UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): October 12, 2025
ADC Therapeutics SA
(Exact Name of Registrant as Specified in Its Charter)
|
Switzerland (State or Other Jurisdiction of Incorporation) |
001-39071 (Commission File Number) |
N/A (IRS Employer Identification Number) |
|
Biopôle Route de la Corniche 3B 1066 Epalinges Switzerland (Address of Principal Executive Offices) (Zip Code) |
+41 21 653 02 00 (Registrant’s Telephone Number) |
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class | Trading Symbol | Name of Each Exchange on Which Registered |
| Common Shares, par value CHF 0.08 per share | ADCT | New York Stock Exchange |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 C.F.R. § 230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 C.F.R. § 240.12b-2). Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 1.01. Entry into a Material Definitive Agreement.
On October 12, 2025, ADC Therapeutics SA (the “Company”) entered into securities purchase agreements for the sale of its equity securities to certain institutional investors in a $60.0 million private placement. In the private placement, the Company will sell 11,250,000 common shares at $4.00 per share and pre-funded warrants to purchase 3,846,153 common shares at $3.90 per pre-funded warrant, which is the price per common share in the private placement minus the exercise price per pre-funded warrant. The private placement is expected to close on October 27, 2025, subject to customary closing conditions.
The purchase agreements provide certain registration rights, pursuant to which the Company has agreed to file a registration statement within 30 business days to register the resale of the common shares sold in the private placement and the common shares issuable upon exercise of the pre-funded warrants sold in the private placement.
The private placement is exempt from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”) under Section 4(a)(2) of the Securities Act in that the private placement is between an issuer and sophisticated investors not involving any public offering. The Company is relying on this exemption from registration based in part on representations made in the purchase agreements for the private placement, a form of which is attached to this Current Report on Form 8-K as Exhibit 10.1.
The pre-funded warrants are exercisable at any time after their original issuance until the tenth anniversary of their original issuance. At any time during the last 90 days of the term, the holder may exchange the pre-funded warrant for, and the Company will issue, a new pre-funded warrant for the number of common shares then remaining under the pre-funded warrant. The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part (but not for less than a common share) by delivering to the Company a duly executed exercise notice and by payment of the aggregate exercise price; provided that any exercise of the pre-funded warrants must be for at least 50,000 common shares (or, if less, the remaining common shares available for purchase under the pre-funded warrants). A holder will not be entitled to exercise any portion of any pre-funded warrants that, upon giving effect to such exercise, would cause the aggregate number of the Company’s common shares beneficially owned by the holder (together with its affiliates and certain attribution parties) to exceed 9.99% (or, 61 days after a written notice from such holder, any other percentage not in excess of 19.99%) of the number of the Company’s common shares outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. The exercise price per common share purchasable upon the exercise of the pre-funded warrants is CHF 0.08 per share, subject to customary adjustments. In lieu of making cash payment of the aggregate exercise price, a holder may elect to exercise the pre-funded warrants on a cashless basis. However, if the Company, at the time of receipt of an exercise notice electing cashless exercise, (i) does not, or has reason to believe that the Company does not, have a sufficient amount of freely distributable equity to fund the nominal value of the number of common shares the Company would be required to deliver upon such cashless exercise, and (ii) (x) holds common shares representing more than 2% of its share capital registered in the commercial register at that time (the “Minimum Stock”) in treasury, then the Company will not be obligated to (but may) deliver more than such number of common shares to the holder as exceeds the Minimum Stock or (y) holds up to the Minimum Stock in treasury, then the Company will not be obligated to deliver any common shares to the holder. The exercise notice will be deemed to be null and void to the extent the holder receives fewer common shares than to which such exercise notice relates. In the event of (i) a sale, lease or other transfer of all or substantially all of the Company’s assets, (ii) a merger or consolidation involving the Company in which the Company is not the surviving entity or in which the Company’s outstanding share capital is converted into or exchanged for shares of capital stock or other securities or property of another entity, or (iii) any sale by holders of the Company’s outstanding voting equity securities in a single transaction or series of related transactions of shares constituting a majority of the Company’s outstanding combined voting power, and (x) if the consideration received by the Company’s shareholders consists solely of cash and/or marketable securities, then holders of the pre-funded warrants will be deemed to have exercised their pre-funded warrants (without regard to the exercise limitations described above) immediately prior to the closing date of such transaction or (y) if the consideration received by the Company’s shareholders does not consist solely of cash and/or marketable securities, then the Company will cause the successor or surviving entity to assume the pre-funded warrants. Subject to applicable laws, the pre-funded warrants may be offered for sale, sold, transferred or assigned without the Company’s consent. The pre-funded warrants are governed by the laws of Switzerland. The foregoing description of the pre-funded warrants does not purport to be complete and is qualified in its entirety by reference to the form of pre-funded warrant, which is attached to this Current Report on Form 8-K as Exhibit 10.2.
Item 2.02. Results of Operations and Financial Condition.
On October 13, 2025, the Company issued a press release and made available a corporate presentation that include the preliminary net product revenues from sales of ZYNLONTA for the quarter ended September 30, 2025 and the preliminary cash and cash equivalents as of September 30, 2025. A copy of the press release is attached as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the corporate presentation is attached as Exhibit 99.2 to this Current Report on Form 8-K and incorporated by reference herein.
The ZYNLONTA net product revenues and cash and cash equivalents figures are preliminary and unaudited and reflect the Company’s estimated financial results. In preparing this information, management made a number of complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Company’s actual financial results for the quarter ended September 30, 2025 have not yet been finalized by management or audited or reviewed by the Company’s independent auditors. The preliminary financial information is not a comprehensive statement of all financial results for the quarter ended September 30, 2025. Subsequent information or events may lead to material differences between the foregoing preliminary financial results and those reported in the Company’s subsequent SEC filings. Accordingly, investors should not place undue reliance on these preliminary financial results.
The information contained in this Item 2.02, Exhibit 99.1 and Exhibit 99.2 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
Item 3.02. Unregistered Sales of Equity Securities.
The information set forth under Item 1.01 of this Current Report on Form 8-K is incorporated by reference in this Item 3.02.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
|
Exhibit Number |
Description |
| 10.1 | Form of securities purchase agreement |
| 10.2 | Form of pre-funded warrant |
| 99.1 | Press release dated October 13, 2025 |
| 99.2 | Corporate presentation dated October 13, 2025 |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| ADC Therapeutics SA | ||
| Date: October 14, 2025 | ||
| By: | /s/ Peter J. Graham | |
| Name: | Peter J. Graham | |
| Title: | Chief Legal Officer | |
Exhibit 10.1
SECURITIES PURCHASE AGREEMENT
This SECURITIES PURCHASE AGREEMENT (this “Agreement”) is dated as of October 12, 2025, by and between ADC Therapeutics SA, a société anonyme domiciled in Epalinges, Canton of Vaud, Switzerland, and organized under the laws of Switzerland (the “Company”), and the undersigned investor (the “Investor”). Substantially concurrently with the execution of this Agreement, the Company is entering into separate purchase agreements (collectively, the “Additional Purchase Agreements”) with certain investors (the “Additional Investors”), severally and not jointly, to purchase securities from the Company in one or more transactions exempt from the registration requirements of the Securities Act (as defined below) pursuant to Section 4(a)(2) thereof (the transactions contemplated by this Agreement and the Additional Purchase Agreements, collectively, the “PIPE Transaction”).
In consideration of the mutual promises made herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto agree as follows:
Section 1. Definitions. For the purposes of this Agreement, capitalized terms not otherwise defined shall have the meanings set forth below:
“Affiliate” means, with respect to any Person, any other Person which directly or indirectly through one or more intermediaries Controls, is controlled by, or is under common Control with, such Person.
“Business Day” means a day, other than a Saturday or Sunday, on which banks in New York City are open for the general transaction of business.
“Common Shares” means the common shares, par value CHF 0.08 per share, of the Company.
“Control” (including the terms “controlling,” “controlled by” or “under common control with”) means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities, by contract or otherwise.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, or any successor statute, and the rules and regulations promulgated thereunder.
“Health Care Laws” means (i) the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §§ 301 et seq.), the Public Health Service Act (42 U.S.C. §§ 201 et seq.); (ii) all applicable federal, state, local and all applicable foreign health care related fraud and abuse laws, including, without limitation, the U.S. Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the U.S. False Statements Law (42 U.S.C. § 1320a-7b(a)), the Civil Monetary Penalties Law (42 U.S.C. §1320a-7a), the U.S. Civil False Claims Act (31 U.S.C. § 3729 et seq.), all criminal laws relating to health care fraud and abuse, including but not limited to 18 U.S.C. §§ 286 and 287, and the health care fraud criminal provisions under the U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) (42 U.S.C. §§ 1320d et seq.), the Physician Payments Sunshine Act (42 U.S.C. § 1320a-7h), the exclusion law (42 U.S.C. §1320a-7); (iii) HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (42 U.S.C. §§ 17921 et seq.); (iv) regulations promulgated pursuant to such statutes; and (v) any and all other applicable federal, state, or foreign health care laws and regulation applicable to the ownership, testing, development, manufacture, packaging, processing, use, distribution, marketing, advertising, labeling, promotion, sale, offer for sale, storage, import, export or disposal of any product manufactured or distributed by the Company.
“NYSE” means the New York Stock Exchange.
“Person” means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
“Placement Agent” means Jefferies LLC.
“Pre-Funded Warrants” means the pre-funded warrants of the Company, in the form attached as Exhibit A hereto.
“Registrable Securities” means (i) the Shares and the Warrant Shares and (ii) any other Common Shares issued as a dividend or other distribution with respect to, in exchange for or in replacement of the Shares or the Warrant Shares; provided, however, that any such Registrable Securities shall cease to be Registrable Securities (and the Company shall not be required to maintain the effectiveness of any, or file another, registration statement hereunder with respect thereto) upon the first to occur of (A) a registration statement with respect to the sale of such Registrable Securities being declared effective by the SEC under the Securities Act and such Registrable Securities having been disposed of or transferred by the holder thereof in accordance with such effective registration statement, (B) such Registrable Securities having been previously sold or transferred in accordance with Rule 144 (or another exemption from the registration requirements of the Securities Act), (C) such securities becoming eligible for resale without volume or manner-of-sale restrictions and without current public information requirements pursuant to Rule 144, (D) such securities are no longer outstanding or (E) the third anniversary of the Closing Date.
“Regulation D” means Regulation D as promulgated by the SEC under the Securities Act.
“Rule 144” means Rule 144 promulgated under the Securities Act.
“SEC” means the U.S. Securities and Exchange Commission.
“SEC Documents” means the documents filed by the Company with the SEC pursuant to the Exchange Act on or after January 1, 2025.
“Securities Act” means the Securities Act of 1933, as amended, or any successor statute, and the rules and regulations promulgated thereunder.
“Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include the location and/or reservation of borrowable shares of Common Shares).
“Trading Day” means a day on which the NYSE is open for trading.
“Transaction Documents” means this Agreement and the Warrants (as defined below).
“Warrant Shares” means the Common Shares issuable upon exercise of the Warrants in full without regard to any exercise limitations therein.
Section 2. Purchase and Sale of the Securities. At the Closing (as defined below), upon the terms of and subject to the conditions set forth in this Agreement, the Company will issue and sell to the Investor, and the Investor will purchase from the Company, (i) the number of Common Shares set forth under the heading “Number of Shares” on the Investor’s signature page to this Agreement (such Common Shares, collectively, the “Shares”), at a price per Share equal to $4.00 and (ii) the number of Pre-Funded Warrants set forth under the heading “Number of Pre-Funded Warrants” on the Investor’s signature page to this Agreement (such Pre-Funded Warrants, collectively, the “Warrants” and, together with the Shares, the “Securities”), at a price per Warrant equal to $3.90, which is the price per Share under this Agreement minus the U.S. dollar equivalent of the exercise price of such Warrant.
Section 3. Closing.
Section 3.1. The closing of the purchase and sale of the Securities pursuant to this Agreement (the “Closing”) shall be held remotely no later than 9:30 a.m. (New York City time) on October 27, 2025 (the “Closing Date”).
Section 3.2. At or prior to the Closing, the Investor shall execute any related agreements or other documents required to be executed under this Agreement, dated on or before the Closing Date, including but not limited to the Investor Questionnaire in the form attached as Annex A hereto (the “Investor Questionnaire”).
Section 3.3. At the Closing, (i) the Investor shall pay the purchase price of the Securities to the Company by wire transfer in immediately available U.S. federal funds to an account designated by the Company in writing not later than two Business Days prior to the Closing Date and (ii) the Company shall issue and deliver to the Investor the Shares in book-entry form and registered in the name of the Investor (or its nominee if specified in writing not later than two Business Days prior to the Closing Date) and the Warrants in electronic form and registered in the name of the Investor (or its nominee if specified in writing not later than two Business Days prior to the Closing Date).
Section 3.4. The book-entry positions evidencing the Shares and the Warrants, in each case, delivered on the Closing Date, shall bear the following legend: “THE SECURITIES REPRESENTED HEREBY (AND ANY SECURITIES ISSUED UPON THE EXERCISE OF THE SECURITIES REPRESENTED HEREBY) HAVE NOT BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED, AND, ACCORDINGLY, MAY NOT BE TRANSFERRED UNLESS (I) SUCH SECURITIES HAVE BEEN REGISTERED FOR SALE PURSUANT TO THE SECURITIES ACT OF 1933, AS AMENDED, (II) SUCH SECURITIES MAY BE SOLD PURSUANT TO RULE 144, OR (III) THE COMPANY HAS RECEIVED AN OPINION OF COUNSEL REASONABLY SATISFACTORY TO IT THAT SUCH TRANSFER MAY LAWFULLY BE MADE WITHOUT REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED.”
Section 4. Representations and Warranties of the Company. The Company represents and warrants to the Investor, as of the date hereof and as of the Closing Date (except to the extent made only as of a specified date, in which case such representation and warranty is made as of such date):
Section 4.1. The Company has filed all reports, schedules, forms, statements and other documents required to be filed by the Company under the Exchange Act. At the time of filing thereof, each SEC Document complied in all material respects with the Exchange Act. At the time of filing thereof, each SEC Document did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading.
Section 4.2. The Common Shares are registered pursuant to Section 12(b) of the Exchange Act and listed for trading on NYSE. There is no suit, action, proceeding or investigation pending or, to the knowledge of the Company, threatened against the Company by NYSE or the SEC, respectively, to prohibit or terminate the listing of the Common Shares on NYSE or to deregister the Common Shares under the Exchange Act.
Section 4.3. The Company has been duly incorporated and is validly existing as a Swiss stock corporation (société anonyme) in good standing (to the extent this concept applies) under the laws of Switzerland, has the corporate power and authority to own its property and to conduct its business, is not in liquidation or receivership or the subject of any insolvency or bankruptcy proceedings and is duly qualified to transact business (and, if applicable, is in good standing) in each jurisdiction in which the conduct of its business or its ownership or leasing of property requires such qualification, except to the extent that the failure to be so qualified or be in good standing (to the extent this concept applies) would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole.
Section 4.4. Each subsidiary of the Company has been duly incorporated, is validly existing as a corporation (and, if applicable, in good standing) under the laws of the jurisdiction of its incorporation, has the corporate power and authority to own its property and to conduct its business, is not in liquidation or receivership or the subject of any insolvency or bankruptcy proceedings and is duly qualified to transact business (and, if applicable, is in good standing) in each jurisdiction in which the conduct of its business or its ownership or leasing of property requires such qualification, except to the extent that the failure to be so qualified or be in good standing (to the extent this concept applies) would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole. Except as otherwise described in the SEC Documents, all of the issued shares of capital stock of each subsidiary of the Company have been duly and validly authorized and issued, are fully paid and non-assessable and such shares are owned directly by the Company, free and clear of all liens, encumbrances, equities or claims.
Section 4.5. This Agreement has been duly authorized, executed and delivered by the Company. Assuming due authorization, execution and delivery of this Agreement by the Investor, this Agreement constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as the enforcement thereof may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar laws relating to or affecting the rights and remedies of creditors or by general equitable principles.
Section 4.6. The Common Shares issued and outstanding prior to the issuance of the Securities have been duly authorized and are validly issued, fully paid and non-assessable.
Section 4.7. The Common Shares conform as to legal matters to the description thereof contained in the SEC Documents. Except as otherwise described in the SEC Documents or in connection with the PIPE Transaction, there are no outstanding rights (including, without limitation, subscription rights), warrants or options to acquire, or instruments, securities or rights convertible into or exchangeable for, any Common Shares or other equity interest in the Company to which the Company or any of its subsidiaries is a party, or any contract, commitment, or arrangement of any kind to which the Company or any of its subsidiaries is a party under which the Company or any of its subsidiaries have committed to issue any Common Shares or grant any such convertible or exchangeable securities or any such rights, warrants or options. Except as otherwise described in the SEC Documents, there are no outstanding rights (including, without limitation, subscription rights), warrants or options to acquire, or instruments, securities or rights convertible into or exchangeable for, any shares of any of the Company’s subsidiaries to which the Company or any of its subsidiaries is a party, or any contract, commitment, or arrangement of any kind to which the Company or any of its subsidiaries is a party under which the Company or any of its subsidiaries have committed to issue any shares, rights, warrants or options or instruments convertible into or exchangeable for, any shares of any subsidiary of the Company.
Section 4.8. The issuance and sale of the Securities will have been duly authorized on the Closing Date. The Shares, when delivered and paid for in the manner contemplated by this Agreement, will, at all times, be validly issued, fully paid and non-assessable. The Shares, when delivered and paid for in the manner contemplated by this Agreement, will be, subject only to any restrictions applicable under the Company’s articles of association or applicable laws, freely transferable except as described in the SEC Documents, and will not be subject to any pre-emptive right, third-party rights or similar rights (including, without limitation, any security interest under articles 24 and 25 of the Swiss Federal Act on Intermediated Securities) granted by the Company or any of its subsidiaries other than as contemplated by this Agreement. The Warrants, when executed and delivered by the Company, will be valid and binding agreements of the Company, enforceable against the Company in accordance with their terms, except as the enforcement thereof may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar laws relating to or affecting the rights and remedies of creditors or by general equitable principles. The Warrant Shares, when issued and delivered upon exercise of the Warrants in accordance therewith, will, at all times, be validly issued, fully paid and non-assessable, and when delivered and paid for in the manner contemplated by the Warrants, will be, subject only to any restrictions applicable under the Company’s articles of association or applicable laws, freely transferable except as otherwise described in the SEC Documents, and will not be subject to any pre-emptive right, third-party rights or similar rights (including, without limitation, any security interest under articles 24 and 25 of the Swiss Federal Act on Intermediated Securities) granted by the Company or any of its subsidiaries other than as contemplated by the Warrants. The Company has a valid conditional share capital for financing purposes, out of which the Warrant Shares may be issued in compliance with the Company's articles of association (Statuts) and then delivered. On the Closing Date, the Company will have duly reserved in its conditional share capital for financing purposes the number of Warrant Shares that are issuable and deliverable upon the exercise of the Warrants.
Section 4.9. The execution and delivery by the Company of, and the performance by the Company of its obligations under, the Transaction Documents do not contravene any provision of (i) applicable law, (ii) the articles of association (Statuts) or the organizational regulations (Règlement d’organisation) of the Company or (iii) any agreement or other instrument binding upon the Company or any of its subsidiaries that is material to the Company and its subsidiaries, taken as a whole, or any judgment, order or decree of any governmental body, agency, or court having jurisdiction over the Company or any subsidiary, except that in the case of clauses (i) and (iii) as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company or on the power and ability of the Company to perform its obligations under the Transaction Documents. No consent, approval, authorization or order of, or qualification with, any governmental body or agency is required for the performance by the Company of its obligations under this Agreement, except (A) such as have been obtained or waived or as may be required by the securities or blue sky laws of the various states in connection with the offer and sale of the Securities and the Warrant Shares, (B) the registration of the share capital increase for the Transaction with the Commercial Register of the Canton of Vaud, (C) the listing of the Shares and the Warrant Shares on the NYSE and (D) the registration of the Registrable Securities as contemplated by Section 7.9, subject to (A) to (B) being obtained or done on or prior to the Closing Date.
Section 4.10. There has not occurred any material adverse effect on the Company and its subsidiaries, taken as a whole, or any development involving a prospective material adverse effect on the Company and its subsidiaries, taken as a whole, in the condition, financial or otherwise, or in the earnings, business or operations of the Company and its subsidiaries, taken as a whole, from that set forth in the SEC Documents or otherwise disclosed to the Investor.
Section 4.11. There are no legal or governmental proceedings pending or, to the knowledge of the Company, threatened to which the Company or any of its subsidiaries is a party or to which any of the properties of the Company or any of its subsidiaries is subject, other than proceedings accurately described in all material respects in the SEC Documents and proceedings that would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, or a material adverse effect on the power or ability of the Company to perform its obligations under this Agreement or to consummate the transactions contemplated by the Transaction Documents. There are no orders, writs, injunctions, judgments or decrees outstanding of any court or government agency or instrumentality and binding upon the Company or any of its subsidiaries, other than proceedings accurately described in all material respects in the SEC Documents and orders, writs, injunctions, judgments or decrees that would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, or a material adverse effect on the power or ability of the Company to perform its obligations under this Agreement or to consummate the transactions contemplated by the Transaction Documents. Neither the Company nor any subsidiary, nor to the knowledge of the Company, any director or officer of the Company or any subsidiary, is, or within the last ten years has been, the subject of any action involving a claim of violation of or liability under federal or state securities laws relating to the Company or such subsidiary or a claim of breach of fiduciary duty relating to the Company or such subsidiary.
Section 4.12. The Company is not, and after giving effect to the offering and sale of the Securities contemplated hereby and the application of the proceeds thereof will not be, required to register as an “investment company” as such term is defined in the Investment Company Act of 1940, as amended.
Section 4.13. The Company and its subsidiaries (i) are in compliance with any and all applicable foreign, federal, state, cantonal and local laws and regulations relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants or contaminants (“Environmental Laws”), (ii) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses and (iii) are in compliance with all terms and conditions of any such permit, license or approval, except where such noncompliance with Environmental Laws, failure to receive required permits, licenses or other approvals or failure to comply with the terms and conditions of such permits, licenses or approvals would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole.
Section 4.14. There are no costs or liabilities associated with Environmental Laws (including, without limitation, any capital or operating expenditures required for clean up, closure of properties or compliance with Environmental Laws or any permit, license or approval, any related constraints on operating activities and any potential liabilities to third parties) which would, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole.
Section 4.15. None of the Company or its subsidiaries or controlled Affiliates, or any director or officer, or, to the Company’s knowledge, any employee, agent or representative of the Company or of any of its subsidiaries or controlled Affiliates, has taken or will take any action in furtherance of an offer, payment, promise to pay, or authorization or approval of the payment, giving or receipt of money, property, gifts or anything else of value, directly or indirectly, to any government official (including any officer or employee of a government or government-owned or controlled entity or of a public international organization, or any person acting in an official capacity for or on behalf of any of the foregoing, or any political party or party official or candidate for political office) (“Government Official”) in order to influence official action, or to any person in violation of any applicable anti-corruption laws.
The Company and its subsidiaries and controlled Affiliates have conducted their businesses in compliance with applicable anti-corruption laws and have instituted and maintained and will continue to maintain policies and procedures reasonably designed to promote and achieve compliance with such laws and with the representations and warranties contained herein. Neither the Company nor its subsidiaries will use, directly or indirectly, the proceeds of the offering in furtherance of an offer, payment, promise to pay, or authorization of the payment or giving of money, or anything else of value, to any person in violation of any applicable anti-corruption laws.
Section 4.16. The operations of the Company and its subsidiaries are and have been conducted at all times in material compliance with all applicable financial recordkeeping and reporting requirements, including, to the extent applicable, those of the Bank Secrecy Act, as amended by Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), and the applicable anti-money laundering statutes of jurisdictions where the Company and its subsidiaries conduct business, the rules and regulations thereunder and any related or similar rules, regulations or guidelines issued, administered or enforced by any governmental agency (collectively, the “Anti-Money Laundering Laws”), and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or any of its subsidiaries with respect to the Anti-Money Laundering Laws is pending or, to the knowledge of the Company, threatened.
Section 4.17. Neither the Company nor any of its subsidiaries, nor any director or officer thereof, nor, to the Company’s knowledge, any employee, agent, controlled Affiliate or representative of the Company or any of its subsidiaries, is a Person that is, or is owned or controlled by a Person that is (i) the subject of any sanctions administered or enforced by the U.S. Department of Treasury’s Office of Foreign Assets Control, the United Nations Security Council, the European Union, His Majesty’s Treasury, the Swiss State Secretariat of Economic Affairs, the Swiss Directorate of International Law or other relevant sanctions authority (collectively, “Sanctions”), or (ii) located, organized or resident in a country or territory that is the subject of Sanctions (including, without limitation, Cuba, Iran, North Korea, Syria, the Crimea Region located in Ukraine, and the so-called Donetsk People’s Republic, the so-called Luhansk People’s Republic and any other Covered Region of Ukraine as may be determined by the U.S. Secretary of the Treasury pursuant to Executive Order 14065). The Company will not, directly or indirectly, use the proceeds of the sale of the Securities, or lend, contribute or otherwise make available such proceeds to any subsidiary, joint venture partner or other Person (i) to fund or facilitate any activities or business of or with any Person or in any country or territory that, at the time of such funding or facilitation, is the subject of Sanctions or (ii) in any other manner that will result in a violation of Sanctions by any Person (including any Person participating in the offering, whether as underwriter, advisor, investor or otherwise). Since April 24, 2019, the Company and its subsidiaries have not engaged in, are not now engaged in, and will not engage in, any dealings or transactions with any Person, or in any country or territory, that at the time of the dealing or transaction is or was the subject of Sanctions.
