as constituting a trade secret, if materially possible by affixing a special “trade secret” label on the medium containing the information in question.
1.95.“Travelers’ Territory” means the entire Territory with the exception of the LMIC Territory.
1.96.“Valid Claim” means, with respect to a particular country and Licensed Product, either:
(a)a claim of an issued and unexpired Licensed Patent Right licensed to Valneva that Covers the Licensed Product and that has not been held permanently revoked, unenforceable or invalid by a final decision of a court or other governmental agency of competent jurisdiction, which decision can no longer be appealed or was not appealed within the time allowed, and that is not admitted to be invalid or unenforceable through reissue, disclaimer or otherwise (i.e., only to the extent the subject matter is disclaimed or is sought to be deleted or amended through reissue), or
(b)a claim of a pending patent application within the Licensed Patent Rights that has not been abandoned, finally rejected or expired without the possibility of appeal or refiling, provided however, that the term Valid Claim shall exclude any such pending claim in a patent application that has not been granted within [***] following the earliest priority filing date for such application unless and until such claim is granted (from and after which time the same would be deemed a Valid Claim).
The Parties agree that the claims of Licensed Patent Rights whose term of protection extends or, as the case may be, would be extended beyond [***] shall no longer be considered as Valid Claims within the meaning of this Section beyond that date.
1.97.“Valneva Diligence Obligations” means Valneva’s Development and Regulatory Approval diligence obligations under Section
5.3.1 and Valneva’s Commercialization diligence obligations under Section
5.3.2.
1.98.“Valneva Know-How” means (a) any Know-How that (i) is Controlled by Valneva or any of its Affiliates before or on the Effective Date and (ii) is useful or necessary for the Development, Manufacture, Commercialization or other Exploitation of any Licensed Products, and (b) any Know-How Developed (i) solely by or on behalf of Valneva or its Representatives or jointly by or on behalf of the Parties or their Representatives before the End of Phase 2; and (c) any Know-How Developed (i) solely by or on behalf of either Party or its Representatives or (ii) jointly by or on behalf of the Parties or their Representatives after the End of Phase 2, in either case during the Term in the conduct of activities under the Agreement.
1.99.“Valneva Patent Rights” means any Patent Rights Controlled by Valneva that are useful or necessary for the Development, Manufacture, Commercialization or other Exploitation of any Licensed Products, including Patent Rights claiming or disclosing any invention included in Valneva Know-How.
1.100.“Valneva Technology” means Valneva Patent Rights and Valneva Know-How.
The following terms are defined in the section of this Agreement listed opposite each term:
Defined Term Section in Agreement
Active Ingredient 1.11
|
|
|
|
|
|
| Agreement |
Preamble |
| Alliance Managers |
|
| Audited Party |
|
| Auditing Party |
|
| Binding Development Milestones |
1.26 |
| Commercial Milestone Event |
3.4 |
| Commercial Milestone Payment |
|
| Debtor |
|
| Development Milestone Payment |
|
| Direct Cost FTE Rate |
|
| EU |
|
| Expert Determination |
|
| Force Majeure |
|
| FTO |
3.5.4 |
| Fully Loaded FTE Rate |
|
| Global Health License |
|
| Global Health Partner(s) |
|
| Global Trade Control Laws |
|
| GSK |
Preamble |
| GSK License |
Preamble |
| Indemnified Party |
|
| Indemnifying Party |
|
| Infringement Claim |
|
| Liabilities |
|
Licensed Activities 6.2.7
LimmaTech Preamble
LimmaTech Indemnified Party 10.2
Manufacturing Process 4.2.5
Party or Parties Preamble
Regulatory Exclusivity 1.87
Term 9.1
Third Party Licensor 1.58
Up-Front Payment 3.1
US 1.33
Valneva Preamble
Valneva Indemnified Party 10.3
VAT 3.8.4
2.LICENSE GRANTS AND TECHNOLOGY TRANSFER.
2.1.Exclusive License from LimmaTech to Valneva. Subject to the terms and conditions of this Agreement, effective as of the Effective Date, LimmaTech hereby grants, and will cause its Affiliates to grant, to Valneva an exclusive (even as to LimmaTech except to the extent necessary to the LimmaTech Phase 2 Clinical Trials), royalty-bearing (to the extent set forth in Section
3.5 below), sublicensable (under the conditions set out in Section
2.3), license under the LimmaTech Technology and the LimmaTech Materials to Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized and otherwise Exploit or have Exploited Licensed Products in the Field of Use in the Territory, provided, however, that - and unless otherwise agreed - in the Global Health Market the Licensed Products shall be Commercialized and otherwise Exploited by Third Party Global Health Partner(s) under a sublicense to be granted in accordance with Section
2.3.2.
2.2.Exclusive Sublicense from LimmaTech to Valneva. Subject to the terms and conditions of this Agreement, effective as of the Effective Date, LimmaTech hereby grants, and will cause its Affiliates to grant, to Valneva an exclusive (even as to LimmaTech except to the extent necessary to the LimmaTech Phase 2 Clinical Trials), royalty-bearing (to the extent set forth in Section
3.5 below), sublicensable (under the conditions set out in Section
2.3) sublicense under the Platform Technology to Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized and otherwise Exploit or have Exploited Licensed Products in the Field of Use in the Territory, subject to Section
2.3.2.
2.3.Sublicensing.
2.3.1.Permitted Sublicenses. Valneva will have the right to grant sublicenses under this Agreement, without LimmaTech’s prior consent, (i) to Subcontractors and Affiliates (to the extent needed) and (ii) after first Regulatory Approval, to any Person of its choice for the Commercialization of Licensed Products in certain countries of the Territory, such countries to be selected by Valneva at its sole discretion. For clarity, a sublicense for Commercialization of Licensed Product in the entire Territory would require LimmaTech’ prior written consent and be subject to the process set forth in Section
2.3.3.
2.3.2.Sublicensing to a Global Health Partner in the LMIC Territory.
Subject to compliance with the terms set forth in this Section 2.3.2, Valneva may sublicense any and all of its rights under this Agreement to one or more Persons for the purpose of Developing, Manufacturing and/or Commercializing the Licensed Product in the LMIC Territory (respectively, the “Global Health License” and the “Global Health Partner(s)”). Certain Global Health Partner(s) [***]. The [***] Global Health Partner(s) on the Effective Date are listed in Exhibit 2.3.2 Part A (the “[***]”). Valneva shall promptly notify LimmaTech of its choice of Global Health Partner(s). If one or more of the Global Health Partners chosen by Valneva is not [***], LimmaTech will notify Valneva, within twenty (20) Business Days after LimmaTech’s receipt of Valneva’s notification, of (i) its approval of or disagreement with such Global Health Partner(s), such approval not to be unreasonably withheld, delayed or conditioned, and (ii) where applicable, the reasons underlying its disagreement. During negotiations with Global Health Partner(s), Valneva commits itself to use Commercially Reasonable Efforts to include the terms and conditions identified in Exhibit 2.3.2 Part B in the Global Health License, without limitation to such other terms and conditions as the negotiating parties may deem appropriate to include in such license so long as they are consistent with the terms and conditions of Exhibit 2.3.2 Part B and any Binding Obligation of the Parties. Valneva shall keep LimmaTech informed of the progress of negotiations with any Global Health Partner(s) on a regular basis and upon reasonable request by LimmaTech. Any deviations from the terms and conditions identified in Exhibit 2.3.2 Part B will require both Parties’prior written consent, such consent not to be unreasonably withheld, delayed or conditioned. Valneva agrees to use Commercially Reasonable Efforts to have the Global Health License entered into no later than at the End of Phase 2. For clarity, with respect to LimmaTech’s obligations under this Section 2.3.2 not to unreasonably withhold its consent, the Parties agree that it would be reasonable for LimmaTech to withhold its consent if a Third Party Licensor who has the right to approve the Global Health Partner and/or the Global Health License does not grant such approval in accordance with the terms of the respective LimmaTech Third Party Agreement.
LimmaTech will request, and to the extent available will share with Valneva, a written statement from such Third Party Licensor (including the reasons for non approval). Notwithstanding the foregoing, LimmaTech shall use Commercially Reasonable Efforts to obtain approval from its Third Party Licensors.
2.3.3.Other Sublicenses. Apart from Sections
2.3.1 and
2.3.2 above, Valneva may only grant sublicenses with the written consent of LimmaTech (and understands that LimmaTech itself may need to obtain consent from its Third Party Licensors prior to granting such consent to Valneva), such consent of LimmaTech not to be unreasonably withheld, conditioned or delayed. Valneva will provide LimmaTech with a copy of each agreement containing such a sublicense (including sublicenses under Section
2.3.1 and
2.3.2) within [***] of its execution, with reasonable redactions that will enable LimmaTech to reasonably monitor compliance with the terms and conditions of this Agreement, such sublicenses being Confidential Information under Article
7 below. In all cases, no sublicense will diminish, reduce or eliminate any obligation of Valneva, as the sublicensing Party, under this Agreement, and Valneva will remain responsible for its obligations under this Agreement and will be responsible for the performance of the relevant sublicensee as if such sublicensee were the sublicensing Party hereunder (including reporting obligations imposed upon Valneva in accordance with this Agreement). Each sublicense granted by Valneva, as the sublicensing Party, to any rights licensed to it hereunder will terminate immediately upon the termination of the original license with respect to such rights.
2.4.Non-Exclusive License from Valneva to LimmaTech. Subject to the terms and conditions of this Agreement, effective as of the Effective Date, Valneva hereby grants to LimmaTech a non-exclusive, royalty-free, fully paid-up license in the Territory, with no right to grant sublicenses other than to Affiliates and Subcontractors under Section
4.2.2, under the Valneva Technology solely to the extent necessary to perform LimmaTech’s activities for the execution of the LimmaTech Phase 2 Clinical Trials.
2.5.Right of Reference. LimmaTech hereby grants to Valneva a Right of Reference to all Development Data (including any regulatory filings or Regulatory Approvals) Controlled by LimmaTech or its Affiliates that relates to any Licensed Product, in all cases solely for the Development, Manufacture, Commercialization and Exploitation of the Licensed Product pursuant to the terms and conditions of this Agreement, and LimmaTech will provide a signed statement to this effect, if requested by Valneva. Valneva may grant such Right of Reference to its Affiliates and Sublicensees.
2.6.Exclusivity Obligation of LimmaTech. During the Term, except to the extent necessary for LimmaTech to perform its activities under the Development Plan, LimmaTech shall not, and shall cause its Affiliates not to (a) directly or indirectly, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized or otherwise Exploit or have Exploited any Licensed Product, alone or in Combination, for itself, an Affiliate or with or on behalf of a Third Party, or (b) license, authorize, appoint, or otherwise enable any Third Party to perform any of the activities under clause (a); provided, however, that in the event of a Change of Control of LimmaTech the foregoing limitations shall not apply to the acquiring company with respect to any research, development or commercialization efforts that such acquiring company had on-going as of the date of such Change of Control. Except as provided in this Agreement, such acquiring company will not use, in any manner, any Licensed Technology for the Exploitation, alone or with any Third Party, of any Licensed Product.
2.7.Exclusivity Obligation of Valneva. Valneva shall not, and shall cause its Affiliates not to, directly or indirectly:
(a)commercialize, have commercialized, or manufacture or have manufactured for commercialization purposes any product in the Field of Use which is not a Licensed Product during the Term;
(b)develop, have developed, or manufacture or have manufactured for development purposes any product in the Field of Use that is not a Licensed Product up to and including [***]. If Valneva decides to undertake such development or manufacturing activities after that date, it will notify LimmaTech within [***] of the commencement of such activities;
(c)acquire a competing product to the Licensed Product for use in the Field of Use up to and including [***]. If Valneva decides to acquire a competing product after that date, it will notify LimmaTech within [***] of such acquisition with a view to organizing a transition period to be negotiated in good faith between the Parties.
In case of a Change of Control of Valneva, Valneva will, and will cause its Representatives to ensure that such relevant surviving entity of such Change of Control and its Affiliates meet the exclusivity obligation of Valneva under this Section
2.7.
2.8.No Implied Rights. Except as expressly provided in this Agreement, neither Party will be deemed to have granted the other Party (by implication, estoppel or otherwise) any right, title, license or other interest in or with respect to any Patent Rights, Know-How or other Intellectual Property Rights or information Controlled by such Party.
2.9.Safe Harbor. This Agreement is not intended to restrict or waive any rights that the Parties may otherwise have under Applicable Law, including the “safe-harbor” (35 U.S.C. § 271(e)(1)), the Bolar Exemption and the research exemption.
2.10.Initial Data Transfer. Within a reasonable time not to exceed [***] following the Effective Date and to the extent LimmaTech is allowed to do so under Applicable Law, LimmaTech will disclose to Valneva true, accurate and complete copies of all Development Data to the extent Controlled by LimmaTech on the Effective Date and in its current (electronic or other) format as Valneva may reasonably request (including by download of digital files to a secure website or e-room designated and controlled by Valneva).
2.11.Samples of Tangible Materials. Within a reasonable time not to exceed [***] following the Effective Date, LimmaTech will provide to Valneva, in compliance with Applicable Law, any Platform Materials and LimmaTech Materials, including research grade samples Controlled by and available at LimmaTech and incorporating all or part of the Licensed Patent Rights discovered, created, conceived, developed or reduced to practice and Controlled by LimmaTech on the Effective Date, except for Materials that are needed for LimmaTech to perform its activities under the Development Plan. Nothing in this Section 2.11 shall limit or in any way change LimmaTech’s obligations under this Agreement with respect to any Human Material it provides or uses under this Agreement.
2.12.Continuing Disclosure and Knowledge Transfer. On a Calendar Quarter basis, or more frequently at the reasonable request of Valneva prior to the End of Phase 2, LimmaTech, to the extent not previously provided to Valneva, will provide to Valneva, through the JSC, a written summary of all Licensed Technology that is licensed or developed by LimmaTech or that otherwise comes into the Control of LimmaTech that relates to the Development of any Licensed Product, alone or in Combination. Further, LimmaTech will make appropriate personnel (directly, or through an Affiliate) available to Valneva at reasonable times and places and upon reasonable prior notice for the purpose of assisting Valneva to understand and use the Licensed Technology in connection with Valneva’s Development, Manufacture, Commercialization and use of Licensed Products.
2.13.Development Data Transfer. LimmaTech shall provide to Valneva copies of all Development Data from any and all completed Clinical Trials for the Licensed Product, including the LimmaTech Phase 2 Clinical Trials, in electronic form or other mutually agreeable alternate form on timelines agreed to by the JSC, and a complete copy of such Development Data shall be provided to Valneva as soon as reasonably possible following interim readouts and completion of each LimmaTech Phase 2 Clinical Trial, or as otherwise agreed by the JSC. As soon as reasonably feasible, LimmaTech will ensure that all patient authorizations and consents required under Applicable Law in connection with the LimmaTech Phase 2 Clinical Trials permit such sharing of Development Data with Valneva.
2.14.Technology Transfer. Notwithstanding Sections
2.10, 2.11, 2.12 and
2.13, LimmaTech will use Commercially Reasonable Efforts to provide Valneva with all reasonable assistance necessary or desirable (a) to effect the timely and orderly transfer of the Licensed Technology in accordance with the Development Plan, (b) to enable Valneva to perform its obligations under Article 5; and (c) for Valneva to exercise its rights under the licenses and sublicenses granted in Section
2.1. Without limiting LimmaTech’s obligations set forth elsewhere under this Agreement, LimmaTech will perform all technology transfer activities as set forth in the Development Plan, and will cause such activities to be performed by the specific individuals identified for such purpose in the Development Plan.
3.PAYMENTS AND COSTS.
3.1.Up-Front Payment. Valneva will make a one-time, non-refundable, non-creditable payment to LimmaTech of ten million Euros (€10,000,000) (the “Up-Front Payment”), within [***] of receipt of LimmaTech’s invoice (such invoice to be delivered on or following the Effective Date of this Agreement) for the rights and (sub)licenses granted by LimmaTech to Valneva hereunder.
3.2.Development Costs. The Parties will collaborate on the Development of the Licensed Product in accordance with the Development Plan and the Development Budget, and pursuant to Article
4. Subject to Section
9.4.1(ii) in the event of termination before the End of Phase 2, LimmaTech’s financial contribution to the Development Costs must amount and is limited to the Development Costs Cap, which has to be spent in accordance with the Development Budget. Valneva will bear all Development Costs not expressly allocated to LimmaTech under the Development Budget in Exhibit
1.21.
3.2.1.Accounting for and Refunding of Development Costs. Each Party shall inform the Joint Steering Committee (JSC) of any potentially required deviation from the amount of Development Costs to be incurred by it as agreed between the Parties under the Development Budget for the performance of LimmaTech Phase 2 Clinical Trials at least [***] prior to incurring the costs in question, for the JSC to review and agree on such deviation. In addition, and in any event, within [***] after the end of each calendar month during each Calendar Year and until the Development Costs Cap has been fully spent in accordance with the Development Budget, the Parties shall provide each other with detailed, activity-based statements of the Development Costs incurred during such month in the performance of the LimmaTech Phase 2 Clinical Trials pursuant to this Section
3.2, provided, however, that no break-down on FTEs is required, as - unless otherwise agreed - the number of FTEs is as agreed in the Development Budget. The statements shall be in a format to be agreed between the Parties. The information contained in these statements shall be such as to enable the Parties to transparently monitor and calculate the amount of Development Costs incurred. Once the Development Costs Cap has been reached, all further Development Costs incurred by LimmaTech in performance of its activities under the Development Plan and in accordance with the Development Budget until the End of Phase 2 will be fully refunded by Valneva within [***] of the invoice sent by LimmaTech to Valneva for this purpose. Within [***] of the other Party’s request, the accounting Party will provide copies of invoices or other appropriate supporting documentation with respect to the incurred Development Costs until the Development Costs Cap has been fully spent in accordance with the Development Budget. Further, Valneva will inform LimmaTech of all costs related to the Manufacturing and characterization of the new S. sonnei GMP Drug Substance Phase 2 lot, including the technology transfer to a CDMO. The Parties will treat all above-mentioned information in accordance with the confidentiality provisions of Article
7.
3.2.2.Audits of Development Costs. Upon [***] prior notice from either Party (“Auditing Party”), the other Party (“Audited Party”) will permit an independent certified public accounting firm of nationally recognized standing selected by the Auditing Party and reasonably acceptable to the Audited Party, to examine, at the Auditing Party’s sole expense, the relevant books and records of the Audited Party and its Affiliates as may be reasonably necessary to verify the amounts reported by the Audited Party in accordance with Section 3.2.1. An audit by a Party under this Section
3.2.2 may not occur more than [***] and this right to request an audit shall expire [***] after the completion of the LimmaTech Phase 2 Clinical Trials. The respective accounting firm will be provided access to such books and records at the Audited Party’s or its Affiliates’ facility(ies) where such books and records are normally kept, and such examination will be conducted during the Audited Party’s normal business hours. The Audited Party may require the accounting firm to sign a reasonably acceptable non-disclosure agreement before providing the accounting firm with access to the Audited Party’s or its Affiliates’ facilities or records. Upon completion of the audit, the accounting firm will provide both Parties with a written report disclosing any discrepancies in the reports submitted by the Audited Party pursuant to Section
3.2.1 and the specific details concerning any discrepancies. No other information will be provided to the Auditing Party.
3.2.3.Third Party Grants. In the event LimmaTech obtains a Third Party offer for a grant or other funding to support the Development under this Agreement prior to the End of Phase 2, it shall inform Valneva of such offer, and the Parties shall discuss in good faith on acceptable terms and conditions of such funding, at which point a decision will be made on whether both Parties are willing to commit to such funding. Provided the Parties agree to accept such funding, fifty percent (50%) of such funding will be used to reduce LimmaTech’s financial contribution to the Development Costs, and fifty percent (50%) of such funding will be used to support the Development financed by Valneva.
3.3.Development Milestone Payments. Valneva will pay LimmaTech the non-refundable, non- creditable, one-time amounts set forth below, subject to the following conditions: (i) Valneva shall notify LimmaTech within [***] of the first occurrence of each event described below for the first Licensed Product Covered by a Valid Claim in the applicable country of Development or Commercialization in the Territory to achieve such event (each, a “Development Milestone Payment”) and (ii) the amount due for each Development Milestone Payment shall be paid within [***] after Valneva’s receipt of an invoice sent by LimmaTech for this purpose.
|
|
|
|
|
|
|
|
|
|
Development Event |
Development
Milestone Payment
|
(i) |
Upon the earlier of (i) full enrolment of the 1st controlled human infection model (CHIM) study; and (ii) transfer of the IND from LimmaTech to Valneva |
[***] |
(ii) |
[***] |
[***] |
(iii) |
[***] |
[***] |
(iv) |
Initiation of first Phase 3 Clinical Trial |
[***] |
(v) |
1st Regulatory Approval in US |
[***] |
(vi) |
1st Regulatory Approval in EU |
[***] |
|
|
|
|
|
|
|
|
|
|
Total potential Development Milestone Payments |
[***] |
(vii) |
Development Milestone Payment at sale of Priority Review
Voucher(s) (PRV) (if any)
|
[***] of Valneva’s Proceeds |
Each of the Development Milestone Payments set forth above will be payable one time only (regardless of the number of Licensed Products with respect to which, or the number of times with respect to any Licensed Product, the specified Development Event occurs). No Development Milestone Payment will be payable by Valneva for any subsequent Licensed Product regardless of the number of Licensed Products Developed. For clarification, if one Licensed Product replaces another Licensed Product in Development, then such replacement Licensed Product will only be subject to Development Milestone Payment(s) that has(have) not previously been triggered by one or more prior Licensed Products. If a Development Milestone is skipped (e.g., Phase 3 Clinical Trials are initiated even though the first Development Milestone has not been reached) the skipped Development Milestone shall become payable together with the respective next achieved Development Milestone. “Initiation of first Phase 3 Clinical Trial” shall occur once the first patient has been recruited for such first Phase 3 Clinical Trial; and “Proceeds” for the PRV Development Milestone Payment shall mean (i) the gross proceeds received by Valneva from a Third Party for the sale of Valneva’s Priority Review Voucher awarded by the FDA, and/or for the sale of Valneva’s corresponding vouchers from other governmental agencies, and linked to the Licensed Products in the Field of Use, or (ii), if such proceeds are received other than in cash, the value of the respective consideration received for such sale, to be determined by a Third Party expert consult to the extent the Parties cannot agree on such value, in either case less the following expenses: (x) the amount owed to [***] (which Valneva will pay to LimmaTech within [***] after Valneva’s receipt of an invoice from LimmaTech, provided that Valneva shall inform LimmaTech of the receipt of such Proceeds within [***]); and (y) the reasonable legal expenses incurred by Valneva or its Affiliates in connection with such sale.
The maximum amount payable to LimmaTech by Valneva under this Agreement with respect to all Development Milestone Payments if all Development Events occur will be [***], plus [***] of Valneva’s Proceeds in the event of the sale of a Priority Review Voucher as described in the above paragraph.
3.4.Commercial Milestone Payments. Valneva will pay LimmaTech the non-refundable, non- creditable, one-time amount set forth below, in accordance with to the following conditions: (i) Valneva will notify LimmaTech within [***] of the day on which one of the two commercial milestone events identified below is reached for the first time (“Commercial Milestone Event”); and (ii) the amount due for such Commercial Milestone Event (“Commercial Milestone Payment”) shall be paid within [***] after Valneva’s receipt of an invoice sent by LimmaTech for this purpose.
|
|
|
|
|
|
| Commercial Milestone Event |
Commercial Milestone Payment |
|
(1)Global cumulative Net Sales in the Travelers’ Territory for the first time reach [***]); or
(2)Global annual Net Sales in the Travelers’ Territory for the first time reach [***],
whatever is earlier.
|
[***] |
| Total potential Commercial Milestone Payments |
[***] |
The Commercial Milestone Payment shall be paid only one time during the Agreement regardless of how many times the Licensed Products achieve a Commercial Milestone Event. The maximum amount payable by Valneva under this Agreement with respect to the Commercial Milestone Payment will be [***].
3.5.Royalty Payments for the Travelers’ Territory.
3.5.1.Royalties Rates. Valneva will pay LimmaTech royalties amounting to a percentage (as specified in the table below) of the annual aggregate Traveler’s Territory-wide Net Sales, on a Licensed Product-by-Licensed Product basis, during each Calendar Year of the applicable Royalty Term for each Licensed Product.
|
|
|
|
|
|
From the First Commercial Sale up to and including [***] |
[***] |
[***] up to and including [***] |
[***] |
[***] up to and including [***] |
[***] |
[***] up to the end of the Royalty Term |
[***] |
3.5.2.Royalties Rates Reductions. If, as a result of renegotiations, LimmaTech Third Party Agreements are modified so as to result in a reduction in the royalty rates owed by LimmaTech to its Third Party Licensors, the royalty rates specified in Section
3.5.1 shall be modified so that the resulting benefit – calculated after deduction of all contributions made by LimmaTech in order to get such royalty rates reduction, such contributions to be evidenced by written records – shall [***] between the Parties. By way of example, if renegotiations result in a royalty reduction of [***], Valneva shall be entitled to a royalty reduction of [***]. LimmaTech shall notify Valneva within [***] of the completion of such renegotiations to allow the Parties to then amend the royalty rates due under Section
3.5.1 accordingly. Valneva shall reimburse [***] of reasonable legal expenses incurred by LimmaTech in connection with the above-mentioned renegotiations, which are payable within [***] from date of receipt of invoice. For clarity, LimmaTech is under no obligation to enter into such renegotiations.
3.5.3.Fully Paid-Up, Royalty Free License. Following expiration of the Royalty Term for any Licensed Product in a given country in the Traveler’s Territory, no further royalties will be payable in respect of sales of such Licensed Product in such country and, thereafter the license granted to Valneva under Sections
2.1 and
2.2 with respect to such Licensed Product in such country will automatically become fully paid-up, irrevocable, non-exclusive and royalty-free.
3.5.4.Adjustment for Additional Third Party License. If the Parties agree that it is necessary for Valneva to license one or more Patent Rights from one or more Third Parties in order to obtain freedom to operate (“FTO”) with respect to the Licensed Technology to Develop, Manufacture, Commercialize or Exploit any Licensed Product (excluding any license related to Active Ingredients in a Combination which are not licensed under this Agreement) as set
out in the Development Plan at the time of the Effective Date, whether directly or through any Valneva Affiliate or Sublicensee in any country in the Traveler’s Territory, then Valneva may, in coordination with LimmaTech, negotiate and obtain a license under such Patent Rights. If the Parties disagree on the need for an additional Third Party license in order to obtain FTO, the Parties shall, within thirty (30) calendar days of notification by one Party to the other of the interest of such license, jointly select an outside patent attorney to assess if there is an FTO issue and the need for an additional Third Party license. The cost of such patent attorney shall be borne by the Party whose opinion was not followed by the outside patent attorney.
On a country-by-country basis in the Travelers’ Territory, any royalty otherwise payable to LimmaTech under this Agreement with respect to Net Sales of any Licensed Product in the applicable country will be reduced [***] of the royalties payable to Third Parties pursuant to such additional Third Party FTO license, provided that such deduction shall never exceed [***].
3.5.5.No Adjustment for LimmaTech Third Party Agreements. With respect to the Travelers’ Territory, LimmaTech will be solely responsible for (i) all payment obligations (including any royalty or other obligations that relate to any of the Licensed Products or Platform Technology) under LimmaTech Third Party Agreements that are in effect on or prior to the Effective Date and (ii) all payments to inventors of Licensed Technology (other than inventors that are
Representatives of Valneva); and to inventors of Valneva Technology (provided the inventors are Representatives of LimmaTech), including payments under inventorship compensation Applicable Law.
3.6.Participation or Royalty Payments for the LMIC Territory.
3.6.1.Commercialization by the Global Health Partner(s) within the LMIC Territory. With respect to any payments received from the Global Health Partner(s) in performance and in consideration of the Global Health License(s), Valneva shall make the following payments to LimmaTech:
(a)From all payments (including both cash payments and the monetary equivalent of any other consideration as, where applicable, reasonably determined, in good faith, by Valneva) received from the Global Health Partner(s) in consideration for sales of the Licensed Product during the Royalty Term, Valneva will:
(i)reimburse LimmaTech all LMIC related payments owed [***] as outlined in Exhibit 3.6.1, For clarity, Valneva shall reimburse LimmaTech for such payments, even if the payments received from the Global Health Partners under Section 3.6.1(a) do not fully cover such LMIC related payments. For further clarity, LimmaTech will be solely responsible for (x) any additional obligations (including any royalty or other obligations that relate to any of the Licensed Products or Platform Technology) under LimmaTech Third Party Agreements that are not listed in Exhibit 3.6.1 and (y) all payments to inventors of Licensed Technology (other than inventors that are Representatives of Valneva); and to inventors of Valneva Technology (provided the inventors are Representatives of LimmaTech), including payments under inventorship compensation Applicable Law; and
(ii)pay to LimmaTech a participation in the amount of [***] of the remainder of such payments received from the Global Health Partner(s). Excluded from the payments received from the Global Health Partner(s) for purposes of the calculations under this Section
3.6.1(a) are only (a) VAT; and (b) the supply price of drug substance or drug product (DP) of Licensed Product.
If the Global Health Partner(s) sell the Licensed Product as part of a bundle including both a Licensed Product and other pharmaceutical products or medical devices (“Bundling Sale”), the gross amount invoiced for the Licensed Product so treated will be deemed to be the weighted average unit invoice price in the same Calendar Quarter for the Licensed Product sold separately and the rebate or discount with respect to the Licensed Product may not exceed the weighted average rebate or discount on all other products sold as part of the bundle or package.
If Valneva was to bundle the Global Health License with any other license (including a cross license arrangement, or a combination of licenses), the fair market value of the license to the Global Health Partners(s) in the LMIC Territory would have to be ascertained to determine the just amount from which LimmaTech’s participation would be paid. The Parties would cooperate in good faith to determine such fair market value, by, e.g., benchmarking against the payments made for similar licenses granted in the LMIC Territory.
