UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities
Exchange Act of 1934
Date of Report (Date of earliest event reported): March 8, 2025
Alumis Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-42143 | 86-1771129 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
280 East Grand Avenue
South San Francisco, California 94080
(Address of principal executive offices)
Registrant’s telephone number, including area code: (650) 231-6625
N/A
(Former name or former address, if changed since last report.)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name
of each exchange on which registered |
||
| Common Stock, $0.0001 par value per share | ALMS | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 8.01. | Other Events. |
On March 8, 2025, Alumis Inc. (the “Company”) issued a press release titled “Late-Breaking ESK-001 Phase 2 OLE Data Presented at 2025 AAD Annual Meeting Demonstrate Robust Clinical Responses Over 52 Weeks in Psoriasis” announcing positive 52-week data from the open-label extension of the Company’s Phase 2 STRIDE clinical trial evaluating ESK-001 in patients with moderate-to-severe plaque psoriasis.
On March 8, 2025, the Company also presented the results of these data during a late-breaking session at the 2025 American Academy of Dermatology Association (AAD) Annual Meeting in Orlando, Florida.
A copy of the press release and the presentation are furnished as Exhibit 99.1 and Exhibit 99.2, respectively, to this Current Report on Form 8-K (the “Report”). The information set forth in this Report, including without limitation the press release and the presentation, is not deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as may be expressly set forth by specific reference in such a filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit No. | Description | |
| 99.1 | Press Release dated March 8, 2025. | |
| 99.2 | Presentation titled “ESK-001, a Highly Selective Oral TYK2 Inhibitor: 52-Week Phase 2 Study Results In Moderate-to-Severe Plaque Psoriasis.” | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL Document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Alumis Inc. | ||
| By: | /s/ Sara Klein | |
| Sara Klein | ||
| Chief Legal Officer | ||
Dated: March 10, 2025
Exhibit 99.1

Late-Breaking ESK-001 Phase 2 OLE Data Presented at 2025 AAD Annual Meeting
Demonstrate Robust Clinical Responses Over 52-Weeks in Psoriasis
| - | ESK-001 is a highly selective, next-generation oral tyrosine kinase 2 (TYK2) inhibitor currently under development in moderate-to-severe plaque psoriasis and systemic lupus |
| - | Phase 2 OLE data of ESK-001 at 40 mg BID demonstrated sustained or increasing clinical responses through week 52 on PASI 90, PASI 100, and sPGA 0 |
| - | ESK-001 was generally well-tolerated at one year, with no new safety findings |
| - | Data further support ESK-001’s potential to offer a highly differentiated and best-in-class treatment option for people with moderate-to-severe plaque psoriasis |
| - | Phase 3 ONWARD program ongoing with topline data expected in Q1 2026 |
SOUTH SAN FRANCISCO, Calif., March 8, 2025 – Alumis Inc. (Nasdaq: ALMS), a clinical-stage biopharmaceutical company developing oral therapies using a precision approach to optimize clinical outcomes and significantly improve the lives of patients with immune-mediated diseases, today announced positive 52-week data from the open-label extension (OLE) of its Phase 2 STRIDE clinical trial evaluating ESK-001 in patients with moderate-to-severe plaque psoriasis. The results were presented during a late-breaking session at the 2025 American Academy of Dermatology Association (AAD) Annual Meeting in Orlando, Florida.
These data demonstrated that patients receiving 40 mg twice daily dosing of ESK-001 achieved long-term sustained or increasing clinical responses through Week 52 compared to Week 12 (using modified non-responder imputation, n=80) as measured by PASI 90 (61.3% vs. 52.4%), PASI 100 (38.8% vs. 26.8%), and sPGA 0 (38.8% vs. 32.9%). At Week 52, patients maintained robust clinical improvements in control of itch (NRS≤4, 81.3%) and quality-of-life (DLQI0/1, 61.3%). Treatment with ESK-001 continued to be generally well tolerated at week 52, with safety and tolerability consistent with previously reported Week 16 and Week 28 data and no new safety findings.
“We’re excited to see that ESK-001 continues to demonstrate a favorable clinical profile for the potential treatment of moderate-to-severe plaque psoriasis, with the ability to improve clinical outcomes as well as patients’ reported symptoms and quality of life," said Dr. Jörn Drappa, Alumis’ Chief Medical Officer. "We believe in the potential of ESK-001 to fill a critical gap in psoriasis patient care as an oral therapy that is well tolerated and may provide biologic-like clinical responses."
ESK-001 is a highly selective, next-generation oral tyrosine kinase 2 (TYK2) inhibitor designed to correct immune dysregulation across a spectrum of diseases driven by proinflammatory mediators, including IL-23, IL-17, and type 1 interferon (IFN). Its selective targeting is designed to deliver maximal inhibition while minimizing off-target binding and effects.
“These long-term data further support the highly differentiated profile of ESK-001 and reinforce its potential as a best-in-class TYK2 inhibitor for the oral treatment of moderate-to-severe plaque psoriasis," said Martin Babler, President and Chief Executive Officer of Alumis. "We continue to progress and enroll patients with moderate-to-severe psoriasis in the pivotal Phase 3 ONWARD studies and expect to report topline data in the first quarter of 2026.”

About ESK-001
Alumis' lead clinical candidate, ESK-001, is a highly selective, next-generation oral TYK2 inhibitor that is designed to correct immune dysregulation across a spectrum of diseases driven by proinflammatory mediators, including IL-23, IL-17, and type 1 interferon (IFN). ESK-001's selective targeting is designed to deliver maximal inhibition while minimizing off-target binding and effects.
ESK-001 is currently being evaluated in the Phase 3 ONWARD clinical program, which consists of two parallel global Phase 3, multi-center, randomized, double-blind placebo-controlled 24-week clinical trials, ONWARD1 and ONWARD2, designed to evaluate the efficacy and safety of ESK-001 in adult patients with moderate-to-severe plaque psoriasis. Each trial will enroll approximately 840 patients randomized 2:1:1 to receive either ESK-001 40 mg twice-daily, placebo or apremilast. The co-primary efficacy endpoints will be the proportion of patients with moderate-to-severe plaque psoriasis achieving a 75% improvement in the Psoriasis Area and Severity Index (PASI 75) and sPGA score 0/1 of ESK-001 compared to placebo at Week 16. Patients completing Week 24 will have the opportunity to participate in a long-term extension (LTE) trial, ONWARD3, that will evaluate durability and maintenance of response and long-term safety.
The Phase 3 clinical program is supported by positive data from the Phase 2 STRIDE clinical trial (NCT05600036) and the long-term OLE extension (CT05739435), which is currently ongoing. Interim 28-week OLE data presented at the 2024 European Academy of Dermatology & Venereology (EADV) Congress demonstrated a dose-dependent sustained increase across all PASI scores over time, with the majority of patients reaching PASI 75 at the 40 mg twice daily dose. ESK-001 continued to show a favorable safety profile in the OLE. Treatment emergent adverse event (TEAE) frequency and severity were similar across study arms, with the most common being upper respiratory tract infections, nasopharyngitis, and headaches, and the majority mild-to-moderate and self-limited.
In parallel with the Phase 3 clinical program, Alumis is developing a once-daily modified release oral formulation of ESK-001 designed to replace the current immediate release oral formulation that is dosed twice daily.
