UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported) June 24, 2025
Hoth Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 001-38803 | 82-1553794 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I. R. S. Employer Identification No.) |
1177 Avenue of the Americas, 5th Floor, Suite 066
New York, NY 10036
(Address of principal executive offices, including ZIP code)
(646) 756-2997
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common stock, $0.0001 par value | HOTH | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 7.01. Regulation FD Disclosure.
Hoth Therapeutics, Inc. (the “Company”) has prepared presentation materials (the “Presentation Materials”) that management intends to use from time to time on and after June 24, 2025 in presentations about the Company’s business. The Presentation Materials are furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information contained in the Presentation Materials is summary information that should be considered within the context of the Company’s filings with the Securities and Exchange Commission and other public announcements that the Company may make by press release or otherwise from time to time. The Presentation Materials speak as of the date of this Current Report on Form 8-K. While the Company may elect to update the Presentation Materials in the future or reflect events and circumstances occurring or existing after the date of this Current Report on Form 8-K, the Company specifically disclaims any obligation to do so.
The information in this Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K is furnished and shall not be deemed to be “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section. The information in this Item 7.01 and Exhibit 99.1 of this Current Report on Form 8-K shall not be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date of this Current Report on Form 8-K, regardless of any general incorporation language in any such filing.
Item 9.01. Financial Statements and Exhibits.
d) Exhibits
| Exhibit No. | Description | |
| 99.1 | Presentation Materials dated June 2025 | |
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL) |
-
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: June 24, 2025 | Hoth Therapeutics, Inc. |
| /s/ Robb Knie | |
| Robb Knie | |
| Chief Executive Officer |
-2-
Exhibit 99.1

HT - 001 The first therapy for EGFR inhibitor induced cutaneous toxicities Innovating for Everyone A significant unmet need Cancer patients on EGFR inhibitors experiencing severe dermatological side effects are limited to dose - reduction or stopping treatment entirely

Safe Harbor Statement

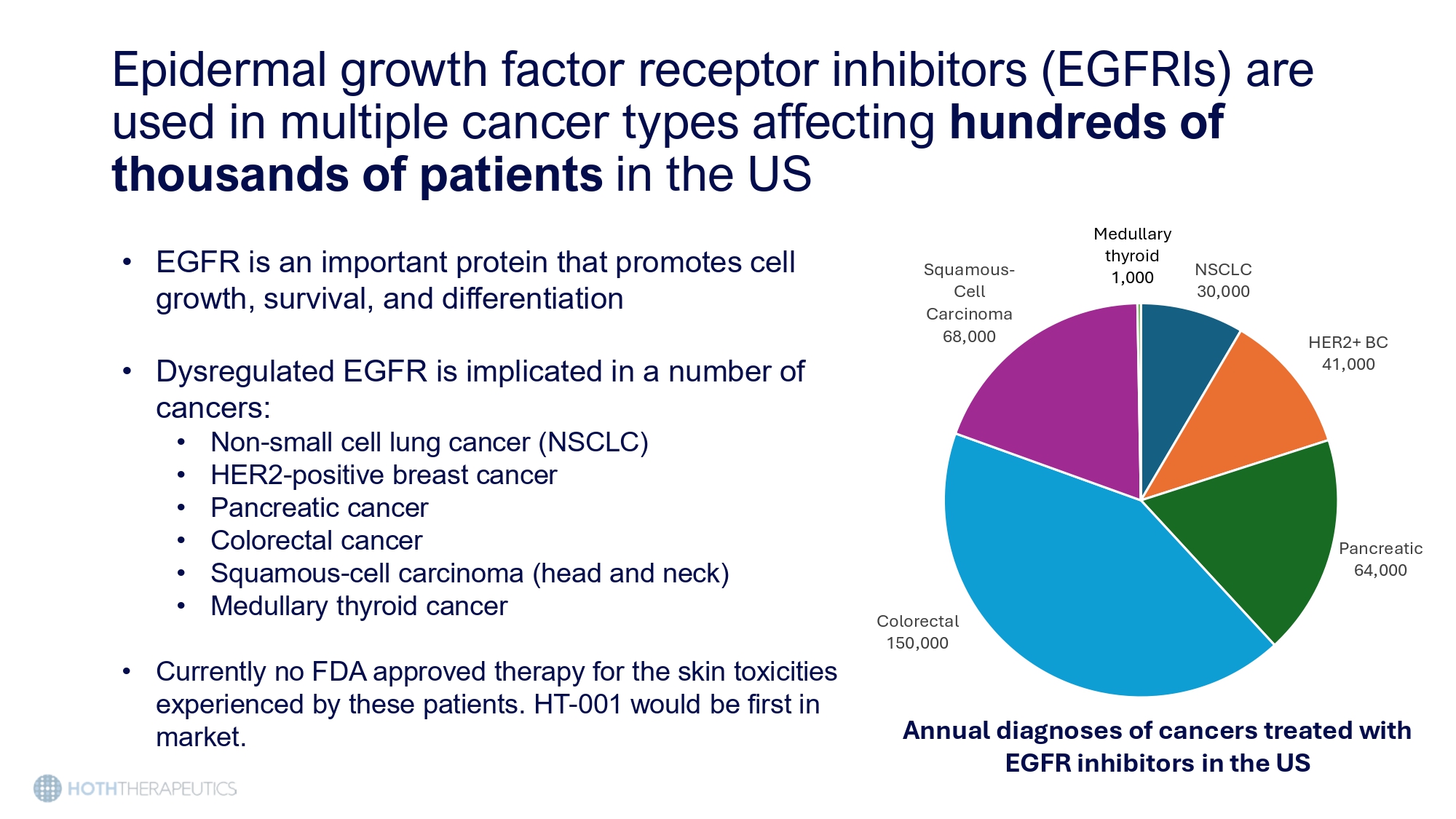
Epidermal growth factor receptor inhibitors (EGFRIs) are used in multiple cancer types affecting hundreds of thousands of patients in the US • EGFR is an important protein that promotes cell growth, survival, and differentiation • Dysregulated EGFR is implicated in a number of cancers: • Non - small cell lung cancer (NSCLC) • HER2 - positive breast cancer • Pancreatic cancer • Colorectal cancer • Squamous - cell carcinoma (head and neck) • Medullary thyroid cancer • Currently no FDA approved therapy for the skin toxicities experienced by these patients. HT - 001 would be first in market.
