UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 25, 2025 |
Acrivon Therapeutics, Inc.
(Exact name of Registrant as Specified in Its Charter)
Delaware |
001-41551 |
82-5125532 |
||
(State or Other Jurisdiction |
(Commission File Number) |
(IRS Employer |
||
|
|
|
|
|
|
480 Arsenal Way Suite 100 |
|
|||
Watertown, Massachusetts |
|
02472 |
||
(Address of Principal Executive Offices) |
|
(Zip Code) |
||
Registrant’s Telephone Number, Including Area Code: (617) 207-8979 |
Not Applicable |
|
|
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Securities registered pursuant to Section 12(b) of the Act:
|
|
Trading |
|
|
Common Stock, par value $0.001 per share |
|
ACRV |
|
The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On March 25, 2025, Acrivon Therapeutics, Inc. (the “Company”) disclosed a preliminary cash and cash equivalents and short-term investments balance of approximately $184.6 million as of December 31, 2024. Because the Company’s consolidated financial statements for the twelve months ended December 31, 2024 have not yet been finalized, the preliminary statement of the Company’s cash and cash equivalents and short-term investments as of December 31, 2024 in this Item 2.02 is subject to change, and the Company’s actual cash and cash equivalents and short-term investments as of December 31, 2024 may differ materially from this preliminary estimate. Accordingly, you should not place undue reliance on this preliminary estimate.
The information in this Item 2.02 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 7.01 Regulation FD Disclosure.
On March 25, 2025, the Company hosted a virtual corporate R&D event from approximately 4:00 p.m. to 5:15 p.m. ET. The agenda featured presentations by the Company’s leadership team and external key opinion leaders, followed by an interactive Q&A session. In connection with this event, the Company posted to the “Investors & Media” section of the Company’s website at ir.acrivon.com, the presentation used in the event (the “R&D Event Presentation”).
A copy of the R&D Event Presentation is attached hereto as Exhibit 99.1, and is incorporated by reference into this Item 7.01 of this Current Report on Form 8-K.
The information in this Item 7.01, including Exhibit 99.1 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as expressly set forth by specific reference in such filing.
Item 8.01 Other Events.
Program Updates
On March 25, 2025, the Company presented an interim data extract from the Electronic Data Capture (EDC) clinical database that was done on February 25, 2025, including 20 Oncosignature-positive, or BM+, endometrial cancer patients treated with ACR-368 monotherapy and 38 Oncosignature-negative, or BM-, patients treated with ACR-368 plus ultra low dose gemcitabine (LDG) that were efficacy-evaluable by RECIST (2 BM- had treatment discontinued without scan).
All BM+ patients had progressed after prior platinum-based chemotherapy and prior anti-PD-1, and the median and mean prior lines of therapy for these patients were 2 and 2.6, respectively. A majority of these BM+ patients were refractory to the last prior line of therapy, with aggressive, generally heavily pre-treated tumors: 12 had refractory disease (best overall response of PD in the last prior line of therapy), 6 had relapsed disease, and 2 unknown. Amongst these 20 BM+ patients, 15 were either serous or carcinosarcomas, 13 were pMMR (2 dMMR, 5 not tested), and 11 p53 mutated (3 wild-type; 6 unknown). In patients that had relapsed after the prior line of therapy (N=6), the confirmed overall response rate, or ORR, was 50% and the DCR was 100%. Amongst the 12 patients with tumors refractory to the last prior line of therapy (ORR = 0%) we observed meaningful ACR-368 clinical activity with a confirmed ORR of 33% and disease control rate (DCR) of 75%. The ACR-368 OncoSignature accurately identified patients whose tumors are sensitive to ACR-368, with 80% of BM+ patients demonstrating tumor shrinkage. Among all 20 BM+ patients the confirmed ORR was 35% and the DCR was 80%. Overall, these results demonstrate significant anti-tumor activity and disease control in BM+ patients with aggressive, refractory tumors that did not respond at all (0 % ORR) to the last line of prior therapy, and with a confirmed ORR more than double (35%) than the best ORR observed in the last prior line of therapy (15%) for all BM+ patients.
