false2024FYIDEXX LABORATORIES INC /DE0000874716P1YP5Y1111111111111111P2YP2Yhttp://fasb.org/us-gaap/2024#AccountsPayableCurrenthttp://fasb.org/us-gaap/2024#AccountsPayableCurrenthttp://fasb.org/us-gaap/2024#AccruedLiabilitiesCurrenthttp://fasb.org/us-gaap/2024#AccruedLiabilitiesCurrentiso4217:USDxbrli:sharesiso4217:USDxbrli:sharesxbrli:pureidxx:arrangementidxx:stageiso4217:EURidxx:amendementidxx:segment00008747162024-01-012024-12-3100008747162024-06-3000008747162025-02-120000874716idxx:JonathanW.AyersMember2024-10-012024-12-310000874716idxx:JonathanW.AyersMember2024-01-012024-12-3100008747162024-10-012024-12-3100008747162024-12-3100008747162023-12-310000874716us-gaap:ProductMember2024-01-012024-12-310000874716us-gaap:ProductMember2023-01-012023-12-310000874716us-gaap:ProductMember2022-01-012022-12-310000874716us-gaap:ServiceMember2024-01-012024-12-310000874716us-gaap:ServiceMember2023-01-012023-12-310000874716us-gaap:ServiceMember2022-01-012022-12-3100008747162023-01-012023-12-3100008747162022-01-012022-12-3100008747162021-12-3100008747162022-12-310000874716us-gaap:CommonStockMember2021-12-310000874716us-gaap:AdditionalPaidInCapitalMember2021-12-310000874716idxx:DeferredStockUnitsMember2021-12-310000874716us-gaap:RetainedEarningsMember2021-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-310000874716us-gaap:TreasuryStockCommonMember2021-12-310000874716us-gaap:RetainedEarningsMember2022-01-012022-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000874716us-gaap:TreasuryStockCommonMember2022-01-012022-12-310000874716us-gaap:CommonStockMember2022-01-012022-12-310000874716us-gaap:AdditionalPaidInCapitalMember2022-01-012022-12-310000874716idxx:DeferredStockUnitsMember2022-01-012022-12-310000874716us-gaap:CommonStockMember2022-12-310000874716us-gaap:AdditionalPaidInCapitalMember2022-12-310000874716idxx:DeferredStockUnitsMember2022-12-310000874716us-gaap:RetainedEarningsMember2022-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2022-12-310000874716us-gaap:TreasuryStockCommonMember2022-12-310000874716us-gaap:RetainedEarningsMember2023-01-012023-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000874716us-gaap:TreasuryStockCommonMember2023-01-012023-12-310000874716us-gaap:CommonStockMember2023-01-012023-12-310000874716us-gaap:AdditionalPaidInCapitalMember2023-01-012023-12-310000874716idxx:DeferredStockUnitsMember2023-01-012023-12-310000874716us-gaap:CommonStockMember2023-12-310000874716us-gaap:AdditionalPaidInCapitalMember2023-12-310000874716idxx:DeferredStockUnitsMember2023-12-310000874716us-gaap:RetainedEarningsMember2023-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2023-12-310000874716us-gaap:TreasuryStockCommonMember2023-12-310000874716us-gaap:RetainedEarningsMember2024-01-012024-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310000874716us-gaap:TreasuryStockCommonMember2024-01-012024-12-310000874716us-gaap:CommonStockMember2024-01-012024-12-310000874716us-gaap:AdditionalPaidInCapitalMember2024-01-012024-12-310000874716idxx:DeferredStockUnitsMember2024-01-012024-12-310000874716us-gaap:CommonStockMember2024-12-310000874716us-gaap:AdditionalPaidInCapitalMember2024-12-310000874716idxx:DeferredStockUnitsMember2024-12-310000874716us-gaap:RetainedEarningsMember2024-12-310000874716us-gaap:AccumulatedOtherComprehensiveIncomeMember2024-12-310000874716us-gaap:TreasuryStockCommonMember2024-12-310000874716srt:MinimumMember2024-01-012024-12-310000874716srt:MaximumMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsRecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsRecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsRecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VetLabConsumablesMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VetLabConsumablesMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VetLabConsumablesMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RapidAssayProductsMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RapidAssayProductsMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RapidAssayProductsMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:ReferenceLaboratoryDiagnosticAndConsultingServicesMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:ReferenceLaboratoryDiagnosticAndConsultingServicesMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:ReferenceLaboratoryDiagnosticAndConsultingServicesMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsServiceAndAccessoriesMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsServiceAndAccessoriesMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticsServiceAndAccessoriesMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticCapitalInstrumentsMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticCapitalInstrumentsMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CAGDiagnosticCapitalInstrumentsMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VeterinarySoftwareServicesAndDiagnosticImagingSystemsMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VeterinarySoftwareServicesAndDiagnosticImagingSystemsMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:VeterinarySoftwareServicesAndDiagnosticImagingSystemsMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:RecurringRevenueMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:SystemsAndHardwareMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:SystemsAndHardwareMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:SystemsAndHardwareMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2022-01-012022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2022-01-012022-12-310000874716us-gaap:CorporateNonSegmentMember2024-01-012024-12-310000874716us-gaap:CorporateNonSegmentMember2023-01-012023-12-310000874716us-gaap:CorporateNonSegmentMember2022-01-012022-12-310000874716country:US2024-01-012024-12-310000874716country:US2023-01-012023-12-310000874716country:US2022-01-012022-12-310000874716country:CA2024-01-012024-12-310000874716country:CA2023-01-012023-12-310000874716country:CA2022-01-012022-12-310000874716idxx:LatinAmericaAndCaribbeanMember2024-01-012024-12-310000874716idxx:LatinAmericaAndCaribbeanMember2023-01-012023-12-310000874716idxx:LatinAmericaAndCaribbeanMember2022-01-012022-12-310000874716srt:AmericasMember2024-01-012024-12-310000874716srt:AmericasMember2023-01-012023-12-310000874716srt:AmericasMember2022-01-012022-12-310000874716country:DE2024-01-012024-12-310000874716country:DE2023-01-012023-12-310000874716country:DE2022-01-012022-12-310000874716country:GB2024-01-012024-12-310000874716country:GB2023-01-012023-12-310000874716country:GB2022-01-012022-12-310000874716country:FR2024-01-012024-12-310000874716country:FR2023-01-012023-12-310000874716country:FR2022-01-012022-12-310000874716country:ES2024-01-012024-12-310000874716country:ES2023-01-012023-12-310000874716country:ES2022-01-012022-12-310000874716country:IT2024-01-012024-12-310000874716country:IT2023-01-012023-12-310000874716country:IT2022-01-012022-12-310000874716country:CH2024-01-012024-12-310000874716country:CH2023-01-012023-12-310000874716country:CH2022-01-012022-12-310000874716country:NL2024-01-012024-12-310000874716country:NL2023-01-012023-12-310000874716country:NL2022-01-012022-12-310000874716idxx:EuropetheMiddleEastandAfricaOtherMember2024-01-012024-12-310000874716idxx:EuropetheMiddleEastandAfricaOtherMember2023-01-012023-12-310000874716idxx:EuropetheMiddleEastandAfricaOtherMember2022-01-012022-12-310000874716us-gaap:EMEAMember2024-01-012024-12-310000874716us-gaap:EMEAMember2023-01-012023-12-310000874716us-gaap:EMEAMember2022-01-012022-12-310000874716country:AU2024-01-012024-12-310000874716country:AU2023-01-012023-12-310000874716country:AU2022-01-012022-12-310000874716country:JP2024-01-012024-12-310000874716country:JP2023-01-012023-12-310000874716country:JP2022-01-012022-12-310000874716country:CN2024-01-012024-12-310000874716country:CN2023-01-012023-12-310000874716country:CN2022-01-012022-12-310000874716idxx:AsiaPacificOtherMember2024-01-012024-12-310000874716idxx:AsiaPacificOtherMember2023-01-012023-12-310000874716idxx:AsiaPacificOtherMember2022-01-012022-12-310000874716srt:AsiaPacificMember2024-01-012024-12-310000874716srt:AsiaPacificMember2023-01-012023-12-310000874716srt:AsiaPacificMember2022-01-012022-12-310000874716idxx:ExtendedWarrantiesAndPostContractSupportRevenueMembersrt:MinimumMember2024-01-012024-12-310000874716idxx:ExtendedWarrantiesAndPostContractSupportRevenueMembersrt:MaximumMember2024-01-012024-12-310000874716idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-01-012024-12-310000874716idxx:FreeOrDiscountedInstrumentsAndSystemsMember2023-12-310000874716idxx:FreeOrDiscountedInstrumentsAndSystemsMember2024-01-012024-12-310000874716idxx:FreeOrDiscountedInstrumentsAndSystemsMember2024-12-310000874716idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2023-12-310000874716idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-01-012024-12-310000874716idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-310000874716idxx:RebateAndUpFrontConsiderationsArrangementsMember2023-12-310000874716idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-01-012024-12-310000874716idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162025-01-01idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162026-01-01idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162027-01-01idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162028-01-01idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162029-01-01idxx:RebateAndUpFrontConsiderationsArrangementsMember2024-12-3100008747162025-01-01idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-3100008747162026-01-01idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-3100008747162027-01-01idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-3100008747162028-01-01idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-3100008747162029-01-01idxx:CustomerCommitmentArrangementsMultiYearArrangementsMember2024-12-310000874716idxx:SalesTypeReagentRentalArrangementsMember2023-12-310000874716idxx:SalesTypeReagentRentalArrangementsMember2024-01-012024-12-310000874716idxx:SalesTypeReagentRentalArrangementsMember2024-12-310000874716idxx:OperatingTypeReagentRentalArrangementsMember2024-01-012024-12-310000874716idxx:OperatingTypeReagentRentalArrangementsMember2023-01-012023-12-310000874716idxx:ReagentRentalArrangementsMember2024-12-3100008747162025-01-01idxx:ReagentRentalArrangementsMember2024-12-3100008747162026-01-01idxx:ReagentRentalArrangementsMember2024-12-3100008747162027-01-01idxx:ReagentRentalArrangementsMember2024-12-3100008747162028-01-01idxx:ReagentRentalArrangementsMember2024-12-3100008747162029-01-01idxx:ReagentRentalArrangementsMember2024-12-310000874716idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2023-12-310000874716idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-3100008747162025-01-01idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-3100008747162026-01-01idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-3100008747162027-01-01idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-3100008747162028-01-01idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-3100008747162029-01-01idxx:ExtendedWarrantiesAndPostContractSupportRevenueMember2024-12-310000874716srt:MinimumMember2024-12-310000874716srt:MaximumMember2024-12-310000874716idxx:PerpetualIntellectualPropertyLicenseMember2024-01-012024-12-310000874716idxx:PerpetualIntellectualPropertyLicenseMember2024-12-310000874716idxx:LicenseIntellectualPropertyRightsMember2022-01-012022-12-310000874716idxx:LicenseIntellectualPropertyRightsMember2022-12-310000874716idxx:LicenseIntellectualPropertyRightsMember2023-01-012023-03-310000874716idxx:PerpetualIntellectualPropertyLicenseMember2022-01-012022-12-310000874716idxx:PerpetualIntellectualPropertyLicenseMember2022-12-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMember2024-01-012024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMember2024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMemberus-gaap:TechnologyBasedIntangibleAssetsMember2024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMemberus-gaap:CustomerRelationshipsMember2024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMemberus-gaap:NoncompeteAgreementsMember2024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMemberus-gaap:TrademarksMember2024-03-310000874716country:USidxx:PrivatelyOwnedSoftwareAndDataPlatformBusinessMemberus-gaap:OtherIntangibleAssetsMember2024-03-310000874716country:CAidxx:InternationalWaterTestingCompanyMember2022-07-012022-09-300000874716country:CAidxx:InternationalWaterTestingCompanyMember2022-09-300000874716country:CAidxx:InternationalWaterTestingCompanyMemberus-gaap:TechnologyBasedIntangibleAssetsMember2022-09-300000874716country:CAidxx:InternationalWaterTestingCompanyMemberus-gaap:CustomerRelationshipsMember2022-09-300000874716country:CAidxx:InternationalWaterTestingCompanyMemberus-gaap:OtherIntangibleAssetsMember2022-09-300000874716us-gaap:PerformanceSharesMember2024-01-012024-12-310000874716idxx:TwoThousandEighteenStockIncentivePlanMember2018-12-310000874716idxx:StockOptionsAndStockAppreciationRightsMember2018-01-012018-12-310000874716idxx:AwardsOtherThanStockOptionsAndStockAppreciationRightsMember2018-01-012018-12-310000874716idxx:TwoThousandEighteenStockIncentivePlanMember2024-12-310000874716us-gaap:CostOfSalesMember2024-01-012024-12-310000874716us-gaap:CostOfSalesMember2023-01-012023-12-310000874716us-gaap:CostOfSalesMember2022-01-012022-12-310000874716idxx:OperatingExpensesMember2024-01-012024-12-310000874716idxx:OperatingExpensesMember2023-01-012023-12-310000874716idxx:OperatingExpensesMember2022-01-012022-12-310000874716idxx:StockOptionsGrantedToEmployeesWithRatableVestingMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2019-01-012019-12-310000874716idxx:StockOptionsGrantedToEmployeesWithRatableVestingMemberus-gaap:ShareBasedCompensationAwardTrancheTwoMember2019-01-012019-12-310000874716us-gaap:EmployeeStockOptionMember2019-01-012019-12-310000874716us-gaap:EmployeeStockOptionMember2024-01-012024-12-310000874716us-gaap:EmployeeStockOptionMember2023-01-012023-12-310000874716us-gaap:EmployeeStockOptionMember2022-01-012022-12-310000874716idxx:RestrictedStockUnitsGrantedToEmployeesWithRatableVestingMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2019-01-012019-12-310000874716idxx:RestrictedStockUnitsGrantedToEmployeesWithRatableVestingMemberus-gaap:ShareBasedCompensationAwardTrancheTwoMember2019-01-012019-12-310000874716idxx:RestrictedStockUnitsGrantedToEmployeesWithRatableVestingMemberus-gaap:ShareBasedCompensationAwardTrancheThreeMember2019-01-012019-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2023-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2024-01-012024-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2024-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2023-01-012023-12-310000874716us-gaap:RestrictedStockUnitsRSUMember2022-01-012022-12-310000874716us-gaap:PerformanceSharesMember2024-02-142024-02-140000874716us-gaap:PerformanceSharesMember2023-12-310000874716us-gaap:PerformanceSharesMember2024-12-310000874716us-gaap:PerformanceSharesMember2023-01-012023-12-310000874716us-gaap:PerformanceSharesMember2022-01-012022-12-3100008747162014-01-012014-12-310000874716idxx:DeferredStockUnitsMember2024-12-310000874716idxx:DeferredStockUnitsMember2023-12-310000874716idxx:DeferredStockUnitsMember2024-01-012024-12-310000874716idxx:NineteenNinetySevenEmployeeStockPurchasePlanMember2024-12-310000874716idxx:NineteenNinetySevenEmployeeStockPurchasePlanMember2024-01-012024-12-310000874716idxx:NineteenNinetySevenEmployeeStockPurchasePlanMember2023-01-012023-12-310000874716idxx:NineteenNinetySevenEmployeeStockPurchasePlanMember2022-01-012022-12-310000874716idxx:ReagentRentalArrangementsMember2024-01-012024-12-310000874716idxx:ReagentRentalArrangementsMember2023-01-012023-12-310000874716idxx:ReagentRentalArrangementsMember2022-01-012022-12-310000874716srt:MinimumMemberus-gaap:BuildingAndBuildingImprovementsMember2024-12-310000874716srt:MaximumMemberus-gaap:BuildingAndBuildingImprovementsMember2024-12-310000874716srt:MinimumMemberus-gaap:MachineryAndEquipmentMember2024-12-310000874716srt:MaximumMemberus-gaap:MachineryAndEquipmentMember2024-12-310000874716srt:MinimumMemberus-gaap:OfficeEquipmentMember2024-12-310000874716srt:MaximumMemberus-gaap:OfficeEquipmentMember2024-12-310000874716srt:MinimumMemberidxx:ComputerHardwareAndSoftwareMember2024-12-310000874716srt:MaximumMemberidxx:ComputerHardwareAndSoftwareMember2024-12-310000874716us-gaap:LandAndLandImprovementsMember2024-12-310000874716us-gaap:LandAndLandImprovementsMember2023-12-310000874716us-gaap:BuildingAndBuildingImprovementsMember2024-12-310000874716us-gaap:BuildingAndBuildingImprovementsMember2023-12-310000874716us-gaap:LeaseholdImprovementsMember2024-12-310000874716us-gaap:LeaseholdImprovementsMember2023-12-310000874716us-gaap:MachineryAndEquipmentMember2024-12-310000874716us-gaap:MachineryAndEquipmentMember2023-12-310000874716us-gaap:OfficeEquipmentMember2024-12-310000874716us-gaap:OfficeEquipmentMember2023-12-310000874716idxx:ComputerHardwareAndSoftwareMember2024-12-310000874716idxx:ComputerHardwareAndSoftwareMember2023-12-310000874716us-gaap:ConstructionInProgressMember2024-12-310000874716us-gaap:ConstructionInProgressMember2023-12-310000874716idxx:SoftwareDevelopedForInternalUseMember2024-01-012024-12-310000874716idxx:SoftwareDevelopedForInternalUseMember2023-01-012023-12-310000874716idxx:SoftwareDevelopedForInternalUseMember2022-01-012022-12-310000874716us-gaap:IntellectualPropertyMember2024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2021-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2021-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2021-12-310000874716us-gaap:CorporateNonSegmentMember2021-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2022-12-310000874716us-gaap:CorporateNonSegmentMember2022-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2023-12-310000874716us-gaap:CorporateNonSegmentMember2023-12-310000874716us-gaap:OperatingSegmentsMemberidxx:CompanionAnimalGroupSegmentMember2024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:WaterSegmentMember2024-12-310000874716us-gaap:OperatingSegmentsMemberidxx:LivestockAndPoultryDiagnosticsSegmentMember2024-12-310000874716us-gaap:CorporateNonSegmentMember2024-12-310000874716us-gaap:CustomerRelatedIntangibleAssetsMembersrt:MinimumMember2024-12-310000874716us-gaap:CustomerRelatedIntangibleAssetsMembersrt:MaximumMember2024-12-310000874716idxx:ProductRightsMembersrt:MinimumMember2024-12-310000874716idxx:ProductRightsMembersrt:MaximumMember2024-12-310000874716us-gaap:NoncompeteAgreementsMembersrt:MinimumMember2024-12-310000874716us-gaap:NoncompeteAgreementsMembersrt:MaximumMember2024-12-310000874716us-gaap:CustomerRelatedIntangibleAssetsMember2024-12-310000874716us-gaap:CustomerRelatedIntangibleAssetsMember2023-12-310000874716idxx:ProductRightsMember2024-12-310000874716idxx:ProductRightsMember2023-12-310000874716us-gaap:NoncompeteAgreementsMember2024-12-310000874716us-gaap:NoncompeteAgreementsMember2023-12-310000874716us-gaap:RevolvingCreditFacilityMemberus-gaap:LineOfCreditMember2022-10-202022-10-200000874716us-gaap:RevolvingCreditFacilityMemberus-gaap:LineOfCreditMember2022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMember2022-10-200000874716us-gaap:LineOfCreditMember2022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberus-gaap:PrimeRateMembersrt:MinimumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberus-gaap:PrimeRateMembersrt:MaximumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberus-gaap:SecuredOvernightFinancingRateSofrMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberus-gaap:SecuredOvernightFinancingRateSofrMembersrt:MinimumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberus-gaap:SecuredOvernightFinancingRateSofrMembersrt:MaximumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberidxx:DailySecuredOvernightFinancingRateSOFRMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberidxx:DailySecuredOvernightFinancingRateSOFRMembersrt:MinimumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMemberidxx:DailySecuredOvernightFinancingRateSOFRMembersrt:MaximumMember2022-10-202022-10-200000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMember2023-03-310000874716srt:MinimumMemberidxx:RevolvingCreditFacilityIndividualBorrowingsMember2022-10-202022-10-200000874716srt:MaximumMemberidxx:RevolvingCreditFacilityIndividualBorrowingsMember2022-10-202022-10-200000874716us-gaap:RevolvingCreditFacilityMember2024-12-310000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMember2024-12-310000874716us-gaap:RevolvingCreditFacilityMember2023-12-310000874716us-gaap:SecuredDebtMemberus-gaap:LineOfCreditMember2023-12-310000874716us-gaap:RevolvingCreditFacilityMember2024-01-012024-12-310000874716idxx:A2025SeriesBNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:A2026SeniorNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:A2025SeriesCNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:Prudential2030SeriesDNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:A2027SeriesBNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:A2029SeriesCNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:MetLife2030SeriesDNotesMemberus-gaap:SeniorNotesMember2024-12-310000874716idxx:A2023SeriesANotesAnd2025SeriesBNotesMemberus-gaap:SeniorNotesMember2013-12-310000874716idxx:A2023SeriesANotesMemberus-gaap:SeniorNotesMember2013-12-310000874716idxx:A2025SeriesBNotesMemberus-gaap:SeniorNotesMember2013-12-310000874716idxx:A2023SeriesANotesMemberus-gaap:SeniorNotesMember2023-12-012023-12-310000874716idxx:A2026SeniorNotesMemberus-gaap:SeniorNotesMember2014-09-300000874716idxx:NYLife2013NoteAgreementAndTheNYLife2014NoteAgreementMemberus-gaap:SeniorNotesMember2020-04-100000874716idxx:A2021SeriesANotesAnd2024SeriesBNotesMemberus-gaap:SeniorNotesMember2014-07-310000874716idxx:A2021SeriesANotesMemberus-gaap:SeniorNotesMember2014-07-310000874716idxx:A2024SeriesBNotesMemberus-gaap:SeniorNotesMember2014-07-310000874716idxx:A2021SeriesANotesMemberus-gaap:SeniorNotesMember2021-07-012021-07-310000874716idxx:A2024SeriesBNotesMemberus-gaap:SeniorNotesMember2024-07-012024-07-310000874716idxx:A2025SeriesCNotesMemberus-gaap:SeniorNotesMember2015-06-300000874716idxx:Prudential2030SeriesDNotesMemberus-gaap:SeniorNotesMember2020-04-100000874716idxx:Prudential2030SeriesDNotesMemberus-gaap:SeniorNotesMember2020-04-140000874716idxx:A2022SeriesANotesand2027SeriesBNotesMemberus-gaap:SeniorNotesMember2014-12-190000874716idxx:A2022SeriesANotesMemberus-gaap:SeniorNotesMember2014-12-190000874716idxx:A2022SeriesANotesMemberus-gaap:SeniorNotesMember2014-12-192014-12-190000874716idxx:A2027SeriesBNotesMemberus-gaap:SeniorNotesMember2014-12-190000874716idxx:A2027SeriesBNotesMemberus-gaap:SeniorNotesMember2014-12-192014-12-190000874716idxx:A2022SeriesANotesMemberus-gaap:SeniorNotesMember2022-02-012022-02-280000874716idxx:A2029SeriesCNotesMemberus-gaap:SeniorNotesMember2019-03-140000874716idxx:MetLife2030SeriesDNotesMemberus-gaap:SeniorNotesMember2020-03-220000874716idxx:MetLife2030SeriesDNotesMemberus-gaap:SeniorNotesMember2020-03-230000874716idxx:MetLife2030SeriesDNotesMemberus-gaap:SeniorNotesMember2020-04-020000874716us-gaap:SeniorNotesMember2024-01-012024-12-310000874716us-gaap:StateAndLocalJurisdictionMember2024-12-310000874716us-gaap:EmployeeStockOptionMember2024-01-012024-12-310000874716us-gaap:EmployeeStockOptionMember2023-01-012023-12-310000874716us-gaap:EmployeeStockOptionMember2022-01-012022-12-310000874716us-gaap:StockCompensationPlanMember2024-01-012024-12-310000874716us-gaap:StockCompensationPlanMember2023-01-012023-12-310000874716us-gaap:StockCompensationPlanMember2022-01-012022-12-3100008747162004-01-012020-06-3000008747162024-04-012024-06-300000874716us-gaap:OperatingSegmentsMember2024-01-012024-12-310000874716us-gaap:MaterialReconcilingItemsMember2024-01-012024-12-310000874716us-gaap:OperatingSegmentsMember2023-01-012023-12-310000874716us-gaap:MaterialReconcilingItemsMember2023-01-012023-12-310000874716us-gaap:OperatingSegmentsMember2022-01-012022-12-310000874716us-gaap:MaterialReconcilingItemsMember2022-01-012022-12-310000874716country:US2024-12-310000874716country:US2023-12-310000874716country:BR2024-12-310000874716country:BR2023-12-310000874716country:CA2024-12-310000874716country:CA2023-12-310000874716srt:AmericasMember2024-12-310000874716srt:AmericasMember2023-12-310000874716country:DE2024-12-310000874716country:DE2023-12-310000874716country:CH2024-12-310000874716country:CH2023-12-310000874716country:GB2024-12-310000874716country:GB2023-12-310000874716country:NL2024-12-310000874716country:NL2023-12-310000874716country:FR2024-12-310000874716country:FR2023-12-310000874716idxx:EuropetheMiddleEastandAfricaOtherMember2024-12-310000874716idxx:EuropetheMiddleEastandAfricaOtherMember2023-12-310000874716us-gaap:EMEAMember2024-12-310000874716us-gaap:EMEAMember2023-12-310000874716country:AU2024-12-310000874716country:AU2023-12-310000874716country:JP2024-12-310000874716country:JP2023-12-310000874716idxx:OtherAsiaPacificMember2024-12-310000874716idxx:OtherAsiaPacificMember2023-12-310000874716srt:AsiaPacificMember2024-12-310000874716srt:AsiaPacificMember2023-12-310000874716us-gaap:EstimateOfFairValueFairValueDisclosureMember2024-12-310000874716us-gaap:CarryingReportedAmountFairValueDisclosureMember2024-12-310000874716us-gaap:EstimateOfFairValueFairValueDisclosureMember2023-12-310000874716us-gaap:CarryingReportedAmountFairValueDisclosureMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2024-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2024-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2024-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2024-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2024-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2024-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2024-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2024-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2024-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMember2024-12-310000874716us-gaap:FairValueMeasurementsRecurringMember2024-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:MoneyMarketFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:EquityMutualFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:EquityMutualFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:EquityMutualFundsMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberidxx:EquityMutualFundsMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CrossCurrencyInterestRateContractMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberus-gaap:InterestRateSwapMember2023-12-310000874716us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:DeferredCompensationMember2023-12-310000874716us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:DeferredCompensationMember2023-12-310000874716us-gaap:FairValueInputsLevel3Memberus-gaap:FairValueMeasurementsRecurringMemberidxx:DeferredCompensationMember2023-12-310000874716us-gaap:FairValueMeasurementsRecurringMemberidxx:DeferredCompensationMember2023-12-310000874716srt:MaximumMemberus-gaap:MoneyMarketFundsMember2024-01-012024-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:SecuredDebtMember2023-03-310000874716us-gaap:ForeignExchangeContractMember2024-01-012024-12-310000874716us-gaap:InterestRateSwapMember2024-01-012024-12-310000874716us-gaap:InterestRateSwapMember2023-03-310000874716us-gaap:ForeignExchangeContractMembersrt:MinimumMember2024-01-012024-12-310000874716us-gaap:ForeignExchangeContractMembersrt:MaximumMember2024-01-012024-12-310000874716us-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2024-01-012024-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-01-012023-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-01-012022-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:DesignatedAsHedgingInstrumentMember2024-01-012024-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-01-012023-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:DesignatedAsHedgingInstrumentMember2022-01-012022-12-310000874716idxx:SeriesCSeniorNoteMember2015-06-300000874716idxx:CrossCurrencyInterestRateContract1Memberus-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716idxx:CrossCurrencyInterestRateContract1Memberus-gaap:DesignatedAsHedgingInstrumentMembersrt:ScenarioForecastMember2025-06-182025-06-180000874716idxx:CrossCurrencyInterestRateContract2Memberus-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716idxx:CrossCurrencyInterestRateContract2Memberus-gaap:DesignatedAsHedgingInstrumentMembersrt:ScenarioForecastMember2028-03-312028-03-310000874716idxx:CrossCurrencyInterestRateContract3Memberus-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716idxx:CrossCurrencyInterestRateContract3Memberus-gaap:DesignatedAsHedgingInstrumentMembersrt:ScenarioForecastMember2028-06-302028-06-300000874716idxx:CrossCurrencyInterestRateContract4Memberus-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716idxx:CrossCurrencyInterestRateContract4Memberus-gaap:DesignatedAsHedgingInstrumentMembersrt:ScenarioForecastMember2029-06-292029-06-290000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-06-300000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-04-012023-06-300000874716us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMember2024-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMember2023-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMember2024-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherCurrentAssetsMember2023-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMember2024-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMember2023-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMember2024-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentAssetsMember2023-12-310000874716us-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716us-gaap:DesignatedAsHedgingInstrumentMember2023-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccruedLiabilitiesMember2024-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:AccruedLiabilitiesMember2023-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentLiabilitiesMember2024-12-310000874716us-gaap:CrossCurrencyInterestRateContractMemberus-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:OtherNoncurrentLiabilitiesMember2023-12-310000874716us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMember2024-12-310000874716us-gaap:CashFlowHedgingMemberus-gaap:DesignatedAsHedgingInstrumentMember2023-12-310000874716idxx:ForeignCurrencyBorrowingsDesignatedAsNetInvestmentHedgeOnBalanceSheetMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:LongTermDebtMember2024-12-310000874716idxx:ForeignCurrencyBorrowingsDesignatedAsNetInvestmentHedgeOnBalanceSheetMemberus-gaap:DesignatedAsHedgingInstrumentMemberus-gaap:LongTermDebtMember2023-12-3100008747162024-12-030000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2022-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ForeignExchangeContractMember2022-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2022-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberidxx:SeriesCSeniorNoteMember2022-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberus-gaap:CrossCurrencyInterestRateContractMember2022-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2022-12-310000874716idxx:AccumulatedCumulativeTranslationAdjustmentAttributableToParentMember2022-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-01-012023-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ForeignExchangeContractMember2023-01-012023-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2023-01-012023-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberidxx:SeriesCSeniorNoteMember2023-01-012023-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberus-gaap:CrossCurrencyInterestRateContractMember2023-01-012023-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-01-012023-12-310000874716idxx:AccumulatedCumulativeTranslationAdjustmentAttributableToParentMember2023-01-012023-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2023-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ForeignExchangeContractMember2023-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2023-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberidxx:SeriesCSeniorNoteMember2023-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberus-gaap:CrossCurrencyInterestRateContractMember2023-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2023-12-310000874716idxx:AccumulatedCumulativeTranslationAdjustmentAttributableToParentMember2023-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-01-012024-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ForeignExchangeContractMember2024-01-012024-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2024-01-012024-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberidxx:SeriesCSeniorNoteMember2024-01-012024-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberus-gaap:CrossCurrencyInterestRateContractMember2024-01-012024-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2024-01-012024-12-310000874716idxx:AccumulatedCumulativeTranslationAdjustmentAttributableToParentMember2024-01-012024-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2024-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ForeignExchangeContractMember2024-12-310000874716us-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:InterestRateSwapMember2024-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberidxx:SeriesCSeniorNoteMember2024-12-310000874716us-gaap:AociDerivativeQualifyingAsHedgeExcludedComponentParentMemberus-gaap:CrossCurrencyInterestRateContractMember2024-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMember2024-12-310000874716idxx:AccumulatedCumulativeTranslationAdjustmentAttributableToParentMember2024-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000874716us-gaap:ForeignExchangeContractMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000874716us-gaap:InterestRateSwapMemberus-gaap:AccumulatedGainLossNetCashFlowHedgeParentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000874716us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2024-01-012024-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2023-01-012023-12-310000874716us-gaap:AccumulatedDefinedBenefitPlansAdjustmentMemberus-gaap:ReclassificationOutOfAccumulatedOtherComprehensiveIncomeMember2022-01-012022-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Mark One) |
|
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2024
|
|
|
|
|
|
|
|
|
|
|
|
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _______________ to _______________
COMMISSION FILE NUMBER: 0-19271
IDEXX LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
| Delaware |
|
|
01-0393723 |
(State or other jurisdiction of incorporation
or organization) |
|
|
(IRS Employer Identification No.) |
|
|
|
|
| One IDEXX Drive |
Westbrook, |
Maine |
04092 |
| (Address of principal executive offices) |
|
|
(ZIP Code) |
207-556-0300
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
|
|
|
|
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered |
| Common Stock, $0.10 par value per share |
IDXX |
NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Large accelerated filer |
☒ |
Accelerated filer |
☐ |
Non-accelerated filer |
☐ |
Smaller reporting company |
☐ |
Emerging growth company |
☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☒
Based on the closing sale price on June 30, 2024, of the registrant’s Common Stock, the last business day of the registrant’s most recently completed second fiscal quarter, as reported by the NASDAQ Global Select Market, the aggregate market value of the voting stock held by non-affiliates of the registrant was $40,083,530,200. For these purposes, the registrant considers its directors and executive officers to be its only affiliates.
The number of shares outstanding of the registrant’s Common Stock was 81,328,233 on February 12, 2025.
DOCUMENTS INCORPORATED BY REFERENCE
Part III—Specifically identified portions of the Company’s definitive Proxy Statement to be filed in connection with the Company’s 2025 annual meeting of stockholders (the “2025 Annual Meeting”), to be held on May 7, 2025, are incorporated herein by reference.
GLOSSARY OF TERMS AND SELECTED ABBREVIATIONS
|
|
|
|
|
|
|
|
|
| Term/Abbreviation |
|
Definition |
|
|
|
AI |
|
Artificial intelligence |
| AOCI |
|
Accumulated other comprehensive income or loss |
| ASC |
|
Accounting Standards Codification |
| ASU |
|
Accounting Standards Update |
| CAG |
|
Companion Animal Group, a reporting segment that provides veterinarians diagnostic products and services and information management solutions that enhance the health and well-being of pets. |
| cGMP |
|
The FDA’s current Good Manufacturing Practice regulations. |
| Clinical visits |
|
The reason for the visit involves an interaction between a clinician and a pet. |
| Credit Facility |
|
Our $1.25 billion five-year unsecured credit facility under an amended and restated credit agreement; consisting of i) $1 billion revolving credit facility, also referred to as line of credit, and ii) $250 million three-year term loan. |
Customer commitment arrangements |
|
Customer contractual arrangements that provide customers incentives in exchange for multi-year commitments to purchase annual minimum amounts of products and services. |
| EPA |
|
U.S. Environmental Protection Agency |
| EPS |
|
Earnings per share, if not specifically stated, EPS refers to earnings per share on a diluted basis. |
| EU |
|
European Union |
| FASB |
|
U.S. Financial Accounting Standards Board |
| FDA |
|
U.S. Food and Drug Administration |
| IVLS |
|
IDEXX VetLab Station, connects and integrates the diagnostic information from all the IDEXX VetLab analyzers and thus provides reference laboratory information management system capability. |
| Kits and consumables |
|
Rapid assay kits and IDEXX VetLab consumables |
| LPD |
|
Livestock, Poultry and Dairy, a reporting segment that provides diagnostic products and services for livestock and poultry health and ensures the quality and safety of milk and improves producer efficiency. |
| OPTI Medical |
|
OPTI Medical Systems, Inc., a wholly-owned subsidiary of IDEXX Laboratories Inc., located in Roswell, Georgia. This business provides point-of-care and laboratory diagnostics (including electrolyte and blood gas analyzers and related consumable products) for the human medical diagnostics sector. The Roswell facility also manufactures electrolytes slides (instrument consumables) to run Catalyst One®, Catalyst Dx®, and blood gas analyzers and consumables for the veterinary market; also referred to as OPTI. |
| Organic revenue growth |
|
A non-GAAP financial measure that represents the percentage change in revenue, compared to the same period for the prior year, net of the effect of changes in foreign currency exchange rates, certain business acquisitions and divestitures. Organic revenue growth should be considered in addition to, and not as a replacement for or as a superior measure to, revenues reported in accordance with U.S. GAAP, and may not be comparable to similarly titled measures reported by other companies. |
| Ortho |
|
Ortho-Clinical Diagnostics, Inc., a subsidiary of QuidelOrtho Corporation, a supplier of dry slide consumables used in our Catalyst One and Catalyst Dx Chemistry Analyzers and VetTest Chemistry Analyzer. |
| Prime rate |
|
The prime rate is an interest rate determined by individual banks. It is often used as a reference rate for many types of loans. |
| PACS |
|
Picture archiving and communication software, our software solution for accessing, storing, and sharing diagnostic images. |
| PCR |
|
Polymerase chain reaction, a technique used to amplify small segments of DNA. |
| R&D |
|
Research and Development |
| Reagent rentals |
|
Instruments being placed at customer sites at little or no cost in exchange for a long-term customer commitment to purchase instrument consumables. |
| Reported revenue growth |
|
The percentage change in revenue reported in accordance with U.S. GAAP, compared to the same period in the prior year. |
| S&P |
|
Standard & Poor’s |
| S&P 500 Health Care Index |
|
The index for the S&P 500 Health Care (U.S. companies) measures the performance of companies that are classified as members in the Global Industry Classification Standard of health care services sub-industry. |
| S&P 500 Index |
|
The S&P 500 Index is a U.S. stock market index based on the market capitalization of 500 large companies having common stock listed on the New York Stock Exchange or NASDAQ, including IDEXX. |
|
|
|
|
|
|
|
|
|
| SaaS |
|
Software-as-a-service |
| SDMA |
|
Symmetrical dimethyl arginine, a biomarker that detects kidney disease. |
| SEC |
|
U.S. Securities and Exchange Commission |
| Senior Note Agreements |
|
Note purchase agreements for the private placement of senior notes, referred to as senior notes or long-term debt. |
| SOFR |
|
The secured overnight financing rate as administered by the Federal Reserve Board of New York (or a successor administrator of the secured overnight financing rate) |
| U.S. GAAP |
|
Accounting principles generally accepted in the United States of America |
| USDA |
|
U.S. Department of Agriculture |
| Water |
|
Water, a reporting segment that provides water microbiology testing products. |
IDEXX LABORATORIES, INC.
Annual Report on Form 10-K
Table of Contents
|
|
|
|
|
|
|
|
|
| Item No. |
|
Page No. |
|
|
|
|
PART I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PART II |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PART III |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PART IV |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The terms “IDEXX,” “Company,” “registrant,” “we,” “us,” and “our” included in this Annual Report on Form 10-K mean IDEXX Laboratories, Inc. and all subsidiaries that are consolidated under U.S. GAAP.
We have included certain terms and abbreviations used throughout this Annual Report on Form 10-K in the "Glossary of Terms and Selected Abbreviations.”
Our name, logo and the following terms used in this Annual Report on Form 10-K are either registered trademarks or trademarks of IDEXX Laboratories, Inc. in the United States and/or other countries: 4Dx®, Alertys®, Animana®, Catalyst Dx®, Catalyst One®, Coag Dx™, Colilert®, Colisure®, Cornerstone®, Enterolert®, ezyVet®, Feline Triple®, Filta-Max®, Filta-Max xpress®, IDEXX DecisionIQ™, IDEXX inVue Dx™, IDEXX I-Vision CR®, IDEXX I-Vision DR®, IDEXX I-Vision Mobile™, IDEXX Neo®, IDEXX-PACS™, IDEXX SDMA®, IDEXX VetAutoread™, IDEXX VetLab®, IDEXX VetLab® UA™, IDEXX VetMedStat®, LaserCyte®, LaserCyte® Dx, OPTI®, Pet Health Network®, Petly® Plans, ProCyte Dx®, Pseudalert®, Quanti-Tray®, rVetLink®, SediVue Dx®, SNAP®, SNAPduo®, SNAP Pro®, SNAPshot Dx®, TECTA®, VetConnect®, VetLINK®, VetLyte®, Vet Radar®, VetStat®, and VetTest®. VetAutoread is a trademark of QBC Diagnostics.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
This Annual Report on Form 10-K for the year ended December 31, 2024, contains statements which, to the extent they are not statements of historical fact, constitute “forward-looking statements.” Such forward-looking statements about our business and expectations within the meaning of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), include statements relating to, among other things, global trends in companion animal healthcare and demand for our products and services; our expectations regarding supply chain and logistics challenges; our expectations regarding the labor supply; future revenue growth rates; future tax benefits; the impact of tax legislation and regulatory action; revenue recognition timing and amounts; business trends, earnings and other measures of financial performance; the effect of economic downturns and inflation on our business performance; tariffs; the projected effect of patent and license expirations; the projected impact of foreign currency exchange rates and hedging activities; realizability of assets; future cash flow and uses of cash; future repurchases of common stock; future levels of indebtedness and capital spending; the working capital and liquidity outlook; interest expense; warranty expense; share-based compensation expense; the adoption and projected impact of new accounting standards; critical accounting estimates; deductibility of goodwill; future commercial and operational efforts; future incorporation of artificial intelligence into our products, services and business processes; future product launches; projected cost and completion of capital investments; and competition. Forward-looking statements can be identified by the use of words such as “expects,” “may,” “anticipates,” “intends,” “would,” “will,” “plans,” “believes,” “estimates,” “should,” “project,” and similar words and expressions. These forward-looking statements are intended to provide our current expectations or forecasts of future events, are based on current estimates, projections, beliefs, and assumptions, and are not guarantees of future performance. Actual events or results may differ materially from those described in the forward-looking statements. These forward-looking statements involve a number of risks and uncertainties, including, among other things, the matters described under the headings “Business,” “Risk Factors,” “Legal Proceedings,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Quantitative and Qualitative Disclosures About Market Risk” in this Annual Report on Form 10-K. Any forward-looking statements represent our estimates only as of the day this Annual Report on Form 10-K was first filed with the Securities and Exchange Commission (“SEC”) and should not be relied upon as representing our estimates as of any subsequent date. From time to time, oral or written forward-looking statements may also be included in other materials released to the public and they are subject to the risks and uncertainties described or cross-referenced in this section. While we may elect to update forward-looking statements at some point in the future, we specifically disclaim any obligation to do so, even if our estimates or expectations change.
PART I
ITEM 1. BUSINESS
COMPANY OVERVIEW
IDEXX was incorporated in Delaware in 1983. We develop, manufacture, and distribute products and provide services primarily for the companion animal veterinary, livestock and poultry, dairy and water testing industries. We also provide human medical point-of-care and laboratory diagnostics. Our primary products and services are:
•Point-of-care veterinary diagnostic products, comprised of instruments, consumables, and rapid assay test kits;
•Veterinary reference laboratory diagnostic and consulting services;
•Practice management and diagnostic imaging systems and services used by veterinarians;
•Health monitoring, biological materials testing, laboratory diagnostic instruments, and services used by the biomedical research community;
•Diagnostic and health-monitoring products for livestock, poultry, and dairy;
•Products that test water for certain microbiological contaminants; and
•Point-of-care electrolytes and blood gas analyzers.
Our Purpose is to be a great company that creates exceptional long-term value for our customers, employees, and stockholders by enhancing the health and well-being of pets, people, and livestock.
DESCRIPTION OF BUSINESS BY SEGMENT
We operate primarily through three business segments: Companion Animal Group, Water quality products, and Livestock, Poultry and Dairy. Our Other operating segment combines and presents our human medical diagnostic products business with our out-licensing arrangements because they do not meet the quantitative or qualitative thresholds for reportable segments.
Companion Animal Group (“CAG”) - Diagnostic and information management-based products and services for the companion animal veterinary industry, including in-clinic diagnostic solutions, outside reference laboratory services, and veterinary software and services.
CAG Diagnostics
We provide diagnostic capabilities that meet veterinarians’ diverse needs through a variety of modalities, including in-clinic diagnostic solutions and outside reference laboratory services. Regardless of modality utilized, veterinarians are provided with clinically relevant data which is integrated within our information management technologies. The result is a comprehensive view of patient diagnostic information that is easily accessible by both the veterinarian and pet owner.
In-Clinic Diagnostic Solutions. Our in-clinic diagnostic solutions are comprised of our IDEXX VetLab suite of in-clinic chemistry, hematology, immunoassay, electrolyte, urinalysis, cytology, blood gas, and coagulation analyzers, as well as associated consumable products that provide real-time reference lab quality diagnostic results. Several of these in-clinic analyzers, including the Catalyst One Chemistry analyzer, ProCyte One hematology analyzer, SediVue Dx Analyzer, and IDEXX inVue Dx Cellular Analyzer, utilize proprietary artificial intelligence (“AI”) capabilities in their image capture systems to analyze samples. IDEXX in-clinic analyzers feature load-and-go sample handling and integration with a cloud-enabled software ecosystem, including the IDEXX VetLab Station and VetConnect PLUS. Our in-clinic diagnostic solutions also include a broad range of single-use, IDEXX SNAP rapid assay test kits that provide quick, accurate, and convenient point-of-care diagnostic test results for a variety of companion animal disease-causing pathogens and health conditions. Additionally, we offer extended maintenance agreements in connection with the sale of our instruments.
Blood and Urine Chemistry. Our blood and urine chemistry analyzers are used by veterinarians to measure levels of certain enzymes and other substances in blood or urine for monitoring health status and assisting in diagnosing physiologic conditions. We actively sell the Catalyst One Chemistry Analyzer and continue to support the Catalyst Dx Analyzer, both of which perform chemistry, immunoassay, and electrolyte tests. We also support the VetStat Electrolyte and Blood Gas analyzer.
Sales of consumables to customers who use our chemistry analyzers provide the majority of our instrument consumables revenues from our installed base of IDEXX VetLab instruments.
Hematology. Our hematology analyzers assess the cellular components of blood, including red blood cells, white blood cells, and platelets (also called a complete blood count). These analyzers include the ProCyte One and ProCyte Dx hematology analyzers. We also sell the Coag Dx Analyzer, which permits the detection and diagnosis of blood clotting disorders. We continue to offer consumables to support analyzers that are not actively marketed including the LaserCyte Dx Hematology Analyzer.
Urinalysis. The SediVue Dx Analyzer provides a complete urine sediment analysis. This platform features proprietary AI capabilities. The IDEXX VetLab UA Analyzer provides rapid, automated capture of semi-quantitative chemical urinalysis from IDEXX UA strips and is validated specifically for veterinary use.
Cytology. During the fourth quarter of 2024, we launched in North America our new cellular analyzer, IDEXX inVue Dx, which detects the most common cytologic changes found in blood. The IDEXX inVue Dx Cellular Analyzer uses advanced optics and AI technology in a slide-free, load-and-go platform.
Rapid Assay. The SNAP rapid assays are single-use, handheld test kits that can work without the use of instrumentation, although many kits may also be activated with results automatically captured and interpreted by the SNAP Pro Analyzer. This device improves medical care by allowing veterinarians to share the test results with the pet owner on the SNAP Pro Analyzer screen, or via VetConnect PLUS. The principal canine SNAP rapid assay tests include SNAP 4Dx Plus, which tests for the seven vector-borne diseases causing pathogens, including Lyme disease as well as canine heartworm, and SNAP Heartworm RT, which tests for heartworm. Sales of our canine vector-borne disease tests are typically greater in the first half of our fiscal year due to seasonality of disease testing in the veterinary practices in the Northern Hemisphere. The principal feline SNAP rapid assay tests include SNAP Feline Triple, which tests for feline immunodeficiency virus (“FIV”) (which is similar to the virus that leads to AIDS in humans), feline leukemia virus (“FeLV”) and heartworm, and SNAP FIV/FeLV Combo Test, which tests for FIV and FeLV.
IDEXX VetLab Station. The IDEXX VetLab Station (“IVLS”) connects and integrates the diagnostic information from all the IDEXX VetLab analyzers, and thus provides reference laboratory information management system capability. IVLS also sends all results created on connected instruments instantly to VetConnect PLUS. We sell IVLS as an integral component for our in-clinic analyzer suite. In 2024, we upgraded our IVLS to work easier and faster than our previous version, providing two times faster performance on common workflows and access to historical results five times faster.
Integrated Diagnostic Information Management. VetConnect PLUS is a cloud-based technology that enables veterinarians to access and analyze patients’ data from all of IDEXX’s diagnostic modalities. These integrated diagnostic results provide the veterinarian with a visualization of patient-specific information, allowing the veterinarian to easily see and trend diagnostic results, enabling greater medical insight and enhanced decision-making through IDEXX DecisionIQ, an analytical tool incorporated in VetConnect PLUS that utilizes proprietary technology, including AI, to aid practitioners in making medical diagnoses. In addition, VetConnect PLUS provides instant mobile or browser-based access to results, which can be printed or emailed to pet owners and other veterinarians.
Reference Laboratory Diagnostic and Consulting Services. We offer commercial reference laboratory diagnostic and consulting services to veterinarians in many developed geographies worldwide, including customers in the U.S., Europe, Canada, Australia, Japan, New Zealand, South Africa, and South Korea, through a network of approximately 80 laboratories. Customers use our services by submitting samples by courier or overnight delivery to one of our facilities. Most test results have same-day or next-day turnaround times. Our diagnostic laboratory business also provides health monitoring and diagnostic testing services to biomedical research customers in North America, Europe, and Asia.
Our reference laboratories offer a large selection of tests and diagnostic panels to detect a number of disease states and other conditions in animals, including all tests that can be run in-clinic at the veterinary practice with our instruments or rapid assays. This menu of tests also includes a number of specialized tests that we have developed that allow practitioners to diagnose increasingly relevant diseases and conditions in dogs and cats, including parasites, heart disease, allergies, pancreatitis, diabetes, renal disease, and infectious diseases. We also offer cancer screening to aid in diagnosis, assist in therapy selection, and support therapy management and monitoring.
IDEXX Telemedicine. Additionally, we provide specialized veterinary consultation, telemedicine, and advisory services, including radiology, dental radiography, cardiology, internal medicine, and ultrasound consulting. These services enable veterinarians to obtain diagnostic interpretations, and radiology and cardiology assessments. IDEXX Telemedicine services are accessed through IDEXX VetMedStat, a cloud-based software platform for case submission and interpretation that embeds proprietary AI capabilities aiding analysis of images and electrocardiogram results.
Veterinary Software and Services & Diagnostic Imaging Systems
Veterinary Software and Services. We develop, market, and sell a portfolio of software and services for independent veterinary clinics and corporate groups. This portfolio includes:
Practice management systems. We provide software, hardware, and integrated services that run key functions of veterinary clinics, including managing patient electronic health records, scheduling, client communication, billing, and inventory management. Our practice management systems offerings include cloud-based ezyVet, Animana, and IDEXX Neo, and on-premises Cornerstone. To support the software system needs of practices, IDEXX provides integrated services including Payment Solutions, Data Backup & Recovery, and Practice Supplies.
Third-party integrations strengthen our practice management systems value proposition by improving user workflows and can quickly add new functionality to the practice management systems. Our commercial application programming interfaces and partner management processes allow controlled access to the practice management systems platform while providing an enhanced user experience. Our large practice management systems installed base provides access to veterinary channel transaction activity, enabling a syndicated data offering. Industry pharmaceutical and nutrition partners leverage our data to understand channel market performance and to provide behavioral insights.
Software applications that extend workflow capabilities for practices and groups. We are able to improve overall patient management and workflow optimization through coordination and tracking of every step of a patient during a hospital stay. Our SmartFlow cloud offering works in conjunction with major veterinary practice management systems, including ezyVet, Cornerstone, Animana, IDEXX Neo, and certain third-party practice management systems, and VetRadar provides workflow capability for ezyVet.
Client marketing and wellness plan management. In addition, we offer cloud-based client communication (Pet Health Network Pro, Pet Health Network 3D, and Vello) and preventive care plan management software (Petly Plans) designed to strengthen the relationship between the veterinarian and the pet owner. To support the communication needs between general practices and specialty referral practices, IDEXX offers rVetLink software. Lastly, IDEXX Enterprise provides centralized management and reporting capabilities for groups of veterinary practices.
Diagnostic Imaging Systems. Our diagnostic imaging systems capture radiographic images in digital form, replacing traditional x-ray film and the film development process, which generally requires the use of hazardous chemicals and darkrooms. We market and sell two diagnostic imaging systems primarily used in small animal veterinary applications: IDEXX ImageVue DR50 and IDEXX ImageVue DR30.
Our diagnostic imaging systems employ picture archiving and communication system (“PACS”) software called IDEXX-PACS, which facilitates radiographic image capture and review. IDEXX Web PACS is our cloud-based software-as-a-service (“SaaS”) offering for viewing, accessing, storing, and sharing multi-modality diagnostic images. IDEXX Web PACS is integrated with Cornerstone, ezyVet, IDEXX Neo, and IDEXX VetConnect PLUS to provide centralized access to diagnostic imaging results alongside patient diagnostic results from any internet-connected device. IDEXX Web PACS uses proprietary AI capabilities to enable optimal sharing, analysis, and storage of diagnostic images.
We believe that the breadth of our full diagnostic solution, including novel products and services developed and made available only by IDEXX, as well as the seamless software integration of our offering, provide a differentiated competitive advantage by giving veterinarians the tools and services to offer advanced veterinary medical care. We believe that with the use of our products and services, veterinary practices significantly improve the quality of veterinary care provided to their patients, increase staff efficiencies, and better communicate the value of this medical care to the pet owner. We believe that these capabilities, enabled by the use of IDEXX products and services, improve the effectiveness and financial health of the veterinary practice.
Water quality products (“Water”) - Water provides innovative testing solutions for easy, rapid, and accurate detection and quantification of various microbiological parameters in water.
Water testing. Our principal products are the Colilert, Colilert-18, and Colisure tests, which detect the presence of total coliforms and E. coli in water. These organisms are broadly used as microbial indicators for potential fecal contamination in water. Our products utilize nutrient-indicators that produce a change in color or fluorescence when metabolized by target microbes in the sample. Our water tests are used by government laboratories, water utilities, and private certified laboratories to test drinking water in compliance with regulatory standards, including U.S. Environmental Protection Agency (“EPA”) standards. The tests also are used in evaluating water used in production processes (for example, in beverage and pharmaceutical applications) and in evaluating bottled water, recreational water, wastewater, and water from private wells. We also sell consumables, parts, and accessories to be used with many of our water testing products. IDEXX also offers the following products:
Enterolert. Our Enterolert products detect the presence of enterococci in drinking, waste, and recreational waters. Enterococci, bacteria normally found in human and animal waste, are organisms broadly used as microbial indicators for potential fecal contamination in water.
Pseudalert. Our Pseudalert products detect the presence of Pseudomonas aeruginosa in pool, spa, and bottled water. Pseudomonas aeruginosa is a pathogen that can cause “hot-tub rash,” “swimmer’s ear,” and potentially fatal infections in individuals with weakened immune systems.
Filta-Max and Filta-Max xpress. Our Filta-Max and Filta-Max xpress products are used in the detection of Cryptosporidium and Giardia in water. Cryptosporidium and Giardia are parasites that can cause potentially fatal gastrointestinal illness if ingested. We also distribute certain water testing kits manufactured by Thermo Fisher Scientific, Inc. that complement our Cryptosporidium and Giardia testing products.
Legiolert. Our Legiolert product is a simple culture method test for the detection of Legionella pneumophila, the most common Legionella species in water and the primary cause of Legionnaires’ disease. The Legiolert test is designed to be used on potable or non-potable water sources with results in seven days.
Quanti-Tray products. Our Quanti-Tray products, when used in conjunction with our Colilert, Colilert-18, Colisure, Enterolert, Pseudalert, Heterotrophic Plate Count (“HPC”) or Legiolert products, provide users quantitative measurements of microbial contamination rather than a presence/absence indication. Our Quanti-Tray Sealer PLUS, and Quanti-Tray Sealer 2X are used with the Quanti-Tray products for the determination of bacterial density in water samples. Our SimPlate and EasyDisc for HPC products detect the total number of the most common bacteria in a water sample.
IDEXX Tecta Systems. Our IDEXX Tecta system instruments automate several steps in water testing workflows, allowing multiple samples to be tested simultaneously, with remote notification capability. Tecta detects total coliforms and E. coli, E. coli only, fecal coliforms, and enterococci.
Livestock, Poultry and Dairy (“LPD”) - LPD provides diagnostic tests, services, and related instrumentation that are used to manage the health status of livestock and poultry, to improve producer efficiency, and to ensure the quality and safety of milk.
Livestock, Poultry, Herd Health Screening and Production Management. Our livestock and poultry diagnostic products are purchased by government and private laboratories that provide testing services to livestock veterinarians, producers, and processors, and also directly by livestock veterinarians and producers. Our principal livestock and poultry diagnostic products include tests for Bovine Viral Diarrhea Virus (“BVDV”), Porcine Reproductive and Respiratory Syndrome (“PRRS”), Transmittable Spongiform Encephalopathies (“TSE”), and African Swine Fever (“ASFV”). BVDV is a common and contagious viral infection that suppresses the immune system, making the animal susceptible to a host of other infections, and impacting beef and dairy production yields as a result. PRRS is a contagious virus causing reproductive problems and respiratory diseases in swine, leading to increased piglet mortality, reduced growth, and vulnerability to secondary infections. TSE is a transmissible disease in which infected animals exhibit behavioral disturbances and a progressive loss of physical condition that precedes death. Our RealPCR ASFV Test is a real-time polymerase chain reaction (“PCR”) assay that provides early and accurate detection of ASFV supporting prevention, control, and eradication programs by veterinarians and producers. We also sell our Alertys Milk Pregnancy Test, Alertys Ruminant Pregnancy Test, Alertys On-Farm Pregnancy Test, and Rapid Visual Pregnancy Test for cattle and other ruminants, which can detect pregnancy 28 days after breeding using milk, serum, or whole blood samples. We also offer herd health screening services to livestock veterinarians and producers.
Dairy products. Our principal dairy products use our SNAP test platform and are used by dairy producers and processors worldwide to detect antibiotic drug residue in milk. Our primary product lines are SNAP Beta-Lactam ST and SNAPduo ST Plus, which detect certain beta-lactam and tetracycline antibiotic residues.
Other - Our Other operating segment combines and presents our human medical diagnostic products and services business (“OPTI Medical”) with our out-licensing arrangements because they do not meet the quantitative or qualitative thresholds for reportable segments.
OPTI Medical. Through OPTI Medical, we sell point-of-care analyzers and related consumables for use in human medical hospitals and clinics to measure electrolytes, blood gases, acid-base balance, glucose, lactate, blood urea nitrogen and ionized calcium, and to calculate other parameters such as base excess and anion gap. These OPTI analyzers are used primarily in emergency rooms, operating rooms, cardiac monitoring areas, and other locations where time-critical diagnostic testing is performed within the hospital setting. The OPTI CCA-TS2 Analyzer runs whole blood, plasma, and serum samples on single-use disposable cassettes that contain various configurations of analytes. Previously we also provided human testing solutions for the detection of SARS-CoV-2, the virus that causes COVID-19. During the first quarter of 2023, we discontinued actively marketing our COVID-19 testing products and services.
Other Activities. We own certain drug delivery technology intellectual property, that we continue to seek to commercialize through agreements with third parties, such as pharmaceutical companies, which are included in the Other segment.
Additional information about our products and services can be found on our website. Information contained on or connected to our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered part of this Annual Report on Form 10-K or any other filing we make with the SEC.
MARKETING AND DISTRIBUTION
We market, sell, and service our products worldwide through our marketing, customer service, sales, and technical service groups, as well as through independent distributors and other resellers. We maintain a sales presence in the U.S. and in major regions worldwide including Africa, Asia Pacific, Canada, Europe, and Latin America.
Generally, we select the appropriate distribution channel for our products based on the type of product, technical service requirements, number and concentration of customers, regulatory requirements, and other factors. We market our companion animal diagnostic products to veterinarians directly in the U.S. Outside the U.S., we sell our companion animal diagnostic products through our direct sales force and, in certain countries, through distributors and other resellers. We sell our veterinary reference laboratory diagnostic and consulting services worldwide, generally through our direct sales force. We market our diagnostic imaging products primarily through our direct sales force in the U.S. and Canada. We market our software products primarily through our direct sales force in the U.S., Canada, Europe, and Australia. We market our Water and LPD products primarily through our direct sales force in the U.S. and Canada. Outside the U.S. and Canada, we market these products through our direct sales force and, in certain countries, through selected independent distributors. We sell our OPTI products and services both directly and through independent human medical product distributors.
RESEARCH AND DEVELOPMENT
Our business includes the development and introduction of new products and services and may involve entry into new business areas. We maintain active research and development programs in each of our three primary business segments. Our research and development expenses, which consist of salaries, employee benefits, certain licensing agreements, materials and external consulting and development costs, were $219.8 million for the year ended December 31, 2024, or 5.6% of our consolidated revenue, $191.0 million for the year ended December 31, 2023, or 5.2% of our consolidated revenue and $254.8 million for the year ended December 31, 2022, or 7.6% of our consolidated revenue.
INTELLECTUAL PROPERTY, INCLUDING PATENTS AND LICENSES
We actively seek to protect our intellectual property rights and the competitive position of our innovative offerings through various approaches, including the use of patents, copyrights, trademarks, and trade secrets. We file applications for and obtain patents, copyrights, and trademarks in the U.S. and other countries as we believe appropriate, and we seek to protect our trade secrets and proprietary know-how. We also license patents and technologies from third parties.
While we consider ownership of these intellectual property rights in the aggregate to be important to us, we do not believe that any single patent, copyright, trademark, trade secret, or license is material to our business as a whole, except for licenses related to our Catalyst instruments and consumables, and we primarily rely on the innovative skills, technical expertise, customer focus and knowledge, and marketing abilities of our employees for our business success. In addition, a range of factors help to mitigate the future potential effects of the expiration of any of our patents, licenses, and other intellectual property rights on our results of operations and financial position. These factors include, but are not limited to:
•publications, including peer-reviewed third-party studies, that demonstrate the performance and benefits of our products and services;
•our brand strength and reputation in the marketplace;
•the breadth, quality and integration of our product and service offerings;
•our existing customer relationships and our customer service and support;
•our sales force;
•our online ordering platform that enables direct ordering of (including establishing automatic reorder schedules for) our consumables, tests and other products by our customers;
•the applicable regulatory approval status for certain products;
•our continued investments in innovative product and service improvements that result in new technologies, features, functionalities, enhancements, integrations, and/or additional patents and other intellectual property rights;
•our investment in diagnostic innovations that results in new product and service offerings that are patentable and that expand the test menu for our in-clinic instruments and/or reference laboratory business; and
•our significant know-how, scale and investments related to the product design and manufacturing processes of associated product offerings and certain supply arrangements for consumables that are compatible with our instruments.
We own or have license rights to various patents, patent applications, and technologies in the U.S. and other countries. These patents, patent applications, and technologies relate to intellectual property embodied in certain of our CAG products and services (including our Catalyst instruments and consumables and certain reference laboratory tests), LPD diagnostic products, and Water testing products, as well as other subject matters relevant to the manufacture and commercialization of these products and services. Although certain of these patents and licenses are expected to expire in future years, the expiration of these patents and licenses, individually or in the aggregate, is not expected to have a material effect on our financial position or future operations. In addition, we face robust competition as other companies have been successful in bringing competitive products and services to market, despite the protections afforded by these patent and license rights.
In addition to seeking patent protection as appropriate, we also protect certain intellectual property (such as software and our manufacturing know-how) through U.S. and international trade secret and copyright laws. We seek to protect our trade secrets and proprietary know-how in many ways, including through confidentiality and invention assignment agreements with our employees and third parties with which we do business (such as vendors, strategic partners, and consultants) and internal confidentiality processes and procedures.
To the extent some of our products and services may now, or in the future, embody technologies protected by patents, copyrights, or trade secrets of others, we may be required to obtain licenses to such technologies in order to continue to sell our products and services. These licenses may not be available on commercially reasonable terms or at all. Our failure to obtain any such licenses may delay or prevent the sale of certain new or existing products and services. Refer to “Part I, Item 1A. Risk Factors.”
PRODUCTION AND SUPPLY
Certain instruments that we sell are manufactured by third parties. We rely on third parties in our supply chain to supply us, and our direct suppliers, with certain important components, raw materials, and consumables used in or with our products. In some cases, these third parties are sole or single-source suppliers. From time to time, we seek to qualify alternative suppliers.
Instruments and consumables. Significant products supplied by sole and single-source providers include certain Catalyst Dx and Catalyst One consumables (other than electrolyte consumables and the fructosamine, thyroxine, canine C-reactive protein, progesterone, SDMA, and Bile Acid slides), LaserCyte Dx consumables, ProCyte Dx Analyzers and consumables, InVue Dx cytology consumables, SediVue Dx urinalysis instruments and consumables, and certain components of our internally manufactured analyzers.
Certain Catalyst chemistry slides are supplied by Ortho-Clinical Diagnostics, Inc. (“Ortho”) under supply agreements that are currently set to expire in December of 2044. The terms of the agreement allow for early termination in certain circumstances. In the event of a notice of non-extension, the agreement will continue for a period of twelve years through December 31 of the twelfth year after such notice, up to 2044. We are required to purchase all of our requirements for our current menu of Catalyst chemistry slides from Ortho to the extent Ortho is able to supply those requirements. The agreements provide for pricing based on purchase volumes and a fixed annual inflationary adjustment. The agreements also prohibit Ortho from promoting and selling these chemistry slides in the veterinary sector, excluding the EU, other than to IDEXX.
We purchase other analyzers and consumables under supply agreements with terms extending through 2034, which in some cases may be extended at our option. We have minimum purchase obligations under some of these agreements, and our failure to satisfy these obligations may result in loss of some or all of our rights under these agreements. Refer to “Part I, Item 1A. Risk Factors.”
Other components. We purchase certain other products, raw materials, and components from sole and single-source suppliers. These products include certain diagnostic imaging systems and certain components used in our SNAP rapid assay and dairy devices, livestock and poultry testing kits and water testing products.
We have been successful in ensuring an uninterrupted supply of products purchased from sole and single-source suppliers. However, there can be no assurance that uninterrupted supply can be maintained if these agreements terminate for any reason, or our suppliers otherwise are unable to satisfy our requirements for products. Refer to “Part I, Item 1A. Risk Factors.”
Product quality and safety. We believe that product quality and safety are essential to our business. We conduct our operations within an Integrated Management System, which encompasses our Quality Assurance program, to help ensure compliance with applicable regulations, product safety requirements and standards, and customer requirements. This Integrated Management System includes strict manufacturing processes and procedures, employee training, ongoing process improvement, product quality risk management procedures, incident investigation and corrective action procedures, and internal and third-party auditing. Our manufacturing and distribution facilities in Westbrook, Maine; Scarborough, Maine; Roswell, Georgia; Memphis, Tennessee; and the Netherlands, Switzerland, France, and the United Kingdom are certified to the ISO 9001 quality standard, and certain of our other facilities are certified to the environmental (ISO 14001) and testing and calibration laboratory (ISO 17025) quality standards. ISO quality standards are internationally recognized manufacturing standards established by the International Organization for Standardization, which are audited and certified by third-party auditors in addition to our internal self-audits. We also require our key suppliers to have quality management systems that comply with recognized industry standards, such as ISO 9001, and are aligned with our quality requirements, and we regularly conduct audits with our Tier 1 suppliers to verify control systems meet all our requirements. In addition, we make available instructions and other information to help ensure the proper and safe use of our products.
BACKLOG
We do not generally maintain a significant backlog of orders and believe that our backlog at any particular date historically has not been indicative of future sales.
