|
☐
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
☒
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2024
|
|
☐
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from ______ to ______
|
|
☐
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 or 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
Israel
|
65 Yigal Alon St.
Tel Aviv 6744316, Israel Tel: +972 3 7177050 |
|
|
(Jurisdiction of incorporation or organization)
|
(Address of principal executive offices)
|
|
Title of each class to be registered
|
|
Trading Symbol(s)
|
|
Name of each exchange on which each
class is to be registered |
|
Ordinary shares, no par value NIS per share
|
|
PRFX
|
|
The Nasdaq Stock Market LLC
|
|
|
Large accelerated filer ☐
|
|
Accelerated filer ☐
|
|
Non-accelerated filer ☒
|
|
|
|
|
|
|
|
Emerging Growth Company ☒
|
|
|
|
|
Page |
| 1 | ||
| 1 | ||
| 2 | ||
| 4 | ||
|
|
| |
|
|
5 | |
|
|
|
|
| 5 | ||
| 5 | ||
| 5 | ||
|
A. |
[RESERVED] |
5 |
|
B. |
Capitalization and Indebtedness |
5 |
|
C. |
Reasons for the Offer and Use of Proceeds |
5 |
|
D. |
Risk Factors |
5 |
| 48 | ||
|
A. |
History and Development of the Company |
48 |
|
B. |
Business Overview |
49 |
|
C. |
Organizational Structure |
75 |
|
D. |
Property, Plants and Equipment |
75 |
| 75 | ||
| 75 | ||
|
A. |
Operating Results |
80 |
|
B. |
Liquidity and Capital Resources |
87 |
|
C. |
Research and Development, Patents and Licenses |
87 |
|
D. |
Trend Information |
87 |
|
E. |
Critical Accounting Estimates |
88 |
| 89 | ||
|
A. |
Directors and Senior Management |
89 |
|
B. |
Compensation |
91 |
|
C. |
Board Practices |
94 |
|
D. |
Employees |
111 |
|
E. |
Share Ownership |
111 |
|
F |
Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation |
112 |
| 113 | ||
|
A. |
Major Shareholders |
113 |
|
B. |
Related Party Transactions |
115 |
|
C. |
Interests of Experts and Counsel |
115 |
| 116 | ||
|
A. |
Consolidated Statements and Other Financial Information |
116 |
|
B. |
Significant Changes |
116 |
| 116 | ||
|
A. |
Offer and Listing Details |
116 |
|
B. |
Plan of Distribution |
116 |
|
C. |
Markets |
116 |
|
D. |
Selling Shareholders |
116 |
|
E. |
Dilution |
116 |
|
F. |
Expenses of the Issue |
116 |
| 117 | ||
|
A. |
Share Capital |
117 |
|
B. |
Articles of Association |
117 |
|
C. |
Material Contracts |
117 |
|
D. |
Exchange Controls |
117 |
|
E. |
Taxation |
117 |
|
F. |
Dividends and Paying Agents |
129 |
|
G. |
Statement by Experts |
129 |
|
H. |
Documents on Display |
129 |
|
I. |
Subsidiary Information |
129 |
|
J. |
Annual Report to Security Holders. |
129 |
| 130 | ||
| 130 | ||
|
A. |
Debt Securities |
130 |
|
B. |
Warrants and rights |
130 |
|
C. |
Other Securities |
130 |
|
D. |
American Depositary Shares |
130 |
|
|
|
|
|
|
130 | |
|
|
|
|
| 130 | ||
| 130 | ||
| 131 | ||
| 132 | ||
| 132 |
| 132 | ||
| 132 | ||
| 133 | ||
| 133 | ||
| 133 | ||
| 134 | ||
| 134 | ||
| 135 | ||
| 135 | ||
|
|
|
|
|
|
136 | |
|
|
|
|
| 136 | ||
| 136 | ||
| 136 | ||
| 139 | ||
|
|
● |
our ability to continue as a going concern;
|
|
|
● |
our history of losses and need for additional capital to fund our operations, and
our ability to obtain additional capital on acceptable terms, or at all; |
|
|
● |
our dependence on the success of our initial product candidate, PRF-110; |
|
|
● |
the outcomes of preclinical studies, clinical trials and other research regarding
PRF-110 and future product candidates; |
|
|
● |
our limited experience managing clinical trials; |
|
|
● |
our ability to retain key personnel and recruit additional employees; |
|
|
● |
our reliance on third parties for the conduct of clinical trials, product manufacturing
and development; |
|
|
● |
the impact of competition and new technologies; |
|
|
● |
our ability to comply with regulatory requirements relating to the development and
marketing of our product candidates; |
|
|
● |
our ability to establish and maintain strategic partnerships and other corporate collaborations;
|
|
|
● |
the implementation of our business model and strategic plans for our business and
product candidates; |
|
|
● |
the scope of protection we are able to establish and maintain for intellectual
property rights covering our product candidates and our ability to operate our business without infringing the intellectual property rights
of others; |
|
|
● |
the overall global economic environment; |
|
|
● |
our ability to develop an active trading market for our ordinary shares and whether
the market price of our ordinary shares is volatile; |
|
|
● |
statements as to the impact of the political and security situation
in Israel on our business, including due to the current security situation in Israel; and |
|
|
● |
those factors referred to in “Item 3.D. Risk Factors,” “Item 4.
Information on the Company,” and “Item 5. Operating and Financial Review and Prospects”, as well as in this Annual Report
on Form 20-F generally. |
|
|
Not applicable. |
|
|
Not applicable. |
|
A. |
[RESERVED] |
|
B. |
Capitalization and Indebtedness
Not applicable. |
|
C. |
Reasons for the Offer and Use of Proceeds
Not applicable. |
|
D. |
Risk Factors |
| |
● |
Our limited operating history may make it difficult for you to assess our future viability. We have never
generated revenues and may never be profitable; |
|
|
● |
The report of our independent registered public accounting firm contains an explanatory
paragraph regarding substantial doubt about our ability to continue as a going concern; |
|
|
● |
We have incurred significant losses and negative cash flows from operations since
our inception and expect to incur losses for the foreseeable future. We may never achieve or maintain profitability; |
|
|
● |
We will need substantial additional funding, which may not be available to us on acceptable
terms or at all. If we are unable to raise capital when needed, we may be forced to delay, reduce and/or eliminate our research and drug
development programs or future commercialization efforts; and |
|
|
● |
Raising additional capital may cause dilution to our shareholders, restrict our operations
or require us to relinquish rights to our product candidates. |
|
|
● |
We are dependent on the success of our initial product candidate, PRF-110. If we are unable to obtain
approval for and ultimately commercialize PRF-110 or experience significant delays in doing so, our business will be materially harmed.
|
|
|
● |
We have not yet commercialized any products or technologies, and we may never become
profitable; |
|
|
● |
If we are unable to successfully complete our clinical trial programs for PRF-110,
or if such clinical trials take longer to complete than we project, our ability to execute our current business strategy will be adversely
affected; |
|
|
● |
We have limited experience in conducting and managing clinical trials necessary to
obtain regulatory approvals. If our drug candidates and technologies do not receive the necessary regulatory approvals, we will be unable
to commercialize our products; |
|
|
● |
If third parties on which we will have to rely for clinical trials do not perform
as contractually required or as we expect, we may not be able to obtain regulatory approval for or commercialize our products; and
|
|
|
● |
If our competitors develop and market products that are less expensive or more effective
than our product, our revenues and results may be harmed and our commercial opportunities may be reduced or eliminated; |
|
|
● |
We recently entered into a new line of business that offers an AI software solution, which subjects us to additional risks.
|
|
|
● |
The market for our DeepSolar solution is new and unproven, may experience limited growth. |
|
|
● |
The market for AI-based software applications is relatively new and unproven and may decline or experience limited growth. Concerns
over the use of AI, including from regulators, the public and our customers, may hinder the adoption of AI technologies, which would adversely
affect our ability to fully realize the potential of the DeepSolar solution. |
|
|
● |
If we are not able to enhance our existing solution or introduce new solutions that achieve market acceptance and keep pace with
technological developments, our business, results of operations and financial condition could be harmed. |
|
|
● |
Our sales efforts involve considerable time and expense and the sales cycle is often long and unpredictable. |
|
|
● |
If we fail to scale our business operations or otherwise manage future growth of the DeepSolar business effectively as we attempt
to grow our company, we may not be able to market and sell the DeepSolar solution successfully. |
|
|
● |
We may face intense competition and expect competition to increase in the future, which could prohibit us from developing a customer
base and generating revenue. |
|
|
● |
Our DeepSolar business could be significantly disrupted if we lose key members of the DeepSolar team. |
|
|
●
|
If we are unable to maintain patent protection for our products, our competitors could
develop and commercialize products and technology similar or identical to our product candidates, and our ability to successfully commercialize
any product candidates we may develop, and our science may be adversely affected. |
|
|
● |
Conditions in the Middle East and in Israel may harm our operations. |
|
|
● |
Our business and operations would suffer in the event of IT system failures, cybersecurity attacks, data
breaches, or vulnerabilities in our or our third-party vendors’ information security program or defenses. |
|
|
● |
We may not be able to successfully identify and execute strategic alliances or other relationships with
third parties or to successfully manage the impacts of acquisitions, dispositions or relationships on our operations. |
|
|
●
|
If we fail to regain compliance with the Nasdaq minimum listing requirements, our
ordinary shares will be subject to delisting. Our ability to publicly or privately sell equity securities and the liquidity of our ordinary
shares could be adversely affected if our ordinary shares are delisted; |
|
|
● |
We are currently operating in a period of economic uncertainty and capital markets disruption,
which has been significantly impacted by geopolitical instability due to the ongoing military conflict between Israel and Hamas and Russia
and Ukraine; |
|
|
● |
Because we are not subject to compliance with rules requiring the adoption of certain
corporate governance measures, our shareholders have limited protections against interested director transactions, conflicts of interest
and similar matters; and |
|
|
● |
If we are unable to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act as they apply to
a foreign private issuer that is listed on a U.S. exchange, or our internal control over financial reporting is not effective, the reliability
of our financial statements may be questioned and our share price may suffer. |
|
|
● |
We have identified a material weakness in
our internal control over financial reporting. If our remediation of the material weakness is not effective, or we fail to develop and
maintain effective internal controls over financial reporting, our ability to produce timely and accurate financial statements or comply
with applicable laws and regulations could be impaired. |
|
|
● |
initiate and manage clinical trials for PRF-110; |
|
|
● |
integrate and expand our DeepSolar business; |
|
|
● |
seek regulatory approvals; |
|
|
● |
implement internal systems and infrastructures; |
|
|
● |
hire management and other personnel; and |
|
|
● |
progress PRF-110 towards commercialization. |
|
|
● |
the costs, timing and outcome of manufacturing clinical trial and commercial quantities
of PRF-110 |
|
|
● |
the scope, progress, results and costs of our current and future clinical trials of
PRF-110 for our current targeted uses; |
|
|
● |
the costs, timing and outcome of regulatory review of PRF-110; |
|
|
● |
the extent to which we acquire or invest in businesses, products and technologies,
including entering into or maintaining licensing or collaboration arrangements for PRF-110 on favorable terms, although we currently have
no commitments or agreements to complete any such transactions; |
|
|
● |
the costs and timing of future commercialization activities, including sales, marketing,
manufacturing and distribution, for any of our product candidates for which we receive marketing approval, to the extent that such sales,
marketing, manufacturing and distribution are not the responsibility of any collaborator that we may have at such time; |
|
● |
the cost to continue the development of the DeepSolar technology to develop a wider
portfolio of solutions; | |
|
● |
the cost of establishing a sales, marketing, and technical support infrastructure
to support the ramp up of the DeepSolar solution; | |
|
|
● |
the amount of revenue, if any, received from commercial sales of PRF-110, should it
receive marketing approval, or from the DeepSolar solution; |
|
|
● |
the costs of preparing, filing and prosecuting patent applications, maintaining, defending
and enforcing our intellectual property rights and defending intellectual property-related claims; |
|
|
● |
our ability to establish strategic collaborations, licensing or other arrangements
and the financial terms of any such agreements, including the timing and amount of any future milestone, royalty or other payments due
under any such agreement; |
|
|
● |
our headcount growth and associated costs as we expand our business operations and
our research and development activities; |
|
|
● |
the costs of operating as a public company; |
|
|
● |
maintaining minimum shareholders’ equity requirements under the Nasdaq rules;
and |
|
|
● |
the impact of the current war between Israel and Hamas which may exacerbate the magnitude
of the factors discussed above. |
|
● |
the outcome of further research and development of PRF-110 following the announcement
in December 2024 that the Phase 3 clinical trial did not satisfy the study’s primary endpoint; |
|
|
●
|
establishing supply arrangements with third-party raw materials and components suppliers,
and drug product manufacturers who can manufacture clinical trial and commercial quantities of PRF-110, and developing, validating and
maintaining a commercially-viable manufacturing process that is compliant with current Good Manufacturing Practices, or cGMP, at a scale
sufficient to meet anticipated demand, which will ultimately enable us to reduce our cost of manufacturing; |
|
|
● |
successfully initiating patient enrollment and completion of additional clinical trials
on a timely basis; |
|
|
● |
our ability to demonstrate PRF-110’s safety, tolerability and efficacy to the
FDA and any comparable foreign regulatory authority for marketing approval; |
|
|
● |
timely receipt of marketing approvals for PRF-110; |
|
|
● |
maintaining patent protection, trade secret protection and regulatory exclusivity,
both in the U.S. and internationally; |
|
|
● |
successfully defending and enforcing our proprietary rights in our intellectual property
portfolio; |
|
|
● |
avoiding and successfully defending against any claims that we have infringed, misappropriated
or otherwise violated any intellectual property of any third party; |
|
|
● |
the performance of any future collaborations; |
|
|
● |
our ability to timely complete any post-marketing approval commitments required by
the FDA or other applicable regulatory authorities; |
|
|
● |
establishing scaled production arrangements with third-party manufacturers to obtain
finished products that are compliant with cGMP and appropriately packaged for commercialization; |
|
|
● |
successful launch of commercial sales following any marketing approval; |
|
|
● |
maintaining an acceptable safety profile following any marketing approval; |
|
|
● |
commercial acceptance by patients, the medical community and third-party payors;
|
|
|
● |
the availability of coverage and adequate reimbursement and pricing by third-party
payors and government authorities; |
|
|
● |
the availability, perceived advantages, relative cost, relative safety and relative
efficacy of alternative and competing treatments; and |
|
|
● |
our ability to compete with other post-operative pain, or POP, treatments. |
|
|
● |
the timing of regulatory approvals in the requested countries, for the applications we seek; |
|
|
● |
competitive market environment; |
|
|
● |
the establishment and demonstration in the medical community of the safety and clinical
efficacy of our products and their potential advantages over existing therapeutic products; |
|
|
● |
our ability to enter into strategic agreements with pharmaceutical and biotechnology
companies with strong marketing and sales capabilities; |
|
|
● |
the adequacy and success of distribution and marketing efforts; and |
|
|
● |
the pricing and reimbursement policies of government and third-party payors, such
as insurance companies, health maintenance organizations and other plan administrators. |
|
|
● |
obtaining regulatory approvals (e.g., an Investigational New Drug, or IND, application)
to commence a clinical trial; |
|
|
● |
reaching agreement on acceptable terms with prospective contract research organizations,
or CROs, and trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and
trial sites; |
|
|
● |
slower than expected rates of patient recruitment due to narrow screening requirements
and competing clinical studies; |
|
|
● |
the inability of patients to meet protocol requirements imposed by the FDA or other
regulatory authorities; |
|
|
● |
the need or desire to modify our manufacturing process; |
|
|
● |
delays, suspension, or termination of the clinical trials due to the institutional
review board responsible for overseeing the study at a particular study site; and |
|
|
● |
governmental or regulatory delays or “clinical holds” requiring suspension
or termination of the trials. |
|
|
● |
assist us in developing, testing and obtaining regulatory approval; |
|
|
● |
manufacture our drug candidates; and |
|
|
● |
market and distribute our products. |
|
|
● |
perceptions by members of the health care community, including physicians, of the
safety and efficacy of our product; |
|
|
● |
the potential advantages that our product offers over existing treatment methods or
other products that may be developed; |
|
|
● |
the cost-effectiveness of our product relative to competing products; |
|
|
● |
the availability of government or third-party pay or reimbursement for our products;
and |
|
|
● |
the effectiveness of our or our partners’ sales, marketing and distribution
efforts. |
|
|
● |
litigation involving patients taking our drug; |
|
|
● |
restrictions on such drugs, manufacturers or manufacturing processes; |
|
|
● |
restrictions on the labeling or marketing of a drug; |
|
|
● |
restrictions on drug distribution or use; |
|
|
● |
requirements to conduct post-marketing studies or clinical trials; |
|
|
● |
warning letters or untitled letters; |
|
|
● |
withdrawal of the drugs from the market; |
|
|
● |
refusal to approve pending applications or supplements to approved applications that
we submit; |
|
|
● |
recall of drugs; |
|
|
● |
fines, restitution or disgorgement of profits or revenues; |
|
|
● |
suspension or withdrawal of marketing approvals; |
|
|
● |
damage to relationships with any potential collaborators; |
|
|
● |
exclusion from or restrictions on coverage by third-party payors; |
|
|
● |
unfavorable press coverage and damage to our reputation; |
|
|
● |
refusal to permit the import or export of drugs; |
|
|
● |
drug seizure; or |
|
|
● |
injunctions or the imposition of civil or criminal penalties. |
|
|
● |
decreased demand for a product; |
|
|
● |
damage to our reputation; |
|
|
● |
withdrawal of clinical trial volunteers; and |
|
|
● |
loss of revenues. |
|
|
● |
diversion of management’s attention, available cash, and other resources from
our existing business; |
|
|
● |
unanticipated liabilities or contingencies; |
|
|
● |
compliance with additional regulatory burdens; |
|
|
● |
potential damage to existing customer relationships, lack of customer acceptance or
inability to attract new customers; and |
|
|
● |
the inability to compete effectively in the new line of business. |
|
|
● |
we have not recruited adequate numbers
of effective sales and marketing personnel; |
|
|
|
|
|
|
● |
the challenge of sales personnel to obtain
access to potential customers; |
|
|
|
|
|
|
● |
unforeseen costs and expenses associated
with creating an independent sales and marketing organization. |
|
|
● |
result in costly litigation; |
|
|
● |
divert management’s attention and resources; |
|
|
● |
cause product shipment delays; and |
|
|
● |
require us to enter into royalty or licensing agreements. Such royalty or licensing
agreements, if required, may not be available on terms acceptable to us, if at all. |
|
|
● |
others may be able to make products that are similar to our product candidates or
utilize similar science or technology but that are not covered by the claims of the patents that we may own or license from our licensors
or that incorporate certain research in our product candidates that is in the public domain; |
|
|
● |
we might not have been the first to file patent applications covering our inventions;
|
|
|
● |
others may independently develop similar or alternative technologies or duplicate
any of our technologies without infringing our intellectual property rights; |
|
|
● |
issued patents that we hold rights to may be held invalid or unenforceable,
including as a result of legal challenges by our competitors or other third parties; |
|
|
●
|
our competitors or other third parties might conduct research and development activities
in countries where do not have patent rights and then use the information learned from such activities to develop competitive products
for sale in our major commercial markets; |
|
|
● |
the patents of others may harm our business if, for example, we are found to have
infringed those patents or if those patents serve as prior art to our patents which could potentially invalidate our patents; and
|
|
|
● |
we may choose not to file a patent in order to maintain certain trade secrets or know-how,
and a third party may subsequently file a patent covering such intellectual property, which could ultimately result in public disclosure
of the intellectual property if the third party’s patent application is published or issues to a patent. |
|
|
● |
the potential disruption of our ongoing business; |
|
|
● |
the distraction of management away from the ongoing oversight of our existing business
activities; |
|
|
● |
incurring additional indebtedness; |
|
|
● |
the anticipated benefits and cost savings of those transactions not being realized
fully, or at all, or taking longer to realize than anticipated; |
|
|
● |
an increase in the scope and complexity of our operations; and |
|
|
● |
the loss or reduction of control over certain of our assets. |
|
|
● |
less liquid trading market for our securities; |
|
|
● |
more limited market quotations for our securities; |
|
|
● |
determination that our ordinary shares and/or warrants are a “penny stock”
that requires brokers to adhere to more stringent rules and possibly resulting in a reduced level of trading activity in the secondary
trading market for our securities; |
|
|
● |
more limited research coverage by stock analysts; |
|
|
● |
loss of reputation; and |
|
|
● |
more difficult and more expensive equity financings in the future. |
|
|
● |
changes or developments in laws or regulations governing our business; |
|
|
● |
announcements of regulatory approvals or the failure to obtain them, or specific label
indications or patient populations for their use, or changes or delays in the regulatory review process; |
|
|
● |
unsatisfactory results of preclinical studies or clinical trials; |
|
|
● |
adverse actions taken by regulatory agencies with respect to our manufacturing supply
chain or sales and marketing activities; |
|
|
● |
announcements of innovations or new products by us or our competitors; |
|
|
● |
any intellectual property infringement, misappropriation or other actions in which
we may become involved; |
|
|
● |
any adverse changes to our relationships with manufacturers or suppliers; |
|
|
● |
announcements concerning our competitors; |
|
|
● |
achievement of expected product sales and profitability or our failure to meet expectations;
|
|
|
● |
our commencement of, or involvement in, litigation; and |
|
|
● |
any changes in our board of directors or management. |
|
ITEM 4.
INFORMATION ON THE COMPANY |
|
A. |
History and Development of the Company |
|
B. |
Business Overview |
|
|
● |
addressing unmet medical needs; |
|
|
● |
de-risked drug development by using long-established APIs and our patented, proprietary
extended release drug-delivery system; |
|
|
● |
reduced development costs; |
|
|
● |
rapid preclinical and clinical testing; |
|
|
● |
well understood paths to approval: and |
|
|
● |
the potential to disrupt current practices. |
|
|
● |
PRF-110 is highly viscous and thus stays in place when placed into a surgical wound
bed. |
|
|
● |
PRF-110 remains within the surgical site when the skin is closed, without being toxic
or proinflammatory. |
|
|
● |
PRF-110 is easy to administer and its use is consistent with current surgical practice.
|
|
|
● |
PRF-110 is highly uniform and resistant to degradation in the wound, resulting is
sustained,/extended release of the analgesic. |
|
|
● |
Ropivacaine, the active drug used in PRF-110, is a safe and well-characterized local
anesthetic. |
|
|
● |
The components that make up the remainder of the PRF-110 formulation are classified
as GRAS (Generally Regarded As Safe) by the FDA. |
|
|
● |
We have amassed a human toxicology portfolio for PRF-110, demonstrating that there
are no PRF-110-associated serious adverse events in either healthy controls or in surgical patients. |
|
|
● |
Based on extensive toxicology and pharmacokinetic studies, as well as positive Phase
2 results, the FDA has granted our company an IND for PRF-110 and approved the initiation of Phase 3 trials for the treatment of post-operative
pain. |
|
|
● |
Unlike many drug trials that take months to years to complete and which are complex
and whose endpoints are difficult to interpret, the planned trials are expected to last for 72 hours with a seven day and a one-month
follow-up, with primary endpoint of pain measurement on the familiar scale of 0 (no pain) to 10 (worst imaginable pain). |
|
|
● |
If and when approved for commercial sale, we intend to capitalize on the opportunity
and carry out post-approval trials in a number of additional surgical indications, including breast augmentation/reduction, bariatric
procedures, hysterectomy, cholecystectomy as well as orthopedic procedures including joint replacements and open fracture repair. We intend
to capitalize on these opportunities to become the leader in opiate-free, long-acting local and regional analgesia. |
|
|
● |
Hospitals; |
|
|
● |
Free-standing surgical centers; and |
|
|
● |
Surgical offices. |
|
|
● |
Posimir by Durect (DRRX). A bupivacaine collagen matrix was recently approved by the
FDA for only arthroscopic subacromial decompression (niche market ~600,000 annual procedures in the U.S.). |
|
|
● |
Xaracoll by Innocoll, a surgically implantable and bioresorbable bupivacaine-collagen
matrix, FDA approved the product for only open inguinal hernia repair. |
|
|
● |
Allay Therapeutics ATX-101, a product based on bupivacaine is in development stage.
|
|
|
●
|
TLC590, from the Taiwan Liposome Company, is a liposomal formulation of ropivacaine
that completed Phase II trials in patients following hernia surgery.
|
|
|
●
|
Cali Biosciences developing an injectable ropivacaine formulation CPL-01 which is currently in
phase III for bunion and hernia; and |
|
|
|
|
|
|
● |
Vertex pharmaceuticals, which completed phase III clinical studies in abdominoplasty
and bunionectomy with VX-548, a selective NaV1.8 inhibitor. |
|
|
● |
completion of preclinical laboratory tests, animal studies and formulation studies
in compliance with the FDA’s good laboratory practice, or GLP, regulations; |
|
|
● |
submission to the FDA of an IND, which must take effect before human clinical trials
begin; |
|
|
● |
approval by an independent institutional review board, or IRB, representing each clinical
site before each clinical trial may be initiated; |
|
|
● |
performance of adequate and well-controlled human clinical trials in accordance with
good clinical practices, or GCP, to establish the safety and efficacy of the proposed drug product for each proposed indication;
|
|
|
● |
preparation and submission to the FDA of an NDA requesting marketing for one or more
proposed indications; |
|
|
● |
review by an FDA advisory committee, where appropriate or if applicable; |
|
|
● |
satisfactory completion of one or more FDA inspections of the manufacturing facility
or facilities at which the product, or components thereof, are produced to assess compliance with current Good Manufacturing Practices,
or cGMP, requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength,
quality and purity; |
|
|
● |
satisfactory completion of FDA audits of clinical trial sites to assure compliance
with GCPs and the integrity of the clinical data; |
|
|
● |
payment of user fees and securing FDA approval of the NDA; and |
|
|
● |
compliance with any post-approval requirements, including the potential requirement
to implement a Risk Evaluation and Mitigation Strategy, or REMS, and the potential requirement to conduct post-approval studies.
