UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): April 3, 2025
ALDEYRA THERAPEUTICS, INC.
(Exact name of Registrant as specified in its charter)
| Delaware | 001-36332 | 20-1968197 | ||
| (State or other jurisdiction of incorporation) |
(Commission File No.) |
(IRS Employer Identification No.) |
131 Hartwell Avenue, Suite 320
Lexington, MA 02421
(Address of principal executive offices and zip code)
Registrant’s telephone number, including area code: (781) 761-4904
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading |
Name of each exchange on which registered |
||
| Common Stock, $0.001 par value per share | ALDX | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01. | Regulation FD Disclosure. |
As reported under Item 8.01 of this Current Report on Form 8-K, on April 3, 2025, Aldeyra Therapeutics, Inc. (“Aldeyra” or the “Company”) issued a press release (the “Press Release”) to provide a regulatory update regarding reproxalap, an investigational drug candidate, for the treatment of dry eye disease. The Company will hold a conference call regarding this announcement on April 3, 2025. A copy of the supplemental presentation which will be referenced during the conference call and posted on the Company’s website is furnished herewith as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated by reference herein.
This information in this Item 7.01 of this Current Report on Form 8-K shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that Section, or incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as shall be expressly set forth by specific reference in any such filing.
| Item 8.01. | Other Events. |
On April 3, 2025, Aldeyra issued the Press Release to announce that it had received a Complete Response Letter (“2025 Complete Response Letter”) from the U.S. Food & Drug Administration (“FDA”) regarding the Company’s resubmitted New Drug Application (“resubmitted NDA”) for reproxalap, an investigational drug candidate, for the treatment of dry eye disease. In the 2025 Complete Response Letter, the FDA stated that the NDA “failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms. The Press Release is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated by reference herein.
The following risk factor is provided to supplement Aldeyra’s risk factors previously disclosed under the heading “Risk Factors” in Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2024.
Aldeyra’s success in obtaining regulatory approval of reproxalap from the FDA depends on Aldeyra’s ability to address the issues raised by the FDA in the 2025 Complete Response Letter, and address any issues the FDA may raise in the future.
Aldeyra resubmitted an NDA for reproxalap for the treatment of the signs and symptoms of dry eye disease in October 2024. In November 2024, the FDA accepted the reproxalap NDA for filing and set a Prescription Drug User Fee Act date of April 2, 2025. On April 3, 2025, Aldeyra announced that it had received the 2025 Complete Response Letter from the FDA. In the 2025 Complete Response Letter, the FDA stated that the NDA “failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms. Aldeyra expects to announce results from two additional dry eye disease trials in the second quarter of 2025. There can be no assurance that the results from either trial will be positive. Pending positive results and discussions with the FDA, Aldeyra intends to resubmit the NDA mid-year 2025. In connection with the review of a potential NDA resubmission, the FDA could require additional studies or clinical trials, and the submission of the results of those studies or clinical trials before a potential NDA resubmission will be reconsidered, which would require Aldeyra to expend more resources than Aldeyra planned or that are available to Aldeyra, and could substantially delay acceptance and/or approval, if any, of a potential NDA resubmission. Any such requirement would increase Aldeyra’s costs and delay approval and commercialization of reproxalap for the treatment of dry eye disease and would have a material adverse effect on Aldeyra’s business and financial condition.
Even if reproxalap is approved for the treatment of dry eye disease, the FDA may limit use to certain patient populations, include extensive warnings on the product labeling, or require costly ongoing requirements for post-marketing clinical studies and surveillance or other risk management measures to monitor the safety or efficacy of reproxalap.