COMPETITION
We compete with many companies ranging from large human and animal health pharmaceutical and medical diagnostics companies to small businesses focused on animal health. Our companion animal veterinary diagnostic products and services compete with both reference laboratory service and in-clinic product providers. Our competitors vary in our different business areas and regions. In some cases, academic institutions, governmental agencies, and other public and private research organizations conduct research activities and may commercialize products or services, which could compete with our products, on their own or through joint ventures. Several of our direct and potential competitors have substantially greater financial and managerial resources than us, as well as greater experience in manufacturing, marketing, research and development, and obtaining regulatory approvals than we do. For more information on risks related to our competition, refer to “Part I, Item 1A. Risk Factors."
Competitive factors in our different business areas are detailed below:
•Companion animal diagnostic offerings. We compete primarily on the basis of ease of use and speed of our products, diagnostic accuracy, product quality, breadth of our product line and services, differentiated product innovations, fully integrated technology, information management capability, enhancement of veterinary practice efficiency, availability of medical consultation, effectiveness of our sales and distribution channels, quality of our technical and customer service, and our pricing relative to the value of our products and services in comparison with competitive products and services. Our major competitors in most geographic locations in North America are Mars, Incorporated brands Antech Diagnostics and Heska; and Zoetis Inc. (including its wholly-owned subsidiary Abaxis, Inc.). We also compete in certain international geographies with Zoetis Inc.; Mars, Incorporated brands including Heska, Antech Diagnostics, scil animal care, and Asia Veterinary Diagnostics; Fujifilm Holdings Corporation; Arkray, Inc.; Mindray; and BioNote, Inc.
•Water, livestock, poultry, and dairy testing products. We compete primarily on the basis of the ease of use, speed, accuracy, product quality, and other performance characteristics of our products and services (including differentiated tests), the breadth of our product line and services, the effectiveness of our sales and distribution channels, the quality of our technical and customer service, our ability to receive regulatory approvals from governing agencies and our pricing relative to the value of our products in comparison with competitive products and services. Our competitors include smaller regional companies and larger companies with livestock and poultry diagnostics and water testing solution products, such as Neogen Corporation, Charm Sciences, Inc., BioChek, Innovative Diagnostics and Thermo Fisher Scientific Inc.
•Veterinary Software, Services and Diagnostic Imaging Systems. We compete primarily on the basis of functionality, system workflows, performance characteristics, effectiveness of our implementation, training process and customer service, information handling capabilities, advances in technologies, enhancement of veterinary practice efficiency, and our pricing relative to the value of our products and services in comparison with competitive products and services. We sell these products primarily in North America and Europe. Our largest competitor in North America and the U.K. is Covetrus, Inc., which offers several systems and leverages its animal health distribution business in sales and service. We also compete with numerous highly focused smaller companies throughout the geographies in which we offer veterinary software, including those offering cloud-based solutions. Our competitors in the diagnostic imaging systems sector include Sound-Eklin, Antech Diagnostics, FUJIFILM, and Heska.
•Human point-of-care medical diagnostic products. We compete primarily on the basis of the ease of use, menu, convenience, international distribution and service, instrument reliability, and our pricing relative to the value of our products. We compete primarily with large human medical diagnostics companies such as Radiometer A/S, Siemens Medical Solutions Diagnostics, Instrumentation Laboratory Company, Abbott Diagnostics, a division of Abbott Laboratories, and Roche Diagnostics Corporation.
GOVERNMENT REGULATION
Many of our products are subject to comprehensive regulation by U.S. and foreign regulatory agencies that relates to, among other things, product approvals, product registrations, manufacturing, import, export, distribution, marketing and promotion, labeling, recordkeeping, testing, quality, storage, product disposal, environmental compliance, and workplace safety. The following is a description of the principal regulations affecting our businesses.
Veterinary diagnostic products. Our veterinary diagnostic products, including instruments, such as Catalyst One and ProCyte One, as well as their corresponding consumables, are veterinary medical devices under the jurisdiction of the FDA under the Food, Drug and Cosmetics Act (the “FDC Act”). Other products under FDA jurisdiction include our rapid assay products such as SNAP devices and enzyme-linked immunosorbent assay plates. While the sale of these products does not require premarket approval by the FDA and does not subject us to FDA inspections or the FDA's current Good Manufacturing Practice regulations (“cGMP”), the FDA Act specifies that these products must not be adulterated, mislabeled, or misbranded.
A subset of our veterinary diagnostic products, including our diagnostic test kits for companion and food animal infectious diseases, as well as most of our livestock and poultry products and many of our rapid assay products, are licensed and regulated in the U.S. by the Center for Veterinary Biologics within the United States Department of Agriculture (“USDA”) Animal and Plant Health Inspection Service (“APHIS”). These products must be approved by APHIS before they may be sold in and from the U.S. The APHIS regulatory approval process involves the submission of product validation data, including manufacturing process and facility documentation. Following regulatory licensure to market a product, APHIS requires that each product serial be submitted for test review before release to customers. In addition, APHIS requires special approval to market products where test results are used for government-mandated disease management programs. A number of foreign governments accept APHIS approval to support product registration for sale, distribution, and use within their countries. However, compliance with extensive country-specific regulatory processes is required in connection with importing and marketing diagnostic products in Japan, Germany, Canada, Brazil, the Netherlands, China, and many other countries. We are also required to have a facility license from APHIS to manufacture USDA-licensed products.
We have a facility license for our manufacturing facility in Westbrook, Maine which also covers our distribution center in Memphis, Tennessee. Our LPD manufacturing facility in Montpellier, France is a USDA-permitted site, and has been approved by APHIS to manufacture specified USDA-permitted products.
Water testing products. Our water tests are generally not subject to formal premarket regulatory approval. However, before a test can be used as part of a water quality monitoring program in the U.S. that is regulated by the EPA, the test method must first be approved by the EPA. The EPA approval process involves submission of extensive product performance data in accordance with an EPA-approved protocol, evaluation of the data by the EPA, and publication for public comment of any proposed approval in the Federal Register before final approval. Our Tecta Systems, Colilert, Colilert-18, Colisure, Quanti-Tray, Filta-Max xpress, Enterolert, and SimPlate for heterotrophic plate counts products have been approved by the EPA for use under various regulatory programs. Water testing products are subject to similarly extensive regulatory processes in other countries around the world.
Dairy testing products. Dairy products used in National Conference on Interstate Milk Shipments (“NCIMS”) milk-monitoring programs in the U.S. are regulated by the FDA as veterinary medical devices. Before these products can be sold in the U.S., performance data must be submitted in accordance with an FDA-approved protocol administered by an independent body, such as the Association of Analytical Chemists Research Institute (“AOAC RI”). While some foreign countries accept AOAC RI certification as part of their regulatory approval process, many countries have separate regulatory processes. All test methods used for drug residue testing require validation by the FDA and acceptance by the NCIMS. IDEXX has two FDA-validated test products, SNAP NBL and SNAP Tetra, for sale in the U.S.
Human point-of-care electrolyte and blood gas analyzers. Our OPTI instrument systems are classified by the FDA as Class I and/or Class II medical devices, and their design, manufacture, and marketing are regulated by the FDA. Accordingly, we must comply with cGMP in the manufacture of our OPTI products. The FDA’s Quality System regulations set forth standards for our product design and manufacturing processes, require the maintenance of certain records, and provide for inspections of our facilities by the FDA. New OPTI products categorized as Class I and/or Class II medical devices would require notification of and review by the FDA via a 510(k) application before marketing or sale of such products. These OPTI products are also subject to the regulations governing the manufacture and marketing of medical devices in other countries in which they are sold, including the EU Medical Device Regulation and In Vitro Diagnostic Medical Devices Regulation.
Other Chemical, Environmental, and Human Health Safety Regulations. All IDEXX products must comply with applicable global product regulations, including those governing consumer product safety and materials requirements such as the Europeans Union's Electromagnetic Compatibility (“EMC”) Directive, the European Regulation for Registration, Evaluation, Authorization and Restriction of Chemical Substances (“REACH”), the Restriction of Hazardous Substances (“RoHS”) Directive, and the Waste Electrical and Electronic Equipment (“WEEE”) Directive. These complex regulatory requirements create risk to IDEXX’s ability to market and sell our products, our business, and our financial performance.
In the European Union, our analyzers and certain associated equipment are subject to the requirements of the RoHS Directive, which regulates and restricts certain hazardous substances in electrical and electronic equipment. Other countries, including China, the United Arab Emirates, and Turkey, have implemented or anticipate implementing regulatory regimes similar to the RoHS Directive. Our veterinary diagnostic instrument systems are not subject to regulation under the EU Medical Device Regulation or In Vitro Diagnostic Medical Device Regulation, which are both strictly applicable to human use products. However, these systems are required to meet CE certification, which require compliance with the RoHS Directive, the EMC Directive, and other safety requirements. Most countries in which we sell our products impose similar registrations and/or certification requirements.
The European Union was among the first to regulate and restrict the use of certain substances that we currently use in our products. These requirements include the Biocidal Products Regulation, which requires the use of only approved biocides in our products imported to or used in the European Union, and REACH, which regulates and restricts the use of certain chemicals in the European Union. Compliance with these regulations (and similar regulations that have been or may be adopted elsewhere, such as Australia, China, Turkey, Korea, and other countries) may require registration, notification, or certification regarding regulated substances, imposition of import restrictions, or in certain cases the redesign or reformulation of our products. As part of the microplastics restrictions pursuant to REACH, some of our products, including Companion Animal products, may be subject to derogation, such as labeling and reporting.
In the U.S., the EPA regulates chemical use similarly to the European Union. In addition, certain states have their own chemical regulations, such as California's Proposition 65, which requires businesses to provide warnings to California residents about significant risk of exposures to chemicals in products that are known to cause cancer, birth defects, or other reproductive harm.
PFAS (per- and polyfluoroalkyl substances), which may be contained in certain IDEXX products, are a subject of increasing regulatory attention. The European Union had proposed draft regulations regarding PFAS, which include restrictions and/or phase-out requirements. In the U.S., the EPA has published a PFAS rule under the Toxic Substance Control Act, requiring manufacturers of PFAS and importers of products containing PFAS to file certain information from January 1, 2011, including PFAS chemical identity and structure, production, imports of products containing PFAS, use, byproducts, exposure, disposal, and health and environmental effects. In addition, a number of states, including Maine, where we manufacture many of our products, have also developed product reporting and/or phase-out requirements. The Maine statute prohibits the sale in Maine of non-exempt products containing intentionally-added PFAS after January 1, 2032, unless the Maine Department of Environmental Protection has made an unavoidable use determination, and requires reporting after the applicable sales ban takes effect of the presence of PFAS in products that have received unavoidable use determinations. However, these bans and reporting requirements in Maine are currently subject to statutory exemptions for veterinary products and medical devices regulated by or under the jurisdiction of the FDA, USDA, or EPA, as well as products that are used for public health, or for environmental or water quality testing.
In addition to the foregoing, our business is generally subject to various U.S. and foreign regulatory authorities, including the U.S. Federal Trade Commission (the “FTC”) and other anti-competition authorities, and we are also subject to anti-bribery and anti-corruption laws, such as the U.S. Foreign Corrupt Practices Act and the UK Bribery Act, import and export laws and regulations, including U.S. import and export control and sanctions laws, and laws and regulations governing the collection, use, retention, sharing and security of data such as the EU General Data Protection Regulation and the EU Data Act. Our products that use generative or other forms of artificial intelligence may also be subject to new and emerging law and regulations, including the European Union’s Artificial Intelligence Act enacted in August 2024. Development or acquisition of new products and technologies may subject us to additional areas of government regulation. These may involve medical device, water-quality and other regulations of the FDA, the EPA, the USDA, the FTC, and other federal agencies, as well as state, local and foreign governments. For more information about the risks associated with various U.S. and foreign government regulation, refer to “Various U.S. and foreign government regulations could limit or delay our ability to market and sell our products or otherwise negatively impact our business” under “Part I, Item 1A. Risk Factors.”
HUMAN CAPITAL
As innovation and customer focus are important parts of our strategy to create long-term value, we aim to attract, motivate, develop, and retain talented employees at all levels who are aligned with and passionate about our Purpose by:
•Building and sustaining an inclusive, ethical culture that welcomes, respects and supports all employees;
•Offering competitive and locally relevant compensation and benefits; and
•Providing growth and development opportunities for all our employees.
Because our strategy includes developing strong, deep relationships with our veterinary customers around the world, we have focused on growing our companion animal diagnostics field-based organization globally.
Inclusive, Ethical Culture. IDEXX promotes an inclusive, ethical culture that values the different skills, perspectives and backgrounds that each employee brings in pursuit of our Purpose. We believe that this culture helps drive both innovation and a better understanding of our increasingly global customer base aligned with long-term value creation. We employ inclusive recruitment practices to source highly qualified candidates, regardless of race, sex, gender, religion, national origin, or any other characteristic protected by law. We encourage and promote an inclusive culture at IDEXX, including through executive sponsorship of employee-led communities that support all our employees.
As of December 31, 2024, we had approximately 11,000 regular full-time and part-time employees in roughly 32 countries around the world. We are an equal opportunity employer, and we aim to have talent management processes that provide equal career advancement opportunities for all our employees.
Our policies strictly prohibit any form of employment discrimination, and pursuant to applicable law, we annually collect certain demographic data regarding our U.S. workforce, which we report in our EEO-1 report to the U.S. Equal Opportunity Commission. Our summarized EEO-1 survey data is disclosed on our website. The information contained on or connected to our website is not incorporated by reference into this Annual Report on Form 10-K and should not be considered part of this Annual Report on Form 10-K or any other filing we make with the SEC.
In addition, we believe that maintaining a workforce that reflects our global, culturally diverse customer base is consistent with and supports our business strategy. Our CAG business, which represents 92% of our 2024 revenues, serves veterinarians, and according to the American Veterinary Medical Association, men and women held 32% and 67%, respectively, of U.S. veterinary positions as of December 31, 2023. As of December 31, 2024, men and women represented 41% and 58%, respectively, of our global employee population, and approximately 50% and 50%, respectively, of our global people managers.
Compensation, Benefits and Well-being. We offer fair, competitive compensation and a wide array of competitive and locally relevant benefits (which vary by country and region) that support our employees’ overall well-being, including comprehensive health and welfare insurance; generous time-off and leave; retirement and financial support. We have recognition programs for the purpose of acknowledging the achievements of individual and team performance. We recognize that offering best-in-class well-being programs is an important element of an effective total rewards strategy and use benchmarking and our understanding of our workforce to identify and implement holistic well-being programs that we believe are aligned with our culture and meet our employees’ needs. Accordingly, our well-being programs have strong utilization by our employee base, and we review these programs from time to time to ensure they are achieving the anticipated outcomes. We provide free counseling for employees and their dependents globally through our mental wellness partner. Interactive holistic well-being resources are available to employees globally, including monthly educational webinars, ergonomic support, team fitness challenges, nutrition programs, and self-guided courses on a broad range of topics. In addition, all employees have access to financial education and our employees, their spouses, and adult dependents in North America can engage with a financial coach for help reaching their personal financial goals.
We are committed to enabling managers, teams, and employees to identify the most effective work arrangements to accomplish their goals. For some teams this consists of 100% onsite work, while others employ remote or hybrid models. Our goal is to promote a positive workplace environment where everyone can contribute to their fullest potential. We strive to build a collaborative culture through in-person and virtual events, including town halls, in-office celebrations, and employee-led communities.
Ensuring the health, safety, and well-being of our employees is a top priority at IDEXX. We provide our employees with the training, tools, and resources they need to safeguard their health, and we empower them to put safety first. Our Environmental Health & Safety (“EH&S”) team oversees the IDEXX EH&S management system and our company-wide safety programs, ensuring that all of our locations implement health and safety processes to maintain and improve employee safety, reduce workplace risks, and drive continuous improvement.
Growth and Development. We are steadfast in our focus on cultivating the leaders of tomorrow and making career development opportunities more accessible across the company. We offer in-person and virtual training; our goal is to ensure robust training development options are accessible to all levels, geographies, and in multiple languages. Our career development programs are designed to build capabilities and enable career progression. We offer multiple leadership development programs to address the needs of employees at different stages of their professional careers, including new people managers, frontline leaders, mid-level leaders and business leaders, with the goal of building their capabilities and enhancing their readiness for the next step in their leadership journey. We also encourage employees to enhance their career development through individual development plans, job-related courses and degree programs.
Employee Turnover and Engagement. We monitor employee turnover and engagement to identify opportunities to strengthen our approach to human capital management. During 2024, our overall voluntary employee turnover rate was approximately 8.5%. Our voluntary turnover among managerial employees was approximately 5%.
We regularly conduct company-wide employee surveys through a third party to collect employee input on our culture, their experiences, and workplace conditions. These survey results provide insights that help us to improve employee engagement. Our most recent global employee survey received a high level of response, and we maintained a strong level of engagement, consistent with prior years. We leverage the insights gained from the survey to develop a roadmap for improving engagement and retention.
ENVIRONMENTAL, HEALTH, AND SAFETY AND CLIMATE CHANGE MATTERS
Our operations are subject to various environmental, health, and safety laws and regulations concerning, among other things, the generation, handling, transportation, and disposal of hazardous substances or wastes, the cleanup of hazardous substance releases, emissions or discharges into the air or water, and occupational safety and health. The ongoing costs of complying with such laws and regulations is significant and may increase in the future, particularly in the European Union, where we are required to comply with increasingly extensive regulations.
We continue to monitor legislative and regulatory actions and their potential impacts on the areas where we conduct business.
In addition to monitoring and managing compliance with environmental, health and safety laws and regulations, we strive to reduce our environmental footprint, although our greenhouse gas emissions from our operations are not significant. We have developed and are implementing a greenhouse gas emissions reduction strategy. To monitor our progress in meeting our environmental goals, we track and publicly report our environmental impact metrics, including greenhouse gas emissions and energy consumption. In pursuit of our goals, we have developed initiatives and multi-year projects to reduce our greenhouse gas emissions, integrate sustainability considerations for new product development and design, and reduce the amount of plastic and other material waste from current products and packaging. Our most recent Corporate Responsibility Report, which is available on our website, is aligned with the Sustainability Accounting Standards Board and the Task Force on Climate-Related Financial Disclosures frameworks and provides our most recent sustainability highlights for our products and operations.
AVAILABLE INFORMATION
Our principal executive offices are located at One IDEXX Drive, Westbrook, Maine 04092, our telephone number is 207-556-0300, and our internet address is www.idexx.com. References to our website in this Annual Report on Form 10-K are inactive textual references only and the content of our website, including our Corporate Responsibility Report, should not be deemed incorporated by reference for any purpose.
We make available free of charge at www.idexx.com our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we file such information with, or furnish it to, the SEC. In addition, copies of our reports filed electronically with or furnished electronically to the SEC may be accessed at www.sec.gov.
Our Corporate Governance Guidelines and our Code of Ethics are also available on our website at www.idexx.com.
ITEM 1A. RISK FACTORS
You should consider carefully the risks and uncertainties described below in addition to the other information included or incorporated by reference in this Annual Report on Form 10-K in evaluating our company and our business. Our future operating results involve a number of risks and uncertainties, and actual events or results may differ materially from those discussed in this Annual Report on Form 10-K. Factors that could cause or contribute to such differences include, but are not limited to, the factors discussed below, as well as those factors discussed elsewhere herein. Any of these factors, in whole or in part, could materially and adversely affect our business, financial condition, operating results, prospects, and stock price.
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
Because our business lines are highly attractive, they are also highly competitive. Our failure to successfully execute certain strategies within this competitive environment could have a material negative impact on our future growth and profitability
The companion animal healthcare industry is highly competitive, and we anticipate increasing levels of competition from both existing competitors and new sector entrants given our performance and the industry’s strong growth and returns. Our ability to maintain or enhance our growth rates and our profitability depends on our successful execution of many elements of our strategy, including:
•Developing, manufacturing, and marketing innovative new or improved and cost competitive in-clinic laboratory analyzers that drive sales of IDEXX VetLab instruments, grow our installed base of instruments, and increase demand for related recurring sales of consumable products, services, and accessories;
•Developing and introducing new or improved innovative diagnostic tests and services for both our reference laboratories and in-clinic applications that provide valuable medical information to our customers and effectively differentiate our products and services from those of our competitors;
•Developing and introducing new or improved innovative, data-insightful software solutions that enable our veterinary customers to improve practice management and efficiency, staff productivity, and client communications and that increase the value to our veterinary customers of our other companion animal products and services by enhancing the integration of the information and transactions of these products and services and supporting the interpretation and management of diagnostic information derived from these products and services;
•Maintaining premium pricing, including by effectively implementing price increases, for our products and services through, among other things, effective communication and promotion of their value in an environment where many of our competitors promote, market, and sell lesser offerings at lower prices;
•Providing our veterinary customers with the medical and business tools, information, and resources that enable them to grow their practices and increase utilization of our diagnostic products and services, through increased pet visits, use of preventive care protocols, enhanced practice of real-time care, and improved practice efficiency;
•Achieving cost improvements in our worldwide network of reference laboratories by implementing global best practices, including lean processing techniques, incorporating technological enhancements, including laboratory automation and a global laboratory information management system, employing purchasing strategies to maximize leverage of our global scale, increasing the leverage of existing infrastructure and consolidating testing in high volume laboratory hubs;
•Achieving cost improvements in the manufacture and service of our in-clinic laboratory analyzers by employing the benefits of economies of scale in both negotiating supply contracts and leveraging manufacturing overhead, and by improving reliability of our instruments;
•Continuing to expand, develop, and advance the productivity of our companion animal diagnostic sales, marketing, customer support, and logistics organizations in the U.S. and international regions in support of, among other things, our all-direct sales strategies;
•Attracting, developing, and retaining key leadership and talent necessary to support all elements of our strategy;
•Strengthening our sales and marketing activities to continue to grow our profitability both in and outside the U.S.;
•Identifying, completing, and integrating acquisitions that enhance our existing businesses, create new businesses for us or expand the geographic areas in which we do business;
•Continuing to incorporate AI, machine learning, and automation into our products and services and associated business processes, such as customer support and software development;
•Developing and implementing new technology and licensing strategies; and
•Continuing to effectively manage our growth and expansion on a global scale through, among other things, designing and implementing cost-effective improvements to our processes, procedures, and infrastructure.
If we are unsuccessful in implementing and executing on some or all of these strategies, our rate of growth or profitability may be negatively impacted.
Our dependence on third-party suppliers could negatively affect our ability to sell certain products or deliver our cloud-based software solutions, which could negatively affect our operating results
We rely on third-party suppliers to provide components and raw materials (including biological materials) for our manufactured products, manufacture some of the products that we sell, provide the cloud-based infrastructure through which we deliver our cloud-based software solutions, and perform certain services, including package-delivery services. Actions taken by third-party suppliers in operating their business, as well as any disruptions to their business operations (or their suppliers' business operations), could disrupt our supply chain or operations and materially negatively impact our ability to supply the market, substantially decrease sales, lead to higher costs, and damage our reputation with our customers. Longer-term disruptions could potentially result in the permanent loss of our customers, which could reduce our recurring revenues and long-term profitability.
Our supply chain and our cost of goods also may be adversely impacted by unanticipated price increases due to factors such as inflation (including wage inflation), supply restrictions beyond our control or the control of our suppliers, the imposition of tariffs or duties, or regulatory requirements regarding the importation, exportation, composition, or production processes of the goods or materials provided by our suppliers. If current suppliers fail to supply sufficient goods or materials that comply with applicable regulatory requirements to us on a timely basis, or at all, or are unable to comply with any due diligence requests that may be required pursuant to applicable law or regulation, we could experience inventory shortages and disruptions in our supply of goods or materials.
For examples of some of the events that could result in disruption to our supply chain or operations, and negatively impact our operating results, refer to “Various U.S. and foreign government regulations could limit or delay our ability to market and sell our products or otherwise negatively impact our business,” “We depend on the continuous and reliable operation and security of our information technology systems and our products and services that incorporate or rely on information technology, and any disruption or significant cybersecurity breach or other incident could adversely affect our business” and “Factors and events beyond our control could disrupt our operations, supply chain, and logistics network and adversely affect our business” below.
In addition, we currently purchase many products, components, and materials from sole or single sources, such as Ortho. Some of these products are proprietary and, therefore, cannot be readily or easily replaced by alternative sources. These products, components, and materials are used in our instruments, consumables and accessories; livestock and poultry diagnostic tests, dairy testing products; and water testing products. Even if products, components, and materials were to become available to us from alternative suppliers, we likely would incur additional costs and delays in identifying and qualifying replacement materials, and there can be no assurance that replacements would be available to us on acceptable terms, or at all. In certain cases, we may be required to obtain regulatory approval to use alternative suppliers, and this process of approval could delay production of our products or development of product candidates indefinitely.
We seek to mitigate sole and single-source suppliers risks on a risk-prioritized basis and in a variety of ways, including, when possible, by identifying and qualifying alternative suppliers, developing applicable in-house manufacturing capabilities and expertise, and entering into escrow arrangements for manufacturing information for certain single or sole-sourced products. We also seek to enter into long-term contracts that provide for an uninterrupted supply of products at predictable or fixed prices. However, there can be no assurance that we will successfully implement any of these mitigating activities or that, if implemented, any of them will be effective in preventing any delay or other disruption in our ability to supply our customers. In addition, suppliers may decline to enter into long-term contracts for any number of reasons, which would require us to purchase products, components, or raw materials via short-term contracts or on a purchase order basis. There can be no assurance that suppliers with which we do not have long-term contracts will continue to supply our requirements, will always fulfill their obligations under those contracts, or will not experience disruptions in their ability to supply our requirements. In cases where we purchase sole and single-source products, components, or raw materials under purchase orders, we are more susceptible to unanticipated cost increases or changes in other terms of supply. In addition, under some contracts with suppliers we have minimum purchase obligations, and our failure to satisfy those obligations may result in loss of some or all of our rights under these contracts or require us to compensate the supplier.
If we are unable to obtain adequate quantities of products, components, or raw materials in the future from sole and single-source suppliers, or if such sole and single-source suppliers are unable to obtain the components or other materials required to manufacture the products, we may be unable to supply our customers, which could have a material adverse effect on our results of operations and damage our reputation, and any longer-term disruptions could potentially result in the permanent loss of customers, which could reduce our recurring revenues and long-term profitability.
Our biologic products are complex and difficult to manufacture, which could negatively affect our ability to supply our customers
Many of our rapid assay, livestock and poultry diagnostic, water, and dairy products are biologic products that include biological materials, such as antibodies, cells, and sera. Manufacturing biologic products is highly complex due to the inherent variability of biological materials and the difficulty of controlling the interactions of these materials with other components of the products, samples, and the environment. There can be no assurance that we will be able to maintain adequate sources of biological materials or that we will be able to consistently manufacture biologic products that satisfy applicable product release criteria and regulatory requirements. Further, products that meet release criteria at the time of manufacture may fall out of specification while in customer inventory, which could require us to incur expenses associated with recalling products and providing customers with new products, either of which could damage customer relations and our reputation. Our inability to produce or obtain necessary biological materials or to successfully manufacture biologic products that incorporate such materials could result in our inability to supply our customers with these products, which would have an adverse effect on our results of operations and damage our reputation.
Issues in the use of AI in our product offerings may result in reputational harm or liability
We have built, and expect to continue to build, AI into many of our product and service offerings, and we expect this element of our business to grow. We envision a future in which responsible AI operating in our devices, applications, and the cloud, helps our customers be more productive in their business activities and interactions with consumers. As with many disruptive innovations, AI presents risks and challenges that could affect its adoption, and therefore our business. AI algorithms and models may be flawed. Datasets may be insufficient or contain information that is non-representative, infringing, or otherwise subject to legal challenge. Existing and potential government regulation related to AI use may also foreclose certain areas of AI use, cause us to modify how we use AI, and increase the burden and cost of research and development in this area, and failure to properly remediate AI usage issues may cause public confidence in AI to be undermined, which could slow adoption of AI in our offerings. The rapid evolution of AI will require the application of resources to develop, test, and maintain our products and services to help ensure that AI is implemented in a manner to minimize unintended, harmful impact and to comply with applicable law. Furthermore, over the last two years, there have been multiple class action lawsuits filed against large language model developers in the Northern District of California, the Southern District of New York, and the Middle District of Tennessee concerning alleged copyright and other intellectual property violations with respect to the information used to train AI models. The outcomes of these litigations may impair our ability to provide our AI technologies.
Various U.S. and foreign government regulations could limit or delay our ability to market and sell our products or otherwise negatively impact our business
As a global business, we sell products and services in more than 175 countries and operate in an increasingly complex legal and regulatory environment. In the U.S., the manufacture and sale of certain of our products are regulated by federal agencies, such as the USDA, the FDA, and the EPA. Our diagnostic tests for animal health applications that involve the detection of infectious diseases, including most rapid assay canine and feline SNAP tests and livestock and poultry diagnostic tests, must be approved by the USDA prior to sale in the U.S. Our dairy testing products, as well as the manufacture and sale of our OPTI line of human point-of-care electrolytes and blood gas analyzers, require approval by the FDA before they may be sold commercially in the U.S. The methods used by our water testing products must be approved by the EPA, as a part of its water quality monitoring program, before they can be used by customers in the U.S.
Delays by government agencies in approving new products or product upgrades or taking action with respect to other regulatory matters could have a negative impact on our growth and profitability. The ability of government agencies to review and approve new products or product upgrades or take other actions can be affected by various factors, including government budget and funding levels, ability to hire and retain key and other personnel, staffing shortages, public health emergencies, and statutory, regulatory, and policy changes. If a prolonged government shutdown or other disruption of normal business operations occurs, it could significantly impact the ability of the USDA, FDA, EPA and other agencies to timely review and process our regulatory submissions, including with respect to new product candidates, which could have a material adverse effect on our business. Similarly, a prolonged government shutdown or other disruption could prevent the timely review of patent applications by the U.S. Patent and Trademark Office, which could delay the issuance of U.S. patents to which we would otherwise be entitled.
We are also subject to chemical regulation in the U.S., such as California’s Proposition 65, which requires businesses to provide warnings to California residents about significant risk of exposures to chemicals in products that are known to cause cancer, birth defects, or other reproductive harm. In addition, governmental authorities in the U.S. are increasingly focused on preventing environmental contamination from per- and polyfluoroalkyl substances (“PFAS”), which may be contained in certain IDEXX products. For example, current law in Maine prohibits the sale in Maine of non-exempt products containing intentionally-added PFAS after January 1, 2032, unless the Maine Department of Environmental Protection has made an unavoidable use determination, and requires reporting after the applicable sales ban takes effect of the presence of PFAS in products that have received unavoidable use determinations. However, these bans and reporting requirements in Maine are currently subject to statutory exemptions for veterinary products and medical devices regulated by or under the jurisdiction of the FDA, USDA or EPA, as well as products that are used for public health, or for environmental or water quality testing. In addition, federal and state governments and agencies are in various stages of considering and/or implementing laws and regulations requiring the reporting, restriction and/or phase-out of PFAS in products.
The manufacture, import, and sale of our products, as well as our research and development processes, are subject to similar and sometimes more stringent laws in many foreign countries. For example, the European Union regulates the use of certain substances that we currently use in our products or processes. These regulations include, but are not limited to, the Biocidal Products Regulation, which requires the use of approved biocides in our products prior to being manufactured, used, or sold in the European Union; the European Regulation for Registration, Evaluation, Authorization and Restriction of Chemical Substances, or REACH, which regulates and restricts the use of chemicals in the European Union; the Restriction of Hazardous Substances (“RoHS”) Directive, which regulates and restricts certain hazardous substances in electrical and electronic equipment; the Electromagnetic Compatibility Directive; and the Waste Electrical and Electronic Equipment Directive. In addition, European Union regulatory authorities and certain European countries are contemplating regulations to restrict and phase-out PFAS.
Compliance with these and similar laws, directives and regulations in the U.S. and abroad may require registration of the applicable substances, performance of due diligence across our supply chain and reporting thereon, and/or the redesign or reformulation of our products, and may reduce or eliminate the availability of certain parts and components used in our products and services or lead us to change our suppliers in the event our suppliers are unable to comply with our due diligence requests or the applicable regulations in a timely and cost-effective manner. In extreme situations, compliance with these laws, directives and regulations may require us to eliminate or discontinue the use of a part or component in one or more products, but the redesign or reformulation of such products without such parts or components may not be possible, or cause us to relocate the production of certain products. Any redesign or reformulation, change in our suppliers, restrictions in our supply of parts and components, or relocation may negatively affect the availability or performance of our products and services, add testing lead-times for products and reformulated products, reduce our margins, result in additional costs, or have other similar effects. Any inability to redesign or reformulate one or more of our products may preclude us from marketing and selling such products in the applicable regions. In addition, the costs to comply with these regulations may be significant. Any of these could adversely affect our business, financial condition, or results of operations. These legal and regulatory requirements are complex and subject to change, and we continue to evaluate their impact.
In addition, some foreign governments require us to register or certify our products before they can be distributed or sold, and these product registration requirements, which vary among the applicable jurisdictions and change from time to time, are often complex and require us to engage in lengthy and costly processes and provide confidential, proprietary information about those products to foreign regulatory agencies. For example, compliance with extensive country-specific regulatory processes is required in connection with importing, marketing, and selling our diagnostic products in Japan, Germany, Canada, Brazil, the Netherlands, China, and many other countries. There can be no assurance that we will be able to obtain or maintain any product registration required by one or more foreign governments. Any inability to obtain or maintain a required product registration in a jurisdiction could adversely affect our ability to market and sell the applicable product in that jurisdiction, which could have a negative effect on our business, financial condition, and results of operations. There can also be no assurance that confidential, proprietary information provided to foreign regulatory agencies may not be accessed by unauthorized persons or otherwise stolen, which could negatively impact our ability to protect our proprietary rights in our innovative products and our future success. For more information about the risks related to the protection of our proprietary rights in our products and services, refer to “Our success is heavily dependent on our continued differentiated product and service innovation” below.
There has been a recent focus in the U.S. on laws and regulations related to artificial intelligence, which cover, among other things, algorithm accountability, privacy, and transparency. For example, use of artificial intelligence and machine learning may be subject to laws and evolving regulations regarding, among other things, data bias and anti-discrimination. For example, the Federal Trade Commission (“FTC”) enforces consumer protection laws such as Section 5 of the FTC Act, which prohibits unfair and deceptive practices. AI-related legislation has also been introduced in a number of U.S. state legislatures. In August 2024, the European Union Artificial Intelligence Act, which establishes requirements for the provision and use of products that leverage artificial intelligence, machine learning, and similar technologies was enacted. This will take effect in stages beginning in February 2025. Additionally, other countries have proposed legal frameworks to regulate artificial intelligence, which is a trend that may continue to increase. Any failure or perceived failure by us to comply with such requirements could have an adverse impact on our business.
We are also subject to a variety of federal, state, local, and international laws and regulations governing our global business practices. For example, jurisdictions in which we operate prohibit bribery and corruption, anti-competitive behavior, and money laundering; impose trade compliance requirements and restrictions, such as prohibitions on doing business with certain entities or individuals; determine rules impacting the importation and exportation of our products; and regulate immigration and travel. These legal, regulatory, and sometimes politically motivated requirements differ among jurisdictions around the world and are rapidly changing and increasingly complex. The costs associated with compliance with these legal and regulatory requirements and adjusting to changing legal and political environments are significant and likely to increase in the future.
Any failure by us to comply with applicable legal and regulatory requirements, or to adjust to changing legal and political environments, could result in fines, penalties, and sanctions; product recalls; suspensions or discontinuations of, or limitations or restrictions on, our ability to design, manufacture, market, import, export or sell our products; and damage to our reputation. Any of these could negatively impact our business.
Our success is heavily dependent on our continued differentiated product and service innovation
We believe our future success significantly depends on our ability to continue, on a cost-effective and timely basis, to enhance our existing differentiated product and service offerings, to continue to incorporate AI, machine learning and automation into our products and services and associated business processes and to develop and introduce new and innovative differentiated products and services. As a result, we invest substantial funds and efforts into R&D, investigating new products and technologies being developed by third parties, and obtaining certain such new products and technologies through licenses or acquisitions. There can be no assurance that our R&D, licensing, or acquisition efforts will achieve expected results, when or whether any of our products or services now under development will be launched, or whether we may be able to develop, license or otherwise acquire new products or technologies or successfully incorporate AI capabilities into our products, services or associated business processes. We also cannot predict whether any product or service offering, once launched, will achieve market acceptance, or achieve sales and revenue consistent with our expectations.
We rely on a combination of patent, trade secret, trademark, and copyright laws to protect our proprietary rights. We also license patents and technologies from third parties to enable the use of third-party technologies in the development, production, and provision of our products and services. If we do not have adequate protection of our proprietary rights or are unable to license third-party patents and technologies on reasonable terms, our business may be adversely affected by competitors who utilize substantially equivalent technologies to compete with us.
We cannot ensure that we will obtain issued patents, that any patents issued or licensed to us will remain valid, or that any patents owned or licensed by us will provide protection against competitors with similar technologies. Even if our patents cover products or services sold by our competitors, the time and expense of litigating to enforce our patent rights could be substantial and could have an adverse effect on our results of operations. In addition, expiration of patent rights could result in substantial new competition for products or services previously covered by those patent rights.
In the past, we have received notices claiming that our products or services infringe third-party patents, and we may receive such notices in the future. Patent litigation is complex and expensive, and the outcome of patent litigation can be difficult to predict. We cannot ensure that we will win a patent litigation case or negotiate an acceptable resolution of such a case. If we lose, we may be prohibited from selling certain products or services and/or we may be required to pay damages and/or ongoing royalties as a result of the lawsuit. Any such result could have an adverse effect on our results of operations.
Increased competition from and technological advances by our competitors could negatively affect our operating results
We face intense competition, and we expect that future competition will become even more intense as new products, services and technologies become available, the use of AI and machine learning expands, and new competitors enter the space. Our competitors in the veterinary diagnostic sector in the United States and abroad include companies that develop, manufacture, and sell veterinary diagnostic tests; commercial veterinary reference laboratories; certain large and well-funded animal health pharmaceutical companies; and corporate hospital chains that operate reference laboratories that serve both their hospitals and unaffiliated hospitals, such as VCA Inc., which is wholly-owned by Mars, Incorporated, another operator of corporate hospital chains that also sells veterinary in-clinic diagnostic instruments. Consolidation among our competitors and our customers may intensify the competition we face. While we believe that our offerings are competitively differentiated due to our innovative products and services that offer an integrated, comprehensive diagnostic solution and the quality of our technical and customer service, there can be no assurance that increased consolidation among our competitors or customers (as well as any resulting veterinary diagnostic vertical integration among our customers) would not have a negative impact on our ability to compete successfully. For more information regarding the risks presented by consolidation and reference laboratory vertical integration among our customers, refer to “Consolidation in our customer base, including through increased corporate hospital ownership, and prevalence of buying consortiums could negatively affect our business” below.
Competition could negatively affect our sales and profitability in a number of ways. New competitors may emerge through the development of innovative new technology (such as the use of AI and machine learning), the acquisition of rights to use existing technologies or the use of existing technologies when patents protecting such existing technologies expire. New or existing competitors may introduce new, innovative, and competitive products and services more quickly, successfully and effectively, and these products and services could be superior, or be perceived by our customers to be superior, to our products and services or lead to the obsolescence of one or more of our products or services. Business combinations and mergers among our competitors may result in competitors that are better positioned to create, market, and sell more compelling product and service offerings. While an important aspect of our strategy is to continue, on a cost-effective and timely basis, to enhance our existing products and services (including through the incorporation of AI capabilities) and to develop and introduce new and innovative products and services, there can be no assurance that we will be able to successfully develop or introduce such products and services or that those products or services will be superior to our competitors’ products or services or otherwise achieve customer acceptance. Some of our competitors and potential competitors may choose to differentiate themselves by offering products and services perceived in the eyes of customers as similar, at substantially lower sales prices, which could have an adverse effect on our results of operations through loss of sales and/or revenues or a decision to lower our own sales prices to remain competitive. In addition, our ability to attract and retain customers depends on the effectiveness of our customer marketing and incentive programs, and multiple competitors could bundle product and service offerings through co-marketing or other arrangements, which could enhance their ability to compete with our broad product and service offerings. Certain of our competitors and potential competitors, including large diagnostic and pharmaceutical companies, also have substantially greater financial and managerial resources than us, as well as greater experience in manufacturing, marketing, research and development, and obtaining regulatory approvals than we do.
Consolidation in our customer base, including through increased corporate hospital ownership, and prevalence of buying consortiums could negatively affect our business
Veterinarians are our primary customers for our CAG products and services, and the veterinary services industry in the U.S. and abroad has become increasingly consolidated in recent years. In the United States, the number of owners of veterinary hospitals has been declining, and an increasing percentage of veterinary hospitals are owned by corporations that are in the business of acquiring veterinary hospitals and/or opening new veterinary hospitals nationally or regionally. Major corporate hospital owners in the U.S. include Mars, Incorporated (owner of Banfield Pet Hospitals, Blue Pearl Veterinary Partners, and VCA Inc.), and National Veterinary Associates, and are joined by dozens of other consolidators. A similar trend exists in other regions such as Canada, Europe, Australia, New Zealand, China, and Brazil. Furthermore, an increasing percentage of individually owned veterinary hospitals in the U.S. are participating in buying consortiums. Corporate owners of veterinary hospitals and buying consortiums often seek to improve profitability by leveraging the buying power they derive from their scale to obtain favorable pricing from suppliers, which could have a negative impact on our profitability and results of operations. While we have strong supplier relationships with several corporate hospital groups and buying consortiums, decisions by larger corporate owners and buying consortiums to shift their purchasing of products and services away from us and to a competitor would have a negative impact on our results of operations from the loss of future business. Under these circumstances, we may receive customer contract resolution payments, which would generally be recorded in other operating income in the period received. Nonetheless, the loss of future revenue may still be significant and adversely affect our future revenue growth rate and profitability. In addition, certain corporate owners also operate reference laboratories that serve both their hospitals and unaffiliated hospitals and, in some cases, sell veterinary in-clinic diagnostic instruments.
Any hospitals acquired by these companies generally attempt to shift all or a large portion of their testing to the reference laboratories operated by these companies, and may attempt to shift their in-clinic diagnostic testing, as well, and there can be no assurance that hospitals that otherwise become affiliated with these companies would not shift all or a portion of their diagnostic testing. Furthermore, because these companies compete with us in the reference laboratory services marketplace, hospitals acquired by these companies or those that establish other affiliations with these companies may cease to be customers or potential customers of our other companion animal products and services, which would cause our sales of these products and services to decline.
Changes in testing patterns could negatively affect our operating results
Demand for our companion animal, livestock and poultry diagnostic tests and our dairy and water testing products could be negatively impacted by a number of factors impacting testing practices. The introduction or broad market acceptance of vaccines or preventatives for the diseases and conditions for which we sell diagnostic tests and services could result in a decline in testing. Changes in accepted medical protocols regarding the diagnosis of certain diseases and conditions could have a similar effect. Eradication or substantial declines in the prevalence of certain diseases also could lead to a decline in diagnostic testing for such diseases. Our livestock and poultry products business in particular is subject to fluctuations resulting from changes in disease prevalence. The outbreak of certain diseases (such as African swine fever) among livestock or poultry, or the adverse impact of climate change-related events (such as hurricanes, earthquakes, fires, and floods), could lead to the widespread death or precautionary destruction of such animals in the affected regions, reducing herd or flock sizes, which could reduce the demand for our testing products for such animals. Changes in government regulations or in the availability of government funds available for monitoring programs could negatively affect sales of our products that are driven by compliance testing, such as our livestock and poultry, dairy and water products. In addition, changes and trends in local dairy, poultry, or other food sectors around the world could negatively affect the related production markets resulting in a decline in demand for our testing products. Actual or perceived economic weakness, whether due to inflation or other factors, may also reduce demand for our companion animal, water, livestock, poultry, and dairy products and services, and public health-related guidance and directives, including stay-at-home orders that may be deployed to combat public health issues, and severe weather conditions could result in a decrease in companion animal clinical visits, the delay of elective procedures and wellness visits and disruption of veterinary clinic operations, all of which would have a negative effect on veterinary service providers and result in declines in demand for our CAG products and services. Declines in testing for any reason, including the reasons described above, along with lost opportunities associated with a reduction in veterinary visits, could have an adverse effect on our results of operations.
We sell many products through distributors, which presents risks that could negatively affect our operating results
Some of our international product sales occur through third-party distributors. As a result, we are dependent on these distributors to promote and create demand for our products. Our distributors often offer products from several different companies, including our competitors, and may promote our competitors’ products over our own products. We have limited ability, if any, to cause our distributors to devote adequate resources to promoting, marketing, selling, and supporting our products or to maintain certain inventory levels, and changes in our distributors’ inventory levels, compared to comparable prior periods, could negatively impact our revenue growth rates. There can be no assurance that we will be successful in maintaining and strengthening our relationships with our distributors or establishing relationships with new distributors who have the ability to market, sell, and support our products effectively. We may rely on one or more key distributors for a product or a region, and the loss of these distributors could reduce our revenue. Distributors may face financial difficulties, including bankruptcy, which could harm our collection of accounts receivable and financial results. While we maintain a rigorous distribution compliance program, violations of anti-corruption or similar laws by our distributors could have a material impact on our business and reputation, and any termination of a distributor relationship may result in increased competition in the applicable jurisdiction. Failure to manage the risks associated with our use of distributors may reduce sales, increase expenses, and weaken our competitive position, any of which could have a negative effect on our operating results.
GENERAL RISKS
We depend on the efforts of key personnel and talent to succeed and compete effectively
Our continued success is substantially dependent on our ability to attract, develop, and retain highly capable and skilled employees and leaders. As we continue to grow our business, expand our geographic scope, and develop and offer innovative, new products and services, we require an engaged, qualified workforce and the organizational talent necessary to ensure effective succession for our senior leadership and other key personnel. Competition for experienced leaders and employees, particularly for persons with specialized skills, can be intense.
Our ability to recruit and retain such talent will depend on a number of factors, including compensation and benefits, work location, flexibility regarding virtual and hybrid work arrangements, work environment, corporate culture, and development opportunities. Furthermore, a more competitive labor market has made it more difficult and costly to attract qualified labor, and prolonged shortages could adversely affect our ability to achieve our business objectives.
The loss of the services of, or our failure to recruit or develop and implement effective succession plans for, our senior leadership, other key personnel, and employees may significantly delay or prevent the achievement of our strategic objectives, disrupt our operations, and adversely affect our business and our future success. In addition, even if we effectively develop and implement succession plans and make key leadership transitions, we cannot provide assurances as to whether we may experience management or other challenges in connection with any of those leadership transitions that could adversely affect our future success and could otherwise materially adversely affect our business, reputation, results of operations, and financial condition.
We depend on the continuous and reliable operation and security of our information technology systems and our products and services that incorporate or rely on information technology, and any disruption or significant cybersecurity breach or other incident could adversely affect our business
We rely on our information systems, as well as third-party information systems, to provide access to our web-based products and services, keep financial records, analyze results of operations, process orders and shipments, manage inventory, store confidential or proprietary information, and operate other critical functions. In addition, some of our products and services include information systems that collect and use data on behalf of customers (e.g., veterinary practice management systems and customer communication tools and services), and some products and services rely on third-party providers for cloud computing and storage. Although we maintain security policies and measures, employ system backup measures, and engage in redundancy planning and processes, such policies, measures, planning and processes, as well as our current disaster recovery plans, may be ineffective or inadequate to address all eventualities. Further, our information systems and our business partners’ and suppliers’ information systems have experienced, and will likely continue to experience, attacks by hackers and other threat actors and other cybersecurity breaches and incidents, including, among other things, computer viruses and malware, ransomware, denial of service actions, phishing schemes, the compromise, misappropriation and/or unauthorized acquisition or disclosure of confidential or otherwise protected information and similar events through the internet (including via devices and applications connected to the internet), and through email attachments and persons with access to these information systems, such as our employees or third parties with whom we do business. The continued use of remote and hybrid work arrangements may additionally result in some increased risk associated with our employees accessing our data and systems remotely. In addition, security industry experts and government officials have warned about the risks of threat actors, such as hackers, nation state actors, and organized groups, targeting U.S. organizations, and recent developments in the cyber threat landscape include the growing use of AI, which could enable or create more sophisticated cybersecurity attacks and increase attack volume and frequency.
As information systems and the use of software and related applications by us, our business partners, suppliers, and customers become more cloud-based and connected to the “Internet of Things” (“IoT”), there has been an increase in global cybersecurity vulnerabilities and threats, including more sophisticated and targeted cyber-related attacks that pose a risk to the security of our information systems and networks and the security, confidentiality, availability and integrity of data and information. We process credit card payments electronically over secure networks and offer IoT products and services, such as our connected devices (e.g., IDEXX VetLab instruments). Any such attack or breach could compromise our networks and the information stored thereon could be accessed, publicly disclosed, lost, or stolen. While we have implemented network security and internal control measures, especially for the purpose of protecting our connected products and services from cyberattacks, and invested in our data and information technology infrastructure, these efforts have not always been successful in preventing, and there can be no assurance that these efforts (or any future investments or efforts) will prevent, a system disruption, attack, or security breach.
Our software and some of our other products and services incorporate or rely on information technology and systems that are highly technical and complex, and the timely development, testing, release and deployment of software updates are important for defending against security vulnerabilities. If we or our business partners and suppliers fail to timely develop, test and deploy software updates, the security of our and our customers’ networks and information systems may be at risk. Vulnerabilities may persist even after a software update is released if the update fails to address the vulnerability’s root cause or is otherwise insufficient, testing of the update delays its timely deployment, there is a failure to install the most recent updates, or customers persist in using solutions that are end of life and no longer receive updates. Furthermore, updates or other software changes can lead to software or system inoperability or inadvertently introduce security vulnerabilities, including vulnerabilities that had been previously remedied or from malicious code injected in a “supply chain” cyberattack.
In addition, in some cases, we rely on third-party providers to develop, test and release software updates on a timely basis, and there can be no assurance that these third-party providers will timely or effectively remedy vulnerabilities.
We, and some of our third-party vendors, have experienced cybersecurity attacks and incidents in the past and will likely experience further attacks and incidents in the future, potentially with more frequency. To our knowledge, none have resulted in any material adverse impact to the Company, our business strategy, results of operations or financial condition. We have adopted measures to mitigate potential risks associated with information technology disruptions and cybersecurity threats; however, given the unpredictability of the timing, nature and scope of such disruptions and the evolving nature of cybersecurity threats, which vary in technique and sources, if we or our business partners or suppliers were to experience a system disruption, attack or security breach or incident that impacts any of our critical functions, or our customers were to experience a system disruption, attack or security breach or incident via any of our software or connected products and services, we could potentially be subject to production downtimes, operational and/or productivity delays, other detrimental impacts on our operations or ability to provide products and services to our customers, the compromise, misappropriation and/or unauthorized acquisition or disclosure of confidential or otherwise protected information, destruction or corruption of data, security breaches, other manipulation or misuse of our systems or networks, financial losses and additional costs from remedial actions, repairs to infrastructure, physical systems or data processing systems, increased cybersecurity and information technology protection costs, loss of business or potential liability, and/or damage to our reputation, any of which could have a material adverse effect on our business strategy, competitive position, results of operations, cash flows, financial condition, or prospects. Additionally, the post-acquisition integration process of acquired companies that may have less sophisticated information systems, cybersecurity practices, or training, may result in an increased risk of cybersecurity incidents. Our customers and/or employees could also face negative consequences such as the compromise of sensitive or critical information or systems. Furthermore, access to, public disclosure of, or other loss of data or information (including any of our confidential or proprietary information or personal data or information) as a result of an attack or security breach or incident has given, and in the future may give, rise to notification obligations to individuals, regulators, customers, employees, and others, and could result in governmental actions or private claims or proceedings, any of which could damage our reputation, cause a loss of confidence in our products and services, damage our ability to develop (and protect our rights to) our differentiated technologies and have a material adverse effect on the Company, our business strategy, financial condition, results of operations or prospects. While we maintain a cyber risk insurance policy intended to address risk of loss due to certain types of cybersecurity events, it may not cover any or all claims, costs or losses associated with such events. For more information regarding personal data and information privacy and data protection risks, refer to “Our operations and reputation may be impaired if we, our products, or our services do not comply with our global privacy policy or evolving laws and regulations regarding data privacy and protection” below.
Factors and events beyond our control could disrupt our operations, supply chain, and logistics network and adversely affect our business
Our business and results of operations could be negatively affected by certain factors and events beyond our control, such as natural disasters, severe weather conditions and/or climate change-related events (such as hurricanes, earthquakes, fires, and floods); public health issues (such as outbreaks, epidemics, or pandemics); civil or military unrest; geopolitical conditions and developments; war, terrorism, armed conflict, or other man-made disasters (including cyberwar, cyberterrorism, or state-sponsored attacks); inflationary pressures, which may increase costs for materials and finished goods; adverse or uncertain macroeconomic conditions, including fears of a global economic downturn or recession; increases in wages that drive up prices; rising interest rates; workforce disruptions; labor shortages or stoppages; the imposition of regulations, trade protection measures, tariffs, duties, import/export restrictions, quotas or embargoes on key components; transportation failures affecting the supply and shipment of materials and finished goods; and the unavailability of raw materials. Any of these events could result in, among other things, damage to or the temporary closure of one or more of our manufacturing or distribution facilities or reference laboratories (damage to one of our facilities or the manufacturing equipment we use could be costly and may require substantial lead-time to repair or replace); damage to or closure of one or more facilities of our third-party business partners or suppliers on which we rely; a temporary lack of an adequate work force in one or more sites; an interruption in power supply; a temporary or long-term disruption in our supply chain or logistics network (including a disruption to our ability to obtain critical components for the manufacture of our products); a temporary disruption in our ability to deliver (or delays in the delivery of) our products or services; and short- or long-term damage to our customers’ businesses (which would adversely impact customer demand for our products and services). For our veterinary customers, these events may negatively affect the number of patient visits and elective procedures, and the volume of diagnostic utilization, and may otherwise disrupt clinical operations, which could adversely affect our revenues. For more information regarding the risks presented by disruption to our suppliers’ operations and supply chain, refer to “Our dependence on third-party suppliers could negatively affect our ability to sell certain products or deliver our cloud-based software solutions, which could negatively affect our operating results” above.
We manufacture many of our significant companion animal products, including our rapid assay products and certain instruments and consumables, many of our water testing products and certain of our livestock, poultry, and dairy testing products in Southern Maine. Certain of our companion animal products, as well as our human point-of-care products, are manufactured in Roswell, Georgia. We also manufacture certain of our livestock and poultry testing products in Bern, Switzerland and Montpellier, France. In addition, we maintain major distribution facilities in North America and in the Netherlands and reference laboratories in multiple locations, including Memphis, Tennessee; Kornwestheim, Germany; West Sacramento, California; Elmhurst, Illinois; North Grafton, Massachusetts; Brisbane, Australia; Markham, Ontario; Wetherby, U.K.; and Tokyo, Japan. Interruption of operations at any of our facilities, including the ones described above, due to the occurrence of one or more of the events described above could have an adverse effect on our results of operations.
While we maintain plans to continue business under such circumstances, there can be no assurance that such plans will be successful in fully or partially mitigating the effects of such events. We also maintain property and business interruption insurance to insure against the financial impact of certain events of this nature. However, this insurance may be insufficient to compensate us for the full amount of any losses that we may incur. In addition, such insurance will not compensate us for potential long-term competitive or reputational effects of being out of the market for the period of any interruption in operations.
Since our business is global in nature, geopolitical risks and other risks associated with doing business internationally could negatively affect our business, financial condition, and operating results
For the year ended December 31, 2024, approximately 35% of our overall revenue and approximately 33%, 82% and 48% of our CAG, LPD, and Water revenues, respectively, were attributable to sales of products and services to customers outside the U.S. Although we intend to continue to expand our international operations and business, we may not be able to successfully promote, market, import, export, sell, or distribute our products and services outside the U.S. Various risks associated with foreign operations may impact our international sales and operations, including, but not limited to, disruptions in transportation of our products or our supply chain; fluctuations in oil prices; increased border protection and restriction on travel; the differing product and service needs of foreign customers; difficulties in building, staffing, and managing foreign operations (including a geographically dispersed workforce); differing protection of intellectual property; trade protection measures, quotas, embargoes, import/export restrictions, capital controls, tariffs, duties, and regulatory and licensing requirements; natural and other disasters; public health issues (such as outbreaks, epidemics, or the prospect of a pandemic); ongoing instability or changes in a country’s or region’s regulatory, economic, or political conditions, including inflation, recession, interest rate fluctuations, and actual or anticipated military or political conflicts; geopolitical crises, including terrorism, war, armed conflict, or civil or military unrest; other unfavorable geopolitical conditions; security concerns; and local business and cultural factors that differ from our normal standards and practices, including business practices prohibited by the Foreign Corrupt Practices Act and other anti-corruption laws and regulations.
In addition, to market and sell many of our products outside the U.S., we are subject to product approval and registration requirements that often require us to provide confidential, proprietary information about those products to foreign regulatory agencies. There can be no assurance that the confidential, proprietary information provided to foreign regulatory agencies to comply with product approval and registration requirements may not be accessed by unauthorized persons or otherwise stolen, which could negatively affect our ability to protect our proprietary rights in our innovative products and our future success. We also may forgo marketing and selling some of our products in certain foreign jurisdictions due to the risk of intellectual property theft, which could negatively affect our ability to expand our international operations and business. For more information about the risks related to the protection of our proprietary rights in our products and services, refer to “Our success is heavily dependent on our continued differentiated product and service innovation” above.
Further, prices that we charge to foreign customers may be different than the prices we charge for the same products in the U.S. due to competitive, market or other factors, or changes in foreign currency exchange rates. Our results of operations are also susceptible to changes in foreign currency exchange rates. As a result, the mix of domestic and international sales in a particular period could have an adverse impact on our results of operations for that period.
Climate change, or legal, regulatory or market measures to address climate change, could adversely affect our business, financial condition, and results of operation
We operate in many regions around the world where our businesses and the activities of our customers and suppliers could be disrupted by climate change. We are exposed to physical risks (such as extreme weather conditions or rising sea levels), risks in transitioning to a low-carbon economy (such as additional legal or regulatory requirements, changes in technology, market risk and reputational risk) and social and human effects (such as harm to health and well-being) associated with climate change.
These risks can be either acute (short-term) or chronic (long-term).
Potential physical risks from climate change may include altered distribution and intensity of rainfall, prolonged droughts or flooding, increased frequency of wildfires and other natural disasters, rising sea levels, and a rising heat index, any of which could cause negative impacts to our and our customers’ and suppliers’ businesses. Increased frequency and severity of extreme weather events could adversely impact our suppliers, manufacturing locations, logistics, and/or customers. Such impacts may include losses incurred as a result of physical damage to facilities, loss or spoilage of inventory, and business interruption and disruption (e.g., decrease in companion animal clinical visits and diagnostic utilization). Other potential physical impacts due to climate change include reduced access to high-quality water in certain regions and the loss of biodiversity, which could impact future product development. These risks could disrupt our operations and supply chain, which may result in increased costs, or adversely affect our revenues.
New legal or regulatory requirements may be enacted to prevent, mitigate, or adapt to the implications of a changing climate and its effects on the environment. These regulations, which may differ across jurisdictions, could result in our being subject to new or expanded carbon pricing or taxes, increased compliance costs, restrictions on greenhouse gas emissions, investment in new technologies, increased carbon disclosure and transparency, investments in developing data gathering and reporting systems, upgrade of facilities to meet new building codes, increased energy costs, and the redesign of utility systems, which could increase our operating costs. Our supply chain would likely be subject to these same transitional risks and would likely pass along any increased costs to us, which may impact our ability to procure goods or services required for the operation of our business at the quantities and levels we require.
A weak worldwide economy, or actual or perceived economic weakness in any significant geography, could result in reduced demand for our products and services or increased customer credit risk
A substantial percentage of our sales are made worldwide to the companion animal veterinary industry. Demand for our companion animal diagnostic products and services is driven in part by the number of patient visits to veterinary hospitals and the practices of veterinarians with respect to the recommendations for diagnostic testing, as well as pet owner compliance with these recommendations. Pet owners generally pay cash out of pocket for health care services for their pets from veterinary practices. Actual or perceived economic weakness, whether due to inflation or other factors, in any of our significant geographies could cause pet owners in those regions to forgo or defer visits to veterinary hospitals or affect their willingness to approve certain diagnostic tests, comply with a treatment plan or, even more fundamentally, continue to own a pet. In addition, concerns about the financial resources of pet owners could cause veterinarians to be less likely to recommend certain diagnostic tests, and concerns about the economy may cause veterinarians to defer purchasing capital items such as our instruments and systems. These conditions, if they continue, could result in a decrease in sales or decrease in sales growth, of diagnostic products and services, which could have an adverse effect on our results of operations.
Demand for our water products is driven in part by the availability of funds at government laboratories, water utilities, and private certified laboratories that utilize our products. Availability of funds also affects demand by government laboratories and cattle, swine and poultry producers that utilize our livestock and poultry diagnostic products, and by users of our human diagnostic products and services. Actual or perceived economic weakness and the other factors described above have caused and could continue to cause our customers to reduce their investment in such testing, which could have an adverse effect on our results of operations.
A weak economy may also cause deterioration in the financial condition of our distributors and customers, which could inhibit their ability to pay us amounts owed for products delivered or services provided in a timely fashion or at all.
We are subject to risks associated with public health issues, including pandemics, which could have a material adverse effect on our financial condition and results of operations
We are subject to risks associated with public health issues, including pandemics and other events beyond our control. Public health issues and crises may adversely impact our operations, supply chain and logistics network if the locations where we operate, manufacture or distribute our products; where our raw materials or product components are sourced, manufactured or distributed; or where our third-party distributors, suppliers and other service providers operate, are disrupted, temporarily closed or experience worker shortages for a sustained period of time. In addition, public health issues and crises may adversely impact our customers’ businesses due to business lockdowns, decreased companion animal clinical visits, labor shortages, the delay of elective procedures and wellness visits, and disruption of veterinary clinic and other customer operations, all of which could cause a decline in demand for our products and services.
These disruptions could also cause economic slowdowns or increased economic uncertainty.
A future public health issue, pandemic, or outbreak could lead to delays in the manufacturing and supply of products, or adversely affect the ability of the USDA, FDA, EPA or other government agencies to timely review and process our regulatory submissions, which could have a material adverse effect on our business and results of operations. Moreover, any future public health issue, pandemic, or outbreak could result in the imposition of new governmental restrictions, quarantine requirements or other public health measures, which could result in closures or other restrictions that significantly disrupt our operations or those of our third-party distributors, suppliers or other service providers, or otherwise adversely affect our customers’ businesses or operations, or result in actual or perceived economic weakness or slowdowns in one or more of our key geographies, any of which could adversely affect our financial condition.
Our operations and reputation may be impaired if we, our products, or our services do not comply with our global privacy policy or evolving laws and regulations regarding data privacy and protection
Our business operations involves the receipt, storage and use of information, including personal data, about our customers, pet owners, suppliers, and employees. We collect and use personal data in a variety of ways. We offer products and services that collect and use personal data, including veterinary practice management systems, online customer communication tools and services, VetConnect PLUS, and two-way integration technology. Some of these products and services rely on third-party providers for cloud computing and storage. We also engage in e-commerce through various websites and collect contact and other personal data from our customers and website visitors. In addition, we transfer information, including personal data, among IDEXX, our subsidiaries and third parties with which we have commercial relations for business purposes. Our collection, transfer, protection, security, retention, storage, disclosure, sharing and use of personal data are subject to expanding and increasingly complex laws and regulations in the U.S. and abroad. In addition, these laws and regulations continue to develop and are subject to frequent revisions (and generally have become more stringent over time), are subject to differing interpretations, may be applied inconsistently from jurisdiction to jurisdiction and could be deemed to be inconsistent with our current global privacy policy and data protection practices.
While we maintain a program to monitor, assess, and comply with applicable global data privacy laws, compliance with these evolving requirements can be costly, require us to change our business practices in a manner adverse to our business or delay or impede the development and offering of innovative products and services. Additionally, public perception and standards related to the privacy of personal data can shift rapidly, in ways that may affect our reputation or influence regulators in the U.S. and abroad to expand or adopt more stringent regulations and laws. As a global company, we are tasked with navigating a patchwork of privacy laws, both in the U.S. and abroad, which may have conflicting requirements that can make compliance challenging. Some examples of privacy laws include (but are not limited to) the California Consumer Privacy Act, as amended by the California Privacy Rights Act (“CPRA”), various U.S. state consumer privacy laws that apply to our business operations within a respective state and/or a U.S. federal privacy law that may be passed in the future; and the European Union’s General Data Protection Regulation (“GDPR”) and similar requirements adopted by the United Kingdom (“UK”) following the UK’s withdrawal from the European Union (“EU”) and the European Economic Area (“EEA”), which impose stringent operational requirements for controllers and processors of personal data of individuals in the EEA and UK. Additional examples include the China Personal Information Protection Law, the Brazilian General Data Protection Law, the South African Protection of Personal Information Act, the Amendments to the Japanese Act on the Protection of Personal Information, the New Zealand Privacy Act, the Australian Privacy and Other Legislation Amendment Bill and the India Digital Personal Data Protection Act.
An additional area of complexity concerns the restrictions on transfers of personal data from certain countries to others. We currently rely on a mixture of mechanisms to transfer certain personal data from the EEA, Switzerland, and the UK to the U.S. and other third countries including the EU-U.S. Data Privacy Framework (the “EU-U.S. DPF”), the Swiss-U.S. Data Privacy Framework (the “Swiss-U.S. DPF”), and the UK Extension to the EU-U.S. DPF. We expect the EU-U.S. DPF adequacy decision, adopted in July of 2023, to be challenged and international transfers to the U.S. and to other jurisdictions more generally to continue to be subject to enhanced scrutiny by regulators. Any transfers by us or our vendors of personal data are subject to potential regulatory scrutiny and may increase our exposure under the GDPR, UK GDPR, and similar laws which contain cross-border personal data transfer heightened requirements and restrictions (such as the new Chinese government standard contract for cross-border personal data transfers).
Any failure or perceived failure by us, the third parties with whom we work or our products and services to comply with all applicable privacy-related laws and regulations, as well as our contractual obligations, could result in damage to our reputation or legal proceedings or actions against us by governmental entities or others, any of which could have an adverse effect on our business.
In addition, concerns about our practices with regard to the collection, use, retention, disclosure, or security of personal data or other privacy-related matters, even if unfounded and even if we are in compliance with applicable laws and regulations, could damage our reputation and harm our business.
Failure to meet current or evolving environmental, social, and governance regulations, standards, or expectations or to achieve our environmental, social, or governance goals could adversely affect our business, results of operations, financial condition, reputation, or stock price
Regulators, as well as our investors, customers, employees, and other stakeholders, are increasingly focused on environmental, social, and governance matters, including climate-related issues; human capital matters; and responsible sourcing, human rights, and supply chain. Regulators in the U.S., Europe, and elsewhere are considering or have proposed or adopted various laws, directives or regulations regarding these matters, which include specific, target-driven disclosure requirements or obligations, including the European Union’s Corporate Sustainability Reporting Directive and Corporate Sustainability Due Diligence Directive and California’s Climate Corporate Data Accountability Act and Climate-Related Financial Risk Act. Furthermore, our investors, customers and other stakeholders have additional (and sometimes different or contradictory) expectations.
