0001610618false00016106182025-06-232025-06-23
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): June 23, 2025
Cidara Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Delaware |
|
001-36912 |
|
46-1537286 |
(State or Other Jurisdiction of
Incorporation or Organization) |
|
(Commission File Number) |
|
(I.R.S. Employer
Identification Number) |
6310 Nancy Ridge Drive, Suite 101
San Diego, California 92121
(858) 752-6170
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligations of the registrant under any of the following provisions:
|
|
|
|
|
|
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
|
|
|
|
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
|
|
|
|
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
| Common Stock, Par Value $0.0001 Per Share |
|
CDTX |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events.
On June 23, 2025, Cidara Therapeutics, Inc. (the “Company”) announced positive topline results from its randomized, double-blind, placebo-controlled Phase 2b NAVIGATE trial evaluating CD388 for the prevention of seasonal influenza in healthy unvaccinated adults aged 18 to 64. The study met its primary endpoint, demonstrating a statistically significant prevention efficacy (“PE”) for each of three dose groups in individuals who received a single dose of CD388 at the beginning of the flu season and were evaluated for laboratory and clinically confirmed influenza over 24 weeks. The study also met all secondary endpoints, including efficacy at 37.8 and 37.2 degree Celsius temperature thresholds, as well as maintenance of PE up to 28 weeks with statistical significance. Over the same period, CD388 was well-tolerated at all doses with no unexpected dose-limiting treatment-emergent adverse events observed.
Primary Efficacy Analyses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
150 mg
N= 11753
n (%)
|
300 mg
N = 11923
n (%)
|
450 mg
N = 11873
n (%)
|
Placebo
N = 11723
n (%)
|
|
Primary
Endpoint1
|
Number of Participants Protocol-Defined ILI2 |
14 (1.2%) |
13 (1.1%) |
8 (0.7%) |
33 (2.8%) |
| Prevention Efficacy |
57.7% |
61.3% |
76.1% |
– |
| 95% CI (%) |
21.1, 78.9 |
27.0, 81.2 |
49.3, 89.9 |
– |
| p-value |
0.0050 |
0.0024 |
<0.0001 |
– |
|
ILI, influenza like illness; CI, confidence interval
1.Statistical significance for grouped 300mg + 450mg dose groups was met (PE = 68.6%, p<0.0001), allowing testing of individual dose groups.
2.ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥38.0°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough) or ≥1 respiratory symptom and ≥1 systemic symptom (headache, feeling feverish, body aches/pains, fatigue).
3.Sample size (N) indicates evaluable population at time of primary analysis data cut (April 30, 2025).
|
Key Secondary Endpoints:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
150 mg
N= 11753
n (%)
|
300 mg
N = 11923
n (%)
|
450 mg
N = 11873
n (%)
|
Placebo
N = 11723
n (%)
|
Secondary
Endpoints |
Number of Participants with ≥ 37.8 °C Temp1 |
15 (1.3%) |
15 (1.3%) |
8 (0.7%) |
33 (2.8%) |
| Prevention Efficacy |
54.7% |
55.3% |
76.1% |
|
| 95% CI (%) |
16.7, 77.4 |
18.0, 77.8 |
49.3, 89.9 |
|
| p-value |
0.0084 |
0.0073 |
<0.0001 |
|
Number of Participants with ≥ 37.2 °C Temp2 |
22 (1.9%) |
21 (1.8%) |
12 (1.0%) |
41 (3.5%) |
| Prevention Efficacy |
46.5% |
49.6% |
71.1% |
|
| 95% CI (%) |
10.2, 69.3 |
14.8, 71.9 |
45.8, 86.1 |
|
| p-value |
0.0148 |
0.0083 |
<0.0001 |
|
|
ILI, influenza like illness; CI, confidence interval
1.CDC definition: ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥37.8°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough).
2.ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥37.2°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough) or ≥1 respiratory symptom and ≥1 systemic symptom (headache, feeling feverish, body aches/pains, fatigue).
3.Sample size (N) indicates evaluable population at time of primary analysis data cut (April 30, 2025).
|
Safety and tolerability data were similar in all arms with no safety signals observed. No drug-related serious adverse events were observed, and treatment-emergent adverse events showed no dose-dependent pattern between CD388 and placebo groups. Injection site reaction rates were similar across all CD388 dose groups and placebo.
The Company expects to present additional results from the NAVIGATE trial at upcoming scientific conferences in 2025.
The Company has submitted an end of Phase 2 meeting request to the U.S. Food and Drug Administration (the “FDA”) to review the Phase 2b results and further discuss the Phase 3 trial design and start time.