Preliminary analyses of the 38 BM- patients, who are heavily pretreated (median of 3 prior lines of therapy) show a confirmed ORR of ~13% with the ACR-368 + LDG combination, which is comparable to the best ORR in the last prior line of therapy (median = 3) in these patients, which was 17%. Based on the totality of the preclinical and observed clinical data, the Company believes this supports significant LDG sensitization to ACR-368 in BM- patients. The Company expects a similar sensitization in BM+ patients which could be explored in a future all-comer study of ACR-368 + LDG. At ESMO 2024 (September 14, 2024 R&D event and press release), the Company reported that endometrial cancer was its prioritized indication, as it represents the first potential registrational opportunity for ACR-368. The Company remains confident in this strategy based on emerging clinical data, competitive positioning given limited treatment options, and the strong commercial opportunity in both second- and front-line settings. The Company’s blinded KOL market research estimates that there are approximately 27,000 U.S. patients annually in the second-line setting alone for endometrial cancer.
Due to increased competition and a smaller market opportunity, the Company set a high internal clinical bar for ovarian cancer, which preliminary data suggests is unlikely to be met. Bladder cancer is also being deprioritized due to lower than preclinically predicted BM+ rate, leading to challenging enrollment with single digit BM+ patient enrollment to date. The Company has now officially deprioritized ovarian and bladder cancers, reallocating all clinical resources to ACR-368 in endometrial cancer and ACR-2316.
The Company's second clinical stage asset, ACR-2316, is in an ongoing Phase 1 monotherapy clinical trial in patients with advanced solid tumors. The trial was initiated during the third quarter of 2024, two quarters ahead of the Company's original timelines, and the Company is currently completing Dose Level (DL) 3 after clearing of DL1 and DL2 by the Safety Review Committee, without safety concerns or dose-limiting toxicities. Based on pharmacokinetic (PK) analysis in the first two DL cohorts, the Company has observed encouraging dose proportionality. Moreover, using internal MS-based AP3 profiling to support the clinical trial of ACR-2316, the Company is already detecting drug target engagement in peripheral blood mononuclear cells (PBMCs) in DL1. In addition, initial clinical activity has been observed in a patient in DL3, with significant decrease in size of metastatic lesions throughout the chest, abdomen and pelvis. This patient (who had received 3 prior lines of therapy including chemotherapy and anti-PD-1) remains on therapy. Using AP3-based Indication Finding and AP3-based analyses of in-house and publicly available data, the Company is enrolling selected high unmet need solid tumor types predicted sensitive to ACR-2316 in the Phase 1 trial.
The Company believes that its cash, cash equivalents and short-term investments as of December 31, 2024 will be sufficient to fund the Company’s operations into 2027.
Item 9.01 Financial Statements and Exhibits.
|
Exhibit Number |
|
Description |
99.1 |
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Acrivon Therapeutics, Inc. |
|
|
|
|
Date: |
March 25, 2025 |
By: |
/s/ Peter Blume-Jensen |
|
|
|
Name: Peter Blume-Jensen, M.D., Ph.D. |

CORPORATE R&D EVENT March 25, 2025

Forward-looking statements Certain information contained in this presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 regarding our future results of operations or financial condition, business strategy and plans and objectives of management for future operations. In some cases, you can identify forward-looking statements because they contain words such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” or “would” or the negative of these words or other similar terms or expressions. Our forward-looking statements are based primarily on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and results of operations. The outcome of the events described in the forward-looking statements is subject to risks and uncertainties, including the factors described in our filings with the U.S. Securities and Exchange Commission. New risks and uncertainties emerge from time to time, and it is not possible for us to predict all risks and uncertainties that could have an impact on the forward-looking statements contained in this presentation. The results, events, and circumstances reflected in the forward-looking statements may not be achieved or occur, and actual results, events, or circumstances could differ materially from those described in the forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which are made only as of the date of this presentation. We undertake no obligation to update any forward-looking statements or to reflect new information or the occurrence of unanticipated events, except as required by law.