We strive to align our environmental, social and governance goals with our long-term business-strategy and Purpose. Some stakeholders, however, including government regulators, may disagree with some or all of our goals and initiatives. The focus of our various stakeholders may also change and evolve over time, and they may have very different (and sometimes contradictory) views on which matters should be prioritized or even undertaken at all. Meeting these emerging and evolving regulations, standards and expectations will require us to make strategic choices and investments, incur compliance costs, and create new practices, processes, and procedures. In addition, if we fail to comply with applicable regulations, or our practices do not meet evolving investor, customer or other stakeholder expectations and standards, then our reputation, ability to attract, retain employees, relationships with some customers and attractiveness as an investment, business partner, acquiror or product or service provider could be negatively impacted, which could adversely affect our business, results of operations, financial condition, reputation, or stock price.
Our ability to achieve any of these goals is subject to numerous risks, many of which are outside of our control and depend in part on third-party performance or data, and there can be no assurance that we will achieve them. Our failure to adequately update, accomplish or accurately track and report on these goals on a timely basis, or at all, could adversely affect our reputation and expose us to increased scrutiny from the investment community, special interest groups and enforcement authorities.
Future operating results could be negatively affected by changes in tax rates, the adoption of new tax legislation or exposure to additional tax liabilities
The nature of our global operations subjects us to local, state, regional and federal tax laws in jurisdictions around the world. Our future tax expense could be affected by changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities or changes in tax laws or their interpretation. Specifically, many jurisdictions have committed to adopting the Organisation for Economic Co-operation and Development (“OECD”) Pillar Two Global Minimum Tax. Pillar Two is designed to ensure large multinational enterprises pay a minimum effective tax of at least 15% on income in each jurisdiction. As of December 31, 2024, various countries have enacted aspects of Pillar Two while committing to enact additional aspects in future years. While we do not expect these rules to have a material impact on our effective tax rate, we continue to monitor these initiatives on a global basis. Additionally, tax law enacted by the Tax Cuts and Jobs Act includes provisions that are scheduled to take effect January 1, 2026, that will increase our effective tax rate should they not be extended.
While we have taken, and may take further, actions intended to align our corporate structure and intercompany relationships with supporting our growth in international geographies and maintaining operational and tax efficiency and continue to consider all of these developments within our overall tax strategy, changes in tax law in the U.S. and other countries in which we operate or have a presence may materially and adversely impact our income tax liability, provision for income taxes, effective tax rate and results of operation, and there can be no assurance that any actions we take to maintain operational and tax efficiency will effectively mitigate these impacts. Moreover, these actions may increase our operating costs, and if ineffectual, could increase our income tax liabilities and our global effective tax rate.
Our income tax filings are subject to examination by various tax authorities, and the final determination of tax audits could be materially different from that which is reflected in historical income tax provisions and accruals. Significant judgment is required in determining our worldwide provision for income taxes.
We regularly assess our exposures related to our worldwide provision for income taxes to determine the adequacy of our provision for taxes. Any reduction in these contingent liabilities or additional assessments would increase or decrease income, respectively, in the period such determination is made. If the ultimate determination of taxes owed is for an amount in excess of amounts previously accrued, our business, results of operations and financial condition could be materially adversely affected.
Strengthening of the rate of exchange for the U.S. dollar has a negative effect on our business
We are a global business, with 35% of our revenue during the year ended December 31, 2024, attributable to sales of products and services to customers outside of the U.S. Any strengthening of the rate of exchange for the U.S. dollar against foreign currencies, and in particular the euro, British pound, Canadian dollar, Chinese renminbi, Japanese yen, Australian dollar and Brazilian real, adversely affects our results, as it reduces the dollar value of sales and profits that are made in those currencies. The strengthening of the U.S. dollar has a greater adverse effect on the profits from products manufactured or sourced in U.S. dollars that are exported to international geographies and a lesser effect on profits from foreign sourced products and services due to a natural hedge from international expenses denominated in the corresponding foreign currencies.
For the year ended December 31, 2024, approximately 22% of our consolidated revenue was derived from products manufactured or sourced in U.S. dollars and sold internationally in local currencies, compared to 21% for the years ended December 31, 2023, and December 31, 2022. A strengthening U.S. dollar could also negatively impact the ability of customers outside the U.S. to pay for purchases denominated in U.S. dollars as well as affect our overall competitiveness in international geographies. The accumulated impacts from any continued, longer-term growth in the value of the U.S. dollar against foreign currencies may have a material adverse effect on our operating results. Refer to “Part II, Item 7A. Quantitative and Qualitative Disclosures About Market Risk” included in this Annual Report on Form 10-K for additional information regarding currency impact.
Our foreign currency hedging activities (refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 19. Hedging Instruments” in the accompanying Notes to consolidated financial statements), which are designed to minimize and delay, but not to eliminate, the effects of foreign currency fluctuations, may not sufficiently offset the adverse financial effect of unfavorable movements in foreign exchange rates on our financial results over the limited time the hedges are in place. In addition, our hedging activities involve costs and risks, such as transactions costs and the risk that our hedging counterparties will default on their obligations.
We primarily hedge intercompany product purchases and sales denominated in the euro, British pound, Canadian dollar, Japanese yen, and Australian dollar. Other foreign currency exposures related to foreign sourced services and emerging markets may not be practical to hedge. In certain cases, these exposures are not offset by foreign currency denominated costs. As we primarily use foreign currency exchange contracts with durations of less than 24 months and enter into contracts to hedge incremental portions of anticipated foreign currency transactions on a quarterly basis for the current and following year, the effectiveness of our foreign currency hedging activities to offset longer-term appreciation in the value of the U.S. dollar against non-U.S. currencies may be limited. Factors that could affect the effectiveness of our hedging activities include accuracy of sales and other forecasts, volatility of currency markets, and the cost and availability of hedging instruments. Since our hedging activities are designed to minimize volatility, they not only temporarily reduce the negative impact of a stronger U.S. dollar, but they also temporarily reduce the positive impact of a weaker U.S. dollar. Our future financial results could be significantly affected by a strengthening value of the U.S. dollar in relation to the foreign currencies in which we conduct business. The degree to which our financial results are affected for any given time period will depend in part upon our hedging activities.
Restrictions in our debt agreements or our inability to obtain financing on favorable terms may increase our cost of borrowing and limit our activities
Our ability to make scheduled payments and satisfy our other obligations under our Credit Facility and senior notes depends on our future operating performance and on economic, financial, competitive, and other factors beyond our control. Our business may not generate sufficient cash flows to meet these obligations or generate sufficient levels of earnings to satisfy the applicable affirmative, negative, and financial covenants. Our failure to comply with these covenants and the other terms of the Credit Facility and senior notes could result in an event of default and acceleration of our obligations under these agreements, which may require us to seek additional financing or restructure existing debt on unfavorable terms. In addition, adverse changes in credit markets could increase our cost of borrowing and make it more difficult for us to obtain financing, which could limit our ability to execute certain strategies and have an adverse effect on our revenue growth and profitability.
Our senior notes require payment of a prepayment penalty in the event that we elect to repay the notes prior to their stated maturity dates. Should we elect to repay some or all of the outstanding principal balance on our senior notes prior to their stated maturity dates, the prepayment penalty we incur could adversely affect our results of operations and cash flows.
We fund our operations, capital purchase requirements and strategic growth needs through cash on hand, funds generated from operations, amounts available under our Credit Facility and senior note financings. If we are unable to obtain financing on favorable terms, we could face restrictions that would limit our ability to execute certain strategies, which could have an adverse effect on our revenue growth and profitability.
Borrowings under our Credit Facility bear interest at variable rates, including rates based on the Secured Overnight Financing Rate (“SOFR”), exposing us to interest rate risk. The recent rise in interest rates has increased our cost of borrowing. If interest rates were to continue to increase, our debt service obligations under our variable-rate Credit Facility would increase even if the principal amount borrowed remained the same.
RISKS RELATED TO AN INVESTMENT IN OUR SECURITIES
Fluctuations in our quarterly or annual results may cause our stock price to decline
Our prior operating results have fluctuated due to a number of factors, many of which are beyond our control, including seasonality of certain product lines; changes in our accounting estimates; the impact of acquisitions; timing of distributor purchases, product launches, operating expenditures, and customer marketing and incentive programs; changes in the number and type of competitors and their product and service offerings; changes in our sales and distribution model; changes in the economy affecting consumer spending; and other matters. Similarly, our future operating results may vary significantly from quarter to quarter or year to year due to these and other factors. If our operating results or projected operating results do not meet the expectations of securities analysts or investors in future periods, our stock price may fall.
The market price of our common stock may be highly volatile, and you may not be able to resell your shares at or above the price you paid
The trading price of our common stock may be volatile. Securities markets worldwide experience significant price and volume fluctuations. This market volatility, as well as other general economic, market, or political conditions, could reduce the market price of our common stock rapidly and unexpectedly, in spite of our operating performance. Factors that may impact the market price of our common stock include the factors described in this “Risk Factors” section and elsewhere in this Form 10-K, as well as:
•Our stock repurchase program and changes in our capital structure or cost of capital, including the issuance of additional debt;
•Public announcements (including the timing of these announcements) regarding our business, financial performance and prospects or new products or services, product enhancements or technological advances by our competitors or us;
•Trading activity in our stock, including portfolio transactions in our stock by the company, our executive officers and directors, and significant stockholders; trading activity that results from the ordinary course rebalancing of stock indices in which we may be included, such as the S&P 500 Index; trading activity related to our inclusion in, or removal from, any stock indices; and short interest in our common stock, which could be significant from time to time;
•Investor perception of the company and the industry and sectors in which we operate, including changes in earnings estimates or buy/sell recommendations by securities analysts; and whether or not we meet earnings estimates of securities analysts; and
•General financial, domestic, international, economic, and market conditions, including overall fluctuations in the U.S. equity and credit markets, which may experience extreme volatility that, in some cases, is unrelated or disproportionate to the operating performance of particular companies.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
ITEM 1C. CYBERSECURITY
Our Cybersecurity Risk Management Program
Like other companies, we currently inhabit an environment of increasing global cybersecurity vulnerabilities and threats. We aim to effectively assess, identify, and manage material risks from these cybersecurity threats through our cybersecurity risk management program.
Our cybersecurity risk management program includes processes that incorporate and utilize certain principles from the National Institute of Standards and Technology Cybersecurity Framework and the Center for Internet Security – Top 18 Critical Security Controls – Control Level Framework. The program aims to protect and preserve the security, availability, integrity, confidentiality, and privacy of our information systems and information residing on those systems and includes controls and procedures for the prevention, identification, containment, and remediation of cybersecurity threats through the use of various technologies, tools, policies, standards, and practices. Features of our cybersecurity risk management program include:
•An expectation, set forth in our Code of Ethics, that all employees are responsible for protecting our data, operations and environment from unauthorized access and use;
•Regular cybersecurity risk assessments and benchmarking;
•Policies and processes related to the detection and reporting of and response to cybersecurity events;
•Cybersecurity training for all newly hired employees upon onboarding;
•Individualized, biannual employee information security assessments, coupled with tailored follow-on employee trainings;
•Phishing tests conducted at least quarterly on a global basis, with additional periodic phishing tests conducted with high-risk employee groups;
•Channels for employees to report suspicious emails or other activity and the actual or suspected loss, theft, improper use of or access to IDEXX systems or information;
•Deployment and ongoing assessment of the effectiveness of technological tools aimed at preventing, detecting, and mitigating cybersecurity threats;
•Policies and procedures to assess third-party service provider cybersecurity risks and security controls and measures (as part of our procurement process and periodically thereafter);
•Periodic performance of cybersecurity tabletop exercises;
•Regular review of and, as applicable, updates to our cyber incident response plan and protocols, system backup measures, redundancy planning and disaster recovery plans; and
•Maintenance of a cyber risk insurance policy to help address risk of loss due to certain types of cybersecurity events.
A review of cybersecurity risks is integrated into our annual enterprise risk assessment that occurs as part of our annual strategic planning process and is included in our quarterly disclosure controls and procedures. Our annual enterprise risk assessment process involves the identification and assessment by senior line-of-business and functional leaders, as well as our Chief Information Security Officer (“CISO”) and Chief Information Officer (“CIO”), of the risks relevant to their lines of business and functional areas, the materiality of those risks, our risk tolerances and our plans to manage and mitigate the risks to the extent prudent and feasible.
From time to time, we engage third parties, including assessors, consultants, legal counsel, and others to conduct penetration testing, assess our program, provide recommendations for improvement, and advise us on best practices.
Material Effects from Risks of Cybersecurity Threats
We do not believe any risks from cybersecurity threats (including from any prior cybersecurity incidents) have materially affected or are reasonably likely to materially affect us, including our business strategy, results of operations, or financial condition. There can be no assurances, however, that we or our business partners or suppliers will not experience a future system disruption, attack or security breach that materially impacts our business, operations, results of operations, or financial condition. For more information refer to “Item 1A. Risk Factors, General Risks, We depend on the continuous and reliable operation and security of our information technology systems and our products and services that incorporate or rely on information technology, and any disruption or significant cybersecurity breach or other incident could adversely affect our business.”
Governance of our Cybersecurity Risk Management Program
Role of Management
Our cybersecurity risk management program and activities are led by a dedicated CISO who is also our Vice President of Information Technology. Our CISO reports to our Senior Vice President and CIO, and oversees a team of information security professionals within the Information Security Group. Our CISO joined IDEXX in 2024 and has more than twenty years of business and technical experience leading information technology teams, including cybersecurity teams, at high tech, marketing and healthcare companies. Our CISO, in close collaboration with our CIO is responsible for our cybersecurity-related governance programs, overseeing testing of our compliance with standards and remediation of known risks, and leads our employee training program.
Our CISO is responsible for providing information regarding our cybersecurity risk management program, as well as cybersecurity risks and incidents, to a senior management-level cybersecurity steering committee. Within our cybersecurity risk governance model, the steering committee, which includes our CIO, CISO, General Counsel, Chief Compliance Officer, Chief Audit Executive, Chief Human Resources Officer and other senior functional and business leaders, meets quarterly, and more frequently as warranted, to review and discuss, among other things, our cybersecurity risk assessments, prioritization of initiatives, training plans and incident response plan, protocols and testing. This committee regularly provides updates on its discussions and decisions to our Chief Executive Officer.
Role of the Board of Directors
The Audit Committee has responsibility for overseeing our cybersecurity risk management. In accordance with the Audit Committee’s charter, the Audit Committee at least annually reviews and discusses with management, including the CIO and CISO, our processes, policies, procedures, and protocols related to cybersecurity and information security. In addition, the Audit Committee regularly reviews and discusses with management, including the CIO and CISO, cybersecurity program assessments and audits, planned improvements and the status of any information security initiatives, as well as risks from cybersecurity threats pertinent to us and any previous cybersecurity incidents experienced by us, including any material impact or reasonably likely material impact on the Company, our business strategy, results of operations, or financial condition. The Audit Committee provides reports to the Board at each regularly scheduled Board meeting of the matters it has recently addressed, including relating to the oversight of our cybersecurity risk management, and the full Board may participate, as warranted, in the Audit Committee’s sessions on cybersecurity risk management. Outside advisors also may meet from time to time with the Audit Committee or Board, as warranted, to review and discuss cybersecurity matters.
ITEM 2. PROPERTIES
Our worldwide headquarters and principal executive offices are located in Westbrook, Maine where we engage in manufacturing, research and development, marketing, sales, and general and administrative support functions.
Primary Facility Locations
|
|
|
|
|
|
|
|
|
| Location |
Functions |
Own/Lease |
| Westbrook, Maine |
Worldwide Headquarters, principal executive offices |
Own |
| Hoofddorp, Netherlands |
Distribution center, warehousing, International administrative offices |
Lease |
Baar, Switzerland |
EMEA administrative offices |
Lease |
Scarborough, Maine |
Water, LPD and Supply Chain |
Own |
| Memphis, Tennessee |
Distribution Center and Reference Lab |
Lease |
North Grafton, Massachusetts |
Reference Lab |
Own |
West Sacramento, California |
Reference Lab |
Own |
| Kornwestheim, Germany |
Reference Lab |
Own |
| Wetherby, United Kingdom |
Reference Lab |
Own |
Markham, Ontario, Canada |
Reference Lab |
Lease |
Elmhurst, Illinois |
Reference Lab |
Lease |
| Newmarket, United Kingdom |
Water manufacturing |
Lease |
| Bern, Switzerland |
LPD manufacturing |
Lease |
| Montpellier, France |
LPD manufacturing |
Lease |
| Roswell, Georgia |
CAG and OPTI Medical manufacturing |
Lease |
Including the locations above, we have over 50 reference laboratories throughout the United States and over 25 reference laboratories internationally, including locations in Europe, Canada, Australia, New Zealand, Asia, and South Africa. The majority of our reference laboratories are leased, with the remainder being owned. We also lease space in various locations worldwide for administrative support, manufacturing, sales, distribution, and storage. We believe that our leased and owned properties are generally in good condition, are well-maintained, and are generally suitable and adequate to carry on our business.
ITEM 3. LEGAL PROCEEDINGS
Due to the nature of our activities, we are at times subject to pending and threatened legal actions that arise out of the ordinary course of business. In the opinion of management, based in part upon advice of legal counsel, the disposition of any such currently pending or threatened matters is not expected to have a material effect on our results of operations, financial condition, or cash flows. However, the results of legal actions cannot be predicted with certainty. Therefore, it is possible that our results of operations, financial condition or cash flows could be materially adversely affected in any particular period by the unfavorable resolution of one or more legal actions.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
PART II
ITEM 5. MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is quoted on the NASDAQ Global Select Market under the symbol IDXX.
Holders of Common Stock
As of February 18, 2025, there were 313 holders of record of our common stock. Because the majority of our common stock is held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by these record holders.
Purchases of Equity Securities by the Issuer
During the three months ended December 31, 2024, we repurchased shares of common stock as described below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Period |
|
Total Number of Shares Purchased
(a) |
|
Average Price Paid per Share
(b) |
|
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs (1)
(c)
|
|
Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs (1)
(d)
|
|
|
|
|
|
|
|
|
|
| October 1, 2024 to October 31, 2024 |
|
177,643 |
|
|
$ |
469.14 |
|
|
177,640 |
|
|
6,519,346 |
|
| November 1, 2024 to November 30, 2024 |
|
183,300 |
|
|
$ |
427.23 |
|
|
183,300 |
|
|
6,336,046 |
|
| December 1, 2024 to December 31, 2024 |
|
202,602 |
|
|
$ |
429.80 |
|
|
202,500 |
|
|
6,133,546 |
|
| Total |
|
563,545 |
|
(2) |
|
|
563,440 |
|
|
6,133,546 |
|
|
|
|
|
|
|
|
|
|
(1)As of December 31, 2024, our Board of Directors had approved the repurchase of up to 78 million shares of our common stock in the open market or in negotiated transactions pursuant to the Company’s share repurchase program, which amount includes the approval of an additional 5 million shares on December 3, 2024. The initial program was approved and announced on August 13, 1999, and the maximum number of shares that may be purchased under the program has been increased by the Board of Directors on numerous occasions. There is no specified expiration date for this repurchase program and it may be suspended or discontinued at any time. There were no other repurchase programs outstanding during the three months ended December 31, 2024, and no repurchase programs expired during the period.
(2)During the three months ended December 31, 2024, we received 105 shares of our common stock that were surrendered by employees in payment for the required withholding taxes due on the vesting of restricted stock units and settlement of deferred stock units. In the above table, these shares are included in columns (a) and (b) but excluded from columns (c) and (d). These shares do not reduce the number of shares that may yet be purchased under the repurchase program.
During the year ended December 31, 2024, we repurchased approximately 1.74 million shares of our common stock in transactions made pursuant to our repurchase program and received approximately 0.02 million shares of our common stock that were surrendered by employees in payment for the minimum required withholding taxes due on the vesting of restricted stock units and settlement of deferred stock units. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 20. Repurchases of Common Stock” to the consolidated financial statements for the year ended December 31, 2024, included in this Annual Report on Form 10-K for further information.
Dividends
We have never declared or paid any cash dividends on our common stock. From time to time our Board of Directors may consider the declaration of a dividend. However, we have no intention to declare or pay a dividend at this time.
Stock Performance
This graph compares our total stockholder returns, the Total Return for the Standard & Poor’s (“S&P”) 500 Index, the Total Return for the S&P 500 Health Care Index, and the Total Return for the NASDAQ Stock Market Index (U.S. Companies) (the “NASDAQ Index”) prepared by the Center for Research in Security Prices. This graph assumes the investment of $100 on December 31, 2019, in IDEXX’s common stock, the S&P 500 Index, the S&P 500 Health Care Index, and the NASDAQ Index and assumes dividends, if any, are reinvested. Measurement points are the last trading days of the years ended December 2019 to 2024. Historic stock price performance should not be relied on as being indicative of future stock price performance.
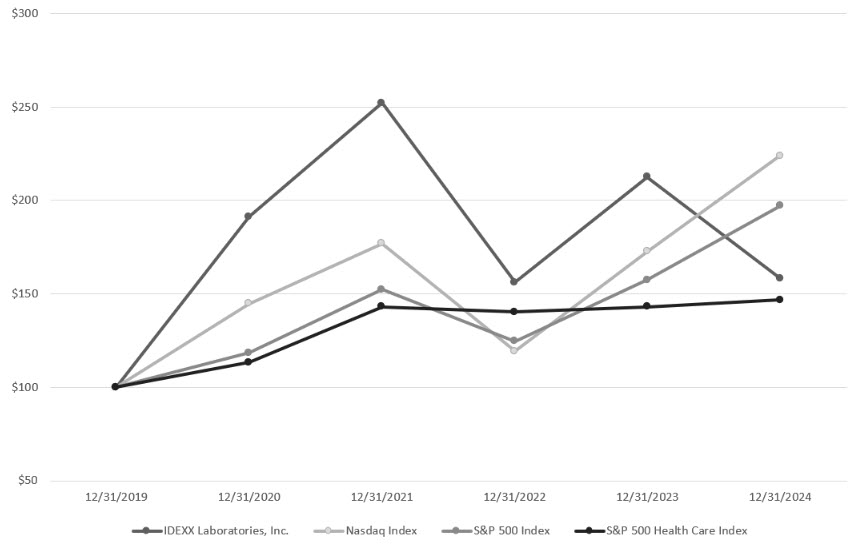
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2019 |
|
2020 |
|
2021 |
|
2022 |
|
2023 |
|
2024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| IDEXX Laboratories, Inc. |
|
$100.00 |
|
$191.43 |
|
$252.16 |
|
$156.23 |
|
$212.56 |
|
$158.33 |
| NASDAQ Index |
|
$100.00 |
|
$144.92 |
|
$177.06 |
|
$119.45 |
|
$172.77 |
|
$223.87 |
| S&P 500 Index |
|
$100.00 |
|
$118.40 |
|
$152.39 |
|
$124.79 |
|
$157.59 |
|
$197.02 |
| S&P 500 Health Care Index |
|
$100.00 |
|
$113.45 |
|
$143.09 |
|
$140.29 |
|
$143.18 |
|
$146.87 |
ITEM 6. [RESERVED]
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 10‑K. The discussion of our financial condition and results of operations and liquidity and capital resources for the year ended December 31, 2022, and year-over-year comparisons between 2023 and 2022, is included in our Annual Report on Form 10-K for the year ended December 31, 2023, within Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations, and is incorporated by reference herein.
We have included certain terms and abbreviations used throughout this Annual Report on Form 10-K in the “Glossary of Terms and Selected Abbreviations.”
Description of Business Segments. We operate primarily through three business segments: diagnostic and information management-based products and services for the companion animal veterinary industry, which we refer to as the Companion Animal Group (“CAG”); water quality products (“Water”); and diagnostic products and services for livestock and poultry health and to ensure the quality and safety of milk and improve producer efficiency, which we refer to as Livestock, Poultry and Dairy (“LPD”). Our Other operating segment combines and presents our human medical diagnostic products and services business (“OPTI Medical”) with our out-licensing arrangements because they do not meet the quantitative or qualitative thresholds for reportable segments. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 3. Revenue and Note 17. Segment Reporting” to the consolidated financial statements for the year ended December 31, 2024, included in this Annual Report on Form 10-K, for financial information about our segments, including our product and service categories, and our geographic areas.
The following is a discussion of the strategic and operating factors that we believe have the most significant effect on the performance of our business.
Companion Animal Group
Our strategy is to provide veterinarians with the highest quality diagnostic information, software products and services, and medical evidence to support more advanced medical care and information management solutions that help demonstrate the value of diagnostics to pet owners and enable efficient and effective practice management. By doing so, we are able to build a mutually successful relationship with our veterinarian customers based on healthy pets, loyal customers, staff efficiency, and expanding practice revenues.
CAG Diagnostics. We provide diagnostic capabilities that meet veterinarians’ diverse needs through a variety of modalities, including in-clinic diagnostic solutions and outside reference laboratory services. Veterinarians that utilize our full line of diagnostic modalities obtain a single view of a patient’s diagnostic results, which allows them to track and evaluate trends and achieve greater medical insight.
Our diagnostic capabilities generate both recurring and non-recurring revenues. Revenues related to capital placements of our in-clinic IDEXX VetLab suite of instruments and our SNAP Pro Analyzer are non-recurring in nature in that they are sold to a particular customer only once. Revenues from the associated IDEXX VetLab consumables, SNAP rapid assay test kits, reference laboratory and consulting services, and extended maintenance agreements and accessories related to our IDEXX VetLab instruments, and our SNAP Pro Analyzer are recurring in nature, in that they are regularly purchased by our customers, typically as they perform diagnostic testing as part of ongoing veterinary care services. Our recurring revenues, most prominently IDEXX VetLab consumables and rapid assay test kits, have significantly higher gross margins than those provided by our instrument sales. Therefore, the sales mix of recurring and non-recurring revenues in a particular period will impact our gross margins.
Diagnostic Capital Revenue. Revenues related to the placement of the IDEXX VetLab suite of instruments are non-recurring in nature, in that the customer will buy an instrument once over its respective product life cycle, but will purchase consumables for that instrument on a recurring basis as they use that instrument for diagnostic testing purposes. Many instruments are placed through our customer commitment arrangements in exchange for multi-year customer commitments to purchase recurring products and services.
Below is a table showing the installed base units of our premium diagnostic instruments as of the years ended December 31, 2024, 2023, and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (units in thousands) |
|
Installed Base (1) |
| Instrument |
|
December 31, 2024 |
|
December 31, 2023 |
|
December 31, 2022 |
| Catalyst |
|
74.1 |
|
69.1 |
|
63.1 |
| Premium Hematology |
|
51.8 |
|
47.8 |
|
43.1 |
| SediVue |
|
21.3 |
|
18.1 |
|
15.6 |
(1) IDEXX InVue Dx was launched in the fourth quarter of 2024, with ten instrument placements.
Our long-term success in the continuing growth of our CAG recurring diagnostic products and services is dependent upon: growing volumes at existing customers by increasing their utilization of existing and new test offerings, acquiring new customers, maintaining high customer loyalty and retention, and realizing price increases based on our differentiated products and the growing value of our diagnostic offering. We continuously seek opportunities to enhance the care that veterinary professionals give to their patients and clients through supporting the implementation of real-time care testing workflows, which is performing tests and sharing test results with the client at the time of the patient visit. Our latest generation of chemistry, hematology, cytology, and urinalysis instruments demonstrates this commitment by offering enhanced ease of use, faster time to results, broader test menu, and connectivity to various information technology platforms that enhance the value of the diagnostic information generated by the instruments. In addition, we provide marketing tools and customer support that help drive efficiencies in veterinary practice processes and allow practices to increase the number of clients they see on a daily basis.
With all of our instrument product lines, we seek to differentiate our products from our competitors’ products based on time-to-result, ease-of-use, throughput, breadth of diagnostic menu, flexibility of menu selection, accuracy, reliability, ability to handle compromised samples, analytical capability of diagnostics software, integration with the IVLS and VetConnect PLUS, client communications capabilities, education and training, and superior sales and customer service. Our success depends, in part, on our ability to differentiate our products in a way that justifies a premium price.
Recurring Diagnostic Revenue. Revenues from our IDEXX VetLab consumable products, our SNAP rapid assay test kits, outside reference laboratory and consulting services, and extended maintenance agreements and accessories related to our CAG Diagnostics instruments are considered recurring in nature. For the year ended December 31, 2024, recurring diagnostic revenue, which is both highly durable and profitable, accounted for approximately 80% of our consolidated revenue.
Our in-clinic diagnostic solutions, consisting of our IDEXX VetLab consumable products and SNAP rapid assay test kits, provide real-time reference lab quality diagnostic results for a variety of companion animal diseases and health conditions. Our outside reference laboratories provide veterinarians with the benefits of a more comprehensive list of diagnostic tests and access to consultations with board-certified veterinary specialists and pathologists, combined with the benefit of same-day or next-day turnaround times for most tests.
We derive substantial revenues and margins from the sale of consumables that are used in IDEXX VetLab instruments, and the multi-year consumable revenue stream is significantly more valuable than the placement of the instrument. Our strategy is to increase diagnostic testing within veterinary practices by placing IDEXX VetLab instruments and increasing instrument utilization of consumables. Utilization can increase due to a greater number of patient samples being run or to an increase in the number of tests being run per patient sample. Our strategy is to increase both drivers. To increase utilization, we seek to educate veterinarians about best medical practices that emphasize the importance of chemistry, hematology, and urinalysis testing for a variety of diagnostic purposes, as well as by introducing new testing capabilities that were previously not available to veterinarians.
Our in-clinic diagnostic solutions also include SNAP rapid assay tests that address important medical needs for particular diseases prevalent in the companion animal population. We seek to differentiate these tests from those of other in-clinic test providers and reference laboratory diagnostic service providers based on critically important sensitivity and specificity, as demonstrated by peer-reviewed third-party research, as well as overall superior performance and ease of use by providing our customers with combination tests that test a single sample for up to six diseases at once, including the ability to utilize our SNAP Pro Analyzer. We further augment our product development and customer service efforts with sales and marketing programs that enhance medical awareness and understanding regarding certain diseases and the importance of diagnostic testing.
The prevalence of in-clinic testing, as opposed to outside reference laboratories such as IDEXX Reference Laboratories, may vary by region. We attempt to differentiate our reference laboratory testing services from those of competitive reference laboratories and competitive in-clinic offerings primarily on the basis of a differentiated test menu, technology employed, quality, turnaround time, customer service, and tools such as VetConnect PLUS that demonstrate the complementary manner in which our laboratory services work with our in-clinic offerings.
Profitability in our lab business is supported, in part, by our expanding business scale globally. Profit improvements also reflect benefits from price increases and our ability to achieve operational efficiencies. When possible, we utilize core reference laboratories to service samples from other states or countries, expanding our customer reach without an associated expansion in our reference laboratory footprint. New laboratories may operate at a loss until testing volumes achieve sufficient scale. Acquired laboratories frequently operate less profitably than our existing laboratories and acquired laboratories may not achieve the profitability of our existing laboratory network for several years until we complete the implementation of operating improvements and efficiencies. Therefore, in the short term, new and acquired reference laboratories generally may have a negative effect on our operating margin.
Recurring reference lab revenue growth is achieved both through increased testing volumes with existing customers and through the acquisition of new customers, net of customer losses. We believe the increased number of customer visits by our sales professionals as a result of the growth in our field sales organization has led to increased reference laboratory opportunities with customers who already use one of our in-clinic diagnostic modalities. Recurring reference laboratory diagnostic and consulting revenues have also increased as a result of our customer commitment arrangements, and customer gains from reference laboratory acquisitions, customer list acquisitions, and the opening of new reference laboratories, including laboratories that are co-located with large practice customers.
Veterinary Software, Services and Diagnostic Imaging Systems. Our portfolio of practice management offerings is designed to serve the full range of customers primarily within the North American, Australian, New Zealand, and European regions. Cornerstone, ezyVet, IDEXX Neo, and Animana practice management systems provide integrated information solutions, backed by customer support and education. These practice management systems support the veterinarian’s ability to practice better medicine and achieve the practice’s business objectives, including a quality client experience, staff efficiency, and practice effectiveness and profitability. We market Cornerstone, ezyVet, and IDEXX Neo practice management systems to customers primarily in North America, Australia, and New Zealand. We market our Animana offering to customers primarily throughout Europe.
Our diagnostic imaging systems offer a convenient radiographic solution that provides superior image quality and the ability to share images with clients virtually anywhere. IDEXX imaging software enables enhanced diagnostic features and streamlined integration with our other products and services. Our digital radiography systems enable low-dose radiation image capture without sacrificing clear, high-quality diagnostic images, reducing the risk posed by excess radiation exposure for veterinary professionals.
Recurring Revenue. Animana, ezyVet, and IDEXX Neo practice management systems are subscription-based SaaS offerings designed to provide flexible pricing and a durable, recurring revenue stream, while utilizing cloud technology instead of a client server platform. We also offer add-on subscription services such as Pet Health Network Pro, Vello, Petly Plans, and credit card processing. Our Cornerstone customer base continues to be an important driver of growth through diagnostic integrations and add-on subscription services. We continue to make investments to enhance the customer experience of all of our license-based software offerings. Our large practice management systems installed base provides access to veterinary channel transaction activity, enabling a syndicated data offering.
With our SmartFlow and Vet Radar cloud technology, we are able to improve overall patient management through coordination and tracking of every step in a patient workflow. Our Pet Health Network Pro and Vello software provide online client communication and engagement functionality integrated into practice management system workflow.
Placements of imaging systems are important to the growth of revenue streams that are recurring in nature, including extended maintenance agreements and IDEXX Web PACS, which is our cloud-based SaaS offering for viewing, accessing, storing, and sharing multi-modality diagnostic images. We derive relatively higher margins from our subscription-based products. IDEXX Web PACS is integrated with Cornerstone, ezyVet, IDEXX Neo, and IDEXX VetConnect PLUS to provide centralized access to diagnostic imaging results alongside patient diagnostic results from any internet connected device.
Systems and Hardware. We differentiate our practice management systems through enhanced functionality, ease of use, and embedded integration with in-clinic IDEXX VetLab instruments and outside reference laboratory test results.
We offer software, hardware, and integrated services that run key functions of veterinary clinics, including managing patient electronic health records, scheduling, client communication, billing, and inventory management. We also provide installation and advisory services associated with our systems and hardware placements.
Our diagnostic imaging systems capture radiographic images in digital form, replacing traditional x-ray film and the film development process, which generally requires the use of hazardous chemicals and darkrooms. We market and sell two diagnostic imaging systems primarily used in small animal veterinary applications: the IDEXX ImageVue DR50 and the IDEXX ImageVue DR30.
Water
Our strategy in the water testing business is to develop, manufacture, market, and sell products that test primarily for the presence of microbial contamination in water matrices, including drinking water supplies, with superior performance, supported by exceptional customer service. Our customers primarily consist of water utilities, government laboratories, and private certified laboratories that highly value strong relationships and customer support. We expect that future growth in this business will be partially dependent on our ability to increase international sales. Growth also will be dependent on our ability to enhance and broaden our product line. Most water microbiological testing is driven by regulation, and, in many countries, a test may not be used for compliance testing unless it has been approved by the applicable regulatory body and integrated into customers’ testing protocols. As a result, we maintain an active regulatory program that involves applying for a growing number of regulatory approvals in a number of countries, primarily in Europe. Further, we seek to receive regulatory approvals from governing agencies as a means to differentiate our products from the competition.
Livestock, Poultry and Dairy
We develop, manufacture, market, and sell a broad range of tests and perform services for various livestock diseases and conditions, and have active research and development and in-licensing programs in this area. Our strategy is to offer differentiated tests with superior performance characteristics for use in government programs to control or eradicate disease and disease outbreaks and in livestock and poultry producers’ disease, reproductive, and herd health and production management programs. Our Alertys Ruminant Pregnancy Test, Rapid Visual Pregnancy Test and Alertys On-Farm Pregnancy Test for cattle can detect pregnancy 28 days after breeding. These tests provide a quick and accurate identifier using whole blood samples.
Disease outbreaks are episodic and unpredictable, and certain diseases that are prevalent at one time may be substantially contained or eradicated at a later time. In response to outbreaks, testing initiatives may lead to exceptional demand for certain products in certain periods. Conversely, successful eradication programs may result in significantly decreased demand for certain products. In addition, increases in government funding may lead to increased demand for certain products and budgetary constraints may lead to decreased demand for certain products. As a result, the performance in certain sectors of this business can fluctuate.
Our strategy in the dairy testing business is to develop, manufacture, and sell antibiotic residue and contaminant testing products that satisfy applicable regulatory requirements or dairy processor standards for testing of milk and provide reliable field performance. The manufacture of these testing products leverages the SNAP platform and production assets that also support our rapid assay business, which also leverages the SNAP platform. The dairy SNAP products incorporate customized reagents for antibiotic and contaminant detection.
Other
OPTI Medical. Our strategy in the OPTI Medical business for the human market is to develop, manufacture, and sell electrolyte and blood gas analyzers, and related consumable products, for the medical point-of-care diagnostics sector worldwide, with a focus on small to mid-sized hospitals. We seek to differentiate our products based on ease of use, convenience, international distribution and service, and instrument reliability. Similar to our veterinary instruments and consumables strategy, a substantial portion of the revenues from this product line is derived from the sale of consumables for use on the installed base of electrolyte and blood gas analyzers.
Previously, we also provided human testing solutions for the detection of SARS-CoV-2, the virus that causes COVID-19. During the first quarter of 2023, we discontinued actively marketing our COVID-19 testing products and services.
Our facility in Roswell, Georgia, develops and manufactures the OPTI product lines using the same or similar technology to support the electrolyte requirements of certain CAG products. We leverage this facility’s know-how, intellectual property, and manufacturing capability to continue to expand the menu and instrument capability of the VetStat and Catalyst platforms for veterinary applications, while reducing our cost of consumables by leveraging experience and economies of scale.
CRITICAL ACCOUNTING ESTIMATES AND ASSUMPTIONS
The discussion and analysis of our financial condition and results of operations is based upon the consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We evaluate our estimates on an ongoing basis. We base our estimates on historical experience and on various assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 2. Summary of Significant Accounting Policies” to the consolidated financial statements included in this Annual Report on Form 10-K for a description of the significant accounting policies used in preparation of these consolidated financial statements.
We believe the following critical accounting estimates and assumptions may have a material impact on reported financial condition and operating performance and involve significant levels of judgment to account for highly uncertain matters or are susceptible to significant change.
Revenue Recognition
Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 3. Revenue” to the consolidated financial statements for the year ended December 31, 2024, included in this Annual Report on Form 10-K for additional information about our revenue recognition policy and criteria for recognizing revenue.
We enter into contracts where customers purchase combinations of IDEXX products and services. Determining whether products and services are considered distinct performance obligations that should be accounted for separately requires judgment. We determine the transaction price for a contract based on the total consideration we expect to receive in exchange for the transferred goods or services. To the extent the transaction price includes variable consideration, such as volume rebates or expected price adjustments, we apply judgment in constraining the estimated variable consideration due to factors that may cause reversal of revenue recognized. We evaluate constraints based on our historical and projected experience with similar customer contracts.
We allocate revenue to each performance obligation in proportion to the relative standalone selling prices and recognize revenue when control of the related goods or services is transferred for each obligation. We utilize the observable standalone selling price when available, which represents the price charged for the promised product or service when sold separately. When standalone selling prices for our products or services are not directly observable, we determine the standalone selling prices using relevant information available and apply suitable estimation methods including, but not limited to, the cost plus a margin approach.
Our customer commitment arrangements that include free or discounted instruments and systems, such as our IDEXX 360 program, provide customers with free or discounted instruments or systems upon entering into multi-year arrangements to purchase annual minimum amounts of products and services. We allocate total consideration, including future committed purchases and expected price adjustments, based on relative standalone selling prices, to identified performance obligations and recognize instrument revenue and cost at the time of installation and customer acceptance in advance of billing the customer, which is also when the customer obtains control of the instrument based on legal title transfer. Our right to future consideration related to instrument revenue is recorded as a contract asset within other current and long-term assets. The contract asset is transferred to accounts receivable when customers are billed for products and services over the term of the arrangement. We estimate, based on historical experience, and apply judgment to predict the amounts of future customer purchases and expected price adjustments related to these multi-year arrangements. Differences between estimated and actual customer purchases may impact the timing and amount of revenue recognition during the term of the customer arrangement, and a 10% change in these estimates would have increased or reduced contract assets and cumulative recognized revenue related to these programs by approximately $7.5 million as of December 31, 2024.
Our customer commitment arrangements that include up-front consideration paid to customers provide customers with incentives in the form of IDEXX Points or, from time to time, cash, upon entering into multi-year arrangements to purchase annual minimum amounts of future products and services. If a customer breaches their agreement, they are required to refund all or a portion of the up-front consideration, or make other repayments, remedial actions, or both.
Up-front incentives to customers are not made in exchange for distinct goods or services and are capitalized as consideration paid to customers within other current and long-term assets, which are subsequently recognized as a reduction to revenue over the term of the customer arrangement. If these up-front incentives are subsequently utilized to purchase instruments, we allocate total consideration, including future committed purchases less up-front incentives and estimates of expected price adjustments, based on relative standalone selling prices, to identified performance obligations and recognize instrument revenue and cost at the time of installation and customer acceptance. We estimate, based on historical experience, and apply judgment to predict the amounts of future customer purchases and expected price adjustments related to these multi-year arrangements. Differences between estimated and actual customer purchases may impact the timing and amount of revenue recognition during the term of the customer arrangement, and a 10% change in these estimates would have increased or reduced cumulative recognized revenue related to these programs by approximately $1.1 million as of December 31, 2024.
Our rebate arrangements provide customers the opportunity to earn future rebates based on the volume of products and services they purchase over the term of the arrangement. We account for the customer’s right to earn rebates on future purchases as a separate performance obligation and determine the standalone selling price based on an estimate of rebates the customer will earn over the term of the arrangement. Total consideration allocated to identified performance obligations is limited to goods and services that the customer is presently obligated to purchase and does not include estimates of future purchases that are optional. We allocate total consideration to identified performance obligations, including the customer’s right to earn rebates on future purchases, which is deferred and subsequently recognized upon the purchase of products and services. We estimate, based on historical experience, and apply judgment to predict the amounts of future customer rebates related to these multi-year arrangements. Differences between estimated and actual customer rebates may impact the timing and amount of revenue recognition during the term of the customer arrangement, and a 10% change in these estimates would have increased or reduced deferred revenue and cumulative recognized revenue related to these programs by approximately $0.3 million as of December 31, 2024.
Future market conditions and changes in product offerings may cause us to change marketing strategies to increase or decrease customer incentive offerings, possibly resulting in incremental reductions of revenue in future periods compared to reductions in the current or prior periods. Additionally, certain customer arrangements require us to estimate, based on historical experience, and apply judgment to predict the amounts of future customer purchases, customer rebates and other incentive payments, and price adjustments related to multi-year arrangements. Differences between estimated and actual customer purchases may impact the timing and amount of revenue recognition as described above.
Income Taxes
The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the estimated future tax effects of temporary differences between book and tax treatment of assets and liabilities and carryforwards to the extent they are realizable.
We assess our current and projected earnings by jurisdiction to determine whether or not our earnings during the periods when the temporary differences become deductible will be sufficient to realize the related future tax benefits. Should we determine that we would not be able to realize all or part of our net deferred tax asset in a particular jurisdiction in the future, an adjustment to the deferred tax asset would be charged to income in the period such determination was made.
For those jurisdictions where tax carryforwards are likely to expire unused or the projected operating results indicate that realization is not more-likely-than-not, a valuation allowance is recorded to offset the deferred tax asset within that jurisdiction. In assessing the need for a valuation allowance, we consider future taxable income and ongoing prudent and feasible tax planning strategies. In the event that we determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, a reduction of the valuation allowance would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, a reduction to the deferred tax asset would be charged to income in the period such determination was made.
Our net taxable temporary differences and tax carryforwards are recorded using the enacted tax rates expected to apply to taxable income in the periods in which the deferred tax liability or asset is expected to be settled or realized. Should the expected applicable tax rates change in the future, an adjustment to our deferred taxes would be credited or charged, as appropriate, to income in the period such determination was made.
We periodically assess our exposures related to our worldwide provision for income taxes and believe that we have appropriately accrued taxes for contingencies. Any reduction of these contingent liabilities or additional assessment would increase or decrease income, respectively, in the period such determination was made.
We record a liability for uncertain tax positions that do not meet the more-likely-than-not standard as prescribed by the authoritative guidance for income tax accounting. We record tax benefits for only those positions that we believe will more-likely-than-not be sustained. For positions that we believe that it is more-likely-than-not that we will prevail, we record a benefit considering the amounts and probabilities that could be realized upon ultimate settlement. If our judgment as to the likely resolution of the uncertainty changes, if the uncertainty is ultimately settled, or if the statute of limitations related to the uncertainty expires, the effects of the change would be recognized in the period in which the change, resolution, or expiration occurs. Our net liability for uncertain tax positions was $18.1 million as of December 31, 2024, and $22.3 million as of December 31, 2023. We also accrue for estimated interest expense and penalties on our uncertain tax positions. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 14. Income Taxes” in the accompanying Notes to consolidated financial statements for more information.
RECENT ACCOUNTING PRONOUNCEMENTS
Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 2. Summary of Significant Accounting Policies (v) and (w)” to the consolidated financial statements for the year ended December 31, 2024, included in this Annual Report on Form 10-K for a complete discussion of recent accounting pronouncements adopted and not adopted.
RESULTS OF OPERATIONS AND TRENDS
Effects of Certain Factors on Results of Operations
CAG Trends. Global trends in companion animal healthcare, including growth in demand for clinical services, continue to support solid growth for companion animal diagnostic products and services across regions. In the U.S., average diagnostics revenue per practice grew approximately 4% on a same-store basis during 2024, faster than approximately 3% growth in overall practice revenues. U.S. same-store clinical visits at veterinary practices declined approximately 2% in 2024, impacted by ongoing veterinary practice capacity challenges from the influx of higher volumes during the pandemic, as well as macroeconomic headwinds. Our initial 2025 financial outlook anticipates a decline in U.S. same-store clinical growth levels reflecting these near-term sector and macroeconomic dynamics. Our products and services include solutions that help improve staff productivity and create additional capacity at veterinary clinics. For example, during 2024, we upgraded our IDEXX VetLab Station software and also launched our new slide-free cellular analyzer, IDEXX inVue Dx.
Supply Chain and Logistics Challenges. We believe that building and maintaining a well-managed and disciplined infrastructure has helped minimize impacts of supply chain constraints, including product and component availability issues, logistics challenges, including extended shipping periods and delays, and inflationary pressures. Our proactive approach to managing our operational processes, including forward planning with a focus on working closely with our suppliers and logistics partners, enables us to maintain continued high levels of product and service availability and customer service. We believe we are well-positioned to enable sustained high growth in our businesses and to effectively manage the impacts of potentially relatively higher costs in certain areas to support these growth plans. However, there can be no assurance as to the duration or severity of the supply chain and logistics challenges or the effectiveness of our mitigating activities.
Effects of Economic Conditions. Demand for our products and services is vulnerable to changes in the economic environment, including slow economic growth, inflationary pressure, high unemployment, and credit availability. Negative or cautious consumer sentiment can lead to reduced or delayed consumer spending, resulting in a decreased number of patient visits to veterinary clinics or lower use of diagnostics. Unfavorable economic conditions can impact sales of instruments, diagnostic imaging, and practice management systems, which are larger capital purchases for veterinarians. In the U.S., we monitor patient visits and clinic revenue data provided by a subset of our CAG customers. Although this data is a limited sample, and may be susceptible to short-term impacts, we believe that this data provides a fair and meaningful long-term representation of the trend in patient visit activity in the U.S., providing us insight regarding demand for our products and services.
Economic conditions can also affect the purchasing decisions of our Water and LPD business customers. Water testing volumes may be susceptible to declines in discretionary testing for existing home and commercial sales and in mandated testing as a result of decreases in home and commercial construction. In addition, fiscal difficulties can also reduce government funding for water and herd health screening services.
We believe that the diversity of our products and services and the geographic diversity of our customers partially mitigate the potential effects of the economic environment and negative consumer sentiment on our revenue growth rates.
Effect of Geopolitical Conflicts. The overall financial performance of our business may be impacted by geopolitical instability and macroeconomic conditions. International conflicts and tensions may result in uncertainty in the markets, volatility in exchange rates, tariffs, and inflationary trends.
Currency Impact. For the year ended December 31, 2024, approximately 22% of our consolidated revenue was derived from products manufactured or sourced in U.S. dollars and sold internationally in local currencies, compared to 21% for both the years ended December 31, 2023, and December 31, 2022. Strengthening of the rate of exchange for the U.S. dollar relative to other currencies has a negative impact on our revenues derived in currencies other than the U.S. dollar and on profits of products manufactured or purchased in U.S. dollars and sold internationally, and a weakening of the U.S. dollar has the opposite effect. Similarly, to the extent that the U.S. dollar is stronger in current or future periods relative to the exchange rates in effect in the corresponding prior periods, our growth rate will be negatively affected. The impact of foreign currency denominated operating expenses and foreign currency denominated supply contracts partly offsets this exposure. Additionally, our designated hedges of intercompany inventory purchases and sales help delay the impact of certain exchange rate fluctuations on non-U.S. denominated revenues. Refer to “Part II, Item 7A. Quantitative and Qualitative Disclosures About Market Risk” included in this Annual Report on Form 10-K for additional information regarding currency impact. Our future income tax expense could also be affected by changes in the mix of earnings, including as a result of changes in the rate of exchange for the U.S. dollar relative to currencies in countries with differing statutory tax rates. Refer to “Part I, Item 1A. Risk Factors” included in this Annual Report on Form 10-K for additional information regarding tax impacts.
Distributor Purchasing and Inventories. When selling our products through distributors, changes in distributors’ inventory levels can impact our reported sales, and these changes may be affected by many factors, which may not be directly related to underlying demand for our products by veterinary practices, which are the end users. If during a quarter or year, distributors’ inventories grew by less than those inventories grew in the comparable period of the prior year, then changes in distributors’ inventories would have an unfavorable impact on our reported sales growth in the current period. Conversely, if during a quarter or year, distributors’ inventories grew by more than those inventories grew in the comparable period of the prior year, then changes in distributors’ inventories would have a favorable impact on our reported sales growth in the current period.
In certain countries, we sell our products through third-party distributors and may be unable to obtain data for sales to end users. We do not believe the impact of changes in these distributors’ inventories had or would have a material impact on our growth rates. Refer to “Part I, Item 1. Business, Marketing and Distribution” included in this Annual Report on Form 10-K for additional information regarding distribution channels.
Effects of Patent Expiration. Although certain patents and licenses of patents and technologies from third parties periodically expire, the expiration of these patents or licenses, individually or in the aggregate, is not expected to have a material effect on our financial position or future operations due to a range of factors as described in “Part I, Item 1. Intellectual Property, Including Patents and License.”
Non-GAAP Financial Measures. The following revenue analysis and discussion focuses on organic revenue growth, and references in this analysis and discussion to “revenue,” “revenues” or “revenue growth” are references to “organic revenue growth.” Organic revenue growth is a non-GAAP financial measure and represents the percentage change in revenue during the current year, compared to the same period for the prior year, net of the effect of changes in foreign currency exchange rates, certain business acquisitions, and divestitures. Organic revenue growth should be considered in addition to, and not as a replacement for, or as a superior measure to, revenue growth reported in accordance with U.S. GAAP, and may not be comparable to similarly titled measures reported by other companies. Management believes that reporting organic revenue growth provides useful information to investors by facilitating easier comparisons of our revenue performance with prior and future periods and to the performance of our peers.
We exclude from organic revenue growth the effect of changes in foreign currency exchange rates because changes in foreign currency exchange rates are not under management’s control, are subject to volatility, and can obscure underlying business trends. We calculate the impact on revenue resulting from changes in foreign currency exchange rates by applying the difference between the weighted average exchange rates during the current year period and the comparable prior year period to foreign currency denominated revenues for the prior year period.
We also exclude from organic revenue growth the effect of certain business acquisitions and divestitures because the nature, size, and number of these transactions can vary dramatically from period to period, and because they either require or generate cash as an inherent consequence of the transaction, and therefore can also obscure underlying business and operating trends. We consider acquisitions to be a business when all three elements of inputs, processes, and outputs are present, consistent with ASU 2017-01, “Business Combinations: (Topic 805) Clarifying the Definition of a Business.” In a business combination, if substantially all the fair value of the assets acquired is concentrated in a single identifiable asset or group of similar identifiable assets, we do not consider these assets to be a business. A typical acquisition that we do not consider a business is a customer list asset acquisition, which does not have all elements necessary to operate a business, such as employees or infrastructure.
We believe the efforts required to convert and retain these acquired customers are similar in nature to our existing customer base and therefore are included in organic revenue growth.
We also use Adjusted EBITDA, gross debt, net debt, gross debt to Adjusted EBITDA ratio, and net debt to Adjusted EBITDA ratio, all of which are non-GAAP financial measures that should be considered in addition to, and not as a replacement for, financial measures presented according to U.S. GAAP. Management believes that reporting these non-GAAP financial measures provides supplemental analysis to help investors further evaluate our business performance and available borrowing capacity under our Credit Facility.
Comparisons to Prior Periods. Our fiscal years end on December 31. Unless otherwise stated, the analysis and discussion of our financial condition, results of operations and liquidity, including references to growth and organic growth and increases and decreases, are being compared to the equivalent prior year period.
Twelve Months Ended December 31, 2024, Compared to Twelve Months Ended December 31, 2023
Total Company
The following table presents revenue by operating segment by U.S. and non-U.S., or international, geographies:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
|
|
|
|
|
|
|
|
|
Net Revenue
(dollars in thousands)
|
|
2024 |
|
2023 |
|
Dollar Change |
|
Reported Revenue Growth (1) |
|
Percentage Change from Currency |
|
Percentage Change from Acquisitions |
|
Organic Revenue Growth (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CAG |
|
$ |
3,574,044 |
|
|
$ |
3,352,356 |
|
|
$ |
221,688 |
|
|
6.6 |
% |
|
(0.2 |
%) |
|
0.4 |
% |
|
6.4 |
% |
| United States |
|
2,409,152 |
|
|
2,282,507 |
|
|
126,645 |
|
|
5.5 |
% |
|
— |
|
|
0.6 |
% |
|
5.0 |
% |
| International |
|
1,164,892 |
|
|
1,069,849 |
|
|
95,043 |
|
|
8.9 |
% |
|
(0.7 |
%) |
|
— |
|
|
9.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Water |
|
$ |
185,112 |
|
|
$ |
168,149 |
|
|
$ |
16,963 |
|
|
10.1 |
% |
|
(0.5 |
%) |
|
— |
|
|
10.6 |
% |
| United States |
|
95,347 |
|
|
83,838 |
|
|
11,509 |
|
|
13.7 |
% |
|
— |
|
|
— |
|
|
13.7 |
% |
| International |
|
89,765 |
|
|
84,311 |
|
|
5,454 |
|
|
6.5 |
% |
|
(1.0 |
%) |
|
— |
|
|
7.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| LPD |
|
$ |
122,060 |
|
|
$ |
121,659 |
|
|
$ |
401 |
|
|
0.3 |
% |
|
(0.9 |
%) |
|
— |
|
|
1.2 |
% |
| United States |
|
22,250 |
|
|
18,961 |
|
|
3,289 |
|
|
17.3 |
% |
|
— |
|
|
— |
|
|
17.3 |
% |
| International |
|
99,810 |
|
|
102,698 |
|
|
(2,888) |
|
|
(2.8 |
%) |
|
(1.0 |
%) |
|
— |
|
|
(1.8 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Other |
|
$ |
16,288 |
|
|
$ |
18,789 |
|
|
$ |
(2,501) |
|
|
(13.3 |
%) |
|
— |
|
|
— |
|
|
(13.3 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total Company |
|
$ |
3,897,504 |
|
|
$ |
3,660,953 |
|
|
$ |
236,551 |
|
|
6.5 |
% |
|
(0.3 |
%) |
|
0.4 |
% |
|
6.4 |
% |
| United States |
|
2,533,174 |
|
|
2,391,427 |
|
|
141,747 |
|
|
5.9 |
% |
|
— |
|
|
0.5 |
% |
|
5.4 |
% |
| International |
|
1,364,330 |
|
|
1,269,526 |
|
|
94,804 |
|
|
7.5 |
% |
|
(0.8 |
%) |
|
— |
|
|
8.2 |
% |
(1)Reported revenue growth and organic revenue growth may not recalculate due to rounding.
Total Company Revenue. The increase in organic revenue reflects growth in CAG Diagnostics recurring revenue, including benefits from higher realized prices and, to a lesser extent, increased volumes, supported by new business gains and sustained high customer retention rates, offsetting constraints from macroeconomic and sector headwinds. Increases in our recurring veterinary software, services, and diagnostic imaging revenue, supported by higher volumes and price gains, also contributed to increased revenue. Higher revenue in our Water business was primarily due to the benefit of realized price increases and higher testing volumes in the U.S. and Europe. The increase in LPD revenue was primarily due to higher realized prices and volume growth in the U.S. and Europe, partially offset by lower testing levels in Asia Pacific. The decrease in Other revenue was primarily due to lower volumes of our OPTI Medical instruments and consumables. The impact of a business acquisition increased total revenue growth by 0.4%, while the change in foreign currency exchange rates decreased total revenue growth by 0.3%.
The following table presents our consolidated Company results of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
|
Total Company - Results of Operations
(dollars in thousands)
|
|
2024 |
|
Percent of Revenue |
|
2023 |
|
Percent of Revenue |
|
Amount |
|
Percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenues |
|
$ |
3,897,504 |
|
|
|
|
$ |
3,660,953 |
|
|
|
|
$ |
236,551 |
|
|
6.5 |
% |
| Cost of revenue |
|
1,518,577 |
|
|
|
|
1,470,983 |
|
|
|
|
47,594 |
|
|
3.2 |
% |
| Gross profit |
|
2,378,927 |
|
|
61.0 |
% |
|
2,189,970 |
|
|
59.8 |
% |
|
188,957 |
|
|
8.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
588,507 |
|
|
15.1 |
% |
|
566,066 |
|
|
15.5 |
% |
|
22,441 |
|
|
4.0 |
% |
| General and administrative |
|
442,291 |
|
|
11.3 |
% |
|
335,825 |
|
|
9.2 |
% |
|
106,466 |
|
|
31.7 |
% |
| Research and development |
|
219,792 |
|
|
5.6 |
% |
|
190,951 |
|
|
5.2 |
% |
|
28,841 |
|
|
15.1 |
% |
| Total operating expenses |
|
1,250,590 |
|
|
32.1 |
% |
|
1,092,842 |
|
|
29.9 |
% |
|
157,748 |
|
|
14.4 |
% |
| Income from operations |
|
$ |
1,128,337 |
|
|
29.0 |
% |
|
$ |
1,097,128 |
|
|
30.0 |
% |
|
$ |
31,209 |
|
|
2.8 |
% |
Gross Profit. Gross profit increased due to higher revenue and a 120 basis point increase in the gross profit margin. The increase in the gross profit margin reflected favorable business mix, the benefit from price realization offsetting inflationary cost impacts, lower instrument costs, and software and services margin gains. The change in foreign currency exchange rates on the gross profit margin was not significant.
Operating Expenses. Sales and marketing expense increased primarily due to higher personnel-related, meeting, and travel costs. General and administrative expense increased primarily due to a $61.5 million expense related to an ongoing litigation matter, the comparison to the prior year benefit of a $16 million customer contract resolution gain, higher information technology and outside services, and acquisition-related costs. Research and development expense increased primarily due to higher project costs. The change in foreign currency exchange rates was not significant to operating expense growth.
Companion Animal Group
The following table presents revenue by product and service category for CAG:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
|
|
|
|
|
|
|
|
|
Net Revenue
(dollars in thousands)
|
|
2024 |
|
2023 |
|
Dollar Change |
|
Reported Revenue Growth (1) |
|
Percentage Change from Currency |
|
Percentage Change from Acquisitions |
|
Organic Revenue Growth (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| CAG Diagnostics recurring revenue: |
|
$ |
3,129,492 |
|
|
$ |
2,935,425 |
|
|
$ |
194,067 |
|
|
6.6 |
% |
|
(0.2 |
%) |
|
— |
|
|
6.8 |
% |
| IDEXX VetLab consumables |
|
1,303,250 |
|
|
1,188,261 |
|
|
114,989 |
|
|
9.7 |
% |
|
(0.3 |
%) |
|
— |
|
|
10.0 |
% |
| Rapid assay products |
|
359,754 |
|
|
344,494 |
|
|
15,260 |
|
|
4.4 |
% |
|
(0.3 |
%) |
|
— |
|
|
4.7 |
% |
| Reference laboratory diagnostic and consulting services |
|
1,336,121 |
|
|
1,278,617 |
|
|
57,504 |
|
|
4.5 |
% |
|
(0.1 |
%) |
|
— |
|
|
4.6 |
% |
| CAG Diagnostics services and accessories |
|
130,367 |
|
|
124,053 |
|
|
6,314 |
|
|
5.1 |
% |
|
(0.4 |
%) |
|
— |
|
|
5.5 |
% |
| CAG Diagnostics capital - instruments |
|
$ |
131,928 |
|
|
$ |
137,603 |
|
|
$ |
(5,675) |
|
|
(4.1 |
%) |
|
(0.8 |
%) |
|
— |
|
|
(3.4 |
%) |
| Veterinary software, services and diagnostic imaging systems: |
|
$ |
312,624 |
|
|
$ |
279,328 |
|
|
$ |
33,296 |
|
|
11.9 |
% |
|
(0.1 |
%) |
|
4.7 |
% |
|
7.3 |
% |
| Recurring revenue |
|
250,359 |
|
|
214,597 |
|
|
35,762 |
|
|
16.7 |
% |
|
— |
|
|
6.0 |
% |
|
10.7 |
% |
Systems and hardware |
|
62,265 |
|
|
64,731 |
|
|
(2,466) |
|
|
(3.8 |
%) |
|
(0.1 |
%) |
|
0.3 |
% |
|
(4.0 |
%) |
| Net CAG revenue |
|
$ |
3,574,044 |
|
|
$ |
3,352,356 |
|
|
$ |
221,688 |
|
|
6.6 |
% |
|
(0.2 |
%) |
|
0.4 |
% |
|
6.4 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) Reported revenue growth and organic revenue growth may not recalculate due to rounding. |
CAG Diagnostics Recurring Revenue. The increase in CAG Diagnostics recurring revenue was primarily due to higher realized prices and, to a lesser extent, increased volumes, supported by new business gains and sustained high customer retention rates, offsetting constraints from macroeconomic and sector headwinds. The change in foreign currency exchange rates decreased CAG Diagnostics recurring revenue growth by 0.2%.
The increase in IDEXX VetLab consumables revenue was primarily due to higher price realization and, to a lesser extent, higher sales volumes, supported by the expansion of our installed base of instruments and our expanded menu of available tests. The change in foreign currency exchange rates decreased revenue growth by 0.3%.
The increase in rapid assay revenue resulted primarily from higher price realization, partially offset by lower volumes driven by lower clinical visits in the U.S. The change in foreign currency exchange rates decreased revenue growth by 0.3%.
The increase in reference laboratory diagnostic and consulting services revenue was due to higher global price realization and higher testing volumes, primarily in the U.S. and, to a lesser extent, Europe and Asia. The change in foreign currency exchange rates decreased revenue growth by 0.1%.
The increase in CAG Diagnostics services and accessories revenue was primarily a result of the 9% increase in our installed base of premium instruments. The change in foreign currency exchange rates decreased revenue growth by 0.4%.
CAG Diagnostics Capital – Instrument Revenue. The decrease in instrument revenue was primarily due to program effects on pricing, partially offset by higher ProCyte One and SediVue Dx placements. The change in foreign currency exchange rates decreased revenue growth by 0.8%.
Veterinary Software, Services, and Diagnostic Imaging Systems Revenue. The increase in recurring revenue was primarily due to higher subscription and support services volume from our expanded SaaS installed base and higher realized prices. The impact of a business acquisition increased recurring revenue growth by 6.0%. The decrease in our systems and hardware revenue was primarily due to lower digital imaging system sales and lower hardware sales associated with the transition to cloud-based software placements, partially offset by higher realized prices. The impact of a business acquisition increased systems and hardware revenue growth by 0.3%.
The change in foreign currency exchange rates decreased systems and hardware revenue growth by 0.1%.
The following table presents the CAG segment results of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
Results of Operations
(dollars in thousands) |
|
2024 |
|
Percent of Revenue |
|
2023 |
|
Percent of Revenue |
|
Amount |
|
Percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenues |
|
$ |
3,574,044 |
|
|
|
|
$ |
3,352,356 |
|
|
|
|
$ |
221,688 |
|
|
6.6 |
% |
| Cost of revenue |
|
1,394,864 |
|
|
|
|
1,349,930 |
|
|
|
|
44,934 |
|
|
3.3 |
% |
| Gross profit |
|
2,179,180 |
|
|
61.0 |
% |
|
2,002,426 |
|
|
59.7 |
% |
|
176,754 |
|
|
8.8 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
536,171 |
|
|
15.0 |
% |
|
517,258 |
|
|
15.4 |
% |
|
18,913 |
|
|
3.7 |
% |
| General and administrative |
|
402,198 |
|
|
11.3 |
% |
|
299,701 |
|
|
8.9 |
% |
|
102,497 |
|
|
34.2 |
% |
| Research and development |
|
202,395 |
|
|
5.7 |
% |
|
172,727 |
|
|
5.2 |
% |
|
29,668 |
|
|
17.2 |
% |
| Total operating expenses |
|
1,140,764 |
|
|
31.9 |
% |
|
989,686 |
|
|
29.5 |
% |
|
151,078 |
|
|
15.3 |
% |
| Income from operations |
|
$ |
1,038,416 |
|
|
29.1 |
% |
|
$ |
1,012,740 |
|
|
30.2 |
% |
|
$ |
25,676 |
|
|
2.5 |
% |
Gross Profit. Gross profit increased primarily due to higher revenue and a 130 basis point increase in the gross profit margin. The increase in the gross profit margin reflected favorable business mix, the benefit from price realization offsetting inflationary cost impacts, lower instrument costs, and software and services margin gains. The change in foreign currency exchange rates on the gross profit margin was not significant.
Operating Expenses. Sales and marketing expense increased primarily due to higher personnel-related, meeting, and travel costs. General and administrative expense increased primarily due to a $61.5 million expense related to an ongoing litigation matter, the comparison to the prior year benefit of a $16 million customer contract resolution gain, higher information technology and outside services, and acquisition-related costs. Research and development expense increased primarily due to higher project costs. The change in foreign currency exchange rates was not significant to operating expense growth.