|
|
Phase 1:
|
The drug is initially introduced into healthy human subjects or, in certain indications
such as cancer, patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution,
excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
|
|
Phase 2: |
The drug is administered to a limited patient population to identify possible adverse
effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage
tolerance and optimal dosage.
|
|
Phase 3: |
The drug is administered to an expanded patient population, generally at geographically
dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and
safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information
for the labeling of the product.
|
|
Phase 4: |
Post-approval studies, which are conducted following initial approval, are typically
conducted to gain additional experience and data from treatment of patients in the intended therapeutic indication. |
|
|
● |
restrictions on the marketing or manufacturing of the product, including total or
partial suspension of production, complete withdrawal of the product from the market or product recalls; |
|
|
● |
fines, warning letters or holds on post-approval clinical trials; |
|
|
● |
refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension
or revocation of product license approvals; |
|
|
● |
product seizure or detention, or refusal to permit the import or export of products;
or |
|
|
● |
injunctions or the imposition of civil or criminal penalties. |
|
|
● |
Compliance with the EU’s stringent pharmacovigilance and safety reporting rules,
pursuant to which inter alia post-authorization studies and additional monitoring obligations
can be imposed, has to be ensured. |
|
|
● |
The manufacturing of authorized drugs, for which a separate manufacturer’s license
is mandatory, must also be conducted in strict compliance with the EMA’s GMP requirements and comparable requirements of other regulatory
bodies in the EU, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure
their safety and identity. |
|
|
● |
The marketing and promotion of authorized drugs, including industry-sponsored continuing
medical education and advertising directed toward the prescribers of drugs, cooperation with healthcare professionals and advertising
of drugs directed to the general public, are strictly regulated in the EU notably under Directive 2001/83EC, as amended, and EU member
state laws. |
|
|
• |
PV Production Analytics: MyDeepSolar goes beyond conventional energy monitoring by factoring
in true irradiance levels. This enables precise performance assessments, ensuring that energy production is optimized under all conditions.
|
|
|
• |
Hardware Malfunction Alerts: The application continuously monitors inverters, panels, and
other critical components, automatically generating real-time alerts for any malfunctions. These notifications are delivered promptly,
reducing downtime and preventing further losses. |
|
|
• |
Losses Breakdown Analysis: MyDeepSolar quantifies energy losses caused by underperforming
devices, soiling levels, and other inefficiencies. This granular analysis empowers homeowners to identify and prioritize corrective actions
effectively. |
|
|
• |
Daily Prioritized Task Lists: The software provides actionable recommendations in real time,
tailored to specific needs such as cleaning schedules, hardware repairs, or system adjustments. Each task is quantified in terms of its
ROI impact, helping users make informed, data-driven decisions. |
|
|
• |
Comprehensive System Visibility: MyDeepSolar offers high-resolution diagnostics at the string
and panel levels, enabling users to understand exactly what is happening across their solar field and address issues with pinpoint accuracy.
|
|
|
• |
Seamless Integration: The application is fully compatible with existing monitoring solutions
and hardware, requiring no additional site visits or hardware installations. This frictionless process ensures easy adoption for homeowners.
|
|
|
• |
Cost Optimization: By automating diagnostics and reducing reliance on manual monitoring or
expensive analyst services, MyDeepSolar delivers savings on operational and maintenance costs. |

|
|
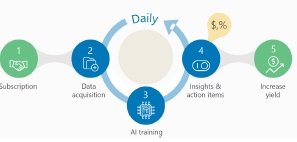
• |
the smart live monitoring feature which offers alerts, availability dashboards, and plots for raw data analysis, ensuring real-time
visibility into system performance; |
|
|
• |
performance analytics which delivers a full breakdown of losses, highlighting gaps between planned and actual outputs; |
|
|
• |
event management which prioritizes fault checks to address issues promptly and efficiently; |
|
|
• |
automatic verification which ensures full gain recovery by continuously validating system performance; |
|
|
• |
the report generator feature which provides detailed reports for any specified time range, enabling thorough analysis and informed
decision-making; and |
|
|
• |
management dashboards with customer-specific performance ratios and tailored insights to support strategic planning and operational
excellence. |
|
C. |
Organizational Structure |
|
D. |
Property, Plant and Equipment |
|
ITEM 4A.
UNRESOLVED STAFF COMMENTS |
|
ITEM
5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
| ● |
Implement our acquisition of the DeepSolar business; |
|
|
● |
continue the ongoing and planned preclinical and clinical development of our drug
candidates; |
|
|
● |
build a portfolio of drug candidates through the acquisition or in-license of drugs,
drug candidates or technologies; |
|
|
● |
initiate preclinical studies and clinical trials for any additional drug candidates
that we may pursue in the future; |
|
|
● |
seek marketing approvals for our current and future drug candidates that successfully
complete clinical trials; |
|
|
● |
establish a sales, marketing and distribution infrastructure to commercialize any
drug candidate for which we may obtain marketing approval; |
|
|
● |
develop, maintain, expand and protect our intellectual property portfolio; |
|
|
● |
implement operational, financial and management systems; and |
|
|
● |
attract, hire and retain additional administrative, clinical, regulatory and scientific
personnel. |
|
|
● |
employee-related expenses, including salaries, benefits and stock-based compensation
expense; |
|
|
● |
fees paid to consultants for services directly related to our drug development and
regulatory effort; |
|
|
● |
expenses incurred under agreements with contract research organizations, as well as CMOs and consultants
that conduct preclinical studies and clinical trials; |
|
|
● |
costs associated with preclinical activities and development activities; and
|
|
|
● |
costs associated with technology and intellectual property licenses. |
|
|
● |
number of clinical trials required for approval and any requirement for extension
trials; |
|
|
● |
per patient trial costs; |
|
|
● |
number of patients that participate in the clinical trials; |
|
|
● |
number of sites included in the clinical trials; |
|
|
● |
countries in which the clinical trial is conducted; |
|
|
● |
length of time required to enroll eligible patients; |
|
|
● |
potential additional safety monitoring or other studies requested by regulatory agencies;
and |
|
|
● |
efficacy and safety profile of the drug candidate. |
|
A. |
Operating Results |
|
|
Year ended December 31, |
|
||||||||||
|
|
|
2024 |
|
|
2023 |
|
|
2022 |
|
|||
|
Statements of comprehensive loss data: |
|
(US$ thousands) |
|
|||||||||
|
Research and development |
11,705 |
6,035 |
4,422 |
|||||||||
|
General and administrative |
2,968 |
3,549 |
4,447 |
|||||||||
|
Total operating loss |
14,673 |
9,584 |
8,869 |
|||||||||
|
financial (income) expenses, net |
(93 |
) |
(248 |
) |
86 |
|||||||
|
Loss before taxes |
14,580 |
9,336 |
8,783 |
|||||||||
|
Income tax expense |
8 |
8 |
9 |
|||||||||
|
Net loss |
14,588 |
9,344 |
8,792 |
|||||||||
|
JOBS Act Exemptions and Foreign Private Issuer Status |
|
|
● |
the sections of the Exchange Act regulating the solicitation of proxies, consents
or authorizations in respect of a security registered under the Exchange Act; |
|
|
● |
the sections of the Exchange Act requiring insiders to file public reports of their
stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; |
|
|
● |
the rules under the Exchange Act requiring the filing with the SEC of quarterly reports
on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified
significant events; and |
|
|
● |
Regulation FD, which regulates selective disclosures of material information by issuers.
|
|
B. |
Liquidity and Capital Resources. |
|
|
● |
the costs, timing and outcome of manufacturing clinical trial and commercial quantities
of PRF-110 |
|
|
● |
the scope, progress, results and costs of our current and future clinical trials of
PRF-110 for our current targeted uses; |
|
|
● |
the costs, timing and outcome of regulatory review of PRF-110; |
|
|
● |
the extent to which we acquire or invest in businesses, products and technologies,
including entering into or maintaining licensing or collaboration arrangements for PRF-110 on favorable terms, although we currently have
no commitments or agreements to complete any such transactions; |
|
|
●
|
the costs and timing of future commercialization activities, including sales, marketing,
manufacturing and distribution, for any of our product candidates for which we receive marketing approval, to the extent that such sales,
marketing, manufacturing and distribution are not the responsibility of any collaborator that we may have at such time; |
|
|
● |
the cost to continue the development of the DeepSolar technology to develop a wider
portfolio of solutions; |
|
|
|
|
● |
the cost of establishing a sales, marketing, and technical support infrastructure to support the ramp up
of the DeepSolar solution; |
|
|
● |
the amount of revenue, if any, received from commercial sales of PRF-110, should it receive marketing approval,
or from DeepSolar solution; |
|
|
● |
the costs of preparing, filing and prosecuting patent applications, maintaining, defending
and enforcing our intellectual property rights and defending intellectual property-related claims; |
|
|
● |
our ability to establish strategic collaborations, licensing or other arrangements
and the financial terms of any such agreements, including the timing and amount of any future milestone, royalty or other payments due
under any such agreement; |
|
|
● |
our headcount growth and associated costs as we expand our business operations and
our research and development activities; |
|
|
● |
the costs of operating as a public company; |
|
|
● |
maintaining minimum shareholders’ equity requirements under the Nasdaq rules;
and |
|
|
|
|
● |
the impact of the current war between Israel and Hamas which may exacerbate the magnitude of the factors
discussed above. |
|
|
|
Payments due by period
|
||||||||||||||||||
|
|
|
(US$ thousands)
|
||||||||||||||||||
|
|
Less than
1 year |
1-3 Years
|
3-5 Years
|
More than 5 years
|
Total
|
|||||||||||||||
|
Obligations under master clinical research agreements(1) |
1,730 |
-
|
-
|
-
|
1,730
|
|||||||||||||||
|
Years ended December 31, |
||||||||||||
|
(US$ thousands) |
||||||||||||
|
|
2024 |
2023 |
2022 |
|||||||||
|
Net cash used in operating activities |
(12,621 |
) |
(6,679 |
) |
(6,459 |
) | ||||||
|
Net cash provided by (used in) investing activities |
(13 |
) |
5,991 |
(6,006 |
) | |||||||
|
Net cash provided by financing activities |
8,863 |
4,616 |
- |
|||||||||
|
Effect of Exchange rate changes on cash, cash equivalents and restricted cash |
6 |
2 |
- |
|||||||||
|
(Decrease) Increase in cash and cash equivalents and restricted cash |
(3,765 |
) |
3,930 |
(12,465 |
) | |||||||
|
Cash and cash equivalents and restricted cash, at the beginning of the year |
8,036 |
4,106 |
16,571 |
|||||||||
|
Cash and cash equivalents and restricted cash, at the end of the year |
4,271 |
8,036 |
4,106 |
|||||||||
|
C. |
Research and Development, Patents and Licenses |
|
D. |
Trend Information |
|
E. |
Critical Accounting Estimates |
|
ITEM 6.
DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
|
A. |
Directors and Senior Management |
|
Name |
|
Age |
|
Position |
|
Senior Management |
|
|
|
|
|
Dr. Ehud Geller |
|
78 |
|
Interim Chief Executive Officer, Executive Chairman of the Board, and Director |
| Eyal Broder | 54 | Interim Chief Financial Officer | ||
|
Prof. Eli Hazum |
|
77 |
|
Chief Technology Officer and Director |
|
Dr. Sigal Aviel |
|
61 |
|
Chief Operating Officer |
|
Rita Keynan |
|
56 |
|
Vice President of Pharmaceutical Operations |
|
|
|
|
|
|
|
Non-Employee Director |
|
|
|
|
|
|
|
|
||
|
Efi Cohen-Arazi(1) (2) (3) (4) |
|
70 |
|
Director |
|
Dr. Ellen S. Baron(1) (2) (3)(4) |
|
72 |
|
External Director |
|
Augustine Lawlor(1) (2) (3)(4) |
|
69 |
|
External Director |
|
(1) |
Member of the Compensation Committee |
|
(2) |
Member of the Audit Committee |
|
(3) |
Independent Director under Israeli Law |
|
(4) |
Independent Director under the Nasdaq Listing Rules |
|
B. |
Compensation |
|
|
Salaries, fees, commissions, and bonuses |
Pension, retirement and similar benefits |
Value of Options Granted(1) |
|||||||||
|
|
(in thousands of U.S. dollars) |
(in thousands of U.S. dollars) |
(in thousands of U.S. dollars)
|
|||||||||
|
All senior management and directors as a group, consisting of 8 persons |
1,348
|
198 |
304 |
|||||||||
|
(1) |
Consists of amounts recognized as share-based compensation expense for the year ended
December 31, 2024. Assumptions and key variables used in the calculation of such amounts are discussed in Note 10 of our financial statements.
|
|
Name and Position(1)
|
Salary |
Social
Benefits
(2) |
Bonuses |
Value of Options
Granted
(3) |
All Other
Compensation(4)
|
Total |
||||||||||||||||||
|
|
(in thousands of U.S. dollars) |
|||||||||||||||||||||||
|
Ehud Geller,
Interim Chief Executive Officer and Chairman |
- |
- |
- |
31 |
150 |
181 | ||||||||||||||||||
|
Sigal Aviel
Chief Operating Officer |
281 |
49 |
- |
17 |
- |
347 | ||||||||||||||||||
|
Rita Keynan
Vice President of Pharmaceutical Operations |
230 | 57 |
- |
68 |
- |
355 | ||||||||||||||||||
|
Eyal Broder,
Interim Chief Financial Officer |
153 | 50 | 32 | - |
- |
235 | ||||||||||||||||||
|
Eli Hazum
Chief Technology Officer |
- |
- |
- |
23 |
144 |
167 | ||||||||||||||||||
|
(1) |
All executive officers listed in the table were employed on a full-time basis during 2024. |
|
(2) |
“Social Benefits” include payments to the National Insurance Institute,
advanced education funds, managers’ insurance and pension funds, vacation pay and recuperation pay as mandated by Israeli law.
|
|
(3) |
Consists of amounts recognized as share-based compensation expense for the year ended
December 31, 2024. Assumptions and key variables used in the calculation of such amounts are discussed in Note 10 of our financial statements.
|
|
(4) |
“All Other Compensation” includes chairman of the board of directors’ annual fee and
directors’ consulting related fees. |
|
C. |
Board Practices |
|
|
● |
at least a majority of the shares of non-controlling shareholders or shareholders
that do not have a personal interest in the approval voted at the meeting are voted in favor (disregarding abstentions); or |
|
|
● |
the total number of shares of non-controlling shareholders or shareholders that do
not have a personal interest in the approval voted against the proposal does not exceed 2% of the aggregate voting rights in the company.
|
|
|
● |
an employment relationship; |
|
|
● |
a business or professional relationship maintained on a regular basis; |
|
|
● |
control; and |
|
|
● |
service as an office holder, excluding service as a director in a private company
prior to the first offering of its shares to the public if such director was appointed as a director of the private company in order to
serve as an external director following the initial public offering. |
|
|
●
|
such majority includes at least a majority of the shares held by shareholders who
are non-controlling shareholders and do not have a personal interest in the election of the external director (other than a personal interest
not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions, to which we refer
as a disinterested majority; or |
|
|
● |
the total number of shares voted by non-controlling shareholders and by shareholders
who do not have a personal interest in the election of the external director, against the election of the external director, does not
exceed 2% of the aggregate voting rights in the company. |
|
|
●
|
his or her service for each such additional term is recommended by one or more shareholders
holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where
the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate
voting rights in the company. In such event, the external director so reappointed may not be a Related or Competing Shareholder, as defined
below, or a relative of such shareholder, at the time of the appointment, and is not and has not had any affiliation with a Related or
Competing Shareholder, at such time or during the two years preceding such person’s reappointment to serve an additional term as
external director. The term “Related or Competing Shareholder” means a shareholder proposing the reappointment or a shareholder
holding 5% or more of the outstanding shares or voting rights of the company, provided, that at the time of the reappointment, such shareholder,
the controlling shareholder of such shareholder, or a company controlled by such shareholder, have a business relationship with the company
or are competitors of the company; |
|
|
● |
the external director proposed his or her own nomination, and such nomination was
approved in accordance with the requirements described above; |
|
|
● |
his or her service for each such additional term is recommended by the board of directors
and is approved at a shareholders meeting by the same majority required for the initial election of an external director (as described
above). |
|
|
●
|
he or she meets the qualifications for being appointed as an external director, except
for the requirement that the director be an Israeli resident (which does not apply to companies whose securities have been offered outside
of Israel or are listed outside of Israel); and |
|
|
● |
he or she has not served as a director of the company for a period exceeding nine
consecutive years, provided that, for this purpose, a break of less than two years in service shall not be deemed to interrupt the continuation
of the service. |
|
|
● |
retaining and terminating our independent auditors, subject to the ratification of
the board of directors, and in the case of retention, to that of the shareholders; |
|
|
● |
pre-approving of audit and non-audit services and related fees and terms, to be provided
by the independent auditors; |
|
|
● |
overseeing the accounting and financial reporting processes of the Company and audits
of our financial statements, the effectiveness of our internal control over financial reporting and making such reports as may be required
of an audit committee under the rules and regulations promulgated under the Exchange Act; |
|
|
● |
reviewing with management and our independent auditor our annual and quarterly financial
statements prior to publication or filing (or submission, as the case may be) to the SEC; |
|
|
●
|
recommending to the board of directors the retention and termination of the internal
auditor, and the internal auditor’s engagement fees and terms, in accordance with the Companies Law as well as approving the yearly
or periodic work plan proposed by the internal auditor; |
|
|
● |
reviewing with our general counsel and/or external counsel, as deem necessary, legal
and regulatory matters that could have a material impact on the financial statements; |
|
|
● |
identifying irregularities in our business administration, inter alia, by consulting
with the internal auditor or with the independent auditor, and suggesting corrective measures to the board of directors; and |
|
|
●
|
reviewing policies and procedures with respect to transactions (other than transactions
related to the compensation or terms of services) between the company and officers and directors, or affiliates of officers or directors,
or transactions that are not in the ordinary course of the Company’s business and deciding whether to approve such acts and transactions
if so required under the Companies Law. |
|
|
●
|
determining whether there are deficiencies or irregularities in the business management
practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to
the board of directors to improve such practices; |
|
|
● |
determining the approval process for transactions with a controlling shareholder or
in which a controlling shareholder has a personal interest; |
|
|
● |
determining whether to approve certain related party transactions (including transactions
in which an office holder has a personal interest and whether such transaction is extraordinary or material under Companies Law) (see
“- Approval of Related Party Transactions under Israeli Law”); |
|
|
● |
where the board of directors approves the working plan of the internal auditor, to
examine such working plan before its submission to the board of directors and proposing amendments thereto; |
|
|
● |
examining our internal controls and internal auditor’s performance, including
whether the internal auditor has sufficient resources and tools to dispose of its responsibilities; |
|
|
● |
examining the scope of our auditor’s work and compensation and submitting a
recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the appointment
of our auditor; and |
|
|
● |
establishing procedures for the handling of employees’ complaints as to the
management of our business and the protection to be provided to such employees. |
|
|
● |
Officers’ interests are as closely as possible aligned with our interests;
|
|
|
● |
The correlation between pay and performance will be enhanced; |
|
|
● |
We will be able to recruit and retain top level executives capable of leading us to further business success,
facing the challenges ahead; |
|
|
● |
Our officers will be motivated to achieve a high level of business performance without taking unreasonable
risks. Therefore, the variable compensation component may not be based on extreme business performance goals which might potentially impose
unreasonable risks on our officers; and |
|
|
● |
An appropriate balance between different compensation elements (e.g., fixed vs. variable, short-term vs.
long-term and cash payments vs. equity-based compensation). |
|
|
●
|
recommending whether a compensation policy should continue in effect, if the then-current
policy has a term of greater than five years from the company’s initial public offering, or otherwise three years (approval of either
a new compensation policy or the continuation of an existing compensation policy must in any case occur five years from the company’s
initial public offering, or otherwise every three years); |
|
|
● |
recommending to the board of directors periodic updates to the compensation policy;
|
|
|
● |
assessing implementation of the compensation policy; |
|
|
● |
determining whether to approve the terms of compensation of certain office holders
which, according to the Companies Law, require the committee’s approval; and |
|
|
● |
determining whether the compensation terms of a candidate for the position of the
chief executive officer of the company needs to be brought to approval of the shareholders according to the Companies Law. |
|
|
● |
the responsibilities set forth in the compensation policy; |
|
|
● |
reviewing and approving the granting of options and other incentive awards to the
extent such authority is delegated by our board of directors; and |
|
|
● |
reviewing, evaluating and making recommendations regarding the compensation and benefits
for our non-employee directors. |
|
|
● |
overseeing our corporate governance functions on behalf of the board; |
|
|
● |
making recommendations to the board regarding corporate governance issues; |
|
|
● |
identifying and evaluating candidates to serve as our directors consistent with the
criteria approved by the board; |
|
|
● |
reviewing and evaluating the performance of the board; |
|
|
● |
serving as a focal point for communication between director candidates, non-committee
directors and our management; selecting or recommending to the board for selection candidates to the board; and |
|
|
● |
making other recommendations to the board regarding affairs relating to our directors.
|
|
|
● |
a person (or a relative of a person) who holds more than 5% of the company’s
outstanding shares or voting rights; |
|
|
● |
a person (or a relative of a person) who has the power to appoint a director or the
general manager of the company; |
|
|
● |
an office holder or director (or a relative of an officer or director) of the company;
or |
|
|
● |
a member of the company’s independent accounting firm, or anyone on its behalf.
|
|
|
● |
information on the advisability of a given action brought for his or her approval
or performed by virtue of his or her position; and |
|
|
● |
all other important information pertaining to these actions. |
|
|
● |
refrain from any act involving a conflict of interest between the performance of his
or her duties to the company and his or her other duties or personal affairs; |
|
|
● |
refrain from any activity that is competitive with the company; |
|
|
● |
refrain from exploiting any business opportunity of the company to receive a personal
gain for himself or herself or others; and |
|
|
● |
disclose to the company any information or documents relating to the company’s
affairs which the office holder received as a result of his or her position as an office holder. |
|
|
● |
a transaction other than in the ordinary course of business; |
|
|
● |
a transaction that is not on market terms; or |
|
|
● |
a transaction that may have a material impact on the company’s profitability,
assets, or liabilities. |
|
|
● |
an amendment of the articles of association of the company; |
|
|
● |
an increase in the company’s authorized share capital; |
|
|
● |
a merger; or |
|
|
● |
the approval of related party transactions and acts of office holders that require shareholder approval.
|
|
|
●
|
financial liability imposed on him or her in favor of another person pursuant to a
judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder
with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the
board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount
or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking must detail
the abovementioned foreseen events and amount or criteria; |
|
|
●
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder: (i) as
a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding,
provided that (a) no indictment was filed against such office holder as a result of such investigation or proceeding and (b) no financial
liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or,
if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and
(ii) in connection with a monetary sanction; |
|
|
● |
expenses associated with an administrative procedure, as defined in the Israeli Securities
Law, conducted regarding an office holder, including reasonable litigation expenses and reasonable attorneys’ fees; and |
|
|
● |
reasonable litigation expenses, including attorneys’ fees, incurred by the office
holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party or in connection
with criminal proceedings in which the office holder was acquitted or as a result of a conviction for an offense that does not require
proof of criminal intent. |
|
|
● |
a breach of duty of care to the company or to a third party, including a breach arising out of the negligent
conduct of the office holder; |
|
|
● |
a breach of fiduciary duty to the company, to the extent that the office holder acted in good faith and
had a reasonable basis to believe that the act would not prejudice the company; |
|
|
● |
a monetary liability imposed on the office holder in favor of a third party; and |
|
|
● |
expenses incurred by an office holder in connection with an administrative procedure, including reasonable
litigation expenses and reasonable attorneys’ fees. |
|
|
● |
a breach of fiduciary duty, except for indemnification and insurance for a breach of the fiduciary duty
to the company and to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not
prejudice the company; |
|
|
● |
a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent
conduct of the office holder; |
|
|
● |
an act or omission committed with intent to derive illegal personal benefit; or |
|
|
● |
a fine or forfeit levied against the office holder. |
|
D. |
Employees. |
|
E. |
Share Ownership. |
|
F. |
Disclosure of a Registrant’s Action to Recover Erroneously
Awarded Compensation |
|
ITEM 7.
MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
|
A. |
Major Shareholders |
|
|
● |
each of our directors and senior management; |
|
|
● |
all of our directors and senior management as a group; and |
|
|
● |
each person (or group of affiliated persons) known by us to be the beneficial owner of 5% or more of the
outstanding ordinary shares. |
|
|
Ordinary Shares Beneficially Owned |
Percentage Owned** |
||||||
|
Senior Management and Directors |
||||||||
|
Dr. Ehud Geller(1) |
21,546 |
1.2 |
% | |||||
|
Eyal Broder (2) |
1,733 |
* | ||||||
|
Dr. Sigal Aviel(3) |
5,045 |
* |
||||||
|
Rita Keynan(4) |
3,952 |
* |
||||||
|
Prof. Eli Hazum(5) |
2,368 |
* |
||||||
|
Ellen S. Baron(6) |
765 |
* |
||||||
|
Augustine Lawlor(7) |
765 |
* |
||||||
|
Efi Cohen-Arazi(8) |
1,327 |
* |
||||||
|
All senior management and directors as a group (8 persons) |
37,501 |
2.0 |
% | |||||
|
More than 5% Shareholders |
||||||||
|
Bladeranger Ltd (9) |
402,561 |
19.1 |
% | |||||
|
* |
Less than 1% |
|
|
|
|
** |
Based on 1,885,001 ordinary shares outstanding. |
|
(1)
|
Consists of 13,720 beneficially owned by the Zori Medica III Ltd., or Zori Medica
III, which includes Zori Medica III Ltd FBO Medica III Investments (International) L.P. which holds 4,637 ordinary shares, Zori Medica
III Ltd. FBO Medica III Investments (Israel) (B) L.P. which holds 2,381 ordinary shares, Zori Medica III Ltd. FBO Poalim Medica III Investments
L.P. which holds 2,198 ordinary shares, Zori Medica III Ltd. FBO Medica III Investments (S.F.) L.P. which holds 1,832 ordinary shares,
Zori Medica III Ltd. FBO Medica III Investments (Israel) L.P. which holds 1,686 ordinary shares, and Zori Medica III Ltd. FBO Medica III
Investments (P.F.) L.P. which holds 986 ordinary shares and Dr. Ehud Geller who holds options to purchase 7,826 ordinary shares that are
currently exercisable average exercise price of $50.0 per share and expiring on October 10, 2034 or will be exercisable within 60 days
from April 1, 2025. Zori Medica III Management L.P., an entity held 50% by Dr. Ehud Geller and 50% by Batsheva Elran, is the managing
entity of Zori Medica III. The principal business address of Zori Medica III is 60C Medinat Hayehudim, Herzliya, 4676670, Israel. Does
not include options to purchase 188 ordinary shares approved for issuance to Mr. Geller. Such options are exercisable at $141.4 per share
and expiring on June 8, 2033, that vest in more than 60 days from April 1, 2025. |
|
(2) |
Consists of options to purchase 900 ordinary shares exercisable at $6.0 per share
and expiring on October 10, 2034 and options to purchase 833 ordinary shares exercisable at $3.1 per share and expiring on February 20,
2035. Does not include options to purchase 9,167 ordinary shares exercisable at $3.1 per share and expiring on February 20, 2035, that
vest in more than 60 days from April 1, 2025. |
|
(3)
|
Consists of options to purchase 214 ordinary shares exercisable at a weighted average
exercise price of $57.6 per share and expiring on May 23, 2029 and 521 ordinary shares exercisable at a weighted average exercise price
of $136.8 expiring on November 23, 2032 and 3,060 ordinary shares exercisable at a weighted average exercise price of $6.0 expiring on
November 23, 2032. And 1,250 ordinary shares exercisable at a weighted average exercise price of $3.1 expiring on February 20, 2035 Does
not include options to purchase 13,750 ordinary shares exercisable at $3.1 per share and expiring on February 20, 2035, that vest in more
than 60 days from April 1, 2025. |
|
(4)
|
Consists of options to purchase 996 ordinary shares exercisable at $136.8 per share
and expiring on November 23, 2032 and options to purchase 2,040 ordinary shares exercisable at $6.0 per share and expiring on November
23, 2032 and options to purchase 917 ordinary shares exercisable at $3.1 per share and expiring on February 20, 2035. Does not include
options to purchase 10,083 ordinary shares exercisable at $3.1 per share and expiring on February 20, 2035, that vest in more than 60
days from April 1, 2025. |
|
(5)
|
Consists of options to purchase 250 ordinary shares exercisable at $1,080 per share
and expiring on 23 February 2031, options to purchase 562 ordinary shares exercisable at $141.4 per share and expiring on June 8, 2033
and options to purchase 1,556 ordinary shares exercisable at $6.0 per share and expiring on October 10, 2034. Does not include options
to purchase 188 ordinary shares exercisable at $141.4 per share and expiring on June 8, 2033, that vest in more than 60 days from April
1, 2025. |
|
(6)
|
Consists of options to purchase 250 ordinary shares exercisable at $1,080 per share
and expiring on February 23, 2031 and options to purchase 515 ordinary shares exercisable at $6.0 per share and expiring on July 18, 2034.
Does not include options to purchase 402 ordinary shares exercisable at $141.4 per share and expiring on July 18, 2034, that vest in more
than 60 days from April 1, 2025. |
|
(7)
|
Consists of options to purchase 250 ordinary shares exercisable at $1,080 per share
and expiring on February 23, 2031 and options to purchase 515 ordinary shares exercisable at $6.0 per share and expiring on July 18, 2034.
Does not include options to purchase 402 ordinary shares exercisable at $141.4 per share and expiring on July 18, 2034, that vest in more
than 60 days from April 1, 2025. |
|
(8)
|
Consists of options to purchase 250 ordinary shares exercisable at $1,080 per share
and expiring on February 23, 2031 and options to purchase 515 ordinary shares exercisable at $6.0 per share and expiring on July 18, 2034
and 562 ordinary shares exercisable at $141.4 per share and expiring on June 8, 2033 Does not include options to purchase 590 ordinary
shares average exercisable at $49.1 per share and expiring on July 18, 2034, that vest in more than 60 days from April 1, 2025.
|
|
(9) |
The securities are directly held by BladeRanger, an Israeli public company. Consists
of 178,769 ordinary shares and a prefunded warrant to purchase 223,792 ordinary shares. The pre-funded warrant is subject to a beneficial
ownership limitation of 9.99%, which such limitation restricts the holder from exercising that portion of the warrant that would result
in the shareholder and its affiliates owning, after exercise, a number of shares in excess of the beneficial ownership limitation. The
address of BladeRanger is 1 Hayasmin St., Ramat-Efal, Israel. See also “Item 4.B. Business Overview - DeepSolar AI Analytics Software
Business – Business Acquisition Agreement.” |
|
B. |
Related Party Transactions |
|
C. |
Interests of Experts and Counsel
Not applicable. |
|
ITEM 8.
FINANCIAL INFORMATION. |
|
A. |
Consolidated Statements and Other Financial Information.
|
|
B. |
Significant Changes |
|
ITEM
9. THE OFFER AND LISTING |
|
A. |
Offer and Listing Details
On September 1, 2020, our ordinary shares commenced trading on the Nasdaq Capital
Market under the symbol “PRFX.” |
|
B. |
Plan of Distribution
Not applicable. |
|
C. |
Markets
Our ordinary shares are listed on the Nasdaq Capital Market. |
|
D. |
Selling Shareholders
Not applicable. |
|
E. |
Dilution
Not applicable. |
|
F. |
Expenses of the Issue
Not applicable. |
|
ITEM
10. ADDITIONAL INFORMATION |
|
A. |
Share Capital
Not applicable. |
|
B. |
Memorandum and Articles of Association |
|
C. |
Material Contracts |
|
D. |
Exchange Controls |
|
E. |
Taxation. |
|
|
● |
amortization over an eight-year period of the cost of patents and rights to use a
patent and know-how which were purchased in good faith and are used for the development or advancement of the Industrial Enterprise;
|
|
|
● |
deduction over a three-year period of expenses incurred in connection with the issuance and listing of
shares on a stock market; and |
|
|
● |
under certain conditions, an election to file tax returns with related Israeli Industrial Companies.
|
|
|
●
|
owns a Preferred Enterprise, which is defined as an “Industrial Enterprise”
(as defined under the Investment Law) that is classified as either a “Competitive Enterprise” (as defined under the Investment
Law) or a “Competitive Enterprise in the Field of Renewable Energy” (as defined under the Investment Law); |
|
|
● |
is controlled and managed from Israel; |
|
|
● |
is not a “Family Company,” a “Home Company,” or a “Kibbutz” (collective
community) as defined under the Income Tax Ordinance; |
|
|
● |
keeps acceptable books of account and files reports in accordance with the provisions of the Investment
Law and the Income Tax Ordinance; and |
|
|
● |
was not, and certain officers of which were not, convicted of certain crimes in the 10 years prior to the
tax year with respect to which benefits are being claimed. |
|
F. |
Dividends and Paying Agents
Not applicable. |
|
G. |
Statement by Experts
Not applicable. |
|
H. |
Documents on Display |
|
I. |
Subsidiary Information.
Not applicable. |
|
J. |
Annual Report to Security Holders.
Not applicable. |
|
ITEM
11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
|
ITEM
12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
|
A. |
Debt Securities.
Not applicable. |
|
B. |
Warrants and rights.
Not applicable. |
|
C. |
Other Securities.
Not applicable. |
|
D. |
American Depositary Shares
Not applicable. |
|
ITEM 13.
DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
|
|
None. |
|
ITEM
14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
|
|
There are no material modifications to the rights of security holders. |
|
ITEM 15.
CONTROLS AND PROCEDURES |
|
ITEM 16A. AUDIT
COMMITTEE FINANCIAL EXPERT |
|
ITEM 16B. CODE OF ETHICS
|
|
ITEM
16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES |
|
|
Year Ended December 31,
|
|||||||
|
|
2024
|
2023
|
||||||
|
(USD in thousands) |
|
|||||||
|
Audit fees (1) |
120 |
120 |
||||||
|
Audit-related fees(2) |
83 |
122 |
||||||
|
Tax fees |
- |
- |
||||||
|
All other fees |
- |
- |
||||||
|
Total |
203 |
242 |
||||||
|
(1)
|
The audit fees for the years ended December 31, 2024 and 2023 include professional services rendered in
connection with the audit of our annual financial statements and the review of our interim financial statements, statutory audits of the
Company.
|
|
(2) |
Issuance of consents and assistance with review of documents filed with the SEC. |
|
ITEM
16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
|
ITEM
16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
|
ITEM 16F.
CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
|
ITEM 16G.
CORPORATE GOVERNANCE |
|
|
●
|
Shareholder approval. We
will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than
seeking approval for corporate actions in accordance with Nasdaq Listing Rule 5635. In particular, under this Nasdaq Listing Rule, shareholder
approval is generally required for: (i) an acquisition of shares or assets of another company that involves the issuance of 20% or more
of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target
company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption or amendment of
equity compensation arrangements; and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into,
or exercisable for, equity) of a listed company via a private placement (or via sales by directors, officers or 5% shareholders) if such
equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Companies Law, shareholder
approval is required (subject to certain limited exceptions) for, among other things: (a) transactions with directors concerning the terms
of their service (including indemnification, exemption, and insurance for their service or for any other position that they may hold at
a company), for which approvals of the compensation committee, board of directors, and shareholders are all required; (b) extraordinary
transactions with controlling shareholders of publicly held companies, which require the special approval described below under “Disclosure
of Personal Interests of Controlling Shareholders and Approval of Certain Transactions;” (c) terms of office and employment or other
engagement of our controlling shareholder, if any, or such controlling shareholder’s relative, which require the special approval
described below under “Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions;”
(d) approval of transactions with Company’s Chief Executive Officer with respect to his or hers compensation, whether in accordance
with the approved compensation policy of the Company or not in accordance with the approved compensation policy of the Company, or transactions
with officers of the Company not in accordance with the approved compensation policy; and (e) approval of the compensation policy of the
Company for office holders. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging
companies. |
|
|
●
|
Nomination of our directors.
Israeli law and our amended articles of association do not require director nominations to be made by a nominating committee of our board
of directors consisting solely of independent directors, as required under the Listing Rules of the Nasdaq Stock Market. We rely on the
exemption available to foreign private issuers under the Nasdaq Listing Rules and follow Israeli law and practice with regard to the process
of nominating directors, in accordance with which directors are recommended by our board of directors for election by our shareholders
(other than directors elected by our board of directors to fill a vacancy). |
|
|
●
|
Quorum requirement. Under
our amended and restated articles of association and as permitted under the Companies Law, a quorum for any meeting of shareholders shall
be the presence of at least two shareholders present in person, by proxy or by a written ballot, who hold at least 25% of the voting power
of our shares (or if a higher percentage is required by law, such higher percentage) instead of 33 1/3% of the issued share capital required
under the Nasdaq Listing Rules. If within half an hour from the time designated for the meeting a quorum is not present, them will stand
adjourned to the same day in the following week, at the same time and place. If a quorum is not present at the adjourned meeting within
half hour from the time designated for its start, the meeting shall take place with any number of participants. |
|
|
●
|
Periodic reports. As opposed
to making periodic reports to shareholders and proxy solicitation materials available to shareholders in the manner specified by the Nasdaq
Marketplace Rules, the Companies Law does not require us to distribute periodic reports directly to shareholders, and the generally accepted
business practice in Israel is not to distribute such reports to shareholders but to make such reports available through a public website.
We will only mail such reports to shareholders upon request; and |
|
|
●
|
Compensation of officers.
We follow Israeli law and practice with respect to the approval of officer compensation. While our compensation committee currently complies
with the provisions of the Nasdaq Listing Rules relating to composition requirements and Israeli law generally requires that the compensation
of the chief executive officer and all other executive officers be approved, or recommended to the board for approval, by the compensation
committee (and in certain instances, shareholder approval is required), Israeli law includes relief from compensation committee approval
in certain instances. For details regarding the approvals required under the Israeli Companies Law and regulation promulgated thereunder
for the approval of compensation of the chief executive officer, all other executive officers and directors, see
Item 6C “Directors, Senior Management and Employees- Board Practices - Approval of Related Party Transactions under Israeli Law
- Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions”. |
|
ITEM
16H. MINE SAFETY DISCLOSURE |
|
|
Not applicable. |
|
|
Not applicable. |
|
ITEM 18. FINANCIAL STATEMENTS
|
|
ITEM 19. EXHIBITS.
|
|
Exhibit No.
|
Exhibit Description
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
137
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
*
|
Filed herewith.
|
|
#
|
Management contract or compensatory plan.
|
|
† |
Portions of this exhibit have been omitted in accordance with Item 601(a)(5) of Regulation S-K. The Registrant undertakes to furnish a copy of all omitted schedules and exhibits to the SEC upon its request. |
|
^ |
Portions of this exhibit (indicated by asterisks) have been omitted under rules of the U.S. Securities and Exchange Commission permitting the confidential treatment of select information. |
138
|
PAINREFORM LTD.
|
||
|
Date: April 7, 2025
|
By:
|
/s/ Ehud Geller |
|
Ehud Geller
|
||
|
Interim Chief Executive Officer, Executive Chairman of the Board and Director
|
||
|
Page
|
|
|
F-2
|
|
|
(Firm Name: Kesselman & Kesselman / PCAOB ID No. 1309)
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6 - F-7
|
|
|
F-8 - F-28
|

|
As of December 31,
|
||||||||||||
|
Note
|
2024
|
2023
|
||||||||||
|
Assets
|
||||||||||||
|
Current assets:
|
||||||||||||
|
Cash and cash equivalents
|
|
4,261
|
|
8,026
|
||||||||
|
Restricted cash
|
2f
|
|
10
|
10
|
||||||||
|
Prepaid clinical trial expenses and deferred clinical trial costs
|
6b
|
|
-
|
1,514
|
||||||||
|
Prepaid expenses and other current assets
|
3
|
157
|
249
|
|||||||||
|
Total current assets
|
$ |
4,428
|
$ |
9,799
|
||||||||
|
Non-current assets
|
||||||||||||
|
Operating lease right of use asset
|
6a
|
|
62
|
93
|
||||||||
|
Property and equipment, net
|
35
|
38
|
||||||||||
|
Total Non-current assets
|
$ |
97
|
$ |
131
|
||||||||
|
Total assets
|
$
|
4,525
|
$
|
9,930
|
||||||||
|
Liabilities and shareholders’ equity
|
||||||||||||
|
Current liabilities: |
||||||||||||
|
Trade payables
|
|
296
|
|
221
|
||||||||
|
Employees and related liabilities
|
197
|
465
|
||||||||||
|
Operating lease liability
|
45
|
56
|
||||||||||
|
Accrued expenses
|
4
|
1,904
|
1,668
|
|||||||||
|
Total current liabilities
|
$
|
2,442
|
$
|
2,410
|
||||||||
|
Non-current liabilities:
|
||||||||||||
|
Operating lease liability
|
-
|
30
|
||||||||||
|
Provision for unrecognized tax positions
|
5f
|
|
259
|
251
|
||||||||
|
Total non-current liabilities
|
259
|
281
|
||||||||||
|
Total liabilities
|
$
|
2,701
|
$
|
2,691
|
||||||||
|
Commitments
|
6
|
|||||||||||
|
Shareholders’ Equity:
|
7
|
|||||||||||
|
Ordinary shares, No par value; Authorized: 2,500,000 and 208,333 shares as of December 31, 2024, and 2023, respectively; Issued and outstanding: 1,471,412 and 165,489 shares as of December 31, 2024, and 2023, respectively (*)
|
|
-
|
|
-
|
||||||||
|
Additional paid-in capital (*)
|
58,275
|
49,102
|
||||||||||
|
Accumulated deficit
|
(56,451
|
)
|
(41,863
|
)
|
||||||||
|
Total shareholders’ equity
|
$ |
1,824
|
$ |
7,239
|
||||||||
|
Total liabilities and shareholders’ equity
|
$
|
4,525
|
$
|
9,930
|
||||||||
|
For the Year Ended
December 31, |
||||||||||||||||
|
Note
|
2024
|
2023
|
2022
|
|||||||||||||
|
Operating expenses:
|
||||||||||||||||
|
Research and development expenses
|
8a
|
|
|
(11,705
|
)
|
|
(6,035
|
)
|
|
(4,422
|
)
|
|||||
|
General and administrative expenses
|
8b
|
|
(2,968
|
)
|
(3,549
|
)
|
(4,447
|
)
|
||||||||
|
Operating loss
|
$ |
(14,673
|
)
|
$ |
(9,584
|
)
|
$ |
(8,869
|
)
|
|||||||
|
Financial income, net
|
8c
|
|
93
|
248
|
86
|
|
||||||||||
|
Loss before taxes
|
(14,580
|
)
|
(9,336
|
)
|
(8,783
|
)
|
||||||||||
|
Income tax expenses
|
(8
|
)
|
(8
|
)
|
(9
|
)
|
||||||||||
|
Net comprehensive loss
|
$
|
(14,588
|
)
|
$
|
(9,344
|
)
|
$
|
(8,792
|
)
|
|||||||
|
Basic and diluted net loss per share
|
2o
|
|
$
|
(32.16
|
)
|
$
|
(63.13
|
)
|
$
|
(63.46
|
)
|
|||||
|
Weighted average number of Ordinary Share used in computing basic and diluted net loss per share (*)
|
453,544
|
148,013
|
138,548
|
|||||||||||||
|
Ordinary shares
|
Additional
paid-in |
Accumulated
|
Total
shareholders’ |
|||||||||||||
|
Number
|
Capital
|
Deficit
|
equity
|
|||||||||||||
|
Balance as of January 1, 2022
|
137,914
|
$
|
41,809
|
$
|
(23,727
|
)
|
$
|
18,082
|
||||||||
|
Share-based compensation to employees and directors
|
-
|
1,389
|
-
|
1,389
|
||||||||||||
|
Share-based compensation to service providers
|
-
|
342
|
-
|
342
|
||||||||||||
|
Share issuance to service providers
|
634
|
|||||||||||||||
|
Net comprehensive loss
|
-
|
-
|
(8,792
|
)
|
(8,792
|
)
|
||||||||||
|
Balance as of December 31, 2022
|
138,548
|
$
|
43,540
|
$
|
(32,519
|
)
|
$
|
11,021
|
||||||||
|
Share-based compensation to employees and directors
|
-
|
804
|
-
|
804
|
||||||||||||
|
Share issuance to service providers
|
362
|
-
|
-
|
*
|
||||||||||||
|
Issuance of common stock and pre-funded warrants upon private placement, net of underwriting commissions and other offering costs.
|
19,496
|
1,450
|
-
|
1,450
|
||||||||||||
|
Issuance and exercise of common stock warrants upon private placement, net of underwriting commissions and other offering costs.
|
7,038
|
3,308
|
3,308
|
|||||||||||||
|
Net comprehensive loss
|
-
|
-
|
(9,344
|
)
|
(9,344
|
)
|
||||||||||
|
Balance as of December 31, 2023
|
165,489
|
$
|
49,102
|
$
|
(41,863
|
)
|
$
|
7,239
|
||||||||
|
Share-based compensation to employees and directors
|
-
|
310
|
-
|
310
|
||||||||||||
|
Exercise of prefunded warrants (Note 7b)
|
12,412
|
-
|
-
|
-
|
||||||||||||
|
Issuance of common stock, warrants and prefunded warrants upon private placement, net of underwriting commissions and other offering costs. (Note 7b)
|
208,334
|
3,340
|
-
|
3,340
|
||||||||||||
|
Exercise of warrants and issuance of warrants upon private placement, net of underwriting commissions and other offering costs. (Note 7b)
|
247,325
|
1,305
|
-
|
1,305
|
||||||||||||
|
Exercise of warrants (Note 7b)
|
494,650
|
2,912
|
-
|
2,912
|
||||||||||||
|
Issuance of Ordinary shares, net of offering costs – At-the-market (Note 7b)
|
343,202
|
1,306
|
-
|
1,306
|
||||||||||||
|
Net loss and comprehensive loss
|
-
|
-
|
(14,588
|
)
|
(14,588
|
)
|
||||||||||
|
Balance as of December 31, 2024
|
1,471,412
|
$
|
58,275
|
$
|
(56,451
|
)
|
$
|
1,824
|
||||||||
|
For the Year Ended
December 31, |
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Cash flows from operating activities
|
||||||||||||
|
Net loss
|
$
|
(14,588
|
) |
$
|
(9,344
|
) |
$
|
(8,792
|
) | |||
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Depreciation
|
16
|
15
|
15
|
|||||||||
|
Exchange rate differences on cash, cash equivalents and restricted cash
|
(6
|
) |
(2
|
) |
-
|
|||||||
|
Share-based compensation to employees and directors
|
310
|
804
|
1,389
|
|||||||||
|
Net change in operating lease asset and liability
|
(10
|
) |
(9
|
) |
-
|
|||||||
|
Share-based compensation to service providers
|
-
|
-
|
342
|
|||||||||
|
Warrant issuance costs
|
-
|
368
|
-
|
|||||||||
|
Interest income (expenses)
|
-
|
85
|
(85
|
) | ||||||||
|
Change in warrant liability valuation
|
-
|
(1,726
|
) |
-
|
||||||||
|
Loss from inducement offer letter agreement (Note 7c)
|
-
|
1,502
|
-
|
|||||||||
|
Changes in operating assets and liabilities:
|
||||||||||||
|
Prepaid expenses and other current assets
|
1,605
|
330
|
356
|
|||||||||
|
Trade payables
|
76
|
12
|
73
|
|||||||||
|
Employees, related liabilities and accrued expenses
|
(24
|
) |
1,286
|
243
|
||||||||
|
Net cash used in operating activities
|
$ |
(12,621
|
) |
$ |
(6,679
|
) |
$ |
(6,459
|
) | |||
|
Cash flows from investing activities
|
||||||||||||
|
Purchase of property and equipment
|
(13
|
) |
(9
|
) |
(6
|
) | ||||||
|
Proceeds from short term deposits
|
-
|
7,000
|
||||||||||
|
Purchase of short-term deposit
|
-
|
(1,000
|
) |
(6,000
|
) | |||||||
|
Net cash provided by (used in) investing activities
|
$ |
(13
|
) |
$ |
5,991
|
$ |
(6,006
|
) | ||||
|
Cash flows from financing activities
|
||||||||||||
|
Proceeds from issuance of shares, warrants and prefunded warrants (Note 7b) |
4,000
|
1,703
|
-
|
|||||||||
|
Proceeds from exercise of warrants (Note 7b) |
3,165
|
2,511
|
-
|
|||||||||
|
Proceeds from exercise and issuance of warrants (Note 7b)
|
1,583
|
1,334
|
-
|
|||||||||
|
Proceeds from issuance of Ordinary shares At-the market offering (Note 7b)
|
1,350
|
-
|
-
|
|||||||||
|
Issuance costs
|
(1,235
|
) |
(932
|
) |
-
|
|||||||
|
Net cash provided by financing activities
|
$ |
8,863
|
$ |
4,616
|
$ |
-
|
||||||
|
Effect of Exchange rate changes on cash, cash equivalents and restricted cash
|
6
|
2
|
-
|
|||||||||
|
Net increase (decrease) in cash, cash equivalents and restricted cash
|
(3,765
|
) |
3,930
|
(12,465
|
) | |||||||
|
Cash, cash equivalents and restricted cash at the beginning of the year
|
8,036
|
4,106
|
16,571
|
|||||||||
|
Cash, cash equivalents and restricted cash at the end of the year
|
$
|
4,271
|
$
|
8,036
|
$
|
4,106
|
||||||
|
December 31, |
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Cash and cash equivalents
|
|
4,261
|
|
8,026
|
|
4,096
|
||||||
|
Restricted cash
|
10
|
10
|
10
|
|||||||||
|
Total cash, cash equivalents, and restricted cash
|
$
|
4,271
|
$
|
8,036
|
$
|
4,106
|
||||||
|
Supplemental cash flow information:
|
||||||||||||
|
Acquisition of right-of-use assets by means of lease liabilities
|
$
|
-
|
$
|
113
|
$
|
-
|
||||||
|
a.
|
PainReform Ltd. (“the Company”) was incorporated and started business operations in November 2007. The Company is a specialty pharmaceutical company focused on the reformulation of established therapeutics. The Company’s proprietary extended-release drug-delivery system is designed to provide an extended period of post-surgical pain relief without the need for repeated dose administration while reducing the potential need for the use of opiates. In November 2024, the Company reported partial topline data from Phase 3 clinical trial of PRF-110. The data showed statistically significant superiority over placebo in reducing pain during the first 48 hours post-surgery, However, the data for the final 24 hours of the 72-hour follow-up was unclear. In December 2024 the company concluded that the data could not be clarified to meet the study’s endpoint at 72 hours. The Company initiated Research and Development activities better understand and refine the pharmaco-kinetics and pharmaco-dynamics of PRF-110 based on the data received from the study. These efforts are intended to potentially resolve this issue to support future clinical trials.