(b)In case Valneva Manufactures drug substance or drug product of Licensed Products for the Global Health Partner(s), it will pay LimmaTech - in addition to the payments under (a) above - royalties amounting to a percentage (as specified in the table below) of the mark-up on costs of goods (COGS) agreed with the Global Health Partner(s). The Parties agree that the markup taken into account for the calculation of the royalties due under this Section shall in no event be less than [***], so that even if Valneva and the Global Health Partner(s) agree, for example, on a mark-up of [***], a percentage of [***] will be applied for the purpose of calculating the royalties due under this Section. For illustration purposes, if COGS were [***] and the mark-up was [***], LimmaTech would receive [***]. If COGS were [***] and the mark-up was only [***], LimmaTech would not only receive [***] but EUR [***].
|
|
|
|
|
|
From the DS or DP sale up to and including [***] |
[***] |
From [***] up to and including [***] |
[***] |
From [***] up to and including [***] |
[***] |
From [***] up to the end of the Royalty Term |
[***] |
3.6.2.Commercialization by Valneva within the LMIC Territory private market. In the event that Valneva Commercializes the Licensed Product(s) by means of its own commercial
infrastructure or through a Third Party distributor in the LMIC Territory private market, it will pay LimmaTech royalties amounting to a percentage (as specified in the table below) of the annual aggregate Net Sales thus achieved in the LMIC Territory private market (thus excluding the Global Health Market), on a Licensed Product-by-Licensed Product basis, during each Calendar Year of the applicable Royalty Term for each Licensed Product:
|
|
|
|
|
|
|
|
|
|
LMIC non- GAVI |
LMIC GAVI |
From the First Commercial Sale up to and including [***] |
[***] |
[***] |
From [***] up to and including [***] |
[***] |
[***] |
From [***] up to and including [***] |
[***] |
[***] |
From [***] up to the end of the Royalty Term |
[***] |
[***] |
3.6.3.Participation or Royalty Rates Reductions. If, as a result of renegotiations, LimmaTech Third Party Agreements are modified so as to result in a reduction in the royalty rates owed by LimmaTech to its Third Party Licensors, the participation and/or royalty rates specified in Sections
3.6.1 and
3.6.2 shall be modified so that the resulting benefit – calculated after deduction of all contributions made by LimmaTech in order to get such royalty rates reduction, such contributions to be evidenced by written records – shall be shared [***] between the Parties. By way of example, if renegotiations result in a royalty reduction of [***], Valneva shall be entitled to a royalty reduction of [***]. LimmaTech shall notify Valneva within fifteen (15) Business Days of the completion of such renegotiations to allow the Parties to then amend the participation and/or royalty rates due under Sections
3.6.1 and
3.6.2 accordingly. Valneva shall reimburse [***] of reasonable legal expenses incurred by LimmaTech in connection with the above-mentioned renegotiations, which are payable within [***] from date of receipt of invoice. For clarity, LimmaTech is under no obligation to enter into such re-negotiations.
3.6.4.Fully Paid-Up, Royalty Free License. Following expiration of the Royalty Term for any Licensed Product in a given country in the LMIC Territory, no further participations and/or royalties will be payable in respect of sales of such Licensed Product in such country and, thereafter the license granted to Valneva under Sections
2.1 and
2.2 with respect to such Licensed Product in such country will automatically become fully paid-up, irrevocable, nonexclusive and royalty-free.
3.6.5.Adjustment for Additional Third Party License. If the Parties agree that it is necessary for Valneva to license one or more Patent Rights from one or more Third Parties in order to obtain freedom to operate with respect to the Licensed Technology to Develop, Manufacture, Commercialize or Exploit any Licensed Product (excluding any license related to Active Ingredients in a Combination which are not licensed under this Agreement) as set out in the Development Plan at the time of the Effective Date, whether directly or through any Valneva Affiliate or Sublicensee, in any country in the LMIC Territory, then Valneva may, in coordination with LimmaTech, negotiate and obtain a license under such Patent Rights. If the
Parties disagree on the need for an additional Third Party license in order to obtain FTO, the Parties shall, within [***] of notification by one Party to the other of the interest of such license, jointly select an outside patent attorney to assess if there is an FTO issue and the need for an additional Third Party license. The cost of such patent attorney shall be borne by the Party whose opinion was not followed by the outside patent attorney.
On a country-by-country basis in the LMIC Territory, any participation and/or royalty otherwise payable to LimmaTech under this Agreement with respect to Net Sales of any Licensed Product in the applicable country will be reduced by [***] of the royalties payable to Third Parties pursuant to such additional Third Party FTO license, provided that such deduction shall never exceed [***] of Net Sales.
3.7.Compensation for Costs Incurred by LimmaTech Beyond 31 December 2046. If the term of protection of Platform Patent Rights extends or, as the case may be, is extended beyond 31 December 2046 (in which case, pursuant to Section
1.96, the corresponding claims can no longer be considered as Valid Claims beyond that date), Valneva will compensate LimmaTech for reasonable costs LimmaTech would incur with respect to the considered Platform Patent Rights in performance of LimmaTech Third Party Agreements.
3.8.Reports and Payments.
3.8.1.No Cumulative Royalties. The obligation to pay royalties under this Agreement will be imposed only once with respect to any sale of any Licensed Product.
3.8.2.Blended Royalty. The Parties acknowledge and agree that the Licensed Technology licensed under this Agreement may justify royalty rates and/or royalty terms of differing amounts for sales of Licensed Products in the Territory, which rates could be applied separately to Licensed Products involving the exercise of Licensed Patent Rights in the Territory and/or the incorporation of Licensed Know-How, and that if such royalties were calculated separately, royalties relating to the Licensed Patent Rights in the Territory and royalties relating to the Licensed Know-How comprised in the Licensed Technology would last for different terms. The Parties have determined in light of such considerations and for reasons of mutual convenience that blended royalty rates for the Licensed Patent Rights and the Licensed KnowHow licensed hereunder will apply during a single Royalty Term (which blended royalty rates would be advantageous for both Parties) for sales of Licensed Products in the Territory. Consequently, the Parties have agreed to adopt the royalty rates set forth in Sections
3.5 and
3.6 with respect to the sales of Licensed Products in the Territory as blended royalty rates.
3.8.3.Royalty Report and Payments. Within [***] days of the end of each Calendar Quarter, Valneva will deliver to LimmaTech a report setting forth, for that Calendar Quarter, all information reasonably required to determine the royalty and participation payments to be made under Sections 3.5 and 3.6. The information shall include, inter alia, with respect to (and clearly distinguishing) the Traveler’s Territory and the LMIC Territory (inside and outside of the Global Health Market), in both cases, on a Licensed Product-by-Licensed Product and country-by-country basis: (a) the gross sales and Net Sales of each Licensed Product in each country’s currency, including an accounting of deductions taken into account in the calculation of the Net Sales; (b) the payments received by Valneva from Global Health Partner(s) under Section 3.6.1(a); (c) in case of the supply by Valneva of drug substance or drug product to Global Health Partner(s), the drug substance or drug product revenue, the mark-up on costs of drug substance or drug product and a certification confirming the accuracy of the numbers communicated; (d) the applicable rate to convert from each country’s currency to Euro; (e) the basis for any adjustments to the royalty and/or participation payable for the sale of any such Licensed Product; and (f) the royalty and/or participation due hereunder for the sale of each such Licensed Product. No such reports will be due for any such Licensed Product (i) before the First Commercial Sale of such Licensed Product or (ii) after the Royalty Term for such Licensed Product has expired in all countries in the Territory. The total royalty and/or participation due for the sale of all such Licensed Products during such Calendar Quarter shall be paid within [***] after Valneva’s receipt of an invoice sent by LimmaTech for this purpose. With respect to the LMIC Territory, the Calendar Quarterly report shall include the corresponding report by the Global Health Partner(s) prepared under the Global Health License for the preceding Calendar Quarter.
3.8.4.Taxes and Withholding.
(a)It is understood and agreed between the Parties that any payments made by Valneva to LimmaTech under this Agreement are exclusive of any value added or similar tax (“VAT”) imposed upon such payments. Where VAT is properly added to a payment made under this Agreement, the Party making the payment will pay the amount of VAT only on receipt of a valid tax invoice issued in accordance with the laws and regulations of the country in which the VAT is chargeable without reduction in the amount otherwise payable to LimmaTech. In addition, the Parties shall co-operate in accordance with Applicable Law to minimize VAT in connection with this Agreement, as applicable.
(b)Of all payments to be made under Article
3 Valneva may retain taxes and other duties payable under the tax laws of Austria or any EU country where Valneva or its permitted assignee is located and may forward such retained payments to the competent tax authorities only if the following conditions are met:
(i)the respective tax is an income tax and no use tax, franchise tax, sales tax or other tax;
(ii)LimmaTech is the debtor of such income taxes under Applicable Law;
(iii)Valneva is required by Applicable Law to retain the tax from LimmaTech and to forward such tax to the competent tax authorities; and
In any event, the Parties shall reasonably support each other to address potential tax claims related to this Agreement. In this respect, the Parties undertake to cooperate to reduce or mitigate any withholding tax liability to the extent possible under the provisions granted by Applicable Law. To this effect, LimmaTech must in particular provide Valneva with a valid tax residence certificate (ZS-QU2) approved by the tax authorities of LimmaTech’s state of residence, prior to any payment.
(c)Notwithstanding anything in this Agreement to the contrary, if an action (including but not limited to a re-domiciliation or similar action, any assignment or sublicense of its rights or obligations under this Agreement, or any failure to comply with Applicable Law or filing or record retention requirements) by a Party leads to the imposition of withholding tax liability or VAT on the other Party that would not have been imposed in the absence of such action or in an increase in such liability above the liability that would have been imposed in the absence of such action, then (i) the sum payable by that Party (in respect of which such deduction or withholding is required to be made) shall be increased to the extent necessary to ensure that the other Party receives a sum equal to the sum which it would have received had no such action occurred, (ii) otherwise, the sum payable by that Party (in respect of which such deduction or withholding is required to be made) shall be made to the other Party after deduction of the amount required to be so deducted or withheld, which deducted or withheld amount shall be remitted in accordance with Applicable Law.
(d)Tax Cooperation. Upon request, each Party shall use Commercially Reasonable Efforts to cooperate with the other Party to mitigate, reduce or eliminate adverse tax consequences to such other Party from changes in Applicable Law, the use of present or future Affiliates of either Party to engage in transactions described in or contemplated by this Agreement, or from other activities or transactions described in or contemplated by this Agreement.
3.8.5.Currency. All amounts payable and calculations under this Agreement will be in Euros. As applicable, Net Sales and any royalty deductions will be translated into Euros using the spot rate published in the Wall Street Journal for the last Business Day of the applicable Calendar Quarter. As regards more specifically the amounts of the Development Costs (including FTEs) of which LimmaTech shall keep Valneva informed under Section
3.2.1, these shall be translated into Euros in application of the exchange rate mechanism applied by the Swiss Federal Office for Customs and Border Security FOCBS (https://www.rates.bazg.admin.ch/home).
3.8.6.Method of Payment. Except as permitted pursuant to Section
3.8.5, each payment hereunder will be made by electronic transfer in immediately available funds via either a bank wire transfer, an ACH (automated clearing house) mechanism, or any other means of electronic funds transfer, at Valneva’s election, to such bank account as LimmaTech will designate in writing to Valneva at least [***] before the payment is due. All invoice or billing related questions should be referred to Valneva’s Accounting Department at [***] or by email to: [***].
3.8.7.Record Keeping. Valneva will keep, and will cause its Affiliates and Sublicensees to keep, books and accounts of record in connection with the sale of Licensed Products in sufficient detail to permit accurate determination of all figures necessary for verification of royalties and Commercial Milestone Payments to be paid hereunder. Valneva and its Affiliates will maintain such records for a period of at least [***] after the end of the Calendar Quarter in which they were generated.
3.8.8.LimmaTech Audits. Upon [***] prior notice from LimmaTech, Valneva will permit an independent certified public accounting firm of nationally recognized standing selected by LimmaTech and reasonably acceptable to Valneva, to examine, at LimmaTech’s sole expense, the relevant books and records of Valneva and its Affiliates as may be reasonably necessary to verify the amounts reported by Valneva in accordance with Section
3.8.3 and the payment of royalties and Sales Milestone Payments hereunder. An examination by LimmaTech under this Section
3.8.8 will occur not more than once in any Calendar Year and will be limited to the pertinent books and records for any Calendar Year ending not more than three (3) years before the date of the request. The accounting firm will be provided access to such books and records at Valneva’s or its Affiliates’ facility(ies) where such books and records are normally kept and such examination will be conducted during Valneva’s normal business hours. Valneva may require the accounting firm to sign a reasonably acceptable nondisclosure agreement before providing the accounting firm with access to Valneva’s or its Affiliates’ facilities or records. LimmaTech shall submit to Valneva, along with any notice of an audit under this Section
3.8.8, a written list identifying all Patent Rights that LimmaTech believes in good faith are relevant to the audit request. Upon completion of the audit, the accounting firm will provide both Valneva and LimmaTech a written report disclosing any discrepancies in the reports submitted by Valneva or the royalties or Sales Milestone Payments paid by Valneva, and, in each case, the specific details concerning any discrepancies. No other information will be provided to LimmaTech.
3.8.9.Underpayments/Overpayments following LimmaTech Audits. If such accounting firm concludes that additional royalties or Sales Milestone Payments were due to LimmaTech, then Valneva will pay to LimmaTech the additional royalties or Sales Milestone Payments within [***] of the date Valneva receives such accountant’s written report. Further, if the amount of such underpayments exceeds more than [***] of the amount that was properly payable to LimmaTech, then Valneva will reimburse LimmaTech for LimmaTech’s out-of-pocket costs in connection with the audit. If such accounting firm concludes that Valneva overpaid royalties or Sales Milestone Payments to LimmaTech, then LimmaTech will refund such overpayments to Valneva, within [***] of the date LimmaTech receives such accountant’s report.
3.8.10.Confidentiality. Notwithstanding any provision of this Agreement to the contrary, all reports and financial information of Valneva, its Affiliates or its Sublicensees which are provided to or subject to review by LimmaTech under this Article
3 will be deemed to be Valneva’s Confidential Information and subject to the provisions of Article
7.
4.DEVELOPMENT PLAN
4.1.Scope of the Development Plan. Valneva and LimmaTech will collaborate in the Development of a Licensed Product as set forth in the Development Plan attached hereto as Exhibit 1.26, and in accordance with the terms and conditions set forth in this Article 4. The Development Plan provides for the activities to be performed by each Party, timelines for such activities, and milestones. With respect to the Development Costs, the Development Budget provides for the budgets for each such activity until the End of Phase 2, including estimated FTEs, costs of Materials required for such activities, and costs to be spent. The Development Plan may be amended from time to time in accordance with Section 4.3.
4.2.Allocation of Responsibilities.
4.2.1.General. Each Party will use Commercially Reasonable Efforts to perform its obligations under the Development Plan in a professional and timely manner. Further, each Party will perform its obligations under the Development Plan in compliance with all Applicable Law relating to its activities under the Development Plan.
4.2.2.Development Obligations; Subcontractors. For the execution of the LimmaTech Phase 2 Clinical Trials, the Parties will devote sufficient internal personnel to conduct the activities assigned to the respective Party under the Development Plan and necessary to conduct those studies. Until the End of Phase 2, neither Party will subcontract any of its responsibilities without the other Party’s prior written consent, such consent to be subject to terms and conditions agreed with such Subcontractor and such consent not to be unreasonably withheld, delayed or conditioned. The approval requirement does not apply with respect to Subcontractors of LimmaTech listed in Exhibit 4.2.2 hereto, [***]. The engagement of any Subcontractor in compliance with this Section
4.2.2 shall not relieve the Party engaging such Subcontractor of its obligations under this Agreement. Any agreement between a Party or its Affiliate and a Subcontractor pertaining to activities under the Development Plan shall be consistent with the provisions of this Agreement including (i) terms and conditions relating to Intellectual Property Rights under Article
6 and (ii) terms and conditions under which such Third Party is obligated to preserve the confidentiality of any Confidential Information received by such Third Party hereunder that are at least as restrictive as those described in Article
7. Furthermore, unless otherwise agreed in writing, prior to or at the time of engagement of any Subcontractor to perform any obligations hereunder, the Party engaging a Subcontractor or its Affiliate shall cause such Subcontractor to agree in writing to be bound by terms providing for the other Party rights no less favorable to such other Party than the rights granted in this Agreement. For clarity, after the End of Phase 2, Valneva is free to engage Subcontractors for the performance of its obligations under the Agreement.
4.2.3.Personnel Matters. Each Party acknowledges and agrees that it is solely responsible for the compensation of the personnel assigned to implement its respective obligations under the Development Plan, for the execution of the Phase 2 Clinical Trials and the technology transfer and shall be responsible for withholding all national, state, local or other applicable taxes and similar items for such personnel. Each Party also shall be responsible for all other employer- related obligations, including providing appropriate insurance coverage and employee benefits, and making all other deductions required by law affecting the gross wages of each Party’s employee. One Party’s personnel assigned to the other Party’s activities are not nor shall they be deemed to be employees of such other Party.
4.2.4.Valneva Oversight of Development Activities. Subject to the JSC process as defined in Section
4.3, Valneva will oversee and retain final decision-making authority with respect to all Development activities performed under this Agreement, in accordance with the terms of this Agreement.
4.2.5.Manufacturing Technology Transfer. As of the Effective Date, LimmaTech shall cooperate with Valneva, and use Commercially Reasonable Efforts to facilitate the transfer to Valneva and/or, at Valneva’s election, to an Affiliate or CMO or CDMO, as agreed in the JSC, of all Licensed Know-How as reasonably necessary for Valneva to implement the then-current process for the Manufacture of the Licensed Product (the “Manufacturing Process”) at Valneva’s facilities and/or those of an Affiliate or CMO or CDMO, as agreed in the JSC (such transfer and implementation, as more fully described in the Development Plan). For the Phase 3 Clinical Trial for the Travelers’ Territory, Valneva and LimmaTech will confirm a suitable CMO or CDMO, with Valneva having the ultimate decision to choose a CMO or CDMO or to Manufacture the Licensed Product itself or have it Manufactured by a Valneva Affiliate.
The costs for the transition of the Manufacturing Process are included in the Development Costs set forth in the Development Budget, that can be amended from time to time pursuant to Section
4.3.
Once this transition has been successfully completed, Valneva will be responsible for Manufacturing the Licensed Product. Until such successful transition, LimmaTech shall be responsible for the procurement and supply of the Clinical Trial Materials required for the execution of LimmaTech Phase 2 Clinical Trials, which direct costs are part of the Development Costs and Valneva will reimburse LimmaTech pursuant to the Development
Budget. For clarity, LimmaTech’s obligations hereunder are limited to transfer Clinical Trial Materials and the Manufacturing Process “as is” and to the extent available at LimmaTech, its Affiliates or Subcontractors at the Effective Date. LimmaTech is under no obligation to improve the Manufacturing Process (except as set forth in the Development Plan) or to Manufacture additional Clinical Trial Materials.
4.2.6.LimmaTech Disclosure and Knowledge Transfer Obligations. Without limiting LimmaTech’s obligations pursuant to Section
2.9, Section
2.10, Section
2.11, Section
2.12, Section
2.13, Section
2.14 or Section
4.2.5, during the Development Term, LimmaTech will:
(a)furnish to Valneva through the Alliance Managers in a timely manner true, accurate and complete copies of all new Development Data developed in connection with the Development Plan, in each case in such format as Valneva may reasonably request (including by download of digital files to a secure website or e-room designated and controlled by Valneva);
(b)provide to Valneva through the Alliance Managers written summaries of all activities, discoveries, Developments and results attained by LimmaTech under the Development Plan no less frequently than every three (3) months, i.e., prior to any meeting of the JSC;
(c)promptly notify Valneva of any suspected or actual misconduct, issues pertaining to data integrity or any other information that could reasonably signify or result in a lack of confidence in the accuracy or collection methods of data, each as such may relate to the activities being conducted under the Development Plan;
(d)transfer the IND for the Licensed Product to Valneva following completion of the study Subject recruitment into LimmaTech Phase 2 Clinical Trials or as otherwise set forth in the Development Plan or agreed by the JSC pursuant to Section
4.3; and
(e)provide Valneva with all reasonable assistance necessary or desirable (i) to effect the timely and orderly transfer of Licensed Technology to Valneva for Valneva’s use under the Development Plan, (ii) to effect the timely and orderly transfer of Licensed Technology to Valneva in order to enable Valneva to perform its obligations under Section
5.1; and (iii) for Valneva to exercise its rights under the licenses and sublicenses set forth in Article
2 that are effective at any given time during the Term.
4.3.Governance.
4.3.1.Joint Steering Committee.
(a)Formation; Composition. As soon as practical, but no later than [***] after the Effective Date, the Parties shall establish a Joint Steering Committee (JSC), comprised of individuals with appropriate decision-making authority, to provide oversight and decision-making regarding the activities of the Parties under this Agreement.
The JSC shall consist of three (3) representatives from the executive leadership of each of the Parties, each with the requisite seniority to enable such person to make decisions on behalf of the Parties with respect to the issues falling within its jurisdiction. One of the three representatives of each Party shall be the Program Director. From time to time, each Party may substitute one (1) or more of its representatives to the JSC on written notice to the other Party. Valneva shall select from its representatives the chairperson for the JSC. From time to time, Valneva may change the representative who will serve as chairperson on written notice to LimmaTech. The JSC shall meet at least [***] a year, or as otherwise agreed to by the Parties, and such meetings may be conducted by telephone, video-conference or in person as determined by the JSC members, provided that with respect to in person meetings, unless otherwise agreed the location of such meetings shall alternate between locations designated by Valneva and locations designated by LimmaTech, and at least one meeting per year shall be in-person, unless otherwise agreed to by the Parties.
(b)Responsibilities. The purposes of the JSC shall be to:
(i)oversee the Development, and other Exploitation of the Licensed Product in the Territory;
(ii)coordinate the Parties’ activities under this Agreement;
(iii)receive prior to each JSC meeting written reports or presentations from both Parties of their respective progress with the Phase 2 Clinical Trials, and from Valneva regarding any later Clinical Trials, key milestones and the Regulatory Approval process;
(iv)exchange Development Data;
(v)agree on updates and amendments to the Development Plan;
(vi)review and serve as a forum for discussing the Phase 2 Clinical Trials and any later Clinical Trials, and discuss, prepare and approve amendments thereto, which approval will be reflected in the applicable minutes of the JSC meeting;
(vii)monitor and assess the progress of activities under any and all Clinical Trials, including Clinical Trial progress, enrollment and results;
(viii)monitor and assess the progress of activities related to the transfer of the Manufacturing Process, if applicable;
(ix)serve as a forum where the Parties inform each other on the identification of and negotiations with potential Global Health Partners;
(x)facilitate the flow of information between the Parties with respect to Development and regulatory activities of the Licensed Products in the Territory;
(xi)review the information provided by LimmaTech pursuant to Section 3.2 to ensure that the planned Development Costs are consistent with the costs set forth in the LimmaTech Phase 2 Clinical Trials and, if LimmaTech is contemplating costs that deviate from such Plan, make recommendations with respect thereto;
(xii)coordinate Intellectual Property Rights aspects;
(xiii)form such other committees and sub-committees as the JSC may deem appropriate, provided that such committees and sub-committees may make recommendations to the JSC but may not be delegated JSC decision-making authority;
(xiv)address such other matters relating to the activities of the Parties under the Agreement as either Party may bring before the JSC, including any matters that are expressly for the JSC to decide as provided in this Agreement;
(xv)establish secure access methods (such as secure databases) for each Party to access regulatory documentation and other JSC related information as contemplated under this Agreement;
(xvi)attempt to resolve any disputes between the Parties with respect to the performance of activities under the Agreement pursuant to Section
4.3.2; and
(xvii)perform such other functions as are set forth herein or as the Parties may mutually agree in writing, except where in conflict with any provision of this Agreement.
(c)JSC Term. The JSC will be dissolved upon 1st Regulatory Approval in the US or the EU unless the Parties otherwise agree in writing.
4.3.2.General Provisions.
(a)Meetings and Minutes. Meetings of the JSC may be called by either Party on no less than [***] notice. Each Party shall make all proposals for agenda items and shall provide all appropriate information with respect to such proposed items at least [***] in advance of the applicable meeting; provided that under exigent circumstances requiring input by the JSC, a Party may provide its agenda items to the other Party within a shorter period of time in advance of the meeting, or may propose that there not be a specific agenda for a particular meeting, so long as the other Party consents to such later addition of such agenda items or the absence of a specific agenda for such meeting, such consent not to be unreasonably withheld or delayed. The chairperson of the JSC shall prepare and circulate for review and approval of the Parties minutes of each meeting within thirty (30) calendar days after the meeting. The Parties shall agree on the minutes of each meeting promptly, but in no event later than the next meeting of the JSC, and such approved minutes shall be signed by each Alliance Manager (as defined in Section
4.3.3(b)).
(b)Procedural Rules. The JSC shall have the right to adopt such standing rules as shall be necessary for its work, to the extent that such rules are not inconsistent with this Agreement; provided that such rules shall not be subject to a deciding vote of either Party under Section 4.3.2(c) below. A quorum of the JSC shall exist whenever there is present at a meeting at least one (1) representative appointed by each Party. Representatives of the Parties on the JSC may attend a meeting either in person or by telephone, video conference or similar means in which each participant can hear what is said by, and be heard by, the other participants. Representation by proxy shall be allowed. The JSC shall take action by consensus of the representatives present at a meeting at which a quorum exists, with each Party having a single vote irrespective of the number of representatives of such Party in attendance, or by a written resolution signed by at least one (1) representative appointed by each Party. Employees or consultants of either Party that are not representatives of the Parties on the JSC may attend meetings of the JSC; provided that such attendees (i) shall not vote or otherwise participate in the decision-making process of the JSC, and (ii) are bound by obligations of confidentiality and non-disclosure equivalent to those set forth in Article 7.
(c)Dispute Resolution.
(i)If the JSC, with the assistance of the Alliance Managers, cannot, or does not, reach consensus on an issue at a meeting or within a period of [***] thereafter, then the matter may be referred by either Party to its respective CEO (or its C-level delegate), who shall meet in person or via video conference within [***] to attempt to resolve such matter in good faith. If the CEOs (or their C-level delegates) fail to reach agreement as to such matter for a period in excess of ten (10) Business Days from their initial meeting, Valneva shall have the right to resolve the matter and shall not be subject to resolution pursuant to Sectio
n 11.13; provided, however, that Valneva shall not have the right (a) to resolve disputes with respect to assigning any additional material obligations to LimmaTech; (b) to unilaterally reduce its diligence obligations under this Agreement; (c) to unilaterally adopt a decision that would cause significant delay of the Development timelines set forth in the Development Plan; or (d) modify the Binding Development Milestones listed in the Development Plan as of the Effective Date.
(ii)Disputes arising between the Parties in connection with or relating to this Agreement or any document or instrument delivered in connection herewith, and that are outside of the jurisdiction of the JSC and not within a Party’s sole decision-making authority, shall be resolved pursuant to Section
11.13.
(d)Limitations on Authority. Notwithstanding any provision of this Section
4.3 to the contrary,
(a) each Party will retain the rights, powers and discretion granted to it under this Agreement and no such rights, powers, or discretion will be delegated to or vested in the JSC unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing, (b) the JSC will not have the power to amend this Agreement or otherwise modify or waive compliance with this Agreement in any manner and (c) neither Party will require the other Party to (i) breach any obligation or agreement that such other Party may have with or to a Third Party or (ii) perform any activities that are materially different or greater in scope or more costly than those provided for in the Development Plan then in effect.
4.3.3.Collaboration Management.
(a)Program Directors. Each Party will appoint a program director to oversee all activities conducted under the Development Plan (each, a “Program Director” and together the “Program Directors”). Each Party may change its designated Program Director at any time upon written notice to the other Party. The Program Directors will coordinate the efforts of their respective Party in conducting activities under the Development Plan.
(b)Alliance Managers. Each Party will appoint a single individual to act as the primary point of contact between the Parties to support the activities under the Development Plan and, more generally, the Agreement (the “Alliance Managers”). Each Party may change its designated Alliance Manager at any time upon written notice to the other Party. The Alliance Managers will:
(i)use good faith efforts to attend (either in person or by telecommunications) all meetings of the JSC, but will be non-voting members at such meetings;
(ii)be responsible for setting dates and agendas for JSC meetings, and for capturing and distributing the associated minutes; and
(iii)be the first point of referral for all matters of conflict resolution and bring disputes to the attention of the JSC in a timely manner.
5.LICENSED PRODUCT DEVELOPMENT AND COMMERCIALIZATION.
5.1.General. Subject to the provisions of Section
2.3.2, Article
4 and Section
5.3, Valneva will have sole authority over and control of the Development, Manufacture, Regulatory Approval and Commercialization of Licensed Products.
5.2.LimmaTech Phase 2 Clinical Trials.
5.2.1.LimmaTech shall continue to act as the sponsor of the LimmaTech Phase 2 Clinical Trials and shall hold the IND relating thereto, subject to Section
4.2.6(d). Pursuant to Section
3.2, LimmaTech will contribute to the Development Costs associated with the LimmaTech Phase 2 Clinical Trials up to the Development Costs Cap.
5.2.2.LimmaTech shall ensure that the LimmaTech Phase 2 Clinical Trials are performed in accordance with this Agreement, the applicable protocols and all Applicable Law, including GCP. In the event that any Regulatory Authority, ethics committee or institutional review board has questions related to a protocol or the conduct of the LimmaTech Phase 2 Clinical Trials LimmaTech will immediately notify Valneva of such questions and will work with Valneva to respond to such questions.
5.2.3.LimmaTech shall ensure that all directions from any Regulatory Authority and/or ethics committee with jurisdiction over the LimmaTech Phase 2 Clinical Trials are followed. Valneva shall have the right (but no obligation) to participate in any discussions with a Regulatory Authority regarding the LimmaTech Phase 2 Clinical Trials.
5.2.4.LimmaTech shall ensure that all reports and related documentation required for the LimmaTech Phase 2 Clinical Trials are maintained in good scientific manner and in compliance with Applicable Law.
5.3.Diligence.
5.3.1.Development Diligence. Valneva’s Development diligence obligations include that it will use its Commercially Reasonable Efforts (i) to Develop a Licensed Product in the Field of Use in accordance with the Development Plan and not halt or delay such Development without reasonable objective cause, such as, but not limited to, if required by Regulatory Authorities or in the event of a Force Majeure; (ii) to seek Regulatory Approval for a Licensed Product in the Field of Use in the Major Market Countries; (iii) to collaborate with LimmaTech to select a Global Health Partner and negotiate and enter into a Global Health License in accordance with Section
2.3.2; (iv) to ensure that the Global Health Partner with whom a Global Health License has been entered into complies with its own development diligence obligations under such Global Health License by exercising against it the rights provided for in such Global Health License when necessary; and (v) to support the request for a Priority Review Voucher for Licensed Products at the FDA.