Any regulatory approval of reproxalap, once obtained, may be withdrawn. Ultimately, the failure to obtain and maintain regulatory approvals would prevent reproxalap from being marketed and would have a material adverse effect on Aldeyra’s business.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements, including statements regarding the outcome and expected timing of discussions with the FDA; the FDA’s potential acceptance and/or approval of a potential NDA resubmission for reproxalap; the adequacy of the data of the ongoing trials or other additional studies or clinical trials conducted in connection with a potential NDA resubmission; a potential NDA resubmission or the supplemental responses to the FDA; and the Company’s ability to successfully commercialize (alone or with others) reproxalap. Any statements about the Company’s expectations, beliefs, plans, predictions, forecasts, objectives, assumptions, or future events or performance are not historical facts and may be forward-looking. These statements are often, but not always, made through the use of words or phrases such as “anticipates,” “believes,” “can,” “could,” “may,” “predicts,” “potential,” “should,” “will,” “estimate,” “plans,” “projects,” “continuing,” “ongoing,” “expects,” “intends,” and similar words or phrases. Although the Company believes that the expectations reflected in these forward-looking statements are reasonable, these statements are not guarantees of future performance and involve risks and uncertainties which are subject to change based on various important factors, some of which are beyond the Company’s control. The Company has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect its business, financial condition and results of operations. These forward-looking statements speak only as of the date of this Current Report on Form 8-K and are subject to a number of risks, uncertainties and assumptions including, without limitation, risks and factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of the Company’s Annual Report on Form 10-K for the year ended December 31, 2024, which is on file with the SEC and available on the SEC’s website at www.sec.gov. Additional factors may be described in those sections of Aldeyra’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, expected to be filed with the SEC in the second quarter of 2025. The Company does not undertake any obligation to update any forward-looking statements made in this Current Report on Form 8-K as a result of new information, future events or otherwise.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit No. |
Description | |
| 99.1 | Aldeyra Therapeutics, Inc. Presentation dated April 3, 2025 | |
| 99.2 | Aldeyra Therapeutics, Inc. Press Release dated April 3, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ALDEYRA THERAPEUTICS, INC. | ||
| By: | /s/ Todd C. Brady |
|
| Name: | Todd C. Brady, M.D., Ph.D. | |
| Title: | Chief Executive Officer | |
Dated April 3, 2025

Exhibit 99.1 C O N F E R E N C E C A L L Reproxalap for the Potential Treatment of Dry Eye Disease: Regulatory Update April 3, 2025 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2025

2 Disclaimers and Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, statements regarding Aldeyra's future expectations, plans and prospects, including, without limitation, statements regarding: the cost of ongoing clinical trials; Aldeyra’ cash runway; the outcome, expected timing, and results of ongoing clinical trials; the outcome and expected timing of discussions with the FDA; FDA agreement with the clinical development and regulatory plan for reproxalap; the outcome and expected timing and results of the clinical development and regulatory plan; the outcome and timing of the FDA’s acceptance, review and/or approval of a potential NDA resubmission for reproxalap and the adequacy of the data included in the potential NDA resubmission or the supplemental responses to the FDA; the potential for and timing of regulatory approval and commencement of commercialization of reproxalap; Aldeyra's expectations regarding the exercise of the AbbVie option; the potential profile and benefit of reproxalap in dry eye disease and allergic conjunctivitis and its other product candidates in the indications for which they are developed; the goals, opportunity and potential for reproxalap and its other product candidates, anticipated clinical or regulatory milestones for ADX-2191, ADX-248, ADX-743, ADX-631, ADX- 629 and ADX-246, including expectations regarding the results of scheduled FDA meetings and discussions, clinical trial initiations and completions, and the timing and nature of NDA or other submissions to the FDA; Aldeyra's business, research, development and regulatory plans or expectations; political, economic, legal, social and health risks that may affect Aldeyra’s business or the global economy; the structure, timing and success of Aldeyra’s planned or pending clinical trials; and expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” could, “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development of, and clinical and regulatory plans or expectations for Aldeyra’s investigational new drugs (including reproxalap, ADX-2191, ADX-629, ADX-248, ADX-743, ADX-631, and ADX-246), and systems-based approaches, later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from earlier clinical trials, portions of clinical trials, or pooled clinical data may not accurately predict results of subsequent trials or the remainder of a clinical trial for the same or different indications, inconsistent expectations regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing or post-hoc review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, which regulatory review timeline may be flexible and subject to change based on the regulator's workload and other potential review issues, preclinical and clinical results, regulatory developments in the United States and other countries, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, enrolment, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed, and actual results may differ materially from such statements. The information in this presentation is provided only as of April 03, 2025, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

3 Aldeyra Received a Complete Response Letter for Reproxalap for the Treatment of Dry Eye Disease The Complete Response Letter stated that the NDA “failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms. Results from two ongoing trials of reproxalap in dry eye disease are expected to be available this quarter. A Type A meeting with the FDA to discuss the letter and the ongoing clinical trials is expected to be held within approximately 30 days. Per draft FDA guidance, efficacy in dry eye disease may be demonstrated with two symptom trials and two sign trials. Aldeyra previously conducted two dry eye chamber trials for ocular redness (a dry eye disease sign) and dry eye disease field trials (environmental exposure) trials for symptoms. No manufacturing or safety issues were identified in the letter. Regulatory review timelines are flexible and subject to change based on the regulator's workload and other potential review issues. The timing of clinical trials depends, in part, the availability of clinical research facilities and staffing, and the ability to recruit patients. Topical ocular reproxalap is an investigational drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site discomfort is the most commonly reported adverse event in clinical trials.

