Document
Xencor Presents Initial Data for XmAb819, a First-in-class ENPP3 x CD3 Bispecific T-Cell Engager, in Development for Clear Cell Renal Cell Carcinoma
-- XmAb819 is well-tolerated in heavily pretreated patients with advanced ccRCC --
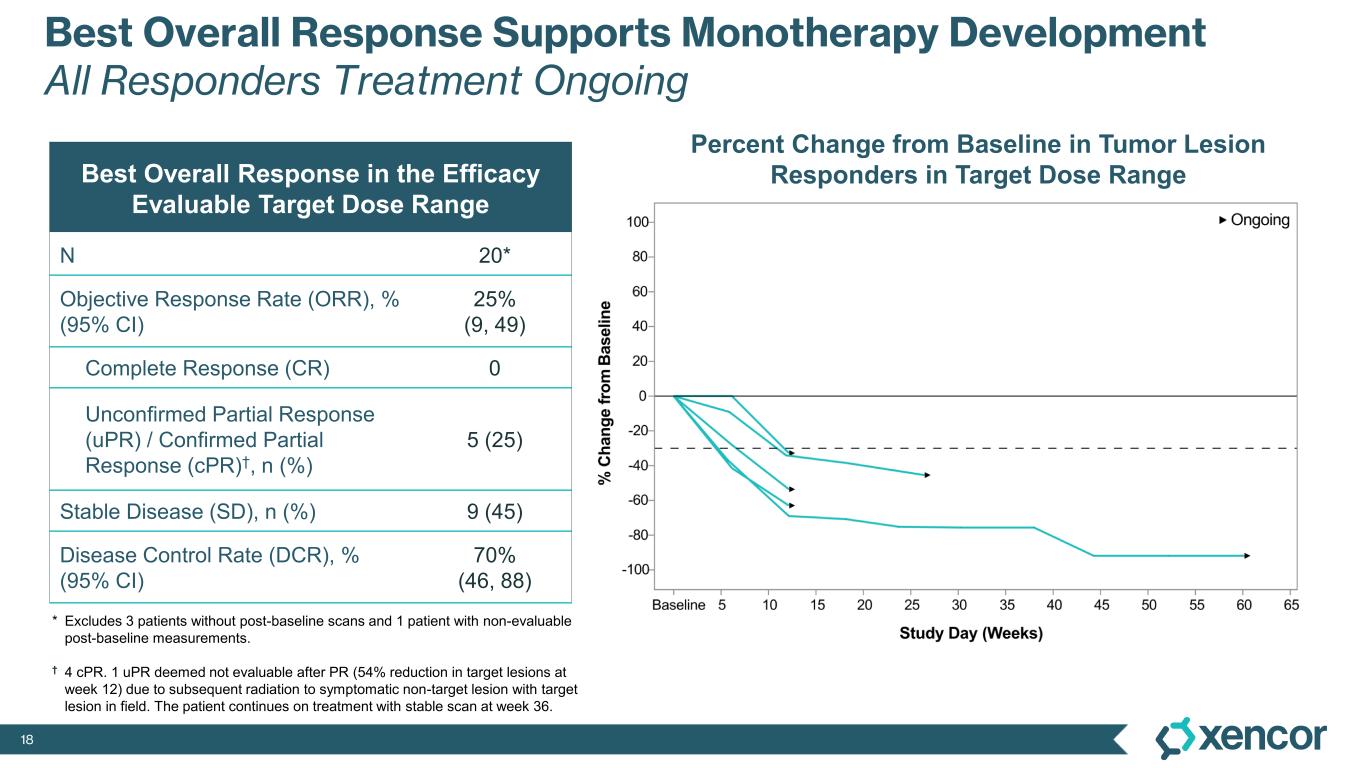
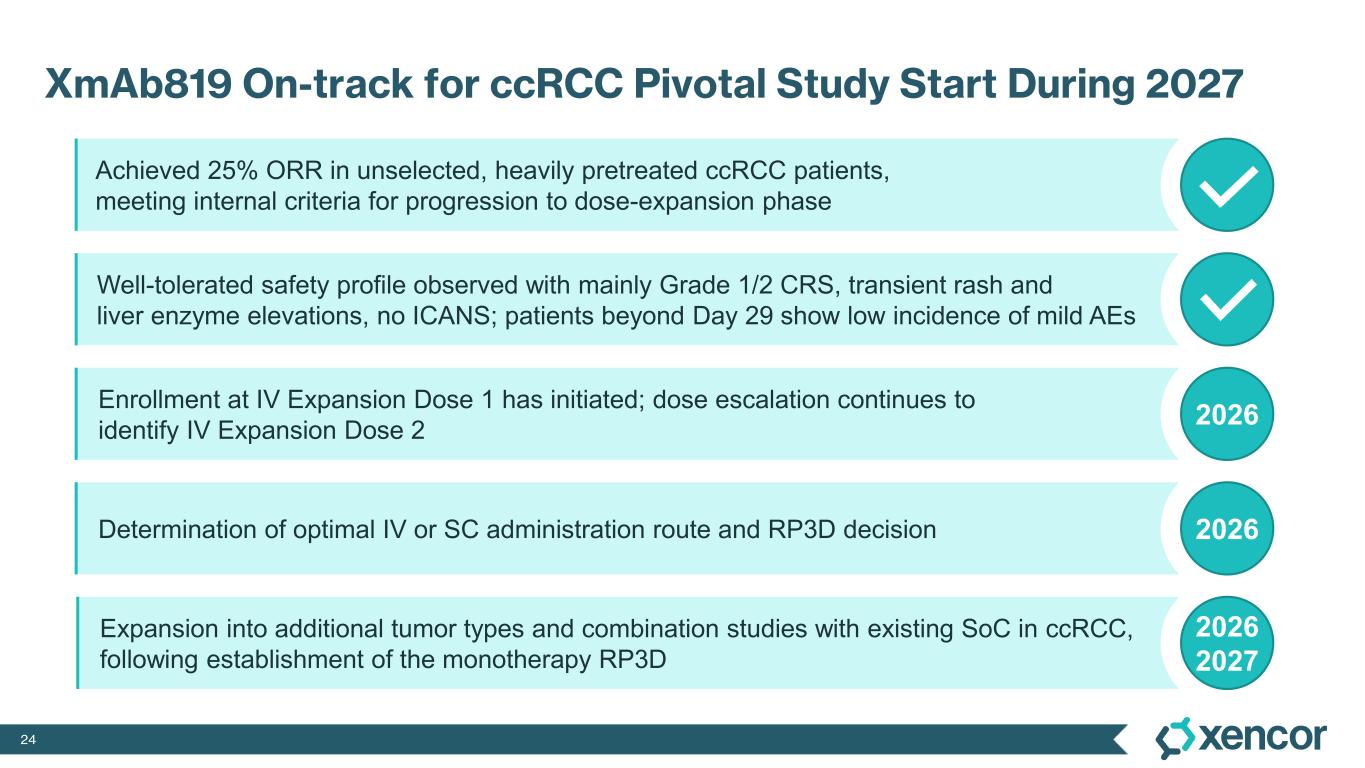
-- 25% overall response rate (ORR) observed within the target dose range --
-- First dose-expansion cohort has been selected; dose escalation continues to identify dose for second expansion cohort --
-- Management hosting webcast and conference call today at 1:30 p.m. ET / 10:30 a.m. PT –
PASADENA, Calif.--Oct. 24, 2025-- Xencor, Inc. (NASDAQ:XNCR), a clinical-stage biopharmaceutical company developing engineered antibodies for the treatment of cancer and autoimmune diseases, today announced initial results from the ongoing Phase 1 dose-escalation study of XmAb819, a ENPP3 x CD3 T-cell engaging bispecific antibody, in patients with advanced clear cell renal cell carcinoma (ccRCC). The results were presented in a poster at the AACR-NCI-EORTC Conference on Molecular Targets and Cancer Therapeutics in Boston, Massachusetts.
“We are excited to be developing XmAb819 as a novel first-in-class ENPP3 T-cell engager that could potentially offer a much-needed new therapeutic modality for patients with advanced clear cell renal cell carcinoma and clinicians. In our first clinical presentation of dose-escalation data, XmAb819 demonstrated compelling anti-tumor activity and a well-tolerated safety profile in very heavily pre-treated patients,” said Bassil Dahiyat, Ph.D., president and chief executive officer at Xencor. “We are on-track with our first dose-expansion cohort now selected and are enrolling patients as we continue to dose escalate. We are confident that we will be able to select a recommended Phase 3 dose during 2026 to support the initiation of our first pivotal study in advanced ccRCC during 2027.”
The poster will be archived under "Events & Presentations" in the Investors section of the Company's website located at www.xencor.com.