LimmaTech’s Development diligence obligations include that it will use its Commercially Reasonable Efforts (i) to complete the LimmaTech Phase 2 Clinical Trials in accordance with its obligations and activities under this Agreement and the Development Plan; (ii) to collaborate with Valneva to select a Global Health Partner and negotiate a Global Health License in accordance with Section
2.3.2; and (iii) to support the request for a Priority Review Voucher for Licensed Products at the FDA.
5.3.2.Commercial Diligence. Valneva’s Commercial diligence obligations include that it will use its Commercially Reasonable Efforts (i) to Commercialize a given Licensed Product in the Field of Use in each Major Market Country in the Territory where Valneva has received Regulatory Approval for such Licensed Product in such indication; and (ii) to ensure that the Global Health Partner with whom a Global Health License has been entered into complies with its own diligence obligations under such Global Health License by exercising against it the rights provided for in such Global Health License when necessary.
5.3.3.Exceptions to Diligence Obligations. Notwithstanding any provision of this Agreement to the contrary, Valneva or LimmaTech will be relieved of their diligence obligations to the extent that:
(i)Valneva or LimmaTech receives, generates, or otherwise becomes aware of, any safety, tolerability or other data indicating or signaling that a Licensed Product has or would have an unacceptable risk-benefit profile or is otherwise not suitable for initiation or continuation of Clinical Trials;
(ii)Valneva or LimmaTech receives any notice, information or correspondence from any applicable Regulatory Authority, or any applicable Regulatory Authority takes any action, that indicates that a Licensed Product is unlikely to receive Regulatory Approval;
(iii)LimmaTech fails to fulfill its Development or other obligations under this Agreement and such failure prevents Valneva from fulfilling the Valneva Diligence Obligations; or
(iv)the transfer of the Manufacturing Process by LimmaTech to Valneva’s facilities or those of an Affiliate or CMO or CDMO designated by Valneva is not completed within the timelines set forth in the Development Plan, and such delay leads to a shift of timelines in the Development Plan;
provided, however, that in the event of (a) Section
5.3.3(i) and
(ii), such exceptions only apply if Valneva terminates this Agreement in accordance with Section
9.3 within a reasonable time period – not to exceed [***] - after having learned of the event justifying such exceptions, and (b) Section
5.3.3(iii) and
(iv), such exceptions only apply to the corresponding time adjustment needed to cover the delay incurred by the respective event.
5.3.4.Deemed Satisfaction of Valneva Diligence Obligations. Without in any way expanding Valneva’s obligations under this Agreement, Valneva’s achievement of any Development Event in accordance with the timelines of the Development Plan and entitling LimmaTech to receive a specific Development Milestone Payment described in Section
3.3 will be conclusive evidence that Valneva has satisfied all Valneva Diligence Obligations in the Travelers’ Territory and under this Agreement up to the date that such Development Event is achieved.
For the avoidance of doubt, the first paragraph of this Section
5.3.4 is intended only as an example of diligence constituting satisfaction of the Valneva Diligence Obligations. Valneva may fully satisfy the Valneva Diligence Obligations without achieving any the specific diligence example set forth in this Section
5.3.4, provided that Valneva otherwise complies with the provisions of Section
5.3.1 or Section
5.3.2, as applicable.
5.3.5.Performance by the Parties’ Affiliates or Valneva’s Sublicensees. For avoidance of doubt, any actions taken by the Parties’ Affiliates or Valneva’s Sublicensees (or their respective Subcontractors) under this Agreement shall be treated as actions taken by, as the case may be, either Party in regard to satisfaction of the requirements of this Section
5.3.
5.4.Regulatory Matters.
5.4.1.Regulatory Reporting. Except as necessary for LimmaTech to complete the LimmaTech Phase 2 Clinical Trials, Valneva or its designated Affiliate(s) will have the sole authority and responsibility to make or file all filings, reports and communications with all Regulatory Authorities with respect to any Licensed Product in the Field of Use in the Territory, including all reports required to be filed in order to obtain or maintain any Regulatory Approvals granted for Licensed Products in the Field of Use in the Territory and adverse drug experience reports. Upon Valneva’s request, LimmaTech will provide Valneva with any data or other information in LimmaTech’s possession and otherwise provide reasonable assistance to Valneva in connection with any such filings, reports and communications.
5.4.2.Regulatory Approvals. Unless otherwise expressly agreed with the Global Health Partner(s), Valneva or its designated Affiliate(s) will have the sole authority to prepare and file applications, in its own name, for Regulatory Approval for Licensed Products in the Field of Use in the Territory, including communicating with any Regulatory Authority both prior to and following Regulatory Approval. To the extent reasonably possible under the applicable regulatory timelines, Valneva (i) will make available to LimmaTech copies of drafts of all regulatory filings and material correspondences with Regulatory Authorities to enable LimmaTech, if it so desires and at its own costs and expenses, to review and comment on them, and (ii) will take such comments into reasonable consideration in its dealings with the Regulatory Authorities. Further, LimmaTech shall have the right to participate in meetings with Regulatory Authorities as a silent observer at its own expense. As set forth in Section
4.2.6(d), LimmaTech will assign to Valneva any and all INDs, Regulatory Approvals or any other rights or permissions granted by any Regulatory Authority, together with all other regulatory filings and Development Data, to the extent such assignment is permissible under Applicable Law. Further, LimmaTech will take all actions and provide all assistance reasonably requested by Valneva to effect the assignments in this Section
5.4.2 immediately following the End of Phase 2, or at such time as directed by Valneva. Such assistance will be refunded by Valneva at the Fully Loaded FTE Rate.
5.4.3.Cooperation. If reasonably requested by Valneva, LimmaTech shall assist and cooperate with Valneva in connection with the preparation of filings, reports and communications to Regulatory Authorities with respect to any Licensed Product in the Field of Use in the Territory, at Valneva’s sole expense. LimmaTech will and will cause its Affiliates to cooperate with Valneva and all Valneva Representatives in the event of any inspection by a Regulatory Authority related to any Licensed Product or any activities to be performed under this Agreement. Any extraordinary cooperation following the End of Phase 2 which requires a Representative of LimmaTech to spend more than [***] in one calendar month or more than [***] in a [***] period will be refunded by Valneva at the Fully Loaded FTE Rate based on the number of hours effectively fulfilled, as evidenced by written records.
5.4.4.Right of Reference. Valneva hereby grants to LimmaTech a Right of Reference to all Development Data (including any regulatory filings or Regulatory Approvals) Controlled by Valneva, its Affiliates or Sublicensees in the Travelers’ Territory that relate to any Licensed Product alone (i.e. excluding any Combination), in all cases solely for the development, manufacture, commercialization and exploitation of products outside the Field of Use. Pursuant to the terms and conditions of this Agreement, Valneva will provide a signed statement to this effect, if requested by LimmaTech. LimmaTech may grant such Right of Reference to its Affiliates and licensees.
5.4.5.Pharmacovigilance. The Parties will have in place and will maintain during the Term systems, procedures, training programs and documentation needed to perform and comply with their pharmacovigilance regulatory obligations, and each Party shall promptly inform the other Party of any safety issues that may arise and that need to be reported under Applicable Law. The Parties will enter into a separate pharmacovigilance agreement, if needed, and such pharmacovigilance agreement shall be coordinated with the Global Health Partner(s) and potential other Sublicensees.
5.5.Commercialization Activities.
5.5.1.General. Subject to Section
5.1, including the rights of the Global Health Partner(s) under the Global Health License(s), Valneva will have sole and exclusive control over all matters relating to the Commercialization of the Licensed Products in the Field of Use in the Territory, including sole and exclusive control over (a) pricing of Licensed Products and (b) the negotiation of Licensed Product pricing with Regulatory Authorities and other Third Parties, in each case in the Field of Use in the Territory.
5.5.2.Branding. Valneva or its designated Affiliates or Sublicensees will select and own all Trademarks and Copyrights used in connection with the Commercialization of any and all Licensed Products in the Field of Use in the Territory (other than LimmaTech’s corporate names and logos). Neither LimmaTech nor its Affiliates will use or seek to register, anywhere in the world, any Trademark which is confusingly similar to any Trademark used by or on behalf of Valneva, its Affiliates or Sublicensees in connection with any Licensed Product.
5.6.Manufacturing. Subject to Section
5.1 and to the provisions of the Development Plan, Valneva will have the exclusive right and responsibility to Manufacture Licensed Products for the Territory itself or through one or more Affiliates or Third Parties selected by Valneva in its sole discretion.
5.7.Progress Reporting Post JSC Dissolution. After the dissolution of the JSC under Sectio
n 4.3.1(c), Valneva will provide LimmaTech with (i) one (1) written report per [***] summarizing Valneva’s activities to Commercialize the Licensed Products; and (ii) annual marketing plans including rolling forward estimates on the sales of Licensed Products both in the Travelers’ Territory and in the LMIC Territory in the next [***], such marketing plans to be provided within the first [***] of the Calendar Year to which they relate. Any information or written report provided by Valneva to LimmaTech pursuant to this Section
5.7 will be deemed to be Valneva’s Confidential Information and subject to the provisions of Article
7.
6.INTELLECTUAL PROPERTY.
6.1.Platform Patent Rights. The Parties acknowledge that the rights that LimmaTech grants to Valneva in the Platform Patent Rights under the Agreement are limited in light of the rights that LimmaTech has in said Platform Patent Rights pursuant to the corresponding LimmaTech Third Party Agreements. In this case, Valneva’s rights in the Platform Patent Rights are limited as follows:
6.1.1.Management and Enforcement of Platform Patent Rights. LimmaTech or the Third Party Licensor, at LimmaTech’s discretion, shall have the sole right, at its expense, using legal counsel of its choosing including professional staff employed by the Third Party Licensor, to:
(a)prepare, file, obtain, prosecute (including any oppositions, interferences, reissue proceedings and re-examinations), maintain all Platform Patent Rights throughout the Territory, whereby Valneva shall have the right to assume management of all the Platform Patent Rights in accordance with Section 6.2.2(a)(ii), in countries where the Third Party Licensor has confirmed in writing to LimmaTech that (i) it does not have any interest in filing or pursuing further any application and (ii) consents to LimmaTech or a Third Party licensee taking over, and LimmaTech itself has notified Valneva of its choice not to pursue the management of the relevant Platform Patent Rights; and
(b)take any actions it deems appropriate in its sole discretion to stop infringement of a Platform Patent Right by any Third Party, or to grant the infringing party adequate rights and licenses under the Platform Patent Rights necessary for continuing its activities.
6.1.2.Suspected Infringement.
(a)If Valneva or LimmaTech has reason to believe that a Third Party may be infringing any of the Platform Patent Rights, it shall duly notify the other Party in writing thereof, identifying the suspected infringer and the suspected infringement and furnishing the information upon which such suspicion is based.
(b)In the event of alleged infringement of the national or regional equivalents of patent WO 21/255684 (see description further outlined in Exhibit 1.79), if the Third Party Licensor elects not to bring a suit against an alleged infringer or not to defend against an infringement claim (of which, when applicable, LimmaTech will inform Valneva within a reasonable time), Valneva shall have the primary right to commence such action or defense, at its own cost and expense, of which it shall notify LimmaTech, who shall in turn notify the Third Party Licensor, both of whom shall then have the right to participate in and be represented in any such proceedings by their own counsel at their own expense. If Valneva elects not to bring a suit against an alleged infringer or not to defend against an infringement claim, it shall promptly notify LimmaTech of such decision, and in any event within [***] after receipt of the Third Party infringement notice, and LimmaTech shall then have the right, but not the obligation to take action or bring suit with respect to such Third Party infringement at its own expense. The Party not bringing the suit shall provide reasonable assistance to the Party suing, as requested and useful for the purpose of this Section
6.1.2(b). Section
6.2.3(c) shall apply mutatis mutandis.
(c)Where the Third Party Licensor pursues an infringement action with the support of LimmaTech and Valneva, any damages, profits or other compensation recovered by such proceedings will be split between the Parties and the Third Party Licensor, with [***] going to the Third Party Licensor, [***] going to Valneva, and [***] going to LimmaTech. If the Third Party Licensor elects not to pursue an infringement action, Section
6.2.3(b) shall apply mutatis mutandis.
6.2.LimmaTech Technology.
6.2.1.Ownership of LimmaTech Technology. Notwithstanding any provision of this Agreement to the contrary, LimmaTech will own all right, title and interest in and to LimmaTech Technology.
6.2.2.Filing, Prosecution and Maintenance of LimmaTech Patent Rights.
(a)Management of LimmaTech Patent Rights until the End of Phase 2.
(i)Pursuant to Section
1.55, LimmaTech will have the first right to file LimmaTech Patent Rights until the End of Phase 2, LimmaTech will inform Valneva of the applications filed within [***] of their effective filing.
(ii)Until the End of Phase 2, unless otherwise expressly provided for in the Agreement and provided that LimmaTech has filed the LimmaTech Patent Rights in accordance with Section
6.2.2(a)(i), LimmaTech will have the first right to prosecute and maintain the LimmaTech Patent Rights in the Territory using counsel of its own choice reasonably acceptable to Valneva, whose consent shall not be unreasonably withheld, conditioned or delayed. LimmaTech will keep Valneva advised on the status of the preparation, prosecution and maintenance of all patent applications and issued patents included within the LimmaTech Patent Rights. Further, LimmaTech will (i) allow Valneva a reasonable opportunity and reasonable time to review and provide comment regarding relevant substantive communications to LimmaTech and drafts of any substantive responses or other proposed substantive filings before any applicable filings are submitted to any relevant patent office (or Governmental Authority) in a Major Market Country and (ii) reflect any reasonable and timely comments offered by Valneva in any final filings submitted by LimmaTech to any relevant patent office (or Governmental Authority) in a Major Market Country unless LimmaTech believes doing so may delay issuance or otherwise compromise patent coverage for the Licensed Products. Unless otherwise provided for in this Agreement, LimmaTech will be solely responsible for all costs incurred in connection with its prosecution and maintenance of the LimmaTech Patent Rights.
(iii)If LimmaTech elects not to file a patent application included in the LimmaTech Patent Rights in any country or elects to cease the prosecution or maintenance of all patent applications and patents of a particular LimmaTech Patent Rights in any country, LimmaTech will provide Valneva with written notice of its decision not less than [***] before any action is required to avoid abandonment or lapse. If Valneva elects to file or continue such prosecution and maintenance at its costs, (i) Valneva will promptly identify and engage the attorneys and agents who will conduct further activities and LimmaTech will reasonably cooperate to promptly transfer the necessary files and execute the necessary forms regarding such transfer, (ii) except as set forth in (i) above, LimmaTech will have no responsibility with respect to the filing, prosecution or maintenance of, or any expenses incurred in connection with, any such LimmaTech Patent Rights following LimmaTech’s notice. Valneva will not disclose any of LimmaTech’s Confidential Information in connection with such filing, prosecution or maintenance without LimmaTech’s prior written approval. Valneva will keep LimmaTech advised on the status of the preparation, filing, prosecution, and maintenance of all such LimmaTech Patent Rights and will reasonably consider any comments made by LimmaTech in connection therewith.
(b)Management of LimmaTech Patent Rights after the End of Phase 2. After the End of Phase 2, Valneva has the right to take over the prosecution and maintenance of those of LimmaTech Patent Rights that solely Cover the Licensed Products in the Field of Use, at Valneva’s sole costs and under corresponding conditions as the primary right of management enjoyed by LimmaTech until the End of Phase 2. If the case arises, Valneva will notify LimmaTech of its intention to take over the management of LimmaTech Patent Rights so that LimmaTech can take the necessary steps to ensure the planned management takeover.
(c)Patent Term Restoration and Extension. Valneva will have the exclusive right, but not the obligation, to seek, at its sole expense, in LimmaTech’s name if so required, patent term extensions, and supplemental protection certificates and the like available under the Applicable Law, including 35 U.S.C. § 156 and applicable foreign counterparts, in any country in the Territory in relation to the LimmaTech Patent Rights. LimmaTech and Valneva will cooperate in connection with all such activities. Valneva, its agents and attorneys will give due consideration to all suggestions and comments of LimmaTech regarding any such activities, but in the event of a disagreement between the Parties, Valneva will have the final decisionmaking authority; provided, however, that Valneva will seek (or allow LimmaTech to seek) to extend any LimmaTech Patent Rights at LimmaTech’s request, including through the use of supplemental protection certificates and the like, unless in Valneva’s reasonable legal determination such LimmaTech Patent Rights may not be extended under the Applicable Law without limiting Valneva’s right to extend any other Patent Rights.
(d)Clarifications. For clarity, (i) prosecution under this Section
6.2.2 includes opposition, revocation, post-grant review or other patent office proceedings, unless such proceedings are concurrent with Third Party litigation under Section
6.2.3, in which case the provisions of Section
6.2.3 shall govern the Parties’ rights and obligations with respect to such proceedings, and (ii) Third Party declaratory judgment actions or other court actions relating to Patent Rights shall be governed by Section
6.2.3, and by Section
6.2.4 if applicable.
(e)Liability. To the extent that a Party is obtaining, prosecuting or maintaining Patent Rights or otherwise exercising its rights under this Section 6.2.1, such Party, and its Affiliates and/or Representatives, will not be liable to the other Party in respect of any act, omission, default or neglect on the part of any such Party, or its Affiliates and/or Representatives, in connection with such activities undertaken in good faith.
(f)Recording. If Valneva deems it necessary or desirable to register or record this Agreement or evidence of this Agreement with any patent office or other appropriate Governmental Authority(ies) in one or more jurisdictions in the Territory, LimmaTech will reasonably cooperate to execute and deliver to Valneva any documents accurately reflecting or evidencing this Agreement that are necessary or desirable, in Valneva’s reasonable judgment, to complete such registration or recordation.
(g)Joint Research Agreement. The Agreement shall be understood to be a joint research agreement under 35 U.S.C § 103(c)(3) entered into for the purpose of researching, identifying and Developing the Licensed Products.
6.2.3.Enforcement and Defense of LimmaTech Patent Rights.
(a)Enforcement. Each Party will promptly notify the other in the event of any actual, potential or suspected infringement of a patent under the LimmaTech Patent Rights by any Third Party (“Third Party Infringement”).
As between Valneva and LimmaTech, if the infringing activities are inside the Field of Use, Valneva will have the primary right, but not the obligation, to institute litigation or take other steps to remedy such Third Party Infringement in the Territory, and any such litigation or steps will be at Valneva’s expense. If Valneva decides to initiate proceedings, LimmaTech will have the right, but not the obligation, to voluntarily intervene and be represented in any such proceedings by its own counsel at its own expense.
If Valneva elects not to bring a suit against an alleged Third Party Infringer, it shall promptly notify LimmaTech of such decision, and in any event within [***] after receipt of the Third Party Infringement notice, and LimmaTech shall then have the right, but not the obligation to take action or bring suit with respect to such Third Party Infringement at its own expense. If LimmaTech decides to initiate proceedings, Valneva will have the right, but not the obligation, to voluntarily intervene and be represented in any such proceedings by its own counsel at its own expense.
The Party not bringing (and not voluntarily intervening in) the suit shall provide reasonable assistance to the Party suing, as requested and useful for the purpose of this clause.
Where the infringing activities are outside the Field of Use, LimmaTech and the Third Party Licensor shall have the primary right, but not the obligation, to institute litigation or take over steps to remedy infringement in connection with the LimmaTech Patent Rights, corresponding to Valneva’s primary right in the Territory inside the Field of Use.
The competent Party will use Commercially Reasonable Efforts to conduct the defense actions in the best interest of the respective LimmaTech Patent Rights. Subject hereto, neither Party will incur any liability to the other Party (other than that related to a Party’s indemnification obligation pursuant to Article 10) as a consequent of any litigation initiated or pursued pursuant to this Section 6.2.3(a) or any unfavorable decision resulting therefrom, including any decision holding any LimmaTech Patent Rights invalid or unenforceable.
(b)Recoveries. Any recoveries obtained by either Party in the Travelers’ Territory inside the Field of Use as a result of any proceedings with regard to a Third Party Infringement under this Section
6.2.3 shall be allocated as follows:
(i)such recovery shall first be used to reimburse each Party for all reasonable costs incurred in connection with such proceeding;
(ii)such recovery shall then be used to compensate each Party for the respective damages suffered from a Third Party Infringement (in the case of damage suffered by LimmaTech, as calculated at the royalty rate) provided that in the event the remaining portion of the recovery is not sufficient to compensate each Party’s damages, such compensation shall be shared on a pro-rata basis depending on the amount of the respective damage suffered; and
(iii)the remaining portion of such recovery, if any, shall be equally shared between LimmaTech and Valneva.
(c)Settlements. Valneva will not, without the prior written consent of LimmaTech, enter into any compromise or settlement relating to such litigation that (i) admits the invalidity or unenforceability of any LimmaTech Patent Rights; or (ii) requires Valneva to abandon any LimmaTech Patent Rights. LimmaTech, upon request of Valneva, agrees to timely commence or to join in any such litigation, at Valneva’s expense, and in any event to cooperate with Valneva in such litigation or steps at Valneva’s expense. LimmaTech will have the right to consult with Valneva about such litigation and to participate in and be represented by independent counsel in such litigation at LimmaTech’s own expense.
6.2.4.Other Actions by Third Parties. Each Party will promptly notify the other Party in the event of any legal or administrative action by any Third Party involving any LimmaTech Patent Rights of which it becomes aware, including any nullity, revocation, interference, reexamination or compulsory license proceeding. Valneva will have the first right, but no obligation, to defend against any such action involving any LimmaTech Patent Rights, in its own name (to the extent permitted by Applicable Law), and any such defense will be at Valneva’s expense, subject to LimmaTech’s indemnification obligations under Article
10. LimmaTech, upon Valneva’s request, agrees to join in any such action at Valneva’s expense and in any event to cooperate with Valneva at Valneva’s expense. If Valneva elects not to defend against any such action involving LimmaTech Patent Rights, then LimmaTech will have the right to defend such action, in its own name, and any such defense will be at LimmaTech’s expense.
6.2.5.Purple Book Listings. To the extent of any LimmaTech Patent Rights Covering a Licensed Product, the Parties shall cooperate with each other to enable Valneva to make filings with Regulatory Authorities, as required or allowed in connection with (i) in the US, the FDA’s Purple Book and the Biologics Price Competition and Innovation Act and (ii) outside the US, under the national implementations of Article 10.1(a)(iii) of Directive 2001/EC/83 or other international equivalents thereof. Valneva shall consider LimmaTech’s reasonable requests in connection therewith, including meeting any submission deadlines, in each case, to the extent required or permitted by Applicable Law.
6.2.6.Paragraph IV Type Notices. Notwithstanding any provision of this Agreement to the contrary, each Party will immediately (but in no event later than [***] following receipt or discovery, whichever occurs first) give written notice to the other of any certification of which it becomes aware filed pursuant to any statutory or regulatory requirement in any country in the Territory similar to 21 U.S.C. § 355(b)(2)(A)(iv) or § 355(j)(2)(A)(vii)(IV) (or any amendment or successor statute thereto) claiming that any LimmaTech Patent Rights Covering any Licensed Product is invalid or that infringement will not arise from the Development, Manufacture, use or Commercialization in the Territory of such Licensed Product by a Third Party. Upon the giving or receipt of such notice, Valneva will have the primary right, but not the obligation, to bring an infringement action against such Third Party. In connection with any action brought by Valneva under this Section
6.2.6, LimmaTech, upon Valneva’s request, will reasonably cooperate with Valneva in any such action at Valneva’s expense and will timely commence or join in any such action at Valneva’s request and expense. In the event of any conflict between the terms of this Section
6.2.6 and the terms of Section
6.2.3(a), the terms of this Section
6.2.6 will control and govern.
6.2.7.Notice of Infringement. If the Development, Manufacture, Commercialization or use of any Licensed Product, the practice of any LimmaTech Technology, or the exercise of any other right granted by LimmaTech to Valneva hereunder (collectively, the “Licensed Activities”) by Valneva or any of its Affiliates or Sublicensees is alleged by a Third Party to infringe, misappropriate or otherwise violate such Third Party’s Patent Rights or other Intellectual Property Rights or that Valneva or LimmaTech otherwise identifies any Third Party’s Patent Rights or other Intellectual Property Rights that may be relevant to such activities, Valneva or LimmaTech – as the case may be – will, promptly upon becoming aware of such allegation or identification, notify the other Party in writing.
6.2.8.Third Party Infringement Suits. Each of the Parties will promptly notify the other in the event that any Third Party files any suit or brings any other action alleging patent infringement by Valneva or LimmaTech or any of their respective Affiliates or Sublicensees with respect to the Development, Manufacture, Commercialization or use of any Licensed Product or the practice of any LimmaTech Technology (any such suit or other action referred to herein as an “Infringement Claim”). Notwithstanding anything to the contrary in Article 10, in the case of any Infringement Claim against Valneva (including its Affiliates or Sublicensees) alone or against both Valneva and LimmaTech (including its Affiliates), Valneva will have the primary right, but not the obligation, to control the defense of such Infringement Claim, including control over any related litigation, settlement, appeal or other disposition arising in connection therewith. LimmaTech, upon request of Valneva, agrees to cooperate with Valneva at Valneva’s expense. LimmaTech will have the right to consult with Valneva concerning any Infringement Claim and to participate in and be represented by independent counsel in any associated litigation in which LimmaTech is a party at LimmaTech’s own expense. In the case of any Infringement Claim against LimmaTech alone, Valneva will have the right to consult with LimmaTech concerning such Infringement Claim and Valneva, upon request of LimmaTech, will reasonably cooperate with LimmaTech at LimmaTech’s expense. Sections 6.2.3(b) and 6.2.3(c) shall apply mutatis mutandis.
6.2.9.Misappropriation Actions Relating to LimmaTech Know-How. Each Party will promptly notify the other in the event of any actual, potential or suspected misappropriation of any LimmaTech Know-How by any Third Party. As between Valneva and LimmaTech, Valneva will have the sole right, but not the obligation, to institute litigation or take other steps to remedy misappropriation in connection therewith, and any such litigation or steps will be at Valneva’s expense. Valneva will not, without the prior written consent of LimmaTech, enter into any compromise or settlement relating to such litigation that (a) admits that all or any portion of the LimmaTech Know-How is not protectable under Applicable Law, including relevant Trade Secret Applicable Law, or (b) requires Valneva to abandon protection for any LimmaTech Know-How. In order to establish standing, LimmaTech, upon request of Valneva, agrees to timely commence or to join in any such litigation, at Valneva’s expense, and in any event to cooperate with Valneva in such litigation or steps at Valneva’s expense. LimmaTech will have the right to consult with Valneva about such litigation and to participate in and be represented by independent counsel in such litigation at LimmaTech’s own expense. Neither Party will incur any liability to the other Party (other than that related to a Party’s indemnification obligation pursuant to Article
10) as a consequent of any litigation initiated or pursued pursuant to this Section
6.2.9 or any unfavorable decision resulting therefrom.
6.3.Valneva Technology.
6.3.1.Ownership of Valneva Technology. Notwithstanding any provision of this Agreement to the contrary, Valneva will own all rights, titles and interests in and to Valneva Technology. LimmaTech agrees to assign and hereby irrevocably assigns, and will cause its Representatives to assign to Valneva all rights, titles and interests throughout the world in and to any and all Valneva Technology generated after the Effective Date. Further, LimmaTech will, and will cause its Representatives to, execute any and all assignments, applications for domestic and foreign patents and other documents and to do such other acts (including the execution and delivery of instruments of further assurance or confirmation) reasonably requested by Valneva to assign such Valneva Technology to Valneva and to permit Valneva to practice and enforce such Valneva Technology.
6.3.2.License from Valneva to LimmaTech over Valneva Technology. Valneva grants LimmaTech an upfront fully paid-up, royalty-free, exclusive, worldwide, sublicensable in multiple tiers, license under the Valneva Patent Rights (a) made (i) solely by or on behalf of LimmaTech or its Representatives or (ii) jointly by or on behalf of the Parties or their Representatives, in either case after the Effective Date and during the Term; and (b) that relate to the Licensed Product and not to the Combination (i.e., excluding any rights covering Active Ingredients in a Combination which are not licensed under this Agreement), for use outside the Field of Use. Further, Valneva grants LimmaTech an upfront fully paid-up, royalty-free, non-exclusive, worldwide, sublicensable pursuant to Section 4.2.2, license for use of the Valneva Technology inside the Field of Use solely for the performance of the Phase 2 Clinical Trials.
6.3.3.Filing, Prosecution and Maintenance. Valneva will have the sole right, but no obligation, using legal counsel of its choosing and at its expense, to prepare, file, obtain, prosecute (including any oppositions, interferences, reissue proceedings and re-examinations), maintain and discontinue all Patent Rights that it owns or to which it otherwise has control of prosecution rights, including the Valneva Patent Rights, in its sole discretion. At least quarterly through the JSC until the End of Phase 2, and thereafter upon LimmaTech’s reasonable request not more than once per [***], Valneva will provide a status report listing the status of all patent applications and issued patents included within the Valneva Patent Rights that Valneva is prosecuting and maintaining. Valneva will be solely responsible for all costs incurred in connection with prosecution and maintenance of the Valneva Patent Rights following the end of the Development Term.
6.3.4.Enforcement and defense. Valneva will have the sole right, but no obligation, to take action to obtain a discontinuance of infringement or bring suit against a Third Party infringing or challenging the validity or enforceability of any Valneva Patent Rights inside the Field of Use. Where the infringing activities relate to the Licensed Product and not to the Combination (i.e., excluding any rights covering Active Ingredients in a Combination which are not licensed under this Agreement) and are outside the Field of Use, LimmaTech and the Third Party Licensor shall have the primary right, but not the obligation, to institute litigation or take over steps to remedy infringement in connection with the Valneva Patent Rights relating to the License Product and not to the Combination (i.e., excluding any rights covering Active Ingredients in a Combination which are not licensed under this Agreement).
6.3.5.Misappropriation Actions. Valneva will have the sole right, but no obligation, to take action to obtain a discontinuance of misappropriation or bring suit against a Third Party that is misappropriating, or that is suspected of misappropriating, any Valneva Know-How.
7.CONFIDENTIALITY, PUBLICATION AND DATA PROTECTION.
7.1.Obligation of Confidentiality. Except to the extent expressly authorized by this Agreement, the Parties agree that, from the Effective Date and for as long as the Confidential Information remains confidential and has not been made public other than by breach of this obligation of confidentiality, the Receiving Party will: (a) keep the Disclosing Party’s Confidential Information confidential and implement all appropriate and reasonable safeguards to this effect; (b) not disclose, or permit the disclosure of, the Disclosing Party’s Confidential Information; and (c) not use, or permit to be used, the Disclosing Party’s Confidential Information for any purpose other than to permit the Receiving Party to exercise its rights, and perform its obligations under the terms of this Agreement.