4 Aldeyra is Well Positioned to Execute on Potential NDA Resubmission Milestones • Top-line results from dry eye disease field and chamber clinical trials are expected in Q2 2025. With $101M in cash, cash • The majority of the costs for the additional trials equivalents, and marketable were incurred in 2024; total 2025 costs of trials securities as of 12/31/2024, are approximately $6M. Aldeyra is well positioned to • Contingent on positive results and FDA execute on potential NDA discussions, NDA resubmission is anticipated resubmission milestones. mid-2025. • The review period for the potential NDA resubmission expected to be six months. Regulatory review timelines are flexible and subject to change based on the regulator's workload and other potential review issues. The timing of clinical trials depends, in part, the availability of clinical research facilities and staffing, and the ability to recruit patients. Topical ocular reproxalap is an investigational drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site discomfort is the most commonly reported adverse event in clinical trials.

5 Dry Eye Disease Field Trial Design Design Randomized, double-masked, parallel group, vehicle-controlled, environmental exposure Dosing Reproxalap or vehicle Further information Four-times-daily dosing for 4 weeks, followed by two-times-daily dosing for 2 weeks can be found on www.clinicaltrials.gov: Size 421 patients (approximately 210 per arm) Trial #NCT0642444. Primary Endpoint Subject-reported ocular discomfort score from Week 1 to Week 6 Other Endpoints Safety Screening Treatment Reproxalap Vehicle Vehicle Week -2 Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Randomization Further information can be found on www.clinicaltrials.gov: Trial #NCT0642444. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.

6 Pooled Data from Two Completed Field Trials Suggested Consistent Symptom Control Relative to Vehicle P<0.0001 Pooled Analysis Weeks 1-6 RENEW-Part 1 The reproxalap (Induction-Maintenance) improvement from baseline was Phase 2 Formulation 10X more than that of vehicle. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials. Prior results may not be indicative of results of ongoing or future trials involving our product candidates.