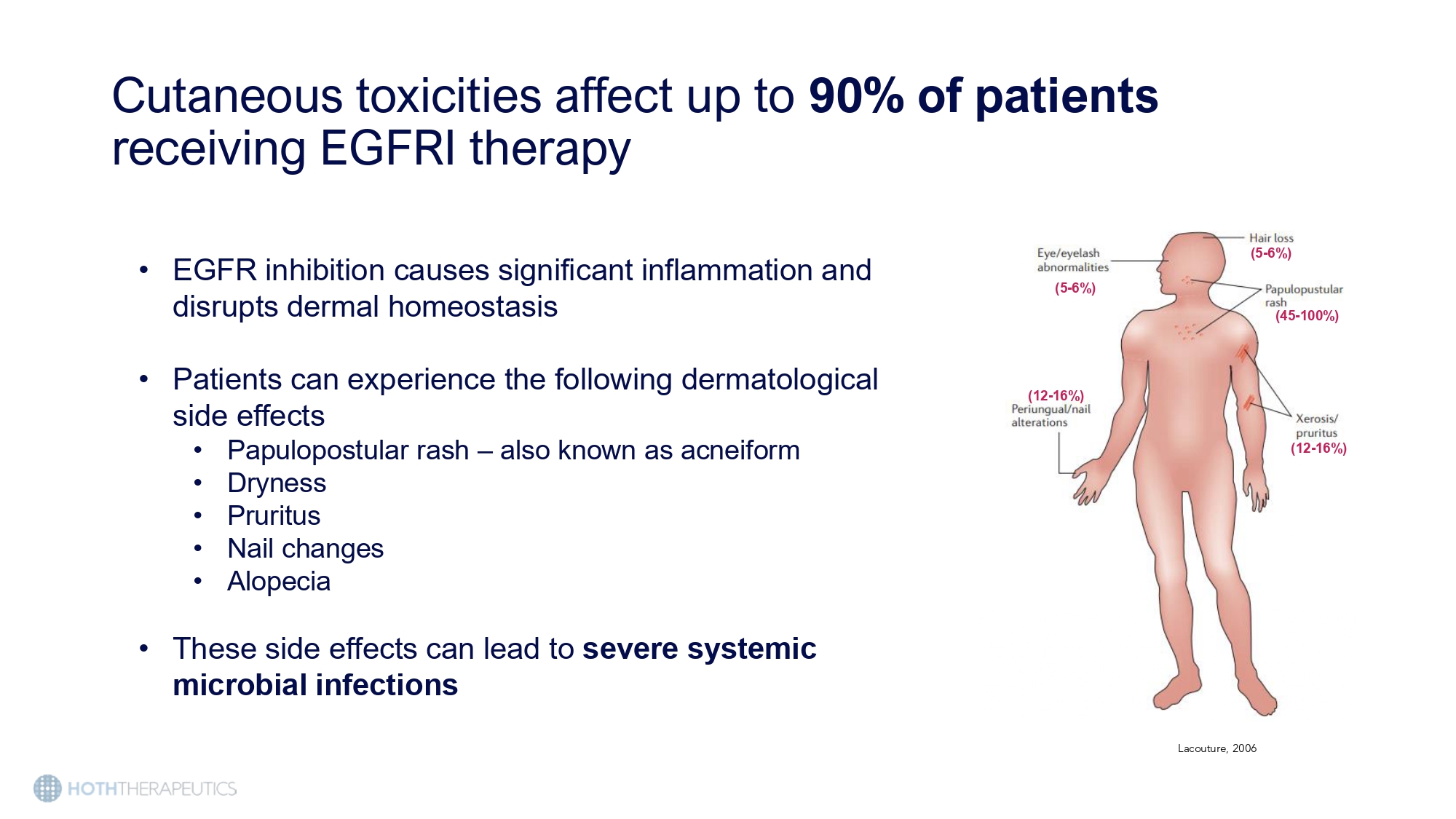
NSCLC 30,000 HER2+ BC 41,000 Pancreatic 64,000 Colorectal 150,000 Squamous - Cell Carcinoma 68,000 Medullary thyroid 1,000 Annual diagnoses of cancers treated with EGFR inhibitors in the US Cutaneous toxicities affect up to 90% of patients receiving EGFRI therapy Lacouture, 2006 • EGFR inhibition causes significant inflammation and disrupts dermal homeostasis • Patients can experience the following dermatological side effects • Papulopostular rash – also known as acneiform • Dryness • Pruritus • Nail changes • Alopecia • These side effects can lead to severe systemic microbial infections (45 - 100%) (12 - 16%) (12 - 16%) (5 - 6%) (5 - 6%)