Peter Blume-Jensen, M.D., Ph.D. CEO, President and Founder; Inventor of the AP3 Platform Kristina Masson, Ph.D., M.B.A. Co-Founder and EVP, Business Operations; President and CEO, Acrivon AB William Bradley, M.D. Attending Physician, Department of Radiology, Massachusetts General Hospital; Clinical Instructor, Harvard Medical School; Senior Clinical Advisor, Acrivon Acrivon TEAM Participants Adam Levy, Ph.D., M.B.A. Senior Vice President of Investor Relations and Corporate Affairs Eric Devroe, Ph.D. Chief Operating Officer Jean-Marie Cuillerot, M.D. Chief Medical Officer

Key opinion leader Participants Mansoor Raza Mirza, M.D. Chief Oncologist at Copenhagen University Hospital, Denmark; Board of Directors, Gynecologic Cancer Inter-Group (GCIG); Medical Director of the Nordic Society of Gynecologic Oncology-Clinical Trial Unit (NSGO-CTU); Vice President of the European Society of Gynecological Oncology (ESGO) Robert Coleman, M.D. Co-director of the Gynecologic Oncology Group (GOG) Partners Foundation, Inc.; Chief Medical Officer at Vaniam Group; Gynecologic Oncologist at Texas Oncology, US Oncology Network Jesper Olsen, Ph.D. Professor at the University of Copenhagen; Executive Director at the Novo Nordisk Foundation Center for Protein Research; Academic Co-founder of Acrivon

agenda AP3 Generative Phosphoproteomics: Transforming drug discovery with proteome-wide SAR ACR-368 Phase 2 program update ACR-2316 Phase 1 program update Brief update on preclinical cell cycle program Extended runway guidance Q&A
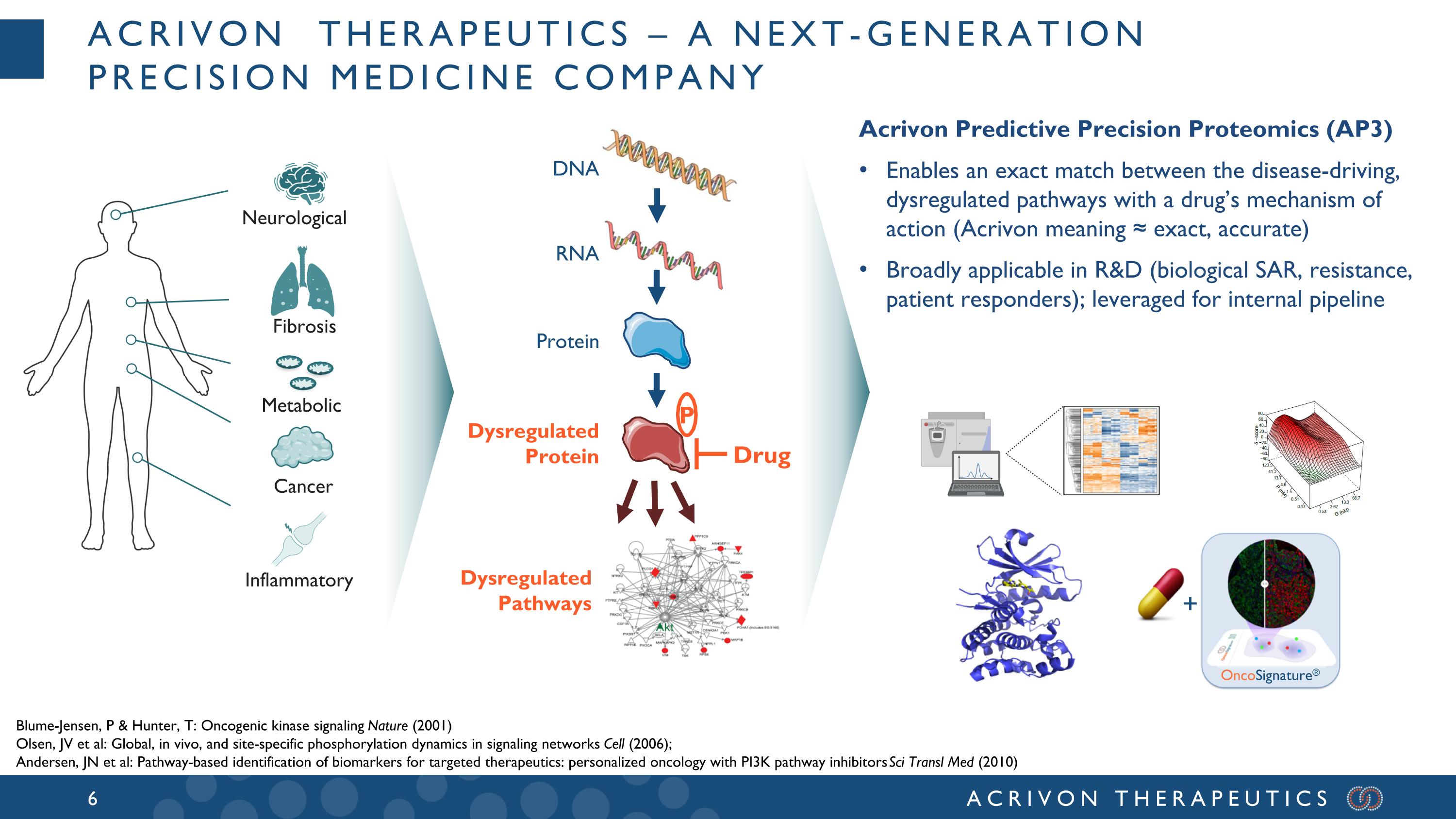
Acrivon Therapeutics – a next-generation precision medicine company Neurological Cancer Inflammatory Fibrosis Drug P Metabolic Acrivon Predictive Precision Proteomics (AP3) Enables an exact match between the disease-driving, dysregulated pathways with a drug’s mechanism of action (Acrivon meaning ≈ exact, accurate) Broadly applicable in R&D (biological SAR, resistance, patient responders); leveraged for internal pipeline DNA RNA Protein Dysregulated Protein Dysregulated Pathways + OncoSignature® Blume-Jensen, P & Hunter, T: Oncogenic kinase signaling Nature (2001) Olsen, JV et al: Global, in vivo, and site-specific phosphorylation dynamics in signaling networks Cell (2006); Andersen, JN et al: Pathway-based identification of biomarkers for targeted therapeutics: personalized oncology with PI3K pathway inhibitors Sci Transl Med (2010)
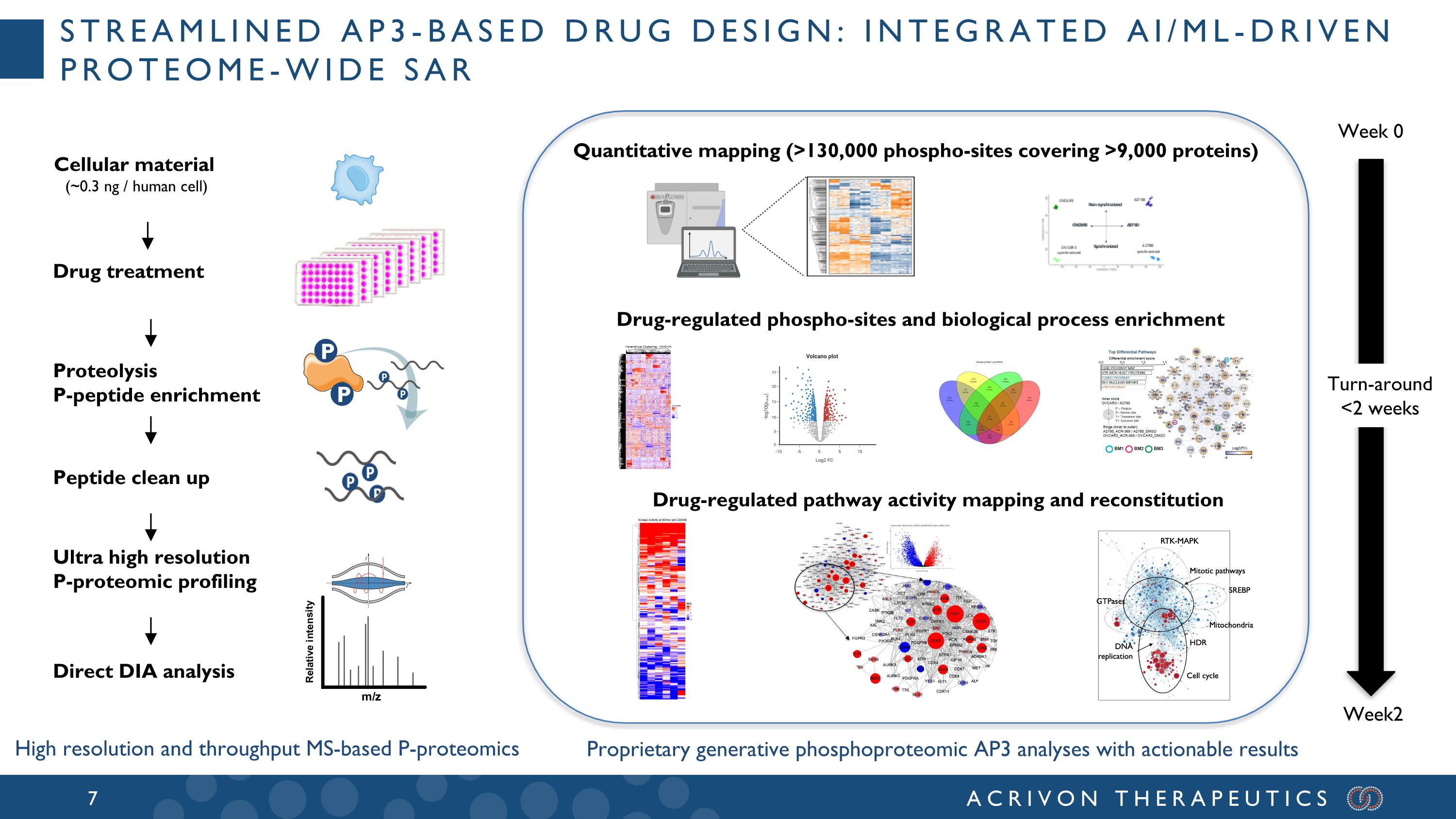
Streamlined AP3-based drug design: integrated AI/ML-driven proteome-wide SAR Peptide clean up Direct DIA analysis Drug treatment Proteolysis P-peptide enrichment Ultra high resolution P-proteomic profiling Week 0 Week2 Turn-around <2 weeks Quantitative mapping (>130,000 phospho-sites covering >9,000 proteins) Drug-regulated phospho-sites and biological process enrichment Drug-regulated pathway activity mapping and reconstitution P P Proprietary generative phosphoproteomic AP3 analyses with actionable results High resolution and throughput MS-based P-proteomics Cellular material (~0.3 ng / human cell)
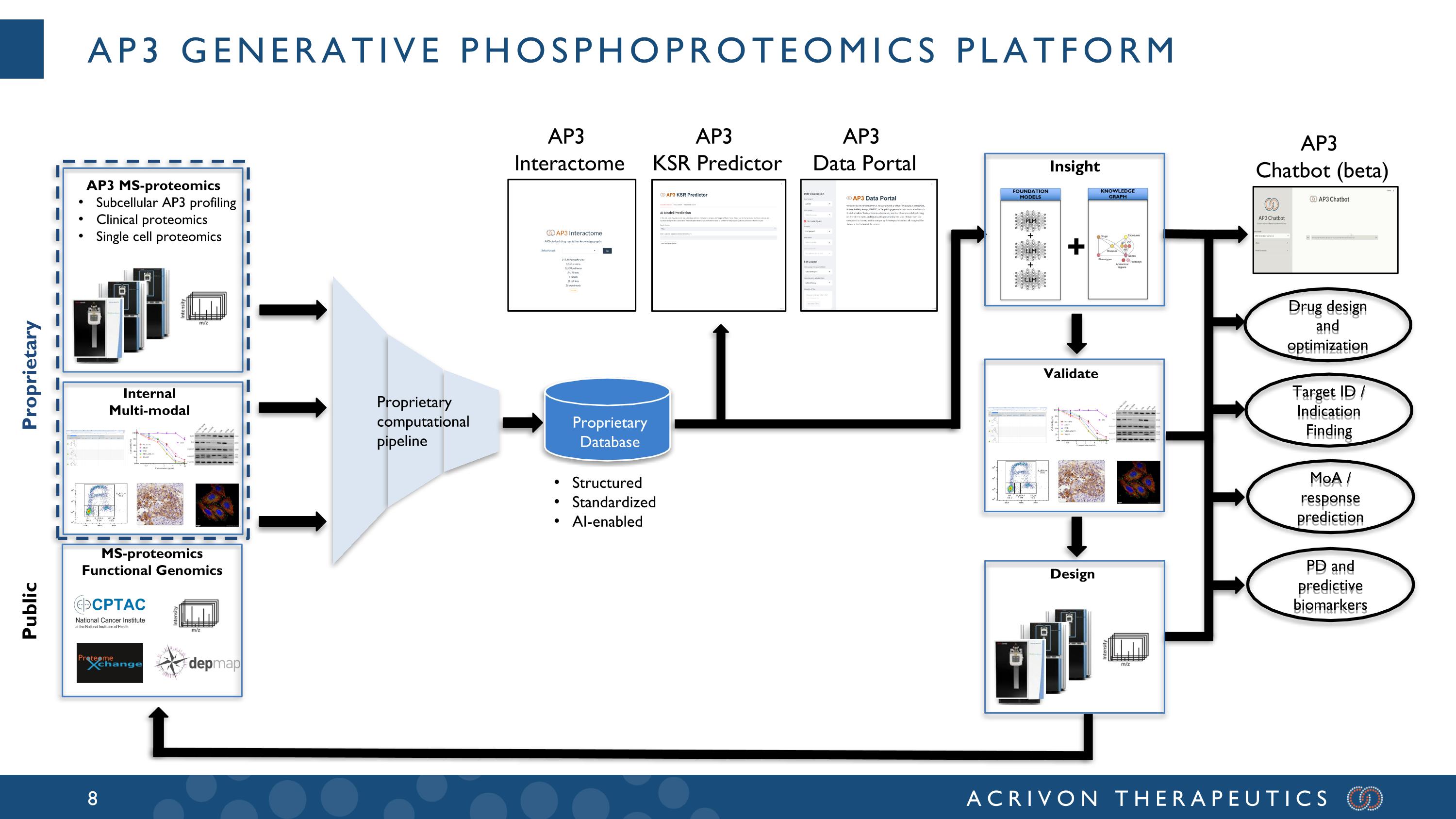
AP3 Generative phosphoproteomics platform Proprietary Database Proprietary computational pipeline Drug design and optimization Target ID / Indication Finding MoA / response prediction PD and predictive biomarkers Structured Standardized AI-enabled AP3 Interactome AP3 KSR Predictor AP3 Data Portal AP3 Chatbot (beta) MS-proteomics Functional Genomics Internal Multi-modal AP3 MS-proteomics Subcellular AP3 profiling Clinical proteomics Single cell proteomics Proprietary Public Validate Design + Insight
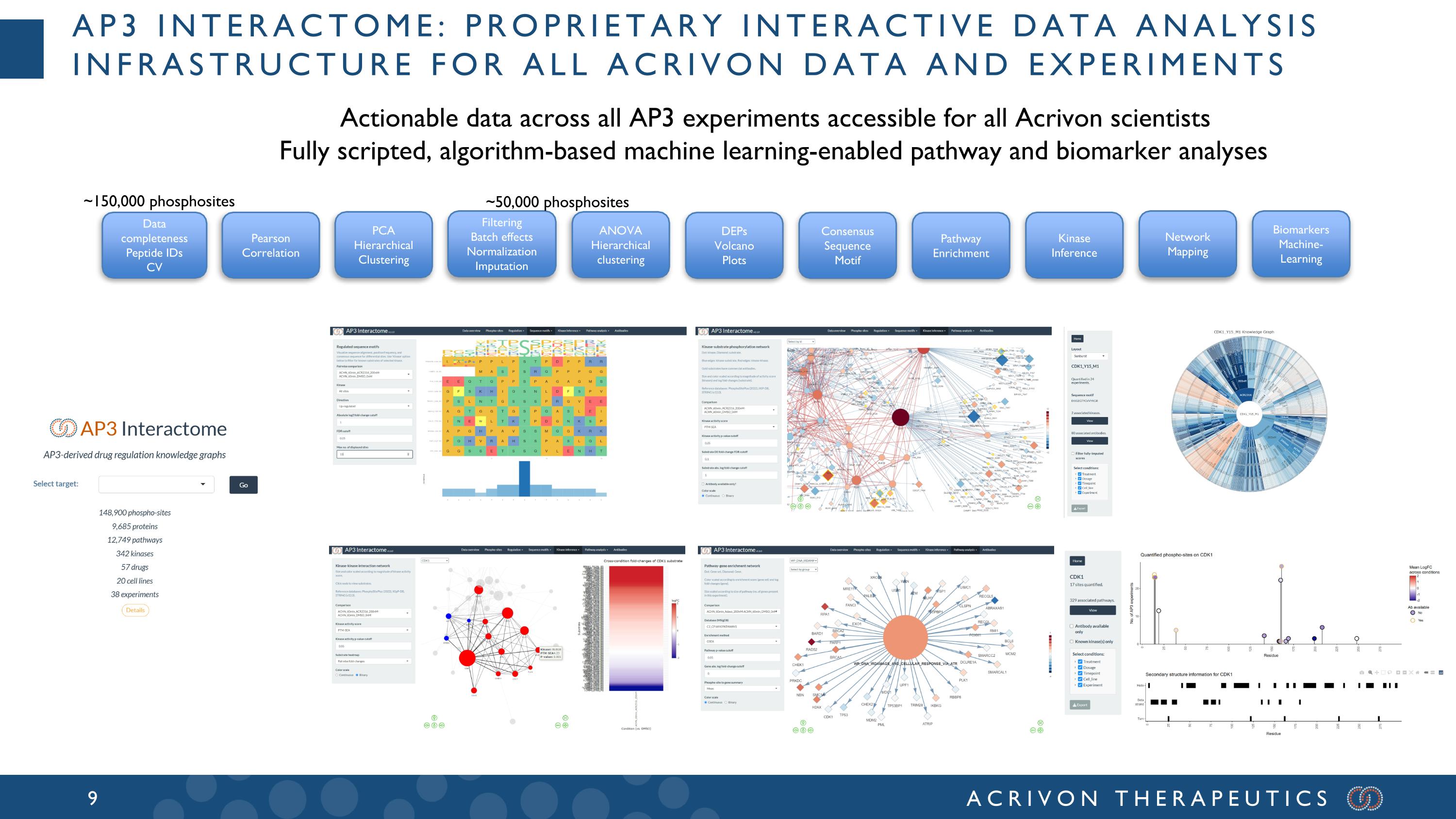
Data completeness Peptide IDs CV Pearson Correlation PCA Hierarchical Clustering DEPs Volcano Plots ANOVA Hierarchical clustering Consensus Sequence Motif Kinase Inference Pathway Enrichment Network Mapping Biomarkers Machine-Learning Filtering Batch effects Normalization Imputation ~150,000 phosphosites ~50,000 phosphosites Ap3 interactome: Proprietary Interactive Data analysis infrastructure for all Acrivon data and experiments Actionable data across all AP3 experiments accessible for all Acrivon scientists Fully scripted, algorithm-based machine learning-enabled pathway and biomarker analyses