On June 23, 2025, the Company will host a conference call and webcast and present certain materials related to the Company (the “Presentation”). A copy of the Presentation is attached hereto as Exhibit 99.1 and incorporated herein by reference.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, including without limitation statements regarding potential benefits of and future plans for CD388, plans for engaging with the FDA and the impact of such discussions, a planned Phase 3 trial of CD388 and the expected timing for presenting additional results from the NAVIGATE trial. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including risks and uncertainties associated with the Company’s business and finances in general and those risks described under the caption “Risk Factors” in the Company’s Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2025, filed with the Securities and Exchange Commission (the “SEC”) on May 8, 2025 and other filings that the Company makes with the SEC in the future. Any forward-looking statements contained in this Current Report on Form 8-K speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
|
|
|
|
|
|
|
|
| Exhibit No. |
|
Description |
| 99.1 |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
|
| |
Cidara Therapeutics, Inc. |
| |
|
|
| Date: June 23, 2025 |
|
|
/s/ Jeffrey Stein, Ph.D. |
| |
|
|
Jeffrey Stein, Ph.D. |
| |
|
|
President and Chief Executive Officer
(Principal Executive Officer) |
EX-99.1
2
ex9912025-06x23.htm
PRESENTATION MATERIALS
ex9912025-06x23
NASDAQ: CDTX NAVIGATE Ph 2B June 23, 2025 JUNE 2025