Section 4.18. Subsequent to December 31, 2024, except as described in the SEC Documents, in connection with the PIPE Transaction or otherwise disclosed to the Investor, (i) the Company and its subsidiaries have not incurred any material liability or obligation, direct or contingent, nor entered into any material transaction, (ii) the Company has not purchased any of its outstanding share capital other than from its employees or other service providers in connection with the termination of their service pursuant to the terms of the equity compensation plans, nor declared, paid or otherwise made any dividend or distribution of any kind on its share capital and (iii) except for the issuance of Common Shares into treasury, there has not been any material change in the share capital (other than the exercise or settlement of equity awards or grants or forfeiture of equity awards granted pursuant to the equity compensation plans), short-term debt or long-term debt of the Company and its subsidiaries.
Section 4.19. The Company and its subsidiaries have good and marketable title to all real property and good and marketable title to all personal property (other than intellectual property, which is addressed exclusively in Section 4.20) owned by them which is material to the business of the Company and its subsidiaries, in each case free and clear of all liens, encumbrances and defects except such as are described in the SEC Documents or such as do not materially affect the value of such property and do not materially interfere with the use made and proposed to be made of such property by the Company and its subsidiaries. Any real property and buildings held under lease by the Company and its subsidiaries are held by them under valid, subsisting and enforceable leases with such exceptions as are not material and do not materially interfere with the use made and proposed to be made of such property and buildings by the Company and its subsidiaries, in each case except as described in the SEC Documents.
Section 4.20. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, (i) the Company and its subsidiaries own or have a valid license to all patents, inventions, copyrights, know how (including trade secrets and other unpatented and/or unpatentable proprietary or confidential information, systems or procedures), trademarks, service marks and trade names (collectively, “Intellectual Property Rights”) used in or reasonably necessary to the conduct of their businesses as now operated by them, and as proposed to be operated in the future (including upon the commercialization of the Company’s and its subsidiaries’ products or services), in each case as described in the SEC Documents (the “Company Intellectual Property”), (ii) to the Company’s knowledge, there are no third parties who have rights to any Company Intellectual Property, except for customary reversionary rights of third-party licensors, (iii) the Company Intellectual Property owned by the Company and its subsidiaries and, to the Company’s knowledge, the Company Intellectual Property licensed to the Company and its subsidiaries, are subsisting and, to the Company’s knowledge, valid and enforceable, (iv) there is no pending or, to the Company’s knowledge, threatened action, suit, proceeding or claim by others challenging the validity, scope or enforceability of any Company Intellectual Property, and, to the Company’s knowledge, the Company is unaware of any facts which would form a reasonable basis for any such action, suit, proceeding or claim, (v) there is no pending or, to the Company’s knowledge, threatened in writing action, suit, proceeding or claim by others challenging the Company’s rights in or to any Company Intellectual Property, and the Company is unaware of any facts which would form a reasonable basis for any such action, suit, proceeding or claim, (vi) neither the Company nor any of its subsidiaries has received any written notice alleging any infringement, misappropriation or other violation of Intellectual Property Rights, (vii) to the Company’s knowledge, no third party is infringing, misappropriating or otherwise violating, or has infringed, misappropriated or otherwise violated, any Company Intellectual Property owned by the Company, (viii) to the Company’s knowledge, (A) neither the Company nor any of its subsidiaries infringes, misappropriates or otherwise violates, or has infringed, misappropriated or otherwise violated, any third-party Intellectual Property Rights, and (B) the commercialization of the products or services described in the SEC Documents as under development by the Company will not infringe, misappropriate, or otherwise violate any third-party Intellectual Property Rights, (ix) to the Company’s knowledge, the Company and its subsidiaries have complied with the terms of each agreement to which they are a party and pursuant to which Intellectual Property Rights have been licensed to the Company or its subsidiaries, and all such agreements are in full force and effect, (x) to the Company’s knowledge, during the prosecution of the patents and patent applications included in the Company Intellectual Property, the Company and its subsidiaries have complied with the duty of candor and good faith with respect to such patents and patent applications as required by the United States Patent and Trademark Office and all foreign offices having similar requirements, (xi) all employees or contractors engaged in the development of Company Intellectual Property on behalf of the Company or any subsidiary of the Company have executed an invention assignment agreement whereby such employees or contractors presently assign all of their right, title and interest in and to such Company Intellectual Property to the Company or the applicable subsidiary, and no such agreement has been breached or violated, (xii) to the Company’s knowledge, none of the Company Intellectual Property has been obtained, or is being used, by the Company or its subsidiary in violation of any contractual obligation binding on the Company or its subsidiaries or any of their respective officers, directors or employees or otherwise in violation of the rights of any persons and (xiii) the Company and its subsidiaries use, and have used, commercially reasonable efforts to appropriately maintain all information intended to be maintained as a trade secret.
Section 4.21. No labor dispute with the employees of the Company or any of its subsidiaries exists, except as described in the SEC Documents, or, to the knowledge of the Company, is imminent, in either case, that could have a material adverse effect on the Company and its subsidiaries, taken as a whole; and the Company is not aware of any existing, threatened or imminent labor disturbance by the employees of any of its principal suppliers, manufacturers or contractors that could have a material adverse effect on the Company and its subsidiaries, taken as a whole.
Section 4.22. The Company and each of its subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as the Company reasonably believes are prudent and customary in the businesses in which they are engaged, except where the failure to be so insured would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole.
Neither the Company nor any of its subsidiaries has been refused any insurance coverage sought or applied for three years preceding the date of this Agreement. Neither the Company nor any of its subsidiaries has any reason to believe that it will not be able to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, except as described in the SEC Documents.
Section 4.23. The Company and its subsidiaries, taken as a whole, possess all certificates, authorizations and permits issued by the appropriate federal, state or foreign regulatory authorities necessary to conduct their respective businesses, except where the failure to obtain such certificates, authorization and permits would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, and neither the Company nor any of its subsidiaries has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit which, singly or in the aggregate, if the subject of an unfavorable decision, ruling or finding, would have a material adverse effect on the Company and its subsidiaries, taken as a whole, except as described in the SEC Documents.
Section 4.24. The Company and its subsidiaries on a consolidated basis maintain a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with generally accepted accounting principles as applied in the United States (“GAAP”) and to maintain asset accountability, (iii) access to assets is permitted only in accordance with management’s general or specific authorization, (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences and (v) the interactive data in eXtensible Business Reporting Language included in the SEC Documents fairly presents the information called for in all material respects and is prepared in accordance with the SEC’s rules and guidelines applicable thereto.
Section 4.25. Except as described in each of the SEC Documents, since the end of the Company’s most recent audited fiscal year, there has been (i) no material weakness in the Company’s internal control over financial reporting (whether or not remediated) and (ii) no change in the Company’s internal control over financial reporting that has materially and adversely affected, or is reasonably likely to materially and adversely affect, the Company’s internal control over financial reporting.
Section 4.26. The Company and each of its subsidiaries have filed all federal, state, local and foreign tax returns required to be filed through the date of this Agreement or have requested extensions thereof (except where the failure to file would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole) and have paid all taxes required to be paid thereon (except for cases in which the failure to file or pay would not have a material adverse effect on the Company and its subsidiaries, taken as a whole, or, except as currently being contested in good faith and for which reserves required by GAAP have been created in the financial statements of the Company), and no tax deficiency has been determined adversely to the Company or any of its subsidiaries which has had (nor does the Company nor any of its subsidiaries have any notice or knowledge of any tax deficiency which could reasonably be expected to be determined adversely to the Company or its subsidiaries and which could reasonably be expected to have) a material adverse effect on the Company and its subsidiaries, taken as a whole.
Section 4.27. The consolidated financial statements included in the SEC Documents, together with the related schedules and notes thereto, present fairly in all material respects the consolidated financial position of the Company as of the dates shown and its results of operations and cash flows for the periods shown, and, except as otherwise disclosed in the SEC Documents, such financial statements have been prepared in conformity with GAAP applied on a consistent basis throughout the periods covered thereby except for any normal year-end adjustments in the Company’s quarterly financial statements and except as otherwise noted therein.
Section 4.28. PricewaterhouseCoopers SA, who has audited certain financial statements of the Company, is (i) an independent registered public accounting firm with respect to the Company within the meaning of the Securities Act and the applicable rules and regulations thereunder adopted by the SEC and the Public Company Accounting Oversight Board (United States) and (ii) an independent statutory auditor with respect to the Company and a state regulated audit firm (société d’audit réglementée par l’État) under the applicable provisions of the Swiss Code of Obligations, and the Swiss Audit Oversight Act (Loi fédérale sur l’agrément et la surveillance des réviseurs) and any ordinances promulgated thereunder, respectively.
Section 4.29. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, (i) the pre-clinical studies and clinical trials conducted by or, to the knowledge of the Company, on behalf of or sponsored by the Company or its subsidiaries or in which the Company or its subsidiaries have participated, that are described in the SEC Documents or the results of which are referred to in the SEC Documents, as applicable, were, and if still pending are, being conducted in accordance with the protocols submitted to the U.S. Food and Drug Administration (the “FDA”), the Swiss Agency for Therapeutic Products (“swissmedic”), and other applicable regulatory authorities (including, without limitation, any foreign, federal, state or local governmental or regulatory authority performing functions similar to those performed by the FDA, FOPH, swissethics and swissmedic) (collectively, the “Regulatory Authorities”), the applicable rules and regulations of the Regulatory Authorities, and current Good Clinical Practices and Good Laboratory Practices, (ii) the descriptions in the SEC Documents of the results of such studies and trials are accurate and fairly present the data derived therefrom, (iii) the Company has no knowledge of any other studies or trials not described in the SEC Documents, the results of which call into question the results described or referred to in the SEC Documents, (iv) the Company and its subsidiaries have operated at all times and are currently in material compliance with all applicable statutes, rules and regulations of the Regulatory Authorities and (v) neither the Company nor any of its subsidiaries have received any written notices, correspondence or other communications from the Regulatory Authorities or any other governmental agency requiring or threatening the termination, modification or suspension of any pre-clinical studies or clinical trials that are described in the SEC Documents or the results of which are referred to in the SEC Documents, other than ordinary course communications with respect to modifications in connection with the design and implementation of such studies or trials, and, to the Company’s best knowledge, there are no reasonable grounds for the same.
Section 4.30. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, the Company has not failed to file with the Regulatory Authorities any required filing, declaration, listing, registration, report or submission that is a responsibility with the Company with respect to the Company’s product candidates that are described or referred to in the SEC Documents. All such filings, declarations, listings, registrations, reports or submissions were to the Company’s knowledge in compliance with applicable laws when filed. To the Company’s knowledge no deficiencies regarding compliance with applicable law have been asserted by any applicable Regulatory Authority with respect to any such filings, declarations, listings, registrations, reports or submissions.
Section 4.31. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, the Company and its subsidiaries are, and at all times have been, in compliance with all applicable Health Care Laws. Neither the Company nor its subsidiaries has received written notice of any claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from any court or arbitrator or governmental or regulatory authority or third party alleging that it is in violation of any Health Care Laws and, to the Company’s knowledge, no such claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action is threatened. Neither the Company nor its subsidiaries, nor their respective officers, directors, employees, contractors or agents, is a party to any corporate integrity agreements, monitoring agreements, consent decrees, settlement orders, or similar agreements with or imposed by any governmental or regulatory authority. Additionally, neither the Company nor any of its employees, officers, directors, contractors or agents, nor its subsidiaries or any of the subsidiary’s employees, officers, directors, contractors or agents, has been excluded, suspended or debarred from participation in any U.S. federal health care program (as defined in 42 U.S.C. § 1320a-7b(f)) or human clinical research or, to the knowledge of the Company, is subject to a governmental inquiry, investigation, proceeding, or other similar action that could reasonably be expected to result in such debarment, suspension, or exclusion. The Company and its subsidiaries have filed, obtained, maintained or submitted all material reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments as required by the Health Care Laws, and all such reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments were timely, complete, accurate and not misleading on the date filed in all material respects (or were corrected or supplemented by a subsequent submission).
Section 4.32. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, (i) there has been no security breach or incident, unauthorized access or disclosure, or other compromise of the Company and its subsidiaries’ information technology assets and equipment, computers, systems, networks, hardware, software, websites, applications, technology, data and databases, including Personal Data (defined below), the data and information of their respective customers and employees, and any sensitive, confidential or regulated data maintained, processed or stored by the Company and its subsidiaries (collectively, “IT Systems and Data”); (ii) the IT Systems and Data are adequate for, and operate and perform as required in connection with the operation of the business of the Company and its subsidiaries as currently conducted; (iii) the Company and its subsidiaries have used commercially reasonable efforts to implement and maintain, and have implemented and maintained, commercially reasonable information technology, information security, cyber security and data protection controls, policies and procedures, including oversight, access controls, encryption, physical, technological and administrative safeguards and controls, and business continuity/disaster recovery and security plans that are designed to protect against and prevent security breaches, unauthorized use or access, disablement, misappropriation, modification, or other compromise or misuse of the IT Systems and Data, and maintain and protect their material confidential information and the integrity, continuous operation, redundancy and security of all IT Systems and Data used in connection with the operation of the Company’s and its subsidiaries’ businesses, which are reasonably consistent with industry standards. “Personal Data” means (i) a natural person’s name, street address, telephone number, e-mail address, photograph, social security number or tax identification number, driver’s license number, passport number, credit card number, bank information, or customer or account number; (ii) “personal data” as defined by GDPR (defined below); (iii) any information which would qualify as “protected health information” under HIPAA; and (iv) any other piece of information that (A) is regulated by an applicable privacy law, regulation or contract and (B) allows the identification of such natural person, or his or her family, or permits the collection or analysis of any data related to an identified person’s health or sexual orientation.
Section 4.33. Except as described in the SEC Documents and except as would not, individually or in the aggregate, be reasonably expected to have a material adverse effect on the Company and its subsidiaries, taken as a whole, (i) the Company and each of its subsidiaries have complied, and are presently in compliance, with all applicable internal and external privacy policies, contractual obligations, applicable state, federal and international data privacy and security laws and regulations, including, without limitation, HIPAA, the European Union General Data Protection Regulation (“GDPR”) (EU 2016/679), and other statutes, judgments, orders, rules and regulations of any applicable court or arbitrator or other governmental or regulatory authority (collectively, the “Data Security Obligations”), (ii) the Company has not received from any applicable governmental authority any written notification of or written complaint alleging non-compliance by the Company or its subsidiaries with any Data Security Obligation, (iii) there is no action, suit or proceeding by or before any applicable court or governmental agency, authority or body pending or threatened in writing alleging non-compliance by the Company or its subsidiaries, (iv) to ensure compliance with the Data Security Obligations, the Company and its subsidiaries have at all times had in place, comply with, and take appropriate steps reasonably designed to ensure compliance in all material respects with their policies and procedures relating to data privacy and security and the collection, storage, use, disclosure, handling and analysis of Personal Data that is subject to the Data Security Obligations (the “Policies”) and (v) the Company and its subsidiaries have made all disclosures of its then-current Policies to users or customers required by applicable laws and regulatory rules or requirements, and none of such disclosures made or contained in any Policy have, to the knowledge of the Company, been inaccurate or in violation of any applicable laws and regulatory rules or requirements.
Section 4.34. The Company maintains disclosure controls and procedures that comply with the requirements of the Exchange Act. Such disclosure controls and procedures have been designed to ensure that material information relating to the Company is made known to the Company’s principal executive officer and principal financial officer by others within the Company and such disclosure controls and procedures are effective at the reasonable assurance level.
Section 4.35. Other than the Placement Agent, no Person will have, as a result of the transactions contemplated by this Agreement, any valid right, interest or claim against or upon the Company or, to the Company’s knowledge, the Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company.
Section 4.36. Neither the Company nor any of its subsidiaries nor any Person acting on their behalf has conducted any general solicitation or general advertising (as those terms are used in Regulation D) in connection with the offer or sale of the Securities.
Section 4.37. Neither the Company nor any of its subsidiaries nor any Person acting on their behalf has, directly or indirectly, made any offers or sales of any Company security or solicited any offers to buy any Company security, under circumstances that would adversely affect reliance by the Company on Section 4(a)(2) for the exemption from the registration requirements of the Securities Act for the transactions contemplated hereby or would require registration of the Securities under the Securities Act or cause this offering of the Securities to require approval of stockholders of the Company for purposes of the Securities Act or any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of the NYSE.
Section 4.38. Assuming the accuracy of the Investor’s representations and warranties set forth in Section 5, the offer and sale of the Securities by the Company to the Investor, and the issuance of the Warrant Shares upon exercise of the Warrants, is exempt from the registration requirements of the Securities Act. The issuance and sale of the Securities by the Company to the Investor, and the issuance of the Warrant Shares upon exercise of the Warrants, do not contravene the rules and regulations of the NYSE applicable to the Company. The Company is in compliance with applicable NYSE continued listing requirements.
Section 4.39. The Company is not, and has never been, an issuer identified in, or subject to, Rule 144(i)(1) of the Securities Act.
Section 4.40. The Company acknowledges and agrees that, during the period beginning on the date of this Agreement and ending on the Closing Date, the Company will not enter into any Additional Purchase Agreements (or any side letter) with terms and conditions that are more advantageous to the investors thereunder than the terms and conditions set forth in this Agreement, unless such terms and conditions are also offered to the Investor. The Additional Purchase Agreements have not been amended or modified following the date of this Agreement. In addition, no amendment shall be made to an Additional Purchase Agreement, and no consideration shall be offered or paid to any Additional Investor to amend or consent to a waiver or modification of any provision of any of such Additional Investor’s Additional Purchase Agreement, unless the same amendment or consideration (other than the reimbursement of legal fees), as the case may be, also is offered to the Investor.
Section 5. Representations and Warranties of the Investors. The Investor represents and warrants to the Company, as of the date hereof and as of the Closing Date (except to the extent made only as of a specified date, in which case such representation and warranty is made as of such date):
Section 5.1. The Investor has been duly incorporated or formed, as applicable, and is validly existing and in good standing (to the extent this concept applies) under the laws of its jurisdiction of incorporation and is not in liquidation or receivership or the subject of any insolvency or bankruptcy proceedings, except to the extent that the failure to be so qualified or be in good standing (to the extent this concept applies) would not have a material adverse effect on the power and ability of the Investor to perform its obligations under this Agreement.
Section 5.2. This Agreement has been duly authorized, executed and delivered by the Investor. Assuming due authorization, execution and delivery of this Agreement by the Company, this Agreement constitutes a legal, valid and binding obligation of the Investor, enforceable against the Investor in accordance with its terms, except as the enforcement thereof may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar laws relating to or affecting the rights and remedies of creditors or by general equitable principles.
Section 5.3. The execution and delivery by the Investor of, and the performance by the Investor of its obligations under, this Agreement do not contravene any provision of (i) applicable law, (ii) certificate of incorporation, bylaws or other constitutive document of the Investor, or (iii) any agreement, or other instrument binding upon the Investor or any of its subsidiaries that is material to the Investor, or any judgment, order or decree of any governmental body, agency, or court having jurisdiction over the Investor or any subsidiary, except that in the case of clauses (i) and (iii) as would not, individually, or in the aggregate, reasonably be expected to have a material adverse effect on the power and ability of the Investor to perform its obligations under this Agreement.
No consent, approval, authorization or order of, or qualification with, any governmental body or agency is required on behalf of the Investor for the performance by the Investor of its obligations under this Agreement, except such as have been obtained or waived.
Section 5.4. The Securities will be acquired for the Investor’s own account, not as nominee or agent, and not with a view to the resale or distribution of any part thereof in violation of the Securities Act, and the Investor has no present intention of selling, granting any participation in, or otherwise distributing the same in violation of the Securities Act without prejudice, however, to the Investor’s right at all times to sell or otherwise dispose of all or any part of such Securities in compliance with applicable federal and state securities laws. Nothing contained herein shall be deemed a representation or warranty by the Investor to hold the Securities for any period of time. The Investor is not a broker-dealer registered with the SEC under the Exchange Act or an entity engaged in a business that would require it to be so registered.
Section 5.5. The Investor acknowledges that it can bear the economic risk and complete loss of its investment in the Securities and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment contemplated hereby.
Section 5.6. The Investor has had an opportunity to receive, review and understand all information related to the Company requested by it and to ask questions of and receive answers from the Company regarding the Company, its business and the terms and conditions of the offering of the Securities, and has had the opportunity to conduct and complete its own independent due diligence. The Investor has received copies of the SEC Documents, which were made available to the Investor through the SEC’s EDGAR system. Based on the information the Investor has deemed appropriate, and without reliance upon the Placement Agent, it has independently made its own analysis and decision to enter into the Transaction Documents. The Investor is relying exclusively on its own sources of information, investment analysis and due diligence (including professional advice it deems appropriate) with respect to the execution, delivery and performance of the Transaction Documents, the Securities and the business, condition (financial and otherwise), management, operations, properties and prospects of the Company, including, but not limited to, all business, legal, regulatory, accounting, credit and tax matters. The Investor has not relied on any advice furnished by or on behalf of the Placement Agent in connection with the transactions contemplated hereby. Neither such inquiries nor any other due diligence investigation conducted by the Investor shall modify, limit or otherwise affect the Investor’s right to rely on the Company’s representations and warranties contained in this Agreement or on the truth, accuracy and completeness of the SEC Documents.
Section 5.7. The Investor understands that the Securities are “restricted securities” under the U.S. federal securities laws inasmuch as they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such securities may be resold without registration under the Securities Act only in certain limited circumstances.
Section 5.8. The Investor is either an institution that is an “accredited investor” within the meaning of Rule 501(a)(1), (a)(2), (a)(3), (a)(7), (a)(8), (a)(9), (a)(12) or (a)(13) under the Securities Act or a “qualified institutional buyer” within the meaning of Rule 144A under the Securities Act, and the Investor is an “institutional account” within the meaning of FINRA Rule 4512(c) and has executed and delivered to the Company its Investor Questionnaire, which the Investor represents and warrants is true, correct and complete. The Investor is a sophisticated institutional investor with sufficient knowledge, sophistication and experience in business, including transactions involving private investments in public equity, to properly evaluate the risks and merits of its purchase of the Securities. The Investor has determined based on its own independent review and such professional advice as it deems appropriate that its purchase of the Securities and participation in the transactions contemplated by this Agreement (a) are fully consistent with its financial needs, objectives and condition, (b) comply and are fully consistent with all investment policies, guidelines and other restrictions applicable to the Investor and (c) are a fit, proper and suitable investment for the Investor, notwithstanding the substantial risks inherent in investing in or holding the Securities.
Section 5.9.
The Investor hereby acknowledges and agrees that it has independently evaluated the merits of its decision to purchase the Securities, and that (i) the Placement Agent is acting solely as placement agent in connection with the execution, delivery and performance of this Agreement and is not acting as an underwriter or in any other capacity and is not and shall not be construed as a fiduciary for the Investor, the Company or any other person or entity in connection with the execution, delivery and performance of the Transaction Documents, (b) the Placement Agent has not made and will not make any representation or warranty, whether express or implied, of any kind or character and have not provided any advice or recommendation in connection with the execution, delivery and performance of the Transaction Documents, (c) the Placement Agent will not have any responsibility with respect to (i) any representations, warranties or agreements made by any person or entity under or in connection with the execution, delivery and performance of the Transaction Documents, or the execution, legality, validity or enforceability (with respect to any person) thereof, or (ii) the business, affairs, financial condition, operations, properties or prospects of, or any other matter concerning the Company and (d) the Placement Agent will not have any liability or obligation (including without limitation, for or with respect to any losses, claims, damages, obligations, penalties, judgments, awards, liabilities, costs, expenses or disbursements incurred by the Investor, the Company or any other person or entity), whether in contract, tort or otherwise, to the Investor, or to any person claiming through it, in respect of the execution, delivery and performance of the Transaction Documents.
Section 5.10. The Investor did not learn of the investment in the Securities as a result of any general solicitation or general advertising (as those terms are used in Regulation D).
Section 5.11. Other than the Placement Agent, no Person will have, as a result of the transactions contemplated by this Agreement, any valid right, interest or claim against or upon the Company for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of such Investor.
Section 5.12. Other than consummating the transactions contemplated hereunder, the Investor has not, nor has any Person acting on behalf of or pursuant to any understanding with the Investor, directly or indirectly, executed any purchases or sales, including Short Sales, of the securities of the Company during the period commencing as of the time that the Investor was first contacted by the Company, the Placement Agent or any other Person regarding the transactions contemplated hereby and ending on the date hereof. Notwithstanding the foregoing, in the case of the Investor that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of the Investor’s assets and the portfolio managers have no direct knowledge of the investment decision made by the portfolio managers managing other portions of the Investor’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Securities. The Investor, its Affiliates and, to the knowledge of such Investor, authorized representatives and advisors of the Investor who are aware of the transactions contemplated hereby, maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction). Notwithstanding the foregoing, for avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to the identification of the availability of, or securing of, available shares to borrow in order to effect Short Sales or similar transactions in the future.
Section 6. Conditions to Closing.
Section 6.1. Conditions to the Investors’ Obligations. The obligation of the Investor to purchase Securities at the Closing is subject to the fulfillment to the Investor’s satisfaction, on or prior to the Closing Date, of the following conditions, any of which may be waived by the Investor:
(a) The representations and warranties made by the Company in Section 4 hereof shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality or by material adverse effect, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date. The Company shall have performed in all material respects all obligations and covenants herein required to be performed by it on or prior to the Closing Date.
(b) The Company shall have obtained any and all consents, permits, approvals, registrations and waivers necessary for consummation of the purchase and sale of the Securities and the consummation of the other transactions contemplated by the Transaction Documents, all of which shall be in full force and effect.
(c) The Company shall have submitted with the NYSE a Supplemental Listing Application with respect to the Shares and the Warrant Shares.
(d) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated by the Transaction Documents.
(e) No stop order or suspension of trading shall have been imposed by the NYSE, the SEC or any other governmental or regulatory body with respect to public trading in the Common Shares.