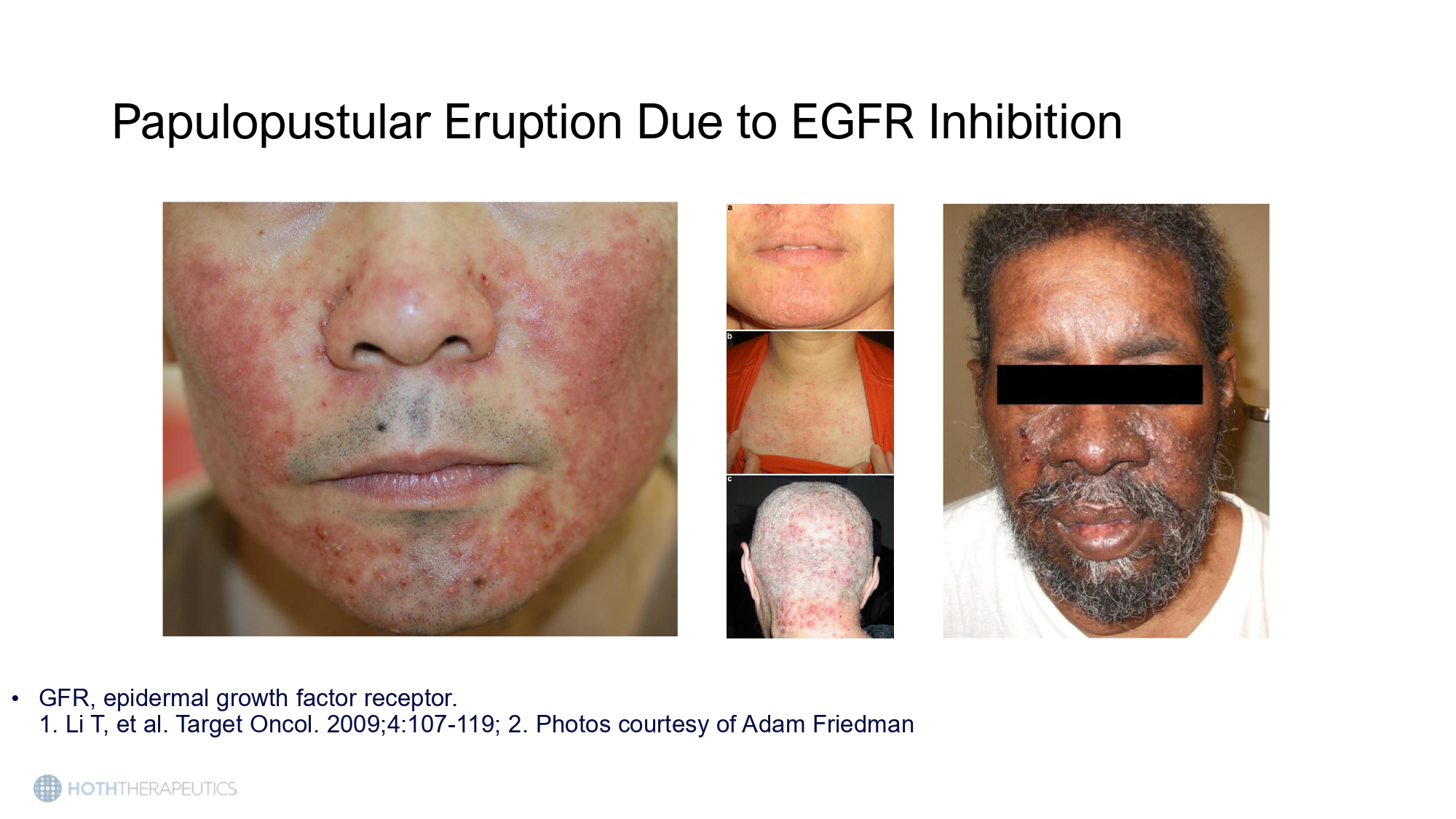
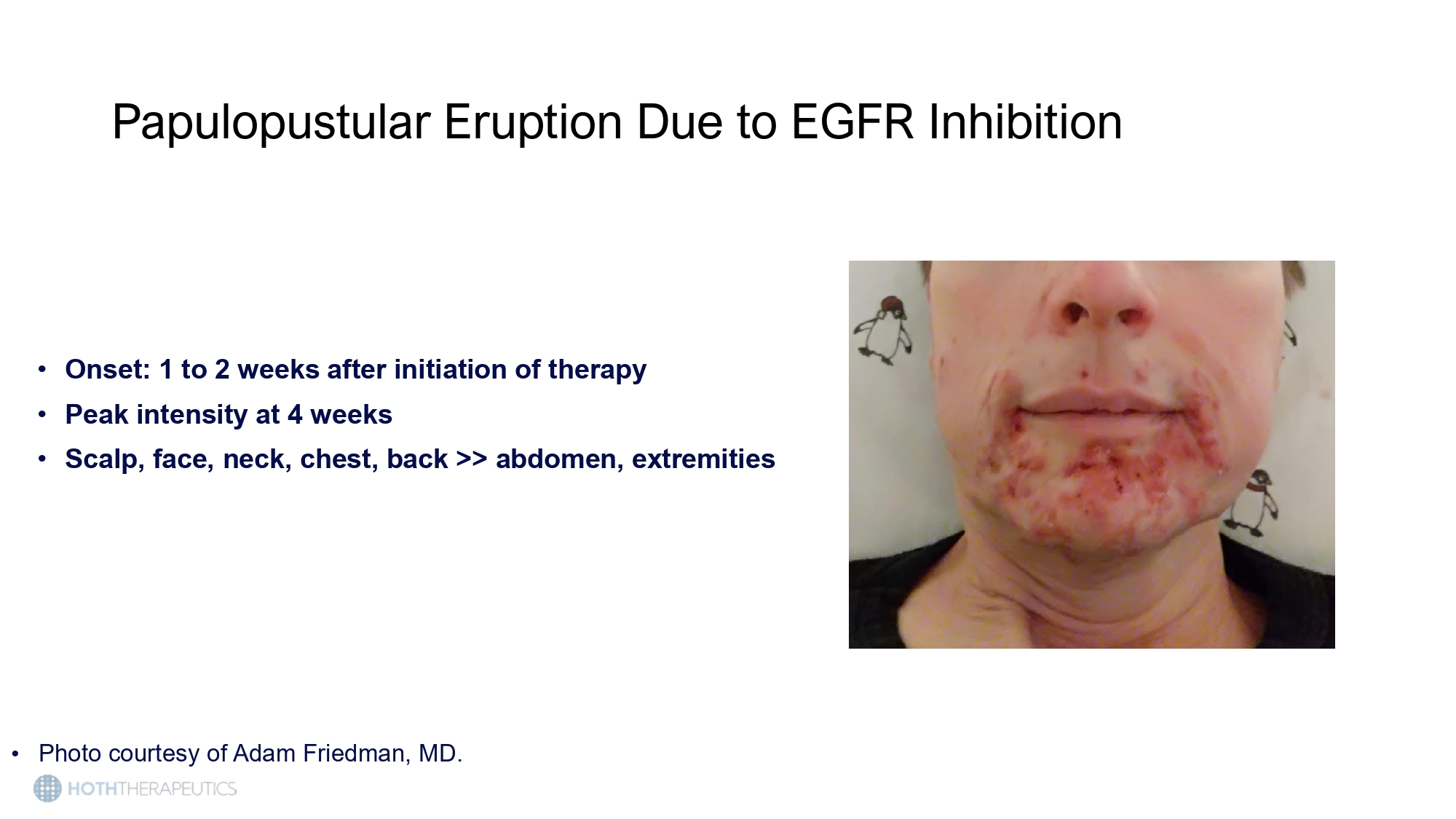
• GFR, epidermal growth factor receptor. 1. Li T, et al. Target Oncol. 2009;4:107 - 119; 2. Photos courtesy of Adam Friedman Papulopustular Eruption Due to EGFR Inhibition Papulopustular Eruption Due to EGFR Inhibition • Onset: 1 to 2 weeks after initiation of therapy • Peak intensity at 4 weeks • Scalp, face, neck, chest, back >> abdomen, extremities • Photo courtesy of Adam Friedman, MD.
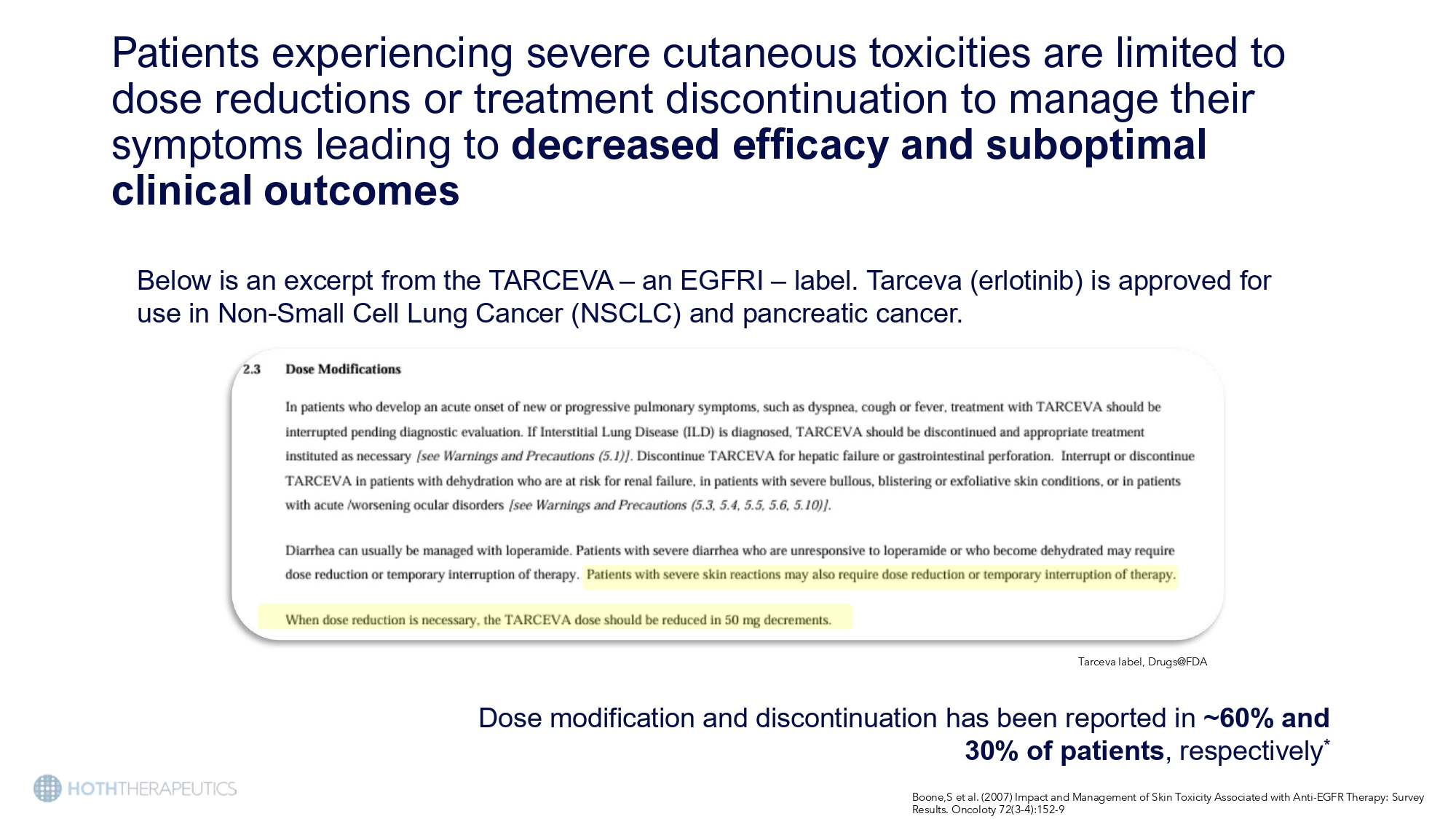
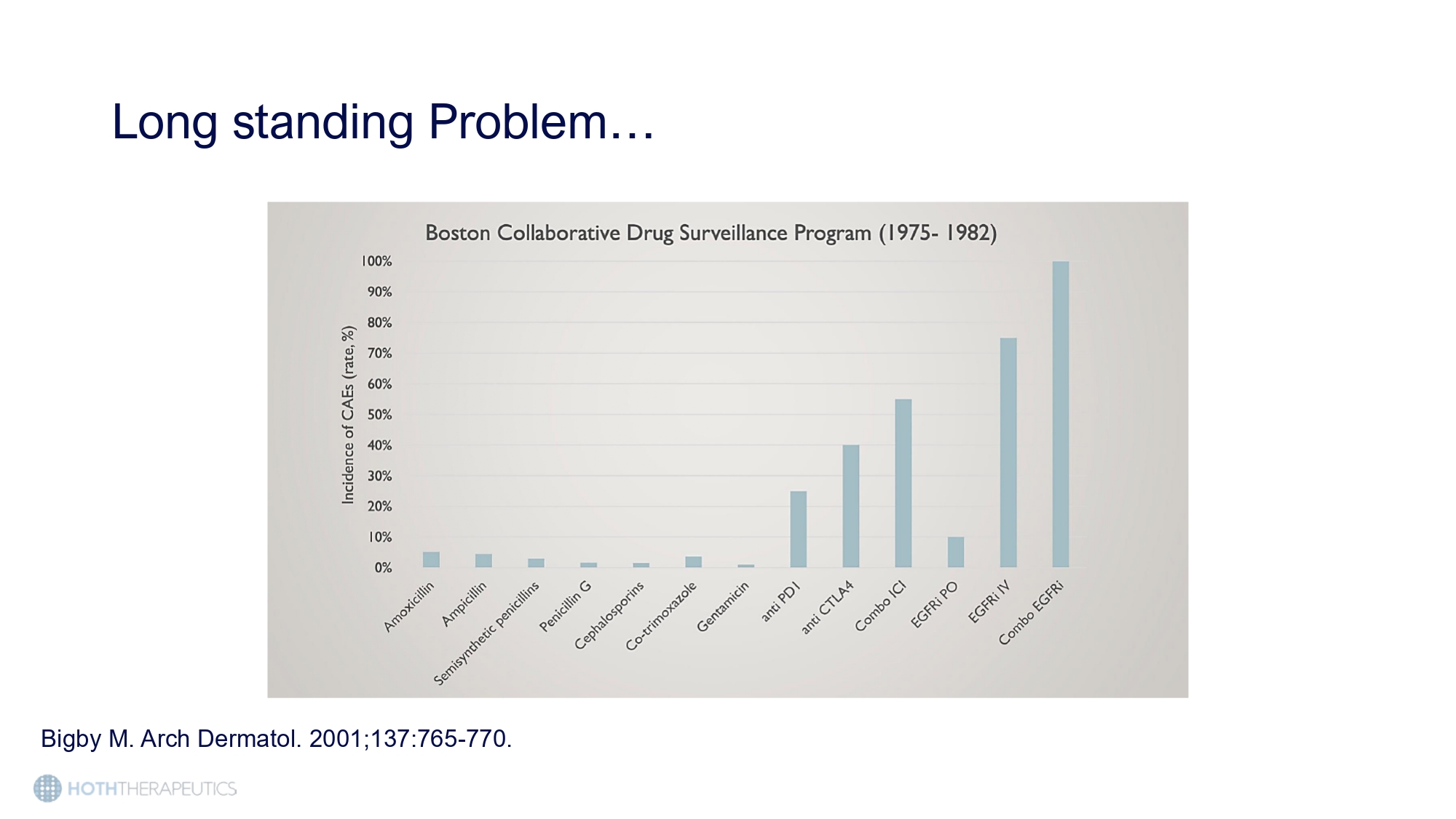
Patients experiencing severe cutaneous toxicities are limited to dose reductions or treatment discontinuation to manage their symptoms leading to decreased efficacy and suboptimal clinical outcomes Below is an excerpt from the TARCEVA – an EGFRI – label. Tarceva (erlotinib) is approved for use in Non - Small Cell Lung Cancer (NSCLC) and pancreatic cancer. Tarceva label, Drugs@FDA Dose modification and discontinuation has been reported in ~60% and 30% of patients , respectively * Boone,S et al. (2007) Impact and Management of Skin Toxicity Associated with Anti - EGFR Therapy: Survey Results. Oncoloty 72(3 - 4):152 - 9 Long standing Problem… Bigby M. Arch Dermatol. 2001;137:765 - 770.