7.2.Limitations. Notwithstanding the foregoing provisions of Section
7.1, the Receiving Party's above obligations of confidentiality shall not apply to the extent that the Receiving Party can demonstrate that any of the Disclosing Party's Confidential Information:
(a)was already known by the Receiving Party (other than under an obligation of confidentiality to the Disclosing Party) at the time of disclosure by or on behalf of the Disclosing Party;
(b)was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party;
(c)became generally available to the public or otherwise part of the public domain after its disclosure to the Receiving Party, other than through any act or omission of the Receiving Party in breach of its obligations under this Agreement;
(d)was disclosed to the Receiving Party by a Third Party who did not obtain such information from the Disclosing Party and is not acting in breach of an obligation of confidentiality; or
(e)was independently discovered or developed by or on behalf of the Receiving Party without use of, reference to or reliance upon any Confidential Information disclosed by the Disclosing Party.
7.3.Authorized Disclosure.
7.3.1.Disclosure to Party Representatives. Notwithstanding the foregoing provisions of Section
7.1, the Receiving Party may disclose Confidential Information belonging to the Disclosing Party to the Receiving Party’s Representatives who (a) have a need to know such Confidential Information in connection with the performance of the Receiving Party’s obligations or the exercise of the Receiving Party’s rights under this Agreement and (b) are bound by nondisclosure and non-use obligations with respect to such Confidential Information that are substantially similar to those set forth in this Article
7.
7.3.2.Disclosure to Third Parties. Notwithstanding the foregoing provisions of Section
7.1, each Party may disclose Confidential Information belonging to the other Party, and may refer to the existence of, or performance under, this Agreement, to the extent such disclosure is reasonably necessary:
(a)to Governmental Authorities or Regulatory Authorities (i) to the extent desirable to obtain or maintain INDs or Regulatory Approvals for any Licensed Product within the Territory, and
(11)in order to respond to inquiries, requests or investigations relating to Licensed Products or this Agreement;
(b)to comply with (a) Applicable Law, including the rules and regulations promulgated by any Governmental Authority, securities exchange or securities regulator in any country in the Territory;
(c)to such Party's attorneys, independent accountants or financial advisors for the sole purpose of enabling such attorneys, independent accountants or financial advisors to provide advice to the Receiving Party, on the condition that such attorneys, independent accountants and financial advisors agree to be bound by the confidentiality and non-use obligations contained in this Agreement;
(d)to outside consultants (including any professional advisor), potential acquisition partners (including any potential successors in interest), private investors or financing sources, contractors, advisory boards, managed care organizations, and non-clinical and clinical investigators, in each case to the extent desirable to Develop, register or market any Licensed Product; provided that the Receiving Party will obtain the same confidentiality obligations from such Third Parties as it obtains with respect to its own similar types of confidential information;
(e)in connection with filing or prosecuting Licensed Technology, Valneva Technology or Trademark rights as permitted by this Agreement;
(f)in connection with prosecuting or defending litigation pursuant to Sections
6.2 or
6.3;
(g)subject to the provisions of Section
7.5, in connection with or included in scientific publications relating to Licensed Products, including abstracts, posters, journal articles and the like, and posting results of and other information about Clinical Trials to clinicaltrials.gov and similar websites;
(h)the Parties may disclose Confidential Information of the Disclosing Party (including the terms of the Agreement) to any Subcontractor and, in case of Valneva, Sublicensee who has agreed in writing to non-disclosure and non-use provisions with respect to such Confidential Information that are at least as restrictive as those set forth in this Article
7;
(i)LimmaTech may disclose certain parts of Valneva’s Confidential Information (including the terms of and the performance under this Agreement, but excluding any of Valneva’s Trade Secrets) to Third Party Licensors and to its shareholders to only the extent required to meet its obligations (e.g., on reporting) towards such Third Party Licensors and shareholders, provided that LimmaTech will obtain the same confidentiality obligations from such Third Party Licensors and shareholders as it obtains with respect to its own similar types of confidential information; and
(j)to the extent necessary in order to enforce its rights under this Agreement.
If a Party deems it reasonably necessary to disclose Confidential Information belonging to the other Party pursuant to clauses (a) through (j) of this Section
7.3.2, then the Disclosing Party will to the extent possible give reasonable advance written notice of such disclosure to the other Party and take such measures to ensure confidential treatment of such information as is reasonably required by the other Party, at the other Party’s expense.
7.3.3.Required Disclosures. If a Party is required by judicial, governmental or administrative process, including to comply with Applicable Law (including stock exchange rules) or pursuant to Section 7.3.2(a) and (b), to disclose (including to make public announcements as referred to in Section 7.5.1) Confidential Information that is subject to the non-disclosure provisions of Section 7.3.1 above, such Party shall to the extent reasonably possible under the circumstances provide the other Party with reasonable advance notice of the disclosure that is being sought in order to provide the other Party an opportunity to challenge or limit the disclosure obligations. Confidential Information that is disclosed by judicial, governmental or administrative process in accordance with this Section 7.3.3 shall remain otherwise subject to the confidentiality and non-use provisions of this Article 7, and the Disclosing Party shall, at its own expense, seek such confidential treatment of confidential portions of this Agreement and such other terms, as may be reasonably requested by the other Party and limit its disclosure of such Confidential Information to only that required to comply with Applicable Law.
7.4.Unauthorized Use or Disclosure. The Receiving Party shall furnish the Disclosing Party with written notice immediately of it becoming aware and indicating details of any unauthorized use or disclosure of any of the Disclosing Party’s Confidential Information by any Representatives of the Receiving Party, and shall take all actions reasonably required in order to prevent any further unauthorized use or disclosure of the Disclosing Party’s Confidential Information. Notwithstanding the foregoing, the Receiving Party remains responsible and liable for any unauthorized use by the Representatives of the Receiving Party.
7.5.Public Announcements; Publications.
7.5.1.Announcements. Upon or shortly following the Effective Date, the Parties will issue a joint press release substantially in the form attached to this Agreement as Exhibit 7.5.1. With respect to any future disclosure, neither Party will make any public announcement regarding this Agreement without the prior written approval of the other Party, except as permitted under Section
7.3.3.
7.5.2.Publications. Subject to Section
7.3.3, in case Valneva wants to publish, release or otherwise make public Development Data or other Confidential Information generated under this Agreement or relating to the Licensed Products, Valneva shall (i) submit any such publication to LimmaTech for approval, at least [***] prior to publication or other release; and (ii) ensure that LimmaTech or a Third Party Licensor (as advised by LimmaTech) is referred to as licensor of Valneva. If LimmaTech determines that the proposed publication contains Confidential Information of LimmaTech or the Third Party Licensor, LimmaTech may object to the proposed publication, and may require one or more of the following: (i) deletion from the proposed publication of any Confidential Information of LimmaTech or the Third Party Licensor; and/or (ii) amendment of the proposed publication to mask/code commercially sensitive Confidential Information of LimmaTech or the Third Party Licensor. LimmaTech will reply within [***] from notice by Valneva. Valneva shall not release or distribute any publication or information related to the Licensed Products prior written approval by LimmaTech, such approval not to be unreasonably withheld after expiry of the [***] answer period of LimmaTech. Subject to Applicable Law, Valneva shall not publish the names of Third Party Licensors without the prior written approval of LimmaTech, unless this information is already available to the public without breach of this Agreement.
7.6.Obligations in Connection with Change of Control. If LimmaTech is subject to a Change of Control, LimmaTech will, and it will cause its Representatives to, ensure that no Confidential Information of Valneva is released to (a) any Affiliate of LimmaTech that becomes an Affiliate as a result of the Change of Control or (b) any other Representatives of LimmaTech (or of the relevant surviving entity of such Change of Control) who become LimmaTech Representatives as a result of the Change of Control, unless such Affiliate or other Representatives, as applicable, have signed individual confidentiality agreements which include equivalent obligations to those set out in this Article
7. If any Change of Control of LimmaTech occurs, LimmaTech will promptly notify Valneva, share with Valneva the policies and procedures it plans to implement in order to protect the confidentiality of Valneva’s Confidential Information prior to such implementation and make any adjustments to such policies and procedures that are reasonably requested by Valneva. In case of a Change of Control of Valneva, this Section
7.6 shall apply mutatis mutandis to such relevant surviving entity of such Change of Control.
7.7.Data Protection. For the purpose of this Agreement, the terms personal data, controller(s), data subject(s) and supervisory authority(ies) shall have the meaning set forth by the EU Regulation 2016/679 of 27 April 2016 (known as the “GDPR”).
The Parties shall comply with all applicable personal data protection laws and regulations for their activities performed under this Agreement which lead to the processing of personal data.
Any personal data processed under this Agreement shall be processed solely for the purpose(s) set out in this Agreement and in accordance with the terms of this Section 7.7.
The Parties agree that they shall be considered as separate (or distinct) controllers, in respect of all the personal data that they respectively process in the context of this Agreement and for the purpose(s) set out therein. Should this status have to be adapted in light of any future circumstances, the Parties agree to reflect this change in due course in this Agreement or in a separate agreement.
The Parties:
-Shall not process more personal data than necessary for the purpose(s) set out in this Agreement;
-Shall implement the appropriate technical and organizational measures to ensure that personal data
is being processed in a sufficiently secure and confidential manner;
-Shall not share any personal data with Third Parties (except insofar as allowed in this Agreement and/or as required by Applicable Law and/or as required by competent authorities);
-Shall not retain personal data for longer than necessary to achieve the purpose(s) set out this Agreement.
To the extent that the activities performed under this Agreement would lead to any transfer(s) of personal data outside of the EU, such transfer(s) shall take place in accordance with Applicable Law and be based on an adequacy decision from the European Commission and/or any other appropriate safeguards required.
The Parties shall cooperate with each other and provide reasonable assistance, to the extent necessary and as allowed by law and this Agreement, to address any personal data protection-related matters and/or requests raised by regulatory authorities and/or supervisory authorities and/or data subjects and/or a Party under this Agreement.
8.REPRESENTATIONS, WARRANTIES AND COVENANTS.
8.1.Mutual Representations and Warranties. Each of LimmaTech and Valneva hereby represents and warrants to the other Party that:
8.1.1.it is duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization;
8.1.2.the execution, delivery and performance of this Agreement by such Party has been duly authorized by all requisite action under the provisions of its charter, bylaws and other organizational documents, and does not require any action or approval by any of its shareholders or other holders of its voting securities or voting interests;
8.1.3.subject to the obligations and covenants under Third Party Agreements listed in Exhibit 8.8.1, it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder;
8.1.4.this Agreement has been duly executed and is a legal, valid and binding obligation on each Party, enforceable against such Party in accordance with its terms; and
8.1.5.the execution, delivery and performance by such Party of this Agreement and its compliance with the terms and provisions hereof does not and will not conflict with or result in a breach of or default under any Binding Obligation of such Party existing as of the Effective Date.
8.2.Mutual Covenants. Each of LimmaTech and Valneva hereby covenants to the other Party that, from the Effective Date until expiration or termination of this Agreement:
8.2.1.it will perform its obligations under this Agreement in compliance with Applicable Law;
8.2.2.subject to the obligations and covenants under Third Party Agreements listed in Exhibit 8.8.1, it will cooperate with the other Party and use Commercially Reasonable Efforts to make all registrations, filings and applications, to give all notices and to obtain as soon as practicable all governmental or other consents, transfers, approvals, orders, qualifications authorizations, permits and waivers, if any, and to do all other things necessary or desirable for the consummation of the transactions as contemplated hereby including the collection of Human Material; and
8.2.3.it will inform and train its Representatives of/in the special treatment reserved for Confidential Information that may be qualified as Trade Secrets and will ensure that Trade Secrets are only shared in application of the need-to-know principle and under a strict obligation of confidentiality.
8.3.Representations and Warranties of LimmaTech. LimmaTech hereby represents and warrants to Valneva that as of the Effective Date:
8.3.1.LimmaTech is the sole and exclusive owner of the LimmaTech Technology, all of which is free and clear of any claims, liens, charges or encumbrances;
8.3.2.subject to the obligations and covenants under Third Party Agreements listed in Exhibit 8.8.1, LimmaTech has the full title, right, power and authority to grant all of the right, title and interest in the licenses, sublicenses and other rights (including the right to use the Platform Materials) granted or to be granted to Valneva or the Global Health Partner under this Agreement;
8.3.3.(a) Exhibit 1.55 and Exhibit 1.79 set forth a true and complete list of all Licensed Patent Rights, i.e. Patent Rights (i) owned by LimmaTech or its Affiliates or (ii) to which LimmaTech or its Affiliates have been granted or otherwise transferred any right to practice under, in each case that relate to the Licensed Products or the Parties’ activities under the Development Plan, (b) each such Patent Rights remains in full force and effect and (c) LimmaTech or its Affiliates have timely paid, or caused the appropriate Third Parties to pay, all filing and renewal fees payable with respect to such Patent Rights;
8.3.4.to LimmaTech’s knowledge, as of the Effective Date, LimmaTech has disclosed to Valneva all material scientific and technical information and all information relating to safety and efficacy known to it or its Affiliates with respect to the Licensed Products;
8.3.5.to LimmaTech’s knowledge, the Licensed Patent Rights, are valid and enforceable patents and no Third Party (a) is infringing any Licensed Patent Rights or (b) has challenged or threatened to challenge the ownership, scope, validity or enforceability of, or LimmaTech’s or any Third Party Licensor’s rights in or to, any Licensed Patent Rights (including, by way of example, through the institution or written threat of institution of interference, nullity or similar invalidity proceedings before the US Patent and Trademark Office or any analogous foreign Governmental Authority);
8.3.6.to LimmaTech’s knowledge, LimmaTech, its Affiliates and Third Parties and Representatives acting on LimmaTech’s behalf in connection with this Agreement, and Third Party Licensors have complied in all material respects with all Applicable Law, including any disclosure requirements, in connection with the filing, prosecution and maintenance of the Licensed Patent Rights;
8.3.7.to LimmaTech’s knowledge, LimmaTech, its Affiliates and all Third Parties and Representatives acting on LimmaTech’s behalf, have complied in all material respects with the Applicable Law and accepted pharmaceutical industry business practices;
8.3.8.subject to the obligations and covenants under the Third Party Agreements listed in Exhibit 8.8.1, LimmaTech has independently developed all the Licensed Technology or otherwise has a valid right to use, and to permit Valneva, Valneva’s Affiliates and Valneva’s Sublicensees to use, the Licensed Technology for all permitted purposes under this Agreement;
8.3.9.LimmaTech has obtained from all inventors of LimmaTech Patent Rights, valid and enforceable agreements assigning to LimmaTech each such inventor’s entire right, title and interest in and to all such LimmaTech Patent Rights, and, to LimmaTech’s knowledge, Third
Party Licensors have obtained similar agreements from the inventors of the Platform Patent Rights;
8.3.10.to LimmaTech’s knowledge, no Licensed Technology is subject to any funding agreement with any government or Governmental Authority;
8.3.11.subject to Section 8.8.1, and except as otherwise set forth herein, neither LimmaTech nor any of its Affiliates are party to or otherwise subject to any agreement or arrangement which (a) limits the ownership or licensed or sublicensed rights of Valneva or its Affiliates with respect to, or (b) limits the ability of Valneva or its Affiliates to grant a license, sublicense or access, or (c) provide or provide access or other rights in, to or under, any Intellectual Property Right or Materials (including any Patent Rights, Know-How or other data or information), in each case, that would, but for such agreement or arrangement, be included in the rights licensed or assigned to Valneva or its Affiliates pursuant to this Agreement;
8.3.12.subject to the obligations and covenants under the Third Party Agreements listed in Exhibit 8.8.1, there are no LimmaTech Third Party Agreements and no Third Party has any right, title or interest in or to, or any license under, any LimmaTech Technology for the use, Development, Manufacture, Commercialization or Exploitation by LimmaTech or Valneva (or their respective Affiliates or Sublicensees) of any Licensed Product;
8.3.13.to LimmaTech’s knowledge, the Development, Manufacture, Commercialization or other Exploitation by Valneva (or its respective Affiliates or Sublicensees) of any Licensed Product
(a) does not and will not infringe any issued patent of any Third Party or (b) will not infringe the claims of any published Third Party’s patent application when and if such claims issue;
8.3.14.there is no (a) claim, demand, suit, proceeding, arbitration, inquiry, investigation or other legal action of any nature, civil, criminal, regulatory or otherwise, pending or, to the knowledge of LimmaTech, threatened against LimmaTech or any of its Affiliates or (b) judgment or settlement against or owed by LimmaTech or any of its Affiliates, in each case in connection with the Licensed Technology, any Licensed Product or relating to the transactions contemplated by this Agreement for the Development, Manufacture, Commercialization or other Exploitation by Valneva (or their respective Affiliates or Sublicensees) of any Licensed Product;
8.3.15.LimmaTech has valid and enforceable agreements with all Persons acting by or on behalf of LimmaTech or its Affiliates under this Agreement which require such Persons to assign to LimmaTech their entire right, title and interest in and to all LimmaTech Technology;
8.3.16.LimmaTech is not, and to LimmaTech’s knowledge, no Third Party Licensor, Representative of LimmaTech or Third Party acting on behalf of LimmaTech (in each case, as applicable) is debarred by any Regulatory Authority or the subject of debarment proceedings by any Regulatory Authority and, in the course of the discovery or pre-clinical Development of any Licensed Product, LimmaTech has not, and to LimmaTech's knowledge, no Third Party Licensor, Representative of LimmaTech or any Third Party acting on behalf of LimmaTech (in each case, as applicable) has used any employee or consultant that is debarred by any Regulatory Authority or is the subject of debarment proceedings by any Regulatory Authority; and
8.3.17.LimmaTech has no knowledge of (a) any prior art or other facts that LimmaTech believes would result in the invalidity or unenforceability of any issued or pending claims included in the Licensed Patent Rights, (b) any inequitable conduct or fraud on any patent office with respect to any of the Licensed Patent Rights or (c) any Person (other than Persons identified in the applicable patent applications or patents, as inventors of inventions disclosed in the Licensed Patent Rights) who claims to be an inventor of an invention disclosed in the Licensed Patent Rights.
8.4.Accuracy of LimmaTech's Representations and Warranties.
8.4.1.LimmaTech will take no action which would render any representation or warranty contained in Section
8.1 or Section
8.3 inaccurate or untrue in any material respect.
8.4.2.LimmaTech will promptly notify Valneva of any lawsuits, claims, administrative actions, regulatory inquiries or investigations, or other proceedings asserted or commenced against LimmaTech or its Representatives involving in any material way the ability of LimmaTech to deliver the rights, licenses and sublicenses granted herein.
8.4.3.LimmaTech will promptly notify Valneva in writing of any facts or circumstances which come to LimmaTech’s attention and which cause, or through the passage of time may cause, any of the representations and warranties contained in Section
8.1, Section
8.3 and Section
11.11 to be untrue or misleading in any material respect at any time during the Term; and in addition to the foregoing, with regard to any of the representations under Section
11.11, LimmaTech will suspend all affected activities (including making any related payments) under this Agreement, unless and until Valneva determines that LimmaTech may resume such activities.
8.5.Valneva Representations and Warranties. Valneva hereby represents and warrants to LimmaTech that, as at the Effective Date:
8.5.1.there are no proceedings or allegations that have been brought or made by or on behalf of Valneva or any of its Affiliates in the European Patent Office or any country of the Territory relating to the Licensed Patent Rights such as but not limited to an infringement claim.
8.5.2.Valneva is not, and to Valneva’s knowledge, no Representative of Valneva or Third Party acting on behalf of Valneva (in each case, as applicable) is debarred by any Regulatory Authority or the subject of debarment proceedings by any Regulatory Authority.
8.6.Accuracy of Valneva's Representations and Warranties.
8.6.1.Valneva will take no action which would render any representation or warranty contained in Section
8.1 or Section
8.5 inaccurate or untrue in any material respect.
8.6.2.Valneva will promptly notify LimmaTech of any lawsuits, claims, administrative actions, regulatory inquiries or investigations, or other proceedings asserted or commenced against Valneva or its Representatives involving in any material way the ability of Valneva to discharge its obligations hereunder.
8.6.3.Valneva will promptly notify LimmaTech in writing of any facts or circumstances which come to Valneva’s attention and which cause, or through the passage of time may cause, any of the representations and warranties contained in Section
8.1, Section
8.5 and Section
11.11 to be untrue or misleading in any material respect at any time during the Term; and in addition to the foregoing, with regard to any of the representations under Section
11.11, Valneva will suspend all affected activities (including making any related payments) under this Agreement, unless and until LimmaTech determines that Valneva may resume such activities.
8.7.LimmaTech Covenants. In addition to the covenants made by LimmaTech elsewhere in this Agreement, LimmaTech hereby covenants to Valneva that, from the Effective Date until expiration or termination of this Agreement:
8.7.1.LimmaTech will not exercise its right to terminate the GSK License under Section 9.3 or 9.8 of the GSK License or on any other grounds without Valneva’s prior written consent;
8.7.2.LimmaTech will not, and will cause its Affiliates not to (a) license, sell, assign (other than in a connection with a permitted license under this Agreement, or permitted assignment of this Agreement by LimmaTech pursuant to Section
11.1) or otherwise transfer to any Person (other than to Valneva or its Affiliates or Sublicensees pursuant to the terms of this Agreement) any Licensed Technology (or agree to do any of the foregoing) or (b) incur or permit to exist, with respect to any Licensed Technology, any lien, encumbrance, charge, security interest, mortgage, liability, assignment, grant of license or other Binding Obligation that is or would be inconsistent with the licenses and other rights granted (or that may be granted) to Valneva or its Affiliates under this Agreement;
8.7.3.LimmaTech will not, and will cause its Affiliates not to commence legal proceedings, or support any Third Party in commencing or conducting any legal proceedings, against Valneva, its Affiliates or its Sublicensees to enforce the Licensed Technology against the Development, Manufacture and Commercialization of the Licensed Products in the Territory during the Term in accordance with this Agreement;
8.7.4.LimmaTech will not (a) take any action that diminishes the rights under the Licensed Technology granted (or that may be granted) to Valneva or Valneva’s Affiliates under this Agreement or (b) fail to take any action that is reasonably necessary to avoid diminishing the rights under the Licensed Technology granted (or that may be granted) to Valneva or Valneva’s Affiliates under this Agreement;
8.7.5.with respect to Human Material used, including collection or transfer, by LimmaTech, its Affiliates or Subcontractors in conducting activities under this Agreement, (a) such use shall be solely as described in the Development Plan and shall be within the scope of and consistent with LimmaTech’s ethical approval policies, (b) LimmaTech will, and will cause its Affiliates or Subcontractors to, handle and use the Human Material in accordance with the Applicable Law and the ICF, (c) LimmaTech will provide the ICF to Valneva upon request by Valneva, (d) LimmaTech will only allow its employees, contractors or agents trained in handling similar materials or data in their assigned job functions to handle the Human Material, (e) the Human Material will be used for research purposes only and not be used for treatment of or administration to humans and (f) if LimmaTech procures any Human Material from a Third Party such as a sample bank, LimmaTech shall ensure that the collection and transfer of such Human Material and the use thereof for purposes of the Development Plan is in accordance with the Applicable Law and recognized international standards for the protection of human research subjects;
8.7.6.LimmaTech will (a) not enter into any LimmaTech Third Party Agreement that adversely affects (i) the rights granted (or that may be granted) to Valneva, Valneva’s Affiliates or Sublicensees hereunder or (ii) LimmaTech’s ability to fully perform its obligations hereunder; (b) not amend or otherwise modify any LimmaTech Third Party Agreement or consent or waive rights with respect thereto in any manner that (i) adversely affects the rights granted (or that may be granted) to Valneva or Valneva’s Affiliates or Sublicensees hereunder or (ii) LimmaTech’s ability to fully perform its obligations hereunder; (c) promptly furnish Valneva with true and complete copies of all LimmaTech Third Party Agreements and related amendments executed following the Effective Date; (d) remain, and cause its Affiliates to remain, in compliance in all material respects with all LimmaTech Third Party Agreements; and (e) furnish Valneva with copies of all notices received by LimmaTech or its Representatives relating to any alleged breach or default by LimmaTech or its Representatives under any LimmaTech Third Party Agreement within [***] after receipt thereof;
8.7.7.LimmaTech will not enter into or otherwise allow itself or its Representatives to be subject to any agreement or arrangement which (a) limits the ownership or the licensed or sublicensed rights of Valneva or its Affiliates; (b) limits the ability of Valneva or its Affiliates to grant a license, sublicense or access; or (c) provides access or other rights in, to or under, any Intellectual Property Right or Materials (including any Patent Rights, Know-How or other data or information), in each case, that would, but for such agreement or arrangement, be included in the rights licensed or assigned (or that may be licensed or assigned) to Valneva or its Affiliates pursuant to this Agreement;
8.7.8.LimmaTech will maintain valid and enforceable agreements with all Persons acting by or on behalf of LimmaTech or its Affiliates under this Agreement which require such Persons to assign to LimmaTech their entire right, title and interest in and to all LimmaTech Technology and Valneva Technology;
8.7.9.LimmaTech has made or will make any payments owing to any inventor of any LimmaTech Technology or to any inventor of any Valneva Technology (as identified in the applicable patent application) who is a Representative of LimmaTech or any other Person that is required in connection with the creation or exploitation or transfer of rights to such LimmaTech Technology or Valneva Technology (to the extent the inventor(s) is/are Representatives of LimmaTech); and
8.7.10.LimmaTech will promptly notify Valneva in the event that it learns of:
(a)any prior art or other facts that LimmaTech believes would result in the invalidity or unenforceability of any issued or pending claims included in any of the Licensed Patent Rights;
(b)any inequitable conduct or fraud on any patent office with respect to any of the Licensed Patent Rights;
(c)any Person (other than Persons identified in the applicable patent applications or patents, as inventors of inventions disclosed in the Licensed Patent Rights) who claims to be an inventor of an invention disclosed in the Licensed Patent Rights; or
(d)any lawsuits, claims, administrative actions, government inquiries or investigations, or other proceedings related to the activities contemplated under this Agreement.
8.8.Valneva Covenants. Valneva hereby covenants to LimmaTech that, from the Effective Date until expiration or termination of this Agreement,
8.8.1.it will comply with the obligations and covenants that are relevant to a sublicensee under the LimmaTech Third Party Agreements listed in Exhibit 8.8.1 hereto. Valneva will reasonably ensure that LimmaTech is able to comply with its non-financial obligations under the Third Party Agreements where such compliance is contingent on Valneva’s actions. For such purpose, and upon LimmaTech’s written request, Valneva will reasonably support LimmaTech in preparing the reports, and meeting its other obligations under the Third Party Agreements.
8.8.2.it will comply in all material respects with the Applicable Law with respect to the Development, Manufacture and Commercialization of the Licensed Products.
8.9.Representation by Legal Counsel. Each Party hereto represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof. In interpreting and applying the terms and provisions of this Agreement, the Parties agree that no presumption will exist or be implied against the Party which drafted such terms and provisions.
8.10.Disclaimer. THE FOREGOING REPRESENTATIONS AND WARRANTIES OF EACH PARTY ARE IN LIEU OF ANY OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTIES OF MERCHANTABILITY OR ANY IMPLIED WARRANTIES OF FITNESS FOR A PARTICULAR PURPOSE, ALL OF WHICH ARE HEREBY SPECIFICALLY EXCLUDED AND DISCLAIMED.
9.TERM AND TERMINATION.
9.1.Term. The term of this Agreement (“Term”) will commence on the Effective Date and extend on a country-by-country basis (in the Territory), unless this Agreement is terminated earlier in accordance with this Article
9, until the last to expire of any Royalty Term for any Licensed Product in such country in the Territory. Notwithstanding any provision of this Agreement to the contrary, upon expiration of this Agreement, Valneva will retain a fully paid-up, irrevocable, non-exclusive, royalty-free license to each Licensed Product as set forth in Sections
3.5.3 and
3.6.4.
9.2.Termination for Cause by LimmaTech. LimmaTech may terminate this Agreement for cause and in its entirety (however, exclusively limited to the LMIC Territory in the event of termination under Section
9.2(b)), at any time during the Term, in the following cases:
(a)Material Breach. If Valneva commits a Material Breach of its obligations under this Agreement and such Material Breach remains uncured within the time periods set forth in (i) and (ii) below, in either case to be calculated from Valneva’s receipt of LimmaTech’s written notice asserting the Material Breach:
(i)[***] for a Material Breach that is a failure of Valneva to make an undisputed payment owed to LimmaTech under this Agreement. If the payment to which LimmaTech claims to be entitled under this Agreement is disputed in good faith (i.e., by setting forth reasonable arguments why no or accurate payment is not due), the cure period will be tolled pending resolution of any bona fide dispute between the Parties as to whether such payment is due; and
(ii)[***] for all other Material Breaches; provided, however, that if any breach is not reasonably curable within ninety (90) calendar days and if Valneva is using Commercially Reasonable Efforts to cure such breach, such termination will be delayed for a time period to be agreed by both Parties in order to permit Valneva a reasonable period of time to cure such breach. For clarity, Valneva’s breach of (i) its diligence obligations under Section 5.3; (ii) the Commercially Reasonable Efforts obligations referred to in Section 2.3.2 (including the obligation to use Commercially Reasonable Efforts to include the terms and conditions identified in Exhibit 2.3.2 Part B in the Global Health License, and the obligation not to deviate from the terms and conditions identified in Exhibit 2.3.2 Part B without both Parties written consent); and/or (iii) its representations, warranties and covenants under Article 8 may constitute a Material Breach.
(b)No Global Health License. If Valneva (a) has not entered into a Global Health License by the End of Phase 2; and (b) either (x) is not in ongoing contract negotiations (i.e., beyond term sheet stage and at contract drafting stage) with a potential Global Health Partners in accordance with Section
2.3.2; or (y) is in ongoing contract negotiations by the End of Phase 2, but has not entered into a Global Health License within [***] after the End of Phase 2, LimmaTech is entitled to terminate this Agreement with respect to the LMIC Territory within the same time periods as set forth in Section
9.2(a)(ii).
(c)Challenge of Licensed Patent Rights. If Valneva or any of its Affiliates or Sublicensees challenges the Licensed Patent Rights during the Term or supports any Third Party in any such attack on the Licensed Patent Rights. Any such termination shall only become effective if Valneva, its Affiliate or its Sublicensee has not taken the necessary steps to withdraw such action before the end of a [***] cure period following LimmaTech’s written notice to this effect. Valneva shall inform LimmaTech of the steps taken in this respect as soon as possible and in any event within [***] of the date on which they were actually taken. In the event a Sublicensee challenges the validity of a Licensed Patent Right and has not withdrawn the proceeding initiated to that effect within the aforementioned [***] period, LimmaTech may terminate this Agreement if Valneva does not terminate such sublicense agreement within [***].