Section 6.2. Conditions to Obligations of the Company. The obligation of the Company to issue and sell the Securities at the Closing is subject to the fulfillment to the Company’s satisfaction, on or prior to the Closing Date, of the following conditions, any of which may be waived by the Company:
(a) The representations and warranties made by the Investor in Section 5 hereof shall be true and correct in all material respects (except for those representations and warranties which are qualified as to materiality or material adverse effect, in which case such representations and warranties shall be true and correct in all respects) as of the date hereof and on the Closing Date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct as of such earlier date. The Investor shall have performed in all material respects all obligations and covenants herein required to be performed by them on or prior to the Closing Date.
(b) No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the transactions contemplated by the Transaction Documents.
(c) The Investor shall have provided to the Company the information set out in the Investor Questionnaire.
Section 6.3. Termination of Obligations to Effect Closing; Effects. The obligations of the Company, on the one hand, and the Investor, on the other hand, to effect the Closing shall terminate as follows:
(a) Upon the mutual written consent of the Company and Investor;
(b) By the Company if any of the conditions set forth in Section 6.2 shall have become incapable of fulfillment, and shall not have been waived by the Company; or
(c) By the Investor if any of the conditions set forth in Section 6.1 shall have become incapable of fulfillment, and shall not have been waived by the Investor.
Section 7. Covenants and Agreements of the Company.
Section 7.1. Disclosure of Transactions. No later than 9:00 a.m., New York City time, on October 13, 2025 (the “Disclosure Time”), the Company shall issue one or more press releases and/or file with the SEC one or more Current Reports on Form 8-K (including all exhibits thereto, the “Disclosure Document”) disclosing (i) all material terms of the transactions contemplated by the Transaction Documents and (ii) all material non-public information concerning the Company disclosed to the Investor by the Company or its representatives prior to the issuance or filing of the Disclosure Document. Upon the issuance or filing of the Disclosure Document, the Investor will not be in possession of any material non-public information concerning the Company disclosed to the Investor by the Company or its representatives. Upon the earlier of the (i) the Disclosure Time and (ii) the issuance or filing, as applicable, of the Disclosure Document satisfying clauses (i) or (ii) of the foregoing sentence, the Investor shall no longer be subject to any confidentiality or similar obligations under any current agreement, whether written or oral, with the Company or any of its officers, directors, affiliates, employees or agents, including, without limitation, the Placement Agent. The Company understands and confirms that the Investor will rely on the foregoing representations in effecting securities transactions. After the issuance or filing of the Disclosure Document, the Company shall not provide the Investor with any material non-public information concerning the Company without the Investor’s prior written consent.
Notwithstanding anything in this Agreement to the contrary, the Company shall not publicly disclose the name of the Investor or any of its Affiliates or advisers, or include the name of the Investor or any of its Affiliates or advisers, in any press release or filing with the SEC or any regulatory agency, without the prior written consent of the Investor, except as required by the federal securities law in connection with any registration statement contemplated by this Agreement or to the extent such disclosure is required by law or the applicable rules or regulations of the NYSE, in which case the Company shall allow the Investor, to the extent reasonably practicable in the circumstances, reasonable time to comment on such disclosure in advance of such disclosure.
Section 7.2. NYSE Listing. From the date hereof until such time as the Shares and the Warrant Shares no longer constitute Registrable Securities, the Company will use commercially reasonable efforts to continue the listing and trading of its Common Shares on the NYSE and, in accordance therewith, will use commercially reasonable efforts to comply in all respects with the Company’s reporting, filing and other obligations under the NYSE listing standards.
Section 7.3. Removal of Legends. In connection with any sale or disposition of the Shares or the Warrant Shares by the Investor pursuant to Rule 144 or pursuant to an effective registration statement, if requested by the Investor, the Company shall use its reasonable efforts to request that the Company’s transfer agent remove any restrictive legends related to the book-entry positions representing such Shares or Warrant Shares, provided that the Company has received customary representations and other documentation reasonably acceptable to the Company in connection therewith. Subject to receipt by the Company of customary representations and other documentation (which shall not include a legal opinion) reasonably acceptable to the Company in connection therewith, upon the earliest of such time as the Shares and Warrant Shares (a) have been sold or transferred pursuant to an effective registration statement, (b) have been sold pursuant to Rule 144 or (c) are eligible for resale under Rule 144 or any successor provision without volume or manner-of-sale restrictions and without current public information requirements, the Company shall (i) deliver to the Company’s transfer agent instructions to remove any restrictive legends related to the book-entry positions representing such Shares or Warrant Shares, as applicable, and (ii) cause its counsel to deliver to the Company’s transfer agent one or more opinions to the effect that the removal of such legends in such circumstances may be effected under the Securities Act. The Company shall be responsible for the fees of its transfer agent.
Section 7.4. Transfer Restrictions. The Investor agrees that it will sell, transfer or otherwise dispose of the Securities and the Warrant Shares only in compliance with all applicable state and federal securities laws.
Section 7.5. Reservation of Warrant Shares. Until the expiration of the Warrants, the Company shall take all action necessary to have authorized and reserved the Warrant Shares (without taking into account any limitations on exercise of the Warrants).
Section 7.6. Fees. The Company shall be responsible for the payment of any placement agent fees, financial advisory fees or broker’s commissions (other than for Persons engaged by the Investor) relating to or arising out of the transactions contemplated hereby, including any fees or commissions payable to the Placement Agent.
Section 7.7. Subsequent Equity Sales. The Company shall not, and shall use its commercially reasonable efforts to ensure that no controlled Affiliate of the Company shall, sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in Section 2 of the Securities Act) that will be integrated with the offer or sale of the Securities in a manner that would require the registration under the Securities Act of the sale of the Securities to the Investor or cause this offering of the Securities to require approval of stockholders of the Company for purposes of the Securities Act or any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of the NYSE. The Company shall not take any action or steps that would adversely affect reliance by the Company on Section 4(a)(2) for the exemption from the registration requirements of the Securities Act for the transactions contemplated hereby or require registration of the Securities under the Securities Act.
Section 7.8. Confidentiality After the Date Hereof. The Investor covenants that until the earlier of the Disclosure Time and such time as all information provided to the Investor that constitutes “material non-public information” under applicable securities laws is publicly disclosed by the Company, the Investor will maintain the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction), other than to such Investor’s outside attorney, accountant, auditor or investment advisor only to the extent necessary to permit evaluation of the investment, and the performance of the necessary or required tax, accounting, financial, legal, or administrative tasks and services and other than as may be required by law.
Section 7.9. Registration Rights and Rule 144.
(a) The Company shall use its reasonable best efforts to register the resale by the Investor of the Registrable Securities on a registration statement on Form S-3 (the “Registration Statement”) no later than 30 Business Days after the Closing Date, and shall provide the Investor with a copy of such draft Registration Statement for review not less than two Business Days before filing. The Company shall use its reasonable best efforts to have the Registration Statement declared effective as soon as practicable, but in no event later than five Business Days after the SEC has notified the Company that it will not review, or has completed its review, of the Registration Statement; provided that the Company’s obligations to include the Registrable Securities in the Registration Statement are contingent upon the Investor furnishing in writing to the Company such information regarding the Investor or its permitted assigns, the securities of the Company held by the Investor and the intended method of disposition of the Registrable Securities (which shall be limited to non-underwritten public offerings) as shall be reasonably requested by the Company to effect the registration of the Registrable Securities, and the Investor shall execute such documents in connection with such registration as the Company may reasonably request that are customary of a selling stockholder in similar situations; provided, further, that the Investor shall not in connection with the foregoing be required to execute any lock-up or similar agreement or otherwise be subject to any contractual restriction on the ability to transfer the Registrable Securities. Upon notification by the SEC that any Registration Statement has been declared effective by the SEC, within one Business Day thereafter, the Company shall file a final prospectus to the extent required by Rule 424 under the Securities Act. For the avoidance of doubt, the Registration Statement may register the securities issued and issuable (including pursuant to warrants issued) pursuant to the Additional Purchase Agreements in addition to the Investor’s Registrable Securities (but not any other securities). The Company shall use its reasonable best efforts to keep the Registration Statement effective (and the related prospectus current) with respect to the Registrable Securities as long as the Investor holds Registrable Securities. Neither the Company nor any affiliate thereof shall identify the Investor as an underwriter in any public disclosure or filing with the SEC applicable trading market without the prior written consent of such Investor, and any Investor being deemed an underwriter by the SEC shall not relieve the Company of any obligations it has under this Agreement; provided that if the Investor is required to be named as an underwriter and the Investor does not consent to being so named, the Company shall be relieved of its obligations to register the Registrable Securities of the Investor.
(b) Notwithstanding anything to the contrary contained herein, the Company may, upon written notice to any holder of Registrable Securities included in a Registration Statement, suspend the use of any Registration Statement, including any prospectus that forms a part of a Registration Statement, if the Company (i) determines that it would be required to make disclosure of material information in the Registration Statement that the Company has a bona fide business purpose for preserving as confidential, (ii) determines it must amend or supplement the Registration Statement or the related prospectus so that such Registration Statement or prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the prospectus in light of the circumstances under which they were made, not misleading or (iii) has experienced or is experiencing some other material nonpublic event, including a pending transaction involving the Company, the disclosure of which at such time, in the good faith judgment of the Company, would adversely affect the Company; provided, however, that in no event shall holders of Registrable Securities be suspended from selling Registrable Securities pursuant to the Registration Statement for a period that exceeds 30 consecutive Trading Days or 60 total Trading Days in any 365-day period. Upon disclosure of such information or the termination of the condition described above, the Company shall provide prompt notice to holders whose Registrable Securities are included in the Registration Statement, and shall promptly terminate any suspension of sales it has put into effect and shall take such other reasonable actions to permit registered sales of Registrable Securities as contemplated hereby. The Investor may deliver written notice (an “Opt-Out Notice”) to the Company requesting that the Investor not receive notices from the Company otherwise required by this Section 7.9; provided, however, that the Investor may later revoke any such Opt-Out Notice in writing.
Following receipt of an Opt-Out Notice from the Investor (unless subsequently revoked), (a) the Company shall not deliver any notices pursuant to this Section 7.9 to the Investor and the Investor shall no longer be entitled to the rights associated with any such notice and (b) each time prior to the Investor’s intended use of an effective Registration Statement, the Investor will notify the Company in writing at least two Business Days in advance of such intended use, and if a notice of a suspension was previously delivered (or would have been delivered but for the provisions of this Section 7.9) and the related suspension period remains in effect, the Company will so notify the Investor, within one Business Day of the Investor’s notification to the Company, by delivering to the Investor a copy of such previous notice of a suspension, and thereafter will provide the Investor with the related notice of the conclusion of such suspension immediately upon the conclusion thereof (which notices shall not contain any material nonpublic information or subject the Investor to any duty of confidentiality).
(c) With a view to making available to the Investors the benefits of Rule 144 (or its successor rule) and any other rule or regulation of the SEC that may at any time permit the Investor to sell Registrable Securities to the public without registration, the Company covenants and agrees to: (i) make and keep public information available, as those terms are understood and defined in Rule 144, until the earlier of (A) six months after such date as all of the Registrable Securities may be sold without restriction by the holders thereof pursuant to Rule 144 or any other rule of similar effect or (B) such date as there are no longer Registrable Securities; (ii) file with the SEC in a timely manner all reports and other documents required of the Company under the Exchange Act; and (iii) furnish electronically to each Investor upon request, as long as the Investor owns any Registrable Securities, a written statement by the Company that it has complied with the reporting requirements of the Securities Act.
Section 8. Indemnification.
Section 8.1. Indemnification by the Company. The Company agrees to indemnify and hold harmless, to the fullest extent permitted by law, the Investor, the officers, directors, partners, members and employees of the Investor, each Person who controls the Investor (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and the officers, directors, partners, members and employees of each such controlling Person (each, an “Investor Indemnified Party”), against any losses, claims, damages, liabilities and expenses (including reasonable attorney fees) to which such Investor Indemnified Party may become subject under the Securities Act, the Exchange Act, or any other federal or state statutory law or regulation, insofar as such losses, claims, damages, liabilities or expenses (or actions in respect thereof as contemplated below) arise out of or are based in whole or in part upon any breach of this Agreement or any Transaction Document by the Company or any untrue statement or alleged untrue statement or omission or alleged omission of any material fact contained in any Registration Statement, any preliminary prospectus or final prospectus thereto, or any amendment or supplement thereof, and will reimburse each Investor Indemnified Party for legal and other expenses reasonably incurred as such expenses are reasonably incurred by such Investor Indemnified Party in connection with investigating, defending, settling, compromising or paying such loss, claim, damage, liability, expense or action; provided, however, that the Company will not be liable in any such case to the extent that any such loss, claim, damage, liability or expense arises out of or is based upon (a) the failure of such Investor Indemnified Party to comply with the covenants and agreements contained herein, (b) the inaccuracy of any representations made by such Investor Indemnified Party herein, (c) an untrue statement or alleged untrue statement or omission or alleged omission so made in conformity with information furnished by an Investor Indemnified Party in writing specifically for use in the Registration Statement or a prospectus, (d) the use by an Investor Indemnified Party of an outdated or defective prospectus after the Company has notified such Investor in writing that such prospectus is outdated or defective or (e) an Investor Indemnified Party’s failure to send or give a copy of the prospectus or supplement (as then amended or supplemented), if required (and not exempted), to the persons asserting an untrue statement or omission or alleged untrue statement or omission at or prior to the written confirmation of the sale of Registrable Securities.
Section 8.2. Indemnification by the Investor.
The Investor agrees to indemnify and hold harmless, to the fullest extent permitted by law, the Company, the officers, directors, partners, members and employees of the Company, each Person who controls the Company (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and the officers, directors, partners, members and employees of each such controlling Person (each, a “Company Indemnified Party”), against any losses, claims, damages, liabilities and expenses (including reasonable attorney fees), to which such Company Indemnified Party may become subject under the Securities Act, the Exchange Act, or any other federal or state statutory law or regulation, insofar as such losses, claims, damages, liabilities or expenses (or actions in respect thereof as contemplated below) arise out of or are based in whole or in part on any untrue statement or alleged untrue statement or omission or alleged omission of any material fact relating to the Investor contained in any Registration Statement, any preliminary prospectus or final prospectus thereto, or any amendment or supplement thereof, and will reimburse each Company Indemnified Party for legal and other expenses reasonably incurred as such expenses are reasonably incurred by such Company Indemnified Party in connection with investigating, defending, settling, compromising or paying such loss, claim, damage, liability, expense or action; provided, however, that the Investor will be liable in any such case only to the extent that such untrue statement or omission relating to the Investor is contained in information furnished in writing by the Investor to the Company specifically for inclusion in the Registration Statement or prospectus or amendment or supplement thereto. In no event shall the liability of the Investor be greater than the dollar amount of the proceeds (net of all expenses paid by the Investor in connection with any claim relating to this Section 8.2 and the amount of any damages the Investor has otherwise been required to pay by reason of such untrue statement or omission) received by the Investor upon the sale of the Registrable Securities included in such Registration Statement giving rise to such indemnification obligation.
Section 8.3. Indemnification Procedure. Promptly after any indemnified party hereunder (the “Indemnified Party”) has received notice of any indemnifiable claim hereunder, or the commencement of any action, suit or proceeding by a third Person, which the Indemnified Party believes in good faith is an indemnifiable claim under this Agreement, the Indemnified Party shall give the indemnitor hereunder (the “Indemnifying Party”) written notice of such claim or the commencement of such action, suit or proceeding, but failure to so notify the Indemnifying Party will not relieve the Indemnifying Party from any liability it may have to such Indemnified Party hereunder except to the extent that the Indemnifying Party is materially prejudiced by such failure. Such notice shall state the nature and the basis of such claim to the extent then known. The Indemnifying Party shall have the right to defend and settle, at its own expense and by its own counsel who shall be reasonably acceptable to the Indemnified Party, any such matter as long as the Indemnifying Party pursues the same diligently and in good faith. If the Indemnifying Party undertakes to defend or settle, it shall promptly notify the Indemnified Party of its intention to do so, and the Indemnified Party shall cooperate with the Indemnifying Party and its counsel in all commercially reasonable respects in the defense thereof and the settlement thereof. Such cooperation shall include, but shall not be limited to, furnishing the Indemnifying Party with any books, records and other information reasonably requested by the Indemnifying Party and in the Indemnified Party’s possession or control. Such cooperation of the Indemnified Party shall be at the cost of the Indemnifying Party. After the Indemnifying Party has notified the Indemnified Party of its intention to undertake to defend or settle any such asserted liability, and for so long as the Indemnifying Party diligently pursues such defense, the Indemnifying Party shall not be liable for any additional legal expenses incurred by the Indemnified Party in connection with any defense or settlement of such asserted liability; provided, however, that the Indemnified Party shall be entitled (a) at its expense, to participate in the defense of such asserted liability and the negotiations of the settlement thereof and (b) if (i) the Indemnifying Party has failed to assume the defense or employ counsel reasonably acceptable to the Indemnified Party or (ii) the defendants in any such action include both the Indemnified Party and the Indemnifying Party and counsel to the Indemnified Party shall have concluded that there may be reasonable defenses available to the Indemnified Party that are different from or in addition to those available to the Indemnifying Party or if the interests of the Indemnified Party reasonably may be deemed to conflict with the interests of the Indemnifying Party, then the Indemnified Party shall have the right to select a separate counsel and to assume such legal defense and otherwise to participate in the defense of such action, with the expenses and fees of such separate counsel and other expenses related to such participation to be reimbursed by the Indemnifying Party as incurred. Notwithstanding any other provision of this Agreement, the Indemnifying Party shall not settle any indemnified claim without the consent of the Indemnified Party, unless the settlement thereof imposes no liability or obligation on, and includes a complete release from liability of, and does not include any admission of wrongdoing or malfeasance by, the Indemnified Party.
Section 9. Miscellaneous.
Section 9.1. Successors and Assigns. This Agreement may not be assigned by a party hereto without the prior written consent of the Company or the Investor, as applicable. The provisions of this Agreement shall inure to the benefit of and be binding upon the respective permitted successors and assigns of the parties.
Section 9.2. Counterparts; Email. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via electronic mail (including PDF or any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
Section 9.3. Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
Section 9.4. Notices. All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given upon the earlier of actual receipt, or (a) personal delivery to the party to be notified or (b) when sent, if sent by electronic mail during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day. All communications shall be sent to the respective parties at their electronic mail address or address as set forth below, or to such electronic mail address or address as subsequently modified by written notice given in accordance with this Section 9.4.
If to the Company:
ADC Therapeutics SA
c/o ADC Therapeutics America, Inc.
430 Mountain Avenue, 4th Floor
Murray Hill, New Jersey 07974
Attention: Peter Graham
Email: peter.graham@adctherapeutics.com
With a copy (which will not constitute notice) to:
Davis Polk & Wardwell LLP
450 Lexington Avenue
New York, New York 10017
Attention: Yasin Keshvargar
Email: yasin.keshvargar@davispolk.com
If to the Investor:
to the contact information set forth on the signature page hereto.
Section 9.5. Expenses. The parties hereto shall pay their own costs and expenses in connection herewith regardless of whether the transactions contemplated hereby are consummated.
Section 9.6. Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Investor; provided that, notwithstanding the foregoing, Section 7.9 may be amended and the observance of any term of Section 7.9 may be waived (either generally or in a particular instance and either retroactively or prospectively) with the written consent of the Company and the Persons holding a majority of all securities sold in the PIPE Transaction then entitled to registration rights substantially similar to those set forth in Section 7.9.
Section 9.7. Publicity. Except as set forth above, no public release or announcement concerning the transactions contemplated hereby shall be issued by the Company or the Investor without the prior written consent of the Company (in the case of a release or announcement by the Investor) or the Investor (in the case of a release or announcement by the Company) (which consents shall not be unreasonably withheld), except as such release or announcement may be required by law or the applicable rules or regulations of any securities exchange or securities market, in which case (other than with respect to any filings by the Investor required by Section 13 or Section 16 of the Exchange Act) the Company or the Investor, as the case may be, shall allow the Investor or the Company, as applicable, to the extent reasonably practicable in the circumstances, reasonable time to comment on such release or announcement in advance of such issuance.
Section 9.8. Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the maximum extent permitted by applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the parties hereby waive any provision of law which renders any provision hereof prohibited or unenforceable in any respect.
Section 9.9. No Third-Party Beneficiaries. The Placement Agent shall be a third-party beneficiary of (i) the representations and warranties of the Company in Section 4 hereof and the representations and warranties of the Investor in Section 5 hereof and (ii) Section 9.10 hereof. This Agreement is intended for the benefit of the parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, except as otherwise set forth in Section 8 and this Section 9.9.
Section 9.10. Exculpation of the Placement Agent. Each party hereto agrees for the express benefit of the Placements Agent and its Affiliates and representatives that:
(a) None of the Placement Agent, its Affiliates or any of its representatives (i) has any duties or obligations with respect to the transactions contemplated by the Transaction Documents, other than those specifically set forth herein or in the engagement letter, dated as of October 9, 2025, between the Company and the Placement Agent (the “Placement Agent Engagement Letter”), (ii) shall be liable for any improper payment made in accordance with the information provided by the Company, (iii) makes any representation or warranty, or has any responsibilities as to the validity, accuracy, value or genuineness of any information, certificates or documentation delivered by or on behalf of the Company pursuant to the Transaction Documents or in connection with any of the transactions contemplated hereby and thereby or (iv) shall be liable (x) for any action taken, suffered or omitted by any of them in good faith and reasonably believed to be authorized or within the discretion or rights or powers conferred upon it by this Agreement or any Transaction Document or (y) for anything which any of them may do or refrain from doing in connection with the Transaction Documents, except in each case, for such party’s own gross negligence, willful misconduct or bad faith.
(b) The Placement Agent and its Affiliates and representatives shall be entitled to (i) rely on, and shall be protected in acting upon, any certificate, instrument, notice, letter, opinion or any other document or security delivered to any of them by or on behalf of the Company, and (ii) be indemnified by the Company for acting as the Placement Agent hereunder pursuant to the indemnification provisions set forth in the Placement Agent Engagement Letter.
Section 9.11. Entire Agreement. The Transaction Documents, including the signature pages and exhibits thereto, and any oral or written agreement between the Company and the Investor regarding confidentiality matters that was entered into in connection with the transactions contemplated hereby, constitute the entire agreement among the parties hereof with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, both oral and written, between the parties with respect to the subject matter hereof and thereof.
Section 9.12. Further Assurances. The parties shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the agreements herein contained.
Section 9.13. Governing Law; Consent to Jurisdiction; Waiver of Jury Trial. This Agreement shall be governed by, and construed in accordance with, the internal laws of the State of New York without regard to the choice of law principles thereof (other than Sections 5-1401 and 5-1402 of the General Obligations Law). Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the courts of the State of New York located in New York County and the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement.
Each of the parties hereto irrevocably consents to the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum. EACH OF THE PARTIES HERETO WAIVES ANY RIGHT TO REQUEST A TRIAL BY JURY IN ANY LITIGATION WITH RESPECT TO THIS AGREEMENT AND REPRESENTS THAT COUNSEL HAS BEEN CONSULTED SPECIFICALLY AS TO THIS WAIVER.
[remainder of page intentionally left blank]
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
|
ADC Therapeutics SA
|
|||
| By: | |||
| Name: | Ameet Mallik | ||
| Title | Chief Executive Officer | ||
[Signature Page to Securities Purchase Agreement]
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized officers to execute this Agreement as of the date first above written.
| By: | |||
| Name: | |||
| Title: | |||
Number of Shares:
Number of Pre-Funded Warrants:
Notice Information:
[Signature Page to Securities Purchase Agreement]
Exhibit A
Form of Warrant
[attached]
Exhibit A
Annex A
Investor Questionnaire
This Investor Questionnaire (“Questionnaire”) must be completed by each potential investor in connection with the offer and sale of the securities (the “Securities”) by ADC Therapeutics SA (the “Corporation”). The Securities are being offered and sold by the Corporation without registration under the Securities Act of 1933, as amended (the “Securities Act”), and the securities laws of certain states, in reliance on the exemptions contained in Section 4(a)(2) of the Securities Act and in reliance on similar exemptions under applicable state laws. The Corporation must determine that a potential investor meets certain suitability requirements before offering or selling the Securities to such investor. The purpose of this Questionnaire is to assure the Corporation that each investor will meet the applicable suitability requirements. The information supplied by you will be used in determining whether you meet such criteria, and reliance upon the private offering exemptions from registration is based in part on the information herein supplied.
This Questionnaire does not constitute an offer to sell or a solicitation of an offer to buy any security. By signing this Questionnaire, you will be authorizing the Corporation to provide a completed copy of this Questionnaire to such parties as the Corporation deems appropriate in order to ensure that the offer and sale of the Securities will not result in a violation of the Securities Act or the securities laws of any state and that you otherwise satisfy the suitability standards applicable to purchasers of the Securities. All potential investors must answer all applicable questions and complete, date and sign this Questionnaire. Please print or type your responses and attach additional sheets of paper if necessary to complete your answers to any item.