2 Forward-looking Statements These slides contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The words “may,” “will,” “estimate,” “plan”, “anticipate,” “expect,” “potential,” “could,” “project,” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding Cidara’s research and development efforts; preclinical and clinical development activities; plans, projections and expectations for and the potential effectiveness, safety and benefits CD388; whether CD388 may have significant advantages beyond and in addition to flu vaccines; and advancement of its strategic plans. Projections, assumptions and estimates of the future performance of the markets in which Cidara operates are necessarily subject to a high degree of uncertainty and risk, including, Cidara's ability to obtain additional financing; the success and timing of Cidara’s preclinical studies, clinical trials and other research and development activities; receipt of necessary regulatory approvals for development, as well as changes to applicable regulatory laws in the United States and foreign countries; changes in Cidara's plans to develop its product candidates; Cidara's ability to obtain and maintain intellectual property protection for its product candidates; and the loss of key scientific or management personnel. These and other risks and uncertainties are described more fully in Cidara's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, filed with the United States Securities and Exchange Commission ("SEC“’) on March 6, 2025, and in Cidara’s other filings with the SEC. Additional risks and uncertainties may emerge from time to time, and it is not possible for Cidara’s management to predict all risk factors and uncertainties. Cidara cautions that the foregoing list of factors is not exclusive and not to place undue reliance upon any forward-looking statements which speak only as of the date of this presentation. Except as required by law, Cidara does not undertake any obligation to update publicly any forward- looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in its expectations.