On February 17, 2025, the Company entered into a business transaction agreement (the “Agreement”) with BladeRanger Ltd. (“BLRN”), a publicly traded Israeli company, to acquire all business operations related to its AI-driven solar analytics platform, DeepSolar. Under the terms of the Agreement, the Company acquired all rights, title, and interest in the associated agreements, intellectual property, accounts receivable, equipment, customer relationships, the "MyDeepSolar" application and platform, and all other related assets. as defined in the Agreement (Note 11). The acquisition was closed on March 5, 2025.
|
|
b.
|
Liquidity
Since its inception, the Company has devoted substantially all its efforts to research and development, clinical trials, and capital raising activities. The Company is still in its development and clinical stage and has not yet generated revenues.
The Company has incurred significant losses and negative cash flows from operations and incurred losses of $14,588, $9,344 and $8,792 for the years ended December 31, 2024, 2023 and 2022, respectively. During the years ended December 31, 2024, 2023 and 2022, the Company had negative operating cash outflows of $12,621, $6,679, and $6,459, respectively. As of December 31, 2024, the Company’s accumulated deficit was $56,451. The Company has funded its operations to date primarily through equity financing and has cash on hand (including restricted cash) of $4,271 as of December 31, 2024.
On April 15, 2024 the Company sold (i) 18,646 ordinary shares, (ii) 189,688 prefunded warrants, and (iii) 208,335 investor warrants at a combined offering price of $19.20 per share (or $19.199 per prefunded warrant), along with (iv) 14,583 underwriter warrants priced at $24.00. Gross proceeds from the offering were approximately $4.0 million, with net proceeds of $3.3 million.(Note 7b)
On September 10, 2024, the Company entered into an inducement agreement with certain holders, under which the holders agreed to exercise 247,325 warrants at a reduced price of $6.40 per share. In exchange, the Company issued new warrants to purchase up to 494,650 ordinary shares at the same exercise price. The transaction generated gross proceeds of approximately $1.6 million.(Note 7b)
Between October 24 and December 31, 2024, the Company issued 343,202 ordinary shares through an At-the-Market (ATM) program, raising approximately $1.35 million in gross proceeds. (Note 7b)
On December 18, 2024, all 494,650 warrants issued in the September 2024 inducement transaction were exercised at $6.40 per share, resulting in gross proceeds of approximately $3.17 million. (Note 7b)
The Company expects to continue incurring losses, and negative cash flows from operations until its activities including product, PRF-110 and the commercialization of the DeepSolar solution reaches profitability. Based on the Company’s current cash position and projected operating requirements, there is uncertainty regarding the ability to meet the financial obligations for at least 12 months following the issuance of these financial statements. While management is actively exploring various financing and strategic alternatives, there can be no assurance that such measures will be successful in alleviating this uncertainty.
Management's plans include continued raising capital through sale of additional equity securities, debt or capital inflows from strategic partnerships and generating revenues from the commercialization of the DeepSolar solution. There are no assurances, however, that the Company will successfully obtain the level of financing needed for its operations. If the Company is unsuccessful in raising capital, it may need to reduce activities, curtail, or abandon some or all of its operations, which could materially harm the Company’s business, financial condition and results of operations. These factors raise substantial doubt about its ability to continue as a going concern.
These financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business and do not include any adjustments that might result from the outcome of this uncertainty.
|
|
c.
|
On September 6, 2024, the Company effected a 1-for-6 reverse share split of our authorized and outstanding ordinary shares, reducing the par value from NIS 0.30 per share to no par value per share. Subsequently, on November 20, 2024, the Company implemented an additional 1-for-4 reverse share split of our authorized and outstanding ordinary shares.
As a result, the cumulative impact of the two reverse splits in 2024 is equivalent to a 1-for-24 reverse share split (the "Reverse Split"). As a result of rounding of fractional shares as part of the splits, 93,475 shares were added, bringing the Company’s total outstanding shares on a post-split basis to 1,471,412.
All related share and per share data have been retroactively applied to the financial statements and their related notes for all periods presented.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
d.
|
On October 7, 2023, an unprecedented attack was launched against Israel, which thrust Israel into a state of war. The Company is continuing the development of its product and commercialization of the DeepSolar solution. At this time, the Company's management does not expect this situation to have a material impact on its operations or its business results.
|
|
e.
|
The Company reports its financial results in U.S. dollars. A portion of research and development and general and administrative expenses of our Israeli operations are incurred in New Israeli Shekel ("NIS"). As a result, the Company is exposed to exchange rate risks that may materially and adversely affect our financial results. If the NIS appreciates against the U.S. dollar, or if the value of the NIS decline against the U.S. dollar, at a time when the rate of inflation in the cost of Israeli goods and services exceed the rate of decline in the relative value of the NIS, then the U.S. dollar-denominated cost of our operations in Israel would increase and our results of operations could be materially and adversely affected. Inflation in Israel compounds the adverse impact of a devaluation of the NIS against the U.S. dollar by further increasing the amount of our Israeli expenses. Israeli inflation may also (in the future) outweigh the positive effect of any appreciation of the U.S. dollar relative to the NIS, if, and to the extent that, it outpaces such appreciation or precedes such appreciation. The Israeli rate of inflation did not have a material adverse effect on the Company’s financial condition during 2024 ,2023 and 2023. Given our general lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of the NIS in relation to the U.S. dollar (and/or from inflation of such non-U.S. currencies), the Company may be exposed to material adverse effects from such movements. the Company cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the U.S. dollar against the NIS.
|
|
a.
|
Basis of presentation:
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”).
The significant accounting policies described below have been applied consistently in relation to all the periods presented, unless otherwise stated.
|
|
b.
|
Use of estimate in preparation of financial statements:
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates, judgments and assumptions that affect the amounts reported in the financial statements and accompanying notes. The Company evaluates on an ongoing basis its assumptions. Estimates are primarily used for, but not limited to, clinical trial accrual expenses, and valuation allowances. The Company’s management believes that the estimates, judgments, and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of expenses during the reporting periods. Actual results could differ from those estimates.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| c. |
Financial statements in United States dollars:
The Company’s functional currency is the U.S. dollar (“dollar” or “$”) since the dollar is the currency of the primary economic environment in which the Company has operated and expects to continue to operate in the foreseeable future. Transactions and balances originally denominated in U.S. dollars are presented at their original amounts. Balances in non-U.S. dollar currencies are translated into U.S. dollars using historical and current exchange rates for non-monetary and monetary balances, respectively. For non-U.S. dollar transactions and other items in the statements of income (indicated below), the following exchange rates are used: (i) for transactions - exchange rates at transaction dates or average exchange rates; and (ii) for other items (derived from non-monetary balance sheet items such as depreciation) - historical exchange rates. Currency transaction gains and losses are presented in financial income or expenses, as appropriate.
|
|
d.
|
Cash and cash equivalents:
Cash equivalents are short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less at acquisition.
|
|
e.
|
Short term deposit:
Bank deposits with original maturity dates of more than three months but at balance sheet date are less than one year are included in short-term deposits. The fair value of bank deposits approximates the carrying value since they bear interest at rates close to the prevailing market rates.
|
|
f.
|
Restricted cash:
As of December 31, 2024 and 2023, the Company’s restricted cash consisted of immaterial bank deposits that are denominated in NIS. Restricted deposits are presented at cost including accrued interest. These bank deposits are used as securities for the Company's credit cards.
|
|
g.
|
Fair Value Measurements:
The carrying values of Company’s financial assets and liabilities, including cash and cash equivalents, restricted cash, other current assets, trade payables and other accounts payable approximate their fair value due to the short-term maturity of these instruments. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. Assets and liabilities recorded at fair value in the financial statements are categorized as follows:
|
|
As of December 31, 2024, and 2023 no assets or liabilities are measured at their fair value.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
h.
|
Property and equipment, net:
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following rates:
|
|
%
|
||||
|
Computers, software and electronic equipment
|
33
|
|||
|
Furniture and office equipment
|
7
|
|||
|
i.
|
Research and development expenses:
Research and development costs include costs of payroll and related expenses of employees, subcontractors and consultants and other costs related to the Company's operation of its planned clinical trials. Research and development expenses are charged to the statements of comprehensive loss as incurred.
Clinical trial costs are a significant component of research and development expenses and include costs associated with third-party contractors. The Company outsources its clinical trial activities utilizing external entities such as clinical research organizations, independent clinical investigators, and other third-party service providers to assist the Company with the execution of its clinical trials.
Clinical trial expenses are charged to research and development expense as incurred. The Company accrues for expenses resulting from obligations under contracts with its clinical research organization (CRO). The financial terms of these contracts are subject to negotiations, which vary from contract to contract and may result in payment flows that do not match the periods over which services are provided. The Company’s objective is to reflect the appropriate trial expense in the financial statements by matching the appropriate expenses with the period in which services and efforts are expended. In the event advance payments are made to a CRO, the payments are recorded as prepaid clinical trial expenses and deferred clinical trial costs, which will be recognized as expenses as services are rendered.
|
| j. |
Employee severance benefits:
The Company is required to make severance payments upon dismissal of an Israeli employee or upon termination of employment in certain circumstances.
In accordance with the current employment terms with all of its employees (Section 14 of the Israeli Severance Pay Law, 1963) located in Israel, the Company makes regular deposits with certain insurance companies for accounts controlled by each applicable employee in order to secure the employee’s full retirement benefit and severance obligation. The Company is relieved from any severance pay liability with respect to each such employee after it makes the payments on behalf of the employee. The liability accrued in respect of these employees and the amounts funded, as of the respective agreement dates, are not reflected on the Company’s balance sheet, as the amounts funded are not under the control and management of the Company and the pension or severance pay risks have been irrevocably transferred to the applicable insurance companies. The amounts of severance payment expenses were $73, $73 and $60 for the years ended December 31, 2024, 2023 and 2022, respectively.
|
|
k.
|
Legal and other contingencies:
Certain conditions may exist as of the date of the financial statements, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company’s management assesses such contingent liabilities, if any, and such assessment inherently involves an exercise of judgment. In assessing loss contingencies related to legal proceedings that are pending against the Company or unasserted claims that may result in such proceedings, the Company’s management evaluates the perceived merits of any legal proceedings or unasserted claims as well as the perceived merits of the amount of relief sought or expected to be sought. Management applies the guidance in ASC 450-20, “Loss Contingencies” when assessing losses resulting from contingencies. If the assessment of a contingency indicates that it is probable that a material loss has been incurred and the amount of the liability can be reasonable estimated, then the estimated liability is recorded as accrued expenses in the Company’s financial statements. Legal costs incurred in connection with loss contingencies are expensed as incurred.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
l.
|
Income taxes:
The Company accounts for income taxes using the asset and liability method whereby deferred tax asset and liability account balances are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, if necessary, to reduce deferred tax assets to their estimated realizable value if it is more likely than not that a portion or all the deferred tax assets will not be realized, based on the weight of available positive and negative evidence. As of December 31, 2024, and 2023, the Company had a full valuation allowance on its deferred tax assets.
The Company implements a two-step approach to recognize and measure uncertain tax positions. The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes. The second step is to measure the tax positions as the largest amount that is more than 50% (cumulative basis) likely to be realized upon ultimate settlement. As of December 31, 2024 and 2023, the total gross amount of provision for unrecognized tax positions was $259 and $251, respectively (Note 5f). The Company recognizes interest and penalties, if any, related to unrecognized tax positions in tax expenses.
|
|
m.
|
Concentrations of credit risk:
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash, cash equivalents and restricted cash. Cash and cash equivalents and restricted cash are invested in a major bank in Israel and the United States.
Management believes that the banks that hold the Company’s cash, cash equivalent and restricted cash are financially sound and, accordingly, minimal credit risk exists with respect to this cash, cash equivalent and restricted cash.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
n.
|
Derivative warrant liability
Financial equity instruments that do not meet the US GAAP criteria for equity classification are classified as a liability at fair value and are adjusted to fair value at each reporting period. Changes in fair value are recognized in the Company’s statements of comprehensive loss in accordance with ASC 815, “Accounting for Derivative Financial Instruments”.
|
|
o.
|
Basic and diluted loss per share:
Basic loss per share is computed on the basis of the net loss for the period divided by the weighted average number of Ordinary Shares, fully vested pre-funded warrant and vested Ordinary Shares issuable for little or no further consideration outstanding during the period. Diluted loss per share is based upon the weighted average number of ordinary shares and of potential Ordinary Shares outstanding when dilutive. Potential Ordinary Shares include outstanding stock options, restricted shares and warrants, which are included under the treasury stock method when dilutive.
For the years ended December 31, 2024, 2023 and 2022, all outstanding share options, restricted shares, and warrants (except for fully vested pre-funded warrants) have been excluded from the calculation of the diluted net loss per share as all such securities are anti-dilutive for all years presented.
The loss and the weighted average number of shares used in computing basic and diluted net loss per share is as follows:
|
|
Year ended
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Numerator:
|
||||||||||||
|
Net loss applicable to shareholders of ordinary shares
|
$
|
(14,588
|
)
|
$
|
(9,344
|
)
|
$
|
(8,792
|
)
|
|||
|
Denominator:
|
||||||||||||
|
Shares of Ordinary Share and restricted shares used in computing basic and diluted net loss per share (*)
|
453,544
|
148,013
|
138,548
|
|||||||||
|
Net loss per share of ordinary share, basic and diluted
|
$
|
(32.16
|
)
|
$
|
(63.13
|
)
|
$
|
(63.46
|
)
|
|||
|
(*) All share amounts have been retroactively adjusted to reflect a 1-for-24 reverse share split (Note 1c).
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
p.
|
Share-based compensation:
Share-based compensation to employees, officers, directors, and non-employees is accounted for in accordance with ASC 718, “Compensation - Share Compensation” (“ASC 718”), which requires estimation of the fair value of share-based payment awards on the grant date. The Company accounts for share-based compensation awards classified as equity awards using the grant-date fair value method. The value of the portion of the award that is ultimately expected to vest is recognized as an expense on a straight-line basis over the requisite service period. The Company has elected to recognize forfeitures as they occur.
The fair value of each share option granted is estimated using the Black-Scholes option pricing model, which incorporates a number of assumptions, including the expected share price volatility, the expected option term, and the risk-free interest rate. Expected volatility is calculated based on comparable public companies in the same industry. The expected share option term is calculated using the “simplified” method when the required conditions are met. The risk-free interest rate is derived from the yield of U.S. Treasury bonds with an equivalent term. The Company grants share-equivalents (“Share-Based Compensation”) in consideration for services rendered (Note 7).
The expected dividend yield assumption is based on the Company’s historical experience and expectation of no future dividend pay outs. The Company has historically not paid cash dividends and has no foreseeable plans to pay cash dividends in the future.
The Company elected to recognize Share-Based Compensation cost for awards with only service conditions that have a graded vesting schedule using the straight-line method based on the multiple-option award approach.
|
|
q.
|
Deferred offering costs
The Company capitalizes certain legal and other third-party fees that are directly related to the Company’s in-process equity financings until such financings are consummated. After the consummation of such equity financings, these costs are recorded as a reduction of the respective gross proceeds. Should a planned equity financing be abandoned, terminated, or significantly delayed, the deferred offering costs are written off to operating expenses. As of December 31, 2024 and 2023, there were no deferred offering costs.
|
|
r.
|
Segment Reporting
The Company has one operating and reportable segment. An operating segment is defined as a component that engages in business activities whose operating results are reviewed by the chief operating decision maker, who is the Company’s Chief Executive Officer, for the purpose of assessing performance and allocating resources and for which discrete financial information is available.
|
|
s.
|
Leases
In accordance with Accounting Standards Codification (“ASC”) 842, Leases, the Company determines whether an arrangement is or contains a lease at the inception of the arrangement and whether such a lease is classified as a financing lease or operating lease at the commencement date of the lease.
Leases consist real estate property that are classified as operating leases with rental payment linked to the index. The Company recorded right of use (“ROU”) asset and a lease liability of the Company obligation to make the lease payments. The ROU asset and the liability are included in non-current assets, current liabilities and non-current liabilities on the balance sheet. Operating lease ROU and liabilities are recognized at the commencement date based on the present value of lease payments over the lease term, which may include options to extend or terminate the lease, when it is reasonably certain at the commencement date whether the Company will or will not exercise the option to renew or terminate the lease.
In previous periods the Company elected the short-term lease recognition exemption for all leases with a term shorter than 12 months period. This means that for those leases, the Company did not recognize ROU assets or lease liabilities but recognizes lease expenses over the lease term on a straight-line basis (Note 6a).
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
|
t. |
Recently Adopted Accounting Pronouncements
In November 2023, the FASB issued ASU No. 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures. This ASU improves reportable segment disclosure requirements, primarily through enhanced disclosures about significant segment expenses. The key amendments include: (a)2-23 introduce a new requirement to disclose significant segment expenses regularly provided to the chief operating decision maker (“CODM”), (b) extend certain annual disclosures to interim periods, (c) clarify single reportable segment entities must apply ASC 280 in its entirety, (d) permit more than one measure of segment profit or loss to be reported under certain conditions, and (e) require disclosure of the title and position of the CODM. This ASU is effective for public entities with fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. The Company adopted ASU 2023-07 on January 1, 2024 and such adoption did not impact the Company’s financial position, results of operations, cash flows or net loss per share (See Note 9).
|
| u. |
Recently Issued Accounting Pronouncements Not Yet Adopted
In November 2024, the FASB issued ASU No. 2024-03 Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40). The ASU improves the disclosures about a public business entity’s expense and provides more detailed information about the types of expenses in commonly presented expense captions. The amendments require that at each interim and annual reporting period an entity will, inter alia, disclose amounts of purchases of inventory, employee compensation, depreciation and amortization included in each relevant expense caption (such as cost of sales, SG&A and research and development). The ASU is effective for fiscal years beginning after December 15, 2026, and interim periods within fiscal years beginning after December 15, 2027. Early adoption is permitted. The Company is currently evaluating this ASU to determine its impact on the Company's disclosures.
In December 2023, FASB issued an Accounting Standard Update No. 2023-09 “Income Taxes (Topic 740)” to enhance the transparency and decision usefulness of income tax disclosure. The amendments in this Update mandate public entities to disclose specific categories in the rate reconciliation and additional information for reconciling items that meet quantitative threshold in the annual tax rate reconciliations. This update requires to present a table showing percentages and currency amounts, outlining tax related aspects such as state/local income tax, foreign tax effect, changes in tax law, credits, valuation allowances, non-taxable and non-deductible items, unrecognized tax benefits. Items that impact tax calculations by 5% and more are required to be disclosed separately, with certain categories required to be disaggregated by jurisdiction or nature. Reconciling items are categorized based on state/local, foreign, or federal/national tax levels. Some items can be presented on a net basis, while others need gross presentation. Entities must provide explanations of the major state/local jurisdictions affecting taxes and explain individual reconciling items. Additionally, the amendments in this Update require that all entities must disclose amount of income taxes paid disaggregated by federal(national) state and by individual jurisdictions in which income taxes paid if equal to or greater than 5% of total income taxes paid. The amendments also require entities to disclose income from continuing operations before income tax expense, and income tax expenses categorized by federal/national, state, and foreign levels. Moreover, certain previous disclosure requirements, like estimating changes in unrecognized tax benefits and cumulative temporary differences in deferred tax liabilities, are eliminated.
The ASU will be effective for fiscal years beginning after December 15, 2025, and allows adoption on a prospective basis, with a retrospective option. The Company is in the process of assessing the impacts and method of adoption.
|
|
December 31,
|
||||||||
|
2024
|
2023
|
|||||||
|
Receivables from governmental authorities
|
$
|
119
|
$
|
45
|
||||
|
Prepaid expenses
|
38
|
204
|
||||||
|
$
|
157
|
$
|
249
|
|||||
|
December 31,
|
||||||||
|
2024
|
2023
|
|||||||
|
Directors’ fees
|
$
|
54
|
$
|
34
|
||||
|
Manufacturing and trials expenses
|
1,710
|
1,486
|
||||||
|
Advisors and legal expenses
|
140
|
148
|
||||||
|
$
|
1,904
|
$
|
1,668
|
|||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
NOTE 5:- TAXES ON INCOME
|
| a. |
Tax rates applicable to the Company:
|
| b. |
Net operating loss carry forward:
|
| c. |
As of December 31, 2024, the Company had final tax assessments for tax years prior to and including the tax year ended December 31, 2019.
|
| d. |
Deferred income taxes:
|
|
December 31,
|
||||||||
|
2024
|
2023
|
|||||||
|
Net operating loss carry forward
|
$
|
7,964
|
$
|
5,698
|
||||
|
Research and development expenses
|
2,245
|
1,179
|
||||||
|
Other
|
37
|
48
|
||||||
|
Less: Valuation allowance
|
(10,246
|
)
|
(6,925
|
)
|
||||
|
Net deferred tax asset
|
$
|
-
|
$
|
-
|
||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| e. |
Reconciliation of theoretical tax expenses to actual expenses
|
| f. |
Uncertain tax positions:
|
|
December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Opening balance
|
$
|
251
|
$
|
243
|
$
|
234
|
||||||
|
Tax positions taken in the current year
|
-
|
-
|
-
|
|||||||||
|
Interest and Exchange rate differences
|
8
|
8
|
9
|
|||||||||
|
Closing balance
|
$
|
259
|
$
|
251
|
$
|
243
|
||||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
NOTE 6:- COMMITMENTS:
|
| a. |
On August 1, 2023, the Company entered into a one-year lease agreement for its principal offices in Tel Aviv, Israel, later extended to July 31, 2025. As of the reporting date, no further extension has been decided, and the Company is exploring alternatives. In accordance with its accounting policy, the Company recognized ROU assets and lease liabilities. Monthly rent is $5, linked to the CPI. In 2024, lease expenses totaled $66, with $33 in lease liability cash payments. The weighted average remaining lease term is 0.5 years, and the discount rate is 8.5%.
|
| b. |
On November 13, 2020, and December 3, 2020, the Company entered into a Master Clinical Research Organization Agreement (the “First Agreement”) and a Master Clinical Trial Agreement (the “Second Agreement”) with Lotus Clinical Research (“Lotus”) as the Company’s clinical research organization.