9.3.Termination by Valneva.
9.3.1.Termination without Cause or for Failure of Results.
(a)General. At any time during the Term, however not earlier than after completion of the first Phase 2 CHIM study (Sonnei), Valneva may terminate this Agreement for the LMIC Territory, or in its entirety, without cause, for any or no reason, as follows:
(i)upon [***] written notice if the Licensed Product is still under Development, and
(ii)upon [***] written notice if the Licensed Product has received Regulatory Approval in a country within the Territory.
(b)Phase 2 Clinical Trials Fail. Valneva may terminate the Agreement in its entirety within [***] of receipt of the final results of the Phase 2 Clinical Trials in the event that the expected result is not met, by giving [***] prior written notice to LimmaTech.
9.3.2.Termination for Cause. Valneva may terminate this Agreement for cause with respect to one or more Licensed Products in one or more countries or in the entire Travelers’ Territory; and/or for the entire LMIC Territory, or may terminate this Agreement in its entirety, at any time during the Term, by giving written notice to LimmaTech in the event that LimmaTech commits a Material Breach of its obligations under this Agreement and such Material Breach remains uncured for [***], measured from the date written notice of such Material Breach is given to LimmaTech; provided, however, that if any breach is not reasonably curable within ninety (90) calendar days and if LimmaTech is using its Commercially Reasonable Efforts to cure such breach, such termination will be delayed for a time period to be agreed by both Parties in order to permit LimmaTech a reasonable period of time to cure such breach. For clarity, LimmaTech’s breach of its diligence obligations under Section 5.3 and/or of its representations, warranties and covenants under Article 8 may constitute a Material Breach.
9.4.Effects of Termination.
9.4.1.Consequences.
(a)Termination for Cause by LimmaTech; Termination without Cause or for Failure of Results by Valneva. In the event that LimmaTech terminates this Agreement for cause pursuant to Section
9.2 or Valneva terminates this Agreement without cause or for failure of results pursuant to Section
9.3.1, the following will apply:
(i)Except as otherwise expressly provided herein, all rights and obligations of each Party hereunder, will cease (including all rights and licenses and sublicenses granted by either Party to the other Party hereunder), and all rights Controlled by LimmaTech and granted to Valneva for performance of its activities under this Agreement shall automatically revert back to LimmaTech.
(ii)If such termination occurs at any time before the End of Phase 2 (before the relevant budget under the Development Budget has been spent), the Parties agree that LimmaTech’s financial contribution to the Development Costs shall be limited to [***] of the total Development Costs incurred by both Parties at the time of termination, even if such [***] is less than the Development Costs Cap that LimmaTech would have had to bear pursuant to Section
3.2 if the Agreement had continued beyond the End of Phase 2. Valneva shall refund LimmaTech the amount of the Development Costs actually incurred by LimmaTech up to termination in excess of the aforementioned [***] within [***] of the invoice sent by LimmaTech to Valneva for this purpose. Sample calculation: If termination occurs before the End of Phase 2, at a time when the total Development Costs spent by both Parties amount to thirty million Euros (€30,000,000) and LimmaTech has spent six million five hundred thousand Euros (€6,500,000), Valneva will have to reimburse LimmaTech [***].
(iii)If such termination occurs at any time before expiration of a [***] period following the End of Phase 2, LimmaTech will automatically benefit from a royalty-free, non-exclusive, worldwide, sublicensable in multiple tiers, license under the Valneva Technology related to the Licensed Product (excluding any license related to Active Ingredients in a Combination which are not licensed under this Agreement), for the continued Development, Manufacture and Commercialization of the Licensed Products inside the Field of Use. The license thus
granted will last as long as Valneva Technology is protected by Intellectual Property Rights and/or remains Confidential.
In case of termination in accordance with Section
9.3.1, and provided Valneva has not yet completed the then current CMC work package led by Valneva as described in the Development Plan, Valneva undertakes to continue performing this CMC work package, at its own costs and expenses, in good faith and in accordance with the Development Plan until completion and to deliver the results and materials thereof to LimmaTech free of charge. Such results and materials shall be provided in a form suitable for tech transfer, within a reasonable time not to exceed [***] following completion of the considered CMC work package.
To the extent assignable under the terms agreed between the parties and upon LimmaTech’s prior written consent, sublicenses granted by Valneva under this Agreement will remain in full force and effect and shall be assigned by Valneva to LimmaTech, who shall then succeed to Valneva’s rights and obligations under such sublicense(s).
(iv)If such termination occurs at any time after expiration of a [***] period following the End of Phase 2:
(aa) LimmaTech may request by written notice to Valneva a royalty-bearing, nonexclusive, worldwide, sublicensable in multiple tiers, license under the Valneva Technology related to the Licensed Product (excluding any license related to the Active Ingredient in a Combination which are not licensed under this Agreement), for the continued Development, Manufacture and Commercialization of the Licensed Products inside the Field of Use, such license to become automatically effective upon LimmaTech’s request for such license. Further, promptly upon LimmaTech’s request following the effective date of termination, Valneva shall make available to LimmaTech the information, documents, Materials, Development Data and/or supplies or drug product in Valneva’s control, as requested by LimmaTech, and as necessary for LimmaTech to effectively continue the licensed activities. Within [***] of Valneva’s receipt of the written notice from LimmaTech, the Parties will use Commercially Reasonable Efforts to negotiate the payments to be made (including the royalties to be paid) by LimmaTech to Valneva in consideration for the license granted to the Valneva Technology and for the transfer of information, documents, Materials, Development Data and/or supplies or drug product as requested by LimmaTech, and as necessary for LimmaTech to effectively continue the licensed activities, taking into account the particular circumstances that led to the termination of the Agreement pursuant to this Section 9.4.1(a) and with retroactive effect to the day of the effective commencement of the considered license. If the Parties fail to reach agreement, they will refer the matter for resolution by Expert Determination, as defined in Section 11.13.4 below. If LimmaTech rejects the financial terms determined by the expert in accordance with Section 11.13.4, the license granted hereunder shall automatically terminate, and all information, documents, Materials, Development Data and/or supplies or drug product transferred to LimmaTech, if any, will be retransferred to Valneva.
If Materials, supplies or drug product have been used or destroyed and cannot be given back, LimmaTech shall indemnify Valneva for the loss of such Materials, supplies or drug product, at the latest book value available at the time of transfer to Valneva, the date of the book value assessment and the date of transfer should not be further apart than [***].
(bb) LimmaTech has an option to obtain an exclusive, sublicensable in multiple tiers, license to the Valneva Technology related to the Licensed Product (excluding any license related to the Active Ingredient in a Combination which are not licensed under this Agreement), for the continued Development, Manufacture and Commercialization of the Licensed Products inside the Field of Use and in the territory affected by the termination. Such exclusive license would be in lieu of the non-exclusive license under Section
9.4.1(a)(iv)(aa)(aa) above and it would last as long as Valneva Technology is protected by Intellectual Property Rights and/or remains Confidential. LimmaTech may exercise such option within [***] following the effective date of termination. Upon exercise of such option the Parties will use Commercially Reasonable Efforts to negotiate the increase in royalties to be paid by LimmaTech to Valneva in consideration for the exclusivity of the license granted to the Valneva Technology and in consideration of the items listed in Section
9.4.1(a)(iv)(aa). If the Parties fail to reach agreement, they will refer the matter for resolution by Expert Determination in accordance with Section
11.13.4 below.
(b)Termination for Cause by Valneva.
In the event that LimmaTech commits a Material Breach justifying termination of this Agreement for cause pursuant to Section
9.3.2, Valneva may, at its discretion, either:
(A)terminate this Agreement in its entirety:
a.except as otherwise expressly provided herein, all rights and obligations of each
Party hereunder, will cease (including all rights and licenses and sublicenses granted by either Party to the other Party hereunder);
b.Valneva may claim damages in relation to the breach of this Agreement by LimmaTech in accordance with the dispute resolution rules in Section
11.13;
if LimmaTech notifies Valneva of its intent to pursue the Development, Manufacture and Commercialization of the Licensed Product in the Field of Use, LimmaTech may request by written notice to Valneva a royalty-bearing, non-exclusive, worldwide, sublicensable in multiple tiers, license under the Valneva Technology related to the Licensed Product (excluding any license related to the Active Ingredient in a Combination which are not licensed under this Agreement), for the continued Development, Manufacture and Commercialization of the Licensed Products inside the Field of Use. The Parties will negotiate the payments to be made (including the royalties to be paid) by LimmaTech to Valneva in consideration for said license, on the same terms and within the same timeframe as set forth in Section
(iv). Or
(B)elect to keep this Agreement in force. In such case:
a.all licenses and sublicenses granted under this Agreement by LimmaTech to Valneva with respect to such Licensed Product will remain in effect in accordance with their terms;
b.all payment obligations under Article
3 shall remain in effect, provided that with respect to milestones and royalties arising after the date on which Valneva could have terminated the Agreement, Valneva may, if Valneva also claims damages in relation to the breach of this Agreement by LimmaTech, suspend the payment of such milestone and royalty payments until the amount of damages suffered or incurred by Valneva (if any) - as has been agreed between the Parties or determined by an arbitration panel in accordance with Section 11.13 - have been set off against such milestone and royalty payments.
9.4.2.Accrued Rights. Expiration or termination of this Agreement for any reason will be without prejudice to any right which will have accrued to the benefit of either Party prior to such expiration or termination, including damages arising from any breach under this Agreement. Expiration or termination of this Agreement will not relieve either Party from any obligation which is expressly indicated to survive such expiration or termination, including the obligation set forth in Section
9.4.1(ii).
9.4.3.Survival Period. The following sections, together with any sections that expressly survive (including any irrevocable licenses and sublicenses granted hereunder), will survive expiration or termination of this Agreement for any reason: Sections
1 (Definitions),
3.8.4 (Taxes and Withholding),
3.8.7 (Record Keeping),
3.8.8 (LimmaTech Audits),
3.8.9
(Underpayments/Overpayments),
3.8.10 (Confidentiality), 6.3.2 (License from Valneva to LimmaTech over Valneva Technology),
7 (Confidentiality), 8.7.9 (LimmaTech Covenants concerning payments made or to be made to any inventor of LimmaTech Technology or Valneva Technology who are Representatives of LimmaTech),
9.4 (Effects of Termination),
9.5 (Provision for Insolvency),
10.1 (No Consequential Damages),
10.2 (Indemnification by Valneva),
10.3 (Indemnification by LimmaTech),
11 (Miscellaneous) and, to the extent this Agreement expires or is terminated, either in whole or in part, for any reason except by LimmaTech for cause pursuant to Section
9.2 or by Valneva without cause pursuant to Section
9.3.1: Article
6 (Intellectual Property).
9.5.Provision for Insolvency.
9.5.1.Termination Right. LimmaTech will be deemed a “Debtor” under this Agreement if, at any time during the Term, (a) a case is commenced by or against LimmaTech under Book XX of the Belgian Code of Economic Law, (b) LimmaTech files for or is subject to bankruptcy or liquidation, (c) LimmaTech assigns all or a substantial portion of its assets for the benefit of creditors, (d) a receiver or custodian is appointed for LimmaTech’s business or (e) a substantial portion of LimmaTech’s business is subject to attachment or similar process; provided, however, that in the case of any involuntary case under the Bankruptcy Code, LimmaTech will not be deemed a Debtor if the case is dismissed within [***] after the commencement thereof. If LimmaTech is deemed a Debtor, then Valneva may terminate this Agreement by providing written notice to LimmaTech. If Valneva terminates this Agreement pursuant this Section 9.5.1, then: (i) all licenses granted to Valneva under this Agreement will become irrevocable, and Valneva will have no further obligations to LimmaTech under this Agreement other than (A) those obligations that expressly survive termination in accordance with Section 9.4.3 and (B) the payment obligations under Article 3, such payments to be made in accordance with and subject to the other terms of this Agreement governing payments; (ii) such termination will not be construed to limit LimmaTech’s right to receive payments that accrued before the effective date of such termination; (iii) Valneva will have the right to offset, against any payment owing to LimmaTech as provided for under clause (i), above, any damages found or agreed by the Parties to be owed by LimmaTech to Valneva; and (iv) nothing in this Section 9.5.1 will limit any other remedy Valneva may have for any breach by LimmaTech of this Agreement.
9.5.2.Rights to Intellectual Property. All rights and licenses now or hereafter granted by LimmaTech to Valneva under or pursuant to any Section of this Agreement, including Sections
2.1, 2.2, 2.3, 2.5, 2.9, 2.11 and
2.12 hereof, are Intellectual Property Rights. The Parties hereto acknowledge and agree that the payments provided for under Sections
3.1 and
3.3 and all other payments by Valneva to LimmaTech hereunder, other than royalty payments pursuant to Section
3.4, do not constitute royalties or relate to licenses of Intellectual Property Rights hereunder. If bankruptcy proceedings are commenced against LimmaTech and Valneva’s appointed bankruptcy trustee elects to continue this Agreement and to retain its rights under this Agreement, then LimmaTech (in any capacity, including debtor-in-possession) and its successors and assigns (including any bankruptcy trustee) will provide to Valneva all Intellectual Property Rights to the extent licensed hereunder, and agrees to grant and hereby grants to Valneva and its Affiliates a right to access and to obtain possession of and to benefit from and, in the case of any Materials or other tangible item of which there is a fixed or limited quantity, to obtain a pro rata portion of, each of the following to the extent related to any Licensed Product, or otherwise related to any right or license granted under or pursuant to this Agreement: (i) copies of pre-clinical and clinical research data and results; (ii) all of the following (to the extent that any of the following are so related): Licensed Product samples; (iii) LimmaTech Technology, (iv) laboratory notes and notebooks; (v) Licensed Product data or filings, and (vi) Rights of Reference in respect of regulatory filings and approvals, all of which constitute “embodiments” of Intellectual Property Rights, and (viii) all other embodiments of such Intellectual Property Rights, whether any of the foregoing are in LimmaTech’s possession or control or in the possession and control of any Third Party but which LimmaTech has the right to access or benefit from and to make available to Valneva. LimmaTech will not interfere with the exercise by Valneva or its Affiliates of rights and licenses to Intellectual Property Rights licensed hereunder and embodiments thereof in accordance with this Agreement and agrees to use Commercially Reasonable Efforts to assist Valneva and its Affiliates to obtain such Intellectual Property Rights and embodiments thereof in the possession or control of Third Parties as reasonably necessary or desirable for Valneva or its Affiliates or Sublicensees to exercise such rights and licenses in accordance with this Agreement.
9.5.3.No Limitation of Rights. All rights, powers and remedies of Valneva provided in this Section
9.5 are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at Applicable Law in the event of the commencement of an insolvency case involving LimmaTech.
10.LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE.
10.1.No Consequential Damages. Except with respect to liability arising from a breach of Article
6 or
7, from any willful misconduct, intentionally wrongful act or gross negligence, or to the extent such Party may be required to indemnify the other Party under this Article
10, in no event will either Party or its Representatives be liable to the other Party under this Agreement for any special (only as related to indirect, incidental or consequential damages), indirect, incidental, consequential or punitive damages, whether in contract, warranty, tort, negligence, strict liability or otherwise, including loss of profits or revenue or loss of business opportunity suffered by the other Party or any of its Representatives. Without limiting the generality of the foregoing, “consequential damages” will be deemed to include, and neither Party will be liable to the other Party or any of such other Party’s Representatives or stockholders for any damages based on or measured by loss of projected or speculative future sales of the Licensed Products, any unearned royalties or participations under Sections
3.5, 3.6.1 and
3.6.2 or any other unearned, speculative or otherwise contingent payments provided for in this Agreement.
10.2.Indemnification by Valneva. Valneva will indemnify, defend and hold harmless LimmaTech, its Affiliates and each of its and its Affiliates’ employees, officers, directors and agents (each, a “LimmaTech Indemnified Party”) from and against any and all claims, causes, or allegations (whether threatened or pending), judgments, expenses, damages, liabilities, obligations, fees (including the reasonable fees of attorneys and other consulting or testifying professionals), costs and losses (collectively, “Liabilities”) that the LimmaTech Indemnified Party may be required to pay to one or more Third Parties resulting from or arising out of:
(a)Development, Manufacture, Commercialization or use of any Licensed Product by, on behalf of, or under the authority of, Valneva;
(b)the Material Breach by Valneva or any of its Representatives of any of its representations, warranties or covenants hereunder; or
(c)any other grossly negligent, willful or intentionally wrongful (i) act, (ii) error or (iii) omission on the part of Valneva, or any officer, director, employee, agent or representative of Valneva;
except, in each case, to the extent caused by the negligence, recklessness or intentional acts of LimmaTech or any LimmaTech Indemnified Party.
10.3.Indemnification by LimmaTech. LimmaTech will indemnify, defend and hold harmless Valneva, its Affiliates and Sublicensees and each of its and their respective employees, officers, directors and agents (each, a “Valneva Indemnified Party”) from and against any and all Liabilities that the Valneva Indemnified Party may be required to pay to one or more Third Parties resulting from or arising out of:
(a)Development, Manufacture or use of any Licensed Product by, on behalf of, or under the authority of, LimmaTech in connection with the performance of the LimmaTech Phase 2 Clinical Trials, with the exception of any Development, Manufacture or use which Valneva required LimmaTech to perform against LimmaTech’s will by using its right to resolve matters in dispute under Section 4.3.2(c);
(b)the Material Breach by LimmaTech or any of its Representatives of any of its representations, warranties or covenants hereunder; or
(c)any other grossly negligent, wilful or intentionally wrongful (i) act, (ii) error or (iii) omission on the part of LimmaTech, or any officer, director, employee, agent or representative of LimmaTech;
(d)Third Party claims based on a product developed, manufactured or commercialized by LimmaTech, its Affiliates or any Third Party Licensors outside the Field of Use;
except, in each case, to the extent caused by the negligence, recklessness or intentional acts of Valneva or any Valneva Indemnified Party.
10.4.Procedure.
10.4.1.Notice. Each Party will notify the other Party in writing in the event it becomes aware of a claim for which indemnification may be sought hereunder. In the event that any Third Party asserts a claim or other proceeding (including any governmental investigation) with respect to any matter for which a Party (the “Indemnified Party”) is entitled to indemnification hereunder (a “Third Party Claim”), then the Indemnified Party will promptly notify the Party obligated to indemnify the Indemnified Party (the “Indemnifying Party”) thereof; provided, however, that no delay on the part of the Indemnified Party in notifying the Indemnifying Party will relieve the Indemnifying Party from any obligation hereunder unless (and then only to the extent that) the Indemnifying Party is prejudiced thereby.
10.4.2.Control. Subject to Valneva’s right to control the defense of any Infringement Claim under Section 6.2.8 (even where LimmaTech is the Indemnifying Party), the Indemnifying Party will have the right, exercisable by notice to the Indemnified Party within [***] after receipt of notice from the Indemnified Party of the commencement of or assertion of any Third Party Claim, to assume direction and control of the defense, litigation, settlement, appeal or other disposition of the Third Party Claim (including the right to settle the claim solely for monetary consideration) with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party; provided that (a) the Indemnifying Party has sufficient financial resources, in the reasonable judgment of the Indemnified Party, to satisfy the amount of any adverse monetary judgment that is sought, (b) the Third Party Claim seeks solely monetary damages and (c) the Indemnifying Party expressly agrees in writing that as between the Indemnifying Party and the Indemnified Party, the Indemnifying Party will be solely obligated to satisfy and discharge the Third Party Claim in full (the conditions set forth in clauses (a), (b) and (c) above are collectively referred to as the “Litigation Conditions”). Within [***] after the Indemnifying Party has given notice to the Indemnified Party of its exercise of its right to defend a Third Party Claim, the Indemnified Party will give notice to the Indemnifying Party of any objection thereto based upon the Litigation Conditions. If the Indemnified Party reasonably so objects, the Indemnified Party will continue to defend the Third Party Claim, at the expense of the Indemnifying Party, until such time as such objection is withdrawn. If no such notice is given, or if any such objection is withdrawn, the Indemnifying Party will be entitled, at its sole cost and expense, to assume direction and control of such defense, with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party. During such time as the Indemnifying Party is controlling the defense of such Third Party Claim, the Indemnified Party will cooperate, and will cause its Affiliates and agents to cooperate upon request of the Indemnifying Party, in the defense or prosecution of the Third Party Claim, including by furnishing such records, information and testimony and attending such conferences, discovery proceedings, hearings, trials or appeals as may reasonably be requested by the Indemnifying Party. In the event that (i) LimmaTech is the Indemnifying Party but Valneva exercises its right to control the defense of an Infringement Claim pursuant to Section 6.2.8, (ii) the Indemnifying Party does not satisfy the Litigation Conditions or (iii) the Indemnifying Party does not notify the Indemnified Party of its intent to defend any Third Party Claim within ten (10) Business Days after notice thereof, the Indemnified Party may (without further notice to the Indemnifying Party) undertake the defense thereof with counsel of its choice and at the Indemnifying Party’s expense (including reasonable, out-of-pocket attorneys’ fees and costs and expenses of enforcement or defense). The Indemnifying Party or the Indemnified Party, as the case may be, will have the right to join in (including the right to conduct discovery, interview and examine witnesses and participate in all settlement conferences), but not control, at its own expense, the defense of any Third Party Claim that the other party is defending as provided in this Agreement.
10.4.3.Settlement. The Indemnifying Party will not, without the prior written consent of the Indemnified Party, enter into any compromise or settlement that commits the Indemnified Party to take, or to forbear to take, any action. The Indemnified Party will have the sole and exclusive right to settle any Third Party Claim, on such terms and conditions as it deems reasonably appropriate, to the extent such Third Party Claim involves equitable or other non-monetary relief, but will not have the right to settle such Third Party Claim to the extent such Third Party Claim involves monetary damages without the prior written consent of the Indemnifying Party. Each of the Indemnifying Party and the Indemnified Party will not make any admission of liability in respect of any Third Party Claim without the prior written consent of the other party, and the Indemnified Party will use reasonable efforts to mitigate Liabilities arising from such Third Party Claim.
10.4.4.Insurance. Each Party further agrees to obtain and maintain, during the Term, commercial general liability insurance, including products liability insurance and information privacy and security liability insurance, with “A”-rated insurance carriers to cover its liability and indemnification obligations under this Agreement, in each case with limits of not less than [***] combined single limit coverage and [***] in global cyber insurance, and other insurance as is reasonable and customary for similar companies, all of which may be satisfied through a combination of primary and excess insurances. Terms of this policy may be reviewed by the
other Party upon its request. If any such coverage is issued on a “claims made” basis, Valneva shall take out and maintain at its own expense, tail insurance on such coverage beginning on the date the relevant insurance obligation ends and ending not less than [***] thereafter. All deductibles and retentions will be the responsibility of the named insured. Neither Party’s insurance will be construed to create a limit of liability with respect to its indemnification obligations under this Article
10. Such insurance certificate shall name both GSK and the Governors of the University of Alberta as additional insured as their respective interests may appear and shall include a certification that such insurance coverage includes contractual coverage for Valneva’s liability under the Agreement. Upon LimmaTech’s request, Valneva commits to provide LimmaTech with a certificate of insurance confirming conformity with this Section 10.4.4.
10.4.5.Assignment. This Agreement may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party, such consent not to be unreasonably withheld, conditioned or delayed; provided, however, that each of the Parties may, without such consent, but with prior notification, assign this Agreement and its rights and obligations hereunder to any of its Affiliates or in case of transfer or sale of all or substantially all of the portion of its business to which this Agreement relates or in the event of its merger or consolidation with a Third Party. Any permitted assignee will assume all obligations of its assignor under this Agreement in writing concurrent with the assignment. Any purported assignment in violation of this Section
11.1 will be void. Except as otherwise provided herein, this Agreement shall be binding upon and inure to the benefit of the Parties and their successors and permitted assignors under this Section
11.1.
10.4.6.Change of Control. The Parties will inform each other in writing promptly (and in any event within [***]) following the entering into of a definitive agreement with respect to a Change of Control of a Party.
10.4.7.Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of the Agreement.
10.4.8.Force Majeure. If the performance of any part of this Agreement by either Party, or any obligation under this Agreement, is prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable control, and which had not been foreseeable at the time of conclusion of this Agreement (“Force Majeure”), of the Party liable to perform, unless conclusive evidence to the contrary is provided, the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, interference or delay, provided that the affected Party shall use Commercially Reasonable Efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed. When such circumstances arise and persist for a period of at least [***], the Parties shall discuss what, if any, modification of the terms of this Agreement may be required in order to arrive at an equitable solution.
10.4.9.Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein will be deemed to encompass references to either or both genders, and the use of the singular will be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” will be deemed to be followed by the phrase “without limitation”, (c) the word “will” will be construed to have the same meaning and effect as the word “shall”, (d) any definition of or reference to any agreement, instrument or other document herein will be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any Person will be construed to include the Person’s successors and assigns, (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, will be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Sections or Exhibits will be construed to refer to Sections or Exhibits of this Agreement, and references to this Agreement include all Exhibits hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and will include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like will require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or article, section or other division thereof, will be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, and (k) the term “or” will be interpreted in the inclusive sense commonly associated with the term “and/or”.
10.4.10.Notices. All notices which are required or permitted hereunder shall be in writing and sufficient if delivered personally, sent by internationally-recognized overnight courier or sent by registered or certified mail, postage prepaid, addressed as follows:
a.if to LimmaTech, addressed to:
LimmaTech Biologics AG
Attention: CEO
Address [***]
with copy to: [***]
Grabenstrasse 3, CH-9852 Schlieren, Switzerland
b.If to Valneva, addressed to:
Valneva Austria GmbH
Attention: CEO
Address: [***]
with copy to: [***]
or to such other address(es) as the Party to whom notice is to be given may have furnished to the other Party in writing in accordance herewith. Any such notice shall be deemed to have been given: (a) when delivered if personally delivered on a Business Day (or if delivered or sent on a non-Business Day, then on the next Business Day); (b) on the Business Day after dispatch if sent by nationally-recognized overnight courier; or (c) on the fifth (5th) Business Day following the date of mailing, if sent by registered mail.
10.4.11.Amendment. No amendment, modification or supplement of any provision of this Agreement will be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
10.4.12.Waiver. The failure by either Party hereto to assert any of its rights hereunder, including the right to terminate this Agreement due to a breach or default by the other Party hereto, shall not be deemed to constitute a waiver by that Party of its right thereafter to enforce each and every provision of this Agreement in accordance with its terms.
10.4.13.Severability. If any provision of this Agreement is determined by any court or administrative tribunal of competent jurisdiction to be invalid or unenforceable, the Parties shall negotiate in good faith a replacement provision that is commercially equivalent, to the maximum extent permitted by Applicable Law, to such invalid or unenforceable provision. The invalidity or unenforceability of any provision of this Agreement shall not affect the validity or enforceability of the other provisions of this Agreement. Nor shall the invalidity or unenforceability of any provision of this Agreement in one country or jurisdiction affect the validity or enforceability of such provision in any other country or jurisdiction in which such provision would otherwise be valid or enforceable.
10.4.14.Descriptive Headings. The headings to the articles hereof are not a part of this Agreement but are merely for convenience to assist in locating and reading the articles hereof.
10.4.15.Global Trade Control Laws. The Parties acknowledge that certain activities covered by or performed under this Agreement may be subject to laws, regulations or orders regarding economic sanctions, import controls or export controls (“Global Trade Control Laws”). Each of the Parties will perform all activities under this Agreement in compliance with all applicable Global Trade Control Laws. Furthermore, with respect to the activities performed under this Agreement, each of the Parties represents, warrants and covenants that:
10.4.1.Each Party will not, for activities under this Agreement, (i) engage in any such activities in a Restricted Market; (ii) involve individuals ordinarily resident in a Restricted Market; or (iii) include companies, organizations, or governmental entities from or located in a Restricted Market. “Restricted Market” for purposes of this Agreement means the Crimean Peninsula, Cuba, the Donbass Region, Iran, North Korea, Sudan, and Syria, or any other country or region sanctioned by the US or EU.
10.4.2.Each Party represents and warrants that it is not a Restricted Party and is not owned or controlled by a Restricted Party. With respect to activities performed under this Agreement, neither Party will engage or delegate to any Restricted Parties for any activities under this Agreement. Each Party will screen all relevant Third Parties involved by such Party in the activities under this Agreement under the relevant Restricted Party Lists. “Restricted Parties” for purposes of this Agreement means any individual or entity on any of the following “Restricted Party Lists”: the list of sanctioned entities maintained by the United Nations; the Specially Designated Nationals List and the Sectoral Sanctions Identifications List of the U.S. Treasury Department’s Office of Foreign Assets Control; the U.S. Denied Persons List, the U.S. Entity List, and the U.S. Unverified List of the U.S. Department of Commerce; entities subject to restrictive measures and the Consolidated List of Persons, Groups and Entities Subject to E.U.
Financial Sanctions, as implemented by the E.U. Common Foreign & Security Policy; the List of Excluded Individuals / Entities published by the U.S. Health and Human Services’ Office of Inspector General; any lists of prohibited or debarred parties established under the U.S. Federal Food Drug and Cosmetic Act; the list of parties suspended or debarred from contracting with the U.S. government; and similar lists of restricted parties maintained by the Governmental Authorities of the countries that have jurisdiction over the activities conducted under this Agreement.
10.4.3.Neither Party will knowingly transfer to the other Party any goods, software, technology or services that are (i) controlled under the U.S. International Traffic in Arms Regulations or at a level other than EAR99 under the U.S. Export Administration Regulations; or (ii) specifically identified as an E.U. Dual Use Item or on an applicable export control list of another country.
10.5.Anti Bribery and Anti-Corruption
10.5.1.Anti Bribery and Anti-Corruption. Both Parties shall comply fully at all times with the Applicable Law, including applicable anti-corruption laws. Each Party shall be entitled to terminate this Agreement for cause in accordance with Sections
9.2 and
9.3.2 (as applicable), if the other Party fails to perform its obligations under this Section.