PART A. BACKGROUND INFORMATION
Name of Beneficial Owner of the Securities:
Name of Holder: ______________________________________________________________________
Address: ______________________________________________________________________
Type of entity: _________________________
State of formation: _____________________ Approximate date of formation: __________________
Telephone number: _______________________
Email: _______________________
Were you formed for the purpose of investing in the securities being offered? Yes No
PART B. INVESTOR STATUS
QUALIFIED INSTITUTIONAL BUYER STATUS
(Please check the applicable subparagraphs):
| ☐ | We are a “qualified institutional buyer” (as defined in Rule 144A under the Securities Act). |
| OR |
| B. | ACCREDITED INVESTOR STATUS (Please check the applicable subparagraphs): |
| 1. | ☐ We are an “accredited investor” (within the meaning of Rule 501(a) under the Securities Act) and have marked and initialed the appropriate box on the following page indicating the provision under which we qualify as an “accredited investor.” |
| 2. | ☐ We are not a natural person. |
Appendix I
Rule 501(a), in relevant part, states that an “accredited investor” shall mean any person who comes within any of the below listed categories, or who the issuer reasonably believes comes within any of the below listed categories, at the time of the sale of the securities to that person. The Investor has indicated, by marking and initialing the appropriate box below, the provision(s) below which apply to the Investor and under which the Investor accordingly qualifies as an “accredited investor.”
| ☐ | Any bank, registered broker or dealer, insurance company, registered investment company, business development company, or small business investment company; |
| ☐ | Any plan established and maintained by a state, its political subdivisions, or any agency or instrumentality of a state or its political subdivisions for the benefit of its employees, if such plan has total assets in excess of $5,000,000; |
| ☐ | Any employee benefit plan, within the meaning of the Employee Retirement Income Security Act of 1974, if a bank, savings and loan association, insurance company, or registered investment adviser makes the investment decisions, or if the plan has total assets in excess of $5,000,000, or, if a self-directed plan, with investment decisions made solely by persons that are accredited investors; |
| ☐ | Any private business development company; |
| ☐ | Any organization described in Section 501(c)(3) of the Internal Revenue Code, corporation, Massachusetts or similar business trust, or partnership, or limited liability company, not formed for the specific purpose of acquiring the securities offered, with total assets in excess of $5,000,000; |
| ☐ | Any director, executive officer, or general partner of the issuer of the securities being offered or sold, or any director, executive officer, or general partner of a general partner of that issuer; |
| ☐ | Any natural person whose individual net worth, or joint net worth with that person’s spouse or spousal equivalent, exceeds $1,000,000. For purposes of calculating a natural person’s net worth: (a) the person’s primary residence shall not be included as an asset; (b) indebtedness that is secured by the person’s primary residence, up to the estimated fair market value of the primary residence at the time of the sale of securities, shall not be included as a liability (except that if the amount of such indebtedness outstanding at the time of sale of securities exceeds the amount outstanding 60 calendar days before such time, other than as a result of the acquisition of the primary residence, the amount of such excess shall be included as a liability); and (c) indebtedness that is secured by the person’s primary residence in excess of the estimated fair market value of the primary residence at the time of the sale of securities shall be included as a liability; |
| ☐ | Any natural person who had an individual income in excess of $200,000 in each of the two most recent years or joint income with that person’s spouse or spousal equivalent in excess of $300,000 in each of those years and has a reasonable expectation of reaching the same income level in the current year; |
| ☐ | Any trust with total assets in excess of $5,000,000, not formed for the specific purpose of acquiring the securities offered, whose purchase is directed by a sophisticated person; |
| ☐ | Any entity in which all of the equity owners are accredited investors; |
| ☐ | Any entity of a type not listed first, second, third, fourth, ninth or tenth bullets of this section, not formed for the specific purpose of acquiring the securities offered, owning investments in excess of $5,000,000; |
| ☐ | Any natural person holding in good standing one or more professional certifications or designations or credentials from an accredited educational institution that the Securities and Exchange Commission has designated as qualifying an individual for accredited investor status; |
| ☐ | Any natural person who is a “knowledgeable employee” as defined in rule 3c-5(a)(4) under the Investment Company Act of 1940 of the issuer of the securities being offered or sold where the issuer would be an investment |
Appendix I
company, as defined in section 3 of such act, but for the exclusion provided by either section 3(c)(1) or section 3(c)(7) of such act;
| ☐ | Any “family office,” as defined in rule 202(a)(11)(G)-1 under the Investment Advisers Act of 1940, as amended, with assets under management in excess of $5,000,000, not formed to acquire the securities offered, and whose prospective investment is directed by a person who has such knowledge and experience in financial and business matters that such family office is capable of evaluating the merits and risks of the prospective investment; or |
| ☐ | Any “family client,” as defined in rule 202(a)(11)(G)-1 under the Investment Advisers Act of 1940, as amended, of a family office meeting the requirements set forth above and whose prospective investment in the issuer is directed by such family office pursuant to the requirements set forth above. |
| 3a. | Please indicate whether or not the Investor is, or is acting (directly or indirectly) on behalf of, (i) an employee benefit plan (within the meaning of Section 3(3) of ERISA), whether or not such plan is subject to ERISA, (ii) a plan, individual retirement account or other arrangement that is described in Section 4975(e)(1) of the Code, whether or not such plan, account or arrangement is subject to Section 4975 of the Code, (iii) an insurance company using general account assets, if such general account assets are deemed to include the assets of any of the foregoing types of plans, accounts or arrangements for purposes of Title I of ERISA or Section 4975 of the Code under Section 401(c)(1)(A) of ERISA or the regulations promulgated thereunder, or (iv) an entity which is deemed to hold the assets of any of the foregoing types of plans, accounts or arrangements (each of the foregoing described in clauses (i), (ii), (iii) and (iv) being referred to as a “Plan Investor”). |
| ☐ | Yes |
| ☐ | No |
| 3b. | If question 3a above was answered “Yes,” please indicate whether or not the Plan Investor is subject to Title I of ERISA or Section 4975 of the Code. |
| ☐ | Yes |
| ☐ | No |
| 3c. | If question 3b above was answered “Yes,” please indicate what percentage of the Plan Investor’s assets invested in the Company are the assets of “benefit plan investors” as defined in Section 3(42) of ERISA. |
| __________________________% |
| By: | ||
| Name: | ||
| Title: | ||
Appendix I
Exhibit 10.2
THIS WARRANT AND THE SHARES ISSUABLE UPON EXERCISE HEREOF HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR THE SECURITIES LAWS OF ANY STATE AND MAY NOT BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED EXCEPT PURSUANT TO A REGISTRATION STATEMENT RELATED THERETO THAT IS IN EFFECT UNDER THE SECURITIES ACT AND ANY APPLICABLE STATE SECURITIES LAWS OR PURSUANT TO AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT AND SUCH LAWS.
WARRANT
TO PURCHASE COMMON SHARES OF
ADC THERAPEUTICS SA
| No. W-PF-[#] | October 27, 2025 |
The undersigned, ADC Therapeutics SA, a Swiss stock corporation (société anonyme) organized under the laws of Switzerland (together with its successors and assigns, the “Company”), hereby grants to [INVESTOR], subject to the terms and conditions herein, the right to subscribe for and purchase, at the Exercise Price per share, the Warrant Shares.
Section 1. Definitions. Capitalized terms used but not otherwise defined in this Warrant shall have the following meanings:
“Affiliates” means any affiliates (as defined in Rule 405 under the Securities Act) of the Warrantholder.
“Articles of Association” means the Articles of Association of the Company, as may be amended, modified, supplemented or restated from time to time in accordance with its terms and pursuant to applicable law.
“Business Day” means any day other than Saturday, Sunday or any other day on which banking institutions in the City of New York or in Lausanne, Switzerland are closed for business.
“Common Shares” means the Company’s common shares, par value CHF 0.08 per share, as presently constituted under the Articles of Association, and any class and/or series of Company capital stock for or into which such common shares may be converted or exchanged in a reorganization, recapitalization or similar transaction.
“Effective Date” means October 27, 2025.
“Exchange” means the New York Stock Exchange, or if the Common Shares are no longer listed on the New York Stock Exchange, the other securities exchange or market on which the Common Shares are then being principally traded.
“Exchange Act” means the United States Securities Exchange Act of 1934, as amended.
“Exercise Date” means, for any given exercise of this Warrant, the date on which the conditions to such exercise as set forth in Section 3(a) shall have been satisfied at or prior to 5:00 P.M., Eastern Time, on a Business Day, including, without limitation, the receipt by the Company of the applicable Exercise Price, it being understood that, if the conditions to such exercise are satisfied after 5:00 P.M., Eastern Time on a Business Day or are satisfied outside of a Business Day, then the Exercise Date shall be deemed to be the next Business Day.
“Exercise Price” means CHF 0.08 per Warrant Share.
“Liquid Sale” means a Merger Event in which the consideration received by the Company’s shareholders consists solely of cash and/or Marketable Securities.
“Marketable Securities,” in connection with a Merger Event, means securities meeting all of the following requirements: (i) the issuer of such securities is then subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act and is then current in its filing of all required reports and other information under the Securities Act and the Exchange Act; (ii) the class and series of such securities that would be received by the Warrantholder in such Merger Event were the Warrantholder to exercise this Warrant on or prior to the closing of such Merger Event is then traded on a national securities exchange or over-the-counter market; and (iii) following the closing of such Merger Event, the Warrantholder would not be restricted from publicly re-selling all of such securities that would be received by the Warrantholder in such Merger Event were the Warrantholder to exercise this Warrant on or prior to the closing of such Merger Event, except to the extent that any such restriction (x) arises solely under federal or state securities laws, rules or regulations, and (y) does not extend beyond six months from the closing of such Merger Event.
“Merger Event” means any of the following: (i) a sale, lease or other transfer of all or substantially all assets of the Company, (ii) any merger or consolidation involving the Company in which the Company is not the surviving entity or in which the outstanding shares of the Company’s capital stock are otherwise converted into or exchanged for shares of capital stock or other equity securities or property of another entity, or (iii) any sale by holders of the outstanding voting equity securities of the Company in a single transaction or series of related transactions of shares constituting a majority of the outstanding combined voting power of the Company.
“Person” means an individual, corporation, limited liability company, partnership, joint venture, association, trust, unincorporated organization or any other entity.
“Purchase Price” means USD 3.90 multiplied by the number of Warrant Shares on the Effective Date.
“Securities Act” means the United States Securities Act of 1933, as amended.
“Trading Day” means a day on which the Exchange is open for trading.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Shares are then listed or quoted on an Exchange, the daily volume weighted average price of the Common Shares for such date (or the nearest preceding date) on such market as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)), (b) if the Common Shares are not then listed on an Exchange, the volume weighted average price of the Common Shares for such date (or the nearest preceding date) on OTCQB or OTCQX as applicable, (c) if the Common Shares are not then listed or quoted for trading on OTCQB or OTCQX and if prices for the Common Shares are then reported on The Pink Open Market (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per Common Share so reported, or (d) in all other cases, the fair market value of a Common Share as determined by the Company’s board of directors in good faith.
“Warrant” means this warrant and any other warrants of like tenor issued in substitution or exchange for any thereof pursuant to the terms of this Warrant.
“Warrantholder” means the initial holder of the Warrant or its registered assign.
“Warrant Shares” means initially [NUMBER] Common Shares, and thereafter subject to adjustment as set forth herein, including reduction for each such Common Share as to which this Warrant has been exercised hereunder.
Section 2. Term.
(a) Term. The right to subscribe for and purchase Common Shares as granted herein shall commence on the Effective Date and, subject to Section 6(a), shall be exercisable until 4:00 P.M. (Zurich time) on the tenth anniversary of the Effective Date (such period, the “Term”). The Warrantholder shall not be entitled to the return or refund of all, or any portion, of the Purchase Price for any reason whatsoever, including in the event this Warrant shall not have been exercised during the Term.
(b) Replacement Warrant. At any time during the last 90 calendar days before the end of the Term, the Warrantholder shall have the right to request the Company, upon the Warrantholder’s return of this Warrant, to exchange this Warrant for a new warrant for the Warrant Shares then remaining under this Warrant, in form and substance substantially similar to this Warrant (with a subsequent ten year exercise period) (the “Replacement Warrant”). Within ten Business Days after the receipt of such request and the physical surrender of this Warrant to the Company, the Company shall issue and deliver the Replacement Warrant to the Warrantholder.
Section 3. Exercise of the Purchase Rights.
(a) Exercise. The purchase rights represented by this Warrant are exercisable by the Warrantholder, in whole or in part (but not in relation to any fraction of a Common Share), at any time and from time to time, during the Term by tendering to the Company (by electronic mail in accordance with Section 12(c)) a duly completed and executed notice of exercise in the form attached as Exhibit A hereto (each, a “Notice of Exercise”), and payment of the applicable Exercise Price for all Warrant Shares to which the Notice of Exercise relates; provided that any exercise of the purchase rights represented by this Warrant must be for at least the lesser of (x) 50,000 Warrant Shares and (y) all remaining Warrant Shares available for purchase under this Warrant. Once received by the Company, a Notice of Exercise may not be revoked without the Company’s written consent.
(b) Exercise Price. The Warrantholder must pay the applicable Exercise Price for all Warrant Shares to which the applicable Notice of Exercise relates, in cash and without any deductions (including for bank fees) or withholdings, on the Exercise Date via wire transfer of immediately available funds to the bank account set out in Exhibit A hereto or to any other bank account designated by the Company to the Warrantholder in writing from time to time, unless the cashless exercise procedure specified in Section 3(g) below is specified in the applicable Notice of Exercise.
(c) Issuance of Warrant Shares. The Warrant Shares to be delivered upon the exercise of Warrants shall be, at the election of the Company, existing Common Shares held by the Company or newly issued Common Shares from the Company’s capital range (Kapitalband) or newly issued Common Shares from the Company’s conditional share capital (bedingtes Aktienkapital). Such Warrant Shares shall be in uncertificated form in accordance with article 973c CO as uncertificated securities (Wertrechte). Nothing in this Warrant shall be construed as requiring the Company to hold a certain amount of Common Shares in treasury at any time.
(d) Delivery of Common Shares. In the event of any exercise of the rights represented by this Warrant in accordance with and subject to the terms and conditions hereof, the Common Shares so purchased shall be delivered by the Company by causing the Company’s transfer agent (“Transfer Agent”) to electronically transmit the Common Shares issuable upon such exercise to the Warrantholder by crediting such Warrantholder’s account at the Transfer Agent within two Business Days of the Exercise Date (the “Delivery Deadline”); provided that the Company shall not be in default if the Company is unable to deliver Common Shares by the Delivery Deadline due to the relevant bank’s failure to issue, or delay in issuing, a confirmation of payment of the Exercise Price.
(e) Surrender and Cancellation. Except as provided in Section 2(b), the Warrantholder shall not be required to physically surrender this Warrant to the Company until the Warrantholder has purchased all of the Common Shares available hereunder and this Warrant has been exercised in full, in which case the Warrantholder shall surrender this Warrant to the Company for cancellation within three Business Days following the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant shall lower the number of Warrant Shares available hereunder by an amount equal to the applicable number of Warrant Shares purchased. The Warrantholder and the Company shall maintain records showing the number of Common Shares purchased and the remaining number of Common Shares available hereunder. The Warrantholder and any assignee of the Warrantholder, by acceptance of this Warrant, acknowledge and agree that, by reason of the provisions of this paragraph, following the purchase of a portion of the Common Shares available hereunder, the Warrant Shares (and, therefore, the number of Common Shares available for purchase hereunder) at any given time may be less than the amount stated herein.
(f) Limitations on Exercise. The Warrantholder shall not have the right to exercise the rights represented by this Warrant, and the Company shall not effect any exercise of this Warrant, to the extent that after giving effect to such exercise, such Warrantholder (together with such Warrantholder’s Affiliates, any Persons acting as a group (as such term is used for purposes of Section 13(d) of the Exchange Act) together with the Warrantholder or its Affiliates, and any other Persons whose beneficial ownership of Common Shares is aggregated with the Warrantholder for purposes of Section 13(d) of the Exchange Act (such Persons, collectively, “Attribution Parties”)) would beneficially own in excess of 9.99% (the “Maximum Percentage”) of Common Shares outstanding immediately after giving effect to such exercise. For purposes of the foregoing sentence, the aggregate number of Common Shares beneficially owned by the Warrantholder and its Affiliates and Attribution Parties shall include the number of Common Shares issuable upon exercise of the rights represented by this Warrant with respect to which the determination of such sentence is being made, but shall exclude Common Shares which would be issuable upon (i) exercise of the remaining, unexercised portion of this Warrant beneficially owned by the Warrantholder or any of its Affiliates or Attribution Parties and (ii) exercise or conversion of the unexercised or unconverted portion of any other securities of the Company beneficially owned by the Warrantholder or any of its Affiliates or Attribution Parties (including, without limitation, any convertible notes or convertible preferred stock or warrants) subject to a limitation on conversion or exercise analogous to the limitation contained herein.
Except as set forth in the preceding sentence, for purposes of this paragraph, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act, it being acknowledged that the Company is not representing to the Warrantholder that such calculation is in compliance with Section 13(d) of the Exchange Act. To the extent that the limitation contained in this Section 3(f) applies, the determination of whether this Warrant is exercisable and of which portion of this Warrant is exercisable shall be in the sole discretion of the Warrantholder and the submission of a Notice of Exercise shall be deemed to be the Warrantholder determination of whether this Warrant is exercisable and of which portion of this Warrant is exercisable, and the Company shall have no obligation to verify or confirm the accuracy of such determination. For purposes of this Warrant, in determining the number of outstanding Common Shares, the Warrantholder may rely on the number of outstanding Common Shares as reflected in (i) the Company’s most recent Form 10-K or Form 10-Q or other public filing by the Company with the SEC, as the case may be, (ii) a more recent public announcement by the Company or (iii) any other notice by the Company setting forth the number of Common Shares outstanding. Upon the written or oral request of the Warrantholder, which request indicates that it is being made pursuant to this Warrant, the Company shall within two Business Days confirm orally or in writing to the Warrantholder the number of Common Shares then outstanding. Upon delivery of a written notice to the Company, the Warrantholder may from time to time increase or decrease the Maximum Percentage to any other percentage not in excess of 19.99% as specified in such notice; provided that any such increase in the Maximum Percentage will not be effective until the sixty-first (61st) day after such notice is delivered to the Company. For purposes of clarity, the Common Shares issuable pursuant to the terms of this Warrant in excess of the Maximum Percentage shall not be deemed to be beneficially owned by the Warrantholder for any purpose including for purposes of Section 13(d) or Rule 16a-1(a)(1) of the Exchange Act. The provisions of this paragraph shall be construed and implemented in a manner otherwise than in strict conformity with the terms of this Section 3(f) to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended beneficial ownership limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such limitation. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
(g) Cashless Exercise. Notwithstanding anything to the contrary set forth herein but subject to Section 3(h), this Warrant may be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the Warrantholder shall be entitled to receive a number of Warrant Shares equal to the quotient obtained by dividing [(A-B) (X)] by (A), where:
(A) = as applicable: (i) the VWAP on the date of the applicable Notice of Exercise if the date of such Notice of Exercise is a Trading Day and such Notice of Exercise is both executed and delivered pursuant to Section 2(a) hereof after the close of “regular trading hours” on such Trading Day or (ii) in all other cases, the VWAP on the Trading Day immediately preceding the date of the applicable Notice of Exercise;
(B) = the Exercise Price of this Warrant, as adjusted hereunder; and
(X) = the number of Warrant Shares that would be issuable upon exercise of this Warrant in accordance with the terms of this Warrant if such exercise were by means of a cash exercise rather than a cashless exercise.
If Warrant Shares are issued in such a cashless exercise, the parties acknowledge and agree that for the purpose of Rule 144 under the Securities Act (as in effect on the Effective Date), the holding period of the Warrant Shares being issued may be tacked on to the Warrants being exercised. The Company agrees not to take any position contrary to this Section 3(g).
(h) Limitations on Cashless Exercise. In addition to any other limitations pursuant to this Warrant on the exercise of the rights represented by this Warrant, the following shall apply with respect to every cashless exercise pursuant to Section 3(g): If the Company, at the time of its receipt of an Exercise Notice electing a cashless exercise, (i) does not, or has reason to believe that it does not, have a sufficient amount of freely distributable equity to fund the nominal value of the number of Warrant Shares that it would be required to deliver upon such cashless exercise and (ii) (1) holds Common Shares representing more than 2.00% of its share capital registered in the commercial register at that time (the “Minimum Stock”) in treasury, then the Company shall not be obliged (but still has the right, at its sole discretion) to deliver more than such number of Warrant Shares to the Warrantholder as exceeds the Minimum Stock and (2) holds up to the Minimum Stock in treasury, then the Company shall not be obliged to deliver any Warrant Shares to the Warrantholder.
The respective Exercise Notice shall be deemed to be null and void to the extent the Warrantholder receives fewer Warrant Shares pursuant to this Section 3(h) than such Exercise Notice relates to. Nothing in this Section 3(h) shall prevent the Warrantholder from duly exercising the rights represented by this Warrant with payment of the Exercise Price in cash.
Section 4. No Rights as Shareholder. Without limitation of any provision hereof, the Warrantholder agrees that this Warrant does not entitle the Warrantholder to any voting rights or other rights as a shareholder of the Company prior to the delivery of any Common Shares to the Warrantholder upon exercise of any of the purchase rights set forth in this Warrant.
Section 5. Warrantholder Registry. The Company shall maintain a registry showing the name and address of the registered holder of this Warrant. The Warrantholder’s initial address, for purposes of such registry, is set forth in Section 12(c) below. The Warrantholder may change such address by giving written notice of such changed address to the Company.
Section 6. Adjustments and Other Events. The Exercise Price and the number of Common Shares purchasable hereunder are subject to adjustment from time to time, as follows; provided that no single event shall cause an adjustment or distribution under more than one subsection of this Section 6 so as to result in duplication.
(a) Merger Event. If, during the Term, a Merger Event that is a Liquid Sale occurs with respect to the Company, the Warrantholder shall be deemed to have exercised the then-remaining purchase rights represented by this Warrant in full (without regard for Section 3(f)) immediately prior to the closing of such Liquid Sale, with the applicable Exercise Price to be withheld by the Company from the proceeds to which the Warrantholder would otherwise be entitled in connection with such Liquid Sale. If, during the Term, a Merger Event that is not a Liquid Sale occurs with respect to the Company, the Company shall cause the successor or surviving entity to assume this Warrant and the obligations of the Company hereunder on the closing of such Merger Event (with such appropriate adjustment to accommodate any changes in the jurisdiction of incorporation), and thereafter this Warrant shall represent the right to purchase the same number and type of securities or other property as the Warrantholder would have received in consideration for the Common Shares issuable hereunder had it exercised this Warrant in full (without regard for Section 3(f)) as of immediately prior to such closing, at an aggregate Exercise Price no greater than the aggregate Exercise Price in effect as of immediately prior to such closing, and subject to further adjustment from time to time in accordance with the provisions of this Warrant. The provisions of this Section 6(a) shall similarly apply to successive Merger Events.
(b) Reclassification of Shares. Except for Merger Events subject to Section 6(a), if the Company at any time shall, by combination, reclassification, exchange or subdivision of securities or otherwise, change any of the securities as to which purchase rights under this Warrant exist into the same or a different number of securities of any other class or classes of securities, this Warrant shall thereafter represent the right to acquire such number and kind of securities as would have been issuable as the result of such change with respect to the securities which were subject to the purchase rights under this Warrant immediately prior to such combination, reclassification, exchange, subdivision or other change. The provisions of this Section 6(b) shall similarly apply to successive combination, reclassification, exchange, subdivision or other change.
(c) Subdivision or Combination of Shares. If the Company at any time shall combine or subdivide its Common Shares, (i) in the case of a subdivision, the Exercise Price shall be proportionately decreased and the number of shares for which this Warrant is exercisable shall be proportionately increased, or (ii) in the case of a combination, the Exercise Price shall be proportionately increased and the number of shares for which this Warrant is exercisable shall be proportionately decreased.
(d) Dividends. If the Company at any time while this Warrant is outstanding and unexpired shall:
(i) pay a dividend with respect to the Common Shares payable in additional Common Shares, then the Exercise Price shall be adjusted, from and after the date of determination of shareholders entitled to receive such dividend, to that price determined by multiplying the Exercise Price in effect immediately prior to such date of determination by a fraction (A) the numerator of which shall be the total number of Common Shares outstanding immediately prior to such dividend or distribution, and (B) the denominator of which shall be the total number of Common Shares outstanding immediately after such dividend or distribution, and the number of Common Shares for which this Warrant is exercisable shall be proportionately increased; or
(ii) make any other dividend or distribution on or with respect to Common Shares, except any dividend or distribution specifically provided for in any other clause of this Section 6, then, in each such case, provision shall be made by the Company such that the Warrantholder shall receive a proportionate share of any such dividend or distribution as though it were the holder of the Common Shares (or other capital stock for which the Common Shares is convertible) as of the record date fixed for the determination of the shareholders of the Company entitled to receive such dividend or distribution without regard for Section 3(f) (a “Dividend Compensation Payment”).
(e) Limitations on Adjustments. Notwithstanding anything to the contrary in this Section 6, (i) all calculations under this Section 6 shall be made to the nearest one-hundredth (1/100th) of a cent or to the nearest one-tenth (l/10th) of a share, as the case may be, (ii) no adjustment in the Exercise Price shall be made if the amount of such adjustment would be less than $0.01, (iii) no adjustment in the number of Common Shares into which this Warrant is exercisable shall be made if the amount of such adjustment would be less than one (1) Common Share, and (iv) if an adjustment in Exercise Price would reduce the Exercise Price to an amount below the then-applicable par value of the Common Shares, then such adjustment in the Exercise Price shall reduce the Exercise Price to such par value of the Common Shares.
Section 7. Transfers. Subject to compliance with applicable federal and state securities laws, this Warrant and all rights hereunder are transferable, in whole or in part, without charge to the holder hereof (except for transfer taxes) by executing an assignment declaration in wet ink in the form attached as Exhibit B hereto (the “Transfer Notice”) and the payment to the Company of all transfer taxes and other governmental charges imposed on such transfer. Until the Company receives a copy of such duly signed Transfer Notice and, if applicable, such transfer taxes and other governmental charges, the Company may treat the registered owner hereof as the owner for all purposes. Upon receipt of a copy of such duly signed Transfer Notice and, if applicable, such transfer taxes and other governmental charges, the Company shall update its registry of warrantholders.
Section 8. Tax Matters.