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| a. |
Ordinary shares:
The ordinary shares confer upon their holders the right to participate and vote in general shareholder meetings of the Company and to share in the distribution of dividends, if any, declared by the Company, and rights to receive a distribution of assets upon liquidation. On August 13, 2024, an Extraordinary Meeting of the General Shareholders of the Company was held, during which a resolution was approved to change the par value of the Company’s ordinary shares from 0.3 NIS per share to zero (0) NIS per share. Following this resolution, all references to share capital in the Company’s financial statements, regulatory filings, and related disclosures reflect ordinary shares with a par value of zero. This change has no impact on the number of shares outstanding or on the rights attached to the shares. At the same meeting, the shareholders also approved to increase the Company’s authorized share capital to 2,500,000 shares, with no Par value and to amend the Company’s articles of association.
|
| b. |
Share activity:
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| c. |
Warrants and warrants units:
|
|
Type
|
Issuance Date
|
Number of
warrants
|
Exercise price(*)
|
Exercisable through
|
|
Warrants to underwriters
|
September 3, 2020
|
125,000
|
$2,400.0
|
September 1, 2025
|
|
Warrants to underwriters
|
October 5, 2020
|
375,000
|
$2,112.0
|
September 3, 2025
|
|
IPO warrants
|
September 3, 2020
|
2,812,170
|
$2,112.0
|
September 3, 2025
|
|
PIPE warrants
|
March 11, 2021
|
232,500
|
$1,104.0
|
September 10, 2026
|
|
Warrants to PIPE placement agent
|
March 11,2021
|
52,173
|
$1,214.4
|
March 8, 2026
|
|
December 2023 warrants
|
December 28, 2023
|
32,753
|
$85.4
|
December 28, 2028
|
|
Warrants issued to underwriters
|
April 15, 2024
|
350,000
|
$24.0
|
April 15, 2029
|
|
Warrants issued to underwriters
|
September 11,2024
|
69,251
|
$8.0
|
September 11,2029
|
|
Warrants issued to underwriters
|
December 18, 2024
|
34,625
|
$8.0
|
December 18, 2029
|
|
|
|
|
|
|
|
TOTAL
|
|
4,083,472
|
|
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| d. |
Share-based compensation:
|
| 1. |
The 2008 Plan
|
| 2. |
The 2019 Plan
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| 3. |
The following tables summarizes information about options granted to employees and directors:
|
|
Number of
options
|
Weighted
average
exercise
price
|
Weighted
average
remaining
contractual
life
|
||||||||||
|
USD
|
||||||||||||
|
Options outstanding at beginning of year
|
642
|
$
|
57.60
|
0.25
|
||||||||
|
Changes during the year:
|
||||||||||||
|
Options granted
|
-
|
-
|
-
|
|||||||||
|
Options exercised
|
-
|
-
|
-
|
|||||||||
|
Options expired
|
(642
|
)
|
57.60
|
-
|
||||||||
|
Options outstanding at end of year
|
-
|
$
|
-
|
-
|
||||||||
|
Options exercisable at end of year
|
-
|
$
|
-
|
-
|
||||||||
|
Number of
options
|
Weighted
average
exercise
price
|
Weighted
average
remaining
contractual
life
|
||||||||||
|
USD
|
||||||||||||
|
Options outstanding at beginning of year
|
7,837
|
$
|
286.56
|
8.69
|
||||||||
|
Changes during the year:
|
||||||||||||
|
Options granted
|
18,488
|
6.00
|
9.65
|
|||||||||
|
Options expired
|
(3,773
|
) |
96.34
|
9.75
|
||||||||
|
Options exercised
|
-
|
-
|
-
|
|||||||||
|
Options forfeited
|
-
|
-
|
-
|
|||||||||
|
Options outstanding at end of year
|
22,552
|
$
|
88.32
|
9.17
|
||||||||
|
Options exercisable at end of year
|
20,094
|
$
|
94.59
|
9.15
|
||||||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| 4. |
The following table sets forth the assumptions that were used in determining the fair value of options granted to employees in 2019 plan for the years ended on December 31, 2024, 2023 and 2022:
|
|
2024
|
2023*
|
|
2022*
|
|||||||||
|
Expected term (years)
|
5.00-6.41
|
5.00-6.41
|
5.28-6.07
|
|||||||||
|
Risk-free interest rates
|
4.68
|
%
|
3.82%-3.87
|
%
|
2.69%-3.88
|
%
|
||||||
|
Volatility
|
97.24
|
%
|
90.43
|
%
|
79.3%-82.6
|
%
|
||||||
|
Dividend yield
|
-
|
-
|
-
|
|||||||||
|
Exercise price
|
$
|
6.0
|
$
|
5.89
|
$
|
5.70-10.60
|
||||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| a. |
Research and development expenses:
|
|
Year ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Subcontractors and consultants
|
$
|
793
|
$
|
1,001
|
$
|
2,228
|
||||||
|
Payroll and related expenses
|
543
|
699
|
766
|
|||||||||
|
Share-based compensation expense
|
80
|
73
|
285
|
|||||||||
|
Clinical trials expenses
|
10,289
|
4,262
|
1,121
|
|||||||||
|
Other expenses
|
-
|
-
|
22
|
|||||||||
|
$
|
11,705
|
$
|
6,035
|
$
|
4,422
|
|||||||
| b. |
General and administrative expenses:
|
|
Year ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Professional services
|
$
|
1,390
|
$
|
1,209
|
$
|
1,489
|
||||||
|
Payroll and related expenses
|
815
|
877
|
780
|
|||||||||
|
D&O insurance
|
263
|
394
|
653
|
|||||||||
|
Rent and office maintenance
|
146
|
191
|
249
|
|||||||||
|
Share-based compensation expense
|
230
|
731
|
1,104
|
|||||||||
|
Other expenses
|
124
|
147
|
172
|
|||||||||
|
$
|
2,968
|
$
|
3,549
|
$
|
4,447
|
|||||||
| c. |
Other financial income (expenses), net:
|
|
Year ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Interest income
|
101
|
406
|
160
|
|||||||||
|
Issuance expenses
|
-
|
(368
|
)
|
-
|
||||||||
|
Bank fees
|
(14
|
)
|
(16
|
)
|
(13
|
)
|
||||||
|
Loss from Inducement offer letter agreement (Note 7c)
|
- |
(1,502
|
)
|
-
|
||||||||
|
Change in fair value of derivative warrant liability (Note7c)
|
- |
1,726
|
-
|
|||||||||
|
Exchange rate differences
|
$
|
6
|
2
|
$
|
(61
|
)
|
||||||
|
Total other financial expenses, net
|
$
|
93
|
248
|
$
|
86
|
|||||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
For the Year Ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Payroll and related Expenses
|
$
|
1,358
|
$
|
1,576
|
1,546
|
|||||||
|
Clinical Trial Expenses
|
10,289
|
4,262
|
1,121
|
|||||||||
|
Other segment items *
|
2,941
|
3,506
|
6,125
|
|||||||||
|
Segment Loss
|
$
|
14,588
|
$
|
9,344
|
8,792
|
|||||||
| a. |
On June 8, 2023, the Company’s shareholders approved the grant of options to purchase an aggregate of 2,250 shares to two current board members, and to the chairman of the board of directors. The valuation of the option on the grant date was $174. (Note 7d2)
|
| b. |
On July 18, 2024, the Company’s shareholders approved the grant of options to purchase an aggregate of 9,765 shares to four board members, and to the chairman of the board of directors. The valuation of the option on the grant date was $27. (Note 7d2)
|
| c. |
On October 10, 2024, the Company’s shareholders approved the grant of options to purchase an aggregate of 1,556 shares to a board member, and to the chairman of the board of directors. The valuation of the option on the grant date was $1. (Note 7d2)
|
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
|
Year ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Employees accrued salaries and bonuses
|
$
|
244
|
$
|
324
|
$
|
359
|
||||||
|
Directors accrued fees expenses
|
54
|
33
|
33
|
|||||||||
|
$
|
298
|
$
|
357
|
$
|
392
|
|||||||
|
Year ended December 31,
|
||||||||||||
|
2024
|
2023
|
2022
|
||||||||||
|
Amounts charged to:
|
||||||||||||
|
Research and development payroll expenses
|
$
|
528
|
$
|
528
|
$
|
702
|
||||||
|
General and administrative payroll and directors’ fees expenses
|
$
|
1,322
|
$
|
1,676
|
$
|
2,091
|
||||||
PAINREFORM LTD.
NOTES TO FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| 1. |
On January 2, 2025, the Board of Directors of PainReform approved an increase to the Company’s ATM program, pursuant to a written board’s approval dated October 14, 2024. The program allows offerings of up to $4.0 million, of which the Company raised approximately $0.9 million between January 1 and March 31, 2025.
|
| 2. |
On February 17, 2025, the Company entered into a business acquisition agreement (the “Agreement”) with BladeRanger Ltd. (“BLRN”), a public Israeli company, to acquire 100% of the business activities associated with its AI-based solar analytics platform, DeepSolar. As part of the acquisition, the company received all rights, title and interest in agreements, intellectual property, accounts receivable, equipment, customer relationships, the "MyDeepSolar" application and platform, and all related assets, as defined in the Agreement.
|
| 3. |
On February 20, 2025, the Company’s Board of Directors approved the following resolutions:
|
|
|
1. |
INTERPRETATION
|
|
|
1.1 | “Acquired Agreements” |
means the agreements/accounts set forth in Schedule 1.1
attached hereto as assigned to the Buyer, subject to receipt of applicable assignment consents no later than the Closing (including all rights and obligations of Seller in, to and under the same, other than Excluded Liabilities). It is
clarified that Schedule1.1 includes all of the Business’s accounts/clients during the three (3) year period preceding the Closing and that some of these accounts/clients are no longer
active. It is agreed that in the event that following the Closing, Buyer finds out about an account/client of the Business that should have been included in Schedule 1.1 in accordance with
the clarification above, Schedule 1.1 will be automatically amended to include such account/client as of the Closing.
|
|
|
1.2 | “Acquired Assets” | (i) | The Acquired Agreements. |
|
|
(ii) |
The Acquired IP.
|
|
|
(iii) |
The Acquired AR (as defined below).
|
|
|
(iv) |
The equipment set forth in Schedule 1.2(a)
|
|
|
(v) |
All Seller’s reputation and customer relations associated with the Business
|
|
|
(vi) |
And all rights, title and interest in, to or arising from any of the foregoing assets, properties and rights (whether real, personal or mixed, tangible or intangible, wherever located).
|
|
|
1.3 | “Acquired IP” | means the Seller IP as set forth in Schedule 1.3 attached hereto, including the “DeepSolar™” trade name and the Acquired Technology. The Acquired IP includes also all income, royalties, damages and payments due or payable to Seller as of the Closing or thereafter with respect thereto (including damages and payments for past, present or future infringements or misappropriations thereof, the right to sue and recover for past, present or future infringements or misappropriations thereof and any and all corresponding rights that now or hereafter may be secured throughout the world, and all copies and tangible embodiments thereof). |
|
|
1.4 | “Acquired Technology” | means all designs, concepts, discoveries, inventions, products, computer programs, procedures, codes, software, improvements, developments, drawings, notes, documents, information and materials that were conceived, reduced to practice, invented, developed or created by Seller, whether alone or jointly with others, which are set forth in Schedule 1.4, and all know how, web sites, domains, and all documents and files related to the Acquired Technology, including all source files (source codes) of the Acquired Technology, all as set forth in Schedule 1.4 attached hereto. |
|
|
1.5 | “Affiliate” | means any Person that, directly or indirectly through one or more intermediaries, controls or is controlled by or is under common control with a Person as such terms are used in and construed
under Rule 405 under the Securities Act. |
|
|
1.6 | “Current Available Resources” | means, the amount of cash on hand which Buyer as of the date of the Closing, which the Board of Directors of the Buyer, in its reasonable discretion, deems available, taking into account all
debts, liabilities of Buyer then due and the amounts which Buyer, in its reasonable discretion, deems necessary to expend or retain for working capital or to place into reserve for customary and usual claims with respect with the Buyer’s
operations.
|
|
|
1.7 | “Business” |
means the business of Seller, operated through the Acquired Assets, as conducted and proposed to be conducted as of immediately prior to the Closing Date.
|
|
|
1.8 | “Business Milestone” |
means ***. |
|
|
1.9 | “Commission” | means the United States Securities and Exchange Commission. |
|
|
1.10 | “Exchange Act” |
means the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder. |
|
|
1.11 | “Exempt Issuance” |
means the issuance of (a) Shares or options to purchase Shares to employees, officers, or directors of the Buyer pursuant to any stock or option plan of the Buyer duly adopted for such purpose, and (b) securities upon the exercise or exchange of or conversion of any Securities issued hereunder and/or other securities exercisable or exchangeable for or convertible into Ordinary Shares issued and outstanding on the date of this Agreement (including those under clause (a), provided that such securities have not been amended since the date of this Agreement to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock splits or combinations) or to extend the term of such securities. |
|
|
1.12 | “Free and Clear” | means free and clear of any liens, mortgage, foreclosure, right of first refusal, claims, encumbrances and any other similar right, whether contractual or in accordance with any applicable law (collectively, “Liens”). |
|
|
1.13 | “IIA” | means the Israeli Innovation Authority. |
|
|
1.14 | “IIA and MOE Programs” | means the grant programs of the IIA and Ministry of Economy and Industry, as listed in Schedule 1.10 |
|
|
1.15 | “Liens” | means a lien, charge, pledge, security interest, encumbrance, right of first refusal, pre-emptive right or other restriction. |
|
|
1.16 |
“Milestone Based Pre-Funded Warrant”
|
means a warrant to purchase one (1) Ordinary Share exercisable upon the fulfillment of the Business Milestone during a period of five and
half (5.5) years from the date of grant, with an exercise price equal to US$ 0.01, which shall be in the form of Schedule 1.16 attached hereto.
|
|
|
1.17 | “NASDAQ” | means the Nasdaq Capital Market . |
|
|
1.18 | “Ordinary Shares” | means Ordinary Shares, with no par value, of the Buyer. |
|
|
1.19 | “Ordinary Share Equivalents” | means any securities of the Buyer or the Subsidiaries which would entitle the holder thereof to acquire at any time Ordinary Shares, including, without limitation, any debt, preferred stock, right, option, warrant or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Ordinary Shares. |
|
|
1.20 | “Person” | means an individual or corporation, partnership, trust, incorporated or unincorporated association, joint venture, limited liability company, joint stock company, government (or an agency or subdivision thereof) or other entity of any kind. |
|
|
1.21 | “Pre-Funded Warrant” | means a warrant to purchase one (1) Ordinary Share, with an exercise price equal to US$ 0.01, which shall be in the form of Schedule 1.20 attached hereto. |
| 1.22 | "PRFX" | means ordinary share with no par value of the Buyer (also “Ordinary Share”). |
| 1.23 |
“Prospectus Supplement” |
means the supplement to the Prospectus complying with Rule 424(b) of the Securities Act that is filed with the Commission and delivered by the Buyer to Seller at the Closing.
|
|
|
1.24 | “Rule 144” |
means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the
same purpose and effect as such Rule.
|
|
|
1.25 | “Rule 424” | means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended or interpreted from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same purpose and effect as such Rule. |
|
|
1.26 | “Securities” | means the Shares, Warrants, and the Underlying Shares, and all securities issuable in connection therewith. |
|
|
1.27 | “Securities Act” | means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder. |
|
|
1.28 | “Shares” | means the Ordinary Shares purchased pursuant to Section Error! Reference source not found. of this Agreement. |
|
|
1.29 |
“Short Sales” | means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include locating and/or borrowing Ordinary Shares). |
|
|
1.30 |
“Subsidiary” | means any subsidiary of the Buyer as set forth in the SEC Reports, including any direct or indirect subsidiary of the Buyer formed or acquired after the date hereof. |
|
|
1.31 | “Transaction Documents” | means this Agreement, the Warrants, and all exhibits and schedules thereto and hereto and any other documents or agreements executed by any party hereto in connection with the transactions contemplated hereunder. |
|
|
1.32 | “Transfer Agent” | means Equiniti Trust Company, LLC, the current transfer agent of the Buyer, with a mailing address of 90 Park Ave, New York, NY 10016, USA, and any successor transfer agent of the Buyer. |
|
|
1.33 | “Underlying Shares” | means the Shares and the Ordinary Shares issued and issuable upon exercise of the Warrants. |
|
|
1.34 | “Seller Intellectual Property” | means the patents, trademarks, brand names, service marks, trade names, copyrights, domain names, trade secrets, know-how, inventions, designs, processes, works of authorship, computer programs and technical data and information and other intellectual property which were developed or will be developed until the Closing by Seller in relation to the Business. |
|
|
1.35 |
“Shrink-Wrap Licenses” | standard “shrink-wrap” or “click-through” or off-the- shelf software licenses. |
|
|
1.36 | “TASE” | means the Tel-Aviv Stock Exchange. |
|
|
1.37 | “Trading Day” | means a day on which the principal Trading Market is open for trading. |
|
|
1.38 | “Trading Market” | means any of the New York Stock Exchange, the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market or the Nasdaq Global Select Market, and any successor to any of the foregoing markets or exchanges. |
|
|
1.39 | “Transferred Employees” | means all of those employees of Seller listed in Schedule 1.15 attached hereto, who have actually signed the New Employment Agreements with Buyer prior to the Closing Date. |
|
|
1.40 | “VWAP” | means, for any security as of any date, the daily dollar volume-weighted average price for such security on the Trading Market during regular trading hours as reported by Bloomberg through its “Historical Prices – Px Table with Average Daily Volume” functions. |
|
|
1.41 | “Warrant-A” | means a warrant to purchase one (1) Ordinary Share exercisable upon either: (i) ***, OR (ii) *** (“Warrant A Milestone”). Subject to the aforesaid, the warrant is exercisable during a period of five and half (5.5) years from the date of grant at an exercise price equal to the average trading closing price of PRFX on the 5 days prior to the Board of Directors of the Buyer resolution to issue such warrant, which shall be in the form of Schedule 1.10 attached hereto |
|
|
1.42 | “Warrant-B” | means a warrant to purchase one (1) Ordinary Share, which shall vest only if during the two (2) year period following the Closing Date, either: (i) *** or (ii) *** (“Warrant B Milestone”), at an exercise price equal to US$ 6.4 (subject to adjustment for share splits, reverse share splits, reclassifications and the like). Once vested, the Warrants shall have a three (3) year exercise period, and which shall be in the form of Schedule 1.10 attached hereto |
|
|
1.43 | “Warrants” | means Warrants-A and Warrants-B, collectively. |
|
|
1.44 | “Warrant Shares” | means the Ordinary Shares issuable upon exercise of the Warrants. |
|
|
2. |
PURCHASE OF ACQUIRED ASSETS
|
|
|
2.1 |
At the Closing (as defined in Section 4 below) of the transaction envisaged under this Agreement, Seller shall assign, sell, transfer and convey to Buyer all rights, title and interest, in and to all the Acquired Assets, as of the Closing
Date, and Buyer shall purchase, acquire and accept from Seller, all rights, title and interest in the Acquired Assets, in consideration for the Consideration, as specified in Section 3 below.
|
|
|
2.2 |
Buyer does not hereby and will not assume or become liable for and shall not be obligated to pay or satisfy any obligation, debt or liability whatsoever, whether fixed, contingent or otherwise, of Seller (including any indebtedness or
other claim, liability, obligation or tax) arising out of the ownership or use or sale of the Acquired Assets prior to the Closing Date, whether or not disclosed on the Schedules attached hereto and regardless of when or by whom asserted,
other than Assumed Liabilities (collectively, the “Excluded Liabilities”). Without limiting the foregoing and for the avoidance of doubt, the Excluded Liabilities shall remain the responsibility and
obligation of Seller prior and after Closing, and such responsibility and obligation of Seller shall in no event be limited or otherwise affected by any provision of this Agreement.
|
|
|
2.3 |
For purposes of this Agreement, and without derogating from the generality of Section 2.2 above, “Excluded Liabilities” also includes (i) Seller liabilities or obligations under this Agreement; (ii)
any and all tax liability or obligation of Seller or its shareholders, (iii) Seller’s liabilities or obligations with respect to any debt or indebtedness of any kind; (iv) the Excluded AR (as defined below); (v) any accrued expenses of
Seller; (vi) any other current liabilities incurred by Seller; (vii) liabilities or obligations of Seller arising by reason of any violation or alleged violation of any federal, state, local or foreign law, rule or regulation, or any other
requirement of any governmental authority in the operation of the Business prior to the Closing Date; (viii) Seller’s liabilities or obligations arising out of or related to any breach or alleged breach by Seller of any Acquired Agreement
when such liability or obligation is asserted with respect to the period before the Closing Date; (ix) Seller's liabilities or obligations relating to any other legal action, claim, right of action or proceeding arising out of or in
connection with the conduction of Seller's business prior to the Closing Date, or any other conduct of Seller or its officers, directors, employees, consultants, agents or advisors on or prior to the Closing Date; (x) Seller liabilities or
obligations relating to its employees, other than Buyer Employee Liabilities (as defined below); (xi) any liability of Seller to any of its shareholders or security holders or any affiliate thereof; (xii) any liability to indemnify, reimburse
or advance amounts to any officer, director, manager, employee or agent of Seller, other than Buyer Employee Liabilities; (xiii) any liability under any contract which is not an Acquired Agreement, including any liability arising out of or
relating to any employment or similar agreement to which Seller is a party or otherwise bound; (xiv) Seller’s liabilities or obligations for tort claims, known or unknown, and any related claims and litigation arising prior to, on or after
the Closing Date with respect to such tort claims; and (xv) any liability or obligation of Seller not expressly assumed by Buyer under this Agreement.
|
|
|
2.4 |
Following the Closing, Seller hereby agrees to sign any and all other instruments of sale, transfer, conveyance and assignment documents reasonably required in order to more effectively transfer and assign to Buyer all and any rights in
the Acquired Assets.
|
|
|
2.5 |
For avoidance of any doubt, it is hereby clarified that, following the transfer of the Acquired Assets from Seller to Buyer, Buyer will be the sole and exclusive owner and will hold all and any of the rights and title of the Acquired
Assets with effect from the Closing Date.
|
|
|
2.6 |
Notwithstanding anything to the contrary hereunder, Acquired Assets shall not include any assets which are not expressly purchased by Buyer under this Agreement, and Seller shall retain all right, title, interest and claims in any such
assets, including the following: (i) Seller’s cash and cash equivalents, including holdings or investments; (ii) tax assets and credits; (iii) the Excluded AR; (iv) Seller’s financial, tax and personnel records (even if copies of which were
provided by Seller to Buyer); (v) all contracts that are not Acquired Agreements; (vi) Seller’s rights under or pursuant to this Agreement and the other transaction documents; and (vii) all claims, refunds, causes of action, rights of
recovery, rights of set-off and rights of recoupment to the extent relating to the assets described in the foregoing clauses (collectively, the “Excluded Assets”).
Without limiting the foregoing and for the avoidance of doubt, the Acquired Assets shall in no event include the Excluded Assets, and the Excluded Assets shall remain at the ownership of and the responsibility and obligation of Seller as an
Excluded Liability, and such ownership, responsibility and obligation of Seller shall in no event be limited or otherwise affected by any provision of this Agreement.
|
|
|
2.7 |
ASSUMPTION OF LIABILITIES
|
|
|
(a) |
Assumed Liabilities. At the Closing, subject to Section 2.7(e) below, the Buyer will irrevocably assume the following Liabilities, solely in respect of periods from and after the Closing, as
additional consideration for the Acquired Assets (collectively, the “Assumed Liabilities”):
|
|
|
(b) |
the Seller’s obligations under Transferred Contracts as assigned to the Buyer solely in respect of periods from and after the Closing and that do not relate in any respect to pre-Closing periods nor arise from breaches by the Seller
occurring prior to Closing. Notwithstanding, the Seller’s obligations under Transferred Contracts as assigned to the Buyer with respect to the contracts detailed in Schedule 2.7(B) to the agreement (excluding section 1 to Schedule )
shall commence from January 1st 2025;
|
|
|
(c) |
all Seller’s obligations and liabilities directly relating to the Acquired Assets, the ownership, use and/or operation of the Acquired Assets solely in respect of periods from and after the Closing and that do not relate in any respect to
pre-Closing periods nor arise from breaches by the Seller occurring prior to Closing;
|
|
|
(d) |
the Seller’s obligations and liabilities under the IIA and MOE Programs in accordance with the approvals received from the IIA and MOE; For avoidance of doubt the Buyer’s monetary liabilities solely in connection with the transfer of the
IIA and MOE Programs shall not exceed USD 15,000.
|
|
|
(e) |
Notwithstanding the above the Buyer assumed the Seller’s liabilities to the suppliers listed in Schedule 2.7(e) for the period commencing as of January 1, 2025 and forward subject to the Closing (it is clarified that the Buyer
shall not be liable for any Seller’s liabilities regarding the suppliers listed in Schedule 2.7(e) prior to January 1, 2025).
|
|
|
3. |
CONSIDERATION FOR THE ACQUIRED ASSETS
|
|
|
3.1 |
Issuance of Securities in the Buyer. The sole consideration to the Seller for the sale and transfer to the Buyer of the Acquired Assets shall be the issuance:
|
|
|
(a) |
at Closing of the following securities of the Buyer:
|
|
|
(i) |
178,769 Ordinary Shares representing 9.9% of the issued and outstanding share capital of Buyer (after such issuance) (the “Shares”).
|
|
|
(ii) |
223,792 Pre-Funded Warrants, which shall at any time be subject to the Equity Blocker (as defined below).
|
|
|
(iii) |
470,463 Milestone Pre-Funded Warrants, which shall at any time be subject to the Equity Blocker (as defined below).
|
|
|
3.2 |
Within ten (10) days from the date of this Agreement, the Buyer shall convene a general meeting of its shareholders. The agenda of such general meeting shall include a separate resolution proposing an increase in the authorized share
capital. Immediately Upon approval of the increase in the authorized share capital at such general meeting, the Buyer shall issue to the Seller the following securities:
|
|
|
(i) |
214,541 Milestone Pre-Funded Warrants, which shall at any time be subject to the Equity Blocker (as defined below).
|
|
|
(ii) |
1,087,565 Warrants-A to purchase 1,087,565 Ordinary Shares, which shall at any time be subject to the Equity Blocker.
|
|
|
(iii) |
1,087,565 Warrants-B to purchase 1,087,565 Ordinary Shares, which shall at any time be subject to the Equity Blocker.
|
|
|
(iv) |
The increase of the registered share capital of the Buyer is expected to be completed within no later than sixty (60) days from the date hereof.
|
|
|
3.3 |
The completion of the Business Milestone, Warrant A Milestone or Warrant B Milestone shall be assessed independently, without dependency on each other, so that achieving or failing to achieve one shall not impact eligibility for
recognition or compensation related to the other milestone.
|
|
|
3.4 |
It is hereby agreed that the Seller may not exercise any of the Pre-Funded Warrants, Milestone Pre- Funded Warrants, Warrants-A or Warrants-B held by it (or any assignee or transferee of the Seller), if, following such exercise, the Seller
(including any assignee or transferee) holds shares of the Buyer which exceed 9.99% of the issued and outstanding share capital of the Buyer (the “Equity Blocker”).
|
|
|
3.5 |
The consideration in securities (Shares, Pre-Funded Warrant, Milestone Pre-Funded Warrants, Warrants) shall be subject to applicable VAT, which shall be paid in cash by the Buyer to the Seller on the date of the securities allocation.
|
|
|
4. |
THE CLOSING
|
|
|
4.1 |
The closing of the transactions contemplated by this Agreement (the “Closing”) will take remotely via the exchange of documents and signatures, on any Trading Day on which all conditions set forth in
Sections 5 and 6 below have been satisfied or waived by the respective Party, but in no event later than February 28, 2025, or any other date as shall be mutually agreed upon by the Parties (the “Closing Date”).
|
|
|
4.2 |
The Closing Date shall be determined by mutual agreement within 4 days after the Seller has obtained all Required Consents (as defined below). If Seller has not been able to obtain all Required Consents within 30 days after signing this
Agreement, this Agreement shall be of no further force and effect, without any further liability on the part of any Party, and the Parties shall consider negotiating a replacement agreement.
|
|
|
4.3 |
At the Closing (or as regards subsection (a), by the Closing Date), the following deliverables shall be made:
|
|
|
(a) |
Executed copies of the New Employment Agreements between Buyer and each of the Transferred Employees.