3 Agenda Topic Discussant Introduction Jeff Stein, PhD NAVIGATE Phase 2b Trial Data Cut Nicole Davarpanah, MD, JD Q&A Discussion Cidara Management
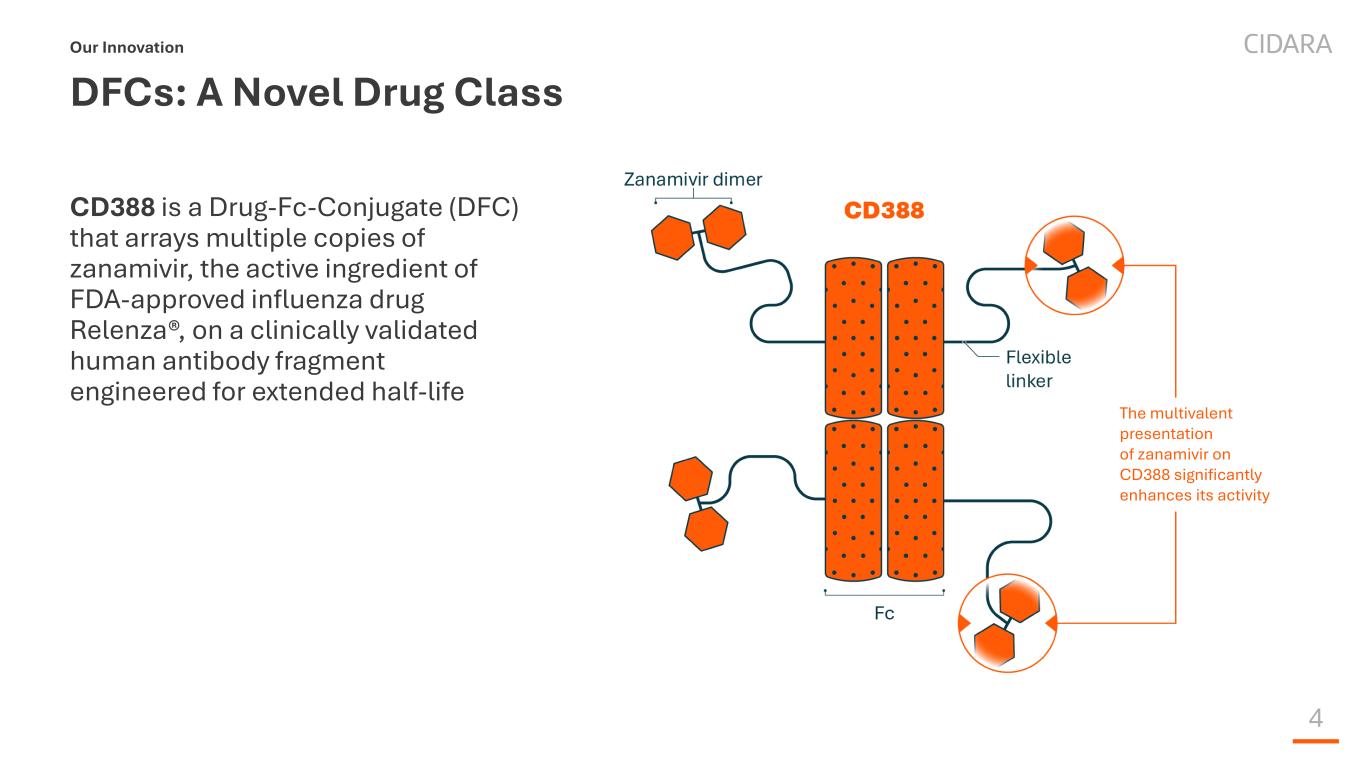
4 DFCs: A Novel Drug Class CD388 is a Drug-Fc-Conjugate (DFC) that arrays multiple copies of zanamivir, the active ingredient of FDA-approved influenza drug Relenza®, on a clinically validated human antibody fragment engineered for extended half-life Our Innovation The multivalent presentation of zanamivir on CD388 significantly enhances its activity

5 Executive Summary The NAVIGATE Study Met Primary and All Secondary Endpoints NAVIGATE Ph 2B Trial Abbreviations: Ph, phase. • The primary endpoint of Prevention Efficacy (PE) of protocol-defined Influenza-like illness (ILI) events at 24 weeks was met with statistical significance at each dose group • All secondary endpoints also met statistical significance in each dose group • Single doses of 450mg, 300mg and 150mg of CD388 conferred 76, 61 and 58% protection, respectively, from symptomatic influenza over 24 weeks compared to placebo • Placebo attack rate was 2.8% for primary endpoint • Safety and tolerability data were similar in all arms with no dose/safety relationship observed • Meeting request with the FDA has been submitted
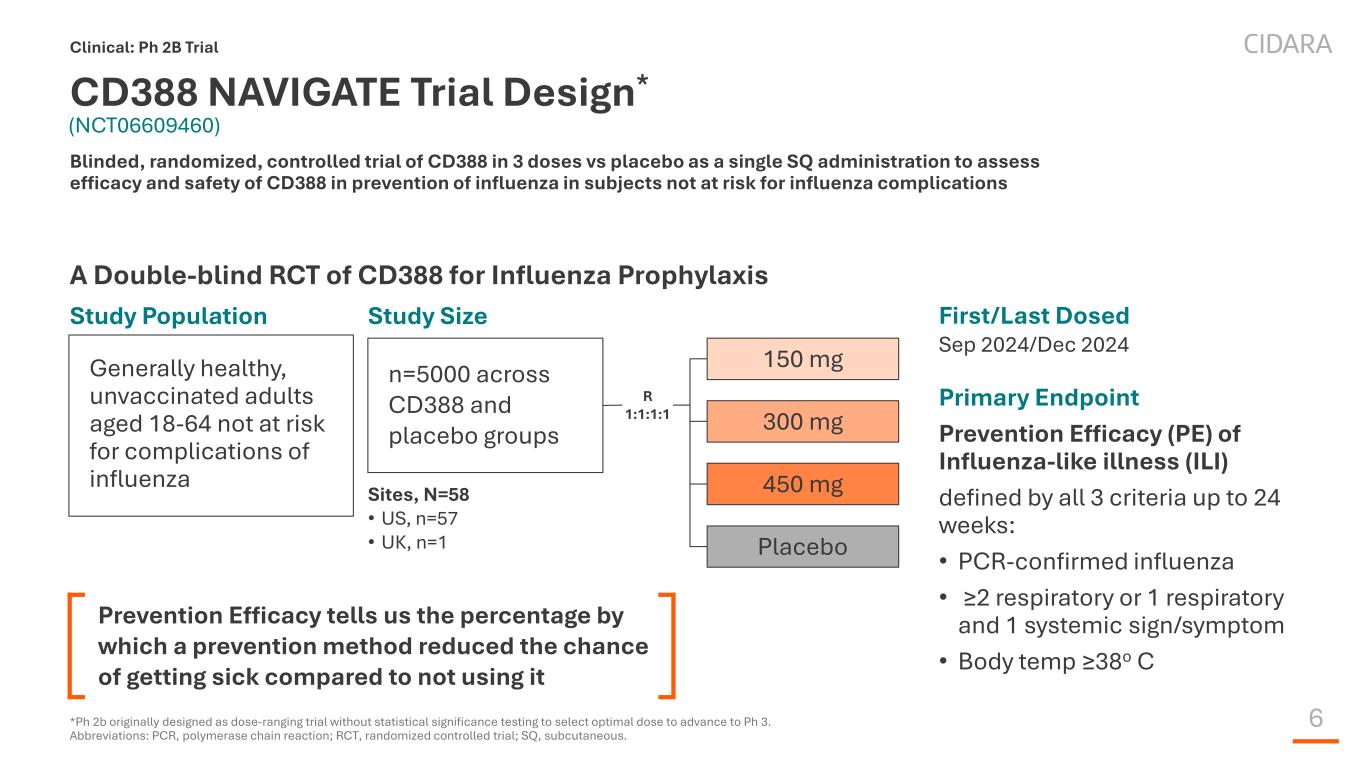
6 CD388 NAVIGATE Trial Design* A Double-blind RCT of CD388 for Influenza Prophylaxis Clinical: Ph 2B Trial Blinded, randomized, controlled trial of CD388 in 3 doses vs placebo as a single SQ administration to assess efficacy and safety of CD388 in prevention of influenza in subjects not at risk for influenza complications Primary Endpoint Prevention Efficacy (PE) of Influenza-like illness (ILI) defined by all 3 criteria up to 24 weeks: • PCR-confirmed influenza • ≥2 respiratory or 1 