Safety and Efficacy of GH001 in Treatment-Resistant Depression: Results From a Phase 2b, Double-Blind,
Randomized Controlled Trial Michael E. Thase,1,2* Bernhard T. Baune,3 Narcís Cardoner,4 Rosa Maria Dueñas Herrero,5 Luboš Janů,6 John R. Kelly,7 Shane J. McInerney,8 Alexander Nawka,9 Tomáš Páleníček,10 Andreas Reif,11 Víctor Pérez
Sola,12-15 Madhukar H. Trivedi,16 Velichka Valcheva,17 Eduard Vieta,18 Wiesław J. Cubała19 1Department of Psychiatry, University of Pennsylvania, Philadelphia, PA, USA; 2Corporal Michael J. Crescenz Veterans Affairs Medical Center,
Philadelphia, PA, USA; 3Department of Psychiatry, University of Muenster, Muenster, Germany; 4Hospital Santa Creu i Sant Pau, Mental Health Research Group, Institut de Recerca Sant Pau, Universitat Autònoma de Barcelona, CIBERSAM
Barcelona, Spain; 5Parc Sanitari Sant Joan de Deu Hospital de Dia de Numancia, Barcelona, Spain; 6A-Shine SRO, Pilsen, Czechia; 7Department of Psychiatry, Tallaght University Hospital, Dublin, Ireland; 8Department of Psychiatry,
University Hospital Galway, Galway, Ireland; 9Institut Neuropsychiatrické Péče, Praha, Czechia; 10Psyon s.r.o., Prague, Czechia; 11Goethe University Frankfurt, University Hospital, Department of Psychiatry, Psychosomatic Medicine and
Psychotherapy, Frankfurt, Germany; 12Mental Health Institute, Hospital del Mar, Barcelona, Spain; 13Neurosciences Research Group, Hospital del Mar Research Institute (IMIM), Barcelona, Spain; 14Department of Psychiatry and Department of
Experimental and Health Sciences, Pompeu Fabra University, Barcelona, Spain; 15Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM G21), Instituto de Salud Carlos III, Madrid, Spain; 16Department of Psychiatry, University
of Texas Southwestern Medical Center, Dallas, TX, USA; 17GH Research, Dublin, Ireland; 18Hospital Clinic de Barcelona, Institute of Neuroscience, University of Barcelona, IDIBAPS, CIBERSAM, Barcelona, Catalonia, Spain; 19Department of
Psychiatry, Faculty of Medicine, Medical University of Gdańsk, Gdańsk, Poland *Presenting Author: Michael E. Thase; thase@pennmedicine.upenn.edu Methods Trial Design This Phase 2b multicenter trial consisted of two parts (Figure
1): Double-blind part (described here): a randomized, double-blind, placebo-controlled trial with follow-up to 7 days postdose. Patients were randomized in a 1:1 ratio to receive an individualized dosing regimen (IDR) of up to three
escalating doses of GH001 (6, 12, and 18 mg) or placebo on a single day; there was a 1-hour interval between doses Open-label extension (OLE): a 6-month trial with up to five GH001 re-treatments administered depending on the patient’s
clinical status Patients were required to meet the trial criteria for TRD as assessed by a trial psychiatrist: Recurrent or single MDD episode without psychotic features, with current episode of ≤2 years Current major depressive
episode based upon the Massachusetts General Hospital – Structured Assessment for Evaluation of Risk (MGH-SAFER) criteria interview 17-Item Hamilton Depression Rating Scale (HAM-D-17) total score ≥20 Nonresponse to ≥2 and ≤5 oral
antidepressant treatments Assessments The primary endpoint of the double-blind part of this trial was mean change in Montgomery–Åsberg Depression Rating Scale (MADRS) from baseline to Day 8, as assessed by a blinded rater Secondary
endpoints included change in global disease severity as assessed by the Clinical Global Impression – Severity (CGI-S) Scale, anxiety as assessed by the Hamilton Anxiety Rating Scale (HAM-A), and quality of life as assessed by the Quality