|
|
|
(b) |
Buyer will provide a duly signed copy of a written resolution of Buyer’s Board of Directors, approving and authorizing Buyer’s execution of this Agreement, substantially in the form attached as Schedule
4.3(b).
|
|
|
(c) |
Seller will provide a duly signed copy of a written resolution of Seller’s Board of Directors, approving and authorizing Seller’s execution of this Agreement, substantially in the form attached as Schedule
4.3(c).
|
|
|
(d) |
Seller will provide a duly signed copy of a mutual waiver and release letter by and between Seller and each of the Transferred Employees, with respect to any employment or other relationship between the parties prior to the Closing Date,
in the form attached as Schedule
4.3Error! Reference source not found.. |
|
|
(e) |
The Buyer shall issue to the Seller the Securities, all subject to the Equity Blocker.
|
|
|
4.4 |
All transactions occurring at the Closing as specified above shall be deemed to take place simultaneously, and no transaction shall be deemed to have been completed and no document or certificate shall be deemed to have been delivered
until all transactions are completed and all documents delivered.
|
|
|
4.5 |
As part of the Closing, any (A)(i) invoices and accounts receivables and (ii) expenses and trade payables, in each case, with respect to the Acquired Assets in relation to the period prior to the Closing Date will, after the Closing Date,
be set by Seller and belong to it (the “Excluded AR” and “Excluded AP”, respectively), and any (B)(i) invoices and accounts receivables and (ii) expenses and
trade payables with respect to the Acquired Assets following the Closing Date will, after the Closing Date, be set by Buyer and belong to it (the “Acquired AR” and “Assumed
AP”; respectively).
|
|
|
4.6 |
Under certain circumstances, subject to Buyer's sole discretion, Buyer shall have the right to request Seller’s assistance, and Seller hereby undertakes to make reasonable commercial efforts to assist in the transfer and/or billing, with
regards to certain suppliers/customers under the Acquired Agreements until the complete transfer of such suppliers/customer to Buyer.
|
|
|
4.7 |
If there shall be technical delays with customers’ invoicing, then according to Buyer’s request, the Seller will invoice such customers after the Closing Date, and the Buyer will invoice the Seller on a back-to-back basis, until the
technical delays will be resolved.
|
|
|
5. |
CONDITIONS PRECEDENT TO BUYER’S OBLIGATIONS TO CLOSE
|
|
|
5.1 |
The representations and warranties of Seller set forth in this Agreement shall be true and correct in all material respects (unless qualified by materiality in which case such representation shall be true and correct in all respects) as of
the Closing Date as though made on the Closing Date (except to the extent that any such representation or warranty by its terms is limited to a specified date, in which case as of such specified date). Seller shall have performed or complied
in all material respects with its obligations contained in this Agreement required to be performed by it hereunder at or prior to the Closing.
|
|
|
5.2 |
There shall exist no valid Order (as defined below), statute, rule, regulation, executive order, stay, decree, judgment, or injunction which prohibits or prevents the consummation of the transactions contemplated by this Agreement.
|
|
|
5.3 |
Seller shall have delivered to Buyer a certificate dated as of the Closing Date, executed by the Seller, certifying that the conditions specified above have been satisfied, substantially in the form attached hereto as Schedule 5.3.
|
|
|
5.4 |
Seller shall have delivered or caused to be delivered to Buyer the deliverables that Seller is required to deliver under Section 4.2.
|
|
|
6. |
CONDITIONS PRECEDENT TO SELLER'S OBLIGATIONS TO CLOSE
|
|
|
6.1 |
All representations and warranties made by Buyer in this Agreement shall be true and correct in all material respects (unless qualified by materiality in which case such representation shall be true and correct in all respects) as of the
Closing Date as though made on the Closing Date (except to the extent that any such representation or warranty by its terms is limited to a specified date, in which case as of such specified date).
|
|
|
6.2 |
The Closing shall not result in the Seller exceeding the Equity Blocker.
|
|
|
6.3 |
Buyer shall have delivered or caused to be delivered to Seller the deliverables that Buyer is required to deliver under Section 4.2.
|
|
|
6.4 |
From the date hereof to the Closing Date, trading in the Shares shall not have been suspended by the Commission or the Buyer’s principal Trading Market, and, at any time prior to the Closing Date, trading in securities generally as
reported by Bloomberg L.P. shall not have been suspended or limited, or minimum prices shall not have been established on securities whose trades are reported by such service, or on any Trading Market, nor shall a banking moratorium have been
declared either by the United States or New York State authorities nor shall there have occurred any material outbreak or escalation of hostilities or other national or international calamity of such magnitude in its effect on, or any
material adverse change in, any financial market which, in each case, in the reasonable judgment of the Seller, makes it impracticable or inadvisable to purchase the Securities at the Closing.
|
|
|
7. |
SELLER'S REPRESENTATIONS AND WARRANTIES
|
|
|
(a) |
Seller is duly organized and validly existing under the laws of the State of Israel. Seller has full power and authority to enter into this Agreement and to perform its obligations hereunder.
|
|
|
(b) |
Seller is not a party to, subject to or bound by any note, bond, mortgage, indenture, deed of trust, agreement, lien, contract or other instrument or obligation or any statute, law, rule, regulation, judgment, order, writ, injunction, or
decree of any court, administrative or regulatory body, governmental agency, arbitrator, mediator or similar body, franchise or license, which would (i) conflict with or be breached or violated or the rights or obligations thereunder
accelerated, increased, extinguished or terminated (whether or not with notice or lapse of time or both) by the execution or delivery by Seller of this Agreement or (ii) prevent the carrying out of the transactions contemplated hereby, except
for the Required Consents. No permit, consent, waiver, approval or authorization of, or declaration to or filing or registration with, any third person or governmental authority is required in connection with the execution or delivery of this
Agreement by Seller, or the consummation by Seller of the transactions contemplated hereby. Any such required permit, consent, waiver, approval or authorization of, or declaration to or filing or registration (an item listed in Schedule 7.1, a “Required Consents”). As of the Closing Date, Seller has obtained all Required Consents. The execution of this Agreement and the consummation
of the transactions contemplated hereby will not, merely by virtue of the Seller executing this Agreement or consummating these transactions, result in the creation of any Liens against Seller or any of the Acquired Assets, or rights or
privileges of any of them, whether tangible or intangible, and whether owned or possessed or granted by Seller.
|
|
|
(a) |
Seller has good and marketable title to all of the Acquired Assets, Free and Clear and other than the Required Consents there exists no restriction on the transfer of such property contemplated hereunder (subject to applicable rules and
regulations under the IIA and MOE Programs).
|
|
|
(b) |
Other than the Excluded Assets and employees, the Acquired Assets, including the Acquired Agreements, constitute all assets (of any type, whether tangible or intangible) and property rights used by or at the direction of Seller in the
conduct of the Business.
|
|
|
(c) |
Upon the Closing Date, good and marketable title to the Acquired Assets shall be vested in Buyer Free and Clear (subject to applicable rules and regulations under the IIA and MOE Programs).
|
|
|
7.3 |
Schedule 1.3 contains a true, complete and accurate list of each of the following items of the registered Seller IP: patents, patent applications, statutory invention registrations and
invention disclosures; and registered trademarks, including but not limited to the DeepSolar™, service marks, domain names, trade names, registered copyrights and applications for and registrations of such copyrights which are included within
the Acquired IP. Seller has good, valid and legal title to, and is the sole and exclusive owner of all right, title and interest in and to Seller IP, Free and Clear (other than in connection with the Shrink-Wrap Licenses or IIA and MOE).
|
|
|
7.4 |
Except with respect to Shrink-Wrap Licenses, Seller IP includes all rights in Intellectual Property used in or necessary for the operations or conduct of the Business (as such operations are currently conducted).
|
|
|
7.5 |
(i) To the best knowledge of the Seller: the operation of the Business by Seller has not infringed, misappropriated or otherwise violated, and does not infringe, misappropriate or otherwise violate, any Intellectual Property of any person;
(ii) the use of Seller Intellectual Property does not conflict with any rights of any person. There is not and has not been any unauthorized use or disclosure, infringement, misappropriation or other violation of any Seller Intellectual
Property by any person. There has been no claim for infringement of intellectual property made or threatened, by or against Seller, and Seller has not received notice of any such claim; and (iii) there has not been any unauthorized disclosure
of any Intellectual Property by Seller and/or by any employees or officers of Seller.
|
|
|
7.6 |
Seller maintains policies and procedures regarding data security and privacy that are commercially reasonable and, in any event, in compliance with all its obligations to its customers and under applicable laws, and to the extent
applicable, the data protection or privacy laws of any other country with respect to controlling, processing and otherwise handling or using Personal Data (as such terms is defined in the GDPR) or otherwise (the “GDPR”). There has been no security breach relating to, violation of any security policy regarding, or unauthorized access or unauthorized use of, any data used by Seller or stored in the Business. The use and dissemination of
any and all data and information concerning individuals by their businesses is in compliance with all applicable privacy policies, terms of use, customer agreements and law, in all material respects. Other than the Required Consents, the
transactions contemplated to be consummated hereunder as of the Closing Date will not constitute a violation by Seller of any privacy policy, terms of use, customer agreements or applicable law relating to the use, dissemination, or transfer
of any such data or information.
|
|
|
7.7 |
Other than IIA and MOE, no institution provided facilities or funding for the development of any Seller IP. No institution has any rights in or with respect to any developments of any Seller IP made by any current or former employee or
contractor of Seller that relates in any manner to Seller IP.
|
|
|
7.8 |
There is no, and there has not been in the past three (3) years, (a) any suit, claim, litigation, proceeding (administrative, judicial, or in arbitration, mediation or alternative dispute resolution), governmental authority investigation,
or other litigation action (any of the foregoing, “Action”) pending or threatened (i) against Seller or involving the Business, any of the Acquired Assets, or (ii) in connection with the Business, any
of the shareholders, directors, officers, agents, or other personnel of Seller, including with respect to each of clauses (i) and (ii), any Action challenging, enjoining, or preventing this Agreement or the consummation of the transactions
contemplated hereby; or (b) Seller is not and has not been subject to any Order. “Order” shall mean any judgment, order, writ, injunction, or decree of any court or other governmental authority.
|
|
|
7.9 |
Seller has, at all times, maintained insurance as required by law or under any agreement to which Seller is or has been a party. All of such insurance policies are in full force and effect (with respect to the applicable coverage periods),
and Seller is not in default with respect to any of its obligations under any of such insurance policies.
|
|
|
7.10 |
Seller has conducted at all times its business relating to the Acquired Assets only in the ordinary course of business and in compliance, in all material respects, with all applicable laws consistent with its past practice and used
commercially reasonable efforts to preserve intact such assets, except as contemplated by this Agreement or the transactions contemplated hereby and (ii) there has been no event, circumstance, change, effect or occurrence that has had or
would reasonably be expected to have a material adverse effect on the value of the trade secrets of Seller or on the Acquired Assets, business, financial condition or results of operations of the Business or (b) prevents, or would be
reasonably likely to prevent the transactions contemplated hereby (a “Material Adverse Effect”).
|
|
|
7.11 |
There has not been in connection with the Business, and the Acquired Assets a default, breach or violation of any applicable law. To the knowledge of Seller, all of the suppliers/customers under the Acquired Agreements were and are in
compliance with such agreements.
|
|
|
7.13 |
Seller has provided Buyer with any and all information, instruments and accessories, including, but not limited to, programs, software, tools, licenses, know how, work methods, management interfaces etc. required to enable Buyer to operate
the Business and the Acquired Assets in the same manner, but not less than a reasonable manner, as they were operated by Seller prior to the Closing and the Closing Date.
|
|
|
7.14 |
No finder, broker, agent, or other intermediary, acting on behalf of Seller thereof is entitled to a commission, fee, or other compensation or obligation in connection with the negotiation or consummation of this Agreement or any of the
transactions contemplated hereby.
|
|
|
7.15 |
No insolvency proceeding of any character including bankruptcy, receivership, reorganization, composition or arrangement with creditors, voluntary or involuntary, affecting, Seller (other than as a creditor) or any of the Acquired Assets
are pending or are being contemplated by Seller, or are being threatened against Seller by any other Person, and Seller has not made any assignment for the benefit of creditors or taken any action in contemplation of which that would
constitute the basis for the institution of such insolvency proceedings. Immediately after giving effect to the consummation of the transactions contemplated by this Agreement, Seller will be able to pay the Excluded Liabilities as they
become due.
|
|
|
7.16 |
No representation or warranty made by Seller in this Agreement or in any of the schedules, attachments or the Exhibits hereto or any agreements contemplated hereby contain any untrue statement of material fact or omit a material fact
necessary to make each statement contained herein or therein, in light of the circumstances under which they were made, not misleading. There is no fact which has not been disclosed to Buyer of which Seller has knowledge and which would
reasonably likely have a Material Adverse Effect.
|
|
|
7.17 |
The Seller is acquiring the Securities as principal for its own account and has no direct or indirect arrangement or understandings with any other persons to distribute or regarding the distribution of such Securities (this representation
and warranty not limiting such Seller’s right to sell the Securities pursuant to a registration statement or otherwise in compliance with applicable federal and state securities laws). The Seller understands that the Securities are
“restricted securities” and have not been registered under the Securities Act or any applicable state securities law and is acquiring the Securities hereunder in the ordinary course of its business. The Seller is acquiring such Securities as
principal for his, her or its own account and not with a view to or for distributing or reselling such Securities or any part thereof in violation of the Securities Act or any applicable state securities law, has no present intention of
distributing any of such Securities in violation of the Securities Act or any applicable state securities law and has no direct or indirect arrangement or understandings with any other persons to distribute or regarding the distribution of
such Securities in violation of the Securities Act or any applicable state securities law (this representation and warranty not limiting such Seller’s right to sell such Securities pursuant to a registration statement or otherwise in
compliance with applicable federal and state securities laws).
|
|
|
7.18 |
The Seller is not purchasing the Securities as a result of a registration statement or any advertisement, article, notice or other communication regarding the Securities published in any newspaper, magazine or similar media or
broadcast over television or radio or presented ay any seminar or any other general solicitation or general advertisement.
|
|
|
7.19 |
Neither the Seller nor any person or entity with whom the Seller will share beneficial ownership of the Securities (each, a “Seller Covered Person”) is subject to any of the “Bad Actor” disqualifications described in Rule
506(d)(1)(i) to (viii) under the Securities Act (a “Seller Disqualification Event”). The Seller will notify the Company in writing, prior to the applicable Closing Date of (i) any Seller Disqualification Event relating to any Seller
Covered Person and (ii) any event that would, with the passage of time, reasonably be expected to become a Seller Disqualification Event relating to any Seller Covered Person, in each case of which such Seller is aware.
|
|
|
7.20 |
The Seller either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the
Securities, and has so evaluated the merits and risks of such investment. The Seller is able to bear the economic risk of an investment in the Securities and, at the present time, is able to afford a complete loss of such investment.
|
|
|
7.21 |
The Seller acknowledges that it has had the opportunity to review the Transaction Documents (including all exhibits and schedules thereto) and the SEC Reports and has been afforded (i) the opportunity to ask such questions as it has deemed
necessary of, and to receive answers from, representatives of the Company concerning the terms and conditions of the offering of the Securities and the merits and risks of investing in the Securities.
|
|
|
7.22 |
Such Seller is an entity duly incorporated or formed, validly existing and in good standing under the laws of the State of Israel with full right, corporate, partnership, limited liability company or similar power and authority to enter
into and to consummate the transactions contemplated by the Transaction Documents and otherwise to carry out its obligations hereunder and thereunder. The execution and delivery of the Transaction Documents and performance by the Seller of
the transactions contemplated by the Transaction Documents have been duly authorized by all necessary corporate, partnership, limited liability company or similar action, as applicable, on the part of the Seller. Each Transaction Document to
which it is a party has been duly executed by the Seller, and when delivered by the Seller in accordance with the terms hereof, will constitute the valid and legally binding obligation of the Seller, enforceable against it in accordance with
its terms, except: (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting enforcement of creditors’ rights generally, (ii) as limited by
laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be limited by applicable law. The Seller’s execution, delivery and
performance of this Agreement and the other Transaction Documents and the consummation by it of the transactions contemplated hereby do not and will not (i) conflict with or violate any provision of the Seller’s certificate or articles of
incorporation, bylaws or other organizational or charter documents, or (ii) conflict with or result in a violation of any law, rule, regulation, order, judgment, injunction, decree or other restriction of any court or governmental authority
to which the Seller is subject (including federal and state securities laws and regulations), or by which any property or asset of the Seller is bound or affected.
|
|
|
7.23 |
The Seller is not subject to any of the “Bad Actor” disqualifications described in Rule 506(d)(1)(i) to (viii) under the Securities Act. The Seller is not required to be registered as a broker-dealer under Section 15 of the Exchange Act
and the Seller is not a broker-dealer, nor an affiliate of a broker- dealer. If the Seller is not a U.S. Person, the Seller (i) acknowledges that the certificate(s) representing or evidencing the Shares contains a customary restrictive legend
restricting the offer, sale or transfer of any Shares except in accordance with the provisions of Regulation S, pursuant to registration under the Securities Act, or pursuant to an available exemption from registration, (ii) agrees that all
offers and sales by the Seller of Shares shall be made pursuant to an effective registration statement under the Securities Act or pursuant to an exemption from, or a transaction not subject to the registration requirements of, the Securities
Act, (iii) represents that the offer to purchase the Shares was made to the Seller outside of the United States, and the Seller was, at the time of the offer and will be, at the time of the sale and is now, outside the United States, (iv) has
not engaged in or directed any unsolicited offers to purchase Shares in the United States, (v) is neither a U.S. Person nor a Distributor (as such terms are defined in Rule 902(k) and 902(d), respectively, of Regulation S), (vi) has purchased
the Shares for its own account and not for the account or benefit of any U.S. Person, (vii) has not pre- arranged any sale with a purchaser in the United States, and (ix) is familiar with and understands the terms and conditions and
requirements contained in Regulation S, specifically, without limitation, each purchaser understands that the statutory basis for the exemption claimed for the sale of the Shares would not be present if the sale, although in technical
compliance with Regulation S, is part of a plan or scheme to evade the registration provisions of the Securities Act.
|
|
|
7.24 |
The Seller understands that the Shares are being offered and sold to it in reliance upon specific exemptions from the registration requirements of United States federal and state securities laws and the prospectus requirements of the laws
of the State of Israel and that the Company is relying upon the truth and accuracy of, and the Seller’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of the Seller set forth herein in order
to determine the availability of such exemptions and the eligibility of the Seller to acquire the Shares.
|
|
|
7.25 |
The Seller, either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the
Shares, and has so evaluated the merits and risks of such investment. The Seller is able to bear the economic risk of an investment in the Shares and, at the present time, is able to afford a complete loss of such investment.
|
|
|
8. |
BUYER’S REPRESENTATIONS AND WARRANTIES
|
|
|
8.1 |
Buyer is a company duly organized and validly existing under the laws of the State of Israel. Buyer has all requisite power and authority to execute and deliver this Agreement and any related agreements contemplated herein, to perform its
obligations hereunder and thereunder and to consummate the transactions contemplated hereby and thereby. This Agreement constitutes a valid and binding obligation of Buyer, enforceable against them in accordance with its respective terms,
except to the extent that enforceability may be limited by bankruptcy, insolvency and other similar laws affecting the rights and remedies of creditors generally.
|
|
|
8.2 |
The execution and delivery of this Agreement and any related agreements contemplated herein do not or will not, and the consummation of the transactions contemplated hereby and thereby will not, conflict with, or result in any violation of
or default (with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of any obligation under (a) any provision of Buyer’s organizational documents, or (b) any agreement or
instrument, permit, license, judgment, order, statute, law, ordinance, rule or regulation applicable to Buyer or their properties or assets, except where such violations or conflicts would not reasonably be expected to prevent or materially
delay Buyer from consummating the transactions contemplated hereunder.
|
|
|
8.3 |
No approval, order, authorization waiver or consent of, or registration, declaration, notice or filing with any court or other federal, state, local or other governmental authority or any third party is required for the execution, delivery
and performance by Buyer of this Agreement and any related agreements contemplated herein, or the consummation by Buyer of the transactions contemplated hereby and thereby, other than: (i) the filings required pursuant to this Agreement, (ii)
the filing with the Commission of the Registration Statement (as defined below), (iii) receipt of corporate approvals, (iv) the filing of Form D with the Commission and such filings as are required to be made under applicable state securities
laws, and (v) any required filing with NASDAQ or NASDAQ approval (collectively, the “Required Approvals”).
|
|
|
8.4 |
The Buyer has the requisite corporate power and authority to enter into and to consummate the transactions contemplated by this Agreement and each of the other Transaction Documents to which it is a party and otherwise to carry out its
obligations hereunder and thereunder. The execution and delivery of this Agreement and each of the other Transaction Documents by the Buyer and the consummation by the Buyer of the transactions contemplated hereby and thereby have been duly
authorized by all necessary action on the part of the Buyer and no further action is required by the Buyer, the Board of Directors or the Buyer’s shareholders in connection herewith or therewith except for the Required Approvals. This
Agreement and each other Transaction Document to which the Buyer is a party has been (or upon delivery will have been) duly executed by the Buyer and, when delivered in accordance with the terms hereof and thereof, will constitute the valid
and binding obligation of the Buyer enforceable against the Buyer in accordance with its terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general
application affecting enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and
contribution provisions may be limited by applicable law or public policy.
|
|
|
8.5 |
As of the date hereof the authorized, issued and outstanding share capital of the Buyer was as set forth in Schedule 8.5 attached hereto. All of the issued and outstanding share capital of the Buyer have been duly authorized and
validly issued and are fully paid and non-assessable; none of the outstanding shares of capital stock of the Buyer was issued in violation of the preemptive or other similar rights of any security holder of the Buyer. No Person has any right
of first refusal, preemptive right, right of participation, or any similar right to participate in the transactions contemplated by the Transaction Documents. Except as set forth in the SEC Reports, as contemplated by this Agreement or with
respect to awards of restricted stock units under the Buyer’s equity incentive plans since its most recently filed periodic report under the Exchange Act, there are no outstanding options, warrants, scrip rights to subscribe to, calls or
commitments of any character whatsoever relating to, or securities, rights or obligations convertible into or exercisable or exchangeable for, or giving any Person any right to subscribe for or acquire, any Ordinary Shares, or contracts,
commitments, understandings or arrangements by which the Buyer or any Subsidiary is or may become bound to issue additional Ordinary Shares or Ordinary Share Equivalents (including, for the avoidance of doubt, as a result of entering into
this Agreement). There are no outstanding securities or instruments of the Buyer or any Subsidiary with any provision that adjusts the exercise, conversion, exchange or reset price of such security or instrument upon an issuance of securities
by the Buyer or any Subsidiary. There are no outstanding securities or instruments of the Buyer or any Subsidiary that contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by
which the Buyer or any Subsidiary is or may become bound to redeem a security of the Buyer or such Subsidiary. The Buyer does not have any stock appreciation rights or “phantom stock” plans or agreements or any similar plan or agreement. All
of the outstanding shares of the Buyer are duly authorized, validly issued, fully paid and nonassessable, have been issued in compliance with the Israeli Companies Law, 5759-1999 (the “Companies Law”), as amended, and the regulations
promulgated thereunder, and all federal and state securities laws, and none of such outstanding shares was issued in violation of any preemptive rights or similar rights to subscribe for or purchase securities. There are no shareholders
agreements, voting agreements or other similar agreements with respect to the Buyer’s share capital to which the Buyer is a party or between or among any of the Buyer’s shareholders.
|
|
|
8.6 |
The Buyer has the requisite corporate power and authority to enter into and to consummate the transactions contemplated by this Agreement and each of the other Transaction Documents and otherwise to carry out its obligations hereunder and
thereunder. The execution and delivery of this Agreement and each of the other Transaction Documents by the Buyer and the consummation by it of the transactions contemplated hereby and thereby have been duly authorized by all necessary action
on the part of the Buyer and no further action is required by the Buyer, the Board of Directors or the Buyer’s shareholders in connection herewith or therewith other than in connection with the Required Approvals. This Agreement and each
other Transaction Document to which it is a party has been (or upon delivery will have been) duly executed by the Buyer and, when delivered in accordance with the terms hereof and thereof, will constitute the valid and binding obligation of
the Buyer enforceable against the Buyer in accordance with its terms, except (i) as limited by general equitable principles and applicable bankruptcy, insolvency, reorganization, moratorium and other laws of general application affecting
enforcement of creditors’ rights generally, (ii) as limited by laws relating to the availability of specific performance, injunctive relief or other equitable remedies and (iii) insofar as indemnification and contribution provisions may be
limited by applicable law.
|
|
|
8.7 |
The Buyer has filed all reports, schedules, forms, statements and other documents required to be filed by the Buyer under the Securities Act and the Exchange Act, including pursuant to Section 13(a) or 15(d) thereof, for the two years
preceding the date hereof (or such shorter period as the Buyer was required by law or regulation to file such material) (the foregoing materials, including the exhibits thereto and documents incorporated by reference therein, and including
all reports on Form 6-K, being collectively referred to herein as the “SEC Reports”) on a timely basis or has received a valid extension of such time of filing and has filed any such SEC Reports
prior to the expiration of any such extension. .