10.5.2.Ethical Standards and Human Rights. Each Party warrants that in relation to the activities to be performed under this Agreement, (i) it does not employ, engage or otherwise use any child labor in circumstances such that the activities performed by any such child labor could reasonably be foreseen to cause either physical or emotional impairment to the development of such child; (ii) it does not use forced labor in any form (prison, indentured, bonded or otherwise) and its employees are not required to lodge papers or deposits on starting work; (iii) it provides a safe and healthy workplace, presenting no immediate hazards to its employees. Any housing provided by a Party to its employees is safe for habitation. Each Party provides access to clean water, food, and emergency healthcare to the employees in the event of accidents or incidents at the Party’s workplace; (iv) it does not discriminate against any employees on any ground (including race, religion, disability or gender); (v) it does not engage in or support the use of corporal punishment, mental, physical, sexual or verbal abuse and does not use cruel or abusive disciplinary practices in the workplace; (vi) it pays each employee at least the minimum wage under Appliable Laws, and provides each employee with all legally mandated benefits; (vii) complies with the laws on working hours and employment rights in the countries in which it operates; and (viii) is respectful of its employees’ right to join and form independent trade unions and freedom of association.
10.5.3.Ethical Care of Animals. To the extent applicable, each Party shall comply with the Applicable Law for the care, welfare, and ethical treatment of animals. In conducting any research involving the use of animals, the Parties shall comply with the “3R Principles”, i.e., reducing the number of animals used, replacing animals with non-animal methods whenever possible and refining the research techniques used. Each Party shall comply, as a minimum, with the following core principles regarding the care, welfare and ethical treatment of animals: (i) ensure access to species appropriate food and water; (ii) ensure access to species specific housing, including species appropriate temperature and humidity levels; (iii) ensure access to veterinary care; (iv) ensure ability to demonstrate species specific behavior; (iv) ensure that the study design is reviewed by institutional ethical review panel; (v) commit to minimizing pain and distress during in vivo studies; and (vi) appropriately train the staff which will work with animals. A Party may, at its own costs and expenses, conduct reasonable inspections of the facilities of the other Party on reasonable notice to confirm adherence to the above principles and guidelines. To the extent that any material deficiencies are identified as the result of such inspection, the respective Party shall take reasonable and practical corrective measures to remedy any such material deficiencies.
10.6.Dispute Resolution.
10.6.1.Escalation. Unless otherwise set forth in this Agreement, in the event of any dispute arising out of or in connection with this Agreement, including any alleged breach under this Agreement or any dispute relating to the validity, performance, construction or interpretation of this Agreement, the Parties shall refer such dispute to their respective CEO (or its C-level delegate), who shall meet in person or via video conference within [***] to attempt to resolve such matter in good faith. If the CEOs (or their C-level delegates) fail to reach agreement as to such matter for a period in excess of [***] from their initial meeting, either Party may submit the dispute to arbitration in accordance with Section
11.13.3 below.
10.6.2.Governing Law. This Agreement is governed by and construed in accordance with, and all disputes arising under or in connection with this Agreement shall be resolved in accordance with, laws of Belgium, without regard to conflict of law principles thereof.
10.6.3.Arbitration. Any dispute arising out of or in connection with this Agreement, including any issue relating to the validity, performance, construction or interpretation of this Agreement, which cannot be resolved amicably between the Parties after following the procedure set forth in Section
11.13.1 shall be submitted to and settled by arbitration in accordance with the arbitration rules of the World Intellectual Property Organization (“WIPO”) in effect on the date of the commencement of the arbitration proceedings. The existence, nature and details of any such dispute and all communications between the Parties related thereto, shall be considered Confidential Information of the Parties and shall be treated in accordance with the terms of Article
7 above. Any Confidential Information may be disclosed by either Party to counsel, experts or other advisors on the arbitration under obligations of confidentiality. The decision of the arbitrators shall be final and binding upon the Parties. The location of arbitration will be Brussels, Belgium. The arbitration will be heard and determined by three (3) arbitrators, with one arbitrator being appointed by each Party and the third arbitrator being appointed by the WIPO. The language of the arbitration proceeding will be English. Notwithstanding the provisions of this Section
11.13, each Party shall have the right to seek interim injunctive relief in any court of competent jurisdiction as such Party deems necessary to preserve its rights and to protect its interests.
10.6.4.Expert Determination. When the Agreement provides for Expert Determination if the Parties fail to agree on a specific matter, after having followed the escalation process in Section 11.13.1, the following procedure shall be carried out: Either Party may submit such failure to agree for final resolution to expert determination, to be held in English, in accordance with the Expert Determination Rules of the WIPO and as further specified in this Section 11.13.4 (“Expert Determination”). For clarity, such failure to agree shall not be brought before arbitration under Section 11.13.3. Within [***] following a Party’s receipt of notice from the other Party that it wants to move to Expert Determination, the Parties shall meet and shall attempt to agree on one (1) independent Third Party expert with at least ten (10) years of experience in the licensing, development and commercialization of pharmaceutical vaccines or other pharmaceutical products. If the Parties cannot agree on such expert within such time period, then the expert will be appointed by the WIPO Arbitration and Mediation Center after consultation with the Parties. Within [***] of the expert’s appointment, the Parties will provide the expert with a copy of the Agreement (including the Exhibits annexed thereto) and will arrange a meeting in order to (a) inform the expert of the Expert Determination terms set forth below and (b) agree on timelines for the Expert Determination:
(i)Each Party will provide the expert with a complete, written proposal of such Party’s solution of the matter on which the Parties have failed to agree, along with any documentary or other evidence it wishes to provide in support for such proposal;
(ii)Each Party will share its proposal with the other Party, who can then comment on such proposal;
(iii)If deemed necessary, the expert will organize a meeting with both Parties (but never with one Party without the other) to inform the expert’s determination and to perform independent research and analysis. Insofar as possible, the timeline for (i) to (iii) shall not be longer than [***].
(iv)The expert will select one of the Parties’ proposals without modification, as far as possible no later than the expiry of the [***] period referred to above. The expert will choose such proposal which the expert believes is most consistent with the intent of the Parties when this Agreement was entered into provided the expert may not alter the terms of this Agreement. The expert will deliver his or her decision regarding the disputed matter in writing, which decision will be binding and conclusive upon both Parties as if the applicable payments and royalty rates had been specified in this Agreement. The Party whose proposal is not selected by the expert is responsible for any reasonable and predetermined fees of the expert, customary in the industry, and the costs and expenses of the Expert Determination.
10.6.5.Entire Agreement. This Agreement, together with all Exhibits attached hereto, constitutes the entire agreement between the Parties regarding the subject matter hereof, and supersedes all prior agreements, understandings and communications between the Parties, with respect to the subject matter hereof. No modification or amendment of this Agreement shall be binding upon the Parties unless in writing and executed by the duly authorized representative of each of the Parties; this shall also apply to any change of this.
10.6.6.Independent Contractors. The Parties are independent contractors and this Agreement shall not constitute or give rise to an employer-employee, agency, partnership or joint venture relationship among the Parties and each Party's performance hereunder is that of a separate, independent entity.
10.6.7.Counterparts. This Agreement may be executed in any number of counterparts, by original or electronic (including "pdf") signature, each of which shall be deemed an original but all of which together shall constitute one and the same instrument.
10.6.8.No Third Party Rights or Obligations. None of the provisions of this Agreement shall be for the benefit of or enforceable by any Third Party which shall be a Third-Party beneficiary to this Agreement.
10.6.9.Electronic Signatures. The Parties agree that electronic signatures, within the meaning of the European “Electronic identification and trust services” regulation, known as the “eIDAS regulation” of 1 July 2016, have the same legal value and evidentiary force as original handwritten signatures, and that in the event of a dispute or claim arising out of this Agreement, each of the Parties hereby waives the right to assert any defense and/or waiver based on the signature of this Agreement by electronic signature.
Each electronically signed copy of this Agreement shall also be admissible in evidence and shall be fully binding on each Party that signed it.
After electronic signature of this Agreement by all the Parties, it is understood that the certificate of realization of the electronic signatures is transmitted by the Party initiating the electronic signature circuit \to all the other Parties.
(Signature page follows.)
IN WITNESS WHEREOF, authorized Representatives of the Parties have duly executed this Agreement to be effective as of the Effective Date.
VALNEVA AUSTRIA GMBH LIMMATECH Biologics AG
Date: 31-07-2024 Date: 31-07-2024
Name: [***] Name: [***]
Title: [***] Title: [***]
EXHIBIT 1.21
Shigella Development Budget
[***]
EXHIBIT 1.26
Development Plan
[***]
EXHIBIT 1.49
Licensed Know-How
[***]
EXHIBIT 1.54
LIMMATECH MATERIALS
[***]
EXHIBIT 1.55
LimmaTech Patent Rights
[***]
EXHIBIT 1.58
LimmaTech Third Party Agreements
[***]
EXHIBIT 2.3.2 Part A
[***] GLOBAL HEALTH PARTNER(S) ON THE EFFECTIVE DATE MINIMUM TERMS AND CONDITIONS REASONABLE AND NECESSARY TO BE INCLUDED IN THE GLOBAL HEALTH LICENSE relating to the Development, Manufacture and Commercialization of the Licensed Product in the LMIC Territory
[***]
EXHIBIT 2.3.2 Part B
[***]
EXHIBIT 3.6.1
Payments to Third Party Licensors for the LMIC Territory
[***]
EXHIBIT 4.2.2
Current Subcontractors of LimmaTech
[***]
EXHIBIT 7.5.1
Draft Joint Press Release
Draft (v7)
Valneva and LimmaTech Enter into a Strategic Partnership to Accelerate the Development of the World’s Most Clinically Advanced Tetraval ent Shigella Vaccine Candidate
■Valneva obtains exclusive worldwide license for LimmaTech’s S4V Shigella vaccine candidate and adds an attractive Phase 2 clinical asset to Valneva's R&D pipeline
■LimmaTech to receive upfront payment, is eligible for future milestone and royalty payments, and will collaborate on S4V clinical development through Phase 2
■Valneva will host a live webcast on this announcement at 3 p.m. CEST/9 a.m. EDT today. Please refer to this link: https://edge.media-server.com/mmc/p/ck932u2n
Saint-Herblain, (France) and Schlieren (Zurich), August XX, 2024 - Valneva SE (Nasdaq: VALN; Euronext Paris: VIA), a specialty vaccine company and LimmaTech Biologics AG. a clinical-stage biotech company developing vaccines for the prevention of life-threatening diseases, today announced that the companies have entered into a strategic partnership and exclusive licensing agreement for the development, manufacturing and commercialization of Sh gella4V (S4V), a tetravelant bioconjugate vaccine candidate against shigellosis.
Shigellosis, caused by Shigella bacteria, is the second leading cause of fetal diarrheal disease worldwide. It is estimated that up to 165 million cases of disease and an estimated 600,000 deaths ate attributed to Shigella each year, particularly among children in Low- and Midde-lncome Countries (LMICs). No approved Shigella vaccine is currently available and the development of Shigella vaccines has been identified as a priority by the World Health Organization (WHO). Shigellosis also affects international travelers from high-income countries and deployed military personnel in endemic regions. The global market for a vaccine against Shigella is estimated to exceed $500 million annually.
Under the terms of the agreement with Valneva, LimmaTech will receive an upfront payment of €10 million and be eligible to receive additional regulatory, development and sales-based milestone payments as well as low double-digit royalties on sales. LimmaTech will be responsible for conducting a Phase 2 Controlled Human Infection Model (CHIM) and a Phase 2 pediatric study in LMIC=. Both clinical trials are expected to begin in the second half of 2024. Vanneva will assume assume all further development, including CMC (chemistry, manufacturing and controls) and regulatory activities and be responsible for its commercialization worldwide if approved.
Thomas Lingelbach, Chief Executive Officer of Valneva, commented, “We are very pleased to partner with LimmaTech to advance a promising program in an area of high unmet medical need. The Shigella vaccine candidate enables a potential first-in-class vaccine solution for both LMICs and travelers and, as such, represents a potentially highly synergistic product for Valneva. The anticipated development path follows a staggered and risk-mitigated strategy, and hence allows an efficient capital allocation in line with our communicated plan of having a new R&D program in Phase 3 by 2027.”
Dr. Franz-Werner Haas, Chief Executive Officer of LimmaTech, said, “Having developed the S4V Shigella vaccine candidate from its early discovery phase to the promising clinical data we achieved to date, we are excited to accelerate the program with our partnership with Valneva. Their proven expertise in late-stage development and commercialization of vaccines will expedite potential market approval and bring a Shigella vaccine to people in need. This agreement underscores our capabilities to leverage LimmaTech’s proficiency in vaccine development with the best path to develop programs rapidly. We continue to expand our pipeline of vaccine candidates to combat microbial-based infectious diseases, providing protection against antimicrobial resistance, a dramatically increasing global health threat.”
LimmaTech initiated the tetravalent Shigella vaccine candidate and continued to lead its development as part of its ongoing collaboration with GSK, and later in-licensed the vaccine candidate from GSK. In February 2024, LimmaTech reported positive interim Phase 1/2 data for the S4V vaccine candidate, including a favorable safety and tolerability profile as well as robust data on immunogenicity against the four most common pathogenic Shigella serotypes, S. flexneri 2a, 3a, 6, and S. sonnei. The results of the completed Phase 1/2 study confirmed the interim data.
About Shigellosis
Shigellosis is a global health threat caused by the Gram-negative Shigella bacteria. It is estimated that up to 165 million infections are due to Shigella of which 62.3 million occur in children younger than five years. Diarrheal infection is one of the major causes of morbidity and mortality in numerous countries as well as in travelers and deployed military personnel in endemic regions. There are an estimated 600,000 deaths attributed to Shigella each year and it is the second leading cause for diarrheal deaths. The standard treatment for shigellosis is oral rehydration and antibiotic therapy, however, the bacteria have acquired resistance to many antibiotics with numerous reports of outbreaks of multidrug-resistant strains, making treatment extremely difficult. Currently, no licensed Shigella vaccine is available.
About Valneva SE
We are a specialty vaccine company that develops, manufactures, and commercializes prophylactic vaccines for infectious diseases addressing unmet medical needs. We take a highly specialized and targeted approach, applying our deep expertise across multiple vaccine modalities, focused on providing either first-, best- or only-in-class vaccine solutions.
We have a strong track record, having advanced multiple vaccines from early R&D to approvals, and currently market three proprietary travel vaccines, including the world’s first and only chikungunya vaccine, as well as certain third-party vaccines.
Revenues from our growing commercial business help fuel the continued advancement of our vaccine pipeline. This includes the only Lyme disease vaccine candidate in advanced clinical development, which is partnered with Pfizer, as well as vaccine candidates against the Zika virus and other global public health threats.
More information is available at www.valneva.com.
About LimmaTech Biologics AG
LimmaTech Biologics is at the forefront of combating the global antimicrobial resistance epidemic based on its unparalleled track record in vaccine technology and clinical candidate development. The company is leveraging its proprietary self-adjuvanting and multi-antigen vaccine platform alongside additional disease-specific vaccine approaches to prevent increasingly untreatable microbial infections. With decades of expertise and an expanding, robust pipeline, the LimmaTech team is dedicated to generating protective solutions to deliver transformative value worldwide. LimmaTech Biologics is backed by specialist healthcare investors, including Adjuvant Capital, AXA IM Alts, Novo Holdings REPAIR Impact Fund, and Tenmile.
|
|
|
|
|
|
|
Valneva Investor and Media Contacts
Laetitia Bachelot-Fontaine
VP Global Communications & European Investor Relations
M +33 (0)6 4516 7099
laetitia.bachelot-fontaine@valneva.com
|
Joshua Drumm, Ph.D.
VP Global Investor Relations
M +001 917 815 4520
joshua.drumm@valneva.com
|
For LimmaTech
LimmaTech Biologics AG
Franz-Werner Haas, CEO
E-mail: media@lmtbio.com
For media enquiries
Trophic Communications
Sara Ortiz or Jacob Verghese
Phone: +49 151 7441 6179
E-mail: limmatech@trophic.eu
Forward-Looking Statements
This press release contains certain forward-looking statements relating to the business of Valneva, including with respect to business partnerships, the progress, timing, results and completiori of research, development and clinical trials for product candidates, to regulatory approval of product candidates and review of existing products. In addition, even if the actual results or development of Valneva are consistent with the forward-looking statements contained in this press release, those results or developments of Valneva may not be sustained in the future. In some cases, you can identify forward-looking statements by words such as “could,’ ’should," “may." ’expects,' "anticipates,' ’believes.' ‘intends," ’estimates,’ ’aims ' ‘targets.' or similar words. These forward-looking statements are based largely on the current expectations of Valneva as of the date of this press release and are subject to a number of known and unknown risks and uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievement expressed or implied by these forward-looking statements In particular, the expectations of Valneva could be affected by, among other things, uncertainties and delays involved in the development and manufacture of vaccines, unexpected clinical trial results, unexpected regulatory actions or delays, competition in general, currency fluctuations, the impact of 1he global and European financing env'ronment, and 1he ability to obtain or maintain patent or other proprietary intellectual property protection. Success in preclinical studies or earier clinical trials may not be indicative of results in future clinical trials.
In light of these risks and uncertainties, there can be no assurance that the forward-looking statements made in this press release will in fact be realized Valneva is providing this information as of the date of this press release and disclaims any intention or obligation to publicly update or revise any forward-looking statements, whether as a result of nevi information future events or otherwise Additional Non-financial Obligations and Covenants under LimmaTech Third Party Agreements
EXHIBIT 8.8.1
[***]
EX-4.16
7
exhibit416-dlacontractreda.htm
EX-4.16
Document
Exhibit 4.16
CERTAIN INFORMATION HAS BEEN EXCLUDED FROM THIS AGREEMENT (INDICATED BY “[***]”) BECAUSE SUCH INFORMATION (I) IS NOT MATERIAL AND (II) IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SOLICITATION/CONTRACT/ORDER FOR COMMERCIAL ITEMS
OFFEROR TO COMPLETE BLOCKS 12, 17, 23, 24, & 30
|
1. REQUISITION NUMBER
1000186747
|
PAGE 1 OF 9 |
|
2. CONTRACT NO.
SPE2DP-25-D-0002
|
3. AWARD/EFFECTIVE
DATE
2025 JAN 29
|
4. ORDER NUMBER |
5. SOLICIATION NUMBER
SPE2DP-24-R-0002
|
6. SOLICIATION ISSUE
DATE
2024 NOV 07
|
7. FOR SOLICITATION INFORMATION CALL: |
a. NAME |
b. TELEPHONE NUMBER (No collect calls) |
8. OFFER DUE DATE/
LOCAL TIME |
|
9. ISSUED BY CODE
DLA TROOP SUPPORT
MEDICAL SUPPLY CHAIN PHARM FSA
700 ROBBINS AVENUE
PHILADELPHIA PA 19111
USA
|
SPE2DP |
10. THIS QCQUISITION IS
SMALL BUSINESS
HUBZONE SMALL BUSINESS
SERVICE-DISABLED
VETERAN-OWNED SMALL BUSINESS
|
UNRESTRICTED OR SET ASIDE: _____ % FOR:
WOMEN-OWNED SMALL BUSINESS
(WOSB) ELIGIBLE UNDER THE WOMEN-OWNED SMALL BUSINESS PROGRAM
EDWPSB NAICS: 325414
8 (A) SIZE STANDARD: 1250
|
|
|
Local Admin: [***] PCPQEA6 Tel: [***] Fax: [***]
Email: [***]
|
|
11. DELIVERY FOR FOB DESTINA TION UNLESS BLOCK IS
MARKED
SEE SCHEDULE
|
12. DISCOUNT TERMS
Net 30 days
|
13.a. THIS CONTRACT IS A RATED ORDER UNDER DPAS (15 CFR 700) |
13b. RATING |
|
14. METHOD OF SOLICITATION
RFQ IFB RFB
|
|
15. DELIVER TO
SEE SCHEDULE
|
|
16. ADMINISTERED BY CODE
SEE BLOCK B
Critically: PAS: None
|
SPE2DP |
|
|
17a. CONTRACTOR/ CODE
OFFEROR |
43FMI |
FACILITY
CODE
|
|
18a. PAYMENT WILL BE MADE BY CODE |
SL4701 |
|
|
|
|
VALNEVA USA, INC.
4550 MONTGOMERY AVE STE 460
BETHESDA MD 20814-3304
USA
TELEPHONE NO. 3015564500
|
DEF FIN AND ACCOUNTING SVC
BSM
P O BOX 182317
COLUMBUS OH 43218-2317
USA
|
|
17b. CHECK IF REMITTANCE IS DIFFERENT AND PUT SUCH ADDRESS IN OFFER |
18b. SUBMIT INVOICES TO ADDRESS SHOWN IN BLOCK 18a UNLESS BLOCK
BELOW IS CHECKED. SEE ADDENDUM |
19.
ITEM NO. |
20.
SCHEDULE OF SUPPLIES/SERVICES |
21.
QUANTITY |
22.
UNIT |
23.
UNIT PRICE |
24.
AMOUNT |
|
See Schedule |
|
|
|
|
25. ACCOUNTING AND APPROPRIATION DATA |
26. TOTAL AWARD AMOUNT (For Govt. Use Only)
$[***]
|
|
27b. SOLICITATION INCORPORATES BY REFERENCE FAR 52.212-1, 52.212-4. FAR 52.212-3 AND 52.212-5 ARE ATTACHED. ADDENDA ARE ARE NOT ATTACHED.
27b. CONTRACT/PURCHASE ORDER INCORPORATES BY REFERENCE FAR 52.212-4. FAR 52.212-5 IS ATTACHED. ADDENDA ARE ARE NOT ATTACHED.
|
|
28. CONTRACTOR IS REQUIRED TO SIGN THIS DOCUMENT AND RETURN 1
COPIES TO ISSUING OFFICE. CONTRACTOR AGREES TO FURNISH AND
DELIVER ALL ITEMS SET FORTH OR OTHERWISE IDENTIFIED ABOVE AND ON ANY ADDITIONAL SHEETS SUBJECT TO THE TERMS AND CONDITIONS SPECIFIED
|
29. AWARD OF CONTRACT: REF. _SPE2DP-24-R-0002_____________________ OFFER
DATED 2024-Dec-16 . YOUR OFFER ON SOLICIATION (BLOCK 5), INCLUDING ANY ADDITIONS OR CHANGES WHICH ARE SET FORTH,
HEREIN IS ACCEPTED AS TO ITEMS: JEV
|
|
30a. SIGNATURE OF OFFEROR/CONTRACTOR
[***]
|
31a. UNITED STATES OF AMERICA (SIGNATURE OF CONTRACTING OFFICER)
[***]
|
|
30b. NAME AND TITLE OF SIGNER (Type or Print)
[***]
|
30c. DATE SIGNED |
31b. NAME OF CONTRACTING OFFICER (Type or Print)
[***]
|
31c. DATE SIGNED
2025 JAN 29
|
AUTHORIZED FOR LOCAL REPRODUCTION STANDARD FORM 1449 (REV. 2/2012)
PREVIOUS EDITION IS NOT USABLE Prescribed by GSA – FAR (48 CFR) 53.212
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
19.
ITEM NO. |
20.
SCHEDULE OF SUPPLIES/SERVICES |
21.
QUANTITY |
22.
UNIT |
23.
UNIT PRICE |
24.
AMOUNT |
|
|
|
|
|
|
|
32a. QUANTITY IN COLUMN 21 HAS BEEN
RECEIVED INSPECTED ACCEPTED, AND CONFORMS TO THE CONTRACT, EXCEPT AS NOTED:
|
32b. SIGNATURE OF AUTHORIZED GOVERNMENT
REPRESENTATIVE |
32c. DATE |
32D. PRINTED NAME AND TITLE OF AUTHORIZED GOVERNMENT
REPRESENTATIVE |
32e. MAILING ADDRESS OF AUTHORIZED GOVERNMENT REPRESENTATIVE |
32f. TELEPHONE NUMBER OF AUTHORIZED GOVERNMENT REPRESENTATIVE |
32g. E-MAIL OF AUTHORIZED GOVERNMENT REPRESENTATIVE |
33. SHIP NUMBER |
34. VOUCHER NUMBER |
35. AMOUNT VERIFIED
CORRECT FOR |
36. PAYMENT
COMPLETE PARTIAL FINAL
|
37. CHECK NUMBER |
PARTIAL FINAL |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
38. S/R ACCOUNT NO. |
39. S/R VOUCHER NUMBER |
40. PAID BY |
41a. I CERTIFY THIS ACCOUNT IS CORRECT AND PROPER FOR PAYMENT |
42a. RECEIVED BY (Print) |
41b. SIGNATURE AND TITLE OF CERTIFYING OFFICER |
41c. DATE |
42b. RECEIVED AT (Location) |
42c. DATE REC'D (YY/MM/DD) |
42d. TOTAL CONTAINERS |
STANDARD FORM 1449 (REV.
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 4 OF 9 PAGES |
SECTION B - SUPPLIES OR SERVICES AND PRICES OR COSTS
**********
2/2012) BACK The following documents are hereby incorporated by reference into this contract: Solicitation SPE2DP-24-R-0002 amendments 0001-0002, and offer submitted by Valneva USA, Inc. in response to Solicitation SPE2DP-24-R-0002, which is being accepted by the Government to form this contract.
IMPORTANT NOTE: DISREGARD BLOCK 26. TOTAL AWARD AMOUNT ON PAGE 1 OF THE AWARD DOCUMENT.
The Government is only bound to order the guaranteed minimum quantity. See below for the guaranteed minimum quantity, unit price, and guaranteed minimum dollar value.
**********
This is a Firm Fixed Price, Indefinite Delivery/Indefinite Quantity type contract for:
Item: Japanese Encephalitis Virus Vaccine, Purified, Inactivated, Single-Dose Prefilled Syringe (JEV)
NSN: 6505-01-607-7018
Medical Procurement Item Description: JEV shall be made in accordance with MPID Number 4 dated 30 January 2020
Quantities & Unit Price
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minimum Quantity |
Estimated Quantity |
Maximum Quantity |
Unit Price |
Guaranteed Minimum Dollar Value |
Maximum Contract Dollar Value |
Base Year |
200,060 |
[***] |
300,300 |
$164.16 |
$32,841,849.60 |
$49,297,248 |
The Government is only bound to order the guaranteed minimum quantity of 200,060 each at $164.16 for a guaranteed minimum dollar value of $32,841,849.60.
2. The estimated quantity refers to the Government's good faith estimate of the requirements during a specified contract tier. The estimated quantity does not obligate the government to order a specific quantity, it is provided for informational purposes only.
3. The Contract Maximum Quantity is 300,300 each. The Government is not obligated to order the maximum Contract Quantity; however, the Government has the legal right to order up to this quantity prior to the final expiration of the resultant contract. The Government reserves the right to place orders up to the Maximum Contract Quantity, but not to exceed [***] each in any given [***] period. The contract maximum dollar value is $49,297,248.
4. The effective ordering period for this contract shall be from the date of award through one year thereafter. The Government may issue delivery orders during the effective ordering period up to the contract maximum quantity in accordance with Clause 52.216-19 Order Limitations. Delivery orders will be placed via DD Form 1155.
5. Delivery shall be FOB Destination and delivered within 120 days from the effective date of the delivery order (DD Form 1155).
6. Delivery Destination (which shall be indicated on each delivery order):
DODAAC: SV3100
DLA Distribution Susquehanna
U Avenue, Bldg. 89, Door 6
New Cumberland, PA 17070 Attn: [***]
7. Inspection / Acceptance: Destination
8. Place of Performance:
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 5 OF 9 PAGES |
SECTION B - SUPPLIES OR SERVICES AND PRICES OR COSTS (CONTINUED)
Valneva SE
Oakbank Park Road
Livingston EH53 0TG
Scotland
9. In accordance with 52.211-16 the Variation in Quantity is 2% increase / 0% decrease. This increase/decrease shall apply to all CLINs on the delivery order (DD Form 1155).
SECTION F - DELIVERIES OR PERFORMANCE
52.247-34 F.O.B. DESTINATION (JAN 1991) FAR
SECTION I - CONTRACT CLAUSES
52.204-19 INCORPORATION BY REFERENCE OF REPRESENTATIONS AND CERTIFICATIONS (DEC 2014) FAR
252.204-7009 LIMITATIONS ON THE USE OR DISCLOSURE OF THIRD-PARTY CONTRACTOR REPORTED CYBER INCIDENT INFORMATION (JAN 2023) DFARS
252.225-7001 BUY AMERICAN AND BALANCE OF PAYMENTS PROGRAM - BASIC (FEB 2024) DFARS
252.225-7002 QUALIFYING COUNTRY SOURCES AS SUBCONTRACTORS (MAR 2022) DFARS
252.225-7012 PREFERENCE FOR CERTAIN DOMESTIC COMMODITIES (APR 2022) DFARS
(a) Definitions. As used in this clause --
“Component” means any item supplied to the Government as part of an end product or of another component.
“End product” means supplies delivered under a line item of this contract.
"Qualifying country" means a country with a reciprocal defense procurement memorandum of understanding or international agreement with the United States in which both countries agree to remove barriers to purchases of supplies produced in the other country or services performed by sources of the other country, and the memorandum or agreement complies, where applicable, with the requirements of section 36 of the Arms Export Control Act (22 U.S.C. 2776) and with 10 U.S.C. 2457. Accordingly, the following are qualifying countries:
Australia
Austria
Belgium
Canada
Czech Republic
Denmark
Egypt
Estonia
Finland
France
Germany
Greece
Israel
Italy
Japan
Latvia
Lithuania
Luxembourg
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 6 OF 9 PAGES |
SECTION I - CONTRACT CLAUSES (CONTINUED)
Netherlands
Norway
Poland
Portugal
Slovenia
Spain
Sweden
Switzerland
Turkey
United Kingdom of Great Britain and Northern Ireland.
“Structural component of a tent” --
(1) Means a component that contributes to the form and stability of the tent (e.g., poles, frames, flooring, guy ropes, pegs); and
(2) Does not include equipment such as heating, cooling, or lighting.
“United States” means the 50 States, the District of Columbia, and outlying areas.
“U.S.-flag vessel” means a vessel of the United States or belonging to the United States, including any vessel registered or having national status under the laws of the United States.
(b) The Contractor shall deliver under this contract only such of the following items, either as end products or components, that have been grown, reprocessed, reused, or produced in the United States:
(1) Food.
(2) Clothing and the materials and components thereof, other than sensors, electronics, or other items added to, and not normally associated with, clothing and the materials components thereof. Clothing includes items such as outerwear, headwear, underwear, nightwear. footwear, hosiery, handwear, belts, badges, and insignia.
(3)(i) Tents and structural components of tents;
(ii) Tarpaulins; or
(iii) Covers.
(4) Cotton and other natural fiber products.
(5) Woven silk or woven silk blends.
(6) Spun silk yarn for cartridge cloth.