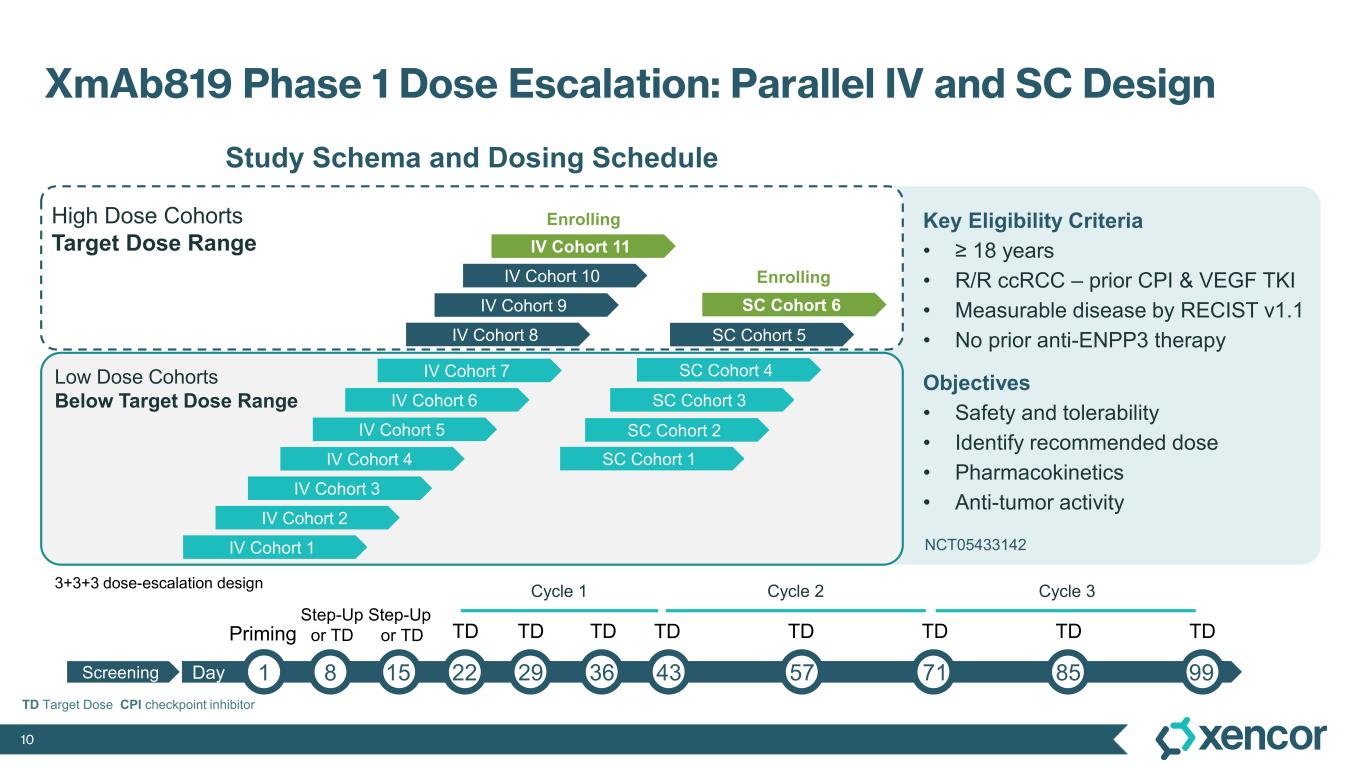
Phase 1 Study of XmAb819 in Advanced ccRCC
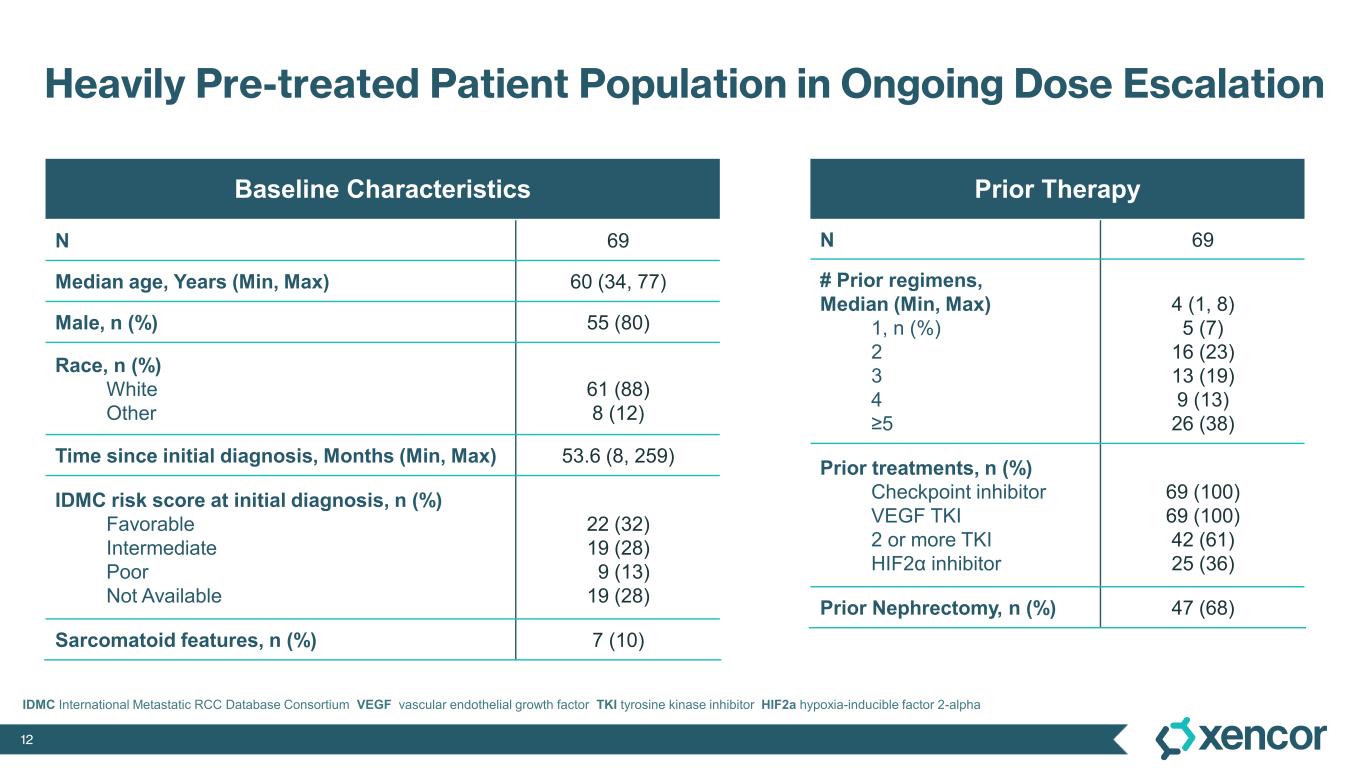
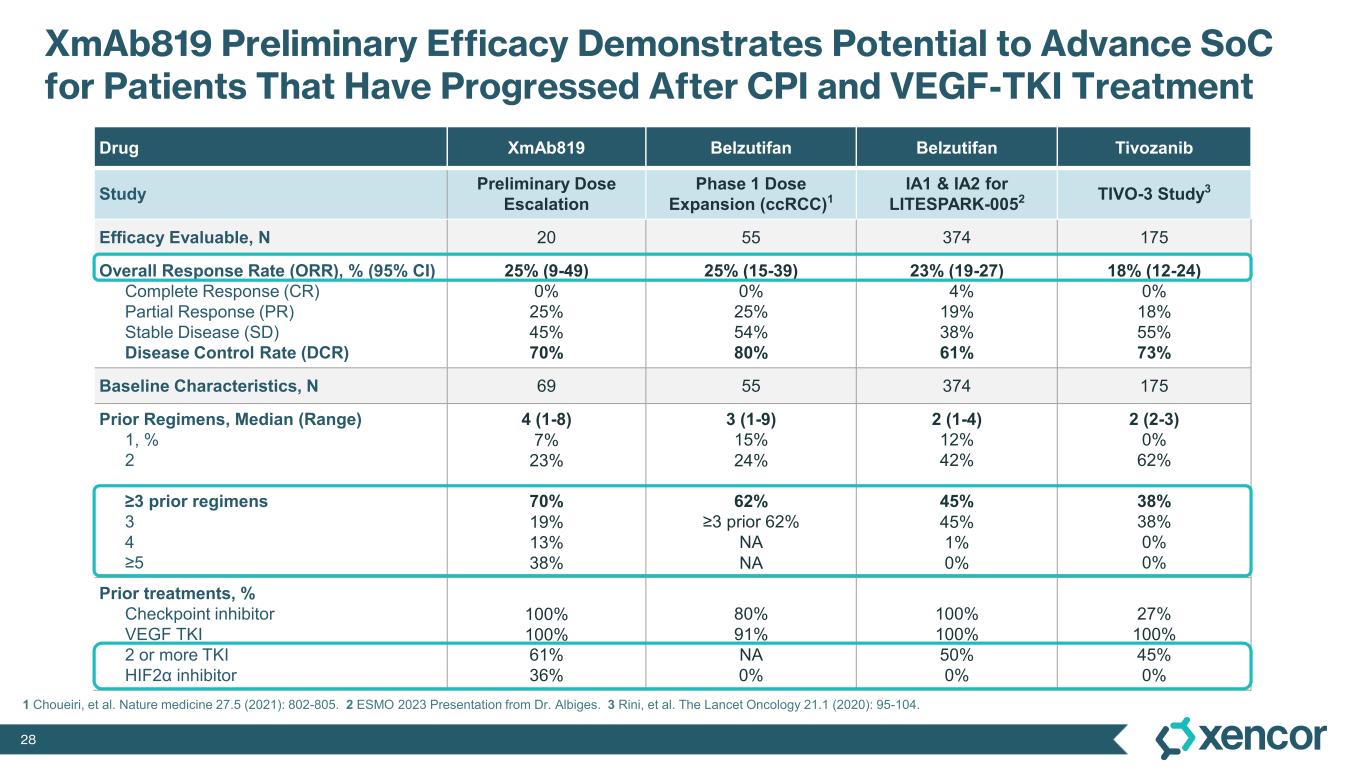
The Phase 1 clinical trial is a multicenter, open-label, dose-escalation and dose-expansion study designed to evaluate XmAb819 as monotherapy in patients with advanced ccRCC. As of the September 19, 2025 data cut-off, 69 patients received XmAb819 across 10 intravenous (IV) dose cohorts and 5 subcutaneous (SC) dose cohorts. Patients received a median of 4 prior lines of therapy (range 1-8). All patients received prior anti-PD1 therapy and prior VEGF-TKI therapy, and 36% of patients were previously treated with a HIF2α inhibitor. Efficacy was assessed by investigators using RECIST v1.1.