|
|
|
8.8 |
Except as set forth in the SEC Reports, the Buyer is in compliance with the provisions of the rules and regulations promulgated by the Trading Market on which any of the securities of the Buyer are listed or designated and has no reason to
believe that it will not in the foreseeable future continue to be in compliance with all such listing and maintenance requirements. The Buyer is in compliance with applicable Nasdaq continued listing requirements within the terms of the
deficiency letter received by the Buyer from Nasdaq on November 7, 2024. There are no proceedings pending or threatened against the Buyer relating to the continued listing of the Ordinary Shares on Nasdaq, and, other than the deficiency
letter received by the Buyer from Nasdaq on November 7, 2024, the Buyer has not received any notice of, nor is there any reasonable basis for, the delisting of the Ordinary Shares from Nasdaq.
|
|
|
8.9 |
The Buyer is not, and is not an Affiliate of, and immediately after receipt of payment for the Securities, will not be or be an Affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended. The
Buyer shall conduct its business in a manner so that it will not become an “investment company” subject to registration under the Investment Company Act of 1940, as amended.
|
|
|
8.10 |
Except as described in the SEC Reports, no Person has any right to cause the Buyer or any Subsidiary to effect the registration under the Securities Act of any securities of the Buyer or any Subsidiary, except for the Seller. As of the
date of this Agreement and the Closing Date, the Buyer nor any Subsidiary is or has ever been a “shell company” (as defined in Rule 405 of the Securities Act).
|
|
|
8.11 |
Assuming the accuracy of the Seller’ representations and warranties set forth in Section 7 hereof, neither the Buyer, nor any of its Affiliates, nor any Person acting on its or their behalf has, directly or indirectly, made any offers or
sales of any security or solicited any offers to buy any security, under circumstances that would cause this offering of the Securities to be integrated with prior offerings by the Buyer for purposes of (i) the Securities Act which would
require the registration of the Shares, Warrants or Underlying Shares under the Securities Act, or (ii) any applicable shareholder approval provisions of any Trading Market on which any of the securities of the Buyer are listed or designated.
|
|
|
8.12 |
The Buyer acknowledges and agrees that the Seller is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated thereby. The Buyer further acknowledges that the
Seller is not acting as a financial advisor or fiduciary of the Buyer (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated thereby and any advice given by the Seller or any of its
representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely incidental to the Seller’s purchase of the Securities. The Buyer further represents to the Seller that the Buyer’s
decision to enter into this Agreement and the other Transaction Documents has been based solely on the independent evaluation of the transactions contemplated hereby by the Buyer and its representatives.
|
|
|
8.13 |
The Buyer has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Buyer to
facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or, paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any Person any compensation for soliciting
another to purchase any other securities of the Buyer, other than, in the case of clauses (ii) and (iii).
|
|
|
8.14 |
The issuance and sale of the Securities hereunder does not contravene the rules and regulations of any Trading Market upon which the Buyer’s securities are traded or listed.
|
|
|
8.15 |
With respect to the Securities to be offered and sold hereunder in reliance on Rule 506 under the Securities Act, none of the Buyer, any of its predecessors, any affiliated issuer, any director, executive officer, other officer of the
Buyer participating in the offering hereunder, any beneficial owner of 20% or more of the Buyer’s outstanding voting equity securities, calculated on the basis of voting power, nor any pro moter (as that term is defined in Rule 405 under the
Securities Act) connected with the Buyer in any capacity at the time of sale (each, an “Issuer Covered Person” and, together, “Issuer Covered Persons”) is subject to any of the “Bad Actor” disqualifications described in
Rule 506(d)(1)(i) to (viii) under the Securities Act (a “Disqualification Event”), except for a Disqualification Event covered by Rule 506(d)(2) or (d)(3). The Buyer has exercised reasonable care to determine whether any Issuer Covered
Person is subject to a Disqualification Event. The Buyer has complied, to the extent applicable, with its disclosure obligations under Rule 506(e), and has furnished to the Seller a copy of any disclosures provided thereunder. The Buyer will
notify the Seller in writing, prior to the Closing Date of (i) any Disqualification Event relating to any Issuer Covered Person and (ii) any event that would, with the passage of time, become a Disqualification Event relating to any Issuer
Covered Person.
|
|
|
8.16 |
The Buyer’s accounting firm is PwC Israel. To the knowledge and belief of the Buyer, such accounting firm (i) is a registered public accounting firm as required by the Exchange Act and (ii) shall express its opinion with respect to the
financial statements to be included in the Registration Statement and other Buyer filings with the Commission and will express its opinion for the fiscal year ending December 31, 2024.
|
|
|
8.17 |
There are no disagreements of any kind presently existing, or reasonably anticipated by the Buyer to arise, between the Buyer and the accountants and lawyers formerly or presently employed by the Buyer and the Buyer is current with respect
to any fees owed to its accountants and lawyers which could affect the Buyer’s ability to perform any of its obligations under any of the Transaction Documents.
|
|
|
8.18 |
Neither the Buyer nor any Subsidiary or any related entities (i) is required to register as a “broker” or “dealer” in accordance with the provisions of the Exchange Act or (ii) directly or indirectly through one or more intermediaries,
controls or is a “person associated with a member” or “associated person of a member” (within the meaning set forth in the FINRA Manual).
|
|
|
8.19 |
From the time of the initial filing of the Buyer’s first registration statement with the Commission through the date hereof, the Buyer has been and is an “emerging growth company,” as defined in Section 2(a) of the Securities Act.
|
|
|
8.20 |
The Buyer qualifies as a “foreign private issuer” within the meaning of Rule 405 under the Securities Act.
|
|
|
8.21 |
The Buyer and the Subsidiaries are in compliance with any and all applicable requirements of the Sarbanes-Oxley Act of 2002, as amended, that are effective as of the date hereof and as of the Closing Date, and any and all applicable rules
and regulations promulgated by the Commission thereunder that are effective as of the date hereof and as of the Closing Date.
|
|
|
8.22 |
No finder, broker, agent, or other intermediary, acting on behalf of Buyer, is entitled to a commission, fee, or other compensation or obligation in connection with the negotiation or consummation of this Agreement or any of the
transactions contemplated.
|
|
|
8.23 |
Without derogating from the representations and warranties in Section 7 above, Buyer is an experienced buyer, and is knowledgeable, sophisticated and experienced in making decisions with respect to purchases of the type contemplated by
this Agreement. The Buyer acknowledges that it has sufficient experience and knowledge of the Business in order to evaluate and bear the risks involved in this Agreement.
|
|
|
8.24 |
For a period of 12 months following the Closing, the Buyer undertakes to continue the development of the Acquired Assets and the Business in accordance with the business plan attached hereto as Schedule 8.6 (the “Business Plan”). The Buyer herein commits to a minimum utilization amount of US$50,000 per month in order to develop the Acquired Assets and the Business, all in accordance with the Business Plan.
|
|
|
9. |
. MUTUAL DUE DILIGENCE
|
|
|
9.1 |
Prior to the Closing Date, the Buyer and its authorized representatives (including its attorneys and accountants), received access to, reviewed and inspected, books and records, contracts, personal files, financial data and other
information requested by it in the course of the due diligence process conducted by it, including without limitations, with regard to Seller's respective business, financial condition, assets, liabilities and employees (and employees related
matters).
|
|
|
9.2 |
As of the date of this Agreement, Buyer and its authorized representatives received access to all information requested by it in the course of the due diligence process except in respect to the items listed in Schedule 9.1.
|
|
|
9.3 |
Each Party has experience in evaluating transactions of this type and in the purchase or, as applicable, sale of assets and assumption of liabilities of the same general nature as the Acquired Assets. Each party acknowledges that it can
bear the risks of the transaction contemplated hereunder, taking into account the provisions of this Agreement, and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of
the transactions contemplated under this Agreement.
|
|
|
9.4 |
Each Party represents that it has received all information it has requested and considered necessary or appropriate for deciding whether to enter into this Agreement and purchase or, as applicable, sell the Acquired Assets and consummate
the transaction contemplated hereby.
|
|
|
9.5 |
Nothing herein shall effect or impair the right of any Party to rely and act upon the representation and warranties of the counter party expressly provided in this Agreement.
|
|
|
10. |
TRANSFERRED EMPLOYEES
|
|
|
10.1 |
Buyer shall notify the Seller of the identity of the Transferred Employees, at least 10 days prior to the Closing Date. Following such notification, Seller shall terminate the employment or consultancy, as applicable, of all Transferred
Employees effective as of Closing Date in accordance with the terms of employment of such employees with Seller and to the fullest extent dictated by the applicable law, and shall, immediately after Closing, take such action as is necessary
to (i) release of any amounts accumulated with respect to any pension program to which Seller has made contributions or been obligated to make contributions with respect to such employee, (ii) transfer ownership of any Insurance Managers’ (bituach minhalim) or advanced study (keren hishtalmut) policies to such Transferred Employees, (iii) make payments to such Transferred Employees with respect to
amounts that may be due to such terminated employees in connection with the Seller's election to redeem the pre- termination notice period under the applicable contract or law, (iv) make payments to such Transferred Employees with respect to
amounts that would be due under applicable law or contract with respect to accumulated but unused recuperation days, and vacation or (iv) make any other payments to such Transferred Employees that would be due in connection with their
employment with Seller or in connection with such termination of employment or consultancy thereof.
|
|
|
10.2 |
In connection with the foregoing, Seller shall complete and file any necessary forms (including Form 161) including with respect to deceleration according to law, extension order, contract, agreement, or similar arrangement (gmar heshbon) for all such Transferred Employees. It is hereby acknowledged and agreed that to the extent that any of the amounts accumulated under any of the insurance or other funds to which Seller has
previously made contributions with respect to any such Transferred Employee (including any pension fund, Managers’ Insurance, or advanced study fund) are not sufficient at Closing to cover all such amounts to which any Transferred Employee is
entitled through the Closing Date under applicable law or contract (the “Seller Existing Funds”), Seller shall, without any consideration or adjustment of the Consideration, make cash payments with
respect to any such deficiency so that Seller Existing Funds are sufficient at Closing to cover all such amounts to which any Transferred Employee is entitled through the Closing Date (whether by law or contract). Attached hereto as Schedule 10.2 are the termination of employment agreements executed between Seller and the Transferred Employees.
|
|
|
10.3 |
Seller shall make best efforts to assist Buyer in the process of hiring all the Transferred Employees.
|
|
|
10.4 |
In no event will Seller seek to enforce any provision or law that purports to restrict such Transferred Employee from providing services to Buyer on or after the Closing Date, and Seller hereby waives its rights under any such provision or
law.
|
|
|
10.5 |
It is hereby agreed and clarified that Buyer shall employ each Transferred Employee under the terms provided for in the New Employment Agreement entered into with such Transferred Employee and that all obligations and liabilities to
Transferred Employees from and after the Closing Date (“Buyer Employee Liabilities”) shall be borne by Buyer only. It being understood, however, that Buyer shall not and shall not be obligated to give
any such Transferred Employees credit for their employment with Seller for purposes of vacation programs, sick pay programs and other ordinary course accruals benefit programs, and any claims by any employee (or on their behalf) for claims
regarding the period in which such employee(s) worked at the Seller shall be included in the Excluded Liabilities, and shall remain the responsibility and obligation of Seller which is not affected by any provision of this Agreement.
|
|
|
11. |
SELLER COVENANTS
|
|
|
11.1 |
Support. Seller will ensure that during the first thirty (30) days following the Closing, Mr. Chagay Climor and [ ] will cooperate, free of charge of any kind, with Buyer to support the Business and its transfer to Buyer,
upon Buyer’s reasonable request.
|
|
|
11.2 |
Non-competition. Seller agrees that it shall not, for a period of three (3) years after the Closing, directly or indirectly, compete, assist in or provide financial resources to any activity which competes with the Business (“Competing Business”), as now carried on, anywhere in the world; provided, that the foregoing shall not prohibit Seller from (i) owning 5% or less of the outstanding equity or debt securities of a publicly
traded entity.
|
|
|
11.3 |
Non-Disclosure. Following the Closing, Seller shall, and shall cause its directors and shareholders to keep confidential and not to disclose or use for its benefit or for the benefit of any other person, any and all trade secrets,
or confidential matters concerning the Business or the Acquired Assets, including unpublished financial information, secrets, customer lists and credit records, employee data, sales representatives and their territories, mailing lists, any
Intellectual Property and any other research or business information, in each case, concerning the Business, or the Acquired Assets which Seller currently treats as confidential (whether or not a trade secret under applicable law) (the “Business Confidential Information”); provided however that Seller may retain copies of such Business Confidential Information solely for accounting, legal compliance and litigation of claims. If Seller is
obligated to disclose any such Business Confidential Information pursuant to applicable law, regulation or legal process, then Seller shall, if lawfully permitted, provide Buyer with prompt written notice before any such disclosure sufficient
to enable Buyer either to seek a protective order preventing or prohibiting such disclosure. If Buyer does not obtain such protective order, then Seller will furnish only that portion of such Business Confidential Information that is legally
required, and will exercise reasonable efforts to obtain assurance that confidential treatment will be accorded such disclosed information.
|
|
|
11.4 |
Non-solicitation. Seller agrees that, during a period of three (3) years after the Closing, it shall not (i) directly or indirectly, solicit, encourage to leave employment, or hire any officer or employee of Buyer or any person who
at the time of proposed hire by Seller had been an officer or employee of Buyer within the previous 12 months, (ii) induce or attempt to induce, or assist anyone else to induce or attempt to induce, any customer or vendor of the Business to
reduce or discontinue its business with Buyer or disclose to anyone else the name and/or requirements of any such customer; or (iii) approach or attempt to approach, or assist anyone else to approach or attempt to approach, any customer of
the Business in order to suggest any services competing with the Business
|
|
|
11.5 |
Enforceability. Seller hereby acknowledges the broad territorial scope of the covenant contained above, but acknowledges and agrees that the restrictions are reasonable and enforceable in view of, among other things, (i) the narrow
range of activities prohibited, (ii) the national and international markets in which the Business now, or in the future will, operate and in which the products of the Business are, or will be, sold, (iii) the confidential, proprietary and
trade secret information of the Business to which Seller had and/or may have had access, (iv) the fact that a business which competes with the Business could greatly benefit if it were to obtain the confidential information of the Business,
(v) the sale and/or transfer of the Acquired IP pursuant to this Agreement, and (vi) the fact that Seller would have an unfair competitive advantage if any it were allowed to engage in the competitive activities prohibited above in light of
the confidential, proprietary and trade secret information that Seller had as of the Closing Date. In addition, Seller agrees that in the event of any breach thereof, the harm to Buyer will be irreparable and without adequate remedy at law
and therefore that injunctive relief with respect thereto will be appropriate. In the event that a court of competent jurisdiction determines, in an Action brought by or on behalf of Buyer or Seller, that any of the foregoing provisions are
unenforceable as stated, the Parties intend that such restrictions be modified to permit the maximum enforceable restriction on Seller’s competition with the Business.
|
|
|
11.6 |
Use of Business Confidential Information; Prohibition on Insider Trading. Seller hereby acknowledges that Buyer securities are listed for trading on NASDAQ and Buyer hereby acknowledges that Seller securities are listed for trading
on TASE. Furthermore, each Party acknowledges that the other Party (i) Business Confidential Information may be considered as “inside information” pursuant to the applicable laws and regulations; and (b) is required to make certain
disclosures and publications under applicable laws which may include this Agreement and/or the Parties' discussions, such disclosure not to be deemed a breach of this Agreement. Additionally, reasonable disclosure of the contents and the
existence of this Agreement by a Party in connection with a due diligence inquiry and subject to customary confidentiality undertakings shall not be considered to be a breach of this Agreement
|
|
|
12. |
OTHER AGREEMENTS OF THE PARTIES
|
|
|
12.1 |
Removal of Legends.
|
|
|
(a) |
The Securities may only be disposed of in compliance with state and federal securities laws. In connection with any transfer of Securities other than pursuant to an effective registration statement or Rule 144, to the Buyer or to an
Affiliate of the Seller or in connection with a pledge as contemplated in Section 12.1(b), the Buyer may require the transferor thereof to provide to the Buyer an opinion of counsel selected by the transferor and reasonably acceptable to the
Buyer, the form and substance of which opinion shall be reasonably satisfactory to the Buyer, to the effect that such transfer does not require registration of such transferred Securities under the Securities Act.
|
|
|
(b) |
The Seller agrees to the imprinting, so long as is required by this Section 4.1, of a legend on any of the Securities in the following form:
|
|
|
(c) |
The Buyer acknowledges and agrees that the Seller may from time to time pledge pursuant to a bona fide margin agreement with a registered broker-dealer or grant a security interest in some or all of the Securities to a financial
institution that is an “accredited investor” as defined in Rule 501(a) under the Securities Act and, if required under the terms of such arrangement, the Seller may transfer pledged or secured Securities to the pledgees or secured parties.
Such a pledge or transfer would not be subject to approval of the Buyer and no legal opinion of legal counsel of the pledgee, secured party or pledgor shall be required in connection therewith. Further, no notice shall be required of such
pledge. The Buyer will execute and deliver such reasonable documentation as a pledgee or secured party of Securities may reasonably request in connection with a pledge or transfer of the Securities, including, if the Securities are included
for registration in a registration statement, the preparation and filing of any required prospectus supplement under Rule 424(b)(3) under the Securities Act or other applicable provision of the Securities Act to appropriately amend the list
of selling shareholders thereunder.
|
|
|
(d) |
Certificates evidencing the Underlying Shares shall not contain any legend (“Unlegended Shares”) (including the legend set forth in Section 12.1(b) hereof): (i) while a registration statement
covering the resale of such security is effective under the Securities Act, or (ii) following any sale of such Underlying Shares pursuant to Rule 144, or (iii) if such Underlying Shares are eligible for sale under Rule 144 without information
requirements, or (iv) if such legend is not required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the Commission). The Buyer shall cause its counsel, at the
expense of the Buyer, to issue not more than one (1) legal opinion per calendar quarter to the Transfer Agent or the Seller promptly if required by the Transfer Agent to effect the removal of the legend hereunder, or if requested by the
Seller, respectively. If all or any portion of the Warrants are exercised at a time when there is an effective registration statement to cover the resale of the Underlying Shares, or if such Underlying Shares may be sold and the Buyer is then
in compliance with the current public information required under Rule 144 without the requirement for the Buyer to be in compliance with the current public information required under Rule 144 as to such Underlying Shares and without volume or
manner-of-sale restrictions under Rule 144 or if such legend is not otherwise required under applicable requirements of the Securities Act (including judicial interpretations and pronouncements issued by the staff of the Commission) then such
Underlying Shares shall be issued free of all legends. The Buyer agrees that following such time as such legend is no longer required under this Section 12.1(d), the Buyer will, no later than the later of (i) one (1) Trading Day and (ii) the
number of Trading Days comprising the Standard Settlement Period (as defined below) following the delivery by the Seller to the Buyer or the Transfer Agent of a certificate representing Underlying Shares, as applicable, issued with a
restrictive legend (such date, the “Legend Removal Date”), deliver or cause to be delivered to the Seller a certificate representing such shares that is free from all restrictive and other legends. The
Buyer may not make any notation on its records or give instructions to the Transfer Agent that enlarge the restrictions on transfer set forth in this Section 12. Certificates for Underlying Shares subject to legend removal hereunder shall be
transmitted by the Transfer Agent to the Seller by crediting the account of the Seller’s prime broker with the Depository Trust Company System as directed by the Seller. As used herein, “Standard Settlement
Period” means the standard settlement period, expressed in a number of Trading Days, on the Buyer’s primary Trading Market with respect to the Ordinary Shares as in effect on the date of delivery of a certificate representing
Underlying Shares, as applicable, issued with a restrictive legend.
|
|
|
12.2 |
Furnishing of Information.
|
|
|
12.3 |
Integration. The Buyer shall not sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the Securities Act) that would be integrated with the offer or sale of the
Securities in a manner that would require the registration under the Securities Act of the sale of the Securities or that would be integrated with the offer or sale of the Securities for purposes of the rules and regulations of any Trading
Market such that it would require shareholder approval prior to the closing of such other transaction or to effectuate such other transaction unless shareholder approval is obtained before the closing of such subsequent transaction or
effectuation of such other transaction.
|
|
|
12.4 |
Securities Laws Disclosure; Publicity. The Buyer shall (a) not later than the Business Day following the Closing Date, issue a press release disclosing the material terms of the transactions contemplated hereby, and (b) file a
Report on Form 6-K, including the Transaction Documents as exhibits thereto, with the Commission within the time required by the Exchange Act. From and after the issuance of such press release, the Buyer represents to the Seller that it shall
have publicly disclosed all material, non-public information delivered to any of the Seller by the Buyer or any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agents, in connection with the
transactions contemplated by the Transaction Documents. In addition, effective upon the issuance of such press release, the Buyer acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether
written or oral, between the Buyer or any of its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agents, on the one hand, and the Seller or any of its Affiliates on the other hand, shall terminate and be
of no further force or effect. The Buyer understands and confirms that the Seller shall be relying on the foregoing covenant in effecting transactions in securities of the Buyer. The Buyer and Seller shall consult with each other in issuing
any other press releases with respect to the transactions contemplated hereby, and neither the Buyer nor the Seller shall issue any such press release nor otherwise make any such public statement without the prior consent of the Buyer, with
respect to any press release of the Seller, or without the prior consent of the Seller, with respect to any press release of the Buyer, which consent shall not unreasonably be withheld or delayed, except if such disclosure is required by law,
in which case the disclosing party shall promptly provide the other party with prior notice of such public statement or communication. Notwithstanding the foregoing, the Buyer shall not publicly disclose the name of the Seller, or include the
name of the Seller in any filing with the Commission or any regulatory agency or Trading Market, without the prior written consent of the Seller, except (a) as required by federal securities law in connection with the filing of final
Transaction Documents with the Commission and (b) to the extent such disclosure is required by law or Trading Market regulations, in which case the Buyer shall provide the Seller with prior notice of such disclosure permitted under this
clause (b) and reasonably cooperate with the Seller regarding such disclosure.
|
|
|
12.5 |
Shareholder Rights Plan. No claim will be made or enforced by the Buyer or, with the consent of the Buyer, any other Person, that the Seller is an “Acquiring Person” under any control share acquisition, business combination,
poison pill (including any distribution under a rights agreement) or similar anti- takeover plan or arrangement in effect or hereafter adopted by the Buyer, or that the Seller could be deemed to trigger the provisions of any such plan or
arrangement, by virtue of receiving Securities under the Transaction Documents or under any other agreement between the Buyer and the Seller.
|
|
|
12.6 |
Non-Public Information. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, which shall be disclosed pursuant to Section Error! R
eference source not found., the Buyer covenants and agrees that neither it, nor any other Person acting on its behalf will provide the Seller or its agents or counsel with any information that constitutes, or the Buyer reasonably
believes constitutes, material non-public information, unless prior thereto the Seller shall have consented in writing to the receipt of such information and agreed in writing with the Buyer to keep such information confidential. The Buyer
understands and confirms that the Seller shall be relying on the foregoing covenant in effecting transactions in securities of the Buyer. To the extent that the Buyer or any of its Subsidiaries, or any of their respective officers, directors,
agents, employees or Affiliates delivers any material, non-public information to the Seller without the Seller’s consent, the Buyer hereby covenants and agrees that the Seller shall not have any duty of confidentiality to the Buyer or any of
its Subsidiaries, or any of their respective officers, directors, employees, Affiliates or agents, including, without limitation, the Placement Agent, or a duty to the Buyer or any of its Subsidiaries, or any of their respective officers,
directors, employees, Affiliates or agents, including, without limitation, the Placement Agent, not to trade on the basis of, such material, non-public information, provided that the Seller shall remain subject to applicable law. To the
extent that any notice provided pursuant to any Transaction Document constitutes, or contains, material, non-public information regarding the Buyer or any Subsidiary, the Buyer shall simultaneously with the delivery of such notice file such
notice with the Commission pursuant to a Report on Form 6-K. The Buyer understands and confirms that the Seller shall be relying on the foregoing covenant in effecting transactions in securities of the Buyer.
|
|
|
12.7 |
Reservation of Ordinary Shares. As of the date hereof, the Buyer has reserved and the Buyer shall continue to reserve and keep available at all times, free of preemptive rights, a sufficient number of Ordinary Shares for the purpose
of enabling the Buyer to deliver Shares at Closing according to this Agreement and any Underlying Shares pursuant to any exercise of Warrants.
|
|
|
12.8 |
Listing of Ordinary Shares. From the date hereof until two (2) years after the Closing Date, the Buyer hereby agrees to use reasonable best efforts to maintain the listing or quotation of the Ordinary Shares on the Trading Market on
which it is currently listed, and within sixty (60) days from the Closing, the Buyer shall apply to list or quote all of the Underlying Shares on such Trading Market (the “Listing of the Underlying Shares”)
and shall promptly secure the listing of all of the Underlying Shares on such Trading Market. The Buyer further agrees, if the Buyer applies to have the Ordinary Shares traded on any other Trading Market, it will then include in such
application all of the Underlying Shares, and will take such other action as is necessary to cause all of the Underlying Shares to be listed or quoted on such other Trading Market as promptly as possible. Until at least the Listing of the
Underlying Shares, the Buyer will take all action reasonably necessary to continue the listing and trading of its Ordinary Shares on a Trading Market and will comply in all material respects with the Buyer’s reporting, filing and other
obligations under the bylaws or rules of the Trading Market. The Buyer agrees to maintain the eligibility of the Ordinary Shares for electronic transfer through the Depository Trust Company or another established clearing corporation,
including, without limitation, by timely payment of fees to the Depository Trust Company or such other established clearing corporation in connection with such electronic transfer.
|
|
|
12.9 |
Subsequent Equity Sales.