Water
The following table presents the Water segment results of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
Results of Operations
(dollars in thousands) |
|
2024 |
|
Percent of Revenue |
|
2023 |
|
Percent of Revenue |
|
Amount |
|
Percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenues |
|
$ |
185,112 |
|
|
|
|
$ |
168,149 |
|
|
|
|
$ |
16,963 |
|
|
10.1 |
% |
| Cost of revenue |
|
55,101 |
|
|
|
|
52,148 |
|
|
|
|
2,953 |
|
|
5.7 |
% |
| Gross profit |
|
130,011 |
|
|
70.2 |
% |
|
116,001 |
|
|
69.0 |
% |
|
14,010 |
|
|
12.1 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
23,149 |
|
|
12.5 |
% |
|
21,249 |
|
|
12.6 |
% |
|
1,900 |
|
|
8.9 |
% |
| General and administrative |
|
16,873 |
|
|
9.1 |
% |
|
15,655 |
|
|
9.3 |
% |
|
1,218 |
|
|
7.8 |
% |
| Research and development |
|
5,456 |
|
|
2.9 |
% |
|
4,757 |
|
|
2.8 |
% |
|
699 |
|
|
14.7 |
% |
| Total operating expenses |
|
45,478 |
|
|
24.6 |
% |
|
41,661 |
|
|
24.8 |
% |
|
3,817 |
|
|
9.2 |
% |
| Income from operations |
|
$ |
84,533 |
|
|
45.7 |
% |
|
$ |
74,340 |
|
|
44.2 |
% |
|
$ |
10,193 |
|
|
13.7 |
% |
Revenue. The increase in our Water business revenue was primarily due to higher realized prices and higher volumes. The increase in volumes in the U.S. and Europe was largely from our Colilert test products and related accessories used in coliform and E. coli testing, and, to a lesser extent, placements of our Tecta system instruments. The change in foreign currency exchange rates decreased revenue growth by 0.5%.
Gross Profit. Gross profit for Water increased due to higher revenue, and a 120 basis point increase in the gross profit margin. The increase in the gross profit margin was primarily due to higher realized prices and lower freight costs, partially offset by higher product costs. The change in foreign currency exchange rates on the gross profit margin was not significant.
Operating Expenses. Sales and marketing expense increased primarily due to higher personnel-related costs and marketing expenses. General and administrative expense increased primarily due to higher personnel-related costs and an increase in bad debt expense. Research and development expense increased primarily due to higher project and outside services costs. The change in foreign currency exchange rates was not significant to operating expense growth.
Livestock, Poultry and Dairy
The following table presents the LPD segment results of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
Results of Operations
(dollars in thousands) |
|
2024 |
|
Percent of Revenue |
|
2023 |
|
Percent of Revenue |
|
Amount |
|
Percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenues |
|
$ |
122,060 |
|
|
|
|
$ |
121,659 |
|
|
|
|
$ |
401 |
|
|
0.3 |
% |
| Cost of revenue |
|
59,500 |
|
|
|
|
56,219 |
|
|
|
|
3,281 |
|
|
5.8 |
% |
| Gross profit |
|
62,560 |
|
|
51.3 |
% |
|
65,440 |
|
|
53.8 |
% |
|
(2,880) |
|
|
(4.4 |
%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
28,027 |
|
|
23.0 |
% |
|
25,798 |
|
|
21.2 |
% |
|
2,229 |
|
|
8.6 |
% |
| General and administrative |
|
16,716 |
|
|
13.7 |
% |
|
17,174 |
|
|
14.1 |
% |
|
(458) |
|
|
(2.7 |
%) |
| Research and development |
|
11,184 |
|
|
9.2 |
% |
|
12,493 |
|
|
10.3 |
% |
|
(1,309) |
|
|
(10.5 |
%) |
| Total operating expenses |
|
55,927 |
|
|
45.8 |
% |
|
55,465 |
|
|
45.6 |
% |
|
462 |
|
|
0.8 |
% |
| Income from operations |
|
$ |
6,633 |
|
|
5.4 |
% |
|
$ |
9,975 |
|
|
8.2 |
% |
|
$ |
(3,342) |
|
|
(33.5 |
%) |
Revenue. The increase in revenue was primarily due to volume growth in the U.S and Europe, and higher realized prices, partially offset by lower testing and herd health screening levels in Asia Pacific. The change in foreign currency exchange rates decreased revenue growth by 0.9%.
Gross Profit. The decrease in LPD gross profit was primarily due to a 250 basis point decrease in the gross profit margin. The decrease in the gross profit margin was primarily due to higher product and distribution costs and unfavorable business mix, partially offset by higher realized prices. The change in foreign currency exchange rates decreased the gross profit margin by approximately 30 basis points, including the impact of higher hedge gains in the current year compared to the prior year.
Operating Expenses. Sales and marketing expense increased primarily due to higher personnel-related costs. General and administrative expense decreased primarily due to lower bad debt expense and lower personnel-related costs. Research and development expense decreased primarily due to lower project costs. The change in foreign currency exchange rates was not significant to operating expense growth.
Other
The following table presents the Other results of operations:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Change |
Results of Operations
(dollars in thousands) |
|
2024 |
|
Percent of Revenue |
|
2023 |
|
Percent of Revenue |
|
Amount |
|
Percentage |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Revenues |
|
$ |
16,288 |
|
|
|
|
$ |
18,789 |
|
|
|
|
$ |
(2,501) |
|
|
(13.3 |
%) |
| Cost of revenue |
|
9,112 |
|
|
|
|
12,686 |
|
|
|
|
(3,574) |
|
|
(28.2 |
%) |
| Gross profit |
|
7,176 |
|
|
44.1 |
% |
|
6,103 |
|
|
32.5 |
% |
|
1,073 |
|
|
17.6 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
| Sales and marketing |
|
1,160 |
|
|
7.1 |
% |
|
1,761 |
|
|
9.4 |
% |
|
(601) |
|
|
(34.1 |
%) |
| General and administrative |
|
6,504 |
|
|
39.9 |
% |
|
3,295 |
|
|
17.5 |
% |
|
3,209 |
|
|
97.4 |
% |
| Research and development |
|
757 |
|
|
4.6 |
% |
|
974 |
|
|
5.2 |
% |
|
(217) |
|
|
(22.3 |
%) |
| Total operating expenses |
|
8,421 |
|
|
51.7 |
% |
|
6,030 |
|
|
32.1 |
% |
|
2,391 |
|
|
39.7 |
% |
| Income from operations |
|
$ |
(1,245) |
|
|
(7.6 |
%) |
|
$ |
73 |
|
|
0.4 |
% |
|
$ |
(1,318) |
|
|
(1,805.5 |
%) |
Revenue. The decrease in Other revenue was primarily due to lower sales of our OPTI Medical instruments and consumables, partially offset by higher realized prices.
Gross Profit. Gross profit increased due to a 1,160 basis point increase in the gross profit margin, which offset the impact of lower revenues. The increase in the gross profit margin was primarily due to lower product costs and higher realized prices. The overall change in foreign currency exchange rates had an immaterial impact on the gross profit margin.
Operating Expenses. Sales and marketing expense decreased due to lower personnel-related costs. General and administrative expense increased primarily due to higher foreign currency transaction losses compared to the prior year. Foreign exchange losses on settlements for all operating segments are reported within our Other segment. Research and development expense decreased primarily due to lower development activities that were not related to our three primary business segments.
Non-Operating Items
Interest Expense and Income. Interest expense was $31.2 million for the year ended December 31, 2024, as compared to $41.6 million for the prior year. The decrease in interest expense was primarily due to lower average debt levels and, to a lesser extent, lower interest rates. Interest income was $12.7 million for the year ended December 31, 2024, compared to $5.6 million for the prior year. This increase in interest income is primarily due to the increase in money market investments throughout the current year, as compared to the prior year.
Provision for Income Taxes. Our effective income tax rate was 20.0% for the year ended December 31, 2024, and 20.4% for the year ended December 31, 2023. Our effective tax rate for the year ended December 31, 2024, was lower than the prior year primarily due to increased benefits related to share-based compensation. Our projected effective tax rate for 2025 is approximately 21.5%. This increase in the projected 2025 effective tax rate is primarily due to estimated reductions in tax benefits, compared to 2024, from share-based compensation and from the release of tax reserves related to uncertain tax positions.
LIQUIDITY AND CAPITAL RESOURCES
We fund the capital needs of our business through cash on hand, funds generated from operations, proceeds from long-term senior note financings, and amounts available under our Credit Facility. We generate cash primarily through the payments made by customers for our companion animal, livestock, poultry, dairy, and water products and services, consulting services, and other various systems and services. Our cash disbursements are primarily related to compensation and benefits for our employees, inventory and supplies, repurchase of our common stock, taxes, research and development, capital expenditures, rents, occupancy-related charges, interest expense, and business acquisitions. As of December 31, 2024, we had $288.3 million of cash and cash equivalents, compared to $453.9 million as of December 31, 2023. Working capital totaled $332.0 million as of December 31, 2024, compared to $543.7 million as of December 31, 2023. The change in working capital is primarily due to lower cash and higher current amount payable of our Senior Notes. As of December 31, 2024, we had a remaining borrowing availability of $998.1 million under our $1.25 billion Credit Facility, with $250.0 million in outstanding borrowings under the Credit Facility. The general availability of funds under our Credit Facility is reduced by $1.9 million for outstanding letters of credit. We believe that, if necessary, we could obtain additional borrowings to fund our growth objectives. We further believe that current cash and cash equivalents, funds generated from operations, and committed borrowing availability will be sufficient to fund our operations, capital purchase requirements, and anticipated growth needs for the next twelve months. We believe that these resources, coupled with our ability, as needed, to obtain additional financing, will also be sufficient to fund our business as currently conducted for the foreseeable future. We may enter into new financing arrangements or refinance or retire existing debt in the future depending on market conditions. Should we require more capital in the U.S. than is generated by our operations, for example to fund significant discretionary activities, we could elect to raise capital in the U.S. through the incurrence of debt or equity issuances, which we may not be able to complete on favorable terms or at all. In addition, these alternatives could result in increased interest expense or other dilution of our earnings.
We manage our worldwide cash requirements considering available funds among all of our subsidiaries. Our foreign cash and cash equivalents are generally available without restrictions to fund ordinary business operations outside the U.S.
The following table presents cash, cash equivalents, and marketable securities held domestically and by our foreign subsidiaries:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
Cash and cash equivalents
(in thousands)
|
|
2024 |
|
2023 |
|
|
|
|
|
| U.S. |
|
$ |
145,118 |
|
|
$ |
324,434 |
|
| Foreign |
|
143,148 |
|
|
129,498 |
|
| Total |
|
$ |
288,266 |
|
|
$ |
453,932 |
|
|
|
|
|
|
| Total cash, cash equivalents and marketable securities held in U.S. dollars by our foreign subsidiaries |
|
$ |
10,623 |
|
|
$ |
13,170 |
|
Of the $288.3 million of cash and cash equivalents held as of December 31, 2024, $148.7 million was held as bank deposits at a diversified group of institutions, primarily systemically important banks, and $139.6 million was held in a U.S. government money market fund. Of the $453.9 million of cash and cash equivalents held as of December 31, 2023, $163.1 million was held as bank deposits at a diversified group of institutions, primarily systemically important banks, and $290.8 million was held in a U.S. government money market fund. Cash and cash equivalents as of December 31, 2024, included approximately USD $1.0 million in cash denominated in non-U.S. currencies held in a country with currency control restrictions, which limit our ability to transfer funds outside of the country in which they are held without incurring costs. The currency control restricted cash is generally available for use within the country where it is held.
The following table presents additional key information concerning working capital:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Three Months Ended |
|
|
December 31, 2024 |
|
September 30, 2024 |
|
June 30,
2024
|
|
March 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
|
|
|
|
|
|
Days sales outstanding (1) |
|
47.1 |
|
|
48.9 |
|
|
47.3 |
|
|
45.7 |
|
|
46.1 |
|
Inventory turns (2) |
|
1.3 |
|
|
1.3 |
|
|
1.4 |
|
|
1.3 |
|
|
1.3 |
|
(1) Days sales outstanding represents the average of the accounts receivable balances at the beginning and end of each quarter divided by revenue for that quarter, the result of which is then multiplied by 91.25 days.
(2) Inventory turns are calculated as the ratio our inventory-related cost of revenue for the quarter multiplied by four, divided by the average inventory balances at the beginning and end of each quarter.
Sources and Uses of Cash
The following table presents cash provided (used):
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
Dollar Change |
|
|
|
|
|
|
|
| Net cash provided by operating activities |
|
$ |
929,001 |
|
|
$ |
906,510 |
|
|
$ |
22,491 |
|
| Net cash used by investing activities |
|
(207,062) |
|
|
(125,254) |
|
|
(81,808) |
|
| Net cash used by financing activities |
|
(878,073) |
|
|
(441,996) |
|
|
(436,077) |
|
| Net effect of changes in exchange rates on cash |
|
(9,532) |
|
|
2,126 |
|
|
(11,658) |
|
| Net change in cash and cash equivalents |
|
$ |
(165,666) |
|
|
$ |
341,386 |
|
|
$ |
(507,052) |
|
Operating Activities. The increase in cash provided by operating activities of $22.5 million during 2024, compared to 2023, was primarily due to higher net income, net of noncash revenues and expenses, including a $61.5 million charge related to an ongoing litigation matter, and a comparative benefit from the use of cash during the prior year for a $15.0 million milestone payment to license intellectual property. Noncash adjustments to net income for net deferred tax benefits were comparatively lower for the current year. These relative increases in cash from operations were partially offset by higher income tax and annual employee incentive program payments during the current year.
The following table presents cash flow impacts from changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
Dollar Change |
|
|
|
|
|
|
|
| Accounts receivable |
|
$ |
(28,280) |
|
|
$ |
(53,871) |
|
|
$ |
25,591 |
|
| Inventories |
|
(28,001) |
|
|
(28,651) |
|
|
650 |
|
| Accounts payable |
|
8,086 |
|
|
(557) |
|
|
8,643 |
|
| Deferred revenue |
|
(4,378) |
|
|
(3,032) |
|
|
(1,346) |
|
| Other assets and liabilities |
|
(80,665) |
|
|
13,682 |
|
|
(94,347) |
|
| Total change in cash due to changes in operating assets and liabilities |
|
$ |
(133,238) |
|
|
$ |
(72,429) |
|
|
$ |
(60,809) |
|
Cash used due to changes in operating assets and liabilities during the year ended December 31, 2024, compared to the same period in the prior year, increased $60.8 million. Cash used for other assets and liabilities increased $94.3 million primarily due to higher tax payments and higher annual employee incentive program payments, compared to the prior year. Uses of cash were partially offset by higher non-cash operating expenses recorded as accrued liabilities, including a $61.5 million accrued expense related to an ongoing litigation matter, and by a comparative benefit from the use of cash during the prior year for a $15.0 million milestone payment to license intellectual property. Cash used for accounts receivable decreased $25.6 million compared to the prior year primarily due to the timing of customer payments received, partially offset by higher revenue.
We have historically experienced proportionally lower net cash flows from operating activities during the first quarter and proportionally higher cash flows from operating activities for the remainder of the year and for the annual period driven primarily by payments related to annual employee incentive programs in the first quarter following the year for which the bonuses were earned.
Investing Activities. Cash used by investing activities was $207.1 million during 2024, compared to $125.3 million used during 2023. The increase in cash used by investing activities during 2024, compared to 2023, was primarily due to the acquisition of a software business during the current year.
Our total capital expenditure plan for 2025 is estimated to be approximately $160.0 million, which includes capital investments in manufacturing and operations facilities to support growth, as well as investments in customer-facing software development.
Financing Activities. Cash used by financing activities was $878.1 million during 2024, compared to $442.0 million used during 2023. The increase in cash used was primarily due to $837.0 million of repurchases of our common stock during the current year, including the 1% excise tax paid on certain stock repurchases, compared to $71.9 million used for repurchases during the prior year. This increase in cash used by financing was partially offset by no borrowings or repayments under our Credit Facility during the current year, compared to repayments of $329.0 million under our Credit Facility during the prior year.
Repurchases of our common stock vary depending upon the level of other investing and deployment activities, as well as share price and prevailing interest rates. We believe that the repurchase of our common stock is a favorable means of returning value to our stockholders, and we also repurchase our stock to offset the dilutive effect of our share-based compensation programs. We primarily fund our share repurchases with cash generated from operations, as well as from various capital market activities, including the committed available financing through our Credit Facility. Cash used to repurchase shares of our common stock increased by $765.1 million during 2024, compared to 2023. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 20. Repurchases of Common Stock” for additional information about our share repurchases. We currently anticipate approximately $1.5 billion of share repurchases in 2025, subject to market conditions.
The aggregate principal amounts of our 2025 Series C Notes will become due and payable on June 18, 2025, and the aggregate principal amounts of our 2025 Series B Notes will become due and payable on December 11, 2025. We anticipate paying off our 2025 Series C Notes for €88.9 million when due in June 2025, and our 2025 Series B Notes for $75.0 million when due in December 2025, with available cash on hand, borrowings under our Credit Facility, or proceeds from the issuance of new notes, or a combination thereof. Should we elect to prepay any of our senior notes, such aggregate prepayment will include the applicable make-whole amount(s), as defined within the applicable Senior Note Agreements. Additionally, in the event of a change in control of the Company or upon the disposition of certain assets of the Company, the proceeds of which are not reinvested (as defined in the Senior Note Agreements), we may be required to prepay all or a portion of the senior notes.
Under the $1.25 billion Credit Facility, the $1.0 billion unsecured credit line matures on December 9, 2026, and requires no scheduled prepayments before that date. On October 20, 2022, pursuant to the terms of the Credit Facility, the term lenders thereunder provided us, as borrower, an incremental term loan in an aggregate principal amount of $250.0 million (the “Term Loan”). The Term Loan matures on October 20, 2025. The net proceeds of the Term Loan were used to repay previously incurred revolver borrowings under the Credit Facility. The Term Loan is subject to the same affirmative and negative covenants and events of default as the borrowings previously incurred pursuant to the Credit Facility. The applicable interest rate for the Term Loan is consistent with our line of credit, and is calculated at a per annum rate equal to either (at our option) (1) a prime rate plus a margin ranging from 0.0% to 0.375% based on our consolidated leverage ratio, (2) an adjusted term SOFR rate, plus 0.10%, plus a margin ranging from 0.875% to 1.375% based on our consolidated leverage ratio, or (3) an adjusted daily simple SOFR rate, plus 0.10%, plus a margin ranging from 0.875% to 1.375% based on our consolidated leverage ratio. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 13, Debt” for additional information about our applicable interest rates on our Credit Facility. Under the Credit Facility, we also pay quarterly commitment fees ranging from 0.075% to 0.25%, based on our leverage ratio, on any unused commitment.
Under the Credit Facility, the net repayment and borrowing activity resulted in increased cash used of $329.0 million during 2024, compared to 2023. As of December 31, 2024, we had $250.0 million outstanding on our line of credit, all of which was on our $250.0 million Term Loan under the Credit Facility. As of December 31, 2023, we had $250.0 million outstanding on our line of credit and a $250.0 million Term Loan, all of which was on our $250.0 million Term Loan under the Credit Facility. The general availability of funds under the Credit Facility was further reduced by $1.9 million and $1.5 million for letters of credit that were issued primarily in connection with our workers' compensation policy as of December 31, 2024, and December 31, 2023, respectively. The Credit Facility contains affirmative, negative, and financial covenants customary for financings of this type. The negative covenants include restrictions on liens, indebtedness of subsidiaries of the Company, fundamental changes, investments, transactions with affiliates, and certain restrictive agreements and violations of laws and regulations. The financial covenant is a consolidated leverage ratio test that requires our ratio of debt to earnings before interest, taxes, depreciation, amortization, and share-based compensation not to exceed 3.5-to-1. As of December 31, 2024, we were in compliance with the covenants of the Credit Facility. The obligations under the Credit Facility may be accelerated upon the occurrence of an event of default under the Credit Facility, which includes customary events of default including payment defaults, defaults in the performance of the affirmative, negative and financial covenants, the inaccuracy of representations or warranties, bankruptcy and insolvency related defaults, defaults relating to judgments, certain events related to employee pension benefit plans under the Employee Retirement Income Security Act of 1974, (“ERISA”), the failure to pay specified indebtedness, cross-acceleration to specified indebtedness, and a change of control default.
The obligations under the senior notes may be accelerated upon the occurrence of an event of default under the applicable Senior Note Agreements, each of which includes customary events of default including payment defaults, defaults in the performance of the affirmative, negative and financial covenants, the inaccuracy of representations or warranties, bankruptcy and insolvency-related defaults, defaults relating to judgments, certain events related to employee pension benefit plans under ERISA, the failure to pay specified indebtedness, and cross-acceleration to specified indebtedness.
Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 13, Debt” for additional information about our Credit Facility, Senior Notes, and Senior Note Agreements.
Effect of Currency Translation on Cash. The net effect of changes in foreign currency exchange rates are related to changes in exchange rates between the U.S. dollar and the functional currencies of our foreign subsidiaries. These changes will fluctuate each year as the value of the U.S. dollar relative to the value of foreign currencies changes. The value of a currency depends on many factors, including interest rates, and the issuing governments' debt levels and strength of economy.
Off-Balance Sheet Arrangements. We have no off-balance sheet arrangements or variable interest entities except for letters of credit and third-party guarantees, as reflected in “Part II, Item 8. Financial Statements and Supplementary Data, Note 13 Debt” and “Part II, Item 8. Financial Statements and Supplementary Data. Note 16. Commitments, Contingencies and Guarantees” to the consolidated financial statements for the year ended December 31, 2024, included in this Annual Report on Form 10-K, respectively.
Financial Covenant. The financial covenant is a consolidated leverage ratio test that requires our ratio of debt to earnings before interest, taxes, depreciation, amortization, and share-based compensation, as defined in the Senior Note Agreements and Credit Facility, not to exceed 3.5-to-1. As of December 31, 2024, we were in compliance with the covenants of the Senior Note Agreements and Credit Facility. The following details our consolidated leverage ratio calculation:
|
|
|
|
|
|
| (in thousands) |
Twelve Months Ended |
| Trailing 12 Months Adjusted EBITDA: |
December 31, 2024 |
|
|
| Net income attributable to stockholders |
$ |
887,867 |
|
| Interest expense |
31,205 |
|
| Provision for income taxes |
221,964 |
|
| Depreciation and amortization |
129,936 |
|
| Acquisition-related expense |
204 |
|
| Share-based compensation expense |
60,295 |
|
| Extraordinary and other non-recurring non-cash charges |
250 |
|
| Adjusted EBITDA |
$ |
1,331,721 |
|
|
|
|
|
| (dollars in thousands) |
Twelve Months Ended |
| Debt to Adjusted EBITDA Ratio: |
December 31, 2024 |
|
|
| Line of credit |
$ |
250,000 |
|
| Current and long-term portion of long-term debt |
617,573 |
|
| Total debt |
867,573 |
|
| Acquisition-related consideration payable |
2,587 |
|
| Deferred financing costs |
230 |
|
| Gross debt |
$ |
870,390 |
|
| Gross debt to Adjusted EBITDA ratio |
0.65 |
|
|
|
| Cash and cash equivalents |
$ |
(288,266) |
|
| Net debt |
$ |
582,124 |
|
| Net debt to Adjusted EBITDA ratio |
0.44 |
|
Commitments, Contingencies and Guarantees
For more information regarding our commitments, contingencies, and guarantees, refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 16. Commitments, Contingencies and Guarantees.”
For more information on our future lease payments, refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 8. Lease Commitments” for our minimum lease payment schedule. The expected timing of payments of our leases may be different in future years, depending on decisions to extend lease terms and/or enter into additional leases in the preceding years.
For more information on our future Swiss defined benefit pension plans payments, refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 23. IDEXX Retirement and Incentive Savings Plan” for our future benefits expected to be paid.
As of December 31, 2024, current liabilities include $250.0 million outstanding borrowing on our Credit Facility and the current portion of long-term debt of $167.8 million recorded as current liabilities. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 13. Debt” for more information about our Credit Facility and for more information on our repayment of our Senior Notes.
We also have purchase obligations that include agreements and purchase orders to purchase goods or services that are contractually enforceable and that specify all significant terms, including fixed or minimum quantities, pricing, and approximate timing of purchases. As of December 31, 2024, we had approximately $211.0 million in purchase obligations due in 2025. Our purchase obligations beyond 2025 are approximately $168.7 million. These purchase obligation amounts do not include amounts recorded in accounts payable, as of December 31, 2024.
The expected timing of payments of our purchase obligations is estimated based on current information. Timing of payments and actual amounts paid may be different, depending on the time of receipt of goods or services, or changes to agreed-upon amounts for some obligations.
Additionally, we have agreements with third parties that we have entered into in the ordinary course of business under which we are obligated to indemnify such third parties for and against various risks and losses. The precise terms of such indemnities vary with the nature of the agreement. In many cases, we limit the maximum amount of our indemnification obligations, but in some cases those obligations may be theoretically unlimited. We have not incurred material expenses in discharging any of these indemnification obligations and, based on our analysis of the nature of the risks involved, we believe that the fair value of these agreements is minimal. Accordingly, we did not record any liabilities for these obligations as of December 31, 2024, and 2023, and do not anticipate any future payments for these guarantees.
As of December 31, 2024, we paid our remaining obligation associated with the deemed repatriation tax resulting from the Tax Cuts and Jobs Act of 2017 for $21.8 million. For information on our unrecognized tax benefits, refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 14. Income Taxes.”
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Our market risk consists primarily of foreign currency exchange risk, interest rate risk, and effects of inflation. Our functional currency is the U.S. dollar and our primary manufacturing operations and inventory supply contracts are in the U.S. or in U.S. dollars, but we distribute our products worldwide both through direct export and through our foreign subsidiaries. Our primary foreign currency transaction risk consists of intercompany purchases and sales of products, and we attempt to mitigate this risk through our hedging program described below. For the year ended December 31, 2024, approximately 22% of our consolidated revenue was derived from products manufactured or sourced in U.S. dollars and sold internationally in local currencies, compared to 21% for both the years ended December 31, 2023, and 2022. The functional currency of most of our subsidiaries is their local currency.
Our foreign currency exchange impacts are comprised of three components: 1) local currency revenues and expenses; 2) the impact of hedge contracts; and 3) intercompany and monetary balances for our subsidiaries that are denominated in a currency that is different from the functional currency used by each subsidiary.
The following table presents the estimated foreign currency exchange impact on our revenues, operating profit, and diluted earnings per share for the current period and compared to the respective prior-year period:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in thousands, except per share amounts) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Revenue (decrease) increase |
|
$ |
(9,471) |
|
|
$ |
(4,603) |
|
|
$ |
(108,812) |
|
|
|
|
|
|
|
|
Operating profit (decrease) increase, excluding hedge activity and exchange impacts on settlement of foreign currency denominated transactions |
|
$ |
(4,253) |
|
|
$ |
(5,489) |
|
|
$ |
(56,420) |
|
| Hedge gains (losses) - current period |
|
5,932 |
|
|
3,512 |
|
|
25,733 |
|
Foreign currency transaction (losses) - current period |
|
(4,527) |
|
|
(1,078) |
|
|
(3,408) |
|
Operating profit (decrease) increase - current period |
|
$ |
(2,848) |
|
|
$ |
(3,055) |
|
|
$ |
(34,095) |
|
|
|
|
|
|
|
|
Hedge (gains) losses - prior period |
|
(3,512) |
|
|
(25,733) |
|
|
7,121 |
|
Foreign currency transaction losses - prior period |
|
1,078 |
|
|
3,408 |
|
|
2,111 |
|
Operating profit (decrease) increase - compared to prior period |
|
$ |
(5,282) |
|
|
$ |
(25,380) |
|
|
$ |
(24,863) |
|
|
|
|
|
|
|
|
Diluted earnings per share (decrease) increase - compared to prior period |
|
$ |
(0.05) |
|
|
$ |
(0.24) |
|
|
$ |
(0.22) |
|
At our current foreign exchange rate assumptions, we anticipate year-over-year changes will reduce our revenues, decrease our operating profit, and diluted earnings per share in the year ending December 31, 2025, by approximately $80 million, $22 million, and $0.21 per share, respectively. These unfavorable impacts to our operating profit and diluted earnings per share include net year-over-year impacts of foreign currency hedging activity, which is expected to increase total company operating profit by approximately $19 million and diluted earnings per share by $0.18 during the year ending December 31, 2025. The actual impact of changes in the value of the U.S. dollar against foreign currencies in which we transact may materially differ from our expectations described above. The above estimate assumes that the value of the U.S. dollar relative to other currencies will reflect the euro at $1.02, the British pound at $1.23, the Canadian dollar at $0.68, the Australian dollar at $0.61; the Japanese yen at ¥160, the Chinese renminbi at RMB 7.43, and the Brazilian real at R$6.21 to the U.S. dollar for the full year of 2025.
The foreign currency exchange impacts on our revenue and operating income will be different from our 2025 estimates if actual foreign exchange rates are different from our assumptions. Excluding the impact of intercompany and trade balances denominated in currencies other than the functional subsidiary currencies, a 1% strengthening of the U.S. dollar would reduce revenue by approximately $13 million and operating income by approximately $5 million, net of hedge positions.
The primary purpose of our foreign currency hedging activities is to protect against the volatility associated with foreign currency transactions. We also utilize natural hedges to mitigate our transaction and commitment exposures. Our corporate policy prescribes the range of allowable hedging activity. We enter into foreign currency exchange contracts with large, well-capitalized multinational financial institutions and we do not hold or engage in transactions involving derivative instruments for purposes other than risk management.
Our accounting policies for these contracts are based on our designation of such instruments as hedging transactions. If a hedging instrument qualifies for hedge accounting, changes in the fair value of the hedging instrument from the effective portion of the hedge are deferred in accumulated other comprehensive income, net of tax, and reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. We immediately record in earnings the extent to which a hedge instrument is not effective in achieving offsetting changes in fair value. We primarily utilize foreign currency exchange contracts with durations of less than 24 months.
Our subsidiaries enter into foreign currency exchange contracts to manage the exchange risk associated with their forecasted intercompany inventory purchases and sales for the next year. From time to time, we may also enter into other foreign currency exchange contracts, cross currency swaps, or foreign-denominated debt issuances to minimize the impact of foreign currency fluctuations associated with specific balance sheet exposures, including net investments in certain foreign subsidiaries. Refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 19. Hedging Instruments” to the consolidated financial statements of this Annual Report on Form 10-K for details regarding euro-denominated notes that we designated as a hedge of our euro net investment in certain foreign subsidiaries.
Our foreign currency hedging strategy is consistent with prior periods and there were no material changes in our market risk exposure during the year ended December 31, 2024. We enter into foreign currency exchange contracts designated as cash flow hedges for amounts that are less than the full value of forecasted intercompany purchases and sales and for amounts that are equivalent to, or less than, other significant transactions. As a result, no significant ineffectiveness has resulted or been recognized in the statements of income for the years ended December 31, 2024, 2023, and 2022. Our hedging strategy related to intercompany inventory purchases and sales is to employ the full amount of our hedges for the succeeding year at the conclusion of our budgeting process for that year. Quarterly, we enter into contracts to hedge incremental portions of anticipated foreign currency transactions for the current and following year. Accordingly, our risk with respect to foreign currency exchange rate fluctuations may vary throughout each annual cycle.
We enter into hedge agreements where we believe we have meaningful exposure to foreign currency exchange risk, with the exception of certain emerging markets where it is not practical to hedge our exposure. We target to hedge approximately 75% to 85% of the estimated exposure from intercompany product purchases and sales denominated in the euro, British pound, Canadian dollar, Japanese yen, and Australian dollar. We have additional unhedged foreign currency exposures related to foreign services and in emerging markets where it is not practical to hedge. The notional amount of foreign currency exchange contracts to hedge forecasted intercompany purchases and sales totaled $325.7 million as of December 31, 2024, and $294.0 million as of December 31, 2023. As of December 31, 2024, we had $12.8 million of net unrealized gains on foreign currency exchange contracts reflected within accumulated other comprehensive income, net of related tax. For more information on our hedge agreements refer to “Part II, Item 8. Financial Statements and Supplementary Data, Note 19. Hedging Instruments.”
For additional information, refer to “Part I, Item 1A. Risk Factors:” “Since our business is global in nature, geopolitical risks and other risks associated with doing business internationally could negatively affect our business, financial condition, and operating results” and “Strengthening of the rate of exchange for the U.S. dollar has a negative effect on our business,” and “Part II, Item 8. Financial Statements and Supplementary Data, Note 2. Summary of Significant Accounting Policies.”
Interest Rate Risk and Effects of Inflation
We incur interest expense on our borrowings outstanding on our Senior Notes at a fixed rate. We have a Credit Facility with a syndicate of multinational banks, which matures on December 9, 2026, and requires no scheduled prepayments before that date. Although the Credit Facility does not mature until December 9, 2026, all individual borrowings under the terms of the Credit Facility have a stated term between 1 and 180 days, including the $250.0 million Term Loan that matures on October 20, 2025. The variable rates are based on SOFR, with rolling maturities of one and three month increments.
We entered into an interest rate swap to manage the effect of variable interest obligations on amounts borrowed under the terms of the Credit Facility. Beginning on March 31, 2023, the variable interest rate associated with $250.0 million of borrowings outstanding under the Credit Facility became effectively fixed at 3.9%, plus the applicable credit spread, through October 20, 2025. Borrowings outstanding under the Credit Facility as of December 31, 2024, were $250.0 million.
During 2024, we experienced inflationary pressure on our operating costs. During 2025, we expect to continue to face higher costs for labor, commodities, energy, and transportation, as well as increased prices from suppliers. Furthermore, additional tariffs on imported goods may increase our costs to purchase products, components, and materials. We may not be able to offset these higher costs through productivity initiatives and price increases, which may materially and adversely affect our business, results of operations, and financial condition.
Any price increases we may impose may lead to declines in sales volume or loss of business, if competitors do not similarly adjust their prices, or customers refuse to purchase at the higher prices.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The response to this item is submitted as a separate section of this report commencing on page F-1.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
ITEM 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining disclosure controls and procedures, as defined by the SEC in its Rules 13a-15(e) and 15d-15(e) under the Exchange Act. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC's rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of December 31, 2024, our Chief Executive Officer and Chief Financial Officer have concluded that, as of such date, the Company’s disclosure controls and procedures were effective at the reasonable assurance level.
Report of Management on Internal Control Over Financial Reporting
We are responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. GAAP and includes those policies and procedures that:
•Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company;
•Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
•Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material adverse effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. In addition, projections of any evaluation of effectiveness to future periods are subject to risk that controls may become inadequate because of changes in conditions and that the degree of compliance with the policies and procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of internal control over financial reporting based on the framework in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation, our management concluded that, as of December 31, 2024, our internal control over financial reporting was effective.
The effectiveness of the Company's internal control over financial reporting as of December 31, 2024, has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during the three months ended December 31, 2024, that materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Certifications
The certifications with respect to disclosure controls and procedures and internal control over financial reporting of the Company’s Chief Executive Officer and Chief Financial Officer are attached as Exhibits 31.1 and 31.2 to this Annual Report on Form 10-K.
ITEM 9B. OTHER INFORMATION
Rule 10b5-1 Trading Plan Elections
Jonathan W. Ayers resigned as an independent director of the Company on November 8, 2024. As previously reported, Mr. Ayers entered into a Rule 10b5-1 trading arrangement (the “plan”) on August 29, 2024. Mr. Ayers terminated the plan effective November 25, 2024, and no trades were executed under the plan.
Except as noted above, during the three months ended December 31, 2024, none of our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act) adopted, modified, or terminated any “Rule 10b5-1 trading arrangement” or “non-Rule 10b5-1 trading arrangement” (as such terms are defined in Item 408(a) of Regulation S-K of the Securities Act of 1933).
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this Item with respect to Directors, executive officers, and compliance with Section 16(a) of the Exchange Act, our code of ethics and corporate governance is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Proposal One – Election of Directors,” “Executive Compensation – Executive Officers,” “Executive Compensation - Equity Award Grant Policy,” “Stock Ownership Information – Delinquent Section 16(a) Reports,” “Corporate Governance – Corporate Governance Guidelines and Code of Ethics,” and “Corporate Governance – Board Committees” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on
Form 10-K.
Insider Trading Arrangements and Policies
We have adopted an insider trading policy (the “Insider Trading Policy”) and related procedures, which govern the purchase, sale, and other dispositions of our securities by our directors, officers, employees, and other covered persons, as well as by IDEXX itself. We believe that our Insider Trading Policy and related procedures are reasonably designed to promote compliance with applicable insider trading laws, rules and regulations, and the Nasdaq Stock Market listing standards applicable to us. The Insider Trading Policy prohibits our directors, officers, employees, and other covered persons from trading in our securities while in possession of material non-public information about us. This Policy also generally prohibits disclosure of material non-public information about us to others, with some limited exceptions.
The foregoing summary of the Insider Trading Policy does not purport to be complete and is qualified in its entirety by reference to the full text of the Insider Trading Policy filed with this Annual Report on Form 10-K as Exhibit 19.1.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Executive Compensation – Compensation Discussion and Analysis,” “Executive Compensation – Executive Compensation Tables,” “Executive Compensation – Potential Payments Upon Termination or Change in Control,” “Corporate Governance – Non-Employee Director Compensation,” “Corporate Governance – 2024 Non-Employee Director Compensation Table,” “Executive Compensation – CEO Pay Ratio,” “Executive Compensation – Pay Versus Performance,” “Corporate Governance – Compensation and Talent Committee Interlocks and Insider Participation,” and “Compensation and Talent Committee Report” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this Item with respect to Item 201(d) of Regulation S-K is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the section entitled “Executive Compensation – Equity Compensation Plan Information” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K. The information required by this Item with respect to Item 403 of Regulation S-K is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the section entitled “Stock Ownership Information” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Corporate Governance – Related Person Transactions” and “Corporate Governance – Director Independence” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this Item is omitted from this Annual Report on Form 10-K and, pursuant to Regulation 14A of the Exchange Act, is incorporated herein by reference from the sections entitled “Audit Committee Matters – Independent Auditors’ Fees” and “Audit Committee Matters – Independent Auditor Fee Approval Policy” in the Company’s definitive Proxy Statement with respect to its 2025 Annual Meeting, which Proxy Statement will be filed with the SEC within 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
|
|
|
|
|
|
|
|
|
| The following documents are filed as part of this Form 10-K: |
|
|
|
| (a)(1) and (a)(2) |
The financial statements set forth in the Index to Consolidated Financial Statements and the Consolidated Financial Statement Schedule are filed as a part of this Annual Report on Form 10-K commencing on page F-1. |
|
|
|
| (a)(3) and (b) |
The exhibits listed in the accompanying Exhibit Index are filed as part of this Annual Report on Form 10-K and either filed herewith or incorporated by reference herein, as applicable. |
ITEM 16. FORM 10-K SUMMARY
None.
FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
AND
CONSOLIDATED FINANCIAL STATEMENT SCHEDULE
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of IDEXX Laboratories, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of IDEXX Laboratories, Inc. and its subsidiaries (the "Company") as of December 31, 2024 and 2023, and the related consolidated statements of income, of comprehensive income, of stockholders’ equity and of cash flows for each of the three years in the period ended December 31, 2024, including the related notes (collectively referred to as the "consolidated financial statements"). We also have audited the Company's internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2024 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2024, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the Report of Management on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue Recognition from Certain Product and Service Revenue
As described in Note 3 to the consolidated financial statements, the Company recognized product and service revenue of $3.9 billion for the year ended December 31, 2024, of which a majority relates to certain product and service revenue. Management recognizes revenue when, or as, performance obligations under the terms of a contract are satisfied, which occurs when control of the promised products or services is transferred to a customer, and it is probable that the Company will collect substantially all of the consideration to which they will be entitled, based on the customer’s intent and ability to pay the promised consideration. Management excludes sales, use, value-added, and other taxes they collect on behalf of third parties from revenue. Revenue is measured as the amount of consideration expected to be received in exchange for transferring products or services to a customer. To accurately present the consideration received in exchange for promised products or services, the Company applies the five-step revenue recognition model.
The principal consideration for our determination that performing procedures relating to revenue recognition from certain product and service revenue is a critical audit matter is a high degree of auditor effort in performing procedures related to the Company’s revenue recognition from certain product and service revenue.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the revenue recognition process, including controls over the recording of certain product and service revenue at the amount of consideration management expects to receive in exchange for transferring products or services to the customer. These procedures also included, among others (i) testing the completeness, accuracy, and occurrence of revenue recognized for a sample of revenue transactions by obtaining and inspecting source documents, such as purchase orders, invoices, contracts, proof of delivery and acceptance for instruments, proof of shipment, proof of lab report or proof of lab test, and subsequent payment receipts and (ii) for a sample of outstanding customer invoice balances as of December 31, 2024, obtaining and inspecting source documents, such as invoices, proof of delivery and acceptance for instruments, proof of shipment, proof of lab report or proof of lab test, and subsequent payment receipts.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
February 21, 2025
We have served as the Company’s auditor since 2002.
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
| ASSETS |
|
|
|
| Current Assets: |
|
|
|
| Cash and cash equivalents |
$ |
288,266 |
|
|
$ |
453,932 |
|
Accounts receivable, net of allowance of $12,585 in 2024 and $9,501 in 2023 |
473,575 |
|
|
457,445 |
|
| Inventories |
381,877 |
|
|
380,282 |
|
| Other current assets |
256,179 |
|
|
203,595 |
|
| Total current assets |
1,399,897 |
|
|
1,495,254 |
|
| Long-Term Assets: |
|
|
|
| Property and equipment, net |
713,123 |
|
|
702,177 |
|
| Operating lease right-of-use assets |
116,129 |
|
|
115,499 |
|
| Goodwill |
405,100 |
|
|
365,961 |
|
| Intangible assets, net |
111,676 |
|
|
84,500 |
|
| Other long-term assets |
547,518 |
|
|
496,534 |
|
| Total long-term assets |
1,893,546 |
|
|
1,764,671 |
|
| TOTAL ASSETS |
$ |
3,293,443 |
|
|
$ |
3,259,925 |
|
|
|
|
|
| LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
| Current Liabilities: |
|
|
|
| Accounts payable |
$ |
114,211 |
|
|
$ |
110,643 |
|
| Accrued liabilities |
502,119 |
|
|
478,712 |
|
| Credit facility |
250,000 |
|
|
250,000 |
|
| Current portion of long-term debt |
167,787 |
|
|
74,997 |
|
| Current portion of deferred revenue |
33,799 |
|
|
37,195 |
|
| Total current liabilities |
1,067,916 |
|
|
951,547 |
|
| Long-Term Liabilities: |
|
|
|
| Deferred income tax liabilities |
11,312 |
|
|
7,235 |
|
| Long-term debt, net of current portion |
449,786 |
|
|
622,883 |
|
| Long-term deferred revenue, net of current portion |
26,939 |
|
|
28,533 |
|
Long-term operating lease liabilities, net of current portion |
97,836 |
|
|
99,671 |
|
| Other long-term liabilities |
44,341 |
|
|
65,526 |
|
| Total long-term liabilities |
630,214 |
|
|
823,848 |
|
| Total liabilities |
1,698,130 |
|
|
1,775,395 |
|
|
|
|
|
| Commitments and Contingencies (Note 16) |
|
|
|
|
|
|
|
| Stockholders’ Equity: |
|
|
|
Common stock, $0.10 par value: Authorized: 120,000 shares; Issued: 107,836 shares in 2024 and 107,506 shares in 2023; Outstanding: 81,604 shares in 2024 and 83,032 shares in 2023 |
10,784 |
|
|
10,751 |
|
| Additional paid-in capital |
1,673,863 |
|
|
1,569,565 |
|
Deferred stock units: Outstanding: 60 units in 2024 and 59 units in 2023 |
5,885 |
|
|
5,530 |
|
| Retained earnings |
5,332,438 |
|
|
4,444,571 |
|
| Accumulated other comprehensive loss |
(93,645) |
|
|
(71,206) |
|
Treasury stock, at cost: 26,232 shares in 2024 and 24,474 shares in 2023 |
(5,334,012) |
|
|
(4,474,681) |
|
| Total IDEXX Laboratories, Inc. stockholders’ equity |
1,595,313 |
|
|
1,484,530 |
|
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ |
3,293,443 |
|
|
$ |
3,259,925 |
|
|
|
|
|
| The accompanying notes are an integral part of these consolidated financial statements. |
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
| Revenue: |
|
|
|
|
|
|
| Product revenue |
|
$ |
2,248,178 |
|
|
$ |
2,089,936 |
|
|
$ |
1,928,773 |
|
| Service revenue |
|
1,649,326 |
|
|
1,571,017 |
|
|
1,438,551 |
|
| Total revenue |
|
3,897,504 |
|
|
3,660,953 |
|
|
3,367,324 |
|
| Cost of Revenue: |
|
|
|
|
|
|
| Cost of product revenue |
|
716,078 |
|
|
717,951 |
|
|
656,511 |
|
| Cost of service revenue |
|
802,499 |
|
|
753,032 |
|
|
706,475 |
|
| Total cost of revenue |
|
1,518,577 |
|
|
1,470,983 |
|
|
1,362,986 |
|
| Gross profit |
|
2,378,927 |
|
|
2,189,970 |
|
|
2,004,338 |
|
| Expenses: |
|
|
|
|
|
|
| Sales and marketing |
|
588,507 |
|
|
566,066 |
|
|
524,505 |
|
| General and administrative |
|
442,291 |
|
|
335,825 |
|
|
326,248 |
|
| Research and development |
|
219,792 |
|
|
190,951 |
|
|
254,820 |
|
Total operating expenses |
|
1,250,590 |
|
|
1,092,842 |
|
|
1,105,573 |
|
| Income from operations |
|
1,128,337 |
|
|
1,097,128 |
|
|
898,765 |
|
| Interest expense |
|
(31,205) |
|
|
(41,581) |
|
|
(39,858) |
|
| Interest income |
|
12,699 |
|
|
5,629 |
|
|
1,065 |
|
| Income before provision for income taxes |
|
1,109,831 |
|
|
1,061,176 |
|
|
859,972 |
|
| Provision for income taxes |
|
221,964 |
|
|
216,134 |
|
|
180,883 |
|
Net income |
|
$ |
887,867 |
|
|
$ |
845,042 |
|
|
$ |
679,089 |
|
|
|
|
|
|
|
|
| Earnings per Share: |
|
|
|
|
|
|
| Basic |
|
$ |
10.77 |
|
|
$ |
10.17 |
|
|
$ |
8.12 |
|
| Diluted |
|
$ |
10.67 |
|
|
$ |
10.06 |
|
|
$ |
8.03 |
|
| Weighted Average Shares Outstanding: |
|
|
|
|
|
|
| Basic |
|
82,467 |
|
|
83,066 |
|
|
83,623 |
|
| Diluted |
|
83,246 |
|
|
83,978 |
|
|
84,600 |
|
|
|
|
|
|
|
|
| The accompanying notes are an integral part of these consolidated financial statements. |
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Net income |
|
$ |
887,867 |
|
|
$ |
845,042 |
|
|
$ |
679,089 |
|
| Other comprehensive income (loss), net of tax: |
|
|
|
|
|
|
| Foreign currency translation adjustments |
|
(46,958) |
|
|
17,725 |
|
|
(25,692) |
|
Defined benefit plans (loss), net of tax benefit of $(130) in 2024, $(267) in 2023, and $(613) in 2022 |
|
(701) |
|
|
(1,302) |
|
|
(3,282) |
|
Reclassification adjustment for defined benefit plans included in net income, net of tax of $154 in 2024, $111 in 2023, and $99 in 2022 |
|
352 |
|
|
519 |
|
|
506 |
|
Unrealized gain (loss) from Euro-denominated notes, net of tax expense (benefit) of $1,280 in 2024, $(811) in 2023, and $1,411 in 2022 |
|
4,105 |
|
|
(2,601) |
|
|
4,525 |
|
Unrealized gain (loss) from investments, net of tax expense (benefit) of $— in 2024, $3 in 2023, and $(14) in 2022 |
|
1 |
|
|
8 |
|
|
(46) |
|
Reclassification adjustment from investments included in net income, net of tax of $51 in 2024, $— in 2023, and $— in 2022 |
|
163 |
|
|
— |
|
|
— |
|
| Unrealized gain (loss) on derivative instruments: |
|
|
|
|
|
|
Unrealized gain (loss) from foreign currency exchange contracts, net of tax expense (benefit) of $7,660 in 2024, $(143) in 2023, and $5,954 in 2022 |
|
19,418 |
|
|
(792) |
|
|
14,851 |
|
Unrealized gain (loss) from cross currency swaps, net of tax expense (benefit) of $1,864 in 2024, $(1,754) in 2023, and $1,190 in 2022 |
|
5,981 |
|
|
(5,629) |
|
|
3,817 |
|
Unrealized gain (loss) from interest rate swap, net of tax expense (benefit) of $600 in 2024, $976 in 2023, and $— in 2022 |
|
1,927 |
|
|
3,131 |
|
|
— |
|
Reclassification adjustment from derivative instruments for (gains) losses included in net income, net of tax (expense) benefit of $(2,473) in 2024, $(1,699) in 2023, and $(6,742) in 2022 |
|
(6,727) |
|
|
(4,469) |
|
|
(18,991) |
|
| Unrealized gain (loss) on derivative instruments |
|
20,599 |
|
|
(7,759) |
|
|
(323) |
|
| Other comprehensive gain (loss), net of tax |
|
(22,439) |
|
|
6,590 |
|
|
(24,312) |
|
| Comprehensive income |
|
$ |
865,428 |
|
|
$ |
851,632 |
|
|
$ |
654,777 |
|
|
|
|
|
|
|
|
| The accompanying notes are an integral part of these consolidated financial statements. |
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except per share amounts)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock |
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Shares |
|
$0.10 Par Value |
|
Additional Paid-in Capital |
|
Deferred Stock Units |
|
Retained Earnings |
|
Accumulated Other Comprehensive Loss |
|
Treasury Stock |
|
Total Stockholders’ Equity |
| Balance as of December 31, 2021 |
106,878 |
|
|
$ |
10,688 |
|
|
$ |
1,377,320 |
|
|
$ |
5,719 |
|
|
$ |
2,920,440 |
|
|
$ |
(53,484) |
|
|
$ |
(3,570,691) |
|
|
$ |
689,992 |
|
| Net income |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
679,089 |
|
|
— |
|
|
— |
|
|
679,089 |
|
| Other comprehensive gain, net of tax |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(24,312) |
|
|
— |
|
|
(24,312) |
|
| Repurchases of common stock, net of issuances |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(821,421) |
|
|
(821,421) |
|
| Common stock issued under stock plans, net |
315 |
|
|
31 |
|
|
36,195 |
|
|
(607) |
|
|
— |
|
|
— |
|
|
— |
|
|
35,619 |
|
| Share-based compensation cost |
— |
|
|
— |
|
|
49,700 |
|
|
70 |
|
|
— |
|
|
— |
|
|
— |
|
|
49,770 |
|
| Balance as of December 31, 2022 |
107,193 |
|
|
10,719 |
|
|
1,463,215 |
|
|
5,182 |
|
|
3,599,529 |
|
|
(77,796) |
|
|
(4,392,112) |
|
|
608,737 |
|
| Net income |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
845,042 |
|
|
— |
|
|
— |
|
|
845,042 |
|
| Other comprehensive loss, net of tax |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
6,590 |
|
|
— |
|
|
6,590 |
|
| Repurchases of common stock, net of issuances |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(82,569) |
|
|
(82,569) |
|
| Common stock issued under stock plans, net |
313 |
|
|
32 |
|
|
46,639 |
|
|
320 |
|
|
— |
|
|
— |
|
|
— |
|
|
46,991 |
|
| Share-based compensation cost |
— |
|
|
— |
|
|
59,711 |
|
|
28 |
|
|
— |
|
|
— |
|
|
— |
|
|
59,739 |
|
| Balance as of December 31, 2023 |
107,506 |
|
|
10,751 |
|
|
1,569,565 |
|
|
5,530 |
|
|
4,444,571 |
|
|
(71,206) |
|
|
(4,474,681) |
|
|
1,484,530 |
|
| Net income |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
887,867 |
|
|
— |
|
|
— |
|
|
887,867 |
|
| Other comprehensive loss, net of tax |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(22,439) |
|
|
— |
|
|
(22,439) |
|
| Repurchases of common stock, net of issuances |
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(859,331) |
|
|
(859,331) |
|
| Common stock issued under stock plans, net |
330 |
|
|
33 |
|
|
44,010 |
|
|
348 |
|
|
— |
|
|
— |
|
|
— |
|
|
44,391 |
|
| Share-based compensation cost |
— |
|
|
— |
|
|
60,288 |
|
|
7 |
|
|
— |
|
|
— |
|
|
— |
|
|
60,295 |
|
| Balance as of December 31, 2024 |
107,836 |
|
|
$ |
10,784 |
|
|
$ |
1,673,863 |
|
|
$ |
5,885 |
|
|
$ |
5,332,438 |
|
|
$ |
(93,645) |
|
|
$ |
(5,334,012) |
|
|
$ |
1,595,313 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| The accompanying notes are an integral part of these consolidated financial statements. |
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Cash Flows from Operating Activities: |
|
|
|
|
|
|
| Net income |
|
$ |
887,867 |
|
|
$ |
845,042 |
|
|
$ |
679,089 |
|
| Adjustments to reconcile net income to net cash provided by operating activities: |
|
|
|
|
|
|
| Depreciation and amortization |
|
129,936 |
|
|
114,908 |
|
|
111,900 |
|
Impairment charges |
|
250 |
|
|
1,484 |
|
|
2,346 |
|
| Provision for uncollectible accounts |
|
6,867 |
|
|
5,552 |
|
|
5,829 |
|
| Deferred income taxes |
|
(24,194) |
|
|
(48,306) |
|
|
(35,065) |
|
| Share-based compensation expense |
|
60,295 |
|
|
59,739 |
|
|
49,770 |
|
| Other |
|
1,218 |
|
|
520 |
|
|
2,645 |
|
| Changes in assets and liabilities: |
|
|
|
|
|
|
| Accounts receivable |
|
(28,280) |
|
|
(53,871) |
|
|
(41,398) |
|
| Inventories |
|
(28,001) |
|
|
(28,651) |
|
|
(121,731) |
|
| Accounts payable |
|
8,086 |
|
|
(557) |
|
|
3,467 |
|
| Deferred revenue |
|
(4,378) |
|
|
(3,032) |
|
|
(11,019) |
|
| Other assets and liabilities |
|
(80,665) |
|
|
13,682 |
|
|
(102,849) |
|
| Net cash provided by operating activities |
|
929,001 |
|
|
906,510 |
|
|
542,984 |
|
| Cash Flows from Investing Activities: |
|
|
|
|
|
|
| Purchases of property and equipment |
|
(120,922) |
|
|
(133,631) |
|
|
(148,838) |
|
| Acquisitions of intangible assets |
|
(10,000) |
|
|
— |
|
|
(10,000) |
|
| Equity investments |
|
(1,004) |
|
|
— |
|
|
(25,000) |
|
| Acquisitions of businesses, net of cash acquired |
|
(76,694) |
|
|
— |
|
|
(11,512) |
|
Proceeds from net investment hedges |
|
1,558 |
|
|
8,377 |
|
|
— |
|
| Net cash used by investing activities |
|
(207,062) |
|
|
(125,254) |
|
|
(195,350) |
|
| Cash Flows from Financing Activities: |
|
|
|
|
|
|
(Payments) borrowings on credit facility, net |
|
— |
|
|
(329,000) |
|
|
505,500 |
|
| Payments of senior notes |
|
(75,000) |
|
|
(75,000) |
|
|
(75,000) |
|
| Debt issuance costs |
|
— |
|
|
— |
|
|
(435) |
|
Repurchases of common stock |
|
(837,034) |
|
|
(71,920) |
|
|
(819,711) |
|
| Proceeds from exercises of stock options and employee stock purchase plans |
|
44,492 |
|
|
47,034 |
|
|
35,747 |
|
Payments of acquisition-related contingent consideration and holdbacks |
|
— |
|
|
(3,135) |
|
|
(6,431) |
|
| Shares withheld for statutory tax withholding payments on restricted stock |
|
(10,531) |
|
|
(9,975) |
|
|
(10,606) |
|
| Net cash used by financing activities |
|
(878,073) |
|
|
(441,996) |
|
|
(370,936) |
|
| Net effect of changes in exchange rates on cash |
|
(9,532) |
|
|
2,126 |
|
|
(8,606) |
|
Net increase (decrease) in cash and cash equivalents |
|
(165,666) |
|
|
341,386 |
|
|
(31,908) |
|
| Cash and cash equivalents at beginning of period |
|
453,932 |
|
|
112,546 |
|
|
144,454 |
|
| Cash and cash equivalents at end of period |
|
$ |
288,266 |
|
|
$ |
453,932 |
|
|
$ |
112,546 |
|
|
|
|
|
|
|
|
| The accompanying notes are an integral part of these consolidated financial statements. |
|
|
IDEXX LABORATORIES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. NATURE OF BUSINESS, BASIS OF PRESENTATION AND PRINCIPLES OF CONSOLIDATION
The accompanying consolidated financial statements of IDEXX Laboratories, Inc. and its subsidiaries have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and with the requirements of Regulation S-X.
These statements include the accounts of IDEXX Laboratories, Inc., and our wholly-owned and majority-owned subsidiaries (“IDEXX,” the “Company,” “we,” or “our”). We do not have any variable interest entities for which we are the primary beneficiary. All intercompany transactions and balances have been eliminated in consolidation.
We have included certain terms and abbreviations used throughout these financial statements in the “Glossary of Terms and Selected Abbreviations.”
We develop, manufacture, and distribute products and provide services primarily for the companion animal veterinary, livestock and poultry, dairy, and water testing industries. We also sell human medical point-of-care products and laboratory diagnostics. Our principal line of business, which we refer to as our Companion Animal Group (“CAG”) operating segment, provides diagnostic capabilities and information management solutions for the companion animal veterinary industry, as well as the biomedical research community. Our principal regions for these products and services are North America, Europe, Australia, and Japan, but we also sell to customers and distributors in many other countries. Our Water operating segment provides innovative testing solutions for the quality and safety of water principally in the U.S. and Europe, and Brazil, but we also sell to customers in many other countries. Our Livestock, Poultry and Dairy (“LPD”) operating segment provides diagnostic tests and related instrumentation and performs services that are used to manage the health status of livestock and poultry, to improve producer efficiency, and to ensure the quality and safety of milk. Our principal regions for these products and services are Europe, the United States, Brazil, China, and Japan, but we also sell to customers in many other countries. We also operate a smaller operating segment that is comprised of our human medical diagnostic products and services business (“OPTI Medical”). Financial information about our OPTI Medical operating segment is combined and presented with our out-licensing arrangements remaining from our pharmaceutical business in an “Other” category because they do not meet the quantitative or qualitative thresholds for reportable segments. Refer to “Note 3. Revenue” for additional information regarding disaggregated revenue by segment, major product and service categories, and geographical areas. Refer to “Note 17. Segment Reporting” for additional information regarding our reportable operating segments.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Estimates
The preparation of these consolidated financial statements in accordance with U.S. GAAP requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures. On an ongoing basis, we evaluate these estimates, including those related to reserves for accounts receivable, customer contract assets and lease receivables; goodwill and other intangible assets; income taxes; inventory valuation; revenue recognition, including product returns and customer contracts with multiple performance obligations; share-based compensation; warranty reserves; self-insurance reserves; fair value measurements; and loss contingencies. We accrue contingent liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ materially from these estimates.
(b) Cash and Cash Equivalents
We consider all highly liquid investments with original maturities of ninety days or less to be cash equivalents. Cash and cash equivalents consist primarily of demand deposits, money market funds, and short-duration agency bonds and commercial paper as described above, which management believes are subject to minimal credit and market risk.
There is no restricted cash on our consolidated balance sheets for the years ended December 31, 2024, and 2023.
(c) Inventories – Refer to Note 7
(d) Property and Equipment – Refer to Note 9
(e) Goodwill and Other Intangible Assets – Refer to Note 11
(f) Warranty Reserves
We provide a standard twelve-month warranty on all diagnostic instruments sold. We recognize the cost of instrument warranties in cost of product revenue at the time revenue is recognized based on the estimated cost to repair the instrument over its warranty period. Cost of product revenue reflects not only estimated warranty expense for instruments sold in the current period, but also any changes in estimated warranty expense for the portion of the aggregate installed base that is under warranty. Estimated warranty expense is based on a variety of inputs, including historical instrument performance in the customers’ environments, historical and estimated costs incurred in servicing instruments, and projected instrument reliability. Should actual service rates or costs differ from our estimates, revisions to the estimated warranty liability would be required. The liability for warranties is included in accrued liabilities in the accompanying consolidated balance sheets. The amount of warranty reserve during the years ended December 31, 2024, and 2023 was not material.
(g) Income Taxes – Refer to Note 14
(h) Taxes Remitted to Governmental Authorities by IDEXX on Behalf of Customers
We calculate, collect from our customers, and remit to governmental authorities sales, value-added, and excise taxes assessed by governmental authorities in connection with revenue-producing transactions with our customers. We report these taxes on a net basis and do not include these tax amounts in revenue or cost of product or service revenue.
(i) Revenue – Refer to Note 3
(j) Research and Development Costs
Research and development costs, which consist of employee compensation and benefits, certain licensing costs, materials, external consulting, and product development costs, are expensed as incurred. We evaluate our research and development costs for capitalization after the technological feasibility has been established for software and products containing software to be sold; however, no costs were capitalized during the years ended December 31, 2024, 2023, and 2022. Software developed to deliver hosted services to our customers has been designated as internal-use, and we capitalize certain costs incurred in connection with developing or obtaining software designated for internal use. Refer to “Note 9. Property and Equipment, Net” for further information on internal-use software.
(k) Advertising Costs
Advertising costs, which are recognized as sales and marketing expense in the period in which they are incurred, were $3.4 million, $4.2 million, and $5.0 million for the years ended December 31, 2024, 2023, and 2022, respectively.
(l) Legal Costs
Legal costs are considered period costs and, accordingly, are expensed in the year services are provided.
(m) Share-Based Compensation – Refer to Note 5
(n) Lease Commitments – Refer to Note 8
(o) Earnings per Share – Refer to Note 15
(p) Foreign Currency
The functional currency of most of our foreign subsidiaries is their local currency, and a small number of our foreign subsidiaries that conduct business primarily in U.S. dollars have the U.S. dollar as their functional currency. Assets and liabilities of our other foreign subsidiaries, whose functional currency is their local currency, are translated to the U.S. dollar using the exchange rate in effect at the balance sheet date. Revenue and expense accounts are translated to the U.S. dollar using the exchange rate at the date at which those elements are recognized, and where it is impractical to do so, an average exchange rate in effect during the period is used to translate those elements. Cumulative translation gains and losses are shown in the accompanying consolidated balance sheets as a separate component of accumulated other comprehensive income (“AOCI”).
Revenues and expenses denominated in a currency other than the respective subsidiary’s functional currency are recorded at the current exchange rate when the transaction is recognized. Monetary assets and liabilities denominated in a currency other than the respective subsidiary’s functional currency are remeasured at each balance sheet date using the exchange rate in effect at each balance sheet date. These foreign currency gains and losses are included in general and administrative expenses within our Other segment. We recognized aggregate foreign currency losses of $4.5 million, $1.1 million, and $3.4 million for the years ended December 31, 2024, 2023, and 2022, respectively.
(q) Hedging Instruments – Refer to Note 19
(r) Fair Value Measurements – Refer to Note 18
(s) Comprehensive Income
We report all changes in equity, including net income and transactions or other events and circumstances from non-owner sources during the period in which they are recognized. We have chosen to present comprehensive income, which encompasses net income, foreign currency translation adjustments, gains and losses on our net investment hedges, defined benefit plan adjustments, and the difference between the cost and the fair market value of investments in debt and equity securities, forward currency exchange contracts, and interest rate swap contracts in the consolidated statements of comprehensive income. Refer to “Note 21. Accumulated Other Comprehensive Income” for information about the effects on net income of significant amounts reclassified out of each component of AOCI for the years ended December 31, 2024, 2023, and 2022.
(t) Equity and Cost Methods of Accounting for Investments
Investments where we have the ability to exercise significant influence, but do not control the entity, are accounted for under the equity method of accounting. Significant influence generally exists if we have a 20% to 50% ownership interest in the investee, or in the case of a limited partnership or similar entity, at least 3% to 5%. Equity investments in entities for which we do not have the ability to exercise significant influence and whose securities do not have a readily determinable fair value are carried at cost less impairment, if any, adjusted for changes resulting from qualifying observable price changes for the identical investment of the same issuer should they occur.
We evaluate our investments for impairment whenever events or changes in circumstances indicate that the carrying amounts of such investments may be impaired. If a decline in the value of an investment is determined to be other than temporary, a loss is recorded in earnings in the current period.
Our equity investments of $31.0 million and $30.3 million for the years ended December 31, 2024, and December 31, 2023, respectively, are recorded at cost in other long-term assets. Refer to “Note 4. Acquisitions, Asset Purchases and Investments” for additional information regarding our acquisition of certain equity investments.
(u) Concentrations of Risk
Financial Instruments. Financial instruments that potentially subject us to concentrations of credit risk are principally cash, cash equivalents, accounts receivable, contract assets, lease receivables, and derivatives. To mitigate such risk with respect to cash and cash equivalents, we place our cash with highly rated financial institutions, in accounts that are insured by the U.S. government and money market funds invested in government securities. To reduce credit risk with respect to accounts receivable, contract assets, and lease receivables, we routinely assess the financial strength of our most significant customers and monitor the amounts owed to us. Concentrations of credit risk with respect to accounts receivable are limited due to the large number of customers that purchase our products and services, and we have no significant customers that accounted for greater than 10% of our consolidated revenues during the year ended December 31, 2024.
As a result, we believe that accounts receivable, contract assets, and lease receivables credit risk exposure is limited. We maintain allowances for expected credit losses, but historically have not experienced material losses related to an individual customer or group of customers in any particular industry or geographic area.
To mitigate concentration of credit risk with respect to derivatives, we enter into transactions with highly rated financial institutions, enter into master netting arrangements with counterparties to our derivative transactions, and frequently monitor the creditworthiness of our counterparties. Our master netting arrangements reduce our exposure in that they permit outstanding receivables and payables with the counterparties to our derivative transactions to be offset in the event of default. We have not incurred such losses and consider the risk of counterparty default to be minimal.
Sole and Single-Source Suppliers. Many of the third parties that provide us with certain instruments we sell, as well as certain components, raw materials and consumables used in or with our products, are sole or single-source suppliers. Some of the products that we purchase from these suppliers are proprietary or complex in nature, and, therefore, cannot be readily or easily replaced by alternative sources. We have a process of qualifying alternative third-party suppliers for select components and materials, which in certain cases require approvals from various regulatory agencies before we are able to use and sell products using these alternative sources. If we are unable to obtain adequate quantities of inventory, we could face cost increases or delays or discontinuations in product shipments, which could have a material adverse effect on our results of operations. Under certain long-term supply arrangements, we paid the supplier for costs related to molds, dies, and other tools used for the production of products we purchase. These payments are capitalized in other assets, as we do not have title or control of the underlying production assets, and are amortized to cost of sales.
(v) New Accounting Pronouncements Adopted
We adopted ASU 2022-04, “Liabilities - Supplier Finance Programs (Subtopic 405-50): Disclosure of Supplier Finance Program Obligations,” as of January 1, 2023, which adds certain disclosure requirements for a buyer in a supplier finance program. The amendments in this update require that a buyer in a supplier finance program discloses sufficient information about the program to allow a user of financial statements to understand the program's nature, activity during the period, changes from period to period, and potential magnitude. Refer to “Note 12. Accounts Payable, Accrued Liabilities and Other Long-Term Liabilities.”
We adopted ASU 2021-08, “Business Combinations (Topic 805): Accounting for Acquired Contract Assets and Contract Liabilities,” as of January 1, 2023. ASU 2021-08 is intended to improve comparability for both the recognition and measurement of acquired revenue contracts with customers at the date of and after a business combination by providing consistent recognition guidance. The adoption of ASU 2021-08 did not have a material impact on our consolidated financial statements.