ESK-001 is also being evaluated in LUMUS, a Phase 2b clinical trial for the treatment of patients with systemic lupus erythematosus. In addition, Alumis continues to leverage its precision data analytics platform to explore ESK-001’s potential application in other immune-mediated conditions.
About Alumis
Alumis is a clinical-stage biopharmaceutical company developing oral therapies using a precision approach to optimize clinical outcomes and significantly improve the lives of patients with immune-mediated diseases. Leveraging its proprietary precision data analytics platform, Alumis is building a pipeline of molecules with the potential to address a broad range of immune-mediated diseases as monotherapy or combination therapies. Alumis’ most advanced product candidate, ESK-001, is an oral, highly selective, small molecule, allosteric inhibitor of TYK2 that is currently being evaluated for the treatment of patients with moderate-to-severe plaque psoriasis and systemic lupus erythematosus. Alumis is also developing A-005, a clinical-stage, CNS-penetrant, allosteric TYK2 inhibitor for the treatment of neuroinflammatory and neurodegenerative diseases. Beyond TYK2, Alumis’ proprietary precision data analytics platform and drug discovery expertise have led to the identification of additional preclinical programs that exemplify its precision approach. Incubated by Foresite Labs and led by a team of industry veterans experienced in small-molecule compound drug development for immune-mediated diseases, Alumis is pioneering a precision approach to drug development to potentially produce the next generation of treatment to address immune dysfunction. For more information, visit www.alumis.com.

Forward-Looking Statements
This press release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will" and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. These forward-looking statements include, without limitation, statements regarding Alumis’ future plans and prospects including development and commercialization of its pipeline, the potential for ESK-001 to be a best-in-class oral treatment for moderate-to-severe plaque psoriasis, any expectations regarding the safety, efficacy or tolerability of ESK-001 and the potential of ESK-001 to treat moderate-to-severe plaque psoriasis and systemic lupus erythematosus. Any forward-looking statements in this press release are based on Alumis’ current expectations, estimates and projections only as of the date of this release and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Readers are cautioned that actual results, levels of activity, safety, efficacy, performance or events and circumstances could differ materially from those expressed or implied in Alumis’ forward-looking statements due to a variety of risks and uncertainties, which include, without limitation, risks and uncertainties related to Alumis’ ability to advance ESK-001 and to obtain regulatory approval of and ultimately commercialize Alumis’ clinical candidates, the timing and results of preclinical and clinical trials, Alumis’ ability to fund development activities and achieve development goals, Alumis’ ability to protect its intellectual property and other risks and uncertainties described in Alumis’ filings with the Securities and Exchange Commission (SEC), including any future reports Alumis may file with the SEC from time to time. Alumis explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Alumis Contact Information
Teri Dahlman
Red House Communications
teri@redhousecomms.com
##
Exhibit 99.2
1 ESK - 001, a Highly S elective O ral TYK2 Inhibitor : 52 - Week Phase 2 Study Results In Moderate - to - Severe Plaque Psoriasis Andrew Blauvelt 1 , Shahram Jacobs 2 , Michael Bukhalo 3 , Howard Sofe n 4 , Elisa Muscianisi 5 , Grace Ma 5 , Gabriel Lau 5 , Michelle Bettinger 5 , Roman G. Rubio 5 , Elena Hitraya 5 , and Kim Papp 6 1 Blauvelt Consulting LLC, Annapolis, MD , USA ; 2 Unison Clinical Trials, Sherman Oaks, CA , USA ; 3 David Geffen UCLA School of Medicine, Division of Dermatology, Los Angeles, CA, USA; 4 Arlington Dermatology, Rolling Meadows, IL, USA ; 5 Alumis Inc., South San Francisco, CA, USA; 6 Probity Medical Research, Waterloo, ON, Canada .