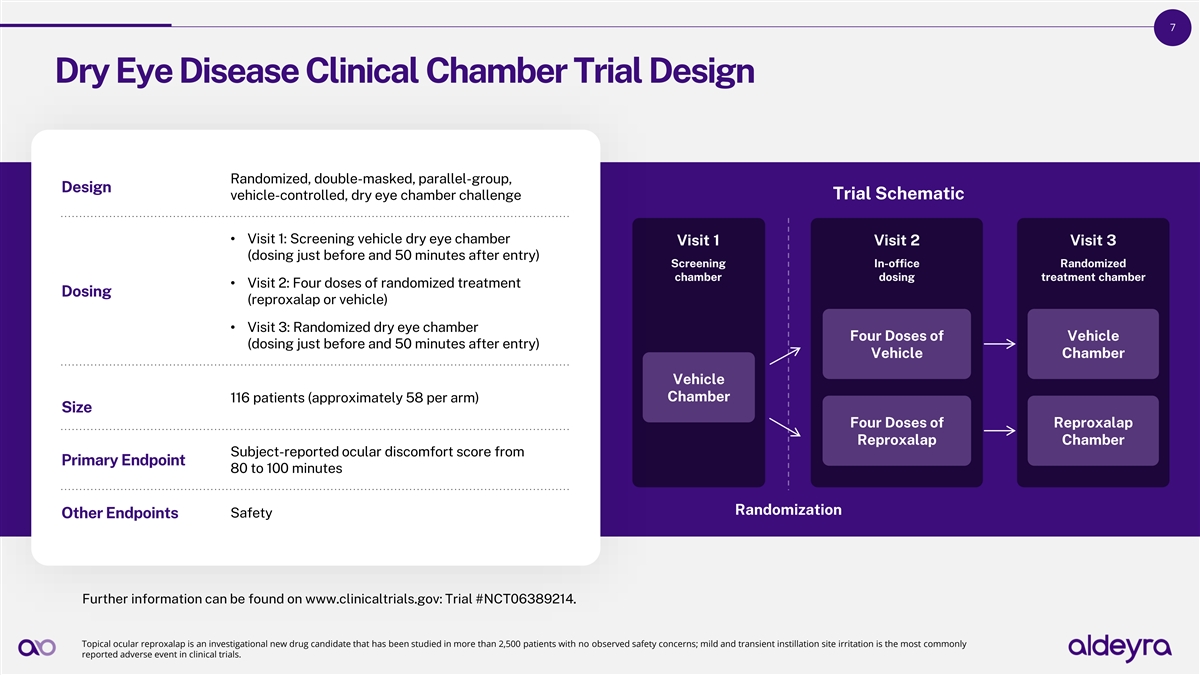
7 Dry Eye Disease Clinical Chamber Trial Design Randomized, double-masked, parallel-group, Design vehicle-controlled, dry eye chamber challenge Trial Schematic • Visit 1: Screening vehicle dry eye chamber Visit 1 Visit 2 Visit 3 (dosing just before and 50 minutes after entry) Screening In-office Randomized chamber dosing treatment chamber • Visit 2: Four doses of randomized treatment Dosing (reproxalap or vehicle) • Visit 3: Randomized dry eye chamber Four Doses of Vehicle (dosing just before and 50 minutes after entry) Vehicle Chamber Vehicle Chamber 116 patients (approximately 58 per arm) Size Four Doses of Reproxalap Reproxalap Chamber Subject-reported ocular discomfort score from Primary Endpoint 80 to 100 minutes Randomization Safety Other Endpoints Further information can be found on www.clinicaltrials.gov: Trial #NCT06389214. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.

8 Pooled Data from Three Completed Trials in the Same Chamber as the Ongoing Trial Supported Rapid Activity of Reproxalap Primary Endpoint Assessment Period P<0.0001 Pooled Analysis The vehicle increase from baseline was 148% higher than that Phase 2 Chamber Trial of reproxalap. TRANQUIILITY TRANQUIILITY-2 Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials. Prior results may not be indicative of results of ongoing or future trials involving our product candidates.

9 Reproxalap Represents a Novel Potential Therapeutic Approach in Dry Eye Disease with Rapid Activity in Clinical Trials Potential advantages for patients and healthcare providers could effect a paradigm shift relative to standard of care. Rapid and sustained Broad Acute reduction of symptom symptomatic ocular redness improvement activity † Dry eye disease afflicts 39 million or more adults in the United States. †Company estimates and Am J Ophthalmol. 2014;157(4):799-806. Topical ocular reproxalap is an investigational new drug candidate that has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.