respiratory and 1 systemic sign/symptom • Body temp ≥38o C Study Population Sites, N=58 • US, n=57 • UK, n=1 150 mg 300 mg 450 mg Placebo n=5000 across CD388 and placebo groups Study Size Generally healthy, unvaccinated adults aged 18-64 not at risk for complications of influenza R 1:1:1:1 *Ph 2b originally designed as dose-ranging trial without statistical significance testing to select optimal dose to advance to Ph 3. Abbreviations: PCR, polymerase chain reaction; RCT, randomized controlled trial; SQ, subcutaneous. First/Last Dosed Sep 2024/Dec 2024 (NCT06609460) Prevention Efficacy tells us the percentage by which a prevention method reduced the chance of getting sick compared to not using it
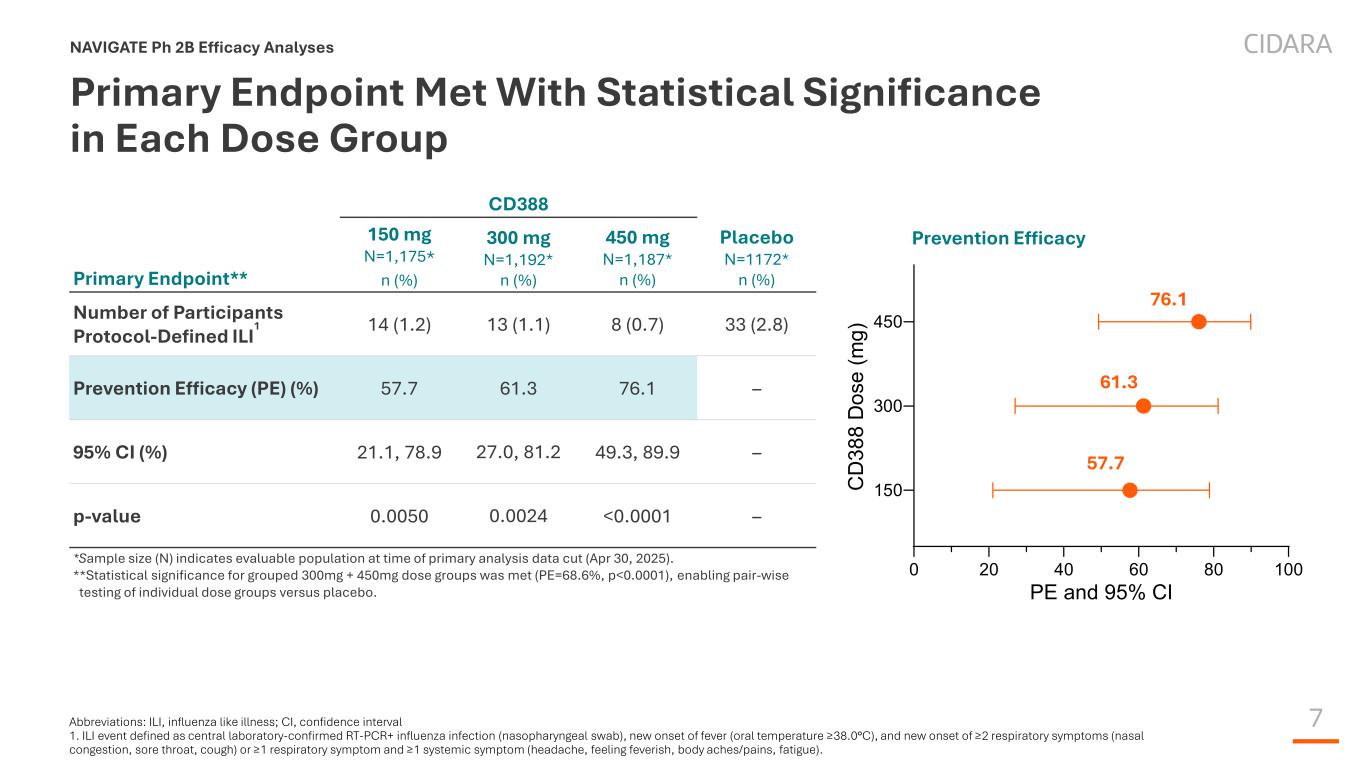
7 Primary Endpoint Met With Statistical Significance in Each Dose Group NAVIGATE Ph 2B Efficacy Analyses CD388 Primary Endpoint** 150 mg N=1,175* n (%) 300 mg N=1,192* n (%) 450 mg N=1,187* n (%) Placebo N=1172* n (%) Number of Participants Protocol-Defined ILI 1 14 (1.2) 13 (1.1) 8 (0.7) 33 (2.8) Prevention Efficacy (PE) (%) 57.7 61.3 76.1 – 95% CI (%) 21.1, 78.9 27.0, 81.2 49.3, 89.9 – p-value 0.0050 0.0024 <0.0001 – *Sample size (N) indicates evaluable population at time of primary analysis data cut (Apr 30, 2025). **Statistical significance for grouped 300mg + 450mg dose groups was met (PE=68.6%, p<0.0001), enabling pair-wise testing of individual dose groups versus placebo. 0 20 40 60 80 100 150 300 450 PE and 95% CI C D 38 8 D os e (m g) Prevention Efficacy 57.7 61.3 76.1 Abbreviations: ILI, influenza like illness; CI, confidence interval 1. ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥38.0°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough) or ≥1 respiratory symptom and ≥1 systemic symptom (headache, feeling feverish, body aches/pains, fatigue).
