UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (date of earliest event reported): October 30, 2025
iBio, Inc.
(Exact name of registrant as specified in charter)
Delaware
(State or other jurisdiction of incorporation)
001-35023 |
26-2797813 |
(Commission File Number) |
(IRS Employer Identification No.) |
11750 Sorrento Valley Road, Suite 200
San Diego, California 92121
(Address of principal executive offices and zip code)
(979) 446-0027
(Registrant’s telephone number including area code)
N/A
(Former Name and Former Address)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12(b) under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
Common Stock, $0.001 par value per share |
IBIO |
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by checkmark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01. Regulation FD Disclosure.
On October 30, 2025, iBio, Inc. (the “Company”) issued a press release announcing new preclinical data from its obese non-human primate (NHP) study evaluating IBIO-610, potentially a first-in-class Activin E antibody candidate supported by preclinical data. The data will be presented by Cory Schwartz, Ph.D., Director of Research and Early Development of the Company, during an oral session at ObesityWeek 2025, taking place November 4, 2025 through November 7, 2025 in Atlanta.
Results from the obese NHP study evaluating IBIO-610 are set forth below:
The pharmacokinetic data, to be presented at ObesityWeek, demonstrates IBIO-610 has an extended half-life in obese NHP of 33.2 days. Based on an allomeric scaling model of half-life extended antibodies, it is predicted IBIO-610 will have a half-life in humans of up to 100 days, reducing the dosing frequency to once every six months, which has the potential to significantly improve patient experience.
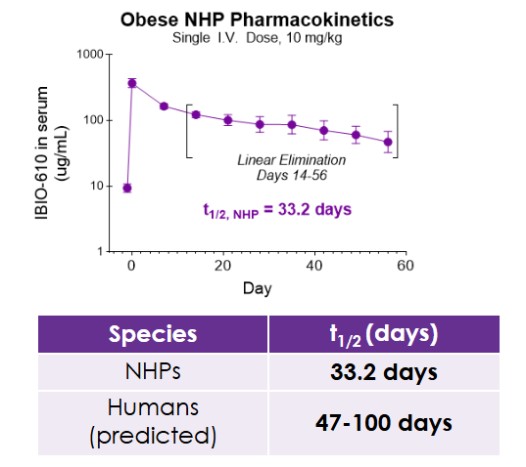
PK of IBIO-610 in NHPs and Allometric Scaling Predicting Human Half-life
The information in this Item 7.01 and in the press release furnished as Exhibit 99.1 to this Current Report on Form 8-K shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended and shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
The press release furnished as Exhibit 99.1 to this Current Report on Form 8-K includes “safe harbor” language pursuant to the Private Securities Litigation Reform Act of 1995, as amended, indicating that certain statements contained therein are “forward-looking” rather than historical.
Item 8.01. Other Events.
On October 30, 2025, the Company issued a press release announcing new preclinical data from its obese NHP study evaluating IBIO-610, potentially a first-in-class Activin E antibody candidate supported by preclinical data. The data will be presented by Cory Schwartz, Ph.D., Director of Research and Early Development of the Company, during an oral session at ObesityWeek 2025, taking place November 4, 2025 through November 7, 2025 in Atlanta.
Results from the obese NHP study evaluating IBIO-610 are set forth below:
The pharmacokinetic data, to be presented at ObesityWeek, demonstrates IBIO-610 has an extended half-life in obese NHP of 33.2 days. Based on an allomeric scaling model of half-life extended antibodies, it is predicted IBIO-610 will have a half-life in humans of up to 100 days, reducing the dosing frequency to once every six months, which has the potential to significantly improve patient experience.