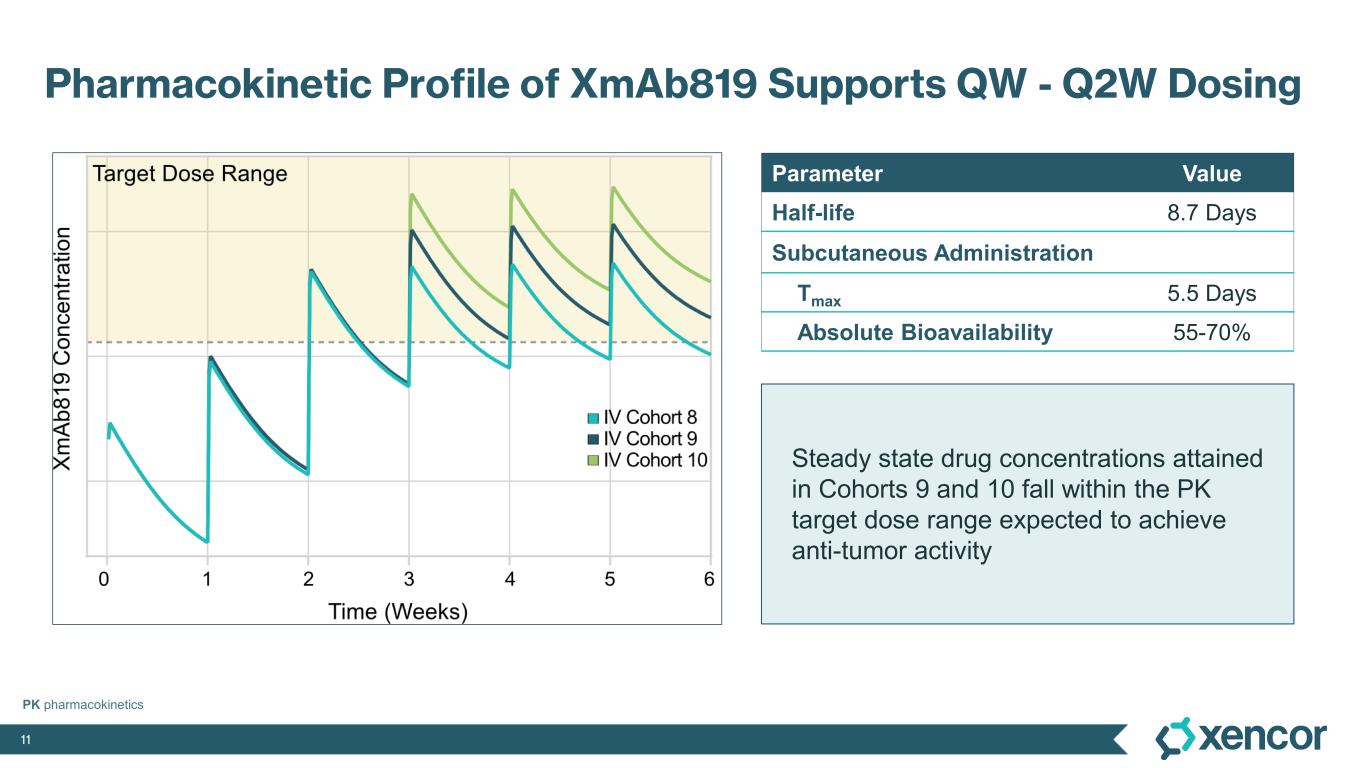
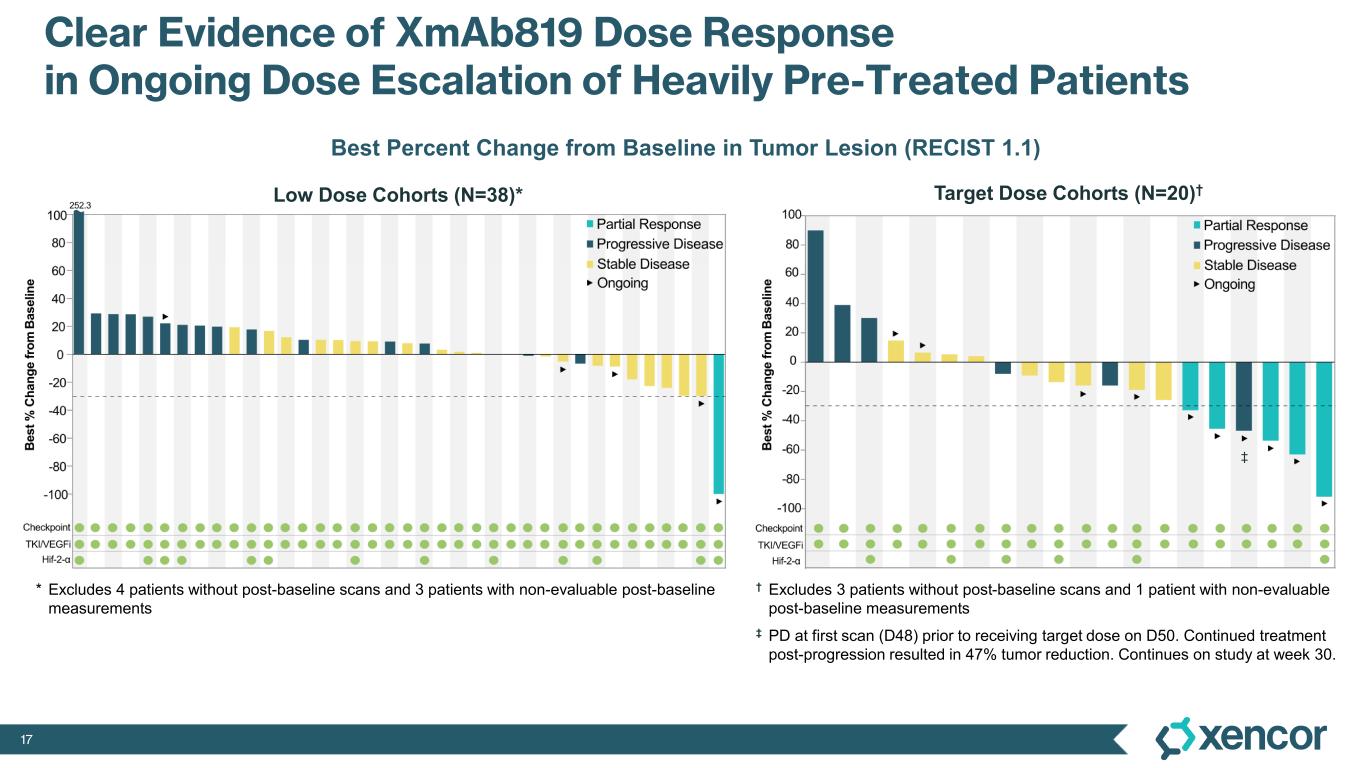
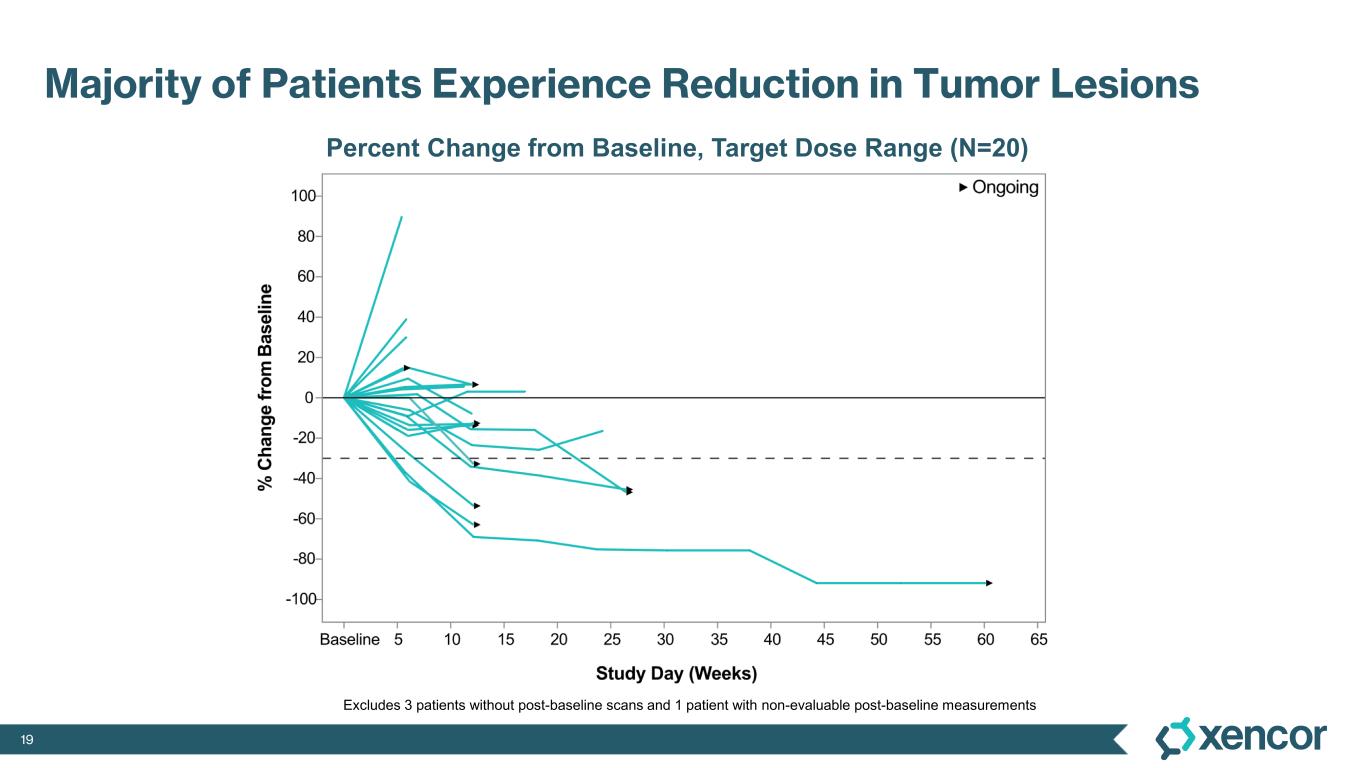
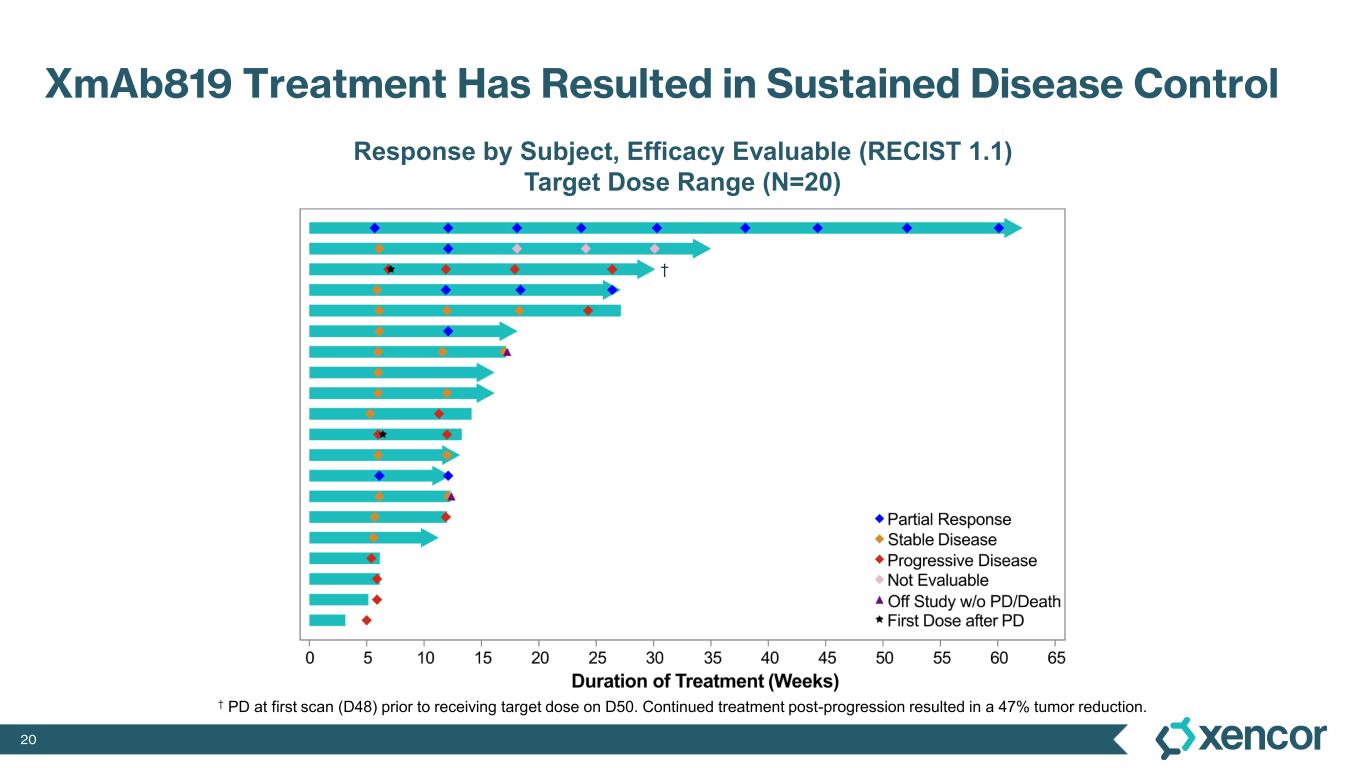
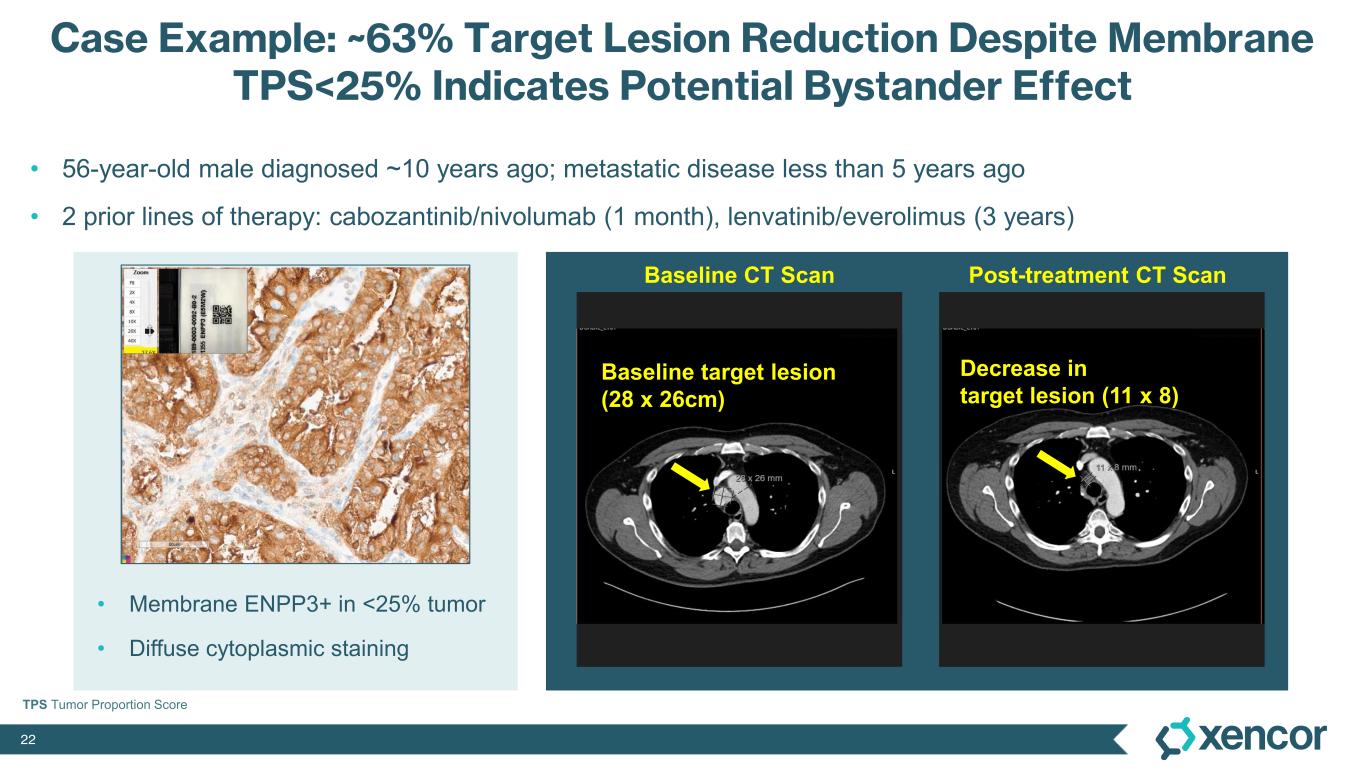
XmAb819 demonstrated evidence of anti-tumor activity and an acceptable safety profile that was generally well tolerated across dose levels. Of the 20 efficacy-evaluable patients treated at the dose levels that were preclinically predicted to be within the target dose range, 25% achieved a partial response (PR) as best response (n=5, 4 confirmed PRs and 1 unconfirmed PR), with a 70% disease control rate (DCR, 14/20). All five responders remain on treatment, and 50% (10/20) of all efficacy-evaluable patients within the target