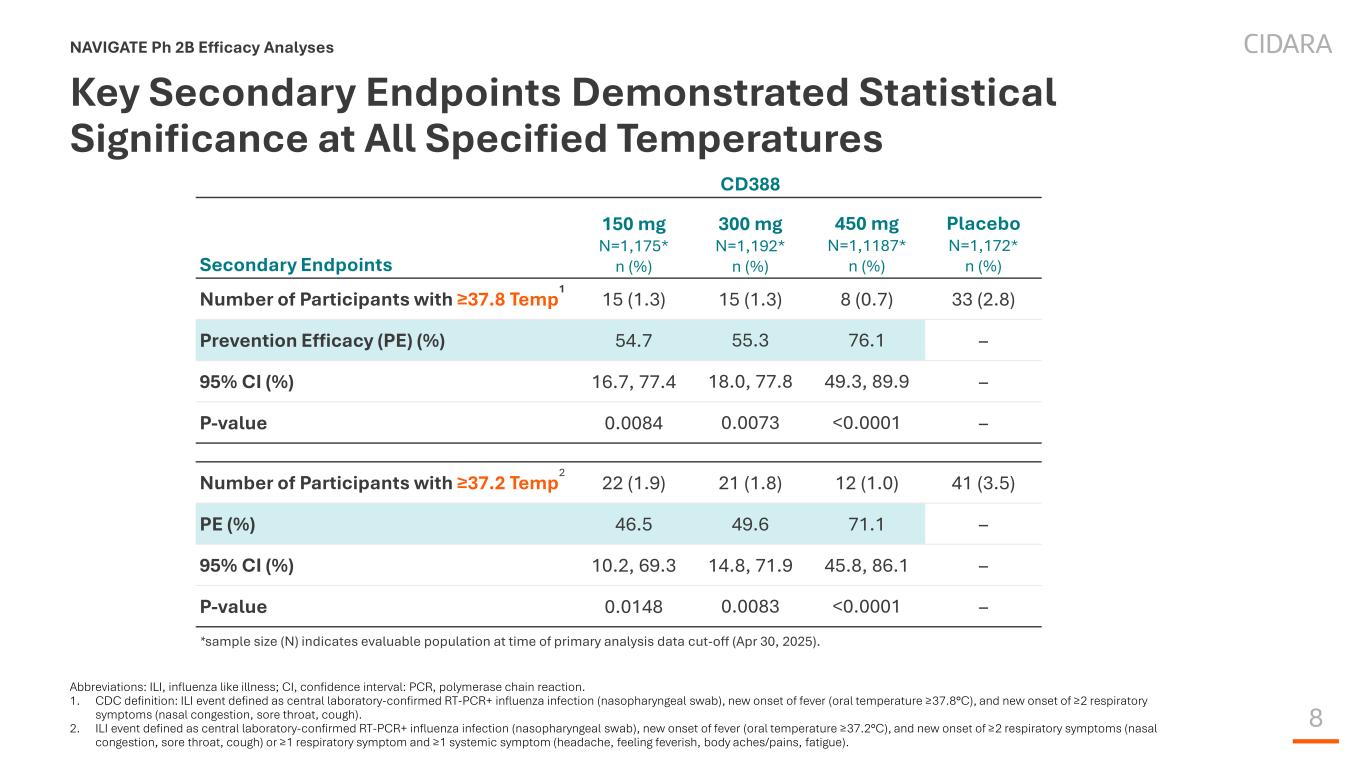
8 Key Secondary Endpoints Demonstrated Statistical Significance at All Specified Temperatures NAVIGATE Ph 2B Efficacy Analyses Abbreviations: ILI, influenza like illness; CI, confidence interval: PCR, polymerase chain reaction. 1. CDC definition: ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥37.8°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough). 2. ILI event defined as central laboratory-confirmed RT-PCR+ influenza infection (nasopharyngeal swab), new onset of fever (oral temperature ≥37.2°C), and new onset of ≥2 respiratory symptoms (nasal congestion, sore throat, cough) or ≥1 respiratory symptom and ≥1 systemic symptom (headache, feeling feverish, body aches/pains, fatigue). CD388 Secondary Endpoints 150 mg N=1,175* n (%) 300 mg N=1,192* n (%) 450 mg N=1,1187* n (%) Placebo N=1,172* n (%) Number of Participants with ≥37.8 Temp 1 15 (1.3) 15 (1.3) 8 (0.7) 33 (2.8) Prevention Efficacy (PE) (%) 54.7 55.3 76.1 – 95% CI (%) 16.7, 77.4 18.0, 77.8 49.3, 89.9 – P-value 0.0084 0.0073 <0.0001 – Number of Participants with ≥37.2 Temp 2 22 (1.9) 21 (1.8) 12 (1.0) 41 (3.5) PE (%) 46.5 49.6 71.1 – 95% CI (%) 10.2, 69.3 14.8, 71.9 45.8, 86.1 – P-value 0.0148 0.0083 <0.0001 – *sample size (N) indicates evaluable population at time of primary analysis data cut-off (Apr 30, 2025).