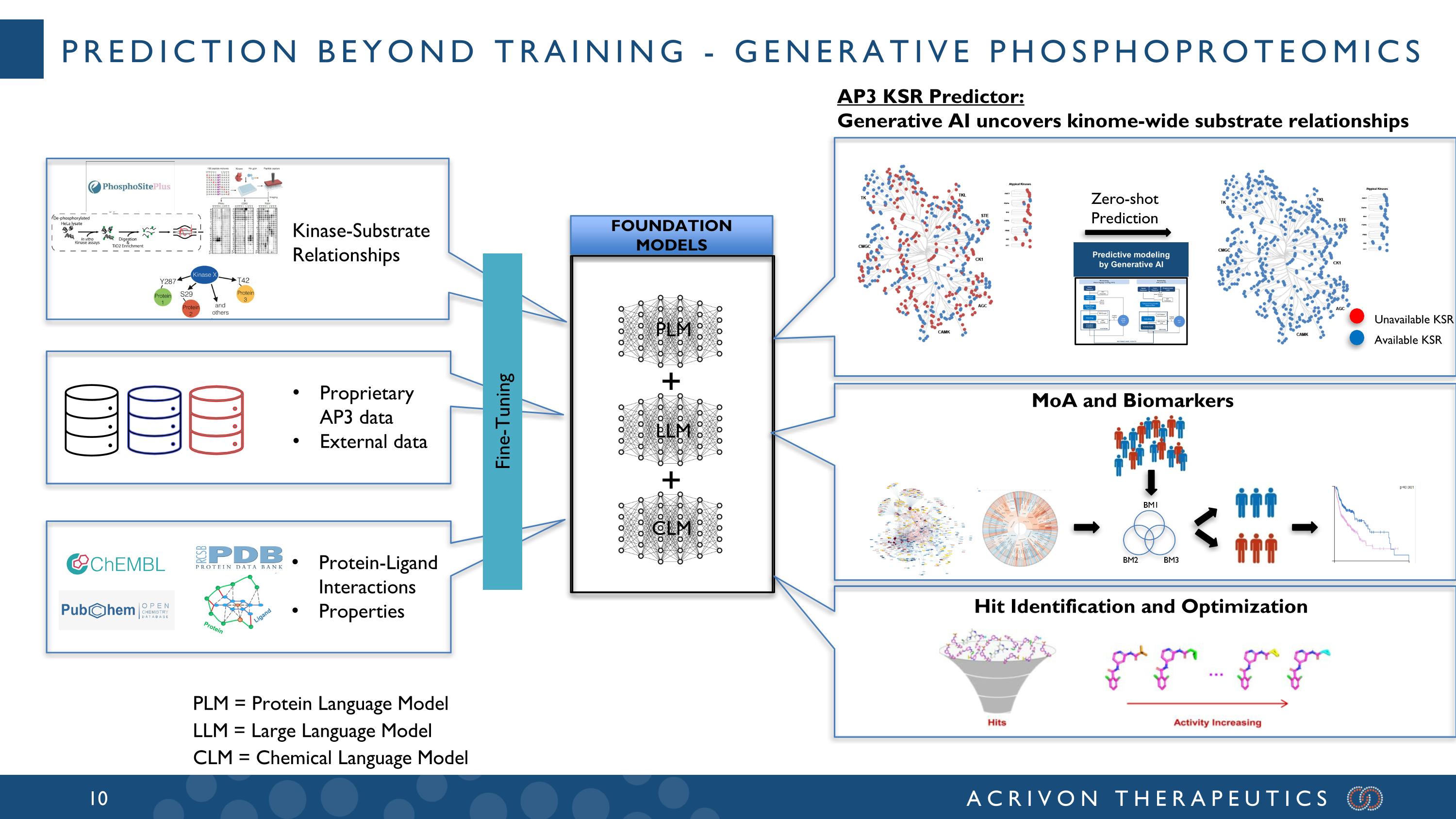
prediction beyond training - Generative phosphoproteomics FOUNDATION MODELS Proprietary AP3 data External data Kinase-Substrate Relationships Protein-Ligand Interactions Properties + + Zero-shot Prediction Available KSR Unavailable KSR AP3 KSR Predictor: Generative AI uncovers kinome-wide substrate relationships Hit Identification and Optimization Fine-Tuning LLM CLM PLM PLM = Protein Language Model LLM = Large Language Model CLM = Chemical Language Model MoA and Biomarkers Predictive modeling by Generative AI
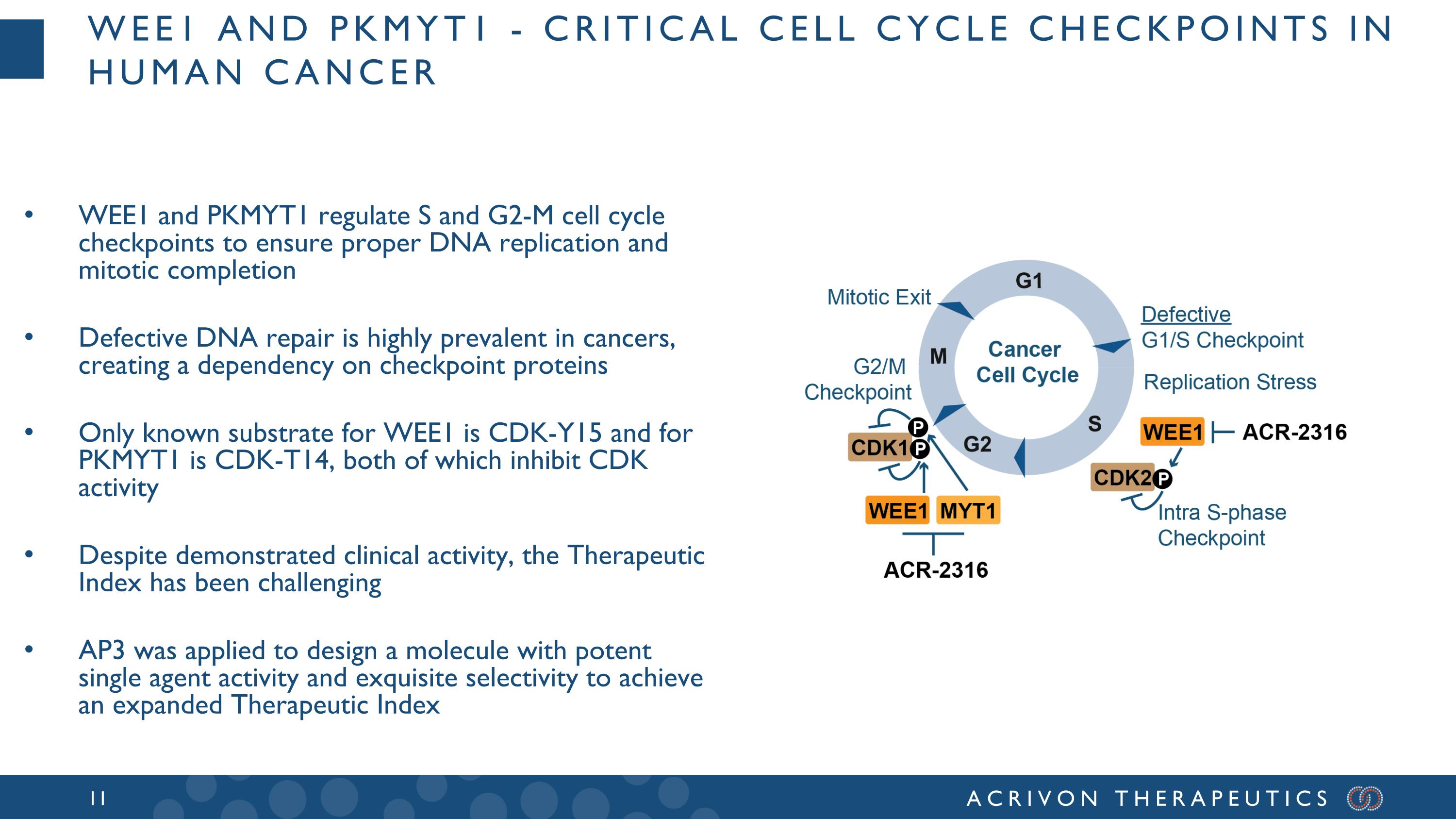
Wee1 and PKMYT1 - critical cell cycle checkpoints in human cancer WEE1 and PKMYT1 regulate S and G2-M cell cycle checkpoints to ensure proper DNA replication and mitotic completion Defective DNA repair is highly prevalent in cancers, creating a dependency on checkpoint proteins Only known substrate for WEE1 is CDK-Y15 and for PKMYT1 is CDK-T14, both of which inhibit CDK activity Despite demonstrated clinical activity, the Therapeutic Index has been challenging AP3 was applied to design a molecule with potent single agent activity and exquisite selectivity to achieve an expanded Therapeutic Index
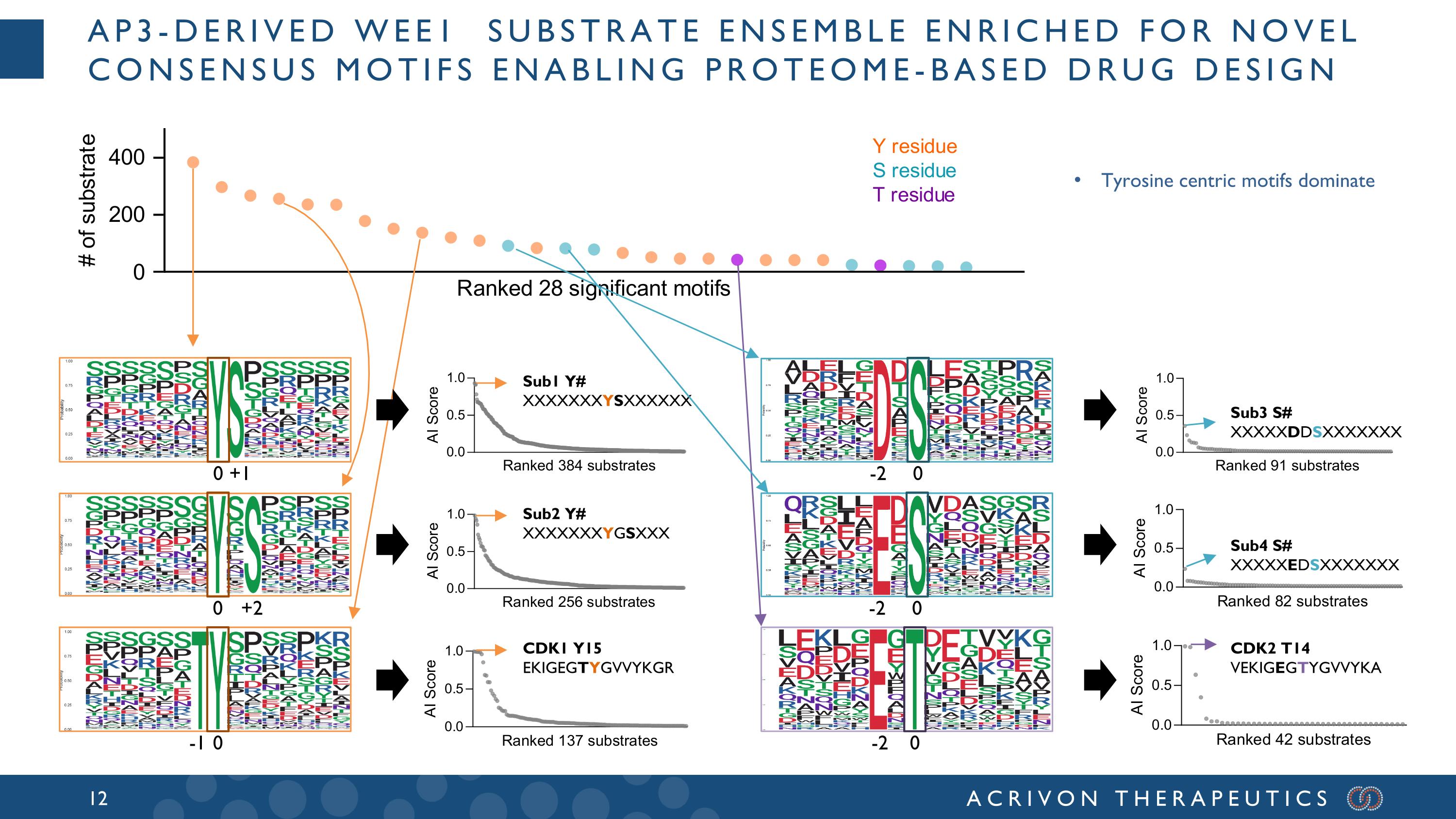
AP3-derived WEE1 substrate ensemble enriched for novel consensus motifs enabling proteome-based drug design 0 +1 0 -1 0 -2 0 +2 CDK1 Y15 EKIGEGTYGVVYKGR Sub1 Y# XXXXXXXYSXXXXXX Sub2 Y# XXXXXXXYGSXXX 0 -2 0 -2 CDK2 T14 VEKIGEGTYGVVYKA Sub3 S# XXXXXDDSXXXXXXX Sub4 S# XXXXXEDSXXXXXXX Tyrosine centric motifs dominate
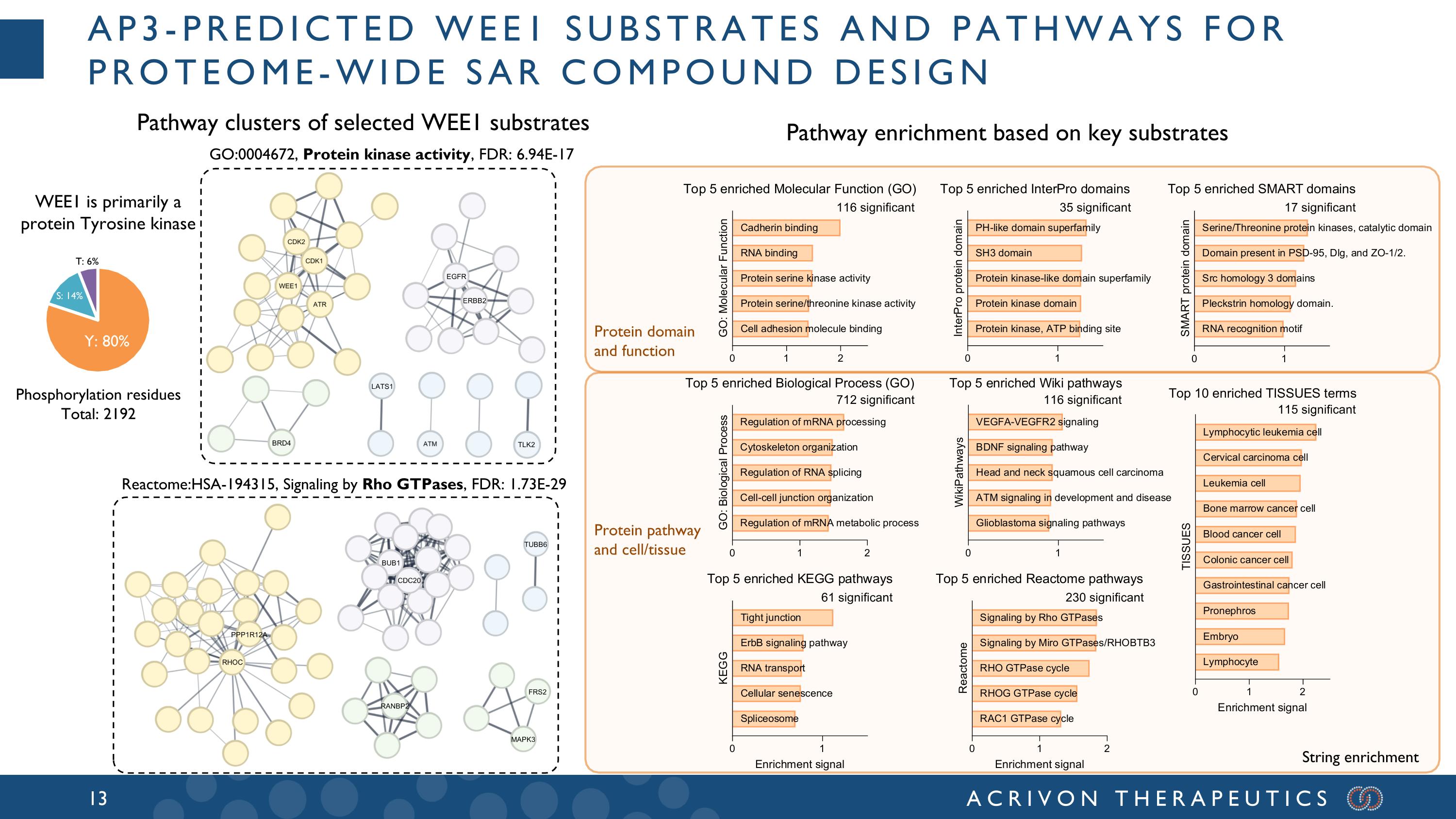
AP3-predicted WEE1 substrates and pathways for proteome-wide SAR compound design Y: 80% S: 14% T: 6% WEE1 is primarily a protein Tyrosine kinase Pathway clusters of selected WEE1 substrates Protein domain and function Protein pathway and cell/tissue Pathway enrichment based on key substrates Reactome:HSA-194315, Signaling by Rho GTPases, FDR: 1.73E-29 String enrichment 116 significant 17 significant 35 significant 712 significant 116 significant 115 significant 61 significant 230 significant GO:0004672, Protein kinase activity, FDR: 6.94E-17 Phosphorylation residues Total: 2192 EGFR ERBB2 LATS1 BRD4 PPP1R12A RANBP2 CDC20 MAPK3 TUBB6 FRS2 BUB1 RHOC TLK2
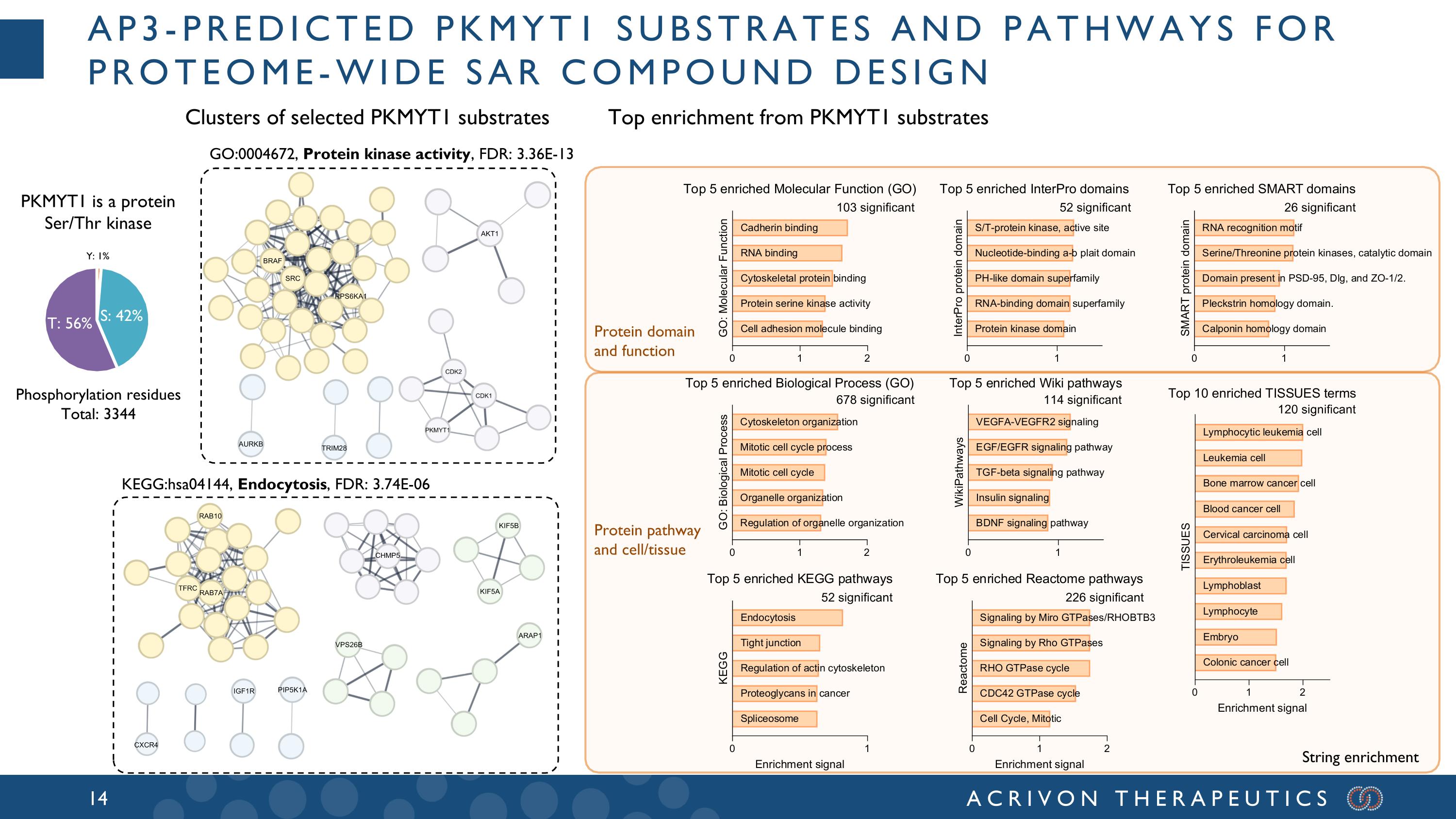
AP3-predicted PKMYT1 substrates and pathways for proteome-wide SAR compound design PKMYT1 is a protein Ser/Thr kinase Clusters of selected PKMYT1 substrates Protein domain and function Protein pathway and cell/tissue Top enrichment from PKMYT1 substrates KEGG:hsa04144, Endocytosis, FDR: 3.74E-06 T: 56% Y: 1% S: 42% 678 significant 103 significant 52 significant 226 significant 114 significant 120 significant 52 significant 26 significant String enrichment GO:0004672, Protein kinase activity, FDR: 3.36E-13 Phosphorylation residues Total: 3344 AURKB AKT1 SRC BRAF TRIM28 RAB7A TFRC RAB10 KIF5A KIF5B VPS26B CXCR4 IGF1R PIP5K1A RPS6KA1 CHMP5 ARAP1
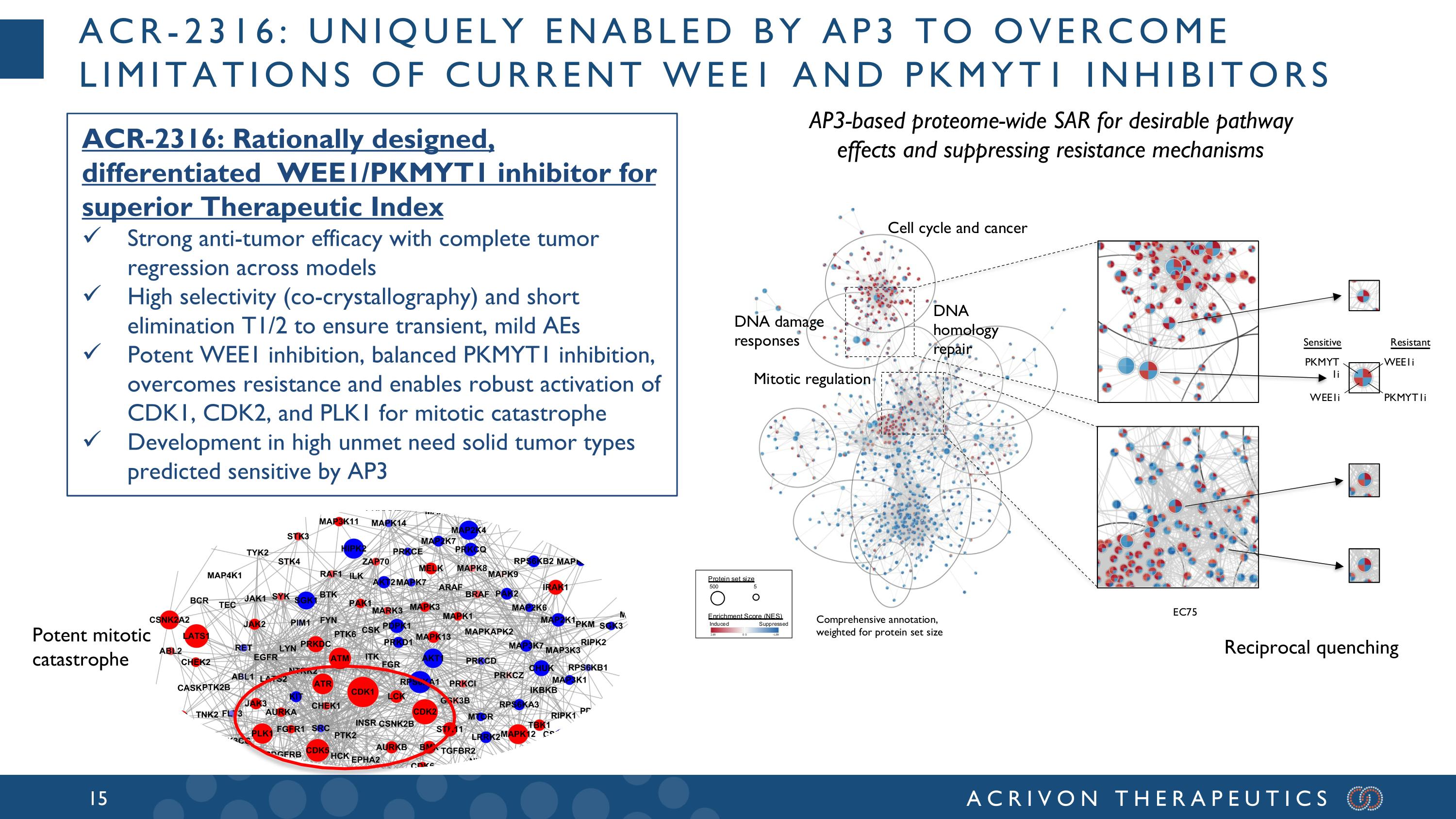
ACR-2316: uniquely enabled by AP3 to overcome limitations of current wee1 and pkmyt1 inhibitors AP3-based proteome-wide SAR for desirable pathway effects and suppressing resistance mechanisms ACR-2316: Rationally designed, differentiated WEE1/PKMYT1 inhibitor for superior Therapeutic Index Strong anti-tumor efficacy with complete tumor regression across models High selectivity (co-crystallography) and short elimination T1/2 to ensure transient, mild AEs Potent WEE1 inhibition, balanced PKMYT1 inhibition, overcomes resistance and enables robust activation of CDK1, CDK2, and PLK1 for mitotic catastrophe Development in high unmet need solid tumor types predicted sensitive by AP3 Reciprocal quenching Potent mitotic catastrophe
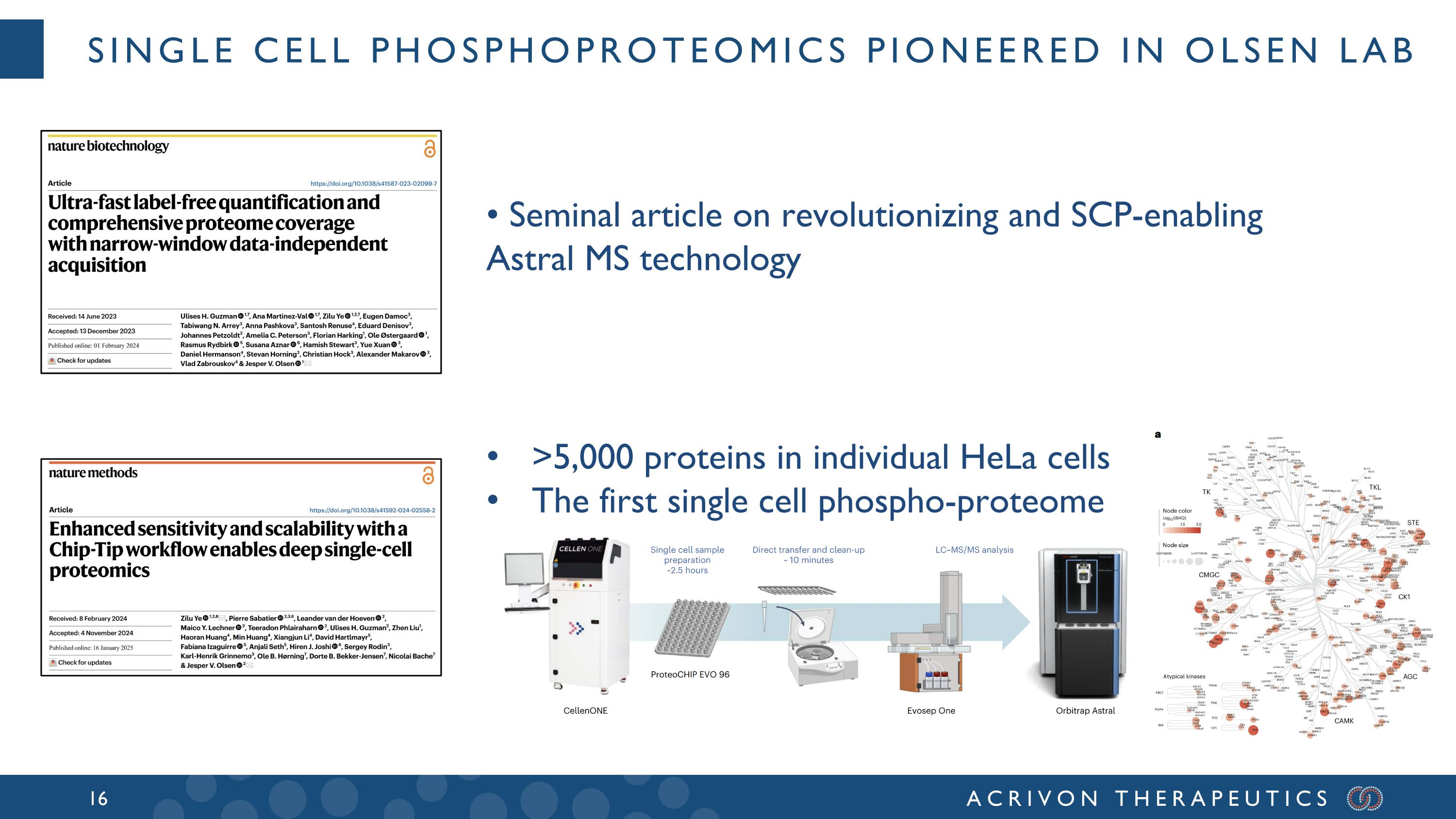
Single cell phosphoproteomics pioneered in Olsen lab • Seminal article on revolutionizing and SCP-enabling Astral MS technology >5,000 proteins in individual HeLa cells The first single cell phospho-proteome

ACR-368 Phase 2 update
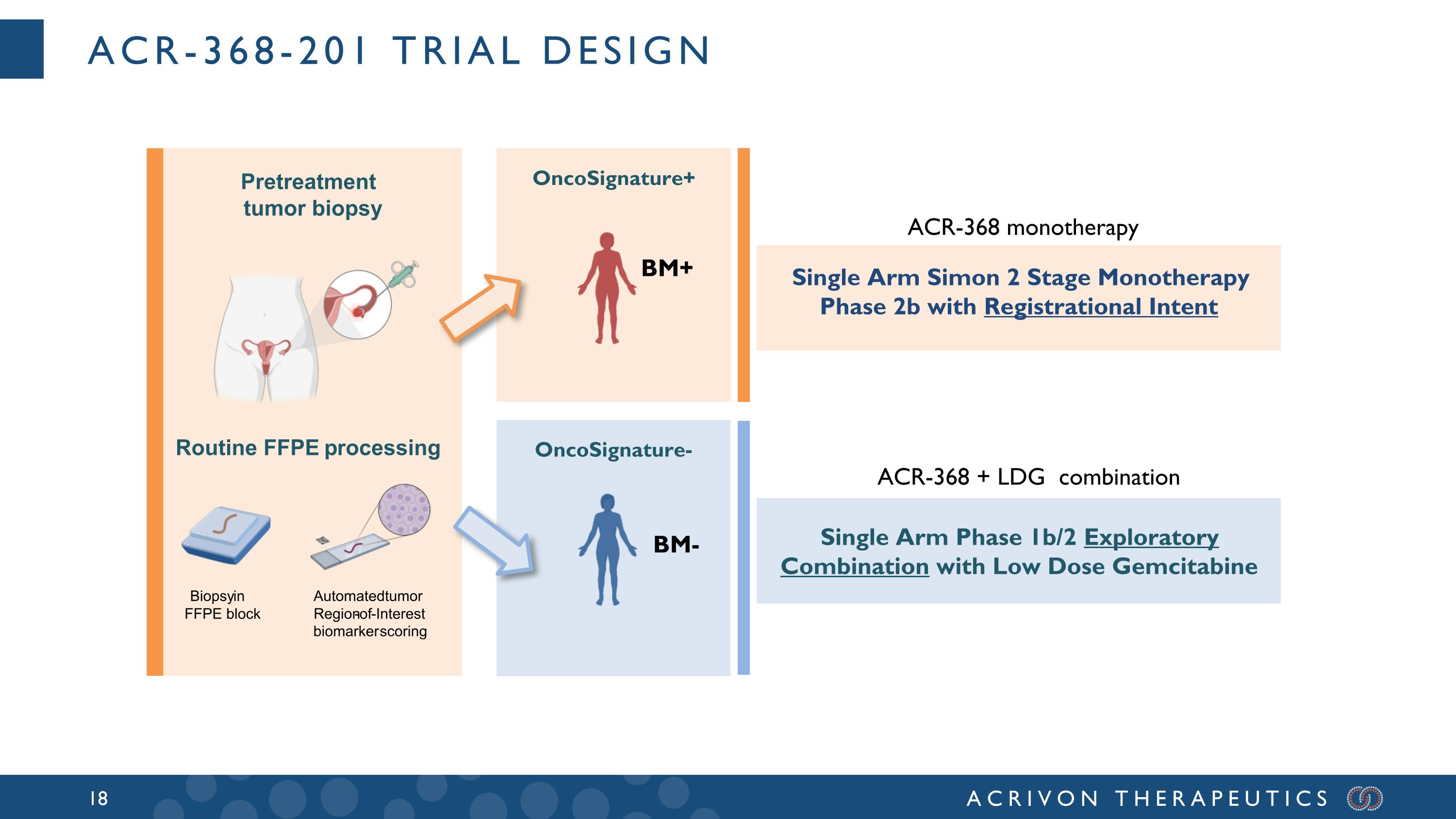
ACR-368-201 Trial design OncoSignature+ Single Arm Simon 2 Stage Monotherapy Phase 2b with Registrational Intent Single Arm Phase 1b/2 Exploratory Combination with Low Dose Gemcitabine OncoSignature- BM+ BM- ACR-368 monotherapy ACR-368 + LDG combination