(7) Synthetic fabric, and coated synthetic fabric, including all textile fibers and yarns that are for use in such fabrics.
(8) Canvas products.
(9) Wool (whether in the form of fiber or yarn or contained in fabrics, materials, or manufactured articles).
(10) Any item of individual equipment (Federal Supply Class 8465) manufactured from or containing fibers, yarns, fabrics, or materials listed in this paragraph (b).
(c) This clause does not apply --
(1) To items listed in section 25.104(a) of the Federal Acquisition Regulation, or other items for which the Government has determined that a satisfactory quality and sufficient quantity cannot be acquired as and when needed at U.S. market prices;
(2) To incidental amounts of cotton, other natural fibers, or wool incorporated in an end product, for which the estimated value of the cotton, other natural fibers, or wool --
(i) Is not more than 10 percent of the total price of the end product; and
(ii) Does not exceed the threshold at Defense Federal Acquisition Regulation Supplement 225.7002-2(a);
(3) To waste and byproducts of cotton or wool fiber for use in the production of propellants and explosives;
(4) To foods, other than fish, shellfish, or seafood, that have been manufactured or processed in the United States, regardless of where the foods (and any component if applicable) were grown or produced. Fish, shellfish, or seafood manufactured or processed in the United States and fish, shellfish, or seafood contained in foods manufactured or processed in the United States shall be provided in accordance with paragraph (d) of this clause;
(5) To chemical warfare protective clothing produced in a qualifying country; or
(6) To fibers and yarns that are for use in synthetic fabric or coated synthetic fabric (but does apply to the synthetic or coated synthetic fabric itself), if --
(i) The fabric is to be used as a component of an end product that is not a textile product. Examples of textile products, made in whole or in part of fabric, include ¾
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 7 OF 9 PAGES |
SECTION I - CONTRACT CLAUSES (CONTINUED)
(A) Draperies, floor coverings, furnishings, and bedding (Federal Supply Group 72, Household and Commercial Furnishings and Appliances);
(B) Items made in whole or in part of fabric in Federal Supply Group 83, Textile/leather/furs/apparel/findings/tents/flags, or Federal Supply Group 84, Clothing, Individual Equipment and Insignia;
(C) Upholstered seats (whether for household, office, or other use); and
(D) Parachutes (Federal Supply Class 1670); or
(ii) The fibers and yarns are para-aramid fibers and continuous filament para-aramid yarns manufactured in a qualifying country.
(d)(1) Fish, shellfish, and seafood delivered under this contract, or contained in foods delivered under this contract --
(i) Shall be taken from the sea by U.S.-flag vessels; or
(ii) If not taken from the sea, shall be obtained from fishing within the United States; and
(2) Any processing or manufacturing of the fish, shellfish, or seafood shall be performed on a U.S.-flag vessel or in the United States.
(End of clause)
52.226-8 ENCOURAGING CONTRACTOR POLICIES TO BAN TEXT MESSAGING WHILE DRIVING (MAY 2024) FAR
52.232-40 PROVIDING ACCELERATED PAYMENTS TO SMALL BUSINESS SUBCONTRACTORS (MAR 2023) FAR
252.232-7006 WIDE AREA WORKFLOW PAYMENT INSTRUCTIONS (JAN 2023) DFARS
As prescribed in 232.7004 (b), use the following clause:
(a) Definitions. As used in this clause -
Department of Defense Activity Address Code (DoDAAC) is a six position code that uniquely identifies a unit, activity, or organization.
Document type means the type of payment request or receiving report available for creation in Wide Area WorkFlow (WAWF).
Local processing office (LPO) is the office responsible for payment certification when payment certification is done external to the entitlement system.
Payment request and receiving report are defined in the clause at 252.232-7003, Electronic Submission of Payment Requests and Receiving Reports.
(b) Electronic invoicing. The WAWF system provides the method to electronically process vendor payment requests and receiving reports, as authorized by Defense Federal Acquisition Regulation System (DFARS) 252.232-7003, Electronic Submission of Payment Requests and Receiving Reports.
(c) WAWF access. To access WAWF, the Contractor shall -
(1) Have a designated electronic business point of contact in the System for Award Management at https://www.sam.gov and
(2) Be registered to use WAWF at https://wawf.eb.mil/ following the step-by-step procedures for self-registration available at this Web site.
(d) WAWF training. The Contractor should follow the training instructions of the WAWF Web-Based Training Course and use the Practice Training Site before submitting payment requests through WAWF. Both can be accessed by selecting the “Web Based Training” link on the WAWF home page at https://wawf.eb.mil/
(e) WAWF methods of document submission. Document submissions may be via Web entry, Electronic Data Interchange, or File Transfer Protocol.
(f) WAWF payment instructions. The Contractor shall use the following information when submitting payment requests and receiving reports in WAWF for this contract or task or delivery order:
(1) Document type. The Contractor shall submit payment requests using the following document type(s):
(i) For cost-type line items, including labor-hour or time-and-materials, submit a cost voucher.
(ii) For fixed price line items -
(A) That require shipment of a deliverable, submit the invoice and receiving report specified by the Contracting Officer.
(Contracting Officer: Insert applicable invoice and receiving report document type(s) for fixed price line items that require shipment of a deliverable.)
Invoice and Receiving Report Combo
(B) For services that do not require shipment of a deliverable, submit either the Invoice 2in1, which meets the requirements for the invoice and receiving report, or the applicable invoice and receiving report, as specified by the Contracting Officer.
n/a
(Contracting Officer: Insert either “Invoice 2in1” or the applicable invoice and receiving report document type(s) for fixed price line items for services.)
(iii) For customary progress payments based on costs incurred, submit a progress payment request.
(iv) For performance based payments, submit a performance based payment request.
(v) For commercial financing, submit a commercial financing request.
(2) Fast Pay requests are only permitted when Federal Acquisition Regulation (FAR) 52.213-1 is included in the contract.
[Note: The Contractor may use a WAWF “combo” document type to create some combinations of invoice and receiving report in one step.]
(3) Document routing. The Contractor shall use the information in the Routing Data Table below only to fill in applicable fields in WAWF when creating
payment requests and receiving reports in the system.
Routing Data Table *
|
|
|
|
|
|
|
|
|
| FIELD NAME in WAWF |
Data to be entered in WAWF |
Guidance |
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 8 OF 9 PAGES |
SECTION I - CONTRACT CLAUSES (CONTINUED)
|
|
|
|
|
|
|
|
|
Field Name in WAWF |
Data to be entered in WAWF |
Guidance |
Pay Official DoDAAC |
SL4701 |
(If blank, see resulting award) |
Issue By DoDAAC |
SPE2DP |
(If blank, see resulting award) |
Admin DoDAAC |
SPE2DP |
(If blank, see resulting award) |
Inspect By DoDAAC |
|
(If blank, see resulting award) |
Ship To Code |
SEE DELIVERY ORDER (DD1155) |
(If blank, see resulting award) |
Ship From Code |
|
(If blank, see resulting award) |
Mark For Code |
|
(If blank, see resulting award) |
Service Approver (DoDAAC) |
|
(If blank, see resulting award) |
Service Acceptor (DoDAAC) |
|
(If blank, see resulting award) |
Accept at Other DoDAAC |
|
(If blank, see resulting award) |
LPO DoDAAC |
|
(If blank, see resulting award) |
DCAA Auditor DoDAAC |
|
(If blank, see resulting award) |
Other DoDAAC(s) |
|
(If blank, see resulting award) |
(* Contracting Officer: Insert applicable DoDAAC information. If multiple ship to/acceptance locations apply, insert “See Schedule” or “Not applicable.”)
(** Contracting Officer: If the contract provides for progress payments or performance-based payments, insert the DoDAAC for the contract administration office assigned the functions under FAR 42.302(a)(13).)
(4) Payment request. The Contractor shall ensure a payment request includes documentation appropriate to the type of payment request in accordance with the payment clause, contract financing clause, or Federal Acquisition Regulation 52.216-7, Allowable Cost and Payment, as applicable.
(5) Receiving report. The Contractor shall ensure a receiving report meets the requirements of DFARS Appendix F.
(g) WAWF point of contact. (1) The Contractor may obtain clarification regarding invoicing in WAWF from the following contracting activity's WAWF point of contact.
DSCPWAWFTEAM@DLA.MIL
(Contracting Officer: Insert applicable information or “Not applicable.”)
(2) Contact the WAWF helpdesk at 866-618-5988, if assistance is needed.
(End of Clause)
252.232-7010 LEVIES ON CONTRACT PAYMENTS (DEC 2006) DFARS
52.233-3 PROTEST AFTER AWARD (AUG 1996) FAR
252.244-7000 SUBCONTRACTS FOR COMMERCIAL PRODUCTS OR COMMERCIAL SERVICES (NOV 2023) DFARS
252.247-7023 TRANSPORATION OF SUPPLIES BY SEA --- BASIC (OCT 2024) DFARS
Basic. As prescribed in 247.574(b) and (b)(1), use the following clause:
(a) Definitions. As used in this clause --
“Foreign-flag vessel” means any vessel that is not a U.S.-flag vessel.
“Ocean transportation” means any water-borne transportation aboard a ship, vessel, boat, barge, ferry, or the like outside the internal waters of the United States as defined in 33 CFR 2.24.
“Subcontractor” means a supplier, materialman, distributor, or vendor at any level below the prime contractor whose contractual obligation to perform results from, or is conditioned upon, award of the prime contract and who is performing any part of the work or other requirement of the prime contract.
"Supplies” means supplies that are clearly identifiable for eventual use by or owned by DoD at the time of transportation by sea, or are otherwise transported by DoD, regardless of ownership or use by DoD. An item is clearly identifiable for eventual use by DoD if, for example, the contract documentation contains a reference to a DoD contract number or a military destination.
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 9 OF 9 PAGES |
SECTION I - CONTRACT CLAUSES (CONTINUED)
“U.S.-flag vessel” means either a vessel belonging to the United States or a vessel of the United States as that term is defined in 46 U.S.C. 116.;
(b)(1) The Contractor shall use U.S.-flag vessels when transporting any supplies by sea under this contract.
(2) A subcontractor transporting supplies by sea under this contract shall use U.S.-flag vessels if --
(i) This contract is a construction contract; or
(ii) The supplies being transported are --
(A) Other than commercial products; or
(B) Commercial products that --
(1) The Contractor is reselling or distributing to the Government without adding value (generally, the Contractor does not add value to items that it subcontracts for f.o.b. destination shipment);
(2) Are shipped in direct support of U.S. military contingency operations, exercises, or forces deployed in humanitarian or peacekeeping operations; or
(3) Are commissary or exchange cargoes transported outside of the Defense Transportation System in accordance with 10 U.S.C. 2643.
(c) The Contractor and its subcontractors may request, via the Contracting Officer, a waiver of the requirement to use a U.S.-flag vessel, or identification of any available U.S.-flag vessels, if the Contractor or a subcontractor sufficiently explains that --
(1) U.S.-flag vessels are not available at a fair and reasonable rate for commercial vessels of the United States; or
(2) U.S.-flag vessels are otherwise not available.
(d) The Contractor must submit any request for use of foreign-flag vessels in writing to the Contracting Officer at least 45 days prior to the sailing date necessary to meet its delivery schedules. The Contracting Officer will process requests submitted after such date(s) as expeditiously as possible, however, if a DoD waiver is not approved prior to shipper's sailing date, this will not of itself constitute a compensable delay under this or any other clause of this contract. Requests shall contain at a minimum --
(1) Type, weight, and cube of cargo;
(2) Required shipping date;
(3) Special handling and discharge requirements;
(4) Loading and discharge points;
(5) Name of shipper and consignee;
(6) Prime contract number; and
(7) A documented description of current, diligent efforts made to secure U.S.-flag vessels, including points of contact (with names and telephone numbers) with at least two U.S.-flag carriers contacted. Copies of quotes will suffice for this purpose. Copies of telephone notes, emails, and other relevant communications will otherwise be considered for this purpose.
(e) The Contractor shall, within 30 days after each shipment covered by this clause, provide the Contracting Officer and the Maritime Administration, Office of Cargo Preference, U.S. Department of Transportation, 400 Seventh Street SW, Washington, DC 20590, one copy of the rated on board vessel operating carrier's ocean bill of lading, which shall contain the following information:
(1) Prime contract number;
(2) Name of vessel;
(3) Vessel flag of registry;
(4) Date of loading;
(5) Port of loading;
(6) Port of final discharge;
(7) Description of commodity;
(8) Gross weight in pounds and cubic feet if available;
(9) Total ocean freight in U.S. dollars; and
(10) Name of the carrier.
(f) If this contract exceeds the simplified acquisition threshold, the Contractor shall provide with its final invoice under this contract a representation that to the best of its knowledge and belief --
(1) No ocean transportation was used in the performance of this contract;
(2) Ocean transportation was used and only U.S.-flag vessels were used for all ocean shipments under the contract;
(3) Ocean transportation was used, and the Contractor had received a prior-approved waiver for U.S.-flag vessels for all foreign-flag ocean transportation; or
(4) Ocean transportation was used and some or all of the shipments were made on foreign-flag vessels without the written consent of DoD. The Contractor shall describe these shipments in the following format:
|
|
|
|
|
|
|
|
|
|
|
|
* |
ITEM DESCRIPTION |
CONTRACT LINE ITEMS |
QUANTITY |
|
|
|
|
TOTAL |
|
|
|
(g) If this contract exceeds the simplified acquisition threshold and the final invoice does not include the required representation, the Government will reject and return it to the Contractor as an improper invoice for the purposes of the Prompt Payment clause of this contract. In the event there has been unauthorized use of foreign-flag vessels in the performance of this contract, the Contracting Officer is entitled to equitably adjust the contract, based on the unauthorized use.
(h) If the Contractor indicated in response to the solicitation provision, Representation of Extent of Transportation by Sea, that it did not anticipate transporting by sea any supplies; however, after the award of this contract, the Contractor learns that supplies will be transported by sea, the Contractor --(1) Shall notify the Contracting Officer of that fact; and
CONTINUED ON NEXT PAGE
|
|
|
|
|
|
|
|
|
| CONTINUATION SHEET |
REFERENCE NO. OF DOCUMENT BEING CONTINUED:
SPE2DP-25-D-0002 |
PAGE 10 OF 9 PAGES |
SECTION I - CONTRACT CLAUSES (CONTINUED)
(2) Hereby agrees to comply with all the terms and conditions of this clause.
(i) Subcontracts. In the award of subcontracts, for the types of supplies described in paragraph (b)(2) of this clause, including subcontracts for commercial products, the Contractor shall flow down the requirements of this clause as follows:
(1) The Contractor shall insert the substance of this clause, including this paragraph (i), in subcontracts that exceed the simplified acquisition threshold in part 2 of the Federal Acquisition Regulation.
(2) The Contractor shall insert the substance of paragraphs (a) through (e) of this clause, and this paragraph (i), in subcontracts that are at or below the simplified acquisition threshold in part 2 of the Federal Acquisition Regulation.
(End of clause)
52.253-1 COMPUTER GENERATED FORMS (JAN 1991) FAR
252.204-7018 PROHIBITION ON THE ACQUISITION OF COVERED DEFENSE TELECOMMUNICATIONS EQUIPMENT OR SERVICES (JAN 2023) DFARS
252.204-7024 NOTICE ON THE USE OF THE SUPPLIER PERFORMANCE RISK SYSTEM (MAR 2023) DFARS
52.204-27 PROHIBITION ON A BYTEDANCE COVERED APPLICATION (JUN 2023) FAR
52.204-30 FEDERAL ACQUISITION SUPPLY CHAIN SECURITY ACT ORDERS -- PROHIBITION (DEC 2023) FAR
SECTION J - LIST OF ATTACHMENTS
List of Attachments
|
|
|
|
|
|
Description |
File Name |
ATTACH_Contractor_Sign ature |
SPE2DP25D0002_Valneva Signed_2025-01-29.pdf |
EX-4.38
8
exhibit438-x2024esoptcseng.htm
EX-4.38
Document
VALNEVA SE
Terms and conditions of the 2024 Employee Stock Option Plan
1. Preliminary statement
1.1The 2024 Employee Stock Option Plan governed by these Terms and Conditions (the “2024 ESOP”) is aiming at promoting the interests of Valneva SE (“Valneva” or “the Company”) by offering an incentive to the Beneficiary Employees (as defined below) to acquire shares in the Company. The objective is to motivate the employees, while allowing them to benefit from increases in the value of Valneva.
Subject to the exclusions set out in the next paragraph, “Beneficiary Employee(s)” shall mean all individuals who, on the working day immediately preceding the Grant Date (as this term is defined in Section 3.2 below), (i) either have an active employment agreement with Valneva or one of its subsidiaries “Valneva Austria GmbH”, “Valneva Canada Inc.”, “Valneva Scotland Ltd.”, “Valneva Sweden AB”, “Valneva France SAS”, “Valneva USA, Inc.” or “Valneva UK Ltd.” (Valneva and its subsidiaries being collectively referred to herein as the “Group” and “active employment” meaning that work is being done and remuneration is being paid), or have an employment agreement with a Group entity and are on maternity, paternity or sick leave, and (ii) belong to employee grade 13 or lower. Further, for US tax reasons, those employees who are US taxpayers shall be included in the 2024 ESOP and be Beneficiary Employees only if they also met the above-mentioned conditions on September 23, 2024 and have been continuously employed by the Group between September 23, 2024 and the working day immediately preceding the Grant Date.
Notwithstanding the foregoing, “Beneficiary Employee(s)” shall not include any individual who, on the working day immediately preceding the Grant Date, (i) is on termination notice with respect to his/her employment with a Group entity (whether on grounds of resignation, dismissal, mutual termination agreement or retirement), without continuing Group employment in another Group entity, (ii) works less than 15% of the normal full working time in the relevant Group entity, (iii) is on educational leave, (iv) is in the legal situation of external workforce (e.g. as consultant), (v) is an intern or trainee, (vi) is a student employed on a short term basis without being included in the Company’s grading system, or (vii) has been on sick leave for a continuous period of one (1) year or more.
1.2 The Company voluntary grants stock options by way of this 2024 ESOP. Such grant shall not give rise to a legal right for the Beneficiary Employees to participate in a subsequent or similar plan. The 2024 ESOP shall not replace any employee stock option plan currently in effect.
2. Granting of Options
2.1 The Board of Directors shall have sole competence over the grant of stock options under the 2024 ESOP (the “Option(s)”). The Board of Directors shall determine the number of Options granted to each Beneficiary Employee and the Strike Price applicable to the subscription of each Share (as such terms are defined in Sections 3.1 and 3.9 below); this information will be provided on an individual basis, by means of a grant letter delivered to each Beneficiary Employee when the Options are granted.
2.2 The grant of Options to the Beneficiary Employees shall be free of charge. However, the exercise of Options is subject to all applicable fees, taxes and duties (see Section 8 below).
3. Exercise of Options
Conversion ratio
3.1 Subject to these Terms and Conditions (including the payment of the Strike Price and the possible adjustment provided for in Section 6.2), each Beneficiary Employee shall be entitled to convert one (1) Option into one (1) Valneva ordinary share (as referenced under ISIN FR0004056851, the “Share(s)”). All Shares resulting from the exercise of Options may be created by the Company through share capital increases, in accordance with French law.
Vesting of Options
3.2 Subject to the opening of an Exercise Period (as this term is defined in Section 3.3 below), one third (1/3) of the Options allocated to the Beneficiary Employees shall become exercisable after a period of twelve (12) months from the date such Options were granted by the Board of Directors of Valneva (the “Grant Date”), an additional one third (1/3) of the Options allocated to the Beneficiary Employees shall become exercisable after a period of twenty-four (24) months from the Grant Date and the remainder shall become exercisable after a period of thirty-six (36) months from the Grant Date. If one third of an allocation is not a whole number, it shall be rounded down for the two first tranches and rounded up for the last tranche.
Exercise periods
3.3 The Beneficiary Employees may exercise their Options only within specific time periods provided for that purpose (the “Exercise Period(s)”). Each Exercise Period will be announced by Valneva’s executive management. Subject to any Lock-Up Period (as defined in Section 7.1 below), there will be up to four (4) Exercise Periods per calendar year, each of them lasting no longer than two (2) weeks. Beneficiary Employees included in any list of insiders will not be allowed to exercise Options, even though an Exercise Period is open.
3.4 The Company reserves the right to postpone, suspend or terminate any Exercise Period, in accordance with applicable laws and regulations.
3.5 Subject to Section 4 of these Terms and Conditions, any Option which was exercisable in an Exercise Period (as per Section 3.2 above), but was not exercised during that Exercise Period, can be exercised by the relevant Beneficiary Employee during any of the following Exercise Periods.
3.6 In the event of a Change of Control (as defined below), all outstanding Options shall become exercisable, and an Exercise Period shall immediately begin, at the time the Change of Control is effective (this process being hereinafter referred to as the “Acceleration”). However, the Company shall retain the right to purchase and/or cancel the concerned Options or Shares with a cash settlement (in accordance with Section 4.5 below), provided that the same value per Share paid in the take-over transaction is applied for calculating the cash compensation amount.
For the purposes of this Section 3.6, “Change of Control” means a transaction by which a single party, or two or more parties acting in concert, take over more than fifty percent (50 %) of the outstanding voting rights of the Company (be it through an acquisition, merger or transfer of essentially all of the assets of the Company).
Declaration of exercise
3.7 The Beneficiary Employees shall exercise their Options by sending a duly completed and signed form to the external services provider managing the plan on behalf of the Company (the “Plan Manager”). This form may be sent as an original or electronically
.
3.8 Properly filled and signed exercise forms referred to in Section 3.7 above must be received by the Plan Manager not earlier than on the first day of the relevant Exercise Period, and no later than 5 p.m., Paris time, on the last day of such Exercise Period. Any form received by the Plan Manager outside this period will be void. In such a case, the relevant Beneficiary Employee may exercise his/her Options during a subsequent Exercise Period, if he/she so wishes (subject to Section 4 below).
Payment of Shares - Strike Price
3.9 The “Strike Price” shall be the amount that each Beneficiary Employee is required to pay at the time of exercising his/her Options, in order to receive the underlying Shares.
Subject to Section 6.2 below, the Strike Price under the 2024 ESOP shall be equal to the higher of (i) one hundred percent (100%) of the volume-weighted average price of the Company's shares on the Euronext Paris regulated market over the period of twenty (20) trading days immediately preceding the Grant Date, and (ii) one hundred percent (100%) of the average closing price of the Company's shares on the Euronext Paris regulated market over the period of twenty (20) trading days immediately preceding the Grant Date.
3.10 The Strike Price must be received in full by the Plan Manager no later than the last day of the relevant Exercise Period.
3.11 By paying the full Strike Price, the Beneficiary Employee shall become the beneficial owner of the resulting Shares at the latest on the last day of the relevant Exercise Period, even though the Shares are held by a custodian on behalf of such Beneficiary Employee.
3.12 Notwithstanding Sections 3.10 and 3.11 of these Terms and Conditions and subject to the provisions of Section 7.1 below, the Company may, in its sole discretion and so long as the 2024 ESOP is managed by a Plan Manager, allow the Beneficiary Employee to exercise his/her Options and immediately sell the resulting Shares, without making any initial payment for the Strike Price. In such a case, it is understood that (i) the Plan Manager shall deduct the Strike Price and any applicable costs, fees and withholding taxes from the selling price, and (ii) if the selling price falls short of the Strike Price and such costs, fees and taxes, the Beneficiary Employee shall pay for the difference.
4. Validity period of Options - Lapse
4.1 The Options may be exercised within a period ending on the tenth (10th) anniversary of the Grant Date. All Options not exercised by that time shall lapse without compensation.
4.2 Upon termination of employment with a Group entity (whether on the grounds of resignation, dismissal, mutual termination agreement or retirement), without continuing Group employment in another Group entity, the Options of the leaving Beneficiary Employee shall lapse without compensation. Notwithstanding the foregoing, a leaving Beneficiary Employee shall retain the right to exercise those Options which were exercisable prior to termination of employment, (i) only during the first Exercise Period which will immediately follow termination of employment, and (ii) on condition that the Company had already opened an Exercise Period under the 2024 ESOP prior to such termination of employment.
For the avoidance of doubt, any leave of a Beneficiary Employee on grounds of (i) maternity/paternity, (ii) education, or (iii) sickness, shall not be considered as termination of employment provided that the relevant employment agreement is only suspended for the duration of the leave and becomes automatically effective again when the Beneficiary Employee is back at work.
4.3 In the event of a Beneficiary Employee’s death, all granted Options not exercisable prior to the date of death shall lapse without compensation. However, any exercisable Options may be exercised pursuant to Section 5.2 below.
4.4 In the event that insolvency proceedings are initiated with respect to the Company, or the Company becomes insolvent, all Options shall lapse without compensation.
4.5 The Company may also cancel an Option (i) pursuant to Section 3.6 above, (ii) through substitution of economically equivalent options, or (iii) if the legal form of the Company changes. In the case of a transaction referred to in Section 3.6 or a change in the legal form of the Company, any exercisable Option with a Strike Price higher than the then-current Valneva’s share price (or, in the event of Change of Control, than the value per share paid in the take-over transaction) shall lapse without compensation. In addition, any acquisition, merger or transfer of essentially all of the assets of the Company which does not result in a Change of Control shall not trigger Acceleration, but may give rise to replacement of the Options by options in the successor company.
4.6 In the event of expiration or lapse of Options, the Company shall not be required to inform the relevant Beneficiary Employees nor to take any other action, and the Beneficiary Employees shall have no right to any compensation.
5. Unassignability of Options
5.1 The Options granted to the Beneficiary Employees under the 2024 ESOP shall not be transferable, negotiable or eligible as collateral, except through transfer by death (i.e. disposition by will or law).
5.2 The Options may only be exercised personally by the Beneficiary Employee during his/her lifetime or by his/her legal representative. During the six (6)-month period immediately following the date of death of a Beneficiary Employee, only his/her heir or the legal representative of the heir, in each case as identified by corresponding documentation submitted to the Company, may declare the exercise of all remaining exercisable Options. The Options shall be deemed immediately exercised if an Exercise Period is opened at the time of the declaration. If there is no Exercise Period opened at the time the exercise is declared, the Options shall be deemed exercised during the first day of the Exercise Period directly subsequent to the declaration. The Shares so received may be further assigned, subject to these Terms and Conditions and any applicable statutory and regulatory provisions.
6. Shareholder’s rights
6.1 Before the Company actually awards the Shares, the Beneficiary Employee shall have no shareholder right in connection with these Shares, and in particular no right to receive dividends. Following the award of the Shares pursuant to these Terms and Conditions, the shareholder rights associated with the Shares, including the right to receive dividends, shall be subject to applicable laws and regulations.
6.2 If the Company proceeds with any of the financial transactions listed in Article L. 228-99 of the French Commercial Code, the rights of Beneficiary Employees shall be protected in accordance with that Article, which may result in a change in the conversion ratio and/or the Strike Price.
7. Disposal of Shares
7.1 The Beneficiary Employees may freely dispose of the Shares received following exercise of the Options. This shall not apply during a period of trading restriction (the “Lock-up Period”), which may be set forth at the Company’s discretion as a result of the then current Company policies dealing with insider information and stock trading by employees and directors.
During a Lock-up Period, the Beneficiary Employee shall not sell nor dispose of its Shares in any way whatsoever, including by means of collateralization or derivative transactions (e.g. options, futures).
8. Fees, taxes and duties
8.1 The Company shall bear all 2024 ESOP set-up and management costs.
8.2 All fees, expenses, taxes and mandatory contributions relating to securities transactions, including the cash settlement option set out in Section 3.12 above, shall be borne by the relevant Beneficiary Employee. If a Group entity is required to withhold and pay the taxes and duties owed by a Beneficiary Employee to the tax authorities, such Beneficiary Employee shall pay the corresponding amount to that entity in due time.
The Beneficiary Employees shall further bear all expenses for personal advice, in particular with respect to legal or tax matters.
9. Miscellaneous
9.1 These Terms and Conditions have been drawn up in French and English. In the event of a conflict between the French and the English version, the English version shall prevail.
9.2 All rights and obligations under the 2024 ESOP shall be governed by French law.
9.3 All disputes shall be submitted to the Paris Commercial Court (France).
9.4 The Company shall have the right to terminate or amend the 2024 ESOP at any time, subject to applicable laws and regulations.
VALNEVA SE
EX-4.41
9
ex441-xslgsop2024.htm
EX-4.41
Document
VALNEVA SE
Terms and conditions of the 2024 Senior Leadership Group Stock Option Plan
1. Preliminary statement
1.1The 2024 Senior Leadership Group Stock Option Plan governed by these Terms and Conditions (the “2024 SLG SOP”) is aiming at promoting the interests of Valneva SE (“Valneva” or “the Company”) by offering an incentive to the Beneficiary Employees (as defined below) to acquire shares in the Company. The objective is to motivate the employees and officers belonging to the Senior Leadership Group, while allowing them to benefit from increases in the value of Valneva. The 2024 SLG SOP is combined with a free share plan so that such employees and officers benefit from both stock options and free ordinary shares.
Subject to the exclusions set out in the next paragraph, “Beneficiary Employee(s)” shall mean all individuals who, on the working day immediately preceding the Grant Date (as this term is defined in Section 3.2 below), (i) either have an active employment or management agreement with Valneva or one of its subsidiaries “Valneva Austria GmbH”, “Valneva Canada Inc.”, “Valneva Scotland Ltd.”, “Valneva Sweden AB”, “Valneva France SAS”, “Valneva USA, Inc.” or “Valneva UK Ltd.” (Valneva and its subsidiaries being collectively referred to herein as the “Group” and “active employment” meaning that work is being done and remuneration is being paid), or have an employment or management agreement with a Group entity and are on maternity, paternity or sick leave, and (ii) belong to employee grade 14 or higher. Further, for US tax reasons, those employees and officers who are US taxpayers shall be included in the 2024 SLG SOP and be Beneficiary Employees only if they also met the above-mentioned conditions on September 23, 2024 and have been continuously employed by the Group between September 23, 2024 and the working day immediately preceding the Grant Date.
Notwithstanding the foregoing, “Beneficiary Employee(s)” shall not include any individual who, on the working day immediately preceding the Grant Date, (i) is on termination notice with respect to his/her employment or office with a Group entity (whether on grounds of resignation, dismissal, mutual termination agreement or retirement), without continuing Group employment in the same or another Group entity, (ii) is on educational leave, (iii) is in the legal situation of external workforce (e.g. as consultant), or (iv) has been on sick leave for a continuous period of one (1) year or more.