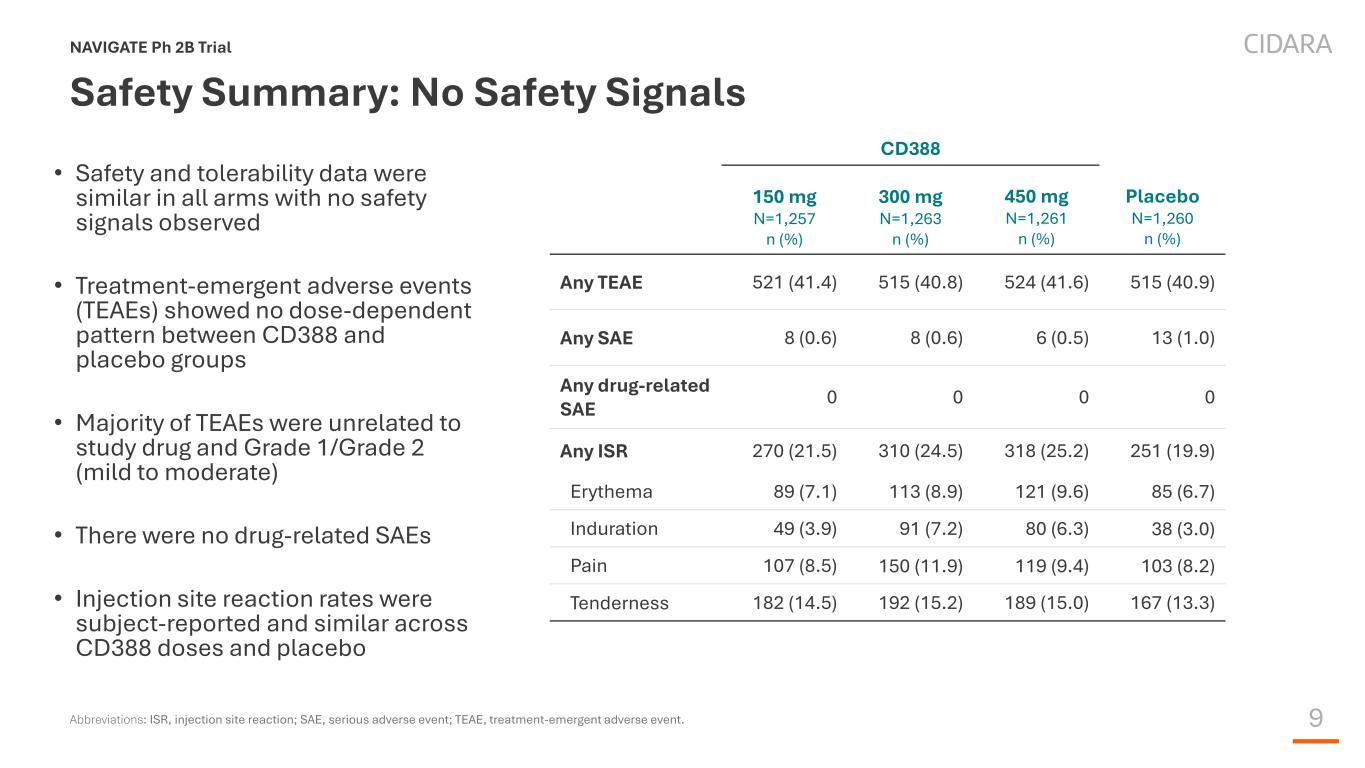
9 Safety Summary: No Safety Signals • Safety and tolerability data were similar in all arms with no safety signals observed • Treatment-emergent adverse events (TEAEs) showed no dose-dependent pattern between CD388 and placebo groups • Majority of TEAEs were unrelated to study drug and Grade 1/Grade 2 (mild to moderate) • There were no drug-related SAEs • Injection site reaction rates were subject-reported and similar across CD388 doses and placebo NAVIGATE Ph 2B Trial Abbreviations: ISR, injection site reaction; SAE, serious adverse event; TEAE, treatment-emergent adverse event. CD388 150 mg N=1,257 n (%) 300 mg N=1,263 n (%) 450 mg N=1,261 n (%) Placebo N=1,260 n (%) Any TEAE 521 (41.4) 515 (40.8) 524 (41.6) 515 (40.9) Any SAE 8 (0.6) 8 (0.6) 6 (0.5) 13 (1.0) Any drug-related SAE 0 0 0 0 Any ISR 270 (21.5) 310 (24.5) 318 (25.2) 251 (19.9) Erythema 89 (7.1) 113 (8.9) 121 (9.6) 85 (6.7) Induration 49 (3.9) 91 (7.2) 80 (6.3) 38 (3.0) Pain 107 (8.5) 150 (11.9) 119 (9.4) 103 (8.2) Tenderness 182 (14.5) 192 (15.2) 189 (15.0) 167 (13.3)
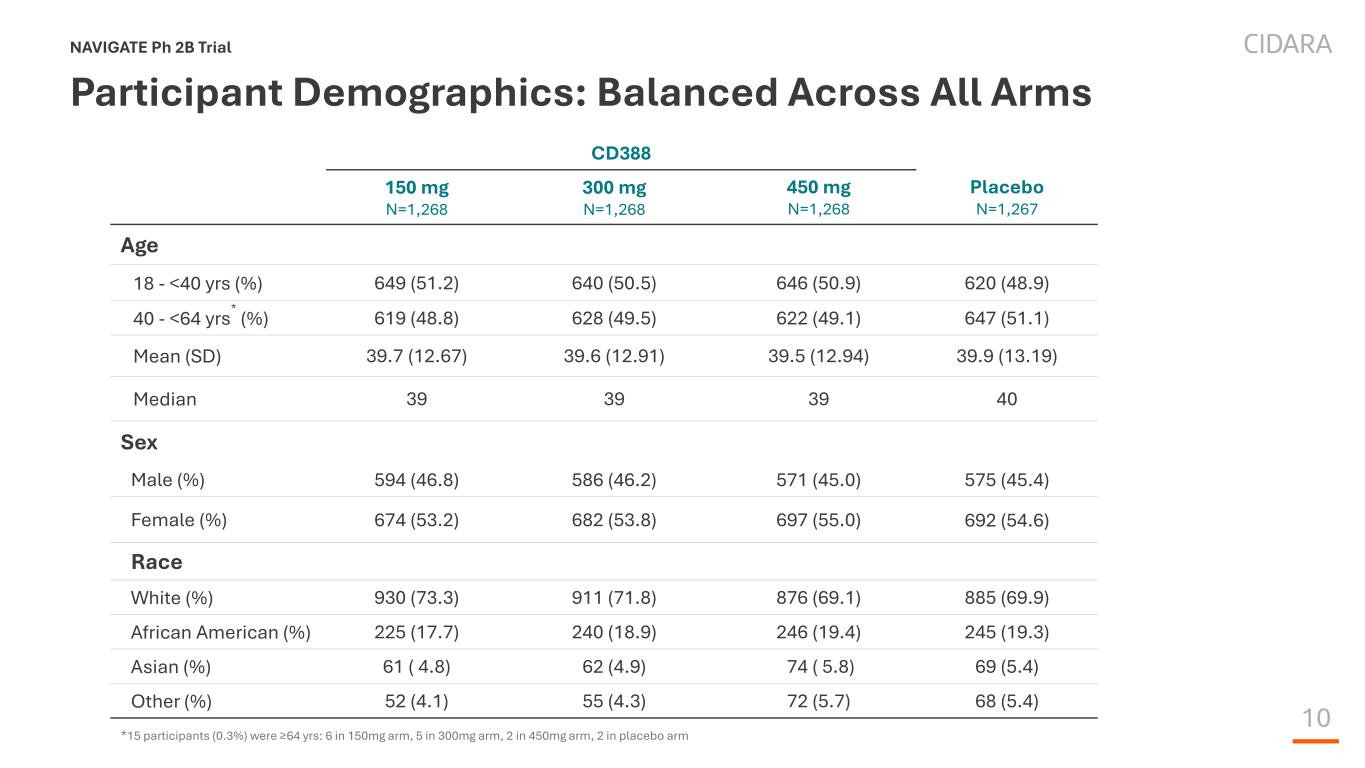