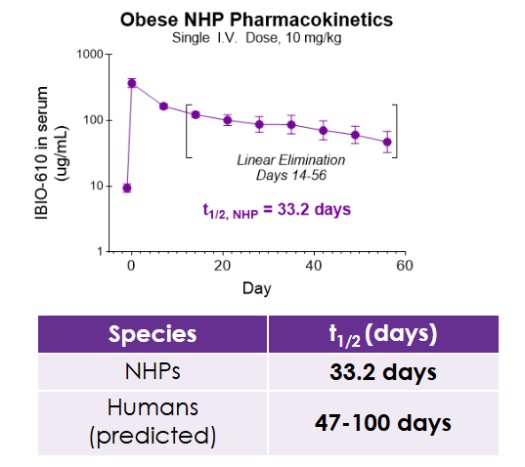
PK of IBIO-610 in NHPs and Allometric Scaling Predicting Human Half-life
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
Exhibit |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Date: October 30, 2025 |
IBIO, INC. |
||
|
|
||
|
By: |
/s/ Marc A. Banjak |
|
|
|
Name: |
Marc A. Banjak |
|
|
Title: |
Chief Legal Counsel |
Exhibit 99.1
iBio Unveils New Non-Human Primate Data on IBIO-610, an Activin E Antibody with Strong Therapeutic Potential for Fat-Selective Weight-Loss and Weight Maintenance
Previously reported data showed that in a diet-induced obesity mouse model, IBIO-610 drives fat-selective, GLP-1-synergistic weight loss and prevents weight regain following GLP-1 treatment discontinuation
New non-human primate data projects a human half-life of up to 100 days, potentially enabling treatment with only twice-yearly dosing
Extended half-life data and differentiated mechanism of action reinforce IBIO-610’s potential as a leading next-generation therapy for obesity and cardiometabolic disease
SAN DIEGO, Oct. 30, 2025 (GLOBE NEWSWIRE) -- iBio, Inc. (NASDAQ: IBIO), an AI-driven innovator of precision antibody therapies, today announced new preclinical data from its obese non-human primate (NHP) study evaluating IBIO-610, potentially a first-in-class Activin E antibody candidate supported by preclinical data. The new data demonstrates an extended half-life of 33.2 days in NHPs and a predicted human half-life of up to 100 days, suggesting the potential for dosing as infrequently as twice per year. The findings will be presented by Cory Schwartz, Ph.D., Director of Research and Early Development of iBio, during an oral session at ObesityWeek® 2025, taking place November 4–7 in Atlanta.
“GLP-1 therapies have transformed the treatment for obesity, but gaps in patient care remain for durable options addressing biology beyond appetite control,” said Martin Brenner, DVM, Ph.D., Chief Executive Officer and Chief Scientific Officer of iBio. “Our AI-enabled discovery platform has accomplished what was long considered extremely difficult - creating potentially a first-in-class long-acting antibody against Activin E. We believe this modality offers deep, sustained pathway blockade with infrequent dosing while leveraging the scalability and reliability of the global antibody manufacturing infrastructure. These advances underscore the differentiated potential of IBIO-610 and the broader power of our platform to unlock challenging, next-generation targets in obesity and cardiometabolic disease.”
The pharmacokinetic data, to be presented at ObesityWeek, demonstrates IBIO-610 has an extended half-life in obese non-human primates of 33.2 days. Based on an allomeric scaling model of half-life extended antibodies1,2, it is predicted IBIO-610 will have a half-life in humans of up to 100 days, reducing the dosing frequency to once every six months, which has the potential to significantly improve patient experience.
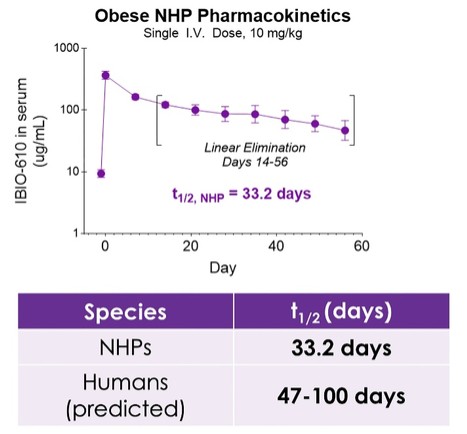
“We are encouraged by these findings, as the combination of extended half-life and strong mechanistic validation in our mouse models underscores the differentiated profile of IBIO-610,” said Dr. Schwartz. “We believe that antibody-mediated targeting of Activin E has the potential to enable more comprehensive pathway modulation than other therapeutic approaches for the treatment of obesity. With its long-acting profile and fat-selective biology, IBIO-610 could not only promote fat-selective weight loss but also serve as an option for individuals transitioning off GLP-1 therapies, helping them maintain results without frequent injections, daily pills, or the side effects associated with GLP-1 agonism.”