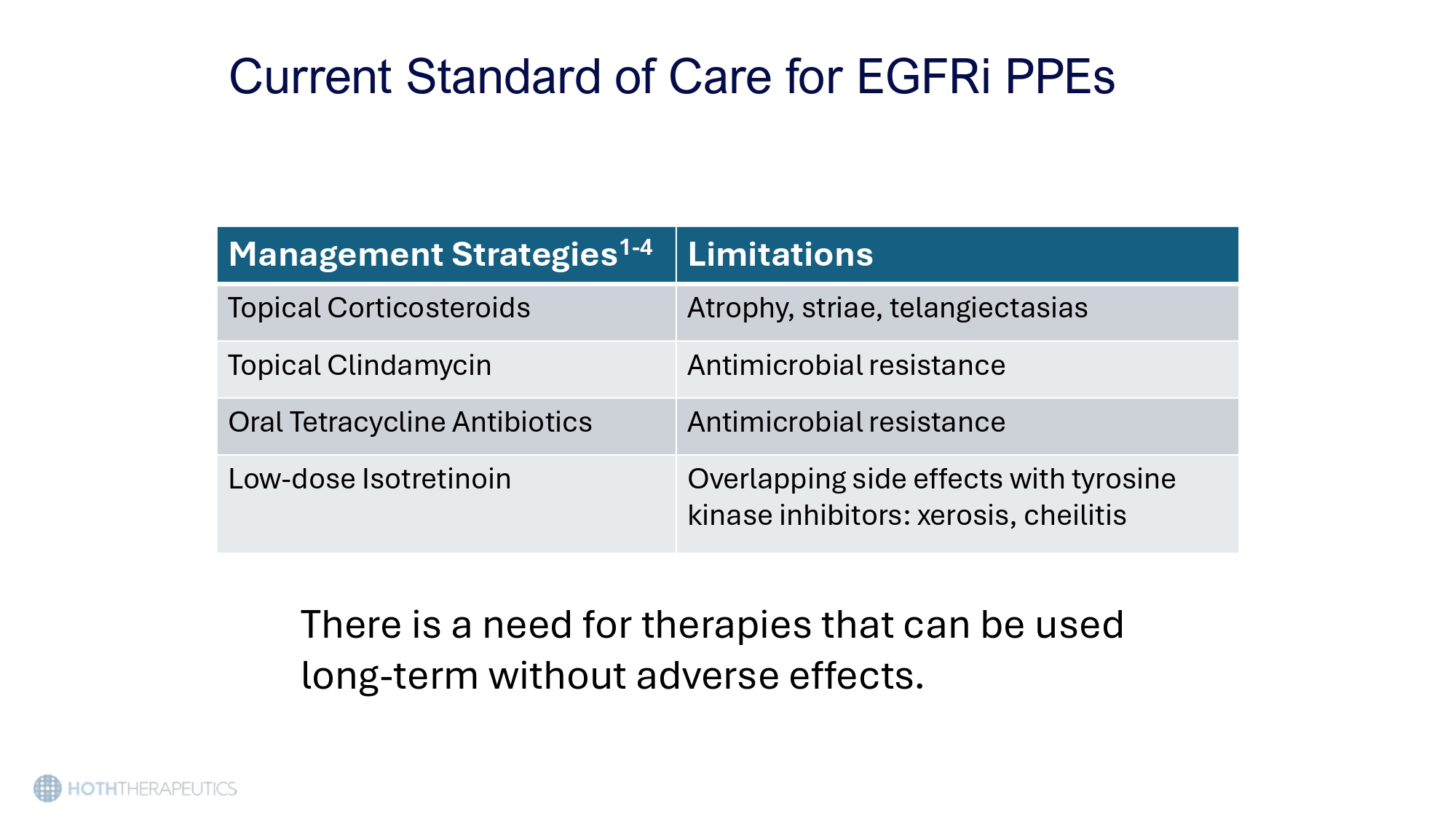
Current Standard of Care for EGFRi PPEs There is a need for therapies that can be used long - term without adverse effects. Limitations Management Strategies 1 - 4 Atrophy, striae, telangiectasias Topical Corticosteroids Antimicrobial resistance Topical Clindamycin Antimicrobial resistance Oral Tetracycline Antibiotics Overlapping side effects with tyrosine kinase inhibitors: xerosis, cheilitis Low - dose Isotretinoin HT - 001 A patient - centric topical solution for EGFRI induced dermal toxicities

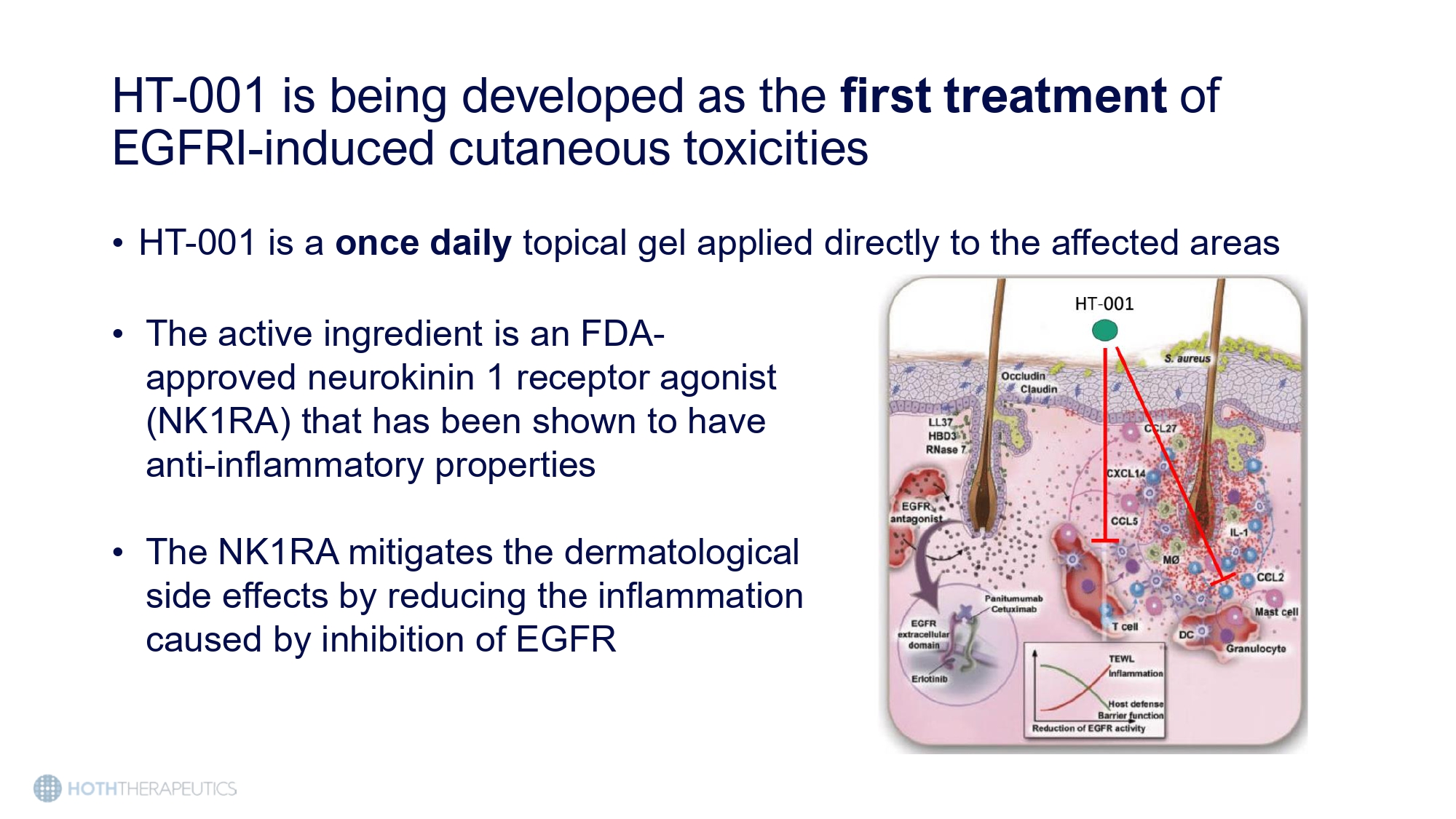
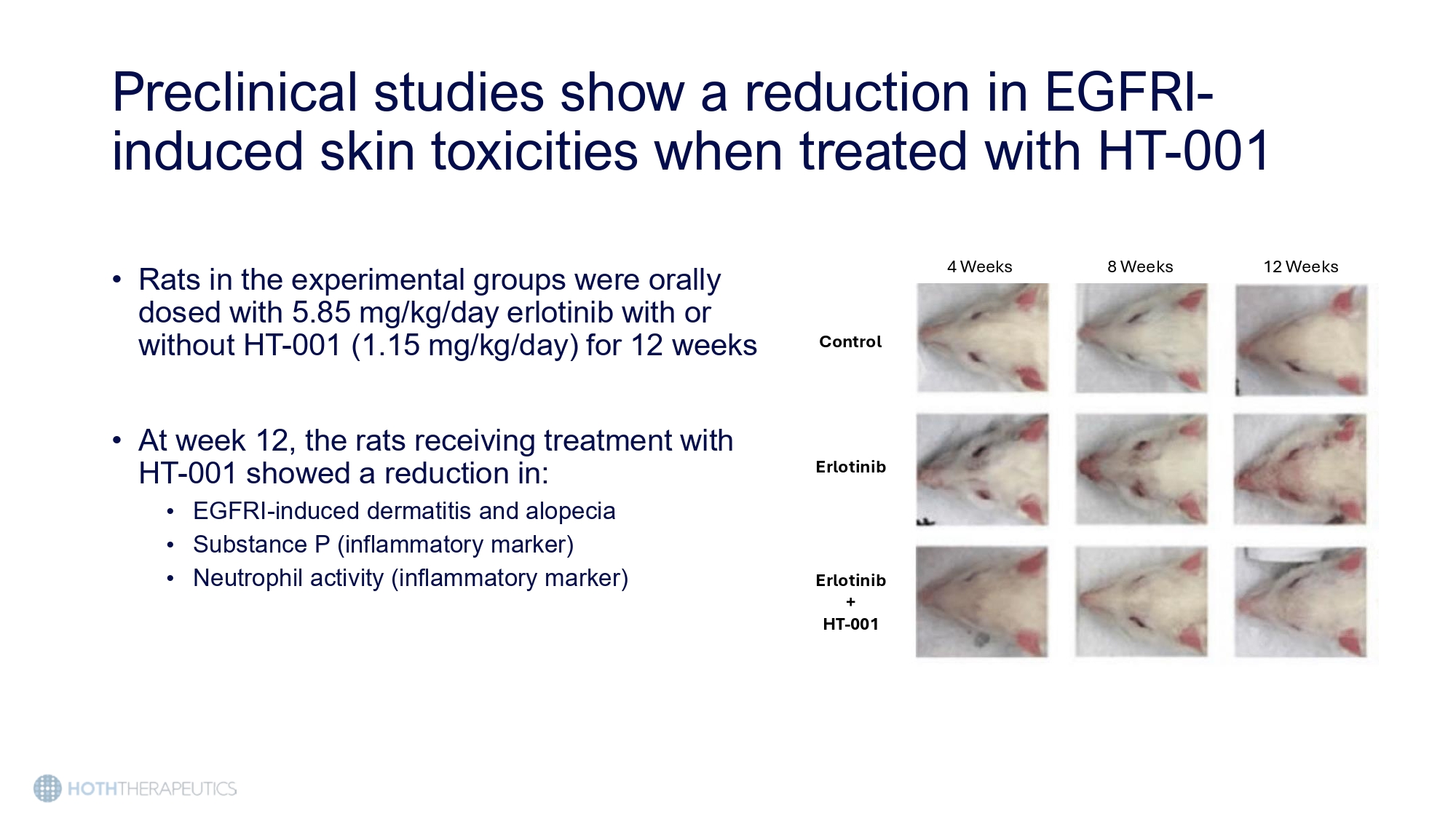
HT - 001 is being developed as the first treatment of EGFRI - induced cutaneous toxicities • HT - 001 is a once daily topical gel applied directly to the affected areas • The active ingredient is an FDA - approved neurokinin 1 receptor