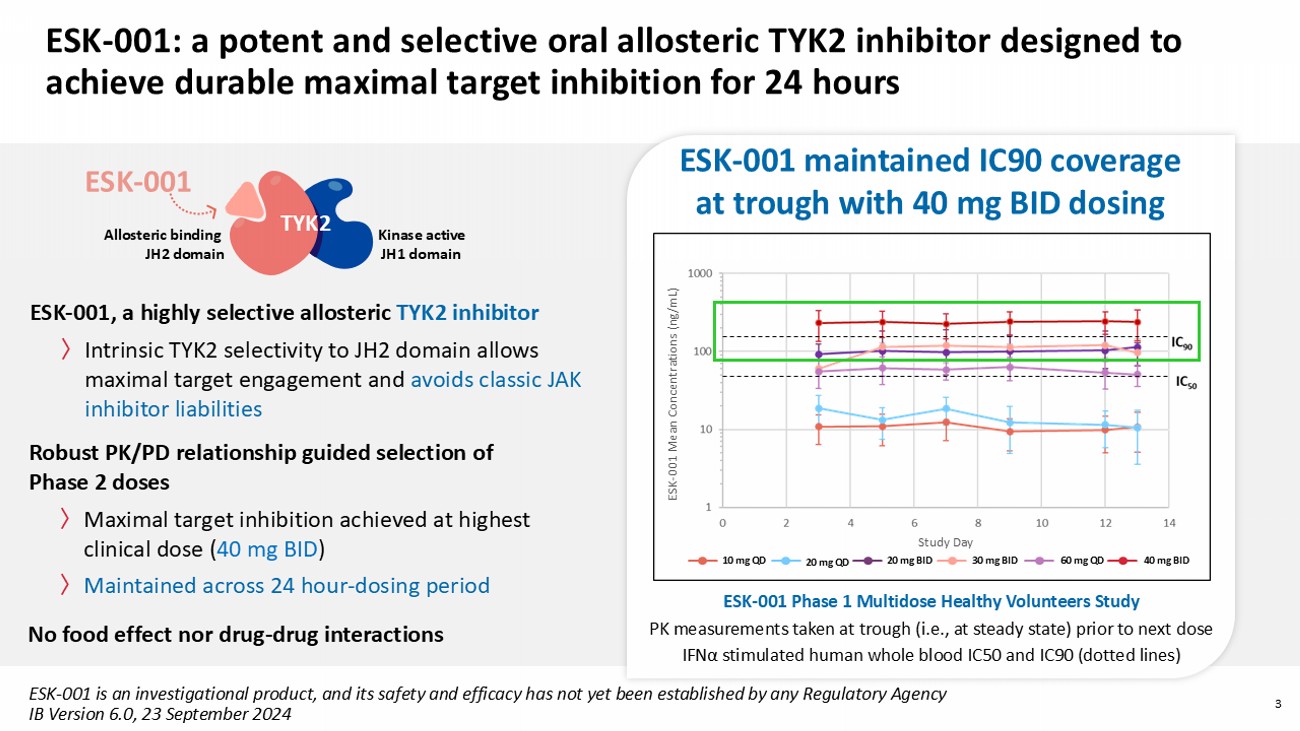
〉 AbbVie: Advisory Board, Investigator 〉 Acelyrin : Investigator 〉 Almirall: Advisory Board, Investigator 〉 Alumis : Advisory Board, Investigator 〉 Amgen: Advisory Board, Investigator 〉 Anaptysbio : Advisory Board 〉 Apogee: Advisory Board 〉 Arcutis : Advisory Board , Investigator 〉 Boehringer Ingelheim: Advisory Board, Investigator 〉 Bristol - Myers Squibb: Advisory Board, Investigator 〉 Celltrion: Advisory Boar d 〉 Corvus: Advisory Board 〉 Dermavant Sciences: Advisory Board , Investigator 〉 Eli Lilly and Company: Advisory Board, Investigator, Speaker 〉 Galderma: Advisory Board, Investigator 〉 GlaxoSmithKline : Advisory Board 2 Disclosure of relationships with industry Andrew Blauvelt, MD, MBA Late Breaking Research: Session 1 〉 Immunovant : Advisory Board 〉 Incyte Corporation: Advisory Board, Investigator 〉 IQVIA: Advisory Board 〉 Janssen Pharmaceuticals: Advisory Board, Investigator 〉 Leo Pharma: Advisory Board, Investigator 〉 Lipidio : Advisory Board, Stock Owner 〉 Merck Serono: Advisory Board, Investigator 〉 Novartis: Advisory Board, Investigator 〉 Oruka : Advisory Board, Stock Owner 〉 Paragon: Advisory Board 〉 Pfizer: Advisory Board, Investigator 〉 Regeneron: Advisory Board , Investigator 〉 Sanofi: Advisory Board, Investigator 〉 Spherix Global Insights: Advisory Board 〉 Sun Pharmaceutical Industries: Advisory Board, Investigator 〉 Syncona : Advisory Board 〉 Takeda Pharmaceuticals: Advisory Board, Investigator 〉 UCB: Advisory Board, Investigator , Speaker 〉 Union: Advisory Board 〉 No patient care recommendations are made 3 ESK - 001: a potent and selective oral allosteric TYK 2 inhibitor designed to achieve durable maximal target inhibition for 24 hours ESK - 001, a highly selective allosteric TYK2 inhibitor 〉 Intrinsic TYK2 selectivity to JH2 domain allows maximal target engagement and avoids classic JAK inhibitor liabilities ESK - 001 Kinase active JH1 domain Allosteric binding JH2 domain TYK2 ESK - 001 Phase 1 Multidose Healthy Volunteers Study PK measurements taken at trough (i . e ., at steady state ) prior to next dose IFN α stimulated human whole blood IC50 and IC90 (dotted lines) ESK - 001 maintained IC90 coverage at trough with 40 mg BID dosing ESK - 001 Mean Concentrations (ng/mL) Study Day 10 mg QD 20 mg QD 20 mg BID 30 mg BID 40 mg BID 60 mg QD Robust PK/PD relationship guided selection of Phase 2 doses 〉 Maximal target inhibition achieved at highest clinical dose ( 40 mg BID ) 〉 Maintained across 24 hour - dosing period ESK - 001 is an investigational product, and its safety and efficacy has not yet been established by any Regulatory Agency IB Version 6.0, 23 September 2024 No food effect nor drug - drug interactions
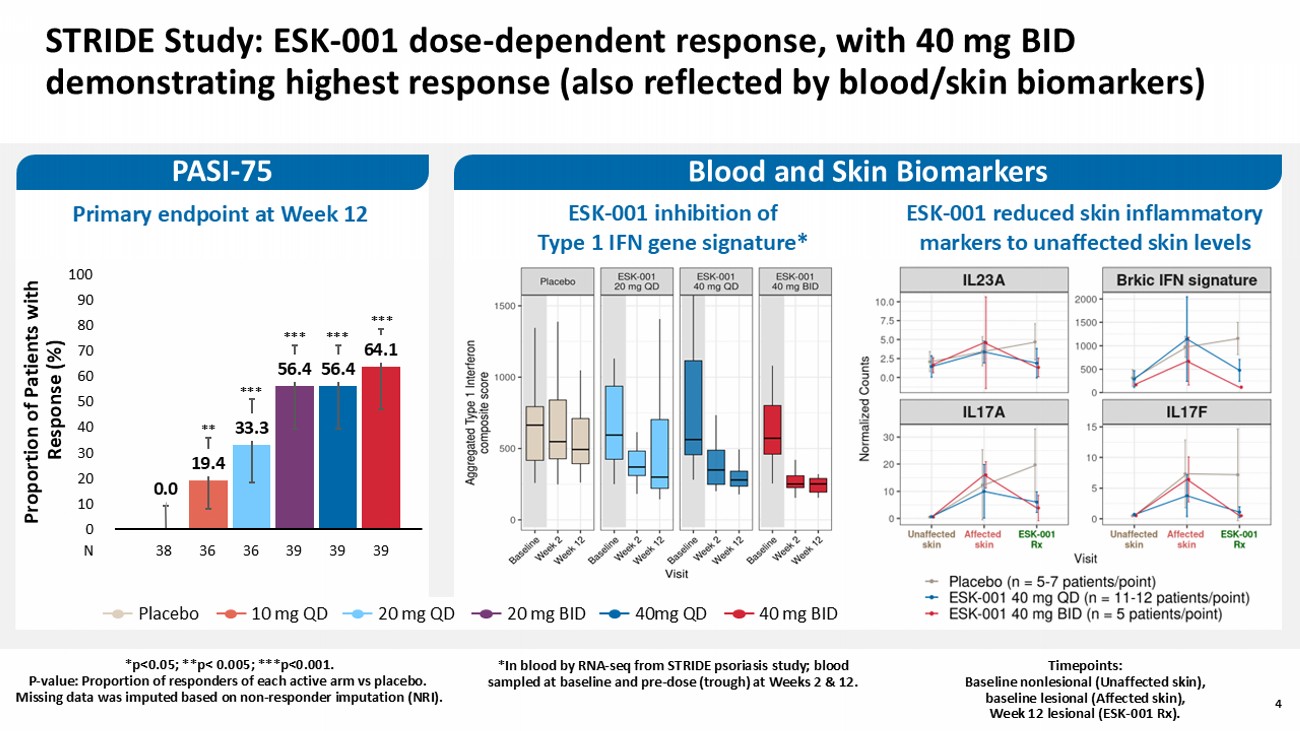
4 STRIDE Study: ESK - 001 dose - dependent response, with 40 mg BID demonstrating highest response (also reflected by blood/skin biomarkers) * PASI - 75 0.0 19.4 33.3 56.4 56.4 64.1 0 10 20 30 40 50 60 70 80 90 100 *** *** *** *** ** Proportion of Patients with Response (%) N 39 36 36 39 39 38 Primary endpoint at Week 12 ESK - 001 inhibition of Type 1 IFN gene signature* ESK - 001 reduced skin inflammatory markers to unaffected skin levels Blood and Skin Biomarkers 4 *p<0.05; **p< 0.005; ***p<0.001. P - value: Proportion of responders of each active arm vs placebo. Missing data wa s imputed based on n on - responder imputation (NRI). *In blood by RNA - seq from STRIDE psoriasis study ; blood sampled at baseline and pre - dose (trough) at W eek s 2 & 12 . 40 mg BID 20 mg BID 40mg QD 20 mg QD 10 mg QD Placebo Timepoints: Baseline nonlesional (Unaffected skin), baseline lesional (Affected skin), Week 12 lesional (ESK - 001 Rx).