of Life Enjoyment and Satisfaction Questionnaire – Short Form (Q-LES-Q-SF) Treatment-emergent adverse events (TEAEs) were assessed throughout the trial Background Treatment-resistant depression (TRD) affects approximately 30% of
patients treated for major depressive disorder (MDD)1 Current therapies for TRD are limited and there is a large unmet need for treatments that offer rapid and sustained effects Mebufotenin (5-methoxy-N,N-dimethyltryptamine [5-MeO-DMT])
is a non-selective serotonin (5-HT) agonist with high affinity for several receptor subtypes, including the 5-HT1A receptor2 GH001 is a synthetic form of mebufotenin for pulmonary inhalation3,4 Early-stage trials in healthy volunteers
and patients with TRD suggest that GH001 is well tolerated and may have the potential to induce an ultra-rapid remission in depressive symptoms3,4 Objective The aim of this double-blind, placebo-controlled trial was to investigate the
safety and efficacy of GH001 in patients with TRD References Kubitz N, et al. PLoS One. 2013;8(10):e76882. Shen H, et al. Curr Drug Metab. 2010;11(8):659-66. Reckweg J, et al. Front Pharmacol. 2021;12:760671. Reckweg JT, et al. Front
Psychiatry. 2023;14:1133414. Acknowledgments This trial was sponsored by GH Research. The sponsor would like to thank the participants in the trial. The sponsor would also like to thank the investigators who conducted this trial. Under
the guidance of the authors, medical writing and editorial support were provided by Brian Brennan, PhD, and Claire Sweeney, PhD, of GH Research, and Jane Phillips, PhD, of OPEN Health. Primary analysis of the trial was conducted by the
contract research organization Worldwide Clinical Trials. Additional analyses were conducted by Rachael MacIsaac, PhD, of GH Research. Disclosures MET: Grants – Acadia, Alkermes, Axsome, Intra-Cellular Therapies, Janssen, National
Institute of Mental Health, Otsuka, Patient-Centered Outcomes Research Institute (PCORI), and Takeda. Advisory Boards – Autobahn Therapeutics, Axsome, Clexio Biosciences, Gerson Lehrman Group, GH Research, Lundbeck, Janssen, Johnson &
Johnson, Luye Pharma, Merck, Object Pharma, Otsuka, Pfizer, Sage, Seelos Therapeutics, Sunovion, and Takeda. Royalties – American Psychiatric Association Foundation, Guilford Publications, Herald House, Wolters Kluwer, and W W Norton
& Company. BTB: Consultant – National Health and Medical Research Council (Australia). Honoraria – Angelini, AstraZeneca, Biogen, BMS, Boehringer Ingelheim, Johnson & Johnson, LivaNova, Lundbeck, Medscape, Otsuka, Pfizer, Roche,
Servier, Sumitomo Pharma, Sunovion, Teva, and Wyeth. Advisory boards – Biogen, Boehringer Ingelheim, Janssen-Cilag, LivaNova, Lundbeck, Medscape, Novartis, Otsuka, and Teva. Research grants from private industries or nonprofit funds –
AstraZeneca, BMBF (Germany), BMG (Germany), DFG (Germany), ERA PerMed, Fay Fuller Foundation, Horizon Europe (European Union), James & Diana Ramsay Foundation (Adelaide), Johnson & Johnson, Lundbeck, La Marató de TV3, National
Health and Medical Research Council (Australia), Sanofi-Synthélabo, and Wellcome Trust (UK). NC: Grants – Spanish Ministry of Health, Spanish Ministry of Science and Innovation (CIBERSAM), Strategic Plan for Health Research and
Innovation (PERIS) 2016–2020, Recercaixa, and La Marató de TV3. Honoraria – Adamed, Elsevier, Exeltis, Janssen, Lundbeck, Pfizer, and Servier. Advisory Boards – Angelini, Esteve, Janssen, Lundbeck, Novartis, Pfizer, and Viatris.