10 • Dry Eye Disease (Reproxalap) Clinical and Completion of Field and Chamber Trials Expected in Q2 2025 Regulatory • Dry Eye Disease (Reproxalap) Potential NDA Resubmission Expected Mid-2025 Milestones • Allergic Conjunctivitis (Reproxalap) Positive Phase 3 INVIGORATE 2 trial top-line results announced • Atopic Dermatitis (ADX-248) ‡ Phase 1 clinical trial initiated • Moderate Alcohol-Associated Hepatitis (ADX-629) ‡ Open-label Phase 2 clinical trial top-line results expected in 2025 • Retinitis Pigmentosa (ADX-2191) ‡ Phase 2/3 clinical trial initiation expected in H1 2025 ▪ Dry Age-Related Macular Degeneration/Geographic Atrophy (ADX-631/ ADX-246) Investigational New Drug application expected to be submitted in 2025 † Regulatory review and discussion timelines ▪Sjögren-Larsson Syndrome (ADX-629) are flexible and subject to change based on Phase 2 clinical trial pediatric cohort top-line results expected in 2025 the regulator’s workload and other potential ‡ review issues. The timing of clinical trials depends, in part, on the availability of ▪ Obesity/Hypertryglyceridemia (ADX-743) clinical research facilities and staffing, the ability to recruit patients, and the number of Investigational New Drug application expected to be submitted in 2025 * patients in the trial. Investigator sponsored.

11 Aldeyra Is a Well-Capitalized Biotechnology Company with a Broad Immunology and Metabolic Pipeline † PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA REVIEW Rasp Platform for Immune-mediated and Metabolic Diseases Dry Eye Disease Reproxalap Option Topical ocular administration Agreement Allergic Conjunctivitis * Sjögren-Larsson Syndrome ADX-629 Oral administration Moderate Alcohol-Associated Hepatitis ADX-248 Atopic Dermatitis Oral administration ADX-743 Obesity/Hypertryglyceridemia Oral administration Dry Age-Related Macular Degeneration/ ADX-631/ADX-246 Geographic Atrophy Intravitreal injection Vitreous Methotrexate Platform for Rare Retinal Inflammatory Diseases ADX-2191 Retinitis Pigmentosa (U.S. FDA Orphan Drug Designation) Intravitreal injection ‡ As of 12/31/2024, cash, cash equivalents, and marketable securities were $101.2M, which Aldeyra believes will be sufficient to fund the Company into 2027. †Regulatory review timelines are flexible and subject to change based on the regulator's workload and other potential review issues. ‡Company guidance as of February 28, 2025; includes continued early and late-stage development of our product candidates in immune-mediated and metabolic diseases. Guidance has not been updated or confirmed since February 28, 2025 and does not include any potential licensing or product revenue associated with reproxalap. *Investigator sponsored.
Exhibit 99.2

|
News Release |
Aldeyra Therapeutics Receives Complete Response Letter from the U.S. Food and Drug Administration for the Reproxalap New Drug Application for the Treatment of Signs and Symptoms of Dry Eye Disease
| • | Top-Line Data from Dry Eye Chamber Trial and Field Trial Expected in Q2 2025 |
| • | Pending Positive Results and Discussions with the FDA, New Drug Application Resubmission Expected Mid-Year 2025 |
Lexington, Mass., April 3, 2025 – Aldeyra Therapeutics, Inc. (Nasdaq: ALDX) (Aldeyra), a biotechnology company devoted to discovering and developing innovative therapies designed to treat immune-mediated and metabolic diseases, today announced receipt of a Complete Response Letter from the U.S. Food and Drug Administration (FDA) for the resubmission of the New Drug Application (NDA) of reproxalap, an investigational drug candidate, for the treatment of dry eye disease. Although no manufacturing or safety issues with reproxalap were identified, the FDA stated in the letter that the NDA “failed to demonstrate efficacy in adequate and well controlled studies in treating ocular symptoms associated with dry eyes” and that “at least one additional adequate and well controlled study to demonstrate a positive effect on the treatment of ocular symptoms of dry eye” should be conducted. The letter identified concerns with the data from the trial submitted to the NDA that may have affected interpretation of the results, which the FDA stated may be related to methodological issues, including a difference in baseline scores across treatment arms.