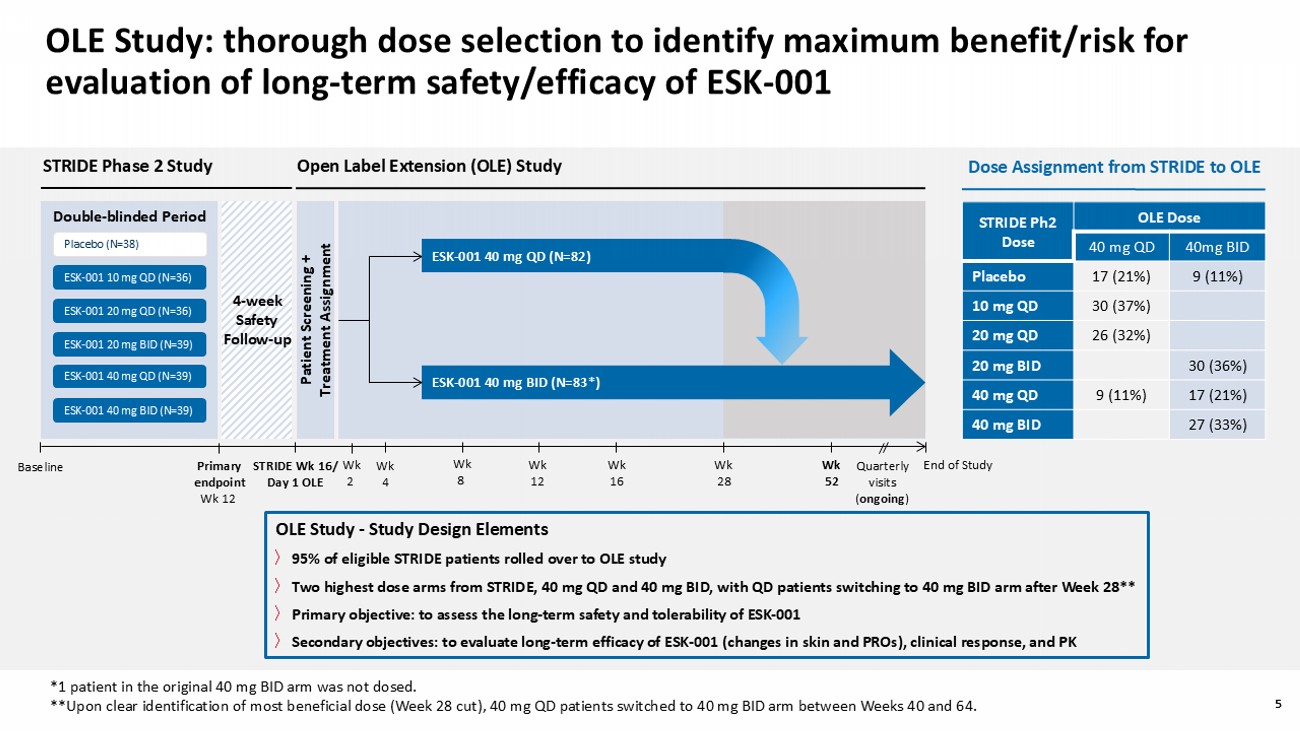
5 OLE Study: thorough dose selection to identify maximum benefit/risk for evaluation of long - term safety/efficacy of ESK - 001 OLE Study - Study Design Elements 〉 95% of eligible STRIDE patients rolled over to OLE study 〉 Two highest dose arms from STRIDE , 40 mg QD and 40 mg BID, with QD patients switching to 40 mg BID arm after Week 28** 〉 Primary objective: to assess the long - term safety and tolerability of ESK - 001 〉 Secondary objectives: to evaluate long - term efficacy of ESK - 001 (changes in skin and PROs), clinical response, and PK *1 patient in the original 40 mg BID arm was not dosed . **Upon clear identification of most beneficial dose (Week 28 cut), 40 mg QD patients switched to 40 mg BID arm between Weeks 40 and 64 .
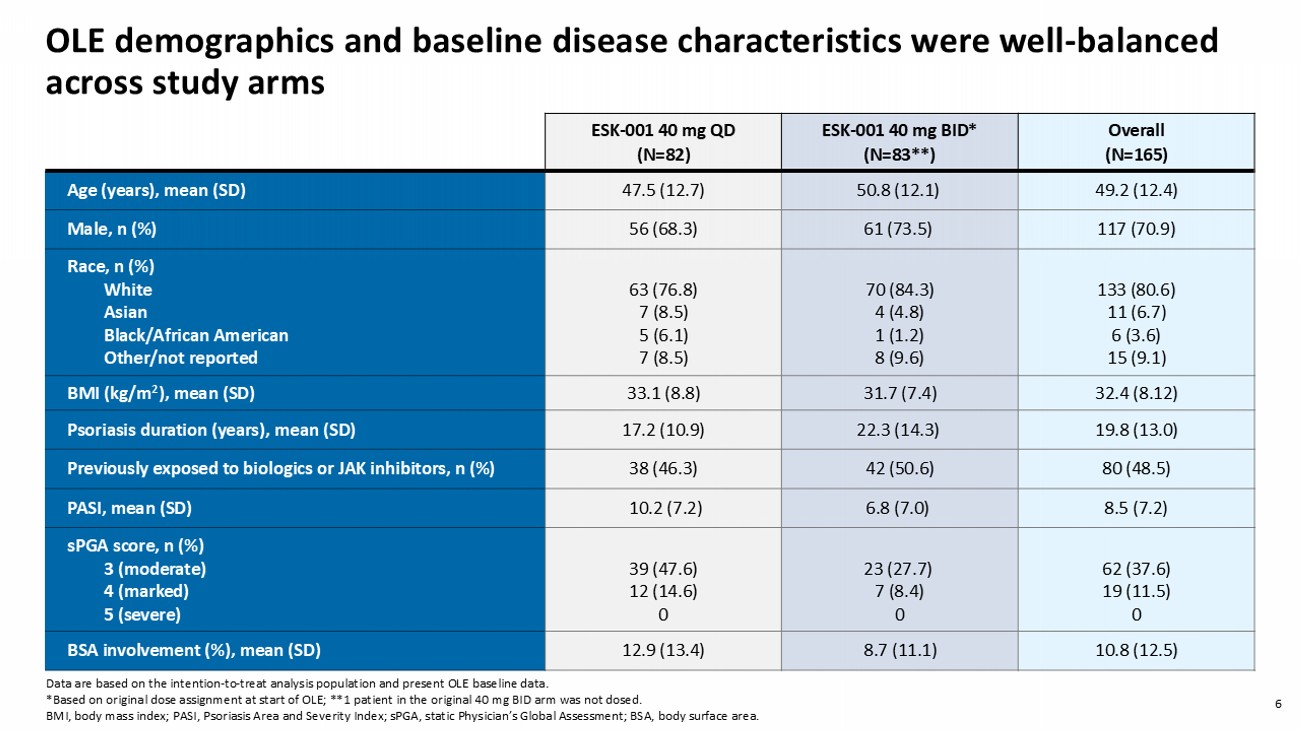
5 Primary endpoint Wk 12 STRIDE Wk 16/ Day 1 OLE STRIDE Phase 2 Study Patient Screening + Treatment Assignment Baseline Open Label Extension (OLE) Study Wk 8 Wk 2 Wk 4 Wk 12 Wk 16 End of Study Quarterly visits ( ongoing ) Wk 28 4 - week Safety Follow - up Double - b linded Period ESK - 001 10 mg QD (N=36) ESK - 001 20 mg QD (N=36) ESK - 001 40 mg QD (N=39) ESK - 001 20 mg BID (N=39) ESK - 001 40 mg BID (N=39) Placebo (N=38) Wk 52 ESK - 001 40 mg BID (N=83*) ESK - 001 40 mg QD (N=82) OLE Dose STRIDE Ph2 Dose 40mg BID 40 mg QD 9 (11%) 17 (21%) Placebo 30 (37%) 10 mg QD 26 (32%) 20 mg QD 30 (36%) 20 mg BID 17 (21%) 9 (11%) 40 mg QD 27 (33%) 40 mg BID Dose Assignment from STRIDE to OLE 6 OLE demographics and baseline disease characteristics were well - balanced across study arms Overall (N=165) ESK - 001 40 mg BID* (N=83**) ESK - 001 40 mg QD (N=82) 49.2 (12.4) 50.8 (12.1) 47.5 (12.7) Age (years), mean (SD) 117 (70.9) 61 (73.5) 56 (68.3) Male, n (%) 133 (80.6) 11 (6.7) 6 (3.6) 15 (9.1) 70 (84.3) 4 (4.8) 1 (1.2) 8 (9.6) 63 (76.8) 7 (8.5) 5 (6.1) 7 (8.5) Race, n (%) White Asian Black/African American Other/not reported 32.4 (8.12) 31.7 (7.4) 33.1 (8.8) BMI (kg/m 2 ), mean (SD) 19.8 (13.0) 22.3 (14.3) 17.2 (10.9) Psoriasis duration (years), mean (SD) 80 (48.5) 42 (50.6) 38 (46.3) Previously exposed to biologics or JAK inhibitors, n (%) 8.5 (7.2) 6.8 (7.0) 10.2 (7.2) PASI, mean (SD) 62 (37.6) 19 (11.5) 0 23 (27.7) 7 (8.4) 0 39 (47.6) 12 (14.6) 0 sPGA score, n (%) 3 (moderate) 4 (marked) 5 (severe) 10.8 (12.5) 8.7 (11.1) 12.9 (13.4) BSA involvement (%), mean (SD) Data are based on the intention - to - treat analysis population and present OLE baseline data. *Based on original dose assignment at start of OLE; **1 patient in the original 40 mg BID arm was not dosed. BMI, body mass index; PASI, Psoriasis Area and Severity Index; sPGA , static Physician’s Global Assessment; BSA, body surface area.
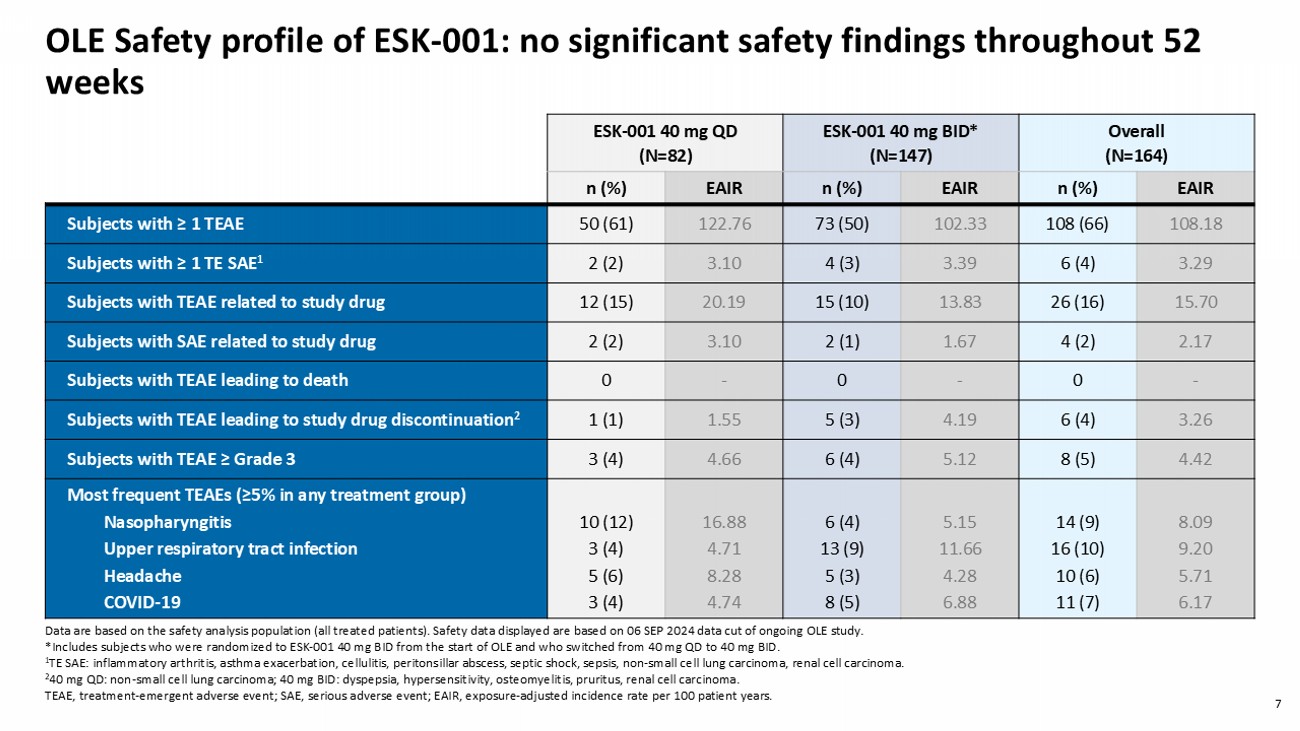
7 OLE Safety profile of ESK - 001: no significant safety findings throughout 52 weeks Overall (N=164) ESK - 001 40 mg BID* (N=147) ESK - 001 40 mg QD (N=82) EAIR n (%) EAIR n (%) EAIR n (%) 108.18 108 (66) 102.33 73 (50) 122.76 50 (61) Subjects with ≥ 1 TEAE 3.29 6 (4) 3.39 4 (3) 3.10 2 (2) Subjects with ≥ 1 TE SAE 1 15.70 26 (16) 13.83 15 (10) 20.19 12 (15) Subjects with TEAE related to study drug 2.17 4 (2) 1.67 2 (1) 3.10 2 (2) Subjects with SAE related to study drug - 0 - 0 - 0 Subjects with TEAE leading to death 3.26 6 (4) 4.19 5 (3) 1.55 1 (1) Subjects with TEAE leading to study drug discontinuation 2 4.42 8 (5) 5.12 6 (4) 4.66 3 (4) Subjects with TEAE ≥ Grade 3 8.09 9.20 5.71 6.17 14 (9) 16 (10) 10 (6) 11 (7) 5.15 11.66 4.28 6.88 6 (4) 13 (9) 5 (3) 8 (5) 16 . 88 4.71 8.28 4.74 10 (12) 3 (4) 5 (6) 3 (4) Most frequent TEAEs (≥5% in any treatment group) Nasopharyngitis Upper respiratory tract infection Headache COVID - 19 Data are based on the safety analysis population (all treated patients). Safety data displayed are based on 06 SEP 2024 data cut of ongoing OLE study. *Includes subjects who were randomized to ESK - 001 40 mg BID from the start of OLE and who switched from 40 mg QD to 40 mg BID. 1 TE SAE: inflammatory arthritis, asthma exacerbation, cellulitis, peritonsillar abscess, septic shock, sepsis, non - small cell lung carcinoma, renal ce ll carcinoma. 2 40 mg QD: non - small cell lung carcinoma ; 40 mg BID: dyspepsia, hypersensitivity, osteomyelitis, pruritus, renal cell carcinoma. TEAE, treatment - emergent adverse event; SAE, serious adverse event; EAIR, exposure - adjusted incidence rate per 100 patient years .