dose range remain on treatment. For one patient with a first-scan assessment of progressive disease, a subsequent 47% reduction in target lesions was reported, and the patient remains on-treatment as of the data cut-off (treatment week 30). All 6 patients within the target dose range with greater than 30% reduction in lesion size remain on treatment as of the data cut-off.
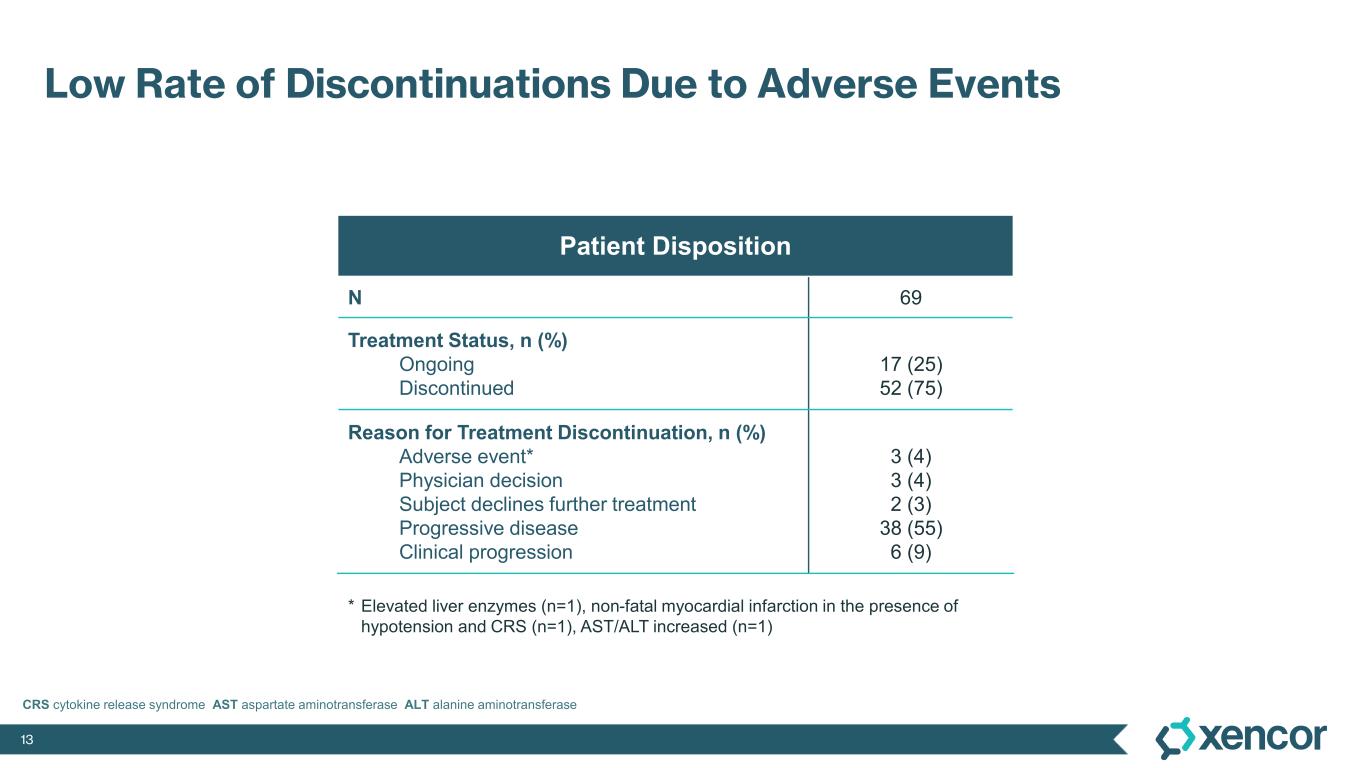
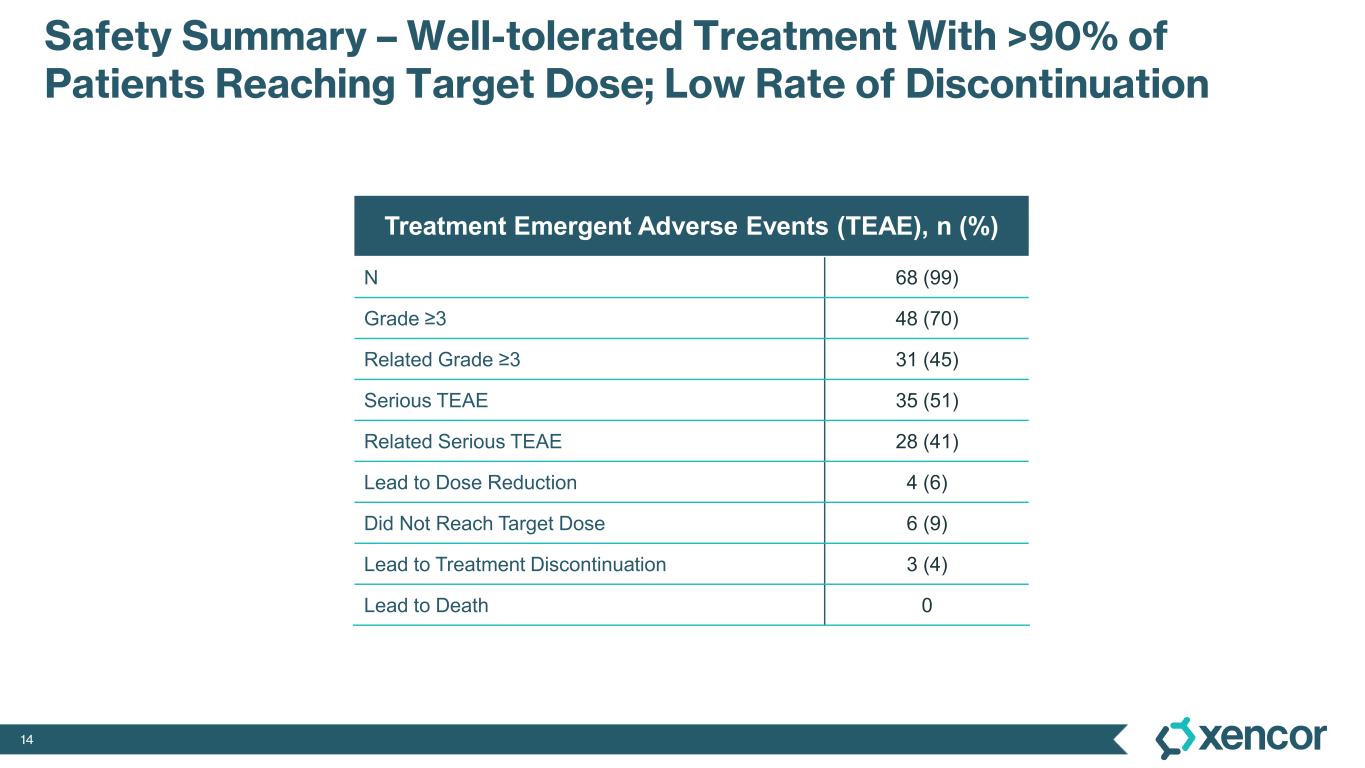
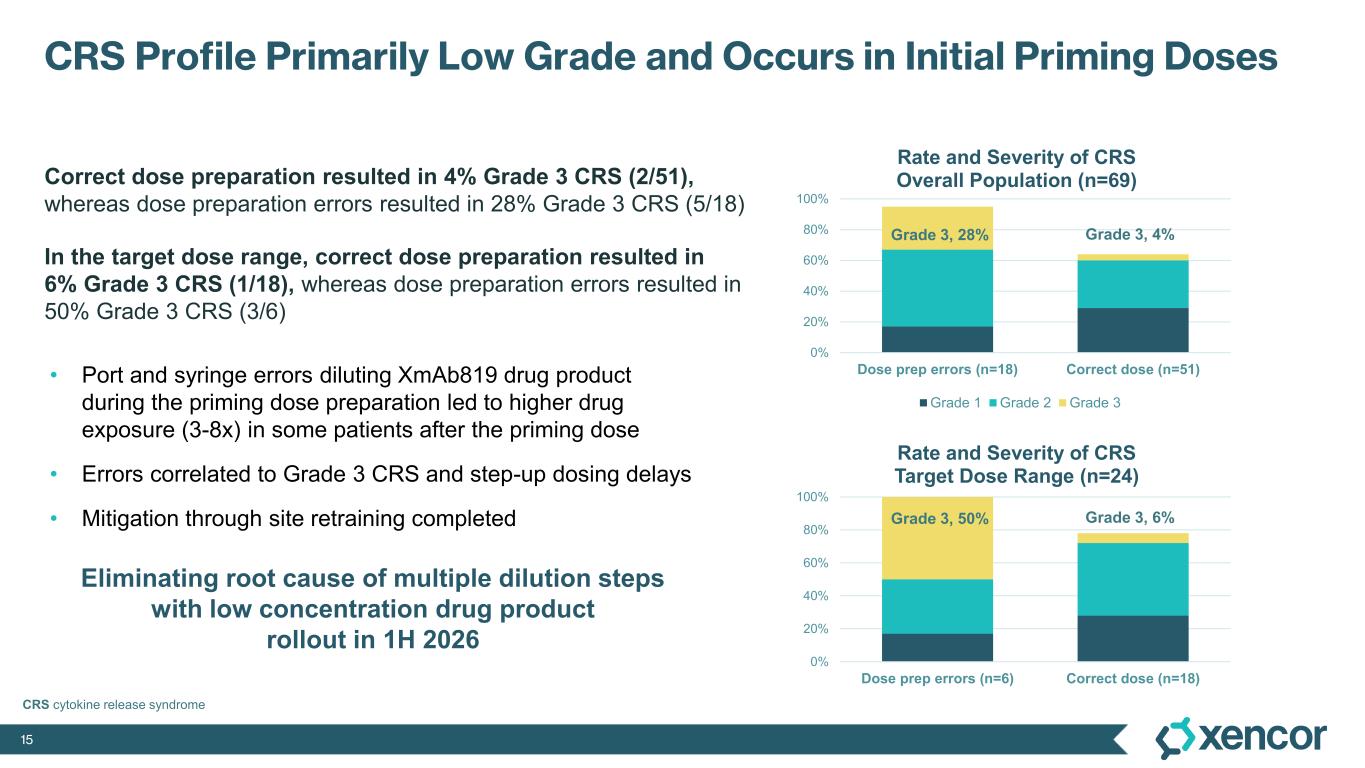
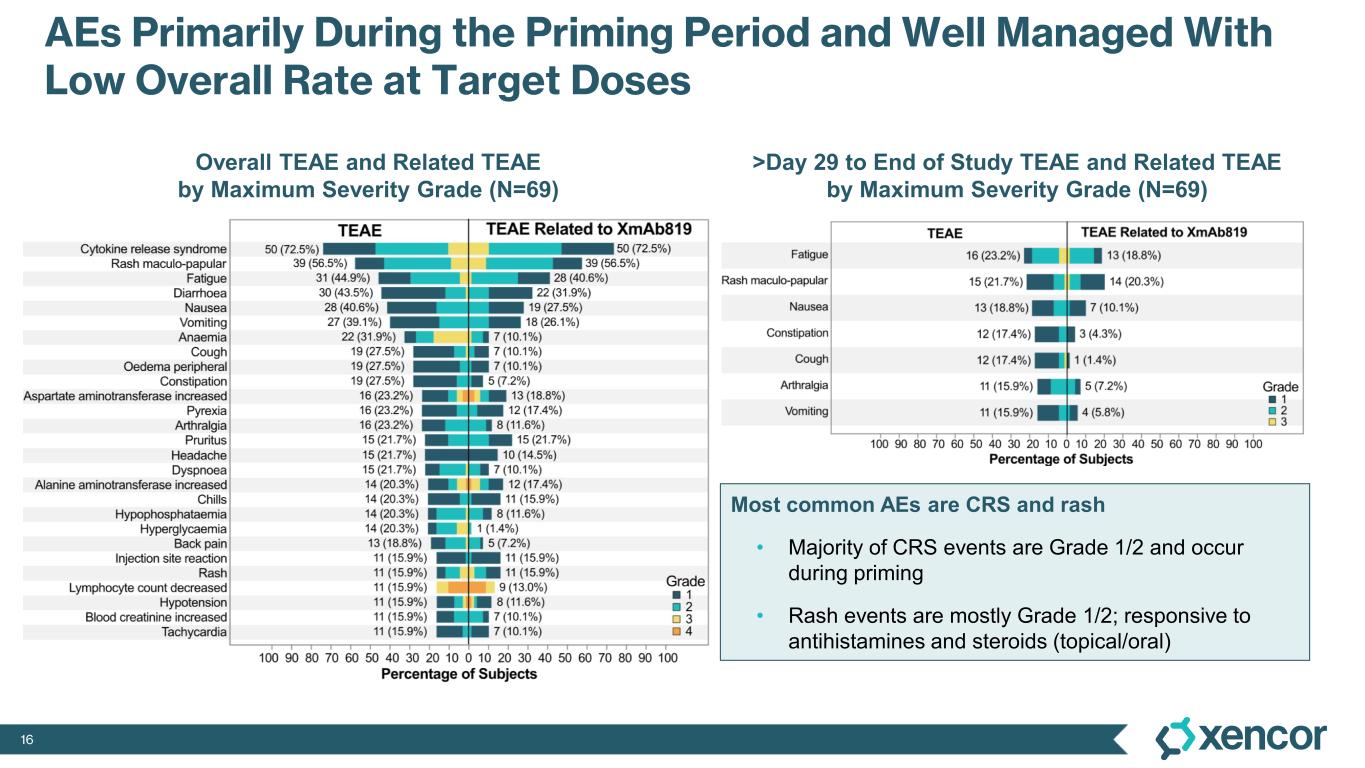
The most common treatment-emergent adverse events (TEAE) were cytokine release syndrome (CRS), rash and gastrointestinal-related toxicities that were primarily Grade 1 or 2 in severity and predominantly associated with prime-step dosing in the first four weeks of treatment. Grade 3 TEAEs related to treatment were rash (16%), liver enzyme elevations (7%) and CRS1 (4%). One dose-limiting toxicity of Grade 4 elevated liver enzymes was deemed related to treatment. No cases of treatment-related immune effector cell-associated neurotoxicity syndrome (ICANS) were observed. No Grade 5 events were reported. Four patients (6%) were dose-reduced due to treatment-related AEs, and three patients (4%) discontinued treatment due to treatment-related AEs, which includes two patients who experienced elevated liver enzymes and one patient who experienced a non-fatal myocardial infarction in the presence of hypotension and CRS.