We adopted ASU 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures,” as of December 31, 2024. ASU 2023-07 is intended to improve reportable segment disclosures. The amendments require disclosure of significant segment expenses that are regularly provided to the chief operating decision maker and included within segment profit and loss. The adoption of ASU 2023-07 did not have a material impact on our consolidated financial statements.
(w) New Accounting Pronouncements Not Yet Adopted
In November 2024, the FASB issued ASU 2024-03, “Disaggregation of Income Statement Expenses”, to provide disaggregated disclosures of specific expense categories underlying all relevant income statement expense line items on an annual and interim basis. The disclosure requirements will be applied on a prospective basis, with the option to apply it retrospectively. The effective date for the standard is for fiscal years beginning after December 15, 2026, and interim periods within fiscal years beginning after December 15, 2027. Early adoption is permitted. We are evaluating ASU 2024-03 to determine its impact on our consolidated financial statements.
In December 2023, the FASB issued ASU 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures,” which includes amendments that enhance income tax disclosures, primarily through standardization and disaggregation of income tax rate reconciliation categories and income taxes paid by jurisdiction. The amendments are effective for annual periods beginning after December 15, 2024, with early adoption permitted, and may be applied either prospectively or retrospectively. We are currently evaluating ASU 2023-09 to assess the impact on our consolidated financial statement disclosures and to determine the transition method in which the new guidance will be adopted.
NOTE 3. REVENUE
Revenue Recognition
We recognize revenue when, or as, performance obligations under the terms of a contract are satisfied, which occurs when control of the promised products or services is transferred to a customer, and it is probable that we will collect substantially all of the consideration to which we will be entitled, based on the customer’s intent and ability to pay the promised consideration. We exclude sales, use, value-added, and other taxes we collect on behalf of third parties from revenue. Revenue is measured as the amount of consideration we expect to receive in exchange for transferring products or services to a customer. To accurately present the consideration received in exchange for promised products or services, we apply the five-step model outlined below:
1.Identification of a contract or agreement with a customer
2.Identification of our performance obligations in the contract or agreement
3.Determination of the transaction price
4.Allocation of the transaction price to the performance obligations
5.Recognition of revenue when, or as, we satisfy a performance obligation
We enter into contracts where customers purchase combinations of IDEXX products and services, which are generally capable of being distinct and accounted for as separate performance obligations. The timing of revenue recognition, billings, and cash collections result in accounts receivable, lease receivables, and contract assets arising when revenue is recognized in advance of billings, and contract liabilities or deferred revenue as a result of receiving consideration in advance of revenue recognition within our consolidated balance sheets. Customer payment terms are typically 30 to 60 days, and these terms vary by location based on local business practices and by customer.
Customer contracts are modified primarily to create new, or change existing, enforceable rights and obligations. Customer contract modifications typically create new performance obligations to deliver additional goods and/or services that are distinct from the goods and/or services transferred before the modification, and the related increase in consideration does not reflect the standalone selling price for the additional goods and/or services. We account for these modifications prospectively as if it were a termination of the existing contract and the creation of a new contract, and we allocate the sum of the remaining consideration of the original contract that has not been recognized as revenue and the incremental consideration promised as part of the modification to the remaining performance obligations.
From time to time we have other types of contract modifications. Contract modifications that create new performance obligations to deliver additional goods and/or services that are distinct from the goods and/or services transferred before the modification, and the related increase in consideration approximates the standalone selling price for the additional goods and/or services, are accounted for as separate contracts. Contract modifications that do not create new performance obligations, but the goods and/or services to be delivered after the contract modification date are distinct from the goods and/or services transferred before the modification, are accounted for as a termination of the existing contract and the creation of a new contract.
Revenues by Product and Service Categories and by Principal Geographic Areas
We present disaggregated revenue for our CAG segment based on major product and service categories. Our Water and LPD segments are comprised of a single major product category.
The following table presents revenue by major product and service categories:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
| CAG segment revenue: |
|
|
|
|
|
|
| CAG Diagnostics recurring revenue: |
|
$ |
3,129,492 |
|
|
$ |
2,935,425 |
|
|
$ |
2,660,280 |
|
| IDEXX VetLab consumables |
|
1,303,250 |
|
|
1,188,261 |
|
|
1,057,236 |
|
| Rapid assay products |
|
359,754 |
|
|
344,494 |
|
|
313,667 |
|
| Reference laboratory diagnostic and consulting services |
|
1,336,121 |
|
|
1,278,617 |
|
|
1,178,113 |
|
| CAG Diagnostics services and accessories |
|
130,367 |
|
|
124,053 |
|
|
111,264 |
|
|
|
|
|
|
|
|
| CAG Diagnostics capital - instruments |
|
131,928 |
|
|
137,603 |
|
|
147,326 |
|
|
|
|
|
|
|
|
Veterinary software, services and diagnostic imaging systems: |
|
312,624 |
|
|
279,328 |
|
|
251,187 |
|
Recurring revenue |
|
250,359 |
|
|
214,597 |
|
|
180,973 |
|
Systems and hardware |
|
62,265 |
|
|
64,731 |
|
|
70,214 |
|
| CAG segment revenue |
|
3,574,044 |
|
|
3,352,356 |
|
|
3,058,793 |
|
|
|
|
|
|
|
|
| Water segment revenue |
|
185,112 |
|
|
168,149 |
|
|
155,720 |
|
| LPD segment revenue |
|
122,060 |
|
|
121,659 |
|
|
122,607 |
|
| Other segment revenue |
|
16,288 |
|
|
18,789 |
|
|
30,204 |
|
|
|
|
|
|
|
|
| Total revenue |
|
$ |
3,897,504 |
|
|
$ |
3,660,953 |
|
|
$ |
3,367,324 |
|
The following table presents revenue by principal geographic area, based on customers’ domiciles:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
| Americas |
|
|
|
|
|
|
| United States |
|
$ |
2,533,174 |
|
|
$ |
2,391,427 |
|
|
$ |
2,182,959 |
|
| Canada |
|
152,885 |
|
|
150,110 |
|
|
142,743 |
|
| Latin America & Caribbean |
|
83,690 |
|
|
83,923 |
|
|
75,035 |
|
|
|
2,769,749 |
|
|
2,625,460 |
|
|
2,400,737 |
|
| Europe, the Middle East and Africa |
|
|
|
|
|
|
| Germany |
|
173,682 |
|
|
149,789 |
|
|
137,876 |
|
| United Kingdom |
|
134,630 |
|
|
121,745 |
|
|
110,400 |
|
| France |
|
110,795 |
|
|
96,797 |
|
|
84,479 |
|
| Spain |
|
61,857 |
|
|
52,332 |
|
|
46,982 |
|
| Italy |
|
58,224 |
|
|
53,787 |
|
|
48,192 |
|
| Switzerland |
|
37,183 |
|
|
34,830 |
|
|
30,646 |
|
| Netherlands |
|
34,047 |
|
|
30,508 |
|
|
26,882 |
|
| Other |
|
196,313 |
|
|
176,396 |
|
|
161,577 |
|
|
|
806,731 |
|
|
716,184 |
|
|
647,034 |
|
| Asia Pacific Region |
|
|
|
|
|
|
| Australia |
|
101,959 |
|
|
95,465 |
|
|
96,408 |
|
| Japan |
|
73,139 |
|
|
75,569 |
|
|
77,661 |
|
| China |
|
35,822 |
|
|
44,168 |
|
|
49,193 |
|
| Other |
|
110,104 |
|
|
104,107 |
|
|
96,291 |
|
|
|
321,024 |
|
|
319,309 |
|
|
319,553 |
|
| Total |
|
$ |
3,897,504 |
|
|
$ |
3,660,953 |
|
|
$ |
3,367,324 |
|
Major Categories of Revenue for our Products and Services
Diagnostic Products and Accessories. Diagnostic products and accessories revenues, including IDEXX VetLab® consumables and accessories, rapid assay, LPD, Water, and OPTI testing products, are predominantly recognized and invoiced at the time of shipment, which is when the customer obtains control of the product based on legal title transfer and we have the right to payment. We also provide customers with certain consumables that are recognized upon utilization by the customer, which is when we have the right to payment and the risks and rewards of ownership transfer. Shipping costs reimbursed by the customer are included in revenue and cost of sales. As a practical expedient, we do not account for shipping activities as a separate performance obligation.
Reference Laboratory Diagnostic and Consulting Services. Laboratory diagnostic and consulting services revenues are recognized and invoiced when the laboratory diagnostic service is performed.
Instruments, Software and Systems. CAG Diagnostics capital instruments, diagnostic imaging systems, veterinary software licenses and computer hardware revenues are recognized and invoiced when the customer obtains control of the products based on legal title transfer and we have the right to payment, which generally occurs at the time of installation and customer acceptance. Our instruments, software, and systems are often included in one of our significant customer programs, as further described below.
SaaS Subscriptions. We offer a variety of veterinary software and diagnostic imaging SaaS subscriptions. We recognize revenue for our SaaS subscriptions over time on a ratable basis over the contract term, beginning on the date our service is made available to the customer. Our subscription contracts vary in term from monthly to two years. Customers typically pay for our subscription contracts in equal monthly amounts over the term of the arrangement. Deferred revenue related to our SaaS subscriptions is not material.
Extended Warranties and Post-Contract Support. CAG Diagnostics capital instruments and diagnostic imaging systems extended warranties typically provide customers with continued coverage for a period of one to five years beyond the first-year standard warranty. Customers can either pay in full for the extended warranty at the time of instrument or system purchase, or can be billed on a quarterly basis over the term of the contract. We recognize revenue associated with extended warranties over time on a ratable basis using a time-elapsed measure of performance over the contract term, which approximates the expected timing in which applicable services are performed.
Veterinary software post-contract support provides customers with access to technical support when and as needed through access to call centers and online customer assistance. Post-contract support contracts typically have a term of twelve months and customers are billed for post-contract support in equal quarterly amounts over the term. We recognize revenue for post-contract support services over time on a ratable basis using a time-elapsed measure of performance over the contract term, which approximates the expected timing in which applicable services are performed.
Contracts with Multiple Performance Obligations
We enter into arrangements with multiple performance obligations where customers purchase a combination of IDEXX products and services. Determining whether products and services are considered distinct performance obligations that should be accounted for separately requires significant judgment. We determine the transaction price for a contract based on the total consideration we expect to receive in exchange for the transferred goods or services. To the extent the transaction price includes variable consideration, such as volume rebates or expected price adjustments, we apply judgment in constraining the estimated variable consideration due to factors that may cause reversal of revenue recognized. We evaluate constraints based on our historical and projected experience with similar customer arrangements.
We allocate revenue to each performance obligation in proportion to the relative standalone selling prices, and recognize revenue when control of the related goods or services is transferred for each obligation. We utilize the observable standalone selling price when available, which represents the price charged for the promised product or service when sold separately. When standalone selling prices for our products or services are not directly observable, we determine the standalone selling prices using relevant information available and apply suitable estimation methods including, but not limited to, the cost plus a margin approach. We recognize revenue as each performance obligation is satisfied, either at a point in time or over time, as described in the revenue categories above. We do not disclose information about remaining performance obligations that are part of arrangements with an original expected duration of one year or less.
The following customer arrangements represent our most significant customer contracts that contain multiple performance obligations:
Customer Commitment Arrangements. We offer customers incentives upon entering into multi-year arrangements to purchase annual minimum amounts of products and services.
Free or Discounted Instruments and Systems. Many of our customer commitment arrangements, such as our IDEXX 360 program, provide customers with free or discounted instruments or systems upon entering into multi-year arrangements to purchase annual minimum amounts of products and services. We allocate total consideration, including future committed purchases and expected price adjustments, based on relative standalone selling prices, to identified performance obligations and recognize instrument revenue and cost at the time of installation and customer acceptance in advance of billing the customer, which is also when the customer obtains control of the instrument based on legal title transfer. Our right to future consideration related to instrument revenue is recorded as a contract asset within other current and long-term assets. The contract asset is transferred to accounts receivable when customers are billed for products and services over the term of the arrangement. We have determined that these arrangements do not include a significant financing component.
On December 31, 2023, our contract assets were $223.1 million, of which approximately $55.6 million was reclassified to accounts receivable when customers were billed for related products and services during the year ended December 31, 2024. Furthermore, as a result of new placements under commitment arrangements, net of subsequent amounts reclassified to accounts receivable and allowances established for credit losses, our contract assets were $246.3 million as of December 31, 2024. We monitor customer purchases over the term of their arrangement to assess the realizability of our contract assets and review estimates of variable consideration. Impairments and revenue adjustments that relate to performance obligations satisfied in prior periods, including cumulative catch-up adjustments to revenue arising from contract modifications, during the years ended December 31, 2024, and 2023, were not material.
Up-Front Consideration Paid to Customers. We provide customers with incentives in the form of IDEXX Points upon entering into multi-year arrangements to purchase annual minimum amounts of future products and services. If a customer breaches their agreement, they are required to refund all or a portion of the up-front consideration, or make other repayments, remedial actions, or both. Up-front incentives to customers in the form of IDEXX Points or, from time to time, cash, are not made in exchange for distinct goods or services and are capitalized as consideration paid to customers within other current and long-term assets, which are subsequently recognized as a reduction to revenue over the term of the customer arrangement. If these up-front incentives are subsequently utilized to purchase instruments, we allocate total consideration, including future committed purchases less up-front incentives and estimates of expected price adjustments, based on relative standalone selling prices, to identified performance obligations and recognize instrument revenue and cost at the time of installation and customer acceptance. To the extent invoiced instrument revenue exceeds recognized instrument revenue, we record deferred revenue as a contract liability, which is subsequently recognized upon the purchase of products and services over the term of the contract. We have determined these arrangements do not include a significant financing component.
On December 31, 2023, our capitalized consideration paid to customers was $168.9 million, of which approximately $54.1 million was recognized as a reduction of revenue during the year ended December 31, 2024. Furthermore, as a result of new payments to customers, net of subsequent recognition, our capitalized consideration paid to customers was $196.6 million as of December 31, 2024. We monitor customer purchases over the term of their arrangement to assess the realizability of our capitalized consideration paid to customers and review estimates of variable consideration. Impairments and revenue adjustments that relate to performance obligations satisfied in prior periods, including cumulative catch-up adjustments to revenue arising from contract modifications, during the years ended December 31, 2024, and 2023, were not material.
Rebate Arrangements. Our rebate arrangements provide customers the opportunity to earn future rebates based on the volume of products and services they purchase over the term of the arrangement. Rebate incentives are typically offered in multi-year arrangements that include customer commitments to purchase annual minimum amounts of products and services or, to a lesser extent, are sometimes offered without future purchase commitments.
We account for the customer’s right to earn rebates on future purchases as a separate performance obligation and determine the standalone selling price based on an estimate of rebates the customer will earn over the term of the arrangement. Total consideration allocated to identified performance obligations is limited to goods and services that the customer is presently obligated to purchase and does not include estimates of future purchases that are optional. We allocate total consideration to identified performance obligations, including the customer’s right to earn rebates on future purchases, which is deferred and subsequently recognized upon the purchase of products and/or services.
On December 31, 2023, our deferred revenue related to rebate and up-front consideration arrangements was $32.9 million, of which approximately $11.0 million was recognized when customers purchased eligible products and services during the year ended December 31, 2024. Furthermore, as a result of new customer purchases under rebate and up-front consideration arrangements, net of subsequent recognition, our deferred revenue was $30.0 million as of December 31, 2024, of which approximately 34%, 26%, 19%, 12%, and 9% are expected to be recognized during 2025, 2026, 2027, 2028, and thereafter, respectively.
For our customer commitment arrangements, we estimate future revenues related to multi-year arrangements to be approximately $4.4 billion, of which approximately 28%, 25%, 21%, 16%, and 10% are expected to be recognized during 2025, 2026, 2027, 2028, and thereafter, respectively. These future revenues relate to performance obligations not yet satisfied, for which customers have committed to future purchases, net of the expected revenue reductions from consideration paid to customers and expected price adjustments, and as a result, are lower than stated contractual commitments by our customers.
Instrument Rental Arrangements. Revenues from instrument rental and reagent rental arrangements are recognized either as operating leases on a ratable basis over the term of the arrangement or as sales-type leases at the time of installation and customer acceptance. Customers typically pay for the right to use instruments under rental arrangements in equal monthly amounts over the term of the rental arrangement. For some arrangements, customers are provided with the right to purchase the instrument at the end of the lease term. Our reagent rental arrangements provide customers the right to use our instruments upon entering into multi-year arrangements to purchase annual minimum amounts of consumables. These types of arrangements include an embedded lease for the right to use our instrument and we determine the amount of lease revenue allocated to the instrument based on relative standalone selling prices. Lease revenues are presented in product revenue on our consolidated income statement. Lease revenue was approximately $13.9 million, $20.7 million, and $20.3 million for the years ended December 31, 2024, 2023, and 2022, respectively, including both operating leases and sales-type leases.
Sales-type Reagent Rental Arrangements. Our reagent rental arrangements that effectively transfer control of instruments to our customers are classified as sales-type leases and we recognize instrument revenue and cost in advance of billing the customer, at the time of installation and customer acceptance. Our right to future consideration related to instrument revenue is recorded as a lease receivable within other current and long-term assets, and is transferred to accounts receivable when customers are billed for products and services over the term of the arrangement. On December 31, 2023, our lease receivable assets were $23.1 million, of which approximately $5.7 million was reclassified to accounts receivable when customers were billed for related products and services during the year ended December 31, 2024. Furthermore, as a result of new placements under sales-type reagent rental arrangements, net of subsequent amounts reclassified to accounts receivable, and allowances established for credit losses, our lease receivable assets were $19.0 million as of December 31, 2024. The impacts of discounting and unearned income as of December 31, 2024, were not material. Profit and loss recognized at the commencement date and interest income during the year ended December 31, 2024, were not material. We monitor customer purchases over the term of their arrangement to assess the realizability of our lease receivable assets. Impairments during the year ended December 31, 2024, were not material.
Operating-type Reagent Rental Arrangements. Our reagent rental arrangements that do not effectively transfer control of instruments to our customers are classified as operating leases and we recognize instrument revenue and costs ratably over the term of the arrangement. The cost of the instrument is capitalized within property and equipment. During the year ended December 31, 2024, we transferred instruments of $14.2 million compared to $14.6 million during the year ended December 31, 2023, from inventory to property and equipment.
We estimate future revenue to be recognized related to our reagent rental arrangements of approximately $69.1 million, of which approximately 26%, 24%, 19%, 16%, and 15% are expected to be recognized during 2025, 2026, 2027, 2028, and thereafter, respectively. These future revenues relate to performance obligations not yet satisfied for which customers have committed to future purchases, net of expected price adjustments, and as a result, may be lower than stated contractual commitments by our customers.
Other Customer Incentive Arrangements. Certain arrangements with customers include discounts or rebates on the sale of products and services applied retrospectively, such as volume rebates achieved by purchasing a specified purchase threshold of goods and services. We account for these discounts as variable consideration and estimate the likelihood of a customer meeting the threshold in order to determine the transaction price using the most predictive approach. We typically use the most-likely-amount method for incentives that are offered to individual customers, and the expected-value method for arrangements that are offered to a broad group of customers. Revenue adjustments that relate to performance obligations satisfied in prior periods, including cumulative catch-up adjustments to revenue arising from contract modifications, during the years ended December 31, 2024, and 2023, were not material. Refund obligations related to customer incentive arrangements are recorded in accrued liabilities for the actual issuance of incentives, incentives earned but not yet issued, and estimates of incentives to be earned in the future.
Combined Arrangements. At times, we combine aspects of customer commitment arrangements, instrument rental arrangements and other incentives within a single customer arrangement. We separate each significant element and include the contract assets, consideration paid to customers, deferred revenues, and estimated future revenues within the most relevant disclosures above. Each customer contract is presented as a net contract asset or net contract liability on our consolidated balance sheets.
Deferred Extended Warranties and Post-Contract Support Revenue
On December 31, 2023, our deferred revenue related to extended warranties and post-contract support was $26.0 million, of which approximately $20.1 million was recognized during the year ended December 31, 2024. Furthermore, as a result of new arrangements, our deferred revenue related to extended warranties and post-contract support was $25.2 million as of December 31, 2024. We do not disclose information about remaining performance obligations that are part of contracts with an original expected duration of one year or less, and do not adjust for the effect of the financing components when the period between customer payment and revenue recognition is one year or less. Deferred revenue related to extended warranties and post-contract support with an original duration of more than one year was $8.7 million as of December 31, 2024, of which approximately 40%, 31%, 17%, 7%, and 5% are expected to be recognized during 2025, 2026, 2027, 2028, and thereafter, respectively. We have determined these arrangements do not include a significant financing component.
IDEXX Points
IDEXX Points may be applied to trade receivables due to us, converted to cash, or applied against the purchase price of IDEXX products and services. We consider IDEXX Points equivalent to cash. IDEXX Points that have not yet been used by customers are included in accrued liabilities until utilized or expired. Breakage is not material because customers can apply IDEXX Points to trade receivables at any time.
Costs to Obtain a Contract
We capitalize sales commissions and the related fringe benefits earned by our sales force when considered incremental and recoverable costs of obtaining a contract that includes future performance obligations. Our contracts include performance obligations related to various goods and services, some of which are satisfied at a point in time and others over time. Commission costs related to performance obligations satisfied at a point in time are expensed at the time of sale, which is when revenue is recognized. Commission costs related to long-term service contracts and performance obligations satisfied over time, including extended warranties and SaaS subscriptions, are deferred and recognized on a systematic basis that is consistent with the transfer of the goods or services to which the asset relates. We apply judgment in estimating the amortization period, which ranges from 3 to 7 years, by taking into consideration our customer contract terms, history of renewals, and expected length of customer relationship, as well as the useful life of the underlying technology and products. Amortization expense is included in sales and marketing expenses in the accompanying consolidated statements of income. Deferred commission costs are periodically reviewed for impairment.
On December 31, 2023, our deferred commission costs, included within other current and long-term assets, were $19.7 million, of which approximately $6.5 million of commission expense was recognized during the year ended December 31, 2024.
Furthermore, as a result of commissions related to new extended warranties and SaaS subscriptions, net of subsequent recognition, our deferred commission costs were $22.0 million as of December 31, 2024. Impairments of deferred commission costs during the years ended December 31, 2024, and 2023, were not material.
NOTE 4. ACQUISITIONS, ASSET PURCHASES AND INVESTMENTS
We believe that our acquisitions of businesses and other assets enhance our existing businesses by either expanding our geographic range, customer base, or existing product and service lines. From time to time, we acquire small reference laboratories or radiology practices that we account for as either asset purchases or business combinations, and we acquire noncontrolling minority interests in business entities, which we recognize as equity investments.
Asset Purchases and Investments
During 2024, we made a $10.0 million payment for a perpetual intellectual property license, which is amortized over 10 years, and included in our CAG segment.
During 2022, we entered into two discrete arrangements to license intellectual property for which we paid $65.0 million and accrued $15.0 million in subsequent payments, all of which was charged to research and development expense. The $15.0 million milestone payment was issued in the first quarter of 2023. These two arrangements were treated as asset acquisitions under U.S. GAAP and resulted in the full amount being expensed to research and development expense as in-process research and development costs with no alternative future use. The acquisitions of these licensing arrangements support new instrument platform advancements. We also made a $10.0 million payment for a perpetual intellectual property license, which will be amortized over 10 years. The research and development expense and amortization expense were recorded in our CAG segment.
During 2022, we also purchased $25.0 million of preferred shares for a noncontrolling minority interest in one of the entities with which we have a license agreement. We have elected to measure the investment as an equity security investment, under ASC 321, “Investments - Equity Securities,” and recorded the investment at cost. The investment is included in other long-term assets.
Business Combinations
During the first quarter of 2024, we acquired the assets of a privately-owned software and data platform business based in the U.S. that extended our practice management system cloud-native workflow and delivers strategic data solutions to our customers and their clients, for approximately $81.1 million, including an estimated contingent payment of $4.4 million. The fair values and the lives of the assets and liabilities acquired are as follows: completed technology of $17.1 million, with a life of 6 years; customer relationship intangibles of $12.5 million, with a life of 10 years; a non-compete agreement of $4.7 million, with a life of 5 years; and a trademark of $0.7 million, with a life of 10 years. We also recognized goodwill of $45.8 million, which represents synergies with our software business, and $0.3 million of net tangible assets, including accounts receivable. Goodwill related to this acquisition is expected to be deductible for tax purposes. Pro forma information has not been presented for this acquisition because such information is not material to the financial statements. The results of operations have been included in our CAG segment since the acquisition date. The acquisition expenses were not significant.
During the third quarter of 2022, we acquired the assets of an international water testing company located in Canada for approximately $12.8 million in cash, including a holdback of approximately $1.3 million, which was paid in the fourth quarter of 2023. This acquisition expanded our product offering in the Water segment. The fair values of the assets and liabilities acquired consist of technology intangibles of approximately $3.4 million, with a life of 10 years; customer relationship intangibles of approximately $1.2 million, with a life of 10 years; approximately $6.9 million of goodwill, representing synergies with our Water testing portfolio; and approximately $1.3 million of tangible assets, including inventory and accounts receivable. Goodwill related to this acquisition is expected to be deductible for tax purposes. Pro forma information has not been presented for this acquisition because such information is not material to the financial statements. The results of operations have been included in our Water segment since the acquisition date. The acquisition expenses were not significant.
NOTE 5. SHARE-BASED COMPENSATION
We provide for various forms of share-based compensation awards to our employees and non-employee directors. Our share-based compensation plans allow for the issuance of a mix of stock options, restricted stock, stock appreciation rights, employee stock purchase rights, and other stock unit awards. With the exception of stock options, the fair value of our awards is equal to the closing stock price of IDEXX common stock on the date of grant. We calculate the fair value of our stock option awards using the Black-Scholes-Merton option-pricing model. For stock options, restricted stock units (“RSUs”), and deferred stock units (“DSUs”), share-based compensation expense is estimated based on awards ultimately expected to vest, reduced for estimated forfeitures, on a straight-line basis over the requisite service period of the award. For performance-based restricted stock units (“PBRSUs”), share-based compensation expense is estimated based on awards ultimately expected to vest, reduced for estimated forfeitures. PBRSUs and certain other awards are expensed on a straight-line basis over the three-year performance and service period as the awards cliff vest at the end of the three-year period.
Stock options permit a holder to buy IDEXX stock upon vesting at the stock option exercise price set on the day of grant. An RSU is an agreement to issue shares of IDEXX stock at the time of vesting. A PBRSU is an agreement to issue shares of IDEXX stock at the time of vesting upon achievement of certain performance goals. DSUs are granted under our Executive Deferred Compensation Plan, which was suspended in February 2013 (the “Suspended Executive Plan”), and non-employee Director Deferred Compensation Plan (the “Director Plan”), and DSUs may be granted under our Deferred Compensation Plan adopted in December 2024 (the “Executive Plan”). DSUs may or may not have vesting conditions depending on the plan under which they are issued. We did not issue any restricted stock or stock appreciation rights during the years ended December 31, 2024, 2023, and 2022, nor were any restricted stock or stock appreciation rights outstanding as of those years then ended.
We primarily issue shares of common stock to satisfy stock option exercises and employee stock purchase rights and to settle RSUs, PBRSUs, and DSUs. We issue shares of treasury stock to settle certain RSUs and upon the exercise of certain stock options, which were not material for the years ended December 31, 2024, 2023, and 2022. The number of shares of common stock and treasury stock issued are equivalent to the number of awards exercised or settled.
With the exception of employee stock purchase rights, equity awards are issued to employees and non-employee directors under the 2018 Stock Incentive Plan (the “2018 Stock Plan”). Our Board of Directors has authorized the issuance of 7.5 million shares of our common stock under the 2018 Stock Plan. Any shares that are subject to awards of stock options or stock appreciation rights will be counted against the share limit as one share for every share granted. Any shares that are issued other than stock options and stock appreciation rights will be counted against the share limit as 2.4 shares for every share granted. If any shares issued under our prior plans are forfeited, settled for cash, or expire, these shares, to the extent of such forfeiture, cash settlement, or expiration, will again be available for issuance under the 2018 Stock Plan. As of December 31, 2024, there were approximately 5.9 million remaining shares available for issuance under the 2018 Stock Plan.
Share-Based Compensation
Share-based compensation costs are classified in the consolidated financial statements consistent with the classification of cash compensation paid to the employees receiving such share-based compensation. The following is a summary of share-based compensation costs and related tax benefits recorded in our consolidated statements of income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Share-based compensation expense included in cost of revenue |
|
$ |
6,014 |
|
|
$ |
5,643 |
|
|
$ |
4,638 |
|
| Share-based compensation expense included in operating expenses |
|
54,281 |
|
|
54,096 |
|
|
45,132 |
|
| Total share-based compensation expense included in consolidated statements of income |
|
60,295 |
|
|
59,739 |
|
|
49,770 |
|
Deferred tax benefit resulting from share-based compensation expense |
|
(6,650) |
|
|
(5,487) |
|
|
(4,658) |
|
| Net share-based compensation expense included in consolidated statements of income, excluding tax benefit from settlement of share-based awards |
|
53,645 |
|
|
54,252 |
|
|
45,112 |
|
| Income tax benefit resulting from settlement of share-based awards |
|
(19,625) |
|
|
(13,703) |
|
|
(12,522) |
|
Net expense related to share-based compensation arrangements included in consolidated statements of income |
|
$ |
34,020 |
|
|
$ |
40,549 |
|
|
$ |
32,590 |
|
There were no material modifications to the terms of outstanding options, RSUs, PBRSUs, or DSUs during the years ended December 31, 2024, 2023, or 2022.
Share-based compensation expense is reduced for an estimate of the number of awards that are expected to be forfeited. We use historical data and other factors to estimate expected employee terminations and to evaluate whether particular groups of employees have significantly different forfeiture expectations. Our share-based awards granted to employees in certain years include a retirement provision based on age and length of service. These grants are subject to accelerated expensing if the grantee meets the retirement definition set forth in the applicable equity and incentive plan documents.
The total unrecognized compensation expense, net of estimated forfeitures, for unvested share-based compensation awards as of December 31, 2024, was $63.8 million, which will be recognized over a weighted average period of approximately 1.3 years.
Stock Options
Prior to December 4, 2019, all options granted to employees primarily vested ratably over five years on each anniversary of the date of grant. Options granted to non-employee directors vested fully on the earlier of the first anniversary of grant or the date of the following annual meeting. Employee grants after December 4, 2019, vest ratably over four years. Vesting of option awards issued is conditional upon continuous service, unless the employee retires under the retirement provision, for grants issued in 2018, 2019, 2022, 2023, and 2024, for which retirees’ grants will vest two additional years following the retirement date. Options granted after May 8, 2013, have a contractual term of ten years. Upon any change in control of the company, 25% of the unvested stock options then outstanding will vest and become exercisable. However, if the acquiring entity does not assume outstanding options, then all options will vest immediately prior to the change in control.
We use the Black-Scholes-Merton option-pricing model to determine the fair value of options granted. Option-pricing models require the input of highly subjective assumptions, particularly for the expected stock price volatility and the expected term of options. Changes in the subjective input assumptions can affect the fair value estimate. Our expected stock price volatility assumptions are based on the historical volatility of our stock over periods that are similar to the expected terms of grants and other relevant factors. We derive the expected term based on historical experience and other relevant factors concerning expected employee behavior with regard to option exercise. The risk-free interest rate is based on U.S. Treasury yields for a maturity approximating the expected term calculated at the date of grant. We have never paid cash dividends on our common stock, and we have no intention to pay a dividend at this time; therefore, we assume that no dividends will be paid over the expected terms of option awards.
We determine the assumptions used in the valuation of option awards as of the date of grant. Differences in the expected stock price volatility, expected term, or risk-free interest rate may necessitate distinct valuation assumptions at those grant dates. As such, we may use different assumptions for options granted throughout the year. The weighted averages of the valuation assumptions used to determine the fair value of each option award on the date of grant and the weighted average estimated fair values were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Expected stock price volatility |
|
32 |
% |
|
32 |
% |
|
30 |
% |
| Expected term, in years |
|
7.0 |
|
6.7 |
|
6.4 |
| Risk-free interest rate |
|
4.3 |
% |
|
3.7 |
% |
|
2.1 |
% |
| Weighted average fair value of options granted |
|
$ |
239.49 |
|
|
$ |
201.48 |
|
|
$ |
166.30 |
|
A summary of the status of options granted under our share-based compensation plans as of December 31, 2024, and changes during the year then ended, are presented in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Options (000) |
|
Weighted Average Exercise Price |
|
Weighted Average Remaining Contractual Term |
|
Aggregate Intrinsic Value ($000) |
|
|
|
|
|
|
|
|
|
| Outstanding as of December 31, 2023 |
|
1,596 |
|
$ |
250.14 |
|
|
|
|
|
| Granted |
|
111 |
|
$ |
555.48 |
|
|
|
|
|
| Exercised |
|
(234) |
|
$ |
123.81 |
|
|
|
|
|
| Forfeited |
|
(11) |
|
$ |
525.30 |
|
|
|
|
|
| Expired |
|
(1) |
|
$ |
529.31 |
|
|
|
|
|
| Outstanding as of December 31, 2024 |
|
1,461 |
|
$ |
291.25 |
|
|
4.5 |
|
$ |
232,648 |
|
|
|
|
|
|
|
|
|
|
| Fully vested as of December 31, 2024 |
|
1,169 |
|
$ |
232.12 |
|
|
3.6 |
|
$ |
232,615 |
|
|
|
|
|
|
|
|
|
|
Fully vested and expected to vest as of December 31, 2024 (1) |
|
1,449 |
|
$ |
289.38 |
|
|
4.4 |
|
$ |
232,647 |
|
|
|
|
|
|
|
|
|
|
(1) Includes options that are vested as of December 31, 2024, and outstanding options that are expected to vest in the future, net of estimated forfeitures. |
The total fair value of options vested were $25.1 million, $22.5 million, and $20.4 million during the years ended December 31, 2024, 2023, and 2022, respectively.
The intrinsic value of stock options exercised represents the amount by which the market price of the common stock exceeded the exercise price before applicable income taxes. The total intrinsic values of stock options exercised were $85.7 million, $75.5 million, and $54.6 million during the years ended December 31, 2024, 2023, and 2022, respectively.
Restricted Stock Units
Prior to December 4, 2019, the majority of RSUs granted to employees vested ratably over five years on each anniversary of the date of grant. The majority of employee grants after December 4, 2019, vest ratably over four years. A minority of certain employee grants cliff vest three years from the date of grant. Vesting as it relates to RSUs issued is conditional upon continuous service, unless the employee retires under the retirement provision, for grants issued in 2018, 2019, 2022, 2023, and 2024, for which they will vest for two additional years following the retirement date. Upon any change in control of the company, 25% of the unvested RSUs then outstanding will vest, provided, however, that if the acquiring entity does not assume the RSUs then all such units will vest immediately prior to the change in control. RSUs granted to non-employee directors vest fully on the first anniversary of the date of grant.
A summary of the status of RSUs granted under our share-based compensation plans as of December 31, 2024, and changes during the period then ended, are presented in the table below:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Units (000) |
|
Weighted Average Grant-Date Fair Value |
|
|
|
|
|
| Nonvested as of December 31, 2023 |
|
141 |
|
$ |
459.11 |
|
| Granted |
|
57 |
|
$ |
550.64 |
|
| Vested |
|
(60) |
|
$ |
406.32 |
|
| Forfeited |
|
(8) |
|
$ |
522.53 |
|
| Nonvested as of December 31, 2024 |
|
130 |
|
$ |
519.38 |
|
|
|
|
|
|
Expected to vest as of December 31, 2024 (1) |
|
123 |
|
$ |
518.59 |
|
|
|
|
|
|
(1) Outstanding units that are expected to vest in the future, net of estimated forfeitures. |
The total fair values of RSUs vested were $32.9 million, $31.1 million, and $32.0 million during the years ended December 31, 2024, 2023, and 2022, respectively. The aggregate intrinsic value of nonvested RSUs was $50.8 million, which is equal to the fair value of IDEXX’s common stock as of December 31, 2024, multiplied by the number of nonvested units expected to vest.
Performance-Based Restricted Stock Units
On February 14, 2024, certain executives were granted performance-based restricted stock units (PBRSUs) that will cliff vest three years from the date of grant. Vesting as it relates to PBRSUs issued is conditional upon continuous service, unless the employee retires under the retirement provision. Upon any change in control of the company, 25% of the unvested PBRSUs then outstanding will vest, provided, however, that if the acquiring entity does not assume the PBRSUs, then all such units will vest immediately prior to the change in control. At the time of grant, we assume the PBRSUs will meet performance goals to vest at 100% of target.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Units (000) |
|
Weighted Average Grant-Date Fair Value |
|
|
|
|
|
| Nonvested as of December 31, 2023 |
|
— |
|
— |
|
| Granted |
|
20 |
|
$ |
560.56 |
|
| Vested |
|
— |
|
— |
|
| Forfeited |
|
— |
|
$ |
560.56 |
|
Performance adjustment (1) |
|
(4) |
|
$ |
560.56 |
|
| Nonvested as of December 31, 2024 |
|
16 |
|
$ |
560.56 |
|
|
|
|
|
|
| Expected to vest as of December 31, 2024 |
|
16 |
|
$ |
560.56 |
|
(1) Adjustment to the number of shares expected to vest based on estimated performance.
The aggregate intrinsic value of nonvested PBRSUs as of December 31, 2024, is equal to the fair value of IDEXX’s common stock as of December 31, 2024, multiplied by the number of nonvested units expected to vest as of December 31, 2024, was $6.6 million. No PBRSUs vested during 2023 and 2024. The total fair value of PBRSUs vested was $0.9 million during the year ended December 31, 2022.
Deferred Stock Units
Under our Director Plan, non-employee directors may defer a portion of their director cash compensation in the form of vested DSUs. Prior to 2014, certain members of our management could elect to defer a portion of their cash compensation in the form of vested DSUs under our Suspended Executive Plan. Under our Executive Plan, certain members of our management may elect to defer a portion of his or her RSUs or PBRSUs in the form of DSUs subject to the vesting conditions set forth in the applicable equity award agreement. Each DSU represents the right to receive one unissued share of our common stock. These recipients receive a number of DSUs equal to the amount of deferred compensation divided by the closing sale price of the common stock on the date of deferral or the number of RSUs or PBRSUs deferred, as applicable.
Also under the Director Plan, non-employee directors are awarded annual grants of either RSUs or DSUs that vest fully on the earlier of the first anniversary of grant or the date of the following annual meeting. Vesting for these annual RSU and DSU grants is conditional upon continuous service.
Vested DSUs are distributed as shares of common stock on the distribution date elected by the participant and pursuant to the terms of the Director Plan, Suspended Executive Plan or Executive Plan, as applicable.
There were approximately 60,000 and 59,000 vested DSUs outstanding under our share-based compensation plans as of December 31, 2024, and 2023, respectively. During 2024, approximately 200 DSUs were distributed as shares of common stock. Unvested DSUs as of December 31, 2024, and 2023, were not material.
Employee Stock Purchase Rights
Employee stock purchase rights are issued under the 1997 Employee Stock Purchase Plan, under which we reserved and may issue up to an aggregate of 4.7 million shares of common stock in periodic offerings. Under this plan, stock is sold to employees at a 15% discount off the closing price of the stock on the last day of each quarter. The dollar value of this discount is equal to the fair value of purchase rights recognized as share-based compensation.
We issued approximately 37,700, 36,300, and 44,600 shares of common stock in connection with the Employee Stock Purchase Plan during the years ended December 31, 2024, 2023, and 2022, respectively. As of December 31, 2024, there were approximately 1.0 million remaining shares available for issuance under the 1997 Employee Stock Purchase Plan.
NOTE 6. CREDIT LOSSES
We are exposed to credit losses primarily through our sales of products and services to our customers. We maintain allowances for credit losses for potentially uncollectible receivables. Additional allowances may be required if either the financial condition of our customers was to deteriorate, or a strengthening U.S. dollar impacts the ability of foreign customers to make payments to us for their U.S. dollar-denominated purchases. We monitor our ongoing credit exposure through active review of counterparty balances against contract terms and due dates. Our activities include timely account reconciliations, dispute resolution, and payment confirmations. We may employ collection agencies and legal counsel to pursue recovery of defaulted receivables. Account balances are charged off against the allowance when we believe it is probable the receivable will not be recovered. We do not have any off-balance sheet credit exposure related to our customers.
Accounts Receivable
We base our estimates on a detailed analysis of specific customer situations and a percentage of our accounts receivable by aging category. Additionally, our estimates are developed based on historical credit loss experience, estimates of recoveries, current economic conditions, and future expectations.
The allowance for credit losses associated with accounts receivable was $12.6 million and $9.5 million as of December 31, 2024, and 2023, respectively. The amount of accounts receivable reflected on the balance sheet is net of this allowance. As of December 31, 2024, approximately 85% of our accounts receivable had not yet reached the invoice due date and approximately 15% was considered past due. As of December 31, 2023, approximately 83% of our accounts receivable had not yet reached the invoice due date and approximately 17% was considered past due. Write-offs and recoveries related to credit losses during the years ended December 31, 2024, 2023, and 2022 were not material.
Contract Assets and Lease Receivables
The allowance for credit losses associated with contract assets and lease receivables was $6.8 million and $6.4 million as of December 31, 2024, and 2023, respectively. The assets reflected on the balance sheet are net of these allowances. Historically, we have experienced low credit loss rates on our customer commitment programs and lease receivables. We apply judgment in determining the customer’s ability and intention to pay, which is based on a variety of factors including the customer’s historical payment experience or, in the case of a new customer, published credit and financial information pertaining to the customer.
NOTE 7. INVENTORIES
Inventories are stated at the lower of cost (first-in, first-out) or net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. We write down the carrying value of inventory for estimated obsolescence by an amount equal to the difference between the cost of inventory and the estimated market value when warranted based on assumptions of future demand, market conditions, remaining shelf life, or product functionality. If actual market conditions or results of estimated functionality are less favorable than those we estimated, additional inventory write-downs may be required, which would have a negative effect on our results of operations.
Unpaid inventory reflected within accounts payable in our consolidated balance sheets was $49.1 million, $58.9 million, and $59.4 million as of December 31, 2024, 2023, and 2022, respectively. Instrument inventory transferred to property and equipment related to rental and operating-type reagent rental programs was $14.2 million, $14.6 million, $17.4 million during the years ended December 31, 2024, 2023, and 2022, respectively.
The components of inventories were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
| Raw materials |
|
$ |
104,195 |
|
|
$ |
106,392 |
|
| Work-in-process |
|
31,907 |
|
|
28,989 |
|
| Finished goods |
|
245,775 |
|
|
244,901 |
|
| Inventories |
|
$ |
381,877 |
|
|
$ |
380,282 |
|
NOTE 8. LEASE COMMITMENTS
The majority of our facilities are occupied under operating lease arrangements with various expiration dates through 2067, some of which include options to extend the life of the lease, and some of which include options to terminate the lease within one year. In certain instances, we are responsible for the real estate taxes and operating expenses related to these facilities. Additionally, we enter into operating leases for certain vehicles and equipment in the normal course of business. We determine the expected term of executed agreements using the non-cancelable lease term plus optional renewal terms under which the failure to renew imposes a significant economic penalty such that we are reasonably certain to exercise the renewal option. The derived expected term is then used in the determination of a financing or operating lease and in the calculation of straight-line rent expense. Rent escalations are considered in the calculation of minimum lease payments in our capital lease tests and in determining straight-line rent expense for operating leases. Minimum lease payments include the fixed lease component of the agreement, as well as fixed rate increases that are initially measured at the lease commencement date. Variable lease payments based on an index, payments associated with non-lease components, and short-term rentals (leases with terms less than twelve months) are expensed as incurred. Consideration is allocated to the lease and non-lease components based on the estimated standalone prices.
We determine if an arrangement is a lease at its inception. Operating leases are included in operating lease right-of-use assets, accrued liabilities, and long-term operating lease liabilities in our consolidated balance sheets. Our financing leases are not material to the financial statements.
Right-of-use assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease liabilities and right-of-use assets are recognized at commencement date based on the present value of lease payments over the lease term. As most of our leases do not provide an explicit rate, we generally use our incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. Our expected lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Rent expense for lease payments is recognized on a straight-line basis over the lease term. The operating lease right-of-use assets also include any rent prepayments, lease incentives upon receipt, and straight-line rent expense impacts, which represent the differences between our operating lease liabilities and right-of-use assets.
Maturities of operating lease liabilities are as follows:
|
|
|
|
|
|
| (in thousands) |
December 31, 2024 |
|
|
| 2025 |
$ |
26,368 |
|
| 2026 |
26,529 |
|
| 2027 |
21,125 |
|
| 2028 |
15,109 |
|
| 2029 |
11,857 |
|
| Thereafter |
39,046 |
|
| Total lease payments |
140,034 |
|
| Less imputed interest |
(20,659) |
|
Total lease liabilities (current and long-term) |
$ |
119,375 |
|
Total minimum future lease payments for leases that have not commenced as of December 31, 2024, are not material.
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
| Weighted average remaining lease term - operating leases |
8.2 years |
|
8.9 years |
|
|
|
|
| Weighted average discount rate - operating leases |
4.4 |
% |
|
4.0 |
% |
Expenses incurred related to operating leases, excluding variable and short-term leases, were approximately $32.4 million and $29.1 million during the years ended December 31, 2024, and 2023, respectively. Total expenses incurred related to operating leases, including variable rent and short-term leases, were approximately $34.0 million and $31.7 million for the years ended December 31, 2024, and 2023, respectively.
Supplemental cash flow information for leases is as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended
December 31, |
| (in thousands) |
2024 |
|
2023 |
|
|
|
|
| Cash paid for amounts included in the measurement of operating lease liabilities |
$ |
27,926 |
|
|
$ |
27,525 |
|
| Right-of-use assets obtained in exchange for operating lease obligations, net of early lease terminations |
$ |
26,010 |
|
|
$ |
19,443 |
|
NOTE 9. PROPERTY AND EQUIPMENT, NET
Property and equipment are stated at cost, net of accumulated depreciation and amortization. The costs of additions and improvements are capitalized, while maintenance and repairs are charged to expense as incurred. When an item is sold or retired, the cost and related accumulated depreciation are relieved, and the resulting gain or loss, if any, is recognized in the consolidated statements of income. We evaluate our property and equipment for impairment periodically or as changes in circumstances or the occurrence of events suggest the remaining value is not recoverable from future cash flows. If the carrying value of our property and equipment is impaired, an impairment charge is recorded for the amount by which the carrying value of the property and equipment exceeds its fair value. We provide for depreciation and amortization primarily using the straight-line method by charges to the consolidated statements of income in amounts that allocate the cost of property and equipment over their estimated useful lives as follows:
|
|
|
|
|
|
|
|
|
| Asset Classification |
|
Estimated Useful Life |
|
|
|
| Buildings and improvements |
|
10 to 40 years |
| Leasehold improvements |
|
Shorter of remaining lease term or useful life of improvements |
| Machinery and equipment |
|
3 to 8 years |
| Office furniture and equipment |
|
3 to 7 years |
| Computer hardware and software |
|
3 to 7 years |
We capitalize interest on the acquisition and construction of significant assets that require a substantial period of time to be made ready for use. The capitalized interest is included in the cost of the completed asset and depreciated over the asset’s estimated useful life. The amount of interest capitalized during the years ended December 31, 2024, and 2023, was not material.
We capitalize certain costs incurred in connection with developing or obtaining software designated for internal use based on three distinct stages of development. Qualifying costs incurred during the application development stage, which consist primarily of internal payroll and direct fringe benefits and external direct project costs, including labor and travel, are capitalized and amortized on a straight-line basis over the estimated useful life of the asset. Costs incurred during the preliminary project and post-implementation and operation phases are expensed as incurred. These costs relate primarily to the determination of performance requirements, data conversion, and training. Software developed to deliver hosted services to our customers has been designated as internal use.
Property and equipment, net, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
| Land and improvements |
|
$ |
23,989 |
|
|
$ |
22,400 |
|
| Buildings and improvements |
|
446,164 |
|
|
432,038 |
|
| Leasehold improvements |
|
119,844 |
|
|
116,347 |
|
| Machinery and equipment |
|
460,750 |
|
|
431,914 |
|
| Office furniture and equipment |
|
80,882 |
|
|
78,941 |
|
| Computer hardware and software |
|
349,372 |
|
|
326,295 |
|
| Construction in progress |
|
67,144 |
|
|
79,059 |
|
|
|
1,548,145 |
|
|
1,486,994 |
|
| Less accumulated depreciation and amortization |
|
835,022 |
|
|
784,817 |
|
| Total property and equipment, net |
|
$ |
713,123 |
|
|
$ |
702,177 |
|
Below are the amounts of depreciation and amortization of property and equipment, capitalized computer software for internal use, unpaid property and equipment reflected in accounts payable and accrued expenses, and rental and reagent rental program instruments transferred from inventory to property and equipment:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
| (in thousands) |
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Depreciation and amortization expense |
|
$ |
112,809 |
|
|
$ |
100,994 |
|
|
$ |
96,746 |
|
Capitalization of internal-use software development costs during the period |
|
$ |
38,777 |
|
|
$ |
37,120 |
|
|
$ |
20,329 |
|
Unpaid property and equipment, reflected in accounts payable and accrued liabilities at end of year |
|
$ |
15,036 |
|
|
$ |
12,061 |
|
|
$ |
18,334 |
|
Rental and operating-type reagent rental program instruments transferred from inventory to property and equipment during the period (Note 3) |
|
$ |
14,190 |
|
|
$ |
14,608 |
|
|
$ |
17,407 |
|
We had no material impairments of fixed assets for the years ended December 31, 2024, 2023, and 2022.
NOTE 10. OTHER CURRENT AND LONG-TERM ASSETS
Other current assets consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
Consideration paid to customers |
|
$ |
61,653 |
|
|
$ |
54,081 |
|
Contract assets, net (1) |
|
60,751 |
|
|
55,111 |
|
| Prepaid expenses |
|
58,626 |
|
|
48,370 |
|
| Taxes receivable |
|
26,990 |
|
|
16,972 |
|
| Other |
|
48,159 |
|
|
29,061 |
|
| Other current assets |
|
$ |
256,179 |
|
|
$ |
203,595 |
|
(1) Contract assets, net, are net of allowances for credit losses.
Other long-term assets consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
Contract assets, net (1) |
|
$ |
185,506 |
|
|
$ |
167,963 |
|
Consideration paid to customers |
|
134,896 |
|
|
114,850 |
|
| Deferred income taxes |
|
125,630 |
|
|
107,364 |
|
| Equity investments |
|
31,004 |
|
|
30,250 |
|
| Investment in long-term product supply arrangements |
|
26,714 |
|
|
25,943 |
|
| Other |
|
43,768 |
|
|
50,164 |
|
| Other long-term assets |
|
$ |
547,518 |
|
|
$ |
496,534 |
|
(1) Contract assets, net, are net of allowances for credit losses.
NOTE 11. GOODWILL AND INTANGIBLE ASSETS, NET
A significant portion of the purchase price for acquired businesses is generally assigned to intangible assets. Intangible assets other than goodwill are initially valued at fair value. If a quoted price in an active market for the asset is not readily available at the measurement date, the fair value of the intangible asset is estimated based on discounted cash flows using market participant assumptions, which are assumptions that are not specific to IDEXX. The selection of appropriate valuation methodologies and the estimation of discounted cash flows require significant assumptions about the timing and amounts of future cash flows, risks, appropriate discount rates, and the useful lives of intangible assets. When significant, we typically utilize independent valuation experts to advise and assist us in determining the fair values of the identified intangible assets acquired in connection with a business acquisition and in determining appropriate amortization methods and periods for those intangible assets. Goodwill is initially valued based on the excess of the purchase price of a business combination over the fair value of acquired net assets recognized and represents the future economic benefits arising from other assets acquired that could not be separately identified and recognized.
Our business combinations regularly include contingent consideration arrangements that require additional consideration to be paid based on the achievement of established objectives, most commonly related to customer retention or revenue growth of the customer base during the post-combination period. We assess contingent consideration on the acquisition date to determine whether it is part of the purchase consideration or should be accounted for separately from the business combination. A liability resulting from contingent consideration is remeasured to fair value at each reporting date until the contingency is resolved, with changes in fair value recognized in earnings if changes in estimates are made after the measurement period. Refer to “Note 18. Fair Value Measurements” for the fair value of contingent consideration.
We assess goodwill for impairment annually, at the reporting unit level in the fourth quarter, and whenever events or circumstances indicate impairment may exist. In evaluating goodwill for impairment, we have the option to first assess the qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount as a basis for determining whether it is necessary to perform the quantitative goodwill impairment test. If, after assessing the totality of events and circumstances, we determine that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, then we assess the fair value of the reporting unit and compare its fair value to its carrying value to determine if the carrying value exceeds its fair value. Any excess of the carrying value of the goodwill above its fair value would be recognized as an impairment loss. In contrast, we can opt to bypass the qualitative assessment for any reporting unit and proceed directly to assessing the fair value of a reporting unit, and compare the fair value of the reporting unit to its carrying value to determine if any impairment exists. Doing so does not preclude us from performing the qualitative assessment in any subsequent period.
A prolonged economic downturn that results in lower long-term growth rates and reduced long-term profitability may reduce the fair value of our reporting units. Industry-specific events or circumstances could have a negative impact on our reporting units and may also reduce the fair value of our reporting units. Should such events occur, and it becomes more likely than not that a reporting unit’s fair value has fallen below its carrying value, we will perform an interim goodwill impairment test, in addition to the annual impairment test. Future impairment tests may result in an impairment of goodwill. An impairment of goodwill would be reported as a non-cash charge to earnings.
In the fourth quarter of 2024, we elected to bypass the qualitative assessment and performed a quantitative assessment of the fair value of all our reporting units and compared the fair value of each reporting unit to the carrying value to determine if any impairment exists.
We estimated the fair values of applicable reporting units using an income approach based on discounted forecasted cash flows. We applied assumptions about the extent and timing of future cash flows, growth rates and discount rates. Model assumptions are based on our projections and best estimates, using appropriate and customary market participant assumptions. In addition, we made certain assumptions in allocating shared assets and liabilities to individual reporting units in determining the carrying value of each reporting unit. We had no goodwill impairments during the years ended December 31, 2024, 2023, or 2022.
Of our total goodwill of $405.1 million, approximately $6.5 million is associated with our remaining pharmaceutical intellectual property out-licensing arrangements within our Other segment. Our sole source of value for this goodwill is royalty revenue associated with the commercialization of certain patented intellectual property, for which the primary royalty obligations expire in 2029. There is no guarantee that we will be able to maintain or increase revenues. A decrease in estimated future revenues could result in an impairment of this goodwill.
The changes in the carrying amount of goodwill for the years ended December 31, 2024, 2023, and 2022, were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
CAG |
|
Water |
|
LPD |
|
Other |
|
Consolidated Total |
| Balance as of December 31, 2021 |
|
$ |
327,171 |
|
|
$ |
11,939 |
|
|
$ |
13,704 |
|
|
$ |
6,531 |
|
|
$ |
359,345 |
|
| Business combinations |
|
1,641 |
|
|
6,857 |
|
|
— |
|
|
— |
|
|
8,498 |
|
| Impact of changes in foreign currency exchange rates |
|
(5,330) |
|
|
(897) |
|
|
179 |
|
|
— |
|
|
(6,048) |
|
| Balance as of December 31, 2022 |
|
$ |
323,482 |
|
|
$ |
17,899 |
|
|
$ |
13,883 |
|
|
$ |
6,531 |
|
|
$ |
361,795 |
|
| Business combinations |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
| Impact of changes in foreign currency exchange rates |
|
2,740 |
|
|
321 |
|
|
1,105 |
|
|
— |
|
|
4,166 |
|
| Balance as of December 31, 2023 |
|
$ |
326,222 |
|
|
$ |
18,220 |
|
|
$ |
14,988 |
|
|
$ |
6,531 |
|
|
$ |
365,961 |
|
| Business combinations |
|
45,782 |
|
|
— |
|
|
— |
|
|
— |
|
|
45,782 |
|
| Impact of changes in foreign currency exchange rates |
|
(5,029) |
|
|
(109) |
|
|
(1,505) |
|
|
— |
|
|
(6,643) |
|
| Balance as of December 31, 2024 |
|
$ |
366,975 |
|
|
$ |
18,111 |
|
|
$ |
13,483 |
|
|
$ |
6,531 |
|
|
$ |
405,100 |
|
Refer to “Note 4. Acquisitions, Asset Purchases and Investments” for information regarding goodwill and other intangible assets recognized in connection with the acquisition of businesses and other assets during the years ended December 31, 2024, 2023, and 2022.
We assess the realizability of intangible assets other than goodwill whenever events or changes in circumstances indicate that the carrying value may not be recoverable. If an impairment review is triggered, we evaluate the carrying value of intangible assets, other than goodwill, based on estimated undiscounted future cash flows over the remaining useful life of the primary asset of the asset group and compare that value to the carrying value of the asset group. The asset group is the lowest level for which identifiable cash flows associated with the intangible asset are largely independent. The cash flows that are used contain our best estimates, using appropriate and customary assumptions and projections at the time. If the net carrying value of the asset group exceeds the related estimated undiscounted future cash flows, an impairment loss to adjust the intangible asset to its fair value would be reported as a non-cash charge to earnings. If necessary, we would calculate the fair value of an intangible asset using the present value of the estimated future cash flows to be generated by the intangible asset and apply a risk-adjusted discount rate. We had no impairments of our intangible assets during the years ended December 31, 2024, 2023, and 2022.
We provide for amortization primarily using the straight-line method by charges to income in amounts that allocate the intangible assets over their estimated useful lives as follows:
|
|
|
|
|
|
|
|
|
| Asset Classification |
|
Estimated Useful Life |
|
|
|
Customer-related intangible assets (1) |
|
3 to 17 years |
Product rights (2) |
|
5 to 15 years |
| Noncompete agreements |
|
3 to 5 years |
Intangible assets other than goodwill consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
Cost |
|
Accumulated Amortization |
|
Net |
|
Cost |
|
Accumulated Amortization |
|
Net |
|
|
|
|
|
|
|
|
|
|
|
|
|
Customer-related intangible assets (1) |
|
$ |
110,860 |
|
|
$ |
44,617 |
|
|
$ |
66,243 |
|
|
$ |
102,217 |
|
|
$ |
37,782 |
|
|
$ |
64,435 |
|
Product rights (2) |
|
55,117 |
|
|
14,161 |
|
|
40,956 |
|
|
27,317 |
|
|
8,606 |
|
|
18,711 |
|
| Noncompete agreements |
|
6,570 |
|
|
2,093 |
|
|
4,477 |
|
|
4,012 |
|
|
2,658 |
|
|
1,354 |
|
Total |
|
$ |
172,547 |
|
|
$ |
60,871 |
|
|
$ |
111,676 |
|
|
$ |
133,546 |
|
|
$ |
49,046 |
|
|
$ |
84,500 |
|
The table above reflect the effects of foreign currency exchange rates, and excludes fully amortized intangible assets.
(1)Customer-related intangible assets are comprised of customer lists and customer relationships acquired from third parties.
(2)Product rights comprise certain technologies, intellectual property, licenses, and trade names acquired from third parties.
Amortization expense of intangible assets other than goodwill was $17.1 million, $13.8 million, and $15.0 million for the years ended December 31, 2024, 2023, and 2022, respectively.
As of December 31, 2024, the aggregate amortization expense associated with intangible assets is estimated to be as follows for each of the next five years and thereafter:
|
|
|
|
|
|
| (in thousands) |
Amortization Expense |
|
|
| 2025 |
$ |
19,040 |
|
| 2026 |
18,779 |
|
| 2027 |
17,438 |
|
| 2028 |
15,245 |
|
| 2029 |
12,013 |
|
| Thereafter |
29,161 |
|
Total amortization |
$ |
111,676 |
|
Future actual amortization expense may differ from estimated amounts due to future intangible asset acquisitions, changes in foreign currency exchange rates, impairments of intangible assets, and other events.
NOTE 12. ACCOUNTS PAYABLE, ACCRUED LIABILITIES AND OTHER LONG-TERM LIABILITIES
Accounts Payable - Supplier Financing Program
We have an agreement with a third party to provide a supplier financing program, which facilitates participating suppliers’ ability to finance payment obligations from us with a designated third-party financial institution. Participating suppliers may, at their sole discretion, make offers to finance one or more of our payment obligations prior to their scheduled due dates at a discounted price. Our obligations to our suppliers, including amounts due and scheduled payment dates, are not impacted by suppliers’ decisions to finance amounts under these arrangements. The terms of payments are consistent with the terms of our trade payables. Activity related to the obligations is presented within operating activities on the consolidated statements of cash flows.
The changes in our outstanding payment obligations under the supplier financing arrangement, which are included in accounts payable on the consolidated balance sheets, are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
For the Years Ended December 31, |
|
2024 |
|
2023 |
|
|
|
|
| Payment obligations outstanding at the beginning of the period |
$ |
9,057 |
|
|
$ |
10,171 |
|
Payment obligation additions during the period |
47,422 |
|
|
45,764 |
|
Payment obligations settled during the period |
(50,512) |
|
|
(46,878) |
|
| Payment obligations outstanding at the end of the period |
$ |
5,967 |
|
|
$ |
9,057 |
|
Accrued liabilities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
| Accrued employee compensation and related expenses |
|
$ |
174,583 |
|
|
$ |
174,375 |
|
| Accrued expenses |
|
165,550 |
|
|
113,596 |
|
| Accrued customer incentives and refund obligations |
|
78,195 |
|
|
84,386 |
|
| Accrued taxes |
|
62,252 |
|
|
86,553 |
|
| Current lease liabilities |
|
21,539 |
|
|
19,802 |
|
| Accrued liabilities |
|
$ |
502,119 |
|
|
$ |
478,712 |
|
Other long-term liabilities consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
| Accrued taxes |
|
$ |
20,898 |
|
|
$ |
39,642 |
|
| Other accrued long-term expenses |
|
23,443 |
|
|
25,884 |
|
| Other long-term liabilities |
|
$ |
44,341 |
|
|
$ |
65,526 |
|
NOTE 13. DEBT
Credit Facility
On October 20, 2022, we, along with IDEXX Distribution, Inc., IDEXX Operations, Inc., OPTI Medical Systems, Inc., IDEXX Laboratories Canada Corporation, IDEXX B.V., IDEXX Laboratories B.V., and IDEXX Laboratories GmbH, each a wholly-owned subsidiary (whether directly or indirectly held) (collectively, the “Borrowers”), together with the lenders party to that certain Existing Credit Agreement (as defined below), JPMorgan Chase Bank, N.A., as administrative agent (“Agent”), and the other parties thereto, entered into Amendment No. 1 (the “Amendment”), to that certain fourth amended and restated credit agreement, dated as of December 9, 2021, relating to a five-year unsecured revolving credit facility in the principal amount of $1.0 billion (the “Existing Credit Agreement,” and as amended by the Amendment, the “Credit Agreement”), among the Borrowers, the lenders, the Agent, JPMorgan Chase Bank, N.A., Toronto Branch, as Toronto agent, and the other parties thereto.
The Amendment amended the Existing Credit Agreement to (i) provide for a borrowing by us effective October 20, 2022, of an incremental term loan in an aggregate principal amount of $250.0 million, (ii) convert all existing borrowings, which have interest rates determined by reference to a LIBOR-based interest rate, to borrowings determined by reference to a SOFR-based interest rate, (iii) provide for an option by us to obtain incremental borrowings under the Credit Agreement of term loans and/or revolving credit commitments of up to an aggregate principal amount of $250.0 million, which would represent an aggregate maximum of up to $1.5 billion, subject to the Borrowers obtaining commitments from existing or new lenders and satisfying other conditions specified in the Credit Agreement, and (iv) add certain implementing mechanics relating to the foregoing.
On October 20, 2022, pursuant to the terms of the Credit Agreement, the term lenders thereunder provided us, as borrower, an incremental term loan in an aggregate principal amount of $250.0 million (the “Term Loan”). The Term Loan matures on October 20, 2025. The net proceeds of the Term Loan were used to repay previously incurred revolver borrowings under the Existing Credit Agreement. The Term Loan is subject to the same affirmative and negative covenants and events of default as the borrowings previously incurred pursuant to the Existing Credit Agreement. The applicable interest rate for the Term Loan is consistent with our line of credit, and is calculated at a per annum rate equal to either (at our option) (i) a prime rate plus a margin ranging from 0.0% to 0.375% based on our consolidated leverage ratio, (ii) an adjusted term SOFR rate, plus 0.10%, plus a margin ranging from 0.875% to 1.375% based on our consolidated leverage ratio, or (iii) an adjusted daily simple SOFR rate, plus 0.10%, plus a margin ranging from 0.875% to 1.375% based on our consolidated leverage ratio. Issuance costs for the incremental Term Loan were immaterial. In March 2023, we entered into an interest rate swap contract to manage the economic effects of $250.0 million of variable interest borrowings under our Credit Facility. Refer to “Note 19. Hedging Instruments” for a discussion of our derivative instruments and hedging activity.
Although the revolving line of credit does not mature until December 9, 2026, and the Term Loan does not mature until October 20, 2025, all individual borrowings under the terms of the Credit Facility with an interest rate based on the prevailing Prime or SOFR rate (as selected by the Borrower) have a stated term between 1 and 180 days. At the end of each term, the obligation is either repaid or rolled over into a new borrowing or replaced by a borrowing based on a specific benchmark rate (where interest is then paid monthly). The Credit Facility contains a subjective material adverse event notification clause, which allows the debt holders to call the loans under the Credit Facility if we fail to provide prompt written notice to the syndicate of such an event. Based on the stated terms and the existence of the subjective material adverse event clause, this Credit Facility is reflected in the current liabilities section of our consolidated balance sheets.
As of December 31, 2024, we had $250.0 million outstanding on our Credit Facility, all of which is the $250.0 million Term Loan, with a weighted average effective interest rate of 6.2%, excluding any impact of our interest rate swap. As of December 31, 2023, we had $250.0 million outstanding on our Credit Facility, all of which is the $250.0 million Term Loan, with a weighted average effective interest rate of 6.0%, excluding any impact of our interest rate swap. The funds available under the Credit Facility reflect a further reduction due to the issuance of letters of credit, which were primarily issued in connection with our workers’ compensation policy, for $1.9 million and $1.5 million in the years ended December 31, 2024, and December 31, 2023, respectively.
The obligations under the Credit Facility may be accelerated upon the occurrence of an event of default under the Credit Facility, which includes customary events of default including payment defaults, defaults in the performance of the affirmative, negative and financial covenants, the inaccuracy of representations or warranties, bankruptcy and insolvency related defaults, defaults relating to judgments, certain events related to employee pension benefit plans under the Employee Retirement Income Security Act of 1974, the failure to pay specified indebtedness, cross-acceleration to specified indebtedness, and a change of control default. The Credit Facility contains affirmative, negative, and financial covenants customary for financings of this type. The negative covenants include restrictions on liens, indebtedness of subsidiaries of the Company, fundamental changes, investments, transactions with affiliates, and certain restrictive agreements. The sole financial covenant is a consolidated leverage ratio test that requires our ratio of debt to earnings before interest, taxes, depreciation, amortization, and share-based compensation, which is defined as the consolidated leverage ratio under the terms of the Credit Facility, not to exceed 3.5-to-1. As of December 31, 2024, we were in compliance with the covenants of the Credit Facility.