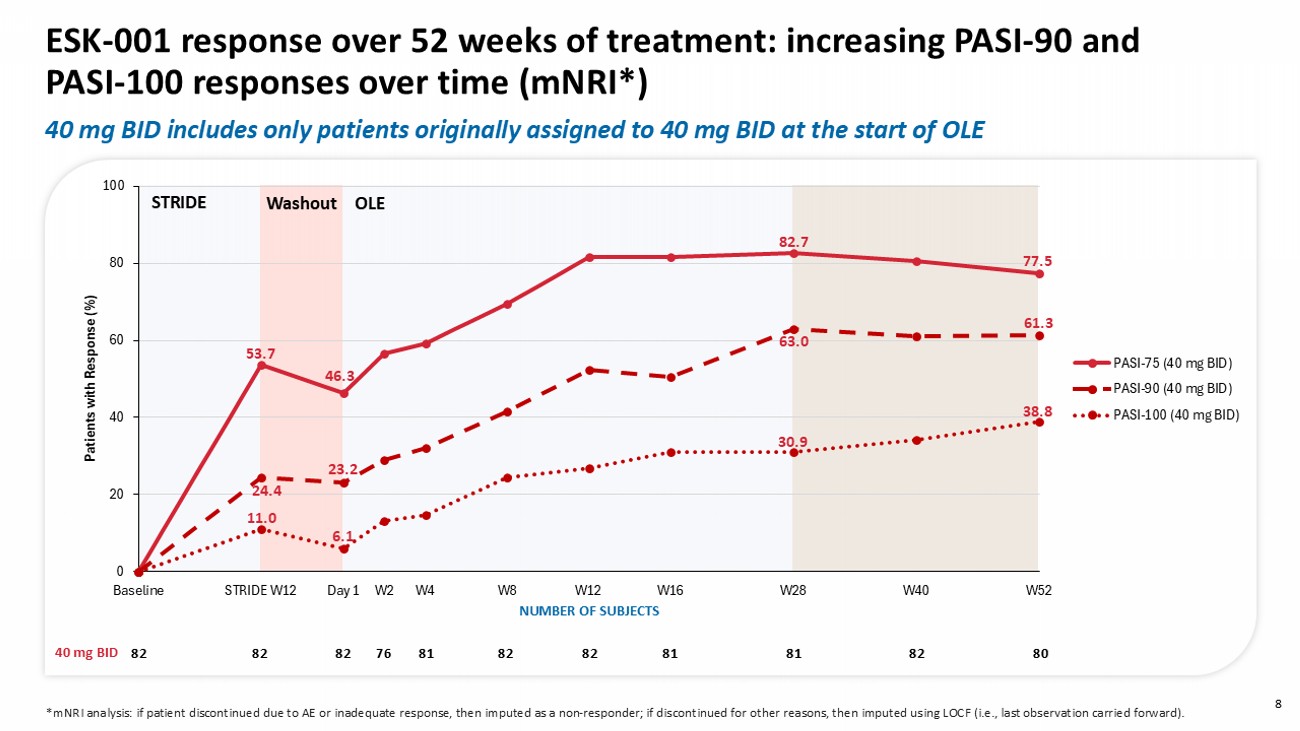
0 20 40 60 80 100 Baseline STRIDE W12 Day 1 W2 W4 W8 W12 W16 W28 W40 W52 Patients with R esponse (%) PASI-75 (40 mg BID) PASI-90 (40 mg BID) PASI-100 (40 mg BID) 53.7 24.4 11.0 46.3 23.2 6.1 82.7 63.0 30.9 77.5 38.8 61.3 Washout STRIDE OLE 8 ESK - 001 response over 52 weeks of treatment: increasing PASI - 90 and PASI - 100 responses over time ( mNRI *) 40 mg BID includes only patients originally assigned to 40 mg BID at the start of OLE * mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF (i.e., last observation carried forward).
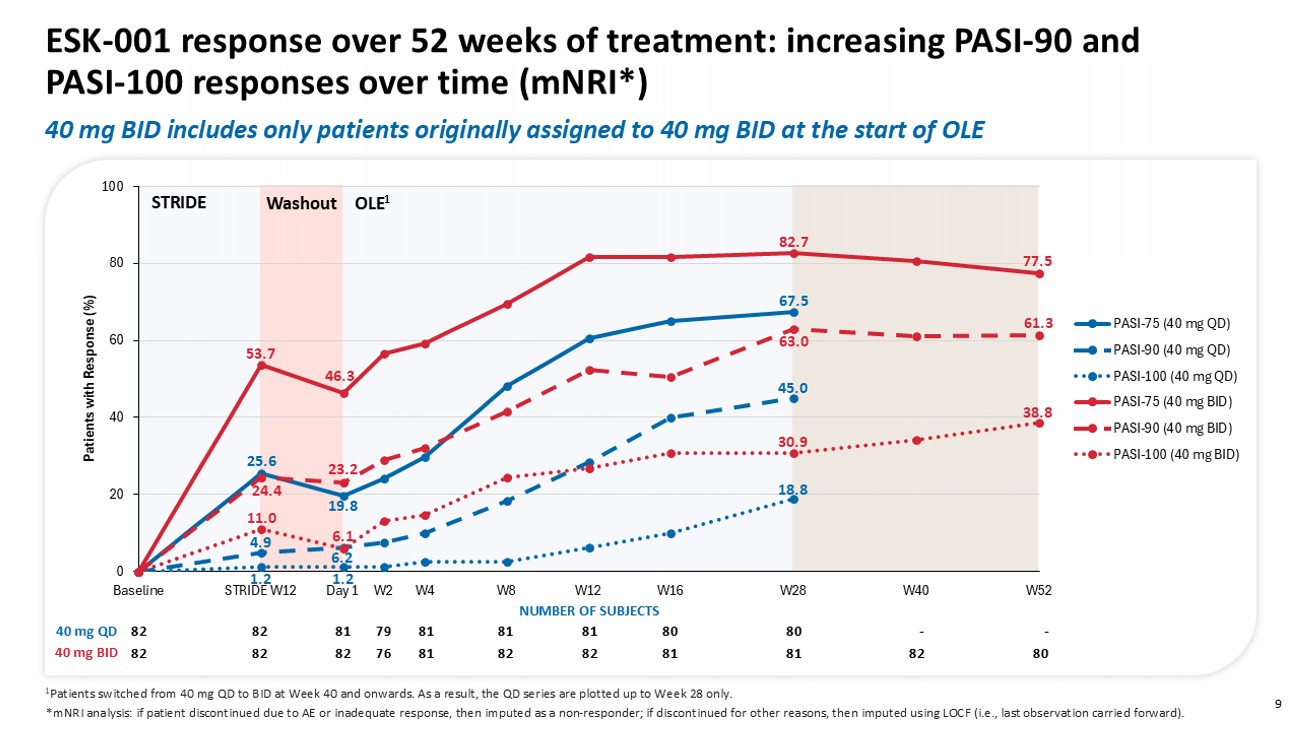
NUMBER OF SUBJECTS 80 82 81 81 82 82 81 76 82 82 82 40 mg BID 0 20 40 60 80 100 Baseline STRIDE W12 Day 1 W2 W4 W8 W12 W16 W28 W40 W52 Patients with R esponse (%) PASI-75 (40 mg QD) PASI-90 (40 mg QD) PASI-100 (40 mg QD) PASI-75 (40 mg BID) PASI-90 (40 mg BID) PASI-100 (40 mg BID) - - 80 80 81 81 81 79 81 82 82 80 82 81 81 82 82 81 76 82 82 82 1 Patients switched from 40 mg QD to BID at Week 40 and onwards. A s a result, the QD series are plotted up to Week 28 only. 40 mg QD 40 mg BID * mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF (i.e., last observation carried forward). NUMBER OF SUBJECTS 25.6 4.9 1.2 19.8 6.2 1.2 67.5 45.0 18.8 53.7 24.4 11.0 46.3 23.2 6.1 82.7 63.0 30.9 77.5 38.8 61.3 Washout STRIDE OLE 1 9 ESK - 001 response over 52 weeks of treatment: increasing PASI - 90 and PASI - 100 responses over time ( mNRI *) 40 mg BID includes only patients originally assigned to 40 mg BID at the start of OLE 0 20 40 60 80 100 Baseline STRIDE W12 Day 1 W2 W4 W8 W12 W16 W28 W40 W52 Patients with R esponse (%) sPGA 0 (40 mg QD) sPGA 0/1 (40 mg QD) sPGA 0 (40 mg BID) sPGA 0/1 (40 mg BID) 10 ESK - 001 response over 52 weeks of treatment: increasing sPGA 0 and 0/1 responses over time ( mNRI *) Washout STRIDE OLE 1 - - 80 80 81 81 81 78 81 82 82 80 82 81 81 82 82 81 76 80 82 82 40 mg QD 40 mg BID NUMBER OF SUBJECTS 61.3 38.8 25.6 51.2 38.8 13.6 67.9 51.3 11.0 2.4 6.3 1.2 18.8 34.6 1 Patients switched from 40 mg QD to BID at Week 40 and onwards.