(a) Withholding. The Company and its paying agent shall be entitled to deduct and withhold taxes on any Dividend Compensation Payment, or any other amount distributed or deemed distributed, to the extent required by applicable law. To the extent that any such amounts are so deducted or withheld and remitted to the applicable governmental authority, such deducted or withheld amounts shall be treated for all purposes of this Warrant as having been paid to the Person in respect of which such deduction or withholding was made. In the event the Company previously remitted any such amounts to a governmental authority on account of taxes required to be deducted or withheld in respect of any Dividend Compensation Payment, the Company shall be entitled (i) to offset any such amounts against any amounts otherwise payable in respect of such Warrant, any Common Shares otherwise required to be issued upon the exercise of such Warrant or any amounts otherwise payable in respect of Common Shares received upon the exercise of such Warrant, or (ii) to require the Person in respect of whom such deduction or withholding was made to reimburse the Company for such amounts (and such Person shall promptly so reimburse the Company upon demand). The Company shall provide a receipt or other evidence of payment of any taxes deducted or withheld reasonably acceptable to the Warrantholder within 30 days after making any deduction or withholding of such taxes and shall collaborate with and provide to the Warrantholder all documentation necessary to obtain a refund of such tax amounts deducted and withheld.
(b) Transfer and Stamp Taxes. Each party shall bear and pay any transfer, stamp, issue, registration, documentary, sales, use, or similar taxes or obligations imposed on such party with respect to any amounts payable in connection with this Warrant.
Section 9. Certain Representations and Agreements of the Company.
The Company represents, covenants and agrees:
(a) This Warrant is, and any Warrant issued in substitution for or replacement of this Warrant shall be, upon issuance, duly authorized and validly issued.
(b) All Warrant Shares issuable upon the exercise of this Warrant pursuant to the terms hereof will be, upon issuance, validly issued, fully paid and non-assessable. The Company has allocated and reserved, and for so long as this Warrant is outstanding the Company will continue to reserve, the Company’s capital range under article 4a or the Company’s conditional share capital under article 4c of the Articles of Association in such an amount as is necessary to deliver such number of Common Shares as are required to settle the exercise in full of this Warrant.
(c) The Company shall take all such actions as may be necessary to ensure that all Warrant Shares are issued without violation by the Company of any applicable law or governmental regulation or any requirements of any securities exchange upon which the Warrant Shares will be listed upon their issuance.
(d) The board of directors of the Company will, as long as any Warrants are outstanding, not make any proposal to the general meeting of shareholders of the Company regarding the amendment or modification of any provision of the Articles of Association in any manner that would materially and adversely affect the powers, preferences or relative participating, optional or other special rights of the Common Shares in a manner which would disproportionately and adversely affect the rights of the Warrantholder.
Section 10. Certain Representations and Agreements of the Warrantholder.
The Warrantholder represents, covenants and agrees:
(a) This Warrant is, and the Common Shares issued upon exercise of this Warrant will be, acquired for the Warrantholder’s own account, not as nominee or agent, and not with a view to the resale or distribution of any part thereof in violation of the Securities Act.
(b) The Warrantholder is an institution that is an “accredited investor” within the meaning of Rule 501(a)(1), (a)(2), (a)(3), (a)(7), (a)(8), (a)(9), (a)(12) or (a)(13) under the Securities Act or a “qualified institutional buyer” within the meaning of Rule 144A under the Securities Act.
(c) No public market exists for the Warrants, and such market may never develop.
Section 11. Exchange of Additional Common Shares. If the legal and factual conditions for an exchange are met and all necessary consents and approvals have been obtained by the Company, the Company will, upon written request of the Warrantholder, consider providing arrangements for the Warrantholder and its Affiliates to exchange or convert within a reasonable timeframe after such conditions are met their Common Shares exceeding 9.99% of outstanding Common Shares for the same amount of non-voting securities (including, without limitation, warrants) that include a 9.99% beneficial ownership blocker.
Section 12. Miscellaneous.
(a) Severability. In the event any one or more of the provisions of this Warrant shall for any reason be held invalid, illegal or unenforceable, the remaining provisions of this Warrant shall be unimpaired, and the invalid, illegal or unenforceable provision shall be replaced by a mutually acceptable valid, legal and enforceable provision, which comes closest to the intention of the parties underlying the invalid, illegal or unenforceable provision.
(b) Successors and Assigns. This Warrant and the rights evidenced hereby shall inure to the benefit of and be binding upon the Company and the Warrantholder and their respective successors and permitted assigns (subject to Section 6 with respect to the Warrantholder); provided that the Company shall not assign its obligations under this Warrant except in connection with a Merger Event as provided in Section 6(a).
(c) Notices. Except as otherwise provided herein, any notice, demand, request, consent, approval, declaration, service of process or other communication that is required, contemplated, or permitted under this Warrant or with respect to the subject matter hereof shall be in writing, and shall be deemed to have been validly served, given, delivered, and received: (i) for personal delivery, upon personal delivery to the party to be notified, or (ii) for email, upon the earlier of (x) confirmation of receipt (other than automatically generated confirmations of receipt), (y) if sent during normal business hours of the recipient, upon delivery and (z) if sent after normal business hours of the recipient, then on the next Business Day, and shall be addressed to the party to be notified as follows: (i) if to the Warrantholder: [●], or to such other address as each party may designate for itself by like notice.
(d) Entire Agreement; Amendments. This Warrant constitutes the entire agreement and understanding of the parties hereto in respect of the subject matter hereof, and supersedes and replaces in their entirety any prior proposals, term sheets, letters, negotiations or other documents or agreements, whether written or oral, with respect to the subject matter hereof. None of the terms of this Warrant may be amended except by an instrument executed by each of the parties hereto.
(e) No Waiver. No omission or delay by the Warrantholder at any time to enforce any right or remedy reserved to it, or to require performance of any of the terms, covenants or provisions hereof by the Company at any time designated, shall be a waiver of any such right or remedy to which the Warrantholder is entitled, nor shall it in any way affect the right of the Warrantholder to enforce such provisions thereafter during the term of this Warrant.
(f) Governing Law, Consent to Jurisdiction and Venue. This Warrant and any claim, controversy or dispute arising out of or related to this Warrant, any of the transactions contemplated hereby, the relationship of the Parties hereunder, or the interpretation and enforcement of the rights and duties of the Parties, whether arising in contract, tort or otherwise, shall be governed by and construed in accordance with the substantive laws of Switzerland, without giving effect to any choice of law or conflict of law provision or rule that would cause the application of the laws of any jurisdiction other than Switzerland. The exclusive place of jurisdiction for any dispute, claim or controversy arising under, out of or in connection with or related to this Warrant (or subsequent amendments thereof), including, without limitation, disputes, claims or controversies regarding its existence, validity, interpretation, performance, breach or termination, shall be the city of Lausanne, Switzerland.
(g) Counterparts. This Warrant and any amendments, waivers, consents or supplements hereto may be executed in any number of counterparts (including by facsimile or electronic delivery (PDF), and by different parties hereto in separate counterparts, each of which when so delivered shall be deemed an original, but all of which counterparts shall constitute but one and the same instrument.
(h) Legends. To the extent required by applicable laws, this Warrant and the Common Shares issuable upon the exercise of this Warrant (and the securities issuable, directly or indirectly, upon conversion of such Common Shares, if any) may be imprinted with a restricted securities legend in substantially the following form:
THIS SECURITY HAS NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR THE SECURITIES LAWS OF ANY STATE AND MAY NOT BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED EXCEPT PURSUANT TO A REGISTRATION STATEMENT RELATED THERETO THAT IS IN EFFECT UNDER THE SECURITIES ACT AND ANY APPLICABLE STATE SECURITIES LAWS OR PURSUANT TO AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT AND SUCH LAWS.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the Company has duly executed this Warrant.
Dated: October 27, 2025.
|
ADC THERAPEUTICS SA
|
|||
| By: | |||
| Name: | |||
| Title: | |||
[Signature Page to Prefunded Warrant]
Dated: October 27, 2025.
Agreed and Acknowledged:
|
[INVESTOR] By:
|
|||
| By: | |||
| Name: | |||
| Title: | |||
[Signature Page to Prefunded Warrant]
EXHIBIT A
FORM OF EXERCISE NOTICE
(To be executed by the registered holder hereof)
Reference is made to (i) the attached Warrant to Purchase Common Shares of ADC Therapeutics SA No. W-PF-_____ (the “Warrant”) and (ii) if applicable, article 4(c) of the articles of association of the Company.
The undersigned registered owner of the Warrant hereby irrevocably exercises the right to purchase represented by the Warrant for, and hereby purchases thereunder, ______ Common Shares (such number of Common Shares, the “Exercise Number”), of ADC THERAPEUTICS SA (the “Company”), as provided for therein in accordance with Section 3 and the other terms and conditions of the Warrant, as well as the articles of association of the Company. All capitalized terms used but not defined in this exercise notice shall have the meanings ascribed thereto in the Warrant. Following this exercise, the Warrant Shares shall be reduced by the Exercise Number.
Payment shall take the form of (check applicable box):
| [__] in cash to the following ADC Therapeutics SA bank account: | ||||
| Account number: | 381032793732 | |||
| Active ACH Blocks/Filters on file: | Yes | |||
| Routing number ACH/EFT: | 021200339 | |||
| Routing number DOM. WIRES: | 026009593 | |||
| SWIFT Code INTL WIRES: | BOFAUS3N (U.S DOMESTIC) BOFAUS6S (FOREIGN CURRENCY) | |||
| Account name: | ADC THERAPEUTICS SA | |||
| Account Address: | BIOPOLE ROUTE DE LA CORNICHE 3 B, EPALINGES 1066 | |||
| Or | ||
| [__] the cancellation of such number of Warrant Shares as is necessary, in accordance with the formula set forth in subsection 3(g), to exercise this Warrant with respect to the maximum number of Warrant Shares purchasable pursuant to the cashless exercise procedure set forth in subsection 3(g). |
Please issue the Common Shares in the name of the undersigned and to the following address:
Issue to: ____________________________
Address: ____________________________
Email Address: ____________________________
DTC Details (if applicable): ____________________________
| Dated: | Name of Holder |
| Signature ____________________________ | |
| Address _____________________________ |
EXHIBIT B
TRANSFER NOTICE
(To transfer or assign the enclosed Warrant execute this form and supply required information. Do not use this form to purchase shares.)
The enclosed Warrant and all rights evidenced thereby are hereby transferred and assigned to:
Name of Transferee: ____________________________________________________________
Address of Transferee: __________________________________________________________
Name of Transferor: _____________________________________________________________
Signature of Transferor: __________________________________________________________
Dated: _______________________________________________________________________
Accepted:
Name of Transferee: _____________________________________________________________
Signature of Transferee: __________________________________________________________
Dated: _______________________________________________________________________
NOTE: The name of the transferor must correspond with the name as it appears on the face of the Warrant. The signature(s) of the individual(s) signing this Transfer Notice on behalf of the transferor must be made in wet ink. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the enclosed Warrant.
Exhibit 99.1

ADC Therapeutics Announces $60 Million Private Placement
LAUSANNE, Switzerland — October 13, 2025 — ADC Therapeutics SA (NYSE: ADCT), a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs), today announced that it has entered into securities purchase agreements for the sale of its equity securities to certain institutional investors in a $60.0 million private investment in public equity (“PIPE”) financing. In the PIPE, ADC Therapeutics is selling 11.3 million common shares at $4.00 per share and pre-funded warrants to purchase 3.8 million common shares at $3.90, per pre-funded warrant, which is the price per common share in the PIPE minus the exercise price of CHF 0.08 per pre-funded warrant.
The PIPE is led by TCGX and includes participation from Redmile Group and other existing investors.
Gross proceeds from the PIPE financing are anticipated to be approximately $60.0 million before deducting placement agent fees and offering expenses. The PIPE is expected to close on October 27, 2025, subject to customary closing conditions. ADC Therapeutics intends to use the net proceeds from the PIPE to invest in the commercial expansion of ZYNLONTA® and strengthen the balance sheet, in addition to funding working capital and general corporate purposes.
“This financing enhances our ability to prepare for and execute the potential relaunch of ZYNLONTA in 2027 and further strengthens our balance sheet relative to our previously disclosed cash runway into 2028,” said Ameet Mallik, Chief Executive Officer of ADC Therapeutics. "We believe we are well-positioned to accelerate our trajectory towards long-term sustainable growth for our company. We look forward to upcoming data catalysts later this year and throughout 2026.”
The company expects net product revenues from sales of ZYNLONTA to be approximately $15.8 million for the third quarter ended September 30, 2025 with cash and cash equivalents totaling $234.7 million as of September 30, 2025. Giving effect to the estimated net proceeds from the PIPE financing of approximately $57.6 million (after deducting placement agent fees and estimated offering expenses), the Company would have had approximately $292.3 million of cash and cash equivalents as of that date.
The offer and sale of the foregoing securities are made in a transaction not involving a public offering, and the foregoing securities have not been registered under the Securities Act of 1933, as amended (the “Securities Act”) or applicable state securities laws, and are being offered and sold in reliance on Section 4(a)(2) of the Securities Act. The securities may not be reoffered or resold in the United States except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act and other applicable securities laws.
ADC Therapeutics has agreed to file a registration statement with the Securities and Exchange Commission registering the resale of the common shares to be sold the PIPE and the common shares issuable upon exercise of the pre-funded warrants to be sold in the PIPE.
Jefferies is acting as placement agent for the PIPE. Davis Polk & Wardwell LLP and Homburger AG are acting as legal advisors to ADC Therapeutics.
This press release shall not constitute an offer to sell or a solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or other jurisdiction.
The Company has uploaded an updated corporate presentation to the Investor portion of its website..
About ZYNLONTA®
ZYNLONTA® is a CD19-directed antibody drug conjugate (ADC). Once bound to a CD19-expressing cell, ZYNLONTA is internalized by the cell, where enzymes release a pyrrolobenzodiazepine (PBD) payload. The potent payload binds to DNA minor groove with little distortion, remaining less visible to DNA repair mechanisms. This ultimately results in cell cycle arrest and tumor cell death.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved ZYNLONTA (loncastuximab tesirine-lpyl) for the treatment of adult patients with relapsed or refractory (r/r) large B-cell lymphoma after two or more lines of systemic therapy, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (NOS), DLBCL arising from low-grade lymphoma and also high-grade B-cell lymphoma. The trial included a broad spectrum of heavily pre-treated patients (median three prior lines of therapy) with difficult-to-treat disease, including patients who did not respond to first-line therapy, patients refractory to all prior lines of therapy, patients with double/triple hit genetics and patients who had stem cell transplant and CAR-T therapy prior to their treatment with ZYNLONTA. This indication is approved by the FDA under accelerated approval and in the European Union under conditional approval based on overall response rate and continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ZYNLONTA is also being evaluated as a therapeutic option in combination studies in other B-cell malignancies and earlier lines of therapy.
About ADC Therapeutics
ADC Therapeutics (NYSE: ADCT) is a commercial-stage global leader and pioneer in the field of antibody drug conjugates (ADCs). The Company is advancing its proprietary ADC technology to transform the treatment paradigm for patients with hematologic malignancies and solid tumors.
ADC Therapeutics' CD19-directed ADC ZYNLONTA (loncastuximab tesirine-lpyl) received accelerated approval by the FDA and conditional approval from the European Commission for the treatment of relapsed or refractory diffuse large B-cell lymphoma after two or more lines of systemic therapy.
ZYNLONTA is also in development in combination with other agents and in earlier lines of therapy. In addition to ZYNLONTA, ADC Therapeutics has multiple ADCs in ongoing clinical and preclinical development.
ADC Therapeutics is based in Lausanne (Biopôle), Switzerland, and has operations in London and New Jersey.
ZYNLONTA® is a registered trademark of ADC Therapeutics SA.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, including statements regarding the anticipated proceeds to be received in the PIPE, the Company’s expected use of proceeds, the expected timing of the closing of the PIPE, the Company’s long-term growth potential, the Company’s expected cash runway into 2028, and the Company’s expected net product revenues from sales of ZYNLONTA for the third quarter ended September 30, 2025 and its cash and cash equivalents as of September 30, 2025. In some cases you can identify forward-looking statements by terminology such as “may”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the success of the Company’s strategic restructuring plan; changes in estimated costs associated with the restructuring plan including the workforce reduction and planned closure of the UK facility; the expected cash runway into 2028 which assumes use of minimum liquidity amount required to be maintained under its loan agreement covenants; whether future LOTIS-7 clinical trial results will be consistent with or different from the LOTIS-7 data presented at EHA and ICML and future compendia and regulatory strategy and opportunity; the timing of the PFS events for LOTIS-5 and the results of the trial and full FDA approval; the Company’s ability to grow ZYNLONTA® revenue in the United States and potential peak revenue; the ability of our partners to commercialize ZYNLONTA® in foreign markets, the timing and amount of future revenue and payments to us from such partnerships and their ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions; the timing and results of the Company’s or its partners’ research and development projects or clinical trials including LOTIS 5 and 7, as well as early pre-clinical research for our exatecan-based ADC targeting PSMA; the timing and results of investigator-initiated trials including those studying FL and MZL and the potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or foreign regulatory authorities; projected revenue and expenses; the Company’s indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company’s activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities; and the uncertainties of international trade policies, including tariffs, sanctions and trade barriers and potential impact they may have on our business, financial condition, and results of operations.
Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward-looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 10-K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward-looking statements. The Company cautions investors not to place undue reliance on the forward-looking statements contained in this document.
The ZYNLONTA net sales and cash and cash equivalents figures included in this press release are preliminary and unaudited and reflect the Company’s estimated financial results. In preparing this information, management made a number of complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Company’s actual financial results for the quarter ended September 30, 2025 have not yet been finalized by management or audited or reviewed by the Company’s independent auditors. The preliminary financial information is not a comprehensive statement of all financial results for the quarter ended September 30, 2025. Subsequent information or events may lead to material differences between the foregoing preliminary financial results and those reported in the Company’s subsequent SEC filings. Accordingly, investors should not place undue reliance on these preliminary financial results.
CONTACTS:
Investors & Media
Nicole Riley
ADC Therapeutics
Nicole.Riley@adctherapeutics.com
+1 862-926-9040
Exhibit 99.2

Corporate Presentation October 2025

2 Forward - Looking Statements This presentation and any accompanying oral presentation have been prepared by ADC Therapeutics SA ("ADC Therapeutics“, “we” or “us”) for informational purposes only and not for any other purpose. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or ADC Therapeutics or any officer, director, employee, agent or advisor of ADC Therapeutics. This presentation does not purport to be all - inclusive or to contain all of the information you may desire. Information provided in this presentation and any accompanying oral presentation speak only as of the date hereof. This presentation contains forward - looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. In some cases you can identify forward - looking statements by terminology such as “may”, “will”, “should”, “would”, “expect”, “intend”, “plan”, “anticipate”, “believe”, “estimate”, “predict”, “potential”, “seem”, “seek”, “future”, “continue”, or “appear” or the negative of these terms or similar expressions, although not all forward - looking statements contain these identifying words. Forward - looking statements are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to: the success of the Company’s strategic restructuring plan; changes in estimated costs associated with the restructuring plan including the workforce reduction and planned closure of the UK facility; the expected cash runway into 2028 which assumes use of minimum liquidity amount required to be maintained under its loan agreement covenants; whether future LOTIS - 7 clinical trial results will be consistent with or different from the LOTIS - 7 data presented at EHA and ICML and future compendia and regulatory strategy and opportunity; the timing of the PFS events for LOTIS - 5 and topline publication and the results of the trial and full FDA approval; the Company’s ability to grow ZYNLONTA® revenue in the United States; the ability of our partners to commercialize ZYNLONTA® in foreign markets, the timing and amount of future revenue and payments to us from such partnerships and their ability to obtain regulatory approval for ZYNLONTA® in foreign jurisdictions; the timing and results of the Company’s or its partners’ research and development projects or clinical trials including LOTIS 5 and 7, as well as early pre - clinical research for our exatecan - based ADC targeting PSMA; the timing and results of investigator - initiated trials including those studying FL and MZL and the potential regulatory and/or compendia strategy and the future opportunity; the timing and outcome of regulatory submissions for the Company’s products or product candidates; actions by the FDA or foreign regulatory authorities; projected peak revenue and expenses; the Company’s indebtedness, including Healthcare Royalty Management and Blue Owl and Oaktree facilities, and the restrictions imposed on the Company’s activities by such indebtedness, the ability to comply with the terms of the various agreements and repay such indebtedness and the significant cash required to service such indebtedness; and the Company’s ability to obtain financial and other resources for its research, development, clinical, and commercial activities; and the uncertainties of international trade policies, including tariffs, sanctions and trade barriers and potential impact they may have on our business, financial condition, and results of operations. Additional information concerning these and other factors that may cause actual results to differ materially from those anticipated in the forward - looking statements is contained in the “Risk Factors” section of the Company's Annual Report on Form 10 - K and in the Company's other periodic and current reports and filings with the U.S. Securities and Exchange Commission. These statements involve known and unknown risks, uncertainties and other factors that may cause actual results, performance, achievements or prospects to be materially different from any future results, performance, achievements or prospects expressed in or implied by such forward - looking statements. The Company cautions investors not to place undue reliance on the forward - looking statements contained in this document. Forward - looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. No assurance can be given that such future results will be achieved. Such forward - looking statements contained in this presentation speak only as of the date of this presentation. The Company expressly disclaim any obligation or undertaking to update these forward - looking statements contained in this presentation to reflect any change in our expectations or any change in events, conditions, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys, and other data derived from third - party sources and our own internal estimates and research. While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, all of the market data included in this presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, although we believe our own internal research is reliable, such research has not been verified by any independent source. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sales of securities in any state jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. The ZYNLONTA net sales and cash and cash equivalents figures included in this presentation are preliminary and unaudited and reflect the Company’s estimated financial results. In preparing this information, management made a number of complex and subjective judgments and estimates about the appropriateness of certain reported amounts and disclosures. The Company’s actual financial results for the quarter ended September 30, 2025 have not yet been finalized by management or audited or reviewed by the Company’s independent auditors. The preliminary financial information is not a comprehensive statement of all financial results for the quarter ended September 30, 2025. Subsequent information or events may lead to material differences between the foregoing preliminary financial results and those reported in the Company’s subsequent SEC filings. Accordingly, investors should not place undue reliance on these preliminary financial results.

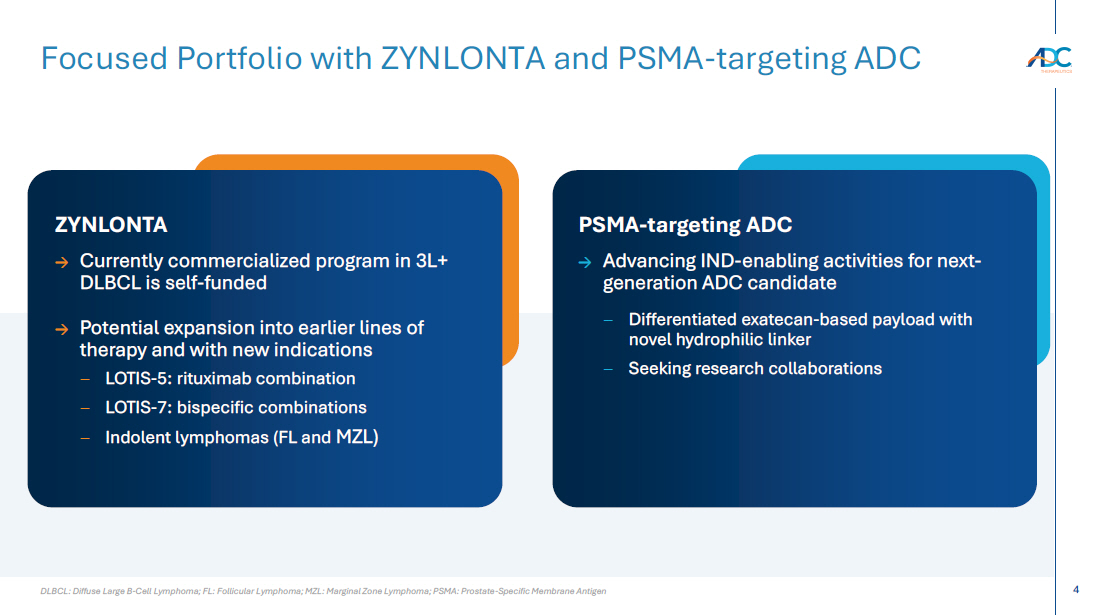
3 A Pioneer and Leader in ADCs Corporate Ca p abil i ty Pipeline Commercial - stage pioneer in the field of ADCs, with specialized capabilities from clinical through commercialization Seeking expansion of FDA - approved ZYNLONTA® while pursuing early - stage PSMA - targeting ADC towards IND Delivering on our strategy supported by an accomplished and multidisciplinary team and a strong balance sheet 4 DLBCL: Diffuse Large B - Cell Lymphoma; FL: Follicular Lymphoma; MZL: Marginal Zone Lymphoma; PSMA: Prostate - Specific Membrane Antigen Focused Portfolio with ZYNLONTA and PSMA - targeting ADC ZYNLONTA → Currently commercialized program in 3L+ DLBCL is self - funded → Potential expansion into earlier lines of therapy and with new indications − LOTIS - 5: rituximab combination − LOTIS - 7: bispecific combinations − Indolent lymphomas (FL and MZL) PSMA - targeting ADC → Advancing IND - enabling activities for next - generation ADC candidate − Differentiated exatecan - based payload with novel hydrophilic linker − Seeking research collaborations 5 Please visit ZYNLONTA.com for complete prescribing information including indication, warnings and precautions.