References
1. |
Nakamura G, Ozeki K, Nagayasu M, Nambu T, Nemoto T, Hosoya KI. Predicting Method for the Human Plasma Concentration-Time Profile of a Monoclonal Antibody from the Half-life of Non-human Primates. Biol Pharm Bull. 2020;43(5):823-830. doi: 10.1248/bpb.b19-01042. PMID: 32378559. |
2. |
Haraya K, Tachibana T. Translational Approach for Predicting Human Pharmacokinetics of Engineered Therapeutic Monoclonal Antibodies with Increased FcRn-Binding Mutations. BioDrugs. 2023 Jan;37(1):99-108. doi: 10.1007/s40259-022-00566-2. Epub 2022 Nov 30. PMID: 36449140; PMCID: PMC9709760. |
About iBio, Inc.
iBio (Nasdaq: IBIO) is a cutting-edge biotech company leveraging AI and advanced computational biology to develop next-generation biopharmaceuticals for cardiometabolic diseases, obesity, cancer and other hard-to-treat diseases. By combining proprietary 3D modeling with innovative drug discovery platforms, iBio is creating a pipeline of breakthrough antibody treatments to address significant unmet medical needs. Our mission is to transform drug discovery, accelerate development timelines, and unlock new possibilities in precision medicine. For more information, visit www.ibioinc.com or follow us on LinkedIn.
Forward-Looking Statements
Certain statements in this press release constitute "forward-looking statements" within the meaning of the federal securities laws. Words such as "may," "might," "will," "should," "believe," "expect," "anticipate," "estimate," "continue," "predict," "forecast," "project," "plan," "intend" or similar expressions, or statements regarding intent, belief, or current expectations, are forward-looking statements. These forward-looking statements are based upon current estimates and assumptions and include statements regarding the therapeutic potential of Activin E as a target for cardiometabolic disorders and obesity; IBIO-610 preclinical data predicting human half-life of up to 100 days, suggesting the potential for dosing as infrequently as twice per year; the presentation of preclinical data during an oral session at ObesityWeek 2025; the Company’s AI-enabled discovery platform creating a potentially first-in-class long-acting antibody against Activin E, offering a deep, sustained pathway blockade with infrequent dosing while leveraging the scalability and reliability of the global antibody manufacturing infrastructure; the potential of IBIO-610 and the broader power of the Company’s platform to unlock challenging, next-generation targets in obesity and cardiometabolic disease; IBIO-610 having a half-life in humans of up to 100 days, reducing the dosing frequency to once every six months, potentially significantly improving patient experience; the potential of the antibody-mediated targeting of Activin E to enable more comprehensive pathway modulation than other therapeutic approaches for the treatment of obesity; and the ability of IBIO-610 to promote fat-selective weight loss and to serve as an option for individuals transitioning off GLP-1 therapies, helping them maintain results without frequent injections, daily pills, or the side effects associated with GLP-1 agonism. While iBio believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based on information available to us on the date of this release. These forward-looking statements are subject to various risks and uncertainties, many of which are difficult to predict that could cause actual results to differ materially from current expectations and assumptions from those set forth or implied by any forward-looking statements. Important factors that could cause actual results to differ materially from current expectations include, among others, the ability of Activin E to be a successful target for cardiometabolic disorders and obesity; the Company’s AI-enabled discovery platform creating a potentially first-in-class long-acting antibody against Activin E, offering a deep, sustained pathway blockade with infrequent dosing while leveraging the scalability and reliability of the global antibody manufacturing infrastructure; the ability of IBIO-610 to promote fat-selective weight loss and to serve as an option for individuals transitioning off GLP-1 therapies, helping them maintain results without frequent injections, daily pills, or the side effects associated with GLP-1 agonism; iBio’s ability to obtain regulatory approvals for commercialization of its product candidates, or to comply with ongoing regulatory requirements; regulatory limitations relating to iBio’s ability to promote or commercialize its product candidates for specific indications; acceptance of iBio’s product candidates in the marketplace and the successful development, marketing or sale of products; and whether iBio will incur unforeseen expenses or liabilities or other market factors; and the other factors discussed in iBio’s filings with the SEC including its Annual Report on Form 10-K for the year ended June 30, 2025 and its subsequent filings with the SEC on Forms 10-Q and 8-K. The information in this release is provided only as of the date of this release, and iBio undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
Corporate Contact:
iBio, Inc.
Investor Relations
ir@ibioinc.com
Media Contacts:
Ignacio Guerrero-Ros, Ph.D., or David Schull
Russo Partners, LLC
Ignacio.guerrero-ros@russopartnersllc.com
David.schull@russopartnersllc.com
(858) 717-2310 or (646) 942-5604

Source: iBio, Inc.

PK of IBIO-610 in NHPs and Allometric Scaling Predicting Human Half-life