Senior Notes
The following describes all of our currently outstanding unsecured senior notes issued and sold in private placements (collectively, the “Senior Notes”) as of December 31, 2024:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (Principal Amount in thousands) |
|
|
|
|
|
|
|
|
|
|
|
| Issue Date |
|
Due Date |
|
Series |
|
Principal Amount |
|
Coupon Rate |
|
Senior Note Agreement |
|
|
|
|
|
|
|
|
|
|
|
| 12/11/2013 |
|
12/11/2025 |
|
2025 Series B Notes |
|
$ |
75,000 |
|
|
4.04 |
% |
|
NY Life 2013 Note Agreement |
| 9/4/2014 |
|
9/4/2026 |
|
2026 Senior Notes |
|
$ |
75,000 |
|
|
3.72 |
% |
|
NY Life 2014 Note Agreement |
| 6/18/2015 |
|
6/18/2025 |
|
2025 Series C Notes |
|
€ |
88,857 |
|
|
1.785 |
% |
|
Prudential 2015 Amended Agreement |
| 4/14/2020 |
|
4/14/2030 |
|
Prudential 2030 Series D Notes |
|
$ |
75,000 |
|
|
2.50 |
% |
|
Prudential 2015 Amended Agreement |
| 2/12/2015 |
|
2/12/2027 |
|
2027 Series B Notes |
|
$ |
75,000 |
|
|
3.72 |
% |
|
MetLife 2014 Note Agreement |
| 3/14/2019 |
|
3/14/2029 |
|
2029 Series C Notes |
|
$ |
100,000 |
|
|
4.19 |
% |
|
MetLife 2014 Note Agreement |
| 4/2/2020 |
|
4/2/2030 |
|
MetLife 2030 Series D Notes |
|
$ |
125,000 |
|
|
2.50 |
% |
|
MetLife 2014 Note Agreement |
The following narrative describes our Senior Note activity:
NY Life 2013 and 2014 Note Agreements, Including Amendments
In December 2013, we issued and sold through a private placement an aggregate principal amount of $150.0 million of unsecured senior notes consisting of $75.0 million of 3.94% Series A Senior Notes due December 11, 2023 (the “2023 Series A Notes”) and $75.0 million of 4.04% Series B Senior Notes due December 11, 2025 (the “2025 Series B Notes”) under a Note Purchase Agreement among the Company, New York Life Insurance Company, and the accredited institutional purchasers named therein (as amended on April 10, 2020, the “NY Life 2013 Note Agreement”). The aggregate principal amount of our 2023 Series A Notes for $75.0 million was repaid in December 2023.
In September 2014, we issued and sold through a private placement an aggregate principal amount of $75.0 million of unsecured 3.72% senior notes due September 4, 2026 (the “2026 Senior Notes”) under a Note Purchase Agreement dated as of July 22, 2014, among the Company, New York Life Insurance Company, and the accredited institutional purchasers named therein (as amended April 10, 2020, the “NY Life 2014 Note Agreement”).
On April 10, 2020, we amended the NY Life 2013 Note Agreement and the NY Life 2014 Note Agreement by entering into two Amendments to the Note Purchase Agreement with New York Life Insurance Company and the other parties thereto, which modified several defined terms, schedules and covenant baskets in the NY Life 2013 Agreement and the NY Life 2014 Note Agreement to create additional operating flexibility, and in particular to align such provisions with similar modifications we made substantially concurrently in our other debt facilities.
Prudential 2015 Amended Agreement, Including Amendments
In July 2014, we issued and sold through a private placement an aggregate principal amount of $125.0 million of unsecured senior notes consisting of $50.0 million of 3.32% Series A Senior Notes due July 21, 2021 (the “2021 Series A Notes”) and $75.0 million of 3.76% Series B Senior Notes due July 21, 2024 (the “2024 Series B Notes”) under a Note Purchase and Private Shelf Agreement among the Company, Prudential Investment Management, Inc. (“Prudential”), and the accredited institutional purchasers named therein (the “Prudential 2014 Note Agreement”). The aggregate principal amount on our Series A Senior Notes for $50.0 million was repaid in July 2021. The aggregate principal amount of our 2024 Series B Notes for $75.0 million was repaid in July 2024.
In June 2015, we entered into an Amended and Restated Multi-Currency Note Purchase and Private Shelf Agreement (the “Original Prudential 2015 Amended Agreement”), among the Company, Prudential, and the accredited institutional purchasers named therein, which amends and restates the Prudential 2014 Note Agreement. Pursuant to the Original Prudential 2015 Amended Agreement, we issued and sold through a private placement an aggregate principal amount of €88.9 million of unsecured 1.785% Series C Senior Notes due June 18, 2025 (the “2025 Series C Notes”).
On May 9, 2019, we entered into the Amendment to Note Purchase and Private Shelf Agreement (the “Prudential First Amendment”) with Prudential and the other parties thereto, which amended certain reporting provisions in the Original Prudential 2015 Amended Agreement.
On April 10, 2020, we entered into the Second Amendment to the Prudential 2015 Amended Agreement (the “Prudential Second Amendment”), in order to (i) increase the facility size to $425.0 million, (ii) extend the facility issuance period to April 10, 2023, (iii) make various implementing and administrative changes in order to facilitate the $75.0 million notes issuance on April 14, 2020, (iv) allow the amount available to be issued under the facility to equal $425.0 million less the amount of notes outstanding from time to time during the issuance period, and (v) modify several defined terms, schedules and covenant baskets in the Original Prudential 2015 Amended Agreement, as amended by the Prudential First Amendment, to create additional operating flexibility, and in particular to align such provisions with similar modifications we made substantially concurrently in our other debt facilities. We refer to the Original Prudential 2015 Agreement, as amended by the Prudential First Amendment and the Prudential Second Amendment, as the “Prudential 2015 Amended Agreement.”
On April 14, 2020, we issued and sold to Prudential and other purchasers $75.0 million of our unsecured senior notes (the “Prudential 2030 Series D Notes”) pursuant to the Prudential Second Amendment. The entire outstanding balance of the Prudential 2030 Series D Notes is due and payable on April 14, 2030, and the Prudential 2030 Series D Notes bear interest at the rate of 2.50% per annum. We used the proceeds received from the Prudential 2030 Series D Notes for general corporate purposes.
MetLife 2014 Note Agreement, Including Amendments
We entered into a Multicurrency Note Purchase and Private Shelf Agreement, dated as of December 19, 2014 (the “Original MetLife 2014 Note Agreement”), among the Company, Metropolitan Life Insurance Company (“MetLife”), and the accredited institutional purchasers named therein pursuant to which we agreed to issue and sell an aggregate principal amount of $150.0 million of unsecured senior notes consisting of $75.0 million of our 3.25% Series A Senior Notes having a seven-year term (the “2022 Series A Notes”), and $75.0 million of our 3.72% Series B Senior Notes having a twelve-year term (“2027 Series B Notes”). The issuance, sale and purchase of these notes occurred in February 2015. The aggregate principal amount of our 2022 Series A Notes for $75.0 million was repaid in February 2022.
On March 14, 2019, we amended the Original MetLife 2014 Note Agreement. Pursuant to the Original MetLife 2014 Note Agreement, as so amended, we issued and sold through a private placement an aggregate principal amount of $100.0 million of unsecured senior notes at a 4.19% per annum rate, due March 14, 2029 (the “2029 Series C Notes”).
On March 23, 2020, we entered into the Second Amendment to the Original MetLife 2014 Note Agreement (the “MetLife Second Amendment”), in order to (i) increase the facility size from $150.0 million to $300.0 million, (ii) extend the facility issuance period to December 20, 2022, (iii) make various implementing and administrative changes in order to facilitate the $125.0 million notes issuance on April 2, 2020, and (iv) allow the amount available to be issued under the facility to equal $300.0 million, less the amounts outstanding on 2029 Series C Notes and MetLife 2030 Series D Notes.
On April 2, 2020, we issued and sold to MetLife and other purchasers $125.0 million of our unsecured senior notes (the “MetLife 2030 Series D Notes”) pursuant to the MetLife Second Amendment. The entire outstanding principal balance of the MetLife 2030 Series D Notes is due and payable on April 2, 2030, and the MetLife 2030 Series D Notes bear interest at the rate of 2.50% per annum. We used the proceeds received from the MetLife 2030 Series D Notes for general corporate purposes.
We refer to the Original MetLife 2014 Agreement, as so amended, as the “MetLife 2014 Agreement,” and together with the NY Life 2013 Note Agreement, NY Life 2014 Note Agreement, and Prudential 2015 Amended Note Agreement, collectively, as the “Senior Note Agreements.”
Senior Note Agreements
The Senior Note Agreements contain affirmative, negative, and financial covenants customary for agreements of this type. The negative covenants include restrictions on liens, indebtedness of our subsidiaries, priority indebtedness, fundamental changes, investments, transactions with affiliates, certain restrictive agreements, and violations of laws and regulations. The sole financial covenant is a consolidated leverage ratio test that requires our ratio of debt to earnings before interest, taxes, depreciation, amortization, and share-based compensation, as defined in the Senior Note Agreements, not to exceed 3.5-to-1. As of December 31, 2024, we were in compliance with the covenants of the Senior Note Agreements.
Should we elect to prepay the Senior Notes, such aggregate prepayment will include the applicable make-whole amount(s), as defined within the applicable Senior Note Agreements. Additionally, in the event of a change in control of the Company or upon the disposition of certain assets of the Company the proceeds of which are not reinvested (as defined in the Senior Note Agreements), we may be required to prepay all or a portion of the Senior Notes. The obligations under the Senior Notes may be accelerated upon the occurrence of an event of default under the applicable Senior Note Agreement, each of which includes customary events of default including payment defaults, defaults in the performance of the affirmative, negative and financial covenants, the inaccuracy of representations or warranties, bankruptcy and insolvency related defaults, defaults relating to judgments, certain events related to employee pension benefit plans under the Employee Retirement Income Security Act of 1974, the failure to pay specified indebtedness, and cross-acceleration to specified indebtedness.
Future maturities of long-term debt as of December 31, 2024, are as follows:
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
|
|
| Years Ending December 31, |
|
Amount |
|
|
|
| 2025 |
|
$ |
167,803 |
|
| 2026 |
|
75,000 |
|
| 2027 |
|
75,000 |
|
| 2028 |
|
— |
|
| 2029 |
|
100,000 |
|
| Thereafter |
|
200,000 |
|
|
|
$ |
617,803 |
|
Total interest paid on all debt (including our Credit Facility) for the years ended December 31, 2024, 2023, and 2022, was $33.8 million, $41.0 million, and $40.3 million, respectively.
NOTE 14. INCOME TAXES
The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the estimated future tax effects of temporary differences between book and tax treatment of assets and liabilities and carryforwards to the extent they are realizable. We record a valuation allowance to reduce our deferred tax assets to the amount that is more-likely-than-not to be realized. In assessing the need for a valuation allowance, we consider future taxable income and ongoing prudent and feasible tax planning strategies. In the event that we determine that we would be able to realize our deferred tax assets in the future in excess of the net recorded amount, a reduction of the valuation allowance would increase income in the period such determination was made. Likewise, should we determine that we would not be able to realize all or part of our net deferred tax asset in the future, a reduction to the deferred tax asset would be charged to income in the period such determination was made.
We record a liability for uncertain tax positions that do not meet the more-likely-than-not standard as prescribed by the authoritative guidance for income tax accounting. We record tax benefits for only those positions that we believe will more-likely-than-not be sustained. Unrecognized tax benefits are the differences between tax positions taken, or expected to be taken, in tax returns, and the benefits recognized for accounting purposes. We classify uncertain tax positions as long-term liabilities.
Significant judgment is required in determining our worldwide provision for income taxes and our income tax filings are regularly under audit by tax authorities. Any audit result differing from amounts recorded would increase or decrease income in the period that we determine such adjustment is likely. Interest expense and penalties associated with the underpayment of income taxes are included in income tax expense.
Earnings before income taxes were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Domestic |
|
$ |
897,336 |
|
|
$ |
889,133 |
|
|
$ |
684,661 |
|
| International |
|
212,495 |
|
|
172,043 |
|
|
175,311 |
|
|
|
$ |
1,109,831 |
|
|
$ |
1,061,176 |
|
|
$ |
859,972 |
|
The provision (benefit) for income taxes comprised the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
| Current |
|
|
|
|
|
|
| Federal |
|
$ |
168,042 |
|
|
$ |
191,274 |
|
|
$ |
150,099 |
|
| State |
|
37,112 |
|
|
40,369 |
|
|
30,529 |
|
| International |
|
41,004 |
|
|
32,797 |
|
|
35,138 |
|
|
|
246,158 |
|
|
264,440 |
|
|
215,766 |
|
| Deferred |
|
|
|
|
|
|
| Federal |
|
(24,642) |
|
|
(36,501) |
|
|
(31,663) |
|
| State |
|
(4,709) |
|
|
(6,462) |
|
|
(5,735) |
|
| International |
|
5,157 |
|
|
(5,343) |
|
|
2,515 |
|
|
|
(24,194) |
|
|
(48,306) |
|
|
(34,883) |
|
|
|
$ |
221,964 |
|
|
$ |
216,134 |
|
|
$ |
180,883 |
|
The provision for income taxes differs from the amounts computed by applying the statutory federal income tax rate as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| U.S. federal statutory rate |
|
21.0 |
% |
|
21.0 |
% |
|
21.0 |
% |
| State income tax, net of federal tax benefit |
|
2.4 |
|
|
2.7 |
|
|
2.3 |
|
| Taxation on international earnings |
|
(0.2) |
|
|
(0.1) |
|
|
0.6 |
|
| Foreign derived intangible income |
|
(1.3) |
|
|
(1.4) |
|
|
(1.7) |
|
| Share-based compensation from settlements |
|
(1.8) |
|
|
(1.3) |
|
|
(1.5) |
|
| Research and development credit |
|
(1.0) |
|
|
(1.2) |
|
|
(1.1) |
|
| Other, net |
|
0.9 |
|
|
0.7 |
|
|
1.4 |
|
| Effective tax rate |
|
20.0 |
% |
|
20.4 |
% |
|
21.0 |
% |
Our effective income tax rate was 20.0% for the year ended December 31, 2024, and 20.4% for the year ended December 31, 2023. Our effective tax rate for the year ended December 31, 2024, was lower primarily due to increased benefits related to share-based compensation.
Income taxes paid, net of refunds received, for the periods ended December 31, 2024, 2023, and 2022, were $307.2 million, $192.5 million, and $239.8 million, respectively.
We have determined that unremitted earnings are not indefinitely reinvested to the extent they can be distributed without incurring a significant tax liability. As such, we have recorded a deferred tax liability for foreign withholding tax that will be incurred with respect to the unremitted earnings upon repatriation. We consider all other outside basis differences to be indefinitely reinvested to the extent reversal would incur a significant tax liability. It is not practicable to calculate a deferred tax liability related to such outside basis differences.
The components of the net deferred tax assets (liabilities) included in the accompanying consolidated balance sheets are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
| Assets |
|
|
|
|
| Accrued expenses |
|
$ |
56,325 |
|
|
$ |
44,043 |
|
| Accounts receivable reserves |
|
3,444 |
|
|
2,700 |
|
| Deferred revenue |
|
5,743 |
|
|
6,427 |
|
| Inventory basis differences |
|
24,040 |
|
|
24,500 |
|
| Property-based differences |
|
16,802 |
|
|
18,036 |
|
| Intangible asset basis differences |
|
41,628 |
|
|
47,089 |
|
| Share-based compensation |
|
14,335 |
|
|
12,061 |
|
| Other |
|
1,878 |
|
|
2,267 |
|
| Net operating loss carryforwards |
|
6,647 |
|
|
9,007 |
|
| Tax credit carryforwards |
|
13,382 |
|
|
12,824 |
|
| Unrealized losses on foreign currency exchange contracts and investments |
|
1,357 |
|
|
821 |
|
| Research and development expenditure differences |
|
92,856 |
|
|
66,705 |
|
| Total assets |
|
278,437 |
|
|
246,480 |
|
| Valuation allowance |
|
(31,927) |
|
|
(34,793) |
|
| Total assets, net of valuation allowance |
|
246,510 |
|
|
211,687 |
|
|
|
|
|
|
| Liabilities |
|
|
|
|
| Customer acquisition costs |
|
(46,892) |
|
|
(39,318) |
|
| Property-based differences |
|
(53,332) |
|
|
(52,235) |
|
| Intangible asset basis differences |
|
(10,443) |
|
|
(6,335) |
|
| Other |
|
(14,867) |
|
|
(11,639) |
|
| Unrealized gains on foreign currency exchange contracts and investments |
|
(6,658) |
|
|
(1,813) |
|
| Total liabilities |
|
(132,192) |
|
|
(111,340) |
|
| Net deferred tax assets |
|
$ |
114,318 |
|
|
$ |
100,347 |
|
As of December 31, 2024, we recorded valuation allowances against certain deferred tax assets related to temporary differences, including intangible asset basis differences, net operating loss (“NOL”), and tax credit carryforwards, as it is more-likely-than-not that they will not be realized or utilized within the carryforward period.
The following table summarizes the changes in valuation allowance for deferred tax assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Balance at beginning of year |
|
$ |
34,793 |
|
|
$ |
39,726 |
|
|
$ |
39,280 |
|
Charges to costs and expense |
|
698 |
|
|
21 |
|
|
2,200 |
|
| Write-off/cash payments |
|
(1,289) |
|
|
(7,846) |
|
|
(1,537) |
|
| Foreign currency translation |
|
(2,275) |
|
|
2,892 |
|
|
(217) |
|
| Balance at the end of the year |
|
$ |
31,927 |
|
|
$ |
34,793 |
|
|
$ |
39,726 |
|
As of December 31, 2024, we have NOLs in certain state and international jurisdictions of approximately $29.5 million available to offset future taxable income. Most of these NOL carryforwards will expire at various dates between 2025 and 2031 and the remainder have indefinite lives. At December 31, 2024, we also had state tax credit carryforwards of $16.9 million that will expire between 2029 and 2044.
The following table summarizes the changes in unrecognized tax positions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Total amounts of unrecognized tax benefits, beginning of period |
|
$ |
22,320 |
|
|
$ |
22,547 |
|
|
$ |
21,789 |
|
Gross increases in unrecognized tax positions as a result of tax positions taken during a prior period |
|
41 |
|
|
6,366 |
|
|
342 |
|
| Gross increases in unrecognized tax positions as a result of tax positions taken in the current period |
|
3,034 |
|
|
3,987 |
|
|
3,197 |
|
Decreases in unrecognized tax positions related to settlements with taxing authorities |
|
(678) |
|
|
(7,535) |
|
|
(1,544) |
|
| Decreases in unrecognized tax positions as a result of a lapse of the applicable statutes of limitations |
|
(6,571) |
|
|
(3,045) |
|
|
(1,237) |
|
| Total amounts of unrecognized tax benefits, end of period |
|
$ |
18,146 |
|
|
$ |
22,320 |
|
|
$ |
22,547 |
|
Of the total unrecognized tax benefits as of December 31, 2024, and 2023, $17.4 million and $21.2 million, respectively, comprise unrecognized tax positions that would, if recognized, affect our effective tax rate. Unrecognized tax benefits of approximately $3.2 million are subject to the lapse in the statutes of limitations during 2025 in various U.S. and international tax jurisdictions.
During the years ended December 31, 2024, 2023, and 2022, we recorded interest expense and penalties related to income taxes of $1.7 million, $2.9 million, and $1.3 million, respectively, as income tax expense in our consolidated statements of income. As of December 31, 2024, 2023, and 2022, we had $2.9 million, $3.8 million, and $4.2 million, respectively, of estimated interest expense and penalties accrued in our consolidated balance sheets.
In the ordinary course of our business, our income tax filings are regularly under audit by tax authorities. While we believe we have appropriately provided for all uncertain tax positions, amounts asserted by taxing authorities could be greater or less than our accrued position. Accordingly, additional provisions on income tax matters, or reductions of previously accrued provisions, could be recorded in the future as we revise our estimates due to changing facts and circumstances or the underlying matters are settled or otherwise resolved. We are currently under tax examinations in various jurisdictions. We anticipate that these examinations will be concluded within the next two years. With few exceptions, we are no longer subject to income tax examinations for years before 2015 in any jurisdiction in which we conduct significant taxable activities.
NOTE 15. EARNINGS PER SHARE
Basic earnings per share is computed by dividing net income attributable to our stockholders by the weighted average number of shares of common stock and vested deferred stock units outstanding during the year. The computation of diluted earnings per share is similar to the computation of basic earnings per share, except that the denominator is increased for the assumed exercise of dilutive options and assumed issuance of unvested restricted stock units and unvested deferred stock units using the treasury stock method unless the effect is anti-dilutive. The treasury stock method assumes that proceeds, including cash received from the exercise of employee stock options and the total unrecognized compensation expense for unvested share-based compensation awards, would be used to purchase our common stock at the average market price during the period. Vested deferred stock units outstanding are included in shares outstanding for basic and diluted earnings per share because the associated shares of our common stock are issuable for no cash consideration, the number of shares of our common stock to be issued is fixed, and issuance is not contingent. Refer to “Note 5. Share-Based Compensation” for additional information regarding deferred stock units.
The following is a reconciliation of weighted average shares outstanding for basic and diluted earnings per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Shares outstanding for basic earnings per share: |
|
82,467 |
|
|
83,066 |
|
|
83,623 |
|
|
|
|
|
|
|
|
| Shares outstanding for diluted earnings per share: |
|
|
|
|
|
|
| Shares outstanding for basic earnings per share |
|
82,467 |
|
|
83,066 |
|
|
83,623 |
|
| Dilutive effect of share-based payment awards |
|
779 |
|
|
912 |
|
|
977 |
|
|
|
83,246 |
|
|
83,978 |
|
|
84,600 |
|
Certain awards and options to acquire shares have been excluded from the calculation of shares outstanding for diluted earnings per share because they were anti-dilutive. The following table presents information concerning those anti-dilutive awards and options:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Weighted average number of shares underlying anti-dilutive options |
|
469 |
|
|
381 |
|
|
263 |
|
| Weighted average number of shares underlying anti-dilutive awards |
|
38 |
|
|
1 |
|
|
46 |
|
NOTE 16. COMMITMENTS, CONTINGENCIES AND GUARANTEES
Commitments
Refer to “Note 8. Lease Commitments” for more information regarding our lease commitments.
In the ordinary course of business we enter into purchase obligations that include agreements and purchase orders to purchase goods or services that are contractually enforceable and that specify all significant terms, including fixed or minimum quantities, pricing, and approximate timing of purchases. As of December 31, 2024, we had approximately $211.0 million in purchase obligations due in 2025. Our purchase obligations beyond 2025 are approximately $168.7 million. The expected timing of payments of our purchase obligations is estimated based on current information. Timing of payments and actual amounts paid may be different, depending on the time of receipt of goods or services, or changes to agreed-upon amounts for some obligations.
Contingencies
We are subject to claims that may arise in the ordinary course of business, including with respect to actual and threatened litigation and other matters. We accrue for loss contingencies when it is probable that future expenditures will be made, and such expenditures can be reasonably estimated. However, the results of legal actions cannot be predicted with certainty, and therefore our actual losses with respect to these contingencies could exceed our accruals. Except for the litigation matter described below, as of December 31, 2024, our accruals with respect to actual and threatened litigation were not material.
We are a defendant in an ongoing litigation matter involving an alleged breach of contract for underpayment of royalty payments made from 2004 through 2017 under an expired patent license agreement. The plaintiff asserted a claim of approximately $50.0 million, inclusive of interest through June 30, 2020, alleging that the incorrect royalty provision was applied to certain licensed products and services throughout the agreement term and that royalties were also due on non-licensed diagnostic services that were provided concurrently with licensed services. The trial court ruled in favor of the plaintiff in September 2020. The appellate court reversed the trial court’s decision regarding the royalty payments in August 2022, and the state supreme court granted the plaintiff’s petition for review. In June 2024, the state supreme court reversed the appellate court, reinstated the trial court decision regarding the royalty payments, and remanded the case to the appellate court to address the remaining issues, including issues related to applicable interest. We will continue to vigorously defend ourselves in this matter; however, litigation is inherently unpredictable, and we cannot predict with certainty the ultimate outcome, timing, or amount of actual loss for this matter. During the second quarter of 2024, we increased our previously established accrual of $27.5 million relating to this matter to $89.0 million, which represents our best estimate at this time of the amount of the probable loss, based on the current status of the case and associated estimated interest. The accrual is included in accrued expenses on the consolidated balance sheets, and included in our CAG reportable segment.
The actual loss associated with this matter may be higher or lower than the amount we have accrued depending on the ultimate outcome of the case.
From time to time, we have received notices alleging that our products infringe third-party proprietary rights, although we are not aware of any pending litigation with respect to such claims. Patent litigation is frequently complex and expensive, and the outcome of patent litigation can be difficult to predict. There can be no assurance that we will prevail in any infringement proceedings that may be commenced against us. If we lose any such litigation, we may be stopped from selling certain products and/or we may be required to pay damages as a result of the litigation.
Guarantees
We enter into agreements with third parties in the ordinary course of business under which we are obligated to indemnify such third parties for and against various risks and losses. The precise terms of such indemnities vary with the nature of the agreement. In many cases, we limit the maximum amount of our indemnification obligations, but in some cases, those obligations may be theoretically unlimited. We have not incurred material expenses in discharging any of these indemnification obligations and, based on our analysis of the nature of the risks involved, we believe that the fair value of potential indemnification under these agreements is minimal. Accordingly, we have recorded no liabilities for these obligations as of December 31, 2024, and 2023.
When acquiring a business, we sometimes assume liability for certain events or occurrences that took place prior to the date of acquisition. As of December 31, 2024, and 2023, we do not have any material pre-acquisition liabilities recorded.
NOTE 17. SEGMENT REPORTING
We operate primarily through three business segments, which are our reportable segments: Companion Animal Group (“CAG”), Water quality products (“Water”), and Livestock, Poultry and Dairy (“LPD”). CAG provides products and services for veterinarians and the biomedical research community, primarily related to diagnostics and information management. Water provides innovative testing solutions for the detection and quantification of various microbiological parameters in water. LPD provides diagnostic tests, services, and related instrumentation that are used to manage the health status of livestock and poultry, to improve producer efficiency, and to ensure the quality and safety of milk. Our Other operating segment combines and presents our human medical diagnostic business (“OPTI Medical”) with our out-licensing arrangements because they do not meet the quantitative or qualitative thresholds for reportable segments. OPTI Medical develops, manufactures, and distributes human medical diagnostic products and services.
Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision-maker (“CODM”) in assessing performance. The CODM, our president and Chief Executive Officer, evaluates the performance of operating segments based on revenues and gross profit. Our CODM reviews budget and actual results of the operating segments and decides how to allocate resources to meet our strategic priorities, and also meets with operating segment leaders on a periodic basis to determine allocation of resources.
The accounting principles used in the preparation of the segment information are the same as those used for the consolidated financial statements. Intersegment revenues, which are not included in the tables below, were not material for the years ended December 31, 2024, 2023, and 2022. Assets are not allocated to segments for internal reporting purposes and are not included in the review performed by the CODM for purposes of assessing segment performance and allocation of resources. Certain corporate expenses are allocated to the segments, including depreciation and amortization. Foreign currency transaction gains and losses for all operating segments are reported within our Other operating segment, and are reconciled in the table below.
The following tables are a summary of reportable segment performance with our Other operating segment to reconcile to the total consolidated for the years ended December 31, 2024, 2023, and 2022:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
CAG |
|
Water |
|
LPD |
|
Total |
| 2024 |
|
|
|
|
|
|
|
|
Total revenues from reportable segments |
|
$ |
3,574,044 |
|
|
$ |
185,112 |
|
|
$ |
122,060 |
|
|
$ |
3,881,216 |
|
| Reconciliation of revenue |
|
|
|
|
|
|
|
|
Other revenues |
|
|
|
|
|
|
|
16,288 |
|
Total consolidated revenue |
|
|
|
|
|
|
|
3,897,504 |
|
Cost of revenue |
|
1,394,864 |
|
|
55,101 |
|
|
59,500 |
|
|
|
| Segment gross profit |
|
$ |
2,179,180 |
|
|
$ |
130,011 |
|
|
$ |
62,560 |
|
|
$ |
2,371,751 |
|
|
|
|
|
|
|
|
|
|
Reconciliation of operating profit (segment profit) |
|
|
|
|
|
|
|
|
Segment gross profit |
|
|
|
|
|
|
|
$ |
2,371,751 |
|
Segment operating expenses |
|
|
|
|
|
|
|
(1,242,169) |
|
Other operating profit (excluding unallocated amounts) |
|
|
|
|
|
|
|
3,282 |
|
Unallocated amounts |
|
|
|
|
|
|
|
|
Foreign currency transaction losses |
|
|
|
|
|
|
|
(4,527) |
|
Interest expense |
|
|
|
|
|
|
|
(31,205) |
|
Interest income |
|
|
|
|
|
|
|
12,699 |
|
| Income before provision for income taxes |
|
|
|
|
|
|
|
$ |
1,109,831 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
CAG |
|
Water |
|
LPD |
|
Total |
| 2023 |
|
|
|
|
|
|
|
|
Total revenues from reportable segments |
|
$ |
3,352,356 |
|
|
$ |
168,149 |
|
|
$ |
121,659 |
|
|
$ |
3,642,164 |
|
Reconciliation of revenue |
|
|
|
|
|
|
|
|
Other revenues |
|
|
|
|
|
|
|
18,789 |
|
Total consolidated revenue |
|
|
|
|
|
|
|
3,660,953 |
|
Cost of revenue |
|
1,349,930 |
|
|
52,148 |
|
|
56,219 |
|
|
|
| Segment gross profit |
|
$ |
2,002,426 |
|
|
$ |
116,001 |
|
|
$ |
65,440 |
|
|
$ |
2,183,867 |
|
|
|
|
|
|
|
|
|
|
Reconciliation of operating profit (segment profit) |
|
|
|
|
|
|
|
|
Segment gross profit |
|
|
|
|
|
|
|
$ |
2,183,867 |
|
Segment operating expenses |
|
|
|
|
|
|
|
(1,086,812) |
|
Other operating profit (excluding unallocated amounts) |
|
|
|
|
|
|
|
1,151 |
|
Unallocated amounts |
|
|
|
|
|
|
|
|
| Foreign currency transaction losses |
|
|
|
|
|
|
|
(1,078) |
|
Interest expense |
|
|
|
|
|
|
|
(41,581) |
|
Interest income |
|
|
|
|
|
|
|
5,629 |
|
| Income before provision for income taxes |
|
|
|
|
|
|
|
$ |
1,061,176 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
CAG |
|
Water |
|
LPD |
|
Total |
| 2022 |
|
|
|
|
|
|
|
|
Total revenues from reportable segments |
|
$ |
3,058,793 |
|
|
$ |
155,720 |
|
|
$ |
122,607 |
|
|
$ |
3,337,120 |
|
Reconciliation of revenue |
|
|
|
|
|
|
|
|
Other revenues |
|
|
|
|
|
|
|
30,204 |
|
Total consolidated revenue |
|
|
|
|
|
|
|
3,367,324 |
|
Cost of revenue |
|
1,252,216 |
|
|
45,861 |
|
|
49,606 |
|
|
|
| Segment gross profit |
|
$ |
1,806,577 |
|
|
$ |
109,859 |
|
|
$ |
73,001 |
|
|
$ |
1,989,437 |
|
|
|
|
|
|
|
|
|
|
Reconciliation of operating profit (segment profit) |
|
|
|
|
|
|
|
|
Segment gross profit |
|
|
|
|
|
|
|
$ |
1,989,437 |
|
Segment operating expenses |
|
|
|
|
|
|
|
(1,096,160) |
|
Other operating profit (excluding unallocated amounts) |
|
|
|
|
|
|
|
8,896 |
|
Unallocated amounts |
|
|
|
|
|
|
|
|
Foreign currency transaction losses |
|
|
|
|
|
|
|
(3,408) |
|
Interest expense |
|
|
|
|
|
|
|
(39,858) |
|
Interest income |
|
|
|
|
|
|
|
1,065 |
|
| Income before provision for income taxes |
|
|
|
|
|
|
|
$ |
859,972 |
|
Refer to “Note 3. Revenue” for a summary of disaggregated revenue by segment and by major product and service category for the years ended December 31, 2024, 2023, and 2022.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
For the Years Ended December 31, |
|
|
CAG |
|
Water |
|
LPD |
|
Other |
|
Consolidated Total |
Depreciation and amortization |
|
|
|
|
|
|
|
|
|
|
2024 |
|
$ |
116,278 |
|
|
$ |
4,734 |
|
|
$ |
3,802 |
|
|
$ |
5,122 |
|
|
$ |
129,936 |
|
2023 |
|
$ |
103,554 |
|
|
$ |
4,117 |
|
|
$ |
3,657 |
|
|
$ |
3,580 |
|
|
$ |
114,908 |
|
2022 |
|
$ |
101,254 |
|
|
$ |
3,020 |
|
|
$ |
3,797 |
|
|
$ |
3,829 |
|
|
$ |
111,900 |
|
Net long-lived assets, consisting of net property and equipment, are subject to geographic risks because they are generally difficult to move and to effectively utilize in another geographic area in a reasonable time period and because they are relatively illiquid. Net long-lived assets by principal geographic areas were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
| Americas |
|
|
|
|
| United States |
|
$ |
553,600 |
|
|
$ |
531,735 |
|
| Brazil |
|
22,139 |
|
|
33,236 |
|
| Canada |
|
8,165 |
|
|
7,032 |
|
|
|
583,904 |
|
|
572,003 |
|
| Europe, the Middle East and Africa |
|
|
|
|
| Germany |
|
52,652 |
|
|
56,115 |
|
| Switzerland |
|
14,722 |
|
|
14,129 |
|
| United Kingdom |
|
11,137 |
|
|
10,900 |
|
| Netherlands |
|
9,484 |
|
|
10,604 |
|
| France |
|
1,769 |
|
|
1,724 |
|
| Other |
|
3,058 |
|
|
2,907 |
|
|
|
92,822 |
|
|
96,379 |
|
| Asia Pacific Region |
|
|
|
|
| Australia |
|
19,307 |
|
|
21,922 |
|
| Japan |
|
9,382 |
|
|
4,892 |
|
| Other |
|
7,708 |
|
|
6,981 |
|
|
|
36,397 |
|
|
33,795 |
|
| Total |
|
$ |
713,123 |
|
|
$ |
702,177 |
|
NOTE 18. FAIR VALUE MEASUREMENTS
U.S. GAAP defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. U.S. GAAP requires an entity to maximize the use of observable inputs, where available, and minimize the use of unobservable inputs when measuring fair value.
We have certain financial assets and liabilities that are measured at fair value on a recurring basis, certain nonfinancial assets and liabilities that may be measured at fair value on a non-recurring basis, and certain financial assets and liabilities that are not measured at fair value in our consolidated balance sheets but for which we disclose the fair value. The fair value disclosures of these assets and liabilities are based on a three-level hierarchy, which is defined as follows:
|
|
|
|
|
|
| Level 1 |
Quoted prices in active markets for identical assets or liabilities that the entity can access at the measurement date. |
| Level 2 |
Observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| Level 3 |
Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. Our assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability. We did not have any transfers between Level 1 and Level 2, or transfers in or out of Level 3, of the fair value hierarchy during the years ended December 31, 2024, and 2023.
Our cross currency swap contracts are measured at fair value on a recurring basis in our accompanying consolidated balance sheets and are classified as derivative instruments. We measure the fair value of our cross currency swap contracts using prevailing market conditions as of the close of business on each balance sheet date. The product of this calculation is then adjusted for counterparty risk.
Our foreign currency exchange contracts are measured at fair value on a recurring basis in our accompanying consolidated balance sheets and are classified as derivative instruments. We measure the fair value of our foreign currency exchange contracts using an income approach, based on prevailing market forward exchange rates less the contract rate multiplied by the notional amount. The product of this calculation is then adjusted for counterparty risk.
Our interest rate swap contract is measured at fair value on a recurring basis in our accompanying consolidated balance sheets and is classified as a derivative instrument. We measure the fair value of our interest rate swap contract using current market interest rates for debt issues with similar remaining years to maturity, adjusted for applicable credit risk.
The amounts outstanding under our unsecured revolving credit facility (“Credit Facility” or “line of credit”) and senior notes (“long-term debt”) are measured at carrying value in our accompanying consolidated balance sheets though we disclose the fair value of these financial instruments. We determine the fair value of the amount outstanding under our Credit Facility and long-term debt using an income approach, utilizing a discounted cash flow analysis based on current market interest rates for debt issues with similar remaining years to maturity, adjusted for applicable credit risk. Our Credit Facility and long-term debt are valued using Level 2 inputs. The estimated fair value of our Credit Facility approximates its carrying value. As of December 31, 2024, the estimated fair value and carrying value of our long-term debt were $594.3 million and $617.8 million, respectively. As of December 31, 2023, the estimated fair value and carrying value of our long-term debt were $670.0 million and $698.2 million, respectively.
The following tables set forth our assets and liabilities that were measured at fair value on a recurring basis by level within the fair value hierarchy:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
|
|
|
|
|
As of December 31, 2024 |
|
Quoted Prices in Active Markets for Identical Assets
(Level 1) |
|
Significant Other Observable Inputs
(Level 2) |
|
Significant Unobservable Inputs
(Level 3) |
|
Balance as of December 31, 2024 |
|
|
|
|
|
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
Money market funds (1) |
|
$ |
139,626 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
139,626 |
|
Cross currency swaps (3) |
|
$ |
— |
|
|
$ |
3,501 |
|
|
$ |
— |
|
|
$ |
3,501 |
|
Foreign currency exchange contracts (3) |
|
$ |
— |
|
|
$ |
16,921 |
|
|
$ |
— |
|
|
$ |
16,921 |
|
Interest rate swap (4) |
|
$ |
— |
|
|
$ |
710 |
|
|
$ |
— |
|
|
$ |
710 |
|
| Liabilities |
|
|
|
|
|
|
|
|
Cross currency swaps (3) |
|
$ |
— |
|
|
$ |
33 |
|
|
$ |
— |
|
|
$ |
33 |
|
Contingent Consideration |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
2,300 |
|
|
$ |
2,300 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
|
|
|
|
|
As of December 31, 2023 |
|
Quoted Prices in Active Markets for Identical Assets
(Level 1) |
|
Significant Other Observable Inputs
(Level 2) |
|
Significant Unobservable Inputs
(Level 3) |
|
Balance as of December 31, 2023 |
|
|
|
|
|
|
|
|
|
| Assets |
|
|
|
|
|
|
|
|
Money market funds (1) |
|
$ |
290,807 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
290,807 |
|
Equity mutual funds (2) |
|
$ |
99 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
99 |
|
|
|
|
|
|
|
|
|
|
Cross currency swaps (3) |
|
$ |
— |
|
|
$ |
664 |
|
|
$ |
— |
|
|
$ |
664 |
|
Foreign currency exchange contracts (3) |
|
$ |
— |
|
|
$ |
1,783 |
|
|
$ |
— |
|
|
$ |
1,783 |
|
Interest rate swap (4) |
|
$ |
— |
|
|
$ |
1,451 |
|
|
|
|
$ |
1,451 |
|
| Liabilities |
|
|
|
|
|
|
|
|
Cross currency swaps (3) |
|
$ |
— |
|
|
$ |
5,041 |
|
|
$ |
— |
|
|
$ |
5,041 |
|
Foreign currency exchange contracts (3) |
|
$ |
— |
|
|
$ |
5,532 |
|
|
$ |
— |
|
|
$ |
5,532 |
|
Deferred compensation (5) |
|
$ |
99 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
99 |
|
|
|
|
|
|
|
|
|
|
(1)Money market funds with an original maturity of less than ninety days are included within cash and cash equivalents. The remaining balance of cash and cash equivalents as of December 31, 2024, consisted of demand deposits.
(2)Equity mutual funds relate to a deferred compensation plan that was assumed as part of a previous business combination. This amount is included within other current assets. Refer to footnote (5) below for a discussion of the related deferred compensation liability. The obligations under the deferred compensation plan were completed in 2024.
(3)Cross currency swaps and foreign currency exchange contracts are included within other current assets; other long-term assets; accrued liabilities; or other long-term liabilities depending on the gain (loss) position and anticipated settlement date.
(4)The interest rate swap is included within other long-term assets, or other long-term liabilities.
(5)A deferred compensation plan assumed as part of a previous business combination is included within accrued liabilities. The fair value of our deferred compensation plan is indexed to the performance of the underlying equity mutual funds discussed in footnote (2) above. The obligations under the deferred compensation plan were completed in 2024.
The estimated fair values of certain financial instruments, including cash and cash equivalents, accounts receivable, and accounts payable, approximate their respective carrying values due to their short maturity.
NOTE 19. HEDGING INSTRUMENTS
Disclosure within this note is presented to provide transparency about how and why we use derivative and non-derivative instruments (collectively “hedging instruments”), how the hedging instruments and related hedged items are accounted for, and how the hedging instruments and related hedged items affect our financial position, results of operations, and cash flows.
We recognize all hedging instrument assets and liabilities on the balance sheet at fair value at the balance sheet date. Hedging instruments that do not qualify for hedge accounting treatment must be recorded at fair value through earnings. To qualify for hedge accounting treatment, cash flow and net investment hedges must be highly effective in offsetting changes to expected future cash flows or fair value on hedged transactions. If the hedging instrument qualifies for hedge accounting, changes in the fair value of the hedging instrument from the effective portion of the hedge are deferred in AOCI, net of tax, and reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. We immediately record in earnings the extent to which a hedging instrument is not effective in achieving offsetting changes in fair value. We de-designate hedging instruments from hedge accounting when the likelihood of the hedged transaction occurring becomes less than probable. For de-designated hedging instruments, the gain or loss from the time of de-designation through maturity of the instrument is recognized in earnings. Any gain or loss in AOCI at the time of de-designation is reclassified into earnings in the same period or periods during which the hedged transaction affects earnings. Refer to “Note 21. Accumulated Other Comprehensive Income” for further information regarding the effect of hedging instruments on the consolidated statements of income for the years ended December 31, 2024, 2023, and 2022.
We enter into master netting arrangements with the counterparties to our derivative transactions which permit certain outstanding receivables and payables to be offset in the event of default. Our derivative contracts do not require either party to post cash collateral. We elect to present our derivative assets and liabilities in the accompanying consolidated balance sheets on a gross basis. All cash flows related to our foreign currency exchange contracts are classified as operating cash flows, which is consistent with the cash flow treatment of the underlying items being hedged.
We are exposed to certain risks related to our ongoing business operations. During the first quarter of 2023, we entered into an interest rate swap to manage the impact of interest rate fluctuations associated with $250.0 million of borrowings under our variable-rate Credit Facility. We have designated the interest rate swap as a cash flow hedge.
Our subsidiaries enter into foreign currency exchange contracts to manage the exchange risk associated with their forecasted intercompany inventory purchases and sales for the next year. From time to time, we may also enter into other foreign currency exchange contracts, cross currency swaps, or foreign-denominated debt issuances to minimize the impact of foreign currency fluctuations associated with specific balance sheet exposures, including net investments in certain foreign subsidiaries.
The primary purpose of our foreign currency hedging activities is to protect against the volatility associated with foreign currency transactions, including transactions denominated in euro, British pound, Japanese yen, Canadian dollar, and Australian dollar. We also utilize natural hedges to mitigate our transaction and commitment exposures. Our corporate policy prescribes the range of allowable hedging activity. We enter into foreign currency exchange contracts with large, well-capitalized multinational financial institutions and we do not hold or engage in transactions involving derivative instruments for purposes other than risk management. Our accounting policies for these contracts are based on our designation of such instruments as hedging transactions.
Refer to “Note 18. Fair Value Measurements” for additional information regarding the fair value of our derivative instruments and “Note 21. Accumulated Other Comprehensive Income” for additional information regarding the effect of derivative instruments designated as cash flow hedges on the consolidated statements of income.
Cash Flow Hedges
We have designated our foreign currency exchange contracts and our interest rate swap as cash flow hedges as these derivative instruments manage our exposure to variability in the cash flows of forecasted transactions attributable to foreign currency exchange and to interest rates on variable interest obligations under the terms of our Credit Facility. Unless noted otherwise, we have also designated our derivative instruments as qualifying for hedge accounting treatment.
We did not de-designate any hedging instruments from hedge accounting treatment during the years ended December 31, 2024, 2023, and 2022. Gains and losses related to hedge ineffectiveness recognized in earnings during the years ended December 31, 2024, 2023, and 2022 were not material. As of December 31, 2024, the estimated amount of gains, net of tax, from our foreign exchange contracts which are expected to be reclassified out of AOCI and into earnings within the next twelve months is $12.8 million if exchange rates do not fluctuate from the levels as of December 31, 2024. As of December 31, 2024, the estimated amount of gains, net of tax, from our interest rate swap contract which are expected to be reclassified out of AOCI and into earnings within the next twelve months is $0.6 million if interest rates do not fluctuate from the levels as of December 31, 2024.
Interest Rate Swap: We entered into an interest rate swap contract to manage the effect of variable interest obligations on amounts borrowed under the terms of the Credit Facility. Beginning on March 31, 2023, the variable interest rate associated with $250.0 million of borrowings outstanding under the Credit Facility became effectively fixed at 3.9% plus the applicable credit spread, through October 20, 2025.
Foreign Currency Exchange Contracts: We target to hedge approximately 75% to 85% of the estimated exposure from intercompany product purchases and sales denominated in the euro, British pound, Canadian dollar, Japanese yen, and Australian dollar. We have additional unhedged foreign currency exposures related to intercompany foreign transactions and emerging markets where it is not practical to hedge. We primarily utilize foreign currency exchange contracts with durations of less than 24 months. Quarterly, we enter into contracts to hedge incremental portions of anticipated foreign currency transactions for the current and following year. As a result, our risk with respect to foreign currency exchange rate fluctuations and the notional value of foreign currency exchange contracts may vary throughout the year. The U.S. dollar is the currency purchased or sold in all of our foreign currency exchange contracts. The notional amount of foreign currency exchange contracts to hedge forecasted intercompany inventory purchases and sales totaled $325.7 million and $294.0 million as of December 31, 2024, and 2023, respectively.
The following table presents the effects of cash flow hedge accounting on our consolidated statements of income and comprehensive income, and provides information regarding the classification on the consolidated statements of income and amounts of pretax gains or losses of derivatives:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
Years Ended December 31, |
|
|
|
|
2024 |
|
2023 |
|
2022 |
|
|
Financial statement line items in which effects of cash flow hedges are recorded |
|
|
|
|
|
|
| Foreign exchange contracts |
|
Cost of revenue |
|
$ |
1,518,577 |
|
|
$ |
1,470,983 |
|
|
$ |
1,362,986 |
|
Amount of gain reclassified from accumulated other comprehensive income into income |
|
|
|
$ |
5,932 |
|
|
$ |
3,512 |
|
|
$ |
25,733 |
|
|
|
|
|
|
|
|
|
|
| Interest rate swap contract |
|
Interest expense |
|
$ |
(31,205) |
|
|
$ |
(41,581) |
|
|
$ |
(39,858) |
|
| Amount of gain reclassified from accumulated other comprehensive income into net income |
|
|
|
$ |
3,268 |
|
|
$ |
2,656 |
|
|
$ |
— |
|
Net Investment Hedges, Euro-Denominated Notes
In June 2015, we issued and sold through a private placement an aggregate principal amount of €88.9 million in euro-denominated 1.785% Series C Senior Notes due June 18, 2025. We have designated these euro-denominated notes as a hedge of our euro net investment in certain foreign subsidiaries to reduce the volatility in stockholders’ equity caused by changes in foreign currency exchange rates in the euro relative to the U.S. dollar. As a result of this designation, gains and losses from the change in translated U.S. dollar value of these euro-denominated notes are recorded in AOCI rather than to earnings. We recorded a gain of $4.1 million, a loss of $2.6 million, and a gain of $4.5 million, net of tax, within AOCI as a result of this net investment hedge for the years ended December 31, 2024, 2023, and 2022, respectively. The related cumulative unrealized gain recorded as of December 31, 2024, will not be reclassified in earnings until the complete or substantially complete liquidation of the net investment in the hedged foreign operations or all or a portion of the hedge no longer qualifies for hedge accounting treatment. Refer to “Note 13. Debt” to the consolidated financial statements included in this Annual Report on Form 10-K for further information regarding the issuance of these euro-denominated notes.
Net Investment Hedges, Cross Currency Swaps
We have entered into several cross currency swap contracts as a hedge of our net investment in foreign operations to offset foreign currency translation gains and losses on the net investment. These cross currency swaps have maturity dates beginning on June 30, 2025, through June 29, 2030.
At maturity of the cross currency swap contracts we will deliver the notional amount of €15 million and will receive approximately $17.5 million from the counterparties on June 18, 2025; we will deliver the notional amount of €35 million and will receive $37.8 million from the counterparties on March 31, 2028; we will deliver the notional amount of €90 million and will receive $98.2 million from the counterparties on June 30, 2028; and we will deliver the notional amount of €20 million and will receive $21.3 million from the counterparties on June 29, 2029. The changes in fair value of the cross currency swap contracts are recorded in AOCI and will be reclassified to earnings when the foreign subsidiaries are sold or substantially liquidated or all or a portion of the hedge no longer qualifies for hedge accounting treatment. We recorded a gain of $6.0 million, a loss of $5.6 million, and a gain of $3.8 million, net of tax, within AOCI as a result of these net investment hedges, during the years ended December 31, 2024, 2023, and 2022, respectively. We will receive quarterly interest payments from the counterparties based on a fixed interest rate until maturity of the cross currency swaps. This interest rate component is excluded from the assessment of hedge effectiveness and, thus, is recognized as a reduction to interest expense over the life of the hedging instrument. We recognized approximately $1.6 million and $2.1 million related to the excluded component as a reduction of interest expense for the years ended December 31, 2024, and 2023, respectively.
During the second quarter of 2023, the notional amount of €90.0 million in cross currency swaps matured, resulting in a net cash receipt of $6.3 million. The receipt of cash is reflected within investing activities on the consolidated statements of cash flows.
Fair Values of Hedging Instruments Designated as Hedges in Consolidated Balance Sheets
The fair values of hedging instruments, their respective classification on the consolidated balance sheets, and amounts subject to offset under master netting arrangements consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
Hedging Assets |
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
|
|
| Derivatives and non-derivatives designated as hedging instruments |
|
Balance Sheet Classification |
|
|
|
|
| Foreign currency exchange contracts |
|
Other current assets |
|
$ |
16,921 |
|
|
$ |
1,783 |
|
| Cross currency swaps |
|
Other current assets |
|
1,839 |
|
|
— |
|
| Interest rate swap contract |
|
Other long-term assets |
|
710 |
|
|
1,451 |
|
| Cross currency swaps |
|
Other long-term assets |
|
1,662 |
|
|
664 |
|
|
|
|
|
|
|
|
Total derivative instruments presented as hedging instruments on the balance sheet |
|
|
|
21,132 |
|
|
3,898 |
|
| Gross amounts subject to master netting arrangements not offset on the balance sheet |
|
|
|
— |
|
|
(1,783) |
|
| Net amount |
|
|
|
$ |
21,132 |
|
|
$ |
2,115 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
|
|
Hedging Liabilities |
|
|
|
|
December 31, 2024 |
|
December 31, 2023 |
|
|
|
|
|
|
|
| Derivatives and non-derivatives designated as hedging instruments |
|
Balance Sheet Classification |
|
|
|
|
| Foreign currency exchange contracts |
|
Accrued liabilities |
|
$ |
— |
|
|
$ |
5,532 |
|
| Cross currency swaps |
|
Other long-term liabilities |
|
33 |
|
|
5,041 |
|
| Total derivative instruments presented as cash flow hedges on the balance sheet |
|
|
|
33 |
|
|
10,573 |
|
Non-derivative foreign currency denominated debt designated as net investment hedge on the balance sheet (1) |
|
Long-term debt |
|
92,803 |
|
|
98,187 |
|
| Total hedging instruments presented on the balance sheet |
|
92,836 |
|
|
108,760 |
|
| Gross amounts subject to master netting arrangements not offset on the balance sheet |
|
|
|
— |
|
|
(1,783) |
|
| Net amount |
|
|
|
$ |
92,836 |
|
|
$ |
106,977 |
|
(1)Amounts represent reported carrying amounts of our foreign currency denominated debt. Refer to “Note 18. Fair Value Measurements” for information regarding the fair value of our long-term debt.
NOTE 20. REPURCHASES OF COMMON STOCK
As of December 31, 2024, our Board of Directors has authorized the repurchase of up to 78.0 million shares of our common stock in the open market or in negotiated transactions pursuant to the Company’s share repurchase program, inclusive of an additional 5.0 million shares approved on December 3, 2024. We believe that the repurchase of our common stock is a favorable means of returning value to our stockholders, and we also repurchase to offset the dilutive effect of our share-based compensation programs. Repurchases of our common stock may vary depending upon the level of other investing and deployment activities, as well as share price and prevailing interest rates. As of December 31, 2024, there were approximately 6.1 million remaining shares available for repurchase under this authorization.
We primarily acquire shares by repurchases in the open market. However, we also acquire shares that are surrendered by employees in payment for the statutory withholding taxes due on the vesting of restricted stock units and the settlement of deferred stock units (“employee surrenders”). We issue shares of treasury stock upon the vesting of certain restricted stock units and upon the exercise of certain stock options. The number of shares of treasury stock issued during the years ended December 31, 2024, 2023, and 2022, was not material.
The Inflation Reduction Act of 2022, which was enacted into law on August 16, 2022, imposed a nondeductible 1% excise tax on the net value of certain stock repurchases made after December 31, 2022. This excise tax is included in the cost of treasury stock acquired in repurchases in the open market for the current year.
The following is a summary of our open market common stock repurchases, reported on a trade date basis, and shares acquired through employee surrenders:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands, except per share amounts) |
|
For the Years Ended December 31, |
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
| Shares repurchased in the open market |
|
1,741 |
|
|
155 |
|
|
1,963 |
|
Shares acquired through employee surrenders for statutory tax withholding |
|
19 |
|
|
20 |
|
|
21 |
|
| Total shares repurchased |
|
1,760 |
|
|
175 |
|
|
1,984 |
|
|
|
|
|
|
|
|
| Cost of shares repurchased in the open market |
|
$ |
848,901 |
|
|
$ |
72,639 |
|
|
$ |
810,942 |
|
| Cost of shares for employee surrenders |
|
10,531 |
|
|
9,974 |
|
|
10,606 |
|
| Total cost of shares |
|
$ |
859,432 |
|
|
$ |
82,613 |
|
|
$ |
821,548 |
|
|
|
|
|
|
|
|
| Average cost per share - open market repurchases |
|
$ |
487.66 |
|
|
$ |
468.84 |
|
|
$ |
413.12 |
|
| Average cost per share - employee surrenders |
|
$ |
556.90 |
|
|
$ |
503.28 |
|
|
$ |
501.89 |
|
| Average cost per share - total |
|
$ |
488.40 |
|
|
$ |
472.74 |
|
|
$ |
414.06 |
|
NOTE 21. ACCUMULATED OTHER COMPREHENSIVE INCOME
The changes in AOCI, net of tax, consisted of the following:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Years Ended December 31, 2024 and 2023 |
|
|
|
|
Unrealized Gain (Loss) on Cash Flow Hedges, Net of Tax |
|
Unrealized Gain (Loss) on Net Investment Hedges, Net of Tax |
|
|
|
|
|
|
| (in thousands) |
|
Unrealized Gain (Loss) on Investments, Net of Tax |
|
Foreign Currency Exchange Contracts |
|
Interest Rate Swap |
|
Euro-Denominated Notes |
|
Cross Currency Swaps |
|
Defined Benefit Plans, Net of Tax |
|
Cumulative Translation Adjustment |
|
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance as of December 31, 2022 |
|
$ |
(172) |
|
|
$ |
839 |
|
|
$ |
— |
|
|
$ |
4,947 |
|
|
$ |
7,057 |
|
|
$ |
(2,776) |
|
|
$ |
(87,691) |
|
|
$ |
(77,796) |
|
Other comprehensive income (loss) before reclassifications |
|
8 |
|
|
(792) |
|
|
3,131 |
|
|
(2,601) |
|
|
(5,629) |
|
|
(1,302) |
|
|
17,725 |
|
|
10,540 |
|
| Amounts reclassified from accumulated other comprehensive income |
|
— |
|
|
(2,444) |
|
|
(2,025) |
|
|
— |
|
|
— |
|
|
519 |
|
|
— |
|
|
(3,950) |
|
Balance as of December 31, 2023 |
|
(164) |
|
|
(2,397) |
|
|
1,106 |
|
|
2,346 |
|
|
1,428 |
|
|
(3,559) |
|
|
(69,966) |
|
|
(71,206) |
|
Other comprehensive income (loss) before reclassifications |
|
1 |
|
|
19,418 |
|
|
1,927 |
|
|
4,105 |
|
|
5,981 |
|
|
(701) |
|
|
(46,958) |
|
|
(16,227) |
|
| Amounts reclassified from accumulated other comprehensive income |
|
163 |
|
|
(4,236) |
|
|
(2,491) |
|
|
— |
|
|
— |
|
|
352 |
|
|
— |
|
|
(6,212) |
|
Balance as of December 31, 2024 |
|
$ |
— |
|
|
$ |
12,785 |
|
|
$ |
542 |
|
|
$ |
6,451 |
|
|
$ |
7,409 |
|
|
$ |
(3,908) |
|
|
$ |
(116,924) |
|
|
$ |
(93,645) |
|
The following table presents components and amounts reclassified out of AOCI to net income:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| (in thousands) |
|
Affected Line Item in the Statements of Income |
|
Amounts Reclassified from AOCI for the Years Ended December 31, |
|
|
|
|
2024 |
|
2023 |
|
2022 |
|
|
|
|
|
|
|
|
|
| Foreign currency exchange contracts |
|
Cost of revenue |
|
$ |
5,932 |
|
|
$ |
3,512 |
|
|
$ |
25,733 |
|
|
|
Provision for income taxes |
|
(1,696) |
|
|
(1,068) |
|
|
(6,742) |
|
| Gains, net of tax |
|
Net income |
|
$ |
4,236 |
|
|
$ |
2,444 |
|
|
$ |
18,991 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Interest rate swap contract |
|
Interest expense |
|
$ |
3,268 |
|
|
$ |
2,656 |
|
|
$ |
— |
|
|
|
Provision for income taxes |
|
(777) |
|
|
(631) |
|
|
— |
|
| Gain, net of tax |
|
Net income |
|
$ |
2,491 |
|
|
$ |
2,025 |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Investments |
|
General and administrative expenses |
|
$ |
(214) |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
Provision for income taxes |
|
51 |
|
|
— |
|
|
— |
|
| Loss, net of tax |
|
Net income |
|
$ |
(163) |
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Defined benefit plans |
|
Cost of revenue and operating expenses |
|
$ |
(506) |
|
|
$ |
(630) |
|
|
$ |
(605) |
|
|
|
Provision for income taxes |
|
154 |
|
|
111 |
|
|
99 |
|
| Loss, net of tax |
|
Net income |
|
$ |
(352) |
|
|
$ |
(519) |
|
|
$ |
(506) |
|
NOTE 22. PREFERRED STOCK
Our Board of Directors is authorized, subject to any limitations prescribed by law, without further stockholder approval, to issue from time to time up to 500,000 shares of Preferred Stock, $1.00 par value per share (“Preferred Stock”), in one or more series. Each such series of Preferred Stock shall have such number of shares, designations, preferences, voting powers, qualifications, and special or relative rights or privileges as shall be determined by the Board of Directors, which may include, among others, dividend rights, voting rights, redemption and sinking fund provisions, liquidation preferences, conversion rights, and preemptive rights. There were no shares of Preferred Stock outstanding as of December 31, 2024, and 2023.
NOTE 23. IDEXX RETIREMENT AND INCENTIVE SAVINGS PLAN
We have established the IDEXX Retirement and Incentive Savings Plan (the “401(k) Plan”). U.S. employees eligible to participate in the 401(k) Plan may contribute specified percentages of their salaries. We match a portion of these contributions, not to exceed 5% of participants’ eligible compensation. We matched $31.0 million, $30.3 million, and $28.3 million for the years ended December 31, 2024, 2023, and 2022, respectively. In addition, we may make contributions to the 401(k) Plan at the discretion of the Board of Directors. There were no discretionary contributions in 2024, 2023, or 2022.
We also have established defined contribution plans for regional employees in Europe and in Canada. With respect to these plans, our contributions over the past three years have not been material.
Defined Benefit Pension Obligations
Our Swiss defined benefit pension plans (“Swiss Plans”) are government-mandated retirement plans that provide employees with a minimum investment return. We account for our Swiss Plans in accordance with ASC 715-30, “Defined Benefit Plans - Pension.” As of December 31, 2024, our Swiss Plans had an unfunded net pension obligation of $5.7 million, with a fair value of plan assets of $15.1 million. The investments of the plan assets are measured using a mix of Level 1, Level 2, and Level 3 inputs. For the year ended December 31, 2024, we recognized $1.3 million in expense related to the Swiss Plans. The expense was reflected in cost of revenue, sales & marketing expense, general and administrative expense, and research and development expense, based on employee classification.