1.2 The Company voluntary grants stock options by way of this 2024 SLG SOP. Such grant shall not give rise to a legal right for the Beneficiary Employees to participate in a subsequent or similar plan. The 2024 SLG SOP shall not replace any employee stock option plan currently in effect.
2. Granting of Options
2.1 The Board of Directors shall have sole competence over the grant of stock options under the 2024 SLG SOP (the “Option(s)”). The Board of Directors shall determine the number of Options granted to each Beneficiary Employee and the Strike Price applicable to the subscription of each Share (as such terms are defined in Sections 3.1 and 3.9 below); this information will be provided on an individual basis, by means of a grant letter delivered to each Beneficiary Employee when the Options are granted.
2.2 The grant of Options to the Beneficiary Employees shall be free of charge. However, the exercise of Options is subject to all applicable fees, taxes and duties (see Section 8 below).
3. Exercise of Options
Conversion ratio
3.1 Subject to these Terms and Conditions (including the payment of the Strike Price and the possible adjustment provided for in Section 6.2), each Beneficiary Employee shall be entitled to convert one (1) Option into one (1) Valneva ordinary share (as referenced under ISIN FR0004056851, the “Share(s)”). All Shares resulting from the exercise of Options may be created by the Company through share capital increases, in accordance with French law.
Vesting of Options
3.2 Subject to the opening of an Exercise Period (as this term is defined in Section 3.3 below), one third (1/3) of the Options allocated to the Beneficiary Employees shall become exercisable after a period of twelve (12) months from the date such Options were granted by the Board of Directors of Valneva (the “Grant Date”), an additional one third (1/3) of the Options allocated to the Beneficiary Employees shall become exercisable after a period of twenty-four (24) months from the Grant Date and the remainder shall become exercisable after a period of thirty-six (36) months from the Grant Date. If one third of an allocation is not a whole number, it shall be rounded down for the two first tranches and rounded up for the last tranche.
Exercise periods
3.3 The Beneficiary Employees may exercise their Options only within specific time periods provided for that purpose (the “Exercise Period(s)”). Each Exercise Period will be announced by Valneva’s executive management. Subject to any Lock-Up Period (as defined in Section 7.1 below), there will be up to four (4) Exercise Periods per calendar year, each of them lasting no longer than two (2) weeks. Beneficiary Employees included in any list of insiders will not be allowed to exercise Options, even though an Exercise Period is open.
3.4 The Company reserves the right to postpone, suspend or terminate any Exercise Period, in accordance with applicable laws and regulations.
3.5 Subject to Section 4 of these Terms and Conditions, any Option which was exercisable in an Exercise Period (as per Section 3.2 above), but was not exercised during that Exercise Period, can be exercised by the relevant Beneficiary Employee during any of the following Exercise Periods.
3.6 In the event of a Change of Control (as defined below), all outstanding Options shall become exercisable, and an Exercise Period shall immediately begin, at the time the Change of Control is effective (this process being hereinafter referred to as the “Acceleration”). However, the Company shall retain the right to purchase and/or cancel the concerned Options or Shares with a cash settlement (in accordance with Section 4.5 below), provided that the same value per Share paid in the take-over transaction is applied for calculating the cash compensation amount.
For the purposes of this Section 3.6, “Change of Control” means a transaction by which a single party, or two or more parties acting in concert, take over more than fifty percent (50 %) of the outstanding voting rights of the Company (be it through an acquisition, merger or transfer of essentially all of the assets of the Company).
Declaration of exercise
3.7 The Beneficiary Employees shall exercise their Options by sending a duly completed and signed form to the external services provider managing the plan on behalf of the Company (the “Plan Manager”). This form may be sent as an original or electronically.
3.8 Properly filled and signed exercise forms referred to in Section 3.7 above must be received by the Plan Manager not earlier than on the first day of the relevant Exercise Period, and no later than 5 p.m., Paris time, on the last day of such Exercise Period. Any form received by the Plan Manager outside this period will be void. In such a case, the relevant Beneficiary Employee may exercise his/her Options during a subsequent Exercise Period, if he/she so wishes (subject to Section 4 below).
Payment of Shares - Strike Price
3.9 The “Strike Price” shall be the amount that each Beneficiary Employee is required to pay at the time of exercising his/her Options, in order to receive the underlying Shares.
Subject to Section 6.2 below, the Strike Price under the 2024 SLG SOP shall be equal to the higher of (i) one hundred percent (100%) of the volume-weighted average price of the Company's shares on the Euronext Paris regulated market over the period of twenty (20) trading days immediately preceding the Grant Date, and (ii) one hundred percent (100%) of the average closing price of the Company's shares on the Euronext Paris regulated market over the period of twenty (20) trading days immediately preceding the Grant Date.
3.10 The Strike Price must be received in full by the Plan Manager no later than the last day of the relevant Exercise Period.
3.11 By paying the full Strike Price, the Beneficiary Employee shall become the beneficial owner of the resulting Shares at the latest on the last day of the relevant Exercise Period, even though the Shares are held by a custodian on behalf of such Beneficiary Employee.
3.12 Notwithstanding Sections 3.10 and 3.11 of these Terms and Conditions and subject to the provisions of Section 7.1 below, the Company may, in its sole discretion and so long as the 2024 SLG SOP is managed by a Plan Manager, allow the Beneficiary Employee to exercise his/her Options and immediately sell the resulting Shares, without making any initial payment for the Strike Price. In such a case, it is understood that (i) the Plan Manager shall deduct the Strike Price and any applicable costs, fees and withholding taxes from the selling price, and (ii) if the selling price falls short of the Strike Price and such costs, fees and taxes, the Beneficiary Employee shall pay for the difference.
4. Validity period of Options - Lapse
4.1 The Options may be exercised within a period ending on the tenth (10th) anniversary of the Grant Date. All Options not exercised by that time shall lapse without compensation.
4.2 Upon termination of employment or office with a Group entity (whether on the grounds of resignation, dismissal, mutual termination agreement or retirement), without continuing Group employment in the same or another Group entity, the Options of the leaving Beneficiary Employee shall lapse without compensation.
For the avoidance of doubt, any leave of a Beneficiary Employee on grounds of (i) maternity/paternity, (ii) education, or (iii) sickness, shall not be considered as termination of employment provided that the relevant employment agreement is only suspended for the duration of the leave and becomes automatically effective again when the Beneficiary Employee is back at work.
4.3 In the event of a Beneficiary Employee’s death, all granted Options not exercisable prior to the date of death shall lapse without compensation. However, any exercisable Options may be exercised pursuant to Section 5.2 below.
4.4 In the event that insolvency proceedings are initiated with respect to the Company, or the Company becomes insolvent, all Options shall lapse without compensation.
4.5 The Company may also cancel an Option (i) pursuant to Section 3.6 above, (ii) through substitution of economically equivalent options, or (iii) if the legal form of the Company changes. In the case of a transaction referred to in Section 3.6 or a change in the legal form of the Company, any exercisable Option with a Strike Price higher than the then-current Valneva’s share price (or, in the event of Change of Control, than the value per share paid in the take-over transaction) shall lapse without compensation. In addition, any acquisition, merger or transfer of essentially all of the assets of the Company which does not result in a Change of Control shall not trigger Acceleration, but may give rise to replacement of the Options by options in the successor company.
4.6 In the event of expiration or lapse of Options, the Company shall not be required to inform the relevant Beneficiary Employees nor to take any other action, and the Beneficiary Employees shall have no right to any compensation.
5. Unassignability of Options
5.1 The Options granted to the Beneficiary Employees under the 2024 SLG SOP shall not be transferable, negotiable or eligible as collateral, except through transfer by death (i.e. disposition by will or law).
5.2 The Options may only be exercised personally by the Beneficiary Employee during his/her lifetime or by his/her legal representative. During the six (6)-month period immediately following the date of death of a Beneficiary Employee, only his/her heir or the legal representative of the heir, in each case as identified by corresponding documentation submitted to the Company, may declare the exercise of all remaining exercisable Options. The Options shall be deemed immediately exercised if an Exercise Period is opened at the time of the declaration. If there is no Exercise Period opened at the time the exercise is declared, the Options shall be deemed exercised during the first day of the Exercise Period directly subsequent to the declaration. The Shares so received may be further assigned, subject to these Terms and Conditions and any applicable statutory and regulatory provisions.
6. Shareholder’s rights
6.1 Before the Company actually awards the Shares, the Beneficiary Employee shall have no shareholder right in connection with these Shares, and in particular no right to receive dividends. Following the award of the Shares pursuant to these Terms and Conditions, the shareholder rights associated with the Shares, including the right to receive dividends, shall be subject to applicable laws and regulations.
6.2 If the Company proceeds with any of the financial transactions listed in Article L. 228-99 of the French Commercial Code, the rights of Beneficiary Employees shall be protected in accordance with that Article, which may result in a change in the conversion ratio and/or the Strike Price.
7. Disposal of Shares
7.1 Subject to the provisions of Section 7.2 below, the Beneficiary Employees may freely dispose of the Shares received following exercise of the Options. This shall not apply during a period of trading restriction (the “Lock-up Period”), which may be set forth at the Company’s discretion as a result of the then current Company policies dealing with insider information and stock trading by employees and directors.
During a Lock-up Period, the Beneficiary Employee shall not sell nor dispose of its Shares in any way whatsoever, including by means of collateralization or derivative transactions (e.g. options, futures).
7.2 As decided by the Company’s Board of Directors, and in compliance with Article L. 225-185 of the French Commercial Code where applicable, the CEO (Directeur Général), the Associate Managing Officers (Directeurs Généraux Délégués) and each of the other members of the Executive Committee must keep not less than 20% of the Shares resulting from exercise of his/her Options until termination of both his/her Executive Committee membership and (as applicable) his/her corporate office, provided, however, that, in accordance with Article L. 225-177 of the French Commercial Code, this obligation shall expire three (3) years after exercise of the Options for those Executive Committee members who are not corporate officers.
8. Fees, taxes and duties
8.1 The Company shall bear all 2024 SLG SOP set-up and management costs.
8.2 All fees, expenses, taxes and mandatory contributions relating to securities transactions, including the cash settlement option set out in Section 3.12 above, shall be borne by the relevant Beneficiary Employee. If a Group entity is required to withhold and pay the taxes and duties owed by a Beneficiary Employee to the tax authorities, such Beneficiary Employee shall pay the corresponding amount to that entity in due time.
The Beneficiary Employees shall further bear all expenses for personal advice, in particular with respect to legal or tax matters.
9. Miscellaneous
9.1 All rights and obligations under the 2024 SLG SOP shall be governed by French law.
9.2 All disputes shall be submitted to the Paris Commercial Court (France).
9.3 The Company shall have the right to terminate or amend the 2024 SLG SOP at any time, subject to applicable laws and regulations.
VALNEVA SE
EX-4.45
10
exhibit4452024-2027fsp.htm
EX-4.45
Document
Valneva SE
(the “Company”)
2024-2027 Free Share Plan
Terms and Conditions
1.Background and purpose of the plan
On December 20, 2023, the General Meeting of Valneva SE’s shareholders decided under its 23rd resolution to grant the Company’s Board of Directors (the “Board”) all powers necessary to decide the granting and issuance of free ordinary shares (“FS”) for the benefit of corporate officers or employees of the Company or its subsidiaries.
In accordance with the powers and authorizations granted by the shareholders' meeting of December 20, 2023, the Board has decided to grant FS to the CEO (Directeur Général), the Associate Managing Officers (Directeurs Généraux Délégués), and those employees of the Company or its subsidiaries whose employment grade is 14 or more (the “Participants”) under a new 2024-2027 Free Share Plan.
The purpose of this 2024-2027 Free Share Plan (the “2024 FSP”) is to provide a long-term incentive program for the Company’s senior leadership group. The 2024 FSP is combined with the grant of stock options under the 2024 Senior Leadership Group Stock Option Plan (“2024 SLG SOP”).
2.Purpose of these Terms and Conditions
The Terms and Conditions set forth herein aim at summarizing and complementing (i) the provisions set out in the Company’s Articles of Association that are relevant to the 2024 FSP, (ii) the resolutions adopted by the General Meeting of the Company’s shareholders on December 20, 2023, and (iii) the decisions made by the Board regarding the granting of free ordinary shares under the 2024 FSP (collectively, the “Constitutional Documents”). In the event of any discrepancy between the Constitutional Documents and these Terms and Conditions, the Constitutional Documents will prevail.
3.FS granting
3.1Allocations
The number of FS granted to each Participant under the 2024 FSP is set forth in the Board’s allocation decision.
The maximum number of FS granted by the Board cannot exceed 3% of the share capital of the Company as of the Grant Date (as defined in Section 3.2 below) or any legal threshold applicable as of the Grant Date.
3.2Tranches
Subject to the vesting conditions set forth below, the FS granted to a Participant under the 2024 FSP will vest in and be delivered to that Participant (“seront définitivement attribuées”) in three tranches.
Each tranche will amount to one third of the total individual allocation. If one third is not a whole number, the FS number will be rounded down for the first two tranches and rounded up for the third tranche.
The tranches will vest in the Participants as follows:
-First and second tranches: two (2) years after the date of the Board decision that initially granted the FS under the 2024 FSP (the “Grant Date”);
-Third Tranche: three (3) years after the Grant Date.
3.3Vesting conditions
Participants must continuously remain a corporate officer or employee (full time or not less than 80%) of the Company or a direct or indirect subsidiary of the Company until vesting of the FS granted under this 2024 FSP, subject to the retirement exception set forth below. If, for any reason other than retirement, the term of office of a corporate officer is not renewed upon its expiration and the Participant concerned is not otherwise employed (full time or not less than 80%) by the Company or a direct or indirect subsidiary of the Company immediately after that expiration, the shares already vested will be kept, but the unvested shares will be lost.
3.4Accelerated vesting after two years
If a Change of Control (as defined below) occurs not earlier than 2 years after the Grant Date, all tranches will vest immediately.
“Change of Control” means that a person or entity other than the Company’s current shareholders has taken control of the Company, “control” having the meaning set forth in Article L. 233-3 of the French Commercial Code.
3.5No compulsory holding period
Following FS vesting in accordance with these Terms and Conditions, and subject to Section 4 below, no compulsory FS holding period will be applicable to the Participants.
3.6Retirement
Participants who will retire in accordance with the age requirements of their applicable retirement regime before complete vesting of their FS under the 2024 FSP will remain entitled to a prorated amount of shares, for each unvested tranche, based on the period from the Grant Date until retirement, as compared to the total duration of the tranche in question; provided, however, that for purposes of this calculation, the duration of the first tranche will be deemed to be one year.
By way of example, a Participant retiring 6 months after the Grant Date will remain entitled to:
-50% of tranche 1;
-25% of tranche 2; and
-16.66% of tranche 3.
If the number of vested shares calculated in accordance with the above is not a whole number, it will be rounded down.
3.7Death
In accordance with Article L. 225-197-3 of the French Commercial Code, heirs can request the vesting of a Participant’s FS within six months after the death of that Participant.
3.8Change of Control occurring less than 2 years after the Grant Date
If a Change of Control takes place less than two years after the Grant Date, and Section III of Article L. 225-197-1 of the French Commercial Code does not apply, the 2024 FSP will be canceled and the Company will indemnify the Participants for the loss of unvested FS granted under this canceled plan, subject however to all required shareholder approvals where corporate officers are concerned. The gross amount of this indemnity will be calculated as though such FS had been vested upon the Change of Control. The conditions and limitations set forth in these Terms and Conditions will apply to this calculation, mutatis mutandis.
4.Additional provisions
As decided by the Company’s Board of Directors, and in compliance with Article L. 225-185 of the French Commercial Code where applicable, the CEO (Directeur Général), the Associate Managing Officers (Directeurs Généraux Délégués) and each of the other members of the Executive Committee must keep not less than 20% of the FS vested under the 2024 FSP until termination of both his/her Executive Committee membership and (as applicable) his/her corporate office.
EX-12.1
11
exhibit121-soxsection302ce.htm
EX-12.1
Document
CERTIFICATION PURSUANT TO RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I Thomas Lingelbach, certify that:
1.I have reviewed this annual report on Form 20-F of Valneva SE (the “Company”);
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and for, the periods presented in this report;
4.The Company's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Company and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the Company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the Company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting; and
5.The Company's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company's auditors and the
audit committee of the Company's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the Company's internal control over financial reporting.
Date: March 24, 2025
By: /s/ Thomas Lingelbach
Thomas Lingelbach
Chief Executive Officer
EX-12.2
12
exhibit122-sox302certifica.htm
EX-12.2
Document
CERTIFICATION PURSUANT TO RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Peter Bühler, certify that:
1.I have reviewed this annual report on Form 20-F of Valneva SE (the “Company”);
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the Company as of, and for, the periods presented in this report;
4.The Company's other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the Company and have:
a.Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b.Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c.Evaluated the effectiveness of the Company's disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d.Disclosed in this report any change in the Company's internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting; and
5.The Company's other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the Company's auditors and the
audit committee of the Company's board of directors (or persons performing the equivalent functions):
a.All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the Company's ability to record, process, summarize and report financial information; and
b.Any fraud, whether or not material, that involves management or other employees who have a significant role in the Company's internal control over financial reporting.
Date: March 24, 2025
By: /s/ Peter Bühler
Peter Bühler
Chief Financial Officer
EX-13.1
13
exhibit131-section906certi.htm
EX-13.1
Document
CERTIFICATION BY THE PRINCIPAL EXECUTIVE OFFICER PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Thomas Lingelbach, Chief Executive Officer of Valneva SE (the “Company”) hereby certifies that, to the best of his knowledge:
1.The Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2024, to which this Certification is attached as Exhibit 13.1 (the “Annual Report”), fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and
2.The information contained in the Annual Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
/s/ Thomas Lingelbach
Thomas Lingelbach
Chief Executive Officer
(Principal Executive Officer)
* This certification accompanies the Form 20-F to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Valneva SE under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 20-F), irrespective of any general incorporation language contained in such filing.
EX-13.2
14
exhibit132-soxsection906ce.htm
EX-13.2
Document
CERTIFICATION BY THE PRINCIPAL FINANCIAL OFFICER PURSUANT TO 18 U.S.C. SECTION 1350, AS ADOPTED PURSUANT TO SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
Pursuant to the requirement set forth in Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350), Peter Bühler, Chief Financial Officer of Valneva SE (the “Company”) hereby certifies that, to the best of his knowledge:
1.The Company’s Annual Report on Form 20-F for the fiscal year ended December 31, 2024, to which this Certification is attached as Exhibit 13.2 (the “Annual Report”), fully complies with the requirements of Section 13(a) or Section 15(d) of the Exchange Act; and
2.The information contained in the Annual Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
/s/ Peter Bühler
Peter Bühler
Chief Financial Officer
(Principal Financial Officer)
* This certification accompanies the Form 20-F to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Valneva SE under the Securities Act of 1933, as amended, or the Exchange Act (whether made before or after the date of the Form 20-F), irrespective of any general incorporation language contained in such filing.
EX-15.1
15
exhibit151deloitteconsent.htm
EX-15.1
Document
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in Registration Statement No. 333-266839 on Form F-3 of our reports dated March 24, 2025, relating to the financial statements of VALNEVA SE and the effectiveness of VALNEVA SE's internal control over financial reporting appearing in this Annual Report on Form 20-F for the year ended December 31, 2024.
/s/ Deloitte & Associés
Paris-La-Défense, France
March 24, 2025
EX-15.2
16
exhibit152-consentletter31.htm
EX-15.2
Document
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statement on Form F-3 (No. 333-266839) of Valneva SE of our reports dated March 24, 2025 relating to the financial statements and the effectiveness of internal control over financial reporting, which appear in this Form 20-F.
PricewaterhouseCoopers Audit
Neuilly-sur-Seine, France
March 24, 2025
PricewaterhouseCoopers Audit, SAS, 63, rue de Villiers 92208 Neuilly-sur-Seine Cedex
Téléphone: +33 (0)1 56 57 58 59, Fax: +33 (0)1 56 57 58 60, www.pwc.fr
Société d’expertise comptable inscrite au tableau de l’ordre de Paris - Ile de France. Société de commissariat aux comptes membre de la compagnie régionale de Versailles et du Centre. Société par Actions Simplifiée au capital de 2 510 460 €. Siège social : 63, rue de Villiers 92200 Neuilly-sur-Seine.
RCS Nanterre 672 006 483. TVA n° FR 76 672 006 483. Siret 672 006 483 00362. Code APE 6920 Z.
Bureaux : Bordeaux, Lille, Lyon, Marseille, Metz, Montpellier, Nantes, Rennes, Rouen, Strasbourg, Toulouse.
EX-19.1
17
exhibit19insidertradingpol.htm
EX-19.1
Document
Policy
for Handling of Insider Information
and Stock Trading by Employees and Directors
1.Preamble
As a result of the listing of the shares of Valneva SE (“the Company”) on NYSE Euronext Paris, transactions in securities issued by the Company are subject to laws and regulations, including but not limited to French Law (in particular the French Financial Market Authority Regulations (hereinafter referred to as “AMF”)and the Regulation (EU) No 596/2014 on market abuse (hereinafter referred to as “MAR”)). These laws and regulations forbid the purchase or sale of securities by persons who are in the possession of Insider Information (as defined in Section 3 below). The abuse of Insider Information is subject to substantial punishments.
Valneva SE and its employees and directors value and depend on the confidence of institutional and private investors in the capital markets. Integrity and ethical conduct are basic conditions to preserve this reputation. Therefore Valneva SE pays utmost attention to the strict adherence to laws, regulations and ethical conduct, in order to avoid even the appearance of improper conduct by its employees, directors or anyone associated with the Company.
2. Scope of application
This Policy for Handling of Insider Information and Stock Trading by Employees or Directors (the “Policy”) applies to any member of the Board of Directors, the Executive Committee, the CEO, and any employee of Valneva SE and its affiliates, as well as any person otherwise working for Valneva SE who receives Insider Information. Each of these persons confirms by written statement that he or she has acknowledged and will observe this Policy and that he or she is aware of the sanctions in case of any violation.
3. Insider Information
Within the general meaning, an insider is any person who, as a member of an administrative, management or supervisory body of an issuer of securities, or otherwise on account of his or her profession, occupation, duties or participation in the capital of such issuer, has access to Insider Information. A person is also an insider if that person has procured such information by committing a punishable action. For the particular purposes of this Policy and without limiting the generality of the foregoing, an “Insider” shall designate a person who has access to Insider Information by virtue of his occupation or responsibilities within or for the Company or its affiliates.
“Insider information” means specific information which directly or indirectly concerns the Company or its securities, which is not publicly known and which, if it became publicly known, would be suitable to significantly influence the price of securities issued by the Company because a reasonable investor would probably use it as part of his/her investment decision.
Insider Information shall be deemed to be of a precise nature, if it indicates
•A set of circumstances that exist or may reasonably come into existence; or
•An event that has occurred or may be expected to occur; or
•Protracted processes and their intermediate steps.
Such information can relate to, without limitation:
◦financial information such as e.g. revenues, losses, budget figures, changes in costs,
◦important research and development results and/or relevant planning,
◦strategic partnerships, co-operations or material license agreements,
◦management changes or restructurings,
◦any measures affecting the Company’s capital, financing transactions or changes in the shareholder structure,
◦mergers or acquisitions.
5. Confidentiality areas
Purpose – Definition
Confidentiality areas can be put in place with respect to projects that may give rise to Insider Information immediately or at some point in the future, based on general experience. The creation of confidentiality areas does not necessarily mean that the information related to such a project already qualifies as Insider Information for the purpose of informing the authorities. However, a confidentiality area may be created for the purpose of limiting the dissemination of confidential information relating to a project (hereinafter referred to as “Confidential Project Information”) and keeping a list of the persons who have received it, in the event that information about this project develops into Insider Information. The number of persons included in a confidentiality area is to be kept as small as possible, as only those persons who have a strict need to deal with a Confidential Project Information within the scope of their work may gain knowledge of such information.
Dissemination of Confidential Project Information
Any Confidential Project Information under a confidentiality area may only be disseminated to those persons within the Company or its affiliates who have a strict need to know within the scope of their work and only if it is necessary for business purposes. Such Confidential Project Information shall be disseminated only to the absolutely necessary extent. Any internal addressees who, at the time of receiving Confidential Project Information, are not included in the confidentiality area to which the information relates, must be reminded by the member of the Executive Committee responsible for the legal function (“Legal EC Member”) of their confidentiality obligations in regard to such information and notified that they become part of such confidentiality area.
Any Confidential Project Information may be disseminated to any persons outside of the Company and its affiliates only if it is necessary for business purposes. Such dissemination has to be limited to the absolutely necessary extent and the addressees have to confirm by way of a duly signed confidentiality agreement that he or she will keep the Confidential Project Information confidential and acknowledges the existence of Insider Information laws. No confidentiality agreement is necessary if the addressee of the Confidential Project Information is de facto under a confidentiality obligation by virtue of the law or a professional code of ethics (e.g. attorneys, auditors).
Any person disseminating Confidential Project Information to other persons outside the corresponding confidentiality area shall immediately inform the Legal EC Member thereof, by providing the following details for record purposes: the contents of the disseminated Confidential Project Information, the name of the informed person(s), the date on which such informed person(s) received the information.
Please note the Legal EC Member need not be notified if the Confidential Project Information is disseminated in Committee and/or Board meetings of the Company, within the scope of institutionalized and predefined information dissemination processes, and if the information is documented in the respective minutes of the meeting.
Handling Confidential Project Information
Documents and external data carriers (e.g. CD ROMs, USB-Sticks) that contain Confidential Project Information must be properly stored to prevent their access by persons who are not involved with processing such information or carriers within their scope of their work. As a result, any such documents and external data carriers must be locked at all times. Offices of those persons who have access to Confidential Project Information must also be locked when these persons leave the office. Data saved electronically, including electronic mail, that contain Confidential Project Information shall also be secured against access by persons who are not involved with processing such information or data or the related IT system, within the scope of their work.
6. Restricted periods and trading bans
Thirty (30) calendar days prior to the planned publication of full quarterly, half-year and annual results and 15 (fifteen) days prior to the planned publication of any preliminary figures, the Company’s and its affiliates’ employees and directors may not purchase or sell securities issued by the Company, whether directly or through a third party. Such restricted period ends when the final full results are published.
In addition, the Legal EC Member, in agreement with the CEO, may announce further restricted periods for certain confidentiality or business areas, or concerning the Company as a whole, if this is deemed necessary for a fair perception on the capital markets. During such additional restricted periods, the Company’s and its affiliates’ employees and directors shall also not purchase or sell securities issued by the Company, whether directly or through a third party.
Persons who are aware of Insider Information may neither purchase nor sell securities issued by the Company, whether directly or through a third party, until this information is published (ad hoc publication). In addition, the Company’s and its affiliates’ employees and directors shall adhere to any trading restrictions that they are informed of as members of a confidential area.
The Legal EC Member may grant exemptions from the trading ban during a restricted period to the Company’s or its affiliates’ employees and\or directors in legitimate cases in either of the following situations:
•on a case-by-case basis due to the existence of exceptional circumstances, such as severe financial difficulty, which require the immediate sale of shares; or
•due to the characteristics of the trading involved for transactions made under, or related to, subscription rights or other entitlement of shares, or transactions where the beneficial interest in the relevant security does not change,
in each case, provided there is assurance that the transaction does not constitute an abuse of insider information according to section 4.
The Legal EC Member shall keep records of all applications that relate to proposed securities transactions within restricted periods by documenting the name of the applicant, as well as the type, scope and reason for the proposed transaction. The Legal EC Member shall also document his decision, as well as the relevant grounds.
7. Directors’ Dealings
According to the Market Abuse Regulation, the CEO, each member of the board of directors, and each employee with general management responsibilities (notably, the members of the Executive Committee), as well as any person having a close relationship with them, must notify the Company as well as the AMF (electronically, through the extranet ONDE - https://onde.amf-france.org/RemiseInformationEmetteur/Client/PTRemiseInformationEmetteur.aspx) promptly within three working days following the transaction date in the event of any exercise of stock options, purchase or sale of securities or other transaction conducted on the person’s own behalf relating to the shares or debt instruments of the Company or to derivatives or other financial instruments linked thereto; provided, however, the total amount of transactions of said person has reached the threshold of EUR 20,000 within the relevant calendar year. For this purpose, each such person shall advise the Legal EC Member immediately of his/her intention to perform any such transaction. The Legal EC Member shall document the information included in such reports and the date of the report.
8. Disclosure obligation of Directors
According to French law (Article L. 225-109 of the French Commercial Code), all Company shares held by the CEO, the members of the board of directors, or employees with general management responsibilities (notably the Executive Committee members), as well as any persons having a close relationship with them, must be held in registered form or with a custodian account holder institution as referred to in the General Rules of the French Market Authority (Articles 322-1 et seq.).
9. Legal EC Member and Insider Register
One member of the Executive Committee shall be responsible for the legal function., Her or his name is to be communicated to all employees and directors of the Company and its affiliates. The Legal EC Member shall monitor compliance with the provisions on the dissemination of the Insider and Confidential Project Information, as well as the organizational measures to prevent the abuse or abusive dissemination of such information by spot checks on an ongoing basis.
In addition, the tasks of the Legal EC Member include:
–to advise and assist management in matters relating to this Policy;
–to submit regular compliance reports to the Board of Directors and the CEO;
–to ensure training and education of employees in matters relating to this Policy and in regard to the prohibition of abuse of Insider Information according to Section 4.
The Legal EC Member shall also keep and regularly update an insider register which contains insiders lists relating to projects involving Insider Information. This register shall provide, with respect to any such project: the full name, the professional and private phone number, the professional and private address, the birth date and the job title of each insider, the reason for which a person is considered as insider and the starting/end date and hour of access to the Insider Information, and the information according to Section 6 on any intended securities transactions during a restricted period.
10. Violations
Any violations of the terms of this Policy and the underlying law can trigger legal consequences resulting from civil law or criminal law. Any breach of the prohibition of abuse of Insider Information may be punished by either the French Market Authority, which may impose fines, or by the courts with high judicial fines and imprisonment.
Beyond that, any behaviour which does not correspond to the regulations of this Policy, may endanger the confidence of investors in Valneva SE and its shares and cause irreparable damage to the Company, its affiliates and the employees. Valneva SE and its affiliates will therefore also ensure compliance with this Policy by disciplinary means in accordance with labour law. The Legal EC Member shall inform the CEO and the Human Resources Department of any violations of this Policy that comes to his / her attention in order to set the necessary disciplinary consequences.
Valneva SE January 2024
/s/ Thomas Lingelbach /s/ Frederic Jacotot
Thomas Lingelbach Frederic Jacotot
CEO General Counsel & Corporate Secretary