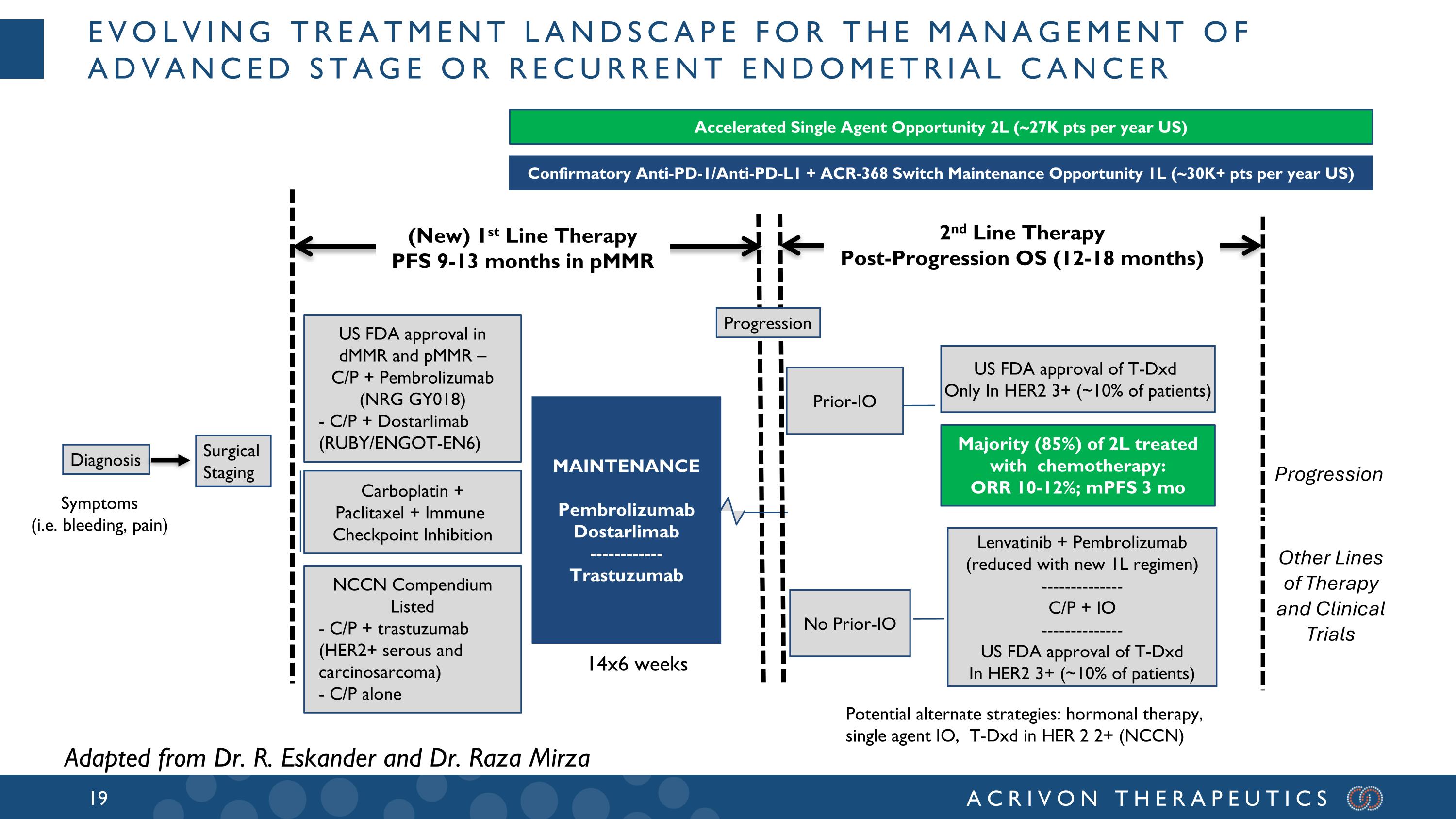
Symptoms (i.e. bleeding, pain) Evolving Treatment Landscape for the Management of Advanced Stage or Recurrent Endometrial Cancer Diagnosis Surgical Staging Carboplatin + Paclitaxel + Immune Checkpoint Inhibition US FDA approval in dMMR and pMMR – C/P + Pembrolizumab (NRG GY018) - C/P + Dostarlimab (RUBY/ENGOT-EN6) NCCN Compendium Listed - C/P + trastuzumab (HER2+ serous and carcinosarcoma) - C/P alone MAINTENANCE Pembrolizumab Dostarlimab ------------ Trastuzumab Other Lines of Therapy and Clinical Trials Prior-IO US FDA approval of T-Dxd Only In HER2 3+ (~10% of patients) Progression Majority (85%) of 2L treated with chemotherapy: ORR 10-12%; mPFS 3 mo Potential alternate strategies: hormonal therapy, single agent IO, T-Dxd in HER 2 2+ (NCCN) Adapted from Dr. R. Eskander and Dr. Raza Mirza Accelerated Single Agent Opportunity 2L (~27K pts per year US) Confirmatory Anti-PD-1/Anti-PD-L1 + ACR-368 Switch Maintenance Opportunity 1L (~30K+ pts per year US) 14x6 weeks Lenvatinib + Pembrolizumab (reduced with new 1L regimen) -------------- C/P + IO -------------- US FDA approval of T-Dxd In HER2 3+ (~10% of patients) No Prior-IO (New) 1st Line Therapy PFS 9-13 months in pMMR 2nd Line Therapy Post-Progression OS (12-18 months) Progression
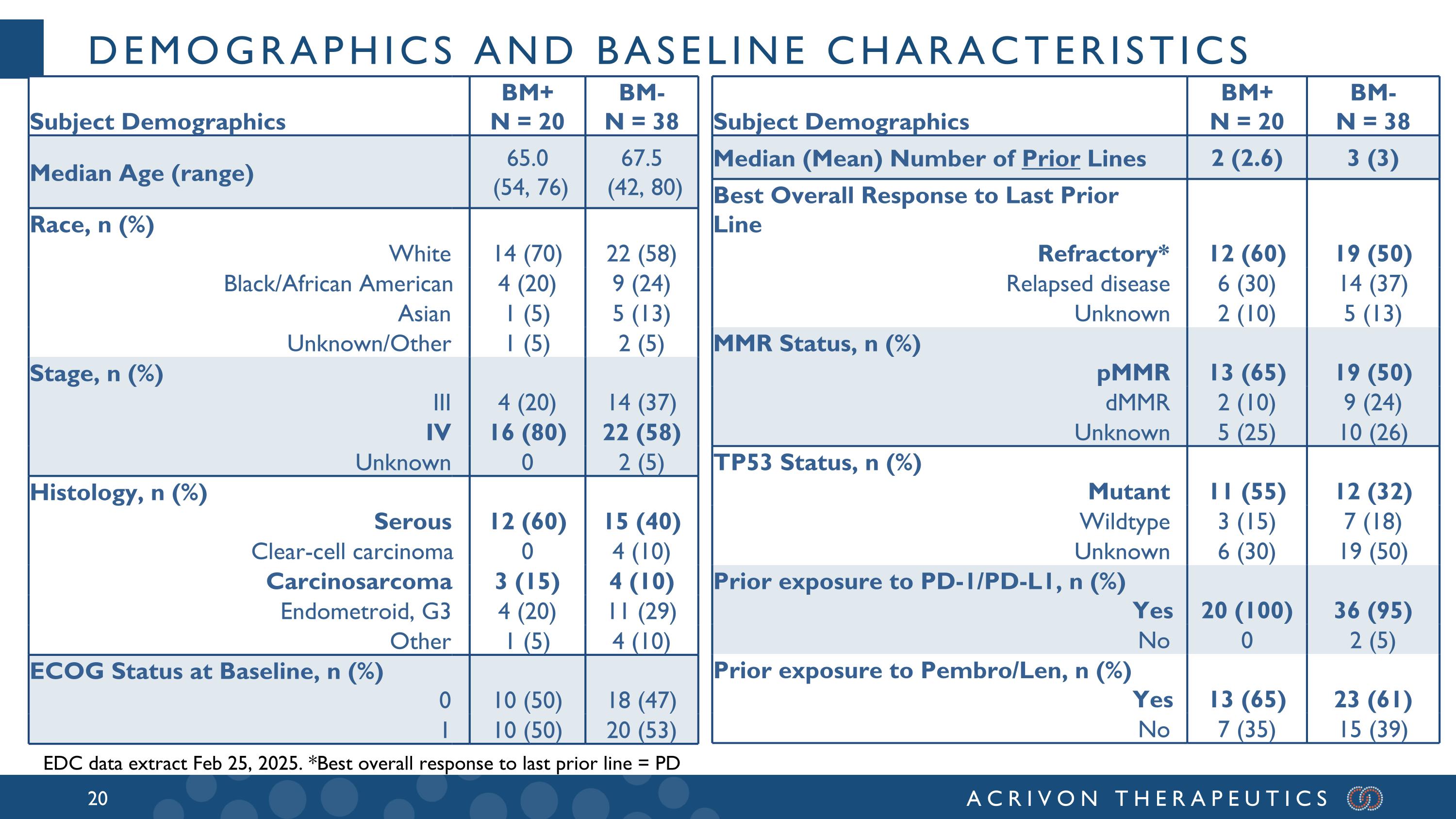
Demographics and baseline characteristics Subject Demographics BM+ N = 20 BM- N = 38 Median Age (range) 65.0 (54, 76) 67.5 (42, 80) Race, n (%) White 14 (70) 22 (58) Black/African American 4 (20) 9 (24) Asian 1 (5) 5 (13) Unknown/Other 1 (5) 2 (5) Stage, n (%) III 4 (20) 14 (37) IV 16 (80) 22 (58) Unknown 0 2 (5) Histology, n (%) Serous 12 (60) 15 (40) Clear-cell carcinoma 0 4 (10) Carcinosarcoma 3 (15) 4 (10) Endometroid, G3 4 (20) 11 (29) Other 1 (5) 4 (10) ECOG Status at Baseline, n (%) 0 10 (50) 18 (47) 1 10 (50) 20 (53) Subject Demographics BM+ N = 20 BM- N = 38 Median (Mean) Number of Prior Lines 2 (2.6) 3 (3) Best Overall Response to Last Prior Line Refractory* 12 (60) 19 (50) Relapsed disease 6 (30) 14 (37) Unknown 2 (10) 5 (13) MMR Status, n (%) pMMR 13 (65) 19 (50) dMMR 2 (10) 9 (24) Unknown 5 (25) 10 (26) TP53 Status, n (%) Mutant 11 (55) 12 (32) Wildtype 3 (15) 7 (18) Unknown 6 (30) 19 (50) Prior exposure to PD-1/PD-L1, n (%) Yes 20 (100) 36 (95) No 0 2 (5) Prior exposure to Pembro/Len, n (%) Yes 13 (65) 23 (61) No 7 (35) 15 (39) EDC data extract Feb 25, 2025. *Best overall response to last prior line = PD
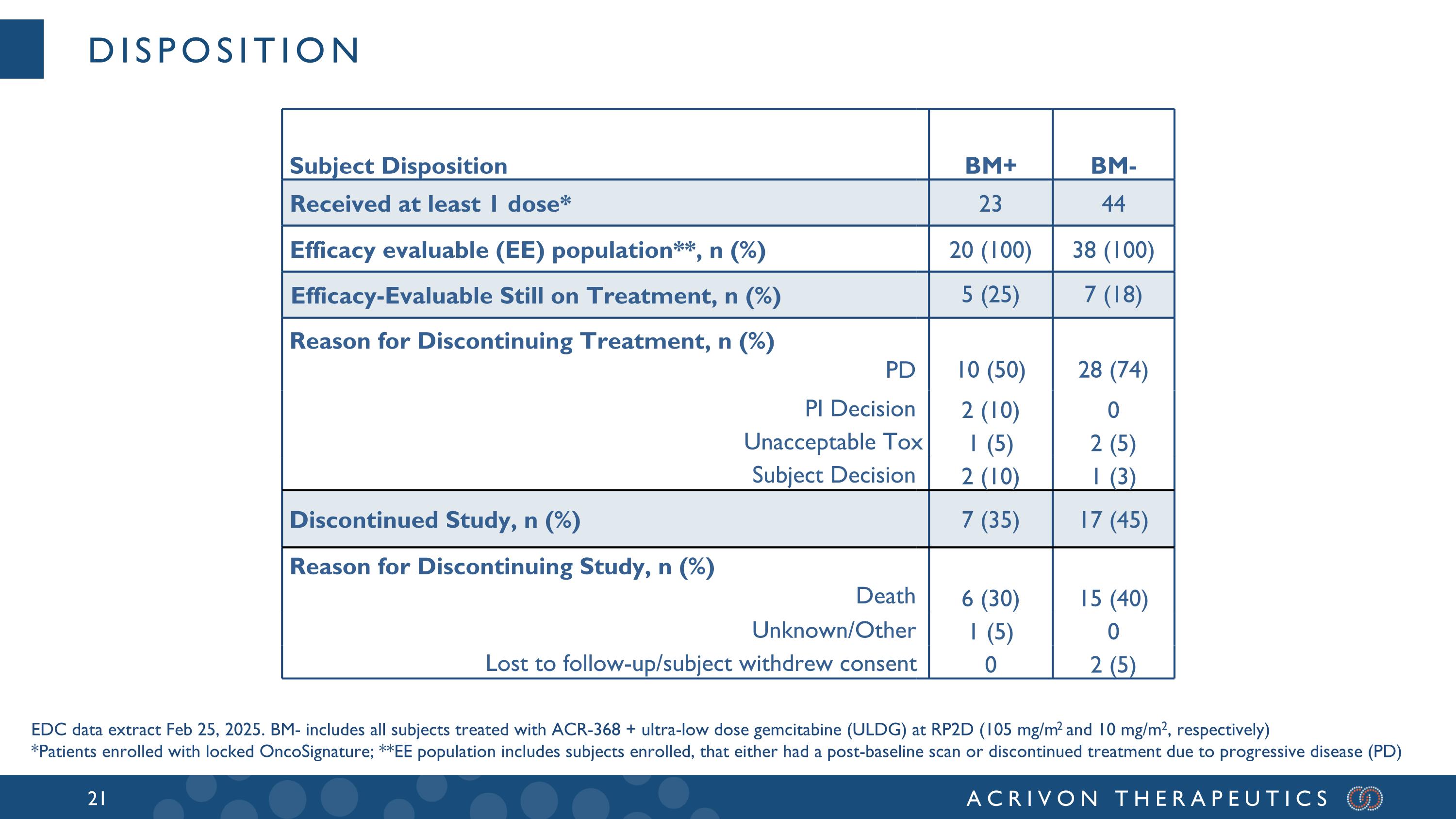
disposition Subject Disposition BM+ BM- Received at least 1 dose* 23 44 Efficacy evaluable (EE) population**, n (%) 20 (100) 38 (100) Efficacy-Evaluable Still on Treatment, n (%) 5 (25) 7 (18) Reason for Discontinuing Treatment, n (%) PD 10 (50) 28 (74) PI Decision 2 (10) 0 Unacceptable Tox 1 (5) 2 (5) Subject Decision 2 (10) 1 (3) Discontinued Study, n (%) 7 (35) 17 (45) Reason for Discontinuing Study, n (%) Death 6 (30) 15 (40) Unknown/Other 1 (5) 0 Lost to follow-up/subject withdrew consent 0 2 (5) EDC data extract Feb 25, 2025. BM- includes all subjects treated with ACR-368 + ultra-low dose gemcitabine (ULDG) at RP2D (105 mg/m2 and 10 mg/m2, respectively) *Patients enrolled with locked OncoSignature; **EE population includes subjects enrolled, that either had a post-baseline scan or discontinued treatment due to progressive disease (PD)
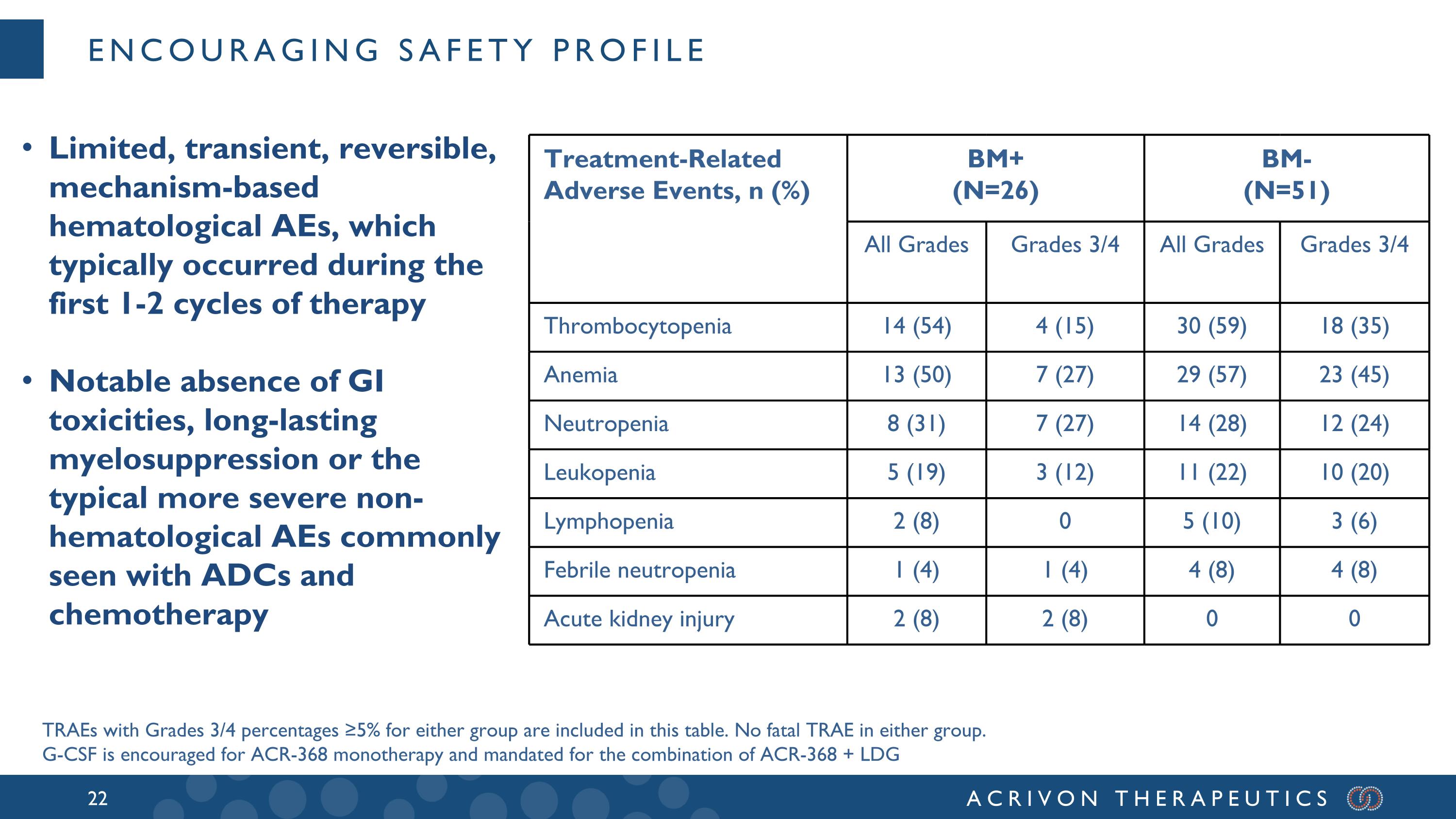
Encouraging safety profile Treatment-Related Adverse Events, n (%) BM+ (N=26) BM- (N=51) All Grades Grades 3/4 All Grades Grades 3/4 Thrombocytopenia 14 (54) 4 (15) 30 (59) 18 (35) Anemia 13 (50) 7 (27) 29 (57) 23 (45) Neutropenia 8 (31) 7 (27) 14 (28) 12 (24) Leukopenia 5 (19) 3 (12) 11 (22) 10 (20) Lymphopenia 2 (8) 0 5 (10) 3 (6) Febrile neutropenia 1 (4) 1 (4) 4 (8) 4 (8) Acute kidney injury 2 (8) 2 (8) 0 0 TRAEs with Grades 3/4 percentages ≥5% for either group are included in this table. No fatal TRAE in either group. G-CSF is encouraged for ACR-368 monotherapy and mandated for the combination of ACR-368 + LDG Limited, transient, reversible, mechanism-based hematological AEs, which typically occurred during the first 1-2 cycles of therapy Notable absence of GI toxicities, long-lasting myelosuppression or the typical more severe non-hematological AEs commonly seen with ADCs and chemotherapy
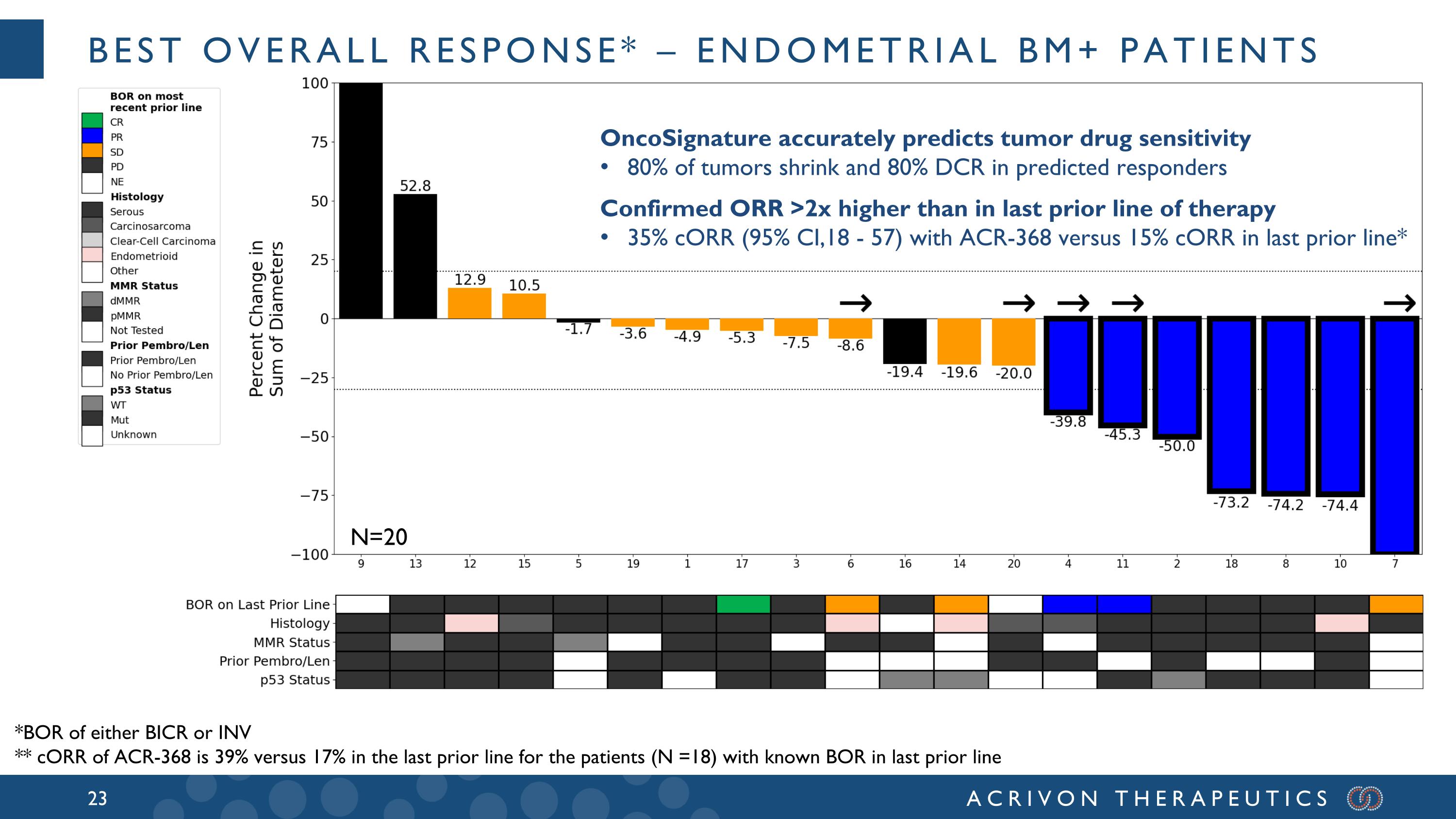