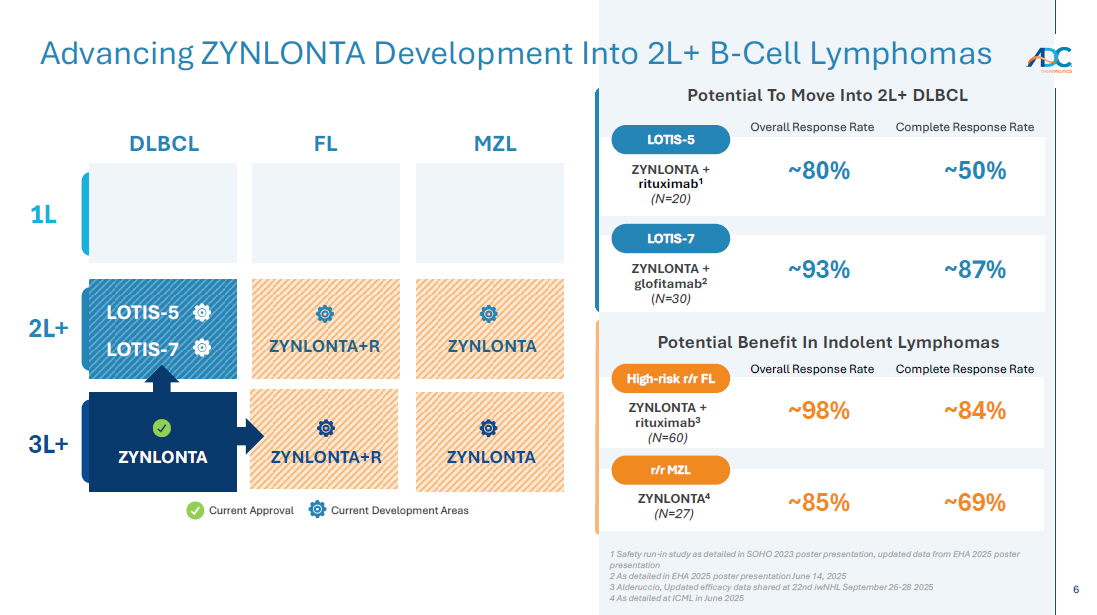
→ ZYNLONTA is a CD19 - directed ADC indicated as monotherapy for the treatment of adult patients with relapsed or refractory large B - cell lymphoma after two or more lines of systemic therapy → Rapid, deep, and durable efficacy – Median time to CR 1.5 months – 48.3% ORR and 24.8% CR – Median duration of response not yet reached for patients in CR at 2 - year follow - up → Manageable safety profile – No CRS or ICANS and no cumulative irreversible toxicities → Accessibility – Simple Q3W dosing with no REMS or inpatient stay requirements ZYNLONTA is Ideally Suited Across Care Settings for Patients with r/r DLBCL 6 Advancing ZYNLONTA Development Into 2L+ B - Cell Lymphomas Current Approval Current Development Areas D L B CL FL MZL 1L 2L+ 3L+ ZYNLONTA+R ZYNLONTA ZYNLONTA+R ZYNLONTA Z Y N L O N T A Potential Benefit In Indolent Lymphomas L OT I S - 5 L OT I S - 7 Potential To Move Into 2L+ DLBCL ZYNLONTA + rituximab 1 (N=20) L O TI S - 5 Overall Response Rate Complete Response Rate ~80% ~50% ZYNLONTA + rituximab 3 (N=60) High - risk r/r FL Overall Response Rate Complete Response Rate ~98% ~84% ZYNLONTA + glofitamab 2 ( N=30) L O TI S - 7 ~93 % ~ 87 % ZYNLONTA 4 (N=27) r/r MZL ~ 85 % ~ 69 % 1 Safety run - in study as detailed in SOHO 2023 poster presentation, updated data from EHA 2025 poster presentation 2 As detailed in EHA 2025 poster presentation June 14, 2025 3 Alderuccio, Updated efficacy data shared at 22nd iwNHL September 26 - 28 2025 4 As detailed at ICML in June 2025 7 Broadly accessible therapies ~75% 2L ~12k patients 3L+ ~6k patients Two Distinct Segments in r/r DLB CL Treatment Options 2L+ treatment choice based on efficacy, safety and accessibility in context of individual patient need » Complex therapies with unique patient management and infrastructure requirements » Broadly accessible outpatient therapies Complex therapies ~25% ~20% CAR T ~5% SCT Complex therapies ~60% 35% Bispecific - mono ~20% CAR T ~5% SCT Current r/r DLBCL U.S. Market ADCs, mAbs, Chemo / other Broadly accessible therapies ~40% ADCs, mAbs, Chemo / other ~10% ZYNLONTA Based on internal market research conducted July to August 2024 (n=160 US Hem/Oncs), and glofitamab ODAC briefing book
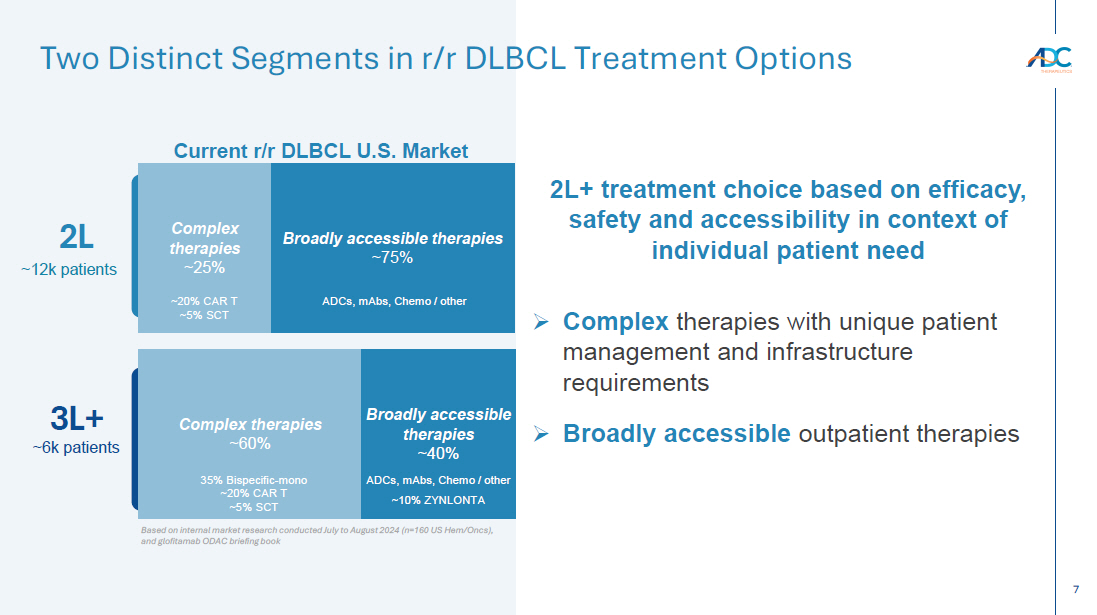
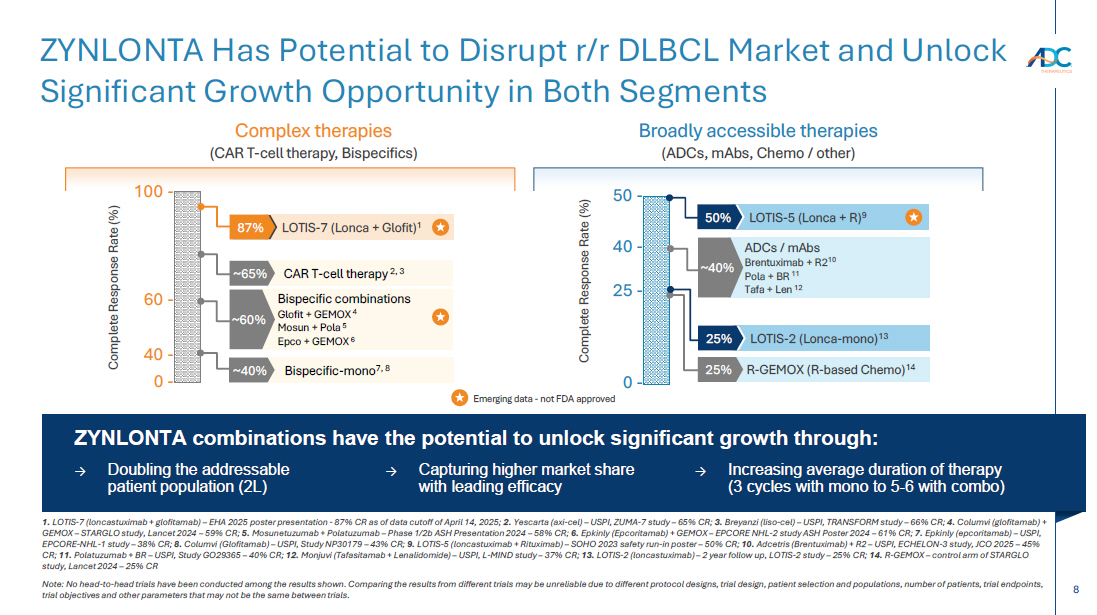
8 1. LOTIS - 7 (loncastuximab + glofitamab) – EHA 2025 poster presentation - 87% CR as of data cutoff of April 14, 2025; 2. Yescarta (axi - cel) – USPI, ZUMA - 7 study – 65% CR; 3. Breyanzi (liso - cel) – USPI, TRANSFORM study – 66% CR; 4. Columvi (glofitamab) + GEMOX – STARGLO study, Lancet 2024 – 59% CR; 5. Mosunetuzumab + Polatuzumab – Phase 1/2b ASH Presentation 2024 – 58% CR; 6. Epkinly (Epcoritamab) + GEMOX – EPCORE NHL - 2 study ASH Poster 2024 – 61% CR; 7. Epkinly (epcoritamab) – USPI, EPCORE - NHL - 1 study – 38% CR; 8. Columvi (Glofitamab) – USPI, Study NP30179 – 43% CR; 9. LOTIS - 5 (loncastuximab + Rituximab) – SOHO 2023 safety run - in poster – 50% CR; 10. Adcetris (Brentuximab) + R2 – USPI, ECHELON - 3 study, JCO 2025 – 45% CR; 11. Polatuzumab + BR – USPI, Study GO29365 – 40% CR; 12. Monjuvi (Tafasitamab + Lenalidomide) – USPI, L - MIND study – 37% CR; 13. LOTIS - 2 (loncastuximab) – 2 year follow up, LOTIS - 2 study – 25% CR; 14. R - GEMOX – control arm of STARGLO study, Lancet 2024 – 25% CR Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unreliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoints, trial objectives and other parameters that may not be the same between trials. ZYNLONTA Has Potential to Disrupt r/r DLBCL Market and Unlock Significant Growth Opportunity in Both Segments Emerging data - not FDA approved 100 - CAR T - cell therapy 2, 3 ~65% Bispecific combinations Glofit + GEMOX 4 Mosun + Pola 5 Epco + GEMOX 6 ~60 % 87% LOTIS - 7 (Lonca + Glofit) 1 Bispecific - mono 7, 8 ~40% Complete Response Rate (%) 40 - 0 - 60 - ZYNLONTA combinations have the potential to unlock significant growth through: → Doubling the addressable patient population (2L) → Capturing higher market share with leading efficacy → Increasing average duration of therapy (3 cycles with mono to 5 - 6 with combo) 50 - 0 - 50% LOTIS - 5 (Lonca + R) 9 ADCs / mAbs Brentuximab + R2 10 Pola + BR 11 Tafa + Len 12 ~40% 25% LOTIS - 2 (Lonca - mono) 13 25% R - GEMOX (R - based Chemo) 14 Complete Response Rate (%) 25 - 40 - Complex therapies (CAR T - cell therapy, Bispecifics) Broadly accessible therapies (ADCs, mAbs, Chemo / other)
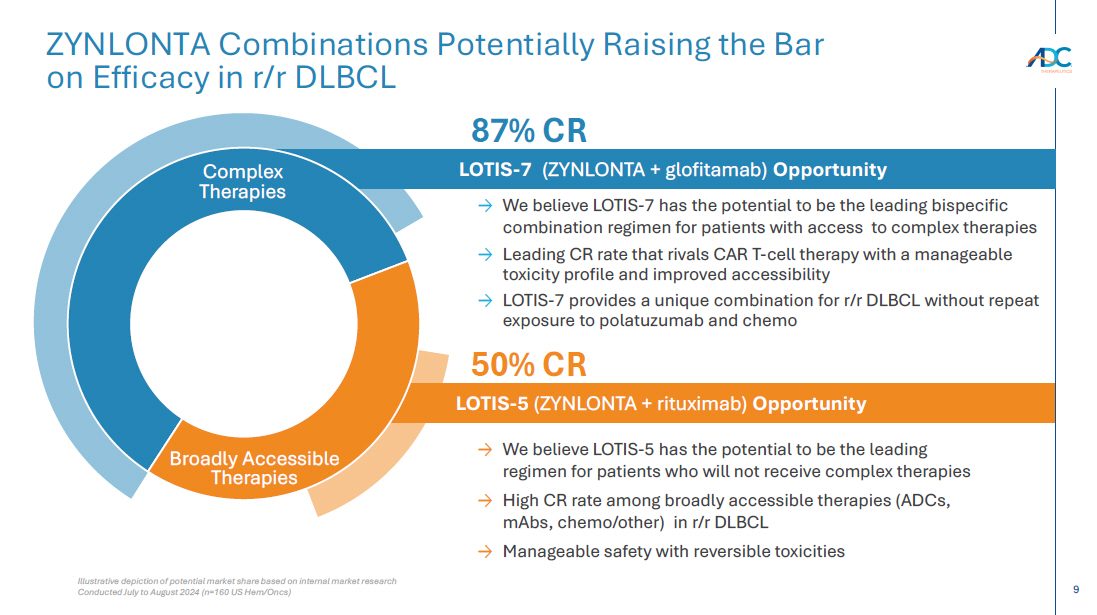
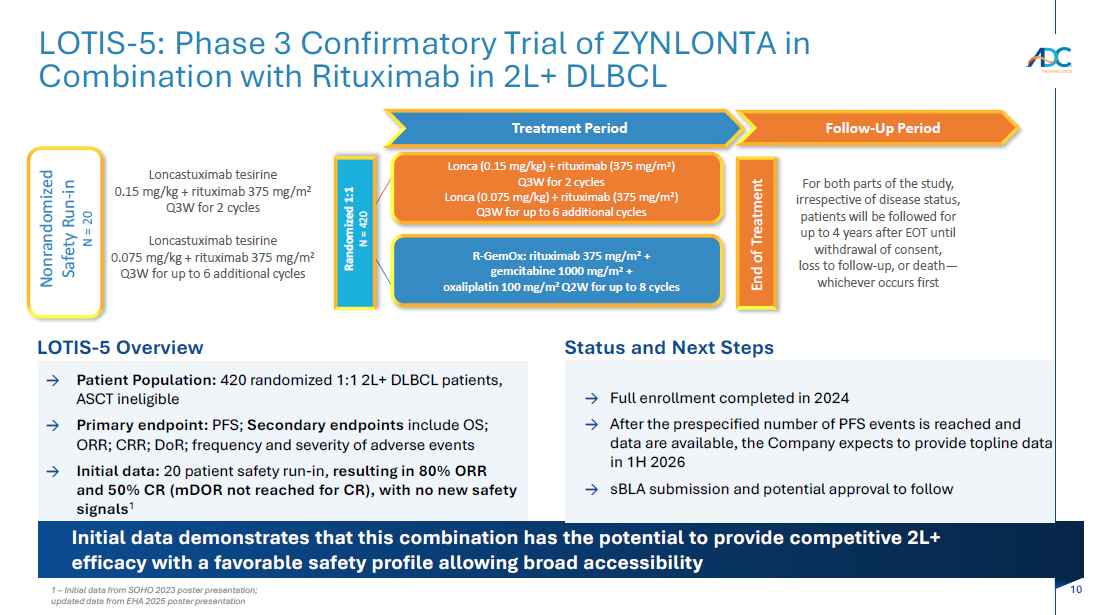
9 LOTIS - 7 (ZYNLONTA + glofitamab) Opportunity LOTIS - 5 (ZYNLONTA + rituximab) Opportunity Illustrative depiction of potential market share based on internal market research Conducted July to August 2024 (n=160 US Hem/Oncs) → We believe LOTIS - 5 has the potential to be the leading regimen for patients who will not receive complex therapies → High CR rate among broadly accessible therapies (ADCs, mAbs, chemo/other) in r/r DLBCL → Manageable safety with reversible toxicities Comp l e x The r api e s → We believe LOTIS - 7 has the potential to be the leading bispecific combination regimen for patients with access to complex therapies → Leading CR rate that rivals CAR T - cell therapy with a manageable toxicity profile and improved accessibility → LOTIS - 7 provides a unique combination for r/r DLBCL without repeat exposure to polatuzumab and chemo 50% CR Broadly Accessible T h e r ap i e s ZYNLONTA Combinations Potentially Raising the Bar on Efficacy in r/r DLBCL 87% CR 10 LOTIS - 5: Phase 3 Confirmatory Trial of ZYNLONTA in Combination with Rituximab in 2L+ DLBCL Initial data demonstrates that this combination has the potential to provide competitive 2L+ efficacy with a favorable safety profile allowing broad accessibility Status and Next Steps → Full enrollment completed in 2024 → After the prespecified number of PFS events is reached and data are available, the Company expects to provide topline data in 1H 2026 → sBLA submission and potential approval to follow LOTIS - 5 Overview → Patient Population: 420 randomized 1:1 2L+ DLBCL patients, ASCT ineligible → Primary endpoint: PFS; Secondary endpoints include OS; ORR; CRR; DoR; frequency and severity of adverse events → Initial data: 20 patient safety run - in, resulting in 80% ORR and 50% CR (mDOR not reached for CR), with no new safety signals 1 Treatment Period Follow - Up Period End of Treatment Randomized 1:1 N = 420 Loncastuximab tesirine 0.15 mg/kg + rituximab 375 mg/m 2 Q3W for 2 cycles Loncastuximab tesirine 0.075 mg/kg + rituximab 375 mg/m 2 Q3W for up to 6 additional cycles Lonca (0.15 mg/kg) + rituximab (375 mg/m 2 ) Q3W for 2 cycles Lonca (0.075 mg/kg) + rituximab (375 mg/m 2 ) Q3W for up to 6 additional cycles R - GemOx: rituximab 375 mg/m 2 + gemcitabine 1000 mg/m 2 + oxaliplatin 100 mg/m 2 Q2W for up to 8 cycles For both parts of the study, irrespective of disease status, patients will be followed for up to 4 years after EOT until withdrawal of consent, loss to follow - up, or death — whichever occurs first Non r an domi z ed Safety Run - in N = 20 1 – Initial data from SOHO 2023 poster presentation; updated data from EHA 2025 poster presentation 11 LOTIS - 7 Combination Rationale and Biological Hypothesis Potent, single agent drugs with distinct and complementary MOAs ZYNLONTA ANTI - CD19 ADC GLOFITAMAB ANTI - CD20/CD3 T - Cell engaging bispecific antibody EFFICACY ▪ Expected to have additive or synergistic efficacy S A F E T Y ▪ No overlapping non - hematologic toxicities expected to yield manageable safety profile ▪ Potentially lower CRS rates/grades given ZYNLONTA use prior to glofitamab may debulk the tumors and reduce peripheral B cells
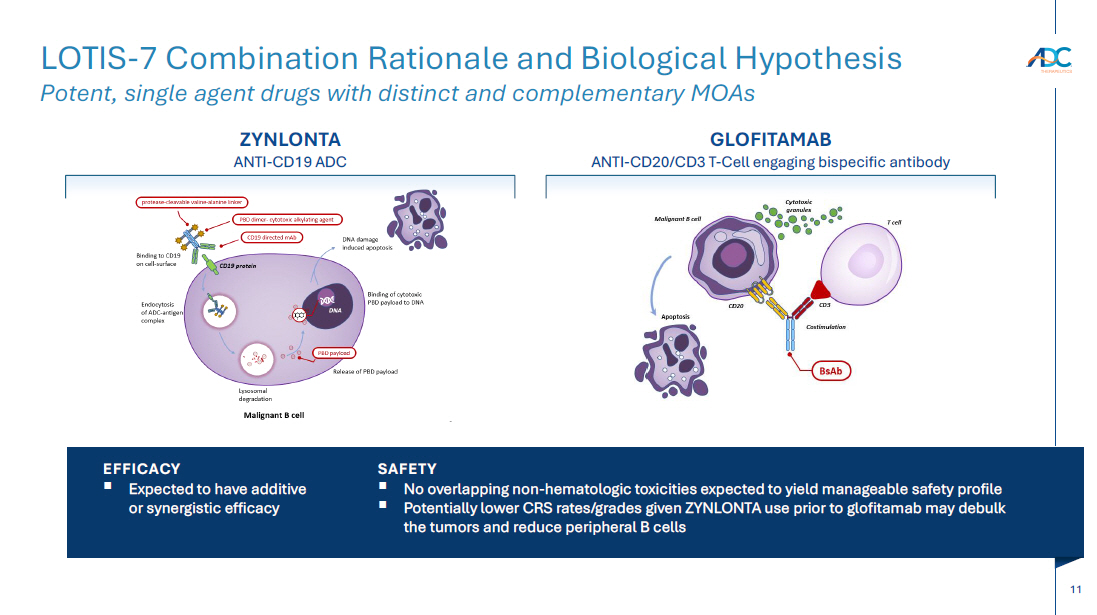
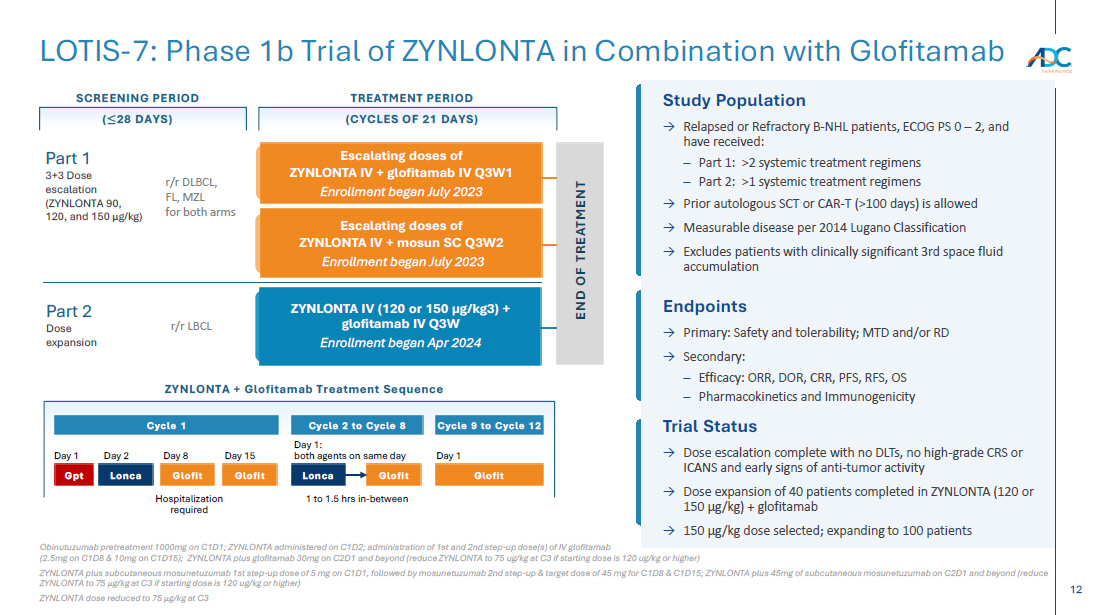
12 END OF TREATMENT Study Population → Relapsed or Refractory B - NHL patients, ECOG PS 0 – 2, and have received: – Part 1: >2 systemic treatment regimens – Part 2: >1 systemic treatment regimens → Prior autologous SCT or CAR - T (>100 days) is allowed → Measurable disease per 2014 Lugano Classification → Excludes patients with clinically significant 3rd space fluid accumulation ZYNLONTA IV (120 or 150 µg/kg3) + glofitamab IV Q3W Enrollment began Apr 2024 Escalating doses of ZYNLONTA IV + mosun SC Q3W2 Enrollment began July 2023 Escalating doses of ZYNLONTA IV + glofitamab IV Q3W1 Enrollment began July 2023 SCREENING PERIOD TREATMENT PERIOD ( ≤ 28 DAYS) (CYCLES OF 21 DAYS) r/r DLBCL, FL, MZL for both arms r/r LBCL Part 1 3+3 Dose escalation (ZYNLONTA 90, 120, and 150 μ g/kg) Part 2 Dose ex p ans i o n Endpoints → Primary: Safety and tolerability; MTD and/or RD → Secondary: – Efficacy: ORR, DOR, CRR, PFS, RFS, OS – Pharmacokinetics and Immunogenicity Trial Status → Dose escalation complete with no DLTs, no high - grade CRS or ICANS and early signs of anti - tumor activity → Dose expansion of 40 patients completed in ZYNLONTA (120 or 150 µg/kg) + glofitamab → 150 µg/kg dose selected; expanding to 100 patients ZYNLONTA + Glofitamab Treatment Sequence Cycle 9 to Cycle 12 Day 1 Hospitalization re quire d G l o f i t Cycle 2 to Cycle 8 Day 1: both agents on same day L o n c a G l o f i t 1 to 1.5 hrs in - between Day 15 G l o f i t Cycle 1 Day 8 G l o f i t Day 2 L o n c a Day 1 G p t Obinutuzumab pretreatment 1000mg on C1D1; ZYNLONTA administered on C1D2; administration of 1st and 2nd step - up dose(s) of IV glofitamab (2.5mg on C1D8 & 10mg on C1D15); ZYNLONTA plus glofitamab 30mg on C2D1 and beyond (reduce ZYNLONTA to 75 ug/kg at C3 if starting dose is 120 ug/kg or higher) ZYNLONTA plus subcutaneous mosunetuzumab 1st step - up dose of 5 mg on C1D1, followed by mosunetuzumab 2nd step - up & target dose of 45 mg for C1D8 & C1D15; ZYNLONTA plus 45mg of subcutaneous mosunetuzumab on C2D1 and beyond (reduce ZYNLONTA to 75 μ g/kg at C3 if starting dose is 120 ug/kg or higher) ZYNLONTA dose reduced to 75 μ g/kg at C3 LOTIS - 7: Phase 1b Trial of ZYNLONTA in Combination with Glofitamab 13 N=41 150 µg/kg N=21 120 µg/kg N=20 IPI Score 19 (46.3%) 10 (47.6%) 9 (45%) 0/1/2 22 (53.7%) 11 (52.4%) 11 (55%) 3/4/5 21 (51.2%) 10 (47.6%) 11 (55%) LDH Level High Ann Arbor stage 6 (14.6%) 3 (14.3%) 3 (15%) I/II 35 (85.3%) 18 (85.7%) 17 (85%) III/IV 4 (9.8%) 2 (9.5%) 2 (10%) Bulky Disease ( ≥10 cm) 2 (1,5) 2 (1,5) 2 (1,4) Median prior lines of therapy (range) Number of prior lines of therapy 20 (48.8%) 10 (47.6%) 10 (50%) 1 21 (51.2%) 11 (52.4%) 10 (50%) ≥ 2 4 (9.8%) 1 (4.8%) 3 (15%) Prior Stem Cell Transplant 8 (19.5%) 4 (19%) 4 (20%) Prior CAR - T Therapy 21 (51.2%) 13 (61.9%) 8 (40%) Refractory to primary therapy 20 (48.8%) 13 (61.9%) 7 (35%) Refractory to last prior therapy LOTIS - 7 Phase 1b Trial: Baseline Patient Characteristics r/r Large B - Cell Lymphoma Treated Population (N=41) as of data cutoff of April 14, 2025 All patients enrolled in US & Europe with majority in US N=41 150 µg/kg N=21 120 µg/kg N=20 71 (26, 85) 74 (26, 85) 70 (50, 82) Median age [years (range)] 23 (56.1%) 12 (57.1%) 11 (55%) Male ECOG Performance Status 23 (56.1%) 14 (66.7%) 9 (45%) 0 17 (41.5%) 7 (33.3%) 10 (50%) 1 1 (2.4%) 0 1 (5%) 2 Large B - Cell Lymphoma Histology 30 (73.2%) 17 (81%) 13 (65%) de novo DLBCL 4 (9.8%) 2 (9.5%) 2 (10%) trFL 6 (14.6%) 2 (9.5%) 4 (20%) HGBCL 1 (2.4%) 0 1 (5%) FL Grade 3b DLBCL Subtype 21 (51.2%) 11 (52.4%) 10 (50%) GCB 13 (31.7%) 8 (38.1%) 5 (25%) non - GCB 8 (19.5%) 5 (23.8%) 3 (15%) Double/Triple hit LBCL = large B - cell lymphoma, DLBCL= diffuse large B - cell lymphoma, HGBCL= high grade B - cell lymphoma, NOS = not otherwise specified, trFL= transformed follicular lymphoma, GCB, germinal center B - cell Data cutoff: 14Apr2025 Note: Data extracted from live clinical database.