agonist (NK1RA) that has been shown to have anti - inflammatory properties • The NK 1 RA mitigates the dermatological side effects by reducing the inflammation caused by inhibition of EGFR Preclinical studies show a reduction in EGFRI - induced skin toxicities when treated with HT - 001 4 Weeks 8 Weeks 12 Weeks Control Erlotinib Erlotinib + HT - 001 • Rats in the experimental groups were orally dosed with 5.85 mg/kg/day erlotinib with or without HT - 001 (1.15 mg/kg/day) for 12 weeks • At week 12, the rats receiving treatment with HT - 001 showed a reduction in: • EGFRI - induced dermatitis and alopecia • Substance P (inflammatory marker) • Neutrophil activity (inflammatory marker)
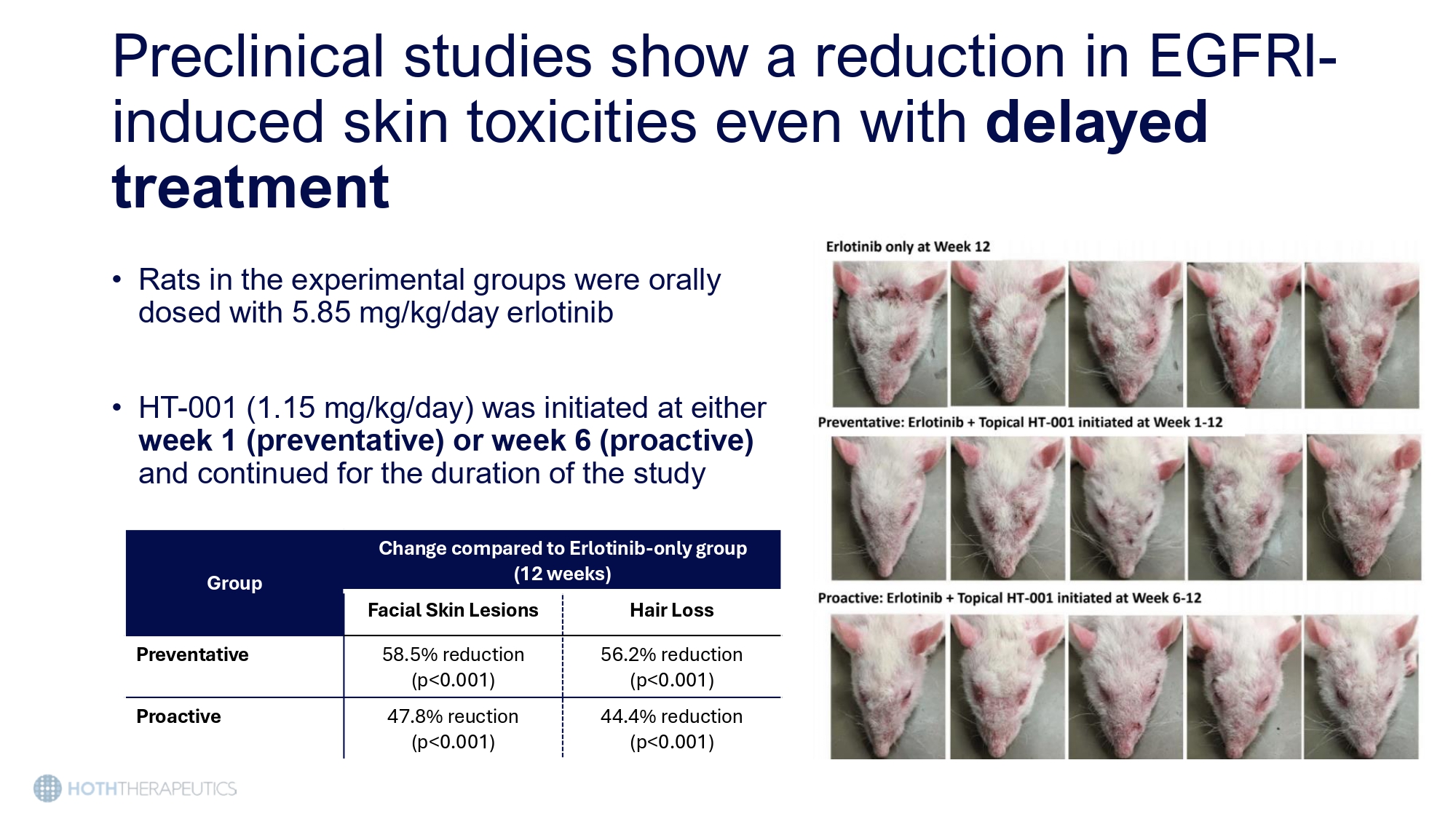
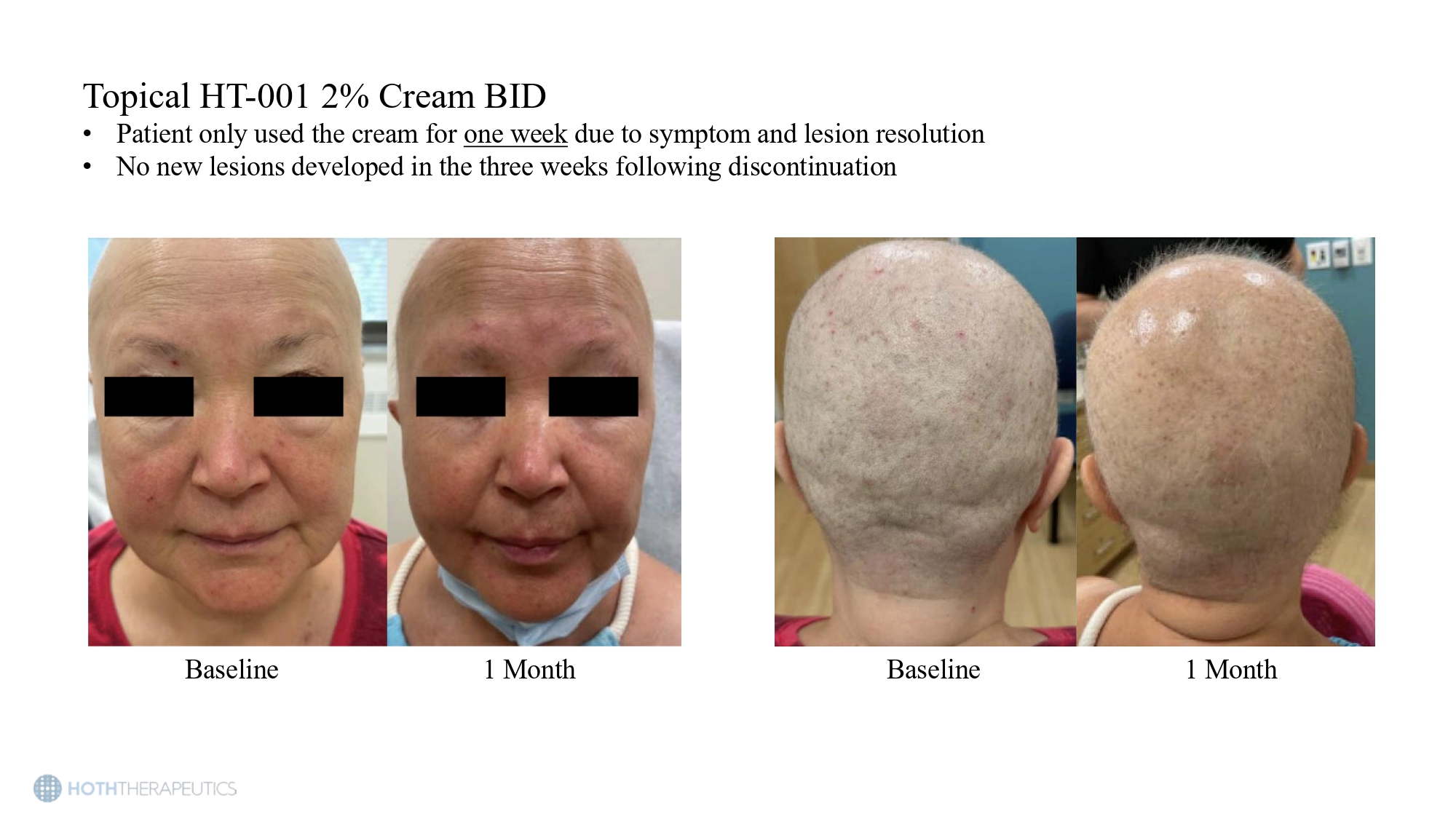