Best overall response* – endometrial BM+ patients OncoSignature accurately predicts tumor drug sensitivity 80% of tumors shrink and 80% DCR in predicted responders Confirmed ORR >2x higher than in last prior line of therapy 35% cORR (95% CI,18 - 57) with ACR-368 versus 15% cORR in last prior line* *BOR of either BICR or INV ** cORR of ACR-368 is 39% versus 17% in the last prior line for the patients (N =18) with known BOR in last prior line N=20
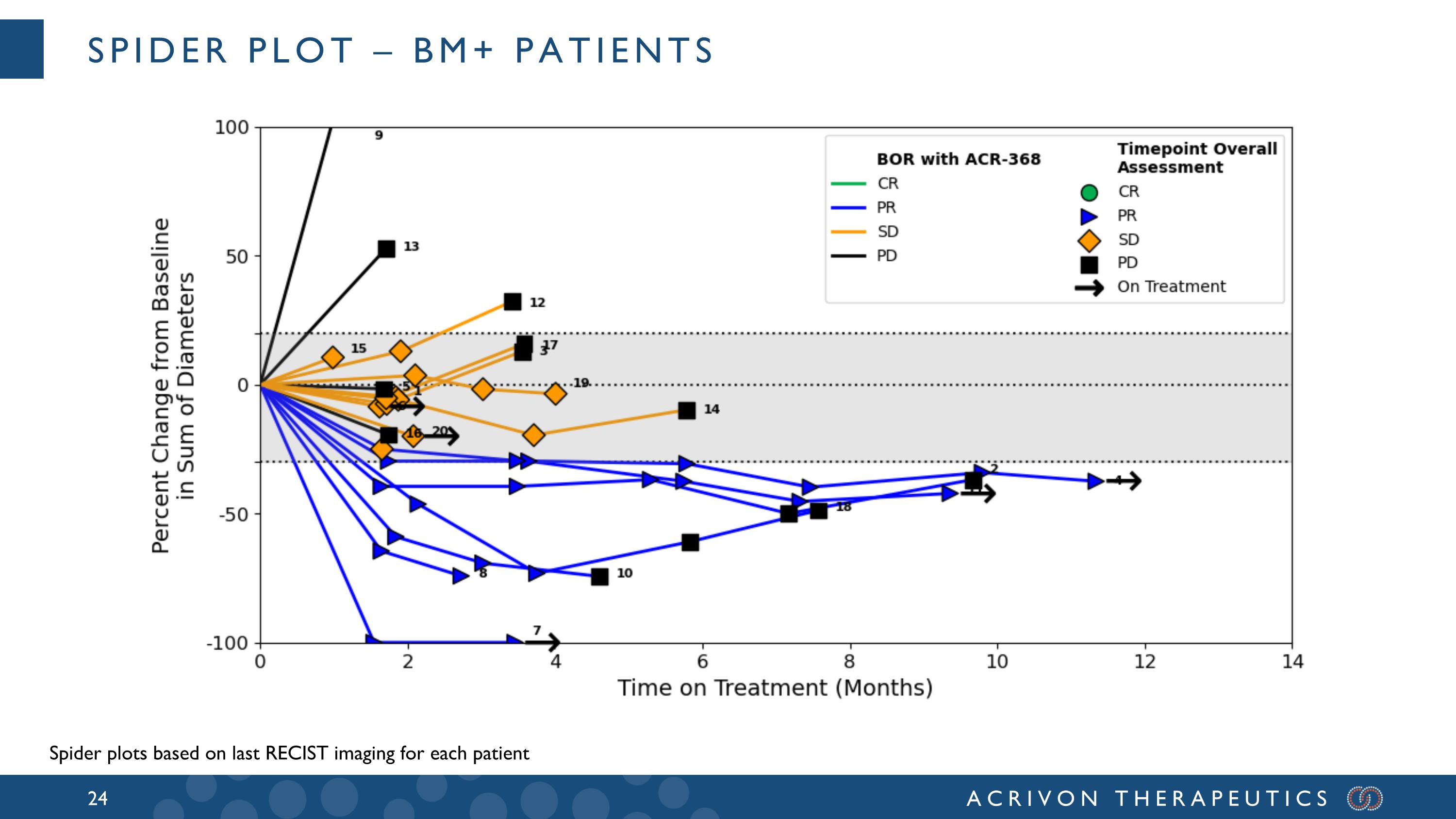
Spider Plot – BM+ patients Spider plots based on last RECIST imaging for each patient
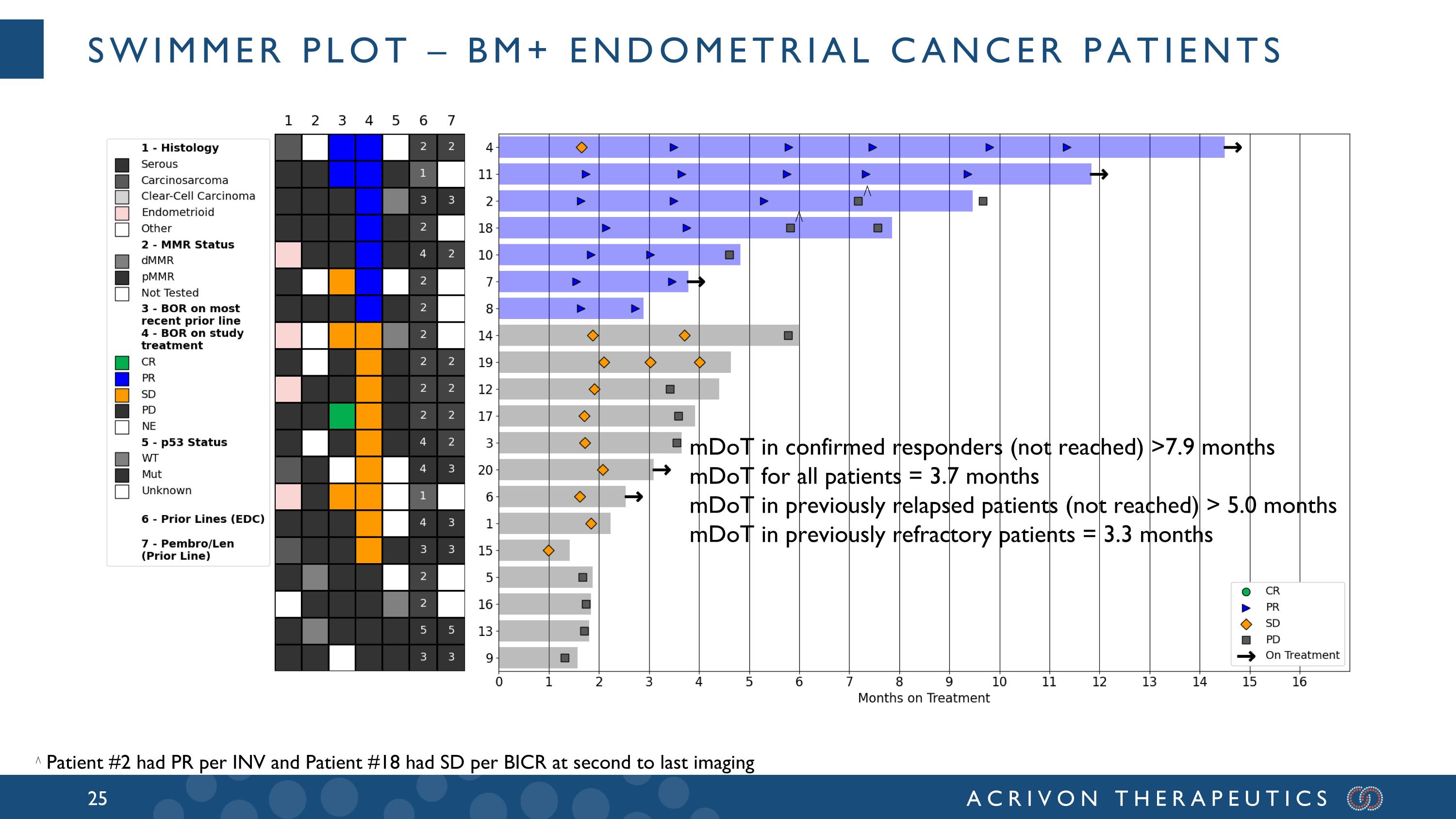
SWIMMER PLOT – BM+ endometrial cancer patients ⋀ ⋀ Patient #2 had PR per INV and Patient #18 had SD per BICR at second to last imaging mDoT in confirmed responders (not reached) >7.9 months mDoT for all patients = 3.7 months mDoT in previously relapsed patients (not reached) > 5.0 months mDoT in previously refractory patients = 3.3 months ⋀
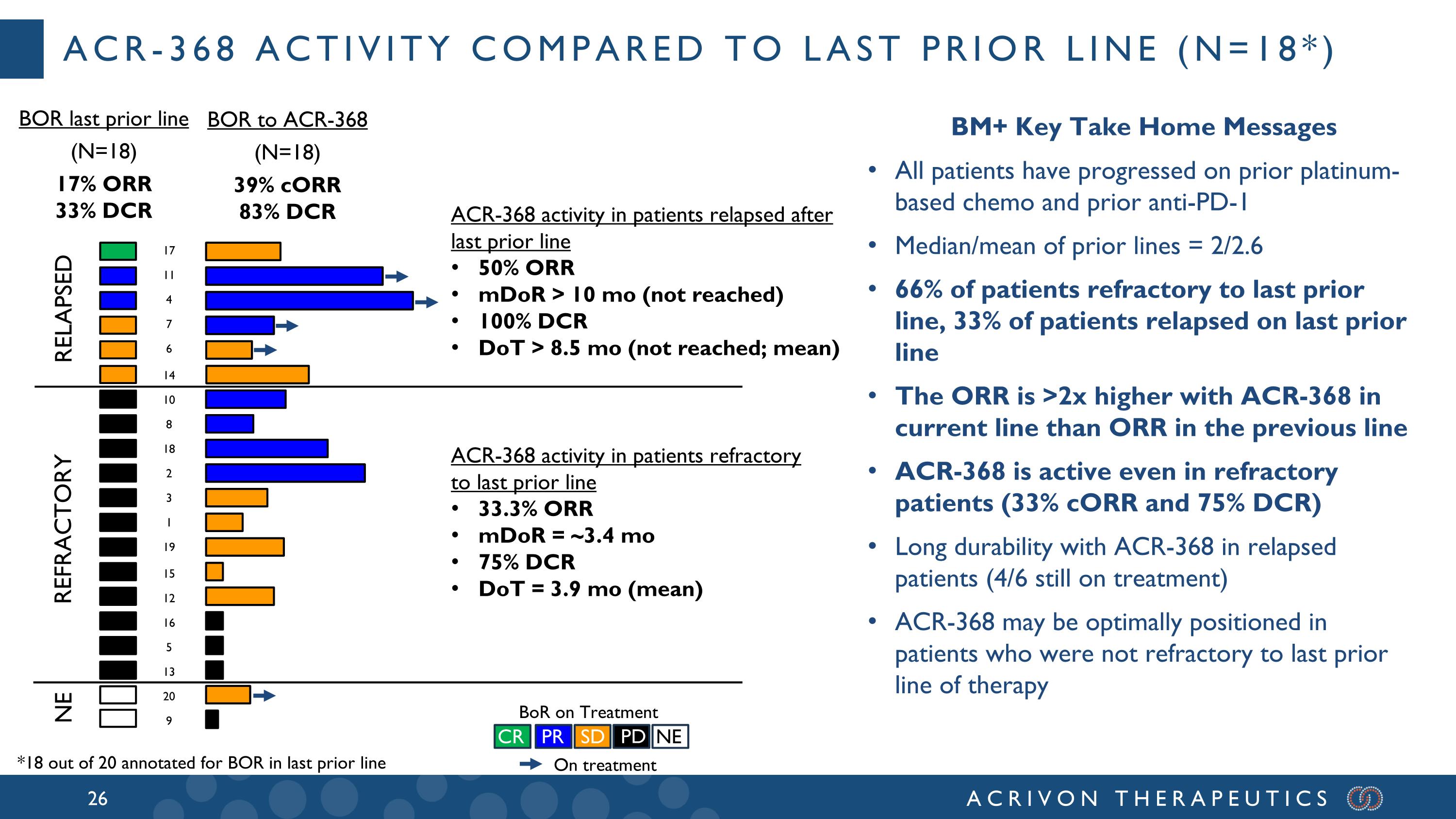
RELAPSED REFRACTORY 17 4 11 7 6 14 10 8 18 2 3 1 19 15 12 16 5 OT OT OT OT BoR on Treatment CR PR SD PD NE ACR-368 activity in patients relapsed after last prior line 50% ORR mDoR > 10 mo (not reached) 100% DCR DoT > 8.5 mo (not reached; mean) ACR-368 activity in patients refractory to last prior line 33.3% ORR mDoR = ~3.4 mo 75% DCR DoT = 3.9 mo (mean) NE 20 13 9 BOR last prior line (N=18) 17% ORR 33% DCR BOR to ACR-368 (N=18) 39% cORR 83% DCR ACR-368 activity compared to last prior line (N=18*) On treatment BM+ Key Take Home Messages All patients have progressed on prior platinum-based chemo and prior anti-PD-1 Median/mean of prior lines = 2/2.6 66% of patients refractory to last prior line, 33% of patients relapsed on last prior line The ORR is >2x higher with ACR-368 in current line than ORR in the previous line ACR-368 is active even in refractory patients (33% cORR and 75% DCR) Long durability with ACR-368 in relapsed patients (4/6 still on treatment) ACR-368 may be optimally positioned in patients who were not refractory to last prior line of therapy *18 out of 20 annotated for BOR in last prior line
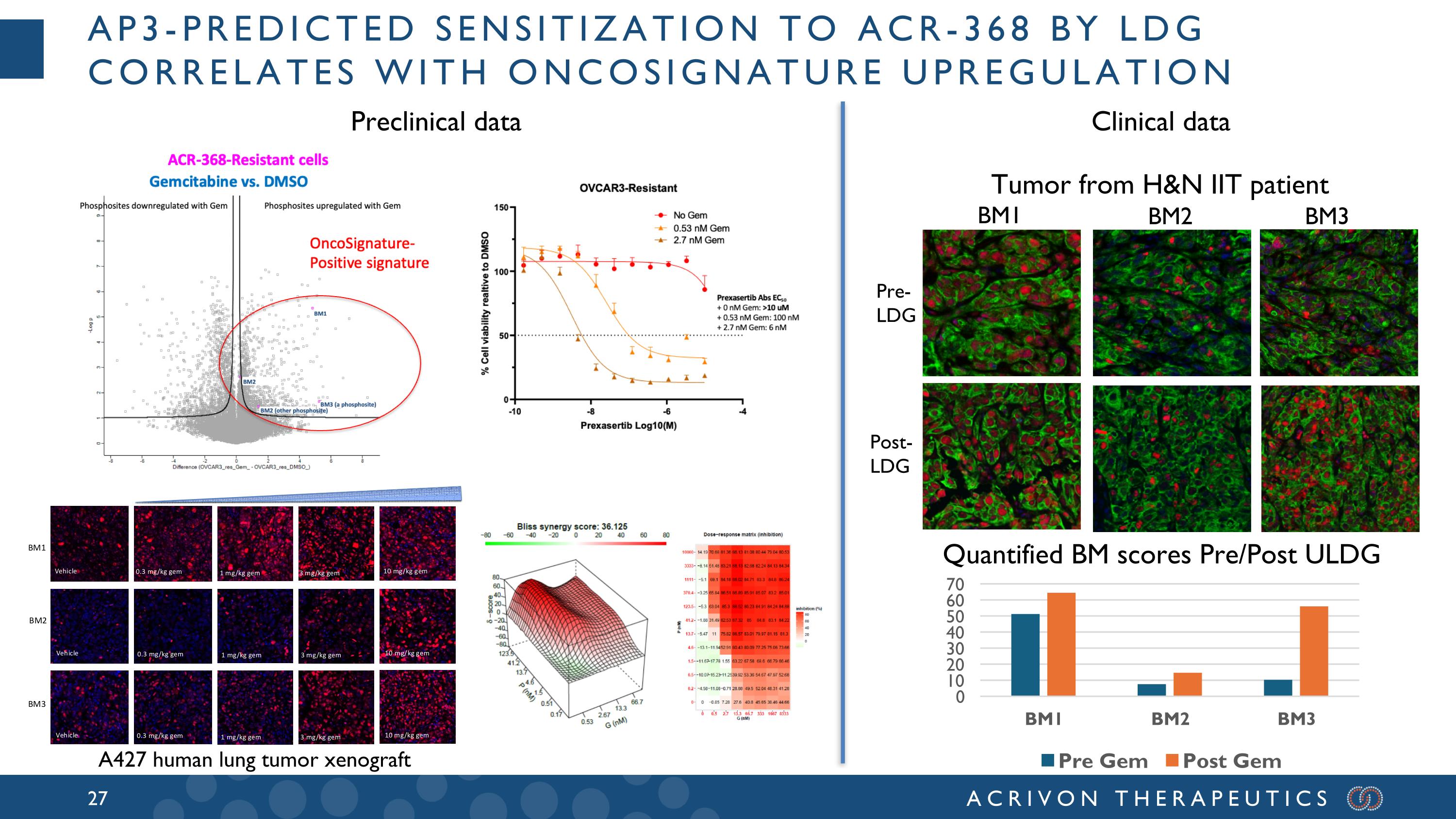
AP3-predicted sensitization to ACR-368 by lDG correlates with OncoSignature upregulation BM2 BM3 Quantified BM scores Pre/Post ULDG Tumor from H&N IIT patient A427 human lung tumor xenograft BM1 Pre- LDG Post- LDG Preclinical data Clinical data
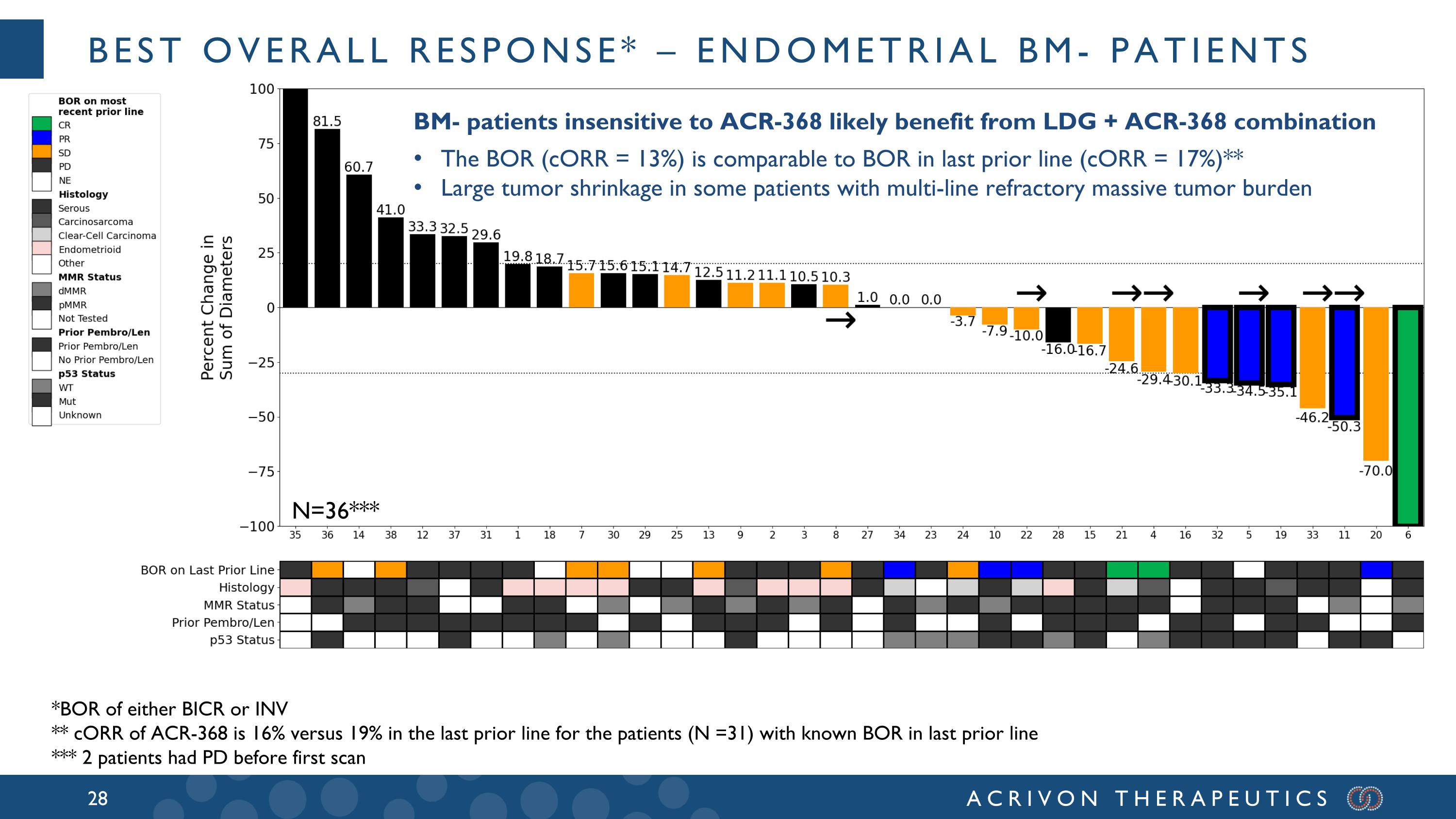
Best overall response* – endometrial BM- patients BM- patients insensitive to ACR-368 likely benefit from LDG + ACR-368 combination The BOR (cORR = 13%) is comparable to BOR in last prior line (cORR = 17%)** Large tumor shrinkage in some patients with multi-line refractory massive tumor burden *BOR of either BICR or INV ** cORR of ACR-368 is 16% versus 19% in the last prior line for the patients (N =31) with known BOR in last prior line *** 2 patients had PD before first scan N=36***
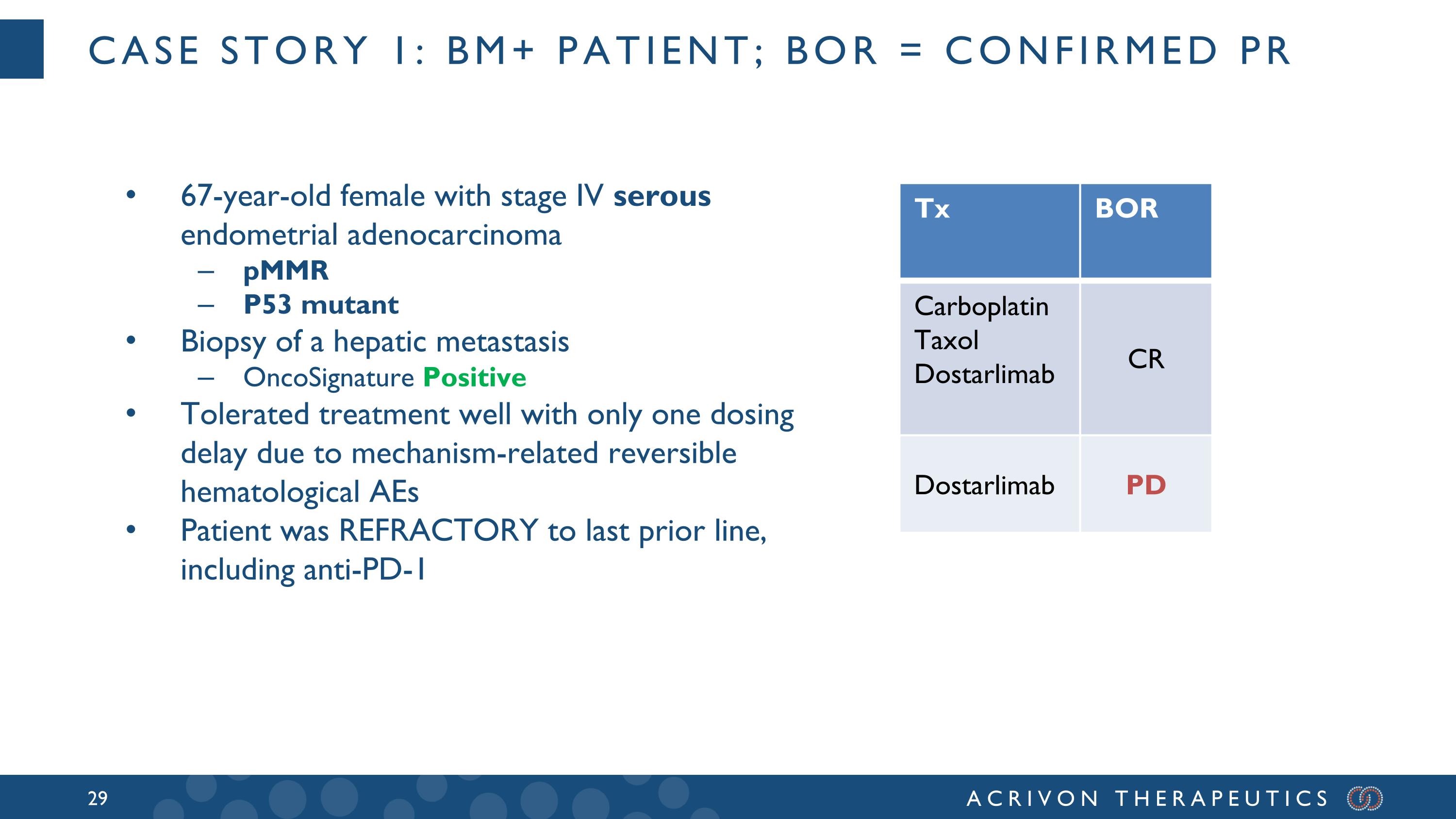
Case story 1: BM+ patient; BOR = confirmed PR Tx BOR Carboplatin Taxol Dostarlimab CR Dostarlimab PD 67-year-old female with stage IV serous endometrial adenocarcinoma pMMR P53 mutant Biopsy of a hepatic metastasis OncoSignature Positive Tolerated treatment well with only one dosing delay due to mechanism-related reversible hematological AEs Patient was REFRACTORY to last prior line, including anti-PD-1
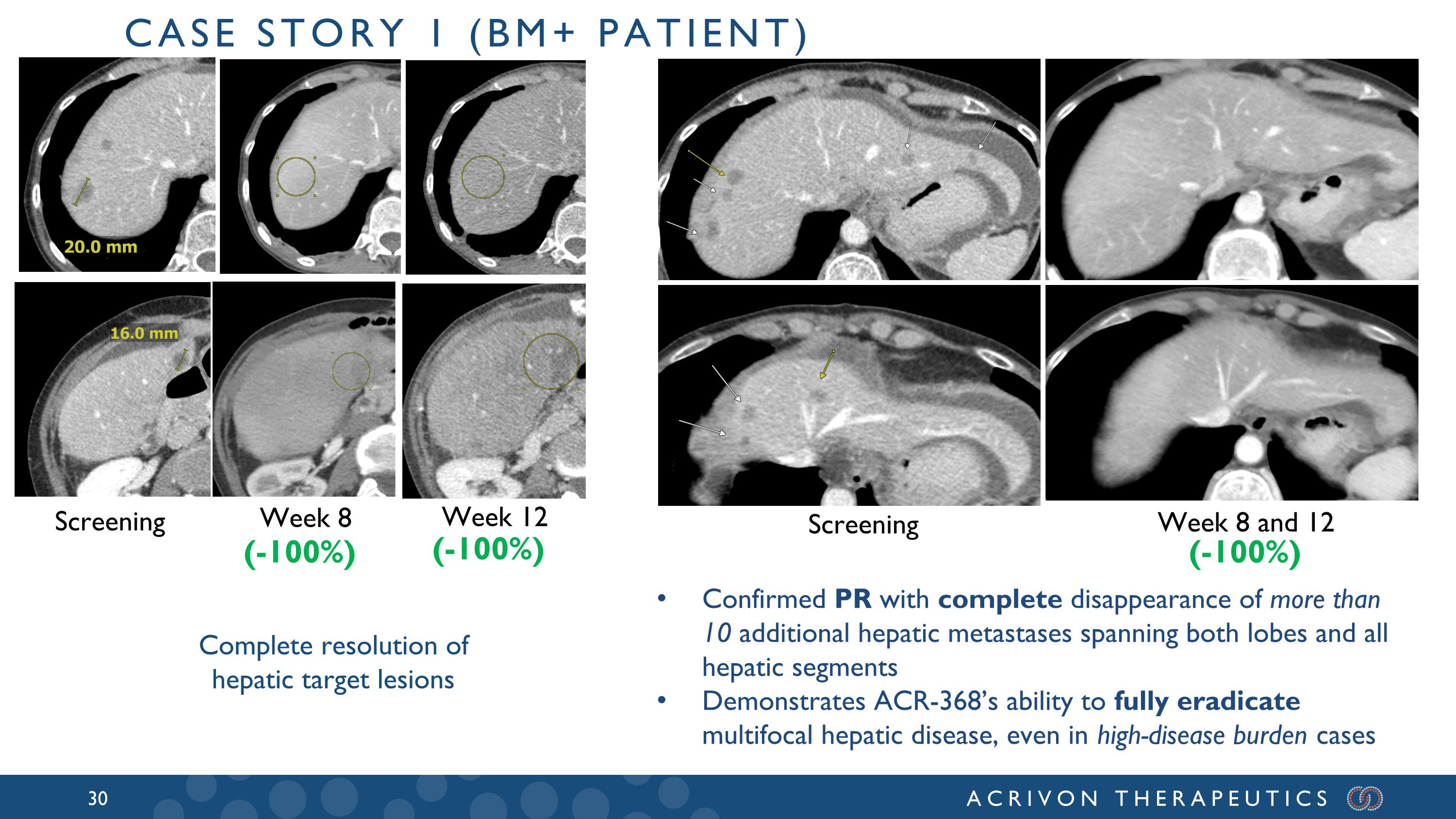
Screening Week 8 Week 12 Complete resolution of hepatic target lesions (-100%) (-100%) Case story 1 (BM+ patient) Screening Week 8 and 12 (-100%) Confirmed PR with complete disappearance of more than 10 additional hepatic metastases spanning both lobes and all hepatic segments Demonstrates ACR-368’s ability to fully eradicate multifocal hepatic disease, even in high-disease burden cases
Case story 2: BM+ patient; BOR = SD 67-year-old female with stage III endometroid endometrial adenocarcinoma pMMR P53 mutant Biopsy of a vaginal mass OncoSignature Positive Dose reduction and delay due to primarily mechanism related reversible hematological AEs Patient was REFRACTORY to prior lines, including anti-PD-1 Tx BOR Carboplatin Taxol Pembrolizumab PD Pembrolizumab Lenvatinib PD
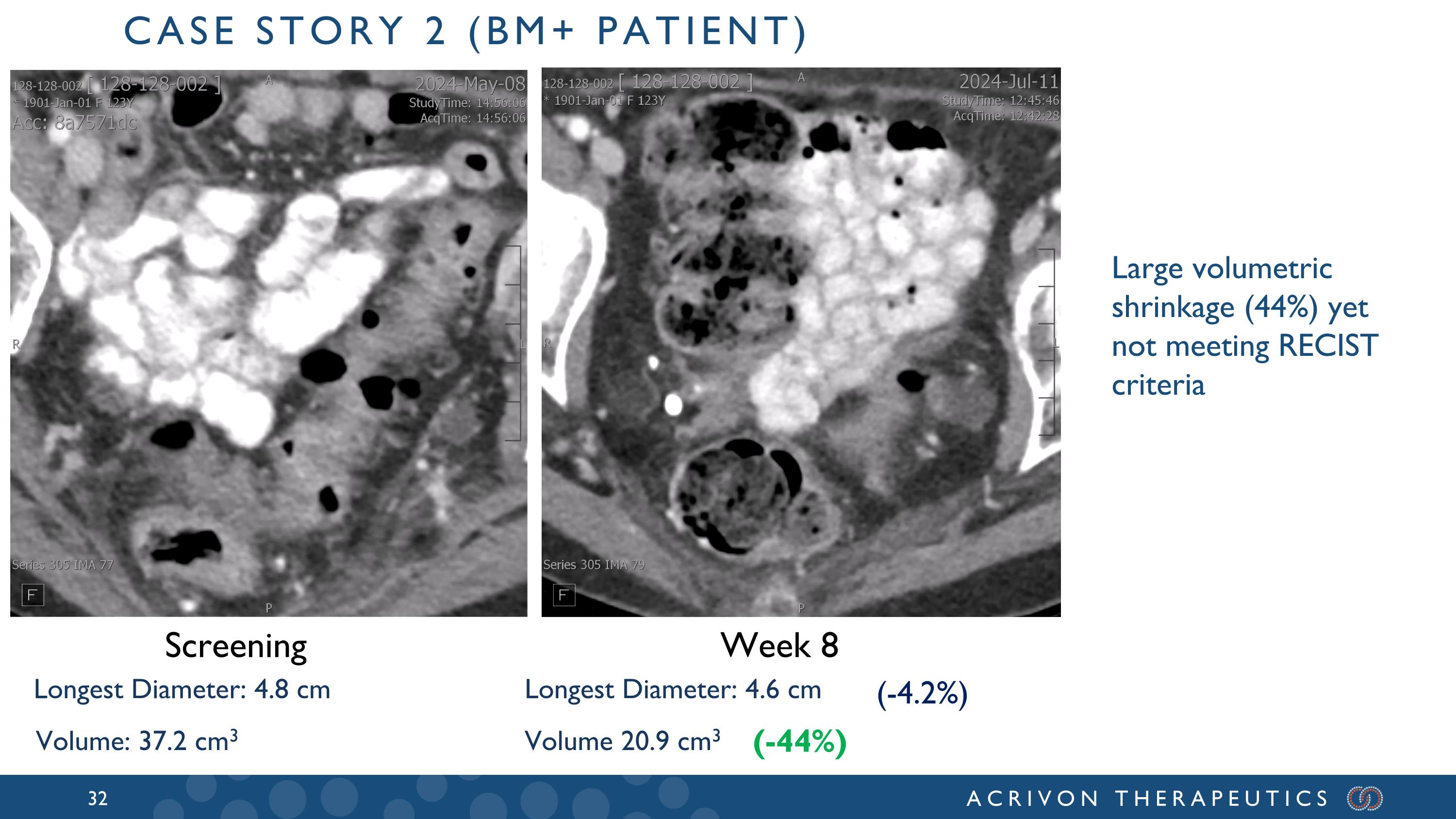
Week 8 Volume 20.9 cm3 (-44%) Screening Longest Diameter: 4.6 cm Volume: 37.2 cm3 Longest Diameter: 4.8 cm (-4.2%) Large volumetric shrinkage (44%) yet not meeting RECIST criteria Case story 2 (BM+ patient)