Future benefits expected to be paid as of December 31, 2024, are as follows:
|
|
|
|
|
|
| (in thousands) |
December 31,
2024 |
|
|
2025 |
$ |
888 |
|
2026 |
$ |
981 |
|
2027 |
$ |
917 |
|
2028 |
$ |
1,063 |
|
2029 |
$ |
951 |
|
2030 through 2034 |
$ |
6,564 |
|
EXHIBIT INDEX
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incorporated by Reference |
| Exhibit No. |
Exhibit Description |
Form |
Exhibit |
Filing Date / Period End Date |
Filed / Furnished Herewith |
|
|
|
|
|
|
| Articles of incorporation and by-laws |
|
|
|
|
|
|
|
|
10-Q |
3(i) |
6/30/06 |
|
|
|
|
|
|
|
|
|
8-K |
3.2 |
7/15/22 |
|
|
|
|
|
|
|
| Instruments defining the rights of security holders, including indenture |
|
|
|
|
|
|
|
|
10-K |
4.1 |
12/31/19 |
|
|
|
|
|
|
|
|
|
8-K |
99.1 |
12/12/13 |
|
|
|
|
|
|
|
|
Note Purchase and Private Shelf Agreement, dated as of July 21, 2014, among the Company, as issuer, Prudential Investment Management, Inc., Pruco Life Insurance Company, The Prudential Insurance Company of America, The Gibraltar Life Insurance Co., Ltd., PAR U Hartford Life Insurance Comfort Trust, The Independent Order of Foresters, Zurich American Insurance Company, Globe Life and Accident Insurance Company, Family Heritage Life Insurance Company of America, MTL Insurance Company, The Lincoln National Life Insurance Company, William Penn Life Insurance Company of New York, Farmers Insurance Exchange and Mid Century Insurance Company, as purchasers |
8-K |
99.1 |
7/25/14 |
|
|
|
|
|
|
|
|
|
8-K |
99.2 |
7/25/14 |
|
|
|
|
|
|
|
|
|
8-K |
10.4 |
4/16/20 |
|
|
|
|
|
|
|
|
|
8-K |
10.5 |
4/16/20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amended and Restated Multi-Currency Note Purchase and Private Shelf Agreement, dated as of June 18, 2015, among the Company, Prudential Investment Management, Inc., Pruco Life Insurance Company, The Prudential Insurance Company of America, The Gibraltar Life Insurance Co., Ltd., PAR U Hartford Life Insurance Comfort Trust, The Independent Order of Foresters, Zurich American Insurance Company, Globe Life and Accident Insurance Company, Family Heritage Life Insurance Company of America, MTL Insurance Company, The Lincoln National Life Insurance Company, William Penn Life Insurance Company of New York, Farmers Insurance Exchange, Mid Century Insurance Company and Farmers New World Life Insurance Company, as purchasers |
8-K |
99.1 |
6/24/15 |
|
|
|
|
|
|
|
|
|
8-K |
10.2 |
4/16/20 |
|
|
|
|
|
|
|
|
|
8-K |
10.3 |
4/16/20 |
|
|
|
|
|
|
|
|
Multicurrency Note Purchase and Private Shelf Agreement, dated as of December 19, 2014, among the Company, as issuer, and Metropolitan Life Insurance Company, MetLife Insurance Company USA, Symetra Life Insurance Company, MetLife Insurance K.K., AXIS Reinsurance Company, and Union Fidelity Life Insurance Company, as purchasers |
8-K |
10.1 |
3/15/19 |
|
|
|
|
|
|
|
|
First Amendment to Multicurrency Note Purchase and Private Shelf Agreement, dated March 14, 2019, among the Company, as issuer, and IDEXX Distribution, Inc., IDEXX Operations, Inc., and OPTI Medical Systems, Inc., each as a subsidiary guarantor, and Metropolitan Life Insurance Company, MetLife Reinsurance Company of Bermuda, Ltd., Brighthouse Life Insurance Company, Symetra Life Insurance Company, and AXIS Reinsurance Company |
8-K |
10.2 |
3/15/19 |
|
|
|
|
|
|
|
|
|
8-K |
10.1 |
3/27/20 |
|
|
|
|
|
|
|
| Material contracts |
|
|
|
|
|
|
|
|
10-K |
10.1 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.2 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.4 |
12/31/07 |
|
|
|
|
|
|
|
|
|
10-K |
10.5 |
12/31/07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
10.5 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.6 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.27 |
12/31/17 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
9/30/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.9 |
12/31/21 |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
10-K |
10.10 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.11 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.8 |
12/31/07 |
|
|
|
|
|
|
|
|
|
10-K |
10.13 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.14 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-Q |
10.2 |
9/30/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.16 |
12/31/21 |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
10-Q |
10.1 |
9/30/02 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
3/31/20 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
10.19 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
9/30/20 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
9/30/23 |
|
|
|
|
|
|
|
|
|
10-Q |
10.3 |
6/30/13 |
|
|
|
|
|
|
|
|
|
10-K |
10.22 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-Q |
10.3 |
6/30/10 |
|
|
|
|
|
|
|
|
|
10-K |
10.19 |
12/31/20 |
|
|
|
|
|
|
|
|
|
S-8 |
99.1 |
12/30/13 |
|
|
|
|
|
|
|
|
|
DEF14A |
Appendix |
3/28/18 |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
10-K |
10.28 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.29 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.21 |
12/31/09 |
|
|
|
|
|
|
|
|
|
10-K |
10.20 |
12/31/15 |
|
|
|
|
|
|
|
|
|
10-K |
10.25 |
12/31/18 |
|
|
|
|
|
|
|
|
|
10-K |
10.28 |
12/31/19 |
|
|
|
|
|
|
|
|
|
10-K |
10.34 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.26 |
12/31/18 |
|
|
|
|
|
|
|
|
|
10-K |
10.32 |
12/31/19 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
3/31/21 |
|
|
|
|
|
|
|
|
|
10-Q |
10.2 |
3/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.41 |
12/31/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
10.42 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.43 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.44 |
12/31/21 |
|
|
|
|
|
|
|
|
|
10-K |
10.27 |
12/31/18 |
|
|
|
|
|
|
|
|
|
8-K |
10.3 |
10/24/19 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
3/31/22 |
|
|
|
|
|
|
|
|
Third Amended and Restated Credit Agreement, dated as of April 14, 2020, among the Company, IDEXX Distribution, Inc., IDEXX Operations, Inc., OPTI Medical Systems, Inc., IDEXX Laboratories Canada Corporation, IDEXX Europe B.V., and IDEXX Holding B.V., as borrowers, the lenders party thereto, JPMorgan Chase Bank, N.A., as administrative agent, JPMorgan Chase Bank, N.A., Toronto Branch, as Toronto agent, and the other parties thereto |
8-K |
10.6 |
4/16/20 |
|
|
|
|
|
|
|
|
Fourth Amended and Restated Credit Agreement, dated as of December 9, 2021, among the Company, IDEXX Distribution, Inc., IDEXX Operations, Inc., OPTI Medical Systems, Inc., IDEXX Laboratories Canada Corporation, IDEXX B.V., IDEXX Laboratories B.V., and IDEXX Laboratories GmbH, as borrowers, the lenders party thereto, JPMorgan Chase Bank, N.A., as administrative agent, JPMorgan Chase Bank, N.A., Toronto Branch, as Toronto agent, and the other parties thereto |
8-K |
10.1 |
12/9/21 |
|
|
|
|
|
|
|
|
Amendment No. 1, dated as of October 20, 2022, to the Fourth Amended and Restated Credit Agreement, among the Company, IDEXX Distribution, Inc., IDEXX Operations, Inc., OPTI Medical Systems, Inc., IDEXX Laboratories Canada Corporation, IDEXX B.V., IDEXX Laboratories B.V., and IDEXX Laboratories GmbH, as borrowers, the lenders party thereto, JPMorgan Chase Bank, N.A., as administrative agent, JPMorgan Chase Bank, N.A., Toronto Branch, as Toronto agent, and the other parties thereto |
8-K |
10.1 |
10/20/22 |
|
|
|
|
|
|
|
|
|
10-K |
10.50 |
12/31/23 |
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
Suspension of Rights Agreement dated May 2, 2024, to the Fourth Amended and Restated Credit Agreement, by and among the Borrowers, JPMorgan Chase Bank, N.A., as Administrative Agent, JPMorgan Chase Bank, N.A., Toronto Branch, as Toronto Agent and the Lenders from time to time party thereto |
10-Q |
10.2 |
06/30/24 |
|
|
|
|
|
|
|
|
|
10-Q |
10.1 |
09/30/24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
8-K |
10.1 |
05/06/24 |
|
|
|
|
|
|
|
| Insider Trading Policy |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
| Subsidiaries of the registrant |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
| Consent of Independent Registered Public Accounting Firm |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
| Rule 13a-14(a)/15-14(a) certifications |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
| Section 1350 certifications |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
Policy relating to recovery of erroneously awarded compensation |
|
|
|
|
|
|
|
|
10-K |
97.1 |
12/31/23 |
|
|
|
|
|
|
|
| Interactive data file |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 101 |
The following financial and related information from IDEXX Laboratories, Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024, formatted in Inline eXtensible Business Reportable Language (iXBRL) includes: (i) the Consolidated Balance Sheet; (ii) the Consolidated Statement of Income; (iii) the Consolidated Statements of Comprehensive Income; (iv) the Consolidated Statement of Changes in Stockholders' Equity (Deficit); (v) the Consolidated Statement of Cash Flows; and, (vi) Notes to Consolidated Financial Statements. |
|
|
|
|
|
|
|
|
|
|
| 104 |
The cover page from the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2024, formatted in Inline XBRL and contained in Exhibit 101. |
|
|
|
|
|
|
|
|
|
|
| * |
Certain portions have been omitted as confidential information. |
|
|
|
|
|
|
|
|
|
|
| ** |
Management contract or compensatory arrangement required to be filed as an exhibit pursuant to Item 15(a)(3) of Form 10-K. |
|
|
|
|
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
|
|
IDEXX LABORATORIES, INC. |
|
|
|
By: /s/ Jonathan J. Mazelsky |
Date: February 21, 2025 |
Jonathan J. Mazelsky |
|
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| SIGNATURE |
|
|
TITLE |
|
DATE |
|
|
|
|
|
| /s/ Jonathan J. Mazelsky |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
February 21, 2025 |
| Jonathan J. Mazelsky |
|
|
|
|
|
|
|
|
| /s/ Brian P. McKeon |
|
Executive Vice President, Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
|
February 21, 2025 |
| Brian P. McKeon |
|
|
|
|
|
|
|
|
| /s/ Lawrence D. Kingsley |
|
Non-Executive Board Chair |
|
February 21, 2025 |
| Lawrence D. Kingsley |
|
|
|
|
|
|
|
|
|
/s/ Irene Chang Britt |
|
Director |
|
February 21, 2025 |
Irene Chang Britt |
|
|
|
|
|
|
|
|
|
| /s/ Bruce L. Claflin |
|
Director |
|
February 21, 2025 |
| Bruce L. Claflin |
|
|
|
|
|
|
|
|
|
| /s/ Stuart M. Essig, PhD |
|
Director |
|
February 21, 2025 |
| Stuart M. Essig, PhD |
|
|
|
|
|
|
|
|
|
| /s/ Daniel M. Junius |
|
Director |
|
February 21, 2025 |
| Daniel M. Junius |
|
|
|
|
|
|
|
|
|
| /s/ Sam A. Samad |
|
Director |
|
February 21, 2025 |
| Sam A. Samad |
|
|
|
|
|
|
|
|
|
| /s/ M. Anne Szostak |
|
Director |
|
February 21, 2025 |
| M. Anne Szostak |
|
|
|
|
|
|
|
|
|
| /s/ Sophie V. Vandebroek, PhD |
|
Director |
|
February 21, 2025 |
| Sophie V. Vandebroek, PhD |
|
|
|
|
EX-10.10
2
exhibit1010.htm
EX-10.10
Document
EXHIBIT 10.10
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY ASTERISKS [**], HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
AMENDMENT NO. 9
THIS AMENDMENT NO. 9 (“Amendment”), effective as of October 31, 2024 (the “Amendment Effective Date”), is between IDEXX Operations, Inc., a Delaware corporation whose principal place of business is at 6100 East Shelby Drive, Memphis Tennessee 38141, U.S.A. (“IDEXX”) and Ortho-Clinical Diagnostics, Inc., a New York corporation with offices at 100 Indigo Creek Drive, Rochester, New York, U.S.A. (“OCD” or “Ortho”).
WHEREAS, Ortho and IDEXX have entered into an agreement effective as of October 16, 2003, which was amended by Amendment Nos. 1 to 8 and the Letter Agreement dated July 28, 2023 (the “2023 Letter Agreement”), regarding supply by Ortho of dry slides for IDEXX veterinary chemistry analyzers (the agreement as amended, including by the 2023 Letter Agreement, collectively, the “Agreement”); and
WHEREAS, the Parties now desire to extend the term of the Agreement and amend the Agreement;
The Parties agree as follows:
1.All capitalized defined terms used in this Amendment but not defined in it have the meanings defined in the Agreement.
2.The definition of “Europe Agreement” in Section 1 of the Agreement is amended to mean the Agreement effective October 17, 2003, between IDEXX B.V. and Ortho, including all amendments thereto.
3.The following is added to Section 1 of the Agreement: IDEXX and Ortho also are each individually referred to as a “Party” and collectively as the “Parties.”
4.Sections 18.01 and 18.02 of the Agreement are deleted in their entirety and replaced with the following:
18.01
Unless terminated earlier pursuant to the terms of this Agreement, this Agreement continues through December 31, 2044; provided that, if either Party sends the other Party a notice of non-extension prior to or on [**] (“Non-Extension Notice”), then this Agreement only will continue through December 31 of the [**] from the date of the Non-Extension Notice (the “Term”). [**].
5. Section 21 of the Agreement is deleted in its entirety and replaced with the following (Section 21, including 21.01 through 21.06):
21. GOVERNING LAW; JURISDICTION; VENUE; PREVAILING PARTY; DAMAGE LIMITATIONS; EQUITABLE RELIEF
21.01 Governing Law. New York and United States law governs the construction, validity and performance of this Agreement and any dispute arising out of or relating to it without regard to conflict of laws principles.
21.02 Jurisdiction and Venue. The state and federal courts in New York, New York, will have exclusive jurisdiction over any dispute arising out of or relating to this Agreement. The Parties consent to personal jurisdiction and venue in those courts. Claims for misappropriation of trade secrets, intellectual property infringement, and breach of confidentiality obligations may also be brought in any court that has jurisdiction over the Parties if the relief sought includes injunctive or other non-monetary relief. A Party that obtains a judgment against the other Party in the courts identified in this Section may enforce that judgment in any court that has jurisdiction over the Parties.
21.03 Jury Trial Waiver. Any controversy that may arise under this Agreement is likely to involve complicated and difficult issues and, therefore, to the fullest extent permitted by applicable law, the Parties irrevocably and unconditionally waive any right that each of them may have to a trial by jury in any litigation or legal proceedings arising out of or relating to this Agreement. The Parties each understand and has considered the implications of this waiver, and each Party makes this waiver voluntarily.
21.04 Prevailing Party. In any litigation arising out of or related to this Agreement, the prevailing Party will be entitled to recover the costs and expenses, including attorneys’ fees, that it reasonably incurred in connection with that litigation.
21.05 [**].
(a) [**]
(i) [**]; or
(ii) [**].
[**].
[**].
(b) [**]. [**].
(c) [**].
(i) [**]:
(a)[**];
(b)[**];
(c)[**];
(d)[**]; or
(e)[**].
(ii) [**].
21.06 Equitable Relief. In connection with a breach or threatened breach of this Agreement, each Party has the right to seek equitable relief, in addition to any other rights and remedies that may be available to that Party at law, at equity, or otherwise.
6. Schedule 5 of the Agreement is deleted in entirety and replaced with the new Schedule 5 attached to this Amendment.
7. [**]. [**].
[**]
•[**];
•[**].
8. Except as modified by this Amendment, the Agreement remains in effect, [**]. This Amendment is to be construed as part of the Agreement. Each reference in the Agreement to “hereof”, “hereunder”, “herein,” “hereby,” the or this “Agreement,” and all other similar references in the Agreement, including any other amendments, from and after the date of this Amendment, is deemed to refer to the Agreement as amended by this Amendment.
9. The Agreement (as amended by this Amendment) constitutes the entire agreement between the Parties and supersedes all prior and contemporaneous agreements, understandings, conditions, representations, warranties, and communications between the Parties relating to the Agreement’s subject matter. The terms of this Amendment will prevail over different or conflicting provisions in the Agreement.
10. This Amendment may be signed electronically and in multiple counterparts, each of which is considered an original, but all of which constitute a single instrument.
*****
[Amendment Signature Page Follows]
[Amendment Signature Page]
IN WITNESS WHEREOF and intending to be legally bound, the Parties have caused this Amendment to be signed by their respective authorized representatives and to be effective as of the Amendment Effective Date.
ORTHO-CLINICAL DIAGNOSTICS, INC. IDEXX OPERATIONS, INC.
By: /s/ Brian Blaser By: /s/ Jeff Chadbourne
Name: Brian Blaser Name: Jeff Chadbourne
Title: President and CEO Title: SVP Finance
Date: Nov-01-2024 Date: Oct-24-2024
Acknowledged and consented to, solely as guarantor pursuant to Section 30 of the Agreement:
IDEXX LABORATORIES, INC.
By: /s/ Jay Mazelsky
Name: Jay Mazelsky
Title: President and CEO
Date: Oct-24-2024
EX-10.18
3
exhibit1018.htm
EX-10.18
Document
EXHIBIT 10.18
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY ASTERISKS [**], HAS BEEN OMITTED BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED.
AMENDMENT NO. 7
THIS AMENDMENT NO. 7 (“Amendment”), effective as of October 31, 2024 (the “Amendment Effective Date”), is between IDEXX B.V., a organized under the laws of the Netherlands (“IDEXX”) and Ortho-Clinical Diagnostics, Inc., a New York corporation with offices at 100 Indigo Creek Drive, Rochester, New York, U.S.A. (“OCD” or “Ortho”).
WHEREAS, Ortho and IDEXX have entered into an agreement effective as of October 16, 2003, which was amended by Amendment Nos. 1 to 6 and the Letter Agreement dated July 28, 2023 (the “2023 Letter Agreement”), regarding supply by Ortho of dry slides for IDEXX veterinary chemistry analyzers (the agreement as amended, including by the 2023 Letter Agreement, collectively, the “Agreement”); and
WHEREAS, the Parties now desire to extend the term of the Agreement and amend the Agreement;
The Parties agree as follows:
1.All capitalized defined terms used in this Amendment but not defined in it have the meanings defined in the Agreement.
2.The following is added to Section 1 of the Agreement: IDEXX and Ortho also are each individually referred to as a “Party” and collectively as the “Parties.”
3.Sections 18.01 and 18.02 of the Agreement are deleted in their entirety and replaced with the following:
18.01 Unless terminated earlier pursuant to the terms of this Agreement, this Agreement continues through December 31, 2044; provided that, if either Party sends the other Party a notice of non-extension prior to or on [**] (“Non-Extension Notice”), then this Agreement only will continue through December 31 of the [**] from the date of the Non-Extension Notice (the “Term”). [**].
4. Section 21 of the Agreement is deleted in its entirety and replaced with the following (Section 21, including 21.01 through 21.06):
21. GOVERNING LAW; JURISDICTION; VENUE; PREVAILING PARTY; DAMAGE LIMITATIONS; EQUITABLE RELIEF
21.01 Governing Law. New York and United States law governs the construction, validity and performance of this Agreement and any dispute arising out of or relating to it without regard to conflict of laws principles.
21.02 Jurisdiction and Venue. The state and federal courts in New York, New York, will have exclusive jurisdiction over any dispute arising out of or relating to this Agreement. The Parties consent to personal jurisdiction and venue in those courts. Claims for misappropriation of trade secrets, intellectual property infringement, and breach of confidentiality obligations may also be brought in any court that has jurisdiction over the Parties if the relief sought includes injunctive or other non-monetary relief. A Party that obtains a judgment against the other Party in the courts identified in this Section may enforce that judgment in any court that has jurisdiction over the Parties.
21.03 Jury Trial Waiver. Any controversy that may arise under this Agreement is likely to involve complicated and difficult issues and, therefore, to the fullest extent permitted by applicable law, the Parties irrevocably and unconditionally waive any right that each of them may have to a trial by jury in any litigation or legal proceedings arising out of or relating to this Agreement. The Parties each understand and has considered the implications of this waiver, and each Party makes this waiver voluntarily.
21.04 Prevailing Party. In any litigation arising out of or related to this Agreement, the prevailing Party will be entitled to recover the costs and expenses, including attorneys’ fees, that it reasonably incurred in connection with that litigation.
21.05 [**].
(a) [**]
(i) [**]; or
(ii) [**].
[**].
[**].
(b) [**]. [**].
(c) [**].
(i) [**]:
(a)[**];
(b)[**];
(c)[**];
(d)[**]; or
(e)[**].
(ii) [**].
21.06 Equitable Relief. In connection with a breach or threatened breach of this Agreement,each Party has the right to seek equitable relief, in addition to any other rights and remedies that may be available to that Party at law, at equity, or otherwise.
5. Schedule 5 of the Agreement is deleted in entirety and replaced with the new Schedule 5 attached to this Amendment.
6. [**]. [**].
[**]
•[**];
•[**].
7. Except as modified by this Amendment, the Agreement remains in effect, [**]. This Amendment is to be construed as part of the Agreement. Each reference in the Agreement to “hereof”, “hereunder”, “herein,” “hereby,” the or this “Agreement,” and all other similar references in the Agreement, including any other amendments, from and after the date of this Amendment, is deemed to refer to the Agreement as amended by this Amendment.
8. The Agreement (as amended by this Amendment) constitutes the entire agreement between the Parties and supersedes all prior and contemporaneous agreements, understandings, conditions, representations, warranties, and communications between the Parties relating to the Agreement’s subject matter. The terms of this Amendment will prevail over different or conflicting provisions in the Agreement.
9. This Amendment may be signed electronically and in multiple counterparts, each of which is considered an original, but all of which constitute a single instrument.
***** [Amendment Signature Page Follows]
[Amendment Signature Page]
IN WITNESS WHEREOF and intending to be legally bound, the Parties have caused this Amendment to be signed by their respective authorized representatives and to be effective as of the Amendment Effective Date.
ORTHO-CLINICAL DIAGNOSTICS, INC. IDEXX B.V.
By: /s/ Brian Blaser By: /s/ Jeff Chadbourne
Name: Brian Blaser Name: Jeff Chadbourne
Title: President and CEO Title: SVP Finance
Date: Nov-01-2024 Date: Oct-24-2024
Acknowledged and consented to, solely as guarantor pursuant to Section 30 of the Agreement:
IDEXX LABORATORIES, INC.
By: /s/ Jay Mazelsky
Name: Jay Mazelsky
Title: President and CEO
Date: Oct-24-2024
EX-10.30
4
exhibit1030.htm
EX-10.30
Document
Exhibit 10.30
IDEXX LABORATORIES, INC.
Executive Incentive Plan
Summary of Bonus Determination Process
As of Performance Year beginning 1/1/2025
Overview
Annual performance-based cash bonuses are paid to the individuals of the Executive Team, pursuant to our Executive Incentive Plan (the 'Executive Incentive Plan'), which program is adopted by the Compensation and Talent Committee historically in February of each year for application to the bonus opportunities of that calendar year. The Executive Team for this purpose includes the Chief Executive Officer and certain other executive officers and senior executives of the Company as designated by the Compensation and Talent Committee. Under the Executive Incentive Plan, the amount of each participating executive’s annual performance-based cash bonus is determined based on the overall performance factor, calculated in accordance with the description below.
Generally, in connection with the establishment of the performance goals under the Executive Incentive Plan relevant for that particular year, the Compensation and Talent Committee also determines, for each participant, the target annual performance-based cash bonus amount for that year, based on a percentage of the participant's annual base salary. The Compensation and Talent Committee may also determine, for each participant who commences employment with the Company, or is promoted in any year, the target annual performance-based cash bonus amount for such participant for that year, which may be prorated in the discretion of the Compensation and Talent Committee. The Compensation and Talent Committee may also limit the maximum amount of the annual performance-based cash bonus payable to any participant to 200% of the participant’s target bonus amount, and historically has done so.
The amount of the annual performance-based cash bonus payable to each participant under the Executive Incentive Plan is determined based upon that participant’s target bonus amount multiplied by an overall performance factor that is calculated using factors having the respective weights as determined by the Compensation and Talent Committee in its sole discretion at the time the factors are determined in February of each year, including:
|
|
|
|
|
|
|
|
| • |
A financial performance factor (determined by measuring Company performance against specific financial metrics selected by the Compensation and Talent Committee); and |
|
|
|
|
|
|
| • |
A non-financial performance factor (determined by measuring the Company’s achievement of non-financial performance goals approved by the Board of Directors that are focused on strengthening and positioning the Company for sustained future growth and profitability). |
Financial Performance Factor
The financial metrics used to calculate the financial performance factor are established annually by the Compensation and Talent Committee. These factors may include any one or more of the following corporate-wide or subsidiary, division, operating unit or individual measures: earnings before interest, taxes, depreciation and amortization (EBITDA), net cash provided by operating activities, free cash flow, earnings per share, earnings per share from continuing operations, operating income, net income, revenues, organic revenues, gross profit, operating margins, gross margins, return on operating assets, return on equity, economic value added, stock price appreciation, total stockholder return, growth rates, cost control, strategic initiatives, market share, product development, before- or after-tax income, attainment by a share of the Company’s common stock of a specified fair market value for a specified period, price-to-earnings growth or return on invested capital of the Company or a subsidiary, affiliate or division of the Company for or within which the Participant is primarily employed, or any other objective measure as deemed appropriate by the Compensation and Talent Committee. Metrics may each be weighted in the determination of the Compensation and Talent Committee at the time the metrics are determined in February of each year.
Each of the chosen metrics is subject to a rating calculated on a sliding scale, ranging from 25% to 200% (with no payout below threshold performance), using that year’s approved budget goal for the applicable metric as 100% of target payout.
Non-Financial Performance Factor
The non-financial performance factor is determined by the Compensation and Talent Committee by measuring achievement of annual goals approved by the Board of Directors and intended to strengthen the business to support long-term performance. Metrics utilized for this factor may include items such as implementation of certain operational initiatives, achievement in advancing functionality of business systems, execution of key management initiatives, goals focused on identified key business strategies, human capital management initiatives, corporate responsibility initiatives or other organizational support activities.
Overall and Individual Performance Factors
In accordance with the weightings applied to the financial and non-financial performance factors, as determined for the applicable year, the incentive plan design results in an overall performance factor for the participants in the Executive Incentive Plan. The Compensation and Talent Committee may also consider the relative contributions made by each participant to the achievement of the Company’s financial and non-financial goals, as well as other factors such as the scope of and tenure in their roles at the Company, in determining the final amount of each award. Subject to the Compensation and Talent Committee’s Charter, the Compensation and Talent Committee may delegate to the CEO the authority to determine the Annual Cash Bonus payable to any senior executive who is not an executive officer, subject to the maximum bonus percentage and any other parameters or conditions approved by the Compensation and Talent Committee.
Adjustments
Financial measures may be adjusted in the discretion of the Compensation and Talent Committee to include or eliminate, as applicable, (i) the effects of differences between actual foreign currency exchange rates and currency exchange rates reflected in the budget of the Company approved by the Board of Directors in or around December of the year prior to the relevant performance year; and (ii) the effects of discrete items including, but not limited to, restructuring charges, debt financing costs, acquisition purchase accounting adjustments, acquisition-related transaction costs and integration expenses, adjustments to finalized pre-acquisition contingencies, litigation-related expenses and payments, gains and losses on the disposition of assets or discontinued operations, non-cash write downs, other unusual, non-recurring or extraordinary items, or otherwise in the discretion of the Compensation and Talent Committee.
Similarly, the non-financial performance goals may be adjusted, in the discretion of the Compensation and Talent Committee, as the Compensation and Talent Committee considers to be necessary or appropriate in accordance with or related to any adjustments made to the financial goals, or as the facts and circumstances may warrant.
The above summary has been prepared for informational purposes only and shall not be deemed to be, and is not considered by the Company to be, a plan document. The process and mechanics of the bonus program described above are subject to change at any time in the discretion of the Compensation and Talent Committee and/or the Board of Directors, as applicable.
EX-10.53
5
exhibit1053.htm
EX-10.53
Document
EXHIBIT 10.53
IDEXX
Deferred Compensation Plan
Effective Date
December 6, 2024
IDEXX Deferred Compensation Plan
Article I
Establishment and Purpose 1
Article II
Definitions 1
Article III
Eligibility and Participation 6
Article IV
Deferrals 6
Article V
Company Contributions 11
Article VI
Payments from Accounts 12
Article VII
Valuation of Account Balances; Investments 15
Article VIII
Administration 16
Article IX
Amendment and Termination 18
Article X
Informal Funding 18
Article XI
Claims 19
Article XII
General Provisions 25
IDEXX Deferred Compensation Plan
Article I
Establishment and Purpose
IDEXX Laboratories, Inc. (the “Company”) has adopted this IDEXX Deferred Compensation Plan, applicable to Compensation deferred under Compensation Deferral Agreements submitted on and after the Effective Date and Company Contributions credited with respect to Plan Years commencing on or after the Effective Date.
The purpose of the Plan is to attract and retain key employees by providing them with an opportunity to defer receipt of a portion of their salary, bonus, and other specified compensation. The Plan is not intended to meet the qualification requirements of Code Section 401(a) but is intended to meet the requirements of Code Section 409A and shall be operated and interpreted consistent with that intent.
The Plan constitutes an unsecured promise by a Participating Employer to pay benefits in the future. Participants in the Plan shall have the status of general unsecured creditors of the Company or the Participating Employer, as applicable. Each Participating Employer shall be solely responsible for payment of the benefits attributable to services performed for it. The Plan is unfunded for Federal tax purposes and is intended to be an unfunded arrangement for eligible employees who are part of a select group of management or highly compensated employees of the Employer within the meaning of Sections 201(2), 301(a)(3) and 401(a)(1) of ERISA and independent contractors. Any amounts set aside to defray the liabilities assumed by the Company or a Participating Employer, as applicable, will remain the general assets of the Company or the Participating Employer, as applicable, and shall remain subject to the claims of the Company’s or the Participating Employer's creditors until such amounts are distributed to the Participants.
Article II
Definitions
2.1 Account. Account means a bookkeeping account maintained by the Committee to record the payment obligation of a Participating Employer to a Participant as determined under the terms of the Plan. The Committee may maintain an Account to record the total obligation to a Participant and component Accounts to reflect amounts payable at different times and in different forms. Subaccounts may be maintained for the purpose of tracking amount subject to different vesting schedules. Reference to an Account means any such Account established by the Committee, as the context requires. Accounts are intended to constitute unfunded obligations within the meaning of Sections 201(2), 301(a)(3) and 401(a)(1) of ERISA.
2.2 Account Balance. Account Balance means, with respect to any Account, the total payment obligation owed to a Participant from such Account as of the most recent Valuation Date.
2.3 Affiliate. Affiliate means a corporation, trade or business that, together with the Company, is treated as a single employer under Code Section 414(b) or (c).
IDEXX Deferred Compensation Plan
2.4 Beneficiary. Beneficiary means a natural person, estate, or trust designated by a Participant in accordance with Section 6.4 hereof to receive payments to which a Beneficiary is entitled in accordance with provisions of the Plan.
2.5 Board of Directors. Board of Directors means, for a Participating Employer organized as a corporation, its board of directors and for a Participating Employer organized as a limited liability company, its board of managers.
2.6 Business Day. Business Day means each day on which the New York Stock Exchange is open for business.
2.7 Change in Control. Change in Control means, with respect to a Participating Employer that is organized as a corporation, any of the following events: (i) a change in the ownership of the Participating Employer, (ii) a change in the effective control of the Participating Employer, or (iii) a change in the ownership of a substantial portion of the assets of the Participating Employer, in each case, as such terms are defined in Code Section 409A(a)(2)(A)(v). Whether a Change in Control has occurred will be determined in manner consistent with the requirements of Section 409A.
2.8 Claimant. Claimant means a Participant or Beneficiary filing a claim under Article XI of this Plan.
2.9 Code. Code means the Internal Revenue Code of 1986, as amended from time to time.
2.10 Code Section 409A. Code Section 409A means section 409A of the Code, and regulations and other guidance issued by the Treasury Department and Internal Revenue Service thereunder.
2.11 Committee. Committee means the Employee Plans Administrative Committee or such other committee appointed by the Company to administer the Plan.
2.12 Company. Company means IDEXX Laboratories, Inc., a Delaware corporation.
2.13 Company Contribution. Company Contribution means a credit by a Participating Employer to a Participant’s Account(s) in accordance with the provisions of Article V of the Plan. Unless the context clearly indicates otherwise, a reference to Company Contribution shall include Earnings attributable to such contribution.
2.14 Compensation. Compensation means a Participant’s salary, bonus, commission, and such other cash compensation approved by the Committee as Compensation that may be deferred under Section 4.2 of this Plan. Compensation may include Stock Units. Compensation excludes any compensation that has been previously deferred under this Plan or any other arrangement subject to Code Section 409A and excluding any compensation that is not U.S. source income.
IDEXX Deferred Compensation Plan
2.15 Compensation Deferral Agreement. Compensation Deferral Agreement means an agreement between a Participant and a Participating Employer that specifies: (i) the amount of each component of Compensation that the Participant has elected to defer to the Plan in accordance with the provisions of Article IV, (ii) the Payment Schedule applicable to the Retirement Account or one or more Flex Accounts established under such Compensation Deferral Agreement and (iii) the allocation of Deferrals among the Participant’s established Accounts.
2.16 Deferral. Deferral means a credit to a Participant’s Account(s) that records that portion of the Participant’s Compensation that the Participant has elected to defer to the Plan in accordance with the provisions of Article IV. Unless the context of the Plan clearly indicates otherwise, a reference to Deferrals includes Earnings attributable to such Deferrals.
2.17 Earnings. Earnings means an adjustment to the value of an Account in accordance with Article VII.
2.18 Effective Date. Effective Date means December 6, 2024.
2.19 Eligible Employee. Eligible Employee means an Employee who is a member of a select group of management or highly compensated employees or an independent contractor who has been notified during an applicable enrollment period of his or her status as an Eligible Employee. The Committee has the discretion to determine which Employees and independent contractors are Eligible Employees for each enrollment.
2.20 Employee. Employee means a common-law employee of an Employer.
2.21 Employer. Employer means the Company and each Affiliate.
2.22 ERISA. ERISA means the Employee Retirement Income Security Act of 1974, as amended from time to time.
2.23 Flex Account. Flex Account means a Separation Account or Specified Date Account (other than a Participant’s PSU Account(s) or RSU Account(s)) established under the terms of a Participant’s Compensation Deferral Agreement. Unless the Committee specifies otherwise during an applicable enrollment, a Participant may maintain no more than five (5) Flex Accounts at any one time.
2.24 Participant. Participant means an individual described in Article III.
IDEXX Deferred Compensation Plan
2.25 Participating Employer. Participating Employer means the Company and each Affiliate who has adopted the Plan with the consent of the Company. Each Participating Employer shall be identified on Schedule A attached hereto.
2.26 Payment Schedule. Payment Schedule means the calendar year when payment of the Retirement Account or any Flex Account will commence and the form in which payment of such Account will be made, as provided in Article VI.
2.27 Performance-Based Compensation. Performance-Based Compensation means Compensation where the amount of, or entitlement to, the Compensation is contingent on the satisfaction of pre-established organizational or individual performance criteria relating to a performance period of at least 12 consecutive months. Organizational or individual performance criteria are considered pre-established if established in writing by not later than 90 days after the commencement of the period of service to which the criteria relate, provided that the outcome is substantially uncertain at the time the criteria are established. Performance-Based Compensation shall not include any Compensation payable upon the Participant’s death or disability (as defined in Treas. Reg. Section 1.409A-1(e)) without regard to the satisfaction of the performance criteria.
2.28 Plan. Plan means “IDEXX Deferred Compensation Plan” as documented herein and as may be amended from time to time hereafter. However, to the extent permitted or required under Code Section 409A, the term Plan may in the appropriate context also mean a portion of the Plan that is treated as a single plan under Treas. Reg. Section 1.409A-1(c), or the Plan or portion of the Plan and any other nonqualified deferred compensation plan or portion thereof that is treated as a single plan under such section.
2.29 Plan Year. Plan Year means January 1 through December 31.
2.30 PSUs. PSUs mean restricted stock units granted in respect of Stock and subject to performance-based vesting conditions.
2.31 PSU Account. PSU Account means a dedicated Specified Date Account established under this Plan to record the deferred portion of a grant of PSUs.
2.32 Retirement Account. Retirement Account means an Account established by the Committee to record Company Contributions and Deferrals allocated to the Retirement Account pursuant to a Participant’s Compensation Deferral Agreement, payable to a Participant upon Separation from Service in accordance with Section 6.3.
2.33 RSUs. RSUs mean restricted stock units granted in respect of Stock and not subject to performance-based vesting conditions.
2.34 RSU Account. RSU Account means a dedicated Specified Date Account established under this Plan to record the deferred portion of a grant of RSUs.
IDEXX Deferred Compensation Plan
2.35 Separation Account. Separation Account means an Account established by the Committee in accordance with a Participant’s Compensation Deferral Agreement to record Deferrals allocated to such Account by the Participant and which are payable upon the Participant’s Separation from Service as set forth in Section 6.3.
2.36 Separation from Service. Separation from Service means an Employee’s termination of employment with the Employer and all Affiliates.
Except in the case of an Employee on a bona fide leave of absence as provided below, an Employee is deemed to have incurred a Separation from Service if the Employer and the Employee reasonably anticipated that the level of services to be performed by the Employee after a date certain would be reduced to 20% or less of the average services rendered by the Employee during the immediately preceding 36-month period (or the total period of employment, if less than 36 months), disregarding periods during which the Employee was on a bona fide leave of absence.
An Employee who is absent from work due to military leave, sick leave, or other bona fide leave of absence shall incur a Separation from Service on the first date immediately following the later of: (i) the date that is six months following the commencement of the leave, or (ii) the expiration of the Employee’s right, if any, to reemployment under statute or contract.
If a Participant ceases to provide services as an Employee and begins providing services as an independent contractor for the Employer, a Separation from Service shall occur only if the parties anticipate that the level of services to be provided as an independent contractor are such that a Separation from Service would have occurred if the Employee had continued to provide services at that level as an Employee. If, in accordance with the preceding sentence, no Separation from Service occurs as of the date the individual’s employment status changes, a Separation from Service shall occur thereafter only upon the first anniversary of the date all contracts with the Employer have expired, provided the Participant does not perform services for the Employer during that time.
For purposes of determining whether a Separation from Service has occurred, the Employer means the Employer as defined in Section 2.21 of the Plan, except that in applying Code sections 1563(a)(1), (2) and (3) for purposes of determining whether another organization is an Affiliate of the Company under Code Section 414(b), and in applying Treasury Regulation Section 1.414(c)-2 for purposes of determining whether another organization is an Affiliate of the Company under Code Section 414(c), “at least 50 percent” shall be used instead of “at least 80 percent” each place it appears in those sections.
2.37 Specified Date Account. Specified Date Account means an Account established by the Committee to record the amounts payable in a future calendar year as specified in the Participant’s Compensation Deferral Agreement. A Specified Date Account also means any PSU Account and any RSU Account.
IDEXX Deferred Compensation Plan
2.38 Stock. Stock means the Company’s common stock, $0.10 par value per share.
2.39 Stock Units. Stock Units mean RSUs or PSUs.
2.39 Unforeseeable Emergency. Unforeseeable Emergency means a severe financial hardship to the Participant resulting from an illness or accident of the Participant, the Participant’s spouse, the Participant’s dependent (as defined in Code Section 152, without regard to Sections 152(b)(1), (b)(2), and (d)(1)(B)), or a Beneficiary; loss of the Participant’s property due to casualty (including the need to rebuild a home following damage to a home not otherwise covered by insurance, for example, as a result of a natural disaster); or other similar extraordinary and unforeseeable circumstances arising as a result of events beyond the control of the Participant. The types of events which may qualify as an Unforeseeable Emergency may be limited by the Committee.
2.40 Valuation Date. Valuation Date means each Business Day.
Article III
Eligibility and Participation
3.1 Eligibility and Participation. All Eligible Employees may enroll in the Plan. Eligible Employees become Participants on the first to occur of (i) the date on which the first Compensation Deferral Agreement becomes irrevocable under Article IV, or (ii) the date Company Contributions are credited to an Account on behalf of such Eligible Employee.
3.2 Duration. Only Eligible Employees may submit Compensation Deferral Agreements during an enrollment period and receive Company Contributions during the Plan Year. A Participant who is no longer an Eligible Employee but has not incurred a Separation from Service will not be allowed to submit Compensation Deferral Agreements but may otherwise exercise all of the rights of a Participant under the Plan with respect to his or her Account(s). On and after a Separation from Service, a Participant shall remain a Participant as long as his or her Account Balance is greater than zero (0). All Participants, regardless of employment status, will continue to be credited with Earnings and during such time may continue to make allocation elections as provided in Section 7.4. An individual shall cease being a Participant in the Plan when his or her Account has been reduced to zero (0).
3.3 Rehires. An Eligible Employee who Separates from Service and who subsequently resumes performing services for an Employer in the same calendar year (regardless of eligibility) will have his or her Compensation Deferral Agreement for such year, if any, reinstated, but his or her eligibility to participate in the Plan in years subsequent to the year of rehire shall be governed by the provisions of Section 3.1.
IDEXX Deferred Compensation Plan
Article IV
Deferrals
1.1Deferral Elections, Generally.
(a)An Eligible Employee may make an initial election to defer Compensation by submitting a Compensation Deferral Agreement during the enrollment periods established by the Committee and in the manner specified by the Committee, but in any event, in accordance with Section 4.2. Unless an earlier date is specified in the Compensation Deferral Agreement, deferral elections with respect to a Compensation source (such as salary, bonus or other Compensation) become irrevocable on the latest date applicable to such Compensation source under Section 4.2.
(b)A Compensation Deferral Agreement that is not timely filed with respect to a service period or component of Compensation, or that is submitted by a Participant who Separates from Service prior to the latest date such agreement would become irrevocable under Section 409A, shall be considered null and void and shall not take effect with respect to such item of Compensation. The Committee may modify or revoke any Compensation Deferral Agreement prior to the date the election becomes irrevocable under the rules of Section 4.2.
(c)The Committee may permit different deferral amounts for each component of Compensation and may establish a minimum or maximum deferral amount for each such component. Unless otherwise specified by the Committee in the Compensation Deferral Agreement, Participants may defer a minimum of five percent (5%) and a maximum of fifty percent (50%) of their base compensation and a minimum of five percent (5%) and a maximum of one hundred percent (100%) of bonus, commissions, Stock Units or other Compensation. Deferrals of Stock Units will be made in whole units, with fractional units rounded down to the nearest whole unit before deferral.
(d)Deferrals of cash Compensation shall be calculated with respect to the gross cash Compensation payable to the Participant prior to any deductions or withholdings but shall be reduced by the Committee as necessary so as not to exceed 100% of the cash Compensation of the Participant remaining after deduction of all required income and employment taxes, required employee benefit deductions, deferrals to 401(k) plans and other deductions required by law. Changes to payroll withholdings that affect the amount of Compensation being deferred to the Plan shall be allowed only to the extent permissible under Code Section 409A.
(e)The Eligible Employee shall specify on his or her Compensation Deferral Agreement the amount of Deferrals and whether to allocate Deferrals to the Retirement Account, to one or more Flex Accounts or, in the case of Stock Units, to a dedicated PSU Account or RSU Account. If no designation is made, Deferrals shall be allocated to the Retirement Account.
IDEXX Deferred Compensation Plan
4.2 Timing Requirements for Compensation Deferral Agreements.
(a) Initial Eligibility. The Committee may permit an Eligible Employee to defer Compensation earned in the first year of eligibility. The Compensation Deferral Agreement must be filed within 30 days after attaining Eligible Employee status and becomes irrevocable not later than the 30th day.
A Compensation Deferral Agreement filed under this paragraph applies to Compensation related to service performed after the date on which the Compensation Deferral Agreement becomes irrevocable.
(b)Prior Year Election. Except as otherwise provided in this Section 4.2, the Committee may permit an Eligible Employee to defer Compensation by filing a Compensation Deferral Agreement no later than December 31 of the year prior to the year in which the services related to such Compensation commence. A Compensation Deferral Agreement filed under this paragraph shall become irrevocable with respect to such Compensation not later than the December 31 filing deadline.
(c)Performance-Based Compensation. The Committee may permit an Eligible Employee to defer Compensation which qualifies as Performance-Based Compensation by filing a Compensation Deferral Agreement no later than the date that is six months before the end of the applicable performance period, provided that:
(i)the Participant performs services continuously from the later of the beginning of the performance period or the date the performance criteria are established through the date the Compensation Deferral Agreement is submitted; and
(ii) the Compensation is not readily ascertainable as of the date the Compensation Deferral Agreement is filed.
Any election to defer Performance-Based Compensation that is made in accordance with this paragraph and that becomes payable as a result of the Participant’s death or disability (as defined in Treas. Reg. Section 1.409A-1(e)) or upon a change in control (as defined in Treas. Reg. Section 1.409A-3(i)(5)) prior to the satisfaction of the performance criteria, will be void, unless it would be considered timely under another rule described in this Section.
IDEXX Deferred Compensation Plan
(d)Certain Forfeitable Rights. With respect to a legally binding right to a payment in a subsequent year that is subject to a forfeiture condition requiring the Participant’s continued services for a period of at least 12 months from the date the Participant obtains the legally binding right, the Committee may permit an Eligible Employee to defer such Compensation by filing a Compensation Deferral Agreement on or before the 30th day after the legally binding right to the Compensation accrues, provided that the Compensation Deferral Agreement is submitted at least 12 months in advance of the earliest date on which the forfeiture condition could lapse. The Compensation Deferral Agreement described in this paragraph becomes irrevocable not later than such 30th day. If the forfeiture condition applicable to the payment lapses before the end of such 12-month period as a result of the Participant’s death or disability (as defined in Treas. Reg. Section 1.409A-3(i)(4)) or upon a change in control (as defined in Treas. Reg. Section 1.409A-3(i)(5)), the Compensation Deferral Agreement will be void, unless it would be considered timely under another rule described in this Section.
(e)“Evergreen” Deferral Elections. The Committee, in its discretion, may provide that Compensation Deferral Agreements will continue in effect for subsequent years or performance periods by communicating that intention to Participants in writing prior to the date Compensation Deferral Agreements become irrevocable under this Section 4.2. An evergreen Compensation Deferral Agreement may be revoked or modified in writing prospectively by the Participant or the Committee with respect to Compensation for which such election remains revocable under this Section 4.2.
A Compensation Deferral Agreement is deemed to be revoked for subsequent years if the Participant is not an Eligible Employee as of the last permissible date for making elections under this Section 4.2 or if the Compensation Deferral Agreement is cancelled in accordance with Section 4.4.
4.3 Allocation of Deferrals. A Compensation Deferral Agreement may allocate cash Deferrals to the Retirement Account or to one or more Flex Accounts. A Compensation Deferral Agreement may allocate Deferrals of Stock Units to the Retirement Account, a dedicated PSU Account (in the case of PSUs) or a dedicated RSU Account (in the case of RSUs). In the event a Participant’s Compensation Deferral Agreement allocates a component of Compensation to a Specified Date Account that commences payment in the year such Compensation is earned or, if later, the year it becomes 100% vested, the Compensation Deferral Agreement shall be deemed to allocate the Deferral to the Participant’s Specified Date Account having the next earliest payment year. If the Participant has no other Specified Date Accounts, the Committee will allocate the Deferral to the Retirement Account.
4.4 Cancellation of Deferrals. The Committee, in its sole discretion, may cancel a Participant’s Deferrals: (i) for the balance of the Plan Year in which an Unforeseeable
IDEXX Deferred Compensation Plan
Emergency occurs, and (ii) during periods in which the Participant is disabled (meaning the Participant is unable to perform the duties of his or her position or any substantially similar position due to a mental or physical impairment that can be expected to result in death or last for a continuous period of at least six months), provided cancellation occurs by the later of the end of the taxable year of the Participant or the 15th day of the third month following the date the Participant incurs the disability (as defined in this clause (ii)).
Article V
Company Contributions
5.1 Discretionary Company Contributions. A Participating Employer may, from time to time in its sole and absolute discretion, credit discretionary Company Contributions in the form of matching, profit-sharing or other contributions to any Participant in any amount determined by the Participating Employer. The fact that a discretionary Company Contribution is credited in one year shall not obligate the Participating Employer to continue to make such Company Contributions in subsequent years. Company Contributions are credited to the Participant’s Retirement Account.
Make-Up Matching Contribution. Company Contributions may take the form of “make-up” matching contributions, at the same matching contribution rate provided under the Company’s 401(k) plan with respect to cash Deferrals to the extent they reduce 401(k) plan compensation below the limitation set forth in Code Section 401(a)(17). A Participant is not required to make any elective deferrals to such 401(k) plan as a condition to receiving a make-up matching Company Contributions under this Plan. However, the Committee may require that a Participant must meet the same conditions for receiving a matching contribution under the 401(k) plan, including, for example, any requirement to be employed on the last day of the plan year.
Supplemental Matching Contribution. Company Contributions may take the form of “supplemental” matching contributions, at the same contribution rate provided under the Company’s 401(k) plan, applied to the portion of the Participant’s cash Compensation (including amounts deferred under this Plan) that exceeds the amount of compensation taken into account in determining the maximum amount of matching contribution under the terms of such 401(k) plan. A Participant is not required to make any elective deferrals to such 401(k) plan as a condition to receiving supplemental matching Company Contributions under this Plan. However, the Committee may require that a Participant must meet the same conditions for receiving a matching contribution under the 401(k) plan, including, for example, any requirement to be employed on the last day of the plan year.
Supplemental Non-Elective Contribution. Company Contributions may take the form of supplemental non-elective contributions at the same rate such non-elective contributions are made to a Participant’s tax-qualified profit-sharing plan account, applied to the portion of the Participant’s cash Compensation (including amounts deferred under this Plan) that exceeds the amount of compensation taken into account in determining the amount of the non-elective contribution under the terms of such profit-sharing plan.
IDEXX Deferred Compensation Plan
The Committee may require that a Participant must meet the same conditions for receiving a non-elective contribution under the profit-sharing plan, including, for example, any requirement to be employed on the last day of the plan year.
5.2 Vesting. Company Contributions vest according to the schedule specified by the Committee on or before the time the contributions are made. Make-up and supplemental contributions related to employer contributions to an Employer’s tax-qualified plan will vest at the same rate provided for the related contribution under such tax-qualified plan.
All Company Contributions become 100% vested, if while employed by an Employer, a Participant dies, the Participant is unable to perform the duties of his or her position or any substantially similar position due to a mental or physical impairment that can be expected to result in death or last for a continuous period of at least six months, his or her Employer experiences a change in control as determined by the Company or the Participant attains age 60. The Company retains the sole discretion to accelerate vesting for any Participant at any time prior to the commencement of payment from an Account.
Article VI
Payments from Accounts
6.1 General Rules. A Participant’s Accounts become payable upon the first to occur of the payment years or events applicable to such Account under Sections 6.2 (if elected) through 6.5.
Payment events and Payment Schedules elected by the Participant shall be set forth in a valid Compensation Deferral Agreement that establishes the Account to which such elections apply in accordance with Article IV or in a valid modification election applicable to such Account as described in Section 6.9.
Payment amounts are based on the vested Account Balances as of the first Valuation Date of the month in which actual payment will be made. In the case of an Account Balance for a PSU Account, RSU Account or other subaccount recording deferrals of Stock Units that are paid in Stock, the Account Balance refers to the number of units in such Account (plus any Earnings).
Notwithstanding anything to the contrary herein or in any Compensation Deferral Agreement, with respect to deferrals of Stock Units:
(a) the Plan and Compensation Deferral Agreements become effective with respect to Stock Units only to the extent the Stock Units are or become vested, and the Plan does not create any right to any payment or benefit with respect to any unvested Stock Units; and (b) the payment date will not, in any event, be earlier than the earliest date that the Stock Units would be settled under the agreement pursuant to which the Stock Units were granted, which is, in all cases, the earliest payment date permitted under the Plan and the applicable Compensation Deferral Agreement with respect to such Stock Units (and any associated Earnings).
IDEXX Deferred Compensation Plan
6.2 Specified Date Accounts.
Commencement. Payment of a Specified Date Account (other than a PSU Account or RSU Account) is made in the third calendar year following the Plan Year in which such Specified Date Account is established, unless the Participant elects a later calendar year.
PSU Accounts and RSU Accounts are payable in the fifth calendar year following the date of grant, unless the Participants elects a later year.
Form of Payment. Payment of a Specified Date Account (including PSU Accounts and RSU Accounts) will be made in a lump sum, unless the Participant elected to receive such Account in a designated number of annual installments not to exceed ten (10) installment payments.
6.3 Separation from Service.
(a) Lump Sum Payment When Separation from Service Occurs Prior to Age 60 or Within 24 Months Following a Change in Control. In the event that a Participant incurs a Separation from Service (i) prior to attaining age 60, other than due to the Participant’s death, or (ii) within 24 months following a Change in Control (regardless of attained age), the Participant will receive all unpaid vested Account Balances (regardless of prior payment commencement under Section 6.2) in a single lump sum in the calendar year next following the calendar year in which Separation from Service occurs, provided that actual payment may not occur earlier than the seventh month following the month in which the Participant incurs his or her Separation from Service.
(b) Payments When Separation from Service Occurs After Attaining Age 60. Upon a Participant’s Separation from Service on or after attaining age 60, other than due to the Participant’s death and other than a Separation from Service within 24 months following a Change in Control, the Participant is entitled to receive the Account Balance of his or her (i) Retirement Account, (ii) Separation Accounts, and (iii) all Specified Date Accounts that are Flex Accounts that commence payment under Section 6.2 in a calendar year after the calendar year in which Separation from Service occurs. For the avoidance of doubt, if the Participant’s Separation from Service occurs on or after attaining age 60, PSU Accounts and RSU Accounts are not paid as a result of the Participant’s Separation from Service but continue to pay according to the Payment Schedule elected under Section 6.2 and not under this Section 6.3(b).
IDEXX Deferred Compensation Plan
Commencement. The Participant’s Retirement Account and any Separation Account will be paid or commence payment in the calendar year next following the calendar year in which Separation from Service occurs, unless the Participant elected a later calendar year for an Account. In no event will actual payment be made earlier than the seventh month following the month in which the Participant incurs his or her Separation from Service. The Participant’s election for the Retirement Account will apply to all Specified Date Accounts that are Flex Accounts payable under this Section 6.3(b).
Form of Payment. Payment of the Participant’s Retirement Account and any Separation Account will be made in a lump sum, unless the Participant elected to receive such Account in a designated number of annual installments not to exceed ten (10) installment payments. All Specified Date Accounts payable under this Section 6.3(b) will be paid at the same time and in the same form of payment elected for the Retirement Account.
6.4 Death. Notwithstanding anything to the contrary in this Article VI, upon the death of the Participant (regardless of whether such Participant is an Employee at the time of death), all remaining vested Account Balances shall be paid to his or her Beneficiary in a single lump sum no later than December 31 of the calendar year following the year of the Participant’s death.
(a) Designation of Beneficiary in General. The Participant shall designate a Beneficiary in the manner and on such terms and conditions as the Committee may prescribe. No such designation shall become effective unless filed with the Committee during the Participant’s lifetime. Any designation shall remain in effect until a new designation is filed with the Committee; provided, however, that in the event a Participant designates his or her spouse as a Beneficiary, such designation shall be automatically revoked upon the dissolution of the marriage unless, following such dissolution, the Participant submits a new designation naming the former spouse as a Beneficiary. A Participant may from time to time change his or her designated Beneficiary without the consent of a previously-designated Beneficiary by filing a new designation with the Committee.
(b) No Beneficiary. If a designated Beneficiary does not survive the Participant, or if there is no valid Beneficiary designation, amounts payable under the Plan upon the death of the Participant shall be paid to the Participant’s spouse, or if there is no surviving spouse, then to the duly appointed and currently acting personal representative of the Participant’s estate.
IDEXX Deferred Compensation Plan
6.5 Unforeseeable Emergency. A Participant who experiences an Unforeseeable Emergency may submit a written request to the Committee to receive payment of all or any portion of his or her vested Accounts. If the emergency need cannot be relieved by cessation of Deferrals to the Plan, the Committee may approve an emergency payment therefrom not to exceed the amount reasonably necessary to satisfy the need, taking into account the additional compensation that is available to the Participant as the result of cancellation of deferrals to the Plan, including amounts necessary to pay any taxes or penalties that the Participant reasonably anticipates will result from the payment. The amount of the emergency payment shall be subtracted from the Retirement Account and any Separation Accounts pro rata until fully distributed, then from the Specified Date Accounts, starting with the Account having the latest commencement date until fully distributed, then continuing in this manner with the next latest Account until the full amount of the distribution is made. Emergency payments shall be paid in a single lump sum within the 90-day period following the date the Committee approves the payment. The Committee may specify under a uniform policy that Company Contributions may not be made available for distribution under this Section 6.5.
6.6 Administrative Cash-Out of Small Balances. Notwithstanding anything to the contrary in this Article VI, the Committee may at any time and without regard to whether a payment event has occurred, direct in writing (no later than the date of the payment) an immediate lump sum payment of the Participant’s Accounts if the balance of such Accounts, combined with any other amounts required to be treated as deferred under a single plan pursuant to Code Section 409A, does not exceed the applicable dollar amount under Code Section 402(g)(1)(B), provided any other such aggregated amounts are also distributed in a lump sum at the same time.
6.7 Acceleration of or Delay in Payments. Notwithstanding anything to the contrary in this Article VI, the Committee, in its sole and absolute discretion, may elect to accelerate the time or form of payment of an Account, provided such acceleration is permitted under Treas. Reg. Section 1.409A-3(j)(4). The Committee may also, in its sole and absolute discretion, delay the time for payment of an Account, to the extent permitted under Treas. Reg. Section 1.409A-2(b)(7).
6.8 Rules Applicable to Installment Payments. If a Payment Schedule specifies annual installment payments, payments will be made commencing in the designated calendar year for the applicable payment under this Article VI (as may be modified under Section 6.9) with subsequent installments paid in successive calendar years until the number of installment payments specified in the applicable Payment Schedule has been paid. The amount of each installment payment shall be determined by dividing (a) by (b), where (a) equals the vested Account Balance as of the first Valuation Date in the month actual payment will be made and (b) equals the remaining number of annual installment payments. Distributions of Stock will be based on the preceding formula, except the number of units in the Account shall be used instead of a dollar amount and any fractional units resulting from the preceding formula shall be retained in the Account. For purposes of Section 6.9, installment payments will be treated as a single payment. Accounts payable in installments will continue to be credited with Earnings in accordance with Article VII hereof until the Account is completely distributed.
IDEXX Deferred Compensation Plan
6.9 Modifications to Payment Schedules. A Participant may modify the Payment Schedule elected by him or her with respect to an Account, consistent with the permissible Payment Schedules available under the Plan for the applicable payment event, provided such modification complies with the requirements of this Section 6.9.
(a) Maximum of Two Elections Per Account. A Participant may submit no more than two Payment Schedule modifications per Account.
(b) Time of Election. The modification election must be submitted to the Committee not less than 12 months prior to the first day of the calendar year payments would have commenced under the Payment Schedule in effect prior to modification (the “Prior Election”).
(c) Date of Payment under Modified Payment Schedule. The date on which payments are to commence under the modified Payment Schedule must be no earlier than the fifth anniversary of the date payments would have commenced under the Prior Election. Under no circumstances may a modification election result in an acceleration of payments in violation of Code Section 409A. If the Participant modifies only the form, and not the commencement date for payment, payments shall commence in the fifth calendar year following the calendar year payment would have commenced under the Prior Election.
(d) Irrevocability; Effective Date. A modification election is irrevocable when filed and becomes effective 12 months after the filing date.
(e) Effect on Accounts. An election to modify a Payment Schedule is limited to the designated Account(s) and payment time or event to which such Payment Schedule applies and shall not be construed to affect any Payment Schedule for an alternative payment time or event applicable to such Account(s) or any Payment Schedule applicable to any other Account.
Article VII
Valuation of Account Balances; Investments
7.1 Valuation. Deferrals shall be credited to appropriate Accounts as of the date such Compensation would have been paid to the Participant absent the Compensation Deferral Agreement. Valuation of Accounts shall be performed under procedures approved by the Committee.
7.2 Earnings Credit. Each Account will be credited with Earnings on each Business Day, based upon the Participant’s investment allocation among a menu of investment options selected in advance by the Committee, in accordance with the provisions of this Article VII (“investment allocation”).
IDEXX Deferred Compensation Plan
7.3 Investment Options. The Committee will determine investment options. The Committee, in its sole discretion, shall be permitted to add or remove investment options from the Plan menu from time to time, provided that any such additions or removals of investment options shall not be effective with respect to any period prior to the effective date of such change.
7.4 Investment Allocations. A Participant’s investment allocation constitutes a deemed, not actual, investment among the investment options comprising the investment menu. At no time shall a Participant have any real or beneficial ownership in any investment option included in the investment menu, nor shall the Participating Employer or any trustee acting on its behalf have any obligation to purchase actual securities as a result of a Participant’s investment allocation. A Participant’s investment allocation shall be used solely for purposes of adjusting the value of a Participant’s Account Balances.
A Participant shall specify an investment allocation for each of his or her Accounts in accordance with procedures established by the Committee. Allocation among the investment options must be designated in increments of 1%. The Participant’s investment allocation will become effective on the same Business Day or, in the case of investment allocations received after a time specified by the Committee, the next Business Day.
A Participant may change an investment allocation on any Business Day, both with respect to future credits to the Plan and with respect to existing Account Balances, in accordance with procedures adopted by the Committee. Changes shall become effective on the same Business Day or, in the case of investment allocations received after a time specified by the Committee, the next Business Day, and shall be applied prospectively.
7.5 Unallocated Deferrals and Accounts. If the Participant fails to make an investment allocation with respect to an Account, such Account shall be invested in an investment option, the primary objective of which is the preservation of capital, as determined by the Committee.
7.6 Company Stock. Deferrals of Stock Units will be credited to a Participant’s Account in the designated number of units with each unit equal in value to one share of Stock. A Participant may not allocate Stock Units to another investment option under the Plan. A Participant may not allocate cash Deferrals into units of Stock.
Dividend equivalents payable with respect to Stock Units will be credited as provided in the equity compensation plan and award agreement under which such units were granted and treated as Earnings for employment tax purposes and for purposes of determining the time and form of payment of such dividend equivalents from the Plan. Notwithstanding the foregoing, dividend equivalents declared and payable prior to the vesting date of the related Stock Units will not be deferred under this Plan.
IDEXX Deferred Compensation Plan
7.7 Valuations Final After 180 Days. The Participant shall have 180 days following the Valuation Date on which the Participant failed to receive the full amount of Earnings and to file a claim under Article XI for the correction of such error.
Article VIII
Administration
8.1 Plan Administration. This Plan shall be administered by the Committee which shall have discretionary authority to make, amend, interpret and enforce all appropriate rules and regulations for the administration of this Plan and to utilize its discretion to decide or resolve any and all questions, including but not limited to eligibility for benefits and interpretations of this Plan and its terms, as may arise in connection with the Plan. Claims for benefits shall be filed with the Committee and resolved in accordance with the claims procedures in Article XI.
8.2 Administration Upon Change in Control. Upon a change in control affecting the Company, the Committee, as constituted immediately prior to such change in control, shall continue to act as the Committee. The Committee, by a vote of a majority of its members, shall have the authority (but shall not be obligated) to appoint an independent third party to act as the Committee. For purposes of this Section 8.2, a “change in control” means a change in control within the meaning of the rabbi trust agreement associated with the Plan or if no such definition is provided, the term shall have the meaning under Code Section 409A.
Upon such change in control, the Company may not remove the Committee or its members, unless a majority of Participants and Beneficiaries with Account Balances consent to the removal and replacement of the Committee. Notwithstanding the foregoing, the Committee shall not have authority to direct investment of trust assets under any rabbi trust described in Section 10.2.
The Participating Employers shall, with respect to the Committee identified under this Section: (i) pay all reasonable expenses and fees of the Committee, (ii) indemnify the Committee (including individuals serving as Committee members) against any costs, expenses and liabilities including, without limitation, attorneys’ fees and expenses arising in connection with the performance of the Committee’s duties hereunder, except with respect to matters resulting from the Committee’s gross negligence or willful misconduct, and (iii) supply full and timely information to the Committee on all matters related to the Plan, any rabbi trust, Participants, Beneficiaries and Accounts as the Committee may reasonably require.
8.3 Withholding. The Participating Employer shall have the right to withhold from any payment due under the Plan (or with respect to any amounts credited to the Plan) any taxes required by law to be withheld in respect of such payment (or credit). Withholdings with respect to amounts credited to the Plan shall be deducted from Compensation that has not been deferred to the Plan.
IDEXX Deferred Compensation Plan
8.4 Indemnification. The Participating Employers shall indemnify and hold harmless each employee, officer, director, agent or organization, to whom or to which are delegated duties, responsibilities, and authority under the Plan or otherwise with respect to administration of the Plan, including, without limitation, the Committee, its delegees and its agents, against all claims, liabilities, fines and penalties, and all expenses reasonably incurred by or imposed upon him or it (including but not limited to reasonable attorney fees) which arise as a result of his, her or its actions or failure to act in connection with the operation and administration of the Plan to the extent lawfully allowable and to the extent that such claim, liability, fine, penalty, or expense is not paid for by liability insurance purchased or paid for by the Participating Employer. Notwithstanding the foregoing, the Participating Employer shall not indemnify any person or organization if his, her or its actions or failure to act are due to gross negligence or willful misconduct or for any such amount incurred through any settlement or compromise of any action, unless the Participating Employer consents in writing to such settlement or compromise.
8.5 Delegation of Authority. In the administration of this Plan, the Committee may, from time to time, employ agents and delegate to them such administrative duties as it sees fit, and may from time to time consult with legal counsel who shall be legal counsel to the Company.
8.6 Binding Decisions or Actions. The decision or action of the Committee in respect of any question arising out of or in connection with the administration, interpretation and application of the Plan and the rules and regulations thereunder shall be final and conclusive and binding upon all persons having any interest in the Plan.
Article IX
Amendment and Termination
9.1 Amendment and Termination. The Company may at any time and from time to time amend the Plan or may terminate the Plan as provided in this Article IX. Each Participating Employer may also terminate its participation in the Plan.
9.2 Amendments. The Company, by action taken by its Board of Directors, may amend the Plan at any time and for any reason, provided that any such amendment shall not reduce the vested Account Balances of any Participant accrued as of the date of any such amendment or restatement (as if the Participant had incurred a voluntary Separation from Service on such date). The Board of Directors of the Company may delegate to the Compensation and Talent Committee of the Board of Directors (the “C&T Committee”) the authority to amend the Plan without the consent of the Board of Directors for the purpose of: (i) conforming the Plan to the requirements of law; (ii) facilitating the administration of the Plan; (iii) clarifying provisions based on the C&T Committee’s interpretation of the Plan documents; and (iv) making such other amendments as the Board of Directors may authorize. No amendment is needed to revise the list of Participating Employers set forth on Schedule A attached hereto.
IDEXX Deferred Compensation Plan
9.3 Termination. The Company, by action taken by its Board of Directors, may terminate the Plan and pay Participants and Beneficiaries their Account Balances in a single lump sum at any time, to the extent and in accordance with Treas. Reg. Section 1.409A-3(j)(4)(ix).
9.4 Accounts Taxable Under Code Section 409A. The Plan is intended to constitute a plan of deferred compensation that meets the requirements for deferral of income taxation under Code Section 409A. The Committee, pursuant to its authority to interpret the Plan, may sever from the Plan or any Compensation Deferral Agreement any provision or exercise of a right that otherwise would result in a violation of Code Section 409A.
Article X
Informal Funding
10.1 General Assets. Obligations established under the terms of the Plan may be satisfied from the general funds of the Participating Employers, or a trust described in this Article X. No Participant, spouse or Beneficiary shall have any right, title or interest whatever in assets of the Participating Employers. Nothing contained in this Plan, and no action taken pursuant to its provisions, shall create or be construed to create a trust of any kind, or a fiduciary relationship, between the Participating Employers and any Employee, spouse, or Beneficiary. To the extent that any person acquires a right to receive payments hereunder, such rights are no greater than the right of an unsecured general creditor of the Participating Employer.
10.2 Rabbi Trust. A Participating Employer may, in its sole discretion, establish a grantor trust, commonly known as a rabbi trust, as a vehicle for accumulating assets to pay benefits under the Plan. Payments under the Plan may be paid from the general assets of the Participating Employer or from the assets of any such rabbi trust. Payment from any such source shall reduce the obligation owed to the Participant or Beneficiary under the Plan.
If a rabbi trust is in existence upon the occurrence of a “change in control,” as defined in such trust, the Participating Employer shall, upon such change in control, and on each anniversary of the change in control, contribute in cash or liquid securities such amounts as are necessary so that the value of assets after making the contributions exceed 100% of the total value of all Account Balances.
Article XI
Claims
IDEXX Deferred Compensation Plan
11.1 Filing a Claim. Any controversy or claim arising out of or relating to the Plan shall be filed in writing with the Committee which shall, in turn, deliver such claim to the C&T Committee. The C&T Committee shall make all determinations concerning such claim. Any claim filed with the Committee and any decision by the C&T Committee denying such claim shall be in writing and shall be delivered to the Participant or Beneficiary filing the claim (the “Claimant”). Notice of a claim for payments shall be delivered to the Committee within 90 days of the latest date upon which the payment could have been timely made in accordance with the terms of the Plan and Code Section 409A, and if not paid, the Participant or Beneficiary must file a claim under this Article XI not later than 180 days after such latest date. If the Participant or Beneficiary fails to file a timely claim, the Participant forfeits any amounts to which he or she may have been entitled to receive under the claim.
(a) In General. Notice of a denial of benefits (other than claims based on disability) will be provided within 90 days of the Committee’s receipt of the Claimant’s claim for benefits. If the C&T Committee determines that it needs additional time to review the claim, the C&T Committee will provide the Claimant with a notice of the extension before the end of the initial 90-day period. The extension will not be more than 90 days from the end of the initial 90-day period and the notice of extension will explain the special circumstances that require the extension and the date by which the C&T Committee expects to make a decision.
(b) Disability Benefits. Notice of denial of claims based on disability will be provided within forty-five (45) days of the Committee’s receipt of the Claimant’s claim for disability benefits. If the C&T Committee determines that it needs additional time to review the disability claim, the C&T Committee will provide the Claimant with a notice of the extension before the end of the initial 45-day period. If the C&T Committee determines that a decision cannot be made within the first extension period due to matters beyond the control of the C&T Committee, the time period for making a determination may be further extended for an additional 30 days. If such an additional extension is necessary, the C&T Committee shall notify the Claimant prior to the expiration of the initial 30-day extension. Any notice of extension shall indicate the circumstances necessitating the extension of time, the date by which the C&T Committee expects to furnish a notice of decision, the specific standards on which such entitlement to a benefit is based, the unresolved issues that prevent a decision on the claim and any additional information needed to resolve those issues. A Claimant will be provided a minimum of 45 days to submit any necessary additional information to the C&T Committee. In the event that a 30-day extension is necessary due to a Claimant’s failure to submit information necessary to decide a claim, the period for furnishing a notice of decision shall be tolled from the date on which the notice of the extension is sent to the Claimant until the earlier of the date the Claimant responds to the request for additional information or the response deadline.
IDEXX Deferred Compensation Plan
(c) Contents of Notice. If a claim for benefits is completely or partially denied, notice of such denial shall be in writing. Any electronic notification shall comply with the standards imposed by Department of Labor Regulation 29 CFR 2520.104b-1(c)(1)(i), (iii), and (iv). The notice of denial shall set forth the specific reasons for denial in plain language. The notice shall: (i) cite the pertinent provisions of the Plan document, and (ii) explain, where appropriate, how the Claimant can perfect the claim, including a description of any additional material or information necessary to complete the claim and why such material or information is necessary. The claim denial also shall include an explanation of the claims review procedures and the time limits applicable to such procedures, including the right to appeal the decision, the deadline by which such appeal must be filed and a statement of the Claimant’s right to bring a civil action under Section 502(a) of ERISA following an adverse decision on appeal and the specific date by which such a civil action must commence under Section 11.4. In the case of a complete or partial denial of a disability benefit claim, the notice shall provide such information and shall be communicated in the manner required under applicable Department of Labor regulations.
11.2 Appeal of Denied Claims. A Claimant whose claim has been completely or partially denied shall be entitled to appeal the claim denial by filing a written appeal with the Committee, which shall, in turn deliver such appeal to the C&T Committee (the “Appeals Committee”). A Claimant who timely requests a review of the denied claim (or his or her authorized representative) may review, upon request and free of charge, copies of all documents, records and other information relevant to the denial and may submit written comments, documents, records and other information relating to the claim to the Committee. All written comments, documents, records, and other information shall be considered “relevant” if the information: (i) was relied upon in making a benefits determination, (ii) was submitted, considered or generated in the course of making a benefits decision regardless of whether it was relied upon to make the decision, or (iii) demonstrates compliance with administrative processes and safeguards established for making benefit decisions. The review shall consider all comments, documents, records, and other information submitted by the Claimant relating to the claim, without regard to whether such information was submitted or considered in the initial benefit determination. The Appeals Committee may, in its sole discretion and if it deems appropriate or necessary, decide to hold a hearing with respect to the claim appeal.