10 Participant Demographics: Balanced Across All Arms NAVIGATE Ph 2B Trial CD388 150 mg N=1,268 300 mg N=1,268 450 mg N=1,268 Placebo N=1,267 Age 18 - <40 yrs (%) 649 (51.2) 640 (50.5) 646 (50.9) 620 (48.9) 40 - <64 yrs* (%) 619 (48.8) 628 (49.5) 622 (49.1) 647 (51.1) Mean (SD) 39.7 (12.67) 39.6 (12.91) 39.5 (12.94) 39.9 (13.19) Median 39 39 39 40 Sex Male (%) 594 (46.8) 586 (46.2) 571 (45.0) 575 (45.4) Female (%) 674 (53.2) 682 (53.8) 697 (55.0) 692 (54.6) Race White (%) 930 (73.3) 911 (71.8) 876 (69.1) 885 (69.9) African American (%) 225 (17.7) 240 (18.9) 246 (19.4) 245 (19.3) Asian (%) 61 ( 4.8) 62 (4.9) 74 ( 5.8) 69 (5.4) Other (%) 52 (4.1) 55 (4.3) 72 (5.7) 68 (5.4) *15 participants (0.3%) were ≥64 yrs: 6 in 150mg arm, 5 in 300mg arm, 2 in 450mg arm, 2 in placebo arm

11 Next Steps • Full primary analysis details will be submitted to upcoming scientific conferences in 2025 • Dose for Phase 3 will be selected after full PK data is assessed • Drug supply for any dose is available to begin Phase 3 • End of Phase 2 FDA meeting request has been submitted to continue discussion on Phase 3 study design and start time NAVIGATE Ph 2B Trial

Thank You cidara.com