Case story 3: bm- patient; BOR = confirmed PR 73-year-old female with stage IV serous endometrial adenocarcinoma dMMR P53 mutant Biopsy of a hepatic metastasis Oncosignature Negative Tolerated treatment well at reduced dosage with mechanism related reversible hematological AEs BOR in last prior line was PD Tx BOR Carboplatin Taxol PR Pembrolizumab PD
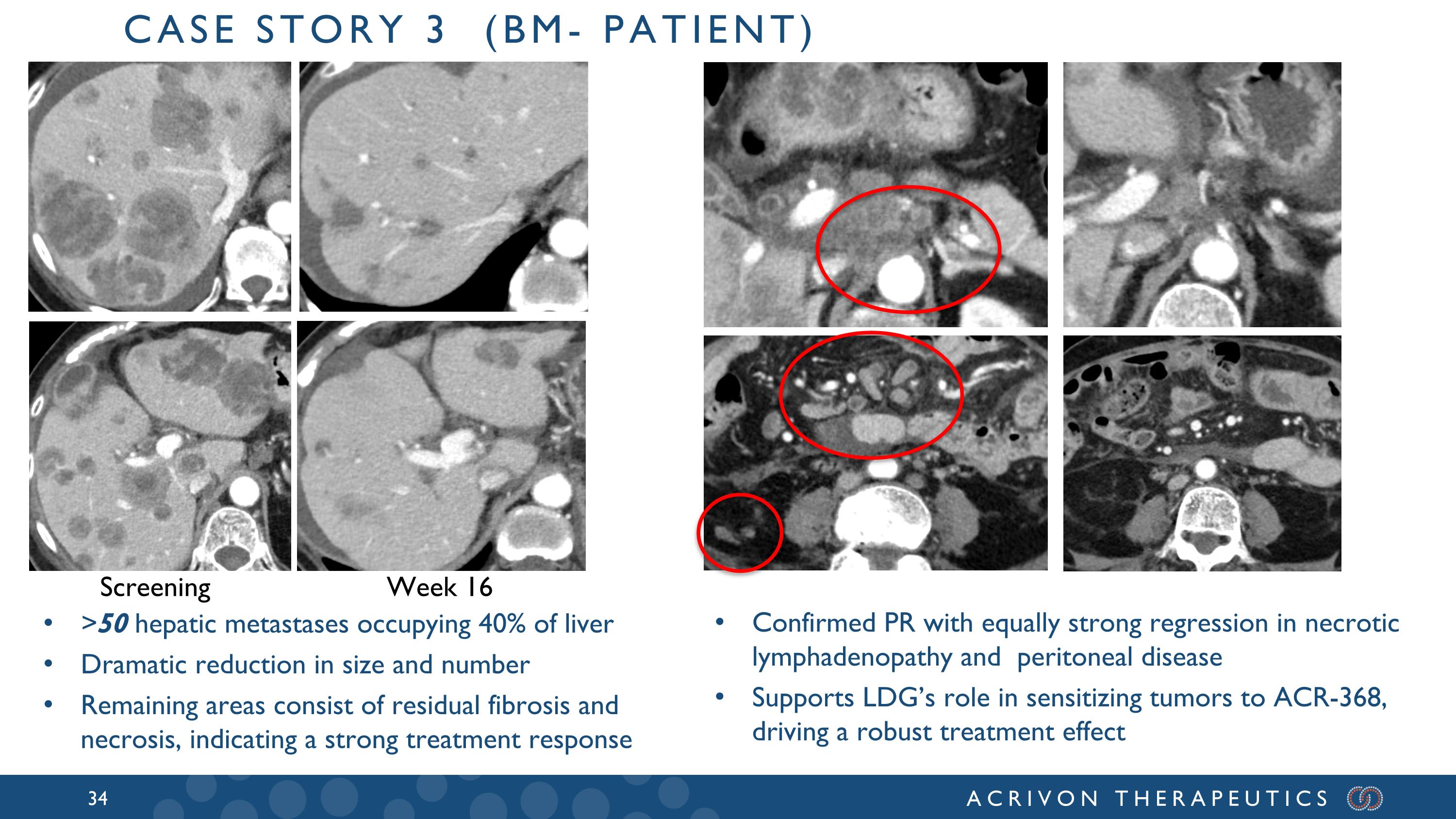
>50 hepatic metastases occupying 40% of liver Dramatic reduction in size and number Remaining areas consist of residual fibrosis and necrosis, indicating a strong treatment response Screening Week 16 Case story 3 (bm- patient) Confirmed PR with equally strong regression in necrotic lymphadenopathy and peritoneal disease Supports LDG’s role in sensitizing tumors to ACR-368, driving a robust treatment effect
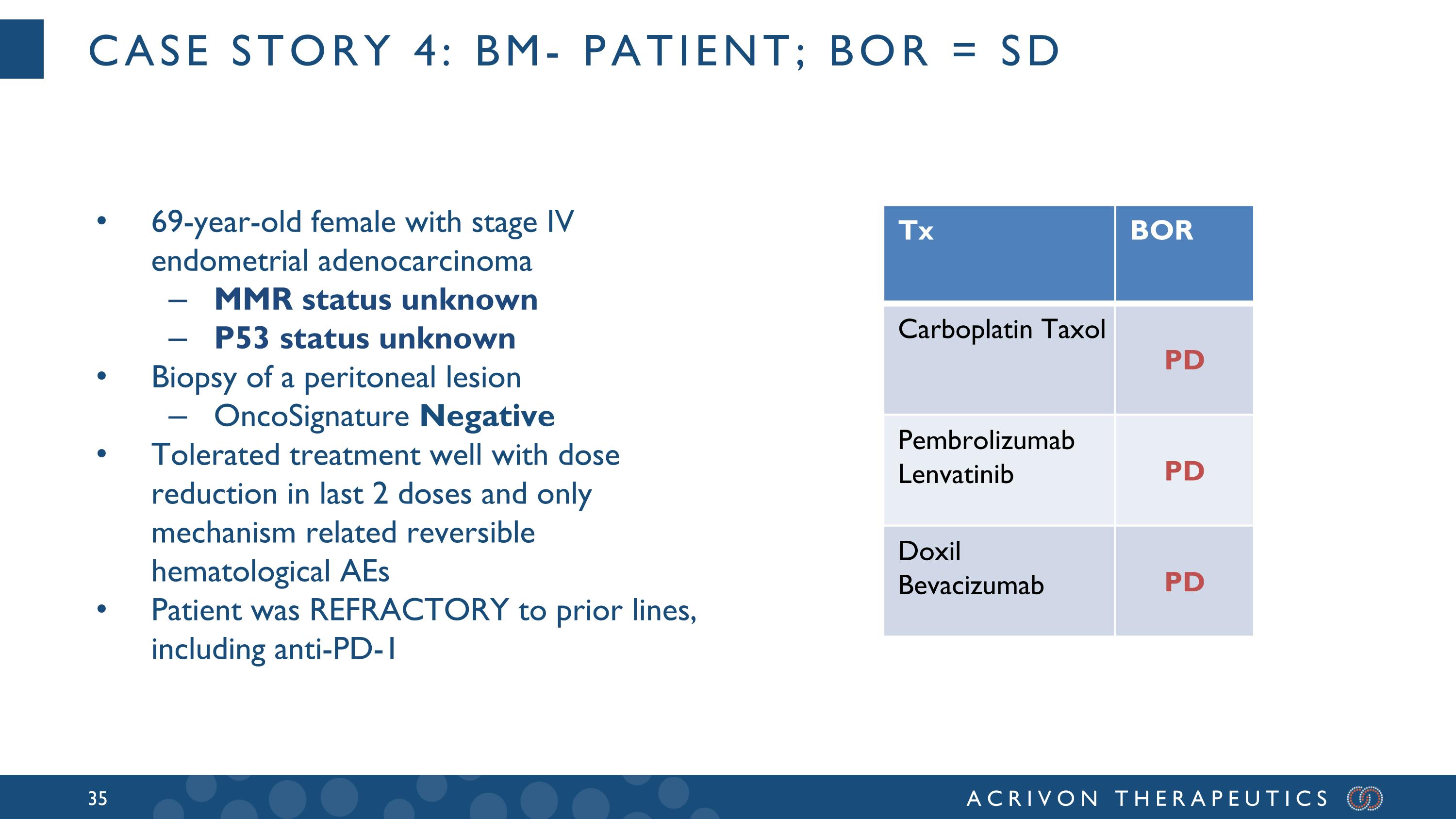
Case story 4: BM- patient; BOR = SD 69-year-old female with stage IV endometrial adenocarcinoma MMR status unknown P53 status unknown Biopsy of a peritoneal lesion OncoSignature Negative Tolerated treatment well with dose reduction in last 2 doses and only mechanism related reversible hematological AEs Patient was REFRACTORY to prior lines, including anti-PD-1 Tx BOR Carboplatin Taxol PD Pembrolizumab Lenvatinib PD Doxil Bevacizumab PD
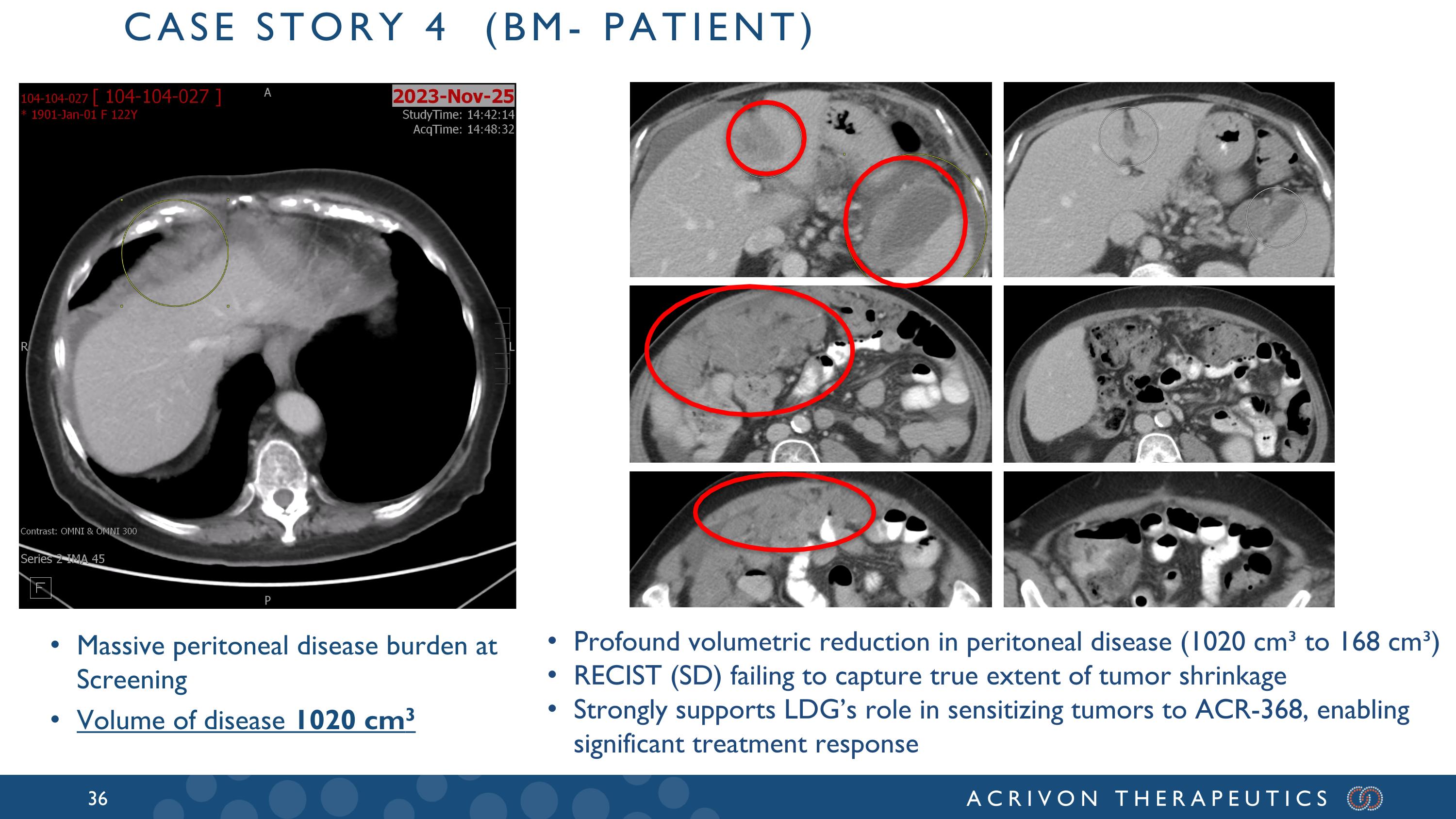
Massive peritoneal disease burden at Screening Volume of disease 1020 cm3 Case story 4 (bm- patient) Profound volumetric reduction in peritoneal disease (1020 cm³ to 168 cm³) RECIST (SD) failing to capture true extent of tumor shrinkage Strongly supports LDG’s role in sensitizing tumors to ACR-368, enabling significant treatment response
Full focus on endometrial cancer; ovarian and bladder cancer being deprioritized Based on our emerging positive clinical data, competitive positioning, and attractive commercial opportunity, endometrial cancer is prioritized as first potential registration opportunity Tumor type predicted sensitive by AP3 Indication Finding prior to clinical entry Breakthrough Device Designation (BDD) received January 21, 2025 Huge unmet need in 2nd line (ORR ~12% and PFS ~3 months) ~27,000 est. new cases/year in 2nd line stage III/IV recurrent or locally advanced, all histopathologies* Preclinical data show LDG sensitization also in BM+ tumors opening for potential 2nd line all-comer ACR-368 + LDG Acrivon continues pursuing registrational intent for ACR-368 in ≥2nd line BM+ patients (post new frontline) and our confirmatory trial strategy (switch maintenance w/ anti-PD-1) in frontline, supported by strong preclinical data Ovarian and bladder cancer deprioritized Due to increased competition and small market opportunity, the clinical bar for ovarian cancer is high and based on our preliminary data after 23 BM+ patients it is not met In bladder cancer, we observed a lower than predicted BM+ fraction resulting in challenging enrollment All clinical resources are now focused on ACR-368 in endometrial cancer and ACR-2316 * Blinded, proprietary third-party market research with endometrial KOLs conducted August-September 2024
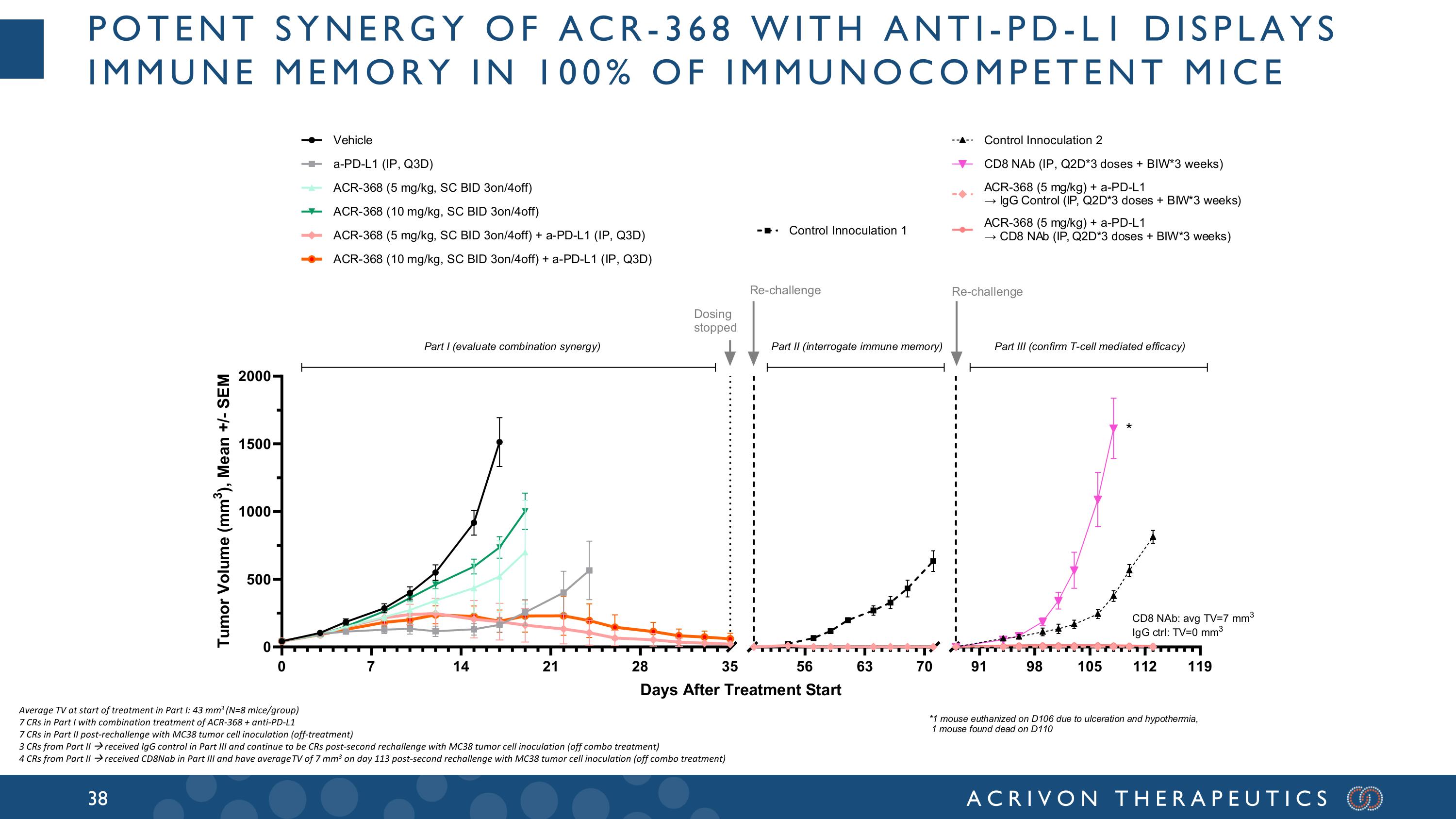
Potent synergy of ACR-368 with anti-PD-L1 displays immune Memory in 100% of immunocompetent Mice Average TV at start of treatment in Part I: 43 mm3 (N=8 mice/group) 7 CRs in Part I with combination treatment of ACR-368 + anti-PD-L1 7 CRs in Part II post-rechallenge with MC38 tumor cell inoculation (off-treatment) 3 CRs from Part II received IgG control in Part III and continue to be CRs post-second rechallenge with MC38 tumor cell inoculation (off combo treatment) 4 CRs from Part II received CD8Nab in Part III and have average TV of 7 mm3 on day 113 post-second rechallenge with MC38 tumor cell inoculation (off combo treatment)

ACR-2316 Phase 1 update
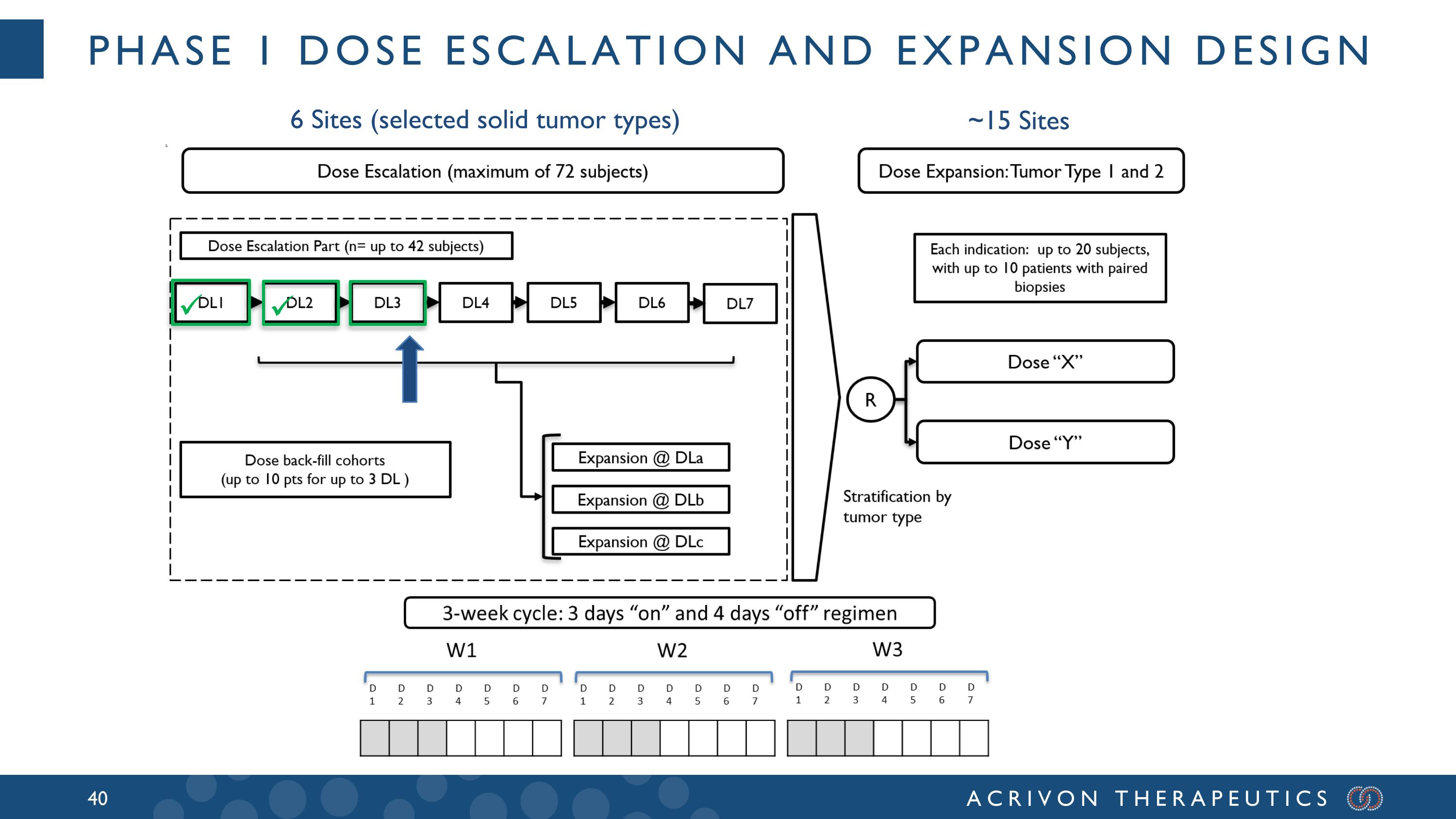
Phase 1 Dose Escalation and Expansion Design ~15 Sites 6 Sites (selected solid tumor types)
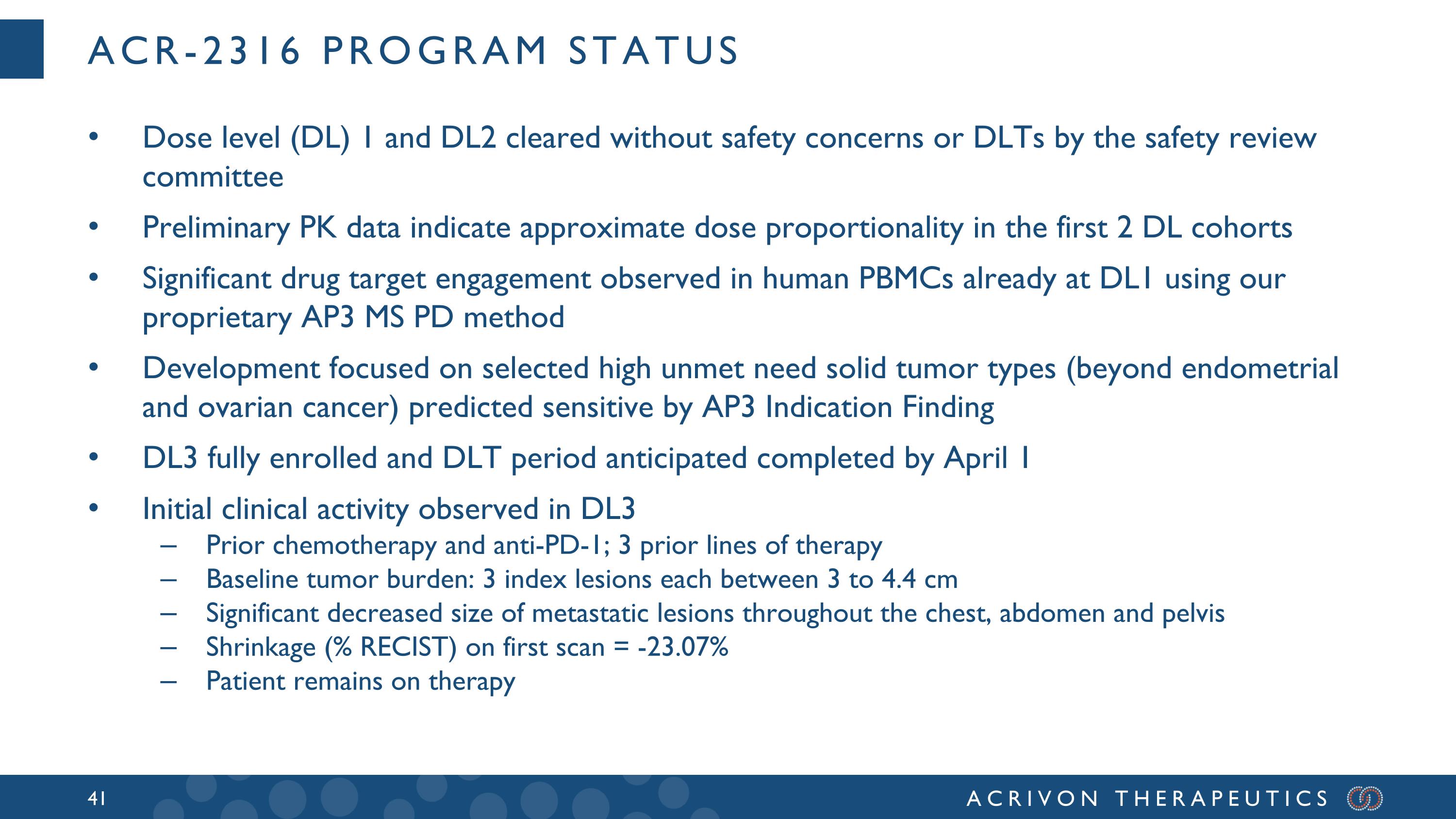
ACR-2316 program status Dose level (DL) 1 and DL2 cleared without safety concerns or DLTs by the safety review committee Preliminary PK data indicate approximate dose proportionality in the first 2 DL cohorts Significant drug target engagement observed in human PBMCs already at DL1 using our proprietary AP3 MS PD method Development focused on selected high unmet need solid tumor types (beyond endometrial and ovarian cancer) predicted sensitive by AP3 Indication Finding DL3 fully enrolled and DLT period anticipated completed by April 1 Initial clinical activity observed in DL3 Prior chemotherapy and anti-PD-1; 3 prior lines of therapy Baseline tumor burden: 3 index lesions each between 3 to 4.4 cm Significant decreased size of metastatic lesions throughout the chest, abdomen and pelvis Shrinkage (% RECIST) on first scan = -23.07% Patient remains on therapy