1 While 51 patients received the correct priming dose of XmAb819, higher than expected serum levels of XmAb819 were observed in 18 patients, which was investigated during early 2025 and linked to priming dose preparation errors that resulted from use of certain ports and syringes during drug dilution. Of the 51 patients receiving the correct priming dose, 2 (4%) experienced Grade 3 CRS. Of the 18 patients that were found to have a 3- to 8-fold higher than expected concentration of study drug post-priming dose, 5 (28%) experienced Grade 3 CRS. Mitigation of dosing errors through site retraining is complete, and the root cause of these errors will be eliminated through the introduction of a low concentration formulation to be implemented during the first half of 2026.
Webcast Details
Xencor will host a webcast today at 1:30 a.m. ET (10:30 a.m. PT) to discuss the initial results outlined in this news release. The live webcast may be accessed through this link and through “Events & Presentations” in the Investors section of the Company’s website, located at investors.xencor.com. A recording will be available for at least 30 days.
About XmAb819
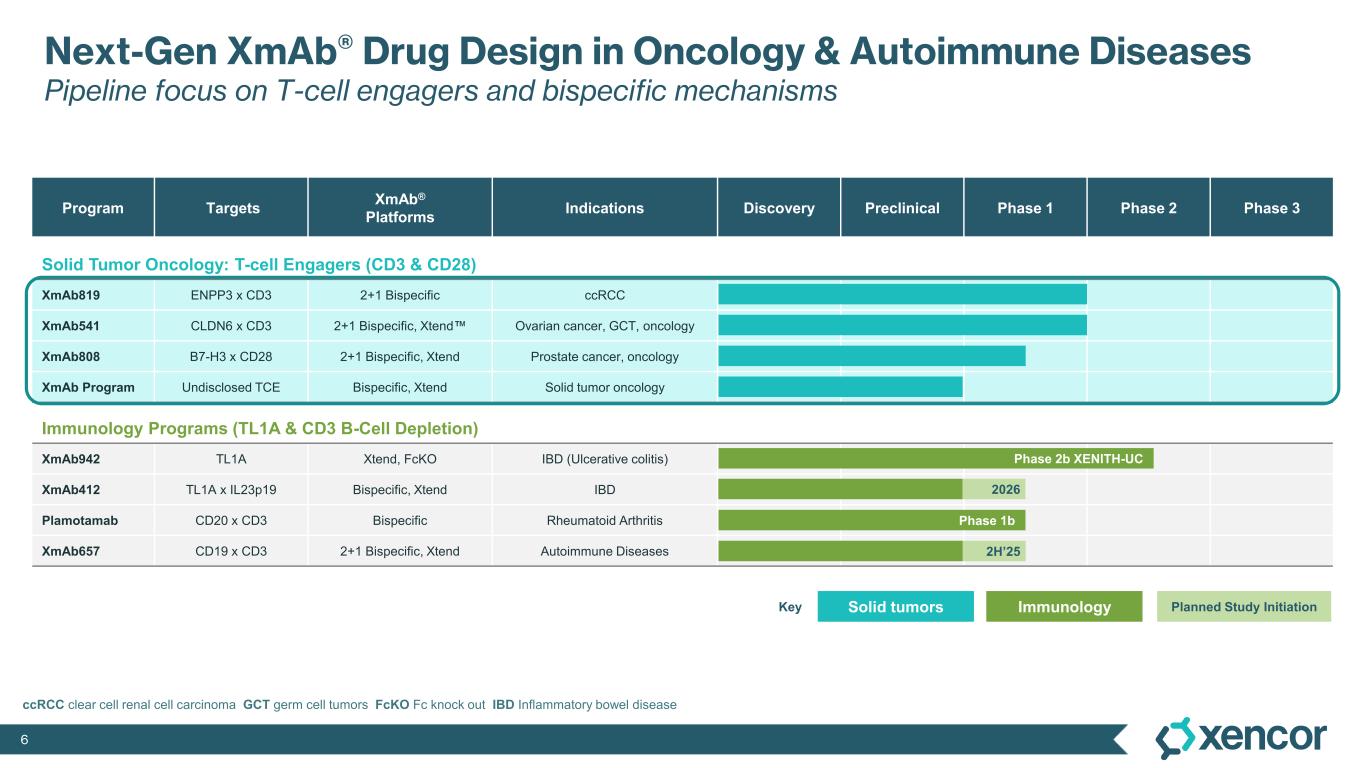
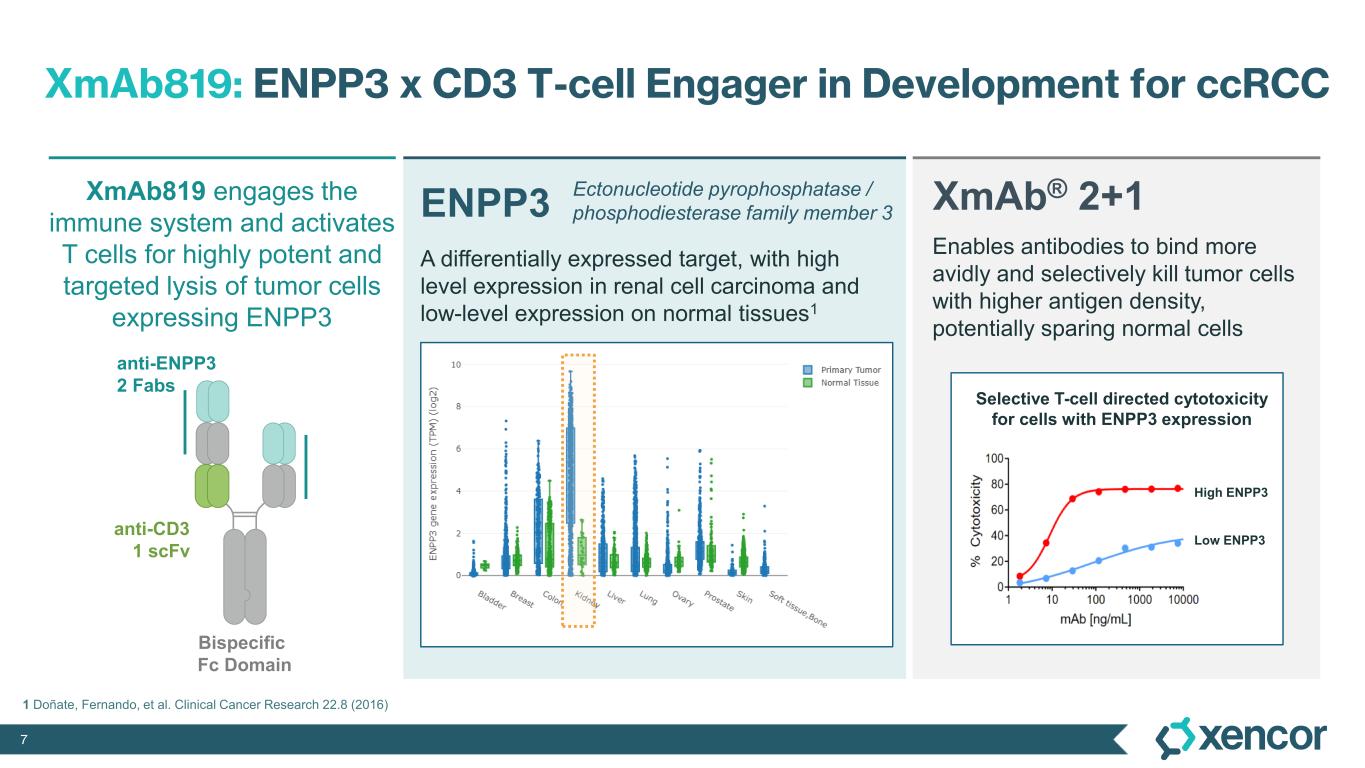
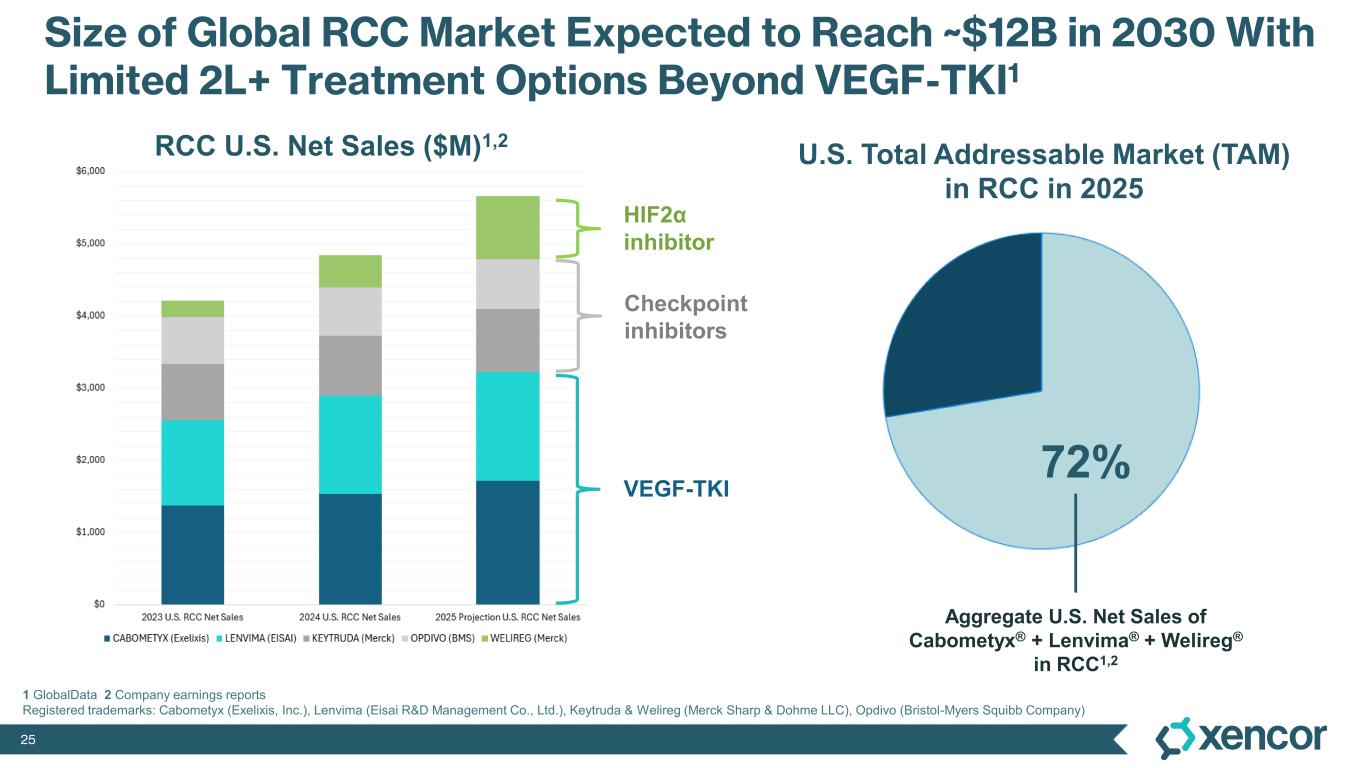
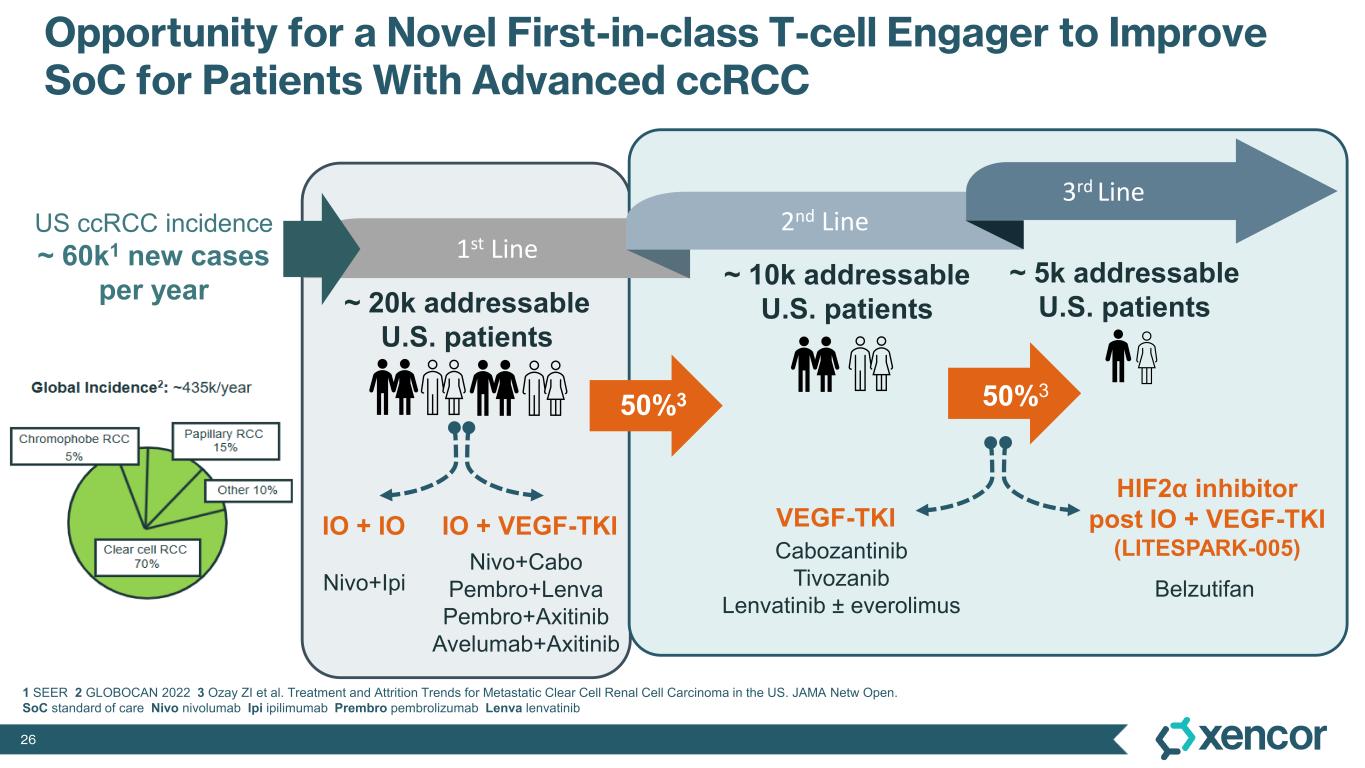
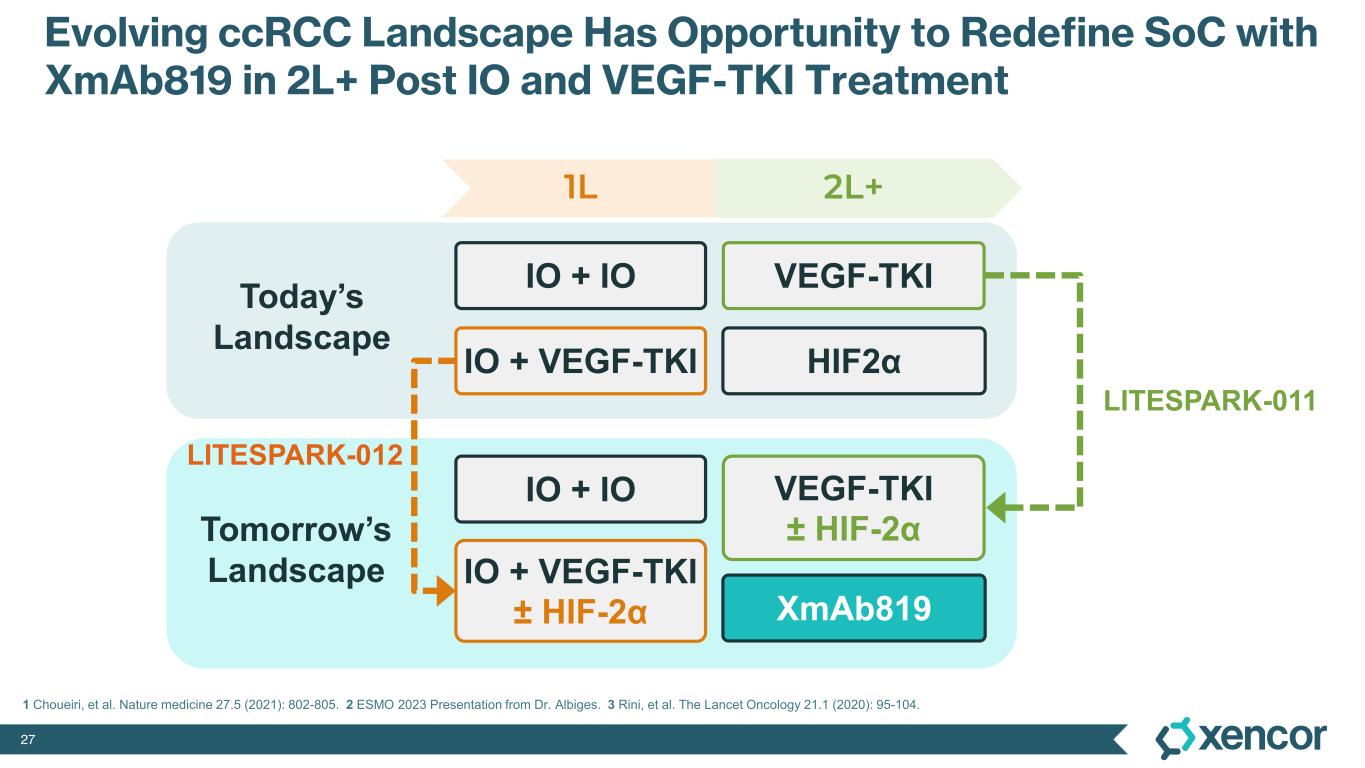
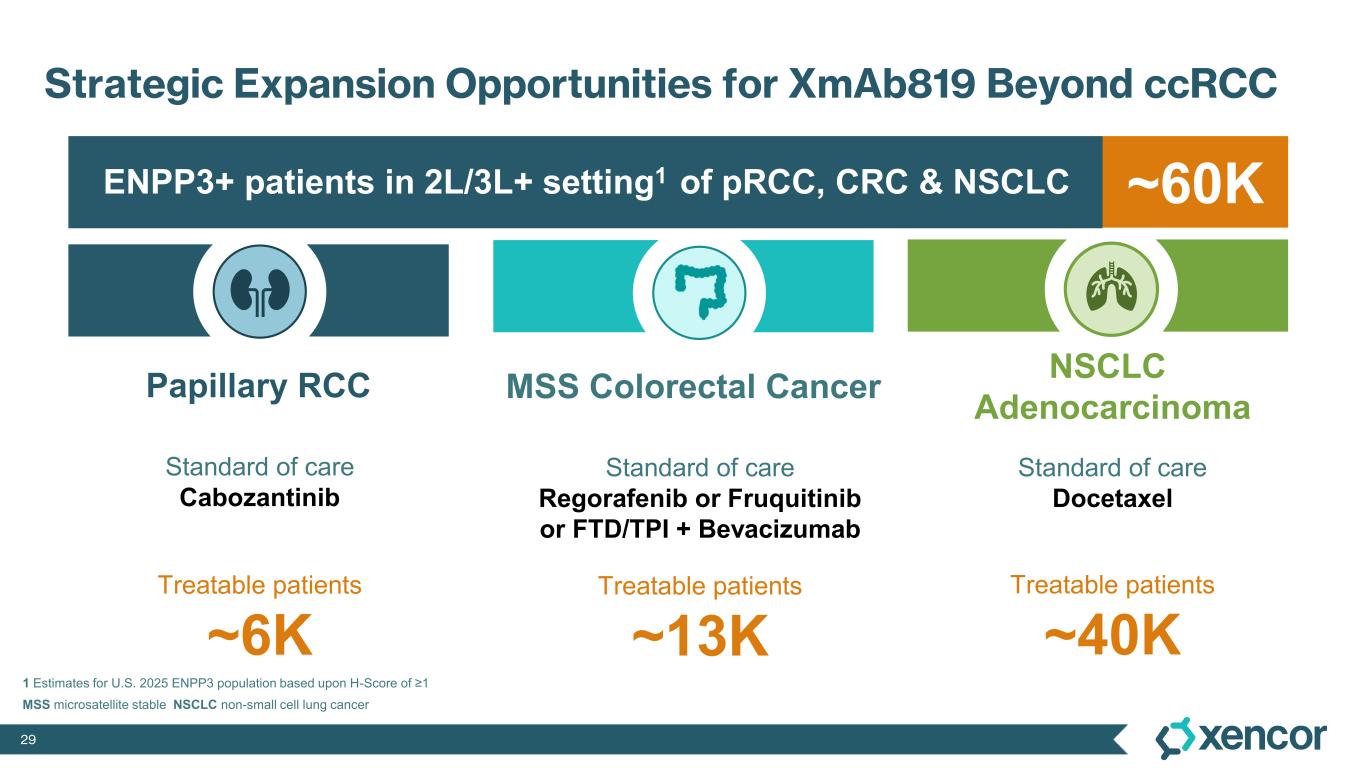
XmAb819 is a first-in-class, tumor-targeted, T-cell engaging XmAb 2+1 bispecific antibody in development for patients with clear cell renal cell carcinoma (ccRCC). XmAb819 engages the immune system and activates T cells for highly potent and targeted lysis of tumor cells expressing ENPP3, an antigen highly expressed on kidney cancers. ENPP3 is a differentially expressed target, with high level expression in renal cell carcinoma (RCC) and low-level expression on normal tissues.