Lectures/ Meetings – Janssen, Lundbeck, and Pfizer. RMDH: Principal investigator – Beckley Psytech and GH Research. Subinvestigator – Compass. LJ: Principal investigator – GH Research. JRK: Principal investigator – Compass, GH Research,
and Transcend Therapeutics. Consultant – Clerkenwell Health. Grant funding – Health Research Board (ILP-POR-2022-030, DIFA-2023-005, KTA-2024-002). SJM: Principal investigator – GH Research and Transcend Therapeutics. Honoraria –
Janssen and Lundbeck. AN: Principal investigator – GH Research. TP: Principal investigator – Compass, GH Research, MAPS, and Ketabon. Shares – Psychedelická klinika s.r.o., Společnost pro podporu neurovědního výzkumu s.r.o., and AVI-X
Aviation Experts s.r.o. Founder – PSYRES (Psychedelic Research Foundation). Consultant – CB21 Pharma and GH Research. AR: Honoraria for lectures and/or advisory boards – AbbVie, Boehringer Ingelheim, Cyclerion, Compass, GH Research,
Janssen, LivaNova, Medice, MSD, Newron, Sage/Biogen, and Shire/Takeda. Research grants – Medice and Janssen. VPS: Consultant, honoraria, or grants – AB-Biotics, AstraZeneca, Bristol Myers Squibb, CIBERSAM, FIS-ISCiii, Janssen Cilag,
Lundbeck, Medtronic, Otsuka, and Servier. MHT: Advisory boards – Alto Neuroscience and Base Point Health Management. Consultant – Axsome, Biogen, Daiichi Sankyo, GH Research, Legion Health, Neurocrine Biosciences, Otsuka Pharmaceutical
Europe, Otsuka Pharmaceutical Development & Commercialization, Otsuka Pharmaceutical, PureTech, and Takeda. Advisor – Cerebral Therapeutics, Circular Genomics, and Seaport Therapeutics. Scientific advisor – GreenLight VitalSign6.
Board of Directors – Charities2Love. VV: Employee and stock option holder of GH Research. EV: Grants – AB-Biotics, AbbVie, Almirall, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celon, Cephalon, Dainippon Sumitomo Pharma,
Elan, Ferrer, GH Research, GlaxoSmithKline, Janssen, Lilly, Lundbeck, Orion, Otsuka, Pfizer, Sanofi Aventis, Servier, Sunovion, and Takeda. Honoraria – Abbott, AbbVie, Angelini, AstraZeneca, Bristol Myers Squibb, Cambridge University
Press, Elsevier, Farmindustria, Ferrer, Galenica, GlaxoSmithKline, Janssen, Johnson & Johnson, Lilly, Lundbeck, Oxford University Press, Otsuka, Pfizer, Sanofi Aventis, and Viatris. Advisory boards – AbbVie, Angelini, AstraZeneca,
Biogen, Biohaven, Bristol Myers Squibb, Celon, Compass, Ferrer, GH Research, Gedeon Richter, HMNC, Idorsia, Janssen, Johnson & Johnson, Jazz, Lilly, Lundbeck, Merck Sharp & Dohme, Novartis, Organon, Otsuka, Pfizer,
Roche, Sage, Sanofi Aventis, Servier, Shire, Sunovion, Takeda, and Teva. WJC: Grants – Acadia, Angelini, Beckley Psytech, GH Research, HMNC Brain Health, Intra-Cellular Therapies, Janssen, MSD, Neumora, Novartis, Otsuka, Recognify Life
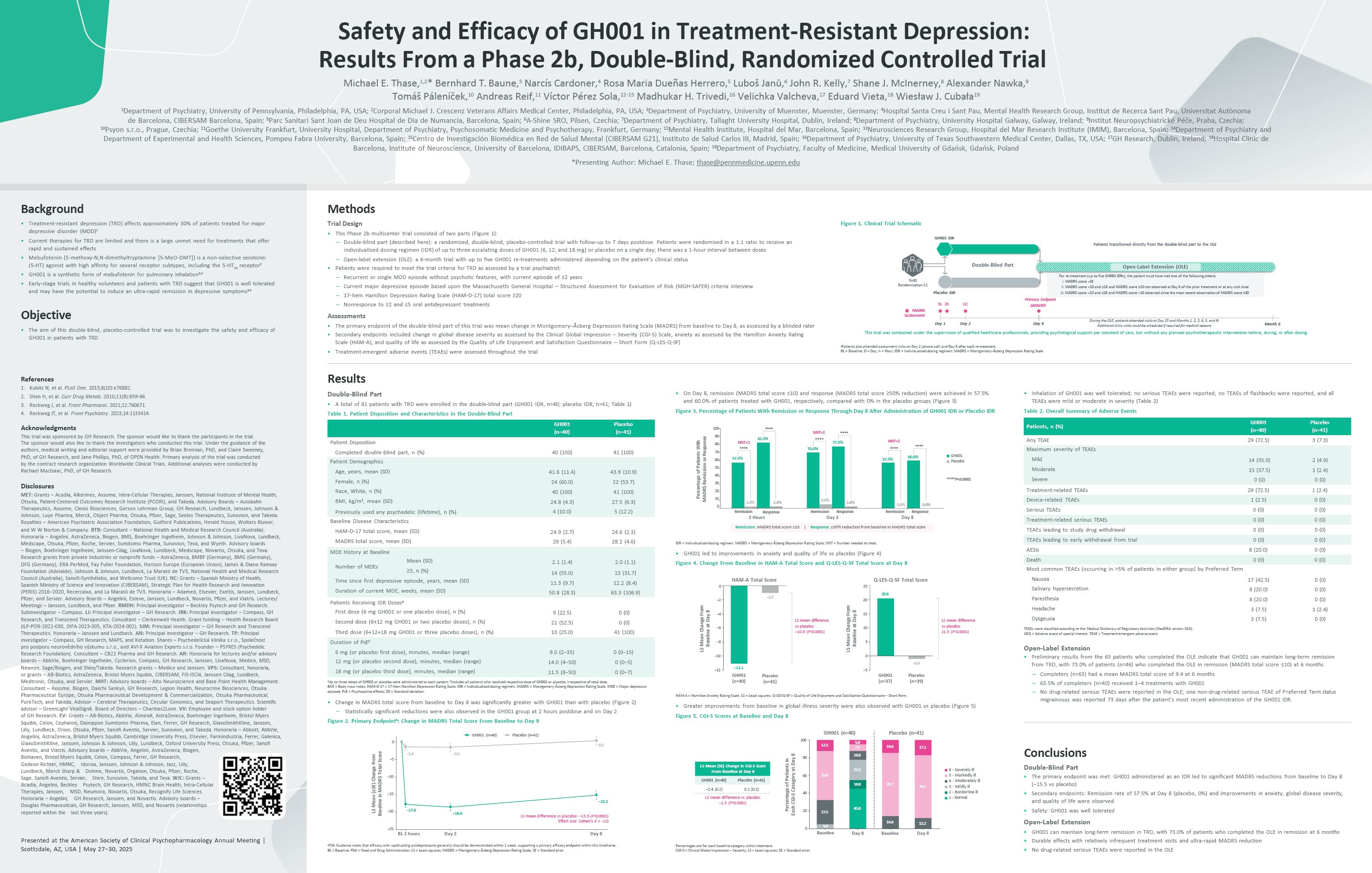