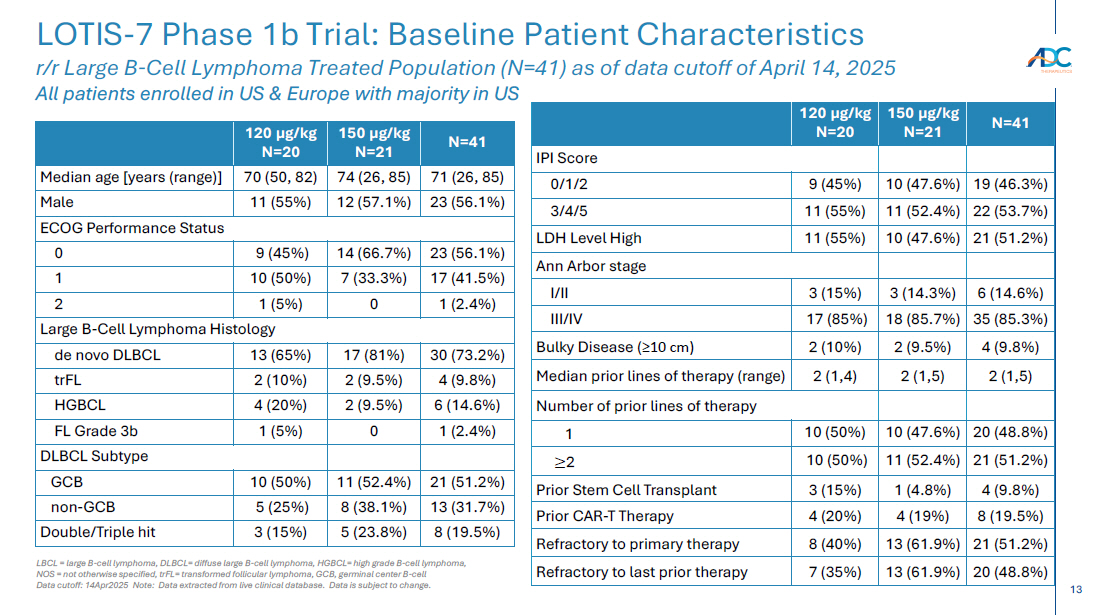
Data is subject to change.
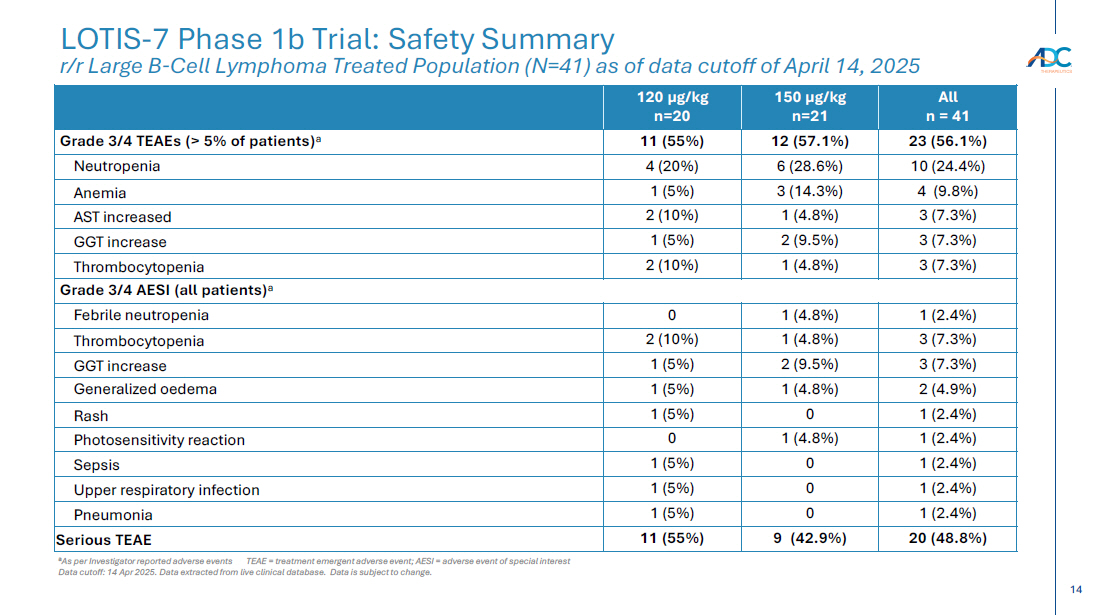
14 LOTIS - 7 Phase 1b Trial: Safety Summary r/r Large B - Cell Lymphoma Treated Population (N=41) as of data cutoff of April 14, 2025 a As per Investigator reported adverse events TEAE = treatment emergent adverse event; AESI = adverse event of special interest Data cutoff: 14 Apr 2025. Data extracted from live clinical database. Data is subject to change. All n = 41 150 µg/kg n=21 120 µg/kg n=20 23 (56.1%) 12 (57.1%) 11 (55%) Grade 3/4 TEAEs (> 5% of patients) a 10 (24.4%) 6 (28.6%) 4 (20%) Neutropenia 4 (9.8%) 3 (14.3%) 1 (5%) Anemia 3 (7.3%) 1 (4.8%) 2 (10%) AST increased 3 (7.3%) 2 (9.5%) 1 (5%) GGT increase 3 (7.3%) 1 (4.8%) 2 (10%) Thrombocytopenia Grade 3/4 AESI (all patients) a 1 (2.4%) 1 (4.8%) 0 Febrile neutropenia 3 (7.3%) 1 (4.8%) 2 (10%) Thrombocytopenia 3 (7.3%) 2 (9.5%) 1 (5%) GGT increase 2 (4.9%) 1 (4.8%) 1 (5%) Generalized oedema 1 (2.4%) 0 1 (5%) Rash 1 (2.4%) 1 (4.8%) 0 Photosensitivity reaction 1 (2.4%) 0 1 (5%) Sepsis 1 (2.4%) 0 1 (5%) Upper respiratory infection 1 (2.4%) 0 1 (5%) Pneumonia 20 (48.8%) 9 (42.9%) 11 (55%) Serious TEAE 15 LOTIS - 7 Phase 1b Trial: Safety Summary r/r Large B - Cell Lymphoma Treated Population (N=41) as of data cutoff of April 14, 2025 a As per Investigator reported adverse events TEAE = treatment emergent adverse event; AESI = adverse event of special interest Data cutoff: 14 Apr 2025.
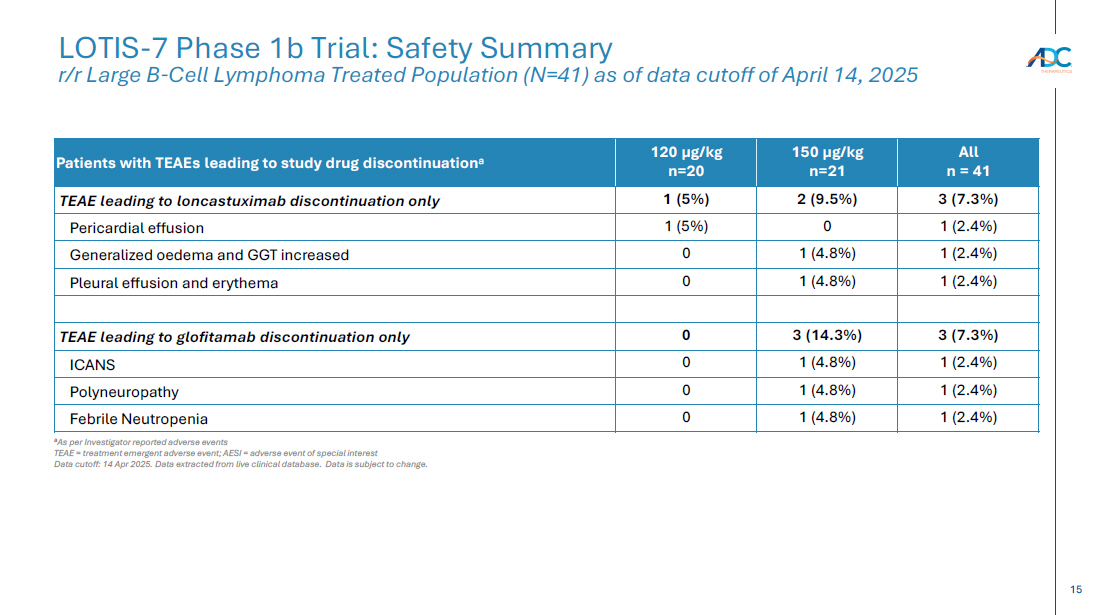
Data extracted from live clinical database. Data is subject to change. All n = 41 150 µg/kg n=21 120 µg/kg n=20 Patients with TEAEs leading to study drug discontinuation a 3 (7.3%) 2 (9.5%) 1 (5%) TEAE leading to loncastuximab discontinuation only 1 (2.4%) 0 1 (5%) Pericardial effusion 1 (2.4%) 1 (4.8%) 0 Generalized oedema and GGT increased 1 (2.4%) 1 (4.8%) 0 Pleural effusion and erythema 3 (7.3%) 3 (14.3%) 0 TEAE leading to glofitamab discontinuation only 1 (2.4%) 1 (4.8%) 0 ICANS 1 (2.4%) 1 (4.8%) 0 Polyneuropathy 1 (2.4%) 1 (4.8%) 0 Febrile Neutropenia 16 LOTIS - 7 Phase 1b Trial: CRS/ICANS Profile & Management r/r Large B - Cell Lymphoma Treated Population (N=41) as of data cutoff of April 14, 2025 a Number of patients who experienced at least 1 event per ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells; worst grade reported if applicable Data Cutoff 14 Apr 2025.
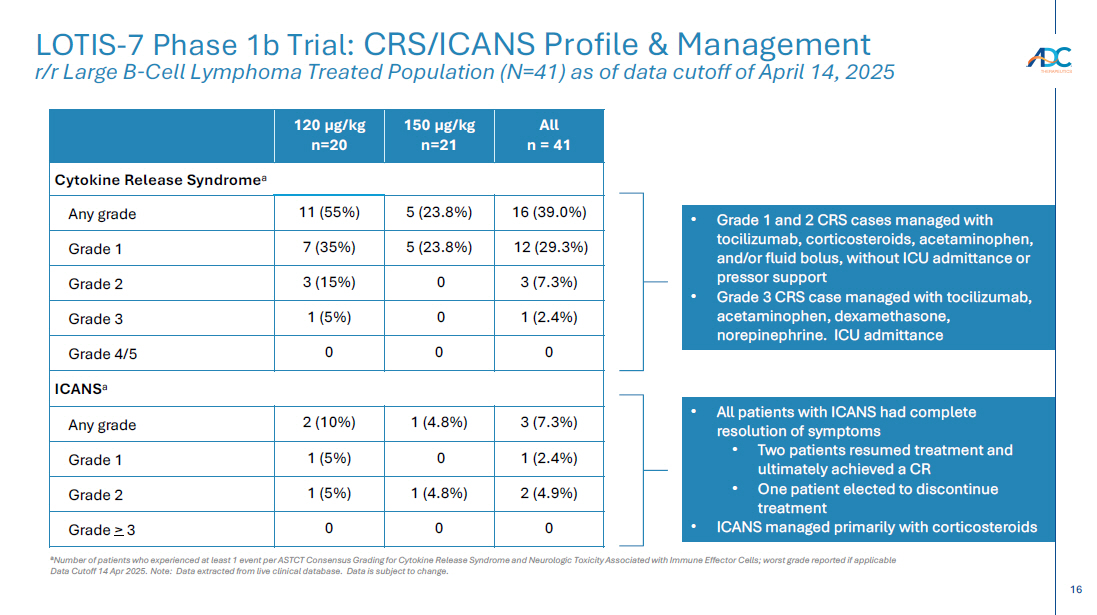
Note: Data extracted from live clinical database. Data is subject to change. All n = 41 150 µg/kg n=21 120 µg/kg n=20 Cytokine Release Syndrome a 16 (39.0%) 5 (23.8%) 11 (55%) Any grade 12 (29.3%) 5 (23.8%) 7 (35%) Grade 1 3 (7.3%) 0 3 (15%) Grade 2 1 (2.4%) 0 1 (5%) Grade 3 0 0 0 Grade 4/5 ICANS a 3 (7.3%) 1 (4.8%) 2 (10%) Any grade 1 (2.4%) 0 1 (5%) Grade 1 2 (4.9%) 1 (4.8%) 1 (5%) Grade 2 0 0 0 Grade > 3 • Grade 1 and 2 CRS cases managed with tocilizumab, corticosteroids, acetaminophen, and/or fluid bolus, without ICU admittance or pressor support • Grade 3 CRS case managed with tocilizumab, acetaminophen, dexamethasone, norepinephrine. ICU admittance • All patients with ICANS had complete resolution of symptoms • Two patients resumed treatment and ultimately achieved a CR • One patient elected to discontinue treatment • ICANS managed primarily with corticosteroids 17 Total 150 µg/kg 120 µg/kg % n=30 % n=15 % n=15 93.3% 28 93.3% 14 93.3% 14 ORR (CR + PR) 86.7% 26 86.7% 13 86.7% 13 Complete Response (CR) 6.7% 2 6.7% 1 6.7% 1 Partial Response (PR) 3.3% 1 0% 0 6.7% 1 Stable Disease 3.3% 1 6.7% 1 0% 0 Progressive Disease As of data cut off 14 Apr 2025.
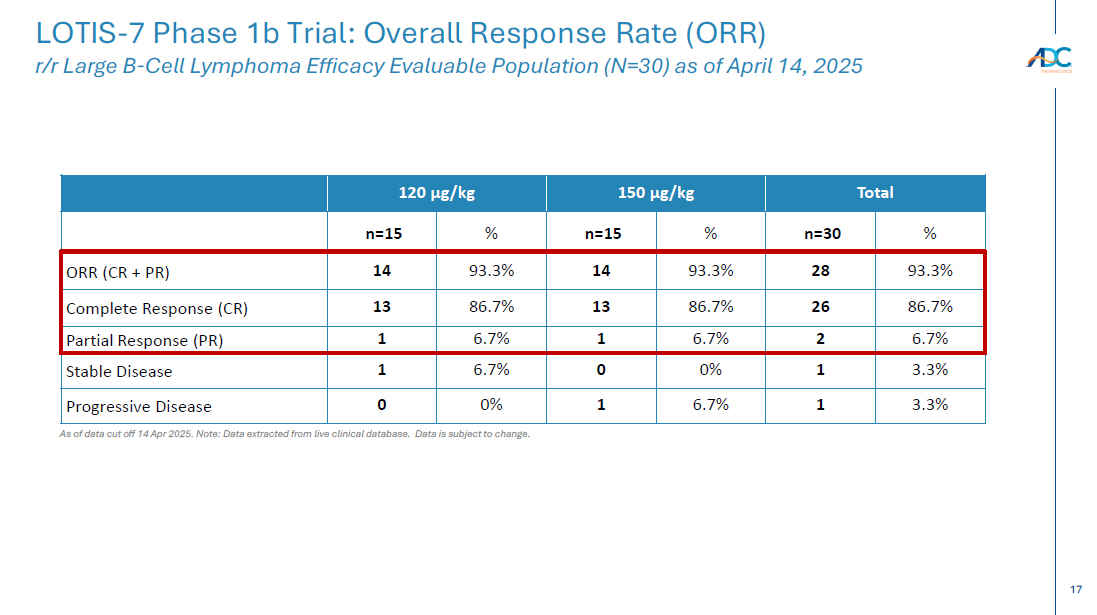
Note: Data extracted from live clinical database. Data is subject to change.
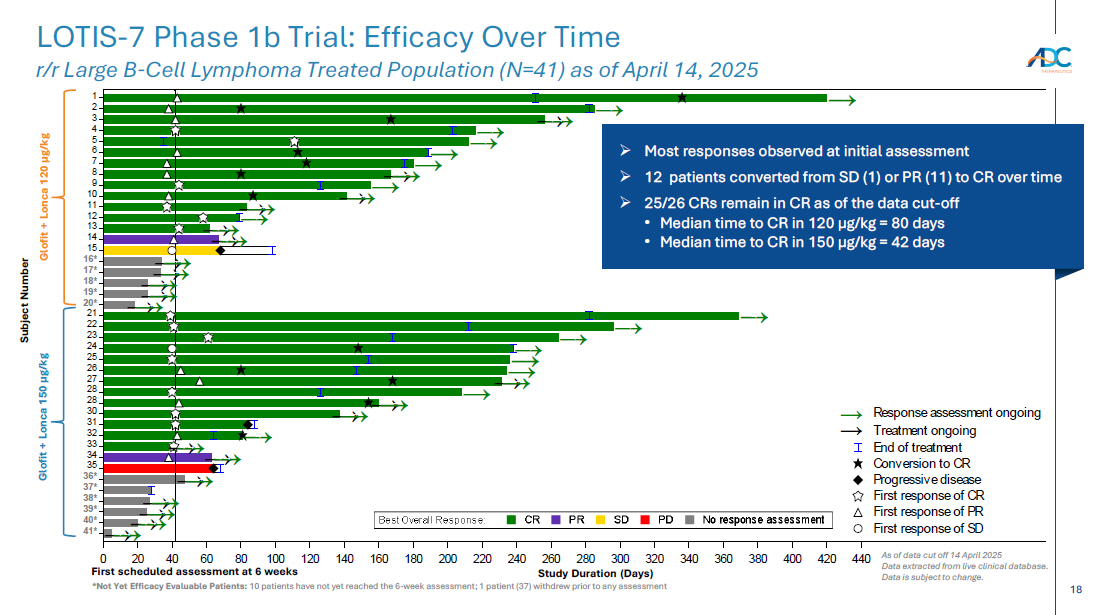
LOTIS - 7 Phase 1b Trial: Overall Response Rate (ORR) r/r Large B - Cell Lymphoma Efficacy Evaluable Population (N=30) as of April 14, 2025 18 → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → → 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 First scheduled assessment at 6 weeks Study Duration (Days) First response of CR First response of PR First response of SD Progressive disease Conversion to CR End of treatment Treatment ongoing Response assessment ongoing First scheduled assessment at 6 weeks → → 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16* 17* 18* 19* 20* 21 22 23 24 25 26 27 28 28 30 31 32 33 34 35 36* 37* 38* 39* 40* 41* Glofit + Lonca 150 µg/kg As of data cut off 14 April 2025 Data extracted from live clinical database. Data is subject to change. LOTIS - 7 Phase 1b Trial: Efficacy Over Time r/r Large B - Cell Lymphoma Treated Population (N=41) as of April 14, 2025 Subject Number Glofit + Lonca 120 µg/kg » Most responses observed at initial assessment » 12 patients converted from SD (1) or PR (11) to CR over time » 25/26 CRs remain in CR as of the data cut - off • Median time to CR in 120 µg/kg = 80 days • Median time to CR in 150 µg/kg = 42 days *Not Yet Efficacy Evaluable Patients: 10 patients have not yet reached the 6 - week assessment; 1 patient (37) withdrew prior to any assessment 19 ^ Data do not include all 2L MZL regimens such as B+O, R - CHOP, R - CVP, or B - R because inclusion based on either front - line data or studies in mixed histologies; 1.
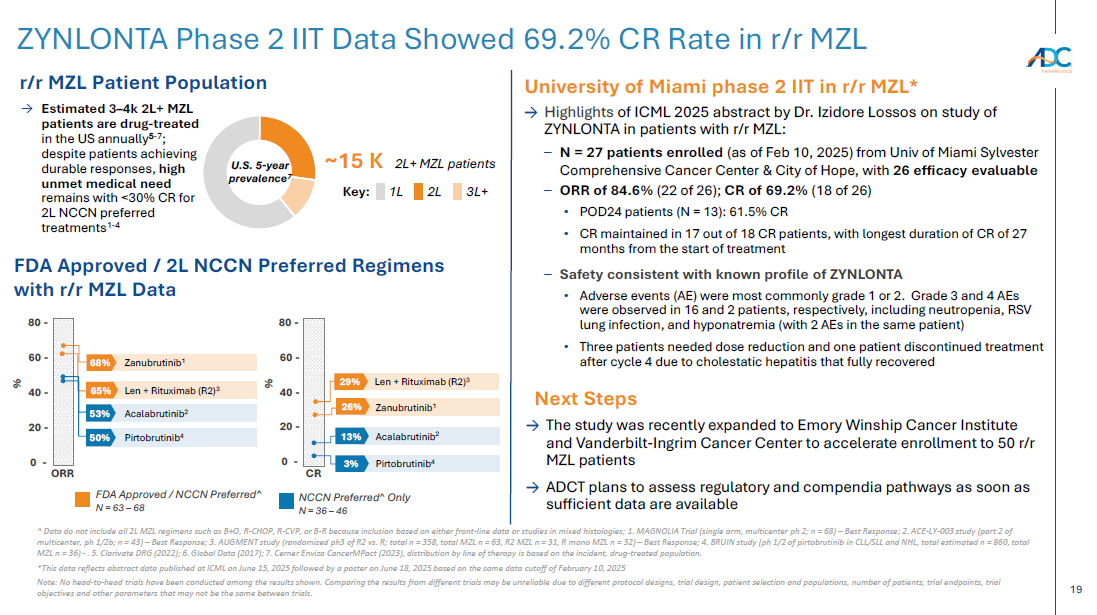
MAGNOLIA Trial (single arm, multicenter ph 2; n = 68) – Best Response; 2. ACE - LY - 003 study (part 2 of multicenter, ph 1/2b; n = 43) – Best Response; 3. AUGMENT study (randomized ph3 of R2 vs. R; total n = 358, total MZL n = 63, R2 MZL n = 31, R mono MZL n = 32) – Best Response; 4. BRUIN study (ph 1/2 of pirtobrutinib in CLL/SLL and NHL, total estimated n = 860, total MZL n = 36) - . 5. Clarivate DRG (2022); 6. Global Data (2017); 7. Cerner Enviza CancerMPact (2023), distribution by line of therapy is based on the incident, drug - treated population. *This data reflects abstract data published at ICML on June 15, 2025 followed by a poster on June 18, 2025 based on the same data cutoff of February 10, 2025 Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unreliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoints, trial objectives and other parameters that may not be the same between trials. ZYNLONTA Phase 2 IIT Data Showed 69.2% CR Rate in r/r MZL O RR % 65% Len + Rituximab (R2) 3 53% Acalabrutinib 2 CR 13% Acalabrutinib 2 3% Pirtobrutinib 4 0 - 0 - % 60 - 68% Zanubrutinib 1 60 - 40 - 40 - 20 - 20 - 50% Pirtobrutinib 4 29% Len + Rituximab (R2) 3 Zanubrutinib 1 prevalence 7 durable responses, high unmet medical need remains with <30% CR for 2L NCCN preferred treatments 1 - 4 ~15 K 2L+ MZL patients Key: 1L 2L 3L+ FDA Approved / 2L NCCN Preferred Regimens with r/r MZL Data 80 - 80 - FDA Approved / NCCN Preferred^ N = 63 – 68 NCCN Preferred^ Only N = 36 – 46 r/r MZL Patient Population → Estimated 3 – 4k 2L+ MZL patients are drug - treated in the US annually 5 - 7 ; despite patients achieving U.S. 5 - year University of Miami phase 2 IIT in r/r MZL* → Highlights of ICML 2025 abstract by Dr. Izidore Lossos on study of ZYNLONTA in patients with r/r MZL: – N = 27 patients enrolled (as of Feb 10, 2025) from Univ of Miami Sylvester Comprehensive Cancer Center & City of Hope, with 26 efficacy evaluable – ORR of 84.6% (22 of 26); CR of 69.2% (18 of 26) • POD24 patients (N = 13): 61.5% CR • CR maintained in 17 out of 18 CR patients, with longest duration of CR of 27 months from the start of treatment – Safety consistent with known profile of ZYNLONTA • Adverse events (AE) were most commonly grade 1 or 2.