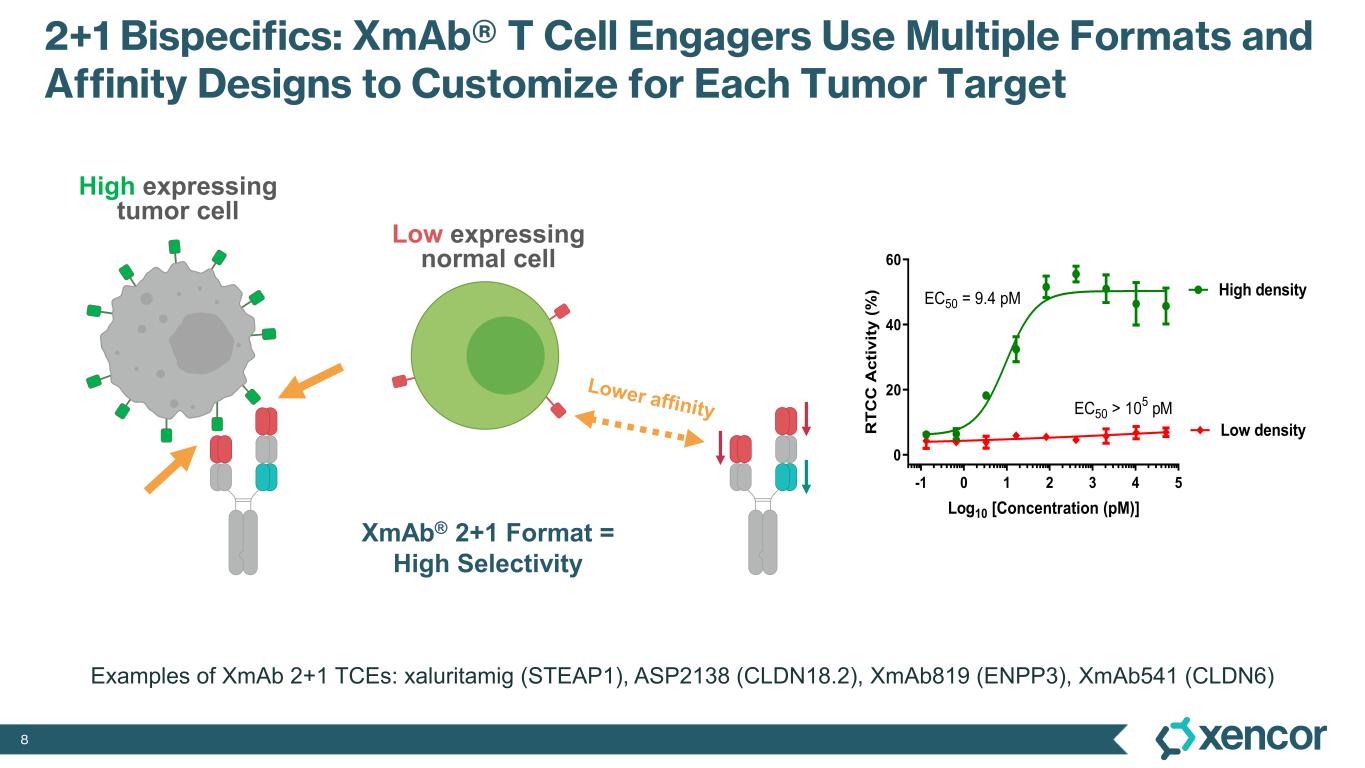
With two tumor-antigen binding domains and one T-cell binding domain, Xencor’s XmAb® 2+1 format enables antibodies to bind more avidly and selectively kill tumor cells with higher antigen density, potentially sparing normal cells. Xencor is conducting a Phase 1 study to evaluate XmAb819 in patients with advanced ccRCC.
About Xencor
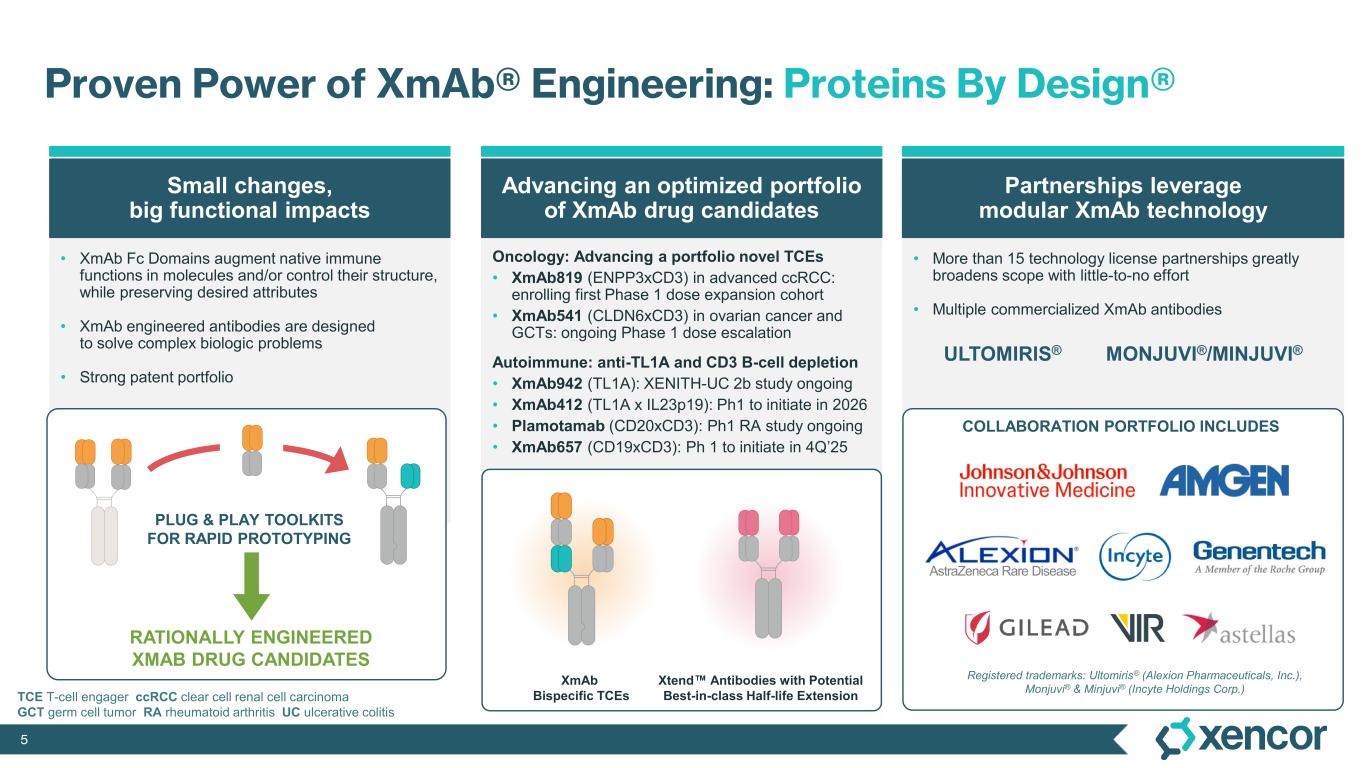
Xencor is a clinical-stage biopharmaceutical company developing engineered antibodies for the treatment of patients with cancer and autoimmune diseases. More than 20 candidates engineered with Xencor's XmAb® technology are in clinical development, and multiple XmAb medicines are marketed by partners. Xencor's XmAb engineering technology enables small changes to a protein’s structure that result in new mechanisms of therapeutic action. For more information, please visit www.xencor.com.
Forward-Looking Statements
Certain statements contained in this press release may constitute forward-looking statements within the meaning of applicable securities laws. Forward-looking statements include statements that are not purely statements of historical fact, and can generally be identified by the use of words such as “potential,” “can,” “will,” “plan,” “may,” “could,” “would,” “expect,” “anticipate,” “seek,” “look forward,” “believe,” “committed,” “investigational,” “indicates,” “supports,” and similar terms, or by express or implied discussions relating to Xencor’s business, including, but not limited to, statements regarding expectations for clinical progress, planned presentations of clinical data, planned and in process clinical trials, the quotations from Xencor's president and chief executive officer, and other statements that are not purely statements of historical fact. Such statements are made on the basis of the current beliefs, expectations, and assumptions of the management of Xencor and are subject to significant known and unknown risks, uncertainties and other factors that may cause actual results, performance or achievements and the timing of events to be materially different from those implied by such statements, and therefore these statements should not be read as guarantees of future performance or results. Such risks include, without limitation, the risks associated with the process of discovering, developing, manufacturing and commercializing drugs that are safe and effective for use as human therapeutics and other risks, including the ability of publicly disclosed preliminary clinical trial data to support continued clinical development and regulatory approval for specific treatments and the risks, uncertainties and other factors described under the heading “Risk Factors” in Xencor's annual report on Form 10-K for the year ended December 31, 2024 as well as Xencor's subsequent filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, as amended to date. All forward-looking statements are qualified in their entirety by this cautionary statement and Xencor undertakes no obligation to revise or update these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
Contact
Charles Liles
cliles@xencor.com
(626) 737-8118
For media:
Cassidy McClain
Inizio Evoke
cassidy.mcclain@inizioevoke.com
(619) 694-6291