Sciences. Honoraria – Angelini, GH Research, Janssen, and Novartis. Advisory boards – Douglas Pharmaceuticals, GH Research, Janssen, MSD, and Novartis (relationships reported within the last three years). Figure 1. Clinical Trial
Schematic This trial was conducted under the supervision of qualified healthcare professionals, providing psychological support per standard of care, but without any planned psychotherapeutic intervention before, during, or after
dosing N=81 Randomization 1:1 GH001 IDR Placebo IDR Open-Label Extension (OLE) For re-treatment (up to five GH001 IDRs), the patient must have met one of the following criteria: MADRS score >18 MADRS score >10 and ≤18 and
MADRS score ≤10 not observed at Day 8 of the prior treatment or at any visit since MADRS score >10 and ≤18 and MADRS score >18 observed since the most recent observation of MADRS score ≤10 During the OLE, patients attended visits
at Day 15 and Months 1, 2, 3, 4, 5, and 6a Additional clinic visits could be scheduled if required for medical reasons MADRS assessment Month 6 Primary Endpoint BL 2h D2 ΔMADRS Day 1 Day 2 Day 8 Double-Blind Part Patients
transitioned directly from the double-blind part to the OLE aPatients also attended assessment visits on Day 2 (phone call) and Day 8 after each re-treatment. BL = Baseline; D = Day; h = Hour; IDR = Individualized dosing regimen; MADRS
= Montgomery–Åsberg Depression Rating Scale. Results Double-Blind Part A total of 81 patients with TRD were enrolled in the double-blind part (GH001 IDR, n=40; placebo IDR, n=41; Table 1) Table 1. Patient Disposition and
Characteristics in the Double-Blind Part GH001 (n=40) Placebo (n=41) Patient Disposition Completed double-blind part, n (%) 40 (100) 41 (100) Patient Demographics Age, years, mean (SD) Female, n (%) Race, White, n (%) BMI, kg/m2,
mean (SD) Previously used any psychedelic (lifetime), n (%) 41.6 (11.4) 24 (60.0) 40 (100) 24.8 (4.3) 4 (10.0) 43.9 (10.9) 22 (53.7) 41 (100) 27.5 (6.3) 5 (12.2) Baseline Disease Characteristics HAM-D-17 total score, mean (SD)
MADRS total score, mean (SD) 24.9 (2.7) 29 (5.4) 24.6 (2.3) 28.2 (4.6) MDE History at Baseline Mean (SD) Number of MDEs ≥3, n (%) Time since first depressive episode, years, mean (SD) Duration of current MDE, weeks, mean (SD) 2.1
(1.4) 14 (35.0) 11.3 (9.7) 50.8 (28.3) 2.0 (1.1) 13 (31.7) 12.2 (8.4) 63.3 (106.9) Patients Receiving IDR Dosesa First dose (6 mg GH001 or one placebo dose), n (%) Second dose (6+12 mg GH001 or two placebo doses), n (%) Third
dose (6+12+18 mg GH001 or three placebo doses), n (%) 9 (22.5) 21 (52.5) 10 (25.0) 0 (0) 0 (0) 41 (100) Duration of PsEb 6 mg (or placebo first dose), minutes, median (range) 12 mg (or placebo second dose), minutes, median
(range) 18 mg (or placebo third dose), minutes, median (range) 9.0 (2−35) 14.0 (4−50) 11.5 (8−50) 0 (0−15) 0 (0−5) 0 (0−7) aUp to three doses of GH001 or placebo were administered to each patient. bIncludes all patients who