(a)In General. Appeal of a denied benefits claim (other than a disability benefits claim) must be filed in writing with the Committee no later than 60 days after receipt of the written notification of such claim denial. The Appeals Committee shall make its decision regarding the merits of the denied claim within 60 days following receipt of the appeal by the Committee (or within 120 days after such receipt, in a case where there are special circumstances requiring extension of time for reviewing the appealed claim). If an extension of time for reviewing the appeal is required because of special circumstances, written notice of the extension shall be furnished to the Claimant prior to the commencement of the extension. The notice will indicate the special circumstances requiring the extension of time and the date by which the Appeals Committee expects to render the determination on review. The review will consider comments, documents, records and other information submitted by the Claimant relating to the claim without regard to whether such information was submitted or considered in the initial benefit determination.
IDEXX Deferred Compensation Plan
(b)Disability Benefits. Appeal of a denied disability benefits claim must be filed in writing with the Committee no later than 180 days after receipt of the written notification of such claim denial. The review shall be conducted in accordance with applicable Department of Labor regulations.
The Appeals Committee shall make its decision regarding the merits of the denied claim within 45 days following receipt of the appeal by the Committee (or within 90 days after such receipt, in a case where there are special circumstances requiring extension of time for reviewing the appealed claim). If an extension of time for reviewing the appeal is required because of special circumstances, written notice of the extension shall be furnished to the Claimant prior to the commencement of the extension. The notice will indicate the special circumstances requiring the extension of time and the date by which the Appeals Committee expects to render the determination on review. Following its review of any additional information submitted by the Claimant, the Appeals Committee shall render a decision on its review of the denied claim.
(c)Contents of Notice. If a benefits claim is completely or partially denied on review, notice of such denial shall be in writing. Any electronic notification shall comply with the standards imposed by Department of Labor Regulation 29 CFR 2520.104b-1(c)(1)(i), (iii), and (iv). Such notice shall set forth the reasons for denial in plain language.
The decision on review shall set forth: (i) the specific reason or reasons for the denial, (ii) specific references to the pertinent Plan provisions on which the denial is based, (iii) a statement that the Claimant is entitled to receive, upon request and free of charge, reasonable access to and copies of all documents, records, or other information relevant (as defined above) to the Claimant’s claim, and (iv) a statement of the Claimant’s right to bring an action under Section 502(a) of ERISA, following an adverse decision on review and the specific date by which such a civil action must commence under Section 11.4.
For the denial of a disability benefit, the notice will also include such additional information and be communicated in the manner required under applicable Department of Labor regulations.
11.3 Claims Appeals Upon Change in Control. Upon a change in control, the Appeals Committee, as constituted immediately prior to such change in control, shall continue to act as the Appeals Committee. The Company may not remove any member of the
IDEXX Deferred Compensation Plan
Appeals Committee but may replace resigning members if 2/3rds of the members of the Board of Directors of the Company and a majority of Participants and Beneficiaries with Account Balances consent to the replacement. For purposes of this Section 11.3, a “change in control” means a change in control within the meaning of the rabbi trust agreement associated with the Plan or if no such definition is provided, the term shall have the meaning under Code Section 409A.
The Appeals Committee shall have the exclusive authority at the appeals stage to interpret the terms of the Plan and resolve appeals under the Claims Procedure.
Each Participating Employer shall, with respect to the Appeals Committee identified under this Section: (i) pay its proportionate share of all reasonable expenses and fees of the Appeals Committee, (ii) indemnify the Appeals Committee (including individual committee members) against any costs, expenses and liabilities including, without limitation, attorneys’ fees and expenses arising in connection with the performance of the Appeals Committee hereunder, except with respect to matters resulting from the Appeals Committee’s gross negligence or willful misconduct, and (iii) supply full and timely information to the Appeals Committee on all matters related to the Plan, any rabbi trust, Participants, Beneficiaries and Accounts as the Appeals Committee may reasonably require.
11.4 Legal Action. A Claimant may not bring any legal action, including commencement of any arbitration, relating to a claim for benefits under the Plan unless and until the Claimant has followed the claims procedures under the Plan and exhausted his or her administrative remedies under Sections 11.1 and 11.2. No such legal action may be brought more than twelve (12) months following the notice of denial of benefits under Section 11.2, or if no appeal is filed by the applicable appeals deadline, twelve (12) months following the appeals deadline.
If a Participant or Beneficiary prevails in a legal proceeding brought under the Plan to enforce the rights of such Participant or any other similarly situated Participant or Beneficiary, in whole or in part, the Participating Employer shall reimburse such Participant or Beneficiary for all legal costs, expenses, attorneys’ fees and such other liabilities incurred as a result of such proceedings. If the legal proceeding is brought in connection with a change in control as defined in Section 11.3, the Participant or Beneficiary may file a claim directly with the trustee for reimbursement of such costs, expenses and fees. For purposes of the preceding sentence, the amount of the claim shall be treated as if it were an addition to the Participant’s or Beneficiary’s Account Balance and will be included in determining the Participating Employer’s trust funding obligation under Section 10.2.
11.5 Discretion of Appeals Committee. All interpretations, determinations and decisions of the Appeals Committee with respect to any claim shall be made in its sole discretion and shall be final and conclusive.
IDEXX Deferred Compensation Plan
11.6 Arbitration.
(a)Prior to Change in Control. If, prior to a change in control as defined in Section 11.3, any claim or controversy between a Participating Employer and a Participant or Beneficiary is not resolved through the claims procedure set forth in Article XI, such claim shall be submitted to and resolved exclusively by expedited binding arbitration by a single arbitrator. Arbitration shall be conducted in accordance with the following procedures:
The complaining party shall promptly send written notice to the other party identifying the matter in dispute and the proposed remedy. Following the giving of such notice, the parties shall meet and attempt in good faith to resolve the matter. In the event the parties are unable to resolve the matter within 21 days, the parties shall meet and attempt in good faith to select a single arbitrator acceptable to both parties. If a single arbitrator is not selected by mutual consent within ten Business Days following the giving of the written notice of dispute, an arbitrator shall be selected from a list of nine persons each of whom shall be an attorney who is either engaged in the active practice of law or recognized arbitrator and who, in either event, is experienced in serving as an arbitrator in disputes between employers and employees, which list shall be provided by the main office of either JAMS, the American Arbitration Association (“AAA”) or the Federal Mediation and Conciliation Service. If, within three Business Days of the parties’ receipt of such list, the parties are unable to agree on an arbitrator from the list, then the parties shall each strike names alternatively from the list, with the first to strike being determined by the flip of a coin. After each party has had four strikes, the remaining name on the list shall be the arbitrator. If such person is unable to serve for any reason, the parties shall repeat this process until an arbitrator is selected.
Unless the parties agree otherwise, within 60 days of the selection of the arbitrator, a hearing shall be conducted before such arbitrator at a time and a place agreed upon by the parties. In the event the parties are unable to agree upon the time or place of the arbitration, the time and place shall be designated by the arbitrator after consultation with the parties. Within 30 days of the conclusion of the arbitration hearing, the arbitrator shall issue an award, accompanied by a written decision explaining the basis for the arbitrator’s award.
In any arbitration hereunder, the Participating Employer shall pay all administrative fees of the arbitration and all fees of the arbitrator, except that the Participant or Beneficiary may, if he/she/it wishes, pay up to one-half of those amounts. Each party shall pay its own attorneys’ fees, costs, and expenses, unless the arbitrator orders otherwise. The prevailing party in such arbitration, as determined by the arbitrator, and in any enforcement or other court proceedings, shall be entitled, to the extent permitted by law, to reimbursement from the other party for all of the prevailing party’s costs (including but not limited to the arbitrator’s compensation), expenses, and attorneys’ fees.
IDEXX Deferred Compensation Plan
The arbitrator shall have no authority to add to or to modify this Plan, shall apply all applicable law, and shall have no lesser and no greater remedial authority than would a court of law resolving the same claim or controversy. The arbitrator shall, upon an appropriate motion, dismiss any claim without an evidentiary hearing if the party bringing the motion establishes that it would be entitled to summary judgment if the matter had been pursued in court litigation.
The parties shall be entitled to discovery as follows: Each party may take no more than three depositions. The Participating Employer may depose the Participant or Beneficiary plus two other witnesses, and the Participant or Beneficiary may depose the Participating Employer, pursuant to Rule 30(b)(6) of the Federal Rules of Civil Procedure, plus two other witnesses. Each party may make such reasonable document discovery requests as are allowed in the discretion of the arbitrator.
The decision of the arbitrator shall be final, binding, and non-appealable, and may be enforced as a final judgment in any court of competent jurisdiction.
This arbitration provision of the Plan shall extend to claims against any parent, subsidiary, or affiliate of each party, and, when acting within such capacity, any officer, director, shareholder, Participant, Beneficiary, or agent of any party, or of any of the above, and shall apply as well to claims arising out of state and federal statutes and local ordinances as well as to claims arising under the common law or under this Plan.
Notwithstanding the foregoing, and unless otherwise agreed between the parties, either party may apply to a court for provisional relief, including a temporary restraining order or preliminary injunction, on the ground that the arbitration award to which the applicant may be entitled may be rendered ineffectual without provisional relief.
Any arbitration hereunder shall be conducted in accordance with the Federal Arbitration Act: provided, however, that, in the event of any inconsistency between the rules and procedures of the Act and the terms of this Plan, the terms of this Plan shall prevail.
If any of the provisions of this Section 11.6(a) are determined to be unlawful or otherwise unenforceable, in the whole part, such determination shall not affect the validity of the remainder of this section and this section shall be reformed to the extent necessary to carry out its provisions to the greatest extent possible and to insure that the resolution of all conflicts between the parties, including those arising out of statutory claims, shall be resolved by neutral, binding arbitration.
IDEXX Deferred Compensation Plan
If a court should find that the provisions of this Section 11.6(a) are not absolutely binding, then the parties intend any arbitration decision and award to be fully admissible in evidence in any subsequent action, given great weight by any finder of fact and treated as determinative to the maximum extent permitted by law.
The parties do not agree to arbitrate any putative class action or any other representative action. The parties agree to arbitrate only the claims(s) of a single Participant or Beneficiary.
(b)Upon Change in Control. Upon a change in control as defined in Section 11.3, Section 11.6(a) shall not apply and any legal action initiated by a Participant or Beneficiary to enforce his or her rights under the Plan may be brought in any court of competent jurisdiction. Notwithstanding the Appeals Committee’s discretion under Sections 11.3 and 11.5, the court shall apply a de novo standard of review to any prior claims decision under Sections 11.1 through 11.3 or any other determination made by the Company, its Board of Directors, a Participating Employer, the Committee, the C&T Committee, or the Appeals Committee.
Article XII
General Provisions
12.1 Assignment. No interest of any Participant, spouse or Beneficiary under this Plan and no benefit payable hereunder shall be assigned as security for a loan, and any such purported assignment shall be null, void and of no effect, nor shall any such interest or any such benefit be subject in any manner, either voluntarily or involuntarily, to anticipation, sale, transfer, assignment or encumbrance by or through any Participant, spouse or Beneficiary. Notwithstanding anything to the contrary herein, however, the Committee has the discretion to make payments to an alternate payee in accordance with the terms of a domestic relations order (as defined in Code Section 414(p)(1)(B)).
The Company may assign any or all of its liabilities under this Plan in connection with any restructuring, recapitalization, sale of assets or other similar transactions affecting a Participating Employer without the consent of the Participant.
12.2 No Legal or Equitable Rights or Interest. No Participant or other person shall have any legal or equitable rights or interest in this Plan that are not expressly granted in this Plan. Participation in this Plan does not give any person any right to be retained in the service of the Participating Employer. The right and power of a Participating Employer to dismiss or discharge an Employee is expressly reserved. The Participating Employers make no representations or warranties as to the tax consequences to a Participant or a Participant’s beneficiaries resulting from a deferral of income pursuant to the Plan.
12.3 No Employment Contract. Nothing contained herein shall be construed to constitute a contract of employment between an Employee and a Participating Employer.
IDEXX Deferred Compensation Plan
12.4 Notice. Any notice or filing required or permitted to be delivered to the Committee under this Plan shall be delivered in writing, in person, or through such electronic means as is established by the Committee. Notice shall be deemed given as of the date of delivery or, if delivery is made by mail, as of the date shown on the postmark on the receipt for registration or certification. Written transmission shall be sent by certified mail to:
IDEXX LABORATORIES, INC.
ONE IDEXX DRIVE
WESTBROOK, ME 04092
ATTN: HUMAN RESOURCES
Any notice or filing required or permitted to be given to a Participant under this Plan shall be sufficient if in writing or hand-delivered or sent by mail to the last known address of the Participant.
12.5 Headings. The headings of Sections are included solely for convenience of reference, and if there is any conflict between such headings and the text of this Plan, the text shall control.
12.6 Invalid or Unenforceable Provisions. If any provision of this Plan shall be held invalid or unenforceable, such invalidity or unenforceability shall not affect any other provisions hereof and the Committee may elect in its sole discretion to construe such invalid or unenforceable provisions in a manner that conforms to applicable law or as if such provisions, to the extent invalid or unenforceable, had not been included.
12.7 Facility of Payment to a Minor. If a distribution is to be made to a minor, or to a person who is otherwise incompetent, then the Committee may, in its discretion, make such distribution: (i) to the legal guardian, or if none, to a parent of a minor payee with whom the payee maintains his or her residence, or (ii) to the conservator or committee or, if none, to the person having custody of an incompetent payee. Any such distribution shall fully discharge the Committee, the Company, and the Plan from further liability on account thereof.
12.8 Governing Law. To the extent not preempted by ERISA, the laws of the State of Maine shall govern the construction and administration of the Plan.
12.9 Compliance With Code Section 409A; No Guarantee. This Plan is intended to be administered in compliance with Code Section 409A and each provision of the Plan shall be interpreted consistent with Code Section 409A. Although intended to comply with Code Section 409A, this Plan shall not constitute a guarantee to any Participant or Beneficiary that the Plan in form or in operation will result in the deferral of federal or state income tax liabilities or that the Participant or Beneficiary will not be subject to the additional taxes imposed under Section 409A. None of the Board of Directors, the Committee, the C&T Committee, or any Employer shall have any legal obligation to a Participant with respect to taxes imposed under Code Section 409A.
IDEXX Deferred Compensation Plan
12.10 Clawback/Recoupment. Notwithstanding any other provisions in the Plan or any other agreement between the Company and the Participant to the contrary, the Plan, Accounts and any amounts paid hereunder shall be subject to repayment or forfeiture by the Participant to the Company in accordance with the terms and conditions of the Company’s Amended & Restated Clawback Policy or any other “clawback” or mandatory recoupment policy adopted by the Company from time to time.
IN WITNESS WHEREOF, the undersigned executed this Plan as of the 19 day of December, 2024, to be effective as of the Effective Date.
IDEXX LABORATORIES, INC.
By: Michael Johnson (Print Name)
Its: CHRO (Title)
/s/ Michael Johnson (Signature)
IDEXX Deferred Compensation Plan
Schedule A
Participating Employers
IDEXX Laboratories, Inc.
IDEXX Operations, Inc.
IDEXX Distribution, Inc.
OPTI Medical Systems, Inc.
IDEXX Holdings, Inc.
EX-10.56
6
exhibit1056.htm
EX-10.56
Document
Exhibit 10.56
FORM OF CHANGE IN CONTROL AGREEMENT
THIS CHANGE IN CONTROL AGREEMENT is made as of [ ], 20[ ] (this “Agreement”), by and between IDEXX Laboratories, Inc., a Delaware corporation (the “Company”), and (the “Executive”) [and amends and restates in its entirety the [Amended and Restated] Executive Employment Agreement by and between the Company and the Executive dated as of [ ]].
The Compensation and Talent Committee of the Board of Directors of the Company (the “Committee”) has determined that it is in the best interests of the Company and its shareholders to assure that the Company will have the continued dedication of the Executive, notwithstanding the possibility, threat or occurrence of a Change in Control (as defined below) of the Company. The Committee believes it is imperative to diminish the inevitable distraction of the Executive by virtue of the personal uncertainties and risks created by a pending or threatened Change in Control and to encourage the Executive’s full attention and dedication to the Company currently and in the event of any threatened or pending Change in Control, and to provide the Executive with compensation and benefits arrangements upon a Change in Control which ensure that the compensation and benefits expectations of the Executive will be satisfied and which are competitive with those of other corporations. Therefore, in order to accomplish these objectives and in consideration of the mutual covenants and promises contained in this Agreement, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged by the parties to this Agreement, the Company and Executive agree as follows:
1.Certain Definitions.
(a)“Effective Date” shall mean the first date during the Change in Control Period (as defined below) on which a Change in Control occurs. Anything in this Agreement to the contrary notwithstanding, if a Change in Control occurs and if the Executive’s employment with the Company is terminated prior to the date on which the Change in Control occurs, and if it is reasonably demonstrated by the Executive that such termination of employment (i) was at the request of a third party who has taken steps reasonably calculated to effect a Change in Control or (ii) otherwise arose in connection with or anticipation of a Change in Control, then for all purposes of this Agreement the “Effective Date” shall mean the date immediately prior to the date of such termination of employment.
(b)“Change in Control” shall have the meaning ascribed to such term in the Company’s 2018 Stock Incentive Plan, as amended or amended and restated from time to time. Notwithstanding the foregoing, for any payments or benefits hereunder that are subject to Section 409A of the Internal Revenue Code of 1986, as amended (the “Code”), a Change in Control must constitute a “change in control event” within the meaning of Treasury Regulation Section 1.409A-3(i)(5)(i).
(c)“Change in Control Period” shall mean the period commencing on the date hereof and ending on December 31, 2024; provided, that on each anniversary of such date (such date and each anniversary thereof shall be hereinafter referred to as the “Renewal Date”), unless previously terminated, the Change in Control Period shall be automatically extended so as to terminate one year from such Renewal Date, unless at least 120 days prior to the Renewal Date the Company shall give notice to the Executive that the Change in Control Period shall not be so extended.
2.Employment Period. The Company hereby agrees to continue the Executive in its employ, and the Executive hereby agrees to remain in the employ of the Company subject to the terms and conditions of this Agreement, for the period commencing on the Effective Date and ending on the earlier of (a) the second anniversary of such date or (b) the termination of the Executive’s employment pursuant to Section 4 hereof (the “Employment Period”). Except as provided in Section 1(a), nothing in this Agreement shall, prior to the Effective Date, impose upon the Company any obligation to retain the Executive as an employee. In addition, nothing in this Agreement shall restrict the Executive from terminating the Executive’s employment with the Company, and no such termination by the Executive shall be deemed a breach of this Agreement.
3.Terms of Employment.
(a)Position and Duties.
(i)During the Employment Period, (A) the Executive’s position (including status, offices, titles and reporting requirements), authority, duties and responsibilities shall be at least commensurate in all material respects with the most significant of those held, exercised and assigned at any time during the 120-day period immediately preceding the Effective Date and (B) the Executive’s services shall be performed at the location where the Executive was employed immediately preceding the Effective Date or any office or location that does not increase the Executive’s one-way commute by more than 35 miles.
(ii)During the Employment Period, and excluding any periods of vacation and sick leave to which the Executive is entitled, the Executive agrees to devote reasonable attention and time during normal business hours to the business and affairs of the Company and, to the extent necessary to discharge the responsibilities assigned to the Executive hereunder, to use the Executive’s reasonable best efforts to perform faithfully and efficiently such responsibilities. During the Employment Period it shall not be a violation of this Agreement for the Executive to (A) serve on corporate, civic or charitable boards or committees, (B) deliver lectures, fulfill speaking engagements or teach at educational institutions, and (C) manage personal investments, so long as such activities do not significantly interfere with the performance of the Executive’s responsibilities as an employee of the Company or the terms of this Agreement. It is expressly understood and agreed that, to the extent that any such activities have been conducted by the Executive prior to the Effective Date, the continued conduct of such activities (or the conduct of activities similar in nature and scope thereto) subsequent to the Effective Date shall not thereafter be deemed to interfere with the performance of the Executive’s responsibilities to the Company.
(b)Compensation.
(i)Base Salary. During the Employment Period, the Executive shall receive an annual base salary (“Annual Base Salary”), which shall be paid at a monthly rate, at least equal to twelve times the highest monthly base salary paid or payable, including any base salary which has been earned but deferred, to the Executive by the Company and its Affiliated Companies (as defined below) in respect of the 12-month period immediately preceding the month in which the Effective Date occurs. During the Employment Period, the Annual Base Salary shall be reviewed no more than 12 months after the last salary increase awarded to the Executive prior to the Effective Date and thereafter at least annually. Any increase in Annual Base Salary shall not serve to limit or reduce any other obligation to the Executive under this Agreement. The Executive’s Annual Base Salary shall not be reduced after any such increase, and the term “Annual Base Salary,” as utilized in this Agreement, shall refer to the Executive’s Annual Base Salary as so increased. As used in this Agreement, the term “Affiliated Companies” shall include any company controlled by, controlling or under common control with the Company.
(ii)Annual Bonus. In addition to Annual Base Salary, during the Employment Period, the Executive shall be entitled to receive such annual bonus as may be determined by the Board of Directors of the Company (the “Board”) or the Committee, as the case may be, but in no event shall the target bonus opportunity, expressed as a percentage of Annual Base Salary, be less than the target bonus opportunity in respect of the full fiscal year immediately preceding the Effective Date. Any annual bonus shall be paid no later than March 15 of the year following the year in which such bonus is earned; provided, that the Executive must be employed by the Company on the date of payment to be entitled to receive an Annual Bonus.
(iii)Incentive Plans. During the Employment Period, the Executive shall be entitled to participate in all incentive plans, practices, policies and programs applicable generally to other peer executives of the Company and its Affiliated Companies, but in no event shall such plans, practices, policies and programs provide the Executive with benefits which are less favorable, in the aggregate, than the most favorable of such plans, practices, policies and programs in effect for the Executive at any time during the 120-day period immediately preceding the Effective Date or, if more favorable to the Executive, those provided generally at any time after the Effective Date to other peer executives of the Company and its Affiliated Companies.
(iv)Welfare Benefit, Savings and Retirement Plans. During the Employment Period, the Executive and/or the Executive’s family, as the case may be, shall be eligible for participation in and shall receive all benefits under welfare benefit, savings and retirement plans, practices, policies and programs provided by the Company and its Affiliated Companies (including, without limitation, medical, prescription, dental, disability, employee life, group life, split-dollar life, accidental death and travel accident insurance plans and programs) to the extent applicable generally to other peer executives of the Company, but in no event shall such plans, practices, policies and programs provide the Executive with benefits which are less favorable, in the aggregate, than the most favorable of such plans, practices, policies and programs in effect for the Executive at any time during the 120-day period immediately preceding the Effective Date or, if more favorable to the Executive, those provided generally at any time after the Effective Date to other peer executives of the Company and its Affiliated Companies.
(v)Expenses. During the Employment Period, the Executive shall be entitled to receive reimbursement for all reasonable expenses incurred by the Executive in accordance with the policies, practices and procedures of the Company in effect immediately prior to the Effective Date.
(vi)Vacation. During the Employment Period, the Executive shall be entitled to paid vacation in accordance with the plans, policies, programs and practices of the Company and its Affiliated Companies, but in no event shall such plans, practices, policies and programs provide the Executive with benefits which are less favorable, in the aggregate, than the most favorable of such plans, practices, policies and programs in effect for the Executive at any time during the 120-day period immediately preceding the Effective Date or, if more favorable to the Executive, those provided generally at any time after the Effective Date to other peer executives of the Company and its Affiliated Companies.
(c)Equity Awards.
(i)Time-Based Awards. Immediately prior to the consummation of a Change in Control, each then-outstanding award for common stock of the Company, including without limitation any stock option, stock appreciation right, restricted stock unit award, restricted stock award or other stock-based award, subject only to time-based vesting conditions (each, a “Time-Based Award”), held by the Executive shall become immediately exercisable, vested, realizable, or deliverable, or free from restrictions applicable to the Award as to 25% of the number of shares as to which each such Time-Based Award would otherwise be subject to restrictions or not then be exercisable, vested, realizable, or deliverable (rounded down to the nearest whole share) (such shares, the “Accelerated Shares”), and the number of shares as to which each such Time-Based Award shall become exercisable, vested, realizable, deliverable and free from restrictions on each vesting date set forth in the Executive’s applicable award agreement shall be reduced proportionately by the Accelerated Shares. In addition, all such Time-Based Awards held by the Executive shall immediately become fully exercisable, vested, realizable, deliverable and free from restrictions if and when, within 24 months after a Change in Control, the Executive’s employment with the Company (or the acquiring or succeeding entity) is involuntarily terminated by the Company (or such acquiring or succeeding entity) other than for Cause (as defined below) or is terminated by the Executive for Good Reason (as defined below). Notwithstanding the provisions of this Section 3(c)(i), if any such outstanding Time-Based Award is terminated in connection with a Change in Control, such award shall become fully exercisable, vested, realizable, deliverable and free from restrictions immediately before the occurrence of the Change in Control.
(ii)Performance-Based Awards. Notwithstanding anything to the contrary herein, each then-outstanding award for common stock of the Company, including without limitation any stock option, stock appreciation right, restricted stock unit award, restricted stock award or other stock-based award, subject to performance-based vesting conditions (each, a “Performance-Based Award”), held by the Executive shall be subject to the terms and conditions set forth in the award agreement for such Performance-Based Award upon the occurrence of a Change in Control.
4.Termination of Employment.
(a)Death or Disability. The Executive’s employment shall terminate automatically upon the Executive’s death during the Employment Period. If the Company determines in good faith that the Disability of the Executive has occurred during the Employment Period (pursuant to the definition of Disability set forth below), it may give to the Executive written notice in accordance with Section 13(c) of this Agreement of its intention to terminate the Executive’s employment. In such event, the Executive’s employment with the Company shall terminate effective on the 30th day after receipt of such notice by the Executive (the “Disability Effective Date”); provided, that, within the 30 days after such receipt, the Executive shall not have returned to full-time performance of the Executive’s duties. For purposes of this Agreement, “Disability” shall mean the Executive is unable to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment that can be expected to result in death or can be expected to last for a continuous period of not less than 12 months, as determined by a physician selected by the Company or its insurers and reasonably acceptable to the Executive or the Executive’s legal representative.
(b)Cause. Subject to Section 4(d), the Company may terminate the Executive’s employment during the Employment Period for Cause. For purposes of this Agreement, “Cause” shall mean (i) the willful failure of the Executive to perform substantially the Executive’s duties with the Company (other than any such failure resulting from incapacity due to physical or mental illness), which failure is not cured within 30 days after a written demand for substantial performance is delivered to the Executive by the Board which specifically identifies the manner in which the Board believes that the Executive has not substantially performed the Executive’s duties, or (ii) the willful engaging by the Executive in illegal conduct or gross misconduct which is materially and demonstrably injurious to the Company. For purposes of this provision, no act or failure to act, on the part of the Executive, shall be considered “willful” unless it is done, or omitted to be done, by the Executive in bad faith or without reasonable belief that the Executive’s action or omission was in the best interests of the Company.
(c)Good Reason. The Executive’s employment may be terminated by the Executive with or without Good Reason. For purposes of this Agreement, “Good Reason” shall mean one or more of the following conditions arising without the consent of the Executive:
(i)a material diminution in the Executive’s Annual Base Salary;
(ii)a material diminution in the Executive’s authority, duties, or responsibilities[; provided, that, for the avoidance of doubt, if at any time, the Executive shall cease to be the [ ] of the Company, the entity surviving any Change in Control (if not the Company) or person that ultimately controls the Company or such surviving entity, then a material diminution of the Executive’s authority, duties, or responsibilities shall be deemed have occurred]1;
(iii)a material diminution in the budget over which the Executive retains authority;
(iv)a change in the geographic location at which the Executive must perform services that results in an increase in the one-way commute of the Executive by more than 35 miles; or
(v)any other action or inaction that constitutes a material breach by the Company of this Agreement.
(d)Notice of Termination.
(i)Any termination by the Company for Cause, or by the Executive for Good Reason, shall be effected by Notice of Termination to the other party hereto given in accordance with Section 13(c) of this Agreement. For purposes of this Agreement, a “Notice of Termination” means a written notice which (A) indicates the specific termination provision in this Agreement relied upon, (B) to the extent applicable, sets forth in reasonable detail the facts and circumstances claimed to provide a basis for termination of the Executive’s employment under the provision so indicated and (C) if the Date of Termination (as defined below) is other than the date of receipt of such notice, specifies the termination date (which date shall be not more than thirty days after the giving of such notice). The failure by the Executive or the Company to set forth in the Notice of Termination any fact or circumstance which contributes to a showing of Good Reason or Cause shall not waive any right of the Executive or the Company, respectively, hereunder or preclude the Executive or the Company, respectively, from asserting such fact or circumstances in enforcing the Executive’s or the Company’s rights hereunder.
(ii)Any Notice of Termination for Cause must be given within 60 days of the Board learning of the event(s) or circumstance(s) which the Board believes constitute(s) Cause. Prior to any Notice of Termination for Cause being given (and prior to any termination for Cause being effective), the Executive shall be entitled to a hearing before the
1 Bracketed proviso only to be included for the Chief Financial Officer or General Counsel, and applicable title to be inserted.
Board at which the Executive may, at the Executive’s election, be represented by counsel and at which the Executive shall have a reasonable opportunity to be heard. Such hearing shall be held on not less than 15 days prior written notice to the Executive stating the Board’s intention to terminate the Executive for Cause and stating, in detail, the particular event(s) or circumstance(s) which the Board believes constitute(s) Cause for termination.
(iii)Any Notice of Termination for Good Reason must be given to the Company within 60 days of the initial existence of one or more conditions described in Section 4(c) which the Executive believes constitute(s) Good Reason. Upon such Notice of Termination for Good Reason, the Company shall be entitled to a period of 30 days during which it may remedy the condition(s) and not be required to pay benefits under this Agreement.
(e)Date of Termination. “Date of Termination” means (i) if the Executive’s employment is terminated by the Company for Cause, or by the Executive for Good Reason, the date of receipt of the Notice of Termination or any later date specified therein, as the case may be, subject, in the case of termination by the Company, for Cause, to the Company’s compliance with Section 4(d)(ii) and in the case of termination by the Executive for Good Reason, to the Executive’s compliance with Section 4(d)(iii); (ii) if the Executive’s employment is terminated by the Company other than for Cause or Disability, the Date of Termination shall be the date on which the Company notifies the Executive of such termination; and (iii) if the Executive’s employment is terminated by reason of death or Disability, the Date of Termination shall be the date of death of the Executive or the Disability Effective Date, as the case may be.
5.Obligations of the Company Upon Termination.
(a)Good Reason; Other Than for Cause, Death or Disability. If, during the Employment Period, the Company shall terminate the Executive’s employment other than for Cause, Death or Disability or the Executive shall terminate employment for Good Reason:
(i)the Company shall pay to the Executive in a lump sum in cash the following amounts, subject, in each case, to Sections 10, 11 and 12 hereof:
A.the sum of (1) the Executive’s Annual Base Salary through the Date of Termination to the extent not theretofore paid, (2) the product of (x) the target bonus for the then current fiscal year and (y) a fraction, the numerator of which is the number of days in the then current fiscal year through the Date of Termination, and the denominator of which is 365 and (3) any compensation previously deferred by the Executive (together with any accrued interest or earnings thereon) and any accrued vacation pay, in each case to the extent not theretofore paid (the sum of the amounts described in clauses (1), (2), and (3) shall be hereinafter referred to as the “Accrued Obligations”); and
B.the amount equal to the product of (1) two and (2) the sum of (x) the Executive’s Annual Base Salary and (y) the Average Annual Bonus. The Average Annual Bonus is equal to the average of the bonus paid (or payable) to the Executive for the three prior full fiscal years (or, if fewer, the number of full fiscal years the Executive was employed by the Company prior to the Effective Date); provided, that if the Executive was not eligible to participate in an annual bonus program for at least one full fiscal year, the Average Annual Bonus shall be the Executive’s target bonus for the year in which termination of employment occurs.
(ii)for 24 months after the Executive’s Date of Termination, or such longer period as may be provided by the terms of the appropriate plan, program, practice or policy, the Company shall continue benefits to the Executive and/or the Executive’s family at least equal to those which would have been provided to them in accordance with the plans, programs, practices and policies described in Section 3(b)(iv) of this Agreement (excluding any savings and/or retirement plans) if the Executive’s employment had not been terminated or, if more favorable to the Executive, as in effect generally at any time thereafter with respect to other peer executives of the Company and its Affiliated Companies and their families, provided, that if the Executive becomes reemployed with another employer and is eligible to receive medical or other welfare benefits under another employer-provided plan, the medical and other welfare benefits described herein shall be secondary to those provided under such other plan during such applicable period of eligibility. For purposes of determining eligibility (but not the time of commencement of benefits) of the Executive for retiree benefits pursuant to such plans, practices, programs and policies, the Executive shall be considered to have remained employed until 24 months after the Date of Termination and to have retired on the last day of such period;
(iii)to the extent not theretofore paid or provided, the Company shall timely pay or provide to the Executive any other amounts or benefits required to be paid or provided or which the Executive is eligible to receive under any plan, program, policy or practice or contract or agreement of the Company and its Affiliated Companies (such other amounts and benefits shall be hereinafter referred to as the “Other Benefits”); and
(iv)the Company shall timely reimburse the Executive up to $12,500 each year (an aggregate of $25,000) for expenses incurred in connection with outplacement services and relocation costs incurred in connection with obtaining new employment outside the state of their then-current principal residence until the earlier of (i) 24 months following the termination of Executive’s employment or (ii) the date the Executive secures full time employment.
(b)Death. If the Executive’s employment is terminated by reason of the Executive’s death during the Employment Period, this Agreement shall terminate without further obligations to the Executive’s legal representatives under this Agreement, other than for payment of Accrued Obligations and the timely payment or provision of Other Benefits. Accrued Obligations shall be paid to the Executive’s estate or beneficiary, as applicable, in a lump sum in cash within 30 days of the Date of Termination.
(c)Disability. If the Executive’s employment is terminated by reason of the Executive’s Disability during the Employment Period, this Agreement shall terminate without further obligations to the Executive, other than for payment of Accrued Obligations and the timely payment or provision of Other Benefits. Accrued Obligations shall be paid to the Executive in a lump sum in cash within 30 days of the Date of Termination.
(d)Cause; Other than for Good Reason. If the Executive’s employment shall be terminated for Cause during the Employment Period, this Agreement shall terminate without further obligations to the Executive other than the obligation to pay to the Executive (i) his or her Annual Base Salary through the Date of Termination, (ii) the amount of any compensation previously deferred by the Executive, and (iii) Other Benefits, in each case to the extent theretofore unpaid or not yet provided. If the Executive voluntarily terminates employment during the Employment Period, excluding a termination for Good Reason, this Agreement shall terminate without further obligations to the Executive, other than for Accrued Obligations and the timely payment or provision of Other Benefits. In such case, all Accrued Obligations shall be paid to the Executive in a lump sum in cash within 30 days of the Date of Termination.
(e)Time of Payment. Amounts payable under this Section 5 following an Executive’s termination of employment, other than those expressly payable on a deferred basis, will be paid in the payroll period next following the payroll period in which termination of employment occurs, except as otherwise provided in Sections 10, 11 or 12.
6.Non-exclusivity of Rights. Nothing in this Agreement shall prevent or limit the Executive’s continuing or future participation in any plan, program, policy or practice provided by the Company or any of its Affiliated Companies and for which the Executive may qualify, nor, subject to Section 13(g), shall anything herein limit or otherwise affect such rights as the Executive may have under any contract or agreement with the Company or any of its Affiliated Companies. Amounts which are vested benefits or which the Executive is otherwise entitled to receive under any plan, policy, practice or program of or any contract or agreement with the Company or any of its Affiliated Companies at or subsequent to the Date of Termination shall be payable in accordance with such plan, policy, practice or program or contract or agreement except as explicitly modified by this Agreement.
7.Full Settlement. The Company’s obligation to make the payments provided for in this Agreement and otherwise to perform its obligations hereunder shall not be affected by any set-off, counterclaim, recoupment, defense or other claim, right or action which the Company may have against the Executive (under this Agreement or otherwise) or others. In no event shall the Executive be obligated to seek other employment or take any other action by way of mitigation of the amounts payable to the Executive under any of the provisions of this Agreement and, except as otherwise provided in this Agreement, such amounts shall not be reduced whether or not the Executive obtains other employment.
8.Confidential Information. The Executive shall hold in a fiduciary capacity for the benefit of the Company all secret or confidential information, knowledge or data relating to the Company or any of its Affiliated Companies, and their respective businesses, which shall have been obtained by the Executive during the Executive’s employment by the Company or any of its Affiliated Companies and which shall not be or become public knowledge (other than by acts by the Executive or representatives of the Executive in violation of this Agreement) (“Confidential Information”). After termination of the Executive’s employment with the Company, the Executive shall not, without the prior written consent of the Company or as may otherwise be required by law or legal process, communicate or divulge any such information, knowledge or data to anyone other than the Company and those designated by it. In no event shall an asserted violation of the provisions of this Section 8 constitute a basis for deferring or withholding any amounts or benefits otherwise payable or to be provided to the Executive under this Agreement. Nothing in this Agreement, any invention and non-disclosure agreement executed by and between the Executive and the Company, any non-compete agreement executed by and between the Executive and the Company, or any policy or procedure of the Company, shall be construed to prevent disclosure of Confidential Information as may be required or permitted by applicable law or regulation; especially with respect to a federal or state administrative agency (e.g., Equal Employment Opportunity Commission, equivalent state employment agency, Securities and Exchange Commission, etc.) and including as part of filing a charge or complaint with such federal or state administrative agency, or pursuant to the valid order of a court of competent jurisdiction or an authorized government agency, provided that the disclosure does not exceed the extent of disclosure required or permitted by such law, regulation, or order. The Executive does not need the prior authorization of, or to provide notice to, any representative of the Company to file a charge or complaint with, or otherwise participate in an investigation or proceeding that may be commenced by, a federal or state administrative agency. With respect, specifically, to an order of a court of competent jurisdiction, Executive will promptly provide the General Counsel of the Company with written notice of any such order. If the Company chooses to seek a protective order or other remedy, Executive will cooperate fully with the Company. If the Company does not obtain a protective order or other remedy or waives compliance with certain provisions of this Agreement, Executive will furnish only that portion of the Confidential Information which, in the written opinion of counsel, is legally required to be disclosed and will use its best efforts to obtain assurances that confidential treatment will be accorded to such disclosed Confidential Information. In addition, nothing in this Agreement in any way prohibits or is intended to restrict or impede, and shall not be interpreted or understood as restricting or impeding, Executive from exercising Executive’s rights under Section 7 of the National Labor Relations Act (NLRA) or otherwise disclosing information as permitted by law. Nothing in this Agreement in any way prohibits or is intended to restrict or impede, the Executive’s right to receive an award from any federal or state administrative agency for information provided under any protected whistleblower or similar program.
9.Successors.
(a)This Agreement is personal to the Executive and without the prior written consent of the Company shall not be assignable by the Executive otherwise than by will or the laws of descent and distribution. This Agreement shall inure to the benefit of and be enforceable by the Executive’s legal representatives.
(b)This Agreement shall inure to the benefit of and be binding upon the Company and its successors and assigns.
(c)The Company will require any successor (whether direct or indirect, by purchase, merger, consolidation or otherwise) to all or substantially all of the business and/or assets of the Company to assume expressly and agree to perform this Agreement in the same manner and to the same extent that the Company would be required to perform it if no such succession had taken place. As used in this Agreement, “Company” shall mean the Company as hereinbefore defined and any successor to its business and/or assets as aforesaid.
10.Section 409A.
(a)This Agreement is intended to comply with Section 409A of the Code and the regulations promulgated thereunder (“Section 409A”) or an exemption thereunder and shall be construed and administered in accordance with Section 409A. Notwithstanding any other provision of this Agreement, payments provided under this Agreement may only be made upon an event and in a manner that complies with Section 409A or an applicable exemption. Any payments under this Agreement that may be excluded from Section 409A either as separation pay due to an involuntary separation from service or as a short-term deferral shall be excluded from Section 409A to the maximum extent possible. For purposes of Section 409A, each installment payment provided under this Agreement shall be treated as a separate payment. Any payments to be made under this Agreement upon a termination of employment shall only be made upon a “separation from service” under Section 409A. Notwithstanding the foregoing, the Company makes no representations that the payments and benefits provided under this Agreement comply with Section 409A, and in no event shall the Company be liable for all or any portion of any taxes, penalties, interest, or other expenses that may be incurred by the Executive on account of non-compliance with Section 409A.
(b)Notwithstanding any other provision of this Agreement, if any payment or benefit provided to the Executive in connection with the Executive’s termination of employment is determined to constitute “nonqualified deferred compensation” within the meaning of Section 409A and the Executive is determined to be a “specified employee” within the meaning of Section 409A, then such payment or benefit shall not be paid until the first payroll date following the date that is six months from the Termination Date or, if earlier, on the Executive’s death (the “Specified Employee Payment Date”). The aggregate of any payments that would otherwise have been paid before the Specified Employee Payment Date shall be paid to the Executive in a lump sum on the Specified Employee Payment Date and thereafter, any remaining payments shall be paid without delay in accordance with their original schedule.
(c)To the extent required by Section 409A, each reimbursement or in-kind benefit provided under this Agreement shall be provided in accordance with the following: (i) the amount of expenses eligible for reimbursement, or in-kind benefits provided, during each calendar year cannot affect the expenses eligible for reimbursement, or in-kind benefits to be provided, in any other calendar year; (ii) any reimbursement of an eligible expense shall be paid to the Executive on or before the last day of the calendar year following the calendar year in which the expense was incurred; and (iii) any right to reimbursements or in-kind benefits under this Agreement shall not be subject to liquidation or exchange for another benefit.
11.Section 280G. The Executive hereby agrees to the terms set forth in Exhibit A to this Agreement.
12.Release. As a condition of receipt of any payments or benefits under Section 5 of this Agreement, the Executive (or, in the event of the Executive’s termination due to death or Disability, the Executive’s estate, beneficiaries or other representatives, as applicable) shall be required to sign a customary release prepared by and provided by the Company (the “Release”) and to abide by the provisions thereof. The Release shall contain a release and waiver of any claims the Executive or the Executive’s estate, beneficiaries and other representatives may have against the Company and its officers, directors, affiliates and/or representatives, and shall release those entities and persons from any liability for such claims including, but not limited to, all employment discrimination claims. Except as otherwise provided in Section 10, payments and benefits under Section 5 of this Agreement will be paid on the 90th day following the Executive’s termination of employment, provided that the Executive has executed and submitted the Release and the statutory period during which the Executive is entitled to revoke the Release has expired on or before that 90th day without the Executive revoking the Release. Notwithstanding anything to the contrary herein, if the Executive fails to timely execute and submit the Release or the Executive revokes the Release after its timely execution and submission, the Executive’s right to receive any payments or benefits under Section 5 of this Agreement will be forfeited.
13.Miscellaneous.
(a)This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware, without reference to principles of conflict of laws. The captions of this Agreement are not part of the provisions hereof and shall have no force or effect.
(b)This Agreement may not be amended or modified otherwise than by a written agreement executed by the parties hereto or their respective successors and legal representatives.
(c)All notices and other communications hereunder shall be in writing and shall be given by hand delivery to the other party or by registered or certified mail, return receipt
requested, postage prepaid, or by email, read receipt requested, addressed as follows (or such other addresses as specified by the parties by like notice):
If to the Executive:
at the address and e-mail on file in the Company’s records.
If to the Company:
IDEXX Laboratories, Inc.
One IDEXX Drive
Westbrook, ME 04092
Attention: General Counsel
Email: GeneralCounsel@idexx.com
Notice and communications shall be effective when actually received by the addressee.
(d)The invalidity or unenforceability of any provision of this Agreement shall not affect the validity or enforceability of any other provision of this Agreement.
(e)The Company may withhold from any amounts payable under this Agreement such Federal, state, local or foreign taxes as shall be required to be withheld pursuant to any applicable law or regulation.
(f)The Executive’s or the Company’s failure to insist upon strict compliance with any provision of this Agreement or the failure to assert any right the Executive or the Company may have hereunder, including, without limitation the right of the Executive to terminate employment for Good Reason pursuant to Section 4(c) of this Agreement, shall not be deemed to be a waiver of such provision or right or any other provision or right of this Agreement.
(g)The Executive and the Company acknowledge that, except as may otherwise be provided under any other written agreement between the Executive and the Company, the employment of the Executive by the Company is “at will” and, subject to Section 1(a) hereof, prior to the Effective Date, the Executive’s employment and/or this Agreement may be terminated by either the Executive or the Company, by written notice to the other, at any time prior to the Effective Date, in which case the Executive shall have no further rights or obligations under this Agreement. From and after the Effective Date, this Agreement shall supersede any other agreement between the parties with respect to the subject matter hereof.
(h)Any dispute, controversy or claim arising under or in connection with this Agreement shall be settled exclusively by arbitration, conducted before a panel of three arbitrators in Portland, Maine, in accordance with the rules of the American Arbitration Association then in effect. Judgment may be entered on the arbitrator’s award in any court of competent jurisdiction. The Company and the Executive shall separately pay for their respective counsel fees and expenses and the arbitration panel shall allocate the costs and expenses of the arbitration between the Executive and the Company; provided, that if the Executive substantially prevails on a material item that was subject to arbitration, the Company shall bear all expenses and other costs of the arbitration and all reasonable attorneys’ fees and expenses borne by the Executive.
(i)This Agreement constitutes the entire agreement between the parties with respect to the subject matter of this Agreement and, except as otherwise provided herein, supersedes all prior communications, agreements and understandings, written or oral, with the Company or any of its affiliates or predecessors with respect to the terms and conditions of the Executive’s employment. Notwithstanding the provisions of the preceding sentence, this Agreement does not supersede any agreement between the Executive and the Company regarding non-disclosure and developments or any non-competition agreement between the Executive and the Company. In addition, the Executive shall remain subject to the post-termination non-compete obligations under any non-compete agreement with the Company notwithstanding any terms of such agreement that would relieve the Executive of such obligations upon termination of the Executive’s employment with the Company other than for Cause.
(j)This Agreement may be executed by .pdf or facsimile signatures, or any other electronic signatures (including without limitation DocuSign or AdobeSign), in any number of counterparts, each of which shall be deemed an original, but all such counterparts shall together constitute one and the same instrument.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, the Executive has hereunto set the Executive’s hand and, pursuant to authorization from the Committee, the Company has caused these presents to be executed in its name on its behalf, all as of the day and year first above written.
EXECUTIVE:
[NAME]
COMPANY:
IDEXX Laboratories, Inc.
By:
Name:
Title:
[Signature Page to Change in Control Agreement]
Exhibit A
Section 280G
This Exhibit A sets forth the terms and provisions applicable to the Executive as referenced in Section 11 of the agreement to which this Exhibit A is attached (the “Agreement”). This Exhibit A shall be subject in all respects to the terms and conditions of the Agreement. All capitalized terms that are used but not defined in this Exhibit A shall have the meanings ascribed to such terms in the Agreement.
(a) If the Executive would otherwise be eligible to receive a payment or benefit pursuant to the terms of the Agreement or any equity or equity-based compensation or other agreement with the Company or any subsidiary or otherwise in connection with, or arising out of, the Executive’s employment with the Company or any subsidiary or a change in ownership or effective control of the Company or of a substantial portion of its assets (any such payment or benefit, a “Parachute Payment”), that a nationally recognized United States public accounting firm selected by the Company (the “Accounting Firm”) determines, but for this sentence, would be subject to excise tax imposed by Section 4999 of the Code (the “Excise Tax”), subject to clause (c) below, then the Company shall pay to the Executive whichever of the following two alternative forms of payment would result in the Executive’s receipt, on an after-tax basis, of the greater amount of the Parachute Payment notwithstanding that all or some portion of the Parachute Payment may be subject to the Excise Tax: (1) payment in full of the entire amount of the Parachute Payment, or (2) payment of only a part of the Parachute Payment so that the Executive receives the largest payment possible without the imposition of the Excise Tax.
(b) If a reduction in the Parachute Payment is necessary pursuant to clause (a), then the reduction shall occur in the following order: (1) reduction of cash payments (with such reduction being applied to the payments in the reverse order in which they would otherwise be made, that is, later payments shall be reduced before earlier payments) and (2) cancellation of acceleration of vesting of equity or equity-based awards; provided, that to the extent permitted by Section 409A and Sections 280G and 4999 of the Code, if a different reduction procedure would be permitted without violating Section 409A or losing the benefit of the reduction under Sections 280G and 4999 of the Code, the Executive may designate a different order of reduction.
(c) For purposes of determining whether any of the Parachute Payments (collectively, the “Total Payments”) will be subject to the Excise Tax and the amount of such Excise Tax, (i) the Total Payments shall be treated as “parachute payments” within the meaning of Section 280G(b)(2) of the Code, and all “parachute payments” in excess of the “base amount” (as defined under Section 280G(b)(3) of the Code) shall be treated as subject to the Excise Tax, unless and except to the extent that, in the opinion of the Accounting Firm, such Total Payments (in whole or in part): (1) do not constitute “parachute payments,” (2) represent reasonable compensation for services actually rendered within the meaning of Section 280G(b)(4) of the Code in excess of the “base amount,” or (3) are otherwise not subject to the Excise Tax, and (ii) the value of any non-cash benefits or any deferred payment or benefit shall be determined by the Accounting Firm in accordance with the principles of Section 280G of the Code.
(d) All determinations hereunder shall be made by the Accounting Firm, which determinations shall be final and binding upon the Company and the Executive.
(e) The federal tax returns filed by the Executive (and any filing made by a consolidated tax group which includes the Company) shall be prepared and filed on a basis consistent with the determination of the Accounting Firm with respect to the Excise Tax payable by the Executive. The Executive shall make proper payment of the amount of any Excise Tax, and at the request of the Company, provide to the Company true and correct copies (with any amendments) of the Executive’s federal income tax return as filed with the Internal Revenue Service, and such other documents reasonably requested by the Company, evidencing such payment (provided, that the Executive may delete information unrelated to the Parachute Payment or the Excise Tax and provided, further, that the Company at all times shall treat such returns as confidential and use such return only for purpose contemplated by this paragraph).
(f) In the event of any controversy with the Internal Revenue Service (or other taxing authority) with regard to the Excise Tax, the Executive shall permit the Company to control issues related to the Excise Tax (at its expense). In the event that the issues are interrelated to the Excise Tax, the Executive and the Company shall cooperate in good faith so as not to jeopardize resolution of either issue. In the event of any conference with any taxing authority as to the Excise Tax or associated income taxes, the Executive shall permit a representative of the Company to accompany the Executive, and the Executive and the Executive’s representative shall cooperate in good faith with the Company and its representative.
(g) The Company shall be responsible for all charges of the Accounting Firm.
(h) The Company and the Executive shall promptly deliver to each other copies of any written communications, and summaries of any verbal communications, with any taxing authority regarding the Excise Tax covered by this Exhibit A.
(i) The provisions of this Exhibit A shall survive the termination of the Executive’s employment with the Company for any reason and the termination of the Agreement.
EX-19.1
7
exhibit191.htm
EX-19.1
Document
EXHIBIT 19.1
Updated as of January 1, 2025
INSIDER TRADING POLICY
From time to time, employees1 and members of the Board of Directors of IDEXX Laboratories, Inc. (“Board Members”) may possess or have access to material information concerning the business or prospects of IDEXX Laboratories, Inc. and its subsidiaries (collectively, “IDEXX” or the “Company”) that, for valid business reasons, has not yet been disclosed to the public. If an employee or Board Member engages in any transaction in IDEXX securities when aware of or in possession of material, nonpublic information concerning IDEXX, he, she or they may have violated the federal securities laws and may be subject to civil and criminal penalties (up to and including imprisonment), including the possibility of monetary damages, which, in certain instances, can be tripled. This potential liability applies to all employees of IDEXX, not just to our Board Members, officers or senior managers. In addition, litigation involving charges of insider trading can result in adverse publicity, embarrassment and reputational harm for IDEXX and its Board Members and employees.
IDEXX has adopted this Insider Trading Policy to help its employees and Board Members comply with the insider trading prohibitions under the federal securities laws, to prevent even the appearance of improper insider trading and to protect IDEXX’s reputation for integrity and ethical conduct.
Insider Trading Policy Statement
It is IDEXX’s policy that no employee or Board Member may:
•buy, sell or otherwise trade, directly or indirectly, in IDEXX securities while aware or in possession of any material, nonpublic information concerning IDEXX;
•“tip” or provide any material, nonpublic information concerning IDEXX to another person unless required as part of that employee’s regular duties for IDEXX and authorized by the General Counsel; or
•buy, sell or otherwise trade, directly or indirectly, in securities of any other company (including, without limitation, a current or prospective IDEXX customer, supplier, joint venture participant, partner, or party to a potential transaction) while aware or in possession
1 For purposes of this Insider Trading Policy, “employees” include any and all permanent employees and temporary employees (including interns, seasonal employees and employees on probationary periods), whether full-time or part-time, of IDEXX Laboratories, Inc. or any of its subsidiaries and certain IDEXX contractors, consultants or other third parties as the General Counsel may determine should be subject to this Insider Trading Policy.
of any material, nonpublic information concerning that other company and that was obtained in the course of his, her or their employment, service or relationship with IDEXX.2
There is no exception to this policy, even for hardship (such as personal financial emergency).
If a person ceases to be an employee or a Board Member at a time when he, she or they are aware or in possession of material, nonpublic information concerning IDEXX or another company, the prohibition on buying, selling or otherwise trading, directly or indirectly, in IDEXX securities or securities of that other company shall continue to apply to such person until that information has become public or is no longer material. In all other respects, however, the procedures set forth in the Addendum to this Insider Trading Policy will cease to apply to a Board Member’s or employee’s transactions in IDEXX securities upon the expiration of any quiet period that is applicable to his, her or their transactions at the time he, she or they cease to be a Board Member or employee.
Certain Exceptions
The prohibition on buying, selling or otherwise trading, directly or indirectly, in IDEXX securities while aware or in possession of any material, nonpublic information concerning IDEXX does not apply to:
•the exercise of stock options (i.e., purchase of the underlying IDEXX shares), although this exception does not apply to a cashless exercise/same-day sale of stock options or a sale of shares obtained upon the exercise of stock options, each of which is subject to the prohibition;
•the purchase of IDEXX shares through participation in IDEXX’s employee stock purchase plan;
•the receipt of IDEXX shares issued upon the vesting of restricted stock units;
•the withholding of otherwise issuable IDEXX shares to satisfy tax withholding requirements upon the vesting of restricted stock units;
•the receipt of a distribution of deferred stock units in the form of IDEXX shares in accordance with an IDEXX deferred compensation plan; and
•trades made pursuant to a Rule 10b5-1 trading plan that complies with the requirements described under “Approved 10b5-1 Plans” in the Addendum to this Insider Trading Policy.
Who Is Required to Comply with the IDEXX Insider Trading Policy?
All IDEXX employees located anywhere in the world and all Board Members are required to comply with this Insider Trading Policy, regardless of whether they own any IDEXX securities (such as IDEXX stock, stock options or restricted stock units).
2 For example, an IDEXX employee may obtain in the course of his, her or their employment with IDEXX information about a major contract being negotiated with the other company. It is important to remember that information that is not material to IDEXX may nevertheless be material to the other company.
This Insider Trading Policy also applies to any family member of an employee or Board Member sharing the same address or who is financially dependent upon such employee or Board Member and to any corporation, partnership, trust or other entity owned or controlled by an employee or Board Member or such a family member of an employee or Board Member.
Definition of Material, Nonpublic Information or Inside Information
Information is considered to be “material” if there is a substantial likelihood that a reasonable investor would consider it important in deciding whether to buy, sell or hold IDEXX securities.
Some examples of the types of information that may be considered “material” include:
•revenue or earnings estimates;
•a significant merger or acquisition;
•a product launch or delay or other significant product development, invention or discovery;
•securities offerings;
•a significant cybersecurity breach at IDEXX; and
•significant developments relating to a major contract.
“Nonpublic information” is any information that has not been broadly disseminated to the investing public. We consider information to have been broadly disseminated to the investing public when:
•it has been publicly disclosed through an appropriate channel (such as an SEC filing or a press release distributed to national wire services); and
•a sufficient amount of time has elapsed since the public disclosure to permit the investing public to absorb and evaluate the information.
When IDEXX information is material and has not been broadly disseminated to the investing public, it is called “material, nonpublic information” or “inside information.”
The most dangerous time to engage in transactions in IDEXX securities is in advance of the public release of inside information relating to IDEXX, such as quarterly or year-end results or other important news, and the safest time is the period shortly following the release and publication of such information. Even after such information has been released to the public, it is important to wait until a sufficient amount of time has elapsed for the information to be absorbed and evaluated by the investing public.
If you are unsure whether any information constitutes material, nonpublic information concerning IDEXX or any other company, please contact the General Counsel’s office at GeneralCounsel@idexx.com.
Maintain the Confidentiality of All Nonpublic Information
For competitive, security and other business reasons, as well as to comply with federal securities laws, all individuals subject to this Insider Trading Policy must maintain the confidentiality of any nonpublic or confidential information obtained during the course of your employment or affiliation with IDEXX.
To comply with this requirement, unless expressly authorized by the General Counsel in writing or in compliance with an effective, Company-approved non-disclosure or confidentiality agreement, do not disclose any nonpublic or confidential information obtained during the course of your employment or affiliation with IDEXX:
•to any other IDEXX employee, unless that employee has a need to know such information and is otherwise authorized or permitted to know such information;
•to any person who is not an IDEXX employee or Board Member – including, but not limited to, any friends, family, social acquaintances, business acquaintances or “expert network firms”; or
•by posting or publishing any such information on social media – including, but not limited to, Facebook, Twitter or Instagram.
As noted above, you are not permitted to provide information to “expert network firms.” These firms seek industry sources to arrange consultations with their clients, which can include private equity funds, hedge funds and other institutional investors who are considering investment in our industry. Expert network firms may seek to engage you as a consultant due to your knowledge of IDEXX, our overall industry or the market. Your provision of consulting services to any “expert network firm” creates the risk that you will use or disclose nonpublic or confidential information in violation of this Insider Trading Policy and may represent a conflict of interest under the IDEXX Code of Ethics. As a result, you may not act as a consultant for any expert network firm.
Consequences of Any Violation of the Insider Trading Policy
In addition to any civil and criminal penalties (up to and including imprisonment) that may be imposed due to any violation of federal securities laws, any IDEXX employee or Board Member who violates this Insider Trading Policy shall become subject to disciplinary action, up to and including termination of employment.
IDEXX Securities Transactions by IDEXX
From time to time, IDEXX engages in transactions in IDEXX securities, and it is IDEXX’s policy to comply with applicable securities laws when engaging in such transactions.
Questions
If employees or Board Members have any questions about this Insider Trading Policy or are unsure whether a transaction in IDEXX securities or securities of another company will inadvertently give rise to an insider trading violation or a violation of this Insider Trading Policy, they should contact the General Counsel’s Office for guidance at GeneralCounsel@idexx.com.
EX-21
8
exhibit21-202410xk.htm
EX-21
Document
Exhibit 21
|
|
|
|
|
|
| IDEXX LABORATORIES, INC. |
| SUBSIDIARIES OF THE REGISTRANT |
|
|
| Legal Entity Name |
Jurisdiction of Incorporation or Formation |
|
|
| 342 Saco Street, LLC |
Maine |
| Beijing IDEXX Laboratories Co. Limited |
China |
| IDEXX Animana B.V. |
Netherlands |
| IDEXX Brasil Laboratórios Ltda. |
Brazil |
| IDEXX Brasil Participações Ltda. |
Brazil |
| IDEXX Diagnostic Limited |
Ireland |
| IDEXX Diavet AG |
Schwyz |
| IDEXX Distribution, Inc. |
Massachusetts |
| IDEXX GmbH |
Germany |
| IDEXX B.V. |
Netherlands |
| IDEXX Holding GmbH |
Germany |
| IDEXX Holdings GmbH |
Zug |
| IDEXX Holdings II GmbH |
Zug |
| IDEXX Holdings, Inc. |
Delaware |
| IDEXX Laboratories II (NZ) ULC |
New Zealand |
| IDEXX Laboratories (Proprietary) Limited |
South Africa |
| IDEXX Laboratories (Shanghai) Company Limited |
China |
| IDEXX Laboratories B.V. |
Netherlands |
| IDEXX Laboratories Canada 1, ULC |
Nova Scotia |
| IDEXX Laboratories Canada 2, ULC |
Nova Scotia |
| IDEXX Laboratories Canada Corporation |
Nova Scotia |
| IDEXX Laboratories Canada LP |
Ontario |
| IDEXX Laboratories Co., Ltd. |
Thailand |
| IDEXX Laboratories Danmark ApS |
Denmark |
| IDEXX Laboratories GmbH |
Zug |
| IDEXX Laboratories Hong Kong Limited |
Hong Kong |
| IDEXX Laboratories Inc. |
Taiwan Province of China |
| IDEXX Laboratories Italia S.r.l. |
Italy |
| IDEXX Laboratories, KK |
Japan |
| IDEXX Laboratories Limited |
England & Wales |
| IDEXX Laboratories Ltd. |
Korea, Republic of |
| IDEXX Laboratories Norge AS |
Norway |
| IDEXX Laboratories Oy |
Finland |
| IDEXX Laboratories Philippines, Inc. |
Philippines |
| IDEXXLAB, Unipessoal Lda. |
Portugal |
|
|
|
|
|
|
| IDEXX Laboratories Private Limited |
India |
| IDEXX Laboratories Pty Limited |
New South Wales |
| IDEXX Laboratories Singapore Pte. Ltd. |
Singapore |
| IDEXX Laboratories Sp. z o.o. |
Poland |
| IDEXX Laboratories SRL |
Belgium |
| IDEXX Laboratories Sverige AB |
Sweden |
| IDEXX Laboratories, S.de R.L. de C.V. |
Mexico |
| IDEXX Laboratories s.r.o. |
Czech Republic |
| IDEXX Laboratories Slovakia s.r.o. |
Slovak Republic |
| IDEXX Laboratorios, S.L. |
Spain |
| IDEXX Montpellier SAS |
France |
| IDEXX Operations, Inc. |
Delaware |
| IDEXX Pharmaceuticals, LLC |
Delaware |
| IDEXX Real Estate Holdings GmbH |
Germany |
| IDEXX Real Estate Holdings, LLC |
Maine |
| IDEXX SARL |
France |
| IDEXX Switzerland GmbH |
Bern/Berne |
| IDEXX Technologies GmbH |
Bern/Berne |
| IDEXX Technologies Limited |
England & Wales |
| IDEXX UK Acquisition Limited |
England & Wales |
| IDEXX Vet Med Labor GmbH |
Austria |
| Laboratoire IDEXX SARL |
France |
| labor-zentral.ch AG für veterinärmedizinische Diagnostik |
Switzerland |
| OPTI Medical Systems, Inc. |
Delaware |
| Vet Med Labor GmbH |
Germany |
EX-23
9
idexxfy24consent.htm
EX-23
Document
Exhibit 23
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in the Registration Statements on Form S-3 (Nos. 333-36007 and 333-70846) and Form S-8 (Nos. 333-11201, 333-11199, 333-36009, 333-56685, 333-78765, 333-78769, 333-43172, 333-43170, 333-27733, 333-105426, 333-108905, 333-108906, 333-146479, 333-160083, 333-160085, 333-193136 and 333-204418) of IDEXX Laboratories, Inc. of our report dated February 21, 2025 relating to the financial statements and the effectiveness of internal control over financial reporting, which appears in this Form 10-K.
|
|
|
|
|
|
| /s/PricewaterhouseCoopers LLP |
|
| Boston, Massachusetts |
|
| February 21, 2025 |
|
EX-31.1
10
exhibit31124.htm
EX-31.1
Document
Exhibit 31.1
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Jonathan J. Mazelsky, certify that:
1)I have reviewed this report on Form 10-K for the year ended December 31, 2024 of IDEXX Laboratories, Inc.;
2)Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3)Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4)The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report, based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5)The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Date: February 21, 2025 |
/s/ Jonathan J. Mazelsky |
|
Jonathan J. Mazelsky |
|
President and Chief Executive Officer |
|
(Principal Executive Officer)
|
EX-31.2
11
exhibit31224.htm
EX-31.2
Document
Exhibit 31.2
CERTIFICATION PURSUANT TO
RULES 13a-14(a) AND 15d-14(a) UNDER THE SECURITIES EXCHANGE ACT OF 1934,
AS ADOPTED PURSUANT TO SECTION 302 OF THE SARBANES-OXLEY ACT OF 2002
I, Brian P. McKeon, certify that:
1)I have reviewed this report on Form 10-K for the year ended December 31, 2024 of IDEXX Laboratories, Inc.;
2)Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3)Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4)The registrant’s other certifying officer and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a) Designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b) Designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c) Evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report, based on such evaluation; and
d) Disclosed in this report any change in the registrant’s internal control over financial reporting that occurred during the registrant’s most recent fiscal quarter (the registrant’s fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant’s internal control over financial reporting; and
5)The registrant’s other certifying officer and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a) All significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b) Any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
|
|
|
|
|
|
| Date: February 21, 2025 |
/s/ Brian P. McKeon |
|
Brian P. McKeon |
|
Executive Vice President, Chief Financial Officer |
|
and Treasurer |
|
(Principal Financial Officer)
|
EX-32.1
12
exhibit32124.htm
EX-32.1
Document
Exhibit 32.1
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350
AS ADOPTED BY
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the report on Form 10-K of IDEXX Laboratories, Inc. (the “Company”) for the year ended December 31, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned certifies, pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
/s/ Jonathan J. Mazelsky |
| February 21, 2025 |
Jonathan J. Mazelsky
President and Chief Executive Officer |
A signed original of this written statement required by Section 906, has been provided to IDEXX Laboratories, Inc. and will be retained by IDEXX Laboratories, Inc. and furnished to the Securities and Exchange Commission or its staff upon request.
EX-32.2
13
exhibit32224.htm
EX-32.2
Document
Exhibit 32.2
CERTIFICATION PURSUANT TO 18 U.S.C. SECTION 1350
AS ADOPTED BY
SECTION 906 OF THE SARBANES-OXLEY ACT OF 2002
In connection with the report on Form 10-K of IDEXX Laboratories, Inc. (the “Company”) for the year ended December 31, 2024 as filed with the Securities and Exchange Commission on the date hereof (the “Report”), the undersigned certifies, pursuant to 18 U.S.C. Section 1350, as adopted by Section 906 of the Sarbanes-Oxley Act of 2002, that:
(1)The Report fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934; and
(2)The information contained in the Report fairly presents, in all material respects, the financial condition and results of operations of the Company.
|
|
|
|
|
|
|
/s/ Brian P. McKeon |
| February 21, 2025 |
Brian P. McKeon
Executive Vice President, Chief Financial Officer
and Treasurer |
A signed original of this written statement required by Section 906, has been provided to IDEXX Laboratories, Inc. and will be retained by IDEXX Laboratories, Inc. and furnished to the Securities and Exchange Commission or its staff upon request.