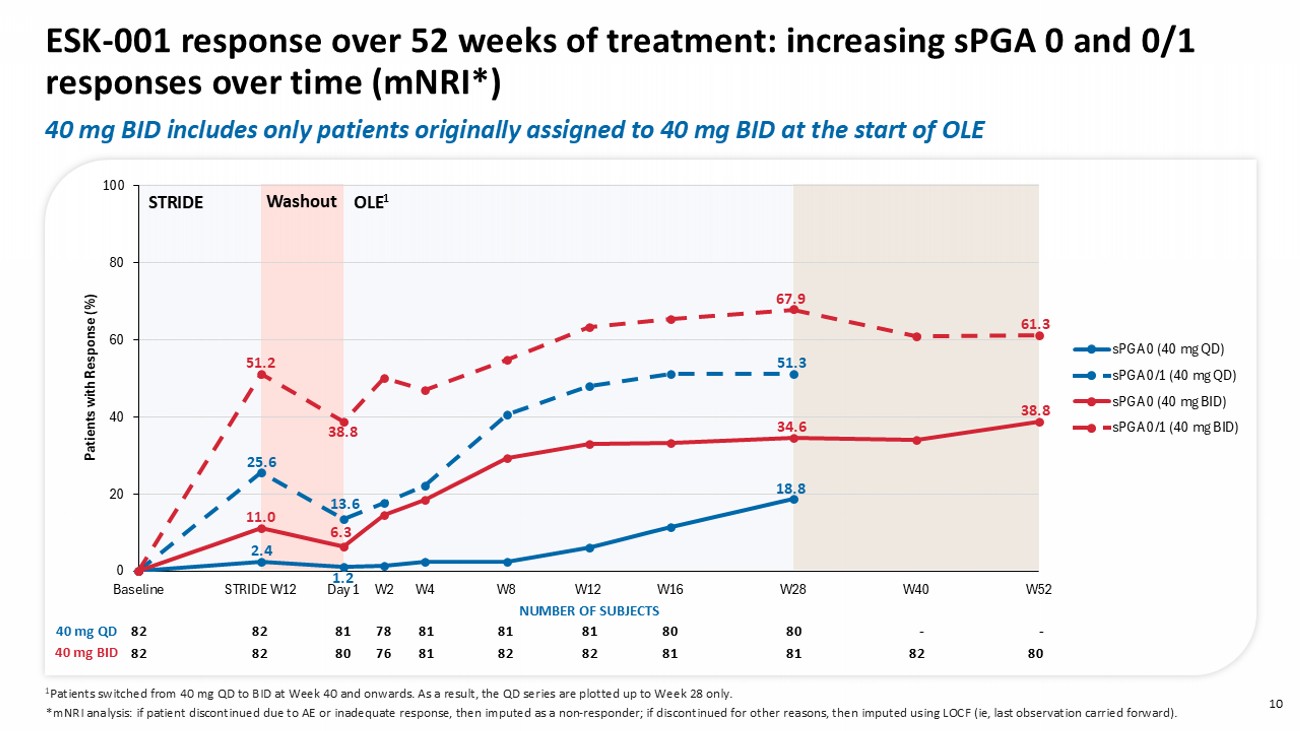
A s a result, the QD series are plotted up to Week 28 only. * mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF ( ie , last observation carried forward). 40 mg BID includes only patients originally assigned to 40 mg BID at the start of OLE 11 Of the original OLE 40 mg BID, 62% patients showed continued improvement in PASI response at Week 52 compared to Week 12 *Of the 18 non - responders at Week 52, 11 discontinued study early.