received respective dose of GH001 or placebo, irrespective of total dose. BMI = Body mass index; HAM-D-17 = 17-Item Hamilton Depression Rating Scale; IDR = Individualized dosing regimen; MADRS = Montgomery-Åsberg Depression Rating Scale;
MDE = Major depressive episode; PsE = Psychoactive effects; SD = Standard deviation. Change in MADRS total score from baseline to Day 8 was significantly greater with GH001 than with placebo (Figure 2) – Statistically significant
reductions were also observed in the GH001 group at 2 hours postdose and on Day 2 Figure 2. Primary Endpointa: Change in MADRS Total Score From Baseline to Day 8 LS mean difference vs placebo: −15.5 (P<0.0001) Effect size: Cohen’s d =
−2.0 0 −1.4 −1.5 −18.6 0.3 −15.2 LS Mean (±SE) Change From Baseline in MADRS Total Score −5 −10 −15 −25 −20 −17.8 BL 2 hours Day 2 Day 8 GH001 (n=40) Placebo (n=41) aFDA Guidance notes that efficacy with rapid-acting
antidepressants generally should be demonstrated within 1 week, supporting a primary efficacy endpoint within this timeframe. BL = Baseline; FDA = Food and Drug Administration; LS = Least squares; MADRS = Montgomery-Åsberg Depression
Rating Scale; SE = Standard error. Presented at the American Society of Clinical Psychopharmacology Annual Meeting | Scottsdale, AZ, USA | May 27−30, 2025 On Day 8, remission (MADRS total score ≤10) and response (MADRS total score ≥50%
reduction) were achieved in 57.5% and 60.0% of patients treated with GH001, respectively, compared with 0% in the placebo groups (Figure 3) Figure 3. Percentage of Patients With Remission or Response Through Day 8 After Administration of
GH001 IDR or Placebo IDR GH001 Placebo ****P<0.0001 Remission: MADRS total score ≤10 | Response: ≥50% reduction from baseline in MADRS total score 2.4% 4.9% 2.4% Percentage of Patients With MADRS Remission or
Response 100 90 80 70 60 50 40 30 20 10 0 2 Hours Remission 2.4% Response Remission Response Day 2 0.0% 0.0% Remission Response Day 8 **** 82.5% **** 60.0% NNT=2 **** 57.5% NNT=2 **** ****
77.5% 70.0% NNT=2 **** 57.5% IDR = Individualized dosing regimen; MADRS = Montgomery-Åsberg Depression Rating Scale; NNT = Number needed to treat. GH001 led to improvements in anxiety and quality of life vs placebo (Figure
4) Figure 4. Change From Baseline in HAM-A Total Score and Q-LES-Q-SF Total Score at Day 8 −11.1 GH001 (n=40) −1.0 Placebo (n=41) LS Mean Change From Baseline at Day 8 −6 −10 −12 LS mean difference vs placebo: −10.0
(P<0.0001) 20.6 −0.9 GH001 Placebo (n=37) (n=39) LS Mean Change From Baseline at Day 8 HAM-A Total Score Q-LES-Q-SF Total Score 0 25 −2 20 −4 15 10 −8 5 0 −5 LS mean difference vs placebo: 21.5 (P<0.0001) HAM-A =
Hamilton Anxiety Rating Scale; LS = Least squares; Q-LES-Q-SF = Quality of Life Enjoyment and Satisfaction Questionnaire – Short Form. Greater improvements from baseline in global illness severity were also observed with GH001 vs placebo