Preclinical studies show a reduction in EGFRI - induced skin toxicities even with delayed treatment • Rats in the experimental groups were orally dosed with 5.85 mg/kg/day erlotinib • HT - 001 (1.15 mg/kg/day) was initiated at either week 1 (preventative) or week 6 (proactive) and continued for the duration of the study Change compared to Erlotinib - only group (12 weeks) Group Hair Loss Facial Skin Lesions 56.2% reduction (p<0.001) 58.5% reduction (p<0.001) Preventative 44.4% reduction (p<0.001) 47.8% reuction (p<0.001) Proactive Topical HT - 001 2% Cream BID • Patient only used the cream for one week due to symptom and lesion resolution • No new lesions developed in the three weeks following discontinuation Baseline 1 Month Baseline 1 Month

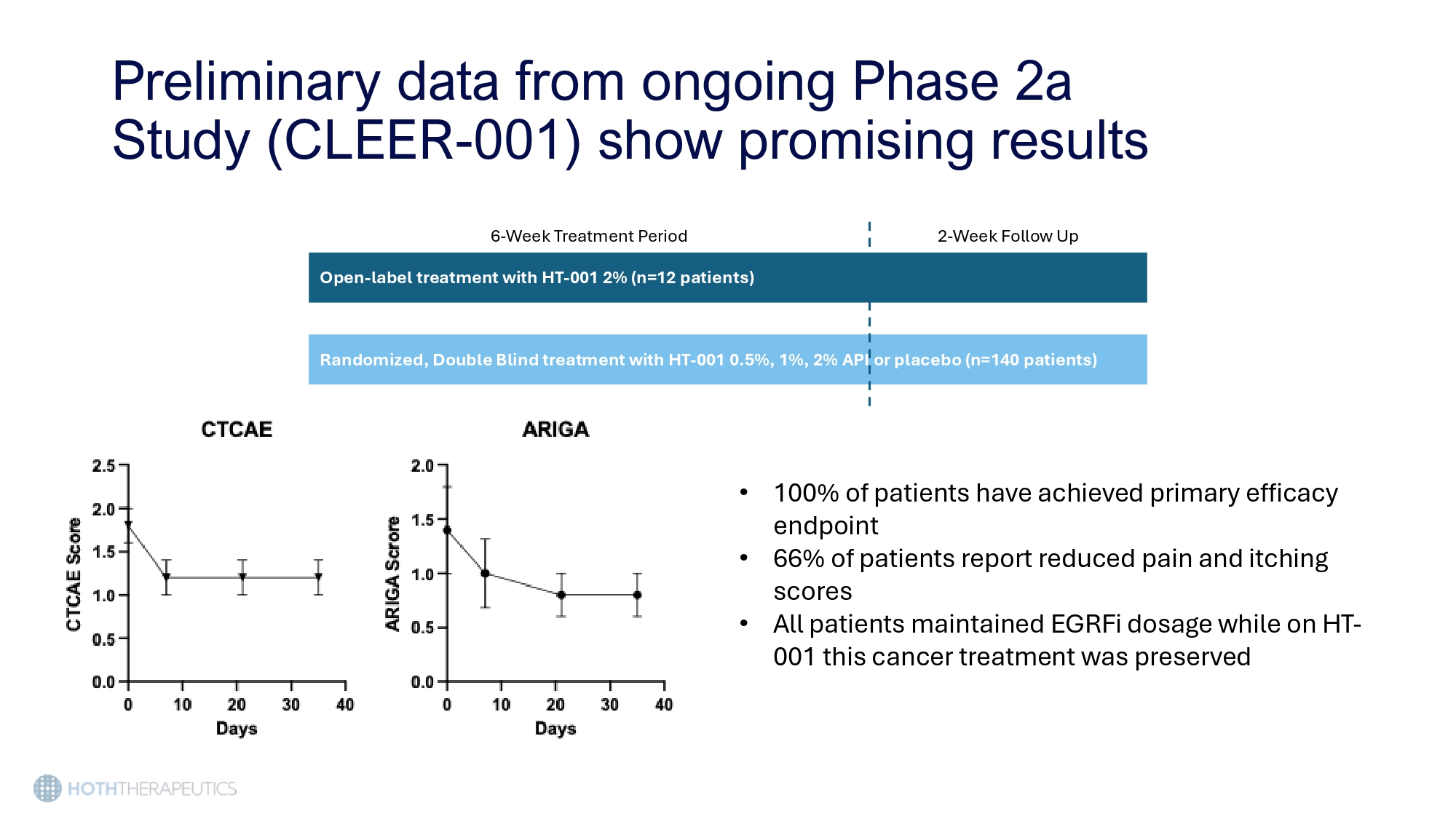
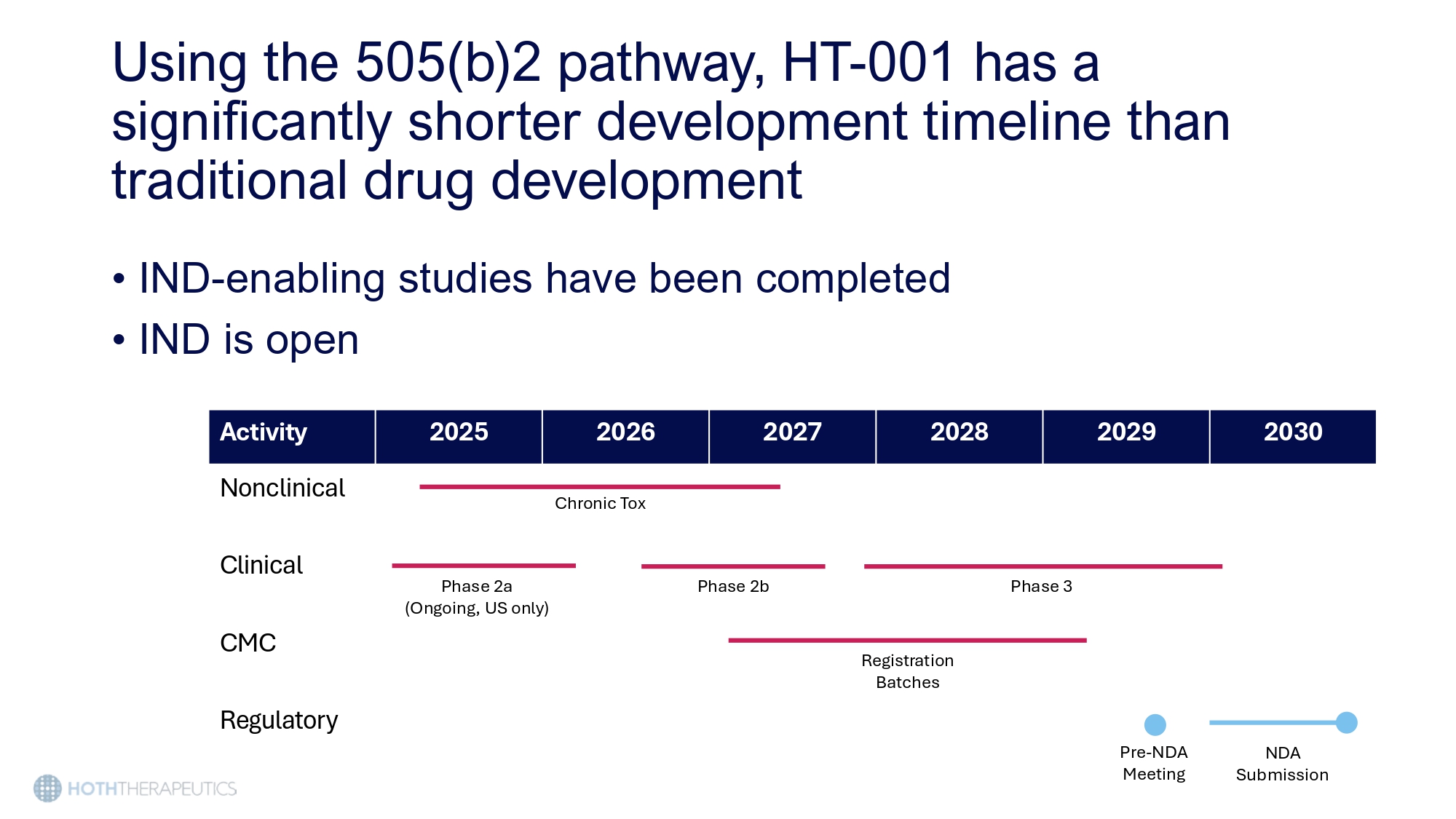
Preliminary data from ongoing Phase 2a Study (CLEER - 001) show promising results 6 - Week Treatment Period 2 - Week Follow Up Open - label treatment with HT - 001 2% (n=12 patients) Randomized, Double Blind treatment with HT - 001 0.5%, 1%, 2% API or placebo (n=140 patients) • 100% of patients have achieved primary efficacy endpoint • 66% of patients report reduced pain and itching scores • All patients maintained EGRFi dosage while on HT - 001 this cancer treatment was preserved Using the 505(b)2 pathway, HT - 001 has a significantly shorter development timeline than traditional drug development • IND - enabling studies have been completed • IND is open 2030 2029 2028 2027 2026 2025 Activity Nonclinical Chronic Tox Clinical e 3 Phas ase 2b Ph ) Phase 2a (Ongoing, US only CMC istration Batches Reg Pre - NDA Meeting Regulatory NDA Submission

Innovating for everyone Contact Information: Robb Knie, Chief Executive Officer partnering@hoththerapeutics.com (646) 756 - 2997