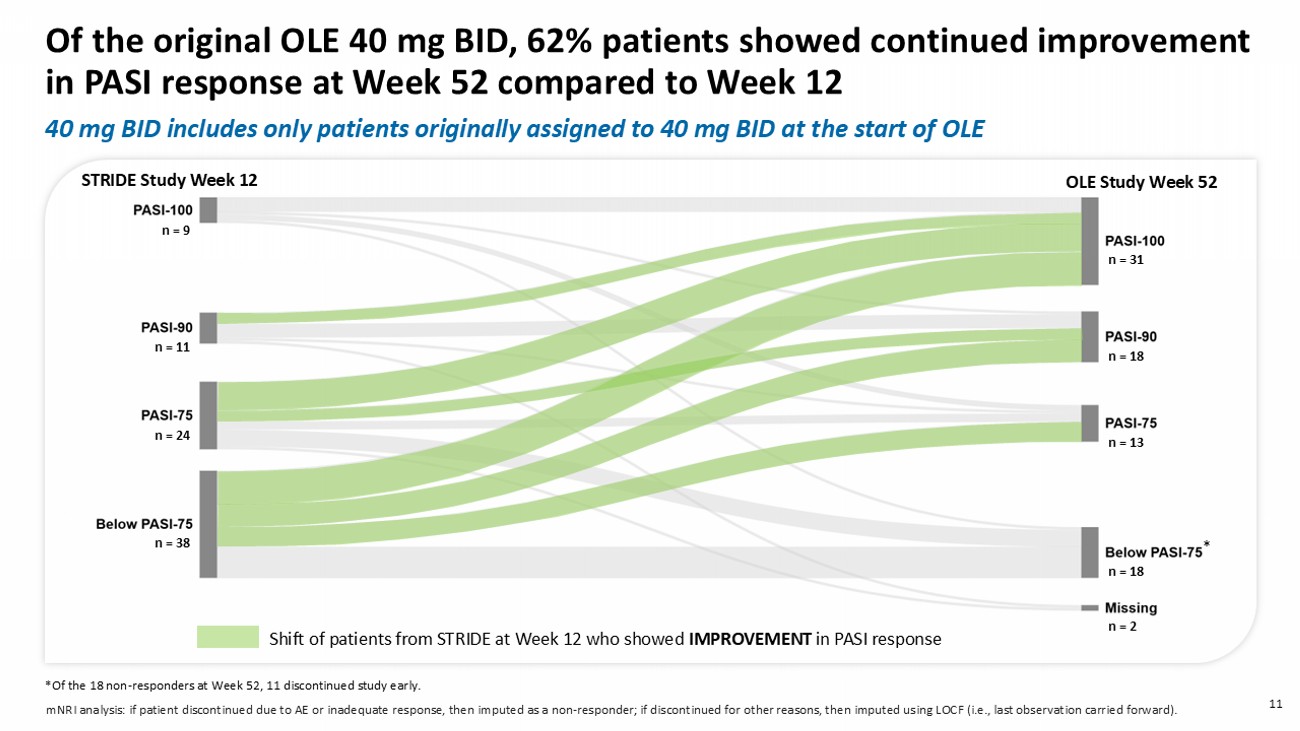
mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF (i.e., last observation carried forward). n = 38 n = 24 n = 11 n = 9 n = 18 n = 13 n = 18 n = 31 n = 2 * STRIDE Study Week 12 OLE Study Week 52 40 mg BID includes only patients originally assigned to 40 mg BID at the start of OLE Shift of patients from STRIDE at Week 12 who showed IMPROVEMENT in PASI response n = 38 n = 24 n = 11 n = 9 n = 18 n = 13 n = 18 n = 31 * STRIDE Study Week 12 OLE Study Week 52 Shift of patients from STRIDE at Week 12 who showed IMPROVEMENT in PASI response Of the original OLE 40 mg BID, 80 % of ≥PASI - 75 responders in STRIDE main tain ed their ≥PASI - 75 response at Week 52 12 40 mg BID includes only patients originally assigned to 40 mg BID at the start of OLE *Of the 18 non - responders at Week 52, 11 discontinued study early.
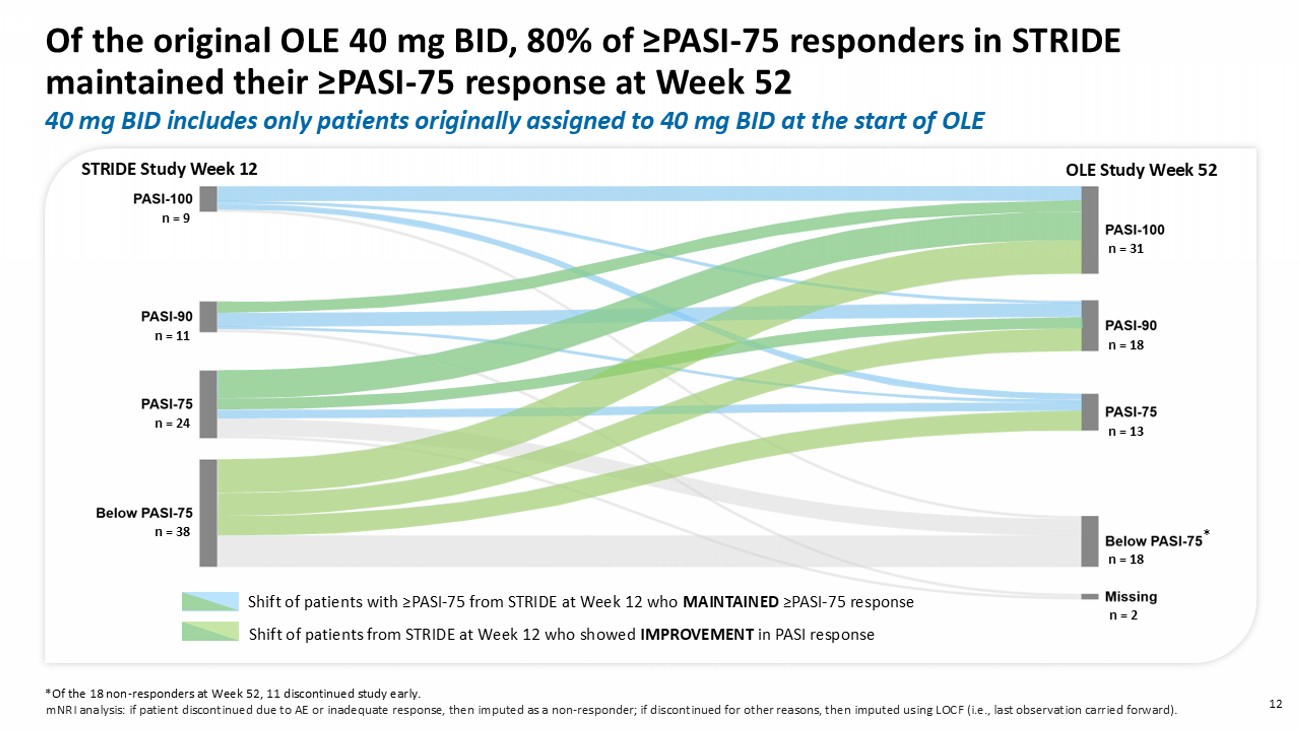
mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF (i.e., last observation carried forward). Shift of patients with ≥ PASI - 75 from STRIDE at Week 12 who MAINTAINED ≥ PASI - 75 response n = 2 Based on the modified intention - to - treat analysis population.

mNRI analysis: if patient discontinued due to AE or inadequate response, then imputed as a non - responder; if discontinued for other reasons, then imputed using LOCF (i.e., last observation carried forward). DLQI, Dermatology Life Quality Index; NRS, numerical rating scale. 13 Patient - reported outcomes throughout Week 52 for original 40 mg BID 58.5 61.3 0 20 40 60 80 100 Proportion of Patients (%) DLQI 0/1 73.2 81.3 0 20 40 60 80 100 Proportion of Patients (%) Average pruritus NRS score <4 STRIDE W12 (N=82) OLE W52 (N=80) Robust and rapid improvements in quality of life and control of itch maintained over time STRIDE W12 (N=82) OLE W52 (N=80)
14 ESK - 001 OLE: 52 - week summary and conclusions Safety summary 〉 ESK - 001 (40 mg BID) remained generally safe and well - tolerated over 52 weeks; majority of TEAEs were mild - to - moderate in severity and self - limited; no safety signals to date Efficacy summary 〉 40 mg BID patients showed high levels of response throughout the study 〉 PASI - 75 (77.5%), PASI - 90 (61.3%) and PASI - 100 (38.8%) ( mNRI ) scores at Week 52 〉 80% of ≥ PASI - 75 responders at Week 12 in STRIDE maintained or improved their response at Week 52; 62% improved response over 52 weeks of treatment 〉 Rapid and sustained improvement in DLQI 0/1 and itch ESK - 001 pivotal program status 〉 ONWARD Phase 3 development program in plaque psoriasis ongoing, with over 600 patients enrolled to date 〉 Modified release (once daily) formulation development ongoing