(Figure 5) Figure 5. CGI-S Scores at Baseline and Day 8 6 - Severely ill 5 - Markedly ill 4 - Moderately ill 3 - Mildly ill 2 - Borderline ill 1 - Normal Percentage of Patients in Each CGI-S Category at Day
8 100 80 60 40 20 0 5.0 Baseline 27.5 55.0 12.5 14.6 70.7 14.6 70.7 45.0 10.0 22.5 GH001 (n=40) Placebo (n=41) 5.0 7.5 17.1 10.0 Day 8 Baseline 12.2 Day 8 Percentages are for each baseline category within
treatment. CGI-S = Clinical Global Impression – Severity; LS = Least squares; SE = Standard error. Inhalation of GH001 was well tolerated; no serious TEAEs were reported, no TEAEs of flashbacks were reported, and all TEAEs were mild or
moderate in severity (Table 2) Table 2. Overall Summary of Adverse Events Patients, n (%) GH001 (n=40) Placebo (n=41) Any TEAE 29 (72.5) 3 (7.3) Maximum severity of TEAEs Mild Moderate Severe 14 (35.0) 15 (37.5) 0 (0) 2
(4.9) 1 (2.4) 0 (0) Treatment-related TEAEs 29 (72.5) 1 (2.4) Device-related TEAEs 1 (2.5) 0 (0) Serious TEAEs 0 (0) 0 (0) Treatment-related serious TEAEs 0 (0) 0 (0) TEAEs leading to study drug withdrawal 0 (0) 0 (0) TEAEs
leading to early withdrawal from trial 0 (0) 0 (0) AESIs 8 (20.0) 0 (0) Death 0 (0) 0 (0) 17 (42.5) 8 (20.0) 8 (20.0) 3 (7.5) 3 (7.5) 0 (0) 0 (0) 0 (0) 1 (2.4) 0 (0) Most common TEAEs (occurring in >5% of patients in
either group) by Preferred Term Nausea Salivary hypersecretion Paresthesia Headache Dysgeusia TEAEs were classified according to the Medical Dictionary of Regulatory Activities (MedDRA version 26.0). AESI = Adverse event of special
interest; TEAE = Treatment-emergent adverse event. Open-Label Extension Preliminary results from the 63 patients who completed the OLE indicate that GH001 can maintain long-term remission from TRD, with 73.0% of patients (n=46) who
completed the OLE in remission (MADRS total score ≤10) at 6 months Completers (n=63) had a mean MADRS total score of 9.4 at 6 months 63.5% of completers (n=40) received 1−4 treatments with GH001 No drug-related serious TEAEs were
reported in the OLE; one non-drug-related serious TEAE of Preferred Term status migrainosus was reported 73 days after the patient's most recent administration of the GH001 IDR. Conclusions Double-Blind Part The primary endpoint was
met: GH001 administered as an IDR led to significant MADRS reductions from baseline to Day 8 (−15.5 vs placebo) Secondary endpoints: Remission rate of 57.5% at Day 8 (placebo, 0%) and improvements in anxiety, global disease severity, and
quality of life were observed Safety: GH001 was well tolerated Open-Label Extension GH001 can maintain long-term remission in TRD, with 73.0% of patients who completed the OLE in remission at 6 months Durable effects with relatively
infrequent treatment visits and ultra-rapid MADRS reduction No drug-related serious TEAEs were reported in the OLE LS Mean (SE) Change in CGI-S Score From Baseline at Day 8 GH001 (n=40) Placebo (n=41) −2.4 (0.2) 0.1 (0.2) LS mean
difference vs placebo: −2.5 (P<0.0001)