Per draft FDA dry eye disease guidance, to be considered for regulatory approval in the United States, efficacy in dry eye disease may be demonstrated with two symptom trials and two sign trials. Among other clinical trials of reproxalap, Aldeyra previously conducted two trials for ocular redness (a dry eye disease sign) in a dry eye chamber, and two dry eye disease symptom field (environmental exposure) trials, which were submitted as part of an initial NDA in November 2022. In November 2023, the FDA issued a Complete Response Letter to the initial NDA stating that at least one additional symptom trial was required. Following discussions with the FDA, and as part of a comprehensive strategy designed to account for disease heterogeneity and potential differences in clinical sites and environment, Aldeyra initiated three clinical trials assessing dry eye disease symptoms: a dry eye chamber trial, a clinical trial in a different dry eye chamber, and a six-week field trial. In August 2024, Aldeyra announced the achievement of the primary endpoint in the first dry eye chamber clinical trial of reproxalap, and the NDA was resubmitted in October 2024. A Type A meeting is expected to be held within approximately 30 days to discuss the Complete Response Letter for the resubmitted NDA and the ongoing clinical trials of reproxalap in dry eye disease.
In the second quarter of 2025, Aldeyra expects to announce top-line results from the ongoing dry eye disease field trial and the ongoing chamber clinical trial. Subject to positive results and discussions with the FDA, Aldeyra intends to resubmit the NDA mid-year 2025. The review period for the potential NDA resubmission is expected to be six months.
As of December 31, 2024, Aldeyra reported cash, cash equivalents, and marketable securities of $101 million. With a majority of costs for the ongoing dry eye clinical trials having occurred in 2024, the full-year 2025 costs of the trials are expected to be approximately $6 million.
“Pending positive results from the ongoing clinical trials and discussions with the FDA, we look forward to a potential NDA resubmission mid-year 2025,” stated Todd C. Brady, M.D., Ph.D., President and Chief Executive Officer of Aldeyra Therapeutics. “Reproxalap remains the only late-stage topical ocular therapy suitable for chronic administration to have potentially demonstrated acute reduction in ocular redness, as well as reduction in ocular discomfort, highlighting rapid and broad activity for both the signs and symptoms of dry eye disease.”
Conference Call & Webcast Information
Aldeyra will host a conference call at 5:00 p.m. ET today, April 3, 2025, to provide a regulatory update on reproxalap. The dial-in numbers are (833) 470-1428 for domestic callers and (404) 975-4839 for international callers. The access code is 287309. A live audio webcast of the conference call also will be accessible from the “Investors & Media” section of Aldeyra’s website at ir.aldeyra.com. A live webcast of the conference call will be available on the Investor Relations page of the company’s website at https://ir.aldeyra.com. After the live webcast, the event will remain archived on the Aldeyra Therapeutics website for 90 days.
About Reproxalap
Reproxalap is an investigational new drug candidate in development for the treatment of dry eye disease and allergic conjunctivitis, two of the largest markets in ophthalmology. Reproxalap is a first-in-class small-molecule modulator of RASP, which are elevated in ocular and systemic inflammatory diseases. The mechanism of action of reproxalap has been supported by the demonstration of statistically significant and clinically relevant activity in multiple physiologically distinct late-phase clinical indications. Reproxalap has been studied in more than 2,500 patients with no observed safety concerns; mild and transient instillation site irritation is the most commonly reported adverse event in clinical trials.
About Aldeyra
Aldeyra Therapeutics is a biotechnology company devoted to discovering innovative therapies designed to treat immune-mediated and metabolic diseases. Our approach is to develop pharmaceuticals that modulate protein systems, instead of directly inhibiting or activating single protein targets, with the goal of optimizing multiple pathways at once while minimizing toxicity. Our product candidates include RASP (reactive aldehyde species) modulators ADX-629, ADX-248, ADX-743, ADX-631, ADX-246, and chemically related molecules for the potential treatment of systemic and retinal immune-mediated and metabolic diseases.
Our late-stage product candidates are reproxalap, a RASP modulator for the potential treatment of dry eye disease and allergic conjunctivitis, and ADX-2191, a novel formulation of intravitreal methotrexate for the potential treatment of retinitis pigmentosa. For additional information, please visit www.aldeyra.com.