|
|
|
(a) |
From the date hereof until ninety (90) days after the Closing Date, neither the Buyer nor any Subsidiary shall (i) issue, enter into any agreement to issue or announce the issuance or proposed issuance of any Ordinary Shares or Ordinary
Share Equivalents or (ii) file a registration statement or any amendment or supplement thereto, other than as contemplated pursuant to this Agreement.
|
|
|
(b) |
Notwithstanding the foregoing, Section 12.9(a) shall not apply in respect of any Exempt Issuance or any at-the-market offering of the Buyer (an “ATM”); provided
however, that in the event of an ATM, the price under which such shares are being sold on the ATM is more than US$***.
|
|
|
12.10 |
Certain Transactions and Confidentiality. Other than consummating the transactions contemplated hereunder, the Seller has not, nor has any Person acting on behalf of or pursuant to any understanding with the Seller, directly or
indirectly executed any purchases or sales, including Short Sales, of the securities of the Buyer during the period commencing as of the time that the Seller first received a term sheet (written or oral) from the Buyer or any other Person
representing the Buyer setting forth the material terms or pricing terms of the transactions contemplated hereunder and ending immediately prior to the execution hereof. Other than to other Persons party to this Agreement or to Seller’s
representatives, including, without limitation, its officers, directors, employees, Affiliates or agents, the Seller has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence
and terms of this transaction). Notwithstanding the foregoing, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to locating or borrowing shares in order
to effect Short Sales or similar transactions in the future.
|
|
|
12.11 |
Exercise Procedures. The form of Notice of Exercise included in the Warrants set forth the totality of the procedures required of the Seller in order to exercise the Warrants. No additional legal opinion, other information or
instructions shall be required of the Seller to exercise their Warrants. Without limiting the preceding sentences, no ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or
notarization) of any Notice of Exercise form be required in order to exercise the Warrants. The Buyer shall honor exercises of the Warrants and shall deliver the Underlying Shares in accordance with the terms, conditions and time periods set
forth in the Transaction Documents. Notwithstanding anything contained in this Agreement to the contrary, in no event shall the exercise price of any Warrant be adjusted below the par value of the Ordinary Shares.
|
|
|
12.12 |
Form D; Blue Sky Filings. To the extent required, the Buyer agrees to timely file a Form D with respect to the Securities as required under Regulation D and to provide a copy thereof, promptly upon request of the Seller. The Buyer
shall take such action as the Buyer shall reasonably determine is necessary in order to obtain an exemption for, or to qualify the Securities for, sale to the Seller at the Closing under applicable securities or “Blue Sky” laws of the states
of the United States and shall provide evidence of such actions promptly upon request of the Seller.
|
|
|
12.13 |
Registration Statement. As soon as practicable (and in any event within 60 calendar days of this Agreement), the Buyer shall file a registration statement on Form F-1 or Form F-3 providing for the resale by the Seller of the
Underlying Shares. The Buyer shall use commercially reasonable efforts to cause such registration statement to become effective (a) within three Trading Days after the Buyer or its counsel has been advised that the staff of the Commission has
“no review” or no further comments or (b) within 90 calendar days following the Closing Date, and to keep such registration statement effective at all times until the Seller no longer owns any Securities.
|
|
|
13. |
INDEMNITY, LIMITATION OF LIABILITY
|
|
|
13.1 |
Survival of Representations and Warranties and Covenants. The representations and warranties and covenants of the Parties made herein shall survive the Closing and continue in effect for a period of eighteen (18) months following
Closing Date, except that representations and warranties in Sections 7.1a, 7.2a, 7.15 and 7.16 (“Seller Fundamental Reps”) shall survive the Closing until the expiration of all applicable statutes of
limitations with respect to the matters addressed therein (including any extensions or tolling thereof). Any indemnity claims under this Agreement must be asserted by written notice within the applicable survival period contemplated by this
Section, and if such a notice is duly and in good faith given, the survival period for such matter shall continue until the claim is fully resolved.
|
|
|
13.2 |
Indemnification by Seller. Seller shall hold Buyer and its shareholders, directors, officers, partners, employees, successors, assigns, representatives and agents of each of them in their capacities as such (the “Buyer Indemnified Persons”), harmless and indemnify each of them from and against, and Seller waives any claim for contribution or indemnity from any of the Buyer Indemnified Persons with respect to, any
and all claims, losses, damages, liabilities, expenses or costs (“Losses”), plus reasonable attorneys’ fees, and expenses incurred in connection with Losses and/or enforcement of this Agreement and
interest on the amount of such Losses at the prime rate, as it appears in Buyer's bank, from the date that such Losses were incurred until the date of payment to the Indemnified Party (as defined below), determined based upon a 365-day year
(in all, “Indemnified Losses”) incurred by any of them resulting from or arising out of:
|
|
|
(a) |
Any breach of, or inaccuracy in, any representation or warranty made by Seller in this Agreement;
|
|
|
(b) |
Any nonfulfillment, non-performance, no observance or other breach or violation, or default in performance by Seller of, any covenant contained in this Agreement;
|
|
|
(c) |
The Excluded Liabilities or the failure at any time of Seller to pay or discharge or to have paid or discharged the same in full; and
|
|
|
(d) |
Any breach of the restricted obligations contained in Section 12 above.
|
|
|
13.4 |
Indemnification by Buyer. Buyer shall hold Seller and Seller's shareholders, directors, officers, partners, employees, successors, assigns, representatives and agents in their capacities as such (the “Seller Indemnified Persons”) harmless and indemnify each of them from and against any and all Indemnified Losses incurred by any of them, resulting from or arising out of:
|
|
|
(a) |
Any breach of, or inaccuracy in, any representation or warranty made by Buyer in this Agreement;
|
|
|
(b) |
Any nonfulfillment, non-performance, no observance or other breach or violation, or default in performance, by Buyer of any covenant or agreement of Buyer contained in this Agreement (together, the “Buyer
Liabilities”).
|
|
|
13.5 |
Notice of Claim. In the event that Buyer seeks indemnification on behalf of a Buyer Indemnified Person in accordance with this Agreement, or Seller seeks indemnification on behalf of a Seller Indemnified Person in accordance with
this Agreement, such Party seeking indemnification (the “Indemnified Party”) shall give reasonably prompt written notice to the indemnifying Party (the “Indemnifying
Party”) specifying the facts constituting the basis for such claim, the provisions in this Agreement that are alleged to invoke such indemnity claim, and the amount, to the extent known, of the claim asserted; provided, however,
that the right of a Person to be indemnified hereunder shall not be adversely affected by a failure to give such notice unless, and then only to the extent that, an Indemnifying Party is actually and materially damaged thereby. Subject to the
terms hereof, the Indemnifying Party shall pay (by wire transfer of immediately available funds) the amount of any valid indemnity claim not more than 30 days after the Indemnified Party provides notice to the Indemnifying Party of such
amount.
|
|
|
13.6 |
Right to Contest Claims of Third Persons
|
|
|
(a) |
If an Indemnified Party is entitled to indemnification hereunder because of a claim asserted by any claimant other than an Indemnified Person hereunder (a “Third Person”), the Indemnified Party shall
give the Indemnifying Party reasonably prompt notice thereof after such assertion is actually known to the Indemnified Party (together with all information provided by the Third Person as part of such claim); provided, however,
that the right of a Person to be indemnified hereunder in respect of claims made by a Third Person shall not be adversely affected by a failure to give such notice unless, and then only to the extent that, an Indemnifying Party is actually
and materially prejudiced thereby. Except as otherwise provided in this Section, the Indemnifying Party shall have the right, upon written notice to the Indemnified Party (a “Defense Notice”) within
thirty (30) days after receipt from the Indemnified Party of notice of such claim, and using counsel reasonably satisfactory to the Indemnified Party, to investigate, contest, or settle the claim alleged by such Third Person (a “Third Person Claim”), provided that the Indemnifying Party has unconditionally acknowledged to the Indemnified Party in writing its obligation to indemnify the Persons to be indemnified hereunder with
respect to such Third Person Claim and to discharge any cost or expense arising out of such investigation, contest or settlement. The Indemnified Party may thereafter participate in (but not control) the defense of any such Third Person Claim
with its own counsel at its own expense, unless separate representation is necessary to avoid a conflict of interest (it being understood that the mere fact of the indemnification hereunder shall not be deemed as a conflict), in which case
such representation shall be at the expense (to the extent reasonable) of the Indemnifying Party. Unless and until the Indemnifying Party so acknowledges its obligation to indemnify, the Indemnified Party shall have the right, at its option,
to assume and control defense of the matter and to look to the Indemnifying Party for the full amount of the reasonable costs of defense. In the event that the Indemnifying Party shall fail to give the Defense Notice within said thirty (30)
day period, (i) the Indemnified Party shall be entitled to have the control over said defense and settlement of the subject claim, (ii) the Indemnifying Party will cooperate with and make available to the Indemnified Party such assistance and
materials as it may reasonably request, and (iii) the Indemnifying Party shall have the right at its expense to participate in the defense assisted by counsel of its own choosing, and the Indemnifying Party, if it is required to provide
indemnification under this Agreement, will be liable for all costs and settlement amounts paid or incurred in connection therewith.
|
|
|
(b) |
In the event that the Indemnifying Party delivers a Defense Notice with respect to such Third Party Claim within thirty (30) days after receipt thereof and thereby elects to conduct the defense of the subject claim, (i) the Indemnifying
Party shall be entitled to have control over said defense and, subject to the provisions set forth below, settlement of the subject claim, (ii) the Indemnified Party will cooperate with and make available to the Indemnifying Party such
assistance and materials as it may reasonably request, and (iii) the Indemnified Party shall have the rights at its expense to participate in the defense assisted by counsel of its own choosing. In such an event, the Indemnifying Party will
not settle the subject claim without the prior written consent of the Indemnified Party, which consent will not be unreasonably withheld, conditioned or delayed unless (i) there is no finding or admission of any violation of Law or any
violation of the rights of any Indemnified Party; (ii) the sole relief provided is monetary damages that are paid in full by the Indemnifying Party; and (iii) the Indemnified Party shall have no liability with respect to any compromise or
settlement of such Third Person Claims effected without its consent, in which cases the consent of the Indemnified Party shall not be required.
|
|
|
(c) |
Notwithstanding anything to the contrary contained in this Section, the Indemnifying Party shall not be entitled to control, but may participate in, and the Indemnified Party shall be entitled to have sole control, including the right to
select defense counsel, over the defense or settlement of any claim (i) that seeks a temporary restraining order, a preliminary or permanent injunction or specific performance against the Indemnified Party or that seeks a remedy, action or
consequence other than monetary damages, (ii) that involves criminal allegations against the Indemnified Party, (iii) that, if successful, would set a precedent that would materially interfere with, or have a material adverse effect on, the
business or financial condition of the Indemnified Party or (iv) that imposes liability on the part of the Indemnified Party for which the Indemnified Party is not entitled to indemnification hereunder. In such event, the Indemnifying Party
will still be subject to its obligations hereunder but the Indemnified Party will not settle the subject claim without the prior written consent of the Indemnifying Party, which consent will not be unreasonably withheld, conditioned or
delayed.
|
|
|
(d) |
Except as set forth in the Disclosure Schedule, no information or knowledge acquired (or capable of being acquired), or investigations conducted, by any Party or its representatives, of the other Party (and with respect to Buyer, also the
Business, the Purchased Assets or the Buyer Liabilities or otherwise), shall in any way limit, or constitute a waiver of, or a defense to, any claim for indemnification under this Agreement.
|
|
|
13.7 |
Indemnity Caps.
|
|
|
(a) |
Except with respect to indemnity claims (i) based on fraud or willful and intentional misrepresentation or willful misconduct, or (ii) for any Excluded Liability, the maximum amount payable by Seller with respect to the indemnity claims
set forth in this Section 13, shall be limited to an amount of US$ 2 million, to be payable solely in the form of cancellation of the applicable portion of Warrant-A and Warrant-B, the value of which shall be equal to the amount to be paid as
indemnification.
|
|
|
(b) |
Except with respect to indemnity claims (i) based on fraud or willful and intentional misrepresentation or willful misconduct, or (ii) for any Buyer Liability, the maximum amount payable by Buyer with respect to the indemnity claims set
forth in this Section 13, shall be limited to an amount of US$ 2 million, to be payable solely in the form of the issuance of additional Pre- paid warrants of the Company.
|
|
|
13.8 |
Indemnity Basket. Except with respect to indemnity claims (i) based on fraud or willful and intentional misrepresentation or willful misconduct, or (ii) for any Excluded Liability (in the case of indemnity claims by Buyer
Indemnified Persons) or for any Buyer Liabilities (in the case of indemnity claims by Seller Indemnified Persons), no indemnity claim under Section 13 shall be brought against the Indemnifying Party until the aggregate of all Losses equals
US$ 100,000, in which case claims may be brought for the full amount of such Losses.
|
|
|
14. |
FURTHER ASSURANCE
|
|
|
15. |
NO PARTNERSHIP OR AGENCY
|
|
|
16. |
SEVERABILITY
|
|
|
17. |
NO WAIVER
|
|
|
18. |
NOTICE
|
|
|
Attention:
Email:
|
|
|
With a required copy to
(which shall not
constitute notice):
|
DTKGG Law Offices
B.S.R. 4 Tower, 33 Floor,
7 Metsada Street, Bnei Brak 5126112, Israel
Attention: rkantor@dtkgg.com
Email: Ronen Kantor, Adv.
|
|
|
|
|
|
| If to Seller : |
1 Hayasmin St, Ramat Efal, Israel | |
|
Attention: Ronen Tsioni. CEO
Email: ronen.tsioni@bladeranger.com
|
||
|
With a required copy to
(which shall not
constitute notice):
|
Victor Tshuva & Co., Law Offices
40 Tuval Street, Ramat Gan, Israel
Attention: Victor Tshuva, Adv./Asaf Peled, Adv.
Email: victor@vtlaw.com; asaf@vtlaw.com
|
|
|
19. |
EXPENSES
|
|
|
20. |
GOVERNING LAW AND JURISDICTION
|
|
|
21. |
ASSIGNMENT
|
|
|
22. |
ENTIRE AGREEMENT; AMENDMENT
|
|
Buyer
By:
Name & Title:
|
Seller
By:
Name & Title:
|
|
Warrant Shares: 223,792
|
Initial Exercise Date: [ ], 2025
|
|
|
c) |
Mechanics of Exercise.
|
|
PAINREFORM LTD.
|
|
By:__________________________________________
Name:
Title:
|
|
Name:
|
|
|
(Please Print)
|
|
|
Address:
|
|
|
Phone Number:
Email Address:
|
(Please Print)
______________________________________
______________________________________
|
|
Dated: _______________ __, ______
|
|
|
Holder’s Signature:
|
|
|
Holder’s Address:
|
|
Warrant Shares: 470,463
|
Warrant Date: [ ], 2025
|
|
|
c) |
Mechanics of Exercise.
|
|
PAINREFORM LTD.
|
|
By:__________________________________________
Name:
Title:
|
|
Name:
|
|
|
(Please Print)
|
|
|
Address:
|
|
|
Phone Number:
Email Address:
|
(Please Print)
______________________________________
______________________________________
|
|
Dated: _______________ __, ______
|
|
|
Holder’s Signature:
|
|
|
Holder’s Address:
|
|
Warrant Shares: 1,087,565
|
Warrant Date: [ ], 2025
|
|
|
a. |
Warrant A to purchase 1,087,565 out of the Warrant Shares shall vest upon the successful achievement of the Warrant A Milestone;
|
|
|
a) |
Exercise of Warrant. Subject to (i) the Closing of the Acquisition Agreement,
(ii) the provisions of Section 4(e) herein, and (iii) the successful achievement of the Warrant A Milestone, exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the date
of successful achievement of the Warrant A Milestone (the “Milestone Date”) and on or before the Warrant A Termination, by delivery to the Company of a duly executed facsimile copy or PDF copy submitted by e-mail (or e-mail attachment) of the
Notice of Exercise in the form annexed hereto (the “Notice of Exercise”). Within the earlier of (i) two (2) Trading Days and (ii) the number of Trading
Days comprising the Standard Settlement Period (as defined in Section 4(d)(i) herein) following the date of exercise as aforesaid, the Holder shall deliver the aggregate Warrant A Exercise Price for the shares specified in the applicable Notice
of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 4(c) below is specified in the applicable Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required.
Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been
exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this
Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number
of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1)
Business Day of receipt of such notice.
|
|
|
d) |
Mechanics of Exercise.
|
|
PAINREFORM LTD.
|
|
By:__________________________________________
Name:
Title:
|
|
Name:
|
|
|
(Please Print)
|
|
|
Address:
|
|
|
Phone Number:
Email Address:
|
(Please Print)
______________________________________
______________________________________
|
|
Dated: _______________ __, ______
|
|
|
Holder’s Signature:
|
|
|
Holder’s Address:
|
|
Warrant Shares: 1,087,565
|
Warrant Date: [ ], 2025
|
|
|
a. |
Warrant B to purchase 1,087,565 out of the Warrant Shares shall vest upon the successful achievement of the Warrant B Milestone;
|
|
|
a) |
Exercise of Warrant. Subject to (i) the Closing of the Acquisition Agreement, (ii) the provisions of Section 4(e) herein, and (iii) the successful achievement of the Warrant B Milestone, exercise of the purchase rights
represented by this Warrant may be made, in whole or in part, at any time or times on or after the date of successful achievement of the Warrant B Milestone (the “Milestone Date”) and on or before the Warrant B Termination, by delivery to
the Company of a duly executed facsimile copy or PDF copy submitted by e-mail (or e-mail attachment) of the Notice of Exercise in the form annexed hereto (the “Notice of Exercise”). Within the earlier of (i) two (2) Trading Days and
(ii) the number of Trading Days comprising the Standard Settlement Period (as defined in Section 4(d)(i) herein) following the date of exercise as aforesaid, the Holder shall deliver the aggregate Warrant B Exercise Price for the shares
specified in the applicable Notice of Exercise by wire transfer or cashier’s check drawn on a United States bank unless the cashless exercise procedure specified in Section 4(c) below is specified in the applicable Notice of Exercise. No ink-original Notice of Exercise shall be required, nor shall any medallion guarantee (or other type of guarantee or notarization) of any Notice of Exercise be required. Notwithstanding anything
herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which
case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date on which the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in
purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares
purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. The Company shall deliver any objection to any Notice of Exercise within one (1) Business Day of
receipt of such notice.
|
|
|
d) |
Mechanics of Exercise.
|
|
PAINREFORM LTD.
|
|
By:__________________________________________
Name:
Title:
|
|
Name:
|
|
|
(Please Print)
|
|
|
Address:
|
|
|
Phone Number:
Email Address:
|
(Please Print)
______________________________________
______________________________________
|
|
Dated: _______________ __, ______
|
|
|
Holder’s Signature:
|
|
|
Holder’s Address:
|
| 1. |
Trading on Material Nonpublic Information
|
| 2. |
Short Sales
|
| 3. |
Publicly Traded Options
|
| 4. |
Standing Orders
|
| 5. |
Hedging Transactions
|
| 6. |
Margin Accounts and Pledges
|
| 7. |
Short-Term Trading
|
| 8. |
Tipping
|
| 9. |
Advice Concerning Trading
|
| 10. |
Confidentiality of Nonpublic Information
|
| 11. |
Post-Termination Transactions
|
| 1. |
Liability for Insider Trading
|
| 2. |
Liability for Tipping
|
| 3. |
Liability of Control Persons
|
| 4. |
Possible Disciplinary Actions
|
| 1. |
Black-Out Periods and Trading Window
|
| a. |
Financial Black-Out Period. The period beginning at the close of
market on the 20th day of the last month of a fiscal quarter and ending at the end of one full Trading Day after the date of public disclosure of the
financial results for that fiscal quarter is a particularly sensitive period of time for transactions in the Company’s Securities from the perspective of compliance with applicable securities laws. This sensitivity arises because directors,
officers and certain employees involved in the preparation of the financial results will often possess Material Nonpublic Information about the expected financial results for the quarter during that period. Accordingly, this period of time is
referred to as a “financial black-out” period. Accordingly, all officers, directors and employees are prohibited from trading during such period.
|
| b. |
Clinical Information Black-Out Period. It is common for
pharmaceutical companies to come into possession of information concerning (i) the early results of clinical trials of product candidates, (ii) reported results of clinical trials of product candidates from Company personnel or from
contractors, and/or (iii) information that results from the analyses of clinical trial results pertaining to product candidates. This information is highly sensitive due to the fact that certain Insiders may possess Material Nonpublic
Information concerning the early results of the clinical trials, the yet-unreported results of the clinical trials, or the scientific or medical inferences or conclusions that can be drawn from the early results or yet-unreported results of
clinical trials. The periods of time during which the Company has received (i) information concerning the early results of clinical trials of product candidates, (ii) reported results of clinical trials of product candidates from Company
personnel or contractors, and/or (iii) information that results from the analyses of clinical trial results pertaining to product candidates, are referred to as “clinical information black-out periods.” All directors and officers (and those
other Insiders identified by the Company from time to time and who have been notified that they have been so identified) are prohibited from trading in the Company’s Securities during clinical information black-out periods and should not disclose to others the fact of such suspension of trading.
|
| c. |
Special Black-Out Periods. In addition, from time to time
Material Nonpublic Information regarding the Company may be pending or there may be material developments known to the Company and not yet disclosed to the public. The Company may impose a special “black-out” period on all directors and
officers (and those other Insiders identified by the Company from time to time and who have been notified that they have been so identified) prohibiting them from trading in the Company’s Securities during a special black-out period and such persons should not disclose to others the fact of such suspension of trading.
|
| d. |
Mandatory Trading Window Related to Financial Information. To
ensure compliance with this Policy and applicable federal and state securities laws, the Company requires that all Insiders refrain from conducting transactions involving the purchase or sale of the Company’s Securities other than during the
period (the “Trading Window”) commencing at the open of market on the second Trading Day following the date of public disclosure of the
financial results for a particular fiscal quarter or year and continuing until the close of market on the 20th day of the last month of the fiscal quarter. During the Trading Window, if the Company is in a clinical information black-out
period or special black-out period, the Company requires that all directors and officers and those certain identified Insiders refrain from conducting transactions involving the purchase or sale of the Company’s Securities even though the
Trading Window may otherwise be open. The prohibition against trading during the financial black-out period, clinical information black-out period and special black-out period encompasses the fulfillment of “limit orders” by any broker for an
Insider and the brokers with whom any such limit order is placed must be so instructed at the time it is placed.
|
| 2. |
Pre-Clearance of Trades
|
| 3. |
Individual Responsibility
|
|
|
(a) |
financial results;
|
|
|
(b) |
news of major clinical or development milestones;
|
|
|
(c) |
early indications of clinical trial results;
|
|
|
(d) |
known but unannounced clinical trial results;
|
|
|
(e) |
known but unannounced analyses of clinical trial results;
|
|
|
(f) |
significant communications to or from regulatory agencies, or other significant
regulatory developments;
|
|
|
(g) |
significant developments related to intellectual property;
|
|
|
(h) |
significant developments related to collaboration relationships;
|
|
|
(i) |
proposals, plans or agreements, even if preliminary in nature, involving mergers, acquisitions, divestitures, recapitalizations, strategic alliances, licensing
arrangements, or purchases or sales of substantial assets;
|
|
|
(j) |
impending bankruptcy or financial liquidity problems;
|
|
|
(k) |
share splits;
|
|
|
(l) |
new equity or debt offerings;
|
|
|
(m) |
positive or negative developments in outstanding litigation;
|
|
|
(n) |
significant litigation exposure due to actual or threatened litigation; and
|
|
|
(1) |
changes in senior management, the Company’s auditors or the board of directors.
|
| 1. |
Share Option Exercises
|
| 2. |
Restricted Share Awards
|
| 3. |
Gifts
|
| 4. |
Blind Trusts and Pre-Arranged Trading Programs
|
|
1.
|
I have reviewed this annual report on Form 20-F of PainReform Ltd.:
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the
circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of
operations and cash flows of the company as of, and for, the periods presented in this report;
|
|
4.
|
The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and
15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
|
|
|
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated
subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this
report based on such evaluation; and
|
|
|
d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the
company’s internal control over financial reporting; and
|
|
5.
|
The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit
committee of the company’s board of directors (or persons performing the equivalent functions):
|
|
|
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report
financial information; and
|
|
|
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
|
|
1.
|
I have reviewed this annual report on Form 20-F of PainReform Ltd.:
|
|
2.
|
Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the
circumstances under which such statements were made, not misleading with respect to the period covered by this report;
|
|
3.
|
Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of
operations and cash flows of the company as of, and for, the periods presented in this report;
|
|
4.
|
The company’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and
15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the company and have:
|
|
|
a) |
Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the company, including its consolidated
subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
|
|
|
b) |
Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and
the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
|
|
|
c) |
Evaluated the effectiveness of the company’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this
report based on such evaluation; and
|
|
|
d) |
Disclosed in this report any change in the company’s internal control over financial reporting that occurred during the period covered by the annual report that has materially affected, or is reasonably likely to materially affect, the
company’s internal control over financial reporting; and
|
|
5.
|
The company’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the company’s auditors and the audit
committee of the company’s board of directors (or persons performing the equivalent functions):
|
|
|
a) |
All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the company’s ability to record, process, summarize and report
financial information; and
|
|
|
b) |
Any fraud, whether or not material, that involves management or other employees who have a significant role in the company’s internal control over financial reporting.
|

| /s/ Kesselman & Kesselman | |
|
Tel-Aviv, Israel
|
Kesselman & Kesselman
|
|
April 7, 2025
|
Certified Public Accountants (lsr.)
|
|
A member firm of PricewaterhouseCoopers International Limited
|