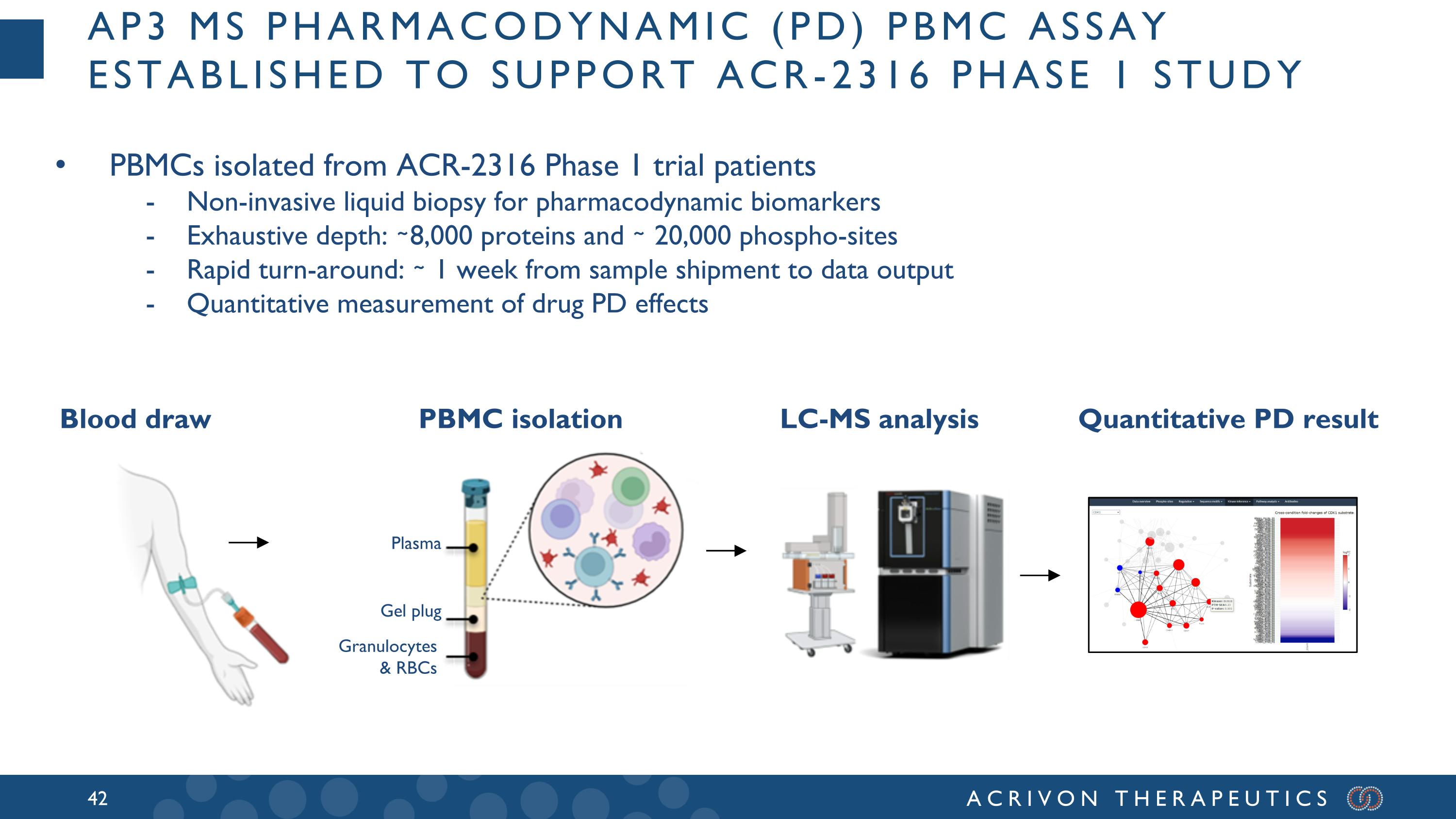
AP3 MS Pharmacodynamic (PD) PBMC Assay established to support ACR-2316 Phase 1 study Blood draw PBMC isolation LC-MS analysis Quantitative PD result PBMCs isolated from ACR-2316 Phase 1 trial patients Non-invasive liquid biopsy for pharmacodynamic biomarkers Exhaustive depth: ∼8,000 proteins and ∼ 20,000 phospho-sites Rapid turn-around: ∼ 1 week from sample shipment to data output Quantitative measurement of drug PD effects Plasma Gel plug Granulocytes & RBCs
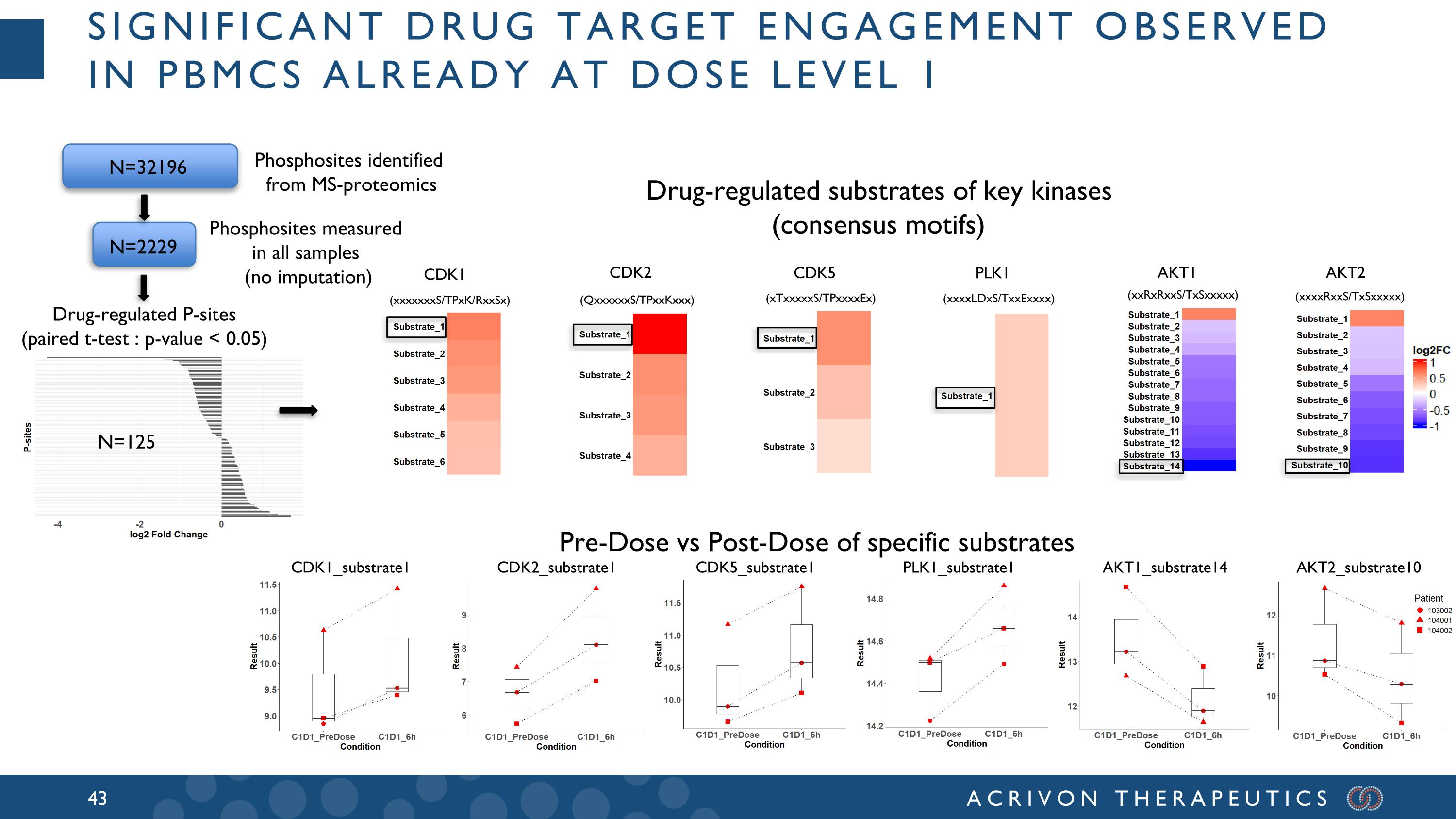
significant drug target engagement observed in pBMCs already at dose level 1 CDK1 CDK2 CDK5 PLK1 AKT1 AKT2 Drug-regulated P-sites (paired t-test : p-value < 0.05) (xxxxxxxS/TPxK/RxxSx) (QxxxxxxS/TPxxKxxx) (xTxxxxxS/TPxxxxEx) (xxxxRxxS/TxSxxxxx) (xxxxLDxS/TxxExxxx) (xxRxRxxS/TxSxxxxx) N=32196 N=2229 Phosphosites identified from MS-proteomics Phosphosites measured in all samples (no imputation) Drug-regulated substrates of key kinases (consensus motifs) CDK1_substrate1 CDK2_substrate1 CDK5_substrate1 PLK1_substrate1 AKT1_substrate14 AKT2_substrate10 Pre-Dose vs Post-Dose of specific substrates N=125

Preclinical program
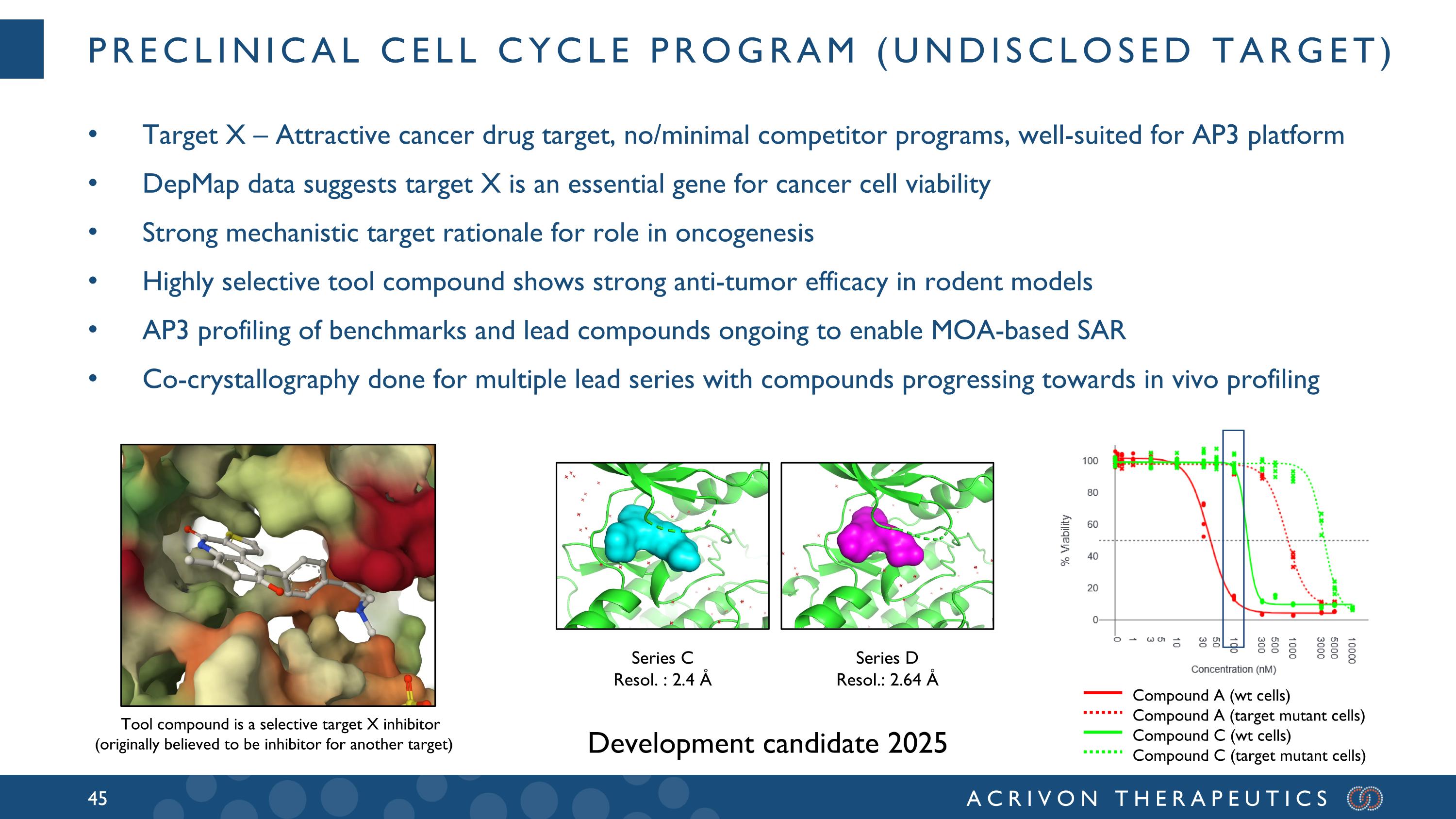
Preclinical Cell Cycle program (Undisclosed Target) Target X – Attractive cancer drug target, no/minimal competitor programs, well-suited for AP3 platform DepMap data suggests target X is an essential gene for cancer cell viability Strong mechanistic target rationale for role in oncogenesis Highly selective tool compound shows strong anti-tumor efficacy in rodent models AP3 profiling of benchmarks and lead compounds ongoing to enable MOA-based SAR Co-crystallography done for multiple lead series with compounds progressing towards in vivo profiling Compound A (wt cells) Compound A (target mutant cells) Compound C (wt cells) Compound C (target mutant cells) Series D Resol.: 2.64 Å Series C Resol. : 2.4 Å Tool compound is a selective target X inhibitor (originally believed to be inhibitor for another target) Development candidate 2025
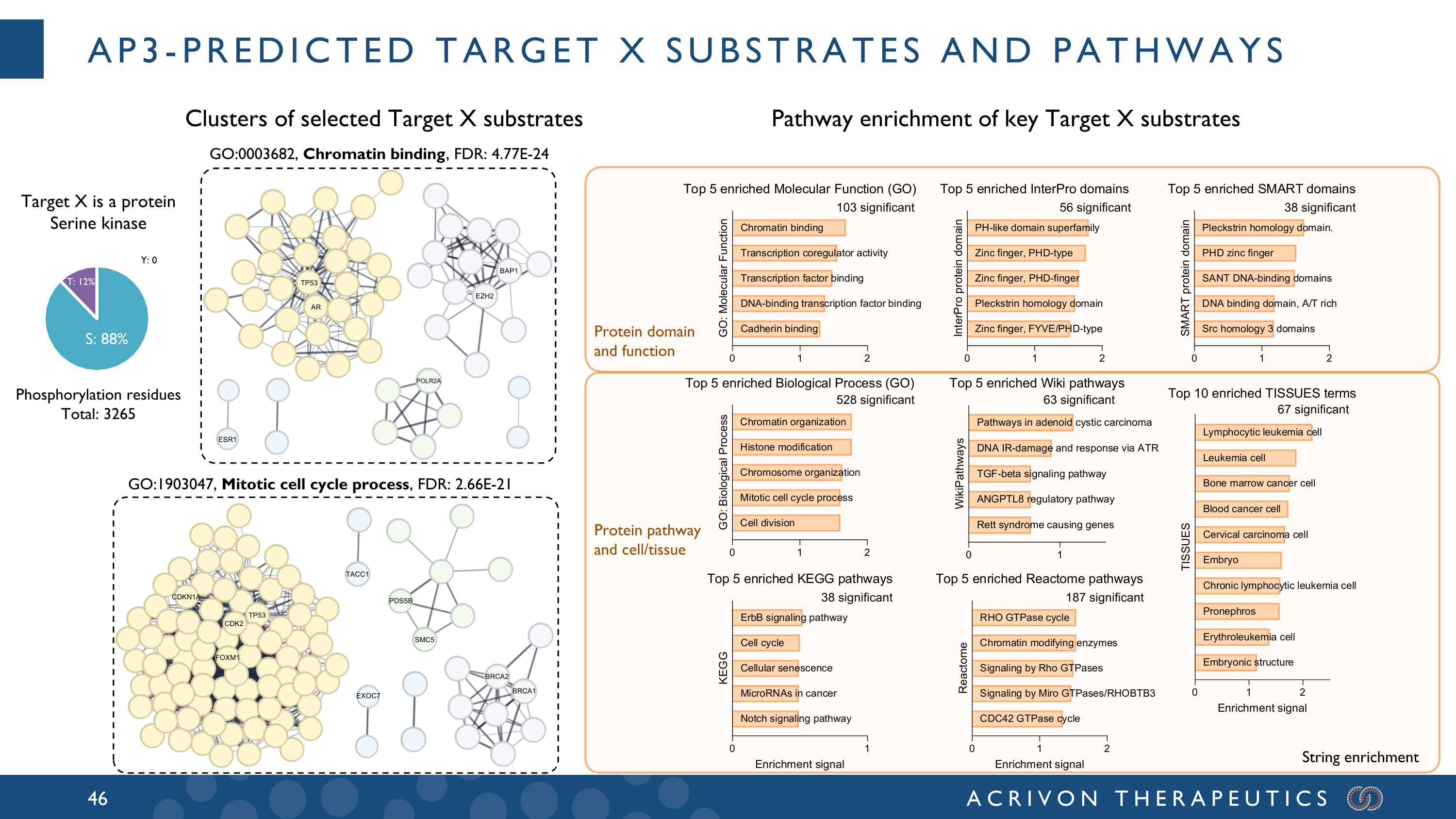
AP3-predicted Target X substrates and pathways Target X is a protein Serine kinase Clusters of selected Target X substrates Protein domain and function Protein pathway and cell/tissue Pathway enrichment of key Target X substrates GO:1903047, Mitotic cell cycle process, FDR: 2.66E-21 T: 12% Y: 0 S: 88% 528 significant 103 significant 38 significant 187 significant 63 significant 67 significant 56 significant 38 significant String enrichment GO:0003682, Chromatin binding, FDR: 4.77E-24 Phosphorylation residues Total: 3265 ESR1 TP53 AR EZH2 BAP1 TACC1 SMC5 BRCA2 BRCA1 FOXM1 CDKN1A TP53 CDK2 EXOC7 PDS5B
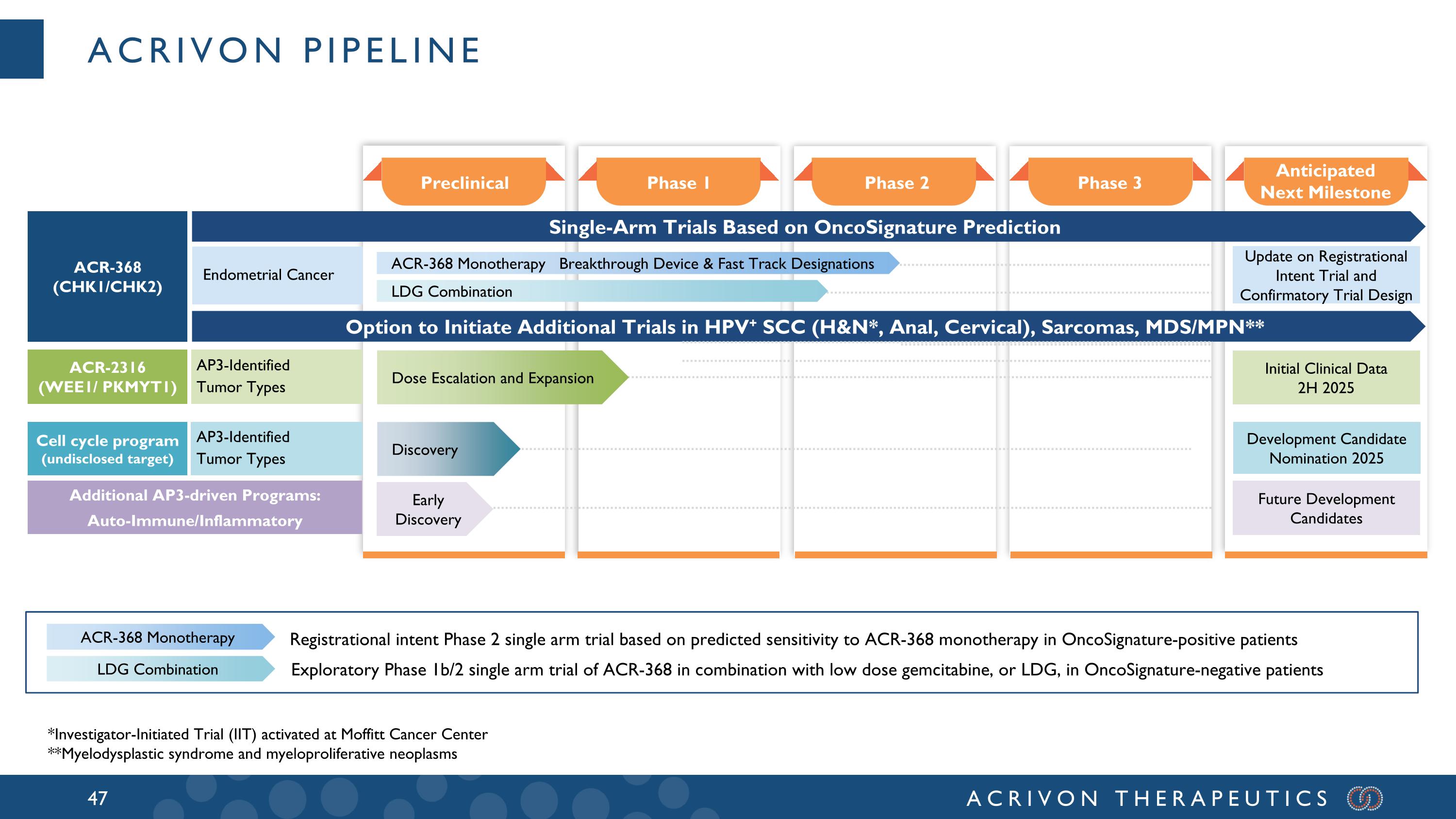
Acrivon Pipeline Anticipated Next Milestone Phase 3 Phase 2 Phase 1 Preclinical ACR-368 (CHK1/CHK2) Single-Arm Trials Based on OncoSignature Prediction Option to Initiate Additional Trials in HPV+ SCC (H&N*, Anal, Cervical), Sarcomas, MDS/MPN** Initial Clinical Data 2H 2025 Update on Registrational Intent Trial and Confirmatory Trial Design Endometrial Cancer AP3-Identified Tumor Types Future Development Candidates Additional AP3-driven Programs: Auto-Immune/Inflammatory ACR-368 Monotherapy LDG Combination Registrational intent Phase 2 single arm trial based on predicted sensitivity to ACR-368 monotherapy in OncoSignature-positive patients Exploratory Phase 1b/2 single arm trial of ACR-368 in combination with low dose gemcitabine, or LDG, in OncoSignature-negative patients LDG Combination ACR-368 Monotherapy Breakthrough Device & Fast Track Designations ACR-2316 (WEE1/ PKMYT1) Early Discovery Dose Escalation and Expansion AP3-Identified Tumor Types Cell cycle program (undisclosed target) Development Candidate Nomination 2025 Discovery *Investigator-Initiated Trial (IIT) activated at Moffitt Cancer Center **Myelodysplastic syndrome and myeloproliferative neoplasms

FINANCIAL HIGHLIGHTS Cash and Investments $184.6M Projected runway into2027 Fully Diluted Shares Outstanding 43.8M Current operating plan, assuming no additional financing Balance sheet 31-Dec-2024 Including shares, pre-funded warrants, and equity grants outstanding 31-Dec-2024 Additional runway compared to previous guidance