Safe Harbor Statement
This release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Aldeyra’s future expectations, plans, and prospects, including without limitation statements regarding: the goals, opportunity, and potential for reproxalap; the costs, outcome and expected timing and the results of the ongoing dry eye disease clinical trials; the outcome and expected timing of discussions with the FDA the potential and the timing of a potential NDA resubmission; the outcome and timing of the FDA’s acceptance, review, or approval of the potential NDA resubmission for reproxalap and the adequacy of the data included in the initial NDA and resubmitted NDA, and expected to be included in the potential resubmitted NDA; the likelihood and timing of the exercise of the Option; and Aldeyra’s expectations regarding the labeling for reproxalap, if approved. Aldeyra intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by terms such as, but not limited to, “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “on track,” “scheduled,” “target,” “design,” “estimate,” “predict,” “contemplates,” “likely,” “potential,” “continue,” “ongoing,” “aim,” “plan,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. Such forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions, and uncertainties. Aldeyra is at an early stage of development and may not ever have any products that generate significant revenue. All of Aldeyra’s development timelines may be subject to adjustment depending on recruitment rate, regulatory review, preclinical and clinical results, funding, and other factors that could delay the initiation, enrollment, or completion of clinical trials.
Important factors that could cause actual results to differ materially from those reflected in Aldeyra’s forward-looking statements include, among others, the timing of enrollment, commencement and completion of Aldeyra’s clinical trials, the timing and success of preclinical studies and clinical trials conducted by Aldeyra and its development partners; delay in or failure to obtain regulatory approval of Aldeyra’s product candidates, including as a result of the FDA not accepting Aldeyra’s regulatory filings, issuing a complete response letter, or requiring additional clinical trials or data prior to review or approval of such filings or in connection with resubmissions of such filings; the ability to maintain regulatory approval of Aldeyra’s product candidates, and the labeling for any approved products; the risk that prior results, such as signals of safety, activity, or durability of effect, observed from preclinical or clinical trials, will not be replicated or will not continue in ongoing or future studies or clinical trials involving Aldeyra’s product candidates in clinical trials focused on the same or different indications; the scope, progress, expansion, and costs of developing and commercializing Aldeyra’s product candidates; uncertainty as to Aldeyra’s ability to commercialize (alone or with others) and obtain reimbursement for Aldeyra’s product candidates following regulatory approval, if any; the size and growth of the potential markets and pricing for Aldeyra’s product candidates and the ability to serve those markets; Aldeyra’s expectations regarding Aldeyra’s expenses and future revenue, the timing of future revenue, the sufficiency or use of Aldeyra’s cash resources and needs for additional financing; the rate and degree of market acceptance of any of Aldeyra’s product candidates; Aldeyra’s expectations regarding competition; Aldeyra’s anticipated growth strategies; Aldeyra’s ability to attract or retain key personnel; Aldeyra’s commercialization, marketing and manufacturing capabilities and strategy; Aldeyra’s ability to establish and maintain development partnerships; Aldeyra’s ability to successfully integrate acquisitions into its business; Aldeyra’s expectations regarding federal, state, and foreign regulatory requirements; political, economic, legal, social, and health risks, public health measures, and war or other military actions, that may affect Aldeyra’s business or the global economy; regulatory developments in the United States and foreign countries; Aldeyra’s ability to obtain and maintain intellectual property protection for its product candidates; the anticipated trends and challenges in Aldeyra’s business and the market in which it operates; and other factors that are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2024, which is on file with the Securities and Exchange Commission (SEC) and available on the SEC’s website at https://www.sec.gov/. Additional factors may be described in those sections of Aldeyra’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, expected to be filed with the SEC in the second quarter of 2025, and Aldeyra’s other filings with the SEC.
In addition to the risks described above and in Aldeyra’s other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra’s results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this release is provided only as of the date of this release, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this release on account of new information, future events, or otherwise, except as required by law.
| Investor & Media Contact: |
| Laura Nichols |
| Tel: (781) 257-3060 investorrelations@aldeyra.com |