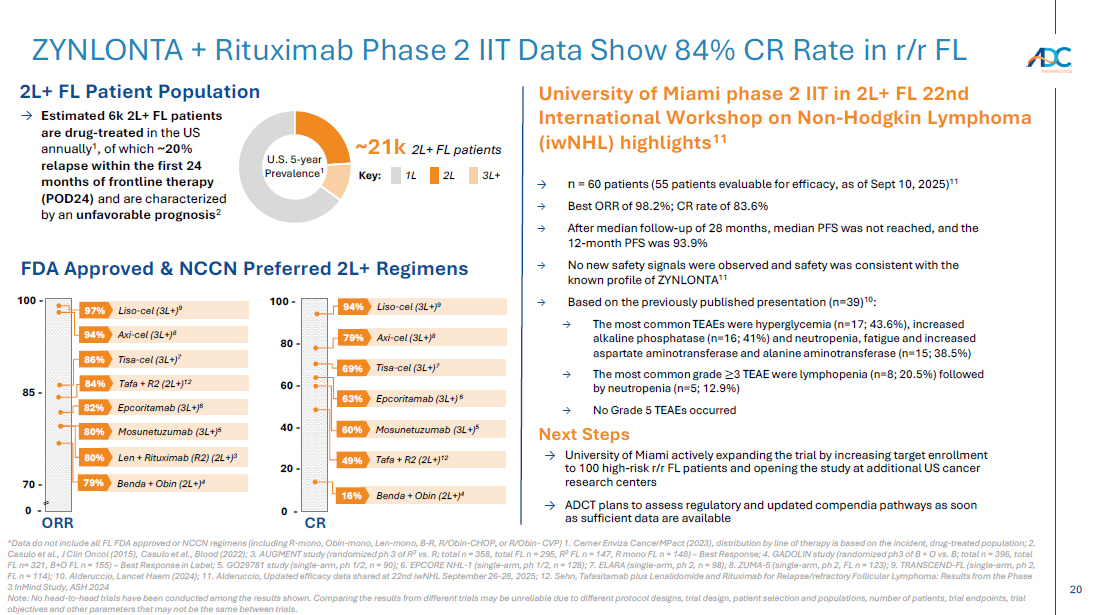
Grade 3 and 4 AEs were observed in 16 and 2 patients, respectively, including neutropenia, RSV lung infection, and hyponatremia (with 2 AEs in the same patient) • Three patients needed dose reduction and one patient discontinued treatment after cycle 4 due to cholestatic hepatitis that fully recovered Next Steps → The study was recently expanded to Emory Winship Cancer Institute and Vanderbilt - Ingrim Cancer Center to accelerate enrollment to 50 r/r MZL patients → ADCT plans to assess regulatory and compendia pathways as soon as sufficient data are available 26% 20 ZYNLONTA + Rituximab Phase 2 IIT Data Show 84% CR Rate in r/r FL U.S. 5 - year Pre v ale n c e 1 ~21k 2L+ FL patients Key: 1L 2L 3L+ 2L+ FL Patient Population → Estimated 6k 2L+ FL patients are drug - treated in the US annually 1 , of which ~20% relapse within the first 24 months of frontline therapy (POD24) and are characterized by an unfavorable prognosis 2 FDA Approved & NCCN Preferred 2L+ Regimens ^Data do not include all FL FDA approved or NCCN regimens (including R - mono, Obin - mono, Len - mono, B - R, R/Obin - CHOP, or R/Obin - CVP) 1. Cerner Enviza CancerMPact (2023), distribution by line of therapy is based on the incident, drug - treated population; 2. Casulo et al., J Clin Oncol (2015), Casulo et al., Blood (2022); 3. AUGMENT study (randomized ph 3 of R 2 vs. R; total n = 358, total FL n = 295, R 2 FL n = 147, R mono FL n = 148) – Best Response; 4. GADOLIN study (randomized ph3 of B + O vs. B; total n = 396, total FL n= 321, B+O FL n = 155) – Best Response in Label; 5. GO29781 study (single - arm, ph 1/2, n = 90); 6. EPCORE NHL - 1 (single - arm, ph 1/2, n = 128); 7. ELARA (single - arm, ph 2, n = 98); 8. ZUMA - 5 (single - arm, ph 2, FL n = 123); 9. TRANSCEND - FL (single - arm, ph 2, FL n = 114); 10. Alderuccio, Lancet Haem (2024); 11. Alderuccio, Updated efficacy data shared at 22nd iwNHL September 26 - 28, 2025; 12. Sehn, Tafasitamab plus Lenalidomide and Rituximab for Relapse/refractory Follicular Lymphoma: Results from the Phase 3 InMind Study, ASH 2024 Note: No head - to - head trials have been conducted among the results shown. Comparing the results from different trials may be unreliable due to different protocol designs, trial design, patient selection and populations, number of patients, trial endpoints, trial objectives and other parameters that may not be the same between trials. 16% Benda + Obin (2L+) 4 80 - 60 - 40 - 20 - 100 - 79% Axi - cel (3L+) 8 94% Liso - cel (3L+) 9 Tisa - cel (3L+) 7 69% 63% Epcoritamab (3L+) 6 0 - 85 - OR R CR 70 - 100 - 94% Axi - cel (3L+) 8 97% Liso - cel (3L+) 9 86% Tisa - cel (3L+) 7 82% Epcoritamab (3L+) 6 80% Mosunetuzumab (3L+) 5 60% Mosunetuzumab (3L+) 5 0 - 84% Tafa + R2 (2L+) 12 49% Tafa + R2 (2L+) 12 79% Benda + Obin (2L+) 4 Len + Rituximab (R2) (2L+) 3 80% University of Miami phase 2 IIT in 2L+ FL 22nd International Workshop on Non - Hodgkin Lymphoma (iwNHL) highlights 11 Next Steps → University of Miami actively expanding the trial by increasing target enrollment to 100 high - risk r/r FL patients and opening the study at additional US cancer research centers → ADCT plans to assess regulatory and updated compendia pathways as soon as sufficient data are available → n = 60 patients (55 patients evaluable for efficacy, as of Sept 10, 2025) 11 → Best ORR of 98.2%; CR rate of 83.6% → After median follow - up of 28 months, median PFS was not reached, and the 12 - month PFS was 93.9% → No new safety signals were observed and safety was consistent with the known profile of ZYNLONTA 11 → Based on the previously published presentation (n=39) 10 : → → → The most common TEAEs were hyperglycemia (n=17; 43.6%), increased alkaline phosphatase (n=16; 41%) and neutropenia, fatigue and increased aspartate aminotransferase and alanine aminotransferase (n=15; 38.5%) The most common grade ≥ 3 TEAE were lymphopenia (n=8; 20.5%) followed by neutropenia (n=5; 12.9%) No Grade 5 TEAEs occurred 21 2L+ Indolent lymphomas $100M - 200M ZYNLONTA U.S. Peak Revenue Potential of $600M - 1B $600M - 1B Peak Revenue in DLBCL & Indolent Lymphomas ZYNLONTA 2L+ ZYNLONTA plus bispecific $500M - 800M L O T I S - 7 2L+ ZYNLONTA plus rituximab $200M - 300M* L O T I S - 5 3L+ Monotherapy CURRENT INDICATION Peak revenue projection assumes both compendia listing and regulatory approval Note: ZYNLONTA Monotherapy is FDA approved under accelerated approval; other potential indications in development *Based on quantitative market research study of 150 physicians Note: The Company does not promote ZYNLONTA for unapproved uses
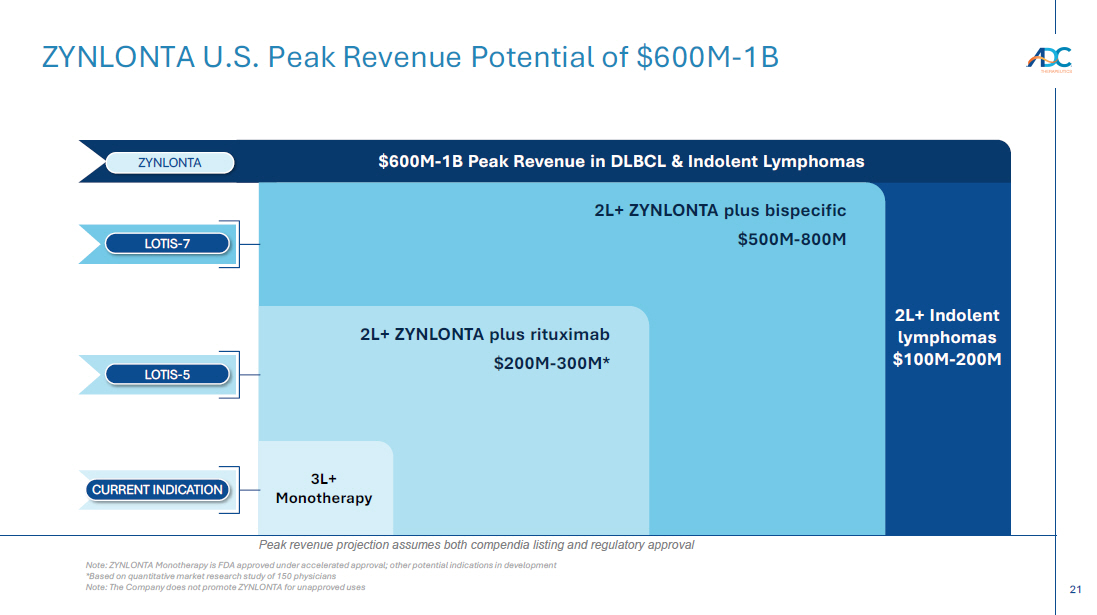
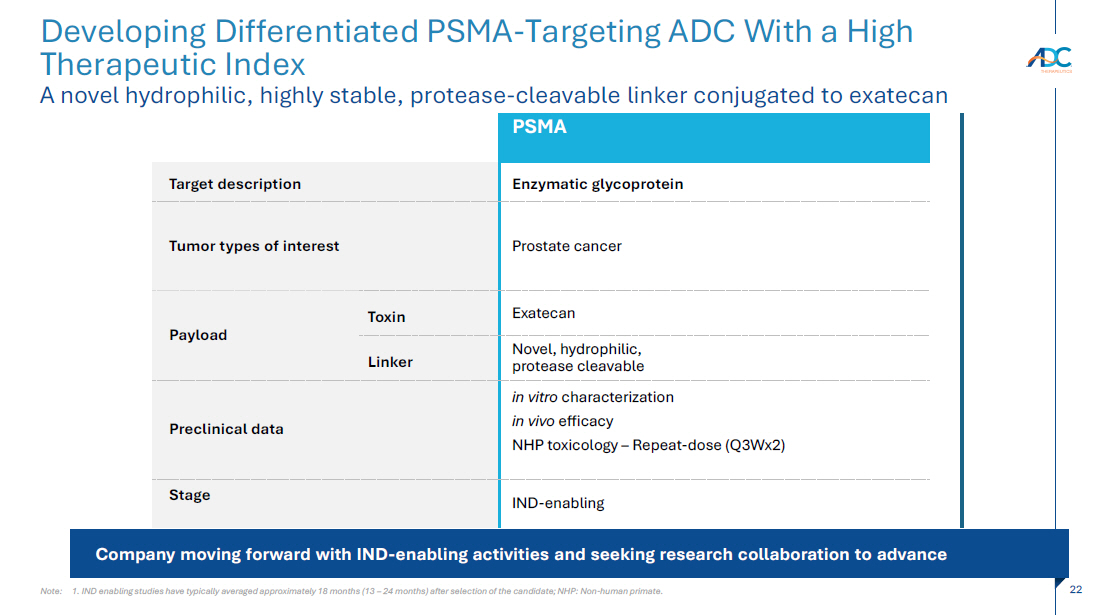
22 N ot e: 1. IND enabling studies have typically averaged approximately 18 months (13 – 24 months) after selection of the candidate; NHP: Non - human primate.
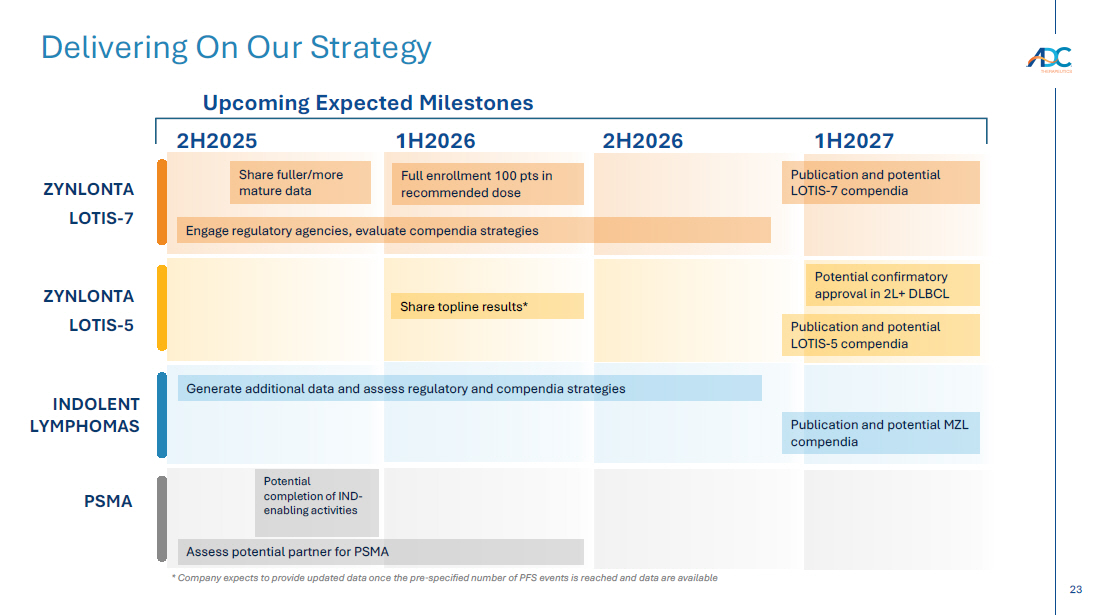
Developing Differentiated PSMA - Targeting ADC With a High Therapeutic Index A novel hydrophilic, highly stable, protease - cleavable linker conjugated to exatecan PSMA Enzymatic glycoprotein Target description Prostate cancer Tumor types of interest Exatecan Toxin Payload Novel, hydrophilic, protease cleavable Linker in vitro characterization in vivo efficacy NHP toxicology – Repeat - dose (Q3Wx2) Preclinical data IND - enabling Stage Company moving forward with IND - enabling activities and seeking research collaboration to advance 23 Delivering On Our Strategy ZY N LO N T A LO T I S - 7 Share fuller/more mature data ZY N LO N T A LO T I S - 5 Engage regulatory agencies, evaluate compendia strategies IN DOLE N T L Y MPHO M AS Generate additional data and assess regulatory and compendia strategies Upcoming Expected Milestones 2H2025 1H2026 2H 2 0 2 6 1H 2 0 2 7 Publication and potential LOTIS - 7 compendia Potential confirmatory approval in 2L+ DLBCL Publication and potential MZL compendia Potential completion of IND - enabling activities Assess potential partner for PSMA * Company expects to provide updated data once the pre - specified number of PFS events is reached and data are available Full enrollment 100 pts in recommended dose PSMA Publication and potential LOTIS - 5 compendia Share topline results* 24 Preliminary Results For the quarter ended September 30, 2025 * Assumes use of minimum liquidity amount required to be maintained under its loan agreement covenants; Note: Financials are preliminary and unaudited and reflect the Company’s estimated financial results.

» Net product revenues from sales of ZYNLONTA expected to be approximately $15.8 million for the third quarter ended September 30, 2025 » Cash and cash equivalents of $234.7 million as of September 30, 2025 26 Anticipated milestones set forth in this chart are subject to further future adjustment.

Thank You
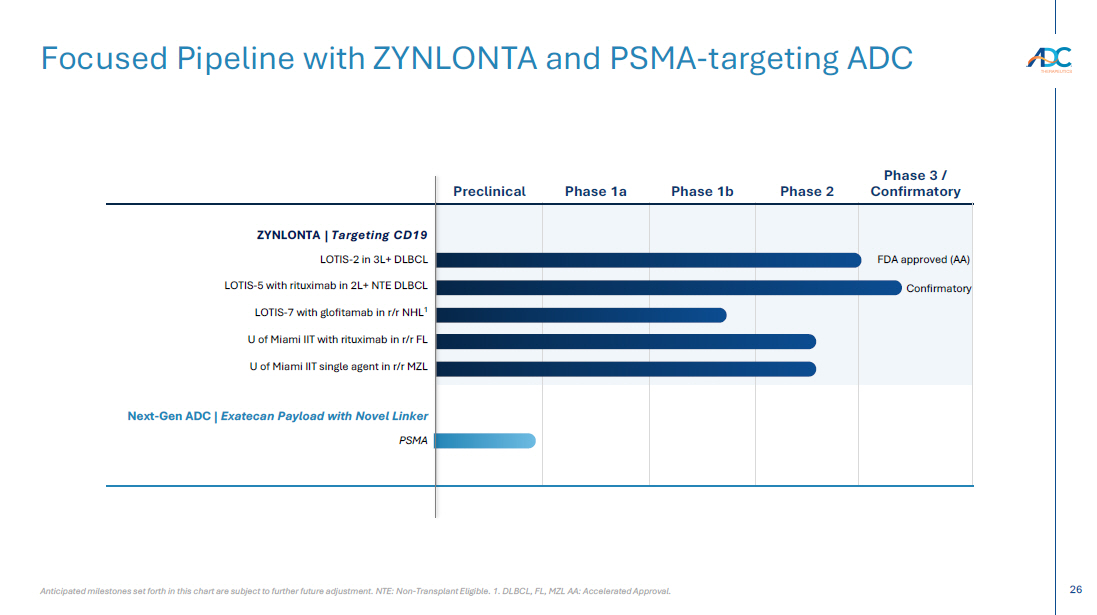
NTE: Non - Transplant Eligible. 1. DLBCL, FL, MZL AA: Accelerated Approval. Focused Pipeline with ZYNLONTA and PSMA - targeting ADC Preclinical Phase 1a Phase 1b Phase 2 Phase 3 / Conf i r m at ory ZYNLONTA | Targeting CD19 LOTIS - 2 in 3L+ DLBCL LOTIS - 5 with rituximab in 2L+ NTE DLBCL LOTIS - 7 with glofitamab in r/r NHL 1 U of Miami IIT with rituximab in r/r FL U of Miami IIT single agent in r/r MZL Next - Gen ADC | Exatecan Payload with Novel Linker P SMA FDA approved (AA) C o n fir mato ry 27 Both Lonca and Glofit Have Demonstrated Durable Complete Responses as Single Agents in Heavily Pretreated 3L+ Patients LOTIS - 2 (Lonca monotherapy) 2 - Yr Follow - up Analysis 1 Glofitamab (monotherapy) 3 - Yr Follow - up Analysis 3 1.
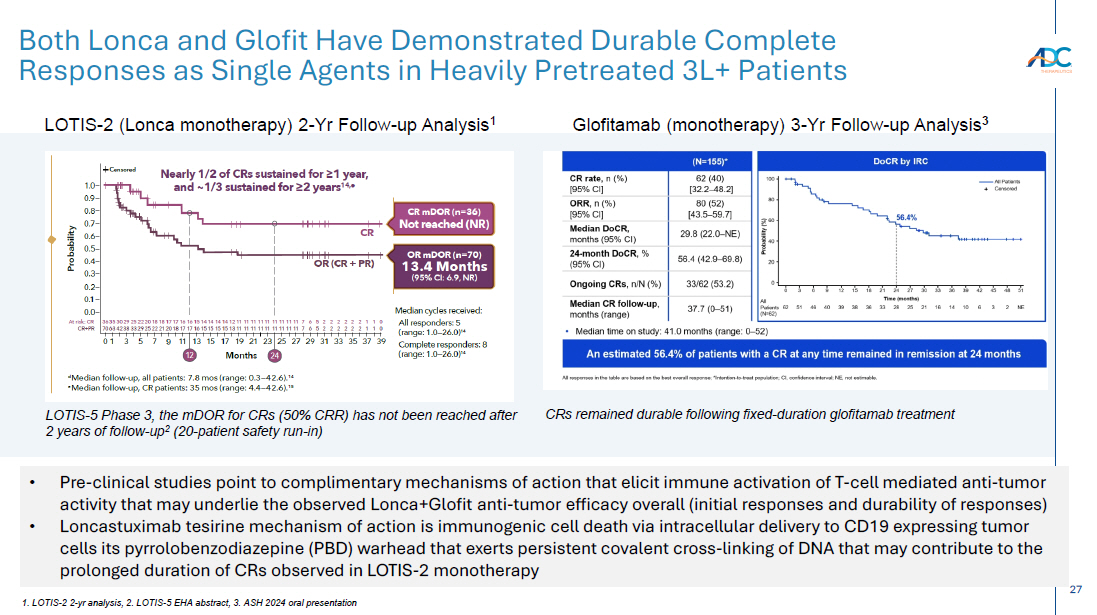
LOTIS - 2 2 - yr analysis, 2. LOTIS - 5 EHA abstract, 3.
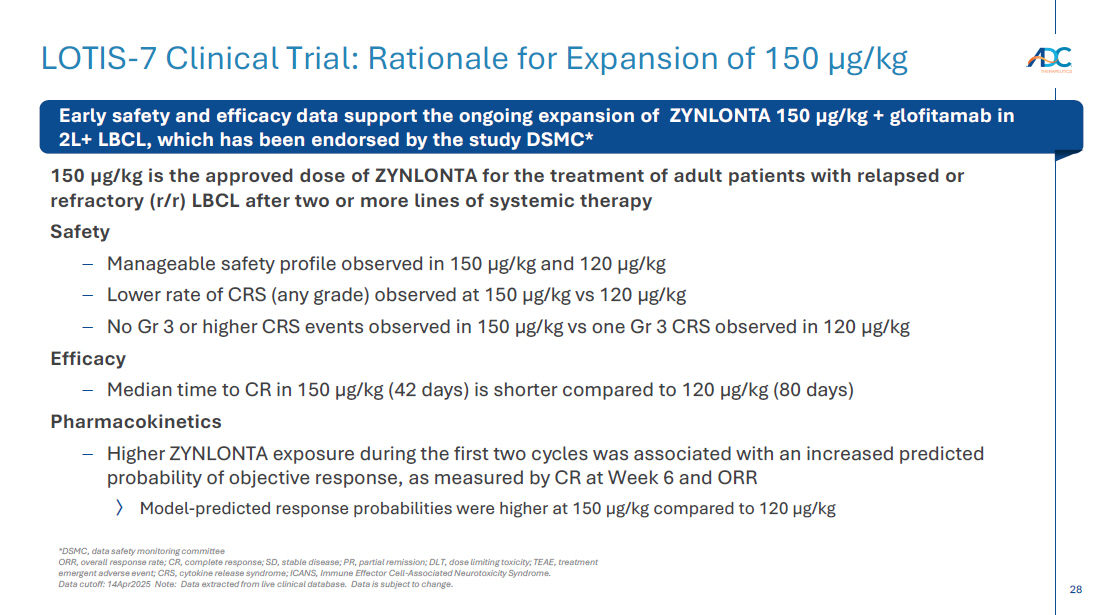
ASH 2024 oral presentation • Pre - clinical studies point to complimentary mechanisms of action that elicit immune activation of T - cell mediated anti - tumor activity that may underlie the observed Lonca+Glofit anti - tumor efficacy overall (initial responses and durability of responses) • Loncastuximab tesirine mechanism of action is immunogenic cell death via intracellular delivery to CD19 expressing tumor cells its pyrrolobenzodiazepine (PBD) warhead that exerts persistent covalent cross - linking of DNA that may contribute to the prolonged duration of CRs observed in LOTIS - 2 monotherapy LOTIS - 5 Phase 3, the mDOR for CRs (50% CRR) has not been reached after 2 years of follow - up 2 (20 - patient safety run - in) CRs remained durable following fixed - duration glofitamab treatment 28 LOTIS - 7 Clinical Trial: Rationale for Expansion of 150 µg/kg Early safety and efficacy data support the ongoing expansion of ZYNLONTA 150 µg/kg + glofitamab in 2L+ LBCL, which has been endorsed by the study DSMC* 150 µg/kg is the approved dose of ZYNLONTA for the treatment of adult patients with relapsed or refractory (r/r) LBCL after two or more lines of systemic therapy Safety – Manageable safety profile observed in 150 µg/kg and 120 µg/kg – Lower rate of CRS (any grade) observed at 150 µg/kg vs 120 µg/kg – No Gr 3 or higher CRS events observed in 150 µg/kg vs one Gr 3 CRS observed in 120 µg/kg Efficacy – Median time to CR in 150 µg/kg (42 days) is shorter compared to 120 µg/kg (80 days) Pharmacokinetics – Higher ZYNLONTA exposure during the first two cycles was associated with an increased predicted probability of objective response, as measured by CR at Week 6 and ORR ࠒ Model - predicted response probabilities were higher at 150 µg/kg compared to 120 µg/kg *DSMC, data safety monitoring committee ORR, overall response rate; CR, complete response; SD, stable disease; PR, partial remission; DLT, dose limiting toxicity; TEAE, treatment emergent adverse event; CRS, cytokine release syndrome; ICANS, Immune Effector Cell - Associated Neurotoxicity Syndrome. Data cutoff: 14Apr2025 Note: Data extracted from live clinical database. Data is subject to change.